User login
Benefits of simultaneous resection of liver metastasis restricted to patients with KRAS wild-type tumors
Key clinical point: Simultaneous vs delayed resection of synchronous liver metastasis (SLM) was associated with longer survival rates in patients with colorectal cancer (CRC) with KRAS wild-type tumors but not in those with KRAS sequence variation.
Major finding: Perioperative complications within 60 days of surgery were not significantly different in the simultaneous vs delayed resection group (P = .89), but simultaneous vs delayed resection was associated with better 5-year overall survival (hazard ratio [HR] 1.61; P < .001) and cancer-specific survival (HR 1.62; P = .003) in patients with KRAS wild-type tumors, but not in those with overall KRAS sequence variation.
Study details: This retrospective study included 1569 patients with CRC with or without KRAS sequence variation who underwent simultaneous (n = 512) or delayed (n = 1057) resection of SLM.
Disclosures: This study was supported by a grant from Shanghai Shenkang Clinical Science and Technology Innovation Project, China. No conflicts of interest were reported.
Source: Wu Y et al. Association of simultaneous vs delayed resection of liver metastasis with complications and survival among adults with colorectal cancer. JAMA Netw Open. 2022;5(9):e2231956 (Sep 19). Doi: 10.1001/jamanetworkopen.2022.31956
Key clinical point: Simultaneous vs delayed resection of synchronous liver metastasis (SLM) was associated with longer survival rates in patients with colorectal cancer (CRC) with KRAS wild-type tumors but not in those with KRAS sequence variation.
Major finding: Perioperative complications within 60 days of surgery were not significantly different in the simultaneous vs delayed resection group (P = .89), but simultaneous vs delayed resection was associated with better 5-year overall survival (hazard ratio [HR] 1.61; P < .001) and cancer-specific survival (HR 1.62; P = .003) in patients with KRAS wild-type tumors, but not in those with overall KRAS sequence variation.
Study details: This retrospective study included 1569 patients with CRC with or without KRAS sequence variation who underwent simultaneous (n = 512) or delayed (n = 1057) resection of SLM.
Disclosures: This study was supported by a grant from Shanghai Shenkang Clinical Science and Technology Innovation Project, China. No conflicts of interest were reported.
Source: Wu Y et al. Association of simultaneous vs delayed resection of liver metastasis with complications and survival among adults with colorectal cancer. JAMA Netw Open. 2022;5(9):e2231956 (Sep 19). Doi: 10.1001/jamanetworkopen.2022.31956
Key clinical point: Simultaneous vs delayed resection of synchronous liver metastasis (SLM) was associated with longer survival rates in patients with colorectal cancer (CRC) with KRAS wild-type tumors but not in those with KRAS sequence variation.
Major finding: Perioperative complications within 60 days of surgery were not significantly different in the simultaneous vs delayed resection group (P = .89), but simultaneous vs delayed resection was associated with better 5-year overall survival (hazard ratio [HR] 1.61; P < .001) and cancer-specific survival (HR 1.62; P = .003) in patients with KRAS wild-type tumors, but not in those with overall KRAS sequence variation.
Study details: This retrospective study included 1569 patients with CRC with or without KRAS sequence variation who underwent simultaneous (n = 512) or delayed (n = 1057) resection of SLM.
Disclosures: This study was supported by a grant from Shanghai Shenkang Clinical Science and Technology Innovation Project, China. No conflicts of interest were reported.
Source: Wu Y et al. Association of simultaneous vs delayed resection of liver metastasis with complications and survival among adults with colorectal cancer. JAMA Netw Open. 2022;5(9):e2231956 (Sep 19). Doi: 10.1001/jamanetworkopen.2022.31956
Low rectal cancer: Laparoscopic-assisted surgery safe in terms of short-term oncological outcomes
Key clinical point: Laparoscopic surgery for low rectal cancer when performed by experienced surgeons yielded short-term pathological outcomes, comparable to open surgery, along with a higher rate of sphincter preservation and a shorter duration of hospitalization.
Major finding: Laparoscopic vs open surgery was associated with similar rates of mesorectal excision (P = .78), negative circumferential resection margin (P = .09), negative distal resection margin (P = .36), postoperative complications (P = .07), and median number of retrieved lymph nodes (P = .39), along with a higher sphincter preservation rate (P = .03) and shorter hospitalization duration (P = .008).
Study details: Findings are from LASRE, a noninferiority randomized clinical trial, including 1039 patients scheduled for curative-intent resection of low rectal cancer who were randomly assigned to undergo laparoscopic (n = 712) or open (n = 358) surgery.
Disclosures: This study was supported by the Key Clinical Specialty Discipline Construction Program of the National Health and Family Planning Commission of China. The authors declared no conflicts of interest.
Source: Jiang WZ et al. Short-term outcomes of laparoscopy-assisted vs open surgery for patients with low rectal cancer: The LASRE randomized clinical trial. JAMA Oncol. 2022 (Sep 15). Doi: 10.1001/jamaoncol.2022.4079
Key clinical point: Laparoscopic surgery for low rectal cancer when performed by experienced surgeons yielded short-term pathological outcomes, comparable to open surgery, along with a higher rate of sphincter preservation and a shorter duration of hospitalization.
Major finding: Laparoscopic vs open surgery was associated with similar rates of mesorectal excision (P = .78), negative circumferential resection margin (P = .09), negative distal resection margin (P = .36), postoperative complications (P = .07), and median number of retrieved lymph nodes (P = .39), along with a higher sphincter preservation rate (P = .03) and shorter hospitalization duration (P = .008).
Study details: Findings are from LASRE, a noninferiority randomized clinical trial, including 1039 patients scheduled for curative-intent resection of low rectal cancer who were randomly assigned to undergo laparoscopic (n = 712) or open (n = 358) surgery.
Disclosures: This study was supported by the Key Clinical Specialty Discipline Construction Program of the National Health and Family Planning Commission of China. The authors declared no conflicts of interest.
Source: Jiang WZ et al. Short-term outcomes of laparoscopy-assisted vs open surgery for patients with low rectal cancer: The LASRE randomized clinical trial. JAMA Oncol. 2022 (Sep 15). Doi: 10.1001/jamaoncol.2022.4079
Key clinical point: Laparoscopic surgery for low rectal cancer when performed by experienced surgeons yielded short-term pathological outcomes, comparable to open surgery, along with a higher rate of sphincter preservation and a shorter duration of hospitalization.
Major finding: Laparoscopic vs open surgery was associated with similar rates of mesorectal excision (P = .78), negative circumferential resection margin (P = .09), negative distal resection margin (P = .36), postoperative complications (P = .07), and median number of retrieved lymph nodes (P = .39), along with a higher sphincter preservation rate (P = .03) and shorter hospitalization duration (P = .008).
Study details: Findings are from LASRE, a noninferiority randomized clinical trial, including 1039 patients scheduled for curative-intent resection of low rectal cancer who were randomly assigned to undergo laparoscopic (n = 712) or open (n = 358) surgery.
Disclosures: This study was supported by the Key Clinical Specialty Discipline Construction Program of the National Health and Family Planning Commission of China. The authors declared no conflicts of interest.
Source: Jiang WZ et al. Short-term outcomes of laparoscopy-assisted vs open surgery for patients with low rectal cancer: The LASRE randomized clinical trial. JAMA Oncol. 2022 (Sep 15). Doi: 10.1001/jamaoncol.2022.4079
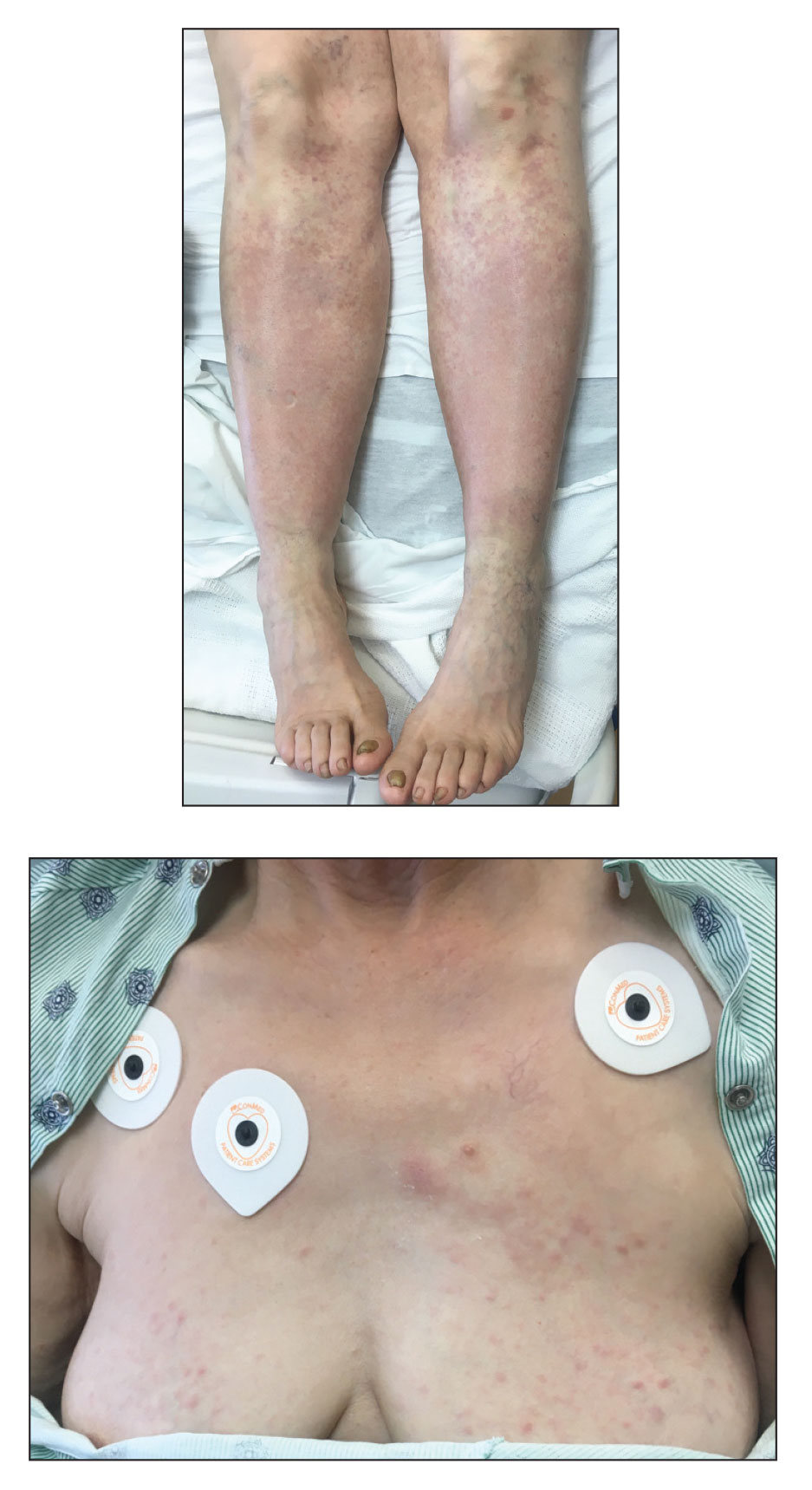
Nonblanching Rash on the Legs and Chest
The Diagnosis: Leukemia Cutis
Hematoxylin and eosin staining revealed an infiltration of monomorphic atypical myeloid cells with cleaved nuclei within the dermis, with a relatively uninvolved epidermis (Figure, A). The cells formed aggregates in single-file lines along dermal collagen bundles. Occasional Auer rods, which are crystal aggregates of the enzyme myeloperoxidase, a marker unique to cells of the myeloid lineage (Figure, B) were appreciated.
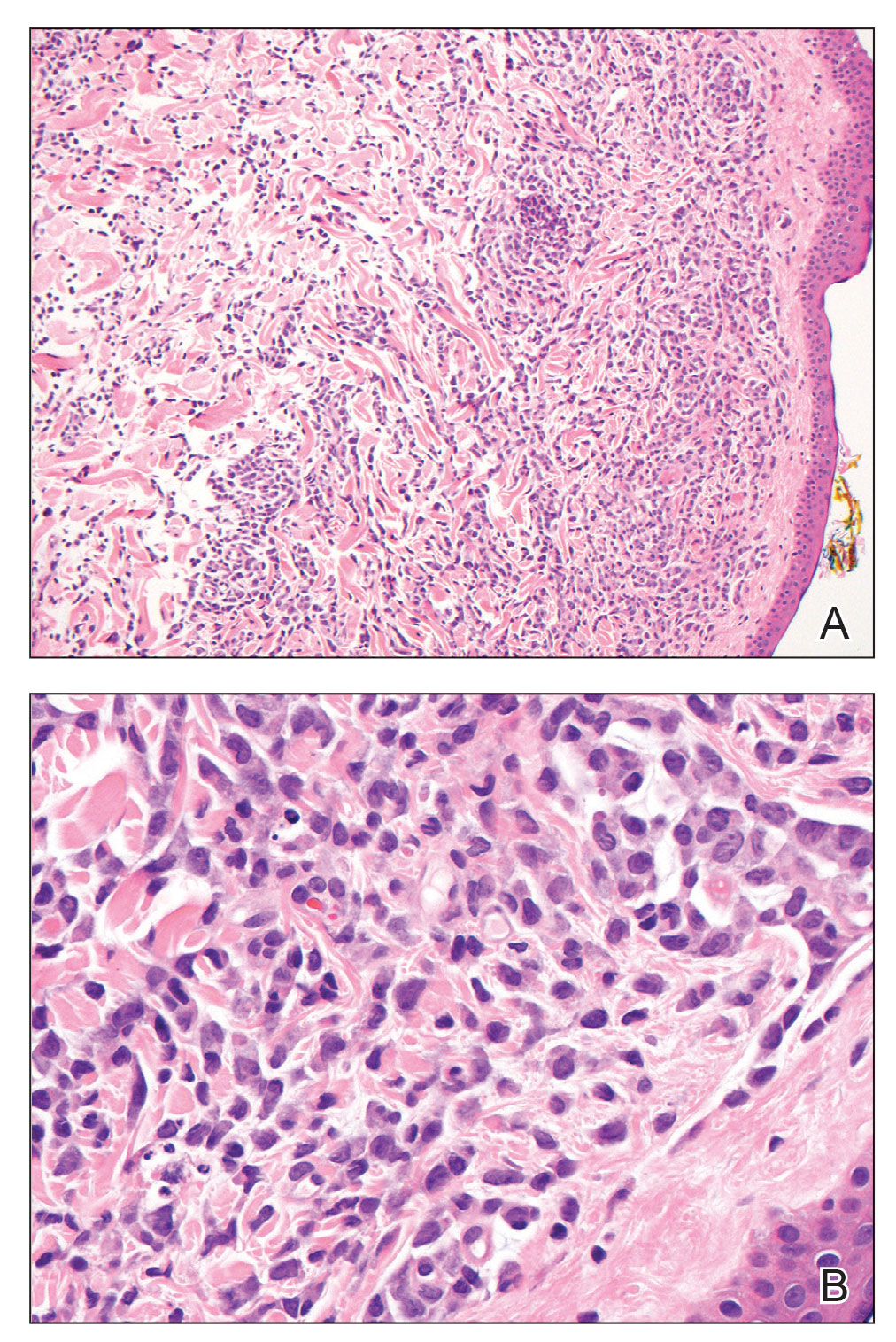
Immunohistochemical staining for myeloperoxidase was weakly positive; however, flow cytometric evaluation of the bone marrow aspirate revealed that approximately 20% of all CD45+ cells were myeloid blasts. These findings confirmed the diagnosis of recurrent acute myeloid leukemia (AML). The diagnosis of AML can be confirmed with a bone marrow biopsy demonstrating more than 20% of the total cells in blast form as well as evidence that the cells are of myeloid origin, which can be inferred by the presence of Auer rods, positive myeloperoxidase staining, or immunophenotyping. In our patient, the Auer rods, myeloperoxidase staining, and atypical myeloid cells on skin biopsy, in conjunction with the bone marrow biopsy results, confirmed leukemia cutis.
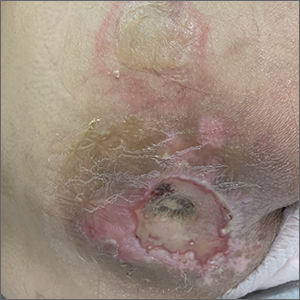
Leukemia cutis is the infiltration of neoplastic proliferating leukocytes in the epidermis, dermis, or subcutis from a primary or more commonly metastatic malignancy. Leukemic cutaneous involvement is seen in up to 13% of leukemia patients and most commonly is seen in monocytic or myelomonocytic forms of AML.1 It may present anywhere on the body but mostly is found on the back, trunk, and head. It also may have a predilection for areas with a history of trauma or inflammation. The lesions most often are firm, erythematous to violaceous papules and nodules, though leukemia cutis can present with hemorrhagic ulcers, purpura, or other cutaneous manifestations of concomitant thrombocytopenia such as petechiae and ecchymoses.2 Involvement of the lower extremities mimicking venous stasis dermatitis has been described.3,4
Treatment of leukemia cutis requires targeting the underlying leukemia2 under the guidance of hematology and oncology as well as the use of chemotherapeutic agents.5 The presence of leukemia cutis is a poor prognostic sign, and a discussion regarding goals of care often is appropriate. Our patient initially responded to FLAG (fludarabine, cytarabine, filgrastim) chemotherapy induction and consolidation, which was followed by midostaurin maintenance. However, she ultimately regressed, requiring decitabine and gilteritinib treatment, and died 9 months later from the course of the disease.
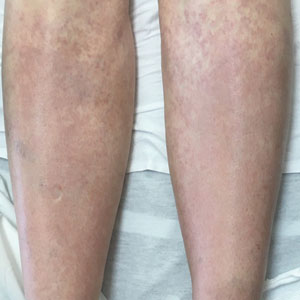
Although typically asymptomatic and presenting on the lower limbs, capillaritis (also known as the pigmented purpuric dermatoses) consists of a set of cutaneous conditions that often are chronic and relapsing in nature, as opposed to our patient’s subacute presentation. These benign conditions have several distinct morphologies; some are characterized by pigmented macules or pinpoint red-brown petechiae that most often are found on the legs but also are seen on the trunk and upper extremities.6 Of the various clinical presentations of capillaritis, our patient’s skin findings may be most consistent with pigmented purpuric lichenoid dermatitis of Gougerot and Blum, in which purpuric red-brown papules coalesce into plaques, though her lesions were not raised. The other pigmented purpuric dermatoses can present with cayenne pepper–colored petechiae, golden-brown macules, pruritic purpuric patches, or red-brown annular patches,6 which were not seen in our patient.
Venous stasis dermatitis also favors the lower extremities7; however, it classically includes the medial malleolus and often presents with scaling and hyperpigmentation from hemosiderin deposition.8 It often is associated with pruritus, as opposed to the nonpruritic nonpainful lesions in leukemia cutis. Other signs of venous insufficiency also may be appreciated, including edema or varicose veins,7 which were not evident in our patient.
Leukocytoclastic vasculitis, a small vessel vasculitis, also appears as palpable or macular purpura, which classically is asymptomatic and erupts on the shins approximately 1 week after an inciting exposure,9 such as medications, pathogens, or autoimmune diseases. One of the least distinctive vasculitides is polyarteritis nodosa, a form of medium vessel vasculitis, which presents most often with palpable purpura or painful nodules on the lower extremities and may be accompanied by livedo reticularis or digital necrosis.9 Acute leukemia may be accompanied by inflammatory paraneoplastic conditions including vasculitis, which is thought to be due to leukemic cells infiltrating and damaging blood vessels.10
Pretibial myxedema is closely associated with Graves disease and shares some features seen in the presentation of our patient’s leukemia cutis. It is asymptomatic, classically affects the pretibial regions, and most commonly affects older adults and women.11,12 Pretibial myxedema presents with thick indurated plaques rather than patches. Our patient did not demonstrate ophthalmopathy, which nearly always precedes pretibial myxedema.12 The most common form of pretibial myxedema is nonpitting, though nodular, plaquelike, polypoid, and elephantiasic forms also exist.11 Pretibial myxedema classically favors the shins; however, it also can affect the ankles, dorsal aspects of the feet, and toes. The characteristic induration of the skin is believed to be the result of excess fibroblast production of glycosaminoglycans in the dermis and subcutis likely triggered by stimulation of fibroblast thyroid stimulating hormone receptors.11
- Bakst RL, Tallman MS, Douer D, et al. How I treat extramedullary acute myeloid leukemia. Blood. 2011;118:3785-3793.
- Bolognia JL, Schaffer JV, Duncan KO, et al. Other lymphoproliferative and myeloproliferative diseases. In: Bolognia JL, Schaffer JV, Duncan KO, et al, eds. Dermatology Essentials. 2nd ed. Elsevier; 2014:973-977.
- Papadavid E, Panayiotides I, Katoulis A, et al. Stasis dermatitis-like leukaemic infiltration in a patient with myelodysplastic syndrome. Clin Exp Dermatol. 2008;33:298-300.
- Chang HY, Wong KM, Bosenberg M, et al. Myelogenous leukemia cutis resembling stasis dermatitis. J Am Acad Dermatol. 2003;49:128-129.
- Aguilera SB, Zarraga M, Rosen L. Leukemia cutis in a patient with acute myelogenous leukemia: a case report and review of the literature. Cutis. 2010;85:31-36.
- Kim DH, Seo SH, Ahn HH, et al. Characteristics and clinical manifestations of pigmented purpuric dermatosis. Ann Dermatol. 2015;27:404-410.
- Bolognia JL, Schaffer JV, Duncan KO, et al. Other eczematous eruptions. In: Bolognia JL, Schaffer JV, Duncan KO, et al, eds. Dermatology Essentials. 2nd ed. Elsevier; 2014:103-108.
- Krooks JA, Weatherall AG. Leukemia cutis in acute myeloid leukemia signifies a poor prognosis. Cutis. 2018;102:266, 271-272.
- Wetter DA, Dutz JP, Shinkai K, et al. Cutaneous vasculitis. In: Bolognia JL, Schaffer JV, Lorenzo C, eds. Dermatology. 4th ed. Elsevier; 2018:409-439.
- Jones D, Dorfman DM, Barnhill RL, et al. Leukemic vasculitis: a feature of leukemia cutis in some patients. Am J Clin Pathol. 1997;107:637-642.
- Fatourechi V. Pretibial myxedema: pathophysiology and treatment options. Am J Clin Dermatol. 2005;6:295-309.
- Fatourechi V, Pajouhi M, Fransway AF. Dermopathy of Graves disease (pretibial myxedema). review of 150 cases. Medicine (Baltimore). 1994;73:1-7.
The Diagnosis: Leukemia Cutis
Hematoxylin and eosin staining revealed an infiltration of monomorphic atypical myeloid cells with cleaved nuclei within the dermis, with a relatively uninvolved epidermis (Figure, A). The cells formed aggregates in single-file lines along dermal collagen bundles. Occasional Auer rods, which are crystal aggregates of the enzyme myeloperoxidase, a marker unique to cells of the myeloid lineage (Figure, B) were appreciated.

Immunohistochemical staining for myeloperoxidase was weakly positive; however, flow cytometric evaluation of the bone marrow aspirate revealed that approximately 20% of all CD45+ cells were myeloid blasts. These findings confirmed the diagnosis of recurrent acute myeloid leukemia (AML). The diagnosis of AML can be confirmed with a bone marrow biopsy demonstrating more than 20% of the total cells in blast form as well as evidence that the cells are of myeloid origin, which can be inferred by the presence of Auer rods, positive myeloperoxidase staining, or immunophenotyping. In our patient, the Auer rods, myeloperoxidase staining, and atypical myeloid cells on skin biopsy, in conjunction with the bone marrow biopsy results, confirmed leukemia cutis.
Leukemia cutis is the infiltration of neoplastic proliferating leukocytes in the epidermis, dermis, or subcutis from a primary or more commonly metastatic malignancy. Leukemic cutaneous involvement is seen in up to 13% of leukemia patients and most commonly is seen in monocytic or myelomonocytic forms of AML.1 It may present anywhere on the body but mostly is found on the back, trunk, and head. It also may have a predilection for areas with a history of trauma or inflammation. The lesions most often are firm, erythematous to violaceous papules and nodules, though leukemia cutis can present with hemorrhagic ulcers, purpura, or other cutaneous manifestations of concomitant thrombocytopenia such as petechiae and ecchymoses.2 Involvement of the lower extremities mimicking venous stasis dermatitis has been described.3,4
Treatment of leukemia cutis requires targeting the underlying leukemia2 under the guidance of hematology and oncology as well as the use of chemotherapeutic agents.5 The presence of leukemia cutis is a poor prognostic sign, and a discussion regarding goals of care often is appropriate. Our patient initially responded to FLAG (fludarabine, cytarabine, filgrastim) chemotherapy induction and consolidation, which was followed by midostaurin maintenance. However, she ultimately regressed, requiring decitabine and gilteritinib treatment, and died 9 months later from the course of the disease.
Although typically asymptomatic and presenting on the lower limbs, capillaritis (also known as the pigmented purpuric dermatoses) consists of a set of cutaneous conditions that often are chronic and relapsing in nature, as opposed to our patient’s subacute presentation. These benign conditions have several distinct morphologies; some are characterized by pigmented macules or pinpoint red-brown petechiae that most often are found on the legs but also are seen on the trunk and upper extremities.6 Of the various clinical presentations of capillaritis, our patient’s skin findings may be most consistent with pigmented purpuric lichenoid dermatitis of Gougerot and Blum, in which purpuric red-brown papules coalesce into plaques, though her lesions were not raised. The other pigmented purpuric dermatoses can present with cayenne pepper–colored petechiae, golden-brown macules, pruritic purpuric patches, or red-brown annular patches,6 which were not seen in our patient.
Venous stasis dermatitis also favors the lower extremities7; however, it classically includes the medial malleolus and often presents with scaling and hyperpigmentation from hemosiderin deposition.8 It often is associated with pruritus, as opposed to the nonpruritic nonpainful lesions in leukemia cutis. Other signs of venous insufficiency also may be appreciated, including edema or varicose veins,7 which were not evident in our patient.
Leukocytoclastic vasculitis, a small vessel vasculitis, also appears as palpable or macular purpura, which classically is asymptomatic and erupts on the shins approximately 1 week after an inciting exposure,9 such as medications, pathogens, or autoimmune diseases. One of the least distinctive vasculitides is polyarteritis nodosa, a form of medium vessel vasculitis, which presents most often with palpable purpura or painful nodules on the lower extremities and may be accompanied by livedo reticularis or digital necrosis.9 Acute leukemia may be accompanied by inflammatory paraneoplastic conditions including vasculitis, which is thought to be due to leukemic cells infiltrating and damaging blood vessels.10
Pretibial myxedema is closely associated with Graves disease and shares some features seen in the presentation of our patient’s leukemia cutis. It is asymptomatic, classically affects the pretibial regions, and most commonly affects older adults and women.11,12 Pretibial myxedema presents with thick indurated plaques rather than patches. Our patient did not demonstrate ophthalmopathy, which nearly always precedes pretibial myxedema.12 The most common form of pretibial myxedema is nonpitting, though nodular, plaquelike, polypoid, and elephantiasic forms also exist.11 Pretibial myxedema classically favors the shins; however, it also can affect the ankles, dorsal aspects of the feet, and toes. The characteristic induration of the skin is believed to be the result of excess fibroblast production of glycosaminoglycans in the dermis and subcutis likely triggered by stimulation of fibroblast thyroid stimulating hormone receptors.11
The Diagnosis: Leukemia Cutis
Hematoxylin and eosin staining revealed an infiltration of monomorphic atypical myeloid cells with cleaved nuclei within the dermis, with a relatively uninvolved epidermis (Figure, A). The cells formed aggregates in single-file lines along dermal collagen bundles. Occasional Auer rods, which are crystal aggregates of the enzyme myeloperoxidase, a marker unique to cells of the myeloid lineage (Figure, B) were appreciated.

Immunohistochemical staining for myeloperoxidase was weakly positive; however, flow cytometric evaluation of the bone marrow aspirate revealed that approximately 20% of all CD45+ cells were myeloid blasts. These findings confirmed the diagnosis of recurrent acute myeloid leukemia (AML). The diagnosis of AML can be confirmed with a bone marrow biopsy demonstrating more than 20% of the total cells in blast form as well as evidence that the cells are of myeloid origin, which can be inferred by the presence of Auer rods, positive myeloperoxidase staining, or immunophenotyping. In our patient, the Auer rods, myeloperoxidase staining, and atypical myeloid cells on skin biopsy, in conjunction with the bone marrow biopsy results, confirmed leukemia cutis.
Leukemia cutis is the infiltration of neoplastic proliferating leukocytes in the epidermis, dermis, or subcutis from a primary or more commonly metastatic malignancy. Leukemic cutaneous involvement is seen in up to 13% of leukemia patients and most commonly is seen in monocytic or myelomonocytic forms of AML.1 It may present anywhere on the body but mostly is found on the back, trunk, and head. It also may have a predilection for areas with a history of trauma or inflammation. The lesions most often are firm, erythematous to violaceous papules and nodules, though leukemia cutis can present with hemorrhagic ulcers, purpura, or other cutaneous manifestations of concomitant thrombocytopenia such as petechiae and ecchymoses.2 Involvement of the lower extremities mimicking venous stasis dermatitis has been described.3,4
Treatment of leukemia cutis requires targeting the underlying leukemia2 under the guidance of hematology and oncology as well as the use of chemotherapeutic agents.5 The presence of leukemia cutis is a poor prognostic sign, and a discussion regarding goals of care often is appropriate. Our patient initially responded to FLAG (fludarabine, cytarabine, filgrastim) chemotherapy induction and consolidation, which was followed by midostaurin maintenance. However, she ultimately regressed, requiring decitabine and gilteritinib treatment, and died 9 months later from the course of the disease.
Although typically asymptomatic and presenting on the lower limbs, capillaritis (also known as the pigmented purpuric dermatoses) consists of a set of cutaneous conditions that often are chronic and relapsing in nature, as opposed to our patient’s subacute presentation. These benign conditions have several distinct morphologies; some are characterized by pigmented macules or pinpoint red-brown petechiae that most often are found on the legs but also are seen on the trunk and upper extremities.6 Of the various clinical presentations of capillaritis, our patient’s skin findings may be most consistent with pigmented purpuric lichenoid dermatitis of Gougerot and Blum, in which purpuric red-brown papules coalesce into plaques, though her lesions were not raised. The other pigmented purpuric dermatoses can present with cayenne pepper–colored petechiae, golden-brown macules, pruritic purpuric patches, or red-brown annular patches,6 which were not seen in our patient.
Venous stasis dermatitis also favors the lower extremities7; however, it classically includes the medial malleolus and often presents with scaling and hyperpigmentation from hemosiderin deposition.8 It often is associated with pruritus, as opposed to the nonpruritic nonpainful lesions in leukemia cutis. Other signs of venous insufficiency also may be appreciated, including edema or varicose veins,7 which were not evident in our patient.
Leukocytoclastic vasculitis, a small vessel vasculitis, also appears as palpable or macular purpura, which classically is asymptomatic and erupts on the shins approximately 1 week after an inciting exposure,9 such as medications, pathogens, or autoimmune diseases. One of the least distinctive vasculitides is polyarteritis nodosa, a form of medium vessel vasculitis, which presents most often with palpable purpura or painful nodules on the lower extremities and may be accompanied by livedo reticularis or digital necrosis.9 Acute leukemia may be accompanied by inflammatory paraneoplastic conditions including vasculitis, which is thought to be due to leukemic cells infiltrating and damaging blood vessels.10
Pretibial myxedema is closely associated with Graves disease and shares some features seen in the presentation of our patient’s leukemia cutis. It is asymptomatic, classically affects the pretibial regions, and most commonly affects older adults and women.11,12 Pretibial myxedema presents with thick indurated plaques rather than patches. Our patient did not demonstrate ophthalmopathy, which nearly always precedes pretibial myxedema.12 The most common form of pretibial myxedema is nonpitting, though nodular, plaquelike, polypoid, and elephantiasic forms also exist.11 Pretibial myxedema classically favors the shins; however, it also can affect the ankles, dorsal aspects of the feet, and toes. The characteristic induration of the skin is believed to be the result of excess fibroblast production of glycosaminoglycans in the dermis and subcutis likely triggered by stimulation of fibroblast thyroid stimulating hormone receptors.11
- Bakst RL, Tallman MS, Douer D, et al. How I treat extramedullary acute myeloid leukemia. Blood. 2011;118:3785-3793.
- Bolognia JL, Schaffer JV, Duncan KO, et al. Other lymphoproliferative and myeloproliferative diseases. In: Bolognia JL, Schaffer JV, Duncan KO, et al, eds. Dermatology Essentials. 2nd ed. Elsevier; 2014:973-977.
- Papadavid E, Panayiotides I, Katoulis A, et al. Stasis dermatitis-like leukaemic infiltration in a patient with myelodysplastic syndrome. Clin Exp Dermatol. 2008;33:298-300.
- Chang HY, Wong KM, Bosenberg M, et al. Myelogenous leukemia cutis resembling stasis dermatitis. J Am Acad Dermatol. 2003;49:128-129.
- Aguilera SB, Zarraga M, Rosen L. Leukemia cutis in a patient with acute myelogenous leukemia: a case report and review of the literature. Cutis. 2010;85:31-36.
- Kim DH, Seo SH, Ahn HH, et al. Characteristics and clinical manifestations of pigmented purpuric dermatosis. Ann Dermatol. 2015;27:404-410.
- Bolognia JL, Schaffer JV, Duncan KO, et al. Other eczematous eruptions. In: Bolognia JL, Schaffer JV, Duncan KO, et al, eds. Dermatology Essentials. 2nd ed. Elsevier; 2014:103-108.
- Krooks JA, Weatherall AG. Leukemia cutis in acute myeloid leukemia signifies a poor prognosis. Cutis. 2018;102:266, 271-272.
- Wetter DA, Dutz JP, Shinkai K, et al. Cutaneous vasculitis. In: Bolognia JL, Schaffer JV, Lorenzo C, eds. Dermatology. 4th ed. Elsevier; 2018:409-439.
- Jones D, Dorfman DM, Barnhill RL, et al. Leukemic vasculitis: a feature of leukemia cutis in some patients. Am J Clin Pathol. 1997;107:637-642.
- Fatourechi V. Pretibial myxedema: pathophysiology and treatment options. Am J Clin Dermatol. 2005;6:295-309.
- Fatourechi V, Pajouhi M, Fransway AF. Dermopathy of Graves disease (pretibial myxedema). review of 150 cases. Medicine (Baltimore). 1994;73:1-7.
- Bakst RL, Tallman MS, Douer D, et al. How I treat extramedullary acute myeloid leukemia. Blood. 2011;118:3785-3793.
- Bolognia JL, Schaffer JV, Duncan KO, et al. Other lymphoproliferative and myeloproliferative diseases. In: Bolognia JL, Schaffer JV, Duncan KO, et al, eds. Dermatology Essentials. 2nd ed. Elsevier; 2014:973-977.
- Papadavid E, Panayiotides I, Katoulis A, et al. Stasis dermatitis-like leukaemic infiltration in a patient with myelodysplastic syndrome. Clin Exp Dermatol. 2008;33:298-300.
- Chang HY, Wong KM, Bosenberg M, et al. Myelogenous leukemia cutis resembling stasis dermatitis. J Am Acad Dermatol. 2003;49:128-129.
- Aguilera SB, Zarraga M, Rosen L. Leukemia cutis in a patient with acute myelogenous leukemia: a case report and review of the literature. Cutis. 2010;85:31-36.
- Kim DH, Seo SH, Ahn HH, et al. Characteristics and clinical manifestations of pigmented purpuric dermatosis. Ann Dermatol. 2015;27:404-410.
- Bolognia JL, Schaffer JV, Duncan KO, et al. Other eczematous eruptions. In: Bolognia JL, Schaffer JV, Duncan KO, et al, eds. Dermatology Essentials. 2nd ed. Elsevier; 2014:103-108.
- Krooks JA, Weatherall AG. Leukemia cutis in acute myeloid leukemia signifies a poor prognosis. Cutis. 2018;102:266, 271-272.
- Wetter DA, Dutz JP, Shinkai K, et al. Cutaneous vasculitis. In: Bolognia JL, Schaffer JV, Lorenzo C, eds. Dermatology. 4th ed. Elsevier; 2018:409-439.
- Jones D, Dorfman DM, Barnhill RL, et al. Leukemic vasculitis: a feature of leukemia cutis in some patients. Am J Clin Pathol. 1997;107:637-642.
- Fatourechi V. Pretibial myxedema: pathophysiology and treatment options. Am J Clin Dermatol. 2005;6:295-309.
- Fatourechi V, Pajouhi M, Fransway AF. Dermopathy of Graves disease (pretibial myxedema). review of 150 cases. Medicine (Baltimore). 1994;73:1-7.
A 67-year-old woman with history of atrial fibrillation and leukemia presented with a nonpruritic nonpainful rash of 10 days' duration that began on the distal lower extremities (top) and then spread superiorly. She reported having a sore throat and mouth, cough, night sweats, unintentional weight loss, and lymphadenopathy. Physical examination revealed pink-purple nonblanching macules and patches on the lower extremities extending from the ankles to the knees. She also had firm pink papules on the chest (bottom) and back. Punch biopsies of the skin on the chest and leg were obtained for histologic examination and immunohistochemical staining.
VA Center Dramatically Shrinks Wait Times for Bone Marrow Biopsies
SAN DIEGO–The Louis Stokes Cleveland VA Medical Center in Ohio dramatically reduced wait times for bone marrow biopsies and treatment by ditching the radiology department and opening a weekly clinic devoted to the procedures, a cancer care team reported at the annual meeting of the Association of VA Hematology/Oncology (AVAHO) September 16 to 18, 2022.
The average time from biopsy order to procedure fell by more than two-thirds from 23.1 days to 7.0 days, and the time from order to diagnosis dipped from 27.8 days to 11.6 days. The time from treatment fell from 54.8 days to 20.2 days.
The new strategy aims to avoid sending patients to the radiology department and treat them in a clinic within the cancer center instead. “It’s great to be able to keep as many hematology/oncology–related things such as infusion, scheduling, and procedures within our department. It provides continuity for the veteran, and it’s helpful for them from that aspect,” said nurse practitioner Kyle Stimpert, MSN, RN, ACNP, of VA Northeast Ohio Healthcare System.
As the cancer team reported in an abstract presented at the AVAHO meeting, “bone marrow biopsies often need to be performed expeditiously to alleviate patient concerns and quickly determine a diagnosis and treatment plan. However, with increasing subspecialization, there are fewer hematology/oncology providers available to perform this procedure.”
The Cleveland VA tried to address this problem by sending patients to interventional radiology, but it still took weeks for bone marrow biopsies to be performed: From August 4, 2020, to August 12, 2021, when 140 biopsies were performed, the average time from order to procedure was 23.1 days. The time from order to diagnosis was 27.8 days, and from order to treatment was 54.8 days.
The bone marrow biopsies provide insight into diseases such as hematologic malignancies and myelodysplastic syndromes, Stimpert said. The procedures may lead to diagnoses or reveal how treatment is progressing.
In 2021, new leadership sought to shrink the wait times. “We put together a small team and started brainstorming,” said oncology clinical nurse specialist Alecia Smalheer, MSN, APRN, OCN, in an interview. With the help of staff who’d come from other facilities, she said, “we were able to see what was being done in surrounding community hospitals and come up with a model and a checklist.”
The team modified a space to create a new weekly, half-day bone marrow biopsy clinic. They also worked on procedures, documentation, education of patients, and training of staff, Smalheer said.
After implementation in the summer of 2021, the biopsy clinic performed 89 procedures through August 31, 2022. The average time from order to procedure was 7.0 days. The time to diagnosis was 11.6 days, and the time to treatment was 20.2 days. The differences between the pre-implementation and postimplementation periods were statistically significant. (P < .001 for each).
The biopsy clinic now sees about 3 to 4 patients a week. “Just yesterday, I had a vet whose cancer was going down. I was able to just do this bone marrow right there, and it was amazing. He didn’t have to go home [and come back],” Stimpert said. “A lot of patients travel a far distance or on oxygen, or it’s hard for them to get around. Coming to the facility for repeat appointments can just take a lot out of them. So it’s really nice to be able to get it all done in one visit.”
There are multiple benefits to shortening wait times, Smalheer said. “They can start treatment much sooner… but it also alleviates some of the emotional distress of waiting. They still have some waiting to do, but it’s definitely not as long.”
And, Stimpert added, patients are familiar with the infusion center and will see faces they know.
As for cost, the biopsy clinic may save money due to several factors related to how and where the biopsy procedures are performed, Stimpert said.
No disclosures are reported.
SAN DIEGO–The Louis Stokes Cleveland VA Medical Center in Ohio dramatically reduced wait times for bone marrow biopsies and treatment by ditching the radiology department and opening a weekly clinic devoted to the procedures, a cancer care team reported at the annual meeting of the Association of VA Hematology/Oncology (AVAHO) September 16 to 18, 2022.
The average time from biopsy order to procedure fell by more than two-thirds from 23.1 days to 7.0 days, and the time from order to diagnosis dipped from 27.8 days to 11.6 days. The time from treatment fell from 54.8 days to 20.2 days.
The new strategy aims to avoid sending patients to the radiology department and treat them in a clinic within the cancer center instead. “It’s great to be able to keep as many hematology/oncology–related things such as infusion, scheduling, and procedures within our department. It provides continuity for the veteran, and it’s helpful for them from that aspect,” said nurse practitioner Kyle Stimpert, MSN, RN, ACNP, of VA Northeast Ohio Healthcare System.
As the cancer team reported in an abstract presented at the AVAHO meeting, “bone marrow biopsies often need to be performed expeditiously to alleviate patient concerns and quickly determine a diagnosis and treatment plan. However, with increasing subspecialization, there are fewer hematology/oncology providers available to perform this procedure.”
The Cleveland VA tried to address this problem by sending patients to interventional radiology, but it still took weeks for bone marrow biopsies to be performed: From August 4, 2020, to August 12, 2021, when 140 biopsies were performed, the average time from order to procedure was 23.1 days. The time from order to diagnosis was 27.8 days, and from order to treatment was 54.8 days.
The bone marrow biopsies provide insight into diseases such as hematologic malignancies and myelodysplastic syndromes, Stimpert said. The procedures may lead to diagnoses or reveal how treatment is progressing.
In 2021, new leadership sought to shrink the wait times. “We put together a small team and started brainstorming,” said oncology clinical nurse specialist Alecia Smalheer, MSN, APRN, OCN, in an interview. With the help of staff who’d come from other facilities, she said, “we were able to see what was being done in surrounding community hospitals and come up with a model and a checklist.”
The team modified a space to create a new weekly, half-day bone marrow biopsy clinic. They also worked on procedures, documentation, education of patients, and training of staff, Smalheer said.
After implementation in the summer of 2021, the biopsy clinic performed 89 procedures through August 31, 2022. The average time from order to procedure was 7.0 days. The time to diagnosis was 11.6 days, and the time to treatment was 20.2 days. The differences between the pre-implementation and postimplementation periods were statistically significant. (P < .001 for each).
The biopsy clinic now sees about 3 to 4 patients a week. “Just yesterday, I had a vet whose cancer was going down. I was able to just do this bone marrow right there, and it was amazing. He didn’t have to go home [and come back],” Stimpert said. “A lot of patients travel a far distance or on oxygen, or it’s hard for them to get around. Coming to the facility for repeat appointments can just take a lot out of them. So it’s really nice to be able to get it all done in one visit.”
There are multiple benefits to shortening wait times, Smalheer said. “They can start treatment much sooner… but it also alleviates some of the emotional distress of waiting. They still have some waiting to do, but it’s definitely not as long.”
And, Stimpert added, patients are familiar with the infusion center and will see faces they know.
As for cost, the biopsy clinic may save money due to several factors related to how and where the biopsy procedures are performed, Stimpert said.
No disclosures are reported.
SAN DIEGO–The Louis Stokes Cleveland VA Medical Center in Ohio dramatically reduced wait times for bone marrow biopsies and treatment by ditching the radiology department and opening a weekly clinic devoted to the procedures, a cancer care team reported at the annual meeting of the Association of VA Hematology/Oncology (AVAHO) September 16 to 18, 2022.
The average time from biopsy order to procedure fell by more than two-thirds from 23.1 days to 7.0 days, and the time from order to diagnosis dipped from 27.8 days to 11.6 days. The time from treatment fell from 54.8 days to 20.2 days.
The new strategy aims to avoid sending patients to the radiology department and treat them in a clinic within the cancer center instead. “It’s great to be able to keep as many hematology/oncology–related things such as infusion, scheduling, and procedures within our department. It provides continuity for the veteran, and it’s helpful for them from that aspect,” said nurse practitioner Kyle Stimpert, MSN, RN, ACNP, of VA Northeast Ohio Healthcare System.
As the cancer team reported in an abstract presented at the AVAHO meeting, “bone marrow biopsies often need to be performed expeditiously to alleviate patient concerns and quickly determine a diagnosis and treatment plan. However, with increasing subspecialization, there are fewer hematology/oncology providers available to perform this procedure.”
The Cleveland VA tried to address this problem by sending patients to interventional radiology, but it still took weeks for bone marrow biopsies to be performed: From August 4, 2020, to August 12, 2021, when 140 biopsies were performed, the average time from order to procedure was 23.1 days. The time from order to diagnosis was 27.8 days, and from order to treatment was 54.8 days.
The bone marrow biopsies provide insight into diseases such as hematologic malignancies and myelodysplastic syndromes, Stimpert said. The procedures may lead to diagnoses or reveal how treatment is progressing.
In 2021, new leadership sought to shrink the wait times. “We put together a small team and started brainstorming,” said oncology clinical nurse specialist Alecia Smalheer, MSN, APRN, OCN, in an interview. With the help of staff who’d come from other facilities, she said, “we were able to see what was being done in surrounding community hospitals and come up with a model and a checklist.”
The team modified a space to create a new weekly, half-day bone marrow biopsy clinic. They also worked on procedures, documentation, education of patients, and training of staff, Smalheer said.
After implementation in the summer of 2021, the biopsy clinic performed 89 procedures through August 31, 2022. The average time from order to procedure was 7.0 days. The time to diagnosis was 11.6 days, and the time to treatment was 20.2 days. The differences between the pre-implementation and postimplementation periods were statistically significant. (P < .001 for each).
The biopsy clinic now sees about 3 to 4 patients a week. “Just yesterday, I had a vet whose cancer was going down. I was able to just do this bone marrow right there, and it was amazing. He didn’t have to go home [and come back],” Stimpert said. “A lot of patients travel a far distance or on oxygen, or it’s hard for them to get around. Coming to the facility for repeat appointments can just take a lot out of them. So it’s really nice to be able to get it all done in one visit.”
There are multiple benefits to shortening wait times, Smalheer said. “They can start treatment much sooner… but it also alleviates some of the emotional distress of waiting. They still have some waiting to do, but it’s definitely not as long.”
And, Stimpert added, patients are familiar with the infusion center and will see faces they know.
As for cost, the biopsy clinic may save money due to several factors related to how and where the biopsy procedures are performed, Stimpert said.
No disclosures are reported.
In VA Oncology, Discussion Groups Are Transforming the Workplace
SAN DIEGO—From coast to coast, 10 US Department of Veterans Affairs (VA) medical centers are holding meetings designed to help clinicians and colleagues talk openly about touchy workplace topics, such as compassion, burnout, and medical errors. New data suggests that “Schwartz Rounds” have tremendous power to change how medical professionals cope, communicate, and commiserate.
At the VA Connecticut Healthcare System (VACHS), nearly all (98%) respondents who took part in Schwartz Round sessions rated them as either good or excellent, 89% reported feeling less isolated in their work with patients, 98% had new insights into the perspectives and experiences of colleagues, and 93% felt more open to communicating with colleagues, reported oncologist Edward Perry, MD, of VA Connecticut and Yale University School of Medicine, in a presentation here at the annual meeting of the Association of VA Hematology/Oncology (AVAHO) held September 16 to 18, 2022.
Schwartz Rounds have been around for 25 years and are named after the late Ken Schwartz, a 40-year-old Boston health care attorney who wrote movingly in 1995 about the “exquisite compassion” he experienced while being treated for advanced lung cancer—and the risk that the rapidly evolving health care system would lose its sense of empathy.
The Boston-based nonprofit Schwartz Center for Compassionate Healthcare facilitates Schwartz Rounds, which are now held at about 600 health care organizations around the world. That number includes the 10 VA medical centers, mostly in the Northeast (Massachusetts, New York, Connecticut, and New Hampshire) but also in California, Illinois, and Minnesota.
Site teams work with the Schwartz Center to plan the rounds and gather data about their effectiveness. “Unlike traditional clinical or ethics rounds, this is not a didactic or problem-solving session. The focus is not on what happened, but how those who were involved felt. In other words, the human dimension of medicine,” Dr. Perry said. “There are no right or wrong answers. Everything that is said during short rounds is confidential. We do encourage people to continue discussion of the general themes afterward but not to share any specifics of what was discussed.”
At VACHS, Schwartz rounds began shortly before the COVID-19 pandemic, Perry said, and they’ve been held virtually since the first meeting. “Schwartz Rounds are open to all employees, trainees, and students at the institution. Anyone with a VA badge is welcome to attend,” he said. “We're averaging about 150 attendees per session.”
Speakers have addressed social/emotional topics, including delivering bad news to patients, maintaining compassion during adversity, and providing compassionate care to patients with substance use disorders.
The VACHS survey of Schwartz Rounds participants had a 50% response rate, with about 400 people responding to each question. Nearly all (98%) said they planned to attend the rounds again, and 55% agreed that a specific discussion “suggests that changes may be needed in departmental or institutional policies or practices.”
The administration has agreed to continue the Schwartz Rounds in light of the positive results, Perry said. As he noted, the Schwartz Center charges dues and initiation fees. The Marjorie Stanzler Financial Aid Fund underwrites the initiation fees for qualifying organizations, including VA facilities.
As for lessons, he said the topics of Schwartz Rounds “should be emotionally resonant. They should involve multiple disciplines and perspectives. They should illuminate an issue or experience that is not often discussed. And should inspire participants to share their own experiences and highlight instances of compassionate care—or barriers to providing compassionate care.”
Dr. Perry has no disclosures.
SAN DIEGO—From coast to coast, 10 US Department of Veterans Affairs (VA) medical centers are holding meetings designed to help clinicians and colleagues talk openly about touchy workplace topics, such as compassion, burnout, and medical errors. New data suggests that “Schwartz Rounds” have tremendous power to change how medical professionals cope, communicate, and commiserate.
At the VA Connecticut Healthcare System (VACHS), nearly all (98%) respondents who took part in Schwartz Round sessions rated them as either good or excellent, 89% reported feeling less isolated in their work with patients, 98% had new insights into the perspectives and experiences of colleagues, and 93% felt more open to communicating with colleagues, reported oncologist Edward Perry, MD, of VA Connecticut and Yale University School of Medicine, in a presentation here at the annual meeting of the Association of VA Hematology/Oncology (AVAHO) held September 16 to 18, 2022.
Schwartz Rounds have been around for 25 years and are named after the late Ken Schwartz, a 40-year-old Boston health care attorney who wrote movingly in 1995 about the “exquisite compassion” he experienced while being treated for advanced lung cancer—and the risk that the rapidly evolving health care system would lose its sense of empathy.
The Boston-based nonprofit Schwartz Center for Compassionate Healthcare facilitates Schwartz Rounds, which are now held at about 600 health care organizations around the world. That number includes the 10 VA medical centers, mostly in the Northeast (Massachusetts, New York, Connecticut, and New Hampshire) but also in California, Illinois, and Minnesota.
Site teams work with the Schwartz Center to plan the rounds and gather data about their effectiveness. “Unlike traditional clinical or ethics rounds, this is not a didactic or problem-solving session. The focus is not on what happened, but how those who were involved felt. In other words, the human dimension of medicine,” Dr. Perry said. “There are no right or wrong answers. Everything that is said during short rounds is confidential. We do encourage people to continue discussion of the general themes afterward but not to share any specifics of what was discussed.”
At VACHS, Schwartz rounds began shortly before the COVID-19 pandemic, Perry said, and they’ve been held virtually since the first meeting. “Schwartz Rounds are open to all employees, trainees, and students at the institution. Anyone with a VA badge is welcome to attend,” he said. “We're averaging about 150 attendees per session.”
Speakers have addressed social/emotional topics, including delivering bad news to patients, maintaining compassion during adversity, and providing compassionate care to patients with substance use disorders.
The VACHS survey of Schwartz Rounds participants had a 50% response rate, with about 400 people responding to each question. Nearly all (98%) said they planned to attend the rounds again, and 55% agreed that a specific discussion “suggests that changes may be needed in departmental or institutional policies or practices.”
The administration has agreed to continue the Schwartz Rounds in light of the positive results, Perry said. As he noted, the Schwartz Center charges dues and initiation fees. The Marjorie Stanzler Financial Aid Fund underwrites the initiation fees for qualifying organizations, including VA facilities.
As for lessons, he said the topics of Schwartz Rounds “should be emotionally resonant. They should involve multiple disciplines and perspectives. They should illuminate an issue or experience that is not often discussed. And should inspire participants to share their own experiences and highlight instances of compassionate care—or barriers to providing compassionate care.”
Dr. Perry has no disclosures.
SAN DIEGO—From coast to coast, 10 US Department of Veterans Affairs (VA) medical centers are holding meetings designed to help clinicians and colleagues talk openly about touchy workplace topics, such as compassion, burnout, and medical errors. New data suggests that “Schwartz Rounds” have tremendous power to change how medical professionals cope, communicate, and commiserate.
At the VA Connecticut Healthcare System (VACHS), nearly all (98%) respondents who took part in Schwartz Round sessions rated them as either good or excellent, 89% reported feeling less isolated in their work with patients, 98% had new insights into the perspectives and experiences of colleagues, and 93% felt more open to communicating with colleagues, reported oncologist Edward Perry, MD, of VA Connecticut and Yale University School of Medicine, in a presentation here at the annual meeting of the Association of VA Hematology/Oncology (AVAHO) held September 16 to 18, 2022.
Schwartz Rounds have been around for 25 years and are named after the late Ken Schwartz, a 40-year-old Boston health care attorney who wrote movingly in 1995 about the “exquisite compassion” he experienced while being treated for advanced lung cancer—and the risk that the rapidly evolving health care system would lose its sense of empathy.
The Boston-based nonprofit Schwartz Center for Compassionate Healthcare facilitates Schwartz Rounds, which are now held at about 600 health care organizations around the world. That number includes the 10 VA medical centers, mostly in the Northeast (Massachusetts, New York, Connecticut, and New Hampshire) but also in California, Illinois, and Minnesota.
Site teams work with the Schwartz Center to plan the rounds and gather data about their effectiveness. “Unlike traditional clinical or ethics rounds, this is not a didactic or problem-solving session. The focus is not on what happened, but how those who were involved felt. In other words, the human dimension of medicine,” Dr. Perry said. “There are no right or wrong answers. Everything that is said during short rounds is confidential. We do encourage people to continue discussion of the general themes afterward but not to share any specifics of what was discussed.”
At VACHS, Schwartz rounds began shortly before the COVID-19 pandemic, Perry said, and they’ve been held virtually since the first meeting. “Schwartz Rounds are open to all employees, trainees, and students at the institution. Anyone with a VA badge is welcome to attend,” he said. “We're averaging about 150 attendees per session.”
Speakers have addressed social/emotional topics, including delivering bad news to patients, maintaining compassion during adversity, and providing compassionate care to patients with substance use disorders.
The VACHS survey of Schwartz Rounds participants had a 50% response rate, with about 400 people responding to each question. Nearly all (98%) said they planned to attend the rounds again, and 55% agreed that a specific discussion “suggests that changes may be needed in departmental or institutional policies or practices.”
The administration has agreed to continue the Schwartz Rounds in light of the positive results, Perry said. As he noted, the Schwartz Center charges dues and initiation fees. The Marjorie Stanzler Financial Aid Fund underwrites the initiation fees for qualifying organizations, including VA facilities.
As for lessons, he said the topics of Schwartz Rounds “should be emotionally resonant. They should involve multiple disciplines and perspectives. They should illuminate an issue or experience that is not often discussed. And should inspire participants to share their own experiences and highlight instances of compassionate care—or barriers to providing compassionate care.”
Dr. Perry has no disclosures.
Commentary: Postpartum hemorrhage and acute chest pain obstetric emergencies, October 2022
The three PPH articles examine the use of preventive B-Lynch suture, risk factors for failure of intrauterine tamponade, and trend changes in risk factors for PPH. Kuwabara and colleagues looked at the effectiveness of preventative B-Lynch sutures in patients at high risk for PPH. Their retrospective observational study included 38 of 663 patients who underwent cesarean section (CS) who received the B-Lynch procedure at their tertiary perinatal medical center in Gifu, Japan, between January 2019 and May 2021. Overall, 92% of patients who received the B-Lynch suture showed no apparent postoperative bleeding within 2 hours after the CS. A total of 24 patients required blood transfusion, none required hysterectomy, and only one patient with a twin pregnancy required additional treatment because of secondary PPH 5 days after the CS. This suggests that earlier use of B-Lynch sutures could be considered in patients at high risk for atony.
Gibier and colleagues examined risk factors for uterine tamponade failure in women with PPH. This was a population-based retrospective cohort study of 1761 women with deliveries complicated by PPH who underwent intrauterine tamponade within 24 hours of PPH to manage persistent bleeding. They noted that the intrauterine tamponade failure rate was 11.1%. Risk for intrauterine tamponade failure was higher in women with CS (adjusted odds ratio [aOR] 4.2; 95% CI 2.9-6.0), preeclampsia (aOR 2.3; 95% CI 1.3-3.9), and uterine rupture (aOR 14.1; 95% CI 2.4-83.0). They concluded that CS, preeclampsia, and uterine rupture were significant risk factors for failures in this procedure.
Sade and colleagues examined trend changes in the individual contribution of risk factors for PPH over more than two decades. Their population-based, retrospective, nested, case-control study included 285,992 pregnancies and suggested that, in their hospital setting in Israel, risks from perineal or vaginal tears were increasing while large-for-gestational-age fetuses decreased and other risk factors remained stable.
Finally, Wu and colleagues examined incidence and outcomes of AHRCP diseases during pregnancy and the puerperium. This observational analysis examined 41,174,101 patients hospitalized for pregnancy and during the puerperium in the National Inpatient Sample (NIS) database from January 1, 2008, to December 31, 2017. The study noted that 40,285 patients were diagnosed with AHRCP diseases during this period. The NIS is the largest publicly available all-payer database in the United States. The investigators found that the incidence of AHRCP diseases increased significantly between 2002 and 2017, especially pulmonary embolism in the puerperium. Although mortality showed a downward trend, it is still at a high level. They suggested that we should strengthen monitoring and management of AHRCP in pregnancy and puerperium, especially for Black women, those in the lowest-income households, and parturients over 35 years of age.
The three PPH articles examine the use of preventive B-Lynch suture, risk factors for failure of intrauterine tamponade, and trend changes in risk factors for PPH. Kuwabara and colleagues looked at the effectiveness of preventative B-Lynch sutures in patients at high risk for PPH. Their retrospective observational study included 38 of 663 patients who underwent cesarean section (CS) who received the B-Lynch procedure at their tertiary perinatal medical center in Gifu, Japan, between January 2019 and May 2021. Overall, 92% of patients who received the B-Lynch suture showed no apparent postoperative bleeding within 2 hours after the CS. A total of 24 patients required blood transfusion, none required hysterectomy, and only one patient with a twin pregnancy required additional treatment because of secondary PPH 5 days after the CS. This suggests that earlier use of B-Lynch sutures could be considered in patients at high risk for atony.
Gibier and colleagues examined risk factors for uterine tamponade failure in women with PPH. This was a population-based retrospective cohort study of 1761 women with deliveries complicated by PPH who underwent intrauterine tamponade within 24 hours of PPH to manage persistent bleeding. They noted that the intrauterine tamponade failure rate was 11.1%. Risk for intrauterine tamponade failure was higher in women with CS (adjusted odds ratio [aOR] 4.2; 95% CI 2.9-6.0), preeclampsia (aOR 2.3; 95% CI 1.3-3.9), and uterine rupture (aOR 14.1; 95% CI 2.4-83.0). They concluded that CS, preeclampsia, and uterine rupture were significant risk factors for failures in this procedure.
Sade and colleagues examined trend changes in the individual contribution of risk factors for PPH over more than two decades. Their population-based, retrospective, nested, case-control study included 285,992 pregnancies and suggested that, in their hospital setting in Israel, risks from perineal or vaginal tears were increasing while large-for-gestational-age fetuses decreased and other risk factors remained stable.
Finally, Wu and colleagues examined incidence and outcomes of AHRCP diseases during pregnancy and the puerperium. This observational analysis examined 41,174,101 patients hospitalized for pregnancy and during the puerperium in the National Inpatient Sample (NIS) database from January 1, 2008, to December 31, 2017. The study noted that 40,285 patients were diagnosed with AHRCP diseases during this period. The NIS is the largest publicly available all-payer database in the United States. The investigators found that the incidence of AHRCP diseases increased significantly between 2002 and 2017, especially pulmonary embolism in the puerperium. Although mortality showed a downward trend, it is still at a high level. They suggested that we should strengthen monitoring and management of AHRCP in pregnancy and puerperium, especially for Black women, those in the lowest-income households, and parturients over 35 years of age.
The three PPH articles examine the use of preventive B-Lynch suture, risk factors for failure of intrauterine tamponade, and trend changes in risk factors for PPH. Kuwabara and colleagues looked at the effectiveness of preventative B-Lynch sutures in patients at high risk for PPH. Their retrospective observational study included 38 of 663 patients who underwent cesarean section (CS) who received the B-Lynch procedure at their tertiary perinatal medical center in Gifu, Japan, between January 2019 and May 2021. Overall, 92% of patients who received the B-Lynch suture showed no apparent postoperative bleeding within 2 hours after the CS. A total of 24 patients required blood transfusion, none required hysterectomy, and only one patient with a twin pregnancy required additional treatment because of secondary PPH 5 days after the CS. This suggests that earlier use of B-Lynch sutures could be considered in patients at high risk for atony.
Gibier and colleagues examined risk factors for uterine tamponade failure in women with PPH. This was a population-based retrospective cohort study of 1761 women with deliveries complicated by PPH who underwent intrauterine tamponade within 24 hours of PPH to manage persistent bleeding. They noted that the intrauterine tamponade failure rate was 11.1%. Risk for intrauterine tamponade failure was higher in women with CS (adjusted odds ratio [aOR] 4.2; 95% CI 2.9-6.0), preeclampsia (aOR 2.3; 95% CI 1.3-3.9), and uterine rupture (aOR 14.1; 95% CI 2.4-83.0). They concluded that CS, preeclampsia, and uterine rupture were significant risk factors for failures in this procedure.
Sade and colleagues examined trend changes in the individual contribution of risk factors for PPH over more than two decades. Their population-based, retrospective, nested, case-control study included 285,992 pregnancies and suggested that, in their hospital setting in Israel, risks from perineal or vaginal tears were increasing while large-for-gestational-age fetuses decreased and other risk factors remained stable.
Finally, Wu and colleagues examined incidence and outcomes of AHRCP diseases during pregnancy and the puerperium. This observational analysis examined 41,174,101 patients hospitalized for pregnancy and during the puerperium in the National Inpatient Sample (NIS) database from January 1, 2008, to December 31, 2017. The study noted that 40,285 patients were diagnosed with AHRCP diseases during this period. The NIS is the largest publicly available all-payer database in the United States. The investigators found that the incidence of AHRCP diseases increased significantly between 2002 and 2017, especially pulmonary embolism in the puerperium. Although mortality showed a downward trend, it is still at a high level. They suggested that we should strengthen monitoring and management of AHRCP in pregnancy and puerperium, especially for Black women, those in the lowest-income households, and parturients over 35 years of age.
Sacral blistering
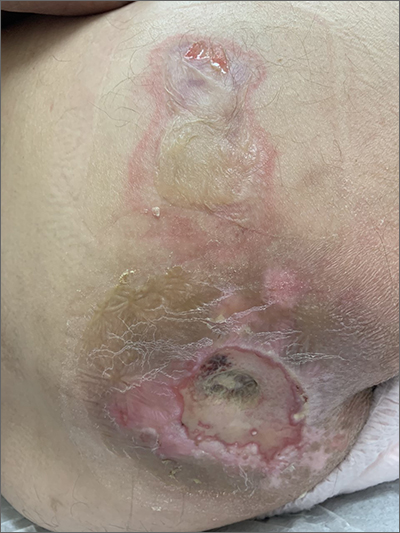
This patient had sustained multiple pressure injuries. The superior aspect of this image shows bullous change with intact dermis, which would classify that area of injury as a Stage 2 pressure injury.1 An older injury in the coccygeal area was through the dermis (Stage 3), with some eschar seen at the base, making that area unstageable.1 (There may have been deeper injury under the eschar.)
This patient was at heightened risk for pressure injury because of his paraplegia.2 Fortunately, he had some preserved sensation. However, his rotator cuff surgery made it harder for him to smoothly transfer to and from the wheelchair, leading to sheer forces against his skin. Social determinates of health care pose an additional risk for pressure injuries. Without a properly fitted wheelchair and cushion, there is an increased risk of localized pressure over both bony prominences and parts of the body that come into contact with mechanical elements of the wheelchair.
Treatment for all pressure injuries includes relief of pressure on the affected area. In this patient’s case, he had to stay in bed (and out of the wheelchair) so that he could heal.
This patient was very knowledgeable about his condition and pressure injuries. He had already arranged for a wheelchair fitting and a visit with the wound care team. His Stage 2 injury had a bullous change instead of absent epithelium, so rather than an adherent hydrocolloid dressing (which would likely remove the loosened epithelium), he was provided with a nonadherent dressing to the area, then an absorbent foam overdressing.
An image of the patient’s deeper sacral injury was shared with the wound care team, which recommended filling the area with a silver rope dressing for its absorptive filler and antibacterial properties. The area was then covered with an absorbent foam. The patient planned to follow up with the Wound Care Clinic for reevaluation and ongoing treatment.
Images and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine Kalamazoo.
1. 2019 Guideline. The National Pressure Injury Advisory Panel. Accessed October 9, 2022. https://npiap.com/page/2019Guideline
2. Ricci JA, Bayer LR, Orgill DP. Evidence-based medicine: the evaluation and treatment of pressure injuries. Plast Reconstr Surg. 2017;139:275e-286e. doi: 10.1097/PRS.0000000000002850

This patient had sustained multiple pressure injuries. The superior aspect of this image shows bullous change with intact dermis, which would classify that area of injury as a Stage 2 pressure injury.1 An older injury in the coccygeal area was through the dermis (Stage 3), with some eschar seen at the base, making that area unstageable.1 (There may have been deeper injury under the eschar.)
This patient was at heightened risk for pressure injury because of his paraplegia.2 Fortunately, he had some preserved sensation. However, his rotator cuff surgery made it harder for him to smoothly transfer to and from the wheelchair, leading to sheer forces against his skin. Social determinates of health care pose an additional risk for pressure injuries. Without a properly fitted wheelchair and cushion, there is an increased risk of localized pressure over both bony prominences and parts of the body that come into contact with mechanical elements of the wheelchair.
Treatment for all pressure injuries includes relief of pressure on the affected area. In this patient’s case, he had to stay in bed (and out of the wheelchair) so that he could heal.
This patient was very knowledgeable about his condition and pressure injuries. He had already arranged for a wheelchair fitting and a visit with the wound care team. His Stage 2 injury had a bullous change instead of absent epithelium, so rather than an adherent hydrocolloid dressing (which would likely remove the loosened epithelium), he was provided with a nonadherent dressing to the area, then an absorbent foam overdressing.
An image of the patient’s deeper sacral injury was shared with the wound care team, which recommended filling the area with a silver rope dressing for its absorptive filler and antibacterial properties. The area was then covered with an absorbent foam. The patient planned to follow up with the Wound Care Clinic for reevaluation and ongoing treatment.
Images and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine Kalamazoo.

This patient had sustained multiple pressure injuries. The superior aspect of this image shows bullous change with intact dermis, which would classify that area of injury as a Stage 2 pressure injury.1 An older injury in the coccygeal area was through the dermis (Stage 3), with some eschar seen at the base, making that area unstageable.1 (There may have been deeper injury under the eschar.)
This patient was at heightened risk for pressure injury because of his paraplegia.2 Fortunately, he had some preserved sensation. However, his rotator cuff surgery made it harder for him to smoothly transfer to and from the wheelchair, leading to sheer forces against his skin. Social determinates of health care pose an additional risk for pressure injuries. Without a properly fitted wheelchair and cushion, there is an increased risk of localized pressure over both bony prominences and parts of the body that come into contact with mechanical elements of the wheelchair.
Treatment for all pressure injuries includes relief of pressure on the affected area. In this patient’s case, he had to stay in bed (and out of the wheelchair) so that he could heal.
This patient was very knowledgeable about his condition and pressure injuries. He had already arranged for a wheelchair fitting and a visit with the wound care team. His Stage 2 injury had a bullous change instead of absent epithelium, so rather than an adherent hydrocolloid dressing (which would likely remove the loosened epithelium), he was provided with a nonadherent dressing to the area, then an absorbent foam overdressing.
An image of the patient’s deeper sacral injury was shared with the wound care team, which recommended filling the area with a silver rope dressing for its absorptive filler and antibacterial properties. The area was then covered with an absorbent foam. The patient planned to follow up with the Wound Care Clinic for reevaluation and ongoing treatment.
Images and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine Kalamazoo.
1. 2019 Guideline. The National Pressure Injury Advisory Panel. Accessed October 9, 2022. https://npiap.com/page/2019Guideline
2. Ricci JA, Bayer LR, Orgill DP. Evidence-based medicine: the evaluation and treatment of pressure injuries. Plast Reconstr Surg. 2017;139:275e-286e. doi: 10.1097/PRS.0000000000002850
1. 2019 Guideline. The National Pressure Injury Advisory Panel. Accessed October 9, 2022. https://npiap.com/page/2019Guideline
2. Ricci JA, Bayer LR, Orgill DP. Evidence-based medicine: the evaluation and treatment of pressure injuries. Plast Reconstr Surg. 2017;139:275e-286e. doi: 10.1097/PRS.0000000000002850
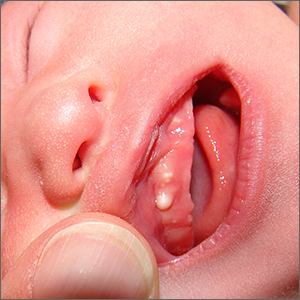
Newborn with white oral lesions
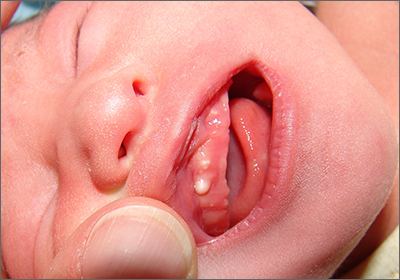
These lesions, called Bohn nodules, manifest on the buccal or lingual portion of the maxillary alveolar ridge and, less frequently, on the mandibular alveolar ridge. Because Bohn nodules are white and have a firm consistency, they are often confused with teeth. They can be differentiated by location, as teeth usually erupt from the distal aspect of the alveolar ridge.
Bohn nodules are epithelial cysts that are filled with keratin, which gives them their white color. They are caused by portions of epithelium that get trapped under surrounding epithelial cells. Bohn nodules usually resolve when the overlying epithelium ruptures and releases the keratinaceous material (usually by the time the child is 3 months of age).1
These nodules can be confused with neonatal or supernumerary teeth. Neonatal teeth can be abnormally small and pointed; they are true deciduous teeth that have erupted early. If they are removed, the child will not replace them until the time of their adult tooth eruption. Additionally, there are supernumerary teeth, which are extra teeth that are often abnormally shaped and loosely adherent. These abnormal teeth warrant extraction to avoid trauma to the tongue or aspiration.2
In this case, the family was advised regarding the benign nature of the nodules and the expectation that they would spontaneously resolve.
Images and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine Kalamazoo.
1. Gupta N, Ramji S. Bohn's nodules: an under-recognised entity. Arch Dis Child Fetal Neonatal Ed. 2013;98:F464. doi: 10.1136/archdischild-2012-302922
2. DeSeta M, Holden E, Siddik D, et al. Natal and neonatal teeth: a review and case series. Br Dent J. 2022;232:449-453. doi: 10.1038/s41415-022-4091-3

These lesions, called Bohn nodules, manifest on the buccal or lingual portion of the maxillary alveolar ridge and, less frequently, on the mandibular alveolar ridge. Because Bohn nodules are white and have a firm consistency, they are often confused with teeth. They can be differentiated by location, as teeth usually erupt from the distal aspect of the alveolar ridge.
Bohn nodules are epithelial cysts that are filled with keratin, which gives them their white color. They are caused by portions of epithelium that get trapped under surrounding epithelial cells. Bohn nodules usually resolve when the overlying epithelium ruptures and releases the keratinaceous material (usually by the time the child is 3 months of age).1
These nodules can be confused with neonatal or supernumerary teeth. Neonatal teeth can be abnormally small and pointed; they are true deciduous teeth that have erupted early. If they are removed, the child will not replace them until the time of their adult tooth eruption. Additionally, there are supernumerary teeth, which are extra teeth that are often abnormally shaped and loosely adherent. These abnormal teeth warrant extraction to avoid trauma to the tongue or aspiration.2
In this case, the family was advised regarding the benign nature of the nodules and the expectation that they would spontaneously resolve.
Images and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine Kalamazoo.

These lesions, called Bohn nodules, manifest on the buccal or lingual portion of the maxillary alveolar ridge and, less frequently, on the mandibular alveolar ridge. Because Bohn nodules are white and have a firm consistency, they are often confused with teeth. They can be differentiated by location, as teeth usually erupt from the distal aspect of the alveolar ridge.
Bohn nodules are epithelial cysts that are filled with keratin, which gives them their white color. They are caused by portions of epithelium that get trapped under surrounding epithelial cells. Bohn nodules usually resolve when the overlying epithelium ruptures and releases the keratinaceous material (usually by the time the child is 3 months of age).1
These nodules can be confused with neonatal or supernumerary teeth. Neonatal teeth can be abnormally small and pointed; they are true deciduous teeth that have erupted early. If they are removed, the child will not replace them until the time of their adult tooth eruption. Additionally, there are supernumerary teeth, which are extra teeth that are often abnormally shaped and loosely adherent. These abnormal teeth warrant extraction to avoid trauma to the tongue or aspiration.2
In this case, the family was advised regarding the benign nature of the nodules and the expectation that they would spontaneously resolve.
Images and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine Kalamazoo.
1. Gupta N, Ramji S. Bohn's nodules: an under-recognised entity. Arch Dis Child Fetal Neonatal Ed. 2013;98:F464. doi: 10.1136/archdischild-2012-302922
2. DeSeta M, Holden E, Siddik D, et al. Natal and neonatal teeth: a review and case series. Br Dent J. 2022;232:449-453. doi: 10.1038/s41415-022-4091-3
1. Gupta N, Ramji S. Bohn's nodules: an under-recognised entity. Arch Dis Child Fetal Neonatal Ed. 2013;98:F464. doi: 10.1136/archdischild-2012-302922
2. DeSeta M, Holden E, Siddik D, et al. Natal and neonatal teeth: a review and case series. Br Dent J. 2022;232:449-453. doi: 10.1038/s41415-022-4091-3
SCLC Guidelines
Chronic Kidney Disease in People with Type 2 Diabetes
We know from the literature and in practice that type 2 diabetes (T2D) is one of the most common risk factors for developing chronic kidney disease (CKD). How prevalent is this overlap, and are certain patients more at risk than others?
Dr. McGill: That’s correct, in fact, 20% to 40% of people with T2D have identifiable CKD, and the rest are at risk for developing CKD in the future. All patients with T2D should recognize that risk and undergo annual screening for CKD. If an individual has prediabetes, then step up the screening to perhaps twice a year to see if the person has progressed. At that point, we can think about intervening with a medication to prevent the onset of diabetes, particularly if the patient is unable to adopt significant lifestyle changes.
In your day-to-day practice, what therapeutic approach do you take in managing patients with T2D and CKD?
Dr. McGill: The earliest and arguably the most important treatment for the care of CKD in T2D is glucose control. Establishing and maintaining blood glucose levels near the normal range is our strongest weapon for preventing CKD. Another treatment avenue is controlling blood pressure. The American Diabetes Association and other groups recommend that blood pressure be less than 130/80 mm Hg. It is critical that we treat hypertension effectively to achieve those numbers.
We also have therapies, such as the SGLT2 inhibitors, that offer protection from progression of CKD and from hospitalization for heart failure. Deployment of these newer agents is important for people who have more advanced diabetes or other serious health conditions.
What is the rate of disease progression, related complications, or even mortality for these patients?
Dr. McGill: People with CKD and T2D are at risk for many things. One risk is progression of kidney disease all the way to end-stage kidney disease, which requires dialysis or transplantation. Another huge risk is cardiovascular events such as myocardial infarction (MI) and stroke.
Persons with kidney disease, for reasons we don't understand, are at higher risk of MI and stroke than people who do not have kidney disease. Therefore, the risks of early mortality and events that adversely affect quality of life are greatly increased.
Can you please discuss the economic burdens associated with T2D and CKD, and whether any interventions are in place to help offset those costs?
Dr. McGill: Diabetes itself is wickedly expensive. We have excellent treatments for diabetes today, but they are very costly. The best approach for the prevention of diabetes is to be screened. When a patient presents with prediabetes, it’s important that they take important measures, such as weight loss, exercising 150 minutes per week, or reducing 500 calories a day from their diet, all of which have been shown to forestall the onset of diabetes.
Once diabetes develops, achieving near-normal glucose control can either be very inexpensive with one or more generic drugs, or it can be terribly expensive with the newer branded drugs. Both options can help with the achievement of near-normal glucose levels, but the newer drugs are better for weight loss and provide protection from heart and kidney disease.
It is important to consider where the patient is along the disease spectrum, and to educate them on the benefits of taking a proactive approach to their health. Don’t wait for diabetes to develop before doing something about it. We have to take action earlier, and more definitively.
We do everything we can to help patients with the high cost of diabetes medications. Pharma companies offer various coupons and patient assistance programs, but it's really important that we get people on the right therapy. In order for that to happen, they have to come to office visits and get lab tests done.
Is there anything else you would like to share on this topic?
Dr. McGill: Once a person has been diagnosed with diabetes, then excellent glucose control from the onset has been shown to prevent later complications, and early treatment is inexpensive. As people progress through their journey with diabetes and blood sugars go up, we have excellent therapies that help manage high glucose and help with weight loss.
We have to be realistic and rethink our approach in some ways, but as long as people develop good health care habits and visit the doctor once or twice a year specifically to address diabetes and blood pressure, we might be able to avoid long-term complications.
We know from the literature and in practice that type 2 diabetes (T2D) is one of the most common risk factors for developing chronic kidney disease (CKD). How prevalent is this overlap, and are certain patients more at risk than others?
Dr. McGill: That’s correct, in fact, 20% to 40% of people with T2D have identifiable CKD, and the rest are at risk for developing CKD in the future. All patients with T2D should recognize that risk and undergo annual screening for CKD. If an individual has prediabetes, then step up the screening to perhaps twice a year to see if the person has progressed. At that point, we can think about intervening with a medication to prevent the onset of diabetes, particularly if the patient is unable to adopt significant lifestyle changes.
In your day-to-day practice, what therapeutic approach do you take in managing patients with T2D and CKD?
Dr. McGill: The earliest and arguably the most important treatment for the care of CKD in T2D is glucose control. Establishing and maintaining blood glucose levels near the normal range is our strongest weapon for preventing CKD. Another treatment avenue is controlling blood pressure. The American Diabetes Association and other groups recommend that blood pressure be less than 130/80 mm Hg. It is critical that we treat hypertension effectively to achieve those numbers.
We also have therapies, such as the SGLT2 inhibitors, that offer protection from progression of CKD and from hospitalization for heart failure. Deployment of these newer agents is important for people who have more advanced diabetes or other serious health conditions.
What is the rate of disease progression, related complications, or even mortality for these patients?
Dr. McGill: People with CKD and T2D are at risk for many things. One risk is progression of kidney disease all the way to end-stage kidney disease, which requires dialysis or transplantation. Another huge risk is cardiovascular events such as myocardial infarction (MI) and stroke.
Persons with kidney disease, for reasons we don't understand, are at higher risk of MI and stroke than people who do not have kidney disease. Therefore, the risks of early mortality and events that adversely affect quality of life are greatly increased.
Can you please discuss the economic burdens associated with T2D and CKD, and whether any interventions are in place to help offset those costs?
Dr. McGill: Diabetes itself is wickedly expensive. We have excellent treatments for diabetes today, but they are very costly. The best approach for the prevention of diabetes is to be screened. When a patient presents with prediabetes, it’s important that they take important measures, such as weight loss, exercising 150 minutes per week, or reducing 500 calories a day from their diet, all of which have been shown to forestall the onset of diabetes.
Once diabetes develops, achieving near-normal glucose control can either be very inexpensive with one or more generic drugs, or it can be terribly expensive with the newer branded drugs. Both options can help with the achievement of near-normal glucose levels, but the newer drugs are better for weight loss and provide protection from heart and kidney disease.
It is important to consider where the patient is along the disease spectrum, and to educate them on the benefits of taking a proactive approach to their health. Don’t wait for diabetes to develop before doing something about it. We have to take action earlier, and more definitively.
We do everything we can to help patients with the high cost of diabetes medications. Pharma companies offer various coupons and patient assistance programs, but it's really important that we get people on the right therapy. In order for that to happen, they have to come to office visits and get lab tests done.
Is there anything else you would like to share on this topic?
Dr. McGill: Once a person has been diagnosed with diabetes, then excellent glucose control from the onset has been shown to prevent later complications, and early treatment is inexpensive. As people progress through their journey with diabetes and blood sugars go up, we have excellent therapies that help manage high glucose and help with weight loss.
We have to be realistic and rethink our approach in some ways, but as long as people develop good health care habits and visit the doctor once or twice a year specifically to address diabetes and blood pressure, we might be able to avoid long-term complications.
We know from the literature and in practice that type 2 diabetes (T2D) is one of the most common risk factors for developing chronic kidney disease (CKD). How prevalent is this overlap, and are certain patients more at risk than others?
Dr. McGill: That’s correct, in fact, 20% to 40% of people with T2D have identifiable CKD, and the rest are at risk for developing CKD in the future. All patients with T2D should recognize that risk and undergo annual screening for CKD. If an individual has prediabetes, then step up the screening to perhaps twice a year to see if the person has progressed. At that point, we can think about intervening with a medication to prevent the onset of diabetes, particularly if the patient is unable to adopt significant lifestyle changes.
In your day-to-day practice, what therapeutic approach do you take in managing patients with T2D and CKD?
Dr. McGill: The earliest and arguably the most important treatment for the care of CKD in T2D is glucose control. Establishing and maintaining blood glucose levels near the normal range is our strongest weapon for preventing CKD. Another treatment avenue is controlling blood pressure. The American Diabetes Association and other groups recommend that blood pressure be less than 130/80 mm Hg. It is critical that we treat hypertension effectively to achieve those numbers.
We also have therapies, such as the SGLT2 inhibitors, that offer protection from progression of CKD and from hospitalization for heart failure. Deployment of these newer agents is important for people who have more advanced diabetes or other serious health conditions.
What is the rate of disease progression, related complications, or even mortality for these patients?
Dr. McGill: People with CKD and T2D are at risk for many things. One risk is progression of kidney disease all the way to end-stage kidney disease, which requires dialysis or transplantation. Another huge risk is cardiovascular events such as myocardial infarction (MI) and stroke.
Persons with kidney disease, for reasons we don't understand, are at higher risk of MI and stroke than people who do not have kidney disease. Therefore, the risks of early mortality and events that adversely affect quality of life are greatly increased.
Can you please discuss the economic burdens associated with T2D and CKD, and whether any interventions are in place to help offset those costs?
Dr. McGill: Diabetes itself is wickedly expensive. We have excellent treatments for diabetes today, but they are very costly. The best approach for the prevention of diabetes is to be screened. When a patient presents with prediabetes, it’s important that they take important measures, such as weight loss, exercising 150 minutes per week, or reducing 500 calories a day from their diet, all of which have been shown to forestall the onset of diabetes.
Once diabetes develops, achieving near-normal glucose control can either be very inexpensive with one or more generic drugs, or it can be terribly expensive with the newer branded drugs. Both options can help with the achievement of near-normal glucose levels, but the newer drugs are better for weight loss and provide protection from heart and kidney disease.
It is important to consider where the patient is along the disease spectrum, and to educate them on the benefits of taking a proactive approach to their health. Don’t wait for diabetes to develop before doing something about it. We have to take action earlier, and more definitively.
We do everything we can to help patients with the high cost of diabetes medications. Pharma companies offer various coupons and patient assistance programs, but it's really important that we get people on the right therapy. In order for that to happen, they have to come to office visits and get lab tests done.
Is there anything else you would like to share on this topic?
Dr. McGill: Once a person has been diagnosed with diabetes, then excellent glucose control from the onset has been shown to prevent later complications, and early treatment is inexpensive. As people progress through their journey with diabetes and blood sugars go up, we have excellent therapies that help manage high glucose and help with weight loss.
We have to be realistic and rethink our approach in some ways, but as long as people develop good health care habits and visit the doctor once or twice a year specifically to address diabetes and blood pressure, we might be able to avoid long-term complications.