User login
Moderate-to-severe atopic dermatitis: No increased infection risk with long-term dupilumab use
Key clinical point: In patients with moderate-to-severe atopic dermatitis (AD), continuous long-term dupilumab treatment was not associated with an increased risk for overall systemic/cutaneous infections.
Major finding: At 4 years, the overall infection rate was 71.27 number of patients with ≥1 event per 100 patient-years (nP/100 PY), with most infections being mild to moderate in severity, and only a very small number of infections resulted in treatment discontinuation (0.34 nP/100 PY). The rate of total skin infections decreased from 28.10 to 11.48 nP/100 PY from week 16 to year 4.
Study details: Findings are from the analysis of the LIBERTY AD OLE study including 2677 patients with moderate-to-severe AD who received dupilumab, of which 13.1% completed treatment up to week 204.
Disclosures: This research was sponsored by Sanofi and Regeneron Pharmaceuticals, Inc. Four authors declared being employees and shareholders of Regeneron Pharmaceuticals. Three authors declared being employees or holding stock options in Sanofi. The other authors reported ties with several sources, including Regeneron and Sanofi.
Source: Blauvelt A et al. No increased risk of overall infection in adults with moderate-to-severe atopic dermatitis treated for up to 4 years with dupilumab. Adv Ther. 2022 (Nov 1). Doi: 10.1007/s12325-022-02322-y
Key clinical point: In patients with moderate-to-severe atopic dermatitis (AD), continuous long-term dupilumab treatment was not associated with an increased risk for overall systemic/cutaneous infections.
Major finding: At 4 years, the overall infection rate was 71.27 number of patients with ≥1 event per 100 patient-years (nP/100 PY), with most infections being mild to moderate in severity, and only a very small number of infections resulted in treatment discontinuation (0.34 nP/100 PY). The rate of total skin infections decreased from 28.10 to 11.48 nP/100 PY from week 16 to year 4.
Study details: Findings are from the analysis of the LIBERTY AD OLE study including 2677 patients with moderate-to-severe AD who received dupilumab, of which 13.1% completed treatment up to week 204.
Disclosures: This research was sponsored by Sanofi and Regeneron Pharmaceuticals, Inc. Four authors declared being employees and shareholders of Regeneron Pharmaceuticals. Three authors declared being employees or holding stock options in Sanofi. The other authors reported ties with several sources, including Regeneron and Sanofi.
Source: Blauvelt A et al. No increased risk of overall infection in adults with moderate-to-severe atopic dermatitis treated for up to 4 years with dupilumab. Adv Ther. 2022 (Nov 1). Doi: 10.1007/s12325-022-02322-y
Key clinical point: In patients with moderate-to-severe atopic dermatitis (AD), continuous long-term dupilumab treatment was not associated with an increased risk for overall systemic/cutaneous infections.
Major finding: At 4 years, the overall infection rate was 71.27 number of patients with ≥1 event per 100 patient-years (nP/100 PY), with most infections being mild to moderate in severity, and only a very small number of infections resulted in treatment discontinuation (0.34 nP/100 PY). The rate of total skin infections decreased from 28.10 to 11.48 nP/100 PY from week 16 to year 4.
Study details: Findings are from the analysis of the LIBERTY AD OLE study including 2677 patients with moderate-to-severe AD who received dupilumab, of which 13.1% completed treatment up to week 204.
Disclosures: This research was sponsored by Sanofi and Regeneron Pharmaceuticals, Inc. Four authors declared being employees and shareholders of Regeneron Pharmaceuticals. Three authors declared being employees or holding stock options in Sanofi. The other authors reported ties with several sources, including Regeneron and Sanofi.
Source: Blauvelt A et al. No increased risk of overall infection in adults with moderate-to-severe atopic dermatitis treated for up to 4 years with dupilumab. Adv Ther. 2022 (Nov 1). Doi: 10.1007/s12325-022-02322-y
Exposure to wildfire air pollution increases atopic dermatitis risk in older adults
Key clinical point: Air pollution due to a wildfire increased the rate of clinic visits for atopic dermatitis (AD), especially at a 0-week lag, in adults aged ≥65 years.
Major finding: In adults aged ≥65 years, the adjusted rate of clinic visits for AD during a week with a wildfire was 1.4 (95% CI 1.1-1.9) times the rate during weeks without wildfire and every 1-unit increase in the mean weekly smoke plume density score increased the rate of clinic visits for AD by 1.3 (95% CI 1.1-1.6) times.
Study details: This study analyzed the data of outpatient dermatology visits for AD (5529 visits) and itch (1319 visits).
Disclosures: This study did not report the source of funding. Dr. Grimes declared receiving grants from the University of California, San Francisco.
Source: Fadadu RP et al. Association of exposure to wildfire air pollution with exacerbations of atopic dermatitis and itch among older adults. JAMA Netw Open. 2022;5(10):e2238594 (Oct 26). Doi: 10.1001/jamanetworkopen.2022.38594
Key clinical point: Air pollution due to a wildfire increased the rate of clinic visits for atopic dermatitis (AD), especially at a 0-week lag, in adults aged ≥65 years.
Major finding: In adults aged ≥65 years, the adjusted rate of clinic visits for AD during a week with a wildfire was 1.4 (95% CI 1.1-1.9) times the rate during weeks without wildfire and every 1-unit increase in the mean weekly smoke plume density score increased the rate of clinic visits for AD by 1.3 (95% CI 1.1-1.6) times.
Study details: This study analyzed the data of outpatient dermatology visits for AD (5529 visits) and itch (1319 visits).
Disclosures: This study did not report the source of funding. Dr. Grimes declared receiving grants from the University of California, San Francisco.
Source: Fadadu RP et al. Association of exposure to wildfire air pollution with exacerbations of atopic dermatitis and itch among older adults. JAMA Netw Open. 2022;5(10):e2238594 (Oct 26). Doi: 10.1001/jamanetworkopen.2022.38594
Key clinical point: Air pollution due to a wildfire increased the rate of clinic visits for atopic dermatitis (AD), especially at a 0-week lag, in adults aged ≥65 years.
Major finding: In adults aged ≥65 years, the adjusted rate of clinic visits for AD during a week with a wildfire was 1.4 (95% CI 1.1-1.9) times the rate during weeks without wildfire and every 1-unit increase in the mean weekly smoke plume density score increased the rate of clinic visits for AD by 1.3 (95% CI 1.1-1.6) times.
Study details: This study analyzed the data of outpatient dermatology visits for AD (5529 visits) and itch (1319 visits).
Disclosures: This study did not report the source of funding. Dr. Grimes declared receiving grants from the University of California, San Francisco.
Source: Fadadu RP et al. Association of exposure to wildfire air pollution with exacerbations of atopic dermatitis and itch among older adults. JAMA Netw Open. 2022;5(10):e2238594 (Oct 26). Doi: 10.1001/jamanetworkopen.2022.38594
Atopic dermatitis: Dupilumab serum levels not associated with treatment response or adverse effects
Key clinical point: In patients with atopic dermatitis (AD), serum dupilumab levels at week 16 were not associated with treatment response or adverse effects due to dupilumab during the first year of treatment.
Major finding: Serum dupilumab levels at 16 weeks were not associated with the prediction of treatment response at 52 weeks (≥90% improvement in the Eczema Area and Severity Index; odds ratio [OR] 0.96; P = .34) or adverse events during the first year of treatment (OR 1.01; P = .83).
Study details: Findings are from a prospective clinical cohort study including 295 patients with AD who started dupilumab and had treatment week 16 serum samples available.
Disclosures: This study was funded by AbbVie, Eli Lilly, and other sources. The authors declared receiving consulting fees, speaking fees, investigator fees, or research funding from several sources.
Source: Spekhorst LS et al. Association of serum dupilumab levels at 16 weeks with treatment response and adverse effects in patients with atopic dermatitis: A prospective clinical cohort study from the BioDay registry. JAMA Dermatol. 2022 (Nov 2). Doi: 10.1001/jamadermatol.2022.4639
Key clinical point: In patients with atopic dermatitis (AD), serum dupilumab levels at week 16 were not associated with treatment response or adverse effects due to dupilumab during the first year of treatment.
Major finding: Serum dupilumab levels at 16 weeks were not associated with the prediction of treatment response at 52 weeks (≥90% improvement in the Eczema Area and Severity Index; odds ratio [OR] 0.96; P = .34) or adverse events during the first year of treatment (OR 1.01; P = .83).
Study details: Findings are from a prospective clinical cohort study including 295 patients with AD who started dupilumab and had treatment week 16 serum samples available.
Disclosures: This study was funded by AbbVie, Eli Lilly, and other sources. The authors declared receiving consulting fees, speaking fees, investigator fees, or research funding from several sources.
Source: Spekhorst LS et al. Association of serum dupilumab levels at 16 weeks with treatment response and adverse effects in patients with atopic dermatitis: A prospective clinical cohort study from the BioDay registry. JAMA Dermatol. 2022 (Nov 2). Doi: 10.1001/jamadermatol.2022.4639
Key clinical point: In patients with atopic dermatitis (AD), serum dupilumab levels at week 16 were not associated with treatment response or adverse effects due to dupilumab during the first year of treatment.
Major finding: Serum dupilumab levels at 16 weeks were not associated with the prediction of treatment response at 52 weeks (≥90% improvement in the Eczema Area and Severity Index; odds ratio [OR] 0.96; P = .34) or adverse events during the first year of treatment (OR 1.01; P = .83).
Study details: Findings are from a prospective clinical cohort study including 295 patients with AD who started dupilumab and had treatment week 16 serum samples available.
Disclosures: This study was funded by AbbVie, Eli Lilly, and other sources. The authors declared receiving consulting fees, speaking fees, investigator fees, or research funding from several sources.
Source: Spekhorst LS et al. Association of serum dupilumab levels at 16 weeks with treatment response and adverse effects in patients with atopic dermatitis: A prospective clinical cohort study from the BioDay registry. JAMA Dermatol. 2022 (Nov 2). Doi: 10.1001/jamadermatol.2022.4639
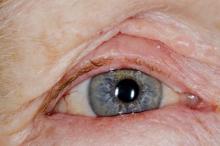
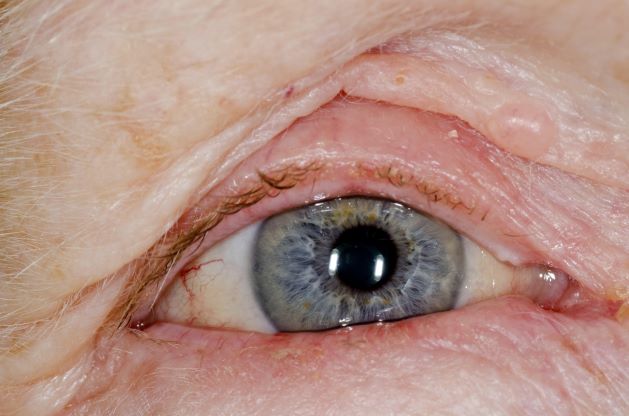
Red swollen eyelids
This patient's symptoms are consistent with a diagnosis of blepharitis.
Blepharitis is an inflammatory disorder of the eyelids that is frequently associated with bacterial colonization of the eyelid. Anatomically, it can be categorized as anterior blepharitis or posterior blepharitis. Anterior blepharitis refers to inflammation primarily positioned around the skin, eyelashes, and lash follicles and is usually further divided into staphylococcal and seborrheic variants. Posterior blepharitis involves the meibomian gland orifices, meibomian glands, tarsal plate, and blepharo-conjunctival junction.
Blepharitis can be acute or chronic. It is frequently associated with systemic diseases, such as rosacea, atopy, and seborrheic dermatitis, as well as ocular diseases, such as dry eye syndromes, chalazion, trichiasis, ectropion and entropion, infectious or other inflammatory conjunctivitis, and keratitis. Moreover, high rates of blepharitis have been reported in patients treated with dupilumab for atopic dermatitis.
Eye irritation, itching, erythema of the lids, flaking of the lid margins, and/or changes in the eyelashes are common presenting symptoms in patients with blepharitis. Other symptoms may include:
• Burning
• Watering
• Foreign-body sensation
• Crusting and mattering of the lashes and medial canthus
• Red lids
• Red eyes
• Photophobia
• Pain
• Decreased vision
• Visual fluctuations
• Heat, cold, alcohol, and spicy-food intolerance
The differential diagnosis for blepharitis includes bacterial keratitis, which is a serious ocular disorder that can lead to vision loss if not properly treated. Bacterial keratitis progresses rapidly and can result in corneal destruction within 24-48 hours with some particularly virulent bacteria. Patients with bacterial keratitis typically report rapid onset of pain, photophobia, and decreased vision.
Ocular rosacea should also be considered in the differential diagnosis of blepharitis, and the two conditions can co-occur. Patients with ocular rosacea may experience facial symptoms (eg, recurrent flushing episodes, persistent and/or recurrent midfacial erythema, papular and pustular lesions) in addition to ocular symptoms, which can range from minor irritation, foreign-body sensation, and blurry vision to severe ocular surface disruption and inflammatory keratitis.
Bacterial conjunctivitis involves inflammation of the bulbar and/or palpebral conjunctiva, whereas blepharitis involves inflammation of the eyelids only. Other conditions to consider in the diagnosis of blepharitis can be found here.
Given the unprecedented efficacy seen in clinical trials, dupilumab is emerging as a first-line therapeutic for moderate to severe atopic dermatitis. However, clinicians should be alert to ocular complications among their patients with atopic dermatitis who are being treated with dupilumab. In some patients, this may be because of preexisting meibomian gland disease and ocular surface disease. After a diagnosis of ocular complications, the continued use of dupilumab should be jointly evaluated by the ophthalmologist and dermatologist or allergist on the basis of the ocular risk vs systemic benefit. Treatment for blepharitis typically includes strict eyelid hygiene and topical antibiotic ointment; oral antibiotics can be beneficial for refractory disease.
William D. James, MD, Professor, Department of Dermatology, University of Pennsylvania, Philadelphia.
Disclosure: William D. James, MD, has disclosed the following relevant financial relationships:
Received income in an amount equal to or greater than $250 from: Elsevier.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
This patient's symptoms are consistent with a diagnosis of blepharitis.
Blepharitis is an inflammatory disorder of the eyelids that is frequently associated with bacterial colonization of the eyelid. Anatomically, it can be categorized as anterior blepharitis or posterior blepharitis. Anterior blepharitis refers to inflammation primarily positioned around the skin, eyelashes, and lash follicles and is usually further divided into staphylococcal and seborrheic variants. Posterior blepharitis involves the meibomian gland orifices, meibomian glands, tarsal plate, and blepharo-conjunctival junction.
Blepharitis can be acute or chronic. It is frequently associated with systemic diseases, such as rosacea, atopy, and seborrheic dermatitis, as well as ocular diseases, such as dry eye syndromes, chalazion, trichiasis, ectropion and entropion, infectious or other inflammatory conjunctivitis, and keratitis. Moreover, high rates of blepharitis have been reported in patients treated with dupilumab for atopic dermatitis.
Eye irritation, itching, erythema of the lids, flaking of the lid margins, and/or changes in the eyelashes are common presenting symptoms in patients with blepharitis. Other symptoms may include:
• Burning
• Watering
• Foreign-body sensation
• Crusting and mattering of the lashes and medial canthus
• Red lids
• Red eyes
• Photophobia
• Pain
• Decreased vision
• Visual fluctuations
• Heat, cold, alcohol, and spicy-food intolerance
The differential diagnosis for blepharitis includes bacterial keratitis, which is a serious ocular disorder that can lead to vision loss if not properly treated. Bacterial keratitis progresses rapidly and can result in corneal destruction within 24-48 hours with some particularly virulent bacteria. Patients with bacterial keratitis typically report rapid onset of pain, photophobia, and decreased vision.
Ocular rosacea should also be considered in the differential diagnosis of blepharitis, and the two conditions can co-occur. Patients with ocular rosacea may experience facial symptoms (eg, recurrent flushing episodes, persistent and/or recurrent midfacial erythema, papular and pustular lesions) in addition to ocular symptoms, which can range from minor irritation, foreign-body sensation, and blurry vision to severe ocular surface disruption and inflammatory keratitis.
Bacterial conjunctivitis involves inflammation of the bulbar and/or palpebral conjunctiva, whereas blepharitis involves inflammation of the eyelids only. Other conditions to consider in the diagnosis of blepharitis can be found here.
Given the unprecedented efficacy seen in clinical trials, dupilumab is emerging as a first-line therapeutic for moderate to severe atopic dermatitis. However, clinicians should be alert to ocular complications among their patients with atopic dermatitis who are being treated with dupilumab. In some patients, this may be because of preexisting meibomian gland disease and ocular surface disease. After a diagnosis of ocular complications, the continued use of dupilumab should be jointly evaluated by the ophthalmologist and dermatologist or allergist on the basis of the ocular risk vs systemic benefit. Treatment for blepharitis typically includes strict eyelid hygiene and topical antibiotic ointment; oral antibiotics can be beneficial for refractory disease.
William D. James, MD, Professor, Department of Dermatology, University of Pennsylvania, Philadelphia.
Disclosure: William D. James, MD, has disclosed the following relevant financial relationships:
Received income in an amount equal to or greater than $250 from: Elsevier.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
This patient's symptoms are consistent with a diagnosis of blepharitis.
Blepharitis is an inflammatory disorder of the eyelids that is frequently associated with bacterial colonization of the eyelid. Anatomically, it can be categorized as anterior blepharitis or posterior blepharitis. Anterior blepharitis refers to inflammation primarily positioned around the skin, eyelashes, and lash follicles and is usually further divided into staphylococcal and seborrheic variants. Posterior blepharitis involves the meibomian gland orifices, meibomian glands, tarsal plate, and blepharo-conjunctival junction.
Blepharitis can be acute or chronic. It is frequently associated with systemic diseases, such as rosacea, atopy, and seborrheic dermatitis, as well as ocular diseases, such as dry eye syndromes, chalazion, trichiasis, ectropion and entropion, infectious or other inflammatory conjunctivitis, and keratitis. Moreover, high rates of blepharitis have been reported in patients treated with dupilumab for atopic dermatitis.
Eye irritation, itching, erythema of the lids, flaking of the lid margins, and/or changes in the eyelashes are common presenting symptoms in patients with blepharitis. Other symptoms may include:
• Burning
• Watering
• Foreign-body sensation
• Crusting and mattering of the lashes and medial canthus
• Red lids
• Red eyes
• Photophobia
• Pain
• Decreased vision
• Visual fluctuations
• Heat, cold, alcohol, and spicy-food intolerance
The differential diagnosis for blepharitis includes bacterial keratitis, which is a serious ocular disorder that can lead to vision loss if not properly treated. Bacterial keratitis progresses rapidly and can result in corneal destruction within 24-48 hours with some particularly virulent bacteria. Patients with bacterial keratitis typically report rapid onset of pain, photophobia, and decreased vision.
Ocular rosacea should also be considered in the differential diagnosis of blepharitis, and the two conditions can co-occur. Patients with ocular rosacea may experience facial symptoms (eg, recurrent flushing episodes, persistent and/or recurrent midfacial erythema, papular and pustular lesions) in addition to ocular symptoms, which can range from minor irritation, foreign-body sensation, and blurry vision to severe ocular surface disruption and inflammatory keratitis.
Bacterial conjunctivitis involves inflammation of the bulbar and/or palpebral conjunctiva, whereas blepharitis involves inflammation of the eyelids only. Other conditions to consider in the diagnosis of blepharitis can be found here.
Given the unprecedented efficacy seen in clinical trials, dupilumab is emerging as a first-line therapeutic for moderate to severe atopic dermatitis. However, clinicians should be alert to ocular complications among their patients with atopic dermatitis who are being treated with dupilumab. In some patients, this may be because of preexisting meibomian gland disease and ocular surface disease. After a diagnosis of ocular complications, the continued use of dupilumab should be jointly evaluated by the ophthalmologist and dermatologist or allergist on the basis of the ocular risk vs systemic benefit. Treatment for blepharitis typically includes strict eyelid hygiene and topical antibiotic ointment; oral antibiotics can be beneficial for refractory disease.
William D. James, MD, Professor, Department of Dermatology, University of Pennsylvania, Philadelphia.
Disclosure: William D. James, MD, has disclosed the following relevant financial relationships:
Received income in an amount equal to or greater than $250 from: Elsevier.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 71-year-old woman was referred for an ophthalmologic examination by her dermatologist. The patient reports recent onset of red, swollen eyelids; ocular itching; and a burning sensation. Prior medical history includes severe atopic dermatitis, type 2 diabetes, and osteoarthritis. Current medications include metformin 1000 mg/d, celecoxib 200 mg/d, and clobetasol propionate 0.05% cream twice daily. The patient began receiving subcutaneous dupilumab 300 mg/once every 2 weeks about 6 weeks earlier.
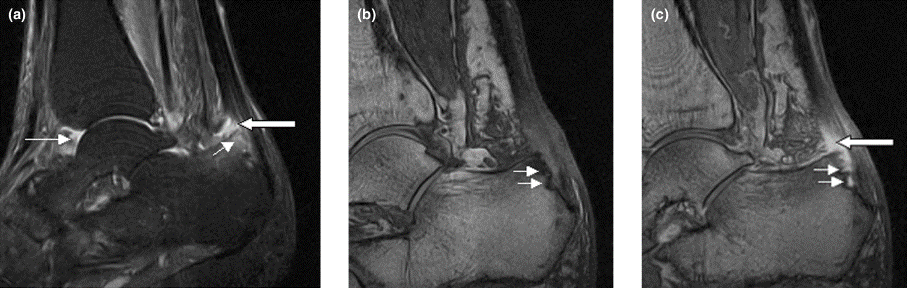
Right ankle pain and swelling
This patient's findings are consistent with a diagnosis of psoriatic enthesitis.
Enthesitis is a hallmark manifestation of psoriatic arthritis (PsA). Approximately 30% of patients with psoriasis are estimated to be affected by PsA, which belongs to the spondyloarthritis (SpA) family of inflammatory rheumatic diseases.
An enthesis is an attachment site of ligaments, tendons, and joint capsules to bone and is a key inflammatory target in SpA. It is a complex structure that dissipates biomechanical stress to preserve homeostasis. Entheses are anatomically and functionally integrated with bursa, fibrocartilage, and synovium in a synovial entheseal complex; biomechanical stress in this area may trigger inflammation. Enthesitis is an early manifestation of PsA that has been associated with radiographic peripheral/axial joint damage and severe disease, as well as reduced quality of life.
Enthesitis can be difficult to diagnose in clinical practice. Symptoms include tenderness, soreness, and pain at entheses on palpation, often without overt clinical evidence of inflammation. In contrast, dactylitis, another hallmark manifestation of PsA, can be recognized by swelling of an entire digit that is different from adjacent digits. Fibromyalgia frequently coexists with enthesitis, and it can be difficult to distinguish the two given the anatomic overlap between the tender points of fibromyalgia and many entheseal sites. Long-lasting morning stiffness and a sustained response to a course of steroids is more suggestive of enthesitis, whereas a higher number of somatoform symptoms is more suggestive of fibromyalgia.
Enthesitis is included in the Classification Criteria for Psoriatic Arthritis (CASPAR) as a hallmark of PsA. While it can be diagnosed clinically, imaging studies may be required, particularly in patients in whom symptoms may be difficult to discern. Evidence of enthesitis by conventional radiography includes bone cortex irregularities, erosions, entheseal soft tissue calcifications, and new bone formation; however, entheseal bone changes detected with conventional radiography appear relatively late in the disease process. Ultrasound is highly sensitive for assessing inflammation and can detect various features of enthesitis, such as increased thickness of tendon insertion, hypoechogenicity, erosions, enthesophytes, and subclinical enthesitis in people with PsA. MRI has the advantage of identifying perientheseal inflammation with adjacent bone marrow edema. Fat-suppressed MRI with or without gadolinium enhancement is a highly sensitive method for visualizing active enthesitis and can identify perientheseal inflammation with adjacent bone marrow edema.
Delayed treatment of PsA can result in irreversible joint damage and reduced quality of life; thus, patients with psoriasis should be closely monitored for early signs of its development, such as enthesitis. A thorough evaluation of the key clinical features of PsA (psoriasis, arthritis, enthesitis, dactylitis, and spondylitis), including evaluation of severity of each feature and impact on physical function and quality of life, is encouraged at each clinical encounter. Because patients may not understand the link between psoriasis and joint pain, specific probing questions can be helpful. Screening questionnaires to detect early signs and symptoms of PsA are available, such as the Psoriasis Epidemiology Screening Tool (PEST), Psoriatic Arthritis Screening and Evaluation (PASE) questionnaire, and Toronto Psoriatic Arthritis Screening (ToPAS) questionnaire. These and many others may be used to help dermatologists detect early signs and symptoms of PsA. Although these questionnaires all have limitations in sensitivity and specificity for the diagnosis of PsA, their use can still improve early diagnosis.
The treatment of PsA focuses on achieving the least amount of disease activity and inflammation possible; optimizing functional status, quality of life, and well-being; and preventing structural damage. Treatment decisions are based on the specific domains affected. Nonsteroidal anti-inflammatory drugs and corticosteroid injections are first-line treatments for enthesitis. Early use of tumor necrosis factor inhibitors (TNF) (adalimumab, certolizumab pegol, etanercept, infliximab, and golimumab) is recommended. Alternative biologic disease-modifying agents are indicated when these TNF inhibitors provide an inadequate response. They include ustekinumab (dual interleukin [IL]-12 and IL-23 inhibitor), secukinumab (IL-17A inhibitor), and apremilast (phosphodiesterase-4 inhibitor) and may be considered for patients with predominantly entheseal manifestations of PsA or dactylitis. Biological disease-modifying agents approved for PsA that have shown efficacy for enthesitis include ixekizumab (which targets IL-17A), abatacept (a T-cell inhibitor), guselkumab (monoclonal antibody), and ustekinumab (monoclonal antibody). Tofacitinib and upadacitinib, both oral Janus kinase inhibitors, may also be considered.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
This patient's findings are consistent with a diagnosis of psoriatic enthesitis.
Enthesitis is a hallmark manifestation of psoriatic arthritis (PsA). Approximately 30% of patients with psoriasis are estimated to be affected by PsA, which belongs to the spondyloarthritis (SpA) family of inflammatory rheumatic diseases.
An enthesis is an attachment site of ligaments, tendons, and joint capsules to bone and is a key inflammatory target in SpA. It is a complex structure that dissipates biomechanical stress to preserve homeostasis. Entheses are anatomically and functionally integrated with bursa, fibrocartilage, and synovium in a synovial entheseal complex; biomechanical stress in this area may trigger inflammation. Enthesitis is an early manifestation of PsA that has been associated with radiographic peripheral/axial joint damage and severe disease, as well as reduced quality of life.
Enthesitis can be difficult to diagnose in clinical practice. Symptoms include tenderness, soreness, and pain at entheses on palpation, often without overt clinical evidence of inflammation. In contrast, dactylitis, another hallmark manifestation of PsA, can be recognized by swelling of an entire digit that is different from adjacent digits. Fibromyalgia frequently coexists with enthesitis, and it can be difficult to distinguish the two given the anatomic overlap between the tender points of fibromyalgia and many entheseal sites. Long-lasting morning stiffness and a sustained response to a course of steroids is more suggestive of enthesitis, whereas a higher number of somatoform symptoms is more suggestive of fibromyalgia.
Enthesitis is included in the Classification Criteria for Psoriatic Arthritis (CASPAR) as a hallmark of PsA. While it can be diagnosed clinically, imaging studies may be required, particularly in patients in whom symptoms may be difficult to discern. Evidence of enthesitis by conventional radiography includes bone cortex irregularities, erosions, entheseal soft tissue calcifications, and new bone formation; however, entheseal bone changes detected with conventional radiography appear relatively late in the disease process. Ultrasound is highly sensitive for assessing inflammation and can detect various features of enthesitis, such as increased thickness of tendon insertion, hypoechogenicity, erosions, enthesophytes, and subclinical enthesitis in people with PsA. MRI has the advantage of identifying perientheseal inflammation with adjacent bone marrow edema. Fat-suppressed MRI with or without gadolinium enhancement is a highly sensitive method for visualizing active enthesitis and can identify perientheseal inflammation with adjacent bone marrow edema.
Delayed treatment of PsA can result in irreversible joint damage and reduced quality of life; thus, patients with psoriasis should be closely monitored for early signs of its development, such as enthesitis. A thorough evaluation of the key clinical features of PsA (psoriasis, arthritis, enthesitis, dactylitis, and spondylitis), including evaluation of severity of each feature and impact on physical function and quality of life, is encouraged at each clinical encounter. Because patients may not understand the link between psoriasis and joint pain, specific probing questions can be helpful. Screening questionnaires to detect early signs and symptoms of PsA are available, such as the Psoriasis Epidemiology Screening Tool (PEST), Psoriatic Arthritis Screening and Evaluation (PASE) questionnaire, and Toronto Psoriatic Arthritis Screening (ToPAS) questionnaire. These and many others may be used to help dermatologists detect early signs and symptoms of PsA. Although these questionnaires all have limitations in sensitivity and specificity for the diagnosis of PsA, their use can still improve early diagnosis.
The treatment of PsA focuses on achieving the least amount of disease activity and inflammation possible; optimizing functional status, quality of life, and well-being; and preventing structural damage. Treatment decisions are based on the specific domains affected. Nonsteroidal anti-inflammatory drugs and corticosteroid injections are first-line treatments for enthesitis. Early use of tumor necrosis factor inhibitors (TNF) (adalimumab, certolizumab pegol, etanercept, infliximab, and golimumab) is recommended. Alternative biologic disease-modifying agents are indicated when these TNF inhibitors provide an inadequate response. They include ustekinumab (dual interleukin [IL]-12 and IL-23 inhibitor), secukinumab (IL-17A inhibitor), and apremilast (phosphodiesterase-4 inhibitor) and may be considered for patients with predominantly entheseal manifestations of PsA or dactylitis. Biological disease-modifying agents approved for PsA that have shown efficacy for enthesitis include ixekizumab (which targets IL-17A), abatacept (a T-cell inhibitor), guselkumab (monoclonal antibody), and ustekinumab (monoclonal antibody). Tofacitinib and upadacitinib, both oral Janus kinase inhibitors, may also be considered.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
This patient's findings are consistent with a diagnosis of psoriatic enthesitis.
Enthesitis is a hallmark manifestation of psoriatic arthritis (PsA). Approximately 30% of patients with psoriasis are estimated to be affected by PsA, which belongs to the spondyloarthritis (SpA) family of inflammatory rheumatic diseases.
An enthesis is an attachment site of ligaments, tendons, and joint capsules to bone and is a key inflammatory target in SpA. It is a complex structure that dissipates biomechanical stress to preserve homeostasis. Entheses are anatomically and functionally integrated with bursa, fibrocartilage, and synovium in a synovial entheseal complex; biomechanical stress in this area may trigger inflammation. Enthesitis is an early manifestation of PsA that has been associated with radiographic peripheral/axial joint damage and severe disease, as well as reduced quality of life.
Enthesitis can be difficult to diagnose in clinical practice. Symptoms include tenderness, soreness, and pain at entheses on palpation, often without overt clinical evidence of inflammation. In contrast, dactylitis, another hallmark manifestation of PsA, can be recognized by swelling of an entire digit that is different from adjacent digits. Fibromyalgia frequently coexists with enthesitis, and it can be difficult to distinguish the two given the anatomic overlap between the tender points of fibromyalgia and many entheseal sites. Long-lasting morning stiffness and a sustained response to a course of steroids is more suggestive of enthesitis, whereas a higher number of somatoform symptoms is more suggestive of fibromyalgia.
Enthesitis is included in the Classification Criteria for Psoriatic Arthritis (CASPAR) as a hallmark of PsA. While it can be diagnosed clinically, imaging studies may be required, particularly in patients in whom symptoms may be difficult to discern. Evidence of enthesitis by conventional radiography includes bone cortex irregularities, erosions, entheseal soft tissue calcifications, and new bone formation; however, entheseal bone changes detected with conventional radiography appear relatively late in the disease process. Ultrasound is highly sensitive for assessing inflammation and can detect various features of enthesitis, such as increased thickness of tendon insertion, hypoechogenicity, erosions, enthesophytes, and subclinical enthesitis in people with PsA. MRI has the advantage of identifying perientheseal inflammation with adjacent bone marrow edema. Fat-suppressed MRI with or without gadolinium enhancement is a highly sensitive method for visualizing active enthesitis and can identify perientheseal inflammation with adjacent bone marrow edema.
Delayed treatment of PsA can result in irreversible joint damage and reduced quality of life; thus, patients with psoriasis should be closely monitored for early signs of its development, such as enthesitis. A thorough evaluation of the key clinical features of PsA (psoriasis, arthritis, enthesitis, dactylitis, and spondylitis), including evaluation of severity of each feature and impact on physical function and quality of life, is encouraged at each clinical encounter. Because patients may not understand the link between psoriasis and joint pain, specific probing questions can be helpful. Screening questionnaires to detect early signs and symptoms of PsA are available, such as the Psoriasis Epidemiology Screening Tool (PEST), Psoriatic Arthritis Screening and Evaluation (PASE) questionnaire, and Toronto Psoriatic Arthritis Screening (ToPAS) questionnaire. These and many others may be used to help dermatologists detect early signs and symptoms of PsA. Although these questionnaires all have limitations in sensitivity and specificity for the diagnosis of PsA, their use can still improve early diagnosis.
The treatment of PsA focuses on achieving the least amount of disease activity and inflammation possible; optimizing functional status, quality of life, and well-being; and preventing structural damage. Treatment decisions are based on the specific domains affected. Nonsteroidal anti-inflammatory drugs and corticosteroid injections are first-line treatments for enthesitis. Early use of tumor necrosis factor inhibitors (TNF) (adalimumab, certolizumab pegol, etanercept, infliximab, and golimumab) is recommended. Alternative biologic disease-modifying agents are indicated when these TNF inhibitors provide an inadequate response. They include ustekinumab (dual interleukin [IL]-12 and IL-23 inhibitor), secukinumab (IL-17A inhibitor), and apremilast (phosphodiesterase-4 inhibitor) and may be considered for patients with predominantly entheseal manifestations of PsA or dactylitis. Biological disease-modifying agents approved for PsA that have shown efficacy for enthesitis include ixekizumab (which targets IL-17A), abatacept (a T-cell inhibitor), guselkumab (monoclonal antibody), and ustekinumab (monoclonal antibody). Tofacitinib and upadacitinib, both oral Janus kinase inhibitors, may also be considered.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 42-year-old woman with a 20-year history of plaque psoriasis presents with complaints of a 3-month history of pain, tenderness, and swelling in her right ankle and foot, of unknown origin. Physical examination reveals active psoriasis, with a Psoriasis Area and Severity Index (PASI) score of 6.7 and psoriatic nail dystrophy, including onycholysis, pitting, and hyperkeratosis. Tenderness and swelling are noted at the back of the heel. The patient denies any other complaints. Laboratory tests are normal, including negative rheumatoid factor and antinuclear factor. MRI reveals soft tissue and bone marrow edema below the Achilles insertion.
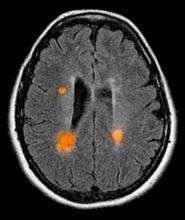
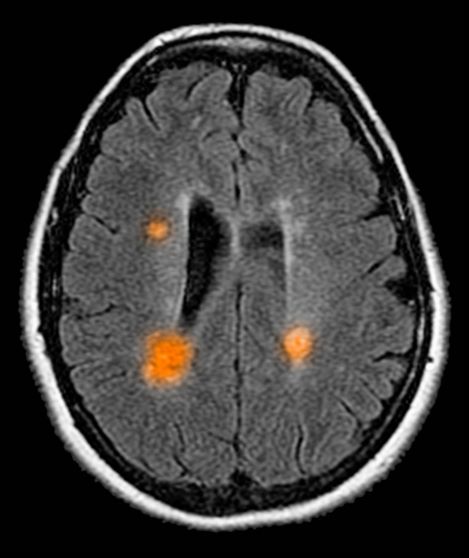
Decline in ambulatory function
Based on this patient's history and presentation, the likely diagnosis is primary progressive multiple sclerosis (PPMS). PPMS represents around 10% of MS cases and tends to develop about a decade later than relapsing MS. Unlike other forms of MS, this phenotype progresses steadily instead of in an episodic fashion like relapsing forms of MS. Most patients with PPMS present with gait difficulty because lesions often develop on the spinal cord. While relapsing-remitting MS (RRMS) is much more common among women than men, men with MS are more likely to have the progressive form.
Although this patient's MRI ultimately points to multiple sclerosis, his functional deficits may initially suggest other conditions in the differential diagnosis. Brainstem gliomas typically manifest in unsteady gait, weakness, double vision, difficulty swallowing, dysarthria, headache, drowsiness, nausea, and vomiting. Transverse myelitis often presents with rapid-onset weakness, sensory deficits, and bowel/bladder dysfunction. Musculoskeletal and neurologic symptoms are common in Lyme disease. B12 deficiency can present with worsening weakness and a sensory ataxia that can present as balance difficulties, but it would not cause focal lesions on the MRI, nor would it present with bladder symptoms. In addition, the patient's steady decline in function rules out RRMS.
PPMS is diagnosed with confirmation of gradual change in functional ability (often ambulation) over time without remission or relapse. These criteria include 1 full year of worsening neurologic function without asymptomatic periods as well as two of these signs of disease: brain lesion, two or more spinal cord lesions, and oligoclonal bands or elevated Immunoglobulin G index. These timing-specific criteria can delay diagnosis, as seen here.
Ocrelizumab is the only FDA-approved disease-modifying therapy (DMT) proven to alter disease progression in ambulatory patients with PPMS. American Academy of Neurology guidelines recommend ocrelizumab for patients with PPMS who are likely to benefit from this therapy. While it is thought that DMTs are more effective at targeting inflammation in RRMS than nerve degeneration in PPMS, these agents may show benefit for patients with active PPMS (relapse and/or evidence of new MRI activity) rather than inactive disease. A recent PPMS study concluded that among patients with relapse or disease activity, DMTs were associated with a significant reduction of long-term disability risk. Together with immunomodulatory therapy, rehabilitation can help manage symptoms.
Krupa Pandey, MD, Director, Multiple Sclerosis Center, Department of Neurology & Neuroscience Institute, Hackensack University Medical Center; Neurologist, Department of Neurology, Hackensack Meridian Health, Hackensack, NJ.
Krupa Pandey, MD, has serve(d) as a speaker or a member of a speakers bureau for: Bristol-Myers Squibb; Biogen; Alexion; Genentech; Sanofi-Genzyme.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Based on this patient's history and presentation, the likely diagnosis is primary progressive multiple sclerosis (PPMS). PPMS represents around 10% of MS cases and tends to develop about a decade later than relapsing MS. Unlike other forms of MS, this phenotype progresses steadily instead of in an episodic fashion like relapsing forms of MS. Most patients with PPMS present with gait difficulty because lesions often develop on the spinal cord. While relapsing-remitting MS (RRMS) is much more common among women than men, men with MS are more likely to have the progressive form.
Although this patient's MRI ultimately points to multiple sclerosis, his functional deficits may initially suggest other conditions in the differential diagnosis. Brainstem gliomas typically manifest in unsteady gait, weakness, double vision, difficulty swallowing, dysarthria, headache, drowsiness, nausea, and vomiting. Transverse myelitis often presents with rapid-onset weakness, sensory deficits, and bowel/bladder dysfunction. Musculoskeletal and neurologic symptoms are common in Lyme disease. B12 deficiency can present with worsening weakness and a sensory ataxia that can present as balance difficulties, but it would not cause focal lesions on the MRI, nor would it present with bladder symptoms. In addition, the patient's steady decline in function rules out RRMS.
PPMS is diagnosed with confirmation of gradual change in functional ability (often ambulation) over time without remission or relapse. These criteria include 1 full year of worsening neurologic function without asymptomatic periods as well as two of these signs of disease: brain lesion, two or more spinal cord lesions, and oligoclonal bands or elevated Immunoglobulin G index. These timing-specific criteria can delay diagnosis, as seen here.
Ocrelizumab is the only FDA-approved disease-modifying therapy (DMT) proven to alter disease progression in ambulatory patients with PPMS. American Academy of Neurology guidelines recommend ocrelizumab for patients with PPMS who are likely to benefit from this therapy. While it is thought that DMTs are more effective at targeting inflammation in RRMS than nerve degeneration in PPMS, these agents may show benefit for patients with active PPMS (relapse and/or evidence of new MRI activity) rather than inactive disease. A recent PPMS study concluded that among patients with relapse or disease activity, DMTs were associated with a significant reduction of long-term disability risk. Together with immunomodulatory therapy, rehabilitation can help manage symptoms.
Krupa Pandey, MD, Director, Multiple Sclerosis Center, Department of Neurology & Neuroscience Institute, Hackensack University Medical Center; Neurologist, Department of Neurology, Hackensack Meridian Health, Hackensack, NJ.
Krupa Pandey, MD, has serve(d) as a speaker or a member of a speakers bureau for: Bristol-Myers Squibb; Biogen; Alexion; Genentech; Sanofi-Genzyme.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Based on this patient's history and presentation, the likely diagnosis is primary progressive multiple sclerosis (PPMS). PPMS represents around 10% of MS cases and tends to develop about a decade later than relapsing MS. Unlike other forms of MS, this phenotype progresses steadily instead of in an episodic fashion like relapsing forms of MS. Most patients with PPMS present with gait difficulty because lesions often develop on the spinal cord. While relapsing-remitting MS (RRMS) is much more common among women than men, men with MS are more likely to have the progressive form.
Although this patient's MRI ultimately points to multiple sclerosis, his functional deficits may initially suggest other conditions in the differential diagnosis. Brainstem gliomas typically manifest in unsteady gait, weakness, double vision, difficulty swallowing, dysarthria, headache, drowsiness, nausea, and vomiting. Transverse myelitis often presents with rapid-onset weakness, sensory deficits, and bowel/bladder dysfunction. Musculoskeletal and neurologic symptoms are common in Lyme disease. B12 deficiency can present with worsening weakness and a sensory ataxia that can present as balance difficulties, but it would not cause focal lesions on the MRI, nor would it present with bladder symptoms. In addition, the patient's steady decline in function rules out RRMS.
PPMS is diagnosed with confirmation of gradual change in functional ability (often ambulation) over time without remission or relapse. These criteria include 1 full year of worsening neurologic function without asymptomatic periods as well as two of these signs of disease: brain lesion, two or more spinal cord lesions, and oligoclonal bands or elevated Immunoglobulin G index. These timing-specific criteria can delay diagnosis, as seen here.
Ocrelizumab is the only FDA-approved disease-modifying therapy (DMT) proven to alter disease progression in ambulatory patients with PPMS. American Academy of Neurology guidelines recommend ocrelizumab for patients with PPMS who are likely to benefit from this therapy. While it is thought that DMTs are more effective at targeting inflammation in RRMS than nerve degeneration in PPMS, these agents may show benefit for patients with active PPMS (relapse and/or evidence of new MRI activity) rather than inactive disease. A recent PPMS study concluded that among patients with relapse or disease activity, DMTs were associated with a significant reduction of long-term disability risk. Together with immunomodulatory therapy, rehabilitation can help manage symptoms.
Krupa Pandey, MD, Director, Multiple Sclerosis Center, Department of Neurology & Neuroscience Institute, Hackensack University Medical Center; Neurologist, Department of Neurology, Hackensack Meridian Health, Hackensack, NJ.
Krupa Pandey, MD, has serve(d) as a speaker or a member of a speakers bureau for: Bristol-Myers Squibb; Biogen; Alexion; Genentech; Sanofi-Genzyme.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 59-year-old man presents with worsening decline in ambulatory function and worsening bladder function. He reports "difficulty getting around" for the past year and a half, which he theorized might be because of arthritis, aging, or many years of biking. He presented to his primary care physician 2 months ago and was referred to rheumatology. His height is 5 ft 11 in and his weight is 166 lb (BMI 23.1). The patient subsequently reported a decreased attention span to the rheumatologist. He has no other significant medical or surgical history, though his brother has psoriatic arthritis. MRI shows multiple brain lesions without gadolinium enhancement and multiple spinal cord lesions.
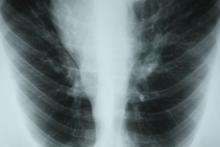
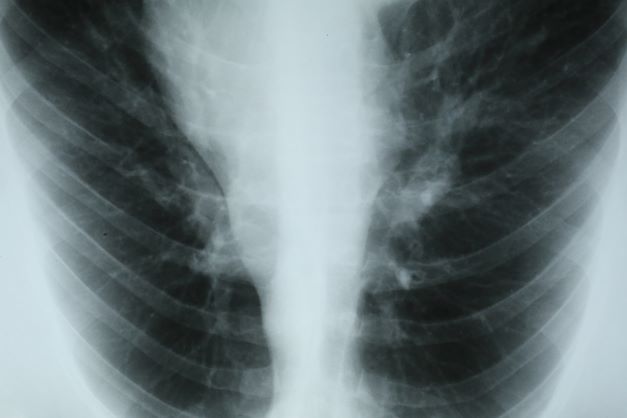
Chest tightness and wheezing
This patient's physical examination and imaging findings are consistent with a diagnosis of acute severe asthma. Agitation, breathlessness during rest, and a respiratory rate > 30 breaths/min are some manifestations of an acute severe episode. During severe episodes, accessory muscles of respiration are usually used, and suprasternal retractions are often present. The heart rate is > 120 beats/min and the respiratory rate is > 30 breaths/min. Loud biphasic (expiratory and inspiratory) wheezing can be heard, and pulsus paradoxus is often present (20-40 mm Hg). Oxyhemoglobin saturation with room air is < 91%. As the severity increases, the patient increasingly assumes a hunched-over sitting position with the hands supporting the torso, termed the tripod position.
Asthma is a chronic, heterogenous inflammatory airway disorder characterized by variable expiratory flow; airway wall thickening; respiratory symptoms; and exacerbations, which sometimes require hospitalization. According to the World Health Organization, asthma affected an estimated 262 million people in 2019. The presence of airway hyperresponsiveness or bronchial hyperreactivity in asthma is an exaggerated response to various exogenous and endogenous stimuli. Mechanisms implicated in the development of asthma include direct stimulation of airway smooth muscle and indirect stimulation by pharmacologically active substances from mediator-secreting cells, such as mast cells or nonmyelinated sensory neurons. The degree of airway hyperresponsiveness is associated with the clinical severity of asthma.
Acute severe asthma is a life-threatening emergency characterized by severe airflow limitation that is unresponsive to the typical appropriate bronchodilator therapy. As a result of pathophysiologic changes, airflow is severely restricted in severe asthma, leading to premature closure of the airway on expiration; impaired gas exchange; and dynamic hyperinflation, or air-trapping. In such cases, urgent action is essential to thwart serious outcomes, including mechanical ventilation and death.
Asthma severity is defined by the level of treatment required to control a patient's symptoms and exacerbations. According to the 2022 Global Initiative for Asthma (GINA) guidelines, a severe asthma exacerbation describes a patient who talks in words (rather than sentences); leans forward; is agitated; uses accessory respiratory muscles; and has a respiratory rate > 30 breaths/min, heart rate > 120 beats/min, oxygen saturation on air < 90%, and peak expiratory flow ≤ 50% of their best or of predicted value. Given the heterogeneity of asthma, patients with acute severe asthma may present with a variety of signs and symptoms, including dyspnea, chest tightness, cough and wheezing, agitation, drowsiness or signs of confusion, and significant breathlessness at rest.
Exposure to external agents, such as indoor and outdoor allergens, air pollutants, and respiratory tract infections (primarily viral), are the most common causes of asthma exacerbations, which vary in severity. Numerous other factors can trigger an asthma exacerbation, including exercise, weather changes, certain foods, additives, drugs, extreme emotional expressions, rhinitis, sinusitis, polyposis, gastroesophageal reflux, menstruation, and pregnancy. Importantly, a patient with known asthma of any level of severity can experience an asthma exacerbation, including patients with mild or well-controlled asthma.
Patients with a history of poorly controlled asthma or a recent exacerbation are at risk for an acute asthma exacerbation. Other risk factors include poor perception of airflow limitation, regular or overuse of short-acting beta agonists, incorrect inhaler technique, and suboptimal adherence to therapy. Comorbidities associated with risk for an acute asthma exacerbation include obesity, chronic rhinosinusitis, inducible laryngeal obstruction (vocal cord dysfunction), gastroesophageal reflux disease, chronic obstructive pulmonary disease, obstructive sleep apnea, bronchiectasis, cardiac disease, and kyphosis due to osteoporosis (followed by corticosteroid overuse). The lack of a written asthma action plan and socioeconomic factors are also associated with increased risk for a severe exacerbation.
In the emergency department setting, pharmacologic therapy of acute severe asthma should consist of a short-acting beta agonist, ipratropium bromide, systemic corticosteroids (oral or intravenous), and controlled oxygen therapy. Clinicians may also consider intravenous magnesium sulfate and high-dose inhaled corticosteroids. Once stable, patients should be treated with optimal asthma-controlling therapy, as outlined in GINA guidelines. Optimizing patients' inhaler technique and adherence to therapy are imperative, and comorbidities should be appropriately managed. Nonpharmacologic interventions, such as smoking cessation, pulmonary rehabilitation, exercise, weight loss, and influenza/COVID-19 vaccination, are also recommended as indicated.
Zab Mosenifar, MD, Medical Director, Women's Lung Institute; Executive Vice Chairman, Department of Medicine, Cedars Sinai Medical Center, Los Angeles, California.
Zab Mosenifar, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
This patient's physical examination and imaging findings are consistent with a diagnosis of acute severe asthma. Agitation, breathlessness during rest, and a respiratory rate > 30 breaths/min are some manifestations of an acute severe episode. During severe episodes, accessory muscles of respiration are usually used, and suprasternal retractions are often present. The heart rate is > 120 beats/min and the respiratory rate is > 30 breaths/min. Loud biphasic (expiratory and inspiratory) wheezing can be heard, and pulsus paradoxus is often present (20-40 mm Hg). Oxyhemoglobin saturation with room air is < 91%. As the severity increases, the patient increasingly assumes a hunched-over sitting position with the hands supporting the torso, termed the tripod position.
Asthma is a chronic, heterogenous inflammatory airway disorder characterized by variable expiratory flow; airway wall thickening; respiratory symptoms; and exacerbations, which sometimes require hospitalization. According to the World Health Organization, asthma affected an estimated 262 million people in 2019. The presence of airway hyperresponsiveness or bronchial hyperreactivity in asthma is an exaggerated response to various exogenous and endogenous stimuli. Mechanisms implicated in the development of asthma include direct stimulation of airway smooth muscle and indirect stimulation by pharmacologically active substances from mediator-secreting cells, such as mast cells or nonmyelinated sensory neurons. The degree of airway hyperresponsiveness is associated with the clinical severity of asthma.
Acute severe asthma is a life-threatening emergency characterized by severe airflow limitation that is unresponsive to the typical appropriate bronchodilator therapy. As a result of pathophysiologic changes, airflow is severely restricted in severe asthma, leading to premature closure of the airway on expiration; impaired gas exchange; and dynamic hyperinflation, or air-trapping. In such cases, urgent action is essential to thwart serious outcomes, including mechanical ventilation and death.
Asthma severity is defined by the level of treatment required to control a patient's symptoms and exacerbations. According to the 2022 Global Initiative for Asthma (GINA) guidelines, a severe asthma exacerbation describes a patient who talks in words (rather than sentences); leans forward; is agitated; uses accessory respiratory muscles; and has a respiratory rate > 30 breaths/min, heart rate > 120 beats/min, oxygen saturation on air < 90%, and peak expiratory flow ≤ 50% of their best or of predicted value. Given the heterogeneity of asthma, patients with acute severe asthma may present with a variety of signs and symptoms, including dyspnea, chest tightness, cough and wheezing, agitation, drowsiness or signs of confusion, and significant breathlessness at rest.
Exposure to external agents, such as indoor and outdoor allergens, air pollutants, and respiratory tract infections (primarily viral), are the most common causes of asthma exacerbations, which vary in severity. Numerous other factors can trigger an asthma exacerbation, including exercise, weather changes, certain foods, additives, drugs, extreme emotional expressions, rhinitis, sinusitis, polyposis, gastroesophageal reflux, menstruation, and pregnancy. Importantly, a patient with known asthma of any level of severity can experience an asthma exacerbation, including patients with mild or well-controlled asthma.
Patients with a history of poorly controlled asthma or a recent exacerbation are at risk for an acute asthma exacerbation. Other risk factors include poor perception of airflow limitation, regular or overuse of short-acting beta agonists, incorrect inhaler technique, and suboptimal adherence to therapy. Comorbidities associated with risk for an acute asthma exacerbation include obesity, chronic rhinosinusitis, inducible laryngeal obstruction (vocal cord dysfunction), gastroesophageal reflux disease, chronic obstructive pulmonary disease, obstructive sleep apnea, bronchiectasis, cardiac disease, and kyphosis due to osteoporosis (followed by corticosteroid overuse). The lack of a written asthma action plan and socioeconomic factors are also associated with increased risk for a severe exacerbation.
In the emergency department setting, pharmacologic therapy of acute severe asthma should consist of a short-acting beta agonist, ipratropium bromide, systemic corticosteroids (oral or intravenous), and controlled oxygen therapy. Clinicians may also consider intravenous magnesium sulfate and high-dose inhaled corticosteroids. Once stable, patients should be treated with optimal asthma-controlling therapy, as outlined in GINA guidelines. Optimizing patients' inhaler technique and adherence to therapy are imperative, and comorbidities should be appropriately managed. Nonpharmacologic interventions, such as smoking cessation, pulmonary rehabilitation, exercise, weight loss, and influenza/COVID-19 vaccination, are also recommended as indicated.
Zab Mosenifar, MD, Medical Director, Women's Lung Institute; Executive Vice Chairman, Department of Medicine, Cedars Sinai Medical Center, Los Angeles, California.
Zab Mosenifar, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
This patient's physical examination and imaging findings are consistent with a diagnosis of acute severe asthma. Agitation, breathlessness during rest, and a respiratory rate > 30 breaths/min are some manifestations of an acute severe episode. During severe episodes, accessory muscles of respiration are usually used, and suprasternal retractions are often present. The heart rate is > 120 beats/min and the respiratory rate is > 30 breaths/min. Loud biphasic (expiratory and inspiratory) wheezing can be heard, and pulsus paradoxus is often present (20-40 mm Hg). Oxyhemoglobin saturation with room air is < 91%. As the severity increases, the patient increasingly assumes a hunched-over sitting position with the hands supporting the torso, termed the tripod position.
Asthma is a chronic, heterogenous inflammatory airway disorder characterized by variable expiratory flow; airway wall thickening; respiratory symptoms; and exacerbations, which sometimes require hospitalization. According to the World Health Organization, asthma affected an estimated 262 million people in 2019. The presence of airway hyperresponsiveness or bronchial hyperreactivity in asthma is an exaggerated response to various exogenous and endogenous stimuli. Mechanisms implicated in the development of asthma include direct stimulation of airway smooth muscle and indirect stimulation by pharmacologically active substances from mediator-secreting cells, such as mast cells or nonmyelinated sensory neurons. The degree of airway hyperresponsiveness is associated with the clinical severity of asthma.
Acute severe asthma is a life-threatening emergency characterized by severe airflow limitation that is unresponsive to the typical appropriate bronchodilator therapy. As a result of pathophysiologic changes, airflow is severely restricted in severe asthma, leading to premature closure of the airway on expiration; impaired gas exchange; and dynamic hyperinflation, or air-trapping. In such cases, urgent action is essential to thwart serious outcomes, including mechanical ventilation and death.
Asthma severity is defined by the level of treatment required to control a patient's symptoms and exacerbations. According to the 2022 Global Initiative for Asthma (GINA) guidelines, a severe asthma exacerbation describes a patient who talks in words (rather than sentences); leans forward; is agitated; uses accessory respiratory muscles; and has a respiratory rate > 30 breaths/min, heart rate > 120 beats/min, oxygen saturation on air < 90%, and peak expiratory flow ≤ 50% of their best or of predicted value. Given the heterogeneity of asthma, patients with acute severe asthma may present with a variety of signs and symptoms, including dyspnea, chest tightness, cough and wheezing, agitation, drowsiness or signs of confusion, and significant breathlessness at rest.
Exposure to external agents, such as indoor and outdoor allergens, air pollutants, and respiratory tract infections (primarily viral), are the most common causes of asthma exacerbations, which vary in severity. Numerous other factors can trigger an asthma exacerbation, including exercise, weather changes, certain foods, additives, drugs, extreme emotional expressions, rhinitis, sinusitis, polyposis, gastroesophageal reflux, menstruation, and pregnancy. Importantly, a patient with known asthma of any level of severity can experience an asthma exacerbation, including patients with mild or well-controlled asthma.
Patients with a history of poorly controlled asthma or a recent exacerbation are at risk for an acute asthma exacerbation. Other risk factors include poor perception of airflow limitation, regular or overuse of short-acting beta agonists, incorrect inhaler technique, and suboptimal adherence to therapy. Comorbidities associated with risk for an acute asthma exacerbation include obesity, chronic rhinosinusitis, inducible laryngeal obstruction (vocal cord dysfunction), gastroesophageal reflux disease, chronic obstructive pulmonary disease, obstructive sleep apnea, bronchiectasis, cardiac disease, and kyphosis due to osteoporosis (followed by corticosteroid overuse). The lack of a written asthma action plan and socioeconomic factors are also associated with increased risk for a severe exacerbation.
In the emergency department setting, pharmacologic therapy of acute severe asthma should consist of a short-acting beta agonist, ipratropium bromide, systemic corticosteroids (oral or intravenous), and controlled oxygen therapy. Clinicians may also consider intravenous magnesium sulfate and high-dose inhaled corticosteroids. Once stable, patients should be treated with optimal asthma-controlling therapy, as outlined in GINA guidelines. Optimizing patients' inhaler technique and adherence to therapy are imperative, and comorbidities should be appropriately managed. Nonpharmacologic interventions, such as smoking cessation, pulmonary rehabilitation, exercise, weight loss, and influenza/COVID-19 vaccination, are also recommended as indicated.
Zab Mosenifar, MD, Medical Director, Women's Lung Institute; Executive Vice Chairman, Department of Medicine, Cedars Sinai Medical Center, Los Angeles, California.
Zab Mosenifar, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 32-year-old Black man presents to the emergency department with severe dyspnea, chest tightness, and wheezing. The patient is sitting forward in the tripod position and appears agitated and confused. Use of accessory respiratory muscles and suprasternal retractions are noted. He reports an approximate 2-week history of rhinorrhea, cough, and mild fever, for which he has been taking an over-the-counter nonsteroidal anti-inflammatory agent and cough suppressant. His prior medical history is notable for obesity, type 2 diabetes, allergic rhinitis, mild asthma, and hypercholesterolemia. The patient is a current smoker (17 pack-years). Pertinent physical examination reveals a respiratory rate of 48 breaths/min, heart rate of 135 beats/min, 87% oxygen saturation, and peak expiratory flow of 300 L/min. Low biphasic wheezing can be heard. Rapid antigen and PCR tests for SARS-CoV-2 detected by nasopharyngeal swabs both come back negative. Chest radiography is ordered and shows pulmonary hyperinflation with bronchial wall thickening.
New and Improved Devices Add More Therapeutic Options for Treatment of Migraine
Since the mid-2010s, the US Food and Drug Administration (FDA) has approved or cleared no fewer than 10 migraine treatments in the form of orals, injectables, nasal sprays, and devices. The medical achievements of the last decade in the field of migraine have been nothing less than stunning for physicians and their patients, whether they relied on off-label medications or those sanctioned by the FDA to treat patients living with migraine.
That said, the newer orals and injectables cannot help everyone living with migraine. The small molecule calcitonin gene-related peptide (CGRP) receptor antagonists (gepants) and the monoclonal antibodies that target the CGRP ligand or receptor, while well received by patients and physicians alike, have drawbacks for some patients, including lack of efficacy, slow response rate, and adverse events that prevent some patients from taking them. The gepants, which are oral medications—as opposed to the CGRP monoclonal antibody injectables—can occasionally cause enough nausea, drowsiness, and constipation for patients to choose to discontinue their use.
Certain patients have other reasons to shun orals and injectables. Some cannot swallow pills while others fear or do not tolerate injections. Insurance companies limit the quantity of acute care medications, so some patients cannot treat every migraine attack. Then there are those who have failed so many therapies in the past that they will not try the latest one. Consequently, some lie in bed, vomiting until the pain is gone, and some take too many over-the-counter or migraine-specific products, which make migraine symptoms worse if they develop medication overuse headache. And lastly, there are patients who have never walked through a physician’s door to secure a migraine diagnosis and get appropriate treatment.
Non interventional medical devices cleared by the FDA now allow physicians to offer relief to patients with migraine. They work either through various types of electrical neuromodulation to nerves outside the brain or they apply magnetic stimulation to the back of the brain itself to reach pain-associated pathways. A 2019 report on pain management from the US Department of Health and Human Services noted that some randomized control trials (RCTs) and other studies “have demonstrated that noninvasive vagal nerve stimulation can be effective in ameliorating pain in various types of cluster headaches and migraines.”
At least 3 devices, 1 designed to stimulate both the occipital and trigeminal nerves (eCOT-NS, Relivion, Neurolief Ltd), 1 that stimulates the vagus nerve noninvasively (nVNS, gammaCORE, electroCore), and 1 that stimulates peripheral nerves in the upper arm (remote electrical neuromodulation [REN], Nerivio, Theranica Bio-Electronics Ltd), are FDA cleared to treat episodic and chronic migraine. nVNS is also cleared to treat migraine, episodic cluster headache acutely, and chronic cluster acutely in connection with medication.
Real-world studies on all migraine treatments, especially the devices, are flooding PubMed. As for a physician’s observation, we will get to that shortly.
The Devices
Nerivio
Theranica Bio-Electronics Ltd makes a REN called Nerivio, which was FDA cleared in January 2021 to treat episodic migraine acutely in adults and adolescents. Studies have shown its effectiveness for chronic migraine patients who are treated acutely, and it has also helped patients with menstrual migraine. The patient wears the device on the upper arm. Sensory fibers, once stimulated in the arm, send an impulse to the brainstem to affect the serotonin- and norepinephrine-modulated descending inhibitory pathway to disrupt incoming pain messaging. Theranica has applied to the FDA for clearance to treat patients with chronic migraine, as well as for prevention.
Relivion
Neurolief Ltd created the external combined occipital and trigeminal nerve stimulation device (eCOT-NS), which stimulates both the occipital and trigeminal nerves. It has multiple output electrodes, which are placed on the forehead to stimulate the trigeminal supraorbital and supratrochlear nerve branches bilaterally, and over the occipital nerves in the back of the head. It is worn like a tiara as it must be in good contact with the forehead and the back of the head simultaneously. It is FDA cleared to treat acute migraine.
gammaCORE
gammaCORE is a nVNS device that is FDA cleared for acute and preventive treatment of migraine in adolescents and adults, and acute and preventive treatment of episodic cluster headache in adults. It is also cleared to treat chronic cluster headache acutely along with medication. The patient applies gel to the device’s 2 electrical contacts and then locates the vagus nerve on the side of the neck and applies the electrodes to the area that will be treated. Patients can adjust the stimulation’s intensity so that they can barely feel the stimulation; it has not been reported to be painful. nVNS is also an FDA cleared treatment for paroxysmal hemicrania and hemicrania continua.
SAVI Dual
The s-TMS (SAVI Dual, formerly called the Spring TMS and the sTMS mini), made by eNeura, is a single-pulse, transcranial magnetic stimulation applied to the back of the head to stimulate the occipital lobes in the brain. It was FDA cleared for acute and preventive care of migraine in adolescents over 12 years and for adults in February 2019. The patient holds a handheld magnetic device against their occiput, and when the tool is discharged, a brief magnetic pulse interrupts the pattern of neuronal firing (probably cortical spreading depression) that can trigger migraine and the visual aura associated with migraine in one-third of patients.
Cefaly
The e-TNS (Cefaly) works by external trigeminal nerve stimulation of the supraorbital and trochlear nerves bilaterally in the forehead. It gradually and automatically increases in intensity and can be controlled by the patient. It is FDA cleared for acute and preventive treatment of migraine, and, unlike the other devices, it is sold over the counter without a prescription. According to the company website, there are 3 devices: 1 is for acute treatment, 1 is for preventive treatment, and 1 device has 2 settings for both acute and preventive treatment.
The Studies
While most of the published studies on devices are company-sponsored, these device makers have underwritten numerous, sometimes very well-designed, studies on their products. A review by VanderPluym et al described those studies and their various risks of bias.
There are at least 10 studies on REN published so far. These include 2 randomized, sham-controlled trials looking at pain freedom and pain relief at 2 hours after stimulation begins. Another study detailed treatment reports from many patients in which 66.5% experienced pain relief at 2 hours post treatment initiation in half of their treatments. A subgroup of 16% of those patients were prescribed REN by their primary care physicians. Of that group, 77.8% experienced pain relief in half their treatments. That figure was very close to another study that found that 23 of 31 (74.2%) of the study patients treated virtually by non headache providers found relief in 50% of their headaches. REN comes with an education and behavioral medicine app that is used during treatment. A study done by the company shows that when a patient uses the relaxation app along with the standard stimulation, they do considerably better than with stimulation alone.
The eCOT-NS has also been tested in an RCT. At 2 hours, the responder rate was twice as high as in the sham group (66.7% vs 32%). Overall headache relief at 2 hours was higher in the responder group (76% vs 31.6%). In a study collecting real-world data on the efficacy of eCOT-NS in the preventive treatment of migraine (abstract data were presented at the American Headache Society meeting in June 2022), there was a 65.3% reduction in monthly migraine days (MMD) from baseline through 6 months. Treatment reduced MMD by 10.0 (from 15.3 to 5.3—a 76.8% reduction), and reduced acute medication use days (12.5 at baseline to 2.9) at 6 months.
Users of nVNS discussed their experiences with the device, which is the size of a large bar of soap, in a patient registry. They reported 192 attacks, with a mean pain score starting at 2.7 and dropping to 1.3 after 30 minutes. The pain levels of 70% of the attacks dropped to either mild or nonexistent. In a multicenter study on nNVS, 48 patients and 44 sham patients with episodic and chronic cluster headache showed no significant difference in the primary endpoint of pain freedom at 15 minutes between the nVNS and sham. There was also no difference in the chronic cluster headache group. But the episodic cluster subgroup showed a difference; nVNS was superior to sham, 48% to 6% (P
The e-TNS device is cleared for treating adults with migraine, acutely and preventively. It received initial clearance in 2017; in 2020, Cefaly Technology received clearance from the FDA to sell its products over the counter. The device, which resembles a large diamond that affixes to the forehead, has received differing reviews between various patient reports (found online at major retailer sites) and study results. In a blinded, intent-to-treat study involving 538 patients, 25.5% of the verum group reported they were pain-free at 2 hours; 18.3% in the sham group reported the same. Additionally, 56.4% of the subjects in the verum group reported they were free of the most bothersome migraine symptoms, as opposed to 42.3% of the sham group.
Adverse Events
The adverse events observed with these devices were, overall, relatively mild, and disappeared once the device was shut off. A few nVNS users said they experienced discomfort at the application site. With REN, 59 of 12,368 patients reported device-related issues; the vast majority were considered mild and consisted mostly of a sensation of warmth under the device. Of the 259 e-TNS users, 8.5% reported minor and reversible occurrences, such as treatment-related discomfort, paresthesia, and burning.
Patients in the Clinic
A few observations from the clinic regarding these devices:
Some devices are easier to use than others. I know this, because at a recent demonstration session in a course for physicians on headache treatment, I agreed to be the person on whom the device was demonstrated. The physician applying the device had difficulty aligning the device’s sensors with the appropriate nerves. Making sure your patients use these devices correctly is essential, and you or your staff should demonstrate their use to the patient. No doubt, this could be time-consuming in some cases, and patients who are reading the device’s instructions while in pain will likely get frustrated if they cannot get the device to work.
Some patients who have failed every medication class can occasionally find partial relief with these devices. One longtime patient of mine came to me severely disabled from chronic migraine and medication overuse headache but was somewhat better with 2 preventive medications. Triptans worked acutely, but she developed nearly every side effect imaginable. I was able to reverse her medication overuse headache, but the gepants, although they worked somewhat, took too long to take effect. We agreed the next step would be to use REN for each migraine attack, combined with acute care medication if necessary. (She uses REN alone for a milder headache and adds a gepant with naproxen if necessary.) She has found using the relaxation module on the REN app increases her chances of eliminating the migraine. She is not pain free all the time, but she appreciates the pain-free intervals.
One chronic cluster patient has relied on subcutaneous sumatriptan and breathing 100% oxygen at 12 liters per minute through a mask over his nose and mouth for acute relief from his headaches. His headache pain can climb from a 3 to a 10 in a matter of minutes. It starts behind and a bit above the right eye where he feels a tremendous pressure building up. He says that at times it feels like a screwdriver has been thrust into his eye and is being turned. Along with the pain, the eye becomes red, the pupil constricts, and the eyelid droops. He also has dripping from the right nostril, which stuffs up when the pain abates. The pain lasts for 1 to 2 hours, then returns 3 to 5 times a day for 5 days a week, on average. The pain never goes away for more than 3 weeks in a year’s time, hence the reason for his chronic cluster headache diagnosis. He is now using nVNS as soon as he feels the pain coming on. If the device does not provide sufficient relief, he uses oxygen or takes the sumatriptan injection.
Some patients who get cluster headaches think of suicide if the pain cannot be stopped; but in my experience, most can become pain free, or at least realize some partial relief from a variety of treatments (sometimes given at the same time).
Doctors often do not think of devices as options, and some doctors think devices do not work even though they have no experience with using them. Devices can give good relief on their own, and when a severe headache needs stronger treatment, medications added to a device usually work better than either treatment alone.
Since the mid-2010s, the US Food and Drug Administration (FDA) has approved or cleared no fewer than 10 migraine treatments in the form of orals, injectables, nasal sprays, and devices. The medical achievements of the last decade in the field of migraine have been nothing less than stunning for physicians and their patients, whether they relied on off-label medications or those sanctioned by the FDA to treat patients living with migraine.
That said, the newer orals and injectables cannot help everyone living with migraine. The small molecule calcitonin gene-related peptide (CGRP) receptor antagonists (gepants) and the monoclonal antibodies that target the CGRP ligand or receptor, while well received by patients and physicians alike, have drawbacks for some patients, including lack of efficacy, slow response rate, and adverse events that prevent some patients from taking them. The gepants, which are oral medications—as opposed to the CGRP monoclonal antibody injectables—can occasionally cause enough nausea, drowsiness, and constipation for patients to choose to discontinue their use.
Certain patients have other reasons to shun orals and injectables. Some cannot swallow pills while others fear or do not tolerate injections. Insurance companies limit the quantity of acute care medications, so some patients cannot treat every migraine attack. Then there are those who have failed so many therapies in the past that they will not try the latest one. Consequently, some lie in bed, vomiting until the pain is gone, and some take too many over-the-counter or migraine-specific products, which make migraine symptoms worse if they develop medication overuse headache. And lastly, there are patients who have never walked through a physician’s door to secure a migraine diagnosis and get appropriate treatment.
Non interventional medical devices cleared by the FDA now allow physicians to offer relief to patients with migraine. They work either through various types of electrical neuromodulation to nerves outside the brain or they apply magnetic stimulation to the back of the brain itself to reach pain-associated pathways. A 2019 report on pain management from the US Department of Health and Human Services noted that some randomized control trials (RCTs) and other studies “have demonstrated that noninvasive vagal nerve stimulation can be effective in ameliorating pain in various types of cluster headaches and migraines.”
At least 3 devices, 1 designed to stimulate both the occipital and trigeminal nerves (eCOT-NS, Relivion, Neurolief Ltd), 1 that stimulates the vagus nerve noninvasively (nVNS, gammaCORE, electroCore), and 1 that stimulates peripheral nerves in the upper arm (remote electrical neuromodulation [REN], Nerivio, Theranica Bio-Electronics Ltd), are FDA cleared to treat episodic and chronic migraine. nVNS is also cleared to treat migraine, episodic cluster headache acutely, and chronic cluster acutely in connection with medication.
Real-world studies on all migraine treatments, especially the devices, are flooding PubMed. As for a physician’s observation, we will get to that shortly.
The Devices
Nerivio
Theranica Bio-Electronics Ltd makes a REN called Nerivio, which was FDA cleared in January 2021 to treat episodic migraine acutely in adults and adolescents. Studies have shown its effectiveness for chronic migraine patients who are treated acutely, and it has also helped patients with menstrual migraine. The patient wears the device on the upper arm. Sensory fibers, once stimulated in the arm, send an impulse to the brainstem to affect the serotonin- and norepinephrine-modulated descending inhibitory pathway to disrupt incoming pain messaging. Theranica has applied to the FDA for clearance to treat patients with chronic migraine, as well as for prevention.
Relivion
Neurolief Ltd created the external combined occipital and trigeminal nerve stimulation device (eCOT-NS), which stimulates both the occipital and trigeminal nerves. It has multiple output electrodes, which are placed on the forehead to stimulate the trigeminal supraorbital and supratrochlear nerve branches bilaterally, and over the occipital nerves in the back of the head. It is worn like a tiara as it must be in good contact with the forehead and the back of the head simultaneously. It is FDA cleared to treat acute migraine.
gammaCORE
gammaCORE is a nVNS device that is FDA cleared for acute and preventive treatment of migraine in adolescents and adults, and acute and preventive treatment of episodic cluster headache in adults. It is also cleared to treat chronic cluster headache acutely along with medication. The patient applies gel to the device’s 2 electrical contacts and then locates the vagus nerve on the side of the neck and applies the electrodes to the area that will be treated. Patients can adjust the stimulation’s intensity so that they can barely feel the stimulation; it has not been reported to be painful. nVNS is also an FDA cleared treatment for paroxysmal hemicrania and hemicrania continua.
SAVI Dual
The s-TMS (SAVI Dual, formerly called the Spring TMS and the sTMS mini), made by eNeura, is a single-pulse, transcranial magnetic stimulation applied to the back of the head to stimulate the occipital lobes in the brain. It was FDA cleared for acute and preventive care of migraine in adolescents over 12 years and for adults in February 2019. The patient holds a handheld magnetic device against their occiput, and when the tool is discharged, a brief magnetic pulse interrupts the pattern of neuronal firing (probably cortical spreading depression) that can trigger migraine and the visual aura associated with migraine in one-third of patients.
Cefaly
The e-TNS (Cefaly) works by external trigeminal nerve stimulation of the supraorbital and trochlear nerves bilaterally in the forehead. It gradually and automatically increases in intensity and can be controlled by the patient. It is FDA cleared for acute and preventive treatment of migraine, and, unlike the other devices, it is sold over the counter without a prescription. According to the company website, there are 3 devices: 1 is for acute treatment, 1 is for preventive treatment, and 1 device has 2 settings for both acute and preventive treatment.
The Studies
While most of the published studies on devices are company-sponsored, these device makers have underwritten numerous, sometimes very well-designed, studies on their products. A review by VanderPluym et al described those studies and their various risks of bias.
There are at least 10 studies on REN published so far. These include 2 randomized, sham-controlled trials looking at pain freedom and pain relief at 2 hours after stimulation begins. Another study detailed treatment reports from many patients in which 66.5% experienced pain relief at 2 hours post treatment initiation in half of their treatments. A subgroup of 16% of those patients were prescribed REN by their primary care physicians. Of that group, 77.8% experienced pain relief in half their treatments. That figure was very close to another study that found that 23 of 31 (74.2%) of the study patients treated virtually by non headache providers found relief in 50% of their headaches. REN comes with an education and behavioral medicine app that is used during treatment. A study done by the company shows that when a patient uses the relaxation app along with the standard stimulation, they do considerably better than with stimulation alone.
The eCOT-NS has also been tested in an RCT. At 2 hours, the responder rate was twice as high as in the sham group (66.7% vs 32%). Overall headache relief at 2 hours was higher in the responder group (76% vs 31.6%). In a study collecting real-world data on the efficacy of eCOT-NS in the preventive treatment of migraine (abstract data were presented at the American Headache Society meeting in June 2022), there was a 65.3% reduction in monthly migraine days (MMD) from baseline through 6 months. Treatment reduced MMD by 10.0 (from 15.3 to 5.3—a 76.8% reduction), and reduced acute medication use days (12.5 at baseline to 2.9) at 6 months.
Users of nVNS discussed their experiences with the device, which is the size of a large bar of soap, in a patient registry. They reported 192 attacks, with a mean pain score starting at 2.7 and dropping to 1.3 after 30 minutes. The pain levels of 70% of the attacks dropped to either mild or nonexistent. In a multicenter study on nNVS, 48 patients and 44 sham patients with episodic and chronic cluster headache showed no significant difference in the primary endpoint of pain freedom at 15 minutes between the nVNS and sham. There was also no difference in the chronic cluster headache group. But the episodic cluster subgroup showed a difference; nVNS was superior to sham, 48% to 6% (P
The e-TNS device is cleared for treating adults with migraine, acutely and preventively. It received initial clearance in 2017; in 2020, Cefaly Technology received clearance from the FDA to sell its products over the counter. The device, which resembles a large diamond that affixes to the forehead, has received differing reviews between various patient reports (found online at major retailer sites) and study results. In a blinded, intent-to-treat study involving 538 patients, 25.5% of the verum group reported they were pain-free at 2 hours; 18.3% in the sham group reported the same. Additionally, 56.4% of the subjects in the verum group reported they were free of the most bothersome migraine symptoms, as opposed to 42.3% of the sham group.
Adverse Events
The adverse events observed with these devices were, overall, relatively mild, and disappeared once the device was shut off. A few nVNS users said they experienced discomfort at the application site. With REN, 59 of 12,368 patients reported device-related issues; the vast majority were considered mild and consisted mostly of a sensation of warmth under the device. Of the 259 e-TNS users, 8.5% reported minor and reversible occurrences, such as treatment-related discomfort, paresthesia, and burning.
Patients in the Clinic
A few observations from the clinic regarding these devices:
Some devices are easier to use than others. I know this, because at a recent demonstration session in a course for physicians on headache treatment, I agreed to be the person on whom the device was demonstrated. The physician applying the device had difficulty aligning the device’s sensors with the appropriate nerves. Making sure your patients use these devices correctly is essential, and you or your staff should demonstrate their use to the patient. No doubt, this could be time-consuming in some cases, and patients who are reading the device’s instructions while in pain will likely get frustrated if they cannot get the device to work.
Some patients who have failed every medication class can occasionally find partial relief with these devices. One longtime patient of mine came to me severely disabled from chronic migraine and medication overuse headache but was somewhat better with 2 preventive medications. Triptans worked acutely, but she developed nearly every side effect imaginable. I was able to reverse her medication overuse headache, but the gepants, although they worked somewhat, took too long to take effect. We agreed the next step would be to use REN for each migraine attack, combined with acute care medication if necessary. (She uses REN alone for a milder headache and adds a gepant with naproxen if necessary.) She has found using the relaxation module on the REN app increases her chances of eliminating the migraine. She is not pain free all the time, but she appreciates the pain-free intervals.
One chronic cluster patient has relied on subcutaneous sumatriptan and breathing 100% oxygen at 12 liters per minute through a mask over his nose and mouth for acute relief from his headaches. His headache pain can climb from a 3 to a 10 in a matter of minutes. It starts behind and a bit above the right eye where he feels a tremendous pressure building up. He says that at times it feels like a screwdriver has been thrust into his eye and is being turned. Along with the pain, the eye becomes red, the pupil constricts, and the eyelid droops. He also has dripping from the right nostril, which stuffs up when the pain abates. The pain lasts for 1 to 2 hours, then returns 3 to 5 times a day for 5 days a week, on average. The pain never goes away for more than 3 weeks in a year’s time, hence the reason for his chronic cluster headache diagnosis. He is now using nVNS as soon as he feels the pain coming on. If the device does not provide sufficient relief, he uses oxygen or takes the sumatriptan injection.
Some patients who get cluster headaches think of suicide if the pain cannot be stopped; but in my experience, most can become pain free, or at least realize some partial relief from a variety of treatments (sometimes given at the same time).
Doctors often do not think of devices as options, and some doctors think devices do not work even though they have no experience with using them. Devices can give good relief on their own, and when a severe headache needs stronger treatment, medications added to a device usually work better than either treatment alone.
Since the mid-2010s, the US Food and Drug Administration (FDA) has approved or cleared no fewer than 10 migraine treatments in the form of orals, injectables, nasal sprays, and devices. The medical achievements of the last decade in the field of migraine have been nothing less than stunning for physicians and their patients, whether they relied on off-label medications or those sanctioned by the FDA to treat patients living with migraine.
That said, the newer orals and injectables cannot help everyone living with migraine. The small molecule calcitonin gene-related peptide (CGRP) receptor antagonists (gepants) and the monoclonal antibodies that target the CGRP ligand or receptor, while well received by patients and physicians alike, have drawbacks for some patients, including lack of efficacy, slow response rate, and adverse events that prevent some patients from taking them. The gepants, which are oral medications—as opposed to the CGRP monoclonal antibody injectables—can occasionally cause enough nausea, drowsiness, and constipation for patients to choose to discontinue their use.
Certain patients have other reasons to shun orals and injectables. Some cannot swallow pills while others fear or do not tolerate injections. Insurance companies limit the quantity of acute care medications, so some patients cannot treat every migraine attack. Then there are those who have failed so many therapies in the past that they will not try the latest one. Consequently, some lie in bed, vomiting until the pain is gone, and some take too many over-the-counter or migraine-specific products, which make migraine symptoms worse if they develop medication overuse headache. And lastly, there are patients who have never walked through a physician’s door to secure a migraine diagnosis and get appropriate treatment.
Non interventional medical devices cleared by the FDA now allow physicians to offer relief to patients with migraine. They work either through various types of electrical neuromodulation to nerves outside the brain or they apply magnetic stimulation to the back of the brain itself to reach pain-associated pathways. A 2019 report on pain management from the US Department of Health and Human Services noted that some randomized control trials (RCTs) and other studies “have demonstrated that noninvasive vagal nerve stimulation can be effective in ameliorating pain in various types of cluster headaches and migraines.”
At least 3 devices, 1 designed to stimulate both the occipital and trigeminal nerves (eCOT-NS, Relivion, Neurolief Ltd), 1 that stimulates the vagus nerve noninvasively (nVNS, gammaCORE, electroCore), and 1 that stimulates peripheral nerves in the upper arm (remote electrical neuromodulation [REN], Nerivio, Theranica Bio-Electronics Ltd), are FDA cleared to treat episodic and chronic migraine. nVNS is also cleared to treat migraine, episodic cluster headache acutely, and chronic cluster acutely in connection with medication.
Real-world studies on all migraine treatments, especially the devices, are flooding PubMed. As for a physician’s observation, we will get to that shortly.
The Devices
Nerivio
Theranica Bio-Electronics Ltd makes a REN called Nerivio, which was FDA cleared in January 2021 to treat episodic migraine acutely in adults and adolescents. Studies have shown its effectiveness for chronic migraine patients who are treated acutely, and it has also helped patients with menstrual migraine. The patient wears the device on the upper arm. Sensory fibers, once stimulated in the arm, send an impulse to the brainstem to affect the serotonin- and norepinephrine-modulated descending inhibitory pathway to disrupt incoming pain messaging. Theranica has applied to the FDA for clearance to treat patients with chronic migraine, as well as for prevention.
Relivion
Neurolief Ltd created the external combined occipital and trigeminal nerve stimulation device (eCOT-NS), which stimulates both the occipital and trigeminal nerves. It has multiple output electrodes, which are placed on the forehead to stimulate the trigeminal supraorbital and supratrochlear nerve branches bilaterally, and over the occipital nerves in the back of the head. It is worn like a tiara as it must be in good contact with the forehead and the back of the head simultaneously. It is FDA cleared to treat acute migraine.
gammaCORE
gammaCORE is a nVNS device that is FDA cleared for acute and preventive treatment of migraine in adolescents and adults, and acute and preventive treatment of episodic cluster headache in adults. It is also cleared to treat chronic cluster headache acutely along with medication. The patient applies gel to the device’s 2 electrical contacts and then locates the vagus nerve on the side of the neck and applies the electrodes to the area that will be treated. Patients can adjust the stimulation’s intensity so that they can barely feel the stimulation; it has not been reported to be painful. nVNS is also an FDA cleared treatment for paroxysmal hemicrania and hemicrania continua.
SAVI Dual
The s-TMS (SAVI Dual, formerly called the Spring TMS and the sTMS mini), made by eNeura, is a single-pulse, transcranial magnetic stimulation applied to the back of the head to stimulate the occipital lobes in the brain. It was FDA cleared for acute and preventive care of migraine in adolescents over 12 years and for adults in February 2019. The patient holds a handheld magnetic device against their occiput, and when the tool is discharged, a brief magnetic pulse interrupts the pattern of neuronal firing (probably cortical spreading depression) that can trigger migraine and the visual aura associated with migraine in one-third of patients.
Cefaly
The e-TNS (Cefaly) works by external trigeminal nerve stimulation of the supraorbital and trochlear nerves bilaterally in the forehead. It gradually and automatically increases in intensity and can be controlled by the patient. It is FDA cleared for acute and preventive treatment of migraine, and, unlike the other devices, it is sold over the counter without a prescription. According to the company website, there are 3 devices: 1 is for acute treatment, 1 is for preventive treatment, and 1 device has 2 settings for both acute and preventive treatment.
The Studies
While most of the published studies on devices are company-sponsored, these device makers have underwritten numerous, sometimes very well-designed, studies on their products. A review by VanderPluym et al described those studies and their various risks of bias.
There are at least 10 studies on REN published so far. These include 2 randomized, sham-controlled trials looking at pain freedom and pain relief at 2 hours after stimulation begins. Another study detailed treatment reports from many patients in which 66.5% experienced pain relief at 2 hours post treatment initiation in half of their treatments. A subgroup of 16% of those patients were prescribed REN by their primary care physicians. Of that group, 77.8% experienced pain relief in half their treatments. That figure was very close to another study that found that 23 of 31 (74.2%) of the study patients treated virtually by non headache providers found relief in 50% of their headaches. REN comes with an education and behavioral medicine app that is used during treatment. A study done by the company shows that when a patient uses the relaxation app along with the standard stimulation, they do considerably better than with stimulation alone.
The eCOT-NS has also been tested in an RCT. At 2 hours, the responder rate was twice as high as in the sham group (66.7% vs 32%). Overall headache relief at 2 hours was higher in the responder group (76% vs 31.6%). In a study collecting real-world data on the efficacy of eCOT-NS in the preventive treatment of migraine (abstract data were presented at the American Headache Society meeting in June 2022), there was a 65.3% reduction in monthly migraine days (MMD) from baseline through 6 months. Treatment reduced MMD by 10.0 (from 15.3 to 5.3—a 76.8% reduction), and reduced acute medication use days (12.5 at baseline to 2.9) at 6 months.
Users of nVNS discussed their experiences with the device, which is the size of a large bar of soap, in a patient registry. They reported 192 attacks, with a mean pain score starting at 2.7 and dropping to 1.3 after 30 minutes. The pain levels of 70% of the attacks dropped to either mild or nonexistent. In a multicenter study on nNVS, 48 patients and 44 sham patients with episodic and chronic cluster headache showed no significant difference in the primary endpoint of pain freedom at 15 minutes between the nVNS and sham. There was also no difference in the chronic cluster headache group. But the episodic cluster subgroup showed a difference; nVNS was superior to sham, 48% to 6% (P
The e-TNS device is cleared for treating adults with migraine, acutely and preventively. It received initial clearance in 2017; in 2020, Cefaly Technology received clearance from the FDA to sell its products over the counter. The device, which resembles a large diamond that affixes to the forehead, has received differing reviews between various patient reports (found online at major retailer sites) and study results. In a blinded, intent-to-treat study involving 538 patients, 25.5% of the verum group reported they were pain-free at 2 hours; 18.3% in the sham group reported the same. Additionally, 56.4% of the subjects in the verum group reported they were free of the most bothersome migraine symptoms, as opposed to 42.3% of the sham group.
Adverse Events
The adverse events observed with these devices were, overall, relatively mild, and disappeared once the device was shut off. A few nVNS users said they experienced discomfort at the application site. With REN, 59 of 12,368 patients reported device-related issues; the vast majority were considered mild and consisted mostly of a sensation of warmth under the device. Of the 259 e-TNS users, 8.5% reported minor and reversible occurrences, such as treatment-related discomfort, paresthesia, and burning.
Patients in the Clinic
A few observations from the clinic regarding these devices:
Some devices are easier to use than others. I know this, because at a recent demonstration session in a course for physicians on headache treatment, I agreed to be the person on whom the device was demonstrated. The physician applying the device had difficulty aligning the device’s sensors with the appropriate nerves. Making sure your patients use these devices correctly is essential, and you or your staff should demonstrate their use to the patient. No doubt, this could be time-consuming in some cases, and patients who are reading the device’s instructions while in pain will likely get frustrated if they cannot get the device to work.
Some patients who have failed every medication class can occasionally find partial relief with these devices. One longtime patient of mine came to me severely disabled from chronic migraine and medication overuse headache but was somewhat better with 2 preventive medications. Triptans worked acutely, but she developed nearly every side effect imaginable. I was able to reverse her medication overuse headache, but the gepants, although they worked somewhat, took too long to take effect. We agreed the next step would be to use REN for each migraine attack, combined with acute care medication if necessary. (She uses REN alone for a milder headache and adds a gepant with naproxen if necessary.) She has found using the relaxation module on the REN app increases her chances of eliminating the migraine. She is not pain free all the time, but she appreciates the pain-free intervals.
One chronic cluster patient has relied on subcutaneous sumatriptan and breathing 100% oxygen at 12 liters per minute through a mask over his nose and mouth for acute relief from his headaches. His headache pain can climb from a 3 to a 10 in a matter of minutes. It starts behind and a bit above the right eye where he feels a tremendous pressure building up. He says that at times it feels like a screwdriver has been thrust into his eye and is being turned. Along with the pain, the eye becomes red, the pupil constricts, and the eyelid droops. He also has dripping from the right nostril, which stuffs up when the pain abates. The pain lasts for 1 to 2 hours, then returns 3 to 5 times a day for 5 days a week, on average. The pain never goes away for more than 3 weeks in a year’s time, hence the reason for his chronic cluster headache diagnosis. He is now using nVNS as soon as he feels the pain coming on. If the device does not provide sufficient relief, he uses oxygen or takes the sumatriptan injection.
Some patients who get cluster headaches think of suicide if the pain cannot be stopped; but in my experience, most can become pain free, or at least realize some partial relief from a variety of treatments (sometimes given at the same time).
Doctors often do not think of devices as options, and some doctors think devices do not work even though they have no experience with using them. Devices can give good relief on their own, and when a severe headache needs stronger treatment, medications added to a device usually work better than either treatment alone.
Quality of Life and Population Health in Behavioral Health Care: A Retrospective, Cross-Sectional Study
From Milwaukee County Behavioral Health Services, Milwaukee, WI.
Abstract
Objectives: The goal of this study was to determine whether a single-item quality of life (QOL) measure could serve as a useful population health–level metric within the Quadruple Aim framework in a publicly funded behavioral health system.
Design: This was a retrospective, cross-sectional study that examined the correlation between the single-item QOL measure and several other key measures of the social determinants of health and a composite measure of acute service utilization for all patients receiving mental health and substance use services in a community behavioral health system.
Methods: Data were collected for 4488 patients who had at least 1 assessment between October 1, 2020, and September 30, 2021. Data on social determinants of health were obtained through patient self-report; acute service use data were obtained from electronic health records.
Results: Statistical analyses revealed results in the expected direction for all relationships tested. Patients with higher QOL were more likely to report “Good” or better self-rated physical health, be employed, have a private residence, and report recent positive social interactions, and were less likely to have received acute services in the previous 90 days.
Conclusion: A single-item QOL measure shows promise as a general, minimally burdensome whole-system metric that can function as a target for population health management efforts in a large behavioral health system. Future research should explore whether this QOL measure is sensitive to change over time and examine its temporal relationship with other key outcome metrics.
Keywords: Quadruple Aim, single-item measures, social determinants of health, acute service utilization metrics.
The Triple Aim for health care—improving the individual experience of care, increasing the health of populations, and reducing the costs of care—was first proposed in 2008.1 More recently, some have advocated for an expanded focus to include a fourth aim: the quality of staff work life.2 Since this seminal paper was published, many health care systems have endeavored to adopt and implement the Quadruple Aim3,4; however, the concepts representing each of the aims are not universally defined,3 nor are the measures needed to populate the Quadruple Aim always available within the health system in question.5
Although several assessment models and frameworks that provide guidance to stakeholders have been developed,6,7 it is ultimately up to organizations themselves to determine which measures they should deploy to best represent the different quadrants of the Quadruple Aim.6 Evidence suggests, however, that quality measurement, and the administrative time required to conduct it, can be both financially and emotionally burdensome to providers and health systems.8-10 Thus, it is incumbent on organizations to select a set of measures that are not only meaningful but as parsimonious as possible.6,11,12
Quality of life (QOL) is a potential candidate to assess the aim of population health. Brief health-related QOL questions have long been used in epidemiological surveys, such as the Behavioral Risk Factor Surveillance System survey.13 Such questions are also a key component of community health frameworks, such as the County Health Rankings developed by the University of Wisconsin Population Health Institute.14 Furthermore, Humana recently revealed that increasing the number of physical and mental health “Healthy Days” (which are among the Centers for Disease Control and Prevention’s Health-Related Quality of Life questions15) among the members enrolled in their insurance plan would become a major goal for the organization.16,17 Many of these measures, while brief, focus on QOL as a function of health, often as a self-rated construct (from “Poor” to “Excellent”) or in the form of days of poor physical or mental health in the past 30 days,15 rather than evaluating QOL itself; however, several authors have pointed out that health status and QOL are related but distinct concepts.18,19
Brief single-item assessments focused specifically on QOL have been developed and implemented within nonclinical20 and clinical populations, including individuals with cancer,21 adults with disabilities,22 individuals with cystic fibrosis,23 and children with epilepsy.24 Despite the long history of QOL assessment in behavioral health treatment,25 single-item measures have not been widely implemented in this population.
Milwaukee County Behavioral Health Services (BHS), a publicly funded, county-based behavioral health care system in Milwaukee, Wisconsin, provides inpatient and ambulatory treatment, psychiatric emergency care, withdrawal management, care management, crisis services, and other support services to individuals in Milwaukee County. In 2018 the community services arm of BHS began implementing a single QOL question from the World Health Organization’s WHOQOL-BREF26: On a 5-point rating scale of “Very Poor” to “Very Good,” “How would you rate your overall quality of life right now?” Previous research by Atroszko and colleagues,20 which used a similar approach with the same item from the WHOQOL-BREF, reported correlations in the expected direction of the single-item QOL measure with perceived stress, depression, anxiety, loneliness, and daily hours of sleep. This study’s sample, however, comprised opportunistically recruited college students, not a clinical population. Further, the researchers did not examine the relationship of QOL with acute service utilization or other measures of the social determinants of health, such as housing, employment, or social connectedness.
The following study was designed to extend these results by focusing on a clinical population—individuals with mental health or substance use issues—being served in a large, publicly funded behavioral health system in Milwaukee, Wisconsin. The objective of this study was to determine whether a single-item QOL measure could be used as a brief, parsimonious measure of overall population health by examining its relationship with other key outcome measures for patients receiving services from BHS. This study was reviewed and approved by BHS’s Institutional Review Board.
Methods
All patients engaged in nonacute community services are offered a standardized assessment that includes, among other measures, items related to QOL, housing status, employment status, self-rated physical health, and social connectedness. This assessment is administered at intake, discharge, and every 6 months while patients are enrolled in services. Patients who received at least 1 assessment between October 1, 2020, and September 30, 2021, were included in the analyses. Patients receiving crisis, inpatient, or withdrawal management services alone (ie, did not receive any other community-based services) were not offered the standard assessment and thus were not included in the analyses. If patients had more than 1 assessment during this time period, QOL data from the last assessment were used. Data on housing (private residence status, defined as adults living alone or with others without supervision in a house or apartment), employment status, self-rated physical health, and social connectedness (measured by asking people whether they have had positive interactions with family or friends in the past 30 days) were extracted from the same timepoint as well.
Also included in the analyses were rates of acute service utilization, in which any patient with at least 1 visit to BHS’s psychiatric emergency department, withdrawal management facility, or psychiatric inpatient facility in the 90 days prior to the date of the assessment received a code of “Yes,” and any patient who did not receive any of these services received a code of “No.” Chi-square analyses were conducted to determine the relationship between QOL rankings (“Very Poor,” “Poor,” “Neither Good nor Poor,” “Good,” and “Very Good”) and housing, employment, self-rated physical health, social connectedness, and 90-day acute service use. All acute service utilization data were obtained from BHS’s electronic health records system. All data used in the study were stored on a secure, password-protected server. All analyses were conducted with SPSS software (SPSS 28; IBM).
Results
Data were available for 4488 patients who received an assessment between October 1, 2020, and September 30, 2021 (total numbers per item vary because some items had missing data; see supplementary eTables 1-3 for sample size per item). Demographics of the patient sample are listed in Table 1; the demographics of the patients who were missing data for specific outcomes are presented in eTables 1-3.
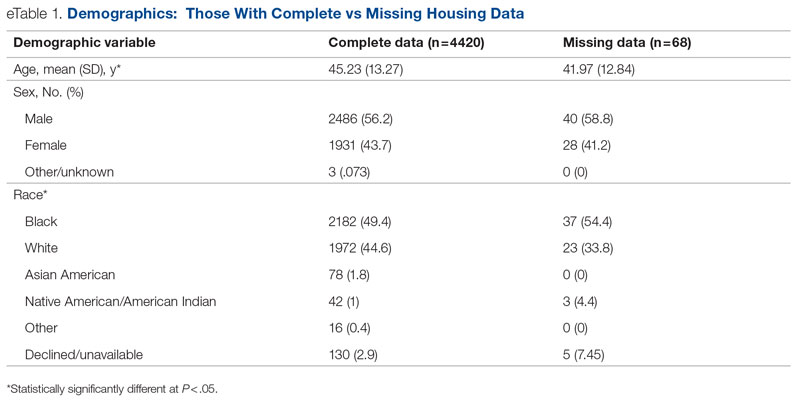
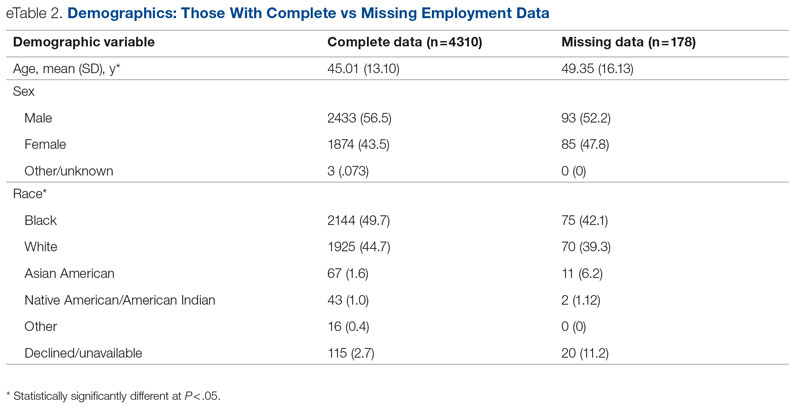
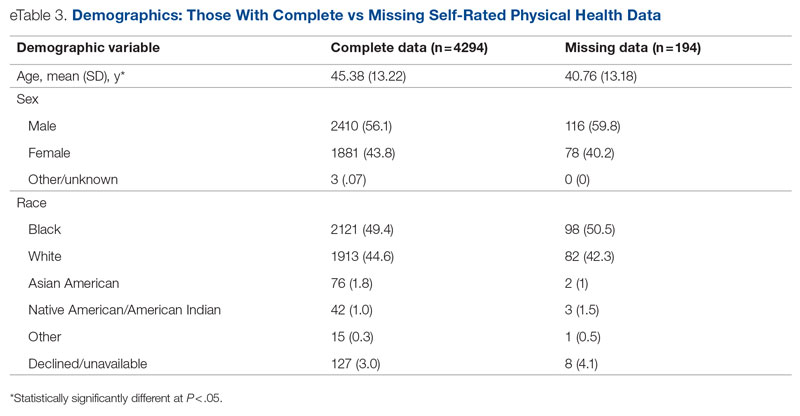
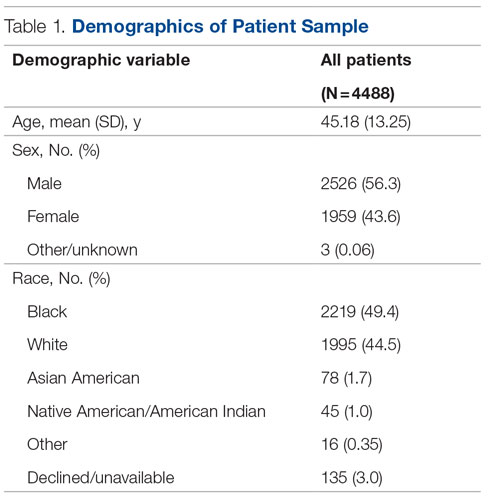
Statistical analyses revealed results in the expected direction for all relationships tested (Table 2). As patients’ self-reported QOL improved, so did the likelihood of higher rates of self-reported “Good” or better physical health, which was 576% higher among individuals who reported “Very Good” QOL relative to those who reported “Very Poor” QOL. Similarly, when compared with individuals with “Very Poor” QOL, individuals who reported “Very Good” QOL were 21.91% more likely to report having a private residence, 126.7% more likely to report being employed, and 29.17% more likely to report having had positive social interactions with family and friends in the past 30 days. There was an inverse relationship between QOL and the likelihood that a patient had received at least 1 admission for an acute service in the previous 90 days, such that patients who reported “Very Good” QOL were 86.34% less likely to have had an admission compared to patients with “Very Poor” QOL (2.8% vs 20.5%, respectively). The relationships among the criterion variables used in this study are presented in Table 3.
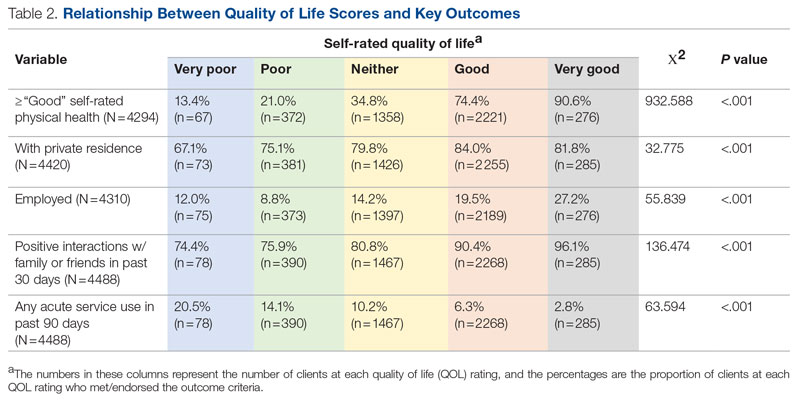
Discussion
The results of this preliminary analysis suggest that self-rated QOL is related to key health, social determinants of health, and acute service utilization metrics. These data are important for several reasons. First, because QOL is a diagnostically agnostic measure, it is a cross-cutting measure to use with clinically diverse populations receiving an array of different services. Second, at 1 item, the QOL measure is extremely brief and therefore minimally onerous to implement for both patients and administratively overburdened providers. Third, its correlation with other key metrics suggests that it can function as a broad population health measure for health care organizations because individuals with higher QOL will also likely have better outcomes in other key areas. This suggests that it has the potential to broadly represent the overall status of a population of patients, thus functioning as a type of “whole system” measure, which the Institute for Healthcare Improvement describes as “a small set of measures that reflect a health system’s overall performance on core dimensions of quality guided by the Triple Aim.”7 These whole system measures can help focus an organization’s strategic initiatives and efforts on the issues that matter most to the patients and community it serves.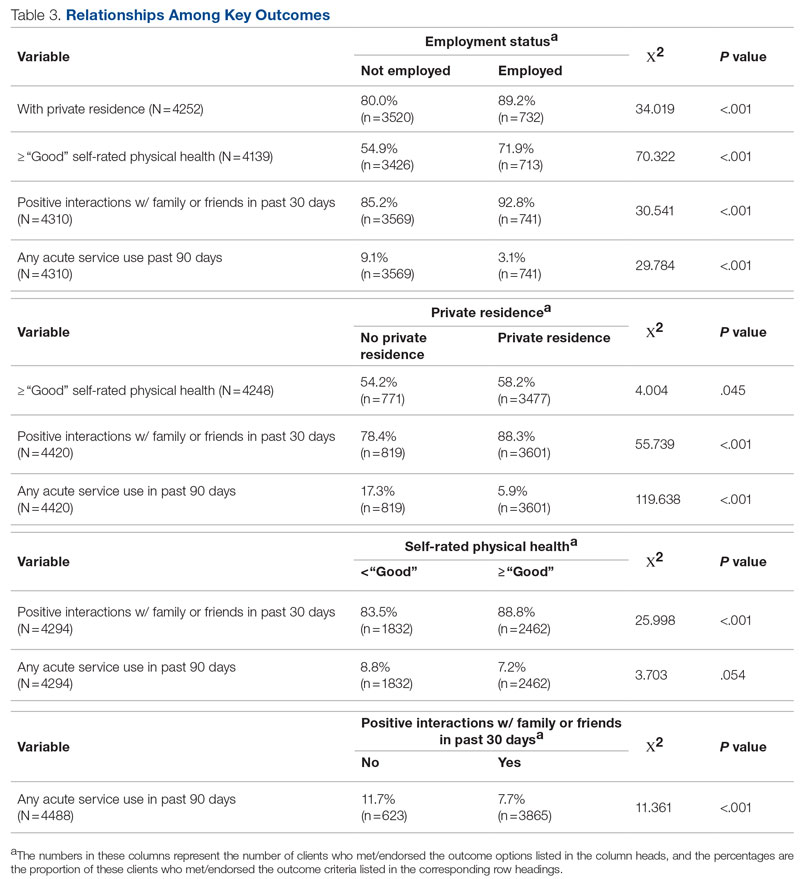
The relationship of QOL to acute service utilization deserves special mention. As an administrative measure, utilization is not susceptible to the same response bias as the other self-reported variables. Furthermore, acute services are costly to health systems, and hospital readmissions are associated with payment reductions in the Centers for Medicare and Medicaid Services (CMS) Hospital Readmissions Reduction Program for hospitals that fail to meet certain performance targets.27 Thus, because of its alignment with federal mandates, improved QOL (and potentially concomitant decreases in acute service use) may have significant financial implications for health systems as well.
This study was limited by several factors. First, it was focused on a population receiving publicly funded behavioral health services with strict eligibility requirements, one of which stipulated that individuals must be at 200% or less of the Federal Poverty Level; therefore, the results might not be applicable to health systems with a more clinically or socioeconomically diverse patient population. Second, because these data are cross-sectional, it was not possible to determine whether QOL improved over time or whether changes in QOL covaried longitudinally with the other metrics under observation. For example, if patients’ QOL improved from the first to last assessment, did their employment or residential status improve as well, or were these patients more likely to be employed at their first assessment? Furthermore, if there was covariance, did changes in employment, housing status, and so on precede changes in QOL or vice versa? Multiple longitudinal observations would help to address these questions and will be the focus of future analyses.
Conclusion
This preliminary study suggests that a single-item QOL measure may be a valuable population health–level metric for health systems. It requires little administrative effort on the part of either the clinician or patient. It is also agnostic with regard to clinical issue or treatment approach and can therefore admit of a range of diagnoses or patient-specific, idiosyncratic recovery goals. It is correlated with other key health, social determinants of health, and acute service utilization indicators and can therefore serve as a “whole system” measure because of its ability to broadly represent improvements in an entire population. Furthermore, QOL is patient-centered in that data are obtained through patient self-report, which is a high priority for CMS and other health care organizations.28 In summary, a single-item QOL measure holds promise for health care organizations looking to implement the Quadruple Aim and assess the health of the populations they serve in a manner that is simple, efficient, and patient-centered.
Acknowledgments: The author thanks Jennifer Wittwer for her thoughtful comments on the initial draft of this manuscript and Gary Kraft for his help extracting the data used in the analyses.
Corresponding author: Walter Matthew Drymalski, PhD; [email protected]
Disclosures: None reported.
1. Berwick DM, Nolan TW, Whittington J. The triple aim: care, health, and cost. Health Aff (Millwood). 2008;27(3):759-769. doi:10.1377/hlthaff.27.3.759
2. Bodenheimer T, Sinsky C. From triple to quadruple aim: care of the patient requires care of the provider. Ann Fam Med. 2014;12(6):573-576. doi:10.1370/afm.1713
3. Hendrikx RJP, Drewes HW, Spreeuwenberg M, et al. Which triple aim related measures are being used to evaluate population management initiatives? An international comparative analysis. Health Policy. 2016;120(5):471-485. doi:10.1016/j.healthpol.2016.03.008
4. Whittington JW, Nolan K, Lewis N, Torres T. Pursuing the triple aim: the first 7 years. Milbank Q. 2015;93(2):263-300. doi:10.1111/1468-0009.12122
5. Ryan BL, Brown JB, Glazier RH, Hutchison B. Examining primary healthcare performance through a triple aim lens. Healthc Policy. 2016;11(3):19-31.
6. Stiefel M, Nolan K. A guide to measuring the Triple Aim: population health, experience of care, and per capita cost. Institute for Healthcare Improvement; 2012. Accessed November 1, 2022. https://nhchc.org/wp-content/uploads/2019/08/ihiguidetomeasuringtripleaimwhitepaper2012.pdf
7. Martin L, Nelson E, Rakover J, Chase A. Whole system measures 2.0: a compass for health system leaders. Institute for Healthcare Improvement; 2016. Accessed November 1, 2022. http://www.ihi.org:80/resources/Pages/IHIWhitePapers/Whole-System-Measures-Compass-for-Health-System-Leaders.aspx
8. Casalino LP, Gans D, Weber R, et al. US physician practices spend more than $15.4 billion annually to report quality measures. Health Aff (Millwood). 2016;35(3):401-406. doi:10.1377/hlthaff.2015.1258
9. Rao SK, Kimball AB, Lehrhoff SR, et al. The impact of administrative burden on academic physicians: results of a hospital-wide physician survey. Acad Med. 2017;92(2):237-243. doi:10.1097/ACM.0000000000001461
10. Woolhandler S, Himmelstein DU. Administrative work consumes one-sixth of U.S. physicians’ working hours and lowers their career satisfaction. Int J Health Serv. 2014;44(4):635-642. doi:10.2190/HS.44.4.a
11. Meyer GS, Nelson EC, Pryor DB, et al. More quality measures versus measuring what matters: a call for balance and parsimony. BMJ Qual Saf. 2012;21(11):964-968. doi:10.1136/bmjqs-2012-001081
12. Vital Signs: Core Metrics for Health and Health Care Progress. Washington, DC: National Academies Press; 2015. doi:10.17226/19402
13. Centers for Disease Control and Prevention. BRFSS questionnaires. Accessed November 1, 2022. https://www.cdc.gov/brfss/questionnaires/index.htm
14. County Health Rankings and Roadmaps. Measures & data sources. University of Wisconsin Population Health Institute. Accessed November 1, 2022. https://www.countyhealthrankings.org/explore-health-rankings/measures-data-sources
15. Centers for Disease Control and Prevention. Healthy days core module (CDC HRQOL-4). Accessed November 1, 2022. https://www.cdc.gov/hrqol/hrqol14_measure.htm
16. Cordier T, Song Y, Cambon J, et al. A bold goal: more healthy days through improved community health. Popul Health Manag. 2018;21(3):202-208. doi:10.1089/pop.2017.0142
17. Slabaugh SL, Shah M, Zack M, et al. Leveraging health-related quality of life in population health management: the case for healthy days. Popul Health Manag. 2017;20(1):13-22. doi:10.1089/pop.2015.0162
18. Karimi M, Brazier J. Health, health-related quality of life, and quality of life: what is the difference? Pharmacoeconomics. 2016;34(7):645-649. doi:10.1007/s40273-016-0389-9
19. Smith KW, Avis NE, Assmann SF. Distinguishing between quality of life and health status in quality of life research: a meta-analysis. Qual Life Res. 1999;8(5):447-459. doi:10.1023/a:1008928518577
20. Atroszko PA, Baginska P, Mokosinska M, et al. Validity and reliability of single-item self-report measures of general quality of life, general health and sleep quality. In: CER Comparative European Research 2015. Sciemcee Publishing; 2015:207-211.
21. Singh JA, Satele D, Pattabasavaiah S, et al. Normative data and clinically significant effect sizes for single-item numerical linear analogue self-assessment (LASA) scales. Health Qual Life Outcomes. 2014;12:187. doi:10.1186/s12955-014-0187-z
22. Siebens HC, Tsukerman D, Adkins RH, et al. Correlates of a single-item quality-of-life measure in people aging with disabilities. Am J Phys Med Rehabil. 2015;94(12):1065-1074. doi:10.1097/PHM.0000000000000298
23. Yohannes AM, Dodd M, Morris J, Webb K. Reliability and validity of a single item measure of quality of life scale for adult patients with cystic fibrosis. Health Qual Life Outcomes. 2011;9:105. doi:10.1186/1477-7525-9-105
24. Conway L, Widjaja E, Smith ML. Single-item measure for assessing quality of life in children with drug-resistant epilepsy. Epilepsia Open. 2017;3(1):46-54. doi:10.1002/epi4.12088
25. Barry MM, Zissi A. Quality of life as an outcome measure in evaluating mental health services: a review of the empirical evidence. Soc Psychiatry Psychiatr Epidemiol. 1997;32(1):38-47. doi:10.1007/BF00800666
26. Skevington SM, Lotfy M, O’Connell KA. The World Health Organization’s WHOQOL-BREF quality of life assessment: psychometric properties and results of the international field trial. Qual Life Res. 2004;13(2):299-310. doi:10.1023/B:QURE.0000018486.91360.00
27. Centers for Medicare & Medicaid Services. Hospital readmissions reduction program (HRRP). Accessed November 1, 2022. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program
28. Centers for Medicare & Medicaid Services. Patient-reported outcome measures. CMS Measures Management System. Published May 2022. Accessed November 1, 2022. https://www.cms.gov/files/document/blueprint-patient-reported-outcome-measures.pdf
From Milwaukee County Behavioral Health Services, Milwaukee, WI.
Abstract
Objectives: The goal of this study was to determine whether a single-item quality of life (QOL) measure could serve as a useful population health–level metric within the Quadruple Aim framework in a publicly funded behavioral health system.
Design: This was a retrospective, cross-sectional study that examined the correlation between the single-item QOL measure and several other key measures of the social determinants of health and a composite measure of acute service utilization for all patients receiving mental health and substance use services in a community behavioral health system.
Methods: Data were collected for 4488 patients who had at least 1 assessment between October 1, 2020, and September 30, 2021. Data on social determinants of health were obtained through patient self-report; acute service use data were obtained from electronic health records.
Results: Statistical analyses revealed results in the expected direction for all relationships tested. Patients with higher QOL were more likely to report “Good” or better self-rated physical health, be employed, have a private residence, and report recent positive social interactions, and were less likely to have received acute services in the previous 90 days.
Conclusion: A single-item QOL measure shows promise as a general, minimally burdensome whole-system metric that can function as a target for population health management efforts in a large behavioral health system. Future research should explore whether this QOL measure is sensitive to change over time and examine its temporal relationship with other key outcome metrics.
Keywords: Quadruple Aim, single-item measures, social determinants of health, acute service utilization metrics.
The Triple Aim for health care—improving the individual experience of care, increasing the health of populations, and reducing the costs of care—was first proposed in 2008.1 More recently, some have advocated for an expanded focus to include a fourth aim: the quality of staff work life.2 Since this seminal paper was published, many health care systems have endeavored to adopt and implement the Quadruple Aim3,4; however, the concepts representing each of the aims are not universally defined,3 nor are the measures needed to populate the Quadruple Aim always available within the health system in question.5
Although several assessment models and frameworks that provide guidance to stakeholders have been developed,6,7 it is ultimately up to organizations themselves to determine which measures they should deploy to best represent the different quadrants of the Quadruple Aim.6 Evidence suggests, however, that quality measurement, and the administrative time required to conduct it, can be both financially and emotionally burdensome to providers and health systems.8-10 Thus, it is incumbent on organizations to select a set of measures that are not only meaningful but as parsimonious as possible.6,11,12
Quality of life (QOL) is a potential candidate to assess the aim of population health. Brief health-related QOL questions have long been used in epidemiological surveys, such as the Behavioral Risk Factor Surveillance System survey.13 Such questions are also a key component of community health frameworks, such as the County Health Rankings developed by the University of Wisconsin Population Health Institute.14 Furthermore, Humana recently revealed that increasing the number of physical and mental health “Healthy Days” (which are among the Centers for Disease Control and Prevention’s Health-Related Quality of Life questions15) among the members enrolled in their insurance plan would become a major goal for the organization.16,17 Many of these measures, while brief, focus on QOL as a function of health, often as a self-rated construct (from “Poor” to “Excellent”) or in the form of days of poor physical or mental health in the past 30 days,15 rather than evaluating QOL itself; however, several authors have pointed out that health status and QOL are related but distinct concepts.18,19
Brief single-item assessments focused specifically on QOL have been developed and implemented within nonclinical20 and clinical populations, including individuals with cancer,21 adults with disabilities,22 individuals with cystic fibrosis,23 and children with epilepsy.24 Despite the long history of QOL assessment in behavioral health treatment,25 single-item measures have not been widely implemented in this population.
Milwaukee County Behavioral Health Services (BHS), a publicly funded, county-based behavioral health care system in Milwaukee, Wisconsin, provides inpatient and ambulatory treatment, psychiatric emergency care, withdrawal management, care management, crisis services, and other support services to individuals in Milwaukee County. In 2018 the community services arm of BHS began implementing a single QOL question from the World Health Organization’s WHOQOL-BREF26: On a 5-point rating scale of “Very Poor” to “Very Good,” “How would you rate your overall quality of life right now?” Previous research by Atroszko and colleagues,20 which used a similar approach with the same item from the WHOQOL-BREF, reported correlations in the expected direction of the single-item QOL measure with perceived stress, depression, anxiety, loneliness, and daily hours of sleep. This study’s sample, however, comprised opportunistically recruited college students, not a clinical population. Further, the researchers did not examine the relationship of QOL with acute service utilization or other measures of the social determinants of health, such as housing, employment, or social connectedness.
The following study was designed to extend these results by focusing on a clinical population—individuals with mental health or substance use issues—being served in a large, publicly funded behavioral health system in Milwaukee, Wisconsin. The objective of this study was to determine whether a single-item QOL measure could be used as a brief, parsimonious measure of overall population health by examining its relationship with other key outcome measures for patients receiving services from BHS. This study was reviewed and approved by BHS’s Institutional Review Board.
Methods
All patients engaged in nonacute community services are offered a standardized assessment that includes, among other measures, items related to QOL, housing status, employment status, self-rated physical health, and social connectedness. This assessment is administered at intake, discharge, and every 6 months while patients are enrolled in services. Patients who received at least 1 assessment between October 1, 2020, and September 30, 2021, were included in the analyses. Patients receiving crisis, inpatient, or withdrawal management services alone (ie, did not receive any other community-based services) were not offered the standard assessment and thus were not included in the analyses. If patients had more than 1 assessment during this time period, QOL data from the last assessment were used. Data on housing (private residence status, defined as adults living alone or with others without supervision in a house or apartment), employment status, self-rated physical health, and social connectedness (measured by asking people whether they have had positive interactions with family or friends in the past 30 days) were extracted from the same timepoint as well.
Also included in the analyses were rates of acute service utilization, in which any patient with at least 1 visit to BHS’s psychiatric emergency department, withdrawal management facility, or psychiatric inpatient facility in the 90 days prior to the date of the assessment received a code of “Yes,” and any patient who did not receive any of these services received a code of “No.” Chi-square analyses were conducted to determine the relationship between QOL rankings (“Very Poor,” “Poor,” “Neither Good nor Poor,” “Good,” and “Very Good”) and housing, employment, self-rated physical health, social connectedness, and 90-day acute service use. All acute service utilization data were obtained from BHS’s electronic health records system. All data used in the study were stored on a secure, password-protected server. All analyses were conducted with SPSS software (SPSS 28; IBM).
Results
Data were available for 4488 patients who received an assessment between October 1, 2020, and September 30, 2021 (total numbers per item vary because some items had missing data; see supplementary eTables 1-3 for sample size per item). Demographics of the patient sample are listed in Table 1; the demographics of the patients who were missing data for specific outcomes are presented in eTables 1-3.




Statistical analyses revealed results in the expected direction for all relationships tested (Table 2). As patients’ self-reported QOL improved, so did the likelihood of higher rates of self-reported “Good” or better physical health, which was 576% higher among individuals who reported “Very Good” QOL relative to those who reported “Very Poor” QOL. Similarly, when compared with individuals with “Very Poor” QOL, individuals who reported “Very Good” QOL were 21.91% more likely to report having a private residence, 126.7% more likely to report being employed, and 29.17% more likely to report having had positive social interactions with family and friends in the past 30 days. There was an inverse relationship between QOL and the likelihood that a patient had received at least 1 admission for an acute service in the previous 90 days, such that patients who reported “Very Good” QOL were 86.34% less likely to have had an admission compared to patients with “Very Poor” QOL (2.8% vs 20.5%, respectively). The relationships among the criterion variables used in this study are presented in Table 3.

Discussion
The results of this preliminary analysis suggest that self-rated QOL is related to key health, social determinants of health, and acute service utilization metrics. These data are important for several reasons. First, because QOL is a diagnostically agnostic measure, it is a cross-cutting measure to use with clinically diverse populations receiving an array of different services. Second, at 1 item, the QOL measure is extremely brief and therefore minimally onerous to implement for both patients and administratively overburdened providers. Third, its correlation with other key metrics suggests that it can function as a broad population health measure for health care organizations because individuals with higher QOL will also likely have better outcomes in other key areas. This suggests that it has the potential to broadly represent the overall status of a population of patients, thus functioning as a type of “whole system” measure, which the Institute for Healthcare Improvement describes as “a small set of measures that reflect a health system’s overall performance on core dimensions of quality guided by the Triple Aim.”7 These whole system measures can help focus an organization’s strategic initiatives and efforts on the issues that matter most to the patients and community it serves.
The relationship of QOL to acute service utilization deserves special mention. As an administrative measure, utilization is not susceptible to the same response bias as the other self-reported variables. Furthermore, acute services are costly to health systems, and hospital readmissions are associated with payment reductions in the Centers for Medicare and Medicaid Services (CMS) Hospital Readmissions Reduction Program for hospitals that fail to meet certain performance targets.27 Thus, because of its alignment with federal mandates, improved QOL (and potentially concomitant decreases in acute service use) may have significant financial implications for health systems as well.
This study was limited by several factors. First, it was focused on a population receiving publicly funded behavioral health services with strict eligibility requirements, one of which stipulated that individuals must be at 200% or less of the Federal Poverty Level; therefore, the results might not be applicable to health systems with a more clinically or socioeconomically diverse patient population. Second, because these data are cross-sectional, it was not possible to determine whether QOL improved over time or whether changes in QOL covaried longitudinally with the other metrics under observation. For example, if patients’ QOL improved from the first to last assessment, did their employment or residential status improve as well, or were these patients more likely to be employed at their first assessment? Furthermore, if there was covariance, did changes in employment, housing status, and so on precede changes in QOL or vice versa? Multiple longitudinal observations would help to address these questions and will be the focus of future analyses.
Conclusion
This preliminary study suggests that a single-item QOL measure may be a valuable population health–level metric for health systems. It requires little administrative effort on the part of either the clinician or patient. It is also agnostic with regard to clinical issue or treatment approach and can therefore admit of a range of diagnoses or patient-specific, idiosyncratic recovery goals. It is correlated with other key health, social determinants of health, and acute service utilization indicators and can therefore serve as a “whole system” measure because of its ability to broadly represent improvements in an entire population. Furthermore, QOL is patient-centered in that data are obtained through patient self-report, which is a high priority for CMS and other health care organizations.28 In summary, a single-item QOL measure holds promise for health care organizations looking to implement the Quadruple Aim and assess the health of the populations they serve in a manner that is simple, efficient, and patient-centered.
Acknowledgments: The author thanks Jennifer Wittwer for her thoughtful comments on the initial draft of this manuscript and Gary Kraft for his help extracting the data used in the analyses.
Corresponding author: Walter Matthew Drymalski, PhD; [email protected]
Disclosures: None reported.
From Milwaukee County Behavioral Health Services, Milwaukee, WI.
Abstract
Objectives: The goal of this study was to determine whether a single-item quality of life (QOL) measure could serve as a useful population health–level metric within the Quadruple Aim framework in a publicly funded behavioral health system.
Design: This was a retrospective, cross-sectional study that examined the correlation between the single-item QOL measure and several other key measures of the social determinants of health and a composite measure of acute service utilization for all patients receiving mental health and substance use services in a community behavioral health system.
Methods: Data were collected for 4488 patients who had at least 1 assessment between October 1, 2020, and September 30, 2021. Data on social determinants of health were obtained through patient self-report; acute service use data were obtained from electronic health records.
Results: Statistical analyses revealed results in the expected direction for all relationships tested. Patients with higher QOL were more likely to report “Good” or better self-rated physical health, be employed, have a private residence, and report recent positive social interactions, and were less likely to have received acute services in the previous 90 days.
Conclusion: A single-item QOL measure shows promise as a general, minimally burdensome whole-system metric that can function as a target for population health management efforts in a large behavioral health system. Future research should explore whether this QOL measure is sensitive to change over time and examine its temporal relationship with other key outcome metrics.
Keywords: Quadruple Aim, single-item measures, social determinants of health, acute service utilization metrics.
The Triple Aim for health care—improving the individual experience of care, increasing the health of populations, and reducing the costs of care—was first proposed in 2008.1 More recently, some have advocated for an expanded focus to include a fourth aim: the quality of staff work life.2 Since this seminal paper was published, many health care systems have endeavored to adopt and implement the Quadruple Aim3,4; however, the concepts representing each of the aims are not universally defined,3 nor are the measures needed to populate the Quadruple Aim always available within the health system in question.5
Although several assessment models and frameworks that provide guidance to stakeholders have been developed,6,7 it is ultimately up to organizations themselves to determine which measures they should deploy to best represent the different quadrants of the Quadruple Aim.6 Evidence suggests, however, that quality measurement, and the administrative time required to conduct it, can be both financially and emotionally burdensome to providers and health systems.8-10 Thus, it is incumbent on organizations to select a set of measures that are not only meaningful but as parsimonious as possible.6,11,12
Quality of life (QOL) is a potential candidate to assess the aim of population health. Brief health-related QOL questions have long been used in epidemiological surveys, such as the Behavioral Risk Factor Surveillance System survey.13 Such questions are also a key component of community health frameworks, such as the County Health Rankings developed by the University of Wisconsin Population Health Institute.14 Furthermore, Humana recently revealed that increasing the number of physical and mental health “Healthy Days” (which are among the Centers for Disease Control and Prevention’s Health-Related Quality of Life questions15) among the members enrolled in their insurance plan would become a major goal for the organization.16,17 Many of these measures, while brief, focus on QOL as a function of health, often as a self-rated construct (from “Poor” to “Excellent”) or in the form of days of poor physical or mental health in the past 30 days,15 rather than evaluating QOL itself; however, several authors have pointed out that health status and QOL are related but distinct concepts.18,19
Brief single-item assessments focused specifically on QOL have been developed and implemented within nonclinical20 and clinical populations, including individuals with cancer,21 adults with disabilities,22 individuals with cystic fibrosis,23 and children with epilepsy.24 Despite the long history of QOL assessment in behavioral health treatment,25 single-item measures have not been widely implemented in this population.
Milwaukee County Behavioral Health Services (BHS), a publicly funded, county-based behavioral health care system in Milwaukee, Wisconsin, provides inpatient and ambulatory treatment, psychiatric emergency care, withdrawal management, care management, crisis services, and other support services to individuals in Milwaukee County. In 2018 the community services arm of BHS began implementing a single QOL question from the World Health Organization’s WHOQOL-BREF26: On a 5-point rating scale of “Very Poor” to “Very Good,” “How would you rate your overall quality of life right now?” Previous research by Atroszko and colleagues,20 which used a similar approach with the same item from the WHOQOL-BREF, reported correlations in the expected direction of the single-item QOL measure with perceived stress, depression, anxiety, loneliness, and daily hours of sleep. This study’s sample, however, comprised opportunistically recruited college students, not a clinical population. Further, the researchers did not examine the relationship of QOL with acute service utilization or other measures of the social determinants of health, such as housing, employment, or social connectedness.
The following study was designed to extend these results by focusing on a clinical population—individuals with mental health or substance use issues—being served in a large, publicly funded behavioral health system in Milwaukee, Wisconsin. The objective of this study was to determine whether a single-item QOL measure could be used as a brief, parsimonious measure of overall population health by examining its relationship with other key outcome measures for patients receiving services from BHS. This study was reviewed and approved by BHS’s Institutional Review Board.
Methods
All patients engaged in nonacute community services are offered a standardized assessment that includes, among other measures, items related to QOL, housing status, employment status, self-rated physical health, and social connectedness. This assessment is administered at intake, discharge, and every 6 months while patients are enrolled in services. Patients who received at least 1 assessment between October 1, 2020, and September 30, 2021, were included in the analyses. Patients receiving crisis, inpatient, or withdrawal management services alone (ie, did not receive any other community-based services) were not offered the standard assessment and thus were not included in the analyses. If patients had more than 1 assessment during this time period, QOL data from the last assessment were used. Data on housing (private residence status, defined as adults living alone or with others without supervision in a house or apartment), employment status, self-rated physical health, and social connectedness (measured by asking people whether they have had positive interactions with family or friends in the past 30 days) were extracted from the same timepoint as well.
Also included in the analyses were rates of acute service utilization, in which any patient with at least 1 visit to BHS’s psychiatric emergency department, withdrawal management facility, or psychiatric inpatient facility in the 90 days prior to the date of the assessment received a code of “Yes,” and any patient who did not receive any of these services received a code of “No.” Chi-square analyses were conducted to determine the relationship between QOL rankings (“Very Poor,” “Poor,” “Neither Good nor Poor,” “Good,” and “Very Good”) and housing, employment, self-rated physical health, social connectedness, and 90-day acute service use. All acute service utilization data were obtained from BHS’s electronic health records system. All data used in the study were stored on a secure, password-protected server. All analyses were conducted with SPSS software (SPSS 28; IBM).
Results
Data were available for 4488 patients who received an assessment between October 1, 2020, and September 30, 2021 (total numbers per item vary because some items had missing data; see supplementary eTables 1-3 for sample size per item). Demographics of the patient sample are listed in Table 1; the demographics of the patients who were missing data for specific outcomes are presented in eTables 1-3.




Statistical analyses revealed results in the expected direction for all relationships tested (Table 2). As patients’ self-reported QOL improved, so did the likelihood of higher rates of self-reported “Good” or better physical health, which was 576% higher among individuals who reported “Very Good” QOL relative to those who reported “Very Poor” QOL. Similarly, when compared with individuals with “Very Poor” QOL, individuals who reported “Very Good” QOL were 21.91% more likely to report having a private residence, 126.7% more likely to report being employed, and 29.17% more likely to report having had positive social interactions with family and friends in the past 30 days. There was an inverse relationship between QOL and the likelihood that a patient had received at least 1 admission for an acute service in the previous 90 days, such that patients who reported “Very Good” QOL were 86.34% less likely to have had an admission compared to patients with “Very Poor” QOL (2.8% vs 20.5%, respectively). The relationships among the criterion variables used in this study are presented in Table 3.

Discussion
The results of this preliminary analysis suggest that self-rated QOL is related to key health, social determinants of health, and acute service utilization metrics. These data are important for several reasons. First, because QOL is a diagnostically agnostic measure, it is a cross-cutting measure to use with clinically diverse populations receiving an array of different services. Second, at 1 item, the QOL measure is extremely brief and therefore minimally onerous to implement for both patients and administratively overburdened providers. Third, its correlation with other key metrics suggests that it can function as a broad population health measure for health care organizations because individuals with higher QOL will also likely have better outcomes in other key areas. This suggests that it has the potential to broadly represent the overall status of a population of patients, thus functioning as a type of “whole system” measure, which the Institute for Healthcare Improvement describes as “a small set of measures that reflect a health system’s overall performance on core dimensions of quality guided by the Triple Aim.”7 These whole system measures can help focus an organization’s strategic initiatives and efforts on the issues that matter most to the patients and community it serves.
The relationship of QOL to acute service utilization deserves special mention. As an administrative measure, utilization is not susceptible to the same response bias as the other self-reported variables. Furthermore, acute services are costly to health systems, and hospital readmissions are associated with payment reductions in the Centers for Medicare and Medicaid Services (CMS) Hospital Readmissions Reduction Program for hospitals that fail to meet certain performance targets.27 Thus, because of its alignment with federal mandates, improved QOL (and potentially concomitant decreases in acute service use) may have significant financial implications for health systems as well.
This study was limited by several factors. First, it was focused on a population receiving publicly funded behavioral health services with strict eligibility requirements, one of which stipulated that individuals must be at 200% or less of the Federal Poverty Level; therefore, the results might not be applicable to health systems with a more clinically or socioeconomically diverse patient population. Second, because these data are cross-sectional, it was not possible to determine whether QOL improved over time or whether changes in QOL covaried longitudinally with the other metrics under observation. For example, if patients’ QOL improved from the first to last assessment, did their employment or residential status improve as well, or were these patients more likely to be employed at their first assessment? Furthermore, if there was covariance, did changes in employment, housing status, and so on precede changes in QOL or vice versa? Multiple longitudinal observations would help to address these questions and will be the focus of future analyses.
Conclusion
This preliminary study suggests that a single-item QOL measure may be a valuable population health–level metric for health systems. It requires little administrative effort on the part of either the clinician or patient. It is also agnostic with regard to clinical issue or treatment approach and can therefore admit of a range of diagnoses or patient-specific, idiosyncratic recovery goals. It is correlated with other key health, social determinants of health, and acute service utilization indicators and can therefore serve as a “whole system” measure because of its ability to broadly represent improvements in an entire population. Furthermore, QOL is patient-centered in that data are obtained through patient self-report, which is a high priority for CMS and other health care organizations.28 In summary, a single-item QOL measure holds promise for health care organizations looking to implement the Quadruple Aim and assess the health of the populations they serve in a manner that is simple, efficient, and patient-centered.
Acknowledgments: The author thanks Jennifer Wittwer for her thoughtful comments on the initial draft of this manuscript and Gary Kraft for his help extracting the data used in the analyses.
Corresponding author: Walter Matthew Drymalski, PhD; [email protected]
Disclosures: None reported.
1. Berwick DM, Nolan TW, Whittington J. The triple aim: care, health, and cost. Health Aff (Millwood). 2008;27(3):759-769. doi:10.1377/hlthaff.27.3.759
2. Bodenheimer T, Sinsky C. From triple to quadruple aim: care of the patient requires care of the provider. Ann Fam Med. 2014;12(6):573-576. doi:10.1370/afm.1713
3. Hendrikx RJP, Drewes HW, Spreeuwenberg M, et al. Which triple aim related measures are being used to evaluate population management initiatives? An international comparative analysis. Health Policy. 2016;120(5):471-485. doi:10.1016/j.healthpol.2016.03.008
4. Whittington JW, Nolan K, Lewis N, Torres T. Pursuing the triple aim: the first 7 years. Milbank Q. 2015;93(2):263-300. doi:10.1111/1468-0009.12122
5. Ryan BL, Brown JB, Glazier RH, Hutchison B. Examining primary healthcare performance through a triple aim lens. Healthc Policy. 2016;11(3):19-31.
6. Stiefel M, Nolan K. A guide to measuring the Triple Aim: population health, experience of care, and per capita cost. Institute for Healthcare Improvement; 2012. Accessed November 1, 2022. https://nhchc.org/wp-content/uploads/2019/08/ihiguidetomeasuringtripleaimwhitepaper2012.pdf
7. Martin L, Nelson E, Rakover J, Chase A. Whole system measures 2.0: a compass for health system leaders. Institute for Healthcare Improvement; 2016. Accessed November 1, 2022. http://www.ihi.org:80/resources/Pages/IHIWhitePapers/Whole-System-Measures-Compass-for-Health-System-Leaders.aspx
8. Casalino LP, Gans D, Weber R, et al. US physician practices spend more than $15.4 billion annually to report quality measures. Health Aff (Millwood). 2016;35(3):401-406. doi:10.1377/hlthaff.2015.1258
9. Rao SK, Kimball AB, Lehrhoff SR, et al. The impact of administrative burden on academic physicians: results of a hospital-wide physician survey. Acad Med. 2017;92(2):237-243. doi:10.1097/ACM.0000000000001461
10. Woolhandler S, Himmelstein DU. Administrative work consumes one-sixth of U.S. physicians’ working hours and lowers their career satisfaction. Int J Health Serv. 2014;44(4):635-642. doi:10.2190/HS.44.4.a
11. Meyer GS, Nelson EC, Pryor DB, et al. More quality measures versus measuring what matters: a call for balance and parsimony. BMJ Qual Saf. 2012;21(11):964-968. doi:10.1136/bmjqs-2012-001081
12. Vital Signs: Core Metrics for Health and Health Care Progress. Washington, DC: National Academies Press; 2015. doi:10.17226/19402
13. Centers for Disease Control and Prevention. BRFSS questionnaires. Accessed November 1, 2022. https://www.cdc.gov/brfss/questionnaires/index.htm
14. County Health Rankings and Roadmaps. Measures & data sources. University of Wisconsin Population Health Institute. Accessed November 1, 2022. https://www.countyhealthrankings.org/explore-health-rankings/measures-data-sources
15. Centers for Disease Control and Prevention. Healthy days core module (CDC HRQOL-4). Accessed November 1, 2022. https://www.cdc.gov/hrqol/hrqol14_measure.htm
16. Cordier T, Song Y, Cambon J, et al. A bold goal: more healthy days through improved community health. Popul Health Manag. 2018;21(3):202-208. doi:10.1089/pop.2017.0142
17. Slabaugh SL, Shah M, Zack M, et al. Leveraging health-related quality of life in population health management: the case for healthy days. Popul Health Manag. 2017;20(1):13-22. doi:10.1089/pop.2015.0162
18. Karimi M, Brazier J. Health, health-related quality of life, and quality of life: what is the difference? Pharmacoeconomics. 2016;34(7):645-649. doi:10.1007/s40273-016-0389-9
19. Smith KW, Avis NE, Assmann SF. Distinguishing between quality of life and health status in quality of life research: a meta-analysis. Qual Life Res. 1999;8(5):447-459. doi:10.1023/a:1008928518577
20. Atroszko PA, Baginska P, Mokosinska M, et al. Validity and reliability of single-item self-report measures of general quality of life, general health and sleep quality. In: CER Comparative European Research 2015. Sciemcee Publishing; 2015:207-211.
21. Singh JA, Satele D, Pattabasavaiah S, et al. Normative data and clinically significant effect sizes for single-item numerical linear analogue self-assessment (LASA) scales. Health Qual Life Outcomes. 2014;12:187. doi:10.1186/s12955-014-0187-z
22. Siebens HC, Tsukerman D, Adkins RH, et al. Correlates of a single-item quality-of-life measure in people aging with disabilities. Am J Phys Med Rehabil. 2015;94(12):1065-1074. doi:10.1097/PHM.0000000000000298
23. Yohannes AM, Dodd M, Morris J, Webb K. Reliability and validity of a single item measure of quality of life scale for adult patients with cystic fibrosis. Health Qual Life Outcomes. 2011;9:105. doi:10.1186/1477-7525-9-105
24. Conway L, Widjaja E, Smith ML. Single-item measure for assessing quality of life in children with drug-resistant epilepsy. Epilepsia Open. 2017;3(1):46-54. doi:10.1002/epi4.12088
25. Barry MM, Zissi A. Quality of life as an outcome measure in evaluating mental health services: a review of the empirical evidence. Soc Psychiatry Psychiatr Epidemiol. 1997;32(1):38-47. doi:10.1007/BF00800666
26. Skevington SM, Lotfy M, O’Connell KA. The World Health Organization’s WHOQOL-BREF quality of life assessment: psychometric properties and results of the international field trial. Qual Life Res. 2004;13(2):299-310. doi:10.1023/B:QURE.0000018486.91360.00
27. Centers for Medicare & Medicaid Services. Hospital readmissions reduction program (HRRP). Accessed November 1, 2022. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program
28. Centers for Medicare & Medicaid Services. Patient-reported outcome measures. CMS Measures Management System. Published May 2022. Accessed November 1, 2022. https://www.cms.gov/files/document/blueprint-patient-reported-outcome-measures.pdf
1. Berwick DM, Nolan TW, Whittington J. The triple aim: care, health, and cost. Health Aff (Millwood). 2008;27(3):759-769. doi:10.1377/hlthaff.27.3.759
2. Bodenheimer T, Sinsky C. From triple to quadruple aim: care of the patient requires care of the provider. Ann Fam Med. 2014;12(6):573-576. doi:10.1370/afm.1713
3. Hendrikx RJP, Drewes HW, Spreeuwenberg M, et al. Which triple aim related measures are being used to evaluate population management initiatives? An international comparative analysis. Health Policy. 2016;120(5):471-485. doi:10.1016/j.healthpol.2016.03.008
4. Whittington JW, Nolan K, Lewis N, Torres T. Pursuing the triple aim: the first 7 years. Milbank Q. 2015;93(2):263-300. doi:10.1111/1468-0009.12122
5. Ryan BL, Brown JB, Glazier RH, Hutchison B. Examining primary healthcare performance through a triple aim lens. Healthc Policy. 2016;11(3):19-31.
6. Stiefel M, Nolan K. A guide to measuring the Triple Aim: population health, experience of care, and per capita cost. Institute for Healthcare Improvement; 2012. Accessed November 1, 2022. https://nhchc.org/wp-content/uploads/2019/08/ihiguidetomeasuringtripleaimwhitepaper2012.pdf
7. Martin L, Nelson E, Rakover J, Chase A. Whole system measures 2.0: a compass for health system leaders. Institute for Healthcare Improvement; 2016. Accessed November 1, 2022. http://www.ihi.org:80/resources/Pages/IHIWhitePapers/Whole-System-Measures-Compass-for-Health-System-Leaders.aspx
8. Casalino LP, Gans D, Weber R, et al. US physician practices spend more than $15.4 billion annually to report quality measures. Health Aff (Millwood). 2016;35(3):401-406. doi:10.1377/hlthaff.2015.1258
9. Rao SK, Kimball AB, Lehrhoff SR, et al. The impact of administrative burden on academic physicians: results of a hospital-wide physician survey. Acad Med. 2017;92(2):237-243. doi:10.1097/ACM.0000000000001461
10. Woolhandler S, Himmelstein DU. Administrative work consumes one-sixth of U.S. physicians’ working hours and lowers their career satisfaction. Int J Health Serv. 2014;44(4):635-642. doi:10.2190/HS.44.4.a
11. Meyer GS, Nelson EC, Pryor DB, et al. More quality measures versus measuring what matters: a call for balance and parsimony. BMJ Qual Saf. 2012;21(11):964-968. doi:10.1136/bmjqs-2012-001081
12. Vital Signs: Core Metrics for Health and Health Care Progress. Washington, DC: National Academies Press; 2015. doi:10.17226/19402
13. Centers for Disease Control and Prevention. BRFSS questionnaires. Accessed November 1, 2022. https://www.cdc.gov/brfss/questionnaires/index.htm
14. County Health Rankings and Roadmaps. Measures & data sources. University of Wisconsin Population Health Institute. Accessed November 1, 2022. https://www.countyhealthrankings.org/explore-health-rankings/measures-data-sources
15. Centers for Disease Control and Prevention. Healthy days core module (CDC HRQOL-4). Accessed November 1, 2022. https://www.cdc.gov/hrqol/hrqol14_measure.htm
16. Cordier T, Song Y, Cambon J, et al. A bold goal: more healthy days through improved community health. Popul Health Manag. 2018;21(3):202-208. doi:10.1089/pop.2017.0142
17. Slabaugh SL, Shah M, Zack M, et al. Leveraging health-related quality of life in population health management: the case for healthy days. Popul Health Manag. 2017;20(1):13-22. doi:10.1089/pop.2015.0162
18. Karimi M, Brazier J. Health, health-related quality of life, and quality of life: what is the difference? Pharmacoeconomics. 2016;34(7):645-649. doi:10.1007/s40273-016-0389-9
19. Smith KW, Avis NE, Assmann SF. Distinguishing between quality of life and health status in quality of life research: a meta-analysis. Qual Life Res. 1999;8(5):447-459. doi:10.1023/a:1008928518577
20. Atroszko PA, Baginska P, Mokosinska M, et al. Validity and reliability of single-item self-report measures of general quality of life, general health and sleep quality. In: CER Comparative European Research 2015. Sciemcee Publishing; 2015:207-211.
21. Singh JA, Satele D, Pattabasavaiah S, et al. Normative data and clinically significant effect sizes for single-item numerical linear analogue self-assessment (LASA) scales. Health Qual Life Outcomes. 2014;12:187. doi:10.1186/s12955-014-0187-z
22. Siebens HC, Tsukerman D, Adkins RH, et al. Correlates of a single-item quality-of-life measure in people aging with disabilities. Am J Phys Med Rehabil. 2015;94(12):1065-1074. doi:10.1097/PHM.0000000000000298
23. Yohannes AM, Dodd M, Morris J, Webb K. Reliability and validity of a single item measure of quality of life scale for adult patients with cystic fibrosis. Health Qual Life Outcomes. 2011;9:105. doi:10.1186/1477-7525-9-105
24. Conway L, Widjaja E, Smith ML. Single-item measure for assessing quality of life in children with drug-resistant epilepsy. Epilepsia Open. 2017;3(1):46-54. doi:10.1002/epi4.12088
25. Barry MM, Zissi A. Quality of life as an outcome measure in evaluating mental health services: a review of the empirical evidence. Soc Psychiatry Psychiatr Epidemiol. 1997;32(1):38-47. doi:10.1007/BF00800666
26. Skevington SM, Lotfy M, O’Connell KA. The World Health Organization’s WHOQOL-BREF quality of life assessment: psychometric properties and results of the international field trial. Qual Life Res. 2004;13(2):299-310. doi:10.1023/B:QURE.0000018486.91360.00
27. Centers for Medicare & Medicaid Services. Hospital readmissions reduction program (HRRP). Accessed November 1, 2022. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program
28. Centers for Medicare & Medicaid Services. Patient-reported outcome measures. CMS Measures Management System. Published May 2022. Accessed November 1, 2022. https://www.cms.gov/files/document/blueprint-patient-reported-outcome-measures.pdf
Neurosurgery Operating Room Efficiency During the COVID-19 Era
From the Department of Neurological Surgery, Vanderbilt University Medical Center, Nashville, TN (Stefan W. Koester, Puja Jagasia, and Drs. Liles, Dambrino IV, Feldman, and Chambless), and the Department of Anesthesiology, Vanderbilt University Medical Center, Nashville, TN (Drs. Mathews and Tiwari).
ABSTRACT
Background: The COVID-19 pandemic has had broad effects on surgical care, including operating room (OR) staffing, personal protective equipment (PPE) utilization, and newly implemented anti-infective measures. Our aim was to assess neurosurgery OR efficiency before the COVID-19 pandemic, during peak COVID-19, and during current times.
Methods: Institutional perioperative databases at a single, high-volume neurosurgical center were queried for operations performed from December 2019 until October 2021. March 12, 2020, the day that the state of Tennessee declared a state of emergency, was chosen as the onset of the COVID-19 pandemic. The 90-day periods before and after this day were used to define the pre-COVID-19, peak-COVID-19, and post-peak restrictions time periods for comparative analysis. Outcomes included delay in first-start and OR turnover time between neurosurgical cases. Preset threshold times were used in analyses to adjust for normal leniency in OR scheduling (15 minutes for first start and 90 minutes for turnover). Univariate analysis used Wilcoxon rank-sum test for continuous outcomes, while chi-square test and Fisher’s exact test were used for categorical comparisons. Significance was defined as P < .05.
Results: First-start time was analyzed in 426 pre-COVID-19, 357 peak-restrictions, and 2304 post-peak-restrictions cases. The unadjusted mean delay length was found to be significantly different between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes, 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004). The adjusted average delay length and proportion of cases delayed beyond the 15-minute threshold were not significantly different. The proportion of cases that started early, as well as significantly early past a 15-minute threshold, have not been impacted. There was no significant change in turnover time during peak restrictions relative to the pre-COVID-19 period (88 [100] minutes vs 85 [95] minutes), and turnover time has since remained unchanged (83 [87] minutes).
Conclusion: Our center was able to maintain OR efficiency before, during, and after peak restrictions even while instituting advanced infection-control strategies. While there were significant changes, delays were relatively small in magnitude.
Keywords: operating room timing, hospital efficiency, socioeconomics, pandemic.
The COVID-19 pandemic has led to major changes in patient care both from a surgical perspective and in regard to inpatient hospital course. Safety protocols nationwide have been implemented to protect both patients and providers. Some elements of surgical care have drastically changed, including operating room (OR) staffing, personal protective equipment (PPE) utilization, and increased sterilization measures. Furloughs, layoffs, and reassignments due to the focus on nonelective and COVID-19–related cases challenged OR staffing and efficiency. Operating room staff with COVID-19 exposures or COVID-19 infections also caused last-minute changes in staffing. All of these scenarios can cause issues due to actual understaffing or due to staff members being pushed into highly specialized areas, such as neurosurgery, in which they have very little experience. A further obstacle to OR efficiency included policy changes involving PPE utilization, sterilization measures, and supply chain shortages of necessary resources such as PPE.
Neurosurgery in particular has been susceptible to COVID-19–related system-wide changes given operator proximity to the patient’s respiratory passages, frequency of emergent cases, and varying anesthetic needs, as well as the high level of specialization needed to perform neurosurgical care. Previous studies have shown a change in the makeup of neurosurgical patients seeking care, as well as in the acuity of neurological consult of these patients.1 A study in orthopedic surgery by Andreata et al demonstrated worsened OR efficiency, with significantly increased first-start and turnover times.2 In the COVID-19 era, OR quality and safety are crucially important to both patients and providers. Providing this safe and effective care in an efficient manner is important for optimal neurosurgical management in the long term.3 Moreover, the financial burden of implementing new protocols and standards can be compounded by additional financial losses due to reduced OR efficiency.
Methods
To analyze the effect of COVID-19 on neurosurgical OR efficiency, institutional perioperative databases at a single high-volume center were queried for operations performed from December 2019 until October 2021. March 12, 2020, was chosen as the onset of COVID-19 for analytic purposes, as this was the date when the state of Tennessee declared a state of emergency. The 90-day periods before and after this date were used for comparative analysis for pre-COVID-19, peak COVID-19, and post-peak-restrictions time periods. The peak COVID-19 period was defined as the 90-day period following the initial onset of COVID-19 and the surge of cases. For comparison purposes, post-peak COVID-19 was defined as the months following the first peak until October 2021 (approximately 17 months). COVID-19 burden was determined using a COVID-19 single-institution census of confirmed cases by polymerase chain reaction (PCR) for which the average number of cases of COVID-19 during a given month was determined. This number is a scaled trend, and a true number of COVID-19 cases in our hospital was not reported.
Neurosurgical and neuroendovascular cases were included in the analysis. Outcomes included delay in first-start and OR turnover time between neurosurgical cases, defined as the time from the patient leaving the room until the next patient entered the room. Preset threshold times were used in analyses to adjust for normal leniency in OR scheduling (15 minutes for first start and 90 minutes for turnover, which is a standard for our single-institution perioperative center). Statistical analyses, including data aggregation, were performed using R, version 4.0.1 (R Foundation for Statistical Computing). Patients’ demographic and clinical characteristics were analyzed using an independent 2-sample t-test for interval variables and a chi-square test for categorical variables. Significance was defined as P < .05.
Results
First-Start Time
First-start time was analyzed in 426 pre-COVID-19, 357 peak-COVID-19, and 2304 post-peak-COVID-19 cases. The unadjusted mean delay length was significantly different between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes, 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004) (Table 1). 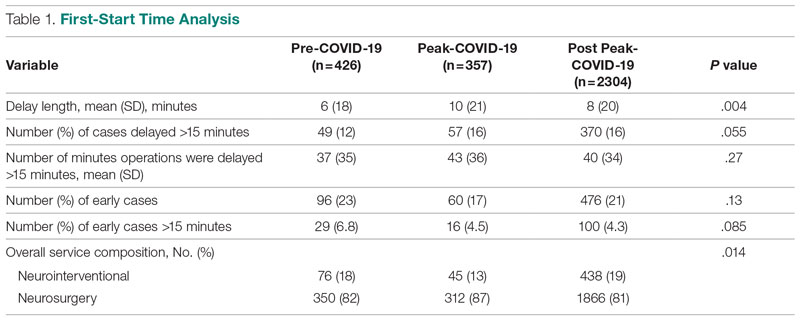
The adjusted average delay length and proportion of cases delayed beyond the 15-minute threshold were not significantly different, but they have been slightly higher since the onset of COVID-19. The proportion of cases that have started early, as well as significantly early past a 15-minute threshold, have also trended down since the onset of the COVID-19 pandemic, but this difference was again not significant. The temporal relationship of first-start delay, both unadjusted and adjusted, from December 2019 to October 2021 is shown in Figure 1. The trend of increasing delay is loosely associated with the COVID-19 burden experienced by our hospital. The start of COVID-19 as well as both COVID-19 peaks have been associated with increased delays in our hospital.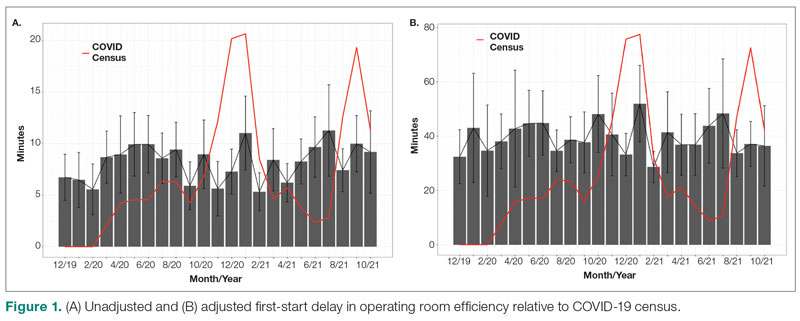
Turnover Time
Turnover time was assessed in 437 pre-COVID-19, 278 peak-restrictions, and 2411 post-peak-restrictions cases. Turnover time during peak restrictions was not significantly different from pre-COVID-19 (88 [100] vs 85 [95]) and has since remained relatively unchanged (83 [87], P = .78). A similar trend held for comparisons of proportion of cases with turnover time past 90 minutes and average times past the 90-minute threshold (Table 2). The temporal relationship between COVID-19 burden and turnover time, both unadjusted and adjusted, from December 2019 to October 2021 is shown in Figure 2. Both figures demonstrate a slight initial increase in turnover time delay at the start of COVID-19, which stabilized with little variation thereafter.
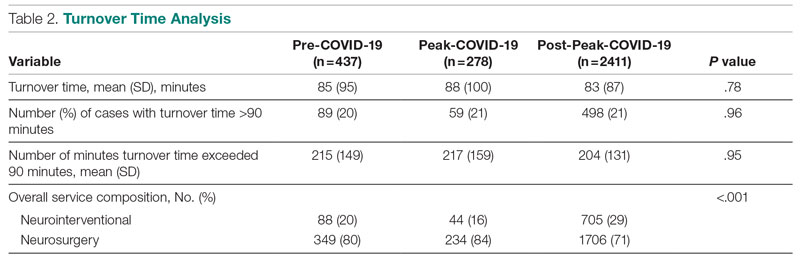
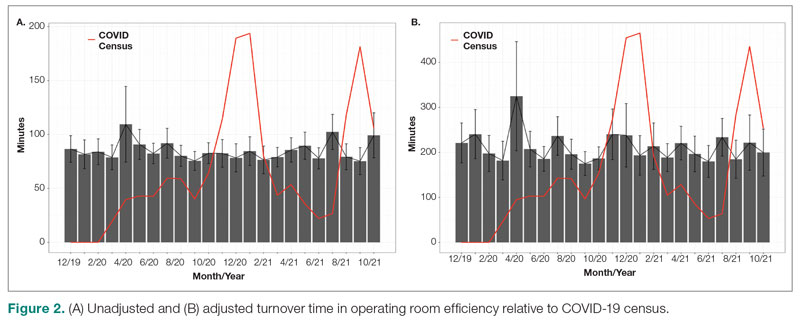
Discussion
We analyzed the OR efficiency metrics of first-start and turnover time during the 90-day period before COVID-19 (pre-COVID-19), the 90 days following Tennessee declaring a state of emergency (peak COVID-19), and the time following this period (post-COVID-19) for all neurosurgical and neuroendovascular cases at Vanderbilt University Medical Center (VUMC). We found a significant difference in unadjusted mean delay length in first-start time between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes for pre-COVID-19, peak-COVID-19, and post-COVID-19: 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004). No significant increase in turnover time between cases was found between these 3 time periods. Based on metrics from first-start delay and turnover time, our center was able to maintain OR efficiency before, during, and after peak COVID-19.
After the Centers for Disease Control and Prevention released guidelines recommending deferring elective procedures to conserve beds and PPE, VUMC made the decision to suspend all elective surgical procedures from March 18 to April 24, 2020. Prior research conducted during the COVID-19 pandemic has demonstrated more than 400 types of surgical procedures with negatively impacted outcomes when compared to surgical outcomes from the same time frame in 2018 and 2019.4 For more than 20 of these types of procedures, there was a significant association between procedure delay and adverse patient outcomes.4 Testing protocols for patients prior to surgery varied throughout the pandemic based on vaccination status and type of procedure. Before vaccines became widely available, all patients were required to obtain a PCR SARS-CoV-2 test within 48 to 72 hours of the scheduled procedure. If the patient’s procedure was urgent and testing was not feasible, the patient was treated as a SARS-CoV-2–positive patient, which required all health care workers involved in the case to wear gowns, gloves, surgical masks, and eye protection. Testing patients preoperatively likely helped to maintain OR efficiency since not all patients received test results prior to the scheduled procedure, leading to cancellations of cases and therefore more staff available for fewer cases.
After vaccines became widely available to the public, testing requirements for patients preoperatively were relaxed, and only patients who were not fully vaccinated or severely immunocompromised were required to test prior to procedures. However, approximately 40% of the population in Tennessee was fully vaccinated in 2021, which reflects the patient population of VUMC.5 This means that many patients who received care at VUMC were still tested prior to procedures.
Adopting adequate safety protocols was found to be key for OR efficiency during the COVID-19 pandemic since performing surgery increased the risk of infection for each health care worker in the OR.6 VUMC protocols identified procedures that required enhanced safety measures to prevent infection of health care workers and avoid staffing shortages, which would decrease OR efficiency. Protocols mandated that only anesthesia team members were allowed to be in the OR during intubation and extubation of patients, which could be one factor leading to increased delays and decreased efficiency for some institutions. Methods for neurosurgeons to decrease risk of infection in the OR include postponing all nonurgent cases, reappraising the necessity for general anesthesia and endotracheal intubation, considering alternative surgical approaches that avoid the respiratory tract, and limiting the use of aerosol-generating instruments.7,8 VUMC’s success in implementing these protocols likely explains why our center was able to maintain OR efficiency throughout the COVID-19 pandemic.
A study conducted by Andreata et al showed a significantly increased mean first-case delay and a nonsignificant increased turnover time in orthopedic surgeries in Northern Italy when comparing surgeries performed during the COVID-19 pandemic to those performed prior to COVID-19.2 Other studies have indicated a similar trend in decreased OR efficiency during COVID-19 in other areas around the world.9,10 These findings are not consistent with our own findings for neurosurgical and neuroendovascular surgeries at VUMC, and any change at our institution was relatively immaterial. Factors that threatened to change OR efficiency—but did not result in meaningful changes in our institutional experience—include delays due to pending COVID-19 test results, safety procedures such as PPE donning, and planning difficulties to ensure the existence of teams with non-overlapping providers in the case of a surgeon being infected.2,11-13
Globally, many surgery centers halted all elective surgeries during the initial COVID-19 spike to prevent a PPE shortage and mitigate risk of infection of patients and health care workers.8,12,14 However, there is no centralized definition of which neurosurgical procedures are elective, so that decision was made on a surgeon or center level, which could lead to variability in efficiency trends.14 One study on neurosurgical procedures during COVID-19 found a 30% decline in all cases and a 23% decline in emergent procedures, showing that the decrease in volume was not only due to cancellation of elective procedures.15 This decrease in elective and emergent surgeries created a backlog of surgeries as well as a loss in health care revenue, and caused many patients to go without adequate health care.10 Looking forward, it is imperative that surgical centers study trends in OR efficiency from COVID-19 and learn how to better maintain OR efficiency during future pandemic conditions to prevent a backlog of cases, loss of health care revenue, and decreased health care access.
Limitations
Our data are from a single center and therefore may not be representative of experiences of other hospitals due to different populations and different impacts from COVID-19. However, given our center’s high volume and diverse patient population, we believe our analysis highlights important trends in neurosurgery practice. Notably, data for patient and OR timing are digitally generated and are entered manually by nurses in the electronic medical record, making it prone to errors and variability. This is in our experience, and if any error is present, we believe it is minimal.
Conclusion
The COVID-19 pandemic has had far-reaching effects on health care worldwide, including neurosurgical care. OR efficiency across the United States generally worsened given the stresses of supply chain issues, staffing shortages, and cancellations. At our institution, we were able to maintain OR efficiency during the known COVID-19 peaks until October 2021. Continually functional neurosurgical ORs are important in preventing delays in care and maintaining a steady revenue in order for hospitals and other health care entities to remain solvent. Further study of OR efficiency is needed for health care systems to prepare for future pandemics and other resource-straining events in order to provide optimal patient care.
Corresponding author: Campbell Liles, MD, Vanderbilt University Medical Center, Department of Neurological Surgery, 1161 21st Ave. South, T4224 Medical Center North, Nashville, TN 37232-2380; [email protected]
Disclosures: None reported.
1. Koester SW, Catapano JS, Ma KL, et al. COVID-19 and neurosurgery consultation call volume at a single large tertiary center with a propensity- adjusted analysis. World Neurosurg. 2021;146:e768-e772. doi:10.1016/j.wneu.2020.11.017
2. Andreata M, Faraldi M, Bucci E, Lombardi G, Zagra L. Operating room efficiency and timing during coronavirus disease 2019 outbreak in a referral orthopaedic hospital in Northern Italy. Int Orthop. 2020;44(12):2499-2504. doi:10.1007/s00264-020-04772-x
3. Dexter F, Abouleish AE, Epstein RH, et al. Use of operating room information system data to predict the impact of reducing turnover times on staffing costs. Anesth Analg. 2003;97(4):1119-1126. doi:10.1213/01.ANE.0000082520.68800.79
4. Zheng NS, Warner JL, Osterman TJ, et al. A retrospective approach to evaluating potential adverse outcomes associated with delay of procedures for cardiovascular and cancer-related diagnoses in the context of COVID-19. J Biomed Inform. 2021;113:103657. doi:10.1016/j.jbi.2020.103657
5. Alcendor DJ. Targeting COVID-19 vaccine hesitancy in rural communities in Tennessee: implications for extending the COVID- 19 pandemic in the South. Vaccines (Basel). 2021;9(11):1279. doi:10.3390/vaccines9111279
6. Perrone G, Giuffrida M, Bellini V, et al. Operating room setup: how to improve health care professionals safety during pandemic COVID- 19: a quality improvement study. J Laparoendosc Adv Surg Tech A. 2021;31(1):85-89. doi:10.1089/lap.2020.0592
7. Iorio-Morin C, Hodaie M, Sarica C, et al. Letter: the risk of COVID-19 infection during neurosurgical procedures: a review of severe acute respiratory distress syndrome coronavirus 2 (SARS-CoV-2) modes of transmission and proposed neurosurgery-specific measures for mitigation. Neurosurgery. 2020;87(2):E178-E185. doi:10.1093/ neuros/nyaa157
8. Gupta P, Muthukumar N, Rajshekhar V, et al. Neurosurgery and neurology practices during the novel COVID-19 pandemic: a consensus statement from India. Neurol India. 2020;68(2):246-254. doi:10.4103/0028-3886.283130
9. Mercer ST, Agarwal R, Dayananda KSS, et al. A comparative study looking at trauma and orthopaedic operating efficiency in the COVID-19 era. Perioper Care Oper Room Manag. 2020;21:100142. doi:10.1016/j.pcorm.2020.100142
10. Rozario N, Rozario D. Can machine learning optimize the efficiency of the operating room in the era of COVID-19? Can J Surg. 2020;63(6):E527-E529. doi:10.1503/cjs.016520
11. Toh KHQ, Barazanchi A, Rajaretnam NS, et al. COVID-19 response by New Zealand general surgical departments in tertiary metropolitan hospitals. ANZ J Surg. 2021;91(7-8):1352-1357. doi:10.1111/ ans.17044
12. Moorthy RK, Rajshekhar V. Impact of COVID-19 pandemic on neurosurgical practice in India: a survey on personal protective equipment usage, testing, and perceptions on disease transmission. Neurol India. 2020;68(5):1133-1138. doi:10.4103/0028- 3886.299173
13. Meneghini RM. Techniques and strategies to optimize efficiencies in the office and operating room: getting through the patient backlog and preserving hospital resources. J Arthroplasty. 2021;36(7S):S49-S51. doi:10.1016/j.arth.2021.03.010
14. Jean WC, Ironside NT, Sack KD, et al. The impact of COVID- 19 on neurosurgeons and the strategy for triaging non-emergent operations: a global neurosurgery study. Acta Neurochir (Wien). 2020;162(6):1229-1240. doi:10.1007/s00701-020- 04342-5
15. Raneri F, Rustemi O, Zambon G, et al. Neurosurgery in times of a pandemic: a survey of neurosurgical services during the COVID-19 outbreak in the Veneto region in Italy. Neurosurg Focus. 2020;49(6):E9. doi:10.3171/2020.9.FOCUS20691
From the Department of Neurological Surgery, Vanderbilt University Medical Center, Nashville, TN (Stefan W. Koester, Puja Jagasia, and Drs. Liles, Dambrino IV, Feldman, and Chambless), and the Department of Anesthesiology, Vanderbilt University Medical Center, Nashville, TN (Drs. Mathews and Tiwari).
ABSTRACT
Background: The COVID-19 pandemic has had broad effects on surgical care, including operating room (OR) staffing, personal protective equipment (PPE) utilization, and newly implemented anti-infective measures. Our aim was to assess neurosurgery OR efficiency before the COVID-19 pandemic, during peak COVID-19, and during current times.
Methods: Institutional perioperative databases at a single, high-volume neurosurgical center were queried for operations performed from December 2019 until October 2021. March 12, 2020, the day that the state of Tennessee declared a state of emergency, was chosen as the onset of the COVID-19 pandemic. The 90-day periods before and after this day were used to define the pre-COVID-19, peak-COVID-19, and post-peak restrictions time periods for comparative analysis. Outcomes included delay in first-start and OR turnover time between neurosurgical cases. Preset threshold times were used in analyses to adjust for normal leniency in OR scheduling (15 minutes for first start and 90 minutes for turnover). Univariate analysis used Wilcoxon rank-sum test for continuous outcomes, while chi-square test and Fisher’s exact test were used for categorical comparisons. Significance was defined as P < .05.
Results: First-start time was analyzed in 426 pre-COVID-19, 357 peak-restrictions, and 2304 post-peak-restrictions cases. The unadjusted mean delay length was found to be significantly different between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes, 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004). The adjusted average delay length and proportion of cases delayed beyond the 15-minute threshold were not significantly different. The proportion of cases that started early, as well as significantly early past a 15-minute threshold, have not been impacted. There was no significant change in turnover time during peak restrictions relative to the pre-COVID-19 period (88 [100] minutes vs 85 [95] minutes), and turnover time has since remained unchanged (83 [87] minutes).
Conclusion: Our center was able to maintain OR efficiency before, during, and after peak restrictions even while instituting advanced infection-control strategies. While there were significant changes, delays were relatively small in magnitude.
Keywords: operating room timing, hospital efficiency, socioeconomics, pandemic.
The COVID-19 pandemic has led to major changes in patient care both from a surgical perspective and in regard to inpatient hospital course. Safety protocols nationwide have been implemented to protect both patients and providers. Some elements of surgical care have drastically changed, including operating room (OR) staffing, personal protective equipment (PPE) utilization, and increased sterilization measures. Furloughs, layoffs, and reassignments due to the focus on nonelective and COVID-19–related cases challenged OR staffing and efficiency. Operating room staff with COVID-19 exposures or COVID-19 infections also caused last-minute changes in staffing. All of these scenarios can cause issues due to actual understaffing or due to staff members being pushed into highly specialized areas, such as neurosurgery, in which they have very little experience. A further obstacle to OR efficiency included policy changes involving PPE utilization, sterilization measures, and supply chain shortages of necessary resources such as PPE.
Neurosurgery in particular has been susceptible to COVID-19–related system-wide changes given operator proximity to the patient’s respiratory passages, frequency of emergent cases, and varying anesthetic needs, as well as the high level of specialization needed to perform neurosurgical care. Previous studies have shown a change in the makeup of neurosurgical patients seeking care, as well as in the acuity of neurological consult of these patients.1 A study in orthopedic surgery by Andreata et al demonstrated worsened OR efficiency, with significantly increased first-start and turnover times.2 In the COVID-19 era, OR quality and safety are crucially important to both patients and providers. Providing this safe and effective care in an efficient manner is important for optimal neurosurgical management in the long term.3 Moreover, the financial burden of implementing new protocols and standards can be compounded by additional financial losses due to reduced OR efficiency.
Methods
To analyze the effect of COVID-19 on neurosurgical OR efficiency, institutional perioperative databases at a single high-volume center were queried for operations performed from December 2019 until October 2021. March 12, 2020, was chosen as the onset of COVID-19 for analytic purposes, as this was the date when the state of Tennessee declared a state of emergency. The 90-day periods before and after this date were used for comparative analysis for pre-COVID-19, peak COVID-19, and post-peak-restrictions time periods. The peak COVID-19 period was defined as the 90-day period following the initial onset of COVID-19 and the surge of cases. For comparison purposes, post-peak COVID-19 was defined as the months following the first peak until October 2021 (approximately 17 months). COVID-19 burden was determined using a COVID-19 single-institution census of confirmed cases by polymerase chain reaction (PCR) for which the average number of cases of COVID-19 during a given month was determined. This number is a scaled trend, and a true number of COVID-19 cases in our hospital was not reported.
Neurosurgical and neuroendovascular cases were included in the analysis. Outcomes included delay in first-start and OR turnover time between neurosurgical cases, defined as the time from the patient leaving the room until the next patient entered the room. Preset threshold times were used in analyses to adjust for normal leniency in OR scheduling (15 minutes for first start and 90 minutes for turnover, which is a standard for our single-institution perioperative center). Statistical analyses, including data aggregation, were performed using R, version 4.0.1 (R Foundation for Statistical Computing). Patients’ demographic and clinical characteristics were analyzed using an independent 2-sample t-test for interval variables and a chi-square test for categorical variables. Significance was defined as P < .05.
Results
First-Start Time
First-start time was analyzed in 426 pre-COVID-19, 357 peak-COVID-19, and 2304 post-peak-COVID-19 cases. The unadjusted mean delay length was significantly different between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes, 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004) (Table 1). 
The adjusted average delay length and proportion of cases delayed beyond the 15-minute threshold were not significantly different, but they have been slightly higher since the onset of COVID-19. The proportion of cases that have started early, as well as significantly early past a 15-minute threshold, have also trended down since the onset of the COVID-19 pandemic, but this difference was again not significant. The temporal relationship of first-start delay, both unadjusted and adjusted, from December 2019 to October 2021 is shown in Figure 1. The trend of increasing delay is loosely associated with the COVID-19 burden experienced by our hospital. The start of COVID-19 as well as both COVID-19 peaks have been associated with increased delays in our hospital.
Turnover Time
Turnover time was assessed in 437 pre-COVID-19, 278 peak-restrictions, and 2411 post-peak-restrictions cases. Turnover time during peak restrictions was not significantly different from pre-COVID-19 (88 [100] vs 85 [95]) and has since remained relatively unchanged (83 [87], P = .78). A similar trend held for comparisons of proportion of cases with turnover time past 90 minutes and average times past the 90-minute threshold (Table 2). The temporal relationship between COVID-19 burden and turnover time, both unadjusted and adjusted, from December 2019 to October 2021 is shown in Figure 2. Both figures demonstrate a slight initial increase in turnover time delay at the start of COVID-19, which stabilized with little variation thereafter.


Discussion
We analyzed the OR efficiency metrics of first-start and turnover time during the 90-day period before COVID-19 (pre-COVID-19), the 90 days following Tennessee declaring a state of emergency (peak COVID-19), and the time following this period (post-COVID-19) for all neurosurgical and neuroendovascular cases at Vanderbilt University Medical Center (VUMC). We found a significant difference in unadjusted mean delay length in first-start time between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes for pre-COVID-19, peak-COVID-19, and post-COVID-19: 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004). No significant increase in turnover time between cases was found between these 3 time periods. Based on metrics from first-start delay and turnover time, our center was able to maintain OR efficiency before, during, and after peak COVID-19.
After the Centers for Disease Control and Prevention released guidelines recommending deferring elective procedures to conserve beds and PPE, VUMC made the decision to suspend all elective surgical procedures from March 18 to April 24, 2020. Prior research conducted during the COVID-19 pandemic has demonstrated more than 400 types of surgical procedures with negatively impacted outcomes when compared to surgical outcomes from the same time frame in 2018 and 2019.4 For more than 20 of these types of procedures, there was a significant association between procedure delay and adverse patient outcomes.4 Testing protocols for patients prior to surgery varied throughout the pandemic based on vaccination status and type of procedure. Before vaccines became widely available, all patients were required to obtain a PCR SARS-CoV-2 test within 48 to 72 hours of the scheduled procedure. If the patient’s procedure was urgent and testing was not feasible, the patient was treated as a SARS-CoV-2–positive patient, which required all health care workers involved in the case to wear gowns, gloves, surgical masks, and eye protection. Testing patients preoperatively likely helped to maintain OR efficiency since not all patients received test results prior to the scheduled procedure, leading to cancellations of cases and therefore more staff available for fewer cases.
After vaccines became widely available to the public, testing requirements for patients preoperatively were relaxed, and only patients who were not fully vaccinated or severely immunocompromised were required to test prior to procedures. However, approximately 40% of the population in Tennessee was fully vaccinated in 2021, which reflects the patient population of VUMC.5 This means that many patients who received care at VUMC were still tested prior to procedures.
Adopting adequate safety protocols was found to be key for OR efficiency during the COVID-19 pandemic since performing surgery increased the risk of infection for each health care worker in the OR.6 VUMC protocols identified procedures that required enhanced safety measures to prevent infection of health care workers and avoid staffing shortages, which would decrease OR efficiency. Protocols mandated that only anesthesia team members were allowed to be in the OR during intubation and extubation of patients, which could be one factor leading to increased delays and decreased efficiency for some institutions. Methods for neurosurgeons to decrease risk of infection in the OR include postponing all nonurgent cases, reappraising the necessity for general anesthesia and endotracheal intubation, considering alternative surgical approaches that avoid the respiratory tract, and limiting the use of aerosol-generating instruments.7,8 VUMC’s success in implementing these protocols likely explains why our center was able to maintain OR efficiency throughout the COVID-19 pandemic.
A study conducted by Andreata et al showed a significantly increased mean first-case delay and a nonsignificant increased turnover time in orthopedic surgeries in Northern Italy when comparing surgeries performed during the COVID-19 pandemic to those performed prior to COVID-19.2 Other studies have indicated a similar trend in decreased OR efficiency during COVID-19 in other areas around the world.9,10 These findings are not consistent with our own findings for neurosurgical and neuroendovascular surgeries at VUMC, and any change at our institution was relatively immaterial. Factors that threatened to change OR efficiency—but did not result in meaningful changes in our institutional experience—include delays due to pending COVID-19 test results, safety procedures such as PPE donning, and planning difficulties to ensure the existence of teams with non-overlapping providers in the case of a surgeon being infected.2,11-13
Globally, many surgery centers halted all elective surgeries during the initial COVID-19 spike to prevent a PPE shortage and mitigate risk of infection of patients and health care workers.8,12,14 However, there is no centralized definition of which neurosurgical procedures are elective, so that decision was made on a surgeon or center level, which could lead to variability in efficiency trends.14 One study on neurosurgical procedures during COVID-19 found a 30% decline in all cases and a 23% decline in emergent procedures, showing that the decrease in volume was not only due to cancellation of elective procedures.15 This decrease in elective and emergent surgeries created a backlog of surgeries as well as a loss in health care revenue, and caused many patients to go without adequate health care.10 Looking forward, it is imperative that surgical centers study trends in OR efficiency from COVID-19 and learn how to better maintain OR efficiency during future pandemic conditions to prevent a backlog of cases, loss of health care revenue, and decreased health care access.
Limitations
Our data are from a single center and therefore may not be representative of experiences of other hospitals due to different populations and different impacts from COVID-19. However, given our center’s high volume and diverse patient population, we believe our analysis highlights important trends in neurosurgery practice. Notably, data for patient and OR timing are digitally generated and are entered manually by nurses in the electronic medical record, making it prone to errors and variability. This is in our experience, and if any error is present, we believe it is minimal.
Conclusion
The COVID-19 pandemic has had far-reaching effects on health care worldwide, including neurosurgical care. OR efficiency across the United States generally worsened given the stresses of supply chain issues, staffing shortages, and cancellations. At our institution, we were able to maintain OR efficiency during the known COVID-19 peaks until October 2021. Continually functional neurosurgical ORs are important in preventing delays in care and maintaining a steady revenue in order for hospitals and other health care entities to remain solvent. Further study of OR efficiency is needed for health care systems to prepare for future pandemics and other resource-straining events in order to provide optimal patient care.
Corresponding author: Campbell Liles, MD, Vanderbilt University Medical Center, Department of Neurological Surgery, 1161 21st Ave. South, T4224 Medical Center North, Nashville, TN 37232-2380; [email protected]
Disclosures: None reported.
From the Department of Neurological Surgery, Vanderbilt University Medical Center, Nashville, TN (Stefan W. Koester, Puja Jagasia, and Drs. Liles, Dambrino IV, Feldman, and Chambless), and the Department of Anesthesiology, Vanderbilt University Medical Center, Nashville, TN (Drs. Mathews and Tiwari).
ABSTRACT
Background: The COVID-19 pandemic has had broad effects on surgical care, including operating room (OR) staffing, personal protective equipment (PPE) utilization, and newly implemented anti-infective measures. Our aim was to assess neurosurgery OR efficiency before the COVID-19 pandemic, during peak COVID-19, and during current times.
Methods: Institutional perioperative databases at a single, high-volume neurosurgical center were queried for operations performed from December 2019 until October 2021. March 12, 2020, the day that the state of Tennessee declared a state of emergency, was chosen as the onset of the COVID-19 pandemic. The 90-day periods before and after this day were used to define the pre-COVID-19, peak-COVID-19, and post-peak restrictions time periods for comparative analysis. Outcomes included delay in first-start and OR turnover time between neurosurgical cases. Preset threshold times were used in analyses to adjust for normal leniency in OR scheduling (15 minutes for first start and 90 minutes for turnover). Univariate analysis used Wilcoxon rank-sum test for continuous outcomes, while chi-square test and Fisher’s exact test were used for categorical comparisons. Significance was defined as P < .05.
Results: First-start time was analyzed in 426 pre-COVID-19, 357 peak-restrictions, and 2304 post-peak-restrictions cases. The unadjusted mean delay length was found to be significantly different between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes, 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004). The adjusted average delay length and proportion of cases delayed beyond the 15-minute threshold were not significantly different. The proportion of cases that started early, as well as significantly early past a 15-minute threshold, have not been impacted. There was no significant change in turnover time during peak restrictions relative to the pre-COVID-19 period (88 [100] minutes vs 85 [95] minutes), and turnover time has since remained unchanged (83 [87] minutes).
Conclusion: Our center was able to maintain OR efficiency before, during, and after peak restrictions even while instituting advanced infection-control strategies. While there were significant changes, delays were relatively small in magnitude.
Keywords: operating room timing, hospital efficiency, socioeconomics, pandemic.
The COVID-19 pandemic has led to major changes in patient care both from a surgical perspective and in regard to inpatient hospital course. Safety protocols nationwide have been implemented to protect both patients and providers. Some elements of surgical care have drastically changed, including operating room (OR) staffing, personal protective equipment (PPE) utilization, and increased sterilization measures. Furloughs, layoffs, and reassignments due to the focus on nonelective and COVID-19–related cases challenged OR staffing and efficiency. Operating room staff with COVID-19 exposures or COVID-19 infections also caused last-minute changes in staffing. All of these scenarios can cause issues due to actual understaffing or due to staff members being pushed into highly specialized areas, such as neurosurgery, in which they have very little experience. A further obstacle to OR efficiency included policy changes involving PPE utilization, sterilization measures, and supply chain shortages of necessary resources such as PPE.
Neurosurgery in particular has been susceptible to COVID-19–related system-wide changes given operator proximity to the patient’s respiratory passages, frequency of emergent cases, and varying anesthetic needs, as well as the high level of specialization needed to perform neurosurgical care. Previous studies have shown a change in the makeup of neurosurgical patients seeking care, as well as in the acuity of neurological consult of these patients.1 A study in orthopedic surgery by Andreata et al demonstrated worsened OR efficiency, with significantly increased first-start and turnover times.2 In the COVID-19 era, OR quality and safety are crucially important to both patients and providers. Providing this safe and effective care in an efficient manner is important for optimal neurosurgical management in the long term.3 Moreover, the financial burden of implementing new protocols and standards can be compounded by additional financial losses due to reduced OR efficiency.
Methods
To analyze the effect of COVID-19 on neurosurgical OR efficiency, institutional perioperative databases at a single high-volume center were queried for operations performed from December 2019 until October 2021. March 12, 2020, was chosen as the onset of COVID-19 for analytic purposes, as this was the date when the state of Tennessee declared a state of emergency. The 90-day periods before and after this date were used for comparative analysis for pre-COVID-19, peak COVID-19, and post-peak-restrictions time periods. The peak COVID-19 period was defined as the 90-day period following the initial onset of COVID-19 and the surge of cases. For comparison purposes, post-peak COVID-19 was defined as the months following the first peak until October 2021 (approximately 17 months). COVID-19 burden was determined using a COVID-19 single-institution census of confirmed cases by polymerase chain reaction (PCR) for which the average number of cases of COVID-19 during a given month was determined. This number is a scaled trend, and a true number of COVID-19 cases in our hospital was not reported.
Neurosurgical and neuroendovascular cases were included in the analysis. Outcomes included delay in first-start and OR turnover time between neurosurgical cases, defined as the time from the patient leaving the room until the next patient entered the room. Preset threshold times were used in analyses to adjust for normal leniency in OR scheduling (15 minutes for first start and 90 minutes for turnover, which is a standard for our single-institution perioperative center). Statistical analyses, including data aggregation, were performed using R, version 4.0.1 (R Foundation for Statistical Computing). Patients’ demographic and clinical characteristics were analyzed using an independent 2-sample t-test for interval variables and a chi-square test for categorical variables. Significance was defined as P < .05.
Results
First-Start Time
First-start time was analyzed in 426 pre-COVID-19, 357 peak-COVID-19, and 2304 post-peak-COVID-19 cases. The unadjusted mean delay length was significantly different between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes, 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004) (Table 1). 
The adjusted average delay length and proportion of cases delayed beyond the 15-minute threshold were not significantly different, but they have been slightly higher since the onset of COVID-19. The proportion of cases that have started early, as well as significantly early past a 15-minute threshold, have also trended down since the onset of the COVID-19 pandemic, but this difference was again not significant. The temporal relationship of first-start delay, both unadjusted and adjusted, from December 2019 to October 2021 is shown in Figure 1. The trend of increasing delay is loosely associated with the COVID-19 burden experienced by our hospital. The start of COVID-19 as well as both COVID-19 peaks have been associated with increased delays in our hospital.
Turnover Time
Turnover time was assessed in 437 pre-COVID-19, 278 peak-restrictions, and 2411 post-peak-restrictions cases. Turnover time during peak restrictions was not significantly different from pre-COVID-19 (88 [100] vs 85 [95]) and has since remained relatively unchanged (83 [87], P = .78). A similar trend held for comparisons of proportion of cases with turnover time past 90 minutes and average times past the 90-minute threshold (Table 2). The temporal relationship between COVID-19 burden and turnover time, both unadjusted and adjusted, from December 2019 to October 2021 is shown in Figure 2. Both figures demonstrate a slight initial increase in turnover time delay at the start of COVID-19, which stabilized with little variation thereafter.


Discussion
We analyzed the OR efficiency metrics of first-start and turnover time during the 90-day period before COVID-19 (pre-COVID-19), the 90 days following Tennessee declaring a state of emergency (peak COVID-19), and the time following this period (post-COVID-19) for all neurosurgical and neuroendovascular cases at Vanderbilt University Medical Center (VUMC). We found a significant difference in unadjusted mean delay length in first-start time between the time periods, but the magnitude of increase in minutes was immaterial (mean [SD] minutes for pre-COVID-19, peak-COVID-19, and post-COVID-19: 6 [18] vs 10 [21] vs 8 [20], respectively; P = .004). No significant increase in turnover time between cases was found between these 3 time periods. Based on metrics from first-start delay and turnover time, our center was able to maintain OR efficiency before, during, and after peak COVID-19.
After the Centers for Disease Control and Prevention released guidelines recommending deferring elective procedures to conserve beds and PPE, VUMC made the decision to suspend all elective surgical procedures from March 18 to April 24, 2020. Prior research conducted during the COVID-19 pandemic has demonstrated more than 400 types of surgical procedures with negatively impacted outcomes when compared to surgical outcomes from the same time frame in 2018 and 2019.4 For more than 20 of these types of procedures, there was a significant association between procedure delay and adverse patient outcomes.4 Testing protocols for patients prior to surgery varied throughout the pandemic based on vaccination status and type of procedure. Before vaccines became widely available, all patients were required to obtain a PCR SARS-CoV-2 test within 48 to 72 hours of the scheduled procedure. If the patient’s procedure was urgent and testing was not feasible, the patient was treated as a SARS-CoV-2–positive patient, which required all health care workers involved in the case to wear gowns, gloves, surgical masks, and eye protection. Testing patients preoperatively likely helped to maintain OR efficiency since not all patients received test results prior to the scheduled procedure, leading to cancellations of cases and therefore more staff available for fewer cases.
After vaccines became widely available to the public, testing requirements for patients preoperatively were relaxed, and only patients who were not fully vaccinated or severely immunocompromised were required to test prior to procedures. However, approximately 40% of the population in Tennessee was fully vaccinated in 2021, which reflects the patient population of VUMC.5 This means that many patients who received care at VUMC were still tested prior to procedures.
Adopting adequate safety protocols was found to be key for OR efficiency during the COVID-19 pandemic since performing surgery increased the risk of infection for each health care worker in the OR.6 VUMC protocols identified procedures that required enhanced safety measures to prevent infection of health care workers and avoid staffing shortages, which would decrease OR efficiency. Protocols mandated that only anesthesia team members were allowed to be in the OR during intubation and extubation of patients, which could be one factor leading to increased delays and decreased efficiency for some institutions. Methods for neurosurgeons to decrease risk of infection in the OR include postponing all nonurgent cases, reappraising the necessity for general anesthesia and endotracheal intubation, considering alternative surgical approaches that avoid the respiratory tract, and limiting the use of aerosol-generating instruments.7,8 VUMC’s success in implementing these protocols likely explains why our center was able to maintain OR efficiency throughout the COVID-19 pandemic.
A study conducted by Andreata et al showed a significantly increased mean first-case delay and a nonsignificant increased turnover time in orthopedic surgeries in Northern Italy when comparing surgeries performed during the COVID-19 pandemic to those performed prior to COVID-19.2 Other studies have indicated a similar trend in decreased OR efficiency during COVID-19 in other areas around the world.9,10 These findings are not consistent with our own findings for neurosurgical and neuroendovascular surgeries at VUMC, and any change at our institution was relatively immaterial. Factors that threatened to change OR efficiency—but did not result in meaningful changes in our institutional experience—include delays due to pending COVID-19 test results, safety procedures such as PPE donning, and planning difficulties to ensure the existence of teams with non-overlapping providers in the case of a surgeon being infected.2,11-13
Globally, many surgery centers halted all elective surgeries during the initial COVID-19 spike to prevent a PPE shortage and mitigate risk of infection of patients and health care workers.8,12,14 However, there is no centralized definition of which neurosurgical procedures are elective, so that decision was made on a surgeon or center level, which could lead to variability in efficiency trends.14 One study on neurosurgical procedures during COVID-19 found a 30% decline in all cases and a 23% decline in emergent procedures, showing that the decrease in volume was not only due to cancellation of elective procedures.15 This decrease in elective and emergent surgeries created a backlog of surgeries as well as a loss in health care revenue, and caused many patients to go without adequate health care.10 Looking forward, it is imperative that surgical centers study trends in OR efficiency from COVID-19 and learn how to better maintain OR efficiency during future pandemic conditions to prevent a backlog of cases, loss of health care revenue, and decreased health care access.
Limitations
Our data are from a single center and therefore may not be representative of experiences of other hospitals due to different populations and different impacts from COVID-19. However, given our center’s high volume and diverse patient population, we believe our analysis highlights important trends in neurosurgery practice. Notably, data for patient and OR timing are digitally generated and are entered manually by nurses in the electronic medical record, making it prone to errors and variability. This is in our experience, and if any error is present, we believe it is minimal.
Conclusion
The COVID-19 pandemic has had far-reaching effects on health care worldwide, including neurosurgical care. OR efficiency across the United States generally worsened given the stresses of supply chain issues, staffing shortages, and cancellations. At our institution, we were able to maintain OR efficiency during the known COVID-19 peaks until October 2021. Continually functional neurosurgical ORs are important in preventing delays in care and maintaining a steady revenue in order for hospitals and other health care entities to remain solvent. Further study of OR efficiency is needed for health care systems to prepare for future pandemics and other resource-straining events in order to provide optimal patient care.
Corresponding author: Campbell Liles, MD, Vanderbilt University Medical Center, Department of Neurological Surgery, 1161 21st Ave. South, T4224 Medical Center North, Nashville, TN 37232-2380; [email protected]
Disclosures: None reported.
1. Koester SW, Catapano JS, Ma KL, et al. COVID-19 and neurosurgery consultation call volume at a single large tertiary center with a propensity- adjusted analysis. World Neurosurg. 2021;146:e768-e772. doi:10.1016/j.wneu.2020.11.017
2. Andreata M, Faraldi M, Bucci E, Lombardi G, Zagra L. Operating room efficiency and timing during coronavirus disease 2019 outbreak in a referral orthopaedic hospital in Northern Italy. Int Orthop. 2020;44(12):2499-2504. doi:10.1007/s00264-020-04772-x
3. Dexter F, Abouleish AE, Epstein RH, et al. Use of operating room information system data to predict the impact of reducing turnover times on staffing costs. Anesth Analg. 2003;97(4):1119-1126. doi:10.1213/01.ANE.0000082520.68800.79
4. Zheng NS, Warner JL, Osterman TJ, et al. A retrospective approach to evaluating potential adverse outcomes associated with delay of procedures for cardiovascular and cancer-related diagnoses in the context of COVID-19. J Biomed Inform. 2021;113:103657. doi:10.1016/j.jbi.2020.103657
5. Alcendor DJ. Targeting COVID-19 vaccine hesitancy in rural communities in Tennessee: implications for extending the COVID- 19 pandemic in the South. Vaccines (Basel). 2021;9(11):1279. doi:10.3390/vaccines9111279
6. Perrone G, Giuffrida M, Bellini V, et al. Operating room setup: how to improve health care professionals safety during pandemic COVID- 19: a quality improvement study. J Laparoendosc Adv Surg Tech A. 2021;31(1):85-89. doi:10.1089/lap.2020.0592
7. Iorio-Morin C, Hodaie M, Sarica C, et al. Letter: the risk of COVID-19 infection during neurosurgical procedures: a review of severe acute respiratory distress syndrome coronavirus 2 (SARS-CoV-2) modes of transmission and proposed neurosurgery-specific measures for mitigation. Neurosurgery. 2020;87(2):E178-E185. doi:10.1093/ neuros/nyaa157
8. Gupta P, Muthukumar N, Rajshekhar V, et al. Neurosurgery and neurology practices during the novel COVID-19 pandemic: a consensus statement from India. Neurol India. 2020;68(2):246-254. doi:10.4103/0028-3886.283130
9. Mercer ST, Agarwal R, Dayananda KSS, et al. A comparative study looking at trauma and orthopaedic operating efficiency in the COVID-19 era. Perioper Care Oper Room Manag. 2020;21:100142. doi:10.1016/j.pcorm.2020.100142
10. Rozario N, Rozario D. Can machine learning optimize the efficiency of the operating room in the era of COVID-19? Can J Surg. 2020;63(6):E527-E529. doi:10.1503/cjs.016520
11. Toh KHQ, Barazanchi A, Rajaretnam NS, et al. COVID-19 response by New Zealand general surgical departments in tertiary metropolitan hospitals. ANZ J Surg. 2021;91(7-8):1352-1357. doi:10.1111/ ans.17044
12. Moorthy RK, Rajshekhar V. Impact of COVID-19 pandemic on neurosurgical practice in India: a survey on personal protective equipment usage, testing, and perceptions on disease transmission. Neurol India. 2020;68(5):1133-1138. doi:10.4103/0028- 3886.299173
13. Meneghini RM. Techniques and strategies to optimize efficiencies in the office and operating room: getting through the patient backlog and preserving hospital resources. J Arthroplasty. 2021;36(7S):S49-S51. doi:10.1016/j.arth.2021.03.010
14. Jean WC, Ironside NT, Sack KD, et al. The impact of COVID- 19 on neurosurgeons and the strategy for triaging non-emergent operations: a global neurosurgery study. Acta Neurochir (Wien). 2020;162(6):1229-1240. doi:10.1007/s00701-020- 04342-5
15. Raneri F, Rustemi O, Zambon G, et al. Neurosurgery in times of a pandemic: a survey of neurosurgical services during the COVID-19 outbreak in the Veneto region in Italy. Neurosurg Focus. 2020;49(6):E9. doi:10.3171/2020.9.FOCUS20691
1. Koester SW, Catapano JS, Ma KL, et al. COVID-19 and neurosurgery consultation call volume at a single large tertiary center with a propensity- adjusted analysis. World Neurosurg. 2021;146:e768-e772. doi:10.1016/j.wneu.2020.11.017
2. Andreata M, Faraldi M, Bucci E, Lombardi G, Zagra L. Operating room efficiency and timing during coronavirus disease 2019 outbreak in a referral orthopaedic hospital in Northern Italy. Int Orthop. 2020;44(12):2499-2504. doi:10.1007/s00264-020-04772-x
3. Dexter F, Abouleish AE, Epstein RH, et al. Use of operating room information system data to predict the impact of reducing turnover times on staffing costs. Anesth Analg. 2003;97(4):1119-1126. doi:10.1213/01.ANE.0000082520.68800.79
4. Zheng NS, Warner JL, Osterman TJ, et al. A retrospective approach to evaluating potential adverse outcomes associated with delay of procedures for cardiovascular and cancer-related diagnoses in the context of COVID-19. J Biomed Inform. 2021;113:103657. doi:10.1016/j.jbi.2020.103657
5. Alcendor DJ. Targeting COVID-19 vaccine hesitancy in rural communities in Tennessee: implications for extending the COVID- 19 pandemic in the South. Vaccines (Basel). 2021;9(11):1279. doi:10.3390/vaccines9111279
6. Perrone G, Giuffrida M, Bellini V, et al. Operating room setup: how to improve health care professionals safety during pandemic COVID- 19: a quality improvement study. J Laparoendosc Adv Surg Tech A. 2021;31(1):85-89. doi:10.1089/lap.2020.0592
7. Iorio-Morin C, Hodaie M, Sarica C, et al. Letter: the risk of COVID-19 infection during neurosurgical procedures: a review of severe acute respiratory distress syndrome coronavirus 2 (SARS-CoV-2) modes of transmission and proposed neurosurgery-specific measures for mitigation. Neurosurgery. 2020;87(2):E178-E185. doi:10.1093/ neuros/nyaa157
8. Gupta P, Muthukumar N, Rajshekhar V, et al. Neurosurgery and neurology practices during the novel COVID-19 pandemic: a consensus statement from India. Neurol India. 2020;68(2):246-254. doi:10.4103/0028-3886.283130
9. Mercer ST, Agarwal R, Dayananda KSS, et al. A comparative study looking at trauma and orthopaedic operating efficiency in the COVID-19 era. Perioper Care Oper Room Manag. 2020;21:100142. doi:10.1016/j.pcorm.2020.100142
10. Rozario N, Rozario D. Can machine learning optimize the efficiency of the operating room in the era of COVID-19? Can J Surg. 2020;63(6):E527-E529. doi:10.1503/cjs.016520
11. Toh KHQ, Barazanchi A, Rajaretnam NS, et al. COVID-19 response by New Zealand general surgical departments in tertiary metropolitan hospitals. ANZ J Surg. 2021;91(7-8):1352-1357. doi:10.1111/ ans.17044
12. Moorthy RK, Rajshekhar V. Impact of COVID-19 pandemic on neurosurgical practice in India: a survey on personal protective equipment usage, testing, and perceptions on disease transmission. Neurol India. 2020;68(5):1133-1138. doi:10.4103/0028- 3886.299173
13. Meneghini RM. Techniques and strategies to optimize efficiencies in the office and operating room: getting through the patient backlog and preserving hospital resources. J Arthroplasty. 2021;36(7S):S49-S51. doi:10.1016/j.arth.2021.03.010
14. Jean WC, Ironside NT, Sack KD, et al. The impact of COVID- 19 on neurosurgeons and the strategy for triaging non-emergent operations: a global neurosurgery study. Acta Neurochir (Wien). 2020;162(6):1229-1240. doi:10.1007/s00701-020- 04342-5
15. Raneri F, Rustemi O, Zambon G, et al. Neurosurgery in times of a pandemic: a survey of neurosurgical services during the COVID-19 outbreak in the Veneto region in Italy. Neurosurg Focus. 2020;49(6):E9. doi:10.3171/2020.9.FOCUS20691