User login
Psychiatrist sentenced to 11 years for sledgehammer attack against another psychiatrist
of her child’s father, who is also a psychiatrist.
Pamela Buchbinder pled guilty to first-degree burglary and assault on September 7, almost exactly 10 years after the November 2012 attack on Michael Weiss, MD. Weiss was beaten with a sledgehammer and stabbed multiple times but survived the attack.
The September plea deal was announced by the Manhattan district attorney, who said that Ms. Buchbinder acknowledged she had enlisted the help of her then-19-year-old cousin Jacob Nolan to kill Dr. Weiss. Ms. Buchbinder was in a custody battle with Dr. Weiss over their then-5-year-old child.
At the Oct. 11 sentencing, Ms. Buchbinder and her defense attorney attempted to withdraw that plea. NBC4 New York reported that Buchbinder claimed she was not in her right mind on the day of the plea because she had received a “contact high” from others in her holding cell who were using synthetic marijuana and that she had not taken her prescribed medications that day.
The judge did not entertain the request and proceeded with the sentencing.
Ms. Buchbinder has been held at Rikers Island prison, in East Elmhurst, N.Y., since she was arrested in 2017, so has already served 5 years of her 11-year sentence. She must also serve 5 years of postrelease probation.
Insurance policy beneficiary
Ms. Buchbinder’s cousin was convicted of second-degree attempted murder in 2016 and was sentenced to 9.5 years in prison.
In a 2017 interview with CBS News, Mr. Nolan, who said he was “bipolar,” claimed Ms. Buchbinder had manipulated him into trying to kill her child’s father by telling him “horror stories” about Weiss. Soon after the interview, Ms. Buchbinder was arrested.
In 2022, the New York Post reported that Ms. Buchbinder had been named a beneficiary of Dr. Weiss’ $1.5 million life insurance policy several days before the attack.
Prosecutors had surveillance footage of Ms. Buchbinder with Nolan at a Manhattan hardware store purchasing the sledgehammer. According to the CBS report, at the time of her arrest, she also was apparently preparing to flee.
She was denied bail and has been held at Rikers Island since her arrest.
Ms. Buchbinder was licensed to practice in New York in 1999. In April 2018, the New York State Board for Professional Medical Conduct issued an interim order that precluded her from practicing medicine in New York.
The interim order will be in effect until the board completes its investigation. As of press time, the board had not updated its files.
A version of this article first appeared on Medscape.com.
of her child’s father, who is also a psychiatrist.
Pamela Buchbinder pled guilty to first-degree burglary and assault on September 7, almost exactly 10 years after the November 2012 attack on Michael Weiss, MD. Weiss was beaten with a sledgehammer and stabbed multiple times but survived the attack.
The September plea deal was announced by the Manhattan district attorney, who said that Ms. Buchbinder acknowledged she had enlisted the help of her then-19-year-old cousin Jacob Nolan to kill Dr. Weiss. Ms. Buchbinder was in a custody battle with Dr. Weiss over their then-5-year-old child.
At the Oct. 11 sentencing, Ms. Buchbinder and her defense attorney attempted to withdraw that plea. NBC4 New York reported that Buchbinder claimed she was not in her right mind on the day of the plea because she had received a “contact high” from others in her holding cell who were using synthetic marijuana and that she had not taken her prescribed medications that day.
The judge did not entertain the request and proceeded with the sentencing.
Ms. Buchbinder has been held at Rikers Island prison, in East Elmhurst, N.Y., since she was arrested in 2017, so has already served 5 years of her 11-year sentence. She must also serve 5 years of postrelease probation.
Insurance policy beneficiary
Ms. Buchbinder’s cousin was convicted of second-degree attempted murder in 2016 and was sentenced to 9.5 years in prison.
In a 2017 interview with CBS News, Mr. Nolan, who said he was “bipolar,” claimed Ms. Buchbinder had manipulated him into trying to kill her child’s father by telling him “horror stories” about Weiss. Soon after the interview, Ms. Buchbinder was arrested.
In 2022, the New York Post reported that Ms. Buchbinder had been named a beneficiary of Dr. Weiss’ $1.5 million life insurance policy several days before the attack.
Prosecutors had surveillance footage of Ms. Buchbinder with Nolan at a Manhattan hardware store purchasing the sledgehammer. According to the CBS report, at the time of her arrest, she also was apparently preparing to flee.
She was denied bail and has been held at Rikers Island since her arrest.
Ms. Buchbinder was licensed to practice in New York in 1999. In April 2018, the New York State Board for Professional Medical Conduct issued an interim order that precluded her from practicing medicine in New York.
The interim order will be in effect until the board completes its investigation. As of press time, the board had not updated its files.
A version of this article first appeared on Medscape.com.
of her child’s father, who is also a psychiatrist.
Pamela Buchbinder pled guilty to first-degree burglary and assault on September 7, almost exactly 10 years after the November 2012 attack on Michael Weiss, MD. Weiss was beaten with a sledgehammer and stabbed multiple times but survived the attack.
The September plea deal was announced by the Manhattan district attorney, who said that Ms. Buchbinder acknowledged she had enlisted the help of her then-19-year-old cousin Jacob Nolan to kill Dr. Weiss. Ms. Buchbinder was in a custody battle with Dr. Weiss over their then-5-year-old child.
At the Oct. 11 sentencing, Ms. Buchbinder and her defense attorney attempted to withdraw that plea. NBC4 New York reported that Buchbinder claimed she was not in her right mind on the day of the plea because she had received a “contact high” from others in her holding cell who were using synthetic marijuana and that she had not taken her prescribed medications that day.
The judge did not entertain the request and proceeded with the sentencing.
Ms. Buchbinder has been held at Rikers Island prison, in East Elmhurst, N.Y., since she was arrested in 2017, so has already served 5 years of her 11-year sentence. She must also serve 5 years of postrelease probation.
Insurance policy beneficiary
Ms. Buchbinder’s cousin was convicted of second-degree attempted murder in 2016 and was sentenced to 9.5 years in prison.
In a 2017 interview with CBS News, Mr. Nolan, who said he was “bipolar,” claimed Ms. Buchbinder had manipulated him into trying to kill her child’s father by telling him “horror stories” about Weiss. Soon after the interview, Ms. Buchbinder was arrested.
In 2022, the New York Post reported that Ms. Buchbinder had been named a beneficiary of Dr. Weiss’ $1.5 million life insurance policy several days before the attack.
Prosecutors had surveillance footage of Ms. Buchbinder with Nolan at a Manhattan hardware store purchasing the sledgehammer. According to the CBS report, at the time of her arrest, she also was apparently preparing to flee.
She was denied bail and has been held at Rikers Island since her arrest.
Ms. Buchbinder was licensed to practice in New York in 1999. In April 2018, the New York State Board for Professional Medical Conduct issued an interim order that precluded her from practicing medicine in New York.
The interim order will be in effect until the board completes its investigation. As of press time, the board had not updated its files.
A version of this article first appeared on Medscape.com.
PsA Guidelines
Randomized, Double-Blind Placebo-Controlled Trial to Assess the Effect of Probiotics on Irritable Bowel Syndrome in Veterans With Gulf War Illness
About 700,000 US military personnel were deployed in Operation Desert Storm (August 1990 to March 1991).1 Almost 30 years since the war, a large number of these veterans continue to experience a complex of symptoms of unknown etiology called Gulf War illness (GWI), which significantly affects health and quality of life (QOL). The lack of clear etiology of the illness has impaired research to find specific treatments and has further exacerbated the stress among veterans. GWI typically includes a mixture of chronic headache, cognitive difficulties, widespread pain, unexplained fatigue, memory and concentration problems, as well as chronic respiratory and gastrointestinal (GI) symptoms.2 Abdominal pain and alteration of bowel habits are also symptoms typical of irritable bowel syndrome (IBS). It has been estimated that IBS occurs in up to 30% of Gulf War veterans.3
The etiology of IBS is unknown. Possible mechanisms include visceral hypersensitivity, altered gut motor function, aberrant brain-gut interaction, and psychological factors, perhaps with a genetic predisposition.4 Gastroenteritis has been reported as a triggering mechanism in up to one-third of patients with IBS.5 Gastroenteritis can alter the gut microbiota and has been reported to be a significant risk factor for the development of IBS.6 In one study of Operation Desert Shield soldiers, > 50% of military personnel developed acute gastroenteritis while on duty.7 A high prevalence of extra-intestinal symptoms also has been reported, including fatigue, headache, joint pains, and anxiety, in Gulf War veterans with IBS. These extra-intestinal symptoms of IBS are consistent with the reported GWI symptoms. Change in gut microbiota also has been associated with many of the extra-intestinal symptoms of IBS, especially fatigue.8,9 Gut microbiota are known to change with travel, stress, and a change in diet, all potential factors that are relevant to Gulf War veterans. This would suggest that an imbalance in the gut microbiota, ie, dysbiosis, may play a role in the pathogenesis of both IBS and GWI. Dysbiosis could be a risk factor for or alternatively a consequence of GWI.
A systematic review highlighted the heterogeneity of the gut microbiota in patients with IBS.10 Overall, Enterobacteriaceae, Lactobacillaceae, and Bacteroides were increased, whereas Clostridiales, Faecalibacterium, and Bifidobacterium were decreased in patients with IBS compared with controls. Gut microbiota also has been associated with cognitive changes, anxiety, and depression—symptoms associated with IBS and are part of the GWI.
If altered gut microbiota contributes to the etiopathogenesis of IBS, its restoration of with probiotics should help. Probiotics are live organisms that when ingested may improve health by promoting the growth of naturally occurring flora and establishing a healthy gut flora. Probiotics have several mechanisms of actions. Probiotics work in the lumen of the gut by producing antibacterial molecules and enhancing the mucosal barrier.11 Probiotics also may produce metabolic compounds that alter the intestinal microbiota and improve intestinal barrier function.12 Probiotics also have been shown to activate receptors in the enteric nervous system with the potential to promote pain relief in the setting of visceral hyperalgesia.13,14 The anti-inflammatory properties of probiotics potentially could modulate the basic pathophysiology of IBS and improve motility, visceral hypersensitivity, and brain-gut interaction.15 Furthermore, significant gut dysbiosis has been shown with GWI; suggesting that probiotics may have a role in its management.16,17
Probiotics have not been studied in Gulf War veterans with IBS. We performed a prospective, double-blind placebo-controlled study to determine the efficacy of a commercially available probiotic containing 8 strains of bacteria (De Simone Formulation; formally known as VSL#3 and Visbiome) on symptoms of IBS and GWI. This probiotic was selected as the overall literature suggested benefit of combination probiotics in IBS, and VSL#3 has been shown to be efficacious in ulcerative colitis and microscopic colitis.18-20
Methods
Veterans who served in Operation Desert Storm (August 1990 to March 1991) and enrolled at the George E. Wahlen Veterans Affairs (VA) Medical Center (GEWVAMC), Salt Lake City, Utah, were eligible for the study. The inclusion criteria were: veterans aged ≥ 35 years; ≥ 2 nonintestinal GWI symptoms (eg, fatigue, joint pains, insomnia, general stiffness, and headache); IBS diagnosis based on the Rome III criteria; IBS symptoms > 6 months; normal gross appearance of the colonic mucosa; negative markers for celiac disease and inflammatory bowel disease (IBD); normal thyroid function; and serum calcium levels.21 Those who had a clinically significant cardiac, pulmonary, hepatic or renal dysfunction; history of/or presence of systemic malignancy; current evidence of celiac disease or IBD; unstable/significant psychiatric disease; recent change in GI medications; current pregnancy; or use of antibiotics or probiotics within the past 1 month were excluded. Subjects were enrolled from a list of veterans with GWI from the GEWVAMC Gulf War registry; referrals to gastroenterology clinics for IBS from internal medicine clinics; and posted advertisements.
Protocol
After written informed consent was obtained, each veteran was verified to have IBS and ≥ 2 GWI symptoms. All veterans had the following tests and panels: complete blood count, erythrocyte sedimentation rate, serum comprehensive metabolic panel, thyroid-stimulating hormone, tissue transglutaminase, stool test for ova and parasite, giardia antigen, and clostridia toxins to exclude organic cause of GI symptoms. Colonoscopy was performed in all veterans to exclude IBD, and to rule out microscopic or lymphocytic colitis.
Randomization was computer generated and maintained by the study pharmacist so that study personnel and patients were blinded to the trial groups. All investigators were blinded and allocation was concealed. The medication was supplied in a numbered container by the pharmacist after patient enrollment. After a 2-week run-in period, veterans were randomized (1:1) to receive either 1 sachet of probiotic (De Simone Formulation; formally known as VSL#3 and Visbiome) or placebo once daily for 8 weeks.
Each probiotic packet contains 900 billion probiotic bacteria per sachet.11 This formulation contained 8 viable strains of bacteria: 4 strains of Lactobacillus (L acidophilus, L plantarum, L paracasei, L delbrueckii subsp. bulgaricus); 3 strains of Bifidobacteria (Bifidobacterium breve, B lactis, B infantis); and 1 strain of Streptococcus thermophilus. This formulation had been commercialized and studied as VSL#3 and is currently available in the United States under the Visbiome trade name. While branding changed during the study, the formulation did not. The investigational medicine (VSL#3, Visbiome, and placebo) were shipped from the manufacturer Dupont/Danisco in Madison, Wisconsin. The subjects received placebo or probiotic (VSL#3/Visbiome) and both were identical in appearance. The medication was supplied in a numbered container by the pharmacist after patient enrollment.
Measures
Veterans completed the bowel disease questionnaire to record baseline bowel habits.22 All veterans recorded daily bowel symptoms to confirm the presence of IBS during the 2-week pretreatment period, at baseline, and at the end of the 8-week treatment. The symptoms assessed included severity of abdominal pain (0, none to 100, severe); severity of bloating (0, none to 100, severe); stool frequency; Bristol stool scale (1, very hard to 7, watery); severity of diarrhea (0, none to 100, severe); severity of constipation (0, none to 100, severe); satisfaction with bowel habits (0, none to 100, severe); and IBS affecting or interfering with life (0, none to 100, severe). The bowel symptom score is the sum of the 5 symptom scores.23,24
IBS-specific QOL (IBS-QOL) was recorded at baseline and at the end of treatment.25 The IBS-QOL consists of a 34-item validated disease-specific questionnaire that measures 8 domains relevant to subjects with IBS: dysphoria, interference with activity, body image, health worry, food avoidance, social reaction, sexual life, and relationships. We used the Somatic Symptom Checklist to detect the following extra-intestinal symptoms that are common among veterans with GWI: headache, backache, wheeziness, insomnia, bad breath, fatigue, general stiffness, dizziness, weakness, sensitivity to hot and cold, palpitation, and tightness in chest. Subjects rated symptoms on a scale of 1 to 5: how often (1, none; 2, monthly; 3, once weekly; 4, several times weekly; 5, daily), and how bothersome (1, not at all to 5, extremely).26
Subjects completed the Posttraumatic Stress Disorder (PTSD) Checklist–Military, which is specific to military experience with 17 items on a 1 to 5 scale (1, not at all to 5, extremely). Scores were summed to produce a total symptom severity score (range, 17-85).27 Subjects also completed the Brief Symptom Inventory 18 (BSI-18) during the baseline evaluation.28 BSI-18 measures subjects’ reported overall psychological distress. It assesses 3 symptoms dimensions (somatization, depression, and anxiety) and a global severity index. The raw scores were transferred to normative T scores based on samples of nonpatient normal men and women.
Symptom data were compared after 8 weeks of treatment. The primary study endpoint was change in bowel symptom score. The secondary endpoints were mean change in symptoms, QOL, extra-intestinal symptoms, and PTSD score. The study was approved by the Salt Lake City Veterans Affairs Medical Center and the University of Utah Institutional Review Board and registered in ClinicalTrials.gov (NCT03078530).
Statistical Methods
Comparisons of the probiotic vs placebo groups for demographic variable were analyzed using a 2-sample t test for continuous variables, and with a χ2 test or Fisher exact test for categorical variables. The primary and secondary outcome variables were recorded daily for 2 weeks as pretreatment baseline and for 2 weeks at the end of treatment. These symptoms were recorded as ordered categorical variables, which were then averaged across the week to produce a continuous measurement for statistical analysis. For the primary outcome of GI symptoms, posttreatment comparisons were made between the study groups using a 2-sample t test of the baseline vs posttreatment values. All P values were calculated for 2-sided comparisons. The planned sample size in our study protocol was to recruit 40 individuals per group in order to achieve 80% power to detect a 30% improvement between baseline and end of treatment in the primary bowel symptom score. This study recruited 53 subjects. With this sample size, the study had 80% power to detect a 0.8 SD in any of the outcomes.
Results
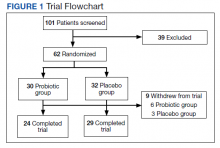
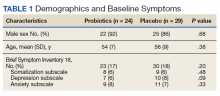
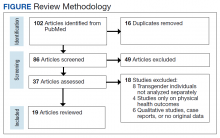
We screened 101 veterans with IBS and GWI; 39 veterans did not fulfill the inclusion/exclusion criteria, 22 declined to participate or did not complete the screening questionnaires and tests, and 9 were lost to follow-up. Sixty-two participants were randomized in a double-blind placebo-controlled study design; 9 dropped out before the end of the study. Data were analyzed from 53 veterans who completed the study, 29 in the placebo group and 24 in the probiotic group (Figure 1). The cohort was primarily male with a mean (SD) age of 55 (8) years (range, 42-73) (Table 1).
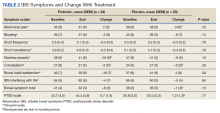
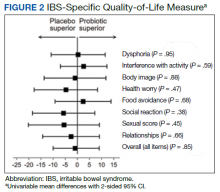
Overall, the treatment was well tolerated. All subjects were contacted every 2 weeks during the study to check for adverse effects, but no serious events were reported. There were no differences at baseline in any of the BSI-18 subscale scores in veterans between the groups. There was a greater mean (SEM) improvement of diarrhea severity in the probiotic group compared with the placebo group: 18 (6), a 31% improvement, vs 6 (5), a 13% improvement, respectively; however, the difference was not statistically significance (P = .13) (Table 2). There also was a greater mean (SEM) improvement in satisfaction of bowel habits in the probiotic group compared with the placebo group: 16 (7), a 35% improvement vs 4 (9), an 8% worsening; this also was not statistically significant (P = .09). There was no difference in the change of IBS-QOL before and after treatment in either group (Figure 2). There was no improvement in any of the symptoms of GWI (all P ≥ .06) (Appendix).
Discussion
GWI is a complex multisystem illness of unknown etiology. There was high prevalence of diarrhea during deployment, and veterans were exposed to several physical, environmental, and mental stresses of the war.3 A change in gut microbiota can occur during deployment due to diet changes, environmental and physical stress, and GI infections.29 These changes would suggest that manipulation of gut microbiota might offer a new modality of treatment of IBS and GWI. We evaluated the effect of a high-potency multistrain probiotic in veterans with IBS and GWI. We did not detect any statistically significant differences between the probiotic and placebo groups on bowel symptom score and individual symptoms of IBS and on QOL. Also, there was no improvement for the other symptoms of GWI. To our knowledge, this is the first study evaluating the effect of probiotics in veterans with IBS and GWI. Our results are consistent with the literature on probiotics and IBS.
The probiotic formulation used in our study has been evaluated in patients with IBS previously. Kim and colleagues found that after 8 weeks of treatment of patients with diarrhea-predominant IBS with VSL#3, there was improvement in bloating, but no effect was found on abdominal pain, gas, or urgency.30 A subsequent study by the same investigators on patients with all types of IBS found that VSL#3 showed no effect on abdominal pain, stool frequency and consistency, or on bloating, but there was improvement in flatulence.31 Another study that evaluated the effect of VSL#3 on symptoms of diarrhea-predominant IBS and QOL found improvement in IBS symptoms from baseline in both the probiotic and the placebo groups, but the difference between the 2 groups was not statistically significant.32 Similarly, Wong and colleagues performed a double-blind, placebo-controlled mechanistic study to evaluate the effect of VSL#3. They found improvement in bowel symptom score, abdominal pain intensity, and satisfaction with bowel habits with both the VSL#3 and placebo group but similar to our study, the differences were not statistically significant.
Several reviews have evaluated the efficacy of probiotics for IBS. A 2010 review found evidence that probiotics trended toward improved IBS symptoms compared with placebo.33 The 2014 follow-up by the same authors demonstrated that overall, probiotics improved global symptoms of IBS and multistrain probiotics were more effective.20 A third meta-analysis from the same group found evidence that multistrain probiotics seemed to have a beneficial effect but could not definitively conclude that probiotics are efficacious in improving IBS symptoms.34 Other authors also have seen inconsistent effects of probiotics compared with placebo on global symptoms, abdominal pain, and bloating after performing systematic reviews of the literature.35-38 Although several reviews support that multistrain probiotics are more effective, they fail to conclude which combinations are more efficacious.
The effect of probiotics on QOL has not been investigated by many studies.37 In our study, we did not find significant improvement in QOL in the probiotic group, which is in line with 2 previous studies that showed no effect on IBS QOL of VSL#3 vs placebo.32,39 Most of the research reports that multistrain probiotics are more effective than using a single strain.34,35,40Bifidobacterium and Lactobacillus are the most commonly used bacteria in the multistrain probiotics that have shown their positive effect on IBS.35,41 The probiotic used in our study contained other species along with these 2 microorganisms.
The dose and duration of treatment of probiotics also has been debated. In one meta-analysis, the investigators found that studies of ≥ 8 weeks were more likely to show a positive effect; 4 of the 7 studies with statistically significant improvement in IBS symptoms were longer than 8 weeks.35 However, another meta-analysis based on 35 randomized controlled trials found that there was not a statistically significant difference between groups treated for > 4 weeks vs < 4 weeks.42 In addition, another meta-analysis of VSL#3 on IBS in children and adults also found no difference in results based on the duration of treatment of probiotics.43 Similar to our study, 3 other studies of VSL#3 treated patients for 8 weeks and found no statistically significant effect.30-32 In the past, VSL#3 has been used at dosages of 450 or 900 billion bacteria per day.
An individual’s response to probiotics may depend on the subtype of IBS. However, most of the studies, like ours, included groups of all subtypes. It may be that probiotics are more effective in patients with moderate-to-severe symptoms. Most of our patients had milder symptoms, and we cannot discount how subjects with more severe disease may have responded to the drug. Interestingly, one study demonstrated that Lactobacillus was more effective in patients with moderately severe abdominal pain compared with mild symptoms.44
In our study, the probiotic did not improve PTSD symptoms or other extra-intestinal symptoms common in IBS and GWI. Similar to our study, Wong and colleagues did not find significant improvement of psychological and sleep scores after treatment with VSL#3.6 Similarly, there is evidence that alteration in gut microbiota is associated with health and diseases, but what specific alterations occur and whether they can be improved with probiotics remains unknown.45
Limitations
The inconsistent response to probiotics in various studies may be due to IBS heterogeneity. Furthermore, there are demographic differences between Gulf War veterans and patients enrolled in other studies: Gulf War veterans are predominantly male, many were deployed abroad and had a history of gastroenteritis during deployment, and were exposed to stressful situations.46 These factors may be involved in triggering or maintaining IBS in Gulf War veterans. A further limitation of our randomized trial is the relatively small sample size.
Conclusions
This study did not demonstrate statistically significant improvement in symptoms of IBS or improvement in QOL after treatment with a multistrain probiotic. We also did not find any improvement in symptoms of GWI or PTSD. There was no difference in psychological scores between the placebo and treatment groups, and it is unlikely that psychological factors confounded the response to treatment in this study.
The effectiveness of a probiotic may depend on the baseline gut microbiome of the individual and depend on the strain, amount, and frequency of bacteria used. A lack of response of the probiotics does not exclude gut viruses and fungi having a role in exacerbating GWI symptoms. It is also possible that the bacteria present or the dose of the probiotic used was not sufficient to improve symptoms. So far, the definitive benefit of probiotics has been demonstrated for only a few preparations, and none are approved by the US Food and Drug Administration for any disease. More research is needed to determine whether probiotics have any role in the treatment of IBS and GWI.
Acknowledgments
AKT received grant support from the US Department of Veterans Affairs and the US Department of Defense (W81XWH-10-1-0593, W81XWH-15-1-0636). We thank Keith G. Tolman, MD, for assistance in editing the initial proposal and for periodic consultation. We thank the manufacturer of the probiotic for supplying the active drug and the placebo. The manufacture of the probiotic had no role in the design and conduct of the study, analysis and interpretation of the data, and in the preparation of the manuscript.
1. O’Shea EF, Cotter PD, Stanton C, Ross RP, Hill C. Production of bioactive substances by intestinal bacteria as a basis for explaining probiotic mechanisms: bacteriocins and conjugated linoleic acid. Int J Food Microbiol. 2012;152(3):189-205. doi:10.1016/j.ijfoodmicro.2011.05.025.
2. Kamiya T, Wang L, Forsythe P, et al. Inhibitory effects of Lactobacillus reuteri on visceral pain induced by colorectal distension in Sprague-Dawley rats. Gut. 2006;55(2):191-196. doi:10.1136/gut.2005.070987.
3. Verdu EF, Bercik P, Verma-Gandhu M, et al. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut. 2006;55(2):182-190. doi:10.1136/gut.2005.066100
4. Ford AC, Harris LA, Lacy BE, Quigley EMM, Moayyedi P. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48(10):1044-1060. doi:10.1111/apt.15001.
5. Niu HL, Xiao JY. The efficacy and safety of probiotics in patients with irritable bowel syndrome: Evidence based on 35 randomized controlled trials. Int J Surg. 2020;75:116-127. doi:10.1016/j.ijsu.2020.01.142.
6. Wong RK, Yang C, Song GH, Wong J, Ho KY. Melatonin regulation as a possible mechanism for probiotic (VSL#3) in irritable bowel syndrome: a randomized double-blinded placebo study. Dig Dis Sci. 2015;60(1):186-194. doi:10.1007/s10620-014-3299-8.
7. Hyams KC, Bourgeois AL, Merrell BR, et al. Diarrheal disease during Operation Desert Shield. N Engl J Med. 1991;325(20):1423-1428. doi:10.1056/NEJM199111143252006 8. Clancy RL, Gleeson M, Cox A, et al. Reversal in fatigued athletes of a defect in interferon gamma secretion after administration of Lactobacillus acidophilus. Br J Sports Med. 2006;40(4):351-354. doi:10.1136/bjsm.2005.024364
9. Sullivan A, Nord CE, Evengard B. Effect of supplement with lactic-acid producing bacteria on fatigue and physical activity in patients with chronic fatigue syndrome. Nutr J. 2009;8:4. doi:10.1186/1475-2891-8-4
10. Pittayanon R, Lau JT, Yuan Y, et al. Gut microbiota in patients with irritable bowel syndrome—a systematic review. Gastroenterology. 2019;157(1):97-108. doi:10.1053/j.gastro.2019.03.049
11. Rao RK, Samak G. Protection and restitution of gut barrier by probiotics: nutritional and clinical implications. Curr Nutr Food Sci. 2013;9(2):99-107. doi:10.2174/1573401311309020004
12. O´Shea EF, Cotter PD, Stanton C, Ross RP, Hill C. Production of bioactive substances by intestinal bacteria as a basis for explaining probiotic mechanisms: bacteriocins and conjugated linoleic acid. Int J Food Microbiol. 2012;152(3):189-205. doi:10.1016/j.ijfoodmicro.2011.05.025
13. Kamiya T, Wang L, Forsythe P, et al. Inhibitory effects of Lactobacillus reuteri on visceral pain induced by colorectal distension in Sprague-Dawley rats. Gut. 2006;55(2):191-196. doi:10.1136/gut.2005.070987
14. Verdu EF, Bercik P, Verma-Gandhu M, et al. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut. 2006;55(2):182-190. doi:10.1136/gut.2005.06610015. O´Mahony L, McCarthy J, Kelly P, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128(3):541-551. doi:10.1053/j.gastro.2004.11.050
16. Alhasson F, Das S, Seth R, et al. Altered gut microbiome in a mouse model of Gulf War Illness causes neuroinflammation and intestinal injury via leaky gut and TLR4 activation. PLoS One. 2017;12(3):e0172914. doi:10.1371/journal.pone.0172914.17. Janulewicz PA, Seth RK, Carlson JM, et al. The gut-microbiome in Gulf War veterans: a preliminary report. Int J Environ Res Public Health. 2019;16(19). doi:10.3390/ijerph16193751
18. Dang X, Xu M, Liu D, Zhou D, Yang W. Assessing the efficacy and safety of fecal microbiota transplantation and probiotic VSL#3 for active ulcerative colitis: a systematic review and meta-analysis. PLoS One. 2020;15(3):e0228846. doi:10.1371/journal.pone.0228846
19. Ford AC, Quigley EM, Lacy BE, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014;109(10):1547-1561; quiz 1546, 1562. doi:10.1038/ajg.2014.202
20. Rohatgi S, Ahuja V, Makharia GK, et al. VSL#3 induces and maintains short-term clinical response in patients with active microscopic colitis: a two-phase randomised clinical trial. BMJ Open Gastroenterol. 2015;2(1):e000018. doi:10.1136/bmjgast-2014-000018
21. Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130(5):1480-1491. doi:10.1053/j.gastro.2005.11.061
22. Talley NJ, Phillips SF, Melton J, 3rd, Wiltgen C, Zinsmeister AR. A patient questionnaire to identify bowel disease. Ann Intern Med. 1989;111(8):671-674. doi:10.7326/0003-4819-111-8-671
23. Bensoussan A, Talley NJ, Hing M, Menzies R, Guo A, Ngu M. Treatment of irritable bowel syndrome with Chinese herbal medicine: a randomized controlled trial. JAMA. 1998;280(18):1585-1589. doi:10.1001/jama.280.18.1585
24. Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11(2):395-402. doi:10.1046/j.1365-2036.1997.142318000.x
25. Patrick DL, Drossman DA, Frederick IO, DiCesare J, Puder KL. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci. 1998;43(2):400-411. doi:10.1023/a:1018831127942
26. Attanasio V, Andrasik F, Blanchard EB, Arena JG. Psychometric properties of the SUNYA revision of the Psychosomatic Symptom Checklist. J Behav Med. 1984;7(2):247-257. doi:10.1007/BF00845390
27. Weathers F, Litz B, Herman D, Huska J, Keane T. The PTSD Checklist (PCL): reliability, validity, and diagnostic utility. Accessed August 25, 2022. https://www.researchgate.net/publication/291448760_The_PTSD_Checklist_PCL_Reliability_validity_and_diagnostic_utility
28. Derogatis L. Brief Symptom Inventory-18 (BSI-18): Administration, Scoring, and Procedure Manual. Ed 3 ed. National Computer Systems; 2000.
29. Stamps BW, Lyon WJ, Irvin AP, Kelley-Loughnane N, Goodson MS. A pilot study of the effect of deployment on the gut microbiome and traveler´s diarrhea susceptibility. Front Cell Infect Microbiol. 2020;10:589297. doi:10.3389/fcimb.2020.589297
30. Kim HJ, Camilleri M, McKinzie S, et al. A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2003;17(7):895-904. doi:10.1046/j.1365-2036.2003.01543.x
31. Kim HJ, Vazquez Roque MI, Camilleri M, et al. A randomized controlled trial of a probiotic combination VSL# 3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol Motil. 2005;17(5):687-696. doi:10.1111/j.1365-2982.2005.00695.x32. Michail S, Kenche H. Gut microbiota is not modified by randomized, double-blind, placebo-controlled trial of vsl#3 in diarrhea-predominant irritable bowel syndrome. Probiotics Antimicrob Proteins. 2011;3(1):1-7. doi:10.1007/s12602-010-9059-y
33. Moayyedi P, Ford AC, Talley NJ, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59(3):325-332. doi:10.1136/gut.2008.167270

34. Ford AC, Harris LA, Lacy BE, Quigley EMM, Moayyedi P. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48(10):1044-1060. doi:10.1111/apt.15001
35. Dale HF, Rasmussen SH, Asiller OO, Lied GA. Probiotics in irritable bowel syndrome: an up-to-date systematic review. Nutrients. 2019;11(9). doi:10.3390/nu11092048
36. Didari T, Mozaffari S, Nikfar S, Abdollahi M. Effectiveness of probiotics in irritable bowel syndrome: Updated systematic review with meta-analysis. World J Gastroenterol. 2015;21(10):3072-84. doi:10.3748/wjg.v21.i10.3072
37. Hungin APS, Mitchell CR, Whorwell P, et al. Systematic review: probiotics in the management of lower gastrointestinal symptoms—an updated evidence-based international consensus. Aliment Pharmacol Ther. 2018;47(8):1054-1070. doi:10.1111/apt.14539
38. Niu HL, Xiao JY. The efficacy and safety of probiotics in patients with irritable bowel syndrome: evidence based on 35 randomized controlled trials. Int J Surg. 2020;75:116-127. doi:10.1016/j.ijsu.2020.01.142
39. Wong RK, Yang C, Song GH, Wong J, Ho KY. Melatonin regulation as a possible mechanism for probiotic (VSL#3) in irritable bowel syndrome: a randomized double-blinded placebo study. Dig Dis Sci. 2015;60(1):186-194. doi:10.1007/s10620-014-3299-8
40. Ford AC, Moayyedi P, Lacy BE, et al. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109(suppl 1):S2-26; quiz S27. doi: 10.1038/ajg.2014.187
41. Simren M, Barbara G, Flint HJ, et al. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013;62(1):159-76. doi:10.1136/gutjnl-2012-302167
42. Ki Cha B, Mun Jung S, Hwan Choi C, et al. The effect of a multispecies probiotic mixture on the symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Clin Gastroenterol. 2012;46(3):220-7. doi:10.1097/MCG.0b013e31823712b1
43. Connell M, Shin A, James-Stevenson T, Xu H, Imperiale TF, Herron J. Systematic review and meta-analysis: Efficacy of patented probiotic, VSL#3, in irritable bowel syndrome. Neurogastroenterol Motil. 2018;30(12):e13427. doi:10.1111/nmo.13427
44. Lyra A, Hillila M, Huttunen T, et al. Irritable bowel syndrome symptom severity improves equally with probiotic and placebo. World J Gastroenterol. 2016;22(48):10631-10642. doi:10.3748/wjg.v22.i48.10631
45. Sanders ME, Guarner F, Guerrant R, et al. An update on the use and investigation of probiotics in health and disease. Gut. 2013;62(5):787-796. doi:10.1136/gutjnl-2012-302504
46. Tuteja AK. Deployment-associated functional gastrointestinal disorders: do we know the etiology? Dig Dis Sci. 2011;56(11):3109-3111. doi:10.1007/s10620-011-1856-y
About 700,000 US military personnel were deployed in Operation Desert Storm (August 1990 to March 1991).1 Almost 30 years since the war, a large number of these veterans continue to experience a complex of symptoms of unknown etiology called Gulf War illness (GWI), which significantly affects health and quality of life (QOL). The lack of clear etiology of the illness has impaired research to find specific treatments and has further exacerbated the stress among veterans. GWI typically includes a mixture of chronic headache, cognitive difficulties, widespread pain, unexplained fatigue, memory and concentration problems, as well as chronic respiratory and gastrointestinal (GI) symptoms.2 Abdominal pain and alteration of bowel habits are also symptoms typical of irritable bowel syndrome (IBS). It has been estimated that IBS occurs in up to 30% of Gulf War veterans.3
The etiology of IBS is unknown. Possible mechanisms include visceral hypersensitivity, altered gut motor function, aberrant brain-gut interaction, and psychological factors, perhaps with a genetic predisposition.4 Gastroenteritis has been reported as a triggering mechanism in up to one-third of patients with IBS.5 Gastroenteritis can alter the gut microbiota and has been reported to be a significant risk factor for the development of IBS.6 In one study of Operation Desert Shield soldiers, > 50% of military personnel developed acute gastroenteritis while on duty.7 A high prevalence of extra-intestinal symptoms also has been reported, including fatigue, headache, joint pains, and anxiety, in Gulf War veterans with IBS. These extra-intestinal symptoms of IBS are consistent with the reported GWI symptoms. Change in gut microbiota also has been associated with many of the extra-intestinal symptoms of IBS, especially fatigue.8,9 Gut microbiota are known to change with travel, stress, and a change in diet, all potential factors that are relevant to Gulf War veterans. This would suggest that an imbalance in the gut microbiota, ie, dysbiosis, may play a role in the pathogenesis of both IBS and GWI. Dysbiosis could be a risk factor for or alternatively a consequence of GWI.
A systematic review highlighted the heterogeneity of the gut microbiota in patients with IBS.10 Overall, Enterobacteriaceae, Lactobacillaceae, and Bacteroides were increased, whereas Clostridiales, Faecalibacterium, and Bifidobacterium were decreased in patients with IBS compared with controls. Gut microbiota also has been associated with cognitive changes, anxiety, and depression—symptoms associated with IBS and are part of the GWI.
If altered gut microbiota contributes to the etiopathogenesis of IBS, its restoration of with probiotics should help. Probiotics are live organisms that when ingested may improve health by promoting the growth of naturally occurring flora and establishing a healthy gut flora. Probiotics have several mechanisms of actions. Probiotics work in the lumen of the gut by producing antibacterial molecules and enhancing the mucosal barrier.11 Probiotics also may produce metabolic compounds that alter the intestinal microbiota and improve intestinal barrier function.12 Probiotics also have been shown to activate receptors in the enteric nervous system with the potential to promote pain relief in the setting of visceral hyperalgesia.13,14 The anti-inflammatory properties of probiotics potentially could modulate the basic pathophysiology of IBS and improve motility, visceral hypersensitivity, and brain-gut interaction.15 Furthermore, significant gut dysbiosis has been shown with GWI; suggesting that probiotics may have a role in its management.16,17
Probiotics have not been studied in Gulf War veterans with IBS. We performed a prospective, double-blind placebo-controlled study to determine the efficacy of a commercially available probiotic containing 8 strains of bacteria (De Simone Formulation; formally known as VSL#3 and Visbiome) on symptoms of IBS and GWI. This probiotic was selected as the overall literature suggested benefit of combination probiotics in IBS, and VSL#3 has been shown to be efficacious in ulcerative colitis and microscopic colitis.18-20
Methods
Veterans who served in Operation Desert Storm (August 1990 to March 1991) and enrolled at the George E. Wahlen Veterans Affairs (VA) Medical Center (GEWVAMC), Salt Lake City, Utah, were eligible for the study. The inclusion criteria were: veterans aged ≥ 35 years; ≥ 2 nonintestinal GWI symptoms (eg, fatigue, joint pains, insomnia, general stiffness, and headache); IBS diagnosis based on the Rome III criteria; IBS symptoms > 6 months; normal gross appearance of the colonic mucosa; negative markers for celiac disease and inflammatory bowel disease (IBD); normal thyroid function; and serum calcium levels.21 Those who had a clinically significant cardiac, pulmonary, hepatic or renal dysfunction; history of/or presence of systemic malignancy; current evidence of celiac disease or IBD; unstable/significant psychiatric disease; recent change in GI medications; current pregnancy; or use of antibiotics or probiotics within the past 1 month were excluded. Subjects were enrolled from a list of veterans with GWI from the GEWVAMC Gulf War registry; referrals to gastroenterology clinics for IBS from internal medicine clinics; and posted advertisements.
Protocol
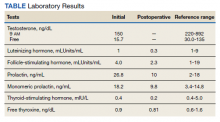
After written informed consent was obtained, each veteran was verified to have IBS and ≥ 2 GWI symptoms. All veterans had the following tests and panels: complete blood count, erythrocyte sedimentation rate, serum comprehensive metabolic panel, thyroid-stimulating hormone, tissue transglutaminase, stool test for ova and parasite, giardia antigen, and clostridia toxins to exclude organic cause of GI symptoms. Colonoscopy was performed in all veterans to exclude IBD, and to rule out microscopic or lymphocytic colitis.
Randomization was computer generated and maintained by the study pharmacist so that study personnel and patients were blinded to the trial groups. All investigators were blinded and allocation was concealed. The medication was supplied in a numbered container by the pharmacist after patient enrollment. After a 2-week run-in period, veterans were randomized (1:1) to receive either 1 sachet of probiotic (De Simone Formulation; formally known as VSL#3 and Visbiome) or placebo once daily for 8 weeks.
Each probiotic packet contains 900 billion probiotic bacteria per sachet.11 This formulation contained 8 viable strains of bacteria: 4 strains of Lactobacillus (L acidophilus, L plantarum, L paracasei, L delbrueckii subsp. bulgaricus); 3 strains of Bifidobacteria (Bifidobacterium breve, B lactis, B infantis); and 1 strain of Streptococcus thermophilus. This formulation had been commercialized and studied as VSL#3 and is currently available in the United States under the Visbiome trade name. While branding changed during the study, the formulation did not. The investigational medicine (VSL#3, Visbiome, and placebo) were shipped from the manufacturer Dupont/Danisco in Madison, Wisconsin. The subjects received placebo or probiotic (VSL#3/Visbiome) and both were identical in appearance. The medication was supplied in a numbered container by the pharmacist after patient enrollment.
Measures
Veterans completed the bowel disease questionnaire to record baseline bowel habits.22 All veterans recorded daily bowel symptoms to confirm the presence of IBS during the 2-week pretreatment period, at baseline, and at the end of the 8-week treatment. The symptoms assessed included severity of abdominal pain (0, none to 100, severe); severity of bloating (0, none to 100, severe); stool frequency; Bristol stool scale (1, very hard to 7, watery); severity of diarrhea (0, none to 100, severe); severity of constipation (0, none to 100, severe); satisfaction with bowel habits (0, none to 100, severe); and IBS affecting or interfering with life (0, none to 100, severe). The bowel symptom score is the sum of the 5 symptom scores.23,24
IBS-specific QOL (IBS-QOL) was recorded at baseline and at the end of treatment.25 The IBS-QOL consists of a 34-item validated disease-specific questionnaire that measures 8 domains relevant to subjects with IBS: dysphoria, interference with activity, body image, health worry, food avoidance, social reaction, sexual life, and relationships. We used the Somatic Symptom Checklist to detect the following extra-intestinal symptoms that are common among veterans with GWI: headache, backache, wheeziness, insomnia, bad breath, fatigue, general stiffness, dizziness, weakness, sensitivity to hot and cold, palpitation, and tightness in chest. Subjects rated symptoms on a scale of 1 to 5: how often (1, none; 2, monthly; 3, once weekly; 4, several times weekly; 5, daily), and how bothersome (1, not at all to 5, extremely).26
Subjects completed the Posttraumatic Stress Disorder (PTSD) Checklist–Military, which is specific to military experience with 17 items on a 1 to 5 scale (1, not at all to 5, extremely). Scores were summed to produce a total symptom severity score (range, 17-85).27 Subjects also completed the Brief Symptom Inventory 18 (BSI-18) during the baseline evaluation.28 BSI-18 measures subjects’ reported overall psychological distress. It assesses 3 symptoms dimensions (somatization, depression, and anxiety) and a global severity index. The raw scores were transferred to normative T scores based on samples of nonpatient normal men and women.
Symptom data were compared after 8 weeks of treatment. The primary study endpoint was change in bowel symptom score. The secondary endpoints were mean change in symptoms, QOL, extra-intestinal symptoms, and PTSD score. The study was approved by the Salt Lake City Veterans Affairs Medical Center and the University of Utah Institutional Review Board and registered in ClinicalTrials.gov (NCT03078530).
Statistical Methods
Comparisons of the probiotic vs placebo groups for demographic variable were analyzed using a 2-sample t test for continuous variables, and with a χ2 test or Fisher exact test for categorical variables. The primary and secondary outcome variables were recorded daily for 2 weeks as pretreatment baseline and for 2 weeks at the end of treatment. These symptoms were recorded as ordered categorical variables, which were then averaged across the week to produce a continuous measurement for statistical analysis. For the primary outcome of GI symptoms, posttreatment comparisons were made between the study groups using a 2-sample t test of the baseline vs posttreatment values. All P values were calculated for 2-sided comparisons. The planned sample size in our study protocol was to recruit 40 individuals per group in order to achieve 80% power to detect a 30% improvement between baseline and end of treatment in the primary bowel symptom score. This study recruited 53 subjects. With this sample size, the study had 80% power to detect a 0.8 SD in any of the outcomes.
Results
We screened 101 veterans with IBS and GWI; 39 veterans did not fulfill the inclusion/exclusion criteria, 22 declined to participate or did not complete the screening questionnaires and tests, and 9 were lost to follow-up. Sixty-two participants were randomized in a double-blind placebo-controlled study design; 9 dropped out before the end of the study. Data were analyzed from 53 veterans who completed the study, 29 in the placebo group and 24 in the probiotic group (Figure 1). The cohort was primarily male with a mean (SD) age of 55 (8) years (range, 42-73) (Table 1).
Overall, the treatment was well tolerated. All subjects were contacted every 2 weeks during the study to check for adverse effects, but no serious events were reported. There were no differences at baseline in any of the BSI-18 subscale scores in veterans between the groups. There was a greater mean (SEM) improvement of diarrhea severity in the probiotic group compared with the placebo group: 18 (6), a 31% improvement, vs 6 (5), a 13% improvement, respectively; however, the difference was not statistically significance (P = .13) (Table 2). There also was a greater mean (SEM) improvement in satisfaction of bowel habits in the probiotic group compared with the placebo group: 16 (7), a 35% improvement vs 4 (9), an 8% worsening; this also was not statistically significant (P = .09). There was no difference in the change of IBS-QOL before and after treatment in either group (Figure 2). There was no improvement in any of the symptoms of GWI (all P ≥ .06) (Appendix).
Discussion
GWI is a complex multisystem illness of unknown etiology. There was high prevalence of diarrhea during deployment, and veterans were exposed to several physical, environmental, and mental stresses of the war.3 A change in gut microbiota can occur during deployment due to diet changes, environmental and physical stress, and GI infections.29 These changes would suggest that manipulation of gut microbiota might offer a new modality of treatment of IBS and GWI. We evaluated the effect of a high-potency multistrain probiotic in veterans with IBS and GWI. We did not detect any statistically significant differences between the probiotic and placebo groups on bowel symptom score and individual symptoms of IBS and on QOL. Also, there was no improvement for the other symptoms of GWI. To our knowledge, this is the first study evaluating the effect of probiotics in veterans with IBS and GWI. Our results are consistent with the literature on probiotics and IBS.
The probiotic formulation used in our study has been evaluated in patients with IBS previously. Kim and colleagues found that after 8 weeks of treatment of patients with diarrhea-predominant IBS with VSL#3, there was improvement in bloating, but no effect was found on abdominal pain, gas, or urgency.30 A subsequent study by the same investigators on patients with all types of IBS found that VSL#3 showed no effect on abdominal pain, stool frequency and consistency, or on bloating, but there was improvement in flatulence.31 Another study that evaluated the effect of VSL#3 on symptoms of diarrhea-predominant IBS and QOL found improvement in IBS symptoms from baseline in both the probiotic and the placebo groups, but the difference between the 2 groups was not statistically significant.32 Similarly, Wong and colleagues performed a double-blind, placebo-controlled mechanistic study to evaluate the effect of VSL#3. They found improvement in bowel symptom score, abdominal pain intensity, and satisfaction with bowel habits with both the VSL#3 and placebo group but similar to our study, the differences were not statistically significant.
Several reviews have evaluated the efficacy of probiotics for IBS. A 2010 review found evidence that probiotics trended toward improved IBS symptoms compared with placebo.33 The 2014 follow-up by the same authors demonstrated that overall, probiotics improved global symptoms of IBS and multistrain probiotics were more effective.20 A third meta-analysis from the same group found evidence that multistrain probiotics seemed to have a beneficial effect but could not definitively conclude that probiotics are efficacious in improving IBS symptoms.34 Other authors also have seen inconsistent effects of probiotics compared with placebo on global symptoms, abdominal pain, and bloating after performing systematic reviews of the literature.35-38 Although several reviews support that multistrain probiotics are more effective, they fail to conclude which combinations are more efficacious.
The effect of probiotics on QOL has not been investigated by many studies.37 In our study, we did not find significant improvement in QOL in the probiotic group, which is in line with 2 previous studies that showed no effect on IBS QOL of VSL#3 vs placebo.32,39 Most of the research reports that multistrain probiotics are more effective than using a single strain.34,35,40Bifidobacterium and Lactobacillus are the most commonly used bacteria in the multistrain probiotics that have shown their positive effect on IBS.35,41 The probiotic used in our study contained other species along with these 2 microorganisms.
The dose and duration of treatment of probiotics also has been debated. In one meta-analysis, the investigators found that studies of ≥ 8 weeks were more likely to show a positive effect; 4 of the 7 studies with statistically significant improvement in IBS symptoms were longer than 8 weeks.35 However, another meta-analysis based on 35 randomized controlled trials found that there was not a statistically significant difference between groups treated for > 4 weeks vs < 4 weeks.42 In addition, another meta-analysis of VSL#3 on IBS in children and adults also found no difference in results based on the duration of treatment of probiotics.43 Similar to our study, 3 other studies of VSL#3 treated patients for 8 weeks and found no statistically significant effect.30-32 In the past, VSL#3 has been used at dosages of 450 or 900 billion bacteria per day.
An individual’s response to probiotics may depend on the subtype of IBS. However, most of the studies, like ours, included groups of all subtypes. It may be that probiotics are more effective in patients with moderate-to-severe symptoms. Most of our patients had milder symptoms, and we cannot discount how subjects with more severe disease may have responded to the drug. Interestingly, one study demonstrated that Lactobacillus was more effective in patients with moderately severe abdominal pain compared with mild symptoms.44
In our study, the probiotic did not improve PTSD symptoms or other extra-intestinal symptoms common in IBS and GWI. Similar to our study, Wong and colleagues did not find significant improvement of psychological and sleep scores after treatment with VSL#3.6 Similarly, there is evidence that alteration in gut microbiota is associated with health and diseases, but what specific alterations occur and whether they can be improved with probiotics remains unknown.45
Limitations
The inconsistent response to probiotics in various studies may be due to IBS heterogeneity. Furthermore, there are demographic differences between Gulf War veterans and patients enrolled in other studies: Gulf War veterans are predominantly male, many were deployed abroad and had a history of gastroenteritis during deployment, and were exposed to stressful situations.46 These factors may be involved in triggering or maintaining IBS in Gulf War veterans. A further limitation of our randomized trial is the relatively small sample size.
Conclusions
This study did not demonstrate statistically significant improvement in symptoms of IBS or improvement in QOL after treatment with a multistrain probiotic. We also did not find any improvement in symptoms of GWI or PTSD. There was no difference in psychological scores between the placebo and treatment groups, and it is unlikely that psychological factors confounded the response to treatment in this study.
The effectiveness of a probiotic may depend on the baseline gut microbiome of the individual and depend on the strain, amount, and frequency of bacteria used. A lack of response of the probiotics does not exclude gut viruses and fungi having a role in exacerbating GWI symptoms. It is also possible that the bacteria present or the dose of the probiotic used was not sufficient to improve symptoms. So far, the definitive benefit of probiotics has been demonstrated for only a few preparations, and none are approved by the US Food and Drug Administration for any disease. More research is needed to determine whether probiotics have any role in the treatment of IBS and GWI.
Acknowledgments
AKT received grant support from the US Department of Veterans Affairs and the US Department of Defense (W81XWH-10-1-0593, W81XWH-15-1-0636). We thank Keith G. Tolman, MD, for assistance in editing the initial proposal and for periodic consultation. We thank the manufacturer of the probiotic for supplying the active drug and the placebo. The manufacture of the probiotic had no role in the design and conduct of the study, analysis and interpretation of the data, and in the preparation of the manuscript.
About 700,000 US military personnel were deployed in Operation Desert Storm (August 1990 to March 1991).1 Almost 30 years since the war, a large number of these veterans continue to experience a complex of symptoms of unknown etiology called Gulf War illness (GWI), which significantly affects health and quality of life (QOL). The lack of clear etiology of the illness has impaired research to find specific treatments and has further exacerbated the stress among veterans. GWI typically includes a mixture of chronic headache, cognitive difficulties, widespread pain, unexplained fatigue, memory and concentration problems, as well as chronic respiratory and gastrointestinal (GI) symptoms.2 Abdominal pain and alteration of bowel habits are also symptoms typical of irritable bowel syndrome (IBS). It has been estimated that IBS occurs in up to 30% of Gulf War veterans.3
The etiology of IBS is unknown. Possible mechanisms include visceral hypersensitivity, altered gut motor function, aberrant brain-gut interaction, and psychological factors, perhaps with a genetic predisposition.4 Gastroenteritis has been reported as a triggering mechanism in up to one-third of patients with IBS.5 Gastroenteritis can alter the gut microbiota and has been reported to be a significant risk factor for the development of IBS.6 In one study of Operation Desert Shield soldiers, > 50% of military personnel developed acute gastroenteritis while on duty.7 A high prevalence of extra-intestinal symptoms also has been reported, including fatigue, headache, joint pains, and anxiety, in Gulf War veterans with IBS. These extra-intestinal symptoms of IBS are consistent with the reported GWI symptoms. Change in gut microbiota also has been associated with many of the extra-intestinal symptoms of IBS, especially fatigue.8,9 Gut microbiota are known to change with travel, stress, and a change in diet, all potential factors that are relevant to Gulf War veterans. This would suggest that an imbalance in the gut microbiota, ie, dysbiosis, may play a role in the pathogenesis of both IBS and GWI. Dysbiosis could be a risk factor for or alternatively a consequence of GWI.
A systematic review highlighted the heterogeneity of the gut microbiota in patients with IBS.10 Overall, Enterobacteriaceae, Lactobacillaceae, and Bacteroides were increased, whereas Clostridiales, Faecalibacterium, and Bifidobacterium were decreased in patients with IBS compared with controls. Gut microbiota also has been associated with cognitive changes, anxiety, and depression—symptoms associated with IBS and are part of the GWI.
If altered gut microbiota contributes to the etiopathogenesis of IBS, its restoration of with probiotics should help. Probiotics are live organisms that when ingested may improve health by promoting the growth of naturally occurring flora and establishing a healthy gut flora. Probiotics have several mechanisms of actions. Probiotics work in the lumen of the gut by producing antibacterial molecules and enhancing the mucosal barrier.11 Probiotics also may produce metabolic compounds that alter the intestinal microbiota and improve intestinal barrier function.12 Probiotics also have been shown to activate receptors in the enteric nervous system with the potential to promote pain relief in the setting of visceral hyperalgesia.13,14 The anti-inflammatory properties of probiotics potentially could modulate the basic pathophysiology of IBS and improve motility, visceral hypersensitivity, and brain-gut interaction.15 Furthermore, significant gut dysbiosis has been shown with GWI; suggesting that probiotics may have a role in its management.16,17
Probiotics have not been studied in Gulf War veterans with IBS. We performed a prospective, double-blind placebo-controlled study to determine the efficacy of a commercially available probiotic containing 8 strains of bacteria (De Simone Formulation; formally known as VSL#3 and Visbiome) on symptoms of IBS and GWI. This probiotic was selected as the overall literature suggested benefit of combination probiotics in IBS, and VSL#3 has been shown to be efficacious in ulcerative colitis and microscopic colitis.18-20
Methods
Veterans who served in Operation Desert Storm (August 1990 to March 1991) and enrolled at the George E. Wahlen Veterans Affairs (VA) Medical Center (GEWVAMC), Salt Lake City, Utah, were eligible for the study. The inclusion criteria were: veterans aged ≥ 35 years; ≥ 2 nonintestinal GWI symptoms (eg, fatigue, joint pains, insomnia, general stiffness, and headache); IBS diagnosis based on the Rome III criteria; IBS symptoms > 6 months; normal gross appearance of the colonic mucosa; negative markers for celiac disease and inflammatory bowel disease (IBD); normal thyroid function; and serum calcium levels.21 Those who had a clinically significant cardiac, pulmonary, hepatic or renal dysfunction; history of/or presence of systemic malignancy; current evidence of celiac disease or IBD; unstable/significant psychiatric disease; recent change in GI medications; current pregnancy; or use of antibiotics or probiotics within the past 1 month were excluded. Subjects were enrolled from a list of veterans with GWI from the GEWVAMC Gulf War registry; referrals to gastroenterology clinics for IBS from internal medicine clinics; and posted advertisements.
Protocol
After written informed consent was obtained, each veteran was verified to have IBS and ≥ 2 GWI symptoms. All veterans had the following tests and panels: complete blood count, erythrocyte sedimentation rate, serum comprehensive metabolic panel, thyroid-stimulating hormone, tissue transglutaminase, stool test for ova and parasite, giardia antigen, and clostridia toxins to exclude organic cause of GI symptoms. Colonoscopy was performed in all veterans to exclude IBD, and to rule out microscopic or lymphocytic colitis.
Randomization was computer generated and maintained by the study pharmacist so that study personnel and patients were blinded to the trial groups. All investigators were blinded and allocation was concealed. The medication was supplied in a numbered container by the pharmacist after patient enrollment. After a 2-week run-in period, veterans were randomized (1:1) to receive either 1 sachet of probiotic (De Simone Formulation; formally known as VSL#3 and Visbiome) or placebo once daily for 8 weeks.
Each probiotic packet contains 900 billion probiotic bacteria per sachet.11 This formulation contained 8 viable strains of bacteria: 4 strains of Lactobacillus (L acidophilus, L plantarum, L paracasei, L delbrueckii subsp. bulgaricus); 3 strains of Bifidobacteria (Bifidobacterium breve, B lactis, B infantis); and 1 strain of Streptococcus thermophilus. This formulation had been commercialized and studied as VSL#3 and is currently available in the United States under the Visbiome trade name. While branding changed during the study, the formulation did not. The investigational medicine (VSL#3, Visbiome, and placebo) were shipped from the manufacturer Dupont/Danisco in Madison, Wisconsin. The subjects received placebo or probiotic (VSL#3/Visbiome) and both were identical in appearance. The medication was supplied in a numbered container by the pharmacist after patient enrollment.
Measures
Veterans completed the bowel disease questionnaire to record baseline bowel habits.22 All veterans recorded daily bowel symptoms to confirm the presence of IBS during the 2-week pretreatment period, at baseline, and at the end of the 8-week treatment. The symptoms assessed included severity of abdominal pain (0, none to 100, severe); severity of bloating (0, none to 100, severe); stool frequency; Bristol stool scale (1, very hard to 7, watery); severity of diarrhea (0, none to 100, severe); severity of constipation (0, none to 100, severe); satisfaction with bowel habits (0, none to 100, severe); and IBS affecting or interfering with life (0, none to 100, severe). The bowel symptom score is the sum of the 5 symptom scores.23,24
IBS-specific QOL (IBS-QOL) was recorded at baseline and at the end of treatment.25 The IBS-QOL consists of a 34-item validated disease-specific questionnaire that measures 8 domains relevant to subjects with IBS: dysphoria, interference with activity, body image, health worry, food avoidance, social reaction, sexual life, and relationships. We used the Somatic Symptom Checklist to detect the following extra-intestinal symptoms that are common among veterans with GWI: headache, backache, wheeziness, insomnia, bad breath, fatigue, general stiffness, dizziness, weakness, sensitivity to hot and cold, palpitation, and tightness in chest. Subjects rated symptoms on a scale of 1 to 5: how often (1, none; 2, monthly; 3, once weekly; 4, several times weekly; 5, daily), and how bothersome (1, not at all to 5, extremely).26
Subjects completed the Posttraumatic Stress Disorder (PTSD) Checklist–Military, which is specific to military experience with 17 items on a 1 to 5 scale (1, not at all to 5, extremely). Scores were summed to produce a total symptom severity score (range, 17-85).27 Subjects also completed the Brief Symptom Inventory 18 (BSI-18) during the baseline evaluation.28 BSI-18 measures subjects’ reported overall psychological distress. It assesses 3 symptoms dimensions (somatization, depression, and anxiety) and a global severity index. The raw scores were transferred to normative T scores based on samples of nonpatient normal men and women.
Symptom data were compared after 8 weeks of treatment. The primary study endpoint was change in bowel symptom score. The secondary endpoints were mean change in symptoms, QOL, extra-intestinal symptoms, and PTSD score. The study was approved by the Salt Lake City Veterans Affairs Medical Center and the University of Utah Institutional Review Board and registered in ClinicalTrials.gov (NCT03078530).
Statistical Methods
Comparisons of the probiotic vs placebo groups for demographic variable were analyzed using a 2-sample t test for continuous variables, and with a χ2 test or Fisher exact test for categorical variables. The primary and secondary outcome variables were recorded daily for 2 weeks as pretreatment baseline and for 2 weeks at the end of treatment. These symptoms were recorded as ordered categorical variables, which were then averaged across the week to produce a continuous measurement for statistical analysis. For the primary outcome of GI symptoms, posttreatment comparisons were made between the study groups using a 2-sample t test of the baseline vs posttreatment values. All P values were calculated for 2-sided comparisons. The planned sample size in our study protocol was to recruit 40 individuals per group in order to achieve 80% power to detect a 30% improvement between baseline and end of treatment in the primary bowel symptom score. This study recruited 53 subjects. With this sample size, the study had 80% power to detect a 0.8 SD in any of the outcomes.
Results
We screened 101 veterans with IBS and GWI; 39 veterans did not fulfill the inclusion/exclusion criteria, 22 declined to participate or did not complete the screening questionnaires and tests, and 9 were lost to follow-up. Sixty-two participants were randomized in a double-blind placebo-controlled study design; 9 dropped out before the end of the study. Data were analyzed from 53 veterans who completed the study, 29 in the placebo group and 24 in the probiotic group (Figure 1). The cohort was primarily male with a mean (SD) age of 55 (8) years (range, 42-73) (Table 1).
Overall, the treatment was well tolerated. All subjects were contacted every 2 weeks during the study to check for adverse effects, but no serious events were reported. There were no differences at baseline in any of the BSI-18 subscale scores in veterans between the groups. There was a greater mean (SEM) improvement of diarrhea severity in the probiotic group compared with the placebo group: 18 (6), a 31% improvement, vs 6 (5), a 13% improvement, respectively; however, the difference was not statistically significance (P = .13) (Table 2). There also was a greater mean (SEM) improvement in satisfaction of bowel habits in the probiotic group compared with the placebo group: 16 (7), a 35% improvement vs 4 (9), an 8% worsening; this also was not statistically significant (P = .09). There was no difference in the change of IBS-QOL before and after treatment in either group (Figure 2). There was no improvement in any of the symptoms of GWI (all P ≥ .06) (Appendix).
Discussion
GWI is a complex multisystem illness of unknown etiology. There was high prevalence of diarrhea during deployment, and veterans were exposed to several physical, environmental, and mental stresses of the war.3 A change in gut microbiota can occur during deployment due to diet changes, environmental and physical stress, and GI infections.29 These changes would suggest that manipulation of gut microbiota might offer a new modality of treatment of IBS and GWI. We evaluated the effect of a high-potency multistrain probiotic in veterans with IBS and GWI. We did not detect any statistically significant differences between the probiotic and placebo groups on bowel symptom score and individual symptoms of IBS and on QOL. Also, there was no improvement for the other symptoms of GWI. To our knowledge, this is the first study evaluating the effect of probiotics in veterans with IBS and GWI. Our results are consistent with the literature on probiotics and IBS.
The probiotic formulation used in our study has been evaluated in patients with IBS previously. Kim and colleagues found that after 8 weeks of treatment of patients with diarrhea-predominant IBS with VSL#3, there was improvement in bloating, but no effect was found on abdominal pain, gas, or urgency.30 A subsequent study by the same investigators on patients with all types of IBS found that VSL#3 showed no effect on abdominal pain, stool frequency and consistency, or on bloating, but there was improvement in flatulence.31 Another study that evaluated the effect of VSL#3 on symptoms of diarrhea-predominant IBS and QOL found improvement in IBS symptoms from baseline in both the probiotic and the placebo groups, but the difference between the 2 groups was not statistically significant.32 Similarly, Wong and colleagues performed a double-blind, placebo-controlled mechanistic study to evaluate the effect of VSL#3. They found improvement in bowel symptom score, abdominal pain intensity, and satisfaction with bowel habits with both the VSL#3 and placebo group but similar to our study, the differences were not statistically significant.
Several reviews have evaluated the efficacy of probiotics for IBS. A 2010 review found evidence that probiotics trended toward improved IBS symptoms compared with placebo.33 The 2014 follow-up by the same authors demonstrated that overall, probiotics improved global symptoms of IBS and multistrain probiotics were more effective.20 A third meta-analysis from the same group found evidence that multistrain probiotics seemed to have a beneficial effect but could not definitively conclude that probiotics are efficacious in improving IBS symptoms.34 Other authors also have seen inconsistent effects of probiotics compared with placebo on global symptoms, abdominal pain, and bloating after performing systematic reviews of the literature.35-38 Although several reviews support that multistrain probiotics are more effective, they fail to conclude which combinations are more efficacious.
The effect of probiotics on QOL has not been investigated by many studies.37 In our study, we did not find significant improvement in QOL in the probiotic group, which is in line with 2 previous studies that showed no effect on IBS QOL of VSL#3 vs placebo.32,39 Most of the research reports that multistrain probiotics are more effective than using a single strain.34,35,40Bifidobacterium and Lactobacillus are the most commonly used bacteria in the multistrain probiotics that have shown their positive effect on IBS.35,41 The probiotic used in our study contained other species along with these 2 microorganisms.
The dose and duration of treatment of probiotics also has been debated. In one meta-analysis, the investigators found that studies of ≥ 8 weeks were more likely to show a positive effect; 4 of the 7 studies with statistically significant improvement in IBS symptoms were longer than 8 weeks.35 However, another meta-analysis based on 35 randomized controlled trials found that there was not a statistically significant difference between groups treated for > 4 weeks vs < 4 weeks.42 In addition, another meta-analysis of VSL#3 on IBS in children and adults also found no difference in results based on the duration of treatment of probiotics.43 Similar to our study, 3 other studies of VSL#3 treated patients for 8 weeks and found no statistically significant effect.30-32 In the past, VSL#3 has been used at dosages of 450 or 900 billion bacteria per day.
An individual’s response to probiotics may depend on the subtype of IBS. However, most of the studies, like ours, included groups of all subtypes. It may be that probiotics are more effective in patients with moderate-to-severe symptoms. Most of our patients had milder symptoms, and we cannot discount how subjects with more severe disease may have responded to the drug. Interestingly, one study demonstrated that Lactobacillus was more effective in patients with moderately severe abdominal pain compared with mild symptoms.44
In our study, the probiotic did not improve PTSD symptoms or other extra-intestinal symptoms common in IBS and GWI. Similar to our study, Wong and colleagues did not find significant improvement of psychological and sleep scores after treatment with VSL#3.6 Similarly, there is evidence that alteration in gut microbiota is associated with health and diseases, but what specific alterations occur and whether they can be improved with probiotics remains unknown.45
Limitations
The inconsistent response to probiotics in various studies may be due to IBS heterogeneity. Furthermore, there are demographic differences between Gulf War veterans and patients enrolled in other studies: Gulf War veterans are predominantly male, many were deployed abroad and had a history of gastroenteritis during deployment, and were exposed to stressful situations.46 These factors may be involved in triggering or maintaining IBS in Gulf War veterans. A further limitation of our randomized trial is the relatively small sample size.
Conclusions
This study did not demonstrate statistically significant improvement in symptoms of IBS or improvement in QOL after treatment with a multistrain probiotic. We also did not find any improvement in symptoms of GWI or PTSD. There was no difference in psychological scores between the placebo and treatment groups, and it is unlikely that psychological factors confounded the response to treatment in this study.
The effectiveness of a probiotic may depend on the baseline gut microbiome of the individual and depend on the strain, amount, and frequency of bacteria used. A lack of response of the probiotics does not exclude gut viruses and fungi having a role in exacerbating GWI symptoms. It is also possible that the bacteria present or the dose of the probiotic used was not sufficient to improve symptoms. So far, the definitive benefit of probiotics has been demonstrated for only a few preparations, and none are approved by the US Food and Drug Administration for any disease. More research is needed to determine whether probiotics have any role in the treatment of IBS and GWI.
Acknowledgments
AKT received grant support from the US Department of Veterans Affairs and the US Department of Defense (W81XWH-10-1-0593, W81XWH-15-1-0636). We thank Keith G. Tolman, MD, for assistance in editing the initial proposal and for periodic consultation. We thank the manufacturer of the probiotic for supplying the active drug and the placebo. The manufacture of the probiotic had no role in the design and conduct of the study, analysis and interpretation of the data, and in the preparation of the manuscript.
1. O’Shea EF, Cotter PD, Stanton C, Ross RP, Hill C. Production of bioactive substances by intestinal bacteria as a basis for explaining probiotic mechanisms: bacteriocins and conjugated linoleic acid. Int J Food Microbiol. 2012;152(3):189-205. doi:10.1016/j.ijfoodmicro.2011.05.025.
2. Kamiya T, Wang L, Forsythe P, et al. Inhibitory effects of Lactobacillus reuteri on visceral pain induced by colorectal distension in Sprague-Dawley rats. Gut. 2006;55(2):191-196. doi:10.1136/gut.2005.070987.
3. Verdu EF, Bercik P, Verma-Gandhu M, et al. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut. 2006;55(2):182-190. doi:10.1136/gut.2005.066100
4. Ford AC, Harris LA, Lacy BE, Quigley EMM, Moayyedi P. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48(10):1044-1060. doi:10.1111/apt.15001.
5. Niu HL, Xiao JY. The efficacy and safety of probiotics in patients with irritable bowel syndrome: Evidence based on 35 randomized controlled trials. Int J Surg. 2020;75:116-127. doi:10.1016/j.ijsu.2020.01.142.
6. Wong RK, Yang C, Song GH, Wong J, Ho KY. Melatonin regulation as a possible mechanism for probiotic (VSL#3) in irritable bowel syndrome: a randomized double-blinded placebo study. Dig Dis Sci. 2015;60(1):186-194. doi:10.1007/s10620-014-3299-8.
7. Hyams KC, Bourgeois AL, Merrell BR, et al. Diarrheal disease during Operation Desert Shield. N Engl J Med. 1991;325(20):1423-1428. doi:10.1056/NEJM199111143252006 8. Clancy RL, Gleeson M, Cox A, et al. Reversal in fatigued athletes of a defect in interferon gamma secretion after administration of Lactobacillus acidophilus. Br J Sports Med. 2006;40(4):351-354. doi:10.1136/bjsm.2005.024364
9. Sullivan A, Nord CE, Evengard B. Effect of supplement with lactic-acid producing bacteria on fatigue and physical activity in patients with chronic fatigue syndrome. Nutr J. 2009;8:4. doi:10.1186/1475-2891-8-4
10. Pittayanon R, Lau JT, Yuan Y, et al. Gut microbiota in patients with irritable bowel syndrome—a systematic review. Gastroenterology. 2019;157(1):97-108. doi:10.1053/j.gastro.2019.03.049
11. Rao RK, Samak G. Protection and restitution of gut barrier by probiotics: nutritional and clinical implications. Curr Nutr Food Sci. 2013;9(2):99-107. doi:10.2174/1573401311309020004
12. O´Shea EF, Cotter PD, Stanton C, Ross RP, Hill C. Production of bioactive substances by intestinal bacteria as a basis for explaining probiotic mechanisms: bacteriocins and conjugated linoleic acid. Int J Food Microbiol. 2012;152(3):189-205. doi:10.1016/j.ijfoodmicro.2011.05.025
13. Kamiya T, Wang L, Forsythe P, et al. Inhibitory effects of Lactobacillus reuteri on visceral pain induced by colorectal distension in Sprague-Dawley rats. Gut. 2006;55(2):191-196. doi:10.1136/gut.2005.070987
14. Verdu EF, Bercik P, Verma-Gandhu M, et al. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut. 2006;55(2):182-190. doi:10.1136/gut.2005.06610015. O´Mahony L, McCarthy J, Kelly P, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128(3):541-551. doi:10.1053/j.gastro.2004.11.050
16. Alhasson F, Das S, Seth R, et al. Altered gut microbiome in a mouse model of Gulf War Illness causes neuroinflammation and intestinal injury via leaky gut and TLR4 activation. PLoS One. 2017;12(3):e0172914. doi:10.1371/journal.pone.0172914.17. Janulewicz PA, Seth RK, Carlson JM, et al. The gut-microbiome in Gulf War veterans: a preliminary report. Int J Environ Res Public Health. 2019;16(19). doi:10.3390/ijerph16193751
18. Dang X, Xu M, Liu D, Zhou D, Yang W. Assessing the efficacy and safety of fecal microbiota transplantation and probiotic VSL#3 for active ulcerative colitis: a systematic review and meta-analysis. PLoS One. 2020;15(3):e0228846. doi:10.1371/journal.pone.0228846
19. Ford AC, Quigley EM, Lacy BE, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014;109(10):1547-1561; quiz 1546, 1562. doi:10.1038/ajg.2014.202
20. Rohatgi S, Ahuja V, Makharia GK, et al. VSL#3 induces and maintains short-term clinical response in patients with active microscopic colitis: a two-phase randomised clinical trial. BMJ Open Gastroenterol. 2015;2(1):e000018. doi:10.1136/bmjgast-2014-000018
21. Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130(5):1480-1491. doi:10.1053/j.gastro.2005.11.061
22. Talley NJ, Phillips SF, Melton J, 3rd, Wiltgen C, Zinsmeister AR. A patient questionnaire to identify bowel disease. Ann Intern Med. 1989;111(8):671-674. doi:10.7326/0003-4819-111-8-671
23. Bensoussan A, Talley NJ, Hing M, Menzies R, Guo A, Ngu M. Treatment of irritable bowel syndrome with Chinese herbal medicine: a randomized controlled trial. JAMA. 1998;280(18):1585-1589. doi:10.1001/jama.280.18.1585
24. Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11(2):395-402. doi:10.1046/j.1365-2036.1997.142318000.x
25. Patrick DL, Drossman DA, Frederick IO, DiCesare J, Puder KL. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci. 1998;43(2):400-411. doi:10.1023/a:1018831127942
26. Attanasio V, Andrasik F, Blanchard EB, Arena JG. Psychometric properties of the SUNYA revision of the Psychosomatic Symptom Checklist. J Behav Med. 1984;7(2):247-257. doi:10.1007/BF00845390
27. Weathers F, Litz B, Herman D, Huska J, Keane T. The PTSD Checklist (PCL): reliability, validity, and diagnostic utility. Accessed August 25, 2022. https://www.researchgate.net/publication/291448760_The_PTSD_Checklist_PCL_Reliability_validity_and_diagnostic_utility
28. Derogatis L. Brief Symptom Inventory-18 (BSI-18): Administration, Scoring, and Procedure Manual. Ed 3 ed. National Computer Systems; 2000.
29. Stamps BW, Lyon WJ, Irvin AP, Kelley-Loughnane N, Goodson MS. A pilot study of the effect of deployment on the gut microbiome and traveler´s diarrhea susceptibility. Front Cell Infect Microbiol. 2020;10:589297. doi:10.3389/fcimb.2020.589297
30. Kim HJ, Camilleri M, McKinzie S, et al. A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2003;17(7):895-904. doi:10.1046/j.1365-2036.2003.01543.x
31. Kim HJ, Vazquez Roque MI, Camilleri M, et al. A randomized controlled trial of a probiotic combination VSL# 3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol Motil. 2005;17(5):687-696. doi:10.1111/j.1365-2982.2005.00695.x32. Michail S, Kenche H. Gut microbiota is not modified by randomized, double-blind, placebo-controlled trial of vsl#3 in diarrhea-predominant irritable bowel syndrome. Probiotics Antimicrob Proteins. 2011;3(1):1-7. doi:10.1007/s12602-010-9059-y
33. Moayyedi P, Ford AC, Talley NJ, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59(3):325-332. doi:10.1136/gut.2008.167270

34. Ford AC, Harris LA, Lacy BE, Quigley EMM, Moayyedi P. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48(10):1044-1060. doi:10.1111/apt.15001
35. Dale HF, Rasmussen SH, Asiller OO, Lied GA. Probiotics in irritable bowel syndrome: an up-to-date systematic review. Nutrients. 2019;11(9). doi:10.3390/nu11092048
36. Didari T, Mozaffari S, Nikfar S, Abdollahi M. Effectiveness of probiotics in irritable bowel syndrome: Updated systematic review with meta-analysis. World J Gastroenterol. 2015;21(10):3072-84. doi:10.3748/wjg.v21.i10.3072
37. Hungin APS, Mitchell CR, Whorwell P, et al. Systematic review: probiotics in the management of lower gastrointestinal symptoms—an updated evidence-based international consensus. Aliment Pharmacol Ther. 2018;47(8):1054-1070. doi:10.1111/apt.14539
38. Niu HL, Xiao JY. The efficacy and safety of probiotics in patients with irritable bowel syndrome: evidence based on 35 randomized controlled trials. Int J Surg. 2020;75:116-127. doi:10.1016/j.ijsu.2020.01.142
39. Wong RK, Yang C, Song GH, Wong J, Ho KY. Melatonin regulation as a possible mechanism for probiotic (VSL#3) in irritable bowel syndrome: a randomized double-blinded placebo study. Dig Dis Sci. 2015;60(1):186-194. doi:10.1007/s10620-014-3299-8
40. Ford AC, Moayyedi P, Lacy BE, et al. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109(suppl 1):S2-26; quiz S27. doi: 10.1038/ajg.2014.187
41. Simren M, Barbara G, Flint HJ, et al. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013;62(1):159-76. doi:10.1136/gutjnl-2012-302167
42. Ki Cha B, Mun Jung S, Hwan Choi C, et al. The effect of a multispecies probiotic mixture on the symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Clin Gastroenterol. 2012;46(3):220-7. doi:10.1097/MCG.0b013e31823712b1
43. Connell M, Shin A, James-Stevenson T, Xu H, Imperiale TF, Herron J. Systematic review and meta-analysis: Efficacy of patented probiotic, VSL#3, in irritable bowel syndrome. Neurogastroenterol Motil. 2018;30(12):e13427. doi:10.1111/nmo.13427
44. Lyra A, Hillila M, Huttunen T, et al. Irritable bowel syndrome symptom severity improves equally with probiotic and placebo. World J Gastroenterol. 2016;22(48):10631-10642. doi:10.3748/wjg.v22.i48.10631
45. Sanders ME, Guarner F, Guerrant R, et al. An update on the use and investigation of probiotics in health and disease. Gut. 2013;62(5):787-796. doi:10.1136/gutjnl-2012-302504
46. Tuteja AK. Deployment-associated functional gastrointestinal disorders: do we know the etiology? Dig Dis Sci. 2011;56(11):3109-3111. doi:10.1007/s10620-011-1856-y
1. O’Shea EF, Cotter PD, Stanton C, Ross RP, Hill C. Production of bioactive substances by intestinal bacteria as a basis for explaining probiotic mechanisms: bacteriocins and conjugated linoleic acid. Int J Food Microbiol. 2012;152(3):189-205. doi:10.1016/j.ijfoodmicro.2011.05.025.
2. Kamiya T, Wang L, Forsythe P, et al. Inhibitory effects of Lactobacillus reuteri on visceral pain induced by colorectal distension in Sprague-Dawley rats. Gut. 2006;55(2):191-196. doi:10.1136/gut.2005.070987.
3. Verdu EF, Bercik P, Verma-Gandhu M, et al. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut. 2006;55(2):182-190. doi:10.1136/gut.2005.066100
4. Ford AC, Harris LA, Lacy BE, Quigley EMM, Moayyedi P. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48(10):1044-1060. doi:10.1111/apt.15001.
5. Niu HL, Xiao JY. The efficacy and safety of probiotics in patients with irritable bowel syndrome: Evidence based on 35 randomized controlled trials. Int J Surg. 2020;75:116-127. doi:10.1016/j.ijsu.2020.01.142.
6. Wong RK, Yang C, Song GH, Wong J, Ho KY. Melatonin regulation as a possible mechanism for probiotic (VSL#3) in irritable bowel syndrome: a randomized double-blinded placebo study. Dig Dis Sci. 2015;60(1):186-194. doi:10.1007/s10620-014-3299-8.
7. Hyams KC, Bourgeois AL, Merrell BR, et al. Diarrheal disease during Operation Desert Shield. N Engl J Med. 1991;325(20):1423-1428. doi:10.1056/NEJM199111143252006 8. Clancy RL, Gleeson M, Cox A, et al. Reversal in fatigued athletes of a defect in interferon gamma secretion after administration of Lactobacillus acidophilus. Br J Sports Med. 2006;40(4):351-354. doi:10.1136/bjsm.2005.024364
9. Sullivan A, Nord CE, Evengard B. Effect of supplement with lactic-acid producing bacteria on fatigue and physical activity in patients with chronic fatigue syndrome. Nutr J. 2009;8:4. doi:10.1186/1475-2891-8-4
10. Pittayanon R, Lau JT, Yuan Y, et al. Gut microbiota in patients with irritable bowel syndrome—a systematic review. Gastroenterology. 2019;157(1):97-108. doi:10.1053/j.gastro.2019.03.049
11. Rao RK, Samak G. Protection and restitution of gut barrier by probiotics: nutritional and clinical implications. Curr Nutr Food Sci. 2013;9(2):99-107. doi:10.2174/1573401311309020004
12. O´Shea EF, Cotter PD, Stanton C, Ross RP, Hill C. Production of bioactive substances by intestinal bacteria as a basis for explaining probiotic mechanisms: bacteriocins and conjugated linoleic acid. Int J Food Microbiol. 2012;152(3):189-205. doi:10.1016/j.ijfoodmicro.2011.05.025
13. Kamiya T, Wang L, Forsythe P, et al. Inhibitory effects of Lactobacillus reuteri on visceral pain induced by colorectal distension in Sprague-Dawley rats. Gut. 2006;55(2):191-196. doi:10.1136/gut.2005.070987
14. Verdu EF, Bercik P, Verma-Gandhu M, et al. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut. 2006;55(2):182-190. doi:10.1136/gut.2005.06610015. O´Mahony L, McCarthy J, Kelly P, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128(3):541-551. doi:10.1053/j.gastro.2004.11.050
16. Alhasson F, Das S, Seth R, et al. Altered gut microbiome in a mouse model of Gulf War Illness causes neuroinflammation and intestinal injury via leaky gut and TLR4 activation. PLoS One. 2017;12(3):e0172914. doi:10.1371/journal.pone.0172914.17. Janulewicz PA, Seth RK, Carlson JM, et al. The gut-microbiome in Gulf War veterans: a preliminary report. Int J Environ Res Public Health. 2019;16(19). doi:10.3390/ijerph16193751
18. Dang X, Xu M, Liu D, Zhou D, Yang W. Assessing the efficacy and safety of fecal microbiota transplantation and probiotic VSL#3 for active ulcerative colitis: a systematic review and meta-analysis. PLoS One. 2020;15(3):e0228846. doi:10.1371/journal.pone.0228846
19. Ford AC, Quigley EM, Lacy BE, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014;109(10):1547-1561; quiz 1546, 1562. doi:10.1038/ajg.2014.202
20. Rohatgi S, Ahuja V, Makharia GK, et al. VSL#3 induces and maintains short-term clinical response in patients with active microscopic colitis: a two-phase randomised clinical trial. BMJ Open Gastroenterol. 2015;2(1):e000018. doi:10.1136/bmjgast-2014-000018
21. Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130(5):1480-1491. doi:10.1053/j.gastro.2005.11.061
22. Talley NJ, Phillips SF, Melton J, 3rd, Wiltgen C, Zinsmeister AR. A patient questionnaire to identify bowel disease. Ann Intern Med. 1989;111(8):671-674. doi:10.7326/0003-4819-111-8-671
23. Bensoussan A, Talley NJ, Hing M, Menzies R, Guo A, Ngu M. Treatment of irritable bowel syndrome with Chinese herbal medicine: a randomized controlled trial. JAMA. 1998;280(18):1585-1589. doi:10.1001/jama.280.18.1585
24. Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11(2):395-402. doi:10.1046/j.1365-2036.1997.142318000.x
25. Patrick DL, Drossman DA, Frederick IO, DiCesare J, Puder KL. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci. 1998;43(2):400-411. doi:10.1023/a:1018831127942
26. Attanasio V, Andrasik F, Blanchard EB, Arena JG. Psychometric properties of the SUNYA revision of the Psychosomatic Symptom Checklist. J Behav Med. 1984;7(2):247-257. doi:10.1007/BF00845390
27. Weathers F, Litz B, Herman D, Huska J, Keane T. The PTSD Checklist (PCL): reliability, validity, and diagnostic utility. Accessed August 25, 2022. https://www.researchgate.net/publication/291448760_The_PTSD_Checklist_PCL_Reliability_validity_and_diagnostic_utility
28. Derogatis L. Brief Symptom Inventory-18 (BSI-18): Administration, Scoring, and Procedure Manual. Ed 3 ed. National Computer Systems; 2000.
29. Stamps BW, Lyon WJ, Irvin AP, Kelley-Loughnane N, Goodson MS. A pilot study of the effect of deployment on the gut microbiome and traveler´s diarrhea susceptibility. Front Cell Infect Microbiol. 2020;10:589297. doi:10.3389/fcimb.2020.589297
30. Kim HJ, Camilleri M, McKinzie S, et al. A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2003;17(7):895-904. doi:10.1046/j.1365-2036.2003.01543.x
31. Kim HJ, Vazquez Roque MI, Camilleri M, et al. A randomized controlled trial of a probiotic combination VSL# 3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol Motil. 2005;17(5):687-696. doi:10.1111/j.1365-2982.2005.00695.x32. Michail S, Kenche H. Gut microbiota is not modified by randomized, double-blind, placebo-controlled trial of vsl#3 in diarrhea-predominant irritable bowel syndrome. Probiotics Antimicrob Proteins. 2011;3(1):1-7. doi:10.1007/s12602-010-9059-y
33. Moayyedi P, Ford AC, Talley NJ, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59(3):325-332. doi:10.1136/gut.2008.167270

34. Ford AC, Harris LA, Lacy BE, Quigley EMM, Moayyedi P. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48(10):1044-1060. doi:10.1111/apt.15001
35. Dale HF, Rasmussen SH, Asiller OO, Lied GA. Probiotics in irritable bowel syndrome: an up-to-date systematic review. Nutrients. 2019;11(9). doi:10.3390/nu11092048
36. Didari T, Mozaffari S, Nikfar S, Abdollahi M. Effectiveness of probiotics in irritable bowel syndrome: Updated systematic review with meta-analysis. World J Gastroenterol. 2015;21(10):3072-84. doi:10.3748/wjg.v21.i10.3072
37. Hungin APS, Mitchell CR, Whorwell P, et al. Systematic review: probiotics in the management of lower gastrointestinal symptoms—an updated evidence-based international consensus. Aliment Pharmacol Ther. 2018;47(8):1054-1070. doi:10.1111/apt.14539
38. Niu HL, Xiao JY. The efficacy and safety of probiotics in patients with irritable bowel syndrome: evidence based on 35 randomized controlled trials. Int J Surg. 2020;75:116-127. doi:10.1016/j.ijsu.2020.01.142
39. Wong RK, Yang C, Song GH, Wong J, Ho KY. Melatonin regulation as a possible mechanism for probiotic (VSL#3) in irritable bowel syndrome: a randomized double-blinded placebo study. Dig Dis Sci. 2015;60(1):186-194. doi:10.1007/s10620-014-3299-8
40. Ford AC, Moayyedi P, Lacy BE, et al. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109(suppl 1):S2-26; quiz S27. doi: 10.1038/ajg.2014.187
41. Simren M, Barbara G, Flint HJ, et al. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013;62(1):159-76. doi:10.1136/gutjnl-2012-302167
42. Ki Cha B, Mun Jung S, Hwan Choi C, et al. The effect of a multispecies probiotic mixture on the symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Clin Gastroenterol. 2012;46(3):220-7. doi:10.1097/MCG.0b013e31823712b1
43. Connell M, Shin A, James-Stevenson T, Xu H, Imperiale TF, Herron J. Systematic review and meta-analysis: Efficacy of patented probiotic, VSL#3, in irritable bowel syndrome. Neurogastroenterol Motil. 2018;30(12):e13427. doi:10.1111/nmo.13427
44. Lyra A, Hillila M, Huttunen T, et al. Irritable bowel syndrome symptom severity improves equally with probiotic and placebo. World J Gastroenterol. 2016;22(48):10631-10642. doi:10.3748/wjg.v22.i48.10631
45. Sanders ME, Guarner F, Guerrant R, et al. An update on the use and investigation of probiotics in health and disease. Gut. 2013;62(5):787-796. doi:10.1136/gutjnl-2012-302504
46. Tuteja AK. Deployment-associated functional gastrointestinal disorders: do we know the etiology? Dig Dis Sci. 2011;56(11):3109-3111. doi:10.1007/s10620-011-1856-y
Spontaneous ecchymoses
A 65-YEAR-OLD WOMAN was brought into the emergency department by her daughter for spontaneous bruising, fatigue, and weakness of several weeks’ duration. She denied taking any medications or illicit drugs and had not experienced any falls or trauma. On a daily basis, she smoked 5 to 7 cigarettes and drank 6 or 7 beers, as had been her custom for several years. The patient lived alone and was grieving the death of her beloved dog, who had died a month earlier. She reported that since the death of her dog, her diet, which hadn’t been especially good to begin with, had deteriorated; it now consisted of beer and crackers.
On admission, she was mildly tachycardic (105 beats/min) with a blood pressure of 125/66 mm Hg. Physical examination revealed a frail-appearing woman who was in no acute distress but was unable to stand without assistance. She had diffuse ecchymoses and perifollicular, purpuric, hyperkeratotic papules and plaques on both of her legs (FIGURES 1A and 1B). In addition, she had faint perifollicular purpuric macules on her upper back. An oral examination revealed poor dentition.
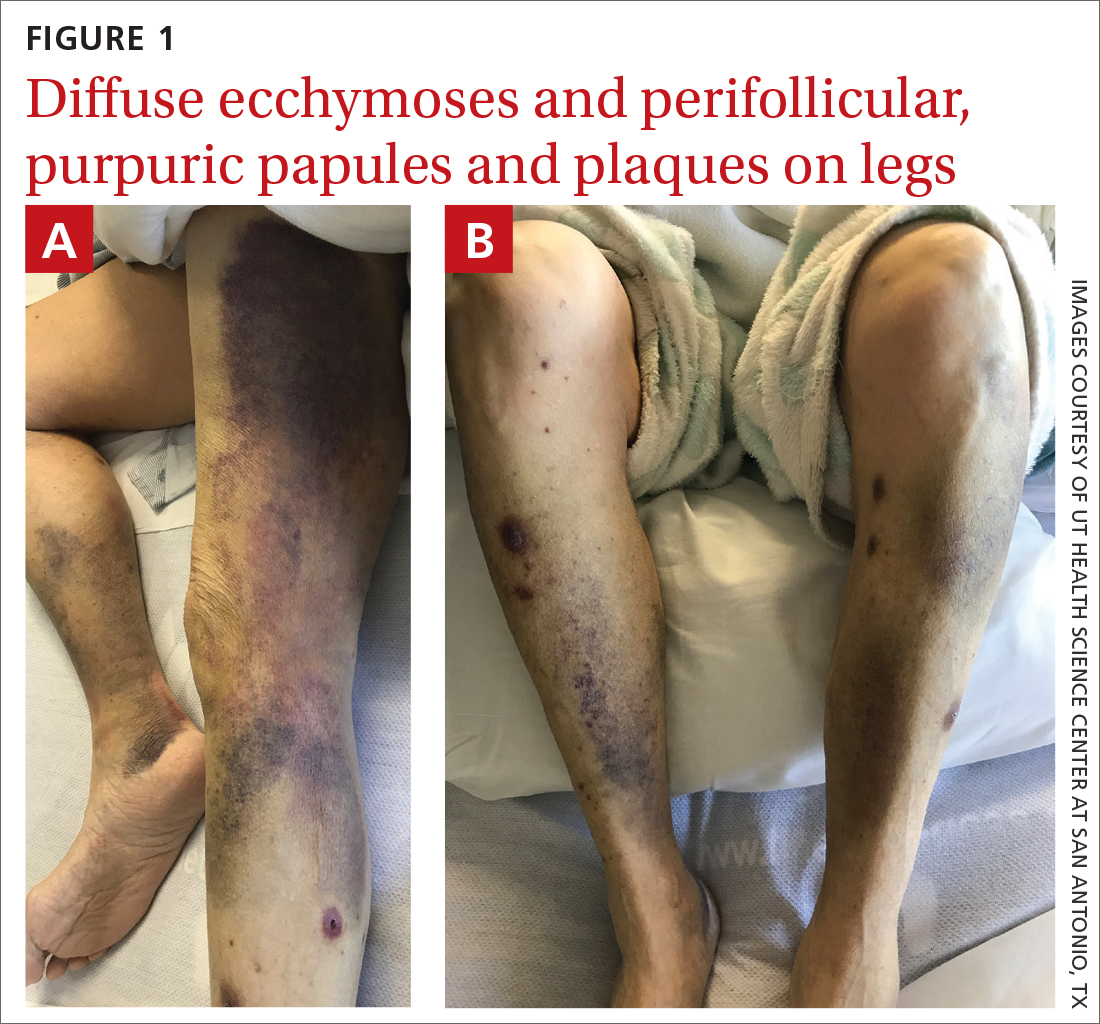
A punch biopsy was performed on her leg, and it revealed noninflammatory dermal hemorrhage without evidence of vasculitis or vasculopathy.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Scurvy
Based on the patient’s appearance and her dietary history, we suspected scurvy, so a serum vitamin C level was ordered. The results took several days to return. In the meantime, additional lab work revealed hyponatremia (sodium, 129 mmol/L; normal range, 135-145 mmol/L), hypokalemia (potassium, 3 mmol/L; normal range, 3.5-5.2 mmol/L), hypophosphatemia (phosphorus, 2.3 mg/dL; normal range, 2.8-4.5 mg/dL); low serum vitamin D (6 ng/mL; normal range, 20-40 ng/mL); and macrocytic anemia (hemoglobin, 7.4 g/dL; normal range, 11-18 g/dL) with a mean corpuscular volume of 101.1 fL (normal range, 80-100 fL). Her iron panel showed normal serum iron and total iron binding capacity with a normal ferritin level (294 ng/mL; normal range, 30-300 ng/mL). A peripheral blood smear test uncovered mild anisocytosis and polychromasia, with no schistocytes. A fecal immunochemical test was negative.
Several days after admission, the results of the patient’s vitamin C test came back. Her levels were undetectable (< 5 µmol/L; normal range, 11-23 µmol/L), confirming that the patient had scurvy.
A health hazard to marinersthat is still around today
Scurvy is a condition that arises from a deficiency of vitamin C, or ascorbic acid. The first known case of scurvy was in 1550 BC.1 Hippocrates termed the condition “ileos ematitis” and stated that “the mouth feels bad; the gums are detached from the teeth; blood runs from the nostrils … ulcerations on the legs … skin is thin.”1 Scurvy was a major health hazard of mariners between the 15thand 18th centuries.2 Today, the deficiency is uncommon in industrialized countries because there are many sources of vitamin C available through diet and vitamin supplements.3 In the United States, the prevalence of vitamin C deficiency is approximately 7%.4
An essential nutrient in humans, vitamin C is required as a cofactor in the synthesis of mature collagen.3 Collagen is found in skin, bone, and endothelium. Inadequate collagen levels can result in poor dermal support of vessels and tissue fragility, leading to hemorrhage, which can occur in nearly any organ system.
Vitamin C deficiency occurs when serum concentration falls below 11.4
Continue to: Scurvy manifests after 8 to 12 weeks
Scurvy manifests after 8 to 12 weeks of inadequate vitamin C intake.1 Patients may initially experience malaise and irritability. Anemia is common. Dermatologic findings include hyperkeratotic lesions, ecchymoses, poor wound healing, gingival swelling with loss of teeth, petechiae, and corkscrew hairs. Perifollicular hemorrhage is a characteristic finding of scurvy, generally seen on the lower extremities, where the capillaries are under higher hydrostatic pressure.3 Patients may also have musculoskeletal involvement with osteopenia or hemarthroses, which may be seen on imaging.3,5 Cardiorespiratory, gastrointestinal, ophthalmologic, and neurologic findings have also been reported.3
Differential is broad; zero in on patient’s history
The differential diagnosis for hemorrhagic skin lesions is extensive and includes scurvy, coagulopathies, trauma, vasculitis, and vasculopathies.
The presence of perifollicular hemorrhage with corkscrew hairs and a dietary history of inadequate vitamin C intake can differentiate scurvy from other conditions. Serum testing revealing low plasma vitamin C will support the diagnosis, but this is an insensitive test, as values increase with recent intake. Leukocyte ascorbic acid concentrations are more representative of total body stores, but impractical for routine use.6 Skin biopsy is not necessary but may help to rule out other conditions.
Ascorbic acid will facilitate a speedy recovery
Treatment of scurvy includes vitamin C replacement. Response is rapid, with improvement to lethargy within several days and disappearance of other manifestations within several weeks.3 Recommendations on supplementation doses and forms vary, but adults require 300 to 1000 mg/d of ascorbic acid for at least 1 week or until clinical symptoms resolve and stores are repleted.3,5,7
During our patient’s hospital stay, she remained stable and improved clinically with vitamin supplementation (ascorbic acid 1 g/d for 3 days, 500 mg/d after that) and physical therapy. She was counseled on a healthy diet, which would include citrus fruits, tomatoes, and leafy vegetables. The patient was also advised to refrain from drinking alcohol and was given information on an alcohol abstinence program.
At her 1-month follow-up, her condition had improved with near resolution of the skin lesions. She reported that she had given up cigarettes and alcohol. She said she’d also begun eating more citrus fruits and leafy vegetables.
1. Maxfield L, Crane JS. Vitamin C deficiency (scurvy). In: StatPearls. StatPearls Publishing; 2020. Accessed on September 13, 2022. www.ncbi.nlm.nih.gov/books/NBK493187/
2. Worral S. A nightmare disease haunted ships during age of discovery. National Geographic. January 15, 2017. Accessed September 21, 2022. www.nationalgeographic.com/science/article/scurvy-disease-discovery-jonathan-lamb
3. Hirschmann JV, Raugi GJ. Adult Scurvy. J Am Acad Dermatol. 1999;41:895-906. doi: 10.1016/s0190-9622(99)70244-6
4. Schleicher RL, Carroll MD, Ford ES, et al. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003-2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr. 2009;90:1252-1263. doi: 10.3945/ajcn.2008.27016
5. Agarwal A, Shaharyar A, Kumar A, et al. Scurvy in pediatric age group – A disease often forgotten? J Clin Orthop Trauma. 2015;6:101-107. doi: 10.1016/j.jcot.2014.12.003
6. Scurvy and its prevention and control in major emergencies. World Health Organization. February 23, 1999. Accessed September 13, 2022. www.who.int/publications/i/item/WHO-NHD-99.11
7. Weinstein M, Babyn P, Zlotkin S. An orange a day keeps the doctor away: scurvy in the year 2000. Pediatrics. 2001;108:E55. doi: 10.1542/peds.108.3.e55
A 65-YEAR-OLD WOMAN was brought into the emergency department by her daughter for spontaneous bruising, fatigue, and weakness of several weeks’ duration. She denied taking any medications or illicit drugs and had not experienced any falls or trauma. On a daily basis, she smoked 5 to 7 cigarettes and drank 6 or 7 beers, as had been her custom for several years. The patient lived alone and was grieving the death of her beloved dog, who had died a month earlier. She reported that since the death of her dog, her diet, which hadn’t been especially good to begin with, had deteriorated; it now consisted of beer and crackers.
On admission, she was mildly tachycardic (105 beats/min) with a blood pressure of 125/66 mm Hg. Physical examination revealed a frail-appearing woman who was in no acute distress but was unable to stand without assistance. She had diffuse ecchymoses and perifollicular, purpuric, hyperkeratotic papules and plaques on both of her legs (FIGURES 1A and 1B). In addition, she had faint perifollicular purpuric macules on her upper back. An oral examination revealed poor dentition.

A punch biopsy was performed on her leg, and it revealed noninflammatory dermal hemorrhage without evidence of vasculitis or vasculopathy.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Scurvy
Based on the patient’s appearance and her dietary history, we suspected scurvy, so a serum vitamin C level was ordered. The results took several days to return. In the meantime, additional lab work revealed hyponatremia (sodium, 129 mmol/L; normal range, 135-145 mmol/L), hypokalemia (potassium, 3 mmol/L; normal range, 3.5-5.2 mmol/L), hypophosphatemia (phosphorus, 2.3 mg/dL; normal range, 2.8-4.5 mg/dL); low serum vitamin D (6 ng/mL; normal range, 20-40 ng/mL); and macrocytic anemia (hemoglobin, 7.4 g/dL; normal range, 11-18 g/dL) with a mean corpuscular volume of 101.1 fL (normal range, 80-100 fL). Her iron panel showed normal serum iron and total iron binding capacity with a normal ferritin level (294 ng/mL; normal range, 30-300 ng/mL). A peripheral blood smear test uncovered mild anisocytosis and polychromasia, with no schistocytes. A fecal immunochemical test was negative.
Several days after admission, the results of the patient’s vitamin C test came back. Her levels were undetectable (< 5 µmol/L; normal range, 11-23 µmol/L), confirming that the patient had scurvy.
A health hazard to marinersthat is still around today
Scurvy is a condition that arises from a deficiency of vitamin C, or ascorbic acid. The first known case of scurvy was in 1550 BC.1 Hippocrates termed the condition “ileos ematitis” and stated that “the mouth feels bad; the gums are detached from the teeth; blood runs from the nostrils … ulcerations on the legs … skin is thin.”1 Scurvy was a major health hazard of mariners between the 15thand 18th centuries.2 Today, the deficiency is uncommon in industrialized countries because there are many sources of vitamin C available through diet and vitamin supplements.3 In the United States, the prevalence of vitamin C deficiency is approximately 7%.4
An essential nutrient in humans, vitamin C is required as a cofactor in the synthesis of mature collagen.3 Collagen is found in skin, bone, and endothelium. Inadequate collagen levels can result in poor dermal support of vessels and tissue fragility, leading to hemorrhage, which can occur in nearly any organ system.
Vitamin C deficiency occurs when serum concentration falls below 11.4
Continue to: Scurvy manifests after 8 to 12 weeks
Scurvy manifests after 8 to 12 weeks of inadequate vitamin C intake.1 Patients may initially experience malaise and irritability. Anemia is common. Dermatologic findings include hyperkeratotic lesions, ecchymoses, poor wound healing, gingival swelling with loss of teeth, petechiae, and corkscrew hairs. Perifollicular hemorrhage is a characteristic finding of scurvy, generally seen on the lower extremities, where the capillaries are under higher hydrostatic pressure.3 Patients may also have musculoskeletal involvement with osteopenia or hemarthroses, which may be seen on imaging.3,5 Cardiorespiratory, gastrointestinal, ophthalmologic, and neurologic findings have also been reported.3
Differential is broad; zero in on patient’s history
The differential diagnosis for hemorrhagic skin lesions is extensive and includes scurvy, coagulopathies, trauma, vasculitis, and vasculopathies.
The presence of perifollicular hemorrhage with corkscrew hairs and a dietary history of inadequate vitamin C intake can differentiate scurvy from other conditions. Serum testing revealing low plasma vitamin C will support the diagnosis, but this is an insensitive test, as values increase with recent intake. Leukocyte ascorbic acid concentrations are more representative of total body stores, but impractical for routine use.6 Skin biopsy is not necessary but may help to rule out other conditions.
Ascorbic acid will facilitate a speedy recovery
Treatment of scurvy includes vitamin C replacement. Response is rapid, with improvement to lethargy within several days and disappearance of other manifestations within several weeks.3 Recommendations on supplementation doses and forms vary, but adults require 300 to 1000 mg/d of ascorbic acid for at least 1 week or until clinical symptoms resolve and stores are repleted.3,5,7
During our patient’s hospital stay, she remained stable and improved clinically with vitamin supplementation (ascorbic acid 1 g/d for 3 days, 500 mg/d after that) and physical therapy. She was counseled on a healthy diet, which would include citrus fruits, tomatoes, and leafy vegetables. The patient was also advised to refrain from drinking alcohol and was given information on an alcohol abstinence program.
At her 1-month follow-up, her condition had improved with near resolution of the skin lesions. She reported that she had given up cigarettes and alcohol. She said she’d also begun eating more citrus fruits and leafy vegetables.
A 65-YEAR-OLD WOMAN was brought into the emergency department by her daughter for spontaneous bruising, fatigue, and weakness of several weeks’ duration. She denied taking any medications or illicit drugs and had not experienced any falls or trauma. On a daily basis, she smoked 5 to 7 cigarettes and drank 6 or 7 beers, as had been her custom for several years. The patient lived alone and was grieving the death of her beloved dog, who had died a month earlier. She reported that since the death of her dog, her diet, which hadn’t been especially good to begin with, had deteriorated; it now consisted of beer and crackers.
On admission, she was mildly tachycardic (105 beats/min) with a blood pressure of 125/66 mm Hg. Physical examination revealed a frail-appearing woman who was in no acute distress but was unable to stand without assistance. She had diffuse ecchymoses and perifollicular, purpuric, hyperkeratotic papules and plaques on both of her legs (FIGURES 1A and 1B). In addition, she had faint perifollicular purpuric macules on her upper back. An oral examination revealed poor dentition.

A punch biopsy was performed on her leg, and it revealed noninflammatory dermal hemorrhage without evidence of vasculitis or vasculopathy.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Scurvy
Based on the patient’s appearance and her dietary history, we suspected scurvy, so a serum vitamin C level was ordered. The results took several days to return. In the meantime, additional lab work revealed hyponatremia (sodium, 129 mmol/L; normal range, 135-145 mmol/L), hypokalemia (potassium, 3 mmol/L; normal range, 3.5-5.2 mmol/L), hypophosphatemia (phosphorus, 2.3 mg/dL; normal range, 2.8-4.5 mg/dL); low serum vitamin D (6 ng/mL; normal range, 20-40 ng/mL); and macrocytic anemia (hemoglobin, 7.4 g/dL; normal range, 11-18 g/dL) with a mean corpuscular volume of 101.1 fL (normal range, 80-100 fL). Her iron panel showed normal serum iron and total iron binding capacity with a normal ferritin level (294 ng/mL; normal range, 30-300 ng/mL). A peripheral blood smear test uncovered mild anisocytosis and polychromasia, with no schistocytes. A fecal immunochemical test was negative.
Several days after admission, the results of the patient’s vitamin C test came back. Her levels were undetectable (< 5 µmol/L; normal range, 11-23 µmol/L), confirming that the patient had scurvy.
A health hazard to marinersthat is still around today
Scurvy is a condition that arises from a deficiency of vitamin C, or ascorbic acid. The first known case of scurvy was in 1550 BC.1 Hippocrates termed the condition “ileos ematitis” and stated that “the mouth feels bad; the gums are detached from the teeth; blood runs from the nostrils … ulcerations on the legs … skin is thin.”1 Scurvy was a major health hazard of mariners between the 15thand 18th centuries.2 Today, the deficiency is uncommon in industrialized countries because there are many sources of vitamin C available through diet and vitamin supplements.3 In the United States, the prevalence of vitamin C deficiency is approximately 7%.4
An essential nutrient in humans, vitamin C is required as a cofactor in the synthesis of mature collagen.3 Collagen is found in skin, bone, and endothelium. Inadequate collagen levels can result in poor dermal support of vessels and tissue fragility, leading to hemorrhage, which can occur in nearly any organ system.
Vitamin C deficiency occurs when serum concentration falls below 11.4
Continue to: Scurvy manifests after 8 to 12 weeks
Scurvy manifests after 8 to 12 weeks of inadequate vitamin C intake.1 Patients may initially experience malaise and irritability. Anemia is common. Dermatologic findings include hyperkeratotic lesions, ecchymoses, poor wound healing, gingival swelling with loss of teeth, petechiae, and corkscrew hairs. Perifollicular hemorrhage is a characteristic finding of scurvy, generally seen on the lower extremities, where the capillaries are under higher hydrostatic pressure.3 Patients may also have musculoskeletal involvement with osteopenia or hemarthroses, which may be seen on imaging.3,5 Cardiorespiratory, gastrointestinal, ophthalmologic, and neurologic findings have also been reported.3
Differential is broad; zero in on patient’s history
The differential diagnosis for hemorrhagic skin lesions is extensive and includes scurvy, coagulopathies, trauma, vasculitis, and vasculopathies.
The presence of perifollicular hemorrhage with corkscrew hairs and a dietary history of inadequate vitamin C intake can differentiate scurvy from other conditions. Serum testing revealing low plasma vitamin C will support the diagnosis, but this is an insensitive test, as values increase with recent intake. Leukocyte ascorbic acid concentrations are more representative of total body stores, but impractical for routine use.6 Skin biopsy is not necessary but may help to rule out other conditions.
Ascorbic acid will facilitate a speedy recovery
Treatment of scurvy includes vitamin C replacement. Response is rapid, with improvement to lethargy within several days and disappearance of other manifestations within several weeks.3 Recommendations on supplementation doses and forms vary, but adults require 300 to 1000 mg/d of ascorbic acid for at least 1 week or until clinical symptoms resolve and stores are repleted.3,5,7
During our patient’s hospital stay, she remained stable and improved clinically with vitamin supplementation (ascorbic acid 1 g/d for 3 days, 500 mg/d after that) and physical therapy. She was counseled on a healthy diet, which would include citrus fruits, tomatoes, and leafy vegetables. The patient was also advised to refrain from drinking alcohol and was given information on an alcohol abstinence program.
At her 1-month follow-up, her condition had improved with near resolution of the skin lesions. She reported that she had given up cigarettes and alcohol. She said she’d also begun eating more citrus fruits and leafy vegetables.
1. Maxfield L, Crane JS. Vitamin C deficiency (scurvy). In: StatPearls. StatPearls Publishing; 2020. Accessed on September 13, 2022. www.ncbi.nlm.nih.gov/books/NBK493187/
2. Worral S. A nightmare disease haunted ships during age of discovery. National Geographic. January 15, 2017. Accessed September 21, 2022. www.nationalgeographic.com/science/article/scurvy-disease-discovery-jonathan-lamb
3. Hirschmann JV, Raugi GJ. Adult Scurvy. J Am Acad Dermatol. 1999;41:895-906. doi: 10.1016/s0190-9622(99)70244-6
4. Schleicher RL, Carroll MD, Ford ES, et al. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003-2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr. 2009;90:1252-1263. doi: 10.3945/ajcn.2008.27016
5. Agarwal A, Shaharyar A, Kumar A, et al. Scurvy in pediatric age group – A disease often forgotten? J Clin Orthop Trauma. 2015;6:101-107. doi: 10.1016/j.jcot.2014.12.003
6. Scurvy and its prevention and control in major emergencies. World Health Organization. February 23, 1999. Accessed September 13, 2022. www.who.int/publications/i/item/WHO-NHD-99.11
7. Weinstein M, Babyn P, Zlotkin S. An orange a day keeps the doctor away: scurvy in the year 2000. Pediatrics. 2001;108:E55. doi: 10.1542/peds.108.3.e55
1. Maxfield L, Crane JS. Vitamin C deficiency (scurvy). In: StatPearls. StatPearls Publishing; 2020. Accessed on September 13, 2022. www.ncbi.nlm.nih.gov/books/NBK493187/
2. Worral S. A nightmare disease haunted ships during age of discovery. National Geographic. January 15, 2017. Accessed September 21, 2022. www.nationalgeographic.com/science/article/scurvy-disease-discovery-jonathan-lamb
3. Hirschmann JV, Raugi GJ. Adult Scurvy. J Am Acad Dermatol. 1999;41:895-906. doi: 10.1016/s0190-9622(99)70244-6
4. Schleicher RL, Carroll MD, Ford ES, et al. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003-2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr. 2009;90:1252-1263. doi: 10.3945/ajcn.2008.27016
5. Agarwal A, Shaharyar A, Kumar A, et al. Scurvy in pediatric age group – A disease often forgotten? J Clin Orthop Trauma. 2015;6:101-107. doi: 10.1016/j.jcot.2014.12.003
6. Scurvy and its prevention and control in major emergencies. World Health Organization. February 23, 1999. Accessed September 13, 2022. www.who.int/publications/i/item/WHO-NHD-99.11
7. Weinstein M, Babyn P, Zlotkin S. An orange a day keeps the doctor away: scurvy in the year 2000. Pediatrics. 2001;108:E55. doi: 10.1542/peds.108.3.e55
Does an early COPD diagnosis improve long-term outcomes?
EVIDENCE SUMMARY
Early Dx didn’t improve smoking cessation rates or treatment outcomes
A 2016 evidence report and systematic review for the US Preventive Services Task Force (USPSTF) identified no studies directly comparing the effectiveness of COPD screening on patient outcomes, so the authors looked first at studies on the outcomes of screening, followed by studies exploring the effects of early treatment.1
The authors identified 5 fair-quality RCTs (N = 1694) addressing the effect of screening asymptomatic patients for COPD with spirometry on the outcome of smoking cessation. One trial (n = 561) found better 12-month smoking cessation rates in patients who underwent spirometry screening and were given their “lung age” (13.6% vs 6.4% not given a lung age; P < .005; number needed to treat [NNT] = 14). However, a similar study (n = 542) published a year later found no significant difference in quit rates with or without “lung age” discussions (10.9% vs 13%, respectively; P not significant). In the other 3 studies, screening produced no significant effect on smoking cessation rates.1
As for possible early treatment benefits, the review authors identified only 1 RCT (n = 1175) that included any patients with mild COPD (defined as COPD with a forced expiratory volume in 1 second [FEV1] ≥ 80% of predicted normal value). It assessed treatment with inhaled corticosteroids (ICS) in patients with mild COPD who continued to smoke. The trial did not record symptoms (if any) at intake. ICS therapy reduced the frequency of COPD exacerbations (relative risk = 0.63; 95% CI, 0.47-0.85), although patients with milder COPD benefitted little in absolute terms (by 0.02 exacerbations/year).1 The review authors further noted that data were insufficient to make definitive statements about the effect of ICS on dyspnea or health-related quality of life.
But later diagnosis is associated with poorer outcomes
Two recent, large retrospective observational cohort studies, however, have examined the impact of an early vs late COPD diagnosis in patients with dyspnea or other symptoms of COPD.2,3 A later diagnosis was associated with worse outcomes.
In the first study, researchers in Sweden identified patients older than 40 years who had received a new diagnosis of COPD between 2000 and 2014.2 They examined electronic health record data for 6 different “indicators” of COPD during the 5 years prior to date of diagnosis: pneumonia, other respiratory disease, oral steroids, antibiotics for respiratory infection, prescribed drugs for respiratory symptoms, and lung function measurement. Researchers categorized patients as early diagnosis (if they had ≤ 2 indicators prior to diagnosis) or late diagnosis (≥ 3 indicators prior to diagnosis). Compared with early diagnosis (n = 3870), late diagnosis (n = 8827) was associated with
- a higher annual rate of exacerbations within the first 2 years after diagnosis (2.67 vs 1.41; hazard ratio [HR] = 1.89; 95% CI, 1.83-1.96; P < .0001; number of early diagnoses needed to prevent 1 exacerbation in 1 year = 79),
- shorter time to first exacerbation (HR = 1.61; 95% CI, 1.54-1.69; P < .0001), and
- higher direct health care costs (by €1500 per year; no P value given).
Mortality was not different between the groups (HR = 1.04; 95% CI, 0.98-1.11; P = .18).
The second investigation was a similarly designed retrospective observational cohort study using a large UK database.3 Researchers enrolled patients who were at least 40 years old and received a new diagnosis of COPD between 2011 and 2014.
Continue to: Researchers examined electronic...
Researchers examined electronic health record data in the 5 years prior to diagnosis for 7 possible indicators of early COPD: pneumonia, respiratory disease other than pneumonia, chest radiograph, prescription of oral steroids, prescription of antibiotics for lung infection, prescription to manage respiratory disease symptoms, and lung function measurement. Researchers categorized patients as early diagnosis (≥ 2 indicators prior to diagnosis) or late diagnosis (≥ 3 indicators prior to diagnosis). Compared with early diagnosis (n = 3375), late diagnosis (n = 6783) was associated with a higher annual rate of exacerbations over 3-year follow-up (1.09 vs 0.57; adjusted HR = 1.68; 95% CI, 1.59-1.79; P < .0001; or 1 additional exacerbation in 192 patients in 1 year), shorter mean time to first exacerbation (HR = 1.46; 95% CI: 1.38-1.55; P < .0001), and a higher risk of hospitalization within 3 years (rate ratio = 1.18; 95% CI, 1.08-1.28; P = .0001). The researchers did not evaluate for mortality.
Importantly, patients in the late COPD diagnosis group in both trials had higher rates of other severe illnesses that cause dyspnea, including cardiovascular disease and other pulmonary diseases. As a result, dyspnea of other etiologies may have contributed to both the later diagnoses and the poorer clinical outcomes of the late-diagnosis group. Both studies had a high risk of lead-time bias.
Recommendations from others
In 2016, the USPSTF gave a “D” rating (moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits) to screening asymptomatic adults without respiratory symptoms for COPD.4 Likewise, the 2017 Global Initiative for Chronic Obstructive Lung Disease (GOLD) report did not recommend routine screening with spirometry but did advocate trying to make an accurate diagnosis using spirometry in patients with risk factors for COPD and chronic, progressive symptoms.5
Editor’s takeaway
Reasonably good evidence failed to find a benefit from an early COPD diagnosis. Even smoking cessation rates were not improved. Without better disease-modifying treatments, spirometry—the gold standard for confirming a COPD diagnosis—should not be used for screening asymptomatic patients.
1. Guirguis-Blake JM, Senger CA, Webber EM, et al. Screening for chronic obstructive pulmonary disease: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315:1378-1393. doi:10.1001/jama.2016.2654
2. Larsson K, Janson C, Ställberg B, et al. Impact of COPD diagnosis timing on clinical and economic outcomes: the ARCTIC observational cohort study. Int J Chron Obstruct Pulmon Dis. 2019;14:995-1008. doi: 10.2147/COPD.S195382
3. Kostikas K, Price D, Gutzwiller FS, et al. Clinical impact and healthcare resource utilization associated with early versus late COPD diagnosis in patients from UK CPRD database. Int J Chron Obstruct Pulmon Dis. 2020;15:1729-1738. doi: 10.2147/COPD.S255414
4. US Preventive Services Task Force; Siu AL, Bibbins-Domingo K, Grossman DC, et al. Screening for chronic obstructive pulmonary disease: US Preventive Services Task Force recommendation statement. JAMA. 2016;315:1372-1377. doi: 10.1001/jama.2016.2638
5. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195:557-582. doi: 10.1164/rccm.201701-0218PP
EVIDENCE SUMMARY
Early Dx didn’t improve smoking cessation rates or treatment outcomes
A 2016 evidence report and systematic review for the US Preventive Services Task Force (USPSTF) identified no studies directly comparing the effectiveness of COPD screening on patient outcomes, so the authors looked first at studies on the outcomes of screening, followed by studies exploring the effects of early treatment.1
The authors identified 5 fair-quality RCTs (N = 1694) addressing the effect of screening asymptomatic patients for COPD with spirometry on the outcome of smoking cessation. One trial (n = 561) found better 12-month smoking cessation rates in patients who underwent spirometry screening and were given their “lung age” (13.6% vs 6.4% not given a lung age; P < .005; number needed to treat [NNT] = 14). However, a similar study (n = 542) published a year later found no significant difference in quit rates with or without “lung age” discussions (10.9% vs 13%, respectively; P not significant). In the other 3 studies, screening produced no significant effect on smoking cessation rates.1
As for possible early treatment benefits, the review authors identified only 1 RCT (n = 1175) that included any patients with mild COPD (defined as COPD with a forced expiratory volume in 1 second [FEV1] ≥ 80% of predicted normal value). It assessed treatment with inhaled corticosteroids (ICS) in patients with mild COPD who continued to smoke. The trial did not record symptoms (if any) at intake. ICS therapy reduced the frequency of COPD exacerbations (relative risk = 0.63; 95% CI, 0.47-0.85), although patients with milder COPD benefitted little in absolute terms (by 0.02 exacerbations/year).1 The review authors further noted that data were insufficient to make definitive statements about the effect of ICS on dyspnea or health-related quality of life.
But later diagnosis is associated with poorer outcomes
Two recent, large retrospective observational cohort studies, however, have examined the impact of an early vs late COPD diagnosis in patients with dyspnea or other symptoms of COPD.2,3 A later diagnosis was associated with worse outcomes.
In the first study, researchers in Sweden identified patients older than 40 years who had received a new diagnosis of COPD between 2000 and 2014.2 They examined electronic health record data for 6 different “indicators” of COPD during the 5 years prior to date of diagnosis: pneumonia, other respiratory disease, oral steroids, antibiotics for respiratory infection, prescribed drugs for respiratory symptoms, and lung function measurement. Researchers categorized patients as early diagnosis (if they had ≤ 2 indicators prior to diagnosis) or late diagnosis (≥ 3 indicators prior to diagnosis). Compared with early diagnosis (n = 3870), late diagnosis (n = 8827) was associated with
- a higher annual rate of exacerbations within the first 2 years after diagnosis (2.67 vs 1.41; hazard ratio [HR] = 1.89; 95% CI, 1.83-1.96; P < .0001; number of early diagnoses needed to prevent 1 exacerbation in 1 year = 79),
- shorter time to first exacerbation (HR = 1.61; 95% CI, 1.54-1.69; P < .0001), and
- higher direct health care costs (by €1500 per year; no P value given).
Mortality was not different between the groups (HR = 1.04; 95% CI, 0.98-1.11; P = .18).
The second investigation was a similarly designed retrospective observational cohort study using a large UK database.3 Researchers enrolled patients who were at least 40 years old and received a new diagnosis of COPD between 2011 and 2014.
Continue to: Researchers examined electronic...
Researchers examined electronic health record data in the 5 years prior to diagnosis for 7 possible indicators of early COPD: pneumonia, respiratory disease other than pneumonia, chest radiograph, prescription of oral steroids, prescription of antibiotics for lung infection, prescription to manage respiratory disease symptoms, and lung function measurement. Researchers categorized patients as early diagnosis (≥ 2 indicators prior to diagnosis) or late diagnosis (≥ 3 indicators prior to diagnosis). Compared with early diagnosis (n = 3375), late diagnosis (n = 6783) was associated with a higher annual rate of exacerbations over 3-year follow-up (1.09 vs 0.57; adjusted HR = 1.68; 95% CI, 1.59-1.79; P < .0001; or 1 additional exacerbation in 192 patients in 1 year), shorter mean time to first exacerbation (HR = 1.46; 95% CI: 1.38-1.55; P < .0001), and a higher risk of hospitalization within 3 years (rate ratio = 1.18; 95% CI, 1.08-1.28; P = .0001). The researchers did not evaluate for mortality.
Importantly, patients in the late COPD diagnosis group in both trials had higher rates of other severe illnesses that cause dyspnea, including cardiovascular disease and other pulmonary diseases. As a result, dyspnea of other etiologies may have contributed to both the later diagnoses and the poorer clinical outcomes of the late-diagnosis group. Both studies had a high risk of lead-time bias.
Recommendations from others
In 2016, the USPSTF gave a “D” rating (moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits) to screening asymptomatic adults without respiratory symptoms for COPD.4 Likewise, the 2017 Global Initiative for Chronic Obstructive Lung Disease (GOLD) report did not recommend routine screening with spirometry but did advocate trying to make an accurate diagnosis using spirometry in patients with risk factors for COPD and chronic, progressive symptoms.5
Editor’s takeaway
Reasonably good evidence failed to find a benefit from an early COPD diagnosis. Even smoking cessation rates were not improved. Without better disease-modifying treatments, spirometry—the gold standard for confirming a COPD diagnosis—should not be used for screening asymptomatic patients.
EVIDENCE SUMMARY
Early Dx didn’t improve smoking cessation rates or treatment outcomes
A 2016 evidence report and systematic review for the US Preventive Services Task Force (USPSTF) identified no studies directly comparing the effectiveness of COPD screening on patient outcomes, so the authors looked first at studies on the outcomes of screening, followed by studies exploring the effects of early treatment.1
The authors identified 5 fair-quality RCTs (N = 1694) addressing the effect of screening asymptomatic patients for COPD with spirometry on the outcome of smoking cessation. One trial (n = 561) found better 12-month smoking cessation rates in patients who underwent spirometry screening and were given their “lung age” (13.6% vs 6.4% not given a lung age; P < .005; number needed to treat [NNT] = 14). However, a similar study (n = 542) published a year later found no significant difference in quit rates with or without “lung age” discussions (10.9% vs 13%, respectively; P not significant). In the other 3 studies, screening produced no significant effect on smoking cessation rates.1
As for possible early treatment benefits, the review authors identified only 1 RCT (n = 1175) that included any patients with mild COPD (defined as COPD with a forced expiratory volume in 1 second [FEV1] ≥ 80% of predicted normal value). It assessed treatment with inhaled corticosteroids (ICS) in patients with mild COPD who continued to smoke. The trial did not record symptoms (if any) at intake. ICS therapy reduced the frequency of COPD exacerbations (relative risk = 0.63; 95% CI, 0.47-0.85), although patients with milder COPD benefitted little in absolute terms (by 0.02 exacerbations/year).1 The review authors further noted that data were insufficient to make definitive statements about the effect of ICS on dyspnea or health-related quality of life.
But later diagnosis is associated with poorer outcomes
Two recent, large retrospective observational cohort studies, however, have examined the impact of an early vs late COPD diagnosis in patients with dyspnea or other symptoms of COPD.2,3 A later diagnosis was associated with worse outcomes.
In the first study, researchers in Sweden identified patients older than 40 years who had received a new diagnosis of COPD between 2000 and 2014.2 They examined electronic health record data for 6 different “indicators” of COPD during the 5 years prior to date of diagnosis: pneumonia, other respiratory disease, oral steroids, antibiotics for respiratory infection, prescribed drugs for respiratory symptoms, and lung function measurement. Researchers categorized patients as early diagnosis (if they had ≤ 2 indicators prior to diagnosis) or late diagnosis (≥ 3 indicators prior to diagnosis). Compared with early diagnosis (n = 3870), late diagnosis (n = 8827) was associated with
- a higher annual rate of exacerbations within the first 2 years after diagnosis (2.67 vs 1.41; hazard ratio [HR] = 1.89; 95% CI, 1.83-1.96; P < .0001; number of early diagnoses needed to prevent 1 exacerbation in 1 year = 79),
- shorter time to first exacerbation (HR = 1.61; 95% CI, 1.54-1.69; P < .0001), and
- higher direct health care costs (by €1500 per year; no P value given).
Mortality was not different between the groups (HR = 1.04; 95% CI, 0.98-1.11; P = .18).
The second investigation was a similarly designed retrospective observational cohort study using a large UK database.3 Researchers enrolled patients who were at least 40 years old and received a new diagnosis of COPD between 2011 and 2014.
Continue to: Researchers examined electronic...
Researchers examined electronic health record data in the 5 years prior to diagnosis for 7 possible indicators of early COPD: pneumonia, respiratory disease other than pneumonia, chest radiograph, prescription of oral steroids, prescription of antibiotics for lung infection, prescription to manage respiratory disease symptoms, and lung function measurement. Researchers categorized patients as early diagnosis (≥ 2 indicators prior to diagnosis) or late diagnosis (≥ 3 indicators prior to diagnosis). Compared with early diagnosis (n = 3375), late diagnosis (n = 6783) was associated with a higher annual rate of exacerbations over 3-year follow-up (1.09 vs 0.57; adjusted HR = 1.68; 95% CI, 1.59-1.79; P < .0001; or 1 additional exacerbation in 192 patients in 1 year), shorter mean time to first exacerbation (HR = 1.46; 95% CI: 1.38-1.55; P < .0001), and a higher risk of hospitalization within 3 years (rate ratio = 1.18; 95% CI, 1.08-1.28; P = .0001). The researchers did not evaluate for mortality.
Importantly, patients in the late COPD diagnosis group in both trials had higher rates of other severe illnesses that cause dyspnea, including cardiovascular disease and other pulmonary diseases. As a result, dyspnea of other etiologies may have contributed to both the later diagnoses and the poorer clinical outcomes of the late-diagnosis group. Both studies had a high risk of lead-time bias.
Recommendations from others
In 2016, the USPSTF gave a “D” rating (moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits) to screening asymptomatic adults without respiratory symptoms for COPD.4 Likewise, the 2017 Global Initiative for Chronic Obstructive Lung Disease (GOLD) report did not recommend routine screening with spirometry but did advocate trying to make an accurate diagnosis using spirometry in patients with risk factors for COPD and chronic, progressive symptoms.5
Editor’s takeaway
Reasonably good evidence failed to find a benefit from an early COPD diagnosis. Even smoking cessation rates were not improved. Without better disease-modifying treatments, spirometry—the gold standard for confirming a COPD diagnosis—should not be used for screening asymptomatic patients.
1. Guirguis-Blake JM, Senger CA, Webber EM, et al. Screening for chronic obstructive pulmonary disease: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315:1378-1393. doi:10.1001/jama.2016.2654
2. Larsson K, Janson C, Ställberg B, et al. Impact of COPD diagnosis timing on clinical and economic outcomes: the ARCTIC observational cohort study. Int J Chron Obstruct Pulmon Dis. 2019;14:995-1008. doi: 10.2147/COPD.S195382
3. Kostikas K, Price D, Gutzwiller FS, et al. Clinical impact and healthcare resource utilization associated with early versus late COPD diagnosis in patients from UK CPRD database. Int J Chron Obstruct Pulmon Dis. 2020;15:1729-1738. doi: 10.2147/COPD.S255414
4. US Preventive Services Task Force; Siu AL, Bibbins-Domingo K, Grossman DC, et al. Screening for chronic obstructive pulmonary disease: US Preventive Services Task Force recommendation statement. JAMA. 2016;315:1372-1377. doi: 10.1001/jama.2016.2638
5. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195:557-582. doi: 10.1164/rccm.201701-0218PP
1. Guirguis-Blake JM, Senger CA, Webber EM, et al. Screening for chronic obstructive pulmonary disease: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315:1378-1393. doi:10.1001/jama.2016.2654
2. Larsson K, Janson C, Ställberg B, et al. Impact of COPD diagnosis timing on clinical and economic outcomes: the ARCTIC observational cohort study. Int J Chron Obstruct Pulmon Dis. 2019;14:995-1008. doi: 10.2147/COPD.S195382
3. Kostikas K, Price D, Gutzwiller FS, et al. Clinical impact and healthcare resource utilization associated with early versus late COPD diagnosis in patients from UK CPRD database. Int J Chron Obstruct Pulmon Dis. 2020;15:1729-1738. doi: 10.2147/COPD.S255414
4. US Preventive Services Task Force; Siu AL, Bibbins-Domingo K, Grossman DC, et al. Screening for chronic obstructive pulmonary disease: US Preventive Services Task Force recommendation statement. JAMA. 2016;315:1372-1377. doi: 10.1001/jama.2016.2638
5. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195:557-582. doi: 10.1164/rccm.201701-0218PP
EVIDENCE-BASED ANSWER:
It depends. A diagnosis of chronic obstructive pulmonary disease (COPD) made using screening spirometry in patients without symptoms does not change the course of the disease or alter smoking rates (strength of recommendation [SOR]: A, preponderance of evidence from multiple randomized controlled trials [RCTs]). However, once a patient develops symptoms of lung disease, a delayed diagnosis is associated with poorer outcomes (SOR: B, cohort studies). Active case finding (including the use of spirometry) is recommended for patients with risk factors for COPD who present with consistent symptoms (SOR: C, expert opinion).
A “no-biopsy” approach to diagnosing celiac disease
ILLUSTRATIVE CASE
A 43-year-old woman presents to the clinic with diffuse, intermittent abdominal discomfort, bloating, and diarrhea that has slowly but steadily worsened over the past few years to now-daily symptoms. She states her overall health is otherwise good. Her review of systems is pertinent only for 8 lbs of unintentional weight loss over the past year and increased fatigue. She takes no supplements or routine over-the-counter or prescription medications, except for low-dose combination oral contraceptives, and is unaware of any family history of gastrointestinal (GI) diseases. She does not drink or smoke. She is up to date with immunizations and with cervical and breast cancer screening. Her body mass index is 23, her vital signs are within normal limits, and her physical exam is normal except for mild, diffuse abdominal tenderness without any masses, organomegaly, or peritoneal signs.
Her diagnostic work-up includes a complete metabolic panel, magnesium level, complete blood count, thyroid-stimulating hormone measurement, cytomegalovirus IgG and IgM serology, and stool studies for fecal leukocytes, ova and parasites, and fecal fat, in addition to a kidney, ureter, and bladder noncontrast computed tomography scan. All diagnostic testing is negative except for slightly elevated fecal fat, thereby decreasing the likelihood of infection, thyroid disorder, electrolyte abnormalities, or malignancy as a source of her symptoms.
She says that based on her online searches, her symptoms seem consistent with CD—with which you concur. However, she is fearful of an endoscopic procedure and asks if there is any other way to diagnose CD.
CD is an immune-mediated disorder in genetically susceptible people that is triggered by dietary gluten, causing damage to the small intestine.1-6 The estimated worldwide prevalence of CD is approximately 1%, with greater prevalence in females.1-6 A strong genetic predisposition also has been noted: prevalence among first-degree relatives is 10% to 44%.2,3,6 Although CD can be diagnosed at any age, in the United States the mean age at diagnosis is in the fifth decade of life.6
The incidence of CD is on the rise due to true increases in disease incidence and prevalence, increased detection through better diagnostic tools, and increased screening of at-risk populations (eg, first-degree relatives, those with specific human leukocyte antigen variant genotypes, and those with certain chromosomal disorders, such as Down syndrome and Turner syndrome).2-6 However, despite the increasing prevalence of CD, most patients remain undiagnosed.1
The diagnosis of CD in adults is typically made with elevated serum tTG-IgA and
STUDY SUMMARY
tTG-IgA titers were highly predictive of CD in 3 distinct cohorts
This 2021 hybrid prospective/retrospective study with 3 distinct cohorts aimed to assess the utility of serum tTG-IgA titers compared to traditional EGD with duodenal biopsy for the diagnosis of CD in adult participants (defined as ≥ 16 years of age). A serum tTG-IgA titer ≥ 10 times the ULN was set as the minimal cutoff value, and standardized duodenal biopsy sampling and evaluation for histologic mucosal changes consistent with
Continue to: Cohort 1 was a...
Cohort 1 was a prospective analysis of adults (N = 740) considered to have a high suspicion for CD, recruited from a single CD subspecialty clinic in the United Kingdom. Patients with a previous diagnosis of CD, those adhering to a gluten-free diet, and those with IgA deficiency were excluded. Study patients had tTG-IgA titers drawn and, within 6 weeks, underwent endoscopy with ≥ 1 biopsy from the duodenal bulb and/or the second part of the duodenum. The PPV of tTG-IgA titers ≥ 10 times the ULN in patients with biopsy-proven CD was 98.7% (95% CI, 97%-99.4%).
Cohort 2 was a retrospective analysis of adult patients (N = 532) considered to have low suspicion for CD. These patients were referred for endoscopy for generalized GI complaints in the same hospital as Cohort 1, but not the subspecialty clinic. Exclusion criteria and timing of IgA titers and endoscopy were identical to those of Cohort 1. The PPV of tTG-IgA titers ≥ 10 times the ULN in patients with biopsy-proven CD was 100%.
Cohort 3 (which included patients in 8 countries) was a retrospective analysis of the performance of multiple assays to enhance the validity of this approach in a wide range of settings. Adult patients (N = 145) with tTG-IgA serology positive for celiac who then underwent endoscopy with 4 to 6 duodenal biopsy samples were included in this analysis. Eleven distinct laboratories performed the tTG-IgA assay. The PPV of tTG-IgA titers ≥ 10 times the ULN in patients with biopsy-proven CD was 95.2% (95% CI, 84.6%-98.6%).
In total, this study included 1417 adult patients; 431 (30%) had tTG-IgA titers ≥ 10 times the ULN. Of those patients, 424 (98%) had histopathologic findings on duodenal biopsy consistent with CD.
Of note, there was no standardization as to the assays used for the tTG-IgA titers: Cohort 1 used 2 different manufacturers’ assays, Cohort 2 used 1 assay, and Cohort 3 used 5 assays. Regardless, the “≥ 10 times the ULN” calculation was based on each manufacturer’s published assay ranges. The lack of assay standardization did create variance in false-positive rates, however: Across all 3 cohorts, the false-positive rate for trusting the “≥ 10 times the ULN” threshold as the sole marker for CD in adults increased from 1% (Cohorts 1 and 2) to 5% (all 3 cohorts).
Continue to: WHAT'S NEW
WHAT’S NEW
Less invasive, less costly diagnosis of celiac disease in adults
In adults with symptoms suggestive of CD, the diagnosis can be made with a high level of certainty if a serum tTG-IgA titer is ≥ 10 times the ULN. Through informed, shared decision making in the presence of such a finding, patients may accept a serologic diagnosis and forgo an invasive EGD with biopsy and its inherent costs and risks. Indeed, if the majority of patients with CD are undiagnosed or underdiagnosed, and there exists a minimally invasive blood test that is highly cost effective in the absence of “red flags,” the overall benefit of this path could be substantial.
CAVEATS
“No biopsy” does not mean no risk/benefit discussion
While the PPVs are quite high, the negative predictive value varied greatly: 13%, 98%, and 10% for Cohorts 1, 2, and 3, respectively. Therefore, although serum tTG-IgA titers ≥ 10 times the ULN are useful for diagnosis, a negative result (serum tTG-IgA titers < 10 times the ULN) should not be used to rule out CD, and other testing should be pursued.
Additionally (although rare), patients with CD who have IgA deficiency may obtain false-negative results using the tTG-IgA ≥ 10 times the ULN diagnostic criterion.7,8
Also, both Cohorts 1 and 2 took place in general or subspecialty GI clinics (Cohort 3’s site types were not specified). However, the objective interpretation of tTG-IgA serology means it could be considered as an additional diagnostic tool for primary care physicians, as well.
Finally, if a primary care physician and their patient decide to go the “no-biopsy” route, it should be with a full discussion of the possible risks and benefits of not pursuing EGD. If there are any potential “red flag” symptoms suggesting the possibility of a more concerning differential diagnosis, EGD evaluation should still be pursued. Such symptoms might include (but not be limited to) chronic dyspepsia, dysphagia, weight loss, and unexplained anemia.7
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Diagnostic guidelines still favor EGD with biopsy for adults
The 2013 American College of Gastroenterology guidelines support the use of EGD and duodenal biopsy to diagnose CD in both low- and high-risk patients, regardless of serologic findings.7 In a 2019 Clinical Practice Update, the American Gastrointestinal Association (AGA) stated that when tTG-IgA titers are ≥ 10 times the ULN and EMAs are positive, the PPV is “virtually 100%” for CD. Yet they still state that in this scenario “EGD and duodenal biopsies may then be performed for purposes of differential diagnosis.”8 Furthermore, the AGA does not discuss informed and shared decision making with patients for the option of a “no-biopsy” diagnosis.8
Additionally, there may be challenges in finding commercial laboratories that report reference ranges with a clear ULN. Although costs for the serum tTG-IgA assay vary, they are less expensive than endoscopy with biopsy and histopathologic examination, and therefore may present less of a financial barrier.
1. Penny HA, Raju SA, Lau MS, et al. Accuracy of a no-biopsy approach for the diagnosis of coeliac disease across different adult cohorts. Gut. 2021;70:876-883. doi: 10.1136/gutjnl-2020-320913
2. Al-Toma A, Volta U, Auricchio R, et al. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United European Gastroenterol J. 2019;7:583-613. doi: 10.1177/2050640619844125
3. Caio G, Volta U, Sapone A, et al. Celiac disease: a comprehensive current review. BMC Med. 2019;17:142. doi: 10.1186/s12916-019-1380-z
4. Lebwohl B, Rubio-Tapia A. Epidemiology, presentation, and diagnosis of celiac disease. Gastroenterology. 2021;160:63-75. doi: 10.1053/j.gastro.2020.06.098
5. Lebwohl B, Sanders DS, Green PHR. Coeliac disease. Lancet. 2018;391:70-81. doi: 10.1016/S0140-6736(17)31796-8
6. Rubin JE, Crowe SE. Celiac disease. Ann Intern Med. 2020;172:ITC1-ITC16. doi: 10.7326/AITC202001070
7. Rubio-Tapia A, Hill ID, Kelly CP, et al; American College of Gastroenterology. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol. 2013;108:656-676; quiz 677. doi: 10.1038/ajg.2013.79
8. Husby S, Murray JA, Katzka DA. AGA clinical practice update on diagnosis and monitoring of celiac disease—changing utility of serology and histologic measures: expert review. Gastroenterology. 2019;156:885-889. doi: 10.1053/j.gastro.2018.12.010
9. Husby S, Koletzko S, Korponay-Szabó I, et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition guidelines for diagnosing coeliac disease 2020. J Pediatr Gastroenterol Nutr. 2020;70:141-156. doi: 10.1097/MPG.0000000000002497
ILLUSTRATIVE CASE
A 43-year-old woman presents to the clinic with diffuse, intermittent abdominal discomfort, bloating, and diarrhea that has slowly but steadily worsened over the past few years to now-daily symptoms. She states her overall health is otherwise good. Her review of systems is pertinent only for 8 lbs of unintentional weight loss over the past year and increased fatigue. She takes no supplements or routine over-the-counter or prescription medications, except for low-dose combination oral contraceptives, and is unaware of any family history of gastrointestinal (GI) diseases. She does not drink or smoke. She is up to date with immunizations and with cervical and breast cancer screening. Her body mass index is 23, her vital signs are within normal limits, and her physical exam is normal except for mild, diffuse abdominal tenderness without any masses, organomegaly, or peritoneal signs.
Her diagnostic work-up includes a complete metabolic panel, magnesium level, complete blood count, thyroid-stimulating hormone measurement, cytomegalovirus IgG and IgM serology, and stool studies for fecal leukocytes, ova and parasites, and fecal fat, in addition to a kidney, ureter, and bladder noncontrast computed tomography scan. All diagnostic testing is negative except for slightly elevated fecal fat, thereby decreasing the likelihood of infection, thyroid disorder, electrolyte abnormalities, or malignancy as a source of her symptoms.
She says that based on her online searches, her symptoms seem consistent with CD—with which you concur. However, she is fearful of an endoscopic procedure and asks if there is any other way to diagnose CD.
CD is an immune-mediated disorder in genetically susceptible people that is triggered by dietary gluten, causing damage to the small intestine.1-6 The estimated worldwide prevalence of CD is approximately 1%, with greater prevalence in females.1-6 A strong genetic predisposition also has been noted: prevalence among first-degree relatives is 10% to 44%.2,3,6 Although CD can be diagnosed at any age, in the United States the mean age at diagnosis is in the fifth decade of life.6
The incidence of CD is on the rise due to true increases in disease incidence and prevalence, increased detection through better diagnostic tools, and increased screening of at-risk populations (eg, first-degree relatives, those with specific human leukocyte antigen variant genotypes, and those with certain chromosomal disorders, such as Down syndrome and Turner syndrome).2-6 However, despite the increasing prevalence of CD, most patients remain undiagnosed.1
The diagnosis of CD in adults is typically made with elevated serum tTG-IgA and
STUDY SUMMARY
tTG-IgA titers were highly predictive of CD in 3 distinct cohorts
This 2021 hybrid prospective/retrospective study with 3 distinct cohorts aimed to assess the utility of serum tTG-IgA titers compared to traditional EGD with duodenal biopsy for the diagnosis of CD in adult participants (defined as ≥ 16 years of age). A serum tTG-IgA titer ≥ 10 times the ULN was set as the minimal cutoff value, and standardized duodenal biopsy sampling and evaluation for histologic mucosal changes consistent with
Continue to: Cohort 1 was a...
Cohort 1 was a prospective analysis of adults (N = 740) considered to have a high suspicion for CD, recruited from a single CD subspecialty clinic in the United Kingdom. Patients with a previous diagnosis of CD, those adhering to a gluten-free diet, and those with IgA deficiency were excluded. Study patients had tTG-IgA titers drawn and, within 6 weeks, underwent endoscopy with ≥ 1 biopsy from the duodenal bulb and/or the second part of the duodenum. The PPV of tTG-IgA titers ≥ 10 times the ULN in patients with biopsy-proven CD was 98.7% (95% CI, 97%-99.4%).
Cohort 2 was a retrospective analysis of adult patients (N = 532) considered to have low suspicion for CD. These patients were referred for endoscopy for generalized GI complaints in the same hospital as Cohort 1, but not the subspecialty clinic. Exclusion criteria and timing of IgA titers and endoscopy were identical to those of Cohort 1. The PPV of tTG-IgA titers ≥ 10 times the ULN in patients with biopsy-proven CD was 100%.
Cohort 3 (which included patients in 8 countries) was a retrospective analysis of the performance of multiple assays to enhance the validity of this approach in a wide range of settings. Adult patients (N = 145) with tTG-IgA serology positive for celiac who then underwent endoscopy with 4 to 6 duodenal biopsy samples were included in this analysis. Eleven distinct laboratories performed the tTG-IgA assay. The PPV of tTG-IgA titers ≥ 10 times the ULN in patients with biopsy-proven CD was 95.2% (95% CI, 84.6%-98.6%).
In total, this study included 1417 adult patients; 431 (30%) had tTG-IgA titers ≥ 10 times the ULN. Of those patients, 424 (98%) had histopathologic findings on duodenal biopsy consistent with CD.
Of note, there was no standardization as to the assays used for the tTG-IgA titers: Cohort 1 used 2 different manufacturers’ assays, Cohort 2 used 1 assay, and Cohort 3 used 5 assays. Regardless, the “≥ 10 times the ULN” calculation was based on each manufacturer’s published assay ranges. The lack of assay standardization did create variance in false-positive rates, however: Across all 3 cohorts, the false-positive rate for trusting the “≥ 10 times the ULN” threshold as the sole marker for CD in adults increased from 1% (Cohorts 1 and 2) to 5% (all 3 cohorts).
Continue to: WHAT'S NEW
WHAT’S NEW
Less invasive, less costly diagnosis of celiac disease in adults
In adults with symptoms suggestive of CD, the diagnosis can be made with a high level of certainty if a serum tTG-IgA titer is ≥ 10 times the ULN. Through informed, shared decision making in the presence of such a finding, patients may accept a serologic diagnosis and forgo an invasive EGD with biopsy and its inherent costs and risks. Indeed, if the majority of patients with CD are undiagnosed or underdiagnosed, and there exists a minimally invasive blood test that is highly cost effective in the absence of “red flags,” the overall benefit of this path could be substantial.
CAVEATS
“No biopsy” does not mean no risk/benefit discussion
While the PPVs are quite high, the negative predictive value varied greatly: 13%, 98%, and 10% for Cohorts 1, 2, and 3, respectively. Therefore, although serum tTG-IgA titers ≥ 10 times the ULN are useful for diagnosis, a negative result (serum tTG-IgA titers < 10 times the ULN) should not be used to rule out CD, and other testing should be pursued.
Additionally (although rare), patients with CD who have IgA deficiency may obtain false-negative results using the tTG-IgA ≥ 10 times the ULN diagnostic criterion.7,8
Also, both Cohorts 1 and 2 took place in general or subspecialty GI clinics (Cohort 3’s site types were not specified). However, the objective interpretation of tTG-IgA serology means it could be considered as an additional diagnostic tool for primary care physicians, as well.
Finally, if a primary care physician and their patient decide to go the “no-biopsy” route, it should be with a full discussion of the possible risks and benefits of not pursuing EGD. If there are any potential “red flag” symptoms suggesting the possibility of a more concerning differential diagnosis, EGD evaluation should still be pursued. Such symptoms might include (but not be limited to) chronic dyspepsia, dysphagia, weight loss, and unexplained anemia.7
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Diagnostic guidelines still favor EGD with biopsy for adults
The 2013 American College of Gastroenterology guidelines support the use of EGD and duodenal biopsy to diagnose CD in both low- and high-risk patients, regardless of serologic findings.7 In a 2019 Clinical Practice Update, the American Gastrointestinal Association (AGA) stated that when tTG-IgA titers are ≥ 10 times the ULN and EMAs are positive, the PPV is “virtually 100%” for CD. Yet they still state that in this scenario “EGD and duodenal biopsies may then be performed for purposes of differential diagnosis.”8 Furthermore, the AGA does not discuss informed and shared decision making with patients for the option of a “no-biopsy” diagnosis.8
Additionally, there may be challenges in finding commercial laboratories that report reference ranges with a clear ULN. Although costs for the serum tTG-IgA assay vary, they are less expensive than endoscopy with biopsy and histopathologic examination, and therefore may present less of a financial barrier.
ILLUSTRATIVE CASE
A 43-year-old woman presents to the clinic with diffuse, intermittent abdominal discomfort, bloating, and diarrhea that has slowly but steadily worsened over the past few years to now-daily symptoms. She states her overall health is otherwise good. Her review of systems is pertinent only for 8 lbs of unintentional weight loss over the past year and increased fatigue. She takes no supplements or routine over-the-counter or prescription medications, except for low-dose combination oral contraceptives, and is unaware of any family history of gastrointestinal (GI) diseases. She does not drink or smoke. She is up to date with immunizations and with cervical and breast cancer screening. Her body mass index is 23, her vital signs are within normal limits, and her physical exam is normal except for mild, diffuse abdominal tenderness without any masses, organomegaly, or peritoneal signs.
Her diagnostic work-up includes a complete metabolic panel, magnesium level, complete blood count, thyroid-stimulating hormone measurement, cytomegalovirus IgG and IgM serology, and stool studies for fecal leukocytes, ova and parasites, and fecal fat, in addition to a kidney, ureter, and bladder noncontrast computed tomography scan. All diagnostic testing is negative except for slightly elevated fecal fat, thereby decreasing the likelihood of infection, thyroid disorder, electrolyte abnormalities, or malignancy as a source of her symptoms.
She says that based on her online searches, her symptoms seem consistent with CD—with which you concur. However, she is fearful of an endoscopic procedure and asks if there is any other way to diagnose CD.
CD is an immune-mediated disorder in genetically susceptible people that is triggered by dietary gluten, causing damage to the small intestine.1-6 The estimated worldwide prevalence of CD is approximately 1%, with greater prevalence in females.1-6 A strong genetic predisposition also has been noted: prevalence among first-degree relatives is 10% to 44%.2,3,6 Although CD can be diagnosed at any age, in the United States the mean age at diagnosis is in the fifth decade of life.6
The incidence of CD is on the rise due to true increases in disease incidence and prevalence, increased detection through better diagnostic tools, and increased screening of at-risk populations (eg, first-degree relatives, those with specific human leukocyte antigen variant genotypes, and those with certain chromosomal disorders, such as Down syndrome and Turner syndrome).2-6 However, despite the increasing prevalence of CD, most patients remain undiagnosed.1
The diagnosis of CD in adults is typically made with elevated serum tTG-IgA and
STUDY SUMMARY
tTG-IgA titers were highly predictive of CD in 3 distinct cohorts
This 2021 hybrid prospective/retrospective study with 3 distinct cohorts aimed to assess the utility of serum tTG-IgA titers compared to traditional EGD with duodenal biopsy for the diagnosis of CD in adult participants (defined as ≥ 16 years of age). A serum tTG-IgA titer ≥ 10 times the ULN was set as the minimal cutoff value, and standardized duodenal biopsy sampling and evaluation for histologic mucosal changes consistent with
Continue to: Cohort 1 was a...
Cohort 1 was a prospective analysis of adults (N = 740) considered to have a high suspicion for CD, recruited from a single CD subspecialty clinic in the United Kingdom. Patients with a previous diagnosis of CD, those adhering to a gluten-free diet, and those with IgA deficiency were excluded. Study patients had tTG-IgA titers drawn and, within 6 weeks, underwent endoscopy with ≥ 1 biopsy from the duodenal bulb and/or the second part of the duodenum. The PPV of tTG-IgA titers ≥ 10 times the ULN in patients with biopsy-proven CD was 98.7% (95% CI, 97%-99.4%).
Cohort 2 was a retrospective analysis of adult patients (N = 532) considered to have low suspicion for CD. These patients were referred for endoscopy for generalized GI complaints in the same hospital as Cohort 1, but not the subspecialty clinic. Exclusion criteria and timing of IgA titers and endoscopy were identical to those of Cohort 1. The PPV of tTG-IgA titers ≥ 10 times the ULN in patients with biopsy-proven CD was 100%.
Cohort 3 (which included patients in 8 countries) was a retrospective analysis of the performance of multiple assays to enhance the validity of this approach in a wide range of settings. Adult patients (N = 145) with tTG-IgA serology positive for celiac who then underwent endoscopy with 4 to 6 duodenal biopsy samples were included in this analysis. Eleven distinct laboratories performed the tTG-IgA assay. The PPV of tTG-IgA titers ≥ 10 times the ULN in patients with biopsy-proven CD was 95.2% (95% CI, 84.6%-98.6%).
In total, this study included 1417 adult patients; 431 (30%) had tTG-IgA titers ≥ 10 times the ULN. Of those patients, 424 (98%) had histopathologic findings on duodenal biopsy consistent with CD.
Of note, there was no standardization as to the assays used for the tTG-IgA titers: Cohort 1 used 2 different manufacturers’ assays, Cohort 2 used 1 assay, and Cohort 3 used 5 assays. Regardless, the “≥ 10 times the ULN” calculation was based on each manufacturer’s published assay ranges. The lack of assay standardization did create variance in false-positive rates, however: Across all 3 cohorts, the false-positive rate for trusting the “≥ 10 times the ULN” threshold as the sole marker for CD in adults increased from 1% (Cohorts 1 and 2) to 5% (all 3 cohorts).
Continue to: WHAT'S NEW
WHAT’S NEW
Less invasive, less costly diagnosis of celiac disease in adults
In adults with symptoms suggestive of CD, the diagnosis can be made with a high level of certainty if a serum tTG-IgA titer is ≥ 10 times the ULN. Through informed, shared decision making in the presence of such a finding, patients may accept a serologic diagnosis and forgo an invasive EGD with biopsy and its inherent costs and risks. Indeed, if the majority of patients with CD are undiagnosed or underdiagnosed, and there exists a minimally invasive blood test that is highly cost effective in the absence of “red flags,” the overall benefit of this path could be substantial.
CAVEATS
“No biopsy” does not mean no risk/benefit discussion
While the PPVs are quite high, the negative predictive value varied greatly: 13%, 98%, and 10% for Cohorts 1, 2, and 3, respectively. Therefore, although serum tTG-IgA titers ≥ 10 times the ULN are useful for diagnosis, a negative result (serum tTG-IgA titers < 10 times the ULN) should not be used to rule out CD, and other testing should be pursued.
Additionally (although rare), patients with CD who have IgA deficiency may obtain false-negative results using the tTG-IgA ≥ 10 times the ULN diagnostic criterion.7,8
Also, both Cohorts 1 and 2 took place in general or subspecialty GI clinics (Cohort 3’s site types were not specified). However, the objective interpretation of tTG-IgA serology means it could be considered as an additional diagnostic tool for primary care physicians, as well.
Finally, if a primary care physician and their patient decide to go the “no-biopsy” route, it should be with a full discussion of the possible risks and benefits of not pursuing EGD. If there are any potential “red flag” symptoms suggesting the possibility of a more concerning differential diagnosis, EGD evaluation should still be pursued. Such symptoms might include (but not be limited to) chronic dyspepsia, dysphagia, weight loss, and unexplained anemia.7
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Diagnostic guidelines still favor EGD with biopsy for adults
The 2013 American College of Gastroenterology guidelines support the use of EGD and duodenal biopsy to diagnose CD in both low- and high-risk patients, regardless of serologic findings.7 In a 2019 Clinical Practice Update, the American Gastrointestinal Association (AGA) stated that when tTG-IgA titers are ≥ 10 times the ULN and EMAs are positive, the PPV is “virtually 100%” for CD. Yet they still state that in this scenario “EGD and duodenal biopsies may then be performed for purposes of differential diagnosis.”8 Furthermore, the AGA does not discuss informed and shared decision making with patients for the option of a “no-biopsy” diagnosis.8
Additionally, there may be challenges in finding commercial laboratories that report reference ranges with a clear ULN. Although costs for the serum tTG-IgA assay vary, they are less expensive than endoscopy with biopsy and histopathologic examination, and therefore may present less of a financial barrier.
1. Penny HA, Raju SA, Lau MS, et al. Accuracy of a no-biopsy approach for the diagnosis of coeliac disease across different adult cohorts. Gut. 2021;70:876-883. doi: 10.1136/gutjnl-2020-320913
2. Al-Toma A, Volta U, Auricchio R, et al. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United European Gastroenterol J. 2019;7:583-613. doi: 10.1177/2050640619844125
3. Caio G, Volta U, Sapone A, et al. Celiac disease: a comprehensive current review. BMC Med. 2019;17:142. doi: 10.1186/s12916-019-1380-z
4. Lebwohl B, Rubio-Tapia A. Epidemiology, presentation, and diagnosis of celiac disease. Gastroenterology. 2021;160:63-75. doi: 10.1053/j.gastro.2020.06.098
5. Lebwohl B, Sanders DS, Green PHR. Coeliac disease. Lancet. 2018;391:70-81. doi: 10.1016/S0140-6736(17)31796-8
6. Rubin JE, Crowe SE. Celiac disease. Ann Intern Med. 2020;172:ITC1-ITC16. doi: 10.7326/AITC202001070
7. Rubio-Tapia A, Hill ID, Kelly CP, et al; American College of Gastroenterology. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol. 2013;108:656-676; quiz 677. doi: 10.1038/ajg.2013.79
8. Husby S, Murray JA, Katzka DA. AGA clinical practice update on diagnosis and monitoring of celiac disease—changing utility of serology and histologic measures: expert review. Gastroenterology. 2019;156:885-889. doi: 10.1053/j.gastro.2018.12.010
9. Husby S, Koletzko S, Korponay-Szabó I, et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition guidelines for diagnosing coeliac disease 2020. J Pediatr Gastroenterol Nutr. 2020;70:141-156. doi: 10.1097/MPG.0000000000002497
1. Penny HA, Raju SA, Lau MS, et al. Accuracy of a no-biopsy approach for the diagnosis of coeliac disease across different adult cohorts. Gut. 2021;70:876-883. doi: 10.1136/gutjnl-2020-320913
2. Al-Toma A, Volta U, Auricchio R, et al. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United European Gastroenterol J. 2019;7:583-613. doi: 10.1177/2050640619844125
3. Caio G, Volta U, Sapone A, et al. Celiac disease: a comprehensive current review. BMC Med. 2019;17:142. doi: 10.1186/s12916-019-1380-z
4. Lebwohl B, Rubio-Tapia A. Epidemiology, presentation, and diagnosis of celiac disease. Gastroenterology. 2021;160:63-75. doi: 10.1053/j.gastro.2020.06.098
5. Lebwohl B, Sanders DS, Green PHR. Coeliac disease. Lancet. 2018;391:70-81. doi: 10.1016/S0140-6736(17)31796-8
6. Rubin JE, Crowe SE. Celiac disease. Ann Intern Med. 2020;172:ITC1-ITC16. doi: 10.7326/AITC202001070
7. Rubio-Tapia A, Hill ID, Kelly CP, et al; American College of Gastroenterology. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol. 2013;108:656-676; quiz 677. doi: 10.1038/ajg.2013.79
8. Husby S, Murray JA, Katzka DA. AGA clinical practice update on diagnosis and monitoring of celiac disease—changing utility of serology and histologic measures: expert review. Gastroenterology. 2019;156:885-889. doi: 10.1053/j.gastro.2018.12.010
9. Husby S, Koletzko S, Korponay-Szabó I, et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition guidelines for diagnosing coeliac disease 2020. J Pediatr Gastroenterol Nutr. 2020;70:141-156. doi: 10.1097/MPG.0000000000002497
PRACTICE CHANGER
Consider a “no-biopsy” approach by evaluating serum immunoglobulin (Ig) A anti-tissue transglutaminase (tTG-IgA) antibody titers in adult patients who present with symptoms concerning for celiac disease (CD). An increase of ≥ 10 times the upper limit of normal (ULN) for tTG-IgA has a positive predictive value (PPV) of ≥ 95% for diagnosing CD when compared with esophagogastroduodenoscopy (EGD) with duodenal biopsy—the current gold standard.
STRENGTH OF RECOMMENDATION
A: Consistent findings from 3 good-quality diagnostic cohorts presented in a single study.1
Penny HA, Raju SA, Lau MS, et al. Accuracy of a no-biopsy approach for the diagnosis of coeliac disease across different adult cohorts. Gut. 2021;70:876-883. doi: 10.1136/gutjnl-2020-320913
COVID-19 vaccine insights: The news beyond the headlines
Worldwide and across many diseases, vaccines have been transformative in reducing mortality—an effect that has been sustained with vaccines that protect against COVID-19.1 Since the first cases of SARS-CoV-2 infection were reported in late 2019, the pace of scientific investigation into the virus and the disease—made possible by unprecedented funding, infrastructure, and public and private partnerships—has been explosive. The result? A vast body of clinical and laboratory evidence about the safety and effectiveness of SARS-CoV-2 vaccines, which quickly became widely available.2-4
In this article, we review the basic underlying virology of SARS-CoV-2; the biotechnological basis of vaccines against COVID-19 that are available in the United States; and recommendations on how to provide those vaccines to your patients. Additional guidance for your practice appears in a select online bibliography, “COVID-19 vaccination resources.”
SIDEBAR
COVID-19 vaccination resources
Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States
Centers for Disease Control and Prevention
www.cdc.gov/vaccines/covid-19/clinical-considerations/interimconsiderations-us.html
COVID-19 ACIP vaccine recommendations
Advisory Committee on Immunization Practices (ACIP)
www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html
MMWR COVID-19 reports
Morbidity and Mortality Weekly Report
www.cdc.gov/mmwr/Novel_Coronavirus_Reports.html
A literature hub for tracking up-to-date scientific information about the 2019 novel coronavirus
National Center for Biotechnology Information of the National Library of Medicine
www.ncbi.nlm.nih.gov/research/coronavirus
Understanding COVID-19 vaccines
National Institutes of Health COVID-19 Research
https://covid19.nih.gov/treatments-and-vaccines/covid-19-vaccines
How COVID-19 affects pregnancy
National Institutes of Health COVID-19 Research
SARS-CoV-2 virology
As the SARS-CoV-2 virus approaches the host cell, normal cell proteases on the surface membrane cause a change in the shape of the SARS-CoV-2 spike protein. That spike protein conformation change allows the virus to avoid detection by the host’s immune system because its receptor-binding site is effectively hidden until just before entry into the cell.5,6 This process is analogous to a so-called lock-and-key method of entry, in which the key (ie, spike protein conformation) is hidden by the virus until the moment it is needed, thereby minimizing exposure of viral contents to the cell. As the virus spreads through the population, it adapts to improve infectivity and transmissibility and to evade developing immunity.7
After the spike protein changes shape, it attaches to an angiotensin-converting enzyme 2 (ACE-2) receptor on the host cell, allowing the virus to enter that cell. ACE-2 receptors are located in numerous human tissues: nasopharynx, lung, gastrointestinal tract, heart, thymus, lymph nodes, bone marrow, brain, arterial and venous endothelial cells, and testes.5 The variety of tissues that contain ACE-2 receptors explains the many sites of infection and location of symptoms with which SARS-CoV-2 infection can manifest, in addition to the respiratory system.
Basic mRNA vaccine immunology
Although messenger RNA (mRNA) vaccines seem novel, they have been in development for more than 30 years.8
mRNA encodes the protein for the antigen of interest and is delivered to the host muscle tissue. There, mRNA is translated into the antigen, which stimulates an immune response. Host enzymes then rapidly degrade the mRNA in the vaccine, and it is quickly eliminated from the host.
mRNA vaccines are attractive vaccine candidates, particularly in their application to emerging infectious diseases, for several reasons:
- They are nonreplicating.
- They do not integrate into the host genome.
- They are highly effective.
- They can produce antibody and cellular immunity.
- They can be produced (and modified) quickly on a large scale without having to grow the virus in eggs.
Continue to: Vaccines against SARS-CoV-2
Vaccines against SARS-CoV-2
Two vaccines (from Pfizer-BioNTech [Comirnaty] and from Moderna [Spikevax]) are US Food and Drug Administration (FDA)–approved for COVID-19; both utilize mRNA technology. Two other vaccines, which do not use mRNA technology, have an FDA emergency use authorization (from Janssen Biotech, of Johnson & Johnson [Janssen COVID-19 Vaccine] and from Novavax [Novavax COVID-19 Vaccine, Adjuvanted]).9
Pfizer-BioNTech and Moderna vaccines. The mRNA of these vaccines encodes the entire spike protein in its pre-fusion conformation, which is the antigen that is replicated in the host, inducing an immune response.10-12 (Recall the earlier lock-and-key analogy: This conformation structure ingeniously replicates the exposed 3-dimensional key to the host’s immune system.)
The Janssen vaccine utilizes a viral vector (a nonreplicating adenovirus that functions as carrier) to deliver its message to the host for antigen production (again, the spike protein) and an immune response.
The Novavax vaccine uses a recombinant nanoparticle protein composed of the full-length spike protein.13,14 In this review, we focus on the 2 available mRNA vaccines, (1) given their FDA-authorized status and (2) because Centers for Disease Control and Prevention (CDC) recommendations indicate a preference for mRNA vaccination over viral-vectored vaccination. However, we also address key points about the Janssen (Johnson & Johnson) vaccine.
Efficacy of COVID-19 vaccines
The first study to document the safety and efficacy of a SARS-CoV-2 vaccine (the Pfizer-BioNTech vaccine) was published just 12 months after the onset of the pandemic.10 This initial trial demonstrated a 95% efficacy in preventing symptomatic, laboratory-confirmed COVID-19 at 3-month follow-up.10 Clinical trial data on the efficacy of COVID-19 vaccines have continued to be published since that first landmark trial.
Continue to: Data from trials...
Data from trials in Israel that became available early in 2021 showed that, in mRNA-vaccinated adults, mechanical ventilation rates declined strikingly, particularly in patients > 70 years of age.15,16 This finding was corroborated by data from a surveillance study of multiple US hospitals, which showed that mRNA vaccines were > 90% effective in preventing hospitalization in adults > 65 years of age.17
Data published in May 2021 showed that the Pfizer-BioNTech and Moderna vaccines were 94% effective in preventing COVID-19-related hospitalization.18 During the end of the Delta wave of the pandemic and the emergence of the Omicron variant of SARS-CoV-2, unvaccinated people were 5 times as likely to be infected as vaccinated people.19
In March 2022, data from 21 US medical centers in 18 states demonstrated that adults who had received 3 doses of the vaccine were 94% less likely to be intubated or die than those who were unvaccinated.16 A July 2022 retrospective cohort study of 231,037 subjects showed that the risk of hospitalization for acute myocardial infarction or for stroke after COVID-19 infection was reduced by more than half in fully vaccinated (ie, 2 doses of an mRNA vaccine or the viral vector [Janssen/Johnson & Johnson] vaccine) subjects, compared to unvaccinated subjects.20 The efficacy of the vaccines is summarized in TABLE 1.21-24
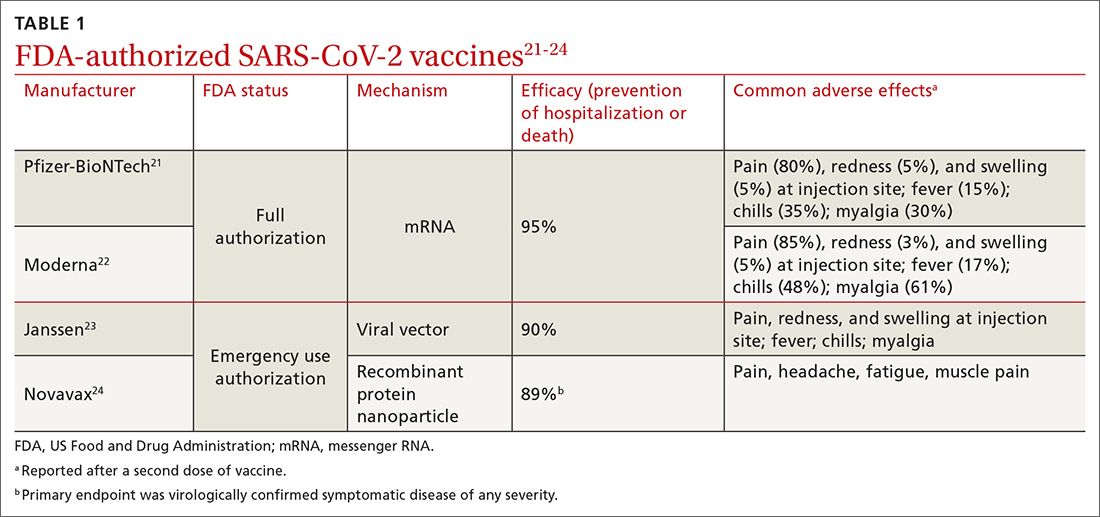
Even in patients who have natural infection, several studies have shown that COVID-19 vaccination after natural infection increases the level and durability of immune response to infection and reinfection and improves clinical outcomes.9,20,25,26 In summary, published literature shows that (1) mRNA vaccines are highly effective at preventing infection and (2) they augment immunity achieved by infection with circulating virus.
Breakthrough infection. COVID-19 mRNA vaccines are associated with breakthrough infection (ie, infections in fully vaccinated people), a phenomenon influenced by the predominant viral variant circulating, the level of vaccine uptake in the studied population, and the timing of vaccination.27,28 Nevertheless, vaccinated people who experience breakthrough infection are much less likely to be hospitalized and die compared to those who are unvaccinated, and vaccination with an mRNA vaccine is more effective than immunity acquired from natural infection.29
Continue to: Vaccine adverse effects
Vaccine adverse effects: Common, rare, myths
Both early mRNA vaccine trials reported common minor adverse effects after vaccination (TABLE 121-24). These included redness and soreness at the injection site, fatigue, myalgias, fever, and nausea, and tended to be more common after the second dose. These adverse effects are similar to common adverse effects seen with other vaccines. Counseling information about adverse effects can be found on the CDC website.a
Two uncommon but serious adverse effects of COVID-19 vaccination are myocarditis or pericarditis after mRNA vaccination and thrombosis with thrombocytopenia syndrome (TTS), which occurs only with the Janssen vaccine.30,31
Myocarditis and pericarditis, particularly in young males (12 to 18 years), and mostly after a second dose of vaccine, was reported in May 2021. Since then, several studies have shown that the risk of myocarditis is slightly higher in males < 40 years of age, with a predicted case rate ranging from 1 to 10 excess cases for every 1 million patients vaccinated.30,32 This risk must be balanced against the rate of myocarditis associated with SARS-CoV-2 infection.
A large study in the United States demonstrated that the risk of myocarditis for those who contract COVID-19 is 16 times higher than it is for those who are disease free.33 Observational safety data from April 2022 showed that men ages 18 to 29 years had 7 to 8 times the risk of heart complications after natural infection, compared to men of those ages who had been vaccinated.34 In this study of 40 US health care systems, the incidence of myocarditis or pericarditis in that age group ranged from 55 to 100 cases for every 100,000 people after infection and from 6 to 15 cases for every 100,000 people after a second dose of an mRNA vaccine.34
A risk–benefit analysis conducted by the Advisory Committee on Immunization Practices (ACIP) ultimately supported the conclusions that (1) the risk of myocarditis secondary to vaccination is small and (2) clear benefits of preventing infection, hospitalization, death, and continued transmission outweigh that risk.35 Study of this question, utilizing vaccine safety and reporting systems around the world, has continued.
Continue to: There is emerging evidence...
There is emerging evidence that extending the interval between the 2 doses of vaccine decreases the risk of myocarditis, particularly in male adolescents.36 That evidence ultimately led the CDC to recommend that it might be optimal that an extended interval (ie, waiting 8 weeks between the first and second dose of vaccine), in particular for males ages 12 to 39 years, could be beneficial in decreasing the risk of myocarditis.
TTS. A population risk–benefit analysis of TTS was conducted by ACIP while use of the Janssen vaccine was paused in the United States in December 2021.36 The analysis determined that, although the risk of TTS was largely in younger women (18 to 49 years; 7 cases for every 1 million vaccine doses administered), benefits of the vaccine in preventing death, hospitalization, and a stay in the intensive care unit (ICU)—particularly if vaccination was delayed or there was a high rate of community infection—clearly outweighed risks. (The CDC estimated an incidence of 2 cases of TTS with more than 3 million doses of Janssen vaccine administered; assuming moderate transmission kinetics, more than 3500 hospitalizations and more than 350 deaths were prevented by vaccination.36) Ultimately, after the CDC analysis was released, vaccination utilizing the Janssen product resumed; however, the CDC offered the caveat that the Janssen vaccine should be used only in specific situations36 (eg, when there has been a severe reaction to mRNA vaccine or when access to mRNA or recombinant nanoparticle vaccine is limited).
Myths surrounding vaccination
Myth #1: SARS-CoV-2 vaccines contain tissue from aborted fetuses. This myth, which emerged during development of the vaccines, is often a conflation of the use of embryonic cell lines obtained decades ago to produce vaccines (a common practice—not only for vaccines but common pharmaceuticals and foods).37 There are no fetal cells or tissue in any SARS-CoV-2 vaccines, and the vaccines have been endorsed by several faith organizations.38
Myth #2: SARS-CoV-2 vaccines can cause sterility in men and women. This myth originated from a report in early December 2020 seeking to link a similarity in a protein involved in placental–uterine binding and a portion of the receptor-binding domain antigen produced by the vaccine.39 No studies support this myth; COVID-19 vaccines are recommended in pregnancy by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine.40,41
Myth #3: mRNA SARS-CoV-2 vaccines alter a recipient’s DNA. mRNA vaccines are broken down by cellular enzymes. They cannot be integrated into the host genome.8
Continue to: Boosters and vaccine mix-and-match
Boosters and vaccine mix-and-match
As the COVID-19 pandemic persists, with new variants of concern emerging, it has also become clear that immunity wanes. In July 2021, the first report was published after a cluster of breakthrough infections occurred in a town in Massachusetts.42 There was no recommendation, at the time, for a booster; the Delta variant was the predominant circulating strain. In this outbreak, there were 469 cases, 74% of which were in people who had received 2 doses of an mRNA vaccine.42 Five patients were hospitalized; none died.42 A key takeaway from this outbreak was that vaccination prevented death, even in the face of fairly wide breakthrough infection.
Newer data show that, although vaccine effectiveness against hospitalization was greater than 90% for the first 2 months after a third dose, it waned to 78% by 4 months.43 Published data, combined with real-world experience, show that boosters provide additional reduction in the risk of death and hospitalization. This has led to a recommendation that all patients ≥ 5 years of age receive a booster.19,26,43-48 The CDC now recommends that people who are ages 12 years and older receive a bivalent booster (containing both wild-type and Omicron-variant antigens) ≥ 2 months after their most recent booster or completed series.
Future booster recommendations will consider the durability of the immune response over time (measured against the original immunizing virus) and the mutation rate of the virus.49
Given the limited supply of vaccine early in the pandemic, and the potential for future limitations, there was early interest in studying so-called mix-and-match SARS-CoV-2 vaccination—that is, receiving one product as a first series and then a different product as a booster, also known as heterologous booster vaccination. Although it is preferred that the 2 doses of the primary series be of the same vaccine product, studies that have examined this question support heterologous boosting as an acceptable approach to protective immunity50 (TABLE 251).
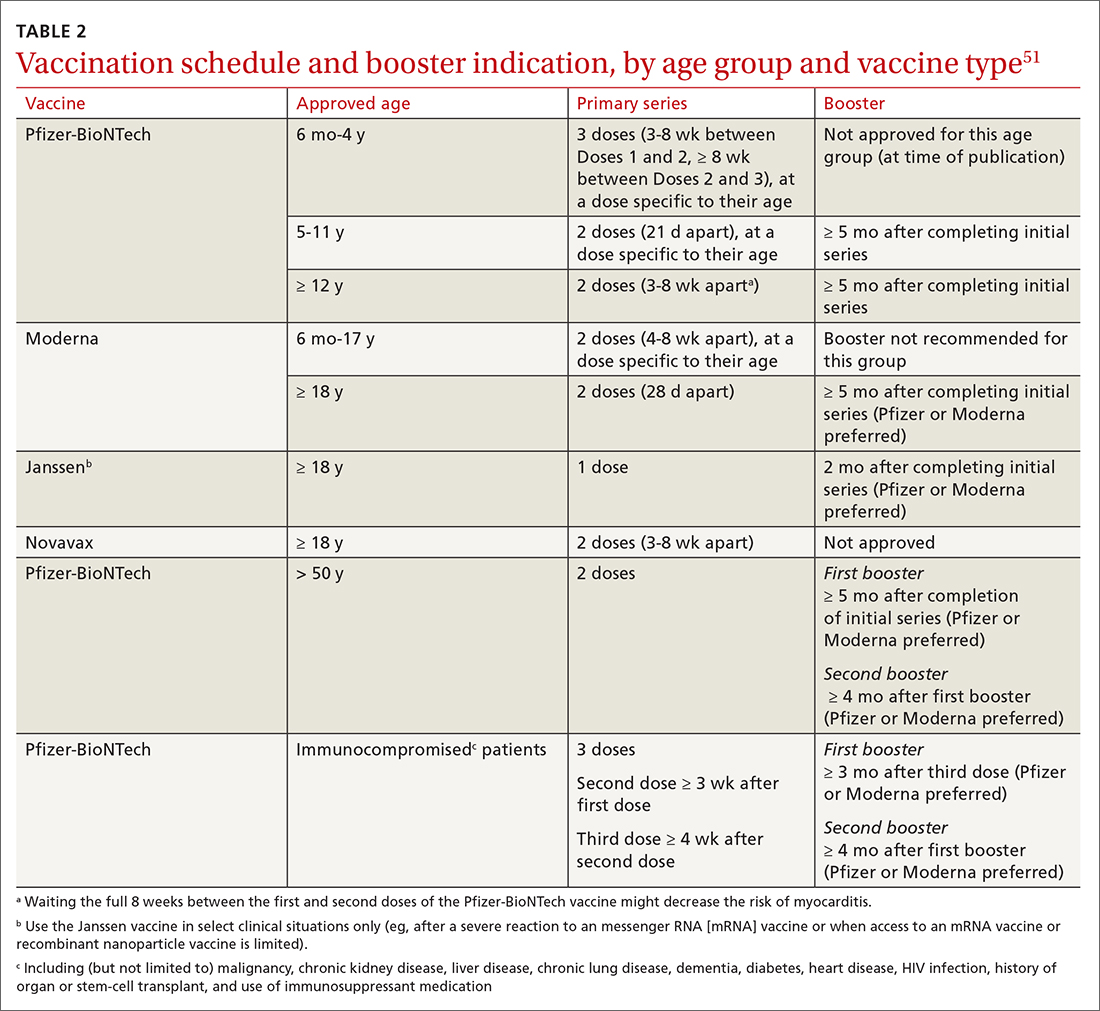
Vaccination in special populations
Three groups of patients have unique host characteristics that are important to consider when providing COVID-19 vaccination in your practice: pregnant patients, children, and patients in the broad category of “immunocompromised status.”
Continue to: Pregnant patients
Pregnant patients with SARS-CoV-2 infection are more likely to be hospitalized and have a higher risk of a stay in the ICU and need for mechanical ventilation. In a study of the course of illness in symptomatic pregnant patients who were hospitalized, 16.2% were admitted to an ICU and 8.5% were mechanically ventilated.52 CDC observational data have consistently supported the finding that (1) pregnant patients infected with SARS-CoV-2 are at increased risk of preterm labor and (2) their newborns are at increased risk of low birth weight and requiring admission to the neonatal ICU.53
A systematic review of 46 studies in pregnant and lactating patients showed no increased risk of adverse effects from COVID-19 vaccination.54 Furthermore, data from multiple studies demonstrate that immunoglobulin G antibodies cross the placenta to protect the infant at birth (ie, are found in umbilical cord blood and neonatal blood) and are found in breast milk. The precise kinetics and durability of these antibodies are unknown.
Pregnant patients were initially excluded from vaccine trials (although there were some patients ultimately found to be pregnant, or who became pregnant, during the trial). Careful examination of vaccine safety and efficacy data has supported the American College of Obstetricians and Gynecologists and European Board and College of Obstetrics and Gynaecology (EBCOG) recommendation that all pregnant patients be vaccinated. Furthermore, EBCOG recommends vaccination during the period of breastfeeding.55
Children. A major challenge during the pandemic has been to understand (1) the effect that infection with SARS-CoV-2 has on children and (2) the role of children in transmission of the virus. Although most children with COVID-19 have mild symptoms, a few require hospitalization and mechanical ventilation and some develop life-threatening multisystem inflammatory syndrome.56 In a large, retrospective study of more than 12,000 children with COVID-19, 5.3% required hospitalization and almost 20% of that subset were admitted to the ICU.57
Various hypotheses have been put forward to describe and explain the differences in disease expression between children and adults. These include:
- the absence of comorbidities often seen in adults
- evidence that pediatric patients might have reduced expression of ACE-2
- a more active T-cell response in infected children, due to an active thymus.56
Continue to: Although the number of children affected...
Although the number of children affected by severe SARS-CoV-2 infection is less than the number of adults, there have been important trends observed in infection and hospitalization as different variants have arisen.58 The Delta and Omicron variants have both been associated with a disturbing trend in the rate of hospitalization of pediatric patients, particularly from birth to 4 years—patients who were ineligible for vaccination at the time of the study.58 Ultimately, these data, combined with multiple studies of vaccine effectiveness in this age group, have led to an emergency use authorization for the Pfizer-BioNTech vaccination in pediatric populations and a recommendation from the American Academy of Pediatrics that all children ages 6 months and older be vaccinated.59,60
Immunocompromised patients. Patients broadly classified as immunocompromised have raised unique concerns. These patients have conditions such as malignancy, primary or secondary immunodeficiency, diabetes, and autoimmune disease; are taking certain classes of medication; or are of older age.61 Early in the pandemic, data showed that immunocompromised hosts could shed virus longer than hosts with an intact immune system—adding to their risk of transmitting SARS-CoV-2 and of viral adaptation for immune escape.62 Antibody response to vaccination was also less robust in this group.
There are limited data that demonstrate a short-lived reduction in risk of infection (in that study, Omicron was the prominent variant) with a fourth dose of an mRNA vaccine.63 Based on these data and FDA approval, the CDC recommends (1) an additional third primary dose and (2) a second booster for people who are moderately or severely immunocompromised. For those ages 50 years or older, a second booster is now required for their vaccination to be considered up to date.b
Predictions (or, why is a COVID-19 vaccine important?)
What does the future hold for our struggle with COVID-19? Perhaps we can learn lessons from the study of the 4 known seasonal coronaviruses, which cause the common cold and circulate annually.64 First, only relative immunity is produced after infection with a seasonal coronavirus.64 Studies of antibodies to seasonal coronaviruses seem to suggest that, although antibody titers remain high, correlation with decreased infection is lacking.65 Second, a dominant strain or 2 emerges each season, probably as a result of genetic variation and selective pressure for immune escape from neutralizing antibodies or cellular immunity.
The complex relationship among competing immune response duration, emergence of viral immune escape, increasing viral transmissibility, and societal viral source control (through vaccination, masking, distancing, testing, isolation, and treatment) widens the confidence bounds on our estimates of what the future holds. Late in 2020, the CDC began reporting wastewater surveillance data as a method for monitoring, and predicting changes in, community spread.66 During Spring 2022, the CDC reported an increase in detection of SARS-CoV-2 from a third of wastewater sampling sites around the United States. This observation coincided with (1) appearance of still more transmissible BA.2 and, later, BA.2.12.1 variants and (2) general relaxing of masking and social distancing guidelines, following the decline of the Omicron variant.
Continue to: At approximately that time...
At approximately that time, application to the FDA for a fourth shot (or a second booster) by Pfizer-BioNTech had been approved for adults > 50 years of age, at > 4 months after their previous vaccination.57 In view of warning signs from wastewater surveillance, priorities for vaccination should be to:
- increase uptake in the hesitant
- get boosters to the eligible
- prepare to tackle either seasonal or sporadic recurrence of COVID-19—whichever scenario the future brings.
As an example of how these priorities have been put into action, in September 2022, the FDA approved, and the CDC recommended, new bivalent boosters for everyone ≥ 12 years of age (Pfizer-BioNTech) or for all those ≥ 18 years of age (Moderna), to be administered ≥ 2 months after receipt of their most recent booster or primary series.
a www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html
b Visit www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html for more guidance on COVID-19 vaccination for immunocompromised patients.
CORRESPONDENCE
John L. Kiley, MD, 3551 Roger Brooke Drive, Fort Sam Houston, TX 78234; [email protected]
1. Orenstein W, Offitt P, Edwards KM, Plotkin S. Plotkin’s Vaccines. 7th ed. Elsevier; 2017:1-15.
2. Operation Warp Speed: implications for global vaccine security. Lancet Glob Health. 2021;9:e1017-e1021. doi: 10.1016/S2214-109X(21)00140-6
3. Lurie N, Saville M, Hatchett R, et al. Developing Covid-19 vaccines at pandemic speed. N Engl J Med. 2020;382:1969-1973. doi: 10.1056/NEJMp2005630
4. Slaoui M, Hepburn M. Developing safe and effective Covid vaccines—Operation Warp Speed’s strategy and approach. N Engl J Med. 2020;383:1701-1703. doi: 10.1056/NEJMp2027405
5. Hu B, Guo H, Zhou P, et al. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19:141-154. doi: 10.1038/s41579-020-00459-7
6. Hussain I, Pervaiz N, Khan A, et al. Evolutionary and structural analysis of SARS-CoV-2 specific evasion of host immunity. Genes Immun. 2020;21:409-419. doi: 10.1038/s41435-020-00120-6
7. Rando HM, Wellhausen N, Ghosh S, et al; COVID-19 Review Consortium. Identification and development of therapeutics for COVID-19. mSystems. 2021;6:e0023321. doi: 10.1128/mSystems.00233-21
8. Pardi N, Hogan MJ, Porter FW, et al. mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261-279. doi: 10.1038/nrd.2017.243
9. National Center for Immunization and Respiratory Diseases. Use of COVID-19 vaccines in the United States: interim clinical considerations. Centers for Disease Control and Prevention. Updated August 22, 2022. Accessed August 27, 2022. www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html#references
10. Polack FP, Thomas SJ, Kitchin N, et al; . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603-2615. doi: 10.1056/NEJMoa2034577
11. Heinz FX, Stiasny K. Distinguishing features of current COVID-19 vaccines: knowns and unknowns of antigen presentation and modes of action. NPJ Vaccines. 2021;6:104. doi: 10.1038/s41541-021-00369-6
12. Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403-416. doi: 10.1056/NEJMoa2035389
13. Keech C, Albert G, Cho I, et al. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383:2320-2332. doi: 10.1056/NEJMoa2026920
14. Heath PT, Galiza EP, Baxter DN, et al; . Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med. 2021;385:1172-1183. doi: 10.1056/NEJMoa2107659
15. Rinott E, Youngster I, Lewis YE. Reduction in COVID-19 patients requiring mechanical ventilation following implementation of a national COVID-19 vaccination program—Israel, December 2020–February 2021. MMWR Morb Mortal Wkly Rep. 2021;70:326-328. doi: 10.15585/mmwr.mm7009e3
16. Tenforde MW, Self WH, Gaglani M, et al; IVY Network. Effectiveness of mRNA vaccination in preventing COVID-19-associated invasive mechanical ventilation and death—United States, March 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:459-465. doi: 10.15585/mmwr.mm7112e1
17. Moline HL, Whitaker M, Deng L, et al. Effectiveness of COVID-19 vaccines in preventing hospitalization among adults aged ≥ 65 years—COVID-NET, 13 States, February–April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1088-1093. doi: 10.15585/mmwr.mm7032e
18. Tenforde MW, Olson SM, Self WH, et al; ; . Effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 among hospitalized adults aged ≥ 65 years—United States, January–March 2021. MMWR Morb Mortal Wkly Rep. 2021;70:674-679. doi: 10.15585/mmwr.mm7018e1
19. Johnson AG, Amin AB, Ali AR, et al. COVID-19 incidence and death rates among unvaccinated and fully vaccinated adults with and without booster doses during periods of Delta and Omicron variant emergence—25 U.S. jurisdictions, April 4–December 25, 2021. MMWR Morb Mortal Wkly Rep. 2022;71:132-138. doi: 10.15585/mmwr.mm7104e2
20. Kim Y-E, Huh K, Park Y-J, et al. Association between vaccination and acute myocardial infarction and ischemic stroke after COVID-19 infection. JAMA. Published online July 22, 2022. doi: 10.1001/jama.2022.12992
21. Centers for Disease Control and Prevention. Pfizer-BioNTech COVID-19 vaccine reactions & adverse events. Updated June 21, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html
22. Centers for Disease Control and Prevention. The Moderna COVID-19 vaccine’s local reactions, systemic reactions, adverse events, and serious adverse events. Updated June 21, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html
23. Centers for Disease Control and Prevention. The Janssen COVID-19 vaccine’s local Reactions, Systemic reactions, adverse events, and serious adverse events. Updated August 12, 2021. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/janssen/reactogenicity.html
24. Centers for Disease Control and Prevention. Novavax COVID-19 vaccine local reactions, systemic reactions, adverse events, and serious adverse events. Updated August 31, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/novavax/reactogenicity.html
25. Greaney AJ, Loes AN, Gentles LE, et al. Antibodies elicited by mRNA-1273 vaccination bind more broadly to the receptor binding domain than do those from SARS-CoV-2 infection. Sci Transl Med. 2021;13:eabi9915. doi: 10.1126/scitranslmed.abi9915
26. Hall V, Foulkes S, Insalata F, et al. Protection against SARS-CoV-2 after Covid-19 vaccination and previous infection. N Engl J Med. 2022;386:1207-1220. doi: 10.1056/NEJMoa2118691
27. Klompas M. Understanding breakthrough infections following mRNA SARS-CoV-2 avccination. JAMA. 2021;326:2018-2020. doi: 10.1001/jama.2021.19063
28. Kustin T, Harel N, Finkel U, et al. Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals. Nat Med. 2021;27:1379-1384. doi: 10.1038/s41591-021-01413-7
29. Yu Y, Esposito D, Kang Z, et al. mRNA vaccine-induced antibodies more effective than natural immunity in neutralizing SARS-CoV-2 and its high affinity variants. Sci Rep. 2022;12:2628. doi: 10.1038/s41598-022-06629-2
30. Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:977-982. doi: 10.15585/mmwr.mm7027e2
31. MacNeil JR, Su JR, Broder KR, et al. Updated recommendations from the Advisory Committee on Immunization Practices for use of the Janssen (Johnson & Johnson) COVID-19 vaccine after reports of thrombosis with thrombocytopenia syndrome among vaccine recipients—United States, April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:651-656. doi: 10.15585/mmwr.mm7017e4
32. Patone M, Mei XW, Handunnetthi L, et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2022;28:410-422. doi: 10.1038/s41591-021-01630-0
33. Boehmer TK, Kompaniyets L, Lavery AM, et al. Association between COVID-19 and myocarditis using hospital-based administrative data—United States, March 2020–January 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1228-1232. doi: 10.15585/mmwr.mm7035e5
34. Block JP, Boehmer TK, Forrest CB, et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination—PCORnet, United States, January 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:517-523. doi: 10.15585/mmwr.mm7114e1
35. Rosemblum H. COVID-19 vaccines in adults: benefit–risk discussion. Centers for Disease Control and Prevention. July 22, 2021. Accessed September 21, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-07/05-COVID-Rosenblum-508.pdf
36. Buchan SA, Seo CY, Johnson C, et al. Epidemiology of myocarditis and pericarditis following mRNA vaccines in Ontario, Canada: by vaccine product, schedule and interval. medRxiv. 2021:12.02.21267156.
37. Wong A. The ethics of HEK 293. Natl Cathol Bioeth Q. 2006;6:473-495. doi: 10.5840/ncbq20066331
38. North Dakota Health. COVID-19 vaccines & fetal cell lines. Updated December 1, 2021. Accessed September 21, 2022. www.health.nd.gov/sites/www/files/documents/COVID%20Vaccine%20Page/COVID-19_Vaccine_Fetal_Cell_Handout.pdf
39. Abbasi J. Widespread misinformation about infertility continues to create COVID-19 vaccine hesitancy. JAMA. 2022;327:1013-1015. doi: 10.1001/jama.2022.2404
40. Halasa NB, Olson SM, Staat MA, et al; ; . Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19-associated hospitalization in infants aged < 6 months—17 States, July 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:264-270. doi: 10.15585/mmwr.mm7107e3
41. American College of Obstetricians and Gynecologists. ACOG and SMFM recommend COVID-19 vaccination for pregnant individuals. July 30, 2021. Accessed September 21, 2022. www.acog.org/news/news-releases/2021/07/acog-smfm-recommend-covid-19-vaccination-for-pregnant-individuals#:~:text=%E2%80%9CACOG%20is%20recommending%20vaccination%20of,complications%2C%20and%20because%20it%20isvaccines
42. Brown CM, Vostok J, Johnson H, et al. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings—Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1059-1062. doi: 10.15585/mmwr.mm7031e2
43. Ferdinands JM, Rao S, Dixon BE, et al. Waning 2-dose and 3-dose effectiveness of mRNA against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance—VISION Network, 10 states, August 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:255-263. doi: 10.15585/mmwr.mm7107e2
44. Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Effect of mRNA vaccine boosters against SARS-CoV-2 Omicron infection in Qatar. N Engl J Med. 2022;386:1804-1816. doi: 10.1056/NEJMoa2200797
45. Arbel R, Hammerman A, Sergienko R, et al. BNT162b2 vaccine booster and mortality due to Covid-19. N Engl J Med. 2021;385:2413-2420. doi: 10.1056/NEJMoa2115624
46. Bar-On YM, Goldberg Y, Mandel M, et al. Protection against Covid-19 by BNT162b2 booster across age groups. N Engl J Med. 2021;385:2421-2430. doi: 10.1056/NEJMoa2115926
47. Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385:1393-1400. doi: 10.1056/NEJMoa2114255
48. Mbaeyi S, Oliver SE, Collins JP, et al. The Advisory Committee on Immunization Practices’ interim recommendations for additional primary and booster doses of COVID-19 vaccines—United States, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1545-1552. doi: 10.15585/mmwr.mm7044e2
49. Chen X, Chen Z, Azman AS, et al. Neutralizing antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants induced by natural infection or vaccination: a systematic review and pooled analysis. Clin Infect Dis. 2022;74:734-742. doi: 10.1093/cid/ciab646
50. Atmar RL, Lyke KE, Deming ME, et al; . Homologous and heterologous Covid-19 booster vaccinations. N Engl J Med. 2022;386:1046-1057. doi: 10.1056/NEJMoa2116414
51. Centers for Disease Control and Prevention. Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. Updated September 2, 2022. Accessed September 21, 2022. www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html
52. Ackerman CM, Nguyen JL, Ambati S, et al. Clinical and pregnancy outcomes of coronavirus disease 2019 among hospitalized pregnant women in the United States. Open Forum Infect Dis. 2022;9:ofab429. doi: 10.1093/ofid/ofab429
53. Osterman MJK, Valenzuela CP, Martin JA. Maternal and infant characteristics among women with confirmed or presumed cases of coronavirus disease (COVID-19) during pregnancy. National Center for Health Statistics. National Vital Statistics System. Updated August 11, 2022. Accessed September 21, 2022. www.cdc.gov/nchs/covid19/technical-linkage.htm
54. De Rose DU, Salvatori G, Dotta A, et al. SARS-CoV-2 vaccines during pregnancy and breastfeeding: a systematic review of maternal and neonatal outcomes. Viruses. 2022;14:539. doi: 10.3390/v14030539
55. Martins I, Louwen F, Ayres-de-Campos D, et al. EBCOG position statement on COVID-19 vaccination for pregnant and breastfeeding women. Eur J Obstet Gynecol Reprod Biol. 2021;262:256-258. doi: 10.1016/j.ejogrb.2021.05.021
56. Chou J, Thomas PG, Randolph AG. Immunology of SARS-CoV-2 infection in children. Nat Immunol. 2022;23:177-185. doi: 10.1038/s41590-021-01123-9
57. Parcha V, Booker KS, Kalra R, et al. A retrospective cohort study of 12,306 pediatric COVID-19 patients in the United States. Sci Rep. 2021;11:10231. doi: 10.1038/s41598-021-89553-1
58. Marks KJ, Whitaker M, Anglin O, et al; . Hospitalizations of children and adolescents with laboratory-confirmed COVID-19—COVID-NET, 14 states, July 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:271-278. doi: 10.15585/mmwr.mm7107e4
59. Price AM, Olson SM, Newhams MM, et al; . BNT162b2 protection against the Omicron variant in children and adolescents. N Engl J Med. 2022;386:1899-1909. doi: 10.1056/NEJMoa2202826
60. Maldonado YA, O’Leary ST, Banerjee R, et al; Committee on Infectious Diseases, American Academy of Pediatrics. COVID-19 vaccines in children and adolescents. Pediatrics. 2021;148:e2021052336. doi: 10.1542/peds.2021-052336
61. Lontok K. How effective are COVID-19 vaccines in immunocompromised people? American Society for Microbiology. August 12, 2021. Accessed September 21, 2022. https://asm.org/Articles/2021/August/How-Effective-Are-COVID-19-Vaccines-in-Immunocompr
62. Meiring S, Tempia S, Bhiman JN, et al; . Prolonged shedding of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) at high viral loads among hospitalized immunocompromised persons living with human immunodeficiency virus, South Africa. Clin Infect Dis. 2022;75:e144-e156. doi: 10.1093/cid/ciac077
63. Bar-On YM, Goldberg Y, Mandel M, et al. Protection by 4th dose of BNT162b2 against Omicron in Israel. medRxiv. 2022: 02.01.22270232. doi: 10.1101/2022.02.01.22270232
64. Monto AS, DeJonge PM, Callear AP, et al. Coronavirus occurrence and transmission over 8 years in the HIVE cohort of households in Michigan. J Infect Dis. 2020;222:9-16. doi: 10.1093/infdis/jiaa161
65. Petrie JG, Bazzi LA, McDermott AB, et al. Coronavirus occurrence in the Household Influenza Vaccine Evaluation (HIVE) cohort of Michigan households: reinfection frequency and serologic responses to seasonal and severe acute respiratory syndrome coronaviruses. J Infect Dis. 2021;224:49-59. doi: 10.1093/infdis/jiab161
66. Kirby AE, Walters MS, Jennings WC, et al. Using wastewater surveillance data to support the COVID-19 response—United States, 2020–2021. MMWR Morb Mortal Wkly Rep. 2021;70:1242-1244. doi: 10.15585/mmwr.mm7036a2
Worldwide and across many diseases, vaccines have been transformative in reducing mortality—an effect that has been sustained with vaccines that protect against COVID-19.1 Since the first cases of SARS-CoV-2 infection were reported in late 2019, the pace of scientific investigation into the virus and the disease—made possible by unprecedented funding, infrastructure, and public and private partnerships—has been explosive. The result? A vast body of clinical and laboratory evidence about the safety and effectiveness of SARS-CoV-2 vaccines, which quickly became widely available.2-4
In this article, we review the basic underlying virology of SARS-CoV-2; the biotechnological basis of vaccines against COVID-19 that are available in the United States; and recommendations on how to provide those vaccines to your patients. Additional guidance for your practice appears in a select online bibliography, “COVID-19 vaccination resources.”
SIDEBAR
COVID-19 vaccination resources
Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States
Centers for Disease Control and Prevention
www.cdc.gov/vaccines/covid-19/clinical-considerations/interimconsiderations-us.html
COVID-19 ACIP vaccine recommendations
Advisory Committee on Immunization Practices (ACIP)
www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html
MMWR COVID-19 reports
Morbidity and Mortality Weekly Report
www.cdc.gov/mmwr/Novel_Coronavirus_Reports.html
A literature hub for tracking up-to-date scientific information about the 2019 novel coronavirus
National Center for Biotechnology Information of the National Library of Medicine
www.ncbi.nlm.nih.gov/research/coronavirus
Understanding COVID-19 vaccines
National Institutes of Health COVID-19 Research
https://covid19.nih.gov/treatments-and-vaccines/covid-19-vaccines
How COVID-19 affects pregnancy
National Institutes of Health COVID-19 Research
SARS-CoV-2 virology
As the SARS-CoV-2 virus approaches the host cell, normal cell proteases on the surface membrane cause a change in the shape of the SARS-CoV-2 spike protein. That spike protein conformation change allows the virus to avoid detection by the host’s immune system because its receptor-binding site is effectively hidden until just before entry into the cell.5,6 This process is analogous to a so-called lock-and-key method of entry, in which the key (ie, spike protein conformation) is hidden by the virus until the moment it is needed, thereby minimizing exposure of viral contents to the cell. As the virus spreads through the population, it adapts to improve infectivity and transmissibility and to evade developing immunity.7
After the spike protein changes shape, it attaches to an angiotensin-converting enzyme 2 (ACE-2) receptor on the host cell, allowing the virus to enter that cell. ACE-2 receptors are located in numerous human tissues: nasopharynx, lung, gastrointestinal tract, heart, thymus, lymph nodes, bone marrow, brain, arterial and venous endothelial cells, and testes.5 The variety of tissues that contain ACE-2 receptors explains the many sites of infection and location of symptoms with which SARS-CoV-2 infection can manifest, in addition to the respiratory system.
Basic mRNA vaccine immunology
Although messenger RNA (mRNA) vaccines seem novel, they have been in development for more than 30 years.8
mRNA encodes the protein for the antigen of interest and is delivered to the host muscle tissue. There, mRNA is translated into the antigen, which stimulates an immune response. Host enzymes then rapidly degrade the mRNA in the vaccine, and it is quickly eliminated from the host.
mRNA vaccines are attractive vaccine candidates, particularly in their application to emerging infectious diseases, for several reasons:
- They are nonreplicating.
- They do not integrate into the host genome.
- They are highly effective.
- They can produce antibody and cellular immunity.
- They can be produced (and modified) quickly on a large scale without having to grow the virus in eggs.
Continue to: Vaccines against SARS-CoV-2
Vaccines against SARS-CoV-2
Two vaccines (from Pfizer-BioNTech [Comirnaty] and from Moderna [Spikevax]) are US Food and Drug Administration (FDA)–approved for COVID-19; both utilize mRNA technology. Two other vaccines, which do not use mRNA technology, have an FDA emergency use authorization (from Janssen Biotech, of Johnson & Johnson [Janssen COVID-19 Vaccine] and from Novavax [Novavax COVID-19 Vaccine, Adjuvanted]).9
Pfizer-BioNTech and Moderna vaccines. The mRNA of these vaccines encodes the entire spike protein in its pre-fusion conformation, which is the antigen that is replicated in the host, inducing an immune response.10-12 (Recall the earlier lock-and-key analogy: This conformation structure ingeniously replicates the exposed 3-dimensional key to the host’s immune system.)
The Janssen vaccine utilizes a viral vector (a nonreplicating adenovirus that functions as carrier) to deliver its message to the host for antigen production (again, the spike protein) and an immune response.
The Novavax vaccine uses a recombinant nanoparticle protein composed of the full-length spike protein.13,14 In this review, we focus on the 2 available mRNA vaccines, (1) given their FDA-authorized status and (2) because Centers for Disease Control and Prevention (CDC) recommendations indicate a preference for mRNA vaccination over viral-vectored vaccination. However, we also address key points about the Janssen (Johnson & Johnson) vaccine.
Efficacy of COVID-19 vaccines
The first study to document the safety and efficacy of a SARS-CoV-2 vaccine (the Pfizer-BioNTech vaccine) was published just 12 months after the onset of the pandemic.10 This initial trial demonstrated a 95% efficacy in preventing symptomatic, laboratory-confirmed COVID-19 at 3-month follow-up.10 Clinical trial data on the efficacy of COVID-19 vaccines have continued to be published since that first landmark trial.
Continue to: Data from trials...
Data from trials in Israel that became available early in 2021 showed that, in mRNA-vaccinated adults, mechanical ventilation rates declined strikingly, particularly in patients > 70 years of age.15,16 This finding was corroborated by data from a surveillance study of multiple US hospitals, which showed that mRNA vaccines were > 90% effective in preventing hospitalization in adults > 65 years of age.17
Data published in May 2021 showed that the Pfizer-BioNTech and Moderna vaccines were 94% effective in preventing COVID-19-related hospitalization.18 During the end of the Delta wave of the pandemic and the emergence of the Omicron variant of SARS-CoV-2, unvaccinated people were 5 times as likely to be infected as vaccinated people.19
In March 2022, data from 21 US medical centers in 18 states demonstrated that adults who had received 3 doses of the vaccine were 94% less likely to be intubated or die than those who were unvaccinated.16 A July 2022 retrospective cohort study of 231,037 subjects showed that the risk of hospitalization for acute myocardial infarction or for stroke after COVID-19 infection was reduced by more than half in fully vaccinated (ie, 2 doses of an mRNA vaccine or the viral vector [Janssen/Johnson & Johnson] vaccine) subjects, compared to unvaccinated subjects.20 The efficacy of the vaccines is summarized in TABLE 1.21-24

Even in patients who have natural infection, several studies have shown that COVID-19 vaccination after natural infection increases the level and durability of immune response to infection and reinfection and improves clinical outcomes.9,20,25,26 In summary, published literature shows that (1) mRNA vaccines are highly effective at preventing infection and (2) they augment immunity achieved by infection with circulating virus.
Breakthrough infection. COVID-19 mRNA vaccines are associated with breakthrough infection (ie, infections in fully vaccinated people), a phenomenon influenced by the predominant viral variant circulating, the level of vaccine uptake in the studied population, and the timing of vaccination.27,28 Nevertheless, vaccinated people who experience breakthrough infection are much less likely to be hospitalized and die compared to those who are unvaccinated, and vaccination with an mRNA vaccine is more effective than immunity acquired from natural infection.29
Continue to: Vaccine adverse effects
Vaccine adverse effects: Common, rare, myths
Both early mRNA vaccine trials reported common minor adverse effects after vaccination (TABLE 121-24). These included redness and soreness at the injection site, fatigue, myalgias, fever, and nausea, and tended to be more common after the second dose. These adverse effects are similar to common adverse effects seen with other vaccines. Counseling information about adverse effects can be found on the CDC website.a
Two uncommon but serious adverse effects of COVID-19 vaccination are myocarditis or pericarditis after mRNA vaccination and thrombosis with thrombocytopenia syndrome (TTS), which occurs only with the Janssen vaccine.30,31
Myocarditis and pericarditis, particularly in young males (12 to 18 years), and mostly after a second dose of vaccine, was reported in May 2021. Since then, several studies have shown that the risk of myocarditis is slightly higher in males < 40 years of age, with a predicted case rate ranging from 1 to 10 excess cases for every 1 million patients vaccinated.30,32 This risk must be balanced against the rate of myocarditis associated with SARS-CoV-2 infection.
A large study in the United States demonstrated that the risk of myocarditis for those who contract COVID-19 is 16 times higher than it is for those who are disease free.33 Observational safety data from April 2022 showed that men ages 18 to 29 years had 7 to 8 times the risk of heart complications after natural infection, compared to men of those ages who had been vaccinated.34 In this study of 40 US health care systems, the incidence of myocarditis or pericarditis in that age group ranged from 55 to 100 cases for every 100,000 people after infection and from 6 to 15 cases for every 100,000 people after a second dose of an mRNA vaccine.34
A risk–benefit analysis conducted by the Advisory Committee on Immunization Practices (ACIP) ultimately supported the conclusions that (1) the risk of myocarditis secondary to vaccination is small and (2) clear benefits of preventing infection, hospitalization, death, and continued transmission outweigh that risk.35 Study of this question, utilizing vaccine safety and reporting systems around the world, has continued.
Continue to: There is emerging evidence...
There is emerging evidence that extending the interval between the 2 doses of vaccine decreases the risk of myocarditis, particularly in male adolescents.36 That evidence ultimately led the CDC to recommend that it might be optimal that an extended interval (ie, waiting 8 weeks between the first and second dose of vaccine), in particular for males ages 12 to 39 years, could be beneficial in decreasing the risk of myocarditis.
TTS. A population risk–benefit analysis of TTS was conducted by ACIP while use of the Janssen vaccine was paused in the United States in December 2021.36 The analysis determined that, although the risk of TTS was largely in younger women (18 to 49 years; 7 cases for every 1 million vaccine doses administered), benefits of the vaccine in preventing death, hospitalization, and a stay in the intensive care unit (ICU)—particularly if vaccination was delayed or there was a high rate of community infection—clearly outweighed risks. (The CDC estimated an incidence of 2 cases of TTS with more than 3 million doses of Janssen vaccine administered; assuming moderate transmission kinetics, more than 3500 hospitalizations and more than 350 deaths were prevented by vaccination.36) Ultimately, after the CDC analysis was released, vaccination utilizing the Janssen product resumed; however, the CDC offered the caveat that the Janssen vaccine should be used only in specific situations36 (eg, when there has been a severe reaction to mRNA vaccine or when access to mRNA or recombinant nanoparticle vaccine is limited).
Myths surrounding vaccination
Myth #1: SARS-CoV-2 vaccines contain tissue from aborted fetuses. This myth, which emerged during development of the vaccines, is often a conflation of the use of embryonic cell lines obtained decades ago to produce vaccines (a common practice—not only for vaccines but common pharmaceuticals and foods).37 There are no fetal cells or tissue in any SARS-CoV-2 vaccines, and the vaccines have been endorsed by several faith organizations.38
Myth #2: SARS-CoV-2 vaccines can cause sterility in men and women. This myth originated from a report in early December 2020 seeking to link a similarity in a protein involved in placental–uterine binding and a portion of the receptor-binding domain antigen produced by the vaccine.39 No studies support this myth; COVID-19 vaccines are recommended in pregnancy by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine.40,41
Myth #3: mRNA SARS-CoV-2 vaccines alter a recipient’s DNA. mRNA vaccines are broken down by cellular enzymes. They cannot be integrated into the host genome.8
Continue to: Boosters and vaccine mix-and-match
Boosters and vaccine mix-and-match
As the COVID-19 pandemic persists, with new variants of concern emerging, it has also become clear that immunity wanes. In July 2021, the first report was published after a cluster of breakthrough infections occurred in a town in Massachusetts.42 There was no recommendation, at the time, for a booster; the Delta variant was the predominant circulating strain. In this outbreak, there were 469 cases, 74% of which were in people who had received 2 doses of an mRNA vaccine.42 Five patients were hospitalized; none died.42 A key takeaway from this outbreak was that vaccination prevented death, even in the face of fairly wide breakthrough infection.
Newer data show that, although vaccine effectiveness against hospitalization was greater than 90% for the first 2 months after a third dose, it waned to 78% by 4 months.43 Published data, combined with real-world experience, show that boosters provide additional reduction in the risk of death and hospitalization. This has led to a recommendation that all patients ≥ 5 years of age receive a booster.19,26,43-48 The CDC now recommends that people who are ages 12 years and older receive a bivalent booster (containing both wild-type and Omicron-variant antigens) ≥ 2 months after their most recent booster or completed series.
Future booster recommendations will consider the durability of the immune response over time (measured against the original immunizing virus) and the mutation rate of the virus.49
Given the limited supply of vaccine early in the pandemic, and the potential for future limitations, there was early interest in studying so-called mix-and-match SARS-CoV-2 vaccination—that is, receiving one product as a first series and then a different product as a booster, also known as heterologous booster vaccination. Although it is preferred that the 2 doses of the primary series be of the same vaccine product, studies that have examined this question support heterologous boosting as an acceptable approach to protective immunity50 (TABLE 251).

Vaccination in special populations
Three groups of patients have unique host characteristics that are important to consider when providing COVID-19 vaccination in your practice: pregnant patients, children, and patients in the broad category of “immunocompromised status.”
Continue to: Pregnant patients
Pregnant patients with SARS-CoV-2 infection are more likely to be hospitalized and have a higher risk of a stay in the ICU and need for mechanical ventilation. In a study of the course of illness in symptomatic pregnant patients who were hospitalized, 16.2% were admitted to an ICU and 8.5% were mechanically ventilated.52 CDC observational data have consistently supported the finding that (1) pregnant patients infected with SARS-CoV-2 are at increased risk of preterm labor and (2) their newborns are at increased risk of low birth weight and requiring admission to the neonatal ICU.53
A systematic review of 46 studies in pregnant and lactating patients showed no increased risk of adverse effects from COVID-19 vaccination.54 Furthermore, data from multiple studies demonstrate that immunoglobulin G antibodies cross the placenta to protect the infant at birth (ie, are found in umbilical cord blood and neonatal blood) and are found in breast milk. The precise kinetics and durability of these antibodies are unknown.
Pregnant patients were initially excluded from vaccine trials (although there were some patients ultimately found to be pregnant, or who became pregnant, during the trial). Careful examination of vaccine safety and efficacy data has supported the American College of Obstetricians and Gynecologists and European Board and College of Obstetrics and Gynaecology (EBCOG) recommendation that all pregnant patients be vaccinated. Furthermore, EBCOG recommends vaccination during the period of breastfeeding.55
Children. A major challenge during the pandemic has been to understand (1) the effect that infection with SARS-CoV-2 has on children and (2) the role of children in transmission of the virus. Although most children with COVID-19 have mild symptoms, a few require hospitalization and mechanical ventilation and some develop life-threatening multisystem inflammatory syndrome.56 In a large, retrospective study of more than 12,000 children with COVID-19, 5.3% required hospitalization and almost 20% of that subset were admitted to the ICU.57
Various hypotheses have been put forward to describe and explain the differences in disease expression between children and adults. These include:
- the absence of comorbidities often seen in adults
- evidence that pediatric patients might have reduced expression of ACE-2
- a more active T-cell response in infected children, due to an active thymus.56
Continue to: Although the number of children affected...
Although the number of children affected by severe SARS-CoV-2 infection is less than the number of adults, there have been important trends observed in infection and hospitalization as different variants have arisen.58 The Delta and Omicron variants have both been associated with a disturbing trend in the rate of hospitalization of pediatric patients, particularly from birth to 4 years—patients who were ineligible for vaccination at the time of the study.58 Ultimately, these data, combined with multiple studies of vaccine effectiveness in this age group, have led to an emergency use authorization for the Pfizer-BioNTech vaccination in pediatric populations and a recommendation from the American Academy of Pediatrics that all children ages 6 months and older be vaccinated.59,60
Immunocompromised patients. Patients broadly classified as immunocompromised have raised unique concerns. These patients have conditions such as malignancy, primary or secondary immunodeficiency, diabetes, and autoimmune disease; are taking certain classes of medication; or are of older age.61 Early in the pandemic, data showed that immunocompromised hosts could shed virus longer than hosts with an intact immune system—adding to their risk of transmitting SARS-CoV-2 and of viral adaptation for immune escape.62 Antibody response to vaccination was also less robust in this group.
There are limited data that demonstrate a short-lived reduction in risk of infection (in that study, Omicron was the prominent variant) with a fourth dose of an mRNA vaccine.63 Based on these data and FDA approval, the CDC recommends (1) an additional third primary dose and (2) a second booster for people who are moderately or severely immunocompromised. For those ages 50 years or older, a second booster is now required for their vaccination to be considered up to date.b
Predictions (or, why is a COVID-19 vaccine important?)
What does the future hold for our struggle with COVID-19? Perhaps we can learn lessons from the study of the 4 known seasonal coronaviruses, which cause the common cold and circulate annually.64 First, only relative immunity is produced after infection with a seasonal coronavirus.64 Studies of antibodies to seasonal coronaviruses seem to suggest that, although antibody titers remain high, correlation with decreased infection is lacking.65 Second, a dominant strain or 2 emerges each season, probably as a result of genetic variation and selective pressure for immune escape from neutralizing antibodies or cellular immunity.
The complex relationship among competing immune response duration, emergence of viral immune escape, increasing viral transmissibility, and societal viral source control (through vaccination, masking, distancing, testing, isolation, and treatment) widens the confidence bounds on our estimates of what the future holds. Late in 2020, the CDC began reporting wastewater surveillance data as a method for monitoring, and predicting changes in, community spread.66 During Spring 2022, the CDC reported an increase in detection of SARS-CoV-2 from a third of wastewater sampling sites around the United States. This observation coincided with (1) appearance of still more transmissible BA.2 and, later, BA.2.12.1 variants and (2) general relaxing of masking and social distancing guidelines, following the decline of the Omicron variant.
Continue to: At approximately that time...
At approximately that time, application to the FDA for a fourth shot (or a second booster) by Pfizer-BioNTech had been approved for adults > 50 years of age, at > 4 months after their previous vaccination.57 In view of warning signs from wastewater surveillance, priorities for vaccination should be to:
- increase uptake in the hesitant
- get boosters to the eligible
- prepare to tackle either seasonal or sporadic recurrence of COVID-19—whichever scenario the future brings.
As an example of how these priorities have been put into action, in September 2022, the FDA approved, and the CDC recommended, new bivalent boosters for everyone ≥ 12 years of age (Pfizer-BioNTech) or for all those ≥ 18 years of age (Moderna), to be administered ≥ 2 months after receipt of their most recent booster or primary series.
a www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html
b Visit www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html for more guidance on COVID-19 vaccination for immunocompromised patients.
CORRESPONDENCE
John L. Kiley, MD, 3551 Roger Brooke Drive, Fort Sam Houston, TX 78234; [email protected]
Worldwide and across many diseases, vaccines have been transformative in reducing mortality—an effect that has been sustained with vaccines that protect against COVID-19.1 Since the first cases of SARS-CoV-2 infection were reported in late 2019, the pace of scientific investigation into the virus and the disease—made possible by unprecedented funding, infrastructure, and public and private partnerships—has been explosive. The result? A vast body of clinical and laboratory evidence about the safety and effectiveness of SARS-CoV-2 vaccines, which quickly became widely available.2-4
In this article, we review the basic underlying virology of SARS-CoV-2; the biotechnological basis of vaccines against COVID-19 that are available in the United States; and recommendations on how to provide those vaccines to your patients. Additional guidance for your practice appears in a select online bibliography, “COVID-19 vaccination resources.”
SIDEBAR
COVID-19 vaccination resources
Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States
Centers for Disease Control and Prevention
www.cdc.gov/vaccines/covid-19/clinical-considerations/interimconsiderations-us.html
COVID-19 ACIP vaccine recommendations
Advisory Committee on Immunization Practices (ACIP)
www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html
MMWR COVID-19 reports
Morbidity and Mortality Weekly Report
www.cdc.gov/mmwr/Novel_Coronavirus_Reports.html
A literature hub for tracking up-to-date scientific information about the 2019 novel coronavirus
National Center for Biotechnology Information of the National Library of Medicine
www.ncbi.nlm.nih.gov/research/coronavirus
Understanding COVID-19 vaccines
National Institutes of Health COVID-19 Research
https://covid19.nih.gov/treatments-and-vaccines/covid-19-vaccines
How COVID-19 affects pregnancy
National Institutes of Health COVID-19 Research
SARS-CoV-2 virology
As the SARS-CoV-2 virus approaches the host cell, normal cell proteases on the surface membrane cause a change in the shape of the SARS-CoV-2 spike protein. That spike protein conformation change allows the virus to avoid detection by the host’s immune system because its receptor-binding site is effectively hidden until just before entry into the cell.5,6 This process is analogous to a so-called lock-and-key method of entry, in which the key (ie, spike protein conformation) is hidden by the virus until the moment it is needed, thereby minimizing exposure of viral contents to the cell. As the virus spreads through the population, it adapts to improve infectivity and transmissibility and to evade developing immunity.7
After the spike protein changes shape, it attaches to an angiotensin-converting enzyme 2 (ACE-2) receptor on the host cell, allowing the virus to enter that cell. ACE-2 receptors are located in numerous human tissues: nasopharynx, lung, gastrointestinal tract, heart, thymus, lymph nodes, bone marrow, brain, arterial and venous endothelial cells, and testes.5 The variety of tissues that contain ACE-2 receptors explains the many sites of infection and location of symptoms with which SARS-CoV-2 infection can manifest, in addition to the respiratory system.
Basic mRNA vaccine immunology
Although messenger RNA (mRNA) vaccines seem novel, they have been in development for more than 30 years.8
mRNA encodes the protein for the antigen of interest and is delivered to the host muscle tissue. There, mRNA is translated into the antigen, which stimulates an immune response. Host enzymes then rapidly degrade the mRNA in the vaccine, and it is quickly eliminated from the host.
mRNA vaccines are attractive vaccine candidates, particularly in their application to emerging infectious diseases, for several reasons:
- They are nonreplicating.
- They do not integrate into the host genome.
- They are highly effective.
- They can produce antibody and cellular immunity.
- They can be produced (and modified) quickly on a large scale without having to grow the virus in eggs.
Continue to: Vaccines against SARS-CoV-2
Vaccines against SARS-CoV-2
Two vaccines (from Pfizer-BioNTech [Comirnaty] and from Moderna [Spikevax]) are US Food and Drug Administration (FDA)–approved for COVID-19; both utilize mRNA technology. Two other vaccines, which do not use mRNA technology, have an FDA emergency use authorization (from Janssen Biotech, of Johnson & Johnson [Janssen COVID-19 Vaccine] and from Novavax [Novavax COVID-19 Vaccine, Adjuvanted]).9
Pfizer-BioNTech and Moderna vaccines. The mRNA of these vaccines encodes the entire spike protein in its pre-fusion conformation, which is the antigen that is replicated in the host, inducing an immune response.10-12 (Recall the earlier lock-and-key analogy: This conformation structure ingeniously replicates the exposed 3-dimensional key to the host’s immune system.)
The Janssen vaccine utilizes a viral vector (a nonreplicating adenovirus that functions as carrier) to deliver its message to the host for antigen production (again, the spike protein) and an immune response.
The Novavax vaccine uses a recombinant nanoparticle protein composed of the full-length spike protein.13,14 In this review, we focus on the 2 available mRNA vaccines, (1) given their FDA-authorized status and (2) because Centers for Disease Control and Prevention (CDC) recommendations indicate a preference for mRNA vaccination over viral-vectored vaccination. However, we also address key points about the Janssen (Johnson & Johnson) vaccine.
Efficacy of COVID-19 vaccines
The first study to document the safety and efficacy of a SARS-CoV-2 vaccine (the Pfizer-BioNTech vaccine) was published just 12 months after the onset of the pandemic.10 This initial trial demonstrated a 95% efficacy in preventing symptomatic, laboratory-confirmed COVID-19 at 3-month follow-up.10 Clinical trial data on the efficacy of COVID-19 vaccines have continued to be published since that first landmark trial.
Continue to: Data from trials...
Data from trials in Israel that became available early in 2021 showed that, in mRNA-vaccinated adults, mechanical ventilation rates declined strikingly, particularly in patients > 70 years of age.15,16 This finding was corroborated by data from a surveillance study of multiple US hospitals, which showed that mRNA vaccines were > 90% effective in preventing hospitalization in adults > 65 years of age.17
Data published in May 2021 showed that the Pfizer-BioNTech and Moderna vaccines were 94% effective in preventing COVID-19-related hospitalization.18 During the end of the Delta wave of the pandemic and the emergence of the Omicron variant of SARS-CoV-2, unvaccinated people were 5 times as likely to be infected as vaccinated people.19
In March 2022, data from 21 US medical centers in 18 states demonstrated that adults who had received 3 doses of the vaccine were 94% less likely to be intubated or die than those who were unvaccinated.16 A July 2022 retrospective cohort study of 231,037 subjects showed that the risk of hospitalization for acute myocardial infarction or for stroke after COVID-19 infection was reduced by more than half in fully vaccinated (ie, 2 doses of an mRNA vaccine or the viral vector [Janssen/Johnson & Johnson] vaccine) subjects, compared to unvaccinated subjects.20 The efficacy of the vaccines is summarized in TABLE 1.21-24

Even in patients who have natural infection, several studies have shown that COVID-19 vaccination after natural infection increases the level and durability of immune response to infection and reinfection and improves clinical outcomes.9,20,25,26 In summary, published literature shows that (1) mRNA vaccines are highly effective at preventing infection and (2) they augment immunity achieved by infection with circulating virus.
Breakthrough infection. COVID-19 mRNA vaccines are associated with breakthrough infection (ie, infections in fully vaccinated people), a phenomenon influenced by the predominant viral variant circulating, the level of vaccine uptake in the studied population, and the timing of vaccination.27,28 Nevertheless, vaccinated people who experience breakthrough infection are much less likely to be hospitalized and die compared to those who are unvaccinated, and vaccination with an mRNA vaccine is more effective than immunity acquired from natural infection.29
Continue to: Vaccine adverse effects
Vaccine adverse effects: Common, rare, myths
Both early mRNA vaccine trials reported common minor adverse effects after vaccination (TABLE 121-24). These included redness and soreness at the injection site, fatigue, myalgias, fever, and nausea, and tended to be more common after the second dose. These adverse effects are similar to common adverse effects seen with other vaccines. Counseling information about adverse effects can be found on the CDC website.a
Two uncommon but serious adverse effects of COVID-19 vaccination are myocarditis or pericarditis after mRNA vaccination and thrombosis with thrombocytopenia syndrome (TTS), which occurs only with the Janssen vaccine.30,31
Myocarditis and pericarditis, particularly in young males (12 to 18 years), and mostly after a second dose of vaccine, was reported in May 2021. Since then, several studies have shown that the risk of myocarditis is slightly higher in males < 40 years of age, with a predicted case rate ranging from 1 to 10 excess cases for every 1 million patients vaccinated.30,32 This risk must be balanced against the rate of myocarditis associated with SARS-CoV-2 infection.
A large study in the United States demonstrated that the risk of myocarditis for those who contract COVID-19 is 16 times higher than it is for those who are disease free.33 Observational safety data from April 2022 showed that men ages 18 to 29 years had 7 to 8 times the risk of heart complications after natural infection, compared to men of those ages who had been vaccinated.34 In this study of 40 US health care systems, the incidence of myocarditis or pericarditis in that age group ranged from 55 to 100 cases for every 100,000 people after infection and from 6 to 15 cases for every 100,000 people after a second dose of an mRNA vaccine.34
A risk–benefit analysis conducted by the Advisory Committee on Immunization Practices (ACIP) ultimately supported the conclusions that (1) the risk of myocarditis secondary to vaccination is small and (2) clear benefits of preventing infection, hospitalization, death, and continued transmission outweigh that risk.35 Study of this question, utilizing vaccine safety and reporting systems around the world, has continued.
Continue to: There is emerging evidence...
There is emerging evidence that extending the interval between the 2 doses of vaccine decreases the risk of myocarditis, particularly in male adolescents.36 That evidence ultimately led the CDC to recommend that it might be optimal that an extended interval (ie, waiting 8 weeks between the first and second dose of vaccine), in particular for males ages 12 to 39 years, could be beneficial in decreasing the risk of myocarditis.
TTS. A population risk–benefit analysis of TTS was conducted by ACIP while use of the Janssen vaccine was paused in the United States in December 2021.36 The analysis determined that, although the risk of TTS was largely in younger women (18 to 49 years; 7 cases for every 1 million vaccine doses administered), benefits of the vaccine in preventing death, hospitalization, and a stay in the intensive care unit (ICU)—particularly if vaccination was delayed or there was a high rate of community infection—clearly outweighed risks. (The CDC estimated an incidence of 2 cases of TTS with more than 3 million doses of Janssen vaccine administered; assuming moderate transmission kinetics, more than 3500 hospitalizations and more than 350 deaths were prevented by vaccination.36) Ultimately, after the CDC analysis was released, vaccination utilizing the Janssen product resumed; however, the CDC offered the caveat that the Janssen vaccine should be used only in specific situations36 (eg, when there has been a severe reaction to mRNA vaccine or when access to mRNA or recombinant nanoparticle vaccine is limited).
Myths surrounding vaccination
Myth #1: SARS-CoV-2 vaccines contain tissue from aborted fetuses. This myth, which emerged during development of the vaccines, is often a conflation of the use of embryonic cell lines obtained decades ago to produce vaccines (a common practice—not only for vaccines but common pharmaceuticals and foods).37 There are no fetal cells or tissue in any SARS-CoV-2 vaccines, and the vaccines have been endorsed by several faith organizations.38
Myth #2: SARS-CoV-2 vaccines can cause sterility in men and women. This myth originated from a report in early December 2020 seeking to link a similarity in a protein involved in placental–uterine binding and a portion of the receptor-binding domain antigen produced by the vaccine.39 No studies support this myth; COVID-19 vaccines are recommended in pregnancy by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine.40,41
Myth #3: mRNA SARS-CoV-2 vaccines alter a recipient’s DNA. mRNA vaccines are broken down by cellular enzymes. They cannot be integrated into the host genome.8
Continue to: Boosters and vaccine mix-and-match
Boosters and vaccine mix-and-match
As the COVID-19 pandemic persists, with new variants of concern emerging, it has also become clear that immunity wanes. In July 2021, the first report was published after a cluster of breakthrough infections occurred in a town in Massachusetts.42 There was no recommendation, at the time, for a booster; the Delta variant was the predominant circulating strain. In this outbreak, there were 469 cases, 74% of which were in people who had received 2 doses of an mRNA vaccine.42 Five patients were hospitalized; none died.42 A key takeaway from this outbreak was that vaccination prevented death, even in the face of fairly wide breakthrough infection.
Newer data show that, although vaccine effectiveness against hospitalization was greater than 90% for the first 2 months after a third dose, it waned to 78% by 4 months.43 Published data, combined with real-world experience, show that boosters provide additional reduction in the risk of death and hospitalization. This has led to a recommendation that all patients ≥ 5 years of age receive a booster.19,26,43-48 The CDC now recommends that people who are ages 12 years and older receive a bivalent booster (containing both wild-type and Omicron-variant antigens) ≥ 2 months after their most recent booster or completed series.
Future booster recommendations will consider the durability of the immune response over time (measured against the original immunizing virus) and the mutation rate of the virus.49
Given the limited supply of vaccine early in the pandemic, and the potential for future limitations, there was early interest in studying so-called mix-and-match SARS-CoV-2 vaccination—that is, receiving one product as a first series and then a different product as a booster, also known as heterologous booster vaccination. Although it is preferred that the 2 doses of the primary series be of the same vaccine product, studies that have examined this question support heterologous boosting as an acceptable approach to protective immunity50 (TABLE 251).

Vaccination in special populations
Three groups of patients have unique host characteristics that are important to consider when providing COVID-19 vaccination in your practice: pregnant patients, children, and patients in the broad category of “immunocompromised status.”
Continue to: Pregnant patients
Pregnant patients with SARS-CoV-2 infection are more likely to be hospitalized and have a higher risk of a stay in the ICU and need for mechanical ventilation. In a study of the course of illness in symptomatic pregnant patients who were hospitalized, 16.2% were admitted to an ICU and 8.5% were mechanically ventilated.52 CDC observational data have consistently supported the finding that (1) pregnant patients infected with SARS-CoV-2 are at increased risk of preterm labor and (2) their newborns are at increased risk of low birth weight and requiring admission to the neonatal ICU.53
A systematic review of 46 studies in pregnant and lactating patients showed no increased risk of adverse effects from COVID-19 vaccination.54 Furthermore, data from multiple studies demonstrate that immunoglobulin G antibodies cross the placenta to protect the infant at birth (ie, are found in umbilical cord blood and neonatal blood) and are found in breast milk. The precise kinetics and durability of these antibodies are unknown.
Pregnant patients were initially excluded from vaccine trials (although there were some patients ultimately found to be pregnant, or who became pregnant, during the trial). Careful examination of vaccine safety and efficacy data has supported the American College of Obstetricians and Gynecologists and European Board and College of Obstetrics and Gynaecology (EBCOG) recommendation that all pregnant patients be vaccinated. Furthermore, EBCOG recommends vaccination during the period of breastfeeding.55
Children. A major challenge during the pandemic has been to understand (1) the effect that infection with SARS-CoV-2 has on children and (2) the role of children in transmission of the virus. Although most children with COVID-19 have mild symptoms, a few require hospitalization and mechanical ventilation and some develop life-threatening multisystem inflammatory syndrome.56 In a large, retrospective study of more than 12,000 children with COVID-19, 5.3% required hospitalization and almost 20% of that subset were admitted to the ICU.57
Various hypotheses have been put forward to describe and explain the differences in disease expression between children and adults. These include:
- the absence of comorbidities often seen in adults
- evidence that pediatric patients might have reduced expression of ACE-2
- a more active T-cell response in infected children, due to an active thymus.56
Continue to: Although the number of children affected...
Although the number of children affected by severe SARS-CoV-2 infection is less than the number of adults, there have been important trends observed in infection and hospitalization as different variants have arisen.58 The Delta and Omicron variants have both been associated with a disturbing trend in the rate of hospitalization of pediatric patients, particularly from birth to 4 years—patients who were ineligible for vaccination at the time of the study.58 Ultimately, these data, combined with multiple studies of vaccine effectiveness in this age group, have led to an emergency use authorization for the Pfizer-BioNTech vaccination in pediatric populations and a recommendation from the American Academy of Pediatrics that all children ages 6 months and older be vaccinated.59,60
Immunocompromised patients. Patients broadly classified as immunocompromised have raised unique concerns. These patients have conditions such as malignancy, primary or secondary immunodeficiency, diabetes, and autoimmune disease; are taking certain classes of medication; or are of older age.61 Early in the pandemic, data showed that immunocompromised hosts could shed virus longer than hosts with an intact immune system—adding to their risk of transmitting SARS-CoV-2 and of viral adaptation for immune escape.62 Antibody response to vaccination was also less robust in this group.
There are limited data that demonstrate a short-lived reduction in risk of infection (in that study, Omicron was the prominent variant) with a fourth dose of an mRNA vaccine.63 Based on these data and FDA approval, the CDC recommends (1) an additional third primary dose and (2) a second booster for people who are moderately or severely immunocompromised. For those ages 50 years or older, a second booster is now required for their vaccination to be considered up to date.b
Predictions (or, why is a COVID-19 vaccine important?)
What does the future hold for our struggle with COVID-19? Perhaps we can learn lessons from the study of the 4 known seasonal coronaviruses, which cause the common cold and circulate annually.64 First, only relative immunity is produced after infection with a seasonal coronavirus.64 Studies of antibodies to seasonal coronaviruses seem to suggest that, although antibody titers remain high, correlation with decreased infection is lacking.65 Second, a dominant strain or 2 emerges each season, probably as a result of genetic variation and selective pressure for immune escape from neutralizing antibodies or cellular immunity.
The complex relationship among competing immune response duration, emergence of viral immune escape, increasing viral transmissibility, and societal viral source control (through vaccination, masking, distancing, testing, isolation, and treatment) widens the confidence bounds on our estimates of what the future holds. Late in 2020, the CDC began reporting wastewater surveillance data as a method for monitoring, and predicting changes in, community spread.66 During Spring 2022, the CDC reported an increase in detection of SARS-CoV-2 from a third of wastewater sampling sites around the United States. This observation coincided with (1) appearance of still more transmissible BA.2 and, later, BA.2.12.1 variants and (2) general relaxing of masking and social distancing guidelines, following the decline of the Omicron variant.
Continue to: At approximately that time...
At approximately that time, application to the FDA for a fourth shot (or a second booster) by Pfizer-BioNTech had been approved for adults > 50 years of age, at > 4 months after their previous vaccination.57 In view of warning signs from wastewater surveillance, priorities for vaccination should be to:
- increase uptake in the hesitant
- get boosters to the eligible
- prepare to tackle either seasonal or sporadic recurrence of COVID-19—whichever scenario the future brings.
As an example of how these priorities have been put into action, in September 2022, the FDA approved, and the CDC recommended, new bivalent boosters for everyone ≥ 12 years of age (Pfizer-BioNTech) or for all those ≥ 18 years of age (Moderna), to be administered ≥ 2 months after receipt of their most recent booster or primary series.
a www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html
b Visit www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html for more guidance on COVID-19 vaccination for immunocompromised patients.
CORRESPONDENCE
John L. Kiley, MD, 3551 Roger Brooke Drive, Fort Sam Houston, TX 78234; [email protected]
1. Orenstein W, Offitt P, Edwards KM, Plotkin S. Plotkin’s Vaccines. 7th ed. Elsevier; 2017:1-15.
2. Operation Warp Speed: implications for global vaccine security. Lancet Glob Health. 2021;9:e1017-e1021. doi: 10.1016/S2214-109X(21)00140-6
3. Lurie N, Saville M, Hatchett R, et al. Developing Covid-19 vaccines at pandemic speed. N Engl J Med. 2020;382:1969-1973. doi: 10.1056/NEJMp2005630
4. Slaoui M, Hepburn M. Developing safe and effective Covid vaccines—Operation Warp Speed’s strategy and approach. N Engl J Med. 2020;383:1701-1703. doi: 10.1056/NEJMp2027405
5. Hu B, Guo H, Zhou P, et al. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19:141-154. doi: 10.1038/s41579-020-00459-7
6. Hussain I, Pervaiz N, Khan A, et al. Evolutionary and structural analysis of SARS-CoV-2 specific evasion of host immunity. Genes Immun. 2020;21:409-419. doi: 10.1038/s41435-020-00120-6
7. Rando HM, Wellhausen N, Ghosh S, et al; COVID-19 Review Consortium. Identification and development of therapeutics for COVID-19. mSystems. 2021;6:e0023321. doi: 10.1128/mSystems.00233-21
8. Pardi N, Hogan MJ, Porter FW, et al. mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261-279. doi: 10.1038/nrd.2017.243
9. National Center for Immunization and Respiratory Diseases. Use of COVID-19 vaccines in the United States: interim clinical considerations. Centers for Disease Control and Prevention. Updated August 22, 2022. Accessed August 27, 2022. www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html#references
10. Polack FP, Thomas SJ, Kitchin N, et al; . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603-2615. doi: 10.1056/NEJMoa2034577
11. Heinz FX, Stiasny K. Distinguishing features of current COVID-19 vaccines: knowns and unknowns of antigen presentation and modes of action. NPJ Vaccines. 2021;6:104. doi: 10.1038/s41541-021-00369-6
12. Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403-416. doi: 10.1056/NEJMoa2035389
13. Keech C, Albert G, Cho I, et al. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383:2320-2332. doi: 10.1056/NEJMoa2026920
14. Heath PT, Galiza EP, Baxter DN, et al; . Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med. 2021;385:1172-1183. doi: 10.1056/NEJMoa2107659
15. Rinott E, Youngster I, Lewis YE. Reduction in COVID-19 patients requiring mechanical ventilation following implementation of a national COVID-19 vaccination program—Israel, December 2020–February 2021. MMWR Morb Mortal Wkly Rep. 2021;70:326-328. doi: 10.15585/mmwr.mm7009e3
16. Tenforde MW, Self WH, Gaglani M, et al; IVY Network. Effectiveness of mRNA vaccination in preventing COVID-19-associated invasive mechanical ventilation and death—United States, March 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:459-465. doi: 10.15585/mmwr.mm7112e1
17. Moline HL, Whitaker M, Deng L, et al. Effectiveness of COVID-19 vaccines in preventing hospitalization among adults aged ≥ 65 years—COVID-NET, 13 States, February–April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1088-1093. doi: 10.15585/mmwr.mm7032e
18. Tenforde MW, Olson SM, Self WH, et al; ; . Effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 among hospitalized adults aged ≥ 65 years—United States, January–March 2021. MMWR Morb Mortal Wkly Rep. 2021;70:674-679. doi: 10.15585/mmwr.mm7018e1
19. Johnson AG, Amin AB, Ali AR, et al. COVID-19 incidence and death rates among unvaccinated and fully vaccinated adults with and without booster doses during periods of Delta and Omicron variant emergence—25 U.S. jurisdictions, April 4–December 25, 2021. MMWR Morb Mortal Wkly Rep. 2022;71:132-138. doi: 10.15585/mmwr.mm7104e2
20. Kim Y-E, Huh K, Park Y-J, et al. Association between vaccination and acute myocardial infarction and ischemic stroke after COVID-19 infection. JAMA. Published online July 22, 2022. doi: 10.1001/jama.2022.12992
21. Centers for Disease Control and Prevention. Pfizer-BioNTech COVID-19 vaccine reactions & adverse events. Updated June 21, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html
22. Centers for Disease Control and Prevention. The Moderna COVID-19 vaccine’s local reactions, systemic reactions, adverse events, and serious adverse events. Updated June 21, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html
23. Centers for Disease Control and Prevention. The Janssen COVID-19 vaccine’s local Reactions, Systemic reactions, adverse events, and serious adverse events. Updated August 12, 2021. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/janssen/reactogenicity.html
24. Centers for Disease Control and Prevention. Novavax COVID-19 vaccine local reactions, systemic reactions, adverse events, and serious adverse events. Updated August 31, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/novavax/reactogenicity.html
25. Greaney AJ, Loes AN, Gentles LE, et al. Antibodies elicited by mRNA-1273 vaccination bind more broadly to the receptor binding domain than do those from SARS-CoV-2 infection. Sci Transl Med. 2021;13:eabi9915. doi: 10.1126/scitranslmed.abi9915
26. Hall V, Foulkes S, Insalata F, et al. Protection against SARS-CoV-2 after Covid-19 vaccination and previous infection. N Engl J Med. 2022;386:1207-1220. doi: 10.1056/NEJMoa2118691
27. Klompas M. Understanding breakthrough infections following mRNA SARS-CoV-2 avccination. JAMA. 2021;326:2018-2020. doi: 10.1001/jama.2021.19063
28. Kustin T, Harel N, Finkel U, et al. Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals. Nat Med. 2021;27:1379-1384. doi: 10.1038/s41591-021-01413-7
29. Yu Y, Esposito D, Kang Z, et al. mRNA vaccine-induced antibodies more effective than natural immunity in neutralizing SARS-CoV-2 and its high affinity variants. Sci Rep. 2022;12:2628. doi: 10.1038/s41598-022-06629-2
30. Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:977-982. doi: 10.15585/mmwr.mm7027e2
31. MacNeil JR, Su JR, Broder KR, et al. Updated recommendations from the Advisory Committee on Immunization Practices for use of the Janssen (Johnson & Johnson) COVID-19 vaccine after reports of thrombosis with thrombocytopenia syndrome among vaccine recipients—United States, April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:651-656. doi: 10.15585/mmwr.mm7017e4
32. Patone M, Mei XW, Handunnetthi L, et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2022;28:410-422. doi: 10.1038/s41591-021-01630-0
33. Boehmer TK, Kompaniyets L, Lavery AM, et al. Association between COVID-19 and myocarditis using hospital-based administrative data—United States, March 2020–January 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1228-1232. doi: 10.15585/mmwr.mm7035e5
34. Block JP, Boehmer TK, Forrest CB, et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination—PCORnet, United States, January 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:517-523. doi: 10.15585/mmwr.mm7114e1
35. Rosemblum H. COVID-19 vaccines in adults: benefit–risk discussion. Centers for Disease Control and Prevention. July 22, 2021. Accessed September 21, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-07/05-COVID-Rosenblum-508.pdf
36. Buchan SA, Seo CY, Johnson C, et al. Epidemiology of myocarditis and pericarditis following mRNA vaccines in Ontario, Canada: by vaccine product, schedule and interval. medRxiv. 2021:12.02.21267156.
37. Wong A. The ethics of HEK 293. Natl Cathol Bioeth Q. 2006;6:473-495. doi: 10.5840/ncbq20066331
38. North Dakota Health. COVID-19 vaccines & fetal cell lines. Updated December 1, 2021. Accessed September 21, 2022. www.health.nd.gov/sites/www/files/documents/COVID%20Vaccine%20Page/COVID-19_Vaccine_Fetal_Cell_Handout.pdf
39. Abbasi J. Widespread misinformation about infertility continues to create COVID-19 vaccine hesitancy. JAMA. 2022;327:1013-1015. doi: 10.1001/jama.2022.2404
40. Halasa NB, Olson SM, Staat MA, et al; ; . Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19-associated hospitalization in infants aged < 6 months—17 States, July 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:264-270. doi: 10.15585/mmwr.mm7107e3
41. American College of Obstetricians and Gynecologists. ACOG and SMFM recommend COVID-19 vaccination for pregnant individuals. July 30, 2021. Accessed September 21, 2022. www.acog.org/news/news-releases/2021/07/acog-smfm-recommend-covid-19-vaccination-for-pregnant-individuals#:~:text=%E2%80%9CACOG%20is%20recommending%20vaccination%20of,complications%2C%20and%20because%20it%20isvaccines
42. Brown CM, Vostok J, Johnson H, et al. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings—Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1059-1062. doi: 10.15585/mmwr.mm7031e2
43. Ferdinands JM, Rao S, Dixon BE, et al. Waning 2-dose and 3-dose effectiveness of mRNA against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance—VISION Network, 10 states, August 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:255-263. doi: 10.15585/mmwr.mm7107e2
44. Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Effect of mRNA vaccine boosters against SARS-CoV-2 Omicron infection in Qatar. N Engl J Med. 2022;386:1804-1816. doi: 10.1056/NEJMoa2200797
45. Arbel R, Hammerman A, Sergienko R, et al. BNT162b2 vaccine booster and mortality due to Covid-19. N Engl J Med. 2021;385:2413-2420. doi: 10.1056/NEJMoa2115624
46. Bar-On YM, Goldberg Y, Mandel M, et al. Protection against Covid-19 by BNT162b2 booster across age groups. N Engl J Med. 2021;385:2421-2430. doi: 10.1056/NEJMoa2115926
47. Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385:1393-1400. doi: 10.1056/NEJMoa2114255
48. Mbaeyi S, Oliver SE, Collins JP, et al. The Advisory Committee on Immunization Practices’ interim recommendations for additional primary and booster doses of COVID-19 vaccines—United States, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1545-1552. doi: 10.15585/mmwr.mm7044e2
49. Chen X, Chen Z, Azman AS, et al. Neutralizing antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants induced by natural infection or vaccination: a systematic review and pooled analysis. Clin Infect Dis. 2022;74:734-742. doi: 10.1093/cid/ciab646
50. Atmar RL, Lyke KE, Deming ME, et al; . Homologous and heterologous Covid-19 booster vaccinations. N Engl J Med. 2022;386:1046-1057. doi: 10.1056/NEJMoa2116414
51. Centers for Disease Control and Prevention. Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. Updated September 2, 2022. Accessed September 21, 2022. www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html
52. Ackerman CM, Nguyen JL, Ambati S, et al. Clinical and pregnancy outcomes of coronavirus disease 2019 among hospitalized pregnant women in the United States. Open Forum Infect Dis. 2022;9:ofab429. doi: 10.1093/ofid/ofab429
53. Osterman MJK, Valenzuela CP, Martin JA. Maternal and infant characteristics among women with confirmed or presumed cases of coronavirus disease (COVID-19) during pregnancy. National Center for Health Statistics. National Vital Statistics System. Updated August 11, 2022. Accessed September 21, 2022. www.cdc.gov/nchs/covid19/technical-linkage.htm
54. De Rose DU, Salvatori G, Dotta A, et al. SARS-CoV-2 vaccines during pregnancy and breastfeeding: a systematic review of maternal and neonatal outcomes. Viruses. 2022;14:539. doi: 10.3390/v14030539
55. Martins I, Louwen F, Ayres-de-Campos D, et al. EBCOG position statement on COVID-19 vaccination for pregnant and breastfeeding women. Eur J Obstet Gynecol Reprod Biol. 2021;262:256-258. doi: 10.1016/j.ejogrb.2021.05.021
56. Chou J, Thomas PG, Randolph AG. Immunology of SARS-CoV-2 infection in children. Nat Immunol. 2022;23:177-185. doi: 10.1038/s41590-021-01123-9
57. Parcha V, Booker KS, Kalra R, et al. A retrospective cohort study of 12,306 pediatric COVID-19 patients in the United States. Sci Rep. 2021;11:10231. doi: 10.1038/s41598-021-89553-1
58. Marks KJ, Whitaker M, Anglin O, et al; . Hospitalizations of children and adolescents with laboratory-confirmed COVID-19—COVID-NET, 14 states, July 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:271-278. doi: 10.15585/mmwr.mm7107e4
59. Price AM, Olson SM, Newhams MM, et al; . BNT162b2 protection against the Omicron variant in children and adolescents. N Engl J Med. 2022;386:1899-1909. doi: 10.1056/NEJMoa2202826
60. Maldonado YA, O’Leary ST, Banerjee R, et al; Committee on Infectious Diseases, American Academy of Pediatrics. COVID-19 vaccines in children and adolescents. Pediatrics. 2021;148:e2021052336. doi: 10.1542/peds.2021-052336
61. Lontok K. How effective are COVID-19 vaccines in immunocompromised people? American Society for Microbiology. August 12, 2021. Accessed September 21, 2022. https://asm.org/Articles/2021/August/How-Effective-Are-COVID-19-Vaccines-in-Immunocompr
62. Meiring S, Tempia S, Bhiman JN, et al; . Prolonged shedding of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) at high viral loads among hospitalized immunocompromised persons living with human immunodeficiency virus, South Africa. Clin Infect Dis. 2022;75:e144-e156. doi: 10.1093/cid/ciac077
63. Bar-On YM, Goldberg Y, Mandel M, et al. Protection by 4th dose of BNT162b2 against Omicron in Israel. medRxiv. 2022: 02.01.22270232. doi: 10.1101/2022.02.01.22270232
64. Monto AS, DeJonge PM, Callear AP, et al. Coronavirus occurrence and transmission over 8 years in the HIVE cohort of households in Michigan. J Infect Dis. 2020;222:9-16. doi: 10.1093/infdis/jiaa161
65. Petrie JG, Bazzi LA, McDermott AB, et al. Coronavirus occurrence in the Household Influenza Vaccine Evaluation (HIVE) cohort of Michigan households: reinfection frequency and serologic responses to seasonal and severe acute respiratory syndrome coronaviruses. J Infect Dis. 2021;224:49-59. doi: 10.1093/infdis/jiab161
66. Kirby AE, Walters MS, Jennings WC, et al. Using wastewater surveillance data to support the COVID-19 response—United States, 2020–2021. MMWR Morb Mortal Wkly Rep. 2021;70:1242-1244. doi: 10.15585/mmwr.mm7036a2
1. Orenstein W, Offitt P, Edwards KM, Plotkin S. Plotkin’s Vaccines. 7th ed. Elsevier; 2017:1-15.
2. Operation Warp Speed: implications for global vaccine security. Lancet Glob Health. 2021;9:e1017-e1021. doi: 10.1016/S2214-109X(21)00140-6
3. Lurie N, Saville M, Hatchett R, et al. Developing Covid-19 vaccines at pandemic speed. N Engl J Med. 2020;382:1969-1973. doi: 10.1056/NEJMp2005630
4. Slaoui M, Hepburn M. Developing safe and effective Covid vaccines—Operation Warp Speed’s strategy and approach. N Engl J Med. 2020;383:1701-1703. doi: 10.1056/NEJMp2027405
5. Hu B, Guo H, Zhou P, et al. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19:141-154. doi: 10.1038/s41579-020-00459-7
6. Hussain I, Pervaiz N, Khan A, et al. Evolutionary and structural analysis of SARS-CoV-2 specific evasion of host immunity. Genes Immun. 2020;21:409-419. doi: 10.1038/s41435-020-00120-6
7. Rando HM, Wellhausen N, Ghosh S, et al; COVID-19 Review Consortium. Identification and development of therapeutics for COVID-19. mSystems. 2021;6:e0023321. doi: 10.1128/mSystems.00233-21
8. Pardi N, Hogan MJ, Porter FW, et al. mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261-279. doi: 10.1038/nrd.2017.243
9. National Center for Immunization and Respiratory Diseases. Use of COVID-19 vaccines in the United States: interim clinical considerations. Centers for Disease Control and Prevention. Updated August 22, 2022. Accessed August 27, 2022. www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html#references
10. Polack FP, Thomas SJ, Kitchin N, et al; . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603-2615. doi: 10.1056/NEJMoa2034577
11. Heinz FX, Stiasny K. Distinguishing features of current COVID-19 vaccines: knowns and unknowns of antigen presentation and modes of action. NPJ Vaccines. 2021;6:104. doi: 10.1038/s41541-021-00369-6
12. Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403-416. doi: 10.1056/NEJMoa2035389
13. Keech C, Albert G, Cho I, et al. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383:2320-2332. doi: 10.1056/NEJMoa2026920
14. Heath PT, Galiza EP, Baxter DN, et al; . Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med. 2021;385:1172-1183. doi: 10.1056/NEJMoa2107659
15. Rinott E, Youngster I, Lewis YE. Reduction in COVID-19 patients requiring mechanical ventilation following implementation of a national COVID-19 vaccination program—Israel, December 2020–February 2021. MMWR Morb Mortal Wkly Rep. 2021;70:326-328. doi: 10.15585/mmwr.mm7009e3
16. Tenforde MW, Self WH, Gaglani M, et al; IVY Network. Effectiveness of mRNA vaccination in preventing COVID-19-associated invasive mechanical ventilation and death—United States, March 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:459-465. doi: 10.15585/mmwr.mm7112e1
17. Moline HL, Whitaker M, Deng L, et al. Effectiveness of COVID-19 vaccines in preventing hospitalization among adults aged ≥ 65 years—COVID-NET, 13 States, February–April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1088-1093. doi: 10.15585/mmwr.mm7032e
18. Tenforde MW, Olson SM, Self WH, et al; ; . Effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 among hospitalized adults aged ≥ 65 years—United States, January–March 2021. MMWR Morb Mortal Wkly Rep. 2021;70:674-679. doi: 10.15585/mmwr.mm7018e1
19. Johnson AG, Amin AB, Ali AR, et al. COVID-19 incidence and death rates among unvaccinated and fully vaccinated adults with and without booster doses during periods of Delta and Omicron variant emergence—25 U.S. jurisdictions, April 4–December 25, 2021. MMWR Morb Mortal Wkly Rep. 2022;71:132-138. doi: 10.15585/mmwr.mm7104e2
20. Kim Y-E, Huh K, Park Y-J, et al. Association between vaccination and acute myocardial infarction and ischemic stroke after COVID-19 infection. JAMA. Published online July 22, 2022. doi: 10.1001/jama.2022.12992
21. Centers for Disease Control and Prevention. Pfizer-BioNTech COVID-19 vaccine reactions & adverse events. Updated June 21, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html
22. Centers for Disease Control and Prevention. The Moderna COVID-19 vaccine’s local reactions, systemic reactions, adverse events, and serious adverse events. Updated June 21, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html
23. Centers for Disease Control and Prevention. The Janssen COVID-19 vaccine’s local Reactions, Systemic reactions, adverse events, and serious adverse events. Updated August 12, 2021. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/janssen/reactogenicity.html
24. Centers for Disease Control and Prevention. Novavax COVID-19 vaccine local reactions, systemic reactions, adverse events, and serious adverse events. Updated August 31, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/novavax/reactogenicity.html
25. Greaney AJ, Loes AN, Gentles LE, et al. Antibodies elicited by mRNA-1273 vaccination bind more broadly to the receptor binding domain than do those from SARS-CoV-2 infection. Sci Transl Med. 2021;13:eabi9915. doi: 10.1126/scitranslmed.abi9915
26. Hall V, Foulkes S, Insalata F, et al. Protection against SARS-CoV-2 after Covid-19 vaccination and previous infection. N Engl J Med. 2022;386:1207-1220. doi: 10.1056/NEJMoa2118691
27. Klompas M. Understanding breakthrough infections following mRNA SARS-CoV-2 avccination. JAMA. 2021;326:2018-2020. doi: 10.1001/jama.2021.19063
28. Kustin T, Harel N, Finkel U, et al. Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals. Nat Med. 2021;27:1379-1384. doi: 10.1038/s41591-021-01413-7
29. Yu Y, Esposito D, Kang Z, et al. mRNA vaccine-induced antibodies more effective than natural immunity in neutralizing SARS-CoV-2 and its high affinity variants. Sci Rep. 2022;12:2628. doi: 10.1038/s41598-022-06629-2
30. Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:977-982. doi: 10.15585/mmwr.mm7027e2
31. MacNeil JR, Su JR, Broder KR, et al. Updated recommendations from the Advisory Committee on Immunization Practices for use of the Janssen (Johnson & Johnson) COVID-19 vaccine after reports of thrombosis with thrombocytopenia syndrome among vaccine recipients—United States, April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:651-656. doi: 10.15585/mmwr.mm7017e4
32. Patone M, Mei XW, Handunnetthi L, et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2022;28:410-422. doi: 10.1038/s41591-021-01630-0
33. Boehmer TK, Kompaniyets L, Lavery AM, et al. Association between COVID-19 and myocarditis using hospital-based administrative data—United States, March 2020–January 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1228-1232. doi: 10.15585/mmwr.mm7035e5
34. Block JP, Boehmer TK, Forrest CB, et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination—PCORnet, United States, January 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:517-523. doi: 10.15585/mmwr.mm7114e1
35. Rosemblum H. COVID-19 vaccines in adults: benefit–risk discussion. Centers for Disease Control and Prevention. July 22, 2021. Accessed September 21, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-07/05-COVID-Rosenblum-508.pdf
36. Buchan SA, Seo CY, Johnson C, et al. Epidemiology of myocarditis and pericarditis following mRNA vaccines in Ontario, Canada: by vaccine product, schedule and interval. medRxiv. 2021:12.02.21267156.
37. Wong A. The ethics of HEK 293. Natl Cathol Bioeth Q. 2006;6:473-495. doi: 10.5840/ncbq20066331
38. North Dakota Health. COVID-19 vaccines & fetal cell lines. Updated December 1, 2021. Accessed September 21, 2022. www.health.nd.gov/sites/www/files/documents/COVID%20Vaccine%20Page/COVID-19_Vaccine_Fetal_Cell_Handout.pdf
39. Abbasi J. Widespread misinformation about infertility continues to create COVID-19 vaccine hesitancy. JAMA. 2022;327:1013-1015. doi: 10.1001/jama.2022.2404
40. Halasa NB, Olson SM, Staat MA, et al; ; . Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19-associated hospitalization in infants aged < 6 months—17 States, July 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:264-270. doi: 10.15585/mmwr.mm7107e3
41. American College of Obstetricians and Gynecologists. ACOG and SMFM recommend COVID-19 vaccination for pregnant individuals. July 30, 2021. Accessed September 21, 2022. www.acog.org/news/news-releases/2021/07/acog-smfm-recommend-covid-19-vaccination-for-pregnant-individuals#:~:text=%E2%80%9CACOG%20is%20recommending%20vaccination%20of,complications%2C%20and%20because%20it%20isvaccines
42. Brown CM, Vostok J, Johnson H, et al. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings—Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1059-1062. doi: 10.15585/mmwr.mm7031e2
43. Ferdinands JM, Rao S, Dixon BE, et al. Waning 2-dose and 3-dose effectiveness of mRNA against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance—VISION Network, 10 states, August 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:255-263. doi: 10.15585/mmwr.mm7107e2
44. Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Effect of mRNA vaccine boosters against SARS-CoV-2 Omicron infection in Qatar. N Engl J Med. 2022;386:1804-1816. doi: 10.1056/NEJMoa2200797
45. Arbel R, Hammerman A, Sergienko R, et al. BNT162b2 vaccine booster and mortality due to Covid-19. N Engl J Med. 2021;385:2413-2420. doi: 10.1056/NEJMoa2115624
46. Bar-On YM, Goldberg Y, Mandel M, et al. Protection against Covid-19 by BNT162b2 booster across age groups. N Engl J Med. 2021;385:2421-2430. doi: 10.1056/NEJMoa2115926
47. Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385:1393-1400. doi: 10.1056/NEJMoa2114255
48. Mbaeyi S, Oliver SE, Collins JP, et al. The Advisory Committee on Immunization Practices’ interim recommendations for additional primary and booster doses of COVID-19 vaccines—United States, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1545-1552. doi: 10.15585/mmwr.mm7044e2
49. Chen X, Chen Z, Azman AS, et al. Neutralizing antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants induced by natural infection or vaccination: a systematic review and pooled analysis. Clin Infect Dis. 2022;74:734-742. doi: 10.1093/cid/ciab646
50. Atmar RL, Lyke KE, Deming ME, et al; . Homologous and heterologous Covid-19 booster vaccinations. N Engl J Med. 2022;386:1046-1057. doi: 10.1056/NEJMoa2116414
51. Centers for Disease Control and Prevention. Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. Updated September 2, 2022. Accessed September 21, 2022. www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html
52. Ackerman CM, Nguyen JL, Ambati S, et al. Clinical and pregnancy outcomes of coronavirus disease 2019 among hospitalized pregnant women in the United States. Open Forum Infect Dis. 2022;9:ofab429. doi: 10.1093/ofid/ofab429
53. Osterman MJK, Valenzuela CP, Martin JA. Maternal and infant characteristics among women with confirmed or presumed cases of coronavirus disease (COVID-19) during pregnancy. National Center for Health Statistics. National Vital Statistics System. Updated August 11, 2022. Accessed September 21, 2022. www.cdc.gov/nchs/covid19/technical-linkage.htm
54. De Rose DU, Salvatori G, Dotta A, et al. SARS-CoV-2 vaccines during pregnancy and breastfeeding: a systematic review of maternal and neonatal outcomes. Viruses. 2022;14:539. doi: 10.3390/v14030539
55. Martins I, Louwen F, Ayres-de-Campos D, et al. EBCOG position statement on COVID-19 vaccination for pregnant and breastfeeding women. Eur J Obstet Gynecol Reprod Biol. 2021;262:256-258. doi: 10.1016/j.ejogrb.2021.05.021
56. Chou J, Thomas PG, Randolph AG. Immunology of SARS-CoV-2 infection in children. Nat Immunol. 2022;23:177-185. doi: 10.1038/s41590-021-01123-9
57. Parcha V, Booker KS, Kalra R, et al. A retrospective cohort study of 12,306 pediatric COVID-19 patients in the United States. Sci Rep. 2021;11:10231. doi: 10.1038/s41598-021-89553-1
58. Marks KJ, Whitaker M, Anglin O, et al; . Hospitalizations of children and adolescents with laboratory-confirmed COVID-19—COVID-NET, 14 states, July 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:271-278. doi: 10.15585/mmwr.mm7107e4
59. Price AM, Olson SM, Newhams MM, et al; . BNT162b2 protection against the Omicron variant in children and adolescents. N Engl J Med. 2022;386:1899-1909. doi: 10.1056/NEJMoa2202826
60. Maldonado YA, O’Leary ST, Banerjee R, et al; Committee on Infectious Diseases, American Academy of Pediatrics. COVID-19 vaccines in children and adolescents. Pediatrics. 2021;148:e2021052336. doi: 10.1542/peds.2021-052336
61. Lontok K. How effective are COVID-19 vaccines in immunocompromised people? American Society for Microbiology. August 12, 2021. Accessed September 21, 2022. https://asm.org/Articles/2021/August/How-Effective-Are-COVID-19-Vaccines-in-Immunocompr
62. Meiring S, Tempia S, Bhiman JN, et al; . Prolonged shedding of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) at high viral loads among hospitalized immunocompromised persons living with human immunodeficiency virus, South Africa. Clin Infect Dis. 2022;75:e144-e156. doi: 10.1093/cid/ciac077
63. Bar-On YM, Goldberg Y, Mandel M, et al. Protection by 4th dose of BNT162b2 against Omicron in Israel. medRxiv. 2022: 02.01.22270232. doi: 10.1101/2022.02.01.22270232
64. Monto AS, DeJonge PM, Callear AP, et al. Coronavirus occurrence and transmission over 8 years in the HIVE cohort of households in Michigan. J Infect Dis. 2020;222:9-16. doi: 10.1093/infdis/jiaa161
65. Petrie JG, Bazzi LA, McDermott AB, et al. Coronavirus occurrence in the Household Influenza Vaccine Evaluation (HIVE) cohort of Michigan households: reinfection frequency and serologic responses to seasonal and severe acute respiratory syndrome coronaviruses. J Infect Dis. 2021;224:49-59. doi: 10.1093/infdis/jiab161
66. Kirby AE, Walters MS, Jennings WC, et al. Using wastewater surveillance data to support the COVID-19 response—United States, 2020–2021. MMWR Morb Mortal Wkly Rep. 2021;70:1242-1244. doi: 10.15585/mmwr.mm7036a2
PRACTICE RECOMMENDATIONS
› Vaccinate all adults (≥ 18 years) against COVID-19, based on recommendations for the initial series and boosters. A
› Vaccinate patients against COVID-19 with evidence-based assurance that doing so reduces disease-related risk of hospitalization, myocardial infarction, stroke, need for mechanical ventilation, and death. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Mental Health Outcomes Among Transgender Veterans and Active-Duty Service Members in the United States: A Systematic Review
According to the United States Transgender Survey, 39% of respondents reported experiencing serious psychological distress (based on the Kessler 6 Psychological Distress Scale) in the past 30 days compared with 5% in the general population.1 Additionally, 40% of respondents attempted suicide in their lifetime, compared with 5% in the general population.1 Almost half of respondents reported being sexually assaulted at some time in their life, and 10% reported being sexually assaulted in the past year.1
Studies have also shown that veterans and active-duty service members experience worse mental health outcomes and are at increased risk for suicide than civilians and nonveterans.2-5 About 1 in 4 active-duty service members meet the criteria for diagnosis of a mental illness.4 Service members were found to have higher rates of probable anxiety and posttraumatic stress disorder (PTSD) compared with the general population.2,6 In 2018, veteran suicide deaths accounted for about 13% of all deaths by suicide in the US even though veterans only accounted for about 7% of the adult population in that year.5,7 Also in 2018, about 17 veterans committed suicide per day.5 According to the Health Related Behaviors Survey of active-duty service members, about 18% reported thinking about attempting suicide some time in their lives compared with 4% of the general population.2,3 Additionally, 5% of service members reported previous suicide attempts compared with 0.5% in the general population.2,3 It is clear that transgender individuals, veterans, and service members have certain mental health outcomes that are worse than that of the general population.1-7
Transgender individuals along with LGB (lesbian, gay, bisexual) individuals have long faced discrimination and unfair treatment in the military.8-11 In the 1920s, the first written policies were established that banned gay men from serving in the military.9 The US Department of Defense (DoD) continued these policies until in 1993, the “Don’t Ask Don’t Tell” policy was established, which had the façade of being more inclusive for LGB individuals but forced LGB service members to hide their sexual identity and continued the anti-LGBTQ messages that previous policies had created.8,10,11 In 2010, “Don’t Ask Don’t Tell” was repealed, which allowed LGB individuals to serve in the military without concealing their sexual orientation and without fear of discharge based on their sexual identity.11 This repeal did not allow transgender individuals to serve their country as the DoD categorized transgender identity as a medical and mental health disorder.8,11
In 2016, the ban on transgender individuals serving in the military was lifted, and service members could no longer be discharged or turned away from joining the military based on gender identity.8,12 However, in 2018, this order was reversed. The new policy stated that new service members must meet requirements and standards of their sex assigned at birth, and individuals with a history of gender dysphoria or those who have received gender-affirming medical or surgical treatment were prohibited to serve in the military.8,13 This policy did not apply to service members who joined before it took effect. Finally, in April 2021, the current policy took effect, permitting transgender individuals to openly serve in the military. The current policy states that service members cannot be discharged or denied reenlistment based on their gender identity and provides support to receive gender-affirming medical care.14 Although transgender individuals are now accepted in military service, there is still much progress needed to promote equity among transgender service members.
In 2015, according to the Health Related Behaviors Survey of active-duty service members, 0.6% of service members identified as transgender, the same percentage as US adults who identify as transgender.2,15 Previous research has shown that the prevalence of gender identity disorder among veterans is higher than that among the general US population.16 Many studies have shown that worse mental health outcomes exist among LGBTQ veterans and service members compared with heterosexual, cisgender veterans and service members.17-24 However, fewer studies have focused solely on mental health outcomes among transgender veterans and active-duty service members, and there exists no current literature review on this topic. In this article, we present data from the existing literature on mental health outcomes in transgender veterans and active-duty service members. We hypothesize, based on the current literature, that transgender veterans and service members have worse mental health outcomes than their cisgender counterparts. Key terms used in this paper are defined in the Key Definitions.25-27
Methods
We conducted a systematic review of articles presenting data on mental health outcomes in transgender veterans and active-duty service members. The National Library of Medicine PubMed database was searched using the following search terms in various combinations: mental health outcomes, transgender, veterans, military, active duty, substance use, and sexual trauma. The literature search was performed in August 2021 and included articles published through July 31, 2021. Methodology, size, demographics, measures, and main findings were extracted from each article. All studies were eligible for inclusion regardless of sample size. Studies that examined the LGBTQ population without separating transgender individuals were excluded. Studies that examined mental health outcomes including, but not limited to, PTSD, depression, suicidality, anxiety, and substance use disorders (SUDs) in addition to sexual trauma were included. Studies that only examined physical health outcomes were excluded. Qualitative studies, case reports, and papers that did not present original data were excluded (Figure).
Results
Our search resulted in 86 publications. After excluding 65 articles that did not meet the inclusion criteria, 19 studies were included in this review. The Appendix shows the summary of findings from each study, including the study size and results. All studies were conducted in the United States. Most papers used a cross-sectional study design. Most of the studies focused on transgender veterans, but some included data on transgender active-duty service members.
We separated the findings into the following categories based on the variables measured: mental health, including depression, anxiety, PTSD, and serious mental illness; suicidality and self-harm; substance use; and military sexual trauma (MST). Many studies overlapped multiple categories.
Mental Health
Most of the studies included reported that transgender veterans have statistically significant worse mental health outcomes compared with cisgender veterans.28-30 In addition, transgender active-duty service members were found to have worse mental health outcomes than cisgender active-duty service members.31 MST and discrimination were associated with worse mental health outcomes among transgender veterans.32,33 One study showed a different result than others and found that transgender older adults with prior military service had higher psychological health-related quality of life and lower depressive symptoms than those without prior military service (P = .02 and .04, respectively).34 Another study compared transgender veterans with active-duty service members and found that transgender veterans reported higher rates of depression (64.6% vs 30.9%; χ2 = 11.68; P = .001) and anxiety (41.3% vs 18.2%; χ2 = 6.54; P = .01) compared with transgender service members.35
Suicidality and Self-harm
Eleven of the 19 studies included measured suicidality and/or self-harm as an outcome. Transgender veterans and active-duty service members were found to have higher odds of suicidality than their cisgender counterparts.16,28,29,31 In addition, transgender veterans may die by suicide at a younger age than cisgender veterans.36 Stigma and gender-related discrimination were found to be associated with suicidal ideation.33,37-39 Transgender veterans were less likely than transgender nonveterans to report nonsuicidal self-injury (NSSI).40
Substance Use
Two studies focused on substance use, while 5 other studies included substance use in their measures. One of these 2 studies that focused only on substance use outcomes found that transgender veterans were more likely than cisgender veterans to have any SUD (7.2% vs 3.9%; P < .001), in addition to specifically cannabis (3.4% vs 1.5%; P < .001), amphetamine (1.1% vs 0.3%; P < .001), and cocaine use disorders (1.5% vs 1.1%; P < .001).41
Another study reported that transgender veterans had lower odds of self-reported alcohol use but had greater odds of having alcohol-related diagnoses compared with cisgender veterans.42 Of the other studies, it was found that a higher percentage of transgender veterans were diagnosed with an SUD compared with transgender active-duty service members, and transgender veterans were more likely than cisgender veterans to be diagnosed with alcohol use disorder.29,31 Additionally, rural transgender veterans had increased odds of tobacco use disorder compared with transgender veterans who lived in urban areas.43
Military Sexual Trauma
Five of the studies included examined MST, defined as sexual assault or sexual harassment that is experienced during military service.44 Studies found that 15% to 17% of transgender veterans experienced MST.32,45 Transgender veterans were more likely to report MST than cisgender veterans.28,29 MST was found to be consistently associated with depression and PTSD.32,45 A high percentage (83.9%) of transgender active-duty service members reported experiencing sexual harassment and almost one-third experienced sexual assault.46
Discussion
Outcomes examined in this review included MST, substance use, suicidality, and symptoms of depression, anxiety, and PTSD among transgender active-duty service members and veterans. To our knowledge, no other review on this topic exists. There is a review of the health and well-being of LGBTQ veterans and service members, but a majority of the included studies did not separate transgender individuals from LGB persons.17 This review of transgender individuals showed similar results to the review of LGBTQ individuals.17 This review also presented similar results to previous studies that indicated that transgender individuals in the general population have worse mental health outcomes compared with their cisgender counterparts, in addition to studies that showed that veterans and active-duty service members have worse mental health outcomes compared with civilians and nonveterans.1-5 The population of focus in this review faced a unique set of challenges, being that they belonged to both of these subsets of the population, both of which experienced worse mental health outcomes, according to the literature.
Studies included in our review found that transgender veterans and service members have worse mental health outcomes than cisgender veterans and service members.28-31 This outcome was predicted based on previous data collection among transgender individuals, veterans, and active-duty service members. One of the studies included found different results and concluded that prior military service was a protective factor against poorer mental health outcomes.34 This could be, in part, due to veterans’ access to care through the US Department of Veterans Affairs (VA) system. It has been found that transgender veterans use VA services at higher rates than the general population of veterans and that barriers to care were found more for medical treatment than for mental health treatment.47 One study found that almost 70% of transgender veterans who used VA services were satisfied with their mental health care.48 In contrast, another study included in our review found that transgender veterans had worse mental health outcomes than transgender service members, possibly showing that even with access to care, the burden of stigma and discrimination worsens mental health over time.31 Although it has been shown that transgender veterans may feel comfortable disclosing their gender identity to their health care professional, many barriers to care have been identified, such as insensitivity and lack of knowledge about transgender care among clinicians.49-51 With this information, it would be useful to ensure proper training for health care professionals on providing gender-affirming care.
Most of the studies also found that transgender veterans and service members had greater odds of suicidal thoughts and events than cisgender veterans and service members.16,28,29,35 On the contrary, transgender veterans were less likely than transgender nonveterans to report NSSI, which could be for various reasons.40 Transgender veterans may report less NSSI but experience it at similar rates, or veteran status may be a protective factor for NSSI.
Very few studies included SUDs in their measurements, but it was found that transgender veterans were more likely than cisgender veterans to have any drug and alcohol use disorder.29,41 In addition, transgender veterans were more likely than transgender service members to be diagnosed with an SUD, again showing that over time and after time of service, mental health may worsen due to the burden of stigma and discrimination.31 Studies that examined MST found that transgender veterans were more likely than cisgender veterans to report MST, which replicates previous data that found high rates of sexual assault experienced among transgender individuals.1,28,29
There is a lack of literature surrounding transgender veterans and active-duty service members, especially with regard to gender-affirming care provided to these populations. To the best of our knowledge, there exists only one original study that examines the effect of gender-affirming hormone therapy and surgery on mental health outcomes among transgender veterans.52 Further research in this area is needed, specifically longitudinal studies examining the effects of gender-affirming medical care on various outcomes, including mental health. Few longitudinal studies exist that examine the mental health effects of gender-affirming hormone therapy on transgender individuals in the general population.53-60 Most of these studies have shown a significant improvement in parameters of depression and anxiety following hormonal treatment, although long-term large follow-up studies to understand whether these improvements persist over time are missing also in the general population. However, as previously described, transgender veterans and service members are a unique subset of the transgender population and require separate data collection. Hence, further research is required to provide optimal care for this population. In addition, early screening for symptoms of mental illness, substance use, and MST is important to providing optimal care.
Limitations
This review was limited due to the lack of data collected from transgender veterans and service members. The studies included did not allow for standardized comparisons and did not use identical measures. Some papers compared transgender veterans with transgender nonveterans, some transgender veterans and/or service members with cisgender veterans and/or service members, and some transgender veterans with transgender service members. There were some consistent results found across the studies, but some studies showed contradictory results or no significant differences within a certain category. It is difficult to compare such different study designs and various participant populations. Additional research is required to verify and replicate these results.
Conclusions
Although this review was limited due to the lack of consistent study designs in the literature examining the mental health of transgender veterans and active-duty service members, overall results showed that transgender veterans and service members experience worse mental health outcomes than their cisgender counterparts. With this knowledge and exploring the history of discrimination that this population has faced, improved systems must be put into place to better serve this population and improve health outcomes. Additional research is required to examine the effects of gender-affirming care on mental health among transgender veterans and service members.
1. James SE, Herman JL, Rankin S, Keisling M, Mottet L, Anafi M. The Report of the 2015 U.S. Transgender Survey. National Center for Transgender Equality. December 2016. Accessed August 22, 2022. https://www.ustranssurvey.org
2. Meadows SO, Engel CC, Collins RL, et al. 2015 Department of Defense Health Related Behaviors Survey (HRBS). Rand Health Q. 2018;8(2):434.
3. Lipari R, Piscopo K, Kroutil LA, Miller GK. Suicidal thoughts and behavior among adults: results from the 2014 National Survey on Drug Use and Health. NSDUH Data Review. 2015:1-14. https://www.samhsa.gov/data/sites/default/files/NSDUH-FRR2-2014/NSDUH-FRR2-2014.pdf
4. Kessler RC, Heeringa SG, Stein MB, et al. Thirty-day prevalence of DSM-IV mental disorders among nondeployed soldiers in the US Army: results from the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS). JAMA Psychiatry. 2014;71(5):504-513. doi:10.1001/jamapsychiatry.2014.28
5. U.S. Department of Veterans Affairs Office of Mental Health and Suicide Prevention. 2020 National Veteran Suicide Prevention Annual Report. November 2020. Accessed August 22, 2022. https://www.mentalhealth.va.gov/docs/data-sheets/2020/2020-National-Veteran-Suicide-Prevention-Annual-Report-11-2020-508.pdf
6. Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602. doi:10.1001/archpsyc.62.6.593
7. Vespa J. Those who SERVED: America’s veterans from World War II to the war on terror. The United States Census Bureau. June 2, 2020. Accessed August 22, 2022. https://www.census.gov/library/publications/2020/demo/acs-43.html
8. Seibert DC, Keller N, Zapor L, Archer H. Military transgender care. J Am Assoc Nurse Pract. 2020;32(11):764-770. doi:10.1097/JXX.0000000000000519
9. Rigby WC. Military penal law: A brief survey of the 1920 revision of the Articles of War. J Crim Law Criminol. 1921;12(1):84.
10. Department of Defense Directive Number 1332.14: Enlisted Administrative Separations. December 21, 1993. Accessed August 22, 2022. https://biotech.law.lsu.edu/blaw/dodd/corres/pdf/d133214wch1_122193/d133214p.pdf
11. Aford B, Lee SJ. Toward complete inclusion: lesbian, gay, bisexual, and transgender military service members after repeal of Don’t Ask, Don’t Tell. Soc Work. 2016;61(3):257-265. doi:10.1093/sw/sww033
12. Department of Defense Instruction 1300.28: In-Service Transition for Transgender Service Members. June 30, 2016. Accessed August 22, 2022. https://dod.defense.gov/Portals/1/features/2016/0616_policy/DoD-Instruction-1300.28.pdf
13. Department of Defense. Directive-type Memorandum (DTM)-19-004 - Military Service by Transgender Persons and Persons with Gender Dysphoria. March 12. 2019. Accessed August 22, 2022. https://health.mil/Reference-Center/Policies/2020/03/17/Military-Service-by-Transgender-Persons-and-Persons-with-Gender-Dysphoria
14. US Department of Defense Instruction 1300.28: In-Service Transition for Transgender Service Members. April 30, 2021. Accessed August 22, 2022. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/130028p.pdf
15. Flores AR, Herman JL, Gates GJ, Brown TNT. How many adults identify as transgender in the United States? The Williams Institute; 2016. Accessed August 22, 2022. https://williamsinstitute.law.ucla.edu/publications/trans-adults-united-states/
16. Blosnich JR, Brown GR, Shipherd Phd JC, Kauth M, Piegari RI, Bossarte RM. Prevalence of gender identity disorder and suicide risk among transgender veterans utilizing veterans health administration care. Am J Public Health. 2013;103(10):e27-e32. doi:10.2105/AJPH.2013.301507
17. Mark KM, McNamara KA, Gribble R, et al. The health and well-being of LGBTQ serving and ex-serving personnel: a narrative review. Int Rev Psychiatry. 2019;31(1):75-94. doi:10.1080/09540261.2019.1575190
18. Blosnich J, Foynes MM, Shipherd JC. Health disparities among sexual minority women veterans. J Womens Health (Larchmt). 2013;22(7):631-636. doi:10.1089/jwh.2012.4214
19. Blosnich JR, Bossarte RM, Silenzio VM. Suicidal ideation among sexual minority veterans: results from the 2005-2010 Massachusetts Behavioral Risk Factor Surveillance Survey. Am J Public Health. 2012;102(suppl 1):S44-S47. doi:10.2105/AJPH.2011.300565
20. Blosnich JR, Gordon AJ, Fine MJ. Associations of sexual and gender minority status with health indicators, health risk factors, and social stressors in a national sample of young adults with military experience. Ann Epidemiol. 2015;25(9):661-667. doi:10.1016/j.annepidem.2015.06.001
21. Cochran BN, Balsam K, Flentje A, Malte CA, Simpson T. Mental health characteristics of sexual minority veterans. J Homosex. 2013;60(2-3):419-435. doi:10.1080/00918369.2013.744932
22. Lehavot K, Browne KC, Simpson TL. Examining sexual orientation disparities in alcohol misuse among women veterans. Am J Prev Med. 2014;47(5):554-562. doi:10.1016/j.amepre.2014.07.002
23. Scott RL, Lasiuk GC, Norris CM. Depression in lesbian, gay, and bisexual members of the Canadian Armed Forces. LGBT Health. 2016;3(5):366-372. doi:10.1089/lgbt.2016.0050
24. Wang J, Dey M, Soldati L, Weiss MG, Gmel G, Mohler-Kuo M. Psychiatric disorders, suicidality, and personality among young men by sexual orientation. Eur Psychiatry. 2014;29(8):514-522. doi:10.1016/j.eurpsy.2014.05.001
25. American Psychological Association. Gender. APA Style. September 2019. Updated July 2022. Accessed August 22, 2022. https://apastyle.apa.org/style-grammar-guidelines/bias-free-language/gender
26. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed., American Psychiatric Association; 2013.
27. Deutsch MB. Overview of gender-affirming treatments and procedures. UCSF Transgender Care. June 17, 2016. Accessed August 22, 2022. https://transcare.ucsf.edu/guidelines/overview
28. Brown GR, Jones KT. Health correlates of criminal justice involvement in 4,793 transgender veterans. LGBT Health. 2015;2(4):297-305. doi:10.1089/lgbt.2015.0052
29. Brown GR, Jones KT. Mental health and medical health disparities in 5135 transgender veterans receiving healthcare in the Veterans Health Administration: a case-control study. LGBT Health. 2016;3(2):122-131. doi:10.1089/lgbt.2015.0058
30. Downing J, Conron K, Herman JL, Blosnich JR. Transgender and cisgender US veterans have few health differences. Health Aff (Millwood). 2018;37(7):1160-1168. doi:10.1377/hlthaff.2018.0027
31. Holloway IW, Green D, Pickering C, et al. Mental health and health risk behaviors of active duty sexual minority and transgender service members in the United States military. LGBT Health. 2021;8(2):152-161. doi:10.1089/lgbt.2020.0031
32. Beckman K, Shipherd J, Simpson T, Lehavot K. Military sexual assault in transgender veterans: results from a nationwide survey. J Trauma Stress. 2018;31(2):181-190. doi:10.1002/jts.22280
33. Blosnich JR, Marsiglio MC, Gao S, Gordon AJ, Shipherd JC, Kauth M, Brown GR, Fine MJ. Mental health of transgender veterans in US states with and without discrimination and hate crime legal protection. Am J Public Health. 2016;106(3):534-540. doi:10.2105/AJPH.2015.302981
34. Hoy-Ellis CP, Shiu C, Sullivan KM, Kim HJ, Sturges AM, Fredriksen-Goldsen KI. Prior military service, identity stigma, and mental health among transgender older adults. Gerontologist. 2017;57(suppl 1):S63-S71. doi:10.1093/geront/gnw173
35. Hill BJ, Bouris A, Barnett JT, Walker D. Fit to serve? Exploring mental and physical health and well-being among transgender active-duty service members and veterans in the U.S. military. Transgend Health. 2016;1(1):4-11. Published 2016 Jan 1. doi:10.1089/trgh.2015.0002
36. Blosnich JR, Brown GR, Wojcio S, Jones KT, Bossarte RM. Mortality among veterans with transgender-related diagnoses in the Veterans Health Administration, FY2000-2009. LGBT Health. 2014;1(4):269-276. doi:10.1089/lgbt.2014.0050
37. Carter SP, Allred KM, Tucker RP, Simpson TL, Shipherd JC, Lehavot K. Discrimination and suicidal ideation among transgender veterans: the role of social support and connection. LGBT Health. 2019;6(2):43-50. doi:10.1089/lgbt.2018.0239
38. Lehavot K, Simpson TL, Shipherd JC. Factors associated with suicidality among a national sample of transgender veterans. Suicide Life Threat Behav. 2016;46(5):507-524. doi:10.1111/sltb.12233
39. Tucker RP, Testa RJ, Reger MA, Simpson TL, Shipherd JC, Lehavot K. Current and military-specific gender minority stress factors and their relationship with suicide ideation in transgender veterans. Suicide Life Threat Behav. 2019;49(1):155-166. doi:10.1111/sltb.12432
40. Aboussouan A, Snow A, Cerel J, Tucker RP. Non-suicidal self-injury, suicide ideation, and past suicide attempts: Comparison between transgender and gender diverse veterans and non-veterans. J Affect Disord. 2019;259:186-194. doi:10.1016/j.jad.2019.08.046
41. Frost MC, Blosnich JR, Lehavot K, Chen JA, Rubinsky AD, Glass JE, Williams EC. Disparities in documented drug use disorders between transgender and cisgender U.S. Veterans Health Administration patients. J Addict Med. 2021;15(4):334-340. doi:10.1097/ADM.0000000000000769
42. Williams EC, Frost MC, Rubinsky AD, et al. Patterns of alcohol use among transgender patients receiving care at the Veterans Health Administration: overall and relative to nontransgender patients. J Stud Alcohol Drugs. 2021;82(1):132-141. doi:10.15288/jsad.2021.82.132
43. Bukowski LA, Blosnich J, Shipherd JC, Kauth MR, Brown GR, Gordon AJ. Exploring rural disparities in medical diagnoses among veterans with transgender-related diagnoses utilizing Veterans Health Administration care. Med Care. 2017;55(suppl 9):S97-S103. doi:10.1097/MLR.0000000000000745
44. U.S. Department of Veterans Affairs. Military Sexual Trauma. Updated August 1, 2022. Accessed August 22, 2022. https://www.mentalhealth.va.gov/mentalhealth/msthome/index.asp
45. Lindsay JA, Keo-Meier C, Hudson S, Walder A, Martin LA, Kauth MR. Mental health of transgender veterans of the Iraq and Afghanistan conflicts who experienced military sexual trauma. J Trauma Stress. 2016;29(6):563-567. doi:10.1002/jts.22146
46. Schuyler AC, Klemmer C, Mamey MR, et al. Experiences of sexual harassment, stalking, and sexual assault during military service among LGBT and Non-LGBT service members. J Trauma Stress. 2020;33(3):257-266. doi:10.1002/jts.22506
47. Shipherd JC, Mizock L, Maguen S, Green KE. Male-to-female transgender veterans and VA health care utilization. Int J Sexual Health. 2012;24(1):78-87. doi:10.1080/19317611.2011.639440
48. Lehavot K, Katon JG, Simpson TL, Shipherd JC. Transgender veterans’ satisfaction with care and unmet health needs. Med Care. 2017;55(suppl 9):S90-S96. doi:10.1097/MLR.0000000000000723
49. Kauth MR, Barrera TL, Latini DM. Lesbian, gay, and transgender veterans’ experiences in the Veterans Health Administration: positive signs and room for improvement. Psychol Serv. 2019;16(2):346-351. doi:10.1037/ser0000232

50. Rosentel K, Hill BJ, Lu C, Barnett JT. Transgender veterans and the Veterans Health Administration: exploring the experiences of transgender veterans in the Veterans Affairs Healthcare System. Transgend Health. 2016;1(1):108-116. Published 2016 Jun 1. doi:10.1089/trgh.2016.0006
51. Dietert M, Dentice D, Keig Z. Addressing the needs of transgender military veterans: better access and more comprehensive care. Transgend Health. 2017;2(1):35-44. Published 2017 Mar 1. doi:10.1089/trgh.2016.0040
52. Tucker RP, Testa RJ, Simpson TL, Shipherd JC, Blosnich JR, Lehavot K. Hormone therapy, gender affirmation surgery, and their association with recent suicidal ideation and depression symptoms in transgender veterans. Psychol Med. 2018;48(14):2329-2336. doi:10.1017/S0033291717003853
53. Colizzi M, Costa R, Todarello O. Transsexual patients’ psychiatric comorbidity and positive effect of cross-sex hormonal treatment on mental health: results from a longitudinal study. Psychoneuroendocrinology. 2014;39:65-73. doi:10.1016/j.psyneuen.2013.09.029
54. Heylens G, Verroken C, De Cock S, T’Sjoen G, De Cuypere G. Effects of different steps in gender reassignment therapy on psychopathology: a prospective study of persons with a gender identity disorder. J Sex Med. 2014;11(1):119-126. doi:10.1111/jsm.12363
55. Fisher AD, Castellini G, Ristori J, et al. Cross-sex hormone treatment and psychobiological changes in transsexual persons: two-year follow-up data. J Clin Endocrinol Metab. 2016;101(11):4260-4269. doi:10.1210/jc.2016-1276
56. Aldridge Z, Patel S, Guo B, et al. Long-term effect of gender-affirming hormone treatment on depression and anxiety symptoms in transgender people: a prospective cohort study. Andrology. 2021;9(6):1808-1816. doi:10.1111/andr.12884
57. Costantino A, Cerpolini S, Alvisi S, Morselli PG, Venturoli S, Meriggiola MC. A prospective study on sexual function and mood in female-to-male transsexuals during testosterone administration and after sex reassignment surgery. J Sex Marital Ther. 2013;39(4):321-335. doi:10.1080/0092623X.2012.736920
58. Keo-Meier CL, Herman LI, Reisner SL, Pardo ST, Sharp C, Babcock JC. Testosterone treatment and MMPI-2 improvement in transgender men: a prospective controlled study. J Consult Clin Psychol. 2015;83(1):143-156. doi:10.1037/a0037599
59. Turan S‚ , Aksoy Poyraz C, Usta Sag˘lam NG, et al. Alterations in body uneasiness, eating attitudes, and psychopathology before and after cross-sex hormonal treatment in patients with female-to-male gender dysphoria. Arch Sex Behav. 2018;47(8):2349-2361. doi:10.1007/s10508-018-1189-4
60. Oda H, Kinoshita T. Efficacy of hormonal and mental treatments with MMPI in FtM individuals: cross-sectional and longitudinal studies. BMC Psychiatry. 2017;17(1):256. Published 2017 Jul 17. doi:10.1186/s12888-017-1423-y
According to the United States Transgender Survey, 39% of respondents reported experiencing serious psychological distress (based on the Kessler 6 Psychological Distress Scale) in the past 30 days compared with 5% in the general population.1 Additionally, 40% of respondents attempted suicide in their lifetime, compared with 5% in the general population.1 Almost half of respondents reported being sexually assaulted at some time in their life, and 10% reported being sexually assaulted in the past year.1
Studies have also shown that veterans and active-duty service members experience worse mental health outcomes and are at increased risk for suicide than civilians and nonveterans.2-5 About 1 in 4 active-duty service members meet the criteria for diagnosis of a mental illness.4 Service members were found to have higher rates of probable anxiety and posttraumatic stress disorder (PTSD) compared with the general population.2,6 In 2018, veteran suicide deaths accounted for about 13% of all deaths by suicide in the US even though veterans only accounted for about 7% of the adult population in that year.5,7 Also in 2018, about 17 veterans committed suicide per day.5 According to the Health Related Behaviors Survey of active-duty service members, about 18% reported thinking about attempting suicide some time in their lives compared with 4% of the general population.2,3 Additionally, 5% of service members reported previous suicide attempts compared with 0.5% in the general population.2,3 It is clear that transgender individuals, veterans, and service members have certain mental health outcomes that are worse than that of the general population.1-7
Transgender individuals along with LGB (lesbian, gay, bisexual) individuals have long faced discrimination and unfair treatment in the military.8-11 In the 1920s, the first written policies were established that banned gay men from serving in the military.9 The US Department of Defense (DoD) continued these policies until in 1993, the “Don’t Ask Don’t Tell” policy was established, which had the façade of being more inclusive for LGB individuals but forced LGB service members to hide their sexual identity and continued the anti-LGBTQ messages that previous policies had created.8,10,11 In 2010, “Don’t Ask Don’t Tell” was repealed, which allowed LGB individuals to serve in the military without concealing their sexual orientation and without fear of discharge based on their sexual identity.11 This repeal did not allow transgender individuals to serve their country as the DoD categorized transgender identity as a medical and mental health disorder.8,11
In 2016, the ban on transgender individuals serving in the military was lifted, and service members could no longer be discharged or turned away from joining the military based on gender identity.8,12 However, in 2018, this order was reversed. The new policy stated that new service members must meet requirements and standards of their sex assigned at birth, and individuals with a history of gender dysphoria or those who have received gender-affirming medical or surgical treatment were prohibited to serve in the military.8,13 This policy did not apply to service members who joined before it took effect. Finally, in April 2021, the current policy took effect, permitting transgender individuals to openly serve in the military. The current policy states that service members cannot be discharged or denied reenlistment based on their gender identity and provides support to receive gender-affirming medical care.14 Although transgender individuals are now accepted in military service, there is still much progress needed to promote equity among transgender service members.
In 2015, according to the Health Related Behaviors Survey of active-duty service members, 0.6% of service members identified as transgender, the same percentage as US adults who identify as transgender.2,15 Previous research has shown that the prevalence of gender identity disorder among veterans is higher than that among the general US population.16 Many studies have shown that worse mental health outcomes exist among LGBTQ veterans and service members compared with heterosexual, cisgender veterans and service members.17-24 However, fewer studies have focused solely on mental health outcomes among transgender veterans and active-duty service members, and there exists no current literature review on this topic. In this article, we present data from the existing literature on mental health outcomes in transgender veterans and active-duty service members. We hypothesize, based on the current literature, that transgender veterans and service members have worse mental health outcomes than their cisgender counterparts. Key terms used in this paper are defined in the Key Definitions.25-27
Methods
We conducted a systematic review of articles presenting data on mental health outcomes in transgender veterans and active-duty service members. The National Library of Medicine PubMed database was searched using the following search terms in various combinations: mental health outcomes, transgender, veterans, military, active duty, substance use, and sexual trauma. The literature search was performed in August 2021 and included articles published through July 31, 2021. Methodology, size, demographics, measures, and main findings were extracted from each article. All studies were eligible for inclusion regardless of sample size. Studies that examined the LGBTQ population without separating transgender individuals were excluded. Studies that examined mental health outcomes including, but not limited to, PTSD, depression, suicidality, anxiety, and substance use disorders (SUDs) in addition to sexual trauma were included. Studies that only examined physical health outcomes were excluded. Qualitative studies, case reports, and papers that did not present original data were excluded (Figure).
Results
Our search resulted in 86 publications. After excluding 65 articles that did not meet the inclusion criteria, 19 studies were included in this review. The Appendix shows the summary of findings from each study, including the study size and results. All studies were conducted in the United States. Most papers used a cross-sectional study design. Most of the studies focused on transgender veterans, but some included data on transgender active-duty service members.
We separated the findings into the following categories based on the variables measured: mental health, including depression, anxiety, PTSD, and serious mental illness; suicidality and self-harm; substance use; and military sexual trauma (MST). Many studies overlapped multiple categories.
Mental Health
Most of the studies included reported that transgender veterans have statistically significant worse mental health outcomes compared with cisgender veterans.28-30 In addition, transgender active-duty service members were found to have worse mental health outcomes than cisgender active-duty service members.31 MST and discrimination were associated with worse mental health outcomes among transgender veterans.32,33 One study showed a different result than others and found that transgender older adults with prior military service had higher psychological health-related quality of life and lower depressive symptoms than those without prior military service (P = .02 and .04, respectively).34 Another study compared transgender veterans with active-duty service members and found that transgender veterans reported higher rates of depression (64.6% vs 30.9%; χ2 = 11.68; P = .001) and anxiety (41.3% vs 18.2%; χ2 = 6.54; P = .01) compared with transgender service members.35
Suicidality and Self-harm
Eleven of the 19 studies included measured suicidality and/or self-harm as an outcome. Transgender veterans and active-duty service members were found to have higher odds of suicidality than their cisgender counterparts.16,28,29,31 In addition, transgender veterans may die by suicide at a younger age than cisgender veterans.36 Stigma and gender-related discrimination were found to be associated with suicidal ideation.33,37-39 Transgender veterans were less likely than transgender nonveterans to report nonsuicidal self-injury (NSSI).40
Substance Use
Two studies focused on substance use, while 5 other studies included substance use in their measures. One of these 2 studies that focused only on substance use outcomes found that transgender veterans were more likely than cisgender veterans to have any SUD (7.2% vs 3.9%; P < .001), in addition to specifically cannabis (3.4% vs 1.5%; P < .001), amphetamine (1.1% vs 0.3%; P < .001), and cocaine use disorders (1.5% vs 1.1%; P < .001).41
Another study reported that transgender veterans had lower odds of self-reported alcohol use but had greater odds of having alcohol-related diagnoses compared with cisgender veterans.42 Of the other studies, it was found that a higher percentage of transgender veterans were diagnosed with an SUD compared with transgender active-duty service members, and transgender veterans were more likely than cisgender veterans to be diagnosed with alcohol use disorder.29,31 Additionally, rural transgender veterans had increased odds of tobacco use disorder compared with transgender veterans who lived in urban areas.43
Military Sexual Trauma
Five of the studies included examined MST, defined as sexual assault or sexual harassment that is experienced during military service.44 Studies found that 15% to 17% of transgender veterans experienced MST.32,45 Transgender veterans were more likely to report MST than cisgender veterans.28,29 MST was found to be consistently associated with depression and PTSD.32,45 A high percentage (83.9%) of transgender active-duty service members reported experiencing sexual harassment and almost one-third experienced sexual assault.46
Discussion
Outcomes examined in this review included MST, substance use, suicidality, and symptoms of depression, anxiety, and PTSD among transgender active-duty service members and veterans. To our knowledge, no other review on this topic exists. There is a review of the health and well-being of LGBTQ veterans and service members, but a majority of the included studies did not separate transgender individuals from LGB persons.17 This review of transgender individuals showed similar results to the review of LGBTQ individuals.17 This review also presented similar results to previous studies that indicated that transgender individuals in the general population have worse mental health outcomes compared with their cisgender counterparts, in addition to studies that showed that veterans and active-duty service members have worse mental health outcomes compared with civilians and nonveterans.1-5 The population of focus in this review faced a unique set of challenges, being that they belonged to both of these subsets of the population, both of which experienced worse mental health outcomes, according to the literature.
Studies included in our review found that transgender veterans and service members have worse mental health outcomes than cisgender veterans and service members.28-31 This outcome was predicted based on previous data collection among transgender individuals, veterans, and active-duty service members. One of the studies included found different results and concluded that prior military service was a protective factor against poorer mental health outcomes.34 This could be, in part, due to veterans’ access to care through the US Department of Veterans Affairs (VA) system. It has been found that transgender veterans use VA services at higher rates than the general population of veterans and that barriers to care were found more for medical treatment than for mental health treatment.47 One study found that almost 70% of transgender veterans who used VA services were satisfied with their mental health care.48 In contrast, another study included in our review found that transgender veterans had worse mental health outcomes than transgender service members, possibly showing that even with access to care, the burden of stigma and discrimination worsens mental health over time.31 Although it has been shown that transgender veterans may feel comfortable disclosing their gender identity to their health care professional, many barriers to care have been identified, such as insensitivity and lack of knowledge about transgender care among clinicians.49-51 With this information, it would be useful to ensure proper training for health care professionals on providing gender-affirming care.
Most of the studies also found that transgender veterans and service members had greater odds of suicidal thoughts and events than cisgender veterans and service members.16,28,29,35 On the contrary, transgender veterans were less likely than transgender nonveterans to report NSSI, which could be for various reasons.40 Transgender veterans may report less NSSI but experience it at similar rates, or veteran status may be a protective factor for NSSI.
Very few studies included SUDs in their measurements, but it was found that transgender veterans were more likely than cisgender veterans to have any drug and alcohol use disorder.29,41 In addition, transgender veterans were more likely than transgender service members to be diagnosed with an SUD, again showing that over time and after time of service, mental health may worsen due to the burden of stigma and discrimination.31 Studies that examined MST found that transgender veterans were more likely than cisgender veterans to report MST, which replicates previous data that found high rates of sexual assault experienced among transgender individuals.1,28,29
There is a lack of literature surrounding transgender veterans and active-duty service members, especially with regard to gender-affirming care provided to these populations. To the best of our knowledge, there exists only one original study that examines the effect of gender-affirming hormone therapy and surgery on mental health outcomes among transgender veterans.52 Further research in this area is needed, specifically longitudinal studies examining the effects of gender-affirming medical care on various outcomes, including mental health. Few longitudinal studies exist that examine the mental health effects of gender-affirming hormone therapy on transgender individuals in the general population.53-60 Most of these studies have shown a significant improvement in parameters of depression and anxiety following hormonal treatment, although long-term large follow-up studies to understand whether these improvements persist over time are missing also in the general population. However, as previously described, transgender veterans and service members are a unique subset of the transgender population and require separate data collection. Hence, further research is required to provide optimal care for this population. In addition, early screening for symptoms of mental illness, substance use, and MST is important to providing optimal care.
Limitations
This review was limited due to the lack of data collected from transgender veterans and service members. The studies included did not allow for standardized comparisons and did not use identical measures. Some papers compared transgender veterans with transgender nonveterans, some transgender veterans and/or service members with cisgender veterans and/or service members, and some transgender veterans with transgender service members. There were some consistent results found across the studies, but some studies showed contradictory results or no significant differences within a certain category. It is difficult to compare such different study designs and various participant populations. Additional research is required to verify and replicate these results.
Conclusions
Although this review was limited due to the lack of consistent study designs in the literature examining the mental health of transgender veterans and active-duty service members, overall results showed that transgender veterans and service members experience worse mental health outcomes than their cisgender counterparts. With this knowledge and exploring the history of discrimination that this population has faced, improved systems must be put into place to better serve this population and improve health outcomes. Additional research is required to examine the effects of gender-affirming care on mental health among transgender veterans and service members.
According to the United States Transgender Survey, 39% of respondents reported experiencing serious psychological distress (based on the Kessler 6 Psychological Distress Scale) in the past 30 days compared with 5% in the general population.1 Additionally, 40% of respondents attempted suicide in their lifetime, compared with 5% in the general population.1 Almost half of respondents reported being sexually assaulted at some time in their life, and 10% reported being sexually assaulted in the past year.1
Studies have also shown that veterans and active-duty service members experience worse mental health outcomes and are at increased risk for suicide than civilians and nonveterans.2-5 About 1 in 4 active-duty service members meet the criteria for diagnosis of a mental illness.4 Service members were found to have higher rates of probable anxiety and posttraumatic stress disorder (PTSD) compared with the general population.2,6 In 2018, veteran suicide deaths accounted for about 13% of all deaths by suicide in the US even though veterans only accounted for about 7% of the adult population in that year.5,7 Also in 2018, about 17 veterans committed suicide per day.5 According to the Health Related Behaviors Survey of active-duty service members, about 18% reported thinking about attempting suicide some time in their lives compared with 4% of the general population.2,3 Additionally, 5% of service members reported previous suicide attempts compared with 0.5% in the general population.2,3 It is clear that transgender individuals, veterans, and service members have certain mental health outcomes that are worse than that of the general population.1-7
Transgender individuals along with LGB (lesbian, gay, bisexual) individuals have long faced discrimination and unfair treatment in the military.8-11 In the 1920s, the first written policies were established that banned gay men from serving in the military.9 The US Department of Defense (DoD) continued these policies until in 1993, the “Don’t Ask Don’t Tell” policy was established, which had the façade of being more inclusive for LGB individuals but forced LGB service members to hide their sexual identity and continued the anti-LGBTQ messages that previous policies had created.8,10,11 In 2010, “Don’t Ask Don’t Tell” was repealed, which allowed LGB individuals to serve in the military without concealing their sexual orientation and without fear of discharge based on their sexual identity.11 This repeal did not allow transgender individuals to serve their country as the DoD categorized transgender identity as a medical and mental health disorder.8,11
In 2016, the ban on transgender individuals serving in the military was lifted, and service members could no longer be discharged or turned away from joining the military based on gender identity.8,12 However, in 2018, this order was reversed. The new policy stated that new service members must meet requirements and standards of their sex assigned at birth, and individuals with a history of gender dysphoria or those who have received gender-affirming medical or surgical treatment were prohibited to serve in the military.8,13 This policy did not apply to service members who joined before it took effect. Finally, in April 2021, the current policy took effect, permitting transgender individuals to openly serve in the military. The current policy states that service members cannot be discharged or denied reenlistment based on their gender identity and provides support to receive gender-affirming medical care.14 Although transgender individuals are now accepted in military service, there is still much progress needed to promote equity among transgender service members.
In 2015, according to the Health Related Behaviors Survey of active-duty service members, 0.6% of service members identified as transgender, the same percentage as US adults who identify as transgender.2,15 Previous research has shown that the prevalence of gender identity disorder among veterans is higher than that among the general US population.16 Many studies have shown that worse mental health outcomes exist among LGBTQ veterans and service members compared with heterosexual, cisgender veterans and service members.17-24 However, fewer studies have focused solely on mental health outcomes among transgender veterans and active-duty service members, and there exists no current literature review on this topic. In this article, we present data from the existing literature on mental health outcomes in transgender veterans and active-duty service members. We hypothesize, based on the current literature, that transgender veterans and service members have worse mental health outcomes than their cisgender counterparts. Key terms used in this paper are defined in the Key Definitions.25-27
Methods
We conducted a systematic review of articles presenting data on mental health outcomes in transgender veterans and active-duty service members. The National Library of Medicine PubMed database was searched using the following search terms in various combinations: mental health outcomes, transgender, veterans, military, active duty, substance use, and sexual trauma. The literature search was performed in August 2021 and included articles published through July 31, 2021. Methodology, size, demographics, measures, and main findings were extracted from each article. All studies were eligible for inclusion regardless of sample size. Studies that examined the LGBTQ population without separating transgender individuals were excluded. Studies that examined mental health outcomes including, but not limited to, PTSD, depression, suicidality, anxiety, and substance use disorders (SUDs) in addition to sexual trauma were included. Studies that only examined physical health outcomes were excluded. Qualitative studies, case reports, and papers that did not present original data were excluded (Figure).
Results
Our search resulted in 86 publications. After excluding 65 articles that did not meet the inclusion criteria, 19 studies were included in this review. The Appendix shows the summary of findings from each study, including the study size and results. All studies were conducted in the United States. Most papers used a cross-sectional study design. Most of the studies focused on transgender veterans, but some included data on transgender active-duty service members.
We separated the findings into the following categories based on the variables measured: mental health, including depression, anxiety, PTSD, and serious mental illness; suicidality and self-harm; substance use; and military sexual trauma (MST). Many studies overlapped multiple categories.
Mental Health
Most of the studies included reported that transgender veterans have statistically significant worse mental health outcomes compared with cisgender veterans.28-30 In addition, transgender active-duty service members were found to have worse mental health outcomes than cisgender active-duty service members.31 MST and discrimination were associated with worse mental health outcomes among transgender veterans.32,33 One study showed a different result than others and found that transgender older adults with prior military service had higher psychological health-related quality of life and lower depressive symptoms than those without prior military service (P = .02 and .04, respectively).34 Another study compared transgender veterans with active-duty service members and found that transgender veterans reported higher rates of depression (64.6% vs 30.9%; χ2 = 11.68; P = .001) and anxiety (41.3% vs 18.2%; χ2 = 6.54; P = .01) compared with transgender service members.35
Suicidality and Self-harm
Eleven of the 19 studies included measured suicidality and/or self-harm as an outcome. Transgender veterans and active-duty service members were found to have higher odds of suicidality than their cisgender counterparts.16,28,29,31 In addition, transgender veterans may die by suicide at a younger age than cisgender veterans.36 Stigma and gender-related discrimination were found to be associated with suicidal ideation.33,37-39 Transgender veterans were less likely than transgender nonveterans to report nonsuicidal self-injury (NSSI).40
Substance Use
Two studies focused on substance use, while 5 other studies included substance use in their measures. One of these 2 studies that focused only on substance use outcomes found that transgender veterans were more likely than cisgender veterans to have any SUD (7.2% vs 3.9%; P < .001), in addition to specifically cannabis (3.4% vs 1.5%; P < .001), amphetamine (1.1% vs 0.3%; P < .001), and cocaine use disorders (1.5% vs 1.1%; P < .001).41
Another study reported that transgender veterans had lower odds of self-reported alcohol use but had greater odds of having alcohol-related diagnoses compared with cisgender veterans.42 Of the other studies, it was found that a higher percentage of transgender veterans were diagnosed with an SUD compared with transgender active-duty service members, and transgender veterans were more likely than cisgender veterans to be diagnosed with alcohol use disorder.29,31 Additionally, rural transgender veterans had increased odds of tobacco use disorder compared with transgender veterans who lived in urban areas.43
Military Sexual Trauma
Five of the studies included examined MST, defined as sexual assault or sexual harassment that is experienced during military service.44 Studies found that 15% to 17% of transgender veterans experienced MST.32,45 Transgender veterans were more likely to report MST than cisgender veterans.28,29 MST was found to be consistently associated with depression and PTSD.32,45 A high percentage (83.9%) of transgender active-duty service members reported experiencing sexual harassment and almost one-third experienced sexual assault.46
Discussion
Outcomes examined in this review included MST, substance use, suicidality, and symptoms of depression, anxiety, and PTSD among transgender active-duty service members and veterans. To our knowledge, no other review on this topic exists. There is a review of the health and well-being of LGBTQ veterans and service members, but a majority of the included studies did not separate transgender individuals from LGB persons.17 This review of transgender individuals showed similar results to the review of LGBTQ individuals.17 This review also presented similar results to previous studies that indicated that transgender individuals in the general population have worse mental health outcomes compared with their cisgender counterparts, in addition to studies that showed that veterans and active-duty service members have worse mental health outcomes compared with civilians and nonveterans.1-5 The population of focus in this review faced a unique set of challenges, being that they belonged to both of these subsets of the population, both of which experienced worse mental health outcomes, according to the literature.
Studies included in our review found that transgender veterans and service members have worse mental health outcomes than cisgender veterans and service members.28-31 This outcome was predicted based on previous data collection among transgender individuals, veterans, and active-duty service members. One of the studies included found different results and concluded that prior military service was a protective factor against poorer mental health outcomes.34 This could be, in part, due to veterans’ access to care through the US Department of Veterans Affairs (VA) system. It has been found that transgender veterans use VA services at higher rates than the general population of veterans and that barriers to care were found more for medical treatment than for mental health treatment.47 One study found that almost 70% of transgender veterans who used VA services were satisfied with their mental health care.48 In contrast, another study included in our review found that transgender veterans had worse mental health outcomes than transgender service members, possibly showing that even with access to care, the burden of stigma and discrimination worsens mental health over time.31 Although it has been shown that transgender veterans may feel comfortable disclosing their gender identity to their health care professional, many barriers to care have been identified, such as insensitivity and lack of knowledge about transgender care among clinicians.49-51 With this information, it would be useful to ensure proper training for health care professionals on providing gender-affirming care.
Most of the studies also found that transgender veterans and service members had greater odds of suicidal thoughts and events than cisgender veterans and service members.16,28,29,35 On the contrary, transgender veterans were less likely than transgender nonveterans to report NSSI, which could be for various reasons.40 Transgender veterans may report less NSSI but experience it at similar rates, or veteran status may be a protective factor for NSSI.
Very few studies included SUDs in their measurements, but it was found that transgender veterans were more likely than cisgender veterans to have any drug and alcohol use disorder.29,41 In addition, transgender veterans were more likely than transgender service members to be diagnosed with an SUD, again showing that over time and after time of service, mental health may worsen due to the burden of stigma and discrimination.31 Studies that examined MST found that transgender veterans were more likely than cisgender veterans to report MST, which replicates previous data that found high rates of sexual assault experienced among transgender individuals.1,28,29
There is a lack of literature surrounding transgender veterans and active-duty service members, especially with regard to gender-affirming care provided to these populations. To the best of our knowledge, there exists only one original study that examines the effect of gender-affirming hormone therapy and surgery on mental health outcomes among transgender veterans.52 Further research in this area is needed, specifically longitudinal studies examining the effects of gender-affirming medical care on various outcomes, including mental health. Few longitudinal studies exist that examine the mental health effects of gender-affirming hormone therapy on transgender individuals in the general population.53-60 Most of these studies have shown a significant improvement in parameters of depression and anxiety following hormonal treatment, although long-term large follow-up studies to understand whether these improvements persist over time are missing also in the general population. However, as previously described, transgender veterans and service members are a unique subset of the transgender population and require separate data collection. Hence, further research is required to provide optimal care for this population. In addition, early screening for symptoms of mental illness, substance use, and MST is important to providing optimal care.
Limitations
This review was limited due to the lack of data collected from transgender veterans and service members. The studies included did not allow for standardized comparisons and did not use identical measures. Some papers compared transgender veterans with transgender nonveterans, some transgender veterans and/or service members with cisgender veterans and/or service members, and some transgender veterans with transgender service members. There were some consistent results found across the studies, but some studies showed contradictory results or no significant differences within a certain category. It is difficult to compare such different study designs and various participant populations. Additional research is required to verify and replicate these results.
Conclusions
Although this review was limited due to the lack of consistent study designs in the literature examining the mental health of transgender veterans and active-duty service members, overall results showed that transgender veterans and service members experience worse mental health outcomes than their cisgender counterparts. With this knowledge and exploring the history of discrimination that this population has faced, improved systems must be put into place to better serve this population and improve health outcomes. Additional research is required to examine the effects of gender-affirming care on mental health among transgender veterans and service members.
1. James SE, Herman JL, Rankin S, Keisling M, Mottet L, Anafi M. The Report of the 2015 U.S. Transgender Survey. National Center for Transgender Equality. December 2016. Accessed August 22, 2022. https://www.ustranssurvey.org
2. Meadows SO, Engel CC, Collins RL, et al. 2015 Department of Defense Health Related Behaviors Survey (HRBS). Rand Health Q. 2018;8(2):434.
3. Lipari R, Piscopo K, Kroutil LA, Miller GK. Suicidal thoughts and behavior among adults: results from the 2014 National Survey on Drug Use and Health. NSDUH Data Review. 2015:1-14. https://www.samhsa.gov/data/sites/default/files/NSDUH-FRR2-2014/NSDUH-FRR2-2014.pdf
4. Kessler RC, Heeringa SG, Stein MB, et al. Thirty-day prevalence of DSM-IV mental disorders among nondeployed soldiers in the US Army: results from the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS). JAMA Psychiatry. 2014;71(5):504-513. doi:10.1001/jamapsychiatry.2014.28
5. U.S. Department of Veterans Affairs Office of Mental Health and Suicide Prevention. 2020 National Veteran Suicide Prevention Annual Report. November 2020. Accessed August 22, 2022. https://www.mentalhealth.va.gov/docs/data-sheets/2020/2020-National-Veteran-Suicide-Prevention-Annual-Report-11-2020-508.pdf
6. Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602. doi:10.1001/archpsyc.62.6.593
7. Vespa J. Those who SERVED: America’s veterans from World War II to the war on terror. The United States Census Bureau. June 2, 2020. Accessed August 22, 2022. https://www.census.gov/library/publications/2020/demo/acs-43.html
8. Seibert DC, Keller N, Zapor L, Archer H. Military transgender care. J Am Assoc Nurse Pract. 2020;32(11):764-770. doi:10.1097/JXX.0000000000000519
9. Rigby WC. Military penal law: A brief survey of the 1920 revision of the Articles of War. J Crim Law Criminol. 1921;12(1):84.
10. Department of Defense Directive Number 1332.14: Enlisted Administrative Separations. December 21, 1993. Accessed August 22, 2022. https://biotech.law.lsu.edu/blaw/dodd/corres/pdf/d133214wch1_122193/d133214p.pdf
11. Aford B, Lee SJ. Toward complete inclusion: lesbian, gay, bisexual, and transgender military service members after repeal of Don’t Ask, Don’t Tell. Soc Work. 2016;61(3):257-265. doi:10.1093/sw/sww033
12. Department of Defense Instruction 1300.28: In-Service Transition for Transgender Service Members. June 30, 2016. Accessed August 22, 2022. https://dod.defense.gov/Portals/1/features/2016/0616_policy/DoD-Instruction-1300.28.pdf
13. Department of Defense. Directive-type Memorandum (DTM)-19-004 - Military Service by Transgender Persons and Persons with Gender Dysphoria. March 12. 2019. Accessed August 22, 2022. https://health.mil/Reference-Center/Policies/2020/03/17/Military-Service-by-Transgender-Persons-and-Persons-with-Gender-Dysphoria
14. US Department of Defense Instruction 1300.28: In-Service Transition for Transgender Service Members. April 30, 2021. Accessed August 22, 2022. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/130028p.pdf
15. Flores AR, Herman JL, Gates GJ, Brown TNT. How many adults identify as transgender in the United States? The Williams Institute; 2016. Accessed August 22, 2022. https://williamsinstitute.law.ucla.edu/publications/trans-adults-united-states/
16. Blosnich JR, Brown GR, Shipherd Phd JC, Kauth M, Piegari RI, Bossarte RM. Prevalence of gender identity disorder and suicide risk among transgender veterans utilizing veterans health administration care. Am J Public Health. 2013;103(10):e27-e32. doi:10.2105/AJPH.2013.301507
17. Mark KM, McNamara KA, Gribble R, et al. The health and well-being of LGBTQ serving and ex-serving personnel: a narrative review. Int Rev Psychiatry. 2019;31(1):75-94. doi:10.1080/09540261.2019.1575190
18. Blosnich J, Foynes MM, Shipherd JC. Health disparities among sexual minority women veterans. J Womens Health (Larchmt). 2013;22(7):631-636. doi:10.1089/jwh.2012.4214
19. Blosnich JR, Bossarte RM, Silenzio VM. Suicidal ideation among sexual minority veterans: results from the 2005-2010 Massachusetts Behavioral Risk Factor Surveillance Survey. Am J Public Health. 2012;102(suppl 1):S44-S47. doi:10.2105/AJPH.2011.300565
20. Blosnich JR, Gordon AJ, Fine MJ. Associations of sexual and gender minority status with health indicators, health risk factors, and social stressors in a national sample of young adults with military experience. Ann Epidemiol. 2015;25(9):661-667. doi:10.1016/j.annepidem.2015.06.001
21. Cochran BN, Balsam K, Flentje A, Malte CA, Simpson T. Mental health characteristics of sexual minority veterans. J Homosex. 2013;60(2-3):419-435. doi:10.1080/00918369.2013.744932
22. Lehavot K, Browne KC, Simpson TL. Examining sexual orientation disparities in alcohol misuse among women veterans. Am J Prev Med. 2014;47(5):554-562. doi:10.1016/j.amepre.2014.07.002
23. Scott RL, Lasiuk GC, Norris CM. Depression in lesbian, gay, and bisexual members of the Canadian Armed Forces. LGBT Health. 2016;3(5):366-372. doi:10.1089/lgbt.2016.0050
24. Wang J, Dey M, Soldati L, Weiss MG, Gmel G, Mohler-Kuo M. Psychiatric disorders, suicidality, and personality among young men by sexual orientation. Eur Psychiatry. 2014;29(8):514-522. doi:10.1016/j.eurpsy.2014.05.001
25. American Psychological Association. Gender. APA Style. September 2019. Updated July 2022. Accessed August 22, 2022. https://apastyle.apa.org/style-grammar-guidelines/bias-free-language/gender
26. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed., American Psychiatric Association; 2013.
27. Deutsch MB. Overview of gender-affirming treatments and procedures. UCSF Transgender Care. June 17, 2016. Accessed August 22, 2022. https://transcare.ucsf.edu/guidelines/overview
28. Brown GR, Jones KT. Health correlates of criminal justice involvement in 4,793 transgender veterans. LGBT Health. 2015;2(4):297-305. doi:10.1089/lgbt.2015.0052
29. Brown GR, Jones KT. Mental health and medical health disparities in 5135 transgender veterans receiving healthcare in the Veterans Health Administration: a case-control study. LGBT Health. 2016;3(2):122-131. doi:10.1089/lgbt.2015.0058
30. Downing J, Conron K, Herman JL, Blosnich JR. Transgender and cisgender US veterans have few health differences. Health Aff (Millwood). 2018;37(7):1160-1168. doi:10.1377/hlthaff.2018.0027
31. Holloway IW, Green D, Pickering C, et al. Mental health and health risk behaviors of active duty sexual minority and transgender service members in the United States military. LGBT Health. 2021;8(2):152-161. doi:10.1089/lgbt.2020.0031
32. Beckman K, Shipherd J, Simpson T, Lehavot K. Military sexual assault in transgender veterans: results from a nationwide survey. J Trauma Stress. 2018;31(2):181-190. doi:10.1002/jts.22280
33. Blosnich JR, Marsiglio MC, Gao S, Gordon AJ, Shipherd JC, Kauth M, Brown GR, Fine MJ. Mental health of transgender veterans in US states with and without discrimination and hate crime legal protection. Am J Public Health. 2016;106(3):534-540. doi:10.2105/AJPH.2015.302981
34. Hoy-Ellis CP, Shiu C, Sullivan KM, Kim HJ, Sturges AM, Fredriksen-Goldsen KI. Prior military service, identity stigma, and mental health among transgender older adults. Gerontologist. 2017;57(suppl 1):S63-S71. doi:10.1093/geront/gnw173
35. Hill BJ, Bouris A, Barnett JT, Walker D. Fit to serve? Exploring mental and physical health and well-being among transgender active-duty service members and veterans in the U.S. military. Transgend Health. 2016;1(1):4-11. Published 2016 Jan 1. doi:10.1089/trgh.2015.0002
36. Blosnich JR, Brown GR, Wojcio S, Jones KT, Bossarte RM. Mortality among veterans with transgender-related diagnoses in the Veterans Health Administration, FY2000-2009. LGBT Health. 2014;1(4):269-276. doi:10.1089/lgbt.2014.0050
37. Carter SP, Allred KM, Tucker RP, Simpson TL, Shipherd JC, Lehavot K. Discrimination and suicidal ideation among transgender veterans: the role of social support and connection. LGBT Health. 2019;6(2):43-50. doi:10.1089/lgbt.2018.0239
38. Lehavot K, Simpson TL, Shipherd JC. Factors associated with suicidality among a national sample of transgender veterans. Suicide Life Threat Behav. 2016;46(5):507-524. doi:10.1111/sltb.12233
39. Tucker RP, Testa RJ, Reger MA, Simpson TL, Shipherd JC, Lehavot K. Current and military-specific gender minority stress factors and their relationship with suicide ideation in transgender veterans. Suicide Life Threat Behav. 2019;49(1):155-166. doi:10.1111/sltb.12432
40. Aboussouan A, Snow A, Cerel J, Tucker RP. Non-suicidal self-injury, suicide ideation, and past suicide attempts: Comparison between transgender and gender diverse veterans and non-veterans. J Affect Disord. 2019;259:186-194. doi:10.1016/j.jad.2019.08.046
41. Frost MC, Blosnich JR, Lehavot K, Chen JA, Rubinsky AD, Glass JE, Williams EC. Disparities in documented drug use disorders between transgender and cisgender U.S. Veterans Health Administration patients. J Addict Med. 2021;15(4):334-340. doi:10.1097/ADM.0000000000000769
42. Williams EC, Frost MC, Rubinsky AD, et al. Patterns of alcohol use among transgender patients receiving care at the Veterans Health Administration: overall and relative to nontransgender patients. J Stud Alcohol Drugs. 2021;82(1):132-141. doi:10.15288/jsad.2021.82.132
43. Bukowski LA, Blosnich J, Shipherd JC, Kauth MR, Brown GR, Gordon AJ. Exploring rural disparities in medical diagnoses among veterans with transgender-related diagnoses utilizing Veterans Health Administration care. Med Care. 2017;55(suppl 9):S97-S103. doi:10.1097/MLR.0000000000000745
44. U.S. Department of Veterans Affairs. Military Sexual Trauma. Updated August 1, 2022. Accessed August 22, 2022. https://www.mentalhealth.va.gov/mentalhealth/msthome/index.asp
45. Lindsay JA, Keo-Meier C, Hudson S, Walder A, Martin LA, Kauth MR. Mental health of transgender veterans of the Iraq and Afghanistan conflicts who experienced military sexual trauma. J Trauma Stress. 2016;29(6):563-567. doi:10.1002/jts.22146
46. Schuyler AC, Klemmer C, Mamey MR, et al. Experiences of sexual harassment, stalking, and sexual assault during military service among LGBT and Non-LGBT service members. J Trauma Stress. 2020;33(3):257-266. doi:10.1002/jts.22506
47. Shipherd JC, Mizock L, Maguen S, Green KE. Male-to-female transgender veterans and VA health care utilization. Int J Sexual Health. 2012;24(1):78-87. doi:10.1080/19317611.2011.639440
48. Lehavot K, Katon JG, Simpson TL, Shipherd JC. Transgender veterans’ satisfaction with care and unmet health needs. Med Care. 2017;55(suppl 9):S90-S96. doi:10.1097/MLR.0000000000000723
49. Kauth MR, Barrera TL, Latini DM. Lesbian, gay, and transgender veterans’ experiences in the Veterans Health Administration: positive signs and room for improvement. Psychol Serv. 2019;16(2):346-351. doi:10.1037/ser0000232

50. Rosentel K, Hill BJ, Lu C, Barnett JT. Transgender veterans and the Veterans Health Administration: exploring the experiences of transgender veterans in the Veterans Affairs Healthcare System. Transgend Health. 2016;1(1):108-116. Published 2016 Jun 1. doi:10.1089/trgh.2016.0006
51. Dietert M, Dentice D, Keig Z. Addressing the needs of transgender military veterans: better access and more comprehensive care. Transgend Health. 2017;2(1):35-44. Published 2017 Mar 1. doi:10.1089/trgh.2016.0040
52. Tucker RP, Testa RJ, Simpson TL, Shipherd JC, Blosnich JR, Lehavot K. Hormone therapy, gender affirmation surgery, and their association with recent suicidal ideation and depression symptoms in transgender veterans. Psychol Med. 2018;48(14):2329-2336. doi:10.1017/S0033291717003853
53. Colizzi M, Costa R, Todarello O. Transsexual patients’ psychiatric comorbidity and positive effect of cross-sex hormonal treatment on mental health: results from a longitudinal study. Psychoneuroendocrinology. 2014;39:65-73. doi:10.1016/j.psyneuen.2013.09.029
54. Heylens G, Verroken C, De Cock S, T’Sjoen G, De Cuypere G. Effects of different steps in gender reassignment therapy on psychopathology: a prospective study of persons with a gender identity disorder. J Sex Med. 2014;11(1):119-126. doi:10.1111/jsm.12363
55. Fisher AD, Castellini G, Ristori J, et al. Cross-sex hormone treatment and psychobiological changes in transsexual persons: two-year follow-up data. J Clin Endocrinol Metab. 2016;101(11):4260-4269. doi:10.1210/jc.2016-1276
56. Aldridge Z, Patel S, Guo B, et al. Long-term effect of gender-affirming hormone treatment on depression and anxiety symptoms in transgender people: a prospective cohort study. Andrology. 2021;9(6):1808-1816. doi:10.1111/andr.12884
57. Costantino A, Cerpolini S, Alvisi S, Morselli PG, Venturoli S, Meriggiola MC. A prospective study on sexual function and mood in female-to-male transsexuals during testosterone administration and after sex reassignment surgery. J Sex Marital Ther. 2013;39(4):321-335. doi:10.1080/0092623X.2012.736920
58. Keo-Meier CL, Herman LI, Reisner SL, Pardo ST, Sharp C, Babcock JC. Testosterone treatment and MMPI-2 improvement in transgender men: a prospective controlled study. J Consult Clin Psychol. 2015;83(1):143-156. doi:10.1037/a0037599
59. Turan S‚ , Aksoy Poyraz C, Usta Sag˘lam NG, et al. Alterations in body uneasiness, eating attitudes, and psychopathology before and after cross-sex hormonal treatment in patients with female-to-male gender dysphoria. Arch Sex Behav. 2018;47(8):2349-2361. doi:10.1007/s10508-018-1189-4
60. Oda H, Kinoshita T. Efficacy of hormonal and mental treatments with MMPI in FtM individuals: cross-sectional and longitudinal studies. BMC Psychiatry. 2017;17(1):256. Published 2017 Jul 17. doi:10.1186/s12888-017-1423-y
1. James SE, Herman JL, Rankin S, Keisling M, Mottet L, Anafi M. The Report of the 2015 U.S. Transgender Survey. National Center for Transgender Equality. December 2016. Accessed August 22, 2022. https://www.ustranssurvey.org
2. Meadows SO, Engel CC, Collins RL, et al. 2015 Department of Defense Health Related Behaviors Survey (HRBS). Rand Health Q. 2018;8(2):434.
3. Lipari R, Piscopo K, Kroutil LA, Miller GK. Suicidal thoughts and behavior among adults: results from the 2014 National Survey on Drug Use and Health. NSDUH Data Review. 2015:1-14. https://www.samhsa.gov/data/sites/default/files/NSDUH-FRR2-2014/NSDUH-FRR2-2014.pdf
4. Kessler RC, Heeringa SG, Stein MB, et al. Thirty-day prevalence of DSM-IV mental disorders among nondeployed soldiers in the US Army: results from the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS). JAMA Psychiatry. 2014;71(5):504-513. doi:10.1001/jamapsychiatry.2014.28
5. U.S. Department of Veterans Affairs Office of Mental Health and Suicide Prevention. 2020 National Veteran Suicide Prevention Annual Report. November 2020. Accessed August 22, 2022. https://www.mentalhealth.va.gov/docs/data-sheets/2020/2020-National-Veteran-Suicide-Prevention-Annual-Report-11-2020-508.pdf
6. Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602. doi:10.1001/archpsyc.62.6.593
7. Vespa J. Those who SERVED: America’s veterans from World War II to the war on terror. The United States Census Bureau. June 2, 2020. Accessed August 22, 2022. https://www.census.gov/library/publications/2020/demo/acs-43.html
8. Seibert DC, Keller N, Zapor L, Archer H. Military transgender care. J Am Assoc Nurse Pract. 2020;32(11):764-770. doi:10.1097/JXX.0000000000000519
9. Rigby WC. Military penal law: A brief survey of the 1920 revision of the Articles of War. J Crim Law Criminol. 1921;12(1):84.
10. Department of Defense Directive Number 1332.14: Enlisted Administrative Separations. December 21, 1993. Accessed August 22, 2022. https://biotech.law.lsu.edu/blaw/dodd/corres/pdf/d133214wch1_122193/d133214p.pdf
11. Aford B, Lee SJ. Toward complete inclusion: lesbian, gay, bisexual, and transgender military service members after repeal of Don’t Ask, Don’t Tell. Soc Work. 2016;61(3):257-265. doi:10.1093/sw/sww033
12. Department of Defense Instruction 1300.28: In-Service Transition for Transgender Service Members. June 30, 2016. Accessed August 22, 2022. https://dod.defense.gov/Portals/1/features/2016/0616_policy/DoD-Instruction-1300.28.pdf
13. Department of Defense. Directive-type Memorandum (DTM)-19-004 - Military Service by Transgender Persons and Persons with Gender Dysphoria. March 12. 2019. Accessed August 22, 2022. https://health.mil/Reference-Center/Policies/2020/03/17/Military-Service-by-Transgender-Persons-and-Persons-with-Gender-Dysphoria
14. US Department of Defense Instruction 1300.28: In-Service Transition for Transgender Service Members. April 30, 2021. Accessed August 22, 2022. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/130028p.pdf
15. Flores AR, Herman JL, Gates GJ, Brown TNT. How many adults identify as transgender in the United States? The Williams Institute; 2016. Accessed August 22, 2022. https://williamsinstitute.law.ucla.edu/publications/trans-adults-united-states/
16. Blosnich JR, Brown GR, Shipherd Phd JC, Kauth M, Piegari RI, Bossarte RM. Prevalence of gender identity disorder and suicide risk among transgender veterans utilizing veterans health administration care. Am J Public Health. 2013;103(10):e27-e32. doi:10.2105/AJPH.2013.301507
17. Mark KM, McNamara KA, Gribble R, et al. The health and well-being of LGBTQ serving and ex-serving personnel: a narrative review. Int Rev Psychiatry. 2019;31(1):75-94. doi:10.1080/09540261.2019.1575190
18. Blosnich J, Foynes MM, Shipherd JC. Health disparities among sexual minority women veterans. J Womens Health (Larchmt). 2013;22(7):631-636. doi:10.1089/jwh.2012.4214
19. Blosnich JR, Bossarte RM, Silenzio VM. Suicidal ideation among sexual minority veterans: results from the 2005-2010 Massachusetts Behavioral Risk Factor Surveillance Survey. Am J Public Health. 2012;102(suppl 1):S44-S47. doi:10.2105/AJPH.2011.300565
20. Blosnich JR, Gordon AJ, Fine MJ. Associations of sexual and gender minority status with health indicators, health risk factors, and social stressors in a national sample of young adults with military experience. Ann Epidemiol. 2015;25(9):661-667. doi:10.1016/j.annepidem.2015.06.001
21. Cochran BN, Balsam K, Flentje A, Malte CA, Simpson T. Mental health characteristics of sexual minority veterans. J Homosex. 2013;60(2-3):419-435. doi:10.1080/00918369.2013.744932
22. Lehavot K, Browne KC, Simpson TL. Examining sexual orientation disparities in alcohol misuse among women veterans. Am J Prev Med. 2014;47(5):554-562. doi:10.1016/j.amepre.2014.07.002
23. Scott RL, Lasiuk GC, Norris CM. Depression in lesbian, gay, and bisexual members of the Canadian Armed Forces. LGBT Health. 2016;3(5):366-372. doi:10.1089/lgbt.2016.0050
24. Wang J, Dey M, Soldati L, Weiss MG, Gmel G, Mohler-Kuo M. Psychiatric disorders, suicidality, and personality among young men by sexual orientation. Eur Psychiatry. 2014;29(8):514-522. doi:10.1016/j.eurpsy.2014.05.001
25. American Psychological Association. Gender. APA Style. September 2019. Updated July 2022. Accessed August 22, 2022. https://apastyle.apa.org/style-grammar-guidelines/bias-free-language/gender
26. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed., American Psychiatric Association; 2013.
27. Deutsch MB. Overview of gender-affirming treatments and procedures. UCSF Transgender Care. June 17, 2016. Accessed August 22, 2022. https://transcare.ucsf.edu/guidelines/overview
28. Brown GR, Jones KT. Health correlates of criminal justice involvement in 4,793 transgender veterans. LGBT Health. 2015;2(4):297-305. doi:10.1089/lgbt.2015.0052
29. Brown GR, Jones KT. Mental health and medical health disparities in 5135 transgender veterans receiving healthcare in the Veterans Health Administration: a case-control study. LGBT Health. 2016;3(2):122-131. doi:10.1089/lgbt.2015.0058
30. Downing J, Conron K, Herman JL, Blosnich JR. Transgender and cisgender US veterans have few health differences. Health Aff (Millwood). 2018;37(7):1160-1168. doi:10.1377/hlthaff.2018.0027
31. Holloway IW, Green D, Pickering C, et al. Mental health and health risk behaviors of active duty sexual minority and transgender service members in the United States military. LGBT Health. 2021;8(2):152-161. doi:10.1089/lgbt.2020.0031
32. Beckman K, Shipherd J, Simpson T, Lehavot K. Military sexual assault in transgender veterans: results from a nationwide survey. J Trauma Stress. 2018;31(2):181-190. doi:10.1002/jts.22280
33. Blosnich JR, Marsiglio MC, Gao S, Gordon AJ, Shipherd JC, Kauth M, Brown GR, Fine MJ. Mental health of transgender veterans in US states with and without discrimination and hate crime legal protection. Am J Public Health. 2016;106(3):534-540. doi:10.2105/AJPH.2015.302981
34. Hoy-Ellis CP, Shiu C, Sullivan KM, Kim HJ, Sturges AM, Fredriksen-Goldsen KI. Prior military service, identity stigma, and mental health among transgender older adults. Gerontologist. 2017;57(suppl 1):S63-S71. doi:10.1093/geront/gnw173
35. Hill BJ, Bouris A, Barnett JT, Walker D. Fit to serve? Exploring mental and physical health and well-being among transgender active-duty service members and veterans in the U.S. military. Transgend Health. 2016;1(1):4-11. Published 2016 Jan 1. doi:10.1089/trgh.2015.0002
36. Blosnich JR, Brown GR, Wojcio S, Jones KT, Bossarte RM. Mortality among veterans with transgender-related diagnoses in the Veterans Health Administration, FY2000-2009. LGBT Health. 2014;1(4):269-276. doi:10.1089/lgbt.2014.0050
37. Carter SP, Allred KM, Tucker RP, Simpson TL, Shipherd JC, Lehavot K. Discrimination and suicidal ideation among transgender veterans: the role of social support and connection. LGBT Health. 2019;6(2):43-50. doi:10.1089/lgbt.2018.0239
38. Lehavot K, Simpson TL, Shipherd JC. Factors associated with suicidality among a national sample of transgender veterans. Suicide Life Threat Behav. 2016;46(5):507-524. doi:10.1111/sltb.12233
39. Tucker RP, Testa RJ, Reger MA, Simpson TL, Shipherd JC, Lehavot K. Current and military-specific gender minority stress factors and their relationship with suicide ideation in transgender veterans. Suicide Life Threat Behav. 2019;49(1):155-166. doi:10.1111/sltb.12432
40. Aboussouan A, Snow A, Cerel J, Tucker RP. Non-suicidal self-injury, suicide ideation, and past suicide attempts: Comparison between transgender and gender diverse veterans and non-veterans. J Affect Disord. 2019;259:186-194. doi:10.1016/j.jad.2019.08.046
41. Frost MC, Blosnich JR, Lehavot K, Chen JA, Rubinsky AD, Glass JE, Williams EC. Disparities in documented drug use disorders between transgender and cisgender U.S. Veterans Health Administration patients. J Addict Med. 2021;15(4):334-340. doi:10.1097/ADM.0000000000000769
42. Williams EC, Frost MC, Rubinsky AD, et al. Patterns of alcohol use among transgender patients receiving care at the Veterans Health Administration: overall and relative to nontransgender patients. J Stud Alcohol Drugs. 2021;82(1):132-141. doi:10.15288/jsad.2021.82.132
43. Bukowski LA, Blosnich J, Shipherd JC, Kauth MR, Brown GR, Gordon AJ. Exploring rural disparities in medical diagnoses among veterans with transgender-related diagnoses utilizing Veterans Health Administration care. Med Care. 2017;55(suppl 9):S97-S103. doi:10.1097/MLR.0000000000000745
44. U.S. Department of Veterans Affairs. Military Sexual Trauma. Updated August 1, 2022. Accessed August 22, 2022. https://www.mentalhealth.va.gov/mentalhealth/msthome/index.asp
45. Lindsay JA, Keo-Meier C, Hudson S, Walder A, Martin LA, Kauth MR. Mental health of transgender veterans of the Iraq and Afghanistan conflicts who experienced military sexual trauma. J Trauma Stress. 2016;29(6):563-567. doi:10.1002/jts.22146
46. Schuyler AC, Klemmer C, Mamey MR, et al. Experiences of sexual harassment, stalking, and sexual assault during military service among LGBT and Non-LGBT service members. J Trauma Stress. 2020;33(3):257-266. doi:10.1002/jts.22506
47. Shipherd JC, Mizock L, Maguen S, Green KE. Male-to-female transgender veterans and VA health care utilization. Int J Sexual Health. 2012;24(1):78-87. doi:10.1080/19317611.2011.639440
48. Lehavot K, Katon JG, Simpson TL, Shipherd JC. Transgender veterans’ satisfaction with care and unmet health needs. Med Care. 2017;55(suppl 9):S90-S96. doi:10.1097/MLR.0000000000000723
49. Kauth MR, Barrera TL, Latini DM. Lesbian, gay, and transgender veterans’ experiences in the Veterans Health Administration: positive signs and room for improvement. Psychol Serv. 2019;16(2):346-351. doi:10.1037/ser0000232

50. Rosentel K, Hill BJ, Lu C, Barnett JT. Transgender veterans and the Veterans Health Administration: exploring the experiences of transgender veterans in the Veterans Affairs Healthcare System. Transgend Health. 2016;1(1):108-116. Published 2016 Jun 1. doi:10.1089/trgh.2016.0006
51. Dietert M, Dentice D, Keig Z. Addressing the needs of transgender military veterans: better access and more comprehensive care. Transgend Health. 2017;2(1):35-44. Published 2017 Mar 1. doi:10.1089/trgh.2016.0040
52. Tucker RP, Testa RJ, Simpson TL, Shipherd JC, Blosnich JR, Lehavot K. Hormone therapy, gender affirmation surgery, and their association with recent suicidal ideation and depression symptoms in transgender veterans. Psychol Med. 2018;48(14):2329-2336. doi:10.1017/S0033291717003853
53. Colizzi M, Costa R, Todarello O. Transsexual patients’ psychiatric comorbidity and positive effect of cross-sex hormonal treatment on mental health: results from a longitudinal study. Psychoneuroendocrinology. 2014;39:65-73. doi:10.1016/j.psyneuen.2013.09.029
54. Heylens G, Verroken C, De Cock S, T’Sjoen G, De Cuypere G. Effects of different steps in gender reassignment therapy on psychopathology: a prospective study of persons with a gender identity disorder. J Sex Med. 2014;11(1):119-126. doi:10.1111/jsm.12363
55. Fisher AD, Castellini G, Ristori J, et al. Cross-sex hormone treatment and psychobiological changes in transsexual persons: two-year follow-up data. J Clin Endocrinol Metab. 2016;101(11):4260-4269. doi:10.1210/jc.2016-1276
56. Aldridge Z, Patel S, Guo B, et al. Long-term effect of gender-affirming hormone treatment on depression and anxiety symptoms in transgender people: a prospective cohort study. Andrology. 2021;9(6):1808-1816. doi:10.1111/andr.12884
57. Costantino A, Cerpolini S, Alvisi S, Morselli PG, Venturoli S, Meriggiola MC. A prospective study on sexual function and mood in female-to-male transsexuals during testosterone administration and after sex reassignment surgery. J Sex Marital Ther. 2013;39(4):321-335. doi:10.1080/0092623X.2012.736920
58. Keo-Meier CL, Herman LI, Reisner SL, Pardo ST, Sharp C, Babcock JC. Testosterone treatment and MMPI-2 improvement in transgender men: a prospective controlled study. J Consult Clin Psychol. 2015;83(1):143-156. doi:10.1037/a0037599
59. Turan S‚ , Aksoy Poyraz C, Usta Sag˘lam NG, et al. Alterations in body uneasiness, eating attitudes, and psychopathology before and after cross-sex hormonal treatment in patients with female-to-male gender dysphoria. Arch Sex Behav. 2018;47(8):2349-2361. doi:10.1007/s10508-018-1189-4
60. Oda H, Kinoshita T. Efficacy of hormonal and mental treatments with MMPI in FtM individuals: cross-sectional and longitudinal studies. BMC Psychiatry. 2017;17(1):256. Published 2017 Jul 17. doi:10.1186/s12888-017-1423-y
Support for Policy Changes for Therapy Related to Homefront Missions
Recent natural disasters, civil disorder, and the COVID-19 pandemic response created an unprecedented demand for the US National Guard and Reserve components as well as active-duty personnel to serve on homefront missions critical to our nation. At times, those serving in these capacities are front and center to the most tragic events confronting our nation, and they frequently encounter tremendous suffering.
Recognizing the potential for these missions to create psychological sequela for those who serve on them, the authority for the Veterans Health Administration (VHA) vet centers to provide readjustment counseling services was broadened on December 30, 2021. Vet centers are community-based counseling centers that have traditionally served combat veterans, and broadening services reflects a major change in mission. Revised VHA Directive 1500(2) specifies that those who “served on active duty in response to a national emergency or major disaster declared by the President” or “served on active duty in the National Guard of a State under orders of the chief executive of that State in response to a disaster or civil disorder in such State” may now receive therapy at vet centers.1,2
As a result of this recent policy change, National Guard and active-duty Reserve service members now have parity with combat veterans to obtain therapy for symptoms arising as a result of their activation for service on homefront missions. As they seek care, we need to be ready so that these service members can obtain the best therapy services possible. Soldiers who served on homefront missions comprise a new cohort of service members now eligible for vet center therapy. Soldiers who served on homefront missions may present with issues that differ from those of combat veterans and veterans who have experienced military sexual trauma (MST), the populations treated by vet centers and other VHA mental health care clinics prior to this broadened authority. This article highlights some suggestions for service delivery to best meet the needs of this population.
Discussion
Available evidence-based therapies to treat posttraumatic stress disorder (PTSD) are effective regardless of whether the trauma occurred in combat, on the homefront, or in a civilian setting. The vet centers and VHA mental health services already have staff trained to deliver these therapy modalities and, in this sense, are ready to provide trauma-focused therapy treatment to soldiers with PTSD who served on homefront missions.
The broadened authority for the vet centers to provide readjustment services is necessary, as it corrects for a critical gap in services, but the importance of ensuring adequate staffing to meet the expected increased demand for services cannot be underscored. According to clinical practice guidelines for the treatment of PTSD, developed by the US Department of Veterans Affairs (VA) and the US Department of Defense (DoD), the therapies with the strongest evidence-based backing are prolonged exposure-based therapy (PE), cognitive processing therapy (CPT), and eye movement and desensitization reprocessing (EMDR).3 These therapy modalities, based on findings from clinical trials, are predicated on seeing a client for a sufficient number of sessions. Attendance at these sessions is recommended at least weekly to ensure adequate intensity of service delivery.4-7 According to the National Center for PTSD, PE typically involves 8 to 15 weekly or twice weekly sessions; CPT requires 8 to 14 or more weekly sessions, and EMDR is usually 4 to 12 weekly sessions.4-7
Ensuring adequate staffing is critical to offer these therapies at least weekly as the efficacies of these therapies are otherwise not proven if return session visits are stretched out over multiple weeks or months. The most recent clinical research has demonstrated that PTSD recovery can be expedited and there are lower patient dropout rates when sessions are massed or compressed so that multiple sessions are administered over 1 week.8-12 Providing these therapies in a massed format has shown to be as effective as when these therapies are provided weekly.
As the authority to treat soldiers serving on homefront missions is new, epidemiologic data do not yet exist to estimate the proportion of this population who will need treatment or present with PTSD, depression, anxiety, a substance use disorder, and/or comorbid conditions. Those with PTSD can benefit from PTSD evidence-based therapies already available for treatment. Others may benefit from treatments that are proven effective for their mental health diagnoses.
Therapists with experience primarily treating patients with PTSD related to combat or MST will need to be sensitive to the unique experiences of the National Guard and Reserve service members. For example, this component of soldiers served on COVID-19–related missions that provided food service support to nursing homes residents who were locked down from family members. As a result, they developed bonds with residents who later died. This may have been the first time that these soldiers witnessed death. If such a soldier is assessed and does not have PTSD but is nonetheless distressed, then the soldier may need alternate therapies, such as grief counseling. This need may be more pronounced for those soldiers who lost loved ones to COVID-19 while they served on these missions.
New Jersey Army National Guard soldiers provided food service support at the Woodland Behavioral and Nursing Center in Andover, New Jersey. These soldiers witnessed the unfortunate conditions in this facility, which included stacked bodies in a makeshift morgue during the height of the pandemic; however, they did not have the ability to make changes. The facility is under investigation for abuse and neglect of its residents.13
New Jersey National Guard soldiers supporting that facility and similar ones may have experienced moral injury, defined as “…perpetrating, failing to prevent, or bearing witness to acts that transgress deeply held moral beliefs and expectations.”14 Importantly, when these soldiers present for therapy and express moral injury, their therapists need to be open to spiritual discourse. However, vet centers do not have chaplains on staff, so therapists must refer patients to chaplaincy services.
Among therapists with existing cultural competency for treating members of the military, some nuances exist for National Guard and Reserve service members. National Guard and Reserve component personnel already may feel that their problems are less important than those experienced by active-duty service members. Now that these soldiers have the eligibility to receive therapy, therapists may have to make extra efforts to both reassure this population that they are welcomed and to validate their need for services.
Special outreach efforts to those who served on historical National Guard and active-duty Reserve missions are a way to show good faith in serving these soldiers because they may have untreated PTSD or other undiagnosed mental health disorders related to earlier deployments, such as hurricane recovery missions. A study of disaster survivors found that the prevalence rate of severe and very severe psychological impact after a natural disaster was about 34%.15 Another epidemiologic study found that the prevalence rate of PTSD was 10% to 20% among disaster rescue workers.16 Specific data about the psychological problems of National Guard and Reserve components serving in disaster recovery are unavailable but is an area for future research.
Therapists who have treated active-duty service members and veterans who worked in mortuary services in a combat zone are used to hearing graphic details of horrifying scenes, but homefront experiences are different. Soldiers on homefront mortuary-based missions frequently reported being unable to forget the faces or the smell of dead bodies as they were stacked up and overwhelming the systems. Experienced vet center therapists should be prepared for the challenges in treating this new cohort of patients.
Conclusions
Now that National Guard and Reserve component soldiers who have responded to national and local emergencies are eligible for therapy, we need to be prepared to provide these services. In addition to addressing systemic staffing concerns, therapists need to be aware of the unique challenges faced by those who have served on homefront missions. These homefront missions have the potential to hit home for therapists.
1. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1550(2): readjustment counseling service. January 26, 2021. Accessed September 1, 2022. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=9168
2. US Department of Veterans Affairs. Vet centers (readjustment counseling: vet center eligibility. Updated January 3, 2022. Accessed September 1, 2022. https://www.vetcenter.va.gov/eligibility.asp
3. US Department of Defense, US Department of Veterans Affairs. VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress reaction, version 3.0, 2017. Accessed September 1, 2022. https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGFinal012418.pdf
4. US Department of Veterans Affairs, National Center for PTSD. Prolonged exposure (PE) therapy. Updated August 10, 2022. Accessed September 1, 2022. https://www.ptsd.va.gov/understand_tx/prolonged_exposure.asp
5. US Department of Veterans Affairs, National Center for PTSD. Cognitive processing therapy (CPT) for PTSD: how to help your loved one during treatment. Accessed September 1, 2022. https://www.ptsd.va.gov/publications/print/CPT_familyhandout.pdf
6. US Department of Veterans Affairs, National Center for PTSD. A provider’s guide to brief cognitive behavioral therapy. Accessed September 1, 2022. https://www.mirecc.va.gov/visn16/docs/Therapists_Guide_to_Brief_CBTManual.pdf
7. US Department of Veterans Affairs, National Center for PTSD. Eye movement desensitization and reprocessing (EMDR) for PTSD. Accessed September 1, 2022. https://www.ptsd.va.gov/understand_tx/emdr.asp
8. Wachen JS, Dondanville KA, Evans WR, Morris K, Cole A. Adjusting the timeframe of evidence-based therapies for PTSD-massed treatments. Curr Treat Options Psych. 2019;6(2):107-118. doi:10.1007/s40501-019-00169-9
9. Dell L, Sbisa AM, Forbes A, et al. Effect of massed v. standard prolonged exposure therapy on PTSD in military personnel and veterans: a non-inferiority randomised controlled trial [published online ahead of print, 2022 Apr 20]. Psychol Med. 2022;1-8. doi:10.1017/S0033291722000927
10. Held P, Kovacevic M, Petrey K, et al. Treating posttraumatic stress disorder at home in a single week using 1-week virtual massed cognitive processing therapy. J Trauma Stress. 2022;35(4):1215-1225. doi:10.1002/jts.22831
11. Yamokoski C, Flores H, Facemire V, Maieritsch K, Perez S, Fedynich A. Feasibility of an intensive outpatient treatment program for posttraumatic stress disorder within the veterans health care administration [published online ahead of print, 2022 Mar 7]. Psychol Serv. 2022;10.1037/ser0000628. doi:10.1037/ser0000628
12. Galovski TE, Werner KB, Weaver TL, et al. Massed cognitive processing therapy for posttraumatic stress disorder in women survivors of intimate partner violence. Psychol Trauma. 2022;14(5):769-779. doi:10.1037/tra0001100
13. Fallon S. NJ to send monitors into troubled nursing home that stacked bodies in makeshift morgue. Updated March 10, 2022. Accessed September 1, 2022. https://www.northjersey.com/story/news/health/2022/03/09/sussex-county-nj-nursing-home-monitors-covid-morgue/9447243002/
14. Litz BT, Stein N, Delaney E, et al. Moral injury and moral repair in war veterans: a preliminary model and intervention strategy. Clin Psychol Rev. 2009;29(8):695-706. doi:10.1016/j.cpr.2009.07.003009
15. Norris FH, Friedman MJ, Watson PJ, Byrne CM, Diaz E, Kaniasty K. 60,000 disaster victims speak: Part I. An empirical review of the empirical literature, 1981-2001. Psychiatry. 2002;65(3):207-239. doi:10.1521/psyc.65.3.207.20173
16. Galea S, Nandi A, Vlahov D. The epidemiology of post-traumatic stress disorder after disasters. Epidemiol Rev. 2005;27:78-91. doi:10.1093/epirev/mxi003
Recent natural disasters, civil disorder, and the COVID-19 pandemic response created an unprecedented demand for the US National Guard and Reserve components as well as active-duty personnel to serve on homefront missions critical to our nation. At times, those serving in these capacities are front and center to the most tragic events confronting our nation, and they frequently encounter tremendous suffering.
Recognizing the potential for these missions to create psychological sequela for those who serve on them, the authority for the Veterans Health Administration (VHA) vet centers to provide readjustment counseling services was broadened on December 30, 2021. Vet centers are community-based counseling centers that have traditionally served combat veterans, and broadening services reflects a major change in mission. Revised VHA Directive 1500(2) specifies that those who “served on active duty in response to a national emergency or major disaster declared by the President” or “served on active duty in the National Guard of a State under orders of the chief executive of that State in response to a disaster or civil disorder in such State” may now receive therapy at vet centers.1,2
As a result of this recent policy change, National Guard and active-duty Reserve service members now have parity with combat veterans to obtain therapy for symptoms arising as a result of their activation for service on homefront missions. As they seek care, we need to be ready so that these service members can obtain the best therapy services possible. Soldiers who served on homefront missions comprise a new cohort of service members now eligible for vet center therapy. Soldiers who served on homefront missions may present with issues that differ from those of combat veterans and veterans who have experienced military sexual trauma (MST), the populations treated by vet centers and other VHA mental health care clinics prior to this broadened authority. This article highlights some suggestions for service delivery to best meet the needs of this population.
Discussion
Available evidence-based therapies to treat posttraumatic stress disorder (PTSD) are effective regardless of whether the trauma occurred in combat, on the homefront, or in a civilian setting. The vet centers and VHA mental health services already have staff trained to deliver these therapy modalities and, in this sense, are ready to provide trauma-focused therapy treatment to soldiers with PTSD who served on homefront missions.
The broadened authority for the vet centers to provide readjustment services is necessary, as it corrects for a critical gap in services, but the importance of ensuring adequate staffing to meet the expected increased demand for services cannot be underscored. According to clinical practice guidelines for the treatment of PTSD, developed by the US Department of Veterans Affairs (VA) and the US Department of Defense (DoD), the therapies with the strongest evidence-based backing are prolonged exposure-based therapy (PE), cognitive processing therapy (CPT), and eye movement and desensitization reprocessing (EMDR).3 These therapy modalities, based on findings from clinical trials, are predicated on seeing a client for a sufficient number of sessions. Attendance at these sessions is recommended at least weekly to ensure adequate intensity of service delivery.4-7 According to the National Center for PTSD, PE typically involves 8 to 15 weekly or twice weekly sessions; CPT requires 8 to 14 or more weekly sessions, and EMDR is usually 4 to 12 weekly sessions.4-7
Ensuring adequate staffing is critical to offer these therapies at least weekly as the efficacies of these therapies are otherwise not proven if return session visits are stretched out over multiple weeks or months. The most recent clinical research has demonstrated that PTSD recovery can be expedited and there are lower patient dropout rates when sessions are massed or compressed so that multiple sessions are administered over 1 week.8-12 Providing these therapies in a massed format has shown to be as effective as when these therapies are provided weekly.
As the authority to treat soldiers serving on homefront missions is new, epidemiologic data do not yet exist to estimate the proportion of this population who will need treatment or present with PTSD, depression, anxiety, a substance use disorder, and/or comorbid conditions. Those with PTSD can benefit from PTSD evidence-based therapies already available for treatment. Others may benefit from treatments that are proven effective for their mental health diagnoses.
Therapists with experience primarily treating patients with PTSD related to combat or MST will need to be sensitive to the unique experiences of the National Guard and Reserve service members. For example, this component of soldiers served on COVID-19–related missions that provided food service support to nursing homes residents who were locked down from family members. As a result, they developed bonds with residents who later died. This may have been the first time that these soldiers witnessed death. If such a soldier is assessed and does not have PTSD but is nonetheless distressed, then the soldier may need alternate therapies, such as grief counseling. This need may be more pronounced for those soldiers who lost loved ones to COVID-19 while they served on these missions.
New Jersey Army National Guard soldiers provided food service support at the Woodland Behavioral and Nursing Center in Andover, New Jersey. These soldiers witnessed the unfortunate conditions in this facility, which included stacked bodies in a makeshift morgue during the height of the pandemic; however, they did not have the ability to make changes. The facility is under investigation for abuse and neglect of its residents.13
New Jersey National Guard soldiers supporting that facility and similar ones may have experienced moral injury, defined as “…perpetrating, failing to prevent, or bearing witness to acts that transgress deeply held moral beliefs and expectations.”14 Importantly, when these soldiers present for therapy and express moral injury, their therapists need to be open to spiritual discourse. However, vet centers do not have chaplains on staff, so therapists must refer patients to chaplaincy services.
Among therapists with existing cultural competency for treating members of the military, some nuances exist for National Guard and Reserve service members. National Guard and Reserve component personnel already may feel that their problems are less important than those experienced by active-duty service members. Now that these soldiers have the eligibility to receive therapy, therapists may have to make extra efforts to both reassure this population that they are welcomed and to validate their need for services.
Special outreach efforts to those who served on historical National Guard and active-duty Reserve missions are a way to show good faith in serving these soldiers because they may have untreated PTSD or other undiagnosed mental health disorders related to earlier deployments, such as hurricane recovery missions. A study of disaster survivors found that the prevalence rate of severe and very severe psychological impact after a natural disaster was about 34%.15 Another epidemiologic study found that the prevalence rate of PTSD was 10% to 20% among disaster rescue workers.16 Specific data about the psychological problems of National Guard and Reserve components serving in disaster recovery are unavailable but is an area for future research.
Therapists who have treated active-duty service members and veterans who worked in mortuary services in a combat zone are used to hearing graphic details of horrifying scenes, but homefront experiences are different. Soldiers on homefront mortuary-based missions frequently reported being unable to forget the faces or the smell of dead bodies as they were stacked up and overwhelming the systems. Experienced vet center therapists should be prepared for the challenges in treating this new cohort of patients.
Conclusions
Now that National Guard and Reserve component soldiers who have responded to national and local emergencies are eligible for therapy, we need to be prepared to provide these services. In addition to addressing systemic staffing concerns, therapists need to be aware of the unique challenges faced by those who have served on homefront missions. These homefront missions have the potential to hit home for therapists.
Recent natural disasters, civil disorder, and the COVID-19 pandemic response created an unprecedented demand for the US National Guard and Reserve components as well as active-duty personnel to serve on homefront missions critical to our nation. At times, those serving in these capacities are front and center to the most tragic events confronting our nation, and they frequently encounter tremendous suffering.
Recognizing the potential for these missions to create psychological sequela for those who serve on them, the authority for the Veterans Health Administration (VHA) vet centers to provide readjustment counseling services was broadened on December 30, 2021. Vet centers are community-based counseling centers that have traditionally served combat veterans, and broadening services reflects a major change in mission. Revised VHA Directive 1500(2) specifies that those who “served on active duty in response to a national emergency or major disaster declared by the President” or “served on active duty in the National Guard of a State under orders of the chief executive of that State in response to a disaster or civil disorder in such State” may now receive therapy at vet centers.1,2
As a result of this recent policy change, National Guard and active-duty Reserve service members now have parity with combat veterans to obtain therapy for symptoms arising as a result of their activation for service on homefront missions. As they seek care, we need to be ready so that these service members can obtain the best therapy services possible. Soldiers who served on homefront missions comprise a new cohort of service members now eligible for vet center therapy. Soldiers who served on homefront missions may present with issues that differ from those of combat veterans and veterans who have experienced military sexual trauma (MST), the populations treated by vet centers and other VHA mental health care clinics prior to this broadened authority. This article highlights some suggestions for service delivery to best meet the needs of this population.
Discussion
Available evidence-based therapies to treat posttraumatic stress disorder (PTSD) are effective regardless of whether the trauma occurred in combat, on the homefront, or in a civilian setting. The vet centers and VHA mental health services already have staff trained to deliver these therapy modalities and, in this sense, are ready to provide trauma-focused therapy treatment to soldiers with PTSD who served on homefront missions.
The broadened authority for the vet centers to provide readjustment services is necessary, as it corrects for a critical gap in services, but the importance of ensuring adequate staffing to meet the expected increased demand for services cannot be underscored. According to clinical practice guidelines for the treatment of PTSD, developed by the US Department of Veterans Affairs (VA) and the US Department of Defense (DoD), the therapies with the strongest evidence-based backing are prolonged exposure-based therapy (PE), cognitive processing therapy (CPT), and eye movement and desensitization reprocessing (EMDR).3 These therapy modalities, based on findings from clinical trials, are predicated on seeing a client for a sufficient number of sessions. Attendance at these sessions is recommended at least weekly to ensure adequate intensity of service delivery.4-7 According to the National Center for PTSD, PE typically involves 8 to 15 weekly or twice weekly sessions; CPT requires 8 to 14 or more weekly sessions, and EMDR is usually 4 to 12 weekly sessions.4-7
Ensuring adequate staffing is critical to offer these therapies at least weekly as the efficacies of these therapies are otherwise not proven if return session visits are stretched out over multiple weeks or months. The most recent clinical research has demonstrated that PTSD recovery can be expedited and there are lower patient dropout rates when sessions are massed or compressed so that multiple sessions are administered over 1 week.8-12 Providing these therapies in a massed format has shown to be as effective as when these therapies are provided weekly.
As the authority to treat soldiers serving on homefront missions is new, epidemiologic data do not yet exist to estimate the proportion of this population who will need treatment or present with PTSD, depression, anxiety, a substance use disorder, and/or comorbid conditions. Those with PTSD can benefit from PTSD evidence-based therapies already available for treatment. Others may benefit from treatments that are proven effective for their mental health diagnoses.
Therapists with experience primarily treating patients with PTSD related to combat or MST will need to be sensitive to the unique experiences of the National Guard and Reserve service members. For example, this component of soldiers served on COVID-19–related missions that provided food service support to nursing homes residents who were locked down from family members. As a result, they developed bonds with residents who later died. This may have been the first time that these soldiers witnessed death. If such a soldier is assessed and does not have PTSD but is nonetheless distressed, then the soldier may need alternate therapies, such as grief counseling. This need may be more pronounced for those soldiers who lost loved ones to COVID-19 while they served on these missions.
New Jersey Army National Guard soldiers provided food service support at the Woodland Behavioral and Nursing Center in Andover, New Jersey. These soldiers witnessed the unfortunate conditions in this facility, which included stacked bodies in a makeshift morgue during the height of the pandemic; however, they did not have the ability to make changes. The facility is under investigation for abuse and neglect of its residents.13
New Jersey National Guard soldiers supporting that facility and similar ones may have experienced moral injury, defined as “…perpetrating, failing to prevent, or bearing witness to acts that transgress deeply held moral beliefs and expectations.”14 Importantly, when these soldiers present for therapy and express moral injury, their therapists need to be open to spiritual discourse. However, vet centers do not have chaplains on staff, so therapists must refer patients to chaplaincy services.
Among therapists with existing cultural competency for treating members of the military, some nuances exist for National Guard and Reserve service members. National Guard and Reserve component personnel already may feel that their problems are less important than those experienced by active-duty service members. Now that these soldiers have the eligibility to receive therapy, therapists may have to make extra efforts to both reassure this population that they are welcomed and to validate their need for services.
Special outreach efforts to those who served on historical National Guard and active-duty Reserve missions are a way to show good faith in serving these soldiers because they may have untreated PTSD or other undiagnosed mental health disorders related to earlier deployments, such as hurricane recovery missions. A study of disaster survivors found that the prevalence rate of severe and very severe psychological impact after a natural disaster was about 34%.15 Another epidemiologic study found that the prevalence rate of PTSD was 10% to 20% among disaster rescue workers.16 Specific data about the psychological problems of National Guard and Reserve components serving in disaster recovery are unavailable but is an area for future research.
Therapists who have treated active-duty service members and veterans who worked in mortuary services in a combat zone are used to hearing graphic details of horrifying scenes, but homefront experiences are different. Soldiers on homefront mortuary-based missions frequently reported being unable to forget the faces or the smell of dead bodies as they were stacked up and overwhelming the systems. Experienced vet center therapists should be prepared for the challenges in treating this new cohort of patients.
Conclusions
Now that National Guard and Reserve component soldiers who have responded to national and local emergencies are eligible for therapy, we need to be prepared to provide these services. In addition to addressing systemic staffing concerns, therapists need to be aware of the unique challenges faced by those who have served on homefront missions. These homefront missions have the potential to hit home for therapists.
1. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1550(2): readjustment counseling service. January 26, 2021. Accessed September 1, 2022. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=9168
2. US Department of Veterans Affairs. Vet centers (readjustment counseling: vet center eligibility. Updated January 3, 2022. Accessed September 1, 2022. https://www.vetcenter.va.gov/eligibility.asp
3. US Department of Defense, US Department of Veterans Affairs. VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress reaction, version 3.0, 2017. Accessed September 1, 2022. https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGFinal012418.pdf
4. US Department of Veterans Affairs, National Center for PTSD. Prolonged exposure (PE) therapy. Updated August 10, 2022. Accessed September 1, 2022. https://www.ptsd.va.gov/understand_tx/prolonged_exposure.asp
5. US Department of Veterans Affairs, National Center for PTSD. Cognitive processing therapy (CPT) for PTSD: how to help your loved one during treatment. Accessed September 1, 2022. https://www.ptsd.va.gov/publications/print/CPT_familyhandout.pdf
6. US Department of Veterans Affairs, National Center for PTSD. A provider’s guide to brief cognitive behavioral therapy. Accessed September 1, 2022. https://www.mirecc.va.gov/visn16/docs/Therapists_Guide_to_Brief_CBTManual.pdf
7. US Department of Veterans Affairs, National Center for PTSD. Eye movement desensitization and reprocessing (EMDR) for PTSD. Accessed September 1, 2022. https://www.ptsd.va.gov/understand_tx/emdr.asp
8. Wachen JS, Dondanville KA, Evans WR, Morris K, Cole A. Adjusting the timeframe of evidence-based therapies for PTSD-massed treatments. Curr Treat Options Psych. 2019;6(2):107-118. doi:10.1007/s40501-019-00169-9
9. Dell L, Sbisa AM, Forbes A, et al. Effect of massed v. standard prolonged exposure therapy on PTSD in military personnel and veterans: a non-inferiority randomised controlled trial [published online ahead of print, 2022 Apr 20]. Psychol Med. 2022;1-8. doi:10.1017/S0033291722000927
10. Held P, Kovacevic M, Petrey K, et al. Treating posttraumatic stress disorder at home in a single week using 1-week virtual massed cognitive processing therapy. J Trauma Stress. 2022;35(4):1215-1225. doi:10.1002/jts.22831
11. Yamokoski C, Flores H, Facemire V, Maieritsch K, Perez S, Fedynich A. Feasibility of an intensive outpatient treatment program for posttraumatic stress disorder within the veterans health care administration [published online ahead of print, 2022 Mar 7]. Psychol Serv. 2022;10.1037/ser0000628. doi:10.1037/ser0000628
12. Galovski TE, Werner KB, Weaver TL, et al. Massed cognitive processing therapy for posttraumatic stress disorder in women survivors of intimate partner violence. Psychol Trauma. 2022;14(5):769-779. doi:10.1037/tra0001100
13. Fallon S. NJ to send monitors into troubled nursing home that stacked bodies in makeshift morgue. Updated March 10, 2022. Accessed September 1, 2022. https://www.northjersey.com/story/news/health/2022/03/09/sussex-county-nj-nursing-home-monitors-covid-morgue/9447243002/
14. Litz BT, Stein N, Delaney E, et al. Moral injury and moral repair in war veterans: a preliminary model and intervention strategy. Clin Psychol Rev. 2009;29(8):695-706. doi:10.1016/j.cpr.2009.07.003009
15. Norris FH, Friedman MJ, Watson PJ, Byrne CM, Diaz E, Kaniasty K. 60,000 disaster victims speak: Part I. An empirical review of the empirical literature, 1981-2001. Psychiatry. 2002;65(3):207-239. doi:10.1521/psyc.65.3.207.20173
16. Galea S, Nandi A, Vlahov D. The epidemiology of post-traumatic stress disorder after disasters. Epidemiol Rev. 2005;27:78-91. doi:10.1093/epirev/mxi003
1. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1550(2): readjustment counseling service. January 26, 2021. Accessed September 1, 2022. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=9168
2. US Department of Veterans Affairs. Vet centers (readjustment counseling: vet center eligibility. Updated January 3, 2022. Accessed September 1, 2022. https://www.vetcenter.va.gov/eligibility.asp
3. US Department of Defense, US Department of Veterans Affairs. VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress reaction, version 3.0, 2017. Accessed September 1, 2022. https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGFinal012418.pdf
4. US Department of Veterans Affairs, National Center for PTSD. Prolonged exposure (PE) therapy. Updated August 10, 2022. Accessed September 1, 2022. https://www.ptsd.va.gov/understand_tx/prolonged_exposure.asp
5. US Department of Veterans Affairs, National Center for PTSD. Cognitive processing therapy (CPT) for PTSD: how to help your loved one during treatment. Accessed September 1, 2022. https://www.ptsd.va.gov/publications/print/CPT_familyhandout.pdf
6. US Department of Veterans Affairs, National Center for PTSD. A provider’s guide to brief cognitive behavioral therapy. Accessed September 1, 2022. https://www.mirecc.va.gov/visn16/docs/Therapists_Guide_to_Brief_CBTManual.pdf
7. US Department of Veterans Affairs, National Center for PTSD. Eye movement desensitization and reprocessing (EMDR) for PTSD. Accessed September 1, 2022. https://www.ptsd.va.gov/understand_tx/emdr.asp
8. Wachen JS, Dondanville KA, Evans WR, Morris K, Cole A. Adjusting the timeframe of evidence-based therapies for PTSD-massed treatments. Curr Treat Options Psych. 2019;6(2):107-118. doi:10.1007/s40501-019-00169-9
9. Dell L, Sbisa AM, Forbes A, et al. Effect of massed v. standard prolonged exposure therapy on PTSD in military personnel and veterans: a non-inferiority randomised controlled trial [published online ahead of print, 2022 Apr 20]. Psychol Med. 2022;1-8. doi:10.1017/S0033291722000927
10. Held P, Kovacevic M, Petrey K, et al. Treating posttraumatic stress disorder at home in a single week using 1-week virtual massed cognitive processing therapy. J Trauma Stress. 2022;35(4):1215-1225. doi:10.1002/jts.22831
11. Yamokoski C, Flores H, Facemire V, Maieritsch K, Perez S, Fedynich A. Feasibility of an intensive outpatient treatment program for posttraumatic stress disorder within the veterans health care administration [published online ahead of print, 2022 Mar 7]. Psychol Serv. 2022;10.1037/ser0000628. doi:10.1037/ser0000628
12. Galovski TE, Werner KB, Weaver TL, et al. Massed cognitive processing therapy for posttraumatic stress disorder in women survivors of intimate partner violence. Psychol Trauma. 2022;14(5):769-779. doi:10.1037/tra0001100
13. Fallon S. NJ to send monitors into troubled nursing home that stacked bodies in makeshift morgue. Updated March 10, 2022. Accessed September 1, 2022. https://www.northjersey.com/story/news/health/2022/03/09/sussex-county-nj-nursing-home-monitors-covid-morgue/9447243002/
14. Litz BT, Stein N, Delaney E, et al. Moral injury and moral repair in war veterans: a preliminary model and intervention strategy. Clin Psychol Rev. 2009;29(8):695-706. doi:10.1016/j.cpr.2009.07.003009
15. Norris FH, Friedman MJ, Watson PJ, Byrne CM, Diaz E, Kaniasty K. 60,000 disaster victims speak: Part I. An empirical review of the empirical literature, 1981-2001. Psychiatry. 2002;65(3):207-239. doi:10.1521/psyc.65.3.207.20173
16. Galea S, Nandi A, Vlahov D. The epidemiology of post-traumatic stress disorder after disasters. Epidemiol Rev. 2005;27:78-91. doi:10.1093/epirev/mxi003
A Veteran Presenting for Low Testosterone and Lower Urinary Tract Symptoms
►Anish Bhatnagar, MD, Chief Medical Resident, Veterans Affairs Boston Healthcare System (VABHS) and Beth Israel Deaconess Medical Center (BIDMC): The patient noted erectile dysfunction starting 4 years ago, with accompanied decreased libido. However, until recently, he was able to achieve acceptable erectile capacity with medications. As part of his previous evaluations for erectile dysfunction, the patient had 2 total testosterone levels checked 6 months apart, both low at 150 ng/dL and 38.3 ng/dL (reference range, 220-892). The results of additional hormone studies are shown in the Table. Dr. Ananthakrishnan, can you help us interpret these laboratory results and tell us what tests you might order next?
►Sonia Ananthakrishnan, MD, Section of Endocrinology, Diabetes and Nutrition, Boston Medical Center (BMC) and Assistant Professor of Medicine, Boston University School of Medicine (BUSM): When patients present with signs of hypogonadism and an initial low morning testosterone levels, the next test should be a confirmatory repeat morning testosterone level as was done in this case. If this level is also low (for most assays < 300 ng/dL), further evaluation for primary vs secondary hypogonadism should be pursued with measurement of luteinizing hormone and follicle-stimulating hormone levels. Secondary hypogonadism should be suspected when these levels are low or inappropriately normal in the setting of a low testosterone level as in this patient. This patient does not appear to be on any medication or have reversible illnesses that we traditionally think of as possibly causing these hormone irregularities. Key examples include medications such as gonadotropin-releasing hormone analogs, glucocorticoids, and opioids, as well as conditions such as hyperprolactinemia, sleep apnea, diabetes mellitus, anorexia nervosa, or other chronic systemic illnesses, including cirrhosis or lung disease. In this setting, further evaluation of the patient’s anterior pituitary function should be undertaken. Initial screening tests showed mildly elevated prolactin and low normal thyroid-stimulating hormone levels, with a relatively normal free thyroxine. Given these abnormalities in the context of the patient’s total testosterone level < 150 ng/dL, magnetic resonance imaging (MRI) of the anterior pituitary is indicated, and what I would recommend next for evaluation of pituitary and/or hypothalamic tumor or infiltrative disease.1
►Dr. Bhatnagar: An MRI of the brain showed a large 2.7-cm sellar mass, with suprasellar extension and mass effect on the optic chiasm and pituitary infundibulum, partial extension into the right sphenoid sinus, and invasion into the right cavernous sinus. These findings were consistent with a pituitary macroadenoma. The patient was subsequently evaluated by a neurosurgeon who felt that because of the extension and compression of the mass, the patient would benefit from surgical resection.
Given his lower urinary tract symptoms, a prostate-specific antigen level was checked and returned elevated at 11.5 ng/mL. In the setting of these abnormalities, the patient underwent MRI of the abdomen, which noted a new 5.6-cm enhancing mass in the upper pole of his solitary right kidney, highly concerning for new RCC. After a multidisciplinary discussion, urology scheduled the patient for partial right nephrectomy first, with plans for pituitary resection only if the patient had adequate recovery following the urologic procedure.
Dr. Rifkin, this patient went straight from imaging to presumed diagnosis to planned surgical intervention without a confirmatory biopsy. In a patient who already has chronic kidney disease stage 4, why would we not want to pursue biopsy prior to this invasive procedure on his solitary kidney? In addition, given his baseline advanced renal disease, why pursue partial nephrectomy to delay initiation of hemodialysis instead of total nephrectomy and beginning hemodialysis?
►Ian Rifkin, MBBCh, PhD, MSc, Chief, Renal Section, VABHS, Section of Nephrology, BMC, and Associate Professor of Medicine, BUSM: In most cases, imaging alone is used to make a presumptive diagnosis of benign vs malignant renal masses. In one study, RCC was identified by MRI with 85% sensitivity and 76% specificity.2 However, as imaging and biopsy techniques have advanced, there are progressing discussions regarding the utility of biopsy. That being said, there are a number of situations in which patients currently undergo biopsy, particularly when there is diagnostic uncertainty.3 In this patient, with a history of RCC and imaging findings concerning for RCC, biopsy is unnecessary given the high clinical suspicion.
Regarding the choice of partial vs total nephrectomy, there are 2 important distinctions to be made. The first is that though it was previously felt that early initiation of dialysis improves survival, newer studies suggest that early initiation based off of glomerular filtration rate (GFR) offers no survival benefits compared to delayed initiation.4 Second, though there is less clinical data to support this, there is a signal toward the use of partial nephrectomy decreasing mortality compared to radical nephrectomy in management of RCC.5 In this patient, partial nephrectomy may not only increase rates of survival, but also delay initiation of dialysis.
►Dr. Bhatnagar: Prior to undergoing partial right nephrectomy, a morning cortisol level was found to be 5.8 μg/dL with an associated corticotropin (ACTH) level of 26 pg/mL. Dr. Ananthakrishnan, how would you interpret these laboratory results and what might you recommend prior to surgery?
►Dr. Ananthakrishnan: In a healthy patient, surgery often results in a several-fold increase in the secretion of cortisol to balance the unique stressors surgery places on the body.6 This patient is at increased risk for complete or partial adrenal insufficiency in the setting of both his pituitary macroadenoma as well as his previous left nephrectomy, which could have affected his left adrenal gland as well. Thus, this patient may not be able to mount the appropriate cortisol response needed to counter the stresses of surgery. His cortisol level is abnormally low for a morning value, with a relatively normal ACTH reference range of 6 to 50 pg/mL. He may have some degree of adrenal insufficiency, and thus will benefit from perioperative steroids.
►Dr. Bhatnagar: The patient was started on hydrocortisone and underwent a successful laparoscopic partial right nephrectomy. During the procedure, an estimated 2.5 L of blood was lost, with transfusion of 3 units of packed red blood cells. A surgical drain was left in the peritoneum. Postoperatively, he developed hypotension, requiring vasopressors and prolonged continuation of stress dosing of hydrocortisone. Over the next 4 days, the patient was weaned off vasopressors, and his creatinine level was noted to increase from a baseline of 1.8 mg/dL to 4.4 mg/dL.
Dr. Rifkin, how do you think about renal recovery in the patient postnephrectomy, and should we be concerned with the dramatic rise in his creatinine level?
►Dr. Rifkin: Removal of renal mass will result in an initial reduction of GFR proportional to the amount of functional renal tissue removed. However, in as early as 1 week, the residual nephrons begin to compensate through various mechanisms, such as modulation of efferent and afferent arterioles and renal tissue growth by hypertrophy and hyperplasia.7 In the acute setting, it may be difficult to distinguish an acute renal injury vs physiological GFR reduction postnephron loss, but often the initially elevated creatinine level may normalize/stabilize over time. Other markers of kidney function should concomitantly be monitored, including urine output, electrolyte/acid-base status, and urine sediment examination. In this patient, although his creatinine level may be elevated over the first few days, if his urine output remains robust and the urine sediment examination is normal, my concern for permanent kidney injury would be lessened.
►Dr. Bhatnagar: During the first 4 postoperative days the patient produced approximately 1 L of urine per day with a stable creatinine level. It is over this same time that the hydrocortisone was discontinued given improving hemodynamics. However, throughout postoperative day 5, the patient’s creatinine level acutely rose to a peak of 5.8 mg/dL. In addition, his urine output dramatically dropped to < 5 mL per hour, with blood clots noted in his Foley catheter. Dr. Rifkin, what is your differential for causing this acute change in both his creatinine level and urine output this far out from his procedure, and what might you do to help further evaluate?
►Dr. Rifkin: The most common cause of acute kidney injury in hospitalized patients is acute tubular necrosis (ATN).8 However, in this patient, who was recovering well postoperatively, was hemodynamically stable with a robust urine output, and in whom no apparent cause for ATN could be identified, other diagnoses were more likely. Considering the abrupt onset of oligo-anuria, the most likely diagnosis was urinary tract obstruction, particularly given the frank blood and blood clots that were present in the urine. Additional possibilities might be a late surgical complication or infection. Surgical complications could range from direct damage to the renal parenchyma to urinary leakage into the peritoneum from the site of anastomosis or tissue injury. Infections introduced either intraoperatively or developed postoperatively could also cause this sudden drop in urine output, though one would expect more systemic symptoms with this. Given that this patient has a surgical drain in place in the peritoneum, I would recommend testing the creatinine level in the peritoneal fluid drainage. If it is comparable to serum levels, this would argue against a urine leak, as we would expect the level to be significantly elevated in a leak. In addition, he should have imaging of the urinary tract followed by procedures to decompress the presumed obstructed urinary tract. These procedures might include either cystoscopy with ureteral stent placement or percutaneous nephrostomy, depending on the result of the imaging.
►Dr. Bhatnagar: The creatinine level obtained from the surgical drain was roughly equivalent to the serum creatinine, decreasing suspicion for a urine leak as the cause of his findings. Cystoscopy with ureteral stent placement was performed with subsequent increase in both urine output and concomitant decrease in serum creatinine.
Around this time, the patient also began to note blurry vision. Evaluation revealed difficulty with visual field confrontation in the right lower quadrant, right eye ptosis, right eye impaired adduction, absent abduction and impaired upgaze, but intact downgaze. Diplopia was present with gaze in all directions. His constellation of physical examination findings were concerning for a pathologic lesion partially involving cranial nerves II and III, with definitive involvement of cranial nerve VI, but sparing of cranial nerve IV. Repeat MRI of the brain showed hemorrhage into the sellar mass, with ongoing mass effect on the optic chiasm and extension into the sinuses (eAppendix). These findings were consistent with pituitary apoplexy. Dr. Ananthakrishnan, can you tell us more about pituitary apoplexy?
►Dr. Ananthakrishnan: Pituitary apoplexy is a clinical syndrome resulting from acute hemorrhage or infarction of the pituitary gland. It typically occurs in patients with preexisting pituitary adenomas and is characterized by the onset of headache, fever, vomiting, meningismus, decreased consciousness, and sometimes death. In addition, given the location of the pituitary gland within the sella, rapid changes in size can result in compression of cranial nerves III, IV, and VI, as well as the optic chiasm, resulting in ophthalmoplegia and visual disturbances as seen in this patient.9
There are a multitude of causes of pituitary apoplexy, including alterations in coagulopathy, pituitary stimulation (eg, dynamic pituitary hormone testing), and both acute increases and decreases in blood flow.10 This patient likely had an ischemic event due to changes in vascular perfusion, spurred by both his blood loss intraoperatively and ongoing hematuria. Management of pituitary apoplexy is dependent on the patient’s hemodynamics, mass effect symptoms, electrolyte balances, and hormone dysfunction. The decision for conservative management vs surgical intervention should be made in consultation with both neurosurgery and endocrinology. Once the patient is hemodynamically stable, the next step in evaluating this patient should be repeating his hormone studies.
►Dr. Bhatnagar: An assessment of pituitary function was consistent with values obtained preoperatively. After multidisciplinary discussions, surgery was deferred, and hydrocortisone was reinitiated to reduce inflammation caused by bleeding into the mass. As the ophthalmoplegia improved, this was transitioned to dexamethasone.
Twelve days after admission, he was discharged to a subacute rehabilitation center, with improvement in his ophthalmoplegia and stabilization of his creatinine level and urine output.
1. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536-2559. doi:10.1210/jc.2009-2354
2. Kay FU, Canvasser NE, Xi Y, et al. Diagnostic performance and interreader agreement of a standardized MR imaging approach in the prediction of small renal mass histology. Radiology. 2018;287(2):543-553. doi:10.1148/radiol.2018171557
3. Sahni VA, Silverman SG. Biopsy of renal masses: when and why. Cancer Imaging. 2009;9(1):44-55. doi:10.1102/1470-7330.2009.0005
4. Cooper BA, Branley P, Bulfone L, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363(7):609-619. doi:10.1056/NEJMoa1000552
5. Kunath F, Schmidt S, Krabbe L-M, et al. Partial nephrectomy versus radical nephrectomy for clinical localised renal masses. Cochrane Database Syst Rev. 2017;5(5):CD012045. doi:10.1002/14651858.CD012045.pub2
6. Kehlet H, Binder C. Adrenocortical function and clinical course during and after surgery in unsupplemented glucocorticoid-treated patients. Br J Anaesth. 1973;45(10):1043-1048. doi:10.1093/bja/45.10.1043
7. Chapman D, Moore R, Klarenbach S, Braam B. Residual renal function after partial or radical nephrectomy for renal cell carcinoma. Can Urol Assoc J. 2010;4(5):337-343. doi:10.5489/cuaj.909
8. Rahman M, Shad F, Smith MC. Acute kidney injury: a guide to diagnosis and management. Am Fam Physician. 2012;86(7):631-639.
9. Ranabir S, Baruah MP. Pituitary apoplexy. Indian J Endocrinol Metab. 2011;15(suppl 3):S188-S196. doi:10.4103/2230-8210.84862
10. Glezer A, Bronstein MD. Pituitary apoplexy: pathophysiology, diagnosis and management. Arch Endocrinol Metab. 2015;59(3):259-264. doi:10.1590/2359-3997000000047
►Anish Bhatnagar, MD, Chief Medical Resident, Veterans Affairs Boston Healthcare System (VABHS) and Beth Israel Deaconess Medical Center (BIDMC): The patient noted erectile dysfunction starting 4 years ago, with accompanied decreased libido. However, until recently, he was able to achieve acceptable erectile capacity with medications. As part of his previous evaluations for erectile dysfunction, the patient had 2 total testosterone levels checked 6 months apart, both low at 150 ng/dL and 38.3 ng/dL (reference range, 220-892). The results of additional hormone studies are shown in the Table. Dr. Ananthakrishnan, can you help us interpret these laboratory results and tell us what tests you might order next?
►Sonia Ananthakrishnan, MD, Section of Endocrinology, Diabetes and Nutrition, Boston Medical Center (BMC) and Assistant Professor of Medicine, Boston University School of Medicine (BUSM): When patients present with signs of hypogonadism and an initial low morning testosterone levels, the next test should be a confirmatory repeat morning testosterone level as was done in this case. If this level is also low (for most assays < 300 ng/dL), further evaluation for primary vs secondary hypogonadism should be pursued with measurement of luteinizing hormone and follicle-stimulating hormone levels. Secondary hypogonadism should be suspected when these levels are low or inappropriately normal in the setting of a low testosterone level as in this patient. This patient does not appear to be on any medication or have reversible illnesses that we traditionally think of as possibly causing these hormone irregularities. Key examples include medications such as gonadotropin-releasing hormone analogs, glucocorticoids, and opioids, as well as conditions such as hyperprolactinemia, sleep apnea, diabetes mellitus, anorexia nervosa, or other chronic systemic illnesses, including cirrhosis or lung disease. In this setting, further evaluation of the patient’s anterior pituitary function should be undertaken. Initial screening tests showed mildly elevated prolactin and low normal thyroid-stimulating hormone levels, with a relatively normal free thyroxine. Given these abnormalities in the context of the patient’s total testosterone level < 150 ng/dL, magnetic resonance imaging (MRI) of the anterior pituitary is indicated, and what I would recommend next for evaluation of pituitary and/or hypothalamic tumor or infiltrative disease.1
►Dr. Bhatnagar: An MRI of the brain showed a large 2.7-cm sellar mass, with suprasellar extension and mass effect on the optic chiasm and pituitary infundibulum, partial extension into the right sphenoid sinus, and invasion into the right cavernous sinus. These findings were consistent with a pituitary macroadenoma. The patient was subsequently evaluated by a neurosurgeon who felt that because of the extension and compression of the mass, the patient would benefit from surgical resection.
Given his lower urinary tract symptoms, a prostate-specific antigen level was checked and returned elevated at 11.5 ng/mL. In the setting of these abnormalities, the patient underwent MRI of the abdomen, which noted a new 5.6-cm enhancing mass in the upper pole of his solitary right kidney, highly concerning for new RCC. After a multidisciplinary discussion, urology scheduled the patient for partial right nephrectomy first, with plans for pituitary resection only if the patient had adequate recovery following the urologic procedure.
Dr. Rifkin, this patient went straight from imaging to presumed diagnosis to planned surgical intervention without a confirmatory biopsy. In a patient who already has chronic kidney disease stage 4, why would we not want to pursue biopsy prior to this invasive procedure on his solitary kidney? In addition, given his baseline advanced renal disease, why pursue partial nephrectomy to delay initiation of hemodialysis instead of total nephrectomy and beginning hemodialysis?
►Ian Rifkin, MBBCh, PhD, MSc, Chief, Renal Section, VABHS, Section of Nephrology, BMC, and Associate Professor of Medicine, BUSM: In most cases, imaging alone is used to make a presumptive diagnosis of benign vs malignant renal masses. In one study, RCC was identified by MRI with 85% sensitivity and 76% specificity.2 However, as imaging and biopsy techniques have advanced, there are progressing discussions regarding the utility of biopsy. That being said, there are a number of situations in which patients currently undergo biopsy, particularly when there is diagnostic uncertainty.3 In this patient, with a history of RCC and imaging findings concerning for RCC, biopsy is unnecessary given the high clinical suspicion.
Regarding the choice of partial vs total nephrectomy, there are 2 important distinctions to be made. The first is that though it was previously felt that early initiation of dialysis improves survival, newer studies suggest that early initiation based off of glomerular filtration rate (GFR) offers no survival benefits compared to delayed initiation.4 Second, though there is less clinical data to support this, there is a signal toward the use of partial nephrectomy decreasing mortality compared to radical nephrectomy in management of RCC.5 In this patient, partial nephrectomy may not only increase rates of survival, but also delay initiation of dialysis.
►Dr. Bhatnagar: Prior to undergoing partial right nephrectomy, a morning cortisol level was found to be 5.8 μg/dL with an associated corticotropin (ACTH) level of 26 pg/mL. Dr. Ananthakrishnan, how would you interpret these laboratory results and what might you recommend prior to surgery?
►Dr. Ananthakrishnan: In a healthy patient, surgery often results in a several-fold increase in the secretion of cortisol to balance the unique stressors surgery places on the body.6 This patient is at increased risk for complete or partial adrenal insufficiency in the setting of both his pituitary macroadenoma as well as his previous left nephrectomy, which could have affected his left adrenal gland as well. Thus, this patient may not be able to mount the appropriate cortisol response needed to counter the stresses of surgery. His cortisol level is abnormally low for a morning value, with a relatively normal ACTH reference range of 6 to 50 pg/mL. He may have some degree of adrenal insufficiency, and thus will benefit from perioperative steroids.
►Dr. Bhatnagar: The patient was started on hydrocortisone and underwent a successful laparoscopic partial right nephrectomy. During the procedure, an estimated 2.5 L of blood was lost, with transfusion of 3 units of packed red blood cells. A surgical drain was left in the peritoneum. Postoperatively, he developed hypotension, requiring vasopressors and prolonged continuation of stress dosing of hydrocortisone. Over the next 4 days, the patient was weaned off vasopressors, and his creatinine level was noted to increase from a baseline of 1.8 mg/dL to 4.4 mg/dL.
Dr. Rifkin, how do you think about renal recovery in the patient postnephrectomy, and should we be concerned with the dramatic rise in his creatinine level?
►Dr. Rifkin: Removal of renal mass will result in an initial reduction of GFR proportional to the amount of functional renal tissue removed. However, in as early as 1 week, the residual nephrons begin to compensate through various mechanisms, such as modulation of efferent and afferent arterioles and renal tissue growth by hypertrophy and hyperplasia.7 In the acute setting, it may be difficult to distinguish an acute renal injury vs physiological GFR reduction postnephron loss, but often the initially elevated creatinine level may normalize/stabilize over time. Other markers of kidney function should concomitantly be monitored, including urine output, electrolyte/acid-base status, and urine sediment examination. In this patient, although his creatinine level may be elevated over the first few days, if his urine output remains robust and the urine sediment examination is normal, my concern for permanent kidney injury would be lessened.
►Dr. Bhatnagar: During the first 4 postoperative days the patient produced approximately 1 L of urine per day with a stable creatinine level. It is over this same time that the hydrocortisone was discontinued given improving hemodynamics. However, throughout postoperative day 5, the patient’s creatinine level acutely rose to a peak of 5.8 mg/dL. In addition, his urine output dramatically dropped to < 5 mL per hour, with blood clots noted in his Foley catheter. Dr. Rifkin, what is your differential for causing this acute change in both his creatinine level and urine output this far out from his procedure, and what might you do to help further evaluate?
►Dr. Rifkin: The most common cause of acute kidney injury in hospitalized patients is acute tubular necrosis (ATN).8 However, in this patient, who was recovering well postoperatively, was hemodynamically stable with a robust urine output, and in whom no apparent cause for ATN could be identified, other diagnoses were more likely. Considering the abrupt onset of oligo-anuria, the most likely diagnosis was urinary tract obstruction, particularly given the frank blood and blood clots that were present in the urine. Additional possibilities might be a late surgical complication or infection. Surgical complications could range from direct damage to the renal parenchyma to urinary leakage into the peritoneum from the site of anastomosis or tissue injury. Infections introduced either intraoperatively or developed postoperatively could also cause this sudden drop in urine output, though one would expect more systemic symptoms with this. Given that this patient has a surgical drain in place in the peritoneum, I would recommend testing the creatinine level in the peritoneal fluid drainage. If it is comparable to serum levels, this would argue against a urine leak, as we would expect the level to be significantly elevated in a leak. In addition, he should have imaging of the urinary tract followed by procedures to decompress the presumed obstructed urinary tract. These procedures might include either cystoscopy with ureteral stent placement or percutaneous nephrostomy, depending on the result of the imaging.
►Dr. Bhatnagar: The creatinine level obtained from the surgical drain was roughly equivalent to the serum creatinine, decreasing suspicion for a urine leak as the cause of his findings. Cystoscopy with ureteral stent placement was performed with subsequent increase in both urine output and concomitant decrease in serum creatinine.
Around this time, the patient also began to note blurry vision. Evaluation revealed difficulty with visual field confrontation in the right lower quadrant, right eye ptosis, right eye impaired adduction, absent abduction and impaired upgaze, but intact downgaze. Diplopia was present with gaze in all directions. His constellation of physical examination findings were concerning for a pathologic lesion partially involving cranial nerves II and III, with definitive involvement of cranial nerve VI, but sparing of cranial nerve IV. Repeat MRI of the brain showed hemorrhage into the sellar mass, with ongoing mass effect on the optic chiasm and extension into the sinuses (eAppendix). These findings were consistent with pituitary apoplexy. Dr. Ananthakrishnan, can you tell us more about pituitary apoplexy?
►Dr. Ananthakrishnan: Pituitary apoplexy is a clinical syndrome resulting from acute hemorrhage or infarction of the pituitary gland. It typically occurs in patients with preexisting pituitary adenomas and is characterized by the onset of headache, fever, vomiting, meningismus, decreased consciousness, and sometimes death. In addition, given the location of the pituitary gland within the sella, rapid changes in size can result in compression of cranial nerves III, IV, and VI, as well as the optic chiasm, resulting in ophthalmoplegia and visual disturbances as seen in this patient.9
There are a multitude of causes of pituitary apoplexy, including alterations in coagulopathy, pituitary stimulation (eg, dynamic pituitary hormone testing), and both acute increases and decreases in blood flow.10 This patient likely had an ischemic event due to changes in vascular perfusion, spurred by both his blood loss intraoperatively and ongoing hematuria. Management of pituitary apoplexy is dependent on the patient’s hemodynamics, mass effect symptoms, electrolyte balances, and hormone dysfunction. The decision for conservative management vs surgical intervention should be made in consultation with both neurosurgery and endocrinology. Once the patient is hemodynamically stable, the next step in evaluating this patient should be repeating his hormone studies.
►Dr. Bhatnagar: An assessment of pituitary function was consistent with values obtained preoperatively. After multidisciplinary discussions, surgery was deferred, and hydrocortisone was reinitiated to reduce inflammation caused by bleeding into the mass. As the ophthalmoplegia improved, this was transitioned to dexamethasone.
Twelve days after admission, he was discharged to a subacute rehabilitation center, with improvement in his ophthalmoplegia and stabilization of his creatinine level and urine output.
►Anish Bhatnagar, MD, Chief Medical Resident, Veterans Affairs Boston Healthcare System (VABHS) and Beth Israel Deaconess Medical Center (BIDMC): The patient noted erectile dysfunction starting 4 years ago, with accompanied decreased libido. However, until recently, he was able to achieve acceptable erectile capacity with medications. As part of his previous evaluations for erectile dysfunction, the patient had 2 total testosterone levels checked 6 months apart, both low at 150 ng/dL and 38.3 ng/dL (reference range, 220-892). The results of additional hormone studies are shown in the Table. Dr. Ananthakrishnan, can you help us interpret these laboratory results and tell us what tests you might order next?
►Sonia Ananthakrishnan, MD, Section of Endocrinology, Diabetes and Nutrition, Boston Medical Center (BMC) and Assistant Professor of Medicine, Boston University School of Medicine (BUSM): When patients present with signs of hypogonadism and an initial low morning testosterone levels, the next test should be a confirmatory repeat morning testosterone level as was done in this case. If this level is also low (for most assays < 300 ng/dL), further evaluation for primary vs secondary hypogonadism should be pursued with measurement of luteinizing hormone and follicle-stimulating hormone levels. Secondary hypogonadism should be suspected when these levels are low or inappropriately normal in the setting of a low testosterone level as in this patient. This patient does not appear to be on any medication or have reversible illnesses that we traditionally think of as possibly causing these hormone irregularities. Key examples include medications such as gonadotropin-releasing hormone analogs, glucocorticoids, and opioids, as well as conditions such as hyperprolactinemia, sleep apnea, diabetes mellitus, anorexia nervosa, or other chronic systemic illnesses, including cirrhosis or lung disease. In this setting, further evaluation of the patient’s anterior pituitary function should be undertaken. Initial screening tests showed mildly elevated prolactin and low normal thyroid-stimulating hormone levels, with a relatively normal free thyroxine. Given these abnormalities in the context of the patient’s total testosterone level < 150 ng/dL, magnetic resonance imaging (MRI) of the anterior pituitary is indicated, and what I would recommend next for evaluation of pituitary and/or hypothalamic tumor or infiltrative disease.1
►Dr. Bhatnagar: An MRI of the brain showed a large 2.7-cm sellar mass, with suprasellar extension and mass effect on the optic chiasm and pituitary infundibulum, partial extension into the right sphenoid sinus, and invasion into the right cavernous sinus. These findings were consistent with a pituitary macroadenoma. The patient was subsequently evaluated by a neurosurgeon who felt that because of the extension and compression of the mass, the patient would benefit from surgical resection.
Given his lower urinary tract symptoms, a prostate-specific antigen level was checked and returned elevated at 11.5 ng/mL. In the setting of these abnormalities, the patient underwent MRI of the abdomen, which noted a new 5.6-cm enhancing mass in the upper pole of his solitary right kidney, highly concerning for new RCC. After a multidisciplinary discussion, urology scheduled the patient for partial right nephrectomy first, with plans for pituitary resection only if the patient had adequate recovery following the urologic procedure.
Dr. Rifkin, this patient went straight from imaging to presumed diagnosis to planned surgical intervention without a confirmatory biopsy. In a patient who already has chronic kidney disease stage 4, why would we not want to pursue biopsy prior to this invasive procedure on his solitary kidney? In addition, given his baseline advanced renal disease, why pursue partial nephrectomy to delay initiation of hemodialysis instead of total nephrectomy and beginning hemodialysis?
►Ian Rifkin, MBBCh, PhD, MSc, Chief, Renal Section, VABHS, Section of Nephrology, BMC, and Associate Professor of Medicine, BUSM: In most cases, imaging alone is used to make a presumptive diagnosis of benign vs malignant renal masses. In one study, RCC was identified by MRI with 85% sensitivity and 76% specificity.2 However, as imaging and biopsy techniques have advanced, there are progressing discussions regarding the utility of biopsy. That being said, there are a number of situations in which patients currently undergo biopsy, particularly when there is diagnostic uncertainty.3 In this patient, with a history of RCC and imaging findings concerning for RCC, biopsy is unnecessary given the high clinical suspicion.
Regarding the choice of partial vs total nephrectomy, there are 2 important distinctions to be made. The first is that though it was previously felt that early initiation of dialysis improves survival, newer studies suggest that early initiation based off of glomerular filtration rate (GFR) offers no survival benefits compared to delayed initiation.4 Second, though there is less clinical data to support this, there is a signal toward the use of partial nephrectomy decreasing mortality compared to radical nephrectomy in management of RCC.5 In this patient, partial nephrectomy may not only increase rates of survival, but also delay initiation of dialysis.
►Dr. Bhatnagar: Prior to undergoing partial right nephrectomy, a morning cortisol level was found to be 5.8 μg/dL with an associated corticotropin (ACTH) level of 26 pg/mL. Dr. Ananthakrishnan, how would you interpret these laboratory results and what might you recommend prior to surgery?
►Dr. Ananthakrishnan: In a healthy patient, surgery often results in a several-fold increase in the secretion of cortisol to balance the unique stressors surgery places on the body.6 This patient is at increased risk for complete or partial adrenal insufficiency in the setting of both his pituitary macroadenoma as well as his previous left nephrectomy, which could have affected his left adrenal gland as well. Thus, this patient may not be able to mount the appropriate cortisol response needed to counter the stresses of surgery. His cortisol level is abnormally low for a morning value, with a relatively normal ACTH reference range of 6 to 50 pg/mL. He may have some degree of adrenal insufficiency, and thus will benefit from perioperative steroids.
►Dr. Bhatnagar: The patient was started on hydrocortisone and underwent a successful laparoscopic partial right nephrectomy. During the procedure, an estimated 2.5 L of blood was lost, with transfusion of 3 units of packed red blood cells. A surgical drain was left in the peritoneum. Postoperatively, he developed hypotension, requiring vasopressors and prolonged continuation of stress dosing of hydrocortisone. Over the next 4 days, the patient was weaned off vasopressors, and his creatinine level was noted to increase from a baseline of 1.8 mg/dL to 4.4 mg/dL.
Dr. Rifkin, how do you think about renal recovery in the patient postnephrectomy, and should we be concerned with the dramatic rise in his creatinine level?
►Dr. Rifkin: Removal of renal mass will result in an initial reduction of GFR proportional to the amount of functional renal tissue removed. However, in as early as 1 week, the residual nephrons begin to compensate through various mechanisms, such as modulation of efferent and afferent arterioles and renal tissue growth by hypertrophy and hyperplasia.7 In the acute setting, it may be difficult to distinguish an acute renal injury vs physiological GFR reduction postnephron loss, but often the initially elevated creatinine level may normalize/stabilize over time. Other markers of kidney function should concomitantly be monitored, including urine output, electrolyte/acid-base status, and urine sediment examination. In this patient, although his creatinine level may be elevated over the first few days, if his urine output remains robust and the urine sediment examination is normal, my concern for permanent kidney injury would be lessened.
►Dr. Bhatnagar: During the first 4 postoperative days the patient produced approximately 1 L of urine per day with a stable creatinine level. It is over this same time that the hydrocortisone was discontinued given improving hemodynamics. However, throughout postoperative day 5, the patient’s creatinine level acutely rose to a peak of 5.8 mg/dL. In addition, his urine output dramatically dropped to < 5 mL per hour, with blood clots noted in his Foley catheter. Dr. Rifkin, what is your differential for causing this acute change in both his creatinine level and urine output this far out from his procedure, and what might you do to help further evaluate?
►Dr. Rifkin: The most common cause of acute kidney injury in hospitalized patients is acute tubular necrosis (ATN).8 However, in this patient, who was recovering well postoperatively, was hemodynamically stable with a robust urine output, and in whom no apparent cause for ATN could be identified, other diagnoses were more likely. Considering the abrupt onset of oligo-anuria, the most likely diagnosis was urinary tract obstruction, particularly given the frank blood and blood clots that were present in the urine. Additional possibilities might be a late surgical complication or infection. Surgical complications could range from direct damage to the renal parenchyma to urinary leakage into the peritoneum from the site of anastomosis or tissue injury. Infections introduced either intraoperatively or developed postoperatively could also cause this sudden drop in urine output, though one would expect more systemic symptoms with this. Given that this patient has a surgical drain in place in the peritoneum, I would recommend testing the creatinine level in the peritoneal fluid drainage. If it is comparable to serum levels, this would argue against a urine leak, as we would expect the level to be significantly elevated in a leak. In addition, he should have imaging of the urinary tract followed by procedures to decompress the presumed obstructed urinary tract. These procedures might include either cystoscopy with ureteral stent placement or percutaneous nephrostomy, depending on the result of the imaging.
►Dr. Bhatnagar: The creatinine level obtained from the surgical drain was roughly equivalent to the serum creatinine, decreasing suspicion for a urine leak as the cause of his findings. Cystoscopy with ureteral stent placement was performed with subsequent increase in both urine output and concomitant decrease in serum creatinine.
Around this time, the patient also began to note blurry vision. Evaluation revealed difficulty with visual field confrontation in the right lower quadrant, right eye ptosis, right eye impaired adduction, absent abduction and impaired upgaze, but intact downgaze. Diplopia was present with gaze in all directions. His constellation of physical examination findings were concerning for a pathologic lesion partially involving cranial nerves II and III, with definitive involvement of cranial nerve VI, but sparing of cranial nerve IV. Repeat MRI of the brain showed hemorrhage into the sellar mass, with ongoing mass effect on the optic chiasm and extension into the sinuses (eAppendix). These findings were consistent with pituitary apoplexy. Dr. Ananthakrishnan, can you tell us more about pituitary apoplexy?
►Dr. Ananthakrishnan: Pituitary apoplexy is a clinical syndrome resulting from acute hemorrhage or infarction of the pituitary gland. It typically occurs in patients with preexisting pituitary adenomas and is characterized by the onset of headache, fever, vomiting, meningismus, decreased consciousness, and sometimes death. In addition, given the location of the pituitary gland within the sella, rapid changes in size can result in compression of cranial nerves III, IV, and VI, as well as the optic chiasm, resulting in ophthalmoplegia and visual disturbances as seen in this patient.9
There are a multitude of causes of pituitary apoplexy, including alterations in coagulopathy, pituitary stimulation (eg, dynamic pituitary hormone testing), and both acute increases and decreases in blood flow.10 This patient likely had an ischemic event due to changes in vascular perfusion, spurred by both his blood loss intraoperatively and ongoing hematuria. Management of pituitary apoplexy is dependent on the patient’s hemodynamics, mass effect symptoms, electrolyte balances, and hormone dysfunction. The decision for conservative management vs surgical intervention should be made in consultation with both neurosurgery and endocrinology. Once the patient is hemodynamically stable, the next step in evaluating this patient should be repeating his hormone studies.
►Dr. Bhatnagar: An assessment of pituitary function was consistent with values obtained preoperatively. After multidisciplinary discussions, surgery was deferred, and hydrocortisone was reinitiated to reduce inflammation caused by bleeding into the mass. As the ophthalmoplegia improved, this was transitioned to dexamethasone.
Twelve days after admission, he was discharged to a subacute rehabilitation center, with improvement in his ophthalmoplegia and stabilization of his creatinine level and urine output.
1. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536-2559. doi:10.1210/jc.2009-2354
2. Kay FU, Canvasser NE, Xi Y, et al. Diagnostic performance and interreader agreement of a standardized MR imaging approach in the prediction of small renal mass histology. Radiology. 2018;287(2):543-553. doi:10.1148/radiol.2018171557
3. Sahni VA, Silverman SG. Biopsy of renal masses: when and why. Cancer Imaging. 2009;9(1):44-55. doi:10.1102/1470-7330.2009.0005
4. Cooper BA, Branley P, Bulfone L, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363(7):609-619. doi:10.1056/NEJMoa1000552
5. Kunath F, Schmidt S, Krabbe L-M, et al. Partial nephrectomy versus radical nephrectomy for clinical localised renal masses. Cochrane Database Syst Rev. 2017;5(5):CD012045. doi:10.1002/14651858.CD012045.pub2
6. Kehlet H, Binder C. Adrenocortical function and clinical course during and after surgery in unsupplemented glucocorticoid-treated patients. Br J Anaesth. 1973;45(10):1043-1048. doi:10.1093/bja/45.10.1043
7. Chapman D, Moore R, Klarenbach S, Braam B. Residual renal function after partial or radical nephrectomy for renal cell carcinoma. Can Urol Assoc J. 2010;4(5):337-343. doi:10.5489/cuaj.909
8. Rahman M, Shad F, Smith MC. Acute kidney injury: a guide to diagnosis and management. Am Fam Physician. 2012;86(7):631-639.
9. Ranabir S, Baruah MP. Pituitary apoplexy. Indian J Endocrinol Metab. 2011;15(suppl 3):S188-S196. doi:10.4103/2230-8210.84862
10. Glezer A, Bronstein MD. Pituitary apoplexy: pathophysiology, diagnosis and management. Arch Endocrinol Metab. 2015;59(3):259-264. doi:10.1590/2359-3997000000047
1. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536-2559. doi:10.1210/jc.2009-2354
2. Kay FU, Canvasser NE, Xi Y, et al. Diagnostic performance and interreader agreement of a standardized MR imaging approach in the prediction of small renal mass histology. Radiology. 2018;287(2):543-553. doi:10.1148/radiol.2018171557
3. Sahni VA, Silverman SG. Biopsy of renal masses: when and why. Cancer Imaging. 2009;9(1):44-55. doi:10.1102/1470-7330.2009.0005
4. Cooper BA, Branley P, Bulfone L, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363(7):609-619. doi:10.1056/NEJMoa1000552
5. Kunath F, Schmidt S, Krabbe L-M, et al. Partial nephrectomy versus radical nephrectomy for clinical localised renal masses. Cochrane Database Syst Rev. 2017;5(5):CD012045. doi:10.1002/14651858.CD012045.pub2
6. Kehlet H, Binder C. Adrenocortical function and clinical course during and after surgery in unsupplemented glucocorticoid-treated patients. Br J Anaesth. 1973;45(10):1043-1048. doi:10.1093/bja/45.10.1043
7. Chapman D, Moore R, Klarenbach S, Braam B. Residual renal function after partial or radical nephrectomy for renal cell carcinoma. Can Urol Assoc J. 2010;4(5):337-343. doi:10.5489/cuaj.909
8. Rahman M, Shad F, Smith MC. Acute kidney injury: a guide to diagnosis and management. Am Fam Physician. 2012;86(7):631-639.
9. Ranabir S, Baruah MP. Pituitary apoplexy. Indian J Endocrinol Metab. 2011;15(suppl 3):S188-S196. doi:10.4103/2230-8210.84862
10. Glezer A, Bronstein MD. Pituitary apoplexy: pathophysiology, diagnosis and management. Arch Endocrinol Metab. 2015;59(3):259-264. doi:10.1590/2359-3997000000047