User login
One Expert’s Approach in Transplant-Ineligible, Newly Diagnosed Multiple Myeloma
1. The treatment of multiple myeloma has evolved significantly in recent years. What are some of the most important things you consider in the treatment of your newly diagnosed, transplant-ineligible patients?
We’ve seen great progress in the treatment of multiple myeloma over the last decade, and outcomes continue to improve for many patients.1 Still, it is important to keep in mind that more than 34,000 patients will be diagnosed and more than 12,000 people will die from the disease this year.2 We may have the greatest opportunity to impact the course of disease in the treatment of newly diagnosed patients due to the nature of this cancer:
- Multiple myeloma is characterized by relapse, and we know the length of remission generally decreases with each relapse and subsequent line of therapy.3
- Patients often become refractory to treatment over time.
When I meet with a patient who has been diagnosed with multiple myeloma, the first thing I consider is their eligibility for autologous stem cell transplant (ASCT). In my opinion, the introduction of ASCT is one of the biggest advancements in the last few decades, and we’ve found that ASCT followed by maintenance therapy with targeted tools improves progression-free survival (PFS).4
Unfortunately, many newly diagnosed patients are not eligible for ASCT–either because of comorbidities or other complexities related to the presentation of their disease.
For patients who are transplant-ineligible (TIE), it is important to have treatment options that are proven effective in extending PFS and overall survival (OS), and capable of producing deep and durable responses.
2. What are the challenges associated with treating newly diagnosed patients who are not eligible for ASCT?
We still consider multiple myeloma to be an incurable disease but, in my opinion, the treatment of TIE patients is less challenging today than a decade ago due to the emergence of novel therapies. That said, TIE patients are typically older and present with more advanced disease and comorbidities, including diabetes or cardiovascular events.5
A retrospective analysis published in 2020 by Rafael Fonseca examined frontline treatment patterns and attrition rates by line of therapy among newly diagnosed multiple myeloma (NDMM) patients who are TIE. More than 22,000 patients were identified from three patient-level databases between 2000 and 2018 - the OPTUM Commercial Claims database, the OPTUM Electronic Medical Records database, and the Surveillance, Epidemiology, and End Results-Meidcare Linked database. Patients included had to have a multiple myeloma diagnosis on or after January 1, 2007. Results showed that attrition rates among newly diagnosed, TIE patients with multiple myeloma increase with each line of therapy, with the proportion of patients who receive a second line of therapy decreasing by 50 percent with each subsequent line.3
3. Can you provide more detail on the goals of therapy for newly diagnosed, transplant-ineligible patients?
When I discuss treatment goals with TIE patients, I feel it is important to emphasize managing side effects and achieving deep and durable responses. I have the benefit of being in an academic setting, where I regularly exchange information with my colleagues about what we’re learning from the clinical studies in which we participate. Choosing which treatment to administer is complex and involves other considerations. For example, if two regimens have comparable efficacy, I may recommend the regimen with a more established safety profile or more robust evidence so I can properly anticipate and manage toxicities in my patients. Overall survival is one of the most important endpoints I consider, in addition to depth of response and PFS. In recent years, we’ve seen increasing evidence pointing to the importance of using a proven effective treatment in frontline patients that are ineligible for transplant.
4. A key study in newly-diagnosed, transplant-ineligible multiple myeloma is the Phase 3 MAIA study. Can you share the key takeaways from this study and discuss how the results have shaped treatment for this patient population?
Of course. The MAIA study is a randomized Phase 3 study evaluating DARZALEX® (daratumumab) intravenous injection in combination with lenalidomide and dexamethasone (D-Rd) compared with Rd in 737 adult patients with newly diagnosed, transplant-ineligible multiple myeloma. The median age of patients participating in the MAIA study was 73 (range 45-90), an important consideration since the median age for multiple myeloma diagnosis is approximately 66-70 years of age.6 The study evaluated PFS as the primary endpoint, and overall survival as a key secondary endpoint, and supported the FDA approval of DARZALEX® in combination with lenalidomide and dexamethasone for adult patients with newly diagnosed, multiple myeloma who are ineligible for ASCT.
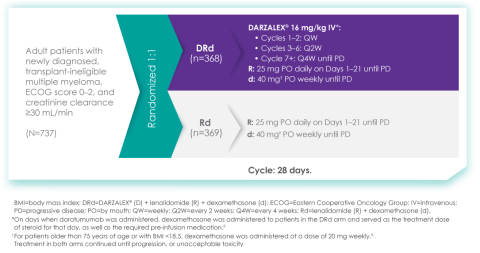
MAIA study design7
The baseline demographic and disease characteristics were similar between the 2 treatment groups. Forty-four percent of the patients were ≥75 years of age. Fifty-two percent (52%) of patients were male, 92% White, 4% Black or African American, and 1% Asian. Three percent (3%) of patients reported an ethnicity of Hispanic or Latino. Thirty-four (34%) had an Eastern Cooperative Oncology Group (ECOG) performance score of 0, 50% had an ECOG performance score of 1, and 17% had an ECOG performance score of ≥2. Twenty-seven percent had International Staging System (ISS) Stage I, 43% had ISS Stage II, and 29% had ISS Stage III disease.
Select Important Safety Information:
CONTRAINDICATIONS
DARZALEX® is contraindicated in patients with a history of severe hypersensitivity (eg, anaphylactic reactions) to daratumumab or any of the components of the formulation.
WARNINGS AND PRECAUTIONS
Infusion-Related Reactions: DARZALEX® can cause severe and/or serious infusion-related reactions including anaphylactic reactions.
These reactions can be life-threatening, and fatal outcomes have been reported. Please scroll down to read Important Safety Information for DARZALEX®.
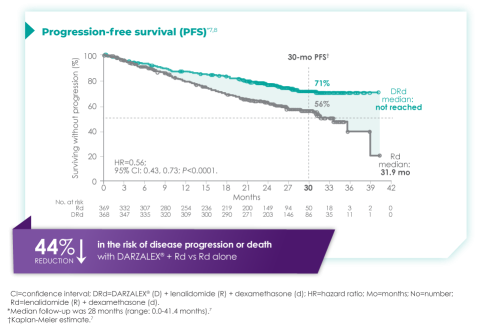
Primary findings from the study, which were published in 2019, showed an improvement in PFS in patients receiving D-Rd compared with those receiving Rd alone.7 The median PFS was not reached in the D-Rd arm and was reached at 31.9 months in the Rd arm (HR 0.56; 95% CI 0.43-0.73; P<0.0001).7 At a median of 30 months of follow-up, the data showed the clinical benefit of D-Rd therapy, with a 44% reduction in the risk of disease progression or death in patients receiving D-Rd compared with Rd alone.7
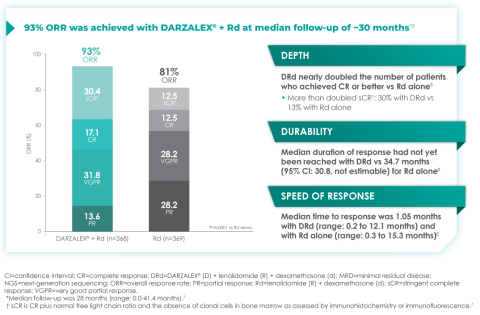
Additionally, 70.6% of patients (95% CI, 65.0-75.4) had no progressive disease with D-Rd treatment at median 30 months of follow-up, compared with 55.6% (95% CI, 49.5-61.3) of patients in the Rd group.7
In terms of depth of response, the percentage of patients with a complete response or better was 47.6% in patients receiving D-Rd compared with 24.9% in the Rd group.7
Overall response rate with D-Rd in TIE NDMM at ~30 months of follow-up8
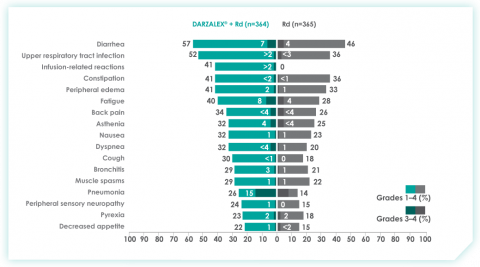
An overview of the most frequent adverse events at 30-months of follow-up are provided below. The most frequent adverse reactions were reported in ≥20% of patients, with at least a 5% greater frequency in the D-Rd arm compared with Rd alone.8
Most frequent adverse events at ~30 months of follow-up with D-Rd in TIE NDMM8
Most frequent hematologic laboratory abnormalities with D-Rd in TIE NDMM at ~30 months8
Serious adverse reactions with a 2% greater incidence in the D-Rd arm compared with the Rd arm were pneumonia (D-Rd 15% vs Rd 8%), bronchitis (D-Rd 4% vs Rd 2%), and dehydration (D-Rd 2% vs Rd <1).
• Discontinuation rates due to any adverse event: 7% with D-Rd vs 16% with Rd
• Infusion-related reactions (IRRs) with D-Rd occurred in 41% of patients; 2% were Grade 3 and <1% were Grade 4
• IRRs of any grade or severity may require management by interruption, modification, and/or discontinuation of the infusion
• Most IRRs occurred during first infusion
5. Thanks for that overview. In addition to these results, The Lancet Oncology has published updated overall survival data from a 5-year follow-up on the MAIA study. Can you provide an overview of these data and insights on their potential for patients?
The MAIA trial was an important study, and for me, the results were practice changing. We see that after a median of nearly 5 years of follow-up, D-Rd significantly improved OS in TIE NDMM patients who were treated to progression compared with Rd alone (66.3% vs. 53.1% [HR=0.68; 95% CI, 0.53-0.86; P=0.0013]).9 This equates to approximately a 32% reduction in death when DARZALEX® was added to a two-drug regimen, which is a meaningful consideration when selecting the most appropriate regimens for my newly diagnosed, transplant-ineligible patients.9
Overall survival data at ~5 years with D-Rd compared to Rd alone in TIE NDMM9
Importantly, efficacy that resulted from longer treatment with D-Rd is also supported by approximately 5 years of safety evaluation. Below is information from a follow-up analysis of the MAIA study. This information is not included in the current Prescribing Information and has not been evaluated by the FDA. Treatment-emergent adverse events are reported as observed. These analyses have not been adjusted for multiple comparisons and no conclusions should be drawn. In what I’ve observed through published data and in my practice, longer treatment has not revealed new safety signals.
Most frequent treatment-emergent adverse events (any grade reported in ≥30% of patients and/or Grade 3/4 reported in ≥10% of patients) at ~5 years9
Select Important Safety Information:
DARZALEX® can cause severe and/or serious infusion-related reactions including anaphylactic reactions. These reactions can be life threatening, and fatal outcomes have been reported. In clinical trials (monotherapy and combination: N=2066), infusion-related reactions occurred in 37% of patients with the Week 1 (16 mg/kg) infusion, 2% with the Week 2 infusion, and cumulatively 6% with subsequent infusions. Less than 1% of patients had a Grade 3/4 infusion-related reaction at Week 2 or subsequent infusions. The median time to onset was 1.5 hours (range: 0 to 73 hours). Nearly all reactions occurred during infusion or within 4 hours of completing DARZALEX®. Severe reactions have occurred, including bronchospasm, hypoxia, dyspnea, hypertension, tachycardia, headache, laryngeal edema, pulmonary edema, and ocular adverse reactions, including choroidal effusion, acute myopia, and acute angle closure glaucoma. Signs and symptoms may include respiratory symptoms, such as nasal congestion, cough, throat irritation, as well as chills, vomiting, and nausea. Less common signs and symptoms were wheezing, allergic rhinitis, pyrexia, chest discomfort, pruritus, hypotension and blurred vision. Please scroll down to see Important Safety Information for DARZALEX®.
6. Does the availability of OS data influence your decisions on treatment selection in TIE NDMM?
Overall survival absolutely remains the gold standard and informs my practice. Prior to OS data being available, I will often look at other efficacy endpoints that are available sooner. In MAIA, I was encouraged by efficacy endpoints in earlier data, which were later confirmed by the latest data on OS.
7. The MAIA study shows that treating to disease progression or unacceptable toxicity is important. How does that impact your approach to treatment?
It's important to keep in mind that the MAIA trial was designed to evaluate treatment until progression or unacceptable toxicity. The results revealed a significant difference between the DR-d and Rd treatment arms, but results observed in this study are contingent on this treatment approach. From a clinical perspective, unless there is considerable toxicity, I advocate for treating with D-Rd to progression.
In the clinic, we also see that TIE patients who have higher frailty scores are more likely to discontinue treatment prior to progression.10 There can be other reasons too – such as a patient simply wanting to have a break from treatment. These conversations are not always easy, but it is important to have an honest dialogue with patients.
8. What can we learn from studies like the MAIA trial that included a wide range of patient populations including patients who are elderly, frail, or had high cytogenetic risk?
Several patient subgroups were analyzed as part of the MAIA study. It is important to note that these subgroup analyses are not included in the Prescribing Information for DARZALEX®. These analyses were not adjusted for multiple comparisons, and there are insufficient numbers of patients per subgroup to make definitive conclusions of efficacy among the subgroups.
As mentioned above, the MAIA study evaluated a wide range of patients (n=737). The baseline demographic and disease characteristics were similar between the D-Rd and Rd treatment groups and the median age was 73 (range: 45-90) years, with 44% of the patients ≥75 years of age.
In the various patient subgroups that were analyzed as part of the MAIA study, it was found that at ~3-years of follow-up the PFS numerically favored DRd compared with Rd alone in most subgroups (see table below).
Median progression-free survival by sub-population at ~3 year follow-up8
The MAIA trial also included patients who were frail and a post hoc analysis was conducted in this subgroup of patients. These analyses are not included in the Prescribing Information for DARZALEX®. These analyses were conducted post hoc and there are insufficient numbers of patients per subgroup to make definitive conclusions of efficacy among the subgroups.
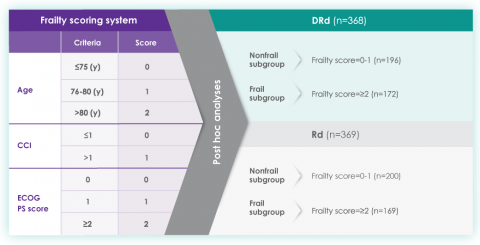
A frailty assessment was performed retrospectively using age, the Charlson Comorbidity Index (CCI) – which is calculated based on a retrospective review of the patient’s medical history to predict the 10-year mortality – and the baseline Eastern Cooperative Oncology Group (ECOG) performance status score, used to measure a patient’s level of functioning in terms of their ability to care for themselves, daily activity, and physical activity. The frailty scores were then added up to classify patients into fit (0), intermediate (1), or frail (≥2). Frailty status was further simplified into 2 categories: non-frail (0-1) and frail (≥2). The median age in the frail subgroup was 77 years (range: 57-80 years), with 88% of patients having ECOG performance score ≥1. CCI was calculated based on retrospective review of each patient’s medical history.12
The charts below illustrate the frailty scoring system with an overview of the patient population included in the 3-year post hoc analysis, PFS rate, and adverse events.
MAIA post hoc subgroup analysis by frailty status score12
The retrospective assessment of frailty score was a limitation of this study. Retrospective CCI calculations were based on reported medical history, which may contain missing data and result in underestimating or overestimating the number of patients in each frailty subgroup. The ECOG PS score parameter used for frailty score calculations in the study is more subjective, with susceptibility to intra- and inter-observer bias, compared with the ADL (activities of daily living) and IADL (instrumental activities of daily living) scales used in the IMWG scoring system. While the frailty scale used in the study is based on parameters that are routinely assessed in clinical practice for clinical use, the use of comprehensive frailty assessments that more accurately reflect biological or functional frailty will remain important for the further optimization of treatment strategies for frail patients. Patients with an ECOG PS score ≥3 and patients with comorbidities that may interfere with the study procedures were excluded from MAIA; the inclusion and exclusion criteria for the study limits the generalizability of these results to more frail patients seen in clinical practice.
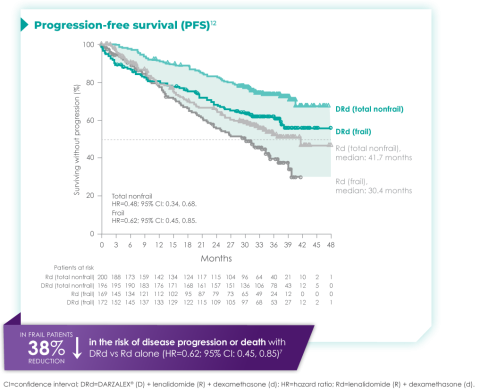
Progression-free survival in a ~3-year subgroup analysis of frail patients following treatment with D-Rd in TIE NDMM12
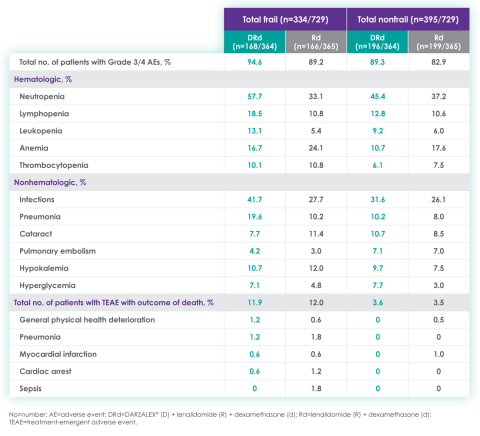
Most frequent Grade 3/4 treatment-emergent adverse events (≥10%) in frail patients at ~3 year follow-up of MAIA trial12
Please see additional Important Safety Information for DARZALEX® below.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
DARZALEX® is contraindicated in patients with a history of severe hypersensitivity (eg, anaphylactic reactions) to daratumumab or any of the components of the formulation.
WARNINGS AND PRECAUTIONS
Infusion-Related Reactions
DARZALEX® can cause severe and/or serious infusion-related reactions including anaphylactic reactions. These reactions can be life‑threatening, and fatal outcomes have been reported. In clinical trials (monotherapy and combination: N=2066), infusion-related reactions occurred in 37% of patients with the Week 1 (16 mg/kg) infusion, 2% with the Week 2 infusion, and cumulatively 6% with subsequent infusions. Less than 1% of patients had a Grade 3/4 infusion-related reaction at Week 2 or subsequent infusions. The median time to onset was 1.5 hours (range: 0 to 73 hours). Nearly all reactions occurred during infusion or within 4 hours of completing DARZALEX®. Severe reactions have occurred, including bronchospasm, hypoxia, dyspnea, hypertension, tachycardia, headache, laryngeal edema, pulmonary edema, and ocular adverse reactions, including choroidal effusion, acute myopia, and acute angle closure glaucoma. Signs and symptoms may include respiratory symptoms, such as nasal congestion, cough, throat irritation, as well as chills, vomiting, and nausea. Less common signs and symptoms were wheezing, allergic rhinitis, pyrexia, chest discomfort, pruritus, hypotension and blurred vision.
When DARZALEX® dosing was interrupted in the setting of ASCT (CASSIOPEIA) for a median of 3.75 months (range: 2.4 to 6.9 months), upon re-initiation of DARZALEX®, the incidence of infusion-related reactions was 11% for the first infusion following ASCT. Infusion-related reactions occurring at re-initiation of DARZALEX® following ASCT were consistent in terms of symptoms and severity (Grade 3 or 4: <1%) with those reported in previous studies at Week 2 or subsequent infusions. In EQUULEUS, patients receiving combination treatment (n=97) were administered the first 16 mg/kg dose at Week 1 split over two days, ie, 8 mg/kg on Day 1 and Day 2, respectively. The incidence of any grade infusion-related reactions was 42%, with 36% of patients experiencing infusion-related reactions on Day 1 of Week 1, 4% on Day 2 of Week 1, and 8% with subsequent infusions.
Pre-medicate patients with antihistamines, antipyretics, and corticosteroids. Frequently monitor patients during the entire infusion. Interrupt DARZALEX® infusion for reactions of any severity and institute medical management as needed. Permanently discontinue DARZALEX® therapy if an anaphylactic reaction or life-threatening (Grade 4) reaction occurs and institute appropriate emergency care. For patients with Grade 1, 2, or 3 reactions, reduce the infusion rate when re-starting the infusion.
To reduce the risk of delayed infusion-related reactions, administer oral corticosteroids to all patients following DARZALEX® infusions. Patients with a history of chronic obstructive pulmonary disease may require additional post-infusion medications to manage respiratory complications. Consider prescribing short- and long-acting bronchodilators and inhaled corticosteroids for patients with chronic obstructive pulmonary disease.
Ocular adverse reactions, including acute myopia and narrowing of the anterior chamber angle due to ciliochoroidal effusions with potential for increased intraocular pressure or glaucoma, have occurred with DARZALEX infusion. If ocular symptoms occur, interrupt DARZALEX infusion and seek immediate ophthalmologic evaluation prior to restarting DARZALEX.
Interference With Serological Testing
Daratumumab binds to CD38 on red blood cells (RBCs) and results in a positive indirect antiglobulin test (indirect Coombs test). Daratumumab-mediated positive indirect antiglobulin test may persist for up to 6 months after the last daratumumab infusion. Daratumumab bound to RBCs masks detection of antibodies to minor antigens in the patient’s serum. The determination of a patient’s ABO and Rh blood type is not impacted. Notify blood transfusion centers of this interference with serological testing and inform blood banks that a patient has received DARZALEX®. Type and screen patients prior to starting DARZALEX®.
Neutropenia and Thrombocytopenia
DARZALEX® may increase neutropenia and thrombocytopenia induced by background therapy. Monitor complete blood cell counts periodically during treatment according to manufacturer’s prescribing information for background therapies. Monitor patients with neutropenia for signs of infection. Consider withholding DARZALEX® until recovery of neutrophils or for recovery of platelets.
Interference With Determination of Complete Response
Daratumumab is a human immunoglobulin G (IgG) kappa monoclonal antibody that can be detected on both the serum protein electrophoresis (SPE) and immunofixation (IFE) assays used for the clinical monitoring of endogenous M-protein. This interference can impact the determination of complete response and of disease progression in some patients with IgG kappa myeloma protein.
Embryo-Fetal Toxicity
Based on the mechanism of action, DARZALEX® can cause fetal harm when administered to a pregnant woman. DARZALEX® may cause depletion of fetal immune cells and decreased bone density. Advise pregnant women of the potential risk to a fetus. Advise females with reproductive potential to use effective contraception during treatment with DARZALEX® and for 3 months after the last dose.
The combination of DARZALEX® with lenalidomide, pomalidomide, or thalidomide is contraindicated in pregnant women because lenalidomide, pomalidomide, and thalidomide may cause birth defects and death of the unborn child. Refer to the lenalidomide, pomalidomide, or thalidomide prescribing information on use during pregnancy.
ADVERSE REACTIONS
The most frequently reported adverse reactions (incidence ≥20%) were: upper respiratory infection, neutropenia, infusion‑related reactions, thrombocytopenia, diarrhea, constipation, anemia, peripheral sensory neuropathy, fatigue, peripheral edema, nausea, cough, pyrexia, dyspnea, and asthenia. The most common hematologic laboratory abnormalities (≥40%) with DARZALEX® are: neutropenia, lymphopenia, thrombocytopenia, leukopenia, and anemia.
INDICATIONS
DARZALEX® (daratumumab) is indicated for the treatment of adult patients with multiple myeloma:
- In combination with lenalidomide and dexamethasone in newly diagnosed patients who are ineligible for autologous stem cell transplant and in patients with relapsed or refractory multiple myeloma who have received at least one prior therapy
- In combination with bortezomib, melphalan, and prednisone in newly diagnosed patients who are ineligible for autologous stem cell transplant
- In combination with bortezomib, thalidomide, and dexamethasone in newly diagnosed patients who are eligible for autologous stem cell transplant
- In combination with bortezomib and dexamethasone in patients who have received at least one prior therapy
- In combination with carfilzomib and dexamethasone in patients with relapsed or refractory multiple myeloma who have received one to three prior lines of therapy
- In combination with pomalidomide and dexamethasone in patients who have received at least two prior therapies including lenalidomide and a proteasome inhibitor
- As monotherapy in patients who have received at least three prior lines of therapy including a proteasome inhibitor (PI) and an immunomodulatory agent or who are double-refractory to a PI and an immunomodulatory agent
Please click here to see the full Prescribing Information.
1. Richardson PG, San Miguel JF, Moreau P, et al. Interpreting clinical trial data in multiple myeloma: translating findings to the real-world setting. Blood Cancer J. 2018;8(11). doi:10.1038/s41408-018-0141-0
2. Key Statistics About Multiple Myeloma. Cancer.org. Published 2019. https://www.cancer.org/cancer/multiple-myeloma/about/key-statistics.html
3. Fonseca R, Usmani SZ, Mehra M, et al. Frontline treatment patterns and attrition rates by subsequent lines of therapy in patients with newly diagnosed multiple myeloma. BMC Cancer. 2020;20(1). doi:10.1186/s12885-020-07503-y
4. Devarakonda S, Efebera Y, Sharma N. Role of Stem Cell Transplantation in Multiple Myeloma. Cancers. 2021;13(4):863. doi:10.3390/cancers13040863
5. Derudas D, Capraro F, Martinelli G, Cerchione C. How I manage frontline transplant-ineligible multiple myeloma. Hematol Rep. 2020;12(s1). doi:10.4081/hr.2020.8956
6. Kazandjian D. Multiple myeloma epidemiology and survival: A unique malignancy. Semin Oncl. 2016;43(6):676-681. doi:10.1053/j.seminoncol.2016.11.004
7. Facon T, Kumar S, Plesner T, et al. Daratumumab plus lenalidomide and dexamethasone for untreated myeloma. N. Engl. J. Med. 2019;380(22):2104-2115. doi:10.1056/nejmoa1817249
8. DARZALEX® [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.
9. Facon T, Kumar SK, Plesner T, et al. Daratumumab, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone alone in newly diagnosed multiple myeloma (MAIA): overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(11):1582-1596. doi:10.1016/s1470-2045(21)00466-6
10. Facon T, Dimopoulos MA, Meuleman N, et al. A simplified frailty scale predicts outcomes in transplant-ineligible patients with newly diagnosed multiple myeloma treated in the FIRST (MM-020) trial. Leukemia. 2019;34(1):224-233. doi:10.1038/s41375-019-0539-0
11. Facon T, Kumar SK, Plesner T, et al. Supplement to: Daratumumab, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone alone in newly diagnosed multiple myeloma (MAIA): overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(11):1582-1596.
12. Facon T, Cook G, Usmani SZ, et al. Daratumumab plus lenalidomide and dexamethasone in transplant-ineligible newly diagnosed multiple myeloma: frailty subgroup analysis of MAIA. Leukemia. 2022;36(4):1066-1077. doi:10.1038/s41375-021-01488-8
© Janssen Biotech, Inc. 2022 All rights reserved. 12/22 cp-333446v1
1. The treatment of multiple myeloma has evolved significantly in recent years. What are some of the most important things you consider in the treatment of your newly diagnosed, transplant-ineligible patients?
We’ve seen great progress in the treatment of multiple myeloma over the last decade, and outcomes continue to improve for many patients.1 Still, it is important to keep in mind that more than 34,000 patients will be diagnosed and more than 12,000 people will die from the disease this year.2 We may have the greatest opportunity to impact the course of disease in the treatment of newly diagnosed patients due to the nature of this cancer:
- Multiple myeloma is characterized by relapse, and we know the length of remission generally decreases with each relapse and subsequent line of therapy.3
- Patients often become refractory to treatment over time.
When I meet with a patient who has been diagnosed with multiple myeloma, the first thing I consider is their eligibility for autologous stem cell transplant (ASCT). In my opinion, the introduction of ASCT is one of the biggest advancements in the last few decades, and we’ve found that ASCT followed by maintenance therapy with targeted tools improves progression-free survival (PFS).4
Unfortunately, many newly diagnosed patients are not eligible for ASCT–either because of comorbidities or other complexities related to the presentation of their disease.
For patients who are transplant-ineligible (TIE), it is important to have treatment options that are proven effective in extending PFS and overall survival (OS), and capable of producing deep and durable responses.
2. What are the challenges associated with treating newly diagnosed patients who are not eligible for ASCT?
We still consider multiple myeloma to be an incurable disease but, in my opinion, the treatment of TIE patients is less challenging today than a decade ago due to the emergence of novel therapies. That said, TIE patients are typically older and present with more advanced disease and comorbidities, including diabetes or cardiovascular events.5
A retrospective analysis published in 2020 by Rafael Fonseca examined frontline treatment patterns and attrition rates by line of therapy among newly diagnosed multiple myeloma (NDMM) patients who are TIE. More than 22,000 patients were identified from three patient-level databases between 2000 and 2018 - the OPTUM Commercial Claims database, the OPTUM Electronic Medical Records database, and the Surveillance, Epidemiology, and End Results-Meidcare Linked database. Patients included had to have a multiple myeloma diagnosis on or after January 1, 2007. Results showed that attrition rates among newly diagnosed, TIE patients with multiple myeloma increase with each line of therapy, with the proportion of patients who receive a second line of therapy decreasing by 50 percent with each subsequent line.3
3. Can you provide more detail on the goals of therapy for newly diagnosed, transplant-ineligible patients?
When I discuss treatment goals with TIE patients, I feel it is important to emphasize managing side effects and achieving deep and durable responses. I have the benefit of being in an academic setting, where I regularly exchange information with my colleagues about what we’re learning from the clinical studies in which we participate. Choosing which treatment to administer is complex and involves other considerations. For example, if two regimens have comparable efficacy, I may recommend the regimen with a more established safety profile or more robust evidence so I can properly anticipate and manage toxicities in my patients. Overall survival is one of the most important endpoints I consider, in addition to depth of response and PFS. In recent years, we’ve seen increasing evidence pointing to the importance of using a proven effective treatment in frontline patients that are ineligible for transplant.
4. A key study in newly-diagnosed, transplant-ineligible multiple myeloma is the Phase 3 MAIA study. Can you share the key takeaways from this study and discuss how the results have shaped treatment for this patient population?
Of course. The MAIA study is a randomized Phase 3 study evaluating DARZALEX® (daratumumab) intravenous injection in combination with lenalidomide and dexamethasone (D-Rd) compared with Rd in 737 adult patients with newly diagnosed, transplant-ineligible multiple myeloma. The median age of patients participating in the MAIA study was 73 (range 45-90), an important consideration since the median age for multiple myeloma diagnosis is approximately 66-70 years of age.6 The study evaluated PFS as the primary endpoint, and overall survival as a key secondary endpoint, and supported the FDA approval of DARZALEX® in combination with lenalidomide and dexamethasone for adult patients with newly diagnosed, multiple myeloma who are ineligible for ASCT.
MAIA study design7
The baseline demographic and disease characteristics were similar between the 2 treatment groups. Forty-four percent of the patients were ≥75 years of age. Fifty-two percent (52%) of patients were male, 92% White, 4% Black or African American, and 1% Asian. Three percent (3%) of patients reported an ethnicity of Hispanic or Latino. Thirty-four (34%) had an Eastern Cooperative Oncology Group (ECOG) performance score of 0, 50% had an ECOG performance score of 1, and 17% had an ECOG performance score of ≥2. Twenty-seven percent had International Staging System (ISS) Stage I, 43% had ISS Stage II, and 29% had ISS Stage III disease.
Select Important Safety Information:
CONTRAINDICATIONS
DARZALEX® is contraindicated in patients with a history of severe hypersensitivity (eg, anaphylactic reactions) to daratumumab or any of the components of the formulation.
WARNINGS AND PRECAUTIONS
Infusion-Related Reactions: DARZALEX® can cause severe and/or serious infusion-related reactions including anaphylactic reactions.
These reactions can be life-threatening, and fatal outcomes have been reported. Please scroll down to read Important Safety Information for DARZALEX®.
Primary findings from the study, which were published in 2019, showed an improvement in PFS in patients receiving D-Rd compared with those receiving Rd alone.7 The median PFS was not reached in the D-Rd arm and was reached at 31.9 months in the Rd arm (HR 0.56; 95% CI 0.43-0.73; P<0.0001).7 At a median of 30 months of follow-up, the data showed the clinical benefit of D-Rd therapy, with a 44% reduction in the risk of disease progression or death in patients receiving D-Rd compared with Rd alone.7
Additionally, 70.6% of patients (95% CI, 65.0-75.4) had no progressive disease with D-Rd treatment at median 30 months of follow-up, compared with 55.6% (95% CI, 49.5-61.3) of patients in the Rd group.7
In terms of depth of response, the percentage of patients with a complete response or better was 47.6% in patients receiving D-Rd compared with 24.9% in the Rd group.7
Overall response rate with D-Rd in TIE NDMM at ~30 months of follow-up8
An overview of the most frequent adverse events at 30-months of follow-up are provided below. The most frequent adverse reactions were reported in ≥20% of patients, with at least a 5% greater frequency in the D-Rd arm compared with Rd alone.8
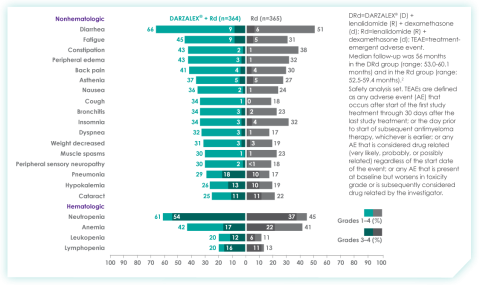
Most frequent adverse events at ~30 months of follow-up with D-Rd in TIE NDMM8
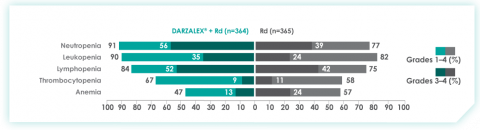
Most frequent hematologic laboratory abnormalities with D-Rd in TIE NDMM at ~30 months8
Serious adverse reactions with a 2% greater incidence in the D-Rd arm compared with the Rd arm were pneumonia (D-Rd 15% vs Rd 8%), bronchitis (D-Rd 4% vs Rd 2%), and dehydration (D-Rd 2% vs Rd <1).
• Discontinuation rates due to any adverse event: 7% with D-Rd vs 16% with Rd
• Infusion-related reactions (IRRs) with D-Rd occurred in 41% of patients; 2% were Grade 3 and <1% were Grade 4
• IRRs of any grade or severity may require management by interruption, modification, and/or discontinuation of the infusion
• Most IRRs occurred during first infusion
5. Thanks for that overview. In addition to these results, The Lancet Oncology has published updated overall survival data from a 5-year follow-up on the MAIA study. Can you provide an overview of these data and insights on their potential for patients?
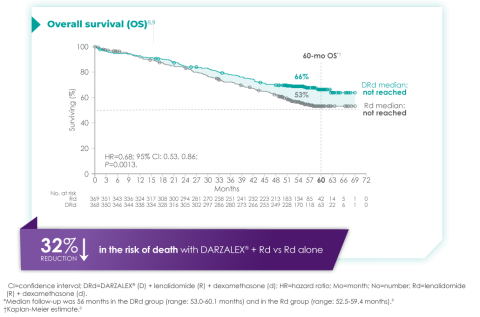
The MAIA trial was an important study, and for me, the results were practice changing. We see that after a median of nearly 5 years of follow-up, D-Rd significantly improved OS in TIE NDMM patients who were treated to progression compared with Rd alone (66.3% vs. 53.1% [HR=0.68; 95% CI, 0.53-0.86; P=0.0013]).9 This equates to approximately a 32% reduction in death when DARZALEX® was added to a two-drug regimen, which is a meaningful consideration when selecting the most appropriate regimens for my newly diagnosed, transplant-ineligible patients.9
Overall survival data at ~5 years with D-Rd compared to Rd alone in TIE NDMM9
Importantly, efficacy that resulted from longer treatment with D-Rd is also supported by approximately 5 years of safety evaluation. Below is information from a follow-up analysis of the MAIA study. This information is not included in the current Prescribing Information and has not been evaluated by the FDA. Treatment-emergent adverse events are reported as observed. These analyses have not been adjusted for multiple comparisons and no conclusions should be drawn. In what I’ve observed through published data and in my practice, longer treatment has not revealed new safety signals.
Most frequent treatment-emergent adverse events (any grade reported in ≥30% of patients and/or Grade 3/4 reported in ≥10% of patients) at ~5 years9
Select Important Safety Information:
DARZALEX® can cause severe and/or serious infusion-related reactions including anaphylactic reactions. These reactions can be life threatening, and fatal outcomes have been reported. In clinical trials (monotherapy and combination: N=2066), infusion-related reactions occurred in 37% of patients with the Week 1 (16 mg/kg) infusion, 2% with the Week 2 infusion, and cumulatively 6% with subsequent infusions. Less than 1% of patients had a Grade 3/4 infusion-related reaction at Week 2 or subsequent infusions. The median time to onset was 1.5 hours (range: 0 to 73 hours). Nearly all reactions occurred during infusion or within 4 hours of completing DARZALEX®. Severe reactions have occurred, including bronchospasm, hypoxia, dyspnea, hypertension, tachycardia, headache, laryngeal edema, pulmonary edema, and ocular adverse reactions, including choroidal effusion, acute myopia, and acute angle closure glaucoma. Signs and symptoms may include respiratory symptoms, such as nasal congestion, cough, throat irritation, as well as chills, vomiting, and nausea. Less common signs and symptoms were wheezing, allergic rhinitis, pyrexia, chest discomfort, pruritus, hypotension and blurred vision. Please scroll down to see Important Safety Information for DARZALEX®.
6. Does the availability of OS data influence your decisions on treatment selection in TIE NDMM?
Overall survival absolutely remains the gold standard and informs my practice. Prior to OS data being available, I will often look at other efficacy endpoints that are available sooner. In MAIA, I was encouraged by efficacy endpoints in earlier data, which were later confirmed by the latest data on OS.
7. The MAIA study shows that treating to disease progression or unacceptable toxicity is important. How does that impact your approach to treatment?
It's important to keep in mind that the MAIA trial was designed to evaluate treatment until progression or unacceptable toxicity. The results revealed a significant difference between the DR-d and Rd treatment arms, but results observed in this study are contingent on this treatment approach. From a clinical perspective, unless there is considerable toxicity, I advocate for treating with D-Rd to progression.
In the clinic, we also see that TIE patients who have higher frailty scores are more likely to discontinue treatment prior to progression.10 There can be other reasons too – such as a patient simply wanting to have a break from treatment. These conversations are not always easy, but it is important to have an honest dialogue with patients.
8. What can we learn from studies like the MAIA trial that included a wide range of patient populations including patients who are elderly, frail, or had high cytogenetic risk?
Several patient subgroups were analyzed as part of the MAIA study. It is important to note that these subgroup analyses are not included in the Prescribing Information for DARZALEX®. These analyses were not adjusted for multiple comparisons, and there are insufficient numbers of patients per subgroup to make definitive conclusions of efficacy among the subgroups.
As mentioned above, the MAIA study evaluated a wide range of patients (n=737). The baseline demographic and disease characteristics were similar between the D-Rd and Rd treatment groups and the median age was 73 (range: 45-90) years, with 44% of the patients ≥75 years of age.
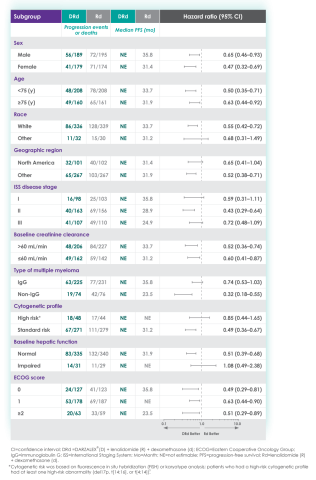
In the various patient subgroups that were analyzed as part of the MAIA study, it was found that at ~3-years of follow-up the PFS numerically favored DRd compared with Rd alone in most subgroups (see table below).
Median progression-free survival by sub-population at ~3 year follow-up8
The MAIA trial also included patients who were frail and a post hoc analysis was conducted in this subgroup of patients. These analyses are not included in the Prescribing Information for DARZALEX®. These analyses were conducted post hoc and there are insufficient numbers of patients per subgroup to make definitive conclusions of efficacy among the subgroups.
A frailty assessment was performed retrospectively using age, the Charlson Comorbidity Index (CCI) – which is calculated based on a retrospective review of the patient’s medical history to predict the 10-year mortality – and the baseline Eastern Cooperative Oncology Group (ECOG) performance status score, used to measure a patient’s level of functioning in terms of their ability to care for themselves, daily activity, and physical activity. The frailty scores were then added up to classify patients into fit (0), intermediate (1), or frail (≥2). Frailty status was further simplified into 2 categories: non-frail (0-1) and frail (≥2). The median age in the frail subgroup was 77 years (range: 57-80 years), with 88% of patients having ECOG performance score ≥1. CCI was calculated based on retrospective review of each patient’s medical history.12
The charts below illustrate the frailty scoring system with an overview of the patient population included in the 3-year post hoc analysis, PFS rate, and adverse events.
MAIA post hoc subgroup analysis by frailty status score12
The retrospective assessment of frailty score was a limitation of this study. Retrospective CCI calculations were based on reported medical history, which may contain missing data and result in underestimating or overestimating the number of patients in each frailty subgroup. The ECOG PS score parameter used for frailty score calculations in the study is more subjective, with susceptibility to intra- and inter-observer bias, compared with the ADL (activities of daily living) and IADL (instrumental activities of daily living) scales used in the IMWG scoring system. While the frailty scale used in the study is based on parameters that are routinely assessed in clinical practice for clinical use, the use of comprehensive frailty assessments that more accurately reflect biological or functional frailty will remain important for the further optimization of treatment strategies for frail patients. Patients with an ECOG PS score ≥3 and patients with comorbidities that may interfere with the study procedures were excluded from MAIA; the inclusion and exclusion criteria for the study limits the generalizability of these results to more frail patients seen in clinical practice.
Progression-free survival in a ~3-year subgroup analysis of frail patients following treatment with D-Rd in TIE NDMM12
Most frequent Grade 3/4 treatment-emergent adverse events (≥10%) in frail patients at ~3 year follow-up of MAIA trial12
Please see additional Important Safety Information for DARZALEX® below.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
DARZALEX® is contraindicated in patients with a history of severe hypersensitivity (eg, anaphylactic reactions) to daratumumab or any of the components of the formulation.
WARNINGS AND PRECAUTIONS
Infusion-Related Reactions
DARZALEX® can cause severe and/or serious infusion-related reactions including anaphylactic reactions. These reactions can be life‑threatening, and fatal outcomes have been reported. In clinical trials (monotherapy and combination: N=2066), infusion-related reactions occurred in 37% of patients with the Week 1 (16 mg/kg) infusion, 2% with the Week 2 infusion, and cumulatively 6% with subsequent infusions. Less than 1% of patients had a Grade 3/4 infusion-related reaction at Week 2 or subsequent infusions. The median time to onset was 1.5 hours (range: 0 to 73 hours). Nearly all reactions occurred during infusion or within 4 hours of completing DARZALEX®. Severe reactions have occurred, including bronchospasm, hypoxia, dyspnea, hypertension, tachycardia, headache, laryngeal edema, pulmonary edema, and ocular adverse reactions, including choroidal effusion, acute myopia, and acute angle closure glaucoma. Signs and symptoms may include respiratory symptoms, such as nasal congestion, cough, throat irritation, as well as chills, vomiting, and nausea. Less common signs and symptoms were wheezing, allergic rhinitis, pyrexia, chest discomfort, pruritus, hypotension and blurred vision.
When DARZALEX® dosing was interrupted in the setting of ASCT (CASSIOPEIA) for a median of 3.75 months (range: 2.4 to 6.9 months), upon re-initiation of DARZALEX®, the incidence of infusion-related reactions was 11% for the first infusion following ASCT. Infusion-related reactions occurring at re-initiation of DARZALEX® following ASCT were consistent in terms of symptoms and severity (Grade 3 or 4: <1%) with those reported in previous studies at Week 2 or subsequent infusions. In EQUULEUS, patients receiving combination treatment (n=97) were administered the first 16 mg/kg dose at Week 1 split over two days, ie, 8 mg/kg on Day 1 and Day 2, respectively. The incidence of any grade infusion-related reactions was 42%, with 36% of patients experiencing infusion-related reactions on Day 1 of Week 1, 4% on Day 2 of Week 1, and 8% with subsequent infusions.
Pre-medicate patients with antihistamines, antipyretics, and corticosteroids. Frequently monitor patients during the entire infusion. Interrupt DARZALEX® infusion for reactions of any severity and institute medical management as needed. Permanently discontinue DARZALEX® therapy if an anaphylactic reaction or life-threatening (Grade 4) reaction occurs and institute appropriate emergency care. For patients with Grade 1, 2, or 3 reactions, reduce the infusion rate when re-starting the infusion.
To reduce the risk of delayed infusion-related reactions, administer oral corticosteroids to all patients following DARZALEX® infusions. Patients with a history of chronic obstructive pulmonary disease may require additional post-infusion medications to manage respiratory complications. Consider prescribing short- and long-acting bronchodilators and inhaled corticosteroids for patients with chronic obstructive pulmonary disease.
Ocular adverse reactions, including acute myopia and narrowing of the anterior chamber angle due to ciliochoroidal effusions with potential for increased intraocular pressure or glaucoma, have occurred with DARZALEX infusion. If ocular symptoms occur, interrupt DARZALEX infusion and seek immediate ophthalmologic evaluation prior to restarting DARZALEX.
Interference With Serological Testing
Daratumumab binds to CD38 on red blood cells (RBCs) and results in a positive indirect antiglobulin test (indirect Coombs test). Daratumumab-mediated positive indirect antiglobulin test may persist for up to 6 months after the last daratumumab infusion. Daratumumab bound to RBCs masks detection of antibodies to minor antigens in the patient’s serum. The determination of a patient’s ABO and Rh blood type is not impacted. Notify blood transfusion centers of this interference with serological testing and inform blood banks that a patient has received DARZALEX®. Type and screen patients prior to starting DARZALEX®.
Neutropenia and Thrombocytopenia
DARZALEX® may increase neutropenia and thrombocytopenia induced by background therapy. Monitor complete blood cell counts periodically during treatment according to manufacturer’s prescribing information for background therapies. Monitor patients with neutropenia for signs of infection. Consider withholding DARZALEX® until recovery of neutrophils or for recovery of platelets.
Interference With Determination of Complete Response
Daratumumab is a human immunoglobulin G (IgG) kappa monoclonal antibody that can be detected on both the serum protein electrophoresis (SPE) and immunofixation (IFE) assays used for the clinical monitoring of endogenous M-protein. This interference can impact the determination of complete response and of disease progression in some patients with IgG kappa myeloma protein.
Embryo-Fetal Toxicity
Based on the mechanism of action, DARZALEX® can cause fetal harm when administered to a pregnant woman. DARZALEX® may cause depletion of fetal immune cells and decreased bone density. Advise pregnant women of the potential risk to a fetus. Advise females with reproductive potential to use effective contraception during treatment with DARZALEX® and for 3 months after the last dose.
The combination of DARZALEX® with lenalidomide, pomalidomide, or thalidomide is contraindicated in pregnant women because lenalidomide, pomalidomide, and thalidomide may cause birth defects and death of the unborn child. Refer to the lenalidomide, pomalidomide, or thalidomide prescribing information on use during pregnancy.
ADVERSE REACTIONS
The most frequently reported adverse reactions (incidence ≥20%) were: upper respiratory infection, neutropenia, infusion‑related reactions, thrombocytopenia, diarrhea, constipation, anemia, peripheral sensory neuropathy, fatigue, peripheral edema, nausea, cough, pyrexia, dyspnea, and asthenia. The most common hematologic laboratory abnormalities (≥40%) with DARZALEX® are: neutropenia, lymphopenia, thrombocytopenia, leukopenia, and anemia.
INDICATIONS
DARZALEX® (daratumumab) is indicated for the treatment of adult patients with multiple myeloma:
- In combination with lenalidomide and dexamethasone in newly diagnosed patients who are ineligible for autologous stem cell transplant and in patients with relapsed or refractory multiple myeloma who have received at least one prior therapy
- In combination with bortezomib, melphalan, and prednisone in newly diagnosed patients who are ineligible for autologous stem cell transplant
- In combination with bortezomib, thalidomide, and dexamethasone in newly diagnosed patients who are eligible for autologous stem cell transplant
- In combination with bortezomib and dexamethasone in patients who have received at least one prior therapy
- In combination with carfilzomib and dexamethasone in patients with relapsed or refractory multiple myeloma who have received one to three prior lines of therapy
- In combination with pomalidomide and dexamethasone in patients who have received at least two prior therapies including lenalidomide and a proteasome inhibitor
- As monotherapy in patients who have received at least three prior lines of therapy including a proteasome inhibitor (PI) and an immunomodulatory agent or who are double-refractory to a PI and an immunomodulatory agent
Please click here to see the full Prescribing Information.
1. Richardson PG, San Miguel JF, Moreau P, et al. Interpreting clinical trial data in multiple myeloma: translating findings to the real-world setting. Blood Cancer J. 2018;8(11). doi:10.1038/s41408-018-0141-0
2. Key Statistics About Multiple Myeloma. Cancer.org. Published 2019. https://www.cancer.org/cancer/multiple-myeloma/about/key-statistics.html
3. Fonseca R, Usmani SZ, Mehra M, et al. Frontline treatment patterns and attrition rates by subsequent lines of therapy in patients with newly diagnosed multiple myeloma. BMC Cancer. 2020;20(1). doi:10.1186/s12885-020-07503-y
4. Devarakonda S, Efebera Y, Sharma N. Role of Stem Cell Transplantation in Multiple Myeloma. Cancers. 2021;13(4):863. doi:10.3390/cancers13040863
5. Derudas D, Capraro F, Martinelli G, Cerchione C. How I manage frontline transplant-ineligible multiple myeloma. Hematol Rep. 2020;12(s1). doi:10.4081/hr.2020.8956
6. Kazandjian D. Multiple myeloma epidemiology and survival: A unique malignancy. Semin Oncl. 2016;43(6):676-681. doi:10.1053/j.seminoncol.2016.11.004
7. Facon T, Kumar S, Plesner T, et al. Daratumumab plus lenalidomide and dexamethasone for untreated myeloma. N. Engl. J. Med. 2019;380(22):2104-2115. doi:10.1056/nejmoa1817249
8. DARZALEX® [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.
9. Facon T, Kumar SK, Plesner T, et al. Daratumumab, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone alone in newly diagnosed multiple myeloma (MAIA): overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(11):1582-1596. doi:10.1016/s1470-2045(21)00466-6
10. Facon T, Dimopoulos MA, Meuleman N, et al. A simplified frailty scale predicts outcomes in transplant-ineligible patients with newly diagnosed multiple myeloma treated in the FIRST (MM-020) trial. Leukemia. 2019;34(1):224-233. doi:10.1038/s41375-019-0539-0
11. Facon T, Kumar SK, Plesner T, et al. Supplement to: Daratumumab, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone alone in newly diagnosed multiple myeloma (MAIA): overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(11):1582-1596.
12. Facon T, Cook G, Usmani SZ, et al. Daratumumab plus lenalidomide and dexamethasone in transplant-ineligible newly diagnosed multiple myeloma: frailty subgroup analysis of MAIA. Leukemia. 2022;36(4):1066-1077. doi:10.1038/s41375-021-01488-8
© Janssen Biotech, Inc. 2022 All rights reserved. 12/22 cp-333446v1
1. The treatment of multiple myeloma has evolved significantly in recent years. What are some of the most important things you consider in the treatment of your newly diagnosed, transplant-ineligible patients?
We’ve seen great progress in the treatment of multiple myeloma over the last decade, and outcomes continue to improve for many patients.1 Still, it is important to keep in mind that more than 34,000 patients will be diagnosed and more than 12,000 people will die from the disease this year.2 We may have the greatest opportunity to impact the course of disease in the treatment of newly diagnosed patients due to the nature of this cancer:
- Multiple myeloma is characterized by relapse, and we know the length of remission generally decreases with each relapse and subsequent line of therapy.3
- Patients often become refractory to treatment over time.
When I meet with a patient who has been diagnosed with multiple myeloma, the first thing I consider is their eligibility for autologous stem cell transplant (ASCT). In my opinion, the introduction of ASCT is one of the biggest advancements in the last few decades, and we’ve found that ASCT followed by maintenance therapy with targeted tools improves progression-free survival (PFS).4
Unfortunately, many newly diagnosed patients are not eligible for ASCT–either because of comorbidities or other complexities related to the presentation of their disease.
For patients who are transplant-ineligible (TIE), it is important to have treatment options that are proven effective in extending PFS and overall survival (OS), and capable of producing deep and durable responses.
2. What are the challenges associated with treating newly diagnosed patients who are not eligible for ASCT?
We still consider multiple myeloma to be an incurable disease but, in my opinion, the treatment of TIE patients is less challenging today than a decade ago due to the emergence of novel therapies. That said, TIE patients are typically older and present with more advanced disease and comorbidities, including diabetes or cardiovascular events.5
A retrospective analysis published in 2020 by Rafael Fonseca examined frontline treatment patterns and attrition rates by line of therapy among newly diagnosed multiple myeloma (NDMM) patients who are TIE. More than 22,000 patients were identified from three patient-level databases between 2000 and 2018 - the OPTUM Commercial Claims database, the OPTUM Electronic Medical Records database, and the Surveillance, Epidemiology, and End Results-Meidcare Linked database. Patients included had to have a multiple myeloma diagnosis on or after January 1, 2007. Results showed that attrition rates among newly diagnosed, TIE patients with multiple myeloma increase with each line of therapy, with the proportion of patients who receive a second line of therapy decreasing by 50 percent with each subsequent line.3
3. Can you provide more detail on the goals of therapy for newly diagnosed, transplant-ineligible patients?
When I discuss treatment goals with TIE patients, I feel it is important to emphasize managing side effects and achieving deep and durable responses. I have the benefit of being in an academic setting, where I regularly exchange information with my colleagues about what we’re learning from the clinical studies in which we participate. Choosing which treatment to administer is complex and involves other considerations. For example, if two regimens have comparable efficacy, I may recommend the regimen with a more established safety profile or more robust evidence so I can properly anticipate and manage toxicities in my patients. Overall survival is one of the most important endpoints I consider, in addition to depth of response and PFS. In recent years, we’ve seen increasing evidence pointing to the importance of using a proven effective treatment in frontline patients that are ineligible for transplant.
4. A key study in newly-diagnosed, transplant-ineligible multiple myeloma is the Phase 3 MAIA study. Can you share the key takeaways from this study and discuss how the results have shaped treatment for this patient population?
Of course. The MAIA study is a randomized Phase 3 study evaluating DARZALEX® (daratumumab) intravenous injection in combination with lenalidomide and dexamethasone (D-Rd) compared with Rd in 737 adult patients with newly diagnosed, transplant-ineligible multiple myeloma. The median age of patients participating in the MAIA study was 73 (range 45-90), an important consideration since the median age for multiple myeloma diagnosis is approximately 66-70 years of age.6 The study evaluated PFS as the primary endpoint, and overall survival as a key secondary endpoint, and supported the FDA approval of DARZALEX® in combination with lenalidomide and dexamethasone for adult patients with newly diagnosed, multiple myeloma who are ineligible for ASCT.
MAIA study design7
The baseline demographic and disease characteristics were similar between the 2 treatment groups. Forty-four percent of the patients were ≥75 years of age. Fifty-two percent (52%) of patients were male, 92% White, 4% Black or African American, and 1% Asian. Three percent (3%) of patients reported an ethnicity of Hispanic or Latino. Thirty-four (34%) had an Eastern Cooperative Oncology Group (ECOG) performance score of 0, 50% had an ECOG performance score of 1, and 17% had an ECOG performance score of ≥2. Twenty-seven percent had International Staging System (ISS) Stage I, 43% had ISS Stage II, and 29% had ISS Stage III disease.
Select Important Safety Information:
CONTRAINDICATIONS
DARZALEX® is contraindicated in patients with a history of severe hypersensitivity (eg, anaphylactic reactions) to daratumumab or any of the components of the formulation.
WARNINGS AND PRECAUTIONS
Infusion-Related Reactions: DARZALEX® can cause severe and/or serious infusion-related reactions including anaphylactic reactions.
These reactions can be life-threatening, and fatal outcomes have been reported. Please scroll down to read Important Safety Information for DARZALEX®.
Primary findings from the study, which were published in 2019, showed an improvement in PFS in patients receiving D-Rd compared with those receiving Rd alone.7 The median PFS was not reached in the D-Rd arm and was reached at 31.9 months in the Rd arm (HR 0.56; 95% CI 0.43-0.73; P<0.0001).7 At a median of 30 months of follow-up, the data showed the clinical benefit of D-Rd therapy, with a 44% reduction in the risk of disease progression or death in patients receiving D-Rd compared with Rd alone.7
Additionally, 70.6% of patients (95% CI, 65.0-75.4) had no progressive disease with D-Rd treatment at median 30 months of follow-up, compared with 55.6% (95% CI, 49.5-61.3) of patients in the Rd group.7
In terms of depth of response, the percentage of patients with a complete response or better was 47.6% in patients receiving D-Rd compared with 24.9% in the Rd group.7
Overall response rate with D-Rd in TIE NDMM at ~30 months of follow-up8
An overview of the most frequent adverse events at 30-months of follow-up are provided below. The most frequent adverse reactions were reported in ≥20% of patients, with at least a 5% greater frequency in the D-Rd arm compared with Rd alone.8
Most frequent adverse events at ~30 months of follow-up with D-Rd in TIE NDMM8
Most frequent hematologic laboratory abnormalities with D-Rd in TIE NDMM at ~30 months8
Serious adverse reactions with a 2% greater incidence in the D-Rd arm compared with the Rd arm were pneumonia (D-Rd 15% vs Rd 8%), bronchitis (D-Rd 4% vs Rd 2%), and dehydration (D-Rd 2% vs Rd <1).
• Discontinuation rates due to any adverse event: 7% with D-Rd vs 16% with Rd
• Infusion-related reactions (IRRs) with D-Rd occurred in 41% of patients; 2% were Grade 3 and <1% were Grade 4
• IRRs of any grade or severity may require management by interruption, modification, and/or discontinuation of the infusion
• Most IRRs occurred during first infusion
5. Thanks for that overview. In addition to these results, The Lancet Oncology has published updated overall survival data from a 5-year follow-up on the MAIA study. Can you provide an overview of these data and insights on their potential for patients?
The MAIA trial was an important study, and for me, the results were practice changing. We see that after a median of nearly 5 years of follow-up, D-Rd significantly improved OS in TIE NDMM patients who were treated to progression compared with Rd alone (66.3% vs. 53.1% [HR=0.68; 95% CI, 0.53-0.86; P=0.0013]).9 This equates to approximately a 32% reduction in death when DARZALEX® was added to a two-drug regimen, which is a meaningful consideration when selecting the most appropriate regimens for my newly diagnosed, transplant-ineligible patients.9
Overall survival data at ~5 years with D-Rd compared to Rd alone in TIE NDMM9
Importantly, efficacy that resulted from longer treatment with D-Rd is also supported by approximately 5 years of safety evaluation. Below is information from a follow-up analysis of the MAIA study. This information is not included in the current Prescribing Information and has not been evaluated by the FDA. Treatment-emergent adverse events are reported as observed. These analyses have not been adjusted for multiple comparisons and no conclusions should be drawn. In what I’ve observed through published data and in my practice, longer treatment has not revealed new safety signals.
Most frequent treatment-emergent adverse events (any grade reported in ≥30% of patients and/or Grade 3/4 reported in ≥10% of patients) at ~5 years9
Select Important Safety Information:
DARZALEX® can cause severe and/or serious infusion-related reactions including anaphylactic reactions. These reactions can be life threatening, and fatal outcomes have been reported. In clinical trials (monotherapy and combination: N=2066), infusion-related reactions occurred in 37% of patients with the Week 1 (16 mg/kg) infusion, 2% with the Week 2 infusion, and cumulatively 6% with subsequent infusions. Less than 1% of patients had a Grade 3/4 infusion-related reaction at Week 2 or subsequent infusions. The median time to onset was 1.5 hours (range: 0 to 73 hours). Nearly all reactions occurred during infusion or within 4 hours of completing DARZALEX®. Severe reactions have occurred, including bronchospasm, hypoxia, dyspnea, hypertension, tachycardia, headache, laryngeal edema, pulmonary edema, and ocular adverse reactions, including choroidal effusion, acute myopia, and acute angle closure glaucoma. Signs and symptoms may include respiratory symptoms, such as nasal congestion, cough, throat irritation, as well as chills, vomiting, and nausea. Less common signs and symptoms were wheezing, allergic rhinitis, pyrexia, chest discomfort, pruritus, hypotension and blurred vision. Please scroll down to see Important Safety Information for DARZALEX®.
6. Does the availability of OS data influence your decisions on treatment selection in TIE NDMM?
Overall survival absolutely remains the gold standard and informs my practice. Prior to OS data being available, I will often look at other efficacy endpoints that are available sooner. In MAIA, I was encouraged by efficacy endpoints in earlier data, which were later confirmed by the latest data on OS.
7. The MAIA study shows that treating to disease progression or unacceptable toxicity is important. How does that impact your approach to treatment?
It's important to keep in mind that the MAIA trial was designed to evaluate treatment until progression or unacceptable toxicity. The results revealed a significant difference between the DR-d and Rd treatment arms, but results observed in this study are contingent on this treatment approach. From a clinical perspective, unless there is considerable toxicity, I advocate for treating with D-Rd to progression.
In the clinic, we also see that TIE patients who have higher frailty scores are more likely to discontinue treatment prior to progression.10 There can be other reasons too – such as a patient simply wanting to have a break from treatment. These conversations are not always easy, but it is important to have an honest dialogue with patients.
8. What can we learn from studies like the MAIA trial that included a wide range of patient populations including patients who are elderly, frail, or had high cytogenetic risk?
Several patient subgroups were analyzed as part of the MAIA study. It is important to note that these subgroup analyses are not included in the Prescribing Information for DARZALEX®. These analyses were not adjusted for multiple comparisons, and there are insufficient numbers of patients per subgroup to make definitive conclusions of efficacy among the subgroups.
As mentioned above, the MAIA study evaluated a wide range of patients (n=737). The baseline demographic and disease characteristics were similar between the D-Rd and Rd treatment groups and the median age was 73 (range: 45-90) years, with 44% of the patients ≥75 years of age.
In the various patient subgroups that were analyzed as part of the MAIA study, it was found that at ~3-years of follow-up the PFS numerically favored DRd compared with Rd alone in most subgroups (see table below).
Median progression-free survival by sub-population at ~3 year follow-up8
The MAIA trial also included patients who were frail and a post hoc analysis was conducted in this subgroup of patients. These analyses are not included in the Prescribing Information for DARZALEX®. These analyses were conducted post hoc and there are insufficient numbers of patients per subgroup to make definitive conclusions of efficacy among the subgroups.
A frailty assessment was performed retrospectively using age, the Charlson Comorbidity Index (CCI) – which is calculated based on a retrospective review of the patient’s medical history to predict the 10-year mortality – and the baseline Eastern Cooperative Oncology Group (ECOG) performance status score, used to measure a patient’s level of functioning in terms of their ability to care for themselves, daily activity, and physical activity. The frailty scores were then added up to classify patients into fit (0), intermediate (1), or frail (≥2). Frailty status was further simplified into 2 categories: non-frail (0-1) and frail (≥2). The median age in the frail subgroup was 77 years (range: 57-80 years), with 88% of patients having ECOG performance score ≥1. CCI was calculated based on retrospective review of each patient’s medical history.12
The charts below illustrate the frailty scoring system with an overview of the patient population included in the 3-year post hoc analysis, PFS rate, and adverse events.
MAIA post hoc subgroup analysis by frailty status score12
The retrospective assessment of frailty score was a limitation of this study. Retrospective CCI calculations were based on reported medical history, which may contain missing data and result in underestimating or overestimating the number of patients in each frailty subgroup. The ECOG PS score parameter used for frailty score calculations in the study is more subjective, with susceptibility to intra- and inter-observer bias, compared with the ADL (activities of daily living) and IADL (instrumental activities of daily living) scales used in the IMWG scoring system. While the frailty scale used in the study is based on parameters that are routinely assessed in clinical practice for clinical use, the use of comprehensive frailty assessments that more accurately reflect biological or functional frailty will remain important for the further optimization of treatment strategies for frail patients. Patients with an ECOG PS score ≥3 and patients with comorbidities that may interfere with the study procedures were excluded from MAIA; the inclusion and exclusion criteria for the study limits the generalizability of these results to more frail patients seen in clinical practice.
Progression-free survival in a ~3-year subgroup analysis of frail patients following treatment with D-Rd in TIE NDMM12
Most frequent Grade 3/4 treatment-emergent adverse events (≥10%) in frail patients at ~3 year follow-up of MAIA trial12
Please see additional Important Safety Information for DARZALEX® below.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
DARZALEX® is contraindicated in patients with a history of severe hypersensitivity (eg, anaphylactic reactions) to daratumumab or any of the components of the formulation.
WARNINGS AND PRECAUTIONS
Infusion-Related Reactions
DARZALEX® can cause severe and/or serious infusion-related reactions including anaphylactic reactions. These reactions can be life‑threatening, and fatal outcomes have been reported. In clinical trials (monotherapy and combination: N=2066), infusion-related reactions occurred in 37% of patients with the Week 1 (16 mg/kg) infusion, 2% with the Week 2 infusion, and cumulatively 6% with subsequent infusions. Less than 1% of patients had a Grade 3/4 infusion-related reaction at Week 2 or subsequent infusions. The median time to onset was 1.5 hours (range: 0 to 73 hours). Nearly all reactions occurred during infusion or within 4 hours of completing DARZALEX®. Severe reactions have occurred, including bronchospasm, hypoxia, dyspnea, hypertension, tachycardia, headache, laryngeal edema, pulmonary edema, and ocular adverse reactions, including choroidal effusion, acute myopia, and acute angle closure glaucoma. Signs and symptoms may include respiratory symptoms, such as nasal congestion, cough, throat irritation, as well as chills, vomiting, and nausea. Less common signs and symptoms were wheezing, allergic rhinitis, pyrexia, chest discomfort, pruritus, hypotension and blurred vision.
When DARZALEX® dosing was interrupted in the setting of ASCT (CASSIOPEIA) for a median of 3.75 months (range: 2.4 to 6.9 months), upon re-initiation of DARZALEX®, the incidence of infusion-related reactions was 11% for the first infusion following ASCT. Infusion-related reactions occurring at re-initiation of DARZALEX® following ASCT were consistent in terms of symptoms and severity (Grade 3 or 4: <1%) with those reported in previous studies at Week 2 or subsequent infusions. In EQUULEUS, patients receiving combination treatment (n=97) were administered the first 16 mg/kg dose at Week 1 split over two days, ie, 8 mg/kg on Day 1 and Day 2, respectively. The incidence of any grade infusion-related reactions was 42%, with 36% of patients experiencing infusion-related reactions on Day 1 of Week 1, 4% on Day 2 of Week 1, and 8% with subsequent infusions.
Pre-medicate patients with antihistamines, antipyretics, and corticosteroids. Frequently monitor patients during the entire infusion. Interrupt DARZALEX® infusion for reactions of any severity and institute medical management as needed. Permanently discontinue DARZALEX® therapy if an anaphylactic reaction or life-threatening (Grade 4) reaction occurs and institute appropriate emergency care. For patients with Grade 1, 2, or 3 reactions, reduce the infusion rate when re-starting the infusion.
To reduce the risk of delayed infusion-related reactions, administer oral corticosteroids to all patients following DARZALEX® infusions. Patients with a history of chronic obstructive pulmonary disease may require additional post-infusion medications to manage respiratory complications. Consider prescribing short- and long-acting bronchodilators and inhaled corticosteroids for patients with chronic obstructive pulmonary disease.
Ocular adverse reactions, including acute myopia and narrowing of the anterior chamber angle due to ciliochoroidal effusions with potential for increased intraocular pressure or glaucoma, have occurred with DARZALEX infusion. If ocular symptoms occur, interrupt DARZALEX infusion and seek immediate ophthalmologic evaluation prior to restarting DARZALEX.
Interference With Serological Testing
Daratumumab binds to CD38 on red blood cells (RBCs) and results in a positive indirect antiglobulin test (indirect Coombs test). Daratumumab-mediated positive indirect antiglobulin test may persist for up to 6 months after the last daratumumab infusion. Daratumumab bound to RBCs masks detection of antibodies to minor antigens in the patient’s serum. The determination of a patient’s ABO and Rh blood type is not impacted. Notify blood transfusion centers of this interference with serological testing and inform blood banks that a patient has received DARZALEX®. Type and screen patients prior to starting DARZALEX®.
Neutropenia and Thrombocytopenia
DARZALEX® may increase neutropenia and thrombocytopenia induced by background therapy. Monitor complete blood cell counts periodically during treatment according to manufacturer’s prescribing information for background therapies. Monitor patients with neutropenia for signs of infection. Consider withholding DARZALEX® until recovery of neutrophils or for recovery of platelets.
Interference With Determination of Complete Response
Daratumumab is a human immunoglobulin G (IgG) kappa monoclonal antibody that can be detected on both the serum protein electrophoresis (SPE) and immunofixation (IFE) assays used for the clinical monitoring of endogenous M-protein. This interference can impact the determination of complete response and of disease progression in some patients with IgG kappa myeloma protein.
Embryo-Fetal Toxicity
Based on the mechanism of action, DARZALEX® can cause fetal harm when administered to a pregnant woman. DARZALEX® may cause depletion of fetal immune cells and decreased bone density. Advise pregnant women of the potential risk to a fetus. Advise females with reproductive potential to use effective contraception during treatment with DARZALEX® and for 3 months after the last dose.
The combination of DARZALEX® with lenalidomide, pomalidomide, or thalidomide is contraindicated in pregnant women because lenalidomide, pomalidomide, and thalidomide may cause birth defects and death of the unborn child. Refer to the lenalidomide, pomalidomide, or thalidomide prescribing information on use during pregnancy.
ADVERSE REACTIONS
The most frequently reported adverse reactions (incidence ≥20%) were: upper respiratory infection, neutropenia, infusion‑related reactions, thrombocytopenia, diarrhea, constipation, anemia, peripheral sensory neuropathy, fatigue, peripheral edema, nausea, cough, pyrexia, dyspnea, and asthenia. The most common hematologic laboratory abnormalities (≥40%) with DARZALEX® are: neutropenia, lymphopenia, thrombocytopenia, leukopenia, and anemia.
INDICATIONS
DARZALEX® (daratumumab) is indicated for the treatment of adult patients with multiple myeloma:
- In combination with lenalidomide and dexamethasone in newly diagnosed patients who are ineligible for autologous stem cell transplant and in patients with relapsed or refractory multiple myeloma who have received at least one prior therapy
- In combination with bortezomib, melphalan, and prednisone in newly diagnosed patients who are ineligible for autologous stem cell transplant
- In combination with bortezomib, thalidomide, and dexamethasone in newly diagnosed patients who are eligible for autologous stem cell transplant
- In combination with bortezomib and dexamethasone in patients who have received at least one prior therapy
- In combination with carfilzomib and dexamethasone in patients with relapsed or refractory multiple myeloma who have received one to three prior lines of therapy
- In combination with pomalidomide and dexamethasone in patients who have received at least two prior therapies including lenalidomide and a proteasome inhibitor
- As monotherapy in patients who have received at least three prior lines of therapy including a proteasome inhibitor (PI) and an immunomodulatory agent or who are double-refractory to a PI and an immunomodulatory agent
Please click here to see the full Prescribing Information.
1. Richardson PG, San Miguel JF, Moreau P, et al. Interpreting clinical trial data in multiple myeloma: translating findings to the real-world setting. Blood Cancer J. 2018;8(11). doi:10.1038/s41408-018-0141-0
2. Key Statistics About Multiple Myeloma. Cancer.org. Published 2019. https://www.cancer.org/cancer/multiple-myeloma/about/key-statistics.html
3. Fonseca R, Usmani SZ, Mehra M, et al. Frontline treatment patterns and attrition rates by subsequent lines of therapy in patients with newly diagnosed multiple myeloma. BMC Cancer. 2020;20(1). doi:10.1186/s12885-020-07503-y
4. Devarakonda S, Efebera Y, Sharma N. Role of Stem Cell Transplantation in Multiple Myeloma. Cancers. 2021;13(4):863. doi:10.3390/cancers13040863
5. Derudas D, Capraro F, Martinelli G, Cerchione C. How I manage frontline transplant-ineligible multiple myeloma. Hematol Rep. 2020;12(s1). doi:10.4081/hr.2020.8956
6. Kazandjian D. Multiple myeloma epidemiology and survival: A unique malignancy. Semin Oncl. 2016;43(6):676-681. doi:10.1053/j.seminoncol.2016.11.004
7. Facon T, Kumar S, Plesner T, et al. Daratumumab plus lenalidomide and dexamethasone for untreated myeloma. N. Engl. J. Med. 2019;380(22):2104-2115. doi:10.1056/nejmoa1817249
8. DARZALEX® [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.
9. Facon T, Kumar SK, Plesner T, et al. Daratumumab, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone alone in newly diagnosed multiple myeloma (MAIA): overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(11):1582-1596. doi:10.1016/s1470-2045(21)00466-6
10. Facon T, Dimopoulos MA, Meuleman N, et al. A simplified frailty scale predicts outcomes in transplant-ineligible patients with newly diagnosed multiple myeloma treated in the FIRST (MM-020) trial. Leukemia. 2019;34(1):224-233. doi:10.1038/s41375-019-0539-0
11. Facon T, Kumar SK, Plesner T, et al. Supplement to: Daratumumab, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone alone in newly diagnosed multiple myeloma (MAIA): overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(11):1582-1596.
12. Facon T, Cook G, Usmani SZ, et al. Daratumumab plus lenalidomide and dexamethasone in transplant-ineligible newly diagnosed multiple myeloma: frailty subgroup analysis of MAIA. Leukemia. 2022;36(4):1066-1077. doi:10.1038/s41375-021-01488-8
© Janssen Biotech, Inc. 2022 All rights reserved. 12/22 cp-333446v1
How the Dobbs decision shapes the ObGyn workforce and training landscape

Six months after the Supreme Court decision that overturned the constitutional right to abortion, trainees across the United States are asking a critical question in the current resident recruitment season: How will the restrictions on abortion access affect my training as an obstetrician-gynecologist, and will they impact my ability to be the kind of provider I want to be in the future?
Among the myriad of downstream effects to patient care, the Dobbs decision will indisputably impact the scope of residency training for those that provide reproductive health services. Almost half of ObGyn residents train in states that have abortion restrictions in place.1 New educational milestones for abortion training, which are a requirement by the Accreditation Council for Graduate Medical Education (ACGME), were proposed quickly after Dobbs, guiding programs to offer opportunities for training in nonrestricted areas or the “combination of didactic activities, including simulation” to meet the training requirement in abortion care.2
Like many providers, residents already are grappling with precarious and risky circumstances, balancing patient safety and patient-driven care amidst pre-existing and newly enforced abortion restrictions. Whether managing a patient with an undesired pregnancy, severe medical comorbidities, unexpected pregnancy complications such as preterm premature rupture of membranes, or bleeding, or substantial fetal anomalies, ObGyn residents cannot gain the experience of providing the full scope of reproductive health care without the ability to offer all possible management options. While some enacted abortion restrictions have exceptions for the health of or life-saving measures for the mother, there is no standard guidance for timing of interventions, leaving providers confused and in fear of legal retribution. At a time when trainees should be learning to provide patient-centered, evidence-based care, they are instead paralyzed by the legal or professional consequences they may face for offering their best medical judgements.
Furthermore, the lack of exposure to dilation and evacuation procedures for residents in restricted practice areas will undoubtably decrease their confidence in managing acute complications, which is one of the critical facets of residency training. In a surgical field where repetition is crucial for technical competence, highlighted by ACGME minimum case requirements, the decreased volume of abortion procedures is a disadvantage for trainees and a disservice for patients. While anti-choice promoters may argue that involvement in surgical management of early pregnancy loss should suffice for ObGyn training in family planning, this piecemeal approach will leave gaps in technical skills.
The fear of legal ramifications, moral injury, and inadequate surgical training may lead to the siphoning of talented trainees to areas in the country with fewer restrictions.3 Dobbs already has demonstrated how limiting abortion access will deepen inequities in reproductive health care service delivery. Approximately 55% of ObGyn trainees and nearly two-thirds of maternal-fetal medicine graduates join the workforce in the state where they received their training.4 Medical students will seek opportunities for high-quality ObGyn training in areas that will help them to be well-prepared, competent physicians—and more often than not, stay in the area or region that they trained in. This will lead to provider shortages in areas where access to reproductive health care and subspecialist providers already is limited, further exacerbating existing health disparities.
During this recruitment season, trainees and residency programs alike will need to reckon with how the ramifications of Dobbs will alter both the immediate and long-term training in comprehensive reproductive health care for the ObGyn workforce. ObGyn trainees have taken a stand in response to the Dobbs decision, and nearly 750 current residents signed onto the statement below as a commitment to high-quality training and patient-centered care. Clinical experience in performing abortions is essential to the provision of comprehensive evidence-based reproductive health care, and access to these procedures is as important for physicians-in-training as it is for patients.
Actions to take to ensure high-quality abortion training in ObGyn residencies include the following:
- Connect with and stay involved with organizations such as the American College of Obstetricians and Gynecologists (ACOG), Physicians for Reproductive Health (PRH), and Medical Students for Choice (MSFC) for initiatives, toolkits, and resources for training at your institutions.
- Seek specific abortion training opportunities through the Leadership Training Academy (offered through PRH) or the Abortion Training Institute (offered through MSFC).
- Ensure that your residency program meets the ACGME criteria of providing opportunities for clinical experiences for abortion care and work with program leadership at a program, state, or regional level to enforce these competencies.
- Reach out to your local American Civil Liberties Union or other local reproductive legal rights organizations if you want to be involved with advocacy around abortion access and training but have concerns about legal protections.
- Have a voice at the table for empowering training opportunities by seeking leadership positions through ACOG, ACGME, Council on Resident Education in Obstetrics and Gynecology and the Association of Professors of Gynecology and Obstetrics, American Medical Association, Student National Medical Association, and subspecialty organizations.
- Vote in every election and promote voting registration and access to your patients, colleagues, and communities. ●
Continue to: The implications of the Dobbs v Jackson Women’s Health Organization decision on the health care and wellbeing of our patients...
On June 24, 2022, the Supreme Court of the United States ruled in a 6-3 majority decision to overturn the constitutional right to abortion protected by Roe v Wade since 1973. As health care providers, we are outraged at the Court’s disregard for an individual’s right to make reproductive decisions for themselves and their families and are deeply concerned about the devastating consequences to reproductive care and outcomes in this country for all people. Reproductive health decisions, including growing a family and whether or not to continue a pregnancy, are complex and incredibly personal. Our role as health care providers is to help guide those decisions with empathy and evidencebased clinical recommendations. This ruling undermines a patient’s right to bodily autonomy, free of impositions from government and political pressures, and it threatens the sanctity of complex medical decision-making between a patient, their family, and their medical team.
As medical professionals, we know that every patient’s situation is unique—banning abortion procedures ties the hands of physicians trying to provide the most medically appropriate options in a compassionate manner. We know that both medical and surgical abortions are safe and can save lives. These procedures can help patients with potentially life-threatening conditions worsened by pregnancy, a poor prognosis for the fetus, or a complication from the pregnancy itself. Physicians use scientific research and individualized approaches to help patients in unique situations, and attempts to legislate personal health decisions compromise the practice of evidence-based medicine.
We also know that this decision will impact some communities more than others. Access to safe abortion care will become dependent on which region of the country a person lives in and whether or not a person has resources to seek this care. Due to continued systemic racism and oppression, patients of color will be disproportionately impacted and likely will suffer worse health outcomes from unsafe abortions. Those that rely on public insurance or who are uninsured will face overwhelming barriers in seeking abortion services. These disparities in reproductive care, which contribute to our nation’s health crises in maternal morbidity and mortality, unintended pregnancy, and neonatal complications, will further entrench health inequities, and patient lives and livelihoods will suffer.
We acknowledge the impact that this decision will have on restricting access to reproductive care. We stand by the fact that abortion care is health care. We vow to uphold the tenets of our profession to place patient autonomy and provision of safe quality medical care at the forefront of our practices.
We, as health care providers and physician trainees, hereby pledge:
- To continue to provide evidence-based, nonjudgmental counseling for all pregnancy options, including abortion, and support our patients through all reproductive health decisions
- To promote equity in providing comprehensive reproductive health care, recognizing the impacts of systemic racism and oppression
- To promote high quality training in providing safe reproductive care in our respective institutions
- To use our voices in our communities to advocate for all our patients to have the freedom to access the safe and compassionate health care they deserve.
Sincerely,
The undersigned 747 ObGyn resident physicians
Please note that we sign this statement on our own behalf as individuals and not on behalf of our respective institutions.
Orchideh Abar, MD
Laurel S. Aberle, MD
Kathleen E. Ackert, DO
Lauryn Adams, MD
Temiloluwa Adejuyigbe, MD
Oluwatoyosi M. Adeoye, MD
Hufriya Y. Aderianwalla, MD
Fareeza Afzal, MD
Adelaide Agyepong, MD
Erin R. Ahart, MD
Noha T. Ahmed, DO
Faria Ahmed, MD
Tracey O. Akanbi, MD
Eloho E. Akpovi, MD
Austin H. Allen, DO
Amanda M. Allen, MD
Alexis L. Allihien, MD
Jorge L. Alsina, MD
Paulina C. Altshuler, DO
Sivani Aluru, MD
Amal Amir, DO
Jon Anderson, DO
Andreas Antono, MD
Annie N. Apple, MD
Janine Appleton, DO
Aarthi Arab, MD
Sydney R. Archer, MD
Youngeun C. Armbuster, MD
Kara Arnold, MD
Blessing C. Aroh, MD
Savannah Pearson Ayala, MD
Archana K. Ayyar, MD
Ann-Sophie Van Backle, DO
Connor R. Baker, MD
Japjot K. Bal, MD
Abigail E. Barger, MD
Kathryn E. Barron, MD
Silvia Bastea, MD
Samantha V.H. Bayer, MD
Kristen Beierwaltes, MD
Gisel Bello, MD
Michelle A. Benassai, MD
Dana Benyas, MD
Alice F. Berenson, MD
Hanna P. Berlin, MD
Abigail L. Bernard, MD
Eli H. Bernstein, MD
Julia T. Berry, MD
Bryce L. Beyer, MD
Caroline Bilbe, MD
Grace E. Binter, DO
Erin E. Bishop, MD
Sierra G. Bishop, MD
Stephanie S. Bista, MD
Tara E. Bjorklund, DO
Alyssa N. Black, MD
Continue to: Kelsey Boghean, DO...
Kelsey Boghean, DO
Areta Bojko, MD
Grace E. Bommarito, DO
Aditi R. Bommireddy, MD
Genna C. Bonfiglio, MD
Mary E. Booker, MD
Kayce L. Booth, MD
Samantha T. Boothe, DO
William Borenzweig, MD
Rebecca M. Borneman, MD
Alexander L. Boscia, MD
Gina M. Botsko, MD
Glenn P. Boyles, MD
Avery C. Bramnik, MD
Sophia N. Brancazio, MD
Katarina M. Braun, MD
Anthony Brausch, MD
Emily L. Brekke, MD
Sara E. Brenner, MD
Bailey A. Brown, DO
Kathryn S. Brown, MD
Denese C. Brown, MD
Abena Bruce, MD
Sabrina C. Brunozzi, MD
Madison Buchman, DO
Deirdre G. Buckley, MD
Rachel L. Budker, MD
Leeann M. Bui, MD
Anthony H. Bui, MD
Jessie Bujouves, MD
Kimberley A. Bullard, MD
Sophia G. Bunde, MD
Emily R. Burdette, MD
Iris Burgard, DO
Korbi M. Burkey, MD
Lindsey K. Burleson, MD
Lindsay M. Burton, MD
Brianna N. Byers, MD
Stephanie Cai, MD
Alexandra S. Calderon, MD
Alexandra G. Caldwell, MD
Natalia Calzada, MD
Tamara Cameo, MD
Arielle Caplin, MD
Angela M. Carracino, DO
Anna L. Carroll, MD
Leigha M. Carryl, MD
Ashlie S. Carter, MD
Stephanie Casey, DO
Chase W. Cataline, DO
Carson L. Catasus, MD
Alena R. Cave, MD
Kelly M. Chacon, MD
Avis L. Chan, MD
Shruthi Chandra, MD
Jennifer Chang, MD
Shannon Chang, DO
Gillian Chase, MD
Cindy Chen, MD
Jessie C. Chen, MD
Jessica T. Chen, MD
Wenjin Cheng, MB
Laura J. Cheng, MD
Lucy Cheng, MD
Monica S. Choo, MD
Jody S. Chou, MD
Hannah C. Christopher, DO
Continue to: David J. Chromey, DO...
David J. Chromey, DO
Grace V. Clark, MD
Celeste Colegrove, MD
Sarah C. Combs, MD
Victoria L. Conniff, MD
Hannah C. Connor, MD
Angela J. Conway, MD
Steffany A. Conyers, MD
Alexandra Cooke, MD
Ashley A. Cooney, MD
Anna Cornelius-Schecter, MD
Alexa M. Corso, DO
Krysten A. Costley, MD
Madeline Coulter, MD
Kelsey Cramer, MD
Anna E. Cronin, MD
Bethany N. Croyle, DO
Carmen A. Cueto, MD
Nicole Cumbo, MD
Mackenzie A. Cummings, MD
Carrie Cummiskey, MD
Hannah M. Cunningham, MD
Sarah D’Souza, DO
Rachael M. D’Auria, MD
Caitlin Dane, MD
Rachel N. Dang, MD
Talin R. Darian, MD
Abigail C. Davies, MD
Berkley Davis, MD
Lois A. Davis, MD
Jennie J. DeBlanc, MD
Ayana G.R. DeGaia, MD, MPH
Katerina N. DeHaan, MD
Rebekka M. Delgado, MD
Brettany C. DeMier, MD
Bonnie W. DePaso, MD
Hemaxi H. Desai, DO
Amberly T. Diep, MD
Abigail K. Dillaha, MD
Sarah K. Dominguez, MD
Abbey P. Donahue, MD
Allan C. Dong, MD
James Doss, MD
Taylor B. Douglas, MD
Abigail G. Downey, MD
Janelle M. Driscoll, MD
Emily Du, MD
Leslie V. Dunmire, MD
Jennifer Duong, DO
Leigh C. Durudogan, MD
Mai N. Dyer, MD, MPH
Rebecca A. Ebbott, MD
Lindsey P. Eck, MD
Molly C. Eckman, MD
Alex Ede, MD, ScM
Claire E. Edelman, MD
Sara E. Edwards, MD
David J. Eggert, DO
Michelle Eide, MD
Etoroabasi Ekpe, MD
Tressa L. Ellett, MD
Laura Peyton Ellis, MD
Kaitlin H. Ellis, MD
Mariah G. Elly, MD
Jennifer Embry, MD
Claire Englert, MD
Brenna Espelien, MD
Kamilah Evans, MD
Joshua A. Ewy, MD
Elana D. Fackler, MD
Lauren E. Falk, MD
Brianna A. Farley, MD
Amanda Stephanie R. Farrell, MD
Sara Fassio, DO
Daniela A. Febres-Cordero, MD
Jasmin E. Feliciano, MD
Alayna H. Feng, MD
Amanda M. Ferraro, MD
Brittany A. Fickau, MD
Brittany H. File, MD
Shannon M. Finner, DO
Mia E. Fischbein, DO
Briah Fischer, MD
Shira Fishbach, MD
Alison C. Fitzgerald, MD
Evan R. Fitzgerald, MD
Margaret R. Flanigan, MD
Kevin C. Flatley, MD
Jordan A. Fletcher, MD
Claudia E. Flores, MD
Lauren A. Forbes, MD
Rana K. Fowlkes, MD
Jennifer M. Franks, MD, MPH
Christina M. Frasik, MD
Haven N. Frazier, DO
Sarah W. Freeman, MD
Emilie O. Fromm, DO
Anna R. Fuchss, MD
Emma K. Gaboury, MD
Madeline H. Ganz, MD
Lex J. Gardner, MD
Keri-Lee Garel, MD
Hailey B. Gaskamp, DO
Brittney A. Gaudet, MD
Gabrielle M. Gear, MD
Eleanor R. Germano, MD
Lauren G. Gernon, MD
Allen Ghareeb, MD
Patricia Giglio Ayers, MD
Jordana L. Gilman, MD
Mianna M. Gilmore, DO
Brian W. Goddard, MD
Julia L. Goldberg, MD
M. Isabel Gonzaga, MD
Fred P. Gonzales, MD
Lillian H. Goodman, MD, MPH
Ashley Goreshnik, MD
Lauren E. Gottshall, MD
Lindsay L. Gould, MD
Kelsea R. Grant, MD
Dorender A. Gray, MD
Sophie Green, MD
Erica A. Green, MD
Danielle C. Greenberg, MD
Kalin J. Gregory-Davis, MD
David M. Greiner, MD
Tyler M. Gresham, MD
Continue to: Nelly Grigorian, MD...
Nelly Grigorian, MD
Erin L. Grimes, MD
Whitney Grither, MD
Jared M. Grootwassink, MD
Maya E. Gross, MD
Paoula Gueorguieva, MD
Margot M. Gurganus, DO
Rachel L. Gutfreund, MD
Andres Gutierrez, MD
Dorothy L. Hakimian, DO
Ashley N. Hamati, DO
Marie M. Hanna-Wagner, MD
Katie Hansen, MD
Courtney Hargreaves, MD
Stephanie Harlow, MD
Kelsey B. Harper, MD
Devon A. Harris, MD
Lauren E. Harris, MD
Emily S. Hart, DO
Sarah A. Hartley, MD
Becky K. Hartman, MD
Abigail K. Hartmann, MD
Charlotte V. Hastings, MD
Cherise Hatch, DO
Jordan Hauck, DO
Sarena Hayer, MD
Jenna M. Heath, MD
Eric D. Helm, MD
Julie A. Hemphill, MD
Ric A.S. Henderson, MD
Nicola A. Hendricks, MD
Andrea A. Henricks, MD
Jesse M. Herman, DO
Alyssa M. Hernandez, DO
Melissa Hernandez, MD
Alyssa R. Hersh, MD
Alexandra Herweck, MD
Brianna Hickey, MD
Allix M. Hillebrand, MD
Alessandra I. Hirsch, MD
Emily A. Hoffberg, MD
Chloe L. Holmes, DO
Cameron M. Holmes, MD
Helena Y. Hong, MD
Wakako Horiuchi, MD
Shweta Hosakoppal, MD
Jaycee E. Housh, MD
Shannon M. Howard, MD
Meredith C. Huszagh, MD
Yihharn P. Hwang, MD
Emma C. Hyde, MD
Brooke Hyman, MD
Hala Ali Ibrahim, MD
Gnendy Indig, MD
Erin E. Isaacson, MD
Shruti S. Iyer, DO
Audrey J. Jaeger, DO
Shobha Jagannatham, MD
Cyrus M. Jalai, MD
Emma V. James, MD
Isabel Janmey, MD
Phoebe Jen, DO
Corey L. Johnson, MD
Crystal J. Johnson, MD
Andrea M. Johnson, MD
Nat C. Jones, MD
Briana L. Jones, DO
Rebecca J. Josephson, MD
Sarah Natasha Jost-Haynes, MD
Continue to: Hannah S. Juhel, MD...
Hannah S. Juhel, MD
Erin Jun, DO
Katherine B. Kaak, MD
Dhara N. Kadakia, MD
Amanda D. Kadesh, MD
Riana K. Kahlon, MD
Nadi N. Kaonga, MD
Moli Karsalia, MD
Stephanie L. Kass, MD
Amanda M. Katz, MD
Chelsea S. Katz, MD
Virginia Kaufman, MD
Gurpinder Kaur, MD
Jessica A. Keesee, MD
Cassandra N. Kelly, MD
Whitney Kelly, DO
Hannah V. Kennedy, MD
Bethany H. Kette, MD
Iman Khan, MD
Maryam M. Khan, MD
Alisa Jion Kim, MD
Tesia G. Kim, MD
Anne E. Kim, MD
Emily H. King, MD
Tarynne E. Kinghorn, MD
Holly T. Kiper, DO
Thomas Kishkovich, MD
Quinn M. Kistenfeger, MD
Sofia E. Klar, DO
Jessica B. Klugman, MD
Hope E. Knochenhauer, MD
Kathleen J. Koenigs, MD
Olga Kontarovich, DO
Alison Kosmacki, MD
Ana E. Kouri, MD
Olga M. Kovalenko, MD
Leigh T. Kowalski, MD
Kayla A. Krajick, MD
Elizabeth S. Kravitz, MD
Shruti Rani Kumar, MD
Alyssa Kurtz, DO
Lauren H. Kus, MD
Arkadiy Kusayev, DO
Amanda E. Lacue, MD
Nava Lalehzari, MD
Amber Lalla, MD
Allie C. Lamari, DO
Kelly L. Lamiman, MD
Stephen Lammers, MD
Monet Lane, MD
Madeline L. Lang, MD
Liana Langdon-Embry, MD
Carolyn Larkins, MD
Leah E. Larson, MD
Matthew W. Lee, MD
Eunjae Lee, MD
Alice Lee, MD
Jared Z. Lee, MD
Charlotte M. Lee, MD
Nicole R. Legro, MD
Aurora Leibold, MD
Rosiris Leon-Rivera, MD, PhD
Anna M. Leone, MD
Keiko M. Leong, MD
Lindsey M. LePoidevin, MD
Molly E. Levine, MD
Khrystyna Levytska, MD
Dana L. Lewis, DO
Jessica L. Li, MD
Kristina Lilja, MD
Deanna M. Lines, DO
Annalise Littman, MD
Julia F. Liu, MD
Tyler B. Lloyd, MD
Alyssa Lo, MD
K’ara A. Locke, MD
Minica Long, MD
Melissa Lopez, MD
Wilfredo A. Lopez, MD
Connie F. Lu, MD
Tyler J. Lueck, MD
Katherine L. Lukas, MD
Davlyn L. Luke, MD
Shani Ma, MD
Colton Mabis, MD
Lauren T. MacNeill, MD
Rachel Madding, MD
Mona Makhamreh, MD
Francesca R. Mancuso, MD
Kelsey L. Manfredi, MD
Valeria Mantilla, MD
Kaitlin M. Mar, MD
Starcher R. Margaret, MD
Audrey M. Marinelli, MD
Brittany A. Marinelli, MD
Emily S. Markovic, MD
Hannah L. Marshall, MD
Aaron Masjedi, MD
Isabelle M. Mason, MD
Akailah T. Mason-Otey, MD
Nicole Massad, MD
Megan M. Masten, MD
Stephanie M. Masters, MD
Anastasia Matthews, MD
Natalia del Mazo, MD
Sara A. McAllaster, MD
Continue to: Nicole McAndrew, DO...
Nicole McAndrew, DO
Madeline G. McCosker, MD
Jamie L. McDowell, DO
Christine E. McGough, MD
Mackenzi R. McHugh, MD
Madeline M. McIntire, MD
Cynthia R. McKinney, MD
Kirsten D. McLane, MD
Shian F. McLeish, MD
Megan I. McNitt, MD
Sarah R. McShane, MD
Grace R. Meade, MD
Nikki Ann R. Medina, DO
Tiffany L. Mei, MD
Jenna Meiman, MD
Anna M. Melicher, MD
Rosa M. Mendez, MD
Riley Mickelsen, MD
Sage A. Mikami, MD
Aletheia B. Millien, MD
Hannah C. Milthorpe, MD
Caroline J. Min, MD
Julie A. Mina, MD
Annie G. Minns, MD
Natalie Mironov, DO
Elizabeth L. Mirsky, MD
Astha Mittal, MD
Rachel E. Mnuk, MD
Silki Modi, MD
Sudarshan J. Mohan, MD
Roxana Mohhebali-Solis, MD
Mugdha V. Mokashi, MD
Jessica A. Montgomery, MD
Ellen Moore, MD
Savannah J. Morehouse, MD
Kristen L. Moriarty, MD
Alexa P. Morrison, MD
Bijan Morshedi, MD
Matthew H. Mossayebi, MD
Kathy Mostajeran, DO
Sharan Mullen, DO
Ellen C. Murphy, MD
Emma Chew Murphy, MD
Lauren M. Murphy, MD
Bria Murray, MD
Erin C. Nacev, MD
Preetha Nandi, MD
Blaire E. Nasstrom, DO
Hallie N. Nelson, MD
Katherine A. Nelson, MD
Margaret S. Nemetz, MD
Daniela Ben Neriah, DO
Cosima M. Neumann, MD
Mollie H. Newbern, DO
Gisella M. Newbery, MD
Stephanie Nguyen, MD
Christine G.T. Nguyen, MD
Desiree Nguyen, MD
Jacqueline W. Nichols, MD
Annika M. Nilsen, MD
Margaret A. Nixon, MD
Emily M. Norkett, MD
Allison N. Nostrant, DO
Susan E. Nourse, MD
Aliya S. Nurani, MD
Emily E. Nuss, MD
Jeanne O. Nwagwu, DO
Kelsey E. O’Hagan, MD
Margaret O’Neill, MD
Emily A. O’Brien, MD
Carly M. O’Connor-Terry, MD, MS
Madison O. Odom, MD
Cynthia I. Okot-Kotber, MD
Sarah P. Oliver, MD
Leanne P. Ondreicka, MD
Ngozika G. Onyiuke, MD
Erika Gonzalez Osorio, MD
Marika L. Osterbur Badhey, MD
Linda A. Otieno, MD
Claire H. Packer, MD
Chloe W. Page, DO
Marissa Palmor, MD
Rishitha Panditi, MD
Katherine A. Panushka, MD
Kelsey J. Pape, MD
Rachel R. Paquette, DO
Hillary C. Park, DO
Kendall M. Parrott, MD
Ekta Partani, MD
Karishma Patel, MD
Shivani Patel, MD
Continue to: Priya Patel, MD...
Priya Patel, MD
Jenna M. Patterson, MD
Ashleigh Pavlovic, MD
Katie M. Peagler, MD
Katherine T. Pellino, MD
Nicholas Per, MD
Elana Perry, MD
Emily J. Peters, MD
Sara E. Peterson, MD
Michelle R. Petrich, MD
Destiny L. Phillips, MD
Chloe Phillips, MD
Megan E. Piacquadio, DO
Sara C. Pierpoint, MD
Celeste M. Pilato, MD
Emma Pindra, MD
Minerva L.R. Pineda, MD
Rebecca Pisan, MD
Alessandra R. Piscina, MD
Rachael Piver, MD
Andrew J. Polio, MD
Hector S. Porragas, MD
Natalie Posever, MD
Allison R. Powell, MD
Mahima V. Prasad, MD
Angelina D. Prat, DO
Rebecca L. Purvis, MD
Teresa L. Qi, MD
Nicholas R. Quam, MD
Candice A. Quarella, MD
Nicholas W. Racchi, DO
Jeannie G. Radoc, MD
Samuel Raine, MD
Anna C. Raines, MD
Stephanie A. Rains, MD
Nicole M. Rainville, DO
Karissa Rajagopal, DO
Kristian R. Ramage, MD
Praveen Ramesh, MD
Tia M. Ramirez, MD
Jania Ramos, MD
Neel K. Rana, MD
Urvi Rana, DO
Indira Ranaweera, MD
Sindhuja Ranganathan, DO
Chloe R. Rasmussen, MD
Laura P. Reguero-Cadilla, MD
Devin M. Reilly, MD
Kimberly E. Reimold, MD
Cory R. Reiter, MD, PhD
Maya E. Reuven, DO
Jessica Reyes-Peterson, MD
Jacqueline Rice, MD
Rebecca L. Richardson, MD
Mikaela J. Rico, DO
Katelyn Rittenhouse, MD
Giuliana A. Rivera Casul, MD
Jill N.T. Roberts, MD
Luke N. Roberts, MD
Esther Robin, MD
Marcella Israel Rocha, MD
Zoe A. Roecker, MD
Hilary E. Rogers, MD
Kelsey A. Roof, MD
Zarah Rosen, MD
Cecilia M. Rossi, MD
Eva S. Rostonics, MD
Felix Rubio, MD
Amela Rugova, MD
Anna J. Rujan, MD
Erika T. Russ, MD
Colin Russell, MD
Ruby L. Russell, MD
Isabella A. Sabatina, MD
Gouri Sadananda, MD
Aashna Saini, MD
Salomeh M. Salari, MD
Ndeye N. Sall, MD
Nicole M. Salvador, MD
Aayushi Sardana, MD
Kendall M. Sarson, MD
Rita Abigail Sartor, MD
Continue to: Haley A. Scarbrough, MD...
Haley A. Scarbrough, MD
Kimberly Schaefer, MD
Demetra Schermerhorn, MD
Ellen C. Schleckman, MD
Maura A. Schlussel, MD
Ellie Schmidt, MD
Alison M. Schmidt, MD
Evan A. Schrader, MD
Morgan A. Schriever, MD
Brianna L. Schumaker Nguyen, DO
Whitney E. Scott, MD
Claire Scrivani, MD
Catherine E. Seaman, MD
Rachel D. Seaman, MD
Danielle J. Seltzer, MD
Joshua R. Shaffer, MD
Emily A. Shaffer, MD
Delia S. Shash, MD
Ishana P. Shetty, MD
Tushar Shetty, MD
Carol Shi, MD
Sarah P. Shim, MD
Emma C. Siewert, MD
Seth M. Sigler, DO
Rebecca L. SigourneyTennyck, MD
Daniella D. Silvino, DO
Andrea M. Simi, MD
Amelia R. Simmons, MD
Amy E. Skeels, DO
Ashley E.S. Keith, MD
Hannah C. Smerker, DO
Katarina Smigoc, MD
Madeline I. Smith, MD
Jessica D. Smith, MD
Melanie R. Smith, MD
Alicia L. Smith, MD
Chloe Smith, MD
Ayanna Smith, MD
Melanie R. Smith, MD
Megan M. Smith, MD
Haverly J. Snyder, MD
Beatrice R. Soderholm, DO
Brianna C. Sohl, MD
Samantha A. Solaru, MD
Michael Solotke, MD
Dara A.H. Som, MD
Alexandra R. Sotiros-Lowry, MD
Melanie Spall, DO
Alicia C. Speak, DO
Lisa M. Spencer, MD
Prakrithi Srinand, MD
Sierra M. Starr, MD
Kathryne E. Staudinger, MD
Emily K. Steele, MD
Morgan R. Steffen, DO
Tricia R. Stepanek, MD
Taylor P. Stewart, MD
Kelsey A. Stewart, MD
Alyssa M. Stiff, MD
Alexandra B. Stiles, MD
Nairi K. Strauch, MD
Margaret J. Stroup, DO
Sean C. Stuart, DO
Hannah M. Stump, MD
Shalini B. Subbarao, MD
Lakshmi Subramani, MD
Heather E. Sweeney, MD
Kristin I. Swope, MD
Suha Syed, MD
Mireya P. Taboada, MD
Eneti S. Tagaloa, MD
Rachel Tang, DO
Adam R. Taylor, MD
Simone R. Thibault, MD
Kimberly A. Thill, MD
Dhanu Thiyag, MD
Andrew T. Thornton, MD
Wendy Tian, MD
Stephanie Tilberry, MD
Amanda L. Tillett, MD
Amanda M. Tjitro, MD
Logan P. Todhunter, DO
David Toffey, MD
Maris K. Toland, MD
Rachel E. Tomassi, MD
Sarah Tounsi, MD
Antonia K. Traina, MD
Taylor Tran, MD
Diem Samantha Tran, DO
Emily C. Trautner, MD
Emma Trawick, MD
Continue to: Elissa Trieu, MD...
Elissa Trieu, MD
Ariel Trilling, MD
Samantha Truong, MD
Mary M. Tsaturian, MD
Athena Tudino, MD
Kati A. Turner, MD
Nicole-Marie Tuzinkiewicz, MD
Gayathri D. Vadlamudi, MD
Stylianos Vagios, MD
Pauline V. Van Dijck, DO
Kaylee A. VanDommelen, MD
Isha B. Vasudeva, MD
Shivani J. Vasudeva, DO
Diana Q. Vazquez Parker, MD
Ridhima Vemula, MD
Elena C. Vinopal, MD
Caroline J. Violette, MD
Pascal T. Vo, DO
Michelle H. Vu, MD
Macy M. Walz, MD
Angelia Wang, MD
Eileen Wang, MD
Courtney Y. Wang, MD
Joyce Wang, MD
Meryl G. Warshafsky, MD
Sophie E.N. Weinstein, MD
Sarah H. Weinstein, MD
Annalyn M. Welp, MD
Shannon M. Wentworth, MD
Erika M. Wert, MD
Rachel C. White, MBchB
Morgan N. Wilhoite, DO
Mercedes Williams, MD
Hayley Williams, MD
Jacquelyn D. Williams, MD
Mary H. Williamson, MD
Elise Wilson, MD
Lauren M. Witchey, MD
Emily A. Wolverton, MD
Stephanie Y. Wong, MD
Jenny Wu, MD
Jackie Xiang, MD
Nancy S. Yang, MD
Kevin P. Yeagle, MD
Halina M. Yee, MD
Alyssa M. Yeung, MD
Samuel K. Yost, MD
Megan Yuen, MD
Nayab Zafar, DO
Cindy X. Zhang, DO
Yingao Zhang, MD
Helen Zhao, MD
Chelsea Zhu, MD
Billie E. Zidel, MD
Ryan A. Zoldowski, MD
- Vinekar K, Karlapudi A, Nathan L, et al. Projected implications of overturning Roe v Wade on abortion training in US obstetrics and gynecology residency programs. Obstet Gynecol. 2022;140:146-149.
- ACGME program requirements for graduate medical education in obstetrics and gynecology summary and impact of interim requirement revisions. ACGME website. Accessed December 18, 2022. https://www.acgme.org/globalassets/pfassets/reviewandcomment/220_obstetricsandgynecology_2022-06-24_impact.pdf
- Crear-Perry J, Hassan A, Daniel S. Advancing birth equity in a post-Dobbs US. JAMA. 2022;328:1689-1690.
- Report on residents. AAMC website. Accessed December 18, 2022. https://www.aamc.org/data-reports/students-residents/interactive-data/report-residents/2021/table-c4-physician-reten tion-state-residency-training-last-completed-gme

Six months after the Supreme Court decision that overturned the constitutional right to abortion, trainees across the United States are asking a critical question in the current resident recruitment season: How will the restrictions on abortion access affect my training as an obstetrician-gynecologist, and will they impact my ability to be the kind of provider I want to be in the future?
Among the myriad of downstream effects to patient care, the Dobbs decision will indisputably impact the scope of residency training for those that provide reproductive health services. Almost half of ObGyn residents train in states that have abortion restrictions in place.1 New educational milestones for abortion training, which are a requirement by the Accreditation Council for Graduate Medical Education (ACGME), were proposed quickly after Dobbs, guiding programs to offer opportunities for training in nonrestricted areas or the “combination of didactic activities, including simulation” to meet the training requirement in abortion care.2
Like many providers, residents already are grappling with precarious and risky circumstances, balancing patient safety and patient-driven care amidst pre-existing and newly enforced abortion restrictions. Whether managing a patient with an undesired pregnancy, severe medical comorbidities, unexpected pregnancy complications such as preterm premature rupture of membranes, or bleeding, or substantial fetal anomalies, ObGyn residents cannot gain the experience of providing the full scope of reproductive health care without the ability to offer all possible management options. While some enacted abortion restrictions have exceptions for the health of or life-saving measures for the mother, there is no standard guidance for timing of interventions, leaving providers confused and in fear of legal retribution. At a time when trainees should be learning to provide patient-centered, evidence-based care, they are instead paralyzed by the legal or professional consequences they may face for offering their best medical judgements.
Furthermore, the lack of exposure to dilation and evacuation procedures for residents in restricted practice areas will undoubtably decrease their confidence in managing acute complications, which is one of the critical facets of residency training. In a surgical field where repetition is crucial for technical competence, highlighted by ACGME minimum case requirements, the decreased volume of abortion procedures is a disadvantage for trainees and a disservice for patients. While anti-choice promoters may argue that involvement in surgical management of early pregnancy loss should suffice for ObGyn training in family planning, this piecemeal approach will leave gaps in technical skills.
The fear of legal ramifications, moral injury, and inadequate surgical training may lead to the siphoning of talented trainees to areas in the country with fewer restrictions.3 Dobbs already has demonstrated how limiting abortion access will deepen inequities in reproductive health care service delivery. Approximately 55% of ObGyn trainees and nearly two-thirds of maternal-fetal medicine graduates join the workforce in the state where they received their training.4 Medical students will seek opportunities for high-quality ObGyn training in areas that will help them to be well-prepared, competent physicians—and more often than not, stay in the area or region that they trained in. This will lead to provider shortages in areas where access to reproductive health care and subspecialist providers already is limited, further exacerbating existing health disparities.
During this recruitment season, trainees and residency programs alike will need to reckon with how the ramifications of Dobbs will alter both the immediate and long-term training in comprehensive reproductive health care for the ObGyn workforce. ObGyn trainees have taken a stand in response to the Dobbs decision, and nearly 750 current residents signed onto the statement below as a commitment to high-quality training and patient-centered care. Clinical experience in performing abortions is essential to the provision of comprehensive evidence-based reproductive health care, and access to these procedures is as important for physicians-in-training as it is for patients.
Actions to take to ensure high-quality abortion training in ObGyn residencies include the following:
- Connect with and stay involved with organizations such as the American College of Obstetricians and Gynecologists (ACOG), Physicians for Reproductive Health (PRH), and Medical Students for Choice (MSFC) for initiatives, toolkits, and resources for training at your institutions.
- Seek specific abortion training opportunities through the Leadership Training Academy (offered through PRH) or the Abortion Training Institute (offered through MSFC).
- Ensure that your residency program meets the ACGME criteria of providing opportunities for clinical experiences for abortion care and work with program leadership at a program, state, or regional level to enforce these competencies.
- Reach out to your local American Civil Liberties Union or other local reproductive legal rights organizations if you want to be involved with advocacy around abortion access and training but have concerns about legal protections.
- Have a voice at the table for empowering training opportunities by seeking leadership positions through ACOG, ACGME, Council on Resident Education in Obstetrics and Gynecology and the Association of Professors of Gynecology and Obstetrics, American Medical Association, Student National Medical Association, and subspecialty organizations.
- Vote in every election and promote voting registration and access to your patients, colleagues, and communities. ●
Continue to: The implications of the Dobbs v Jackson Women’s Health Organization decision on the health care and wellbeing of our patients...
On June 24, 2022, the Supreme Court of the United States ruled in a 6-3 majority decision to overturn the constitutional right to abortion protected by Roe v Wade since 1973. As health care providers, we are outraged at the Court’s disregard for an individual’s right to make reproductive decisions for themselves and their families and are deeply concerned about the devastating consequences to reproductive care and outcomes in this country for all people. Reproductive health decisions, including growing a family and whether or not to continue a pregnancy, are complex and incredibly personal. Our role as health care providers is to help guide those decisions with empathy and evidencebased clinical recommendations. This ruling undermines a patient’s right to bodily autonomy, free of impositions from government and political pressures, and it threatens the sanctity of complex medical decision-making between a patient, their family, and their medical team.
As medical professionals, we know that every patient’s situation is unique—banning abortion procedures ties the hands of physicians trying to provide the most medically appropriate options in a compassionate manner. We know that both medical and surgical abortions are safe and can save lives. These procedures can help patients with potentially life-threatening conditions worsened by pregnancy, a poor prognosis for the fetus, or a complication from the pregnancy itself. Physicians use scientific research and individualized approaches to help patients in unique situations, and attempts to legislate personal health decisions compromise the practice of evidence-based medicine.
We also know that this decision will impact some communities more than others. Access to safe abortion care will become dependent on which region of the country a person lives in and whether or not a person has resources to seek this care. Due to continued systemic racism and oppression, patients of color will be disproportionately impacted and likely will suffer worse health outcomes from unsafe abortions. Those that rely on public insurance or who are uninsured will face overwhelming barriers in seeking abortion services. These disparities in reproductive care, which contribute to our nation’s health crises in maternal morbidity and mortality, unintended pregnancy, and neonatal complications, will further entrench health inequities, and patient lives and livelihoods will suffer.
We acknowledge the impact that this decision will have on restricting access to reproductive care. We stand by the fact that abortion care is health care. We vow to uphold the tenets of our profession to place patient autonomy and provision of safe quality medical care at the forefront of our practices.
We, as health care providers and physician trainees, hereby pledge:
- To continue to provide evidence-based, nonjudgmental counseling for all pregnancy options, including abortion, and support our patients through all reproductive health decisions
- To promote equity in providing comprehensive reproductive health care, recognizing the impacts of systemic racism and oppression
- To promote high quality training in providing safe reproductive care in our respective institutions
- To use our voices in our communities to advocate for all our patients to have the freedom to access the safe and compassionate health care they deserve.
Sincerely,
The undersigned 747 ObGyn resident physicians
Please note that we sign this statement on our own behalf as individuals and not on behalf of our respective institutions.
Orchideh Abar, MD
Laurel S. Aberle, MD
Kathleen E. Ackert, DO
Lauryn Adams, MD
Temiloluwa Adejuyigbe, MD
Oluwatoyosi M. Adeoye, MD
Hufriya Y. Aderianwalla, MD
Fareeza Afzal, MD
Adelaide Agyepong, MD
Erin R. Ahart, MD
Noha T. Ahmed, DO
Faria Ahmed, MD
Tracey O. Akanbi, MD
Eloho E. Akpovi, MD
Austin H. Allen, DO
Amanda M. Allen, MD
Alexis L. Allihien, MD
Jorge L. Alsina, MD
Paulina C. Altshuler, DO
Sivani Aluru, MD
Amal Amir, DO
Jon Anderson, DO
Andreas Antono, MD
Annie N. Apple, MD
Janine Appleton, DO
Aarthi Arab, MD
Sydney R. Archer, MD
Youngeun C. Armbuster, MD
Kara Arnold, MD
Blessing C. Aroh, MD
Savannah Pearson Ayala, MD
Archana K. Ayyar, MD
Ann-Sophie Van Backle, DO
Connor R. Baker, MD
Japjot K. Bal, MD
Abigail E. Barger, MD
Kathryn E. Barron, MD
Silvia Bastea, MD
Samantha V.H. Bayer, MD
Kristen Beierwaltes, MD
Gisel Bello, MD
Michelle A. Benassai, MD
Dana Benyas, MD
Alice F. Berenson, MD
Hanna P. Berlin, MD
Abigail L. Bernard, MD
Eli H. Bernstein, MD
Julia T. Berry, MD
Bryce L. Beyer, MD
Caroline Bilbe, MD
Grace E. Binter, DO
Erin E. Bishop, MD
Sierra G. Bishop, MD
Stephanie S. Bista, MD
Tara E. Bjorklund, DO
Alyssa N. Black, MD
Continue to: Kelsey Boghean, DO...
Kelsey Boghean, DO
Areta Bojko, MD
Grace E. Bommarito, DO
Aditi R. Bommireddy, MD
Genna C. Bonfiglio, MD
Mary E. Booker, MD
Kayce L. Booth, MD
Samantha T. Boothe, DO
William Borenzweig, MD
Rebecca M. Borneman, MD
Alexander L. Boscia, MD
Gina M. Botsko, MD
Glenn P. Boyles, MD
Avery C. Bramnik, MD
Sophia N. Brancazio, MD
Katarina M. Braun, MD
Anthony Brausch, MD
Emily L. Brekke, MD
Sara E. Brenner, MD
Bailey A. Brown, DO
Kathryn S. Brown, MD
Denese C. Brown, MD
Abena Bruce, MD
Sabrina C. Brunozzi, MD
Madison Buchman, DO
Deirdre G. Buckley, MD
Rachel L. Budker, MD
Leeann M. Bui, MD
Anthony H. Bui, MD
Jessie Bujouves, MD
Kimberley A. Bullard, MD
Sophia G. Bunde, MD
Emily R. Burdette, MD
Iris Burgard, DO
Korbi M. Burkey, MD
Lindsey K. Burleson, MD
Lindsay M. Burton, MD
Brianna N. Byers, MD
Stephanie Cai, MD
Alexandra S. Calderon, MD
Alexandra G. Caldwell, MD
Natalia Calzada, MD
Tamara Cameo, MD
Arielle Caplin, MD
Angela M. Carracino, DO
Anna L. Carroll, MD
Leigha M. Carryl, MD
Ashlie S. Carter, MD
Stephanie Casey, DO
Chase W. Cataline, DO
Carson L. Catasus, MD
Alena R. Cave, MD
Kelly M. Chacon, MD
Avis L. Chan, MD
Shruthi Chandra, MD
Jennifer Chang, MD
Shannon Chang, DO
Gillian Chase, MD
Cindy Chen, MD
Jessie C. Chen, MD
Jessica T. Chen, MD
Wenjin Cheng, MB
Laura J. Cheng, MD
Lucy Cheng, MD
Monica S. Choo, MD
Jody S. Chou, MD
Hannah C. Christopher, DO
Continue to: David J. Chromey, DO...
David J. Chromey, DO
Grace V. Clark, MD
Celeste Colegrove, MD
Sarah C. Combs, MD
Victoria L. Conniff, MD
Hannah C. Connor, MD
Angela J. Conway, MD
Steffany A. Conyers, MD
Alexandra Cooke, MD
Ashley A. Cooney, MD
Anna Cornelius-Schecter, MD
Alexa M. Corso, DO
Krysten A. Costley, MD
Madeline Coulter, MD
Kelsey Cramer, MD
Anna E. Cronin, MD
Bethany N. Croyle, DO
Carmen A. Cueto, MD
Nicole Cumbo, MD
Mackenzie A. Cummings, MD
Carrie Cummiskey, MD
Hannah M. Cunningham, MD
Sarah D’Souza, DO
Rachael M. D’Auria, MD
Caitlin Dane, MD
Rachel N. Dang, MD
Talin R. Darian, MD
Abigail C. Davies, MD
Berkley Davis, MD
Lois A. Davis, MD
Jennie J. DeBlanc, MD
Ayana G.R. DeGaia, MD, MPH
Katerina N. DeHaan, MD
Rebekka M. Delgado, MD
Brettany C. DeMier, MD
Bonnie W. DePaso, MD
Hemaxi H. Desai, DO
Amberly T. Diep, MD
Abigail K. Dillaha, MD
Sarah K. Dominguez, MD
Abbey P. Donahue, MD
Allan C. Dong, MD
James Doss, MD
Taylor B. Douglas, MD
Abigail G. Downey, MD
Janelle M. Driscoll, MD
Emily Du, MD
Leslie V. Dunmire, MD
Jennifer Duong, DO
Leigh C. Durudogan, MD
Mai N. Dyer, MD, MPH
Rebecca A. Ebbott, MD
Lindsey P. Eck, MD
Molly C. Eckman, MD
Alex Ede, MD, ScM
Claire E. Edelman, MD
Sara E. Edwards, MD
David J. Eggert, DO
Michelle Eide, MD
Etoroabasi Ekpe, MD
Tressa L. Ellett, MD
Laura Peyton Ellis, MD
Kaitlin H. Ellis, MD
Mariah G. Elly, MD
Jennifer Embry, MD
Claire Englert, MD
Brenna Espelien, MD
Kamilah Evans, MD
Joshua A. Ewy, MD
Elana D. Fackler, MD
Lauren E. Falk, MD
Brianna A. Farley, MD
Amanda Stephanie R. Farrell, MD
Sara Fassio, DO
Daniela A. Febres-Cordero, MD
Jasmin E. Feliciano, MD
Alayna H. Feng, MD
Amanda M. Ferraro, MD
Brittany A. Fickau, MD
Brittany H. File, MD
Shannon M. Finner, DO
Mia E. Fischbein, DO
Briah Fischer, MD
Shira Fishbach, MD
Alison C. Fitzgerald, MD
Evan R. Fitzgerald, MD
Margaret R. Flanigan, MD
Kevin C. Flatley, MD
Jordan A. Fletcher, MD
Claudia E. Flores, MD
Lauren A. Forbes, MD
Rana K. Fowlkes, MD
Jennifer M. Franks, MD, MPH
Christina M. Frasik, MD
Haven N. Frazier, DO
Sarah W. Freeman, MD
Emilie O. Fromm, DO
Anna R. Fuchss, MD
Emma K. Gaboury, MD
Madeline H. Ganz, MD
Lex J. Gardner, MD
Keri-Lee Garel, MD
Hailey B. Gaskamp, DO
Brittney A. Gaudet, MD
Gabrielle M. Gear, MD
Eleanor R. Germano, MD
Lauren G. Gernon, MD
Allen Ghareeb, MD
Patricia Giglio Ayers, MD
Jordana L. Gilman, MD
Mianna M. Gilmore, DO
Brian W. Goddard, MD
Julia L. Goldberg, MD
M. Isabel Gonzaga, MD
Fred P. Gonzales, MD
Lillian H. Goodman, MD, MPH
Ashley Goreshnik, MD
Lauren E. Gottshall, MD
Lindsay L. Gould, MD
Kelsea R. Grant, MD
Dorender A. Gray, MD
Sophie Green, MD
Erica A. Green, MD
Danielle C. Greenberg, MD
Kalin J. Gregory-Davis, MD
David M. Greiner, MD
Tyler M. Gresham, MD
Continue to: Nelly Grigorian, MD...
Nelly Grigorian, MD
Erin L. Grimes, MD
Whitney Grither, MD
Jared M. Grootwassink, MD
Maya E. Gross, MD
Paoula Gueorguieva, MD
Margot M. Gurganus, DO
Rachel L. Gutfreund, MD
Andres Gutierrez, MD
Dorothy L. Hakimian, DO
Ashley N. Hamati, DO
Marie M. Hanna-Wagner, MD
Katie Hansen, MD
Courtney Hargreaves, MD
Stephanie Harlow, MD
Kelsey B. Harper, MD
Devon A. Harris, MD
Lauren E. Harris, MD
Emily S. Hart, DO
Sarah A. Hartley, MD
Becky K. Hartman, MD
Abigail K. Hartmann, MD
Charlotte V. Hastings, MD
Cherise Hatch, DO
Jordan Hauck, DO
Sarena Hayer, MD
Jenna M. Heath, MD
Eric D. Helm, MD
Julie A. Hemphill, MD
Ric A.S. Henderson, MD
Nicola A. Hendricks, MD
Andrea A. Henricks, MD
Jesse M. Herman, DO
Alyssa M. Hernandez, DO
Melissa Hernandez, MD
Alyssa R. Hersh, MD
Alexandra Herweck, MD
Brianna Hickey, MD
Allix M. Hillebrand, MD
Alessandra I. Hirsch, MD
Emily A. Hoffberg, MD
Chloe L. Holmes, DO
Cameron M. Holmes, MD
Helena Y. Hong, MD
Wakako Horiuchi, MD
Shweta Hosakoppal, MD
Jaycee E. Housh, MD
Shannon M. Howard, MD
Meredith C. Huszagh, MD
Yihharn P. Hwang, MD
Emma C. Hyde, MD
Brooke Hyman, MD
Hala Ali Ibrahim, MD
Gnendy Indig, MD
Erin E. Isaacson, MD
Shruti S. Iyer, DO
Audrey J. Jaeger, DO
Shobha Jagannatham, MD
Cyrus M. Jalai, MD
Emma V. James, MD
Isabel Janmey, MD
Phoebe Jen, DO
Corey L. Johnson, MD
Crystal J. Johnson, MD
Andrea M. Johnson, MD
Nat C. Jones, MD
Briana L. Jones, DO
Rebecca J. Josephson, MD
Sarah Natasha Jost-Haynes, MD
Continue to: Hannah S. Juhel, MD...
Hannah S. Juhel, MD
Erin Jun, DO
Katherine B. Kaak, MD
Dhara N. Kadakia, MD
Amanda D. Kadesh, MD
Riana K. Kahlon, MD
Nadi N. Kaonga, MD
Moli Karsalia, MD
Stephanie L. Kass, MD
Amanda M. Katz, MD
Chelsea S. Katz, MD
Virginia Kaufman, MD
Gurpinder Kaur, MD
Jessica A. Keesee, MD
Cassandra N. Kelly, MD
Whitney Kelly, DO
Hannah V. Kennedy, MD
Bethany H. Kette, MD
Iman Khan, MD
Maryam M. Khan, MD
Alisa Jion Kim, MD
Tesia G. Kim, MD
Anne E. Kim, MD
Emily H. King, MD
Tarynne E. Kinghorn, MD
Holly T. Kiper, DO
Thomas Kishkovich, MD
Quinn M. Kistenfeger, MD
Sofia E. Klar, DO
Jessica B. Klugman, MD
Hope E. Knochenhauer, MD
Kathleen J. Koenigs, MD
Olga Kontarovich, DO
Alison Kosmacki, MD
Ana E. Kouri, MD
Olga M. Kovalenko, MD
Leigh T. Kowalski, MD
Kayla A. Krajick, MD
Elizabeth S. Kravitz, MD
Shruti Rani Kumar, MD
Alyssa Kurtz, DO
Lauren H. Kus, MD
Arkadiy Kusayev, DO
Amanda E. Lacue, MD
Nava Lalehzari, MD
Amber Lalla, MD
Allie C. Lamari, DO
Kelly L. Lamiman, MD
Stephen Lammers, MD
Monet Lane, MD
Madeline L. Lang, MD
Liana Langdon-Embry, MD
Carolyn Larkins, MD
Leah E. Larson, MD
Matthew W. Lee, MD
Eunjae Lee, MD
Alice Lee, MD
Jared Z. Lee, MD
Charlotte M. Lee, MD
Nicole R. Legro, MD
Aurora Leibold, MD
Rosiris Leon-Rivera, MD, PhD
Anna M. Leone, MD
Keiko M. Leong, MD
Lindsey M. LePoidevin, MD
Molly E. Levine, MD
Khrystyna Levytska, MD
Dana L. Lewis, DO
Jessica L. Li, MD
Kristina Lilja, MD
Deanna M. Lines, DO
Annalise Littman, MD
Julia F. Liu, MD
Tyler B. Lloyd, MD
Alyssa Lo, MD
K’ara A. Locke, MD
Minica Long, MD
Melissa Lopez, MD
Wilfredo A. Lopez, MD
Connie F. Lu, MD
Tyler J. Lueck, MD
Katherine L. Lukas, MD
Davlyn L. Luke, MD
Shani Ma, MD
Colton Mabis, MD
Lauren T. MacNeill, MD
Rachel Madding, MD
Mona Makhamreh, MD
Francesca R. Mancuso, MD
Kelsey L. Manfredi, MD
Valeria Mantilla, MD
Kaitlin M. Mar, MD
Starcher R. Margaret, MD
Audrey M. Marinelli, MD
Brittany A. Marinelli, MD
Emily S. Markovic, MD
Hannah L. Marshall, MD
Aaron Masjedi, MD
Isabelle M. Mason, MD
Akailah T. Mason-Otey, MD
Nicole Massad, MD
Megan M. Masten, MD
Stephanie M. Masters, MD
Anastasia Matthews, MD
Natalia del Mazo, MD
Sara A. McAllaster, MD
Continue to: Nicole McAndrew, DO...
Nicole McAndrew, DO
Madeline G. McCosker, MD
Jamie L. McDowell, DO
Christine E. McGough, MD
Mackenzi R. McHugh, MD
Madeline M. McIntire, MD
Cynthia R. McKinney, MD
Kirsten D. McLane, MD
Shian F. McLeish, MD
Megan I. McNitt, MD
Sarah R. McShane, MD
Grace R. Meade, MD
Nikki Ann R. Medina, DO
Tiffany L. Mei, MD
Jenna Meiman, MD
Anna M. Melicher, MD
Rosa M. Mendez, MD
Riley Mickelsen, MD
Sage A. Mikami, MD
Aletheia B. Millien, MD
Hannah C. Milthorpe, MD
Caroline J. Min, MD
Julie A. Mina, MD
Annie G. Minns, MD
Natalie Mironov, DO
Elizabeth L. Mirsky, MD
Astha Mittal, MD
Rachel E. Mnuk, MD
Silki Modi, MD
Sudarshan J. Mohan, MD
Roxana Mohhebali-Solis, MD
Mugdha V. Mokashi, MD
Jessica A. Montgomery, MD
Ellen Moore, MD
Savannah J. Morehouse, MD
Kristen L. Moriarty, MD
Alexa P. Morrison, MD
Bijan Morshedi, MD
Matthew H. Mossayebi, MD
Kathy Mostajeran, DO
Sharan Mullen, DO
Ellen C. Murphy, MD
Emma Chew Murphy, MD
Lauren M. Murphy, MD
Bria Murray, MD
Erin C. Nacev, MD
Preetha Nandi, MD
Blaire E. Nasstrom, DO
Hallie N. Nelson, MD
Katherine A. Nelson, MD
Margaret S. Nemetz, MD
Daniela Ben Neriah, DO
Cosima M. Neumann, MD
Mollie H. Newbern, DO
Gisella M. Newbery, MD
Stephanie Nguyen, MD
Christine G.T. Nguyen, MD
Desiree Nguyen, MD
Jacqueline W. Nichols, MD
Annika M. Nilsen, MD
Margaret A. Nixon, MD
Emily M. Norkett, MD
Allison N. Nostrant, DO
Susan E. Nourse, MD
Aliya S. Nurani, MD
Emily E. Nuss, MD
Jeanne O. Nwagwu, DO
Kelsey E. O’Hagan, MD
Margaret O’Neill, MD
Emily A. O’Brien, MD
Carly M. O’Connor-Terry, MD, MS
Madison O. Odom, MD
Cynthia I. Okot-Kotber, MD
Sarah P. Oliver, MD
Leanne P. Ondreicka, MD
Ngozika G. Onyiuke, MD
Erika Gonzalez Osorio, MD
Marika L. Osterbur Badhey, MD
Linda A. Otieno, MD
Claire H. Packer, MD
Chloe W. Page, DO
Marissa Palmor, MD
Rishitha Panditi, MD
Katherine A. Panushka, MD
Kelsey J. Pape, MD
Rachel R. Paquette, DO
Hillary C. Park, DO
Kendall M. Parrott, MD
Ekta Partani, MD
Karishma Patel, MD
Shivani Patel, MD
Continue to: Priya Patel, MD...
Priya Patel, MD
Jenna M. Patterson, MD
Ashleigh Pavlovic, MD
Katie M. Peagler, MD
Katherine T. Pellino, MD
Nicholas Per, MD
Elana Perry, MD
Emily J. Peters, MD
Sara E. Peterson, MD
Michelle R. Petrich, MD
Destiny L. Phillips, MD
Chloe Phillips, MD
Megan E. Piacquadio, DO
Sara C. Pierpoint, MD
Celeste M. Pilato, MD
Emma Pindra, MD
Minerva L.R. Pineda, MD
Rebecca Pisan, MD
Alessandra R. Piscina, MD
Rachael Piver, MD
Andrew J. Polio, MD
Hector S. Porragas, MD
Natalie Posever, MD
Allison R. Powell, MD
Mahima V. Prasad, MD
Angelina D. Prat, DO
Rebecca L. Purvis, MD
Teresa L. Qi, MD
Nicholas R. Quam, MD
Candice A. Quarella, MD
Nicholas W. Racchi, DO
Jeannie G. Radoc, MD
Samuel Raine, MD
Anna C. Raines, MD
Stephanie A. Rains, MD
Nicole M. Rainville, DO
Karissa Rajagopal, DO
Kristian R. Ramage, MD
Praveen Ramesh, MD
Tia M. Ramirez, MD
Jania Ramos, MD
Neel K. Rana, MD
Urvi Rana, DO
Indira Ranaweera, MD
Sindhuja Ranganathan, DO
Chloe R. Rasmussen, MD
Laura P. Reguero-Cadilla, MD
Devin M. Reilly, MD
Kimberly E. Reimold, MD
Cory R. Reiter, MD, PhD
Maya E. Reuven, DO
Jessica Reyes-Peterson, MD
Jacqueline Rice, MD
Rebecca L. Richardson, MD
Mikaela J. Rico, DO
Katelyn Rittenhouse, MD
Giuliana A. Rivera Casul, MD
Jill N.T. Roberts, MD
Luke N. Roberts, MD
Esther Robin, MD
Marcella Israel Rocha, MD
Zoe A. Roecker, MD
Hilary E. Rogers, MD
Kelsey A. Roof, MD
Zarah Rosen, MD
Cecilia M. Rossi, MD
Eva S. Rostonics, MD
Felix Rubio, MD
Amela Rugova, MD
Anna J. Rujan, MD
Erika T. Russ, MD
Colin Russell, MD
Ruby L. Russell, MD
Isabella A. Sabatina, MD
Gouri Sadananda, MD
Aashna Saini, MD
Salomeh M. Salari, MD
Ndeye N. Sall, MD
Nicole M. Salvador, MD
Aayushi Sardana, MD
Kendall M. Sarson, MD
Rita Abigail Sartor, MD
Continue to: Haley A. Scarbrough, MD...
Haley A. Scarbrough, MD
Kimberly Schaefer, MD
Demetra Schermerhorn, MD
Ellen C. Schleckman, MD
Maura A. Schlussel, MD
Ellie Schmidt, MD
Alison M. Schmidt, MD
Evan A. Schrader, MD
Morgan A. Schriever, MD
Brianna L. Schumaker Nguyen, DO
Whitney E. Scott, MD
Claire Scrivani, MD
Catherine E. Seaman, MD
Rachel D. Seaman, MD
Danielle J. Seltzer, MD
Joshua R. Shaffer, MD
Emily A. Shaffer, MD
Delia S. Shash, MD
Ishana P. Shetty, MD
Tushar Shetty, MD
Carol Shi, MD
Sarah P. Shim, MD
Emma C. Siewert, MD
Seth M. Sigler, DO
Rebecca L. SigourneyTennyck, MD
Daniella D. Silvino, DO
Andrea M. Simi, MD
Amelia R. Simmons, MD
Amy E. Skeels, DO
Ashley E.S. Keith, MD
Hannah C. Smerker, DO
Katarina Smigoc, MD
Madeline I. Smith, MD
Jessica D. Smith, MD
Melanie R. Smith, MD
Alicia L. Smith, MD
Chloe Smith, MD
Ayanna Smith, MD
Melanie R. Smith, MD
Megan M. Smith, MD
Haverly J. Snyder, MD
Beatrice R. Soderholm, DO
Brianna C. Sohl, MD
Samantha A. Solaru, MD
Michael Solotke, MD
Dara A.H. Som, MD
Alexandra R. Sotiros-Lowry, MD
Melanie Spall, DO
Alicia C. Speak, DO
Lisa M. Spencer, MD
Prakrithi Srinand, MD
Sierra M. Starr, MD
Kathryne E. Staudinger, MD
Emily K. Steele, MD
Morgan R. Steffen, DO
Tricia R. Stepanek, MD
Taylor P. Stewart, MD
Kelsey A. Stewart, MD
Alyssa M. Stiff, MD
Alexandra B. Stiles, MD
Nairi K. Strauch, MD
Margaret J. Stroup, DO
Sean C. Stuart, DO
Hannah M. Stump, MD
Shalini B. Subbarao, MD
Lakshmi Subramani, MD
Heather E. Sweeney, MD
Kristin I. Swope, MD
Suha Syed, MD
Mireya P. Taboada, MD
Eneti S. Tagaloa, MD
Rachel Tang, DO
Adam R. Taylor, MD
Simone R. Thibault, MD
Kimberly A. Thill, MD
Dhanu Thiyag, MD
Andrew T. Thornton, MD
Wendy Tian, MD
Stephanie Tilberry, MD
Amanda L. Tillett, MD
Amanda M. Tjitro, MD
Logan P. Todhunter, DO
David Toffey, MD
Maris K. Toland, MD
Rachel E. Tomassi, MD
Sarah Tounsi, MD
Antonia K. Traina, MD
Taylor Tran, MD
Diem Samantha Tran, DO
Emily C. Trautner, MD
Emma Trawick, MD
Continue to: Elissa Trieu, MD...
Elissa Trieu, MD
Ariel Trilling, MD
Samantha Truong, MD
Mary M. Tsaturian, MD
Athena Tudino, MD
Kati A. Turner, MD
Nicole-Marie Tuzinkiewicz, MD
Gayathri D. Vadlamudi, MD
Stylianos Vagios, MD
Pauline V. Van Dijck, DO
Kaylee A. VanDommelen, MD
Isha B. Vasudeva, MD
Shivani J. Vasudeva, DO
Diana Q. Vazquez Parker, MD
Ridhima Vemula, MD
Elena C. Vinopal, MD
Caroline J. Violette, MD
Pascal T. Vo, DO
Michelle H. Vu, MD
Macy M. Walz, MD
Angelia Wang, MD
Eileen Wang, MD
Courtney Y. Wang, MD
Joyce Wang, MD
Meryl G. Warshafsky, MD
Sophie E.N. Weinstein, MD
Sarah H. Weinstein, MD
Annalyn M. Welp, MD
Shannon M. Wentworth, MD
Erika M. Wert, MD
Rachel C. White, MBchB
Morgan N. Wilhoite, DO
Mercedes Williams, MD
Hayley Williams, MD
Jacquelyn D. Williams, MD
Mary H. Williamson, MD
Elise Wilson, MD
Lauren M. Witchey, MD
Emily A. Wolverton, MD
Stephanie Y. Wong, MD
Jenny Wu, MD
Jackie Xiang, MD
Nancy S. Yang, MD
Kevin P. Yeagle, MD
Halina M. Yee, MD
Alyssa M. Yeung, MD
Samuel K. Yost, MD
Megan Yuen, MD
Nayab Zafar, DO
Cindy X. Zhang, DO
Yingao Zhang, MD
Helen Zhao, MD
Chelsea Zhu, MD
Billie E. Zidel, MD
Ryan A. Zoldowski, MD

Six months after the Supreme Court decision that overturned the constitutional right to abortion, trainees across the United States are asking a critical question in the current resident recruitment season: How will the restrictions on abortion access affect my training as an obstetrician-gynecologist, and will they impact my ability to be the kind of provider I want to be in the future?
Among the myriad of downstream effects to patient care, the Dobbs decision will indisputably impact the scope of residency training for those that provide reproductive health services. Almost half of ObGyn residents train in states that have abortion restrictions in place.1 New educational milestones for abortion training, which are a requirement by the Accreditation Council for Graduate Medical Education (ACGME), were proposed quickly after Dobbs, guiding programs to offer opportunities for training in nonrestricted areas or the “combination of didactic activities, including simulation” to meet the training requirement in abortion care.2
Like many providers, residents already are grappling with precarious and risky circumstances, balancing patient safety and patient-driven care amidst pre-existing and newly enforced abortion restrictions. Whether managing a patient with an undesired pregnancy, severe medical comorbidities, unexpected pregnancy complications such as preterm premature rupture of membranes, or bleeding, or substantial fetal anomalies, ObGyn residents cannot gain the experience of providing the full scope of reproductive health care without the ability to offer all possible management options. While some enacted abortion restrictions have exceptions for the health of or life-saving measures for the mother, there is no standard guidance for timing of interventions, leaving providers confused and in fear of legal retribution. At a time when trainees should be learning to provide patient-centered, evidence-based care, they are instead paralyzed by the legal or professional consequences they may face for offering their best medical judgements.
Furthermore, the lack of exposure to dilation and evacuation procedures for residents in restricted practice areas will undoubtably decrease their confidence in managing acute complications, which is one of the critical facets of residency training. In a surgical field where repetition is crucial for technical competence, highlighted by ACGME minimum case requirements, the decreased volume of abortion procedures is a disadvantage for trainees and a disservice for patients. While anti-choice promoters may argue that involvement in surgical management of early pregnancy loss should suffice for ObGyn training in family planning, this piecemeal approach will leave gaps in technical skills.
The fear of legal ramifications, moral injury, and inadequate surgical training may lead to the siphoning of talented trainees to areas in the country with fewer restrictions.3 Dobbs already has demonstrated how limiting abortion access will deepen inequities in reproductive health care service delivery. Approximately 55% of ObGyn trainees and nearly two-thirds of maternal-fetal medicine graduates join the workforce in the state where they received their training.4 Medical students will seek opportunities for high-quality ObGyn training in areas that will help them to be well-prepared, competent physicians—and more often than not, stay in the area or region that they trained in. This will lead to provider shortages in areas where access to reproductive health care and subspecialist providers already is limited, further exacerbating existing health disparities.
During this recruitment season, trainees and residency programs alike will need to reckon with how the ramifications of Dobbs will alter both the immediate and long-term training in comprehensive reproductive health care for the ObGyn workforce. ObGyn trainees have taken a stand in response to the Dobbs decision, and nearly 750 current residents signed onto the statement below as a commitment to high-quality training and patient-centered care. Clinical experience in performing abortions is essential to the provision of comprehensive evidence-based reproductive health care, and access to these procedures is as important for physicians-in-training as it is for patients.
Actions to take to ensure high-quality abortion training in ObGyn residencies include the following:
- Connect with and stay involved with organizations such as the American College of Obstetricians and Gynecologists (ACOG), Physicians for Reproductive Health (PRH), and Medical Students for Choice (MSFC) for initiatives, toolkits, and resources for training at your institutions.
- Seek specific abortion training opportunities through the Leadership Training Academy (offered through PRH) or the Abortion Training Institute (offered through MSFC).
- Ensure that your residency program meets the ACGME criteria of providing opportunities for clinical experiences for abortion care and work with program leadership at a program, state, or regional level to enforce these competencies.
- Reach out to your local American Civil Liberties Union or other local reproductive legal rights organizations if you want to be involved with advocacy around abortion access and training but have concerns about legal protections.
- Have a voice at the table for empowering training opportunities by seeking leadership positions through ACOG, ACGME, Council on Resident Education in Obstetrics and Gynecology and the Association of Professors of Gynecology and Obstetrics, American Medical Association, Student National Medical Association, and subspecialty organizations.
- Vote in every election and promote voting registration and access to your patients, colleagues, and communities. ●
Continue to: The implications of the Dobbs v Jackson Women’s Health Organization decision on the health care and wellbeing of our patients...
On June 24, 2022, the Supreme Court of the United States ruled in a 6-3 majority decision to overturn the constitutional right to abortion protected by Roe v Wade since 1973. As health care providers, we are outraged at the Court’s disregard for an individual’s right to make reproductive decisions for themselves and their families and are deeply concerned about the devastating consequences to reproductive care and outcomes in this country for all people. Reproductive health decisions, including growing a family and whether or not to continue a pregnancy, are complex and incredibly personal. Our role as health care providers is to help guide those decisions with empathy and evidencebased clinical recommendations. This ruling undermines a patient’s right to bodily autonomy, free of impositions from government and political pressures, and it threatens the sanctity of complex medical decision-making between a patient, their family, and their medical team.
As medical professionals, we know that every patient’s situation is unique—banning abortion procedures ties the hands of physicians trying to provide the most medically appropriate options in a compassionate manner. We know that both medical and surgical abortions are safe and can save lives. These procedures can help patients with potentially life-threatening conditions worsened by pregnancy, a poor prognosis for the fetus, or a complication from the pregnancy itself. Physicians use scientific research and individualized approaches to help patients in unique situations, and attempts to legislate personal health decisions compromise the practice of evidence-based medicine.
We also know that this decision will impact some communities more than others. Access to safe abortion care will become dependent on which region of the country a person lives in and whether or not a person has resources to seek this care. Due to continued systemic racism and oppression, patients of color will be disproportionately impacted and likely will suffer worse health outcomes from unsafe abortions. Those that rely on public insurance or who are uninsured will face overwhelming barriers in seeking abortion services. These disparities in reproductive care, which contribute to our nation’s health crises in maternal morbidity and mortality, unintended pregnancy, and neonatal complications, will further entrench health inequities, and patient lives and livelihoods will suffer.
We acknowledge the impact that this decision will have on restricting access to reproductive care. We stand by the fact that abortion care is health care. We vow to uphold the tenets of our profession to place patient autonomy and provision of safe quality medical care at the forefront of our practices.
We, as health care providers and physician trainees, hereby pledge:
- To continue to provide evidence-based, nonjudgmental counseling for all pregnancy options, including abortion, and support our patients through all reproductive health decisions
- To promote equity in providing comprehensive reproductive health care, recognizing the impacts of systemic racism and oppression
- To promote high quality training in providing safe reproductive care in our respective institutions
- To use our voices in our communities to advocate for all our patients to have the freedom to access the safe and compassionate health care they deserve.
Sincerely,
The undersigned 747 ObGyn resident physicians
Please note that we sign this statement on our own behalf as individuals and not on behalf of our respective institutions.
Orchideh Abar, MD
Laurel S. Aberle, MD
Kathleen E. Ackert, DO
Lauryn Adams, MD
Temiloluwa Adejuyigbe, MD
Oluwatoyosi M. Adeoye, MD
Hufriya Y. Aderianwalla, MD
Fareeza Afzal, MD
Adelaide Agyepong, MD
Erin R. Ahart, MD
Noha T. Ahmed, DO
Faria Ahmed, MD
Tracey O. Akanbi, MD
Eloho E. Akpovi, MD
Austin H. Allen, DO
Amanda M. Allen, MD
Alexis L. Allihien, MD
Jorge L. Alsina, MD
Paulina C. Altshuler, DO
Sivani Aluru, MD
Amal Amir, DO
Jon Anderson, DO
Andreas Antono, MD
Annie N. Apple, MD
Janine Appleton, DO
Aarthi Arab, MD
Sydney R. Archer, MD
Youngeun C. Armbuster, MD
Kara Arnold, MD
Blessing C. Aroh, MD
Savannah Pearson Ayala, MD
Archana K. Ayyar, MD
Ann-Sophie Van Backle, DO
Connor R. Baker, MD
Japjot K. Bal, MD
Abigail E. Barger, MD
Kathryn E. Barron, MD
Silvia Bastea, MD
Samantha V.H. Bayer, MD
Kristen Beierwaltes, MD
Gisel Bello, MD
Michelle A. Benassai, MD
Dana Benyas, MD
Alice F. Berenson, MD
Hanna P. Berlin, MD
Abigail L. Bernard, MD
Eli H. Bernstein, MD
Julia T. Berry, MD
Bryce L. Beyer, MD
Caroline Bilbe, MD
Grace E. Binter, DO
Erin E. Bishop, MD
Sierra G. Bishop, MD
Stephanie S. Bista, MD
Tara E. Bjorklund, DO
Alyssa N. Black, MD
Continue to: Kelsey Boghean, DO...
Kelsey Boghean, DO
Areta Bojko, MD
Grace E. Bommarito, DO
Aditi R. Bommireddy, MD
Genna C. Bonfiglio, MD
Mary E. Booker, MD
Kayce L. Booth, MD
Samantha T. Boothe, DO
William Borenzweig, MD
Rebecca M. Borneman, MD
Alexander L. Boscia, MD
Gina M. Botsko, MD
Glenn P. Boyles, MD
Avery C. Bramnik, MD
Sophia N. Brancazio, MD
Katarina M. Braun, MD
Anthony Brausch, MD
Emily L. Brekke, MD
Sara E. Brenner, MD
Bailey A. Brown, DO
Kathryn S. Brown, MD
Denese C. Brown, MD
Abena Bruce, MD
Sabrina C. Brunozzi, MD
Madison Buchman, DO
Deirdre G. Buckley, MD
Rachel L. Budker, MD
Leeann M. Bui, MD
Anthony H. Bui, MD
Jessie Bujouves, MD
Kimberley A. Bullard, MD
Sophia G. Bunde, MD
Emily R. Burdette, MD
Iris Burgard, DO
Korbi M. Burkey, MD
Lindsey K. Burleson, MD
Lindsay M. Burton, MD
Brianna N. Byers, MD
Stephanie Cai, MD
Alexandra S. Calderon, MD
Alexandra G. Caldwell, MD
Natalia Calzada, MD
Tamara Cameo, MD
Arielle Caplin, MD
Angela M. Carracino, DO
Anna L. Carroll, MD
Leigha M. Carryl, MD
Ashlie S. Carter, MD
Stephanie Casey, DO
Chase W. Cataline, DO
Carson L. Catasus, MD
Alena R. Cave, MD
Kelly M. Chacon, MD
Avis L. Chan, MD
Shruthi Chandra, MD
Jennifer Chang, MD
Shannon Chang, DO
Gillian Chase, MD
Cindy Chen, MD
Jessie C. Chen, MD
Jessica T. Chen, MD
Wenjin Cheng, MB
Laura J. Cheng, MD
Lucy Cheng, MD
Monica S. Choo, MD
Jody S. Chou, MD
Hannah C. Christopher, DO
Continue to: David J. Chromey, DO...
David J. Chromey, DO
Grace V. Clark, MD
Celeste Colegrove, MD
Sarah C. Combs, MD
Victoria L. Conniff, MD
Hannah C. Connor, MD
Angela J. Conway, MD
Steffany A. Conyers, MD
Alexandra Cooke, MD
Ashley A. Cooney, MD
Anna Cornelius-Schecter, MD
Alexa M. Corso, DO
Krysten A. Costley, MD
Madeline Coulter, MD
Kelsey Cramer, MD
Anna E. Cronin, MD
Bethany N. Croyle, DO
Carmen A. Cueto, MD
Nicole Cumbo, MD
Mackenzie A. Cummings, MD
Carrie Cummiskey, MD
Hannah M. Cunningham, MD
Sarah D’Souza, DO
Rachael M. D’Auria, MD
Caitlin Dane, MD
Rachel N. Dang, MD
Talin R. Darian, MD
Abigail C. Davies, MD
Berkley Davis, MD
Lois A. Davis, MD
Jennie J. DeBlanc, MD
Ayana G.R. DeGaia, MD, MPH
Katerina N. DeHaan, MD
Rebekka M. Delgado, MD
Brettany C. DeMier, MD
Bonnie W. DePaso, MD
Hemaxi H. Desai, DO
Amberly T. Diep, MD
Abigail K. Dillaha, MD
Sarah K. Dominguez, MD
Abbey P. Donahue, MD
Allan C. Dong, MD
James Doss, MD
Taylor B. Douglas, MD
Abigail G. Downey, MD
Janelle M. Driscoll, MD
Emily Du, MD
Leslie V. Dunmire, MD
Jennifer Duong, DO
Leigh C. Durudogan, MD
Mai N. Dyer, MD, MPH
Rebecca A. Ebbott, MD
Lindsey P. Eck, MD
Molly C. Eckman, MD
Alex Ede, MD, ScM
Claire E. Edelman, MD
Sara E. Edwards, MD
David J. Eggert, DO
Michelle Eide, MD
Etoroabasi Ekpe, MD
Tressa L. Ellett, MD
Laura Peyton Ellis, MD
Kaitlin H. Ellis, MD
Mariah G. Elly, MD
Jennifer Embry, MD
Claire Englert, MD
Brenna Espelien, MD
Kamilah Evans, MD
Joshua A. Ewy, MD
Elana D. Fackler, MD
Lauren E. Falk, MD
Brianna A. Farley, MD
Amanda Stephanie R. Farrell, MD
Sara Fassio, DO
Daniela A. Febres-Cordero, MD
Jasmin E. Feliciano, MD
Alayna H. Feng, MD
Amanda M. Ferraro, MD
Brittany A. Fickau, MD
Brittany H. File, MD
Shannon M. Finner, DO
Mia E. Fischbein, DO
Briah Fischer, MD
Shira Fishbach, MD
Alison C. Fitzgerald, MD
Evan R. Fitzgerald, MD
Margaret R. Flanigan, MD
Kevin C. Flatley, MD
Jordan A. Fletcher, MD
Claudia E. Flores, MD
Lauren A. Forbes, MD
Rana K. Fowlkes, MD
Jennifer M. Franks, MD, MPH
Christina M. Frasik, MD
Haven N. Frazier, DO
Sarah W. Freeman, MD
Emilie O. Fromm, DO
Anna R. Fuchss, MD
Emma K. Gaboury, MD
Madeline H. Ganz, MD
Lex J. Gardner, MD
Keri-Lee Garel, MD
Hailey B. Gaskamp, DO
Brittney A. Gaudet, MD
Gabrielle M. Gear, MD
Eleanor R. Germano, MD
Lauren G. Gernon, MD
Allen Ghareeb, MD
Patricia Giglio Ayers, MD
Jordana L. Gilman, MD
Mianna M. Gilmore, DO
Brian W. Goddard, MD
Julia L. Goldberg, MD
M. Isabel Gonzaga, MD
Fred P. Gonzales, MD
Lillian H. Goodman, MD, MPH
Ashley Goreshnik, MD
Lauren E. Gottshall, MD
Lindsay L. Gould, MD
Kelsea R. Grant, MD
Dorender A. Gray, MD
Sophie Green, MD
Erica A. Green, MD
Danielle C. Greenberg, MD
Kalin J. Gregory-Davis, MD
David M. Greiner, MD
Tyler M. Gresham, MD
Continue to: Nelly Grigorian, MD...
Nelly Grigorian, MD
Erin L. Grimes, MD
Whitney Grither, MD
Jared M. Grootwassink, MD
Maya E. Gross, MD
Paoula Gueorguieva, MD
Margot M. Gurganus, DO
Rachel L. Gutfreund, MD
Andres Gutierrez, MD
Dorothy L. Hakimian, DO
Ashley N. Hamati, DO
Marie M. Hanna-Wagner, MD
Katie Hansen, MD
Courtney Hargreaves, MD
Stephanie Harlow, MD
Kelsey B. Harper, MD
Devon A. Harris, MD
Lauren E. Harris, MD
Emily S. Hart, DO
Sarah A. Hartley, MD
Becky K. Hartman, MD
Abigail K. Hartmann, MD
Charlotte V. Hastings, MD
Cherise Hatch, DO
Jordan Hauck, DO
Sarena Hayer, MD
Jenna M. Heath, MD
Eric D. Helm, MD
Julie A. Hemphill, MD
Ric A.S. Henderson, MD
Nicola A. Hendricks, MD
Andrea A. Henricks, MD
Jesse M. Herman, DO
Alyssa M. Hernandez, DO
Melissa Hernandez, MD
Alyssa R. Hersh, MD
Alexandra Herweck, MD
Brianna Hickey, MD
Allix M. Hillebrand, MD
Alessandra I. Hirsch, MD
Emily A. Hoffberg, MD
Chloe L. Holmes, DO
Cameron M. Holmes, MD
Helena Y. Hong, MD
Wakako Horiuchi, MD
Shweta Hosakoppal, MD
Jaycee E. Housh, MD
Shannon M. Howard, MD
Meredith C. Huszagh, MD
Yihharn P. Hwang, MD
Emma C. Hyde, MD
Brooke Hyman, MD
Hala Ali Ibrahim, MD
Gnendy Indig, MD
Erin E. Isaacson, MD
Shruti S. Iyer, DO
Audrey J. Jaeger, DO
Shobha Jagannatham, MD
Cyrus M. Jalai, MD
Emma V. James, MD
Isabel Janmey, MD
Phoebe Jen, DO
Corey L. Johnson, MD
Crystal J. Johnson, MD
Andrea M. Johnson, MD
Nat C. Jones, MD
Briana L. Jones, DO
Rebecca J. Josephson, MD
Sarah Natasha Jost-Haynes, MD
Continue to: Hannah S. Juhel, MD...
Hannah S. Juhel, MD
Erin Jun, DO
Katherine B. Kaak, MD
Dhara N. Kadakia, MD
Amanda D. Kadesh, MD
Riana K. Kahlon, MD
Nadi N. Kaonga, MD
Moli Karsalia, MD
Stephanie L. Kass, MD
Amanda M. Katz, MD
Chelsea S. Katz, MD
Virginia Kaufman, MD
Gurpinder Kaur, MD
Jessica A. Keesee, MD
Cassandra N. Kelly, MD
Whitney Kelly, DO
Hannah V. Kennedy, MD
Bethany H. Kette, MD
Iman Khan, MD
Maryam M. Khan, MD
Alisa Jion Kim, MD
Tesia G. Kim, MD
Anne E. Kim, MD
Emily H. King, MD
Tarynne E. Kinghorn, MD
Holly T. Kiper, DO
Thomas Kishkovich, MD
Quinn M. Kistenfeger, MD
Sofia E. Klar, DO
Jessica B. Klugman, MD
Hope E. Knochenhauer, MD
Kathleen J. Koenigs, MD
Olga Kontarovich, DO
Alison Kosmacki, MD
Ana E. Kouri, MD
Olga M. Kovalenko, MD
Leigh T. Kowalski, MD
Kayla A. Krajick, MD
Elizabeth S. Kravitz, MD
Shruti Rani Kumar, MD
Alyssa Kurtz, DO
Lauren H. Kus, MD
Arkadiy Kusayev, DO
Amanda E. Lacue, MD
Nava Lalehzari, MD
Amber Lalla, MD
Allie C. Lamari, DO
Kelly L. Lamiman, MD
Stephen Lammers, MD
Monet Lane, MD
Madeline L. Lang, MD
Liana Langdon-Embry, MD
Carolyn Larkins, MD
Leah E. Larson, MD
Matthew W. Lee, MD
Eunjae Lee, MD
Alice Lee, MD
Jared Z. Lee, MD
Charlotte M. Lee, MD
Nicole R. Legro, MD
Aurora Leibold, MD
Rosiris Leon-Rivera, MD, PhD
Anna M. Leone, MD
Keiko M. Leong, MD
Lindsey M. LePoidevin, MD
Molly E. Levine, MD
Khrystyna Levytska, MD
Dana L. Lewis, DO
Jessica L. Li, MD
Kristina Lilja, MD
Deanna M. Lines, DO
Annalise Littman, MD
Julia F. Liu, MD
Tyler B. Lloyd, MD
Alyssa Lo, MD
K’ara A. Locke, MD
Minica Long, MD
Melissa Lopez, MD
Wilfredo A. Lopez, MD
Connie F. Lu, MD
Tyler J. Lueck, MD
Katherine L. Lukas, MD
Davlyn L. Luke, MD
Shani Ma, MD
Colton Mabis, MD
Lauren T. MacNeill, MD
Rachel Madding, MD
Mona Makhamreh, MD
Francesca R. Mancuso, MD
Kelsey L. Manfredi, MD
Valeria Mantilla, MD
Kaitlin M. Mar, MD
Starcher R. Margaret, MD
Audrey M. Marinelli, MD
Brittany A. Marinelli, MD
Emily S. Markovic, MD
Hannah L. Marshall, MD
Aaron Masjedi, MD
Isabelle M. Mason, MD
Akailah T. Mason-Otey, MD
Nicole Massad, MD
Megan M. Masten, MD
Stephanie M. Masters, MD
Anastasia Matthews, MD
Natalia del Mazo, MD
Sara A. McAllaster, MD
Continue to: Nicole McAndrew, DO...
Nicole McAndrew, DO
Madeline G. McCosker, MD
Jamie L. McDowell, DO
Christine E. McGough, MD
Mackenzi R. McHugh, MD
Madeline M. McIntire, MD
Cynthia R. McKinney, MD
Kirsten D. McLane, MD
Shian F. McLeish, MD
Megan I. McNitt, MD
Sarah R. McShane, MD
Grace R. Meade, MD
Nikki Ann R. Medina, DO
Tiffany L. Mei, MD
Jenna Meiman, MD
Anna M. Melicher, MD
Rosa M. Mendez, MD
Riley Mickelsen, MD
Sage A. Mikami, MD
Aletheia B. Millien, MD
Hannah C. Milthorpe, MD
Caroline J. Min, MD
Julie A. Mina, MD
Annie G. Minns, MD
Natalie Mironov, DO
Elizabeth L. Mirsky, MD
Astha Mittal, MD
Rachel E. Mnuk, MD
Silki Modi, MD
Sudarshan J. Mohan, MD
Roxana Mohhebali-Solis, MD
Mugdha V. Mokashi, MD
Jessica A. Montgomery, MD
Ellen Moore, MD
Savannah J. Morehouse, MD
Kristen L. Moriarty, MD
Alexa P. Morrison, MD
Bijan Morshedi, MD
Matthew H. Mossayebi, MD
Kathy Mostajeran, DO
Sharan Mullen, DO
Ellen C. Murphy, MD
Emma Chew Murphy, MD
Lauren M. Murphy, MD
Bria Murray, MD
Erin C. Nacev, MD
Preetha Nandi, MD
Blaire E. Nasstrom, DO
Hallie N. Nelson, MD
Katherine A. Nelson, MD
Margaret S. Nemetz, MD
Daniela Ben Neriah, DO
Cosima M. Neumann, MD
Mollie H. Newbern, DO
Gisella M. Newbery, MD
Stephanie Nguyen, MD
Christine G.T. Nguyen, MD
Desiree Nguyen, MD
Jacqueline W. Nichols, MD
Annika M. Nilsen, MD
Margaret A. Nixon, MD
Emily M. Norkett, MD
Allison N. Nostrant, DO
Susan E. Nourse, MD
Aliya S. Nurani, MD
Emily E. Nuss, MD
Jeanne O. Nwagwu, DO
Kelsey E. O’Hagan, MD
Margaret O’Neill, MD
Emily A. O’Brien, MD
Carly M. O’Connor-Terry, MD, MS
Madison O. Odom, MD
Cynthia I. Okot-Kotber, MD
Sarah P. Oliver, MD
Leanne P. Ondreicka, MD
Ngozika G. Onyiuke, MD
Erika Gonzalez Osorio, MD
Marika L. Osterbur Badhey, MD
Linda A. Otieno, MD
Claire H. Packer, MD
Chloe W. Page, DO
Marissa Palmor, MD
Rishitha Panditi, MD
Katherine A. Panushka, MD
Kelsey J. Pape, MD
Rachel R. Paquette, DO
Hillary C. Park, DO
Kendall M. Parrott, MD
Ekta Partani, MD
Karishma Patel, MD
Shivani Patel, MD
Continue to: Priya Patel, MD...
Priya Patel, MD
Jenna M. Patterson, MD
Ashleigh Pavlovic, MD
Katie M. Peagler, MD
Katherine T. Pellino, MD
Nicholas Per, MD
Elana Perry, MD
Emily J. Peters, MD
Sara E. Peterson, MD
Michelle R. Petrich, MD
Destiny L. Phillips, MD
Chloe Phillips, MD
Megan E. Piacquadio, DO
Sara C. Pierpoint, MD
Celeste M. Pilato, MD
Emma Pindra, MD
Minerva L.R. Pineda, MD
Rebecca Pisan, MD
Alessandra R. Piscina, MD
Rachael Piver, MD
Andrew J. Polio, MD
Hector S. Porragas, MD
Natalie Posever, MD
Allison R. Powell, MD
Mahima V. Prasad, MD
Angelina D. Prat, DO
Rebecca L. Purvis, MD
Teresa L. Qi, MD
Nicholas R. Quam, MD
Candice A. Quarella, MD
Nicholas W. Racchi, DO
Jeannie G. Radoc, MD
Samuel Raine, MD
Anna C. Raines, MD
Stephanie A. Rains, MD
Nicole M. Rainville, DO
Karissa Rajagopal, DO
Kristian R. Ramage, MD
Praveen Ramesh, MD
Tia M. Ramirez, MD
Jania Ramos, MD
Neel K. Rana, MD
Urvi Rana, DO
Indira Ranaweera, MD
Sindhuja Ranganathan, DO
Chloe R. Rasmussen, MD
Laura P. Reguero-Cadilla, MD
Devin M. Reilly, MD
Kimberly E. Reimold, MD
Cory R. Reiter, MD, PhD
Maya E. Reuven, DO
Jessica Reyes-Peterson, MD
Jacqueline Rice, MD
Rebecca L. Richardson, MD
Mikaela J. Rico, DO
Katelyn Rittenhouse, MD
Giuliana A. Rivera Casul, MD
Jill N.T. Roberts, MD
Luke N. Roberts, MD
Esther Robin, MD
Marcella Israel Rocha, MD
Zoe A. Roecker, MD
Hilary E. Rogers, MD
Kelsey A. Roof, MD
Zarah Rosen, MD
Cecilia M. Rossi, MD
Eva S. Rostonics, MD
Felix Rubio, MD
Amela Rugova, MD
Anna J. Rujan, MD
Erika T. Russ, MD
Colin Russell, MD
Ruby L. Russell, MD
Isabella A. Sabatina, MD
Gouri Sadananda, MD
Aashna Saini, MD
Salomeh M. Salari, MD
Ndeye N. Sall, MD
Nicole M. Salvador, MD
Aayushi Sardana, MD
Kendall M. Sarson, MD
Rita Abigail Sartor, MD
Continue to: Haley A. Scarbrough, MD...
Haley A. Scarbrough, MD
Kimberly Schaefer, MD
Demetra Schermerhorn, MD
Ellen C. Schleckman, MD
Maura A. Schlussel, MD
Ellie Schmidt, MD
Alison M. Schmidt, MD
Evan A. Schrader, MD
Morgan A. Schriever, MD
Brianna L. Schumaker Nguyen, DO
Whitney E. Scott, MD
Claire Scrivani, MD
Catherine E. Seaman, MD
Rachel D. Seaman, MD
Danielle J. Seltzer, MD
Joshua R. Shaffer, MD
Emily A. Shaffer, MD
Delia S. Shash, MD
Ishana P. Shetty, MD
Tushar Shetty, MD
Carol Shi, MD
Sarah P. Shim, MD
Emma C. Siewert, MD
Seth M. Sigler, DO
Rebecca L. SigourneyTennyck, MD
Daniella D. Silvino, DO
Andrea M. Simi, MD
Amelia R. Simmons, MD
Amy E. Skeels, DO
Ashley E.S. Keith, MD
Hannah C. Smerker, DO
Katarina Smigoc, MD
Madeline I. Smith, MD
Jessica D. Smith, MD
Melanie R. Smith, MD
Alicia L. Smith, MD
Chloe Smith, MD
Ayanna Smith, MD
Melanie R. Smith, MD
Megan M. Smith, MD
Haverly J. Snyder, MD
Beatrice R. Soderholm, DO
Brianna C. Sohl, MD
Samantha A. Solaru, MD
Michael Solotke, MD
Dara A.H. Som, MD
Alexandra R. Sotiros-Lowry, MD
Melanie Spall, DO
Alicia C. Speak, DO
Lisa M. Spencer, MD
Prakrithi Srinand, MD
Sierra M. Starr, MD
Kathryne E. Staudinger, MD
Emily K. Steele, MD
Morgan R. Steffen, DO
Tricia R. Stepanek, MD
Taylor P. Stewart, MD
Kelsey A. Stewart, MD
Alyssa M. Stiff, MD
Alexandra B. Stiles, MD
Nairi K. Strauch, MD
Margaret J. Stroup, DO
Sean C. Stuart, DO
Hannah M. Stump, MD
Shalini B. Subbarao, MD
Lakshmi Subramani, MD
Heather E. Sweeney, MD
Kristin I. Swope, MD
Suha Syed, MD
Mireya P. Taboada, MD
Eneti S. Tagaloa, MD
Rachel Tang, DO
Adam R. Taylor, MD
Simone R. Thibault, MD
Kimberly A. Thill, MD
Dhanu Thiyag, MD
Andrew T. Thornton, MD
Wendy Tian, MD
Stephanie Tilberry, MD
Amanda L. Tillett, MD
Amanda M. Tjitro, MD
Logan P. Todhunter, DO
David Toffey, MD
Maris K. Toland, MD
Rachel E. Tomassi, MD
Sarah Tounsi, MD
Antonia K. Traina, MD
Taylor Tran, MD
Diem Samantha Tran, DO
Emily C. Trautner, MD
Emma Trawick, MD
Continue to: Elissa Trieu, MD...
Elissa Trieu, MD
Ariel Trilling, MD
Samantha Truong, MD
Mary M. Tsaturian, MD
Athena Tudino, MD
Kati A. Turner, MD
Nicole-Marie Tuzinkiewicz, MD
Gayathri D. Vadlamudi, MD
Stylianos Vagios, MD
Pauline V. Van Dijck, DO
Kaylee A. VanDommelen, MD
Isha B. Vasudeva, MD
Shivani J. Vasudeva, DO
Diana Q. Vazquez Parker, MD
Ridhima Vemula, MD
Elena C. Vinopal, MD
Caroline J. Violette, MD
Pascal T. Vo, DO
Michelle H. Vu, MD
Macy M. Walz, MD
Angelia Wang, MD
Eileen Wang, MD
Courtney Y. Wang, MD
Joyce Wang, MD
Meryl G. Warshafsky, MD
Sophie E.N. Weinstein, MD
Sarah H. Weinstein, MD
Annalyn M. Welp, MD
Shannon M. Wentworth, MD
Erika M. Wert, MD
Rachel C. White, MBchB
Morgan N. Wilhoite, DO
Mercedes Williams, MD
Hayley Williams, MD
Jacquelyn D. Williams, MD
Mary H. Williamson, MD
Elise Wilson, MD
Lauren M. Witchey, MD
Emily A. Wolverton, MD
Stephanie Y. Wong, MD
Jenny Wu, MD
Jackie Xiang, MD
Nancy S. Yang, MD
Kevin P. Yeagle, MD
Halina M. Yee, MD
Alyssa M. Yeung, MD
Samuel K. Yost, MD
Megan Yuen, MD
Nayab Zafar, DO
Cindy X. Zhang, DO
Yingao Zhang, MD
Helen Zhao, MD
Chelsea Zhu, MD
Billie E. Zidel, MD
Ryan A. Zoldowski, MD
- Vinekar K, Karlapudi A, Nathan L, et al. Projected implications of overturning Roe v Wade on abortion training in US obstetrics and gynecology residency programs. Obstet Gynecol. 2022;140:146-149.
- ACGME program requirements for graduate medical education in obstetrics and gynecology summary and impact of interim requirement revisions. ACGME website. Accessed December 18, 2022. https://www.acgme.org/globalassets/pfassets/reviewandcomment/220_obstetricsandgynecology_2022-06-24_impact.pdf
- Crear-Perry J, Hassan A, Daniel S. Advancing birth equity in a post-Dobbs US. JAMA. 2022;328:1689-1690.
- Report on residents. AAMC website. Accessed December 18, 2022. https://www.aamc.org/data-reports/students-residents/interactive-data/report-residents/2021/table-c4-physician-reten tion-state-residency-training-last-completed-gme
- Vinekar K, Karlapudi A, Nathan L, et al. Projected implications of overturning Roe v Wade on abortion training in US obstetrics and gynecology residency programs. Obstet Gynecol. 2022;140:146-149.
- ACGME program requirements for graduate medical education in obstetrics and gynecology summary and impact of interim requirement revisions. ACGME website. Accessed December 18, 2022. https://www.acgme.org/globalassets/pfassets/reviewandcomment/220_obstetricsandgynecology_2022-06-24_impact.pdf
- Crear-Perry J, Hassan A, Daniel S. Advancing birth equity in a post-Dobbs US. JAMA. 2022;328:1689-1690.
- Report on residents. AAMC website. Accessed December 18, 2022. https://www.aamc.org/data-reports/students-residents/interactive-data/report-residents/2021/table-c4-physician-reten tion-state-residency-training-last-completed-gme
Have investigators reached the first steps for redefining a diagnostic definition of preeclampsia that includes morbidity?
Thadhani R, Lemoine E, Rana S, et al. Circulating angiogenic factor levels in hypertensive disorders of pregnancy. N Engl J Med. 2022;1. DOI: 10.1056/EVIDoa2200161
EXPERT COMMENTARY
The standard core lecture on preeclampsia given to all medical students frequently begins with an epic, if not potentially apocryphal, statement regarding how this disease has been noted in the annals of medical history since the time of the Ancients. Although contemporary diagnostic criteria for preeclampsia are not that far out of date, they are close. The increased urinary protein loss and hypertension preceding eclamptic seizures was first noted at the end of the 19th century. The blood pressure and proteinuria criteria used for diagnosis was codified in its contemporary form in the late 1940s. Since then, “tweak” rather than “overhaul” probably best describes the updates of the obstetrical community to the definition of preeclampsia. This has just changed.
Details of the study
Thadhani and colleagues prospectively recruited a nationally representative observational cohort of patients hospitalized for hypertension during pregnancy, then followed the patients until either the diagnosis of preeclampsia with severe features or for 2 weeks, whichever came first. At enrollment, circulating levels of the soluble fms-like tyrosine kinase 1 (sFlt-1) and placental growth factor (PlGF) were measured. In a 2-phased design, the first 219 participants were used to define a sFlt-1/PlGF ratio that would predict progression to severe preeclampsia within 2 weeks. The next 556 enrollees served to validate the predictive properties of the ratio. The authors found that a sFlt-1/PlGF ratio of ≥40 predicted progression to preeclampsia with severe features with an area under the curve (AUC) of 0.92.
As products of the trophoblasts, both sFlt-1 and PlGF have been mooted for almost 2 decades as potential predictive, if not diagnostic, aids with respect to preeclampsia. Indeed, both analytes are commercially available in Europe for specifically this purpose and many maternal-fetal medicine practitioners working in the European equivalent American tertiary referral centers use an sFlt-1/PlGF ratio as their primary criteria for a diagnosis of preeclampsia. Within the United States, there was an initial flurry of interest in and an infusion of corporate and federal research support for sFlt-1 and PlGF as diagnostic aids for preeclampsia in the mid-2000s. However, at present, the US Food and Drug Administration (FDA) has not sanctioned these (or any) biomarkers to aid in the diagnosis of preeclampsia. As Thermo-Fisher Scientific (Waltham, Massachusetts) is a supporting partner in this study, it is almost certain that these data will be submitted for review by the FDA as part of an application for a preeclampsia diagnostic. At some point in the near future, American practitioners will potentially be able to join their European colleagues in utilizing these biomarkers in the diagnosis of preeclampsia with severe features. ●
Thadhani and colleagues observed that the majority of both maternal and neonatal morbidity in their study, including 8 of the 9 neonatal deaths and both cases of eclampsia, occurred among patients with a ratio ≥40 at admission. There was an inverse relation between the sFlt-1/PlGF ratio and the admission to delivery interval. Where only 17% of patients in the highest quartile of ratios remained pregnant at 14 days post-enrollment, more than 79% of the lowest quartile were still pregnant. If not a causal relationship, sFlt-1 and PlGF are clearly associated with not only the occurrence of preeclampsia with severe features but also the degree of morbidity.
The implication for the disposition of patient care resources is clear. Patients at higher risk for preeclampsia could be seen in specialty high-risk clinics with an emphasis on increased monitoring. In situations where tertiary care is more remote, plans could be developed should they need to be transported to centers able to provide the appropriate level of care. Conversely, patients screening at lower ratios may be more appropriately managed as outpatients, or at least in less clinically involved accommodations.
Thadhani et al do note that there were false negative cases in which the sFlt-1/PlGF ratio at admission was <40 but patients nonetheless progressed to preeclampsia with severe features. The majority of these cases had concurrent pre-pregnancy, chronic hypertension. This observation suggests not only the potential for insights into the pathophysiology of the hypertensive diseases in pregnancy but also that the interpretation of the sFlt/PlGF ratio may eventually need to be stratified by preexisting conditions.
The final implications for the observations of this study are perhaps the most tantalizing. If there is a causal relation between the level of the sFlt-1/PlGF ratio and the morbidity of preeclampsia with severe features, then lowering the circulating concentration of sFlt-1 would ameliorate not only the morbidity but also the risk of preeclampsia. Work with plasma phoresies has suggested that this might be possible, albeit via a clinical intervention demanding more intensive resources. The potential for a targeted pharmacologic moderation of sFlt-1 levels would hold great promise in that those identified as at increased risk could be offered an intervention widely available to all.
Thadhani R, Lemoine E, Rana S, et al. Circulating angiogenic factor levels in hypertensive disorders of pregnancy. N Engl J Med. 2022;1. DOI: 10.1056/EVIDoa2200161
EXPERT COMMENTARY
The standard core lecture on preeclampsia given to all medical students frequently begins with an epic, if not potentially apocryphal, statement regarding how this disease has been noted in the annals of medical history since the time of the Ancients. Although contemporary diagnostic criteria for preeclampsia are not that far out of date, they are close. The increased urinary protein loss and hypertension preceding eclamptic seizures was first noted at the end of the 19th century. The blood pressure and proteinuria criteria used for diagnosis was codified in its contemporary form in the late 1940s. Since then, “tweak” rather than “overhaul” probably best describes the updates of the obstetrical community to the definition of preeclampsia. This has just changed.
Details of the study
Thadhani and colleagues prospectively recruited a nationally representative observational cohort of patients hospitalized for hypertension during pregnancy, then followed the patients until either the diagnosis of preeclampsia with severe features or for 2 weeks, whichever came first. At enrollment, circulating levels of the soluble fms-like tyrosine kinase 1 (sFlt-1) and placental growth factor (PlGF) were measured. In a 2-phased design, the first 219 participants were used to define a sFlt-1/PlGF ratio that would predict progression to severe preeclampsia within 2 weeks. The next 556 enrollees served to validate the predictive properties of the ratio. The authors found that a sFlt-1/PlGF ratio of ≥40 predicted progression to preeclampsia with severe features with an area under the curve (AUC) of 0.92.

As products of the trophoblasts, both sFlt-1 and PlGF have been mooted for almost 2 decades as potential predictive, if not diagnostic, aids with respect to preeclampsia. Indeed, both analytes are commercially available in Europe for specifically this purpose and many maternal-fetal medicine practitioners working in the European equivalent American tertiary referral centers use an sFlt-1/PlGF ratio as their primary criteria for a diagnosis of preeclampsia. Within the United States, there was an initial flurry of interest in and an infusion of corporate and federal research support for sFlt-1 and PlGF as diagnostic aids for preeclampsia in the mid-2000s. However, at present, the US Food and Drug Administration (FDA) has not sanctioned these (or any) biomarkers to aid in the diagnosis of preeclampsia. As Thermo-Fisher Scientific (Waltham, Massachusetts) is a supporting partner in this study, it is almost certain that these data will be submitted for review by the FDA as part of an application for a preeclampsia diagnostic. At some point in the near future, American practitioners will potentially be able to join their European colleagues in utilizing these biomarkers in the diagnosis of preeclampsia with severe features. ●
Thadhani and colleagues observed that the majority of both maternal and neonatal morbidity in their study, including 8 of the 9 neonatal deaths and both cases of eclampsia, occurred among patients with a ratio ≥40 at admission. There was an inverse relation between the sFlt-1/PlGF ratio and the admission to delivery interval. Where only 17% of patients in the highest quartile of ratios remained pregnant at 14 days post-enrollment, more than 79% of the lowest quartile were still pregnant. If not a causal relationship, sFlt-1 and PlGF are clearly associated with not only the occurrence of preeclampsia with severe features but also the degree of morbidity.
The implication for the disposition of patient care resources is clear. Patients at higher risk for preeclampsia could be seen in specialty high-risk clinics with an emphasis on increased monitoring. In situations where tertiary care is more remote, plans could be developed should they need to be transported to centers able to provide the appropriate level of care. Conversely, patients screening at lower ratios may be more appropriately managed as outpatients, or at least in less clinically involved accommodations.
Thadhani et al do note that there were false negative cases in which the sFlt-1/PlGF ratio at admission was <40 but patients nonetheless progressed to preeclampsia with severe features. The majority of these cases had concurrent pre-pregnancy, chronic hypertension. This observation suggests not only the potential for insights into the pathophysiology of the hypertensive diseases in pregnancy but also that the interpretation of the sFlt/PlGF ratio may eventually need to be stratified by preexisting conditions.
The final implications for the observations of this study are perhaps the most tantalizing. If there is a causal relation between the level of the sFlt-1/PlGF ratio and the morbidity of preeclampsia with severe features, then lowering the circulating concentration of sFlt-1 would ameliorate not only the morbidity but also the risk of preeclampsia. Work with plasma phoresies has suggested that this might be possible, albeit via a clinical intervention demanding more intensive resources. The potential for a targeted pharmacologic moderation of sFlt-1 levels would hold great promise in that those identified as at increased risk could be offered an intervention widely available to all.
Thadhani R, Lemoine E, Rana S, et al. Circulating angiogenic factor levels in hypertensive disorders of pregnancy. N Engl J Med. 2022;1. DOI: 10.1056/EVIDoa2200161
EXPERT COMMENTARY
The standard core lecture on preeclampsia given to all medical students frequently begins with an epic, if not potentially apocryphal, statement regarding how this disease has been noted in the annals of medical history since the time of the Ancients. Although contemporary diagnostic criteria for preeclampsia are not that far out of date, they are close. The increased urinary protein loss and hypertension preceding eclamptic seizures was first noted at the end of the 19th century. The blood pressure and proteinuria criteria used for diagnosis was codified in its contemporary form in the late 1940s. Since then, “tweak” rather than “overhaul” probably best describes the updates of the obstetrical community to the definition of preeclampsia. This has just changed.
Details of the study
Thadhani and colleagues prospectively recruited a nationally representative observational cohort of patients hospitalized for hypertension during pregnancy, then followed the patients until either the diagnosis of preeclampsia with severe features or for 2 weeks, whichever came first. At enrollment, circulating levels of the soluble fms-like tyrosine kinase 1 (sFlt-1) and placental growth factor (PlGF) were measured. In a 2-phased design, the first 219 participants were used to define a sFlt-1/PlGF ratio that would predict progression to severe preeclampsia within 2 weeks. The next 556 enrollees served to validate the predictive properties of the ratio. The authors found that a sFlt-1/PlGF ratio of ≥40 predicted progression to preeclampsia with severe features with an area under the curve (AUC) of 0.92.

As products of the trophoblasts, both sFlt-1 and PlGF have been mooted for almost 2 decades as potential predictive, if not diagnostic, aids with respect to preeclampsia. Indeed, both analytes are commercially available in Europe for specifically this purpose and many maternal-fetal medicine practitioners working in the European equivalent American tertiary referral centers use an sFlt-1/PlGF ratio as their primary criteria for a diagnosis of preeclampsia. Within the United States, there was an initial flurry of interest in and an infusion of corporate and federal research support for sFlt-1 and PlGF as diagnostic aids for preeclampsia in the mid-2000s. However, at present, the US Food and Drug Administration (FDA) has not sanctioned these (or any) biomarkers to aid in the diagnosis of preeclampsia. As Thermo-Fisher Scientific (Waltham, Massachusetts) is a supporting partner in this study, it is almost certain that these data will be submitted for review by the FDA as part of an application for a preeclampsia diagnostic. At some point in the near future, American practitioners will potentially be able to join their European colleagues in utilizing these biomarkers in the diagnosis of preeclampsia with severe features. ●
Thadhani and colleagues observed that the majority of both maternal and neonatal morbidity in their study, including 8 of the 9 neonatal deaths and both cases of eclampsia, occurred among patients with a ratio ≥40 at admission. There was an inverse relation between the sFlt-1/PlGF ratio and the admission to delivery interval. Where only 17% of patients in the highest quartile of ratios remained pregnant at 14 days post-enrollment, more than 79% of the lowest quartile were still pregnant. If not a causal relationship, sFlt-1 and PlGF are clearly associated with not only the occurrence of preeclampsia with severe features but also the degree of morbidity.
The implication for the disposition of patient care resources is clear. Patients at higher risk for preeclampsia could be seen in specialty high-risk clinics with an emphasis on increased monitoring. In situations where tertiary care is more remote, plans could be developed should they need to be transported to centers able to provide the appropriate level of care. Conversely, patients screening at lower ratios may be more appropriately managed as outpatients, or at least in less clinically involved accommodations.
Thadhani et al do note that there were false negative cases in which the sFlt-1/PlGF ratio at admission was <40 but patients nonetheless progressed to preeclampsia with severe features. The majority of these cases had concurrent pre-pregnancy, chronic hypertension. This observation suggests not only the potential for insights into the pathophysiology of the hypertensive diseases in pregnancy but also that the interpretation of the sFlt/PlGF ratio may eventually need to be stratified by preexisting conditions.
The final implications for the observations of this study are perhaps the most tantalizing. If there is a causal relation between the level of the sFlt-1/PlGF ratio and the morbidity of preeclampsia with severe features, then lowering the circulating concentration of sFlt-1 would ameliorate not only the morbidity but also the risk of preeclampsia. Work with plasma phoresies has suggested that this might be possible, albeit via a clinical intervention demanding more intensive resources. The potential for a targeted pharmacologic moderation of sFlt-1 levels would hold great promise in that those identified as at increased risk could be offered an intervention widely available to all.
2023 Update on obstetrics
In the musical Hamilton, there is a line from the song “The Election of 1800” in which, after a tumultuous time, Thomas Jefferson pleads for a sense of normalcy with, “Can we get back to politics?”
Trying to get back to “normal,” whatever that is, characterized the year 2022. Peeking out from under the constant shadow of the COVID-19 pandemic (not really gone, definitely not forgotten) were some blockbuster obstetrical headlines, including those on the CHAP (Chronic Hypertension and Pregnancy) trial and the impact of the Dobbs v Jackson Supreme Court decision. As these have been extensively covered in both OBG Management and other publications, in this Update we simply ask, “Can we get back to obstetrics?” as we focus on some straightforward patient care guidelines.
Thus, we offer updated information on the use of progesterone for preterm birth prevention, management of pregnancies that result from in vitro fertilization (IVF), and headache management in pregnant and postpartum patients.
Society guidance and FDA advisement on the use of progesterone for the prevention of spontaneous preterm birth
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth. ACOG practice bulletin no. 234. Obstet Gynecol. 2021;138:e65-e90.
EPPPIC Group. Evaluating Progestogens for Preventing Preterm birth International Collaborative (EPPPIC): meta-analysis of individual participant data from randomised controlled trials. Lancet. 2021;397:1183-1194.
This is not déjà vu! Progesterone and spontaneous preterm birth (sPTB) is a hot topic again. If you wonder what to tell your patients, you are not alone. Preterm birth (PTB) continues to pose a challenge in obstetrics, with a most recently reported overall rate of 10.49%1 in the United States—a 4% increase from 2019. Preterm birth accounts for approximately 75% of perinatal mortality and more than half of neonatal morbidity.2
What has not changed
A recent practice bulletin from the American College of Obstetricians and Gynecologists (ACOG) notes that some risk factors and screening assessments for PTB remain unchanged, including2:
- A history of PTB increases the risk for subsequent PTB. Risk increases with the number of prior preterm deliveries.
- A short cervix (<25 mm between 16 and 24 weeks’ gestation) is a risk factor for sPTB.
- The cervix should be visualized during the anatomy ultrasound exam (18 0/7 to 22 6/7 weeks’ gestation) in all pregnant patients regardless of prior birth history. If the cervix length (CL) appears shortened on transabdominal imaging, transvaginal (TV) imaging should be performed.
- Patients with a current singleton pregnancy and history of sPTB should have serial TV cervical measurements between 16 0/7 and 24 0/7 weeks’ gestation.2
EPPPIC changes and key takeaway points
In a meta-analysis of data from 31 randomized controlled trials, the EPPPIC (Evaluating Progestogens for Preventing Preterm birth International Collaborative) investigators compared vaginal progesterone, intramuscular 17-hydroxyprogesterone caproate (17-OHPC), or oral progesterone with control or with each other in women at risk for PTB.3 Outcomes included PTB and the associated adverse neonatal and maternal outcomes.
The EPPPIC study’s main findings were:
- Singleton pregnancies at high risk for PTB due to prior sPTB or short cervix who received 17-OHPC or vaginal progesterone were less likely to deliver before 34 weeks’ gestation compared with those who received no treatment.
- There is a benefit to both 17-OHPC and vaginal progesterone in reducing the risk of PTB, with no clear evidence to support one intervention’s effectiveness over the other.
- There is benefit to either 17-OHPC or vaginal progesterone for CL less than 25 mm. The shorter the CL, the greater the absolute risk reduction on PTB.
- In multifetal pregnancies, use of 17-OHPC, when compared with placebo, was shown to increase the risk of preterm premature rupture of membranes. Neither 17-OHPC nor vaginal progesterone was found to reduce the risk of sPTB in multifetal pregnancies.3
What continues to change
While the March 30, 2021, statement from the Society for Maternal-Fetal Medicine (SMFM), “Response to EPPPIC and consideration for the use of progestogens for the prevention of preterm birth” (https://www .smfm.org/publications/383-smfm-stat ement-response-to-epppic-and-consider ations-of-the-use-of-progestogens-for-the -prevention-of-preterm-birth), stands, ACOG has withdrawn its accompanying Practice Advisory on guidance for integrating the EPPPIC findings.
In August 2022, the US Food and Drug Administration (FDA) granted a hearing on the Center for Drug Evaluation and Research’s proposal to withdraw approval for Makena (hydroxyprogesterone caproate injection, 250 mg/mL, once weekly) on the basis that available evidence does not demonstrate that it is effective for its approved indication to reduce the risk of PTB in women with a singleton pregnancy with a history of singleton sPTB.4
The key takeaway points from the FDA hearing (October 17–19, 2022) were:
- A better designed randomized controlled confirmatory trial is needed in the most at-risk patients to determine if Makena is effective for its approved indication.
- Makena and its approved generic equivalents remain on the market until the FDA makes its final decision regarding approval.4
For now, the decision to use intramuscular progesterone in women with a prior sPTB should be based on shared decision-making between the health care provider and patient, with discussion of its benefits, risks, and uncertainties. SMFM currently recommends that women with a singleton pregnancy and a short CL (<25 mm) without a history of prior sPTB be offered treatment with a progesterone. While 17-OHPC and vaginal progesterone appear to offer benefit to women with a singleton pregnancy and either a short CL or a history of sPTB, the greatest benefit and least risk is seen with use of vaginal progesterone. In multifetal pregnancies, there is not enough evidence to recommend the use of progesterone outside of clinical trials.
Although in our practice we still offer 17-OHPC to patients with the counseling noted above, we have focused more on the use of vaginal progesterone in women with singleton pregnancies and a history of sPTB or short CL.
Continue to: Managing pregnancies that result from IVF...
Managing pregnancies that result from IVF
Society for Maternal-Fetal Medicine (SMFM); Ghidini A, Gandhi M, McCoy J, et al; Publications Committee. Society for Maternal-Fetal Medicine consult series #60: management of pregnancies resulting from in vitro fertilization. Am J Obstet Gynecol. 2022;226:B2-B12.
Assisted reproductive technology contributes to 1.6% of all infant births, and although most pregnancies are uncomplicated, some specific risks alter management.5–7 For example, IVF is associated with increased rates of prematurity and its complications, fetal growth restriction, low birth weight, congenital anomalies, genetic abnormalities, and placental abnormalities. In addition, there is doubling of the risk of morbidities to the pregnant IVF patient, including but not limited to hypertensive disorders and diabetes. These complications are thought to be related to both the process of IVF itself as well as to conditions that contribute to subfertility and infertility in the first place.
Genetic screening and diagnostic testing options
IVF pregnancies have a documented increase in chromosomal abnormalities compared with spontaneously conceived pregnancies due to the following factors:
- karyotypic abnormalities in couples with infertility
- microdeletions on the Y chromosome in patients with oligospermia or azoospermia
- de novo chromosomal abnormalities in IVF pregnancies that utilize intracytoplasmic sperm injection (ICSI)
- fragile X mutations in patients with reduced ovarian reserve
- imprinting disorders in patients with fertility issues.
A common misconception is that preimplantation genetic testing renders prenatal genetic screening or testing unnecessary. However, preimplantation testing can be anywhere from 43% to 84% concordant with prenatal diagnostic testing due to biologic and technical factors. Therefore, all pregnancies should be offered the same options of aneuploidy screening as well as diagnostic testing. Pretest counseling should include an increased risk in IVF pregnancies of false-positives for the first-trimester screen and “no-call” results for cell-free fetal DNA. Additionally, diagnostic testing is recommended specifically in cases where mosaic embryos are transferred when euploid embryos are not available.
Counseling on fetal reduction for multifetal pregnancies
The risks of multifetal pregnancies (particularly higher order multiples) are significant and well documented for both the patient and the fetuses. It is therefore recommended that the option of multifetal pregnancy reduction be discussed, including the risks and benefits of reduction versus pregnancy continuation, timing, procedural considerations, and genetic testing options.5,8
Detailed anatomic survey and fetal echocardiogram are indicated
Fetal anomalies, including congenital cardiac defects, occur at a higher rate in IVF pregnancies compared with spontaneously conceived pregnancies (475/10,000 live births vs 317/10,000 live births). Placental anomalies (such as placenta previa, vasa previa, and velamentous cord insertion) are also more common in this population. A detailed anatomic survey is therefore recommended for all IVF pregnancies and it is suggested that a fetal echocardiogram is offered these patients as well.
Pregnancy management and delivery considerations
Despite an increased risk of preterm birth, preeclampsia, and fetal growth restriction in IVF pregnancies (odds ratios range, 1.4–2), serial cervical lengths, serial growth ultrasound exams, and low-dose aspirin are not recommended for the sole indication of IVF. Due to lack of data on the utility of serial exams, a single screening cervical length at the time of anatomic survey and a third-trimester growth assessment are recommended. For aspirin, IVF qualifies as a “moderate” risk factor for preeclampsia; it is therefore recommended if another moderate risk factor is present (for example, nulliparity, obesity, or family history of preeclampsia).9
There is a 2- to 3-fold increased risk of stillbirth in IVF pregnancies; therefore, antenatal surveillance in the third trimester is recommended (weekly starting at 36 weeks for the sole indication of IVF).10 As no specific studies have evaluated the timing of delivery in IVF pregnancies, delivery recommendations include the option of 39-week delivery with shared decision-making with the patient.
While the expected outcome is good for most pregnancies conceived via IVF, there is an increased risk of adverse perinatal outcomes that varies based on individual patient characteristics and IVF technical aspects. Individualized care plans for these patients should include counseling regarding genetic screening and testing options, multifetal reduction in multiple gestations, imaging for fetal anomalies, and fetal surveillance in the third trimester.
Continue to: Evaluating and treating headaches in pregnancy and postpartum...
Evaluating and treating headaches in pregnancy and postpartum
American College of Obstetricians and Gynecologists. Clinical practice guideline no. 3: headaches in pregnancy and postpartum. Obstet Gynecol. 2022;139:944-972.
For obstetricians, headaches are a common and often frustrating condition to treat, as many of the available diagnostic tools and medications are either not recommended or have no data on use in pregnancy and lactation. Additionally, a headache is not always just a headache but could be a sign of a time-sensitive serious complication. An updated guideline from the American College of Obstetricians and Gynecologists approaches the topic of headaches in a stepwise algorithm that promotes efficiency and efficacy in diagnosis and treatment.11
Types of headaches
The primary headache types—migraine, cluster, and tension—are distinguished from each other by patient characteristics, quality, duration, location, and related symptoms. Reassuringly, headache frequency decreases by 30% to 80% during pregnancy, which allows for the option to decrease, change, or stop current medications, ideally prior to pregnancy. Prevention via use of calcium channel blockers, antihistamines, or β-blockers is recommended, as requiring acute treatments more than 2 days per week increases the risk of medication overuse headaches.
Treating acute headache
For patients who present with an acute headache consistent with their usual type, treatment starts with known medications that are compatible with pregnancy and proceeds in a stepwise fashion:
1. Acetaminophen 1,000 mg orally with or without caffeine 130 mg orally (maximum dose, acetaminophen < 3.25–4 g per day, caffeine 200 mg per day)
2. Metoclopramide 10 mg intravenously with or without diphenhydramine 25 mg intravenously (for nausea and to counteract restlessness and offer sedation)
3. If headache continues after steps 1 and 2, consider the following secondary treatment options: magnesium sulfate 1–2 g intravenously, sumatriptan 6 mg subcutaneously or 20-mg nasal spray, ibuprofen 600 mg orally once, or ketorolac 30 mg intravenously once (second trimester only)
4. If continued treatment and/or hospitalization is required after step 3, steroids can be used: prednisone 20 mg 4 times a day for 2 days or methylprednisolone 4-mg dose pack over 6 days
5. Do not use butalbital, opioids, or ergotamines due to lack of efficacy in providing additional pain relief, potential for addiction, risk of medication overuse headaches, and association with fetal/ pregnancy abnormalities.
Consider secondary headache
An acute headache discordant from the patient’s usual type or with concerning symptoms (“red flags”) requires consideration of secondary headaches as well as a comprehensive symptom evaluation, imaging, and consultation as needed. While secondary headaches postpartum are most likely musculoskeletal in nature, the following symptoms need to be evaluated immediately:
- rapid onset/change from baseline
- “thunderclap” nature
- hypertension
- fever
- focal neurologic deficits (blurry vision or blindness, confusion, seizures)
- altered consciousness
- laboratory abnormalities.
The differential diagnosis includes preeclampsia, reversible cerebral vasoconstriction syndrome (RCVS), posterior reversible encephalopathy syndrome (PRES), infection, cerebral venous sinus thrombosis (CVST), post–dural puncture (PDP) headache, idiopathic intracranial hypertension (IIH), and less likely, carotid dissection, subarachnoid hemorrhage, intracranial hemorrhage, pituitary apoplexy, or neoplasm.
Treatment. Individualized treatment depends on the diagnosis. Preeclampsia with severe features is treated with antihypertensive medication, magnesium sulfate, and delivery planning. PDP headache is treated with epidural blood patch, sphenopalatine block, or occipital block with an anesthesiology consultation. If preeclampsia and PDP are ruled out, or if there are more concerning neurologic features, imaging is essential, as 25% of pregnant patients with acute headaches will have a secondary etiology. Magnetic resonance imaging without contrast is preferred due to concerns about gadolinium crossing the placenta and the lack of data on long-term accumulation in fetal tissues. Once diagnosed on imaging, PRES and RCVS are treated with antihypertensives and delivery. CVST is treated with anticoagulation and a thrombophilia workup. IIH may be treated with acetazolamide after 20 weeks or serial lumbar punctures. Intracranial vascular abnormalities may be treated with endoscopic resection and steroids. ●
Calcium channel blockers and antihistamines are recommended for primary headache prevention.
Acetaminophen, caffeine, diphenhydramine, and metoclopramide administered in a stepwise manner are recommended for acute treatment of primary headache in pregnancy. Nonsteroidal antiinflammatory agents and triptans may be added during lactation and postpartum.
Butalbital and opioids are not recommended for acute treatment of headaches in pregnancy and postpartum due to risk of medication overuse headaches, dependence, and neonatal abstinence syndrome.
“Red flag” headache symptoms warrant imaging, prompt treatment of severe hypertension, and timely treatment of potentially life-threatening intracranial conditions.
- Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2021. NCHS Data Brief, no 442. Hyattsville, MD: National Center for Health Statistics. August 2022. Accessed December 15, 2022. https://dx.doi.org/10.15620 /cdc:119632
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth. ACOG practice bulletin no. 234. Obstet Gynecol. 2021;138:e65-e90.
- EPPPIC Group. Evaluating Progestogens for Preventing Preterm birth International Collaborative (EPPPIC): meta-analysis of individual participant data from randomised controlled trials. Lancet. 2021;397:1183-1194.
- US Food and Drug Administration. Proposal to withdraw approval of Makena; notice of opportunity for a hearing. August 17, 2022. Accessed December 15, 2022. https://www. regulations.gov/docket/FDA-2020-N-2029
- Society for Maternal-Fetal Medicine (SMFM); Ghidini A, Gandhi M, McCoy J, et al; Publications Committee. Society for Maternal-Fetal Medicine consult series #60: management of pregnancies resulting from in vitro fertilization. Am J Obstet Gynecol. 2022;226:B2-B12.
- Society for Maternal-Fetal Medicine; Abu-Rustum RS, Combs CA, Davidson CM, et al; Patient Safety and Quality Committee. Society for Maternal-Fetal Medicine special statement: checklist for pregnancies resulting from in vitro fertilization. Am J Obstet Gynecol. 2022;227:B2-B3.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice; Committee on Genetics; US Food and Drug Administration. Committee opinion no. 671: perinatal risks associated with assisted reproductive technology. Obstet Gynecol. 2016;128:e61-e68.
- American College of Obstetricians and Gynecologists. Committee opinion no. 719: multifetal pregnancy reduction. Obstet Gynecol. 2017;130:e158-e163.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 743: low-dose aspirin use during pregnancy. Obstet Gynecol. 2018;132:e44-e52.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice, Society for Maternal-Fetal Medicine. ACOG committee opinion no. 828: indications for outpatient antenatal fetal surveillance. Obstet Gynecol. 2021;137:e177-e197.
- American College of Obstetricians and Gynecologists. Clinical practice guideline no. 3: headaches in pregnancy and postpartum. Obstet Gynecol. 2022;139:944-972.
In the musical Hamilton, there is a line from the song “The Election of 1800” in which, after a tumultuous time, Thomas Jefferson pleads for a sense of normalcy with, “Can we get back to politics?”
Trying to get back to “normal,” whatever that is, characterized the year 2022. Peeking out from under the constant shadow of the COVID-19 pandemic (not really gone, definitely not forgotten) were some blockbuster obstetrical headlines, including those on the CHAP (Chronic Hypertension and Pregnancy) trial and the impact of the Dobbs v Jackson Supreme Court decision. As these have been extensively covered in both OBG Management and other publications, in this Update we simply ask, “Can we get back to obstetrics?” as we focus on some straightforward patient care guidelines.
Thus, we offer updated information on the use of progesterone for preterm birth prevention, management of pregnancies that result from in vitro fertilization (IVF), and headache management in pregnant and postpartum patients.
Society guidance and FDA advisement on the use of progesterone for the prevention of spontaneous preterm birth
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth. ACOG practice bulletin no. 234. Obstet Gynecol. 2021;138:e65-e90.
EPPPIC Group. Evaluating Progestogens for Preventing Preterm birth International Collaborative (EPPPIC): meta-analysis of individual participant data from randomised controlled trials. Lancet. 2021;397:1183-1194.
This is not déjà vu! Progesterone and spontaneous preterm birth (sPTB) is a hot topic again. If you wonder what to tell your patients, you are not alone. Preterm birth (PTB) continues to pose a challenge in obstetrics, with a most recently reported overall rate of 10.49%1 in the United States—a 4% increase from 2019. Preterm birth accounts for approximately 75% of perinatal mortality and more than half of neonatal morbidity.2
What has not changed
A recent practice bulletin from the American College of Obstetricians and Gynecologists (ACOG) notes that some risk factors and screening assessments for PTB remain unchanged, including2:
- A history of PTB increases the risk for subsequent PTB. Risk increases with the number of prior preterm deliveries.
- A short cervix (<25 mm between 16 and 24 weeks’ gestation) is a risk factor for sPTB.
- The cervix should be visualized during the anatomy ultrasound exam (18 0/7 to 22 6/7 weeks’ gestation) in all pregnant patients regardless of prior birth history. If the cervix length (CL) appears shortened on transabdominal imaging, transvaginal (TV) imaging should be performed.
- Patients with a current singleton pregnancy and history of sPTB should have serial TV cervical measurements between 16 0/7 and 24 0/7 weeks’ gestation.2
EPPPIC changes and key takeaway points
In a meta-analysis of data from 31 randomized controlled trials, the EPPPIC (Evaluating Progestogens for Preventing Preterm birth International Collaborative) investigators compared vaginal progesterone, intramuscular 17-hydroxyprogesterone caproate (17-OHPC), or oral progesterone with control or with each other in women at risk for PTB.3 Outcomes included PTB and the associated adverse neonatal and maternal outcomes.
The EPPPIC study’s main findings were:
- Singleton pregnancies at high risk for PTB due to prior sPTB or short cervix who received 17-OHPC or vaginal progesterone were less likely to deliver before 34 weeks’ gestation compared with those who received no treatment.
- There is a benefit to both 17-OHPC and vaginal progesterone in reducing the risk of PTB, with no clear evidence to support one intervention’s effectiveness over the other.
- There is benefit to either 17-OHPC or vaginal progesterone for CL less than 25 mm. The shorter the CL, the greater the absolute risk reduction on PTB.
- In multifetal pregnancies, use of 17-OHPC, when compared with placebo, was shown to increase the risk of preterm premature rupture of membranes. Neither 17-OHPC nor vaginal progesterone was found to reduce the risk of sPTB in multifetal pregnancies.3
What continues to change
While the March 30, 2021, statement from the Society for Maternal-Fetal Medicine (SMFM), “Response to EPPPIC and consideration for the use of progestogens for the prevention of preterm birth” (https://www .smfm.org/publications/383-smfm-stat ement-response-to-epppic-and-consider ations-of-the-use-of-progestogens-for-the -prevention-of-preterm-birth), stands, ACOG has withdrawn its accompanying Practice Advisory on guidance for integrating the EPPPIC findings.
In August 2022, the US Food and Drug Administration (FDA) granted a hearing on the Center for Drug Evaluation and Research’s proposal to withdraw approval for Makena (hydroxyprogesterone caproate injection, 250 mg/mL, once weekly) on the basis that available evidence does not demonstrate that it is effective for its approved indication to reduce the risk of PTB in women with a singleton pregnancy with a history of singleton sPTB.4
The key takeaway points from the FDA hearing (October 17–19, 2022) were:
- A better designed randomized controlled confirmatory trial is needed in the most at-risk patients to determine if Makena is effective for its approved indication.
- Makena and its approved generic equivalents remain on the market until the FDA makes its final decision regarding approval.4
For now, the decision to use intramuscular progesterone in women with a prior sPTB should be based on shared decision-making between the health care provider and patient, with discussion of its benefits, risks, and uncertainties. SMFM currently recommends that women with a singleton pregnancy and a short CL (<25 mm) without a history of prior sPTB be offered treatment with a progesterone. While 17-OHPC and vaginal progesterone appear to offer benefit to women with a singleton pregnancy and either a short CL or a history of sPTB, the greatest benefit and least risk is seen with use of vaginal progesterone. In multifetal pregnancies, there is not enough evidence to recommend the use of progesterone outside of clinical trials.
Although in our practice we still offer 17-OHPC to patients with the counseling noted above, we have focused more on the use of vaginal progesterone in women with singleton pregnancies and a history of sPTB or short CL.
Continue to: Managing pregnancies that result from IVF...
Managing pregnancies that result from IVF
Society for Maternal-Fetal Medicine (SMFM); Ghidini A, Gandhi M, McCoy J, et al; Publications Committee. Society for Maternal-Fetal Medicine consult series #60: management of pregnancies resulting from in vitro fertilization. Am J Obstet Gynecol. 2022;226:B2-B12.
Assisted reproductive technology contributes to 1.6% of all infant births, and although most pregnancies are uncomplicated, some specific risks alter management.5–7 For example, IVF is associated with increased rates of prematurity and its complications, fetal growth restriction, low birth weight, congenital anomalies, genetic abnormalities, and placental abnormalities. In addition, there is doubling of the risk of morbidities to the pregnant IVF patient, including but not limited to hypertensive disorders and diabetes. These complications are thought to be related to both the process of IVF itself as well as to conditions that contribute to subfertility and infertility in the first place.
Genetic screening and diagnostic testing options
IVF pregnancies have a documented increase in chromosomal abnormalities compared with spontaneously conceived pregnancies due to the following factors:
- karyotypic abnormalities in couples with infertility
- microdeletions on the Y chromosome in patients with oligospermia or azoospermia
- de novo chromosomal abnormalities in IVF pregnancies that utilize intracytoplasmic sperm injection (ICSI)
- fragile X mutations in patients with reduced ovarian reserve
- imprinting disorders in patients with fertility issues.
A common misconception is that preimplantation genetic testing renders prenatal genetic screening or testing unnecessary. However, preimplantation testing can be anywhere from 43% to 84% concordant with prenatal diagnostic testing due to biologic and technical factors. Therefore, all pregnancies should be offered the same options of aneuploidy screening as well as diagnostic testing. Pretest counseling should include an increased risk in IVF pregnancies of false-positives for the first-trimester screen and “no-call” results for cell-free fetal DNA. Additionally, diagnostic testing is recommended specifically in cases where mosaic embryos are transferred when euploid embryos are not available.
Counseling on fetal reduction for multifetal pregnancies
The risks of multifetal pregnancies (particularly higher order multiples) are significant and well documented for both the patient and the fetuses. It is therefore recommended that the option of multifetal pregnancy reduction be discussed, including the risks and benefits of reduction versus pregnancy continuation, timing, procedural considerations, and genetic testing options.5,8
Detailed anatomic survey and fetal echocardiogram are indicated
Fetal anomalies, including congenital cardiac defects, occur at a higher rate in IVF pregnancies compared with spontaneously conceived pregnancies (475/10,000 live births vs 317/10,000 live births). Placental anomalies (such as placenta previa, vasa previa, and velamentous cord insertion) are also more common in this population. A detailed anatomic survey is therefore recommended for all IVF pregnancies and it is suggested that a fetal echocardiogram is offered these patients as well.
Pregnancy management and delivery considerations
Despite an increased risk of preterm birth, preeclampsia, and fetal growth restriction in IVF pregnancies (odds ratios range, 1.4–2), serial cervical lengths, serial growth ultrasound exams, and low-dose aspirin are not recommended for the sole indication of IVF. Due to lack of data on the utility of serial exams, a single screening cervical length at the time of anatomic survey and a third-trimester growth assessment are recommended. For aspirin, IVF qualifies as a “moderate” risk factor for preeclampsia; it is therefore recommended if another moderate risk factor is present (for example, nulliparity, obesity, or family history of preeclampsia).9
There is a 2- to 3-fold increased risk of stillbirth in IVF pregnancies; therefore, antenatal surveillance in the third trimester is recommended (weekly starting at 36 weeks for the sole indication of IVF).10 As no specific studies have evaluated the timing of delivery in IVF pregnancies, delivery recommendations include the option of 39-week delivery with shared decision-making with the patient.
While the expected outcome is good for most pregnancies conceived via IVF, there is an increased risk of adverse perinatal outcomes that varies based on individual patient characteristics and IVF technical aspects. Individualized care plans for these patients should include counseling regarding genetic screening and testing options, multifetal reduction in multiple gestations, imaging for fetal anomalies, and fetal surveillance in the third trimester.
Continue to: Evaluating and treating headaches in pregnancy and postpartum...
Evaluating and treating headaches in pregnancy and postpartum
American College of Obstetricians and Gynecologists. Clinical practice guideline no. 3: headaches in pregnancy and postpartum. Obstet Gynecol. 2022;139:944-972.
For obstetricians, headaches are a common and often frustrating condition to treat, as many of the available diagnostic tools and medications are either not recommended or have no data on use in pregnancy and lactation. Additionally, a headache is not always just a headache but could be a sign of a time-sensitive serious complication. An updated guideline from the American College of Obstetricians and Gynecologists approaches the topic of headaches in a stepwise algorithm that promotes efficiency and efficacy in diagnosis and treatment.11
Types of headaches
The primary headache types—migraine, cluster, and tension—are distinguished from each other by patient characteristics, quality, duration, location, and related symptoms. Reassuringly, headache frequency decreases by 30% to 80% during pregnancy, which allows for the option to decrease, change, or stop current medications, ideally prior to pregnancy. Prevention via use of calcium channel blockers, antihistamines, or β-blockers is recommended, as requiring acute treatments more than 2 days per week increases the risk of medication overuse headaches.
Treating acute headache
For patients who present with an acute headache consistent with their usual type, treatment starts with known medications that are compatible with pregnancy and proceeds in a stepwise fashion:
1. Acetaminophen 1,000 mg orally with or without caffeine 130 mg orally (maximum dose, acetaminophen < 3.25–4 g per day, caffeine 200 mg per day)
2. Metoclopramide 10 mg intravenously with or without diphenhydramine 25 mg intravenously (for nausea and to counteract restlessness and offer sedation)
3. If headache continues after steps 1 and 2, consider the following secondary treatment options: magnesium sulfate 1–2 g intravenously, sumatriptan 6 mg subcutaneously or 20-mg nasal spray, ibuprofen 600 mg orally once, or ketorolac 30 mg intravenously once (second trimester only)
4. If continued treatment and/or hospitalization is required after step 3, steroids can be used: prednisone 20 mg 4 times a day for 2 days or methylprednisolone 4-mg dose pack over 6 days
5. Do not use butalbital, opioids, or ergotamines due to lack of efficacy in providing additional pain relief, potential for addiction, risk of medication overuse headaches, and association with fetal/ pregnancy abnormalities.
Consider secondary headache
An acute headache discordant from the patient’s usual type or with concerning symptoms (“red flags”) requires consideration of secondary headaches as well as a comprehensive symptom evaluation, imaging, and consultation as needed. While secondary headaches postpartum are most likely musculoskeletal in nature, the following symptoms need to be evaluated immediately:
- rapid onset/change from baseline
- “thunderclap” nature
- hypertension
- fever
- focal neurologic deficits (blurry vision or blindness, confusion, seizures)
- altered consciousness
- laboratory abnormalities.
The differential diagnosis includes preeclampsia, reversible cerebral vasoconstriction syndrome (RCVS), posterior reversible encephalopathy syndrome (PRES), infection, cerebral venous sinus thrombosis (CVST), post–dural puncture (PDP) headache, idiopathic intracranial hypertension (IIH), and less likely, carotid dissection, subarachnoid hemorrhage, intracranial hemorrhage, pituitary apoplexy, or neoplasm.
Treatment. Individualized treatment depends on the diagnosis. Preeclampsia with severe features is treated with antihypertensive medication, magnesium sulfate, and delivery planning. PDP headache is treated with epidural blood patch, sphenopalatine block, or occipital block with an anesthesiology consultation. If preeclampsia and PDP are ruled out, or if there are more concerning neurologic features, imaging is essential, as 25% of pregnant patients with acute headaches will have a secondary etiology. Magnetic resonance imaging without contrast is preferred due to concerns about gadolinium crossing the placenta and the lack of data on long-term accumulation in fetal tissues. Once diagnosed on imaging, PRES and RCVS are treated with antihypertensives and delivery. CVST is treated with anticoagulation and a thrombophilia workup. IIH may be treated with acetazolamide after 20 weeks or serial lumbar punctures. Intracranial vascular abnormalities may be treated with endoscopic resection and steroids. ●
Calcium channel blockers and antihistamines are recommended for primary headache prevention.
Acetaminophen, caffeine, diphenhydramine, and metoclopramide administered in a stepwise manner are recommended for acute treatment of primary headache in pregnancy. Nonsteroidal antiinflammatory agents and triptans may be added during lactation and postpartum.
Butalbital and opioids are not recommended for acute treatment of headaches in pregnancy and postpartum due to risk of medication overuse headaches, dependence, and neonatal abstinence syndrome.
“Red flag” headache symptoms warrant imaging, prompt treatment of severe hypertension, and timely treatment of potentially life-threatening intracranial conditions.
In the musical Hamilton, there is a line from the song “The Election of 1800” in which, after a tumultuous time, Thomas Jefferson pleads for a sense of normalcy with, “Can we get back to politics?”
Trying to get back to “normal,” whatever that is, characterized the year 2022. Peeking out from under the constant shadow of the COVID-19 pandemic (not really gone, definitely not forgotten) were some blockbuster obstetrical headlines, including those on the CHAP (Chronic Hypertension and Pregnancy) trial and the impact of the Dobbs v Jackson Supreme Court decision. As these have been extensively covered in both OBG Management and other publications, in this Update we simply ask, “Can we get back to obstetrics?” as we focus on some straightforward patient care guidelines.
Thus, we offer updated information on the use of progesterone for preterm birth prevention, management of pregnancies that result from in vitro fertilization (IVF), and headache management in pregnant and postpartum patients.
Society guidance and FDA advisement on the use of progesterone for the prevention of spontaneous preterm birth
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth. ACOG practice bulletin no. 234. Obstet Gynecol. 2021;138:e65-e90.
EPPPIC Group. Evaluating Progestogens for Preventing Preterm birth International Collaborative (EPPPIC): meta-analysis of individual participant data from randomised controlled trials. Lancet. 2021;397:1183-1194.
This is not déjà vu! Progesterone and spontaneous preterm birth (sPTB) is a hot topic again. If you wonder what to tell your patients, you are not alone. Preterm birth (PTB) continues to pose a challenge in obstetrics, with a most recently reported overall rate of 10.49%1 in the United States—a 4% increase from 2019. Preterm birth accounts for approximately 75% of perinatal mortality and more than half of neonatal morbidity.2
What has not changed
A recent practice bulletin from the American College of Obstetricians and Gynecologists (ACOG) notes that some risk factors and screening assessments for PTB remain unchanged, including2:
- A history of PTB increases the risk for subsequent PTB. Risk increases with the number of prior preterm deliveries.
- A short cervix (<25 mm between 16 and 24 weeks’ gestation) is a risk factor for sPTB.
- The cervix should be visualized during the anatomy ultrasound exam (18 0/7 to 22 6/7 weeks’ gestation) in all pregnant patients regardless of prior birth history. If the cervix length (CL) appears shortened on transabdominal imaging, transvaginal (TV) imaging should be performed.
- Patients with a current singleton pregnancy and history of sPTB should have serial TV cervical measurements between 16 0/7 and 24 0/7 weeks’ gestation.2
EPPPIC changes and key takeaway points
In a meta-analysis of data from 31 randomized controlled trials, the EPPPIC (Evaluating Progestogens for Preventing Preterm birth International Collaborative) investigators compared vaginal progesterone, intramuscular 17-hydroxyprogesterone caproate (17-OHPC), or oral progesterone with control or with each other in women at risk for PTB.3 Outcomes included PTB and the associated adverse neonatal and maternal outcomes.
The EPPPIC study’s main findings were:
- Singleton pregnancies at high risk for PTB due to prior sPTB or short cervix who received 17-OHPC or vaginal progesterone were less likely to deliver before 34 weeks’ gestation compared with those who received no treatment.
- There is a benefit to both 17-OHPC and vaginal progesterone in reducing the risk of PTB, with no clear evidence to support one intervention’s effectiveness over the other.
- There is benefit to either 17-OHPC or vaginal progesterone for CL less than 25 mm. The shorter the CL, the greater the absolute risk reduction on PTB.
- In multifetal pregnancies, use of 17-OHPC, when compared with placebo, was shown to increase the risk of preterm premature rupture of membranes. Neither 17-OHPC nor vaginal progesterone was found to reduce the risk of sPTB in multifetal pregnancies.3
What continues to change
While the March 30, 2021, statement from the Society for Maternal-Fetal Medicine (SMFM), “Response to EPPPIC and consideration for the use of progestogens for the prevention of preterm birth” (https://www .smfm.org/publications/383-smfm-stat ement-response-to-epppic-and-consider ations-of-the-use-of-progestogens-for-the -prevention-of-preterm-birth), stands, ACOG has withdrawn its accompanying Practice Advisory on guidance for integrating the EPPPIC findings.
In August 2022, the US Food and Drug Administration (FDA) granted a hearing on the Center for Drug Evaluation and Research’s proposal to withdraw approval for Makena (hydroxyprogesterone caproate injection, 250 mg/mL, once weekly) on the basis that available evidence does not demonstrate that it is effective for its approved indication to reduce the risk of PTB in women with a singleton pregnancy with a history of singleton sPTB.4
The key takeaway points from the FDA hearing (October 17–19, 2022) were:
- A better designed randomized controlled confirmatory trial is needed in the most at-risk patients to determine if Makena is effective for its approved indication.
- Makena and its approved generic equivalents remain on the market until the FDA makes its final decision regarding approval.4
For now, the decision to use intramuscular progesterone in women with a prior sPTB should be based on shared decision-making between the health care provider and patient, with discussion of its benefits, risks, and uncertainties. SMFM currently recommends that women with a singleton pregnancy and a short CL (<25 mm) without a history of prior sPTB be offered treatment with a progesterone. While 17-OHPC and vaginal progesterone appear to offer benefit to women with a singleton pregnancy and either a short CL or a history of sPTB, the greatest benefit and least risk is seen with use of vaginal progesterone. In multifetal pregnancies, there is not enough evidence to recommend the use of progesterone outside of clinical trials.
Although in our practice we still offer 17-OHPC to patients with the counseling noted above, we have focused more on the use of vaginal progesterone in women with singleton pregnancies and a history of sPTB or short CL.
Continue to: Managing pregnancies that result from IVF...
Managing pregnancies that result from IVF
Society for Maternal-Fetal Medicine (SMFM); Ghidini A, Gandhi M, McCoy J, et al; Publications Committee. Society for Maternal-Fetal Medicine consult series #60: management of pregnancies resulting from in vitro fertilization. Am J Obstet Gynecol. 2022;226:B2-B12.
Assisted reproductive technology contributes to 1.6% of all infant births, and although most pregnancies are uncomplicated, some specific risks alter management.5–7 For example, IVF is associated with increased rates of prematurity and its complications, fetal growth restriction, low birth weight, congenital anomalies, genetic abnormalities, and placental abnormalities. In addition, there is doubling of the risk of morbidities to the pregnant IVF patient, including but not limited to hypertensive disorders and diabetes. These complications are thought to be related to both the process of IVF itself as well as to conditions that contribute to subfertility and infertility in the first place.
Genetic screening and diagnostic testing options
IVF pregnancies have a documented increase in chromosomal abnormalities compared with spontaneously conceived pregnancies due to the following factors:
- karyotypic abnormalities in couples with infertility
- microdeletions on the Y chromosome in patients with oligospermia or azoospermia
- de novo chromosomal abnormalities in IVF pregnancies that utilize intracytoplasmic sperm injection (ICSI)
- fragile X mutations in patients with reduced ovarian reserve
- imprinting disorders in patients with fertility issues.
A common misconception is that preimplantation genetic testing renders prenatal genetic screening or testing unnecessary. However, preimplantation testing can be anywhere from 43% to 84% concordant with prenatal diagnostic testing due to biologic and technical factors. Therefore, all pregnancies should be offered the same options of aneuploidy screening as well as diagnostic testing. Pretest counseling should include an increased risk in IVF pregnancies of false-positives for the first-trimester screen and “no-call” results for cell-free fetal DNA. Additionally, diagnostic testing is recommended specifically in cases where mosaic embryos are transferred when euploid embryos are not available.
Counseling on fetal reduction for multifetal pregnancies
The risks of multifetal pregnancies (particularly higher order multiples) are significant and well documented for both the patient and the fetuses. It is therefore recommended that the option of multifetal pregnancy reduction be discussed, including the risks and benefits of reduction versus pregnancy continuation, timing, procedural considerations, and genetic testing options.5,8
Detailed anatomic survey and fetal echocardiogram are indicated
Fetal anomalies, including congenital cardiac defects, occur at a higher rate in IVF pregnancies compared with spontaneously conceived pregnancies (475/10,000 live births vs 317/10,000 live births). Placental anomalies (such as placenta previa, vasa previa, and velamentous cord insertion) are also more common in this population. A detailed anatomic survey is therefore recommended for all IVF pregnancies and it is suggested that a fetal echocardiogram is offered these patients as well.
Pregnancy management and delivery considerations
Despite an increased risk of preterm birth, preeclampsia, and fetal growth restriction in IVF pregnancies (odds ratios range, 1.4–2), serial cervical lengths, serial growth ultrasound exams, and low-dose aspirin are not recommended for the sole indication of IVF. Due to lack of data on the utility of serial exams, a single screening cervical length at the time of anatomic survey and a third-trimester growth assessment are recommended. For aspirin, IVF qualifies as a “moderate” risk factor for preeclampsia; it is therefore recommended if another moderate risk factor is present (for example, nulliparity, obesity, or family history of preeclampsia).9
There is a 2- to 3-fold increased risk of stillbirth in IVF pregnancies; therefore, antenatal surveillance in the third trimester is recommended (weekly starting at 36 weeks for the sole indication of IVF).10 As no specific studies have evaluated the timing of delivery in IVF pregnancies, delivery recommendations include the option of 39-week delivery with shared decision-making with the patient.
While the expected outcome is good for most pregnancies conceived via IVF, there is an increased risk of adverse perinatal outcomes that varies based on individual patient characteristics and IVF technical aspects. Individualized care plans for these patients should include counseling regarding genetic screening and testing options, multifetal reduction in multiple gestations, imaging for fetal anomalies, and fetal surveillance in the third trimester.
Continue to: Evaluating and treating headaches in pregnancy and postpartum...
Evaluating and treating headaches in pregnancy and postpartum
American College of Obstetricians and Gynecologists. Clinical practice guideline no. 3: headaches in pregnancy and postpartum. Obstet Gynecol. 2022;139:944-972.
For obstetricians, headaches are a common and often frustrating condition to treat, as many of the available diagnostic tools and medications are either not recommended or have no data on use in pregnancy and lactation. Additionally, a headache is not always just a headache but could be a sign of a time-sensitive serious complication. An updated guideline from the American College of Obstetricians and Gynecologists approaches the topic of headaches in a stepwise algorithm that promotes efficiency and efficacy in diagnosis and treatment.11
Types of headaches
The primary headache types—migraine, cluster, and tension—are distinguished from each other by patient characteristics, quality, duration, location, and related symptoms. Reassuringly, headache frequency decreases by 30% to 80% during pregnancy, which allows for the option to decrease, change, or stop current medications, ideally prior to pregnancy. Prevention via use of calcium channel blockers, antihistamines, or β-blockers is recommended, as requiring acute treatments more than 2 days per week increases the risk of medication overuse headaches.
Treating acute headache
For patients who present with an acute headache consistent with their usual type, treatment starts with known medications that are compatible with pregnancy and proceeds in a stepwise fashion:
1. Acetaminophen 1,000 mg orally with or without caffeine 130 mg orally (maximum dose, acetaminophen < 3.25–4 g per day, caffeine 200 mg per day)
2. Metoclopramide 10 mg intravenously with or without diphenhydramine 25 mg intravenously (for nausea and to counteract restlessness and offer sedation)
3. If headache continues after steps 1 and 2, consider the following secondary treatment options: magnesium sulfate 1–2 g intravenously, sumatriptan 6 mg subcutaneously or 20-mg nasal spray, ibuprofen 600 mg orally once, or ketorolac 30 mg intravenously once (second trimester only)
4. If continued treatment and/or hospitalization is required after step 3, steroids can be used: prednisone 20 mg 4 times a day for 2 days or methylprednisolone 4-mg dose pack over 6 days
5. Do not use butalbital, opioids, or ergotamines due to lack of efficacy in providing additional pain relief, potential for addiction, risk of medication overuse headaches, and association with fetal/ pregnancy abnormalities.
Consider secondary headache
An acute headache discordant from the patient’s usual type or with concerning symptoms (“red flags”) requires consideration of secondary headaches as well as a comprehensive symptom evaluation, imaging, and consultation as needed. While secondary headaches postpartum are most likely musculoskeletal in nature, the following symptoms need to be evaluated immediately:
- rapid onset/change from baseline
- “thunderclap” nature
- hypertension
- fever
- focal neurologic deficits (blurry vision or blindness, confusion, seizures)
- altered consciousness
- laboratory abnormalities.
The differential diagnosis includes preeclampsia, reversible cerebral vasoconstriction syndrome (RCVS), posterior reversible encephalopathy syndrome (PRES), infection, cerebral venous sinus thrombosis (CVST), post–dural puncture (PDP) headache, idiopathic intracranial hypertension (IIH), and less likely, carotid dissection, subarachnoid hemorrhage, intracranial hemorrhage, pituitary apoplexy, or neoplasm.
Treatment. Individualized treatment depends on the diagnosis. Preeclampsia with severe features is treated with antihypertensive medication, magnesium sulfate, and delivery planning. PDP headache is treated with epidural blood patch, sphenopalatine block, or occipital block with an anesthesiology consultation. If preeclampsia and PDP are ruled out, or if there are more concerning neurologic features, imaging is essential, as 25% of pregnant patients with acute headaches will have a secondary etiology. Magnetic resonance imaging without contrast is preferred due to concerns about gadolinium crossing the placenta and the lack of data on long-term accumulation in fetal tissues. Once diagnosed on imaging, PRES and RCVS are treated with antihypertensives and delivery. CVST is treated with anticoagulation and a thrombophilia workup. IIH may be treated with acetazolamide after 20 weeks or serial lumbar punctures. Intracranial vascular abnormalities may be treated with endoscopic resection and steroids. ●
Calcium channel blockers and antihistamines are recommended for primary headache prevention.
Acetaminophen, caffeine, diphenhydramine, and metoclopramide administered in a stepwise manner are recommended for acute treatment of primary headache in pregnancy. Nonsteroidal antiinflammatory agents and triptans may be added during lactation and postpartum.
Butalbital and opioids are not recommended for acute treatment of headaches in pregnancy and postpartum due to risk of medication overuse headaches, dependence, and neonatal abstinence syndrome.
“Red flag” headache symptoms warrant imaging, prompt treatment of severe hypertension, and timely treatment of potentially life-threatening intracranial conditions.
- Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2021. NCHS Data Brief, no 442. Hyattsville, MD: National Center for Health Statistics. August 2022. Accessed December 15, 2022. https://dx.doi.org/10.15620 /cdc:119632
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth. ACOG practice bulletin no. 234. Obstet Gynecol. 2021;138:e65-e90.
- EPPPIC Group. Evaluating Progestogens for Preventing Preterm birth International Collaborative (EPPPIC): meta-analysis of individual participant data from randomised controlled trials. Lancet. 2021;397:1183-1194.
- US Food and Drug Administration. Proposal to withdraw approval of Makena; notice of opportunity for a hearing. August 17, 2022. Accessed December 15, 2022. https://www. regulations.gov/docket/FDA-2020-N-2029
- Society for Maternal-Fetal Medicine (SMFM); Ghidini A, Gandhi M, McCoy J, et al; Publications Committee. Society for Maternal-Fetal Medicine consult series #60: management of pregnancies resulting from in vitro fertilization. Am J Obstet Gynecol. 2022;226:B2-B12.
- Society for Maternal-Fetal Medicine; Abu-Rustum RS, Combs CA, Davidson CM, et al; Patient Safety and Quality Committee. Society for Maternal-Fetal Medicine special statement: checklist for pregnancies resulting from in vitro fertilization. Am J Obstet Gynecol. 2022;227:B2-B3.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice; Committee on Genetics; US Food and Drug Administration. Committee opinion no. 671: perinatal risks associated with assisted reproductive technology. Obstet Gynecol. 2016;128:e61-e68.
- American College of Obstetricians and Gynecologists. Committee opinion no. 719: multifetal pregnancy reduction. Obstet Gynecol. 2017;130:e158-e163.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 743: low-dose aspirin use during pregnancy. Obstet Gynecol. 2018;132:e44-e52.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice, Society for Maternal-Fetal Medicine. ACOG committee opinion no. 828: indications for outpatient antenatal fetal surveillance. Obstet Gynecol. 2021;137:e177-e197.
- American College of Obstetricians and Gynecologists. Clinical practice guideline no. 3: headaches in pregnancy and postpartum. Obstet Gynecol. 2022;139:944-972.
- Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2021. NCHS Data Brief, no 442. Hyattsville, MD: National Center for Health Statistics. August 2022. Accessed December 15, 2022. https://dx.doi.org/10.15620 /cdc:119632
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Prediction and prevention of spontaneous preterm birth. ACOG practice bulletin no. 234. Obstet Gynecol. 2021;138:e65-e90.
- EPPPIC Group. Evaluating Progestogens for Preventing Preterm birth International Collaborative (EPPPIC): meta-analysis of individual participant data from randomised controlled trials. Lancet. 2021;397:1183-1194.
- US Food and Drug Administration. Proposal to withdraw approval of Makena; notice of opportunity for a hearing. August 17, 2022. Accessed December 15, 2022. https://www. regulations.gov/docket/FDA-2020-N-2029
- Society for Maternal-Fetal Medicine (SMFM); Ghidini A, Gandhi M, McCoy J, et al; Publications Committee. Society for Maternal-Fetal Medicine consult series #60: management of pregnancies resulting from in vitro fertilization. Am J Obstet Gynecol. 2022;226:B2-B12.
- Society for Maternal-Fetal Medicine; Abu-Rustum RS, Combs CA, Davidson CM, et al; Patient Safety and Quality Committee. Society for Maternal-Fetal Medicine special statement: checklist for pregnancies resulting from in vitro fertilization. Am J Obstet Gynecol. 2022;227:B2-B3.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice; Committee on Genetics; US Food and Drug Administration. Committee opinion no. 671: perinatal risks associated with assisted reproductive technology. Obstet Gynecol. 2016;128:e61-e68.
- American College of Obstetricians and Gynecologists. Committee opinion no. 719: multifetal pregnancy reduction. Obstet Gynecol. 2017;130:e158-e163.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 743: low-dose aspirin use during pregnancy. Obstet Gynecol. 2018;132:e44-e52.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice, Society for Maternal-Fetal Medicine. ACOG committee opinion no. 828: indications for outpatient antenatal fetal surveillance. Obstet Gynecol. 2021;137:e177-e197.
- American College of Obstetricians and Gynecologists. Clinical practice guideline no. 3: headaches in pregnancy and postpartum. Obstet Gynecol. 2022;139:944-972.
Complaints of mass in breast
The history and findings in this case are consistent with advanced infiltrating ductal carcinoma.
Breast cancer is the most commonly diagnosed life-threatening cancer and the leading cause of cancer death among women worldwide. In the United States, it is estimated that 287,850 new cases of invasive breast cancer were diagnosed in 2022; in addition, 43,250 deaths because of breast cancer are expected to occur.
Infiltrating ductal carcinoma is the most common type of breast tumor. It accounts for 75% of breast cancers and has a propensity to metastasize via lymphatic vessels. This lesion has no specific histologic characteristics apart from invasion through the basement membrane. This enables it to be differentiated from ductal carcinoma in situ, which remains inside the duct.
All newly diagnosed cases of invasive breast cancers are tested for estrogen receptors, progesterone receptors, and HER2 status. The presence of estrogen and progesterone receptors is tested by immunohistochemistry, whereas HER2 can be tested by either immunohistochemistry or in situ hybridization (usually fluorescent or fluorescence in situ hybridization). Testing results have important treatment implications and prognostic significance.
Surgical treatment of invasive breast cancer consists of either lumpectomy or total mastectomy, followed by radiation therapy. Surgical resection is performed in all patients with nonmetastatic disease.
Systemic therapy options include endocrine therapy, cytotoxic chemotherapy, targeted therapy, and immunotherapy. The choice of systemic therapy is largely determined by subtype. For example, patients with hormone receptor–positive tumors receive endocrine therapy; a minority of these patients may receive chemotherapy as well. Patients with HER2-positive tumors receive HER2–targeted antibody or small-molecule inhibitor therapy combined with chemotherapy. For patients with triple-negative tumors, chemotherapy alone is often used; newer targeted therapies however may improve outcomes. In both the adjuvant and neoadjuvant setting, the primary goal of treatment is to eradicate or control undiscovered distant metastases. In the metastatic setting, the primary goal of treatment is to extend life and alleviate symptoms.
Depending on the individual patient, systemic therapy may be used in the adjuvant or neoadjuvant setting. Systemic therapy is chosen according to breast cancer subtype and recurrence risk, with the treatment for low-risk patients being de-escalated, whereas high-risk patients receive aggressive systemic treatment. When systemic therapy is used in the neoadjuvant setting, treatment response is the most important factor for predicting outcomes and selecting the optimal adjuvant therapy. Novel biological markers enable the selection of appropriate targeted therapy, which can achieve optimal efficacy.
Up-to-date, evidence-based recommendations for pre- and postoperative treatment of breast cancer, including advanced breast cancer, are provided by the National Comprehensive Cancer Network.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are consistent with advanced infiltrating ductal carcinoma.
Breast cancer is the most commonly diagnosed life-threatening cancer and the leading cause of cancer death among women worldwide. In the United States, it is estimated that 287,850 new cases of invasive breast cancer were diagnosed in 2022; in addition, 43,250 deaths because of breast cancer are expected to occur.
Infiltrating ductal carcinoma is the most common type of breast tumor. It accounts for 75% of breast cancers and has a propensity to metastasize via lymphatic vessels. This lesion has no specific histologic characteristics apart from invasion through the basement membrane. This enables it to be differentiated from ductal carcinoma in situ, which remains inside the duct.
All newly diagnosed cases of invasive breast cancers are tested for estrogen receptors, progesterone receptors, and HER2 status. The presence of estrogen and progesterone receptors is tested by immunohistochemistry, whereas HER2 can be tested by either immunohistochemistry or in situ hybridization (usually fluorescent or fluorescence in situ hybridization). Testing results have important treatment implications and prognostic significance.
Surgical treatment of invasive breast cancer consists of either lumpectomy or total mastectomy, followed by radiation therapy. Surgical resection is performed in all patients with nonmetastatic disease.
Systemic therapy options include endocrine therapy, cytotoxic chemotherapy, targeted therapy, and immunotherapy. The choice of systemic therapy is largely determined by subtype. For example, patients with hormone receptor–positive tumors receive endocrine therapy; a minority of these patients may receive chemotherapy as well. Patients with HER2-positive tumors receive HER2–targeted antibody or small-molecule inhibitor therapy combined with chemotherapy. For patients with triple-negative tumors, chemotherapy alone is often used; newer targeted therapies however may improve outcomes. In both the adjuvant and neoadjuvant setting, the primary goal of treatment is to eradicate or control undiscovered distant metastases. In the metastatic setting, the primary goal of treatment is to extend life and alleviate symptoms.
Depending on the individual patient, systemic therapy may be used in the adjuvant or neoadjuvant setting. Systemic therapy is chosen according to breast cancer subtype and recurrence risk, with the treatment for low-risk patients being de-escalated, whereas high-risk patients receive aggressive systemic treatment. When systemic therapy is used in the neoadjuvant setting, treatment response is the most important factor for predicting outcomes and selecting the optimal adjuvant therapy. Novel biological markers enable the selection of appropriate targeted therapy, which can achieve optimal efficacy.
Up-to-date, evidence-based recommendations for pre- and postoperative treatment of breast cancer, including advanced breast cancer, are provided by the National Comprehensive Cancer Network.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are consistent with advanced infiltrating ductal carcinoma.
Breast cancer is the most commonly diagnosed life-threatening cancer and the leading cause of cancer death among women worldwide. In the United States, it is estimated that 287,850 new cases of invasive breast cancer were diagnosed in 2022; in addition, 43,250 deaths because of breast cancer are expected to occur.
Infiltrating ductal carcinoma is the most common type of breast tumor. It accounts for 75% of breast cancers and has a propensity to metastasize via lymphatic vessels. This lesion has no specific histologic characteristics apart from invasion through the basement membrane. This enables it to be differentiated from ductal carcinoma in situ, which remains inside the duct.
All newly diagnosed cases of invasive breast cancers are tested for estrogen receptors, progesterone receptors, and HER2 status. The presence of estrogen and progesterone receptors is tested by immunohistochemistry, whereas HER2 can be tested by either immunohistochemistry or in situ hybridization (usually fluorescent or fluorescence in situ hybridization). Testing results have important treatment implications and prognostic significance.
Surgical treatment of invasive breast cancer consists of either lumpectomy or total mastectomy, followed by radiation therapy. Surgical resection is performed in all patients with nonmetastatic disease.
Systemic therapy options include endocrine therapy, cytotoxic chemotherapy, targeted therapy, and immunotherapy. The choice of systemic therapy is largely determined by subtype. For example, patients with hormone receptor–positive tumors receive endocrine therapy; a minority of these patients may receive chemotherapy as well. Patients with HER2-positive tumors receive HER2–targeted antibody or small-molecule inhibitor therapy combined with chemotherapy. For patients with triple-negative tumors, chemotherapy alone is often used; newer targeted therapies however may improve outcomes. In both the adjuvant and neoadjuvant setting, the primary goal of treatment is to eradicate or control undiscovered distant metastases. In the metastatic setting, the primary goal of treatment is to extend life and alleviate symptoms.
Depending on the individual patient, systemic therapy may be used in the adjuvant or neoadjuvant setting. Systemic therapy is chosen according to breast cancer subtype and recurrence risk, with the treatment for low-risk patients being de-escalated, whereas high-risk patients receive aggressive systemic treatment. When systemic therapy is used in the neoadjuvant setting, treatment response is the most important factor for predicting outcomes and selecting the optimal adjuvant therapy. Novel biological markers enable the selection of appropriate targeted therapy, which can achieve optimal efficacy.
Up-to-date, evidence-based recommendations for pre- and postoperative treatment of breast cancer, including advanced breast cancer, are provided by the National Comprehensive Cancer Network.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 40-year-old woman presents with a left breast mass that she discovered while showering. The patient has no significant medical history and does not take any medications. She has not yet undergone any routine mammography screening. Clinical breast examination reveals a large palpable immobile mass in the inner quadrant of the left breast. The nipple is retracted. Left axial lymphadenopathy is also detected. There are no palpable abnormalities in the right breast. The remainder of the physical examination is unremarkable. Laboratory findings are all within normal range, apart from C-reactive protein, which is elevated (8 mg/L). The patient is 5 ft 4 in and weighs 142 lb.
The patient is sent for a mammogram and needle biopsy of the mass and axial lymph nodes. Mammogram findings include an irregular mass 4.2 cm in size in the left breast with heterogeneous echotexture, abrupt interface, and intramass calcifications as well as overlying skin thickening. The mass is predominately in the inner left breast and is extending into the outside quadrant. Multiple morphologically abnormal lymph nodes are noted in the left axilla. Biopsy results include atypical glands infiltrating the surrounding stroma in an irregular pattern. Immunohistochemistry results show p53 expression.
Characteristics of Matched vs Nonmatched Dermatology Applicants
Dermatology residency continues to be one of the most competitive specialties, with a match rate of 84.7% for US allopathic seniors in the 2019-2020 academic year.1 In the 2019-2020 cycle, dermatology applicants were tied with plastic surgery for the highest median US Medical Licensing Examination (USMLE) Step 1 score compared with other specialties, which suggests that the top medical students are applying, yet only approximately 5 of 6 students are matching.
Factors that have been cited with successful dermatology matching include USMLE Step 1 and Step 2 Clinical Knowledge (CK) scores,2 research accomplishments,3 letters of recommendation,4 medical school performance, personal statement, grades in required clerkships, and volunteer/extracurricular experiences, among others.5
The National Resident Matching Program (NRMP) publishes data each year regarding different academic factors—USMLE scores; number of abstracts, presentations, and papers; work, volunteer, and research experiences—and compares the mean between matched and nonmatched applicants.1 However, the USMLE does not report any demographic information of the applicants and the implication it has for matching. Additionally, the number of couples participating in the couples match continues to increase each year. In the 2019-2020 cycle, 1224 couples participated in the couples match.1 However, NRMP reports only limited data regarding the couples match, and it is not specialty specific.
We aimed to determine the characteristics of matched vs nonmatched dermatology applicants. Secondarily, we aimed to determine any differences among demographics regarding matching rates, academic performance, and research publications. We also aimed to characterize the strategy and outcomes of applicants that couples matched.
Materials and Methods
The Mayo Clinic institutional review board deemed this study exempt. All applicants who applied to Mayo Clinic dermatology residency in Scottsdale, Arizona, during the 2018-2019 cycle were emailed an initial survey (N=475) before Match Day that obtained demographic information, geographic information, gap-year information, USMLE Step 1 score, publications, medical school grades, number of away rotations, and number of interviews. A follow-up survey gathering match data and couples matching data was sent to the applicants who completed the first survey on Match Day. The survey was repeated for the 2019-2020 cycle. In the second survey, Step 2 CK data were obtained. The survey was sent to 629 applicants who applied to Mayo Clinic dermatology residencies in Arizona, Minnesota, and Florida to include a broader group of applicants. For publications, applicants were asked to count only published or accepted manuscripts, not abstracts, posters, conference presentations, or submitted manuscripts. Applicants who did not respond to the second survey (match data) were not included in that part of the analysis. One survey was excluded because of implausible answers (eg, scores outside of range for USMLE Step scores).
Statistical Analysis—For statistical analyses, the applicants from both applications cycles were combined. Descriptive statistics were reported in the form of mean, median, or counts (percentages), as applicable. Means were compared using 2-sided t tests. Group comparisons were examined using χ2 tests for categorical variables. Statistical analyses were performed using the BlueSky Statistics version 6.30. P<.05 was considered significant.
Results
In 2019, a total of 149 applicants completed the initial survey (31.4% response rate), and 112 completed the follow-up survey (75.2% response rate). In 2020, a total of 142 applicants completed the initial survey (22.6% response rate), and 124 completed the follow-up survey (87.3% response rate). Combining the 2 years, after removing 1 survey with implausible answers, there were 290 respondents from the initial survey and 235 from the follow-up survey. The median (SD) age for the total applicants over both years was 27 (3.0) years, and 180 applicants were female (61.9%).
USMLE Scores—The median USMLE Step 1 score was 250, and scores ranged from 196 to 271. The median USMLE Step 2 CK score was 257, and scores ranged from 213 to 281. Higher USMLE Step 1 and Step 2 CK scores and more interviews were associated with higher match rates (Table 1). In addition, students with a dermatology program at their medical school were more likely to match than those without a home dermatology program.
Gender Differences—There were 180 females and 110 males who completed the surveys. Males and females had similar match rates (85.2% vs 89.0%; P=.39)(Table 2).
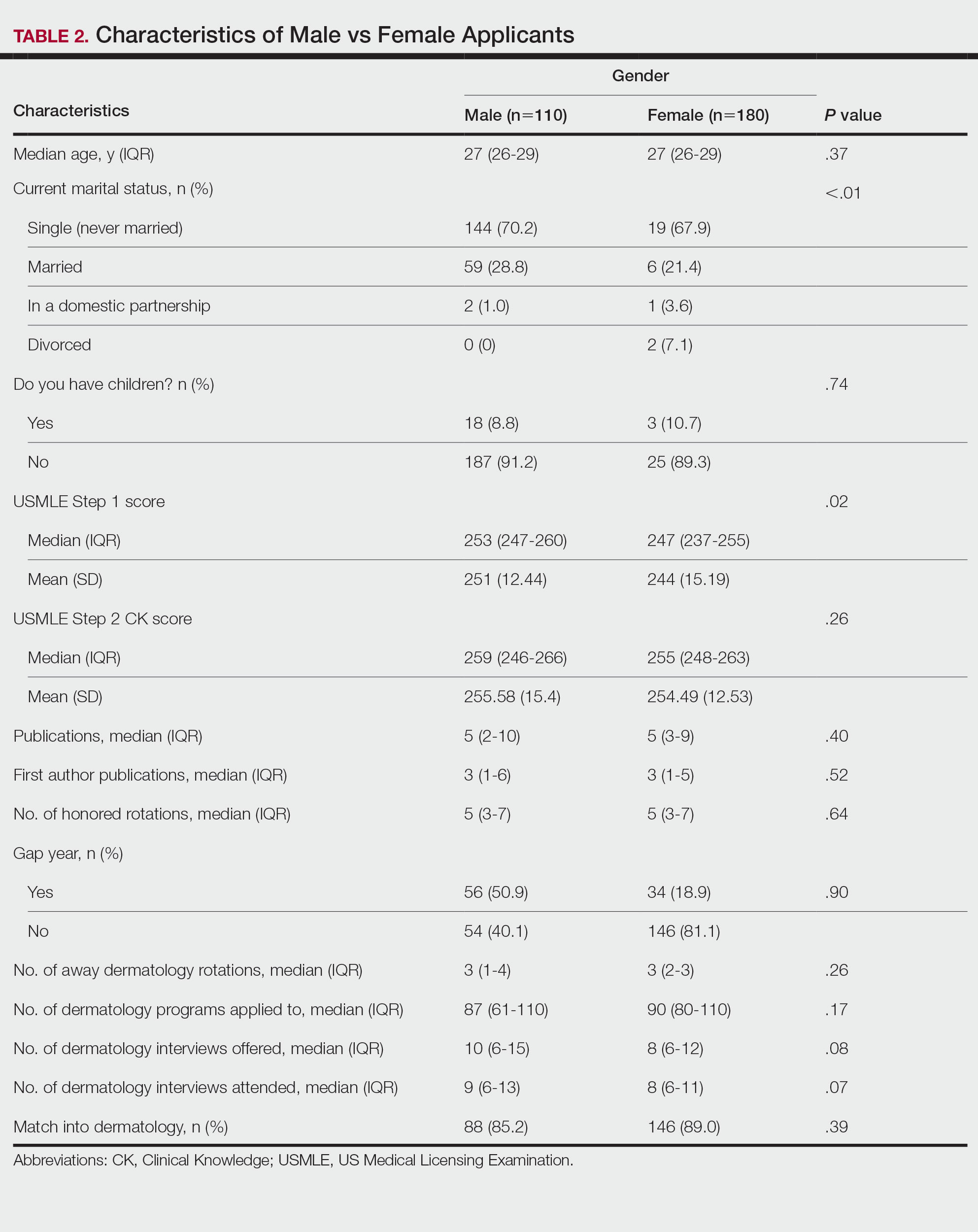
Family Life—In comparing marital status, applicants who were divorced had a higher median age (38.5 years) compared with applicants who were single, married, or in a domestic partnership (all 27 years; P<.01). Differences are outlined in Table 3.
On average, applicants with children (n=27 [15 male, 12 female]; P=.13) were 3 years older than those without (30.5 vs 27; P<.01) and were more likely to be married (88.9% vs 21.5%; P<.01). Applicants with children had a mean USMLE Step 1 score of 241 compared to 251 for those without children (P=.02) and a mean USMLE Step 2 CK score of 246 compared to 258 for those without children (P<.01). Applicants with children had similar debt, number of publications, number of honored rotations, and match rates compared to applicants without children (Figure).
Couples Match—Seventeen individuals in our survey participated in the couples match (7.8%), and all 17 (100%) matched into dermatology. The mean age was 26.7 years, 12 applicants were female, 2 applicants were married, and 1 applicant had children. The mean number of interviews offered was 13.6, and the mean number of interviews attended was 11.3. This was higher than participants who were not couples matching (13.6 vs 9.8 [P=.02] and 11.3 vs 8.9 [P=.04], respectively). Applicants and their partners applied to programs and received interviews in a mean of 10 cities. Sixteen applicants reported that they contacted programs where their partner had interview offers. All participants’ rank lists included programs located in different cities than their partners’ ranked programs, and all but 1 participant ranked programs located in a different state than their partners’ ranked programs. Fifteen participants had options in their rank list for the applicant not to match, even if the partner would match. Similarly, 12 had the option for the applicant to match, even if the partner would not match. Fourteen (82.4%) matched at the same institution as their significant other. Three (17.6%) applicants matched to a program in a different state than the partner’s matched program. Two (11.8%) participants felt their relationship with their partner suffered because of the match, and 1 (5.9%) applicant was undetermined. One applicant described their relationship suffering from “unnecessary tension and anxiety” and noted “difficult conversations” about potentially matching into dermatology in a different location from their partner that could have been “devastating and not something [he or she] should have to choose.”
Comment
Factors for Matching in Dermatology—In our survey, we found the statistically significant factors of matching into dermatology included high USMLE Step 1 and Step 2 CK scores (P<.01), having a home dermatology program (P=.04), and attending a higher number of dermatology interviews (P<.01). These data are similar to NRMP results1; however, the higher likelihood of matching if the medical school has a home dermatology program has not been reported. This finding could be due to multiple factors such as students have less access to academic dermatologists for research projects, letters of recommendations, mentorship, and clinical rotations.
Gender and having children were factors that had no correlation with the match rate. There was a statistical difference of matching based on marital status (P<.01), but this is likely due to the low number of applicants in the divorced category. There were differences among demographics with USMLE Step 1 and Step 2 CK scores, which is a known factor in matching.1,2 Applicants with children had lower USMLE Step 1 and Step 2 CK scores compared to applicants without children. Females also had lower median USMLE Step 1 scores compared to males. This finding may serve as a reminder to programs when comparing USMLE Step examination scores that demographic factors may play a role. The race and ethnicity of applicants likely play a role. It has been reported that underrepresented minorities had lower match rates than White and Asian applicants in dermatology.6 There have been several published articles discussing the lack of diversity in dermatology, with a call to action.7-9
Factors for Couples Matching—The number of applicants participating in the couples match continues to increase yearly. The NMRP does publish data regarding “successful” couples matching but does not specify how many couples match together. There also is little published regarding advice for participation in the couples match. Although we had a limited number of couples that participated in the match, it is interesting to note they had similar strategies, including contacting programs at institutions that had offered interviews to their partners. This strategy may be effective, as dermatology programs offer interviews relatively late compared with other specialties.5 Additionally, this strategy may increase the number of interviews offered and received, as evidenced by the higher number of interviews offered compared with those who were not couples matching. Additionally, this survey highlights the sacrifice often needed by couples in the couples match as revealed by the inclusion of rank-list options in which the couples reside long distance or in which 1 partner does not match. This information may be helpful to applicants who are planning a strategy for the couples match in dermatology. Although this study does not encompass all dermatology applicants in the 2019-2020 cycle, we do believe it may be representative. The USMLE Step 1 scores in this study were similar to the published NRMP data.1,10 According to NRMP data from the 2019-2020 cycle, the mean USMLE Step 1 score was 248 for matched applicants and 239 for unmatched.1 The NRMP reported the mean USMLE Step 2 CK score for matched was 256 and 248 for unmatched, which also is similar to our data. The NRMP reported the mean number of programs ranked was 9.9 for matched and 4.5 for unmatched applicants.1 Again, our data were similar for number of dermatology interviews attended.
Limitations—There are limitations to this study. The main limitation is that the survey is from a single institution and had a limited number of respondents. Given the nature of the study, the accuracy of the data is dependent on the applicants’ honesty in self-reporting academic performance and other variables. There also may be a selection bias given the low response rate. The subanalyses—children and couples matching—were underpowered with the limited number of participants. Further studies that include multiple residency programs and multiple years could be helpful to provide more power and less risk of bias. We did not gather information such as the Medical Student Performance Evaluation letter, letters of recommendation, or personal statements, which do play an important role in the assessment of an applicant. However, because the applicants completed these surveys, and given these are largely blinded to applicants, we did not feel the applicants could accurately respond to those aspects of the application.
Conclusion
Our survey finds that factors associated with matching included a higher USMLE Step 1 score, having a home dermatology program, and a higher number of interviews offered and attended. Some demographics had varying USMLE Step 1 scores but similar match rates.
- National Resident Matching Program. Results and Data: 2020 Main Residency Match. National Resident Matching Program; May 2020. Accessed January 9, 2023. https://www.nrmp.org/wp-content/uploads/2021/12/MM_Results_and-Data_2020-1.pdf
- Gauer JL, Jackson JB. The association of USMLE Step 1 and Step 2 CK scores with residency match specialty and location. Med Educ Online. 2017;22:1358579.
- Wang JV, Keller M. Pressure to publish for residency applicants in dermatology. Dermatol Online J. 2016;22:13030/qt56x1t7ww.
- Wang RF, Zhang M, Kaffenberger JA. Does the dermatology standardized letter of recommendation alter applicants’ chances of matching into residency. J Am Acad Dermatol. 2017;77:e139-e140.
- National Resident Matching Program, Data Release and Research Committee: results of the 2018 NRMP Program Director Survey. Accessed December 19, 2022. https://www.nrmp.org/wp-content/uploads/2021/07/NRMP-2018-Program-Director-Survey-for-WWW.pdf
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role of race and ethnicity in the dermatology applicant match process. J Natl Med Assoc. 2022;113:666-670.
- Chen A, Shinkai K. Rethinking how we select dermatology applicants-turning the tide. JAMA Dermatol. 2017;153:259-260.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49.
- National Resident Matching Program. Charting outcomes in the match: U.S. allopathic seniors. Characteristics of U.S. allopathic seniors who matched to their preferred specialty in the 2018 main residency match. 2nd ed. Accessed December 19, 2022. https://www.nrmp.org/wp-content/uploads/2021/07/Charting-Outcomes-in-the-Match-2018_Seniors-1.pdf
Dermatology residency continues to be one of the most competitive specialties, with a match rate of 84.7% for US allopathic seniors in the 2019-2020 academic year.1 In the 2019-2020 cycle, dermatology applicants were tied with plastic surgery for the highest median US Medical Licensing Examination (USMLE) Step 1 score compared with other specialties, which suggests that the top medical students are applying, yet only approximately 5 of 6 students are matching.
Factors that have been cited with successful dermatology matching include USMLE Step 1 and Step 2 Clinical Knowledge (CK) scores,2 research accomplishments,3 letters of recommendation,4 medical school performance, personal statement, grades in required clerkships, and volunteer/extracurricular experiences, among others.5
The National Resident Matching Program (NRMP) publishes data each year regarding different academic factors—USMLE scores; number of abstracts, presentations, and papers; work, volunteer, and research experiences—and compares the mean between matched and nonmatched applicants.1 However, the USMLE does not report any demographic information of the applicants and the implication it has for matching. Additionally, the number of couples participating in the couples match continues to increase each year. In the 2019-2020 cycle, 1224 couples participated in the couples match.1 However, NRMP reports only limited data regarding the couples match, and it is not specialty specific.
We aimed to determine the characteristics of matched vs nonmatched dermatology applicants. Secondarily, we aimed to determine any differences among demographics regarding matching rates, academic performance, and research publications. We also aimed to characterize the strategy and outcomes of applicants that couples matched.
Materials and Methods
The Mayo Clinic institutional review board deemed this study exempt. All applicants who applied to Mayo Clinic dermatology residency in Scottsdale, Arizona, during the 2018-2019 cycle were emailed an initial survey (N=475) before Match Day that obtained demographic information, geographic information, gap-year information, USMLE Step 1 score, publications, medical school grades, number of away rotations, and number of interviews. A follow-up survey gathering match data and couples matching data was sent to the applicants who completed the first survey on Match Day. The survey was repeated for the 2019-2020 cycle. In the second survey, Step 2 CK data were obtained. The survey was sent to 629 applicants who applied to Mayo Clinic dermatology residencies in Arizona, Minnesota, and Florida to include a broader group of applicants. For publications, applicants were asked to count only published or accepted manuscripts, not abstracts, posters, conference presentations, or submitted manuscripts. Applicants who did not respond to the second survey (match data) were not included in that part of the analysis. One survey was excluded because of implausible answers (eg, scores outside of range for USMLE Step scores).
Statistical Analysis—For statistical analyses, the applicants from both applications cycles were combined. Descriptive statistics were reported in the form of mean, median, or counts (percentages), as applicable. Means were compared using 2-sided t tests. Group comparisons were examined using χ2 tests for categorical variables. Statistical analyses were performed using the BlueSky Statistics version 6.30. P<.05 was considered significant.
Results
In 2019, a total of 149 applicants completed the initial survey (31.4% response rate), and 112 completed the follow-up survey (75.2% response rate). In 2020, a total of 142 applicants completed the initial survey (22.6% response rate), and 124 completed the follow-up survey (87.3% response rate). Combining the 2 years, after removing 1 survey with implausible answers, there were 290 respondents from the initial survey and 235 from the follow-up survey. The median (SD) age for the total applicants over both years was 27 (3.0) years, and 180 applicants were female (61.9%).
USMLE Scores—The median USMLE Step 1 score was 250, and scores ranged from 196 to 271. The median USMLE Step 2 CK score was 257, and scores ranged from 213 to 281. Higher USMLE Step 1 and Step 2 CK scores and more interviews were associated with higher match rates (Table 1). In addition, students with a dermatology program at their medical school were more likely to match than those without a home dermatology program.


Gender Differences—There were 180 females and 110 males who completed the surveys. Males and females had similar match rates (85.2% vs 89.0%; P=.39)(Table 2).

Family Life—In comparing marital status, applicants who were divorced had a higher median age (38.5 years) compared with applicants who were single, married, or in a domestic partnership (all 27 years; P<.01). Differences are outlined in Table 3.


On average, applicants with children (n=27 [15 male, 12 female]; P=.13) were 3 years older than those without (30.5 vs 27; P<.01) and were more likely to be married (88.9% vs 21.5%; P<.01). Applicants with children had a mean USMLE Step 1 score of 241 compared to 251 for those without children (P=.02) and a mean USMLE Step 2 CK score of 246 compared to 258 for those without children (P<.01). Applicants with children had similar debt, number of publications, number of honored rotations, and match rates compared to applicants without children (Figure).

Couples Match—Seventeen individuals in our survey participated in the couples match (7.8%), and all 17 (100%) matched into dermatology. The mean age was 26.7 years, 12 applicants were female, 2 applicants were married, and 1 applicant had children. The mean number of interviews offered was 13.6, and the mean number of interviews attended was 11.3. This was higher than participants who were not couples matching (13.6 vs 9.8 [P=.02] and 11.3 vs 8.9 [P=.04], respectively). Applicants and their partners applied to programs and received interviews in a mean of 10 cities. Sixteen applicants reported that they contacted programs where their partner had interview offers. All participants’ rank lists included programs located in different cities than their partners’ ranked programs, and all but 1 participant ranked programs located in a different state than their partners’ ranked programs. Fifteen participants had options in their rank list for the applicant not to match, even if the partner would match. Similarly, 12 had the option for the applicant to match, even if the partner would not match. Fourteen (82.4%) matched at the same institution as their significant other. Three (17.6%) applicants matched to a program in a different state than the partner’s matched program. Two (11.8%) participants felt their relationship with their partner suffered because of the match, and 1 (5.9%) applicant was undetermined. One applicant described their relationship suffering from “unnecessary tension and anxiety” and noted “difficult conversations” about potentially matching into dermatology in a different location from their partner that could have been “devastating and not something [he or she] should have to choose.”
Comment
Factors for Matching in Dermatology—In our survey, we found the statistically significant factors of matching into dermatology included high USMLE Step 1 and Step 2 CK scores (P<.01), having a home dermatology program (P=.04), and attending a higher number of dermatology interviews (P<.01). These data are similar to NRMP results1; however, the higher likelihood of matching if the medical school has a home dermatology program has not been reported. This finding could be due to multiple factors such as students have less access to academic dermatologists for research projects, letters of recommendations, mentorship, and clinical rotations.
Gender and having children were factors that had no correlation with the match rate. There was a statistical difference of matching based on marital status (P<.01), but this is likely due to the low number of applicants in the divorced category. There were differences among demographics with USMLE Step 1 and Step 2 CK scores, which is a known factor in matching.1,2 Applicants with children had lower USMLE Step 1 and Step 2 CK scores compared to applicants without children. Females also had lower median USMLE Step 1 scores compared to males. This finding may serve as a reminder to programs when comparing USMLE Step examination scores that demographic factors may play a role. The race and ethnicity of applicants likely play a role. It has been reported that underrepresented minorities had lower match rates than White and Asian applicants in dermatology.6 There have been several published articles discussing the lack of diversity in dermatology, with a call to action.7-9
Factors for Couples Matching—The number of applicants participating in the couples match continues to increase yearly. The NMRP does publish data regarding “successful” couples matching but does not specify how many couples match together. There also is little published regarding advice for participation in the couples match. Although we had a limited number of couples that participated in the match, it is interesting to note they had similar strategies, including contacting programs at institutions that had offered interviews to their partners. This strategy may be effective, as dermatology programs offer interviews relatively late compared with other specialties.5 Additionally, this strategy may increase the number of interviews offered and received, as evidenced by the higher number of interviews offered compared with those who were not couples matching. Additionally, this survey highlights the sacrifice often needed by couples in the couples match as revealed by the inclusion of rank-list options in which the couples reside long distance or in which 1 partner does not match. This information may be helpful to applicants who are planning a strategy for the couples match in dermatology. Although this study does not encompass all dermatology applicants in the 2019-2020 cycle, we do believe it may be representative. The USMLE Step 1 scores in this study were similar to the published NRMP data.1,10 According to NRMP data from the 2019-2020 cycle, the mean USMLE Step 1 score was 248 for matched applicants and 239 for unmatched.1 The NRMP reported the mean USMLE Step 2 CK score for matched was 256 and 248 for unmatched, which also is similar to our data. The NRMP reported the mean number of programs ranked was 9.9 for matched and 4.5 for unmatched applicants.1 Again, our data were similar for number of dermatology interviews attended.
Limitations—There are limitations to this study. The main limitation is that the survey is from a single institution and had a limited number of respondents. Given the nature of the study, the accuracy of the data is dependent on the applicants’ honesty in self-reporting academic performance and other variables. There also may be a selection bias given the low response rate. The subanalyses—children and couples matching—were underpowered with the limited number of participants. Further studies that include multiple residency programs and multiple years could be helpful to provide more power and less risk of bias. We did not gather information such as the Medical Student Performance Evaluation letter, letters of recommendation, or personal statements, which do play an important role in the assessment of an applicant. However, because the applicants completed these surveys, and given these are largely blinded to applicants, we did not feel the applicants could accurately respond to those aspects of the application.
Conclusion
Our survey finds that factors associated with matching included a higher USMLE Step 1 score, having a home dermatology program, and a higher number of interviews offered and attended. Some demographics had varying USMLE Step 1 scores but similar match rates.
Dermatology residency continues to be one of the most competitive specialties, with a match rate of 84.7% for US allopathic seniors in the 2019-2020 academic year.1 In the 2019-2020 cycle, dermatology applicants were tied with plastic surgery for the highest median US Medical Licensing Examination (USMLE) Step 1 score compared with other specialties, which suggests that the top medical students are applying, yet only approximately 5 of 6 students are matching.
Factors that have been cited with successful dermatology matching include USMLE Step 1 and Step 2 Clinical Knowledge (CK) scores,2 research accomplishments,3 letters of recommendation,4 medical school performance, personal statement, grades in required clerkships, and volunteer/extracurricular experiences, among others.5
The National Resident Matching Program (NRMP) publishes data each year regarding different academic factors—USMLE scores; number of abstracts, presentations, and papers; work, volunteer, and research experiences—and compares the mean between matched and nonmatched applicants.1 However, the USMLE does not report any demographic information of the applicants and the implication it has for matching. Additionally, the number of couples participating in the couples match continues to increase each year. In the 2019-2020 cycle, 1224 couples participated in the couples match.1 However, NRMP reports only limited data regarding the couples match, and it is not specialty specific.
We aimed to determine the characteristics of matched vs nonmatched dermatology applicants. Secondarily, we aimed to determine any differences among demographics regarding matching rates, academic performance, and research publications. We also aimed to characterize the strategy and outcomes of applicants that couples matched.
Materials and Methods
The Mayo Clinic institutional review board deemed this study exempt. All applicants who applied to Mayo Clinic dermatology residency in Scottsdale, Arizona, during the 2018-2019 cycle were emailed an initial survey (N=475) before Match Day that obtained demographic information, geographic information, gap-year information, USMLE Step 1 score, publications, medical school grades, number of away rotations, and number of interviews. A follow-up survey gathering match data and couples matching data was sent to the applicants who completed the first survey on Match Day. The survey was repeated for the 2019-2020 cycle. In the second survey, Step 2 CK data were obtained. The survey was sent to 629 applicants who applied to Mayo Clinic dermatology residencies in Arizona, Minnesota, and Florida to include a broader group of applicants. For publications, applicants were asked to count only published or accepted manuscripts, not abstracts, posters, conference presentations, or submitted manuscripts. Applicants who did not respond to the second survey (match data) were not included in that part of the analysis. One survey was excluded because of implausible answers (eg, scores outside of range for USMLE Step scores).
Statistical Analysis—For statistical analyses, the applicants from both applications cycles were combined. Descriptive statistics were reported in the form of mean, median, or counts (percentages), as applicable. Means were compared using 2-sided t tests. Group comparisons were examined using χ2 tests for categorical variables. Statistical analyses were performed using the BlueSky Statistics version 6.30. P<.05 was considered significant.
Results
In 2019, a total of 149 applicants completed the initial survey (31.4% response rate), and 112 completed the follow-up survey (75.2% response rate). In 2020, a total of 142 applicants completed the initial survey (22.6% response rate), and 124 completed the follow-up survey (87.3% response rate). Combining the 2 years, after removing 1 survey with implausible answers, there were 290 respondents from the initial survey and 235 from the follow-up survey. The median (SD) age for the total applicants over both years was 27 (3.0) years, and 180 applicants were female (61.9%).
USMLE Scores—The median USMLE Step 1 score was 250, and scores ranged from 196 to 271. The median USMLE Step 2 CK score was 257, and scores ranged from 213 to 281. Higher USMLE Step 1 and Step 2 CK scores and more interviews were associated with higher match rates (Table 1). In addition, students with a dermatology program at their medical school were more likely to match than those without a home dermatology program.


Gender Differences—There were 180 females and 110 males who completed the surveys. Males and females had similar match rates (85.2% vs 89.0%; P=.39)(Table 2).

Family Life—In comparing marital status, applicants who were divorced had a higher median age (38.5 years) compared with applicants who were single, married, or in a domestic partnership (all 27 years; P<.01). Differences are outlined in Table 3.


On average, applicants with children (n=27 [15 male, 12 female]; P=.13) were 3 years older than those without (30.5 vs 27; P<.01) and were more likely to be married (88.9% vs 21.5%; P<.01). Applicants with children had a mean USMLE Step 1 score of 241 compared to 251 for those without children (P=.02) and a mean USMLE Step 2 CK score of 246 compared to 258 for those without children (P<.01). Applicants with children had similar debt, number of publications, number of honored rotations, and match rates compared to applicants without children (Figure).

Couples Match—Seventeen individuals in our survey participated in the couples match (7.8%), and all 17 (100%) matched into dermatology. The mean age was 26.7 years, 12 applicants were female, 2 applicants were married, and 1 applicant had children. The mean number of interviews offered was 13.6, and the mean number of interviews attended was 11.3. This was higher than participants who were not couples matching (13.6 vs 9.8 [P=.02] and 11.3 vs 8.9 [P=.04], respectively). Applicants and their partners applied to programs and received interviews in a mean of 10 cities. Sixteen applicants reported that they contacted programs where their partner had interview offers. All participants’ rank lists included programs located in different cities than their partners’ ranked programs, and all but 1 participant ranked programs located in a different state than their partners’ ranked programs. Fifteen participants had options in their rank list for the applicant not to match, even if the partner would match. Similarly, 12 had the option for the applicant to match, even if the partner would not match. Fourteen (82.4%) matched at the same institution as their significant other. Three (17.6%) applicants matched to a program in a different state than the partner’s matched program. Two (11.8%) participants felt their relationship with their partner suffered because of the match, and 1 (5.9%) applicant was undetermined. One applicant described their relationship suffering from “unnecessary tension and anxiety” and noted “difficult conversations” about potentially matching into dermatology in a different location from their partner that could have been “devastating and not something [he or she] should have to choose.”
Comment
Factors for Matching in Dermatology—In our survey, we found the statistically significant factors of matching into dermatology included high USMLE Step 1 and Step 2 CK scores (P<.01), having a home dermatology program (P=.04), and attending a higher number of dermatology interviews (P<.01). These data are similar to NRMP results1; however, the higher likelihood of matching if the medical school has a home dermatology program has not been reported. This finding could be due to multiple factors such as students have less access to academic dermatologists for research projects, letters of recommendations, mentorship, and clinical rotations.
Gender and having children were factors that had no correlation with the match rate. There was a statistical difference of matching based on marital status (P<.01), but this is likely due to the low number of applicants in the divorced category. There were differences among demographics with USMLE Step 1 and Step 2 CK scores, which is a known factor in matching.1,2 Applicants with children had lower USMLE Step 1 and Step 2 CK scores compared to applicants without children. Females also had lower median USMLE Step 1 scores compared to males. This finding may serve as a reminder to programs when comparing USMLE Step examination scores that demographic factors may play a role. The race and ethnicity of applicants likely play a role. It has been reported that underrepresented minorities had lower match rates than White and Asian applicants in dermatology.6 There have been several published articles discussing the lack of diversity in dermatology, with a call to action.7-9
Factors for Couples Matching—The number of applicants participating in the couples match continues to increase yearly. The NMRP does publish data regarding “successful” couples matching but does not specify how many couples match together. There also is little published regarding advice for participation in the couples match. Although we had a limited number of couples that participated in the match, it is interesting to note they had similar strategies, including contacting programs at institutions that had offered interviews to their partners. This strategy may be effective, as dermatology programs offer interviews relatively late compared with other specialties.5 Additionally, this strategy may increase the number of interviews offered and received, as evidenced by the higher number of interviews offered compared with those who were not couples matching. Additionally, this survey highlights the sacrifice often needed by couples in the couples match as revealed by the inclusion of rank-list options in which the couples reside long distance or in which 1 partner does not match. This information may be helpful to applicants who are planning a strategy for the couples match in dermatology. Although this study does not encompass all dermatology applicants in the 2019-2020 cycle, we do believe it may be representative. The USMLE Step 1 scores in this study were similar to the published NRMP data.1,10 According to NRMP data from the 2019-2020 cycle, the mean USMLE Step 1 score was 248 for matched applicants and 239 for unmatched.1 The NRMP reported the mean USMLE Step 2 CK score for matched was 256 and 248 for unmatched, which also is similar to our data. The NRMP reported the mean number of programs ranked was 9.9 for matched and 4.5 for unmatched applicants.1 Again, our data were similar for number of dermatology interviews attended.
Limitations—There are limitations to this study. The main limitation is that the survey is from a single institution and had a limited number of respondents. Given the nature of the study, the accuracy of the data is dependent on the applicants’ honesty in self-reporting academic performance and other variables. There also may be a selection bias given the low response rate. The subanalyses—children and couples matching—were underpowered with the limited number of participants. Further studies that include multiple residency programs and multiple years could be helpful to provide more power and less risk of bias. We did not gather information such as the Medical Student Performance Evaluation letter, letters of recommendation, or personal statements, which do play an important role in the assessment of an applicant. However, because the applicants completed these surveys, and given these are largely blinded to applicants, we did not feel the applicants could accurately respond to those aspects of the application.
Conclusion
Our survey finds that factors associated with matching included a higher USMLE Step 1 score, having a home dermatology program, and a higher number of interviews offered and attended. Some demographics had varying USMLE Step 1 scores but similar match rates.
- National Resident Matching Program. Results and Data: 2020 Main Residency Match. National Resident Matching Program; May 2020. Accessed January 9, 2023. https://www.nrmp.org/wp-content/uploads/2021/12/MM_Results_and-Data_2020-1.pdf
- Gauer JL, Jackson JB. The association of USMLE Step 1 and Step 2 CK scores with residency match specialty and location. Med Educ Online. 2017;22:1358579.
- Wang JV, Keller M. Pressure to publish for residency applicants in dermatology. Dermatol Online J. 2016;22:13030/qt56x1t7ww.
- Wang RF, Zhang M, Kaffenberger JA. Does the dermatology standardized letter of recommendation alter applicants’ chances of matching into residency. J Am Acad Dermatol. 2017;77:e139-e140.
- National Resident Matching Program, Data Release and Research Committee: results of the 2018 NRMP Program Director Survey. Accessed December 19, 2022. https://www.nrmp.org/wp-content/uploads/2021/07/NRMP-2018-Program-Director-Survey-for-WWW.pdf
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role of race and ethnicity in the dermatology applicant match process. J Natl Med Assoc. 2022;113:666-670.
- Chen A, Shinkai K. Rethinking how we select dermatology applicants-turning the tide. JAMA Dermatol. 2017;153:259-260.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49.
- National Resident Matching Program. Charting outcomes in the match: U.S. allopathic seniors. Characteristics of U.S. allopathic seniors who matched to their preferred specialty in the 2018 main residency match. 2nd ed. Accessed December 19, 2022. https://www.nrmp.org/wp-content/uploads/2021/07/Charting-Outcomes-in-the-Match-2018_Seniors-1.pdf
- National Resident Matching Program. Results and Data: 2020 Main Residency Match. National Resident Matching Program; May 2020. Accessed January 9, 2023. https://www.nrmp.org/wp-content/uploads/2021/12/MM_Results_and-Data_2020-1.pdf
- Gauer JL, Jackson JB. The association of USMLE Step 1 and Step 2 CK scores with residency match specialty and location. Med Educ Online. 2017;22:1358579.
- Wang JV, Keller M. Pressure to publish for residency applicants in dermatology. Dermatol Online J. 2016;22:13030/qt56x1t7ww.
- Wang RF, Zhang M, Kaffenberger JA. Does the dermatology standardized letter of recommendation alter applicants’ chances of matching into residency. J Am Acad Dermatol. 2017;77:e139-e140.
- National Resident Matching Program, Data Release and Research Committee: results of the 2018 NRMP Program Director Survey. Accessed December 19, 2022. https://www.nrmp.org/wp-content/uploads/2021/07/NRMP-2018-Program-Director-Survey-for-WWW.pdf
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role of race and ethnicity in the dermatology applicant match process. J Natl Med Assoc. 2022;113:666-670.
- Chen A, Shinkai K. Rethinking how we select dermatology applicants-turning the tide. JAMA Dermatol. 2017;153:259-260.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49.
- National Resident Matching Program. Charting outcomes in the match: U.S. allopathic seniors. Characteristics of U.S. allopathic seniors who matched to their preferred specialty in the 2018 main residency match. 2nd ed. Accessed December 19, 2022. https://www.nrmp.org/wp-content/uploads/2021/07/Charting-Outcomes-in-the-Match-2018_Seniors-1.pdf
PRACTICE POINTS
- Dermatology residency continues to be one of the most competitive specialties, with a match rate of 84.7% in 2019.
- A high US Medical Licensing Examination (USMLE) Step 1 score and having a home dermatology program and a greater number of interviews may lead to higher likeliness of matching in dermatology.
- Most applicants (82.4%) applied to programs their partner had interviews at, suggesting this may be a helpful strategy.
Liability in robotic gyn surgery
The approach to hysterectomy has been debated, with the need for individualization case by case stressed, and the expertise of the operating surgeon considered.
CASE Was surgeon experience a factor in case complications?
VM is a 46-year-old woman (G5 P4014) reporting persistent uterine bleeding that is refractory to medical therapy. The patient has uterine fibroids, 6 weeks in size on examination, with “mild” prolapse noted. Additional medical diagnoses included vulvitis, ovarian cyst in the past, cystic mastopathy, and prior evidence of pelvic adhesion, noted at the time of ovarian cystectomy. Prior surgical records were not obtained by the operating surgeon, although her obstetric history includes 2 prior vaginal deliveries and 2 cesarean deliveries (CDs). The patient had an umbilical herniorraphy a number of years ago. Her medications include hormonal therapy, for presumed menopause, and medication for depression (she reported “doing well” on medication). She reported smoking 1 PPD and had a prior tubal ligation.
VM was previously evaluated for Lynch Syndrome and informed of the potential for increased risks of colon, endometrial, and several other cancers. She did not have cancer as of the time of planned surgery.
The patient underwent robotic-assisted total laparoscopic hysterectomy and bilateral salpingo-oophorectomy. The operating surgeon did not have a lot of experience with robotic hysterectomies but told the patient preoperatively “I have done a few.” Perioperatively, blood loss was minimal, urine output was recorded as 25 mL, and according to the operative report there were extensive pelvic adhesions and no complications. The “ureters were identified” when the broad ligament was opened at the time of skeletonization of the uterine vessels and documented accordingly. The intraoperative Foley was discontinued at the end of the procedure. The pathology report noted diffuse adenomyosis and uterine fibroids; the uterus weighed 250 g. In addition, a “large hemorrhagic corpus luteum cyst” was noted on the right ovary.
The patient presented for a postoperative visit reporting “leaking” serosanguinous fluid that began 2.5 weeks postoperatively and required her to wear 3 to 4 “Depends” every day. She also reported constipation since beginning her prescribed pain medication. She requested a copy of her medical records and said she was dissatisfied with the care she had received related to the hysterectomy; she was “seeking a second opinion from a urologist.” The urologist suggested evaluation of the “leaking,” and a Foley catheter was placed. When she stood up, however, there was leaking around the catheter, and she reported a “yellowish-green,” foul smelling discharge. She called the urologist’s office, stating, “I think I have a bowel obstruction.” The patient was instructed to proceed to the emergency department at her local hospital. She was released with a diagnosis of constipation. Upon follow-up urologic evaluation, a vulvovaginal fistula was noted. Management was a “simple fistula repair,” and the patient did well subsequently.
The patient brought suit against the hospital and operating gynecologist. In part the hospital records noted, “relatively inexperienced robotic surgeon.” The hospital was taken to task for granting privileges to an individual that had prior privilege “problems.”

Continue to: Medical opinion...
Medical opinion
This case demonstrates a number of issues. (We will discuss the credentials for the surgeon and hospital privileges in the legal considerations section.) From the medical perspective, the rate of urologic injury associated with all hysterectomies is 0.87%.1 Robotic hysterectomy has been reported at 0.92% in a series published from Henry Ford Hospital.1 The lowest rate of urologic injury is associated with vaginal hysterectomy, reported at 0.2%.2 Reported rates of urologic injury by approach to hysterectomy are1:
- robotic, 0.92%
- laparoscopic, 0.90%
- vaginal, 0.33%
- abdominal, 0.96%.
Complications by surgeon type also have been addressed, and the percent of total urologic complications are reported as1:
- ObGyn, 47%
- gyn oncologist, 47%
- urogynecologist, 6%.
Intraoperative conversion to laparotomy from initial robotic approach has been addressed in a retrospective study over a 2-year period, with operative times ranging from 1 hr, 50 min to 9 hrs of surgical time.1 The vast majority of intraoperative complications in a series reported from Finland were managed “within minutes,” and in the series of 83 patients, 5 (6%) required conversion to laparotomy.2 Intraoperative complications reported include failed entry, vascular injury, nerve injury, visceral injury, solid organ injury, tumor fragmentation, and anesthetic-related complications.3 Of note, the vascular injuries included inferior vena cava, common iliac, and external iliac.
Mortality rates in association with benign laparoscopic and robotic procedures have been addressed and noted to be 1:6,456 cases based upon a meta-analysis.4 The analysis included 124,216 patients. Laparoscopic versus robotic mortality rates were not statistically different. Mortality was more common among cases of undiagnosed rare colorectal injury. This mortality is on par with complications from Roux-en-Y gastric bypass procedures. Procedures such as sacrocolpopexy are equated with higher mortality (1:1,246) in comparison with benign hysterectomy.5
Infectious complications following either laparoscopic or robotic hysterectomy were reported at less than 1% and not statistically different for either approach.6 The series authored by Marra et al evaluated 176,016 patients.
Overall, robotic-assisted gynecologic complications are rare. One series was focused on gynecological oncologic cases.7 Specific categories of complications included7:
- patient positioning and pneumoperitoneum
- injury to surrounding organs
- bowel injury
- port site metastasis
- surgical emphysema
- vaginal cuff dehiscence
- anesthesia-related problems.
The authors concluded, “robotic assisted surgery in gynecological oncology is safe and the incidence of complications is low.”7 The major cause of death related to robotic surgery is vascular injury–related. The authors emphasized the importance of knowledge of anatomy, basic principles of “traction and counter-traction” and proper dissection along tissue planes as key to minimizing complications. Consider placement of stents for ureter identification, as appropriate. Barbed-suturing does not prevent dehiscence.
Continue to: Legal considerations...
Legal considerations
Robotic surgery presents many legal issues and promises to raise many more in the future. The law must control new technology while encouraging productive uses, and provide new remedies for harms while respecting traditional legal principles.8 There is no shortage of good ideas about controlling surgical robots,9 automated devices more generally,10 and artificial intelligence.11 Those issues will be important, and watching them unfold will be intriguing.
In the meantime, physicians and other health care professionals, health care facilities, technology companies, and patients must work within current legal structures in implementing and using robotic surgery. These are extraordinarily complex issues, so it is possible only to review the current landscape and speculate what the near future may hold.
Regulating surgical robots
The US Food and Drug Administration (FDA) is the primary regulator of robots used in medicine.12 It has the authority to regulate surgical devices, including surgical robots—which it refers to as “robotically-assisted surgical devices,” or RASD. In 2000, it approved Intuitive Surgical’s daVinci system for use in surgery. In 2017, the FDA expanded its clearance to include the Senhance System of TransEnterix Surgical Inc. for minimally invasive gynecologic surgery.13 In 2021, the FDA cleared the Hominis Surgical System for transvaginal hysterectomy “in certain patients.” However, the FDA emphasized that this clearance is for benign hysterectomy with salpingo-oophorectomy.14 (The FDA has cleared various robotic devices for several other areas of surgical practice, including neurosurgery, orthopedics, and urology.)
The use of robots in cancer surgery is limited. The FDA approved specific RASDs in some “surgical procedures commonly performed in patients with cancer, such as hysterectomy, prostatectomy, and colectomy.”15 However, it cautioned that this clearance was based only on a 30-day patient follow up. More specifically, the FDA “has not evaluated the safety or effectiveness of RASD devices for the prevention or treatment of cancer, based on cancer-related outcomes such as overall survival, recurrence, and disease-free survival.”15
The FDA has clearly warned physicians and patients that the agency has not granted the use of RASDs “for any cancer-related surgery marketing authorization, and therefore the survival benefits to patients compared to traditional surgery have not been established.”15 (This did not apply to the hysterectomy surgery as noted above. More specifically, that clearance did not apply to anything other than 30-day results, nor to the efficacy related to cancer survival.)
States also have some authority to regulate medical practice within their borders.9 When the FDA has approved a device as safe and effective, however, there are limits on what states can do to regulate or impose liability on the approved product. The Supreme Court held that the FDA approval “pre-empted” some state action regarding approved devices.16
Hospitals, of course, regulate what is allowed within the hospital. For example, it may require training before a physician is permitted to use equipment, limit the conditions for which the equipment may be used, or decline to obtain equipment for use in the hospitals.17 In the case of RASDs, however, the high cost of equipment may provide an incentive for hospitals to urge the wide use of the latest robotic acquisition.18
Regulation aims primarily to protect patients, usually from injury or inadequate treatment. Some robotic surgery is likely to be more expensive than the same surgery without robotic assistance. The cost to the patient is not usually part of the FDA’s consideration. Insurance companies (including Medicare and Medicaid), however, do care about costs and will set or negotiate how much the reimbursement will be for a procedure. Third-party payers may decline to cover the additional cost when there is no apparent benefit from using the robot.19 For some institutions, the public perception that it offers “the most modern technology” is an important public message and a strong incentive to have the equipment.20
There are inconsistent studies about the advantages and disadvantages of RADS in gynecologic procedures, although there are few randomized studies.21 The demonstrated advantages are generally identified as somewhat shorter recovery time.22 The ultimate goal will be to minimize risks while maximizing the many potential benefits of robotic surgery.23
Continue to: Liability...
Liability
A recent study by De Ravin and colleagues of robotic surgery liability found a 250% increase in the total number of robotic surgery–related malpractice claims reported in 7 recent years (2014-2021), compared with the prior 7 (2006-2013).24 However, the number of cases varied considerably from year to year. ObGyn had the most significant gain (from 19% to 49% of all claims). During the same time, urology claims declined from 56% to 16%. (The limitations of the study’s data are discussed later in this article.)
De Ravin et al reported the legal bases for the claims, but the specific legal claim was unclear in many cases.24 For example, the vast majority were classified as “negligent surgery.” Many cases made more than 1 legal claim for liability, so the total percentages were greater than 100%. Of the specific claims, many appear unrelated to robotic surgery (misdiagnosis, delayed treatment, or infection). However, there were a significant number of cases that raised issues that were related to robotic surgery. The following are those claims that probably relate to the “robotic” surgery, along with the percentage of cases making such a claim as reported24:
- “Patient not a candidate for surgery performed” appeared in about 13% of the cases.24 Such claims could include that the surgeon should have performed the surgery with traditional laparoscopy or open technique, but instead using a robot led to the injury. Physicians may feel pressure from patients or hospitals, because of the equipment’s cost, to use robotic surgery as it seems to be the modern approach (and therefore better). Neither reason is sufficient for using robotic assistance unless it will benefit the patient.
- “Failure to calibrate or operate robot” was in 11% of the claims.24 Physicians must properly calibrate and otherwise ensure that surgical equipment is operating correctly. In addition, the hospitals supplying the equipment must ensure that the equipment is maintained correctly. Finally, the equipment manufacturer may be liable through “products liability” if the equipment is defective.25 The expanding use of artificial intelligence in medical equipment (including surgical robots) is increasing the complexity of determining what “defective” means.11
- “Training deficiencies or credentialing” liability is a common problem with new technology. Physicians using new technology should be thoroughly trained and, where appropriate, certified in the use of the new technology.26 Early adopters of the technology should be especially cautious because good training may be challenging to obtain. In the study, the claims of inadequate training were particularly high during the early 7 years (35%), but dropped during the later time (4%).24
- “Improper positioning” of the patient or device or patient was raised in 7% of the cases.24
- “Manufacturing problems” were claimed in a small number of cases—13% in 2006-2013, but 2% in 2014-2021.24 These cases raise the complex question of products liability for robotic surgery and artificial intelligence (AI). Products liability has been part of surgical practice for many years. There usually will be liability if there are “defects” in a product, whether or not resulting from negligence. What a “defect” in a computer program means is a complicated issue that will be significant in future liability cases.27
Several other cases reported in the De Ravin study were probably related to robotic surgery. For example, Informed Consent and Failure to Monitor each appeared in more than 30%, of 2014-2021 cases, and Failure to Refer in 16% of the cases.24,27
The outcomes of the reported cases were mostly verdicts (or trial-related settlements) for defendants (doctors and hospitals). The defense prevailed 69% of the time in the early period and 78% of the time in 2014-2021. However, there were substantial damages in some cases. The range of damages in 2006-2013 was $95,000 to $6 million (mean, $2.5 million); in 2014-2021, it was $10,000 to $5 million (mean, $1.3 million).24
An earlier study looked at reported cases against Intuitive Surgical, maker of the daVinci system, from 2000-2017.28 Of the 108 claims in the study, 62% were gynecologic surgeries. Of these claims, 35% were dismissed, but “no other information regarding settlements or trial outcomes was available.” The study did not report the basis for the lawsuits involving gynecologic surgeries.
We should exercise caution in reviewing these studies. Although the studies were of considerable value, the authors note significant limitations of the databases available. The database was Westlaw in the first study discussed (“Robotic surgery: the impact”24) and Bloomberg in the second (“Robotic urologic”28). For example, the “impact” study was based on “jury verdict reports” excluding settlements, and the latter excluded class actions and cases settled. Thus the studies undoubtedly understated the number of claims made (those that resulted in settlement before a lawsuit was filed), cases filed but abandoned, and settlements made before trial.
Despite these limitations, the studies provide valuable insights into current malpractice risks and future directions. It is worth remembering that these cases nearly all involved a single robot, the daVinci, produced by Intuitive Surgical. It is not a “smart” robot and is commonly referred to as a “master-slave” machine. With much more intelligent and independent machines, the future will raise more complex problems in the FDA approval process and malpractice and product liability claims when things go wrong.
Continue to: What’s the verdict?...
What’s the verdict?
The case of VM and operating surgeon Dr. G illustrates several important legal aspects of using surgical robots. It also demonstrates that the presence of the robot assist still requires the surgeon’s careful attention to issues of informed consent, adequate specific training, and thorough follow up. In the following discussion, we divide the case review into the elements of negligence-malpractice (duty and breach, causation, and damages) and conclude with a thought about how to proceed when things have gone wrong.
Dr. G’s statement, “I’ve done a few,” is indefinite, but it may suggest that Dr. G. had not received full, supervised training in the robotic assist he was planning to use. That problem was underlined by the conclusion that Dr. G was a “relatively inexperienced robotic surgeon.” If so, that failure could constitute a breach of the duty of care to the patient. In addition, if it is inaccurate or did not provide information VM reasonably needed in consenting to Dr. G proceeding with the surgery, there could be an issue of whether there was a partial failure of fully informed consent.
The hospital also may have potential liability. It was “taken to task for granting privileges to an individual that had prior privilege ‘problems,’” suggesting that it had not performed adequate review before granting hospital privileges. Furthermore, if Dr. G was not sufficiently practiced or supervised in robotic surgery, the hospital, which allowed Dr. G to proceed, might also be negligent.
VM had a series of problems postsurgery that ultimately resulted in additional care and “simple fistula repair.” Assuming that there was negligence, the next question is whether that failure caused the injury. Causation may be the most difficult part of the case for VM to prove. It would require expert testimony that the inadequate surgery (inappropriate use of robotic surgery or other error during surgery) and follow up resulted in the formation or increase in the likelihood of the fistula.
VM would also have to prove damages. Damages are those costs (the economic value) of injuries that would not have occurred but for negligence. Damages would include most of the cost of the follow-up medical care and any related additional future care required, plus costs that were a consequence of the negligence (such as lost work). In addition, damages would include pain and suffering that resulted from the negligence, subject to caps in some states.
When the patient was dissatisfied and reported a postsurgical problem, the hospital and Dr. G may have had an opportunity to avoid further dissatisfaction, complaints, and ultimately a lawsuit. Effective approaches for dealing with such dissatisfaction may serve the institution’s and physician’s values and financial best interests.
The jury verdict was in favor of the plaintiff. Jurors felt the operating surgeon should have conveyed his experience with robotic surgery more clearly as part of the informed consent process.
“Hey Siri! Perform a type 3 hysterectomy. Please watch out for the ureter!”29
Medicine is still at the frontier of surgical robots. Over future decades, the number and sophistication of these machines will increase substantially. They likely will become much more like robots, guided by AI, and make independent judgments. These have the potential for significant medical progress that improves the treatment of patients. At the same time, the last 20 years suggest that robotic innovation will challenge medicine, the FDA and other regulators, lawmakers, and courts. In the future, regulators and patients should embrace genuine advances in robotic surgery but not be dazzled by these new machines’ luster (or potential for considerable profits).30
The public may be wildly optimistic about the benefits without balancing the risks. The AI that runs them will be essentially invisible and constantly changing. Physicians and regulators must develop new techniques for assessing and controlling the software. Real surgical robots require rigorous testing, cautious promotion, disciplined use, and perpetual review. ●
- Petersen S, Doe S, Rubinfield I, et al. Rate of urologic injury with robotic hysterectomy. J Min Invasc Gynecol. 2018;25:867-871.
- Makinen J, Johansson J, Toma C, et al. Morbidity of 10,110 hysterectomies by type approach. Hum Reprod. 2001;16:1473-1478.
- Karasu A, Kran G, Sanlikan F. Intraoperative complications and conversion to laparotomy in gynecologic robotic surgery. J Investig Surg. 2022;35:912-915.
- Behbehani S, Suarez-Salvador E, Buras M, et al. Mortality rates in benign laparoscopic and robotic surgery: a systematic review and meta-analysis. J Min Invasc. 2020;27:603-612.
- Giurdano S, Victorzon M. Laparoscopic roux-en-Y gastric bypass in elderly patients (60 years or older): a meta-analysis of comparative studies. Scand J Surg. 2018;107:6-11.
- Marra A, Pulg-Asensio M, Edmond M, et al. Infectious complications of laparoscopic and robotic hysterectomy: a systematic literature review and meta-analysis. Int J Gynecol Cancer. 2019;29:518-530.
- Tse KY, Sheung H, Lim P. Robot-assisted gyneaecological cancer surgery-complications and prevention. Best Pract Res Clin Obstet Gynaecol. 2017;25:94-105.
- Hubbard FP. Sophisticated robots: balancing liability, regulation, and innovation. Fla Law Rev. 2014;66:1803-1872. https://scholarship.law.ufl.edu/cgi/viewcontent. cgi?article=1204&context=flr. Accessed December 20, 2022.
- Villanueva A. The legal battle with the future of autonomous surgical robotics. Ind Health Law Rev. 2020;17:367-392. https://journals.iupui.edu/index.php/ihlr/article /download/25051/23544. Accessed December 20, 2022.
- Lemley MA, Casey B. Remedies for robots. U Chi Law Rev. 2019;86:1311-1396. https://chicagounbound.uchicago.edu /cgi/viewcontent.cgi?article=6140&context=uclrev. Accessed December 20, 2022.
- Griffin F. Artificial intelligence and liability in health care. Health Matrix. 2021;31:65-106. https://scholarlycommons. law.case.edu/cgi/viewcontent.cgi?article=1659&context=hea lthmatrix. Accessed December 20, 2022.
- Britton D. Autonomous surgery: the law of autonomous surgical robots. J Law Tech Tex. 2017;1:152-189.
- US Food and Drug Administration. FDA clears new robotically-assisted surgical device for adult patients. October 13, 2017. https://www.fda.gov/news-events/press-announcements /fda-clears-new-robotically-assisted-surgical-device-adult -patients. Accessed December 20, 2022.
- US Food and Drug Administration. FDA authorizes first robotically-assisted surgical device for performing transvaginal hysterectomy. March 1, 2021. https://www.fda .gov/news-events/press-announcements/fda-authorizes -first-robotically-assisted-surgical-device-performing -transvaginal-hysterectomy. Accessed December 20, 2022.
- US Food and Drug Administration. Caution with robotically-assisted surgical devices in mastectomy: FDA Safety Communication, August 20, 2021. https://www.fda.gov/medical-devices/safety-communications/update-caution-robotically-assisted-surgical-devices-mastectomy-fda-safety-communication. Accessed December 22, 2022. Riegel v Medtronic, 552 US 312 (2008).
- Han ES, Advincula AP. Robotic surgery: advancements and inflection points in the field of gynecology. Obstet Gynecol Clin North Am. 2021;48:759-776.
- Witharm H. Robot-assisted surgery: an analysis of the legal and economic implications. Az J Interdisciplinary Studies. 2022;8:19-29. https://journals.librarypublishing.arizona.edu /azjis/article/id/5093/download/pdf/.
- Cameron S. Is daVinci robotic surgery a revolution or a rip-off? Healthline. August 10, 2016. https://www.healthline .com/health-news/is-da-vinci-robotic-surgery-revolution -or-ripoff-021215. Accessed December 20, 2022.
- Perez RE, Schwaitzberg SD. Robotic surgery: finding value in 2019 and beyond. Ann Laparosc Endosc Surg. 2019;4:1-7.
- Gitas G, Hanker L, Rody A, et al. Robotic surgery in gynecology: is the future already here? Minim Invasiv Therapy Allied Technol. 2022;4:1-0.
- Moon AS, Garofalo J, Koirala P, et al. Robotic surgery in gynecology. Surgical Clinics. 2020;100:445-460.
- Simshaw D, Terry N, Hauser K, et al. Regulating healthcare robots: maximizing opportunities while minimizing risks. Richmond J Law Tech. 2015;22:1-38. https://scholar works.iupui.edu/bitstream/handle/1805/11587/simshaw _2015_regulating.pdf?sequence=1&isAllowed=y. Accessed December 20, 2022.
- De Ravin E, Sell EA, Newman JG, et al. Medical malpractice in robotic surgery: a Westlaw database analysis. J Robotic Surg. 2022. https://doi.org/10.1007/s11701-022-01417-6. https:// link.springer.com/article/10.1007/s11701-022-014176#citeas. Accessed December 20, 2022.
- Beglinger C. A broken theory: the malfunction theory of strict products liability and the need for a new doctrine in the field of surgical robotics. Minnesotta Law Rev. 2019;104:1041-1093. . Accessed December 20, 2022.
- Azadi S, Green IC, Arnold A, et al. Robotic surgery: the impact of simulation and other innovative platforms on performance and training. J Minim Invasiv Gynecol. 2021;28:490-495.
- Koerner D. Doctor roboto: The no-man operation. U Tol L Rev. 2019;51:125-146.
- Nik-Ahd F, Souders CP, Zhao H, et al. Robotic urologic surgery: trends in litigation over the last decade. J Robotic Surg. 2019;13:729-734.
- Gültekin CalibriİB, Karabük E, Köse MF. “Hey Siri! Perform a type 3 hysterectomy. Please watch out for the ureter!” What is autonomous surgery and what are the latest developments? J Turk Ger Gynecol Assoc. 2021;22:58-70. https://www.ncbi .nlm.nih.gov/pmc/articles/PMC7944239/.
- Matsuzaki T. Ethical issues of artificial intelligence in medicine. California West Law Rev. 2018;55:255-273. https://scholarlycommons.law.cwsl.edu/cgi/viewcontent. cgi?article=1669&context=cwlr. Accessed December 20, 2022.
The approach to hysterectomy has been debated, with the need for individualization case by case stressed, and the expertise of the operating surgeon considered.
CASE Was surgeon experience a factor in case complications?
VM is a 46-year-old woman (G5 P4014) reporting persistent uterine bleeding that is refractory to medical therapy. The patient has uterine fibroids, 6 weeks in size on examination, with “mild” prolapse noted. Additional medical diagnoses included vulvitis, ovarian cyst in the past, cystic mastopathy, and prior evidence of pelvic adhesion, noted at the time of ovarian cystectomy. Prior surgical records were not obtained by the operating surgeon, although her obstetric history includes 2 prior vaginal deliveries and 2 cesarean deliveries (CDs). The patient had an umbilical herniorraphy a number of years ago. Her medications include hormonal therapy, for presumed menopause, and medication for depression (she reported “doing well” on medication). She reported smoking 1 PPD and had a prior tubal ligation.
VM was previously evaluated for Lynch Syndrome and informed of the potential for increased risks of colon, endometrial, and several other cancers. She did not have cancer as of the time of planned surgery.
The patient underwent robotic-assisted total laparoscopic hysterectomy and bilateral salpingo-oophorectomy. The operating surgeon did not have a lot of experience with robotic hysterectomies but told the patient preoperatively “I have done a few.” Perioperatively, blood loss was minimal, urine output was recorded as 25 mL, and according to the operative report there were extensive pelvic adhesions and no complications. The “ureters were identified” when the broad ligament was opened at the time of skeletonization of the uterine vessels and documented accordingly. The intraoperative Foley was discontinued at the end of the procedure. The pathology report noted diffuse adenomyosis and uterine fibroids; the uterus weighed 250 g. In addition, a “large hemorrhagic corpus luteum cyst” was noted on the right ovary.
The patient presented for a postoperative visit reporting “leaking” serosanguinous fluid that began 2.5 weeks postoperatively and required her to wear 3 to 4 “Depends” every day. She also reported constipation since beginning her prescribed pain medication. She requested a copy of her medical records and said she was dissatisfied with the care she had received related to the hysterectomy; she was “seeking a second opinion from a urologist.” The urologist suggested evaluation of the “leaking,” and a Foley catheter was placed. When she stood up, however, there was leaking around the catheter, and she reported a “yellowish-green,” foul smelling discharge. She called the urologist’s office, stating, “I think I have a bowel obstruction.” The patient was instructed to proceed to the emergency department at her local hospital. She was released with a diagnosis of constipation. Upon follow-up urologic evaluation, a vulvovaginal fistula was noted. Management was a “simple fistula repair,” and the patient did well subsequently.
The patient brought suit against the hospital and operating gynecologist. In part the hospital records noted, “relatively inexperienced robotic surgeon.” The hospital was taken to task for granting privileges to an individual that had prior privilege “problems.”

Continue to: Medical opinion...
Medical opinion
This case demonstrates a number of issues. (We will discuss the credentials for the surgeon and hospital privileges in the legal considerations section.) From the medical perspective, the rate of urologic injury associated with all hysterectomies is 0.87%.1 Robotic hysterectomy has been reported at 0.92% in a series published from Henry Ford Hospital.1 The lowest rate of urologic injury is associated with vaginal hysterectomy, reported at 0.2%.2 Reported rates of urologic injury by approach to hysterectomy are1:
- robotic, 0.92%
- laparoscopic, 0.90%
- vaginal, 0.33%
- abdominal, 0.96%.
Complications by surgeon type also have been addressed, and the percent of total urologic complications are reported as1:
- ObGyn, 47%
- gyn oncologist, 47%
- urogynecologist, 6%.
Intraoperative conversion to laparotomy from initial robotic approach has been addressed in a retrospective study over a 2-year period, with operative times ranging from 1 hr, 50 min to 9 hrs of surgical time.1 The vast majority of intraoperative complications in a series reported from Finland were managed “within minutes,” and in the series of 83 patients, 5 (6%) required conversion to laparotomy.2 Intraoperative complications reported include failed entry, vascular injury, nerve injury, visceral injury, solid organ injury, tumor fragmentation, and anesthetic-related complications.3 Of note, the vascular injuries included inferior vena cava, common iliac, and external iliac.
Mortality rates in association with benign laparoscopic and robotic procedures have been addressed and noted to be 1:6,456 cases based upon a meta-analysis.4 The analysis included 124,216 patients. Laparoscopic versus robotic mortality rates were not statistically different. Mortality was more common among cases of undiagnosed rare colorectal injury. This mortality is on par with complications from Roux-en-Y gastric bypass procedures. Procedures such as sacrocolpopexy are equated with higher mortality (1:1,246) in comparison with benign hysterectomy.5
Infectious complications following either laparoscopic or robotic hysterectomy were reported at less than 1% and not statistically different for either approach.6 The series authored by Marra et al evaluated 176,016 patients.
Overall, robotic-assisted gynecologic complications are rare. One series was focused on gynecological oncologic cases.7 Specific categories of complications included7:
- patient positioning and pneumoperitoneum
- injury to surrounding organs
- bowel injury
- port site metastasis
- surgical emphysema
- vaginal cuff dehiscence
- anesthesia-related problems.
The authors concluded, “robotic assisted surgery in gynecological oncology is safe and the incidence of complications is low.”7 The major cause of death related to robotic surgery is vascular injury–related. The authors emphasized the importance of knowledge of anatomy, basic principles of “traction and counter-traction” and proper dissection along tissue planes as key to minimizing complications. Consider placement of stents for ureter identification, as appropriate. Barbed-suturing does not prevent dehiscence.
Continue to: Legal considerations...
Legal considerations
Robotic surgery presents many legal issues and promises to raise many more in the future. The law must control new technology while encouraging productive uses, and provide new remedies for harms while respecting traditional legal principles.8 There is no shortage of good ideas about controlling surgical robots,9 automated devices more generally,10 and artificial intelligence.11 Those issues will be important, and watching them unfold will be intriguing.
In the meantime, physicians and other health care professionals, health care facilities, technology companies, and patients must work within current legal structures in implementing and using robotic surgery. These are extraordinarily complex issues, so it is possible only to review the current landscape and speculate what the near future may hold.
Regulating surgical robots
The US Food and Drug Administration (FDA) is the primary regulator of robots used in medicine.12 It has the authority to regulate surgical devices, including surgical robots—which it refers to as “robotically-assisted surgical devices,” or RASD. In 2000, it approved Intuitive Surgical’s daVinci system for use in surgery. In 2017, the FDA expanded its clearance to include the Senhance System of TransEnterix Surgical Inc. for minimally invasive gynecologic surgery.13 In 2021, the FDA cleared the Hominis Surgical System for transvaginal hysterectomy “in certain patients.” However, the FDA emphasized that this clearance is for benign hysterectomy with salpingo-oophorectomy.14 (The FDA has cleared various robotic devices for several other areas of surgical practice, including neurosurgery, orthopedics, and urology.)
The use of robots in cancer surgery is limited. The FDA approved specific RASDs in some “surgical procedures commonly performed in patients with cancer, such as hysterectomy, prostatectomy, and colectomy.”15 However, it cautioned that this clearance was based only on a 30-day patient follow up. More specifically, the FDA “has not evaluated the safety or effectiveness of RASD devices for the prevention or treatment of cancer, based on cancer-related outcomes such as overall survival, recurrence, and disease-free survival.”15
The FDA has clearly warned physicians and patients that the agency has not granted the use of RASDs “for any cancer-related surgery marketing authorization, and therefore the survival benefits to patients compared to traditional surgery have not been established.”15 (This did not apply to the hysterectomy surgery as noted above. More specifically, that clearance did not apply to anything other than 30-day results, nor to the efficacy related to cancer survival.)
States also have some authority to regulate medical practice within their borders.9 When the FDA has approved a device as safe and effective, however, there are limits on what states can do to regulate or impose liability on the approved product. The Supreme Court held that the FDA approval “pre-empted” some state action regarding approved devices.16
Hospitals, of course, regulate what is allowed within the hospital. For example, it may require training before a physician is permitted to use equipment, limit the conditions for which the equipment may be used, or decline to obtain equipment for use in the hospitals.17 In the case of RASDs, however, the high cost of equipment may provide an incentive for hospitals to urge the wide use of the latest robotic acquisition.18
Regulation aims primarily to protect patients, usually from injury or inadequate treatment. Some robotic surgery is likely to be more expensive than the same surgery without robotic assistance. The cost to the patient is not usually part of the FDA’s consideration. Insurance companies (including Medicare and Medicaid), however, do care about costs and will set or negotiate how much the reimbursement will be for a procedure. Third-party payers may decline to cover the additional cost when there is no apparent benefit from using the robot.19 For some institutions, the public perception that it offers “the most modern technology” is an important public message and a strong incentive to have the equipment.20
There are inconsistent studies about the advantages and disadvantages of RADS in gynecologic procedures, although there are few randomized studies.21 The demonstrated advantages are generally identified as somewhat shorter recovery time.22 The ultimate goal will be to minimize risks while maximizing the many potential benefits of robotic surgery.23
Continue to: Liability...
Liability
A recent study by De Ravin and colleagues of robotic surgery liability found a 250% increase in the total number of robotic surgery–related malpractice claims reported in 7 recent years (2014-2021), compared with the prior 7 (2006-2013).24 However, the number of cases varied considerably from year to year. ObGyn had the most significant gain (from 19% to 49% of all claims). During the same time, urology claims declined from 56% to 16%. (The limitations of the study’s data are discussed later in this article.)
De Ravin et al reported the legal bases for the claims, but the specific legal claim was unclear in many cases.24 For example, the vast majority were classified as “negligent surgery.” Many cases made more than 1 legal claim for liability, so the total percentages were greater than 100%. Of the specific claims, many appear unrelated to robotic surgery (misdiagnosis, delayed treatment, or infection). However, there were a significant number of cases that raised issues that were related to robotic surgery. The following are those claims that probably relate to the “robotic” surgery, along with the percentage of cases making such a claim as reported24:
- “Patient not a candidate for surgery performed” appeared in about 13% of the cases.24 Such claims could include that the surgeon should have performed the surgery with traditional laparoscopy or open technique, but instead using a robot led to the injury. Physicians may feel pressure from patients or hospitals, because of the equipment’s cost, to use robotic surgery as it seems to be the modern approach (and therefore better). Neither reason is sufficient for using robotic assistance unless it will benefit the patient.
- “Failure to calibrate or operate robot” was in 11% of the claims.24 Physicians must properly calibrate and otherwise ensure that surgical equipment is operating correctly. In addition, the hospitals supplying the equipment must ensure that the equipment is maintained correctly. Finally, the equipment manufacturer may be liable through “products liability” if the equipment is defective.25 The expanding use of artificial intelligence in medical equipment (including surgical robots) is increasing the complexity of determining what “defective” means.11
- “Training deficiencies or credentialing” liability is a common problem with new technology. Physicians using new technology should be thoroughly trained and, where appropriate, certified in the use of the new technology.26 Early adopters of the technology should be especially cautious because good training may be challenging to obtain. In the study, the claims of inadequate training were particularly high during the early 7 years (35%), but dropped during the later time (4%).24
- “Improper positioning” of the patient or device or patient was raised in 7% of the cases.24
- “Manufacturing problems” were claimed in a small number of cases—13% in 2006-2013, but 2% in 2014-2021.24 These cases raise the complex question of products liability for robotic surgery and artificial intelligence (AI). Products liability has been part of surgical practice for many years. There usually will be liability if there are “defects” in a product, whether or not resulting from negligence. What a “defect” in a computer program means is a complicated issue that will be significant in future liability cases.27
Several other cases reported in the De Ravin study were probably related to robotic surgery. For example, Informed Consent and Failure to Monitor each appeared in more than 30%, of 2014-2021 cases, and Failure to Refer in 16% of the cases.24,27
The outcomes of the reported cases were mostly verdicts (or trial-related settlements) for defendants (doctors and hospitals). The defense prevailed 69% of the time in the early period and 78% of the time in 2014-2021. However, there were substantial damages in some cases. The range of damages in 2006-2013 was $95,000 to $6 million (mean, $2.5 million); in 2014-2021, it was $10,000 to $5 million (mean, $1.3 million).24
An earlier study looked at reported cases against Intuitive Surgical, maker of the daVinci system, from 2000-2017.28 Of the 108 claims in the study, 62% were gynecologic surgeries. Of these claims, 35% were dismissed, but “no other information regarding settlements or trial outcomes was available.” The study did not report the basis for the lawsuits involving gynecologic surgeries.
We should exercise caution in reviewing these studies. Although the studies were of considerable value, the authors note significant limitations of the databases available. The database was Westlaw in the first study discussed (“Robotic surgery: the impact”24) and Bloomberg in the second (“Robotic urologic”28). For example, the “impact” study was based on “jury verdict reports” excluding settlements, and the latter excluded class actions and cases settled. Thus the studies undoubtedly understated the number of claims made (those that resulted in settlement before a lawsuit was filed), cases filed but abandoned, and settlements made before trial.
Despite these limitations, the studies provide valuable insights into current malpractice risks and future directions. It is worth remembering that these cases nearly all involved a single robot, the daVinci, produced by Intuitive Surgical. It is not a “smart” robot and is commonly referred to as a “master-slave” machine. With much more intelligent and independent machines, the future will raise more complex problems in the FDA approval process and malpractice and product liability claims when things go wrong.
Continue to: What’s the verdict?...
What’s the verdict?
The case of VM and operating surgeon Dr. G illustrates several important legal aspects of using surgical robots. It also demonstrates that the presence of the robot assist still requires the surgeon’s careful attention to issues of informed consent, adequate specific training, and thorough follow up. In the following discussion, we divide the case review into the elements of negligence-malpractice (duty and breach, causation, and damages) and conclude with a thought about how to proceed when things have gone wrong.
Dr. G’s statement, “I’ve done a few,” is indefinite, but it may suggest that Dr. G. had not received full, supervised training in the robotic assist he was planning to use. That problem was underlined by the conclusion that Dr. G was a “relatively inexperienced robotic surgeon.” If so, that failure could constitute a breach of the duty of care to the patient. In addition, if it is inaccurate or did not provide information VM reasonably needed in consenting to Dr. G proceeding with the surgery, there could be an issue of whether there was a partial failure of fully informed consent.
The hospital also may have potential liability. It was “taken to task for granting privileges to an individual that had prior privilege ‘problems,’” suggesting that it had not performed adequate review before granting hospital privileges. Furthermore, if Dr. G was not sufficiently practiced or supervised in robotic surgery, the hospital, which allowed Dr. G to proceed, might also be negligent.
VM had a series of problems postsurgery that ultimately resulted in additional care and “simple fistula repair.” Assuming that there was negligence, the next question is whether that failure caused the injury. Causation may be the most difficult part of the case for VM to prove. It would require expert testimony that the inadequate surgery (inappropriate use of robotic surgery or other error during surgery) and follow up resulted in the formation or increase in the likelihood of the fistula.
VM would also have to prove damages. Damages are those costs (the economic value) of injuries that would not have occurred but for negligence. Damages would include most of the cost of the follow-up medical care and any related additional future care required, plus costs that were a consequence of the negligence (such as lost work). In addition, damages would include pain and suffering that resulted from the negligence, subject to caps in some states.
When the patient was dissatisfied and reported a postsurgical problem, the hospital and Dr. G may have had an opportunity to avoid further dissatisfaction, complaints, and ultimately a lawsuit. Effective approaches for dealing with such dissatisfaction may serve the institution’s and physician’s values and financial best interests.
The jury verdict was in favor of the plaintiff. Jurors felt the operating surgeon should have conveyed his experience with robotic surgery more clearly as part of the informed consent process.
“Hey Siri! Perform a type 3 hysterectomy. Please watch out for the ureter!”29
Medicine is still at the frontier of surgical robots. Over future decades, the number and sophistication of these machines will increase substantially. They likely will become much more like robots, guided by AI, and make independent judgments. These have the potential for significant medical progress that improves the treatment of patients. At the same time, the last 20 years suggest that robotic innovation will challenge medicine, the FDA and other regulators, lawmakers, and courts. In the future, regulators and patients should embrace genuine advances in robotic surgery but not be dazzled by these new machines’ luster (or potential for considerable profits).30
The public may be wildly optimistic about the benefits without balancing the risks. The AI that runs them will be essentially invisible and constantly changing. Physicians and regulators must develop new techniques for assessing and controlling the software. Real surgical robots require rigorous testing, cautious promotion, disciplined use, and perpetual review. ●
The approach to hysterectomy has been debated, with the need for individualization case by case stressed, and the expertise of the operating surgeon considered.
CASE Was surgeon experience a factor in case complications?
VM is a 46-year-old woman (G5 P4014) reporting persistent uterine bleeding that is refractory to medical therapy. The patient has uterine fibroids, 6 weeks in size on examination, with “mild” prolapse noted. Additional medical diagnoses included vulvitis, ovarian cyst in the past, cystic mastopathy, and prior evidence of pelvic adhesion, noted at the time of ovarian cystectomy. Prior surgical records were not obtained by the operating surgeon, although her obstetric history includes 2 prior vaginal deliveries and 2 cesarean deliveries (CDs). The patient had an umbilical herniorraphy a number of years ago. Her medications include hormonal therapy, for presumed menopause, and medication for depression (she reported “doing well” on medication). She reported smoking 1 PPD and had a prior tubal ligation.
VM was previously evaluated for Lynch Syndrome and informed of the potential for increased risks of colon, endometrial, and several other cancers. She did not have cancer as of the time of planned surgery.
The patient underwent robotic-assisted total laparoscopic hysterectomy and bilateral salpingo-oophorectomy. The operating surgeon did not have a lot of experience with robotic hysterectomies but told the patient preoperatively “I have done a few.” Perioperatively, blood loss was minimal, urine output was recorded as 25 mL, and according to the operative report there were extensive pelvic adhesions and no complications. The “ureters were identified” when the broad ligament was opened at the time of skeletonization of the uterine vessels and documented accordingly. The intraoperative Foley was discontinued at the end of the procedure. The pathology report noted diffuse adenomyosis and uterine fibroids; the uterus weighed 250 g. In addition, a “large hemorrhagic corpus luteum cyst” was noted on the right ovary.
The patient presented for a postoperative visit reporting “leaking” serosanguinous fluid that began 2.5 weeks postoperatively and required her to wear 3 to 4 “Depends” every day. She also reported constipation since beginning her prescribed pain medication. She requested a copy of her medical records and said she was dissatisfied with the care she had received related to the hysterectomy; she was “seeking a second opinion from a urologist.” The urologist suggested evaluation of the “leaking,” and a Foley catheter was placed. When she stood up, however, there was leaking around the catheter, and she reported a “yellowish-green,” foul smelling discharge. She called the urologist’s office, stating, “I think I have a bowel obstruction.” The patient was instructed to proceed to the emergency department at her local hospital. She was released with a diagnosis of constipation. Upon follow-up urologic evaluation, a vulvovaginal fistula was noted. Management was a “simple fistula repair,” and the patient did well subsequently.
The patient brought suit against the hospital and operating gynecologist. In part the hospital records noted, “relatively inexperienced robotic surgeon.” The hospital was taken to task for granting privileges to an individual that had prior privilege “problems.”

Continue to: Medical opinion...
Medical opinion
This case demonstrates a number of issues. (We will discuss the credentials for the surgeon and hospital privileges in the legal considerations section.) From the medical perspective, the rate of urologic injury associated with all hysterectomies is 0.87%.1 Robotic hysterectomy has been reported at 0.92% in a series published from Henry Ford Hospital.1 The lowest rate of urologic injury is associated with vaginal hysterectomy, reported at 0.2%.2 Reported rates of urologic injury by approach to hysterectomy are1:
- robotic, 0.92%
- laparoscopic, 0.90%
- vaginal, 0.33%
- abdominal, 0.96%.
Complications by surgeon type also have been addressed, and the percent of total urologic complications are reported as1:
- ObGyn, 47%
- gyn oncologist, 47%
- urogynecologist, 6%.
Intraoperative conversion to laparotomy from initial robotic approach has been addressed in a retrospective study over a 2-year period, with operative times ranging from 1 hr, 50 min to 9 hrs of surgical time.1 The vast majority of intraoperative complications in a series reported from Finland were managed “within minutes,” and in the series of 83 patients, 5 (6%) required conversion to laparotomy.2 Intraoperative complications reported include failed entry, vascular injury, nerve injury, visceral injury, solid organ injury, tumor fragmentation, and anesthetic-related complications.3 Of note, the vascular injuries included inferior vena cava, common iliac, and external iliac.
Mortality rates in association with benign laparoscopic and robotic procedures have been addressed and noted to be 1:6,456 cases based upon a meta-analysis.4 The analysis included 124,216 patients. Laparoscopic versus robotic mortality rates were not statistically different. Mortality was more common among cases of undiagnosed rare colorectal injury. This mortality is on par with complications from Roux-en-Y gastric bypass procedures. Procedures such as sacrocolpopexy are equated with higher mortality (1:1,246) in comparison with benign hysterectomy.5
Infectious complications following either laparoscopic or robotic hysterectomy were reported at less than 1% and not statistically different for either approach.6 The series authored by Marra et al evaluated 176,016 patients.
Overall, robotic-assisted gynecologic complications are rare. One series was focused on gynecological oncologic cases.7 Specific categories of complications included7:
- patient positioning and pneumoperitoneum
- injury to surrounding organs
- bowel injury
- port site metastasis
- surgical emphysema
- vaginal cuff dehiscence
- anesthesia-related problems.
The authors concluded, “robotic assisted surgery in gynecological oncology is safe and the incidence of complications is low.”7 The major cause of death related to robotic surgery is vascular injury–related. The authors emphasized the importance of knowledge of anatomy, basic principles of “traction and counter-traction” and proper dissection along tissue planes as key to minimizing complications. Consider placement of stents for ureter identification, as appropriate. Barbed-suturing does not prevent dehiscence.
Continue to: Legal considerations...
Legal considerations
Robotic surgery presents many legal issues and promises to raise many more in the future. The law must control new technology while encouraging productive uses, and provide new remedies for harms while respecting traditional legal principles.8 There is no shortage of good ideas about controlling surgical robots,9 automated devices more generally,10 and artificial intelligence.11 Those issues will be important, and watching them unfold will be intriguing.
In the meantime, physicians and other health care professionals, health care facilities, technology companies, and patients must work within current legal structures in implementing and using robotic surgery. These are extraordinarily complex issues, so it is possible only to review the current landscape and speculate what the near future may hold.
Regulating surgical robots
The US Food and Drug Administration (FDA) is the primary regulator of robots used in medicine.12 It has the authority to regulate surgical devices, including surgical robots—which it refers to as “robotically-assisted surgical devices,” or RASD. In 2000, it approved Intuitive Surgical’s daVinci system for use in surgery. In 2017, the FDA expanded its clearance to include the Senhance System of TransEnterix Surgical Inc. for minimally invasive gynecologic surgery.13 In 2021, the FDA cleared the Hominis Surgical System for transvaginal hysterectomy “in certain patients.” However, the FDA emphasized that this clearance is for benign hysterectomy with salpingo-oophorectomy.14 (The FDA has cleared various robotic devices for several other areas of surgical practice, including neurosurgery, orthopedics, and urology.)
The use of robots in cancer surgery is limited. The FDA approved specific RASDs in some “surgical procedures commonly performed in patients with cancer, such as hysterectomy, prostatectomy, and colectomy.”15 However, it cautioned that this clearance was based only on a 30-day patient follow up. More specifically, the FDA “has not evaluated the safety or effectiveness of RASD devices for the prevention or treatment of cancer, based on cancer-related outcomes such as overall survival, recurrence, and disease-free survival.”15
The FDA has clearly warned physicians and patients that the agency has not granted the use of RASDs “for any cancer-related surgery marketing authorization, and therefore the survival benefits to patients compared to traditional surgery have not been established.”15 (This did not apply to the hysterectomy surgery as noted above. More specifically, that clearance did not apply to anything other than 30-day results, nor to the efficacy related to cancer survival.)
States also have some authority to regulate medical practice within their borders.9 When the FDA has approved a device as safe and effective, however, there are limits on what states can do to regulate or impose liability on the approved product. The Supreme Court held that the FDA approval “pre-empted” some state action regarding approved devices.16
Hospitals, of course, regulate what is allowed within the hospital. For example, it may require training before a physician is permitted to use equipment, limit the conditions for which the equipment may be used, or decline to obtain equipment for use in the hospitals.17 In the case of RASDs, however, the high cost of equipment may provide an incentive for hospitals to urge the wide use of the latest robotic acquisition.18
Regulation aims primarily to protect patients, usually from injury or inadequate treatment. Some robotic surgery is likely to be more expensive than the same surgery without robotic assistance. The cost to the patient is not usually part of the FDA’s consideration. Insurance companies (including Medicare and Medicaid), however, do care about costs and will set or negotiate how much the reimbursement will be for a procedure. Third-party payers may decline to cover the additional cost when there is no apparent benefit from using the robot.19 For some institutions, the public perception that it offers “the most modern technology” is an important public message and a strong incentive to have the equipment.20
There are inconsistent studies about the advantages and disadvantages of RADS in gynecologic procedures, although there are few randomized studies.21 The demonstrated advantages are generally identified as somewhat shorter recovery time.22 The ultimate goal will be to minimize risks while maximizing the many potential benefits of robotic surgery.23
Continue to: Liability...
Liability
A recent study by De Ravin and colleagues of robotic surgery liability found a 250% increase in the total number of robotic surgery–related malpractice claims reported in 7 recent years (2014-2021), compared with the prior 7 (2006-2013).24 However, the number of cases varied considerably from year to year. ObGyn had the most significant gain (from 19% to 49% of all claims). During the same time, urology claims declined from 56% to 16%. (The limitations of the study’s data are discussed later in this article.)
De Ravin et al reported the legal bases for the claims, but the specific legal claim was unclear in many cases.24 For example, the vast majority were classified as “negligent surgery.” Many cases made more than 1 legal claim for liability, so the total percentages were greater than 100%. Of the specific claims, many appear unrelated to robotic surgery (misdiagnosis, delayed treatment, or infection). However, there were a significant number of cases that raised issues that were related to robotic surgery. The following are those claims that probably relate to the “robotic” surgery, along with the percentage of cases making such a claim as reported24:
- “Patient not a candidate for surgery performed” appeared in about 13% of the cases.24 Such claims could include that the surgeon should have performed the surgery with traditional laparoscopy or open technique, but instead using a robot led to the injury. Physicians may feel pressure from patients or hospitals, because of the equipment’s cost, to use robotic surgery as it seems to be the modern approach (and therefore better). Neither reason is sufficient for using robotic assistance unless it will benefit the patient.
- “Failure to calibrate or operate robot” was in 11% of the claims.24 Physicians must properly calibrate and otherwise ensure that surgical equipment is operating correctly. In addition, the hospitals supplying the equipment must ensure that the equipment is maintained correctly. Finally, the equipment manufacturer may be liable through “products liability” if the equipment is defective.25 The expanding use of artificial intelligence in medical equipment (including surgical robots) is increasing the complexity of determining what “defective” means.11
- “Training deficiencies or credentialing” liability is a common problem with new technology. Physicians using new technology should be thoroughly trained and, where appropriate, certified in the use of the new technology.26 Early adopters of the technology should be especially cautious because good training may be challenging to obtain. In the study, the claims of inadequate training were particularly high during the early 7 years (35%), but dropped during the later time (4%).24
- “Improper positioning” of the patient or device or patient was raised in 7% of the cases.24
- “Manufacturing problems” were claimed in a small number of cases—13% in 2006-2013, but 2% in 2014-2021.24 These cases raise the complex question of products liability for robotic surgery and artificial intelligence (AI). Products liability has been part of surgical practice for many years. There usually will be liability if there are “defects” in a product, whether or not resulting from negligence. What a “defect” in a computer program means is a complicated issue that will be significant in future liability cases.27
Several other cases reported in the De Ravin study were probably related to robotic surgery. For example, Informed Consent and Failure to Monitor each appeared in more than 30%, of 2014-2021 cases, and Failure to Refer in 16% of the cases.24,27
The outcomes of the reported cases were mostly verdicts (or trial-related settlements) for defendants (doctors and hospitals). The defense prevailed 69% of the time in the early period and 78% of the time in 2014-2021. However, there were substantial damages in some cases. The range of damages in 2006-2013 was $95,000 to $6 million (mean, $2.5 million); in 2014-2021, it was $10,000 to $5 million (mean, $1.3 million).24
An earlier study looked at reported cases against Intuitive Surgical, maker of the daVinci system, from 2000-2017.28 Of the 108 claims in the study, 62% were gynecologic surgeries. Of these claims, 35% were dismissed, but “no other information regarding settlements or trial outcomes was available.” The study did not report the basis for the lawsuits involving gynecologic surgeries.
We should exercise caution in reviewing these studies. Although the studies were of considerable value, the authors note significant limitations of the databases available. The database was Westlaw in the first study discussed (“Robotic surgery: the impact”24) and Bloomberg in the second (“Robotic urologic”28). For example, the “impact” study was based on “jury verdict reports” excluding settlements, and the latter excluded class actions and cases settled. Thus the studies undoubtedly understated the number of claims made (those that resulted in settlement before a lawsuit was filed), cases filed but abandoned, and settlements made before trial.
Despite these limitations, the studies provide valuable insights into current malpractice risks and future directions. It is worth remembering that these cases nearly all involved a single robot, the daVinci, produced by Intuitive Surgical. It is not a “smart” robot and is commonly referred to as a “master-slave” machine. With much more intelligent and independent machines, the future will raise more complex problems in the FDA approval process and malpractice and product liability claims when things go wrong.
Continue to: What’s the verdict?...
What’s the verdict?
The case of VM and operating surgeon Dr. G illustrates several important legal aspects of using surgical robots. It also demonstrates that the presence of the robot assist still requires the surgeon’s careful attention to issues of informed consent, adequate specific training, and thorough follow up. In the following discussion, we divide the case review into the elements of negligence-malpractice (duty and breach, causation, and damages) and conclude with a thought about how to proceed when things have gone wrong.
Dr. G’s statement, “I’ve done a few,” is indefinite, but it may suggest that Dr. G. had not received full, supervised training in the robotic assist he was planning to use. That problem was underlined by the conclusion that Dr. G was a “relatively inexperienced robotic surgeon.” If so, that failure could constitute a breach of the duty of care to the patient. In addition, if it is inaccurate or did not provide information VM reasonably needed in consenting to Dr. G proceeding with the surgery, there could be an issue of whether there was a partial failure of fully informed consent.
The hospital also may have potential liability. It was “taken to task for granting privileges to an individual that had prior privilege ‘problems,’” suggesting that it had not performed adequate review before granting hospital privileges. Furthermore, if Dr. G was not sufficiently practiced or supervised in robotic surgery, the hospital, which allowed Dr. G to proceed, might also be negligent.
VM had a series of problems postsurgery that ultimately resulted in additional care and “simple fistula repair.” Assuming that there was negligence, the next question is whether that failure caused the injury. Causation may be the most difficult part of the case for VM to prove. It would require expert testimony that the inadequate surgery (inappropriate use of robotic surgery or other error during surgery) and follow up resulted in the formation or increase in the likelihood of the fistula.
VM would also have to prove damages. Damages are those costs (the economic value) of injuries that would not have occurred but for negligence. Damages would include most of the cost of the follow-up medical care and any related additional future care required, plus costs that were a consequence of the negligence (such as lost work). In addition, damages would include pain and suffering that resulted from the negligence, subject to caps in some states.
When the patient was dissatisfied and reported a postsurgical problem, the hospital and Dr. G may have had an opportunity to avoid further dissatisfaction, complaints, and ultimately a lawsuit. Effective approaches for dealing with such dissatisfaction may serve the institution’s and physician’s values and financial best interests.
The jury verdict was in favor of the plaintiff. Jurors felt the operating surgeon should have conveyed his experience with robotic surgery more clearly as part of the informed consent process.
“Hey Siri! Perform a type 3 hysterectomy. Please watch out for the ureter!”29
Medicine is still at the frontier of surgical robots. Over future decades, the number and sophistication of these machines will increase substantially. They likely will become much more like robots, guided by AI, and make independent judgments. These have the potential for significant medical progress that improves the treatment of patients. At the same time, the last 20 years suggest that robotic innovation will challenge medicine, the FDA and other regulators, lawmakers, and courts. In the future, regulators and patients should embrace genuine advances in robotic surgery but not be dazzled by these new machines’ luster (or potential for considerable profits).30
The public may be wildly optimistic about the benefits without balancing the risks. The AI that runs them will be essentially invisible and constantly changing. Physicians and regulators must develop new techniques for assessing and controlling the software. Real surgical robots require rigorous testing, cautious promotion, disciplined use, and perpetual review. ●
- Petersen S, Doe S, Rubinfield I, et al. Rate of urologic injury with robotic hysterectomy. J Min Invasc Gynecol. 2018;25:867-871.
- Makinen J, Johansson J, Toma C, et al. Morbidity of 10,110 hysterectomies by type approach. Hum Reprod. 2001;16:1473-1478.
- Karasu A, Kran G, Sanlikan F. Intraoperative complications and conversion to laparotomy in gynecologic robotic surgery. J Investig Surg. 2022;35:912-915.
- Behbehani S, Suarez-Salvador E, Buras M, et al. Mortality rates in benign laparoscopic and robotic surgery: a systematic review and meta-analysis. J Min Invasc. 2020;27:603-612.
- Giurdano S, Victorzon M. Laparoscopic roux-en-Y gastric bypass in elderly patients (60 years or older): a meta-analysis of comparative studies. Scand J Surg. 2018;107:6-11.
- Marra A, Pulg-Asensio M, Edmond M, et al. Infectious complications of laparoscopic and robotic hysterectomy: a systematic literature review and meta-analysis. Int J Gynecol Cancer. 2019;29:518-530.
- Tse KY, Sheung H, Lim P. Robot-assisted gyneaecological cancer surgery-complications and prevention. Best Pract Res Clin Obstet Gynaecol. 2017;25:94-105.
- Hubbard FP. Sophisticated robots: balancing liability, regulation, and innovation. Fla Law Rev. 2014;66:1803-1872. https://scholarship.law.ufl.edu/cgi/viewcontent. cgi?article=1204&context=flr. Accessed December 20, 2022.
- Villanueva A. The legal battle with the future of autonomous surgical robotics. Ind Health Law Rev. 2020;17:367-392. https://journals.iupui.edu/index.php/ihlr/article /download/25051/23544. Accessed December 20, 2022.
- Lemley MA, Casey B. Remedies for robots. U Chi Law Rev. 2019;86:1311-1396. https://chicagounbound.uchicago.edu /cgi/viewcontent.cgi?article=6140&context=uclrev. Accessed December 20, 2022.
- Griffin F. Artificial intelligence and liability in health care. Health Matrix. 2021;31:65-106. https://scholarlycommons. law.case.edu/cgi/viewcontent.cgi?article=1659&context=hea lthmatrix. Accessed December 20, 2022.
- Britton D. Autonomous surgery: the law of autonomous surgical robots. J Law Tech Tex. 2017;1:152-189.
- US Food and Drug Administration. FDA clears new robotically-assisted surgical device for adult patients. October 13, 2017. https://www.fda.gov/news-events/press-announcements /fda-clears-new-robotically-assisted-surgical-device-adult -patients. Accessed December 20, 2022.
- US Food and Drug Administration. FDA authorizes first robotically-assisted surgical device for performing transvaginal hysterectomy. March 1, 2021. https://www.fda .gov/news-events/press-announcements/fda-authorizes -first-robotically-assisted-surgical-device-performing -transvaginal-hysterectomy. Accessed December 20, 2022.
- US Food and Drug Administration. Caution with robotically-assisted surgical devices in mastectomy: FDA Safety Communication, August 20, 2021. https://www.fda.gov/medical-devices/safety-communications/update-caution-robotically-assisted-surgical-devices-mastectomy-fda-safety-communication. Accessed December 22, 2022. Riegel v Medtronic, 552 US 312 (2008).
- Han ES, Advincula AP. Robotic surgery: advancements and inflection points in the field of gynecology. Obstet Gynecol Clin North Am. 2021;48:759-776.
- Witharm H. Robot-assisted surgery: an analysis of the legal and economic implications. Az J Interdisciplinary Studies. 2022;8:19-29. https://journals.librarypublishing.arizona.edu /azjis/article/id/5093/download/pdf/.
- Cameron S. Is daVinci robotic surgery a revolution or a rip-off? Healthline. August 10, 2016. https://www.healthline .com/health-news/is-da-vinci-robotic-surgery-revolution -or-ripoff-021215. Accessed December 20, 2022.
- Perez RE, Schwaitzberg SD. Robotic surgery: finding value in 2019 and beyond. Ann Laparosc Endosc Surg. 2019;4:1-7.
- Gitas G, Hanker L, Rody A, et al. Robotic surgery in gynecology: is the future already here? Minim Invasiv Therapy Allied Technol. 2022;4:1-0.
- Moon AS, Garofalo J, Koirala P, et al. Robotic surgery in gynecology. Surgical Clinics. 2020;100:445-460.
- Simshaw D, Terry N, Hauser K, et al. Regulating healthcare robots: maximizing opportunities while minimizing risks. Richmond J Law Tech. 2015;22:1-38. https://scholar works.iupui.edu/bitstream/handle/1805/11587/simshaw _2015_regulating.pdf?sequence=1&isAllowed=y. Accessed December 20, 2022.
- De Ravin E, Sell EA, Newman JG, et al. Medical malpractice in robotic surgery: a Westlaw database analysis. J Robotic Surg. 2022. https://doi.org/10.1007/s11701-022-01417-6. https:// link.springer.com/article/10.1007/s11701-022-014176#citeas. Accessed December 20, 2022.
- Beglinger C. A broken theory: the malfunction theory of strict products liability and the need for a new doctrine in the field of surgical robotics. Minnesotta Law Rev. 2019;104:1041-1093. . Accessed December 20, 2022.
- Azadi S, Green IC, Arnold A, et al. Robotic surgery: the impact of simulation and other innovative platforms on performance and training. J Minim Invasiv Gynecol. 2021;28:490-495.
- Koerner D. Doctor roboto: The no-man operation. U Tol L Rev. 2019;51:125-146.
- Nik-Ahd F, Souders CP, Zhao H, et al. Robotic urologic surgery: trends in litigation over the last decade. J Robotic Surg. 2019;13:729-734.
- Gültekin CalibriİB, Karabük E, Köse MF. “Hey Siri! Perform a type 3 hysterectomy. Please watch out for the ureter!” What is autonomous surgery and what are the latest developments? J Turk Ger Gynecol Assoc. 2021;22:58-70. https://www.ncbi .nlm.nih.gov/pmc/articles/PMC7944239/.
- Matsuzaki T. Ethical issues of artificial intelligence in medicine. California West Law Rev. 2018;55:255-273. https://scholarlycommons.law.cwsl.edu/cgi/viewcontent. cgi?article=1669&context=cwlr. Accessed December 20, 2022.
- Petersen S, Doe S, Rubinfield I, et al. Rate of urologic injury with robotic hysterectomy. J Min Invasc Gynecol. 2018;25:867-871.
- Makinen J, Johansson J, Toma C, et al. Morbidity of 10,110 hysterectomies by type approach. Hum Reprod. 2001;16:1473-1478.
- Karasu A, Kran G, Sanlikan F. Intraoperative complications and conversion to laparotomy in gynecologic robotic surgery. J Investig Surg. 2022;35:912-915.
- Behbehani S, Suarez-Salvador E, Buras M, et al. Mortality rates in benign laparoscopic and robotic surgery: a systematic review and meta-analysis. J Min Invasc. 2020;27:603-612.
- Giurdano S, Victorzon M. Laparoscopic roux-en-Y gastric bypass in elderly patients (60 years or older): a meta-analysis of comparative studies. Scand J Surg. 2018;107:6-11.
- Marra A, Pulg-Asensio M, Edmond M, et al. Infectious complications of laparoscopic and robotic hysterectomy: a systematic literature review and meta-analysis. Int J Gynecol Cancer. 2019;29:518-530.
- Tse KY, Sheung H, Lim P. Robot-assisted gyneaecological cancer surgery-complications and prevention. Best Pract Res Clin Obstet Gynaecol. 2017;25:94-105.
- Hubbard FP. Sophisticated robots: balancing liability, regulation, and innovation. Fla Law Rev. 2014;66:1803-1872. https://scholarship.law.ufl.edu/cgi/viewcontent. cgi?article=1204&context=flr. Accessed December 20, 2022.
- Villanueva A. The legal battle with the future of autonomous surgical robotics. Ind Health Law Rev. 2020;17:367-392. https://journals.iupui.edu/index.php/ihlr/article /download/25051/23544. Accessed December 20, 2022.
- Lemley MA, Casey B. Remedies for robots. U Chi Law Rev. 2019;86:1311-1396. https://chicagounbound.uchicago.edu /cgi/viewcontent.cgi?article=6140&context=uclrev. Accessed December 20, 2022.
- Griffin F. Artificial intelligence and liability in health care. Health Matrix. 2021;31:65-106. https://scholarlycommons. law.case.edu/cgi/viewcontent.cgi?article=1659&context=hea lthmatrix. Accessed December 20, 2022.
- Britton D. Autonomous surgery: the law of autonomous surgical robots. J Law Tech Tex. 2017;1:152-189.
- US Food and Drug Administration. FDA clears new robotically-assisted surgical device for adult patients. October 13, 2017. https://www.fda.gov/news-events/press-announcements /fda-clears-new-robotically-assisted-surgical-device-adult -patients. Accessed December 20, 2022.
- US Food and Drug Administration. FDA authorizes first robotically-assisted surgical device for performing transvaginal hysterectomy. March 1, 2021. https://www.fda .gov/news-events/press-announcements/fda-authorizes -first-robotically-assisted-surgical-device-performing -transvaginal-hysterectomy. Accessed December 20, 2022.
- US Food and Drug Administration. Caution with robotically-assisted surgical devices in mastectomy: FDA Safety Communication, August 20, 2021. https://www.fda.gov/medical-devices/safety-communications/update-caution-robotically-assisted-surgical-devices-mastectomy-fda-safety-communication. Accessed December 22, 2022. Riegel v Medtronic, 552 US 312 (2008).
- Han ES, Advincula AP. Robotic surgery: advancements and inflection points in the field of gynecology. Obstet Gynecol Clin North Am. 2021;48:759-776.
- Witharm H. Robot-assisted surgery: an analysis of the legal and economic implications. Az J Interdisciplinary Studies. 2022;8:19-29. https://journals.librarypublishing.arizona.edu /azjis/article/id/5093/download/pdf/.
- Cameron S. Is daVinci robotic surgery a revolution or a rip-off? Healthline. August 10, 2016. https://www.healthline .com/health-news/is-da-vinci-robotic-surgery-revolution -or-ripoff-021215. Accessed December 20, 2022.
- Perez RE, Schwaitzberg SD. Robotic surgery: finding value in 2019 and beyond. Ann Laparosc Endosc Surg. 2019;4:1-7.
- Gitas G, Hanker L, Rody A, et al. Robotic surgery in gynecology: is the future already here? Minim Invasiv Therapy Allied Technol. 2022;4:1-0.
- Moon AS, Garofalo J, Koirala P, et al. Robotic surgery in gynecology. Surgical Clinics. 2020;100:445-460.
- Simshaw D, Terry N, Hauser K, et al. Regulating healthcare robots: maximizing opportunities while minimizing risks. Richmond J Law Tech. 2015;22:1-38. https://scholar works.iupui.edu/bitstream/handle/1805/11587/simshaw _2015_regulating.pdf?sequence=1&isAllowed=y. Accessed December 20, 2022.
- De Ravin E, Sell EA, Newman JG, et al. Medical malpractice in robotic surgery: a Westlaw database analysis. J Robotic Surg. 2022. https://doi.org/10.1007/s11701-022-01417-6. https:// link.springer.com/article/10.1007/s11701-022-014176#citeas. Accessed December 20, 2022.
- Beglinger C. A broken theory: the malfunction theory of strict products liability and the need for a new doctrine in the field of surgical robotics. Minnesotta Law Rev. 2019;104:1041-1093. . Accessed December 20, 2022.
- Azadi S, Green IC, Arnold A, et al. Robotic surgery: the impact of simulation and other innovative platforms on performance and training. J Minim Invasiv Gynecol. 2021;28:490-495.
- Koerner D. Doctor roboto: The no-man operation. U Tol L Rev. 2019;51:125-146.
- Nik-Ahd F, Souders CP, Zhao H, et al. Robotic urologic surgery: trends in litigation over the last decade. J Robotic Surg. 2019;13:729-734.
- Gültekin CalibriİB, Karabük E, Köse MF. “Hey Siri! Perform a type 3 hysterectomy. Please watch out for the ureter!” What is autonomous surgery and what are the latest developments? J Turk Ger Gynecol Assoc. 2021;22:58-70. https://www.ncbi .nlm.nih.gov/pmc/articles/PMC7944239/.
- Matsuzaki T. Ethical issues of artificial intelligence in medicine. California West Law Rev. 2018;55:255-273. https://scholarlycommons.law.cwsl.edu/cgi/viewcontent. cgi?article=1669&context=cwlr. Accessed December 20, 2022.
Medication Overuse Headache (MOH): Prevention and Treatment
Medication overuse headache, previously known as rebound headache or medication-induced headache, may be caused by the frequent or excessive use of various acute care medications. When these medications are used too frequently, they can cause headaches rather than relieving them. (Some headache specialists feel that MOH is the result of recurring severe headaches, and the patients’ overuse of medications to relieve them.) These medications, some of which are painkillers or analgesics, include over-the-counter products such as acetaminophen, aspirin, and anti-inflammatories, as well as prescription medications such as triptans, ergots opioids, opioids, and barbiturates. The one category of acute care medication that does not seem to cause MOH is the gepants, such as rimegepant and ubrogepant.
MOH is the fourth most common headache disorder. It is defined by the International Classification of Headache Disorders (ICHD-3) as a headache present 15 days per month, evolving from regular use of strong acute medication (10 or more days of triptans, ergotamines, butalbital medications, opioids, or combination medications or 15 or more days per month of simple analgesics such as aspirin, acetaminophen, or nonsteroidal anti-inflammatories) for 3 months.
Patients are usually not aware they have MOH, and this is the most problematic aspect of the condition. Patients do not realize that the medicine they are taking is making their headaches worse. It can be difficult to explain to the patient exactly what is going on with MOH, and why they are doing the wrong thing by taking the very medication that was prescribed by their doctor to stop a migraine attack. Many doctors do not fully understand MOH either, which can make it difficult to treat patients with this type of headache; therefore, it is imperative to educate both doctors and patients on the causes and treatments of MOH.
One of the most important facets of treating MOH traditionally has been the process of detoxifying patients from their overused medication by gradually or precipitously withdrawing the offending medication. There is variability in how detoxification can be accomplished. Some of my patients stopped medications abruptly and experienced very bad headaches. Others tried reducing dosages on their own and reported experiencing the worst headaches of their lives—some of which lasted for a few weeks. I have found that if patients can endure 2 to 3 weeks of detox, they start to feel better. But because the headaches can worsen before they get better, patients understandably try to avoid the detoxification process.
I start patients on preventive medicine, then slowly increase it to an effective dose, and have them come back in a month for an evaluation. I then have them gradually reduce, but not completely stop, the pain medication before they return. Once I feel their preventive medication is at a therapeutic level, I have them begin a slow detox. After a month of preventive medication, there is a reasonable chance that headaches will start to decrease and be less severe. I tell them that if their headache is less severe try to avoid taking the medicine that they were overusing to prevent perpetuating the MOH.
One plausible physiologic mechanism behind MOH is that chronic exposure to acute care migraine treatment leads to suppression of the serotonergic/norepinephrinergic endogenous antinociceptive system in the upper brain stem, with facilitation of the trigeminal nociceptive process via up-regulation of calcitonin gene-related peptide (CGRP).This increase in CGRP at the end of peripheral nerve terminals in the trigeminovascular system may facilitate pain transmission. An increase in cortical CGRP may cause cortical spreading depression: a wave of excitement traveling through the cortex, followed by a wave of electrical depression seems to cause headache.
Good, effective prevention often helps avoid MOH; medications such as topiramate, nortriptyline, gabapentin, onabotulinumtoxinA, and CGRP monoclonal antibodies or some type of local nerve block have improved MOH in patients, but detoxification is usually necessary is some patients.
Monoclonal antibodies targeting CGRP or its receptor (CGRP-R), given by subcutaneous or intravenous injection or small molecule CGRP receptor antagonists given orally (gepants), seem to be able to treat MOH in some patients without a detoxification. This has been best demonstrated in the monoclonal antibody group, but there is some evidence showing that it may also occur with gepants. These treatments seem to work even when patients are overusing acute care medications; this helps some patients to self-detoxify at their own pace, which is easier for both the patient and the doctor.
Currently, there are 4 monoclonal antibodies against CGRP or the CGRP-R. Erenumab is the only completely human one and the only antibody that blocks the CGRP receptor to prevent the CGRP ligand from docking and exerting its effect. The other 3 (fremanezumab, galcanezumab, and eptinezumab) are humanized monoclonal antibodies that selectively bind to the CGRP ligand, preventing it from docking on its receptor. Patients started on the monoclonal antibodies against CGRP or its receptor usually have fewer headaches in the first week or two of therapy, and this helps make the self-detox easier for the patient.
Further, substantial data have shown that onabotulinumtoxinA reduces the number/frequency of headaches and reduces the need for patients to take acute medication. OnabotulinumtoxinA is currently the only medication approved for preventive treatment of chronic migraine; it has long-term safety data available and has reported efficacy lasting for up to 3 years when given in multiple injection sites every 3 months. Interestingly, although topiramate is used as a preventive medication, a recent study comparing erenumab vs topiramate for reducing monthly migraine days (MMD) showed that erenumab outperformed topiramate with a 50% reduction in MMD, and with fewer reported adverse events.
We are just starting to learn about some other potential cellular mechanisms that could be causing MOH in patients; these data could help create new and improved therapies for treating and possibly preventing MOH in the future. Patient outcomes could also be improved by encouraging the inclusion of MOH as part of a continuing education program for physicians who could potentially be treating new patients presenting with MOH.
Medication overuse headache, previously known as rebound headache or medication-induced headache, may be caused by the frequent or excessive use of various acute care medications. When these medications are used too frequently, they can cause headaches rather than relieving them. (Some headache specialists feel that MOH is the result of recurring severe headaches, and the patients’ overuse of medications to relieve them.) These medications, some of which are painkillers or analgesics, include over-the-counter products such as acetaminophen, aspirin, and anti-inflammatories, as well as prescription medications such as triptans, ergots opioids, opioids, and barbiturates. The one category of acute care medication that does not seem to cause MOH is the gepants, such as rimegepant and ubrogepant.
MOH is the fourth most common headache disorder. It is defined by the International Classification of Headache Disorders (ICHD-3) as a headache present 15 days per month, evolving from regular use of strong acute medication (10 or more days of triptans, ergotamines, butalbital medications, opioids, or combination medications or 15 or more days per month of simple analgesics such as aspirin, acetaminophen, or nonsteroidal anti-inflammatories) for 3 months.
Patients are usually not aware they have MOH, and this is the most problematic aspect of the condition. Patients do not realize that the medicine they are taking is making their headaches worse. It can be difficult to explain to the patient exactly what is going on with MOH, and why they are doing the wrong thing by taking the very medication that was prescribed by their doctor to stop a migraine attack. Many doctors do not fully understand MOH either, which can make it difficult to treat patients with this type of headache; therefore, it is imperative to educate both doctors and patients on the causes and treatments of MOH.
One of the most important facets of treating MOH traditionally has been the process of detoxifying patients from their overused medication by gradually or precipitously withdrawing the offending medication. There is variability in how detoxification can be accomplished. Some of my patients stopped medications abruptly and experienced very bad headaches. Others tried reducing dosages on their own and reported experiencing the worst headaches of their lives—some of which lasted for a few weeks. I have found that if patients can endure 2 to 3 weeks of detox, they start to feel better. But because the headaches can worsen before they get better, patients understandably try to avoid the detoxification process.
I start patients on preventive medicine, then slowly increase it to an effective dose, and have them come back in a month for an evaluation. I then have them gradually reduce, but not completely stop, the pain medication before they return. Once I feel their preventive medication is at a therapeutic level, I have them begin a slow detox. After a month of preventive medication, there is a reasonable chance that headaches will start to decrease and be less severe. I tell them that if their headache is less severe try to avoid taking the medicine that they were overusing to prevent perpetuating the MOH.
One plausible physiologic mechanism behind MOH is that chronic exposure to acute care migraine treatment leads to suppression of the serotonergic/norepinephrinergic endogenous antinociceptive system in the upper brain stem, with facilitation of the trigeminal nociceptive process via up-regulation of calcitonin gene-related peptide (CGRP).This increase in CGRP at the end of peripheral nerve terminals in the trigeminovascular system may facilitate pain transmission. An increase in cortical CGRP may cause cortical spreading depression: a wave of excitement traveling through the cortex, followed by a wave of electrical depression seems to cause headache.
Good, effective prevention often helps avoid MOH; medications such as topiramate, nortriptyline, gabapentin, onabotulinumtoxinA, and CGRP monoclonal antibodies or some type of local nerve block have improved MOH in patients, but detoxification is usually necessary is some patients.
Monoclonal antibodies targeting CGRP or its receptor (CGRP-R), given by subcutaneous or intravenous injection or small molecule CGRP receptor antagonists given orally (gepants), seem to be able to treat MOH in some patients without a detoxification. This has been best demonstrated in the monoclonal antibody group, but there is some evidence showing that it may also occur with gepants. These treatments seem to work even when patients are overusing acute care medications; this helps some patients to self-detoxify at their own pace, which is easier for both the patient and the doctor.
Currently, there are 4 monoclonal antibodies against CGRP or the CGRP-R. Erenumab is the only completely human one and the only antibody that blocks the CGRP receptor to prevent the CGRP ligand from docking and exerting its effect. The other 3 (fremanezumab, galcanezumab, and eptinezumab) are humanized monoclonal antibodies that selectively bind to the CGRP ligand, preventing it from docking on its receptor. Patients started on the monoclonal antibodies against CGRP or its receptor usually have fewer headaches in the first week or two of therapy, and this helps make the self-detox easier for the patient.
Further, substantial data have shown that onabotulinumtoxinA reduces the number/frequency of headaches and reduces the need for patients to take acute medication. OnabotulinumtoxinA is currently the only medication approved for preventive treatment of chronic migraine; it has long-term safety data available and has reported efficacy lasting for up to 3 years when given in multiple injection sites every 3 months. Interestingly, although topiramate is used as a preventive medication, a recent study comparing erenumab vs topiramate for reducing monthly migraine days (MMD) showed that erenumab outperformed topiramate with a 50% reduction in MMD, and with fewer reported adverse events.
We are just starting to learn about some other potential cellular mechanisms that could be causing MOH in patients; these data could help create new and improved therapies for treating and possibly preventing MOH in the future. Patient outcomes could also be improved by encouraging the inclusion of MOH as part of a continuing education program for physicians who could potentially be treating new patients presenting with MOH.
Medication overuse headache, previously known as rebound headache or medication-induced headache, may be caused by the frequent or excessive use of various acute care medications. When these medications are used too frequently, they can cause headaches rather than relieving them. (Some headache specialists feel that MOH is the result of recurring severe headaches, and the patients’ overuse of medications to relieve them.) These medications, some of which are painkillers or analgesics, include over-the-counter products such as acetaminophen, aspirin, and anti-inflammatories, as well as prescription medications such as triptans, ergots opioids, opioids, and barbiturates. The one category of acute care medication that does not seem to cause MOH is the gepants, such as rimegepant and ubrogepant.
MOH is the fourth most common headache disorder. It is defined by the International Classification of Headache Disorders (ICHD-3) as a headache present 15 days per month, evolving from regular use of strong acute medication (10 or more days of triptans, ergotamines, butalbital medications, opioids, or combination medications or 15 or more days per month of simple analgesics such as aspirin, acetaminophen, or nonsteroidal anti-inflammatories) for 3 months.
Patients are usually not aware they have MOH, and this is the most problematic aspect of the condition. Patients do not realize that the medicine they are taking is making their headaches worse. It can be difficult to explain to the patient exactly what is going on with MOH, and why they are doing the wrong thing by taking the very medication that was prescribed by their doctor to stop a migraine attack. Many doctors do not fully understand MOH either, which can make it difficult to treat patients with this type of headache; therefore, it is imperative to educate both doctors and patients on the causes and treatments of MOH.
One of the most important facets of treating MOH traditionally has been the process of detoxifying patients from their overused medication by gradually or precipitously withdrawing the offending medication. There is variability in how detoxification can be accomplished. Some of my patients stopped medications abruptly and experienced very bad headaches. Others tried reducing dosages on their own and reported experiencing the worst headaches of their lives—some of which lasted for a few weeks. I have found that if patients can endure 2 to 3 weeks of detox, they start to feel better. But because the headaches can worsen before they get better, patients understandably try to avoid the detoxification process.
I start patients on preventive medicine, then slowly increase it to an effective dose, and have them come back in a month for an evaluation. I then have them gradually reduce, but not completely stop, the pain medication before they return. Once I feel their preventive medication is at a therapeutic level, I have them begin a slow detox. After a month of preventive medication, there is a reasonable chance that headaches will start to decrease and be less severe. I tell them that if their headache is less severe try to avoid taking the medicine that they were overusing to prevent perpetuating the MOH.
One plausible physiologic mechanism behind MOH is that chronic exposure to acute care migraine treatment leads to suppression of the serotonergic/norepinephrinergic endogenous antinociceptive system in the upper brain stem, with facilitation of the trigeminal nociceptive process via up-regulation of calcitonin gene-related peptide (CGRP).This increase in CGRP at the end of peripheral nerve terminals in the trigeminovascular system may facilitate pain transmission. An increase in cortical CGRP may cause cortical spreading depression: a wave of excitement traveling through the cortex, followed by a wave of electrical depression seems to cause headache.
Good, effective prevention often helps avoid MOH; medications such as topiramate, nortriptyline, gabapentin, onabotulinumtoxinA, and CGRP monoclonal antibodies or some type of local nerve block have improved MOH in patients, but detoxification is usually necessary is some patients.
Monoclonal antibodies targeting CGRP or its receptor (CGRP-R), given by subcutaneous or intravenous injection or small molecule CGRP receptor antagonists given orally (gepants), seem to be able to treat MOH in some patients without a detoxification. This has been best demonstrated in the monoclonal antibody group, but there is some evidence showing that it may also occur with gepants. These treatments seem to work even when patients are overusing acute care medications; this helps some patients to self-detoxify at their own pace, which is easier for both the patient and the doctor.
Currently, there are 4 monoclonal antibodies against CGRP or the CGRP-R. Erenumab is the only completely human one and the only antibody that blocks the CGRP receptor to prevent the CGRP ligand from docking and exerting its effect. The other 3 (fremanezumab, galcanezumab, and eptinezumab) are humanized monoclonal antibodies that selectively bind to the CGRP ligand, preventing it from docking on its receptor. Patients started on the monoclonal antibodies against CGRP or its receptor usually have fewer headaches in the first week or two of therapy, and this helps make the self-detox easier for the patient.
Further, substantial data have shown that onabotulinumtoxinA reduces the number/frequency of headaches and reduces the need for patients to take acute medication. OnabotulinumtoxinA is currently the only medication approved for preventive treatment of chronic migraine; it has long-term safety data available and has reported efficacy lasting for up to 3 years when given in multiple injection sites every 3 months. Interestingly, although topiramate is used as a preventive medication, a recent study comparing erenumab vs topiramate for reducing monthly migraine days (MMD) showed that erenumab outperformed topiramate with a 50% reduction in MMD, and with fewer reported adverse events.
We are just starting to learn about some other potential cellular mechanisms that could be causing MOH in patients; these data could help create new and improved therapies for treating and possibly preventing MOH in the future. Patient outcomes could also be improved by encouraging the inclusion of MOH as part of a continuing education program for physicians who could potentially be treating new patients presenting with MOH.
Cardiac Adverse Events Following COVID-19 Vaccination in Patients With Prior Vaccine-Associated Myocarditis
Vaccinations have substantially reduced morbidity and mortality from many infectious diseases. Despite the clear value of vaccinations in public health, efforts to better understand adverse events (AEs) following immunization are important to sustain public trust and vaccine confidence. Noninfectious inflammation of the heart may manifest as myocarditis or pericarditis, or occasionally, with shared signs and symptoms of each, as myopericarditis. This is a rare AE following some immunizations. Vaccine-associated myocarditis, pericarditis, or myopericarditis (VAMP) has been most clearly associated with smallpox vaccines and mRNA COVID-19 vaccines.1-6 Although extremely rare, VAMP also has been associated with other vaccines.7,8 Limited information exists to guide shared clinical decision making on COVID-19 vaccination in persons with a history of VAMP. It is unknown whether individuals with a history of VAMP are at higher risk for developing a recurrence or experiencing a more severe outcome following COVID-19 vaccination.
Methods
As part of the collaborative public health mission with the Centers for Disease Control and Prevention (CDC) for enhanced vaccine AE surveillance, the Defense Health Agency Immunization Healthcare Division (IHD) maintains a clinical database of service members and beneficiaries referred for suspected AEs following immunizations. A review of all AEs following immunization cases in this database from January 1, 2003, through February 28, 2022, identified individuals meeting the following criteria: (a) VAMP prior to receipt of COVID-19 vaccine; (b) receipt of COVID-19 vaccine in 2021; and (c) medical documentation in available electronic health records sufficient to describe health status at least 30 days following COVID-19 vaccination.9 If medical entries suggested cardiac symptoms following a COVID-19 vaccine, additional information was sought to verify VAMP based on current published criteria.10,11 Both the initial VAMP cases and the suspected COVID-19 VAMP cases were adjudicated by a team of vaccine experts and specialists in immunology, cardiology, and preventive medicine.
This retrospective review was approved and conducted in accordance with the Walter Reed National Military Medical Center Institutional Review Board protocol #20664. All individuals with recurrent VAMP consented to share their health records and clinical details.
Results
Among 9260 cases in the IHD database, 431 met the case definition for VAMP.
Among the 179 patients included in this analysis, 171 (96%) were male. Their median age was 39 years at the time of COVID-19 vaccination.
Within 1 month of receipt of any COVID-19 vaccine, 11 individuals had documented symptoms suggesting cardiac involvement, specifically, chest pain, palpitations, or dyspnea. After cardiac evaluation, 4 patients met the criteria for VAMP after COVID-19 vaccination.10,11 Seven patients either did not meet the criteria for VAMP or had alternative causes for their symptoms.
Two men aged 49 and 50 years with a history of vaccine-associated myocarditis following smallpox vaccination (Dryvax and ACAM2000) developed myocarditis 3 days after their second dose of the Moderna vaccine. One of these patients received a Pfizer-BioNTech booster 10 months later with no recurrence of symptoms. A 55-year-old man with a history of vaccine-associated myocarditis following Dryvax vaccination developed myocarditis 2 days after his Pfizer-BioNTech booster. None of the patients who developed post-COVID-19 VAMP reported residual symptoms from their initial VAMP episode, which occurred 12 to 18 years earlier. All were hospitalized briefly for observation and had complete symptom resolution within 6 weeks.
A 25-year-old man developed pericarditis 4 days after his second Pfizer-BioNTech vaccination. His previous ACAM2000 vaccine-associated myocarditis occurred 3 years earlier, with no residual symptoms. Of note, he had a mild COVID-19 infection 78 days before the onset of his pericarditis. After the onset of his COVID-19 vaccine-associated pericarditis, he continued to experience transient bouts of chest pressure and exertional dyspnea that resolved within 7 months of onset.
The median interval between COVID-19 vaccine doses in those who developed post-COVID-19 VAMP was within the recommended mRNA vaccine dosing intervals of 3 to 4 weeks and was consistent with the median mRNA vaccine dosing intervals among the entire cohort.
Due to the small cohort size and other limitations of this study, the suggested rate of cardiac injury in this review (4 cases in 179 persons, or 2.2%) is an imprecise estimate of risk in a small population (95% CI, 0.1%-4.4%). While this rate may seem higher than expected within the general population after COVID-19 vaccination, it is lower than the estimated lifetime risk of recurrent myocarditis from any cause.6,12
Discussion
To our knowledge, this is the first report describing cardiac outcomes after COVID-19 vaccination among a cohort of individuals with prior history of VAMP. Four cases of COVID-19 VAMP were identified among 179 patients with previous VAMP. All cases had experienced VAMP after the smallpox vaccine several years earlier, with complete resolution of symptoms. Three cases presented with recurrent VAMP after their second dose of an mRNA COVID-19 vaccine, and one after an mRNA booster dose. All fully recovered over the course of several months.
Myocarditis is a heterogeneous inflammatory injury with diverse, sometimes idiopathic, etiologies.13 In contrast to infection-related cardiac injury, prior reports of vaccine-associated myocarditis have suggested a hypersensitivity reaction characterized by patchy eosinophilic infiltrates, a benign clinical course, and good prognosis.2,3
There are several common features between VAMP after smallpox and COVID-19 vaccination. Cases occur predominantly in young men. The onset of symptoms after smallpox vaccine (mean, 10 days) and after mRNA COVID-19 vaccine (mean, 3 days) appears to correspond to the timing of peak postvaccination pro-inflammatory cytokine elevation.14 While all VAMP cases are serious events, the majority of patients appear to have a relatively benign clinical course with rapid and full recovery.13
Patients who have experienced an inflammatory cardiac injury may be at higher risk for recurrence, but quantifying risk of this rare phenomenon is challenging. Cases of VAMP after the COVID-19 vaccine have occasionally been reported in patients with previous cardiac injury unrelated to vaccination.15-17 The cases presented here represent the first report of recurrent VAMP following prior non-COVID-19 vaccinations.
Most patients with prior VAMP in this cohort did not experience cardiac-suggestive symptoms following COVID-19 vaccination. Among 11 patients who developed symptoms, 3 had confirmed myocarditis and 1 had confirmed pericarditis. The clinical course for these patients with recurrent VAMP was observed to be no different in severity or duration from those who experience new-onset VAMP.4 All other patients not meeting criteria for VAMP or having alternative explanations for their symptoms also had a benign clinical course. Nonetheless, of the study cohort of 179, recurrent VAMP was diagnosed in 4 of the 11 who developed cardiac-suggestive symptoms following COVID-19 vaccination. The importance of cardiac evaluation should be emphasized for any patient presenting with chest pain, dyspnea, or other cardiac-suggestive symptoms following vaccination.
Strengths and Limitations
The strength of this review of VAMP recurrence associated with COVID-19 vaccination derives from our large and unique longitudinal database of VAMP among current and prior service members. Additionally, the IHD’s ongoing enhanced vaccine AEs surveillance provides the opportunity to contact patients and review their electronic health records over an extended interval of time.
When interpreting this report’s implications, limitations inherent to any retrospective case review should be considered. The cohort of cases of prior VAMP included primarily healthy, fit, young service members; this population is not representative of the general population. The cohort included prior VAMP cases that generally occurred after smallpox vaccination. Experiences after smallpox vaccine may not apply to cardiac injury from other vaccines or etiologies. By the nature of this review, the population studied at the time of COVID-19 vaccination was somewhat older than those most likely to develop an initial bout of VAMP.2 This review was limited by information available in the electronic health records of a small number of patients. Subclinical cases of VAMP and cases without adequate clinical evaluation also could not be included.
Conclusions
Noninfectious inflammation of the heart (myocarditis, pericarditis, or myopericarditis) is a rare AE following certain vaccines, especially live replicating smallpox vaccine and mRNA COVID-19 vaccines. In this observational analysis, the majority of patients with previous VAMP successfully received a COVID-19 vaccine without recurrence. The 4 patients who were identified with recurrent VAMP following COVID-19 vaccination all recovered with supportive care. While the CDC endorses that individuals with a history of infectious myocarditis may receive COVID-19 vaccine after symptoms have resolved, there is currently insufficient safety data regarding COVID-19 vaccination of those with prior non-COVID-19 VAMP or following subsequent COVID-19 vaccination in those with prior VAMP related to COVID-19.10 For these individuals, COVID-19 vaccination is a precaution.10 Although insufficient to determine a precise level of risk, this report does provide data on which to base the CDC-recommended shared decision-making counseling of these patients. More research is needed to better define factors that increase risk for, or protection from, immune-mediated AEs following immunization, including VAMP. While benefits of vaccination have clearly outweighed risks during the COVID-19 pandemic, such research may optimize future vaccine recommendations.18
1. Decker MD, Garman PM, Hughes H, et al. Enhanced safety surveillance study of ACAM2000 smallpox vaccine among US military service members. Vaccine. 2021;39(39):5541-5547. doi:10.1016/j.vaccine.2021.08.041
2. Engler RJ, Nelson MR, Collins LC Jr, et al. A prospective study of the incidence of myocarditis/pericarditis and new onset cardiac symptoms following smallpox and influenza vaccination. PLoS One. 2015;10(3):e0118283. doi:10.1371/journal.pone.0118283
3. Faix DJ, Gordon DM, Perry LN, et al. Prospective safety surveillance study of ACAM2000 smallpox vaccine in deploying military personnel. Vaccine. 2020;38(46):7323-7330. doi:10.1016/j.vaccine.2020.09.037
4. Montgomery J, Ryan M, Engler R, et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol. 2021;6(10):1202-1206. doi:10.1001/jamacardio.2021.2833
5. Witberg G, Barda N, Hoss S, et al. Myocarditis after Covid-19 vaccination in a large health care organization. N Engl J Med. 2021;385(23):2132-2139. doi:10.1056/NEJMoa2110737
6. Oster ME, Shay DK, Su JR, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327(4):331-340. doi:10.1001/jama.2021.24110
7. Su JR, McNeil MM, Welsh KJ, et al. Myopericarditis after vaccination, Vaccine Adverse Event Reporting System (VAERS), 1990-2018. Vaccine. 2021;39(5):839-845. doi:10.1016/j.vaccine.2020.12.046
8. Mei R, Raschi E, Forcesi E, Diemberger I, De Ponti F, Poluzzi E. Myocarditis and pericarditis after immunization: gaining insights through the Vaccine Adverse Event Reporting System. Int J Cardiol. 2018;273:183-186. doi:10.1016/j.ijcard.2018.09.054
9. Centers for Disease Control and Prevention (CDC). Update: cardiac-related events during the civilian smallpox vaccination program—United States, 2003. MMWR Morb Mortal Wkly Rep. 2003;52(21):492-496.
10. Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70(27):977-982. doi:10.15585/mmwr.mm7027e2
11. Sexson Tejtel SK, Munoz FM, Al-Ammouri I, et al. Myocarditis and pericarditis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2022;40(10):1499-1511. doi:10.1016/j.vaccine.2021.11.074
12. Sagar S, Liu PP, Cooper LT Jr. Myocarditis. Lancet. 2012;379(9817):738-747. doi:10.1016/S0140-6736(11) 60648-X
13. Heymans S, Cooper LT. Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nat Rev Cardiol. 2022;19(2):75-77. doi:10.1038/s41569-021-00662-w
14. Cohen JI, Hohman P, Fulton R, et al. Kinetics of serum cytokines after primary or repeat vaccination with the smallpox vaccine. J Infect Dis. 2010;201(8):1183-1191. doi:10.1086/651453
15. Minocha PK, Better D, Singh RK, Hoque T. Recurrence of acute myocarditis temporally associated with receipt of the mRNA COVID-19 vaccine in an adolescent male. J Pediatr. 2021;238:321-323. doi:10.1016/j.jpeds.2021.06.035
16. Umei TC, Kishino Y, Watanabe K, et al. Recurrence of myopericarditis following mRNA COVID-19 vaccination in a male adolescent. CJC Open. 2022;4(3):350-352. doi:10.1016/j.cjco.2021.12.002
17. Pasha MA, Isaac S, Khan Z. Recurrent myocarditis following COVID-19 infection and the mRNA vaccine. Cureus. 2022;14(7):e26650. doi:10.7759/cureus.26650
18. Block JP, Boehmer TK, Forrest CB, et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination—PCORnet, United States, January 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(14):517-523. Published 2022 Apr 8. doi:10.15585/mmwr.mm7114e1
Vaccinations have substantially reduced morbidity and mortality from many infectious diseases. Despite the clear value of vaccinations in public health, efforts to better understand adverse events (AEs) following immunization are important to sustain public trust and vaccine confidence. Noninfectious inflammation of the heart may manifest as myocarditis or pericarditis, or occasionally, with shared signs and symptoms of each, as myopericarditis. This is a rare AE following some immunizations. Vaccine-associated myocarditis, pericarditis, or myopericarditis (VAMP) has been most clearly associated with smallpox vaccines and mRNA COVID-19 vaccines.1-6 Although extremely rare, VAMP also has been associated with other vaccines.7,8 Limited information exists to guide shared clinical decision making on COVID-19 vaccination in persons with a history of VAMP. It is unknown whether individuals with a history of VAMP are at higher risk for developing a recurrence or experiencing a more severe outcome following COVID-19 vaccination.
Methods
As part of the collaborative public health mission with the Centers for Disease Control and Prevention (CDC) for enhanced vaccine AE surveillance, the Defense Health Agency Immunization Healthcare Division (IHD) maintains a clinical database of service members and beneficiaries referred for suspected AEs following immunizations. A review of all AEs following immunization cases in this database from January 1, 2003, through February 28, 2022, identified individuals meeting the following criteria: (a) VAMP prior to receipt of COVID-19 vaccine; (b) receipt of COVID-19 vaccine in 2021; and (c) medical documentation in available electronic health records sufficient to describe health status at least 30 days following COVID-19 vaccination.9 If medical entries suggested cardiac symptoms following a COVID-19 vaccine, additional information was sought to verify VAMP based on current published criteria.10,11 Both the initial VAMP cases and the suspected COVID-19 VAMP cases were adjudicated by a team of vaccine experts and specialists in immunology, cardiology, and preventive medicine.
This retrospective review was approved and conducted in accordance with the Walter Reed National Military Medical Center Institutional Review Board protocol #20664. All individuals with recurrent VAMP consented to share their health records and clinical details.
Results
Among 9260 cases in the IHD database, 431 met the case definition for VAMP.
Among the 179 patients included in this analysis, 171 (96%) were male. Their median age was 39 years at the time of COVID-19 vaccination.
Within 1 month of receipt of any COVID-19 vaccine, 11 individuals had documented symptoms suggesting cardiac involvement, specifically, chest pain, palpitations, or dyspnea. After cardiac evaluation, 4 patients met the criteria for VAMP after COVID-19 vaccination.10,11 Seven patients either did not meet the criteria for VAMP or had alternative causes for their symptoms.
Two men aged 49 and 50 years with a history of vaccine-associated myocarditis following smallpox vaccination (Dryvax and ACAM2000) developed myocarditis 3 days after their second dose of the Moderna vaccine. One of these patients received a Pfizer-BioNTech booster 10 months later with no recurrence of symptoms. A 55-year-old man with a history of vaccine-associated myocarditis following Dryvax vaccination developed myocarditis 2 days after his Pfizer-BioNTech booster. None of the patients who developed post-COVID-19 VAMP reported residual symptoms from their initial VAMP episode, which occurred 12 to 18 years earlier. All were hospitalized briefly for observation and had complete symptom resolution within 6 weeks.
A 25-year-old man developed pericarditis 4 days after his second Pfizer-BioNTech vaccination. His previous ACAM2000 vaccine-associated myocarditis occurred 3 years earlier, with no residual symptoms. Of note, he had a mild COVID-19 infection 78 days before the onset of his pericarditis. After the onset of his COVID-19 vaccine-associated pericarditis, he continued to experience transient bouts of chest pressure and exertional dyspnea that resolved within 7 months of onset.
The median interval between COVID-19 vaccine doses in those who developed post-COVID-19 VAMP was within the recommended mRNA vaccine dosing intervals of 3 to 4 weeks and was consistent with the median mRNA vaccine dosing intervals among the entire cohort.
Due to the small cohort size and other limitations of this study, the suggested rate of cardiac injury in this review (4 cases in 179 persons, or 2.2%) is an imprecise estimate of risk in a small population (95% CI, 0.1%-4.4%). While this rate may seem higher than expected within the general population after COVID-19 vaccination, it is lower than the estimated lifetime risk of recurrent myocarditis from any cause.6,12
Discussion
To our knowledge, this is the first report describing cardiac outcomes after COVID-19 vaccination among a cohort of individuals with prior history of VAMP. Four cases of COVID-19 VAMP were identified among 179 patients with previous VAMP. All cases had experienced VAMP after the smallpox vaccine several years earlier, with complete resolution of symptoms. Three cases presented with recurrent VAMP after their second dose of an mRNA COVID-19 vaccine, and one after an mRNA booster dose. All fully recovered over the course of several months.
Myocarditis is a heterogeneous inflammatory injury with diverse, sometimes idiopathic, etiologies.13 In contrast to infection-related cardiac injury, prior reports of vaccine-associated myocarditis have suggested a hypersensitivity reaction characterized by patchy eosinophilic infiltrates, a benign clinical course, and good prognosis.2,3
There are several common features between VAMP after smallpox and COVID-19 vaccination. Cases occur predominantly in young men. The onset of symptoms after smallpox vaccine (mean, 10 days) and after mRNA COVID-19 vaccine (mean, 3 days) appears to correspond to the timing of peak postvaccination pro-inflammatory cytokine elevation.14 While all VAMP cases are serious events, the majority of patients appear to have a relatively benign clinical course with rapid and full recovery.13
Patients who have experienced an inflammatory cardiac injury may be at higher risk for recurrence, but quantifying risk of this rare phenomenon is challenging. Cases of VAMP after the COVID-19 vaccine have occasionally been reported in patients with previous cardiac injury unrelated to vaccination.15-17 The cases presented here represent the first report of recurrent VAMP following prior non-COVID-19 vaccinations.
Most patients with prior VAMP in this cohort did not experience cardiac-suggestive symptoms following COVID-19 vaccination. Among 11 patients who developed symptoms, 3 had confirmed myocarditis and 1 had confirmed pericarditis. The clinical course for these patients with recurrent VAMP was observed to be no different in severity or duration from those who experience new-onset VAMP.4 All other patients not meeting criteria for VAMP or having alternative explanations for their symptoms also had a benign clinical course. Nonetheless, of the study cohort of 179, recurrent VAMP was diagnosed in 4 of the 11 who developed cardiac-suggestive symptoms following COVID-19 vaccination. The importance of cardiac evaluation should be emphasized for any patient presenting with chest pain, dyspnea, or other cardiac-suggestive symptoms following vaccination.
Strengths and Limitations
The strength of this review of VAMP recurrence associated with COVID-19 vaccination derives from our large and unique longitudinal database of VAMP among current and prior service members. Additionally, the IHD’s ongoing enhanced vaccine AEs surveillance provides the opportunity to contact patients and review their electronic health records over an extended interval of time.
When interpreting this report’s implications, limitations inherent to any retrospective case review should be considered. The cohort of cases of prior VAMP included primarily healthy, fit, young service members; this population is not representative of the general population. The cohort included prior VAMP cases that generally occurred after smallpox vaccination. Experiences after smallpox vaccine may not apply to cardiac injury from other vaccines or etiologies. By the nature of this review, the population studied at the time of COVID-19 vaccination was somewhat older than those most likely to develop an initial bout of VAMP.2 This review was limited by information available in the electronic health records of a small number of patients. Subclinical cases of VAMP and cases without adequate clinical evaluation also could not be included.
Conclusions
Noninfectious inflammation of the heart (myocarditis, pericarditis, or myopericarditis) is a rare AE following certain vaccines, especially live replicating smallpox vaccine and mRNA COVID-19 vaccines. In this observational analysis, the majority of patients with previous VAMP successfully received a COVID-19 vaccine without recurrence. The 4 patients who were identified with recurrent VAMP following COVID-19 vaccination all recovered with supportive care. While the CDC endorses that individuals with a history of infectious myocarditis may receive COVID-19 vaccine after symptoms have resolved, there is currently insufficient safety data regarding COVID-19 vaccination of those with prior non-COVID-19 VAMP or following subsequent COVID-19 vaccination in those with prior VAMP related to COVID-19.10 For these individuals, COVID-19 vaccination is a precaution.10 Although insufficient to determine a precise level of risk, this report does provide data on which to base the CDC-recommended shared decision-making counseling of these patients. More research is needed to better define factors that increase risk for, or protection from, immune-mediated AEs following immunization, including VAMP. While benefits of vaccination have clearly outweighed risks during the COVID-19 pandemic, such research may optimize future vaccine recommendations.18
Vaccinations have substantially reduced morbidity and mortality from many infectious diseases. Despite the clear value of vaccinations in public health, efforts to better understand adverse events (AEs) following immunization are important to sustain public trust and vaccine confidence. Noninfectious inflammation of the heart may manifest as myocarditis or pericarditis, or occasionally, with shared signs and symptoms of each, as myopericarditis. This is a rare AE following some immunizations. Vaccine-associated myocarditis, pericarditis, or myopericarditis (VAMP) has been most clearly associated with smallpox vaccines and mRNA COVID-19 vaccines.1-6 Although extremely rare, VAMP also has been associated with other vaccines.7,8 Limited information exists to guide shared clinical decision making on COVID-19 vaccination in persons with a history of VAMP. It is unknown whether individuals with a history of VAMP are at higher risk for developing a recurrence or experiencing a more severe outcome following COVID-19 vaccination.
Methods
As part of the collaborative public health mission with the Centers for Disease Control and Prevention (CDC) for enhanced vaccine AE surveillance, the Defense Health Agency Immunization Healthcare Division (IHD) maintains a clinical database of service members and beneficiaries referred for suspected AEs following immunizations. A review of all AEs following immunization cases in this database from January 1, 2003, through February 28, 2022, identified individuals meeting the following criteria: (a) VAMP prior to receipt of COVID-19 vaccine; (b) receipt of COVID-19 vaccine in 2021; and (c) medical documentation in available electronic health records sufficient to describe health status at least 30 days following COVID-19 vaccination.9 If medical entries suggested cardiac symptoms following a COVID-19 vaccine, additional information was sought to verify VAMP based on current published criteria.10,11 Both the initial VAMP cases and the suspected COVID-19 VAMP cases were adjudicated by a team of vaccine experts and specialists in immunology, cardiology, and preventive medicine.
This retrospective review was approved and conducted in accordance with the Walter Reed National Military Medical Center Institutional Review Board protocol #20664. All individuals with recurrent VAMP consented to share their health records and clinical details.
Results
Among 9260 cases in the IHD database, 431 met the case definition for VAMP.
Among the 179 patients included in this analysis, 171 (96%) were male. Their median age was 39 years at the time of COVID-19 vaccination.
Within 1 month of receipt of any COVID-19 vaccine, 11 individuals had documented symptoms suggesting cardiac involvement, specifically, chest pain, palpitations, or dyspnea. After cardiac evaluation, 4 patients met the criteria for VAMP after COVID-19 vaccination.10,11 Seven patients either did not meet the criteria for VAMP or had alternative causes for their symptoms.
Two men aged 49 and 50 years with a history of vaccine-associated myocarditis following smallpox vaccination (Dryvax and ACAM2000) developed myocarditis 3 days after their second dose of the Moderna vaccine. One of these patients received a Pfizer-BioNTech booster 10 months later with no recurrence of symptoms. A 55-year-old man with a history of vaccine-associated myocarditis following Dryvax vaccination developed myocarditis 2 days after his Pfizer-BioNTech booster. None of the patients who developed post-COVID-19 VAMP reported residual symptoms from their initial VAMP episode, which occurred 12 to 18 years earlier. All were hospitalized briefly for observation and had complete symptom resolution within 6 weeks.
A 25-year-old man developed pericarditis 4 days after his second Pfizer-BioNTech vaccination. His previous ACAM2000 vaccine-associated myocarditis occurred 3 years earlier, with no residual symptoms. Of note, he had a mild COVID-19 infection 78 days before the onset of his pericarditis. After the onset of his COVID-19 vaccine-associated pericarditis, he continued to experience transient bouts of chest pressure and exertional dyspnea that resolved within 7 months of onset.
The median interval between COVID-19 vaccine doses in those who developed post-COVID-19 VAMP was within the recommended mRNA vaccine dosing intervals of 3 to 4 weeks and was consistent with the median mRNA vaccine dosing intervals among the entire cohort.
Due to the small cohort size and other limitations of this study, the suggested rate of cardiac injury in this review (4 cases in 179 persons, or 2.2%) is an imprecise estimate of risk in a small population (95% CI, 0.1%-4.4%). While this rate may seem higher than expected within the general population after COVID-19 vaccination, it is lower than the estimated lifetime risk of recurrent myocarditis from any cause.6,12
Discussion
To our knowledge, this is the first report describing cardiac outcomes after COVID-19 vaccination among a cohort of individuals with prior history of VAMP. Four cases of COVID-19 VAMP were identified among 179 patients with previous VAMP. All cases had experienced VAMP after the smallpox vaccine several years earlier, with complete resolution of symptoms. Three cases presented with recurrent VAMP after their second dose of an mRNA COVID-19 vaccine, and one after an mRNA booster dose. All fully recovered over the course of several months.
Myocarditis is a heterogeneous inflammatory injury with diverse, sometimes idiopathic, etiologies.13 In contrast to infection-related cardiac injury, prior reports of vaccine-associated myocarditis have suggested a hypersensitivity reaction characterized by patchy eosinophilic infiltrates, a benign clinical course, and good prognosis.2,3
There are several common features between VAMP after smallpox and COVID-19 vaccination. Cases occur predominantly in young men. The onset of symptoms after smallpox vaccine (mean, 10 days) and after mRNA COVID-19 vaccine (mean, 3 days) appears to correspond to the timing of peak postvaccination pro-inflammatory cytokine elevation.14 While all VAMP cases are serious events, the majority of patients appear to have a relatively benign clinical course with rapid and full recovery.13
Patients who have experienced an inflammatory cardiac injury may be at higher risk for recurrence, but quantifying risk of this rare phenomenon is challenging. Cases of VAMP after the COVID-19 vaccine have occasionally been reported in patients with previous cardiac injury unrelated to vaccination.15-17 The cases presented here represent the first report of recurrent VAMP following prior non-COVID-19 vaccinations.
Most patients with prior VAMP in this cohort did not experience cardiac-suggestive symptoms following COVID-19 vaccination. Among 11 patients who developed symptoms, 3 had confirmed myocarditis and 1 had confirmed pericarditis. The clinical course for these patients with recurrent VAMP was observed to be no different in severity or duration from those who experience new-onset VAMP.4 All other patients not meeting criteria for VAMP or having alternative explanations for their symptoms also had a benign clinical course. Nonetheless, of the study cohort of 179, recurrent VAMP was diagnosed in 4 of the 11 who developed cardiac-suggestive symptoms following COVID-19 vaccination. The importance of cardiac evaluation should be emphasized for any patient presenting with chest pain, dyspnea, or other cardiac-suggestive symptoms following vaccination.
Strengths and Limitations
The strength of this review of VAMP recurrence associated with COVID-19 vaccination derives from our large and unique longitudinal database of VAMP among current and prior service members. Additionally, the IHD’s ongoing enhanced vaccine AEs surveillance provides the opportunity to contact patients and review their electronic health records over an extended interval of time.
When interpreting this report’s implications, limitations inherent to any retrospective case review should be considered. The cohort of cases of prior VAMP included primarily healthy, fit, young service members; this population is not representative of the general population. The cohort included prior VAMP cases that generally occurred after smallpox vaccination. Experiences after smallpox vaccine may not apply to cardiac injury from other vaccines or etiologies. By the nature of this review, the population studied at the time of COVID-19 vaccination was somewhat older than those most likely to develop an initial bout of VAMP.2 This review was limited by information available in the electronic health records of a small number of patients. Subclinical cases of VAMP and cases without adequate clinical evaluation also could not be included.
Conclusions
Noninfectious inflammation of the heart (myocarditis, pericarditis, or myopericarditis) is a rare AE following certain vaccines, especially live replicating smallpox vaccine and mRNA COVID-19 vaccines. In this observational analysis, the majority of patients with previous VAMP successfully received a COVID-19 vaccine without recurrence. The 4 patients who were identified with recurrent VAMP following COVID-19 vaccination all recovered with supportive care. While the CDC endorses that individuals with a history of infectious myocarditis may receive COVID-19 vaccine after symptoms have resolved, there is currently insufficient safety data regarding COVID-19 vaccination of those with prior non-COVID-19 VAMP or following subsequent COVID-19 vaccination in those with prior VAMP related to COVID-19.10 For these individuals, COVID-19 vaccination is a precaution.10 Although insufficient to determine a precise level of risk, this report does provide data on which to base the CDC-recommended shared decision-making counseling of these patients. More research is needed to better define factors that increase risk for, or protection from, immune-mediated AEs following immunization, including VAMP. While benefits of vaccination have clearly outweighed risks during the COVID-19 pandemic, such research may optimize future vaccine recommendations.18
1. Decker MD, Garman PM, Hughes H, et al. Enhanced safety surveillance study of ACAM2000 smallpox vaccine among US military service members. Vaccine. 2021;39(39):5541-5547. doi:10.1016/j.vaccine.2021.08.041
2. Engler RJ, Nelson MR, Collins LC Jr, et al. A prospective study of the incidence of myocarditis/pericarditis and new onset cardiac symptoms following smallpox and influenza vaccination. PLoS One. 2015;10(3):e0118283. doi:10.1371/journal.pone.0118283
3. Faix DJ, Gordon DM, Perry LN, et al. Prospective safety surveillance study of ACAM2000 smallpox vaccine in deploying military personnel. Vaccine. 2020;38(46):7323-7330. doi:10.1016/j.vaccine.2020.09.037
4. Montgomery J, Ryan M, Engler R, et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol. 2021;6(10):1202-1206. doi:10.1001/jamacardio.2021.2833
5. Witberg G, Barda N, Hoss S, et al. Myocarditis after Covid-19 vaccination in a large health care organization. N Engl J Med. 2021;385(23):2132-2139. doi:10.1056/NEJMoa2110737
6. Oster ME, Shay DK, Su JR, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327(4):331-340. doi:10.1001/jama.2021.24110
7. Su JR, McNeil MM, Welsh KJ, et al. Myopericarditis after vaccination, Vaccine Adverse Event Reporting System (VAERS), 1990-2018. Vaccine. 2021;39(5):839-845. doi:10.1016/j.vaccine.2020.12.046
8. Mei R, Raschi E, Forcesi E, Diemberger I, De Ponti F, Poluzzi E. Myocarditis and pericarditis after immunization: gaining insights through the Vaccine Adverse Event Reporting System. Int J Cardiol. 2018;273:183-186. doi:10.1016/j.ijcard.2018.09.054
9. Centers for Disease Control and Prevention (CDC). Update: cardiac-related events during the civilian smallpox vaccination program—United States, 2003. MMWR Morb Mortal Wkly Rep. 2003;52(21):492-496.
10. Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70(27):977-982. doi:10.15585/mmwr.mm7027e2
11. Sexson Tejtel SK, Munoz FM, Al-Ammouri I, et al. Myocarditis and pericarditis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2022;40(10):1499-1511. doi:10.1016/j.vaccine.2021.11.074
12. Sagar S, Liu PP, Cooper LT Jr. Myocarditis. Lancet. 2012;379(9817):738-747. doi:10.1016/S0140-6736(11) 60648-X
13. Heymans S, Cooper LT. Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nat Rev Cardiol. 2022;19(2):75-77. doi:10.1038/s41569-021-00662-w
14. Cohen JI, Hohman P, Fulton R, et al. Kinetics of serum cytokines after primary or repeat vaccination with the smallpox vaccine. J Infect Dis. 2010;201(8):1183-1191. doi:10.1086/651453
15. Minocha PK, Better D, Singh RK, Hoque T. Recurrence of acute myocarditis temporally associated with receipt of the mRNA COVID-19 vaccine in an adolescent male. J Pediatr. 2021;238:321-323. doi:10.1016/j.jpeds.2021.06.035
16. Umei TC, Kishino Y, Watanabe K, et al. Recurrence of myopericarditis following mRNA COVID-19 vaccination in a male adolescent. CJC Open. 2022;4(3):350-352. doi:10.1016/j.cjco.2021.12.002
17. Pasha MA, Isaac S, Khan Z. Recurrent myocarditis following COVID-19 infection and the mRNA vaccine. Cureus. 2022;14(7):e26650. doi:10.7759/cureus.26650
18. Block JP, Boehmer TK, Forrest CB, et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination—PCORnet, United States, January 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(14):517-523. Published 2022 Apr 8. doi:10.15585/mmwr.mm7114e1
1. Decker MD, Garman PM, Hughes H, et al. Enhanced safety surveillance study of ACAM2000 smallpox vaccine among US military service members. Vaccine. 2021;39(39):5541-5547. doi:10.1016/j.vaccine.2021.08.041
2. Engler RJ, Nelson MR, Collins LC Jr, et al. A prospective study of the incidence of myocarditis/pericarditis and new onset cardiac symptoms following smallpox and influenza vaccination. PLoS One. 2015;10(3):e0118283. doi:10.1371/journal.pone.0118283
3. Faix DJ, Gordon DM, Perry LN, et al. Prospective safety surveillance study of ACAM2000 smallpox vaccine in deploying military personnel. Vaccine. 2020;38(46):7323-7330. doi:10.1016/j.vaccine.2020.09.037
4. Montgomery J, Ryan M, Engler R, et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol. 2021;6(10):1202-1206. doi:10.1001/jamacardio.2021.2833
5. Witberg G, Barda N, Hoss S, et al. Myocarditis after Covid-19 vaccination in a large health care organization. N Engl J Med. 2021;385(23):2132-2139. doi:10.1056/NEJMoa2110737
6. Oster ME, Shay DK, Su JR, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327(4):331-340. doi:10.1001/jama.2021.24110
7. Su JR, McNeil MM, Welsh KJ, et al. Myopericarditis after vaccination, Vaccine Adverse Event Reporting System (VAERS), 1990-2018. Vaccine. 2021;39(5):839-845. doi:10.1016/j.vaccine.2020.12.046
8. Mei R, Raschi E, Forcesi E, Diemberger I, De Ponti F, Poluzzi E. Myocarditis and pericarditis after immunization: gaining insights through the Vaccine Adverse Event Reporting System. Int J Cardiol. 2018;273:183-186. doi:10.1016/j.ijcard.2018.09.054
9. Centers for Disease Control and Prevention (CDC). Update: cardiac-related events during the civilian smallpox vaccination program—United States, 2003. MMWR Morb Mortal Wkly Rep. 2003;52(21):492-496.
10. Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70(27):977-982. doi:10.15585/mmwr.mm7027e2
11. Sexson Tejtel SK, Munoz FM, Al-Ammouri I, et al. Myocarditis and pericarditis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2022;40(10):1499-1511. doi:10.1016/j.vaccine.2021.11.074
12. Sagar S, Liu PP, Cooper LT Jr. Myocarditis. Lancet. 2012;379(9817):738-747. doi:10.1016/S0140-6736(11) 60648-X
13. Heymans S, Cooper LT. Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nat Rev Cardiol. 2022;19(2):75-77. doi:10.1038/s41569-021-00662-w
14. Cohen JI, Hohman P, Fulton R, et al. Kinetics of serum cytokines after primary or repeat vaccination with the smallpox vaccine. J Infect Dis. 2010;201(8):1183-1191. doi:10.1086/651453
15. Minocha PK, Better D, Singh RK, Hoque T. Recurrence of acute myocarditis temporally associated with receipt of the mRNA COVID-19 vaccine in an adolescent male. J Pediatr. 2021;238:321-323. doi:10.1016/j.jpeds.2021.06.035
16. Umei TC, Kishino Y, Watanabe K, et al. Recurrence of myopericarditis following mRNA COVID-19 vaccination in a male adolescent. CJC Open. 2022;4(3):350-352. doi:10.1016/j.cjco.2021.12.002
17. Pasha MA, Isaac S, Khan Z. Recurrent myocarditis following COVID-19 infection and the mRNA vaccine. Cureus. 2022;14(7):e26650. doi:10.7759/cureus.26650
18. Block JP, Boehmer TK, Forrest CB, et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination—PCORnet, United States, January 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(14):517-523. Published 2022 Apr 8. doi:10.15585/mmwr.mm7114e1
Scaly facial plaques
This patient was experiencing a flare of his psoriasis. Three factors contributed to the flare: noncompliance with his treatment regimen, decreased sunlight in the winter, and his lithium therapy. Though carcinogenic, certain wavelengths of UV light are beneficial for psoriasis, and the shorter days of winter can cause flaring of psoriasis (or relative flaring). In addition, lithium—the most effective therapy for this patient’s bipolar disorder—can worsen psoriasis.
Psoriasis is a chronic multisystem inflammatory disorder with characteristic skin findings that include well-demarcated micaceous plaques, nail pitting, and sometimes tendon pain and inflammatory arthritis. Severity can range from small, thin plaques that are intermittently noticeable on the elbows or knees to widespread ash-like plaques covering most of the body.
Good topical choices for facial skin include hydrocortisone 2.5% cream or desonide 0.05%. Nonsteroidal topical therapies that are safe for facial skin include tacrolimus 0.1% ointment or pimecrolimus 1% cream.1 These options may be used twice daily until the disease is controlled.
In many cases (as in this one), the patient’s previous psoriasis outbreaks could not be controlled with topical therapy alone. The patient had not responded to a previous methotrexate regimen, and more recently had been clear for several years on systemic ustekinumab, a monoclonal antibody. Dosed every 12 weeks, or sometimes every 8 weeks, ustekinumab is given by subcutaneous injection, usually in the abdomen, through normal skin. Ustekinumab was recently approved for home use with just 4 injections per year for maintenance therapy. However, the infrequency of the injections sometimes leads to noncompliance, as occurred with this patient. He had missed 2 doses since taking over his own dosing regimen.
Ultimately, the patient’s flare resolved when he was transitioned back to in-office treatment with ustekinumab.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Woo SM, Choi JW, Yoon HS, et al. Classification of facial psoriasis based on the distributions of facial lesions. J Am Acad Dermatol. 2008;58:959-63. doi: 10.1016/j.jaad.2008.02.006

This patient was experiencing a flare of his psoriasis. Three factors contributed to the flare: noncompliance with his treatment regimen, decreased sunlight in the winter, and his lithium therapy. Though carcinogenic, certain wavelengths of UV light are beneficial for psoriasis, and the shorter days of winter can cause flaring of psoriasis (or relative flaring). In addition, lithium—the most effective therapy for this patient’s bipolar disorder—can worsen psoriasis.
Psoriasis is a chronic multisystem inflammatory disorder with characteristic skin findings that include well-demarcated micaceous plaques, nail pitting, and sometimes tendon pain and inflammatory arthritis. Severity can range from small, thin plaques that are intermittently noticeable on the elbows or knees to widespread ash-like plaques covering most of the body.
Good topical choices for facial skin include hydrocortisone 2.5% cream or desonide 0.05%. Nonsteroidal topical therapies that are safe for facial skin include tacrolimus 0.1% ointment or pimecrolimus 1% cream.1 These options may be used twice daily until the disease is controlled.
In many cases (as in this one), the patient’s previous psoriasis outbreaks could not be controlled with topical therapy alone. The patient had not responded to a previous methotrexate regimen, and more recently had been clear for several years on systemic ustekinumab, a monoclonal antibody. Dosed every 12 weeks, or sometimes every 8 weeks, ustekinumab is given by subcutaneous injection, usually in the abdomen, through normal skin. Ustekinumab was recently approved for home use with just 4 injections per year for maintenance therapy. However, the infrequency of the injections sometimes leads to noncompliance, as occurred with this patient. He had missed 2 doses since taking over his own dosing regimen.
Ultimately, the patient’s flare resolved when he was transitioned back to in-office treatment with ustekinumab.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

This patient was experiencing a flare of his psoriasis. Three factors contributed to the flare: noncompliance with his treatment regimen, decreased sunlight in the winter, and his lithium therapy. Though carcinogenic, certain wavelengths of UV light are beneficial for psoriasis, and the shorter days of winter can cause flaring of psoriasis (or relative flaring). In addition, lithium—the most effective therapy for this patient’s bipolar disorder—can worsen psoriasis.
Psoriasis is a chronic multisystem inflammatory disorder with characteristic skin findings that include well-demarcated micaceous plaques, nail pitting, and sometimes tendon pain and inflammatory arthritis. Severity can range from small, thin plaques that are intermittently noticeable on the elbows or knees to widespread ash-like plaques covering most of the body.
Good topical choices for facial skin include hydrocortisone 2.5% cream or desonide 0.05%. Nonsteroidal topical therapies that are safe for facial skin include tacrolimus 0.1% ointment or pimecrolimus 1% cream.1 These options may be used twice daily until the disease is controlled.
In many cases (as in this one), the patient’s previous psoriasis outbreaks could not be controlled with topical therapy alone. The patient had not responded to a previous methotrexate regimen, and more recently had been clear for several years on systemic ustekinumab, a monoclonal antibody. Dosed every 12 weeks, or sometimes every 8 weeks, ustekinumab is given by subcutaneous injection, usually in the abdomen, through normal skin. Ustekinumab was recently approved for home use with just 4 injections per year for maintenance therapy. However, the infrequency of the injections sometimes leads to noncompliance, as occurred with this patient. He had missed 2 doses since taking over his own dosing regimen.
Ultimately, the patient’s flare resolved when he was transitioned back to in-office treatment with ustekinumab.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Woo SM, Choi JW, Yoon HS, et al. Classification of facial psoriasis based on the distributions of facial lesions. J Am Acad Dermatol. 2008;58:959-63. doi: 10.1016/j.jaad.2008.02.006
1. Woo SM, Choi JW, Yoon HS, et al. Classification of facial psoriasis based on the distributions of facial lesions. J Am Acad Dermatol. 2008;58:959-63. doi: 10.1016/j.jaad.2008.02.006