User login
Lung cancer screening: New evidence, updated guidance
CASE
A 51-year-old man presents to your office to discuss lung cancer screening. He has a history of hypertension and prediabetes. His father died of lung cancer 5 years ago, at age 77. The patient stopped smoking soon thereafter; prior to that, he smoked 1 pack of cigarettes per day for 20 years. He wants to know if he should be screened for lung cancer.
The relative lack of symptoms during the early stages of lung cancer frequently results in a delayed diagnosis. This, and the speed at which the disease progresses, underscores the need for an effective screening modality. More than half of people with lung cancer die within 1 year of diagnosis.1 Excluding skin cancer, lung cancer is the second most commonly diagnosed cancer, and more people die of lung cancer than of colon, breast, and prostate cancers combined.2 In 2022, it was estimated that there would be 236,740 new cases of lung cancer and 130,180 deaths from lung cancer.1,2 The average age at diagnosis is 70 years.2

Screening modalities: Only 1 has demonstrated mortality benefit
In 1968, Wilson and Junger3 outlined the characteristics of the ideal screening test for the World Health Organization: it should limit risk to the patient, be sensitive for detecting the disease early in its course, limit false-positive results, be acceptable to the patient, and be inexpensive to the health system.3 For decades, several screening modalities for lung cancer were trialed to fit the above guidance, but many of them fell short of the most important outcome: the impact on mortality.
Sputum cytology. The use of sputum cytology, either in combination with or without chest radiography, is not recommended. Several randomized controlled trials (RCTs) have failed to demonstrate improved lung cancer detection or mortality reduction in patients screened with this modality.4
Chest radiography (CXR). Several studies have assessed the efficacy of CXR as a screening modality. The best known was the Prostate, Lung, Colon, Ovarian (PLCO) Trial.5 This multicenter RCT enrolled more than 154,000 participants, half of whom received CXR at baseline and then annually for 3 years; the other half continued usual care (no screening). After 13 years of follow-up, there were no significant differences in lung cancer detection or mortality rates between the 2 groups.5
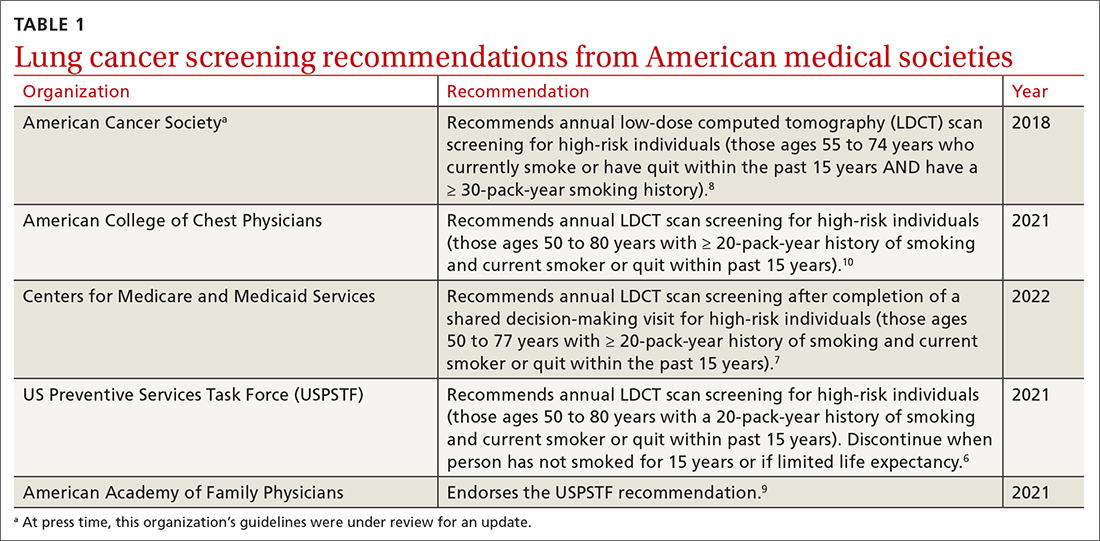
Low-dose computed tomography (LDCT). Several major medical societies recommend LDCT to screen high-risk individuals for lung cancer (TABLE 16-10). Results from 2 major RCTs have guided these recommendations.
The National Lung Screening Trial (NLST) was a multicenter RCT comparing 2 screening tests for lung cancer.11 Approximately 54,000 high-risk participants were enrolled between 2002 and 2004 and were randomized to receive annual screening with either LDCT or single-view CXR. The trial was discontinued prematurely when investigators noted a 20% reduction in lung cancer mortality in the LDCT group vs the CXR group.12 This equates to 3 fewer deaths for every 1000 people screened with LDCT vs CXR. There was also a 6% reduction in all-cause mortality noted in the LDCT vs the CXR group.12
Continue to: The NELSON trial...
The NELSON trial, conducted between 2005 and 2015, studied more than 15,000 current or former smokers ages 50 to 74 years and compared LDCT screening at various intervals to no screening.13 After 10 years, lung cancer–related mortality was reduced by 24% (or 1 less death per 1000 person-years) in men who were screened vs their unscreened counterparts.13 In contrast to the NLST, in the NELSON trial, no significant difference in all-cause mortality was observed. Subgroup analysis of the relatively small population of women included in the NELSON trial suggested a 33% reduction in 10-year mortality; however, the difference was nonsignificant between the screened and unscreened groups.13
Each of these landmark studies had characteristics that could limit the results' generalizability to the US population. In the NELSON trial, more than 80% of the study participants were male. In both trials, there was significant underrepresentation of Black, Asian, Hispanic, and other non-White people.12,13 Furthermore, participants in these studies were of higher socioeconomic status than the general US screening-eligible population.
At this time, LDCT is the only lung cancer screening modality that has shown benefit for both disease-related and all-cause mortality, in the populations that were studied. Based on the NLST, the number needed to screen (NNS) with LDCT to prevent 1 lung cancer–related death is 308. The NNS to prevent 1 death from any cause is 219.6
Updated evidence has led to a consensus on screening criteria
Many national societies endorse annual screening with LDCT in high-risk individuals (TABLE 16-10). Risk assessment for the purpose of lung cancer screening includes a detailed review of smoking history and age. The risk of lung cancer increases with advancing age and with cumulative quantity and duration of smoking, but decreases with increasing time since quitting. Therefore, a detailed smoking history should include total number of pack-years, current smoking status, and, if applicable, when smoking cessation occurred.
In 2021, the US Preventive Services Task Force (USPSTF) updated their 2013 lung cancer screening recommendations, expanding the screening age range and lowering the smoking history threshold for triggering initiation of screening.6 The impetus for the update was emerging evidence from systematic reviews, RCTs, and the Cancer Intervention and Surveillance Modeling Network (CISNET) that could help to determine the optimal age for screening and identify high-risk groups. For example, the NELSON trial, combined with results from CISNET modeling data, showed an empirical benefit for screening those ages 50 to 55 years.6
Continue to: As a result...
As a result, the USPSTF now recommends annual lung cancer screening with LDCT for any adult ages 50 to 80 years who has a 20-pack-year smoking history and currently smokes or has quit within the past 15 years.6 Screening should be discontinued once a person has not smoked for 15 years, develops a health problem that substantially limits life expectancy, or is not willing to have curative lung surgery.6
Expanding the screening eligibility may also address racial and gender disparities in health care. Black people and women who smoke have a higher risk for lung cancer at a lower intensity of smoking.6
Following the USPSTF update, the American College of Chest Physicians and the Centers for Medicare and Medicaid Services published updated guidance that aligns with USPSTF’s recommendations to lower the age and pack-year qualifications for initiating screening.7,10 The American Cancer Society is currently reviewing its 2018 guidelines on lung cancer screening.14 TABLE 16-10 summarizes the guidance on lung cancer screening from these medical societies.
Effective screening could save lives (and money)
A smoker’s risk for lung cancer is 20 times higher than that of a nonsmoker15,16; 55% of lung cancer deaths in women and 70% in men are attributed to smoking.17 Once diagnosed with lung cancer, more than 50% of people will die within 1 year.1 This underpins the need for a lung cancer screening modality that reduces mortality. Large RCTs, including the NLST and NELSON trials, have shown that screening high-risk individuals with LDCT can significantly reduce lung cancer–related death when compared to no screening or screening with CXR alone.11,13
There is controversy surrounding the cost benefit of implementing a nationwide lung cancer screening program. However, recent use of microsimulation models has shown LDCT to be a cost-effective strategy, with an average cost of $81,000 per quality-adjusted life-year, which is below the threshold of $100,000 to be considered cost effective.18 Expanding the upper age limit for screening leads to a greater reduction in mortality but increases treatment costs and overdiagnosis rates, and overall does not improve quality-adjusted life-years.18
Continue to: Potential harms
Potential harms: False-positives and related complications
Screening for lung cancer is not without its risks. Harms from screening typically result from false-positive test results leading to overdiagnosis, anxiety and distress, unnecessary invasive tests or procedures, and increased costs.19 TABLE 26,19-23 lists specific complications from lung cancer screening with LDCT.
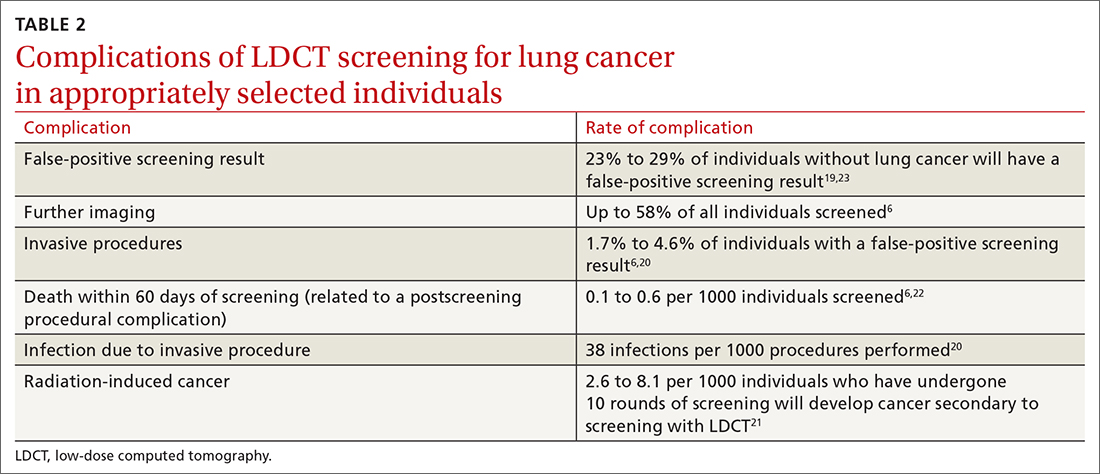
The false-positive rate is not trivial. For every 1000 patients screened, 250 people will have a positive LDCT finding but will not have lung cancer.19 Furthermore, about 1 in every 2000 individuals who screen positive, but who do not have lung cancer, die as a result of complications from the ensuing work-up.6
Annual LDCT screening increases the risk of radiation-induced cancer by approximately 0.05% over 10 years.21 The absolute risk is generally low but not insignificant. However, the mortality benefits previously outlined are significantly more robust in both absolute and relative terms vs the 10-year risk of radiation-induced cancer.
Lastly, it is important to note that the NELSON trial and NLST included a limited number of LDCT scans. Current guidelines for lung cancer screening with LDCT, including those from the USPSTF, recommend screening annually. We do not know the cumulative harm of annual LDCT over a 20- or 30-year period for those who would qualify (ie, current smokers).
If you screen, you must be able to act on the results
Effective screening programs should extend beyond the LDCT scan itself. The studies that have shown a benefit of LDCT were done at large academic centers that had the appropriate radiologic, pathologic, and surgical infrastructure to interpret and act on results and offer further diagnostic or treatment procedures.
Continue to: Prior to screening...
Prior to screening for lung cancer with LDCT, documentation of shared decision-making between the patient and the clinician is necessary.7 This discussion should include the potential benefits and harms of screening, potential results and likelihood of follow-up diagnostic testing, the false-positive rate of LDCT lung cancer screening, and cumulative radiation exposure. In addition, screening should be considered only if the patient is willing to be screened annually, is willing to pursue follow-up scans and procedures (including lung biopsy) if deemed necessary, and does not have comorbid conditions that significantly limit life expectancy.
Smoking cessation: The most important change to make
Smoking cessation is the single most important risk-modifying behavior to reduce one’s chance of developing lung cancer. At age 40, smokers have a 2-fold increase in all-cause mortality compared to age-matched nonsmokers. This rises to a 3-fold increase by the age of 70.16
Smoking cessation reduces the risk of lung cancer by 20% after 5 years, 30% to 50% after 10 years, and up to 70% after 15 years.24 In its guidelines, the American Thoracic Society recommends varenicline (Chantix) for all smokers to assist with smoking cessation.25
CASE
This 51-year-old patient with at least a 20-pack-year history of smoking should be commended for giving up smoking. Based on the USPSTF recommendations, he should be screened annually with LDCT for the next 10 years.
Screening to save more lives
The results of 2 large multicenter RCTs have led to the recent recommendation for lung cancer screening of high-risk adults with the use of LDCT. Screening with LDCT has been shown to reduce disease-related mortality and likely be cost effective in the long term.
Screening with LDCT should be part of a multidisciplinary system that has the infrastructure not only to perform the screening, but also to diagnose and appropriately follow up and treat patients whose results are concerning. The risk of false-positive results leading to increased anxiety, overdiagnosis, and unnecessary procedures points to the importance of proper patient selection, counseling, and shared decision-making. Smoking cessation remains the most important disease-modifying behavior one can make to reduce their risk for lung cancer.
CORRESPONDENCE
Carlton J. Covey, MD, 101 Bodin Circle, David Grant Medical Center, Travis Air Force Base, Fairfield, CA, 94545; [email protected]
1. National Cancer Institute. Cancer Stat Facts: lung and bronchus cancer. Accessed October 12, 2022. https://seer.cancer.gov/statfacts/html/lungb.html
2. American Cancer Society. Key statistics for lung cancer. Accessed October 12, 2022. https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html
3. Wilson JMG, Junger G. Principles and Practice of Screening for Disease. World Health Organization; 1968:21-25, 100. https://apps.who.int/iris/handle/10665/37650
4. Humphrey LL, Teutsch S, Johnson M. Lung cancer screening with sputum cytologic examination, chest radiography, and computed tomography: an update for the United States preventive services task force. Ann Intern Med. 2004;140:740-753. doi: 10.7326/0003-4819-140-9-200405040-00015
5. Oken MM, Hocking WG, Kvale PA, et al. Screening by chest radiograph and lung cancer mortality: the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA. 2011;306:1865-1873. doi: 10.1001/jama.2011.1591
6. US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325:962-970. doi: 10.1001/jama.2021.1117
7. Centers for Medicare & Medicaid Services. Screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439R). Accessed October 14, 2022. www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=304
8. Smith RA, Andrews KS, Brooks D, et al. Cancer screening in the United States, 2018: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2018;68:297-316. doi: 10.3322/caac.21446
9. American Academy of Family Physicians. AAFP updates recommendation on lung cancer screening. Published April 6, 2021. Accessed October 12, 2022. www.aafp.org/news/health-of-the-public/20210406lungcancer.html
10. Mazzone PJ, Silvestri GA, Souter LH, et al. Screening for lung cancer: CHEST Guideline and Expert Panel Report. CHEST. 2021;160:E427-E494. doi: 10.1016/j.chest.2021.06.063
11. The National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. doi: 10.1056/NEJMoa1102873
12. The National Lung Screening Trial Research Team. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368:1980-1991. doi: 10.1056/NEJMoa1209120
13. de Koning HJ, van der Aalst CM, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382:503-513. doi: 10.1056/NEJMoa1911793
14. American Cancer Society. Lung cancer screening guidelines. Accessed October 14, 2022. www.cancer.org/health-care-professionals/american-cancer-society-prevention-early-detection-guidelines/lung-cancer-screening-guidelines.html
15. Pirie K, Peto R, Reeves GK, et al. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet. 2013;381:133-141. doi: 10.1016/S0140-6736(12)61720-6
16. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519. doi: 10.1136/bmj.38142.554479.AE
17. O’Keefe LM, Gemma T, Huxley R, et al. Smoking as a risk factor for lung cancer in women and men: a systematic review and meta-analysis. BMJ Open. 2018;8:e021611. doi: 10.1136/bmjopen-2018-021611
18. Criss SD, Pianpian C, Bastani M, et al. Cost-effectiveness analysis of lung cancer screening in the United States: a comparative modeling study. Ann Intern Med. 2019;171:796-805. doi: 10.7326/M19-0322
19. Lazris A, Roth RA. Lung cancer screening: pros and cons. Am Fam Physician. 2019;99:740-742.
20. Ali MU, Miller J, Peirson L, et al. Screening for lung cancer: a systematic review and meta-analysis. Prev Med. 2016;89:301-314. doi: 10.1016/j.ypmed.2016.04.015
21. Rampinelli C, De Marco P, Origgi D, et al. Exposure to low dose computed tomography for lung cancer screening and risk of cancer: secondary analysis of trial data and risk-benefit analysis. BMJ. 2017;356:j347. doi: 10.1136/bmj.j347
22. Manser RL, Lethaby A, Irving LB, et al. Screening for lung cancer. Cochrane Database Syst Rev. 2013;CD001991. doi: 10.1002/14651858.CD001991.pub3
23. Mazzone PJ, Silvestri GA, Patel S, et al. Screening for lung cancer: CHEST guideline and expert panel report. CHEST. 2018;153:954-985. doi: 10.1016/j.chest.2018.01.016
24. US Public Health Service Office of the Surgeon General; National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking. and Health. Smoking Cessation: A Report of the Surgeon General. US Department of Health and Human Services; 2020. www.ncbi.nlm.nih.gov/books/NBK555591/
25. Leone FT, Zhang Y, Evers-Casey S, et al, on behalf of the American Thoracic Society Assembly on Clinical Problems. Initiating pharmacologic treatment in tobacco-dependent adults: an official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;202:e5-e31. doi: 10.1164/rccm.202005-1982ST
CASE
A 51-year-old man presents to your office to discuss lung cancer screening. He has a history of hypertension and prediabetes. His father died of lung cancer 5 years ago, at age 77. The patient stopped smoking soon thereafter; prior to that, he smoked 1 pack of cigarettes per day for 20 years. He wants to know if he should be screened for lung cancer.
The relative lack of symptoms during the early stages of lung cancer frequently results in a delayed diagnosis. This, and the speed at which the disease progresses, underscores the need for an effective screening modality. More than half of people with lung cancer die within 1 year of diagnosis.1 Excluding skin cancer, lung cancer is the second most commonly diagnosed cancer, and more people die of lung cancer than of colon, breast, and prostate cancers combined.2 In 2022, it was estimated that there would be 236,740 new cases of lung cancer and 130,180 deaths from lung cancer.1,2 The average age at diagnosis is 70 years.2

Screening modalities: Only 1 has demonstrated mortality benefit
In 1968, Wilson and Junger3 outlined the characteristics of the ideal screening test for the World Health Organization: it should limit risk to the patient, be sensitive for detecting the disease early in its course, limit false-positive results, be acceptable to the patient, and be inexpensive to the health system.3 For decades, several screening modalities for lung cancer were trialed to fit the above guidance, but many of them fell short of the most important outcome: the impact on mortality.
Sputum cytology. The use of sputum cytology, either in combination with or without chest radiography, is not recommended. Several randomized controlled trials (RCTs) have failed to demonstrate improved lung cancer detection or mortality reduction in patients screened with this modality.4
Chest radiography (CXR). Several studies have assessed the efficacy of CXR as a screening modality. The best known was the Prostate, Lung, Colon, Ovarian (PLCO) Trial.5 This multicenter RCT enrolled more than 154,000 participants, half of whom received CXR at baseline and then annually for 3 years; the other half continued usual care (no screening). After 13 years of follow-up, there were no significant differences in lung cancer detection or mortality rates between the 2 groups.5
Low-dose computed tomography (LDCT). Several major medical societies recommend LDCT to screen high-risk individuals for lung cancer (TABLE 16-10). Results from 2 major RCTs have guided these recommendations.

The National Lung Screening Trial (NLST) was a multicenter RCT comparing 2 screening tests for lung cancer.11 Approximately 54,000 high-risk participants were enrolled between 2002 and 2004 and were randomized to receive annual screening with either LDCT or single-view CXR. The trial was discontinued prematurely when investigators noted a 20% reduction in lung cancer mortality in the LDCT group vs the CXR group.12 This equates to 3 fewer deaths for every 1000 people screened with LDCT vs CXR. There was also a 6% reduction in all-cause mortality noted in the LDCT vs the CXR group.12
Continue to: The NELSON trial...
The NELSON trial, conducted between 2005 and 2015, studied more than 15,000 current or former smokers ages 50 to 74 years and compared LDCT screening at various intervals to no screening.13 After 10 years, lung cancer–related mortality was reduced by 24% (or 1 less death per 1000 person-years) in men who were screened vs their unscreened counterparts.13 In contrast to the NLST, in the NELSON trial, no significant difference in all-cause mortality was observed. Subgroup analysis of the relatively small population of women included in the NELSON trial suggested a 33% reduction in 10-year mortality; however, the difference was nonsignificant between the screened and unscreened groups.13
Each of these landmark studies had characteristics that could limit the results' generalizability to the US population. In the NELSON trial, more than 80% of the study participants were male. In both trials, there was significant underrepresentation of Black, Asian, Hispanic, and other non-White people.12,13 Furthermore, participants in these studies were of higher socioeconomic status than the general US screening-eligible population.
At this time, LDCT is the only lung cancer screening modality that has shown benefit for both disease-related and all-cause mortality, in the populations that were studied. Based on the NLST, the number needed to screen (NNS) with LDCT to prevent 1 lung cancer–related death is 308. The NNS to prevent 1 death from any cause is 219.6
Updated evidence has led to a consensus on screening criteria
Many national societies endorse annual screening with LDCT in high-risk individuals (TABLE 16-10). Risk assessment for the purpose of lung cancer screening includes a detailed review of smoking history and age. The risk of lung cancer increases with advancing age and with cumulative quantity and duration of smoking, but decreases with increasing time since quitting. Therefore, a detailed smoking history should include total number of pack-years, current smoking status, and, if applicable, when smoking cessation occurred.
In 2021, the US Preventive Services Task Force (USPSTF) updated their 2013 lung cancer screening recommendations, expanding the screening age range and lowering the smoking history threshold for triggering initiation of screening.6 The impetus for the update was emerging evidence from systematic reviews, RCTs, and the Cancer Intervention and Surveillance Modeling Network (CISNET) that could help to determine the optimal age for screening and identify high-risk groups. For example, the NELSON trial, combined with results from CISNET modeling data, showed an empirical benefit for screening those ages 50 to 55 years.6
Continue to: As a result...
As a result, the USPSTF now recommends annual lung cancer screening with LDCT for any adult ages 50 to 80 years who has a 20-pack-year smoking history and currently smokes or has quit within the past 15 years.6 Screening should be discontinued once a person has not smoked for 15 years, develops a health problem that substantially limits life expectancy, or is not willing to have curative lung surgery.6
Expanding the screening eligibility may also address racial and gender disparities in health care. Black people and women who smoke have a higher risk for lung cancer at a lower intensity of smoking.6
Following the USPSTF update, the American College of Chest Physicians and the Centers for Medicare and Medicaid Services published updated guidance that aligns with USPSTF’s recommendations to lower the age and pack-year qualifications for initiating screening.7,10 The American Cancer Society is currently reviewing its 2018 guidelines on lung cancer screening.14 TABLE 16-10 summarizes the guidance on lung cancer screening from these medical societies.
Effective screening could save lives (and money)
A smoker’s risk for lung cancer is 20 times higher than that of a nonsmoker15,16; 55% of lung cancer deaths in women and 70% in men are attributed to smoking.17 Once diagnosed with lung cancer, more than 50% of people will die within 1 year.1 This underpins the need for a lung cancer screening modality that reduces mortality. Large RCTs, including the NLST and NELSON trials, have shown that screening high-risk individuals with LDCT can significantly reduce lung cancer–related death when compared to no screening or screening with CXR alone.11,13
There is controversy surrounding the cost benefit of implementing a nationwide lung cancer screening program. However, recent use of microsimulation models has shown LDCT to be a cost-effective strategy, with an average cost of $81,000 per quality-adjusted life-year, which is below the threshold of $100,000 to be considered cost effective.18 Expanding the upper age limit for screening leads to a greater reduction in mortality but increases treatment costs and overdiagnosis rates, and overall does not improve quality-adjusted life-years.18
Continue to: Potential harms
Potential harms: False-positives and related complications
Screening for lung cancer is not without its risks. Harms from screening typically result from false-positive test results leading to overdiagnosis, anxiety and distress, unnecessary invasive tests or procedures, and increased costs.19 TABLE 26,19-23 lists specific complications from lung cancer screening with LDCT.

The false-positive rate is not trivial. For every 1000 patients screened, 250 people will have a positive LDCT finding but will not have lung cancer.19 Furthermore, about 1 in every 2000 individuals who screen positive, but who do not have lung cancer, die as a result of complications from the ensuing work-up.6
Annual LDCT screening increases the risk of radiation-induced cancer by approximately 0.05% over 10 years.21 The absolute risk is generally low but not insignificant. However, the mortality benefits previously outlined are significantly more robust in both absolute and relative terms vs the 10-year risk of radiation-induced cancer.
Lastly, it is important to note that the NELSON trial and NLST included a limited number of LDCT scans. Current guidelines for lung cancer screening with LDCT, including those from the USPSTF, recommend screening annually. We do not know the cumulative harm of annual LDCT over a 20- or 30-year period for those who would qualify (ie, current smokers).
If you screen, you must be able to act on the results
Effective screening programs should extend beyond the LDCT scan itself. The studies that have shown a benefit of LDCT were done at large academic centers that had the appropriate radiologic, pathologic, and surgical infrastructure to interpret and act on results and offer further diagnostic or treatment procedures.
Continue to: Prior to screening...
Prior to screening for lung cancer with LDCT, documentation of shared decision-making between the patient and the clinician is necessary.7 This discussion should include the potential benefits and harms of screening, potential results and likelihood of follow-up diagnostic testing, the false-positive rate of LDCT lung cancer screening, and cumulative radiation exposure. In addition, screening should be considered only if the patient is willing to be screened annually, is willing to pursue follow-up scans and procedures (including lung biopsy) if deemed necessary, and does not have comorbid conditions that significantly limit life expectancy.
Smoking cessation: The most important change to make
Smoking cessation is the single most important risk-modifying behavior to reduce one’s chance of developing lung cancer. At age 40, smokers have a 2-fold increase in all-cause mortality compared to age-matched nonsmokers. This rises to a 3-fold increase by the age of 70.16
Smoking cessation reduces the risk of lung cancer by 20% after 5 years, 30% to 50% after 10 years, and up to 70% after 15 years.24 In its guidelines, the American Thoracic Society recommends varenicline (Chantix) for all smokers to assist with smoking cessation.25
CASE
This 51-year-old patient with at least a 20-pack-year history of smoking should be commended for giving up smoking. Based on the USPSTF recommendations, he should be screened annually with LDCT for the next 10 years.
Screening to save more lives
The results of 2 large multicenter RCTs have led to the recent recommendation for lung cancer screening of high-risk adults with the use of LDCT. Screening with LDCT has been shown to reduce disease-related mortality and likely be cost effective in the long term.
Screening with LDCT should be part of a multidisciplinary system that has the infrastructure not only to perform the screening, but also to diagnose and appropriately follow up and treat patients whose results are concerning. The risk of false-positive results leading to increased anxiety, overdiagnosis, and unnecessary procedures points to the importance of proper patient selection, counseling, and shared decision-making. Smoking cessation remains the most important disease-modifying behavior one can make to reduce their risk for lung cancer.
CORRESPONDENCE
Carlton J. Covey, MD, 101 Bodin Circle, David Grant Medical Center, Travis Air Force Base, Fairfield, CA, 94545; [email protected]
CASE
A 51-year-old man presents to your office to discuss lung cancer screening. He has a history of hypertension and prediabetes. His father died of lung cancer 5 years ago, at age 77. The patient stopped smoking soon thereafter; prior to that, he smoked 1 pack of cigarettes per day for 20 years. He wants to know if he should be screened for lung cancer.
The relative lack of symptoms during the early stages of lung cancer frequently results in a delayed diagnosis. This, and the speed at which the disease progresses, underscores the need for an effective screening modality. More than half of people with lung cancer die within 1 year of diagnosis.1 Excluding skin cancer, lung cancer is the second most commonly diagnosed cancer, and more people die of lung cancer than of colon, breast, and prostate cancers combined.2 In 2022, it was estimated that there would be 236,740 new cases of lung cancer and 130,180 deaths from lung cancer.1,2 The average age at diagnosis is 70 years.2

Screening modalities: Only 1 has demonstrated mortality benefit
In 1968, Wilson and Junger3 outlined the characteristics of the ideal screening test for the World Health Organization: it should limit risk to the patient, be sensitive for detecting the disease early in its course, limit false-positive results, be acceptable to the patient, and be inexpensive to the health system.3 For decades, several screening modalities for lung cancer were trialed to fit the above guidance, but many of them fell short of the most important outcome: the impact on mortality.
Sputum cytology. The use of sputum cytology, either in combination with or without chest radiography, is not recommended. Several randomized controlled trials (RCTs) have failed to demonstrate improved lung cancer detection or mortality reduction in patients screened with this modality.4
Chest radiography (CXR). Several studies have assessed the efficacy of CXR as a screening modality. The best known was the Prostate, Lung, Colon, Ovarian (PLCO) Trial.5 This multicenter RCT enrolled more than 154,000 participants, half of whom received CXR at baseline and then annually for 3 years; the other half continued usual care (no screening). After 13 years of follow-up, there were no significant differences in lung cancer detection or mortality rates between the 2 groups.5
Low-dose computed tomography (LDCT). Several major medical societies recommend LDCT to screen high-risk individuals for lung cancer (TABLE 16-10). Results from 2 major RCTs have guided these recommendations.

The National Lung Screening Trial (NLST) was a multicenter RCT comparing 2 screening tests for lung cancer.11 Approximately 54,000 high-risk participants were enrolled between 2002 and 2004 and were randomized to receive annual screening with either LDCT or single-view CXR. The trial was discontinued prematurely when investigators noted a 20% reduction in lung cancer mortality in the LDCT group vs the CXR group.12 This equates to 3 fewer deaths for every 1000 people screened with LDCT vs CXR. There was also a 6% reduction in all-cause mortality noted in the LDCT vs the CXR group.12
Continue to: The NELSON trial...
The NELSON trial, conducted between 2005 and 2015, studied more than 15,000 current or former smokers ages 50 to 74 years and compared LDCT screening at various intervals to no screening.13 After 10 years, lung cancer–related mortality was reduced by 24% (or 1 less death per 1000 person-years) in men who were screened vs their unscreened counterparts.13 In contrast to the NLST, in the NELSON trial, no significant difference in all-cause mortality was observed. Subgroup analysis of the relatively small population of women included in the NELSON trial suggested a 33% reduction in 10-year mortality; however, the difference was nonsignificant between the screened and unscreened groups.13
Each of these landmark studies had characteristics that could limit the results' generalizability to the US population. In the NELSON trial, more than 80% of the study participants were male. In both trials, there was significant underrepresentation of Black, Asian, Hispanic, and other non-White people.12,13 Furthermore, participants in these studies were of higher socioeconomic status than the general US screening-eligible population.
At this time, LDCT is the only lung cancer screening modality that has shown benefit for both disease-related and all-cause mortality, in the populations that were studied. Based on the NLST, the number needed to screen (NNS) with LDCT to prevent 1 lung cancer–related death is 308. The NNS to prevent 1 death from any cause is 219.6
Updated evidence has led to a consensus on screening criteria
Many national societies endorse annual screening with LDCT in high-risk individuals (TABLE 16-10). Risk assessment for the purpose of lung cancer screening includes a detailed review of smoking history and age. The risk of lung cancer increases with advancing age and with cumulative quantity and duration of smoking, but decreases with increasing time since quitting. Therefore, a detailed smoking history should include total number of pack-years, current smoking status, and, if applicable, when smoking cessation occurred.
In 2021, the US Preventive Services Task Force (USPSTF) updated their 2013 lung cancer screening recommendations, expanding the screening age range and lowering the smoking history threshold for triggering initiation of screening.6 The impetus for the update was emerging evidence from systematic reviews, RCTs, and the Cancer Intervention and Surveillance Modeling Network (CISNET) that could help to determine the optimal age for screening and identify high-risk groups. For example, the NELSON trial, combined with results from CISNET modeling data, showed an empirical benefit for screening those ages 50 to 55 years.6
Continue to: As a result...
As a result, the USPSTF now recommends annual lung cancer screening with LDCT for any adult ages 50 to 80 years who has a 20-pack-year smoking history and currently smokes or has quit within the past 15 years.6 Screening should be discontinued once a person has not smoked for 15 years, develops a health problem that substantially limits life expectancy, or is not willing to have curative lung surgery.6
Expanding the screening eligibility may also address racial and gender disparities in health care. Black people and women who smoke have a higher risk for lung cancer at a lower intensity of smoking.6
Following the USPSTF update, the American College of Chest Physicians and the Centers for Medicare and Medicaid Services published updated guidance that aligns with USPSTF’s recommendations to lower the age and pack-year qualifications for initiating screening.7,10 The American Cancer Society is currently reviewing its 2018 guidelines on lung cancer screening.14 TABLE 16-10 summarizes the guidance on lung cancer screening from these medical societies.
Effective screening could save lives (and money)
A smoker’s risk for lung cancer is 20 times higher than that of a nonsmoker15,16; 55% of lung cancer deaths in women and 70% in men are attributed to smoking.17 Once diagnosed with lung cancer, more than 50% of people will die within 1 year.1 This underpins the need for a lung cancer screening modality that reduces mortality. Large RCTs, including the NLST and NELSON trials, have shown that screening high-risk individuals with LDCT can significantly reduce lung cancer–related death when compared to no screening or screening with CXR alone.11,13
There is controversy surrounding the cost benefit of implementing a nationwide lung cancer screening program. However, recent use of microsimulation models has shown LDCT to be a cost-effective strategy, with an average cost of $81,000 per quality-adjusted life-year, which is below the threshold of $100,000 to be considered cost effective.18 Expanding the upper age limit for screening leads to a greater reduction in mortality but increases treatment costs and overdiagnosis rates, and overall does not improve quality-adjusted life-years.18
Continue to: Potential harms
Potential harms: False-positives and related complications
Screening for lung cancer is not without its risks. Harms from screening typically result from false-positive test results leading to overdiagnosis, anxiety and distress, unnecessary invasive tests or procedures, and increased costs.19 TABLE 26,19-23 lists specific complications from lung cancer screening with LDCT.

The false-positive rate is not trivial. For every 1000 patients screened, 250 people will have a positive LDCT finding but will not have lung cancer.19 Furthermore, about 1 in every 2000 individuals who screen positive, but who do not have lung cancer, die as a result of complications from the ensuing work-up.6
Annual LDCT screening increases the risk of radiation-induced cancer by approximately 0.05% over 10 years.21 The absolute risk is generally low but not insignificant. However, the mortality benefits previously outlined are significantly more robust in both absolute and relative terms vs the 10-year risk of radiation-induced cancer.
Lastly, it is important to note that the NELSON trial and NLST included a limited number of LDCT scans. Current guidelines for lung cancer screening with LDCT, including those from the USPSTF, recommend screening annually. We do not know the cumulative harm of annual LDCT over a 20- or 30-year period for those who would qualify (ie, current smokers).
If you screen, you must be able to act on the results
Effective screening programs should extend beyond the LDCT scan itself. The studies that have shown a benefit of LDCT were done at large academic centers that had the appropriate radiologic, pathologic, and surgical infrastructure to interpret and act on results and offer further diagnostic or treatment procedures.
Continue to: Prior to screening...
Prior to screening for lung cancer with LDCT, documentation of shared decision-making between the patient and the clinician is necessary.7 This discussion should include the potential benefits and harms of screening, potential results and likelihood of follow-up diagnostic testing, the false-positive rate of LDCT lung cancer screening, and cumulative radiation exposure. In addition, screening should be considered only if the patient is willing to be screened annually, is willing to pursue follow-up scans and procedures (including lung biopsy) if deemed necessary, and does not have comorbid conditions that significantly limit life expectancy.
Smoking cessation: The most important change to make
Smoking cessation is the single most important risk-modifying behavior to reduce one’s chance of developing lung cancer. At age 40, smokers have a 2-fold increase in all-cause mortality compared to age-matched nonsmokers. This rises to a 3-fold increase by the age of 70.16
Smoking cessation reduces the risk of lung cancer by 20% after 5 years, 30% to 50% after 10 years, and up to 70% after 15 years.24 In its guidelines, the American Thoracic Society recommends varenicline (Chantix) for all smokers to assist with smoking cessation.25
CASE
This 51-year-old patient with at least a 20-pack-year history of smoking should be commended for giving up smoking. Based on the USPSTF recommendations, he should be screened annually with LDCT for the next 10 years.
Screening to save more lives
The results of 2 large multicenter RCTs have led to the recent recommendation for lung cancer screening of high-risk adults with the use of LDCT. Screening with LDCT has been shown to reduce disease-related mortality and likely be cost effective in the long term.
Screening with LDCT should be part of a multidisciplinary system that has the infrastructure not only to perform the screening, but also to diagnose and appropriately follow up and treat patients whose results are concerning. The risk of false-positive results leading to increased anxiety, overdiagnosis, and unnecessary procedures points to the importance of proper patient selection, counseling, and shared decision-making. Smoking cessation remains the most important disease-modifying behavior one can make to reduce their risk for lung cancer.
CORRESPONDENCE
Carlton J. Covey, MD, 101 Bodin Circle, David Grant Medical Center, Travis Air Force Base, Fairfield, CA, 94545; [email protected]
1. National Cancer Institute. Cancer Stat Facts: lung and bronchus cancer. Accessed October 12, 2022. https://seer.cancer.gov/statfacts/html/lungb.html
2. American Cancer Society. Key statistics for lung cancer. Accessed October 12, 2022. https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html
3. Wilson JMG, Junger G. Principles and Practice of Screening for Disease. World Health Organization; 1968:21-25, 100. https://apps.who.int/iris/handle/10665/37650
4. Humphrey LL, Teutsch S, Johnson M. Lung cancer screening with sputum cytologic examination, chest radiography, and computed tomography: an update for the United States preventive services task force. Ann Intern Med. 2004;140:740-753. doi: 10.7326/0003-4819-140-9-200405040-00015
5. Oken MM, Hocking WG, Kvale PA, et al. Screening by chest radiograph and lung cancer mortality: the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA. 2011;306:1865-1873. doi: 10.1001/jama.2011.1591
6. US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325:962-970. doi: 10.1001/jama.2021.1117
7. Centers for Medicare & Medicaid Services. Screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439R). Accessed October 14, 2022. www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=304
8. Smith RA, Andrews KS, Brooks D, et al. Cancer screening in the United States, 2018: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2018;68:297-316. doi: 10.3322/caac.21446
9. American Academy of Family Physicians. AAFP updates recommendation on lung cancer screening. Published April 6, 2021. Accessed October 12, 2022. www.aafp.org/news/health-of-the-public/20210406lungcancer.html
10. Mazzone PJ, Silvestri GA, Souter LH, et al. Screening for lung cancer: CHEST Guideline and Expert Panel Report. CHEST. 2021;160:E427-E494. doi: 10.1016/j.chest.2021.06.063
11. The National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. doi: 10.1056/NEJMoa1102873
12. The National Lung Screening Trial Research Team. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368:1980-1991. doi: 10.1056/NEJMoa1209120
13. de Koning HJ, van der Aalst CM, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382:503-513. doi: 10.1056/NEJMoa1911793
14. American Cancer Society. Lung cancer screening guidelines. Accessed October 14, 2022. www.cancer.org/health-care-professionals/american-cancer-society-prevention-early-detection-guidelines/lung-cancer-screening-guidelines.html
15. Pirie K, Peto R, Reeves GK, et al. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet. 2013;381:133-141. doi: 10.1016/S0140-6736(12)61720-6
16. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519. doi: 10.1136/bmj.38142.554479.AE
17. O’Keefe LM, Gemma T, Huxley R, et al. Smoking as a risk factor for lung cancer in women and men: a systematic review and meta-analysis. BMJ Open. 2018;8:e021611. doi: 10.1136/bmjopen-2018-021611
18. Criss SD, Pianpian C, Bastani M, et al. Cost-effectiveness analysis of lung cancer screening in the United States: a comparative modeling study. Ann Intern Med. 2019;171:796-805. doi: 10.7326/M19-0322
19. Lazris A, Roth RA. Lung cancer screening: pros and cons. Am Fam Physician. 2019;99:740-742.
20. Ali MU, Miller J, Peirson L, et al. Screening for lung cancer: a systematic review and meta-analysis. Prev Med. 2016;89:301-314. doi: 10.1016/j.ypmed.2016.04.015
21. Rampinelli C, De Marco P, Origgi D, et al. Exposure to low dose computed tomography for lung cancer screening and risk of cancer: secondary analysis of trial data and risk-benefit analysis. BMJ. 2017;356:j347. doi: 10.1136/bmj.j347
22. Manser RL, Lethaby A, Irving LB, et al. Screening for lung cancer. Cochrane Database Syst Rev. 2013;CD001991. doi: 10.1002/14651858.CD001991.pub3
23. Mazzone PJ, Silvestri GA, Patel S, et al. Screening for lung cancer: CHEST guideline and expert panel report. CHEST. 2018;153:954-985. doi: 10.1016/j.chest.2018.01.016
24. US Public Health Service Office of the Surgeon General; National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking. and Health. Smoking Cessation: A Report of the Surgeon General. US Department of Health and Human Services; 2020. www.ncbi.nlm.nih.gov/books/NBK555591/
25. Leone FT, Zhang Y, Evers-Casey S, et al, on behalf of the American Thoracic Society Assembly on Clinical Problems. Initiating pharmacologic treatment in tobacco-dependent adults: an official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;202:e5-e31. doi: 10.1164/rccm.202005-1982ST
1. National Cancer Institute. Cancer Stat Facts: lung and bronchus cancer. Accessed October 12, 2022. https://seer.cancer.gov/statfacts/html/lungb.html
2. American Cancer Society. Key statistics for lung cancer. Accessed October 12, 2022. https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html
3. Wilson JMG, Junger G. Principles and Practice of Screening for Disease. World Health Organization; 1968:21-25, 100. https://apps.who.int/iris/handle/10665/37650
4. Humphrey LL, Teutsch S, Johnson M. Lung cancer screening with sputum cytologic examination, chest radiography, and computed tomography: an update for the United States preventive services task force. Ann Intern Med. 2004;140:740-753. doi: 10.7326/0003-4819-140-9-200405040-00015
5. Oken MM, Hocking WG, Kvale PA, et al. Screening by chest radiograph and lung cancer mortality: the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA. 2011;306:1865-1873. doi: 10.1001/jama.2011.1591
6. US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325:962-970. doi: 10.1001/jama.2021.1117
7. Centers for Medicare & Medicaid Services. Screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439R). Accessed October 14, 2022. www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=304
8. Smith RA, Andrews KS, Brooks D, et al. Cancer screening in the United States, 2018: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2018;68:297-316. doi: 10.3322/caac.21446
9. American Academy of Family Physicians. AAFP updates recommendation on lung cancer screening. Published April 6, 2021. Accessed October 12, 2022. www.aafp.org/news/health-of-the-public/20210406lungcancer.html
10. Mazzone PJ, Silvestri GA, Souter LH, et al. Screening for lung cancer: CHEST Guideline and Expert Panel Report. CHEST. 2021;160:E427-E494. doi: 10.1016/j.chest.2021.06.063
11. The National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. doi: 10.1056/NEJMoa1102873
12. The National Lung Screening Trial Research Team. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368:1980-1991. doi: 10.1056/NEJMoa1209120
13. de Koning HJ, van der Aalst CM, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382:503-513. doi: 10.1056/NEJMoa1911793
14. American Cancer Society. Lung cancer screening guidelines. Accessed October 14, 2022. www.cancer.org/health-care-professionals/american-cancer-society-prevention-early-detection-guidelines/lung-cancer-screening-guidelines.html
15. Pirie K, Peto R, Reeves GK, et al. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet. 2013;381:133-141. doi: 10.1016/S0140-6736(12)61720-6
16. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519. doi: 10.1136/bmj.38142.554479.AE
17. O’Keefe LM, Gemma T, Huxley R, et al. Smoking as a risk factor for lung cancer in women and men: a systematic review and meta-analysis. BMJ Open. 2018;8:e021611. doi: 10.1136/bmjopen-2018-021611
18. Criss SD, Pianpian C, Bastani M, et al. Cost-effectiveness analysis of lung cancer screening in the United States: a comparative modeling study. Ann Intern Med. 2019;171:796-805. doi: 10.7326/M19-0322
19. Lazris A, Roth RA. Lung cancer screening: pros and cons. Am Fam Physician. 2019;99:740-742.
20. Ali MU, Miller J, Peirson L, et al. Screening for lung cancer: a systematic review and meta-analysis. Prev Med. 2016;89:301-314. doi: 10.1016/j.ypmed.2016.04.015
21. Rampinelli C, De Marco P, Origgi D, et al. Exposure to low dose computed tomography for lung cancer screening and risk of cancer: secondary analysis of trial data and risk-benefit analysis. BMJ. 2017;356:j347. doi: 10.1136/bmj.j347
22. Manser RL, Lethaby A, Irving LB, et al. Screening for lung cancer. Cochrane Database Syst Rev. 2013;CD001991. doi: 10.1002/14651858.CD001991.pub3
23. Mazzone PJ, Silvestri GA, Patel S, et al. Screening for lung cancer: CHEST guideline and expert panel report. CHEST. 2018;153:954-985. doi: 10.1016/j.chest.2018.01.016
24. US Public Health Service Office of the Surgeon General; National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking. and Health. Smoking Cessation: A Report of the Surgeon General. US Department of Health and Human Services; 2020. www.ncbi.nlm.nih.gov/books/NBK555591/
25. Leone FT, Zhang Y, Evers-Casey S, et al, on behalf of the American Thoracic Society Assembly on Clinical Problems. Initiating pharmacologic treatment in tobacco-dependent adults: an official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;202:e5-e31. doi: 10.1164/rccm.202005-1982ST
PRACTICE RECOMMENDATIONS
› Recommend annual lung cancer screening for all highrisk adults ages 50 to 80 years using low-dose computed tomography. A
› Do not pursue lung cancer screening in patients who quit smoking ≥ 15 years ago, have a health problem that limits their life expectancy, or are unwilling to undergo lung surgery. A
› Recommend varenicline as first-line pharmacotherapy for smokers who would like to quit. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
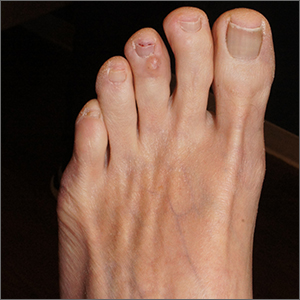
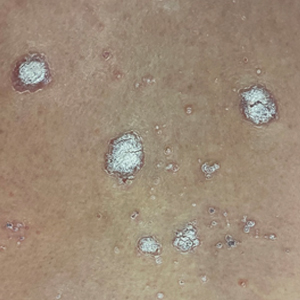
Clear toe lesion
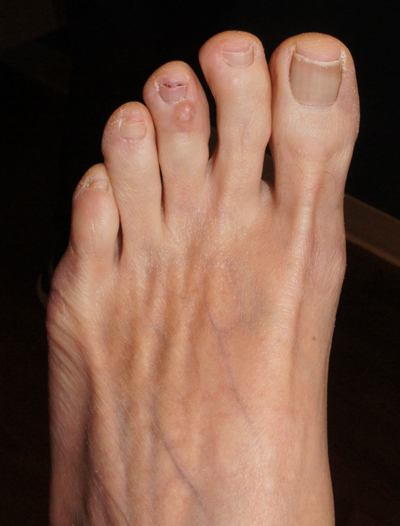
This is a digital mucous cyst, also known as a myxoid cyst. The clear to translucent appearance over a finger or toe joint is usually diagnosed clinically. If uncertain, a biopsy can confirm the diagnosis.
Digital mucous cysts are a type of ganglion cyst that is associated with trauma or arthritis in the toe joint. A microscopic opening in the joint capsule results in a fluid filled cyst in the surrounding tissue. If the cyst is ruptured, thick, gelatinous (sometimes blood-tinged) hyaluronic acid–rich fluid may escape. Sometimes, the cyst applies pressure to the nail matrix, causing a scooped out longitudinal nail deformity.
Digital mucous cysts more commonly affect the fingers than the toes. Although benign, patients may be bothered by the appearance of these cysts and their effect on nails. Observation is a reasonable approach. Rarely, digital mucous cysts resolve spontaneously.
Treatment options include cryotherapy, needle draining and scarification, and surgical excision with flap repair. Surgical excision may be performed quickly in the office and offers the highest cure rate of 95% in 1 study on fingers.1 Cryotherapy is successful in 70% of cases and needle drainage is successful in 39% of cases, but these modalities are quick and require minimal downtime.1
In this case, the patient was not significantly bothered by the lesion and was happy to forego treatment.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Jabbour S, Kechichian E, Haber R, et al. Management of digital mucous cysts: a systematic review and treatment algorithm. Int J Dermatol. 2017;56:701-708. doi: 10.1111/ijd.13583

This is a digital mucous cyst, also known as a myxoid cyst. The clear to translucent appearance over a finger or toe joint is usually diagnosed clinically. If uncertain, a biopsy can confirm the diagnosis.
Digital mucous cysts are a type of ganglion cyst that is associated with trauma or arthritis in the toe joint. A microscopic opening in the joint capsule results in a fluid filled cyst in the surrounding tissue. If the cyst is ruptured, thick, gelatinous (sometimes blood-tinged) hyaluronic acid–rich fluid may escape. Sometimes, the cyst applies pressure to the nail matrix, causing a scooped out longitudinal nail deformity.
Digital mucous cysts more commonly affect the fingers than the toes. Although benign, patients may be bothered by the appearance of these cysts and their effect on nails. Observation is a reasonable approach. Rarely, digital mucous cysts resolve spontaneously.
Treatment options include cryotherapy, needle draining and scarification, and surgical excision with flap repair. Surgical excision may be performed quickly in the office and offers the highest cure rate of 95% in 1 study on fingers.1 Cryotherapy is successful in 70% of cases and needle drainage is successful in 39% of cases, but these modalities are quick and require minimal downtime.1
In this case, the patient was not significantly bothered by the lesion and was happy to forego treatment.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

This is a digital mucous cyst, also known as a myxoid cyst. The clear to translucent appearance over a finger or toe joint is usually diagnosed clinically. If uncertain, a biopsy can confirm the diagnosis.
Digital mucous cysts are a type of ganglion cyst that is associated with trauma or arthritis in the toe joint. A microscopic opening in the joint capsule results in a fluid filled cyst in the surrounding tissue. If the cyst is ruptured, thick, gelatinous (sometimes blood-tinged) hyaluronic acid–rich fluid may escape. Sometimes, the cyst applies pressure to the nail matrix, causing a scooped out longitudinal nail deformity.
Digital mucous cysts more commonly affect the fingers than the toes. Although benign, patients may be bothered by the appearance of these cysts and their effect on nails. Observation is a reasonable approach. Rarely, digital mucous cysts resolve spontaneously.
Treatment options include cryotherapy, needle draining and scarification, and surgical excision with flap repair. Surgical excision may be performed quickly in the office and offers the highest cure rate of 95% in 1 study on fingers.1 Cryotherapy is successful in 70% of cases and needle drainage is successful in 39% of cases, but these modalities are quick and require minimal downtime.1
In this case, the patient was not significantly bothered by the lesion and was happy to forego treatment.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Jabbour S, Kechichian E, Haber R, et al. Management of digital mucous cysts: a systematic review and treatment algorithm. Int J Dermatol. 2017;56:701-708. doi: 10.1111/ijd.13583
1. Jabbour S, Kechichian E, Haber R, et al. Management of digital mucous cysts: a systematic review and treatment algorithm. Int J Dermatol. 2017;56:701-708. doi: 10.1111/ijd.13583
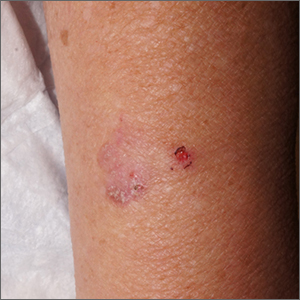
Scaly forearm plaque
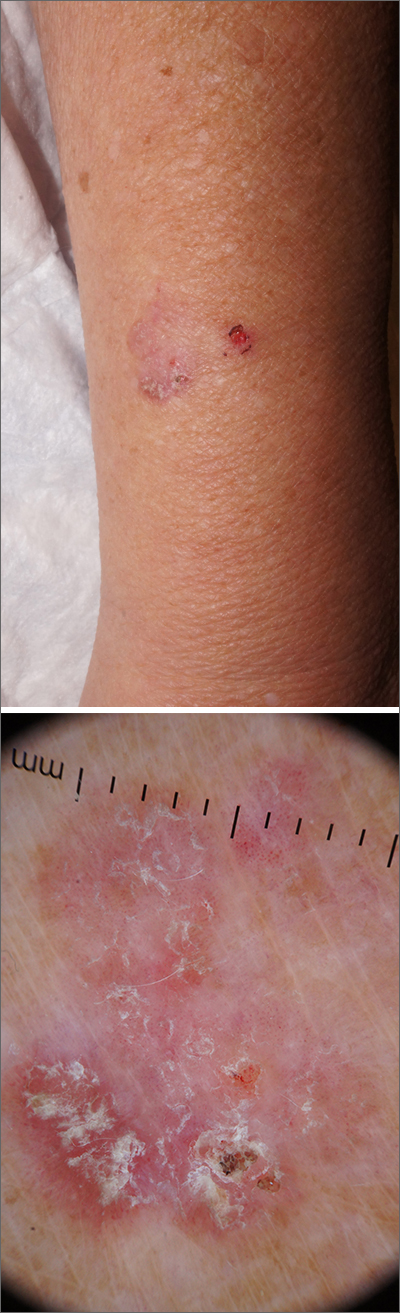
Dermoscopy revealed a keratotic, 2.5-cm scaly plaque with linearly arranged dotted vessels, ulceration, and shiny white lines. A shave biopsy was consistent with a squamous cell carcinoma in situ (SCC in situ)—a pre-invasive keratinocyte carcinoma.
SCC in situ, also known as Bowen’s disease, is a very common skin cancer that can be easily treated. Lesions may manifest anywhere on the skin but are most often found on sun-damaged areas. Actinic keratoses are a pre-malignant precursor of SCC in situ; both are characterized by a sandpapery rough surface on a pink or brown background. Histologically, SCC in situ has atypia of keratinocytes over the full thickness of the epidermis, while actinic keratoses have limited atypia of the upper epidermis only. With this in mind, suspect SCC in situ (over actinic keratosis) when a lesion is thicker than 1 mm, larger in diameter than 5 mm, or painful.1
Treatment options include surgical and nonsurgical modalities. Excision and electrodessication and curettage (EDC) are both effective surgical procedures, with cure rates greater than 90%.2 Nonsurgical options include cryotherapy, 5-fluorouracil (5FU), imiquimod, and photodynamic therapy. Treatment with 5FU or imiquimod involves the application of cream to the lesion for 4 to 6 weeks. Marked inflammation during treatment is to be expected.
In the case described here, the patient underwent EDC in the office and was counseled to continue with complete skin exams twice a year for the next 2 years.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Mills KC, Kwatra SG, Feneran AN, et al. Itch and pain in nonmelanoma skin cancer: pain as an important feature of cutaneous squamous cell carcinoma. Arch Dermatol. 2012;148:1422-1423. doi: 10.1001/archdermatol.2012.3104
2. Reschly MJ, Shenefelt PD. Controversies in skin surgery: electrodessication and curettage versus excision for low-risk, small, well-differentiated squamous cell carcinomas. J Drugs Dermatol. 2010;9:773-776.

Dermoscopy revealed a keratotic, 2.5-cm scaly plaque with linearly arranged dotted vessels, ulceration, and shiny white lines. A shave biopsy was consistent with a squamous cell carcinoma in situ (SCC in situ)—a pre-invasive keratinocyte carcinoma.
SCC in situ, also known as Bowen’s disease, is a very common skin cancer that can be easily treated. Lesions may manifest anywhere on the skin but are most often found on sun-damaged areas. Actinic keratoses are a pre-malignant precursor of SCC in situ; both are characterized by a sandpapery rough surface on a pink or brown background. Histologically, SCC in situ has atypia of keratinocytes over the full thickness of the epidermis, while actinic keratoses have limited atypia of the upper epidermis only. With this in mind, suspect SCC in situ (over actinic keratosis) when a lesion is thicker than 1 mm, larger in diameter than 5 mm, or painful.1
Treatment options include surgical and nonsurgical modalities. Excision and electrodessication and curettage (EDC) are both effective surgical procedures, with cure rates greater than 90%.2 Nonsurgical options include cryotherapy, 5-fluorouracil (5FU), imiquimod, and photodynamic therapy. Treatment with 5FU or imiquimod involves the application of cream to the lesion for 4 to 6 weeks. Marked inflammation during treatment is to be expected.
In the case described here, the patient underwent EDC in the office and was counseled to continue with complete skin exams twice a year for the next 2 years.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

Dermoscopy revealed a keratotic, 2.5-cm scaly plaque with linearly arranged dotted vessels, ulceration, and shiny white lines. A shave biopsy was consistent with a squamous cell carcinoma in situ (SCC in situ)—a pre-invasive keratinocyte carcinoma.
SCC in situ, also known as Bowen’s disease, is a very common skin cancer that can be easily treated. Lesions may manifest anywhere on the skin but are most often found on sun-damaged areas. Actinic keratoses are a pre-malignant precursor of SCC in situ; both are characterized by a sandpapery rough surface on a pink or brown background. Histologically, SCC in situ has atypia of keratinocytes over the full thickness of the epidermis, while actinic keratoses have limited atypia of the upper epidermis only. With this in mind, suspect SCC in situ (over actinic keratosis) when a lesion is thicker than 1 mm, larger in diameter than 5 mm, or painful.1
Treatment options include surgical and nonsurgical modalities. Excision and electrodessication and curettage (EDC) are both effective surgical procedures, with cure rates greater than 90%.2 Nonsurgical options include cryotherapy, 5-fluorouracil (5FU), imiquimod, and photodynamic therapy. Treatment with 5FU or imiquimod involves the application of cream to the lesion for 4 to 6 weeks. Marked inflammation during treatment is to be expected.
In the case described here, the patient underwent EDC in the office and was counseled to continue with complete skin exams twice a year for the next 2 years.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Mills KC, Kwatra SG, Feneran AN, et al. Itch and pain in nonmelanoma skin cancer: pain as an important feature of cutaneous squamous cell carcinoma. Arch Dermatol. 2012;148:1422-1423. doi: 10.1001/archdermatol.2012.3104
2. Reschly MJ, Shenefelt PD. Controversies in skin surgery: electrodessication and curettage versus excision for low-risk, small, well-differentiated squamous cell carcinomas. J Drugs Dermatol. 2010;9:773-776.
1. Mills KC, Kwatra SG, Feneran AN, et al. Itch and pain in nonmelanoma skin cancer: pain as an important feature of cutaneous squamous cell carcinoma. Arch Dermatol. 2012;148:1422-1423. doi: 10.1001/archdermatol.2012.3104
2. Reschly MJ, Shenefelt PD. Controversies in skin surgery: electrodessication and curettage versus excision for low-risk, small, well-differentiated squamous cell carcinomas. J Drugs Dermatol. 2010;9:773-776.
Psoriasiform Dermatitis Associated With the Moderna COVID-19 Messenger RNA Vaccine
To the Editor:
The Moderna COVID-19 messenger RNA (mRNA) vaccine was authorized for use on December 18, 2020, with the second dose beginning on January 15, 2021.1-3 Some individuals who received the Moderna vaccine experienced an intense rash known as “COVID arm,” a harmless but bothersome adverse effect that typically appears within a week and is a localized and transient immunogenic response.4 COVID arm differs from most vaccine adverse effects. The rash emerges not immediately but 5 to 9 days after the initial dose—on average, 1 week later. Apart from being itchy, the rash does not appear to be harmful and is not a reason to hesitate getting vaccinated.
Dermatologists and allergists have been studying this adverse effect, which has been formally termed delayed cutaneous hypersensitivity. Of potential clinical consequence is that the efficacy of the mRNA COVID-19 vaccine may be harmed if postvaccination dermal reactions necessitate systemic corticosteroid therapy. Because this vaccine stimulates an immune response as viral RNA integrates in cells secondary to production of the spike protein of the virus, the skin may be affected secondarily and manifestations of any underlying disease may be aggravated.5 We report a patient who developed a psoriasiform dermatitis after the first dose of the Moderna vaccine.
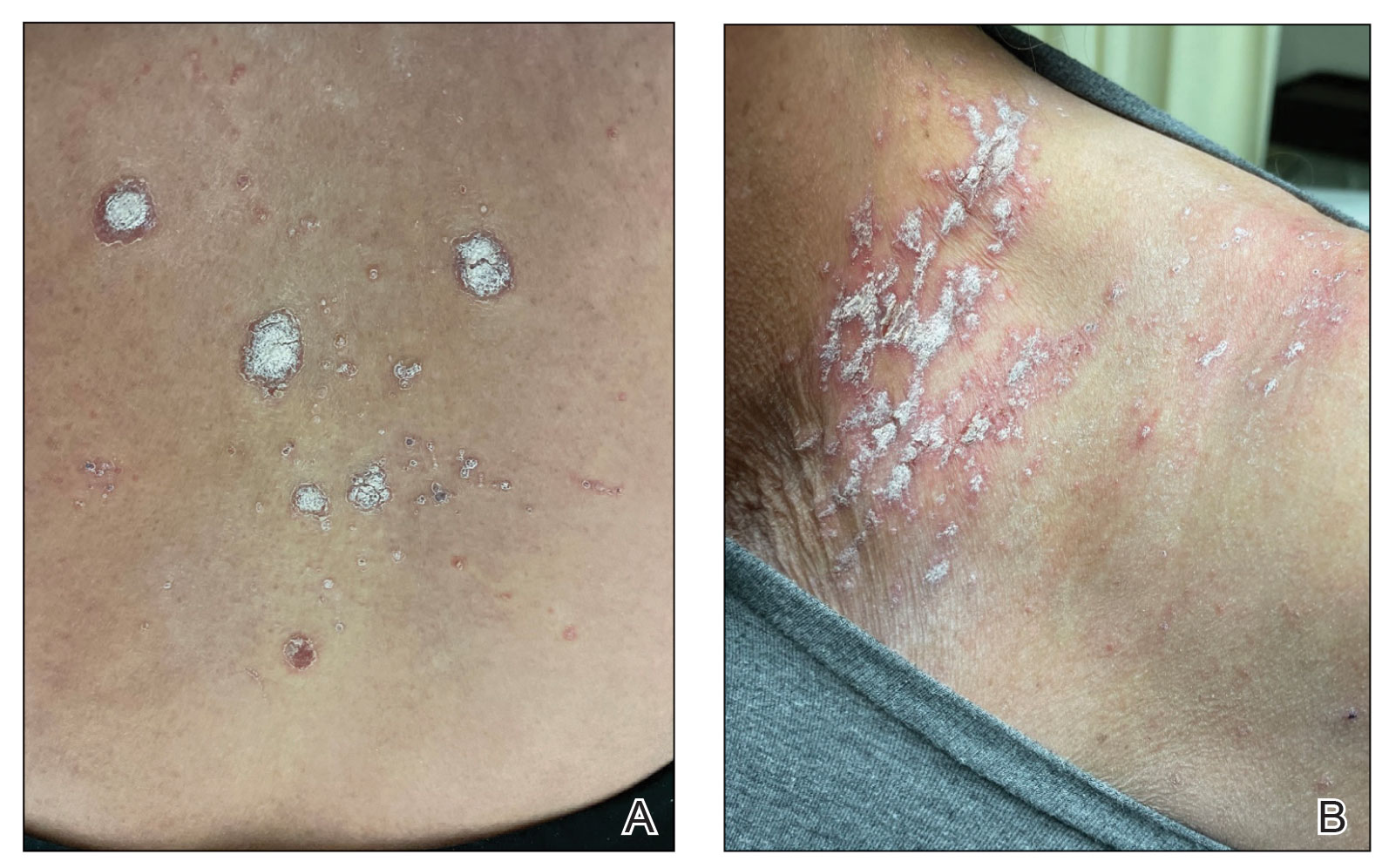
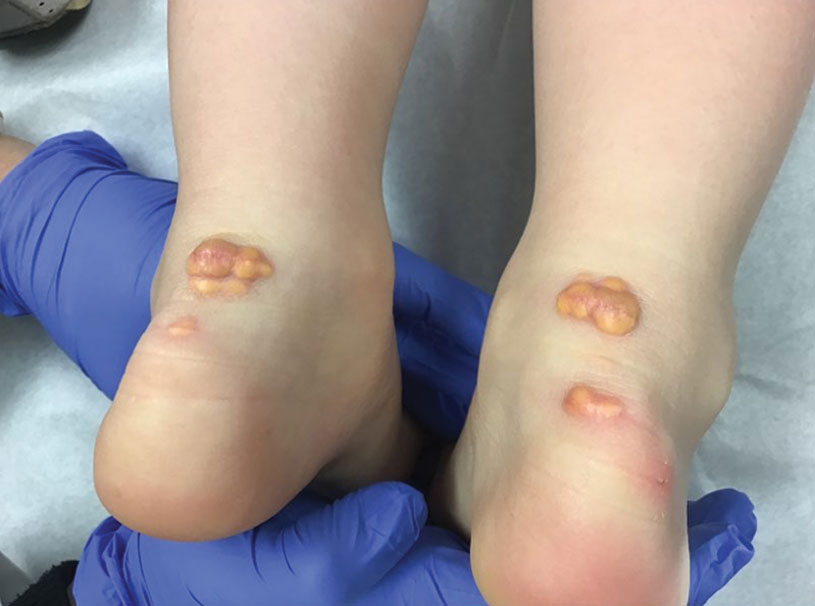
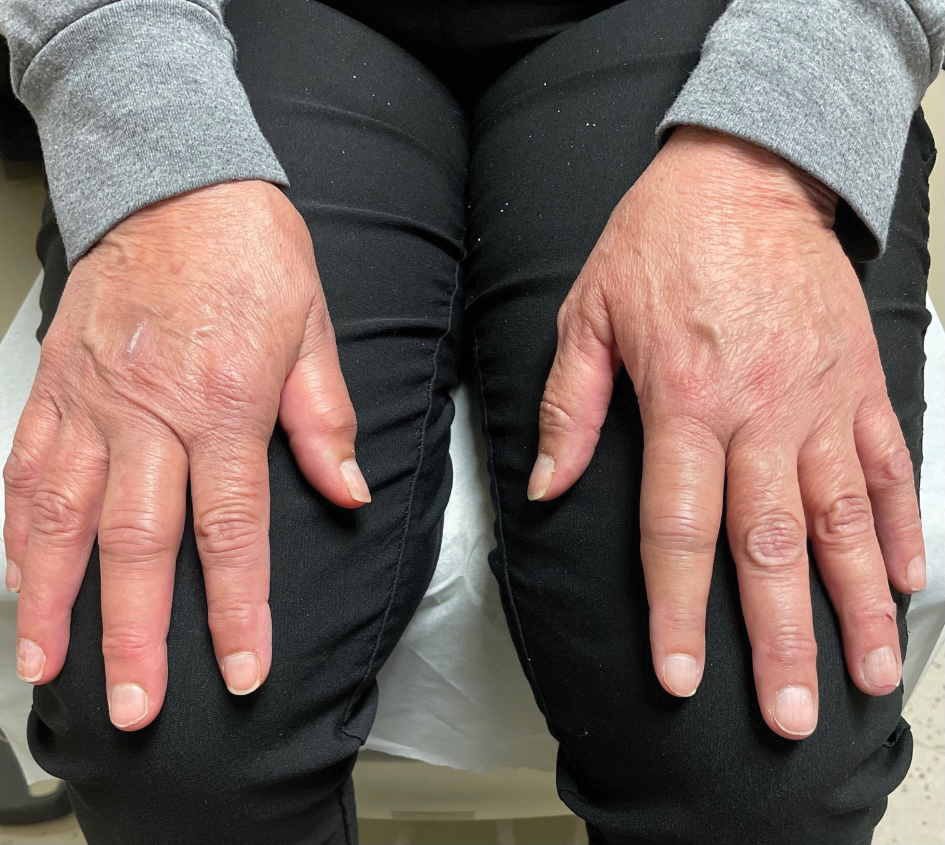
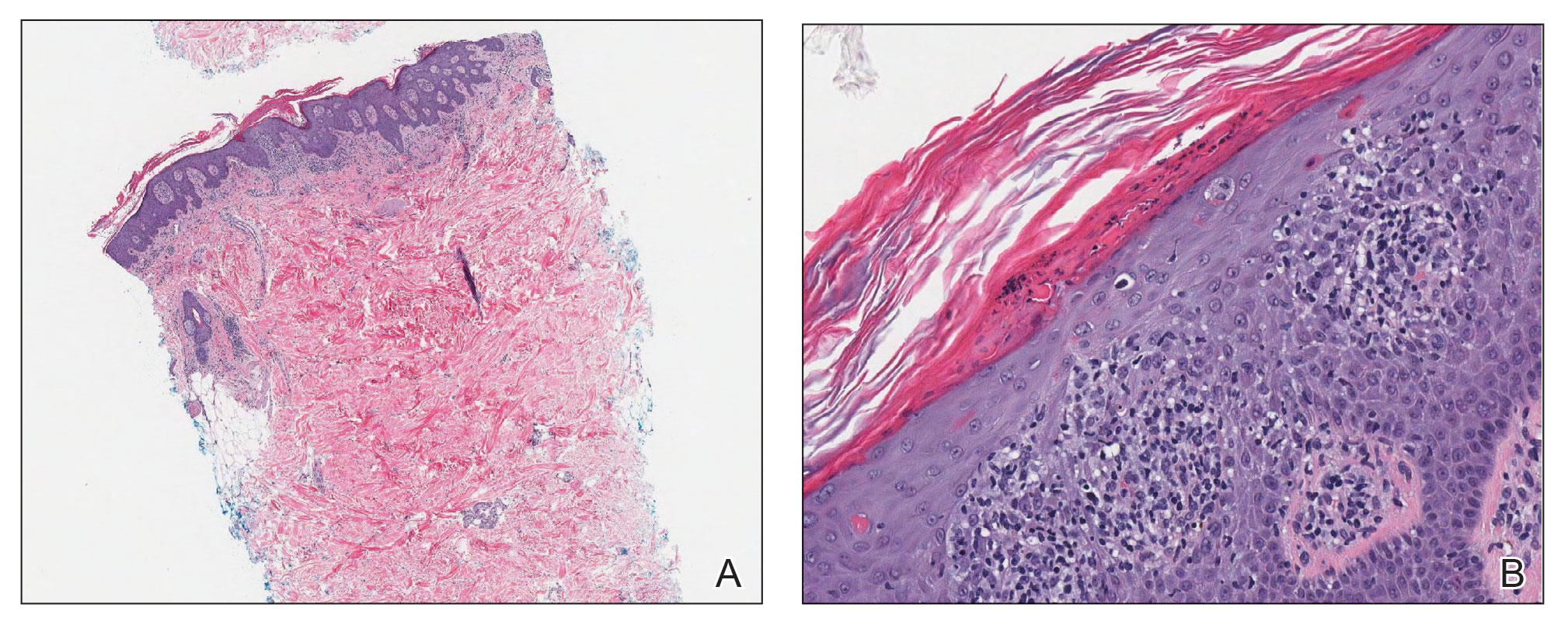
A 65-year-old woman presented to her primary care physician because of the severity of psoriasiform dermatitis that developed 5 days after she received the first dose of the Moderna COVID-19 mRNA vaccine. The patient had a medical history of Sjögren syndrome. Her medication history was negative, and her family history was negative for autoimmune disease. Physical examination by primary care revealed an erythematous scaly rash with plaques and papules on the neck and back (Figure 1). The patient presented again to primary care 2 days later with swollen, painful, discolored digits (Figure 2) and a stiff, sore neck.
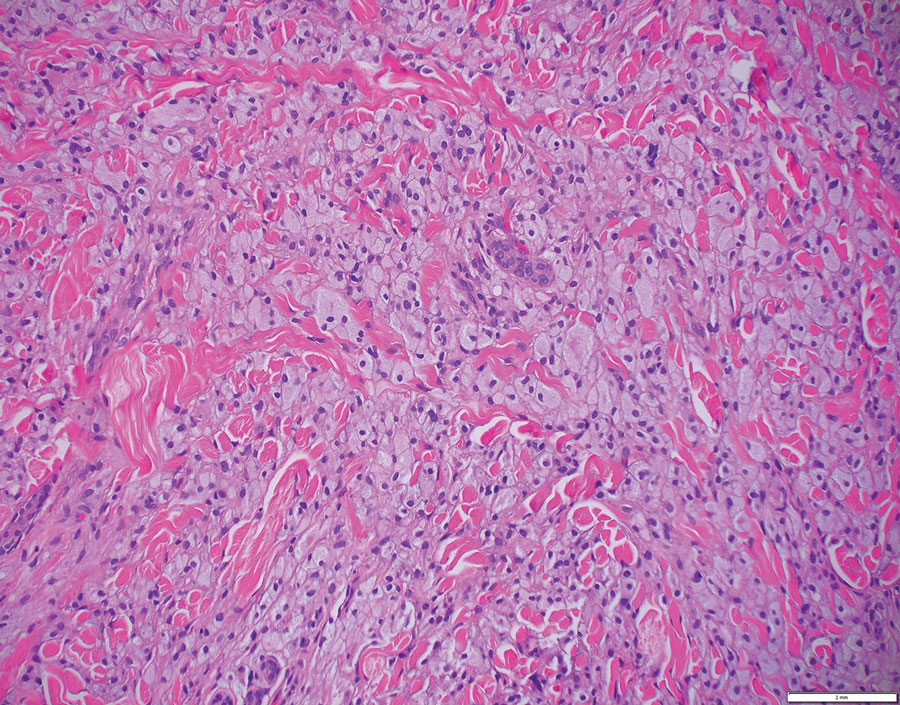
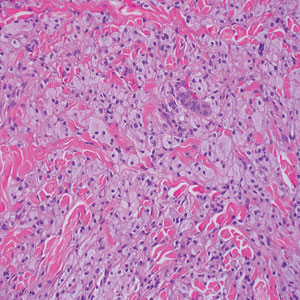
Laboratory results were positive for anti–Sjögren syndrome–related antigens A and B. A complete blood cell count; comprehensive metabolic panel; erythrocyte sedimentation rate; and assays of rheumatoid factor, C-reactive protein, and anti–cyclic citrullinated peptide were within reference range. A biopsy of a lesion on the back showed psoriasiform dermatitis with confluent parakeratosis and scattered necrotic keratinocytes. There was superficial perivascular inflammation with rare eosinophils (Figure 3).
The patient was treated with a course of systemic corticosteroids. The rash resolved in 1 week. She did not receive the second dose due to the rash.
Two mRNA COVID-19 vaccines—Pfizer BioNTech and Moderna—have been granted emergency use authorization by the US Food and Drug Administration.6 The safety profile of the mRNA-1273 vaccine for the median 2-month follow-up showed no safety concerns.3 Minor localized adverse effects (eg, pain, redness, swelling) have been observed more frequently with the vaccines than with placebo. Systemic symptoms, such as fever, fatigue, headache, and muscle and joint pain, also were seen somewhat more often with the vaccines than with placebo; most such effects occurred 24 to 48 hours after vaccination.3,6,7 The frequency of unsolicited adverse events and serious adverse events reported during the 28-day period after vaccination generally was similar among participants in the vaccine and placebo groups.3
There are 2 types of reactions to COVID-19 vaccination: immediate and delayed. Immediate reactions usually are due to anaphylaxis, requiring prompt recognition and treatment with epinephrine to stop rapid progression of life-threatening symptoms. Delayed reactions include localized reactions, such as urticaria and benign exanthema; serum sickness and serum sickness–like reactions; fever; and rare skin, organ, and neurologic sequelae.1,6-8
Cutaneous manifestations, present in 16% to 50% of patients with Sjögren syndrome, are considered one of the most common extraglandular presentations of the syndrome. They are classified as nonvascular (eg, xerosis, angular cheilitis, eyelid dermatitis, annular erythema) and vascular (eg, Raynaud phenomenon, vasculitis).9-11 Our patient did not have any of those findings. She had not taken any medications before the rash appeared, thereby ruling out a drug reaction.
The differential for our patient included post–urinary tract infection immune-reactive arthritis and rash, which is not typical with Escherichia coli infection but is described with infection with Chlamydia species and Salmonella species. Moreover, post–urinary tract infection immune-reactive arthritis and rash appear mostly on the palms and soles. Systemic lupus erythematosus–like rashes have a different histology and appear on sun-exposed areas; our patient’s rash was found mainly on unexposed areas.12
Because our patient received the Moderna vaccine 5 days before the rash appeared and later developed swelling of the digits with morning stiffness, a delayed serum sickness–like reaction secondary to COVID-19 vaccination was possible.3,6
COVID-19 mRNA vaccines developed by Pfizer-BioNTech and Moderna incorporate a lipid-based nanoparticle carrier system that prevents rapid enzymatic degradation of mRNA and facilitates in vivo delivery of mRNA. This lipid-based nanoparticle carrier system is further stabilized by a polyethylene glycol 2000 lipid conjugate that provides a hydrophilic layer, thus prolonging half-life. The presence of lipid polyethylene glycol 2000 in mRNA vaccines has led to concern that this component could be implicated in anaphylaxis.6
COVID-19 antigens can give rise to varying clinical manifestations that are directly related to viral tissue damage or are indirectly induced by the antiviral immune response.13,14 Hyperactivation of the immune system to eradicate COVID-19 may trigger autoimmunity; several immune-mediated disorders have been described in individuals infected with SARS-CoV-2. Dermal manifestations include cutaneous rash and vasculitis.13-16 Crucial immunologic steps occur during SARS-CoV-2 infection that may link autoimmunity to COVID-19.13,14 In preliminary published data on the efficacy of the Moderna vaccine on 45 trial enrollees, 3 did not receive the second dose of vaccination, including 1 who developed urticaria on both legs 5 days after the first dose.1
Introduction of viral RNA can induce autoimmunity that can be explained by various phenomena, including epitope spreading, molecular mimicry, cryptic antigen, and bystander activation. Remarkably, more than one-third of immunogenic proteins in SARS-CoV-2 have potentially problematic homology to proteins that are key to the human adaptive immune system.5
Moreover, SARS-CoV-2 seems to induce organ injury through alternative mechanisms beyond direct viral infection, including immunologic injury. In some situations, hyperactivation of the immune response to SARS-CoV-2 RNA can result in autoimmune disease. COVID-19 has been associated with immune-mediated systemic or organ-selective manifestations, some of which fulfill the diagnostic or classification criteria of specific autoimmune diseases. It is unclear whether those medical disorders are the result of transitory postinfectious epiphenomena.5
A few studies have shown that patients with rheumatic disease have an incidence and prevalence of COVID-19 that is similar to the general population. A similar pattern has been detected in COVID-19 morbidity and mortality rates, even among patients with an autoimmune disease, such as rheumatoid arthritis and Sjögren syndrome.5,17 Furthermore, exacerbation of preexisting rheumatic symptoms may be due to hyperactivation of antiviral pathways in a person with an autoimmune disease.17-19 The findings in our patient suggested a direct role for the vaccine in skin manifestations, rather than for reactivation or development of new systemic autoimmune processes, such as systemic lupus erythematosus.
Exacerbation of psoriasis following COVID-19 vaccination has been described20; however, the case patient did not have a history of psoriasis. The mechanism(s) of such exacerbation remain unclear; COVID-19 vaccine–induced helper T cells (TH17) may play a role.21 Other skin manifestations encountered following COVID-19 vaccination include lichen planus, leukocytoclastic vasculitic rash, erythema multiforme–like rash, and pityriasis rosea–like rash.22-25 The immune mechanisms of these manifestations remain unclear.
The clinical presentation of delayed vaccination reactions can be attributed to the timing of symptoms and, in this case, the immune-mediated background of a psoriasiform reaction. Although adverse reactions to the SARS-CoV-2 mRNA vaccine are rare, more individuals should be studied after vaccination to confirm and better understand this phenomenon.
- Jackson LA, Anderson EJ, Rouphael NG, et al; . An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020;383:1920-1931. doi:10.1056/NEJMoa2022483
- Anderson EJ, Rouphael NG, Widge AT, et al; . Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383:2427-2438. doi:10.1056/NEJMoa2028436
- Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403-416. doi:10.1056/NEJMoa2035389
- Weise E. ‘COVID arm’ rash seen after Moderna vaccine annoying but harmless, doctors say. USA Today. January 27, 2021. Accessed September 4, 2022. https://www.usatoday.com/story/news/health/2021/01/27/covid-arm-moderna-vaccine-rash-harmless-side-effect-doctors-say/4277725001/
- Talotta R, Robertson E. Autoimmunity as the comet tail of COVID-19 pandemic. World J Clin Cases. 2020;8:3621-3644. doi:10.12998/wjcc.v8.i17.3621
- Castells MC, Phillips EJ. Maintaining safety with SARS-CoV-2 vaccines. N Engl J Med. 2021;384:643-649. doi:10.1056/NEJMra2035343
- Polack FP, Thomas SJ, Kitchin N, et al; . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603-2615. doi:10.1056/NEJMoa2034577
- Dooling K, McClung N, Chamberland M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for allocating initial supplies of COVID-19 vaccine—United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1857-1859. doi:10.15585/mmwr.mm6949e1
- Roguedas AM, Misery L, Sassolas B, et al. Cutaneous manifestations of primary Sjögren’s syndrome are underestimated. Clin Exp Rheumatol. 2004;22:632-636.
- Katayama I. Dry skin manifestations in Sjögren syndrome and atopic dermatitis related to aberrant sudomotor function in inflammatory allergic skin diseases. Allergol Int. 2018;67:448-454. doi:10.1016/j.alit.2018.07.001
- Generali E, Costanzo A, Mainetti C, et al. Cutaneous and mucosal manifestations of Sjögren’s syndrome. Clin Rev Allergy Immunol. 2017;53:357-370. doi:10.1007/s12016-017-8639-y
- Chanprapaph K, Tankunakorn J, Suchonwanit P, et al. Dermatologic manifestations, histologic features and disease progression among cutaneous lupus erythematosus subtypes: a prospective observational study in Asians. Dermatol Ther (Heidelb). 2021;11:131-147. doi:10.1007/s13555-020-00471-y
- Ortega-Quijano D, Jimenez-Cauhe J, Selda-Enriquez G, et al. Algorithm for the classification of COVID-19 rashes. J Am Acad Dermatol. 2020;83:e103-e104. doi:10.1016/j.jaad.2020.05.034
- Rahimi H, Tehranchinia Z. A comprehensive review of cutaneous manifestations associated with COVID-19. Biomed Res Int. 2020;2020:1236520. doi:10.1155/2020/1236520
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatol Sci. 2020;98:75-81. doi:10.1016/j.jdermsci.2020.04.011
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743. doi:10.1111/ijd.14937
- Dellavance A, Coelho Andrade LE. Immunologic derangement preceding clinical autoimmunity. Lupus. 2014;23:1305-1308. doi:10.1177/0961203314531346
- Parodi A, Gasparini G, Cozzani E. Could antiphospholipid antibodies contribute to coagulopathy in COVID-19? J Am Acad Dermatol. 2020;83:e249. doi:10.1016/j.jaad.2020.06.003
- Zhou Y, Han T, Chen J, et al. Clinical and autoimmune characteristics of severe and critical cases of COVID-19. Clin Transl Sci. 2020;13:1077-1086. doi:10.1111/cts.12805
- Huang YW, Tsai TF. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med (Lausanne). 2021;8:812010. doi:10.3389/fmed.2021.812010
- Rouai M, Slimane MB, Sassi W, et al. Pustular rash triggered by Pfizer-BioNTech COVID-19 vaccination: a case report. Dermatol Ther. 2022:e15465. doi:10.1111/dth.15465
- Altun E, Kuzucular E. Leukocytoclastic vasculitis after COVID-19 vaccination. Dermatol Ther. 2022;35:e15279. doi:10.1111/dth.15279
- Buckley JE, Landis LN, Rapini RP. Pityriasis rosea-like rash after mRNA COVID-19 vaccination: a case report and review of the literature. JAAD Int. 2022;7:164-168. doi:10.1016/j.jdin.2022.01.009
- Gökçek GE, Öksüm Solak E, Çölgeçen E. Pityriasis rosea like eruption: a dermatological manifestation of Coronavac-COVID-19 vaccine. Dermatol Ther. 2022;35:e15256. doi:10.1111/dth.15256
- Kim MJ, Kim JW, Kim MS, et al. Generalized erythema multiforme-like skin rash following the first dose of COVID-19 vaccine (Pfizer-BioNTech). J Eur Acad Dermatol Venereol. 2022;36:e98-e100. doi:10.1111/jdv.17757
To the Editor:
The Moderna COVID-19 messenger RNA (mRNA) vaccine was authorized for use on December 18, 2020, with the second dose beginning on January 15, 2021.1-3 Some individuals who received the Moderna vaccine experienced an intense rash known as “COVID arm,” a harmless but bothersome adverse effect that typically appears within a week and is a localized and transient immunogenic response.4 COVID arm differs from most vaccine adverse effects. The rash emerges not immediately but 5 to 9 days after the initial dose—on average, 1 week later. Apart from being itchy, the rash does not appear to be harmful and is not a reason to hesitate getting vaccinated.
Dermatologists and allergists have been studying this adverse effect, which has been formally termed delayed cutaneous hypersensitivity. Of potential clinical consequence is that the efficacy of the mRNA COVID-19 vaccine may be harmed if postvaccination dermal reactions necessitate systemic corticosteroid therapy. Because this vaccine stimulates an immune response as viral RNA integrates in cells secondary to production of the spike protein of the virus, the skin may be affected secondarily and manifestations of any underlying disease may be aggravated.5 We report a patient who developed a psoriasiform dermatitis after the first dose of the Moderna vaccine.

A 65-year-old woman presented to her primary care physician because of the severity of psoriasiform dermatitis that developed 5 days after she received the first dose of the Moderna COVID-19 mRNA vaccine. The patient had a medical history of Sjögren syndrome. Her medication history was negative, and her family history was negative for autoimmune disease. Physical examination by primary care revealed an erythematous scaly rash with plaques and papules on the neck and back (Figure 1). The patient presented again to primary care 2 days later with swollen, painful, discolored digits (Figure 2) and a stiff, sore neck.

Laboratory results were positive for anti–Sjögren syndrome–related antigens A and B. A complete blood cell count; comprehensive metabolic panel; erythrocyte sedimentation rate; and assays of rheumatoid factor, C-reactive protein, and anti–cyclic citrullinated peptide were within reference range. A biopsy of a lesion on the back showed psoriasiform dermatitis with confluent parakeratosis and scattered necrotic keratinocytes. There was superficial perivascular inflammation with rare eosinophils (Figure 3).

The patient was treated with a course of systemic corticosteroids. The rash resolved in 1 week. She did not receive the second dose due to the rash.
Two mRNA COVID-19 vaccines—Pfizer BioNTech and Moderna—have been granted emergency use authorization by the US Food and Drug Administration.6 The safety profile of the mRNA-1273 vaccine for the median 2-month follow-up showed no safety concerns.3 Minor localized adverse effects (eg, pain, redness, swelling) have been observed more frequently with the vaccines than with placebo. Systemic symptoms, such as fever, fatigue, headache, and muscle and joint pain, also were seen somewhat more often with the vaccines than with placebo; most such effects occurred 24 to 48 hours after vaccination.3,6,7 The frequency of unsolicited adverse events and serious adverse events reported during the 28-day period after vaccination generally was similar among participants in the vaccine and placebo groups.3
There are 2 types of reactions to COVID-19 vaccination: immediate and delayed. Immediate reactions usually are due to anaphylaxis, requiring prompt recognition and treatment with epinephrine to stop rapid progression of life-threatening symptoms. Delayed reactions include localized reactions, such as urticaria and benign exanthema; serum sickness and serum sickness–like reactions; fever; and rare skin, organ, and neurologic sequelae.1,6-8
Cutaneous manifestations, present in 16% to 50% of patients with Sjögren syndrome, are considered one of the most common extraglandular presentations of the syndrome. They are classified as nonvascular (eg, xerosis, angular cheilitis, eyelid dermatitis, annular erythema) and vascular (eg, Raynaud phenomenon, vasculitis).9-11 Our patient did not have any of those findings. She had not taken any medications before the rash appeared, thereby ruling out a drug reaction.
The differential for our patient included post–urinary tract infection immune-reactive arthritis and rash, which is not typical with Escherichia coli infection but is described with infection with Chlamydia species and Salmonella species. Moreover, post–urinary tract infection immune-reactive arthritis and rash appear mostly on the palms and soles. Systemic lupus erythematosus–like rashes have a different histology and appear on sun-exposed areas; our patient’s rash was found mainly on unexposed areas.12
Because our patient received the Moderna vaccine 5 days before the rash appeared and later developed swelling of the digits with morning stiffness, a delayed serum sickness–like reaction secondary to COVID-19 vaccination was possible.3,6
COVID-19 mRNA vaccines developed by Pfizer-BioNTech and Moderna incorporate a lipid-based nanoparticle carrier system that prevents rapid enzymatic degradation of mRNA and facilitates in vivo delivery of mRNA. This lipid-based nanoparticle carrier system is further stabilized by a polyethylene glycol 2000 lipid conjugate that provides a hydrophilic layer, thus prolonging half-life. The presence of lipid polyethylene glycol 2000 in mRNA vaccines has led to concern that this component could be implicated in anaphylaxis.6
COVID-19 antigens can give rise to varying clinical manifestations that are directly related to viral tissue damage or are indirectly induced by the antiviral immune response.13,14 Hyperactivation of the immune system to eradicate COVID-19 may trigger autoimmunity; several immune-mediated disorders have been described in individuals infected with SARS-CoV-2. Dermal manifestations include cutaneous rash and vasculitis.13-16 Crucial immunologic steps occur during SARS-CoV-2 infection that may link autoimmunity to COVID-19.13,14 In preliminary published data on the efficacy of the Moderna vaccine on 45 trial enrollees, 3 did not receive the second dose of vaccination, including 1 who developed urticaria on both legs 5 days after the first dose.1
Introduction of viral RNA can induce autoimmunity that can be explained by various phenomena, including epitope spreading, molecular mimicry, cryptic antigen, and bystander activation. Remarkably, more than one-third of immunogenic proteins in SARS-CoV-2 have potentially problematic homology to proteins that are key to the human adaptive immune system.5
Moreover, SARS-CoV-2 seems to induce organ injury through alternative mechanisms beyond direct viral infection, including immunologic injury. In some situations, hyperactivation of the immune response to SARS-CoV-2 RNA can result in autoimmune disease. COVID-19 has been associated with immune-mediated systemic or organ-selective manifestations, some of which fulfill the diagnostic or classification criteria of specific autoimmune diseases. It is unclear whether those medical disorders are the result of transitory postinfectious epiphenomena.5
A few studies have shown that patients with rheumatic disease have an incidence and prevalence of COVID-19 that is similar to the general population. A similar pattern has been detected in COVID-19 morbidity and mortality rates, even among patients with an autoimmune disease, such as rheumatoid arthritis and Sjögren syndrome.5,17 Furthermore, exacerbation of preexisting rheumatic symptoms may be due to hyperactivation of antiviral pathways in a person with an autoimmune disease.17-19 The findings in our patient suggested a direct role for the vaccine in skin manifestations, rather than for reactivation or development of new systemic autoimmune processes, such as systemic lupus erythematosus.
Exacerbation of psoriasis following COVID-19 vaccination has been described20; however, the case patient did not have a history of psoriasis. The mechanism(s) of such exacerbation remain unclear; COVID-19 vaccine–induced helper T cells (TH17) may play a role.21 Other skin manifestations encountered following COVID-19 vaccination include lichen planus, leukocytoclastic vasculitic rash, erythema multiforme–like rash, and pityriasis rosea–like rash.22-25 The immune mechanisms of these manifestations remain unclear.
The clinical presentation of delayed vaccination reactions can be attributed to the timing of symptoms and, in this case, the immune-mediated background of a psoriasiform reaction. Although adverse reactions to the SARS-CoV-2 mRNA vaccine are rare, more individuals should be studied after vaccination to confirm and better understand this phenomenon.
To the Editor:
The Moderna COVID-19 messenger RNA (mRNA) vaccine was authorized for use on December 18, 2020, with the second dose beginning on January 15, 2021.1-3 Some individuals who received the Moderna vaccine experienced an intense rash known as “COVID arm,” a harmless but bothersome adverse effect that typically appears within a week and is a localized and transient immunogenic response.4 COVID arm differs from most vaccine adverse effects. The rash emerges not immediately but 5 to 9 days after the initial dose—on average, 1 week later. Apart from being itchy, the rash does not appear to be harmful and is not a reason to hesitate getting vaccinated.
Dermatologists and allergists have been studying this adverse effect, which has been formally termed delayed cutaneous hypersensitivity. Of potential clinical consequence is that the efficacy of the mRNA COVID-19 vaccine may be harmed if postvaccination dermal reactions necessitate systemic corticosteroid therapy. Because this vaccine stimulates an immune response as viral RNA integrates in cells secondary to production of the spike protein of the virus, the skin may be affected secondarily and manifestations of any underlying disease may be aggravated.5 We report a patient who developed a psoriasiform dermatitis after the first dose of the Moderna vaccine.

A 65-year-old woman presented to her primary care physician because of the severity of psoriasiform dermatitis that developed 5 days after she received the first dose of the Moderna COVID-19 mRNA vaccine. The patient had a medical history of Sjögren syndrome. Her medication history was negative, and her family history was negative for autoimmune disease. Physical examination by primary care revealed an erythematous scaly rash with plaques and papules on the neck and back (Figure 1). The patient presented again to primary care 2 days later with swollen, painful, discolored digits (Figure 2) and a stiff, sore neck.

Laboratory results were positive for anti–Sjögren syndrome–related antigens A and B. A complete blood cell count; comprehensive metabolic panel; erythrocyte sedimentation rate; and assays of rheumatoid factor, C-reactive protein, and anti–cyclic citrullinated peptide were within reference range. A biopsy of a lesion on the back showed psoriasiform dermatitis with confluent parakeratosis and scattered necrotic keratinocytes. There was superficial perivascular inflammation with rare eosinophils (Figure 3).

The patient was treated with a course of systemic corticosteroids. The rash resolved in 1 week. She did not receive the second dose due to the rash.
Two mRNA COVID-19 vaccines—Pfizer BioNTech and Moderna—have been granted emergency use authorization by the US Food and Drug Administration.6 The safety profile of the mRNA-1273 vaccine for the median 2-month follow-up showed no safety concerns.3 Minor localized adverse effects (eg, pain, redness, swelling) have been observed more frequently with the vaccines than with placebo. Systemic symptoms, such as fever, fatigue, headache, and muscle and joint pain, also were seen somewhat more often with the vaccines than with placebo; most such effects occurred 24 to 48 hours after vaccination.3,6,7 The frequency of unsolicited adverse events and serious adverse events reported during the 28-day period after vaccination generally was similar among participants in the vaccine and placebo groups.3
There are 2 types of reactions to COVID-19 vaccination: immediate and delayed. Immediate reactions usually are due to anaphylaxis, requiring prompt recognition and treatment with epinephrine to stop rapid progression of life-threatening symptoms. Delayed reactions include localized reactions, such as urticaria and benign exanthema; serum sickness and serum sickness–like reactions; fever; and rare skin, organ, and neurologic sequelae.1,6-8
Cutaneous manifestations, present in 16% to 50% of patients with Sjögren syndrome, are considered one of the most common extraglandular presentations of the syndrome. They are classified as nonvascular (eg, xerosis, angular cheilitis, eyelid dermatitis, annular erythema) and vascular (eg, Raynaud phenomenon, vasculitis).9-11 Our patient did not have any of those findings. She had not taken any medications before the rash appeared, thereby ruling out a drug reaction.
The differential for our patient included post–urinary tract infection immune-reactive arthritis and rash, which is not typical with Escherichia coli infection but is described with infection with Chlamydia species and Salmonella species. Moreover, post–urinary tract infection immune-reactive arthritis and rash appear mostly on the palms and soles. Systemic lupus erythematosus–like rashes have a different histology and appear on sun-exposed areas; our patient’s rash was found mainly on unexposed areas.12
Because our patient received the Moderna vaccine 5 days before the rash appeared and later developed swelling of the digits with morning stiffness, a delayed serum sickness–like reaction secondary to COVID-19 vaccination was possible.3,6
COVID-19 mRNA vaccines developed by Pfizer-BioNTech and Moderna incorporate a lipid-based nanoparticle carrier system that prevents rapid enzymatic degradation of mRNA and facilitates in vivo delivery of mRNA. This lipid-based nanoparticle carrier system is further stabilized by a polyethylene glycol 2000 lipid conjugate that provides a hydrophilic layer, thus prolonging half-life. The presence of lipid polyethylene glycol 2000 in mRNA vaccines has led to concern that this component could be implicated in anaphylaxis.6
COVID-19 antigens can give rise to varying clinical manifestations that are directly related to viral tissue damage or are indirectly induced by the antiviral immune response.13,14 Hyperactivation of the immune system to eradicate COVID-19 may trigger autoimmunity; several immune-mediated disorders have been described in individuals infected with SARS-CoV-2. Dermal manifestations include cutaneous rash and vasculitis.13-16 Crucial immunologic steps occur during SARS-CoV-2 infection that may link autoimmunity to COVID-19.13,14 In preliminary published data on the efficacy of the Moderna vaccine on 45 trial enrollees, 3 did not receive the second dose of vaccination, including 1 who developed urticaria on both legs 5 days after the first dose.1
Introduction of viral RNA can induce autoimmunity that can be explained by various phenomena, including epitope spreading, molecular mimicry, cryptic antigen, and bystander activation. Remarkably, more than one-third of immunogenic proteins in SARS-CoV-2 have potentially problematic homology to proteins that are key to the human adaptive immune system.5
Moreover, SARS-CoV-2 seems to induce organ injury through alternative mechanisms beyond direct viral infection, including immunologic injury. In some situations, hyperactivation of the immune response to SARS-CoV-2 RNA can result in autoimmune disease. COVID-19 has been associated with immune-mediated systemic or organ-selective manifestations, some of which fulfill the diagnostic or classification criteria of specific autoimmune diseases. It is unclear whether those medical disorders are the result of transitory postinfectious epiphenomena.5
A few studies have shown that patients with rheumatic disease have an incidence and prevalence of COVID-19 that is similar to the general population. A similar pattern has been detected in COVID-19 morbidity and mortality rates, even among patients with an autoimmune disease, such as rheumatoid arthritis and Sjögren syndrome.5,17 Furthermore, exacerbation of preexisting rheumatic symptoms may be due to hyperactivation of antiviral pathways in a person with an autoimmune disease.17-19 The findings in our patient suggested a direct role for the vaccine in skin manifestations, rather than for reactivation or development of new systemic autoimmune processes, such as systemic lupus erythematosus.
Exacerbation of psoriasis following COVID-19 vaccination has been described20; however, the case patient did not have a history of psoriasis. The mechanism(s) of such exacerbation remain unclear; COVID-19 vaccine–induced helper T cells (TH17) may play a role.21 Other skin manifestations encountered following COVID-19 vaccination include lichen planus, leukocytoclastic vasculitic rash, erythema multiforme–like rash, and pityriasis rosea–like rash.22-25 The immune mechanisms of these manifestations remain unclear.
The clinical presentation of delayed vaccination reactions can be attributed to the timing of symptoms and, in this case, the immune-mediated background of a psoriasiform reaction. Although adverse reactions to the SARS-CoV-2 mRNA vaccine are rare, more individuals should be studied after vaccination to confirm and better understand this phenomenon.
- Jackson LA, Anderson EJ, Rouphael NG, et al; . An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020;383:1920-1931. doi:10.1056/NEJMoa2022483
- Anderson EJ, Rouphael NG, Widge AT, et al; . Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383:2427-2438. doi:10.1056/NEJMoa2028436
- Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403-416. doi:10.1056/NEJMoa2035389
- Weise E. ‘COVID arm’ rash seen after Moderna vaccine annoying but harmless, doctors say. USA Today. January 27, 2021. Accessed September 4, 2022. https://www.usatoday.com/story/news/health/2021/01/27/covid-arm-moderna-vaccine-rash-harmless-side-effect-doctors-say/4277725001/
- Talotta R, Robertson E. Autoimmunity as the comet tail of COVID-19 pandemic. World J Clin Cases. 2020;8:3621-3644. doi:10.12998/wjcc.v8.i17.3621
- Castells MC, Phillips EJ. Maintaining safety with SARS-CoV-2 vaccines. N Engl J Med. 2021;384:643-649. doi:10.1056/NEJMra2035343
- Polack FP, Thomas SJ, Kitchin N, et al; . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603-2615. doi:10.1056/NEJMoa2034577
- Dooling K, McClung N, Chamberland M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for allocating initial supplies of COVID-19 vaccine—United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1857-1859. doi:10.15585/mmwr.mm6949e1
- Roguedas AM, Misery L, Sassolas B, et al. Cutaneous manifestations of primary Sjögren’s syndrome are underestimated. Clin Exp Rheumatol. 2004;22:632-636.
- Katayama I. Dry skin manifestations in Sjögren syndrome and atopic dermatitis related to aberrant sudomotor function in inflammatory allergic skin diseases. Allergol Int. 2018;67:448-454. doi:10.1016/j.alit.2018.07.001
- Generali E, Costanzo A, Mainetti C, et al. Cutaneous and mucosal manifestations of Sjögren’s syndrome. Clin Rev Allergy Immunol. 2017;53:357-370. doi:10.1007/s12016-017-8639-y
- Chanprapaph K, Tankunakorn J, Suchonwanit P, et al. Dermatologic manifestations, histologic features and disease progression among cutaneous lupus erythematosus subtypes: a prospective observational study in Asians. Dermatol Ther (Heidelb). 2021;11:131-147. doi:10.1007/s13555-020-00471-y
- Ortega-Quijano D, Jimenez-Cauhe J, Selda-Enriquez G, et al. Algorithm for the classification of COVID-19 rashes. J Am Acad Dermatol. 2020;83:e103-e104. doi:10.1016/j.jaad.2020.05.034
- Rahimi H, Tehranchinia Z. A comprehensive review of cutaneous manifestations associated with COVID-19. Biomed Res Int. 2020;2020:1236520. doi:10.1155/2020/1236520
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatol Sci. 2020;98:75-81. doi:10.1016/j.jdermsci.2020.04.011
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743. doi:10.1111/ijd.14937
- Dellavance A, Coelho Andrade LE. Immunologic derangement preceding clinical autoimmunity. Lupus. 2014;23:1305-1308. doi:10.1177/0961203314531346
- Parodi A, Gasparini G, Cozzani E. Could antiphospholipid antibodies contribute to coagulopathy in COVID-19? J Am Acad Dermatol. 2020;83:e249. doi:10.1016/j.jaad.2020.06.003
- Zhou Y, Han T, Chen J, et al. Clinical and autoimmune characteristics of severe and critical cases of COVID-19. Clin Transl Sci. 2020;13:1077-1086. doi:10.1111/cts.12805
- Huang YW, Tsai TF. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med (Lausanne). 2021;8:812010. doi:10.3389/fmed.2021.812010
- Rouai M, Slimane MB, Sassi W, et al. Pustular rash triggered by Pfizer-BioNTech COVID-19 vaccination: a case report. Dermatol Ther. 2022:e15465. doi:10.1111/dth.15465
- Altun E, Kuzucular E. Leukocytoclastic vasculitis after COVID-19 vaccination. Dermatol Ther. 2022;35:e15279. doi:10.1111/dth.15279
- Buckley JE, Landis LN, Rapini RP. Pityriasis rosea-like rash after mRNA COVID-19 vaccination: a case report and review of the literature. JAAD Int. 2022;7:164-168. doi:10.1016/j.jdin.2022.01.009
- Gökçek GE, Öksüm Solak E, Çölgeçen E. Pityriasis rosea like eruption: a dermatological manifestation of Coronavac-COVID-19 vaccine. Dermatol Ther. 2022;35:e15256. doi:10.1111/dth.15256
- Kim MJ, Kim JW, Kim MS, et al. Generalized erythema multiforme-like skin rash following the first dose of COVID-19 vaccine (Pfizer-BioNTech). J Eur Acad Dermatol Venereol. 2022;36:e98-e100. doi:10.1111/jdv.17757
- Jackson LA, Anderson EJ, Rouphael NG, et al; . An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020;383:1920-1931. doi:10.1056/NEJMoa2022483
- Anderson EJ, Rouphael NG, Widge AT, et al; . Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383:2427-2438. doi:10.1056/NEJMoa2028436
- Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403-416. doi:10.1056/NEJMoa2035389
- Weise E. ‘COVID arm’ rash seen after Moderna vaccine annoying but harmless, doctors say. USA Today. January 27, 2021. Accessed September 4, 2022. https://www.usatoday.com/story/news/health/2021/01/27/covid-arm-moderna-vaccine-rash-harmless-side-effect-doctors-say/4277725001/
- Talotta R, Robertson E. Autoimmunity as the comet tail of COVID-19 pandemic. World J Clin Cases. 2020;8:3621-3644. doi:10.12998/wjcc.v8.i17.3621
- Castells MC, Phillips EJ. Maintaining safety with SARS-CoV-2 vaccines. N Engl J Med. 2021;384:643-649. doi:10.1056/NEJMra2035343
- Polack FP, Thomas SJ, Kitchin N, et al; . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603-2615. doi:10.1056/NEJMoa2034577
- Dooling K, McClung N, Chamberland M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for allocating initial supplies of COVID-19 vaccine—United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1857-1859. doi:10.15585/mmwr.mm6949e1
- Roguedas AM, Misery L, Sassolas B, et al. Cutaneous manifestations of primary Sjögren’s syndrome are underestimated. Clin Exp Rheumatol. 2004;22:632-636.
- Katayama I. Dry skin manifestations in Sjögren syndrome and atopic dermatitis related to aberrant sudomotor function in inflammatory allergic skin diseases. Allergol Int. 2018;67:448-454. doi:10.1016/j.alit.2018.07.001
- Generali E, Costanzo A, Mainetti C, et al. Cutaneous and mucosal manifestations of Sjögren’s syndrome. Clin Rev Allergy Immunol. 2017;53:357-370. doi:10.1007/s12016-017-8639-y
- Chanprapaph K, Tankunakorn J, Suchonwanit P, et al. Dermatologic manifestations, histologic features and disease progression among cutaneous lupus erythematosus subtypes: a prospective observational study in Asians. Dermatol Ther (Heidelb). 2021;11:131-147. doi:10.1007/s13555-020-00471-y
- Ortega-Quijano D, Jimenez-Cauhe J, Selda-Enriquez G, et al. Algorithm for the classification of COVID-19 rashes. J Am Acad Dermatol. 2020;83:e103-e104. doi:10.1016/j.jaad.2020.05.034
- Rahimi H, Tehranchinia Z. A comprehensive review of cutaneous manifestations associated with COVID-19. Biomed Res Int. 2020;2020:1236520. doi:10.1155/2020/1236520
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatol Sci. 2020;98:75-81. doi:10.1016/j.jdermsci.2020.04.011
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743. doi:10.1111/ijd.14937
- Dellavance A, Coelho Andrade LE. Immunologic derangement preceding clinical autoimmunity. Lupus. 2014;23:1305-1308. doi:10.1177/0961203314531346
- Parodi A, Gasparini G, Cozzani E. Could antiphospholipid antibodies contribute to coagulopathy in COVID-19? J Am Acad Dermatol. 2020;83:e249. doi:10.1016/j.jaad.2020.06.003
- Zhou Y, Han T, Chen J, et al. Clinical and autoimmune characteristics of severe and critical cases of COVID-19. Clin Transl Sci. 2020;13:1077-1086. doi:10.1111/cts.12805
- Huang YW, Tsai TF. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med (Lausanne). 2021;8:812010. doi:10.3389/fmed.2021.812010
- Rouai M, Slimane MB, Sassi W, et al. Pustular rash triggered by Pfizer-BioNTech COVID-19 vaccination: a case report. Dermatol Ther. 2022:e15465. doi:10.1111/dth.15465
- Altun E, Kuzucular E. Leukocytoclastic vasculitis after COVID-19 vaccination. Dermatol Ther. 2022;35:e15279. doi:10.1111/dth.15279
- Buckley JE, Landis LN, Rapini RP. Pityriasis rosea-like rash after mRNA COVID-19 vaccination: a case report and review of the literature. JAAD Int. 2022;7:164-168. doi:10.1016/j.jdin.2022.01.009
- Gökçek GE, Öksüm Solak E, Çölgeçen E. Pityriasis rosea like eruption: a dermatological manifestation of Coronavac-COVID-19 vaccine. Dermatol Ther. 2022;35:e15256. doi:10.1111/dth.15256
- Kim MJ, Kim JW, Kim MS, et al. Generalized erythema multiforme-like skin rash following the first dose of COVID-19 vaccine (Pfizer-BioNTech). J Eur Acad Dermatol Venereol. 2022;36:e98-e100. doi:10.1111/jdv.17757
PRACTICE POINTS
- The differential diagnosis for a new-onset psoriasiform rash in an elderly patient should include a vaccine-related rash.
- A rash following vaccination that necessitates systemic corticosteroid therapy can decrease vaccine efficacy.
How Low Is Too Low? A Retrospective Analysis of Very Low LDL-C Levels in Veterans
According to the Centers for Disease Control and Prevention (CDC), approximately 795,000 strokes occur in the United States yearly and are the fifth leading cause of death.1 The CDC also states that about 43 million Americans who could benefit from cholesterol medication are currently taking them.2 As of 2019, West Virginia, Ohio, and Kentucky are 3 states with the highest rates of heart disease mortality.3
Low-density lipoprotein cholesterol (LDL-C) accumulates on the walls of blood vessels, which can lead to coronary heart disease. However, some LDL-C is necessary to maintain proper brain function. Guidelines from the American College of Cardiology (ACC) and American Heart Association (AHA) recommend LDL-C goal levels < 70 mg/dL.4 Yet, there is no consensus on how low LDL-C levels should be. According to clinical practice guidelines for dyslipidemia, developed by the US Department of Veterans Affairs (VA) and US Department of Defense, statin medications are first-line agents for lowering LDL-C. The intensity of the statin medication is based on primary or secondary prevention, atherosclerotic cardiovascular disease (ASCVD) risk, and current LDL-C levels prior to treatment.5
Statin medications are used for primary and secondary prevention of ASCVD. In addition, statin medications decrease total cholesterol, LDL-C, and triglycerides while causing a mild increase in high-density lipoprotein cholesterol. Although statin medications are first-line therapy for LDL-C lowering, other medications can be used to assist in decreasing LDL-C. Ezetimibe, fenofibrates, and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors can also be used.5 Statin medications do pose a risk of severe adverse drug reactions (ADRs), such as rhabdomyolysis and myopathy.6
One prospective cohort study looked at 27,937 women and analyzed total cholesterol, LDL-C, high-density lipoprotein cholesterol, triglycerides, and strokes. The study noted a mean 19.3-year follow-up and within that follow-up, 137 hemorrhagic strokes occurred. Based on the study’s results, LDL-C levels < 70 mg/dL had 2.17 times the risk of experiencing a hemorrhagic stroke.7 A meta-analysis of prospective studies analyzed 476,173 patients and 7487 hemorrhagic stroke cases. This review concluded that a 10 mg/dL increase in LDL-C was associated with a 3% lower risk of hemorrhagic stroke.8
An observational study conducted in Asia of Chinese adults found that 22% of all strokes were hemorrhagic. The incidence of the hemorrhagic strokes was higher for patients who had an LDL-C < 1.8 mmol/L than those who had an LDL-C between 1.8 and 2.6 mmol/L. This study also showed that if hypertension was inadequately treated, the risk of hemorrhagic stroke increased. This study concluded that the benefit of reducing ASCVD outweighs the small risk of hemorrhagic strokes.9
Another prospective cohort study included 96,043 stroke-free participants and analyzed LDL-C concentrations and incidence of intracranial hemorrhage. The average LDL-C concentrations were calculated from data collected in 4 separate reporting years, and incidence of intracranial hemorrhage was confirmed through review of medication records. Over a 9-year follow-up period, the study concluded that participants with an LDL-C level of < 70 mg/dL had a significantly higher risk of developing intracranial hemorrhage than participants with LDL-C levels 70 to 99 mg/dL.10
The safety and effects of prolonged very low LDL-C levels are currently unknown. The current study sought to gather information to determine the risks of very low LDL-C levels in a veteran population.
Methods
A retrospective chart review was conducted on patients aged 18 to 90 years receiving care at the Hershel “Woody” Williams Veterans Affairs Medical Center (HWW VAMC) in Huntington, West Virginia, between January 1, 2010, and September 1, 2020. Approval of the current study was obtained through the Marshall University Institutional Review Board, HWW VAMC Research and Development Committee, and Veterans Health Administration (VHA) DATA Access Request Tracker (DART)/VA Informatic and Computing Infrastructure (VINCI). Data were obtained via the VHA Corporate Data Warehouse (CDW) for the HWW VAMC using Microsoft Structured Query Language (SQL) server available in VINCI. Analysis of the data was conducted using STATA v. 15.
Patients were included if they had a diagnosis of hyperlipidemia/dyslipidemia, received treatment with HMG-CoA reductase inhibitors or PCSK9 medications, and had an LDL-C level ≤ 40 mg/dL. The primary outcome was the rate of intracranial hemorrhage that could be caused by very low LDL-C levels. The secondary outcomes included actions taken by clinicians to address LDL-C level < 40 mg/dL, ADRs, duration of therapy, and medication adherence. Patients were excluded if they were aged < 18 or > 90 years, were pregnant during the study period, had hypothyroidism, received chronic anticoagulation medications, or had a triglyceride level > 300 mg/dL.
Results
The study included 3027 patients. Of those patients, 78 patients were female while 2949 were male, and the mean (SD) age was 68.3 (9.4) years. A subsample of 32 patients was analyzed to determine whether an ADR was noted or low LDL-C level was addressed in the chart. The subsample size was determined through chart review and included patients who had a documented intracranial hemorrhage. None of the 32 patients had an ADR documented, and 6 (19%) had the low LDL-C level addressed in the chart by monitoring levels, reducing statin doses, or discontinuing the medication. Of the total population analyzed, 8 patients (0.3%) had a documented intracranial hemorrhage within 1 year following the low LDL-C level.
We also analyzed the intensity of statin related to the low LDL-C level (Table 1).
The most common ADRs were muscle, joint, and leg pain, rash, and cramps (Table 2).
Adherence to the medications and duration of therapy was also analyzed and was found to be similar among the various medications. Lovastatin had the highest percent adherence with 91.2% while atorvastatin had the lowest with 85.5%. It can be noted that lovastatin had a lower documented percentage of ADRs while atorvastatin had a higher documented percentage of ADRs, which can be clinically meaningful when prescribing these medications; however, these similar adherence rates are not influencing the primary outcome of the rate of intracranial hemorrhage due to LDL-C level < 40 mg/dL. Mean duration of therapy lasted between 1 year and > 4 years with 1.1 years for alirocumab and 4.2 for simvastatin. The duration of therapy could be influenced by formulary restrictions during the study time. Nonetheless, patients, regardless of formulary restrictions, have taken these medications for a duration long enough to affect LDL-C levels.
Eight patients of the total sample analyzed had an intracranial hemorrhage within 1 year of having a recorded LDL-C level < 40 mg/dL. Secondarily, 32 patients had clinicians address an LDL-C level < 40 mg/dL through documentation or modifying the medication therapy. The most common ADRs among all medications analyzed were leg and joint pain, rash, and cramps. Of all medications included in this study, the mean duration of therapy was > 1 year, which would allow them to affect LDL-C levels and have those levels monitored and recorded in patients’ charts.
Discussion
When comparing our primary outcome of risk of intracranial hemorrhage with previous literature, the results are consistent with previous outcomes. Previous literature had a smaller sample size but analyzed LDL-C levels < 50 mg/dL and had an outcome of 48 patients experiencing an intracranial hemorrhage within 1 year of an LDL-C level < 50 mg/dL. Due to this study having stricter parameters of LDL-C levels < 40 mg/dL, there were fewer patients with documented intracranial hemorrhages. With there being a risk of intracranial hemorrhage with low LDL-C levels, the results demonstrate the need to monitor and address LDL-C levels.
Limitations
There were several notable limitations to this study. The retrospective, single-center nature coupled with the predominately male study population may affect the generalizability of the study results to patients outside of the facility in which the study was performed. Additionally, the study only included statin medications and PCSK9 inhibitors. With future studies, all lipid-lowering medications could be analyzed. The study was largely reliant on the proper documentation of International Statistical Classification of Diseases, Tenth Revision (ICD-10) codes exclusive to the HWW VAMC, which may exclude patients who first present to outside facilities. Due to time restraints, the incidence of hemorrhage was only analyzed 1 year following an LDL-C level < 40 mg/dL. For considerations for future investigation, the length of time to analyze incidence of hemorrhage could be expanded to be similar to previous studies, and the study could be expanded across the local Veterans Integrated Service Network or VA system. Additionally, the study could have analyzed the percentage of time a patient had an LDL-C level < 40 mg/dL in their lifetime.
Conclusions
These results show there is a risk that patients with an LDL-C level < 40 mg/dL may experience an intracranial hemorrhage. As seen by the results, there is a clinical need for practitioners to routinely monitor and address LDL-C levels. With various guidelines that recommend starting statin medication to reduce risk of ASCVD, it is necessary that practitioners routinely monitor cholesterol levels and adjust the medications according to laboratory results.11
Within 1 year of an LDL-C level < 40 mg/dL, 0.3% of patients had an intracranial hemorrhage. There was no statistical significance between the rate of ADRs among the medications analyzed. High-intensity statin medications were statistically significant in resulting in an LDL-C level < 40 mg/dL compared with moderate- and low-intensity statin medications. Of the 32 subsample of patients, LDL-C levels < 40 mg/mL are not routinely being addressed in the chart by the clinician.
1. Centers for Disease Control and Prevention. Stroke facts. Updated April 5, 2022. Accessed September 21, 2022. https://www.cdc.gov/stroke/facts.htm
2. Centers for Disease Control and Prevention. High cholesterol facts. Updated July 12, 2022. Accessed September 21, 2022. https://www.cdc.gov/cholesterol/facts.htm
3. Centers for Disease Control and Prevention. Heart disease mortality by state. Updated February 25, 2022. Accessed September 21, 2022. https://www.cdc.gov/nchs/pressroom/sosmap/heart_disease_mortality/heart_disease.htm
4. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082-e1143. doi:10.1161/CIR.0000000000000625
5. US Department of Veterans Affairs, US Department of Defense. VA/DoD Clinical Practice Guideline for the Management of Dyslipidemia for Cardiovascular Risk Reduction. Version 4.0. US Department of Veterans Affairs. June 2020. Accessed September 21, 2022. https://www.healthquality.va.gov/guidelines/CD/lipids/VADoDDyslipidemiaCPG5087212020.pdf
6. Tomaszewski M, Ste¸pien´ KM, Tomaszewska J, Czuczwar SJ. Statin-induced myopathies. Pharmacol Rep. 2011;63(4):859-66. doi:10.1016/s1734-1140(11)70601-6
7. Rist PM, Buring JE, Ridker PM, Kase CS, Kurth T, Rexrode KM. Lipid levels and the risk of hemorrhagic stroke among women. Neurology. 2019;92(19):e2286-e2294. doi:10.1212/WNL.0000000000007454
8. Ma C, Na M, Neumann S, Gao X. Low-density lipoprotein cholesterol and risk of hemorrhagic stroke: a systematic review and dose-response meta-analysis of prospective studies. Curr Atheroscler Rep. 2019;21(12):52. Published 2019 Nov 20. doi:10.1007/s11883-019-0815-5
9. Lui DT, Tan KC. Low-density lipoprotein cholesterol and stroke: How low should we go? J Diabetes Investig. 2020;11(6):1379-1381. doi:10.1111/jdi.13310
10. Ma C, Gurol ME, Huang Z, et al. Low-density lipoprotein cholesterol and risk of intracerebral hemorrhage: a prospective study. Neurology. 2019;93(5):e445-e457. doi:10.1212/WNL.0000000000007853
11. American Diabetes Association Professional Practice Committee. 10. Cardiovascular disease and risk management: standards of medical care in diabetes—2022. Diabetes Care. 2022;45(suppl 1):S144–S174. doi:10.2337/dc22-S010
According to the Centers for Disease Control and Prevention (CDC), approximately 795,000 strokes occur in the United States yearly and are the fifth leading cause of death.1 The CDC also states that about 43 million Americans who could benefit from cholesterol medication are currently taking them.2 As of 2019, West Virginia, Ohio, and Kentucky are 3 states with the highest rates of heart disease mortality.3
Low-density lipoprotein cholesterol (LDL-C) accumulates on the walls of blood vessels, which can lead to coronary heart disease. However, some LDL-C is necessary to maintain proper brain function. Guidelines from the American College of Cardiology (ACC) and American Heart Association (AHA) recommend LDL-C goal levels < 70 mg/dL.4 Yet, there is no consensus on how low LDL-C levels should be. According to clinical practice guidelines for dyslipidemia, developed by the US Department of Veterans Affairs (VA) and US Department of Defense, statin medications are first-line agents for lowering LDL-C. The intensity of the statin medication is based on primary or secondary prevention, atherosclerotic cardiovascular disease (ASCVD) risk, and current LDL-C levels prior to treatment.5
Statin medications are used for primary and secondary prevention of ASCVD. In addition, statin medications decrease total cholesterol, LDL-C, and triglycerides while causing a mild increase in high-density lipoprotein cholesterol. Although statin medications are first-line therapy for LDL-C lowering, other medications can be used to assist in decreasing LDL-C. Ezetimibe, fenofibrates, and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors can also be used.5 Statin medications do pose a risk of severe adverse drug reactions (ADRs), such as rhabdomyolysis and myopathy.6
One prospective cohort study looked at 27,937 women and analyzed total cholesterol, LDL-C, high-density lipoprotein cholesterol, triglycerides, and strokes. The study noted a mean 19.3-year follow-up and within that follow-up, 137 hemorrhagic strokes occurred. Based on the study’s results, LDL-C levels < 70 mg/dL had 2.17 times the risk of experiencing a hemorrhagic stroke.7 A meta-analysis of prospective studies analyzed 476,173 patients and 7487 hemorrhagic stroke cases. This review concluded that a 10 mg/dL increase in LDL-C was associated with a 3% lower risk of hemorrhagic stroke.8
An observational study conducted in Asia of Chinese adults found that 22% of all strokes were hemorrhagic. The incidence of the hemorrhagic strokes was higher for patients who had an LDL-C < 1.8 mmol/L than those who had an LDL-C between 1.8 and 2.6 mmol/L. This study also showed that if hypertension was inadequately treated, the risk of hemorrhagic stroke increased. This study concluded that the benefit of reducing ASCVD outweighs the small risk of hemorrhagic strokes.9
Another prospective cohort study included 96,043 stroke-free participants and analyzed LDL-C concentrations and incidence of intracranial hemorrhage. The average LDL-C concentrations were calculated from data collected in 4 separate reporting years, and incidence of intracranial hemorrhage was confirmed through review of medication records. Over a 9-year follow-up period, the study concluded that participants with an LDL-C level of < 70 mg/dL had a significantly higher risk of developing intracranial hemorrhage than participants with LDL-C levels 70 to 99 mg/dL.10
The safety and effects of prolonged very low LDL-C levels are currently unknown. The current study sought to gather information to determine the risks of very low LDL-C levels in a veteran population.
Methods
A retrospective chart review was conducted on patients aged 18 to 90 years receiving care at the Hershel “Woody” Williams Veterans Affairs Medical Center (HWW VAMC) in Huntington, West Virginia, between January 1, 2010, and September 1, 2020. Approval of the current study was obtained through the Marshall University Institutional Review Board, HWW VAMC Research and Development Committee, and Veterans Health Administration (VHA) DATA Access Request Tracker (DART)/VA Informatic and Computing Infrastructure (VINCI). Data were obtained via the VHA Corporate Data Warehouse (CDW) for the HWW VAMC using Microsoft Structured Query Language (SQL) server available in VINCI. Analysis of the data was conducted using STATA v. 15.
Patients were included if they had a diagnosis of hyperlipidemia/dyslipidemia, received treatment with HMG-CoA reductase inhibitors or PCSK9 medications, and had an LDL-C level ≤ 40 mg/dL. The primary outcome was the rate of intracranial hemorrhage that could be caused by very low LDL-C levels. The secondary outcomes included actions taken by clinicians to address LDL-C level < 40 mg/dL, ADRs, duration of therapy, and medication adherence. Patients were excluded if they were aged < 18 or > 90 years, were pregnant during the study period, had hypothyroidism, received chronic anticoagulation medications, or had a triglyceride level > 300 mg/dL.
Results
The study included 3027 patients. Of those patients, 78 patients were female while 2949 were male, and the mean (SD) age was 68.3 (9.4) years. A subsample of 32 patients was analyzed to determine whether an ADR was noted or low LDL-C level was addressed in the chart. The subsample size was determined through chart review and included patients who had a documented intracranial hemorrhage. None of the 32 patients had an ADR documented, and 6 (19%) had the low LDL-C level addressed in the chart by monitoring levels, reducing statin doses, or discontinuing the medication. Of the total population analyzed, 8 patients (0.3%) had a documented intracranial hemorrhage within 1 year following the low LDL-C level.
We also analyzed the intensity of statin related to the low LDL-C level (Table 1).
The most common ADRs were muscle, joint, and leg pain, rash, and cramps (Table 2).
Adherence to the medications and duration of therapy was also analyzed and was found to be similar among the various medications. Lovastatin had the highest percent adherence with 91.2% while atorvastatin had the lowest with 85.5%. It can be noted that lovastatin had a lower documented percentage of ADRs while atorvastatin had a higher documented percentage of ADRs, which can be clinically meaningful when prescribing these medications; however, these similar adherence rates are not influencing the primary outcome of the rate of intracranial hemorrhage due to LDL-C level < 40 mg/dL. Mean duration of therapy lasted between 1 year and > 4 years with 1.1 years for alirocumab and 4.2 for simvastatin. The duration of therapy could be influenced by formulary restrictions during the study time. Nonetheless, patients, regardless of formulary restrictions, have taken these medications for a duration long enough to affect LDL-C levels.
Eight patients of the total sample analyzed had an intracranial hemorrhage within 1 year of having a recorded LDL-C level < 40 mg/dL. Secondarily, 32 patients had clinicians address an LDL-C level < 40 mg/dL through documentation or modifying the medication therapy. The most common ADRs among all medications analyzed were leg and joint pain, rash, and cramps. Of all medications included in this study, the mean duration of therapy was > 1 year, which would allow them to affect LDL-C levels and have those levels monitored and recorded in patients’ charts.
Discussion
When comparing our primary outcome of risk of intracranial hemorrhage with previous literature, the results are consistent with previous outcomes. Previous literature had a smaller sample size but analyzed LDL-C levels < 50 mg/dL and had an outcome of 48 patients experiencing an intracranial hemorrhage within 1 year of an LDL-C level < 50 mg/dL. Due to this study having stricter parameters of LDL-C levels < 40 mg/dL, there were fewer patients with documented intracranial hemorrhages. With there being a risk of intracranial hemorrhage with low LDL-C levels, the results demonstrate the need to monitor and address LDL-C levels.
Limitations
There were several notable limitations to this study. The retrospective, single-center nature coupled with the predominately male study population may affect the generalizability of the study results to patients outside of the facility in which the study was performed. Additionally, the study only included statin medications and PCSK9 inhibitors. With future studies, all lipid-lowering medications could be analyzed. The study was largely reliant on the proper documentation of International Statistical Classification of Diseases, Tenth Revision (ICD-10) codes exclusive to the HWW VAMC, which may exclude patients who first present to outside facilities. Due to time restraints, the incidence of hemorrhage was only analyzed 1 year following an LDL-C level < 40 mg/dL. For considerations for future investigation, the length of time to analyze incidence of hemorrhage could be expanded to be similar to previous studies, and the study could be expanded across the local Veterans Integrated Service Network or VA system. Additionally, the study could have analyzed the percentage of time a patient had an LDL-C level < 40 mg/dL in their lifetime.
Conclusions
These results show there is a risk that patients with an LDL-C level < 40 mg/dL may experience an intracranial hemorrhage. As seen by the results, there is a clinical need for practitioners to routinely monitor and address LDL-C levels. With various guidelines that recommend starting statin medication to reduce risk of ASCVD, it is necessary that practitioners routinely monitor cholesterol levels and adjust the medications according to laboratory results.11
Within 1 year of an LDL-C level < 40 mg/dL, 0.3% of patients had an intracranial hemorrhage. There was no statistical significance between the rate of ADRs among the medications analyzed. High-intensity statin medications were statistically significant in resulting in an LDL-C level < 40 mg/dL compared with moderate- and low-intensity statin medications. Of the 32 subsample of patients, LDL-C levels < 40 mg/mL are not routinely being addressed in the chart by the clinician.
According to the Centers for Disease Control and Prevention (CDC), approximately 795,000 strokes occur in the United States yearly and are the fifth leading cause of death.1 The CDC also states that about 43 million Americans who could benefit from cholesterol medication are currently taking them.2 As of 2019, West Virginia, Ohio, and Kentucky are 3 states with the highest rates of heart disease mortality.3
Low-density lipoprotein cholesterol (LDL-C) accumulates on the walls of blood vessels, which can lead to coronary heart disease. However, some LDL-C is necessary to maintain proper brain function. Guidelines from the American College of Cardiology (ACC) and American Heart Association (AHA) recommend LDL-C goal levels < 70 mg/dL.4 Yet, there is no consensus on how low LDL-C levels should be. According to clinical practice guidelines for dyslipidemia, developed by the US Department of Veterans Affairs (VA) and US Department of Defense, statin medications are first-line agents for lowering LDL-C. The intensity of the statin medication is based on primary or secondary prevention, atherosclerotic cardiovascular disease (ASCVD) risk, and current LDL-C levels prior to treatment.5
Statin medications are used for primary and secondary prevention of ASCVD. In addition, statin medications decrease total cholesterol, LDL-C, and triglycerides while causing a mild increase in high-density lipoprotein cholesterol. Although statin medications are first-line therapy for LDL-C lowering, other medications can be used to assist in decreasing LDL-C. Ezetimibe, fenofibrates, and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors can also be used.5 Statin medications do pose a risk of severe adverse drug reactions (ADRs), such as rhabdomyolysis and myopathy.6
One prospective cohort study looked at 27,937 women and analyzed total cholesterol, LDL-C, high-density lipoprotein cholesterol, triglycerides, and strokes. The study noted a mean 19.3-year follow-up and within that follow-up, 137 hemorrhagic strokes occurred. Based on the study’s results, LDL-C levels < 70 mg/dL had 2.17 times the risk of experiencing a hemorrhagic stroke.7 A meta-analysis of prospective studies analyzed 476,173 patients and 7487 hemorrhagic stroke cases. This review concluded that a 10 mg/dL increase in LDL-C was associated with a 3% lower risk of hemorrhagic stroke.8
An observational study conducted in Asia of Chinese adults found that 22% of all strokes were hemorrhagic. The incidence of the hemorrhagic strokes was higher for patients who had an LDL-C < 1.8 mmol/L than those who had an LDL-C between 1.8 and 2.6 mmol/L. This study also showed that if hypertension was inadequately treated, the risk of hemorrhagic stroke increased. This study concluded that the benefit of reducing ASCVD outweighs the small risk of hemorrhagic strokes.9
Another prospective cohort study included 96,043 stroke-free participants and analyzed LDL-C concentrations and incidence of intracranial hemorrhage. The average LDL-C concentrations were calculated from data collected in 4 separate reporting years, and incidence of intracranial hemorrhage was confirmed through review of medication records. Over a 9-year follow-up period, the study concluded that participants with an LDL-C level of < 70 mg/dL had a significantly higher risk of developing intracranial hemorrhage than participants with LDL-C levels 70 to 99 mg/dL.10
The safety and effects of prolonged very low LDL-C levels are currently unknown. The current study sought to gather information to determine the risks of very low LDL-C levels in a veteran population.
Methods
A retrospective chart review was conducted on patients aged 18 to 90 years receiving care at the Hershel “Woody” Williams Veterans Affairs Medical Center (HWW VAMC) in Huntington, West Virginia, between January 1, 2010, and September 1, 2020. Approval of the current study was obtained through the Marshall University Institutional Review Board, HWW VAMC Research and Development Committee, and Veterans Health Administration (VHA) DATA Access Request Tracker (DART)/VA Informatic and Computing Infrastructure (VINCI). Data were obtained via the VHA Corporate Data Warehouse (CDW) for the HWW VAMC using Microsoft Structured Query Language (SQL) server available in VINCI. Analysis of the data was conducted using STATA v. 15.
Patients were included if they had a diagnosis of hyperlipidemia/dyslipidemia, received treatment with HMG-CoA reductase inhibitors or PCSK9 medications, and had an LDL-C level ≤ 40 mg/dL. The primary outcome was the rate of intracranial hemorrhage that could be caused by very low LDL-C levels. The secondary outcomes included actions taken by clinicians to address LDL-C level < 40 mg/dL, ADRs, duration of therapy, and medication adherence. Patients were excluded if they were aged < 18 or > 90 years, were pregnant during the study period, had hypothyroidism, received chronic anticoagulation medications, or had a triglyceride level > 300 mg/dL.
Results
The study included 3027 patients. Of those patients, 78 patients were female while 2949 were male, and the mean (SD) age was 68.3 (9.4) years. A subsample of 32 patients was analyzed to determine whether an ADR was noted or low LDL-C level was addressed in the chart. The subsample size was determined through chart review and included patients who had a documented intracranial hemorrhage. None of the 32 patients had an ADR documented, and 6 (19%) had the low LDL-C level addressed in the chart by monitoring levels, reducing statin doses, or discontinuing the medication. Of the total population analyzed, 8 patients (0.3%) had a documented intracranial hemorrhage within 1 year following the low LDL-C level.
We also analyzed the intensity of statin related to the low LDL-C level (Table 1).
The most common ADRs were muscle, joint, and leg pain, rash, and cramps (Table 2).
Adherence to the medications and duration of therapy was also analyzed and was found to be similar among the various medications. Lovastatin had the highest percent adherence with 91.2% while atorvastatin had the lowest with 85.5%. It can be noted that lovastatin had a lower documented percentage of ADRs while atorvastatin had a higher documented percentage of ADRs, which can be clinically meaningful when prescribing these medications; however, these similar adherence rates are not influencing the primary outcome of the rate of intracranial hemorrhage due to LDL-C level < 40 mg/dL. Mean duration of therapy lasted between 1 year and > 4 years with 1.1 years for alirocumab and 4.2 for simvastatin. The duration of therapy could be influenced by formulary restrictions during the study time. Nonetheless, patients, regardless of formulary restrictions, have taken these medications for a duration long enough to affect LDL-C levels.
Eight patients of the total sample analyzed had an intracranial hemorrhage within 1 year of having a recorded LDL-C level < 40 mg/dL. Secondarily, 32 patients had clinicians address an LDL-C level < 40 mg/dL through documentation or modifying the medication therapy. The most common ADRs among all medications analyzed were leg and joint pain, rash, and cramps. Of all medications included in this study, the mean duration of therapy was > 1 year, which would allow them to affect LDL-C levels and have those levels monitored and recorded in patients’ charts.
Discussion
When comparing our primary outcome of risk of intracranial hemorrhage with previous literature, the results are consistent with previous outcomes. Previous literature had a smaller sample size but analyzed LDL-C levels < 50 mg/dL and had an outcome of 48 patients experiencing an intracranial hemorrhage within 1 year of an LDL-C level < 50 mg/dL. Due to this study having stricter parameters of LDL-C levels < 40 mg/dL, there were fewer patients with documented intracranial hemorrhages. With there being a risk of intracranial hemorrhage with low LDL-C levels, the results demonstrate the need to monitor and address LDL-C levels.
Limitations
There were several notable limitations to this study. The retrospective, single-center nature coupled with the predominately male study population may affect the generalizability of the study results to patients outside of the facility in which the study was performed. Additionally, the study only included statin medications and PCSK9 inhibitors. With future studies, all lipid-lowering medications could be analyzed. The study was largely reliant on the proper documentation of International Statistical Classification of Diseases, Tenth Revision (ICD-10) codes exclusive to the HWW VAMC, which may exclude patients who first present to outside facilities. Due to time restraints, the incidence of hemorrhage was only analyzed 1 year following an LDL-C level < 40 mg/dL. For considerations for future investigation, the length of time to analyze incidence of hemorrhage could be expanded to be similar to previous studies, and the study could be expanded across the local Veterans Integrated Service Network or VA system. Additionally, the study could have analyzed the percentage of time a patient had an LDL-C level < 40 mg/dL in their lifetime.
Conclusions
These results show there is a risk that patients with an LDL-C level < 40 mg/dL may experience an intracranial hemorrhage. As seen by the results, there is a clinical need for practitioners to routinely monitor and address LDL-C levels. With various guidelines that recommend starting statin medication to reduce risk of ASCVD, it is necessary that practitioners routinely monitor cholesterol levels and adjust the medications according to laboratory results.11
Within 1 year of an LDL-C level < 40 mg/dL, 0.3% of patients had an intracranial hemorrhage. There was no statistical significance between the rate of ADRs among the medications analyzed. High-intensity statin medications were statistically significant in resulting in an LDL-C level < 40 mg/dL compared with moderate- and low-intensity statin medications. Of the 32 subsample of patients, LDL-C levels < 40 mg/mL are not routinely being addressed in the chart by the clinician.
1. Centers for Disease Control and Prevention. Stroke facts. Updated April 5, 2022. Accessed September 21, 2022. https://www.cdc.gov/stroke/facts.htm
2. Centers for Disease Control and Prevention. High cholesterol facts. Updated July 12, 2022. Accessed September 21, 2022. https://www.cdc.gov/cholesterol/facts.htm
3. Centers for Disease Control and Prevention. Heart disease mortality by state. Updated February 25, 2022. Accessed September 21, 2022. https://www.cdc.gov/nchs/pressroom/sosmap/heart_disease_mortality/heart_disease.htm
4. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082-e1143. doi:10.1161/CIR.0000000000000625
5. US Department of Veterans Affairs, US Department of Defense. VA/DoD Clinical Practice Guideline for the Management of Dyslipidemia for Cardiovascular Risk Reduction. Version 4.0. US Department of Veterans Affairs. June 2020. Accessed September 21, 2022. https://www.healthquality.va.gov/guidelines/CD/lipids/VADoDDyslipidemiaCPG5087212020.pdf
6. Tomaszewski M, Ste¸pien´ KM, Tomaszewska J, Czuczwar SJ. Statin-induced myopathies. Pharmacol Rep. 2011;63(4):859-66. doi:10.1016/s1734-1140(11)70601-6
7. Rist PM, Buring JE, Ridker PM, Kase CS, Kurth T, Rexrode KM. Lipid levels and the risk of hemorrhagic stroke among women. Neurology. 2019;92(19):e2286-e2294. doi:10.1212/WNL.0000000000007454
8. Ma C, Na M, Neumann S, Gao X. Low-density lipoprotein cholesterol and risk of hemorrhagic stroke: a systematic review and dose-response meta-analysis of prospective studies. Curr Atheroscler Rep. 2019;21(12):52. Published 2019 Nov 20. doi:10.1007/s11883-019-0815-5
9. Lui DT, Tan KC. Low-density lipoprotein cholesterol and stroke: How low should we go? J Diabetes Investig. 2020;11(6):1379-1381. doi:10.1111/jdi.13310
10. Ma C, Gurol ME, Huang Z, et al. Low-density lipoprotein cholesterol and risk of intracerebral hemorrhage: a prospective study. Neurology. 2019;93(5):e445-e457. doi:10.1212/WNL.0000000000007853
11. American Diabetes Association Professional Practice Committee. 10. Cardiovascular disease and risk management: standards of medical care in diabetes—2022. Diabetes Care. 2022;45(suppl 1):S144–S174. doi:10.2337/dc22-S010
1. Centers for Disease Control and Prevention. Stroke facts. Updated April 5, 2022. Accessed September 21, 2022. https://www.cdc.gov/stroke/facts.htm
2. Centers for Disease Control and Prevention. High cholesterol facts. Updated July 12, 2022. Accessed September 21, 2022. https://www.cdc.gov/cholesterol/facts.htm
3. Centers for Disease Control and Prevention. Heart disease mortality by state. Updated February 25, 2022. Accessed September 21, 2022. https://www.cdc.gov/nchs/pressroom/sosmap/heart_disease_mortality/heart_disease.htm
4. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082-e1143. doi:10.1161/CIR.0000000000000625
5. US Department of Veterans Affairs, US Department of Defense. VA/DoD Clinical Practice Guideline for the Management of Dyslipidemia for Cardiovascular Risk Reduction. Version 4.0. US Department of Veterans Affairs. June 2020. Accessed September 21, 2022. https://www.healthquality.va.gov/guidelines/CD/lipids/VADoDDyslipidemiaCPG5087212020.pdf
6. Tomaszewski M, Ste¸pien´ KM, Tomaszewska J, Czuczwar SJ. Statin-induced myopathies. Pharmacol Rep. 2011;63(4):859-66. doi:10.1016/s1734-1140(11)70601-6
7. Rist PM, Buring JE, Ridker PM, Kase CS, Kurth T, Rexrode KM. Lipid levels and the risk of hemorrhagic stroke among women. Neurology. 2019;92(19):e2286-e2294. doi:10.1212/WNL.0000000000007454
8. Ma C, Na M, Neumann S, Gao X. Low-density lipoprotein cholesterol and risk of hemorrhagic stroke: a systematic review and dose-response meta-analysis of prospective studies. Curr Atheroscler Rep. 2019;21(12):52. Published 2019 Nov 20. doi:10.1007/s11883-019-0815-5
9. Lui DT, Tan KC. Low-density lipoprotein cholesterol and stroke: How low should we go? J Diabetes Investig. 2020;11(6):1379-1381. doi:10.1111/jdi.13310
10. Ma C, Gurol ME, Huang Z, et al. Low-density lipoprotein cholesterol and risk of intracerebral hemorrhage: a prospective study. Neurology. 2019;93(5):e445-e457. doi:10.1212/WNL.0000000000007853
11. American Diabetes Association Professional Practice Committee. 10. Cardiovascular disease and risk management: standards of medical care in diabetes—2022. Diabetes Care. 2022;45(suppl 1):S144–S174. doi:10.2337/dc22-S010
Evaluation of a Pharmacist-Driven Ambulatory Aspirin Deprescribing Protocol
The use of low-dose aspirin for the primary prevention of cardiovascular disease (CVD) morbidity and mortality continues to be controversial, particularly for older adults. Recently published, robust randomized controlled trials have revealed less cardiovascular benefit from aspirin for primary prevention compared with previous trials; additionally, an increased risk of major bleeding events has been notably more prevalent in older adults.1-5 These trials have suggested that preventative aspirin use in older adults confers less benefit than other therapies for decreasing atherosclerotic CVD (ASCVD) risk, including blood pressure (BP) control, cholesterol management, and tobacco cessation.1,6
A recent meta-analysis indicated a composite cardiovascular risk reduction in patients aged 53 to 74 years taking aspirin vs no aspirin; however, this benefit was offset with an even greater increased risk of major bleeding.7 This trend was consistent regardless of stratification by 10-year ASCVD risk or presence of diabetes mellitus (DM) diagnosis.7,8 Additionally, the recently published Aspirin in Reducing Events in the Elderly (ASPREE) trial studied the impacts of aspirin use in healthy adults aged ≥ 70 years and aged ≥ 65 years among Black and Hispanic adults.4 The study concluded that the risk of major bleeding with aspirin use was even higher vs the potential cardiovascular benefit in older adults.4
With this emerging evidence, guidelines have been updated to represent the need for risk vs benefit considerations regarding aspirin use for primary prevention in older adults.1,9,10 The most recent guideline update from the American College of Cardiology and American Heart Association (ACC/AHA) recommends against the routine use of aspirin in patients aged > 70 years or those with bleeding risk factors.1 The guideline recommends considering aspirin use for patients ages 40 to 70 years only after a patient-specific risk vs benefit discussion.1 Furthermore, the 2020 American Diabetes Association guideline recommends considering aspirin use for primary prevention in adults with DM between ages 50 and 70 only after a risk vs benefit discussion of patient-specific bleeding risk factors and ASCVD risk-enhancing factors.10
Despite the demonstrated risks for bleeding with the routine use of aspirin, studies indicate that aspirin continues to be used commonly among older adults, often when unnecessary. In the 2017 National Health Interview Survey, about 23% of adults aged > 40 years in the United States without CVD used aspirin daily, and 23% of these did so without recommendation from a health care professional.11 Furthermore, nearly half of adults ages ≥ 70 years and nearly one-quarter of adults with a history of peptic ulcer disease used aspirin daily.11 Although the most recent guidelines from the ACC/AHA do not recommend a 10-year ASCVD risk threshold for therapy, one study illustrated that 12% of older adult patients were inappropriately prescribed aspirin for primary prevention despite a 10-year ASCVD risk of < 6%.1,12 These studies highlight the large proportion of individuals, particularly older adults, who may be inappropriately taking aspirin for primary prevention.
Deprescribing Program
Deprescribing potentially inappropriate medications (PIMs) is particularly important in the older adult population, as these individuals experience a high risk of adverse effects (AEs), polypharmacy, cognitive decline, and falls related to medication use.6,13-17 Evidence suggests that mortality outcomes are improved with the implementation of targeted deprescribing efforts based on patient-specific factors.18 Additionally, deprescribing unnecessary medications may improve adherence to other essential medications and reduce financial burdens.19 Pharmacists play a crucial role among health care professionals in the implementation of deprescribing practices, and studies have shown that physicians are highly accepting of pharmacists’ deprescribing recommendations.13,20-22
Despite the evidence for the benefits of deprescribing, limited data are available regarding the impact and feasibility of a targeted aspirin deprescribing approach by nonphysician practitioners.23 The objective of this study was to implement and evaluate the success of a pharmacist-driven aspirin deprescribing protocol for older adults in a primary care setting.
This aspirin deprescribing protocol was developed by ambulatory care clinical pharmacist or clinical pharmacist practitioners (CPPs), at the William S. Middleton Memorial Veterans Hospital in Madison, Wisconsin. Within the US Department of Veterans Affairs (VA) health care system, CPPs work under a broad scope of practice with the ability to independently prescribe and monitor medications. The protocol was reviewed by physician stakeholders in both primary care and cardiology and a list was generated, including patients from 2 primary care panels aged ≥ 70 years with aspirin on their medication list, either as a prescription or over-the-counter medication, using the VA Information System Technology and Architecture. A CPP or supervised pharmacy intern identified patients from this list who were appropriate for risk/benefit discussions regarding the discontinuation of aspirin. Patients were excluded from the intervention if they had a history of clinical ASCVD, including myocardial infarction (MI), stable or unstable angina, coronary artery disease (CAD), coronary or other arterial revascularization, cerebrovascular accident (CVA), transient ischemic accident (TIA), or peripheral artery disease (PAD), or another documented indication for aspirin use, including pain, flushing (with niacin use), venous thromboembolism prophylaxis, valvular heart disease, or acute or recurrent pericarditis.
After identifying eligible patients, a CPP or pharmacy intern contacted patients by telephone, following a script to guide conversation. All patients were screened for potential appropriate aspirin indications, particularly any history of MI, CAD, CVA, TIA, PAD, or other clinical ASCVD. The patient was asked about their rationale for taking aspirin and patient-specific ASCVD risk-enhancing factors and bleeding risk factors and educated them on lifestyle modalities to reduce ASCVD risk, using the script as a guide. ASCVD risk-enhancing factors included family history of premature MI, inability to achieve BP goal, DM with the inability to achieve blood glucose or hemoglobin A1c goal, tobacco use, or inadequate statin therapy. Bleeding risk factors included a history of gastrointestinal bleed or peptic ulcer disease, concurrent use of medications that increase bleeding risk, chronic kidney disease, or thrombocytopenia.
Through shared decision making with careful consideration of these factors, we reached a conclusion with each patient to either continue or to deprescribe aspirin. Each discussion was documented in the electronic health record (EHR) using a standard documentation template (eAppendix, available at doi:10.12788/fp.0320). The patient’s medication list also was updated to reflect changes in aspirin use. For patients who declined deprescribing, the CPP or pharmacy intern asked the patient for their primary reason for preferring to continue aspirin, which was subsequently categorized as one of the following: no prior concerns with bleeding, concerns about a future cardiovascular event, wishing to discuss further with their primary care practitioner (PCP), or identifying an appropriate use for aspirin not evident through record review. For the patients who wished to further discuss the issue with their PCP before deprescribing, the patient’s PCP was notified of this preference by a record alert to the note documenting the encounter, and the patient was also encouraged to follow up about this issue. A voicemail was left if the patient did not answer requesting a call back, and a second attempt was made within 2 weeks.
Data Collected
We collected data to assess the proportion of patients for whom aspirin for primary prevention was discontinued. For patients who declined deprescribing, we documented the rationale for continuing aspirin. Additionally, the feasibility of implementation was assessed, including pharmacist time spent on each record review and intervention. Descriptive statistics were generated to evaluate baseline characteristics and intervention outcomes. The time to completion of these tasks was summarized with descriptive statistics.
We reviewed 459 patient records, and 110 were determined eligible for risk/benefit discussions.
Patients had various reasons for declining deprescribing, including 8 (28%) who had no prior concerns with bleeding while on aspirin and 6 (21%) who were concerned about a future cardiovascular event. Of those who declined aspirin deprescribing, 6 (21%) wished to further discuss the issue with their PCP. In 9 (31%) patients an alternative appropriate indication for aspirin was identified through discussion. In these cases, the indication for aspirin was documented and updated in the EHR.
Most patients (87%) contacted reported taking low-dose aspirin 81 mg daily, while 10% reported taking higher doses (range, 162-325) and 3% on an as-needed basis. In all 3 patients who agreed to dose reduction, the initial dose of 325 mg daily was reduced to 81 mg daily.
Results of the time-study analysis for each intervention indicated that a pharmacy intern or pharmacist spent about 2 minutes reviewing the record of each patient to determine eligibility for risk/benefit discussions. The 110 patients identified as eligible were 24% of the 459 records reviewed. An average (range) of 12 (6-20) minutes was spent on the telephone call plus documentation for each patient contacted. Additionally, we estimated that CPPs and pharmacy interns spent an approximate combined 12 hours in the development and review of materials for this program, including the protocol, script, and documentation templates. This also included about 1 hour to identify appropriate parameters for, and generate, the eligible patient list.
Discussion
The implementation of a pharmacist-driven aspirin deprescribing protocol for older adults in a primary care setting led to the discontinuation of inappropriate aspirin use in nearly half of older adults contacted. Furthermore, opportunities were identified to update medication lists to reflect previously self-discontinued aspirin for older adults. Just over one-quarter of those contacted declined to discontinue or reduce their aspirin dose. It is hypothesized that with these targeted deprescribing interventions, overall risk reduction for bleeding and polypharmacy will be observed for older adults.1
In addition to deprescribing aspirin, CPPs used shared decision making to initiate risk/benefit discussions and to educate on targeted lifestyle modifications to lower ASCVD risk. While not all patients agreed to discontinue aspirin, all were provided education that may empower them to engage in future discussions with PCPs regarding appropriate aspirin use. Previous pharmacist-led deprescribing initiatives for proton pump inhibitors and other PIMs have indicated that a large percentage of patients who opt to further discuss a deprescribing concern with their PCPs ultimately resulted in deprescribing outcomes.24,25 Additionally, a recent trial examining pharmacist-led deprescribing of 4 common PIMs in older adults compared the impact of pharmacists leading educational interventions directly to patients with pharmacists making deprescribing recommendations to physicians. Deprescribing was more successful when patients were involved in the decision-making process.26
Limitations
Although this quality improvement initiative resulted in the deprescribing of inappropriate aspirin for many older adults, a limitation is the small sample size within a single institution. The population of male veterans also may limit generalizability to nonmale and nonveteran older adults. As the protocol was initiated within a limited number of primary care teams initially, future implementation into additional primary care teams will increase the number of older adults impacted by risk/benefit discussions regarding aspirin use. This work may not be generalizable to other health care systems. Many patients within the VA receive both their primary and specialty care within the system, which facilitates communication and collaboration between primary and specialty practitioners. The protocol may require workflow adjustments for patients receiving care within multiple systems. Additionally, although the deprescribing protocol was created in collaboration with physicians, CPPs within the VA work under a broad scope of practice that includes independent medication prescribing, deprescribing, and monitoring. This may be a consideration when implementing similar protocols at other sites, as collaborative practice agreements may need to be in place.
Future Directions
The time required to complete these interventions was generally feasible, though this intervention would require some workflow alteration to be incorporated routinely into a CPP’s schedule. The telephone calls were completed as isolated interventions and were not incorporated into existing scheduled primary care appointments. In the future, the aspirin deprescribing protocol could be incorporated into existing pharmacist-led primary care appointments. Based on the outcomes of this study, CPPs are leading an initiative to develop an aspirin deprescribing clinical reminder tool, which may be quickly inserted into a progress note within the EHR and may be incorporated into any primary care visit led by a CPP or PCP.
Conclusions
This study demonstrates that a pharmacist-led aspirin deprescribing protocol in the ambulatory care pharmacy setting was successful in the discontinuation of unnecessary aspirin use in older adults. The protocol also provided opportunities for education on ASCVD risk reduction in all older adults reached. These findings highlight the role of pharmacists in deprescribing PIMs for older adults and identifying opportunities to further streamline risk/benefit discussions on aspirin deprescribing potential within primary care visits.
1. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2019;140(11):e596-e646. doi:10.1161/CIR.0000000000000678
2. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomized, double-blind, placebo-controlled trial. Lancet. 2018;392(10152):1036-1046. doi:10.1016/S0140-6736(18)31924-X
3. Bowman L, Mafham M, et al; ASCEND Study Collaborative Group. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379(16):1529-1539. doi:10.1056/NEJMoa1804988
4. McNeil JJ, Wolfe R, Woods, RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379(16):1509-1518. doi:10.1056/NEJMoa1805819
5. García Rodríguez LA, Martín-Pérez M, Hennekens CH, Rothwell PM, Lanas A. Bleeding risk with long-term low-dose aspirin: a systematic review of observational studies. PloS One. 2016;11(8):e0160046. doi:10.1371/journal.pone.0160046
6. Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment): consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72-83. doi:10.5414/cpp46072
7. Zheng SL, Roddick AJ. Association of aspirin use for primary prevention with cardiovascular events and bleeding events: a systematic review and meta-analysis. JAMA. 2019;321(3):277-287. doi:10.1001/jama.2018.20578
8. Patrono C, Baigent C. Role of aspirin in primary prevention of cardiovascular disease. Nat Rev Cardiol. 2019;16(11):675-686. doi:10.1038/s41569-019-0225-y
9. Bibbins-Domingo K; U.S. Preventative Services Task Force. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164(12):836-845. doi:10.7326/M16-0577
10. American Diabetes Association. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(suppl 1):S14-S31. doi:10.2337/dc20-S002
11. O’Brien CW, Juraschek SP, Wee CC. Prevalence of aspirin use for primary prevention of cardiovascular disease in the United States: results from the 2017 National Health Interview Survey. Ann Intern Med. 2019;171(8):596-598. doi:10.7326/M19-0953
12. Hira RS, Kennedy K, Nambi V, et al. Frequency and practice-level variation in inappropriate aspirin use for the primary prevention of cardiovascular disease: insights from the National Cardiovascular Disease Registry’s Practice Innovation and Clinical Excellence registry. J Am Coll Cardiol. 2015;65(2):111-121. doi:10.1016/j.jacc.2014.10.035
13. Cheong ST, Ng TM, Tan KT. Pharmacist-initiated deprescribing in hospitalized elderly: prevalence and acceptance by physicians. Eur J Hosp Pharm. 2018;25(e1):e35-e39. doi:10.1136/ejhpharm-2017-001251
14. Dyck MJ. Evidence-based administrative guideline: quality improvement in nursing homes. J Gerontol Nurs. 2005;31(2):4-10. doi:10.3928/0098-9134-20050201-04
15. Zullo AR, Gray SL, Holmes HM, Marcum ZA. Screening for medication appropriateness in older adults. Clin Geriatr Med. 2018;34(1):39-54. doi:10.1016/j.cger.2017.09.003
16. American Geriatrics Society. 2019 updated AGS Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694. doi:10.1111/jgs.15767
17. Shah BM, Hajjar ER. Polypharmacy, adverse drug reactions, and geriatric syndromes. Clin Geriatr Med. 2012;28(2):173-186. doi:10.1016/j.cger.2012.01.002
18. Page AT, Clifford RM, Potter K, Schwartz D, Etherton-Beer CD. The feasibility and effect of deprescribing in older adults on mortality and health: a systematic review and meta-analysis. Br J Clin Pharmacol. 2016;82(3):583-623. doi:10.1111/bcp.12975
19. Reeve E, Shakib S, Hendrix I, Roberts MS, Wiese MD. The benefits and harms of deprescribing. Med J Aust. 2014;201(7):386-389. doi:10.5694/mja13.00200
20. Ailabouni NJ, Marcum ZA, Schmader KE, Gray SL. Medication use quality and safety in older adults: 2018 update. J Am Geriatr Soc. 2019;67(12):2458-2462. doi:10.1111/jgs.16243
21. Frank C, Weir E. Deprescribing for older patients. CMAJ. 2014;186(18):1369-1376. doi:10.1503/cmaj.131873
22. Clark CM, LaValley SA, Singh R, Mustafa E, Monte SV, Wahler RG Jr. A pharmacist-led program to facilitate deprescribing in a primary care clinic. J Am Pharm Assoc (2003). 2020;60(1):105-111. doi:10.1016/j.japh.2019.09.011
23. Folks B, Leblanc WG, Staton EW, Pace WD. Reconsidering low-dose aspirin therapy for cardiovascular disease: a study protocol for physician and patient behavioral change. Implement Sci. 2011;6:65. Published 2011 Jun 26. doi:10.1186/1748-5908-6-65
24. Odenthal DR, Philbrick AM, Harris IM. Successful deprescribing of unnecessary proton pump inhibitors in a primary care clinic. J Am Pharm Assoc. 2020;60(1):100-104. doi:10.1016/j.japh.2019.08.012
25. Duncan, P. Duerden M, Payne RA. Deprescribing: a primary care perspective. Eur J Hosp Pharm. 2017;24(1):37-42. doi:10.1136/ejhpharm-2016-000967
26. Martin P, Tamblyn R, Benedetti A, Ahmed S, Tannenbaum C. Effect of a pharmacist-led educational intervention on inappropriate medication prescriptions in older adults: the D-PRESCRIBE randomized clinical trial. JAMA. 2018;320(18):1889-1898. doi:10.1001/jama.2018.16131
The use of low-dose aspirin for the primary prevention of cardiovascular disease (CVD) morbidity and mortality continues to be controversial, particularly for older adults. Recently published, robust randomized controlled trials have revealed less cardiovascular benefit from aspirin for primary prevention compared with previous trials; additionally, an increased risk of major bleeding events has been notably more prevalent in older adults.1-5 These trials have suggested that preventative aspirin use in older adults confers less benefit than other therapies for decreasing atherosclerotic CVD (ASCVD) risk, including blood pressure (BP) control, cholesterol management, and tobacco cessation.1,6
A recent meta-analysis indicated a composite cardiovascular risk reduction in patients aged 53 to 74 years taking aspirin vs no aspirin; however, this benefit was offset with an even greater increased risk of major bleeding.7 This trend was consistent regardless of stratification by 10-year ASCVD risk or presence of diabetes mellitus (DM) diagnosis.7,8 Additionally, the recently published Aspirin in Reducing Events in the Elderly (ASPREE) trial studied the impacts of aspirin use in healthy adults aged ≥ 70 years and aged ≥ 65 years among Black and Hispanic adults.4 The study concluded that the risk of major bleeding with aspirin use was even higher vs the potential cardiovascular benefit in older adults.4
With this emerging evidence, guidelines have been updated to represent the need for risk vs benefit considerations regarding aspirin use for primary prevention in older adults.1,9,10 The most recent guideline update from the American College of Cardiology and American Heart Association (ACC/AHA) recommends against the routine use of aspirin in patients aged > 70 years or those with bleeding risk factors.1 The guideline recommends considering aspirin use for patients ages 40 to 70 years only after a patient-specific risk vs benefit discussion.1 Furthermore, the 2020 American Diabetes Association guideline recommends considering aspirin use for primary prevention in adults with DM between ages 50 and 70 only after a risk vs benefit discussion of patient-specific bleeding risk factors and ASCVD risk-enhancing factors.10
Despite the demonstrated risks for bleeding with the routine use of aspirin, studies indicate that aspirin continues to be used commonly among older adults, often when unnecessary. In the 2017 National Health Interview Survey, about 23% of adults aged > 40 years in the United States without CVD used aspirin daily, and 23% of these did so without recommendation from a health care professional.11 Furthermore, nearly half of adults ages ≥ 70 years and nearly one-quarter of adults with a history of peptic ulcer disease used aspirin daily.11 Although the most recent guidelines from the ACC/AHA do not recommend a 10-year ASCVD risk threshold for therapy, one study illustrated that 12% of older adult patients were inappropriately prescribed aspirin for primary prevention despite a 10-year ASCVD risk of < 6%.1,12 These studies highlight the large proportion of individuals, particularly older adults, who may be inappropriately taking aspirin for primary prevention.
Deprescribing Program
Deprescribing potentially inappropriate medications (PIMs) is particularly important in the older adult population, as these individuals experience a high risk of adverse effects (AEs), polypharmacy, cognitive decline, and falls related to medication use.6,13-17 Evidence suggests that mortality outcomes are improved with the implementation of targeted deprescribing efforts based on patient-specific factors.18 Additionally, deprescribing unnecessary medications may improve adherence to other essential medications and reduce financial burdens.19 Pharmacists play a crucial role among health care professionals in the implementation of deprescribing practices, and studies have shown that physicians are highly accepting of pharmacists’ deprescribing recommendations.13,20-22
Despite the evidence for the benefits of deprescribing, limited data are available regarding the impact and feasibility of a targeted aspirin deprescribing approach by nonphysician practitioners.23 The objective of this study was to implement and evaluate the success of a pharmacist-driven aspirin deprescribing protocol for older adults in a primary care setting.
This aspirin deprescribing protocol was developed by ambulatory care clinical pharmacist or clinical pharmacist practitioners (CPPs), at the William S. Middleton Memorial Veterans Hospital in Madison, Wisconsin. Within the US Department of Veterans Affairs (VA) health care system, CPPs work under a broad scope of practice with the ability to independently prescribe and monitor medications. The protocol was reviewed by physician stakeholders in both primary care and cardiology and a list was generated, including patients from 2 primary care panels aged ≥ 70 years with aspirin on their medication list, either as a prescription or over-the-counter medication, using the VA Information System Technology and Architecture. A CPP or supervised pharmacy intern identified patients from this list who were appropriate for risk/benefit discussions regarding the discontinuation of aspirin. Patients were excluded from the intervention if they had a history of clinical ASCVD, including myocardial infarction (MI), stable or unstable angina, coronary artery disease (CAD), coronary or other arterial revascularization, cerebrovascular accident (CVA), transient ischemic accident (TIA), or peripheral artery disease (PAD), or another documented indication for aspirin use, including pain, flushing (with niacin use), venous thromboembolism prophylaxis, valvular heart disease, or acute or recurrent pericarditis.
After identifying eligible patients, a CPP or pharmacy intern contacted patients by telephone, following a script to guide conversation. All patients were screened for potential appropriate aspirin indications, particularly any history of MI, CAD, CVA, TIA, PAD, or other clinical ASCVD. The patient was asked about their rationale for taking aspirin and patient-specific ASCVD risk-enhancing factors and bleeding risk factors and educated them on lifestyle modalities to reduce ASCVD risk, using the script as a guide. ASCVD risk-enhancing factors included family history of premature MI, inability to achieve BP goal, DM with the inability to achieve blood glucose or hemoglobin A1c goal, tobacco use, or inadequate statin therapy. Bleeding risk factors included a history of gastrointestinal bleed or peptic ulcer disease, concurrent use of medications that increase bleeding risk, chronic kidney disease, or thrombocytopenia.
Through shared decision making with careful consideration of these factors, we reached a conclusion with each patient to either continue or to deprescribe aspirin. Each discussion was documented in the electronic health record (EHR) using a standard documentation template (eAppendix, available at doi:10.12788/fp.0320). The patient’s medication list also was updated to reflect changes in aspirin use. For patients who declined deprescribing, the CPP or pharmacy intern asked the patient for their primary reason for preferring to continue aspirin, which was subsequently categorized as one of the following: no prior concerns with bleeding, concerns about a future cardiovascular event, wishing to discuss further with their primary care practitioner (PCP), or identifying an appropriate use for aspirin not evident through record review. For the patients who wished to further discuss the issue with their PCP before deprescribing, the patient’s PCP was notified of this preference by a record alert to the note documenting the encounter, and the patient was also encouraged to follow up about this issue. A voicemail was left if the patient did not answer requesting a call back, and a second attempt was made within 2 weeks.
Data Collected
We collected data to assess the proportion of patients for whom aspirin for primary prevention was discontinued. For patients who declined deprescribing, we documented the rationale for continuing aspirin. Additionally, the feasibility of implementation was assessed, including pharmacist time spent on each record review and intervention. Descriptive statistics were generated to evaluate baseline characteristics and intervention outcomes. The time to completion of these tasks was summarized with descriptive statistics.
We reviewed 459 patient records, and 110 were determined eligible for risk/benefit discussions.
Patients had various reasons for declining deprescribing, including 8 (28%) who had no prior concerns with bleeding while on aspirin and 6 (21%) who were concerned about a future cardiovascular event. Of those who declined aspirin deprescribing, 6 (21%) wished to further discuss the issue with their PCP. In 9 (31%) patients an alternative appropriate indication for aspirin was identified through discussion. In these cases, the indication for aspirin was documented and updated in the EHR.
Most patients (87%) contacted reported taking low-dose aspirin 81 mg daily, while 10% reported taking higher doses (range, 162-325) and 3% on an as-needed basis. In all 3 patients who agreed to dose reduction, the initial dose of 325 mg daily was reduced to 81 mg daily.
Results of the time-study analysis for each intervention indicated that a pharmacy intern or pharmacist spent about 2 minutes reviewing the record of each patient to determine eligibility for risk/benefit discussions. The 110 patients identified as eligible were 24% of the 459 records reviewed. An average (range) of 12 (6-20) minutes was spent on the telephone call plus documentation for each patient contacted. Additionally, we estimated that CPPs and pharmacy interns spent an approximate combined 12 hours in the development and review of materials for this program, including the protocol, script, and documentation templates. This also included about 1 hour to identify appropriate parameters for, and generate, the eligible patient list.
Discussion
The implementation of a pharmacist-driven aspirin deprescribing protocol for older adults in a primary care setting led to the discontinuation of inappropriate aspirin use in nearly half of older adults contacted. Furthermore, opportunities were identified to update medication lists to reflect previously self-discontinued aspirin for older adults. Just over one-quarter of those contacted declined to discontinue or reduce their aspirin dose. It is hypothesized that with these targeted deprescribing interventions, overall risk reduction for bleeding and polypharmacy will be observed for older adults.1
In addition to deprescribing aspirin, CPPs used shared decision making to initiate risk/benefit discussions and to educate on targeted lifestyle modifications to lower ASCVD risk. While not all patients agreed to discontinue aspirin, all were provided education that may empower them to engage in future discussions with PCPs regarding appropriate aspirin use. Previous pharmacist-led deprescribing initiatives for proton pump inhibitors and other PIMs have indicated that a large percentage of patients who opt to further discuss a deprescribing concern with their PCPs ultimately resulted in deprescribing outcomes.24,25 Additionally, a recent trial examining pharmacist-led deprescribing of 4 common PIMs in older adults compared the impact of pharmacists leading educational interventions directly to patients with pharmacists making deprescribing recommendations to physicians. Deprescribing was more successful when patients were involved in the decision-making process.26
Limitations
Although this quality improvement initiative resulted in the deprescribing of inappropriate aspirin for many older adults, a limitation is the small sample size within a single institution. The population of male veterans also may limit generalizability to nonmale and nonveteran older adults. As the protocol was initiated within a limited number of primary care teams initially, future implementation into additional primary care teams will increase the number of older adults impacted by risk/benefit discussions regarding aspirin use. This work may not be generalizable to other health care systems. Many patients within the VA receive both their primary and specialty care within the system, which facilitates communication and collaboration between primary and specialty practitioners. The protocol may require workflow adjustments for patients receiving care within multiple systems. Additionally, although the deprescribing protocol was created in collaboration with physicians, CPPs within the VA work under a broad scope of practice that includes independent medication prescribing, deprescribing, and monitoring. This may be a consideration when implementing similar protocols at other sites, as collaborative practice agreements may need to be in place.
Future Directions
The time required to complete these interventions was generally feasible, though this intervention would require some workflow alteration to be incorporated routinely into a CPP’s schedule. The telephone calls were completed as isolated interventions and were not incorporated into existing scheduled primary care appointments. In the future, the aspirin deprescribing protocol could be incorporated into existing pharmacist-led primary care appointments. Based on the outcomes of this study, CPPs are leading an initiative to develop an aspirin deprescribing clinical reminder tool, which may be quickly inserted into a progress note within the EHR and may be incorporated into any primary care visit led by a CPP or PCP.
Conclusions
This study demonstrates that a pharmacist-led aspirin deprescribing protocol in the ambulatory care pharmacy setting was successful in the discontinuation of unnecessary aspirin use in older adults. The protocol also provided opportunities for education on ASCVD risk reduction in all older adults reached. These findings highlight the role of pharmacists in deprescribing PIMs for older adults and identifying opportunities to further streamline risk/benefit discussions on aspirin deprescribing potential within primary care visits.
The use of low-dose aspirin for the primary prevention of cardiovascular disease (CVD) morbidity and mortality continues to be controversial, particularly for older adults. Recently published, robust randomized controlled trials have revealed less cardiovascular benefit from aspirin for primary prevention compared with previous trials; additionally, an increased risk of major bleeding events has been notably more prevalent in older adults.1-5 These trials have suggested that preventative aspirin use in older adults confers less benefit than other therapies for decreasing atherosclerotic CVD (ASCVD) risk, including blood pressure (BP) control, cholesterol management, and tobacco cessation.1,6
A recent meta-analysis indicated a composite cardiovascular risk reduction in patients aged 53 to 74 years taking aspirin vs no aspirin; however, this benefit was offset with an even greater increased risk of major bleeding.7 This trend was consistent regardless of stratification by 10-year ASCVD risk or presence of diabetes mellitus (DM) diagnosis.7,8 Additionally, the recently published Aspirin in Reducing Events in the Elderly (ASPREE) trial studied the impacts of aspirin use in healthy adults aged ≥ 70 years and aged ≥ 65 years among Black and Hispanic adults.4 The study concluded that the risk of major bleeding with aspirin use was even higher vs the potential cardiovascular benefit in older adults.4
With this emerging evidence, guidelines have been updated to represent the need for risk vs benefit considerations regarding aspirin use for primary prevention in older adults.1,9,10 The most recent guideline update from the American College of Cardiology and American Heart Association (ACC/AHA) recommends against the routine use of aspirin in patients aged > 70 years or those with bleeding risk factors.1 The guideline recommends considering aspirin use for patients ages 40 to 70 years only after a patient-specific risk vs benefit discussion.1 Furthermore, the 2020 American Diabetes Association guideline recommends considering aspirin use for primary prevention in adults with DM between ages 50 and 70 only after a risk vs benefit discussion of patient-specific bleeding risk factors and ASCVD risk-enhancing factors.10
Despite the demonstrated risks for bleeding with the routine use of aspirin, studies indicate that aspirin continues to be used commonly among older adults, often when unnecessary. In the 2017 National Health Interview Survey, about 23% of adults aged > 40 years in the United States without CVD used aspirin daily, and 23% of these did so without recommendation from a health care professional.11 Furthermore, nearly half of adults ages ≥ 70 years and nearly one-quarter of adults with a history of peptic ulcer disease used aspirin daily.11 Although the most recent guidelines from the ACC/AHA do not recommend a 10-year ASCVD risk threshold for therapy, one study illustrated that 12% of older adult patients were inappropriately prescribed aspirin for primary prevention despite a 10-year ASCVD risk of < 6%.1,12 These studies highlight the large proportion of individuals, particularly older adults, who may be inappropriately taking aspirin for primary prevention.
Deprescribing Program
Deprescribing potentially inappropriate medications (PIMs) is particularly important in the older adult population, as these individuals experience a high risk of adverse effects (AEs), polypharmacy, cognitive decline, and falls related to medication use.6,13-17 Evidence suggests that mortality outcomes are improved with the implementation of targeted deprescribing efforts based on patient-specific factors.18 Additionally, deprescribing unnecessary medications may improve adherence to other essential medications and reduce financial burdens.19 Pharmacists play a crucial role among health care professionals in the implementation of deprescribing practices, and studies have shown that physicians are highly accepting of pharmacists’ deprescribing recommendations.13,20-22
Despite the evidence for the benefits of deprescribing, limited data are available regarding the impact and feasibility of a targeted aspirin deprescribing approach by nonphysician practitioners.23 The objective of this study was to implement and evaluate the success of a pharmacist-driven aspirin deprescribing protocol for older adults in a primary care setting.
This aspirin deprescribing protocol was developed by ambulatory care clinical pharmacist or clinical pharmacist practitioners (CPPs), at the William S. Middleton Memorial Veterans Hospital in Madison, Wisconsin. Within the US Department of Veterans Affairs (VA) health care system, CPPs work under a broad scope of practice with the ability to independently prescribe and monitor medications. The protocol was reviewed by physician stakeholders in both primary care and cardiology and a list was generated, including patients from 2 primary care panels aged ≥ 70 years with aspirin on their medication list, either as a prescription or over-the-counter medication, using the VA Information System Technology and Architecture. A CPP or supervised pharmacy intern identified patients from this list who were appropriate for risk/benefit discussions regarding the discontinuation of aspirin. Patients were excluded from the intervention if they had a history of clinical ASCVD, including myocardial infarction (MI), stable or unstable angina, coronary artery disease (CAD), coronary or other arterial revascularization, cerebrovascular accident (CVA), transient ischemic accident (TIA), or peripheral artery disease (PAD), or another documented indication for aspirin use, including pain, flushing (with niacin use), venous thromboembolism prophylaxis, valvular heart disease, or acute or recurrent pericarditis.
After identifying eligible patients, a CPP or pharmacy intern contacted patients by telephone, following a script to guide conversation. All patients were screened for potential appropriate aspirin indications, particularly any history of MI, CAD, CVA, TIA, PAD, or other clinical ASCVD. The patient was asked about their rationale for taking aspirin and patient-specific ASCVD risk-enhancing factors and bleeding risk factors and educated them on lifestyle modalities to reduce ASCVD risk, using the script as a guide. ASCVD risk-enhancing factors included family history of premature MI, inability to achieve BP goal, DM with the inability to achieve blood glucose or hemoglobin A1c goal, tobacco use, or inadequate statin therapy. Bleeding risk factors included a history of gastrointestinal bleed or peptic ulcer disease, concurrent use of medications that increase bleeding risk, chronic kidney disease, or thrombocytopenia.
Through shared decision making with careful consideration of these factors, we reached a conclusion with each patient to either continue or to deprescribe aspirin. Each discussion was documented in the electronic health record (EHR) using a standard documentation template (eAppendix, available at doi:10.12788/fp.0320). The patient’s medication list also was updated to reflect changes in aspirin use. For patients who declined deprescribing, the CPP or pharmacy intern asked the patient for their primary reason for preferring to continue aspirin, which was subsequently categorized as one of the following: no prior concerns with bleeding, concerns about a future cardiovascular event, wishing to discuss further with their primary care practitioner (PCP), or identifying an appropriate use for aspirin not evident through record review. For the patients who wished to further discuss the issue with their PCP before deprescribing, the patient’s PCP was notified of this preference by a record alert to the note documenting the encounter, and the patient was also encouraged to follow up about this issue. A voicemail was left if the patient did not answer requesting a call back, and a second attempt was made within 2 weeks.
Data Collected
We collected data to assess the proportion of patients for whom aspirin for primary prevention was discontinued. For patients who declined deprescribing, we documented the rationale for continuing aspirin. Additionally, the feasibility of implementation was assessed, including pharmacist time spent on each record review and intervention. Descriptive statistics were generated to evaluate baseline characteristics and intervention outcomes. The time to completion of these tasks was summarized with descriptive statistics.
We reviewed 459 patient records, and 110 were determined eligible for risk/benefit discussions.
Patients had various reasons for declining deprescribing, including 8 (28%) who had no prior concerns with bleeding while on aspirin and 6 (21%) who were concerned about a future cardiovascular event. Of those who declined aspirin deprescribing, 6 (21%) wished to further discuss the issue with their PCP. In 9 (31%) patients an alternative appropriate indication for aspirin was identified through discussion. In these cases, the indication for aspirin was documented and updated in the EHR.
Most patients (87%) contacted reported taking low-dose aspirin 81 mg daily, while 10% reported taking higher doses (range, 162-325) and 3% on an as-needed basis. In all 3 patients who agreed to dose reduction, the initial dose of 325 mg daily was reduced to 81 mg daily.
Results of the time-study analysis for each intervention indicated that a pharmacy intern or pharmacist spent about 2 minutes reviewing the record of each patient to determine eligibility for risk/benefit discussions. The 110 patients identified as eligible were 24% of the 459 records reviewed. An average (range) of 12 (6-20) minutes was spent on the telephone call plus documentation for each patient contacted. Additionally, we estimated that CPPs and pharmacy interns spent an approximate combined 12 hours in the development and review of materials for this program, including the protocol, script, and documentation templates. This also included about 1 hour to identify appropriate parameters for, and generate, the eligible patient list.
Discussion
The implementation of a pharmacist-driven aspirin deprescribing protocol for older adults in a primary care setting led to the discontinuation of inappropriate aspirin use in nearly half of older adults contacted. Furthermore, opportunities were identified to update medication lists to reflect previously self-discontinued aspirin for older adults. Just over one-quarter of those contacted declined to discontinue or reduce their aspirin dose. It is hypothesized that with these targeted deprescribing interventions, overall risk reduction for bleeding and polypharmacy will be observed for older adults.1
In addition to deprescribing aspirin, CPPs used shared decision making to initiate risk/benefit discussions and to educate on targeted lifestyle modifications to lower ASCVD risk. While not all patients agreed to discontinue aspirin, all were provided education that may empower them to engage in future discussions with PCPs regarding appropriate aspirin use. Previous pharmacist-led deprescribing initiatives for proton pump inhibitors and other PIMs have indicated that a large percentage of patients who opt to further discuss a deprescribing concern with their PCPs ultimately resulted in deprescribing outcomes.24,25 Additionally, a recent trial examining pharmacist-led deprescribing of 4 common PIMs in older adults compared the impact of pharmacists leading educational interventions directly to patients with pharmacists making deprescribing recommendations to physicians. Deprescribing was more successful when patients were involved in the decision-making process.26
Limitations
Although this quality improvement initiative resulted in the deprescribing of inappropriate aspirin for many older adults, a limitation is the small sample size within a single institution. The population of male veterans also may limit generalizability to nonmale and nonveteran older adults. As the protocol was initiated within a limited number of primary care teams initially, future implementation into additional primary care teams will increase the number of older adults impacted by risk/benefit discussions regarding aspirin use. This work may not be generalizable to other health care systems. Many patients within the VA receive both their primary and specialty care within the system, which facilitates communication and collaboration between primary and specialty practitioners. The protocol may require workflow adjustments for patients receiving care within multiple systems. Additionally, although the deprescribing protocol was created in collaboration with physicians, CPPs within the VA work under a broad scope of practice that includes independent medication prescribing, deprescribing, and monitoring. This may be a consideration when implementing similar protocols at other sites, as collaborative practice agreements may need to be in place.
Future Directions
The time required to complete these interventions was generally feasible, though this intervention would require some workflow alteration to be incorporated routinely into a CPP’s schedule. The telephone calls were completed as isolated interventions and were not incorporated into existing scheduled primary care appointments. In the future, the aspirin deprescribing protocol could be incorporated into existing pharmacist-led primary care appointments. Based on the outcomes of this study, CPPs are leading an initiative to develop an aspirin deprescribing clinical reminder tool, which may be quickly inserted into a progress note within the EHR and may be incorporated into any primary care visit led by a CPP or PCP.
Conclusions
This study demonstrates that a pharmacist-led aspirin deprescribing protocol in the ambulatory care pharmacy setting was successful in the discontinuation of unnecessary aspirin use in older adults. The protocol also provided opportunities for education on ASCVD risk reduction in all older adults reached. These findings highlight the role of pharmacists in deprescribing PIMs for older adults and identifying opportunities to further streamline risk/benefit discussions on aspirin deprescribing potential within primary care visits.
1. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2019;140(11):e596-e646. doi:10.1161/CIR.0000000000000678
2. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomized, double-blind, placebo-controlled trial. Lancet. 2018;392(10152):1036-1046. doi:10.1016/S0140-6736(18)31924-X
3. Bowman L, Mafham M, et al; ASCEND Study Collaborative Group. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379(16):1529-1539. doi:10.1056/NEJMoa1804988
4. McNeil JJ, Wolfe R, Woods, RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379(16):1509-1518. doi:10.1056/NEJMoa1805819
5. García Rodríguez LA, Martín-Pérez M, Hennekens CH, Rothwell PM, Lanas A. Bleeding risk with long-term low-dose aspirin: a systematic review of observational studies. PloS One. 2016;11(8):e0160046. doi:10.1371/journal.pone.0160046
6. Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment): consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72-83. doi:10.5414/cpp46072
7. Zheng SL, Roddick AJ. Association of aspirin use for primary prevention with cardiovascular events and bleeding events: a systematic review and meta-analysis. JAMA. 2019;321(3):277-287. doi:10.1001/jama.2018.20578
8. Patrono C, Baigent C. Role of aspirin in primary prevention of cardiovascular disease. Nat Rev Cardiol. 2019;16(11):675-686. doi:10.1038/s41569-019-0225-y
9. Bibbins-Domingo K; U.S. Preventative Services Task Force. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164(12):836-845. doi:10.7326/M16-0577
10. American Diabetes Association. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(suppl 1):S14-S31. doi:10.2337/dc20-S002
11. O’Brien CW, Juraschek SP, Wee CC. Prevalence of aspirin use for primary prevention of cardiovascular disease in the United States: results from the 2017 National Health Interview Survey. Ann Intern Med. 2019;171(8):596-598. doi:10.7326/M19-0953
12. Hira RS, Kennedy K, Nambi V, et al. Frequency and practice-level variation in inappropriate aspirin use for the primary prevention of cardiovascular disease: insights from the National Cardiovascular Disease Registry’s Practice Innovation and Clinical Excellence registry. J Am Coll Cardiol. 2015;65(2):111-121. doi:10.1016/j.jacc.2014.10.035
13. Cheong ST, Ng TM, Tan KT. Pharmacist-initiated deprescribing in hospitalized elderly: prevalence and acceptance by physicians. Eur J Hosp Pharm. 2018;25(e1):e35-e39. doi:10.1136/ejhpharm-2017-001251
14. Dyck MJ. Evidence-based administrative guideline: quality improvement in nursing homes. J Gerontol Nurs. 2005;31(2):4-10. doi:10.3928/0098-9134-20050201-04
15. Zullo AR, Gray SL, Holmes HM, Marcum ZA. Screening for medication appropriateness in older adults. Clin Geriatr Med. 2018;34(1):39-54. doi:10.1016/j.cger.2017.09.003
16. American Geriatrics Society. 2019 updated AGS Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694. doi:10.1111/jgs.15767
17. Shah BM, Hajjar ER. Polypharmacy, adverse drug reactions, and geriatric syndromes. Clin Geriatr Med. 2012;28(2):173-186. doi:10.1016/j.cger.2012.01.002
18. Page AT, Clifford RM, Potter K, Schwartz D, Etherton-Beer CD. The feasibility and effect of deprescribing in older adults on mortality and health: a systematic review and meta-analysis. Br J Clin Pharmacol. 2016;82(3):583-623. doi:10.1111/bcp.12975
19. Reeve E, Shakib S, Hendrix I, Roberts MS, Wiese MD. The benefits and harms of deprescribing. Med J Aust. 2014;201(7):386-389. doi:10.5694/mja13.00200
20. Ailabouni NJ, Marcum ZA, Schmader KE, Gray SL. Medication use quality and safety in older adults: 2018 update. J Am Geriatr Soc. 2019;67(12):2458-2462. doi:10.1111/jgs.16243
21. Frank C, Weir E. Deprescribing for older patients. CMAJ. 2014;186(18):1369-1376. doi:10.1503/cmaj.131873
22. Clark CM, LaValley SA, Singh R, Mustafa E, Monte SV, Wahler RG Jr. A pharmacist-led program to facilitate deprescribing in a primary care clinic. J Am Pharm Assoc (2003). 2020;60(1):105-111. doi:10.1016/j.japh.2019.09.011
23. Folks B, Leblanc WG, Staton EW, Pace WD. Reconsidering low-dose aspirin therapy for cardiovascular disease: a study protocol for physician and patient behavioral change. Implement Sci. 2011;6:65. Published 2011 Jun 26. doi:10.1186/1748-5908-6-65
24. Odenthal DR, Philbrick AM, Harris IM. Successful deprescribing of unnecessary proton pump inhibitors in a primary care clinic. J Am Pharm Assoc. 2020;60(1):100-104. doi:10.1016/j.japh.2019.08.012
25. Duncan, P. Duerden M, Payne RA. Deprescribing: a primary care perspective. Eur J Hosp Pharm. 2017;24(1):37-42. doi:10.1136/ejhpharm-2016-000967
26. Martin P, Tamblyn R, Benedetti A, Ahmed S, Tannenbaum C. Effect of a pharmacist-led educational intervention on inappropriate medication prescriptions in older adults: the D-PRESCRIBE randomized clinical trial. JAMA. 2018;320(18):1889-1898. doi:10.1001/jama.2018.16131
1. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2019;140(11):e596-e646. doi:10.1161/CIR.0000000000000678
2. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomized, double-blind, placebo-controlled trial. Lancet. 2018;392(10152):1036-1046. doi:10.1016/S0140-6736(18)31924-X
3. Bowman L, Mafham M, et al; ASCEND Study Collaborative Group. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379(16):1529-1539. doi:10.1056/NEJMoa1804988
4. McNeil JJ, Wolfe R, Woods, RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379(16):1509-1518. doi:10.1056/NEJMoa1805819
5. García Rodríguez LA, Martín-Pérez M, Hennekens CH, Rothwell PM, Lanas A. Bleeding risk with long-term low-dose aspirin: a systematic review of observational studies. PloS One. 2016;11(8):e0160046. doi:10.1371/journal.pone.0160046
6. Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment): consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72-83. doi:10.5414/cpp46072
7. Zheng SL, Roddick AJ. Association of aspirin use for primary prevention with cardiovascular events and bleeding events: a systematic review and meta-analysis. JAMA. 2019;321(3):277-287. doi:10.1001/jama.2018.20578
8. Patrono C, Baigent C. Role of aspirin in primary prevention of cardiovascular disease. Nat Rev Cardiol. 2019;16(11):675-686. doi:10.1038/s41569-019-0225-y
9. Bibbins-Domingo K; U.S. Preventative Services Task Force. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164(12):836-845. doi:10.7326/M16-0577
10. American Diabetes Association. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(suppl 1):S14-S31. doi:10.2337/dc20-S002
11. O’Brien CW, Juraschek SP, Wee CC. Prevalence of aspirin use for primary prevention of cardiovascular disease in the United States: results from the 2017 National Health Interview Survey. Ann Intern Med. 2019;171(8):596-598. doi:10.7326/M19-0953
12. Hira RS, Kennedy K, Nambi V, et al. Frequency and practice-level variation in inappropriate aspirin use for the primary prevention of cardiovascular disease: insights from the National Cardiovascular Disease Registry’s Practice Innovation and Clinical Excellence registry. J Am Coll Cardiol. 2015;65(2):111-121. doi:10.1016/j.jacc.2014.10.035
13. Cheong ST, Ng TM, Tan KT. Pharmacist-initiated deprescribing in hospitalized elderly: prevalence and acceptance by physicians. Eur J Hosp Pharm. 2018;25(e1):e35-e39. doi:10.1136/ejhpharm-2017-001251
14. Dyck MJ. Evidence-based administrative guideline: quality improvement in nursing homes. J Gerontol Nurs. 2005;31(2):4-10. doi:10.3928/0098-9134-20050201-04
15. Zullo AR, Gray SL, Holmes HM, Marcum ZA. Screening for medication appropriateness in older adults. Clin Geriatr Med. 2018;34(1):39-54. doi:10.1016/j.cger.2017.09.003
16. American Geriatrics Society. 2019 updated AGS Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694. doi:10.1111/jgs.15767
17. Shah BM, Hajjar ER. Polypharmacy, adverse drug reactions, and geriatric syndromes. Clin Geriatr Med. 2012;28(2):173-186. doi:10.1016/j.cger.2012.01.002
18. Page AT, Clifford RM, Potter K, Schwartz D, Etherton-Beer CD. The feasibility and effect of deprescribing in older adults on mortality and health: a systematic review and meta-analysis. Br J Clin Pharmacol. 2016;82(3):583-623. doi:10.1111/bcp.12975
19. Reeve E, Shakib S, Hendrix I, Roberts MS, Wiese MD. The benefits and harms of deprescribing. Med J Aust. 2014;201(7):386-389. doi:10.5694/mja13.00200
20. Ailabouni NJ, Marcum ZA, Schmader KE, Gray SL. Medication use quality and safety in older adults: 2018 update. J Am Geriatr Soc. 2019;67(12):2458-2462. doi:10.1111/jgs.16243
21. Frank C, Weir E. Deprescribing for older patients. CMAJ. 2014;186(18):1369-1376. doi:10.1503/cmaj.131873
22. Clark CM, LaValley SA, Singh R, Mustafa E, Monte SV, Wahler RG Jr. A pharmacist-led program to facilitate deprescribing in a primary care clinic. J Am Pharm Assoc (2003). 2020;60(1):105-111. doi:10.1016/j.japh.2019.09.011
23. Folks B, Leblanc WG, Staton EW, Pace WD. Reconsidering low-dose aspirin therapy for cardiovascular disease: a study protocol for physician and patient behavioral change. Implement Sci. 2011;6:65. Published 2011 Jun 26. doi:10.1186/1748-5908-6-65
24. Odenthal DR, Philbrick AM, Harris IM. Successful deprescribing of unnecessary proton pump inhibitors in a primary care clinic. J Am Pharm Assoc. 2020;60(1):100-104. doi:10.1016/j.japh.2019.08.012
25. Duncan, P. Duerden M, Payne RA. Deprescribing: a primary care perspective. Eur J Hosp Pharm. 2017;24(1):37-42. doi:10.1136/ejhpharm-2016-000967
26. Martin P, Tamblyn R, Benedetti A, Ahmed S, Tannenbaum C. Effect of a pharmacist-led educational intervention on inappropriate medication prescriptions in older adults: the D-PRESCRIBE randomized clinical trial. JAMA. 2018;320(18):1889-1898. doi:10.1001/jama.2018.16131
Assessment of Glucagon-like Peptide-1 Receptor Agonists in Veterans Taking Basal/Bolus Insulin Regimens
In 2019, diabetes mellitus (DM) was the seventh leading cause of death in the United States, and currently, about 11% of the American population has a DM diagnosis.1 Most have a diagnosis of type 2 diabetes (T2DM), which has a strong genetic predisposition, and the risk of developing T2DM increases with age, obesity, and lack of physical activity.1,2 Nearly one-quarter of veterans have a diagnosis of DM, and DM is the leading cause of comorbidities, such as blindness, end-stage renal disease, and amputation for patients receiving care from the Veterans Health Administration (VHA).2 The elevated incidence of DM in the veteran population is attributed to a variety of factors, including exposure to herbicides, such as Agent Orange, advanced age, increased risk of obesity, and limited access to high-quality food.3
After diagnosis, both the American Diabetes Association (ADA) and the American Association of Clinical Endocrinologists and American College of Endocrinology (AACE/ACE) emphasize the appropriate use of lifestyle management and pharmacologic therapy for DM care. The use of pharmacologic agents (oral medications, insulin, or noninsulin injectables) is often determined by efficacy, cost, potential adverse effects (AEs), and patient factors and comorbidities.4,5
The initial recommendation for pharmacologic treatment for T2DM differs slightly between expert guidelines. The ADA and AACE/ACE recommend any of the following as initial monotherapy, listed in order to represent a hierarchy of usage: metformin, glucagon-like peptide-1 receptor agonists (GLP-1 RAs), sodium-glucose cotransporter 2 (SGLT-2) inhibitors, or dipeptidyl peptidase-4 (DPP-4) inhibitors, with the first 3 agents carrying the strongest recommendations.4,5 For patients with established atherosclerotic cardiovascular disease (CVD), chronic kidney disease, or heart failure, it is recommended to start a long-acting GLP-1 RA or SGLT-2 inhibitor. For patients with T2DM and hemoglobin A1c (HbA1c) between 7.5% and 9.0% at diagnosis, the AACE/ACE recommend initiation of dual therapy using metformin alongside another first-line agent and recommend the addition of another antidiabetic agent if glycemic goals are not met after regular follow-up. AACE/ACE recommend the consideration of insulin therapy in symptomatic patients with HbA1c > 9.0%.5 In contrast, the ADA recommends metformin as first-line therapy for all patients with T2DM and recommends dual therapy using metformin and another preferred agent (selection based on comorbidities) when HbA1c is 1.5% to 2% above target. The ADA recommends the consideration of insulin with HbA1c > 10% or with evidence of ongoing catabolism or symptoms of hyperglycemia.4 There are several reasons why insulin may be initiated prior to GLP-1 RAs, including profound hyperglycemia at time of diagnosis or implementation of insulin agents prior to commercial availability of GLP-1 RA.
GLP-1 RAs are analogs of the hormone incretin, which increases glucose-dependent insulin secretion, decreases postprandial glucagon secretion, increases satiety, and slows gastric emptying.6,7 When used in combination with noninsulin agents, GLP-1 RAs have demonstrated HbA1c reductions of 0.5% to 1.5%.8 The use of GLP-1 RAs with basal insulin also has been studied extensively.6,8-10 When the combination of GLP-1 RAs and basal insulin was compared with basal/bolus insulin regimens, the use of the GLP-1 RAs resulted in lower HbA1c levels and lower incidence of hypoglycemia.6,9 Data have demonstrated the complementary mechanisms of using basal insulin and GLP 1 RAs in decreasing HbA1c levels, insulin requirements, and weight compared with using basal insulin monotherapy and basal/bolus combinations.6,9-13 Moreover, 3 GLP-1 RA medications currently on the market (liraglutide, dulaglutide, and semaglutide) have displayed cardiovascular and renal benefits, further supporting the use of these medications.2,5
Despite these benefits, GLP-1 RAs may have bothersome AEs and are associated with a high cost.6 In addition, some studies have found that as the length of therapy increases, the positive effects of these agents may diminish.9,11 In one study, which looked at the impact of the addition of exenatide to patients taking basal or basal/bolus insulin regimens, mean changes in weight were −2.4 kg at 0 to 6 months, −4.3 kg at 6 to 12 months, −6.2 kg at 12 to 18 months, and −5.5 kg at 18 to 27 months. After 18 months, an increase in weight was observed, but the increase remained lower than baseline.11 Another study, conducted over 12 months, found no significant decrease in weight or total daily dose (TDD) of insulin when exenatide or liraglutide were added to various insulin regimens (basal or basal/bolus).13 To date, minimal published data exist regarding the addition of newer GLP-1 RAs and the long-term use of these agents beyond 12 months in patients taking basal/bolus insulin regimens. The primary goal of this study was to evaluate the effect of adding GLP-1 RAs to basal/bolus insulin regimens over a 24-month period.
Methods
This study was a retrospective, electronic health record review of all patients on basal and bolus insulin regimens who received additional therapy with a GLP-1 RA at Veteran Health Indiana in Indianapolis from September 1, 2015, to June 30, 2019. Patients meeting inclusion criteria served as their own control. The primary outcome was change in HbA1c at 3, 6, 12, 18, and 24 months after initiation of the GLP-1 RA. Secondary outcomes included change in weight and TDD of insulin at 3, 6, 12, 18, and 24 months after the initiation of the GLP-1 RAs and incidence of patient-reported or laboratory-confirmed hypoglycemia and other AEs.
Patients were included if they were aged ≥ 18 years with a diagnosis of T2DM, had concomitant prescriptions for both a basal insulin (glargine, detemir, or NPH) and a bolus insulin (aspart, lispro, or regular) before receiving add-on therapy with a GLP-1 RA (exenatide, liraglutide, albiglutide, lixisenatide, dulaglutide, or semaglutide) from September 1, 2015, to June 30, 2019, and had baseline and subsequent HbA1c measurements available in the electronic health record. Patients were excluded if they had a diagnosis of type 1 DM (T1DM), were followed by an outside clinician for DM care, or if the GLP-1 RA was discontinued before subsequent HbA1c measurement. The study protocol was approved by the Research and Development Office of Veteran Health Indiana, and the project was deemed exempt from review by the Indiana University Institutional Review Board due to the retrospective nature of the study.
Data analysis was performed using Excel. Change from baseline for each interval was computed, and 1 sample t tests (2-tailed) compared change from baseline to no change. Due to the disparity in the number of patients with data available at each of the time intervals, a mean plot was presented for each group of patients within each interval, allowing mean changes in individual groups to be observed over time.
Results
One hundred twenty-three subjects met inclusion criteria; 16 patients were excluded due to GLP-1 RA discontinuation before follow-up measurement of HbA1c; 14 were excluded due to patients being managed by a clinician outside of the facility; 1 patient was excluded for lack of documentation regarding baseline and subsequent insulin doses. Ninety-two patient charts were reviewed. Participants had a mean age of 64 years, 95% were male, and 89% were White. Mean baseline HbA1c was 9.2%, mean body mass index was 38.9, and the mean TDD of insulin was 184 units.
Since some patients switched between GLP-1 RAs throughout the study and there was variation in timing of laboratory and clinic follow-up,
Discussion
Adding a GLP-1 RA to basal/bolus insulin regimens was associated with a statistically significant decrease in HbA1c at each time point through 18 months. The greatest improvement in glycemic control from baseline was seen at 3 months, with improvements in HbA1c diminishing at each subsequent period. The study also demonstrated a significant decrease in weight at each time point through 18 months. The greatest decrease in weight was observed at both 6 and 12 months. Statistically significant decreases in TDD were observed at 3, 6, and 12 months. Insulin changes after 12 months were not found to be statistically significant.
Few studies have previously evaluated the use of GLP-1 RAs in patients with T2DM who are already taking basal/bolus insulin regimens. Gyorffy and colleagues reported significant improvements in glycemic control at 3 and 6 months in a sample of 54 patients taking basal/bolus insulin when liraglutide or exenatide was added, although statistical significance was not found at the final 12-month time point.13 That study also found a significant decrease in weight at 6 months; however there was not a significant reduction in weight at both 3 and 12 months of GLP-1 RA therapy. There was not a significant decrease in TDD at any of the collected time points. Nonetheless, Gyorffy and colleagues concluded that reduction in TDD leveled off after 12 months, which is consistent with this study’s findings. The small size of the study may have limited the ability to detect statistical significance; however, this study was conducted in a population that was racially diverse and included a higher proportion of women, though average age was similar.13
Yoon and colleagues reported weight loss through 18 months, then saw weight increase, though weights did remain lower than baseline. The study also showed no significant change in TDD of insulin after 12 months of concomitant exenatide and insulin therapy.11 Although these results mirror the outcomes observed in this study, Yoon and colleagues did not differentiate results between basal and basal/bolus insulin groups.11 Seino and colleagues observed no significant change in weight after 36 weeks of GLP-1 RA therapy in Japanese patients when used with basal and basal/bolus insulin regimens. Despite the consideration that the population in the study was not overweight (mean body mass index was 25.6), the results of these studies support the idea that effects of GLP-1 RAs on weight and TDD may diminish over time.14
Within the VHA, GLP-1 RAs are nonformulary medications. Patients must meet certain criteria in order to be approved for these agents, which may include diagnosis of CVD, renal disease, or failure to reach glycemic control with the use of oral agents or insulin. Therefore, participants of this study represent a particular subset of VHA patients, many of whom may have been selected for consideration due to long-standing or uncontrolled T2DM and failure of previous therapies. The baseline demographics support this idea, given poor glycemic control at baseline and high insulin requirements. Once approved for GLP-1 RA therapy, semaglutide is currently the preferred agent within the VHA, with other agents available for select considerations. It should be noted that albiglutide, which was the primary agent selected for some of the patients included in this study, was removed from the market in 2017 for economic considerations.15 In the case for these patients, a conversion to a formulary-preferred GLP-1 RA was made.
Most of the patients included in this study (70%) were maintained on metformin from baseline throughout the study period. Fifty-seven percent of patients were taking TDD of insulin > 150 units. Considering the significant cost of concentrated insulins, the addition of GLP-1 RAs to standard insulin may prove to be beneficial from a cost standpoint. Additional research in this area may be warranted to establish more data regarding this potential benefit of GLP-1 RAs as add-on therapy.
Many adverse drug reactions were reported at different periods; however, most of these were associated with the gastrointestinal system, which is consistent with current literature, drug labeling, and the mechanism of action.16 Hypoglycemia occurred in about one-third of the participants; however, it should be noted that alone, GLP-1 RAs are not associated with a high risk of hypoglycemia. Previous studies have found that GLP-1 RA monotherapy is associated with hypoglycemia in 1.6% to 12.6% of patients.17,18 More likely, the combination of basal/bolus insulin and the GLP-1 RA’s effect on increasing insulin sensitivity through weight loss, improving glucose-dependent insulin secretion, or by decreasing appetite and therefore decreasing carbohydrate intake contributed to the hypoglycemia prevalence.
Limitations and Strengths
Limitations of this study include a small patient population and a gradual reduction in available data as time periods progressed, making even smaller sample sizes for subsequent time periods. A majority of participants were older, males and White race. This could have limited the determination of statistical significance and applicability of the results to other patient populations. Another potential limitation was the retrospective nature of the study design, which may have limited reporting of hypoglycemia and other AEs based on the documentation of the clinician.
Strengths included the study duration and the diversity of GLP-1 RAs used by participants, as the impact of many of these agents has not yet been assessed in the literature. In addition, the retrospective nature of the study allows for a more realistic representation of patient adherence, education, and motivation, which are likely different from those of patients included in prospective clinical trials.
There are no clear guidelines dictating the optimal duration of concomitant GLP-1 RA and insulin therapy; however, our study suggests that there may be continued benefits past short-term use. Also our study suggests that patients with T2DM treated with basal/bolus insulin regimens may glean additional benefit from adding GLP-1 RAs; however, further randomized, controlled studies are warranted, particularly in poorly controlled patients requiring even more aggressive treatment regimens, such as concentrated insulins.
Conclusions
In our study, adding GLP-1 RA to basal/bolus insulin was associated with a significant decrease in HbA1c from baseline through 18 months. An overall decrease in weight and TDD of insulin was observed through 24 months, but the change in weight was not significant past 18 months, and the change in insulin requirement was not significant past 12 months. Hypoglycemia was observed in almost one-third of patients, and gastrointestinal symptoms were the most common AE observed as a result of adding GLP-1 RAs. More studies are needed to better evaluate the durability and cost benefit of GLP-1 RAs, especially in patients with high insulin requirements.
Acknowledgments
This material is the result of work supported with resources and facilities at Veteran Health Indiana in Indianapolis. Study data were collected and managed using REDCap electronic data capture tools hosted at Veteran Health Indiana. The authors also acknowledge George Eckert for his assistance with data analysis.
1. American Diabetes Association. Statistics about diabetes. Accessed August 9, 2022. http://www.diabetes.org/diabetes-basics/statistics
2. US Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development. VA research on: diabetes. Updated January 15, 2021. Accessed August 9, 2022. https://www.research.va.gov/topics/diabetes.cfm
3. Federal Practitioner. Federal Health Care Data Trends 2017, Diabetes mellitus. Accessed August 9, 2022. https://www.fedprac-digital.com/federalpractitioner/data_trends_2017?pg=20#pg20
4. American Diabetes Association Professional Practice Committee. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S125-S143. doi:10.2337/dc22-S009
5. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2019 executive summary. Endocr Pract. 2019;25(1):69-100. doi:10.4158/CS-2018-0535
6. St Onge E, Miller S, Clements E, Celauro L, Barnes K. The role of glucagon-like peptide-1 receptor agonists in the treatment of type 2 diabetes. J Transl Int Med. 2017;5(2):79-89. Published 2017 Jun 30. doi:10.1515/jtim-2017-0015
7. Almandoz JP, Lingvay I, Morales J, Campos C. Switching between glucagon-like peptide-1 receptor agonists: rationale and practical guidance. Clin Diabetes. 2020;38(4):390-402. doi:10.2337/cd19-0100
8. Davies ML, Pham DQ, Drab SR. GLP1-RA add-on therapy in patients with type 2 diabetes currently on a bolus containing insulin regimen. Pharmacotherapy. 2016;36(8):893-905. doi:10.1002/phar.1792
9. Rosenstock J, Guerci B, Hanefeld M, et al. Prandial options to advance basal insulin glargine therapy: testing lixisenatide plus basal insulin versus insulin glulisine either as basal-plus or basal-bolus in type 2 diabetes: the GetGoal Duo-2 Trial Investigators. Diabetes Care. 2016;39(8):1318-1328. doi:10.2337/dc16-0014
10. Levin PA, Mersey JH, Zhou S, Bromberger LA. Clinical outcomes using long-term combination therapy with insulin glargine and exenatide in patients with type 2 diabetes mellitus. Endocr Pract. 2012;18(1):17-25. doi:10.4158/EP11097.OR
11. Yoon NM, Cavaghan MK, Brunelle RL, Roach P. Exenatide added to insulin therapy: a retrospective review of clinical practice over two years in an academic endocrinology outpatient setting. Clin Ther. 2009;31(7):1511-1523. doi:10.1016/j.clinthera.2009.07.021
12. Weissman PN, Carr MC, Ye J, et al. HARMONY 4: randomised clinical trial comparing once-weekly albiglutide and insulin glargine in patients with type 2 diabetes inadequately controlled with metformin with or without sulfonylurea. Diabetologia. 2014;57(12):2475-2484. doi:10.1007/s00125-014-3360-3
13. Gyorffy JB, Keithler AN, Wardian JL, Zarzabal LA, Rittel A, True MW. The impact of GLP-1 receptor agonists on patients with diabetes on insulin therapy. Endocr Pract. 2019;25(9):935-942. doi:10.4158/EP-2019-0023
14. Seino Y, Kaneko S, Fukuda S, et al. Combination therapy with liraglutide and insulin in Japanese patients with type 2 diabetes: a 36-week, randomized, double-blind, parallel-group trial. J Diabetes Investig. 2016;7(4):565-573. doi:10.1111/jdi.12457
15. Optum. Tanzeum (albiglutide)–drug discontinuation. Published 2017. Accessed August 15, 2022. https://professionals.optumrx.com/content/dam/optum3/professional-optumrx/news/rxnews/drug-recalls-shortages/drugwithdrawal_tanzeum_2017-0801.pdf
16. Chun JH, Butts A. Long-acting GLP-1RAs: an overview of efficacy, safety, and their role in type 2 diabetes management. JAAPA. 2020;33(8):3-18. doi:10.1097/01.JAA.0000669456.13763.bd
17. Ozempic semaglutide injection. Prescribing information. Novo Nordisk; 2022. Accessed August 9, 2022. https://www.novo-pi.com/ozempic.pdf
18. Victoza liraglutide injection. Prescribing information. Novo Nordisk; 2021. Accessed August 9, 2022. https://www.novo-pi.com/victoza.pdf
In 2019, diabetes mellitus (DM) was the seventh leading cause of death in the United States, and currently, about 11% of the American population has a DM diagnosis.1 Most have a diagnosis of type 2 diabetes (T2DM), which has a strong genetic predisposition, and the risk of developing T2DM increases with age, obesity, and lack of physical activity.1,2 Nearly one-quarter of veterans have a diagnosis of DM, and DM is the leading cause of comorbidities, such as blindness, end-stage renal disease, and amputation for patients receiving care from the Veterans Health Administration (VHA).2 The elevated incidence of DM in the veteran population is attributed to a variety of factors, including exposure to herbicides, such as Agent Orange, advanced age, increased risk of obesity, and limited access to high-quality food.3
After diagnosis, both the American Diabetes Association (ADA) and the American Association of Clinical Endocrinologists and American College of Endocrinology (AACE/ACE) emphasize the appropriate use of lifestyle management and pharmacologic therapy for DM care. The use of pharmacologic agents (oral medications, insulin, or noninsulin injectables) is often determined by efficacy, cost, potential adverse effects (AEs), and patient factors and comorbidities.4,5
The initial recommendation for pharmacologic treatment for T2DM differs slightly between expert guidelines. The ADA and AACE/ACE recommend any of the following as initial monotherapy, listed in order to represent a hierarchy of usage: metformin, glucagon-like peptide-1 receptor agonists (GLP-1 RAs), sodium-glucose cotransporter 2 (SGLT-2) inhibitors, or dipeptidyl peptidase-4 (DPP-4) inhibitors, with the first 3 agents carrying the strongest recommendations.4,5 For patients with established atherosclerotic cardiovascular disease (CVD), chronic kidney disease, or heart failure, it is recommended to start a long-acting GLP-1 RA or SGLT-2 inhibitor. For patients with T2DM and hemoglobin A1c (HbA1c) between 7.5% and 9.0% at diagnosis, the AACE/ACE recommend initiation of dual therapy using metformin alongside another first-line agent and recommend the addition of another antidiabetic agent if glycemic goals are not met after regular follow-up. AACE/ACE recommend the consideration of insulin therapy in symptomatic patients with HbA1c > 9.0%.5 In contrast, the ADA recommends metformin as first-line therapy for all patients with T2DM and recommends dual therapy using metformin and another preferred agent (selection based on comorbidities) when HbA1c is 1.5% to 2% above target. The ADA recommends the consideration of insulin with HbA1c > 10% or with evidence of ongoing catabolism or symptoms of hyperglycemia.4 There are several reasons why insulin may be initiated prior to GLP-1 RAs, including profound hyperglycemia at time of diagnosis or implementation of insulin agents prior to commercial availability of GLP-1 RA.
GLP-1 RAs are analogs of the hormone incretin, which increases glucose-dependent insulin secretion, decreases postprandial glucagon secretion, increases satiety, and slows gastric emptying.6,7 When used in combination with noninsulin agents, GLP-1 RAs have demonstrated HbA1c reductions of 0.5% to 1.5%.8 The use of GLP-1 RAs with basal insulin also has been studied extensively.6,8-10 When the combination of GLP-1 RAs and basal insulin was compared with basal/bolus insulin regimens, the use of the GLP-1 RAs resulted in lower HbA1c levels and lower incidence of hypoglycemia.6,9 Data have demonstrated the complementary mechanisms of using basal insulin and GLP 1 RAs in decreasing HbA1c levels, insulin requirements, and weight compared with using basal insulin monotherapy and basal/bolus combinations.6,9-13 Moreover, 3 GLP-1 RA medications currently on the market (liraglutide, dulaglutide, and semaglutide) have displayed cardiovascular and renal benefits, further supporting the use of these medications.2,5
Despite these benefits, GLP-1 RAs may have bothersome AEs and are associated with a high cost.6 In addition, some studies have found that as the length of therapy increases, the positive effects of these agents may diminish.9,11 In one study, which looked at the impact of the addition of exenatide to patients taking basal or basal/bolus insulin regimens, mean changes in weight were −2.4 kg at 0 to 6 months, −4.3 kg at 6 to 12 months, −6.2 kg at 12 to 18 months, and −5.5 kg at 18 to 27 months. After 18 months, an increase in weight was observed, but the increase remained lower than baseline.11 Another study, conducted over 12 months, found no significant decrease in weight or total daily dose (TDD) of insulin when exenatide or liraglutide were added to various insulin regimens (basal or basal/bolus).13 To date, minimal published data exist regarding the addition of newer GLP-1 RAs and the long-term use of these agents beyond 12 months in patients taking basal/bolus insulin regimens. The primary goal of this study was to evaluate the effect of adding GLP-1 RAs to basal/bolus insulin regimens over a 24-month period.
Methods
This study was a retrospective, electronic health record review of all patients on basal and bolus insulin regimens who received additional therapy with a GLP-1 RA at Veteran Health Indiana in Indianapolis from September 1, 2015, to June 30, 2019. Patients meeting inclusion criteria served as their own control. The primary outcome was change in HbA1c at 3, 6, 12, 18, and 24 months after initiation of the GLP-1 RA. Secondary outcomes included change in weight and TDD of insulin at 3, 6, 12, 18, and 24 months after the initiation of the GLP-1 RAs and incidence of patient-reported or laboratory-confirmed hypoglycemia and other AEs.
Patients were included if they were aged ≥ 18 years with a diagnosis of T2DM, had concomitant prescriptions for both a basal insulin (glargine, detemir, or NPH) and a bolus insulin (aspart, lispro, or regular) before receiving add-on therapy with a GLP-1 RA (exenatide, liraglutide, albiglutide, lixisenatide, dulaglutide, or semaglutide) from September 1, 2015, to June 30, 2019, and had baseline and subsequent HbA1c measurements available in the electronic health record. Patients were excluded if they had a diagnosis of type 1 DM (T1DM), were followed by an outside clinician for DM care, or if the GLP-1 RA was discontinued before subsequent HbA1c measurement. The study protocol was approved by the Research and Development Office of Veteran Health Indiana, and the project was deemed exempt from review by the Indiana University Institutional Review Board due to the retrospective nature of the study.
Data analysis was performed using Excel. Change from baseline for each interval was computed, and 1 sample t tests (2-tailed) compared change from baseline to no change. Due to the disparity in the number of patients with data available at each of the time intervals, a mean plot was presented for each group of patients within each interval, allowing mean changes in individual groups to be observed over time.
Results
One hundred twenty-three subjects met inclusion criteria; 16 patients were excluded due to GLP-1 RA discontinuation before follow-up measurement of HbA1c; 14 were excluded due to patients being managed by a clinician outside of the facility; 1 patient was excluded for lack of documentation regarding baseline and subsequent insulin doses. Ninety-two patient charts were reviewed. Participants had a mean age of 64 years, 95% were male, and 89% were White. Mean baseline HbA1c was 9.2%, mean body mass index was 38.9, and the mean TDD of insulin was 184 units.
Since some patients switched between GLP-1 RAs throughout the study and there was variation in timing of laboratory and clinic follow-up,
Discussion
Adding a GLP-1 RA to basal/bolus insulin regimens was associated with a statistically significant decrease in HbA1c at each time point through 18 months. The greatest improvement in glycemic control from baseline was seen at 3 months, with improvements in HbA1c diminishing at each subsequent period. The study also demonstrated a significant decrease in weight at each time point through 18 months. The greatest decrease in weight was observed at both 6 and 12 months. Statistically significant decreases in TDD were observed at 3, 6, and 12 months. Insulin changes after 12 months were not found to be statistically significant.
Few studies have previously evaluated the use of GLP-1 RAs in patients with T2DM who are already taking basal/bolus insulin regimens. Gyorffy and colleagues reported significant improvements in glycemic control at 3 and 6 months in a sample of 54 patients taking basal/bolus insulin when liraglutide or exenatide was added, although statistical significance was not found at the final 12-month time point.13 That study also found a significant decrease in weight at 6 months; however there was not a significant reduction in weight at both 3 and 12 months of GLP-1 RA therapy. There was not a significant decrease in TDD at any of the collected time points. Nonetheless, Gyorffy and colleagues concluded that reduction in TDD leveled off after 12 months, which is consistent with this study’s findings. The small size of the study may have limited the ability to detect statistical significance; however, this study was conducted in a population that was racially diverse and included a higher proportion of women, though average age was similar.13
Yoon and colleagues reported weight loss through 18 months, then saw weight increase, though weights did remain lower than baseline. The study also showed no significant change in TDD of insulin after 12 months of concomitant exenatide and insulin therapy.11 Although these results mirror the outcomes observed in this study, Yoon and colleagues did not differentiate results between basal and basal/bolus insulin groups.11 Seino and colleagues observed no significant change in weight after 36 weeks of GLP-1 RA therapy in Japanese patients when used with basal and basal/bolus insulin regimens. Despite the consideration that the population in the study was not overweight (mean body mass index was 25.6), the results of these studies support the idea that effects of GLP-1 RAs on weight and TDD may diminish over time.14
Within the VHA, GLP-1 RAs are nonformulary medications. Patients must meet certain criteria in order to be approved for these agents, which may include diagnosis of CVD, renal disease, or failure to reach glycemic control with the use of oral agents or insulin. Therefore, participants of this study represent a particular subset of VHA patients, many of whom may have been selected for consideration due to long-standing or uncontrolled T2DM and failure of previous therapies. The baseline demographics support this idea, given poor glycemic control at baseline and high insulin requirements. Once approved for GLP-1 RA therapy, semaglutide is currently the preferred agent within the VHA, with other agents available for select considerations. It should be noted that albiglutide, which was the primary agent selected for some of the patients included in this study, was removed from the market in 2017 for economic considerations.15 In the case for these patients, a conversion to a formulary-preferred GLP-1 RA was made.
Most of the patients included in this study (70%) were maintained on metformin from baseline throughout the study period. Fifty-seven percent of patients were taking TDD of insulin > 150 units. Considering the significant cost of concentrated insulins, the addition of GLP-1 RAs to standard insulin may prove to be beneficial from a cost standpoint. Additional research in this area may be warranted to establish more data regarding this potential benefit of GLP-1 RAs as add-on therapy.
Many adverse drug reactions were reported at different periods; however, most of these were associated with the gastrointestinal system, which is consistent with current literature, drug labeling, and the mechanism of action.16 Hypoglycemia occurred in about one-third of the participants; however, it should be noted that alone, GLP-1 RAs are not associated with a high risk of hypoglycemia. Previous studies have found that GLP-1 RA monotherapy is associated with hypoglycemia in 1.6% to 12.6% of patients.17,18 More likely, the combination of basal/bolus insulin and the GLP-1 RA’s effect on increasing insulin sensitivity through weight loss, improving glucose-dependent insulin secretion, or by decreasing appetite and therefore decreasing carbohydrate intake contributed to the hypoglycemia prevalence.
Limitations and Strengths
Limitations of this study include a small patient population and a gradual reduction in available data as time periods progressed, making even smaller sample sizes for subsequent time periods. A majority of participants were older, males and White race. This could have limited the determination of statistical significance and applicability of the results to other patient populations. Another potential limitation was the retrospective nature of the study design, which may have limited reporting of hypoglycemia and other AEs based on the documentation of the clinician.
Strengths included the study duration and the diversity of GLP-1 RAs used by participants, as the impact of many of these agents has not yet been assessed in the literature. In addition, the retrospective nature of the study allows for a more realistic representation of patient adherence, education, and motivation, which are likely different from those of patients included in prospective clinical trials.
There are no clear guidelines dictating the optimal duration of concomitant GLP-1 RA and insulin therapy; however, our study suggests that there may be continued benefits past short-term use. Also our study suggests that patients with T2DM treated with basal/bolus insulin regimens may glean additional benefit from adding GLP-1 RAs; however, further randomized, controlled studies are warranted, particularly in poorly controlled patients requiring even more aggressive treatment regimens, such as concentrated insulins.
Conclusions
In our study, adding GLP-1 RA to basal/bolus insulin was associated with a significant decrease in HbA1c from baseline through 18 months. An overall decrease in weight and TDD of insulin was observed through 24 months, but the change in weight was not significant past 18 months, and the change in insulin requirement was not significant past 12 months. Hypoglycemia was observed in almost one-third of patients, and gastrointestinal symptoms were the most common AE observed as a result of adding GLP-1 RAs. More studies are needed to better evaluate the durability and cost benefit of GLP-1 RAs, especially in patients with high insulin requirements.
Acknowledgments
This material is the result of work supported with resources and facilities at Veteran Health Indiana in Indianapolis. Study data were collected and managed using REDCap electronic data capture tools hosted at Veteran Health Indiana. The authors also acknowledge George Eckert for his assistance with data analysis.
In 2019, diabetes mellitus (DM) was the seventh leading cause of death in the United States, and currently, about 11% of the American population has a DM diagnosis.1 Most have a diagnosis of type 2 diabetes (T2DM), which has a strong genetic predisposition, and the risk of developing T2DM increases with age, obesity, and lack of physical activity.1,2 Nearly one-quarter of veterans have a diagnosis of DM, and DM is the leading cause of comorbidities, such as blindness, end-stage renal disease, and amputation for patients receiving care from the Veterans Health Administration (VHA).2 The elevated incidence of DM in the veteran population is attributed to a variety of factors, including exposure to herbicides, such as Agent Orange, advanced age, increased risk of obesity, and limited access to high-quality food.3
After diagnosis, both the American Diabetes Association (ADA) and the American Association of Clinical Endocrinologists and American College of Endocrinology (AACE/ACE) emphasize the appropriate use of lifestyle management and pharmacologic therapy for DM care. The use of pharmacologic agents (oral medications, insulin, or noninsulin injectables) is often determined by efficacy, cost, potential adverse effects (AEs), and patient factors and comorbidities.4,5
The initial recommendation for pharmacologic treatment for T2DM differs slightly between expert guidelines. The ADA and AACE/ACE recommend any of the following as initial monotherapy, listed in order to represent a hierarchy of usage: metformin, glucagon-like peptide-1 receptor agonists (GLP-1 RAs), sodium-glucose cotransporter 2 (SGLT-2) inhibitors, or dipeptidyl peptidase-4 (DPP-4) inhibitors, with the first 3 agents carrying the strongest recommendations.4,5 For patients with established atherosclerotic cardiovascular disease (CVD), chronic kidney disease, or heart failure, it is recommended to start a long-acting GLP-1 RA or SGLT-2 inhibitor. For patients with T2DM and hemoglobin A1c (HbA1c) between 7.5% and 9.0% at diagnosis, the AACE/ACE recommend initiation of dual therapy using metformin alongside another first-line agent and recommend the addition of another antidiabetic agent if glycemic goals are not met after regular follow-up. AACE/ACE recommend the consideration of insulin therapy in symptomatic patients with HbA1c > 9.0%.5 In contrast, the ADA recommends metformin as first-line therapy for all patients with T2DM and recommends dual therapy using metformin and another preferred agent (selection based on comorbidities) when HbA1c is 1.5% to 2% above target. The ADA recommends the consideration of insulin with HbA1c > 10% or with evidence of ongoing catabolism or symptoms of hyperglycemia.4 There are several reasons why insulin may be initiated prior to GLP-1 RAs, including profound hyperglycemia at time of diagnosis or implementation of insulin agents prior to commercial availability of GLP-1 RA.
GLP-1 RAs are analogs of the hormone incretin, which increases glucose-dependent insulin secretion, decreases postprandial glucagon secretion, increases satiety, and slows gastric emptying.6,7 When used in combination with noninsulin agents, GLP-1 RAs have demonstrated HbA1c reductions of 0.5% to 1.5%.8 The use of GLP-1 RAs with basal insulin also has been studied extensively.6,8-10 When the combination of GLP-1 RAs and basal insulin was compared with basal/bolus insulin regimens, the use of the GLP-1 RAs resulted in lower HbA1c levels and lower incidence of hypoglycemia.6,9 Data have demonstrated the complementary mechanisms of using basal insulin and GLP 1 RAs in decreasing HbA1c levels, insulin requirements, and weight compared with using basal insulin monotherapy and basal/bolus combinations.6,9-13 Moreover, 3 GLP-1 RA medications currently on the market (liraglutide, dulaglutide, and semaglutide) have displayed cardiovascular and renal benefits, further supporting the use of these medications.2,5
Despite these benefits, GLP-1 RAs may have bothersome AEs and are associated with a high cost.6 In addition, some studies have found that as the length of therapy increases, the positive effects of these agents may diminish.9,11 In one study, which looked at the impact of the addition of exenatide to patients taking basal or basal/bolus insulin regimens, mean changes in weight were −2.4 kg at 0 to 6 months, −4.3 kg at 6 to 12 months, −6.2 kg at 12 to 18 months, and −5.5 kg at 18 to 27 months. After 18 months, an increase in weight was observed, but the increase remained lower than baseline.11 Another study, conducted over 12 months, found no significant decrease in weight or total daily dose (TDD) of insulin when exenatide or liraglutide were added to various insulin regimens (basal or basal/bolus).13 To date, minimal published data exist regarding the addition of newer GLP-1 RAs and the long-term use of these agents beyond 12 months in patients taking basal/bolus insulin regimens. The primary goal of this study was to evaluate the effect of adding GLP-1 RAs to basal/bolus insulin regimens over a 24-month period.
Methods
This study was a retrospective, electronic health record review of all patients on basal and bolus insulin regimens who received additional therapy with a GLP-1 RA at Veteran Health Indiana in Indianapolis from September 1, 2015, to June 30, 2019. Patients meeting inclusion criteria served as their own control. The primary outcome was change in HbA1c at 3, 6, 12, 18, and 24 months after initiation of the GLP-1 RA. Secondary outcomes included change in weight and TDD of insulin at 3, 6, 12, 18, and 24 months after the initiation of the GLP-1 RAs and incidence of patient-reported or laboratory-confirmed hypoglycemia and other AEs.
Patients were included if they were aged ≥ 18 years with a diagnosis of T2DM, had concomitant prescriptions for both a basal insulin (glargine, detemir, or NPH) and a bolus insulin (aspart, lispro, or regular) before receiving add-on therapy with a GLP-1 RA (exenatide, liraglutide, albiglutide, lixisenatide, dulaglutide, or semaglutide) from September 1, 2015, to June 30, 2019, and had baseline and subsequent HbA1c measurements available in the electronic health record. Patients were excluded if they had a diagnosis of type 1 DM (T1DM), were followed by an outside clinician for DM care, or if the GLP-1 RA was discontinued before subsequent HbA1c measurement. The study protocol was approved by the Research and Development Office of Veteran Health Indiana, and the project was deemed exempt from review by the Indiana University Institutional Review Board due to the retrospective nature of the study.
Data analysis was performed using Excel. Change from baseline for each interval was computed, and 1 sample t tests (2-tailed) compared change from baseline to no change. Due to the disparity in the number of patients with data available at each of the time intervals, a mean plot was presented for each group of patients within each interval, allowing mean changes in individual groups to be observed over time.
Results
One hundred twenty-three subjects met inclusion criteria; 16 patients were excluded due to GLP-1 RA discontinuation before follow-up measurement of HbA1c; 14 were excluded due to patients being managed by a clinician outside of the facility; 1 patient was excluded for lack of documentation regarding baseline and subsequent insulin doses. Ninety-two patient charts were reviewed. Participants had a mean age of 64 years, 95% were male, and 89% were White. Mean baseline HbA1c was 9.2%, mean body mass index was 38.9, and the mean TDD of insulin was 184 units.
Since some patients switched between GLP-1 RAs throughout the study and there was variation in timing of laboratory and clinic follow-up,
Discussion
Adding a GLP-1 RA to basal/bolus insulin regimens was associated with a statistically significant decrease in HbA1c at each time point through 18 months. The greatest improvement in glycemic control from baseline was seen at 3 months, with improvements in HbA1c diminishing at each subsequent period. The study also demonstrated a significant decrease in weight at each time point through 18 months. The greatest decrease in weight was observed at both 6 and 12 months. Statistically significant decreases in TDD were observed at 3, 6, and 12 months. Insulin changes after 12 months were not found to be statistically significant.
Few studies have previously evaluated the use of GLP-1 RAs in patients with T2DM who are already taking basal/bolus insulin regimens. Gyorffy and colleagues reported significant improvements in glycemic control at 3 and 6 months in a sample of 54 patients taking basal/bolus insulin when liraglutide or exenatide was added, although statistical significance was not found at the final 12-month time point.13 That study also found a significant decrease in weight at 6 months; however there was not a significant reduction in weight at both 3 and 12 months of GLP-1 RA therapy. There was not a significant decrease in TDD at any of the collected time points. Nonetheless, Gyorffy and colleagues concluded that reduction in TDD leveled off after 12 months, which is consistent with this study’s findings. The small size of the study may have limited the ability to detect statistical significance; however, this study was conducted in a population that was racially diverse and included a higher proportion of women, though average age was similar.13
Yoon and colleagues reported weight loss through 18 months, then saw weight increase, though weights did remain lower than baseline. The study also showed no significant change in TDD of insulin after 12 months of concomitant exenatide and insulin therapy.11 Although these results mirror the outcomes observed in this study, Yoon and colleagues did not differentiate results between basal and basal/bolus insulin groups.11 Seino and colleagues observed no significant change in weight after 36 weeks of GLP-1 RA therapy in Japanese patients when used with basal and basal/bolus insulin regimens. Despite the consideration that the population in the study was not overweight (mean body mass index was 25.6), the results of these studies support the idea that effects of GLP-1 RAs on weight and TDD may diminish over time.14
Within the VHA, GLP-1 RAs are nonformulary medications. Patients must meet certain criteria in order to be approved for these agents, which may include diagnosis of CVD, renal disease, or failure to reach glycemic control with the use of oral agents or insulin. Therefore, participants of this study represent a particular subset of VHA patients, many of whom may have been selected for consideration due to long-standing or uncontrolled T2DM and failure of previous therapies. The baseline demographics support this idea, given poor glycemic control at baseline and high insulin requirements. Once approved for GLP-1 RA therapy, semaglutide is currently the preferred agent within the VHA, with other agents available for select considerations. It should be noted that albiglutide, which was the primary agent selected for some of the patients included in this study, was removed from the market in 2017 for economic considerations.15 In the case for these patients, a conversion to a formulary-preferred GLP-1 RA was made.
Most of the patients included in this study (70%) were maintained on metformin from baseline throughout the study period. Fifty-seven percent of patients were taking TDD of insulin > 150 units. Considering the significant cost of concentrated insulins, the addition of GLP-1 RAs to standard insulin may prove to be beneficial from a cost standpoint. Additional research in this area may be warranted to establish more data regarding this potential benefit of GLP-1 RAs as add-on therapy.
Many adverse drug reactions were reported at different periods; however, most of these were associated with the gastrointestinal system, which is consistent with current literature, drug labeling, and the mechanism of action.16 Hypoglycemia occurred in about one-third of the participants; however, it should be noted that alone, GLP-1 RAs are not associated with a high risk of hypoglycemia. Previous studies have found that GLP-1 RA monotherapy is associated with hypoglycemia in 1.6% to 12.6% of patients.17,18 More likely, the combination of basal/bolus insulin and the GLP-1 RA’s effect on increasing insulin sensitivity through weight loss, improving glucose-dependent insulin secretion, or by decreasing appetite and therefore decreasing carbohydrate intake contributed to the hypoglycemia prevalence.
Limitations and Strengths
Limitations of this study include a small patient population and a gradual reduction in available data as time periods progressed, making even smaller sample sizes for subsequent time periods. A majority of participants were older, males and White race. This could have limited the determination of statistical significance and applicability of the results to other patient populations. Another potential limitation was the retrospective nature of the study design, which may have limited reporting of hypoglycemia and other AEs based on the documentation of the clinician.
Strengths included the study duration and the diversity of GLP-1 RAs used by participants, as the impact of many of these agents has not yet been assessed in the literature. In addition, the retrospective nature of the study allows for a more realistic representation of patient adherence, education, and motivation, which are likely different from those of patients included in prospective clinical trials.
There are no clear guidelines dictating the optimal duration of concomitant GLP-1 RA and insulin therapy; however, our study suggests that there may be continued benefits past short-term use. Also our study suggests that patients with T2DM treated with basal/bolus insulin regimens may glean additional benefit from adding GLP-1 RAs; however, further randomized, controlled studies are warranted, particularly in poorly controlled patients requiring even more aggressive treatment regimens, such as concentrated insulins.
Conclusions
In our study, adding GLP-1 RA to basal/bolus insulin was associated with a significant decrease in HbA1c from baseline through 18 months. An overall decrease in weight and TDD of insulin was observed through 24 months, but the change in weight was not significant past 18 months, and the change in insulin requirement was not significant past 12 months. Hypoglycemia was observed in almost one-third of patients, and gastrointestinal symptoms were the most common AE observed as a result of adding GLP-1 RAs. More studies are needed to better evaluate the durability and cost benefit of GLP-1 RAs, especially in patients with high insulin requirements.
Acknowledgments
This material is the result of work supported with resources and facilities at Veteran Health Indiana in Indianapolis. Study data were collected and managed using REDCap electronic data capture tools hosted at Veteran Health Indiana. The authors also acknowledge George Eckert for his assistance with data analysis.
1. American Diabetes Association. Statistics about diabetes. Accessed August 9, 2022. http://www.diabetes.org/diabetes-basics/statistics
2. US Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development. VA research on: diabetes. Updated January 15, 2021. Accessed August 9, 2022. https://www.research.va.gov/topics/diabetes.cfm
3. Federal Practitioner. Federal Health Care Data Trends 2017, Diabetes mellitus. Accessed August 9, 2022. https://www.fedprac-digital.com/federalpractitioner/data_trends_2017?pg=20#pg20
4. American Diabetes Association Professional Practice Committee. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S125-S143. doi:10.2337/dc22-S009
5. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2019 executive summary. Endocr Pract. 2019;25(1):69-100. doi:10.4158/CS-2018-0535
6. St Onge E, Miller S, Clements E, Celauro L, Barnes K. The role of glucagon-like peptide-1 receptor agonists in the treatment of type 2 diabetes. J Transl Int Med. 2017;5(2):79-89. Published 2017 Jun 30. doi:10.1515/jtim-2017-0015
7. Almandoz JP, Lingvay I, Morales J, Campos C. Switching between glucagon-like peptide-1 receptor agonists: rationale and practical guidance. Clin Diabetes. 2020;38(4):390-402. doi:10.2337/cd19-0100
8. Davies ML, Pham DQ, Drab SR. GLP1-RA add-on therapy in patients with type 2 diabetes currently on a bolus containing insulin regimen. Pharmacotherapy. 2016;36(8):893-905. doi:10.1002/phar.1792
9. Rosenstock J, Guerci B, Hanefeld M, et al. Prandial options to advance basal insulin glargine therapy: testing lixisenatide plus basal insulin versus insulin glulisine either as basal-plus or basal-bolus in type 2 diabetes: the GetGoal Duo-2 Trial Investigators. Diabetes Care. 2016;39(8):1318-1328. doi:10.2337/dc16-0014
10. Levin PA, Mersey JH, Zhou S, Bromberger LA. Clinical outcomes using long-term combination therapy with insulin glargine and exenatide in patients with type 2 diabetes mellitus. Endocr Pract. 2012;18(1):17-25. doi:10.4158/EP11097.OR
11. Yoon NM, Cavaghan MK, Brunelle RL, Roach P. Exenatide added to insulin therapy: a retrospective review of clinical practice over two years in an academic endocrinology outpatient setting. Clin Ther. 2009;31(7):1511-1523. doi:10.1016/j.clinthera.2009.07.021
12. Weissman PN, Carr MC, Ye J, et al. HARMONY 4: randomised clinical trial comparing once-weekly albiglutide and insulin glargine in patients with type 2 diabetes inadequately controlled with metformin with or without sulfonylurea. Diabetologia. 2014;57(12):2475-2484. doi:10.1007/s00125-014-3360-3
13. Gyorffy JB, Keithler AN, Wardian JL, Zarzabal LA, Rittel A, True MW. The impact of GLP-1 receptor agonists on patients with diabetes on insulin therapy. Endocr Pract. 2019;25(9):935-942. doi:10.4158/EP-2019-0023
14. Seino Y, Kaneko S, Fukuda S, et al. Combination therapy with liraglutide and insulin in Japanese patients with type 2 diabetes: a 36-week, randomized, double-blind, parallel-group trial. J Diabetes Investig. 2016;7(4):565-573. doi:10.1111/jdi.12457
15. Optum. Tanzeum (albiglutide)–drug discontinuation. Published 2017. Accessed August 15, 2022. https://professionals.optumrx.com/content/dam/optum3/professional-optumrx/news/rxnews/drug-recalls-shortages/drugwithdrawal_tanzeum_2017-0801.pdf
16. Chun JH, Butts A. Long-acting GLP-1RAs: an overview of efficacy, safety, and their role in type 2 diabetes management. JAAPA. 2020;33(8):3-18. doi:10.1097/01.JAA.0000669456.13763.bd
17. Ozempic semaglutide injection. Prescribing information. Novo Nordisk; 2022. Accessed August 9, 2022. https://www.novo-pi.com/ozempic.pdf
18. Victoza liraglutide injection. Prescribing information. Novo Nordisk; 2021. Accessed August 9, 2022. https://www.novo-pi.com/victoza.pdf
1. American Diabetes Association. Statistics about diabetes. Accessed August 9, 2022. http://www.diabetes.org/diabetes-basics/statistics
2. US Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development. VA research on: diabetes. Updated January 15, 2021. Accessed August 9, 2022. https://www.research.va.gov/topics/diabetes.cfm
3. Federal Practitioner. Federal Health Care Data Trends 2017, Diabetes mellitus. Accessed August 9, 2022. https://www.fedprac-digital.com/federalpractitioner/data_trends_2017?pg=20#pg20
4. American Diabetes Association Professional Practice Committee. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S125-S143. doi:10.2337/dc22-S009
5. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2019 executive summary. Endocr Pract. 2019;25(1):69-100. doi:10.4158/CS-2018-0535
6. St Onge E, Miller S, Clements E, Celauro L, Barnes K. The role of glucagon-like peptide-1 receptor agonists in the treatment of type 2 diabetes. J Transl Int Med. 2017;5(2):79-89. Published 2017 Jun 30. doi:10.1515/jtim-2017-0015
7. Almandoz JP, Lingvay I, Morales J, Campos C. Switching between glucagon-like peptide-1 receptor agonists: rationale and practical guidance. Clin Diabetes. 2020;38(4):390-402. doi:10.2337/cd19-0100
8. Davies ML, Pham DQ, Drab SR. GLP1-RA add-on therapy in patients with type 2 diabetes currently on a bolus containing insulin regimen. Pharmacotherapy. 2016;36(8):893-905. doi:10.1002/phar.1792
9. Rosenstock J, Guerci B, Hanefeld M, et al. Prandial options to advance basal insulin glargine therapy: testing lixisenatide plus basal insulin versus insulin glulisine either as basal-plus or basal-bolus in type 2 diabetes: the GetGoal Duo-2 Trial Investigators. Diabetes Care. 2016;39(8):1318-1328. doi:10.2337/dc16-0014
10. Levin PA, Mersey JH, Zhou S, Bromberger LA. Clinical outcomes using long-term combination therapy with insulin glargine and exenatide in patients with type 2 diabetes mellitus. Endocr Pract. 2012;18(1):17-25. doi:10.4158/EP11097.OR
11. Yoon NM, Cavaghan MK, Brunelle RL, Roach P. Exenatide added to insulin therapy: a retrospective review of clinical practice over two years in an academic endocrinology outpatient setting. Clin Ther. 2009;31(7):1511-1523. doi:10.1016/j.clinthera.2009.07.021
12. Weissman PN, Carr MC, Ye J, et al. HARMONY 4: randomised clinical trial comparing once-weekly albiglutide and insulin glargine in patients with type 2 diabetes inadequately controlled with metformin with or without sulfonylurea. Diabetologia. 2014;57(12):2475-2484. doi:10.1007/s00125-014-3360-3
13. Gyorffy JB, Keithler AN, Wardian JL, Zarzabal LA, Rittel A, True MW. The impact of GLP-1 receptor agonists on patients with diabetes on insulin therapy. Endocr Pract. 2019;25(9):935-942. doi:10.4158/EP-2019-0023
14. Seino Y, Kaneko S, Fukuda S, et al. Combination therapy with liraglutide and insulin in Japanese patients with type 2 diabetes: a 36-week, randomized, double-blind, parallel-group trial. J Diabetes Investig. 2016;7(4):565-573. doi:10.1111/jdi.12457
15. Optum. Tanzeum (albiglutide)–drug discontinuation. Published 2017. Accessed August 15, 2022. https://professionals.optumrx.com/content/dam/optum3/professional-optumrx/news/rxnews/drug-recalls-shortages/drugwithdrawal_tanzeum_2017-0801.pdf
16. Chun JH, Butts A. Long-acting GLP-1RAs: an overview of efficacy, safety, and their role in type 2 diabetes management. JAAPA. 2020;33(8):3-18. doi:10.1097/01.JAA.0000669456.13763.bd
17. Ozempic semaglutide injection. Prescribing information. Novo Nordisk; 2022. Accessed August 9, 2022. https://www.novo-pi.com/ozempic.pdf
18. Victoza liraglutide injection. Prescribing information. Novo Nordisk; 2021. Accessed August 9, 2022. https://www.novo-pi.com/victoza.pdf
Preoperative Insulin Intensification to Improve Day of Surgery Blood Glucose Control
Perioperative hyperglycemia, defined as blood glucose levels ≥ 180 mg/dL in the immediate pre- and postoperative period, is associated with increased postoperative morbidity, including infections, preoperative interventions, and in-hospital mortality.1-3 Despite being identified as a barrier to optimal perioperative glycemic control, limited evidence is available on patient or health care practitioner (HCP) adherence to preoperative insulin protocols.4-6
Background
Despite mounting evidence of the advantages of maintaining perioperative glucose levels between 80 and 180 mg/dL, available guidelines vary in their recommendations for long-acting basal insulin dosing.7-10 The Society of Ambulatory Anesthesia suggests using 100% of the prescribed evening dosage of long-acting basal insulin dose on the night before surgery in patients without a history of nocturnal or morning hypoglycemia (category 2A evidence).9 However, the revised 2016 United Kingdom National Health Service consensus guideline recommends using 80% to 100% of the prescribed evening dosage of long-acting basal insulin dose on the night before surgery.7 The 2022 American Diabetes Association references an observational study of patients with type 2 DM (T2DM) treated with evening-only, long-acting glargine insulin, indicating that the optimal basal insulin dose on the evening before surgery is about 75% of the outpatient dose.5,10 However, in a randomized, prospective open trial of patients with DM treated with evening-only long-acting basal insulin, no significant difference was noted in the target day of surgery (DOS) glucose levels among different dosing strategies on the evening before surgery.6 Presently, the optimal dose of long-acting insulin analogs on the evening before surgery is unknown.
Additionally, little is known about the other factors that influence perioperative glycemic control. Several barriers to optimal perioperative care of patients with DM have been identified, including lack of prioritization by HCPs, lack of knowledge about current evidence-based recommendations, and lack of patient information and involvement.4 To determine the effect of patient adherence to preoperative medication instructions on postoperative outcome, a cross-sectional study assessed surgical patients admitted to the postanesthetic care unit (PACU) and found that only 70% of patients with insulin-treated DM took their medications preoperatively. Additionally, 23% of nonadherent patients who omitted their medications either did not understand or forgot preoperative medication management instructions. Preoperative DM medication omission was associated with higher rates of hyperglycemia in the PACU (23.8% vs 3.6%; P = .02).11 Importantly, to our knowledge, the extent of HCP adherence to DM management protocols and the subsequent effect on DOS hyperglycemia has not been examined until now.For patients with DM treated with an evening dose of long-acting basal insulin (ie, either once-daily long-acting basal insulin in the evening or twice-daily long-acting basal insulin, both morning and evening) presenting for elective noncardiac surgery, our aim was to decrease the rate of DOS hyperglycemia from 29% (our baseline) to 15% by intensifying the dose of insulin on the evening before surgery without increasing the rate of hypoglycemia. We also sought to determine the rates of HCP adherence to our insulin protocols as well as patients’ self-reported adherence to HCP instructions over the course of this quality improvement (QI) initiative.
Quality Improvement Program
Our surgical department consists of 11 surgical subspecialties that performed approximately 4400 noncardiac surgeries in 2019. All patients undergoing elective surgery are evaluated in the preoperative clinic, which is staffed by an anesthesiology professional (attending and resident physicians, nurse practitioners, and physician assistants) and internal medicine attending physicians. At the preoperative visit, each patient is evaluated by anesthesiology; medically complex patients may also be referred to an internal medicine professional for further risk stratification and optimization before surgery.
At the preoperative clinic visit, HCPs prepare written patient instructions for the preoperative management of medications, including glucose-lowering medications, based on a DM management protocol that was implemented in 2016 for the preoperative management of insulin, noninsulin injectable agents, and oral hyperglycemic agents. According to this protocol, patients with DM treated with evening long-acting basal insulin (eg, glargine insulin) are instructed to take 50% of their usual evening dose the evening before surgery. A preoperative clinic nurse reviews the final preoperative medication instructions with the patient at the end of the clinic visit. Patients are also instructed to avoid oral intake other than water and necessary medications after midnight before surgery regardless of the time of surgery. On the DOS, the patient’s blood glucose level is measured on arrival to the presurgical area.
Our QI initiative focused only on the dose of self-administered, long-acting basal insulin on the evening before surgery. The effect of the morning of surgery long-acting insulin dose on the DOS glucose levels largely depends on the timing of surgery, which is variable; therefore, we did not target this dose for our initiative. Patients receiving intermediate-acting neutral protamine Hagedorn (NPH) insulin were excluded because our protocol does not recommend a dose reduction for NPH insulin on the evening before surgery.
We developed a comprehensive driver diagram to help elucidate the different factors contributing to DOS hyperglycemia and to guide specific QI interventions.12 Some of the identified contributors to DOS hyperglycemia, such as the length of preoperative fasting and timing of surgery, are unpredictable and were deemed difficult to address preoperatively. Other contributors to DOS hyperglycemia, such as outpatient DM management, often require interventions over several months, which is well beyond the time usually allotted for preoperative evaluation and optimization. On the other hand, immediate preoperative insulin dosing directly affects DOS glycemic control; therefore, improvement of the preoperative insulin management protocol to optimize the dosage on the evening before surgery was considered to be an achievable QI goal with the potential for decreasing the rate of DOS hyperglycemia in patients presenting for elective noncardiac surgery.
We used the Model for Understanding Success in Quality (MUSIQ) as a framework to identify key contextual factors that may affect the success of our QI project.13 Limited resource availability and difficulty with dissemination of protocol changes in the preoperative clinic were determined to be potential barriers to the successful implementation of our QI initiative. Nonetheless, senior leadership support, microsystem QI culture, QI team skills, and physician involvement supported the implementation. The revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) guidelines were followed for this study.14
Interventions
With stakeholder input from anesthesiology, internal medicine, endocrinology, and nursing, we designed an intervention to iteratively change the HCP protocol instructions for long-acting insulin dosing on the evening before surgery. In phase 1 of the study (October 1, 2018, to March 11, 2019), we obtained baseline data on the rates of DOS hyperglycemia (blood glucose ≥ 180 mg/dL) and hypoglycemia (blood glucose < 80 mg/dL), as well as patient and HCP adherence rates to our existing preoperative DM protocol. For phase 2 (March 12, 2019, to July 22, 2019), the preoperative DM management protocol was changed to increase the dose of long-acting basal insulin on the evening before surgery for patients with hemoglobin A1c (HbA1c) levels > 8% from 50% of the usual outpatient dose to 100%. Finally, in phase 3 (July 23, 2019, to March 12, 2020), the protocol was changed to increase the dose of long-acting basal insulin on the evening before surgery for patients with HbA1c levels ≤ 8% from 50% of the usual outpatient dose to 75% while sustaining the phase 2 change. Preoperative HCPs were informed of the protocol changes in person and were provided with electronic and hard copies of each new protocol.
Protocol
We used a prospective cohort design of 424 consecutive patients with DM who presented for preoperative evaluation for elective noncardiac surgery between October 1, 2018, and March 12, 2020. For the subset of 195 patients treated with an evening dose of long-acting basal insulin, we examined the effect of intensification of this preoperative basal insulin dose on DOS hyperglycemia and hypoglycemia, HCP adherence to iterative changes of the protocol, and patient adherence to HCP instructions on preoperative medication dosing. The QI project was concluded when elective surgeries were paused due to the COVID-19 pandemic.
We created a standardized preoperative data collection form that included information on the most recent HbA1c, time, dose, and type of patient-administered insulin on the evening before surgery, and DOS blood glucose level. A preoperative clinic nurse completed the standardized preoperative data collection form. The HCP’s preoperative medication instructions and the preoperative data collection forms were gathered for review and data analysis.
The primary outcome was DOS hyperglycemia (blood glucose levels ≥ 180 mg/dL). We monitored the rate of DOS hypoglycemia (blood glucose levels < 80 mg/dL) as a balancing measure to ensure safety with long-acting basal insulin intensification. Although hypoglycemia is defined as a blood glucose level < 70 mg/dL, a target glucose range of 80 mg/dL to 180 mg/dL is recommended during the perioperative period.8 Therefore, we chose a more conservative definition of hypoglycemia (blood glucose levels < 80 mg/dL) to adhere to the recommended perioperative glucose target range.
Process measures included HCP adherence to each protocol change, which was assessed by comparing written preoperative patient instructions to the current protocol. Similarly, patient adherence to HCP-recommended long-acting basal insulin dosing was assessed by comparing written preoperative patient instructions to the patient’s self-reported time and dose of long-acting basal insulin on the evening before surgery. For any discrepancy between the HCP instructions and protocol or HCP-recommended dose and patient self-reported dose of long-acting basal insulin, a detailed chart review was performed to determine the etiology.
Statistical Analysis
We used the statistical process p-control chart to assess the effect of iterative changes to the preoperative long-acting basal insulin protocol on DOS hyperglycemia. The proportion defective (rate of DOS hyperglycemia) was plotted against time to determine whether the observed variations in the rate of DOS hyperglycemia over time were attributable to random common causes or special causes because of our intervention. The lower control limit (LCL) and upper control limit (UCL) define the limits of expected outcome measures in a stable process prior to introducing changes and were set at 3 SDs from the mean to balance the likelihood of type I (false-positive) and type II (false-negative) errors. Because of the variable interval sample sizes, we used the CRITBINOM function of Microsoft Excel to calculate the exact UCL satisfying the 3 SD limits of 0.99865.15 The Shewhart rules (outliers, runs or shifts, trends, sawtooth) were used to analyze the p-control chart to identify special cause signals resulting from our interventions.16 We used the statistical process t-control chart to record the time (days) between the few occurrences of DOS hypoglycemia because cases of hypoglycemia were rare.
Ethical Consideration
The Human Research Protection Program, Associate Chief of Staff for Research and Development, and Quality, Safety, and Values department reviewed this project in accordance with the Veterans Health Administration Program Guide 1200.21 and determined that it was a nonresearch operations activity; thus, approval by an institutional review board was not needed. The authors declare no competing interests.
Patient Outcomes
We prospectively followed 424 consecutive patients with DM undergoing elective noncardiac surgery from the time of the preoperative clinic evaluation until DOS; 195 patients were on evening
A subgroup analysis of DOS glucose levels in insulin-treated patients with preoperative HbA1c levels > 8% did not demonstrate a change in the rate of
Only 7 of 424 (1.7%) patients with DM and 4 of 195 (2.1%) patients treated with evening, long-acting basal insulin had marked hyperglycemia (DOS glucose levels ≥ 300 mg/dL). Only 1 patient who was not on outpatient insulin treatment had surgery canceled for hyperglycemia.
Overall, 89% of the HCPs followed the preoperative insulin protocol. HCP adherence to the protocol decreased to 77% after the phase 2 change, often related to deviations from the protocol or when a prior version was used. By the end of phase 3, HCP adherence returned to the baseline rate (88%). Patient adherence to medication instructions was not affected by protocol changes (86% throughout the study period). Prospective data collection was briefly interrupted between January 18, 2019, and March 5, 2019, while designing our phase 2 intervention. We were unable to track the total number of eligible patients during this time, but were able to identify 8 insulin-treated patients with DM who underwent elective noncardiac surgery and included their data in phase 1.
Discussion
The management and prevention of immediate perioperative hyperglycemia and glycemic variability have attracted attention as evidence has mounted for their association with postoperative morbidity and mortality.1,2,17 Available guidelines for preventing DOS hyperglycemia vary in their recommendations for preoperative insulin management.7-10 Notably, concerns about iatrogenic hypoglycemia often hinder efforts to lower rates of DOS hyperglycemia.4 We successfully implemented an iterative intensification protocol for preoperative long-acting basal insulin doses on the evening before surgery but did not observe a lower rate of hyperglycemia. Importantly, we also did not observe a higher rate of hypoglycemia on the DOS, as observed in a previous study.5
The observational study by Demma and colleagues found that patients receiving 75% of their evening, long-acting basal insulin dose were significantly more likely to achieve target blood glucose levels of 100 to 180 mg/dL than patients receiving no insulin at all (78% vs 0%; P = .001). However, no significant difference was noted when this group was compared with patients receiving 50% of their evening, long-acting basal insulin doses (78% vs 70%; P = .56). This is more clinically pertinent as it is generally accepted that the evening, long-acting insulin dose should not be entirely withheld on the evening before surgery.5
These findings are consistent with our observation that the rate of DOS hyperglycemia did not decrease with intensification of the evening, long-acting insulin dose from 50% to 100% of the prescribed dose in patients with HbA1c levels > 8% (phase 2) and 50% to 75% of the prescribed dose in patients with HbA1c levels ≤ 8% (phase 3). In the study by Demma and colleagues, few patients presented with preoperative hypoglycemia (2.7%) but all had received 100% of their evening, long-acting basal insulin dose, suggesting a significant increase in the rate of hypoglycemia compared with patients receiving lower doses of insulin (P = .01).5 However, long-term DM control as assessed by HbA1c level was available for < 10% of the patients, making it difficult to evaluate the effect of overall DM control on the results.5 In our study, preoperative HbA1c levels were available for 99.5% of the patients and only those with HbA1c levels > 8% received 100% of their evening, long-acting insulin dose on the evening before surgery. Notably, we did not observe a higher rate of hypoglycemia in this patient population, indicating that preoperative insulin dose intensification is safe for this subgroup.
Although HCP adherence to perioperative DM management protocols has been identified as a predominant barrier to the delivery of optimal perioperative DM care, prior studies of various preoperative insulin protocols to reduce perioperative hyperglycemia have not reported HCP adherence to their insulin protocols or its effect on DOS hyperglycemia.4-6 Additionally, patient adherence to HCP instructions is a key factor identified in our driver diagram that may influence DOS hyperglycemia, a hypothesis that is supported by a prior cross-sectional study showing an increased rate of hyperglycemia in the PACU with omission of preoperative DM medication.11 In our study, patient adherence to preoperative medication management instructions was higher than reported previously and remained consistently high regardless of protocol changes, which may explain why patient adherence did not affect the rate of DOS hyperglycemia.
Although not part of our study protocol, our preoperative HCPs routinely prepare written patient instructions for the preoperative management of medications for all patients, which likely explains higher patient adherence to instructions in our study than seen in the previous study where written instructions were only encouraged.11 However, HCP adherence to the protocol decreased after our phase 2 changes and was associated with a transient increase in DOS hyperglycemia rates. The DOS hyperglycemia rates returned to baseline levels with ongoing QI efforts and education to improve HCP adherence to protocol.
Limitations
Our QI initiative had several limitations. Nearly all patients were male veterans with T2DM, and most were older (range, 50-89 years). This limits the generalizability to women, younger patients, and people with type 1 DM. Additionally, our data collection relied on completion and collection of the preoperative form by different HCPs, allowing for sampling bias if some patients with DM undergoing elective noncardiac surgery were missed. Furthermore, although we could verify HCP adherence to the preoperative DM management protocols by reviewing their written instructions, we relied on patients’ self-reported adherence to the preoperative instructions. Finally, we did not evaluate postoperative blood glucose levels because the effect of intraoperative factors such as fluid, insulin, and glucocorticoid administration on postoperative glucose levels are variable. To the best of our knowledge, no other major systematic changes occurred in the preoperative care of patients with DM during the study period.
Conclusions
The findings of our QI initiative suggest that HCP adherence to preoperative DM management protocols may be a key contributor to DOS hyperglycemia and that ensuring HCP adherence may be as important as preoperative insulin dose adjustments. To our knowledge, this is the first study to report rates of HCP adherence to preoperative DM management protocols and its effect on DOS hyperglycemia. We will focus future QI efforts on optimizing HCP adherence to preoperative DM management protocols at our institution.
Acknowledgments
We thank our endocrinology expert, Dr. Kristina Utzschneider, for her guidance in designing this improvement project and our academic research coach, Dr. Helene Starks, for her help in editing the manuscript.
1. van den Boom W, Schroeder RA, Manning MW, Setji TL, Fiestan GO, Dunson DB. Effect of A1c and glucose on postoperative mortality in noncardiac and cardiac surgeries. Diabetes Care. 2018;41(4):782-788. doi:10.2337/dc17-2232
2. Punthakee Z, Iglesias PP, Alonso-Coello P, et al. Association of preoperative glucose concentration with myocardial injury and death after non-cardiac surgery (GlucoVISION): a prospective cohort study. Lancet Diabetes Endocrinol. 2018;6(10):790-797. doi:10.1016/S2213-8587(18)30205-5
3. Kwon S, Thompson R, Dellinger P, Yanez D, Farrohki E, Flum D. Importance of perioperative glycemic control in general surgery: a report from the Surgical Care and Outcomes Assessment Program. Ann Surg. 2013;257(1):8-14. doi:10.1097/SLA.0b013e31827b6bbc
4. Hommel I, van Gurp PJ, den Broeder AA, et al. Reactive rather than proactive diabetes management in the perioperative period. Horm Metab Res. 2017;49(7):527-533. doi:10.1055/s-0043-105501
5. Demma LJ, Carlson KT, Duggan EW, Morrow JG 3rd, Umpierrez G. Effect of basal insulin dosage on blood glucose concentration in ambulatory surgery patients with type 2 diabetes. J Clin Anesth. 2017;36:184-188. doi:10.1016/j.jclinane.2016.10.003
6. Rosenblatt SI, Dukatz T, Jahn R, et al. Insulin glargine dosing before next-day surgery: comparing three strategies. J Clin Anesth. 2012;24(8):610-617. doi:10.1016/j.jclinane.2012.02.010
7. Dhatariya K, Levy N, Flanagen D, et al; Joint British Diabetes Societies for Inpatient Care. Management of adults with diabetes undergoing surgery and elective procedures: improving standards. Summary. Published 2011. Revised March 2016. Accessed October 31, 2022. https://www.diabetes.org.uk/resources-s3/2017-09/Surgical%20guideline%202015%20-%20summary%20FINAL%20amended%20Mar%202016.pdf
8. American Diabetes Association. 15. Diabetes care in the hospital: standards of medical care in diabetes–2021. Diabetes Care. 2021;44(suppl 1):S211-S220. doi:10.2337/dc21-S015
9. Joshi GP, Chung F, Vann MA, et al; Society for Ambulatory Anesthesia. Society for Ambulatory Anesthesia consensus statement on perioperative blood glucose management in diabetic patients undergoing ambulatory surgery. Anesth Analg. 2010;111(6):1378-1387. doi:10.1213/ANE.0b013e3181f9c288
10. American Diabetes Association Professional Practice Committee. 16. Diabetes care in the hospital: standards of medical care in diabetes–2022. Diabetes Care. 2021;45(suppl 1):S244-S253. doi:10.2337/dc22-S016
11. Notaras AP, Demetriou E, Galvin J, Ben-Menachem E. A cross-sectional study of preoperative medication adherence and early postoperative recovery. J Clin Anesth. 2016;35:129-135. doi:10.1016/j.jclinane.2016.07.007
12. Bennett B, Provost L. What’s your theory? Driver diagram serves as tool for building and testing theories for improvement. Quality Progress. 2015;48(7):36-43. Accessed August 31, 2022. http://www.apiweb.org/QP_whats-your-theory_201507.pdf
13. Kaplan HC, Provost LP, Froehle CM, Margolis PA. The Model for Understanding Success in Quality (MUSIQ): building a theory of context in healthcare quality improvement. BMJ Qual Saf. 2012;21(1):13-20. doi:10.1136/bmjqs-2011-000010
14. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. doi:10.1136/bmjqs-2015-004411
15. Duclos A, Voirin N. The p-control chart: a tool for care improvement. Int J Qual Health Care. 2010;22(5):402-407. doi:10.1093/intqhc/mzq037
16. Cheung YY, Jung B, Sohn JH, Ogrinc G. Quality initiatives: statistical control charts: simplifying the analysis of data for quality improvement. Radiographics. 2012;32(7):2113-2126. doi:10.1148/rg.327125713
17. Simha V, Shah P. Perioperative glucose control in patients with diabetes undergoing elective surgery. JAMA. 2019;321(4):399. doi:10.1001/jama.2018.20922
Perioperative hyperglycemia, defined as blood glucose levels ≥ 180 mg/dL in the immediate pre- and postoperative period, is associated with increased postoperative morbidity, including infections, preoperative interventions, and in-hospital mortality.1-3 Despite being identified as a barrier to optimal perioperative glycemic control, limited evidence is available on patient or health care practitioner (HCP) adherence to preoperative insulin protocols.4-6
Background
Despite mounting evidence of the advantages of maintaining perioperative glucose levels between 80 and 180 mg/dL, available guidelines vary in their recommendations for long-acting basal insulin dosing.7-10 The Society of Ambulatory Anesthesia suggests using 100% of the prescribed evening dosage of long-acting basal insulin dose on the night before surgery in patients without a history of nocturnal or morning hypoglycemia (category 2A evidence).9 However, the revised 2016 United Kingdom National Health Service consensus guideline recommends using 80% to 100% of the prescribed evening dosage of long-acting basal insulin dose on the night before surgery.7 The 2022 American Diabetes Association references an observational study of patients with type 2 DM (T2DM) treated with evening-only, long-acting glargine insulin, indicating that the optimal basal insulin dose on the evening before surgery is about 75% of the outpatient dose.5,10 However, in a randomized, prospective open trial of patients with DM treated with evening-only long-acting basal insulin, no significant difference was noted in the target day of surgery (DOS) glucose levels among different dosing strategies on the evening before surgery.6 Presently, the optimal dose of long-acting insulin analogs on the evening before surgery is unknown.
Additionally, little is known about the other factors that influence perioperative glycemic control. Several barriers to optimal perioperative care of patients with DM have been identified, including lack of prioritization by HCPs, lack of knowledge about current evidence-based recommendations, and lack of patient information and involvement.4 To determine the effect of patient adherence to preoperative medication instructions on postoperative outcome, a cross-sectional study assessed surgical patients admitted to the postanesthetic care unit (PACU) and found that only 70% of patients with insulin-treated DM took their medications preoperatively. Additionally, 23% of nonadherent patients who omitted their medications either did not understand or forgot preoperative medication management instructions. Preoperative DM medication omission was associated with higher rates of hyperglycemia in the PACU (23.8% vs 3.6%; P = .02).11 Importantly, to our knowledge, the extent of HCP adherence to DM management protocols and the subsequent effect on DOS hyperglycemia has not been examined until now.For patients with DM treated with an evening dose of long-acting basal insulin (ie, either once-daily long-acting basal insulin in the evening or twice-daily long-acting basal insulin, both morning and evening) presenting for elective noncardiac surgery, our aim was to decrease the rate of DOS hyperglycemia from 29% (our baseline) to 15% by intensifying the dose of insulin on the evening before surgery without increasing the rate of hypoglycemia. We also sought to determine the rates of HCP adherence to our insulin protocols as well as patients’ self-reported adherence to HCP instructions over the course of this quality improvement (QI) initiative.
Quality Improvement Program
Our surgical department consists of 11 surgical subspecialties that performed approximately 4400 noncardiac surgeries in 2019. All patients undergoing elective surgery are evaluated in the preoperative clinic, which is staffed by an anesthesiology professional (attending and resident physicians, nurse practitioners, and physician assistants) and internal medicine attending physicians. At the preoperative visit, each patient is evaluated by anesthesiology; medically complex patients may also be referred to an internal medicine professional for further risk stratification and optimization before surgery.
At the preoperative clinic visit, HCPs prepare written patient instructions for the preoperative management of medications, including glucose-lowering medications, based on a DM management protocol that was implemented in 2016 for the preoperative management of insulin, noninsulin injectable agents, and oral hyperglycemic agents. According to this protocol, patients with DM treated with evening long-acting basal insulin (eg, glargine insulin) are instructed to take 50% of their usual evening dose the evening before surgery. A preoperative clinic nurse reviews the final preoperative medication instructions with the patient at the end of the clinic visit. Patients are also instructed to avoid oral intake other than water and necessary medications after midnight before surgery regardless of the time of surgery. On the DOS, the patient’s blood glucose level is measured on arrival to the presurgical area.
Our QI initiative focused only on the dose of self-administered, long-acting basal insulin on the evening before surgery. The effect of the morning of surgery long-acting insulin dose on the DOS glucose levels largely depends on the timing of surgery, which is variable; therefore, we did not target this dose for our initiative. Patients receiving intermediate-acting neutral protamine Hagedorn (NPH) insulin were excluded because our protocol does not recommend a dose reduction for NPH insulin on the evening before surgery.
We developed a comprehensive driver diagram to help elucidate the different factors contributing to DOS hyperglycemia and to guide specific QI interventions.12 Some of the identified contributors to DOS hyperglycemia, such as the length of preoperative fasting and timing of surgery, are unpredictable and were deemed difficult to address preoperatively. Other contributors to DOS hyperglycemia, such as outpatient DM management, often require interventions over several months, which is well beyond the time usually allotted for preoperative evaluation and optimization. On the other hand, immediate preoperative insulin dosing directly affects DOS glycemic control; therefore, improvement of the preoperative insulin management protocol to optimize the dosage on the evening before surgery was considered to be an achievable QI goal with the potential for decreasing the rate of DOS hyperglycemia in patients presenting for elective noncardiac surgery.
We used the Model for Understanding Success in Quality (MUSIQ) as a framework to identify key contextual factors that may affect the success of our QI project.13 Limited resource availability and difficulty with dissemination of protocol changes in the preoperative clinic were determined to be potential barriers to the successful implementation of our QI initiative. Nonetheless, senior leadership support, microsystem QI culture, QI team skills, and physician involvement supported the implementation. The revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) guidelines were followed for this study.14
Interventions
With stakeholder input from anesthesiology, internal medicine, endocrinology, and nursing, we designed an intervention to iteratively change the HCP protocol instructions for long-acting insulin dosing on the evening before surgery. In phase 1 of the study (October 1, 2018, to March 11, 2019), we obtained baseline data on the rates of DOS hyperglycemia (blood glucose ≥ 180 mg/dL) and hypoglycemia (blood glucose < 80 mg/dL), as well as patient and HCP adherence rates to our existing preoperative DM protocol. For phase 2 (March 12, 2019, to July 22, 2019), the preoperative DM management protocol was changed to increase the dose of long-acting basal insulin on the evening before surgery for patients with hemoglobin A1c (HbA1c) levels > 8% from 50% of the usual outpatient dose to 100%. Finally, in phase 3 (July 23, 2019, to March 12, 2020), the protocol was changed to increase the dose of long-acting basal insulin on the evening before surgery for patients with HbA1c levels ≤ 8% from 50% of the usual outpatient dose to 75% while sustaining the phase 2 change. Preoperative HCPs were informed of the protocol changes in person and were provided with electronic and hard copies of each new protocol.
Protocol
We used a prospective cohort design of 424 consecutive patients with DM who presented for preoperative evaluation for elective noncardiac surgery between October 1, 2018, and March 12, 2020. For the subset of 195 patients treated with an evening dose of long-acting basal insulin, we examined the effect of intensification of this preoperative basal insulin dose on DOS hyperglycemia and hypoglycemia, HCP adherence to iterative changes of the protocol, and patient adherence to HCP instructions on preoperative medication dosing. The QI project was concluded when elective surgeries were paused due to the COVID-19 pandemic.
We created a standardized preoperative data collection form that included information on the most recent HbA1c, time, dose, and type of patient-administered insulin on the evening before surgery, and DOS blood glucose level. A preoperative clinic nurse completed the standardized preoperative data collection form. The HCP’s preoperative medication instructions and the preoperative data collection forms were gathered for review and data analysis.
The primary outcome was DOS hyperglycemia (blood glucose levels ≥ 180 mg/dL). We monitored the rate of DOS hypoglycemia (blood glucose levels < 80 mg/dL) as a balancing measure to ensure safety with long-acting basal insulin intensification. Although hypoglycemia is defined as a blood glucose level < 70 mg/dL, a target glucose range of 80 mg/dL to 180 mg/dL is recommended during the perioperative period.8 Therefore, we chose a more conservative definition of hypoglycemia (blood glucose levels < 80 mg/dL) to adhere to the recommended perioperative glucose target range.
Process measures included HCP adherence to each protocol change, which was assessed by comparing written preoperative patient instructions to the current protocol. Similarly, patient adherence to HCP-recommended long-acting basal insulin dosing was assessed by comparing written preoperative patient instructions to the patient’s self-reported time and dose of long-acting basal insulin on the evening before surgery. For any discrepancy between the HCP instructions and protocol or HCP-recommended dose and patient self-reported dose of long-acting basal insulin, a detailed chart review was performed to determine the etiology.
Statistical Analysis
We used the statistical process p-control chart to assess the effect of iterative changes to the preoperative long-acting basal insulin protocol on DOS hyperglycemia. The proportion defective (rate of DOS hyperglycemia) was plotted against time to determine whether the observed variations in the rate of DOS hyperglycemia over time were attributable to random common causes or special causes because of our intervention. The lower control limit (LCL) and upper control limit (UCL) define the limits of expected outcome measures in a stable process prior to introducing changes and were set at 3 SDs from the mean to balance the likelihood of type I (false-positive) and type II (false-negative) errors. Because of the variable interval sample sizes, we used the CRITBINOM function of Microsoft Excel to calculate the exact UCL satisfying the 3 SD limits of 0.99865.15 The Shewhart rules (outliers, runs or shifts, trends, sawtooth) were used to analyze the p-control chart to identify special cause signals resulting from our interventions.16 We used the statistical process t-control chart to record the time (days) between the few occurrences of DOS hypoglycemia because cases of hypoglycemia were rare.
Ethical Consideration
The Human Research Protection Program, Associate Chief of Staff for Research and Development, and Quality, Safety, and Values department reviewed this project in accordance with the Veterans Health Administration Program Guide 1200.21 and determined that it was a nonresearch operations activity; thus, approval by an institutional review board was not needed. The authors declare no competing interests.
Patient Outcomes
We prospectively followed 424 consecutive patients with DM undergoing elective noncardiac surgery from the time of the preoperative clinic evaluation until DOS; 195 patients were on evening
A subgroup analysis of DOS glucose levels in insulin-treated patients with preoperative HbA1c levels > 8% did not demonstrate a change in the rate of
Only 7 of 424 (1.7%) patients with DM and 4 of 195 (2.1%) patients treated with evening, long-acting basal insulin had marked hyperglycemia (DOS glucose levels ≥ 300 mg/dL). Only 1 patient who was not on outpatient insulin treatment had surgery canceled for hyperglycemia.
Overall, 89% of the HCPs followed the preoperative insulin protocol. HCP adherence to the protocol decreased to 77% after the phase 2 change, often related to deviations from the protocol or when a prior version was used. By the end of phase 3, HCP adherence returned to the baseline rate (88%). Patient adherence to medication instructions was not affected by protocol changes (86% throughout the study period). Prospective data collection was briefly interrupted between January 18, 2019, and March 5, 2019, while designing our phase 2 intervention. We were unable to track the total number of eligible patients during this time, but were able to identify 8 insulin-treated patients with DM who underwent elective noncardiac surgery and included their data in phase 1.
Discussion
The management and prevention of immediate perioperative hyperglycemia and glycemic variability have attracted attention as evidence has mounted for their association with postoperative morbidity and mortality.1,2,17 Available guidelines for preventing DOS hyperglycemia vary in their recommendations for preoperative insulin management.7-10 Notably, concerns about iatrogenic hypoglycemia often hinder efforts to lower rates of DOS hyperglycemia.4 We successfully implemented an iterative intensification protocol for preoperative long-acting basal insulin doses on the evening before surgery but did not observe a lower rate of hyperglycemia. Importantly, we also did not observe a higher rate of hypoglycemia on the DOS, as observed in a previous study.5
The observational study by Demma and colleagues found that patients receiving 75% of their evening, long-acting basal insulin dose were significantly more likely to achieve target blood glucose levels of 100 to 180 mg/dL than patients receiving no insulin at all (78% vs 0%; P = .001). However, no significant difference was noted when this group was compared with patients receiving 50% of their evening, long-acting basal insulin doses (78% vs 70%; P = .56). This is more clinically pertinent as it is generally accepted that the evening, long-acting insulin dose should not be entirely withheld on the evening before surgery.5
These findings are consistent with our observation that the rate of DOS hyperglycemia did not decrease with intensification of the evening, long-acting insulin dose from 50% to 100% of the prescribed dose in patients with HbA1c levels > 8% (phase 2) and 50% to 75% of the prescribed dose in patients with HbA1c levels ≤ 8% (phase 3). In the study by Demma and colleagues, few patients presented with preoperative hypoglycemia (2.7%) but all had received 100% of their evening, long-acting basal insulin dose, suggesting a significant increase in the rate of hypoglycemia compared with patients receiving lower doses of insulin (P = .01).5 However, long-term DM control as assessed by HbA1c level was available for < 10% of the patients, making it difficult to evaluate the effect of overall DM control on the results.5 In our study, preoperative HbA1c levels were available for 99.5% of the patients and only those with HbA1c levels > 8% received 100% of their evening, long-acting insulin dose on the evening before surgery. Notably, we did not observe a higher rate of hypoglycemia in this patient population, indicating that preoperative insulin dose intensification is safe for this subgroup.
Although HCP adherence to perioperative DM management protocols has been identified as a predominant barrier to the delivery of optimal perioperative DM care, prior studies of various preoperative insulin protocols to reduce perioperative hyperglycemia have not reported HCP adherence to their insulin protocols or its effect on DOS hyperglycemia.4-6 Additionally, patient adherence to HCP instructions is a key factor identified in our driver diagram that may influence DOS hyperglycemia, a hypothesis that is supported by a prior cross-sectional study showing an increased rate of hyperglycemia in the PACU with omission of preoperative DM medication.11 In our study, patient adherence to preoperative medication management instructions was higher than reported previously and remained consistently high regardless of protocol changes, which may explain why patient adherence did not affect the rate of DOS hyperglycemia.
Although not part of our study protocol, our preoperative HCPs routinely prepare written patient instructions for the preoperative management of medications for all patients, which likely explains higher patient adherence to instructions in our study than seen in the previous study where written instructions were only encouraged.11 However, HCP adherence to the protocol decreased after our phase 2 changes and was associated with a transient increase in DOS hyperglycemia rates. The DOS hyperglycemia rates returned to baseline levels with ongoing QI efforts and education to improve HCP adherence to protocol.
Limitations
Our QI initiative had several limitations. Nearly all patients were male veterans with T2DM, and most were older (range, 50-89 years). This limits the generalizability to women, younger patients, and people with type 1 DM. Additionally, our data collection relied on completion and collection of the preoperative form by different HCPs, allowing for sampling bias if some patients with DM undergoing elective noncardiac surgery were missed. Furthermore, although we could verify HCP adherence to the preoperative DM management protocols by reviewing their written instructions, we relied on patients’ self-reported adherence to the preoperative instructions. Finally, we did not evaluate postoperative blood glucose levels because the effect of intraoperative factors such as fluid, insulin, and glucocorticoid administration on postoperative glucose levels are variable. To the best of our knowledge, no other major systematic changes occurred in the preoperative care of patients with DM during the study period.
Conclusions
The findings of our QI initiative suggest that HCP adherence to preoperative DM management protocols may be a key contributor to DOS hyperglycemia and that ensuring HCP adherence may be as important as preoperative insulin dose adjustments. To our knowledge, this is the first study to report rates of HCP adherence to preoperative DM management protocols and its effect on DOS hyperglycemia. We will focus future QI efforts on optimizing HCP adherence to preoperative DM management protocols at our institution.
Acknowledgments
We thank our endocrinology expert, Dr. Kristina Utzschneider, for her guidance in designing this improvement project and our academic research coach, Dr. Helene Starks, for her help in editing the manuscript.
Perioperative hyperglycemia, defined as blood glucose levels ≥ 180 mg/dL in the immediate pre- and postoperative period, is associated with increased postoperative morbidity, including infections, preoperative interventions, and in-hospital mortality.1-3 Despite being identified as a barrier to optimal perioperative glycemic control, limited evidence is available on patient or health care practitioner (HCP) adherence to preoperative insulin protocols.4-6
Background
Despite mounting evidence of the advantages of maintaining perioperative glucose levels between 80 and 180 mg/dL, available guidelines vary in their recommendations for long-acting basal insulin dosing.7-10 The Society of Ambulatory Anesthesia suggests using 100% of the prescribed evening dosage of long-acting basal insulin dose on the night before surgery in patients without a history of nocturnal or morning hypoglycemia (category 2A evidence).9 However, the revised 2016 United Kingdom National Health Service consensus guideline recommends using 80% to 100% of the prescribed evening dosage of long-acting basal insulin dose on the night before surgery.7 The 2022 American Diabetes Association references an observational study of patients with type 2 DM (T2DM) treated with evening-only, long-acting glargine insulin, indicating that the optimal basal insulin dose on the evening before surgery is about 75% of the outpatient dose.5,10 However, in a randomized, prospective open trial of patients with DM treated with evening-only long-acting basal insulin, no significant difference was noted in the target day of surgery (DOS) glucose levels among different dosing strategies on the evening before surgery.6 Presently, the optimal dose of long-acting insulin analogs on the evening before surgery is unknown.
Additionally, little is known about the other factors that influence perioperative glycemic control. Several barriers to optimal perioperative care of patients with DM have been identified, including lack of prioritization by HCPs, lack of knowledge about current evidence-based recommendations, and lack of patient information and involvement.4 To determine the effect of patient adherence to preoperative medication instructions on postoperative outcome, a cross-sectional study assessed surgical patients admitted to the postanesthetic care unit (PACU) and found that only 70% of patients with insulin-treated DM took their medications preoperatively. Additionally, 23% of nonadherent patients who omitted their medications either did not understand or forgot preoperative medication management instructions. Preoperative DM medication omission was associated with higher rates of hyperglycemia in the PACU (23.8% vs 3.6%; P = .02).11 Importantly, to our knowledge, the extent of HCP adherence to DM management protocols and the subsequent effect on DOS hyperglycemia has not been examined until now.For patients with DM treated with an evening dose of long-acting basal insulin (ie, either once-daily long-acting basal insulin in the evening or twice-daily long-acting basal insulin, both morning and evening) presenting for elective noncardiac surgery, our aim was to decrease the rate of DOS hyperglycemia from 29% (our baseline) to 15% by intensifying the dose of insulin on the evening before surgery without increasing the rate of hypoglycemia. We also sought to determine the rates of HCP adherence to our insulin protocols as well as patients’ self-reported adherence to HCP instructions over the course of this quality improvement (QI) initiative.
Quality Improvement Program
Our surgical department consists of 11 surgical subspecialties that performed approximately 4400 noncardiac surgeries in 2019. All patients undergoing elective surgery are evaluated in the preoperative clinic, which is staffed by an anesthesiology professional (attending and resident physicians, nurse practitioners, and physician assistants) and internal medicine attending physicians. At the preoperative visit, each patient is evaluated by anesthesiology; medically complex patients may also be referred to an internal medicine professional for further risk stratification and optimization before surgery.
At the preoperative clinic visit, HCPs prepare written patient instructions for the preoperative management of medications, including glucose-lowering medications, based on a DM management protocol that was implemented in 2016 for the preoperative management of insulin, noninsulin injectable agents, and oral hyperglycemic agents. According to this protocol, patients with DM treated with evening long-acting basal insulin (eg, glargine insulin) are instructed to take 50% of their usual evening dose the evening before surgery. A preoperative clinic nurse reviews the final preoperative medication instructions with the patient at the end of the clinic visit. Patients are also instructed to avoid oral intake other than water and necessary medications after midnight before surgery regardless of the time of surgery. On the DOS, the patient’s blood glucose level is measured on arrival to the presurgical area.
Our QI initiative focused only on the dose of self-administered, long-acting basal insulin on the evening before surgery. The effect of the morning of surgery long-acting insulin dose on the DOS glucose levels largely depends on the timing of surgery, which is variable; therefore, we did not target this dose for our initiative. Patients receiving intermediate-acting neutral protamine Hagedorn (NPH) insulin were excluded because our protocol does not recommend a dose reduction for NPH insulin on the evening before surgery.
We developed a comprehensive driver diagram to help elucidate the different factors contributing to DOS hyperglycemia and to guide specific QI interventions.12 Some of the identified contributors to DOS hyperglycemia, such as the length of preoperative fasting and timing of surgery, are unpredictable and were deemed difficult to address preoperatively. Other contributors to DOS hyperglycemia, such as outpatient DM management, often require interventions over several months, which is well beyond the time usually allotted for preoperative evaluation and optimization. On the other hand, immediate preoperative insulin dosing directly affects DOS glycemic control; therefore, improvement of the preoperative insulin management protocol to optimize the dosage on the evening before surgery was considered to be an achievable QI goal with the potential for decreasing the rate of DOS hyperglycemia in patients presenting for elective noncardiac surgery.
We used the Model for Understanding Success in Quality (MUSIQ) as a framework to identify key contextual factors that may affect the success of our QI project.13 Limited resource availability and difficulty with dissemination of protocol changes in the preoperative clinic were determined to be potential barriers to the successful implementation of our QI initiative. Nonetheless, senior leadership support, microsystem QI culture, QI team skills, and physician involvement supported the implementation. The revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) guidelines were followed for this study.14
Interventions
With stakeholder input from anesthesiology, internal medicine, endocrinology, and nursing, we designed an intervention to iteratively change the HCP protocol instructions for long-acting insulin dosing on the evening before surgery. In phase 1 of the study (October 1, 2018, to March 11, 2019), we obtained baseline data on the rates of DOS hyperglycemia (blood glucose ≥ 180 mg/dL) and hypoglycemia (blood glucose < 80 mg/dL), as well as patient and HCP adherence rates to our existing preoperative DM protocol. For phase 2 (March 12, 2019, to July 22, 2019), the preoperative DM management protocol was changed to increase the dose of long-acting basal insulin on the evening before surgery for patients with hemoglobin A1c (HbA1c) levels > 8% from 50% of the usual outpatient dose to 100%. Finally, in phase 3 (July 23, 2019, to March 12, 2020), the protocol was changed to increase the dose of long-acting basal insulin on the evening before surgery for patients with HbA1c levels ≤ 8% from 50% of the usual outpatient dose to 75% while sustaining the phase 2 change. Preoperative HCPs were informed of the protocol changes in person and were provided with electronic and hard copies of each new protocol.
Protocol
We used a prospective cohort design of 424 consecutive patients with DM who presented for preoperative evaluation for elective noncardiac surgery between October 1, 2018, and March 12, 2020. For the subset of 195 patients treated with an evening dose of long-acting basal insulin, we examined the effect of intensification of this preoperative basal insulin dose on DOS hyperglycemia and hypoglycemia, HCP adherence to iterative changes of the protocol, and patient adherence to HCP instructions on preoperative medication dosing. The QI project was concluded when elective surgeries were paused due to the COVID-19 pandemic.
We created a standardized preoperative data collection form that included information on the most recent HbA1c, time, dose, and type of patient-administered insulin on the evening before surgery, and DOS blood glucose level. A preoperative clinic nurse completed the standardized preoperative data collection form. The HCP’s preoperative medication instructions and the preoperative data collection forms were gathered for review and data analysis.
The primary outcome was DOS hyperglycemia (blood glucose levels ≥ 180 mg/dL). We monitored the rate of DOS hypoglycemia (blood glucose levels < 80 mg/dL) as a balancing measure to ensure safety with long-acting basal insulin intensification. Although hypoglycemia is defined as a blood glucose level < 70 mg/dL, a target glucose range of 80 mg/dL to 180 mg/dL is recommended during the perioperative period.8 Therefore, we chose a more conservative definition of hypoglycemia (blood glucose levels < 80 mg/dL) to adhere to the recommended perioperative glucose target range.
Process measures included HCP adherence to each protocol change, which was assessed by comparing written preoperative patient instructions to the current protocol. Similarly, patient adherence to HCP-recommended long-acting basal insulin dosing was assessed by comparing written preoperative patient instructions to the patient’s self-reported time and dose of long-acting basal insulin on the evening before surgery. For any discrepancy between the HCP instructions and protocol or HCP-recommended dose and patient self-reported dose of long-acting basal insulin, a detailed chart review was performed to determine the etiology.
Statistical Analysis
We used the statistical process p-control chart to assess the effect of iterative changes to the preoperative long-acting basal insulin protocol on DOS hyperglycemia. The proportion defective (rate of DOS hyperglycemia) was plotted against time to determine whether the observed variations in the rate of DOS hyperglycemia over time were attributable to random common causes or special causes because of our intervention. The lower control limit (LCL) and upper control limit (UCL) define the limits of expected outcome measures in a stable process prior to introducing changes and were set at 3 SDs from the mean to balance the likelihood of type I (false-positive) and type II (false-negative) errors. Because of the variable interval sample sizes, we used the CRITBINOM function of Microsoft Excel to calculate the exact UCL satisfying the 3 SD limits of 0.99865.15 The Shewhart rules (outliers, runs or shifts, trends, sawtooth) were used to analyze the p-control chart to identify special cause signals resulting from our interventions.16 We used the statistical process t-control chart to record the time (days) between the few occurrences of DOS hypoglycemia because cases of hypoglycemia were rare.
Ethical Consideration
The Human Research Protection Program, Associate Chief of Staff for Research and Development, and Quality, Safety, and Values department reviewed this project in accordance with the Veterans Health Administration Program Guide 1200.21 and determined that it was a nonresearch operations activity; thus, approval by an institutional review board was not needed. The authors declare no competing interests.
Patient Outcomes
We prospectively followed 424 consecutive patients with DM undergoing elective noncardiac surgery from the time of the preoperative clinic evaluation until DOS; 195 patients were on evening
A subgroup analysis of DOS glucose levels in insulin-treated patients with preoperative HbA1c levels > 8% did not demonstrate a change in the rate of
Only 7 of 424 (1.7%) patients with DM and 4 of 195 (2.1%) patients treated with evening, long-acting basal insulin had marked hyperglycemia (DOS glucose levels ≥ 300 mg/dL). Only 1 patient who was not on outpatient insulin treatment had surgery canceled for hyperglycemia.
Overall, 89% of the HCPs followed the preoperative insulin protocol. HCP adherence to the protocol decreased to 77% after the phase 2 change, often related to deviations from the protocol or when a prior version was used. By the end of phase 3, HCP adherence returned to the baseline rate (88%). Patient adherence to medication instructions was not affected by protocol changes (86% throughout the study period). Prospective data collection was briefly interrupted between January 18, 2019, and March 5, 2019, while designing our phase 2 intervention. We were unable to track the total number of eligible patients during this time, but were able to identify 8 insulin-treated patients with DM who underwent elective noncardiac surgery and included their data in phase 1.
Discussion
The management and prevention of immediate perioperative hyperglycemia and glycemic variability have attracted attention as evidence has mounted for their association with postoperative morbidity and mortality.1,2,17 Available guidelines for preventing DOS hyperglycemia vary in their recommendations for preoperative insulin management.7-10 Notably, concerns about iatrogenic hypoglycemia often hinder efforts to lower rates of DOS hyperglycemia.4 We successfully implemented an iterative intensification protocol for preoperative long-acting basal insulin doses on the evening before surgery but did not observe a lower rate of hyperglycemia. Importantly, we also did not observe a higher rate of hypoglycemia on the DOS, as observed in a previous study.5
The observational study by Demma and colleagues found that patients receiving 75% of their evening, long-acting basal insulin dose were significantly more likely to achieve target blood glucose levels of 100 to 180 mg/dL than patients receiving no insulin at all (78% vs 0%; P = .001). However, no significant difference was noted when this group was compared with patients receiving 50% of their evening, long-acting basal insulin doses (78% vs 70%; P = .56). This is more clinically pertinent as it is generally accepted that the evening, long-acting insulin dose should not be entirely withheld on the evening before surgery.5
These findings are consistent with our observation that the rate of DOS hyperglycemia did not decrease with intensification of the evening, long-acting insulin dose from 50% to 100% of the prescribed dose in patients with HbA1c levels > 8% (phase 2) and 50% to 75% of the prescribed dose in patients with HbA1c levels ≤ 8% (phase 3). In the study by Demma and colleagues, few patients presented with preoperative hypoglycemia (2.7%) but all had received 100% of their evening, long-acting basal insulin dose, suggesting a significant increase in the rate of hypoglycemia compared with patients receiving lower doses of insulin (P = .01).5 However, long-term DM control as assessed by HbA1c level was available for < 10% of the patients, making it difficult to evaluate the effect of overall DM control on the results.5 In our study, preoperative HbA1c levels were available for 99.5% of the patients and only those with HbA1c levels > 8% received 100% of their evening, long-acting insulin dose on the evening before surgery. Notably, we did not observe a higher rate of hypoglycemia in this patient population, indicating that preoperative insulin dose intensification is safe for this subgroup.
Although HCP adherence to perioperative DM management protocols has been identified as a predominant barrier to the delivery of optimal perioperative DM care, prior studies of various preoperative insulin protocols to reduce perioperative hyperglycemia have not reported HCP adherence to their insulin protocols or its effect on DOS hyperglycemia.4-6 Additionally, patient adherence to HCP instructions is a key factor identified in our driver diagram that may influence DOS hyperglycemia, a hypothesis that is supported by a prior cross-sectional study showing an increased rate of hyperglycemia in the PACU with omission of preoperative DM medication.11 In our study, patient adherence to preoperative medication management instructions was higher than reported previously and remained consistently high regardless of protocol changes, which may explain why patient adherence did not affect the rate of DOS hyperglycemia.
Although not part of our study protocol, our preoperative HCPs routinely prepare written patient instructions for the preoperative management of medications for all patients, which likely explains higher patient adherence to instructions in our study than seen in the previous study where written instructions were only encouraged.11 However, HCP adherence to the protocol decreased after our phase 2 changes and was associated with a transient increase in DOS hyperglycemia rates. The DOS hyperglycemia rates returned to baseline levels with ongoing QI efforts and education to improve HCP adherence to protocol.
Limitations
Our QI initiative had several limitations. Nearly all patients were male veterans with T2DM, and most were older (range, 50-89 years). This limits the generalizability to women, younger patients, and people with type 1 DM. Additionally, our data collection relied on completion and collection of the preoperative form by different HCPs, allowing for sampling bias if some patients with DM undergoing elective noncardiac surgery were missed. Furthermore, although we could verify HCP adherence to the preoperative DM management protocols by reviewing their written instructions, we relied on patients’ self-reported adherence to the preoperative instructions. Finally, we did not evaluate postoperative blood glucose levels because the effect of intraoperative factors such as fluid, insulin, and glucocorticoid administration on postoperative glucose levels are variable. To the best of our knowledge, no other major systematic changes occurred in the preoperative care of patients with DM during the study period.
Conclusions
The findings of our QI initiative suggest that HCP adherence to preoperative DM management protocols may be a key contributor to DOS hyperglycemia and that ensuring HCP adherence may be as important as preoperative insulin dose adjustments. To our knowledge, this is the first study to report rates of HCP adherence to preoperative DM management protocols and its effect on DOS hyperglycemia. We will focus future QI efforts on optimizing HCP adherence to preoperative DM management protocols at our institution.
Acknowledgments
We thank our endocrinology expert, Dr. Kristina Utzschneider, for her guidance in designing this improvement project and our academic research coach, Dr. Helene Starks, for her help in editing the manuscript.
1. van den Boom W, Schroeder RA, Manning MW, Setji TL, Fiestan GO, Dunson DB. Effect of A1c and glucose on postoperative mortality in noncardiac and cardiac surgeries. Diabetes Care. 2018;41(4):782-788. doi:10.2337/dc17-2232
2. Punthakee Z, Iglesias PP, Alonso-Coello P, et al. Association of preoperative glucose concentration with myocardial injury and death after non-cardiac surgery (GlucoVISION): a prospective cohort study. Lancet Diabetes Endocrinol. 2018;6(10):790-797. doi:10.1016/S2213-8587(18)30205-5
3. Kwon S, Thompson R, Dellinger P, Yanez D, Farrohki E, Flum D. Importance of perioperative glycemic control in general surgery: a report from the Surgical Care and Outcomes Assessment Program. Ann Surg. 2013;257(1):8-14. doi:10.1097/SLA.0b013e31827b6bbc
4. Hommel I, van Gurp PJ, den Broeder AA, et al. Reactive rather than proactive diabetes management in the perioperative period. Horm Metab Res. 2017;49(7):527-533. doi:10.1055/s-0043-105501
5. Demma LJ, Carlson KT, Duggan EW, Morrow JG 3rd, Umpierrez G. Effect of basal insulin dosage on blood glucose concentration in ambulatory surgery patients with type 2 diabetes. J Clin Anesth. 2017;36:184-188. doi:10.1016/j.jclinane.2016.10.003
6. Rosenblatt SI, Dukatz T, Jahn R, et al. Insulin glargine dosing before next-day surgery: comparing three strategies. J Clin Anesth. 2012;24(8):610-617. doi:10.1016/j.jclinane.2012.02.010
7. Dhatariya K, Levy N, Flanagen D, et al; Joint British Diabetes Societies for Inpatient Care. Management of adults with diabetes undergoing surgery and elective procedures: improving standards. Summary. Published 2011. Revised March 2016. Accessed October 31, 2022. https://www.diabetes.org.uk/resources-s3/2017-09/Surgical%20guideline%202015%20-%20summary%20FINAL%20amended%20Mar%202016.pdf
8. American Diabetes Association. 15. Diabetes care in the hospital: standards of medical care in diabetes–2021. Diabetes Care. 2021;44(suppl 1):S211-S220. doi:10.2337/dc21-S015
9. Joshi GP, Chung F, Vann MA, et al; Society for Ambulatory Anesthesia. Society for Ambulatory Anesthesia consensus statement on perioperative blood glucose management in diabetic patients undergoing ambulatory surgery. Anesth Analg. 2010;111(6):1378-1387. doi:10.1213/ANE.0b013e3181f9c288
10. American Diabetes Association Professional Practice Committee. 16. Diabetes care in the hospital: standards of medical care in diabetes–2022. Diabetes Care. 2021;45(suppl 1):S244-S253. doi:10.2337/dc22-S016
11. Notaras AP, Demetriou E, Galvin J, Ben-Menachem E. A cross-sectional study of preoperative medication adherence and early postoperative recovery. J Clin Anesth. 2016;35:129-135. doi:10.1016/j.jclinane.2016.07.007
12. Bennett B, Provost L. What’s your theory? Driver diagram serves as tool for building and testing theories for improvement. Quality Progress. 2015;48(7):36-43. Accessed August 31, 2022. http://www.apiweb.org/QP_whats-your-theory_201507.pdf
13. Kaplan HC, Provost LP, Froehle CM, Margolis PA. The Model for Understanding Success in Quality (MUSIQ): building a theory of context in healthcare quality improvement. BMJ Qual Saf. 2012;21(1):13-20. doi:10.1136/bmjqs-2011-000010
14. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. doi:10.1136/bmjqs-2015-004411
15. Duclos A, Voirin N. The p-control chart: a tool for care improvement. Int J Qual Health Care. 2010;22(5):402-407. doi:10.1093/intqhc/mzq037
16. Cheung YY, Jung B, Sohn JH, Ogrinc G. Quality initiatives: statistical control charts: simplifying the analysis of data for quality improvement. Radiographics. 2012;32(7):2113-2126. doi:10.1148/rg.327125713
17. Simha V, Shah P. Perioperative glucose control in patients with diabetes undergoing elective surgery. JAMA. 2019;321(4):399. doi:10.1001/jama.2018.20922
1. van den Boom W, Schroeder RA, Manning MW, Setji TL, Fiestan GO, Dunson DB. Effect of A1c and glucose on postoperative mortality in noncardiac and cardiac surgeries. Diabetes Care. 2018;41(4):782-788. doi:10.2337/dc17-2232
2. Punthakee Z, Iglesias PP, Alonso-Coello P, et al. Association of preoperative glucose concentration with myocardial injury and death after non-cardiac surgery (GlucoVISION): a prospective cohort study. Lancet Diabetes Endocrinol. 2018;6(10):790-797. doi:10.1016/S2213-8587(18)30205-5
3. Kwon S, Thompson R, Dellinger P, Yanez D, Farrohki E, Flum D. Importance of perioperative glycemic control in general surgery: a report from the Surgical Care and Outcomes Assessment Program. Ann Surg. 2013;257(1):8-14. doi:10.1097/SLA.0b013e31827b6bbc
4. Hommel I, van Gurp PJ, den Broeder AA, et al. Reactive rather than proactive diabetes management in the perioperative period. Horm Metab Res. 2017;49(7):527-533. doi:10.1055/s-0043-105501
5. Demma LJ, Carlson KT, Duggan EW, Morrow JG 3rd, Umpierrez G. Effect of basal insulin dosage on blood glucose concentration in ambulatory surgery patients with type 2 diabetes. J Clin Anesth. 2017;36:184-188. doi:10.1016/j.jclinane.2016.10.003
6. Rosenblatt SI, Dukatz T, Jahn R, et al. Insulin glargine dosing before next-day surgery: comparing three strategies. J Clin Anesth. 2012;24(8):610-617. doi:10.1016/j.jclinane.2012.02.010
7. Dhatariya K, Levy N, Flanagen D, et al; Joint British Diabetes Societies for Inpatient Care. Management of adults with diabetes undergoing surgery and elective procedures: improving standards. Summary. Published 2011. Revised March 2016. Accessed October 31, 2022. https://www.diabetes.org.uk/resources-s3/2017-09/Surgical%20guideline%202015%20-%20summary%20FINAL%20amended%20Mar%202016.pdf
8. American Diabetes Association. 15. Diabetes care in the hospital: standards of medical care in diabetes–2021. Diabetes Care. 2021;44(suppl 1):S211-S220. doi:10.2337/dc21-S015
9. Joshi GP, Chung F, Vann MA, et al; Society for Ambulatory Anesthesia. Society for Ambulatory Anesthesia consensus statement on perioperative blood glucose management in diabetic patients undergoing ambulatory surgery. Anesth Analg. 2010;111(6):1378-1387. doi:10.1213/ANE.0b013e3181f9c288
10. American Diabetes Association Professional Practice Committee. 16. Diabetes care in the hospital: standards of medical care in diabetes–2022. Diabetes Care. 2021;45(suppl 1):S244-S253. doi:10.2337/dc22-S016
11. Notaras AP, Demetriou E, Galvin J, Ben-Menachem E. A cross-sectional study of preoperative medication adherence and early postoperative recovery. J Clin Anesth. 2016;35:129-135. doi:10.1016/j.jclinane.2016.07.007
12. Bennett B, Provost L. What’s your theory? Driver diagram serves as tool for building and testing theories for improvement. Quality Progress. 2015;48(7):36-43. Accessed August 31, 2022. http://www.apiweb.org/QP_whats-your-theory_201507.pdf
13. Kaplan HC, Provost LP, Froehle CM, Margolis PA. The Model for Understanding Success in Quality (MUSIQ): building a theory of context in healthcare quality improvement. BMJ Qual Saf. 2012;21(1):13-20. doi:10.1136/bmjqs-2011-000010
14. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. doi:10.1136/bmjqs-2015-004411
15. Duclos A, Voirin N. The p-control chart: a tool for care improvement. Int J Qual Health Care. 2010;22(5):402-407. doi:10.1093/intqhc/mzq037
16. Cheung YY, Jung B, Sohn JH, Ogrinc G. Quality initiatives: statistical control charts: simplifying the analysis of data for quality improvement. Radiographics. 2012;32(7):2113-2126. doi:10.1148/rg.327125713
17. Simha V, Shah P. Perioperative glucose control in patients with diabetes undergoing elective surgery. JAMA. 2019;321(4):399. doi:10.1001/jama.2018.20922
Yellow Papules and Plaques on a Child
The Diagnosis: Tuberous Xanthoma
The skin biopsy revealed a nodular collection of foam cells (quiz image [bottom]). Tuberous xanthoma was the most likely diagnosis based on the patient’s history as well as the clinical and histologic findings. Tuberous xanthomas are flat or elevated nodules in the dermis and subcutaneous tissue, commonly occurring on the skin over the joints.1 Smaller nodules and papules often are referred to as tuberoeruptive xanthomas and exist on a continuum with the larger tuberous xanthomas. All xanthomas appear histologically similar, with collections of foam cells present within the dermis.2 Foam cells form when serum lipoproteins diffuse through capillary walls, deposit in the skin or tendons, and are scavenged by monocytes.3 Tuberous xanthomas, along with tendinous, eruptive, and planar xanthomas, are the most likely to be associated with hyperlipidemia.4 They may indicate an underlying disorder of lipid metabolism, such as familial hypercholesterolemia.1,3 This is the most common cause of inheritable cardiovascular disease, with a prevalence of approximately 1:250.2 Premature cardiovascular disease risk increases 2 to 4 times in patients with familial hypercholesterolemia and tendinous xanthomas,1 illustrating that recognition of cutaneous lesions can lead to earlier diagnosis and prevention of patient morbidity and mortality.
Juvenile xanthogranuloma typically presents as smooth yellow papules or nodules on the head and neck, with a characteristic “setting-sun” appearance (ie, yellow center with an erythematous halo) on dermoscopy.5 Histologically, juvenile xanthogranulomas are composed of foam cells and a mixed lymphohistiocytic infiltrate with eosinophils within the dermis. Giant cells with a ring of nuclei surrounded by cytoplasm containing lipid vacuoles (called Touton giant cells) are characteristic (Figure 1). In contrast to tuberous xanthomas, juvenile xanthogranulomas often present within the first year of life.6
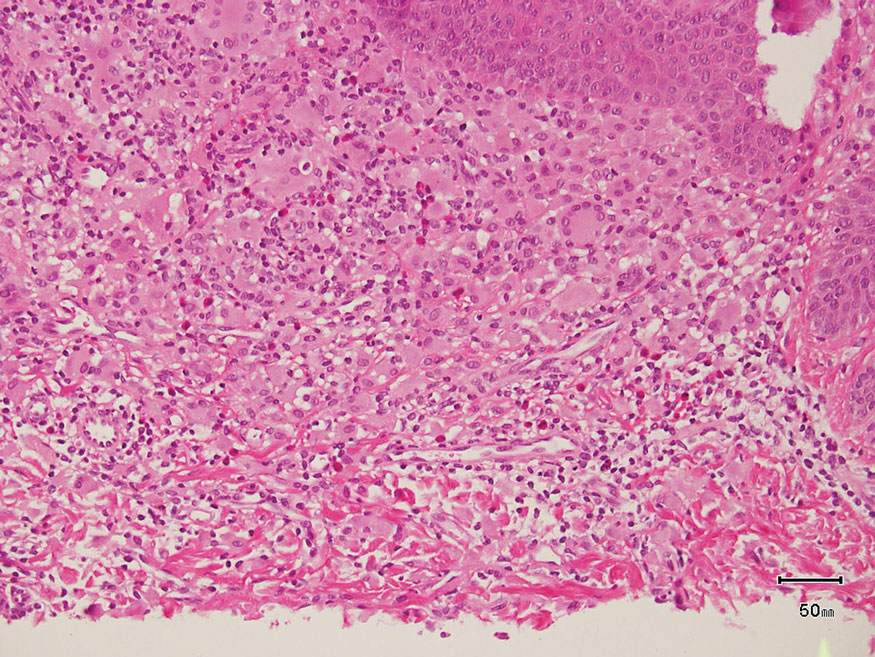
Keloid scars are more prevalent in patients with skin of color. They are characterized by eosinophilic keloidal collagen with a whorled proliferation of fibroblasts on histology (Figure 2).7 They occur spontaneously or at sites of injury and present as bluish-red or flesh-colored firm papules or nodules.8 In our patient, keloid scars were an unlikely diagnosis due to the lack of trauma and the absence of keloidal collagen on histology.
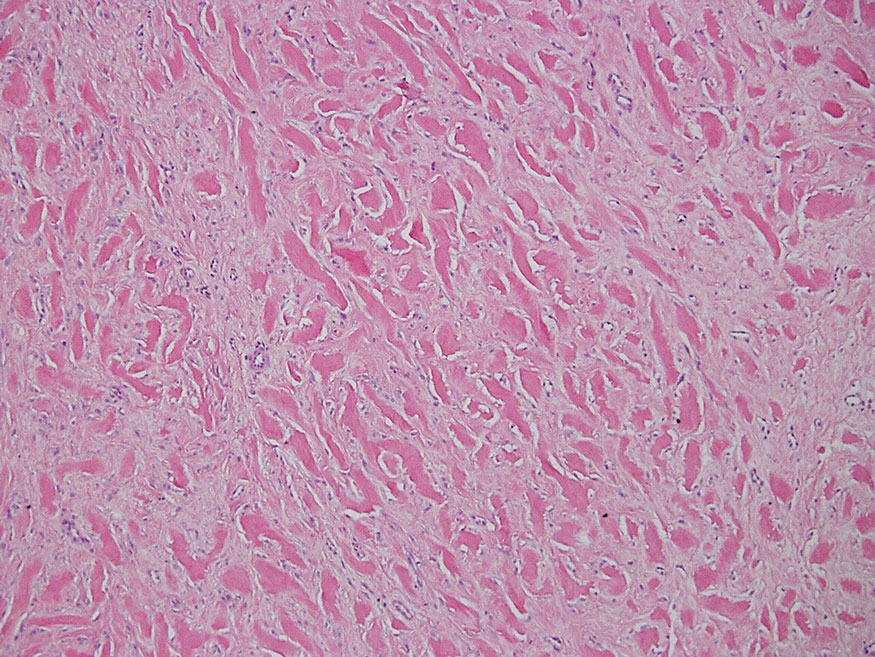
Necrobiosis lipoidica diabeticorum typically presents as an erythematous, yellow-brown, circular plaque on the anterior lower leg in patients with diabetes mellitus; it rarely occurs in children.9 Microscopy shows palisaded granulomas surrounding necrobiotic collagen arranged horizontally in a layer cake–like fashion (Figure 3).9,10 The etiology of necrobiosis lipoidica diabeticorum currently is unknown, though immune complex deposition may contribute to its pathology. It has been associated with type 1 diabetes mellitus, though severity of the lesions is not associated with extent of glycemic control.10
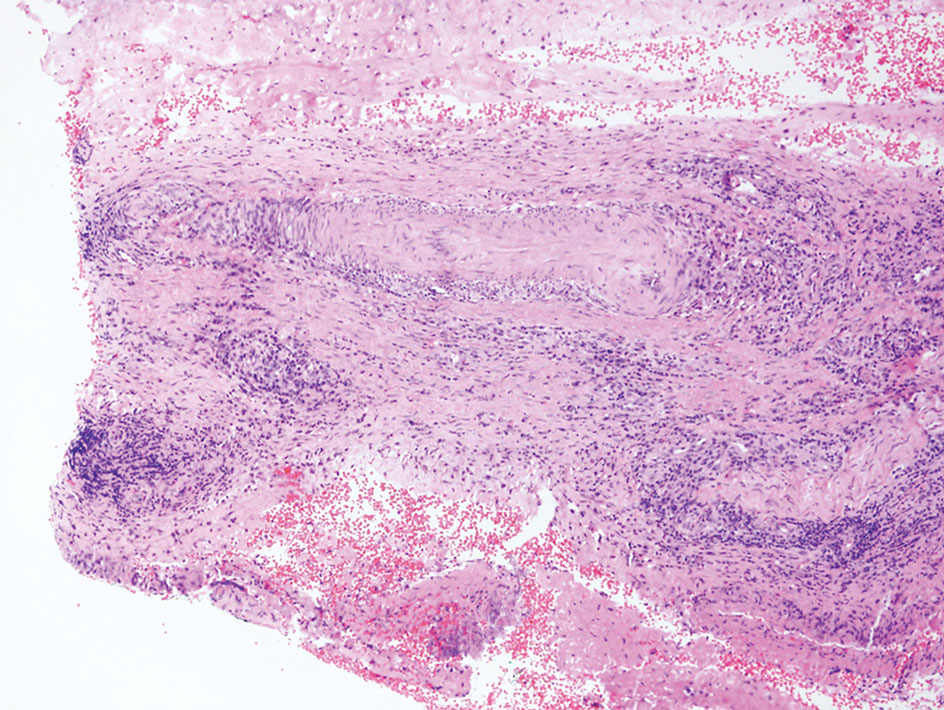
Rosai-Dorfman disease is an uncommon disorder characterized by a proliferation of histiocytes that most often presents as bilateral cervical lymphadenopathy in children and young adults but rarely can present with cutaneous lesions when extranodal involvement is present.11,12 The cutaneous form most commonly presents as red papules or nodules. On histology, the lesions exhibit a nodular dermal proliferation of histiocytes and smaller lymphocytoid cells with a marbled or starry sky–like appearance on low power (Figure 4). On higher magnification, the characteristic finding of emperipolesis can be seen.11 On immunohistochemistry, the histiocytes stain positively for CD68 and S-100. Although the pathogenesis currently is unknown, evidence of clonality indicates the disease may be related to a neoplastic process.12
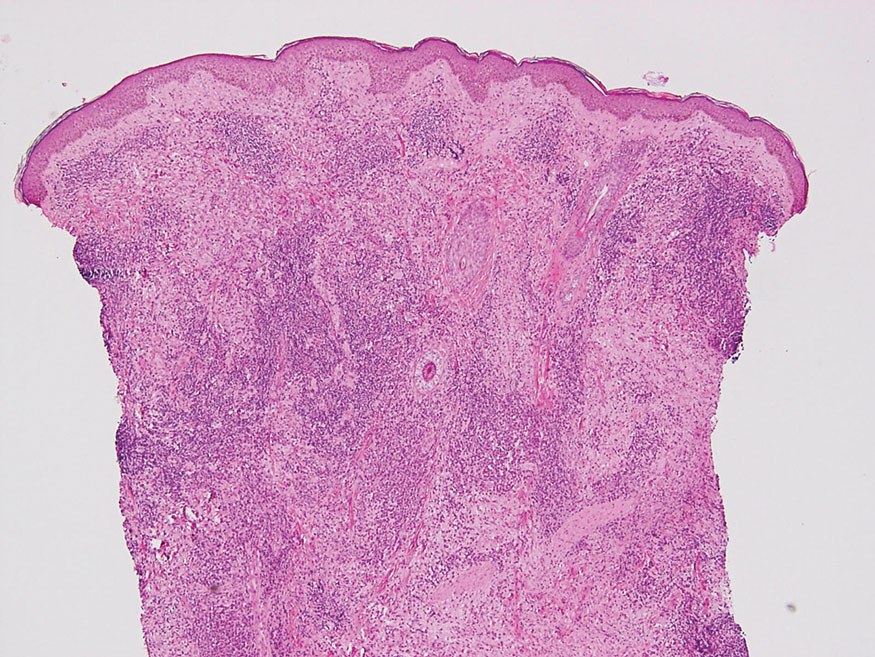
- Zak A, Zeman M, Slaby A, et al. Xanthomas: clinical and pathophysiological relations. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014;158:181-188. doi:10.5507/bp.2014.016
- Ison HE, Clarke SL, Knowles JW. Familial hypercholesterolemia. In: Adam MP, Everman DB, Mirzaa GM, et al, eds. GeneReviews. University of Washington, Seattle; 1993-2022. https://www.ncbi.nlm.nih.gov/books/NBK174884/
- Sathiyakumar V, Jones SR, Martin SS. Xanthomas and lipoprotein disorders. In: Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw Hill; 2019.
- Massangale WT. Xanthomas. In: Bolognia JL, Schaffer JV, Cerroni L, et al, eds. Dermatology. Elsevier; 2018:1634-1643.
- Collie JS, Harper CD, Fillman EP. Juvenile xanthogranuloma. StatPearls. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK526103/
- Hernández-San Martín MJ, Vargas-Mora P, Aranibar L. Juvenile xanthogranuloma: an entity with a wide clinical spectrum. Actas Dermosifiliogr (Engl Ed). 2020;111:725-733. doi:10.1016/j.ad.2020.07.004
- Lee JY, Yang C, Chao S, et al. Histopathological differential diagnosis of keloid and hypertrophic scar. Am J Dermatopathology. 2004;26:379-384.
- Wolff K, Johnson R, Saavedra AP, et al. Benign neoplasms and hyperplasias. In: Wolff K, Johnson R, Saavedra AP, et al, eds. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 8th ed. McGraw Hill; 2017:141-188.
- Bonura C, Frontino G, Rigamonti A, et al. Necrobiosis lipoidica diabeticorum: a pediatric case report. Dermatoendocrinol. 2014;6:E27790. doi:10.4161/derm.27790
- Lepe K, Riley CA, Salazar FJ. Necrobiosis lipoidica. StatPearls. StatPearls Publishing; 2021. https://www-ncbi-nlm-nih-gov.proxy.kumc.edu/books/NBK459318/
- Parrent T, Clark T, Hall D. Cutaneous Rosai-Dorfman disease. Cutis. 2012;90:237-238.
- Bruce-Brand C, Schneider JW, Schubert P. Rosai-Dorfman disease: an overview. J Clin Pathol. 2020;73:697-705. doi:10.1136/jclinpath-2020-206733
The Diagnosis: Tuberous Xanthoma
The skin biopsy revealed a nodular collection of foam cells (quiz image [bottom]). Tuberous xanthoma was the most likely diagnosis based on the patient’s history as well as the clinical and histologic findings. Tuberous xanthomas are flat or elevated nodules in the dermis and subcutaneous tissue, commonly occurring on the skin over the joints.1 Smaller nodules and papules often are referred to as tuberoeruptive xanthomas and exist on a continuum with the larger tuberous xanthomas. All xanthomas appear histologically similar, with collections of foam cells present within the dermis.2 Foam cells form when serum lipoproteins diffuse through capillary walls, deposit in the skin or tendons, and are scavenged by monocytes.3 Tuberous xanthomas, along with tendinous, eruptive, and planar xanthomas, are the most likely to be associated with hyperlipidemia.4 They may indicate an underlying disorder of lipid metabolism, such as familial hypercholesterolemia.1,3 This is the most common cause of inheritable cardiovascular disease, with a prevalence of approximately 1:250.2 Premature cardiovascular disease risk increases 2 to 4 times in patients with familial hypercholesterolemia and tendinous xanthomas,1 illustrating that recognition of cutaneous lesions can lead to earlier diagnosis and prevention of patient morbidity and mortality.
Juvenile xanthogranuloma typically presents as smooth yellow papules or nodules on the head and neck, with a characteristic “setting-sun” appearance (ie, yellow center with an erythematous halo) on dermoscopy.5 Histologically, juvenile xanthogranulomas are composed of foam cells and a mixed lymphohistiocytic infiltrate with eosinophils within the dermis. Giant cells with a ring of nuclei surrounded by cytoplasm containing lipid vacuoles (called Touton giant cells) are characteristic (Figure 1). In contrast to tuberous xanthomas, juvenile xanthogranulomas often present within the first year of life.6

Keloid scars are more prevalent in patients with skin of color. They are characterized by eosinophilic keloidal collagen with a whorled proliferation of fibroblasts on histology (Figure 2).7 They occur spontaneously or at sites of injury and present as bluish-red or flesh-colored firm papules or nodules.8 In our patient, keloid scars were an unlikely diagnosis due to the lack of trauma and the absence of keloidal collagen on histology.

Necrobiosis lipoidica diabeticorum typically presents as an erythematous, yellow-brown, circular plaque on the anterior lower leg in patients with diabetes mellitus; it rarely occurs in children.9 Microscopy shows palisaded granulomas surrounding necrobiotic collagen arranged horizontally in a layer cake–like fashion (Figure 3).9,10 The etiology of necrobiosis lipoidica diabeticorum currently is unknown, though immune complex deposition may contribute to its pathology. It has been associated with type 1 diabetes mellitus, though severity of the lesions is not associated with extent of glycemic control.10

Rosai-Dorfman disease is an uncommon disorder characterized by a proliferation of histiocytes that most often presents as bilateral cervical lymphadenopathy in children and young adults but rarely can present with cutaneous lesions when extranodal involvement is present.11,12 The cutaneous form most commonly presents as red papules or nodules. On histology, the lesions exhibit a nodular dermal proliferation of histiocytes and smaller lymphocytoid cells with a marbled or starry sky–like appearance on low power (Figure 4). On higher magnification, the characteristic finding of emperipolesis can be seen.11 On immunohistochemistry, the histiocytes stain positively for CD68 and S-100. Although the pathogenesis currently is unknown, evidence of clonality indicates the disease may be related to a neoplastic process.12

The Diagnosis: Tuberous Xanthoma
The skin biopsy revealed a nodular collection of foam cells (quiz image [bottom]). Tuberous xanthoma was the most likely diagnosis based on the patient’s history as well as the clinical and histologic findings. Tuberous xanthomas are flat or elevated nodules in the dermis and subcutaneous tissue, commonly occurring on the skin over the joints.1 Smaller nodules and papules often are referred to as tuberoeruptive xanthomas and exist on a continuum with the larger tuberous xanthomas. All xanthomas appear histologically similar, with collections of foam cells present within the dermis.2 Foam cells form when serum lipoproteins diffuse through capillary walls, deposit in the skin or tendons, and are scavenged by monocytes.3 Tuberous xanthomas, along with tendinous, eruptive, and planar xanthomas, are the most likely to be associated with hyperlipidemia.4 They may indicate an underlying disorder of lipid metabolism, such as familial hypercholesterolemia.1,3 This is the most common cause of inheritable cardiovascular disease, with a prevalence of approximately 1:250.2 Premature cardiovascular disease risk increases 2 to 4 times in patients with familial hypercholesterolemia and tendinous xanthomas,1 illustrating that recognition of cutaneous lesions can lead to earlier diagnosis and prevention of patient morbidity and mortality.
Juvenile xanthogranuloma typically presents as smooth yellow papules or nodules on the head and neck, with a characteristic “setting-sun” appearance (ie, yellow center with an erythematous halo) on dermoscopy.5 Histologically, juvenile xanthogranulomas are composed of foam cells and a mixed lymphohistiocytic infiltrate with eosinophils within the dermis. Giant cells with a ring of nuclei surrounded by cytoplasm containing lipid vacuoles (called Touton giant cells) are characteristic (Figure 1). In contrast to tuberous xanthomas, juvenile xanthogranulomas often present within the first year of life.6

Keloid scars are more prevalent in patients with skin of color. They are characterized by eosinophilic keloidal collagen with a whorled proliferation of fibroblasts on histology (Figure 2).7 They occur spontaneously or at sites of injury and present as bluish-red or flesh-colored firm papules or nodules.8 In our patient, keloid scars were an unlikely diagnosis due to the lack of trauma and the absence of keloidal collagen on histology.

Necrobiosis lipoidica diabeticorum typically presents as an erythematous, yellow-brown, circular plaque on the anterior lower leg in patients with diabetes mellitus; it rarely occurs in children.9 Microscopy shows palisaded granulomas surrounding necrobiotic collagen arranged horizontally in a layer cake–like fashion (Figure 3).9,10 The etiology of necrobiosis lipoidica diabeticorum currently is unknown, though immune complex deposition may contribute to its pathology. It has been associated with type 1 diabetes mellitus, though severity of the lesions is not associated with extent of glycemic control.10

Rosai-Dorfman disease is an uncommon disorder characterized by a proliferation of histiocytes that most often presents as bilateral cervical lymphadenopathy in children and young adults but rarely can present with cutaneous lesions when extranodal involvement is present.11,12 The cutaneous form most commonly presents as red papules or nodules. On histology, the lesions exhibit a nodular dermal proliferation of histiocytes and smaller lymphocytoid cells with a marbled or starry sky–like appearance on low power (Figure 4). On higher magnification, the characteristic finding of emperipolesis can be seen.11 On immunohistochemistry, the histiocytes stain positively for CD68 and S-100. Although the pathogenesis currently is unknown, evidence of clonality indicates the disease may be related to a neoplastic process.12

- Zak A, Zeman M, Slaby A, et al. Xanthomas: clinical and pathophysiological relations. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014;158:181-188. doi:10.5507/bp.2014.016
- Ison HE, Clarke SL, Knowles JW. Familial hypercholesterolemia. In: Adam MP, Everman DB, Mirzaa GM, et al, eds. GeneReviews. University of Washington, Seattle; 1993-2022. https://www.ncbi.nlm.nih.gov/books/NBK174884/
- Sathiyakumar V, Jones SR, Martin SS. Xanthomas and lipoprotein disorders. In: Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw Hill; 2019.
- Massangale WT. Xanthomas. In: Bolognia JL, Schaffer JV, Cerroni L, et al, eds. Dermatology. Elsevier; 2018:1634-1643.
- Collie JS, Harper CD, Fillman EP. Juvenile xanthogranuloma. StatPearls. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK526103/
- Hernández-San Martín MJ, Vargas-Mora P, Aranibar L. Juvenile xanthogranuloma: an entity with a wide clinical spectrum. Actas Dermosifiliogr (Engl Ed). 2020;111:725-733. doi:10.1016/j.ad.2020.07.004
- Lee JY, Yang C, Chao S, et al. Histopathological differential diagnosis of keloid and hypertrophic scar. Am J Dermatopathology. 2004;26:379-384.
- Wolff K, Johnson R, Saavedra AP, et al. Benign neoplasms and hyperplasias. In: Wolff K, Johnson R, Saavedra AP, et al, eds. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 8th ed. McGraw Hill; 2017:141-188.
- Bonura C, Frontino G, Rigamonti A, et al. Necrobiosis lipoidica diabeticorum: a pediatric case report. Dermatoendocrinol. 2014;6:E27790. doi:10.4161/derm.27790
- Lepe K, Riley CA, Salazar FJ. Necrobiosis lipoidica. StatPearls. StatPearls Publishing; 2021. https://www-ncbi-nlm-nih-gov.proxy.kumc.edu/books/NBK459318/
- Parrent T, Clark T, Hall D. Cutaneous Rosai-Dorfman disease. Cutis. 2012;90:237-238.
- Bruce-Brand C, Schneider JW, Schubert P. Rosai-Dorfman disease: an overview. J Clin Pathol. 2020;73:697-705. doi:10.1136/jclinpath-2020-206733
- Zak A, Zeman M, Slaby A, et al. Xanthomas: clinical and pathophysiological relations. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014;158:181-188. doi:10.5507/bp.2014.016
- Ison HE, Clarke SL, Knowles JW. Familial hypercholesterolemia. In: Adam MP, Everman DB, Mirzaa GM, et al, eds. GeneReviews. University of Washington, Seattle; 1993-2022. https://www.ncbi.nlm.nih.gov/books/NBK174884/
- Sathiyakumar V, Jones SR, Martin SS. Xanthomas and lipoprotein disorders. In: Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw Hill; 2019.
- Massangale WT. Xanthomas. In: Bolognia JL, Schaffer JV, Cerroni L, et al, eds. Dermatology. Elsevier; 2018:1634-1643.
- Collie JS, Harper CD, Fillman EP. Juvenile xanthogranuloma. StatPearls. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK526103/
- Hernández-San Martín MJ, Vargas-Mora P, Aranibar L. Juvenile xanthogranuloma: an entity with a wide clinical spectrum. Actas Dermosifiliogr (Engl Ed). 2020;111:725-733. doi:10.1016/j.ad.2020.07.004
- Lee JY, Yang C, Chao S, et al. Histopathological differential diagnosis of keloid and hypertrophic scar. Am J Dermatopathology. 2004;26:379-384.
- Wolff K, Johnson R, Saavedra AP, et al. Benign neoplasms and hyperplasias. In: Wolff K, Johnson R, Saavedra AP, et al, eds. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 8th ed. McGraw Hill; 2017:141-188.
- Bonura C, Frontino G, Rigamonti A, et al. Necrobiosis lipoidica diabeticorum: a pediatric case report. Dermatoendocrinol. 2014;6:E27790. doi:10.4161/derm.27790
- Lepe K, Riley CA, Salazar FJ. Necrobiosis lipoidica. StatPearls. StatPearls Publishing; 2021. https://www-ncbi-nlm-nih-gov.proxy.kumc.edu/books/NBK459318/
- Parrent T, Clark T, Hall D. Cutaneous Rosai-Dorfman disease. Cutis. 2012;90:237-238.
- Bruce-Brand C, Schneider JW, Schubert P. Rosai-Dorfman disease: an overview. J Clin Pathol. 2020;73:697-705. doi:10.1136/jclinpath-2020-206733
A 3-year-old girl presented with raised, firm, enlarging, asymptomatic, well-defined, subcutaneous papules, plaques, and nodules on the hands, knees, and posterior ankles of 1 year’s duration. The patient’s mother stated that the lesions began on the ankles (top), and she initially believed them to be due to friction from the child’s shoes until the more recent involvement of the knees and hands. The patient’s father, paternal grandfather, and paternal great-grandfather had a history of elevated cholesterol levels. A shave biopsy was performed (bottom).