User login
Violaceous-Purpuric Targetoid Macules and Patches With Bullae and Ulceration
The Diagnosis: Sweet Syndrome (Acute Febrile Neutrophilic Dermatosis)
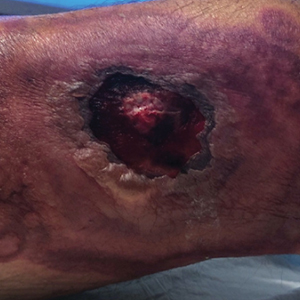
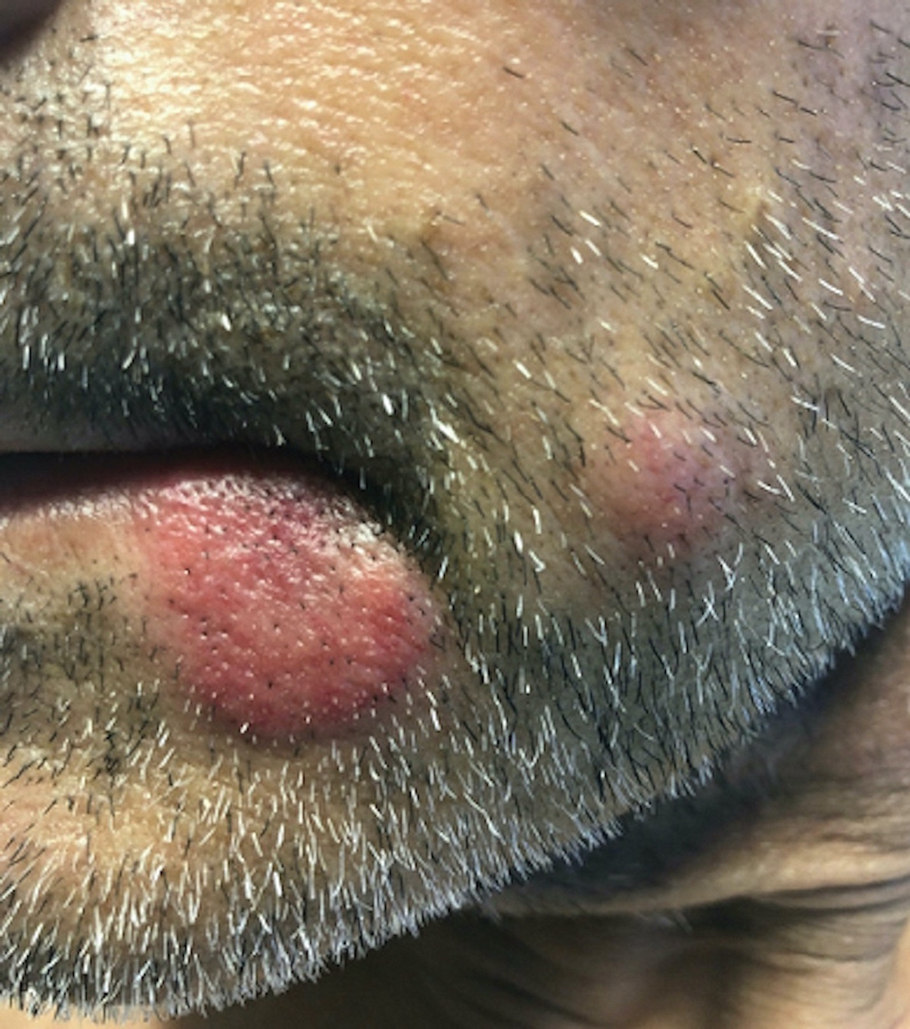
A skin biopsy of the right lower extremity demonstrated diffuse interstitial, perivascular, and periadnexal neutrophilic dermal infiltrate in the reticular dermis (Figure 1), consistent with a diagnosis of Sweet syndrome without evidence of leukemia cutis or infection. The firm erythematous papulonodules with follicular accentuation on the face (Figure 2) also were confirmed as Sweet syndrome on histopathology. Concern for leukemic transformation was confirmed with bone biopsy revealing acute myeloid leukemia (AML). Our patient began a short course of prednisone, and the cutaneous lesions improved during hospitalization; however, he was lost to follow-up.
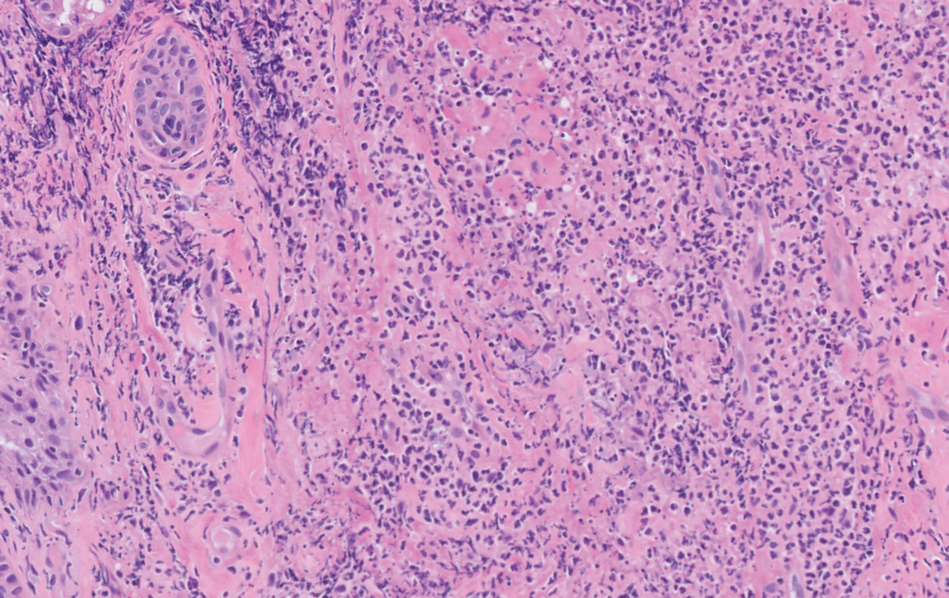
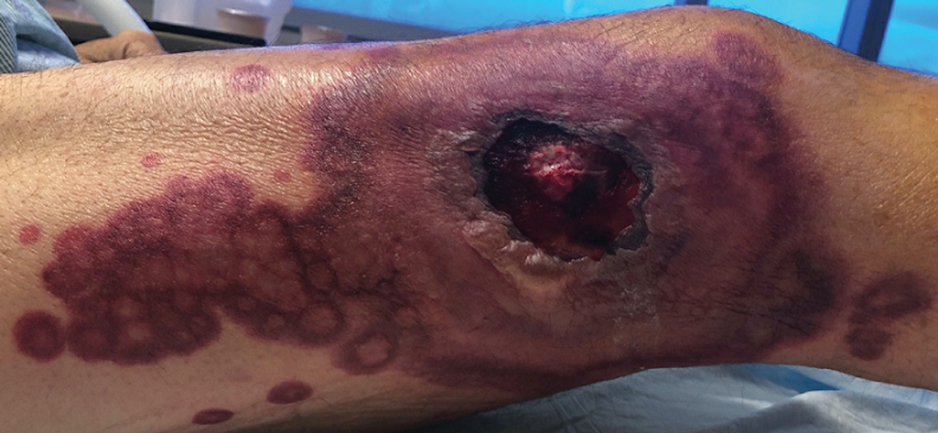
Sweet syndrome (also known as acute febrile neutrophilic dermatosis) is a rare inflammatory skin condition typically characterized by asymmetric, painful, erythematous to violaceous papules, plaques, or nodules involving the arms, face, and neck.1 It most commonly occurs in women and typically presents in patients aged 47 to 57 years. Although the pathogenesis of neutrophilic dermatoses is not completely understood, they are believed to be due to altered expression of inflammatory cytokines, irregular neutrophil function, and a genetic predisposition.2 There are 3 main categories of Sweet syndrome: classical (or idiopathic), drug induced, and malignancy associated.1 The lesions associated with Sweet syndrome vary from a few millimeters to several centimeters and may be annular or targetoid in the later stages. They also may form bullae and ulcerate. Fever, leukocytosis, and elevated acute-phase reactants also are common on presentation.1 Histopathologic analysis demonstrates an intense neutrophilic infiltrate within the reticular dermis with marked leukocytoclasia. Admixed within the neutrophil polymorphs are variable numbers of lymphocytes and histiocytes. Edema in the upper dermis also is characteristic.3 The exact pathogenesis of Sweet syndrome has yet to be elucidated but may involve a combination of cytokine dysregulation, hypersensitivity reactions, and genetics.4 Our case demonstrates 3 distinct morphologies of Sweet syndrome in a single patient, including classic edematous plaques, agminated targetoid plaques, and ulceration. Based on the clinical presentation, diagnostic workup for an undiagnosed malignancy was warranted, which confirmed AML. The malignancy-associated form of Sweet syndrome accounts for a substantial portion of cases, with approximately 21% of patients diagnosed with Sweet syndrome having an underlying malignancy, commonly a hematologic malignancy or myeloproliferative disorder with AML being the most common.1
The differential diagnosis for Sweet syndrome includes cutaneous small vessel vasculitis, which commonly presents with symmetric palpable purpura of the legs. Lesions may be round, port wine–colored plaques and even may form ulcers, vesicles, and targetoid lesions. However, skin biopsy shows polymorphonuclear infiltrate affecting postcapillary venules, fibrinoid deposits, and extravasation of red blood cells.5 Leukemia cutis describes any type of leukemia that manifests in the skin. It typically presents as violaceous or red-brown papules, nodules, and plaques most commonly on the legs. Histopathology varies by immunophenotype but generally demonstrates perivascular or periadnexal involvement or a diffuse, interstitial, or nodular infiltrate of the dermis or subcutis.6 Neutrophilic eccrine hidradenitis describes an aseptic neutrophilic infiltration around eccrine coils and glands. It may present as papules or plaques that usually are erythematous but also may be pigmented. Lesions can be asymptomatic or painful as in Sweet syndrome and are distributed proximally or on the distal extremities. Histopathologic examination demonstrates the degeneration of the eccrine gland and neutrophilic inflammatory infiltrates.7 Lastly, necrotizing fasciitis is a life-threatening infection of the deep soft tissue and fascia, classically caused by group A Streptococcus. The infected site may have erythema, tenderness, fluctuance, necrosis, and bullae.8 Although our patient had a fever, he did not display the tachycardia, hypotension, tachypnea, and rapid deterioration that is common in necrotizing fasciitis.
Sweet syndrome may present with various morphologies within the same patient. Painful, erythematous to violaceous papules, plaques, nodules, bullae, and ulcers may be seen. A workup for an underlying malignancy may be warranted based on clinical presentation. Most patients have a rapid and dramatic response to systemic corticosteroids.
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. doi:10.1186/1750-1172-2-34
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018;79:987-1006. doi:10.1016/J .JAAD.2017.11.064
- Pulido-Pérez A, Bergon-Sendin M, Sacks CA. Images in clinical medicine. N Engl J Med. 2020;16:382. doi:10.1056/NEJMicm1911025
- Marzano AV, Hilbrands L, Le ST, et al. Insights into the pathogenesis of Sweet’s syndrome. Front Immunol. 2019;10:414. doi:10.3389/fimmu.2019.00414
- Goeser MR, Laniosz V, Wetter DA. A practical approach to the diagnosis, evaluation, and management of cutaneous small-vessel vasculitis. Am J Clin Dermatol. 2014;15:299-306. doi:10.1007/s40257-014-0076-6
- Hee Cho-Vega J, Jeffrey Medeiros L, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142. doi:10.1309/WYAC YWF6NGM3WBRT
- Bachmeyer C, Aractingi S. Neutrophilic eccrine hidradenitis. Clin Dermatol. 2000;18:319-330. doi:10.1016/S0738-081X(99)00123-6
- Shimizu T, Tokuda Y. Necrotizing fasciitis. Intern Med. 2010; 49:1051-1057. doi:10.2169/internalmedicine.49.2964
The Diagnosis: Sweet Syndrome (Acute Febrile Neutrophilic Dermatosis)
A skin biopsy of the right lower extremity demonstrated diffuse interstitial, perivascular, and periadnexal neutrophilic dermal infiltrate in the reticular dermis (Figure 1), consistent with a diagnosis of Sweet syndrome without evidence of leukemia cutis or infection. The firm erythematous papulonodules with follicular accentuation on the face (Figure 2) also were confirmed as Sweet syndrome on histopathology. Concern for leukemic transformation was confirmed with bone biopsy revealing acute myeloid leukemia (AML). Our patient began a short course of prednisone, and the cutaneous lesions improved during hospitalization; however, he was lost to follow-up.

Sweet syndrome (also known as acute febrile neutrophilic dermatosis) is a rare inflammatory skin condition typically characterized by asymmetric, painful, erythematous to violaceous papules, plaques, or nodules involving the arms, face, and neck.1 It most commonly occurs in women and typically presents in patients aged 47 to 57 years. Although the pathogenesis of neutrophilic dermatoses is not completely understood, they are believed to be due to altered expression of inflammatory cytokines, irregular neutrophil function, and a genetic predisposition.2 There are 3 main categories of Sweet syndrome: classical (or idiopathic), drug induced, and malignancy associated.1 The lesions associated with Sweet syndrome vary from a few millimeters to several centimeters and may be annular or targetoid in the later stages. They also may form bullae and ulcerate. Fever, leukocytosis, and elevated acute-phase reactants also are common on presentation.1 Histopathologic analysis demonstrates an intense neutrophilic infiltrate within the reticular dermis with marked leukocytoclasia. Admixed within the neutrophil polymorphs are variable numbers of lymphocytes and histiocytes. Edema in the upper dermis also is characteristic.3 The exact pathogenesis of Sweet syndrome has yet to be elucidated but may involve a combination of cytokine dysregulation, hypersensitivity reactions, and genetics.4 Our case demonstrates 3 distinct morphologies of Sweet syndrome in a single patient, including classic edematous plaques, agminated targetoid plaques, and ulceration. Based on the clinical presentation, diagnostic workup for an undiagnosed malignancy was warranted, which confirmed AML. The malignancy-associated form of Sweet syndrome accounts for a substantial portion of cases, with approximately 21% of patients diagnosed with Sweet syndrome having an underlying malignancy, commonly a hematologic malignancy or myeloproliferative disorder with AML being the most common.1

The differential diagnosis for Sweet syndrome includes cutaneous small vessel vasculitis, which commonly presents with symmetric palpable purpura of the legs. Lesions may be round, port wine–colored plaques and even may form ulcers, vesicles, and targetoid lesions. However, skin biopsy shows polymorphonuclear infiltrate affecting postcapillary venules, fibrinoid deposits, and extravasation of red blood cells.5 Leukemia cutis describes any type of leukemia that manifests in the skin. It typically presents as violaceous or red-brown papules, nodules, and plaques most commonly on the legs. Histopathology varies by immunophenotype but generally demonstrates perivascular or periadnexal involvement or a diffuse, interstitial, or nodular infiltrate of the dermis or subcutis.6 Neutrophilic eccrine hidradenitis describes an aseptic neutrophilic infiltration around eccrine coils and glands. It may present as papules or plaques that usually are erythematous but also may be pigmented. Lesions can be asymptomatic or painful as in Sweet syndrome and are distributed proximally or on the distal extremities. Histopathologic examination demonstrates the degeneration of the eccrine gland and neutrophilic inflammatory infiltrates.7 Lastly, necrotizing fasciitis is a life-threatening infection of the deep soft tissue and fascia, classically caused by group A Streptococcus. The infected site may have erythema, tenderness, fluctuance, necrosis, and bullae.8 Although our patient had a fever, he did not display the tachycardia, hypotension, tachypnea, and rapid deterioration that is common in necrotizing fasciitis.
Sweet syndrome may present with various morphologies within the same patient. Painful, erythematous to violaceous papules, plaques, nodules, bullae, and ulcers may be seen. A workup for an underlying malignancy may be warranted based on clinical presentation. Most patients have a rapid and dramatic response to systemic corticosteroids.
The Diagnosis: Sweet Syndrome (Acute Febrile Neutrophilic Dermatosis)
A skin biopsy of the right lower extremity demonstrated diffuse interstitial, perivascular, and periadnexal neutrophilic dermal infiltrate in the reticular dermis (Figure 1), consistent with a diagnosis of Sweet syndrome without evidence of leukemia cutis or infection. The firm erythematous papulonodules with follicular accentuation on the face (Figure 2) also were confirmed as Sweet syndrome on histopathology. Concern for leukemic transformation was confirmed with bone biopsy revealing acute myeloid leukemia (AML). Our patient began a short course of prednisone, and the cutaneous lesions improved during hospitalization; however, he was lost to follow-up.

Sweet syndrome (also known as acute febrile neutrophilic dermatosis) is a rare inflammatory skin condition typically characterized by asymmetric, painful, erythematous to violaceous papules, plaques, or nodules involving the arms, face, and neck.1 It most commonly occurs in women and typically presents in patients aged 47 to 57 years. Although the pathogenesis of neutrophilic dermatoses is not completely understood, they are believed to be due to altered expression of inflammatory cytokines, irregular neutrophil function, and a genetic predisposition.2 There are 3 main categories of Sweet syndrome: classical (or idiopathic), drug induced, and malignancy associated.1 The lesions associated with Sweet syndrome vary from a few millimeters to several centimeters and may be annular or targetoid in the later stages. They also may form bullae and ulcerate. Fever, leukocytosis, and elevated acute-phase reactants also are common on presentation.1 Histopathologic analysis demonstrates an intense neutrophilic infiltrate within the reticular dermis with marked leukocytoclasia. Admixed within the neutrophil polymorphs are variable numbers of lymphocytes and histiocytes. Edema in the upper dermis also is characteristic.3 The exact pathogenesis of Sweet syndrome has yet to be elucidated but may involve a combination of cytokine dysregulation, hypersensitivity reactions, and genetics.4 Our case demonstrates 3 distinct morphologies of Sweet syndrome in a single patient, including classic edematous plaques, agminated targetoid plaques, and ulceration. Based on the clinical presentation, diagnostic workup for an undiagnosed malignancy was warranted, which confirmed AML. The malignancy-associated form of Sweet syndrome accounts for a substantial portion of cases, with approximately 21% of patients diagnosed with Sweet syndrome having an underlying malignancy, commonly a hematologic malignancy or myeloproliferative disorder with AML being the most common.1

The differential diagnosis for Sweet syndrome includes cutaneous small vessel vasculitis, which commonly presents with symmetric palpable purpura of the legs. Lesions may be round, port wine–colored plaques and even may form ulcers, vesicles, and targetoid lesions. However, skin biopsy shows polymorphonuclear infiltrate affecting postcapillary venules, fibrinoid deposits, and extravasation of red blood cells.5 Leukemia cutis describes any type of leukemia that manifests in the skin. It typically presents as violaceous or red-brown papules, nodules, and plaques most commonly on the legs. Histopathology varies by immunophenotype but generally demonstrates perivascular or periadnexal involvement or a diffuse, interstitial, or nodular infiltrate of the dermis or subcutis.6 Neutrophilic eccrine hidradenitis describes an aseptic neutrophilic infiltration around eccrine coils and glands. It may present as papules or plaques that usually are erythematous but also may be pigmented. Lesions can be asymptomatic or painful as in Sweet syndrome and are distributed proximally or on the distal extremities. Histopathologic examination demonstrates the degeneration of the eccrine gland and neutrophilic inflammatory infiltrates.7 Lastly, necrotizing fasciitis is a life-threatening infection of the deep soft tissue and fascia, classically caused by group A Streptococcus. The infected site may have erythema, tenderness, fluctuance, necrosis, and bullae.8 Although our patient had a fever, he did not display the tachycardia, hypotension, tachypnea, and rapid deterioration that is common in necrotizing fasciitis.
Sweet syndrome may present with various morphologies within the same patient. Painful, erythematous to violaceous papules, plaques, nodules, bullae, and ulcers may be seen. A workup for an underlying malignancy may be warranted based on clinical presentation. Most patients have a rapid and dramatic response to systemic corticosteroids.
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. doi:10.1186/1750-1172-2-34
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018;79:987-1006. doi:10.1016/J .JAAD.2017.11.064
- Pulido-Pérez A, Bergon-Sendin M, Sacks CA. Images in clinical medicine. N Engl J Med. 2020;16:382. doi:10.1056/NEJMicm1911025
- Marzano AV, Hilbrands L, Le ST, et al. Insights into the pathogenesis of Sweet’s syndrome. Front Immunol. 2019;10:414. doi:10.3389/fimmu.2019.00414
- Goeser MR, Laniosz V, Wetter DA. A practical approach to the diagnosis, evaluation, and management of cutaneous small-vessel vasculitis. Am J Clin Dermatol. 2014;15:299-306. doi:10.1007/s40257-014-0076-6
- Hee Cho-Vega J, Jeffrey Medeiros L, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142. doi:10.1309/WYAC YWF6NGM3WBRT
- Bachmeyer C, Aractingi S. Neutrophilic eccrine hidradenitis. Clin Dermatol. 2000;18:319-330. doi:10.1016/S0738-081X(99)00123-6
- Shimizu T, Tokuda Y. Necrotizing fasciitis. Intern Med. 2010; 49:1051-1057. doi:10.2169/internalmedicine.49.2964
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. doi:10.1186/1750-1172-2-34
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018;79:987-1006. doi:10.1016/J .JAAD.2017.11.064
- Pulido-Pérez A, Bergon-Sendin M, Sacks CA. Images in clinical medicine. N Engl J Med. 2020;16:382. doi:10.1056/NEJMicm1911025
- Marzano AV, Hilbrands L, Le ST, et al. Insights into the pathogenesis of Sweet’s syndrome. Front Immunol. 2019;10:414. doi:10.3389/fimmu.2019.00414
- Goeser MR, Laniosz V, Wetter DA. A practical approach to the diagnosis, evaluation, and management of cutaneous small-vessel vasculitis. Am J Clin Dermatol. 2014;15:299-306. doi:10.1007/s40257-014-0076-6
- Hee Cho-Vega J, Jeffrey Medeiros L, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142. doi:10.1309/WYAC YWF6NGM3WBRT
- Bachmeyer C, Aractingi S. Neutrophilic eccrine hidradenitis. Clin Dermatol. 2000;18:319-330. doi:10.1016/S0738-081X(99)00123-6
- Shimizu T, Tokuda Y. Necrotizing fasciitis. Intern Med. 2010; 49:1051-1057. doi:10.2169/internalmedicine.49.2964
A 64-year-old man with long-standing myelofibrosis presented with neutropenic fevers as well as progressive painful lesions of 3 days’ duration on the legs. A bone marrow biopsy during this hospitalization demonstrated a recent progression of the patient’s myelofibrosis to acute myeloid leukemia. Physical examination revealed round to oval, violaceous, targetoid plaques. Within a week, new erythematous and nodular lesions appeared on the right arm and left vermilion border. The lesions on the legs enlarged, formed bullae, and ulcerated.
Immunotherapy drug boosts survival in newly diagnosed ALL
NEW ORLEANS – The immunotherapy drug blinatumomab improves survival as a first-line treatment in certain younger adult patients with B-lineage acute lymphoblastic leukemia, investigators have found. The extremely expensive drug is currently Food and Drug Administration approved for B-lineage ALL in relapsed/refractory cases.
“We feel that this represents a new standard of care for these patients and should be incorporated into their standard therapy,” said lead author and hematologist Mark R. Litzow, MD, of Mayo Clinic in Rochester, Minn., in a news briefing at the annual meeting of the American Society of Hematology.
B-lineage ALL, also known as B-cell ALL, represents 75% of cases of the blood cancer in adults according to the Leukemia & Lymphoma Society. It occurs when there’s an overgrowth of immature white blood cells known as B-cell lymphoblasts. “These are the blast cells that don’t function well and cause these patients to develop infections and bleeding,” Dr. Litzow said.
Treatments include chemotherapy and stem-cell transplants. Blinatumomab, a bispecific T-cell engager molecule, is FDA approved for patients with relapsed/refractory B-lineage ALL and those with morphologic complete remission who still have measurable residual disease (MRD).
As the new study notes, some patients who undergo chemotherapy and reach remission have poor survival outcomes even when there’s no sign of MRD. “Even though we can’t find leukemia in the patients’ bone marrow, it’s still hiding there,” Dr. Litzow said.
The new phase 3, randomized trial aims to determine if adding blinatumomab (Blincyto) to first-line chemotherapy improves outcomes. The drug “brings a normal T cell, part of the immune system, in proximity to a leukemia plasma cell and kills it.”
For the study, researchers from 2013 to 2019 recruited 488 patients aged 30-70 years with newly diagnosed BCR::ABL1 negative B-lineage ALL (median age = 51). The subjects underwent chemotherapy, and then were “randomized to receive an additional four cycles of consolidation chemo or two cycles of blin [blinatumomab] for 28 days each cycle followed by three cycles of consolidation chemo, another 4-week cycle of blinatumomab (third cycle of blinatumomab) followed by an additional cycle of chemo and then a fourth cycle of blinatumomab (step 3),” the researchers reported. “Following completion of consolidation chemo +/– blin, patients were given 2.5 years of POMP [prednisone, vincristine, 6-mercaptopurine, and methotrexate] maintenance therapy timed from the start of the intensification cycle (step 4).”
There were 112 patients in each group. Among MRD-negative patients, 56 patients died – 17 in the blinatumomab arm and 39 in the control arm at the third interim efficacy analysis. At a mean follow-up of 43 months, median overall survival for patients in the blinatumomab arm was not reached vs. 71.4 months in the control group (hazard ratio, 0.42, 95% confidence interval, 0.24-0.75; P = .003).
“The patients that got blinatumomab plus chemotherapy had an improved survival over those that got the standard chemotherapy,” Dr. Litzow said.
Dr. Litzow didn’t discuss the drug’s expense in his presentation. According to a 2019 report, when a daily vial of blinatumomab cost $3,464-$3,815, a treatment course of five month-long cycles could run to $535,000. According to drugs.com, the cost now is $4,740 per vial – more than $660,000 for five cycles.
In an interview, Cleveland Clinic hematologist/oncologist Anjali Advani, MD, said the study is “groundbreaking and one of the most exciting studies to come along in the acute lymphoblastic leukemia field.”
The trial “is one of the first studies to show improvement in outcome in a randomized manner with the addition of a novel agent,” she added. “This will change our standard of care for these patients.”
The National Cancer Institute funded the trial and drug manufacturer Amgen provided the medication and support through a cooperative research and development agreement.
Dr. Litzow discloses relationships with Actinium, Jazz, Syndax, Novartis, Astellas, Amgen, Abbvie, Pluristem and Biosight. Other authors have various disclosures with multiple drugmakers. Dr. Advani discloses relationships with Amgen, Jazz, Nkarta, Taiho, Beam, GMI, Kura, Pfizer, OBI, Incyte, Kite, ImmunoGen, GlycoMimetics, SGN, MacroGenics, and Servier.
NEW ORLEANS – The immunotherapy drug blinatumomab improves survival as a first-line treatment in certain younger adult patients with B-lineage acute lymphoblastic leukemia, investigators have found. The extremely expensive drug is currently Food and Drug Administration approved for B-lineage ALL in relapsed/refractory cases.
“We feel that this represents a new standard of care for these patients and should be incorporated into their standard therapy,” said lead author and hematologist Mark R. Litzow, MD, of Mayo Clinic in Rochester, Minn., in a news briefing at the annual meeting of the American Society of Hematology.
B-lineage ALL, also known as B-cell ALL, represents 75% of cases of the blood cancer in adults according to the Leukemia & Lymphoma Society. It occurs when there’s an overgrowth of immature white blood cells known as B-cell lymphoblasts. “These are the blast cells that don’t function well and cause these patients to develop infections and bleeding,” Dr. Litzow said.
Treatments include chemotherapy and stem-cell transplants. Blinatumomab, a bispecific T-cell engager molecule, is FDA approved for patients with relapsed/refractory B-lineage ALL and those with morphologic complete remission who still have measurable residual disease (MRD).
As the new study notes, some patients who undergo chemotherapy and reach remission have poor survival outcomes even when there’s no sign of MRD. “Even though we can’t find leukemia in the patients’ bone marrow, it’s still hiding there,” Dr. Litzow said.
The new phase 3, randomized trial aims to determine if adding blinatumomab (Blincyto) to first-line chemotherapy improves outcomes. The drug “brings a normal T cell, part of the immune system, in proximity to a leukemia plasma cell and kills it.”
For the study, researchers from 2013 to 2019 recruited 488 patients aged 30-70 years with newly diagnosed BCR::ABL1 negative B-lineage ALL (median age = 51). The subjects underwent chemotherapy, and then were “randomized to receive an additional four cycles of consolidation chemo or two cycles of blin [blinatumomab] for 28 days each cycle followed by three cycles of consolidation chemo, another 4-week cycle of blinatumomab (third cycle of blinatumomab) followed by an additional cycle of chemo and then a fourth cycle of blinatumomab (step 3),” the researchers reported. “Following completion of consolidation chemo +/– blin, patients were given 2.5 years of POMP [prednisone, vincristine, 6-mercaptopurine, and methotrexate] maintenance therapy timed from the start of the intensification cycle (step 4).”
There were 112 patients in each group. Among MRD-negative patients, 56 patients died – 17 in the blinatumomab arm and 39 in the control arm at the third interim efficacy analysis. At a mean follow-up of 43 months, median overall survival for patients in the blinatumomab arm was not reached vs. 71.4 months in the control group (hazard ratio, 0.42, 95% confidence interval, 0.24-0.75; P = .003).
“The patients that got blinatumomab plus chemotherapy had an improved survival over those that got the standard chemotherapy,” Dr. Litzow said.
Dr. Litzow didn’t discuss the drug’s expense in his presentation. According to a 2019 report, when a daily vial of blinatumomab cost $3,464-$3,815, a treatment course of five month-long cycles could run to $535,000. According to drugs.com, the cost now is $4,740 per vial – more than $660,000 for five cycles.
In an interview, Cleveland Clinic hematologist/oncologist Anjali Advani, MD, said the study is “groundbreaking and one of the most exciting studies to come along in the acute lymphoblastic leukemia field.”
The trial “is one of the first studies to show improvement in outcome in a randomized manner with the addition of a novel agent,” she added. “This will change our standard of care for these patients.”
The National Cancer Institute funded the trial and drug manufacturer Amgen provided the medication and support through a cooperative research and development agreement.
Dr. Litzow discloses relationships with Actinium, Jazz, Syndax, Novartis, Astellas, Amgen, Abbvie, Pluristem and Biosight. Other authors have various disclosures with multiple drugmakers. Dr. Advani discloses relationships with Amgen, Jazz, Nkarta, Taiho, Beam, GMI, Kura, Pfizer, OBI, Incyte, Kite, ImmunoGen, GlycoMimetics, SGN, MacroGenics, and Servier.
NEW ORLEANS – The immunotherapy drug blinatumomab improves survival as a first-line treatment in certain younger adult patients with B-lineage acute lymphoblastic leukemia, investigators have found. The extremely expensive drug is currently Food and Drug Administration approved for B-lineage ALL in relapsed/refractory cases.
“We feel that this represents a new standard of care for these patients and should be incorporated into their standard therapy,” said lead author and hematologist Mark R. Litzow, MD, of Mayo Clinic in Rochester, Minn., in a news briefing at the annual meeting of the American Society of Hematology.
B-lineage ALL, also known as B-cell ALL, represents 75% of cases of the blood cancer in adults according to the Leukemia & Lymphoma Society. It occurs when there’s an overgrowth of immature white blood cells known as B-cell lymphoblasts. “These are the blast cells that don’t function well and cause these patients to develop infections and bleeding,” Dr. Litzow said.
Treatments include chemotherapy and stem-cell transplants. Blinatumomab, a bispecific T-cell engager molecule, is FDA approved for patients with relapsed/refractory B-lineage ALL and those with morphologic complete remission who still have measurable residual disease (MRD).
As the new study notes, some patients who undergo chemotherapy and reach remission have poor survival outcomes even when there’s no sign of MRD. “Even though we can’t find leukemia in the patients’ bone marrow, it’s still hiding there,” Dr. Litzow said.
The new phase 3, randomized trial aims to determine if adding blinatumomab (Blincyto) to first-line chemotherapy improves outcomes. The drug “brings a normal T cell, part of the immune system, in proximity to a leukemia plasma cell and kills it.”
For the study, researchers from 2013 to 2019 recruited 488 patients aged 30-70 years with newly diagnosed BCR::ABL1 negative B-lineage ALL (median age = 51). The subjects underwent chemotherapy, and then were “randomized to receive an additional four cycles of consolidation chemo or two cycles of blin [blinatumomab] for 28 days each cycle followed by three cycles of consolidation chemo, another 4-week cycle of blinatumomab (third cycle of blinatumomab) followed by an additional cycle of chemo and then a fourth cycle of blinatumomab (step 3),” the researchers reported. “Following completion of consolidation chemo +/– blin, patients were given 2.5 years of POMP [prednisone, vincristine, 6-mercaptopurine, and methotrexate] maintenance therapy timed from the start of the intensification cycle (step 4).”
There were 112 patients in each group. Among MRD-negative patients, 56 patients died – 17 in the blinatumomab arm and 39 in the control arm at the third interim efficacy analysis. At a mean follow-up of 43 months, median overall survival for patients in the blinatumomab arm was not reached vs. 71.4 months in the control group (hazard ratio, 0.42, 95% confidence interval, 0.24-0.75; P = .003).
“The patients that got blinatumomab plus chemotherapy had an improved survival over those that got the standard chemotherapy,” Dr. Litzow said.
Dr. Litzow didn’t discuss the drug’s expense in his presentation. According to a 2019 report, when a daily vial of blinatumomab cost $3,464-$3,815, a treatment course of five month-long cycles could run to $535,000. According to drugs.com, the cost now is $4,740 per vial – more than $660,000 for five cycles.
In an interview, Cleveland Clinic hematologist/oncologist Anjali Advani, MD, said the study is “groundbreaking and one of the most exciting studies to come along in the acute lymphoblastic leukemia field.”
The trial “is one of the first studies to show improvement in outcome in a randomized manner with the addition of a novel agent,” she added. “This will change our standard of care for these patients.”
The National Cancer Institute funded the trial and drug manufacturer Amgen provided the medication and support through a cooperative research and development agreement.
Dr. Litzow discloses relationships with Actinium, Jazz, Syndax, Novartis, Astellas, Amgen, Abbvie, Pluristem and Biosight. Other authors have various disclosures with multiple drugmakers. Dr. Advani discloses relationships with Amgen, Jazz, Nkarta, Taiho, Beam, GMI, Kura, Pfizer, OBI, Incyte, Kite, ImmunoGen, GlycoMimetics, SGN, MacroGenics, and Servier.
AT ASH 2022
Juvenile Dermatomyositis–Associated Panniculitis
To the Editor:
Juvenile dermatomyositis (JDM) is an autoimmune disorder with childhood onset that predominantly affects the muscles and skin, among other organs. Since the recognition of dermatomyositis (DM) more than 100 years ago, a variety of clinical diagnostic criteria have been utilized. Classically, DM presents with muscle weakness and a pathognomonic cutaneous macular, violaceous, erythematous eruption. The juvenile variant is defined by onset prior to 16 years of age. Histologically, these entities are indistinguishable and demonstrate an interface dermatitis with epidermal atrophy. Clinically, JDM has a higher incidence of calcinosis cutis and is not associated with an increased risk for malignancy in contrast to the adult-onset variant.1 Panniculitis is a rare but serious complication in a subset of patients with DM and may represent a precursor to calcinosis cutis.2 We describe a case of JDM-associated panniculitis that was difficult to control with prednisone and rituximab.
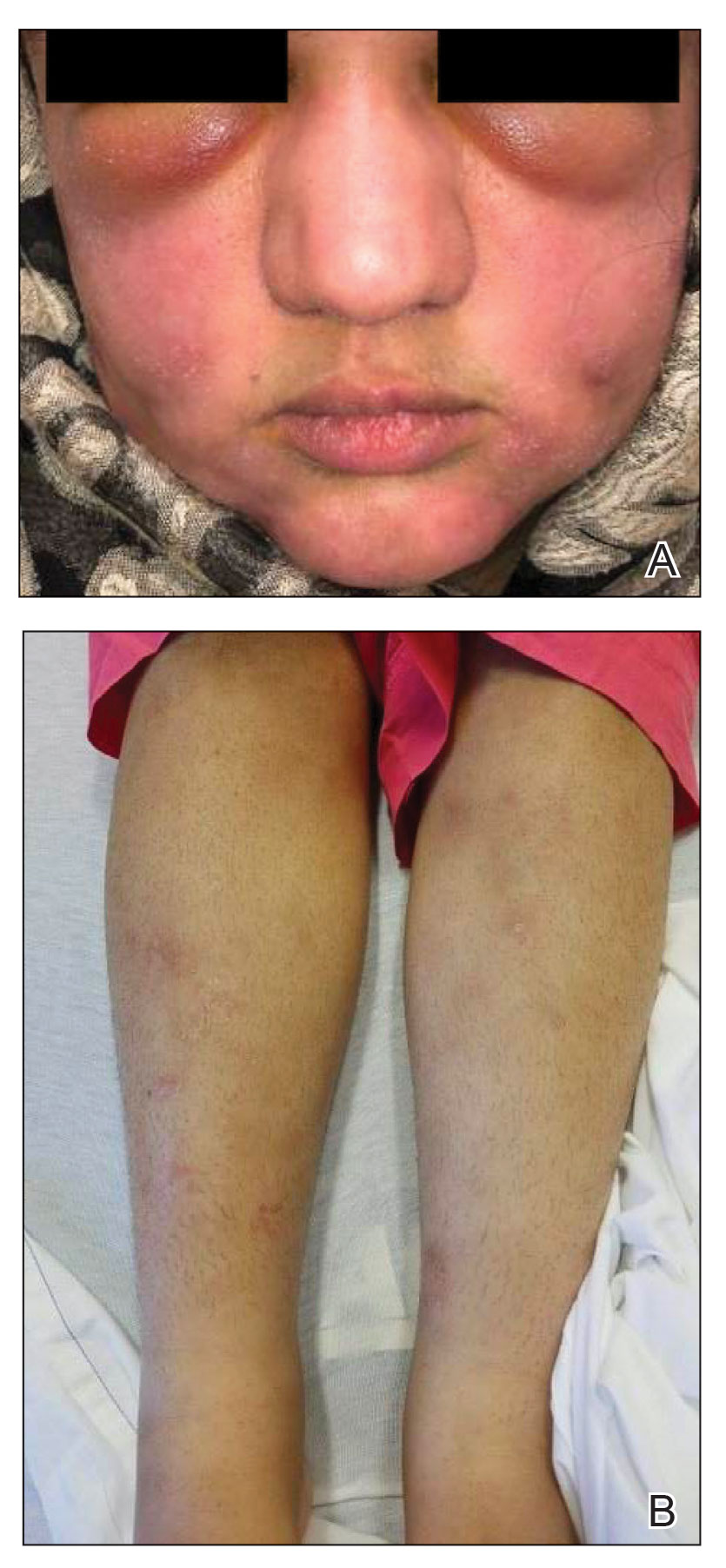
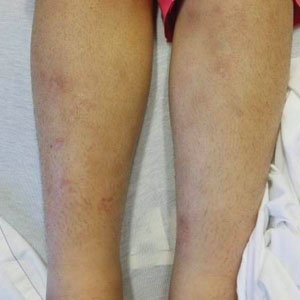
A 21-year-old woman with fever, fatigue, muscle pain, and new-onset swelling of 2 weeks’ duration was admitted to the hospital. She had a 5-year history of intermittent muscle weakness and concomitant rash. Prior to presentation, she had been hospitalized twice for fever of unknown origin, and the source remained undetermined. Physical examination revealed prominent facial and periorbital edema. There was tender nonpitting edema present on all 4 extremities and hyperpigmented indurated nodules on the shins (Figure 1). A full laboratory and imaging workup was performed for autoantibodies and infectious etiologies. The complete blood cell count was notable for pancytopenia, and a thorough infectious workup was negative. Creatine kinase level was within reference range. A biopsy of the right shin was performed, and histopathology revealed a lobular panniculitis with fat necrosis and mixed inflammation with neutrophils with perieccrine involvement as well as an interface dermatitis (Figure 2). Periodic acid–Schiff, Grocott methenamine-silver, and Gram stains were negative. Myositis-specific antibody testing revealed anti-p155/140 autoantibodies, and magnetic resonance imaging did not reveal active myositis within the visualized muscles, consistent with stable nonprogressing DM. A diagnosis of JDM with panniculitis was made. The patient was started on oral prednisone. Subsequently, a trial of rituximab was initiated. Although the patient’s symptoms initially improved, the response was not sustained on rituximab, and the patient was continued on systemic steroids with initiation of cyclosporine.
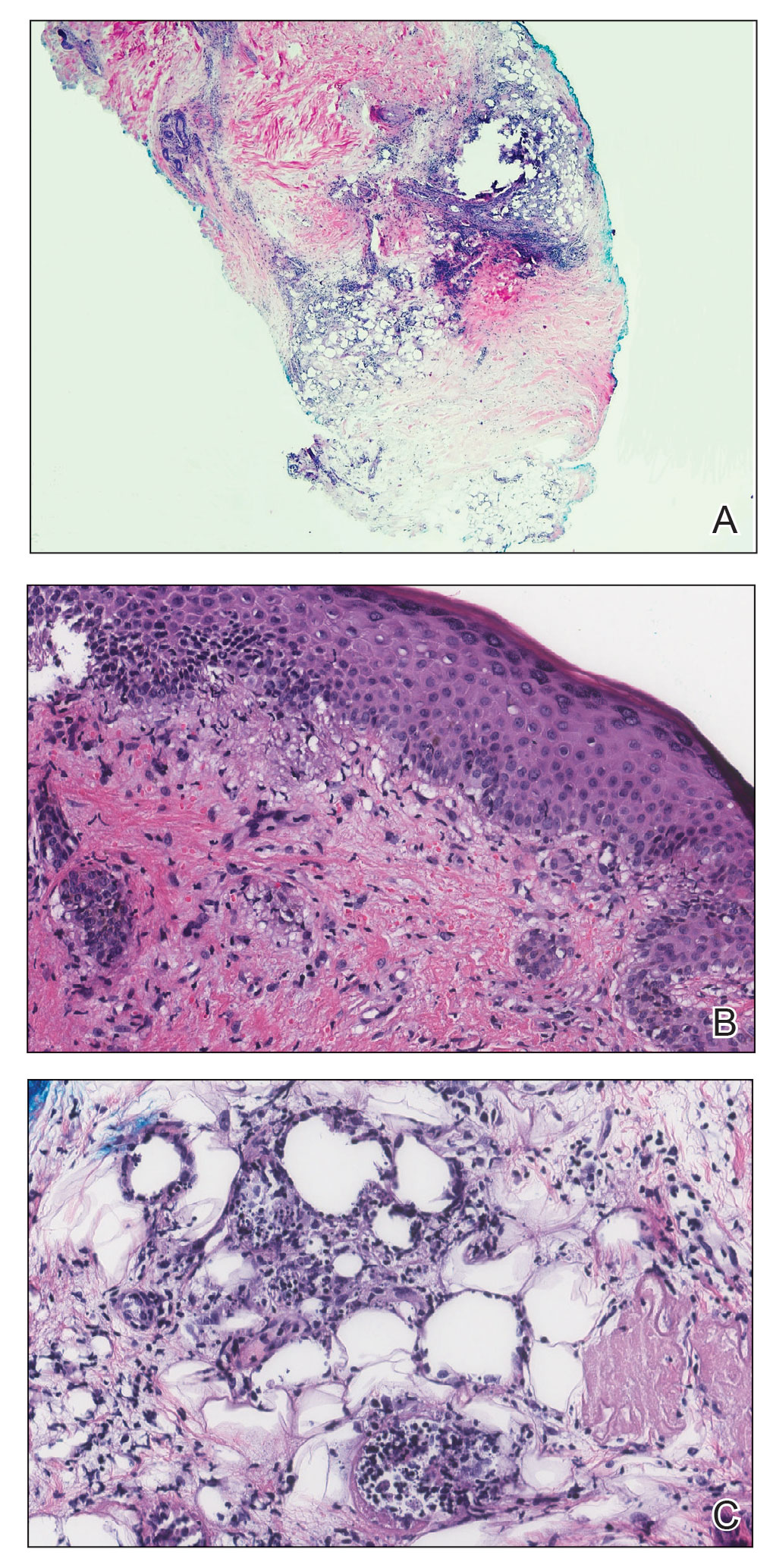
Juvenile dermatomyositis is an autoimmune disorder with childhood onset that involves systemic inflammation of the muscles, skin, and internal organs. It often can present diagnostic and therapeutic challenges.2,3 Bohan and Peter4,5 clinical criteria may help identify potential patients with JDM, but magnetic resonance imaging, electromyography, and muscle biopsy often are required to confirm the diagnosis.6 Skin manifestations include heliotrope rash; V sign; shawl sign; Gottron papules; periorbital edema; and infrequently panniculitis, the subcutaneous inflammation of adipose tissue.3,7
Although panniculitis is found in approximately 10% of skin biopsies in patients with DM, our patient presented with anti-p155/140 antibodies.8-10 Fat involvement in these patients traditionally manifests as lipodystrophy. Panniculitis also may precede calcinosis cutis, a debilitating skin change that may occur in approximately 46% of patients with JDM and can cause severe morbidity.2,6,9
Subcutaneous edema rarely is described in DM-panniculitis, present in only 6% of 86 DM patients in one study.7 The pathophysiology of DM may be due to antibodies that target endothelial cells and activate complement, resulting in the membranolytic attack complex. This leads to microischemia, and microinfarction of the muscle fibers has been suggested to result in edema of the subcutaneous tissue in severe cases.7,11 Microinfarction has been found to be present 2.3 times more often in edematous DM compared with nonedematous DM.7 Subcutaneous edema may be an isolated presentation of DM that arises more quickly with severe disease activity. As such, recommendations have been made to consider edema in future classification schemes.7
Because of the severity of edematous and/or subcutaneous DM, aggressive therapy may be required. First-line therapy consists of corticosteroids with additional immunosuppressants and immunomodulatory agents if adequate response is not achieved.3,12 The effectiveness of rituximab in DM has been suggested.2,12,13 The Rituximab in Myositis (RIM) trial (N=200) was the first double-blind, placebo-controlled, phase 3 clinical trial to assess rituximab’s efficacy in refractory compared with early-onset inflammatory myopathies. Although outcomes were similar in both groups, 83% of patients overall, including the JDM subset, met the definition of improvement.12 In re-examining the RIM trial data and other cases using rituximab to treat inflammatory myopathies, an overall response rate of 78.3% was observed, with 52.1% of patients with DM reporting improvement in skin lesions (N=458, pooled from 48 studies).13 Further analysis of the RIM data revealed that panniculitis affected 10.4% of patients with JDM at baseline, which decreased to 6.8% at 36 weeks of rituximab therapy (N=48).12
As exhibited in our patient, subcutaneous tissue involvement, including calcinosis cutis and panniculitis, is seen more often in JDM than adult DM.2,6 However, panniculitis in anti-p155/140 patients is rare. Our patient also had antibody positivity, which likely predisposed her to a more severe course. Despite not having sustained improvement on rituximab, initiating aggressive therapy earlier in the disease course may be beneficial, and our patient continues with alternative therapies.
- Jorizzo JL, Vleugels RA. Dermatomyositis. In: Bolognia J, Schaffer J, Cerroni L. Dermatology. 4th ed. Elsevier; 2019:681-687.
- Aggarwal R, Loganathan P, Koontz D, et al. Cutaneous improvement in refractory adult and juvenile dermatomyositis after treatment with rituximab. Rheumatology. 2016;56:247-254.
- Santos-Briz A, Calle A, Linos K, et al. Dermatomyositis panniculitis: a clinicopathological and immunohistochemical study of 18 cases. J Eur Acad Dermatol Venereol. 2018;32:1352-1359.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344-347.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med. 1975;292:403-407.
- Sakurai N, Hino-Shishikura A, Nozawa T, et al. Clinical significance of subcutaneous fat and fascial involvement in juvenile dermatomyositis. Mod Rheumatol. 2019;29:808-813.
- Milisenda JC, Doti PI, Prieto-Gonzalez S, et al. Dermatomyositis presenting with severe subcutaneous edema: five additional cases and review of the literature. Semin Arthritis Rheum. 2014;44:228-233.
- Janis JF, Winkelmann RK. Histopathology of the skin in dermatomyositis: a histopathologic study of 55 cases. Arch Dermatol. 1968;97:640-650.
- van Dongen HM, van Vugt RM, Stoof TJ. Extensive persistent panniculitis in the context of dermatomyositis. J Clin Rheumatol. 2020;26:e187-e188.
- Gunawardena H, Wedderburn LR, North J, et al. Clinical associations of autoantibodies to a p155/140 kDa doublet protein in juvenile dermatomyositis. Rheumatology. 2008;47:324-328.
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982.
- Oddis CV, Reed AM, Aggarwal R, et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum. 2013;65:314-324.
- Fasano S, Gordon P, Hajji R, et al. Rituximab in the treatment of inflammatory myopathies: a review. Rheumatology. 2016;56:26-36.
To the Editor:
Juvenile dermatomyositis (JDM) is an autoimmune disorder with childhood onset that predominantly affects the muscles and skin, among other organs. Since the recognition of dermatomyositis (DM) more than 100 years ago, a variety of clinical diagnostic criteria have been utilized. Classically, DM presents with muscle weakness and a pathognomonic cutaneous macular, violaceous, erythematous eruption. The juvenile variant is defined by onset prior to 16 years of age. Histologically, these entities are indistinguishable and demonstrate an interface dermatitis with epidermal atrophy. Clinically, JDM has a higher incidence of calcinosis cutis and is not associated with an increased risk for malignancy in contrast to the adult-onset variant.1 Panniculitis is a rare but serious complication in a subset of patients with DM and may represent a precursor to calcinosis cutis.2 We describe a case of JDM-associated panniculitis that was difficult to control with prednisone and rituximab.

A 21-year-old woman with fever, fatigue, muscle pain, and new-onset swelling of 2 weeks’ duration was admitted to the hospital. She had a 5-year history of intermittent muscle weakness and concomitant rash. Prior to presentation, she had been hospitalized twice for fever of unknown origin, and the source remained undetermined. Physical examination revealed prominent facial and periorbital edema. There was tender nonpitting edema present on all 4 extremities and hyperpigmented indurated nodules on the shins (Figure 1). A full laboratory and imaging workup was performed for autoantibodies and infectious etiologies. The complete blood cell count was notable for pancytopenia, and a thorough infectious workup was negative. Creatine kinase level was within reference range. A biopsy of the right shin was performed, and histopathology revealed a lobular panniculitis with fat necrosis and mixed inflammation with neutrophils with perieccrine involvement as well as an interface dermatitis (Figure 2). Periodic acid–Schiff, Grocott methenamine-silver, and Gram stains were negative. Myositis-specific antibody testing revealed anti-p155/140 autoantibodies, and magnetic resonance imaging did not reveal active myositis within the visualized muscles, consistent with stable nonprogressing DM. A diagnosis of JDM with panniculitis was made. The patient was started on oral prednisone. Subsequently, a trial of rituximab was initiated. Although the patient’s symptoms initially improved, the response was not sustained on rituximab, and the patient was continued on systemic steroids with initiation of cyclosporine.

Juvenile dermatomyositis is an autoimmune disorder with childhood onset that involves systemic inflammation of the muscles, skin, and internal organs. It often can present diagnostic and therapeutic challenges.2,3 Bohan and Peter4,5 clinical criteria may help identify potential patients with JDM, but magnetic resonance imaging, electromyography, and muscle biopsy often are required to confirm the diagnosis.6 Skin manifestations include heliotrope rash; V sign; shawl sign; Gottron papules; periorbital edema; and infrequently panniculitis, the subcutaneous inflammation of adipose tissue.3,7
Although panniculitis is found in approximately 10% of skin biopsies in patients with DM, our patient presented with anti-p155/140 antibodies.8-10 Fat involvement in these patients traditionally manifests as lipodystrophy. Panniculitis also may precede calcinosis cutis, a debilitating skin change that may occur in approximately 46% of patients with JDM and can cause severe morbidity.2,6,9
Subcutaneous edema rarely is described in DM-panniculitis, present in only 6% of 86 DM patients in one study.7 The pathophysiology of DM may be due to antibodies that target endothelial cells and activate complement, resulting in the membranolytic attack complex. This leads to microischemia, and microinfarction of the muscle fibers has been suggested to result in edema of the subcutaneous tissue in severe cases.7,11 Microinfarction has been found to be present 2.3 times more often in edematous DM compared with nonedematous DM.7 Subcutaneous edema may be an isolated presentation of DM that arises more quickly with severe disease activity. As such, recommendations have been made to consider edema in future classification schemes.7
Because of the severity of edematous and/or subcutaneous DM, aggressive therapy may be required. First-line therapy consists of corticosteroids with additional immunosuppressants and immunomodulatory agents if adequate response is not achieved.3,12 The effectiveness of rituximab in DM has been suggested.2,12,13 The Rituximab in Myositis (RIM) trial (N=200) was the first double-blind, placebo-controlled, phase 3 clinical trial to assess rituximab’s efficacy in refractory compared with early-onset inflammatory myopathies. Although outcomes were similar in both groups, 83% of patients overall, including the JDM subset, met the definition of improvement.12 In re-examining the RIM trial data and other cases using rituximab to treat inflammatory myopathies, an overall response rate of 78.3% was observed, with 52.1% of patients with DM reporting improvement in skin lesions (N=458, pooled from 48 studies).13 Further analysis of the RIM data revealed that panniculitis affected 10.4% of patients with JDM at baseline, which decreased to 6.8% at 36 weeks of rituximab therapy (N=48).12
As exhibited in our patient, subcutaneous tissue involvement, including calcinosis cutis and panniculitis, is seen more often in JDM than adult DM.2,6 However, panniculitis in anti-p155/140 patients is rare. Our patient also had antibody positivity, which likely predisposed her to a more severe course. Despite not having sustained improvement on rituximab, initiating aggressive therapy earlier in the disease course may be beneficial, and our patient continues with alternative therapies.
To the Editor:
Juvenile dermatomyositis (JDM) is an autoimmune disorder with childhood onset that predominantly affects the muscles and skin, among other organs. Since the recognition of dermatomyositis (DM) more than 100 years ago, a variety of clinical diagnostic criteria have been utilized. Classically, DM presents with muscle weakness and a pathognomonic cutaneous macular, violaceous, erythematous eruption. The juvenile variant is defined by onset prior to 16 years of age. Histologically, these entities are indistinguishable and demonstrate an interface dermatitis with epidermal atrophy. Clinically, JDM has a higher incidence of calcinosis cutis and is not associated with an increased risk for malignancy in contrast to the adult-onset variant.1 Panniculitis is a rare but serious complication in a subset of patients with DM and may represent a precursor to calcinosis cutis.2 We describe a case of JDM-associated panniculitis that was difficult to control with prednisone and rituximab.

A 21-year-old woman with fever, fatigue, muscle pain, and new-onset swelling of 2 weeks’ duration was admitted to the hospital. She had a 5-year history of intermittent muscle weakness and concomitant rash. Prior to presentation, she had been hospitalized twice for fever of unknown origin, and the source remained undetermined. Physical examination revealed prominent facial and periorbital edema. There was tender nonpitting edema present on all 4 extremities and hyperpigmented indurated nodules on the shins (Figure 1). A full laboratory and imaging workup was performed for autoantibodies and infectious etiologies. The complete blood cell count was notable for pancytopenia, and a thorough infectious workup was negative. Creatine kinase level was within reference range. A biopsy of the right shin was performed, and histopathology revealed a lobular panniculitis with fat necrosis and mixed inflammation with neutrophils with perieccrine involvement as well as an interface dermatitis (Figure 2). Periodic acid–Schiff, Grocott methenamine-silver, and Gram stains were negative. Myositis-specific antibody testing revealed anti-p155/140 autoantibodies, and magnetic resonance imaging did not reveal active myositis within the visualized muscles, consistent with stable nonprogressing DM. A diagnosis of JDM with panniculitis was made. The patient was started on oral prednisone. Subsequently, a trial of rituximab was initiated. Although the patient’s symptoms initially improved, the response was not sustained on rituximab, and the patient was continued on systemic steroids with initiation of cyclosporine.

Juvenile dermatomyositis is an autoimmune disorder with childhood onset that involves systemic inflammation of the muscles, skin, and internal organs. It often can present diagnostic and therapeutic challenges.2,3 Bohan and Peter4,5 clinical criteria may help identify potential patients with JDM, but magnetic resonance imaging, electromyography, and muscle biopsy often are required to confirm the diagnosis.6 Skin manifestations include heliotrope rash; V sign; shawl sign; Gottron papules; periorbital edema; and infrequently panniculitis, the subcutaneous inflammation of adipose tissue.3,7
Although panniculitis is found in approximately 10% of skin biopsies in patients with DM, our patient presented with anti-p155/140 antibodies.8-10 Fat involvement in these patients traditionally manifests as lipodystrophy. Panniculitis also may precede calcinosis cutis, a debilitating skin change that may occur in approximately 46% of patients with JDM and can cause severe morbidity.2,6,9
Subcutaneous edema rarely is described in DM-panniculitis, present in only 6% of 86 DM patients in one study.7 The pathophysiology of DM may be due to antibodies that target endothelial cells and activate complement, resulting in the membranolytic attack complex. This leads to microischemia, and microinfarction of the muscle fibers has been suggested to result in edema of the subcutaneous tissue in severe cases.7,11 Microinfarction has been found to be present 2.3 times more often in edematous DM compared with nonedematous DM.7 Subcutaneous edema may be an isolated presentation of DM that arises more quickly with severe disease activity. As such, recommendations have been made to consider edema in future classification schemes.7
Because of the severity of edematous and/or subcutaneous DM, aggressive therapy may be required. First-line therapy consists of corticosteroids with additional immunosuppressants and immunomodulatory agents if adequate response is not achieved.3,12 The effectiveness of rituximab in DM has been suggested.2,12,13 The Rituximab in Myositis (RIM) trial (N=200) was the first double-blind, placebo-controlled, phase 3 clinical trial to assess rituximab’s efficacy in refractory compared with early-onset inflammatory myopathies. Although outcomes were similar in both groups, 83% of patients overall, including the JDM subset, met the definition of improvement.12 In re-examining the RIM trial data and other cases using rituximab to treat inflammatory myopathies, an overall response rate of 78.3% was observed, with 52.1% of patients with DM reporting improvement in skin lesions (N=458, pooled from 48 studies).13 Further analysis of the RIM data revealed that panniculitis affected 10.4% of patients with JDM at baseline, which decreased to 6.8% at 36 weeks of rituximab therapy (N=48).12
As exhibited in our patient, subcutaneous tissue involvement, including calcinosis cutis and panniculitis, is seen more often in JDM than adult DM.2,6 However, panniculitis in anti-p155/140 patients is rare. Our patient also had antibody positivity, which likely predisposed her to a more severe course. Despite not having sustained improvement on rituximab, initiating aggressive therapy earlier in the disease course may be beneficial, and our patient continues with alternative therapies.
- Jorizzo JL, Vleugels RA. Dermatomyositis. In: Bolognia J, Schaffer J, Cerroni L. Dermatology. 4th ed. Elsevier; 2019:681-687.
- Aggarwal R, Loganathan P, Koontz D, et al. Cutaneous improvement in refractory adult and juvenile dermatomyositis after treatment with rituximab. Rheumatology. 2016;56:247-254.
- Santos-Briz A, Calle A, Linos K, et al. Dermatomyositis panniculitis: a clinicopathological and immunohistochemical study of 18 cases. J Eur Acad Dermatol Venereol. 2018;32:1352-1359.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344-347.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med. 1975;292:403-407.
- Sakurai N, Hino-Shishikura A, Nozawa T, et al. Clinical significance of subcutaneous fat and fascial involvement in juvenile dermatomyositis. Mod Rheumatol. 2019;29:808-813.
- Milisenda JC, Doti PI, Prieto-Gonzalez S, et al. Dermatomyositis presenting with severe subcutaneous edema: five additional cases and review of the literature. Semin Arthritis Rheum. 2014;44:228-233.
- Janis JF, Winkelmann RK. Histopathology of the skin in dermatomyositis: a histopathologic study of 55 cases. Arch Dermatol. 1968;97:640-650.
- van Dongen HM, van Vugt RM, Stoof TJ. Extensive persistent panniculitis in the context of dermatomyositis. J Clin Rheumatol. 2020;26:e187-e188.
- Gunawardena H, Wedderburn LR, North J, et al. Clinical associations of autoantibodies to a p155/140 kDa doublet protein in juvenile dermatomyositis. Rheumatology. 2008;47:324-328.
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982.
- Oddis CV, Reed AM, Aggarwal R, et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum. 2013;65:314-324.
- Fasano S, Gordon P, Hajji R, et al. Rituximab in the treatment of inflammatory myopathies: a review. Rheumatology. 2016;56:26-36.
- Jorizzo JL, Vleugels RA. Dermatomyositis. In: Bolognia J, Schaffer J, Cerroni L. Dermatology. 4th ed. Elsevier; 2019:681-687.
- Aggarwal R, Loganathan P, Koontz D, et al. Cutaneous improvement in refractory adult and juvenile dermatomyositis after treatment with rituximab. Rheumatology. 2016;56:247-254.
- Santos-Briz A, Calle A, Linos K, et al. Dermatomyositis panniculitis: a clinicopathological and immunohistochemical study of 18 cases. J Eur Acad Dermatol Venereol. 2018;32:1352-1359.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344-347.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med. 1975;292:403-407.
- Sakurai N, Hino-Shishikura A, Nozawa T, et al. Clinical significance of subcutaneous fat and fascial involvement in juvenile dermatomyositis. Mod Rheumatol. 2019;29:808-813.
- Milisenda JC, Doti PI, Prieto-Gonzalez S, et al. Dermatomyositis presenting with severe subcutaneous edema: five additional cases and review of the literature. Semin Arthritis Rheum. 2014;44:228-233.
- Janis JF, Winkelmann RK. Histopathology of the skin in dermatomyositis: a histopathologic study of 55 cases. Arch Dermatol. 1968;97:640-650.
- van Dongen HM, van Vugt RM, Stoof TJ. Extensive persistent panniculitis in the context of dermatomyositis. J Clin Rheumatol. 2020;26:e187-e188.
- Gunawardena H, Wedderburn LR, North J, et al. Clinical associations of autoantibodies to a p155/140 kDa doublet protein in juvenile dermatomyositis. Rheumatology. 2008;47:324-328.
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982.
- Oddis CV, Reed AM, Aggarwal R, et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum. 2013;65:314-324.
- Fasano S, Gordon P, Hajji R, et al. Rituximab in the treatment of inflammatory myopathies: a review. Rheumatology. 2016;56:26-36.
Practice Points
- Juvenile dermatomyositis is an autoimmune disorder with childhood onset that predominantly affects the muscles and skin.
- Juvenile dermatomyositis has a higher incidence of calcinosis cutis and is not associated with an increased risk for malignancy in contrast to the adult-onset variant, dermatomyositis (DM).
- Panniculitis is a rare but severe complication of DM, and this subset of DM may be challenging to treat, requiring aggressive therapy.
Skin Manifestations of Complex Regional Pain Syndrome
To the Editor:
Complex regional pain syndrome (CRPS) is a neurologic condition characterized by chronic pain and sensory changes, including allodynia and hyperalgesia, that usually affect the extremities.1,2 The syndrome is defined by the International Association for the Study of Pain (IASP) as a condition that appears regionally after an injury, with a variety of symptoms that often exceed the expected clinical course both in magnitude and duration, causing impairment of motor function and variable progression.3
Although CRPS most often is described following minor peripheral trauma, other precipitating causes include surgery and vascular events.4 Additional features of the condition include autonomic dysfunction, edema, and trophic changes.1 Symptoms of CRPS traditionally present in 3 stages, with notable skin changes most often documented in stages II and III.2
Skin changes are a known manifestation of the syndrome, but reports in the dermatologic literature are scarce. Qureshi and Friedman5 identified only 23 articles in the dermatology literature since 1990 in which skin changes in CRPS were described. We present a patient with a diagnosis of CRPS who developed hyperpigmentation and sclerotic changes, including skin thickening, induration, and skin tightening.
A middle-aged Black woman presented to dermatology for evaluation of progressive hyperpigmentation, hyperhidrosis, and sclerotic changes to the skin. Approximately 3 years prior, the patient was given a diagnosis of CRPS of the hands and feet. Pain symptoms started approximately 3 years prior to the onset of symptoms. Symptoms started in the left hand and eventually spread to the right arm, left leg, and subsequently to the right leg. The first dermatologic change the patient noticed was tightening of the skin in the affected area that led to decreased mobility, which improved over time—partly on its own and partly with physical therapy.
A biopsy performed by an outside dermatologist at the initial presentation demonstrated sclerodermalike changes, which were treated with creams but without improvement. Scleroderma was later ruled out by the same dermatologist. Skin tightening improved over time, with complete resolution approximately 1 year after the onset of symptoms.
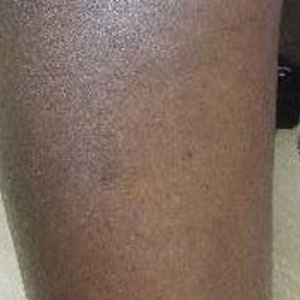
Upon presentation to our clinic, the patient reported continuing intermittent flares of CRPS; however, she said she was most concerned about diffuse hyperpigmentation, which spread to include the face, arms, abdomen, legs (Figure), and buttocks and persisted after skin tightening resolved.
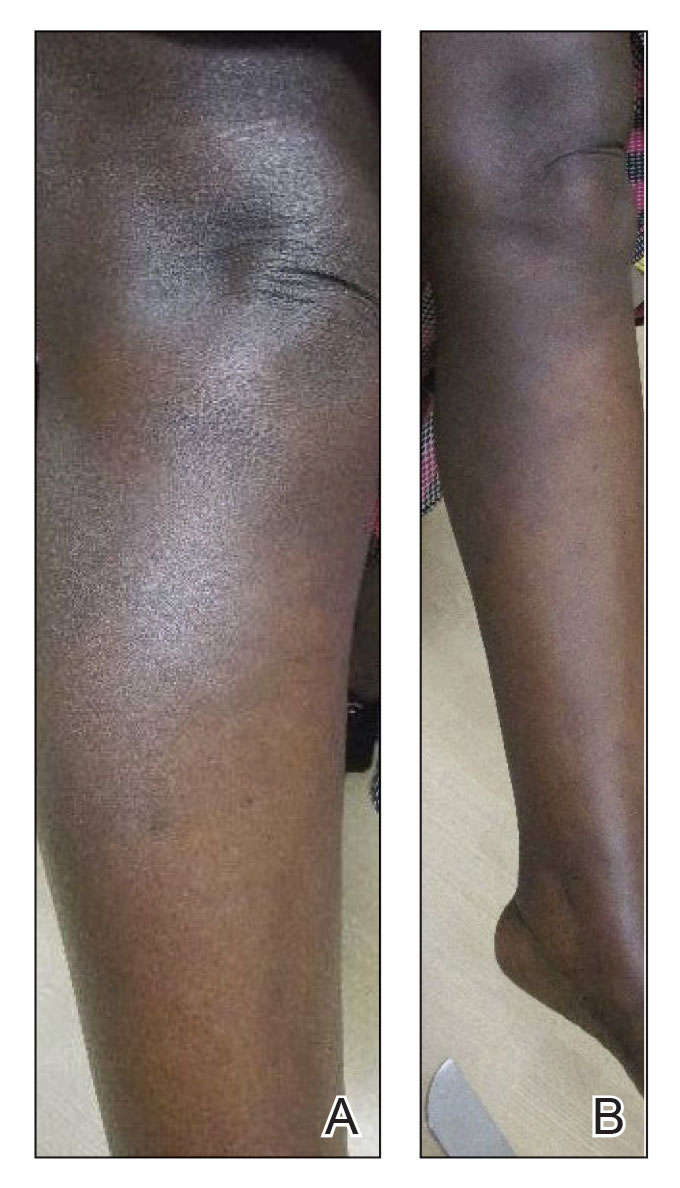
To treat the hyperpigmentation, a decision was made to first focus on a localized area. Facial hyperpigmentation was chosen because it was of greatest concern to the patient. She was instructed to use azelaic acid gel 15% in the morning, tretinoin cream 0.05% at night, and sunscreen daily. The patient had mild improvement in hyperpigmentation after a 4-month period but has been inconsistent in follow-up. She continues to have intermittent flares of CRPS, which may interfere with her response to treatment. In addition to the aforementioned regimen of azelaic acid gel and tretinoin, she has continued to work with a pain specialist to better control the neurologic symptoms and pain associated with her CRPS.
Complex regional pain syndrome, a neurological condition characterized by chronic pain, affects women 3 times more often than men. The syndrome is more common in the fourth and fifth decades of life.1,2
There are 2 subtypes of CRPS. Type I (also known as reflex sympathetic dystrophy) is more common and occurs following minor trauma without peripheral nerve injury. Type II (otherwise known as causalgia) occurs following more notable trauma with injury to a peripheral nerve.1,6 Onset of symptoms most often is secondary to minor peripheral trauma. More common triggers include soft-tissue injury (40%); fractures and subsequent orthopedic surgery (25%); and visceral lesions, such as myocardial infarction and cerebral vascular accident (12%).5 Regardless of the inciting event, prolonged immobilization of a limb has been identified as an important predisposing factor. One study found that 47% of patients who received a diagnosis of CRPS previously underwent immobilization of the same limb.7
The pathogenesis of CRPS has not been fully elucidated. Possible explanations include central nervous system sensitization to thermal, mechanical, and pain stimuli; sympathetic dysfunction leading to vasomotor, pseudomotor, and trophic changes; and inflammatory cytokine release and microcirculatory dysfunction, causing tissue injury.1,2,6
The diagnosis of CRPS is a based on clinical findings. Using the Budapest Criteria established to define CRPS, a clinical diagnosis can be made when all of the following criteria are met: chronic continuing pain disproportionate to any inciting event; 1 or more reported symptoms from 3 or more of the categories of involvement including sensory, vasomotor, pseudomotor, edema, and motor or trophic; 1 or more sign at the time of evaluation in 2 or more of the categories of involvement including sensory, vasomotor, pseudomotor, edema, and motor or trophic.8 Dermatologic findings are a common presenting feature of CRPS and are included in the Budapest Criteria used for diagnosis. In a retrospective chart review (N=26), researchers found that vascular findings were the most common dermatologic manifestation of CRPS—edema in 58% of patients and erythema in 54%.9 Other common manifestations included dermatitis (35%), erythematous papules (23%), and cutaneous atrophy (23%). Hyperpigmentation, which was present in our patient, was seen in 8% of patients in the chart review.9
Complex regional pain syndrome progresses through 3 stages; dermatologic changes are present in each stage and are more severe in later stages. Stage I lasts 2 or 3 months and is characterized by onset of pain, usually burning type, accompanied by allodynia and hyperalgesia. Early vasomotor and pseudomotor changes, such as erythema and edema, may become apparent.1,2 Stage II lasts 3 to 6 months and is characterized by more severe edema and more obvious trophic changes. Functional limitations, such as limited range of motion and muscle weakness, begin to manifest. Stage III—the final and most severe stage—is characterized by obvious hair, skin, and nail changes, as well as functional limitations.1,2 The waxy thickened skin changes and hyperpigmentation observed in our patient are characteristic of stage III. Furthermore, our patient experienced decreased mobility and limited range of motion secondary to tightening of the skin, a characteristic motor change of late-stage CRPS. Although chronic pain and allodynia are the most common characteristics of CRPS, skin changes also can cause notable distress and early dermatologic manifestations can be a chief concern.
Dermatologic management is focused to address the specific skin changes of CRPS. However, traditional treatment of the common dermatologic findings of CRPS is difficult and often unsuccessful; instead, the most successful treatment of skin findings involves controlling the underlying CRPS.9 Current treatment options include removal of any nidus of tissue trauma, sympathetic neural blockade with a local anesthetic, spinal cord stimulation to interrupt dysregulated sympathetic innervation, and physiotherapy or occupational therapy to desensitize skin.1,10
Given the complexity of CRPS and the variability of its presentation, management of the syndrome and its associated dermatologic conditions often requires interdisciplinary care and coordination of multiple specialties. Dermatologists can play an important role in both identification of CRPS and co-management of affected patients. Early diagnosis of CRPS has been universally identified as a key prognostic factor. For that reason, dermatologists should be aware of CRPS and include the syndrome in the differential diagnosis when presented with severe cutaneous findings following trauma either with or without peripheral nerve damage, suggestive of CRPS.
- Sebastin SJ. Complex regional pain syndrome. Indian J Plast Surg. 2011;44:298-307. doi:10.4103/0970-0358.85351
- Kabani R, Brassard A. Dermatological findings in early detection of complex regional pain syndrome. JAMA Dermatol. 2014;150:640-642. doi:10.1001/jamadermatol.2013.7459
- Moseley L. What is complex regional pain syndrome – in plain English. International Association for the Study of Pain website. Published 2009. Accessed December 15, 2022. https://www.iasp-pain.org/publications/relief-news/article/what-is-complex-pain-syndrome-in-plain-english/
- Pak TJ, Martin GM, Magness JL, et al. Reflex sympathetic dystrophy. Review of 140 cases. Minn Med. 1970;53:507-512.
- Qureshi AA, Friedman AJ. Complex regional pain syndrome: what the dermatologist should know. J Drugs Dermatol. 2018;17:532-536.
- Gorodkin R. Complex regional pain syndrome. Rheumatology. 2016;55(suppl 1):i12.
- Araki E, Tanioka M, Miyachi Y, et al. A case of complex regional pain syndrome: an underdiagnosed condition in dermatology. Acta Derm Venereol. 2007;87:440-441. doi:10.2340/00015555-0281
- Pergolizzi JV, LeQuang JA, Nalamachu S, et al. The Budapest criteria for complex regional pain syndrome: the diagnostic challenge. Anaesthesiol Clin Sci Res. 2018;2:1-10. doi:10.35841/anesthesiology.2.1.1-10
- Sundaram S, Webster GF. Vascular diseases are the most common cutaneous manifestations of reflex sympathetic dystrophy. J Am Acad Dermatol. 2001;44:1050-1051. doi:10.1067/mjd.2001.114299
- Taylor RS, Van Buyten J-P, Buchser E. Spinal stimulation for complex regional pain syndrome: a systematic review of the clinical and cost-effectiveness literature and assessment of prognostic factors. Eur J Pain. 2006;10:91-101. doi:10.1016/j.ejpain.2005.02.004
To the Editor:
Complex regional pain syndrome (CRPS) is a neurologic condition characterized by chronic pain and sensory changes, including allodynia and hyperalgesia, that usually affect the extremities.1,2 The syndrome is defined by the International Association for the Study of Pain (IASP) as a condition that appears regionally after an injury, with a variety of symptoms that often exceed the expected clinical course both in magnitude and duration, causing impairment of motor function and variable progression.3
Although CRPS most often is described following minor peripheral trauma, other precipitating causes include surgery and vascular events.4 Additional features of the condition include autonomic dysfunction, edema, and trophic changes.1 Symptoms of CRPS traditionally present in 3 stages, with notable skin changes most often documented in stages II and III.2
Skin changes are a known manifestation of the syndrome, but reports in the dermatologic literature are scarce. Qureshi and Friedman5 identified only 23 articles in the dermatology literature since 1990 in which skin changes in CRPS were described. We present a patient with a diagnosis of CRPS who developed hyperpigmentation and sclerotic changes, including skin thickening, induration, and skin tightening.
A middle-aged Black woman presented to dermatology for evaluation of progressive hyperpigmentation, hyperhidrosis, and sclerotic changes to the skin. Approximately 3 years prior, the patient was given a diagnosis of CRPS of the hands and feet. Pain symptoms started approximately 3 years prior to the onset of symptoms. Symptoms started in the left hand and eventually spread to the right arm, left leg, and subsequently to the right leg. The first dermatologic change the patient noticed was tightening of the skin in the affected area that led to decreased mobility, which improved over time—partly on its own and partly with physical therapy.
A biopsy performed by an outside dermatologist at the initial presentation demonstrated sclerodermalike changes, which were treated with creams but without improvement. Scleroderma was later ruled out by the same dermatologist. Skin tightening improved over time, with complete resolution approximately 1 year after the onset of symptoms.
Upon presentation to our clinic, the patient reported continuing intermittent flares of CRPS; however, she said she was most concerned about diffuse hyperpigmentation, which spread to include the face, arms, abdomen, legs (Figure), and buttocks and persisted after skin tightening resolved.

To treat the hyperpigmentation, a decision was made to first focus on a localized area. Facial hyperpigmentation was chosen because it was of greatest concern to the patient. She was instructed to use azelaic acid gel 15% in the morning, tretinoin cream 0.05% at night, and sunscreen daily. The patient had mild improvement in hyperpigmentation after a 4-month period but has been inconsistent in follow-up. She continues to have intermittent flares of CRPS, which may interfere with her response to treatment. In addition to the aforementioned regimen of azelaic acid gel and tretinoin, she has continued to work with a pain specialist to better control the neurologic symptoms and pain associated with her CRPS.
Complex regional pain syndrome, a neurological condition characterized by chronic pain, affects women 3 times more often than men. The syndrome is more common in the fourth and fifth decades of life.1,2
There are 2 subtypes of CRPS. Type I (also known as reflex sympathetic dystrophy) is more common and occurs following minor trauma without peripheral nerve injury. Type II (otherwise known as causalgia) occurs following more notable trauma with injury to a peripheral nerve.1,6 Onset of symptoms most often is secondary to minor peripheral trauma. More common triggers include soft-tissue injury (40%); fractures and subsequent orthopedic surgery (25%); and visceral lesions, such as myocardial infarction and cerebral vascular accident (12%).5 Regardless of the inciting event, prolonged immobilization of a limb has been identified as an important predisposing factor. One study found that 47% of patients who received a diagnosis of CRPS previously underwent immobilization of the same limb.7
The pathogenesis of CRPS has not been fully elucidated. Possible explanations include central nervous system sensitization to thermal, mechanical, and pain stimuli; sympathetic dysfunction leading to vasomotor, pseudomotor, and trophic changes; and inflammatory cytokine release and microcirculatory dysfunction, causing tissue injury.1,2,6
The diagnosis of CRPS is a based on clinical findings. Using the Budapest Criteria established to define CRPS, a clinical diagnosis can be made when all of the following criteria are met: chronic continuing pain disproportionate to any inciting event; 1 or more reported symptoms from 3 or more of the categories of involvement including sensory, vasomotor, pseudomotor, edema, and motor or trophic; 1 or more sign at the time of evaluation in 2 or more of the categories of involvement including sensory, vasomotor, pseudomotor, edema, and motor or trophic.8 Dermatologic findings are a common presenting feature of CRPS and are included in the Budapest Criteria used for diagnosis. In a retrospective chart review (N=26), researchers found that vascular findings were the most common dermatologic manifestation of CRPS—edema in 58% of patients and erythema in 54%.9 Other common manifestations included dermatitis (35%), erythematous papules (23%), and cutaneous atrophy (23%). Hyperpigmentation, which was present in our patient, was seen in 8% of patients in the chart review.9
Complex regional pain syndrome progresses through 3 stages; dermatologic changes are present in each stage and are more severe in later stages. Stage I lasts 2 or 3 months and is characterized by onset of pain, usually burning type, accompanied by allodynia and hyperalgesia. Early vasomotor and pseudomotor changes, such as erythema and edema, may become apparent.1,2 Stage II lasts 3 to 6 months and is characterized by more severe edema and more obvious trophic changes. Functional limitations, such as limited range of motion and muscle weakness, begin to manifest. Stage III—the final and most severe stage—is characterized by obvious hair, skin, and nail changes, as well as functional limitations.1,2 The waxy thickened skin changes and hyperpigmentation observed in our patient are characteristic of stage III. Furthermore, our patient experienced decreased mobility and limited range of motion secondary to tightening of the skin, a characteristic motor change of late-stage CRPS. Although chronic pain and allodynia are the most common characteristics of CRPS, skin changes also can cause notable distress and early dermatologic manifestations can be a chief concern.
Dermatologic management is focused to address the specific skin changes of CRPS. However, traditional treatment of the common dermatologic findings of CRPS is difficult and often unsuccessful; instead, the most successful treatment of skin findings involves controlling the underlying CRPS.9 Current treatment options include removal of any nidus of tissue trauma, sympathetic neural blockade with a local anesthetic, spinal cord stimulation to interrupt dysregulated sympathetic innervation, and physiotherapy or occupational therapy to desensitize skin.1,10
Given the complexity of CRPS and the variability of its presentation, management of the syndrome and its associated dermatologic conditions often requires interdisciplinary care and coordination of multiple specialties. Dermatologists can play an important role in both identification of CRPS and co-management of affected patients. Early diagnosis of CRPS has been universally identified as a key prognostic factor. For that reason, dermatologists should be aware of CRPS and include the syndrome in the differential diagnosis when presented with severe cutaneous findings following trauma either with or without peripheral nerve damage, suggestive of CRPS.
To the Editor:
Complex regional pain syndrome (CRPS) is a neurologic condition characterized by chronic pain and sensory changes, including allodynia and hyperalgesia, that usually affect the extremities.1,2 The syndrome is defined by the International Association for the Study of Pain (IASP) as a condition that appears regionally after an injury, with a variety of symptoms that often exceed the expected clinical course both in magnitude and duration, causing impairment of motor function and variable progression.3
Although CRPS most often is described following minor peripheral trauma, other precipitating causes include surgery and vascular events.4 Additional features of the condition include autonomic dysfunction, edema, and trophic changes.1 Symptoms of CRPS traditionally present in 3 stages, with notable skin changes most often documented in stages II and III.2
Skin changes are a known manifestation of the syndrome, but reports in the dermatologic literature are scarce. Qureshi and Friedman5 identified only 23 articles in the dermatology literature since 1990 in which skin changes in CRPS were described. We present a patient with a diagnosis of CRPS who developed hyperpigmentation and sclerotic changes, including skin thickening, induration, and skin tightening.
A middle-aged Black woman presented to dermatology for evaluation of progressive hyperpigmentation, hyperhidrosis, and sclerotic changes to the skin. Approximately 3 years prior, the patient was given a diagnosis of CRPS of the hands and feet. Pain symptoms started approximately 3 years prior to the onset of symptoms. Symptoms started in the left hand and eventually spread to the right arm, left leg, and subsequently to the right leg. The first dermatologic change the patient noticed was tightening of the skin in the affected area that led to decreased mobility, which improved over time—partly on its own and partly with physical therapy.
A biopsy performed by an outside dermatologist at the initial presentation demonstrated sclerodermalike changes, which were treated with creams but without improvement. Scleroderma was later ruled out by the same dermatologist. Skin tightening improved over time, with complete resolution approximately 1 year after the onset of symptoms.
Upon presentation to our clinic, the patient reported continuing intermittent flares of CRPS; however, she said she was most concerned about diffuse hyperpigmentation, which spread to include the face, arms, abdomen, legs (Figure), and buttocks and persisted after skin tightening resolved.

To treat the hyperpigmentation, a decision was made to first focus on a localized area. Facial hyperpigmentation was chosen because it was of greatest concern to the patient. She was instructed to use azelaic acid gel 15% in the morning, tretinoin cream 0.05% at night, and sunscreen daily. The patient had mild improvement in hyperpigmentation after a 4-month period but has been inconsistent in follow-up. She continues to have intermittent flares of CRPS, which may interfere with her response to treatment. In addition to the aforementioned regimen of azelaic acid gel and tretinoin, she has continued to work with a pain specialist to better control the neurologic symptoms and pain associated with her CRPS.
Complex regional pain syndrome, a neurological condition characterized by chronic pain, affects women 3 times more often than men. The syndrome is more common in the fourth and fifth decades of life.1,2
There are 2 subtypes of CRPS. Type I (also known as reflex sympathetic dystrophy) is more common and occurs following minor trauma without peripheral nerve injury. Type II (otherwise known as causalgia) occurs following more notable trauma with injury to a peripheral nerve.1,6 Onset of symptoms most often is secondary to minor peripheral trauma. More common triggers include soft-tissue injury (40%); fractures and subsequent orthopedic surgery (25%); and visceral lesions, such as myocardial infarction and cerebral vascular accident (12%).5 Regardless of the inciting event, prolonged immobilization of a limb has been identified as an important predisposing factor. One study found that 47% of patients who received a diagnosis of CRPS previously underwent immobilization of the same limb.7
The pathogenesis of CRPS has not been fully elucidated. Possible explanations include central nervous system sensitization to thermal, mechanical, and pain stimuli; sympathetic dysfunction leading to vasomotor, pseudomotor, and trophic changes; and inflammatory cytokine release and microcirculatory dysfunction, causing tissue injury.1,2,6
The diagnosis of CRPS is a based on clinical findings. Using the Budapest Criteria established to define CRPS, a clinical diagnosis can be made when all of the following criteria are met: chronic continuing pain disproportionate to any inciting event; 1 or more reported symptoms from 3 or more of the categories of involvement including sensory, vasomotor, pseudomotor, edema, and motor or trophic; 1 or more sign at the time of evaluation in 2 or more of the categories of involvement including sensory, vasomotor, pseudomotor, edema, and motor or trophic.8 Dermatologic findings are a common presenting feature of CRPS and are included in the Budapest Criteria used for diagnosis. In a retrospective chart review (N=26), researchers found that vascular findings were the most common dermatologic manifestation of CRPS—edema in 58% of patients and erythema in 54%.9 Other common manifestations included dermatitis (35%), erythematous papules (23%), and cutaneous atrophy (23%). Hyperpigmentation, which was present in our patient, was seen in 8% of patients in the chart review.9
Complex regional pain syndrome progresses through 3 stages; dermatologic changes are present in each stage and are more severe in later stages. Stage I lasts 2 or 3 months and is characterized by onset of pain, usually burning type, accompanied by allodynia and hyperalgesia. Early vasomotor and pseudomotor changes, such as erythema and edema, may become apparent.1,2 Stage II lasts 3 to 6 months and is characterized by more severe edema and more obvious trophic changes. Functional limitations, such as limited range of motion and muscle weakness, begin to manifest. Stage III—the final and most severe stage—is characterized by obvious hair, skin, and nail changes, as well as functional limitations.1,2 The waxy thickened skin changes and hyperpigmentation observed in our patient are characteristic of stage III. Furthermore, our patient experienced decreased mobility and limited range of motion secondary to tightening of the skin, a characteristic motor change of late-stage CRPS. Although chronic pain and allodynia are the most common characteristics of CRPS, skin changes also can cause notable distress and early dermatologic manifestations can be a chief concern.
Dermatologic management is focused to address the specific skin changes of CRPS. However, traditional treatment of the common dermatologic findings of CRPS is difficult and often unsuccessful; instead, the most successful treatment of skin findings involves controlling the underlying CRPS.9 Current treatment options include removal of any nidus of tissue trauma, sympathetic neural blockade with a local anesthetic, spinal cord stimulation to interrupt dysregulated sympathetic innervation, and physiotherapy or occupational therapy to desensitize skin.1,10
Given the complexity of CRPS and the variability of its presentation, management of the syndrome and its associated dermatologic conditions often requires interdisciplinary care and coordination of multiple specialties. Dermatologists can play an important role in both identification of CRPS and co-management of affected patients. Early diagnosis of CRPS has been universally identified as a key prognostic factor. For that reason, dermatologists should be aware of CRPS and include the syndrome in the differential diagnosis when presented with severe cutaneous findings following trauma either with or without peripheral nerve damage, suggestive of CRPS.
- Sebastin SJ. Complex regional pain syndrome. Indian J Plast Surg. 2011;44:298-307. doi:10.4103/0970-0358.85351
- Kabani R, Brassard A. Dermatological findings in early detection of complex regional pain syndrome. JAMA Dermatol. 2014;150:640-642. doi:10.1001/jamadermatol.2013.7459
- Moseley L. What is complex regional pain syndrome – in plain English. International Association for the Study of Pain website. Published 2009. Accessed December 15, 2022. https://www.iasp-pain.org/publications/relief-news/article/what-is-complex-pain-syndrome-in-plain-english/
- Pak TJ, Martin GM, Magness JL, et al. Reflex sympathetic dystrophy. Review of 140 cases. Minn Med. 1970;53:507-512.
- Qureshi AA, Friedman AJ. Complex regional pain syndrome: what the dermatologist should know. J Drugs Dermatol. 2018;17:532-536.
- Gorodkin R. Complex regional pain syndrome. Rheumatology. 2016;55(suppl 1):i12.
- Araki E, Tanioka M, Miyachi Y, et al. A case of complex regional pain syndrome: an underdiagnosed condition in dermatology. Acta Derm Venereol. 2007;87:440-441. doi:10.2340/00015555-0281
- Pergolizzi JV, LeQuang JA, Nalamachu S, et al. The Budapest criteria for complex regional pain syndrome: the diagnostic challenge. Anaesthesiol Clin Sci Res. 2018;2:1-10. doi:10.35841/anesthesiology.2.1.1-10
- Sundaram S, Webster GF. Vascular diseases are the most common cutaneous manifestations of reflex sympathetic dystrophy. J Am Acad Dermatol. 2001;44:1050-1051. doi:10.1067/mjd.2001.114299
- Taylor RS, Van Buyten J-P, Buchser E. Spinal stimulation for complex regional pain syndrome: a systematic review of the clinical and cost-effectiveness literature and assessment of prognostic factors. Eur J Pain. 2006;10:91-101. doi:10.1016/j.ejpain.2005.02.004
- Sebastin SJ. Complex regional pain syndrome. Indian J Plast Surg. 2011;44:298-307. doi:10.4103/0970-0358.85351
- Kabani R, Brassard A. Dermatological findings in early detection of complex regional pain syndrome. JAMA Dermatol. 2014;150:640-642. doi:10.1001/jamadermatol.2013.7459
- Moseley L. What is complex regional pain syndrome – in plain English. International Association for the Study of Pain website. Published 2009. Accessed December 15, 2022. https://www.iasp-pain.org/publications/relief-news/article/what-is-complex-pain-syndrome-in-plain-english/
- Pak TJ, Martin GM, Magness JL, et al. Reflex sympathetic dystrophy. Review of 140 cases. Minn Med. 1970;53:507-512.
- Qureshi AA, Friedman AJ. Complex regional pain syndrome: what the dermatologist should know. J Drugs Dermatol. 2018;17:532-536.
- Gorodkin R. Complex regional pain syndrome. Rheumatology. 2016;55(suppl 1):i12.
- Araki E, Tanioka M, Miyachi Y, et al. A case of complex regional pain syndrome: an underdiagnosed condition in dermatology. Acta Derm Venereol. 2007;87:440-441. doi:10.2340/00015555-0281
- Pergolizzi JV, LeQuang JA, Nalamachu S, et al. The Budapest criteria for complex regional pain syndrome: the diagnostic challenge. Anaesthesiol Clin Sci Res. 2018;2:1-10. doi:10.35841/anesthesiology.2.1.1-10
- Sundaram S, Webster GF. Vascular diseases are the most common cutaneous manifestations of reflex sympathetic dystrophy. J Am Acad Dermatol. 2001;44:1050-1051. doi:10.1067/mjd.2001.114299
- Taylor RS, Van Buyten J-P, Buchser E. Spinal stimulation for complex regional pain syndrome: a systematic review of the clinical and cost-effectiveness literature and assessment of prognostic factors. Eur J Pain. 2006;10:91-101. doi:10.1016/j.ejpain.2005.02.004
PRACTICE POINTS
- Common dermatologic manifestations of complex regional pain syndrome (CRPS), which often are nonspecific and often the presenting symptoms of the syndrome, include allodynia, edema, erythema, hypopigmentation or hyperpigmentation, and petechiae.
- Diagnosis and management of CRPS are the most important steps in treating dermatologic manifestations of the syndrome.
Factors Influencing Patient Preferences for Phototherapy: A Survey Study
Phototherapy—particularly UVB phototherapy, which utilizes UVB rays of specific wavelengths within the UV spectrum—is indicated for a wide variety of dermatoses. In-office and at-home UVB treatments commonly are used, as are salon tanning and sunbathing. When selecting a form of phototherapy, patients are likely to consider safety, cost, effectiveness, insurance issues, and convenience. Research on patient preferences; the reasons for these preferences; and which options patients perceive to be the safest, most cost-effective, efficacious, and convenient is lacking. We aimed to assess the forms of phototherapy that patients would most consider using; the factors influencing patient preferences; and the forms patients perceived as the safest and most cost-effective, efficacious, and convenient.
Methods
Study Participants—We recruited 500 Amazon Mechanical Turk users who were 18 years or older to complete our REDCap-generated survey. The study was approved by the Wake Forest University institutional review board (Winston-Salem, North Carolina).
Evaluation—Participants were asked, “If you were diagnosed with a skin disease that benefited from UV therapy, which of the following forms of UV therapy would you consider choosing?” Participants were instructed to choose all of the forms they would consider using. Available options included in-office UV, at-home UV, home tanning, salon tanning, sunbathing, and other. Participants were asked to select which factors—from safety, cost, effectiveness, issues with insurance, convenience, and other—influenced their decision-making; which form of phototherapy they would most consider along with the factors that influenced their preference for this specific form of phototherapy; and which options they considered to be safest and most cost-effective, efficacious, and convenient. Participants were asked to provide basic sociodemographic information, level of education, income, insurance status (private, Medicare, Medicaid, Veterans Affairs, and uninsured), and distance from the nearest dermatologist.
Statistical Analysis—Descriptive and inferential statistics (χ2 test) were used to analyze the data with a significance set at P<.05.
Results
Five hundred participants completed the survey (Table 1).
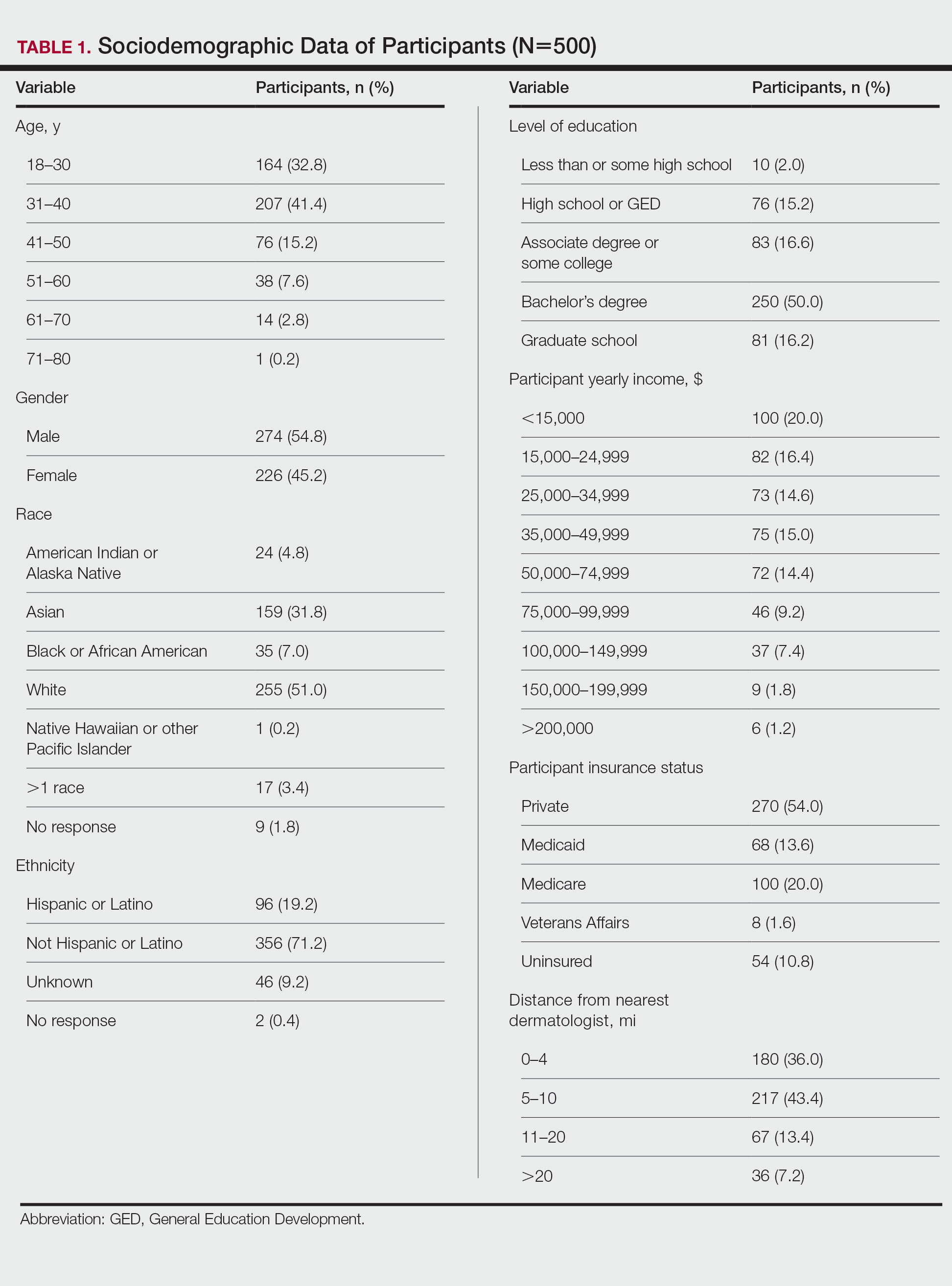
Factors Influencing Patient Preferences—When asked to select all forms of phototherapy they would consider, 186 (37.2%) participants selected in-office UVB, 263 (52.6%) selected at-home UV, 141 (28.2%) selected home tanning, 117 (23.4%) selected salon tanning, 191 (38.2%) selected sunbathing, and 3 (0.6%) selected other. Participants who selected in-office UVB as an option were more likely to also select salon tanning (P<.012). No other relationship was found between the UVB options and the tanning options. When asked which factors influenced their phototherapy preferences, 295 (59%) selected convenience, 266 (53.2%) selected effectiveness, 220 (44%) selected safety, 218 (43.6%) selected cost, 72 (14.4%) selected issues with insurance, and 4 (0.8%) selected other. Forms of Phototherapy Patients Consider Using—When asked which form of phototherapy they would most consider using, 179 (35.8%) participants selected at-home UVB, 108 (21.6%) selected sunbathing, 92 (18.4%) selected in-office UVB, 62 (12.4%) selected home-tanning, 57 (11.4%) selected salon tanning, 1 (0.2%) selected other, and 1 participant provided no response (P<.001).
Reasons for Using Phototherapy—Of the 179 who selected at-home UVB, 125 (70%) cited convenience as a reason. Of the 108 who selected salon tanning as their top choice, 62 (57%) cited cost as a reason. Convenience (P<.001), cost (P<.001), and safety (P=.023) were related to top preference. Issues with insurance did not have a statistically significant relationship with the top preference. However, participant insurance type was related to top phototherapy preference (P=.021), with privately insured patients more likely to select in-office UVB, whereas those with Medicaid and Medicare were more likely to select home or salon tanning. Efficacy was not related to top preference. Furthermore, age, gender, education, income, and distance from nearest dermatologist were not related to top preference.
In-office UVB was perceived to be safest (P<.001) and most efficacious (P<.001). Meanwhile, at-home UVB was selected as most convenient (P<.001). Lastly, sunbathing was determined to be most cost-effective (P<.001)(Table 2). Cost-effectiveness had a relationship (P<.001) with the participant’s insurance, as those with private insurance were more likely to select at-home UVB, whereas those with Medicare or Medicaid were more likely to select the tanning options. Additionally, of the54 uninsured participants in the survey, 29 selected sunbathing as the most cost-effective option.
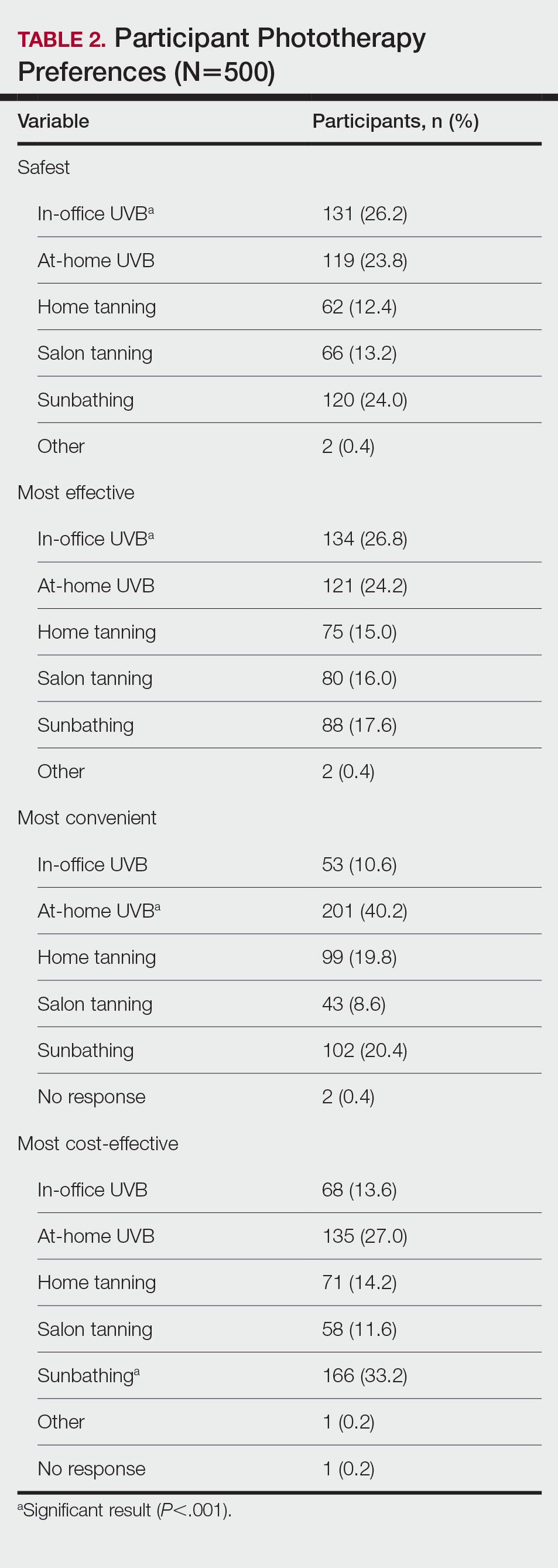
Comment
Phototherapy Treatment—UVB phototherapy at a wavelength of 290 to 320 nm (311–313 nm for narrowband UVB) is used to treat various dermatoses, including psoriasis and atopic dermatitis. UVB alters skin cytokines, induces apoptosis, promotes immunosuppression, causes DNA damage, and decreases the proliferation of dendritic cells and other cells of the innate immune system.1 In-office and at-home UV therapies make use of UVB wavelengths for treatment, while tanning and sunbathing contain not only UVB but also potentially harmful UVA rays. The wavelengths for indoor tanning devices include UVB at 280 to 315 nm and UVA at 315 to 400 nm, which are similar to those of the sun but with a different ratio of UVB to UVA and more intense total UV.2 When in-office and at-home UVB options are not available, various forms of tanning such as salon tanning and sunbathing may be alternatives that are widely used.3 One of the main reasons patients consider alternative phototherapy options is cost, as 1 in-office UVB treatment may cost $140, but a month of unlimited tanning may cost $30 or perhaps nothing if a patient has a gym membership with access to a tanning bed. Lack of insurance benefits covering phototherapy can exacerbate cost burden.4 However, tanning beds are associated with an increased risk for melanoma and nonmelanoma cancers.5,6 Additionally, all forms of phototherapy are associated with photoaging, but it is more intense with tanning and heliotherapy because of the presence of UVA, which penetrates deeper into the dermis.7 Meanwhile, for those who choose UVB therapy, deciding between an in-office and at-home UVB treatment could be a matter of convenience, as patients must consider long trips to the physician’s office; insurance status, as some insurances may not cover at-home UVB; or efficacy, which might be influenced by the presence of a physician or other medical staff. In many cases, patients may not be informed that at-home UVB is an option.
Patient Preferences—At-home UVB therapy was the most popular option in our study population, with most participants (52.6%) considering using it, and 35.9% choosing it as their top choice over all other phototherapy options. Safety, cost, and convenience were all found to be related to the option participants would most consider using. Prior analysis between at-home UVB and in-office UVB for the treatment of psoriasis determined that at-home UVB is as safe and cost-effective as in-office UVB without the inconvenience of the patient having to take time out of the week to visit the physician’s office,8,9 making at-home UVB an option dermatologists may strongly consider for patients who value safety, cost, and convenience. Oddly, efficacy was not related to the top preference, despite being the second highest–cited factor (53.2%) for which forms of phototherapy participants would consider using. For insurance coverage, those with Medicaid and Medicare selected the cheaper tanning options with higher-than-expected frequencies. Although problems with insurance were not related to the top preference, insurance status was related, suggesting that preferences are tied to cost. Of note, while the number of dermatologists that accept Medicare has increased in the last few years, there still remains an uneven distribution of phototherapy clinics. As of 2015, there were 19 million individuals who qualified for Medicare without a clinic within driving distance.10 This problem likely also exists for many Medicaid patients who may not qualify for at-home UVB. In this scenario, tanning or heliotherapy may be effective alternatives.
In-Office vs At-Home Options—Although in-office UVB was the option considered safest (26.2%) and most efficacious (26.8%), it was followed closely by at-home UVB in both categories (safest, 23.8%; most efficacious, 24.2%). Meanwhile, at-home UVB (40.2%) was chosen as the most convenient. Some patients consider tanning options over in-office UVB because of the inconvenience of traveling to an appointment.11 Therefore, at-home tanning may be a convenient alternative for these patients.
Considerations—Although our study was limited to an adult population, issues with convenience exist for the pediatric population as well, as children may need to miss multiple days of school each week to be treated in the office. For these pediatric patients, an at-home unit is preferable; however; issues with insurance coverage remain a challenge.12 Increasing insurance coverage of at-home units for the pediatric population therefore would be most prudent. However, when other options have been exhausted, including in-office UVB, tanning and sunbathing may be viable alternatives because of cost and convenience. In our study, sunbathing (33.2%) was considered the most cost-effective, likely because it does not require expensive equipment or a visit to a salon or physician’s office. Sunbathing has been effective in treating some dermatologic conditions, such as atopic dermatitis.13 However, it may only be effective during certain months and at different latitudes—conditions that make UVB sun rays more accessible—particularly when treating psoriasis.14 Furthermore, sunbathing may not be as cost-effective in patients with average-severity psoriasis compared with conventional psoriasis therapy because of the costs of travel to areas with sufficient UVB rays for treatment.15 Additionally, insurance status was related to which option was selected as the most cost-effective, as 29 (53.7%) of 54 uninsured participants chose sunbathing as the most cost-effective option, while only 92 (34.2%) of 269 privately insured patients selected sunbathing. Therefore, insurance status may be a factor for dermatologists to consider if a patient prefers a treatment that is cost-effective. Overall, dermatologists could perhaps consider guiding patients and optimizing their treatment plans based on the factors most important to the patients while understanding that costs and insurance status may ultimately determine the treatment option.
Limitations—Survey participants were recruited on Amazon Mechanical Turk, which could create sampling bias. Furthermore, these participants were representative of the general public and not exclusively patients on phototherapy, therefore representing the opinions of the general public and not those who may require phototherapy. Furthermore, given the nature of the survey, the study was limited to the adult population.
- Totonchy MB, Chiu MW. UV-based therapy. Dermatol Clin. 2014;32:399-413, ix-x.
- Nilsen LT, Hannevik M, Veierød MB. Ultraviolet exposure from indoor tanning devices: a systematic review. Br J Dermatol. 2016;174:730-740.
- Su J, Pearce DJ, Feldman SR. The role of commercial tanning beds and ultraviolet A light in the treatment of psoriasis. J Dermatolog Treat. 2005;16:324-326.
- Anderson KL, Huang KE, Huang WW, et al. Dermatology residents are prescribing tanning bed treatment. Dermatol Online J. 2016;22:13030/qt19h4k7sx.
- Wehner MR, Shive ML, Chren MM, et al. Indoor tanning and non-melanoma skin cancer: systematic review and meta-analysis. BMJ. 2012;345:e5909.
- Boniol M, Autier P, Boyle P, et al. Cutaneous melanomaattributable to sunbed use: systematic review and meta-analysis. BMJ. 2012;345:E4757.
- Barros NM, Sbroglio LL, Buffara MO, et al. Phototherapy. An Bras Dermatol. 2021;96:397-407.
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomized controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:b1542.
- Koek MB, Sigurdsson V, van Weelden H, et al. Cost effectiveness of home ultraviolet B phototherapy for psoriasis: economic evaluation of a randomized controlled trial (PLUTO study). BMJ. 2010;340:c1490.
- Tan SY, Buzney E, Mostaghimi A. Trends in phototherapy utilization among Medicare beneficiaries in the United States, 2000 to 2015. J Am Acad Dermatol. 2018;79:672-679.
- Felton S, Adinoff B, Jeon-Slaughter H, et al. The significant health threat from tanning bed use as a self-treatment for psoriasis. J Am Acad Dermatol. 2016;74:1015-1017.
- Juarez MC, Grossberg AL. Phototherapy in the pediatric population. Dermatol Clin. 2020;38:91-108.
- Autio P, Komulainen P, Larni HM. Heliotherapy in atopic dermatitis: a prospective study on climatotherapy using the SCORAD index. Acta Derm Venereol. 2002;82:436-440.
- Krzys´cin JW, Jarosławski J, Rajewska-Wie˛ch B, et al. Effectiveness of heliotherapy for psoriasis clearance in low and mid-latitudinal regions: a theoretical approach. J Photochem Photobiol B. 2012;115:35-41.
- Snellman E, Maljanen T, Aromaa A, et al. Effect of heliotherapy on the cost of psoriasis. Br J Dermatol. 1998;138:288-292.
Phototherapy—particularly UVB phototherapy, which utilizes UVB rays of specific wavelengths within the UV spectrum—is indicated for a wide variety of dermatoses. In-office and at-home UVB treatments commonly are used, as are salon tanning and sunbathing. When selecting a form of phototherapy, patients are likely to consider safety, cost, effectiveness, insurance issues, and convenience. Research on patient preferences; the reasons for these preferences; and which options patients perceive to be the safest, most cost-effective, efficacious, and convenient is lacking. We aimed to assess the forms of phototherapy that patients would most consider using; the factors influencing patient preferences; and the forms patients perceived as the safest and most cost-effective, efficacious, and convenient.
Methods
Study Participants—We recruited 500 Amazon Mechanical Turk users who were 18 years or older to complete our REDCap-generated survey. The study was approved by the Wake Forest University institutional review board (Winston-Salem, North Carolina).
Evaluation—Participants were asked, “If you were diagnosed with a skin disease that benefited from UV therapy, which of the following forms of UV therapy would you consider choosing?” Participants were instructed to choose all of the forms they would consider using. Available options included in-office UV, at-home UV, home tanning, salon tanning, sunbathing, and other. Participants were asked to select which factors—from safety, cost, effectiveness, issues with insurance, convenience, and other—influenced their decision-making; which form of phototherapy they would most consider along with the factors that influenced their preference for this specific form of phototherapy; and which options they considered to be safest and most cost-effective, efficacious, and convenient. Participants were asked to provide basic sociodemographic information, level of education, income, insurance status (private, Medicare, Medicaid, Veterans Affairs, and uninsured), and distance from the nearest dermatologist.
Statistical Analysis—Descriptive and inferential statistics (χ2 test) were used to analyze the data with a significance set at P<.05.
Results
Five hundred participants completed the survey (Table 1).

Factors Influencing Patient Preferences—When asked to select all forms of phototherapy they would consider, 186 (37.2%) participants selected in-office UVB, 263 (52.6%) selected at-home UV, 141 (28.2%) selected home tanning, 117 (23.4%) selected salon tanning, 191 (38.2%) selected sunbathing, and 3 (0.6%) selected other. Participants who selected in-office UVB as an option were more likely to also select salon tanning (P<.012). No other relationship was found between the UVB options and the tanning options. When asked which factors influenced their phototherapy preferences, 295 (59%) selected convenience, 266 (53.2%) selected effectiveness, 220 (44%) selected safety, 218 (43.6%) selected cost, 72 (14.4%) selected issues with insurance, and 4 (0.8%) selected other. Forms of Phototherapy Patients Consider Using—When asked which form of phototherapy they would most consider using, 179 (35.8%) participants selected at-home UVB, 108 (21.6%) selected sunbathing, 92 (18.4%) selected in-office UVB, 62 (12.4%) selected home-tanning, 57 (11.4%) selected salon tanning, 1 (0.2%) selected other, and 1 participant provided no response (P<.001).
Reasons for Using Phototherapy—Of the 179 who selected at-home UVB, 125 (70%) cited convenience as a reason. Of the 108 who selected salon tanning as their top choice, 62 (57%) cited cost as a reason. Convenience (P<.001), cost (P<.001), and safety (P=.023) were related to top preference. Issues with insurance did not have a statistically significant relationship with the top preference. However, participant insurance type was related to top phototherapy preference (P=.021), with privately insured patients more likely to select in-office UVB, whereas those with Medicaid and Medicare were more likely to select home or salon tanning. Efficacy was not related to top preference. Furthermore, age, gender, education, income, and distance from nearest dermatologist were not related to top preference.
In-office UVB was perceived to be safest (P<.001) and most efficacious (P<.001). Meanwhile, at-home UVB was selected as most convenient (P<.001). Lastly, sunbathing was determined to be most cost-effective (P<.001)(Table 2). Cost-effectiveness had a relationship (P<.001) with the participant’s insurance, as those with private insurance were more likely to select at-home UVB, whereas those with Medicare or Medicaid were more likely to select the tanning options. Additionally, of the54 uninsured participants in the survey, 29 selected sunbathing as the most cost-effective option.

Comment
Phototherapy Treatment—UVB phototherapy at a wavelength of 290 to 320 nm (311–313 nm for narrowband UVB) is used to treat various dermatoses, including psoriasis and atopic dermatitis. UVB alters skin cytokines, induces apoptosis, promotes immunosuppression, causes DNA damage, and decreases the proliferation of dendritic cells and other cells of the innate immune system.1 In-office and at-home UV therapies make use of UVB wavelengths for treatment, while tanning and sunbathing contain not only UVB but also potentially harmful UVA rays. The wavelengths for indoor tanning devices include UVB at 280 to 315 nm and UVA at 315 to 400 nm, which are similar to those of the sun but with a different ratio of UVB to UVA and more intense total UV.2 When in-office and at-home UVB options are not available, various forms of tanning such as salon tanning and sunbathing may be alternatives that are widely used.3 One of the main reasons patients consider alternative phototherapy options is cost, as 1 in-office UVB treatment may cost $140, but a month of unlimited tanning may cost $30 or perhaps nothing if a patient has a gym membership with access to a tanning bed. Lack of insurance benefits covering phototherapy can exacerbate cost burden.4 However, tanning beds are associated with an increased risk for melanoma and nonmelanoma cancers.5,6 Additionally, all forms of phototherapy are associated with photoaging, but it is more intense with tanning and heliotherapy because of the presence of UVA, which penetrates deeper into the dermis.7 Meanwhile, for those who choose UVB therapy, deciding between an in-office and at-home UVB treatment could be a matter of convenience, as patients must consider long trips to the physician’s office; insurance status, as some insurances may not cover at-home UVB; or efficacy, which might be influenced by the presence of a physician or other medical staff. In many cases, patients may not be informed that at-home UVB is an option.
Patient Preferences—At-home UVB therapy was the most popular option in our study population, with most participants (52.6%) considering using it, and 35.9% choosing it as their top choice over all other phototherapy options. Safety, cost, and convenience were all found to be related to the option participants would most consider using. Prior analysis between at-home UVB and in-office UVB for the treatment of psoriasis determined that at-home UVB is as safe and cost-effective as in-office UVB without the inconvenience of the patient having to take time out of the week to visit the physician’s office,8,9 making at-home UVB an option dermatologists may strongly consider for patients who value safety, cost, and convenience. Oddly, efficacy was not related to the top preference, despite being the second highest–cited factor (53.2%) for which forms of phototherapy participants would consider using. For insurance coverage, those with Medicaid and Medicare selected the cheaper tanning options with higher-than-expected frequencies. Although problems with insurance were not related to the top preference, insurance status was related, suggesting that preferences are tied to cost. Of note, while the number of dermatologists that accept Medicare has increased in the last few years, there still remains an uneven distribution of phototherapy clinics. As of 2015, there were 19 million individuals who qualified for Medicare without a clinic within driving distance.10 This problem likely also exists for many Medicaid patients who may not qualify for at-home UVB. In this scenario, tanning or heliotherapy may be effective alternatives.
In-Office vs At-Home Options—Although in-office UVB was the option considered safest (26.2%) and most efficacious (26.8%), it was followed closely by at-home UVB in both categories (safest, 23.8%; most efficacious, 24.2%). Meanwhile, at-home UVB (40.2%) was chosen as the most convenient. Some patients consider tanning options over in-office UVB because of the inconvenience of traveling to an appointment.11 Therefore, at-home tanning may be a convenient alternative for these patients.
Considerations—Although our study was limited to an adult population, issues with convenience exist for the pediatric population as well, as children may need to miss multiple days of school each week to be treated in the office. For these pediatric patients, an at-home unit is preferable; however; issues with insurance coverage remain a challenge.12 Increasing insurance coverage of at-home units for the pediatric population therefore would be most prudent. However, when other options have been exhausted, including in-office UVB, tanning and sunbathing may be viable alternatives because of cost and convenience. In our study, sunbathing (33.2%) was considered the most cost-effective, likely because it does not require expensive equipment or a visit to a salon or physician’s office. Sunbathing has been effective in treating some dermatologic conditions, such as atopic dermatitis.13 However, it may only be effective during certain months and at different latitudes—conditions that make UVB sun rays more accessible—particularly when treating psoriasis.14 Furthermore, sunbathing may not be as cost-effective in patients with average-severity psoriasis compared with conventional psoriasis therapy because of the costs of travel to areas with sufficient UVB rays for treatment.15 Additionally, insurance status was related to which option was selected as the most cost-effective, as 29 (53.7%) of 54 uninsured participants chose sunbathing as the most cost-effective option, while only 92 (34.2%) of 269 privately insured patients selected sunbathing. Therefore, insurance status may be a factor for dermatologists to consider if a patient prefers a treatment that is cost-effective. Overall, dermatologists could perhaps consider guiding patients and optimizing their treatment plans based on the factors most important to the patients while understanding that costs and insurance status may ultimately determine the treatment option.
Limitations—Survey participants were recruited on Amazon Mechanical Turk, which could create sampling bias. Furthermore, these participants were representative of the general public and not exclusively patients on phototherapy, therefore representing the opinions of the general public and not those who may require phototherapy. Furthermore, given the nature of the survey, the study was limited to the adult population.
Phototherapy—particularly UVB phototherapy, which utilizes UVB rays of specific wavelengths within the UV spectrum—is indicated for a wide variety of dermatoses. In-office and at-home UVB treatments commonly are used, as are salon tanning and sunbathing. When selecting a form of phototherapy, patients are likely to consider safety, cost, effectiveness, insurance issues, and convenience. Research on patient preferences; the reasons for these preferences; and which options patients perceive to be the safest, most cost-effective, efficacious, and convenient is lacking. We aimed to assess the forms of phototherapy that patients would most consider using; the factors influencing patient preferences; and the forms patients perceived as the safest and most cost-effective, efficacious, and convenient.
Methods
Study Participants—We recruited 500 Amazon Mechanical Turk users who were 18 years or older to complete our REDCap-generated survey. The study was approved by the Wake Forest University institutional review board (Winston-Salem, North Carolina).
Evaluation—Participants were asked, “If you were diagnosed with a skin disease that benefited from UV therapy, which of the following forms of UV therapy would you consider choosing?” Participants were instructed to choose all of the forms they would consider using. Available options included in-office UV, at-home UV, home tanning, salon tanning, sunbathing, and other. Participants were asked to select which factors—from safety, cost, effectiveness, issues with insurance, convenience, and other—influenced their decision-making; which form of phototherapy they would most consider along with the factors that influenced their preference for this specific form of phototherapy; and which options they considered to be safest and most cost-effective, efficacious, and convenient. Participants were asked to provide basic sociodemographic information, level of education, income, insurance status (private, Medicare, Medicaid, Veterans Affairs, and uninsured), and distance from the nearest dermatologist.
Statistical Analysis—Descriptive and inferential statistics (χ2 test) were used to analyze the data with a significance set at P<.05.
Results
Five hundred participants completed the survey (Table 1).

Factors Influencing Patient Preferences—When asked to select all forms of phototherapy they would consider, 186 (37.2%) participants selected in-office UVB, 263 (52.6%) selected at-home UV, 141 (28.2%) selected home tanning, 117 (23.4%) selected salon tanning, 191 (38.2%) selected sunbathing, and 3 (0.6%) selected other. Participants who selected in-office UVB as an option were more likely to also select salon tanning (P<.012). No other relationship was found between the UVB options and the tanning options. When asked which factors influenced their phototherapy preferences, 295 (59%) selected convenience, 266 (53.2%) selected effectiveness, 220 (44%) selected safety, 218 (43.6%) selected cost, 72 (14.4%) selected issues with insurance, and 4 (0.8%) selected other. Forms of Phototherapy Patients Consider Using—When asked which form of phototherapy they would most consider using, 179 (35.8%) participants selected at-home UVB, 108 (21.6%) selected sunbathing, 92 (18.4%) selected in-office UVB, 62 (12.4%) selected home-tanning, 57 (11.4%) selected salon tanning, 1 (0.2%) selected other, and 1 participant provided no response (P<.001).
Reasons for Using Phototherapy—Of the 179 who selected at-home UVB, 125 (70%) cited convenience as a reason. Of the 108 who selected salon tanning as their top choice, 62 (57%) cited cost as a reason. Convenience (P<.001), cost (P<.001), and safety (P=.023) were related to top preference. Issues with insurance did not have a statistically significant relationship with the top preference. However, participant insurance type was related to top phototherapy preference (P=.021), with privately insured patients more likely to select in-office UVB, whereas those with Medicaid and Medicare were more likely to select home or salon tanning. Efficacy was not related to top preference. Furthermore, age, gender, education, income, and distance from nearest dermatologist were not related to top preference.
In-office UVB was perceived to be safest (P<.001) and most efficacious (P<.001). Meanwhile, at-home UVB was selected as most convenient (P<.001). Lastly, sunbathing was determined to be most cost-effective (P<.001)(Table 2). Cost-effectiveness had a relationship (P<.001) with the participant’s insurance, as those with private insurance were more likely to select at-home UVB, whereas those with Medicare or Medicaid were more likely to select the tanning options. Additionally, of the54 uninsured participants in the survey, 29 selected sunbathing as the most cost-effective option.

Comment
Phototherapy Treatment—UVB phototherapy at a wavelength of 290 to 320 nm (311–313 nm for narrowband UVB) is used to treat various dermatoses, including psoriasis and atopic dermatitis. UVB alters skin cytokines, induces apoptosis, promotes immunosuppression, causes DNA damage, and decreases the proliferation of dendritic cells and other cells of the innate immune system.1 In-office and at-home UV therapies make use of UVB wavelengths for treatment, while tanning and sunbathing contain not only UVB but also potentially harmful UVA rays. The wavelengths for indoor tanning devices include UVB at 280 to 315 nm and UVA at 315 to 400 nm, which are similar to those of the sun but with a different ratio of UVB to UVA and more intense total UV.2 When in-office and at-home UVB options are not available, various forms of tanning such as salon tanning and sunbathing may be alternatives that are widely used.3 One of the main reasons patients consider alternative phototherapy options is cost, as 1 in-office UVB treatment may cost $140, but a month of unlimited tanning may cost $30 or perhaps nothing if a patient has a gym membership with access to a tanning bed. Lack of insurance benefits covering phototherapy can exacerbate cost burden.4 However, tanning beds are associated with an increased risk for melanoma and nonmelanoma cancers.5,6 Additionally, all forms of phototherapy are associated with photoaging, but it is more intense with tanning and heliotherapy because of the presence of UVA, which penetrates deeper into the dermis.7 Meanwhile, for those who choose UVB therapy, deciding between an in-office and at-home UVB treatment could be a matter of convenience, as patients must consider long trips to the physician’s office; insurance status, as some insurances may not cover at-home UVB; or efficacy, which might be influenced by the presence of a physician or other medical staff. In many cases, patients may not be informed that at-home UVB is an option.
Patient Preferences—At-home UVB therapy was the most popular option in our study population, with most participants (52.6%) considering using it, and 35.9% choosing it as their top choice over all other phototherapy options. Safety, cost, and convenience were all found to be related to the option participants would most consider using. Prior analysis between at-home UVB and in-office UVB for the treatment of psoriasis determined that at-home UVB is as safe and cost-effective as in-office UVB without the inconvenience of the patient having to take time out of the week to visit the physician’s office,8,9 making at-home UVB an option dermatologists may strongly consider for patients who value safety, cost, and convenience. Oddly, efficacy was not related to the top preference, despite being the second highest–cited factor (53.2%) for which forms of phototherapy participants would consider using. For insurance coverage, those with Medicaid and Medicare selected the cheaper tanning options with higher-than-expected frequencies. Although problems with insurance were not related to the top preference, insurance status was related, suggesting that preferences are tied to cost. Of note, while the number of dermatologists that accept Medicare has increased in the last few years, there still remains an uneven distribution of phototherapy clinics. As of 2015, there were 19 million individuals who qualified for Medicare without a clinic within driving distance.10 This problem likely also exists for many Medicaid patients who may not qualify for at-home UVB. In this scenario, tanning or heliotherapy may be effective alternatives.
In-Office vs At-Home Options—Although in-office UVB was the option considered safest (26.2%) and most efficacious (26.8%), it was followed closely by at-home UVB in both categories (safest, 23.8%; most efficacious, 24.2%). Meanwhile, at-home UVB (40.2%) was chosen as the most convenient. Some patients consider tanning options over in-office UVB because of the inconvenience of traveling to an appointment.11 Therefore, at-home tanning may be a convenient alternative for these patients.
Considerations—Although our study was limited to an adult population, issues with convenience exist for the pediatric population as well, as children may need to miss multiple days of school each week to be treated in the office. For these pediatric patients, an at-home unit is preferable; however; issues with insurance coverage remain a challenge.12 Increasing insurance coverage of at-home units for the pediatric population therefore would be most prudent. However, when other options have been exhausted, including in-office UVB, tanning and sunbathing may be viable alternatives because of cost and convenience. In our study, sunbathing (33.2%) was considered the most cost-effective, likely because it does not require expensive equipment or a visit to a salon or physician’s office. Sunbathing has been effective in treating some dermatologic conditions, such as atopic dermatitis.13 However, it may only be effective during certain months and at different latitudes—conditions that make UVB sun rays more accessible—particularly when treating psoriasis.14 Furthermore, sunbathing may not be as cost-effective in patients with average-severity psoriasis compared with conventional psoriasis therapy because of the costs of travel to areas with sufficient UVB rays for treatment.15 Additionally, insurance status was related to which option was selected as the most cost-effective, as 29 (53.7%) of 54 uninsured participants chose sunbathing as the most cost-effective option, while only 92 (34.2%) of 269 privately insured patients selected sunbathing. Therefore, insurance status may be a factor for dermatologists to consider if a patient prefers a treatment that is cost-effective. Overall, dermatologists could perhaps consider guiding patients and optimizing their treatment plans based on the factors most important to the patients while understanding that costs and insurance status may ultimately determine the treatment option.
Limitations—Survey participants were recruited on Amazon Mechanical Turk, which could create sampling bias. Furthermore, these participants were representative of the general public and not exclusively patients on phototherapy, therefore representing the opinions of the general public and not those who may require phototherapy. Furthermore, given the nature of the survey, the study was limited to the adult population.
- Totonchy MB, Chiu MW. UV-based therapy. Dermatol Clin. 2014;32:399-413, ix-x.
- Nilsen LT, Hannevik M, Veierød MB. Ultraviolet exposure from indoor tanning devices: a systematic review. Br J Dermatol. 2016;174:730-740.
- Su J, Pearce DJ, Feldman SR. The role of commercial tanning beds and ultraviolet A light in the treatment of psoriasis. J Dermatolog Treat. 2005;16:324-326.
- Anderson KL, Huang KE, Huang WW, et al. Dermatology residents are prescribing tanning bed treatment. Dermatol Online J. 2016;22:13030/qt19h4k7sx.
- Wehner MR, Shive ML, Chren MM, et al. Indoor tanning and non-melanoma skin cancer: systematic review and meta-analysis. BMJ. 2012;345:e5909.
- Boniol M, Autier P, Boyle P, et al. Cutaneous melanomaattributable to sunbed use: systematic review and meta-analysis. BMJ. 2012;345:E4757.
- Barros NM, Sbroglio LL, Buffara MO, et al. Phototherapy. An Bras Dermatol. 2021;96:397-407.
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomized controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:b1542.
- Koek MB, Sigurdsson V, van Weelden H, et al. Cost effectiveness of home ultraviolet B phototherapy for psoriasis: economic evaluation of a randomized controlled trial (PLUTO study). BMJ. 2010;340:c1490.
- Tan SY, Buzney E, Mostaghimi A. Trends in phototherapy utilization among Medicare beneficiaries in the United States, 2000 to 2015. J Am Acad Dermatol. 2018;79:672-679.
- Felton S, Adinoff B, Jeon-Slaughter H, et al. The significant health threat from tanning bed use as a self-treatment for psoriasis. J Am Acad Dermatol. 2016;74:1015-1017.
- Juarez MC, Grossberg AL. Phototherapy in the pediatric population. Dermatol Clin. 2020;38:91-108.
- Autio P, Komulainen P, Larni HM. Heliotherapy in atopic dermatitis: a prospective study on climatotherapy using the SCORAD index. Acta Derm Venereol. 2002;82:436-440.
- Krzys´cin JW, Jarosławski J, Rajewska-Wie˛ch B, et al. Effectiveness of heliotherapy for psoriasis clearance in low and mid-latitudinal regions: a theoretical approach. J Photochem Photobiol B. 2012;115:35-41.
- Snellman E, Maljanen T, Aromaa A, et al. Effect of heliotherapy on the cost of psoriasis. Br J Dermatol. 1998;138:288-292.
- Totonchy MB, Chiu MW. UV-based therapy. Dermatol Clin. 2014;32:399-413, ix-x.
- Nilsen LT, Hannevik M, Veierød MB. Ultraviolet exposure from indoor tanning devices: a systematic review. Br J Dermatol. 2016;174:730-740.
- Su J, Pearce DJ, Feldman SR. The role of commercial tanning beds and ultraviolet A light in the treatment of psoriasis. J Dermatolog Treat. 2005;16:324-326.
- Anderson KL, Huang KE, Huang WW, et al. Dermatology residents are prescribing tanning bed treatment. Dermatol Online J. 2016;22:13030/qt19h4k7sx.
- Wehner MR, Shive ML, Chren MM, et al. Indoor tanning and non-melanoma skin cancer: systematic review and meta-analysis. BMJ. 2012;345:e5909.
- Boniol M, Autier P, Boyle P, et al. Cutaneous melanomaattributable to sunbed use: systematic review and meta-analysis. BMJ. 2012;345:E4757.
- Barros NM, Sbroglio LL, Buffara MO, et al. Phototherapy. An Bras Dermatol. 2021;96:397-407.
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomized controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:b1542.
- Koek MB, Sigurdsson V, van Weelden H, et al. Cost effectiveness of home ultraviolet B phototherapy for psoriasis: economic evaluation of a randomized controlled trial (PLUTO study). BMJ. 2010;340:c1490.
- Tan SY, Buzney E, Mostaghimi A. Trends in phototherapy utilization among Medicare beneficiaries in the United States, 2000 to 2015. J Am Acad Dermatol. 2018;79:672-679.
- Felton S, Adinoff B, Jeon-Slaughter H, et al. The significant health threat from tanning bed use as a self-treatment for psoriasis. J Am Acad Dermatol. 2016;74:1015-1017.
- Juarez MC, Grossberg AL. Phototherapy in the pediatric population. Dermatol Clin. 2020;38:91-108.
- Autio P, Komulainen P, Larni HM. Heliotherapy in atopic dermatitis: a prospective study on climatotherapy using the SCORAD index. Acta Derm Venereol. 2002;82:436-440.
- Krzys´cin JW, Jarosławski J, Rajewska-Wie˛ch B, et al. Effectiveness of heliotherapy for psoriasis clearance in low and mid-latitudinal regions: a theoretical approach. J Photochem Photobiol B. 2012;115:35-41.
- Snellman E, Maljanen T, Aromaa A, et al. Effect of heliotherapy on the cost of psoriasis. Br J Dermatol. 1998;138:288-292.
Practice Points
- Patients have different priorities when selecting phototherapy, including safety, costs, effectiveness, insurance issues, and convenience.
- By offering and educating patients on all forms of phototherapy, dermatologists may help guide patients to their optimal treatment plan according to patient priorities.
Pyostomatitis Vegetans With Orofacial and Vulvar Granulomatosis in a Pediatric Patient
Case Report
A 7-year-old girl who was otherwise healthy was referred by pediatric gastroenterology for evaluation of cutaneous Crohn disease (CD). The patient had a 4-year history of persistent lip swelling and a 3-year history of asymmetric erythematous labial swelling and perianal erythema with skin tags. She had been applying the calcineurin inhibitor tacrolimus ointment 0.03% 1 or 2 times daily to her lesions with minimal improvement. She did not have a medical history of recurrent or unusual infectious diseases. There was no family history of autoimmune disease.
The patient and her guardian reported intermittent perianal pain but denied constipation, diarrhea, abdominal pain, and blood in the stool. She denied throat and tongue swelling, dysphagia, dyspnea, drooling, facial paralysis, and eyelid edema. She was a well-nourished child whose height and weight percentiles tracked at 30% and 25%, respectively. Physical examination revealed confluent symmetric lip swelling with mild angular cheilitis. Multiple 1- to 2-mm white pustules with pinpoint erosions covered the upper and lower labial mucosa and extended onto the buccal mucosa (Figure 1). She had symmetric erythema and swelling of the left labia majora extending to and involving the left perianal mucosa. Three perianal erythematous skin tags and a perianal fissure were identified.
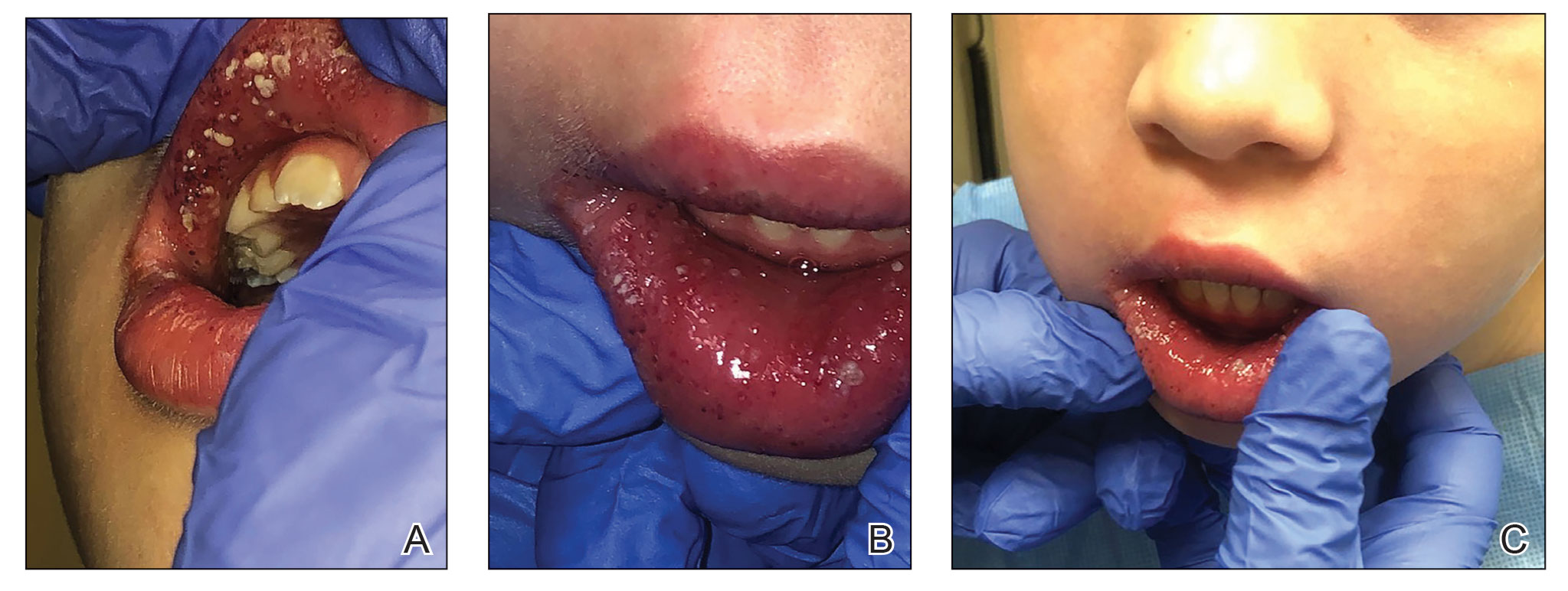
The patient had been assessed 2 years earlier by pediatric dermatology and gastroenterology with an extensive evaluation that favored a diagnosis of cutaneous CD because the combination of orofacial granulomatosis (OFG), vulvar edema, and perianal skin tags is strongly associated.1-3 Contact dermatitis affecting the mouth was considered; however, allergen testing did not demonstrate a trigger.
A trial of a benzoate- and cinnamon-free diet, which has been reported to improve OFG,4 did not provide symptomatic improvement. Topical corticosteroids and tacrolimus reduced the perioral erythema, but the swelling persisted. An infectious cause was considered; however, topical mupirocin had no effect, and amoxicillin resulted in oral candidiasis.
A perianal biopsy revealed a granulomatous dermatitis. Fungal and bacterial cultures were negative. Upper and lower gastrointestinal (GI) endoscopy and a fecal calprotectin assay were not suggestive of inflammatory bowel disease (IBD). A complete blood cell count and QuantiFERON-TB Gold test measuring the immune response to tuberculosis antigens were normal. Chronic granulomatous disease, RAG1/RAG2 deficiency, common variable immunodeficiency, and NOD2 defects were ruled out with normal tests of dihydrorhodamine, quantitative immunoglobulins, and toll-like receptors.
Because of the discomfort associated with the patient’s lesions, she was offered treatment with tumor necrosis factor α inhibitors, including infliximab and adalimumab. These agents had been offered since the onset of symptoms; however, her parents declined systemic medication unless she developed GI involvement. Instead, the tacrolimus concentration was increased to 0.1% applied to the lips, labia, and perianal area, and fluocinonide gel 0.05% applied nightly to the oral pustules was added.
Two months later the patient had notably fewer oral pustules and diminished erythema but only slightly reduced oral, vulvar, and perianal swelling. A trial of oral metronidazole, which has been reported to clear a patient with cutaneous CD,5 was discontinued by her parents after 6 weeks because of a lack of interval improvement.
One year later, a pre-existing perianal skin tag doubled in size and became exquisitely tender. The calprotectin level—previously within reference range at less than 16 μg/g—was now elevated at 149 μg/g (reference range, 1–120 μg/g) and increased to 336 μg/g 3 weeks later. Testing for C-reactive protein, zinc, and stool occult blood; a comprehensive metabolic panel; and a complete blood cell count were unremarkable.
Repeat upper and lower GI endoscopy did not suggest CD. A biopsy using direct immunofluorescence (DIF) was obtained to evaluate for pyostomatitis vegetans (PSV) and rule out
The captured biopsy did not demonstrate the intended pustule; instead, it included less-affected mucosa and was obtained during topical treatment when few pustules and erosions persisted. Pathologic analysis revealed noncaseating granulomas without an increase in microabscesses, neutrophils, or eosinophils (Figure 2). Direct immunofluorescence staining for IgG, IgA, and C3 and indirect immunofluorescence staining for desmoglein-1 and desmoglein-3 antibodies were negative. Although the biopsy did not capture the intended pustule, diagnosis of PV was made based on clinical features and the constellation of cutaneous findings associated with IBD.
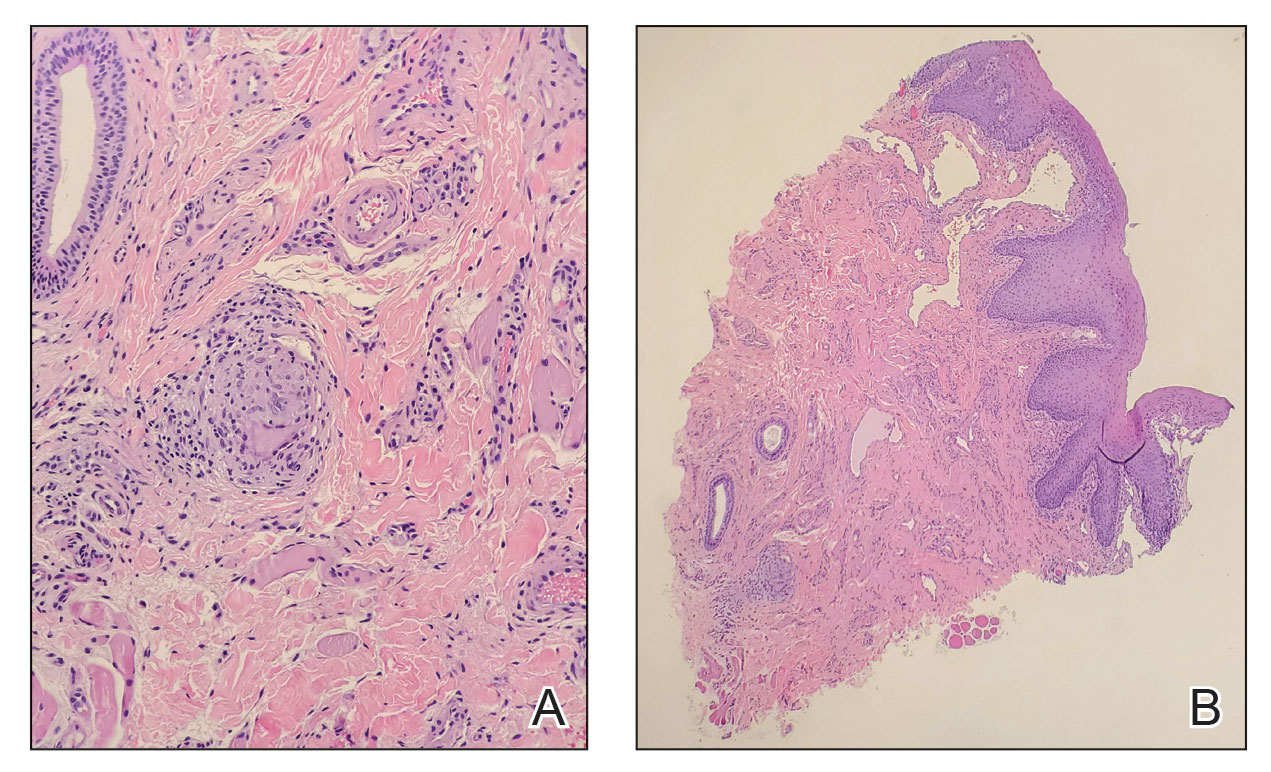
Intralesional triamcinolone, which has been of benefit for pediatric patients with orofacial granulomatosis,1,6,7 was instituted and normalized the vulva and perianal mucosa; however, lip swelling improved only minimally.
Comment
Pyostomatitis vegetans is characterized by multiple white or yellow, friable, miliary pustules that rupture, leaving behind ulcerations and erosions that cause a varying degree of oral pain.8 The disorder can involve any area of the oral mucosa—most often the labia-attached gingiva, soft and hard palates, buccal mucosa, vestibule, and tonsillar areas—but often spares the floor of the mouth and tongue.8-11 The term pyostomatitis vegetans was proposed in 1949 by McCarthy12 when he noted in a patient who presented with the characteristic appearance of the oral mucosa, though cases of vaginal, nasal, and periocular involvement have been reported.8,13,14
Histopathology—Pyostomatitis vegetans displays pseudoepithelial hyperplasia with acanthosis, hyperkeratosis, and intraepithelial or subepithelial microabscesses (or both) with neutrophils and eosinophils.8,9,15 There are a few possible explanations for this patient’s lack of tissue eosinophilia. It has been theorized that the presence of granulomas could mask concurrent PSV16 or that tissue in PSV contains fewer eosinophils as the disorder progresses.11 The oral biopsy obtained from our patient did not capture a pustule, and the condition had noticeably improved with topical tacrolimus at the time of biopsy; therefore, neither neutrophils nor eosinophils were identified. Peripheral eosinophilia, which is present in 42% to 90% of cases of PSV,9,17 can be a diagnostic clue.18 However, PE is associated with IBD,24 which usually occurs with PSV, so the absence of peripheral eosinophilia in our patient may be explained by her lack of bowel disease.
Pathogenesis—The pathogenesis of PSV is unknown. A proposed etiology includes cross-reacting antigens in the bowel and skin secondary to IBD as well as an aberrant immune response to an unidentified factor.8 Pyostomatitis vegetans is considered by many to be the mucosal variant of pyodermatitis vegetans,9,15,19 a neutrophilic dermatosis characterized by asymmetric, crusted, erythematous papulopustules that extend peripherally and coalesce to form large vegetating plaques. These lesions commonly manifest in the axillary folds, groin, and scalp and can involve the face, trunk, and distal extremities.9,18 Infection has been suggested as a cause of PSV, though cultures for pathogenic bacteria, viruses, and fungi consistently show only normal flora.20 Zinc deficiency attributed to malabsorption from CD was reported in an adult with PSV.21 The PSV resolved after 6 weeks of zinc supplementation.
Differential Diagnosis—The main entity in the clinical differential diagnosis for PSV is PVH, which is considered a variant of pemphigus vulgaris. Pemphigus vegetans of Hallopeau presents with pustules and progresses to hyperpigmented vegetative plaques with peripheral hypertrophic granulation tissue.22 The clinical and histological presentation of PVH can be similar to PSV; in PVH, however, DIF demonstrates intercellular IgG and C3 due to circulating IgG autoantibodies specific for desmoglein 3, a cell adhesion molecule.22-24 In PSV, DIF typically is negative for IgG, IgA, and C3.8 Immunohistochemical findings of PSV may overlap with IgA pemphigus, IgG/IgA pemphigus, and IgG pemphigus, which has sparked debate if PSV is an autoimmune blistering disorder or a secondary finding of epithelial injury.9,18,24
Pyostomatitis vegetans is most prevalent in patients aged 20 to 59 years25 but can occur at any age.8,19 Overall, extraintestinal symptoms, including mucocutaneous findings, are common in pediatric patients—in 30% to 71% of children with CD and 21% to 22% of children with ulcerative colitis26—and can predate onset of GI symptoms in 6% of pediatric patients.27
Oral disease is common in CD; manifestations are listed in the Table.28,29 In a prospective study of 48 children with CD, 42% (20/48) had oral manifestations identified at diagnosis28; in a similar study of 25 children, researchers noted that 48% (12/25) had disease-specific oral lesions.29 None of these children recognized the oral findings prior to the onset of systemic symptoms.28 Pyostomatitis vegetans was the least common oral manifestation, reported in 1 of 73 patients in the 2 studies combined.28,29
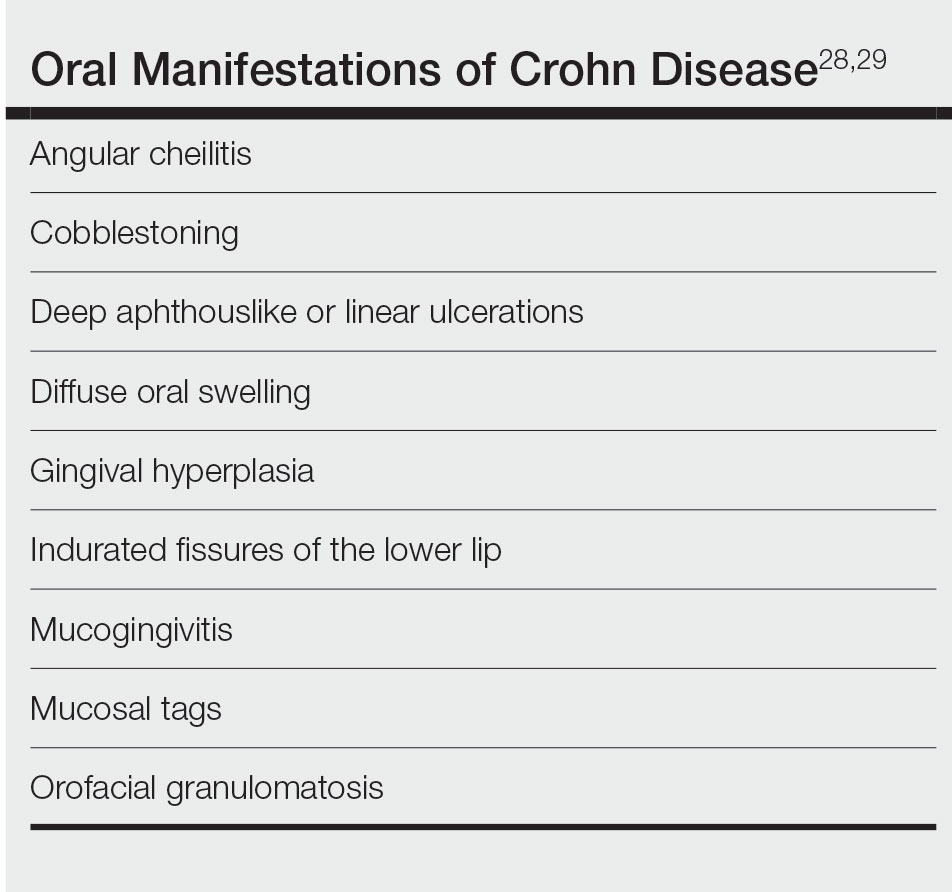
Two recent articles that looked at PSV in pediatric and adolescent populations identified only 9 patients with PSV.24,30 Only 2 patients (siblings) had documented onset of PSV before 12 years of age,31 which suggests an underlying genetic predisposition in young children.
It has been reported that active or subclinical (ie, asymptomatic with positive endoscopic findings) IBD in adults precedes onset of PSV, which may be considered a sign of relapse.9,30 However, PSV is incredibly rare in children and adolescents and can be an early finding of IBD in children.16,31,32
Our patient has not developed GI involvement since her initial presentation 5 years prior, though another pediatric patient developed symptomatic CD 9 years after onset of OFG.5 A retrospective review of pediatric OFG without CD met criteria for CD at a median of 3.1 years (range, 0.4–6.9 years).33 Regrettably, the early presence of PSV has been associated with future progression to CD and a complicated disease course.12,34
Management—Pyoderma stomatitis vegetans is treated with management of underlying IBD,8 with scarce literature available regarding pediatric patients. Oral lesions have been treated with antiseptics and topical corticosteroids, though these have limited benefit.8 In an adult with IBD, topical tacrolimus initially cleared PSV; however, lesions recurred until mesalamine was initiated.35 Systemic steroids were effective in a 16-year-old patient with CD and PSV,12 but recurrence is common after corticosteroids are stopped.34
Some patients benefit from steroid-sparing medications, such as dapsone, azathioprine, sulfamethoxypyridazine, methotrexate, mycophenolate mofetil, and tumor necrosis factor α inhibitors such as infliximab and adalimumab.8,9,15,23,34,36 A 12-year-old patient with pyodermatitis–PSV without intestinal disease was treated with prednisone, dapsone, and azathioprine with improvement but not complete resolution of oral erosions after 18 weeks of treatment.32 A 15-year-old patient with CD and pyodermatitis–PSV did not show improvement on prednisone, dapsone, and azathioprine but rapidly responded to infliximab.23 Infliximab led to complete clearance of oral lesions in an adult with severe fistulizing CD who developed PSV.11 However, 2 adolescent patients with CD developed PSV while on adalimumab,6,34 though 1 did improve after increasing adalimumab from once to twice weekly.6
Conclusion
The case described here—PSV in a prepubertal 7-year-old with multiple cutaneous findings suggestive of CD, including OFG, perianal and vulvar edema with biopsy-proven noncaseating granulomas, anal skin tags, and an elevated calprotectin level, noted during a cutaneous flare without clinical or endoscopically identified underlying bowel involvement—is an extremely rare presentation. Literature regarding management of PSV primarily is found in the form of case reports and focuses on treating underlying IBD. In patients with intestinal disease, treatment with biologic therapy appears most effective.6,23
ADDENDUM
Interestingly, 3 years after the patient’s original presentation to our clinic, chromosomal sequencing analysis to assess for copy number variants and whole exome gene sequencing identified a variant of unknown significance in the heat shock protein family A member 1-like gene, HSPA1L, which has an unknown mode of inheritance, but the literature suggests that both truncating and missense variants could be associated with individuals with ulcerative colitis, CD, and IBD.37,38 Although we cannot use this information to render a molecular diagnosis, it is highly suspicious that this is the cause of her clinical findings. Additionally, the patient currently is aged 10 years with unchanged cutaneous findings and has not developed gastrointestinal findings of IBD.
- Tuxen AJ, Orchard D. Childhood and adolescent orofacial granulomatosis is strongly associated with Crohn’s disease and responds to intralesional corticosteroids. Australas J Dermatol. 2010;51:124-127. doi:10.1111/j.1440-0960.2010.00627.x
- Vaid RM, Cohen BA. Cutaneous Crohn’s disease in the pediatric population. Pediatr Dermatol. 2010;27:279-281. doi:10.1111/j.1525-1470.2010.01138.x
- van de Scheur MR, van der Waal RIF, van der Waal I, et al. Ano-genital granulomatosis: the counterpart of oro-facial granulomatosis. J Eur Acad Dermatol Venereol. 2003;17:184-189. doi:10.1046/j.1468-3083.2003.00573.x
- Campbell HE, Escudier MP, Patel P, et al. Review article: cinnamon- and benzoate-free diet as a primary treatment for orofacial granulomatosis. Aliment Pharmacol Ther. 2011;34:687-701. doi:10.1111/j.1365-2036.2011.04792.x
- Duhra P, Paul CJ. Metastatic Crohn’s disease responding to metronidazole. Br J Dermatol. 1988;119:87-91. doi:10.1111/j.1365-2133.1988.tb07107.x
- Katsanos KH, Torres J, Roda G, et al. Review article: non-malignant oral manifestations in inflammatory bowel diseases. Aliment Pharmacol Ther. 2015;42:40-60. doi:10.1111/apt.13217
- Schmitz BA, Unkel JH. Symptomatic oral Crohn’s disease in an adolescent. J Dent Child (Chic). 2018;85:66-69.
- Femiano F, Lanza A, Buonaiuto C, et al. Pyostomatitis vegetans: a review of the literature. Med Oral Patol Oral Cir Bucal. 2009;14:E114-E117.
- Clark LG, Tolkachjov SN, Bridges AG, et al. Pyostomatitis vegetans (PSV)–pyodermatitis vegetans (PDV): a clinicopathologic study of 7 cases at a tertiary referral center. J Am Acad Dermatol. 2016;75:578-584. doi:10.1016/j.jaad.2016.03.047
- Hansen LS, Silverman S Jr, Daniels TE. The differential diagnosis of pyostomatitis vegetans and its relation to bowel disease. Oral Surg Oral Med Oral Pathol. 1983;55:363-373. doi:10.1016/0030-4220(83)90191-3
- Cataldo E, Covino MC, Tesone PE. Pyostomatitis vegetans. Oral Surg Oral Med Oral Pathol. 1981;52:172-177. doi:10.1016/0030-4220(81)90316-9
- McCarthy FP. Pyostomatitis vegetans; report of three cases. Arch Derm Syphilol. 1949;60:750-764.
- Bens G, Laharie D, Beylot-Barry M, et al. Successful treatment with infliximab and methotrexate of pyostomatitis vegetans associated with Crohn’s disease. Br J Dermatol. 2003;149:181-184. doi:10.1046/j.1365-2133.2003.05385.x
- Leibovitch I, Ooi C, Huilgol SC, et al. Pyodermatitis–pyostomatitis vegetans of the eyelids: case report and review of the literature. Ophthalmology. 2005;112:1809-1813. doi:10.1016/j.ophtha.2005.04.027
- Ruiz-Roca JA, Berini-Aytés L, Gay-Escoda C. Pyostomatitis vegetans. report of two cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:447-454. doi:10.1016/j.tripleo.2003.08.022
- Molnár T, Farkas K, Nagy F, et al. Third case: another pediatric patient with pyostomatitis vegetans and oral granuloma as one of the initial symptoms of Crohn’s disease. Inflamm Bowel Dis. 2011;17:E122-E123. doi:10.1002/ibd.21791
- Leydhecker W, Lund OE. Eye involvement in pyostomatitis vegetans. Klin Monbl Augenheilkd Augenarztl Fortbild. 1962;141:595-602.
- Thornhill MH, Zakrzewska JM, Gilkes JJ. Pyostomatitis vegetans: report of three cases and review of the literature. J Oral Pathol Med. 1992;21:128-133. doi:10.1111/j.1600-0714.1992.tb00996.x
- Chaudhry SI, Philpot NS, Odell EW, et al. Pyostomatitis vegetans associated with asymptomatic ulcerative colitis: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:327-330. doi:10.1016/s1079-2104(99)70217-9
- Konstantopoulou M, O’Dwyer EM, Steele JC, et al. Pyodermatitis–pyostomatitis vegetans complicated by methicillin-resistant Staphylococcus aureus infection. Clin Exp Dermatol. 2005;30:666-668. doi:10.1111/j.1365-2230.2005.01906.x
- Ficarra G, Cicchi P, Amorosi A, et al. Oral Crohn’s disease and pyostomatitis vegetans. an unusual association. Oral Surg Oral Med Oral Pathol. 1993;75:220-224. doi:10.1016/0030-4220(93)90097-n
- Markopoulos AK, Antoniades DZ, Zaraboukas T. Pemphigus vegetans of the oral cavity. Int J Dermatol. 2006;45:425-428. doi:10.1111/j.1365-4632.2004.02480.x
- Nico MMS, Hussein TP, Aoki V, et al. Pyostomatitis vegetans and its relation to inflammatory bowel disease, pyoderma gangrenosum, pyodermatitis vegetans, and pemphigus. J Oral Pathol Med. 2012;41:584-588. doi:10.1111/j.1600-0714.2012.01152.x
- Berzin D, Lahad A, Weiss B, et al. Inflammatory bowel disease presenting with pyodermatitis–pyostomatitis vegetans in a pediatric patient: a case report and review of the literature. Pediatr Dermatol. 2021;38:868-871. doi:10.1111/pde.14625
- Ballo FS, Camisa C, Allen CM. Pyostomatitis vegetans. report of a case and review of the literature. J Am Acad Dermatol. 1989;21:381-387.
- Greuter T, Bertoldo F, Rechner R, et al; Swiss IBD Cohort Study Group. Extraintestinal manifestations of pediatric inflammatory bowel disease: prevalence, presentation, and anti-TNF treatment. J Pediatr Gastroenterol Nutr. 2017;65:200-206. doi:10.1097/MPG.0000000000001455
- Jose FA, Garnett EA, Vittinghoff E, et al. Development of extraintestinal manifestations in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:63-68. doi:10.1002/ibd.20604
- Harty S, Fleming P, Rowland M, et al. A prospective study of the oral manifestations of Crohn’s disease. Clin Gastroenterol Hepatol. 2005;3:886-891. doi:10.1016/s1542-3565(05)00424-6
- Pittock S, Drumm B, Fleming P, et al. The oral cavity in Crohn’s disease. J Pediatr. 2001;138:767-771. doi:10.1067/mpd.2001.113008
- Bardasi G, Romagnoli A, Foschini MP, et al. Pyostomatitis vegetans in a pediatric patient with ulcerative colitis: case report of a rare pediatric inflammatory bowel disease extraintestinal manifestation and review of the literature. Eur J Gastroenterol Hepatol. 2020;32:889-892. doi:10.1097/MEG.0000000000001723
- Mesquita Kde C, Costa IM. Case for diagnosis. An Bras Dermatol. 2012;87:929-931. doi:10.1590/s0365-05962012000600022
- Al-Rimawi HS, Hammad MM, Raweily EA, et al. Pyostomatitis vegetans in childhood. Eur J Pediatr. 1998;157:402-405. doi:10.1007/s004310050838
- Chen KL, Diiorio DA, Chiu YE, et al. Pediatric patients with orofacial granulomatosis likely to subsequently develop intestinal Crohn’s disease: brief report. Pediatr Dermatol. 2020;37:1162-1164. doi:10.1111/pde.14390
- Pazheri F, Alkhouri N, Radhakrishnan K. Pyostomatitis vegetans as an oral manifestation of Crohn’s disease in a pediatric patient. Inflamm Bowel Dis. 2010;16:2007. doi:10.1002/ibd.21245.
- Werchniak AE, Storm CA, Plunkett RW, et al. Treatment of pyostomatitis vegetans with topical tacrolimus. J Am Acad Dermatol. 2005;52:722-723. doi:10.1016/j.jaad.2004.11.041
- Stingeni L, Tramontana M, Bassotti G, et al. Pyodermatitis–pyostomatitis vegetans and antibullous pemphigoid antigen 180 autoantibodies: a casual association? Br J Dermatol. 2015;172:811-813. doi:10.1111/bjd.13297
- Takahashi S, Andreoletti G, Chen R, et al. De novo and rare mutations in the HSPA1L heat shock gene associated with inflammatory bowel disease. Genome Med. 2017;9:8. doi:10.1186/s13073-016-0394-9
- Crowley E, Warner N, Pan J, et al. Prevalence and clinical features of inflammatory bowel diseases associated with monogenic variants, identified by whole-exome sequencing in 1000 children at a single center. Gastroenterology. 2020;158:2208-2220. doi:10.1053/j .gastro.2020.02.023
Case Report
A 7-year-old girl who was otherwise healthy was referred by pediatric gastroenterology for evaluation of cutaneous Crohn disease (CD). The patient had a 4-year history of persistent lip swelling and a 3-year history of asymmetric erythematous labial swelling and perianal erythema with skin tags. She had been applying the calcineurin inhibitor tacrolimus ointment 0.03% 1 or 2 times daily to her lesions with minimal improvement. She did not have a medical history of recurrent or unusual infectious diseases. There was no family history of autoimmune disease.
The patient and her guardian reported intermittent perianal pain but denied constipation, diarrhea, abdominal pain, and blood in the stool. She denied throat and tongue swelling, dysphagia, dyspnea, drooling, facial paralysis, and eyelid edema. She was a well-nourished child whose height and weight percentiles tracked at 30% and 25%, respectively. Physical examination revealed confluent symmetric lip swelling with mild angular cheilitis. Multiple 1- to 2-mm white pustules with pinpoint erosions covered the upper and lower labial mucosa and extended onto the buccal mucosa (Figure 1). She had symmetric erythema and swelling of the left labia majora extending to and involving the left perianal mucosa. Three perianal erythematous skin tags and a perianal fissure were identified.

The patient had been assessed 2 years earlier by pediatric dermatology and gastroenterology with an extensive evaluation that favored a diagnosis of cutaneous CD because the combination of orofacial granulomatosis (OFG), vulvar edema, and perianal skin tags is strongly associated.1-3 Contact dermatitis affecting the mouth was considered; however, allergen testing did not demonstrate a trigger.
A trial of a benzoate- and cinnamon-free diet, which has been reported to improve OFG,4 did not provide symptomatic improvement. Topical corticosteroids and tacrolimus reduced the perioral erythema, but the swelling persisted. An infectious cause was considered; however, topical mupirocin had no effect, and amoxicillin resulted in oral candidiasis.
A perianal biopsy revealed a granulomatous dermatitis. Fungal and bacterial cultures were negative. Upper and lower gastrointestinal (GI) endoscopy and a fecal calprotectin assay were not suggestive of inflammatory bowel disease (IBD). A complete blood cell count and QuantiFERON-TB Gold test measuring the immune response to tuberculosis antigens were normal. Chronic granulomatous disease, RAG1/RAG2 deficiency, common variable immunodeficiency, and NOD2 defects were ruled out with normal tests of dihydrorhodamine, quantitative immunoglobulins, and toll-like receptors.
Because of the discomfort associated with the patient’s lesions, she was offered treatment with tumor necrosis factor α inhibitors, including infliximab and adalimumab. These agents had been offered since the onset of symptoms; however, her parents declined systemic medication unless she developed GI involvement. Instead, the tacrolimus concentration was increased to 0.1% applied to the lips, labia, and perianal area, and fluocinonide gel 0.05% applied nightly to the oral pustules was added.
Two months later the patient had notably fewer oral pustules and diminished erythema but only slightly reduced oral, vulvar, and perianal swelling. A trial of oral metronidazole, which has been reported to clear a patient with cutaneous CD,5 was discontinued by her parents after 6 weeks because of a lack of interval improvement.
One year later, a pre-existing perianal skin tag doubled in size and became exquisitely tender. The calprotectin level—previously within reference range at less than 16 μg/g—was now elevated at 149 μg/g (reference range, 1–120 μg/g) and increased to 336 μg/g 3 weeks later. Testing for C-reactive protein, zinc, and stool occult blood; a comprehensive metabolic panel; and a complete blood cell count were unremarkable.
Repeat upper and lower GI endoscopy did not suggest CD. A biopsy using direct immunofluorescence (DIF) was obtained to evaluate for pyostomatitis vegetans (PSV) and rule out
The captured biopsy did not demonstrate the intended pustule; instead, it included less-affected mucosa and was obtained during topical treatment when few pustules and erosions persisted. Pathologic analysis revealed noncaseating granulomas without an increase in microabscesses, neutrophils, or eosinophils (Figure 2). Direct immunofluorescence staining for IgG, IgA, and C3 and indirect immunofluorescence staining for desmoglein-1 and desmoglein-3 antibodies were negative. Although the biopsy did not capture the intended pustule, diagnosis of PV was made based on clinical features and the constellation of cutaneous findings associated with IBD.

Intralesional triamcinolone, which has been of benefit for pediatric patients with orofacial granulomatosis,1,6,7 was instituted and normalized the vulva and perianal mucosa; however, lip swelling improved only minimally.
Comment
Pyostomatitis vegetans is characterized by multiple white or yellow, friable, miliary pustules that rupture, leaving behind ulcerations and erosions that cause a varying degree of oral pain.8 The disorder can involve any area of the oral mucosa—most often the labia-attached gingiva, soft and hard palates, buccal mucosa, vestibule, and tonsillar areas—but often spares the floor of the mouth and tongue.8-11 The term pyostomatitis vegetans was proposed in 1949 by McCarthy12 when he noted in a patient who presented with the characteristic appearance of the oral mucosa, though cases of vaginal, nasal, and periocular involvement have been reported.8,13,14
Histopathology—Pyostomatitis vegetans displays pseudoepithelial hyperplasia with acanthosis, hyperkeratosis, and intraepithelial or subepithelial microabscesses (or both) with neutrophils and eosinophils.8,9,15 There are a few possible explanations for this patient’s lack of tissue eosinophilia. It has been theorized that the presence of granulomas could mask concurrent PSV16 or that tissue in PSV contains fewer eosinophils as the disorder progresses.11 The oral biopsy obtained from our patient did not capture a pustule, and the condition had noticeably improved with topical tacrolimus at the time of biopsy; therefore, neither neutrophils nor eosinophils were identified. Peripheral eosinophilia, which is present in 42% to 90% of cases of PSV,9,17 can be a diagnostic clue.18 However, PE is associated with IBD,24 which usually occurs with PSV, so the absence of peripheral eosinophilia in our patient may be explained by her lack of bowel disease.
Pathogenesis—The pathogenesis of PSV is unknown. A proposed etiology includes cross-reacting antigens in the bowel and skin secondary to IBD as well as an aberrant immune response to an unidentified factor.8 Pyostomatitis vegetans is considered by many to be the mucosal variant of pyodermatitis vegetans,9,15,19 a neutrophilic dermatosis characterized by asymmetric, crusted, erythematous papulopustules that extend peripherally and coalesce to form large vegetating plaques. These lesions commonly manifest in the axillary folds, groin, and scalp and can involve the face, trunk, and distal extremities.9,18 Infection has been suggested as a cause of PSV, though cultures for pathogenic bacteria, viruses, and fungi consistently show only normal flora.20 Zinc deficiency attributed to malabsorption from CD was reported in an adult with PSV.21 The PSV resolved after 6 weeks of zinc supplementation.
Differential Diagnosis—The main entity in the clinical differential diagnosis for PSV is PVH, which is considered a variant of pemphigus vulgaris. Pemphigus vegetans of Hallopeau presents with pustules and progresses to hyperpigmented vegetative plaques with peripheral hypertrophic granulation tissue.22 The clinical and histological presentation of PVH can be similar to PSV; in PVH, however, DIF demonstrates intercellular IgG and C3 due to circulating IgG autoantibodies specific for desmoglein 3, a cell adhesion molecule.22-24 In PSV, DIF typically is negative for IgG, IgA, and C3.8 Immunohistochemical findings of PSV may overlap with IgA pemphigus, IgG/IgA pemphigus, and IgG pemphigus, which has sparked debate if PSV is an autoimmune blistering disorder or a secondary finding of epithelial injury.9,18,24
Pyostomatitis vegetans is most prevalent in patients aged 20 to 59 years25 but can occur at any age.8,19 Overall, extraintestinal symptoms, including mucocutaneous findings, are common in pediatric patients—in 30% to 71% of children with CD and 21% to 22% of children with ulcerative colitis26—and can predate onset of GI symptoms in 6% of pediatric patients.27
Oral disease is common in CD; manifestations are listed in the Table.28,29 In a prospective study of 48 children with CD, 42% (20/48) had oral manifestations identified at diagnosis28; in a similar study of 25 children, researchers noted that 48% (12/25) had disease-specific oral lesions.29 None of these children recognized the oral findings prior to the onset of systemic symptoms.28 Pyostomatitis vegetans was the least common oral manifestation, reported in 1 of 73 patients in the 2 studies combined.28,29

Two recent articles that looked at PSV in pediatric and adolescent populations identified only 9 patients with PSV.24,30 Only 2 patients (siblings) had documented onset of PSV before 12 years of age,31 which suggests an underlying genetic predisposition in young children.
It has been reported that active or subclinical (ie, asymptomatic with positive endoscopic findings) IBD in adults precedes onset of PSV, which may be considered a sign of relapse.9,30 However, PSV is incredibly rare in children and adolescents and can be an early finding of IBD in children.16,31,32
Our patient has not developed GI involvement since her initial presentation 5 years prior, though another pediatric patient developed symptomatic CD 9 years after onset of OFG.5 A retrospective review of pediatric OFG without CD met criteria for CD at a median of 3.1 years (range, 0.4–6.9 years).33 Regrettably, the early presence of PSV has been associated with future progression to CD and a complicated disease course.12,34
Management—Pyoderma stomatitis vegetans is treated with management of underlying IBD,8 with scarce literature available regarding pediatric patients. Oral lesions have been treated with antiseptics and topical corticosteroids, though these have limited benefit.8 In an adult with IBD, topical tacrolimus initially cleared PSV; however, lesions recurred until mesalamine was initiated.35 Systemic steroids were effective in a 16-year-old patient with CD and PSV,12 but recurrence is common after corticosteroids are stopped.34
Some patients benefit from steroid-sparing medications, such as dapsone, azathioprine, sulfamethoxypyridazine, methotrexate, mycophenolate mofetil, and tumor necrosis factor α inhibitors such as infliximab and adalimumab.8,9,15,23,34,36 A 12-year-old patient with pyodermatitis–PSV without intestinal disease was treated with prednisone, dapsone, and azathioprine with improvement but not complete resolution of oral erosions after 18 weeks of treatment.32 A 15-year-old patient with CD and pyodermatitis–PSV did not show improvement on prednisone, dapsone, and azathioprine but rapidly responded to infliximab.23 Infliximab led to complete clearance of oral lesions in an adult with severe fistulizing CD who developed PSV.11 However, 2 adolescent patients with CD developed PSV while on adalimumab,6,34 though 1 did improve after increasing adalimumab from once to twice weekly.6
Conclusion
The case described here—PSV in a prepubertal 7-year-old with multiple cutaneous findings suggestive of CD, including OFG, perianal and vulvar edema with biopsy-proven noncaseating granulomas, anal skin tags, and an elevated calprotectin level, noted during a cutaneous flare without clinical or endoscopically identified underlying bowel involvement—is an extremely rare presentation. Literature regarding management of PSV primarily is found in the form of case reports and focuses on treating underlying IBD. In patients with intestinal disease, treatment with biologic therapy appears most effective.6,23
ADDENDUM
Interestingly, 3 years after the patient’s original presentation to our clinic, chromosomal sequencing analysis to assess for copy number variants and whole exome gene sequencing identified a variant of unknown significance in the heat shock protein family A member 1-like gene, HSPA1L, which has an unknown mode of inheritance, but the literature suggests that both truncating and missense variants could be associated with individuals with ulcerative colitis, CD, and IBD.37,38 Although we cannot use this information to render a molecular diagnosis, it is highly suspicious that this is the cause of her clinical findings. Additionally, the patient currently is aged 10 years with unchanged cutaneous findings and has not developed gastrointestinal findings of IBD.
Case Report
A 7-year-old girl who was otherwise healthy was referred by pediatric gastroenterology for evaluation of cutaneous Crohn disease (CD). The patient had a 4-year history of persistent lip swelling and a 3-year history of asymmetric erythematous labial swelling and perianal erythema with skin tags. She had been applying the calcineurin inhibitor tacrolimus ointment 0.03% 1 or 2 times daily to her lesions with minimal improvement. She did not have a medical history of recurrent or unusual infectious diseases. There was no family history of autoimmune disease.
The patient and her guardian reported intermittent perianal pain but denied constipation, diarrhea, abdominal pain, and blood in the stool. She denied throat and tongue swelling, dysphagia, dyspnea, drooling, facial paralysis, and eyelid edema. She was a well-nourished child whose height and weight percentiles tracked at 30% and 25%, respectively. Physical examination revealed confluent symmetric lip swelling with mild angular cheilitis. Multiple 1- to 2-mm white pustules with pinpoint erosions covered the upper and lower labial mucosa and extended onto the buccal mucosa (Figure 1). She had symmetric erythema and swelling of the left labia majora extending to and involving the left perianal mucosa. Three perianal erythematous skin tags and a perianal fissure were identified.

The patient had been assessed 2 years earlier by pediatric dermatology and gastroenterology with an extensive evaluation that favored a diagnosis of cutaneous CD because the combination of orofacial granulomatosis (OFG), vulvar edema, and perianal skin tags is strongly associated.1-3 Contact dermatitis affecting the mouth was considered; however, allergen testing did not demonstrate a trigger.
A trial of a benzoate- and cinnamon-free diet, which has been reported to improve OFG,4 did not provide symptomatic improvement. Topical corticosteroids and tacrolimus reduced the perioral erythema, but the swelling persisted. An infectious cause was considered; however, topical mupirocin had no effect, and amoxicillin resulted in oral candidiasis.
A perianal biopsy revealed a granulomatous dermatitis. Fungal and bacterial cultures were negative. Upper and lower gastrointestinal (GI) endoscopy and a fecal calprotectin assay were not suggestive of inflammatory bowel disease (IBD). A complete blood cell count and QuantiFERON-TB Gold test measuring the immune response to tuberculosis antigens were normal. Chronic granulomatous disease, RAG1/RAG2 deficiency, common variable immunodeficiency, and NOD2 defects were ruled out with normal tests of dihydrorhodamine, quantitative immunoglobulins, and toll-like receptors.
Because of the discomfort associated with the patient’s lesions, she was offered treatment with tumor necrosis factor α inhibitors, including infliximab and adalimumab. These agents had been offered since the onset of symptoms; however, her parents declined systemic medication unless she developed GI involvement. Instead, the tacrolimus concentration was increased to 0.1% applied to the lips, labia, and perianal area, and fluocinonide gel 0.05% applied nightly to the oral pustules was added.
Two months later the patient had notably fewer oral pustules and diminished erythema but only slightly reduced oral, vulvar, and perianal swelling. A trial of oral metronidazole, which has been reported to clear a patient with cutaneous CD,5 was discontinued by her parents after 6 weeks because of a lack of interval improvement.
One year later, a pre-existing perianal skin tag doubled in size and became exquisitely tender. The calprotectin level—previously within reference range at less than 16 μg/g—was now elevated at 149 μg/g (reference range, 1–120 μg/g) and increased to 336 μg/g 3 weeks later. Testing for C-reactive protein, zinc, and stool occult blood; a comprehensive metabolic panel; and a complete blood cell count were unremarkable.
Repeat upper and lower GI endoscopy did not suggest CD. A biopsy using direct immunofluorescence (DIF) was obtained to evaluate for pyostomatitis vegetans (PSV) and rule out
The captured biopsy did not demonstrate the intended pustule; instead, it included less-affected mucosa and was obtained during topical treatment when few pustules and erosions persisted. Pathologic analysis revealed noncaseating granulomas without an increase in microabscesses, neutrophils, or eosinophils (Figure 2). Direct immunofluorescence staining for IgG, IgA, and C3 and indirect immunofluorescence staining for desmoglein-1 and desmoglein-3 antibodies were negative. Although the biopsy did not capture the intended pustule, diagnosis of PV was made based on clinical features and the constellation of cutaneous findings associated with IBD.

Intralesional triamcinolone, which has been of benefit for pediatric patients with orofacial granulomatosis,1,6,7 was instituted and normalized the vulva and perianal mucosa; however, lip swelling improved only minimally.
Comment
Pyostomatitis vegetans is characterized by multiple white or yellow, friable, miliary pustules that rupture, leaving behind ulcerations and erosions that cause a varying degree of oral pain.8 The disorder can involve any area of the oral mucosa—most often the labia-attached gingiva, soft and hard palates, buccal mucosa, vestibule, and tonsillar areas—but often spares the floor of the mouth and tongue.8-11 The term pyostomatitis vegetans was proposed in 1949 by McCarthy12 when he noted in a patient who presented with the characteristic appearance of the oral mucosa, though cases of vaginal, nasal, and periocular involvement have been reported.8,13,14
Histopathology—Pyostomatitis vegetans displays pseudoepithelial hyperplasia with acanthosis, hyperkeratosis, and intraepithelial or subepithelial microabscesses (or both) with neutrophils and eosinophils.8,9,15 There are a few possible explanations for this patient’s lack of tissue eosinophilia. It has been theorized that the presence of granulomas could mask concurrent PSV16 or that tissue in PSV contains fewer eosinophils as the disorder progresses.11 The oral biopsy obtained from our patient did not capture a pustule, and the condition had noticeably improved with topical tacrolimus at the time of biopsy; therefore, neither neutrophils nor eosinophils were identified. Peripheral eosinophilia, which is present in 42% to 90% of cases of PSV,9,17 can be a diagnostic clue.18 However, PE is associated with IBD,24 which usually occurs with PSV, so the absence of peripheral eosinophilia in our patient may be explained by her lack of bowel disease.
Pathogenesis—The pathogenesis of PSV is unknown. A proposed etiology includes cross-reacting antigens in the bowel and skin secondary to IBD as well as an aberrant immune response to an unidentified factor.8 Pyostomatitis vegetans is considered by many to be the mucosal variant of pyodermatitis vegetans,9,15,19 a neutrophilic dermatosis characterized by asymmetric, crusted, erythematous papulopustules that extend peripherally and coalesce to form large vegetating plaques. These lesions commonly manifest in the axillary folds, groin, and scalp and can involve the face, trunk, and distal extremities.9,18 Infection has been suggested as a cause of PSV, though cultures for pathogenic bacteria, viruses, and fungi consistently show only normal flora.20 Zinc deficiency attributed to malabsorption from CD was reported in an adult with PSV.21 The PSV resolved after 6 weeks of zinc supplementation.
Differential Diagnosis—The main entity in the clinical differential diagnosis for PSV is PVH, which is considered a variant of pemphigus vulgaris. Pemphigus vegetans of Hallopeau presents with pustules and progresses to hyperpigmented vegetative plaques with peripheral hypertrophic granulation tissue.22 The clinical and histological presentation of PVH can be similar to PSV; in PVH, however, DIF demonstrates intercellular IgG and C3 due to circulating IgG autoantibodies specific for desmoglein 3, a cell adhesion molecule.22-24 In PSV, DIF typically is negative for IgG, IgA, and C3.8 Immunohistochemical findings of PSV may overlap with IgA pemphigus, IgG/IgA pemphigus, and IgG pemphigus, which has sparked debate if PSV is an autoimmune blistering disorder or a secondary finding of epithelial injury.9,18,24
Pyostomatitis vegetans is most prevalent in patients aged 20 to 59 years25 but can occur at any age.8,19 Overall, extraintestinal symptoms, including mucocutaneous findings, are common in pediatric patients—in 30% to 71% of children with CD and 21% to 22% of children with ulcerative colitis26—and can predate onset of GI symptoms in 6% of pediatric patients.27
Oral disease is common in CD; manifestations are listed in the Table.28,29 In a prospective study of 48 children with CD, 42% (20/48) had oral manifestations identified at diagnosis28; in a similar study of 25 children, researchers noted that 48% (12/25) had disease-specific oral lesions.29 None of these children recognized the oral findings prior to the onset of systemic symptoms.28 Pyostomatitis vegetans was the least common oral manifestation, reported in 1 of 73 patients in the 2 studies combined.28,29

Two recent articles that looked at PSV in pediatric and adolescent populations identified only 9 patients with PSV.24,30 Only 2 patients (siblings) had documented onset of PSV before 12 years of age,31 which suggests an underlying genetic predisposition in young children.
It has been reported that active or subclinical (ie, asymptomatic with positive endoscopic findings) IBD in adults precedes onset of PSV, which may be considered a sign of relapse.9,30 However, PSV is incredibly rare in children and adolescents and can be an early finding of IBD in children.16,31,32
Our patient has not developed GI involvement since her initial presentation 5 years prior, though another pediatric patient developed symptomatic CD 9 years after onset of OFG.5 A retrospective review of pediatric OFG without CD met criteria for CD at a median of 3.1 years (range, 0.4–6.9 years).33 Regrettably, the early presence of PSV has been associated with future progression to CD and a complicated disease course.12,34
Management—Pyoderma stomatitis vegetans is treated with management of underlying IBD,8 with scarce literature available regarding pediatric patients. Oral lesions have been treated with antiseptics and topical corticosteroids, though these have limited benefit.8 In an adult with IBD, topical tacrolimus initially cleared PSV; however, lesions recurred until mesalamine was initiated.35 Systemic steroids were effective in a 16-year-old patient with CD and PSV,12 but recurrence is common after corticosteroids are stopped.34
Some patients benefit from steroid-sparing medications, such as dapsone, azathioprine, sulfamethoxypyridazine, methotrexate, mycophenolate mofetil, and tumor necrosis factor α inhibitors such as infliximab and adalimumab.8,9,15,23,34,36 A 12-year-old patient with pyodermatitis–PSV without intestinal disease was treated with prednisone, dapsone, and azathioprine with improvement but not complete resolution of oral erosions after 18 weeks of treatment.32 A 15-year-old patient with CD and pyodermatitis–PSV did not show improvement on prednisone, dapsone, and azathioprine but rapidly responded to infliximab.23 Infliximab led to complete clearance of oral lesions in an adult with severe fistulizing CD who developed PSV.11 However, 2 adolescent patients with CD developed PSV while on adalimumab,6,34 though 1 did improve after increasing adalimumab from once to twice weekly.6
Conclusion
The case described here—PSV in a prepubertal 7-year-old with multiple cutaneous findings suggestive of CD, including OFG, perianal and vulvar edema with biopsy-proven noncaseating granulomas, anal skin tags, and an elevated calprotectin level, noted during a cutaneous flare without clinical or endoscopically identified underlying bowel involvement—is an extremely rare presentation. Literature regarding management of PSV primarily is found in the form of case reports and focuses on treating underlying IBD. In patients with intestinal disease, treatment with biologic therapy appears most effective.6,23
ADDENDUM
Interestingly, 3 years after the patient’s original presentation to our clinic, chromosomal sequencing analysis to assess for copy number variants and whole exome gene sequencing identified a variant of unknown significance in the heat shock protein family A member 1-like gene, HSPA1L, which has an unknown mode of inheritance, but the literature suggests that both truncating and missense variants could be associated with individuals with ulcerative colitis, CD, and IBD.37,38 Although we cannot use this information to render a molecular diagnosis, it is highly suspicious that this is the cause of her clinical findings. Additionally, the patient currently is aged 10 years with unchanged cutaneous findings and has not developed gastrointestinal findings of IBD.
- Tuxen AJ, Orchard D. Childhood and adolescent orofacial granulomatosis is strongly associated with Crohn’s disease and responds to intralesional corticosteroids. Australas J Dermatol. 2010;51:124-127. doi:10.1111/j.1440-0960.2010.00627.x
- Vaid RM, Cohen BA. Cutaneous Crohn’s disease in the pediatric population. Pediatr Dermatol. 2010;27:279-281. doi:10.1111/j.1525-1470.2010.01138.x
- van de Scheur MR, van der Waal RIF, van der Waal I, et al. Ano-genital granulomatosis: the counterpart of oro-facial granulomatosis. J Eur Acad Dermatol Venereol. 2003;17:184-189. doi:10.1046/j.1468-3083.2003.00573.x
- Campbell HE, Escudier MP, Patel P, et al. Review article: cinnamon- and benzoate-free diet as a primary treatment for orofacial granulomatosis. Aliment Pharmacol Ther. 2011;34:687-701. doi:10.1111/j.1365-2036.2011.04792.x
- Duhra P, Paul CJ. Metastatic Crohn’s disease responding to metronidazole. Br J Dermatol. 1988;119:87-91. doi:10.1111/j.1365-2133.1988.tb07107.x
- Katsanos KH, Torres J, Roda G, et al. Review article: non-malignant oral manifestations in inflammatory bowel diseases. Aliment Pharmacol Ther. 2015;42:40-60. doi:10.1111/apt.13217
- Schmitz BA, Unkel JH. Symptomatic oral Crohn’s disease in an adolescent. J Dent Child (Chic). 2018;85:66-69.
- Femiano F, Lanza A, Buonaiuto C, et al. Pyostomatitis vegetans: a review of the literature. Med Oral Patol Oral Cir Bucal. 2009;14:E114-E117.
- Clark LG, Tolkachjov SN, Bridges AG, et al. Pyostomatitis vegetans (PSV)–pyodermatitis vegetans (PDV): a clinicopathologic study of 7 cases at a tertiary referral center. J Am Acad Dermatol. 2016;75:578-584. doi:10.1016/j.jaad.2016.03.047
- Hansen LS, Silverman S Jr, Daniels TE. The differential diagnosis of pyostomatitis vegetans and its relation to bowel disease. Oral Surg Oral Med Oral Pathol. 1983;55:363-373. doi:10.1016/0030-4220(83)90191-3
- Cataldo E, Covino MC, Tesone PE. Pyostomatitis vegetans. Oral Surg Oral Med Oral Pathol. 1981;52:172-177. doi:10.1016/0030-4220(81)90316-9
- McCarthy FP. Pyostomatitis vegetans; report of three cases. Arch Derm Syphilol. 1949;60:750-764.
- Bens G, Laharie D, Beylot-Barry M, et al. Successful treatment with infliximab and methotrexate of pyostomatitis vegetans associated with Crohn’s disease. Br J Dermatol. 2003;149:181-184. doi:10.1046/j.1365-2133.2003.05385.x
- Leibovitch I, Ooi C, Huilgol SC, et al. Pyodermatitis–pyostomatitis vegetans of the eyelids: case report and review of the literature. Ophthalmology. 2005;112:1809-1813. doi:10.1016/j.ophtha.2005.04.027
- Ruiz-Roca JA, Berini-Aytés L, Gay-Escoda C. Pyostomatitis vegetans. report of two cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:447-454. doi:10.1016/j.tripleo.2003.08.022
- Molnár T, Farkas K, Nagy F, et al. Third case: another pediatric patient with pyostomatitis vegetans and oral granuloma as one of the initial symptoms of Crohn’s disease. Inflamm Bowel Dis. 2011;17:E122-E123. doi:10.1002/ibd.21791
- Leydhecker W, Lund OE. Eye involvement in pyostomatitis vegetans. Klin Monbl Augenheilkd Augenarztl Fortbild. 1962;141:595-602.
- Thornhill MH, Zakrzewska JM, Gilkes JJ. Pyostomatitis vegetans: report of three cases and review of the literature. J Oral Pathol Med. 1992;21:128-133. doi:10.1111/j.1600-0714.1992.tb00996.x
- Chaudhry SI, Philpot NS, Odell EW, et al. Pyostomatitis vegetans associated with asymptomatic ulcerative colitis: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:327-330. doi:10.1016/s1079-2104(99)70217-9
- Konstantopoulou M, O’Dwyer EM, Steele JC, et al. Pyodermatitis–pyostomatitis vegetans complicated by methicillin-resistant Staphylococcus aureus infection. Clin Exp Dermatol. 2005;30:666-668. doi:10.1111/j.1365-2230.2005.01906.x
- Ficarra G, Cicchi P, Amorosi A, et al. Oral Crohn’s disease and pyostomatitis vegetans. an unusual association. Oral Surg Oral Med Oral Pathol. 1993;75:220-224. doi:10.1016/0030-4220(93)90097-n
- Markopoulos AK, Antoniades DZ, Zaraboukas T. Pemphigus vegetans of the oral cavity. Int J Dermatol. 2006;45:425-428. doi:10.1111/j.1365-4632.2004.02480.x
- Nico MMS, Hussein TP, Aoki V, et al. Pyostomatitis vegetans and its relation to inflammatory bowel disease, pyoderma gangrenosum, pyodermatitis vegetans, and pemphigus. J Oral Pathol Med. 2012;41:584-588. doi:10.1111/j.1600-0714.2012.01152.x
- Berzin D, Lahad A, Weiss B, et al. Inflammatory bowel disease presenting with pyodermatitis–pyostomatitis vegetans in a pediatric patient: a case report and review of the literature. Pediatr Dermatol. 2021;38:868-871. doi:10.1111/pde.14625
- Ballo FS, Camisa C, Allen CM. Pyostomatitis vegetans. report of a case and review of the literature. J Am Acad Dermatol. 1989;21:381-387.
- Greuter T, Bertoldo F, Rechner R, et al; Swiss IBD Cohort Study Group. Extraintestinal manifestations of pediatric inflammatory bowel disease: prevalence, presentation, and anti-TNF treatment. J Pediatr Gastroenterol Nutr. 2017;65:200-206. doi:10.1097/MPG.0000000000001455
- Jose FA, Garnett EA, Vittinghoff E, et al. Development of extraintestinal manifestations in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:63-68. doi:10.1002/ibd.20604
- Harty S, Fleming P, Rowland M, et al. A prospective study of the oral manifestations of Crohn’s disease. Clin Gastroenterol Hepatol. 2005;3:886-891. doi:10.1016/s1542-3565(05)00424-6
- Pittock S, Drumm B, Fleming P, et al. The oral cavity in Crohn’s disease. J Pediatr. 2001;138:767-771. doi:10.1067/mpd.2001.113008
- Bardasi G, Romagnoli A, Foschini MP, et al. Pyostomatitis vegetans in a pediatric patient with ulcerative colitis: case report of a rare pediatric inflammatory bowel disease extraintestinal manifestation and review of the literature. Eur J Gastroenterol Hepatol. 2020;32:889-892. doi:10.1097/MEG.0000000000001723
- Mesquita Kde C, Costa IM. Case for diagnosis. An Bras Dermatol. 2012;87:929-931. doi:10.1590/s0365-05962012000600022
- Al-Rimawi HS, Hammad MM, Raweily EA, et al. Pyostomatitis vegetans in childhood. Eur J Pediatr. 1998;157:402-405. doi:10.1007/s004310050838
- Chen KL, Diiorio DA, Chiu YE, et al. Pediatric patients with orofacial granulomatosis likely to subsequently develop intestinal Crohn’s disease: brief report. Pediatr Dermatol. 2020;37:1162-1164. doi:10.1111/pde.14390
- Pazheri F, Alkhouri N, Radhakrishnan K. Pyostomatitis vegetans as an oral manifestation of Crohn’s disease in a pediatric patient. Inflamm Bowel Dis. 2010;16:2007. doi:10.1002/ibd.21245.
- Werchniak AE, Storm CA, Plunkett RW, et al. Treatment of pyostomatitis vegetans with topical tacrolimus. J Am Acad Dermatol. 2005;52:722-723. doi:10.1016/j.jaad.2004.11.041
- Stingeni L, Tramontana M, Bassotti G, et al. Pyodermatitis–pyostomatitis vegetans and antibullous pemphigoid antigen 180 autoantibodies: a casual association? Br J Dermatol. 2015;172:811-813. doi:10.1111/bjd.13297
- Takahashi S, Andreoletti G, Chen R, et al. De novo and rare mutations in the HSPA1L heat shock gene associated with inflammatory bowel disease. Genome Med. 2017;9:8. doi:10.1186/s13073-016-0394-9
- Crowley E, Warner N, Pan J, et al. Prevalence and clinical features of inflammatory bowel diseases associated with monogenic variants, identified by whole-exome sequencing in 1000 children at a single center. Gastroenterology. 2020;158:2208-2220. doi:10.1053/j .gastro.2020.02.023
- Tuxen AJ, Orchard D. Childhood and adolescent orofacial granulomatosis is strongly associated with Crohn’s disease and responds to intralesional corticosteroids. Australas J Dermatol. 2010;51:124-127. doi:10.1111/j.1440-0960.2010.00627.x
- Vaid RM, Cohen BA. Cutaneous Crohn’s disease in the pediatric population. Pediatr Dermatol. 2010;27:279-281. doi:10.1111/j.1525-1470.2010.01138.x
- van de Scheur MR, van der Waal RIF, van der Waal I, et al. Ano-genital granulomatosis: the counterpart of oro-facial granulomatosis. J Eur Acad Dermatol Venereol. 2003;17:184-189. doi:10.1046/j.1468-3083.2003.00573.x
- Campbell HE, Escudier MP, Patel P, et al. Review article: cinnamon- and benzoate-free diet as a primary treatment for orofacial granulomatosis. Aliment Pharmacol Ther. 2011;34:687-701. doi:10.1111/j.1365-2036.2011.04792.x
- Duhra P, Paul CJ. Metastatic Crohn’s disease responding to metronidazole. Br J Dermatol. 1988;119:87-91. doi:10.1111/j.1365-2133.1988.tb07107.x
- Katsanos KH, Torres J, Roda G, et al. Review article: non-malignant oral manifestations in inflammatory bowel diseases. Aliment Pharmacol Ther. 2015;42:40-60. doi:10.1111/apt.13217
- Schmitz BA, Unkel JH. Symptomatic oral Crohn’s disease in an adolescent. J Dent Child (Chic). 2018;85:66-69.
- Femiano F, Lanza A, Buonaiuto C, et al. Pyostomatitis vegetans: a review of the literature. Med Oral Patol Oral Cir Bucal. 2009;14:E114-E117.
- Clark LG, Tolkachjov SN, Bridges AG, et al. Pyostomatitis vegetans (PSV)–pyodermatitis vegetans (PDV): a clinicopathologic study of 7 cases at a tertiary referral center. J Am Acad Dermatol. 2016;75:578-584. doi:10.1016/j.jaad.2016.03.047
- Hansen LS, Silverman S Jr, Daniels TE. The differential diagnosis of pyostomatitis vegetans and its relation to bowel disease. Oral Surg Oral Med Oral Pathol. 1983;55:363-373. doi:10.1016/0030-4220(83)90191-3
- Cataldo E, Covino MC, Tesone PE. Pyostomatitis vegetans. Oral Surg Oral Med Oral Pathol. 1981;52:172-177. doi:10.1016/0030-4220(81)90316-9
- McCarthy FP. Pyostomatitis vegetans; report of three cases. Arch Derm Syphilol. 1949;60:750-764.
- Bens G, Laharie D, Beylot-Barry M, et al. Successful treatment with infliximab and methotrexate of pyostomatitis vegetans associated with Crohn’s disease. Br J Dermatol. 2003;149:181-184. doi:10.1046/j.1365-2133.2003.05385.x
- Leibovitch I, Ooi C, Huilgol SC, et al. Pyodermatitis–pyostomatitis vegetans of the eyelids: case report and review of the literature. Ophthalmology. 2005;112:1809-1813. doi:10.1016/j.ophtha.2005.04.027
- Ruiz-Roca JA, Berini-Aytés L, Gay-Escoda C. Pyostomatitis vegetans. report of two cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:447-454. doi:10.1016/j.tripleo.2003.08.022
- Molnár T, Farkas K, Nagy F, et al. Third case: another pediatric patient with pyostomatitis vegetans and oral granuloma as one of the initial symptoms of Crohn’s disease. Inflamm Bowel Dis. 2011;17:E122-E123. doi:10.1002/ibd.21791
- Leydhecker W, Lund OE. Eye involvement in pyostomatitis vegetans. Klin Monbl Augenheilkd Augenarztl Fortbild. 1962;141:595-602.
- Thornhill MH, Zakrzewska JM, Gilkes JJ. Pyostomatitis vegetans: report of three cases and review of the literature. J Oral Pathol Med. 1992;21:128-133. doi:10.1111/j.1600-0714.1992.tb00996.x
- Chaudhry SI, Philpot NS, Odell EW, et al. Pyostomatitis vegetans associated with asymptomatic ulcerative colitis: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:327-330. doi:10.1016/s1079-2104(99)70217-9
- Konstantopoulou M, O’Dwyer EM, Steele JC, et al. Pyodermatitis–pyostomatitis vegetans complicated by methicillin-resistant Staphylococcus aureus infection. Clin Exp Dermatol. 2005;30:666-668. doi:10.1111/j.1365-2230.2005.01906.x
- Ficarra G, Cicchi P, Amorosi A, et al. Oral Crohn’s disease and pyostomatitis vegetans. an unusual association. Oral Surg Oral Med Oral Pathol. 1993;75:220-224. doi:10.1016/0030-4220(93)90097-n
- Markopoulos AK, Antoniades DZ, Zaraboukas T. Pemphigus vegetans of the oral cavity. Int J Dermatol. 2006;45:425-428. doi:10.1111/j.1365-4632.2004.02480.x
- Nico MMS, Hussein TP, Aoki V, et al. Pyostomatitis vegetans and its relation to inflammatory bowel disease, pyoderma gangrenosum, pyodermatitis vegetans, and pemphigus. J Oral Pathol Med. 2012;41:584-588. doi:10.1111/j.1600-0714.2012.01152.x
- Berzin D, Lahad A, Weiss B, et al. Inflammatory bowel disease presenting with pyodermatitis–pyostomatitis vegetans in a pediatric patient: a case report and review of the literature. Pediatr Dermatol. 2021;38:868-871. doi:10.1111/pde.14625
- Ballo FS, Camisa C, Allen CM. Pyostomatitis vegetans. report of a case and review of the literature. J Am Acad Dermatol. 1989;21:381-387.
- Greuter T, Bertoldo F, Rechner R, et al; Swiss IBD Cohort Study Group. Extraintestinal manifestations of pediatric inflammatory bowel disease: prevalence, presentation, and anti-TNF treatment. J Pediatr Gastroenterol Nutr. 2017;65:200-206. doi:10.1097/MPG.0000000000001455
- Jose FA, Garnett EA, Vittinghoff E, et al. Development of extraintestinal manifestations in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:63-68. doi:10.1002/ibd.20604
- Harty S, Fleming P, Rowland M, et al. A prospective study of the oral manifestations of Crohn’s disease. Clin Gastroenterol Hepatol. 2005;3:886-891. doi:10.1016/s1542-3565(05)00424-6
- Pittock S, Drumm B, Fleming P, et al. The oral cavity in Crohn’s disease. J Pediatr. 2001;138:767-771. doi:10.1067/mpd.2001.113008
- Bardasi G, Romagnoli A, Foschini MP, et al. Pyostomatitis vegetans in a pediatric patient with ulcerative colitis: case report of a rare pediatric inflammatory bowel disease extraintestinal manifestation and review of the literature. Eur J Gastroenterol Hepatol. 2020;32:889-892. doi:10.1097/MEG.0000000000001723
- Mesquita Kde C, Costa IM. Case for diagnosis. An Bras Dermatol. 2012;87:929-931. doi:10.1590/s0365-05962012000600022
- Al-Rimawi HS, Hammad MM, Raweily EA, et al. Pyostomatitis vegetans in childhood. Eur J Pediatr. 1998;157:402-405. doi:10.1007/s004310050838
- Chen KL, Diiorio DA, Chiu YE, et al. Pediatric patients with orofacial granulomatosis likely to subsequently develop intestinal Crohn’s disease: brief report. Pediatr Dermatol. 2020;37:1162-1164. doi:10.1111/pde.14390
- Pazheri F, Alkhouri N, Radhakrishnan K. Pyostomatitis vegetans as an oral manifestation of Crohn’s disease in a pediatric patient. Inflamm Bowel Dis. 2010;16:2007. doi:10.1002/ibd.21245.
- Werchniak AE, Storm CA, Plunkett RW, et al. Treatment of pyostomatitis vegetans with topical tacrolimus. J Am Acad Dermatol. 2005;52:722-723. doi:10.1016/j.jaad.2004.11.041
- Stingeni L, Tramontana M, Bassotti G, et al. Pyodermatitis–pyostomatitis vegetans and antibullous pemphigoid antigen 180 autoantibodies: a casual association? Br J Dermatol. 2015;172:811-813. doi:10.1111/bjd.13297
- Takahashi S, Andreoletti G, Chen R, et al. De novo and rare mutations in the HSPA1L heat shock gene associated with inflammatory bowel disease. Genome Med. 2017;9:8. doi:10.1186/s13073-016-0394-9
- Crowley E, Warner N, Pan J, et al. Prevalence and clinical features of inflammatory bowel diseases associated with monogenic variants, identified by whole-exome sequencing in 1000 children at a single center. Gastroenterology. 2020;158:2208-2220. doi:10.1053/j .gastro.2020.02.023
Practice Points
- Pyostomatitis vegetans (PSV) is a rare manifestation of cutaneous Crohn disease in children and can precede the onset of bowel pathology.
- Although topical and intralesional corticosteroids were beneficial in our patient, systemic corticosteroids and tumor necrosis factor α inhibitors, including infliximab and adalimumab, used to treat underlying inflammatory bowel disease appear to be the most efficacious option for treating PSV.
Chronic exposure to heavy metals and breast cancer: Is there a link?
Key clinical point: Chronic exposure to heavy metals was not associated with an increased risk for breast cancer (BC) among never smokers in the general population.
Major finding: Serum levels of cobalt were inversely associated with the risk for BC (odds ratio 0.33; P = .033), with no association being observed between the risk for BC and exposure to other heavy metals.
Study details: Findings are from a prospective cohort study including 150 women with BC and without a smoking history and 150 matched control women without BC and smoking history.
Disclosures: This study was supported by the Tuscany Region, “Bando Ricerca Salute 2018.” The authors declared no conflicts of interest.
Source: Caini S et al. Serum heavy metals and breast cancer risk: A case-control study nested in the Florence cohort of the EPIC (European Prospective Investigation into Cancer and nutrition) study. Sci Total Environ. 2022;160568 (Dec 1). Doi: 10.1016/j.scitotenv.2022.160568
Key clinical point: Chronic exposure to heavy metals was not associated with an increased risk for breast cancer (BC) among never smokers in the general population.
Major finding: Serum levels of cobalt were inversely associated with the risk for BC (odds ratio 0.33; P = .033), with no association being observed between the risk for BC and exposure to other heavy metals.
Study details: Findings are from a prospective cohort study including 150 women with BC and without a smoking history and 150 matched control women without BC and smoking history.
Disclosures: This study was supported by the Tuscany Region, “Bando Ricerca Salute 2018.” The authors declared no conflicts of interest.
Source: Caini S et al. Serum heavy metals and breast cancer risk: A case-control study nested in the Florence cohort of the EPIC (European Prospective Investigation into Cancer and nutrition) study. Sci Total Environ. 2022;160568 (Dec 1). Doi: 10.1016/j.scitotenv.2022.160568
Key clinical point: Chronic exposure to heavy metals was not associated with an increased risk for breast cancer (BC) among never smokers in the general population.
Major finding: Serum levels of cobalt were inversely associated with the risk for BC (odds ratio 0.33; P = .033), with no association being observed between the risk for BC and exposure to other heavy metals.
Study details: Findings are from a prospective cohort study including 150 women with BC and without a smoking history and 150 matched control women without BC and smoking history.
Disclosures: This study was supported by the Tuscany Region, “Bando Ricerca Salute 2018.” The authors declared no conflicts of interest.
Source: Caini S et al. Serum heavy metals and breast cancer risk: A case-control study nested in the Florence cohort of the EPIC (European Prospective Investigation into Cancer and nutrition) study. Sci Total Environ. 2022;160568 (Dec 1). Doi: 10.1016/j.scitotenv.2022.160568
HER2+ metastatic BC: Isolated brain metastasis worsens survival
Key clinical point: Patients with human epidermal growth factor receptor 2-positive (HER2+) metastatic breast cancer (BC) who had an isolated brain metastasis as their first metastatic event reported worse survival outcomes than those with concurrent progressive or stable/responding extracranial disease (ECD).
Major finding: Patients with isolated brain relapse or no evidence of ECD (28.4 months; P = .0028) reported worse overall survival from metastatic diagnosis to death than patients with concurrent progressive ECD (48.8 months) or stable/responding disease (71.5 months).
Study details: Findings are from a retrospective analysis including 126 patients with HER2+ BC, brain metastasis, and known ECD status.
Disclosures: This study was funded by the Duke University Department of Medicine and other sources. Some authors declared receiving royalties or serving as consultants at various sources.
Source: Noteware L et al. Brain metastasis as the first and only metastatic relapse site portends worse survival in patients with advanced HER2 + breast cancer. Breast Cancer Res Treat. 2022 (Nov 20). Doi: 10.1007/s10549-022-06799-7
Key clinical point: Patients with human epidermal growth factor receptor 2-positive (HER2+) metastatic breast cancer (BC) who had an isolated brain metastasis as their first metastatic event reported worse survival outcomes than those with concurrent progressive or stable/responding extracranial disease (ECD).
Major finding: Patients with isolated brain relapse or no evidence of ECD (28.4 months; P = .0028) reported worse overall survival from metastatic diagnosis to death than patients with concurrent progressive ECD (48.8 months) or stable/responding disease (71.5 months).
Study details: Findings are from a retrospective analysis including 126 patients with HER2+ BC, brain metastasis, and known ECD status.
Disclosures: This study was funded by the Duke University Department of Medicine and other sources. Some authors declared receiving royalties or serving as consultants at various sources.
Source: Noteware L et al. Brain metastasis as the first and only metastatic relapse site portends worse survival in patients with advanced HER2 + breast cancer. Breast Cancer Res Treat. 2022 (Nov 20). Doi: 10.1007/s10549-022-06799-7
Key clinical point: Patients with human epidermal growth factor receptor 2-positive (HER2+) metastatic breast cancer (BC) who had an isolated brain metastasis as their first metastatic event reported worse survival outcomes than those with concurrent progressive or stable/responding extracranial disease (ECD).
Major finding: Patients with isolated brain relapse or no evidence of ECD (28.4 months; P = .0028) reported worse overall survival from metastatic diagnosis to death than patients with concurrent progressive ECD (48.8 months) or stable/responding disease (71.5 months).
Study details: Findings are from a retrospective analysis including 126 patients with HER2+ BC, brain metastasis, and known ECD status.
Disclosures: This study was funded by the Duke University Department of Medicine and other sources. Some authors declared receiving royalties or serving as consultants at various sources.
Source: Noteware L et al. Brain metastasis as the first and only metastatic relapse site portends worse survival in patients with advanced HER2 + breast cancer. Breast Cancer Res Treat. 2022 (Nov 20). Doi: 10.1007/s10549-022-06799-7
Breast conserving surgery plus radiotherapy superior to mastectomy in breast ductal carcinoma in situ with microinvasion
Key clinical point: Breast conserving surgery (BCS) plus radiotherapy (RT) demonstrated superior survival outcomes compared to mastectomy in patients with ductal carcinoma in situ with microinvasion (DCIS-MI).
Major finding: Overall survival (hazard ratio [HR] 0.676; P < .001) and breast cancer-specific survival (HR 0.565; P = .017) were significantly improved in the BCS+RT vs mastectomy group.
Study details: This study analyzed the data of 5432 patients with DCIS-MI from the Surveillance, Epidemiology, and End Results (SEER) database, of which 52.17% of patients had received BCS+RT.
Disclosures: This study did not report a source of funding. The authors declared no conflicts of interest.
Source: Xia LY et al. Survival outcomes after breast-conserving surgery plus radiotherapy compared with mastectomy in breast ductal carcinoma in situ with microinvasion. Sci Rep. 2022;12:20132 (Nov 22). Doi: 10.1038/s41598-022-24630-7
Key clinical point: Breast conserving surgery (BCS) plus radiotherapy (RT) demonstrated superior survival outcomes compared to mastectomy in patients with ductal carcinoma in situ with microinvasion (DCIS-MI).
Major finding: Overall survival (hazard ratio [HR] 0.676; P < .001) and breast cancer-specific survival (HR 0.565; P = .017) were significantly improved in the BCS+RT vs mastectomy group.
Study details: This study analyzed the data of 5432 patients with DCIS-MI from the Surveillance, Epidemiology, and End Results (SEER) database, of which 52.17% of patients had received BCS+RT.
Disclosures: This study did not report a source of funding. The authors declared no conflicts of interest.
Source: Xia LY et al. Survival outcomes after breast-conserving surgery plus radiotherapy compared with mastectomy in breast ductal carcinoma in situ with microinvasion. Sci Rep. 2022;12:20132 (Nov 22). Doi: 10.1038/s41598-022-24630-7
Key clinical point: Breast conserving surgery (BCS) plus radiotherapy (RT) demonstrated superior survival outcomes compared to mastectomy in patients with ductal carcinoma in situ with microinvasion (DCIS-MI).
Major finding: Overall survival (hazard ratio [HR] 0.676; P < .001) and breast cancer-specific survival (HR 0.565; P = .017) were significantly improved in the BCS+RT vs mastectomy group.
Study details: This study analyzed the data of 5432 patients with DCIS-MI from the Surveillance, Epidemiology, and End Results (SEER) database, of which 52.17% of patients had received BCS+RT.
Disclosures: This study did not report a source of funding. The authors declared no conflicts of interest.
Source: Xia LY et al. Survival outcomes after breast-conserving surgery plus radiotherapy compared with mastectomy in breast ductal carcinoma in situ with microinvasion. Sci Rep. 2022;12:20132 (Nov 22). Doi: 10.1038/s41598-022-24630-7
Palbociclib+endocrine therapy improves progression-free survival across all subgroups
Key clinical point: Palbociclib plus endocrine therapy (ET) improved progression-free survival (PFS) across all subgroups of patients with hormone receptor-positive/human epidermal growth factor receptor 2-negative (HR+/HER2−) advanced breast cancer (BC).
Major finding: Median PFS was longer in patients receiving palbociclib+letrozole vs placebo+letrozole (hazard ratio [HR] 0.56; 95% CI 0.46-0.69) or palbociclib+fulvestrant vs placebo+fulvestrant (HR 0.50; 95% CI 0.40-0.62), with similar outcomes observed in subgroups of patients reporting a disease-free interval of ≤12 months, visceral disease, or ET resistance.
Study details: Findings are from a post hoc analysis of two phase 3 trials including women with HR+/HER2− advanced BC who were randomly assigned to receive letrozole with palbociclib or placebo (n = 666; PALOMA-2) or fulvestrant with palbociclib or placebo (n = 521; PALOMA-3).
Disclosures: This study was funded by Pfizer Inc. Four authors declared being employees and stockholders of Pfizer, and the other authors reported ties with several sources, including Pfizer.
Source: Rugo HS et al. Effect of palbociclib plus endocrine therapy on time to chemotherapy across subgroups of patients with hormone receptor‒positive/human epidermal growth factor receptor 2‒negative advanced breast cancer: Post hoc analyses from PALOMA-2 and PALOMA-3. Breast. 2022;66:324-331 (Nov 15). Doi: 10.1016/j.breast.2022.11.005
Key clinical point: Palbociclib plus endocrine therapy (ET) improved progression-free survival (PFS) across all subgroups of patients with hormone receptor-positive/human epidermal growth factor receptor 2-negative (HR+/HER2−) advanced breast cancer (BC).
Major finding: Median PFS was longer in patients receiving palbociclib+letrozole vs placebo+letrozole (hazard ratio [HR] 0.56; 95% CI 0.46-0.69) or palbociclib+fulvestrant vs placebo+fulvestrant (HR 0.50; 95% CI 0.40-0.62), with similar outcomes observed in subgroups of patients reporting a disease-free interval of ≤12 months, visceral disease, or ET resistance.
Study details: Findings are from a post hoc analysis of two phase 3 trials including women with HR+/HER2− advanced BC who were randomly assigned to receive letrozole with palbociclib or placebo (n = 666; PALOMA-2) or fulvestrant with palbociclib or placebo (n = 521; PALOMA-3).
Disclosures: This study was funded by Pfizer Inc. Four authors declared being employees and stockholders of Pfizer, and the other authors reported ties with several sources, including Pfizer.
Source: Rugo HS et al. Effect of palbociclib plus endocrine therapy on time to chemotherapy across subgroups of patients with hormone receptor‒positive/human epidermal growth factor receptor 2‒negative advanced breast cancer: Post hoc analyses from PALOMA-2 and PALOMA-3. Breast. 2022;66:324-331 (Nov 15). Doi: 10.1016/j.breast.2022.11.005
Key clinical point: Palbociclib plus endocrine therapy (ET) improved progression-free survival (PFS) across all subgroups of patients with hormone receptor-positive/human epidermal growth factor receptor 2-negative (HR+/HER2−) advanced breast cancer (BC).
Major finding: Median PFS was longer in patients receiving palbociclib+letrozole vs placebo+letrozole (hazard ratio [HR] 0.56; 95% CI 0.46-0.69) or palbociclib+fulvestrant vs placebo+fulvestrant (HR 0.50; 95% CI 0.40-0.62), with similar outcomes observed in subgroups of patients reporting a disease-free interval of ≤12 months, visceral disease, or ET resistance.
Study details: Findings are from a post hoc analysis of two phase 3 trials including women with HR+/HER2− advanced BC who were randomly assigned to receive letrozole with palbociclib or placebo (n = 666; PALOMA-2) or fulvestrant with palbociclib or placebo (n = 521; PALOMA-3).
Disclosures: This study was funded by Pfizer Inc. Four authors declared being employees and stockholders of Pfizer, and the other authors reported ties with several sources, including Pfizer.
Source: Rugo HS et al. Effect of palbociclib plus endocrine therapy on time to chemotherapy across subgroups of patients with hormone receptor‒positive/human epidermal growth factor receptor 2‒negative advanced breast cancer: Post hoc analyses from PALOMA-2 and PALOMA-3. Breast. 2022;66:324-331 (Nov 15). Doi: 10.1016/j.breast.2022.11.005