User login
Anesthetic Choices and Postoperative Delirium Incidence: Propofol vs Sevoflurane
Study 1 Overview (Chang et al)
Objective: To assess the incidence of postoperative delirium (POD) following propofol- vs sevoflurane-based anesthesia in geriatric spine surgery patients.
Design: Retrospective, single-blinded observational study of propofol- and sevoflurane-based anesthesia cohorts.
Setting and participants: Patients eligible for this study were aged 65 years or older admitted to the SMG-SNU Boramae Medical Center (Seoul, South Korea). All patients underwent general anesthesia either via intravenous propofol or inhalational sevoflurane for spine surgery between January 2015 and December 2019. Patients were retrospectively identified via electronic medical records. Patient exclusion criteria included preoperative delirium, history of dementia, psychiatric disease, alcoholism, hepatic or renal dysfunction, postoperative mechanical ventilation dependence, other surgery within the recent 6 months, maintenance of intraoperative anesthesia with combined anesthetics, or incomplete medical record.
Main outcome measures: The primary outcome was the incidence of POD after administration of propofol- and sevoflurane-based anesthesia during hospitalization. Patients were screened for POD regularly by attending nurses using the Nursing Delirium Screening Scale (disorientation, inappropriate behavior, inappropriate communication, hallucination, and psychomotor retardation) during the entirety of the patient’s hospital stay; if 1 or more screening criteria were met, a psychiatrist was consulted for the proper diagnosis and management of delirium. A psychiatric diagnosis was required for a case to be counted toward the incidence of POD in this study. Secondary outcomes included postoperative 30-day complications (angina, myocardial infarction, transient ischemic attack/stroke, pneumonia, deep vein thrombosis, pulmonary embolism, acute kidney injury, or infection) and length of postoperative hospital stay.
Main results: POD occurred in 29 patients (10.3%) out of the total cohort of 281. POD was more common in the sevoflurane group than in the propofol group (15.7% vs 5.0%; P = .003). Using multivariable logistic regression, inhalational sevoflurane was associated with an increased risk of POD as compared to propofol-based anesthesia (odds ratio [OR], 4.120; 95% CI, 1.549-10.954; P = .005). There was no association between choice of anesthetic and postoperative 30-day complications or the length of postoperative hospital stay. Both older age (OR, 1.242; 95% CI, 1.130-1.366; P < .001) and higher pain score at postoperative day 1 (OR, 1.338; 95% CI, 1.056-1.696; P = .016) were associated with increased risk of POD.
Conclusion: Propofol-based anesthesia was associated with a lower incidence of and risk for POD than sevoflurane-based anesthesia in older patients undergoing spine surgery.
Study 2 Overview (Mei et al)
Objective: To determine the incidence and duration of POD in older patients after total knee/hip replacement (TKR/THR) under intravenous propofol or inhalational sevoflurane general anesthesia.
Design: Randomized clinical trial of propofol and sevoflurane groups.
Setting and participants: This study was conducted at the Shanghai Tenth People’s Hospital and involved 209 participants enrolled between June 2016 and November 2019. All participants were 60 years of age or older, scheduled for TKR/THR surgery under general anesthesia, American Society of Anesthesiologists (ASA) class I to III, and assessed to be of normal cognitive function preoperatively via a Mini-Mental State Examination. Participant exclusion criteria included preexisting delirium as assessed by the Confusion Assessment Method (CAM), prior diagnosed neurological diseases (eg, Parkinson’s disease), prior diagnosed mental disorders (eg, schizophrenia), or impaired vision or hearing that would influence cognitive assessments. All participants were randomly assigned to either sevoflurane or propofol anesthesia for their surgery via a computer-generated list. Of these, 103 received inhalational sevoflurane and 106 received intravenous propofol. All participants received standardized postoperative care.
Main outcome measures: All participants were interviewed by investigators, who were blinded to the anesthesia regimen, twice daily on postoperative days 1, 2, and 3 using CAM and a CAM-based scoring system (CAM-S) to assess delirium severity. The CAM encapsulated 4 criteria: acute onset and fluctuating course, agitation, disorganized thinking, and altered level of consciousness. To diagnose delirium, both the first and second criteria must be met, in addition to either the third or fourth criterion. The averages of the scores across the 3 postoperative days indicated delirium severity, while the incidence and duration of delirium was assessed by the presence of delirium as determined by CAM on any postoperative day.
Main results: All eligible participants (N = 209; mean [SD] age 71.2 [6.7] years; 29.2% male) were included in the final analysis. The incidence of POD was not statistically different between the propofol and sevoflurane groups (33.0% vs 23.3%; P = .119, Chi-square test). It was estimated that 316 participants in each arm of the study were needed to detect statistical differences. The number of days of POD per person were higher with propofol anesthesia as compared to sevoflurane (0.5 [0.8] vs 0.3 [0.5]; P = .049, Student’s t-test).
Conclusion: This underpowered study showed a 9.7% difference in the incidence of POD between older adults who received propofol (33.0%) and sevoflurane (23.3%) after THR/TKR. Further studies with a larger sample size are needed to compare general anesthetics and their role in POD.
Commentary
Delirium is characterized by an acute state of confusion with fluctuating mental status, inattention, disorganized thinking, and altered level of consciousness. It is often caused by medications and/or their related adverse effects, infections, electrolyte imbalances, and other clinical etiologies. Delirium often manifests in post-surgical settings, disproportionately affecting older patients and leading to increased risk of morbidity, mortality, hospital length of stay, and health care costs.1 Intraoperative risk factors for POD are determined by the degree of operative stress (eg, lower-risk surgeries put the patient at reduced risk for POD as compared to higher-risk surgeries) and are additive to preexisting patient-specific risk factors, such as older age and functional impairment.1 Because operative stress is associated with risk for POD, limiting operative stress in controlled ways, such as through the choice of anesthetic agent administered, may be a pragmatic way to manage operative risks and optimize outcomes, especially when serving a surgically vulnerable population.
In Study 1, Chang et al sought to assess whether 2 commonly utilized general anesthetics, propofol and sevoflurane, in older patients undergoing spine surgery differentially affected the incidence of POD. In this retrospective, single-blinded observational study of 281 geriatric patients, the researchers found that sevoflurane was associated with a higher risk of POD as compared to propofol. However, these anesthetics were not associated with surgical outcomes such as postoperative 30-day complications or the length of postoperative hospital stay. While these findings added new knowledge to this field of research, several limitations should be kept in mind when interpreting this study’s results. For instance, the sample size was relatively small, with all cases selected from a single center utilizing a retrospective analysis. In addition, although a standardized nursing screening tool was used as a method for delirium detection, hypoactive delirium or less symptomatic delirium may have been missed, which in turn would lead to an underestimation of POD incidence. The latter is a common limitation in delirium research.
In Study 2, Mei et al similarly explored the effects of general anesthetics on POD in older surgical patients. Specifically, using a randomized clinical trial design, the investigators compared propofol with sevoflurane in older patients who underwent TKR/THR, and their roles in POD severity and duration. Although the incidence of POD was higher in those who received propofol compared to sevoflurane, this trial was underpowered and the results did not reach statistical significance. In addition, while the duration of POD was slightly longer in the propofol group compared to the sevoflurane group (0.5 vs 0.3 days), it was unclear if this finding was clinically significant. Similar to many research studies in POD, limitations of Study 2 included a small sample size of 209 patients, with all participants enrolled from a single center. On the other hand, this study illustrated the feasibility of a method that allowed reproducible prospective assessment of POD time course using CAM and CAM-S.
Applications for Clinical Practice and System Implementation
The delineation of risk factors that contribute to delirium after surgery in older patients is key to mitigating risks for POD and improving clinical outcomes. An important step towards a better understanding of these modifiable risk factors is to clearly quantify intraoperative risk of POD attributable to specific anesthetics. While preclinical studies have shown differential neurotoxicity effects of propofol and sevoflurane, their impact on clinically important neurologic outcomes such as delirium and cognitive decline remains poorly understood. Although Studies 1 and 2 both provided head-to-head comparisons of propofol and sevoflurane as risk factors for POD in high-operative-stress surgeries in older patients, the results were inconsistent. That being said, this small incremental increase in knowledge was not unexpected in the course of discovery around a clinically complex research question. Importantly, these studies provided evidence regarding the methodological approaches that could be taken to further this line of research.
The mediating factors of the differences on neurologic outcomes between anesthetic agents are likely pharmacological, biological, and methodological. Pharmacologically, the differences between target receptors, such as GABAA (propofol, etomidate) or NMDA (ketamine), could be a defining feature in the difference in incidence of POD. Additionally, secondary actions of anesthetic agents on glycine, nicotinic, and acetylcholine receptors could play a role as well. Biologically, genes such as CYP2E1, CYP2B6, CYP2C9, GSTP1, UGT1A9, SULT1A1, and NQO1 have all been identified as genetic factors in the metabolism of anesthetics, and variations in such genes could result in different responses to anesthetics.2 Methodologically, routes of anesthetic administration (eg, inhalation vs intravenous), preexisting anatomical structures, or confounding medical conditions (eg, lower respiratory volume due to older age) may influence POD incidence, duration, or severity. Moreover, methodological differences between Studies 1 and 2, such as surgeries performed (spinal vs TKR/THR), patient populations (South Korean vs Chinese), and the diagnosis and monitoring of delirium (retrospective screening and diagnosis vs prospective CAM/CAM-S) may impact delirium outcomes. Thus, these factors should be considered in the design of future clinical trials undertaken to investigate the effects of anesthetics on POD.
Given the high prevalence of delirium and its associated adverse outcomes in the immediate postoperative period in older patients, further research is warranted to determine how anesthetics affect POD in order to optimize perioperative care and mitigate risks in this vulnerable population. Moreover, parallel investigations into how anesthetics differentially impact the development of transient or longer-term cognitive impairment after a surgical procedure (ie, postoperative cognitive dysfunction) in older adults are urgently needed in order to improve their cognitive health.
Practice Points
- Intravenous propofol and inhalational sevoflurane may be differentially associated with incidence, duration, and severity of POD in geriatric surgical patients.
- Further larger-scale studies are warranted to clarify the role of anesthetic choice in POD in order to optimize surgical outcomes in older patients.
–Jared Doan, BS, and Fred Ko, MD
Icahn School of Medicine at Mount Sinai
1. Dasgupta M, Dumbrell AC. Preoperative risk assessment for delirium after noncardiac surgery: a systematic review. J Am Geriatr Soc. 2006;54(10):1578-1589. doi:10.1111/j.1532-5415.2006.00893.x
2. Mikstacki A, Skrzypczak-Zielinska M, Tamowicz B, et al. The impact of genetic factors on response to anaesthetics. Adv Med Sci. 2013;58(1):9-14. doi:10.2478/v10039-012-0065-z
Study 1 Overview (Chang et al)
Objective: To assess the incidence of postoperative delirium (POD) following propofol- vs sevoflurane-based anesthesia in geriatric spine surgery patients.
Design: Retrospective, single-blinded observational study of propofol- and sevoflurane-based anesthesia cohorts.
Setting and participants: Patients eligible for this study were aged 65 years or older admitted to the SMG-SNU Boramae Medical Center (Seoul, South Korea). All patients underwent general anesthesia either via intravenous propofol or inhalational sevoflurane for spine surgery between January 2015 and December 2019. Patients were retrospectively identified via electronic medical records. Patient exclusion criteria included preoperative delirium, history of dementia, psychiatric disease, alcoholism, hepatic or renal dysfunction, postoperative mechanical ventilation dependence, other surgery within the recent 6 months, maintenance of intraoperative anesthesia with combined anesthetics, or incomplete medical record.
Main outcome measures: The primary outcome was the incidence of POD after administration of propofol- and sevoflurane-based anesthesia during hospitalization. Patients were screened for POD regularly by attending nurses using the Nursing Delirium Screening Scale (disorientation, inappropriate behavior, inappropriate communication, hallucination, and psychomotor retardation) during the entirety of the patient’s hospital stay; if 1 or more screening criteria were met, a psychiatrist was consulted for the proper diagnosis and management of delirium. A psychiatric diagnosis was required for a case to be counted toward the incidence of POD in this study. Secondary outcomes included postoperative 30-day complications (angina, myocardial infarction, transient ischemic attack/stroke, pneumonia, deep vein thrombosis, pulmonary embolism, acute kidney injury, or infection) and length of postoperative hospital stay.
Main results: POD occurred in 29 patients (10.3%) out of the total cohort of 281. POD was more common in the sevoflurane group than in the propofol group (15.7% vs 5.0%; P = .003). Using multivariable logistic regression, inhalational sevoflurane was associated with an increased risk of POD as compared to propofol-based anesthesia (odds ratio [OR], 4.120; 95% CI, 1.549-10.954; P = .005). There was no association between choice of anesthetic and postoperative 30-day complications or the length of postoperative hospital stay. Both older age (OR, 1.242; 95% CI, 1.130-1.366; P < .001) and higher pain score at postoperative day 1 (OR, 1.338; 95% CI, 1.056-1.696; P = .016) were associated with increased risk of POD.
Conclusion: Propofol-based anesthesia was associated with a lower incidence of and risk for POD than sevoflurane-based anesthesia in older patients undergoing spine surgery.
Study 2 Overview (Mei et al)
Objective: To determine the incidence and duration of POD in older patients after total knee/hip replacement (TKR/THR) under intravenous propofol or inhalational sevoflurane general anesthesia.
Design: Randomized clinical trial of propofol and sevoflurane groups.
Setting and participants: This study was conducted at the Shanghai Tenth People’s Hospital and involved 209 participants enrolled between June 2016 and November 2019. All participants were 60 years of age or older, scheduled for TKR/THR surgery under general anesthesia, American Society of Anesthesiologists (ASA) class I to III, and assessed to be of normal cognitive function preoperatively via a Mini-Mental State Examination. Participant exclusion criteria included preexisting delirium as assessed by the Confusion Assessment Method (CAM), prior diagnosed neurological diseases (eg, Parkinson’s disease), prior diagnosed mental disorders (eg, schizophrenia), or impaired vision or hearing that would influence cognitive assessments. All participants were randomly assigned to either sevoflurane or propofol anesthesia for their surgery via a computer-generated list. Of these, 103 received inhalational sevoflurane and 106 received intravenous propofol. All participants received standardized postoperative care.
Main outcome measures: All participants were interviewed by investigators, who were blinded to the anesthesia regimen, twice daily on postoperative days 1, 2, and 3 using CAM and a CAM-based scoring system (CAM-S) to assess delirium severity. The CAM encapsulated 4 criteria: acute onset and fluctuating course, agitation, disorganized thinking, and altered level of consciousness. To diagnose delirium, both the first and second criteria must be met, in addition to either the third or fourth criterion. The averages of the scores across the 3 postoperative days indicated delirium severity, while the incidence and duration of delirium was assessed by the presence of delirium as determined by CAM on any postoperative day.
Main results: All eligible participants (N = 209; mean [SD] age 71.2 [6.7] years; 29.2% male) were included in the final analysis. The incidence of POD was not statistically different between the propofol and sevoflurane groups (33.0% vs 23.3%; P = .119, Chi-square test). It was estimated that 316 participants in each arm of the study were needed to detect statistical differences. The number of days of POD per person were higher with propofol anesthesia as compared to sevoflurane (0.5 [0.8] vs 0.3 [0.5]; P = .049, Student’s t-test).
Conclusion: This underpowered study showed a 9.7% difference in the incidence of POD between older adults who received propofol (33.0%) and sevoflurane (23.3%) after THR/TKR. Further studies with a larger sample size are needed to compare general anesthetics and their role in POD.
Commentary
Delirium is characterized by an acute state of confusion with fluctuating mental status, inattention, disorganized thinking, and altered level of consciousness. It is often caused by medications and/or their related adverse effects, infections, electrolyte imbalances, and other clinical etiologies. Delirium often manifests in post-surgical settings, disproportionately affecting older patients and leading to increased risk of morbidity, mortality, hospital length of stay, and health care costs.1 Intraoperative risk factors for POD are determined by the degree of operative stress (eg, lower-risk surgeries put the patient at reduced risk for POD as compared to higher-risk surgeries) and are additive to preexisting patient-specific risk factors, such as older age and functional impairment.1 Because operative stress is associated with risk for POD, limiting operative stress in controlled ways, such as through the choice of anesthetic agent administered, may be a pragmatic way to manage operative risks and optimize outcomes, especially when serving a surgically vulnerable population.
In Study 1, Chang et al sought to assess whether 2 commonly utilized general anesthetics, propofol and sevoflurane, in older patients undergoing spine surgery differentially affected the incidence of POD. In this retrospective, single-blinded observational study of 281 geriatric patients, the researchers found that sevoflurane was associated with a higher risk of POD as compared to propofol. However, these anesthetics were not associated with surgical outcomes such as postoperative 30-day complications or the length of postoperative hospital stay. While these findings added new knowledge to this field of research, several limitations should be kept in mind when interpreting this study’s results. For instance, the sample size was relatively small, with all cases selected from a single center utilizing a retrospective analysis. In addition, although a standardized nursing screening tool was used as a method for delirium detection, hypoactive delirium or less symptomatic delirium may have been missed, which in turn would lead to an underestimation of POD incidence. The latter is a common limitation in delirium research.
In Study 2, Mei et al similarly explored the effects of general anesthetics on POD in older surgical patients. Specifically, using a randomized clinical trial design, the investigators compared propofol with sevoflurane in older patients who underwent TKR/THR, and their roles in POD severity and duration. Although the incidence of POD was higher in those who received propofol compared to sevoflurane, this trial was underpowered and the results did not reach statistical significance. In addition, while the duration of POD was slightly longer in the propofol group compared to the sevoflurane group (0.5 vs 0.3 days), it was unclear if this finding was clinically significant. Similar to many research studies in POD, limitations of Study 2 included a small sample size of 209 patients, with all participants enrolled from a single center. On the other hand, this study illustrated the feasibility of a method that allowed reproducible prospective assessment of POD time course using CAM and CAM-S.
Applications for Clinical Practice and System Implementation
The delineation of risk factors that contribute to delirium after surgery in older patients is key to mitigating risks for POD and improving clinical outcomes. An important step towards a better understanding of these modifiable risk factors is to clearly quantify intraoperative risk of POD attributable to specific anesthetics. While preclinical studies have shown differential neurotoxicity effects of propofol and sevoflurane, their impact on clinically important neurologic outcomes such as delirium and cognitive decline remains poorly understood. Although Studies 1 and 2 both provided head-to-head comparisons of propofol and sevoflurane as risk factors for POD in high-operative-stress surgeries in older patients, the results were inconsistent. That being said, this small incremental increase in knowledge was not unexpected in the course of discovery around a clinically complex research question. Importantly, these studies provided evidence regarding the methodological approaches that could be taken to further this line of research.
The mediating factors of the differences on neurologic outcomes between anesthetic agents are likely pharmacological, biological, and methodological. Pharmacologically, the differences between target receptors, such as GABAA (propofol, etomidate) or NMDA (ketamine), could be a defining feature in the difference in incidence of POD. Additionally, secondary actions of anesthetic agents on glycine, nicotinic, and acetylcholine receptors could play a role as well. Biologically, genes such as CYP2E1, CYP2B6, CYP2C9, GSTP1, UGT1A9, SULT1A1, and NQO1 have all been identified as genetic factors in the metabolism of anesthetics, and variations in such genes could result in different responses to anesthetics.2 Methodologically, routes of anesthetic administration (eg, inhalation vs intravenous), preexisting anatomical structures, or confounding medical conditions (eg, lower respiratory volume due to older age) may influence POD incidence, duration, or severity. Moreover, methodological differences between Studies 1 and 2, such as surgeries performed (spinal vs TKR/THR), patient populations (South Korean vs Chinese), and the diagnosis and monitoring of delirium (retrospective screening and diagnosis vs prospective CAM/CAM-S) may impact delirium outcomes. Thus, these factors should be considered in the design of future clinical trials undertaken to investigate the effects of anesthetics on POD.
Given the high prevalence of delirium and its associated adverse outcomes in the immediate postoperative period in older patients, further research is warranted to determine how anesthetics affect POD in order to optimize perioperative care and mitigate risks in this vulnerable population. Moreover, parallel investigations into how anesthetics differentially impact the development of transient or longer-term cognitive impairment after a surgical procedure (ie, postoperative cognitive dysfunction) in older adults are urgently needed in order to improve their cognitive health.
Practice Points
- Intravenous propofol and inhalational sevoflurane may be differentially associated with incidence, duration, and severity of POD in geriatric surgical patients.
- Further larger-scale studies are warranted to clarify the role of anesthetic choice in POD in order to optimize surgical outcomes in older patients.
–Jared Doan, BS, and Fred Ko, MD
Icahn School of Medicine at Mount Sinai
Study 1 Overview (Chang et al)
Objective: To assess the incidence of postoperative delirium (POD) following propofol- vs sevoflurane-based anesthesia in geriatric spine surgery patients.
Design: Retrospective, single-blinded observational study of propofol- and sevoflurane-based anesthesia cohorts.
Setting and participants: Patients eligible for this study were aged 65 years or older admitted to the SMG-SNU Boramae Medical Center (Seoul, South Korea). All patients underwent general anesthesia either via intravenous propofol or inhalational sevoflurane for spine surgery between January 2015 and December 2019. Patients were retrospectively identified via electronic medical records. Patient exclusion criteria included preoperative delirium, history of dementia, psychiatric disease, alcoholism, hepatic or renal dysfunction, postoperative mechanical ventilation dependence, other surgery within the recent 6 months, maintenance of intraoperative anesthesia with combined anesthetics, or incomplete medical record.
Main outcome measures: The primary outcome was the incidence of POD after administration of propofol- and sevoflurane-based anesthesia during hospitalization. Patients were screened for POD regularly by attending nurses using the Nursing Delirium Screening Scale (disorientation, inappropriate behavior, inappropriate communication, hallucination, and psychomotor retardation) during the entirety of the patient’s hospital stay; if 1 or more screening criteria were met, a psychiatrist was consulted for the proper diagnosis and management of delirium. A psychiatric diagnosis was required for a case to be counted toward the incidence of POD in this study. Secondary outcomes included postoperative 30-day complications (angina, myocardial infarction, transient ischemic attack/stroke, pneumonia, deep vein thrombosis, pulmonary embolism, acute kidney injury, or infection) and length of postoperative hospital stay.
Main results: POD occurred in 29 patients (10.3%) out of the total cohort of 281. POD was more common in the sevoflurane group than in the propofol group (15.7% vs 5.0%; P = .003). Using multivariable logistic regression, inhalational sevoflurane was associated with an increased risk of POD as compared to propofol-based anesthesia (odds ratio [OR], 4.120; 95% CI, 1.549-10.954; P = .005). There was no association between choice of anesthetic and postoperative 30-day complications or the length of postoperative hospital stay. Both older age (OR, 1.242; 95% CI, 1.130-1.366; P < .001) and higher pain score at postoperative day 1 (OR, 1.338; 95% CI, 1.056-1.696; P = .016) were associated with increased risk of POD.
Conclusion: Propofol-based anesthesia was associated with a lower incidence of and risk for POD than sevoflurane-based anesthesia in older patients undergoing spine surgery.
Study 2 Overview (Mei et al)
Objective: To determine the incidence and duration of POD in older patients after total knee/hip replacement (TKR/THR) under intravenous propofol or inhalational sevoflurane general anesthesia.
Design: Randomized clinical trial of propofol and sevoflurane groups.
Setting and participants: This study was conducted at the Shanghai Tenth People’s Hospital and involved 209 participants enrolled between June 2016 and November 2019. All participants were 60 years of age or older, scheduled for TKR/THR surgery under general anesthesia, American Society of Anesthesiologists (ASA) class I to III, and assessed to be of normal cognitive function preoperatively via a Mini-Mental State Examination. Participant exclusion criteria included preexisting delirium as assessed by the Confusion Assessment Method (CAM), prior diagnosed neurological diseases (eg, Parkinson’s disease), prior diagnosed mental disorders (eg, schizophrenia), or impaired vision or hearing that would influence cognitive assessments. All participants were randomly assigned to either sevoflurane or propofol anesthesia for their surgery via a computer-generated list. Of these, 103 received inhalational sevoflurane and 106 received intravenous propofol. All participants received standardized postoperative care.
Main outcome measures: All participants were interviewed by investigators, who were blinded to the anesthesia regimen, twice daily on postoperative days 1, 2, and 3 using CAM and a CAM-based scoring system (CAM-S) to assess delirium severity. The CAM encapsulated 4 criteria: acute onset and fluctuating course, agitation, disorganized thinking, and altered level of consciousness. To diagnose delirium, both the first and second criteria must be met, in addition to either the third or fourth criterion. The averages of the scores across the 3 postoperative days indicated delirium severity, while the incidence and duration of delirium was assessed by the presence of delirium as determined by CAM on any postoperative day.
Main results: All eligible participants (N = 209; mean [SD] age 71.2 [6.7] years; 29.2% male) were included in the final analysis. The incidence of POD was not statistically different between the propofol and sevoflurane groups (33.0% vs 23.3%; P = .119, Chi-square test). It was estimated that 316 participants in each arm of the study were needed to detect statistical differences. The number of days of POD per person were higher with propofol anesthesia as compared to sevoflurane (0.5 [0.8] vs 0.3 [0.5]; P = .049, Student’s t-test).
Conclusion: This underpowered study showed a 9.7% difference in the incidence of POD between older adults who received propofol (33.0%) and sevoflurane (23.3%) after THR/TKR. Further studies with a larger sample size are needed to compare general anesthetics and their role in POD.
Commentary
Delirium is characterized by an acute state of confusion with fluctuating mental status, inattention, disorganized thinking, and altered level of consciousness. It is often caused by medications and/or their related adverse effects, infections, electrolyte imbalances, and other clinical etiologies. Delirium often manifests in post-surgical settings, disproportionately affecting older patients and leading to increased risk of morbidity, mortality, hospital length of stay, and health care costs.1 Intraoperative risk factors for POD are determined by the degree of operative stress (eg, lower-risk surgeries put the patient at reduced risk for POD as compared to higher-risk surgeries) and are additive to preexisting patient-specific risk factors, such as older age and functional impairment.1 Because operative stress is associated with risk for POD, limiting operative stress in controlled ways, such as through the choice of anesthetic agent administered, may be a pragmatic way to manage operative risks and optimize outcomes, especially when serving a surgically vulnerable population.
In Study 1, Chang et al sought to assess whether 2 commonly utilized general anesthetics, propofol and sevoflurane, in older patients undergoing spine surgery differentially affected the incidence of POD. In this retrospective, single-blinded observational study of 281 geriatric patients, the researchers found that sevoflurane was associated with a higher risk of POD as compared to propofol. However, these anesthetics were not associated with surgical outcomes such as postoperative 30-day complications or the length of postoperative hospital stay. While these findings added new knowledge to this field of research, several limitations should be kept in mind when interpreting this study’s results. For instance, the sample size was relatively small, with all cases selected from a single center utilizing a retrospective analysis. In addition, although a standardized nursing screening tool was used as a method for delirium detection, hypoactive delirium or less symptomatic delirium may have been missed, which in turn would lead to an underestimation of POD incidence. The latter is a common limitation in delirium research.
In Study 2, Mei et al similarly explored the effects of general anesthetics on POD in older surgical patients. Specifically, using a randomized clinical trial design, the investigators compared propofol with sevoflurane in older patients who underwent TKR/THR, and their roles in POD severity and duration. Although the incidence of POD was higher in those who received propofol compared to sevoflurane, this trial was underpowered and the results did not reach statistical significance. In addition, while the duration of POD was slightly longer in the propofol group compared to the sevoflurane group (0.5 vs 0.3 days), it was unclear if this finding was clinically significant. Similar to many research studies in POD, limitations of Study 2 included a small sample size of 209 patients, with all participants enrolled from a single center. On the other hand, this study illustrated the feasibility of a method that allowed reproducible prospective assessment of POD time course using CAM and CAM-S.
Applications for Clinical Practice and System Implementation
The delineation of risk factors that contribute to delirium after surgery in older patients is key to mitigating risks for POD and improving clinical outcomes. An important step towards a better understanding of these modifiable risk factors is to clearly quantify intraoperative risk of POD attributable to specific anesthetics. While preclinical studies have shown differential neurotoxicity effects of propofol and sevoflurane, their impact on clinically important neurologic outcomes such as delirium and cognitive decline remains poorly understood. Although Studies 1 and 2 both provided head-to-head comparisons of propofol and sevoflurane as risk factors for POD in high-operative-stress surgeries in older patients, the results were inconsistent. That being said, this small incremental increase in knowledge was not unexpected in the course of discovery around a clinically complex research question. Importantly, these studies provided evidence regarding the methodological approaches that could be taken to further this line of research.
The mediating factors of the differences on neurologic outcomes between anesthetic agents are likely pharmacological, biological, and methodological. Pharmacologically, the differences between target receptors, such as GABAA (propofol, etomidate) or NMDA (ketamine), could be a defining feature in the difference in incidence of POD. Additionally, secondary actions of anesthetic agents on glycine, nicotinic, and acetylcholine receptors could play a role as well. Biologically, genes such as CYP2E1, CYP2B6, CYP2C9, GSTP1, UGT1A9, SULT1A1, and NQO1 have all been identified as genetic factors in the metabolism of anesthetics, and variations in such genes could result in different responses to anesthetics.2 Methodologically, routes of anesthetic administration (eg, inhalation vs intravenous), preexisting anatomical structures, or confounding medical conditions (eg, lower respiratory volume due to older age) may influence POD incidence, duration, or severity. Moreover, methodological differences between Studies 1 and 2, such as surgeries performed (spinal vs TKR/THR), patient populations (South Korean vs Chinese), and the diagnosis and monitoring of delirium (retrospective screening and diagnosis vs prospective CAM/CAM-S) may impact delirium outcomes. Thus, these factors should be considered in the design of future clinical trials undertaken to investigate the effects of anesthetics on POD.
Given the high prevalence of delirium and its associated adverse outcomes in the immediate postoperative period in older patients, further research is warranted to determine how anesthetics affect POD in order to optimize perioperative care and mitigate risks in this vulnerable population. Moreover, parallel investigations into how anesthetics differentially impact the development of transient or longer-term cognitive impairment after a surgical procedure (ie, postoperative cognitive dysfunction) in older adults are urgently needed in order to improve their cognitive health.
Practice Points
- Intravenous propofol and inhalational sevoflurane may be differentially associated with incidence, duration, and severity of POD in geriatric surgical patients.
- Further larger-scale studies are warranted to clarify the role of anesthetic choice in POD in order to optimize surgical outcomes in older patients.
–Jared Doan, BS, and Fred Ko, MD
Icahn School of Medicine at Mount Sinai
1. Dasgupta M, Dumbrell AC. Preoperative risk assessment for delirium after noncardiac surgery: a systematic review. J Am Geriatr Soc. 2006;54(10):1578-1589. doi:10.1111/j.1532-5415.2006.00893.x
2. Mikstacki A, Skrzypczak-Zielinska M, Tamowicz B, et al. The impact of genetic factors on response to anaesthetics. Adv Med Sci. 2013;58(1):9-14. doi:10.2478/v10039-012-0065-z
1. Dasgupta M, Dumbrell AC. Preoperative risk assessment for delirium after noncardiac surgery: a systematic review. J Am Geriatr Soc. 2006;54(10):1578-1589. doi:10.1111/j.1532-5415.2006.00893.x
2. Mikstacki A, Skrzypczak-Zielinska M, Tamowicz B, et al. The impact of genetic factors on response to anaesthetics. Adv Med Sci. 2013;58(1):9-14. doi:10.2478/v10039-012-0065-z
Effectiveness of Colonoscopy for Colorectal Cancer Screening in Reducing Cancer-Related Mortality: Interpreting the Results From Two Ongoing Randomized Trials
Study 1 Overview (Bretthauer et al)
Objective: To evaluate the impact of screening colonoscopy on colon cancer–related death.
Design: Randomized trial conducted in 4 European countries.
Setting and participants: Presumptively healthy men and women between the ages of 55 and 64 years were selected from population registries in Poland, Norway, Sweden, and the Netherlands between 2009 and 2014. Eligible participants had not previously undergone screening. Patients with a diagnosis of colon cancer before trial entry were excluded.
Intervention: Participants were randomly assigned in a 1:2 ratio to undergo colonoscopy screening by invitation or to no invitation and no screening. Participants were randomized using a computer-generated allocation algorithm. Patients were stratified by age, sex, and municipality.
Main outcome measures: The primary endpoint of the study was risk of colorectal cancer and related death after a median follow-up of 10 to 15 years. The main secondary endpoint was death from any cause.
Main results: The study reported follow-up data from 84,585 participants (89.1% of all participants originally included in the trial). The remaining participants were either excluded or data could not be included due to lack of follow-up data from the usual-care group. Men (50.1%) and women (49.9%) were equally represented. The median age at entry was 59 years. The median follow-up was 10 years. Characteristics were otherwise balanced. Good bowel preparation was reported in 91% of all participants. Cecal intubation was achieved in 96.8% of all participants. The percentage of patients who underwent screening was 42% for the group, but screening rates varied by country (33%-60%). Colorectal cancer was diagnosed at screening in 62 participants (0.5% of screening group). Adenomas were detected in 30.7% of participants; 15 patients had polypectomy-related major bleeding. There were no perforations.
The risk of colorectal cancer at 10 years was 0.98% in the invited-to-screen group and 1.2% in the usual-care group (risk ratio, 0.82; 95% CI, 0.7-0.93). The reported number needed to invite to prevent 1 case of colon cancer in a 10-year period was 455. The risk of colorectal cancer–related death at 10 years was 0.28% in the invited-to-screen group and 0.31% in the usual-care group (risk ratio, 0.9; 95% CI, 0.64-1.16). An adjusted per-protocol analysis was performed to account for the estimated effect of screening if all participants assigned to the screening group underwent screening. In this analysis, the risk of colorectal cancer at 10 years was decreased from 1.22% to 0.84% (risk ratio, 0.69; 95% CI, 0.66-0.83).
Conclusion: Based on the results of this European randomized trial, the risk of colorectal cancer at 10 years was lower among those who were invited to undergo screening.
Study 2 Overview (Forsberg et al)
Objective: To investigate the effect of colorectal cancer screening with once-only colonoscopy or fecal immunochemical testing (FIT) on colorectal cancer mortality and incidence.
Design: Randomized controlled trial in Sweden utilizing a population registry.
Setting and participants: Patients aged 60 years at the time of entry were identified from a population-based registry from the Swedish Tax Agency.
Intervention: Individuals were assigned by an independent statistician to once-only colonoscopy, 2 rounds of FIT 2 years apart, or a control group in which no intervention was performed. Patients were assigned in a 1:6 ratio for colonoscopy vs control and a 1:2 ratio for FIT vs control.
Main outcome measures: The primary endpoint of the trial was colorectal cancer incidence and mortality.
Main results: A total of 278,280 participants were included in the study from March 1, 2014, through December 31, 2020 (31,140 in the colonoscopy group, 60,300 in the FIT group, and 186,840 in the control group). Of those in the colonoscopy group, 35% underwent colonoscopy, and 55% of those in the FIT group participated in testing. Colorectal cancer was detected in 0.16% (49) of people in the colonoscopy group and 0.2% (121) of people in the FIT test group (relative risk, 0.78; 95% CI, 0.56-1.09). The advanced adenoma detection rate was 2.05% in the colonoscopy group and 1.61% in the FIT group (relative risk, 1.27; 95% CI, 1.15-1.41). There were 2 perforations noted in the colonoscopy group and 15 major bleeding events. More right-sided adenomas were detected in the colonoscopy group.
Conclusion: The results of the current study highlight similar detection rates in the colonoscopy and FIT group. Should further follow-up show a benefit in disease-specific mortality, such screening strategies could be translated into population-based screening programs.
Commentary
The first colonoscopy screening recommendations were established in the mid 1990s in the United States, and over the subsequent 2 decades colonoscopy has been the recommended method and main modality for colorectal cancer screening in this country. The advantage of colonoscopy over other screening modalities (sigmoidoscopy and fecal-based testing) is that it can examine the entire large bowel and allow for removal of potential precancerous lesions. However, data to support colonoscopy as a screening modality for colorectal cancer are largely based on cohort studies.1,2 These studies have reported a significant reduction in the incidence of colon cancer. Additionally, colorectal cancer mortality was notably lower in the screened populations. For example, one study among health professionals found a nearly 70% reduction in colorectal cancer mortality in those who underwent at least 1 screening colonoscopy.3
There has been a lack of randomized clinical data to validate the efficacy of colonoscopy screening for reducing colorectal cancer–related deaths. The current study by Bretthauer et al addresses an important need and enhances our understanding of the efficacy of colorectal cancer screening with colonoscopy. In this randomized trial involving more than 84,000 participants from Poland, Norway, Sweden, and the Netherlands, there was a noted 18% decrease in the risk of colorectal cancer over a 10-year period in the intention-to-screen population. The reduction in the risk of death from colorectal cancer was not statistically significant (risk ratio, 0.90; 95% CI, 0.64-1.16). These results are surprising and certainly raise the question as to whether previous studies overestimated the effectiveness of colonoscopy in reducing the risk of colorectal cancer–related deaths. There are several limitations to the Bretthauer et al study, however.
Perhaps the most important limitation is the fact that only 42% of participants in the invited-to-screen cohort underwent screening colonoscopy. Therefore, this raises the question of whether the efficacy noted is simply due to a lack of participation in the screening protocol. In the adjusted per-protocol analysis, colonoscopy was estimated to reduce the risk of colorectal cancer by 31% and the risk of colorectal cancer–related death by around 50%. These findings are more in line with prior published studies regarding the efficacy of colorectal cancer screening. The authors plan to repeat this analysis at 15 years, and it is possible that the risk of colorectal cancer and colorectal cancer–related death can be reduced on subsequent follow-up.
While the results of the Bretthauer et al trial are important, randomized trials that directly compare the effectiveness of different colorectal cancer screening strategies are lacking. The Forsberg et al trial, also an ongoing study, seeks to address this vitally important gap in our current data. The SCREESCO trial is a study that compares the efficacy of colonoscopy with FIT every 2 years or no screening. The currently reported data are preliminary but show a similarly low rate of colonoscopy screening in those invited to do so (35%). This is a similar limitation to that noted in the Bretthauer et al study. Furthermore, there is some question regarding colonoscopy quality in this study, which had a very low reported adenoma detection rate.
While the current studies are important and provide quality randomized data on the effect of colorectal cancer screening, there remain many unanswered questions. Should the results presented by Bretthauer et al represent the current real-world scenario, then colonoscopy screening may not be viewed as an effective screening tool compared to simpler, less-invasive modalities (ie, FIT). Further follow-up from the SCREESCO trial will help shed light on this question. However, there are concerns with this study, including a very low participation rate, which could greatly underestimate the effectiveness of screening. Additional analysis and longer follow-up will be vital to fully understand the benefits of screening colonoscopy. In the meantime, screening remains an important tool for early detection of colorectal cancer and remains a category A recommendation by the United States Preventive Services Task Force.4
Applications for Clinical Practice and System Implementation
Current guidelines continue to strongly recommend screening for colorectal cancer for persons between 45 and 75 years of age (category B recommendation for those aged 45 to 49 years per the United States Preventive Services Task Force). Stool-based tests and direct visualization tests are both endorsed as screening options. Further follow-up from the presented studies is needed to help shed light on the magnitude of benefit of these modalities.
Practice Points
- Current guidelines continue to strongly recommend screening for colon cancer in those aged 45 to 75 years.
- The optimal modality for screening and the impact of screening on cancer-related mortality requires longer- term follow-up from these ongoing studies.
–Daniel Isaac, DO, MS
1. Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR. Screening for Colorectal Cancer: An Evidence Update for the U.S. Preventive Services Task Force [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2021 May. Report No.: 20-05271-EF-1.
2. Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325(19):1978-1998. doi:10.1001/jama.2021.4417
3. Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369(12):1095-1105. doi:10.1056/NEJMoa1301969
4. U.S. Preventive Services Task Force. Colorectal cancer: screening. Published May 18, 2021. Accessed November 8, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening
Study 1 Overview (Bretthauer et al)
Objective: To evaluate the impact of screening colonoscopy on colon cancer–related death.
Design: Randomized trial conducted in 4 European countries.
Setting and participants: Presumptively healthy men and women between the ages of 55 and 64 years were selected from population registries in Poland, Norway, Sweden, and the Netherlands between 2009 and 2014. Eligible participants had not previously undergone screening. Patients with a diagnosis of colon cancer before trial entry were excluded.
Intervention: Participants were randomly assigned in a 1:2 ratio to undergo colonoscopy screening by invitation or to no invitation and no screening. Participants were randomized using a computer-generated allocation algorithm. Patients were stratified by age, sex, and municipality.
Main outcome measures: The primary endpoint of the study was risk of colorectal cancer and related death after a median follow-up of 10 to 15 years. The main secondary endpoint was death from any cause.
Main results: The study reported follow-up data from 84,585 participants (89.1% of all participants originally included in the trial). The remaining participants were either excluded or data could not be included due to lack of follow-up data from the usual-care group. Men (50.1%) and women (49.9%) were equally represented. The median age at entry was 59 years. The median follow-up was 10 years. Characteristics were otherwise balanced. Good bowel preparation was reported in 91% of all participants. Cecal intubation was achieved in 96.8% of all participants. The percentage of patients who underwent screening was 42% for the group, but screening rates varied by country (33%-60%). Colorectal cancer was diagnosed at screening in 62 participants (0.5% of screening group). Adenomas were detected in 30.7% of participants; 15 patients had polypectomy-related major bleeding. There were no perforations.
The risk of colorectal cancer at 10 years was 0.98% in the invited-to-screen group and 1.2% in the usual-care group (risk ratio, 0.82; 95% CI, 0.7-0.93). The reported number needed to invite to prevent 1 case of colon cancer in a 10-year period was 455. The risk of colorectal cancer–related death at 10 years was 0.28% in the invited-to-screen group and 0.31% in the usual-care group (risk ratio, 0.9; 95% CI, 0.64-1.16). An adjusted per-protocol analysis was performed to account for the estimated effect of screening if all participants assigned to the screening group underwent screening. In this analysis, the risk of colorectal cancer at 10 years was decreased from 1.22% to 0.84% (risk ratio, 0.69; 95% CI, 0.66-0.83).
Conclusion: Based on the results of this European randomized trial, the risk of colorectal cancer at 10 years was lower among those who were invited to undergo screening.
Study 2 Overview (Forsberg et al)
Objective: To investigate the effect of colorectal cancer screening with once-only colonoscopy or fecal immunochemical testing (FIT) on colorectal cancer mortality and incidence.
Design: Randomized controlled trial in Sweden utilizing a population registry.
Setting and participants: Patients aged 60 years at the time of entry were identified from a population-based registry from the Swedish Tax Agency.
Intervention: Individuals were assigned by an independent statistician to once-only colonoscopy, 2 rounds of FIT 2 years apart, or a control group in which no intervention was performed. Patients were assigned in a 1:6 ratio for colonoscopy vs control and a 1:2 ratio for FIT vs control.
Main outcome measures: The primary endpoint of the trial was colorectal cancer incidence and mortality.
Main results: A total of 278,280 participants were included in the study from March 1, 2014, through December 31, 2020 (31,140 in the colonoscopy group, 60,300 in the FIT group, and 186,840 in the control group). Of those in the colonoscopy group, 35% underwent colonoscopy, and 55% of those in the FIT group participated in testing. Colorectal cancer was detected in 0.16% (49) of people in the colonoscopy group and 0.2% (121) of people in the FIT test group (relative risk, 0.78; 95% CI, 0.56-1.09). The advanced adenoma detection rate was 2.05% in the colonoscopy group and 1.61% in the FIT group (relative risk, 1.27; 95% CI, 1.15-1.41). There were 2 perforations noted in the colonoscopy group and 15 major bleeding events. More right-sided adenomas were detected in the colonoscopy group.
Conclusion: The results of the current study highlight similar detection rates in the colonoscopy and FIT group. Should further follow-up show a benefit in disease-specific mortality, such screening strategies could be translated into population-based screening programs.
Commentary
The first colonoscopy screening recommendations were established in the mid 1990s in the United States, and over the subsequent 2 decades colonoscopy has been the recommended method and main modality for colorectal cancer screening in this country. The advantage of colonoscopy over other screening modalities (sigmoidoscopy and fecal-based testing) is that it can examine the entire large bowel and allow for removal of potential precancerous lesions. However, data to support colonoscopy as a screening modality for colorectal cancer are largely based on cohort studies.1,2 These studies have reported a significant reduction in the incidence of colon cancer. Additionally, colorectal cancer mortality was notably lower in the screened populations. For example, one study among health professionals found a nearly 70% reduction in colorectal cancer mortality in those who underwent at least 1 screening colonoscopy.3
There has been a lack of randomized clinical data to validate the efficacy of colonoscopy screening for reducing colorectal cancer–related deaths. The current study by Bretthauer et al addresses an important need and enhances our understanding of the efficacy of colorectal cancer screening with colonoscopy. In this randomized trial involving more than 84,000 participants from Poland, Norway, Sweden, and the Netherlands, there was a noted 18% decrease in the risk of colorectal cancer over a 10-year period in the intention-to-screen population. The reduction in the risk of death from colorectal cancer was not statistically significant (risk ratio, 0.90; 95% CI, 0.64-1.16). These results are surprising and certainly raise the question as to whether previous studies overestimated the effectiveness of colonoscopy in reducing the risk of colorectal cancer–related deaths. There are several limitations to the Bretthauer et al study, however.
Perhaps the most important limitation is the fact that only 42% of participants in the invited-to-screen cohort underwent screening colonoscopy. Therefore, this raises the question of whether the efficacy noted is simply due to a lack of participation in the screening protocol. In the adjusted per-protocol analysis, colonoscopy was estimated to reduce the risk of colorectal cancer by 31% and the risk of colorectal cancer–related death by around 50%. These findings are more in line with prior published studies regarding the efficacy of colorectal cancer screening. The authors plan to repeat this analysis at 15 years, and it is possible that the risk of colorectal cancer and colorectal cancer–related death can be reduced on subsequent follow-up.
While the results of the Bretthauer et al trial are important, randomized trials that directly compare the effectiveness of different colorectal cancer screening strategies are lacking. The Forsberg et al trial, also an ongoing study, seeks to address this vitally important gap in our current data. The SCREESCO trial is a study that compares the efficacy of colonoscopy with FIT every 2 years or no screening. The currently reported data are preliminary but show a similarly low rate of colonoscopy screening in those invited to do so (35%). This is a similar limitation to that noted in the Bretthauer et al study. Furthermore, there is some question regarding colonoscopy quality in this study, which had a very low reported adenoma detection rate.
While the current studies are important and provide quality randomized data on the effect of colorectal cancer screening, there remain many unanswered questions. Should the results presented by Bretthauer et al represent the current real-world scenario, then colonoscopy screening may not be viewed as an effective screening tool compared to simpler, less-invasive modalities (ie, FIT). Further follow-up from the SCREESCO trial will help shed light on this question. However, there are concerns with this study, including a very low participation rate, which could greatly underestimate the effectiveness of screening. Additional analysis and longer follow-up will be vital to fully understand the benefits of screening colonoscopy. In the meantime, screening remains an important tool for early detection of colorectal cancer and remains a category A recommendation by the United States Preventive Services Task Force.4
Applications for Clinical Practice and System Implementation
Current guidelines continue to strongly recommend screening for colorectal cancer for persons between 45 and 75 years of age (category B recommendation for those aged 45 to 49 years per the United States Preventive Services Task Force). Stool-based tests and direct visualization tests are both endorsed as screening options. Further follow-up from the presented studies is needed to help shed light on the magnitude of benefit of these modalities.
Practice Points
- Current guidelines continue to strongly recommend screening for colon cancer in those aged 45 to 75 years.
- The optimal modality for screening and the impact of screening on cancer-related mortality requires longer- term follow-up from these ongoing studies.
–Daniel Isaac, DO, MS
Study 1 Overview (Bretthauer et al)
Objective: To evaluate the impact of screening colonoscopy on colon cancer–related death.
Design: Randomized trial conducted in 4 European countries.
Setting and participants: Presumptively healthy men and women between the ages of 55 and 64 years were selected from population registries in Poland, Norway, Sweden, and the Netherlands between 2009 and 2014. Eligible participants had not previously undergone screening. Patients with a diagnosis of colon cancer before trial entry were excluded.
Intervention: Participants were randomly assigned in a 1:2 ratio to undergo colonoscopy screening by invitation or to no invitation and no screening. Participants were randomized using a computer-generated allocation algorithm. Patients were stratified by age, sex, and municipality.
Main outcome measures: The primary endpoint of the study was risk of colorectal cancer and related death after a median follow-up of 10 to 15 years. The main secondary endpoint was death from any cause.
Main results: The study reported follow-up data from 84,585 participants (89.1% of all participants originally included in the trial). The remaining participants were either excluded or data could not be included due to lack of follow-up data from the usual-care group. Men (50.1%) and women (49.9%) were equally represented. The median age at entry was 59 years. The median follow-up was 10 years. Characteristics were otherwise balanced. Good bowel preparation was reported in 91% of all participants. Cecal intubation was achieved in 96.8% of all participants. The percentage of patients who underwent screening was 42% for the group, but screening rates varied by country (33%-60%). Colorectal cancer was diagnosed at screening in 62 participants (0.5% of screening group). Adenomas were detected in 30.7% of participants; 15 patients had polypectomy-related major bleeding. There were no perforations.
The risk of colorectal cancer at 10 years was 0.98% in the invited-to-screen group and 1.2% in the usual-care group (risk ratio, 0.82; 95% CI, 0.7-0.93). The reported number needed to invite to prevent 1 case of colon cancer in a 10-year period was 455. The risk of colorectal cancer–related death at 10 years was 0.28% in the invited-to-screen group and 0.31% in the usual-care group (risk ratio, 0.9; 95% CI, 0.64-1.16). An adjusted per-protocol analysis was performed to account for the estimated effect of screening if all participants assigned to the screening group underwent screening. In this analysis, the risk of colorectal cancer at 10 years was decreased from 1.22% to 0.84% (risk ratio, 0.69; 95% CI, 0.66-0.83).
Conclusion: Based on the results of this European randomized trial, the risk of colorectal cancer at 10 years was lower among those who were invited to undergo screening.
Study 2 Overview (Forsberg et al)
Objective: To investigate the effect of colorectal cancer screening with once-only colonoscopy or fecal immunochemical testing (FIT) on colorectal cancer mortality and incidence.
Design: Randomized controlled trial in Sweden utilizing a population registry.
Setting and participants: Patients aged 60 years at the time of entry were identified from a population-based registry from the Swedish Tax Agency.
Intervention: Individuals were assigned by an independent statistician to once-only colonoscopy, 2 rounds of FIT 2 years apart, or a control group in which no intervention was performed. Patients were assigned in a 1:6 ratio for colonoscopy vs control and a 1:2 ratio for FIT vs control.
Main outcome measures: The primary endpoint of the trial was colorectal cancer incidence and mortality.
Main results: A total of 278,280 participants were included in the study from March 1, 2014, through December 31, 2020 (31,140 in the colonoscopy group, 60,300 in the FIT group, and 186,840 in the control group). Of those in the colonoscopy group, 35% underwent colonoscopy, and 55% of those in the FIT group participated in testing. Colorectal cancer was detected in 0.16% (49) of people in the colonoscopy group and 0.2% (121) of people in the FIT test group (relative risk, 0.78; 95% CI, 0.56-1.09). The advanced adenoma detection rate was 2.05% in the colonoscopy group and 1.61% in the FIT group (relative risk, 1.27; 95% CI, 1.15-1.41). There were 2 perforations noted in the colonoscopy group and 15 major bleeding events. More right-sided adenomas were detected in the colonoscopy group.
Conclusion: The results of the current study highlight similar detection rates in the colonoscopy and FIT group. Should further follow-up show a benefit in disease-specific mortality, such screening strategies could be translated into population-based screening programs.
Commentary
The first colonoscopy screening recommendations were established in the mid 1990s in the United States, and over the subsequent 2 decades colonoscopy has been the recommended method and main modality for colorectal cancer screening in this country. The advantage of colonoscopy over other screening modalities (sigmoidoscopy and fecal-based testing) is that it can examine the entire large bowel and allow for removal of potential precancerous lesions. However, data to support colonoscopy as a screening modality for colorectal cancer are largely based on cohort studies.1,2 These studies have reported a significant reduction in the incidence of colon cancer. Additionally, colorectal cancer mortality was notably lower in the screened populations. For example, one study among health professionals found a nearly 70% reduction in colorectal cancer mortality in those who underwent at least 1 screening colonoscopy.3
There has been a lack of randomized clinical data to validate the efficacy of colonoscopy screening for reducing colorectal cancer–related deaths. The current study by Bretthauer et al addresses an important need and enhances our understanding of the efficacy of colorectal cancer screening with colonoscopy. In this randomized trial involving more than 84,000 participants from Poland, Norway, Sweden, and the Netherlands, there was a noted 18% decrease in the risk of colorectal cancer over a 10-year period in the intention-to-screen population. The reduction in the risk of death from colorectal cancer was not statistically significant (risk ratio, 0.90; 95% CI, 0.64-1.16). These results are surprising and certainly raise the question as to whether previous studies overestimated the effectiveness of colonoscopy in reducing the risk of colorectal cancer–related deaths. There are several limitations to the Bretthauer et al study, however.
Perhaps the most important limitation is the fact that only 42% of participants in the invited-to-screen cohort underwent screening colonoscopy. Therefore, this raises the question of whether the efficacy noted is simply due to a lack of participation in the screening protocol. In the adjusted per-protocol analysis, colonoscopy was estimated to reduce the risk of colorectal cancer by 31% and the risk of colorectal cancer–related death by around 50%. These findings are more in line with prior published studies regarding the efficacy of colorectal cancer screening. The authors plan to repeat this analysis at 15 years, and it is possible that the risk of colorectal cancer and colorectal cancer–related death can be reduced on subsequent follow-up.
While the results of the Bretthauer et al trial are important, randomized trials that directly compare the effectiveness of different colorectal cancer screening strategies are lacking. The Forsberg et al trial, also an ongoing study, seeks to address this vitally important gap in our current data. The SCREESCO trial is a study that compares the efficacy of colonoscopy with FIT every 2 years or no screening. The currently reported data are preliminary but show a similarly low rate of colonoscopy screening in those invited to do so (35%). This is a similar limitation to that noted in the Bretthauer et al study. Furthermore, there is some question regarding colonoscopy quality in this study, which had a very low reported adenoma detection rate.
While the current studies are important and provide quality randomized data on the effect of colorectal cancer screening, there remain many unanswered questions. Should the results presented by Bretthauer et al represent the current real-world scenario, then colonoscopy screening may not be viewed as an effective screening tool compared to simpler, less-invasive modalities (ie, FIT). Further follow-up from the SCREESCO trial will help shed light on this question. However, there are concerns with this study, including a very low participation rate, which could greatly underestimate the effectiveness of screening. Additional analysis and longer follow-up will be vital to fully understand the benefits of screening colonoscopy. In the meantime, screening remains an important tool for early detection of colorectal cancer and remains a category A recommendation by the United States Preventive Services Task Force.4
Applications for Clinical Practice and System Implementation
Current guidelines continue to strongly recommend screening for colorectal cancer for persons between 45 and 75 years of age (category B recommendation for those aged 45 to 49 years per the United States Preventive Services Task Force). Stool-based tests and direct visualization tests are both endorsed as screening options. Further follow-up from the presented studies is needed to help shed light on the magnitude of benefit of these modalities.
Practice Points
- Current guidelines continue to strongly recommend screening for colon cancer in those aged 45 to 75 years.
- The optimal modality for screening and the impact of screening on cancer-related mortality requires longer- term follow-up from these ongoing studies.
–Daniel Isaac, DO, MS
1. Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR. Screening for Colorectal Cancer: An Evidence Update for the U.S. Preventive Services Task Force [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2021 May. Report No.: 20-05271-EF-1.
2. Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325(19):1978-1998. doi:10.1001/jama.2021.4417
3. Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369(12):1095-1105. doi:10.1056/NEJMoa1301969
4. U.S. Preventive Services Task Force. Colorectal cancer: screening. Published May 18, 2021. Accessed November 8, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening
1. Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR. Screening for Colorectal Cancer: An Evidence Update for the U.S. Preventive Services Task Force [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2021 May. Report No.: 20-05271-EF-1.
2. Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325(19):1978-1998. doi:10.1001/jama.2021.4417
3. Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369(12):1095-1105. doi:10.1056/NEJMoa1301969
4. U.S. Preventive Services Task Force. Colorectal cancer: screening. Published May 18, 2021. Accessed November 8, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening
Safety and Efficacy of GLP-1 Receptor Agonists and SGLT2 Inhibitors Among Veterans With Type 2 Diabetes
Selecting the best medication regimen for a patient with type 2 diabetes mellitus (T2DM) depends on many factors, such as glycemic control, adherence, adverse effect (AE) profile, and comorbid conditions.1 Selected agents from 2 newer medication classes, glucagon-like peptide 1 receptor agonists (GLP-1 RA) and sodium-glucose cotransporter 2 inhibitors (SGLT2i), have demonstrated cardiovascular and renal protective properties, creating a new paradigm in management.
The American Diabetes Association recommends medications with proven benefit in cardiovascular disease (CVD), such as the GLP-1 RAs liraglutide, injectable semaglutide, or dulaglutide, or the SGLT2i empagliflozin or canagliflozin, as second-line after metformin in patients with established atherosclerotic CVD or indicators of high risk to reduce the risk of major adverse cardiovascular events (MACE).1 SGLT2i are preferred in patients with diabetic kidney disease, and GLP-1 RAs are next in line for selection of agents with proven nephroprotection (liraglutide, injectable semaglutide, dulaglutide). The mechanisms of these benefits are not fully understood but may be due to their extraglycemic effects. The classes likely induce these benefits by different mechanisms: SGLT2i by hemodynamic effects and GLP-1 RAs by anti-inflammatory mechanisms.2 Although there is much interest, evidence is limited regarding the cardiovascular and renal protection benefits of these agents used in combination.
The combined use of GLP-1 RA and SGLT2i agents demonstrated greater benefit than separate use in trials with nonveteran populations.3-7 These studies evaluated effects on hemoglobin A1c (HbA1c) levels, weight loss, blood pressure (BP), and estimated glomerular filtration rate (eGFR). A meta-analysis of 7 trials found that the combination of GLP-1 RA and SGLT2i reduced HbA1c levels, body weight, and systolic blood pressure (SBP).8 All of the changes were statistically significant except for body weight with combination vs SGLT2i alone. Combination therapy was not associated with increased risk of severe hypoglycemia compared with either therapy separately.
The purpose of our study was to evaluate the safety and efficacy of the combined use of GLP-1 RA and SGLT2i in a real-world, US Department of Veterans Affairs (VA) population with T2DM.
Methods
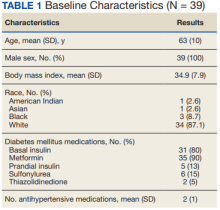
This study was a pre-post, retrospective, single-center chart review. Subjects served as their own control. The project was reviewed and approved by the VA Ann Arbor Healthcare System Institutional Review Board. Subjects prescribed both a GLP-1 RA (semaglutide or liraglutide) and SGLT2i (empagliflozin) between January 1, 2014, and November 10, 2019, were extracted from the Corporate Data Warehouse (CDW) for possible inclusion in the study.
Patients were excluded if they received < 12 weeks of combination GLP-1 RA and SGLT2i therapy or did not have a corresponding 12-week HbA1c level. Patients also were excluded if they had < 12 weeks of monotherapy before starting combination therapy or did not have a baseline HbA1c level, or if the start date of combination therapy was not recorded in the VA electronic health record (EHR). We reviewed data for each patient from 6 months before to 1 year after the second agent was started. Start of the first agent (GLP-1 RA or SGLT2i) was recorded as the date the prescription was picked up in-person or 7 days after release date if mailed to the patient. Start of the second agent (GLP-1 RA or SGLT2i) was defined as baseline and was the date the prescription was picked up in person or 7 days after the release date if mailed.
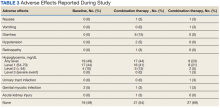
Baseline measures were taken anytime from 8 weeks after the start of the first agent through 2 weeks after the start of the second agent. Data collected included age, sex, race, height, weight, BP, HbA1c levels, serum creatinine (SCr), eGFR, classes of medications for the treatment of T2DM, and the number of prescribed antihypertensive medications. HbA1c levels, SCr, eGFR, weight, and BP also were collected at 12 weeks (within 8-21 weeks); 26 weeks (within 22-35 weeks); and 52 weeks (within 36-57 weeks) of combination therapy. We reviewed progress notes and laboratory results to determine AEs within 26 weeks before initiating second agent (baseline) and 0 to 26 weeks and 26 to 52 weeks after initiating combination therapy.
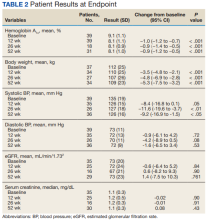
The primary objective was to determine the effect on HbA1c levels at 12 weeks when using a GLP-1 RA and SGLT2i in combination vs separately. Secondary objectives were to determine change from baseline in mean body weight, BP, SCr, and eGFR at 12, 26, and 52 weeks; change in HbA1c levels at 26 and 52 weeks; and incidence of prespecified adverse drug reactions during combination therapy vs separately.
Statistical Analysis
Assuming a SD of 1, 80% power, significance level of P < .05, 2-sided test, and a correlation between baseline and follow-up of 0.5, we determined that a sample size of 34 subjects was required to detect a 0.5% change in baseline HbA1c level at 12 weeks. A t test (or Wilcoxon signed rank test if outcome not normally distributed) was conducted to examine whether the expected change from baseline was different from 0 for continuous outcomes. Median change from baseline was reported for SCr as a nonparametric t test (Wilcoxon signed rank test) was used.
Results
We identified 110 patients for possible study inclusion and 39 met eligibility criteria. After record review, 30 patients were excluded for receiving < 12 weeks of combination therapy or no 12 week HbA1c level; 26 patients were excluded for receiving < 12 weeks of monotherapy before starting combination therapy or no baseline HbA1c level; and 15 patients were excluded for lack of documentation in the VA EHR. Of the 39 patients included, 24 (62%) were prescribed empagliflozin first and then 8 started liraglutide and 16 started semaglutide.
HbA1c levels decreased by 1% after 12 weeks of combination therapy compared with baseline (P < .001), and this reduction was sustained through the duration of the study period (Table 2).
The most common AE during the trial was hypoglycemia, which was mostly mild (level 1) (Table 3).
Discussion
This study evaluated the safety and efficacy of combined use of semaglutide or liraglutide and empagliflozin in a veteran population with T2DM. The retrospective chart review captured real-world practice and outcomes. Combination therapy was associated with a significant reduction in HbA1c levels, body weight, and SBP compared with either agent alone. No significant change was seen in DBP, SCr, or eGFR. Overall, the combination of GLP-1 RA and SGLT2i medications demonstrated a good safety profile with most patients reporting no AEs.
Several other studies have assessed the safety and efficacy of using GLP-1 RA and SGLT2i in combination. The DURATION 8 trial is the only double-blind trial to randomize subjects to receive either exenatide once weekly, dapagliflozin, or the combination of both for up to 52 weeks.3 Other controlled trials required stable background therapy with either SGLT2i or GLP-1 RA before randomization to receive the other class or placebo and had durations between 18 and 30 weeks.4-7 The AWARD 10 trial studied the combination of canagliflozin and dulaglutide, which both have proven CVD benefit.4 Other studies did not restrict SGLT2i or GLP-1 RA background therapy to agents with proven CVD benefit.5-7 The present study evaluated the combination of empagliflozin plus liraglutide or semaglutide, agents that all have proven CVD benefit.
A meta-analysis of 7 trials, including those previously mentioned, was conducted to evaluate the combination of GLP-1 RA and SGLT2i.8 The combination significantly reduced HbA1c levels by 0.61% and 0.85% compared with GLP-1 RA or SGLT2i, respectively. Our trial showed greater HbA1c level reduction of 1% with combination therapy compared with either agent separately. This may have been due in part to a higher baseline HbA1c level in our real-world veteran population. The meta-analysis found the combination decreased body weight 2.6 kg and 1.5 kg compared with GLP-1 RA or SGLT2i, respectively.8 This only reached significance with comparison vs GLP-1 RA alone. Our study demonstrated impressive weight loss of up to about 5 kg after 26 and 52 weeks of combination therapy. This is equivalent to about 5% weight loss from baseline, which is clinically significant.9 Liraglutide and semaglutide are the GLP-1 RAs associated with the greatest weight loss, which may contribute to greater weight loss efficacy seen in the present trial.1
In our trial SBP fell lower compared with the meta-analysis. Combination therapy significantly reduced SBP by 4.1 mm Hg and 2.7 mm Hg compared with GLP-1 RA or SGLT2i, respectively, in the meta-analysis.8 We observed a significant 9 to 12 mm Hg reduction in SBP after 26 to 52 weeks of combination therapy compared with baseline. This reduction occurred despite relatively controlled SBP at baseline (135 mm Hg). Each reduction of 10 mm Hg in SBP significantly reduces the risk of MACE, stroke, and heart failure, making our results clinically significant.10 Neither the meta-analysis nor present study found a significant difference in DBP or eGFR with combination therapy.
AEs were similar in this trial compared with the meta-analysis. Combination treatment with GLP-1 RA and SGLT2i did not increase the incidence of severe hypoglycemia in either study.8 Hypoglycemia was the most common AE in this study, but frequency was similar with combination and separate therapy. Both medication classes are associated with low or no risk of hypoglycemia on their own.1 Baseline medications likely contributed to episodes of hypoglycemia seen in this study: About 80% of patients were prescribed basal insulin, 15% were prescribed a sulfonylurea, and 13% were prescribed prandial insulin. There is limited overlap between the known AEs of GLP-1 RA and SGLT2i, making combination therapy a safe option for use in patients with T2DM.
Our study confirms greater reduction in HbA1c levels, weight, and SBP in veterans taking GLP-1 RA and SGLT2i medications in combination compared with separate use in a real-world setting in a veteran population. The magnitude of change seen in this population appears greater compared with previous studies.
Limitations
There were several limitations to our study. Given the retrospective nature, many patients included in the study did not have bloodwork drawn during the specified time frames. Because of this, many patients were excluded and missing data on renal outcomes limited the power to detect differences. Data regarding AEs were limited to what was recorded in the EHR, which may underrepresent the AEs that patients experienced. Finally, our study size was small, consisting primarily of a White and male population, which may limit generalizability.
Further research is needed to validate these findings in this population and should include a larger study population. The impact of combining GLP-1 RA with SGLT2i on cardiorenal outcomes is an important area of ongoing research.
ConclusionS
The combined use of GLP-1 RA and SGLT2i resulted in significant improvement in HbA1c levels, weight, and SBP compared with separate use in this real-world study of a VA population with T2DM. The combination was well tolerated overall. Awareness of these results can facilitate optimal care and outcomes in the VA population.
Acknowledgments
Serena Kelley, PharmD, and Michael Brenner, PharmD, assisted with study design and initial data collection. Julie Strominger, MS, provided statistical support.
1. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(suppl 1):S111-S124. doi.10.2337/dc21-S009
2. DeFronzo RA. Combination therapy with GLP-1 receptor agonist and SGLT2 inhibitor. Diabetes Obes Metab. 2017;19(10):1353-1362. doi.10.1111/dom.12982
3. Jabbour S, Frias J, Guja C, Hardy E, Ahmed A, Ohman P. Effects of exenatide once weekly plus dapagliflozin, exenatide once weekly, or dapagliflozin, added to metformin monotherapy, on body weight, systolic blood pressure, and triglycerides in patients with type 2 diabetes in the DURATION-8 study. Diabetes Obes Metab. 2018;20(6):1515-1519. doi:10.1111/dom.13206
4. Ludvik B, Frias J, Tinahones F, et al. Dulaglutide as add-on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD-10): a 24-week, randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2018;6(5):370-381. doi:10.1016/S2213-8587(18)30023-8
5. Blonde L, Belousova L, Fainberg U, et al. Liraglutide as add-on to sodium-glucose co-transporter-2 inhibitors in patients with inadequately controlled type 2 diabetes: LIRA-ADD2SGLT2i, a 26-week, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2020;22(6):929-937. doi:10.1111/dom.13978
6. Fulcher G, Matthews D, Perkovic V, et al; CANVAS trial collaborative group. Efficacy and safety of canagliflozin when used in conjunction with incretin-mimetic therapy in patients with type 2 diabetes. Diabetes Obes Metab. 2016;18(1):82-91. doi:10.1111/dom.12589
7. Zinman B, Bhosekar V, Busch R, et al. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7(5):356-367. doi:10.1016/S2213-8587(19)30066-X
8. Mantsiou C, Karagiannis T, Kakotrichi P, et al. Glucagon-like peptide-1 receptor agonists and sodium-glucose co-transporter-2 inhibitors as combination therapy for type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2020;22(10):1857-1868. doi:10.1111/dom.14108
9. US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the management of adult overweight and obesity. Version 3.0. Accessed August 18, 2022. www.healthquality.va.gov/guidelines/CD/obesity/VADoDObesityCPGFinal5087242020.pdf
10. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2015;387(10022):957-967. doi.10.1016/S0140-6736(15)01225-8
Selecting the best medication regimen for a patient with type 2 diabetes mellitus (T2DM) depends on many factors, such as glycemic control, adherence, adverse effect (AE) profile, and comorbid conditions.1 Selected agents from 2 newer medication classes, glucagon-like peptide 1 receptor agonists (GLP-1 RA) and sodium-glucose cotransporter 2 inhibitors (SGLT2i), have demonstrated cardiovascular and renal protective properties, creating a new paradigm in management.
The American Diabetes Association recommends medications with proven benefit in cardiovascular disease (CVD), such as the GLP-1 RAs liraglutide, injectable semaglutide, or dulaglutide, or the SGLT2i empagliflozin or canagliflozin, as second-line after metformin in patients with established atherosclerotic CVD or indicators of high risk to reduce the risk of major adverse cardiovascular events (MACE).1 SGLT2i are preferred in patients with diabetic kidney disease, and GLP-1 RAs are next in line for selection of agents with proven nephroprotection (liraglutide, injectable semaglutide, dulaglutide). The mechanisms of these benefits are not fully understood but may be due to their extraglycemic effects. The classes likely induce these benefits by different mechanisms: SGLT2i by hemodynamic effects and GLP-1 RAs by anti-inflammatory mechanisms.2 Although there is much interest, evidence is limited regarding the cardiovascular and renal protection benefits of these agents used in combination.
The combined use of GLP-1 RA and SGLT2i agents demonstrated greater benefit than separate use in trials with nonveteran populations.3-7 These studies evaluated effects on hemoglobin A1c (HbA1c) levels, weight loss, blood pressure (BP), and estimated glomerular filtration rate (eGFR). A meta-analysis of 7 trials found that the combination of GLP-1 RA and SGLT2i reduced HbA1c levels, body weight, and systolic blood pressure (SBP).8 All of the changes were statistically significant except for body weight with combination vs SGLT2i alone. Combination therapy was not associated with increased risk of severe hypoglycemia compared with either therapy separately.
The purpose of our study was to evaluate the safety and efficacy of the combined use of GLP-1 RA and SGLT2i in a real-world, US Department of Veterans Affairs (VA) population with T2DM.
Methods
This study was a pre-post, retrospective, single-center chart review. Subjects served as their own control. The project was reviewed and approved by the VA Ann Arbor Healthcare System Institutional Review Board. Subjects prescribed both a GLP-1 RA (semaglutide or liraglutide) and SGLT2i (empagliflozin) between January 1, 2014, and November 10, 2019, were extracted from the Corporate Data Warehouse (CDW) for possible inclusion in the study.
Patients were excluded if they received < 12 weeks of combination GLP-1 RA and SGLT2i therapy or did not have a corresponding 12-week HbA1c level. Patients also were excluded if they had < 12 weeks of monotherapy before starting combination therapy or did not have a baseline HbA1c level, or if the start date of combination therapy was not recorded in the VA electronic health record (EHR). We reviewed data for each patient from 6 months before to 1 year after the second agent was started. Start of the first agent (GLP-1 RA or SGLT2i) was recorded as the date the prescription was picked up in-person or 7 days after release date if mailed to the patient. Start of the second agent (GLP-1 RA or SGLT2i) was defined as baseline and was the date the prescription was picked up in person or 7 days after the release date if mailed.
Baseline measures were taken anytime from 8 weeks after the start of the first agent through 2 weeks after the start of the second agent. Data collected included age, sex, race, height, weight, BP, HbA1c levels, serum creatinine (SCr), eGFR, classes of medications for the treatment of T2DM, and the number of prescribed antihypertensive medications. HbA1c levels, SCr, eGFR, weight, and BP also were collected at 12 weeks (within 8-21 weeks); 26 weeks (within 22-35 weeks); and 52 weeks (within 36-57 weeks) of combination therapy. We reviewed progress notes and laboratory results to determine AEs within 26 weeks before initiating second agent (baseline) and 0 to 26 weeks and 26 to 52 weeks after initiating combination therapy.
The primary objective was to determine the effect on HbA1c levels at 12 weeks when using a GLP-1 RA and SGLT2i in combination vs separately. Secondary objectives were to determine change from baseline in mean body weight, BP, SCr, and eGFR at 12, 26, and 52 weeks; change in HbA1c levels at 26 and 52 weeks; and incidence of prespecified adverse drug reactions during combination therapy vs separately.
Statistical Analysis
Assuming a SD of 1, 80% power, significance level of P < .05, 2-sided test, and a correlation between baseline and follow-up of 0.5, we determined that a sample size of 34 subjects was required to detect a 0.5% change in baseline HbA1c level at 12 weeks. A t test (or Wilcoxon signed rank test if outcome not normally distributed) was conducted to examine whether the expected change from baseline was different from 0 for continuous outcomes. Median change from baseline was reported for SCr as a nonparametric t test (Wilcoxon signed rank test) was used.
Results
We identified 110 patients for possible study inclusion and 39 met eligibility criteria. After record review, 30 patients were excluded for receiving < 12 weeks of combination therapy or no 12 week HbA1c level; 26 patients were excluded for receiving < 12 weeks of monotherapy before starting combination therapy or no baseline HbA1c level; and 15 patients were excluded for lack of documentation in the VA EHR. Of the 39 patients included, 24 (62%) were prescribed empagliflozin first and then 8 started liraglutide and 16 started semaglutide.
HbA1c levels decreased by 1% after 12 weeks of combination therapy compared with baseline (P < .001), and this reduction was sustained through the duration of the study period (Table 2).
The most common AE during the trial was hypoglycemia, which was mostly mild (level 1) (Table 3).
Discussion
This study evaluated the safety and efficacy of combined use of semaglutide or liraglutide and empagliflozin in a veteran population with T2DM. The retrospective chart review captured real-world practice and outcomes. Combination therapy was associated with a significant reduction in HbA1c levels, body weight, and SBP compared with either agent alone. No significant change was seen in DBP, SCr, or eGFR. Overall, the combination of GLP-1 RA and SGLT2i medications demonstrated a good safety profile with most patients reporting no AEs.
Several other studies have assessed the safety and efficacy of using GLP-1 RA and SGLT2i in combination. The DURATION 8 trial is the only double-blind trial to randomize subjects to receive either exenatide once weekly, dapagliflozin, or the combination of both for up to 52 weeks.3 Other controlled trials required stable background therapy with either SGLT2i or GLP-1 RA before randomization to receive the other class or placebo and had durations between 18 and 30 weeks.4-7 The AWARD 10 trial studied the combination of canagliflozin and dulaglutide, which both have proven CVD benefit.4 Other studies did not restrict SGLT2i or GLP-1 RA background therapy to agents with proven CVD benefit.5-7 The present study evaluated the combination of empagliflozin plus liraglutide or semaglutide, agents that all have proven CVD benefit.
A meta-analysis of 7 trials, including those previously mentioned, was conducted to evaluate the combination of GLP-1 RA and SGLT2i.8 The combination significantly reduced HbA1c levels by 0.61% and 0.85% compared with GLP-1 RA or SGLT2i, respectively. Our trial showed greater HbA1c level reduction of 1% with combination therapy compared with either agent separately. This may have been due in part to a higher baseline HbA1c level in our real-world veteran population. The meta-analysis found the combination decreased body weight 2.6 kg and 1.5 kg compared with GLP-1 RA or SGLT2i, respectively.8 This only reached significance with comparison vs GLP-1 RA alone. Our study demonstrated impressive weight loss of up to about 5 kg after 26 and 52 weeks of combination therapy. This is equivalent to about 5% weight loss from baseline, which is clinically significant.9 Liraglutide and semaglutide are the GLP-1 RAs associated with the greatest weight loss, which may contribute to greater weight loss efficacy seen in the present trial.1
In our trial SBP fell lower compared with the meta-analysis. Combination therapy significantly reduced SBP by 4.1 mm Hg and 2.7 mm Hg compared with GLP-1 RA or SGLT2i, respectively, in the meta-analysis.8 We observed a significant 9 to 12 mm Hg reduction in SBP after 26 to 52 weeks of combination therapy compared with baseline. This reduction occurred despite relatively controlled SBP at baseline (135 mm Hg). Each reduction of 10 mm Hg in SBP significantly reduces the risk of MACE, stroke, and heart failure, making our results clinically significant.10 Neither the meta-analysis nor present study found a significant difference in DBP or eGFR with combination therapy.
AEs were similar in this trial compared with the meta-analysis. Combination treatment with GLP-1 RA and SGLT2i did not increase the incidence of severe hypoglycemia in either study.8 Hypoglycemia was the most common AE in this study, but frequency was similar with combination and separate therapy. Both medication classes are associated with low or no risk of hypoglycemia on their own.1 Baseline medications likely contributed to episodes of hypoglycemia seen in this study: About 80% of patients were prescribed basal insulin, 15% were prescribed a sulfonylurea, and 13% were prescribed prandial insulin. There is limited overlap between the known AEs of GLP-1 RA and SGLT2i, making combination therapy a safe option for use in patients with T2DM.
Our study confirms greater reduction in HbA1c levels, weight, and SBP in veterans taking GLP-1 RA and SGLT2i medications in combination compared with separate use in a real-world setting in a veteran population. The magnitude of change seen in this population appears greater compared with previous studies.
Limitations
There were several limitations to our study. Given the retrospective nature, many patients included in the study did not have bloodwork drawn during the specified time frames. Because of this, many patients were excluded and missing data on renal outcomes limited the power to detect differences. Data regarding AEs were limited to what was recorded in the EHR, which may underrepresent the AEs that patients experienced. Finally, our study size was small, consisting primarily of a White and male population, which may limit generalizability.
Further research is needed to validate these findings in this population and should include a larger study population. The impact of combining GLP-1 RA with SGLT2i on cardiorenal outcomes is an important area of ongoing research.
ConclusionS
The combined use of GLP-1 RA and SGLT2i resulted in significant improvement in HbA1c levels, weight, and SBP compared with separate use in this real-world study of a VA population with T2DM. The combination was well tolerated overall. Awareness of these results can facilitate optimal care and outcomes in the VA population.
Acknowledgments
Serena Kelley, PharmD, and Michael Brenner, PharmD, assisted with study design and initial data collection. Julie Strominger, MS, provided statistical support.
Selecting the best medication regimen for a patient with type 2 diabetes mellitus (T2DM) depends on many factors, such as glycemic control, adherence, adverse effect (AE) profile, and comorbid conditions.1 Selected agents from 2 newer medication classes, glucagon-like peptide 1 receptor agonists (GLP-1 RA) and sodium-glucose cotransporter 2 inhibitors (SGLT2i), have demonstrated cardiovascular and renal protective properties, creating a new paradigm in management.
The American Diabetes Association recommends medications with proven benefit in cardiovascular disease (CVD), such as the GLP-1 RAs liraglutide, injectable semaglutide, or dulaglutide, or the SGLT2i empagliflozin or canagliflozin, as second-line after metformin in patients with established atherosclerotic CVD or indicators of high risk to reduce the risk of major adverse cardiovascular events (MACE).1 SGLT2i are preferred in patients with diabetic kidney disease, and GLP-1 RAs are next in line for selection of agents with proven nephroprotection (liraglutide, injectable semaglutide, dulaglutide). The mechanisms of these benefits are not fully understood but may be due to their extraglycemic effects. The classes likely induce these benefits by different mechanisms: SGLT2i by hemodynamic effects and GLP-1 RAs by anti-inflammatory mechanisms.2 Although there is much interest, evidence is limited regarding the cardiovascular and renal protection benefits of these agents used in combination.
The combined use of GLP-1 RA and SGLT2i agents demonstrated greater benefit than separate use in trials with nonveteran populations.3-7 These studies evaluated effects on hemoglobin A1c (HbA1c) levels, weight loss, blood pressure (BP), and estimated glomerular filtration rate (eGFR). A meta-analysis of 7 trials found that the combination of GLP-1 RA and SGLT2i reduced HbA1c levels, body weight, and systolic blood pressure (SBP).8 All of the changes were statistically significant except for body weight with combination vs SGLT2i alone. Combination therapy was not associated with increased risk of severe hypoglycemia compared with either therapy separately.
The purpose of our study was to evaluate the safety and efficacy of the combined use of GLP-1 RA and SGLT2i in a real-world, US Department of Veterans Affairs (VA) population with T2DM.
Methods
This study was a pre-post, retrospective, single-center chart review. Subjects served as their own control. The project was reviewed and approved by the VA Ann Arbor Healthcare System Institutional Review Board. Subjects prescribed both a GLP-1 RA (semaglutide or liraglutide) and SGLT2i (empagliflozin) between January 1, 2014, and November 10, 2019, were extracted from the Corporate Data Warehouse (CDW) for possible inclusion in the study.
Patients were excluded if they received < 12 weeks of combination GLP-1 RA and SGLT2i therapy or did not have a corresponding 12-week HbA1c level. Patients also were excluded if they had < 12 weeks of monotherapy before starting combination therapy or did not have a baseline HbA1c level, or if the start date of combination therapy was not recorded in the VA electronic health record (EHR). We reviewed data for each patient from 6 months before to 1 year after the second agent was started. Start of the first agent (GLP-1 RA or SGLT2i) was recorded as the date the prescription was picked up in-person or 7 days after release date if mailed to the patient. Start of the second agent (GLP-1 RA or SGLT2i) was defined as baseline and was the date the prescription was picked up in person or 7 days after the release date if mailed.
Baseline measures were taken anytime from 8 weeks after the start of the first agent through 2 weeks after the start of the second agent. Data collected included age, sex, race, height, weight, BP, HbA1c levels, serum creatinine (SCr), eGFR, classes of medications for the treatment of T2DM, and the number of prescribed antihypertensive medications. HbA1c levels, SCr, eGFR, weight, and BP also were collected at 12 weeks (within 8-21 weeks); 26 weeks (within 22-35 weeks); and 52 weeks (within 36-57 weeks) of combination therapy. We reviewed progress notes and laboratory results to determine AEs within 26 weeks before initiating second agent (baseline) and 0 to 26 weeks and 26 to 52 weeks after initiating combination therapy.
The primary objective was to determine the effect on HbA1c levels at 12 weeks when using a GLP-1 RA and SGLT2i in combination vs separately. Secondary objectives were to determine change from baseline in mean body weight, BP, SCr, and eGFR at 12, 26, and 52 weeks; change in HbA1c levels at 26 and 52 weeks; and incidence of prespecified adverse drug reactions during combination therapy vs separately.
Statistical Analysis
Assuming a SD of 1, 80% power, significance level of P < .05, 2-sided test, and a correlation between baseline and follow-up of 0.5, we determined that a sample size of 34 subjects was required to detect a 0.5% change in baseline HbA1c level at 12 weeks. A t test (or Wilcoxon signed rank test if outcome not normally distributed) was conducted to examine whether the expected change from baseline was different from 0 for continuous outcomes. Median change from baseline was reported for SCr as a nonparametric t test (Wilcoxon signed rank test) was used.
Results
We identified 110 patients for possible study inclusion and 39 met eligibility criteria. After record review, 30 patients were excluded for receiving < 12 weeks of combination therapy or no 12 week HbA1c level; 26 patients were excluded for receiving < 12 weeks of monotherapy before starting combination therapy or no baseline HbA1c level; and 15 patients were excluded for lack of documentation in the VA EHR. Of the 39 patients included, 24 (62%) were prescribed empagliflozin first and then 8 started liraglutide and 16 started semaglutide.
HbA1c levels decreased by 1% after 12 weeks of combination therapy compared with baseline (P < .001), and this reduction was sustained through the duration of the study period (Table 2).
The most common AE during the trial was hypoglycemia, which was mostly mild (level 1) (Table 3).
Discussion
This study evaluated the safety and efficacy of combined use of semaglutide or liraglutide and empagliflozin in a veteran population with T2DM. The retrospective chart review captured real-world practice and outcomes. Combination therapy was associated with a significant reduction in HbA1c levels, body weight, and SBP compared with either agent alone. No significant change was seen in DBP, SCr, or eGFR. Overall, the combination of GLP-1 RA and SGLT2i medications demonstrated a good safety profile with most patients reporting no AEs.
Several other studies have assessed the safety and efficacy of using GLP-1 RA and SGLT2i in combination. The DURATION 8 trial is the only double-blind trial to randomize subjects to receive either exenatide once weekly, dapagliflozin, or the combination of both for up to 52 weeks.3 Other controlled trials required stable background therapy with either SGLT2i or GLP-1 RA before randomization to receive the other class or placebo and had durations between 18 and 30 weeks.4-7 The AWARD 10 trial studied the combination of canagliflozin and dulaglutide, which both have proven CVD benefit.4 Other studies did not restrict SGLT2i or GLP-1 RA background therapy to agents with proven CVD benefit.5-7 The present study evaluated the combination of empagliflozin plus liraglutide or semaglutide, agents that all have proven CVD benefit.
A meta-analysis of 7 trials, including those previously mentioned, was conducted to evaluate the combination of GLP-1 RA and SGLT2i.8 The combination significantly reduced HbA1c levels by 0.61% and 0.85% compared with GLP-1 RA or SGLT2i, respectively. Our trial showed greater HbA1c level reduction of 1% with combination therapy compared with either agent separately. This may have been due in part to a higher baseline HbA1c level in our real-world veteran population. The meta-analysis found the combination decreased body weight 2.6 kg and 1.5 kg compared with GLP-1 RA or SGLT2i, respectively.8 This only reached significance with comparison vs GLP-1 RA alone. Our study demonstrated impressive weight loss of up to about 5 kg after 26 and 52 weeks of combination therapy. This is equivalent to about 5% weight loss from baseline, which is clinically significant.9 Liraglutide and semaglutide are the GLP-1 RAs associated with the greatest weight loss, which may contribute to greater weight loss efficacy seen in the present trial.1
In our trial SBP fell lower compared with the meta-analysis. Combination therapy significantly reduced SBP by 4.1 mm Hg and 2.7 mm Hg compared with GLP-1 RA or SGLT2i, respectively, in the meta-analysis.8 We observed a significant 9 to 12 mm Hg reduction in SBP after 26 to 52 weeks of combination therapy compared with baseline. This reduction occurred despite relatively controlled SBP at baseline (135 mm Hg). Each reduction of 10 mm Hg in SBP significantly reduces the risk of MACE, stroke, and heart failure, making our results clinically significant.10 Neither the meta-analysis nor present study found a significant difference in DBP or eGFR with combination therapy.
AEs were similar in this trial compared with the meta-analysis. Combination treatment with GLP-1 RA and SGLT2i did not increase the incidence of severe hypoglycemia in either study.8 Hypoglycemia was the most common AE in this study, but frequency was similar with combination and separate therapy. Both medication classes are associated with low or no risk of hypoglycemia on their own.1 Baseline medications likely contributed to episodes of hypoglycemia seen in this study: About 80% of patients were prescribed basal insulin, 15% were prescribed a sulfonylurea, and 13% were prescribed prandial insulin. There is limited overlap between the known AEs of GLP-1 RA and SGLT2i, making combination therapy a safe option for use in patients with T2DM.
Our study confirms greater reduction in HbA1c levels, weight, and SBP in veterans taking GLP-1 RA and SGLT2i medications in combination compared with separate use in a real-world setting in a veteran population. The magnitude of change seen in this population appears greater compared with previous studies.
Limitations
There were several limitations to our study. Given the retrospective nature, many patients included in the study did not have bloodwork drawn during the specified time frames. Because of this, many patients were excluded and missing data on renal outcomes limited the power to detect differences. Data regarding AEs were limited to what was recorded in the EHR, which may underrepresent the AEs that patients experienced. Finally, our study size was small, consisting primarily of a White and male population, which may limit generalizability.
Further research is needed to validate these findings in this population and should include a larger study population. The impact of combining GLP-1 RA with SGLT2i on cardiorenal outcomes is an important area of ongoing research.
ConclusionS
The combined use of GLP-1 RA and SGLT2i resulted in significant improvement in HbA1c levels, weight, and SBP compared with separate use in this real-world study of a VA population with T2DM. The combination was well tolerated overall. Awareness of these results can facilitate optimal care and outcomes in the VA population.
Acknowledgments
Serena Kelley, PharmD, and Michael Brenner, PharmD, assisted with study design and initial data collection. Julie Strominger, MS, provided statistical support.
1. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(suppl 1):S111-S124. doi.10.2337/dc21-S009
2. DeFronzo RA. Combination therapy with GLP-1 receptor agonist and SGLT2 inhibitor. Diabetes Obes Metab. 2017;19(10):1353-1362. doi.10.1111/dom.12982
3. Jabbour S, Frias J, Guja C, Hardy E, Ahmed A, Ohman P. Effects of exenatide once weekly plus dapagliflozin, exenatide once weekly, or dapagliflozin, added to metformin monotherapy, on body weight, systolic blood pressure, and triglycerides in patients with type 2 diabetes in the DURATION-8 study. Diabetes Obes Metab. 2018;20(6):1515-1519. doi:10.1111/dom.13206
4. Ludvik B, Frias J, Tinahones F, et al. Dulaglutide as add-on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD-10): a 24-week, randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2018;6(5):370-381. doi:10.1016/S2213-8587(18)30023-8
5. Blonde L, Belousova L, Fainberg U, et al. Liraglutide as add-on to sodium-glucose co-transporter-2 inhibitors in patients with inadequately controlled type 2 diabetes: LIRA-ADD2SGLT2i, a 26-week, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2020;22(6):929-937. doi:10.1111/dom.13978
6. Fulcher G, Matthews D, Perkovic V, et al; CANVAS trial collaborative group. Efficacy and safety of canagliflozin when used in conjunction with incretin-mimetic therapy in patients with type 2 diabetes. Diabetes Obes Metab. 2016;18(1):82-91. doi:10.1111/dom.12589
7. Zinman B, Bhosekar V, Busch R, et al. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7(5):356-367. doi:10.1016/S2213-8587(19)30066-X
8. Mantsiou C, Karagiannis T, Kakotrichi P, et al. Glucagon-like peptide-1 receptor agonists and sodium-glucose co-transporter-2 inhibitors as combination therapy for type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2020;22(10):1857-1868. doi:10.1111/dom.14108
9. US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the management of adult overweight and obesity. Version 3.0. Accessed August 18, 2022. www.healthquality.va.gov/guidelines/CD/obesity/VADoDObesityCPGFinal5087242020.pdf
10. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2015;387(10022):957-967. doi.10.1016/S0140-6736(15)01225-8
1. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(suppl 1):S111-S124. doi.10.2337/dc21-S009
2. DeFronzo RA. Combination therapy with GLP-1 receptor agonist and SGLT2 inhibitor. Diabetes Obes Metab. 2017;19(10):1353-1362. doi.10.1111/dom.12982
3. Jabbour S, Frias J, Guja C, Hardy E, Ahmed A, Ohman P. Effects of exenatide once weekly plus dapagliflozin, exenatide once weekly, or dapagliflozin, added to metformin monotherapy, on body weight, systolic blood pressure, and triglycerides in patients with type 2 diabetes in the DURATION-8 study. Diabetes Obes Metab. 2018;20(6):1515-1519. doi:10.1111/dom.13206
4. Ludvik B, Frias J, Tinahones F, et al. Dulaglutide as add-on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD-10): a 24-week, randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2018;6(5):370-381. doi:10.1016/S2213-8587(18)30023-8
5. Blonde L, Belousova L, Fainberg U, et al. Liraglutide as add-on to sodium-glucose co-transporter-2 inhibitors in patients with inadequately controlled type 2 diabetes: LIRA-ADD2SGLT2i, a 26-week, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2020;22(6):929-937. doi:10.1111/dom.13978
6. Fulcher G, Matthews D, Perkovic V, et al; CANVAS trial collaborative group. Efficacy and safety of canagliflozin when used in conjunction with incretin-mimetic therapy in patients with type 2 diabetes. Diabetes Obes Metab. 2016;18(1):82-91. doi:10.1111/dom.12589
7. Zinman B, Bhosekar V, Busch R, et al. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7(5):356-367. doi:10.1016/S2213-8587(19)30066-X
8. Mantsiou C, Karagiannis T, Kakotrichi P, et al. Glucagon-like peptide-1 receptor agonists and sodium-glucose co-transporter-2 inhibitors as combination therapy for type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2020;22(10):1857-1868. doi:10.1111/dom.14108
9. US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the management of adult overweight and obesity. Version 3.0. Accessed August 18, 2022. www.healthquality.va.gov/guidelines/CD/obesity/VADoDObesityCPGFinal5087242020.pdf
10. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2015;387(10022):957-967. doi.10.1016/S0140-6736(15)01225-8
Make room for continuous glucose monitoring in type 2 diabetes management
A1C has been used to estimate 3-month glycemic control in patients with diabetes. However, A1C monitoring alone does not provide insight into daily glycemic variation, which is valuable in clinical management because tight glycemic control (defined as A1C < 7.0%) has been shown to reduce the risk of microvascular complications. Prior to the approval of glucagon-like peptide-1 receptor agonists and sodium-glucose co-transporter 2 inhibitors by the US Food and Drug Administration for the treatment of type 2 diabetes (T2D), reduction in the risk of macrovascular complications (aside from nonfatal myocardial infarction) was more difficult to achieve than it is now; some patients had a worse outcome with overly aggressive glycemic control.1
Previously, the use of a continuous glucose monitor (CGM) was limited to patients with type 1 diabetes who required basal and bolus insulin. However, technological advances have led to more patient-friendly and affordable devices, making CGMs more available. As such, the American Diabetes Association (ADA), in its 2022 Standards of Medical Care in Diabetes, recommends that clinicians offer continuous glucose monitoring to adults with T2D who require multiple daily injections, and based on a given patient’s ability, preferences, and needs.2
In this article, we discuss, first, the intricacies of CGMs and, second, what the evidence says about their use so that physicians can confidently recommend, and educate patients on, effective utilization of CGMs to obtain an individualized target of glycemic control.
Continuous glucose monitoring: A glossary
CGMs are characterized by who possesses the device and how data are recorded. This review is not about professional CGMs, which are owned by the health care provider and consist of a sensor that is applied in the clinic and returned to clinic for downloading of data1; rather, we focus on the novel category of nonprofessional, or personal, CGMs.
Three words to remember. Every CGM has 3 common components:
- The reader (also known as a receiver) is a handheld device that allows a patient to scan a sensor (see definition below) and instantaneously collect a glucose reading. The patient can use a standalone reader; a smartphone or other smart device with an associated app that serves as a reader; or both.
- A sensor is inserted subcutaneously to measure interstitial glucose. The lifespan of a sensor is 10 to 14 days.
- A transmitter relays information from the sensor to the reader.
The technology behind a CGM
CGM sensors measure interstitial glucose by means of a chemical reaction involving glucose oxidase and an oxidation-reduction cofactor, measuring the generation of hydrogen peroxide.3 Interstitial glucose readings lag behind plasma blood glucose readings by 2 to 21 minutes.4,5 Although this lag time is often not clinically significant, situations such as aerobic exercise and a rapidly changing glucose level might warrant confirmation by means of fingerstick measurement.5 It is common for CGM readings to vary slightly from venipuncture or fingerstick glucose readings.
What CGMs are availableto your patients?
Intermittently scanned CGMs (isCGMs) measure the glucose level continuously; the patient must scan a sensor to display and record the glucose level.6 Prolonged periods without scanning result in gaps in glycemic data.7,8
Continue to: Two isCGM systems...
Two isCGM systems are available: the FreeStyle Libre 14 day and the FreeStyle Libre 2 (both from Abbott).a Both consist of a reader and a disposable sensor, applied to the back of the arm, that is worn for 14 days. If the patient has a compatible smartphone or other smart device, the reader can be replaced by the smart device with the downloaded FreeStyle Libre or FreeStyle Libre 2 app.
To activate a new sensor, the patient applies the sensor, then scans it. Once activated, scanning the sensor provides the current glucose reading and recalls the last 8 hours of data. In addition to providing an instantaneous glucose reading, the display also provides a trend arrow indicating the direction and degree to which the glucose level is changing (TABLE 110,14,15). This feature helps patients avoid hypoglycemic episodes by allowing them to preemptively correct if the arrow indicates a rapidly declining glucose level.
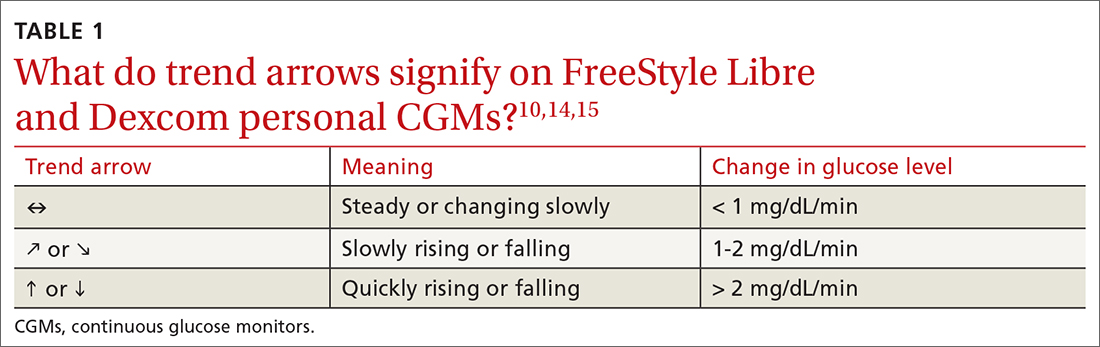
For the first 12 hours after a new sensor is activated, and when a glucose reading is < 70 mg/dL, patients should be instructed to avoid making treatment decisions and encouraged to utilize fingerstick glucose readings. FreeStyle Libre 14 day does not allow a glucose level alarm to be set; the system cannot detect these events without scanning the sensor.10 Bluetooth connectivity does allow FreeStyle Libre 2 users to set a glucose alarm if the reader or smart device is within 20 feet of the sensor. A default alarm is set to activate at 70 mg/dL (“low”) and 240 mg/dL (“high”); low and high alarm settings are also customizable. Because both FreeStyle Libre devices store 8 hours of data, patients must scan the sensor every 8 hours for a comprehensive glycemic report.14
FreeStyle Libre CGMs allow patients to add therapy notes, including time and amount of insulin administered and carbohydrates ingested. Readers for both devices function as a glucometer that is compatible with Abbott FreeStyle Precision Neo test strips.
Real-time CGMs (rtCGMs) measure and display glucose levels continuously for the duration of the life of the sensor, without the need to scan. Three rtCGM systems are available: Dexcom G6, Medtronic Guardian 3, and Senseonics Eversense E3.
Continue to: Dexcom G6...
Dexcom G6 is the first Dexcom CGM that does not require fingerstick calibration and the only rtCGM available in the United States that does not require patient calibration. This system comprises a single-use sensor replaced every 10 days; a transmitter that is transferred to each new sensor and replaced every 3 months; and an optional receiver that can be omitted if the patient prefers to utilize a smart device.
Dexcom G6 never requires a patient to scan a sensor. Instead, the receiver (or smart device) utilizes Bluetooth technology to obtain blood glucose readings if it is positioned within 20 feet of the transmitter. Patients can set both hypoglycemic and hyperglycemic alarms to predict events within 20 minutes. Similar to the functionality of the FreeStyle Libre systems, Dexcom G6 provides the opportunity to log lifestyle events, including insulin dosing, carbohydrate ingestion, exercise, and sick days.15
Medtronic Guardian 3 comprises the multi-use Guardian Connect Transmitter that is replaced annually and a single-use Guardian Sensor that is replaced every 7 days. Guardian 3 requires twice-daily fingerstick glucose calibration, which reduces the convenience of a CGM.
Guardian 3 allows the user to set alarm levels, providing predictive alerts 10 to 60 minutes before set glucose levels are reached. Patients must utilize a smart device to connect through Bluetooth to the CareLink Connect app and remain within 20 feet of the transmitter to provide continuous glucose readings. The CareLink Connect app allows patients to document exercise, calibration of fingerstick readings, meals, and insulin administration.16
Senseonics Eversense E3 consists of a 3.5 mm × 18.3 mm sensor inserted subcutaneously in the upper arm once every 180 days; a removable transmitter that attaches to an adhesive patch placed over the sensor; and a smart device with the Eversense app. The transmitter has a 1-year rechargeable battery and provides the patient with on-body vibration alerts even when they are not near their smart device.
Continue to: The Eversense E3 transmitter...
The Eversense E3 transmitter can be removed and reapplied without affecting the life of the sensor; however, no glucose data will be collected during this time. Once the transmitter is reapplied, it takes 10 minutes for the sensor to begin communicating with the transmitter. Eversense provides predictive alerts as long as 30 minutes before hyperglycemic or hypoglycemic events. The device requires twice-daily fingerstick calibrations.17
A comparison of the specifications and capabilities of the personal CGMs discussed here is provided in TABLE 2.10,14-17
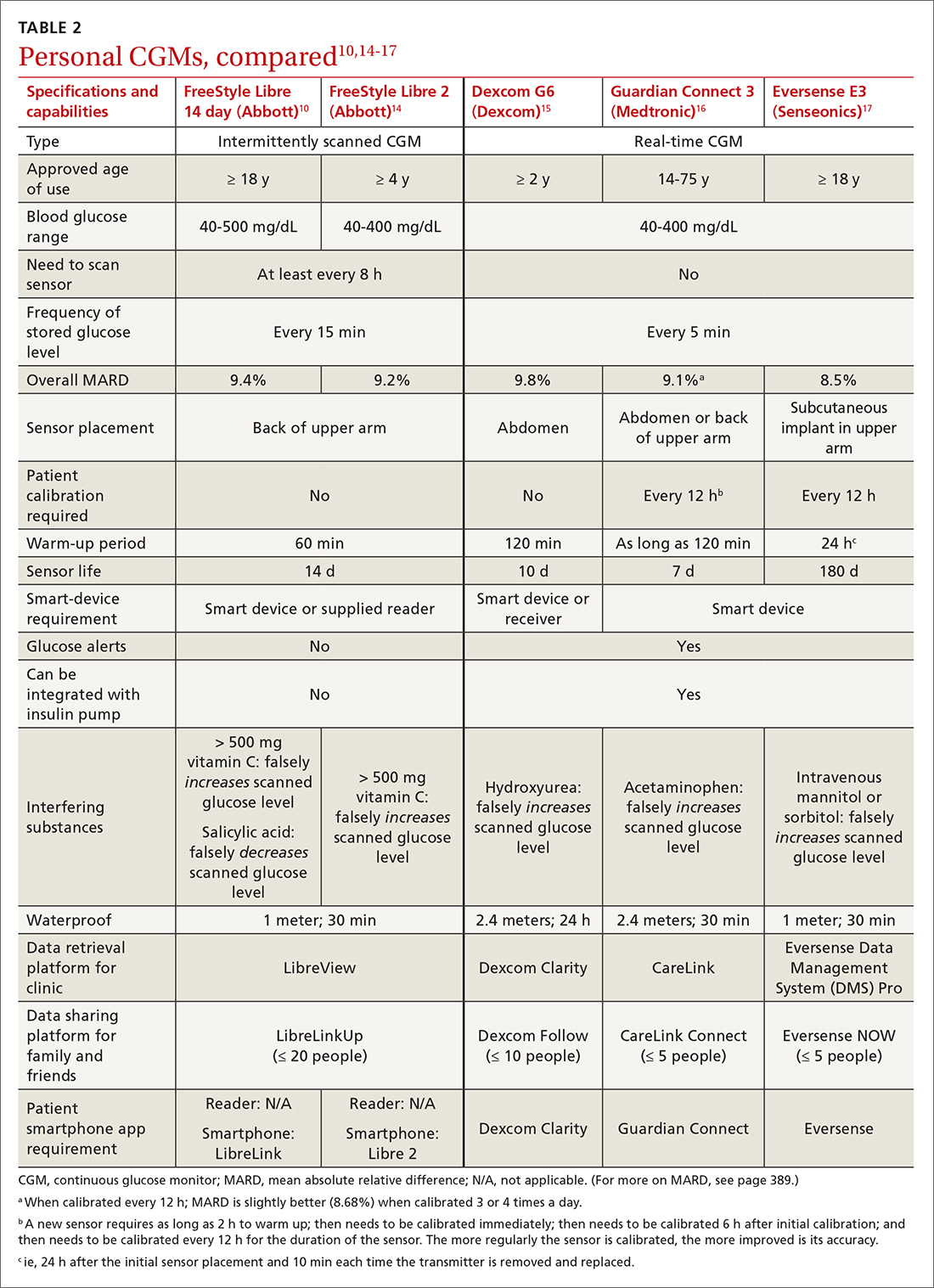
The evidence, reviewed
Clinical outcomes evidence with CGMs in patients with T2D is sparse. Most studies that support improved clinical outcomes enrolled patients with type 1 diabetes who were treated with intensive insulin regimens. Many studies utilized rtCGMs that are capable of incorporating a hypoglycemic alarm, and results might not be generalizable to isCGMs.18,19 In this article, we review only the continuous glucose monitoring literature in which subjects had T2D.
Evidence for is CGMs
The REPLACE trial compared outcomes in patients with T2D who used an isCGM vs those who self-monitored blood glucose (SMBG); both groups were being treated with intensive insulin regimens. Both groups had similar glucose reductions, but the time in the hypoglycemic range (see “Clinical targets,” in the text that follows) was significantly shorter in the isCGM group.20
A randomized controlled trial (RCT) that compared intermittently scanned continuous glucose monitoring and SMBG in patients with T2D who received multiple doses of insulin daily demonstrated a significant A1C reduction of 0.82% with an isCGM and 0.33% with SMBG, with no difference in the rate of hypoglycemic events, over 10 weeks.21
Continue to: Chart review
Chart review. Data extracted from chart reviews in Austria, France, and Germany demonstrated a mean improvement in A1C of 0.9% among patients when using a CGM after using SMBG previously.22
A retrospective review of patients with T2D who were not taking bolus insulin and who used a CGM had a reduction in A1C from 10.1% to 8.6% over 60 to 300 days.23
Evidence for rtCGMs
The DIAMOND study included a subset of patients with T2D who used an rtCGM and were compared to a subset who received usual care. The primary outcome was the change in A1C. A 0.3% greater reduction was observed in the CGM group at 24 weeks. There was no difference in hypoglycemic events between the 2 groups; there were few events in either group.24
An RCT demonstrated a similar reduction in A1C in rtCGM users and in nonusers over 1 year.25 However, patients who used the rtCGM by protocol demonstrated the greatest reduction in A1C. The CGM utilized in this trial required regular fingerstick calibration, which likely led to poorer adherence to protocol than would have been the case had the trial utilized a CGM that did not require calibration.
A prospective trial demonstrated that utilization of an rtCGM only 3 days per month for 3 consecutive months was associated with (1) significant improvement in A1C (a decrease of 1.1% in the CGM group, compared to a decrease of 0.4% in the SMBG group) and (2) numerous lifestyle modifications, including reduction in total caloric intake, weight loss, decreased body mass index, and an increase in total weekly exercise.26 This trial demonstrated that CGMs might be beneficial earlier in the course of disease by reinforcing lifestyle changes.
Continue to: The MOBILE trial
The MOBILE trial demonstrated that use of an rtCGM reduced baseline A1C from 9.1% to 8.0% in the CGM group, compared to 9.0% to 8.4% in the non-CGM group.27
Practical utilization of CGMs
Patient education
Detailed patient education resources—for initial setup, sensor application, methods to ensure appropriate sensor adhesion, and app and platform assistance—are available on each manufacturer’s website.
Clinical targets
In 2019, the Advanced Technologies & Treatments for Diabetes Congress determined that what is known as the time in range metric yields the most practical data to help clinicians manage glycemic control.28 The time in range metric comprises:
- time in the target glucose range (TIR)
- time below the target glucose range (TBR)
- time above the target glucose range (TAR).
TIR glucose ranges are modifiable and based on the A1C goal. For example, if the A1C goal is < 7.0%, the TIR glucose range is 70-180 mg/dL. If a patient maintains TIR > 70% for 3 months, the measured A1C will correlate well with the goal. Each 10% fluctuation in TIR from the goal of 70% corresponds to a difference of approximately 0.5% in A1C. Therefore, TIR of approximately 50% predicts an A1C of 8.0%.28
A retrospective review of 1440 patients with CGM data demonstrated that progression of retinopathy and development of microalbuminuria increased 64% and 40%, respectively, over 10 years for each 10% reduction in TIR—highlighting the importance of TIR and consistent glycemic control.29 Importantly, the CGM sensor must be active ≥ 70% of the wearable time to provide adequate TIR data.30
Continue to: Concerns about accuracy
Concerns about accuracy
There is no universally accepted standard for determining the accuracy of a CGM; however, the mean absolute relative difference (MARD) is the most common statistic referenced. MARD is calculated as the average of the absolute error between all CGM values and matched reference values that are usually obtained from SMBG.31 The lower the MARD percentage, the better the accuracy of the CGM. A MARD of ≤ 10% is considered acceptable for making therapeutic decisions.30
Package labeling for all CGMs recommends that patients have access to a fingerstick glucometer to verify CGM readings when concerns about accuracy exist. If a sensor becomes dislodged, it can malfunction or lose accuracy. Patients should not try to re-apply the sensor; instead, they should remove and discard the sensor and apply a new one. TABLE 210,14-17 compares MARD for CGMs and lists substances that might affect the accuracy of a CGM.
Patient–provider data-sharing platforms
FreeStyle Libre and Libre 2. Providers create a LibreView Practice ID at www.Libre View.com. Patient data-sharing depends on whether they are using a smart device, a reader, or both. Patients can utilize both the smart device and the reader but must upload data from the reader at regular intervals to provide a comprehensive report that will merge data from the smart device (ie, data that have been uploaded automatically) and the reader.7
Dexcom G6. Clinicians create a Dexcom CLARITY account at https://clarity.dexcom.com and add patients to a practice list or gain access to a share code generated by the patient. Patients must download the Dexcom CLARITY app to create an account; once the account is established, readings will be transmitted to the clinic automatically.15 A patient who is utilizing a nonsmart-device reader must upload data manually to their web-based CLARITY account.
Family and caregiver access
Beyond sharing CGM data with clinic staff, an important feature available with FreeStyle Libre and Dexcom systems is the ability to share data with friends and caregivers. The relevant platforms and apps are listed in TABLE 2.10,14-17
Continue to: Insurance coverage, cost, and accessibility
Insurance coverage, cost, and accessibility
Medicare Part B has established criteria by which patients with T2D qualify for a CGM (TABLE 332). A Medicare patient who has been determined to be eligible is responsible for 20% of the out-of-pocket expense of the CGM and supplies once their deductible is met. Once Medicare covers a CGM, the patient is no longer able to obtain fingerstick glucose supplies through Medicare; they must pay the cash price for any fingerstick supplies that are determined to be necessary.32
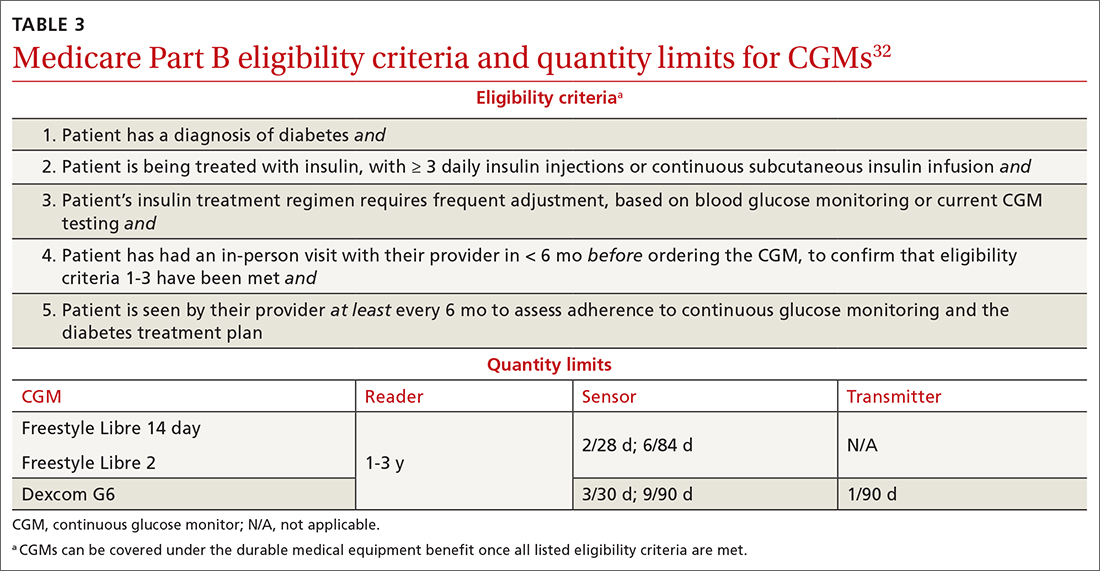
Patients with private insurance can obtain CGM supplies through their preferred pharmacy when the order is written as a prescription (the same as for fingerstick glucometers). That is not the case for patients with Medicare because not all US distributors and pharmacies are contracted to bill Medicare Part B for CGM supplies. A list of distributors and eligible pharmacies can be found on each manufacturer’s website.
Risk–benefit analysis
CGMs are associated with few risks overall. The predominant adverse effect is contact dermatitis; the prevalence of CGM-associated contact dermatitis is difficult to quantify and differs from device to device.
FreeStyle Libre. In a retrospective review of records of patients with diabetes, researchers determined that a cutaneous adverse event occurred in approximately 5.5% of 1036 patients who utilized a FreeStyle Libre sensor.33 Of that percentage, 3.8% of dermatitis cases were determined to be allergic in nature and related to isobornyl acrylate (IBOA), a chemical constituent of the sensor’s adhesive that is not used in the FreeStyle Libre 2. Among patients who wore a sensor and developed allergic contact dermatitis, interventions such as a barrier film were of limited utility in alleviating or preventing further cutaneous eruption.33
Dexcom G6. The prevalence of Dexcom G6–associated allergic contact dermatitis is more difficult to ascertain (the IBOA adhesive was replaced in October 2019) but has been reported to be less common than with FreeStyle Libre,34 a finding that corroborates our anecdotal clinical experience. Although Dexcom sensors no longer contain IBOA, cases of allergic contact dermatitis are still reported.35 We propose that the lower incidence of cutaneous reactions associated with the Dexcom G6 sensor might be due to the absence of IBOA and shorter contact time with skin.
Continue to: In general, patients should be...
In general, patients should be counseled to rotate the location of the sensor and to use only specific barrier products that are recommended on each manufacturer’s website. The use of other barriers that are not specifically recommended might compromise the accuracy of the sensor.
Summing up
As CGM technology improves, it is likely that more and more of your patients will utilize one of these devices. The value of CGMs has been documented, but any endorsement of their use is qualified:
- Data from many older RCTs of patients with T2D who utilize a CGM did not demonstrate a significant reduction in A1C20,24,36; however, real-world observational data do show a greater reduction in A1C.
- From a safety standpoint, contact dermatitis is the primary drawback of CGMs.
- CGMs can provide patients and clinicians with a comprehensive picture of daily glucose trends, which can help patients make lifestyle changes and serve as a positive reinforcement for the effects of diet and exercise. Analysis of glucose trends can also help clinicians confidently make decisions about when to intensify or taper a medication regimen, based on data that is reported more often than 90-day A1C changes.
Health insurance coverage will continue to dictate access to CGM technology for many patients. When a CGM is reimbursable by the patient’s insurance, consider offering it as an option—even for patients who do not require an intensive insulin regimen.
a The US Food and Drug Administration cleared a new Abbott CGM, FreeStyle Libre 3, earlier this year; however, the device is not yet available for purchase. With advances in monitoring technology, several other manufacturers also anticipate introducing novel CGMs. (See “Continuous glucose monitors: The next generation.” )
SIDEBAR
Continuous glucose monitors: The next generation9-13
Expect new continuous glucose monitoring devices to be introduced to US and European health care markets in the near future.
FreeStyle Libre 3 (Abbott) was cleared by the US Food and Drug Administration in May 2022, although it is not yet available for purchase. The manufacturer promotes the device as having the smallest sensor of any continuous glucose monitor (the diameter and thickness of 2 stacked pennies); improved mean absolute relative difference; the ability to provide real-time glucose level readings; and 50% greater range of Bluetooth connectivity (about 10 extra feet).9,10
Dexcom G7 (Dexcom) has a sensor that is 60% smaller than the Dexcom G6 sensor and a 30-minute warm-up time, compared to 120 minutes for the G6.11 The device has received European Union CE mark approval.
Guardian 4 Sensor (Medtronic) does not require fingerstick calibration. The device has also received European Union CE mark approval12 but is available only for investigational use in the United States.
Eversense XL technology is similar to that of the Eversense E3, including a 180-day sensor.13 The device, which has received European Union CE mark approval, includes a removable smart transmitter.
CORRESPONDENCE
Kevin Schleich, PharmD, BCACP, Departments of Pharmaceutical Care and Family Medicine, University of Iowa, 200 Hawkins Drive, 01102-D PFP, Iowa City, IA, 52242; [email protected]
1. Rodríguez-Gutiérrez R, Montori VM. Glycemic control for patients with type 2 diabetes mellitus: our evolving faith in the face of evidence. Circ Cardiovasc Qual Outcomes. 2016;9:504-512. doi: 10.1161/CIRCOUTCOMES.116.002901
2. Draznin B, Aroda VR, Bakris G, et al; . 7. Diabetes technology: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S97-S112. doi: 10.2337/dc22-S007
3. Olczuk D, Priefer R. A history of continuous glucose monitors (CGMs) in self-monitoring of diabetes mellitus. Diabetes Metab Syndr. 2018;12:181-187. doi: 10.1016/j.dsx.2017.09.005
4. Alva S, Bailey T, Brazg R, et al. Accuracy of a 14-day factory-calibrated continuous glucose monitoring system with advanced algorithm in pediatric and adult population with diabetes. J Diabetes Sci Technol. 2022;16:70-77. doi: 10.1177/1932296820958754
5. Zaharieva DP, Turksoy K, McGaugh SM, et al. Lag time remains with newer real-time continuous glucose monitoring technology during aerobic exercise in adults living with type 1 diabetes. Diabetes Technol Ther. 2019;21:313-321. doi: 10.1089/dia.2018.0364
6. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2021. Diabetes Care. 2021;44(suppl 1):S15-S33. doi: 10.2337/dc21-S002
7. FreeStyle Libre systems: The #1 CGM used in the US. Abbott. Updated May 2022. Accessed October 22, 2022. www.freestyleprovider.abbott/us-en/home.html
8. Rowland K. Choosing Wisely: 10 practices to stop—or adopt—to reduce overuse in health care. J Fam Pract. 2020;69:396-400.
9. Tucker ME. FDA clears Abbott Freestyle Libre 3 glucose sensor. MDedge. June 1, 2022. Accessed October 21, 2022. www.mdedge.com/endocrinology/article/255095/diabetes/fda-clears-abbott-freestyle-libre-3-glucose-sensor
10. Manage your diabetes with more confidence. Abbott. Updated May 2022. Accessed October 22, 2022. www.freestyle.abbott/us-en/home.html
11. Whooley S. Dexcom CEO Kevin Sayer says G7 will be ‘wonderful’. Drug Delivery Business News. July 19, 2021. Accessed October 21, 2022. www.drugdeliverybusiness.com/dexcom-ceo-kevin-sayer-says-g7-will-be-wonderful
12. Medtronic secures two CE mark approvals for Guardian 4 Sensor & for InPen MDI Smart Insulin Pen. Medtronic. Press release. May 26, 2021. Accessed October 22, 2022. https://news.medtronic.com/2021-05-26-Medtronic-Secures-Two-CE-Mark-Approvals-for-Guardian-4-Sensor-for-InPen-MDI-Smart-Insulin-Pen
13. Eversense—up to 180 days of freedom [XL CGM System]. Senseonics. Accessed September 14, 2022. https://global.eversensediabetes.com
14. FreeStyle Libre 2 User’s Manual. Abbott. Revised August 24, 2022. Accessed October 2, 2022. https://freestyleserver.com/Payloads/IFU/2022/q3/ART46983-001_rev-A.pdf
15. Dexcom G6 Continuous Glucose Monitoring System user guide. Dexcom. Revised March 2022. Accessed October 21, 2022. https://s3-us-west-2.amazonaws.com/dexcompdf/G6-CGM-Users-Guide.pdf
16. Guardian Connect System user guide. Medtronic. 2020. Accessed October 21, 2022. www.medtronicdiabetes.com/sites/default/files/library/download-library/user-guides/Guardian-Connect-System-User-Guide.pdf
17. Eversense E3 user guides. Senseonics. 2022. Accessed October 22, 2022. www.ascensiadiabetes.com/eversense/user-guides/
18. Battelino T, Conget I, Olsen B, et al; SWITCH Study Group. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012;55:3155-3162. doi: 10.1007/s00125-012-2708-9
19. Weinzimer S, Miller K, Beck R, et al; Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes Research Foundation continuous glucose monitoring (JDRF-CGM) trial. Diabetes Care. 2010;33:17-22. doi: 10.2337/dc09-1502
20. Haak T, Hanaire H, Ajjan R, et al. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8:55-73. doi: 10.1007/s13300-016-0223-6
21. Yaron M, Roitman E, Aharon-Hananel G, et al. Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with type 2 diabetes. Diabetes Care. 2019;42:1178-1184. doi: 10.2337/dc18-0166
22. Kröger J, Fasching P, Hanaire H. Three European retrospective real-world chart review studies to determine the effectiveness of flash glucose monitoring on HbA1c in adults with type 2 diabetes. Diabetes Ther. 2020;11:279-291. doi: 10.1007/s13300-019-00741-9
23. Wright EE, Jr, Kerr MSD, Reyes IJ, et al. Use of flash continuous glucose monitoring is associated with A1C reduction in people with type 2 diabetes treated with basal insulin or noninsulin therapy. Diabetes Spectr. 2021;34:184-189. doi: 10.2337/ds20-0069
24. Beck RW, Riddlesworth TD, Ruedy K, et al; DIAMOND Study Group. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med. 2017;167:365-374. doi: 10.7326/M16-2855
25. Vigersky RA, Fonda SJ, Chellappa M, et al. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care. 2012;35:32-38. doi: 10.2337/dc11-1438
26. Yoo HJ, An HG, Park SY, et al. Use of a real time continuous glucose monitoring system as a motivational device for poorly controlled type 2 diabetes. Diabetes Res Clin Pract. 2008;82:73-79. doi: 10.1016/j.diabres.2008.06.015
27. Martens T, Beck RW, Bailey R, et al; MOBILE Study Group. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA. 2021;325:2262-2272. doi: 10.1001/jama.2021.7444
28. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42:1593-1603. doi: 10.2337/dci19-0028
29. Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019;42:400-405. doi: 10.2337/dc18-1444
30. Freckmann G. Basics and use of continuous glucose monitoring (CGM) in diabetes therapy. Journal of Laboratory Medicine. 2020;44:71-79. doi: 10.1515/labmed-2019-0189
31. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40:1631-1640. doi: 10.2337/dc17-1600
32. Glucose monitors. Centers for Medicare & Medicaid Services. April 22, 2022. Accessed October 22, 2022. www.cms.gov/medicare-coverage-database/view/lcd.aspx?lcdid=33822
33. Pyl J, Dendooven E, Van Eekelen I, et al. Prevalence and prevention of contact dermatitis caused by FreeStyle Libre: a monocentric experience. Diabetes Care. 2020;43:918-920. doi: 10.2337/dc19-1354
34. Smith J, Bleiker T, Narang I. Cutaneous reactions to glucose sensors: a sticky problem [Abstract 677]. Arch Dis Child. 2021;106 (suppl 1):A80.
35. MAUDE Adverse event report: Dexcom, Inc G6 Sensor. U.S. Food & Drug Administration. Updated September 30, 2022. Accessed October 21, 2022. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/detail.cfm?mdrfoi__id=11064819&pc=MDS
36. New JP, Ajjan R, Pfeiffer AFH, et al. Continuous glucose monitoring in people with diabetes: the randomized controlled Glucose Level Awareness in Diabetes Study (GLADIS). Diabet Med. 2015;32:609-617. doi: 10.1111/dme.12713
A1C has been used to estimate 3-month glycemic control in patients with diabetes. However, A1C monitoring alone does not provide insight into daily glycemic variation, which is valuable in clinical management because tight glycemic control (defined as A1C < 7.0%) has been shown to reduce the risk of microvascular complications. Prior to the approval of glucagon-like peptide-1 receptor agonists and sodium-glucose co-transporter 2 inhibitors by the US Food and Drug Administration for the treatment of type 2 diabetes (T2D), reduction in the risk of macrovascular complications (aside from nonfatal myocardial infarction) was more difficult to achieve than it is now; some patients had a worse outcome with overly aggressive glycemic control.1
Previously, the use of a continuous glucose monitor (CGM) was limited to patients with type 1 diabetes who required basal and bolus insulin. However, technological advances have led to more patient-friendly and affordable devices, making CGMs more available. As such, the American Diabetes Association (ADA), in its 2022 Standards of Medical Care in Diabetes, recommends that clinicians offer continuous glucose monitoring to adults with T2D who require multiple daily injections, and based on a given patient’s ability, preferences, and needs.2
In this article, we discuss, first, the intricacies of CGMs and, second, what the evidence says about their use so that physicians can confidently recommend, and educate patients on, effective utilization of CGMs to obtain an individualized target of glycemic control.
Continuous glucose monitoring: A glossary
CGMs are characterized by who possesses the device and how data are recorded. This review is not about professional CGMs, which are owned by the health care provider and consist of a sensor that is applied in the clinic and returned to clinic for downloading of data1; rather, we focus on the novel category of nonprofessional, or personal, CGMs.
Three words to remember. Every CGM has 3 common components:
- The reader (also known as a receiver) is a handheld device that allows a patient to scan a sensor (see definition below) and instantaneously collect a glucose reading. The patient can use a standalone reader; a smartphone or other smart device with an associated app that serves as a reader; or both.
- A sensor is inserted subcutaneously to measure interstitial glucose. The lifespan of a sensor is 10 to 14 days.
- A transmitter relays information from the sensor to the reader.
The technology behind a CGM
CGM sensors measure interstitial glucose by means of a chemical reaction involving glucose oxidase and an oxidation-reduction cofactor, measuring the generation of hydrogen peroxide.3 Interstitial glucose readings lag behind plasma blood glucose readings by 2 to 21 minutes.4,5 Although this lag time is often not clinically significant, situations such as aerobic exercise and a rapidly changing glucose level might warrant confirmation by means of fingerstick measurement.5 It is common for CGM readings to vary slightly from venipuncture or fingerstick glucose readings.
What CGMs are availableto your patients?
Intermittently scanned CGMs (isCGMs) measure the glucose level continuously; the patient must scan a sensor to display and record the glucose level.6 Prolonged periods without scanning result in gaps in glycemic data.7,8
Continue to: Two isCGM systems...
Two isCGM systems are available: the FreeStyle Libre 14 day and the FreeStyle Libre 2 (both from Abbott).a Both consist of a reader and a disposable sensor, applied to the back of the arm, that is worn for 14 days. If the patient has a compatible smartphone or other smart device, the reader can be replaced by the smart device with the downloaded FreeStyle Libre or FreeStyle Libre 2 app.
To activate a new sensor, the patient applies the sensor, then scans it. Once activated, scanning the sensor provides the current glucose reading and recalls the last 8 hours of data. In addition to providing an instantaneous glucose reading, the display also provides a trend arrow indicating the direction and degree to which the glucose level is changing (TABLE 110,14,15). This feature helps patients avoid hypoglycemic episodes by allowing them to preemptively correct if the arrow indicates a rapidly declining glucose level.

For the first 12 hours after a new sensor is activated, and when a glucose reading is < 70 mg/dL, patients should be instructed to avoid making treatment decisions and encouraged to utilize fingerstick glucose readings. FreeStyle Libre 14 day does not allow a glucose level alarm to be set; the system cannot detect these events without scanning the sensor.10 Bluetooth connectivity does allow FreeStyle Libre 2 users to set a glucose alarm if the reader or smart device is within 20 feet of the sensor. A default alarm is set to activate at 70 mg/dL (“low”) and 240 mg/dL (“high”); low and high alarm settings are also customizable. Because both FreeStyle Libre devices store 8 hours of data, patients must scan the sensor every 8 hours for a comprehensive glycemic report.14
FreeStyle Libre CGMs allow patients to add therapy notes, including time and amount of insulin administered and carbohydrates ingested. Readers for both devices function as a glucometer that is compatible with Abbott FreeStyle Precision Neo test strips.
Real-time CGMs (rtCGMs) measure and display glucose levels continuously for the duration of the life of the sensor, without the need to scan. Three rtCGM systems are available: Dexcom G6, Medtronic Guardian 3, and Senseonics Eversense E3.
Continue to: Dexcom G6...
Dexcom G6 is the first Dexcom CGM that does not require fingerstick calibration and the only rtCGM available in the United States that does not require patient calibration. This system comprises a single-use sensor replaced every 10 days; a transmitter that is transferred to each new sensor and replaced every 3 months; and an optional receiver that can be omitted if the patient prefers to utilize a smart device.
Dexcom G6 never requires a patient to scan a sensor. Instead, the receiver (or smart device) utilizes Bluetooth technology to obtain blood glucose readings if it is positioned within 20 feet of the transmitter. Patients can set both hypoglycemic and hyperglycemic alarms to predict events within 20 minutes. Similar to the functionality of the FreeStyle Libre systems, Dexcom G6 provides the opportunity to log lifestyle events, including insulin dosing, carbohydrate ingestion, exercise, and sick days.15
Medtronic Guardian 3 comprises the multi-use Guardian Connect Transmitter that is replaced annually and a single-use Guardian Sensor that is replaced every 7 days. Guardian 3 requires twice-daily fingerstick glucose calibration, which reduces the convenience of a CGM.
Guardian 3 allows the user to set alarm levels, providing predictive alerts 10 to 60 minutes before set glucose levels are reached. Patients must utilize a smart device to connect through Bluetooth to the CareLink Connect app and remain within 20 feet of the transmitter to provide continuous glucose readings. The CareLink Connect app allows patients to document exercise, calibration of fingerstick readings, meals, and insulin administration.16
Senseonics Eversense E3 consists of a 3.5 mm × 18.3 mm sensor inserted subcutaneously in the upper arm once every 180 days; a removable transmitter that attaches to an adhesive patch placed over the sensor; and a smart device with the Eversense app. The transmitter has a 1-year rechargeable battery and provides the patient with on-body vibration alerts even when they are not near their smart device.
Continue to: The Eversense E3 transmitter...
The Eversense E3 transmitter can be removed and reapplied without affecting the life of the sensor; however, no glucose data will be collected during this time. Once the transmitter is reapplied, it takes 10 minutes for the sensor to begin communicating with the transmitter. Eversense provides predictive alerts as long as 30 minutes before hyperglycemic or hypoglycemic events. The device requires twice-daily fingerstick calibrations.17
A comparison of the specifications and capabilities of the personal CGMs discussed here is provided in TABLE 2.10,14-17

The evidence, reviewed
Clinical outcomes evidence with CGMs in patients with T2D is sparse. Most studies that support improved clinical outcomes enrolled patients with type 1 diabetes who were treated with intensive insulin regimens. Many studies utilized rtCGMs that are capable of incorporating a hypoglycemic alarm, and results might not be generalizable to isCGMs.18,19 In this article, we review only the continuous glucose monitoring literature in which subjects had T2D.
Evidence for is CGMs
The REPLACE trial compared outcomes in patients with T2D who used an isCGM vs those who self-monitored blood glucose (SMBG); both groups were being treated with intensive insulin regimens. Both groups had similar glucose reductions, but the time in the hypoglycemic range (see “Clinical targets,” in the text that follows) was significantly shorter in the isCGM group.20
A randomized controlled trial (RCT) that compared intermittently scanned continuous glucose monitoring and SMBG in patients with T2D who received multiple doses of insulin daily demonstrated a significant A1C reduction of 0.82% with an isCGM and 0.33% with SMBG, with no difference in the rate of hypoglycemic events, over 10 weeks.21
Continue to: Chart review
Chart review. Data extracted from chart reviews in Austria, France, and Germany demonstrated a mean improvement in A1C of 0.9% among patients when using a CGM after using SMBG previously.22
A retrospective review of patients with T2D who were not taking bolus insulin and who used a CGM had a reduction in A1C from 10.1% to 8.6% over 60 to 300 days.23
Evidence for rtCGMs
The DIAMOND study included a subset of patients with T2D who used an rtCGM and were compared to a subset who received usual care. The primary outcome was the change in A1C. A 0.3% greater reduction was observed in the CGM group at 24 weeks. There was no difference in hypoglycemic events between the 2 groups; there were few events in either group.24
An RCT demonstrated a similar reduction in A1C in rtCGM users and in nonusers over 1 year.25 However, patients who used the rtCGM by protocol demonstrated the greatest reduction in A1C. The CGM utilized in this trial required regular fingerstick calibration, which likely led to poorer adherence to protocol than would have been the case had the trial utilized a CGM that did not require calibration.
A prospective trial demonstrated that utilization of an rtCGM only 3 days per month for 3 consecutive months was associated with (1) significant improvement in A1C (a decrease of 1.1% in the CGM group, compared to a decrease of 0.4% in the SMBG group) and (2) numerous lifestyle modifications, including reduction in total caloric intake, weight loss, decreased body mass index, and an increase in total weekly exercise.26 This trial demonstrated that CGMs might be beneficial earlier in the course of disease by reinforcing lifestyle changes.
Continue to: The MOBILE trial
The MOBILE trial demonstrated that use of an rtCGM reduced baseline A1C from 9.1% to 8.0% in the CGM group, compared to 9.0% to 8.4% in the non-CGM group.27
Practical utilization of CGMs
Patient education
Detailed patient education resources—for initial setup, sensor application, methods to ensure appropriate sensor adhesion, and app and platform assistance—are available on each manufacturer’s website.
Clinical targets
In 2019, the Advanced Technologies & Treatments for Diabetes Congress determined that what is known as the time in range metric yields the most practical data to help clinicians manage glycemic control.28 The time in range metric comprises:
- time in the target glucose range (TIR)
- time below the target glucose range (TBR)
- time above the target glucose range (TAR).
TIR glucose ranges are modifiable and based on the A1C goal. For example, if the A1C goal is < 7.0%, the TIR glucose range is 70-180 mg/dL. If a patient maintains TIR > 70% for 3 months, the measured A1C will correlate well with the goal. Each 10% fluctuation in TIR from the goal of 70% corresponds to a difference of approximately 0.5% in A1C. Therefore, TIR of approximately 50% predicts an A1C of 8.0%.28
A retrospective review of 1440 patients with CGM data demonstrated that progression of retinopathy and development of microalbuminuria increased 64% and 40%, respectively, over 10 years for each 10% reduction in TIR—highlighting the importance of TIR and consistent glycemic control.29 Importantly, the CGM sensor must be active ≥ 70% of the wearable time to provide adequate TIR data.30
Continue to: Concerns about accuracy
Concerns about accuracy
There is no universally accepted standard for determining the accuracy of a CGM; however, the mean absolute relative difference (MARD) is the most common statistic referenced. MARD is calculated as the average of the absolute error between all CGM values and matched reference values that are usually obtained from SMBG.31 The lower the MARD percentage, the better the accuracy of the CGM. A MARD of ≤ 10% is considered acceptable for making therapeutic decisions.30
Package labeling for all CGMs recommends that patients have access to a fingerstick glucometer to verify CGM readings when concerns about accuracy exist. If a sensor becomes dislodged, it can malfunction or lose accuracy. Patients should not try to re-apply the sensor; instead, they should remove and discard the sensor and apply a new one. TABLE 210,14-17 compares MARD for CGMs and lists substances that might affect the accuracy of a CGM.
Patient–provider data-sharing platforms
FreeStyle Libre and Libre 2. Providers create a LibreView Practice ID at www.Libre View.com. Patient data-sharing depends on whether they are using a smart device, a reader, or both. Patients can utilize both the smart device and the reader but must upload data from the reader at regular intervals to provide a comprehensive report that will merge data from the smart device (ie, data that have been uploaded automatically) and the reader.7
Dexcom G6. Clinicians create a Dexcom CLARITY account at https://clarity.dexcom.com and add patients to a practice list or gain access to a share code generated by the patient. Patients must download the Dexcom CLARITY app to create an account; once the account is established, readings will be transmitted to the clinic automatically.15 A patient who is utilizing a nonsmart-device reader must upload data manually to their web-based CLARITY account.
Family and caregiver access
Beyond sharing CGM data with clinic staff, an important feature available with FreeStyle Libre and Dexcom systems is the ability to share data with friends and caregivers. The relevant platforms and apps are listed in TABLE 2.10,14-17
Continue to: Insurance coverage, cost, and accessibility
Insurance coverage, cost, and accessibility
Medicare Part B has established criteria by which patients with T2D qualify for a CGM (TABLE 332). A Medicare patient who has been determined to be eligible is responsible for 20% of the out-of-pocket expense of the CGM and supplies once their deductible is met. Once Medicare covers a CGM, the patient is no longer able to obtain fingerstick glucose supplies through Medicare; they must pay the cash price for any fingerstick supplies that are determined to be necessary.32

Patients with private insurance can obtain CGM supplies through their preferred pharmacy when the order is written as a prescription (the same as for fingerstick glucometers). That is not the case for patients with Medicare because not all US distributors and pharmacies are contracted to bill Medicare Part B for CGM supplies. A list of distributors and eligible pharmacies can be found on each manufacturer’s website.
Risk–benefit analysis
CGMs are associated with few risks overall. The predominant adverse effect is contact dermatitis; the prevalence of CGM-associated contact dermatitis is difficult to quantify and differs from device to device.
FreeStyle Libre. In a retrospective review of records of patients with diabetes, researchers determined that a cutaneous adverse event occurred in approximately 5.5% of 1036 patients who utilized a FreeStyle Libre sensor.33 Of that percentage, 3.8% of dermatitis cases were determined to be allergic in nature and related to isobornyl acrylate (IBOA), a chemical constituent of the sensor’s adhesive that is not used in the FreeStyle Libre 2. Among patients who wore a sensor and developed allergic contact dermatitis, interventions such as a barrier film were of limited utility in alleviating or preventing further cutaneous eruption.33
Dexcom G6. The prevalence of Dexcom G6–associated allergic contact dermatitis is more difficult to ascertain (the IBOA adhesive was replaced in October 2019) but has been reported to be less common than with FreeStyle Libre,34 a finding that corroborates our anecdotal clinical experience. Although Dexcom sensors no longer contain IBOA, cases of allergic contact dermatitis are still reported.35 We propose that the lower incidence of cutaneous reactions associated with the Dexcom G6 sensor might be due to the absence of IBOA and shorter contact time with skin.
Continue to: In general, patients should be...
In general, patients should be counseled to rotate the location of the sensor and to use only specific barrier products that are recommended on each manufacturer’s website. The use of other barriers that are not specifically recommended might compromise the accuracy of the sensor.
Summing up
As CGM technology improves, it is likely that more and more of your patients will utilize one of these devices. The value of CGMs has been documented, but any endorsement of their use is qualified:
- Data from many older RCTs of patients with T2D who utilize a CGM did not demonstrate a significant reduction in A1C20,24,36; however, real-world observational data do show a greater reduction in A1C.
- From a safety standpoint, contact dermatitis is the primary drawback of CGMs.
- CGMs can provide patients and clinicians with a comprehensive picture of daily glucose trends, which can help patients make lifestyle changes and serve as a positive reinforcement for the effects of diet and exercise. Analysis of glucose trends can also help clinicians confidently make decisions about when to intensify or taper a medication regimen, based on data that is reported more often than 90-day A1C changes.
Health insurance coverage will continue to dictate access to CGM technology for many patients. When a CGM is reimbursable by the patient’s insurance, consider offering it as an option—even for patients who do not require an intensive insulin regimen.
a The US Food and Drug Administration cleared a new Abbott CGM, FreeStyle Libre 3, earlier this year; however, the device is not yet available for purchase. With advances in monitoring technology, several other manufacturers also anticipate introducing novel CGMs. (See “Continuous glucose monitors: The next generation.” )
SIDEBAR
Continuous glucose monitors: The next generation9-13
Expect new continuous glucose monitoring devices to be introduced to US and European health care markets in the near future.
FreeStyle Libre 3 (Abbott) was cleared by the US Food and Drug Administration in May 2022, although it is not yet available for purchase. The manufacturer promotes the device as having the smallest sensor of any continuous glucose monitor (the diameter and thickness of 2 stacked pennies); improved mean absolute relative difference; the ability to provide real-time glucose level readings; and 50% greater range of Bluetooth connectivity (about 10 extra feet).9,10
Dexcom G7 (Dexcom) has a sensor that is 60% smaller than the Dexcom G6 sensor and a 30-minute warm-up time, compared to 120 minutes for the G6.11 The device has received European Union CE mark approval.
Guardian 4 Sensor (Medtronic) does not require fingerstick calibration. The device has also received European Union CE mark approval12 but is available only for investigational use in the United States.
Eversense XL technology is similar to that of the Eversense E3, including a 180-day sensor.13 The device, which has received European Union CE mark approval, includes a removable smart transmitter.
CORRESPONDENCE
Kevin Schleich, PharmD, BCACP, Departments of Pharmaceutical Care and Family Medicine, University of Iowa, 200 Hawkins Drive, 01102-D PFP, Iowa City, IA, 52242; [email protected]
A1C has been used to estimate 3-month glycemic control in patients with diabetes. However, A1C monitoring alone does not provide insight into daily glycemic variation, which is valuable in clinical management because tight glycemic control (defined as A1C < 7.0%) has been shown to reduce the risk of microvascular complications. Prior to the approval of glucagon-like peptide-1 receptor agonists and sodium-glucose co-transporter 2 inhibitors by the US Food and Drug Administration for the treatment of type 2 diabetes (T2D), reduction in the risk of macrovascular complications (aside from nonfatal myocardial infarction) was more difficult to achieve than it is now; some patients had a worse outcome with overly aggressive glycemic control.1
Previously, the use of a continuous glucose monitor (CGM) was limited to patients with type 1 diabetes who required basal and bolus insulin. However, technological advances have led to more patient-friendly and affordable devices, making CGMs more available. As such, the American Diabetes Association (ADA), in its 2022 Standards of Medical Care in Diabetes, recommends that clinicians offer continuous glucose monitoring to adults with T2D who require multiple daily injections, and based on a given patient’s ability, preferences, and needs.2
In this article, we discuss, first, the intricacies of CGMs and, second, what the evidence says about their use so that physicians can confidently recommend, and educate patients on, effective utilization of CGMs to obtain an individualized target of glycemic control.
Continuous glucose monitoring: A glossary
CGMs are characterized by who possesses the device and how data are recorded. This review is not about professional CGMs, which are owned by the health care provider and consist of a sensor that is applied in the clinic and returned to clinic for downloading of data1; rather, we focus on the novel category of nonprofessional, or personal, CGMs.
Three words to remember. Every CGM has 3 common components:
- The reader (also known as a receiver) is a handheld device that allows a patient to scan a sensor (see definition below) and instantaneously collect a glucose reading. The patient can use a standalone reader; a smartphone or other smart device with an associated app that serves as a reader; or both.
- A sensor is inserted subcutaneously to measure interstitial glucose. The lifespan of a sensor is 10 to 14 days.
- A transmitter relays information from the sensor to the reader.
The technology behind a CGM
CGM sensors measure interstitial glucose by means of a chemical reaction involving glucose oxidase and an oxidation-reduction cofactor, measuring the generation of hydrogen peroxide.3 Interstitial glucose readings lag behind plasma blood glucose readings by 2 to 21 minutes.4,5 Although this lag time is often not clinically significant, situations such as aerobic exercise and a rapidly changing glucose level might warrant confirmation by means of fingerstick measurement.5 It is common for CGM readings to vary slightly from venipuncture or fingerstick glucose readings.
What CGMs are availableto your patients?
Intermittently scanned CGMs (isCGMs) measure the glucose level continuously; the patient must scan a sensor to display and record the glucose level.6 Prolonged periods without scanning result in gaps in glycemic data.7,8
Continue to: Two isCGM systems...
Two isCGM systems are available: the FreeStyle Libre 14 day and the FreeStyle Libre 2 (both from Abbott).a Both consist of a reader and a disposable sensor, applied to the back of the arm, that is worn for 14 days. If the patient has a compatible smartphone or other smart device, the reader can be replaced by the smart device with the downloaded FreeStyle Libre or FreeStyle Libre 2 app.
To activate a new sensor, the patient applies the sensor, then scans it. Once activated, scanning the sensor provides the current glucose reading and recalls the last 8 hours of data. In addition to providing an instantaneous glucose reading, the display also provides a trend arrow indicating the direction and degree to which the glucose level is changing (TABLE 110,14,15). This feature helps patients avoid hypoglycemic episodes by allowing them to preemptively correct if the arrow indicates a rapidly declining glucose level.

For the first 12 hours after a new sensor is activated, and when a glucose reading is < 70 mg/dL, patients should be instructed to avoid making treatment decisions and encouraged to utilize fingerstick glucose readings. FreeStyle Libre 14 day does not allow a glucose level alarm to be set; the system cannot detect these events without scanning the sensor.10 Bluetooth connectivity does allow FreeStyle Libre 2 users to set a glucose alarm if the reader or smart device is within 20 feet of the sensor. A default alarm is set to activate at 70 mg/dL (“low”) and 240 mg/dL (“high”); low and high alarm settings are also customizable. Because both FreeStyle Libre devices store 8 hours of data, patients must scan the sensor every 8 hours for a comprehensive glycemic report.14
FreeStyle Libre CGMs allow patients to add therapy notes, including time and amount of insulin administered and carbohydrates ingested. Readers for both devices function as a glucometer that is compatible with Abbott FreeStyle Precision Neo test strips.
Real-time CGMs (rtCGMs) measure and display glucose levels continuously for the duration of the life of the sensor, without the need to scan. Three rtCGM systems are available: Dexcom G6, Medtronic Guardian 3, and Senseonics Eversense E3.
Continue to: Dexcom G6...
Dexcom G6 is the first Dexcom CGM that does not require fingerstick calibration and the only rtCGM available in the United States that does not require patient calibration. This system comprises a single-use sensor replaced every 10 days; a transmitter that is transferred to each new sensor and replaced every 3 months; and an optional receiver that can be omitted if the patient prefers to utilize a smart device.
Dexcom G6 never requires a patient to scan a sensor. Instead, the receiver (or smart device) utilizes Bluetooth technology to obtain blood glucose readings if it is positioned within 20 feet of the transmitter. Patients can set both hypoglycemic and hyperglycemic alarms to predict events within 20 minutes. Similar to the functionality of the FreeStyle Libre systems, Dexcom G6 provides the opportunity to log lifestyle events, including insulin dosing, carbohydrate ingestion, exercise, and sick days.15
Medtronic Guardian 3 comprises the multi-use Guardian Connect Transmitter that is replaced annually and a single-use Guardian Sensor that is replaced every 7 days. Guardian 3 requires twice-daily fingerstick glucose calibration, which reduces the convenience of a CGM.
Guardian 3 allows the user to set alarm levels, providing predictive alerts 10 to 60 minutes before set glucose levels are reached. Patients must utilize a smart device to connect through Bluetooth to the CareLink Connect app and remain within 20 feet of the transmitter to provide continuous glucose readings. The CareLink Connect app allows patients to document exercise, calibration of fingerstick readings, meals, and insulin administration.16
Senseonics Eversense E3 consists of a 3.5 mm × 18.3 mm sensor inserted subcutaneously in the upper arm once every 180 days; a removable transmitter that attaches to an adhesive patch placed over the sensor; and a smart device with the Eversense app. The transmitter has a 1-year rechargeable battery and provides the patient with on-body vibration alerts even when they are not near their smart device.
Continue to: The Eversense E3 transmitter...
The Eversense E3 transmitter can be removed and reapplied without affecting the life of the sensor; however, no glucose data will be collected during this time. Once the transmitter is reapplied, it takes 10 minutes for the sensor to begin communicating with the transmitter. Eversense provides predictive alerts as long as 30 minutes before hyperglycemic or hypoglycemic events. The device requires twice-daily fingerstick calibrations.17
A comparison of the specifications and capabilities of the personal CGMs discussed here is provided in TABLE 2.10,14-17

The evidence, reviewed
Clinical outcomes evidence with CGMs in patients with T2D is sparse. Most studies that support improved clinical outcomes enrolled patients with type 1 diabetes who were treated with intensive insulin regimens. Many studies utilized rtCGMs that are capable of incorporating a hypoglycemic alarm, and results might not be generalizable to isCGMs.18,19 In this article, we review only the continuous glucose monitoring literature in which subjects had T2D.
Evidence for is CGMs
The REPLACE trial compared outcomes in patients with T2D who used an isCGM vs those who self-monitored blood glucose (SMBG); both groups were being treated with intensive insulin regimens. Both groups had similar glucose reductions, but the time in the hypoglycemic range (see “Clinical targets,” in the text that follows) was significantly shorter in the isCGM group.20
A randomized controlled trial (RCT) that compared intermittently scanned continuous glucose monitoring and SMBG in patients with T2D who received multiple doses of insulin daily demonstrated a significant A1C reduction of 0.82% with an isCGM and 0.33% with SMBG, with no difference in the rate of hypoglycemic events, over 10 weeks.21
Continue to: Chart review
Chart review. Data extracted from chart reviews in Austria, France, and Germany demonstrated a mean improvement in A1C of 0.9% among patients when using a CGM after using SMBG previously.22
A retrospective review of patients with T2D who were not taking bolus insulin and who used a CGM had a reduction in A1C from 10.1% to 8.6% over 60 to 300 days.23
Evidence for rtCGMs
The DIAMOND study included a subset of patients with T2D who used an rtCGM and were compared to a subset who received usual care. The primary outcome was the change in A1C. A 0.3% greater reduction was observed in the CGM group at 24 weeks. There was no difference in hypoglycemic events between the 2 groups; there were few events in either group.24
An RCT demonstrated a similar reduction in A1C in rtCGM users and in nonusers over 1 year.25 However, patients who used the rtCGM by protocol demonstrated the greatest reduction in A1C. The CGM utilized in this trial required regular fingerstick calibration, which likely led to poorer adherence to protocol than would have been the case had the trial utilized a CGM that did not require calibration.
A prospective trial demonstrated that utilization of an rtCGM only 3 days per month for 3 consecutive months was associated with (1) significant improvement in A1C (a decrease of 1.1% in the CGM group, compared to a decrease of 0.4% in the SMBG group) and (2) numerous lifestyle modifications, including reduction in total caloric intake, weight loss, decreased body mass index, and an increase in total weekly exercise.26 This trial demonstrated that CGMs might be beneficial earlier in the course of disease by reinforcing lifestyle changes.
Continue to: The MOBILE trial
The MOBILE trial demonstrated that use of an rtCGM reduced baseline A1C from 9.1% to 8.0% in the CGM group, compared to 9.0% to 8.4% in the non-CGM group.27
Practical utilization of CGMs
Patient education
Detailed patient education resources—for initial setup, sensor application, methods to ensure appropriate sensor adhesion, and app and platform assistance—are available on each manufacturer’s website.
Clinical targets
In 2019, the Advanced Technologies & Treatments for Diabetes Congress determined that what is known as the time in range metric yields the most practical data to help clinicians manage glycemic control.28 The time in range metric comprises:
- time in the target glucose range (TIR)
- time below the target glucose range (TBR)
- time above the target glucose range (TAR).
TIR glucose ranges are modifiable and based on the A1C goal. For example, if the A1C goal is < 7.0%, the TIR glucose range is 70-180 mg/dL. If a patient maintains TIR > 70% for 3 months, the measured A1C will correlate well with the goal. Each 10% fluctuation in TIR from the goal of 70% corresponds to a difference of approximately 0.5% in A1C. Therefore, TIR of approximately 50% predicts an A1C of 8.0%.28
A retrospective review of 1440 patients with CGM data demonstrated that progression of retinopathy and development of microalbuminuria increased 64% and 40%, respectively, over 10 years for each 10% reduction in TIR—highlighting the importance of TIR and consistent glycemic control.29 Importantly, the CGM sensor must be active ≥ 70% of the wearable time to provide adequate TIR data.30
Continue to: Concerns about accuracy
Concerns about accuracy
There is no universally accepted standard for determining the accuracy of a CGM; however, the mean absolute relative difference (MARD) is the most common statistic referenced. MARD is calculated as the average of the absolute error between all CGM values and matched reference values that are usually obtained from SMBG.31 The lower the MARD percentage, the better the accuracy of the CGM. A MARD of ≤ 10% is considered acceptable for making therapeutic decisions.30
Package labeling for all CGMs recommends that patients have access to a fingerstick glucometer to verify CGM readings when concerns about accuracy exist. If a sensor becomes dislodged, it can malfunction or lose accuracy. Patients should not try to re-apply the sensor; instead, they should remove and discard the sensor and apply a new one. TABLE 210,14-17 compares MARD for CGMs and lists substances that might affect the accuracy of a CGM.
Patient–provider data-sharing platforms
FreeStyle Libre and Libre 2. Providers create a LibreView Practice ID at www.Libre View.com. Patient data-sharing depends on whether they are using a smart device, a reader, or both. Patients can utilize both the smart device and the reader but must upload data from the reader at regular intervals to provide a comprehensive report that will merge data from the smart device (ie, data that have been uploaded automatically) and the reader.7
Dexcom G6. Clinicians create a Dexcom CLARITY account at https://clarity.dexcom.com and add patients to a practice list or gain access to a share code generated by the patient. Patients must download the Dexcom CLARITY app to create an account; once the account is established, readings will be transmitted to the clinic automatically.15 A patient who is utilizing a nonsmart-device reader must upload data manually to their web-based CLARITY account.
Family and caregiver access
Beyond sharing CGM data with clinic staff, an important feature available with FreeStyle Libre and Dexcom systems is the ability to share data with friends and caregivers. The relevant platforms and apps are listed in TABLE 2.10,14-17
Continue to: Insurance coverage, cost, and accessibility
Insurance coverage, cost, and accessibility
Medicare Part B has established criteria by which patients with T2D qualify for a CGM (TABLE 332). A Medicare patient who has been determined to be eligible is responsible for 20% of the out-of-pocket expense of the CGM and supplies once their deductible is met. Once Medicare covers a CGM, the patient is no longer able to obtain fingerstick glucose supplies through Medicare; they must pay the cash price for any fingerstick supplies that are determined to be necessary.32

Patients with private insurance can obtain CGM supplies through their preferred pharmacy when the order is written as a prescription (the same as for fingerstick glucometers). That is not the case for patients with Medicare because not all US distributors and pharmacies are contracted to bill Medicare Part B for CGM supplies. A list of distributors and eligible pharmacies can be found on each manufacturer’s website.
Risk–benefit analysis
CGMs are associated with few risks overall. The predominant adverse effect is contact dermatitis; the prevalence of CGM-associated contact dermatitis is difficult to quantify and differs from device to device.
FreeStyle Libre. In a retrospective review of records of patients with diabetes, researchers determined that a cutaneous adverse event occurred in approximately 5.5% of 1036 patients who utilized a FreeStyle Libre sensor.33 Of that percentage, 3.8% of dermatitis cases were determined to be allergic in nature and related to isobornyl acrylate (IBOA), a chemical constituent of the sensor’s adhesive that is not used in the FreeStyle Libre 2. Among patients who wore a sensor and developed allergic contact dermatitis, interventions such as a barrier film were of limited utility in alleviating or preventing further cutaneous eruption.33
Dexcom G6. The prevalence of Dexcom G6–associated allergic contact dermatitis is more difficult to ascertain (the IBOA adhesive was replaced in October 2019) but has been reported to be less common than with FreeStyle Libre,34 a finding that corroborates our anecdotal clinical experience. Although Dexcom sensors no longer contain IBOA, cases of allergic contact dermatitis are still reported.35 We propose that the lower incidence of cutaneous reactions associated with the Dexcom G6 sensor might be due to the absence of IBOA and shorter contact time with skin.
Continue to: In general, patients should be...
In general, patients should be counseled to rotate the location of the sensor and to use only specific barrier products that are recommended on each manufacturer’s website. The use of other barriers that are not specifically recommended might compromise the accuracy of the sensor.
Summing up
As CGM technology improves, it is likely that more and more of your patients will utilize one of these devices. The value of CGMs has been documented, but any endorsement of their use is qualified:
- Data from many older RCTs of patients with T2D who utilize a CGM did not demonstrate a significant reduction in A1C20,24,36; however, real-world observational data do show a greater reduction in A1C.
- From a safety standpoint, contact dermatitis is the primary drawback of CGMs.
- CGMs can provide patients and clinicians with a comprehensive picture of daily glucose trends, which can help patients make lifestyle changes and serve as a positive reinforcement for the effects of diet and exercise. Analysis of glucose trends can also help clinicians confidently make decisions about when to intensify or taper a medication regimen, based on data that is reported more often than 90-day A1C changes.
Health insurance coverage will continue to dictate access to CGM technology for many patients. When a CGM is reimbursable by the patient’s insurance, consider offering it as an option—even for patients who do not require an intensive insulin regimen.
a The US Food and Drug Administration cleared a new Abbott CGM, FreeStyle Libre 3, earlier this year; however, the device is not yet available for purchase. With advances in monitoring technology, several other manufacturers also anticipate introducing novel CGMs. (See “Continuous glucose monitors: The next generation.” )
SIDEBAR
Continuous glucose monitors: The next generation9-13
Expect new continuous glucose monitoring devices to be introduced to US and European health care markets in the near future.
FreeStyle Libre 3 (Abbott) was cleared by the US Food and Drug Administration in May 2022, although it is not yet available for purchase. The manufacturer promotes the device as having the smallest sensor of any continuous glucose monitor (the diameter and thickness of 2 stacked pennies); improved mean absolute relative difference; the ability to provide real-time glucose level readings; and 50% greater range of Bluetooth connectivity (about 10 extra feet).9,10
Dexcom G7 (Dexcom) has a sensor that is 60% smaller than the Dexcom G6 sensor and a 30-minute warm-up time, compared to 120 minutes for the G6.11 The device has received European Union CE mark approval.
Guardian 4 Sensor (Medtronic) does not require fingerstick calibration. The device has also received European Union CE mark approval12 but is available only for investigational use in the United States.
Eversense XL technology is similar to that of the Eversense E3, including a 180-day sensor.13 The device, which has received European Union CE mark approval, includes a removable smart transmitter.
CORRESPONDENCE
Kevin Schleich, PharmD, BCACP, Departments of Pharmaceutical Care and Family Medicine, University of Iowa, 200 Hawkins Drive, 01102-D PFP, Iowa City, IA, 52242; [email protected]
1. Rodríguez-Gutiérrez R, Montori VM. Glycemic control for patients with type 2 diabetes mellitus: our evolving faith in the face of evidence. Circ Cardiovasc Qual Outcomes. 2016;9:504-512. doi: 10.1161/CIRCOUTCOMES.116.002901
2. Draznin B, Aroda VR, Bakris G, et al; . 7. Diabetes technology: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S97-S112. doi: 10.2337/dc22-S007
3. Olczuk D, Priefer R. A history of continuous glucose monitors (CGMs) in self-monitoring of diabetes mellitus. Diabetes Metab Syndr. 2018;12:181-187. doi: 10.1016/j.dsx.2017.09.005
4. Alva S, Bailey T, Brazg R, et al. Accuracy of a 14-day factory-calibrated continuous glucose monitoring system with advanced algorithm in pediatric and adult population with diabetes. J Diabetes Sci Technol. 2022;16:70-77. doi: 10.1177/1932296820958754
5. Zaharieva DP, Turksoy K, McGaugh SM, et al. Lag time remains with newer real-time continuous glucose monitoring technology during aerobic exercise in adults living with type 1 diabetes. Diabetes Technol Ther. 2019;21:313-321. doi: 10.1089/dia.2018.0364
6. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2021. Diabetes Care. 2021;44(suppl 1):S15-S33. doi: 10.2337/dc21-S002
7. FreeStyle Libre systems: The #1 CGM used in the US. Abbott. Updated May 2022. Accessed October 22, 2022. www.freestyleprovider.abbott/us-en/home.html
8. Rowland K. Choosing Wisely: 10 practices to stop—or adopt—to reduce overuse in health care. J Fam Pract. 2020;69:396-400.
9. Tucker ME. FDA clears Abbott Freestyle Libre 3 glucose sensor. MDedge. June 1, 2022. Accessed October 21, 2022. www.mdedge.com/endocrinology/article/255095/diabetes/fda-clears-abbott-freestyle-libre-3-glucose-sensor
10. Manage your diabetes with more confidence. Abbott. Updated May 2022. Accessed October 22, 2022. www.freestyle.abbott/us-en/home.html
11. Whooley S. Dexcom CEO Kevin Sayer says G7 will be ‘wonderful’. Drug Delivery Business News. July 19, 2021. Accessed October 21, 2022. www.drugdeliverybusiness.com/dexcom-ceo-kevin-sayer-says-g7-will-be-wonderful
12. Medtronic secures two CE mark approvals for Guardian 4 Sensor & for InPen MDI Smart Insulin Pen. Medtronic. Press release. May 26, 2021. Accessed October 22, 2022. https://news.medtronic.com/2021-05-26-Medtronic-Secures-Two-CE-Mark-Approvals-for-Guardian-4-Sensor-for-InPen-MDI-Smart-Insulin-Pen
13. Eversense—up to 180 days of freedom [XL CGM System]. Senseonics. Accessed September 14, 2022. https://global.eversensediabetes.com
14. FreeStyle Libre 2 User’s Manual. Abbott. Revised August 24, 2022. Accessed October 2, 2022. https://freestyleserver.com/Payloads/IFU/2022/q3/ART46983-001_rev-A.pdf
15. Dexcom G6 Continuous Glucose Monitoring System user guide. Dexcom. Revised March 2022. Accessed October 21, 2022. https://s3-us-west-2.amazonaws.com/dexcompdf/G6-CGM-Users-Guide.pdf
16. Guardian Connect System user guide. Medtronic. 2020. Accessed October 21, 2022. www.medtronicdiabetes.com/sites/default/files/library/download-library/user-guides/Guardian-Connect-System-User-Guide.pdf
17. Eversense E3 user guides. Senseonics. 2022. Accessed October 22, 2022. www.ascensiadiabetes.com/eversense/user-guides/
18. Battelino T, Conget I, Olsen B, et al; SWITCH Study Group. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012;55:3155-3162. doi: 10.1007/s00125-012-2708-9
19. Weinzimer S, Miller K, Beck R, et al; Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes Research Foundation continuous glucose monitoring (JDRF-CGM) trial. Diabetes Care. 2010;33:17-22. doi: 10.2337/dc09-1502
20. Haak T, Hanaire H, Ajjan R, et al. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8:55-73. doi: 10.1007/s13300-016-0223-6
21. Yaron M, Roitman E, Aharon-Hananel G, et al. Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with type 2 diabetes. Diabetes Care. 2019;42:1178-1184. doi: 10.2337/dc18-0166
22. Kröger J, Fasching P, Hanaire H. Three European retrospective real-world chart review studies to determine the effectiveness of flash glucose monitoring on HbA1c in adults with type 2 diabetes. Diabetes Ther. 2020;11:279-291. doi: 10.1007/s13300-019-00741-9
23. Wright EE, Jr, Kerr MSD, Reyes IJ, et al. Use of flash continuous glucose monitoring is associated with A1C reduction in people with type 2 diabetes treated with basal insulin or noninsulin therapy. Diabetes Spectr. 2021;34:184-189. doi: 10.2337/ds20-0069
24. Beck RW, Riddlesworth TD, Ruedy K, et al; DIAMOND Study Group. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med. 2017;167:365-374. doi: 10.7326/M16-2855
25. Vigersky RA, Fonda SJ, Chellappa M, et al. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care. 2012;35:32-38. doi: 10.2337/dc11-1438
26. Yoo HJ, An HG, Park SY, et al. Use of a real time continuous glucose monitoring system as a motivational device for poorly controlled type 2 diabetes. Diabetes Res Clin Pract. 2008;82:73-79. doi: 10.1016/j.diabres.2008.06.015
27. Martens T, Beck RW, Bailey R, et al; MOBILE Study Group. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA. 2021;325:2262-2272. doi: 10.1001/jama.2021.7444
28. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42:1593-1603. doi: 10.2337/dci19-0028
29. Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019;42:400-405. doi: 10.2337/dc18-1444
30. Freckmann G. Basics and use of continuous glucose monitoring (CGM) in diabetes therapy. Journal of Laboratory Medicine. 2020;44:71-79. doi: 10.1515/labmed-2019-0189
31. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40:1631-1640. doi: 10.2337/dc17-1600
32. Glucose monitors. Centers for Medicare & Medicaid Services. April 22, 2022. Accessed October 22, 2022. www.cms.gov/medicare-coverage-database/view/lcd.aspx?lcdid=33822
33. Pyl J, Dendooven E, Van Eekelen I, et al. Prevalence and prevention of contact dermatitis caused by FreeStyle Libre: a monocentric experience. Diabetes Care. 2020;43:918-920. doi: 10.2337/dc19-1354
34. Smith J, Bleiker T, Narang I. Cutaneous reactions to glucose sensors: a sticky problem [Abstract 677]. Arch Dis Child. 2021;106 (suppl 1):A80.
35. MAUDE Adverse event report: Dexcom, Inc G6 Sensor. U.S. Food & Drug Administration. Updated September 30, 2022. Accessed October 21, 2022. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/detail.cfm?mdrfoi__id=11064819&pc=MDS
36. New JP, Ajjan R, Pfeiffer AFH, et al. Continuous glucose monitoring in people with diabetes: the randomized controlled Glucose Level Awareness in Diabetes Study (GLADIS). Diabet Med. 2015;32:609-617. doi: 10.1111/dme.12713
1. Rodríguez-Gutiérrez R, Montori VM. Glycemic control for patients with type 2 diabetes mellitus: our evolving faith in the face of evidence. Circ Cardiovasc Qual Outcomes. 2016;9:504-512. doi: 10.1161/CIRCOUTCOMES.116.002901
2. Draznin B, Aroda VR, Bakris G, et al; . 7. Diabetes technology: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S97-S112. doi: 10.2337/dc22-S007
3. Olczuk D, Priefer R. A history of continuous glucose monitors (CGMs) in self-monitoring of diabetes mellitus. Diabetes Metab Syndr. 2018;12:181-187. doi: 10.1016/j.dsx.2017.09.005
4. Alva S, Bailey T, Brazg R, et al. Accuracy of a 14-day factory-calibrated continuous glucose monitoring system with advanced algorithm in pediatric and adult population with diabetes. J Diabetes Sci Technol. 2022;16:70-77. doi: 10.1177/1932296820958754
5. Zaharieva DP, Turksoy K, McGaugh SM, et al. Lag time remains with newer real-time continuous glucose monitoring technology during aerobic exercise in adults living with type 1 diabetes. Diabetes Technol Ther. 2019;21:313-321. doi: 10.1089/dia.2018.0364
6. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2021. Diabetes Care. 2021;44(suppl 1):S15-S33. doi: 10.2337/dc21-S002
7. FreeStyle Libre systems: The #1 CGM used in the US. Abbott. Updated May 2022. Accessed October 22, 2022. www.freestyleprovider.abbott/us-en/home.html
8. Rowland K. Choosing Wisely: 10 practices to stop—or adopt—to reduce overuse in health care. J Fam Pract. 2020;69:396-400.
9. Tucker ME. FDA clears Abbott Freestyle Libre 3 glucose sensor. MDedge. June 1, 2022. Accessed October 21, 2022. www.mdedge.com/endocrinology/article/255095/diabetes/fda-clears-abbott-freestyle-libre-3-glucose-sensor
10. Manage your diabetes with more confidence. Abbott. Updated May 2022. Accessed October 22, 2022. www.freestyle.abbott/us-en/home.html
11. Whooley S. Dexcom CEO Kevin Sayer says G7 will be ‘wonderful’. Drug Delivery Business News. July 19, 2021. Accessed October 21, 2022. www.drugdeliverybusiness.com/dexcom-ceo-kevin-sayer-says-g7-will-be-wonderful
12. Medtronic secures two CE mark approvals for Guardian 4 Sensor & for InPen MDI Smart Insulin Pen. Medtronic. Press release. May 26, 2021. Accessed October 22, 2022. https://news.medtronic.com/2021-05-26-Medtronic-Secures-Two-CE-Mark-Approvals-for-Guardian-4-Sensor-for-InPen-MDI-Smart-Insulin-Pen
13. Eversense—up to 180 days of freedom [XL CGM System]. Senseonics. Accessed September 14, 2022. https://global.eversensediabetes.com
14. FreeStyle Libre 2 User’s Manual. Abbott. Revised August 24, 2022. Accessed October 2, 2022. https://freestyleserver.com/Payloads/IFU/2022/q3/ART46983-001_rev-A.pdf
15. Dexcom G6 Continuous Glucose Monitoring System user guide. Dexcom. Revised March 2022. Accessed October 21, 2022. https://s3-us-west-2.amazonaws.com/dexcompdf/G6-CGM-Users-Guide.pdf
16. Guardian Connect System user guide. Medtronic. 2020. Accessed October 21, 2022. www.medtronicdiabetes.com/sites/default/files/library/download-library/user-guides/Guardian-Connect-System-User-Guide.pdf
17. Eversense E3 user guides. Senseonics. 2022. Accessed October 22, 2022. www.ascensiadiabetes.com/eversense/user-guides/
18. Battelino T, Conget I, Olsen B, et al; SWITCH Study Group. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012;55:3155-3162. doi: 10.1007/s00125-012-2708-9
19. Weinzimer S, Miller K, Beck R, et al; Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes Research Foundation continuous glucose monitoring (JDRF-CGM) trial. Diabetes Care. 2010;33:17-22. doi: 10.2337/dc09-1502
20. Haak T, Hanaire H, Ajjan R, et al. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8:55-73. doi: 10.1007/s13300-016-0223-6
21. Yaron M, Roitman E, Aharon-Hananel G, et al. Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with type 2 diabetes. Diabetes Care. 2019;42:1178-1184. doi: 10.2337/dc18-0166
22. Kröger J, Fasching P, Hanaire H. Three European retrospective real-world chart review studies to determine the effectiveness of flash glucose monitoring on HbA1c in adults with type 2 diabetes. Diabetes Ther. 2020;11:279-291. doi: 10.1007/s13300-019-00741-9
23. Wright EE, Jr, Kerr MSD, Reyes IJ, et al. Use of flash continuous glucose monitoring is associated with A1C reduction in people with type 2 diabetes treated with basal insulin or noninsulin therapy. Diabetes Spectr. 2021;34:184-189. doi: 10.2337/ds20-0069
24. Beck RW, Riddlesworth TD, Ruedy K, et al; DIAMOND Study Group. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med. 2017;167:365-374. doi: 10.7326/M16-2855
25. Vigersky RA, Fonda SJ, Chellappa M, et al. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care. 2012;35:32-38. doi: 10.2337/dc11-1438
26. Yoo HJ, An HG, Park SY, et al. Use of a real time continuous glucose monitoring system as a motivational device for poorly controlled type 2 diabetes. Diabetes Res Clin Pract. 2008;82:73-79. doi: 10.1016/j.diabres.2008.06.015
27. Martens T, Beck RW, Bailey R, et al; MOBILE Study Group. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA. 2021;325:2262-2272. doi: 10.1001/jama.2021.7444
28. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42:1593-1603. doi: 10.2337/dci19-0028
29. Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019;42:400-405. doi: 10.2337/dc18-1444
30. Freckmann G. Basics and use of continuous glucose monitoring (CGM) in diabetes therapy. Journal of Laboratory Medicine. 2020;44:71-79. doi: 10.1515/labmed-2019-0189
31. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40:1631-1640. doi: 10.2337/dc17-1600
32. Glucose monitors. Centers for Medicare & Medicaid Services. April 22, 2022. Accessed October 22, 2022. www.cms.gov/medicare-coverage-database/view/lcd.aspx?lcdid=33822
33. Pyl J, Dendooven E, Van Eekelen I, et al. Prevalence and prevention of contact dermatitis caused by FreeStyle Libre: a monocentric experience. Diabetes Care. 2020;43:918-920. doi: 10.2337/dc19-1354
34. Smith J, Bleiker T, Narang I. Cutaneous reactions to glucose sensors: a sticky problem [Abstract 677]. Arch Dis Child. 2021;106 (suppl 1):A80.
35. MAUDE Adverse event report: Dexcom, Inc G6 Sensor. U.S. Food & Drug Administration. Updated September 30, 2022. Accessed October 21, 2022. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/detail.cfm?mdrfoi__id=11064819&pc=MDS
36. New JP, Ajjan R, Pfeiffer AFH, et al. Continuous glucose monitoring in people with diabetes: the randomized controlled Glucose Level Awareness in Diabetes Study (GLADIS). Diabet Med. 2015;32:609-617. doi: 10.1111/dme.12713
PRACTICE RECOMMENDATIONS
› Initiate continuous glucose monitoring early in the disease process, based on a patient’s needs or preferences. C
› Interpret a continuous glucose monitor (CGM) report with the understanding that time within target range is the most important factor to evaluate. Minimizing or eliminating time below range is of paramount importance. B
› Advise patients who use a CGM to continue to have access to a glucometer and instruct them on appropriate times when such confirmation might be necessary. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Migraine Workup
T2D Medications II
34-year-old man • chronic lower back pain • peripheral neuropathy • leg spasms with increasing weakness • Dx?
THE CASE
A 34-year-old man was referred to the sports medicine clinic for evaluation of lumbar radiculopathy. He had a 2-year history of chronic lower back pain that started while he was working on power line towers in Puerto Rico. The back pain was achy, burning, shooting, and stabbing in nature. He had been treated with anti-inflammatories by a company health care provider while in Puerto Rico, but he did not have any imaging done.
At that time, he had tingling and burning that radiated down his left leg to his ankle. The patient also had leg spasms—in his left leg more than his right—and needed a cane when walking. His symptoms did not worsen at any particular time of day or with activity. He had no history of eating exotic foods or sustaining any venomous bites/stings. Ultimately, the back pain and leg spasms forced him to leave his job and return home to Louisiana.
Upon presentation to the sports medicine clinic, he explained that things had worsened since his return home. The pain and burning in his left leg had increased and were now present in his right leg, as well (bilateral paresthesias). In addition, he said he was feeling anxious (and described symptoms of forgetfulness, confusion, and agitation), was sleeping less, and was experiencing worsening fatigue.
Work-ups over the course of the previous 2 years had shed little light on the cause of his symptoms. X-rays of his lumbar spine revealed moderate degenerative changes at L5-S1. A lab work-up was negative and included a complete blood count, testing for HIV and herpes, a hepatitis panel, an antinuclear antibody screen, a C-reactive protein test, and a comprehensive metabolic panel. Thyroid-stimulating hormone, creatine kinase, rapid plasma reagin, and human leukocyte antigen B27 tests were also normal.
Magnetic resonance imaging (MRI) revealed a cystic lesion in the right ilium near the sacroiliac joint. A more recent follow-up MRI and computed tomography scan of the pelvis found the cyst to be stable and well marginalized, with no cortical erosion. Attempts at physical therapy had been unsuccessful because of the pain and decreasing muscle strength in his lower extremities. The patient’s primary care provider was treating him with meloxicam 15 mg/d and duloxetine 60 mg/d, but that had not provided any relief.
Our physical examination revealed a patient who was in mild distress and had limited lumbar spine range of motion (secondary to pain in all planes) and significant paraspinal spasms on the right side in both the lumbar and thoracic regions. The patient had reduced vibratory sensation on his left side vs the right, with a 256-Hz tuning fork at the great toe, as well as reduced sensation to fine touch with a cotton swab and a positive Babinski sign bilaterally. Lower extremity reflexes were hyperreflexic on the left compared with the right. He had no pronator drift; Trendelenburg, straight leg raise, Hoover sign, and slump tests were all negative. His gait was antalgic with a cane, as he described bilateral paresthesias.
THE DIAGNOSIS
The differential diagnosis for low back pain is quite extensive and includes simple mechanical low back pain, lumbar radiculopathy, facet arthritis, spinal stenosis, spondylolysis/spondylolisthesis, and referred pain from the hip, knee, or upper back. It can also be caused by referred pain from visceral organs such as the liver, colon, or kidneys. Low back pain can also signal primary or metastatic disease. However, most of these potential diagnoses had been ruled out with imaging and lab tests.
Two things caught our attention. First: Mechanical low back pain and the associated discogenic radiculopathy would be unilateral, manifesting with asymmetric paresthesias and pain. Our patient had weakness in gait and pain and burning in both of his legs. Second: Our patient described decreased sleep and feeling anxious, with symptoms of forgetfulness, confusion, and agitation. These factors prompted us to look beyond the normal differential and consider a potential toxicity. A heavy metal screen was ordered, and the results were positive for arsenic toxicity.
DISCUSSION
Arsenic toxicity is a global health problem that affects millions of people.1,2 Arsenic has been used for centuries in depilatories, cosmetics, poisons, and therapeutic agents. Today it is used as a treatment for leukemia and in several ayurvedic and homeopathic remedies.3-7 It is a common earth element found in ground water and a waste product from mining and the manufacturing of glass, computer chips, wood preservatives, and various pesticides.2,3,7,8
A great masquerader. Once in the body, arsenic can cause many serious ailments ranging from urinary tract, liver, and skin cancers to various peripheral and central nervous system disorders.2 Arsenic can cause symmetrical peripheral neuropathy characterized by sensory nerves being more sensitive than motor nerves.2,3,5,6 Clinically, it causes numbness and paresthesias of the distal extremities, with the lower extremities more severely affected.3,6 Symptoms can develop within 2 hours to 2 years of exposure, with vomiting, diarrhea, or both preceding the onset of the neuropathy.2,3,5,6 Arsenic is linked to forgetfulness, confusion, visual distortion, sleep disturbances, decreased concentration, disorientation, severe agitation, paranoid ideation, emotional lability, and decreases in locomotor activity.3,5,6
Testing and treatment. Arsenic levels in the body are measured by blood and urine testing. Blood arsenic levels are typically detectable immediately after exposure and with continued exposure, but quickly normalize as the metal integrates into the nonvascular tissues. Urine arsenic levels can be detected for weeks. Normal levels for arsenic in both urine and blood are ≤ 12 µg/L.3 Anything greater than 12 µg/L is considered high; critically high values are those above 50 µg/L.3,5 Our patient’s blood arsenic level was 13 µg/L.
Treatment involves removing the source of the arsenic. Chelation therapy should be pursued when urine arsenic levels are greater than 50 µg/L or when removing the source of the arsenic fails to reduce arsenic levels. Chelation therapy should be continued until urine arsenic levels are below 20 µg/L.5,6
Continue to: After discussing potential sources of exposure
After discussing potential sources of exposure, our patient decided to move out of the house he shared with his ex-wife. He started to recover soon after moving out. Two weeks after his clinic visit, he no longer needed a cane to walk, and his blood arsenic level had dropped to 6 µg/L. Two months after his clinic visit, the patient’s blood arsenic level was undetectable. The patient’s peripheral neuropathy symptoms continued to improve.
The source of this patient’s arsenic exposure was never confirmed. The exposure could have occurred in Puerto Rico or in Louisiana. Even though no one else in the Louisiana home became ill, the patient was instructed to contact the local health department and water department to have the water tested. However, when he returned to the clinic for follow-up, he had not followed through.
THE TAKEAWAY
When evaluating causes of peripheral neuropathy, consider the possibility of heavy metal toxicity, which can be easily overlooked by the busy clinician. In this case, the patient initially experienced asymmetric paresthesia that gradually increased to burning pain and weakness, with reduced motor control bilaterally. This was significant because mechanical low back pain and the associated discogenic radiculopathy would be unilateral, manifesting with asymmetric paresthesias and pain.
Our patient’s leg symptoms, the constellation of forgetfulness, confusion, and agitation, and his sleep issues prompted us to look outside our normal differential. Fortunately, once arsenic exposure ceases, patients will gradually improve because arsenic is rapidly cleared from the bloodstream.3,6
CORRESPONDENCE
Charles W. Webb, DO, CAQSM, FAMSSM, FAAFP, Department of Family Medicine, 1501 Kings Highway, PO Box 33932, Shreveport, LA 71130-3932; [email protected]
1. Ahmad SA, Khan MH, Haque M. Arsenic contamination in groundwater in Bangladesh: implications and challenges for healthcare policy. Risk Manag Health Policy. 2018;11:251-261. doi: 10.2147/RMHP.S153188
2. Roh T, Steinmaus C, Marshall G, et al. Age at exposure to arsenic in water and mortality 30-40 years after exposure cessation. Am J Epidemiol. 2018;187:2297-2305. doi: 10.1093/aje/kwy159
3. Baker BA, Cassano VA, Murray C, ACOEM Task Force on Arsenic Exposure. Arsenic exposure, assessment, toxicity, diagnosis, and management. J Occup Environ Med. 2018;60:634-639. doi: 10.1097/JOM.0000000000001485
4. Lasky T, Sun W, Kadry A, Hoffman MK. Mean total arsenic concentrations in chicken 1989-2000 and estimated exposures for consumers of chicken. Environ Health Perspect. 2004;112:18-21. doi: 10.1289/ehp.6407
5. Lindenmeyer G, Hoggett K, Burrow J, et al. A sickening tale. N Engl J Med. 2018;379:75-80. doi: 10.1056/NEJMcps1716775
6. Rodríguez VM, Jímenez-Capdevill ME, Giordano M. The effects of arsenic exposure on the nervous system. Toxicol Lett. 2003;145: 1-18. doi: 10.1016/s0378-4274(03)00262-5
7. Saper RB, Phillips RS, Sehgal A, et al. Lead, mercury, and arsenic in US- and Indian- manufactured ayurvedic medicines sold via the internet. JAMA. 2008;300:915-923. doi: 10.1001/jama.300.8.915
8. Rose M, Lewis J, Langford N, et al. Arsenic in seaweed—forms, concentration and dietary exposure. Food Chem Toxicol. 2007;45:1263-1267. doi: 10.1016/j.fct.2007.01.007
THE CASE
A 34-year-old man was referred to the sports medicine clinic for evaluation of lumbar radiculopathy. He had a 2-year history of chronic lower back pain that started while he was working on power line towers in Puerto Rico. The back pain was achy, burning, shooting, and stabbing in nature. He had been treated with anti-inflammatories by a company health care provider while in Puerto Rico, but he did not have any imaging done.
At that time, he had tingling and burning that radiated down his left leg to his ankle. The patient also had leg spasms—in his left leg more than his right—and needed a cane when walking. His symptoms did not worsen at any particular time of day or with activity. He had no history of eating exotic foods or sustaining any venomous bites/stings. Ultimately, the back pain and leg spasms forced him to leave his job and return home to Louisiana.
Upon presentation to the sports medicine clinic, he explained that things had worsened since his return home. The pain and burning in his left leg had increased and were now present in his right leg, as well (bilateral paresthesias). In addition, he said he was feeling anxious (and described symptoms of forgetfulness, confusion, and agitation), was sleeping less, and was experiencing worsening fatigue.
Work-ups over the course of the previous 2 years had shed little light on the cause of his symptoms. X-rays of his lumbar spine revealed moderate degenerative changes at L5-S1. A lab work-up was negative and included a complete blood count, testing for HIV and herpes, a hepatitis panel, an antinuclear antibody screen, a C-reactive protein test, and a comprehensive metabolic panel. Thyroid-stimulating hormone, creatine kinase, rapid plasma reagin, and human leukocyte antigen B27 tests were also normal.
Magnetic resonance imaging (MRI) revealed a cystic lesion in the right ilium near the sacroiliac joint. A more recent follow-up MRI and computed tomography scan of the pelvis found the cyst to be stable and well marginalized, with no cortical erosion. Attempts at physical therapy had been unsuccessful because of the pain and decreasing muscle strength in his lower extremities. The patient’s primary care provider was treating him with meloxicam 15 mg/d and duloxetine 60 mg/d, but that had not provided any relief.
Our physical examination revealed a patient who was in mild distress and had limited lumbar spine range of motion (secondary to pain in all planes) and significant paraspinal spasms on the right side in both the lumbar and thoracic regions. The patient had reduced vibratory sensation on his left side vs the right, with a 256-Hz tuning fork at the great toe, as well as reduced sensation to fine touch with a cotton swab and a positive Babinski sign bilaterally. Lower extremity reflexes were hyperreflexic on the left compared with the right. He had no pronator drift; Trendelenburg, straight leg raise, Hoover sign, and slump tests were all negative. His gait was antalgic with a cane, as he described bilateral paresthesias.
THE DIAGNOSIS
The differential diagnosis for low back pain is quite extensive and includes simple mechanical low back pain, lumbar radiculopathy, facet arthritis, spinal stenosis, spondylolysis/spondylolisthesis, and referred pain from the hip, knee, or upper back. It can also be caused by referred pain from visceral organs such as the liver, colon, or kidneys. Low back pain can also signal primary or metastatic disease. However, most of these potential diagnoses had been ruled out with imaging and lab tests.
Two things caught our attention. First: Mechanical low back pain and the associated discogenic radiculopathy would be unilateral, manifesting with asymmetric paresthesias and pain. Our patient had weakness in gait and pain and burning in both of his legs. Second: Our patient described decreased sleep and feeling anxious, with symptoms of forgetfulness, confusion, and agitation. These factors prompted us to look beyond the normal differential and consider a potential toxicity. A heavy metal screen was ordered, and the results were positive for arsenic toxicity.
DISCUSSION
Arsenic toxicity is a global health problem that affects millions of people.1,2 Arsenic has been used for centuries in depilatories, cosmetics, poisons, and therapeutic agents. Today it is used as a treatment for leukemia and in several ayurvedic and homeopathic remedies.3-7 It is a common earth element found in ground water and a waste product from mining and the manufacturing of glass, computer chips, wood preservatives, and various pesticides.2,3,7,8
A great masquerader. Once in the body, arsenic can cause many serious ailments ranging from urinary tract, liver, and skin cancers to various peripheral and central nervous system disorders.2 Arsenic can cause symmetrical peripheral neuropathy characterized by sensory nerves being more sensitive than motor nerves.2,3,5,6 Clinically, it causes numbness and paresthesias of the distal extremities, with the lower extremities more severely affected.3,6 Symptoms can develop within 2 hours to 2 years of exposure, with vomiting, diarrhea, or both preceding the onset of the neuropathy.2,3,5,6 Arsenic is linked to forgetfulness, confusion, visual distortion, sleep disturbances, decreased concentration, disorientation, severe agitation, paranoid ideation, emotional lability, and decreases in locomotor activity.3,5,6
Testing and treatment. Arsenic levels in the body are measured by blood and urine testing. Blood arsenic levels are typically detectable immediately after exposure and with continued exposure, but quickly normalize as the metal integrates into the nonvascular tissues. Urine arsenic levels can be detected for weeks. Normal levels for arsenic in both urine and blood are ≤ 12 µg/L.3 Anything greater than 12 µg/L is considered high; critically high values are those above 50 µg/L.3,5 Our patient’s blood arsenic level was 13 µg/L.
Treatment involves removing the source of the arsenic. Chelation therapy should be pursued when urine arsenic levels are greater than 50 µg/L or when removing the source of the arsenic fails to reduce arsenic levels. Chelation therapy should be continued until urine arsenic levels are below 20 µg/L.5,6
Continue to: After discussing potential sources of exposure
After discussing potential sources of exposure, our patient decided to move out of the house he shared with his ex-wife. He started to recover soon after moving out. Two weeks after his clinic visit, he no longer needed a cane to walk, and his blood arsenic level had dropped to 6 µg/L. Two months after his clinic visit, the patient’s blood arsenic level was undetectable. The patient’s peripheral neuropathy symptoms continued to improve.
The source of this patient’s arsenic exposure was never confirmed. The exposure could have occurred in Puerto Rico or in Louisiana. Even though no one else in the Louisiana home became ill, the patient was instructed to contact the local health department and water department to have the water tested. However, when he returned to the clinic for follow-up, he had not followed through.
THE TAKEAWAY
When evaluating causes of peripheral neuropathy, consider the possibility of heavy metal toxicity, which can be easily overlooked by the busy clinician. In this case, the patient initially experienced asymmetric paresthesia that gradually increased to burning pain and weakness, with reduced motor control bilaterally. This was significant because mechanical low back pain and the associated discogenic radiculopathy would be unilateral, manifesting with asymmetric paresthesias and pain.
Our patient’s leg symptoms, the constellation of forgetfulness, confusion, and agitation, and his sleep issues prompted us to look outside our normal differential. Fortunately, once arsenic exposure ceases, patients will gradually improve because arsenic is rapidly cleared from the bloodstream.3,6
CORRESPONDENCE
Charles W. Webb, DO, CAQSM, FAMSSM, FAAFP, Department of Family Medicine, 1501 Kings Highway, PO Box 33932, Shreveport, LA 71130-3932; [email protected]
THE CASE
A 34-year-old man was referred to the sports medicine clinic for evaluation of lumbar radiculopathy. He had a 2-year history of chronic lower back pain that started while he was working on power line towers in Puerto Rico. The back pain was achy, burning, shooting, and stabbing in nature. He had been treated with anti-inflammatories by a company health care provider while in Puerto Rico, but he did not have any imaging done.
At that time, he had tingling and burning that radiated down his left leg to his ankle. The patient also had leg spasms—in his left leg more than his right—and needed a cane when walking. His symptoms did not worsen at any particular time of day or with activity. He had no history of eating exotic foods or sustaining any venomous bites/stings. Ultimately, the back pain and leg spasms forced him to leave his job and return home to Louisiana.
Upon presentation to the sports medicine clinic, he explained that things had worsened since his return home. The pain and burning in his left leg had increased and were now present in his right leg, as well (bilateral paresthesias). In addition, he said he was feeling anxious (and described symptoms of forgetfulness, confusion, and agitation), was sleeping less, and was experiencing worsening fatigue.
Work-ups over the course of the previous 2 years had shed little light on the cause of his symptoms. X-rays of his lumbar spine revealed moderate degenerative changes at L5-S1. A lab work-up was negative and included a complete blood count, testing for HIV and herpes, a hepatitis panel, an antinuclear antibody screen, a C-reactive protein test, and a comprehensive metabolic panel. Thyroid-stimulating hormone, creatine kinase, rapid plasma reagin, and human leukocyte antigen B27 tests were also normal.
Magnetic resonance imaging (MRI) revealed a cystic lesion in the right ilium near the sacroiliac joint. A more recent follow-up MRI and computed tomography scan of the pelvis found the cyst to be stable and well marginalized, with no cortical erosion. Attempts at physical therapy had been unsuccessful because of the pain and decreasing muscle strength in his lower extremities. The patient’s primary care provider was treating him with meloxicam 15 mg/d and duloxetine 60 mg/d, but that had not provided any relief.
Our physical examination revealed a patient who was in mild distress and had limited lumbar spine range of motion (secondary to pain in all planes) and significant paraspinal spasms on the right side in both the lumbar and thoracic regions. The patient had reduced vibratory sensation on his left side vs the right, with a 256-Hz tuning fork at the great toe, as well as reduced sensation to fine touch with a cotton swab and a positive Babinski sign bilaterally. Lower extremity reflexes were hyperreflexic on the left compared with the right. He had no pronator drift; Trendelenburg, straight leg raise, Hoover sign, and slump tests were all negative. His gait was antalgic with a cane, as he described bilateral paresthesias.
THE DIAGNOSIS
The differential diagnosis for low back pain is quite extensive and includes simple mechanical low back pain, lumbar radiculopathy, facet arthritis, spinal stenosis, spondylolysis/spondylolisthesis, and referred pain from the hip, knee, or upper back. It can also be caused by referred pain from visceral organs such as the liver, colon, or kidneys. Low back pain can also signal primary or metastatic disease. However, most of these potential diagnoses had been ruled out with imaging and lab tests.
Two things caught our attention. First: Mechanical low back pain and the associated discogenic radiculopathy would be unilateral, manifesting with asymmetric paresthesias and pain. Our patient had weakness in gait and pain and burning in both of his legs. Second: Our patient described decreased sleep and feeling anxious, with symptoms of forgetfulness, confusion, and agitation. These factors prompted us to look beyond the normal differential and consider a potential toxicity. A heavy metal screen was ordered, and the results were positive for arsenic toxicity.
DISCUSSION
Arsenic toxicity is a global health problem that affects millions of people.1,2 Arsenic has been used for centuries in depilatories, cosmetics, poisons, and therapeutic agents. Today it is used as a treatment for leukemia and in several ayurvedic and homeopathic remedies.3-7 It is a common earth element found in ground water and a waste product from mining and the manufacturing of glass, computer chips, wood preservatives, and various pesticides.2,3,7,8
A great masquerader. Once in the body, arsenic can cause many serious ailments ranging from urinary tract, liver, and skin cancers to various peripheral and central nervous system disorders.2 Arsenic can cause symmetrical peripheral neuropathy characterized by sensory nerves being more sensitive than motor nerves.2,3,5,6 Clinically, it causes numbness and paresthesias of the distal extremities, with the lower extremities more severely affected.3,6 Symptoms can develop within 2 hours to 2 years of exposure, with vomiting, diarrhea, or both preceding the onset of the neuropathy.2,3,5,6 Arsenic is linked to forgetfulness, confusion, visual distortion, sleep disturbances, decreased concentration, disorientation, severe agitation, paranoid ideation, emotional lability, and decreases in locomotor activity.3,5,6
Testing and treatment. Arsenic levels in the body are measured by blood and urine testing. Blood arsenic levels are typically detectable immediately after exposure and with continued exposure, but quickly normalize as the metal integrates into the nonvascular tissues. Urine arsenic levels can be detected for weeks. Normal levels for arsenic in both urine and blood are ≤ 12 µg/L.3 Anything greater than 12 µg/L is considered high; critically high values are those above 50 µg/L.3,5 Our patient’s blood arsenic level was 13 µg/L.
Treatment involves removing the source of the arsenic. Chelation therapy should be pursued when urine arsenic levels are greater than 50 µg/L or when removing the source of the arsenic fails to reduce arsenic levels. Chelation therapy should be continued until urine arsenic levels are below 20 µg/L.5,6
Continue to: After discussing potential sources of exposure
After discussing potential sources of exposure, our patient decided to move out of the house he shared with his ex-wife. He started to recover soon after moving out. Two weeks after his clinic visit, he no longer needed a cane to walk, and his blood arsenic level had dropped to 6 µg/L. Two months after his clinic visit, the patient’s blood arsenic level was undetectable. The patient’s peripheral neuropathy symptoms continued to improve.
The source of this patient’s arsenic exposure was never confirmed. The exposure could have occurred in Puerto Rico or in Louisiana. Even though no one else in the Louisiana home became ill, the patient was instructed to contact the local health department and water department to have the water tested. However, when he returned to the clinic for follow-up, he had not followed through.
THE TAKEAWAY
When evaluating causes of peripheral neuropathy, consider the possibility of heavy metal toxicity, which can be easily overlooked by the busy clinician. In this case, the patient initially experienced asymmetric paresthesia that gradually increased to burning pain and weakness, with reduced motor control bilaterally. This was significant because mechanical low back pain and the associated discogenic radiculopathy would be unilateral, manifesting with asymmetric paresthesias and pain.
Our patient’s leg symptoms, the constellation of forgetfulness, confusion, and agitation, and his sleep issues prompted us to look outside our normal differential. Fortunately, once arsenic exposure ceases, patients will gradually improve because arsenic is rapidly cleared from the bloodstream.3,6
CORRESPONDENCE
Charles W. Webb, DO, CAQSM, FAMSSM, FAAFP, Department of Family Medicine, 1501 Kings Highway, PO Box 33932, Shreveport, LA 71130-3932; [email protected]
1. Ahmad SA, Khan MH, Haque M. Arsenic contamination in groundwater in Bangladesh: implications and challenges for healthcare policy. Risk Manag Health Policy. 2018;11:251-261. doi: 10.2147/RMHP.S153188
2. Roh T, Steinmaus C, Marshall G, et al. Age at exposure to arsenic in water and mortality 30-40 years after exposure cessation. Am J Epidemiol. 2018;187:2297-2305. doi: 10.1093/aje/kwy159
3. Baker BA, Cassano VA, Murray C, ACOEM Task Force on Arsenic Exposure. Arsenic exposure, assessment, toxicity, diagnosis, and management. J Occup Environ Med. 2018;60:634-639. doi: 10.1097/JOM.0000000000001485
4. Lasky T, Sun W, Kadry A, Hoffman MK. Mean total arsenic concentrations in chicken 1989-2000 and estimated exposures for consumers of chicken. Environ Health Perspect. 2004;112:18-21. doi: 10.1289/ehp.6407
5. Lindenmeyer G, Hoggett K, Burrow J, et al. A sickening tale. N Engl J Med. 2018;379:75-80. doi: 10.1056/NEJMcps1716775
6. Rodríguez VM, Jímenez-Capdevill ME, Giordano M. The effects of arsenic exposure on the nervous system. Toxicol Lett. 2003;145: 1-18. doi: 10.1016/s0378-4274(03)00262-5
7. Saper RB, Phillips RS, Sehgal A, et al. Lead, mercury, and arsenic in US- and Indian- manufactured ayurvedic medicines sold via the internet. JAMA. 2008;300:915-923. doi: 10.1001/jama.300.8.915
8. Rose M, Lewis J, Langford N, et al. Arsenic in seaweed—forms, concentration and dietary exposure. Food Chem Toxicol. 2007;45:1263-1267. doi: 10.1016/j.fct.2007.01.007
1. Ahmad SA, Khan MH, Haque M. Arsenic contamination in groundwater in Bangladesh: implications and challenges for healthcare policy. Risk Manag Health Policy. 2018;11:251-261. doi: 10.2147/RMHP.S153188
2. Roh T, Steinmaus C, Marshall G, et al. Age at exposure to arsenic in water and mortality 30-40 years after exposure cessation. Am J Epidemiol. 2018;187:2297-2305. doi: 10.1093/aje/kwy159
3. Baker BA, Cassano VA, Murray C, ACOEM Task Force on Arsenic Exposure. Arsenic exposure, assessment, toxicity, diagnosis, and management. J Occup Environ Med. 2018;60:634-639. doi: 10.1097/JOM.0000000000001485
4. Lasky T, Sun W, Kadry A, Hoffman MK. Mean total arsenic concentrations in chicken 1989-2000 and estimated exposures for consumers of chicken. Environ Health Perspect. 2004;112:18-21. doi: 10.1289/ehp.6407
5. Lindenmeyer G, Hoggett K, Burrow J, et al. A sickening tale. N Engl J Med. 2018;379:75-80. doi: 10.1056/NEJMcps1716775
6. Rodríguez VM, Jímenez-Capdevill ME, Giordano M. The effects of arsenic exposure on the nervous system. Toxicol Lett. 2003;145: 1-18. doi: 10.1016/s0378-4274(03)00262-5
7. Saper RB, Phillips RS, Sehgal A, et al. Lead, mercury, and arsenic in US- and Indian- manufactured ayurvedic medicines sold via the internet. JAMA. 2008;300:915-923. doi: 10.1001/jama.300.8.915
8. Rose M, Lewis J, Langford N, et al. Arsenic in seaweed—forms, concentration and dietary exposure. Food Chem Toxicol. 2007;45:1263-1267. doi: 10.1016/j.fct.2007.01.007
Severe pediatric oral mucositis
A 12-YEAR-OLD BOY presented to the hospital with a 2-day history of fever, cough, and painful blisters on swollen lips. On examination, he had multiple tense blisters with clear fluid on the buccal mucosa and inner lips (FIGURE 1A), as well as multiple discrete ulcers on his posterior pharynx. The patient had no other skin, eye, or urogenital involvement, but he was dehydrated. Respiratory examination was unremarkable. A complete blood count and metabolic panel were normal, as was a C-reactive protein (CRP) test (0.8 mg/L).
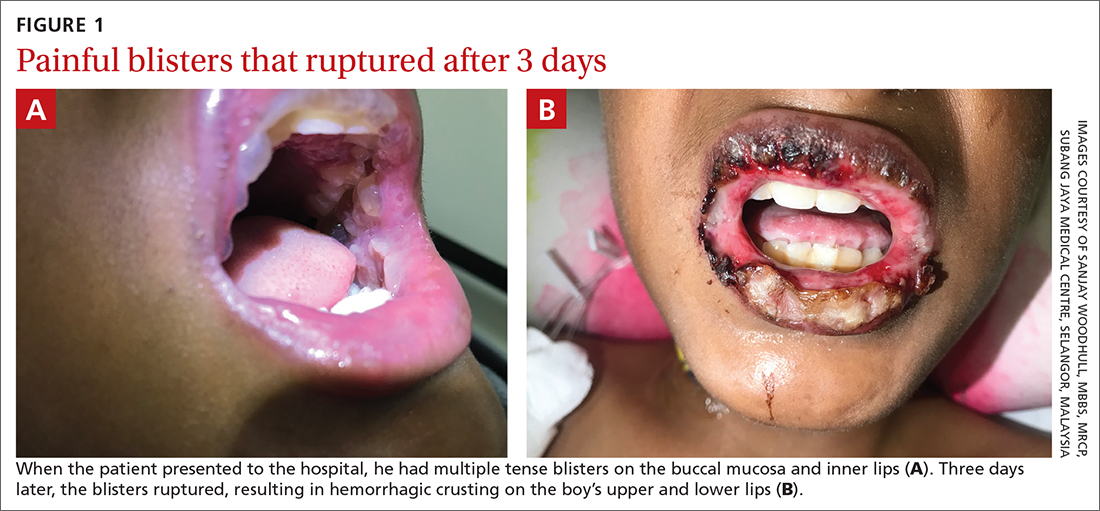
The preliminary diagnosis was primary herpetic gingivostomatitis, and treatment was initiated with intravenous (IV) acyclovir (10 mg/kg every 8 hours), IV fluids, and topical lidocaine gel and topical steroids for analgesia. However, the patient’s fever persisted over the next 4 days, with his temperature fluctuating between 101.3 °F and 104 °F, and he had a worsening productive cough. The blisters ruptured on Day 6 of illness, leaving hemorrhagic crusting on his lips (FIGURE 1B). Herpes simplex virus types 1 and 2 and polymerase chain reaction (PCR) testing were negative.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Mycoplasma pneumoniae–induced rash and mucositis
Further follow-up on Day 6 of illness revealed bibasilar crepitations along with an elevated CRP level of 40.5 mg/L and a positive mycoplasma antibody serology (titer > 1:1280; normal, < 1:80). The patient was given a diagnosis of pneumonia (due to infection with Mycoplasma pneumoniae) and M pneumoniae–induced rash and mucositis (MIRM).
MIRM was first proposed as a distinct clinical entity in 2015 to distinguish it from Stevens-Johnson syndrome and erythema multiforme.1 MIRM is seen more commonly in children and young adults, with a male preponderance.1
A small longitudinal study found that approximately 22.7% of children who have M pneumoniae infections present with mucocutaneous lesions, and of those cases, 6.8% are MIRM.2 Chlamydia pneumoniae is another potential causal organism of mucositis resembling MIRM.3
Pathogenesis. The commonly accepted mechanism of MIRM is an immune response triggered by a distant infection. This leads to tissue damage via polyclonal B cell proliferation and subsequent immune complex deposition, complement activation, and cytokine overproduction. Molecular mimicry between M pneumoniae P1-adhesion molecules and keratinocyte antigens may also contribute to this pathway.
3 criteria to make the diagnosis
Canavan et al1 have proposed the following criteria for the diagnosis of MIRM:
- Clinical symptoms, such as fever and cough, and laboratory findings of M pneumoniae infection (elevated M pneumoniae immunoglobulin M antibodies, positive cultures or PCR for M pneumoniae from oropharyngeal samples or bullae, and/or serial cold agglutinins) AND
- a rash to the mucosa that usually affects ≥ 2 sites (although rare cases may have fewer than 2 mucosal sites involved) AND
- skin detachment of less than 10% of the body surface area.
Continue to: The 3 variants of MIRM include...
The 3 variants of MIRM include:
- Classic MIRM has evidence of all 3 diagnostic criteria plus a nonmucosal rash, such as vesiculobullous lesions (77%), scattered target lesions (48%), papules (14%), macules (12%), and morbilliform eruptions (9%).4
- MIRM sine rash includes all 3 criteria but there is no significant cutaneous, nonmucosal rash. There may be “few fleeting morbilliform lesions or a few vesicles.”4
- Severe MIRM includes the first 2 criteria listed, but the cutaneous rash is extensive, with widespread nonmucosal blisters or flat atypical target lesions.4
Our patient had definitive clinical symptoms, laboratory evidence, and severe oral mucositis without significant cutaneous rash, thereby fulfilling the criteria for a diagnosis of MIRM sine rash variant.
These skin conditions were considered in the differential
The differential diagnosis for sudden onset of severe oral mucosal blisters in children includes herpes gingivostomatitis; hand, foot, and mouth disease
Herpes gingivostomatitis would involve numerous ulcerations of the oral mucosa and tongue, as well as gum hypertrophy.
Hand, foot, and mouth disease is characterized by
Continue to: Erythema multiforme
Erythema multiforme appears as cutaneous target lesions on the limbs that spread in a centripetal manner following herpes simplex virus infection.
SJS/TEN manifests with severe mucositis and is commonly triggered by medications (eg, sulphonamides, beta-lactams, nonsteroidal anti-inflammatory drugs, and antiepileptics).
With antibiotics, the prognosis is good
There are no established guidelines for the treatment of MIRM. Antibiotics and supportive care are universally accepted. Immunosuppressive therapy (eg, systemic steroids) is frequently used in patients with MIRM who have extensive mucosal involvement, in an attempt to decrease inflammation and pain; however, evidence for such an approach is lacking. The hyperimmune reactions of the host to M pneumoniae infection include cytokine overproduction and T-cell activation, which promote both pulmonary and extrapulmonary manifestations. This forms the basis of immunosuppressive therapy, such as systemic corticosteroids, IV immunoglobulin, and cyclosporin A, particularly when MIRM is associated with pneumonia caused by infection with M pneumoniae.1,5,6
The overall prognosis of MIRM is good. Recurrence has been reported in up to 8% of cases, the treatment of which remains the same. Mucocutaneous and ocular sequelae (oral or genital synechiae, corneal ulcerations, dry eyes, loss of eye lashes) have been reported in less than 9% of patients.1 Other rare reported complications following the occurrence of MIRM include persistent cutaneous lesions, B cell lymphopenia, and restrictive lung disease or chronic obliterative bronchitis.
Our patient was started on IV ceftriaxone (50 mg/kg/d), azithromycin (10 mg/kg/d on the first day, then 5 mg/kg/d on the subsequent 5 days), and methylprednisolone (3 mg/kg/d) on Day 6 of illness. Within 3 days, there was marked improvement of mucositis and respiratory symptoms with resolution of fever. He was discharged on Day 10. At his outpatient follow-up 2 weeks later, the patient had made a complete recovery.
1. Canavan TN, Mathes EF, Frieden I, et al. Mycoplasma pneumoniae-induced rash and mucositis as a syndrome distinct from Stevens-Johnson syndrome and erythema multiforme: a systematic review. J Am Acad Dermatol 2015;72:239-245. doi: 10.1016/j.jaad.2014.06.026
2. Sauteur PMM, Theiler M, Buettcher M, et al. Frequency and clinical presentation of mucocutaneous disease due to mycoplasma pneumoniae infection in children with community-acquired pneumonia. JAMA Dermatol. 2020;156:144-150. doi: 10.1001/jamadermatol.2019.3602
3. Mayor-Ibarguren A, Feito-Rodriguez M, González-Ramos J, et al. Mucositis secondary to chlamydia pneumoniae infection: expanding the mycoplasma pneumoniae-induced rash and mucositis concept. Pediatr Dermatol 2017;34:465-472. doi: 10.1111/pde.13140
4. Frantz GF, McAninch SA. Mycoplasma mucositis. StatPearls [Internet]. Updated August 8, 2022. Accessed November 1, 2022. www.ncbi.nlm.nih.gov/books/NBK525960/
5. Yang EA, Kang HM, Rhim JW, et al. Early corticosteroid therapy for Mycoplasma pneumoniae pneumonia irrespective of used antibiotics in children. J Clin Med. 2019;8:726. doi: 10.3390/jcm8050726
6. Li HOY, Colantonio S, Ramien ML. Treatment of Mycoplasma pneumoniae-induced rash and mucositis with cyclosporine. J Cutan Med Surg. 2019;23:608-612. doi: 10.1177/1203475419874444
A 12-YEAR-OLD BOY presented to the hospital with a 2-day history of fever, cough, and painful blisters on swollen lips. On examination, he had multiple tense blisters with clear fluid on the buccal mucosa and inner lips (FIGURE 1A), as well as multiple discrete ulcers on his posterior pharynx. The patient had no other skin, eye, or urogenital involvement, but he was dehydrated. Respiratory examination was unremarkable. A complete blood count and metabolic panel were normal, as was a C-reactive protein (CRP) test (0.8 mg/L).

The preliminary diagnosis was primary herpetic gingivostomatitis, and treatment was initiated with intravenous (IV) acyclovir (10 mg/kg every 8 hours), IV fluids, and topical lidocaine gel and topical steroids for analgesia. However, the patient’s fever persisted over the next 4 days, with his temperature fluctuating between 101.3 °F and 104 °F, and he had a worsening productive cough. The blisters ruptured on Day 6 of illness, leaving hemorrhagic crusting on his lips (FIGURE 1B). Herpes simplex virus types 1 and 2 and polymerase chain reaction (PCR) testing were negative.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Mycoplasma pneumoniae–induced rash and mucositis
Further follow-up on Day 6 of illness revealed bibasilar crepitations along with an elevated CRP level of 40.5 mg/L and a positive mycoplasma antibody serology (titer > 1:1280; normal, < 1:80). The patient was given a diagnosis of pneumonia (due to infection with Mycoplasma pneumoniae) and M pneumoniae–induced rash and mucositis (MIRM).
MIRM was first proposed as a distinct clinical entity in 2015 to distinguish it from Stevens-Johnson syndrome and erythema multiforme.1 MIRM is seen more commonly in children and young adults, with a male preponderance.1
A small longitudinal study found that approximately 22.7% of children who have M pneumoniae infections present with mucocutaneous lesions, and of those cases, 6.8% are MIRM.2 Chlamydia pneumoniae is another potential causal organism of mucositis resembling MIRM.3
Pathogenesis. The commonly accepted mechanism of MIRM is an immune response triggered by a distant infection. This leads to tissue damage via polyclonal B cell proliferation and subsequent immune complex deposition, complement activation, and cytokine overproduction. Molecular mimicry between M pneumoniae P1-adhesion molecules and keratinocyte antigens may also contribute to this pathway.
3 criteria to make the diagnosis
Canavan et al1 have proposed the following criteria for the diagnosis of MIRM:
- Clinical symptoms, such as fever and cough, and laboratory findings of M pneumoniae infection (elevated M pneumoniae immunoglobulin M antibodies, positive cultures or PCR for M pneumoniae from oropharyngeal samples or bullae, and/or serial cold agglutinins) AND
- a rash to the mucosa that usually affects ≥ 2 sites (although rare cases may have fewer than 2 mucosal sites involved) AND
- skin detachment of less than 10% of the body surface area.
Continue to: The 3 variants of MIRM include...
The 3 variants of MIRM include:
- Classic MIRM has evidence of all 3 diagnostic criteria plus a nonmucosal rash, such as vesiculobullous lesions (77%), scattered target lesions (48%), papules (14%), macules (12%), and morbilliform eruptions (9%).4
- MIRM sine rash includes all 3 criteria but there is no significant cutaneous, nonmucosal rash. There may be “few fleeting morbilliform lesions or a few vesicles.”4
- Severe MIRM includes the first 2 criteria listed, but the cutaneous rash is extensive, with widespread nonmucosal blisters or flat atypical target lesions.4
Our patient had definitive clinical symptoms, laboratory evidence, and severe oral mucositis without significant cutaneous rash, thereby fulfilling the criteria for a diagnosis of MIRM sine rash variant.
These skin conditions were considered in the differential
The differential diagnosis for sudden onset of severe oral mucosal blisters in children includes herpes gingivostomatitis; hand, foot, and mouth disease
Herpes gingivostomatitis would involve numerous ulcerations of the oral mucosa and tongue, as well as gum hypertrophy.
Hand, foot, and mouth disease is characterized by
Continue to: Erythema multiforme
Erythema multiforme appears as cutaneous target lesions on the limbs that spread in a centripetal manner following herpes simplex virus infection.
SJS/TEN manifests with severe mucositis and is commonly triggered by medications (eg, sulphonamides, beta-lactams, nonsteroidal anti-inflammatory drugs, and antiepileptics).
With antibiotics, the prognosis is good
There are no established guidelines for the treatment of MIRM. Antibiotics and supportive care are universally accepted. Immunosuppressive therapy (eg, systemic steroids) is frequently used in patients with MIRM who have extensive mucosal involvement, in an attempt to decrease inflammation and pain; however, evidence for such an approach is lacking. The hyperimmune reactions of the host to M pneumoniae infection include cytokine overproduction and T-cell activation, which promote both pulmonary and extrapulmonary manifestations. This forms the basis of immunosuppressive therapy, such as systemic corticosteroids, IV immunoglobulin, and cyclosporin A, particularly when MIRM is associated with pneumonia caused by infection with M pneumoniae.1,5,6
The overall prognosis of MIRM is good. Recurrence has been reported in up to 8% of cases, the treatment of which remains the same. Mucocutaneous and ocular sequelae (oral or genital synechiae, corneal ulcerations, dry eyes, loss of eye lashes) have been reported in less than 9% of patients.1 Other rare reported complications following the occurrence of MIRM include persistent cutaneous lesions, B cell lymphopenia, and restrictive lung disease or chronic obliterative bronchitis.
Our patient was started on IV ceftriaxone (50 mg/kg/d), azithromycin (10 mg/kg/d on the first day, then 5 mg/kg/d on the subsequent 5 days), and methylprednisolone (3 mg/kg/d) on Day 6 of illness. Within 3 days, there was marked improvement of mucositis and respiratory symptoms with resolution of fever. He was discharged on Day 10. At his outpatient follow-up 2 weeks later, the patient had made a complete recovery.
A 12-YEAR-OLD BOY presented to the hospital with a 2-day history of fever, cough, and painful blisters on swollen lips. On examination, he had multiple tense blisters with clear fluid on the buccal mucosa and inner lips (FIGURE 1A), as well as multiple discrete ulcers on his posterior pharynx. The patient had no other skin, eye, or urogenital involvement, but he was dehydrated. Respiratory examination was unremarkable. A complete blood count and metabolic panel were normal, as was a C-reactive protein (CRP) test (0.8 mg/L).

The preliminary diagnosis was primary herpetic gingivostomatitis, and treatment was initiated with intravenous (IV) acyclovir (10 mg/kg every 8 hours), IV fluids, and topical lidocaine gel and topical steroids for analgesia. However, the patient’s fever persisted over the next 4 days, with his temperature fluctuating between 101.3 °F and 104 °F, and he had a worsening productive cough. The blisters ruptured on Day 6 of illness, leaving hemorrhagic crusting on his lips (FIGURE 1B). Herpes simplex virus types 1 and 2 and polymerase chain reaction (PCR) testing were negative.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Mycoplasma pneumoniae–induced rash and mucositis
Further follow-up on Day 6 of illness revealed bibasilar crepitations along with an elevated CRP level of 40.5 mg/L and a positive mycoplasma antibody serology (titer > 1:1280; normal, < 1:80). The patient was given a diagnosis of pneumonia (due to infection with Mycoplasma pneumoniae) and M pneumoniae–induced rash and mucositis (MIRM).
MIRM was first proposed as a distinct clinical entity in 2015 to distinguish it from Stevens-Johnson syndrome and erythema multiforme.1 MIRM is seen more commonly in children and young adults, with a male preponderance.1
A small longitudinal study found that approximately 22.7% of children who have M pneumoniae infections present with mucocutaneous lesions, and of those cases, 6.8% are MIRM.2 Chlamydia pneumoniae is another potential causal organism of mucositis resembling MIRM.3
Pathogenesis. The commonly accepted mechanism of MIRM is an immune response triggered by a distant infection. This leads to tissue damage via polyclonal B cell proliferation and subsequent immune complex deposition, complement activation, and cytokine overproduction. Molecular mimicry between M pneumoniae P1-adhesion molecules and keratinocyte antigens may also contribute to this pathway.
3 criteria to make the diagnosis
Canavan et al1 have proposed the following criteria for the diagnosis of MIRM:
- Clinical symptoms, such as fever and cough, and laboratory findings of M pneumoniae infection (elevated M pneumoniae immunoglobulin M antibodies, positive cultures or PCR for M pneumoniae from oropharyngeal samples or bullae, and/or serial cold agglutinins) AND
- a rash to the mucosa that usually affects ≥ 2 sites (although rare cases may have fewer than 2 mucosal sites involved) AND
- skin detachment of less than 10% of the body surface area.
Continue to: The 3 variants of MIRM include...
The 3 variants of MIRM include:
- Classic MIRM has evidence of all 3 diagnostic criteria plus a nonmucosal rash, such as vesiculobullous lesions (77%), scattered target lesions (48%), papules (14%), macules (12%), and morbilliform eruptions (9%).4
- MIRM sine rash includes all 3 criteria but there is no significant cutaneous, nonmucosal rash. There may be “few fleeting morbilliform lesions or a few vesicles.”4
- Severe MIRM includes the first 2 criteria listed, but the cutaneous rash is extensive, with widespread nonmucosal blisters or flat atypical target lesions.4
Our patient had definitive clinical symptoms, laboratory evidence, and severe oral mucositis without significant cutaneous rash, thereby fulfilling the criteria for a diagnosis of MIRM sine rash variant.
These skin conditions were considered in the differential
The differential diagnosis for sudden onset of severe oral mucosal blisters in children includes herpes gingivostomatitis; hand, foot, and mouth disease
Herpes gingivostomatitis would involve numerous ulcerations of the oral mucosa and tongue, as well as gum hypertrophy.
Hand, foot, and mouth disease is characterized by
Continue to: Erythema multiforme
Erythema multiforme appears as cutaneous target lesions on the limbs that spread in a centripetal manner following herpes simplex virus infection.
SJS/TEN manifests with severe mucositis and is commonly triggered by medications (eg, sulphonamides, beta-lactams, nonsteroidal anti-inflammatory drugs, and antiepileptics).
With antibiotics, the prognosis is good
There are no established guidelines for the treatment of MIRM. Antibiotics and supportive care are universally accepted. Immunosuppressive therapy (eg, systemic steroids) is frequently used in patients with MIRM who have extensive mucosal involvement, in an attempt to decrease inflammation and pain; however, evidence for such an approach is lacking. The hyperimmune reactions of the host to M pneumoniae infection include cytokine overproduction and T-cell activation, which promote both pulmonary and extrapulmonary manifestations. This forms the basis of immunosuppressive therapy, such as systemic corticosteroids, IV immunoglobulin, and cyclosporin A, particularly when MIRM is associated with pneumonia caused by infection with M pneumoniae.1,5,6
The overall prognosis of MIRM is good. Recurrence has been reported in up to 8% of cases, the treatment of which remains the same. Mucocutaneous and ocular sequelae (oral or genital synechiae, corneal ulcerations, dry eyes, loss of eye lashes) have been reported in less than 9% of patients.1 Other rare reported complications following the occurrence of MIRM include persistent cutaneous lesions, B cell lymphopenia, and restrictive lung disease or chronic obliterative bronchitis.
Our patient was started on IV ceftriaxone (50 mg/kg/d), azithromycin (10 mg/kg/d on the first day, then 5 mg/kg/d on the subsequent 5 days), and methylprednisolone (3 mg/kg/d) on Day 6 of illness. Within 3 days, there was marked improvement of mucositis and respiratory symptoms with resolution of fever. He was discharged on Day 10. At his outpatient follow-up 2 weeks later, the patient had made a complete recovery.
1. Canavan TN, Mathes EF, Frieden I, et al. Mycoplasma pneumoniae-induced rash and mucositis as a syndrome distinct from Stevens-Johnson syndrome and erythema multiforme: a systematic review. J Am Acad Dermatol 2015;72:239-245. doi: 10.1016/j.jaad.2014.06.026
2. Sauteur PMM, Theiler M, Buettcher M, et al. Frequency and clinical presentation of mucocutaneous disease due to mycoplasma pneumoniae infection in children with community-acquired pneumonia. JAMA Dermatol. 2020;156:144-150. doi: 10.1001/jamadermatol.2019.3602
3. Mayor-Ibarguren A, Feito-Rodriguez M, González-Ramos J, et al. Mucositis secondary to chlamydia pneumoniae infection: expanding the mycoplasma pneumoniae-induced rash and mucositis concept. Pediatr Dermatol 2017;34:465-472. doi: 10.1111/pde.13140
4. Frantz GF, McAninch SA. Mycoplasma mucositis. StatPearls [Internet]. Updated August 8, 2022. Accessed November 1, 2022. www.ncbi.nlm.nih.gov/books/NBK525960/
5. Yang EA, Kang HM, Rhim JW, et al. Early corticosteroid therapy for Mycoplasma pneumoniae pneumonia irrespective of used antibiotics in children. J Clin Med. 2019;8:726. doi: 10.3390/jcm8050726
6. Li HOY, Colantonio S, Ramien ML. Treatment of Mycoplasma pneumoniae-induced rash and mucositis with cyclosporine. J Cutan Med Surg. 2019;23:608-612. doi: 10.1177/1203475419874444
1. Canavan TN, Mathes EF, Frieden I, et al. Mycoplasma pneumoniae-induced rash and mucositis as a syndrome distinct from Stevens-Johnson syndrome and erythema multiforme: a systematic review. J Am Acad Dermatol 2015;72:239-245. doi: 10.1016/j.jaad.2014.06.026
2. Sauteur PMM, Theiler M, Buettcher M, et al. Frequency and clinical presentation of mucocutaneous disease due to mycoplasma pneumoniae infection in children with community-acquired pneumonia. JAMA Dermatol. 2020;156:144-150. doi: 10.1001/jamadermatol.2019.3602
3. Mayor-Ibarguren A, Feito-Rodriguez M, González-Ramos J, et al. Mucositis secondary to chlamydia pneumoniae infection: expanding the mycoplasma pneumoniae-induced rash and mucositis concept. Pediatr Dermatol 2017;34:465-472. doi: 10.1111/pde.13140
4. Frantz GF, McAninch SA. Mycoplasma mucositis. StatPearls [Internet]. Updated August 8, 2022. Accessed November 1, 2022. www.ncbi.nlm.nih.gov/books/NBK525960/
5. Yang EA, Kang HM, Rhim JW, et al. Early corticosteroid therapy for Mycoplasma pneumoniae pneumonia irrespective of used antibiotics in children. J Clin Med. 2019;8:726. doi: 10.3390/jcm8050726
6. Li HOY, Colantonio S, Ramien ML. Treatment of Mycoplasma pneumoniae-induced rash and mucositis with cyclosporine. J Cutan Med Surg. 2019;23:608-612. doi: 10.1177/1203475419874444
Keeping up with the evidence (and the residents)
I work with medical students nearly every day that I see patients. I recently mentioned to a student that I have a limited working knowledge of the brand names of diabetes medications released in the past 10 years. Just like the M3s, I need the full generic name to know whether a medication is a GLP-1 inhibitor or a DPP-4 inhibitor, because I know that “flozins” are SGLT-2 inhibitors and “glutides” are GLP-1 agonists. The combined efforts of an ambulatory care pharmacist and some flashcards have helped me to better understand how they work and which ones to prescribe when. Meanwhile, the residents are capably counseling on the adverse effects of the latest diabetes agent, while I am googling its generic name.
The premise of science is continuous discovery. In the first 10 months of 2022, the US Food & Drug Administration approved more than 2 dozen new medications, almost 100 new generics, and new indications for dozens more.1,2 The US Preventive Services Task Force (USPSTF) issued 13 new or reaffirmed recommendations in the first 10 months of 2022, and it is just one of dozens of bodies that issue guidelines relevant to primary care.3 PubMed indexes more than a million new articles each year. Learning new information and changing practice are crucial to being an effective clinician.
In this edition of JFP, Covey and Cagle4 write about updates to the USPSTF’s lung cancer screening guidelines. The authors reference changing evidence that led to the revised recommendations. When the original guideline was released in 2013, it drew on the best available evidence at the time.5 The National Lung Screening Trial, which looked at CT scanning compared with chest x-rays as screening tests for lung cancer, was groundbreaking in its methods and results.6 However, it was not without its flaws. It enrolled < 5% Black patients, and so the recommendations for age cutoffs and pack-year cutoffs were made based on the majority White population from the trial.
Black patients experience a higher mortality from lung cancer and are diagnosed at an earlier age and a lower cumulative pack-year exposure than White patients.7 Other studies have explored the social and political factors that lead to these disparities, which range from access to care to racial segregation of neighborhoods and tobacco marketing practices.7 When the USPSTF performed its periodic update of the guideline, it had access to additional research. The updates reflect the new information.
Every physician has a responsibility to find a way to adapt to important new information in medicine. Not using SGLT-2 inhibitors in the management of diabetes would be substandard care, and my patients would suffer for it. Not adopting the new lung cancer screening recommendations would exclude patients most at risk of lung cancer and allow disparities in lung cancer morbidity and mortality to grow.7,8Understanding the evidence behind the recommendations also reminds me that the guidelines will change again. These recommendations are no more static than the first guidelines were. I’ll be ready when the next update comes, and I’ll have the medical students and residents to keep me sharp.
1. US Food & Drug Administration. Novel drug approvals for 2022. Accessed October 27. 2022. www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2022
2. US Food & Drug Administration. First generic drug approvals. Accessed October 27. 2022. www.fda.gov/drugs/drug-and-biologic-approval-and-ind-activity-reports/first-generic-drug-approvals
3. US Preventive Services Task Force. Recommendations. Accessed October 27, 2022. www.uspreventiveservicestaskforce.org/uspstf/topic_search_results?topic_status=P
4. Covey CL, Cagle SD. Lung cancer screening: New evidence, updated guidance. J Fam Pract. 2022;71:398-402;415.
5. US Preventive Services Task Force. Lung cancer: screening. December 31, 2013. Accessed October 27, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening-december-2013
6. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. doi: 10.1056/NEJMoa1102873
7. Pinheiro LC, Groner L, Soroka O, et al. Analysis of eligibility for lung cancer screening by race after 2021 changes to US Preventive Services Task Force screening guidelines. JAMA network open. 2022;5:e2229741. doi: 10.1001/jamanetworkopen.2022.29741
8. US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325:962-970. doi: 10.1001/jama.2021.1117
I work with medical students nearly every day that I see patients. I recently mentioned to a student that I have a limited working knowledge of the brand names of diabetes medications released in the past 10 years. Just like the M3s, I need the full generic name to know whether a medication is a GLP-1 inhibitor or a DPP-4 inhibitor, because I know that “flozins” are SGLT-2 inhibitors and “glutides” are GLP-1 agonists. The combined efforts of an ambulatory care pharmacist and some flashcards have helped me to better understand how they work and which ones to prescribe when. Meanwhile, the residents are capably counseling on the adverse effects of the latest diabetes agent, while I am googling its generic name.
The premise of science is continuous discovery. In the first 10 months of 2022, the US Food & Drug Administration approved more than 2 dozen new medications, almost 100 new generics, and new indications for dozens more.1,2 The US Preventive Services Task Force (USPSTF) issued 13 new or reaffirmed recommendations in the first 10 months of 2022, and it is just one of dozens of bodies that issue guidelines relevant to primary care.3 PubMed indexes more than a million new articles each year. Learning new information and changing practice are crucial to being an effective clinician.
In this edition of JFP, Covey and Cagle4 write about updates to the USPSTF’s lung cancer screening guidelines. The authors reference changing evidence that led to the revised recommendations. When the original guideline was released in 2013, it drew on the best available evidence at the time.5 The National Lung Screening Trial, which looked at CT scanning compared with chest x-rays as screening tests for lung cancer, was groundbreaking in its methods and results.6 However, it was not without its flaws. It enrolled < 5% Black patients, and so the recommendations for age cutoffs and pack-year cutoffs were made based on the majority White population from the trial.
Black patients experience a higher mortality from lung cancer and are diagnosed at an earlier age and a lower cumulative pack-year exposure than White patients.7 Other studies have explored the social and political factors that lead to these disparities, which range from access to care to racial segregation of neighborhoods and tobacco marketing practices.7 When the USPSTF performed its periodic update of the guideline, it had access to additional research. The updates reflect the new information.
Every physician has a responsibility to find a way to adapt to important new information in medicine. Not using SGLT-2 inhibitors in the management of diabetes would be substandard care, and my patients would suffer for it. Not adopting the new lung cancer screening recommendations would exclude patients most at risk of lung cancer and allow disparities in lung cancer morbidity and mortality to grow.7,8Understanding the evidence behind the recommendations also reminds me that the guidelines will change again. These recommendations are no more static than the first guidelines were. I’ll be ready when the next update comes, and I’ll have the medical students and residents to keep me sharp.
I work with medical students nearly every day that I see patients. I recently mentioned to a student that I have a limited working knowledge of the brand names of diabetes medications released in the past 10 years. Just like the M3s, I need the full generic name to know whether a medication is a GLP-1 inhibitor or a DPP-4 inhibitor, because I know that “flozins” are SGLT-2 inhibitors and “glutides” are GLP-1 agonists. The combined efforts of an ambulatory care pharmacist and some flashcards have helped me to better understand how they work and which ones to prescribe when. Meanwhile, the residents are capably counseling on the adverse effects of the latest diabetes agent, while I am googling its generic name.
The premise of science is continuous discovery. In the first 10 months of 2022, the US Food & Drug Administration approved more than 2 dozen new medications, almost 100 new generics, and new indications for dozens more.1,2 The US Preventive Services Task Force (USPSTF) issued 13 new or reaffirmed recommendations in the first 10 months of 2022, and it is just one of dozens of bodies that issue guidelines relevant to primary care.3 PubMed indexes more than a million new articles each year. Learning new information and changing practice are crucial to being an effective clinician.
In this edition of JFP, Covey and Cagle4 write about updates to the USPSTF’s lung cancer screening guidelines. The authors reference changing evidence that led to the revised recommendations. When the original guideline was released in 2013, it drew on the best available evidence at the time.5 The National Lung Screening Trial, which looked at CT scanning compared with chest x-rays as screening tests for lung cancer, was groundbreaking in its methods and results.6 However, it was not without its flaws. It enrolled < 5% Black patients, and so the recommendations for age cutoffs and pack-year cutoffs were made based on the majority White population from the trial.
Black patients experience a higher mortality from lung cancer and are diagnosed at an earlier age and a lower cumulative pack-year exposure than White patients.7 Other studies have explored the social and political factors that lead to these disparities, which range from access to care to racial segregation of neighborhoods and tobacco marketing practices.7 When the USPSTF performed its periodic update of the guideline, it had access to additional research. The updates reflect the new information.
Every physician has a responsibility to find a way to adapt to important new information in medicine. Not using SGLT-2 inhibitors in the management of diabetes would be substandard care, and my patients would suffer for it. Not adopting the new lung cancer screening recommendations would exclude patients most at risk of lung cancer and allow disparities in lung cancer morbidity and mortality to grow.7,8Understanding the evidence behind the recommendations also reminds me that the guidelines will change again. These recommendations are no more static than the first guidelines were. I’ll be ready when the next update comes, and I’ll have the medical students and residents to keep me sharp.
1. US Food & Drug Administration. Novel drug approvals for 2022. Accessed October 27. 2022. www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2022
2. US Food & Drug Administration. First generic drug approvals. Accessed October 27. 2022. www.fda.gov/drugs/drug-and-biologic-approval-and-ind-activity-reports/first-generic-drug-approvals
3. US Preventive Services Task Force. Recommendations. Accessed October 27, 2022. www.uspreventiveservicestaskforce.org/uspstf/topic_search_results?topic_status=P
4. Covey CL, Cagle SD. Lung cancer screening: New evidence, updated guidance. J Fam Pract. 2022;71:398-402;415.
5. US Preventive Services Task Force. Lung cancer: screening. December 31, 2013. Accessed October 27, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening-december-2013
6. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. doi: 10.1056/NEJMoa1102873
7. Pinheiro LC, Groner L, Soroka O, et al. Analysis of eligibility for lung cancer screening by race after 2021 changes to US Preventive Services Task Force screening guidelines. JAMA network open. 2022;5:e2229741. doi: 10.1001/jamanetworkopen.2022.29741
8. US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325:962-970. doi: 10.1001/jama.2021.1117
1. US Food & Drug Administration. Novel drug approvals for 2022. Accessed October 27. 2022. www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2022
2. US Food & Drug Administration. First generic drug approvals. Accessed October 27. 2022. www.fda.gov/drugs/drug-and-biologic-approval-and-ind-activity-reports/first-generic-drug-approvals
3. US Preventive Services Task Force. Recommendations. Accessed October 27, 2022. www.uspreventiveservicestaskforce.org/uspstf/topic_search_results?topic_status=P
4. Covey CL, Cagle SD. Lung cancer screening: New evidence, updated guidance. J Fam Pract. 2022;71:398-402;415.
5. US Preventive Services Task Force. Lung cancer: screening. December 31, 2013. Accessed October 27, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening-december-2013
6. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. doi: 10.1056/NEJMoa1102873
7. Pinheiro LC, Groner L, Soroka O, et al. Analysis of eligibility for lung cancer screening by race after 2021 changes to US Preventive Services Task Force screening guidelines. JAMA network open. 2022;5:e2229741. doi: 10.1001/jamanetworkopen.2022.29741
8. US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325:962-970. doi: 10.1001/jama.2021.1117
Which anticoagulant is safest for frail elderly patients with nonvalvular A-fib?
ILLUSTRATIVE CASE
A frail 76-year-old woman with a history of hypertension and hyperlipidemia presents for evaluation of palpitations. An in-office electrocardiogram reveals that the patient is in AF. Her CHA2DS2-VASc score is 4 and her HAS-BLED score is 2.2,3 Using shared decision making, you decide to start medications for her AF. You plan to initiate a beta-blocker for rate control and must now decide on anticoagulation. Which oral anticoagulant would you prescribe for this patient’s AF, given her frail status?
Frailty is defined as a state of vulnerability with a decreased ability to recover from an acute stressful event.4 The prevalence of frailty varies by the measurements used and the population studied. A 2021 meta-analysis found that frailty prevalence ranges from 12% to 24% worldwide in patients older than 50 years5 and may increase to > 30% among those ages 85 years and older.6 Frailty increases rates of AEs such as falls7 and fracture,8 leading to disability,9 decreased quality of life,10 increased utilization of health care,11 and increased mortality.12 A number of validated approaches are available to screen for and measure frailty.13-18
Given the association with negative health outcomes and high health care utilization, frailty is an important clinical factor for physicians to consider when treating elderly patients. Frailty assessment may allow for more tailored treatment choices for patients, with a potential reduction in complications. Although CHA2DS2-VASc and HAS-BLED scores assist in the decision-making process of whether to start anticoagulation, these tools do not take frailty into consideration or guide anticoagulant choice.2,3 The purpose of this study was to analyze how levels of frailty affect the association of 3 different direct oral anticoagulants (DOACs) vs warfarin with various AEs (death, stroke, or major bleeding).
STUDY SUMMARY
This DOAC rose above the others
This retrospective cohort study compared the safety of 3 DOACs—dabigatran, rivaroxaban, and apixaban—vs warfarin in Medicare beneficiaries with AF, using 1:1 propensity score (PS)–matched analysis. Eligible patients were ages 65 years or older, with a filled prescription for a DOAC or warfarin, no prior oral anticoagulant exposure in the previous 183 days, a diagnostic code of AF, and continuous enrollment in Medicare Parts A, B, and D only. Patients were excluded if they had missing demographic data, received hospice care, resided in a nursing facility at drug initiation, had another indication for anticoagulation, or had a contraindication to either a DOAC or warfarin.
Frailty was measured using a claims-based frailty index (CFI), which applies health care utilization data to estimate a frailty index, with cut points for nonfrailty, prefrailty, and frailty. The CFI score has 93 claims-based variables, including wheelchairs and durable medical equipment, open wounds, diseases such as chronic obstructive pulmonary disease and ischemic heart disease, and transportation services.15-17 In this study, nonfrailty was defined as a CFI < 0.15, prefrailty as a CFI of 0.15 to 0.24, and frailty as a CFI ≥ 0.25.
The primary outcome—a composite endpoint of death, ischemic stroke, or major bleeding—was measured for each of the DOAC–warfarin cohorts in the overall population and stratified by frailty classification. Patients were followed until the occurrence of a study outcome, Medicare disenrollment, the end of the study period, discontinuation of the index drug (defined as > 5 days), change to a different anticoagulant, admission to a nursing facility, enrollment in hospice, initiation of dialysis, or kidney transplant. The authors conducted a PS-matched analysis to reduce any imbalances in clinical characteristics between the DOAC- and warfarin-treated groups, as well as a sensitivity analysis to assess the strength of the data findings using different assumptions.
The authors created 3 DOAC–warfarin cohorts: dabigatran (n = 81,863) vs warfarin (n = 256,722), rivaroxaban (n = 185,011) vs warfarin (n = 228,028), and apixaban (n = 222,478) vs warfarin (n = 206,031). After PS matching, the mean age in all cohorts was 76 to 77 years, about 50% were female, and 91% were White. The mean HAS-BLED score was 2 and the mean CHA2DS2-VASc score was 4. The mean CFI was 0.19 to 0.20, defined as prefrail. Patients classified as frail were older, more likely to be female, and more likely to have greater comorbidities, higher scores on CHA2DS2-VASc and HAS-BLED, and higher health care utilization.
Continue to: In the dabigatran-warfarin...
In the dabigatran–warfarin cohort (median follow-up, 72 days), the event rate of the composite endpoint per 1000 person-years (PY) was 63.5 for dabigatran and 65.6 for warfarin (hazard ratio [HR] = 0.98; 95% CI, 0.92 to 1.05; rate difference [RD] per 1000 PY = –2.2; 95% CI, –6.5 to 2.1). A lower rate of the composite endpoint was associated with dabigatran than warfarin for the nonfrail subgroup but not the prefrail or frail groups.
In the rivaroxaban–warfarin cohort (median follow-up, 82 days), the composite endpoint rate per 1000 PY was 77.8 for rivaroxaban and 83.7 for warfarin (HR = 0.98; 95% CI, 0.94 to 1.02; RD per 1000 PY = –5.9; 95% CI, –9.4 to –2.4). When stratifying by frailty category, both dabigatran and rivaroxaban were associated with a lower composite endpoint rate than warfarin for the nonfrail population only (HR = 0.81; 95% CI, 0.68 to 0.97, and HR = 0.88; 95% CI, 0.77 to 0.99, respectively).
In the apixaban–warfarin cohort (median follow-up, 84 days), the rate of the composite endpoint per 1000 PY was 60.1 for apixaban and 92.3 for warfarin (HR = 0.68; 95% CI, 0.65 to 0.72; RD per 1000 PY = –32.2; 95% CI, –36.1 to –28.3). The beneficial association for apixaban was present in all frailty categories, with an HR of 0.61 (95% CI, 0.52 to 0.71) for nonfrail patients, 0.66 (95% CI, 0.61 to 0.70) for prefrail patients, and 0.73 (95% CI, 0.67 to 0.80) for frail patients. Apixaban was the only DOAC with a relative reduction in the hazard of death, ischemic stroke, or major bleeding among all frailty groups.
WHAT’S NEW
Only apixaban had lower AE rates vs warfarin across frailty levels
Three DOACs (dabigatran, rivaroxaban, and apixaban) reduced the risk of death, ischemic stroke, or major bleeding compared with warfarin in older adults with AF, but only apixaban was associated with a relative reduction of these adverse outcomes in patients of all frailty classifications.
CAVEATS
Important data but RCTs are needed
The power of this observational study is considerable. However, it remains a retrospective observational study. The authors attempted to account for these limitations and potential confounders by performing a PS-matched analysis and sensitivity analysis; however, these findings should be confirmed with randomized controlled trials.
Continue to: Additionally, the study...
Additionally, the study collected data on each of the DOAC–warfarin cohorts for < 90 days. Trials to address long-term outcomes are warranted.
Finally, there was no control group in comparison with anticoagulation. It is possible that choosing not to use an anticoagulant is the best choice for frail elderly patients.
CHALLENGES TO IMPLEMENTATION
Doctors need a practical frailty scale, patients need an affordable Rx
Frailty is not often considered a measurable trait. The approach used in the study to determine the CFI is not a practical clinical tool. Studies comparing a frailty calculation software application or an easily implementable survey may help bring this clinically impactful information to the hands of primary care physicians. The Clinical Frailty Scale—a brief, 7-point scale based on the physician’s clinical impression of the patient—has been found to correlate with other established frailty measures18 and might be an option for busy clinicians. However, the current study did not utilize this measurement, and the validity of its use by primary care physicians in the outpatient setting requires further study.
In addition, cost may be a barrier for patients younger than 65 years or for those older than 65 years who do not qualify for Medicare or do not have Medicare Part D. The average monthly cost of the DOACs ranges from $560 for dabigatran19 to $600 for rivaroxaban20 and $623 for apixaban.21 As always, the choice of anticoagulant therapy is a clinical judgment and a joint decision of the patient and physician.
1. Kim DH, Pawar A, Gagne JJ, et al. Frailty and clinical outcomes of direct oral anticoagulants versus warfarin in older adults with atrial fibrillation: a cohort study. Ann Intern Med. 2021;174:1214-1223. doi: 10.7326/M20-7141
2. Zhu W, He W, Guo L, et al. The HAS-BLED score for predicting major bleeding risk in anticoagulated patients with atrial fibrillation: a systematic review and meta-analysis. Clin Cardiol. 2015;38:555-561. doi: 10.1002/clc.22435
3. Olesen JB, Lip GYH, Hansen ML, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342:d124. doi: 10.1136/bmj.d124
4. Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27:1-15. doi: 10.1016/j.cger.2010.08.009
5. O’Caoimh R, Sezgin D, O’Donovan MR, et al. Prevalence of frailty in 62 countries across the world: a systematic review and meta-analysis of population-level studies. Age Ageing. 2021;50:96-104. doi: 10.1093/ageing/afaa219
6. Campitelli MA, Bronskill SE, Hogan DB, et al. The prevalence and health consequences of frailty in a population-based older home care cohort: a comparison of different measures. BMC Geriatr. 2016;16:133. doi: 10.1186/s12877-016-0309-z
7. Kojima G. Frailty as a predictor of future falls among community-dwelling older people: a systematic review and meta-analysis. J Am Med Dir Assoc. 2015;16:1027-1033. doi: 10.1016/j.jamda. 2015.06.018
8. Kojima G. Frailty as a predictor of fractures among community-dwelling older people: a systematic review and meta-analysis. Bone. 2016;90:116-122. doi: 10.1016/j.bone.2016.06.009
9. Kojima G. Quick and simple FRAIL scale predicts incident activities of daily living (ADL) and instrumental ADL (IADL) disabilities: a systematic review and meta-analysis. J Am Med Dir Assoc. 2018;19:1063-1068. doi: 10.1016/j.jamda.2018.07.019
10. Kojima G, Liljas AEM, Iliffe S. Frailty syndrome: implications and challenges for health care policy. Risk Manag Healthc Policy. 2019;12:23-30. doi: 10.2147/RMHP.S168750
11. Roe L, Normand C, Wren MA, et al. The impact of frailty on healthcare utilisation in Ireland: evidence from The Irish Longitudinal Study on Ageing. BMC Geriatr. 2017;17:203. doi: 10.1186/s12877-017-0579-0
12. Hao Q, Zhou L, Dong B, et al. The role of frailty in predicting mortality and readmission in older adults in acute care wards: a prospective study. Sci Rep. 2019;9:1207. doi: 10.1038/s41598-018-38072-7
13. Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146-M156. doi: 10.1093/gerona/56.3.m146
14. Ryan J, Espinoza S, Ernst ME, et al. Validation of a deficit-accumulation frailty Index in the ASPirin in Reducing Events in the Elderly study and its predictive capacity for disability-free survival. J Gerontol A Biol Sci Med Sci. 2022;77:19-26. doi: 10.1093/gerona/glab225
15. Kim DH, Glynn RJ, Avorn J, et al. Validation of a claims-based frailty index against physical performance and adverse health outcomes in the Health and Retirement Study. J Gerontol A Biol Sci Med Sci. 2019;74:1271-1276. doi: 10.1093/gerona/gly197
16. Kim DH, Schneeweiss S, Glynn RJ, et al. Measuring frailty in Medicare data: development and validation of a claims-based frailty index. J Gerontol A Biol Sci Med Sci. 2018;73:980-987. doi: 10.1093/gerona/glx229
17. Claims-based frailty index. Harvard Dataverse website. 2022. Accessed April 5, 2022. https://dataverse.harvard.edu/dataverse/cfi
18. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489-95. doi: 10.1503/cmaj.050051
19. Dabigatran. GoodRx. Accessed September 26, 2022. www.goodrx.com/dabigatran
20. Rivaroxaban. GoodRx. Accessed September 26, 2022. www.goodrx.com/rivaroxaban
21. Apixaban (Eliquis). GoodRx. Accessed September 26, 2022. www.goodrx.com/eliquis
ILLUSTRATIVE CASE
A frail 76-year-old woman with a history of hypertension and hyperlipidemia presents for evaluation of palpitations. An in-office electrocardiogram reveals that the patient is in AF. Her CHA2DS2-VASc score is 4 and her HAS-BLED score is 2.2,3 Using shared decision making, you decide to start medications for her AF. You plan to initiate a beta-blocker for rate control and must now decide on anticoagulation. Which oral anticoagulant would you prescribe for this patient’s AF, given her frail status?
Frailty is defined as a state of vulnerability with a decreased ability to recover from an acute stressful event.4 The prevalence of frailty varies by the measurements used and the population studied. A 2021 meta-analysis found that frailty prevalence ranges from 12% to 24% worldwide in patients older than 50 years5 and may increase to > 30% among those ages 85 years and older.6 Frailty increases rates of AEs such as falls7 and fracture,8 leading to disability,9 decreased quality of life,10 increased utilization of health care,11 and increased mortality.12 A number of validated approaches are available to screen for and measure frailty.13-18
Given the association with negative health outcomes and high health care utilization, frailty is an important clinical factor for physicians to consider when treating elderly patients. Frailty assessment may allow for more tailored treatment choices for patients, with a potential reduction in complications. Although CHA2DS2-VASc and HAS-BLED scores assist in the decision-making process of whether to start anticoagulation, these tools do not take frailty into consideration or guide anticoagulant choice.2,3 The purpose of this study was to analyze how levels of frailty affect the association of 3 different direct oral anticoagulants (DOACs) vs warfarin with various AEs (death, stroke, or major bleeding).
STUDY SUMMARY
This DOAC rose above the others
This retrospective cohort study compared the safety of 3 DOACs—dabigatran, rivaroxaban, and apixaban—vs warfarin in Medicare beneficiaries with AF, using 1:1 propensity score (PS)–matched analysis. Eligible patients were ages 65 years or older, with a filled prescription for a DOAC or warfarin, no prior oral anticoagulant exposure in the previous 183 days, a diagnostic code of AF, and continuous enrollment in Medicare Parts A, B, and D only. Patients were excluded if they had missing demographic data, received hospice care, resided in a nursing facility at drug initiation, had another indication for anticoagulation, or had a contraindication to either a DOAC or warfarin.
Frailty was measured using a claims-based frailty index (CFI), which applies health care utilization data to estimate a frailty index, with cut points for nonfrailty, prefrailty, and frailty. The CFI score has 93 claims-based variables, including wheelchairs and durable medical equipment, open wounds, diseases such as chronic obstructive pulmonary disease and ischemic heart disease, and transportation services.15-17 In this study, nonfrailty was defined as a CFI < 0.15, prefrailty as a CFI of 0.15 to 0.24, and frailty as a CFI ≥ 0.25.
The primary outcome—a composite endpoint of death, ischemic stroke, or major bleeding—was measured for each of the DOAC–warfarin cohorts in the overall population and stratified by frailty classification. Patients were followed until the occurrence of a study outcome, Medicare disenrollment, the end of the study period, discontinuation of the index drug (defined as > 5 days), change to a different anticoagulant, admission to a nursing facility, enrollment in hospice, initiation of dialysis, or kidney transplant. The authors conducted a PS-matched analysis to reduce any imbalances in clinical characteristics between the DOAC- and warfarin-treated groups, as well as a sensitivity analysis to assess the strength of the data findings using different assumptions.
The authors created 3 DOAC–warfarin cohorts: dabigatran (n = 81,863) vs warfarin (n = 256,722), rivaroxaban (n = 185,011) vs warfarin (n = 228,028), and apixaban (n = 222,478) vs warfarin (n = 206,031). After PS matching, the mean age in all cohorts was 76 to 77 years, about 50% were female, and 91% were White. The mean HAS-BLED score was 2 and the mean CHA2DS2-VASc score was 4. The mean CFI was 0.19 to 0.20, defined as prefrail. Patients classified as frail were older, more likely to be female, and more likely to have greater comorbidities, higher scores on CHA2DS2-VASc and HAS-BLED, and higher health care utilization.
Continue to: In the dabigatran-warfarin...
In the dabigatran–warfarin cohort (median follow-up, 72 days), the event rate of the composite endpoint per 1000 person-years (PY) was 63.5 for dabigatran and 65.6 for warfarin (hazard ratio [HR] = 0.98; 95% CI, 0.92 to 1.05; rate difference [RD] per 1000 PY = –2.2; 95% CI, –6.5 to 2.1). A lower rate of the composite endpoint was associated with dabigatran than warfarin for the nonfrail subgroup but not the prefrail or frail groups.
In the rivaroxaban–warfarin cohort (median follow-up, 82 days), the composite endpoint rate per 1000 PY was 77.8 for rivaroxaban and 83.7 for warfarin (HR = 0.98; 95% CI, 0.94 to 1.02; RD per 1000 PY = –5.9; 95% CI, –9.4 to –2.4). When stratifying by frailty category, both dabigatran and rivaroxaban were associated with a lower composite endpoint rate than warfarin for the nonfrail population only (HR = 0.81; 95% CI, 0.68 to 0.97, and HR = 0.88; 95% CI, 0.77 to 0.99, respectively).
In the apixaban–warfarin cohort (median follow-up, 84 days), the rate of the composite endpoint per 1000 PY was 60.1 for apixaban and 92.3 for warfarin (HR = 0.68; 95% CI, 0.65 to 0.72; RD per 1000 PY = –32.2; 95% CI, –36.1 to –28.3). The beneficial association for apixaban was present in all frailty categories, with an HR of 0.61 (95% CI, 0.52 to 0.71) for nonfrail patients, 0.66 (95% CI, 0.61 to 0.70) for prefrail patients, and 0.73 (95% CI, 0.67 to 0.80) for frail patients. Apixaban was the only DOAC with a relative reduction in the hazard of death, ischemic stroke, or major bleeding among all frailty groups.
WHAT’S NEW
Only apixaban had lower AE rates vs warfarin across frailty levels
Three DOACs (dabigatran, rivaroxaban, and apixaban) reduced the risk of death, ischemic stroke, or major bleeding compared with warfarin in older adults with AF, but only apixaban was associated with a relative reduction of these adverse outcomes in patients of all frailty classifications.
CAVEATS
Important data but RCTs are needed
The power of this observational study is considerable. However, it remains a retrospective observational study. The authors attempted to account for these limitations and potential confounders by performing a PS-matched analysis and sensitivity analysis; however, these findings should be confirmed with randomized controlled trials.
Continue to: Additionally, the study...
Additionally, the study collected data on each of the DOAC–warfarin cohorts for < 90 days. Trials to address long-term outcomes are warranted.
Finally, there was no control group in comparison with anticoagulation. It is possible that choosing not to use an anticoagulant is the best choice for frail elderly patients.
CHALLENGES TO IMPLEMENTATION
Doctors need a practical frailty scale, patients need an affordable Rx
Frailty is not often considered a measurable trait. The approach used in the study to determine the CFI is not a practical clinical tool. Studies comparing a frailty calculation software application or an easily implementable survey may help bring this clinically impactful information to the hands of primary care physicians. The Clinical Frailty Scale—a brief, 7-point scale based on the physician’s clinical impression of the patient—has been found to correlate with other established frailty measures18 and might be an option for busy clinicians. However, the current study did not utilize this measurement, and the validity of its use by primary care physicians in the outpatient setting requires further study.
In addition, cost may be a barrier for patients younger than 65 years or for those older than 65 years who do not qualify for Medicare or do not have Medicare Part D. The average monthly cost of the DOACs ranges from $560 for dabigatran19 to $600 for rivaroxaban20 and $623 for apixaban.21 As always, the choice of anticoagulant therapy is a clinical judgment and a joint decision of the patient and physician.
ILLUSTRATIVE CASE
A frail 76-year-old woman with a history of hypertension and hyperlipidemia presents for evaluation of palpitations. An in-office electrocardiogram reveals that the patient is in AF. Her CHA2DS2-VASc score is 4 and her HAS-BLED score is 2.2,3 Using shared decision making, you decide to start medications for her AF. You plan to initiate a beta-blocker for rate control and must now decide on anticoagulation. Which oral anticoagulant would you prescribe for this patient’s AF, given her frail status?
Frailty is defined as a state of vulnerability with a decreased ability to recover from an acute stressful event.4 The prevalence of frailty varies by the measurements used and the population studied. A 2021 meta-analysis found that frailty prevalence ranges from 12% to 24% worldwide in patients older than 50 years5 and may increase to > 30% among those ages 85 years and older.6 Frailty increases rates of AEs such as falls7 and fracture,8 leading to disability,9 decreased quality of life,10 increased utilization of health care,11 and increased mortality.12 A number of validated approaches are available to screen for and measure frailty.13-18
Given the association with negative health outcomes and high health care utilization, frailty is an important clinical factor for physicians to consider when treating elderly patients. Frailty assessment may allow for more tailored treatment choices for patients, with a potential reduction in complications. Although CHA2DS2-VASc and HAS-BLED scores assist in the decision-making process of whether to start anticoagulation, these tools do not take frailty into consideration or guide anticoagulant choice.2,3 The purpose of this study was to analyze how levels of frailty affect the association of 3 different direct oral anticoagulants (DOACs) vs warfarin with various AEs (death, stroke, or major bleeding).
STUDY SUMMARY
This DOAC rose above the others
This retrospective cohort study compared the safety of 3 DOACs—dabigatran, rivaroxaban, and apixaban—vs warfarin in Medicare beneficiaries with AF, using 1:1 propensity score (PS)–matched analysis. Eligible patients were ages 65 years or older, with a filled prescription for a DOAC or warfarin, no prior oral anticoagulant exposure in the previous 183 days, a diagnostic code of AF, and continuous enrollment in Medicare Parts A, B, and D only. Patients were excluded if they had missing demographic data, received hospice care, resided in a nursing facility at drug initiation, had another indication for anticoagulation, or had a contraindication to either a DOAC or warfarin.
Frailty was measured using a claims-based frailty index (CFI), which applies health care utilization data to estimate a frailty index, with cut points for nonfrailty, prefrailty, and frailty. The CFI score has 93 claims-based variables, including wheelchairs and durable medical equipment, open wounds, diseases such as chronic obstructive pulmonary disease and ischemic heart disease, and transportation services.15-17 In this study, nonfrailty was defined as a CFI < 0.15, prefrailty as a CFI of 0.15 to 0.24, and frailty as a CFI ≥ 0.25.
The primary outcome—a composite endpoint of death, ischemic stroke, or major bleeding—was measured for each of the DOAC–warfarin cohorts in the overall population and stratified by frailty classification. Patients were followed until the occurrence of a study outcome, Medicare disenrollment, the end of the study period, discontinuation of the index drug (defined as > 5 days), change to a different anticoagulant, admission to a nursing facility, enrollment in hospice, initiation of dialysis, or kidney transplant. The authors conducted a PS-matched analysis to reduce any imbalances in clinical characteristics between the DOAC- and warfarin-treated groups, as well as a sensitivity analysis to assess the strength of the data findings using different assumptions.
The authors created 3 DOAC–warfarin cohorts: dabigatran (n = 81,863) vs warfarin (n = 256,722), rivaroxaban (n = 185,011) vs warfarin (n = 228,028), and apixaban (n = 222,478) vs warfarin (n = 206,031). After PS matching, the mean age in all cohorts was 76 to 77 years, about 50% were female, and 91% were White. The mean HAS-BLED score was 2 and the mean CHA2DS2-VASc score was 4. The mean CFI was 0.19 to 0.20, defined as prefrail. Patients classified as frail were older, more likely to be female, and more likely to have greater comorbidities, higher scores on CHA2DS2-VASc and HAS-BLED, and higher health care utilization.
Continue to: In the dabigatran-warfarin...
In the dabigatran–warfarin cohort (median follow-up, 72 days), the event rate of the composite endpoint per 1000 person-years (PY) was 63.5 for dabigatran and 65.6 for warfarin (hazard ratio [HR] = 0.98; 95% CI, 0.92 to 1.05; rate difference [RD] per 1000 PY = –2.2; 95% CI, –6.5 to 2.1). A lower rate of the composite endpoint was associated with dabigatran than warfarin for the nonfrail subgroup but not the prefrail or frail groups.
In the rivaroxaban–warfarin cohort (median follow-up, 82 days), the composite endpoint rate per 1000 PY was 77.8 for rivaroxaban and 83.7 for warfarin (HR = 0.98; 95% CI, 0.94 to 1.02; RD per 1000 PY = –5.9; 95% CI, –9.4 to –2.4). When stratifying by frailty category, both dabigatran and rivaroxaban were associated with a lower composite endpoint rate than warfarin for the nonfrail population only (HR = 0.81; 95% CI, 0.68 to 0.97, and HR = 0.88; 95% CI, 0.77 to 0.99, respectively).
In the apixaban–warfarin cohort (median follow-up, 84 days), the rate of the composite endpoint per 1000 PY was 60.1 for apixaban and 92.3 for warfarin (HR = 0.68; 95% CI, 0.65 to 0.72; RD per 1000 PY = –32.2; 95% CI, –36.1 to –28.3). The beneficial association for apixaban was present in all frailty categories, with an HR of 0.61 (95% CI, 0.52 to 0.71) for nonfrail patients, 0.66 (95% CI, 0.61 to 0.70) for prefrail patients, and 0.73 (95% CI, 0.67 to 0.80) for frail patients. Apixaban was the only DOAC with a relative reduction in the hazard of death, ischemic stroke, or major bleeding among all frailty groups.
WHAT’S NEW
Only apixaban had lower AE rates vs warfarin across frailty levels
Three DOACs (dabigatran, rivaroxaban, and apixaban) reduced the risk of death, ischemic stroke, or major bleeding compared with warfarin in older adults with AF, but only apixaban was associated with a relative reduction of these adverse outcomes in patients of all frailty classifications.
CAVEATS
Important data but RCTs are needed
The power of this observational study is considerable. However, it remains a retrospective observational study. The authors attempted to account for these limitations and potential confounders by performing a PS-matched analysis and sensitivity analysis; however, these findings should be confirmed with randomized controlled trials.
Continue to: Additionally, the study...
Additionally, the study collected data on each of the DOAC–warfarin cohorts for < 90 days. Trials to address long-term outcomes are warranted.
Finally, there was no control group in comparison with anticoagulation. It is possible that choosing not to use an anticoagulant is the best choice for frail elderly patients.
CHALLENGES TO IMPLEMENTATION
Doctors need a practical frailty scale, patients need an affordable Rx
Frailty is not often considered a measurable trait. The approach used in the study to determine the CFI is not a practical clinical tool. Studies comparing a frailty calculation software application or an easily implementable survey may help bring this clinically impactful information to the hands of primary care physicians. The Clinical Frailty Scale—a brief, 7-point scale based on the physician’s clinical impression of the patient—has been found to correlate with other established frailty measures18 and might be an option for busy clinicians. However, the current study did not utilize this measurement, and the validity of its use by primary care physicians in the outpatient setting requires further study.
In addition, cost may be a barrier for patients younger than 65 years or for those older than 65 years who do not qualify for Medicare or do not have Medicare Part D. The average monthly cost of the DOACs ranges from $560 for dabigatran19 to $600 for rivaroxaban20 and $623 for apixaban.21 As always, the choice of anticoagulant therapy is a clinical judgment and a joint decision of the patient and physician.
1. Kim DH, Pawar A, Gagne JJ, et al. Frailty and clinical outcomes of direct oral anticoagulants versus warfarin in older adults with atrial fibrillation: a cohort study. Ann Intern Med. 2021;174:1214-1223. doi: 10.7326/M20-7141
2. Zhu W, He W, Guo L, et al. The HAS-BLED score for predicting major bleeding risk in anticoagulated patients with atrial fibrillation: a systematic review and meta-analysis. Clin Cardiol. 2015;38:555-561. doi: 10.1002/clc.22435
3. Olesen JB, Lip GYH, Hansen ML, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342:d124. doi: 10.1136/bmj.d124
4. Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27:1-15. doi: 10.1016/j.cger.2010.08.009
5. O’Caoimh R, Sezgin D, O’Donovan MR, et al. Prevalence of frailty in 62 countries across the world: a systematic review and meta-analysis of population-level studies. Age Ageing. 2021;50:96-104. doi: 10.1093/ageing/afaa219
6. Campitelli MA, Bronskill SE, Hogan DB, et al. The prevalence and health consequences of frailty in a population-based older home care cohort: a comparison of different measures. BMC Geriatr. 2016;16:133. doi: 10.1186/s12877-016-0309-z
7. Kojima G. Frailty as a predictor of future falls among community-dwelling older people: a systematic review and meta-analysis. J Am Med Dir Assoc. 2015;16:1027-1033. doi: 10.1016/j.jamda. 2015.06.018
8. Kojima G. Frailty as a predictor of fractures among community-dwelling older people: a systematic review and meta-analysis. Bone. 2016;90:116-122. doi: 10.1016/j.bone.2016.06.009
9. Kojima G. Quick and simple FRAIL scale predicts incident activities of daily living (ADL) and instrumental ADL (IADL) disabilities: a systematic review and meta-analysis. J Am Med Dir Assoc. 2018;19:1063-1068. doi: 10.1016/j.jamda.2018.07.019
10. Kojima G, Liljas AEM, Iliffe S. Frailty syndrome: implications and challenges for health care policy. Risk Manag Healthc Policy. 2019;12:23-30. doi: 10.2147/RMHP.S168750
11. Roe L, Normand C, Wren MA, et al. The impact of frailty on healthcare utilisation in Ireland: evidence from The Irish Longitudinal Study on Ageing. BMC Geriatr. 2017;17:203. doi: 10.1186/s12877-017-0579-0
12. Hao Q, Zhou L, Dong B, et al. The role of frailty in predicting mortality and readmission in older adults in acute care wards: a prospective study. Sci Rep. 2019;9:1207. doi: 10.1038/s41598-018-38072-7
13. Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146-M156. doi: 10.1093/gerona/56.3.m146
14. Ryan J, Espinoza S, Ernst ME, et al. Validation of a deficit-accumulation frailty Index in the ASPirin in Reducing Events in the Elderly study and its predictive capacity for disability-free survival. J Gerontol A Biol Sci Med Sci. 2022;77:19-26. doi: 10.1093/gerona/glab225
15. Kim DH, Glynn RJ, Avorn J, et al. Validation of a claims-based frailty index against physical performance and adverse health outcomes in the Health and Retirement Study. J Gerontol A Biol Sci Med Sci. 2019;74:1271-1276. doi: 10.1093/gerona/gly197
16. Kim DH, Schneeweiss S, Glynn RJ, et al. Measuring frailty in Medicare data: development and validation of a claims-based frailty index. J Gerontol A Biol Sci Med Sci. 2018;73:980-987. doi: 10.1093/gerona/glx229
17. Claims-based frailty index. Harvard Dataverse website. 2022. Accessed April 5, 2022. https://dataverse.harvard.edu/dataverse/cfi
18. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489-95. doi: 10.1503/cmaj.050051
19. Dabigatran. GoodRx. Accessed September 26, 2022. www.goodrx.com/dabigatran
20. Rivaroxaban. GoodRx. Accessed September 26, 2022. www.goodrx.com/rivaroxaban
21. Apixaban (Eliquis). GoodRx. Accessed September 26, 2022. www.goodrx.com/eliquis
1. Kim DH, Pawar A, Gagne JJ, et al. Frailty and clinical outcomes of direct oral anticoagulants versus warfarin in older adults with atrial fibrillation: a cohort study. Ann Intern Med. 2021;174:1214-1223. doi: 10.7326/M20-7141
2. Zhu W, He W, Guo L, et al. The HAS-BLED score for predicting major bleeding risk in anticoagulated patients with atrial fibrillation: a systematic review and meta-analysis. Clin Cardiol. 2015;38:555-561. doi: 10.1002/clc.22435
3. Olesen JB, Lip GYH, Hansen ML, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342:d124. doi: 10.1136/bmj.d124
4. Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27:1-15. doi: 10.1016/j.cger.2010.08.009
5. O’Caoimh R, Sezgin D, O’Donovan MR, et al. Prevalence of frailty in 62 countries across the world: a systematic review and meta-analysis of population-level studies. Age Ageing. 2021;50:96-104. doi: 10.1093/ageing/afaa219
6. Campitelli MA, Bronskill SE, Hogan DB, et al. The prevalence and health consequences of frailty in a population-based older home care cohort: a comparison of different measures. BMC Geriatr. 2016;16:133. doi: 10.1186/s12877-016-0309-z
7. Kojima G. Frailty as a predictor of future falls among community-dwelling older people: a systematic review and meta-analysis. J Am Med Dir Assoc. 2015;16:1027-1033. doi: 10.1016/j.jamda. 2015.06.018
8. Kojima G. Frailty as a predictor of fractures among community-dwelling older people: a systematic review and meta-analysis. Bone. 2016;90:116-122. doi: 10.1016/j.bone.2016.06.009
9. Kojima G. Quick and simple FRAIL scale predicts incident activities of daily living (ADL) and instrumental ADL (IADL) disabilities: a systematic review and meta-analysis. J Am Med Dir Assoc. 2018;19:1063-1068. doi: 10.1016/j.jamda.2018.07.019
10. Kojima G, Liljas AEM, Iliffe S. Frailty syndrome: implications and challenges for health care policy. Risk Manag Healthc Policy. 2019;12:23-30. doi: 10.2147/RMHP.S168750
11. Roe L, Normand C, Wren MA, et al. The impact of frailty on healthcare utilisation in Ireland: evidence from The Irish Longitudinal Study on Ageing. BMC Geriatr. 2017;17:203. doi: 10.1186/s12877-017-0579-0
12. Hao Q, Zhou L, Dong B, et al. The role of frailty in predicting mortality and readmission in older adults in acute care wards: a prospective study. Sci Rep. 2019;9:1207. doi: 10.1038/s41598-018-38072-7
13. Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146-M156. doi: 10.1093/gerona/56.3.m146
14. Ryan J, Espinoza S, Ernst ME, et al. Validation of a deficit-accumulation frailty Index in the ASPirin in Reducing Events in the Elderly study and its predictive capacity for disability-free survival. J Gerontol A Biol Sci Med Sci. 2022;77:19-26. doi: 10.1093/gerona/glab225
15. Kim DH, Glynn RJ, Avorn J, et al. Validation of a claims-based frailty index against physical performance and adverse health outcomes in the Health and Retirement Study. J Gerontol A Biol Sci Med Sci. 2019;74:1271-1276. doi: 10.1093/gerona/gly197
16. Kim DH, Schneeweiss S, Glynn RJ, et al. Measuring frailty in Medicare data: development and validation of a claims-based frailty index. J Gerontol A Biol Sci Med Sci. 2018;73:980-987. doi: 10.1093/gerona/glx229
17. Claims-based frailty index. Harvard Dataverse website. 2022. Accessed April 5, 2022. https://dataverse.harvard.edu/dataverse/cfi
18. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489-95. doi: 10.1503/cmaj.050051
19. Dabigatran. GoodRx. Accessed September 26, 2022. www.goodrx.com/dabigatran
20. Rivaroxaban. GoodRx. Accessed September 26, 2022. www.goodrx.com/rivaroxaban
21. Apixaban (Eliquis). GoodRx. Accessed September 26, 2022. www.goodrx.com/eliquis
PRACTICE CHANGER
Consider apixaban, which demonstrated a lower adverse event (AE) rate than warfarin regardless of frailty status, for anticoagulation treatment of older patients with nonvalvular atrial fibrillation (AF); by comparison, AE rates for dabigatran and rivaroxaban were lower vs warfarin only among nonfrail individuals.
STRENGTH OF RECOMMENDATION
C: Based on a retrospective observational cohort study.1
Kim DH, Pawar A, Gagne JJ, et al. Frailty and clinical outcomes of direct oral anticoagulants versus warfarin in older adults with atrial fibrillation: a cohort study. Ann Intern Med. 2021;174:1214-1223. doi: 10.7326/M20-7141