User login
How Well Does the Third Dose of COVID-19 Vaccine Work?
How effective are the COVID-19 vaccines, third time around? Researchers compared 2 large groups of veterans to find out how well a third dose protected against documented infection, symptomatic COVID-19, and COVID-19–related hospitalization, intensive care unit (ICU) admission, and death.
The research, published in Nature, used electronic health records of 65,196 veterans who received BNT162b2 (Pfizer-BioNTech) and 65,196 who received mRNA-1273 (Moderna). They chose to study the 16 weeks between October 20, 2021 and February 8, 2022, which included both Delta- and Omicron-variant waves.
During the follow-up (median, 77 days), 2994 COVID-19 infections were documented, of which 200 were detected as symptomatic, 194 required hospitalization, and 52 required ICU admission. Twenty-two patients died.
In a previous head-to-head trial comparing breakthrough COVID-19 outcomes after the first doses of the 2 vaccines (given when the Alpha and Delta variants were predominant), the researchers had found a low risk of documented infection and severe outcomes, but lower for the Moderna vaccine. They note that few head-to-head comparisons have been made of third-dose effectiveness.
As expected, in this trial, the researchers found a “nearly identical” pattern for the risk of the 2 vaccine groups. Although the risks for all of the measured outcomes over 16 weeks were low for both vaccines ≤ 4% for documented infection and < 0.03% for death in each group—those veterans who received the Pfizer-BioNTech vaccine had an excess of 45 documented infections and 11 hospitalizations per 10,000 persons, compared with the Moderna group. The Pfizer-BioNTech group also had a higher risk of documented infection over 9 weeks of follow-up, during which an Omicron-variant predominated.
Given the high effectiveness of a third dose of both vaccines, either vaccine is strongly recommended, the researchers conclude. They point to “evidence of clear and comparable benefits” for the most severe outcomes: The difference in estimated 16-week risk of death between the 2 groups was two-thousandths of 1 %.
They add that, while the differences in estimated risk for less severe outcomes between the 2 groups were small on the absolute scale, they may be meaningful when considering the population scale at which these vaccines are deployed.
How effective are the COVID-19 vaccines, third time around? Researchers compared 2 large groups of veterans to find out how well a third dose protected against documented infection, symptomatic COVID-19, and COVID-19–related hospitalization, intensive care unit (ICU) admission, and death.
The research, published in Nature, used electronic health records of 65,196 veterans who received BNT162b2 (Pfizer-BioNTech) and 65,196 who received mRNA-1273 (Moderna). They chose to study the 16 weeks between October 20, 2021 and February 8, 2022, which included both Delta- and Omicron-variant waves.
During the follow-up (median, 77 days), 2994 COVID-19 infections were documented, of which 200 were detected as symptomatic, 194 required hospitalization, and 52 required ICU admission. Twenty-two patients died.
In a previous head-to-head trial comparing breakthrough COVID-19 outcomes after the first doses of the 2 vaccines (given when the Alpha and Delta variants were predominant), the researchers had found a low risk of documented infection and severe outcomes, but lower for the Moderna vaccine. They note that few head-to-head comparisons have been made of third-dose effectiveness.
As expected, in this trial, the researchers found a “nearly identical” pattern for the risk of the 2 vaccine groups. Although the risks for all of the measured outcomes over 16 weeks were low for both vaccines ≤ 4% for documented infection and < 0.03% for death in each group—those veterans who received the Pfizer-BioNTech vaccine had an excess of 45 documented infections and 11 hospitalizations per 10,000 persons, compared with the Moderna group. The Pfizer-BioNTech group also had a higher risk of documented infection over 9 weeks of follow-up, during which an Omicron-variant predominated.
Given the high effectiveness of a third dose of both vaccines, either vaccine is strongly recommended, the researchers conclude. They point to “evidence of clear and comparable benefits” for the most severe outcomes: The difference in estimated 16-week risk of death between the 2 groups was two-thousandths of 1 %.
They add that, while the differences in estimated risk for less severe outcomes between the 2 groups were small on the absolute scale, they may be meaningful when considering the population scale at which these vaccines are deployed.
How effective are the COVID-19 vaccines, third time around? Researchers compared 2 large groups of veterans to find out how well a third dose protected against documented infection, symptomatic COVID-19, and COVID-19–related hospitalization, intensive care unit (ICU) admission, and death.
The research, published in Nature, used electronic health records of 65,196 veterans who received BNT162b2 (Pfizer-BioNTech) and 65,196 who received mRNA-1273 (Moderna). They chose to study the 16 weeks between October 20, 2021 and February 8, 2022, which included both Delta- and Omicron-variant waves.
During the follow-up (median, 77 days), 2994 COVID-19 infections were documented, of which 200 were detected as symptomatic, 194 required hospitalization, and 52 required ICU admission. Twenty-two patients died.
In a previous head-to-head trial comparing breakthrough COVID-19 outcomes after the first doses of the 2 vaccines (given when the Alpha and Delta variants were predominant), the researchers had found a low risk of documented infection and severe outcomes, but lower for the Moderna vaccine. They note that few head-to-head comparisons have been made of third-dose effectiveness.
As expected, in this trial, the researchers found a “nearly identical” pattern for the risk of the 2 vaccine groups. Although the risks for all of the measured outcomes over 16 weeks were low for both vaccines ≤ 4% for documented infection and < 0.03% for death in each group—those veterans who received the Pfizer-BioNTech vaccine had an excess of 45 documented infections and 11 hospitalizations per 10,000 persons, compared with the Moderna group. The Pfizer-BioNTech group also had a higher risk of documented infection over 9 weeks of follow-up, during which an Omicron-variant predominated.
Given the high effectiveness of a third dose of both vaccines, either vaccine is strongly recommended, the researchers conclude. They point to “evidence of clear and comparable benefits” for the most severe outcomes: The difference in estimated 16-week risk of death between the 2 groups was two-thousandths of 1 %.
They add that, while the differences in estimated risk for less severe outcomes between the 2 groups were small on the absolute scale, they may be meaningful when considering the population scale at which these vaccines are deployed.
Black Veterans Disproportionately Denied VA Benefits
Black veterans are less likely to have their benefits claims processed and paid than are their White peers because of systemic problems within the US Department of Veterans Affairs, according to a lawsuit filed against the agency.
“A Black veteran who served honorably can walk into the VA, file a disability claim, and be at a significantly higher likelihood of having that claim denied,” said Adam Henderson, a student working with the Yale Law School Veterans Legal Services Clinic, one of several groups connected to the lawsuit.
“The VA has denied countless meritorious applications of Black veterans and thus deprived them and their families of the support that they are entitled to.”
The suit, filed in federal court by the clinic on behalf of Vietnam War veteran Conley Monk Jr., asks for “redress for the harms caused by the failure of VA staff and leaders to administer these benefits programs in a manner free from racial discrimination against Black veterans.”
In a press conference announcing the lawsuit, the effort received backing from Sen. Richard Blumenthal (D, Connecticut) who called it an “unacceptable” situation.
“Black veterans are denied benefits at a very significantly disproportionate rate,” he said. “We know the results. We want to know the reason why.”
The suit stems from an analysis of VA claims records released by the department following an earlier legal action. Between 2001 and 2020, the average denial rate for disability claims filed for Black veterans was 29.5%, significantly above the 24.2% for White veterans.
Attorneys allege the problems date back even further and that VA officials should have known about the racial disparities in the system from previous complaints.
“The negligence of VA leadership, and their failure to train, supervise, monitor and instruct agency officials to take steps to identify and correct racial disparities, led to systematic benefits obstruction for Black veterans,” the suit states.
Monk is a Black disabled Marine Corps veteran who previously sued the military to overturn his less-than-honorable military discharge due to complications from undiagnosed posttraumatic stress disorder.
He was subsequently granted access to a host of veterans benefits but not to retroactive payouts for claims he was denied in the 1970s.
“They didn’t fully compensate me or my family,” he said. “I wasn’t able to give my kids my educational benefits. We should have been receiving checks while they were growing up.”
Along with potential past benefits for Monk, individuals involved with the lawsuit said the move could force the VA to reassess thousands of other unfairly dismissed cases. “For decades [the US government] has allowed racially discriminatory practices to obstruct Black veterans from easily accessing veterans housing, education, and health care benefits with wide-reaching economic consequences for Black veterans and their families,” said Richard Brookshire, executive director of the Black Veterans Project.
“This lawsuit reckons with the shameful history of racism by the Department of Veteran Affairs and seeks to redress long-standing improprieties reverberating across generations of Black military service.”
In a statement, VA press secretary Terrence Hayes did not directly respond to the lawsuit but noted that “throughout history, there have been unacceptable disparities in both VA benefits decisions and military discharge status due to racism, which have wrongly left Black veterans without access to VA care and benefits.”
“We are actively working to right these wrongs, and we will stop at nothing to ensure that all Black veterans get the VA services they have earned and deserve,” he said. “We are currently studying racial disparities in benefits claims decisions, and we will publish the results of that study as soon as they are available.”
Hayes said the department has already begun targeted outreach to Black veterans to help them with claims and is “taking steps to ensure that our claims process combats institutional racism, rather than perpetuating it.”
Black veterans are less likely to have their benefits claims processed and paid than are their White peers because of systemic problems within the US Department of Veterans Affairs, according to a lawsuit filed against the agency.
“A Black veteran who served honorably can walk into the VA, file a disability claim, and be at a significantly higher likelihood of having that claim denied,” said Adam Henderson, a student working with the Yale Law School Veterans Legal Services Clinic, one of several groups connected to the lawsuit.
“The VA has denied countless meritorious applications of Black veterans and thus deprived them and their families of the support that they are entitled to.”
The suit, filed in federal court by the clinic on behalf of Vietnam War veteran Conley Monk Jr., asks for “redress for the harms caused by the failure of VA staff and leaders to administer these benefits programs in a manner free from racial discrimination against Black veterans.”
In a press conference announcing the lawsuit, the effort received backing from Sen. Richard Blumenthal (D, Connecticut) who called it an “unacceptable” situation.
“Black veterans are denied benefits at a very significantly disproportionate rate,” he said. “We know the results. We want to know the reason why.”
The suit stems from an analysis of VA claims records released by the department following an earlier legal action. Between 2001 and 2020, the average denial rate for disability claims filed for Black veterans was 29.5%, significantly above the 24.2% for White veterans.
Attorneys allege the problems date back even further and that VA officials should have known about the racial disparities in the system from previous complaints.
“The negligence of VA leadership, and their failure to train, supervise, monitor and instruct agency officials to take steps to identify and correct racial disparities, led to systematic benefits obstruction for Black veterans,” the suit states.
Monk is a Black disabled Marine Corps veteran who previously sued the military to overturn his less-than-honorable military discharge due to complications from undiagnosed posttraumatic stress disorder.
He was subsequently granted access to a host of veterans benefits but not to retroactive payouts for claims he was denied in the 1970s.
“They didn’t fully compensate me or my family,” he said. “I wasn’t able to give my kids my educational benefits. We should have been receiving checks while they were growing up.”
Along with potential past benefits for Monk, individuals involved with the lawsuit said the move could force the VA to reassess thousands of other unfairly dismissed cases. “For decades [the US government] has allowed racially discriminatory practices to obstruct Black veterans from easily accessing veterans housing, education, and health care benefits with wide-reaching economic consequences for Black veterans and their families,” said Richard Brookshire, executive director of the Black Veterans Project.
“This lawsuit reckons with the shameful history of racism by the Department of Veteran Affairs and seeks to redress long-standing improprieties reverberating across generations of Black military service.”
In a statement, VA press secretary Terrence Hayes did not directly respond to the lawsuit but noted that “throughout history, there have been unacceptable disparities in both VA benefits decisions and military discharge status due to racism, which have wrongly left Black veterans without access to VA care and benefits.”
“We are actively working to right these wrongs, and we will stop at nothing to ensure that all Black veterans get the VA services they have earned and deserve,” he said. “We are currently studying racial disparities in benefits claims decisions, and we will publish the results of that study as soon as they are available.”
Hayes said the department has already begun targeted outreach to Black veterans to help them with claims and is “taking steps to ensure that our claims process combats institutional racism, rather than perpetuating it.”
Black veterans are less likely to have their benefits claims processed and paid than are their White peers because of systemic problems within the US Department of Veterans Affairs, according to a lawsuit filed against the agency.
“A Black veteran who served honorably can walk into the VA, file a disability claim, and be at a significantly higher likelihood of having that claim denied,” said Adam Henderson, a student working with the Yale Law School Veterans Legal Services Clinic, one of several groups connected to the lawsuit.
“The VA has denied countless meritorious applications of Black veterans and thus deprived them and their families of the support that they are entitled to.”
The suit, filed in federal court by the clinic on behalf of Vietnam War veteran Conley Monk Jr., asks for “redress for the harms caused by the failure of VA staff and leaders to administer these benefits programs in a manner free from racial discrimination against Black veterans.”
In a press conference announcing the lawsuit, the effort received backing from Sen. Richard Blumenthal (D, Connecticut) who called it an “unacceptable” situation.
“Black veterans are denied benefits at a very significantly disproportionate rate,” he said. “We know the results. We want to know the reason why.”
The suit stems from an analysis of VA claims records released by the department following an earlier legal action. Between 2001 and 2020, the average denial rate for disability claims filed for Black veterans was 29.5%, significantly above the 24.2% for White veterans.
Attorneys allege the problems date back even further and that VA officials should have known about the racial disparities in the system from previous complaints.
“The negligence of VA leadership, and their failure to train, supervise, monitor and instruct agency officials to take steps to identify and correct racial disparities, led to systematic benefits obstruction for Black veterans,” the suit states.
Monk is a Black disabled Marine Corps veteran who previously sued the military to overturn his less-than-honorable military discharge due to complications from undiagnosed posttraumatic stress disorder.
He was subsequently granted access to a host of veterans benefits but not to retroactive payouts for claims he was denied in the 1970s.
“They didn’t fully compensate me or my family,” he said. “I wasn’t able to give my kids my educational benefits. We should have been receiving checks while they were growing up.”
Along with potential past benefits for Monk, individuals involved with the lawsuit said the move could force the VA to reassess thousands of other unfairly dismissed cases. “For decades [the US government] has allowed racially discriminatory practices to obstruct Black veterans from easily accessing veterans housing, education, and health care benefits with wide-reaching economic consequences for Black veterans and their families,” said Richard Brookshire, executive director of the Black Veterans Project.
“This lawsuit reckons with the shameful history of racism by the Department of Veteran Affairs and seeks to redress long-standing improprieties reverberating across generations of Black military service.”
In a statement, VA press secretary Terrence Hayes did not directly respond to the lawsuit but noted that “throughout history, there have been unacceptable disparities in both VA benefits decisions and military discharge status due to racism, which have wrongly left Black veterans without access to VA care and benefits.”
“We are actively working to right these wrongs, and we will stop at nothing to ensure that all Black veterans get the VA services they have earned and deserve,” he said. “We are currently studying racial disparities in benefits claims decisions, and we will publish the results of that study as soon as they are available.”
Hayes said the department has already begun targeted outreach to Black veterans to help them with claims and is “taking steps to ensure that our claims process combats institutional racism, rather than perpetuating it.”
Exophytic Firm Papulonodule on the Labia in a Patient With Nonspecific Gastrointestinal Symptoms
The Diagnosis: Cutaneous Crohn Disease
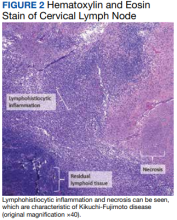
Kinyoun and Grocott-Gomori methenamine-silver staining of the labial biopsy were negative for mycobacteria and fungi, respectively. A complete blood cell count, erythrocyte sedimentation rate, C-reactive protein, celiac disease serologies, stool occult blood, and stool calprotectin laboratory test results were within reference range. Magnetic resonance imaging of the pelvis demonstrated an anal fissure extending from the anal verge at the 6 o’clock position, abnormal T2 bright signal in the skin of the buttocks and perineum extending to the labia, and mild mucosal enhancement of the rectal and anal mucosa. Esophagogastroduodenoscopy and magnetic resonance elastography were unremarkable. Colonoscopy demonstrated scattered superficial erythematous patches and erosions in the rectum. Histologically, there was mild to moderately active colitis in the rectum with no evidence of chronicity. Given our patient’s labial edema and exophytic papulonodule (Figure 1) in the setting of nonspecific gastrointestinal symptoms and granulomatous dermatitis seen on pathology (Figure 2), she was diagnosed with cutaneous Crohn disease (CD).
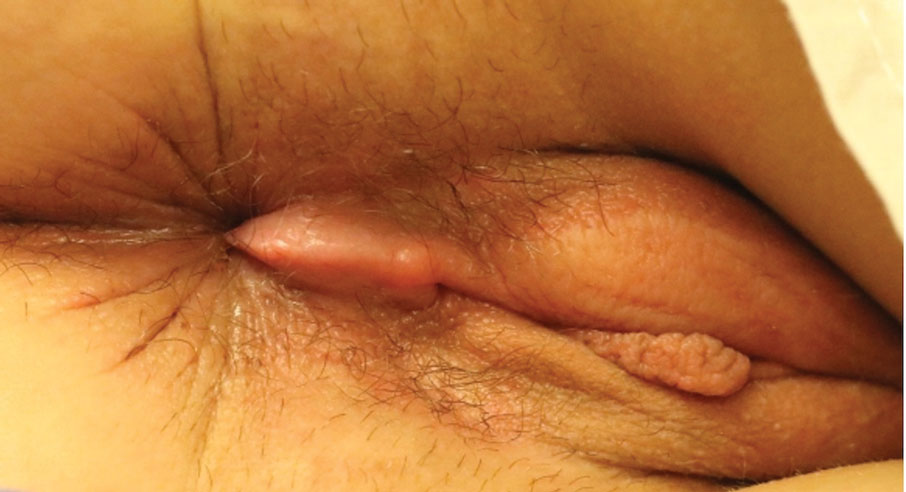
In our patient, labial biopsy was necessary to definitively diagnose CD. Prior to biopsy of the lesion, our patient was diagnosed with irritable bowel syndrome with constipation leading to an anal fissure and skin tag due to lack of laboratory, imaging, and colonoscopy findings commonly associated with CD. Her biopsy results and gastrointestinal symptoms made these diagnoses, as well as condyloma or a large sentinel skin tag, less likely.
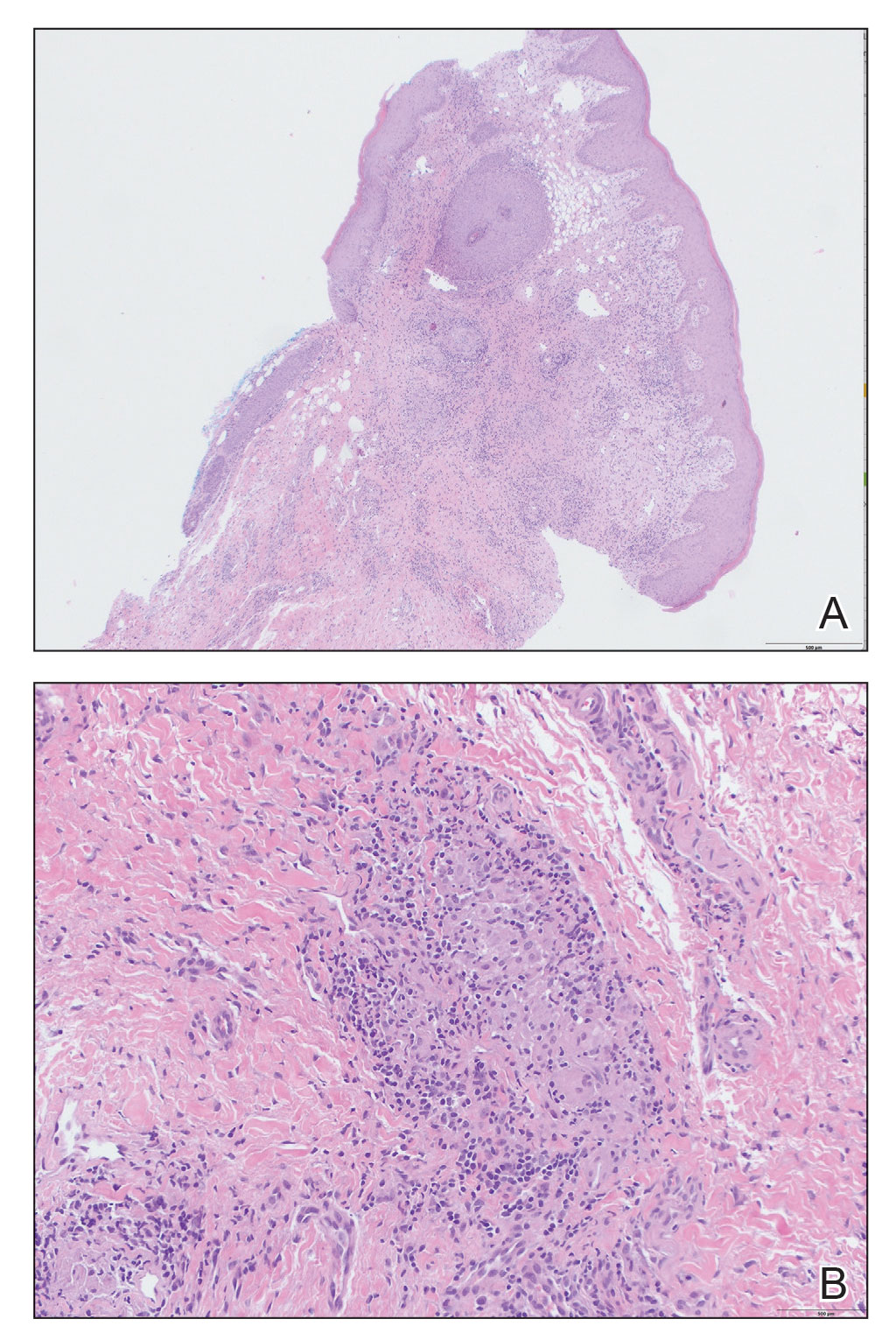
Extraintestinal findings of CD, especially cutaneous manifestations, are relatively frequent and may be present in as many as 44% of patients.1,2 Cutaneous CD often is characterized based on pathogenic mechanisms as either reactive, associated, or CD specific. Reactive cutaneous manifestations include erythema nodosum, pyoderma gangrenosum, and oral aphthae. Associated cutaneous manifestations include vitiligo, palmar erythema, and palmoplantar pustulosis.2 Crohn disease–specific manifestations, including genital or extragenital metastatic CD (MCD), fistulas, and oral involvement, are granulomatous in nature, similar to intestinal CD. Genital manifestations of MCD include edema, erythema, fissures, and/or ulceration of the vulva, penis, or scrotum. Labial swelling is the most common presenting symptom of MCD in females in both pediatric and adult age groups.2 Lymphedema, skin tags, and condylomalike growths also can be seen but are relatively less common.2
Given the labial edema, exophytic papulonodule, and granulomatous dermatitis seen on histopathology, our patient likely fit into the MCD category.2 In adults, most instances of MCD arise in the setting of well-established intestinal CD disease,3 whereas in children 86% of cases occur in patients without concurrent intestinal CD.2
Given the nonspecific and variable presentation of MCD, the differential diagnosis is broad. The differential diagnosis could include infectious etiologies such as condyloma acuminatum (human papillomavirus); syphilitic chancre; or mycobacterial, bacterial, fungal, or parasitic vulvovaginitis. Sexual abuse, sarcoidosis, Behçet disease, or hidradenitis suppurativa, among other diagnoses, also should be considered. Diagnostic workup should include biopsy of the lesion with special stains, polarizing microscopy, and tissue cultures.4 A thorough evaluation for gastrointestinal CD should be completed after diagnosis.3
The clinical course of vulvar CD can be unpredictable, with some cases healing spontaneously but most persisting despite treatment and sometimes prompting surgical removal.2,4 Early recognition is crucial, as long-standing MCD lesions can be therapy resistant.5 Due to the rarity of the condition and lack of data, there is a lack of treatment consensus for MCD. In 2014, the American Academy of Dermatology published treatment guidelines recommending superpotent topical steroids or topical tacrolimus as first-line therapy. Next-line therapy includes oral metronidazole, followed by prednisolone if still symptomatic.3 Treatment-resistant disease can warrant treatment with immunomodulators or tumor necrosis factor α inhibitors. Our patient was started on adalimumab; after just 2 months of therapy, the labial swelling decreased and the exophytic nodule was less firm and smaller.
Metastatic CD is a rare manifestation of cutaneous CD and can be present in the absence of gastrointestinal disease.3 This case demonstrates the importance of recognizing the cutaneous signs of CD and the necessity of lesional biopsy for the diagnosis of MCD, as our patient presented with nonspecific gastrointestinal symptoms and a diagnostic workup, including endoscopies, that proved inconclusive for the diagnosis of CD.
- Antonelli E, Bassotti G, Tramontana M, et al. Dermatological manifestations in inflammatory bowel diseases. J Clin Med. 2021;10:1-16. doi:10.3390/JCM10020364
- Schneider SL, Foster K, Patel D, et al. Cutaneous manifestations of metastatic Crohn’s disease. Pediatr Dermatol. 2018;35:566-574. doi:10.1111/PDE.13565
- Kurtzman DJB, Jones T, Lian F, et al. Metastatic Crohn’s disease: a review and approach to therapy. J Am Acad Dermatol. 2014;71:804-813. doi:10.1016/J.JAAD.2014.04.002
- Barret M, De Parades V, Battistella M, et al. Crohn’s disease of the vulva. J Crohns Colitis. 2014;8:563-570. doi:10.1016/J.CROHNS.2013.10.009
- Aberumand B, Howard J, Howard J. Metastatic Crohn’s disease: an approach to an uncommon but important cutaneous disorder [published online January 3, 2017]. Biomed Res Int. 2017;2017:8192150. doi:10.1155/2017/8192150
The Diagnosis: Cutaneous Crohn Disease
Kinyoun and Grocott-Gomori methenamine-silver staining of the labial biopsy were negative for mycobacteria and fungi, respectively. A complete blood cell count, erythrocyte sedimentation rate, C-reactive protein, celiac disease serologies, stool occult blood, and stool calprotectin laboratory test results were within reference range. Magnetic resonance imaging of the pelvis demonstrated an anal fissure extending from the anal verge at the 6 o’clock position, abnormal T2 bright signal in the skin of the buttocks and perineum extending to the labia, and mild mucosal enhancement of the rectal and anal mucosa. Esophagogastroduodenoscopy and magnetic resonance elastography were unremarkable. Colonoscopy demonstrated scattered superficial erythematous patches and erosions in the rectum. Histologically, there was mild to moderately active colitis in the rectum with no evidence of chronicity. Given our patient’s labial edema and exophytic papulonodule (Figure 1) in the setting of nonspecific gastrointestinal symptoms and granulomatous dermatitis seen on pathology (Figure 2), she was diagnosed with cutaneous Crohn disease (CD).

In our patient, labial biopsy was necessary to definitively diagnose CD. Prior to biopsy of the lesion, our patient was diagnosed with irritable bowel syndrome with constipation leading to an anal fissure and skin tag due to lack of laboratory, imaging, and colonoscopy findings commonly associated with CD. Her biopsy results and gastrointestinal symptoms made these diagnoses, as well as condyloma or a large sentinel skin tag, less likely.

Extraintestinal findings of CD, especially cutaneous manifestations, are relatively frequent and may be present in as many as 44% of patients.1,2 Cutaneous CD often is characterized based on pathogenic mechanisms as either reactive, associated, or CD specific. Reactive cutaneous manifestations include erythema nodosum, pyoderma gangrenosum, and oral aphthae. Associated cutaneous manifestations include vitiligo, palmar erythema, and palmoplantar pustulosis.2 Crohn disease–specific manifestations, including genital or extragenital metastatic CD (MCD), fistulas, and oral involvement, are granulomatous in nature, similar to intestinal CD. Genital manifestations of MCD include edema, erythema, fissures, and/or ulceration of the vulva, penis, or scrotum. Labial swelling is the most common presenting symptom of MCD in females in both pediatric and adult age groups.2 Lymphedema, skin tags, and condylomalike growths also can be seen but are relatively less common.2
Given the labial edema, exophytic papulonodule, and granulomatous dermatitis seen on histopathology, our patient likely fit into the MCD category.2 In adults, most instances of MCD arise in the setting of well-established intestinal CD disease,3 whereas in children 86% of cases occur in patients without concurrent intestinal CD.2
Given the nonspecific and variable presentation of MCD, the differential diagnosis is broad. The differential diagnosis could include infectious etiologies such as condyloma acuminatum (human papillomavirus); syphilitic chancre; or mycobacterial, bacterial, fungal, or parasitic vulvovaginitis. Sexual abuse, sarcoidosis, Behçet disease, or hidradenitis suppurativa, among other diagnoses, also should be considered. Diagnostic workup should include biopsy of the lesion with special stains, polarizing microscopy, and tissue cultures.4 A thorough evaluation for gastrointestinal CD should be completed after diagnosis.3
The clinical course of vulvar CD can be unpredictable, with some cases healing spontaneously but most persisting despite treatment and sometimes prompting surgical removal.2,4 Early recognition is crucial, as long-standing MCD lesions can be therapy resistant.5 Due to the rarity of the condition and lack of data, there is a lack of treatment consensus for MCD. In 2014, the American Academy of Dermatology published treatment guidelines recommending superpotent topical steroids or topical tacrolimus as first-line therapy. Next-line therapy includes oral metronidazole, followed by prednisolone if still symptomatic.3 Treatment-resistant disease can warrant treatment with immunomodulators or tumor necrosis factor α inhibitors. Our patient was started on adalimumab; after just 2 months of therapy, the labial swelling decreased and the exophytic nodule was less firm and smaller.
Metastatic CD is a rare manifestation of cutaneous CD and can be present in the absence of gastrointestinal disease.3 This case demonstrates the importance of recognizing the cutaneous signs of CD and the necessity of lesional biopsy for the diagnosis of MCD, as our patient presented with nonspecific gastrointestinal symptoms and a diagnostic workup, including endoscopies, that proved inconclusive for the diagnosis of CD.
The Diagnosis: Cutaneous Crohn Disease
Kinyoun and Grocott-Gomori methenamine-silver staining of the labial biopsy were negative for mycobacteria and fungi, respectively. A complete blood cell count, erythrocyte sedimentation rate, C-reactive protein, celiac disease serologies, stool occult blood, and stool calprotectin laboratory test results were within reference range. Magnetic resonance imaging of the pelvis demonstrated an anal fissure extending from the anal verge at the 6 o’clock position, abnormal T2 bright signal in the skin of the buttocks and perineum extending to the labia, and mild mucosal enhancement of the rectal and anal mucosa. Esophagogastroduodenoscopy and magnetic resonance elastography were unremarkable. Colonoscopy demonstrated scattered superficial erythematous patches and erosions in the rectum. Histologically, there was mild to moderately active colitis in the rectum with no evidence of chronicity. Given our patient’s labial edema and exophytic papulonodule (Figure 1) in the setting of nonspecific gastrointestinal symptoms and granulomatous dermatitis seen on pathology (Figure 2), she was diagnosed with cutaneous Crohn disease (CD).

In our patient, labial biopsy was necessary to definitively diagnose CD. Prior to biopsy of the lesion, our patient was diagnosed with irritable bowel syndrome with constipation leading to an anal fissure and skin tag due to lack of laboratory, imaging, and colonoscopy findings commonly associated with CD. Her biopsy results and gastrointestinal symptoms made these diagnoses, as well as condyloma or a large sentinel skin tag, less likely.

Extraintestinal findings of CD, especially cutaneous manifestations, are relatively frequent and may be present in as many as 44% of patients.1,2 Cutaneous CD often is characterized based on pathogenic mechanisms as either reactive, associated, or CD specific. Reactive cutaneous manifestations include erythema nodosum, pyoderma gangrenosum, and oral aphthae. Associated cutaneous manifestations include vitiligo, palmar erythema, and palmoplantar pustulosis.2 Crohn disease–specific manifestations, including genital or extragenital metastatic CD (MCD), fistulas, and oral involvement, are granulomatous in nature, similar to intestinal CD. Genital manifestations of MCD include edema, erythema, fissures, and/or ulceration of the vulva, penis, or scrotum. Labial swelling is the most common presenting symptom of MCD in females in both pediatric and adult age groups.2 Lymphedema, skin tags, and condylomalike growths also can be seen but are relatively less common.2
Given the labial edema, exophytic papulonodule, and granulomatous dermatitis seen on histopathology, our patient likely fit into the MCD category.2 In adults, most instances of MCD arise in the setting of well-established intestinal CD disease,3 whereas in children 86% of cases occur in patients without concurrent intestinal CD.2
Given the nonspecific and variable presentation of MCD, the differential diagnosis is broad. The differential diagnosis could include infectious etiologies such as condyloma acuminatum (human papillomavirus); syphilitic chancre; or mycobacterial, bacterial, fungal, or parasitic vulvovaginitis. Sexual abuse, sarcoidosis, Behçet disease, or hidradenitis suppurativa, among other diagnoses, also should be considered. Diagnostic workup should include biopsy of the lesion with special stains, polarizing microscopy, and tissue cultures.4 A thorough evaluation for gastrointestinal CD should be completed after diagnosis.3
The clinical course of vulvar CD can be unpredictable, with some cases healing spontaneously but most persisting despite treatment and sometimes prompting surgical removal.2,4 Early recognition is crucial, as long-standing MCD lesions can be therapy resistant.5 Due to the rarity of the condition and lack of data, there is a lack of treatment consensus for MCD. In 2014, the American Academy of Dermatology published treatment guidelines recommending superpotent topical steroids or topical tacrolimus as first-line therapy. Next-line therapy includes oral metronidazole, followed by prednisolone if still symptomatic.3 Treatment-resistant disease can warrant treatment with immunomodulators or tumor necrosis factor α inhibitors. Our patient was started on adalimumab; after just 2 months of therapy, the labial swelling decreased and the exophytic nodule was less firm and smaller.
Metastatic CD is a rare manifestation of cutaneous CD and can be present in the absence of gastrointestinal disease.3 This case demonstrates the importance of recognizing the cutaneous signs of CD and the necessity of lesional biopsy for the diagnosis of MCD, as our patient presented with nonspecific gastrointestinal symptoms and a diagnostic workup, including endoscopies, that proved inconclusive for the diagnosis of CD.
- Antonelli E, Bassotti G, Tramontana M, et al. Dermatological manifestations in inflammatory bowel diseases. J Clin Med. 2021;10:1-16. doi:10.3390/JCM10020364
- Schneider SL, Foster K, Patel D, et al. Cutaneous manifestations of metastatic Crohn’s disease. Pediatr Dermatol. 2018;35:566-574. doi:10.1111/PDE.13565
- Kurtzman DJB, Jones T, Lian F, et al. Metastatic Crohn’s disease: a review and approach to therapy. J Am Acad Dermatol. 2014;71:804-813. doi:10.1016/J.JAAD.2014.04.002
- Barret M, De Parades V, Battistella M, et al. Crohn’s disease of the vulva. J Crohns Colitis. 2014;8:563-570. doi:10.1016/J.CROHNS.2013.10.009
- Aberumand B, Howard J, Howard J. Metastatic Crohn’s disease: an approach to an uncommon but important cutaneous disorder [published online January 3, 2017]. Biomed Res Int. 2017;2017:8192150. doi:10.1155/2017/8192150
- Antonelli E, Bassotti G, Tramontana M, et al. Dermatological manifestations in inflammatory bowel diseases. J Clin Med. 2021;10:1-16. doi:10.3390/JCM10020364
- Schneider SL, Foster K, Patel D, et al. Cutaneous manifestations of metastatic Crohn’s disease. Pediatr Dermatol. 2018;35:566-574. doi:10.1111/PDE.13565
- Kurtzman DJB, Jones T, Lian F, et al. Metastatic Crohn’s disease: a review and approach to therapy. J Am Acad Dermatol. 2014;71:804-813. doi:10.1016/J.JAAD.2014.04.002
- Barret M, De Parades V, Battistella M, et al. Crohn’s disease of the vulva. J Crohns Colitis. 2014;8:563-570. doi:10.1016/J.CROHNS.2013.10.009
- Aberumand B, Howard J, Howard J. Metastatic Crohn’s disease: an approach to an uncommon but important cutaneous disorder [published online January 3, 2017]. Biomed Res Int. 2017;2017:8192150. doi:10.1155/2017/8192150
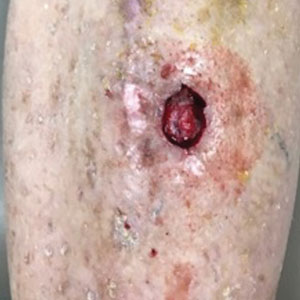
An 18-year-old woman with chronic constipation presented with an enlarging, painful, and edematous “lump” in the perineum of 1 year’s duration. The lesion became firmer and more painful with bowel movements. Physical examination revealed an enlarged right labia majora, as well as a pink to flesh-colored, exophytic, firm papulonodule in the perineum posterior to the right labia. The patient concomitantly was following with gastroenterology due to abdominal pain that worsened with eating, as well as constipation, nausea, weight loss, and rectal bleeding of 5 years’ duration. The patient denied rash, joint arthralgia, or oral ulcers. A biopsy from the labial lesion was performed.
Recent Developments in Mantle Cell Lymphoma: Reflections From ASH 2022
What were the most exciting mantle cell lymphoma (MCL) updates from the recent meeting of the American Society of Hematology (ASH)?
Dr. Martin: The 2022 ASH meeting reported mostly about MCL research, which is great for the MCL community, because clearly, there is a lot of room for improvement. One of the big trials presented at a plenary session—one which we have been eager to see the results from, but maybe did not expect to see quite so soon—was the European MCL Network TRIANGLE trial. This is a 3-arm trial in which 870 patients were randomized. They had treatment-naive MCL and were younger than 66 years, so they were eligible for more intensive chemotherapy.
Arm A was the standard-of-care arm, defined by the prior European MCL Network TRIANGLE Trial. This was 6 alternating cycles of R-CHOP (rituximab, cyclophosphamide, hydroxydaunorubicin hydrochloride [doxorubicin hydrochloride], vincristine, and prednisone) and R-DHAP (rituximab, dexamethasone, cytarabine, cisplatin) – 3 of each followed by autologous stem cell transplant. Arm B was the same regimen with the addition of the first-in-class Bruton tyrosine kinase (BTK) inhibitor ibrutinib to induction followed by 2 years of ibrutinib maintenance. Arm C was the same induction regimen (6 alternating cycles of R-CHOP and R-DHAP plus ibrutinib during induction and maintenance) with no autologous stem cell transplant. Roughly half the patients in the trial, all equally distributed across all arms, received 3 years of maintenance rituximab.
The primary outcome was failure-free survival (FFS). After only 31 months of median follow-up, the trial reported a significant difference in FFS between patients receiving ibrutinib (Arms B and C) and patients who underwent autologous stem cell transplant and did not receive ibrutinib (Arm A).
This clearly shows that 2 years of ibrutinib maintenance significantly improves FFS. FFS was 88% versus 72% (Arm B vs Arm A) at 3 years with a hazard ratio of 0.5. That is a striking hazard ratio, highly statistically significant. Importantly, patients in Arms B and C fared similarly, suggesting that transplant was unnecessary in patients receiving ibrutinib.
What these findings suggest is that in the patient population treated with intensive induction, we are moving beyond autologous stem cell transplant. These results were similar across all subgroups. In fact, outcomes were most striking for patients with higher risk features like high Ki-67 and overexpression of p53.
The patients who need ibrutinib most were those who were most likely to benefit, and that is really encouraging for all of us. There is a clear trend toward an improvement in overall survival with ibrutinib maintenance and there clearly is less toxicity and less treatment-related mortality from avoiding transplant.
It will be important to see this trial published in a peer-reviewed journal with more granular data. But to me, these trial results are groundbreaking. It is a practice-changing trial for sure.
Is there anything else from an investigational approach on the horizon for MCL?
Dr. Martin: Yes. I would like to highlight 2 trials that stand out to me.
First, my colleague Dr. Ruan from Cornell presented on a phase 2 trial of a triplet of acalabrutinib plus lenalidomide plus rituximab with real-time monitoring of minimal residual disease (MRD) in patients with treatment-naive MCL.
This was a small trial with just 24 patients. It was fairly evenly split between low-, medium-, and high-risk MCL international prognostic index (MIPI) scores. All of these patients received the triplet for 1 year of induction followed by an additional year of maintenance with a slightly lower dose of lenalidomide. At the end of 2 years, patients who were in a durable MRD-negative state could stop the oral therapy and just continue with rituximab maintenance.
In a prior trial published in The New England Journal of Medicine, we showed that the lenalidomide plus rituximab regimen has a complete response rate of about 60%. In this new ongoing trial regimen of acalabrutinib plus lenalidomide plus rituximab, we found that at the end of just 1 year of induction treatment, the complete response rate was 83%. With all of the caveats and comparing across trials, this new regimen was clearly active and potentially more active than the prior regimen. It also appeared to be well tolerated without any real significant issues.
I think what this trial plus the TRIANGLE showed us is that BTK inhibitors belong in the front-line setting. That is what patients want. That is what physicians want.
The other trial that I wanted to highlight is an update of something that we saw last year at ASH, specifically a phase 1/2 trial of glofitamab in people with previously treated MCL. The overall response rate was 83% and the complete response rate was 73%. The complete response rate at the first assessment was already almost 50%. These are among patients who have had prior treatment for MCL, including BTK inhibitors.
We are not accustomed to seeing treatments that are so active in the relapsed/refractory MCL patient population, particularly, if they have had a prior BTK inhibitor. So, these results are exciting and promising.
This compares to the ZUMA-2 trial with CAR T-cells. CAR T-cells are also strikingly active in this patient population, but they do have some drawbacks. They have to be administered in a specialized facility and they are associated with fairly high rates of cytokine release syndrome and neurotoxicity.
The rates of grade 3 to 4 cytokine release syndrome and neurotoxicity with glofitamab were low, but not negligible. All cytokine release syndrome events were manageable, and no patients discontinued treatment because of adverse events. This is, potentially, attractive, because it offers an active therapy to a broader subset of patients with MCL who may not be able to access CAR T-cell therapy as easily. A phase 3 trial is in the planning stages, and it is likely that if that trial has positive results, we will see glofitamab approved in the not-too-distant future for people with MCL, and having more options is always great.
Based on these developments, do you see any shifts in your day-to-day practice in the future?
Dr. Martin: I think what has been interesting to me about MCL over the past decade is this idea that not everybody is the same. That should not come as a surprise statement, but MCL does behave differently in different people.
As a physician who treats a lot of patients with MCL, I have seen all of the different ways in which MCL can behave; combine that with the heterogeneity of humanity as a whole. Having guidelines from the NCCN (National Comprehensive Care Network) are helpful, but those guidelines are broad.
Learning how to take all that heterogeneity and variety into account and match the appropriate treatment to each patient is important. What these front-line trials are telling us is that it is OK to do research that does not involve chemotherapy.
In the past, it might have been considered unethical to give a younger patient a treatment without autologous stem cell transplant. But that is clearly not the case now. I think that in real-life practice in the near future, guidelines may actually start to get a little bit easier to follow as we come up with options that are less intensive.
It may be that patients can access treatments that are a little bit easier, that do not involve a transplant. That would be good for people with MCL from all across the country.
What were the most exciting mantle cell lymphoma (MCL) updates from the recent meeting of the American Society of Hematology (ASH)?
Dr. Martin: The 2022 ASH meeting reported mostly about MCL research, which is great for the MCL community, because clearly, there is a lot of room for improvement. One of the big trials presented at a plenary session—one which we have been eager to see the results from, but maybe did not expect to see quite so soon—was the European MCL Network TRIANGLE trial. This is a 3-arm trial in which 870 patients were randomized. They had treatment-naive MCL and were younger than 66 years, so they were eligible for more intensive chemotherapy.
Arm A was the standard-of-care arm, defined by the prior European MCL Network TRIANGLE Trial. This was 6 alternating cycles of R-CHOP (rituximab, cyclophosphamide, hydroxydaunorubicin hydrochloride [doxorubicin hydrochloride], vincristine, and prednisone) and R-DHAP (rituximab, dexamethasone, cytarabine, cisplatin) – 3 of each followed by autologous stem cell transplant. Arm B was the same regimen with the addition of the first-in-class Bruton tyrosine kinase (BTK) inhibitor ibrutinib to induction followed by 2 years of ibrutinib maintenance. Arm C was the same induction regimen (6 alternating cycles of R-CHOP and R-DHAP plus ibrutinib during induction and maintenance) with no autologous stem cell transplant. Roughly half the patients in the trial, all equally distributed across all arms, received 3 years of maintenance rituximab.
The primary outcome was failure-free survival (FFS). After only 31 months of median follow-up, the trial reported a significant difference in FFS between patients receiving ibrutinib (Arms B and C) and patients who underwent autologous stem cell transplant and did not receive ibrutinib (Arm A).
This clearly shows that 2 years of ibrutinib maintenance significantly improves FFS. FFS was 88% versus 72% (Arm B vs Arm A) at 3 years with a hazard ratio of 0.5. That is a striking hazard ratio, highly statistically significant. Importantly, patients in Arms B and C fared similarly, suggesting that transplant was unnecessary in patients receiving ibrutinib.
What these findings suggest is that in the patient population treated with intensive induction, we are moving beyond autologous stem cell transplant. These results were similar across all subgroups. In fact, outcomes were most striking for patients with higher risk features like high Ki-67 and overexpression of p53.
The patients who need ibrutinib most were those who were most likely to benefit, and that is really encouraging for all of us. There is a clear trend toward an improvement in overall survival with ibrutinib maintenance and there clearly is less toxicity and less treatment-related mortality from avoiding transplant.
It will be important to see this trial published in a peer-reviewed journal with more granular data. But to me, these trial results are groundbreaking. It is a practice-changing trial for sure.
Is there anything else from an investigational approach on the horizon for MCL?
Dr. Martin: Yes. I would like to highlight 2 trials that stand out to me.
First, my colleague Dr. Ruan from Cornell presented on a phase 2 trial of a triplet of acalabrutinib plus lenalidomide plus rituximab with real-time monitoring of minimal residual disease (MRD) in patients with treatment-naive MCL.
This was a small trial with just 24 patients. It was fairly evenly split between low-, medium-, and high-risk MCL international prognostic index (MIPI) scores. All of these patients received the triplet for 1 year of induction followed by an additional year of maintenance with a slightly lower dose of lenalidomide. At the end of 2 years, patients who were in a durable MRD-negative state could stop the oral therapy and just continue with rituximab maintenance.
In a prior trial published in The New England Journal of Medicine, we showed that the lenalidomide plus rituximab regimen has a complete response rate of about 60%. In this new ongoing trial regimen of acalabrutinib plus lenalidomide plus rituximab, we found that at the end of just 1 year of induction treatment, the complete response rate was 83%. With all of the caveats and comparing across trials, this new regimen was clearly active and potentially more active than the prior regimen. It also appeared to be well tolerated without any real significant issues.
I think what this trial plus the TRIANGLE showed us is that BTK inhibitors belong in the front-line setting. That is what patients want. That is what physicians want.
The other trial that I wanted to highlight is an update of something that we saw last year at ASH, specifically a phase 1/2 trial of glofitamab in people with previously treated MCL. The overall response rate was 83% and the complete response rate was 73%. The complete response rate at the first assessment was already almost 50%. These are among patients who have had prior treatment for MCL, including BTK inhibitors.
We are not accustomed to seeing treatments that are so active in the relapsed/refractory MCL patient population, particularly, if they have had a prior BTK inhibitor. So, these results are exciting and promising.
This compares to the ZUMA-2 trial with CAR T-cells. CAR T-cells are also strikingly active in this patient population, but they do have some drawbacks. They have to be administered in a specialized facility and they are associated with fairly high rates of cytokine release syndrome and neurotoxicity.
The rates of grade 3 to 4 cytokine release syndrome and neurotoxicity with glofitamab were low, but not negligible. All cytokine release syndrome events were manageable, and no patients discontinued treatment because of adverse events. This is, potentially, attractive, because it offers an active therapy to a broader subset of patients with MCL who may not be able to access CAR T-cell therapy as easily. A phase 3 trial is in the planning stages, and it is likely that if that trial has positive results, we will see glofitamab approved in the not-too-distant future for people with MCL, and having more options is always great.
Based on these developments, do you see any shifts in your day-to-day practice in the future?
Dr. Martin: I think what has been interesting to me about MCL over the past decade is this idea that not everybody is the same. That should not come as a surprise statement, but MCL does behave differently in different people.
As a physician who treats a lot of patients with MCL, I have seen all of the different ways in which MCL can behave; combine that with the heterogeneity of humanity as a whole. Having guidelines from the NCCN (National Comprehensive Care Network) are helpful, but those guidelines are broad.
Learning how to take all that heterogeneity and variety into account and match the appropriate treatment to each patient is important. What these front-line trials are telling us is that it is OK to do research that does not involve chemotherapy.
In the past, it might have been considered unethical to give a younger patient a treatment without autologous stem cell transplant. But that is clearly not the case now. I think that in real-life practice in the near future, guidelines may actually start to get a little bit easier to follow as we come up with options that are less intensive.
It may be that patients can access treatments that are a little bit easier, that do not involve a transplant. That would be good for people with MCL from all across the country.
What were the most exciting mantle cell lymphoma (MCL) updates from the recent meeting of the American Society of Hematology (ASH)?
Dr. Martin: The 2022 ASH meeting reported mostly about MCL research, which is great for the MCL community, because clearly, there is a lot of room for improvement. One of the big trials presented at a plenary session—one which we have been eager to see the results from, but maybe did not expect to see quite so soon—was the European MCL Network TRIANGLE trial. This is a 3-arm trial in which 870 patients were randomized. They had treatment-naive MCL and were younger than 66 years, so they were eligible for more intensive chemotherapy.
Arm A was the standard-of-care arm, defined by the prior European MCL Network TRIANGLE Trial. This was 6 alternating cycles of R-CHOP (rituximab, cyclophosphamide, hydroxydaunorubicin hydrochloride [doxorubicin hydrochloride], vincristine, and prednisone) and R-DHAP (rituximab, dexamethasone, cytarabine, cisplatin) – 3 of each followed by autologous stem cell transplant. Arm B was the same regimen with the addition of the first-in-class Bruton tyrosine kinase (BTK) inhibitor ibrutinib to induction followed by 2 years of ibrutinib maintenance. Arm C was the same induction regimen (6 alternating cycles of R-CHOP and R-DHAP plus ibrutinib during induction and maintenance) with no autologous stem cell transplant. Roughly half the patients in the trial, all equally distributed across all arms, received 3 years of maintenance rituximab.
The primary outcome was failure-free survival (FFS). After only 31 months of median follow-up, the trial reported a significant difference in FFS between patients receiving ibrutinib (Arms B and C) and patients who underwent autologous stem cell transplant and did not receive ibrutinib (Arm A).
This clearly shows that 2 years of ibrutinib maintenance significantly improves FFS. FFS was 88% versus 72% (Arm B vs Arm A) at 3 years with a hazard ratio of 0.5. That is a striking hazard ratio, highly statistically significant. Importantly, patients in Arms B and C fared similarly, suggesting that transplant was unnecessary in patients receiving ibrutinib.
What these findings suggest is that in the patient population treated with intensive induction, we are moving beyond autologous stem cell transplant. These results were similar across all subgroups. In fact, outcomes were most striking for patients with higher risk features like high Ki-67 and overexpression of p53.
The patients who need ibrutinib most were those who were most likely to benefit, and that is really encouraging for all of us. There is a clear trend toward an improvement in overall survival with ibrutinib maintenance and there clearly is less toxicity and less treatment-related mortality from avoiding transplant.
It will be important to see this trial published in a peer-reviewed journal with more granular data. But to me, these trial results are groundbreaking. It is a practice-changing trial for sure.
Is there anything else from an investigational approach on the horizon for MCL?
Dr. Martin: Yes. I would like to highlight 2 trials that stand out to me.
First, my colleague Dr. Ruan from Cornell presented on a phase 2 trial of a triplet of acalabrutinib plus lenalidomide plus rituximab with real-time monitoring of minimal residual disease (MRD) in patients with treatment-naive MCL.
This was a small trial with just 24 patients. It was fairly evenly split between low-, medium-, and high-risk MCL international prognostic index (MIPI) scores. All of these patients received the triplet for 1 year of induction followed by an additional year of maintenance with a slightly lower dose of lenalidomide. At the end of 2 years, patients who were in a durable MRD-negative state could stop the oral therapy and just continue with rituximab maintenance.
In a prior trial published in The New England Journal of Medicine, we showed that the lenalidomide plus rituximab regimen has a complete response rate of about 60%. In this new ongoing trial regimen of acalabrutinib plus lenalidomide plus rituximab, we found that at the end of just 1 year of induction treatment, the complete response rate was 83%. With all of the caveats and comparing across trials, this new regimen was clearly active and potentially more active than the prior regimen. It also appeared to be well tolerated without any real significant issues.
I think what this trial plus the TRIANGLE showed us is that BTK inhibitors belong in the front-line setting. That is what patients want. That is what physicians want.
The other trial that I wanted to highlight is an update of something that we saw last year at ASH, specifically a phase 1/2 trial of glofitamab in people with previously treated MCL. The overall response rate was 83% and the complete response rate was 73%. The complete response rate at the first assessment was already almost 50%. These are among patients who have had prior treatment for MCL, including BTK inhibitors.
We are not accustomed to seeing treatments that are so active in the relapsed/refractory MCL patient population, particularly, if they have had a prior BTK inhibitor. So, these results are exciting and promising.
This compares to the ZUMA-2 trial with CAR T-cells. CAR T-cells are also strikingly active in this patient population, but they do have some drawbacks. They have to be administered in a specialized facility and they are associated with fairly high rates of cytokine release syndrome and neurotoxicity.
The rates of grade 3 to 4 cytokine release syndrome and neurotoxicity with glofitamab were low, but not negligible. All cytokine release syndrome events were manageable, and no patients discontinued treatment because of adverse events. This is, potentially, attractive, because it offers an active therapy to a broader subset of patients with MCL who may not be able to access CAR T-cell therapy as easily. A phase 3 trial is in the planning stages, and it is likely that if that trial has positive results, we will see glofitamab approved in the not-too-distant future for people with MCL, and having more options is always great.
Based on these developments, do you see any shifts in your day-to-day practice in the future?
Dr. Martin: I think what has been interesting to me about MCL over the past decade is this idea that not everybody is the same. That should not come as a surprise statement, but MCL does behave differently in different people.
As a physician who treats a lot of patients with MCL, I have seen all of the different ways in which MCL can behave; combine that with the heterogeneity of humanity as a whole. Having guidelines from the NCCN (National Comprehensive Care Network) are helpful, but those guidelines are broad.
Learning how to take all that heterogeneity and variety into account and match the appropriate treatment to each patient is important. What these front-line trials are telling us is that it is OK to do research that does not involve chemotherapy.
In the past, it might have been considered unethical to give a younger patient a treatment without autologous stem cell transplant. But that is clearly not the case now. I think that in real-life practice in the near future, guidelines may actually start to get a little bit easier to follow as we come up with options that are less intensive.
It may be that patients can access treatments that are a little bit easier, that do not involve a transplant. That would be good for people with MCL from all across the country.
Listeria infection in pregnancy: A potentially serious foodborne illness
CASE Pregnant patient with concerning symptoms of infection
A 28-year-old primigravid woman at 26 weeks’ gestation requests evaluation because of a 3-day history of low-grade fever (38.3 °C), chills, malaise, myalgias, pain in her upper back, nausea, diarrhea, and intermittent uterine contractions. Her symptoms began 2 days after she and her husband dined at a local Mexican restaurant. She specifically recalls eating unpasteurized cheese (queso fresco). Her husband also is experiencing similar symptoms.
- What is the most likely diagnosis?
- What tests should be performed to confirm the diagnosis?
- Does this infection pose a risk to the fetus?
- How should this patient be treated?
Listeriosis, a potentially serious foodborne illness, is an unusual infection in pregnancy. It can cause a number of adverse effects in both the pregnant woman and her fetus, including fetal death in utero. In this article, we review the microbiology and epidemiology of Listeria infection, consider the important steps in diagnosis, and discuss treatment options and prevention measures.
The causative organism in listeriosis
Listeriosis is caused by Listeria monocytogenes, a gram-positive, non–spore-forming bacillus. The organism is catalase positive and oxidase negative, and it exhibits tumbling motility when grown in culture. It can grow at temperatures less than 4 °C, which facilitates foodborne transmission of the bacterium despite adequate refrigeration. Of the 13 serotypes of L monocytogenes, the 1/2a, 1/2b, and 4b are most likely to be associated with human infection. The major virulence factors of L monocytogenes are the internalin surface proteins and the pore-forming listeriolysin O (LLO) cytotoxin. These factors enable the organism to effectively invade host cells.1
The pathogen uses several mechanisms to evade gastrointestinal defenses prior to entry into the bloodstream. It avoids destruction in the stomach by using proton pump inhibitors to elevate the pH of gastric acid. In the duodenum, it survives the antibacterial properties of bile by secreting bile salt hydrolases, which catabolize bile salts. In addition, the cytotoxin listeriolysin S (LLS) disrupts the protective barrier created by the normal gut flora. Once the organism penetrates the gastrointestinal barriers, it disseminates through the blood and lymphatics and then infects other tissues, such as the brain and placenta.1,2
Pathogenesis of infection
The primary reservoir of Listeria is soil and decaying vegetable matter. The organism also has been isolated from animal feed, water, sewage, and many animal species. With rare exceptions, most infections in adults result from inadvertent ingestion of the organism in contaminated food. In certain high-risk occupations, such as veterinary medicine, farming, and laboratory work, infection of the skin or eye can result from direct contact with an infected animal.3
Of note, foodborne illness caused by Listeria has the third highest mortality rate of any foodborne infection, 16% compared with 35% for Vibrio vulnificus and 17% for Clostridium botulinum.2,3 The principal foods that have been linked to listeriosis include:
- soft cheeses, particularly those made from unpasteurized milk
- melon
- hot dogs
- lunch meat, such as bologna
- deli meat, especially chicken
- canned foods, such as smoked seafood, and pâté or meat spreads that are labeled “keep refrigerated”
- unpasteurized milk
- sprouts
- hummus.
In healthy adults, listeriosis is usually a short-lived illness. However, in older adults, immunocompromised patients, and pregnant women, the infection can be devastating. Infection in the pregnant woman also poses major danger to the developing fetus because the organism has a special predilection for placental and fetal tissue.1,3,4
Immunity to Listeria infection depends primarily on T-cell lymphokine activation of macrophages. These latter cells are responsible for clearing the bacterium from the blood. As noted above, the principal virulence factor of L monocytogenes is listeriolysin O, a cholesterol-dependent cytolysin. This substance induces T-cell receptor unresponsiveness, thus interfering with the host immune response to the invading pathogen.1,3-5
Continue to: Clinical manifestations of listeriosis...
Clinical manifestations of listeriosis
Listeria infections may present with various manifestations, depending on the degree of exposure and the underlying immunocompetence of the host (FIGURE). In its most common and simplest form, listeriosis presents as a mild to moderate gastroenteritis following exposure to contaminated food. Symptoms typically develop within 24 hours of exposure and include fever, myalgias, abdominal or back pain, nausea, vomiting, and diarrhea.5
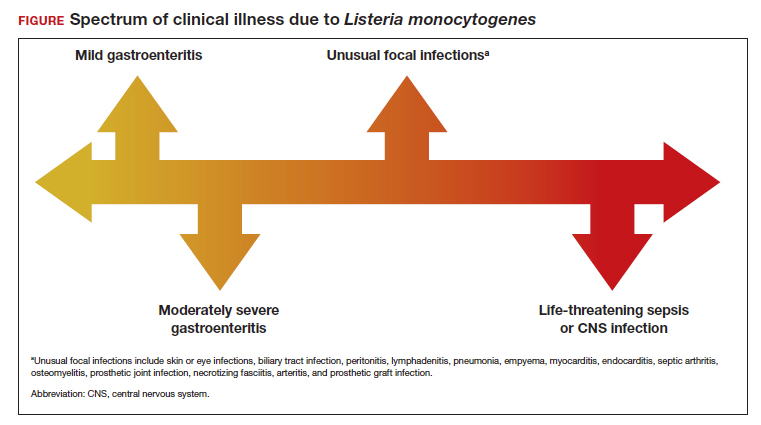
Conversely, in immunocompromised patients, including pregnant women, listeriosis can present as life-threatening sepsis and/or central nervous system (CNS) infection (invasive infection). In this clinical setting, the mean incubation period is 11 days. The manifestations of CNS infection include meningoencephalitis, cerebritis, rhombencephalitis (infection and inflammation of the brain stem), brain abscess, and spinal cord abscess.5
In addition to these 2 clinical presentations, listeriosis can cause unusual focal infections as illustrated in the FIGURE. Some of these infections have unique clinical associations. For example, skin or eye infections may occur as a result of direct inoculation in veterinarians, farmers, and laboratory workers. Listeria peritonitis may occur in patients who are receiving peritoneal dialysis and in those who have cirrhosis. Prosthetic joint and graft infections, of course, may occur in patients who have had invasive procedures for implantation of grafts or prosthetic devices.5
Listeriosis is especially dangerous in pregnancy because it not only can cause serious injury to the mother and even death but it also may pose a major risk to fetal well-being. Possible perinatal complications include fetal death; preterm labor and delivery; and neonatal sepsis, meningitis, and death.5-8
Making the diagnosis
Diagnosis begins with a thorough and focused history to assess for characteristic symptoms and possible Listeria exposure. Exposure should be presumed for patients who report consuming high-risk foods, especially foods recently recalled by the US Food and Drug Administration.
In the asymptomatic pregnant patient, diagnostic testing can be deferred, and the patient should be instructed to return for evaluation if symptoms develop within 2 months of exposure. However, symptomatic, febrile patients require testing. The most valuable testing modality is Gram stain and culture of blood. Gram stain typically will show gram-positive pleomorphic rods with rounded ends. Amniocentesis may be indicated if blood cultures are not definitive. Meconium staining of the amniotic fluid and a positive Gram stain are highly indicative of fetal infection. Cultures of the cerebrospinal fluid are indicated in any individual with focal neurologic findings. Stool cultures are rarely indicated.
When obtaining any of the cultures noted above, the clinician should alert the microbiologist of the concern for listeriosis because L monocytogenes can be confused with common contaminants, such as diphtheroids.5-9
Treatment and follow-up
The treatment of listeriosis in pregnancy depends on the severity of the infection and the immune status of the mother. The TABLE offers several different clinical scenarios and the appropriate treatment for each. As noted, several scenarios may require cultures of the blood, cerebrospinal fluid, and amniotic fluid.7,9,10
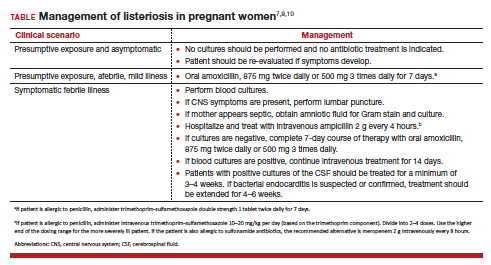
Following treatment of the mother, serial ultrasound examinations should be performed to monitor fetal growth, CNS anatomy, placental morphology, amniotic fluid volume, and umbilical artery Doppler velocimetry. In the presence of fetal growth restriction, oligohydramnios, or abnormal Doppler velocimetry, biophysical profile testing should be performed. After delivery, the placenta should be examined carefully for histologic evidence of Listeria infection, such as miliary abscesses, and cultured for the bacterium.7-9
Prevention measures
Conservative measures for prevention of Listeria infection in pregnant women include the following7,10-12:
- Refrigerate milk and milk products at 40 °F (4.4 °C).
- Thoroughly cook raw food from animal sources.
- Wash raw vegetables carefully before eating.
- Keep uncooked meats separate from cooked meats and vegetables.
- Do not consume any beverages or foods made from unpasteurized milk.
- After handling uncooked foods, carefully wash all utensils and hands.
- Avoid all soft cheeses, such as Mexican-style feta, Brie, Camembert, and blue cheese, even if they are supposedly made from pasteurized milk.
- Reheat until steaming hot all leftover foods or ready-to-eat foods, such as hot dogs.
- Do not let juice from hot dogs or lunch meat packages drip onto other foods, utensils, or food preparation surfaces.
- Do not store opened hot dog packages in the refrigerator for more than 1 week. Do not store unopened packages for longer than 2 weeks.
- Do not store unopened lunch and deli meat packages in the refrigerator for longer than 2 weeks. Do not store opened packages for longer than 3 to 5 days.
- If other immunosuppressive conditions are present in combination with pregnancy, thoroughly heat cold cuts before eating.
- Do not eat raw or even lightly cooked sprouts of any kind. Cook sprouts thoroughly. Rinsing sprouts will not remove Listeria organisms.
- Do not eat refrigerated pâté or meat spreads from a deli counter or the refrigerated section of a grocery store.
- Canned or shelf-stable pâté and meat spreads are safe to eat, but be sure to refrigerate them after opening the packages.
- Do not eat refrigerated smoked seafood. Canned or shelf-stable seafood, particularly when incorporated into a casserole, is safe to eat.
- Eat cut melon immediately. Refrigerate uneaten melon quickly if not eaten. Discard cut melon that is left at room temperature for more than 4 hours.
CASE Diagnosis made and prompt treatment initiated
The most likely diagnosis in this patient is listeriosis. Because the patient is moderately ill and experiencing uterine contractions, she should be hospitalized and monitored for progressive cervical dilation. Blood cultures should be obtained to identify L monocytogenes. In addition, an amniocentesis should be performed, and the amniotic fluid should be cultured for this microorganism. Stool culture and culture of the cerebrospinal fluid are not indicated. The patient should be treated with intravenous ampicillin, 2 g every 4 hours for 14 days. If she is allergic to penicillin, the alternative drug is trimethoprim-sulfamethoxazole, 8 to 10 mg/kg per day in 2 divided doses, for 14 days. Prompt and effective treatment of the mother should prevent infection in the fetus and newborn. ●
- Listeriosis is primarily a foodborne illness caused by Listeria monocytogenes, a gram-positive bacillus.
- Pregnant women, particularly those who are immunocompromised, are especially susceptible to Listeria infection.
- Foods that pose particular risk of transmitting infection include fresh unpasteurized cheeses, processed meats such as hot dogs, refrigerated pâté and meat spreads, refrigerated smoked seafood, unpasteurized milk, and unwashed raw produce.
- The infection may range from a mild gastroenteritis to life-threatening sepsis and meningitis.
- Listeriosis may cause early and late-onset neonatal infection that presents as either meningitis or sepsis.
- Blood and amniotic fluid cultures are essential to diagnose maternal infection. Stool cultures usually are not indicated.
- Mildly symptomatic but afebrile patients do not require treatment.
- Febrile symptomatic patients should be treated with either intravenous ampicillin or trimethoprim-sulfamethoxazole.
- Radoshevich L, Cossart P. Listeria monocytogenes: towards a complete picture of its physiology and pathogenesis. Nat Rev Microbiol. 2018;16:32-46. doi:10.1038/nnrmicro.2017.126.
- Johnson JE, Mylonakis E. Listeria monocytogenes. In: Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 9th ed. Elsevier; 2020:2543-2549.
- Gelfand MS, Swamy GK, Thompson JL. Epidemiology and pathogenesis of Listeria monocytogenes infection. UpToDate. Updated August 23, 2022. Accessed November 9, 2022. https://www.uptodate.com/contents/epidemiology-and-pathogenesis-of-listeria-monocytogenes-infection?sectionName=CLINICAL%20EPIDEMIOLOGY&topicRef=1277&anchor=H4&source=see_link#H4
- Cherubin CE, Appleman MD, Heseltine PN, et al. Epidemiological spectrum and current treatment of listeriosis. Rev Infect Dis. 1991;13:1108-1114.
- Gelfand MS, Swamy GK, Thompson JL. Clinical manifestations and diagnosis of Listeria monocytogenes infection. UpToDate. Updated August 23, 2022. Accessed November 7, 2022. https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-listeriamonocytogenes-infection
- Boucher M, Yonekura ML. Perinatal listeriosis (early-onset): correlation of antenatal manifestations and neonatal outcome. Obstet Gynecol. 1986;68:593-597.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 614: management of pregnant women with presumptive exposure to Listeria monocytogenes. Obstet Gynecol. 2014;124:1241-1244.
- Rouse DJ, Keimig TW, Riley LE, et al. Case 16-2016. A 31-year-old pregnant woman with fever. N Engl J Med. 2016;374:2076-2083.
- Craig AM, Dotters-Katz S, Kuller JA, et al. Listeriosis in pregnancy: a review. Obstet Gynecol Surv. 2019;74: 362-368.
- Gelfand MS, Thompson JL, Swamy GK. Treatment and prevention of Listeria monocytogenes infection. UpToDate. Updated August 23, 2022. Accessed November 9, 2022. https://www.uptodate.com/contents/treatment-and-prevention-of-listeria-monocytogenes-infection?topicRef=1280&source=see_link
- Voetsch AC, Angulo FJ, Jones TF, et al; Centers for Disease Control and Prevention Emerging Infections Program Foodborne Diseases Active Surveillance Networking Group. Reduction in the incidence of invasive listeriosis in Foodborne Diseases Active Surveillance Network sites, 1996-2003. Clin Infect Dis. 2007;44:513-520.
- MacDonald PDM, Whitwan RE, Boggs JD, et al. Outbreak of listeriosis among Mexican immigrants as a result of consumption of illicitly produced Mexican-style cheese. Clin Infect Dis. 2005;40:677-682.
CASE Pregnant patient with concerning symptoms of infection
A 28-year-old primigravid woman at 26 weeks’ gestation requests evaluation because of a 3-day history of low-grade fever (38.3 °C), chills, malaise, myalgias, pain in her upper back, nausea, diarrhea, and intermittent uterine contractions. Her symptoms began 2 days after she and her husband dined at a local Mexican restaurant. She specifically recalls eating unpasteurized cheese (queso fresco). Her husband also is experiencing similar symptoms.
- What is the most likely diagnosis?
- What tests should be performed to confirm the diagnosis?
- Does this infection pose a risk to the fetus?
- How should this patient be treated?
Listeriosis, a potentially serious foodborne illness, is an unusual infection in pregnancy. It can cause a number of adverse effects in both the pregnant woman and her fetus, including fetal death in utero. In this article, we review the microbiology and epidemiology of Listeria infection, consider the important steps in diagnosis, and discuss treatment options and prevention measures.
The causative organism in listeriosis
Listeriosis is caused by Listeria monocytogenes, a gram-positive, non–spore-forming bacillus. The organism is catalase positive and oxidase negative, and it exhibits tumbling motility when grown in culture. It can grow at temperatures less than 4 °C, which facilitates foodborne transmission of the bacterium despite adequate refrigeration. Of the 13 serotypes of L monocytogenes, the 1/2a, 1/2b, and 4b are most likely to be associated with human infection. The major virulence factors of L monocytogenes are the internalin surface proteins and the pore-forming listeriolysin O (LLO) cytotoxin. These factors enable the organism to effectively invade host cells.1
The pathogen uses several mechanisms to evade gastrointestinal defenses prior to entry into the bloodstream. It avoids destruction in the stomach by using proton pump inhibitors to elevate the pH of gastric acid. In the duodenum, it survives the antibacterial properties of bile by secreting bile salt hydrolases, which catabolize bile salts. In addition, the cytotoxin listeriolysin S (LLS) disrupts the protective barrier created by the normal gut flora. Once the organism penetrates the gastrointestinal barriers, it disseminates through the blood and lymphatics and then infects other tissues, such as the brain and placenta.1,2
Pathogenesis of infection
The primary reservoir of Listeria is soil and decaying vegetable matter. The organism also has been isolated from animal feed, water, sewage, and many animal species. With rare exceptions, most infections in adults result from inadvertent ingestion of the organism in contaminated food. In certain high-risk occupations, such as veterinary medicine, farming, and laboratory work, infection of the skin or eye can result from direct contact with an infected animal.3
Of note, foodborne illness caused by Listeria has the third highest mortality rate of any foodborne infection, 16% compared with 35% for Vibrio vulnificus and 17% for Clostridium botulinum.2,3 The principal foods that have been linked to listeriosis include:
- soft cheeses, particularly those made from unpasteurized milk
- melon
- hot dogs
- lunch meat, such as bologna
- deli meat, especially chicken
- canned foods, such as smoked seafood, and pâté or meat spreads that are labeled “keep refrigerated”
- unpasteurized milk
- sprouts
- hummus.
In healthy adults, listeriosis is usually a short-lived illness. However, in older adults, immunocompromised patients, and pregnant women, the infection can be devastating. Infection in the pregnant woman also poses major danger to the developing fetus because the organism has a special predilection for placental and fetal tissue.1,3,4
Immunity to Listeria infection depends primarily on T-cell lymphokine activation of macrophages. These latter cells are responsible for clearing the bacterium from the blood. As noted above, the principal virulence factor of L monocytogenes is listeriolysin O, a cholesterol-dependent cytolysin. This substance induces T-cell receptor unresponsiveness, thus interfering with the host immune response to the invading pathogen.1,3-5
Continue to: Clinical manifestations of listeriosis...
Clinical manifestations of listeriosis
Listeria infections may present with various manifestations, depending on the degree of exposure and the underlying immunocompetence of the host (FIGURE). In its most common and simplest form, listeriosis presents as a mild to moderate gastroenteritis following exposure to contaminated food. Symptoms typically develop within 24 hours of exposure and include fever, myalgias, abdominal or back pain, nausea, vomiting, and diarrhea.5

Conversely, in immunocompromised patients, including pregnant women, listeriosis can present as life-threatening sepsis and/or central nervous system (CNS) infection (invasive infection). In this clinical setting, the mean incubation period is 11 days. The manifestations of CNS infection include meningoencephalitis, cerebritis, rhombencephalitis (infection and inflammation of the brain stem), brain abscess, and spinal cord abscess.5
In addition to these 2 clinical presentations, listeriosis can cause unusual focal infections as illustrated in the FIGURE. Some of these infections have unique clinical associations. For example, skin or eye infections may occur as a result of direct inoculation in veterinarians, farmers, and laboratory workers. Listeria peritonitis may occur in patients who are receiving peritoneal dialysis and in those who have cirrhosis. Prosthetic joint and graft infections, of course, may occur in patients who have had invasive procedures for implantation of grafts or prosthetic devices.5
Listeriosis is especially dangerous in pregnancy because it not only can cause serious injury to the mother and even death but it also may pose a major risk to fetal well-being. Possible perinatal complications include fetal death; preterm labor and delivery; and neonatal sepsis, meningitis, and death.5-8
Making the diagnosis
Diagnosis begins with a thorough and focused history to assess for characteristic symptoms and possible Listeria exposure. Exposure should be presumed for patients who report consuming high-risk foods, especially foods recently recalled by the US Food and Drug Administration.
In the asymptomatic pregnant patient, diagnostic testing can be deferred, and the patient should be instructed to return for evaluation if symptoms develop within 2 months of exposure. However, symptomatic, febrile patients require testing. The most valuable testing modality is Gram stain and culture of blood. Gram stain typically will show gram-positive pleomorphic rods with rounded ends. Amniocentesis may be indicated if blood cultures are not definitive. Meconium staining of the amniotic fluid and a positive Gram stain are highly indicative of fetal infection. Cultures of the cerebrospinal fluid are indicated in any individual with focal neurologic findings. Stool cultures are rarely indicated.
When obtaining any of the cultures noted above, the clinician should alert the microbiologist of the concern for listeriosis because L monocytogenes can be confused with common contaminants, such as diphtheroids.5-9
Treatment and follow-up
The treatment of listeriosis in pregnancy depends on the severity of the infection and the immune status of the mother. The TABLE offers several different clinical scenarios and the appropriate treatment for each. As noted, several scenarios may require cultures of the blood, cerebrospinal fluid, and amniotic fluid.7,9,10

Following treatment of the mother, serial ultrasound examinations should be performed to monitor fetal growth, CNS anatomy, placental morphology, amniotic fluid volume, and umbilical artery Doppler velocimetry. In the presence of fetal growth restriction, oligohydramnios, or abnormal Doppler velocimetry, biophysical profile testing should be performed. After delivery, the placenta should be examined carefully for histologic evidence of Listeria infection, such as miliary abscesses, and cultured for the bacterium.7-9
Prevention measures
Conservative measures for prevention of Listeria infection in pregnant women include the following7,10-12:
- Refrigerate milk and milk products at 40 °F (4.4 °C).
- Thoroughly cook raw food from animal sources.
- Wash raw vegetables carefully before eating.
- Keep uncooked meats separate from cooked meats and vegetables.
- Do not consume any beverages or foods made from unpasteurized milk.
- After handling uncooked foods, carefully wash all utensils and hands.
- Avoid all soft cheeses, such as Mexican-style feta, Brie, Camembert, and blue cheese, even if they are supposedly made from pasteurized milk.
- Reheat until steaming hot all leftover foods or ready-to-eat foods, such as hot dogs.
- Do not let juice from hot dogs or lunch meat packages drip onto other foods, utensils, or food preparation surfaces.
- Do not store opened hot dog packages in the refrigerator for more than 1 week. Do not store unopened packages for longer than 2 weeks.
- Do not store unopened lunch and deli meat packages in the refrigerator for longer than 2 weeks. Do not store opened packages for longer than 3 to 5 days.
- If other immunosuppressive conditions are present in combination with pregnancy, thoroughly heat cold cuts before eating.
- Do not eat raw or even lightly cooked sprouts of any kind. Cook sprouts thoroughly. Rinsing sprouts will not remove Listeria organisms.
- Do not eat refrigerated pâté or meat spreads from a deli counter or the refrigerated section of a grocery store.
- Canned or shelf-stable pâté and meat spreads are safe to eat, but be sure to refrigerate them after opening the packages.
- Do not eat refrigerated smoked seafood. Canned or shelf-stable seafood, particularly when incorporated into a casserole, is safe to eat.
- Eat cut melon immediately. Refrigerate uneaten melon quickly if not eaten. Discard cut melon that is left at room temperature for more than 4 hours.
CASE Diagnosis made and prompt treatment initiated
The most likely diagnosis in this patient is listeriosis. Because the patient is moderately ill and experiencing uterine contractions, she should be hospitalized and monitored for progressive cervical dilation. Blood cultures should be obtained to identify L monocytogenes. In addition, an amniocentesis should be performed, and the amniotic fluid should be cultured for this microorganism. Stool culture and culture of the cerebrospinal fluid are not indicated. The patient should be treated with intravenous ampicillin, 2 g every 4 hours for 14 days. If she is allergic to penicillin, the alternative drug is trimethoprim-sulfamethoxazole, 8 to 10 mg/kg per day in 2 divided doses, for 14 days. Prompt and effective treatment of the mother should prevent infection in the fetus and newborn. ●
- Listeriosis is primarily a foodborne illness caused by Listeria monocytogenes, a gram-positive bacillus.
- Pregnant women, particularly those who are immunocompromised, are especially susceptible to Listeria infection.
- Foods that pose particular risk of transmitting infection include fresh unpasteurized cheeses, processed meats such as hot dogs, refrigerated pâté and meat spreads, refrigerated smoked seafood, unpasteurized milk, and unwashed raw produce.
- The infection may range from a mild gastroenteritis to life-threatening sepsis and meningitis.
- Listeriosis may cause early and late-onset neonatal infection that presents as either meningitis or sepsis.
- Blood and amniotic fluid cultures are essential to diagnose maternal infection. Stool cultures usually are not indicated.
- Mildly symptomatic but afebrile patients do not require treatment.
- Febrile symptomatic patients should be treated with either intravenous ampicillin or trimethoprim-sulfamethoxazole.
CASE Pregnant patient with concerning symptoms of infection
A 28-year-old primigravid woman at 26 weeks’ gestation requests evaluation because of a 3-day history of low-grade fever (38.3 °C), chills, malaise, myalgias, pain in her upper back, nausea, diarrhea, and intermittent uterine contractions. Her symptoms began 2 days after she and her husband dined at a local Mexican restaurant. She specifically recalls eating unpasteurized cheese (queso fresco). Her husband also is experiencing similar symptoms.
- What is the most likely diagnosis?
- What tests should be performed to confirm the diagnosis?
- Does this infection pose a risk to the fetus?
- How should this patient be treated?
Listeriosis, a potentially serious foodborne illness, is an unusual infection in pregnancy. It can cause a number of adverse effects in both the pregnant woman and her fetus, including fetal death in utero. In this article, we review the microbiology and epidemiology of Listeria infection, consider the important steps in diagnosis, and discuss treatment options and prevention measures.
The causative organism in listeriosis
Listeriosis is caused by Listeria monocytogenes, a gram-positive, non–spore-forming bacillus. The organism is catalase positive and oxidase negative, and it exhibits tumbling motility when grown in culture. It can grow at temperatures less than 4 °C, which facilitates foodborne transmission of the bacterium despite adequate refrigeration. Of the 13 serotypes of L monocytogenes, the 1/2a, 1/2b, and 4b are most likely to be associated with human infection. The major virulence factors of L monocytogenes are the internalin surface proteins and the pore-forming listeriolysin O (LLO) cytotoxin. These factors enable the organism to effectively invade host cells.1
The pathogen uses several mechanisms to evade gastrointestinal defenses prior to entry into the bloodstream. It avoids destruction in the stomach by using proton pump inhibitors to elevate the pH of gastric acid. In the duodenum, it survives the antibacterial properties of bile by secreting bile salt hydrolases, which catabolize bile salts. In addition, the cytotoxin listeriolysin S (LLS) disrupts the protective barrier created by the normal gut flora. Once the organism penetrates the gastrointestinal barriers, it disseminates through the blood and lymphatics and then infects other tissues, such as the brain and placenta.1,2
Pathogenesis of infection
The primary reservoir of Listeria is soil and decaying vegetable matter. The organism also has been isolated from animal feed, water, sewage, and many animal species. With rare exceptions, most infections in adults result from inadvertent ingestion of the organism in contaminated food. In certain high-risk occupations, such as veterinary medicine, farming, and laboratory work, infection of the skin or eye can result from direct contact with an infected animal.3
Of note, foodborne illness caused by Listeria has the third highest mortality rate of any foodborne infection, 16% compared with 35% for Vibrio vulnificus and 17% for Clostridium botulinum.2,3 The principal foods that have been linked to listeriosis include:
- soft cheeses, particularly those made from unpasteurized milk
- melon
- hot dogs
- lunch meat, such as bologna
- deli meat, especially chicken
- canned foods, such as smoked seafood, and pâté or meat spreads that are labeled “keep refrigerated”
- unpasteurized milk
- sprouts
- hummus.
In healthy adults, listeriosis is usually a short-lived illness. However, in older adults, immunocompromised patients, and pregnant women, the infection can be devastating. Infection in the pregnant woman also poses major danger to the developing fetus because the organism has a special predilection for placental and fetal tissue.1,3,4
Immunity to Listeria infection depends primarily on T-cell lymphokine activation of macrophages. These latter cells are responsible for clearing the bacterium from the blood. As noted above, the principal virulence factor of L monocytogenes is listeriolysin O, a cholesterol-dependent cytolysin. This substance induces T-cell receptor unresponsiveness, thus interfering with the host immune response to the invading pathogen.1,3-5
Continue to: Clinical manifestations of listeriosis...
Clinical manifestations of listeriosis
Listeria infections may present with various manifestations, depending on the degree of exposure and the underlying immunocompetence of the host (FIGURE). In its most common and simplest form, listeriosis presents as a mild to moderate gastroenteritis following exposure to contaminated food. Symptoms typically develop within 24 hours of exposure and include fever, myalgias, abdominal or back pain, nausea, vomiting, and diarrhea.5

Conversely, in immunocompromised patients, including pregnant women, listeriosis can present as life-threatening sepsis and/or central nervous system (CNS) infection (invasive infection). In this clinical setting, the mean incubation period is 11 days. The manifestations of CNS infection include meningoencephalitis, cerebritis, rhombencephalitis (infection and inflammation of the brain stem), brain abscess, and spinal cord abscess.5
In addition to these 2 clinical presentations, listeriosis can cause unusual focal infections as illustrated in the FIGURE. Some of these infections have unique clinical associations. For example, skin or eye infections may occur as a result of direct inoculation in veterinarians, farmers, and laboratory workers. Listeria peritonitis may occur in patients who are receiving peritoneal dialysis and in those who have cirrhosis. Prosthetic joint and graft infections, of course, may occur in patients who have had invasive procedures for implantation of grafts or prosthetic devices.5
Listeriosis is especially dangerous in pregnancy because it not only can cause serious injury to the mother and even death but it also may pose a major risk to fetal well-being. Possible perinatal complications include fetal death; preterm labor and delivery; and neonatal sepsis, meningitis, and death.5-8
Making the diagnosis
Diagnosis begins with a thorough and focused history to assess for characteristic symptoms and possible Listeria exposure. Exposure should be presumed for patients who report consuming high-risk foods, especially foods recently recalled by the US Food and Drug Administration.
In the asymptomatic pregnant patient, diagnostic testing can be deferred, and the patient should be instructed to return for evaluation if symptoms develop within 2 months of exposure. However, symptomatic, febrile patients require testing. The most valuable testing modality is Gram stain and culture of blood. Gram stain typically will show gram-positive pleomorphic rods with rounded ends. Amniocentesis may be indicated if blood cultures are not definitive. Meconium staining of the amniotic fluid and a positive Gram stain are highly indicative of fetal infection. Cultures of the cerebrospinal fluid are indicated in any individual with focal neurologic findings. Stool cultures are rarely indicated.
When obtaining any of the cultures noted above, the clinician should alert the microbiologist of the concern for listeriosis because L monocytogenes can be confused with common contaminants, such as diphtheroids.5-9
Treatment and follow-up
The treatment of listeriosis in pregnancy depends on the severity of the infection and the immune status of the mother. The TABLE offers several different clinical scenarios and the appropriate treatment for each. As noted, several scenarios may require cultures of the blood, cerebrospinal fluid, and amniotic fluid.7,9,10

Following treatment of the mother, serial ultrasound examinations should be performed to monitor fetal growth, CNS anatomy, placental morphology, amniotic fluid volume, and umbilical artery Doppler velocimetry. In the presence of fetal growth restriction, oligohydramnios, or abnormal Doppler velocimetry, biophysical profile testing should be performed. After delivery, the placenta should be examined carefully for histologic evidence of Listeria infection, such as miliary abscesses, and cultured for the bacterium.7-9
Prevention measures
Conservative measures for prevention of Listeria infection in pregnant women include the following7,10-12:
- Refrigerate milk and milk products at 40 °F (4.4 °C).
- Thoroughly cook raw food from animal sources.
- Wash raw vegetables carefully before eating.
- Keep uncooked meats separate from cooked meats and vegetables.
- Do not consume any beverages or foods made from unpasteurized milk.
- After handling uncooked foods, carefully wash all utensils and hands.
- Avoid all soft cheeses, such as Mexican-style feta, Brie, Camembert, and blue cheese, even if they are supposedly made from pasteurized milk.
- Reheat until steaming hot all leftover foods or ready-to-eat foods, such as hot dogs.
- Do not let juice from hot dogs or lunch meat packages drip onto other foods, utensils, or food preparation surfaces.
- Do not store opened hot dog packages in the refrigerator for more than 1 week. Do not store unopened packages for longer than 2 weeks.
- Do not store unopened lunch and deli meat packages in the refrigerator for longer than 2 weeks. Do not store opened packages for longer than 3 to 5 days.
- If other immunosuppressive conditions are present in combination with pregnancy, thoroughly heat cold cuts before eating.
- Do not eat raw or even lightly cooked sprouts of any kind. Cook sprouts thoroughly. Rinsing sprouts will not remove Listeria organisms.
- Do not eat refrigerated pâté or meat spreads from a deli counter or the refrigerated section of a grocery store.
- Canned or shelf-stable pâté and meat spreads are safe to eat, but be sure to refrigerate them after opening the packages.
- Do not eat refrigerated smoked seafood. Canned or shelf-stable seafood, particularly when incorporated into a casserole, is safe to eat.
- Eat cut melon immediately. Refrigerate uneaten melon quickly if not eaten. Discard cut melon that is left at room temperature for more than 4 hours.
CASE Diagnosis made and prompt treatment initiated
The most likely diagnosis in this patient is listeriosis. Because the patient is moderately ill and experiencing uterine contractions, she should be hospitalized and monitored for progressive cervical dilation. Blood cultures should be obtained to identify L monocytogenes. In addition, an amniocentesis should be performed, and the amniotic fluid should be cultured for this microorganism. Stool culture and culture of the cerebrospinal fluid are not indicated. The patient should be treated with intravenous ampicillin, 2 g every 4 hours for 14 days. If she is allergic to penicillin, the alternative drug is trimethoprim-sulfamethoxazole, 8 to 10 mg/kg per day in 2 divided doses, for 14 days. Prompt and effective treatment of the mother should prevent infection in the fetus and newborn. ●
- Listeriosis is primarily a foodborne illness caused by Listeria monocytogenes, a gram-positive bacillus.
- Pregnant women, particularly those who are immunocompromised, are especially susceptible to Listeria infection.
- Foods that pose particular risk of transmitting infection include fresh unpasteurized cheeses, processed meats such as hot dogs, refrigerated pâté and meat spreads, refrigerated smoked seafood, unpasteurized milk, and unwashed raw produce.
- The infection may range from a mild gastroenteritis to life-threatening sepsis and meningitis.
- Listeriosis may cause early and late-onset neonatal infection that presents as either meningitis or sepsis.
- Blood and amniotic fluid cultures are essential to diagnose maternal infection. Stool cultures usually are not indicated.
- Mildly symptomatic but afebrile patients do not require treatment.
- Febrile symptomatic patients should be treated with either intravenous ampicillin or trimethoprim-sulfamethoxazole.
- Radoshevich L, Cossart P. Listeria monocytogenes: towards a complete picture of its physiology and pathogenesis. Nat Rev Microbiol. 2018;16:32-46. doi:10.1038/nnrmicro.2017.126.
- Johnson JE, Mylonakis E. Listeria monocytogenes. In: Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 9th ed. Elsevier; 2020:2543-2549.
- Gelfand MS, Swamy GK, Thompson JL. Epidemiology and pathogenesis of Listeria monocytogenes infection. UpToDate. Updated August 23, 2022. Accessed November 9, 2022. https://www.uptodate.com/contents/epidemiology-and-pathogenesis-of-listeria-monocytogenes-infection?sectionName=CLINICAL%20EPIDEMIOLOGY&topicRef=1277&anchor=H4&source=see_link#H4
- Cherubin CE, Appleman MD, Heseltine PN, et al. Epidemiological spectrum and current treatment of listeriosis. Rev Infect Dis. 1991;13:1108-1114.
- Gelfand MS, Swamy GK, Thompson JL. Clinical manifestations and diagnosis of Listeria monocytogenes infection. UpToDate. Updated August 23, 2022. Accessed November 7, 2022. https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-listeriamonocytogenes-infection
- Boucher M, Yonekura ML. Perinatal listeriosis (early-onset): correlation of antenatal manifestations and neonatal outcome. Obstet Gynecol. 1986;68:593-597.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 614: management of pregnant women with presumptive exposure to Listeria monocytogenes. Obstet Gynecol. 2014;124:1241-1244.
- Rouse DJ, Keimig TW, Riley LE, et al. Case 16-2016. A 31-year-old pregnant woman with fever. N Engl J Med. 2016;374:2076-2083.
- Craig AM, Dotters-Katz S, Kuller JA, et al. Listeriosis in pregnancy: a review. Obstet Gynecol Surv. 2019;74: 362-368.
- Gelfand MS, Thompson JL, Swamy GK. Treatment and prevention of Listeria monocytogenes infection. UpToDate. Updated August 23, 2022. Accessed November 9, 2022. https://www.uptodate.com/contents/treatment-and-prevention-of-listeria-monocytogenes-infection?topicRef=1280&source=see_link
- Voetsch AC, Angulo FJ, Jones TF, et al; Centers for Disease Control and Prevention Emerging Infections Program Foodborne Diseases Active Surveillance Networking Group. Reduction in the incidence of invasive listeriosis in Foodborne Diseases Active Surveillance Network sites, 1996-2003. Clin Infect Dis. 2007;44:513-520.
- MacDonald PDM, Whitwan RE, Boggs JD, et al. Outbreak of listeriosis among Mexican immigrants as a result of consumption of illicitly produced Mexican-style cheese. Clin Infect Dis. 2005;40:677-682.
- Radoshevich L, Cossart P. Listeria monocytogenes: towards a complete picture of its physiology and pathogenesis. Nat Rev Microbiol. 2018;16:32-46. doi:10.1038/nnrmicro.2017.126.
- Johnson JE, Mylonakis E. Listeria monocytogenes. In: Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 9th ed. Elsevier; 2020:2543-2549.
- Gelfand MS, Swamy GK, Thompson JL. Epidemiology and pathogenesis of Listeria monocytogenes infection. UpToDate. Updated August 23, 2022. Accessed November 9, 2022. https://www.uptodate.com/contents/epidemiology-and-pathogenesis-of-listeria-monocytogenes-infection?sectionName=CLINICAL%20EPIDEMIOLOGY&topicRef=1277&anchor=H4&source=see_link#H4
- Cherubin CE, Appleman MD, Heseltine PN, et al. Epidemiological spectrum and current treatment of listeriosis. Rev Infect Dis. 1991;13:1108-1114.
- Gelfand MS, Swamy GK, Thompson JL. Clinical manifestations and diagnosis of Listeria monocytogenes infection. UpToDate. Updated August 23, 2022. Accessed November 7, 2022. https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-listeriamonocytogenes-infection
- Boucher M, Yonekura ML. Perinatal listeriosis (early-onset): correlation of antenatal manifestations and neonatal outcome. Obstet Gynecol. 1986;68:593-597.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 614: management of pregnant women with presumptive exposure to Listeria monocytogenes. Obstet Gynecol. 2014;124:1241-1244.
- Rouse DJ, Keimig TW, Riley LE, et al. Case 16-2016. A 31-year-old pregnant woman with fever. N Engl J Med. 2016;374:2076-2083.
- Craig AM, Dotters-Katz S, Kuller JA, et al. Listeriosis in pregnancy: a review. Obstet Gynecol Surv. 2019;74: 362-368.
- Gelfand MS, Thompson JL, Swamy GK. Treatment and prevention of Listeria monocytogenes infection. UpToDate. Updated August 23, 2022. Accessed November 9, 2022. https://www.uptodate.com/contents/treatment-and-prevention-of-listeria-monocytogenes-infection?topicRef=1280&source=see_link
- Voetsch AC, Angulo FJ, Jones TF, et al; Centers for Disease Control and Prevention Emerging Infections Program Foodborne Diseases Active Surveillance Networking Group. Reduction in the incidence of invasive listeriosis in Foodborne Diseases Active Surveillance Network sites, 1996-2003. Clin Infect Dis. 2007;44:513-520.
- MacDonald PDM, Whitwan RE, Boggs JD, et al. Outbreak of listeriosis among Mexican immigrants as a result of consumption of illicitly produced Mexican-style cheese. Clin Infect Dis. 2005;40:677-682.
A Better Way to Breathe: Combining Allergy and Pulmonary Care Into One Clinic
Asthma affects more than 300 million people worldwide.1 While many of these cases can achieve control with standard therapy, 5% to 10% of these cases are classified as severe asthma, remaining poorly controlled despite treatment with inhaled corticosteroids (ICS) and long-acting β agonists (LABA).2 These patients also account for the majority of morbidity and mortality associated with the disease, with increased hospitalizations, intensive care unit (ICU) stays, detrimental adverse effects of oral corticosteroids (OCS), and lower quality of life.3-6 Additionally, the financial repercussions of severe asthma are notable; in the United States, the estimated cost of asthma management is $82 billion annually, with $3 billion accounting for asthma-related work/school absences.7
In the past several years, the use of anti-immunoglobulin E (IgE), anti-interleukin-4 (IL-4), and anti-IL-5 biologic agents for severe asthma has been shown to decrease asthma exacerbations, improve lung function, reduce corticosteroid use, and decrease hospitalizations, especially for type 2 helper T cell (TH2-high) asthma.8-10 However, clinicians have observed significant barriers to the implementation and widespread use of biologics, including insurance coverage, long wait times, follow-up, and limited access for lower income groups.11,12
This article describes a unique model for a severe asthma clinic located at the Washington DC Veterans Affairs Medical Center (WDCVAMC) that is dually staffed by an allergist and pulmonologist. This clinic uses biologic agents for patients with difficult-to-treat asthma, many of whom require repeated or prolonged steroid use, in addition to prolonged and recurrent hospitalizations for exacerbations. The objective of this clinic is to provide a standardized approach to the management of severe asthma with the perspective of both an allergist and pulmonologist, thereby reducing the need to schedule appointments with multiple specialties and reducing delays in initiating biologics. This article presents the preliminary findings of 30 months of severe asthma management with various biologic agents, examining the impact of these therapies on hospitalizations, asthma exacerbations, ICU stays, and OCS use. The findings of this study support the benefits of biologics and suggest that the use of these agents within a dually staffed clinic may be a particularly effective model through which to manage severe asthma.
Background
Asthma affects approximately 20 million adults in the United States.13 Veterans are a population particularly impacted by asthma. Between 2015 and 2018, 10.9% of all veterans reported being diagnosed with asthma and 5.1% stated that they currently have asthma, compared with 13.4% and 8.0% of nonveterans, respectively.14 Veterans are susceptible to many of the factors that can trigger exacerbations while engaging in military service, such as chemical and environmental exposures both abroad and domestically.15,16 Additionally, medication adherence is often challenging among the veteran population, particularly with more involved therapy, such as inhaler use.17 Such factors contribute to asthma exacerbations, with 2.9% of veterans reporting at least 1 asthma exacerbation in the past 12 months.14
Over the past several years, the development and use of biologic agents have transformed the management of severe asthma.8 Before the development of biologic agents for severe asthma, treatment options for patients were limited. While OCS are frequently used for asthma exacerbations, they are associated with a multiplicity of undesirable adverse effects, including weight gain, mood lability, gastrointestinal upset, hyperglycemia, risk of bone fractures, and hypertension.18-20 The regular use of OCS are particularly problematic among other medical comorbidities commonly affecting the veteran population, such as diabetes and hypertension.21-22
The WDCVAMC severe allergy clinic used 3 biologic agents: omalizumab (anti-IgE), benralizumab (anti-IL-5), and agent dupilumab (anti-IL-4). These medications have shown significant improvements in quality of life, reduction in asthma exacerbations and hospitalizations, and decreased use of OCS.8,9 While research has firmly established the medical benefits of the use of biologic agents in severe asthma, several barriers exist in implementing widespread use.11,12
In Gelhorn and colleagues’ study examining both physician and patient challenges in the use of biologics for severe asthma, scheduling, administrative time, and insurance costs were found to be major barriers to the use of these medications.12 Patients expressed a preference for the administration of these medications in a specialist’s office but cited long wait times and scheduling difficulties as barriers. One of the most notable challenges from the physician perspective was the difficulty in obtaining reimbursement from insurance companies, requiring them to devote significant portions of time to prior authorizations and documentation.12
This article examines a dual specialty clinic that focuses on the treatment of severe asthma with biologic agents. This model is unique for several reasons. First, given the US Department of Veterans Affairs (VA) health care model, the health care practitioners (HCPs) in this clinic can avoid much of the administrative burden of obtaining reimbursement or working with insurance companies. Additionally, by dedicating specific days to the severe asthma clinic, patients do not experience long wait times to see both an allergist and pulmonologist. By seeing both clinicians, concurrent allergic and pulmonary issues can be addressed in the same visit, rather than delaying treatment by waiting on 2 specialist appointments.
Severe Asthma Clinic
The severe asthma clinic was started in September 2017 by a pulmonologist and an allergist at WDCVAMC. After experiencing substantial delays with the initiation of biologics for their patients and multiple referrals between their clinics, these physicians wanted to start a dually staffed asthma clinic to specifically focus on evaluating and treating severe asthma. A dedicated severe asthma clinic allowed the allergist and pulmonologist to streamline resources and collaborate to advocate for patients with the pharmacy section. Additionally, patients can benefit from the perspective of both specialists, as both the pulmonologist and allergist evaluate each patient and discuss the next steps of management.
This clinic is composed of 4 registered nurses, an allergist, and a pulmonologist. Clinic is held twice monthly through both telemedicine and in-office visits. The VA has strict guidelines for the use of certain biologics, including blood eosinophil count > 150 cells/µL, failure of traditional therapy, and frequent use of OCS. Additionally, to ensure these biologic agents are prescribed to patients that will benefit from them, the patients enrolled in this clinic are already on maximum therapy for their asthma, meaning all other therapeutic options (inhalers and oral medications) are being used. The clinic services all patients with severe asthma, not just patients who are on biologic therapy. Often, patients are referred to the severe allergy clinic late in their disease course given a lack of familiarity with biologic agents from prescribers and both institutional and insurance barriers.
Before the COVID-19 pandemic, spirometry and fractional exhaled nitric oxide (FENO) tests were recorded at each visit. Initially during the pandemic, the clinic transitioned to primarily telemedicine visits due to patients’ hesitance to seek in-person care. More recently, the clinic has transitioned back to primarily office visits; patients are seen in clinic on average every 3 months. At each visit, the patient is seen by both the pulmonologist and allergist. Additionally, the nursing staff reviews inhaler adherence with patients, spacer use, documents, Asthma Control Test (ACT) scores, and schedules follow-up visits.
Every 4 to 8 weeks, patients receive biologics agent at the WDCVAMC infusion center depending on the agent. The infusion center also instructs patients how to handle self-administered medications, like benralizumab, if the patient expresses a preference for taking it at home. Omalizumab has a boxed warning for anaphylaxis, although the other biologics in this study have a low risk of anaphylaxis. All patients receiving omalizumab, benralizumab, and dupilumab were provided with epinephrine injection devices in case of an allergic reaction and were taught how to use them in the clinic.23,24
If patients continued to experience asthma exacerbations after the initiation of a biologic, a change in agent was considered after 4 to 6 months. Additionally, a complete blood count, respiratory allergy panel, and pulmonary function tests (PFTs) were completed.
Clinic Patients
Preliminary data were obtained from a retrospective chart review of 15 patients enrolled in the severe asthma clinic over 30 months. The inclusion criteria for chart review consisted of patients aged > 18 years receiving a biologic agent for > 3 months for the treatment of severe asthma. The outcomes examined included steroid use, emergency department (ED) visits, hospitalizations, FEV1, and ICU stays.
Seven patients used benralizumab, 6 used dupilumab, and 2 used omalizumab (Table).
There was a notable clinical improvement in these patients. Before starting a biologic agent, all the patients in this study were prescribed steroids at least once a year for an asthma exacerbation, with a mean of 4.2 steroid tapers per year.
The initiation of a biologic agent also resulted in fewer ED visits and hospitalizations. Two patients had an ED visit for an asthma exacerbation since starting a biologic agent and 1 patient had a hospital admission for an asthma exacerbation. No patients were hospitalized in the ICU after starting a biologic agent.
Discussion
The 15 patients in this initial data were referred to the severe asthma clinic by pulmonology, ear, nose, and throat (ENT), primary care, and a hospitalist during an in-patient stay. As the enrollment in our clinic grows, an increasing number of patients are referred from the allergy clinic as well. Patients in the severe asthma clinic also are referred by regional centers as news of the clinic is spread by word of mouth to surrounding VA facilities. As our clinic gains the capacity to serve more patients, we hope to contact WDCVAMC primary care, pulmonology, allergy, and ENT departments to raise awareness of the clinic.
Benralizumab and dupilumab were the most used agents in this preliminary data. This finding was largely due to the ability of patients to self-administer benralizumab, which was particularly beneficial during the COVID-19 pandemic. Of note, 5 patients in this study switched from another biologic agent to benralizumab due to the ability to self-administer. Three of 5 patients that required steroids after initiating benralizumab used fewer steroids than they had previously. This finding suggests benralizumab may be the preferred agent when travel time to health care is a challenge, reducing the need for frequent clinic visits and transportation.
This preliminary data supports previous studies that have demonstrated that biologic agents improve clinical outcomes by reducing asthma exacerbations, OCS use, hospitalizations, and ICU stays for patients on all 4 biologic agents. In addition to improving patient health through avoiding complications of prolonged OCS use and hospital stays, the decrease in ED visits and hospitalizations provides a substantial cost reduction to the health care system.
These findings highlight the strength of a unique model of a combined allergy/pulmonary clinic. Before this combined clinic model, both pulmonology and allergy clinics noted delays in the initiation of biologics for patients who were potential candidates. Impediments include referrals between each specialty for evaluation of concurrent pulmonary conditions or allergy testing, overlap in asthma management, and a delay in coordination with the pharmacy department to start biologic agents. A dedicated severe asthma clinic staffed by both an allergist and pulmonologist provides a convenient option for patients to be seen by both specialists, reducing the need for separate appointments with each specialty, transportation to those appointments, and clinical time. This is particularly beneficial in a clinic such as this model, as this clinic serves patients from 4 states and Washington, DC. An additional benefit of this model is trained staff who directly communicate with the pharmacy in the initiation of these agents, allocate time to educating patients in biologic use, and coordinate follow-up.
Limitations
There were several limitations to this report. First, the number of patients examined in this preliminary data set is small. Due to the COVID-19 pandemic, there was a limited ability to see patients in person, and patients were seen exclusively over telemedicine for several months. For this reason, collecting data such as patient surveys and laboratory work following the initiation of a biologic was a challenge. Additionally, during the height of COVID-19, WDCVAMC did not perform aerosolizing procedures, such as PFTs and FENOs; thus, peak flows were obtained instead. Examining metrics, such as FENOs and IgE levels, and expanding PFT data would provide additional insight into the impact of biologic agents on clinical outcomes. Patient survey data in the form of ACTs or satisfaction surveys would also yield important data examining the impact of this clinic design and biologic use on patient experience. As of December 2022, 114 patients are enrolled in the clinic. We are working to collect the above laboratory results and spirometry for these patients so that these results can be published with a more robust data set. Another limitation of the information presented is that it is a retrospective data analysis; the data collected was contingent upon documentation and the assumption that these patients were exclusively receiving care through the VA. For example, steroid use before and after initiation of biologic was taken from asthma clinic notes and the patient’s medication list. Therefore, there is a possibility that not all instances were accounted for if that patient sought care outside the VA or whether it was not documented in a follow-up note.
Conclusions
The model of a combined allergy/pulmonology clinic can be particularly efficacious in the treatment of severe asthma, as it reduces the need for multiple appointments with different specialties, reduces wait time before starting a biologic agent, and offers the perspective of 2 specialists. This kind of model could be an example to many clinics in the VA. With a rapid increase in telemedicine due to the COVID-19 pandemic, multiple physicians consulting simultaneously is becoming a more feasible possibility across multiple specialties. As the use of biologics becomes more widespread, a combined clinic design is an efficient and promising method to improve severe asthma management.
This preliminary data continue to support previous research that shows biologic agents have led to better clinical outcomes through the reduction of asthma exacerbations, hospitalizations, and improved PFTs. While this initial data set highlights the results for 15 patients, there are 86 patients currently enrolled in this clinic. We are collecting additional data to publish more comprehensive results.
1. Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015;16(1):45-56. doi:10.1038/ni.3049
2. Moore WC, Bleecker ER, Curran-Everett D, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119(2):405-413. doi:10.1016/j.jaci.2006.11.639
3. Barnes PJ, Jonsson B, Klim JB. The costs of asthma. Eur Respir J. 1996;9(4):636-642. doi:10.1183/09031936.96.09040636
4. Bourdin A, Charriot J, Boissin C, et al. Will the asthma revolution fostered by biologics also benefit adult ICU patients?. Allergy. 2021;76(8):2395-2406. doi:10.1111/all.14688
5. Lloyd A, Price D, Brown R. The impact of asthma exacerbations on health-related quality of life in moderate to severe asthma patients in the UK. Prim Care Respir J. 2007;16(1):22-27. doi:10.3132/pcrj.2007.00002
6. Eisner MD, Yelin EH, Katz PP, Lactao G, Iribarren C, Blanc PD. Risk factors for work disability in severe adult asthma. Am J Med. 2006;119(10):884-891. doi:10.1016/j.amjmed.2006.01.016
7. Nurmagambetov T, Kuwahara R, Garbe P. The Economic Burden of Asthma in the United States, 2008-2013. Ann Am Thorac Soc. 2018;15(3):348-356. doi:10.1513/AnnalsATS.201703-259OC
8. McGregor MC, Krings JG, Nair P, Castro M. Role of biologics in asthma. Am J Respir Crit Care Med. 2019;199(4):433-445. doi:10.1164/rccm.201810-1944CI
9. Bice JB, Leechawengwongs E, Montanaro A. Biologic targeted therapy in allergic asthma. Ann Allergy Asthma Immunol. 2014;112(2):108-115. doi:10.1016/j.anai.2013.12.013
10. Darveaux J, Busse WW. Biologics in asthma--the next step toward personalized treatment. J Allergy Clin Immunol Pract. 2015;3(2):152-161. doi:10.1016/j.jaip.2014.09.014
11. Inselman JW, Jeffery MM, Maddux JT, Shah ND, Rank MA. Trends and disparities in asthma biologic use in the United States. J Allergy Clin Immunol Pract. 2020;8(2):549-554.e1. doi:10.1016/j.jaip.2019.08.024
12. Gelhorn HL, Balantac Z, Ambrose CS, Chung YN, Stone B. Patient and physician preferences for attributes of biologic medications for severe asthma. Patient Prefer Adherence. 2019;13:1253-1268. Published 2019 Jul 25. doi:10.2147/PPA.S198953
13. Centers for Disease Control and Prevention, National Center for Environmental Health. 2019 National Health Interview Survey (NHIS) data. Accessed December 6, 2022. https://www.cdc.gov/asthma/nhis/2019/data.htm
14. Zelaya CE BP, Moy E. Crude and age-adjusted percent distribution of respondent-assessed health status among adults aged 20 and over, by veteran status and other selected characteristics: United States, 2015-2018. National Center for Health Statistic. Updated June 19, 2020. Accessed December 12, 2022. https://www.cdc.gov/nchs/nhis/veterans_health_statistics/tables.htm
15. Szema AM, Peters MC, Weissinger KM, Gagliano CA, Chen JJ. New-onset asthma among soldiers serving in Iraq and Afghanistan. Allergy Asthma Proc. 2010;31(5):67-71. doi:10.2500/aap.2010.31.3383
16. Yi SW, Hong JS, Ohrr H, Yi JJ. Agent Orange exposure and disease prevalence in Korean Vietnam veterans: the Korean veterans health study. Environ Res. 2014;133:56-65. doi:10.1016/j.envres.2014.04.027
17. Huetsch JC, Uman JE, Udris EM, Au DH. Predictors of adherence to inhaled medications among veterans with COPD. J Gen Intern Med. 2012;27(11):1506-1512. doi:10.1007/s11606-012-2130-5
18. Mundell L, Lindemann R, Douglas J. Monitoring long-term oral corticosteroids. BMJ Open Qual. 2017;6(2):e000209. Published 2017 Nov 8. doi:10.1136/bmjoq-2017-000209
19. Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2005;20(8):1487-1486. doi:10.1359/jbmr.2005.20.8.1486
20. Warrington TP, Bostwick JM. Psychiatric adverse effects of corticosteroids. Mayo Clin Proc. 2006;81(10):1361-1367. doi:10.4065/81.10.1361
21. Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care. 2004;27(suppl 2):B10-B21. doi:10.2337/diacare.27.suppl_2.b10
22. Acharya T, Tringali S, Singh M, Huang J. Resistant hypertension and associated comorbidities in a Veterans Affairs population. J Clin Hypertens (Greenwich). 2014;16(10):741-745. doi:10.1111/jch.12410
23. Dupixent (dupilumab). Prescribing information. Sanofi and Regeneron Pharmaceuticals; 2022. Accessed December 6, 2022. https://www.dupixenthcp.com/asthma/efficacy/safety-data
24. Li L, Wang Z, Cui L, Xu Y, Guan K, Zhao B. Anaphylactic risk related to omalizumab, benralizumab, reslizumab, mepolizumab, and dupilumab. Clin Transl Allergy. 2021;11(4):e12038. Published 2021 Jun 3. doi:10.1002/clt2.12038
Asthma affects more than 300 million people worldwide.1 While many of these cases can achieve control with standard therapy, 5% to 10% of these cases are classified as severe asthma, remaining poorly controlled despite treatment with inhaled corticosteroids (ICS) and long-acting β agonists (LABA).2 These patients also account for the majority of morbidity and mortality associated with the disease, with increased hospitalizations, intensive care unit (ICU) stays, detrimental adverse effects of oral corticosteroids (OCS), and lower quality of life.3-6 Additionally, the financial repercussions of severe asthma are notable; in the United States, the estimated cost of asthma management is $82 billion annually, with $3 billion accounting for asthma-related work/school absences.7
In the past several years, the use of anti-immunoglobulin E (IgE), anti-interleukin-4 (IL-4), and anti-IL-5 biologic agents for severe asthma has been shown to decrease asthma exacerbations, improve lung function, reduce corticosteroid use, and decrease hospitalizations, especially for type 2 helper T cell (TH2-high) asthma.8-10 However, clinicians have observed significant barriers to the implementation and widespread use of biologics, including insurance coverage, long wait times, follow-up, and limited access for lower income groups.11,12
This article describes a unique model for a severe asthma clinic located at the Washington DC Veterans Affairs Medical Center (WDCVAMC) that is dually staffed by an allergist and pulmonologist. This clinic uses biologic agents for patients with difficult-to-treat asthma, many of whom require repeated or prolonged steroid use, in addition to prolonged and recurrent hospitalizations for exacerbations. The objective of this clinic is to provide a standardized approach to the management of severe asthma with the perspective of both an allergist and pulmonologist, thereby reducing the need to schedule appointments with multiple specialties and reducing delays in initiating biologics. This article presents the preliminary findings of 30 months of severe asthma management with various biologic agents, examining the impact of these therapies on hospitalizations, asthma exacerbations, ICU stays, and OCS use. The findings of this study support the benefits of biologics and suggest that the use of these agents within a dually staffed clinic may be a particularly effective model through which to manage severe asthma.
Background
Asthma affects approximately 20 million adults in the United States.13 Veterans are a population particularly impacted by asthma. Between 2015 and 2018, 10.9% of all veterans reported being diagnosed with asthma and 5.1% stated that they currently have asthma, compared with 13.4% and 8.0% of nonveterans, respectively.14 Veterans are susceptible to many of the factors that can trigger exacerbations while engaging in military service, such as chemical and environmental exposures both abroad and domestically.15,16 Additionally, medication adherence is often challenging among the veteran population, particularly with more involved therapy, such as inhaler use.17 Such factors contribute to asthma exacerbations, with 2.9% of veterans reporting at least 1 asthma exacerbation in the past 12 months.14
Over the past several years, the development and use of biologic agents have transformed the management of severe asthma.8 Before the development of biologic agents for severe asthma, treatment options for patients were limited. While OCS are frequently used for asthma exacerbations, they are associated with a multiplicity of undesirable adverse effects, including weight gain, mood lability, gastrointestinal upset, hyperglycemia, risk of bone fractures, and hypertension.18-20 The regular use of OCS are particularly problematic among other medical comorbidities commonly affecting the veteran population, such as diabetes and hypertension.21-22
The WDCVAMC severe allergy clinic used 3 biologic agents: omalizumab (anti-IgE), benralizumab (anti-IL-5), and agent dupilumab (anti-IL-4). These medications have shown significant improvements in quality of life, reduction in asthma exacerbations and hospitalizations, and decreased use of OCS.8,9 While research has firmly established the medical benefits of the use of biologic agents in severe asthma, several barriers exist in implementing widespread use.11,12
In Gelhorn and colleagues’ study examining both physician and patient challenges in the use of biologics for severe asthma, scheduling, administrative time, and insurance costs were found to be major barriers to the use of these medications.12 Patients expressed a preference for the administration of these medications in a specialist’s office but cited long wait times and scheduling difficulties as barriers. One of the most notable challenges from the physician perspective was the difficulty in obtaining reimbursement from insurance companies, requiring them to devote significant portions of time to prior authorizations and documentation.12
This article examines a dual specialty clinic that focuses on the treatment of severe asthma with biologic agents. This model is unique for several reasons. First, given the US Department of Veterans Affairs (VA) health care model, the health care practitioners (HCPs) in this clinic can avoid much of the administrative burden of obtaining reimbursement or working with insurance companies. Additionally, by dedicating specific days to the severe asthma clinic, patients do not experience long wait times to see both an allergist and pulmonologist. By seeing both clinicians, concurrent allergic and pulmonary issues can be addressed in the same visit, rather than delaying treatment by waiting on 2 specialist appointments.
Severe Asthma Clinic
The severe asthma clinic was started in September 2017 by a pulmonologist and an allergist at WDCVAMC. After experiencing substantial delays with the initiation of biologics for their patients and multiple referrals between their clinics, these physicians wanted to start a dually staffed asthma clinic to specifically focus on evaluating and treating severe asthma. A dedicated severe asthma clinic allowed the allergist and pulmonologist to streamline resources and collaborate to advocate for patients with the pharmacy section. Additionally, patients can benefit from the perspective of both specialists, as both the pulmonologist and allergist evaluate each patient and discuss the next steps of management.
This clinic is composed of 4 registered nurses, an allergist, and a pulmonologist. Clinic is held twice monthly through both telemedicine and in-office visits. The VA has strict guidelines for the use of certain biologics, including blood eosinophil count > 150 cells/µL, failure of traditional therapy, and frequent use of OCS. Additionally, to ensure these biologic agents are prescribed to patients that will benefit from them, the patients enrolled in this clinic are already on maximum therapy for their asthma, meaning all other therapeutic options (inhalers and oral medications) are being used. The clinic services all patients with severe asthma, not just patients who are on biologic therapy. Often, patients are referred to the severe allergy clinic late in their disease course given a lack of familiarity with biologic agents from prescribers and both institutional and insurance barriers.
Before the COVID-19 pandemic, spirometry and fractional exhaled nitric oxide (FENO) tests were recorded at each visit. Initially during the pandemic, the clinic transitioned to primarily telemedicine visits due to patients’ hesitance to seek in-person care. More recently, the clinic has transitioned back to primarily office visits; patients are seen in clinic on average every 3 months. At each visit, the patient is seen by both the pulmonologist and allergist. Additionally, the nursing staff reviews inhaler adherence with patients, spacer use, documents, Asthma Control Test (ACT) scores, and schedules follow-up visits.
Every 4 to 8 weeks, patients receive biologics agent at the WDCVAMC infusion center depending on the agent. The infusion center also instructs patients how to handle self-administered medications, like benralizumab, if the patient expresses a preference for taking it at home. Omalizumab has a boxed warning for anaphylaxis, although the other biologics in this study have a low risk of anaphylaxis. All patients receiving omalizumab, benralizumab, and dupilumab were provided with epinephrine injection devices in case of an allergic reaction and were taught how to use them in the clinic.23,24
If patients continued to experience asthma exacerbations after the initiation of a biologic, a change in agent was considered after 4 to 6 months. Additionally, a complete blood count, respiratory allergy panel, and pulmonary function tests (PFTs) were completed.
Clinic Patients
Preliminary data were obtained from a retrospective chart review of 15 patients enrolled in the severe asthma clinic over 30 months. The inclusion criteria for chart review consisted of patients aged > 18 years receiving a biologic agent for > 3 months for the treatment of severe asthma. The outcomes examined included steroid use, emergency department (ED) visits, hospitalizations, FEV1, and ICU stays.
Seven patients used benralizumab, 6 used dupilumab, and 2 used omalizumab (Table).
There was a notable clinical improvement in these patients. Before starting a biologic agent, all the patients in this study were prescribed steroids at least once a year for an asthma exacerbation, with a mean of 4.2 steroid tapers per year.
The initiation of a biologic agent also resulted in fewer ED visits and hospitalizations. Two patients had an ED visit for an asthma exacerbation since starting a biologic agent and 1 patient had a hospital admission for an asthma exacerbation. No patients were hospitalized in the ICU after starting a biologic agent.
Discussion
The 15 patients in this initial data were referred to the severe asthma clinic by pulmonology, ear, nose, and throat (ENT), primary care, and a hospitalist during an in-patient stay. As the enrollment in our clinic grows, an increasing number of patients are referred from the allergy clinic as well. Patients in the severe asthma clinic also are referred by regional centers as news of the clinic is spread by word of mouth to surrounding VA facilities. As our clinic gains the capacity to serve more patients, we hope to contact WDCVAMC primary care, pulmonology, allergy, and ENT departments to raise awareness of the clinic.
Benralizumab and dupilumab were the most used agents in this preliminary data. This finding was largely due to the ability of patients to self-administer benralizumab, which was particularly beneficial during the COVID-19 pandemic. Of note, 5 patients in this study switched from another biologic agent to benralizumab due to the ability to self-administer. Three of 5 patients that required steroids after initiating benralizumab used fewer steroids than they had previously. This finding suggests benralizumab may be the preferred agent when travel time to health care is a challenge, reducing the need for frequent clinic visits and transportation.
This preliminary data supports previous studies that have demonstrated that biologic agents improve clinical outcomes by reducing asthma exacerbations, OCS use, hospitalizations, and ICU stays for patients on all 4 biologic agents. In addition to improving patient health through avoiding complications of prolonged OCS use and hospital stays, the decrease in ED visits and hospitalizations provides a substantial cost reduction to the health care system.
These findings highlight the strength of a unique model of a combined allergy/pulmonary clinic. Before this combined clinic model, both pulmonology and allergy clinics noted delays in the initiation of biologics for patients who were potential candidates. Impediments include referrals between each specialty for evaluation of concurrent pulmonary conditions or allergy testing, overlap in asthma management, and a delay in coordination with the pharmacy department to start biologic agents. A dedicated severe asthma clinic staffed by both an allergist and pulmonologist provides a convenient option for patients to be seen by both specialists, reducing the need for separate appointments with each specialty, transportation to those appointments, and clinical time. This is particularly beneficial in a clinic such as this model, as this clinic serves patients from 4 states and Washington, DC. An additional benefit of this model is trained staff who directly communicate with the pharmacy in the initiation of these agents, allocate time to educating patients in biologic use, and coordinate follow-up.
Limitations
There were several limitations to this report. First, the number of patients examined in this preliminary data set is small. Due to the COVID-19 pandemic, there was a limited ability to see patients in person, and patients were seen exclusively over telemedicine for several months. For this reason, collecting data such as patient surveys and laboratory work following the initiation of a biologic was a challenge. Additionally, during the height of COVID-19, WDCVAMC did not perform aerosolizing procedures, such as PFTs and FENOs; thus, peak flows were obtained instead. Examining metrics, such as FENOs and IgE levels, and expanding PFT data would provide additional insight into the impact of biologic agents on clinical outcomes. Patient survey data in the form of ACTs or satisfaction surveys would also yield important data examining the impact of this clinic design and biologic use on patient experience. As of December 2022, 114 patients are enrolled in the clinic. We are working to collect the above laboratory results and spirometry for these patients so that these results can be published with a more robust data set. Another limitation of the information presented is that it is a retrospective data analysis; the data collected was contingent upon documentation and the assumption that these patients were exclusively receiving care through the VA. For example, steroid use before and after initiation of biologic was taken from asthma clinic notes and the patient’s medication list. Therefore, there is a possibility that not all instances were accounted for if that patient sought care outside the VA or whether it was not documented in a follow-up note.
Conclusions
The model of a combined allergy/pulmonology clinic can be particularly efficacious in the treatment of severe asthma, as it reduces the need for multiple appointments with different specialties, reduces wait time before starting a biologic agent, and offers the perspective of 2 specialists. This kind of model could be an example to many clinics in the VA. With a rapid increase in telemedicine due to the COVID-19 pandemic, multiple physicians consulting simultaneously is becoming a more feasible possibility across multiple specialties. As the use of biologics becomes more widespread, a combined clinic design is an efficient and promising method to improve severe asthma management.
This preliminary data continue to support previous research that shows biologic agents have led to better clinical outcomes through the reduction of asthma exacerbations, hospitalizations, and improved PFTs. While this initial data set highlights the results for 15 patients, there are 86 patients currently enrolled in this clinic. We are collecting additional data to publish more comprehensive results.
Asthma affects more than 300 million people worldwide.1 While many of these cases can achieve control with standard therapy, 5% to 10% of these cases are classified as severe asthma, remaining poorly controlled despite treatment with inhaled corticosteroids (ICS) and long-acting β agonists (LABA).2 These patients also account for the majority of morbidity and mortality associated with the disease, with increased hospitalizations, intensive care unit (ICU) stays, detrimental adverse effects of oral corticosteroids (OCS), and lower quality of life.3-6 Additionally, the financial repercussions of severe asthma are notable; in the United States, the estimated cost of asthma management is $82 billion annually, with $3 billion accounting for asthma-related work/school absences.7
In the past several years, the use of anti-immunoglobulin E (IgE), anti-interleukin-4 (IL-4), and anti-IL-5 biologic agents for severe asthma has been shown to decrease asthma exacerbations, improve lung function, reduce corticosteroid use, and decrease hospitalizations, especially for type 2 helper T cell (TH2-high) asthma.8-10 However, clinicians have observed significant barriers to the implementation and widespread use of biologics, including insurance coverage, long wait times, follow-up, and limited access for lower income groups.11,12
This article describes a unique model for a severe asthma clinic located at the Washington DC Veterans Affairs Medical Center (WDCVAMC) that is dually staffed by an allergist and pulmonologist. This clinic uses biologic agents for patients with difficult-to-treat asthma, many of whom require repeated or prolonged steroid use, in addition to prolonged and recurrent hospitalizations for exacerbations. The objective of this clinic is to provide a standardized approach to the management of severe asthma with the perspective of both an allergist and pulmonologist, thereby reducing the need to schedule appointments with multiple specialties and reducing delays in initiating biologics. This article presents the preliminary findings of 30 months of severe asthma management with various biologic agents, examining the impact of these therapies on hospitalizations, asthma exacerbations, ICU stays, and OCS use. The findings of this study support the benefits of biologics and suggest that the use of these agents within a dually staffed clinic may be a particularly effective model through which to manage severe asthma.
Background
Asthma affects approximately 20 million adults in the United States.13 Veterans are a population particularly impacted by asthma. Between 2015 and 2018, 10.9% of all veterans reported being diagnosed with asthma and 5.1% stated that they currently have asthma, compared with 13.4% and 8.0% of nonveterans, respectively.14 Veterans are susceptible to many of the factors that can trigger exacerbations while engaging in military service, such as chemical and environmental exposures both abroad and domestically.15,16 Additionally, medication adherence is often challenging among the veteran population, particularly with more involved therapy, such as inhaler use.17 Such factors contribute to asthma exacerbations, with 2.9% of veterans reporting at least 1 asthma exacerbation in the past 12 months.14
Over the past several years, the development and use of biologic agents have transformed the management of severe asthma.8 Before the development of biologic agents for severe asthma, treatment options for patients were limited. While OCS are frequently used for asthma exacerbations, they are associated with a multiplicity of undesirable adverse effects, including weight gain, mood lability, gastrointestinal upset, hyperglycemia, risk of bone fractures, and hypertension.18-20 The regular use of OCS are particularly problematic among other medical comorbidities commonly affecting the veteran population, such as diabetes and hypertension.21-22
The WDCVAMC severe allergy clinic used 3 biologic agents: omalizumab (anti-IgE), benralizumab (anti-IL-5), and agent dupilumab (anti-IL-4). These medications have shown significant improvements in quality of life, reduction in asthma exacerbations and hospitalizations, and decreased use of OCS.8,9 While research has firmly established the medical benefits of the use of biologic agents in severe asthma, several barriers exist in implementing widespread use.11,12
In Gelhorn and colleagues’ study examining both physician and patient challenges in the use of biologics for severe asthma, scheduling, administrative time, and insurance costs were found to be major barriers to the use of these medications.12 Patients expressed a preference for the administration of these medications in a specialist’s office but cited long wait times and scheduling difficulties as barriers. One of the most notable challenges from the physician perspective was the difficulty in obtaining reimbursement from insurance companies, requiring them to devote significant portions of time to prior authorizations and documentation.12
This article examines a dual specialty clinic that focuses on the treatment of severe asthma with biologic agents. This model is unique for several reasons. First, given the US Department of Veterans Affairs (VA) health care model, the health care practitioners (HCPs) in this clinic can avoid much of the administrative burden of obtaining reimbursement or working with insurance companies. Additionally, by dedicating specific days to the severe asthma clinic, patients do not experience long wait times to see both an allergist and pulmonologist. By seeing both clinicians, concurrent allergic and pulmonary issues can be addressed in the same visit, rather than delaying treatment by waiting on 2 specialist appointments.
Severe Asthma Clinic
The severe asthma clinic was started in September 2017 by a pulmonologist and an allergist at WDCVAMC. After experiencing substantial delays with the initiation of biologics for their patients and multiple referrals between their clinics, these physicians wanted to start a dually staffed asthma clinic to specifically focus on evaluating and treating severe asthma. A dedicated severe asthma clinic allowed the allergist and pulmonologist to streamline resources and collaborate to advocate for patients with the pharmacy section. Additionally, patients can benefit from the perspective of both specialists, as both the pulmonologist and allergist evaluate each patient and discuss the next steps of management.
This clinic is composed of 4 registered nurses, an allergist, and a pulmonologist. Clinic is held twice monthly through both telemedicine and in-office visits. The VA has strict guidelines for the use of certain biologics, including blood eosinophil count > 150 cells/µL, failure of traditional therapy, and frequent use of OCS. Additionally, to ensure these biologic agents are prescribed to patients that will benefit from them, the patients enrolled in this clinic are already on maximum therapy for their asthma, meaning all other therapeutic options (inhalers and oral medications) are being used. The clinic services all patients with severe asthma, not just patients who are on biologic therapy. Often, patients are referred to the severe allergy clinic late in their disease course given a lack of familiarity with biologic agents from prescribers and both institutional and insurance barriers.
Before the COVID-19 pandemic, spirometry and fractional exhaled nitric oxide (FENO) tests were recorded at each visit. Initially during the pandemic, the clinic transitioned to primarily telemedicine visits due to patients’ hesitance to seek in-person care. More recently, the clinic has transitioned back to primarily office visits; patients are seen in clinic on average every 3 months. At each visit, the patient is seen by both the pulmonologist and allergist. Additionally, the nursing staff reviews inhaler adherence with patients, spacer use, documents, Asthma Control Test (ACT) scores, and schedules follow-up visits.
Every 4 to 8 weeks, patients receive biologics agent at the WDCVAMC infusion center depending on the agent. The infusion center also instructs patients how to handle self-administered medications, like benralizumab, if the patient expresses a preference for taking it at home. Omalizumab has a boxed warning for anaphylaxis, although the other biologics in this study have a low risk of anaphylaxis. All patients receiving omalizumab, benralizumab, and dupilumab were provided with epinephrine injection devices in case of an allergic reaction and were taught how to use them in the clinic.23,24
If patients continued to experience asthma exacerbations after the initiation of a biologic, a change in agent was considered after 4 to 6 months. Additionally, a complete blood count, respiratory allergy panel, and pulmonary function tests (PFTs) were completed.
Clinic Patients
Preliminary data were obtained from a retrospective chart review of 15 patients enrolled in the severe asthma clinic over 30 months. The inclusion criteria for chart review consisted of patients aged > 18 years receiving a biologic agent for > 3 months for the treatment of severe asthma. The outcomes examined included steroid use, emergency department (ED) visits, hospitalizations, FEV1, and ICU stays.
Seven patients used benralizumab, 6 used dupilumab, and 2 used omalizumab (Table).
There was a notable clinical improvement in these patients. Before starting a biologic agent, all the patients in this study were prescribed steroids at least once a year for an asthma exacerbation, with a mean of 4.2 steroid tapers per year.
The initiation of a biologic agent also resulted in fewer ED visits and hospitalizations. Two patients had an ED visit for an asthma exacerbation since starting a biologic agent and 1 patient had a hospital admission for an asthma exacerbation. No patients were hospitalized in the ICU after starting a biologic agent.
Discussion
The 15 patients in this initial data were referred to the severe asthma clinic by pulmonology, ear, nose, and throat (ENT), primary care, and a hospitalist during an in-patient stay. As the enrollment in our clinic grows, an increasing number of patients are referred from the allergy clinic as well. Patients in the severe asthma clinic also are referred by regional centers as news of the clinic is spread by word of mouth to surrounding VA facilities. As our clinic gains the capacity to serve more patients, we hope to contact WDCVAMC primary care, pulmonology, allergy, and ENT departments to raise awareness of the clinic.
Benralizumab and dupilumab were the most used agents in this preliminary data. This finding was largely due to the ability of patients to self-administer benralizumab, which was particularly beneficial during the COVID-19 pandemic. Of note, 5 patients in this study switched from another biologic agent to benralizumab due to the ability to self-administer. Three of 5 patients that required steroids after initiating benralizumab used fewer steroids than they had previously. This finding suggests benralizumab may be the preferred agent when travel time to health care is a challenge, reducing the need for frequent clinic visits and transportation.
This preliminary data supports previous studies that have demonstrated that biologic agents improve clinical outcomes by reducing asthma exacerbations, OCS use, hospitalizations, and ICU stays for patients on all 4 biologic agents. In addition to improving patient health through avoiding complications of prolonged OCS use and hospital stays, the decrease in ED visits and hospitalizations provides a substantial cost reduction to the health care system.
These findings highlight the strength of a unique model of a combined allergy/pulmonary clinic. Before this combined clinic model, both pulmonology and allergy clinics noted delays in the initiation of biologics for patients who were potential candidates. Impediments include referrals between each specialty for evaluation of concurrent pulmonary conditions or allergy testing, overlap in asthma management, and a delay in coordination with the pharmacy department to start biologic agents. A dedicated severe asthma clinic staffed by both an allergist and pulmonologist provides a convenient option for patients to be seen by both specialists, reducing the need for separate appointments with each specialty, transportation to those appointments, and clinical time. This is particularly beneficial in a clinic such as this model, as this clinic serves patients from 4 states and Washington, DC. An additional benefit of this model is trained staff who directly communicate with the pharmacy in the initiation of these agents, allocate time to educating patients in biologic use, and coordinate follow-up.
Limitations
There were several limitations to this report. First, the number of patients examined in this preliminary data set is small. Due to the COVID-19 pandemic, there was a limited ability to see patients in person, and patients were seen exclusively over telemedicine for several months. For this reason, collecting data such as patient surveys and laboratory work following the initiation of a biologic was a challenge. Additionally, during the height of COVID-19, WDCVAMC did not perform aerosolizing procedures, such as PFTs and FENOs; thus, peak flows were obtained instead. Examining metrics, such as FENOs and IgE levels, and expanding PFT data would provide additional insight into the impact of biologic agents on clinical outcomes. Patient survey data in the form of ACTs or satisfaction surveys would also yield important data examining the impact of this clinic design and biologic use on patient experience. As of December 2022, 114 patients are enrolled in the clinic. We are working to collect the above laboratory results and spirometry for these patients so that these results can be published with a more robust data set. Another limitation of the information presented is that it is a retrospective data analysis; the data collected was contingent upon documentation and the assumption that these patients were exclusively receiving care through the VA. For example, steroid use before and after initiation of biologic was taken from asthma clinic notes and the patient’s medication list. Therefore, there is a possibility that not all instances were accounted for if that patient sought care outside the VA or whether it was not documented in a follow-up note.
Conclusions
The model of a combined allergy/pulmonology clinic can be particularly efficacious in the treatment of severe asthma, as it reduces the need for multiple appointments with different specialties, reduces wait time before starting a biologic agent, and offers the perspective of 2 specialists. This kind of model could be an example to many clinics in the VA. With a rapid increase in telemedicine due to the COVID-19 pandemic, multiple physicians consulting simultaneously is becoming a more feasible possibility across multiple specialties. As the use of biologics becomes more widespread, a combined clinic design is an efficient and promising method to improve severe asthma management.
This preliminary data continue to support previous research that shows biologic agents have led to better clinical outcomes through the reduction of asthma exacerbations, hospitalizations, and improved PFTs. While this initial data set highlights the results for 15 patients, there are 86 patients currently enrolled in this clinic. We are collecting additional data to publish more comprehensive results.
1. Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015;16(1):45-56. doi:10.1038/ni.3049
2. Moore WC, Bleecker ER, Curran-Everett D, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119(2):405-413. doi:10.1016/j.jaci.2006.11.639
3. Barnes PJ, Jonsson B, Klim JB. The costs of asthma. Eur Respir J. 1996;9(4):636-642. doi:10.1183/09031936.96.09040636
4. Bourdin A, Charriot J, Boissin C, et al. Will the asthma revolution fostered by biologics also benefit adult ICU patients?. Allergy. 2021;76(8):2395-2406. doi:10.1111/all.14688
5. Lloyd A, Price D, Brown R. The impact of asthma exacerbations on health-related quality of life in moderate to severe asthma patients in the UK. Prim Care Respir J. 2007;16(1):22-27. doi:10.3132/pcrj.2007.00002
6. Eisner MD, Yelin EH, Katz PP, Lactao G, Iribarren C, Blanc PD. Risk factors for work disability in severe adult asthma. Am J Med. 2006;119(10):884-891. doi:10.1016/j.amjmed.2006.01.016
7. Nurmagambetov T, Kuwahara R, Garbe P. The Economic Burden of Asthma in the United States, 2008-2013. Ann Am Thorac Soc. 2018;15(3):348-356. doi:10.1513/AnnalsATS.201703-259OC
8. McGregor MC, Krings JG, Nair P, Castro M. Role of biologics in asthma. Am J Respir Crit Care Med. 2019;199(4):433-445. doi:10.1164/rccm.201810-1944CI
9. Bice JB, Leechawengwongs E, Montanaro A. Biologic targeted therapy in allergic asthma. Ann Allergy Asthma Immunol. 2014;112(2):108-115. doi:10.1016/j.anai.2013.12.013
10. Darveaux J, Busse WW. Biologics in asthma--the next step toward personalized treatment. J Allergy Clin Immunol Pract. 2015;3(2):152-161. doi:10.1016/j.jaip.2014.09.014
11. Inselman JW, Jeffery MM, Maddux JT, Shah ND, Rank MA. Trends and disparities in asthma biologic use in the United States. J Allergy Clin Immunol Pract. 2020;8(2):549-554.e1. doi:10.1016/j.jaip.2019.08.024
12. Gelhorn HL, Balantac Z, Ambrose CS, Chung YN, Stone B. Patient and physician preferences for attributes of biologic medications for severe asthma. Patient Prefer Adherence. 2019;13:1253-1268. Published 2019 Jul 25. doi:10.2147/PPA.S198953
13. Centers for Disease Control and Prevention, National Center for Environmental Health. 2019 National Health Interview Survey (NHIS) data. Accessed December 6, 2022. https://www.cdc.gov/asthma/nhis/2019/data.htm
14. Zelaya CE BP, Moy E. Crude and age-adjusted percent distribution of respondent-assessed health status among adults aged 20 and over, by veteran status and other selected characteristics: United States, 2015-2018. National Center for Health Statistic. Updated June 19, 2020. Accessed December 12, 2022. https://www.cdc.gov/nchs/nhis/veterans_health_statistics/tables.htm
15. Szema AM, Peters MC, Weissinger KM, Gagliano CA, Chen JJ. New-onset asthma among soldiers serving in Iraq and Afghanistan. Allergy Asthma Proc. 2010;31(5):67-71. doi:10.2500/aap.2010.31.3383
16. Yi SW, Hong JS, Ohrr H, Yi JJ. Agent Orange exposure and disease prevalence in Korean Vietnam veterans: the Korean veterans health study. Environ Res. 2014;133:56-65. doi:10.1016/j.envres.2014.04.027
17. Huetsch JC, Uman JE, Udris EM, Au DH. Predictors of adherence to inhaled medications among veterans with COPD. J Gen Intern Med. 2012;27(11):1506-1512. doi:10.1007/s11606-012-2130-5
18. Mundell L, Lindemann R, Douglas J. Monitoring long-term oral corticosteroids. BMJ Open Qual. 2017;6(2):e000209. Published 2017 Nov 8. doi:10.1136/bmjoq-2017-000209
19. Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2005;20(8):1487-1486. doi:10.1359/jbmr.2005.20.8.1486
20. Warrington TP, Bostwick JM. Psychiatric adverse effects of corticosteroids. Mayo Clin Proc. 2006;81(10):1361-1367. doi:10.4065/81.10.1361
21. Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care. 2004;27(suppl 2):B10-B21. doi:10.2337/diacare.27.suppl_2.b10
22. Acharya T, Tringali S, Singh M, Huang J. Resistant hypertension and associated comorbidities in a Veterans Affairs population. J Clin Hypertens (Greenwich). 2014;16(10):741-745. doi:10.1111/jch.12410
23. Dupixent (dupilumab). Prescribing information. Sanofi and Regeneron Pharmaceuticals; 2022. Accessed December 6, 2022. https://www.dupixenthcp.com/asthma/efficacy/safety-data
24. Li L, Wang Z, Cui L, Xu Y, Guan K, Zhao B. Anaphylactic risk related to omalizumab, benralizumab, reslizumab, mepolizumab, and dupilumab. Clin Transl Allergy. 2021;11(4):e12038. Published 2021 Jun 3. doi:10.1002/clt2.12038
1. Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015;16(1):45-56. doi:10.1038/ni.3049
2. Moore WC, Bleecker ER, Curran-Everett D, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119(2):405-413. doi:10.1016/j.jaci.2006.11.639
3. Barnes PJ, Jonsson B, Klim JB. The costs of asthma. Eur Respir J. 1996;9(4):636-642. doi:10.1183/09031936.96.09040636
4. Bourdin A, Charriot J, Boissin C, et al. Will the asthma revolution fostered by biologics also benefit adult ICU patients?. Allergy. 2021;76(8):2395-2406. doi:10.1111/all.14688
5. Lloyd A, Price D, Brown R. The impact of asthma exacerbations on health-related quality of life in moderate to severe asthma patients in the UK. Prim Care Respir J. 2007;16(1):22-27. doi:10.3132/pcrj.2007.00002
6. Eisner MD, Yelin EH, Katz PP, Lactao G, Iribarren C, Blanc PD. Risk factors for work disability in severe adult asthma. Am J Med. 2006;119(10):884-891. doi:10.1016/j.amjmed.2006.01.016
7. Nurmagambetov T, Kuwahara R, Garbe P. The Economic Burden of Asthma in the United States, 2008-2013. Ann Am Thorac Soc. 2018;15(3):348-356. doi:10.1513/AnnalsATS.201703-259OC
8. McGregor MC, Krings JG, Nair P, Castro M. Role of biologics in asthma. Am J Respir Crit Care Med. 2019;199(4):433-445. doi:10.1164/rccm.201810-1944CI
9. Bice JB, Leechawengwongs E, Montanaro A. Biologic targeted therapy in allergic asthma. Ann Allergy Asthma Immunol. 2014;112(2):108-115. doi:10.1016/j.anai.2013.12.013
10. Darveaux J, Busse WW. Biologics in asthma--the next step toward personalized treatment. J Allergy Clin Immunol Pract. 2015;3(2):152-161. doi:10.1016/j.jaip.2014.09.014
11. Inselman JW, Jeffery MM, Maddux JT, Shah ND, Rank MA. Trends and disparities in asthma biologic use in the United States. J Allergy Clin Immunol Pract. 2020;8(2):549-554.e1. doi:10.1016/j.jaip.2019.08.024
12. Gelhorn HL, Balantac Z, Ambrose CS, Chung YN, Stone B. Patient and physician preferences for attributes of biologic medications for severe asthma. Patient Prefer Adherence. 2019;13:1253-1268. Published 2019 Jul 25. doi:10.2147/PPA.S198953
13. Centers for Disease Control and Prevention, National Center for Environmental Health. 2019 National Health Interview Survey (NHIS) data. Accessed December 6, 2022. https://www.cdc.gov/asthma/nhis/2019/data.htm
14. Zelaya CE BP, Moy E. Crude and age-adjusted percent distribution of respondent-assessed health status among adults aged 20 and over, by veteran status and other selected characteristics: United States, 2015-2018. National Center for Health Statistic. Updated June 19, 2020. Accessed December 12, 2022. https://www.cdc.gov/nchs/nhis/veterans_health_statistics/tables.htm
15. Szema AM, Peters MC, Weissinger KM, Gagliano CA, Chen JJ. New-onset asthma among soldiers serving in Iraq and Afghanistan. Allergy Asthma Proc. 2010;31(5):67-71. doi:10.2500/aap.2010.31.3383
16. Yi SW, Hong JS, Ohrr H, Yi JJ. Agent Orange exposure and disease prevalence in Korean Vietnam veterans: the Korean veterans health study. Environ Res. 2014;133:56-65. doi:10.1016/j.envres.2014.04.027
17. Huetsch JC, Uman JE, Udris EM, Au DH. Predictors of adherence to inhaled medications among veterans with COPD. J Gen Intern Med. 2012;27(11):1506-1512. doi:10.1007/s11606-012-2130-5
18. Mundell L, Lindemann R, Douglas J. Monitoring long-term oral corticosteroids. BMJ Open Qual. 2017;6(2):e000209. Published 2017 Nov 8. doi:10.1136/bmjoq-2017-000209
19. Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2005;20(8):1487-1486. doi:10.1359/jbmr.2005.20.8.1486
20. Warrington TP, Bostwick JM. Psychiatric adverse effects of corticosteroids. Mayo Clin Proc. 2006;81(10):1361-1367. doi:10.4065/81.10.1361
21. Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care. 2004;27(suppl 2):B10-B21. doi:10.2337/diacare.27.suppl_2.b10
22. Acharya T, Tringali S, Singh M, Huang J. Resistant hypertension and associated comorbidities in a Veterans Affairs population. J Clin Hypertens (Greenwich). 2014;16(10):741-745. doi:10.1111/jch.12410
23. Dupixent (dupilumab). Prescribing information. Sanofi and Regeneron Pharmaceuticals; 2022. Accessed December 6, 2022. https://www.dupixenthcp.com/asthma/efficacy/safety-data
24. Li L, Wang Z, Cui L, Xu Y, Guan K, Zhao B. Anaphylactic risk related to omalizumab, benralizumab, reslizumab, mepolizumab, and dupilumab. Clin Transl Allergy. 2021;11(4):e12038. Published 2021 Jun 3. doi:10.1002/clt2.12038
Kikuchi-Fujimoto Disease: A Case Report of Fever and Lymphadenopathy in a Young White Man
Kikuchi-Fujimoto disease (KFD) is a rare, usually self-limited cause of cervical lymphadenitis that is more prevalent among patients of Asian descent.1 The pathogenesis of KFD remains unknown. Clinically, KFD may mimic malignant lymphoproliferative disorders, autoimmune diseases such as systemic lupus erythematosus (SLE) lymphadenitis, and infectious conditions such as HIV and tuberculous lymphadenitis. The most common presentation of KFD involves fever and rapidly evolving cervical lymphadenopathy.2,3 Due to its rarity, KFD is not always considered in the differential diagnosis for fever with tender lymphadenopathy, and up to one-third of cases are initially misdiagnosed.2
Definitive diagnosis requires lymph node biopsy. It is critical to achieving a timely diagnosis of KFD to exclude more serious conditions, initiate appropriate treatment, and minimize undue stress for patients. We describe a case of KFD in a patient who was met with delays in obtaining a definitive diagnosis for his symptoms.
Case Presentation
A 27-year-old previously healthy White man presented to the emergency department with subacute, progressive right-sided neck pain and swelling. In the week leading up to presentation, he also noted intermittent fevers, night sweats, and abdominal pain. His symptoms were unrelieved with acetaminophen and aspirin. He reported no sick contacts, recent travel, or animal exposures. He had no known history of autoimmune disease, malignancy, or immunocompromising conditions. Vital signs at the time of presentation were notable for a temperature of 39.0 °C. On examination, he had several firm, mobile, and exquisitely tender lymph nodes in the right upper anterior cervical chain. Abdominal examination was notable for left upper quadrant tenderness with palpable splenomegaly. Due to initial concern that his symptoms represented bacterial lymphadenitis, he was started on broad-spectrum antibiotics and admitted to the hospital for an expedited infectious workup.
Initial laboratory studies were notable for a white blood cell count of 3.7 × 109/L with 57.5% neutrophils and 27.0% lymphocytes on differential.
Computed tomography (CT) of the neck revealed multiple heterogeneously enlarged lymph nodes along the right anterior cervical chain with necrotic changes (Figure 1).
A core needle biopsy of a right-sided cervical lymph node was initially pursued, demonstrating necrotic tissue with minimal residual lymphoid tissue and no definitive evidence of lymphoma. Because these results were nondiagnostic, an excisional biopsy of the right-sided cervical lymph node was pursued 10 days later. Due to the stress of his 2-week hospitalization without a unifying diagnosis, the patient then elected to discharge home with close outpatient follow-up while awaiting his biopsy results. Antibiotics were not continued at the time of discharge as our broad infectious workup failed to yield a causative organism.
Two weeks postdischarge, the patient’s excisional lymph node biopsy returned demonstrating lymphohistiocytic inflammation with plasmacytoid dendritic cells, areas of necrosis, and scattered karyorrhectic nuclear debris, consistent with a diagnosis of KFD (Figure 2).
After 4 months of hydroxychloroquine therapy, the patient’s KFD symptoms resolved, prompting his dose to be reduced and eventually tapered. Repeat testing of his ANA and anti-dsDNA were performed at 1 and 6 months posthospitalization and returned within normal limits. A repeat PET-CT was performed 6 months posthospitalization showing resolution of his hypermetabolic right neck and right supraclavicular lymphadenopathy as well as his splenomegaly. It has now been more than a year since the patient’s initial presentation to the hospital, and he remains symptom-free and off prednisone and hydroxychloroquine.
Discussion
KFD is a rare cause of cervical lymphadenitis that was first described in 1972. Although cases have been reported worldwide, it is seen with higher prevalence in Asian countries. KFD was previously thought to have a female predominance, but recent reviews suggest a female to male ratio close to 1:1.1 The pathogenesis of KFD remains unknown, though some studies have suggested Epstein-Barr virus infection as a potential trigger.4,5 Human herpesvirus (HHV) 6, HHV 7, HHV 8, HSV, HIV, and parvovirus B19 also have been implicated as potential triggers, though no causative relationship has been established.2,5,6 Autoimmunity may also play a role in the pathogenesis of KFD given its histopathologic overlap with SLE lymphadenitis.1,7
The most common presenting symptoms of KFD include fever and tender cervical lymphadenopathy. Many patients also experience constitutional symptoms such as weight loss, night sweats, and fatigue.2 KFD is characterized by enlarged cervical lymph nodes, typically > 2 cm in diameter.3 Cutaneous manifestations of KFD are common and may manifest as nonspecific papules, plaques, nodules, or facial malar erythema.1,2 Case reports also have described KFD manifesting with ataxia, arthritis, parotitis, or ocular pathologies such as conjunctivitis and uveitis.1,2,8,9 Hepatosplenomegaly is a relatively rare manifestation of KFD seen in approximately 3% of cases.10 When present, hepatosplenomegaly may make the diagnosis of KFD especially difficult to distinguish from lymphoproliferative disorders such as lymphoma. Laboratory findings in KFD are nonspecific and include elevated levels of lactate dehydrogenase, erythrocyte sedimentation rate, C-reactive protein, and liver enzymes.3 Both lymphocytosis and lymphopenia have been described.3Definitive diagnosis of KFD is achieved through lymph node biopsy and histologic examination. Histopathologic findings of KFD include areas of coagulative necrosis and histiocytic proliferation within the cortical and paracortical regions of the lymph node. Scattered nuclear debris also may be seen, though this histologic finding also is seen with lymphoma. The absence of neutrophils is characteristic of KFD.2 In our patient, a core needle biopsy was initially pursued but returned nondiagnostic. A PET-CT also was obtained, though KFD may mimic lymphoma on PET as was seen in this patient’s case as well as in prior case reports.11 An excisional lymph node biopsy was ultimately performed and secured the diagnosis of KFD.
Although ultrasound-guided core needle biopsy was unable to yield the diagnosis for our patient, its diagnostic accuracy is still superior to that of fine needle aspiration and is therefore suggested as the primary diagnostic modality when KFD is suspected.12 Core needle biopsy also is less invasive, less time consuming, and perhaps more cost-effective than an open excisional biopsy, which often requires the use of an operating room and monitored anesthesia care.12 Understandably, our patient experienced significant stress while awaiting a final diagnosis. Whenever possible, lymph node biopsy should be prioritized over other diagnostic modalities to achieve a timely and definitive diagnosis.
KFD has no established treatment guidelines. Supportive care with antipyretics and analgesics is the most common initial approach, as KFD is typically a self-limited disease that resolves in 1 to 4 months.2 Patients with severe, persistent symptoms have been successfully treated with corticosteroids and hydroxychloroquine, with monotherapy typically trialed before concomitant use.2,13 After 2 courses of prednisone, our patient was prescribed single-agent hydroxychloroquine due to his recurrent symptoms and debilitating AEs from the steroids. Other case reports have described hydroxychloroquine as a treatment option when steroids fail to provide symptom relief or when there are recurrences of KFD.14-19 Retinopathy can occur as a result of long-term hydroxychloroquine use. As such, patients anticipated to require long-term hydroxychloroquine therapy should receive a baseline eye examination within months of drug initiation and repeat examination after 5 years of therapy.20
After symptom resolution, continued follow-up with a health care professional is recommended due to the potential for KFD recurrence or the development of a new autoimmune disease. The rate of KFD recurrence was previously described as 3%, but a more recent review found the rate of recurrence to be approximately 15% at > 6 months follow-up.1,3 Recurrence is often described during or shortly after the tapering of steroids.13,16,21,22 Recurrent KFD can be diagnosed with repeat lymph node biopsy, which also serves to exclude other disease processes.13,16 However, recurrence also has been diagnosed clinically based on the patient’s symptoms and laboratory investigations.21,22Continued surveillance of patients with KFD is also necessary to monitor for the development of new autoimmune diseases, especially SLE. SLE lymphadenitis shares many histopathologic characteristics with KFD. Case reports have described the development of SLE in patients with a history of KFD.2,7 Other autoimmune conditions described in patients with prior KFD include Sjögren syndrome, Hashimoto thyroiditis, Graves disease, mixed connective tissue disease, and antiphospholipid syndrome.3,23 Among patients with KFD, female sex, painful adenopathy, and cytopenias are significantly associated with the later development of autoimmune disease.23
Patient Perspective
This began for me in September 2020 out of the blue. I woke up one day with a random lymph node in my neck but otherwise felt completely healthy, and within 2 to 3 weeks I had never been more sick in my entire life. It came with bouts of fevers, neck pain from the swelling, stomach pain (I later learned an enlarged spleen was the source), terrible night sweats, violent chills where the shaking was uncontrollable for hours at a time, loss of appetite, and countless other symptoms that have come and gone over the past year.
It did take a little while to get a diagnosis, but I understand the autoimmune field is tricky. For about 4 to 5 weeks, I was told to prepare for a lymphoma diagnosis. I ended up doing 2 rounds of prednisone, one for 3 weeks at the end of 2020 and one for 2 months from March to May. The initial round helped quite a bit, but the second round did not have any effect on the lingering symptoms. In my opinion, prednisone is miserable to be on long term and I do not recommend it. The daily AEs that came with it included mood swings, insomnia, weight gain, and more. I have been on hydroxychloroquine now for almost 2 months and although it has some AEs of its own, it is nowhere near as rough as the prednisone and has helped manage my remaining symptoms quite a bit.
This certainly has not been a fun experience, but I was under great care during my time in the hospital and continue to be under good care through the rheumatology clinic. The one thing that could have made a huge difference would have been the issues involved in getting my surgery scheduled while I was still inpatient, which took quite a while. The pain during that time was so intense and unlike anything I have ever experienced before, and it was only the surgery that finally brought me some relief. To paint you a picture, I have broken bones, split my leg open, and have roughly 40 to 50 hours of tattoo work on me, and I have never experienced the level of pain like I felt in my neck and stomach. I remember feeling like someone had wound up and hit me with a baseball bat. The surgery brought me immense relief and if it had occurred when it was originally supposed to, I would have been spared 3 or so days of this type of pain.
It has been almost 10 months since my surgery and diagnosis, and life has mostly returned to normal for me. I am still on long-term medication as I mentioned, and I still deal with fatigue, spleen pain, and several other symptoms, but it is much more under control these days. I feel very fortunate to have been under and continue to be under such great care.
Conclusions
This case report highlights the importance of recognizing KFD as a rare but possible cause of fever and necrotizing cervical lymphadenopathy. KFD often mimics malignant lymphoproliferative disorders, autoimmune diseases such as SLE lymphadenitis, and infectious conditions such as HIV and tuberculous lymphadenitis. While KFD is seen with higher prevalence in Asian countries and was previously thought to be more predominant in females, the diagnosis should still be considered irrespective of geographic location or patient sex. Lymph node biopsy is the preferred diagnostic approach for patients with suspected KFD. Treatment is typically supportive but may consist of glucocorticoids in severe cases. Hydroxychloroquine may be used in refractory cases or as a steroid-sparing regimen when steroid AEs are poorly tolerated. Long-term follow-up is critical for patients with KFD to monitor for both disease recurrence and the development of autoimmune disease, especially SLE.
Acknowledgments
The authors thank Dr. Jacob Pilley for his detailed review of the patient’s pathology results. The authors also extend their gratitude to the patient, who deepened our understanding of this condition and what it is like to live with it.
1. Bosch X, Guilabert A, Miquel R, Campo E. Enigmatic Kikuchi-Fujimoto disease: a comprehensive review. Am J Clin Pathol. 2004;122(1):141-152. doi:10.1309/YF08-1L4T-KYWV-YVPQ
2. Deaver D, Horna P, Cualing H, Sokol L. Pathogenesis, diagnosis, and management of Kikuchi-Fujimoto disease. Cancer Control. 2014;21(4):313-321. doi:10.1177/107327481402100407
3. Cheng CY, Sheng WH, Lo YC, Chung CS, Chen YC, Chang SC. Clinical presentations, laboratory results and outcomes of patients with Kikuchi’s disease: emphasis on the association between recurrent Kikuchi’s disease and autoimmune diseases. J Microbiol Immunol Infect. 2010;43(5):366-371. doi:10.1016/S1684-1182(10)60058-8
4. Stéphan JL, Jeannoël P, Chanoz J, Gentil-Përret A. Epstein-Barr virus-associated Kikuchi disease in two children. J Pediatr Hematol Oncol. 2001;23(4):240-243. doi:10.1097/00043426-200105000-00012
5. Chiu CF, Chow KC, Lin TY, Tsai MH, Shih CM, Chen LM. Virus infection in patients with histiocytic necrotizing lymphadenitis in Taiwan. Detection of Epstein-Barr virus, type I human T-cell lymphotropic virus, and parvovirus B19. Am J Clin Pathol. 2000;113(6):774-781. doi:10.1309/1A6Y-YCKP-5AVF-QTYR
6. Rosado FG, Tang YW, Hasserjian RP, McClain CM, Wang B, Mosse CA. Kikuchi-Fujimoto lymphadenitis: role of parvovirus B-19, Epstein-Barr virus, human herpesvirus 6, and human herpesvirus 8. Hum Pathol. 2013;44(2):255-259. doi:10.1016/j.humpath.2012.05.016
7. Gordon JK, Magro C, Lu T, et al. Overlap between systemic lupus erythematosus and Kikuchi Fujimoto disease: a clinical pathology conference held by the Department of Rheumatology at Hospital for Special Surgery. HSS J. 2009;5(2):169-177. doi:10.1007/s11420-009-9123-x
8. Lo KB, Papazoglou A, Chua L, Candelario N. Case Report: Kikuchi: The great mimicker. F1000Res. 2018;7:520. Published 2018 Apr 30. doi:10.12688/f1000research.14758.1
9. Galor A, Georgy M, Leder HA, Dunn JP, Peters GB 3rd. Papillary conjunctivitis associated with Kikuchi disease. Cornea. 2008;27(8):944-946. doi:10.1097/ICO.0b013e31816bf488
10. Kucukardali Y, Solmazgul E, Kunter E, Oncul O, Yildirim S, Kaplan M. Kikuchi-Fujimoto disease: analysis of 244 cases. Clin Rheumatol. 2007;26(1):50-54. doi:10.1007/s10067-006-0230-5
11. Lee DH, Lee JH, Shim EJ, et al. Disseminated Kikuchi-Fujimoto disease mimicking malignant lymphoma on positron emission tomography in a child. J Pediatr Hematol Oncol. 2009;31(9):687-689. doi:10.1097/MPH.0b013e31819a5d77
12. Park SG, Koo HR, Jang K, et al. Efficacy of ultrasound-guided needle biopsy in the diagnosis of Kikuchi-Fujimoto disease. Laryngoscope. 2021;131(5):E1519-E1523. doi:10.1002/lary.29160
13. Honda F, Tsuboi H, Toko H, et al. Recurrent Kikuchi-Fujimoto disease successfully treated by the concomitant use of hydroxychloroquine and corticosteroids. Intern Med. 2017;56(24):3373-3377. doi:10.2169/internalmedicine.9205-17
14. Rezayat T, Carroll MB, Ramsey BC, Smith A. A case of relapsing Kikuchi-Fujimoto disease. Case Rep Otolaryngol. 2013;2013:364795. doi:10.1155/2013/364795
15. Rezai K, Kuchipudi S, Chundi V, Ariga R, Loew J, Sha BE. Kikuchi-Fujimoto disease: hydroxychloroquine as a treatment. Clin Infect Dis. 2004;39(12):e124-e126. doi:10.1086/426144
16. Hyun M, So IT, Kim HA, Jung H, Ryu SY. Recurrent Kikuchi’s disease treated by hydroxychloroquine. Infect Chemother. 2016;48(2):127-131. doi:10.3947/ic.2016.48.2.127
17. Lin YC, Huang HH, Nong BR, et al. Pediatric Kikuchi-Fujimoto disease: A clinicopathologic study and the therapeutic effects of hydroxychloroquine. J Microbiol Immunol Infect. 2019;52(3):395-401. doi:10.1016/j.jmii.2017.08.023
18. Lin DY, Villegas MS, Tan PL, Wang S, Shek LP. Severe Kikuchi’s disease responsive to immune modulation. Singapore Med J. 2010;51(1):e18-e21.
19. Quintás-Cardama A, Fraga M, Cozzi SN, Caparrini A, Maceiras F, Forteza J. Fatal Kikuchi-Fujimoto disease: the lupus connection. Ann Hematol. 2003;82(3):186-188. doi:10.1007/s00277-003-0611-7
20. American Academy of Ophthalmology. ACR, AAD, RDS, and AAO 2020 Joint Statement on Hydroxychloroquine Use with Respect to Retinal Toxicity. Updated February 2021. Accessed November 28, 2022. https://www.aao.org/clinical-statement/acr-aad-rds-aao-2020-joint-statement-on-hydroxychl-2
21. Gerwig U, Weidmann RG, Lindner G. Relapsing Kikuchi-Fujimoto disease requiring prolonged steroid therapy. Case Rep Emerg Med. 2019;2019:6405687. Published 2019 Mar 7. doi:10.1155/2019/6405687
22. Faheem B, Kumar V, Ashkar H, Komal F, Sultana Y. Recurrent Kikuchi-Fujimoto disease masquerading as lymphoma successfully treated by anakinra. Cureus. 2020;12(11):e11655. Published 2020 Nov 23. doi:10.7759/cureus.11655
23. Sopeña B, Rivera A, Vázquez-Triñanes C, et al. Autoimmune manifestations of Kikuchi disease. Semin Arthritis Rheum. 2012;41(6):900-906. doi:10.1016/j.semarthrit.2011.11.001
Kikuchi-Fujimoto disease (KFD) is a rare, usually self-limited cause of cervical lymphadenitis that is more prevalent among patients of Asian descent.1 The pathogenesis of KFD remains unknown. Clinically, KFD may mimic malignant lymphoproliferative disorders, autoimmune diseases such as systemic lupus erythematosus (SLE) lymphadenitis, and infectious conditions such as HIV and tuberculous lymphadenitis. The most common presentation of KFD involves fever and rapidly evolving cervical lymphadenopathy.2,3 Due to its rarity, KFD is not always considered in the differential diagnosis for fever with tender lymphadenopathy, and up to one-third of cases are initially misdiagnosed.2
Definitive diagnosis requires lymph node biopsy. It is critical to achieving a timely diagnosis of KFD to exclude more serious conditions, initiate appropriate treatment, and minimize undue stress for patients. We describe a case of KFD in a patient who was met with delays in obtaining a definitive diagnosis for his symptoms.
Case Presentation
A 27-year-old previously healthy White man presented to the emergency department with subacute, progressive right-sided neck pain and swelling. In the week leading up to presentation, he also noted intermittent fevers, night sweats, and abdominal pain. His symptoms were unrelieved with acetaminophen and aspirin. He reported no sick contacts, recent travel, or animal exposures. He had no known history of autoimmune disease, malignancy, or immunocompromising conditions. Vital signs at the time of presentation were notable for a temperature of 39.0 °C. On examination, he had several firm, mobile, and exquisitely tender lymph nodes in the right upper anterior cervical chain. Abdominal examination was notable for left upper quadrant tenderness with palpable splenomegaly. Due to initial concern that his symptoms represented bacterial lymphadenitis, he was started on broad-spectrum antibiotics and admitted to the hospital for an expedited infectious workup.
Initial laboratory studies were notable for a white blood cell count of 3.7 × 109/L with 57.5% neutrophils and 27.0% lymphocytes on differential.
Computed tomography (CT) of the neck revealed multiple heterogeneously enlarged lymph nodes along the right anterior cervical chain with necrotic changes (Figure 1).
A core needle biopsy of a right-sided cervical lymph node was initially pursued, demonstrating necrotic tissue with minimal residual lymphoid tissue and no definitive evidence of lymphoma. Because these results were nondiagnostic, an excisional biopsy of the right-sided cervical lymph node was pursued 10 days later. Due to the stress of his 2-week hospitalization without a unifying diagnosis, the patient then elected to discharge home with close outpatient follow-up while awaiting his biopsy results. Antibiotics were not continued at the time of discharge as our broad infectious workup failed to yield a causative organism.
Two weeks postdischarge, the patient’s excisional lymph node biopsy returned demonstrating lymphohistiocytic inflammation with plasmacytoid dendritic cells, areas of necrosis, and scattered karyorrhectic nuclear debris, consistent with a diagnosis of KFD (Figure 2).
After 4 months of hydroxychloroquine therapy, the patient’s KFD symptoms resolved, prompting his dose to be reduced and eventually tapered. Repeat testing of his ANA and anti-dsDNA were performed at 1 and 6 months posthospitalization and returned within normal limits. A repeat PET-CT was performed 6 months posthospitalization showing resolution of his hypermetabolic right neck and right supraclavicular lymphadenopathy as well as his splenomegaly. It has now been more than a year since the patient’s initial presentation to the hospital, and he remains symptom-free and off prednisone and hydroxychloroquine.
Discussion
KFD is a rare cause of cervical lymphadenitis that was first described in 1972. Although cases have been reported worldwide, it is seen with higher prevalence in Asian countries. KFD was previously thought to have a female predominance, but recent reviews suggest a female to male ratio close to 1:1.1 The pathogenesis of KFD remains unknown, though some studies have suggested Epstein-Barr virus infection as a potential trigger.4,5 Human herpesvirus (HHV) 6, HHV 7, HHV 8, HSV, HIV, and parvovirus B19 also have been implicated as potential triggers, though no causative relationship has been established.2,5,6 Autoimmunity may also play a role in the pathogenesis of KFD given its histopathologic overlap with SLE lymphadenitis.1,7
The most common presenting symptoms of KFD include fever and tender cervical lymphadenopathy. Many patients also experience constitutional symptoms such as weight loss, night sweats, and fatigue.2 KFD is characterized by enlarged cervical lymph nodes, typically > 2 cm in diameter.3 Cutaneous manifestations of KFD are common and may manifest as nonspecific papules, plaques, nodules, or facial malar erythema.1,2 Case reports also have described KFD manifesting with ataxia, arthritis, parotitis, or ocular pathologies such as conjunctivitis and uveitis.1,2,8,9 Hepatosplenomegaly is a relatively rare manifestation of KFD seen in approximately 3% of cases.10 When present, hepatosplenomegaly may make the diagnosis of KFD especially difficult to distinguish from lymphoproliferative disorders such as lymphoma. Laboratory findings in KFD are nonspecific and include elevated levels of lactate dehydrogenase, erythrocyte sedimentation rate, C-reactive protein, and liver enzymes.3 Both lymphocytosis and lymphopenia have been described.3Definitive diagnosis of KFD is achieved through lymph node biopsy and histologic examination. Histopathologic findings of KFD include areas of coagulative necrosis and histiocytic proliferation within the cortical and paracortical regions of the lymph node. Scattered nuclear debris also may be seen, though this histologic finding also is seen with lymphoma. The absence of neutrophils is characteristic of KFD.2 In our patient, a core needle biopsy was initially pursued but returned nondiagnostic. A PET-CT also was obtained, though KFD may mimic lymphoma on PET as was seen in this patient’s case as well as in prior case reports.11 An excisional lymph node biopsy was ultimately performed and secured the diagnosis of KFD.
Although ultrasound-guided core needle biopsy was unable to yield the diagnosis for our patient, its diagnostic accuracy is still superior to that of fine needle aspiration and is therefore suggested as the primary diagnostic modality when KFD is suspected.12 Core needle biopsy also is less invasive, less time consuming, and perhaps more cost-effective than an open excisional biopsy, which often requires the use of an operating room and monitored anesthesia care.12 Understandably, our patient experienced significant stress while awaiting a final diagnosis. Whenever possible, lymph node biopsy should be prioritized over other diagnostic modalities to achieve a timely and definitive diagnosis.
KFD has no established treatment guidelines. Supportive care with antipyretics and analgesics is the most common initial approach, as KFD is typically a self-limited disease that resolves in 1 to 4 months.2 Patients with severe, persistent symptoms have been successfully treated with corticosteroids and hydroxychloroquine, with monotherapy typically trialed before concomitant use.2,13 After 2 courses of prednisone, our patient was prescribed single-agent hydroxychloroquine due to his recurrent symptoms and debilitating AEs from the steroids. Other case reports have described hydroxychloroquine as a treatment option when steroids fail to provide symptom relief or when there are recurrences of KFD.14-19 Retinopathy can occur as a result of long-term hydroxychloroquine use. As such, patients anticipated to require long-term hydroxychloroquine therapy should receive a baseline eye examination within months of drug initiation and repeat examination after 5 years of therapy.20
After symptom resolution, continued follow-up with a health care professional is recommended due to the potential for KFD recurrence or the development of a new autoimmune disease. The rate of KFD recurrence was previously described as 3%, but a more recent review found the rate of recurrence to be approximately 15% at > 6 months follow-up.1,3 Recurrence is often described during or shortly after the tapering of steroids.13,16,21,22 Recurrent KFD can be diagnosed with repeat lymph node biopsy, which also serves to exclude other disease processes.13,16 However, recurrence also has been diagnosed clinically based on the patient’s symptoms and laboratory investigations.21,22Continued surveillance of patients with KFD is also necessary to monitor for the development of new autoimmune diseases, especially SLE. SLE lymphadenitis shares many histopathologic characteristics with KFD. Case reports have described the development of SLE in patients with a history of KFD.2,7 Other autoimmune conditions described in patients with prior KFD include Sjögren syndrome, Hashimoto thyroiditis, Graves disease, mixed connective tissue disease, and antiphospholipid syndrome.3,23 Among patients with KFD, female sex, painful adenopathy, and cytopenias are significantly associated with the later development of autoimmune disease.23
Patient Perspective
This began for me in September 2020 out of the blue. I woke up one day with a random lymph node in my neck but otherwise felt completely healthy, and within 2 to 3 weeks I had never been more sick in my entire life. It came with bouts of fevers, neck pain from the swelling, stomach pain (I later learned an enlarged spleen was the source), terrible night sweats, violent chills where the shaking was uncontrollable for hours at a time, loss of appetite, and countless other symptoms that have come and gone over the past year.
It did take a little while to get a diagnosis, but I understand the autoimmune field is tricky. For about 4 to 5 weeks, I was told to prepare for a lymphoma diagnosis. I ended up doing 2 rounds of prednisone, one for 3 weeks at the end of 2020 and one for 2 months from March to May. The initial round helped quite a bit, but the second round did not have any effect on the lingering symptoms. In my opinion, prednisone is miserable to be on long term and I do not recommend it. The daily AEs that came with it included mood swings, insomnia, weight gain, and more. I have been on hydroxychloroquine now for almost 2 months and although it has some AEs of its own, it is nowhere near as rough as the prednisone and has helped manage my remaining symptoms quite a bit.
This certainly has not been a fun experience, but I was under great care during my time in the hospital and continue to be under good care through the rheumatology clinic. The one thing that could have made a huge difference would have been the issues involved in getting my surgery scheduled while I was still inpatient, which took quite a while. The pain during that time was so intense and unlike anything I have ever experienced before, and it was only the surgery that finally brought me some relief. To paint you a picture, I have broken bones, split my leg open, and have roughly 40 to 50 hours of tattoo work on me, and I have never experienced the level of pain like I felt in my neck and stomach. I remember feeling like someone had wound up and hit me with a baseball bat. The surgery brought me immense relief and if it had occurred when it was originally supposed to, I would have been spared 3 or so days of this type of pain.
It has been almost 10 months since my surgery and diagnosis, and life has mostly returned to normal for me. I am still on long-term medication as I mentioned, and I still deal with fatigue, spleen pain, and several other symptoms, but it is much more under control these days. I feel very fortunate to have been under and continue to be under such great care.
Conclusions
This case report highlights the importance of recognizing KFD as a rare but possible cause of fever and necrotizing cervical lymphadenopathy. KFD often mimics malignant lymphoproliferative disorders, autoimmune diseases such as SLE lymphadenitis, and infectious conditions such as HIV and tuberculous lymphadenitis. While KFD is seen with higher prevalence in Asian countries and was previously thought to be more predominant in females, the diagnosis should still be considered irrespective of geographic location or patient sex. Lymph node biopsy is the preferred diagnostic approach for patients with suspected KFD. Treatment is typically supportive but may consist of glucocorticoids in severe cases. Hydroxychloroquine may be used in refractory cases or as a steroid-sparing regimen when steroid AEs are poorly tolerated. Long-term follow-up is critical for patients with KFD to monitor for both disease recurrence and the development of autoimmune disease, especially SLE.
Acknowledgments
The authors thank Dr. Jacob Pilley for his detailed review of the patient’s pathology results. The authors also extend their gratitude to the patient, who deepened our understanding of this condition and what it is like to live with it.
Kikuchi-Fujimoto disease (KFD) is a rare, usually self-limited cause of cervical lymphadenitis that is more prevalent among patients of Asian descent.1 The pathogenesis of KFD remains unknown. Clinically, KFD may mimic malignant lymphoproliferative disorders, autoimmune diseases such as systemic lupus erythematosus (SLE) lymphadenitis, and infectious conditions such as HIV and tuberculous lymphadenitis. The most common presentation of KFD involves fever and rapidly evolving cervical lymphadenopathy.2,3 Due to its rarity, KFD is not always considered in the differential diagnosis for fever with tender lymphadenopathy, and up to one-third of cases are initially misdiagnosed.2
Definitive diagnosis requires lymph node biopsy. It is critical to achieving a timely diagnosis of KFD to exclude more serious conditions, initiate appropriate treatment, and minimize undue stress for patients. We describe a case of KFD in a patient who was met with delays in obtaining a definitive diagnosis for his symptoms.
Case Presentation
A 27-year-old previously healthy White man presented to the emergency department with subacute, progressive right-sided neck pain and swelling. In the week leading up to presentation, he also noted intermittent fevers, night sweats, and abdominal pain. His symptoms were unrelieved with acetaminophen and aspirin. He reported no sick contacts, recent travel, or animal exposures. He had no known history of autoimmune disease, malignancy, or immunocompromising conditions. Vital signs at the time of presentation were notable for a temperature of 39.0 °C. On examination, he had several firm, mobile, and exquisitely tender lymph nodes in the right upper anterior cervical chain. Abdominal examination was notable for left upper quadrant tenderness with palpable splenomegaly. Due to initial concern that his symptoms represented bacterial lymphadenitis, he was started on broad-spectrum antibiotics and admitted to the hospital for an expedited infectious workup.
Initial laboratory studies were notable for a white blood cell count of 3.7 × 109/L with 57.5% neutrophils and 27.0% lymphocytes on differential.
Computed tomography (CT) of the neck revealed multiple heterogeneously enlarged lymph nodes along the right anterior cervical chain with necrotic changes (Figure 1).
A core needle biopsy of a right-sided cervical lymph node was initially pursued, demonstrating necrotic tissue with minimal residual lymphoid tissue and no definitive evidence of lymphoma. Because these results were nondiagnostic, an excisional biopsy of the right-sided cervical lymph node was pursued 10 days later. Due to the stress of his 2-week hospitalization without a unifying diagnosis, the patient then elected to discharge home with close outpatient follow-up while awaiting his biopsy results. Antibiotics were not continued at the time of discharge as our broad infectious workup failed to yield a causative organism.
Two weeks postdischarge, the patient’s excisional lymph node biopsy returned demonstrating lymphohistiocytic inflammation with plasmacytoid dendritic cells, areas of necrosis, and scattered karyorrhectic nuclear debris, consistent with a diagnosis of KFD (Figure 2).
After 4 months of hydroxychloroquine therapy, the patient’s KFD symptoms resolved, prompting his dose to be reduced and eventually tapered. Repeat testing of his ANA and anti-dsDNA were performed at 1 and 6 months posthospitalization and returned within normal limits. A repeat PET-CT was performed 6 months posthospitalization showing resolution of his hypermetabolic right neck and right supraclavicular lymphadenopathy as well as his splenomegaly. It has now been more than a year since the patient’s initial presentation to the hospital, and he remains symptom-free and off prednisone and hydroxychloroquine.
Discussion
KFD is a rare cause of cervical lymphadenitis that was first described in 1972. Although cases have been reported worldwide, it is seen with higher prevalence in Asian countries. KFD was previously thought to have a female predominance, but recent reviews suggest a female to male ratio close to 1:1.1 The pathogenesis of KFD remains unknown, though some studies have suggested Epstein-Barr virus infection as a potential trigger.4,5 Human herpesvirus (HHV) 6, HHV 7, HHV 8, HSV, HIV, and parvovirus B19 also have been implicated as potential triggers, though no causative relationship has been established.2,5,6 Autoimmunity may also play a role in the pathogenesis of KFD given its histopathologic overlap with SLE lymphadenitis.1,7
The most common presenting symptoms of KFD include fever and tender cervical lymphadenopathy. Many patients also experience constitutional symptoms such as weight loss, night sweats, and fatigue.2 KFD is characterized by enlarged cervical lymph nodes, typically > 2 cm in diameter.3 Cutaneous manifestations of KFD are common and may manifest as nonspecific papules, plaques, nodules, or facial malar erythema.1,2 Case reports also have described KFD manifesting with ataxia, arthritis, parotitis, or ocular pathologies such as conjunctivitis and uveitis.1,2,8,9 Hepatosplenomegaly is a relatively rare manifestation of KFD seen in approximately 3% of cases.10 When present, hepatosplenomegaly may make the diagnosis of KFD especially difficult to distinguish from lymphoproliferative disorders such as lymphoma. Laboratory findings in KFD are nonspecific and include elevated levels of lactate dehydrogenase, erythrocyte sedimentation rate, C-reactive protein, and liver enzymes.3 Both lymphocytosis and lymphopenia have been described.3Definitive diagnosis of KFD is achieved through lymph node biopsy and histologic examination. Histopathologic findings of KFD include areas of coagulative necrosis and histiocytic proliferation within the cortical and paracortical regions of the lymph node. Scattered nuclear debris also may be seen, though this histologic finding also is seen with lymphoma. The absence of neutrophils is characteristic of KFD.2 In our patient, a core needle biopsy was initially pursued but returned nondiagnostic. A PET-CT also was obtained, though KFD may mimic lymphoma on PET as was seen in this patient’s case as well as in prior case reports.11 An excisional lymph node biopsy was ultimately performed and secured the diagnosis of KFD.
Although ultrasound-guided core needle biopsy was unable to yield the diagnosis for our patient, its diagnostic accuracy is still superior to that of fine needle aspiration and is therefore suggested as the primary diagnostic modality when KFD is suspected.12 Core needle biopsy also is less invasive, less time consuming, and perhaps more cost-effective than an open excisional biopsy, which often requires the use of an operating room and monitored anesthesia care.12 Understandably, our patient experienced significant stress while awaiting a final diagnosis. Whenever possible, lymph node biopsy should be prioritized over other diagnostic modalities to achieve a timely and definitive diagnosis.
KFD has no established treatment guidelines. Supportive care with antipyretics and analgesics is the most common initial approach, as KFD is typically a self-limited disease that resolves in 1 to 4 months.2 Patients with severe, persistent symptoms have been successfully treated with corticosteroids and hydroxychloroquine, with monotherapy typically trialed before concomitant use.2,13 After 2 courses of prednisone, our patient was prescribed single-agent hydroxychloroquine due to his recurrent symptoms and debilitating AEs from the steroids. Other case reports have described hydroxychloroquine as a treatment option when steroids fail to provide symptom relief or when there are recurrences of KFD.14-19 Retinopathy can occur as a result of long-term hydroxychloroquine use. As such, patients anticipated to require long-term hydroxychloroquine therapy should receive a baseline eye examination within months of drug initiation and repeat examination after 5 years of therapy.20
After symptom resolution, continued follow-up with a health care professional is recommended due to the potential for KFD recurrence or the development of a new autoimmune disease. The rate of KFD recurrence was previously described as 3%, but a more recent review found the rate of recurrence to be approximately 15% at > 6 months follow-up.1,3 Recurrence is often described during or shortly after the tapering of steroids.13,16,21,22 Recurrent KFD can be diagnosed with repeat lymph node biopsy, which also serves to exclude other disease processes.13,16 However, recurrence also has been diagnosed clinically based on the patient’s symptoms and laboratory investigations.21,22Continued surveillance of patients with KFD is also necessary to monitor for the development of new autoimmune diseases, especially SLE. SLE lymphadenitis shares many histopathologic characteristics with KFD. Case reports have described the development of SLE in patients with a history of KFD.2,7 Other autoimmune conditions described in patients with prior KFD include Sjögren syndrome, Hashimoto thyroiditis, Graves disease, mixed connective tissue disease, and antiphospholipid syndrome.3,23 Among patients with KFD, female sex, painful adenopathy, and cytopenias are significantly associated with the later development of autoimmune disease.23
Patient Perspective
This began for me in September 2020 out of the blue. I woke up one day with a random lymph node in my neck but otherwise felt completely healthy, and within 2 to 3 weeks I had never been more sick in my entire life. It came with bouts of fevers, neck pain from the swelling, stomach pain (I later learned an enlarged spleen was the source), terrible night sweats, violent chills where the shaking was uncontrollable for hours at a time, loss of appetite, and countless other symptoms that have come and gone over the past year.
It did take a little while to get a diagnosis, but I understand the autoimmune field is tricky. For about 4 to 5 weeks, I was told to prepare for a lymphoma diagnosis. I ended up doing 2 rounds of prednisone, one for 3 weeks at the end of 2020 and one for 2 months from March to May. The initial round helped quite a bit, but the second round did not have any effect on the lingering symptoms. In my opinion, prednisone is miserable to be on long term and I do not recommend it. The daily AEs that came with it included mood swings, insomnia, weight gain, and more. I have been on hydroxychloroquine now for almost 2 months and although it has some AEs of its own, it is nowhere near as rough as the prednisone and has helped manage my remaining symptoms quite a bit.
This certainly has not been a fun experience, but I was under great care during my time in the hospital and continue to be under good care through the rheumatology clinic. The one thing that could have made a huge difference would have been the issues involved in getting my surgery scheduled while I was still inpatient, which took quite a while. The pain during that time was so intense and unlike anything I have ever experienced before, and it was only the surgery that finally brought me some relief. To paint you a picture, I have broken bones, split my leg open, and have roughly 40 to 50 hours of tattoo work on me, and I have never experienced the level of pain like I felt in my neck and stomach. I remember feeling like someone had wound up and hit me with a baseball bat. The surgery brought me immense relief and if it had occurred when it was originally supposed to, I would have been spared 3 or so days of this type of pain.
It has been almost 10 months since my surgery and diagnosis, and life has mostly returned to normal for me. I am still on long-term medication as I mentioned, and I still deal with fatigue, spleen pain, and several other symptoms, but it is much more under control these days. I feel very fortunate to have been under and continue to be under such great care.
Conclusions
This case report highlights the importance of recognizing KFD as a rare but possible cause of fever and necrotizing cervical lymphadenopathy. KFD often mimics malignant lymphoproliferative disorders, autoimmune diseases such as SLE lymphadenitis, and infectious conditions such as HIV and tuberculous lymphadenitis. While KFD is seen with higher prevalence in Asian countries and was previously thought to be more predominant in females, the diagnosis should still be considered irrespective of geographic location or patient sex. Lymph node biopsy is the preferred diagnostic approach for patients with suspected KFD. Treatment is typically supportive but may consist of glucocorticoids in severe cases. Hydroxychloroquine may be used in refractory cases or as a steroid-sparing regimen when steroid AEs are poorly tolerated. Long-term follow-up is critical for patients with KFD to monitor for both disease recurrence and the development of autoimmune disease, especially SLE.
Acknowledgments
The authors thank Dr. Jacob Pilley for his detailed review of the patient’s pathology results. The authors also extend their gratitude to the patient, who deepened our understanding of this condition and what it is like to live with it.
1. Bosch X, Guilabert A, Miquel R, Campo E. Enigmatic Kikuchi-Fujimoto disease: a comprehensive review. Am J Clin Pathol. 2004;122(1):141-152. doi:10.1309/YF08-1L4T-KYWV-YVPQ
2. Deaver D, Horna P, Cualing H, Sokol L. Pathogenesis, diagnosis, and management of Kikuchi-Fujimoto disease. Cancer Control. 2014;21(4):313-321. doi:10.1177/107327481402100407
3. Cheng CY, Sheng WH, Lo YC, Chung CS, Chen YC, Chang SC. Clinical presentations, laboratory results and outcomes of patients with Kikuchi’s disease: emphasis on the association between recurrent Kikuchi’s disease and autoimmune diseases. J Microbiol Immunol Infect. 2010;43(5):366-371. doi:10.1016/S1684-1182(10)60058-8
4. Stéphan JL, Jeannoël P, Chanoz J, Gentil-Përret A. Epstein-Barr virus-associated Kikuchi disease in two children. J Pediatr Hematol Oncol. 2001;23(4):240-243. doi:10.1097/00043426-200105000-00012
5. Chiu CF, Chow KC, Lin TY, Tsai MH, Shih CM, Chen LM. Virus infection in patients with histiocytic necrotizing lymphadenitis in Taiwan. Detection of Epstein-Barr virus, type I human T-cell lymphotropic virus, and parvovirus B19. Am J Clin Pathol. 2000;113(6):774-781. doi:10.1309/1A6Y-YCKP-5AVF-QTYR
6. Rosado FG, Tang YW, Hasserjian RP, McClain CM, Wang B, Mosse CA. Kikuchi-Fujimoto lymphadenitis: role of parvovirus B-19, Epstein-Barr virus, human herpesvirus 6, and human herpesvirus 8. Hum Pathol. 2013;44(2):255-259. doi:10.1016/j.humpath.2012.05.016
7. Gordon JK, Magro C, Lu T, et al. Overlap between systemic lupus erythematosus and Kikuchi Fujimoto disease: a clinical pathology conference held by the Department of Rheumatology at Hospital for Special Surgery. HSS J. 2009;5(2):169-177. doi:10.1007/s11420-009-9123-x
8. Lo KB, Papazoglou A, Chua L, Candelario N. Case Report: Kikuchi: The great mimicker. F1000Res. 2018;7:520. Published 2018 Apr 30. doi:10.12688/f1000research.14758.1
9. Galor A, Georgy M, Leder HA, Dunn JP, Peters GB 3rd. Papillary conjunctivitis associated with Kikuchi disease. Cornea. 2008;27(8):944-946. doi:10.1097/ICO.0b013e31816bf488
10. Kucukardali Y, Solmazgul E, Kunter E, Oncul O, Yildirim S, Kaplan M. Kikuchi-Fujimoto disease: analysis of 244 cases. Clin Rheumatol. 2007;26(1):50-54. doi:10.1007/s10067-006-0230-5
11. Lee DH, Lee JH, Shim EJ, et al. Disseminated Kikuchi-Fujimoto disease mimicking malignant lymphoma on positron emission tomography in a child. J Pediatr Hematol Oncol. 2009;31(9):687-689. doi:10.1097/MPH.0b013e31819a5d77
12. Park SG, Koo HR, Jang K, et al. Efficacy of ultrasound-guided needle biopsy in the diagnosis of Kikuchi-Fujimoto disease. Laryngoscope. 2021;131(5):E1519-E1523. doi:10.1002/lary.29160
13. Honda F, Tsuboi H, Toko H, et al. Recurrent Kikuchi-Fujimoto disease successfully treated by the concomitant use of hydroxychloroquine and corticosteroids. Intern Med. 2017;56(24):3373-3377. doi:10.2169/internalmedicine.9205-17
14. Rezayat T, Carroll MB, Ramsey BC, Smith A. A case of relapsing Kikuchi-Fujimoto disease. Case Rep Otolaryngol. 2013;2013:364795. doi:10.1155/2013/364795
15. Rezai K, Kuchipudi S, Chundi V, Ariga R, Loew J, Sha BE. Kikuchi-Fujimoto disease: hydroxychloroquine as a treatment. Clin Infect Dis. 2004;39(12):e124-e126. doi:10.1086/426144
16. Hyun M, So IT, Kim HA, Jung H, Ryu SY. Recurrent Kikuchi’s disease treated by hydroxychloroquine. Infect Chemother. 2016;48(2):127-131. doi:10.3947/ic.2016.48.2.127
17. Lin YC, Huang HH, Nong BR, et al. Pediatric Kikuchi-Fujimoto disease: A clinicopathologic study and the therapeutic effects of hydroxychloroquine. J Microbiol Immunol Infect. 2019;52(3):395-401. doi:10.1016/j.jmii.2017.08.023
18. Lin DY, Villegas MS, Tan PL, Wang S, Shek LP. Severe Kikuchi’s disease responsive to immune modulation. Singapore Med J. 2010;51(1):e18-e21.
19. Quintás-Cardama A, Fraga M, Cozzi SN, Caparrini A, Maceiras F, Forteza J. Fatal Kikuchi-Fujimoto disease: the lupus connection. Ann Hematol. 2003;82(3):186-188. doi:10.1007/s00277-003-0611-7
20. American Academy of Ophthalmology. ACR, AAD, RDS, and AAO 2020 Joint Statement on Hydroxychloroquine Use with Respect to Retinal Toxicity. Updated February 2021. Accessed November 28, 2022. https://www.aao.org/clinical-statement/acr-aad-rds-aao-2020-joint-statement-on-hydroxychl-2
21. Gerwig U, Weidmann RG, Lindner G. Relapsing Kikuchi-Fujimoto disease requiring prolonged steroid therapy. Case Rep Emerg Med. 2019;2019:6405687. Published 2019 Mar 7. doi:10.1155/2019/6405687
22. Faheem B, Kumar V, Ashkar H, Komal F, Sultana Y. Recurrent Kikuchi-Fujimoto disease masquerading as lymphoma successfully treated by anakinra. Cureus. 2020;12(11):e11655. Published 2020 Nov 23. doi:10.7759/cureus.11655
23. Sopeña B, Rivera A, Vázquez-Triñanes C, et al. Autoimmune manifestations of Kikuchi disease. Semin Arthritis Rheum. 2012;41(6):900-906. doi:10.1016/j.semarthrit.2011.11.001
1. Bosch X, Guilabert A, Miquel R, Campo E. Enigmatic Kikuchi-Fujimoto disease: a comprehensive review. Am J Clin Pathol. 2004;122(1):141-152. doi:10.1309/YF08-1L4T-KYWV-YVPQ
2. Deaver D, Horna P, Cualing H, Sokol L. Pathogenesis, diagnosis, and management of Kikuchi-Fujimoto disease. Cancer Control. 2014;21(4):313-321. doi:10.1177/107327481402100407
3. Cheng CY, Sheng WH, Lo YC, Chung CS, Chen YC, Chang SC. Clinical presentations, laboratory results and outcomes of patients with Kikuchi’s disease: emphasis on the association between recurrent Kikuchi’s disease and autoimmune diseases. J Microbiol Immunol Infect. 2010;43(5):366-371. doi:10.1016/S1684-1182(10)60058-8
4. Stéphan JL, Jeannoël P, Chanoz J, Gentil-Përret A. Epstein-Barr virus-associated Kikuchi disease in two children. J Pediatr Hematol Oncol. 2001;23(4):240-243. doi:10.1097/00043426-200105000-00012
5. Chiu CF, Chow KC, Lin TY, Tsai MH, Shih CM, Chen LM. Virus infection in patients with histiocytic necrotizing lymphadenitis in Taiwan. Detection of Epstein-Barr virus, type I human T-cell lymphotropic virus, and parvovirus B19. Am J Clin Pathol. 2000;113(6):774-781. doi:10.1309/1A6Y-YCKP-5AVF-QTYR
6. Rosado FG, Tang YW, Hasserjian RP, McClain CM, Wang B, Mosse CA. Kikuchi-Fujimoto lymphadenitis: role of parvovirus B-19, Epstein-Barr virus, human herpesvirus 6, and human herpesvirus 8. Hum Pathol. 2013;44(2):255-259. doi:10.1016/j.humpath.2012.05.016
7. Gordon JK, Magro C, Lu T, et al. Overlap between systemic lupus erythematosus and Kikuchi Fujimoto disease: a clinical pathology conference held by the Department of Rheumatology at Hospital for Special Surgery. HSS J. 2009;5(2):169-177. doi:10.1007/s11420-009-9123-x
8. Lo KB, Papazoglou A, Chua L, Candelario N. Case Report: Kikuchi: The great mimicker. F1000Res. 2018;7:520. Published 2018 Apr 30. doi:10.12688/f1000research.14758.1
9. Galor A, Georgy M, Leder HA, Dunn JP, Peters GB 3rd. Papillary conjunctivitis associated with Kikuchi disease. Cornea. 2008;27(8):944-946. doi:10.1097/ICO.0b013e31816bf488
10. Kucukardali Y, Solmazgul E, Kunter E, Oncul O, Yildirim S, Kaplan M. Kikuchi-Fujimoto disease: analysis of 244 cases. Clin Rheumatol. 2007;26(1):50-54. doi:10.1007/s10067-006-0230-5
11. Lee DH, Lee JH, Shim EJ, et al. Disseminated Kikuchi-Fujimoto disease mimicking malignant lymphoma on positron emission tomography in a child. J Pediatr Hematol Oncol. 2009;31(9):687-689. doi:10.1097/MPH.0b013e31819a5d77
12. Park SG, Koo HR, Jang K, et al. Efficacy of ultrasound-guided needle biopsy in the diagnosis of Kikuchi-Fujimoto disease. Laryngoscope. 2021;131(5):E1519-E1523. doi:10.1002/lary.29160
13. Honda F, Tsuboi H, Toko H, et al. Recurrent Kikuchi-Fujimoto disease successfully treated by the concomitant use of hydroxychloroquine and corticosteroids. Intern Med. 2017;56(24):3373-3377. doi:10.2169/internalmedicine.9205-17
14. Rezayat T, Carroll MB, Ramsey BC, Smith A. A case of relapsing Kikuchi-Fujimoto disease. Case Rep Otolaryngol. 2013;2013:364795. doi:10.1155/2013/364795
15. Rezai K, Kuchipudi S, Chundi V, Ariga R, Loew J, Sha BE. Kikuchi-Fujimoto disease: hydroxychloroquine as a treatment. Clin Infect Dis. 2004;39(12):e124-e126. doi:10.1086/426144
16. Hyun M, So IT, Kim HA, Jung H, Ryu SY. Recurrent Kikuchi’s disease treated by hydroxychloroquine. Infect Chemother. 2016;48(2):127-131. doi:10.3947/ic.2016.48.2.127
17. Lin YC, Huang HH, Nong BR, et al. Pediatric Kikuchi-Fujimoto disease: A clinicopathologic study and the therapeutic effects of hydroxychloroquine. J Microbiol Immunol Infect. 2019;52(3):395-401. doi:10.1016/j.jmii.2017.08.023
18. Lin DY, Villegas MS, Tan PL, Wang S, Shek LP. Severe Kikuchi’s disease responsive to immune modulation. Singapore Med J. 2010;51(1):e18-e21.
19. Quintás-Cardama A, Fraga M, Cozzi SN, Caparrini A, Maceiras F, Forteza J. Fatal Kikuchi-Fujimoto disease: the lupus connection. Ann Hematol. 2003;82(3):186-188. doi:10.1007/s00277-003-0611-7
20. American Academy of Ophthalmology. ACR, AAD, RDS, and AAO 2020 Joint Statement on Hydroxychloroquine Use with Respect to Retinal Toxicity. Updated February 2021. Accessed November 28, 2022. https://www.aao.org/clinical-statement/acr-aad-rds-aao-2020-joint-statement-on-hydroxychl-2
21. Gerwig U, Weidmann RG, Lindner G. Relapsing Kikuchi-Fujimoto disease requiring prolonged steroid therapy. Case Rep Emerg Med. 2019;2019:6405687. Published 2019 Mar 7. doi:10.1155/2019/6405687
22. Faheem B, Kumar V, Ashkar H, Komal F, Sultana Y. Recurrent Kikuchi-Fujimoto disease masquerading as lymphoma successfully treated by anakinra. Cureus. 2020;12(11):e11655. Published 2020 Nov 23. doi:10.7759/cureus.11655
23. Sopeña B, Rivera A, Vázquez-Triñanes C, et al. Autoimmune manifestations of Kikuchi disease. Semin Arthritis Rheum. 2012;41(6):900-906. doi:10.1016/j.semarthrit.2011.11.001
Follicular Lymphoma Highlights From ASH 2022
Highlights in follicular lymphoma from the 2022 American Society of Hematology (ASH) Annual Meeting are discussed by Dr Thomas Rodgers of the Durham VA Medical Center.
Dr Rodgers begins with a prognostic model designed to evaluate the risk for disease progression in high-risk patients within 24 months of starting first-line treatment with the intention of better individualizing management in this group.
Next, he presents long-term phase 3 data comparing first-line rituximab with a watch-and-wait approach. After 12 years of follow-up, results showed no significant difference in overall survival between watch and wait, rituximab induction, and rituximab induction plus maintenance, suggesting to Dr Rodgers that individualized upfront management can lead to similarly excellent outcomes in patients with low tumor burden.
Turning to relapsed/refractory disease, Dr Rodgers cites a study comparing rituximab plus lenalidomide with rituximab plus placebo. The combination yielded superior results and more durable efficacy than did the control group.
He also discusses studies on the use of novel agent tazemetostat in combination with lenalidomide, and the bispecific monoclonal antibody mosunetuzumab as monotherapy. The US Food and Drug Administration approved mosunetuzumab in December, expanding the armamentarium for patients with follicular lymphoma who have undergone multiple lines of therapy.
--
Thomas Rodgers, MD, Assistant Professor, Department of Hematologic Malignancies and Cellular Therapy, Duke University; Staff Physician, Department of Hematology/Oncology, Durham VA Medical Center, Durham, North Carolina
Thomas Rodgers, MD, has disclosed no relevant financial relationships.
Highlights in follicular lymphoma from the 2022 American Society of Hematology (ASH) Annual Meeting are discussed by Dr Thomas Rodgers of the Durham VA Medical Center.
Dr Rodgers begins with a prognostic model designed to evaluate the risk for disease progression in high-risk patients within 24 months of starting first-line treatment with the intention of better individualizing management in this group.
Next, he presents long-term phase 3 data comparing first-line rituximab with a watch-and-wait approach. After 12 years of follow-up, results showed no significant difference in overall survival between watch and wait, rituximab induction, and rituximab induction plus maintenance, suggesting to Dr Rodgers that individualized upfront management can lead to similarly excellent outcomes in patients with low tumor burden.
Turning to relapsed/refractory disease, Dr Rodgers cites a study comparing rituximab plus lenalidomide with rituximab plus placebo. The combination yielded superior results and more durable efficacy than did the control group.
He also discusses studies on the use of novel agent tazemetostat in combination with lenalidomide, and the bispecific monoclonal antibody mosunetuzumab as monotherapy. The US Food and Drug Administration approved mosunetuzumab in December, expanding the armamentarium for patients with follicular lymphoma who have undergone multiple lines of therapy.
--
Thomas Rodgers, MD, Assistant Professor, Department of Hematologic Malignancies and Cellular Therapy, Duke University; Staff Physician, Department of Hematology/Oncology, Durham VA Medical Center, Durham, North Carolina
Thomas Rodgers, MD, has disclosed no relevant financial relationships.
Highlights in follicular lymphoma from the 2022 American Society of Hematology (ASH) Annual Meeting are discussed by Dr Thomas Rodgers of the Durham VA Medical Center.
Dr Rodgers begins with a prognostic model designed to evaluate the risk for disease progression in high-risk patients within 24 months of starting first-line treatment with the intention of better individualizing management in this group.
Next, he presents long-term phase 3 data comparing first-line rituximab with a watch-and-wait approach. After 12 years of follow-up, results showed no significant difference in overall survival between watch and wait, rituximab induction, and rituximab induction plus maintenance, suggesting to Dr Rodgers that individualized upfront management can lead to similarly excellent outcomes in patients with low tumor burden.
Turning to relapsed/refractory disease, Dr Rodgers cites a study comparing rituximab plus lenalidomide with rituximab plus placebo. The combination yielded superior results and more durable efficacy than did the control group.
He also discusses studies on the use of novel agent tazemetostat in combination with lenalidomide, and the bispecific monoclonal antibody mosunetuzumab as monotherapy. The US Food and Drug Administration approved mosunetuzumab in December, expanding the armamentarium for patients with follicular lymphoma who have undergone multiple lines of therapy.
--
Thomas Rodgers, MD, Assistant Professor, Department of Hematologic Malignancies and Cellular Therapy, Duke University; Staff Physician, Department of Hematology/Oncology, Durham VA Medical Center, Durham, North Carolina
Thomas Rodgers, MD, has disclosed no relevant financial relationships.

The Safety and Efficacy of AUC/MIC-Guided vs Trough-Guided Vancomycin Monitoring Among Veterans
Vancomycin is a commonly used glycopeptide antibiotic used to treat infections caused by gram-positive organisms. Vancomycin is most often used as a parenteral agent for empiric or definitive treatment of methicillin-resistant Staphylococcus aureus (MRSA). It can also be used for the treatment of other susceptible Staphylococcus or Enterococcus species. Adverse effects of parenteral vancomycin include infusion-related reactions, ototoxicity, and nephrotoxicity.1 Higher vancomycin trough levels have been associated with an increased risk of nephrotoxicity.1-4 The major safety concern with vancomycin is acute kidney injury (AKI). Even mild AKI can prolong hospitalizations, increase the cost of health care, and increase morbidity.2
In March 2020, the American Society of Health-System Pharmacists, the Infectious Diseases Society of America (IDSA), the Pediatric Infectious Disease Society, and the Society of Infectious Diseases Pharmacists released a consensus statement and guidelines regarding the optimization of vancomycin dosing and monitoring for patients with suspected or definitive serious MRSA infections. Based on these guidelines, it is recommended to target an individualized area under the curve/minimum inhibitory concentration (AUC/MIC) ratio of 400 to 600 mg × h/L to maximize clinical efficacy and minimize the risk of AKI.2
Before March 2020, the vancomycin monitoring recommendation was to target trough levels of 10 to 20 mg/L. A goal trough of 15 to 20 mg/L was recommended for severe infections, including sepsis, endocarditis, hospital-acquired pneumonia, meningitis, and osteomyelitis, caused by MRSA. A goal trough of 10 to 15 mg/L was recommended for noninvasive infections, such as skin and soft tissue infections and urinary tract infections, caused by MRSA. Targeting these trough levels was thought to achieve an AUC/MIC ≥ 400 mg × h/L.5 Evidence has since shown that trough values may not be an optimal marker for AUC/MIC values.2
The updated vancomycin therapeutic drug monitoring (TDM) guidelines recommend that health systems transition to AUC/MIC-guided monitoring for suspected or confirmed infections caused by MRSA. There is not enough evidence to recommend AUC/MIC-guided monitoring in patients with noninvasive infections or infections caused by other microbes.2
AUC/MIC-guided monitoring can be achieved in 2 ways. The first method is collecting Cmax (peak level) and Cmin (trough level) serum concentrations, preferably during the same dosing interval. Ideally, Cmax should be drawn 1 to 2 hours after the vancomycin infusion and Cmin should be drawn at the end of the dosing interval. First-order pharmacokinetic equations are used to estimate the AUC/MIC with this method. Bayesian software pharmacokinetic modeling based on 1 or 2 vancomycin concentrations with 1 trough level also can be used for monitoring. Preferably, 2 levels would be obtained to estimate the AUC/MIC when using Bayesian modeling.2
The bactericidal activity of vancomycin was achieved with AUC/MIC ratios of ≥ 400 mg × h/L. AUC/MIC ratios of < 400 mg × h/L increase the incidence of resistant and intermediate strains of S aureus. AUC/MIC-guided monitoring assumes an MIC of 1 mg/L. When the MIC is > 1 mg/L, it is less likely that an AUC/MIC ≥ 400 mg × h/L is achievable. Regardless of the TDM method used, AUC/MIC ratios ≥ 400 mg × h/L are not achievable with conventional dosing methods if the vancomycin MIC is > 2 mg/L in patients with normal renal function. Alternative therapy is recommended to be used for these patients.2
There are multiple studies investigating the therapeutic dosing of vancomycin and the associated incidence of AKI. Previous studies have correlated vancomycin AUC/MICs of 400 mg to 600 mg × h/L with clinical effectiveness.2,6 In 2017, Neely and colleagues looked at the therapeutic dosing of vancomycin in 252 adults with ≥ 1 vancomycin level.7 During this prospective trial, they evaluated patients for 1 year and targeted trough concentrations of 10 to 20 mg/L with infection-specific goal ranges of 10 to 15 mg/L and 15 to 20 mg/L for noninvasive and invasive infections, respectively. They also targeted AUC/MIC ratios ≥ 400 mg × h/L regardless of trough concentration using Bayesian estimated AUC/MICs for 2 years. They found only 19% of trough concentrations to be therapeutic compared with 70% of AUC/MICs. A secondary outcome assessed by Neely and colleagues was nephrotoxicity, which was identified in 8% of patients with trough targets and 2% of patients with AUC/MIC targets.8
Previous studies evaluating the use of vancomycin in the veteran population have focused on AKI incidence, general nephrotoxicity, and 30-day readmission rates.4,7,9,10 Poston-Blahnik and colleagues investigated the rates of AKI in 200 veterans using AUC/MIC-guided vancomycin TDM.5 They found an AKI incidence of 42% of patients with AUC/MICs ≥ 550 mg × h/L and 2% of patients with AUC/MICs < 550 mg × h/L.5 Gyamlani and colleagues investigated the rates of AKI in 33,527 veterans and found that serum vancomycin trough levels ≥ 20 mg/L were associated with a higher risk of AKI.8 Prabaker and colleagues investigated the association between vancomycin trough levels and nephrotoxicity, defined as 0.5 mg/L or a 50% increase in serum creatinine (sCr) in 348 veterans. They found nephrotoxicity in 8.9% of patients.10 Patel and colleagues investigated the effect of AKI on 30-day readmission rates in 216 veterans.10 AKI occurred in 8.8% of patients and of those 19.4% were readmitted within 30 days.10 Current literature lacks evidence regarding the comparison of the safety and efficacy of vancomycin trough-guided vs AUC/MIC-guided TDM in the veteran population. Therefore, the objective of this study was to investigate the differences in the safety and efficacy of vancomycin TDM in the veteran population based on the different monitoring methods used.
METHODS
This study was a retrospective, single-center, quasi-experimental chart review conducted at the Sioux Falls Veterans Affairs Health Care System (SFVAHCS) in South Dakota. Data were collected from the Computerized Patient Record System (CPRS). The SFVAHCS transitioned from trough-guided to AUC/MIC-guided TDM in November 2020.
Patients included in this study were veterans aged ≥ 18 years with orders for parenteral vancomycin between February 1, 2020, and October 31, 2020, for the trough-guided TDM group and between December 1, 2020, and August 31, 2021, for the AUC/MIC-guided TDM group. Patients with vancomycin courses initiated during November 2020 were excluded as both TDM methods were being used at that time. Patients were excluded if their vancomycin course began before February 1, 2020, for the trough-guided TDM group or began during November 2020 for the AUC/MIC-guided TDM group. Patients were excluded if their vancomycin course extended past October 31, 2020, for the trough group or past August 31, 2021, for the AUC/MIC group. Patients on dialysis or missing Cmax, Cmin, or sCr levels were excluded.
This study evaluated both safety (AKI incidence) and effectiveness (time spent in therapeutic range and time to therapeutic range). The primary endpoint was presence of vancomycin-induced AKI, which was based on the most recent Kidney Disease: Improving Global Outcomes (KDIGO) AKI definition: increased sCr of ≥ 0.3 mg/dL or by 50% from baseline sustained over 48 hours without any other explanation for the change.11 A secondary endpoint was the absence or presence of AKI.
Additional secondary endpoints included the presence of the initial trough or AUC/MIC of each vancomycin course within the therapeutic range and the percentage of all trough levels or AUC/MICs within therapeutic, subtherapeutic, and supratherapeutic ranges. The therapeutic range for AUC/MIC-guided TDM was 400 to 600 mg × h/L and 10 to 20 mg/L depending on indication for trough-guided TDM (15-20 mg/L for severe infections and 10-15 mg/L for less invasive infections). The percentage of trough levels or AUC/MICs within therapeutic, subtherapeutic, and supratherapeutic ranges were calculated as a ratio of levels within each range to total levels taken for each patient.
For AUC/MIC-guided TDM the Cmax levels were ideally drawn 1 to 2 hours after vancomycin infusion and Cmin levels were ideally drawn 30 minutes before the next dose. First-order pharmacokinetic equations were used to estimate the AUC/MIC.12 If the timing of a vancomycin level was inappropriate, actual levels were extrapolated based on the timing of the blood draw compared with the ideal Cmin or Cmax time. Extrapolated levels were used for both trough-guided and AUC/MIC-guided TDM groups when appropriate. Vancomycin levels were excluded if they were drawn during the vancomycin infusion.
Study participant age, sex, race, weight, baseline estimated glomerular filtration (eGFR) rate, baseline sCr, concomitant nephrotoxic medications, duration of vancomycin course, indication of vancomycin, and acuity of illness based on indication were collected. sCr levels were collected from the initial day vancomycin was ordered through 72 hours following completion of a vancomycin course to evaluate for AKI. Patients’ charts were reviewed for the use of the following nephrotoxic medications: nonsteroidal anti-inflammatories, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, aminoglycosides, piperacillin/tazobactam, loop diuretics, amphotericin B, acyclovir, intravenous contrast, and nephrotoxic chemotherapy (cisplatin). The category of concomitant nephrotoxic medications was also collected including the continuation of a home nephrotoxic medication vs the initiation of a new nephrotoxic medication.
Statistical Analysis
The primary endpoint of the incidence of vancomycin-induced AKI was compared using a Fisher exact test. The secondary endpoint of the percentage of trough levels or AUC/MICs in the therapeutic, subtherapeutic, and supratherapeutic range were compared using a student t test. The secondary endpoint of first level or AUC/MIC within goal range was compared using a χ2 test. Continuous baseline characteristics were reported as a mean and compared using a student t test. Nominal baseline characteristics were reported as a percentage and compared using the χ2 test. P values < .05 were considered statistically significant.
RESULTS
This study included 97 patients, 43 in the AUC/MIC group and 54 in the trough group.
One (2%) patient in the AUC/MIC group and 2 (4%) patients in the trough group experienced vancomycin-induced AKI (P = .10) (Table 2).
DISCUSSION
There was no statistically significant difference between the 2 groups for the vancomycin-induced AKI (P = .10), the primary endpoint, or overall AKI (P = .29), the secondary endpoint. It should be noted that there was more overall AKI in the AUC/MIC group. Veterans in the AUC/MIC group were found to have their first AUC/MIC within the therapeutic range statistically significantly more often than the first trough level in the trough group (P = .04). The percentage of time spent within therapeutic range was statistically significantly higher in the AUC/MIC-guided TDM group (P = .02). The percentage of time spent subtherapeutic of goal range was statistically significantly higher in the trough-guided TDM group (P < .001). There was no statistically significant difference found in the percent of time spent supratherapeutic of goal range (P = .25). However, the observed percentage of time spent supratherapeutic of goal range was higher in the AUC/MIC group. These results indicate that AUC/MIC-guided TDM may be more efficacious with regard to time in therapeutic range and time to therapeutic range.
The finding of increased AKI with AUC/MIC-guided TDM does not align with previous studies.8 The prospective study by Neely and colleagues found that AUC/MIC-guided TDM resulted in more time in the therapeutic range as well as less nephrotoxicity compared with trough-guided TDM, although it was limited by its lack of randomization and did not account for other causes of nephrotoxicity.8 They found that only 19% of trough concentrations were therapeutic compared with 70% of AUC/MICs and found nephrotoxicity in 8% of trough-guided TDM patients compared with 2% of AUC/MIC-guided TDM patients.8
Unlike Nealy and colleagues, our study did not find lower nephrotoxicity associated with AUC/MIC-guided TDM. Multiple factors may have influenced our results. Our AUC/MIC group had significantly more newly started concomitant nephrotoxins and other nephrotoxic medications used during the vancomycin courses compared with the trough-guided group, which may have influenced AKI outcomes. It also should be noted that there was significantly more time spent subtherapeutic of the goal range and significantly less time in the goal range in the trough group compared with the AUC/MIC group. In our study, the trough-guided group had significantly more patients with acute illness compared with the AUC/MIC group (skin, soft tissue, and joint infections were similar between the groups). The group with more acutely ill patients would have been expected to have more nephrotoxicity. However, despite the acute illnesses, patients in the trough-guided group spent more time in the subtherapeutic range. This may explain the increased nephrotoxicity in the AUC/MIC group since those patients spent more time in the therapeutic range.
This study used the most recent KDIGO AKI definition: either an increase in sCr of ≥ 0.3 mg/dL or a 50% increase in sCr from baseline sustained over 48 hours without any other explanation for the change in renal function.11 This AKI definition is stricter than the previous definition, which was used by earlier studies, including Neely and colleagues, to evaluate rates of vancomycin-induced AKI.2,3 Therefore, the rates of overall AKI found in this study may be higher than in previous studies due to the definition of AKI used.
Limitations
This study was limited by its retrospective nature, lack of randomization, and small sample size. To decrease the potential for error in this study, analysis of power and a larger study sample would have been beneficial. During the COVID-19 pandemic, increased pneumonia cases may have hidden bacterial causes and caused an undercount. Nephrotoxicity may also be related to volume depletion, severe systemic illness, dehydration, or hypotension. Screening was completed via chart review for these alternative causes of nephrotoxicity in this study but may not be completely accounted for due to lack of documentation and the retrospective nature of this study.
CONCLUSIONS
This study did not find a significant difference in the rates of vancomycin-induced or overall AKI between AUC/MIC-guided and trough-guided TDM. However, this study may not have been powered to detect a significant difference in the primary endpoint. This study indicated that AUC/MIC-guided TDM of vancomycin resulted in a quicker time to the therapeutic range and a higher percentage of overall time in the therapeutic range as compared with trough-guided TDM. The results of this study indicated that trough-guided monitoring resulted in a higher percentage of time in a subtherapeutic range. This study also found that the first AUC/MIC calculated was within therapeutic range more often than the first trough level collected.
These results indicate that AUC/MIC-guided TDM may be more effective than trough-guided TDM in the veteran population. However, while AUC/MIC-guided TDM may be more effective with regards to time in therapeutic range and time to therapeutic range, this study did not indicate any safety benefit of AUC/MIC-guided over trough-guided TDM with regards to AKI incidence. Our data indicate that AUC/MIC-guided TDM increases the amount of time in the therapeutic range compared with trough-guided TDM and is not more nephrotoxic. The findings of this study support the recommendation to transition to the use of AUC/MIC-guided TDM of vancomycin in the veteran population.
Acknowledgments
This material is the result of work supported with the use of facilities and resources from the Sioux Falls Veterans Affairs Health Care System.
1. Gallagher J, MacDougall C. Glycopeptides and short-acting lipoglycopeptides In: Antibiotics Simplified. Jones & Bartlett Learning; 2018.
2. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835-864. doi:10.1093/ajhp/zxaa036
3. Hermsen ED, Hanson M, Sankaranarayanan J, Stoner JA, Florescu MC, Rupp ME. Clinical outcomes and nephrotoxicity associated with vancomycin trough concentrations during treatment of deep-seated infections. Expert Opin Drug Saf. 2010;9(1):9-14. doi:10.1517/14740330903413514
4. Poston-Blahnik A, Moenster R. Association between vancomycin area under the curve and nephrotoxicity: a single center, retrospective cohort study in a veteran population. Open Forum Infect Dis. 2021;8(5):ofab094. Published 2021 Mar 12. doi:10.1093/ofid/ofab094
5. Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66(1):82-98. doi:10.2146/ajhp080434
6. Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet. 2004;43(13):925-942. doi:10.2165/00003088-200443130-00005
7. Gyamlani G, Potukuchi PK, Thomas F, et al. Vancomycin-Associated Acute Kidney Injury in a Large Veteran Population. Am J Nephrol. 2019;49(2):133-142. doi:10.1159/000496484
8. Neely MN, Kato L, Youn G, et al. Prospective Trial on the Use of Trough Concentration versus Area under the Curve To Determine Therapeutic Vancomycin Dosing. Antimicrob Agents Chemother. 2018;62(2):e02042-17. Published 2018 Jan 25. doi:10.1128/AAC.02042-17
9. Prabaker KK, Tran TP, Pratummas T, Goetz MB, Graber CJ. Elevated vancomycin trough is not associated with nephrotoxicity among inpatient veterans. J Hosp Med. 2012;7(2):91-97. doi:10.1002/jhm.946
10. Patel N, Stornelli N, Sangiovanni RJ, Huang DB, Lodise TP. Effect of vancomycin-associated acute kidney injury on incidence of 30-day readmissions among hospitalized Veterans Affairs patients with skin and skin structure infections. Antimicrob Agents Chemother. 2020;64(10):e01268-20. Published 2020 Sep 21. doi:10.1128/AAC.01268-20
11. Acute Kidney Injury Work Group. Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. 2012;2(suppl 1):1-138.
12. Pai MP, Neely M, Rodvold KA, Lodise TP. Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv Drug Deliv Rev. 2014;77:50-57. doi:10.1016/j.addr.2014.05.016
Vancomycin is a commonly used glycopeptide antibiotic used to treat infections caused by gram-positive organisms. Vancomycin is most often used as a parenteral agent for empiric or definitive treatment of methicillin-resistant Staphylococcus aureus (MRSA). It can also be used for the treatment of other susceptible Staphylococcus or Enterococcus species. Adverse effects of parenteral vancomycin include infusion-related reactions, ototoxicity, and nephrotoxicity.1 Higher vancomycin trough levels have been associated with an increased risk of nephrotoxicity.1-4 The major safety concern with vancomycin is acute kidney injury (AKI). Even mild AKI can prolong hospitalizations, increase the cost of health care, and increase morbidity.2
In March 2020, the American Society of Health-System Pharmacists, the Infectious Diseases Society of America (IDSA), the Pediatric Infectious Disease Society, and the Society of Infectious Diseases Pharmacists released a consensus statement and guidelines regarding the optimization of vancomycin dosing and monitoring for patients with suspected or definitive serious MRSA infections. Based on these guidelines, it is recommended to target an individualized area under the curve/minimum inhibitory concentration (AUC/MIC) ratio of 400 to 600 mg × h/L to maximize clinical efficacy and minimize the risk of AKI.2
Before March 2020, the vancomycin monitoring recommendation was to target trough levels of 10 to 20 mg/L. A goal trough of 15 to 20 mg/L was recommended for severe infections, including sepsis, endocarditis, hospital-acquired pneumonia, meningitis, and osteomyelitis, caused by MRSA. A goal trough of 10 to 15 mg/L was recommended for noninvasive infections, such as skin and soft tissue infections and urinary tract infections, caused by MRSA. Targeting these trough levels was thought to achieve an AUC/MIC ≥ 400 mg × h/L.5 Evidence has since shown that trough values may not be an optimal marker for AUC/MIC values.2
The updated vancomycin therapeutic drug monitoring (TDM) guidelines recommend that health systems transition to AUC/MIC-guided monitoring for suspected or confirmed infections caused by MRSA. There is not enough evidence to recommend AUC/MIC-guided monitoring in patients with noninvasive infections or infections caused by other microbes.2
AUC/MIC-guided monitoring can be achieved in 2 ways. The first method is collecting Cmax (peak level) and Cmin (trough level) serum concentrations, preferably during the same dosing interval. Ideally, Cmax should be drawn 1 to 2 hours after the vancomycin infusion and Cmin should be drawn at the end of the dosing interval. First-order pharmacokinetic equations are used to estimate the AUC/MIC with this method. Bayesian software pharmacokinetic modeling based on 1 or 2 vancomycin concentrations with 1 trough level also can be used for monitoring. Preferably, 2 levels would be obtained to estimate the AUC/MIC when using Bayesian modeling.2
The bactericidal activity of vancomycin was achieved with AUC/MIC ratios of ≥ 400 mg × h/L. AUC/MIC ratios of < 400 mg × h/L increase the incidence of resistant and intermediate strains of S aureus. AUC/MIC-guided monitoring assumes an MIC of 1 mg/L. When the MIC is > 1 mg/L, it is less likely that an AUC/MIC ≥ 400 mg × h/L is achievable. Regardless of the TDM method used, AUC/MIC ratios ≥ 400 mg × h/L are not achievable with conventional dosing methods if the vancomycin MIC is > 2 mg/L in patients with normal renal function. Alternative therapy is recommended to be used for these patients.2
There are multiple studies investigating the therapeutic dosing of vancomycin and the associated incidence of AKI. Previous studies have correlated vancomycin AUC/MICs of 400 mg to 600 mg × h/L with clinical effectiveness.2,6 In 2017, Neely and colleagues looked at the therapeutic dosing of vancomycin in 252 adults with ≥ 1 vancomycin level.7 During this prospective trial, they evaluated patients for 1 year and targeted trough concentrations of 10 to 20 mg/L with infection-specific goal ranges of 10 to 15 mg/L and 15 to 20 mg/L for noninvasive and invasive infections, respectively. They also targeted AUC/MIC ratios ≥ 400 mg × h/L regardless of trough concentration using Bayesian estimated AUC/MICs for 2 years. They found only 19% of trough concentrations to be therapeutic compared with 70% of AUC/MICs. A secondary outcome assessed by Neely and colleagues was nephrotoxicity, which was identified in 8% of patients with trough targets and 2% of patients with AUC/MIC targets.8
Previous studies evaluating the use of vancomycin in the veteran population have focused on AKI incidence, general nephrotoxicity, and 30-day readmission rates.4,7,9,10 Poston-Blahnik and colleagues investigated the rates of AKI in 200 veterans using AUC/MIC-guided vancomycin TDM.5 They found an AKI incidence of 42% of patients with AUC/MICs ≥ 550 mg × h/L and 2% of patients with AUC/MICs < 550 mg × h/L.5 Gyamlani and colleagues investigated the rates of AKI in 33,527 veterans and found that serum vancomycin trough levels ≥ 20 mg/L were associated with a higher risk of AKI.8 Prabaker and colleagues investigated the association between vancomycin trough levels and nephrotoxicity, defined as 0.5 mg/L or a 50% increase in serum creatinine (sCr) in 348 veterans. They found nephrotoxicity in 8.9% of patients.10 Patel and colleagues investigated the effect of AKI on 30-day readmission rates in 216 veterans.10 AKI occurred in 8.8% of patients and of those 19.4% were readmitted within 30 days.10 Current literature lacks evidence regarding the comparison of the safety and efficacy of vancomycin trough-guided vs AUC/MIC-guided TDM in the veteran population. Therefore, the objective of this study was to investigate the differences in the safety and efficacy of vancomycin TDM in the veteran population based on the different monitoring methods used.
METHODS
This study was a retrospective, single-center, quasi-experimental chart review conducted at the Sioux Falls Veterans Affairs Health Care System (SFVAHCS) in South Dakota. Data were collected from the Computerized Patient Record System (CPRS). The SFVAHCS transitioned from trough-guided to AUC/MIC-guided TDM in November 2020.
Patients included in this study were veterans aged ≥ 18 years with orders for parenteral vancomycin between February 1, 2020, and October 31, 2020, for the trough-guided TDM group and between December 1, 2020, and August 31, 2021, for the AUC/MIC-guided TDM group. Patients with vancomycin courses initiated during November 2020 were excluded as both TDM methods were being used at that time. Patients were excluded if their vancomycin course began before February 1, 2020, for the trough-guided TDM group or began during November 2020 for the AUC/MIC-guided TDM group. Patients were excluded if their vancomycin course extended past October 31, 2020, for the trough group or past August 31, 2021, for the AUC/MIC group. Patients on dialysis or missing Cmax, Cmin, or sCr levels were excluded.
This study evaluated both safety (AKI incidence) and effectiveness (time spent in therapeutic range and time to therapeutic range). The primary endpoint was presence of vancomycin-induced AKI, which was based on the most recent Kidney Disease: Improving Global Outcomes (KDIGO) AKI definition: increased sCr of ≥ 0.3 mg/dL or by 50% from baseline sustained over 48 hours without any other explanation for the change.11 A secondary endpoint was the absence or presence of AKI.
Additional secondary endpoints included the presence of the initial trough or AUC/MIC of each vancomycin course within the therapeutic range and the percentage of all trough levels or AUC/MICs within therapeutic, subtherapeutic, and supratherapeutic ranges. The therapeutic range for AUC/MIC-guided TDM was 400 to 600 mg × h/L and 10 to 20 mg/L depending on indication for trough-guided TDM (15-20 mg/L for severe infections and 10-15 mg/L for less invasive infections). The percentage of trough levels or AUC/MICs within therapeutic, subtherapeutic, and supratherapeutic ranges were calculated as a ratio of levels within each range to total levels taken for each patient.
For AUC/MIC-guided TDM the Cmax levels were ideally drawn 1 to 2 hours after vancomycin infusion and Cmin levels were ideally drawn 30 minutes before the next dose. First-order pharmacokinetic equations were used to estimate the AUC/MIC.12 If the timing of a vancomycin level was inappropriate, actual levels were extrapolated based on the timing of the blood draw compared with the ideal Cmin or Cmax time. Extrapolated levels were used for both trough-guided and AUC/MIC-guided TDM groups when appropriate. Vancomycin levels were excluded if they were drawn during the vancomycin infusion.
Study participant age, sex, race, weight, baseline estimated glomerular filtration (eGFR) rate, baseline sCr, concomitant nephrotoxic medications, duration of vancomycin course, indication of vancomycin, and acuity of illness based on indication were collected. sCr levels were collected from the initial day vancomycin was ordered through 72 hours following completion of a vancomycin course to evaluate for AKI. Patients’ charts were reviewed for the use of the following nephrotoxic medications: nonsteroidal anti-inflammatories, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, aminoglycosides, piperacillin/tazobactam, loop diuretics, amphotericin B, acyclovir, intravenous contrast, and nephrotoxic chemotherapy (cisplatin). The category of concomitant nephrotoxic medications was also collected including the continuation of a home nephrotoxic medication vs the initiation of a new nephrotoxic medication.
Statistical Analysis
The primary endpoint of the incidence of vancomycin-induced AKI was compared using a Fisher exact test. The secondary endpoint of the percentage of trough levels or AUC/MICs in the therapeutic, subtherapeutic, and supratherapeutic range were compared using a student t test. The secondary endpoint of first level or AUC/MIC within goal range was compared using a χ2 test. Continuous baseline characteristics were reported as a mean and compared using a student t test. Nominal baseline characteristics were reported as a percentage and compared using the χ2 test. P values < .05 were considered statistically significant.
RESULTS
This study included 97 patients, 43 in the AUC/MIC group and 54 in the trough group.
One (2%) patient in the AUC/MIC group and 2 (4%) patients in the trough group experienced vancomycin-induced AKI (P = .10) (Table 2).
DISCUSSION
There was no statistically significant difference between the 2 groups for the vancomycin-induced AKI (P = .10), the primary endpoint, or overall AKI (P = .29), the secondary endpoint. It should be noted that there was more overall AKI in the AUC/MIC group. Veterans in the AUC/MIC group were found to have their first AUC/MIC within the therapeutic range statistically significantly more often than the first trough level in the trough group (P = .04). The percentage of time spent within therapeutic range was statistically significantly higher in the AUC/MIC-guided TDM group (P = .02). The percentage of time spent subtherapeutic of goal range was statistically significantly higher in the trough-guided TDM group (P < .001). There was no statistically significant difference found in the percent of time spent supratherapeutic of goal range (P = .25). However, the observed percentage of time spent supratherapeutic of goal range was higher in the AUC/MIC group. These results indicate that AUC/MIC-guided TDM may be more efficacious with regard to time in therapeutic range and time to therapeutic range.
The finding of increased AKI with AUC/MIC-guided TDM does not align with previous studies.8 The prospective study by Neely and colleagues found that AUC/MIC-guided TDM resulted in more time in the therapeutic range as well as less nephrotoxicity compared with trough-guided TDM, although it was limited by its lack of randomization and did not account for other causes of nephrotoxicity.8 They found that only 19% of trough concentrations were therapeutic compared with 70% of AUC/MICs and found nephrotoxicity in 8% of trough-guided TDM patients compared with 2% of AUC/MIC-guided TDM patients.8
Unlike Nealy and colleagues, our study did not find lower nephrotoxicity associated with AUC/MIC-guided TDM. Multiple factors may have influenced our results. Our AUC/MIC group had significantly more newly started concomitant nephrotoxins and other nephrotoxic medications used during the vancomycin courses compared with the trough-guided group, which may have influenced AKI outcomes. It also should be noted that there was significantly more time spent subtherapeutic of the goal range and significantly less time in the goal range in the trough group compared with the AUC/MIC group. In our study, the trough-guided group had significantly more patients with acute illness compared with the AUC/MIC group (skin, soft tissue, and joint infections were similar between the groups). The group with more acutely ill patients would have been expected to have more nephrotoxicity. However, despite the acute illnesses, patients in the trough-guided group spent more time in the subtherapeutic range. This may explain the increased nephrotoxicity in the AUC/MIC group since those patients spent more time in the therapeutic range.
This study used the most recent KDIGO AKI definition: either an increase in sCr of ≥ 0.3 mg/dL or a 50% increase in sCr from baseline sustained over 48 hours without any other explanation for the change in renal function.11 This AKI definition is stricter than the previous definition, which was used by earlier studies, including Neely and colleagues, to evaluate rates of vancomycin-induced AKI.2,3 Therefore, the rates of overall AKI found in this study may be higher than in previous studies due to the definition of AKI used.
Limitations
This study was limited by its retrospective nature, lack of randomization, and small sample size. To decrease the potential for error in this study, analysis of power and a larger study sample would have been beneficial. During the COVID-19 pandemic, increased pneumonia cases may have hidden bacterial causes and caused an undercount. Nephrotoxicity may also be related to volume depletion, severe systemic illness, dehydration, or hypotension. Screening was completed via chart review for these alternative causes of nephrotoxicity in this study but may not be completely accounted for due to lack of documentation and the retrospective nature of this study.
CONCLUSIONS
This study did not find a significant difference in the rates of vancomycin-induced or overall AKI between AUC/MIC-guided and trough-guided TDM. However, this study may not have been powered to detect a significant difference in the primary endpoint. This study indicated that AUC/MIC-guided TDM of vancomycin resulted in a quicker time to the therapeutic range and a higher percentage of overall time in the therapeutic range as compared with trough-guided TDM. The results of this study indicated that trough-guided monitoring resulted in a higher percentage of time in a subtherapeutic range. This study also found that the first AUC/MIC calculated was within therapeutic range more often than the first trough level collected.
These results indicate that AUC/MIC-guided TDM may be more effective than trough-guided TDM in the veteran population. However, while AUC/MIC-guided TDM may be more effective with regards to time in therapeutic range and time to therapeutic range, this study did not indicate any safety benefit of AUC/MIC-guided over trough-guided TDM with regards to AKI incidence. Our data indicate that AUC/MIC-guided TDM increases the amount of time in the therapeutic range compared with trough-guided TDM and is not more nephrotoxic. The findings of this study support the recommendation to transition to the use of AUC/MIC-guided TDM of vancomycin in the veteran population.
Acknowledgments
This material is the result of work supported with the use of facilities and resources from the Sioux Falls Veterans Affairs Health Care System.
Vancomycin is a commonly used glycopeptide antibiotic used to treat infections caused by gram-positive organisms. Vancomycin is most often used as a parenteral agent for empiric or definitive treatment of methicillin-resistant Staphylococcus aureus (MRSA). It can also be used for the treatment of other susceptible Staphylococcus or Enterococcus species. Adverse effects of parenteral vancomycin include infusion-related reactions, ototoxicity, and nephrotoxicity.1 Higher vancomycin trough levels have been associated with an increased risk of nephrotoxicity.1-4 The major safety concern with vancomycin is acute kidney injury (AKI). Even mild AKI can prolong hospitalizations, increase the cost of health care, and increase morbidity.2
In March 2020, the American Society of Health-System Pharmacists, the Infectious Diseases Society of America (IDSA), the Pediatric Infectious Disease Society, and the Society of Infectious Diseases Pharmacists released a consensus statement and guidelines regarding the optimization of vancomycin dosing and monitoring for patients with suspected or definitive serious MRSA infections. Based on these guidelines, it is recommended to target an individualized area under the curve/minimum inhibitory concentration (AUC/MIC) ratio of 400 to 600 mg × h/L to maximize clinical efficacy and minimize the risk of AKI.2
Before March 2020, the vancomycin monitoring recommendation was to target trough levels of 10 to 20 mg/L. A goal trough of 15 to 20 mg/L was recommended for severe infections, including sepsis, endocarditis, hospital-acquired pneumonia, meningitis, and osteomyelitis, caused by MRSA. A goal trough of 10 to 15 mg/L was recommended for noninvasive infections, such as skin and soft tissue infections and urinary tract infections, caused by MRSA. Targeting these trough levels was thought to achieve an AUC/MIC ≥ 400 mg × h/L.5 Evidence has since shown that trough values may not be an optimal marker for AUC/MIC values.2
The updated vancomycin therapeutic drug monitoring (TDM) guidelines recommend that health systems transition to AUC/MIC-guided monitoring for suspected or confirmed infections caused by MRSA. There is not enough evidence to recommend AUC/MIC-guided monitoring in patients with noninvasive infections or infections caused by other microbes.2
AUC/MIC-guided monitoring can be achieved in 2 ways. The first method is collecting Cmax (peak level) and Cmin (trough level) serum concentrations, preferably during the same dosing interval. Ideally, Cmax should be drawn 1 to 2 hours after the vancomycin infusion and Cmin should be drawn at the end of the dosing interval. First-order pharmacokinetic equations are used to estimate the AUC/MIC with this method. Bayesian software pharmacokinetic modeling based on 1 or 2 vancomycin concentrations with 1 trough level also can be used for monitoring. Preferably, 2 levels would be obtained to estimate the AUC/MIC when using Bayesian modeling.2
The bactericidal activity of vancomycin was achieved with AUC/MIC ratios of ≥ 400 mg × h/L. AUC/MIC ratios of < 400 mg × h/L increase the incidence of resistant and intermediate strains of S aureus. AUC/MIC-guided monitoring assumes an MIC of 1 mg/L. When the MIC is > 1 mg/L, it is less likely that an AUC/MIC ≥ 400 mg × h/L is achievable. Regardless of the TDM method used, AUC/MIC ratios ≥ 400 mg × h/L are not achievable with conventional dosing methods if the vancomycin MIC is > 2 mg/L in patients with normal renal function. Alternative therapy is recommended to be used for these patients.2
There are multiple studies investigating the therapeutic dosing of vancomycin and the associated incidence of AKI. Previous studies have correlated vancomycin AUC/MICs of 400 mg to 600 mg × h/L with clinical effectiveness.2,6 In 2017, Neely and colleagues looked at the therapeutic dosing of vancomycin in 252 adults with ≥ 1 vancomycin level.7 During this prospective trial, they evaluated patients for 1 year and targeted trough concentrations of 10 to 20 mg/L with infection-specific goal ranges of 10 to 15 mg/L and 15 to 20 mg/L for noninvasive and invasive infections, respectively. They also targeted AUC/MIC ratios ≥ 400 mg × h/L regardless of trough concentration using Bayesian estimated AUC/MICs for 2 years. They found only 19% of trough concentrations to be therapeutic compared with 70% of AUC/MICs. A secondary outcome assessed by Neely and colleagues was nephrotoxicity, which was identified in 8% of patients with trough targets and 2% of patients with AUC/MIC targets.8
Previous studies evaluating the use of vancomycin in the veteran population have focused on AKI incidence, general nephrotoxicity, and 30-day readmission rates.4,7,9,10 Poston-Blahnik and colleagues investigated the rates of AKI in 200 veterans using AUC/MIC-guided vancomycin TDM.5 They found an AKI incidence of 42% of patients with AUC/MICs ≥ 550 mg × h/L and 2% of patients with AUC/MICs < 550 mg × h/L.5 Gyamlani and colleagues investigated the rates of AKI in 33,527 veterans and found that serum vancomycin trough levels ≥ 20 mg/L were associated with a higher risk of AKI.8 Prabaker and colleagues investigated the association between vancomycin trough levels and nephrotoxicity, defined as 0.5 mg/L or a 50% increase in serum creatinine (sCr) in 348 veterans. They found nephrotoxicity in 8.9% of patients.10 Patel and colleagues investigated the effect of AKI on 30-day readmission rates in 216 veterans.10 AKI occurred in 8.8% of patients and of those 19.4% were readmitted within 30 days.10 Current literature lacks evidence regarding the comparison of the safety and efficacy of vancomycin trough-guided vs AUC/MIC-guided TDM in the veteran population. Therefore, the objective of this study was to investigate the differences in the safety and efficacy of vancomycin TDM in the veteran population based on the different monitoring methods used.
METHODS
This study was a retrospective, single-center, quasi-experimental chart review conducted at the Sioux Falls Veterans Affairs Health Care System (SFVAHCS) in South Dakota. Data were collected from the Computerized Patient Record System (CPRS). The SFVAHCS transitioned from trough-guided to AUC/MIC-guided TDM in November 2020.
Patients included in this study were veterans aged ≥ 18 years with orders for parenteral vancomycin between February 1, 2020, and October 31, 2020, for the trough-guided TDM group and between December 1, 2020, and August 31, 2021, for the AUC/MIC-guided TDM group. Patients with vancomycin courses initiated during November 2020 were excluded as both TDM methods were being used at that time. Patients were excluded if their vancomycin course began before February 1, 2020, for the trough-guided TDM group or began during November 2020 for the AUC/MIC-guided TDM group. Patients were excluded if their vancomycin course extended past October 31, 2020, for the trough group or past August 31, 2021, for the AUC/MIC group. Patients on dialysis or missing Cmax, Cmin, or sCr levels were excluded.
This study evaluated both safety (AKI incidence) and effectiveness (time spent in therapeutic range and time to therapeutic range). The primary endpoint was presence of vancomycin-induced AKI, which was based on the most recent Kidney Disease: Improving Global Outcomes (KDIGO) AKI definition: increased sCr of ≥ 0.3 mg/dL or by 50% from baseline sustained over 48 hours without any other explanation for the change.11 A secondary endpoint was the absence or presence of AKI.
Additional secondary endpoints included the presence of the initial trough or AUC/MIC of each vancomycin course within the therapeutic range and the percentage of all trough levels or AUC/MICs within therapeutic, subtherapeutic, and supratherapeutic ranges. The therapeutic range for AUC/MIC-guided TDM was 400 to 600 mg × h/L and 10 to 20 mg/L depending on indication for trough-guided TDM (15-20 mg/L for severe infections and 10-15 mg/L for less invasive infections). The percentage of trough levels or AUC/MICs within therapeutic, subtherapeutic, and supratherapeutic ranges were calculated as a ratio of levels within each range to total levels taken for each patient.
For AUC/MIC-guided TDM the Cmax levels were ideally drawn 1 to 2 hours after vancomycin infusion and Cmin levels were ideally drawn 30 minutes before the next dose. First-order pharmacokinetic equations were used to estimate the AUC/MIC.12 If the timing of a vancomycin level was inappropriate, actual levels were extrapolated based on the timing of the blood draw compared with the ideal Cmin or Cmax time. Extrapolated levels were used for both trough-guided and AUC/MIC-guided TDM groups when appropriate. Vancomycin levels were excluded if they were drawn during the vancomycin infusion.
Study participant age, sex, race, weight, baseline estimated glomerular filtration (eGFR) rate, baseline sCr, concomitant nephrotoxic medications, duration of vancomycin course, indication of vancomycin, and acuity of illness based on indication were collected. sCr levels were collected from the initial day vancomycin was ordered through 72 hours following completion of a vancomycin course to evaluate for AKI. Patients’ charts were reviewed for the use of the following nephrotoxic medications: nonsteroidal anti-inflammatories, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, aminoglycosides, piperacillin/tazobactam, loop diuretics, amphotericin B, acyclovir, intravenous contrast, and nephrotoxic chemotherapy (cisplatin). The category of concomitant nephrotoxic medications was also collected including the continuation of a home nephrotoxic medication vs the initiation of a new nephrotoxic medication.
Statistical Analysis
The primary endpoint of the incidence of vancomycin-induced AKI was compared using a Fisher exact test. The secondary endpoint of the percentage of trough levels or AUC/MICs in the therapeutic, subtherapeutic, and supratherapeutic range were compared using a student t test. The secondary endpoint of first level or AUC/MIC within goal range was compared using a χ2 test. Continuous baseline characteristics were reported as a mean and compared using a student t test. Nominal baseline characteristics were reported as a percentage and compared using the χ2 test. P values < .05 were considered statistically significant.
RESULTS
This study included 97 patients, 43 in the AUC/MIC group and 54 in the trough group.
One (2%) patient in the AUC/MIC group and 2 (4%) patients in the trough group experienced vancomycin-induced AKI (P = .10) (Table 2).
DISCUSSION
There was no statistically significant difference between the 2 groups for the vancomycin-induced AKI (P = .10), the primary endpoint, or overall AKI (P = .29), the secondary endpoint. It should be noted that there was more overall AKI in the AUC/MIC group. Veterans in the AUC/MIC group were found to have their first AUC/MIC within the therapeutic range statistically significantly more often than the first trough level in the trough group (P = .04). The percentage of time spent within therapeutic range was statistically significantly higher in the AUC/MIC-guided TDM group (P = .02). The percentage of time spent subtherapeutic of goal range was statistically significantly higher in the trough-guided TDM group (P < .001). There was no statistically significant difference found in the percent of time spent supratherapeutic of goal range (P = .25). However, the observed percentage of time spent supratherapeutic of goal range was higher in the AUC/MIC group. These results indicate that AUC/MIC-guided TDM may be more efficacious with regard to time in therapeutic range and time to therapeutic range.
The finding of increased AKI with AUC/MIC-guided TDM does not align with previous studies.8 The prospective study by Neely and colleagues found that AUC/MIC-guided TDM resulted in more time in the therapeutic range as well as less nephrotoxicity compared with trough-guided TDM, although it was limited by its lack of randomization and did not account for other causes of nephrotoxicity.8 They found that only 19% of trough concentrations were therapeutic compared with 70% of AUC/MICs and found nephrotoxicity in 8% of trough-guided TDM patients compared with 2% of AUC/MIC-guided TDM patients.8
Unlike Nealy and colleagues, our study did not find lower nephrotoxicity associated with AUC/MIC-guided TDM. Multiple factors may have influenced our results. Our AUC/MIC group had significantly more newly started concomitant nephrotoxins and other nephrotoxic medications used during the vancomycin courses compared with the trough-guided group, which may have influenced AKI outcomes. It also should be noted that there was significantly more time spent subtherapeutic of the goal range and significantly less time in the goal range in the trough group compared with the AUC/MIC group. In our study, the trough-guided group had significantly more patients with acute illness compared with the AUC/MIC group (skin, soft tissue, and joint infections were similar between the groups). The group with more acutely ill patients would have been expected to have more nephrotoxicity. However, despite the acute illnesses, patients in the trough-guided group spent more time in the subtherapeutic range. This may explain the increased nephrotoxicity in the AUC/MIC group since those patients spent more time in the therapeutic range.
This study used the most recent KDIGO AKI definition: either an increase in sCr of ≥ 0.3 mg/dL or a 50% increase in sCr from baseline sustained over 48 hours without any other explanation for the change in renal function.11 This AKI definition is stricter than the previous definition, which was used by earlier studies, including Neely and colleagues, to evaluate rates of vancomycin-induced AKI.2,3 Therefore, the rates of overall AKI found in this study may be higher than in previous studies due to the definition of AKI used.
Limitations
This study was limited by its retrospective nature, lack of randomization, and small sample size. To decrease the potential for error in this study, analysis of power and a larger study sample would have been beneficial. During the COVID-19 pandemic, increased pneumonia cases may have hidden bacterial causes and caused an undercount. Nephrotoxicity may also be related to volume depletion, severe systemic illness, dehydration, or hypotension. Screening was completed via chart review for these alternative causes of nephrotoxicity in this study but may not be completely accounted for due to lack of documentation and the retrospective nature of this study.
CONCLUSIONS
This study did not find a significant difference in the rates of vancomycin-induced or overall AKI between AUC/MIC-guided and trough-guided TDM. However, this study may not have been powered to detect a significant difference in the primary endpoint. This study indicated that AUC/MIC-guided TDM of vancomycin resulted in a quicker time to the therapeutic range and a higher percentage of overall time in the therapeutic range as compared with trough-guided TDM. The results of this study indicated that trough-guided monitoring resulted in a higher percentage of time in a subtherapeutic range. This study also found that the first AUC/MIC calculated was within therapeutic range more often than the first trough level collected.
These results indicate that AUC/MIC-guided TDM may be more effective than trough-guided TDM in the veteran population. However, while AUC/MIC-guided TDM may be more effective with regards to time in therapeutic range and time to therapeutic range, this study did not indicate any safety benefit of AUC/MIC-guided over trough-guided TDM with regards to AKI incidence. Our data indicate that AUC/MIC-guided TDM increases the amount of time in the therapeutic range compared with trough-guided TDM and is not more nephrotoxic. The findings of this study support the recommendation to transition to the use of AUC/MIC-guided TDM of vancomycin in the veteran population.
Acknowledgments
This material is the result of work supported with the use of facilities and resources from the Sioux Falls Veterans Affairs Health Care System.
1. Gallagher J, MacDougall C. Glycopeptides and short-acting lipoglycopeptides In: Antibiotics Simplified. Jones & Bartlett Learning; 2018.
2. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835-864. doi:10.1093/ajhp/zxaa036
3. Hermsen ED, Hanson M, Sankaranarayanan J, Stoner JA, Florescu MC, Rupp ME. Clinical outcomes and nephrotoxicity associated with vancomycin trough concentrations during treatment of deep-seated infections. Expert Opin Drug Saf. 2010;9(1):9-14. doi:10.1517/14740330903413514
4. Poston-Blahnik A, Moenster R. Association between vancomycin area under the curve and nephrotoxicity: a single center, retrospective cohort study in a veteran population. Open Forum Infect Dis. 2021;8(5):ofab094. Published 2021 Mar 12. doi:10.1093/ofid/ofab094
5. Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66(1):82-98. doi:10.2146/ajhp080434
6. Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet. 2004;43(13):925-942. doi:10.2165/00003088-200443130-00005
7. Gyamlani G, Potukuchi PK, Thomas F, et al. Vancomycin-Associated Acute Kidney Injury in a Large Veteran Population. Am J Nephrol. 2019;49(2):133-142. doi:10.1159/000496484
8. Neely MN, Kato L, Youn G, et al. Prospective Trial on the Use of Trough Concentration versus Area under the Curve To Determine Therapeutic Vancomycin Dosing. Antimicrob Agents Chemother. 2018;62(2):e02042-17. Published 2018 Jan 25. doi:10.1128/AAC.02042-17
9. Prabaker KK, Tran TP, Pratummas T, Goetz MB, Graber CJ. Elevated vancomycin trough is not associated with nephrotoxicity among inpatient veterans. J Hosp Med. 2012;7(2):91-97. doi:10.1002/jhm.946
10. Patel N, Stornelli N, Sangiovanni RJ, Huang DB, Lodise TP. Effect of vancomycin-associated acute kidney injury on incidence of 30-day readmissions among hospitalized Veterans Affairs patients with skin and skin structure infections. Antimicrob Agents Chemother. 2020;64(10):e01268-20. Published 2020 Sep 21. doi:10.1128/AAC.01268-20
11. Acute Kidney Injury Work Group. Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. 2012;2(suppl 1):1-138.
12. Pai MP, Neely M, Rodvold KA, Lodise TP. Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv Drug Deliv Rev. 2014;77:50-57. doi:10.1016/j.addr.2014.05.016
1. Gallagher J, MacDougall C. Glycopeptides and short-acting lipoglycopeptides In: Antibiotics Simplified. Jones & Bartlett Learning; 2018.
2. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835-864. doi:10.1093/ajhp/zxaa036
3. Hermsen ED, Hanson M, Sankaranarayanan J, Stoner JA, Florescu MC, Rupp ME. Clinical outcomes and nephrotoxicity associated with vancomycin trough concentrations during treatment of deep-seated infections. Expert Opin Drug Saf. 2010;9(1):9-14. doi:10.1517/14740330903413514
4. Poston-Blahnik A, Moenster R. Association between vancomycin area under the curve and nephrotoxicity: a single center, retrospective cohort study in a veteran population. Open Forum Infect Dis. 2021;8(5):ofab094. Published 2021 Mar 12. doi:10.1093/ofid/ofab094
5. Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66(1):82-98. doi:10.2146/ajhp080434
6. Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet. 2004;43(13):925-942. doi:10.2165/00003088-200443130-00005
7. Gyamlani G, Potukuchi PK, Thomas F, et al. Vancomycin-Associated Acute Kidney Injury in a Large Veteran Population. Am J Nephrol. 2019;49(2):133-142. doi:10.1159/000496484
8. Neely MN, Kato L, Youn G, et al. Prospective Trial on the Use of Trough Concentration versus Area under the Curve To Determine Therapeutic Vancomycin Dosing. Antimicrob Agents Chemother. 2018;62(2):e02042-17. Published 2018 Jan 25. doi:10.1128/AAC.02042-17
9. Prabaker KK, Tran TP, Pratummas T, Goetz MB, Graber CJ. Elevated vancomycin trough is not associated with nephrotoxicity among inpatient veterans. J Hosp Med. 2012;7(2):91-97. doi:10.1002/jhm.946
10. Patel N, Stornelli N, Sangiovanni RJ, Huang DB, Lodise TP. Effect of vancomycin-associated acute kidney injury on incidence of 30-day readmissions among hospitalized Veterans Affairs patients with skin and skin structure infections. Antimicrob Agents Chemother. 2020;64(10):e01268-20. Published 2020 Sep 21. doi:10.1128/AAC.01268-20
11. Acute Kidney Injury Work Group. Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. 2012;2(suppl 1):1-138.
12. Pai MP, Neely M, Rodvold KA, Lodise TP. Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv Drug Deliv Rev. 2014;77:50-57. doi:10.1016/j.addr.2014.05.016
Methacrylate Polymer Powder Dressing for a Lower Leg Surgical Defect
To the Editor:
Surgical wounds on the lower leg are challenging to manage because venous stasis, bacterial colonization, and high tension may contribute to protracted healing. Advances in technology led to the development of novel, polymer-based wound-healing modalities that hold promise for the management of these wounds.
A 75-year-old man presented with a well-differentiated squamous cell carcinoma with a 3-mm depth of invasion on the left pretibial region. His comorbidities were notable for hypertension, hypercholesterolemia, varicose veins, myocardial infarction, peripheral vascular disease, and a 32 pack-year cigarette smoking history. Current medications included clopidogrel bisulfate and warfarin sodium to manage a recently placed coronary artery stent.
The tumor was cleared after 2 stages of Mohs micrographic surgery with excision down to tibialis anterior fascia (Figure 1A). The resultant defect measured 43×33 mm in area and 9 mm in depth (wound size, 12,771 mm3). Reconstructive options were discussed, including random-pattern flap repair and skin graft. Given the patient’s risk of bleeding, the decision was made to forego a flap repair. Additionally, the patient was a heavy smoker and could not comply with the wound care and elevation and ambulation restrictions required for optimal skin graft care. Therefore, a decision was made to proceed with secondary intention healing using a methacrylate polymer powder dressing.
After achieving hemostasis, a novel 10-mg sterile, biologically inert methacrylate polymer powder dressing was poured over the wound in a uniform layer to fill and seal the entire wound surface (Figure 1B). Sterile normal saline 0.1 mL was sprayed onto the powder to activate particle aggregation. No secondary dressing was used, and the patient was permitted to get the dressing wet after 48 hours.
The dressing was changed in a similar fashion 4 weeks after application, following gentle debridement with gauze and normal saline. Eight weeks after surgery, the wound exhibited healthy granulation tissue and measured 5×6 mm in area and 2 mm in depth (wound size, 60 mm3), which represented a 99.5% reduction in wound size (Figure 1C). The dressing was not painful, and there were no reported adverse effects. The patient continued to smoke and ambulate fully throughout this period. No antibiotics were used.
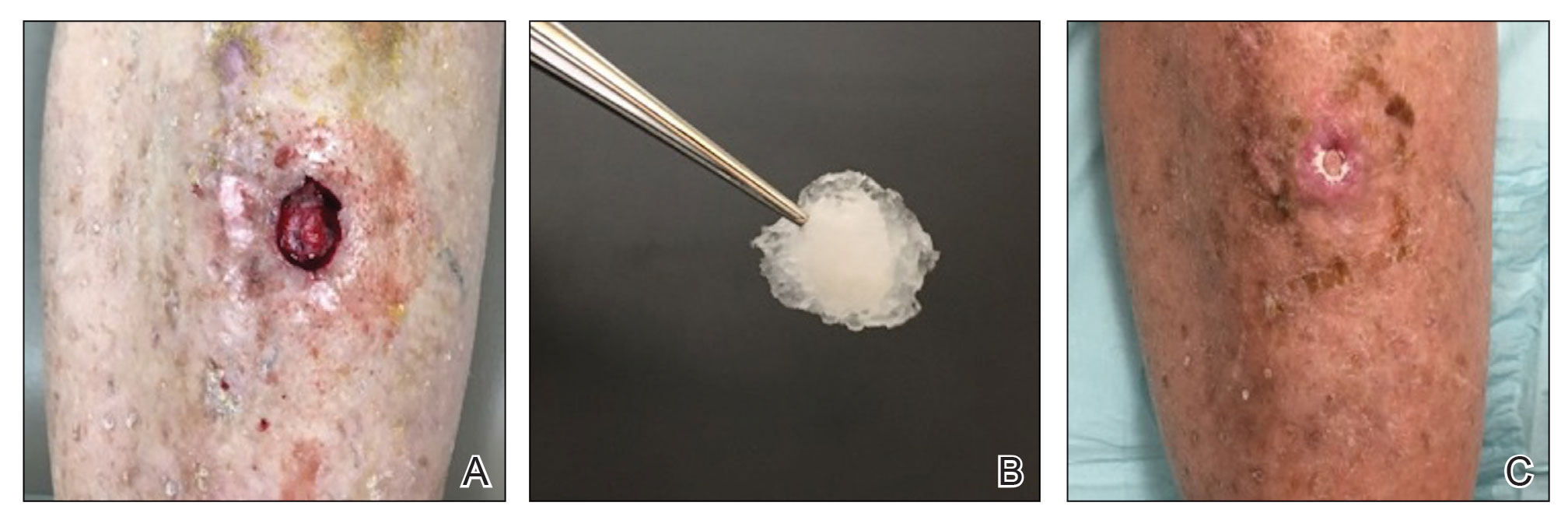
Methacrylate polymer powder dressings are a novel and sophisticated dressing modality with great promise for the management of surgical wounds on the lower limb. The dressing is a sterile powder consisting of 84.8% poly-2-hydroxyethylmethacrylate, 14.9% poly-2-hydroxypropylmethacrylate, and 0.3% sodium deoxycholate. These hydrophilic polymers have a covalent methacrylate backbone with a hydroxyl aliphatic side chain. When saline or wound exudate contacts the powder, the spheres hydrate and nonreversibly aggregate to form a moist, flexible dressing that conforms to the topography of the wound and seals it (Figure 2).1
Once the spheres have aggregated, they are designed to orient in a honeycomb formation with 4- to 10-nm openings that serve as capillary channels (Figure 3). This porous architecture of the polymer is essential for adequate moisture management. It allows for vapor transpiration at a rate of 12 L/m2 per day, which ensures the capillary flow from the moist wound surface is evenly distributed through the dressing, contributing to its 68% water content. Notably, this approximately three-fifths water composition is similar to the water makeup of human skin. Optimized moisture management is theorized to enhance epithelial migration, stimulate angiogenesis, retain growth factors, promote autolytic debridement, and maintain ideal voltage and oxygen gradients for wound healing. The risk for infection is not increased by the existence of these pores, as their small size does not allow for bacterial migration.1
This case demonstrates the effectiveness of using a methacrylate polymer powder dressing to promote timely wound healing in a poorly vascularized lower leg surgical wound. The low maintenance, user-friendly dressing was changed at monthly intervals, which spared the patient the inconvenience and pain associated with the repeated application of more conventional primary and secondary dressings. The dressing was well tolerated and resulted in a 99.5% reduction in wound size. Further studies are needed to investigate the utility of this promising technology.
1. Fitzgerald RH, Bharara M, Mills JL, et al. Use of a nanoflex powder dressing for wound management following debridement for necrotising fasciitis in the diabetic foot. Int Wound J. 2009;6:133-139.
To the Editor:
Surgical wounds on the lower leg are challenging to manage because venous stasis, bacterial colonization, and high tension may contribute to protracted healing. Advances in technology led to the development of novel, polymer-based wound-healing modalities that hold promise for the management of these wounds.
A 75-year-old man presented with a well-differentiated squamous cell carcinoma with a 3-mm depth of invasion on the left pretibial region. His comorbidities were notable for hypertension, hypercholesterolemia, varicose veins, myocardial infarction, peripheral vascular disease, and a 32 pack-year cigarette smoking history. Current medications included clopidogrel bisulfate and warfarin sodium to manage a recently placed coronary artery stent.
The tumor was cleared after 2 stages of Mohs micrographic surgery with excision down to tibialis anterior fascia (Figure 1A). The resultant defect measured 43×33 mm in area and 9 mm in depth (wound size, 12,771 mm3). Reconstructive options were discussed, including random-pattern flap repair and skin graft. Given the patient’s risk of bleeding, the decision was made to forego a flap repair. Additionally, the patient was a heavy smoker and could not comply with the wound care and elevation and ambulation restrictions required for optimal skin graft care. Therefore, a decision was made to proceed with secondary intention healing using a methacrylate polymer powder dressing.
After achieving hemostasis, a novel 10-mg sterile, biologically inert methacrylate polymer powder dressing was poured over the wound in a uniform layer to fill and seal the entire wound surface (Figure 1B). Sterile normal saline 0.1 mL was sprayed onto the powder to activate particle aggregation. No secondary dressing was used, and the patient was permitted to get the dressing wet after 48 hours.
The dressing was changed in a similar fashion 4 weeks after application, following gentle debridement with gauze and normal saline. Eight weeks after surgery, the wound exhibited healthy granulation tissue and measured 5×6 mm in area and 2 mm in depth (wound size, 60 mm3), which represented a 99.5% reduction in wound size (Figure 1C). The dressing was not painful, and there were no reported adverse effects. The patient continued to smoke and ambulate fully throughout this period. No antibiotics were used.

Methacrylate polymer powder dressings are a novel and sophisticated dressing modality with great promise for the management of surgical wounds on the lower limb. The dressing is a sterile powder consisting of 84.8% poly-2-hydroxyethylmethacrylate, 14.9% poly-2-hydroxypropylmethacrylate, and 0.3% sodium deoxycholate. These hydrophilic polymers have a covalent methacrylate backbone with a hydroxyl aliphatic side chain. When saline or wound exudate contacts the powder, the spheres hydrate and nonreversibly aggregate to form a moist, flexible dressing that conforms to the topography of the wound and seals it (Figure 2).1

Once the spheres have aggregated, they are designed to orient in a honeycomb formation with 4- to 10-nm openings that serve as capillary channels (Figure 3). This porous architecture of the polymer is essential for adequate moisture management. It allows for vapor transpiration at a rate of 12 L/m2 per day, which ensures the capillary flow from the moist wound surface is evenly distributed through the dressing, contributing to its 68% water content. Notably, this approximately three-fifths water composition is similar to the water makeup of human skin. Optimized moisture management is theorized to enhance epithelial migration, stimulate angiogenesis, retain growth factors, promote autolytic debridement, and maintain ideal voltage and oxygen gradients for wound healing. The risk for infection is not increased by the existence of these pores, as their small size does not allow for bacterial migration.1

This case demonstrates the effectiveness of using a methacrylate polymer powder dressing to promote timely wound healing in a poorly vascularized lower leg surgical wound. The low maintenance, user-friendly dressing was changed at monthly intervals, which spared the patient the inconvenience and pain associated with the repeated application of more conventional primary and secondary dressings. The dressing was well tolerated and resulted in a 99.5% reduction in wound size. Further studies are needed to investigate the utility of this promising technology.
To the Editor:
Surgical wounds on the lower leg are challenging to manage because venous stasis, bacterial colonization, and high tension may contribute to protracted healing. Advances in technology led to the development of novel, polymer-based wound-healing modalities that hold promise for the management of these wounds.
A 75-year-old man presented with a well-differentiated squamous cell carcinoma with a 3-mm depth of invasion on the left pretibial region. His comorbidities were notable for hypertension, hypercholesterolemia, varicose veins, myocardial infarction, peripheral vascular disease, and a 32 pack-year cigarette smoking history. Current medications included clopidogrel bisulfate and warfarin sodium to manage a recently placed coronary artery stent.
The tumor was cleared after 2 stages of Mohs micrographic surgery with excision down to tibialis anterior fascia (Figure 1A). The resultant defect measured 43×33 mm in area and 9 mm in depth (wound size, 12,771 mm3). Reconstructive options were discussed, including random-pattern flap repair and skin graft. Given the patient’s risk of bleeding, the decision was made to forego a flap repair. Additionally, the patient was a heavy smoker and could not comply with the wound care and elevation and ambulation restrictions required for optimal skin graft care. Therefore, a decision was made to proceed with secondary intention healing using a methacrylate polymer powder dressing.
After achieving hemostasis, a novel 10-mg sterile, biologically inert methacrylate polymer powder dressing was poured over the wound in a uniform layer to fill and seal the entire wound surface (Figure 1B). Sterile normal saline 0.1 mL was sprayed onto the powder to activate particle aggregation. No secondary dressing was used, and the patient was permitted to get the dressing wet after 48 hours.
The dressing was changed in a similar fashion 4 weeks after application, following gentle debridement with gauze and normal saline. Eight weeks after surgery, the wound exhibited healthy granulation tissue and measured 5×6 mm in area and 2 mm in depth (wound size, 60 mm3), which represented a 99.5% reduction in wound size (Figure 1C). The dressing was not painful, and there were no reported adverse effects. The patient continued to smoke and ambulate fully throughout this period. No antibiotics were used.

Methacrylate polymer powder dressings are a novel and sophisticated dressing modality with great promise for the management of surgical wounds on the lower limb. The dressing is a sterile powder consisting of 84.8% poly-2-hydroxyethylmethacrylate, 14.9% poly-2-hydroxypropylmethacrylate, and 0.3% sodium deoxycholate. These hydrophilic polymers have a covalent methacrylate backbone with a hydroxyl aliphatic side chain. When saline or wound exudate contacts the powder, the spheres hydrate and nonreversibly aggregate to form a moist, flexible dressing that conforms to the topography of the wound and seals it (Figure 2).1

Once the spheres have aggregated, they are designed to orient in a honeycomb formation with 4- to 10-nm openings that serve as capillary channels (Figure 3). This porous architecture of the polymer is essential for adequate moisture management. It allows for vapor transpiration at a rate of 12 L/m2 per day, which ensures the capillary flow from the moist wound surface is evenly distributed through the dressing, contributing to its 68% water content. Notably, this approximately three-fifths water composition is similar to the water makeup of human skin. Optimized moisture management is theorized to enhance epithelial migration, stimulate angiogenesis, retain growth factors, promote autolytic debridement, and maintain ideal voltage and oxygen gradients for wound healing. The risk for infection is not increased by the existence of these pores, as their small size does not allow for bacterial migration.1

This case demonstrates the effectiveness of using a methacrylate polymer powder dressing to promote timely wound healing in a poorly vascularized lower leg surgical wound. The low maintenance, user-friendly dressing was changed at monthly intervals, which spared the patient the inconvenience and pain associated with the repeated application of more conventional primary and secondary dressings. The dressing was well tolerated and resulted in a 99.5% reduction in wound size. Further studies are needed to investigate the utility of this promising technology.
1. Fitzgerald RH, Bharara M, Mills JL, et al. Use of a nanoflex powder dressing for wound management following debridement for necrotising fasciitis in the diabetic foot. Int Wound J. 2009;6:133-139.
1. Fitzgerald RH, Bharara M, Mills JL, et al. Use of a nanoflex powder dressing for wound management following debridement for necrotising fasciitis in the diabetic foot. Int Wound J. 2009;6:133-139.
PRACTICE POINTS
- Lower leg surgical wounds are difficult to manage, as venous stasis, bacterial colonization, and high tension may contribute to protracted healing.
- A methacrylate polymer powder dressing is user friendly and facilitates granulation and reduction in size of difficult lower leg wounds.