User login
Disability in medicine: My experience
What does a doctor look like? Throughout history, this concept has shifted due to societal norms and increased access to medical education. Today, the idea of a physician has expanded to incorporate a myriad of people; however, stigma still exists in medicine regarding mental illness and disability. I would like to share my personal journey through high school, college, medical school, and now residency, and how my identity and struggles have shaped me into the physician I am today. There are few conversations around disability—especially disability and mental health—in medicine, and through my own advocacy, I have met many students with disability who feel that medical school is unattainable. Additionally, I have met many medical students, residents, and pre-health advisors who are happy for the experience to learn more about a marginalized group in medicine. My hope in sharing my story is to offer a space for conversation about intersectionality within medical communities and how physicians and physicians in training can facilitate that change, regardless of their position or specialty. Additionally, I hope to shed light on the unique mental health needs of patients with disabilities and how mental health clinicians can address those needs.
Perceived weaknesses turned into strengths
“Why do you walk like that?” “What is that brace on your leg?” The early years of my childhood were marked by these questions and others like them. I was the kid with the limp, the kid with a brace on his leg, and the kid who disappeared multiple times a week for doctor’s appointments or physical therapy. I learned to deflect these questions or give nebulous answers about an accident or injury. The reality is that I was born with cerebral palsy (CP). My CP manifested as hemiparesis on the left side of my body. I was in aggressive physical therapy throughout childhood, received Botox injections for muscle spasticity, and underwent corrective surgery on my left leg to straighten my foot. In childhood, the diagnosis meant nothing more than 2 words that sounded like they belonged to superheroes in comic books. Even with supportive parents and family, I kept my disability a secret, much like the powers and abilities of my favorite superheroes.
However, like all great origin stories, what I once thought were weaknesses turned out to be strengths that pushed me through college, medical school, and now psychiatry residency. Living with a disability has shaped how I see the world and relate to my patients. My experience has helped me connect to my patients in ways others might not. These properties are important in any physician but vital in psychiatry, where many patients feel neglected or stigmatized; this is another reason there should be more doctors with disabilities in medicine. Unfortunately, systemic barriers are still in place that disincentivize those with a disability from pursuing careers in medicine. Stories like mine are important to inspire a reexamination of what a physician should be and how medicine, patients, and communities benefit from this change.
My experience through medical school
My path to psychiatry and residency was shaped by my early experience with the medical field and treatment. From the early days of my diagnosis at age 4, I was told that my brain was “wired differently” and that, because of this disruption in circuitry, I would have difficulty with physical activity. I grew to appreciate the intricacies of the brain and pathology to understand my body. With greater understanding came the existential realization that I would live with a disability for the rest of my life. Rather than dream of a future where I would be “normal,” I focused on adapting my life to my normal. An unfortunate reality of this normal was that no doctor would be able to relate to me, and my health care would focus on limitations rather than possibilities.
I focused on school as a distraction and slowly warmed to the idea of pursuing medicine as a career. The seed was planted years prior by the numerous doctors’ visits and procedures, and was cultivated by a desire to understand pathologies and offer treatment to patients from the perspective of a patient. When I applied to medical school, I did not know how to address my CP. Living as a person with CP was a core reason for my decision to pursue medicine, but I was afraid that a disclosure of disability would preclude any admission to medical school. Research into programs offered little guidance because most institutions only listed vague “physical expectations” of each student. There were times I doubted if I would be accepted anywhere. Many programs I reached out to about my situation seemed unenthusiastic about the prospect of a student with CP, and when I brought up my CP in interviews, the reaction was often of surprise and an admission that they had forgotten about “that part” of my application. Fortunately, I was accepted to medical school, but still struggled with the fear that one day I would be found out and not allowed to continue. No one in my class or school was like me, and a meeting with an Americans with Disabilities Act coordinator who asked me to reexamine the physical competencies of the school before advancing to clinical clerkships only further reinforced this fear. I decided to fly under the radar and not say anything about my disability to my attendings. I slowly worked my way through clerkships by making do with adapted ways to perform procedures and exams with additional practice and maneuvering at home. I found myself drawn to psychiatry because of the similarities I saw in the patients and myself. I empathized with how the patients struggled with chronic conditions that left them feeling separated from society and how they felt that their diagnosis was something they needed to hide. When medical school ended and I decided to pursue psychiatry, I wanted to share my story to inspire others with a disability to consider medicine as a career given their unique experiences. My experience thus far has been uplifting as my journey has echoed so many others.
A need for greater representation
Disability representation in medicine is needed more than ever. According to the CDC, >60 million adults in the United States (1 in 4) live with a disability.1 Although the physical health disparities are often discussed, there is less conversation surrounding mental health for individuals with disabilities. A 2018 study by Cree et al2 found that approximately 17.4 million adults with disabilities experienced frequent mental distress, defined as reporting ≥14 mentally unhealthy days in the past 30 days. Furthermore, compared to individuals without a disability, those with a disability are statistically more likely to have suicidal ideation, suicidal planning, and suicide attempts.3 One way to address this disparity is to recruit medical students with disabilities to become physicians with disabilities. Evidence suggests that physicians who are members of groups that are underrepresented in medicine are more likely to deliver care to underrepresented patients.4 However, medical schools and institutions have been slow to address the disparity. A 2019 survey found an estimated 4.6% of medical students responded “yes” when asked if they had a disability, with most students reporting a psychological or attention/hyperactive disorder.5 Existing barriers include restrictive language surrounding technical standards influenced by long-standing vestiges of what a physician should be.6
An opportunity to connect with patients
I now do not see myself as having a secret identity to hide. Although my CP does not give me any superpowers, it has given me the opportunity to connect with my patients and serve as an example of why medical school recruitment and admissions should expand. Psychiatrists have been on the forefront of change in medicine and can shift the perception of a physician. In doing so, we not only enrich our field but also the lives of our patients who may need it most.
1. Okoro CA, Hollis ND, Cyrus AC, et al. Prevalence of disabilities and health care access by disability status and type among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(32):882-887.
2. Cree RA, Okoro CA, Zack MM, et al. Frequent mental distress among adults, by disability status, disability type, and selected characteristics—United States 2018. MMWR Morb Mortal Wkly Rep. 2020;69(36):1238-1243.
3. Marlow NM, Xie Z, Tanner R, et al. Association between disability and suicide-related outcomes among US adults. Am J Prev Med. 2021;61(6):852-862.
4. Thurmond VB, Kirch DG. Impact of minority physicians on health care. South Med J. 1998;91(11):1009-1013.
5. Meeks LM, Case B, Herzer K, et al. Change in prevalence of disabilities and accommodation practices among US medical schools, 2016 vs 2019. JAMA. 2019;322(20):2022-2024.
6. Stauffer C, Case B, Moreland CJ, et al. Technical standards from newly established medical schools: a review of disability inclusive practices. J Med Educ Curric Dev. 2022;9:23821205211072763.
What does a doctor look like? Throughout history, this concept has shifted due to societal norms and increased access to medical education. Today, the idea of a physician has expanded to incorporate a myriad of people; however, stigma still exists in medicine regarding mental illness and disability. I would like to share my personal journey through high school, college, medical school, and now residency, and how my identity and struggles have shaped me into the physician I am today. There are few conversations around disability—especially disability and mental health—in medicine, and through my own advocacy, I have met many students with disability who feel that medical school is unattainable. Additionally, I have met many medical students, residents, and pre-health advisors who are happy for the experience to learn more about a marginalized group in medicine. My hope in sharing my story is to offer a space for conversation about intersectionality within medical communities and how physicians and physicians in training can facilitate that change, regardless of their position or specialty. Additionally, I hope to shed light on the unique mental health needs of patients with disabilities and how mental health clinicians can address those needs.
Perceived weaknesses turned into strengths
“Why do you walk like that?” “What is that brace on your leg?” The early years of my childhood were marked by these questions and others like them. I was the kid with the limp, the kid with a brace on his leg, and the kid who disappeared multiple times a week for doctor’s appointments or physical therapy. I learned to deflect these questions or give nebulous answers about an accident or injury. The reality is that I was born with cerebral palsy (CP). My CP manifested as hemiparesis on the left side of my body. I was in aggressive physical therapy throughout childhood, received Botox injections for muscle spasticity, and underwent corrective surgery on my left leg to straighten my foot. In childhood, the diagnosis meant nothing more than 2 words that sounded like they belonged to superheroes in comic books. Even with supportive parents and family, I kept my disability a secret, much like the powers and abilities of my favorite superheroes.
However, like all great origin stories, what I once thought were weaknesses turned out to be strengths that pushed me through college, medical school, and now psychiatry residency. Living with a disability has shaped how I see the world and relate to my patients. My experience has helped me connect to my patients in ways others might not. These properties are important in any physician but vital in psychiatry, where many patients feel neglected or stigmatized; this is another reason there should be more doctors with disabilities in medicine. Unfortunately, systemic barriers are still in place that disincentivize those with a disability from pursuing careers in medicine. Stories like mine are important to inspire a reexamination of what a physician should be and how medicine, patients, and communities benefit from this change.
My experience through medical school
My path to psychiatry and residency was shaped by my early experience with the medical field and treatment. From the early days of my diagnosis at age 4, I was told that my brain was “wired differently” and that, because of this disruption in circuitry, I would have difficulty with physical activity. I grew to appreciate the intricacies of the brain and pathology to understand my body. With greater understanding came the existential realization that I would live with a disability for the rest of my life. Rather than dream of a future where I would be “normal,” I focused on adapting my life to my normal. An unfortunate reality of this normal was that no doctor would be able to relate to me, and my health care would focus on limitations rather than possibilities.
I focused on school as a distraction and slowly warmed to the idea of pursuing medicine as a career. The seed was planted years prior by the numerous doctors’ visits and procedures, and was cultivated by a desire to understand pathologies and offer treatment to patients from the perspective of a patient. When I applied to medical school, I did not know how to address my CP. Living as a person with CP was a core reason for my decision to pursue medicine, but I was afraid that a disclosure of disability would preclude any admission to medical school. Research into programs offered little guidance because most institutions only listed vague “physical expectations” of each student. There were times I doubted if I would be accepted anywhere. Many programs I reached out to about my situation seemed unenthusiastic about the prospect of a student with CP, and when I brought up my CP in interviews, the reaction was often of surprise and an admission that they had forgotten about “that part” of my application. Fortunately, I was accepted to medical school, but still struggled with the fear that one day I would be found out and not allowed to continue. No one in my class or school was like me, and a meeting with an Americans with Disabilities Act coordinator who asked me to reexamine the physical competencies of the school before advancing to clinical clerkships only further reinforced this fear. I decided to fly under the radar and not say anything about my disability to my attendings. I slowly worked my way through clerkships by making do with adapted ways to perform procedures and exams with additional practice and maneuvering at home. I found myself drawn to psychiatry because of the similarities I saw in the patients and myself. I empathized with how the patients struggled with chronic conditions that left them feeling separated from society and how they felt that their diagnosis was something they needed to hide. When medical school ended and I decided to pursue psychiatry, I wanted to share my story to inspire others with a disability to consider medicine as a career given their unique experiences. My experience thus far has been uplifting as my journey has echoed so many others.
A need for greater representation
Disability representation in medicine is needed more than ever. According to the CDC, >60 million adults in the United States (1 in 4) live with a disability.1 Although the physical health disparities are often discussed, there is less conversation surrounding mental health for individuals with disabilities. A 2018 study by Cree et al2 found that approximately 17.4 million adults with disabilities experienced frequent mental distress, defined as reporting ≥14 mentally unhealthy days in the past 30 days. Furthermore, compared to individuals without a disability, those with a disability are statistically more likely to have suicidal ideation, suicidal planning, and suicide attempts.3 One way to address this disparity is to recruit medical students with disabilities to become physicians with disabilities. Evidence suggests that physicians who are members of groups that are underrepresented in medicine are more likely to deliver care to underrepresented patients.4 However, medical schools and institutions have been slow to address the disparity. A 2019 survey found an estimated 4.6% of medical students responded “yes” when asked if they had a disability, with most students reporting a psychological or attention/hyperactive disorder.5 Existing barriers include restrictive language surrounding technical standards influenced by long-standing vestiges of what a physician should be.6
An opportunity to connect with patients
I now do not see myself as having a secret identity to hide. Although my CP does not give me any superpowers, it has given me the opportunity to connect with my patients and serve as an example of why medical school recruitment and admissions should expand. Psychiatrists have been on the forefront of change in medicine and can shift the perception of a physician. In doing so, we not only enrich our field but also the lives of our patients who may need it most.
What does a doctor look like? Throughout history, this concept has shifted due to societal norms and increased access to medical education. Today, the idea of a physician has expanded to incorporate a myriad of people; however, stigma still exists in medicine regarding mental illness and disability. I would like to share my personal journey through high school, college, medical school, and now residency, and how my identity and struggles have shaped me into the physician I am today. There are few conversations around disability—especially disability and mental health—in medicine, and through my own advocacy, I have met many students with disability who feel that medical school is unattainable. Additionally, I have met many medical students, residents, and pre-health advisors who are happy for the experience to learn more about a marginalized group in medicine. My hope in sharing my story is to offer a space for conversation about intersectionality within medical communities and how physicians and physicians in training can facilitate that change, regardless of their position or specialty. Additionally, I hope to shed light on the unique mental health needs of patients with disabilities and how mental health clinicians can address those needs.
Perceived weaknesses turned into strengths
“Why do you walk like that?” “What is that brace on your leg?” The early years of my childhood were marked by these questions and others like them. I was the kid with the limp, the kid with a brace on his leg, and the kid who disappeared multiple times a week for doctor’s appointments or physical therapy. I learned to deflect these questions or give nebulous answers about an accident or injury. The reality is that I was born with cerebral palsy (CP). My CP manifested as hemiparesis on the left side of my body. I was in aggressive physical therapy throughout childhood, received Botox injections for muscle spasticity, and underwent corrective surgery on my left leg to straighten my foot. In childhood, the diagnosis meant nothing more than 2 words that sounded like they belonged to superheroes in comic books. Even with supportive parents and family, I kept my disability a secret, much like the powers and abilities of my favorite superheroes.
However, like all great origin stories, what I once thought were weaknesses turned out to be strengths that pushed me through college, medical school, and now psychiatry residency. Living with a disability has shaped how I see the world and relate to my patients. My experience has helped me connect to my patients in ways others might not. These properties are important in any physician but vital in psychiatry, where many patients feel neglected or stigmatized; this is another reason there should be more doctors with disabilities in medicine. Unfortunately, systemic barriers are still in place that disincentivize those with a disability from pursuing careers in medicine. Stories like mine are important to inspire a reexamination of what a physician should be and how medicine, patients, and communities benefit from this change.
My experience through medical school
My path to psychiatry and residency was shaped by my early experience with the medical field and treatment. From the early days of my diagnosis at age 4, I was told that my brain was “wired differently” and that, because of this disruption in circuitry, I would have difficulty with physical activity. I grew to appreciate the intricacies of the brain and pathology to understand my body. With greater understanding came the existential realization that I would live with a disability for the rest of my life. Rather than dream of a future where I would be “normal,” I focused on adapting my life to my normal. An unfortunate reality of this normal was that no doctor would be able to relate to me, and my health care would focus on limitations rather than possibilities.
I focused on school as a distraction and slowly warmed to the idea of pursuing medicine as a career. The seed was planted years prior by the numerous doctors’ visits and procedures, and was cultivated by a desire to understand pathologies and offer treatment to patients from the perspective of a patient. When I applied to medical school, I did not know how to address my CP. Living as a person with CP was a core reason for my decision to pursue medicine, but I was afraid that a disclosure of disability would preclude any admission to medical school. Research into programs offered little guidance because most institutions only listed vague “physical expectations” of each student. There were times I doubted if I would be accepted anywhere. Many programs I reached out to about my situation seemed unenthusiastic about the prospect of a student with CP, and when I brought up my CP in interviews, the reaction was often of surprise and an admission that they had forgotten about “that part” of my application. Fortunately, I was accepted to medical school, but still struggled with the fear that one day I would be found out and not allowed to continue. No one in my class or school was like me, and a meeting with an Americans with Disabilities Act coordinator who asked me to reexamine the physical competencies of the school before advancing to clinical clerkships only further reinforced this fear. I decided to fly under the radar and not say anything about my disability to my attendings. I slowly worked my way through clerkships by making do with adapted ways to perform procedures and exams with additional practice and maneuvering at home. I found myself drawn to psychiatry because of the similarities I saw in the patients and myself. I empathized with how the patients struggled with chronic conditions that left them feeling separated from society and how they felt that their diagnosis was something they needed to hide. When medical school ended and I decided to pursue psychiatry, I wanted to share my story to inspire others with a disability to consider medicine as a career given their unique experiences. My experience thus far has been uplifting as my journey has echoed so many others.
A need for greater representation
Disability representation in medicine is needed more than ever. According to the CDC, >60 million adults in the United States (1 in 4) live with a disability.1 Although the physical health disparities are often discussed, there is less conversation surrounding mental health for individuals with disabilities. A 2018 study by Cree et al2 found that approximately 17.4 million adults with disabilities experienced frequent mental distress, defined as reporting ≥14 mentally unhealthy days in the past 30 days. Furthermore, compared to individuals without a disability, those with a disability are statistically more likely to have suicidal ideation, suicidal planning, and suicide attempts.3 One way to address this disparity is to recruit medical students with disabilities to become physicians with disabilities. Evidence suggests that physicians who are members of groups that are underrepresented in medicine are more likely to deliver care to underrepresented patients.4 However, medical schools and institutions have been slow to address the disparity. A 2019 survey found an estimated 4.6% of medical students responded “yes” when asked if they had a disability, with most students reporting a psychological or attention/hyperactive disorder.5 Existing barriers include restrictive language surrounding technical standards influenced by long-standing vestiges of what a physician should be.6
An opportunity to connect with patients
I now do not see myself as having a secret identity to hide. Although my CP does not give me any superpowers, it has given me the opportunity to connect with my patients and serve as an example of why medical school recruitment and admissions should expand. Psychiatrists have been on the forefront of change in medicine and can shift the perception of a physician. In doing so, we not only enrich our field but also the lives of our patients who may need it most.
1. Okoro CA, Hollis ND, Cyrus AC, et al. Prevalence of disabilities and health care access by disability status and type among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(32):882-887.
2. Cree RA, Okoro CA, Zack MM, et al. Frequent mental distress among adults, by disability status, disability type, and selected characteristics—United States 2018. MMWR Morb Mortal Wkly Rep. 2020;69(36):1238-1243.
3. Marlow NM, Xie Z, Tanner R, et al. Association between disability and suicide-related outcomes among US adults. Am J Prev Med. 2021;61(6):852-862.
4. Thurmond VB, Kirch DG. Impact of minority physicians on health care. South Med J. 1998;91(11):1009-1013.
5. Meeks LM, Case B, Herzer K, et al. Change in prevalence of disabilities and accommodation practices among US medical schools, 2016 vs 2019. JAMA. 2019;322(20):2022-2024.
6. Stauffer C, Case B, Moreland CJ, et al. Technical standards from newly established medical schools: a review of disability inclusive practices. J Med Educ Curric Dev. 2022;9:23821205211072763.
1. Okoro CA, Hollis ND, Cyrus AC, et al. Prevalence of disabilities and health care access by disability status and type among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(32):882-887.
2. Cree RA, Okoro CA, Zack MM, et al. Frequent mental distress among adults, by disability status, disability type, and selected characteristics—United States 2018. MMWR Morb Mortal Wkly Rep. 2020;69(36):1238-1243.
3. Marlow NM, Xie Z, Tanner R, et al. Association between disability and suicide-related outcomes among US adults. Am J Prev Med. 2021;61(6):852-862.
4. Thurmond VB, Kirch DG. Impact of minority physicians on health care. South Med J. 1998;91(11):1009-1013.
5. Meeks LM, Case B, Herzer K, et al. Change in prevalence of disabilities and accommodation practices among US medical schools, 2016 vs 2019. JAMA. 2019;322(20):2022-2024.
6. Stauffer C, Case B, Moreland CJ, et al. Technical standards from newly established medical schools: a review of disability inclusive practices. J Med Educ Curric Dev. 2022;9:23821205211072763.
Discontinuing a long-acting injectable antipsychotic: What to consider
Mr. R, age 29, was diagnosed with schizophrenia 6 years ago. To manage his disorder, he has been receiving paliperidone palmitate long-acting injectable (LAI) 156 mg once a month for 2 years. Prior to maintenance with paliperidone palmitate, Mr. R was stabilized on oral paliperidone 9 mg/d. Though he was originally initiated on paliperidone palmitate due to nonadherence concerns, Mr. R has been adherent with each injection for 1 year.
At a recent visit, Mr. R says he wants to discontinue the injection because he is not interested in receiving an ongoing injectable medication and is not able to continue monthly clinic visits. He wants to take a daily oral antipsychotic again, despite the availability of longer-acting products.
A paucity of evidence exists regarding the discontinuation of LAI antipsychotics and the next steps that follow in treatment. There is neither a consensus nor recognized guidelines advising how and when to discontinue an LAI and restart an oral antipsychotic. A recent systematic review and meta-analysis evaluated different maintenance treatment strategies; however, switching from an LAI antipsychotic to an oral medication was not a focus.1 In this article, we outline a possible approach to discontinuing an LAI antipsychotic and restarting an oral formulation. Before discontinuing an LAI antipsychotic, clinicians should review with the patient the risks and benefits of switching medications, including the risk of decompensation and potential adverse effects.
Switching to an oral antipsychotic
The first step in the discontinuation process is to determine whether the patient will continue the same oral medication as the LAI antipsychotic or if a different oral antipsychotic will be initiated. Next, determining when to initiate the oral medication requires several pieces of information, including the oral dose equivalent of the patient’s current LAI, the half-life of the LAI, and the release mechanism of the LAI (Table 1).2-5 To determine the appropriate time frame for restarting oral treatment, it is also vital to know the date of the last injection.
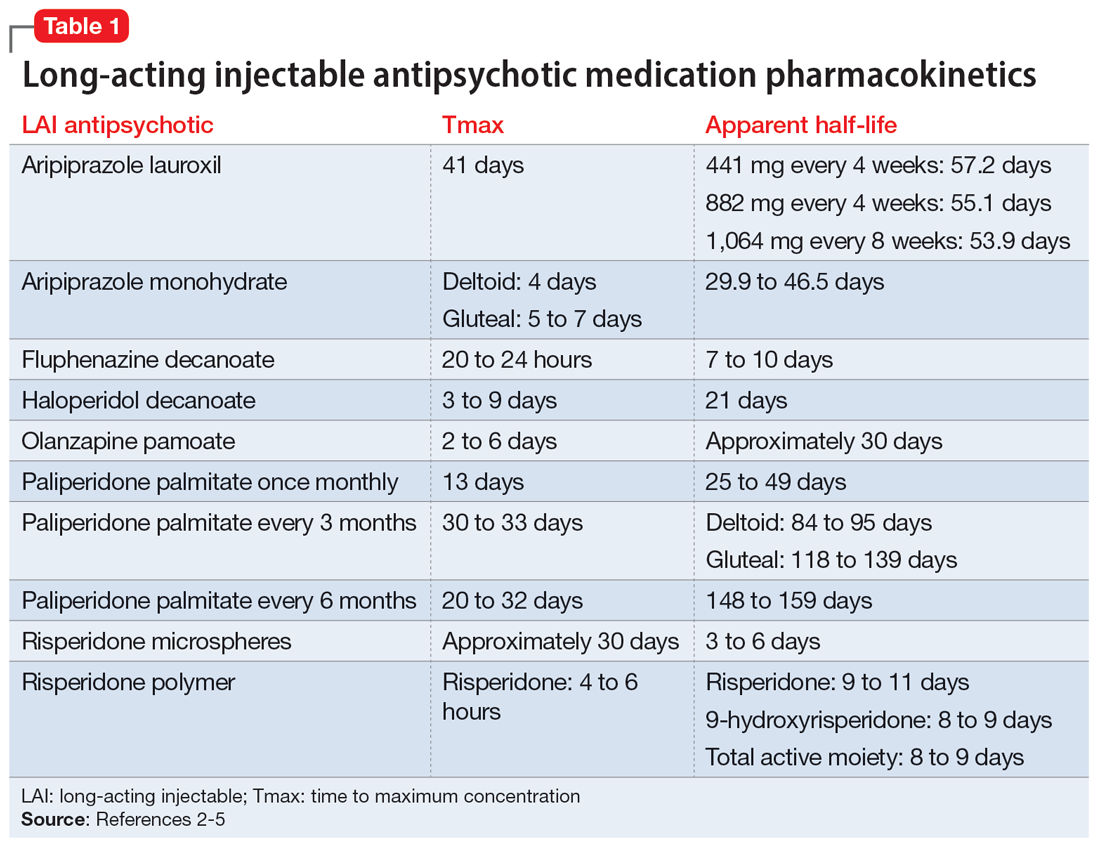
Based on the date of the next injection, the clinician will utilize the LAI’s half-life and its release mechanism to determine the appropriate time to start a new oral antipsychotic. Research demonstrates that in patients who have achieved steady state with a first-generation antipsychotic, plasma concentrations stay relatively consistent for 6 to 7 weeks after the last injection, which suggests oral medications may not need to be initiated until that time.6-9
For many second-generation LAI antipsychotics, oral medications may be initiated at the date of the next injection. Initiation of an oral antipsychotic may require more time between the last injection dose and the date of administration for oral medication due to the pharmacokinetic profile of risperidone microspheres. Once a patient is at steady state with risperidone microspheres, trough levels are not observed until 3 to 4 weeks after discontinuation.10
Previous pharmacokinetic model–based stimulations of active moiety plasma concentrations of risperidone microspheres demonstrate that 2 weeks after an injection of risperidone microspheres, the concentration of active moiety continued to approximate the steady-state concentration for 3 to 5 weeks.11 This is likely due to the product’s delay in release being 3 weeks from the time of injection to the last release phase. Of note, there was a rapid decline in the active moiety concentration; it reached nearly 0 by Week 5.11 The same pharmacokinetic model–based stimulation demonstrated a steady and slow decline of the concentration of active moiety of paliperidone palmitate after discontinuation of the LAI.11
Continue to: No guidance exists for...
No guidance exists for aripiprazole LAI medications; however, based on the pharmacokinetic data, administration of oral medications should be initiated at the date of next injection. Given the long half-life of aripiprazole, a cross-titration of the LAI with oral medication is reasonable.
Monitoring drug levels
In addition to utilizing the pharmacokinetic data from LAI antipsychotics, therapeutic drug levels can be instrumental in determining the dose of oral medication to use and when to begin titration (Table 2).12-14 Obtaining a drug level on the date of the next injection can provide the clinician with data regarding the release of the medication specific to the patient. Based on the level and the current symptomatology, the clinician could choose to start the oral medication at a lower dose and titrate back to the LAI equivalent oral dose, or initiate the oral dose at the LAI equivalent oral dose. Continued therapeutic drug levels can aid in this determination.
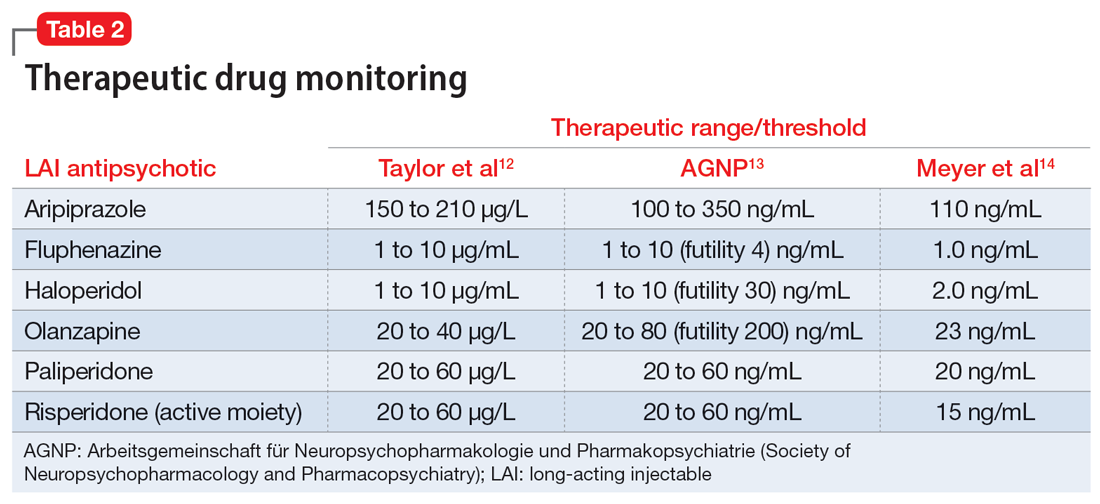
No guidance exists on the appropriate discontinuation of LAI antipsychotics. Utilizing a medication’s half-life and release mechanism, as well as the patient’s previous medication history, date of last injection, and therapeutic drug levels, should be considered when determining the schedule for restarting an oral antipsychotic.
CASE CONTINUED
Based on the current dosing of paliperidone palmitate of 156 mg once a month, Mr. R likely requires 9 mg/d of oral paliperidone upon discontinuation of the LAI. On the date of the next injection, the clinician could decide to initiate a lower dose of paliperidone, such as to 3 mg/d or 6 mg/d, and increase the dose as tolerated over the next 10 to 14 days as the paliperidone palmitate is further metabolized. Additionally, the clinician may consider obtaining a therapeutic drug level to determine the current paliperidone level prior to initiating the oral medication. Each treatment option offers individual risks and benefits. The decision on when and how to initiate the oral medication will be based on the individual patient’s situation and history, as well as the comfort and discretion of the clinician. The clinician should arrange appropriate monitoring for potential increased symptomatology during the transition, and adverse effects should be assessed regularly until steady state is achieved with the targeted oral dose of medication.
Related Resources
- Parmentier BL. Second-generation long-acting injectable antipsychotics: a practical guide. Current Psychiatry. 2020;19(3):24-32.
- Thippaiah SM, Fargason RE, Birur B. Switching antipsychotics: a guide to dose equivalents. Current Psychiatry. 2021;20(4):13-14. doi:10.12788/cp.0103
Drug Brand Names
Aripiprazole lauroxil • Aristada
Aripiprazole monohydrate • Maintena
Haloperidol injection • Haldol decanoate
Olanzapine pamoate • Zyprexa Relprevv
Paliperidone • Invega
Paliperidone palmitate once monthly • Invega Sustenna
Paliperidone palmitate every 3 months • Invega Trinza
Paliperidone palmitate every 6 months • Invega Hafyera
Risperidone microspheres • Risperdal Consta
Risperidone polymer • Perseris
1. Ostuzzi G, Vita G, Bertolini F, et al. Continuing, reducing, switching, or stopping antipsychotics in individuals with schizophrenia-spectrum disorders who are clinically stable: a systematic review and network meta-analysis. Lancet Psychiatry. 2022;9(8):614-624.
2. Correll CU, Kim E, Sliwa JK, et al. Pharmacokinetic characteristics of long-acting injectable antipsychotics for schizophrenia: an overview. CNS Drugs. 2021;35(1):39-59.
3. Spanarello S, La Ferla T. The pharmacokinetics of long-acting antipsychotic medications. Curr Clin Pharmacol. 2014;9(3):310-317.
4. Meyer JM. Understanding depot antipsychotics: an illustrated guide to kinetics. CNS Spectr. 2013;18(Suppl 1):58-68.
5. Invega Hafyera [package insert]. Janssen Pharmaceuticals, Inc; 2021.
6. Gitlin MJ, Midha KK, Fogelson D, et al. Persistence of fluphenazine in plasma after decanoate withdrawal. J Clin Psychopharmacol. 1988;8(1):53-56.
7. Wistedt B, Jørgensen A, Wiles D. A depot neuroleptic withdrawal study. Plasma concentration of fluphenazine and flupenthixol and relapse frequency. Psychopharmacology. 1982;78(4):301-304.
8. Chang WH, Lin SK, Juang DJ, et al. Prolonged haloperidol and reduced haloperidol plasma concentrations after decanoate withdrawal. Schizophr Res. 1993;9(1):35-40.
9. Eklund K, Forsman A. Minimal effective dose and relapse—double-blind trial: haloperidol decanoate vs. placebo. Clin Neuropharmacol. 1991;1(Suppl 2):S7-S15.
10. Wilson WH. A visual guide to expected blood levels of long-acting injectable risperidone in clinical practice. J Psychiatry Pract. 2004;10(6):393-401.
11. Samtani MN, Sheehan JJ, Fu DJ, et al. Management of antipsychotic treatment discontinuation and interruptions using model-based simulations. Clin Pharmacol. 2012;4:25-40.
12. Taylor D, Barnes TRE, Young AH. The Maudsley Prescribing Guidelines in Psychiatry. 13th ed. Wiley-Blackwell; 2018.
13. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-2):9-62.
14. Meyer JM, Stahl SM. The Clinical Use of Antipsychotic Plasma Levels. Cambridge University Press; 2021.
Mr. R, age 29, was diagnosed with schizophrenia 6 years ago. To manage his disorder, he has been receiving paliperidone palmitate long-acting injectable (LAI) 156 mg once a month for 2 years. Prior to maintenance with paliperidone palmitate, Mr. R was stabilized on oral paliperidone 9 mg/d. Though he was originally initiated on paliperidone palmitate due to nonadherence concerns, Mr. R has been adherent with each injection for 1 year.
At a recent visit, Mr. R says he wants to discontinue the injection because he is not interested in receiving an ongoing injectable medication and is not able to continue monthly clinic visits. He wants to take a daily oral antipsychotic again, despite the availability of longer-acting products.
A paucity of evidence exists regarding the discontinuation of LAI antipsychotics and the next steps that follow in treatment. There is neither a consensus nor recognized guidelines advising how and when to discontinue an LAI and restart an oral antipsychotic. A recent systematic review and meta-analysis evaluated different maintenance treatment strategies; however, switching from an LAI antipsychotic to an oral medication was not a focus.1 In this article, we outline a possible approach to discontinuing an LAI antipsychotic and restarting an oral formulation. Before discontinuing an LAI antipsychotic, clinicians should review with the patient the risks and benefits of switching medications, including the risk of decompensation and potential adverse effects.
Switching to an oral antipsychotic
The first step in the discontinuation process is to determine whether the patient will continue the same oral medication as the LAI antipsychotic or if a different oral antipsychotic will be initiated. Next, determining when to initiate the oral medication requires several pieces of information, including the oral dose equivalent of the patient’s current LAI, the half-life of the LAI, and the release mechanism of the LAI (Table 1).2-5 To determine the appropriate time frame for restarting oral treatment, it is also vital to know the date of the last injection.

Based on the date of the next injection, the clinician will utilize the LAI’s half-life and its release mechanism to determine the appropriate time to start a new oral antipsychotic. Research demonstrates that in patients who have achieved steady state with a first-generation antipsychotic, plasma concentrations stay relatively consistent for 6 to 7 weeks after the last injection, which suggests oral medications may not need to be initiated until that time.6-9
For many second-generation LAI antipsychotics, oral medications may be initiated at the date of the next injection. Initiation of an oral antipsychotic may require more time between the last injection dose and the date of administration for oral medication due to the pharmacokinetic profile of risperidone microspheres. Once a patient is at steady state with risperidone microspheres, trough levels are not observed until 3 to 4 weeks after discontinuation.10
Previous pharmacokinetic model–based stimulations of active moiety plasma concentrations of risperidone microspheres demonstrate that 2 weeks after an injection of risperidone microspheres, the concentration of active moiety continued to approximate the steady-state concentration for 3 to 5 weeks.11 This is likely due to the product’s delay in release being 3 weeks from the time of injection to the last release phase. Of note, there was a rapid decline in the active moiety concentration; it reached nearly 0 by Week 5.11 The same pharmacokinetic model–based stimulation demonstrated a steady and slow decline of the concentration of active moiety of paliperidone palmitate after discontinuation of the LAI.11
Continue to: No guidance exists for...
No guidance exists for aripiprazole LAI medications; however, based on the pharmacokinetic data, administration of oral medications should be initiated at the date of next injection. Given the long half-life of aripiprazole, a cross-titration of the LAI with oral medication is reasonable.
Monitoring drug levels
In addition to utilizing the pharmacokinetic data from LAI antipsychotics, therapeutic drug levels can be instrumental in determining the dose of oral medication to use and when to begin titration (Table 2).12-14 Obtaining a drug level on the date of the next injection can provide the clinician with data regarding the release of the medication specific to the patient. Based on the level and the current symptomatology, the clinician could choose to start the oral medication at a lower dose and titrate back to the LAI equivalent oral dose, or initiate the oral dose at the LAI equivalent oral dose. Continued therapeutic drug levels can aid in this determination.

No guidance exists on the appropriate discontinuation of LAI antipsychotics. Utilizing a medication’s half-life and release mechanism, as well as the patient’s previous medication history, date of last injection, and therapeutic drug levels, should be considered when determining the schedule for restarting an oral antipsychotic.
CASE CONTINUED
Based on the current dosing of paliperidone palmitate of 156 mg once a month, Mr. R likely requires 9 mg/d of oral paliperidone upon discontinuation of the LAI. On the date of the next injection, the clinician could decide to initiate a lower dose of paliperidone, such as to 3 mg/d or 6 mg/d, and increase the dose as tolerated over the next 10 to 14 days as the paliperidone palmitate is further metabolized. Additionally, the clinician may consider obtaining a therapeutic drug level to determine the current paliperidone level prior to initiating the oral medication. Each treatment option offers individual risks and benefits. The decision on when and how to initiate the oral medication will be based on the individual patient’s situation and history, as well as the comfort and discretion of the clinician. The clinician should arrange appropriate monitoring for potential increased symptomatology during the transition, and adverse effects should be assessed regularly until steady state is achieved with the targeted oral dose of medication.
Related Resources
- Parmentier BL. Second-generation long-acting injectable antipsychotics: a practical guide. Current Psychiatry. 2020;19(3):24-32.
- Thippaiah SM, Fargason RE, Birur B. Switching antipsychotics: a guide to dose equivalents. Current Psychiatry. 2021;20(4):13-14. doi:10.12788/cp.0103
Drug Brand Names
Aripiprazole lauroxil • Aristada
Aripiprazole monohydrate • Maintena
Haloperidol injection • Haldol decanoate
Olanzapine pamoate • Zyprexa Relprevv
Paliperidone • Invega
Paliperidone palmitate once monthly • Invega Sustenna
Paliperidone palmitate every 3 months • Invega Trinza
Paliperidone palmitate every 6 months • Invega Hafyera
Risperidone microspheres • Risperdal Consta
Risperidone polymer • Perseris
Mr. R, age 29, was diagnosed with schizophrenia 6 years ago. To manage his disorder, he has been receiving paliperidone palmitate long-acting injectable (LAI) 156 mg once a month for 2 years. Prior to maintenance with paliperidone palmitate, Mr. R was stabilized on oral paliperidone 9 mg/d. Though he was originally initiated on paliperidone palmitate due to nonadherence concerns, Mr. R has been adherent with each injection for 1 year.
At a recent visit, Mr. R says he wants to discontinue the injection because he is not interested in receiving an ongoing injectable medication and is not able to continue monthly clinic visits. He wants to take a daily oral antipsychotic again, despite the availability of longer-acting products.
A paucity of evidence exists regarding the discontinuation of LAI antipsychotics and the next steps that follow in treatment. There is neither a consensus nor recognized guidelines advising how and when to discontinue an LAI and restart an oral antipsychotic. A recent systematic review and meta-analysis evaluated different maintenance treatment strategies; however, switching from an LAI antipsychotic to an oral medication was not a focus.1 In this article, we outline a possible approach to discontinuing an LAI antipsychotic and restarting an oral formulation. Before discontinuing an LAI antipsychotic, clinicians should review with the patient the risks and benefits of switching medications, including the risk of decompensation and potential adverse effects.
Switching to an oral antipsychotic
The first step in the discontinuation process is to determine whether the patient will continue the same oral medication as the LAI antipsychotic or if a different oral antipsychotic will be initiated. Next, determining when to initiate the oral medication requires several pieces of information, including the oral dose equivalent of the patient’s current LAI, the half-life of the LAI, and the release mechanism of the LAI (Table 1).2-5 To determine the appropriate time frame for restarting oral treatment, it is also vital to know the date of the last injection.

Based on the date of the next injection, the clinician will utilize the LAI’s half-life and its release mechanism to determine the appropriate time to start a new oral antipsychotic. Research demonstrates that in patients who have achieved steady state with a first-generation antipsychotic, plasma concentrations stay relatively consistent for 6 to 7 weeks after the last injection, which suggests oral medications may not need to be initiated until that time.6-9
For many second-generation LAI antipsychotics, oral medications may be initiated at the date of the next injection. Initiation of an oral antipsychotic may require more time between the last injection dose and the date of administration for oral medication due to the pharmacokinetic profile of risperidone microspheres. Once a patient is at steady state with risperidone microspheres, trough levels are not observed until 3 to 4 weeks after discontinuation.10
Previous pharmacokinetic model–based stimulations of active moiety plasma concentrations of risperidone microspheres demonstrate that 2 weeks after an injection of risperidone microspheres, the concentration of active moiety continued to approximate the steady-state concentration for 3 to 5 weeks.11 This is likely due to the product’s delay in release being 3 weeks from the time of injection to the last release phase. Of note, there was a rapid decline in the active moiety concentration; it reached nearly 0 by Week 5.11 The same pharmacokinetic model–based stimulation demonstrated a steady and slow decline of the concentration of active moiety of paliperidone palmitate after discontinuation of the LAI.11
Continue to: No guidance exists for...
No guidance exists for aripiprazole LAI medications; however, based on the pharmacokinetic data, administration of oral medications should be initiated at the date of next injection. Given the long half-life of aripiprazole, a cross-titration of the LAI with oral medication is reasonable.
Monitoring drug levels
In addition to utilizing the pharmacokinetic data from LAI antipsychotics, therapeutic drug levels can be instrumental in determining the dose of oral medication to use and when to begin titration (Table 2).12-14 Obtaining a drug level on the date of the next injection can provide the clinician with data regarding the release of the medication specific to the patient. Based on the level and the current symptomatology, the clinician could choose to start the oral medication at a lower dose and titrate back to the LAI equivalent oral dose, or initiate the oral dose at the LAI equivalent oral dose. Continued therapeutic drug levels can aid in this determination.

No guidance exists on the appropriate discontinuation of LAI antipsychotics. Utilizing a medication’s half-life and release mechanism, as well as the patient’s previous medication history, date of last injection, and therapeutic drug levels, should be considered when determining the schedule for restarting an oral antipsychotic.
CASE CONTINUED
Based on the current dosing of paliperidone palmitate of 156 mg once a month, Mr. R likely requires 9 mg/d of oral paliperidone upon discontinuation of the LAI. On the date of the next injection, the clinician could decide to initiate a lower dose of paliperidone, such as to 3 mg/d or 6 mg/d, and increase the dose as tolerated over the next 10 to 14 days as the paliperidone palmitate is further metabolized. Additionally, the clinician may consider obtaining a therapeutic drug level to determine the current paliperidone level prior to initiating the oral medication. Each treatment option offers individual risks and benefits. The decision on when and how to initiate the oral medication will be based on the individual patient’s situation and history, as well as the comfort and discretion of the clinician. The clinician should arrange appropriate monitoring for potential increased symptomatology during the transition, and adverse effects should be assessed regularly until steady state is achieved with the targeted oral dose of medication.
Related Resources
- Parmentier BL. Second-generation long-acting injectable antipsychotics: a practical guide. Current Psychiatry. 2020;19(3):24-32.
- Thippaiah SM, Fargason RE, Birur B. Switching antipsychotics: a guide to dose equivalents. Current Psychiatry. 2021;20(4):13-14. doi:10.12788/cp.0103
Drug Brand Names
Aripiprazole lauroxil • Aristada
Aripiprazole monohydrate • Maintena
Haloperidol injection • Haldol decanoate
Olanzapine pamoate • Zyprexa Relprevv
Paliperidone • Invega
Paliperidone palmitate once monthly • Invega Sustenna
Paliperidone palmitate every 3 months • Invega Trinza
Paliperidone palmitate every 6 months • Invega Hafyera
Risperidone microspheres • Risperdal Consta
Risperidone polymer • Perseris
1. Ostuzzi G, Vita G, Bertolini F, et al. Continuing, reducing, switching, or stopping antipsychotics in individuals with schizophrenia-spectrum disorders who are clinically stable: a systematic review and network meta-analysis. Lancet Psychiatry. 2022;9(8):614-624.
2. Correll CU, Kim E, Sliwa JK, et al. Pharmacokinetic characteristics of long-acting injectable antipsychotics for schizophrenia: an overview. CNS Drugs. 2021;35(1):39-59.
3. Spanarello S, La Ferla T. The pharmacokinetics of long-acting antipsychotic medications. Curr Clin Pharmacol. 2014;9(3):310-317.
4. Meyer JM. Understanding depot antipsychotics: an illustrated guide to kinetics. CNS Spectr. 2013;18(Suppl 1):58-68.
5. Invega Hafyera [package insert]. Janssen Pharmaceuticals, Inc; 2021.
6. Gitlin MJ, Midha KK, Fogelson D, et al. Persistence of fluphenazine in plasma after decanoate withdrawal. J Clin Psychopharmacol. 1988;8(1):53-56.
7. Wistedt B, Jørgensen A, Wiles D. A depot neuroleptic withdrawal study. Plasma concentration of fluphenazine and flupenthixol and relapse frequency. Psychopharmacology. 1982;78(4):301-304.
8. Chang WH, Lin SK, Juang DJ, et al. Prolonged haloperidol and reduced haloperidol plasma concentrations after decanoate withdrawal. Schizophr Res. 1993;9(1):35-40.
9. Eklund K, Forsman A. Minimal effective dose and relapse—double-blind trial: haloperidol decanoate vs. placebo. Clin Neuropharmacol. 1991;1(Suppl 2):S7-S15.
10. Wilson WH. A visual guide to expected blood levels of long-acting injectable risperidone in clinical practice. J Psychiatry Pract. 2004;10(6):393-401.
11. Samtani MN, Sheehan JJ, Fu DJ, et al. Management of antipsychotic treatment discontinuation and interruptions using model-based simulations. Clin Pharmacol. 2012;4:25-40.
12. Taylor D, Barnes TRE, Young AH. The Maudsley Prescribing Guidelines in Psychiatry. 13th ed. Wiley-Blackwell; 2018.
13. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-2):9-62.
14. Meyer JM, Stahl SM. The Clinical Use of Antipsychotic Plasma Levels. Cambridge University Press; 2021.
1. Ostuzzi G, Vita G, Bertolini F, et al. Continuing, reducing, switching, or stopping antipsychotics in individuals with schizophrenia-spectrum disorders who are clinically stable: a systematic review and network meta-analysis. Lancet Psychiatry. 2022;9(8):614-624.
2. Correll CU, Kim E, Sliwa JK, et al. Pharmacokinetic characteristics of long-acting injectable antipsychotics for schizophrenia: an overview. CNS Drugs. 2021;35(1):39-59.
3. Spanarello S, La Ferla T. The pharmacokinetics of long-acting antipsychotic medications. Curr Clin Pharmacol. 2014;9(3):310-317.
4. Meyer JM. Understanding depot antipsychotics: an illustrated guide to kinetics. CNS Spectr. 2013;18(Suppl 1):58-68.
5. Invega Hafyera [package insert]. Janssen Pharmaceuticals, Inc; 2021.
6. Gitlin MJ, Midha KK, Fogelson D, et al. Persistence of fluphenazine in plasma after decanoate withdrawal. J Clin Psychopharmacol. 1988;8(1):53-56.
7. Wistedt B, Jørgensen A, Wiles D. A depot neuroleptic withdrawal study. Plasma concentration of fluphenazine and flupenthixol and relapse frequency. Psychopharmacology. 1982;78(4):301-304.
8. Chang WH, Lin SK, Juang DJ, et al. Prolonged haloperidol and reduced haloperidol plasma concentrations after decanoate withdrawal. Schizophr Res. 1993;9(1):35-40.
9. Eklund K, Forsman A. Minimal effective dose and relapse—double-blind trial: haloperidol decanoate vs. placebo. Clin Neuropharmacol. 1991;1(Suppl 2):S7-S15.
10. Wilson WH. A visual guide to expected blood levels of long-acting injectable risperidone in clinical practice. J Psychiatry Pract. 2004;10(6):393-401.
11. Samtani MN, Sheehan JJ, Fu DJ, et al. Management of antipsychotic treatment discontinuation and interruptions using model-based simulations. Clin Pharmacol. 2012;4:25-40.
12. Taylor D, Barnes TRE, Young AH. The Maudsley Prescribing Guidelines in Psychiatry. 13th ed. Wiley-Blackwell; 2018.
13. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-2):9-62.
14. Meyer JM, Stahl SM. The Clinical Use of Antipsychotic Plasma Levels. Cambridge University Press; 2021.
Medication-induced rhabdomyolysis
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Ms. A, age 32, has a history of anxiety, bipolar disorder, and borderline personality disorder. She is undergoing treatment with lamotrigine 200 mg/d at bedtime, aripiprazole 5 mg/d, trazodone 100 mg/d at bedtime, clonazepam 0.5 mg twice a day, and hydroxyzine 25 mg twice a day. She presents to the emergency department with myalgia, left upper and lower extremity numbness, and weakness. These symptoms started at approximately 3
Ms. A’s vital signs are hemodynamically stable, but her pulse is 113 bpm. On examination, she appears anxious and has decreased sensation in her upper and lower extremities, with 3/5 strength on the left side. Her laboratory results indicate mild leukocytosis, hyponatremia (129 mmol/L; reference range 136 to 145 mmol/L), and elevations in serum creatinine (3.7 mg/dL; reference range 0.6 to 1.2 mg/dL), aspartate aminotransferase (654 U/L; reference range 10 to 42 U/L), alanine transaminase (234 U/L; reference range 10 to 60 U/L), and troponin (2.11 ng/mL; reference range 0 to 0.04 ng/mL). A urinalysis reveals darkly colored urine with large red blood cells.
Neurology and Cardiology consultations are requested to rule out stroke and acute coronary syndromes. A computed tomography scan of the head shows no acute intracranial findings. Her creatinine kinase (CK) level is elevated (>42,670 U/L; reference range 22 to 232 U/L), which prompts a search for causes of rhabdomyolysis, a breakdown of muscle tissue that releases muscle fiber contents into the blood. Ms. A reports no history of recent trauma or strenuous exercise. Infectious, endocrine, and other workups are negative. After a consult to Psychiatry, the treating clinicians suspect that the most likely cause for rhabdomyolysis is aripiprazole.
Ms. A is treated with IV isotonic fluids. Aripiprazole is stopped and her CK levels are closely monitored. CK levels continue to trend down, and by Day 6 of hospitalization her CK level is 1,648 U/L. Her transaminase levels also improve; these elevations are considered likely secondary to rhabdomyolysis. Because there is notable improvement in CK and transaminase levels after stopping aripiprazole, Ms. A is discharged and instructed to follow up with a psychiatrist for further management.
Aripiprazole and rhabdomyolysis
According to the National Institute of Mental Health, an estimated 2.8% of the US population has bipolar disorder and 0.24% to 0.64% has schizophrenia.1,2 Antipsychotics are often used to treat these disorders. The prevalence of antipsychotic use in the general adult population is 1.6%.3 The use of second-generation antipsychotics (SGAs) has increased over recent years with the availability of a variety of formulations, such as immediate-release injectable, long-acting injectable, and orally disintegrating tablets in addition to the customary oral tablets. SGAs can cause several adverse effects, including weight gain, hyperlipidemia, diabetes, QTc prolongation, extrapyramidal side effects, myocarditis, agranulocytosis, cataracts, and sexual adverse effects.4
Antipsychotic use is more commonly associated with serotonin syndrome and neuroleptic malignant syndrome than it is with rhabdomyolysis. Rhabdomyolysis as an adverse effect of antipsychotic use has not been well understood or reported. One study found the prevalence of rhabdomyolysis was approximately 10% among patients who received an antipsychotic medication.5 There have been 4 case reports of clozapine use, 6 of olanzapine use, and 3 of aripiprazole use associated with rhabdomyolysis.6-8 Therefore, this would be the fourth case report to describe aripiprazole-associated rhabdomyolysis.
Aripiprazole is FDA-approved for the treatment of schizophrenia. In this case report, we found that aripiprazole could have led to rhabdomyolysis. Aripiprazole is a quinoline derivative that acts by binding to the 5-HT1A and 5-HT2A receptors.9,10 It acts as a partial agonist at 5-HT1A receptors, an antagonist at 5-HT2A receptors, and a partial agonist and stabilizer at the D2 receptor. By binding to the dopamine receptor in its G protein–coupled state, aripiprazole blocks the receptor in the presence of excessive dopamine.11-13 The mechanism of how aripiprazole could cause rhabdomyolysis is unclear. One proposed mechanism is that it can increase the permeability of skeletal muscle by 5-HT2A antagonism. This leads to a decrease in glucose reuptake in the cell and increases the permeability of the cell membrane, leading to elevations in CK levels.14 Another proposed mechanism is that dopamine blockade in the nigrostriatal pathway can result in muscle stiffness, rigidity, parkinsonian-like symptoms, and akathisia, which can result in elevated CK levels.15 There are only 3 other published cases of aripiprazole-induced rhabdomyolysis; we hope this case report will add value to the available literature. More evidence is needed to establish the safety profile of aripiprazole.
1. National Institute of Mental Health. Prevalence of bipolar disorder among adults. Accessed December 21, 2022. https://www.nimh.nih.gov/health/statistics/bipolar-disorder#part_2605
2. National Institute of Mental Health. Schizophrenia. Accessed December 21, 2022. https://www.nimh.nih.gov/health/statistics/schizophrenia#part_2543
3. Dennis JA, Gittner LS, Payne JD, et al. Characteristics of U.S. adults taking prescription antipsychotic medications, National Health and Nutrition Examination Survey 2013-2018. BMC Psychiatry. 2020;20(1):483. doi: 10.1186/s12888-020-02895-4
4. Willner K, Vasan S, Abdijadid S. Atypical antipsychotic agents. In: StatPearls [Internet]. StatPearls Publishing; 2022. Updated May 2, 2022. Accessed December 22, 2022. https://www.ncbi.nlm.nih.gov/books/NBK448156/
5. Packard K, Price P, Hanson A. Antipsychotic use and the risk of rhabdomyolysis. J Pharm Pract 2014;27(5):501-512. doi: 10.1177/0897190013516509
6. Wu YF, Chang KY. Aripiprazole-associated rhabdomyolysis in a patient with schizophrenia. J Neuropsychiatry Clin Neurosci. 2011;23(3):E51.
7. Marzetti E, Bocchino L, Teramo S, et al. Rhabdomyolysis in a patient on aripiprazole with traumatic hip prosthesis luxation. J Neuropsychiatry Clin Neurosci. 2012;24(4):E40-E41.
8. Zhu X, Hu J, Deng S, et al. Rhabdomyolysis and elevated liver enzymes after rapid correction of hyponatremia due to pneumonia and concurrent use of aripiprazole: a case report. Aust N Z J Psychiatry. 2018;52(2):206. doi:10.1177/0004867417743342
9. Stahl SM. Essential Psychopharmacology: Neuroscientific Basis and Practical Application. 2nd ed. Cambridge University Press; 2000.
10. Stahl SM. “Hit-and-run” actions at dopamine receptors, part 1: mechanism of action of atypical antipsychotics. J Clin Psychiatry. 2001;62(9):670-671.
11. Leysen JE, Janssen PM, Schotte A, et al. Interaction of antipsychotic drugs with neurotransmitter receptor sites in vitro and in vivo in relation to pharmacological and clinical effects: role of 5HT2 receptors. Psychopharmacology (Berl). 1993;112(1 Suppl):S40-S54.
12. Millan MJ. Improving the treatment of schizophrenia: focus on serotonin (5-HT)(1A) receptors. J Pharmacol Exp Ther. 2000;295(3):853-861.
13. Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol. 2003;70(2):83-244.
14. Meltzer HY, Cola PA, Parsa M. Marked elevations of serum creatine kinase activity associated with antipsychotic drug treatment. Neuropsychopharmacology. 1996;15(4):395-405.
15. Devarajan S, Dursun SM. Antipsychotic drugs, serum creatine kinase (CPK) and possible mechanisms. Psychopharmacology (Berl). 2000;152(1):122.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Ms. A, age 32, has a history of anxiety, bipolar disorder, and borderline personality disorder. She is undergoing treatment with lamotrigine 200 mg/d at bedtime, aripiprazole 5 mg/d, trazodone 100 mg/d at bedtime, clonazepam 0.5 mg twice a day, and hydroxyzine 25 mg twice a day. She presents to the emergency department with myalgia, left upper and lower extremity numbness, and weakness. These symptoms started at approximately 3
Ms. A’s vital signs are hemodynamically stable, but her pulse is 113 bpm. On examination, she appears anxious and has decreased sensation in her upper and lower extremities, with 3/5 strength on the left side. Her laboratory results indicate mild leukocytosis, hyponatremia (129 mmol/L; reference range 136 to 145 mmol/L), and elevations in serum creatinine (3.7 mg/dL; reference range 0.6 to 1.2 mg/dL), aspartate aminotransferase (654 U/L; reference range 10 to 42 U/L), alanine transaminase (234 U/L; reference range 10 to 60 U/L), and troponin (2.11 ng/mL; reference range 0 to 0.04 ng/mL). A urinalysis reveals darkly colored urine with large red blood cells.
Neurology and Cardiology consultations are requested to rule out stroke and acute coronary syndromes. A computed tomography scan of the head shows no acute intracranial findings. Her creatinine kinase (CK) level is elevated (>42,670 U/L; reference range 22 to 232 U/L), which prompts a search for causes of rhabdomyolysis, a breakdown of muscle tissue that releases muscle fiber contents into the blood. Ms. A reports no history of recent trauma or strenuous exercise. Infectious, endocrine, and other workups are negative. After a consult to Psychiatry, the treating clinicians suspect that the most likely cause for rhabdomyolysis is aripiprazole.
Ms. A is treated with IV isotonic fluids. Aripiprazole is stopped and her CK levels are closely monitored. CK levels continue to trend down, and by Day 6 of hospitalization her CK level is 1,648 U/L. Her transaminase levels also improve; these elevations are considered likely secondary to rhabdomyolysis. Because there is notable improvement in CK and transaminase levels after stopping aripiprazole, Ms. A is discharged and instructed to follow up with a psychiatrist for further management.
Aripiprazole and rhabdomyolysis
According to the National Institute of Mental Health, an estimated 2.8% of the US population has bipolar disorder and 0.24% to 0.64% has schizophrenia.1,2 Antipsychotics are often used to treat these disorders. The prevalence of antipsychotic use in the general adult population is 1.6%.3 The use of second-generation antipsychotics (SGAs) has increased over recent years with the availability of a variety of formulations, such as immediate-release injectable, long-acting injectable, and orally disintegrating tablets in addition to the customary oral tablets. SGAs can cause several adverse effects, including weight gain, hyperlipidemia, diabetes, QTc prolongation, extrapyramidal side effects, myocarditis, agranulocytosis, cataracts, and sexual adverse effects.4
Antipsychotic use is more commonly associated with serotonin syndrome and neuroleptic malignant syndrome than it is with rhabdomyolysis. Rhabdomyolysis as an adverse effect of antipsychotic use has not been well understood or reported. One study found the prevalence of rhabdomyolysis was approximately 10% among patients who received an antipsychotic medication.5 There have been 4 case reports of clozapine use, 6 of olanzapine use, and 3 of aripiprazole use associated with rhabdomyolysis.6-8 Therefore, this would be the fourth case report to describe aripiprazole-associated rhabdomyolysis.
Aripiprazole is FDA-approved for the treatment of schizophrenia. In this case report, we found that aripiprazole could have led to rhabdomyolysis. Aripiprazole is a quinoline derivative that acts by binding to the 5-HT1A and 5-HT2A receptors.9,10 It acts as a partial agonist at 5-HT1A receptors, an antagonist at 5-HT2A receptors, and a partial agonist and stabilizer at the D2 receptor. By binding to the dopamine receptor in its G protein–coupled state, aripiprazole blocks the receptor in the presence of excessive dopamine.11-13 The mechanism of how aripiprazole could cause rhabdomyolysis is unclear. One proposed mechanism is that it can increase the permeability of skeletal muscle by 5-HT2A antagonism. This leads to a decrease in glucose reuptake in the cell and increases the permeability of the cell membrane, leading to elevations in CK levels.14 Another proposed mechanism is that dopamine blockade in the nigrostriatal pathway can result in muscle stiffness, rigidity, parkinsonian-like symptoms, and akathisia, which can result in elevated CK levels.15 There are only 3 other published cases of aripiprazole-induced rhabdomyolysis; we hope this case report will add value to the available literature. More evidence is needed to establish the safety profile of aripiprazole.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Ms. A, age 32, has a history of anxiety, bipolar disorder, and borderline personality disorder. She is undergoing treatment with lamotrigine 200 mg/d at bedtime, aripiprazole 5 mg/d, trazodone 100 mg/d at bedtime, clonazepam 0.5 mg twice a day, and hydroxyzine 25 mg twice a day. She presents to the emergency department with myalgia, left upper and lower extremity numbness, and weakness. These symptoms started at approximately 3
Ms. A’s vital signs are hemodynamically stable, but her pulse is 113 bpm. On examination, she appears anxious and has decreased sensation in her upper and lower extremities, with 3/5 strength on the left side. Her laboratory results indicate mild leukocytosis, hyponatremia (129 mmol/L; reference range 136 to 145 mmol/L), and elevations in serum creatinine (3.7 mg/dL; reference range 0.6 to 1.2 mg/dL), aspartate aminotransferase (654 U/L; reference range 10 to 42 U/L), alanine transaminase (234 U/L; reference range 10 to 60 U/L), and troponin (2.11 ng/mL; reference range 0 to 0.04 ng/mL). A urinalysis reveals darkly colored urine with large red blood cells.
Neurology and Cardiology consultations are requested to rule out stroke and acute coronary syndromes. A computed tomography scan of the head shows no acute intracranial findings. Her creatinine kinase (CK) level is elevated (>42,670 U/L; reference range 22 to 232 U/L), which prompts a search for causes of rhabdomyolysis, a breakdown of muscle tissue that releases muscle fiber contents into the blood. Ms. A reports no history of recent trauma or strenuous exercise. Infectious, endocrine, and other workups are negative. After a consult to Psychiatry, the treating clinicians suspect that the most likely cause for rhabdomyolysis is aripiprazole.
Ms. A is treated with IV isotonic fluids. Aripiprazole is stopped and her CK levels are closely monitored. CK levels continue to trend down, and by Day 6 of hospitalization her CK level is 1,648 U/L. Her transaminase levels also improve; these elevations are considered likely secondary to rhabdomyolysis. Because there is notable improvement in CK and transaminase levels after stopping aripiprazole, Ms. A is discharged and instructed to follow up with a psychiatrist for further management.
Aripiprazole and rhabdomyolysis
According to the National Institute of Mental Health, an estimated 2.8% of the US population has bipolar disorder and 0.24% to 0.64% has schizophrenia.1,2 Antipsychotics are often used to treat these disorders. The prevalence of antipsychotic use in the general adult population is 1.6%.3 The use of second-generation antipsychotics (SGAs) has increased over recent years with the availability of a variety of formulations, such as immediate-release injectable, long-acting injectable, and orally disintegrating tablets in addition to the customary oral tablets. SGAs can cause several adverse effects, including weight gain, hyperlipidemia, diabetes, QTc prolongation, extrapyramidal side effects, myocarditis, agranulocytosis, cataracts, and sexual adverse effects.4
Antipsychotic use is more commonly associated with serotonin syndrome and neuroleptic malignant syndrome than it is with rhabdomyolysis. Rhabdomyolysis as an adverse effect of antipsychotic use has not been well understood or reported. One study found the prevalence of rhabdomyolysis was approximately 10% among patients who received an antipsychotic medication.5 There have been 4 case reports of clozapine use, 6 of olanzapine use, and 3 of aripiprazole use associated with rhabdomyolysis.6-8 Therefore, this would be the fourth case report to describe aripiprazole-associated rhabdomyolysis.
Aripiprazole is FDA-approved for the treatment of schizophrenia. In this case report, we found that aripiprazole could have led to rhabdomyolysis. Aripiprazole is a quinoline derivative that acts by binding to the 5-HT1A and 5-HT2A receptors.9,10 It acts as a partial agonist at 5-HT1A receptors, an antagonist at 5-HT2A receptors, and a partial agonist and stabilizer at the D2 receptor. By binding to the dopamine receptor in its G protein–coupled state, aripiprazole blocks the receptor in the presence of excessive dopamine.11-13 The mechanism of how aripiprazole could cause rhabdomyolysis is unclear. One proposed mechanism is that it can increase the permeability of skeletal muscle by 5-HT2A antagonism. This leads to a decrease in glucose reuptake in the cell and increases the permeability of the cell membrane, leading to elevations in CK levels.14 Another proposed mechanism is that dopamine blockade in the nigrostriatal pathway can result in muscle stiffness, rigidity, parkinsonian-like symptoms, and akathisia, which can result in elevated CK levels.15 There are only 3 other published cases of aripiprazole-induced rhabdomyolysis; we hope this case report will add value to the available literature. More evidence is needed to establish the safety profile of aripiprazole.
1. National Institute of Mental Health. Prevalence of bipolar disorder among adults. Accessed December 21, 2022. https://www.nimh.nih.gov/health/statistics/bipolar-disorder#part_2605
2. National Institute of Mental Health. Schizophrenia. Accessed December 21, 2022. https://www.nimh.nih.gov/health/statistics/schizophrenia#part_2543
3. Dennis JA, Gittner LS, Payne JD, et al. Characteristics of U.S. adults taking prescription antipsychotic medications, National Health and Nutrition Examination Survey 2013-2018. BMC Psychiatry. 2020;20(1):483. doi: 10.1186/s12888-020-02895-4
4. Willner K, Vasan S, Abdijadid S. Atypical antipsychotic agents. In: StatPearls [Internet]. StatPearls Publishing; 2022. Updated May 2, 2022. Accessed December 22, 2022. https://www.ncbi.nlm.nih.gov/books/NBK448156/
5. Packard K, Price P, Hanson A. Antipsychotic use and the risk of rhabdomyolysis. J Pharm Pract 2014;27(5):501-512. doi: 10.1177/0897190013516509
6. Wu YF, Chang KY. Aripiprazole-associated rhabdomyolysis in a patient with schizophrenia. J Neuropsychiatry Clin Neurosci. 2011;23(3):E51.
7. Marzetti E, Bocchino L, Teramo S, et al. Rhabdomyolysis in a patient on aripiprazole with traumatic hip prosthesis luxation. J Neuropsychiatry Clin Neurosci. 2012;24(4):E40-E41.
8. Zhu X, Hu J, Deng S, et al. Rhabdomyolysis and elevated liver enzymes after rapid correction of hyponatremia due to pneumonia and concurrent use of aripiprazole: a case report. Aust N Z J Psychiatry. 2018;52(2):206. doi:10.1177/0004867417743342
9. Stahl SM. Essential Psychopharmacology: Neuroscientific Basis and Practical Application. 2nd ed. Cambridge University Press; 2000.
10. Stahl SM. “Hit-and-run” actions at dopamine receptors, part 1: mechanism of action of atypical antipsychotics. J Clin Psychiatry. 2001;62(9):670-671.
11. Leysen JE, Janssen PM, Schotte A, et al. Interaction of antipsychotic drugs with neurotransmitter receptor sites in vitro and in vivo in relation to pharmacological and clinical effects: role of 5HT2 receptors. Psychopharmacology (Berl). 1993;112(1 Suppl):S40-S54.
12. Millan MJ. Improving the treatment of schizophrenia: focus on serotonin (5-HT)(1A) receptors. J Pharmacol Exp Ther. 2000;295(3):853-861.
13. Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol. 2003;70(2):83-244.
14. Meltzer HY, Cola PA, Parsa M. Marked elevations of serum creatine kinase activity associated with antipsychotic drug treatment. Neuropsychopharmacology. 1996;15(4):395-405.
15. Devarajan S, Dursun SM. Antipsychotic drugs, serum creatine kinase (CPK) and possible mechanisms. Psychopharmacology (Berl). 2000;152(1):122.
1. National Institute of Mental Health. Prevalence of bipolar disorder among adults. Accessed December 21, 2022. https://www.nimh.nih.gov/health/statistics/bipolar-disorder#part_2605
2. National Institute of Mental Health. Schizophrenia. Accessed December 21, 2022. https://www.nimh.nih.gov/health/statistics/schizophrenia#part_2543
3. Dennis JA, Gittner LS, Payne JD, et al. Characteristics of U.S. adults taking prescription antipsychotic medications, National Health and Nutrition Examination Survey 2013-2018. BMC Psychiatry. 2020;20(1):483. doi: 10.1186/s12888-020-02895-4
4. Willner K, Vasan S, Abdijadid S. Atypical antipsychotic agents. In: StatPearls [Internet]. StatPearls Publishing; 2022. Updated May 2, 2022. Accessed December 22, 2022. https://www.ncbi.nlm.nih.gov/books/NBK448156/
5. Packard K, Price P, Hanson A. Antipsychotic use and the risk of rhabdomyolysis. J Pharm Pract 2014;27(5):501-512. doi: 10.1177/0897190013516509
6. Wu YF, Chang KY. Aripiprazole-associated rhabdomyolysis in a patient with schizophrenia. J Neuropsychiatry Clin Neurosci. 2011;23(3):E51.
7. Marzetti E, Bocchino L, Teramo S, et al. Rhabdomyolysis in a patient on aripiprazole with traumatic hip prosthesis luxation. J Neuropsychiatry Clin Neurosci. 2012;24(4):E40-E41.
8. Zhu X, Hu J, Deng S, et al. Rhabdomyolysis and elevated liver enzymes after rapid correction of hyponatremia due to pneumonia and concurrent use of aripiprazole: a case report. Aust N Z J Psychiatry. 2018;52(2):206. doi:10.1177/0004867417743342
9. Stahl SM. Essential Psychopharmacology: Neuroscientific Basis and Practical Application. 2nd ed. Cambridge University Press; 2000.
10. Stahl SM. “Hit-and-run” actions at dopamine receptors, part 1: mechanism of action of atypical antipsychotics. J Clin Psychiatry. 2001;62(9):670-671.
11. Leysen JE, Janssen PM, Schotte A, et al. Interaction of antipsychotic drugs with neurotransmitter receptor sites in vitro and in vivo in relation to pharmacological and clinical effects: role of 5HT2 receptors. Psychopharmacology (Berl). 1993;112(1 Suppl):S40-S54.
12. Millan MJ. Improving the treatment of schizophrenia: focus on serotonin (5-HT)(1A) receptors. J Pharmacol Exp Ther. 2000;295(3):853-861.
13. Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol. 2003;70(2):83-244.
14. Meltzer HY, Cola PA, Parsa M. Marked elevations of serum creatine kinase activity associated with antipsychotic drug treatment. Neuropsychopharmacology. 1996;15(4):395-405.
15. Devarajan S, Dursun SM. Antipsychotic drugs, serum creatine kinase (CPK) and possible mechanisms. Psychopharmacology (Berl). 2000;152(1):122.
Subtle cognitive decline in a patient with depression and anxiety
CASE Anxious and confused
Mr. M, age 53, a surgeon, presents to the emergency department (ED) following a panic attack and concerns from his staff that he appears confused. Specifically, staff members report that in the past 4 months, Mr. M was observed having problems completing some postoperative tasks related to chart documentation. Mr. M has a history of major depressive disorder (MDD), hypertension, hyperlipidemia, and type 2 diabetes.
HISTORY A long-standing diagnosis of depression
Mr. M reports that 30 years ago, he received care from a psychiatrist to address symptoms of MDD. He says that around the time he arrived at the ED, he had noticed subtle but gradual changes in his cognition, which led him to skip words and often struggle to find the correct words. These episodes left him confused. Mr. M started getting anxious about these cognitive issues because they disrupted his work and forced him to reduce his duties. He does not have any known family history of mental illness, is single, and lives alone.
EVALUATION After stroke is ruled out, a psychiatric workup
In the ED, a comprehensive exam rules out an acute cerebrovascular event. A neurologic evaluation notes some delay in processing information and observes Mr. M having difficulty following simple commands. Laboratory investigations, including a comprehensive metabolic panel, are unremarkable. An MRI of Mr. M’s brain, with and without contrast, notes no acute findings. He is discharged from the ED with a diagnosis of MDD.
Before he presented to the ED, Mr. M’s medication regimen included duloxetine 60 mg/d, buspirone 10 mg 3 times a day, and aripiprazole 5 mg/d for MDD and anxiety. After the ED visit, Mr. M’s physician refers him to an outpatient psychiatrist for management of worsening depression and panic attacks. During the psychiatrist’s evaluation, Mr. M reports a decreased interest in activities, decreased motivation, being easily fatigued, and having poor sleep. He denies having a depressed mood, difficulty concentrating, or having problems with his appetite. He also denies suicidal thoughts, both past and present.
Mr. M describes his mood as anxious, primarily surrounding his recent cognitive changes. He does not have a substance use disorder, psychotic illness, mania or hypomania, posttraumatic stress disorder, or obsessive-compulsive disorder. He reports adherence to his psychiatric medications. A mental status exam reveals Mr. M to be anxious. His attention is not well sustained, and he has difficulty describing details of his cognitive struggles, providing vague descriptions such as “skipping thought” and “skipping words.” Mr. M’s affect is congruent to his mood with some restriction and the psychiatrist notes that he is experiencing thought latency, poverty of content of thoughts, word-finding difficulties, and circumlocution. Mr. M denies any perceptual abnormalities, and there is no evidence of delusions.
[polldaddy:11320112]
The authors’ observations
Mr. M’s symptoms are significant for subacute cognitive decline that is subtle but gradual and can be easily missed, especially in the beginning. Though his ED evaluation—including brain imaging—ruled out acute or focal neurologic findings and his primary psychiatric presentation was anxiety, Mr. M’s medical history and mental status exam were suggestive of cognitive deficits.
Collateral information was obtained from his work colleagues, which confirmed both cognitive problems and comorbid anxiety. Additionally, given Mr. M’s high cognitive baseline as a surgeon, the new-onset cognitive changes over 4 months warranted further cognitive and neurologic evaluation. There are many causes of cognitive impairment (vascular, cancer, infection, autoimmune, medications, substances or toxins, neurodegenerative, psychiatric, vitamin deficiencies), all of which need to be considered in a patient with a nonspecific presentation such as Mr. M’s. The psychiatrist confirmed Mr. M’s current medication regimen, and discussed tapering aripiprazole while continuing duloxetine and buspirone.
Continue to: EVALUATION A closer look at cognitive deficits
EVALUATION A closer look at cognitive deficits
Mr. M scores 12/30 on the Montreal Cognitive Assessment (MoCA), indicating moderate cognitive impairment (Table 1). The psychiatrist refers Mr. M to Neurology. During his neurologic evaluation, Mr. M continues to report feeling anxious that “something is wrong” and skips his words. The neurologist confirms Mr. M’s symptoms may have started 2 to 3 months before he presented to the ED. Mr. M reports unusual eating habits, including yogurt and cookies for breakfast, Mexican food for lunch, and more cookies for dinner. He denies having a fever, gaining or losing weight, rashes, headaches, neck stiffness, tingling or weakness or stiffness of limbs, vertigo, visual changes, photophobia, unsteady gait, bowel or bladder incontinence, or tremors.
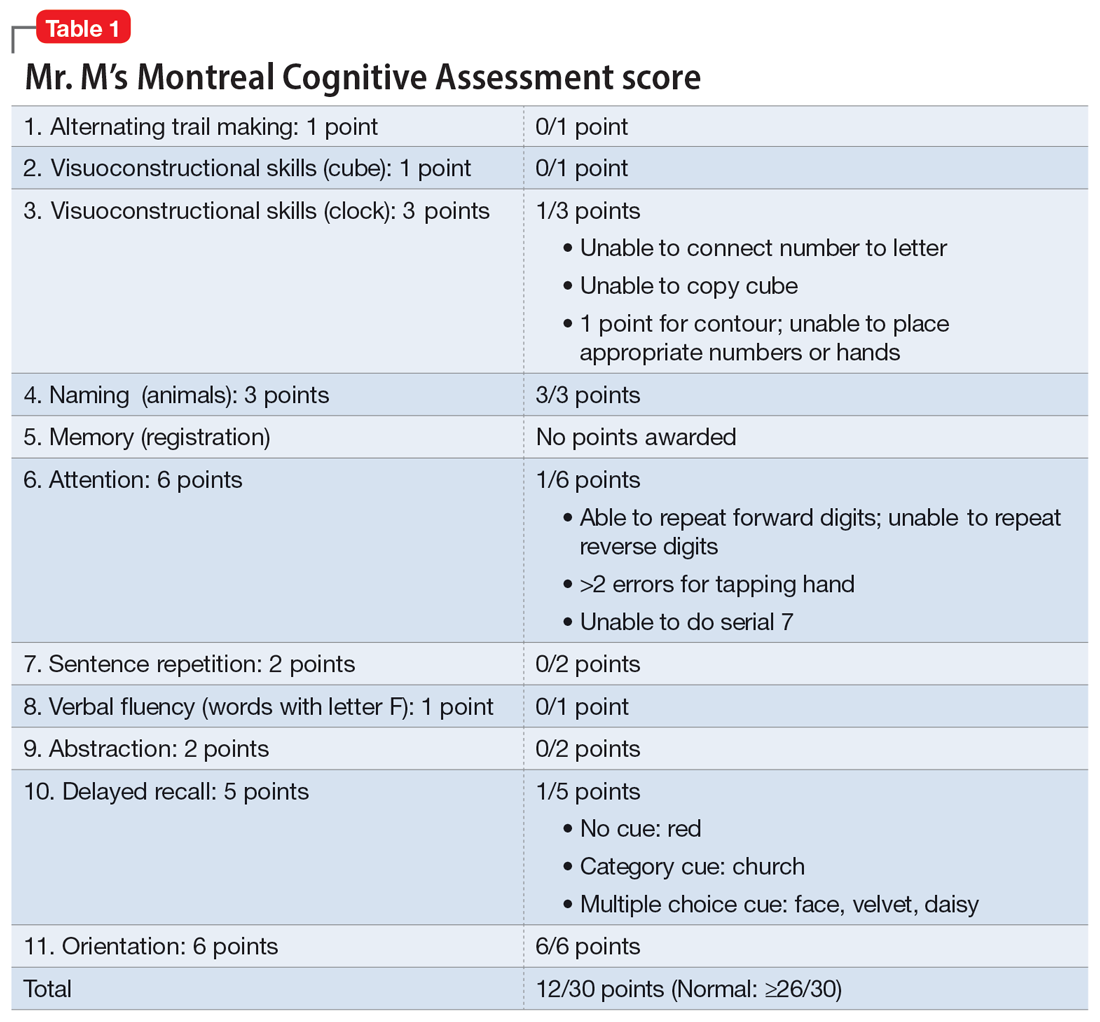
When the neurologist repeats the MoCA, Mr. M again scores 12. The neurologist notes that Mr. M answers questions a little slowly and pauses for thoughts when unable to find an answer. Mr. M has difficulty following some simple commands, such as “touch a finger to your nose.” Other in-office neurologic physical exams (cranial nerves, involuntary movements or tremors, sensation, muscle strength, reflexes, cerebellar signs) are unremarkable except for mildly decreased vibration sense of his toes. The neurologist concludes that Mr. M’s presentation is suggestive of subacute to chronic bradyphrenia and orders additional evaluation, including neuropsychological testing.
[polldaddy:11320114]
The authors’ observations
Physical and neurologic exams were not suggestive of any obvious causes of cognitive decline. Both the mental status exam and 2 serial MoCAs suggested deficits in executive function, language, and memory. Each of the differential diagnoses considered was ruled out with workup or exams (Table 2), which led to a most likely diagnosis of neurodegenerative disorder with PPA. Neuropsychological testing confirmed the diagnosis of nonfluent PPA.
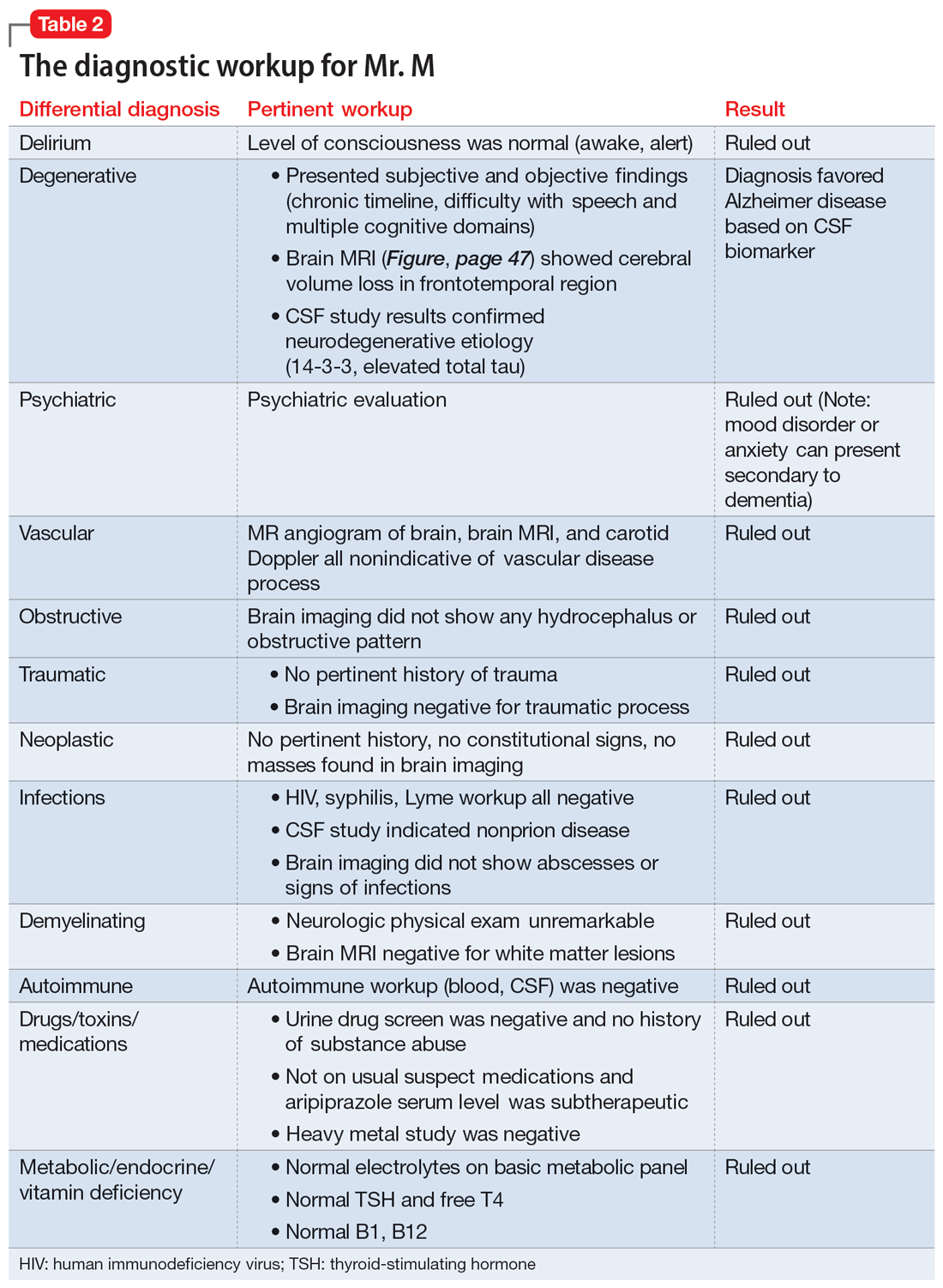
Primary progressive aphasia
PPA is an uncommon, heterogeneous group of disorders stemming from focal degeneration of language-governing centers of the brain.1,2 The estimated prevalence of PPA is 3 in 100,000 cases.2,3 There are 4 major variants of PPA (Table 34), and each presents with distinct language, cognitive, neuroanatomical, and neuropathological characteristics.4 PPA is usually diagnosed in late middle life; however, diagnosis is often delayed due to the relative obscurity of the disorder.4 In Mr. M’s case, it took approximately 4 months of evaluations by various specialists before a diagnosis was confirmed.
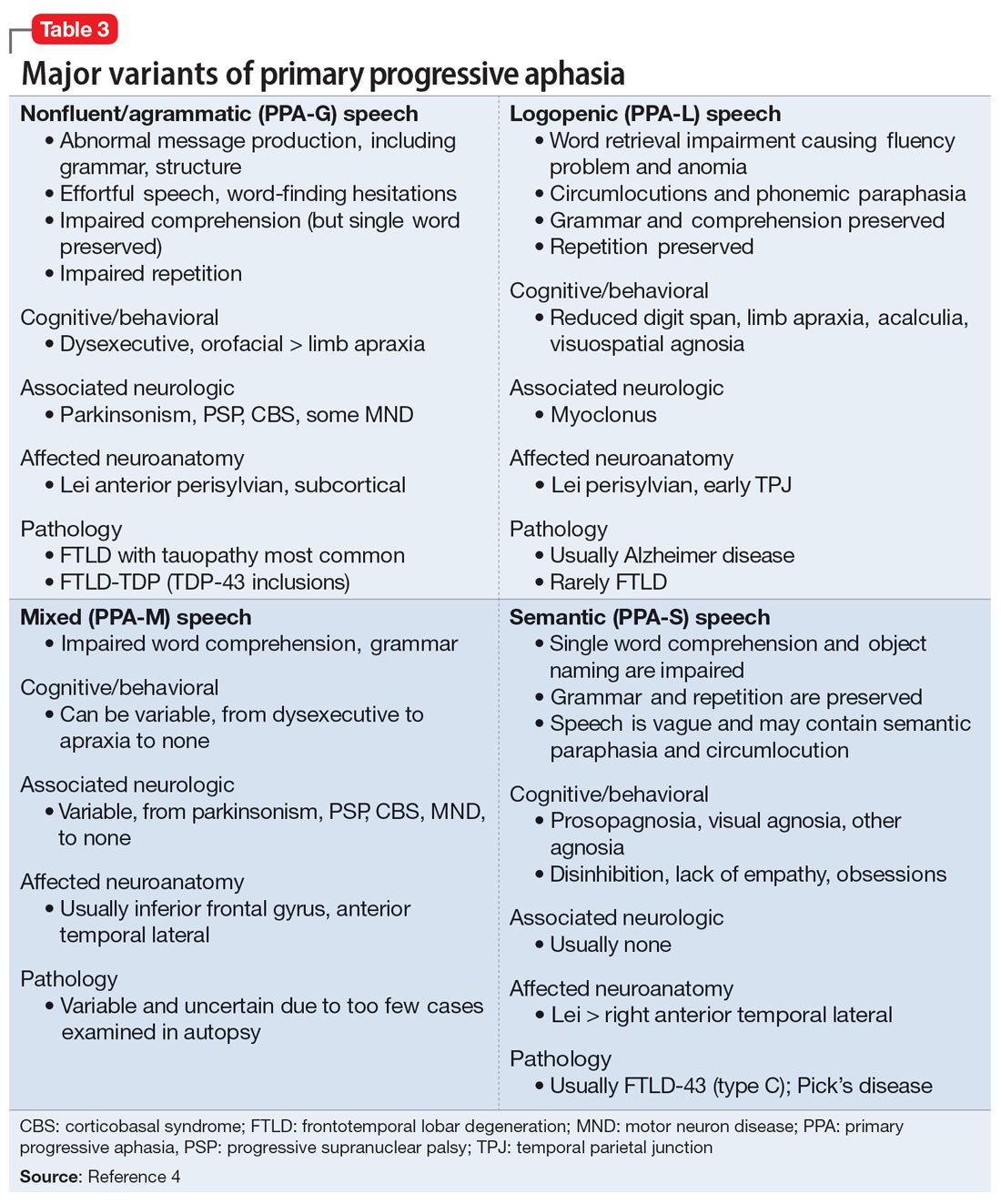
The initial phase of PPA can present as a diagnostic challenge because patients can have difficulty articulating their cognitive and language deficits. PPA can be commonly mistaken for a primary psychiatric disorder such as MDD or anxiety, which can further delay an accurate diagnosis and treatment. Special attention to the mental status exam, close observation of the patient’s language, and assessment of cognitive abilities using standardized screenings such as the MoCA or Mini-Mental State Examination can be helpful in clarifying the diagnosis. It is also important to rule out developmental problems (eg, dyslexia) and hearing difficulties, particularly in older patients.
Continue to: TREATMENT Adjusting the medication regimen
TREATMENT Adjusting the medication regimen
The neurologist completes additional examinations to rule out causes of rare neurodegenerative disorders, including CSF autoimmune disorders, Creutzfeldt-Jakob disease, and Alzheimer disease (AD) (Table 4). Mr. M continues to follow up with his outpatient psychiatrist and his medication regimen is adjusted. Aripiprazole and buspirone are discontinued, and duloxetine is titrated to 60 mg twice a day. During follow-up visits, Mr. M discusses his understanding of his neurologic condition. His concerns shift to his illness and prognosis. During these visits, he continues to deny suicidality.
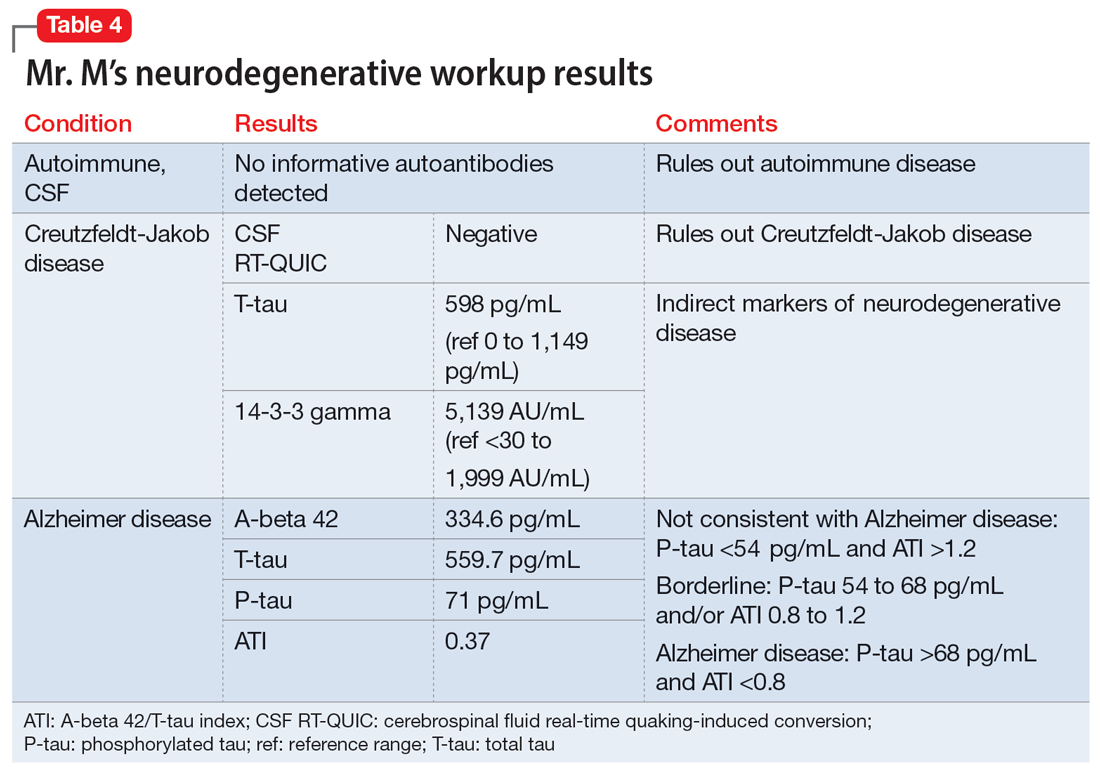
[polldaddy:11320115]
The authors’ observations
Mr. M’s neurodegenerative workup identified an intriguing diagnostic challenge. A repeat brain MRI (Figure) showed atrophy patterns suggestive of frontotemporal lobar degeneration (FTLD). On the other hand, his CSF ATI (A-beta 42/T-tau index, a value used to aid in the diagnosis of AD) was <1, suggesting early-onset AD.5,6 Although significant advances have been made to distinguish AD and FTLD following an autopsy, there are still no reliable or definitive biomarkers to distinguish AD from FTLD (particularly in the early stages of FTLD). This can often leave the confirmatory diagnosis as a question.7
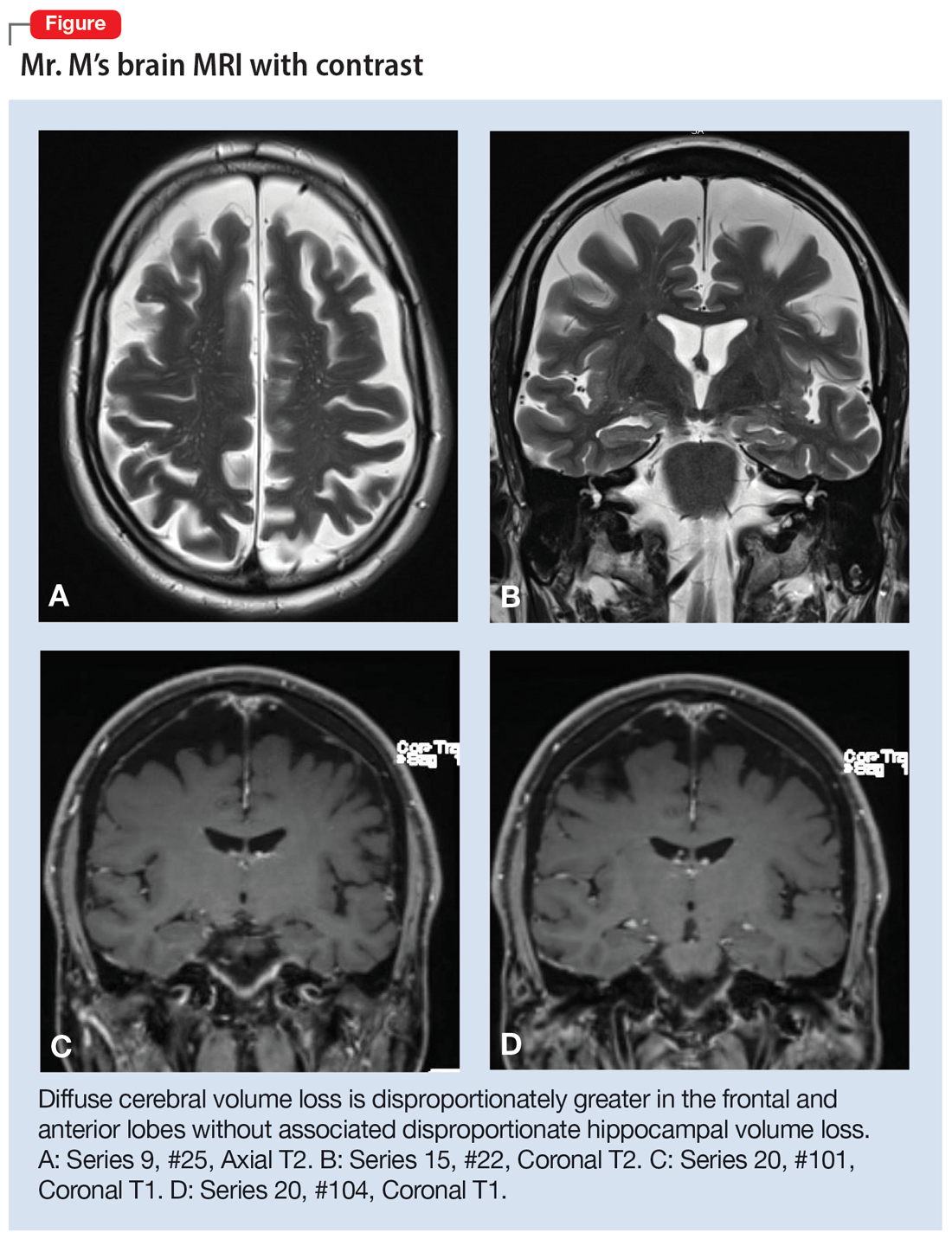
A PPA diagnosis (and other dementias) can have a significant impact on the patient and their family due to the uncertain nature of the progression of the disease and quality-of-life issues related to language and other cognitive deficits. Early identification and accurate diagnosis of PPA and its etiology (ie, AD vs FTLD) is important to avoid unnecessary exposure to medications or the use of polypharmacy to treat an inaccurate diagnosis of a primary psychiatric illness. For example, Mr. M was being treated with 3 psychiatric medications (aripiprazole, buspirone, and duloxetine) for depression and anxiety prior to the diagnosis of PPA.
Nonpharmacologic interventions can play an important role in the management of patients with PPA. These include educating the patient and their family about the diagnosis and discussions about future planning, including appropriate social support, employment, and finances.4 Pharmacologic interventions may be limited, as there are currently no disease-modifying treatments for PPA or FTLD. For patients with nonfluent PPA or AD, cholinesterase inhibitors such as donepezil or N-methyl
Psychiatrists should continue to treat patients with PPA for comorbid anxiety or depression, with appropriate medications and/or supportive therapy to guide the patient through the process of grief. Assessing for suicide risk is also important in patients diagnosed with dementia. A retrospective cohort study of patients age ≥60 with a diagnosis of dementia suggested that the majority of suicides occurred in those with a new dementia diagnosis.9 End-of-life decisions such as advanced directives should be made when the patient still has legal capacity, ideally as soon as possible after diagnosis.10
OUTCOME Remaining engaged in treatment
Mr. M continues to follow-up with the Neurology team. He has also been regularly seeing his psychiatric team for medication management and supportive therapy, and his psychiatric medications have been optimized to reduce polypharmacy. During his sessions, Mr. M discusses his grief and plans for the future. Despite his anxiety about the uncertainty of his prognosis, Mr. M continues to report that he is doing reasonably well and remains engaged in treatment.
Bottom Line
Patients with primary progressive aphasia and rare neurodegenerative disorders may present to an outpatient or emergency setting with symptoms of anxiety and confusion. They are frequently misdiagnosed with a primary psychiatric disorder due to the nature of cognitive and language deficits, particularly in the early stages of the disease. Paying close attention to language and conducting cognitive screening are critical in identifying the true cause of a patient’s symptoms.
Related Resources
- Primary progressive aphasia. National Center for Advancing Translational Sciences. Genetic and Rare Diseases Information Center. https://rarediseases.info.nih.gov/diseases/8541/primary-progressive-aphasia
- Moller MD, Parmenter BA, Lane DW. Neuropsychological testing: A useful but underutilized resource. Current Psychiatry. 2019;18(11):40-46,51.
Drug Brand Names
Aripiprazole • Abilify
Donepezil • Aricept
Duloxetine • Cymbalta
Memantine • Namenda
1. Grossman M. Primary progressive aphasia: clinicopathological correlations. Nat Rev Neurol. 2010;6(2):88-97. doi:10.1038/nrneurol.2009.216
2. Mesulam M-M, Rogalski EJ, Wieneke C, et al. Primary progressive aphasia and the evolving neurology of the language network. Nat Rev Neurol. 2014;10(10):554-569. doi:10.1038/nrneurol.2014.159
3. Coyle-Gilchrist ITS, Dick KM, Patterson K, et al. Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology. 2016;86(18):1736-1743. doi:10.1212/WNL.0000000000002638
4. Marshall CR, Hardy CJD, Volkmer A, et al. Primary progressive aphasia: a clinical approach. J Neurol. 2018;265(6):1474-1490. doi:10.1007/s00415-018-8762-6
5. Blennow K. Cerebrospinal fluid protein biomarkers for Alzheimer’s disease. NeuroRx. 2004;1(2):213-225. doi:10.1602/neurorx.1.2.213
6. Hulstaert F, Blennow K, Ivanoiu A, et al. Improved discrimination of AD patients using beta-amyloid(1-42) and tau levels in CSF. Neurology. 1999;52(8):1555-1562. doi:10.1212/wnl.52.8.1555
7. Thijssen EH, La Joie R, Wolf A, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat Med. 2020;26(3):387-397. doi:10.1038/s41591-020-0762-2
8. Newhart M, Davis C, Kannan V, et al. Therapy for naming deficits in two variants of primary progressive aphasia. Aphasiology. 2009;23(7-8):823-834. doi:10.1080/02687030802661762
9. Seyfried LS, Kales HC, Ignacio RV, et al. Predictors of suicide in patients with dementia. Alzheimers Dement. 2011;7(6):567-573. doi:10.1016/j.jalz.2011.01.006
10. Porteri C. Advance directives as a tool to respect patients’ values and preferences: discussion on the case of Alzheimer’s disease. BMC Med Ethics. 2018;19(1):9. doi:10.1186/s12910-018-0249-6
CASE Anxious and confused
Mr. M, age 53, a surgeon, presents to the emergency department (ED) following a panic attack and concerns from his staff that he appears confused. Specifically, staff members report that in the past 4 months, Mr. M was observed having problems completing some postoperative tasks related to chart documentation. Mr. M has a history of major depressive disorder (MDD), hypertension, hyperlipidemia, and type 2 diabetes.
HISTORY A long-standing diagnosis of depression
Mr. M reports that 30 years ago, he received care from a psychiatrist to address symptoms of MDD. He says that around the time he arrived at the ED, he had noticed subtle but gradual changes in his cognition, which led him to skip words and often struggle to find the correct words. These episodes left him confused. Mr. M started getting anxious about these cognitive issues because they disrupted his work and forced him to reduce his duties. He does not have any known family history of mental illness, is single, and lives alone.
EVALUATION After stroke is ruled out, a psychiatric workup
In the ED, a comprehensive exam rules out an acute cerebrovascular event. A neurologic evaluation notes some delay in processing information and observes Mr. M having difficulty following simple commands. Laboratory investigations, including a comprehensive metabolic panel, are unremarkable. An MRI of Mr. M’s brain, with and without contrast, notes no acute findings. He is discharged from the ED with a diagnosis of MDD.
Before he presented to the ED, Mr. M’s medication regimen included duloxetine 60 mg/d, buspirone 10 mg 3 times a day, and aripiprazole 5 mg/d for MDD and anxiety. After the ED visit, Mr. M’s physician refers him to an outpatient psychiatrist for management of worsening depression and panic attacks. During the psychiatrist’s evaluation, Mr. M reports a decreased interest in activities, decreased motivation, being easily fatigued, and having poor sleep. He denies having a depressed mood, difficulty concentrating, or having problems with his appetite. He also denies suicidal thoughts, both past and present.
Mr. M describes his mood as anxious, primarily surrounding his recent cognitive changes. He does not have a substance use disorder, psychotic illness, mania or hypomania, posttraumatic stress disorder, or obsessive-compulsive disorder. He reports adherence to his psychiatric medications. A mental status exam reveals Mr. M to be anxious. His attention is not well sustained, and he has difficulty describing details of his cognitive struggles, providing vague descriptions such as “skipping thought” and “skipping words.” Mr. M’s affect is congruent to his mood with some restriction and the psychiatrist notes that he is experiencing thought latency, poverty of content of thoughts, word-finding difficulties, and circumlocution. Mr. M denies any perceptual abnormalities, and there is no evidence of delusions.
[polldaddy:11320112]
The authors’ observations
Mr. M’s symptoms are significant for subacute cognitive decline that is subtle but gradual and can be easily missed, especially in the beginning. Though his ED evaluation—including brain imaging—ruled out acute or focal neurologic findings and his primary psychiatric presentation was anxiety, Mr. M’s medical history and mental status exam were suggestive of cognitive deficits.
Collateral information was obtained from his work colleagues, which confirmed both cognitive problems and comorbid anxiety. Additionally, given Mr. M’s high cognitive baseline as a surgeon, the new-onset cognitive changes over 4 months warranted further cognitive and neurologic evaluation. There are many causes of cognitive impairment (vascular, cancer, infection, autoimmune, medications, substances or toxins, neurodegenerative, psychiatric, vitamin deficiencies), all of which need to be considered in a patient with a nonspecific presentation such as Mr. M’s. The psychiatrist confirmed Mr. M’s current medication regimen, and discussed tapering aripiprazole while continuing duloxetine and buspirone.
Continue to: EVALUATION A closer look at cognitive deficits
EVALUATION A closer look at cognitive deficits
Mr. M scores 12/30 on the Montreal Cognitive Assessment (MoCA), indicating moderate cognitive impairment (Table 1). The psychiatrist refers Mr. M to Neurology. During his neurologic evaluation, Mr. M continues to report feeling anxious that “something is wrong” and skips his words. The neurologist confirms Mr. M’s symptoms may have started 2 to 3 months before he presented to the ED. Mr. M reports unusual eating habits, including yogurt and cookies for breakfast, Mexican food for lunch, and more cookies for dinner. He denies having a fever, gaining or losing weight, rashes, headaches, neck stiffness, tingling or weakness or stiffness of limbs, vertigo, visual changes, photophobia, unsteady gait, bowel or bladder incontinence, or tremors.

When the neurologist repeats the MoCA, Mr. M again scores 12. The neurologist notes that Mr. M answers questions a little slowly and pauses for thoughts when unable to find an answer. Mr. M has difficulty following some simple commands, such as “touch a finger to your nose.” Other in-office neurologic physical exams (cranial nerves, involuntary movements or tremors, sensation, muscle strength, reflexes, cerebellar signs) are unremarkable except for mildly decreased vibration sense of his toes. The neurologist concludes that Mr. M’s presentation is suggestive of subacute to chronic bradyphrenia and orders additional evaluation, including neuropsychological testing.
[polldaddy:11320114]
The authors’ observations
Physical and neurologic exams were not suggestive of any obvious causes of cognitive decline. Both the mental status exam and 2 serial MoCAs suggested deficits in executive function, language, and memory. Each of the differential diagnoses considered was ruled out with workup or exams (Table 2), which led to a most likely diagnosis of neurodegenerative disorder with PPA. Neuropsychological testing confirmed the diagnosis of nonfluent PPA.

Primary progressive aphasia
PPA is an uncommon, heterogeneous group of disorders stemming from focal degeneration of language-governing centers of the brain.1,2 The estimated prevalence of PPA is 3 in 100,000 cases.2,3 There are 4 major variants of PPA (Table 34), and each presents with distinct language, cognitive, neuroanatomical, and neuropathological characteristics.4 PPA is usually diagnosed in late middle life; however, diagnosis is often delayed due to the relative obscurity of the disorder.4 In Mr. M’s case, it took approximately 4 months of evaluations by various specialists before a diagnosis was confirmed.

The initial phase of PPA can present as a diagnostic challenge because patients can have difficulty articulating their cognitive and language deficits. PPA can be commonly mistaken for a primary psychiatric disorder such as MDD or anxiety, which can further delay an accurate diagnosis and treatment. Special attention to the mental status exam, close observation of the patient’s language, and assessment of cognitive abilities using standardized screenings such as the MoCA or Mini-Mental State Examination can be helpful in clarifying the diagnosis. It is also important to rule out developmental problems (eg, dyslexia) and hearing difficulties, particularly in older patients.
Continue to: TREATMENT Adjusting the medication regimen
TREATMENT Adjusting the medication regimen
The neurologist completes additional examinations to rule out causes of rare neurodegenerative disorders, including CSF autoimmune disorders, Creutzfeldt-Jakob disease, and Alzheimer disease (AD) (Table 4). Mr. M continues to follow up with his outpatient psychiatrist and his medication regimen is adjusted. Aripiprazole and buspirone are discontinued, and duloxetine is titrated to 60 mg twice a day. During follow-up visits, Mr. M discusses his understanding of his neurologic condition. His concerns shift to his illness and prognosis. During these visits, he continues to deny suicidality.

[polldaddy:11320115]
The authors’ observations
Mr. M’s neurodegenerative workup identified an intriguing diagnostic challenge. A repeat brain MRI (Figure) showed atrophy patterns suggestive of frontotemporal lobar degeneration (FTLD). On the other hand, his CSF ATI (A-beta 42/T-tau index, a value used to aid in the diagnosis of AD) was <1, suggesting early-onset AD.5,6 Although significant advances have been made to distinguish AD and FTLD following an autopsy, there are still no reliable or definitive biomarkers to distinguish AD from FTLD (particularly in the early stages of FTLD). This can often leave the confirmatory diagnosis as a question.7

A PPA diagnosis (and other dementias) can have a significant impact on the patient and their family due to the uncertain nature of the progression of the disease and quality-of-life issues related to language and other cognitive deficits. Early identification and accurate diagnosis of PPA and its etiology (ie, AD vs FTLD) is important to avoid unnecessary exposure to medications or the use of polypharmacy to treat an inaccurate diagnosis of a primary psychiatric illness. For example, Mr. M was being treated with 3 psychiatric medications (aripiprazole, buspirone, and duloxetine) for depression and anxiety prior to the diagnosis of PPA.
Nonpharmacologic interventions can play an important role in the management of patients with PPA. These include educating the patient and their family about the diagnosis and discussions about future planning, including appropriate social support, employment, and finances.4 Pharmacologic interventions may be limited, as there are currently no disease-modifying treatments for PPA or FTLD. For patients with nonfluent PPA or AD, cholinesterase inhibitors such as donepezil or N-methyl
Psychiatrists should continue to treat patients with PPA for comorbid anxiety or depression, with appropriate medications and/or supportive therapy to guide the patient through the process of grief. Assessing for suicide risk is also important in patients diagnosed with dementia. A retrospective cohort study of patients age ≥60 with a diagnosis of dementia suggested that the majority of suicides occurred in those with a new dementia diagnosis.9 End-of-life decisions such as advanced directives should be made when the patient still has legal capacity, ideally as soon as possible after diagnosis.10
OUTCOME Remaining engaged in treatment
Mr. M continues to follow-up with the Neurology team. He has also been regularly seeing his psychiatric team for medication management and supportive therapy, and his psychiatric medications have been optimized to reduce polypharmacy. During his sessions, Mr. M discusses his grief and plans for the future. Despite his anxiety about the uncertainty of his prognosis, Mr. M continues to report that he is doing reasonably well and remains engaged in treatment.
Bottom Line
Patients with primary progressive aphasia and rare neurodegenerative disorders may present to an outpatient or emergency setting with symptoms of anxiety and confusion. They are frequently misdiagnosed with a primary psychiatric disorder due to the nature of cognitive and language deficits, particularly in the early stages of the disease. Paying close attention to language and conducting cognitive screening are critical in identifying the true cause of a patient’s symptoms.
Related Resources
- Primary progressive aphasia. National Center for Advancing Translational Sciences. Genetic and Rare Diseases Information Center. https://rarediseases.info.nih.gov/diseases/8541/primary-progressive-aphasia
- Moller MD, Parmenter BA, Lane DW. Neuropsychological testing: A useful but underutilized resource. Current Psychiatry. 2019;18(11):40-46,51.
Drug Brand Names
Aripiprazole • Abilify
Donepezil • Aricept
Duloxetine • Cymbalta
Memantine • Namenda
CASE Anxious and confused
Mr. M, age 53, a surgeon, presents to the emergency department (ED) following a panic attack and concerns from his staff that he appears confused. Specifically, staff members report that in the past 4 months, Mr. M was observed having problems completing some postoperative tasks related to chart documentation. Mr. M has a history of major depressive disorder (MDD), hypertension, hyperlipidemia, and type 2 diabetes.
HISTORY A long-standing diagnosis of depression
Mr. M reports that 30 years ago, he received care from a psychiatrist to address symptoms of MDD. He says that around the time he arrived at the ED, he had noticed subtle but gradual changes in his cognition, which led him to skip words and often struggle to find the correct words. These episodes left him confused. Mr. M started getting anxious about these cognitive issues because they disrupted his work and forced him to reduce his duties. He does not have any known family history of mental illness, is single, and lives alone.
EVALUATION After stroke is ruled out, a psychiatric workup
In the ED, a comprehensive exam rules out an acute cerebrovascular event. A neurologic evaluation notes some delay in processing information and observes Mr. M having difficulty following simple commands. Laboratory investigations, including a comprehensive metabolic panel, are unremarkable. An MRI of Mr. M’s brain, with and without contrast, notes no acute findings. He is discharged from the ED with a diagnosis of MDD.
Before he presented to the ED, Mr. M’s medication regimen included duloxetine 60 mg/d, buspirone 10 mg 3 times a day, and aripiprazole 5 mg/d for MDD and anxiety. After the ED visit, Mr. M’s physician refers him to an outpatient psychiatrist for management of worsening depression and panic attacks. During the psychiatrist’s evaluation, Mr. M reports a decreased interest in activities, decreased motivation, being easily fatigued, and having poor sleep. He denies having a depressed mood, difficulty concentrating, or having problems with his appetite. He also denies suicidal thoughts, both past and present.
Mr. M describes his mood as anxious, primarily surrounding his recent cognitive changes. He does not have a substance use disorder, psychotic illness, mania or hypomania, posttraumatic stress disorder, or obsessive-compulsive disorder. He reports adherence to his psychiatric medications. A mental status exam reveals Mr. M to be anxious. His attention is not well sustained, and he has difficulty describing details of his cognitive struggles, providing vague descriptions such as “skipping thought” and “skipping words.” Mr. M’s affect is congruent to his mood with some restriction and the psychiatrist notes that he is experiencing thought latency, poverty of content of thoughts, word-finding difficulties, and circumlocution. Mr. M denies any perceptual abnormalities, and there is no evidence of delusions.
[polldaddy:11320112]
The authors’ observations
Mr. M’s symptoms are significant for subacute cognitive decline that is subtle but gradual and can be easily missed, especially in the beginning. Though his ED evaluation—including brain imaging—ruled out acute or focal neurologic findings and his primary psychiatric presentation was anxiety, Mr. M’s medical history and mental status exam were suggestive of cognitive deficits.
Collateral information was obtained from his work colleagues, which confirmed both cognitive problems and comorbid anxiety. Additionally, given Mr. M’s high cognitive baseline as a surgeon, the new-onset cognitive changes over 4 months warranted further cognitive and neurologic evaluation. There are many causes of cognitive impairment (vascular, cancer, infection, autoimmune, medications, substances or toxins, neurodegenerative, psychiatric, vitamin deficiencies), all of which need to be considered in a patient with a nonspecific presentation such as Mr. M’s. The psychiatrist confirmed Mr. M’s current medication regimen, and discussed tapering aripiprazole while continuing duloxetine and buspirone.
Continue to: EVALUATION A closer look at cognitive deficits
EVALUATION A closer look at cognitive deficits
Mr. M scores 12/30 on the Montreal Cognitive Assessment (MoCA), indicating moderate cognitive impairment (Table 1). The psychiatrist refers Mr. M to Neurology. During his neurologic evaluation, Mr. M continues to report feeling anxious that “something is wrong” and skips his words. The neurologist confirms Mr. M’s symptoms may have started 2 to 3 months before he presented to the ED. Mr. M reports unusual eating habits, including yogurt and cookies for breakfast, Mexican food for lunch, and more cookies for dinner. He denies having a fever, gaining or losing weight, rashes, headaches, neck stiffness, tingling or weakness or stiffness of limbs, vertigo, visual changes, photophobia, unsteady gait, bowel or bladder incontinence, or tremors.

When the neurologist repeats the MoCA, Mr. M again scores 12. The neurologist notes that Mr. M answers questions a little slowly and pauses for thoughts when unable to find an answer. Mr. M has difficulty following some simple commands, such as “touch a finger to your nose.” Other in-office neurologic physical exams (cranial nerves, involuntary movements or tremors, sensation, muscle strength, reflexes, cerebellar signs) are unremarkable except for mildly decreased vibration sense of his toes. The neurologist concludes that Mr. M’s presentation is suggestive of subacute to chronic bradyphrenia and orders additional evaluation, including neuropsychological testing.
[polldaddy:11320114]
The authors’ observations
Physical and neurologic exams were not suggestive of any obvious causes of cognitive decline. Both the mental status exam and 2 serial MoCAs suggested deficits in executive function, language, and memory. Each of the differential diagnoses considered was ruled out with workup or exams (Table 2), which led to a most likely diagnosis of neurodegenerative disorder with PPA. Neuropsychological testing confirmed the diagnosis of nonfluent PPA.

Primary progressive aphasia
PPA is an uncommon, heterogeneous group of disorders stemming from focal degeneration of language-governing centers of the brain.1,2 The estimated prevalence of PPA is 3 in 100,000 cases.2,3 There are 4 major variants of PPA (Table 34), and each presents with distinct language, cognitive, neuroanatomical, and neuropathological characteristics.4 PPA is usually diagnosed in late middle life; however, diagnosis is often delayed due to the relative obscurity of the disorder.4 In Mr. M’s case, it took approximately 4 months of evaluations by various specialists before a diagnosis was confirmed.

The initial phase of PPA can present as a diagnostic challenge because patients can have difficulty articulating their cognitive and language deficits. PPA can be commonly mistaken for a primary psychiatric disorder such as MDD or anxiety, which can further delay an accurate diagnosis and treatment. Special attention to the mental status exam, close observation of the patient’s language, and assessment of cognitive abilities using standardized screenings such as the MoCA or Mini-Mental State Examination can be helpful in clarifying the diagnosis. It is also important to rule out developmental problems (eg, dyslexia) and hearing difficulties, particularly in older patients.
Continue to: TREATMENT Adjusting the medication regimen
TREATMENT Adjusting the medication regimen
The neurologist completes additional examinations to rule out causes of rare neurodegenerative disorders, including CSF autoimmune disorders, Creutzfeldt-Jakob disease, and Alzheimer disease (AD) (Table 4). Mr. M continues to follow up with his outpatient psychiatrist and his medication regimen is adjusted. Aripiprazole and buspirone are discontinued, and duloxetine is titrated to 60 mg twice a day. During follow-up visits, Mr. M discusses his understanding of his neurologic condition. His concerns shift to his illness and prognosis. During these visits, he continues to deny suicidality.

[polldaddy:11320115]
The authors’ observations
Mr. M’s neurodegenerative workup identified an intriguing diagnostic challenge. A repeat brain MRI (Figure) showed atrophy patterns suggestive of frontotemporal lobar degeneration (FTLD). On the other hand, his CSF ATI (A-beta 42/T-tau index, a value used to aid in the diagnosis of AD) was <1, suggesting early-onset AD.5,6 Although significant advances have been made to distinguish AD and FTLD following an autopsy, there are still no reliable or definitive biomarkers to distinguish AD from FTLD (particularly in the early stages of FTLD). This can often leave the confirmatory diagnosis as a question.7

A PPA diagnosis (and other dementias) can have a significant impact on the patient and their family due to the uncertain nature of the progression of the disease and quality-of-life issues related to language and other cognitive deficits. Early identification and accurate diagnosis of PPA and its etiology (ie, AD vs FTLD) is important to avoid unnecessary exposure to medications or the use of polypharmacy to treat an inaccurate diagnosis of a primary psychiatric illness. For example, Mr. M was being treated with 3 psychiatric medications (aripiprazole, buspirone, and duloxetine) for depression and anxiety prior to the diagnosis of PPA.
Nonpharmacologic interventions can play an important role in the management of patients with PPA. These include educating the patient and their family about the diagnosis and discussions about future planning, including appropriate social support, employment, and finances.4 Pharmacologic interventions may be limited, as there are currently no disease-modifying treatments for PPA or FTLD. For patients with nonfluent PPA or AD, cholinesterase inhibitors such as donepezil or N-methyl
Psychiatrists should continue to treat patients with PPA for comorbid anxiety or depression, with appropriate medications and/or supportive therapy to guide the patient through the process of grief. Assessing for suicide risk is also important in patients diagnosed with dementia. A retrospective cohort study of patients age ≥60 with a diagnosis of dementia suggested that the majority of suicides occurred in those with a new dementia diagnosis.9 End-of-life decisions such as advanced directives should be made when the patient still has legal capacity, ideally as soon as possible after diagnosis.10
OUTCOME Remaining engaged in treatment
Mr. M continues to follow-up with the Neurology team. He has also been regularly seeing his psychiatric team for medication management and supportive therapy, and his psychiatric medications have been optimized to reduce polypharmacy. During his sessions, Mr. M discusses his grief and plans for the future. Despite his anxiety about the uncertainty of his prognosis, Mr. M continues to report that he is doing reasonably well and remains engaged in treatment.
Bottom Line
Patients with primary progressive aphasia and rare neurodegenerative disorders may present to an outpatient or emergency setting with symptoms of anxiety and confusion. They are frequently misdiagnosed with a primary psychiatric disorder due to the nature of cognitive and language deficits, particularly in the early stages of the disease. Paying close attention to language and conducting cognitive screening are critical in identifying the true cause of a patient’s symptoms.
Related Resources
- Primary progressive aphasia. National Center for Advancing Translational Sciences. Genetic and Rare Diseases Information Center. https://rarediseases.info.nih.gov/diseases/8541/primary-progressive-aphasia
- Moller MD, Parmenter BA, Lane DW. Neuropsychological testing: A useful but underutilized resource. Current Psychiatry. 2019;18(11):40-46,51.
Drug Brand Names
Aripiprazole • Abilify
Donepezil • Aricept
Duloxetine • Cymbalta
Memantine • Namenda
1. Grossman M. Primary progressive aphasia: clinicopathological correlations. Nat Rev Neurol. 2010;6(2):88-97. doi:10.1038/nrneurol.2009.216
2. Mesulam M-M, Rogalski EJ, Wieneke C, et al. Primary progressive aphasia and the evolving neurology of the language network. Nat Rev Neurol. 2014;10(10):554-569. doi:10.1038/nrneurol.2014.159
3. Coyle-Gilchrist ITS, Dick KM, Patterson K, et al. Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology. 2016;86(18):1736-1743. doi:10.1212/WNL.0000000000002638
4. Marshall CR, Hardy CJD, Volkmer A, et al. Primary progressive aphasia: a clinical approach. J Neurol. 2018;265(6):1474-1490. doi:10.1007/s00415-018-8762-6
5. Blennow K. Cerebrospinal fluid protein biomarkers for Alzheimer’s disease. NeuroRx. 2004;1(2):213-225. doi:10.1602/neurorx.1.2.213
6. Hulstaert F, Blennow K, Ivanoiu A, et al. Improved discrimination of AD patients using beta-amyloid(1-42) and tau levels in CSF. Neurology. 1999;52(8):1555-1562. doi:10.1212/wnl.52.8.1555
7. Thijssen EH, La Joie R, Wolf A, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat Med. 2020;26(3):387-397. doi:10.1038/s41591-020-0762-2
8. Newhart M, Davis C, Kannan V, et al. Therapy for naming deficits in two variants of primary progressive aphasia. Aphasiology. 2009;23(7-8):823-834. doi:10.1080/02687030802661762
9. Seyfried LS, Kales HC, Ignacio RV, et al. Predictors of suicide in patients with dementia. Alzheimers Dement. 2011;7(6):567-573. doi:10.1016/j.jalz.2011.01.006
10. Porteri C. Advance directives as a tool to respect patients’ values and preferences: discussion on the case of Alzheimer’s disease. BMC Med Ethics. 2018;19(1):9. doi:10.1186/s12910-018-0249-6
1. Grossman M. Primary progressive aphasia: clinicopathological correlations. Nat Rev Neurol. 2010;6(2):88-97. doi:10.1038/nrneurol.2009.216
2. Mesulam M-M, Rogalski EJ, Wieneke C, et al. Primary progressive aphasia and the evolving neurology of the language network. Nat Rev Neurol. 2014;10(10):554-569. doi:10.1038/nrneurol.2014.159
3. Coyle-Gilchrist ITS, Dick KM, Patterson K, et al. Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology. 2016;86(18):1736-1743. doi:10.1212/WNL.0000000000002638
4. Marshall CR, Hardy CJD, Volkmer A, et al. Primary progressive aphasia: a clinical approach. J Neurol. 2018;265(6):1474-1490. doi:10.1007/s00415-018-8762-6
5. Blennow K. Cerebrospinal fluid protein biomarkers for Alzheimer’s disease. NeuroRx. 2004;1(2):213-225. doi:10.1602/neurorx.1.2.213
6. Hulstaert F, Blennow K, Ivanoiu A, et al. Improved discrimination of AD patients using beta-amyloid(1-42) and tau levels in CSF. Neurology. 1999;52(8):1555-1562. doi:10.1212/wnl.52.8.1555
7. Thijssen EH, La Joie R, Wolf A, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat Med. 2020;26(3):387-397. doi:10.1038/s41591-020-0762-2
8. Newhart M, Davis C, Kannan V, et al. Therapy for naming deficits in two variants of primary progressive aphasia. Aphasiology. 2009;23(7-8):823-834. doi:10.1080/02687030802661762
9. Seyfried LS, Kales HC, Ignacio RV, et al. Predictors of suicide in patients with dementia. Alzheimers Dement. 2011;7(6):567-573. doi:10.1016/j.jalz.2011.01.006
10. Porteri C. Advance directives as a tool to respect patients’ values and preferences: discussion on the case of Alzheimer’s disease. BMC Med Ethics. 2018;19(1):9. doi:10.1186/s12910-018-0249-6
More on psilocybin
I would like to remark on “Psychedelics for treating psychiatric disorders: Are they safe?” (
The Oregon Psilocybin Services that will begin in 2023 are not specific to therapeutic use; this is a common misconception. These are specifically referred to as “psilocybin services” in the Oregon Administrative Rules (OAR), and psilocybin facilitators are required to limit their scope such that they are not practicing psychotherapy or other interventions, even if they do have a medical or psychotherapy background. The intention of the Oregon Psilocybin Services rollout was that these services would not be of the medical model. In the spirit of this, services do not require a medical diagnosis or referral, and services are not a medical or clinical treatment (OAR 333-333-5040). Additionally, services cannot be provided in a health care facility (OAR 441). Facilitators receive robust training as defined by Oregon law, and licensed facilitators provide this information during preparation for services. When discussing this model on a large public scale, I have noticed substantial misconceptions; it is imperative that we refer to these services as they are defined so that individuals with mental health conditions who seek them are aware that such services are different from psilocybin-assisted psychotherapy. Instead, Oregon Psilocybin Services might be better categorized as supported psilocybin use.
I would like to remark on “Psychedelics for treating psychiatric disorders: Are they safe?” (
The Oregon Psilocybin Services that will begin in 2023 are not specific to therapeutic use; this is a common misconception. These are specifically referred to as “psilocybin services” in the Oregon Administrative Rules (OAR), and psilocybin facilitators are required to limit their scope such that they are not practicing psychotherapy or other interventions, even if they do have a medical or psychotherapy background. The intention of the Oregon Psilocybin Services rollout was that these services would not be of the medical model. In the spirit of this, services do not require a medical diagnosis or referral, and services are not a medical or clinical treatment (OAR 333-333-5040). Additionally, services cannot be provided in a health care facility (OAR 441). Facilitators receive robust training as defined by Oregon law, and licensed facilitators provide this information during preparation for services. When discussing this model on a large public scale, I have noticed substantial misconceptions; it is imperative that we refer to these services as they are defined so that individuals with mental health conditions who seek them are aware that such services are different from psilocybin-assisted psychotherapy. Instead, Oregon Psilocybin Services might be better categorized as supported psilocybin use.
I would like to remark on “Psychedelics for treating psychiatric disorders: Are they safe?” (
The Oregon Psilocybin Services that will begin in 2023 are not specific to therapeutic use; this is a common misconception. These are specifically referred to as “psilocybin services” in the Oregon Administrative Rules (OAR), and psilocybin facilitators are required to limit their scope such that they are not practicing psychotherapy or other interventions, even if they do have a medical or psychotherapy background. The intention of the Oregon Psilocybin Services rollout was that these services would not be of the medical model. In the spirit of this, services do not require a medical diagnosis or referral, and services are not a medical or clinical treatment (OAR 333-333-5040). Additionally, services cannot be provided in a health care facility (OAR 441). Facilitators receive robust training as defined by Oregon law, and licensed facilitators provide this information during preparation for services. When discussing this model on a large public scale, I have noticed substantial misconceptions; it is imperative that we refer to these services as they are defined so that individuals with mental health conditions who seek them are aware that such services are different from psilocybin-assisted psychotherapy. Instead, Oregon Psilocybin Services might be better categorized as supported psilocybin use.
Microneedling With Bimatoprost to Treat Hypopigmented Skin Caused by Burn Scars
To the Editor:
Microneedling is a percutaneous collagen induction therapy frequently used in cosmetic dermatology to promote skin rejuvenation and hair growth and to treat scars by taking advantage of the body’s natural wound-healing cascade.1 The procedure works by generating thousands of microscopic wounds in the dermis with minimal damage to the epidermis, thus initiating the wound-healing cascade and subsequently promoting collagen production in a manner safe for all Fitzpatrick classification skin types.1-3 This therapy effectively treats scars by breaking down scarred collagen and replacing it with new healthy collagen. Microneedling also has application in drug delivery by increasing the permeability of the skin; the microwounds generated can serve as a portal for drug delivery.4
Bimatoprost is a prostaglandin analogue typically used to treat hypotrichosis and open-angle glaucoma.5-7 A known side effect of bimatoprost is hyperpigmentation of surrounding skin; the drug increases melanogenesis, melanocyte proliferation, and melanocyte dendricity, resulting in activation of the inflammatory response and subsequent prostaglandin release, which stimulates melanogenesis. This effect is similar to UV radiation–induced inflammation and hyperpigmentation.6,8
Capitalizing on this effect, a novel application of bimatoprost has been proposed—treating vitiligo, in which hypopigmentation results from destruction of melanocytes in certain areas of the skin. Bimatoprost ophthalmic solution 0.3% utilized as an off-label treatment for vitiligo has been shown to notably increase melanogenesis and return pigmentation to hypopigmented areas.8-10
A 32-year-old Black woman presented to our clinic with a 40×15-cm scar that was marked by postinflammatory hypopigmentation from a second-degree burn on the right proximal arm. The patient had been burned 5 months prior by boiling water that was spilled on the arm while cooking. She had immediately sought treatment at an emergency department and subsequently in a burn unit, where the burn was debrided twice; medication was not prescribed to continue treatment. The patient reported that the scarring and hypopigmentation had taken a psychologic toll; her hope was to have pigmentation restored to the affected area to boost her confidence.
Physical examination revealed that the burn wound had healed but visible scarring and severe hypopigmentation due to destroyed melanocytes remained (Figure 1). To inhibit inflammation and stimulate repigmentation, we prescribed the calcineurin inhibitor tacrolimus ointment 0.1% to be applied daily to the affected area. The patient returned to the clinic 1 month later. Perifollicular hyperpigmentation was noted at the site of the scar.
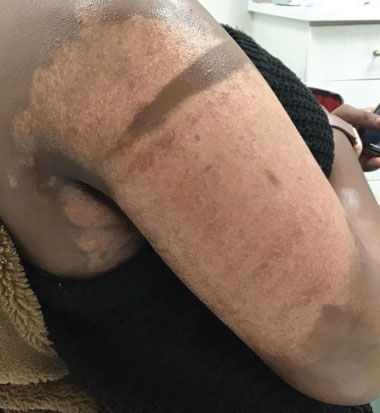
Monthly microneedling sessions with bimatoprost ophthalmic solution 0.3% were started. To avoid damaging any potentially remaining unhealed hypodermis and vasculature, the first microneedling session was performed with 9 needles set at minimal needle depth and frequency. The number of needles and their depth and frequency gradually were increased with each subsequent treatment. The patient continued tacrolimus ointment 0.1% throughout the course of treatment.
For each microneedling procedure, a handheld motorized microneedling device was applied to the skin at a depth of 0.25 mm, which was gradually increased until pinpoint petechiae were achieved. Bimatoprost ophthalmic solution 0.3% was then painted on the skin and allowed to absorb. Microneedling was performed again, ensuring that bimatoprost entered the skin in the area of the burn scar.
Microneedling procedures were performed monthly for 6 months, then once 3 months later, and once more 3 months later—8 treatments in total over the course of 1 year. Improvement in skin pigmentation was noted at each visit (Figure 2). Repigmentation was first noticed surrounding hair follicles; after later visits, it was observed that pigmentation began to spread from hair follicles to fill in remaining skin. The darkest areas of pigmentation were first noted around hair follicles; over time, melanocytes appeared to spontaneously regenerate and fill in surrounding areas as the scar continued to heal. The patient continued use of tacrolimus during the entire course of microneedling treatments and for the following 4 months. Sixteen months after initiation of treatment, the appearance of the skin was texturally smooth and returned to almost its original pigmentation (Figure 3).
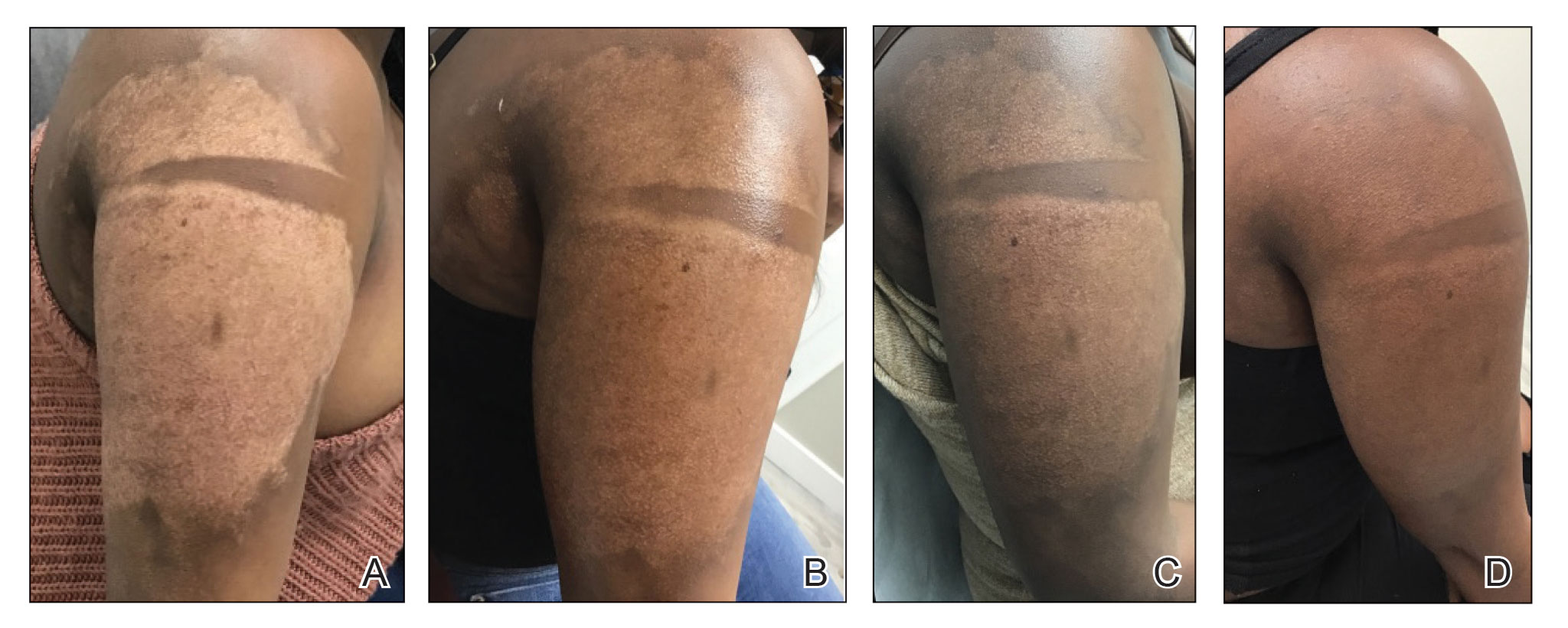
We report a successful outcome in a patient with a hypopigmented burn scar who was treated with bimatoprost administered with traditional microneedling and alongside a tacrolimus regimen. Tacrolimus ointment inhibited the inflammatory response to allow melanocytes to heal and regenerate; bimatoprost and microneedling promoted hyperpigmentation of hair follicles in the affected area, eventually restoring pigmentation to the entire area. Our patient was extremely satisfied with the results of this combination treatment. She has reported feeling more confident going out and wearing short-sleeved clothing. Percutaneous drug delivery of bimatoprost ophthalmic solution 0.3% combined with topical tacrolimus may be an effective treatment for skin repigmentation. Further investigation of this regimen is needed to develop standardized treatment protocols.
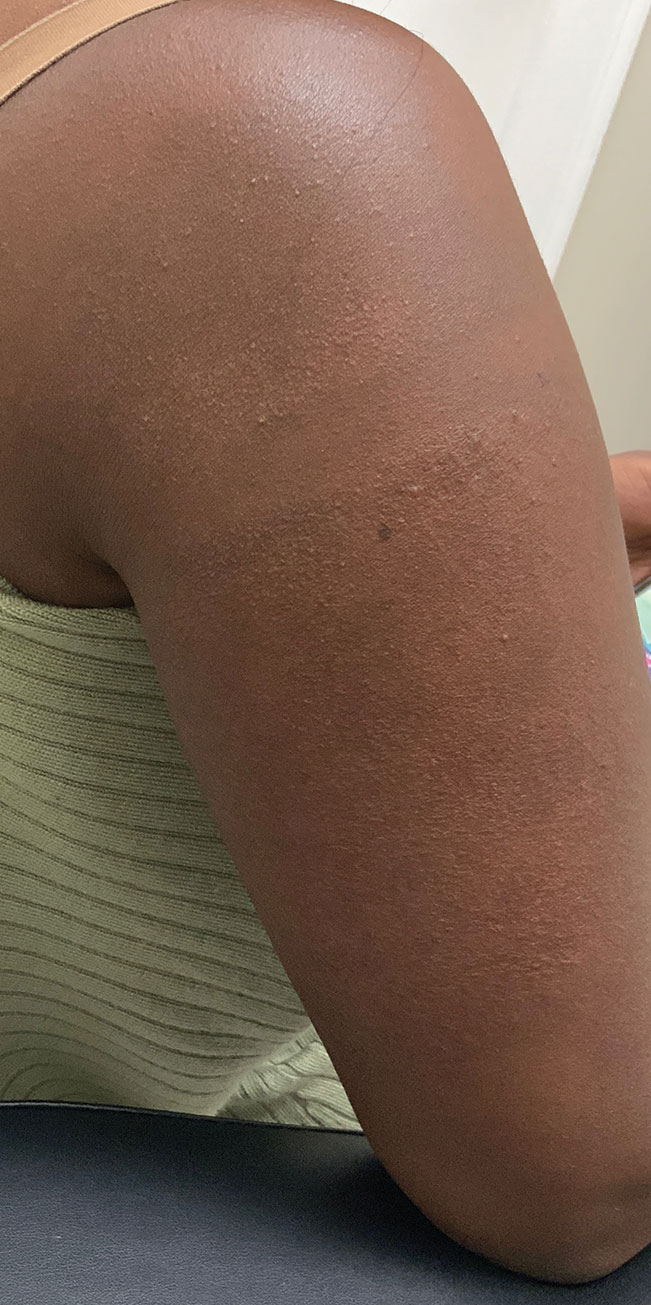
- Juhasz MLW, Cohen JL. Micro-needling for the treatment of scars: an update for clinicians. Clin Cosmet Investig Dermatol. 2020;13:997-1003. doi:10.2147/CCID.S267192
- Alster TS, Li MKY. Micro-needling of scars: a large prospective study with long-term follow-up. Plast Reconstr Surg. 2020;145:358-364. doi:10.1097/PRS.0000000000006462
- Aust MC, Knobloch K, Reimers K, et al. Percutaneous collagen induction therapy: an alternative treatment for burn scars. Burns. 2010;36:836-843. doi:10.1016/j.burns.2009.11.014
- Kim Y-C, Park J-H, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64:1547-1568. doi:10.1016/j.addr.2012.04.005
- Doshi M, Edward DP, Osmanovic S. Clinical course of bimatoprost-induced periocular skin changes in Caucasians. Ophthalmology. 2006;113:1961-1967. doi:10.1016/j.ophtha.2006.05.041
- Kapur R, Osmanovic S, Toyran S, et al. Bimatoprost-induced periocular skin hyperpigmentation: histopathological study. Arch Ophthalmol. 2005;123:1541-1546. doi:10.1001/archopht.123.11.1541
- Priluck JC, Fu S. Latisse-induced periocular skin hyperpigmentation. Arch Ophthalmol. 2010;128:792-793. doi:10.1001/archophthalmol.2010.89
- Grimes PE. Bimatoprost 0.03% solution for the treatment of nonfacial vitiligo. J Drugs Dermatol. 2016;15:703-710.
- Barbulescu C, Goldstein N, Roop D, et al. Harnessing the power of regenerative therapy for vitiligo and alopecia areata. J Invest Dermatol. 2020;140: 29-37. doi:10.1016/j.jid.2019.03.1142
- Kanokrungsee S, Pruettivorawongse D, Rajatanavin N. Clinicaloutcomes of topical bimatoprost for nonsegmental facial vitiligo: a preliminary study. J Cosmet Dermatol. 2021;20:812-818. doi.org/10.1111/jocd.13648
To the Editor:
Microneedling is a percutaneous collagen induction therapy frequently used in cosmetic dermatology to promote skin rejuvenation and hair growth and to treat scars by taking advantage of the body’s natural wound-healing cascade.1 The procedure works by generating thousands of microscopic wounds in the dermis with minimal damage to the epidermis, thus initiating the wound-healing cascade and subsequently promoting collagen production in a manner safe for all Fitzpatrick classification skin types.1-3 This therapy effectively treats scars by breaking down scarred collagen and replacing it with new healthy collagen. Microneedling also has application in drug delivery by increasing the permeability of the skin; the microwounds generated can serve as a portal for drug delivery.4
Bimatoprost is a prostaglandin analogue typically used to treat hypotrichosis and open-angle glaucoma.5-7 A known side effect of bimatoprost is hyperpigmentation of surrounding skin; the drug increases melanogenesis, melanocyte proliferation, and melanocyte dendricity, resulting in activation of the inflammatory response and subsequent prostaglandin release, which stimulates melanogenesis. This effect is similar to UV radiation–induced inflammation and hyperpigmentation.6,8
Capitalizing on this effect, a novel application of bimatoprost has been proposed—treating vitiligo, in which hypopigmentation results from destruction of melanocytes in certain areas of the skin. Bimatoprost ophthalmic solution 0.3% utilized as an off-label treatment for vitiligo has been shown to notably increase melanogenesis and return pigmentation to hypopigmented areas.8-10
A 32-year-old Black woman presented to our clinic with a 40×15-cm scar that was marked by postinflammatory hypopigmentation from a second-degree burn on the right proximal arm. The patient had been burned 5 months prior by boiling water that was spilled on the arm while cooking. She had immediately sought treatment at an emergency department and subsequently in a burn unit, where the burn was debrided twice; medication was not prescribed to continue treatment. The patient reported that the scarring and hypopigmentation had taken a psychologic toll; her hope was to have pigmentation restored to the affected area to boost her confidence.
Physical examination revealed that the burn wound had healed but visible scarring and severe hypopigmentation due to destroyed melanocytes remained (Figure 1). To inhibit inflammation and stimulate repigmentation, we prescribed the calcineurin inhibitor tacrolimus ointment 0.1% to be applied daily to the affected area. The patient returned to the clinic 1 month later. Perifollicular hyperpigmentation was noted at the site of the scar.

Monthly microneedling sessions with bimatoprost ophthalmic solution 0.3% were started. To avoid damaging any potentially remaining unhealed hypodermis and vasculature, the first microneedling session was performed with 9 needles set at minimal needle depth and frequency. The number of needles and their depth and frequency gradually were increased with each subsequent treatment. The patient continued tacrolimus ointment 0.1% throughout the course of treatment.
For each microneedling procedure, a handheld motorized microneedling device was applied to the skin at a depth of 0.25 mm, which was gradually increased until pinpoint petechiae were achieved. Bimatoprost ophthalmic solution 0.3% was then painted on the skin and allowed to absorb. Microneedling was performed again, ensuring that bimatoprost entered the skin in the area of the burn scar.
Microneedling procedures were performed monthly for 6 months, then once 3 months later, and once more 3 months later—8 treatments in total over the course of 1 year. Improvement in skin pigmentation was noted at each visit (Figure 2). Repigmentation was first noticed surrounding hair follicles; after later visits, it was observed that pigmentation began to spread from hair follicles to fill in remaining skin. The darkest areas of pigmentation were first noted around hair follicles; over time, melanocytes appeared to spontaneously regenerate and fill in surrounding areas as the scar continued to heal. The patient continued use of tacrolimus during the entire course of microneedling treatments and for the following 4 months. Sixteen months after initiation of treatment, the appearance of the skin was texturally smooth and returned to almost its original pigmentation (Figure 3).

We report a successful outcome in a patient with a hypopigmented burn scar who was treated with bimatoprost administered with traditional microneedling and alongside a tacrolimus regimen. Tacrolimus ointment inhibited the inflammatory response to allow melanocytes to heal and regenerate; bimatoprost and microneedling promoted hyperpigmentation of hair follicles in the affected area, eventually restoring pigmentation to the entire area. Our patient was extremely satisfied with the results of this combination treatment. She has reported feeling more confident going out and wearing short-sleeved clothing. Percutaneous drug delivery of bimatoprost ophthalmic solution 0.3% combined with topical tacrolimus may be an effective treatment for skin repigmentation. Further investigation of this regimen is needed to develop standardized treatment protocols.

To the Editor:
Microneedling is a percutaneous collagen induction therapy frequently used in cosmetic dermatology to promote skin rejuvenation and hair growth and to treat scars by taking advantage of the body’s natural wound-healing cascade.1 The procedure works by generating thousands of microscopic wounds in the dermis with minimal damage to the epidermis, thus initiating the wound-healing cascade and subsequently promoting collagen production in a manner safe for all Fitzpatrick classification skin types.1-3 This therapy effectively treats scars by breaking down scarred collagen and replacing it with new healthy collagen. Microneedling also has application in drug delivery by increasing the permeability of the skin; the microwounds generated can serve as a portal for drug delivery.4
Bimatoprost is a prostaglandin analogue typically used to treat hypotrichosis and open-angle glaucoma.5-7 A known side effect of bimatoprost is hyperpigmentation of surrounding skin; the drug increases melanogenesis, melanocyte proliferation, and melanocyte dendricity, resulting in activation of the inflammatory response and subsequent prostaglandin release, which stimulates melanogenesis. This effect is similar to UV radiation–induced inflammation and hyperpigmentation.6,8
Capitalizing on this effect, a novel application of bimatoprost has been proposed—treating vitiligo, in which hypopigmentation results from destruction of melanocytes in certain areas of the skin. Bimatoprost ophthalmic solution 0.3% utilized as an off-label treatment for vitiligo has been shown to notably increase melanogenesis and return pigmentation to hypopigmented areas.8-10
A 32-year-old Black woman presented to our clinic with a 40×15-cm scar that was marked by postinflammatory hypopigmentation from a second-degree burn on the right proximal arm. The patient had been burned 5 months prior by boiling water that was spilled on the arm while cooking. She had immediately sought treatment at an emergency department and subsequently in a burn unit, where the burn was debrided twice; medication was not prescribed to continue treatment. The patient reported that the scarring and hypopigmentation had taken a psychologic toll; her hope was to have pigmentation restored to the affected area to boost her confidence.
Physical examination revealed that the burn wound had healed but visible scarring and severe hypopigmentation due to destroyed melanocytes remained (Figure 1). To inhibit inflammation and stimulate repigmentation, we prescribed the calcineurin inhibitor tacrolimus ointment 0.1% to be applied daily to the affected area. The patient returned to the clinic 1 month later. Perifollicular hyperpigmentation was noted at the site of the scar.

Monthly microneedling sessions with bimatoprost ophthalmic solution 0.3% were started. To avoid damaging any potentially remaining unhealed hypodermis and vasculature, the first microneedling session was performed with 9 needles set at minimal needle depth and frequency. The number of needles and their depth and frequency gradually were increased with each subsequent treatment. The patient continued tacrolimus ointment 0.1% throughout the course of treatment.
For each microneedling procedure, a handheld motorized microneedling device was applied to the skin at a depth of 0.25 mm, which was gradually increased until pinpoint petechiae were achieved. Bimatoprost ophthalmic solution 0.3% was then painted on the skin and allowed to absorb. Microneedling was performed again, ensuring that bimatoprost entered the skin in the area of the burn scar.
Microneedling procedures were performed monthly for 6 months, then once 3 months later, and once more 3 months later—8 treatments in total over the course of 1 year. Improvement in skin pigmentation was noted at each visit (Figure 2). Repigmentation was first noticed surrounding hair follicles; after later visits, it was observed that pigmentation began to spread from hair follicles to fill in remaining skin. The darkest areas of pigmentation were first noted around hair follicles; over time, melanocytes appeared to spontaneously regenerate and fill in surrounding areas as the scar continued to heal. The patient continued use of tacrolimus during the entire course of microneedling treatments and for the following 4 months. Sixteen months after initiation of treatment, the appearance of the skin was texturally smooth and returned to almost its original pigmentation (Figure 3).

We report a successful outcome in a patient with a hypopigmented burn scar who was treated with bimatoprost administered with traditional microneedling and alongside a tacrolimus regimen. Tacrolimus ointment inhibited the inflammatory response to allow melanocytes to heal and regenerate; bimatoprost and microneedling promoted hyperpigmentation of hair follicles in the affected area, eventually restoring pigmentation to the entire area. Our patient was extremely satisfied with the results of this combination treatment. She has reported feeling more confident going out and wearing short-sleeved clothing. Percutaneous drug delivery of bimatoprost ophthalmic solution 0.3% combined with topical tacrolimus may be an effective treatment for skin repigmentation. Further investigation of this regimen is needed to develop standardized treatment protocols.

- Juhasz MLW, Cohen JL. Micro-needling for the treatment of scars: an update for clinicians. Clin Cosmet Investig Dermatol. 2020;13:997-1003. doi:10.2147/CCID.S267192
- Alster TS, Li MKY. Micro-needling of scars: a large prospective study with long-term follow-up. Plast Reconstr Surg. 2020;145:358-364. doi:10.1097/PRS.0000000000006462
- Aust MC, Knobloch K, Reimers K, et al. Percutaneous collagen induction therapy: an alternative treatment for burn scars. Burns. 2010;36:836-843. doi:10.1016/j.burns.2009.11.014
- Kim Y-C, Park J-H, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64:1547-1568. doi:10.1016/j.addr.2012.04.005
- Doshi M, Edward DP, Osmanovic S. Clinical course of bimatoprost-induced periocular skin changes in Caucasians. Ophthalmology. 2006;113:1961-1967. doi:10.1016/j.ophtha.2006.05.041
- Kapur R, Osmanovic S, Toyran S, et al. Bimatoprost-induced periocular skin hyperpigmentation: histopathological study. Arch Ophthalmol. 2005;123:1541-1546. doi:10.1001/archopht.123.11.1541
- Priluck JC, Fu S. Latisse-induced periocular skin hyperpigmentation. Arch Ophthalmol. 2010;128:792-793. doi:10.1001/archophthalmol.2010.89
- Grimes PE. Bimatoprost 0.03% solution for the treatment of nonfacial vitiligo. J Drugs Dermatol. 2016;15:703-710.
- Barbulescu C, Goldstein N, Roop D, et al. Harnessing the power of regenerative therapy for vitiligo and alopecia areata. J Invest Dermatol. 2020;140: 29-37. doi:10.1016/j.jid.2019.03.1142
- Kanokrungsee S, Pruettivorawongse D, Rajatanavin N. Clinicaloutcomes of topical bimatoprost for nonsegmental facial vitiligo: a preliminary study. J Cosmet Dermatol. 2021;20:812-818. doi.org/10.1111/jocd.13648
- Juhasz MLW, Cohen JL. Micro-needling for the treatment of scars: an update for clinicians. Clin Cosmet Investig Dermatol. 2020;13:997-1003. doi:10.2147/CCID.S267192
- Alster TS, Li MKY. Micro-needling of scars: a large prospective study with long-term follow-up. Plast Reconstr Surg. 2020;145:358-364. doi:10.1097/PRS.0000000000006462
- Aust MC, Knobloch K, Reimers K, et al. Percutaneous collagen induction therapy: an alternative treatment for burn scars. Burns. 2010;36:836-843. doi:10.1016/j.burns.2009.11.014
- Kim Y-C, Park J-H, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64:1547-1568. doi:10.1016/j.addr.2012.04.005
- Doshi M, Edward DP, Osmanovic S. Clinical course of bimatoprost-induced periocular skin changes in Caucasians. Ophthalmology. 2006;113:1961-1967. doi:10.1016/j.ophtha.2006.05.041
- Kapur R, Osmanovic S, Toyran S, et al. Bimatoprost-induced periocular skin hyperpigmentation: histopathological study. Arch Ophthalmol. 2005;123:1541-1546. doi:10.1001/archopht.123.11.1541
- Priluck JC, Fu S. Latisse-induced periocular skin hyperpigmentation. Arch Ophthalmol. 2010;128:792-793. doi:10.1001/archophthalmol.2010.89
- Grimes PE. Bimatoprost 0.03% solution for the treatment of nonfacial vitiligo. J Drugs Dermatol. 2016;15:703-710.
- Barbulescu C, Goldstein N, Roop D, et al. Harnessing the power of regenerative therapy for vitiligo and alopecia areata. J Invest Dermatol. 2020;140: 29-37. doi:10.1016/j.jid.2019.03.1142
- Kanokrungsee S, Pruettivorawongse D, Rajatanavin N. Clinicaloutcomes of topical bimatoprost for nonsegmental facial vitiligo: a preliminary study. J Cosmet Dermatol. 2021;20:812-818. doi.org/10.1111/jocd.13648
PRACTICE POINTS
- Microneedling is a percutaneous collagen induction therapy that also may be used in drug delivery.
- Hypopigmentation can cause considerable distress for patients with skin of color.
- Percutaneous drug delivery of bimatoprost may be helpful in skin repigmentation.
Development of a Safety Awards Program at a Veterans Affairs Health Care System: A Quality Improvement Initiative
ABSTRACT
Objective: Promoting a culture of safety is a critical component of improving health care quality. Recognizing staff who stop the line for safety can positively impact the growth of a culture of safety. The purpose of this initiative was to demonstrate to staff the importance of speaking up for safety and being acknowledged for doing so.
Methods: Following a review of the literature on safety awards programs and their role in promoting a culture of safety in health care covering the period 2017 to 2020, a formal process was developed and implemented to disseminate safety awards to employees.
Results: During the initial 18 months of the initiative, a total of 59 awards were presented. The awards were well received by the recipients and other staff members. Within this period, adjustments were made to enhance the scope and reach of the program.
Conclusion: Recognizing staff behaviors that support a culture of safety is important for improving health care quality and employee engagement. Future research should focus on a formal evaluation of the impact of safety awards programs on patient safety outcomes.
Keywords: patient safety, culture of safety, incident reporting, near miss.
A key aspect of improving health care quality is promoting and sustaining a culture of safety in the workplace. Improving the quality of health care services and systems involves making informed choices regarding the types of strategies to implement.1 An essential aspect of supporting a safety culture is safety-event reporting. To approach the goal of zero harm, all safety events, whether they result in actual harm or are considered near misses, need to be reported. Near-miss events are errors that occur while care is being provided but are detected and corrected before harm reaches the patient.1-3 Near-miss reporting plays a critical role in helping to identify and correct weaknesses in health care delivery systems and processes.4 However, evidence shows that there are a multitude of barriers to the reporting of near-miss events, such as fear of punitive actions, additional workload, unsupportive work environments, a culture with poor psychological safety, knowledge deficit, and lack of recognition of staff who do report near misses.4-11
According to The Joint Commission (TJC), acknowledging health care team members who recognize and report unsafe conditions that provide insight for improving patient safety is a key method for promoting the reporting of near-miss events.6 As a result, some health care organizations and patient safety agencies have started to institute some form of recognition for their employees in the realm of safety.8-10 The Pennsylvania Patient Safety Authority offers exceptional guidance for creating a safety awards program to promote a culture of safety.12 Furthermore, TJC supports recognizing individuals and health care teams who identify and report near misses, or who have suggestions for initiatives to promote patient safety, with “good catch” awards. Individuals or teams working to promote and sustain a culture of safety should be recognized for their efforts. Acknowledging “good catches” to reward the identification, communication, and resolution of safety issues is an effective strategy for improving patient safety and health care quality.6,8
This quality improvement (QI) initiative was undertaken to demonstrate to staff that, in building an organizational culture of safety, it is important that staff be encouraged to speak up for safety and be acknowledged for doing so. If health care organizations want staff to be motivated to report near misses and improve safety and health care quality, the culture needs to shift from focusing on blame to incentivizing individuals and teams to speak up when they have concerns.8-10 Although deciding which safety actions are worthy of recognition can be challenging, recognizing all safe acts, regardless of how big or small they are perceived to be, is important. This QI initiative aimed to establish a tiered approach to recognize staff members for various categories of safety acts.
METHODS
A review of the literature from January 2017 to May 2020 for peer-reviewed publications regarding how other organizations implemented safety award programs to promote a culture of safety resulted in a dearth of evidence. This prompted us at the Veterans Affairs Connecticut Healthcare System to develop and implement a formal program to disseminate safety awards to employees.
Program Launch and Promotion
In 2020, our institution embarked on a journey to high reliability with the goal of approaching zero harm. As part of efforts to promote a culture of safety, the hospital’s High Reliability Organization (HRO) team worked to develop a safety awards recognition program. Prior to the launch, the hospital’s patient safety committee recognized staff members through the medical center safety event reporting system (the Joint Patient Safety Reporting system [JPSR]) or through direct communication with staff members on safety actions they were engaged in. JPSR is the Veterans Health Administration National Center for Patient Safety incident reporting system for reporting, tracking, and trending of patient incidents in a national database. The award consisted of a certificate presented by the patient safety committee chairpersons to the employee in front of their peers in their respective work area. Hospital leadership was not involved in the safety awards recognition program at that time. No nomination process existed prior to our QI launch.
Once the QI initiative was launched and marketed heavily at staff meetings, we started to receive nominations for actions that were truly exceptional, while many others were submitted for behaviors that were within the day-to-day scope of practice of the staff member. For those early nominations that did not meet criteria for an award, we thanked staff for their submissions with a gentle statement that their nomination did not meet the criteria for an award. After following this practice for a few weeks, we became concerned that if we did not acknowledge the staff who came forward to request recognition for their routine work that supported safety, we could risk losing their engagement in a culture of safety. As such, we decided to create 3 levels of awards to recognize behaviors that went above and beyond while also acknowledging staff for actions within their scope of practice. Additionally, hospital leadership wanted to ensure that all staff recognize that their safety efforts are valued by leadership and that that sense of value will hopefully contribute to a culture of safety over time.
Initially, the single award system was called the “Good Catch Award” to acknowledge staff who go above and beyond to speak up and take action when they have safety concerns. This particular recognition includes a certificate, an encased baseball card that has been personalized by including the staff member’s picture and safety event identified, a stress-release baseball, and a stick of Bazooka gum (similar to what used to come in baseball cards packs). The award is presented to employees in their work area by the HRO and patient safety teams and includes representatives from the executive leadership team (ELT). The safety event identified is described by an ELT member, and all items are presented to the employee. Participation by the leadership team communicates how much the work being done to promote a culture of safety and advance quality health care is appreciated. This action also encourages others in the organization to identify and report safety concerns.13
With the rollout of the QI initiative, the volume of nominations submitted quickly increased (eg, approximately 1 every 2 months before to 3 per month following implementation). Frequently, nominations were for actions believed to be within the scope of the employee’s responsibilities. Our institution’s leadership team quickly recognized that, as an organization, not diminishing the importance of the “Good Catch Award” was important. However, the
The original Good Catch Award was labelled as a Level 1 award. The Level 2 safety recognition award, named the HRO Safety Champion Award, is given to employees who stop the line for a safety concern within their scope of practice and also participate as part of a team to investigate and improve processes to avoid recurring safety concerns in the future. For the Level Two award, a certificate is presented to an employee by the hospital’s HRO lead, HRO physician champion, patient safety manager, immediate supervisor, and peers. With the Level 3 award, the Culture of Safety Appreciation Award, individuals are recognized for addressing safety concerns within their assigned scope of responsibilities. Recognition is bestowed by an email of appreciation sent to the employee, acknowledging their commitment to promoting a culture of safety and quality health care. The recipient’s direct supervisor and other hospital leaders are copied on the message.14 See Table 1 for a

Our institution’s HRO and patient safety teams utilized many additional venues to disseminate information regarding awardees and their actions. These included our monthly HRO newsletter, monthly safety forums, and biweekly Team Connecticut Healthcare system-wide huddles.
Nomination Process
Awards nominations are submitted via the hospital intranet homepage, where there is an “HRO Safety Award Nomination” icon. Once a staff member clicks the icon, a template opens asking for information, such as the reason for the nomination submission, and then walks them through the template using the CAR (C-context, A-actions, and R-results)15 format for describing the situation, identifying actions taken, and specifying the outcome of the action. Emails with award nominations can also be sent to the HRO lead, HRO champion, or Patient Safety Committee co-chairs. Calls for nominations are made at several venues attended by employees as well as supervisors. These include monthly safety forums, biweekly Team Connecticut Healthcare system-wide huddles, supervisory staff meetings, department and unit-based staff meetings, and many other formal and informal settings. This QI initiative has allowed us to capture potential awardees through several avenues, including self-nominations. All nominations are reviewed by a safety awards committee. Each committee member ranks the nomination as a Level 1, 2, or 3 award. For nominations where conflicting scores are obtained, the committee discusses the nomination together to resolve discrepancies.
Needed Resources
Material resources required for this QI initiative include certificate paper, plastic baseball card sleeves, stress-release baseballs, and Bazooka gum. The largest resource investment was the time needed to support the initiative. This included the time spent scheduling the Level 1 and 2 award presentations with staff and leadership. Time was also required to put the individual award packages together, which included printing the paper certificates, obtaining awardee pictures, placing them with their safety stories in a plastic baseball card sleeve, and arranging for the hospital photographer to take pictures of the awardees with their peers and leaders.
RESULTS
Prior to this QI initiative launch, 14 awards were given out over the preceding 2-year period. During the initial 18 months of the initiative (December 2020 to June 2022), 59 awards were presented (Level 1, n = 26; Level 2, n = 22; and Level 3, n = 11). Looking further into the Level 1 awards presented, 25 awardees worked in clinical roles and 1 in a nonclinical position (Table 2). The awardees represented multidisciplinary areas, including medical/surgical (med/surg) inpatient units, anesthesia, operating room, pharmacy, mental health clinics, surgical intensive care, specialty care clinics, and nutrition and food services. With the Level 2 awards, 18 clinical staff and 4 nonclinical staff received awards from the areas of med/surg inpatient, outpatient surgical suites, the medical center director’s office, radiology, pharmacy, primary care, facilities management, environmental management, infection prevention, and emergency services. All Level 3 awardees were from clinical areas, including primary care, hospital education, sterile processing, pharmacies, operating rooms, and med/surg inpatient units.
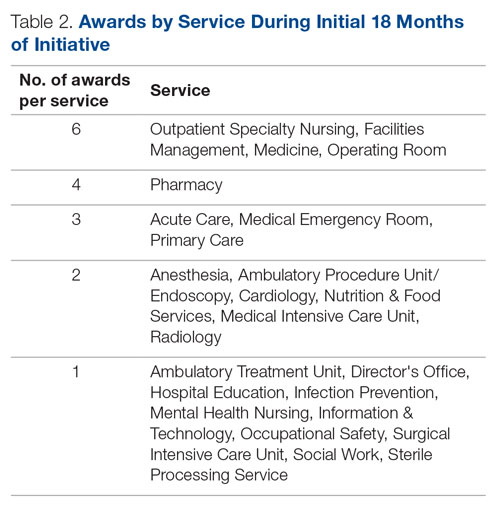
With the inception of this QI initiative, our organization has begun to see trends reflecting increased reporting of both actual and close-call events in JPSR (Figure 1). 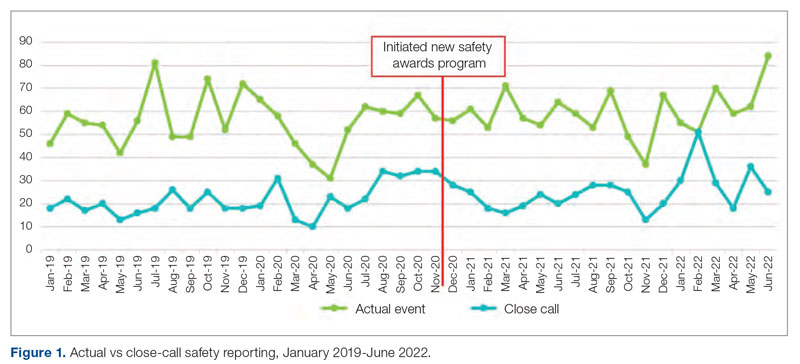
With the inclusion of information regarding awardees and their actions in monthly safety forums, attendance at these forums has increased from an average of 64 attendees per month in 2021 to an average of 131 attendees per month in 2022 (Figure 2).
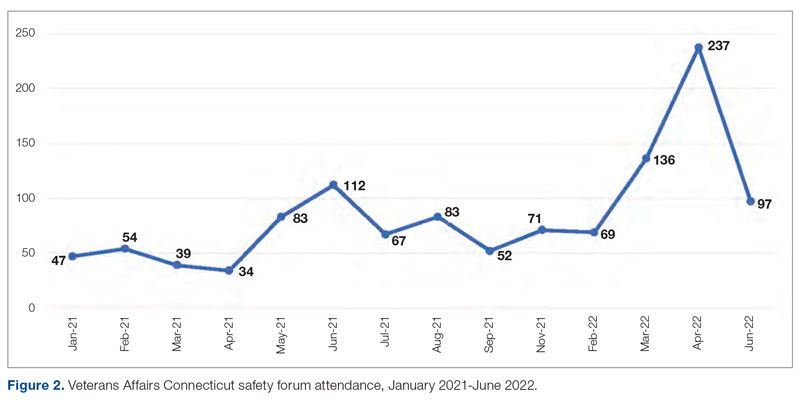
Finally, our organization’s annual All-Employee Survey results have shown incremental increases in staff reporting feeling psychologically safe and not fearing reprisal (Figure 3). It is important to note that there may be other contributing factors to these incremental changes.
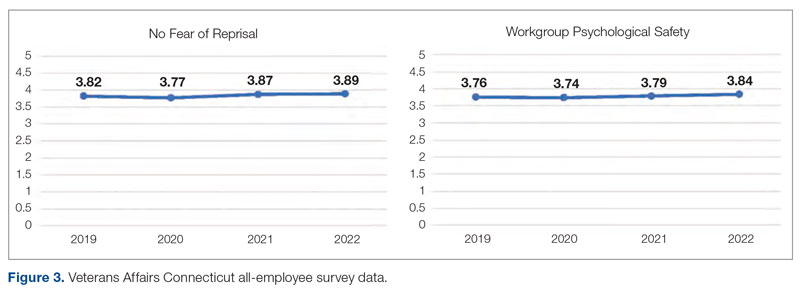
Level 1 – Good Catch Award. M.S. was assigned as a continuous safety monitor, or “sitter,” on one of the med/surg inpatient units. M.S. arrived at the bedside and asked for a report on the patient at a change in shift. The report stated that the patient was sleeping and had not moved in a while. M.S. set about to perform the functions of a sitter and did her usual tasks in cleaning and tidying the room for the patient for breakfast and taking care of all items in the room, in general. M.S. introduced herself to the patient, who she thought might wake up because of her speaking to him. She thought the patient was in an odd position, and knowing that a patient should be a little further up in the bed, she tried with touch to awaken him to adjust his position. M.S. found that the patient was rather chilly to the touch and immediately became concerned. She continued to attempt to rouse the patient. M.S. called for the nurse and began to adjust the patient’s position. M.S. insisted that the patient was cold and “something was wrong.” A set of vitals was taken and a rapid response team code was called. The patient was immediately transferred to the intensive care unit to receive a higher level of care. If not for the diligence and caring attitude of M.S., this patient may have had a very poor outcome.
Reason for criteria being met: The scope of practice of a sitter is to be present in a patient’s room to monitor for falls and overall safety. This employee noticed that the patient was not responsive to verbal or tactile stimuli. Her immediate reporting of her concern to the nurse resulted in prompt intervention. If she had let the patient be, the patient could have died. The staff member went above and beyond by speaking up and taking action when she had a patient safety concern.
Level 2 – HRO Safety Champion Award. A patient presented to an outpatient clinic for monoclonal antibody (mAb) therapy for a COVID-19 infection; the treatment has been scheduled by the patient’s primary care provider. At that time, outpatient mAb therapy was the recommended care option for patients stable enough to receive treatment in this setting, but it is contraindicated in patients who are too unstable to receive mAb therapy in an outpatient setting, such as those with increased oxygen demands. R.L., a staff nurse, assessed the patient on arrival and found that his vital signs were stable, except for a slightly elevated respiratory rate. Upon questioning, the patient reported that he had increased his oxygen use at home from 2 to 4 L via a nasal cannula. R.L. assessed that the patient was too high-risk for outpatient mAb therapy and had the patient checked into the emergency department (ED) to receive a full diagnostic workup and evaluation by Dr. W., an ED provider. The patient required admission to the hospital for a higher level of care in an inpatient unit because of severe COVID-19 infection. Within 48 hours of admission, the patient’s condition further declined, requiring an upgrade to the medical intensive care unit with progressive interventions. Owing to the clinical assessment skills and prompt action of R.L., the patient was admitted to the hospital instead of receiving treatment in a suboptimal care setting and returning home. Had the patient gone home, his rapid decline could have had serious consequences.
Reason for criteria being met: On a cursory look, the patient may have passed as someone sufficiently stable to undergo outpatient treatment. However, the nurse stopped the line, paid close attention, and picked up on an abnormal vital sign and the projected consequences. The nurse brought the patient to a higher level of care in the ED so that he could get the attention he needed. If this patient was given mAb therapy in the outpatient setting, he would have been discharged and become sicker with the COVID-19 illness. As a result of this incident, R.L. is working with the outpatient clinic and ED staff to enahance triage and evaluation of patients referred for outpatient therapy for COVID-19 infections to prevent a similar event from recurring.
Level 3 – Culture of Safety Appreciation Award. While C.C. was reviewing the hazardous item
Reason for criteria being met: The employee works in the hospital education department. It is within her scope of responsibilities to provide ongoing education to staff in order to address potential safety concerns.
DISCUSSION
This QI initiative was undertaken to demonstrate to staff that, in building an organizational culture of safety and advancing quality health care, it is important that staff be encouraged to speak up for safety and be acknowledged for doing so. As part of efforts to continuously build on a safety-first culture, transparency and celebration of successes were demonstrated. This QI initiative demonstrated that a diverse and wide range of employees were reached, from clinical to nonclinical staff, and frontline to supervisory staff, as all were included in the recognition process. While many award nominations were received through the submission of safety concerns to the high-reliability team and patient safety office, several came directly from staff who wanted to recognize their peers for their work, supporting a culture of safety. This showed that staff felt that taking the time to submit a write-up to recognize a peer was an important task. Achieving zero harm for patients and staff alike is a top priority for our institution and guides all decisions, which reinforces that everyone has a responsibility to ensure that safety is always the first consideration. A culture of safety is enhanced by staff recognition. This QI initiative also showed that staff felt valued when they were acknowledged, regardless of the level of recognition they received. The theme of feeling valued came from unsolicited feedback. For example, some direct comments from awardees are presented in the Box.

In addition to endorsing the importance of safe practices to staff, safety award programs can identify gaps in existing standard procedures that can be updated quickly and shared broadly across a health care organization. The authors observed that the existence of the award program gives staff permission to use their voice to speak up when they have questions or concerns related to safety and to proactively engage in safety practices; a cultural shift of this kind informs safety practices and procedures and contributes to a more inspiring workplace. Staff at our organization who have received any of the safety awards, and those who are aware of these awards, have embraced the program readily. At the time of submission of this manuscript, there was a relative paucity of published literature on the details, performance, and impact of such programs. This initiative aims to share a road map highlighting the various dimensions of staff recognition and how the program supports our health care system in fostering a strong, sustainable culture of safety and health care quality. A next step is to formally assess the impact of the awards program on our culture of safety and quality using a psychometrically sound measurement tool, as recommended by TJC,16 such as the
CONCLUSION
A health care organization safety awards program is a strategy for building and sustaining a culture of safety. This QI initiative may be valuable to other organizations in the process of establishing a safety awards program of their own. Future research should focus on a formal evaluation of the impact of safety awards programs on patient safety outcomes.
Corresponding author: John S. Murray, PhD, MPH, MSGH, RN, FAAN, 20 Chapel Street, Unit A502, Brookline, MA 02446; [email protected]
Disclosures: None reported.
1. National Center for Biotechnology Information. Improving healthcare quality in Europe: Characteristics, effectiveness and implementation of different strategies. National Library of Medicine; 2019.
2.
3. Agency for Healthcare Research and Quality. Implementing near-miss reporting and improvement tracking in primary care practices: lessons learned. Agency for Healthcare Research and Quality; 2017.
4. Hamed M, Konstantinidis S. Barriers to incident reporting among nurses: a qualitative systematic review. West J Nurs Res. 2022;44(5):506-523. doi:10.1177/0193945921999449
5. Mohamed M, Abubeker IY, Al-Mohanadi D, et al. Perceived barriers of incident reporting among internists: results from Hamad medical corporation in Qatar. Avicenna J Med. 2021;11(3):139-144. doi:10.1055/s-0041-1734386
6. The Joint Commission. The essential role of leadership in developing a safety culture. The Joint Commission; 2017.
7. Yali G, Nzala S. Healthcare providers’ perspective on barriers to patient safety incident reporting in Lusaka District. J Prev Rehabil Med. 2022;4:44-52. doi:10.21617/jprm2022.417
8. Herzer KR, Mirrer M, Xie Y, et al. Patient safety reporting systems: sustained quality improvement using a multidisciplinary team and “good catch” awards. Jt Comm J Qual Patient Saf. 2012;38(8):339-347. doi:10.1016/s1553-7250(12)38044-6
9. Rogers E, Griffin E, Carnie W, et al. A just culture approach to managing medication errors. Hosp Pharm. 2017;52(4):308-315. doi:10.1310/hpj5204-308
10. Murray JS, Clifford J, Larson S, et al. Implementing just culture to improve patient safety. Mil Med. 2022;0: 1. doi:10.1093/milmed/usac115
11. Paradiso L, Sweeney N. Just culture: it’s more than policy. Nurs Manag. 2019;50(6):38–45. doi:10.1097/01.NUMA.0000558482.07815.ae
12. Wallace S, Mamrol M, Finley E; Pennsylvania Patient Safety Authority. Promote a culture of safety with good catch reports. PA Patient Saf Advis. 2017;14(3).
13. Tan KH, Pang NL, Siau C, et al: Building an organizational culture of patient safety. J Patient Saf Risk Manag. 2019;24:253-261. doi.10.1177/251604351987897
14. Merchant N, O’Neal J, Dealino-Perez C, et al: A high reliability mindset. Am J Med Qual. 2022;37(6):504-510. doi:10.1097/JMQ.0000000000000086
15. Behavioral interview questions and answers. Hudson. Accessed December 23, 2022. https://au.hudson.com/insights/career-advice/job-interviews/behavioural-interview-questions-and-answers/
16. The Joint Commission. Safety culture assessment: Improving the survey process. Accessed December 26, 2022. https://www.jointcommission.org/-/media/tjc/documents/accred-and-cert/safety_culture_assessment_improving_the_survey_process.pdf
17. Reis CT, Paiva SG, Sousa P. The patient safety culture: a systematic review by characteristics of hospital survey on patient safety culture dimensions. Int J Qual Heal Care. 2018;30(9):660-677. doi:10.1093/intqhc/mzy080
18. Fourar YO, Benhassine W, Boughaba A, et al. Contribution to the assessment of patient safety culture in Algerian healthcare settings: the ASCO project. Int J Healthc Manag. 2022;15:52-61. doi.org/10.1080/20479700.2020.1836736
ABSTRACT
Objective: Promoting a culture of safety is a critical component of improving health care quality. Recognizing staff who stop the line for safety can positively impact the growth of a culture of safety. The purpose of this initiative was to demonstrate to staff the importance of speaking up for safety and being acknowledged for doing so.
Methods: Following a review of the literature on safety awards programs and their role in promoting a culture of safety in health care covering the period 2017 to 2020, a formal process was developed and implemented to disseminate safety awards to employees.
Results: During the initial 18 months of the initiative, a total of 59 awards were presented. The awards were well received by the recipients and other staff members. Within this period, adjustments were made to enhance the scope and reach of the program.
Conclusion: Recognizing staff behaviors that support a culture of safety is important for improving health care quality and employee engagement. Future research should focus on a formal evaluation of the impact of safety awards programs on patient safety outcomes.
Keywords: patient safety, culture of safety, incident reporting, near miss.
A key aspect of improving health care quality is promoting and sustaining a culture of safety in the workplace. Improving the quality of health care services and systems involves making informed choices regarding the types of strategies to implement.1 An essential aspect of supporting a safety culture is safety-event reporting. To approach the goal of zero harm, all safety events, whether they result in actual harm or are considered near misses, need to be reported. Near-miss events are errors that occur while care is being provided but are detected and corrected before harm reaches the patient.1-3 Near-miss reporting plays a critical role in helping to identify and correct weaknesses in health care delivery systems and processes.4 However, evidence shows that there are a multitude of barriers to the reporting of near-miss events, such as fear of punitive actions, additional workload, unsupportive work environments, a culture with poor psychological safety, knowledge deficit, and lack of recognition of staff who do report near misses.4-11
According to The Joint Commission (TJC), acknowledging health care team members who recognize and report unsafe conditions that provide insight for improving patient safety is a key method for promoting the reporting of near-miss events.6 As a result, some health care organizations and patient safety agencies have started to institute some form of recognition for their employees in the realm of safety.8-10 The Pennsylvania Patient Safety Authority offers exceptional guidance for creating a safety awards program to promote a culture of safety.12 Furthermore, TJC supports recognizing individuals and health care teams who identify and report near misses, or who have suggestions for initiatives to promote patient safety, with “good catch” awards. Individuals or teams working to promote and sustain a culture of safety should be recognized for their efforts. Acknowledging “good catches” to reward the identification, communication, and resolution of safety issues is an effective strategy for improving patient safety and health care quality.6,8
This quality improvement (QI) initiative was undertaken to demonstrate to staff that, in building an organizational culture of safety, it is important that staff be encouraged to speak up for safety and be acknowledged for doing so. If health care organizations want staff to be motivated to report near misses and improve safety and health care quality, the culture needs to shift from focusing on blame to incentivizing individuals and teams to speak up when they have concerns.8-10 Although deciding which safety actions are worthy of recognition can be challenging, recognizing all safe acts, regardless of how big or small they are perceived to be, is important. This QI initiative aimed to establish a tiered approach to recognize staff members for various categories of safety acts.
METHODS
A review of the literature from January 2017 to May 2020 for peer-reviewed publications regarding how other organizations implemented safety award programs to promote a culture of safety resulted in a dearth of evidence. This prompted us at the Veterans Affairs Connecticut Healthcare System to develop and implement a formal program to disseminate safety awards to employees.
Program Launch and Promotion
In 2020, our institution embarked on a journey to high reliability with the goal of approaching zero harm. As part of efforts to promote a culture of safety, the hospital’s High Reliability Organization (HRO) team worked to develop a safety awards recognition program. Prior to the launch, the hospital’s patient safety committee recognized staff members through the medical center safety event reporting system (the Joint Patient Safety Reporting system [JPSR]) or through direct communication with staff members on safety actions they were engaged in. JPSR is the Veterans Health Administration National Center for Patient Safety incident reporting system for reporting, tracking, and trending of patient incidents in a national database. The award consisted of a certificate presented by the patient safety committee chairpersons to the employee in front of their peers in their respective work area. Hospital leadership was not involved in the safety awards recognition program at that time. No nomination process existed prior to our QI launch.
Once the QI initiative was launched and marketed heavily at staff meetings, we started to receive nominations for actions that were truly exceptional, while many others were submitted for behaviors that were within the day-to-day scope of practice of the staff member. For those early nominations that did not meet criteria for an award, we thanked staff for their submissions with a gentle statement that their nomination did not meet the criteria for an award. After following this practice for a few weeks, we became concerned that if we did not acknowledge the staff who came forward to request recognition for their routine work that supported safety, we could risk losing their engagement in a culture of safety. As such, we decided to create 3 levels of awards to recognize behaviors that went above and beyond while also acknowledging staff for actions within their scope of practice. Additionally, hospital leadership wanted to ensure that all staff recognize that their safety efforts are valued by leadership and that that sense of value will hopefully contribute to a culture of safety over time.
Initially, the single award system was called the “Good Catch Award” to acknowledge staff who go above and beyond to speak up and take action when they have safety concerns. This particular recognition includes a certificate, an encased baseball card that has been personalized by including the staff member’s picture and safety event identified, a stress-release baseball, and a stick of Bazooka gum (similar to what used to come in baseball cards packs). The award is presented to employees in their work area by the HRO and patient safety teams and includes representatives from the executive leadership team (ELT). The safety event identified is described by an ELT member, and all items are presented to the employee. Participation by the leadership team communicates how much the work being done to promote a culture of safety and advance quality health care is appreciated. This action also encourages others in the organization to identify and report safety concerns.13
With the rollout of the QI initiative, the volume of nominations submitted quickly increased (eg, approximately 1 every 2 months before to 3 per month following implementation). Frequently, nominations were for actions believed to be within the scope of the employee’s responsibilities. Our institution’s leadership team quickly recognized that, as an organization, not diminishing the importance of the “Good Catch Award” was important. However, the
The original Good Catch Award was labelled as a Level 1 award. The Level 2 safety recognition award, named the HRO Safety Champion Award, is given to employees who stop the line for a safety concern within their scope of practice and also participate as part of a team to investigate and improve processes to avoid recurring safety concerns in the future. For the Level Two award, a certificate is presented to an employee by the hospital’s HRO lead, HRO physician champion, patient safety manager, immediate supervisor, and peers. With the Level 3 award, the Culture of Safety Appreciation Award, individuals are recognized for addressing safety concerns within their assigned scope of responsibilities. Recognition is bestowed by an email of appreciation sent to the employee, acknowledging their commitment to promoting a culture of safety and quality health care. The recipient’s direct supervisor and other hospital leaders are copied on the message.14 See Table 1 for a

Our institution’s HRO and patient safety teams utilized many additional venues to disseminate information regarding awardees and their actions. These included our monthly HRO newsletter, monthly safety forums, and biweekly Team Connecticut Healthcare system-wide huddles.
Nomination Process
Awards nominations are submitted via the hospital intranet homepage, where there is an “HRO Safety Award Nomination” icon. Once a staff member clicks the icon, a template opens asking for information, such as the reason for the nomination submission, and then walks them through the template using the CAR (C-context, A-actions, and R-results)15 format for describing the situation, identifying actions taken, and specifying the outcome of the action. Emails with award nominations can also be sent to the HRO lead, HRO champion, or Patient Safety Committee co-chairs. Calls for nominations are made at several venues attended by employees as well as supervisors. These include monthly safety forums, biweekly Team Connecticut Healthcare system-wide huddles, supervisory staff meetings, department and unit-based staff meetings, and many other formal and informal settings. This QI initiative has allowed us to capture potential awardees through several avenues, including self-nominations. All nominations are reviewed by a safety awards committee. Each committee member ranks the nomination as a Level 1, 2, or 3 award. For nominations where conflicting scores are obtained, the committee discusses the nomination together to resolve discrepancies.
Needed Resources
Material resources required for this QI initiative include certificate paper, plastic baseball card sleeves, stress-release baseballs, and Bazooka gum. The largest resource investment was the time needed to support the initiative. This included the time spent scheduling the Level 1 and 2 award presentations with staff and leadership. Time was also required to put the individual award packages together, which included printing the paper certificates, obtaining awardee pictures, placing them with their safety stories in a plastic baseball card sleeve, and arranging for the hospital photographer to take pictures of the awardees with their peers and leaders.
RESULTS
Prior to this QI initiative launch, 14 awards were given out over the preceding 2-year period. During the initial 18 months of the initiative (December 2020 to June 2022), 59 awards were presented (Level 1, n = 26; Level 2, n = 22; and Level 3, n = 11). Looking further into the Level 1 awards presented, 25 awardees worked in clinical roles and 1 in a nonclinical position (Table 2). The awardees represented multidisciplinary areas, including medical/surgical (med/surg) inpatient units, anesthesia, operating room, pharmacy, mental health clinics, surgical intensive care, specialty care clinics, and nutrition and food services. With the Level 2 awards, 18 clinical staff and 4 nonclinical staff received awards from the areas of med/surg inpatient, outpatient surgical suites, the medical center director’s office, radiology, pharmacy, primary care, facilities management, environmental management, infection prevention, and emergency services. All Level 3 awardees were from clinical areas, including primary care, hospital education, sterile processing, pharmacies, operating rooms, and med/surg inpatient units.

With the inception of this QI initiative, our organization has begun to see trends reflecting increased reporting of both actual and close-call events in JPSR (Figure 1). 
With the inclusion of information regarding awardees and their actions in monthly safety forums, attendance at these forums has increased from an average of 64 attendees per month in 2021 to an average of 131 attendees per month in 2022 (Figure 2).

Finally, our organization’s annual All-Employee Survey results have shown incremental increases in staff reporting feeling psychologically safe and not fearing reprisal (Figure 3). It is important to note that there may be other contributing factors to these incremental changes.

Level 1 – Good Catch Award. M.S. was assigned as a continuous safety monitor, or “sitter,” on one of the med/surg inpatient units. M.S. arrived at the bedside and asked for a report on the patient at a change in shift. The report stated that the patient was sleeping and had not moved in a while. M.S. set about to perform the functions of a sitter and did her usual tasks in cleaning and tidying the room for the patient for breakfast and taking care of all items in the room, in general. M.S. introduced herself to the patient, who she thought might wake up because of her speaking to him. She thought the patient was in an odd position, and knowing that a patient should be a little further up in the bed, she tried with touch to awaken him to adjust his position. M.S. found that the patient was rather chilly to the touch and immediately became concerned. She continued to attempt to rouse the patient. M.S. called for the nurse and began to adjust the patient’s position. M.S. insisted that the patient was cold and “something was wrong.” A set of vitals was taken and a rapid response team code was called. The patient was immediately transferred to the intensive care unit to receive a higher level of care. If not for the diligence and caring attitude of M.S., this patient may have had a very poor outcome.
Reason for criteria being met: The scope of practice of a sitter is to be present in a patient’s room to monitor for falls and overall safety. This employee noticed that the patient was not responsive to verbal or tactile stimuli. Her immediate reporting of her concern to the nurse resulted in prompt intervention. If she had let the patient be, the patient could have died. The staff member went above and beyond by speaking up and taking action when she had a patient safety concern.
Level 2 – HRO Safety Champion Award. A patient presented to an outpatient clinic for monoclonal antibody (mAb) therapy for a COVID-19 infection; the treatment has been scheduled by the patient’s primary care provider. At that time, outpatient mAb therapy was the recommended care option for patients stable enough to receive treatment in this setting, but it is contraindicated in patients who are too unstable to receive mAb therapy in an outpatient setting, such as those with increased oxygen demands. R.L., a staff nurse, assessed the patient on arrival and found that his vital signs were stable, except for a slightly elevated respiratory rate. Upon questioning, the patient reported that he had increased his oxygen use at home from 2 to 4 L via a nasal cannula. R.L. assessed that the patient was too high-risk for outpatient mAb therapy and had the patient checked into the emergency department (ED) to receive a full diagnostic workup and evaluation by Dr. W., an ED provider. The patient required admission to the hospital for a higher level of care in an inpatient unit because of severe COVID-19 infection. Within 48 hours of admission, the patient’s condition further declined, requiring an upgrade to the medical intensive care unit with progressive interventions. Owing to the clinical assessment skills and prompt action of R.L., the patient was admitted to the hospital instead of receiving treatment in a suboptimal care setting and returning home. Had the patient gone home, his rapid decline could have had serious consequences.
Reason for criteria being met: On a cursory look, the patient may have passed as someone sufficiently stable to undergo outpatient treatment. However, the nurse stopped the line, paid close attention, and picked up on an abnormal vital sign and the projected consequences. The nurse brought the patient to a higher level of care in the ED so that he could get the attention he needed. If this patient was given mAb therapy in the outpatient setting, he would have been discharged and become sicker with the COVID-19 illness. As a result of this incident, R.L. is working with the outpatient clinic and ED staff to enahance triage and evaluation of patients referred for outpatient therapy for COVID-19 infections to prevent a similar event from recurring.
Level 3 – Culture of Safety Appreciation Award. While C.C. was reviewing the hazardous item
Reason for criteria being met: The employee works in the hospital education department. It is within her scope of responsibilities to provide ongoing education to staff in order to address potential safety concerns.
DISCUSSION
This QI initiative was undertaken to demonstrate to staff that, in building an organizational culture of safety and advancing quality health care, it is important that staff be encouraged to speak up for safety and be acknowledged for doing so. As part of efforts to continuously build on a safety-first culture, transparency and celebration of successes were demonstrated. This QI initiative demonstrated that a diverse and wide range of employees were reached, from clinical to nonclinical staff, and frontline to supervisory staff, as all were included in the recognition process. While many award nominations were received through the submission of safety concerns to the high-reliability team and patient safety office, several came directly from staff who wanted to recognize their peers for their work, supporting a culture of safety. This showed that staff felt that taking the time to submit a write-up to recognize a peer was an important task. Achieving zero harm for patients and staff alike is a top priority for our institution and guides all decisions, which reinforces that everyone has a responsibility to ensure that safety is always the first consideration. A culture of safety is enhanced by staff recognition. This QI initiative also showed that staff felt valued when they were acknowledged, regardless of the level of recognition they received. The theme of feeling valued came from unsolicited feedback. For example, some direct comments from awardees are presented in the Box.

In addition to endorsing the importance of safe practices to staff, safety award programs can identify gaps in existing standard procedures that can be updated quickly and shared broadly across a health care organization. The authors observed that the existence of the award program gives staff permission to use their voice to speak up when they have questions or concerns related to safety and to proactively engage in safety practices; a cultural shift of this kind informs safety practices and procedures and contributes to a more inspiring workplace. Staff at our organization who have received any of the safety awards, and those who are aware of these awards, have embraced the program readily. At the time of submission of this manuscript, there was a relative paucity of published literature on the details, performance, and impact of such programs. This initiative aims to share a road map highlighting the various dimensions of staff recognition and how the program supports our health care system in fostering a strong, sustainable culture of safety and health care quality. A next step is to formally assess the impact of the awards program on our culture of safety and quality using a psychometrically sound measurement tool, as recommended by TJC,16 such as the
CONCLUSION
A health care organization safety awards program is a strategy for building and sustaining a culture of safety. This QI initiative may be valuable to other organizations in the process of establishing a safety awards program of their own. Future research should focus on a formal evaluation of the impact of safety awards programs on patient safety outcomes.
Corresponding author: John S. Murray, PhD, MPH, MSGH, RN, FAAN, 20 Chapel Street, Unit A502, Brookline, MA 02446; [email protected]
Disclosures: None reported.
ABSTRACT
Objective: Promoting a culture of safety is a critical component of improving health care quality. Recognizing staff who stop the line for safety can positively impact the growth of a culture of safety. The purpose of this initiative was to demonstrate to staff the importance of speaking up for safety and being acknowledged for doing so.
Methods: Following a review of the literature on safety awards programs and their role in promoting a culture of safety in health care covering the period 2017 to 2020, a formal process was developed and implemented to disseminate safety awards to employees.
Results: During the initial 18 months of the initiative, a total of 59 awards were presented. The awards were well received by the recipients and other staff members. Within this period, adjustments were made to enhance the scope and reach of the program.
Conclusion: Recognizing staff behaviors that support a culture of safety is important for improving health care quality and employee engagement. Future research should focus on a formal evaluation of the impact of safety awards programs on patient safety outcomes.
Keywords: patient safety, culture of safety, incident reporting, near miss.
A key aspect of improving health care quality is promoting and sustaining a culture of safety in the workplace. Improving the quality of health care services and systems involves making informed choices regarding the types of strategies to implement.1 An essential aspect of supporting a safety culture is safety-event reporting. To approach the goal of zero harm, all safety events, whether they result in actual harm or are considered near misses, need to be reported. Near-miss events are errors that occur while care is being provided but are detected and corrected before harm reaches the patient.1-3 Near-miss reporting plays a critical role in helping to identify and correct weaknesses in health care delivery systems and processes.4 However, evidence shows that there are a multitude of barriers to the reporting of near-miss events, such as fear of punitive actions, additional workload, unsupportive work environments, a culture with poor psychological safety, knowledge deficit, and lack of recognition of staff who do report near misses.4-11
According to The Joint Commission (TJC), acknowledging health care team members who recognize and report unsafe conditions that provide insight for improving patient safety is a key method for promoting the reporting of near-miss events.6 As a result, some health care organizations and patient safety agencies have started to institute some form of recognition for their employees in the realm of safety.8-10 The Pennsylvania Patient Safety Authority offers exceptional guidance for creating a safety awards program to promote a culture of safety.12 Furthermore, TJC supports recognizing individuals and health care teams who identify and report near misses, or who have suggestions for initiatives to promote patient safety, with “good catch” awards. Individuals or teams working to promote and sustain a culture of safety should be recognized for their efforts. Acknowledging “good catches” to reward the identification, communication, and resolution of safety issues is an effective strategy for improving patient safety and health care quality.6,8
This quality improvement (QI) initiative was undertaken to demonstrate to staff that, in building an organizational culture of safety, it is important that staff be encouraged to speak up for safety and be acknowledged for doing so. If health care organizations want staff to be motivated to report near misses and improve safety and health care quality, the culture needs to shift from focusing on blame to incentivizing individuals and teams to speak up when they have concerns.8-10 Although deciding which safety actions are worthy of recognition can be challenging, recognizing all safe acts, regardless of how big or small they are perceived to be, is important. This QI initiative aimed to establish a tiered approach to recognize staff members for various categories of safety acts.
METHODS
A review of the literature from January 2017 to May 2020 for peer-reviewed publications regarding how other organizations implemented safety award programs to promote a culture of safety resulted in a dearth of evidence. This prompted us at the Veterans Affairs Connecticut Healthcare System to develop and implement a formal program to disseminate safety awards to employees.
Program Launch and Promotion
In 2020, our institution embarked on a journey to high reliability with the goal of approaching zero harm. As part of efforts to promote a culture of safety, the hospital’s High Reliability Organization (HRO) team worked to develop a safety awards recognition program. Prior to the launch, the hospital’s patient safety committee recognized staff members through the medical center safety event reporting system (the Joint Patient Safety Reporting system [JPSR]) or through direct communication with staff members on safety actions they were engaged in. JPSR is the Veterans Health Administration National Center for Patient Safety incident reporting system for reporting, tracking, and trending of patient incidents in a national database. The award consisted of a certificate presented by the patient safety committee chairpersons to the employee in front of their peers in their respective work area. Hospital leadership was not involved in the safety awards recognition program at that time. No nomination process existed prior to our QI launch.
Once the QI initiative was launched and marketed heavily at staff meetings, we started to receive nominations for actions that were truly exceptional, while many others were submitted for behaviors that were within the day-to-day scope of practice of the staff member. For those early nominations that did not meet criteria for an award, we thanked staff for their submissions with a gentle statement that their nomination did not meet the criteria for an award. After following this practice for a few weeks, we became concerned that if we did not acknowledge the staff who came forward to request recognition for their routine work that supported safety, we could risk losing their engagement in a culture of safety. As such, we decided to create 3 levels of awards to recognize behaviors that went above and beyond while also acknowledging staff for actions within their scope of practice. Additionally, hospital leadership wanted to ensure that all staff recognize that their safety efforts are valued by leadership and that that sense of value will hopefully contribute to a culture of safety over time.
Initially, the single award system was called the “Good Catch Award” to acknowledge staff who go above and beyond to speak up and take action when they have safety concerns. This particular recognition includes a certificate, an encased baseball card that has been personalized by including the staff member’s picture and safety event identified, a stress-release baseball, and a stick of Bazooka gum (similar to what used to come in baseball cards packs). The award is presented to employees in their work area by the HRO and patient safety teams and includes representatives from the executive leadership team (ELT). The safety event identified is described by an ELT member, and all items are presented to the employee. Participation by the leadership team communicates how much the work being done to promote a culture of safety and advance quality health care is appreciated. This action also encourages others in the organization to identify and report safety concerns.13
With the rollout of the QI initiative, the volume of nominations submitted quickly increased (eg, approximately 1 every 2 months before to 3 per month following implementation). Frequently, nominations were for actions believed to be within the scope of the employee’s responsibilities. Our institution’s leadership team quickly recognized that, as an organization, not diminishing the importance of the “Good Catch Award” was important. However, the
The original Good Catch Award was labelled as a Level 1 award. The Level 2 safety recognition award, named the HRO Safety Champion Award, is given to employees who stop the line for a safety concern within their scope of practice and also participate as part of a team to investigate and improve processes to avoid recurring safety concerns in the future. For the Level Two award, a certificate is presented to an employee by the hospital’s HRO lead, HRO physician champion, patient safety manager, immediate supervisor, and peers. With the Level 3 award, the Culture of Safety Appreciation Award, individuals are recognized for addressing safety concerns within their assigned scope of responsibilities. Recognition is bestowed by an email of appreciation sent to the employee, acknowledging their commitment to promoting a culture of safety and quality health care. The recipient’s direct supervisor and other hospital leaders are copied on the message.14 See Table 1 for a

Our institution’s HRO and patient safety teams utilized many additional venues to disseminate information regarding awardees and their actions. These included our monthly HRO newsletter, monthly safety forums, and biweekly Team Connecticut Healthcare system-wide huddles.
Nomination Process
Awards nominations are submitted via the hospital intranet homepage, where there is an “HRO Safety Award Nomination” icon. Once a staff member clicks the icon, a template opens asking for information, such as the reason for the nomination submission, and then walks them through the template using the CAR (C-context, A-actions, and R-results)15 format for describing the situation, identifying actions taken, and specifying the outcome of the action. Emails with award nominations can also be sent to the HRO lead, HRO champion, or Patient Safety Committee co-chairs. Calls for nominations are made at several venues attended by employees as well as supervisors. These include monthly safety forums, biweekly Team Connecticut Healthcare system-wide huddles, supervisory staff meetings, department and unit-based staff meetings, and many other formal and informal settings. This QI initiative has allowed us to capture potential awardees through several avenues, including self-nominations. All nominations are reviewed by a safety awards committee. Each committee member ranks the nomination as a Level 1, 2, or 3 award. For nominations where conflicting scores are obtained, the committee discusses the nomination together to resolve discrepancies.
Needed Resources
Material resources required for this QI initiative include certificate paper, plastic baseball card sleeves, stress-release baseballs, and Bazooka gum. The largest resource investment was the time needed to support the initiative. This included the time spent scheduling the Level 1 and 2 award presentations with staff and leadership. Time was also required to put the individual award packages together, which included printing the paper certificates, obtaining awardee pictures, placing them with their safety stories in a plastic baseball card sleeve, and arranging for the hospital photographer to take pictures of the awardees with their peers and leaders.
RESULTS
Prior to this QI initiative launch, 14 awards were given out over the preceding 2-year period. During the initial 18 months of the initiative (December 2020 to June 2022), 59 awards were presented (Level 1, n = 26; Level 2, n = 22; and Level 3, n = 11). Looking further into the Level 1 awards presented, 25 awardees worked in clinical roles and 1 in a nonclinical position (Table 2). The awardees represented multidisciplinary areas, including medical/surgical (med/surg) inpatient units, anesthesia, operating room, pharmacy, mental health clinics, surgical intensive care, specialty care clinics, and nutrition and food services. With the Level 2 awards, 18 clinical staff and 4 nonclinical staff received awards from the areas of med/surg inpatient, outpatient surgical suites, the medical center director’s office, radiology, pharmacy, primary care, facilities management, environmental management, infection prevention, and emergency services. All Level 3 awardees were from clinical areas, including primary care, hospital education, sterile processing, pharmacies, operating rooms, and med/surg inpatient units.

With the inception of this QI initiative, our organization has begun to see trends reflecting increased reporting of both actual and close-call events in JPSR (Figure 1). 
With the inclusion of information regarding awardees and their actions in monthly safety forums, attendance at these forums has increased from an average of 64 attendees per month in 2021 to an average of 131 attendees per month in 2022 (Figure 2).

Finally, our organization’s annual All-Employee Survey results have shown incremental increases in staff reporting feeling psychologically safe and not fearing reprisal (Figure 3). It is important to note that there may be other contributing factors to these incremental changes.

Level 1 – Good Catch Award. M.S. was assigned as a continuous safety monitor, or “sitter,” on one of the med/surg inpatient units. M.S. arrived at the bedside and asked for a report on the patient at a change in shift. The report stated that the patient was sleeping and had not moved in a while. M.S. set about to perform the functions of a sitter and did her usual tasks in cleaning and tidying the room for the patient for breakfast and taking care of all items in the room, in general. M.S. introduced herself to the patient, who she thought might wake up because of her speaking to him. She thought the patient was in an odd position, and knowing that a patient should be a little further up in the bed, she tried with touch to awaken him to adjust his position. M.S. found that the patient was rather chilly to the touch and immediately became concerned. She continued to attempt to rouse the patient. M.S. called for the nurse and began to adjust the patient’s position. M.S. insisted that the patient was cold and “something was wrong.” A set of vitals was taken and a rapid response team code was called. The patient was immediately transferred to the intensive care unit to receive a higher level of care. If not for the diligence and caring attitude of M.S., this patient may have had a very poor outcome.
Reason for criteria being met: The scope of practice of a sitter is to be present in a patient’s room to monitor for falls and overall safety. This employee noticed that the patient was not responsive to verbal or tactile stimuli. Her immediate reporting of her concern to the nurse resulted in prompt intervention. If she had let the patient be, the patient could have died. The staff member went above and beyond by speaking up and taking action when she had a patient safety concern.
Level 2 – HRO Safety Champion Award. A patient presented to an outpatient clinic for monoclonal antibody (mAb) therapy for a COVID-19 infection; the treatment has been scheduled by the patient’s primary care provider. At that time, outpatient mAb therapy was the recommended care option for patients stable enough to receive treatment in this setting, but it is contraindicated in patients who are too unstable to receive mAb therapy in an outpatient setting, such as those with increased oxygen demands. R.L., a staff nurse, assessed the patient on arrival and found that his vital signs were stable, except for a slightly elevated respiratory rate. Upon questioning, the patient reported that he had increased his oxygen use at home from 2 to 4 L via a nasal cannula. R.L. assessed that the patient was too high-risk for outpatient mAb therapy and had the patient checked into the emergency department (ED) to receive a full diagnostic workup and evaluation by Dr. W., an ED provider. The patient required admission to the hospital for a higher level of care in an inpatient unit because of severe COVID-19 infection. Within 48 hours of admission, the patient’s condition further declined, requiring an upgrade to the medical intensive care unit with progressive interventions. Owing to the clinical assessment skills and prompt action of R.L., the patient was admitted to the hospital instead of receiving treatment in a suboptimal care setting and returning home. Had the patient gone home, his rapid decline could have had serious consequences.
Reason for criteria being met: On a cursory look, the patient may have passed as someone sufficiently stable to undergo outpatient treatment. However, the nurse stopped the line, paid close attention, and picked up on an abnormal vital sign and the projected consequences. The nurse brought the patient to a higher level of care in the ED so that he could get the attention he needed. If this patient was given mAb therapy in the outpatient setting, he would have been discharged and become sicker with the COVID-19 illness. As a result of this incident, R.L. is working with the outpatient clinic and ED staff to enahance triage and evaluation of patients referred for outpatient therapy for COVID-19 infections to prevent a similar event from recurring.
Level 3 – Culture of Safety Appreciation Award. While C.C. was reviewing the hazardous item
Reason for criteria being met: The employee works in the hospital education department. It is within her scope of responsibilities to provide ongoing education to staff in order to address potential safety concerns.
DISCUSSION
This QI initiative was undertaken to demonstrate to staff that, in building an organizational culture of safety and advancing quality health care, it is important that staff be encouraged to speak up for safety and be acknowledged for doing so. As part of efforts to continuously build on a safety-first culture, transparency and celebration of successes were demonstrated. This QI initiative demonstrated that a diverse and wide range of employees were reached, from clinical to nonclinical staff, and frontline to supervisory staff, as all were included in the recognition process. While many award nominations were received through the submission of safety concerns to the high-reliability team and patient safety office, several came directly from staff who wanted to recognize their peers for their work, supporting a culture of safety. This showed that staff felt that taking the time to submit a write-up to recognize a peer was an important task. Achieving zero harm for patients and staff alike is a top priority for our institution and guides all decisions, which reinforces that everyone has a responsibility to ensure that safety is always the first consideration. A culture of safety is enhanced by staff recognition. This QI initiative also showed that staff felt valued when they were acknowledged, regardless of the level of recognition they received. The theme of feeling valued came from unsolicited feedback. For example, some direct comments from awardees are presented in the Box.

In addition to endorsing the importance of safe practices to staff, safety award programs can identify gaps in existing standard procedures that can be updated quickly and shared broadly across a health care organization. The authors observed that the existence of the award program gives staff permission to use their voice to speak up when they have questions or concerns related to safety and to proactively engage in safety practices; a cultural shift of this kind informs safety practices and procedures and contributes to a more inspiring workplace. Staff at our organization who have received any of the safety awards, and those who are aware of these awards, have embraced the program readily. At the time of submission of this manuscript, there was a relative paucity of published literature on the details, performance, and impact of such programs. This initiative aims to share a road map highlighting the various dimensions of staff recognition and how the program supports our health care system in fostering a strong, sustainable culture of safety and health care quality. A next step is to formally assess the impact of the awards program on our culture of safety and quality using a psychometrically sound measurement tool, as recommended by TJC,16 such as the
CONCLUSION
A health care organization safety awards program is a strategy for building and sustaining a culture of safety. This QI initiative may be valuable to other organizations in the process of establishing a safety awards program of their own. Future research should focus on a formal evaluation of the impact of safety awards programs on patient safety outcomes.
Corresponding author: John S. Murray, PhD, MPH, MSGH, RN, FAAN, 20 Chapel Street, Unit A502, Brookline, MA 02446; [email protected]
Disclosures: None reported.
1. National Center for Biotechnology Information. Improving healthcare quality in Europe: Characteristics, effectiveness and implementation of different strategies. National Library of Medicine; 2019.
2.
3. Agency for Healthcare Research and Quality. Implementing near-miss reporting and improvement tracking in primary care practices: lessons learned. Agency for Healthcare Research and Quality; 2017.
4. Hamed M, Konstantinidis S. Barriers to incident reporting among nurses: a qualitative systematic review. West J Nurs Res. 2022;44(5):506-523. doi:10.1177/0193945921999449
5. Mohamed M, Abubeker IY, Al-Mohanadi D, et al. Perceived barriers of incident reporting among internists: results from Hamad medical corporation in Qatar. Avicenna J Med. 2021;11(3):139-144. doi:10.1055/s-0041-1734386
6. The Joint Commission. The essential role of leadership in developing a safety culture. The Joint Commission; 2017.
7. Yali G, Nzala S. Healthcare providers’ perspective on barriers to patient safety incident reporting in Lusaka District. J Prev Rehabil Med. 2022;4:44-52. doi:10.21617/jprm2022.417
8. Herzer KR, Mirrer M, Xie Y, et al. Patient safety reporting systems: sustained quality improvement using a multidisciplinary team and “good catch” awards. Jt Comm J Qual Patient Saf. 2012;38(8):339-347. doi:10.1016/s1553-7250(12)38044-6
9. Rogers E, Griffin E, Carnie W, et al. A just culture approach to managing medication errors. Hosp Pharm. 2017;52(4):308-315. doi:10.1310/hpj5204-308
10. Murray JS, Clifford J, Larson S, et al. Implementing just culture to improve patient safety. Mil Med. 2022;0: 1. doi:10.1093/milmed/usac115
11. Paradiso L, Sweeney N. Just culture: it’s more than policy. Nurs Manag. 2019;50(6):38–45. doi:10.1097/01.NUMA.0000558482.07815.ae
12. Wallace S, Mamrol M, Finley E; Pennsylvania Patient Safety Authority. Promote a culture of safety with good catch reports. PA Patient Saf Advis. 2017;14(3).
13. Tan KH, Pang NL, Siau C, et al: Building an organizational culture of patient safety. J Patient Saf Risk Manag. 2019;24:253-261. doi.10.1177/251604351987897
14. Merchant N, O’Neal J, Dealino-Perez C, et al: A high reliability mindset. Am J Med Qual. 2022;37(6):504-510. doi:10.1097/JMQ.0000000000000086
15. Behavioral interview questions and answers. Hudson. Accessed December 23, 2022. https://au.hudson.com/insights/career-advice/job-interviews/behavioural-interview-questions-and-answers/
16. The Joint Commission. Safety culture assessment: Improving the survey process. Accessed December 26, 2022. https://www.jointcommission.org/-/media/tjc/documents/accred-and-cert/safety_culture_assessment_improving_the_survey_process.pdf
17. Reis CT, Paiva SG, Sousa P. The patient safety culture: a systematic review by characteristics of hospital survey on patient safety culture dimensions. Int J Qual Heal Care. 2018;30(9):660-677. doi:10.1093/intqhc/mzy080
18. Fourar YO, Benhassine W, Boughaba A, et al. Contribution to the assessment of patient safety culture in Algerian healthcare settings: the ASCO project. Int J Healthc Manag. 2022;15:52-61. doi.org/10.1080/20479700.2020.1836736
1. National Center for Biotechnology Information. Improving healthcare quality in Europe: Characteristics, effectiveness and implementation of different strategies. National Library of Medicine; 2019.
2.
3. Agency for Healthcare Research and Quality. Implementing near-miss reporting and improvement tracking in primary care practices: lessons learned. Agency for Healthcare Research and Quality; 2017.
4. Hamed M, Konstantinidis S. Barriers to incident reporting among nurses: a qualitative systematic review. West J Nurs Res. 2022;44(5):506-523. doi:10.1177/0193945921999449
5. Mohamed M, Abubeker IY, Al-Mohanadi D, et al. Perceived barriers of incident reporting among internists: results from Hamad medical corporation in Qatar. Avicenna J Med. 2021;11(3):139-144. doi:10.1055/s-0041-1734386
6. The Joint Commission. The essential role of leadership in developing a safety culture. The Joint Commission; 2017.
7. Yali G, Nzala S. Healthcare providers’ perspective on barriers to patient safety incident reporting in Lusaka District. J Prev Rehabil Med. 2022;4:44-52. doi:10.21617/jprm2022.417
8. Herzer KR, Mirrer M, Xie Y, et al. Patient safety reporting systems: sustained quality improvement using a multidisciplinary team and “good catch” awards. Jt Comm J Qual Patient Saf. 2012;38(8):339-347. doi:10.1016/s1553-7250(12)38044-6
9. Rogers E, Griffin E, Carnie W, et al. A just culture approach to managing medication errors. Hosp Pharm. 2017;52(4):308-315. doi:10.1310/hpj5204-308
10. Murray JS, Clifford J, Larson S, et al. Implementing just culture to improve patient safety. Mil Med. 2022;0: 1. doi:10.1093/milmed/usac115
11. Paradiso L, Sweeney N. Just culture: it’s more than policy. Nurs Manag. 2019;50(6):38–45. doi:10.1097/01.NUMA.0000558482.07815.ae
12. Wallace S, Mamrol M, Finley E; Pennsylvania Patient Safety Authority. Promote a culture of safety with good catch reports. PA Patient Saf Advis. 2017;14(3).
13. Tan KH, Pang NL, Siau C, et al: Building an organizational culture of patient safety. J Patient Saf Risk Manag. 2019;24:253-261. doi.10.1177/251604351987897
14. Merchant N, O’Neal J, Dealino-Perez C, et al: A high reliability mindset. Am J Med Qual. 2022;37(6):504-510. doi:10.1097/JMQ.0000000000000086
15. Behavioral interview questions and answers. Hudson. Accessed December 23, 2022. https://au.hudson.com/insights/career-advice/job-interviews/behavioural-interview-questions-and-answers/
16. The Joint Commission. Safety culture assessment: Improving the survey process. Accessed December 26, 2022. https://www.jointcommission.org/-/media/tjc/documents/accred-and-cert/safety_culture_assessment_improving_the_survey_process.pdf
17. Reis CT, Paiva SG, Sousa P. The patient safety culture: a systematic review by characteristics of hospital survey on patient safety culture dimensions. Int J Qual Heal Care. 2018;30(9):660-677. doi:10.1093/intqhc/mzy080
18. Fourar YO, Benhassine W, Boughaba A, et al. Contribution to the assessment of patient safety culture in Algerian healthcare settings: the ASCO project. Int J Healthc Manag. 2022;15:52-61. doi.org/10.1080/20479700.2020.1836736
Teaching Quality Improvement to Internal Medicine Residents to Address Patient Care Gaps in Ambulatory Quality Metrics
ABSTRACT
Objective: To teach internal medicine residents quality improvement (QI) principles in an effort to improve resident knowledge and comfort with QI, as well as address quality care gaps in resident clinic primary care patient panels.
Design: A QI curriculum was implemented for all residents rotating through a primary care block over a 6-month period. Residents completed Institute for Healthcare Improvement (IHI) modules, participated in a QI workshop, and received panel data reports, ultimately completing a plan-do-study-act (PDSA) cycle to improve colorectal cancer screening and hypertension control.
Setting and participants: This project was undertaken at Tufts Medical Center Primary Care, Boston, Massachusetts, the primary care teaching practice for all 75 internal medicine residents at Tufts Medical Center. All internal medicine residents were included, with 55 (73%) of the 75 residents completing the presurvey, and 39 (52%) completing the postsurvey.
Measurements: We administered a 10-question pre- and postsurvey looking at resident attitudes toward and comfort with QI and familiarity with their panel data as well as measured rates of colorectal cancer screening and hypertension control in resident panels.
Results: There was an increase in the numbers of residents who performed a PDSA cycle (P = .002), completed outreach based on their panel data (P = .02), and felt comfortable in both creating aim statements and designing and implementing PDSA cycles (P < .0001). The residents’ knowledge of their panel data significantly increased. There was no significant improvement in hypertension control, but there was an increase in colorectal cancer screening rates (P < .0001).
Conclusion: Providing panel data and performing targeted QI interventions can improve resident comfort with QI, translating to improvement in patient outcomes.
Keywords: quality improvement, resident education, medical education, care gaps, quality metrics.
As quality improvement (QI) has become an integral part of clinical practice, residency training programs have continued to evolve in how best to teach QI. The Accreditation Council for Graduate Medical Education (ACGME) Common Program requirements mandate that core competencies in residency programs include practice-based learning and improvement and systems-based practice.1 Residents should receive education in QI, receive data on quality metrics and benchmarks related to their patient population, and participate in QI activities. The Clinical Learning Environment Review (CLER) program was established to provide feedback to institutions on 6 focused areas, including patient safety and health care quality. In visits to institutions across the United States, the CLER committees found that many residents had limited knowledge of QI concepts and limited access to data on quality metrics and benchmarks.2
There are many barriers to implementing a QI curriculum in residency programs, and creating and maintaining successful strategies has proven challenging.3 Many QI curricula for internal medicine residents have been described in the literature, but the results of many of these studies focus on resident self-assessment of QI knowledge and numbers of projects rather than on patient outcomes.4-13 As there is some evidence suggesting that patients treated by residents have worse outcomes on ambulatory quality measures when compared with patients treated by staff physicians,14,15 it is important to also look at patient outcomes when evaluating a QI curriculum. Experts in education recommend the following to optimize learning: exposure to both didactic and experiential opportunities, connection to health system improvement efforts, and assessment of patient outcomes in addition to learner feedback.16,17 A study also found that providing panel data to residents could improve quality metrics.18
In this study, we sought to investigate the effects of a resident QI intervention during an ambulatory block on both residents’ self-assessments of QI knowledge and attitudes as well as on patient quality metrics.
Methods
Curriculum
We implemented this educational initiative at Tufts Medical Center Primary Care, Boston, Massachusetts, the primary care teaching practice for all 75 internal medicine residents at Tufts Medical Center. Co-located with the 415-bed academic medical center in downtown Boston, the practice serves more than 40,000 patients, approximately 7000 of whom are cared for by resident primary care physicians (PCPs). The internal medicine residents rotate through the primary care clinic as part of continuity clinic during ambulatory or elective blocks. In addition to continuity clinic, the residents have 2 dedicated 3-week primary care rotations during the course of an academic year. Primary care rotations consist of 5 clinic sessions a week as well as structured teaching sessions. Each resident inherits a panel of patients from an outgoing senior resident, with an average panel size of 96 patients per resident.
Prior to this study intervention, we did not do any formal QI teaching to our residents as part of their primary care curriculum, and previous panel management had focused more on chart reviews of patients whom residents perceived to be higher risk. Residents from all 3 years were included in the intervention. We taught a QI curriculum to our residents from January 2018 to June 2018 during the 3-week primary care rotation, which consisted of the following components:
- Institute for Healthcare Improvement (IHI) module QI 102 completed independently online.
- A 2-hour QI workshop led by 1 of 2 primary care faculty with backgrounds in QI, during which residents were taught basic principles of QI, including how to craft aim statements and design plan-do-study-act (PDSA) cycles, and participated in a hands-on QI activity designed to model rapid cycle improvement (the Paper Airplane Factory19).
- Distribution of individualized reports of residents’ patient panel data by email at the start of the primary care block that detailed patients’ overall rates of colorectal cancer screening and hypertension (HTN) control, along with the average resident panel rates and the average attending panel rates. The reports also included a list of all residents’ patients who were overdue for colorectal cancer screening or whose last blood pressure (BP) was uncontrolled (systolic BP ≥ 140 mm Hg or diastolic BP ≥ 90 mm Hg). These reports were originally designed by our practice’s QI team and run and exported in Microsoft Excel format monthly by our information technology (IT) administrator.
- Instruction on aim statements as a group, followed by the expectation that each resident create an individualized aim statement tailored to each resident’s patient panel rates, with the PDSA cycle to be implemented during the remainder of the primary care rotation, focusing on improvement of colorectal cancer screening and HTN control (see supplementary eFigure 1 online for the worksheet used for the workshop).
- Residents were held accountable for their interventions by various check-ins. At the end of the primary care block, residents were required to submit their completed worksheets showing the intervention they had undertaken and when it was performed. The 2 primary care attendings primarily responsible for QI education would review the resident’s work approximately 1 to 2 months after they submitted their worksheets describing their intervention. These attendings sent the residents personalized feedback based on whether the intervention had been completed or successful as evidenced by documentation in the chart, including direct patient outreach by phone, letter, or portal; outreach to the resident coordinator; scheduled follow-up appointment; or booking or completion of colorectal cancer screening. Along with this feedback, residents were also sent suggestions for next steps. Resident preceptors were copied on the email to facilitate reinforcement of the goals and plans. Finally, the resident preceptors also helped with accountability by going through the residents’ worksheets and patient panel metrics with the residents during biannual evaluations.
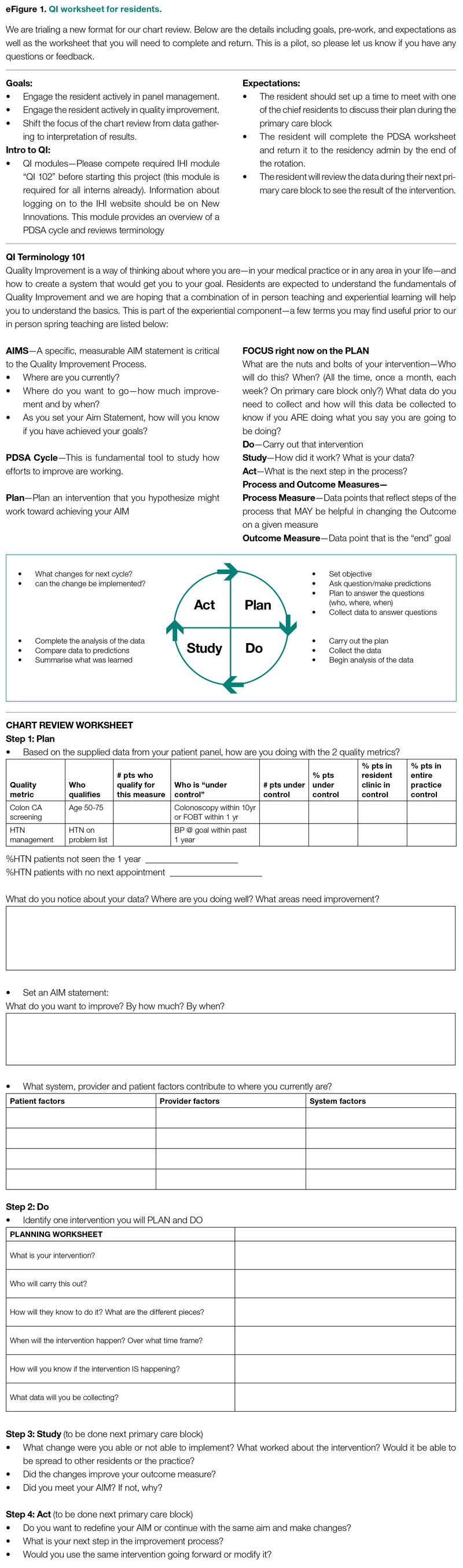
Evaluation
Residents were surveyed with a 10-item questionnaire pre and post intervention regarding their attitudes toward QI, understanding of QI principles, and familiarity with their patient panel data. Surveys were anonymous and distributed via the SurveyMonkey platform (see supplementary eFigure 2 online). Residents were asked if they had ever performed a PDSA cycle, performed patient outreach, or performed an intervention and whether they knew the rates of diabetes, HTN, and colorectal cancer screening in their patient panels. Questions rated on a 5-point Likert scale were used to assess comfort with panel management, developing an aim statement, designing and implementing a PDSA cycle, as well as interest in pursuing QI as a career. For the purposes of analysis, these questions were dichotomized into “somewhat comfortable” and “very comfortable” vs “neutral,” “somewhat uncomfortable,” and “very uncomfortable.” Similarly, we dichotomized the question about interest in QI as a career into “somewhat interested” and “very interested” vs “neutral,” “somewhat disinterested,” and “very disinterested.” As the surveys were anonymous, we were unable to pair the pre- and postintervention surveys and used a chi-square test to evaluate whether there was an association between survey assessments pre intervention vs post intervention and a positive or negative response to the question.
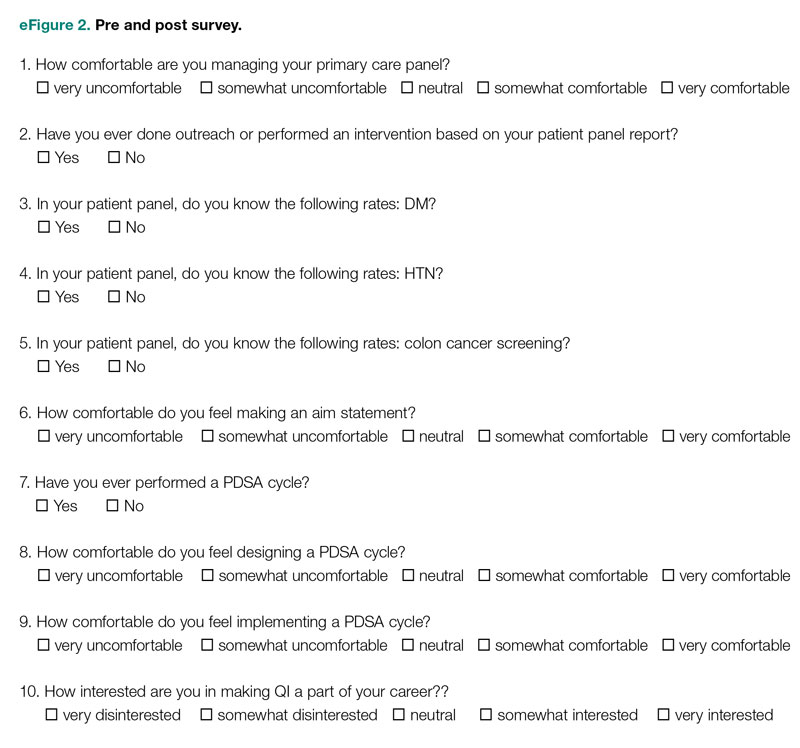
We also examined rates of HTN control and colorectal cancer screening in all 75 resident panels pre and post intervention. The paired t-test was used to determine whether the mean change from pre to post intervention was significant. SAS 9.4 (SAS Institute Inc.) was used for all analyses. Institutional Review Board exemption was obtained from the Tufts Medical Center IRB. There was no funding received for this study.
Results
Respondents
Of the 75 residents, 55 (73%) completed the survey prior to the intervention, and 39 (52%) completed the survey after the intervention.
Panel Knowledge and Intervention
Prior to the intervention, 45% of residents had performed a PDSA cycle, compared with 77% post intervention, which was a significant increase (P = .002) (Table 1). Sixty-two percent of residents had performed outreach or an intervention based on their patient panel reports prior to the intervention, compared with 85% of residents post intervention, which was also a significant increase (P = .02). The increase post intervention was not 100%, as there were residents who either missed the initial workshop or who did not follow through with their planned intervention. Common interventions included the residents giving their coordinators a list of patients to call to schedule appointments, utilizing fellow team members (eg, pharmacists, social workers) for targeted patient outreach, or calling patients themselves to reestablish a connection.
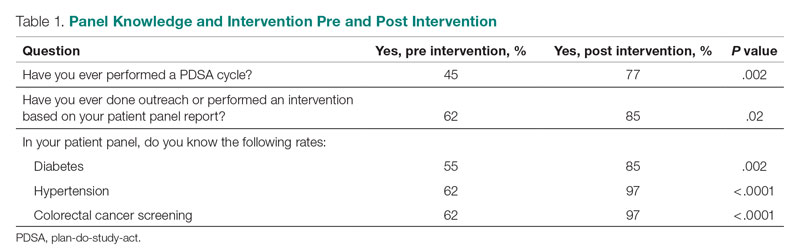
In terms of knowledge of their patient panels, prior to the intervention, 55%, 62%, and 62% of residents knew the rates of patients in their panel with diabetes, HTN, and colorectal cancer screening, respectively. After the intervention, the residents’ knowledge of these rates increased significantly, to 85% for diabetes (P = .002), 97% for HTN (P < .0001), and 97% for colorectal cancer screening (P < .0001).
Comfort With QI Approaches
Prior to the intervention, 82% of residents were comfortable managing their primary care panel, which did not change significantly post intervention (Table 2). The residents’ comfort with designing an aim statement did significantly increase, from 55% to 95% (P < .0001). The residents also had a significant increase in comfort with both designing and implementing a PDSA cycle. Prior to the intervention, 22% felt comfortable designing a PDSA cycle, which increased to 79% (P < .0001) post intervention, and 24% felt comfortable implementing a PDSA cycle, which increased to 77% (P < .0001) post intervention.
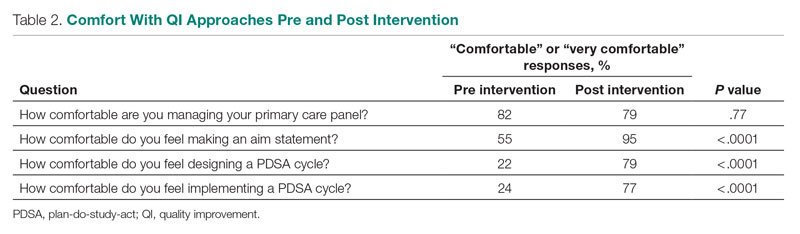
Patient Outcome Measures
The rate of HTN control in the residents' patient panels did not change significantly pre and post intervention (Table 3). The rate of resident patients who were up to date with colorectal cancer screening increased by 6.5% post intervention (P < .0001).
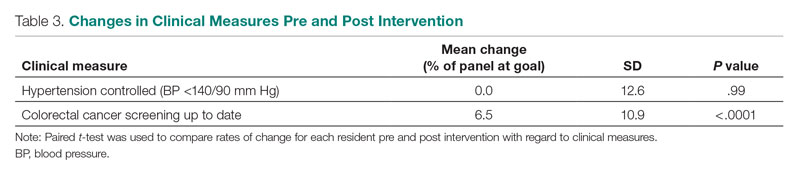
Interest in QI as a Career
As part of the survey, residents were asked how interested they were in making QI a part of their career. Fifty percent of residents indicated an interest in QI pre intervention, and 54% indicated an interest post intervention, which was not a significant difference (P = .72).
Discussion
In this study, we found that integration of a QI curriculum into a primary care rotation improved both residents’ knowledge of their patient panels and comfort with QI approaches, which translated to improvement in patient outcomes. Several previous studies have found improvements in resident self-assessment or knowledge after implementation of a QI curriculum.4-13 Liao et al implemented a longitudinal curriculum including both didactic and experiential components and found an improvement in both QI confidence and knowledge.3 Similarly, Duello et al8 found that a curriculum including both didactic lectures and QI projects improved subjective QI knowledge and comfort. Interestingly, Fok and Wong9 found that resident knowledge could be sustained post curriculum after completion of a QI project, suggesting that experiential learning may be helpful in maintaining knowledge.
Studies also have looked at providing performance data to residents. Hwang et al18 found that providing audit and feedback in the form of individual panel performance data to residents compared with practice targets led to statistically significant improvement in cancer screening rates and composite quality score, indicating that there is tremendous potential in providing residents with their data. While the ACGME mandates that residents should receive data on their quality metrics, on CLER visits, many residents interviewed noted limited access to data on their metrics and benchmarks.1,2
Though previous studies have individually looked at teaching QI concepts, providing panel data, or targeting select metrics, our study was unique in that it reviewed both self-reported resident outcomes data as well as actual patient outcomes. In addition to finding increased knowledge of patient panels and comfort with QI approaches, we found a significant increase in colorectal cancer screening rates post intervention. We thought this finding was particularly important given some data that residents' patients have been found to have worse outcomes on quality metrics compared with patients cared for by staff physicians.14,15 Given that having a resident physician as a PCP has been associated with failing to meet quality measures, it is especially important to focus targeted quality improvement initiatives in this patient population to reduce disparities in care.
We found that residents had improved knowledge on their patient panels as a result of this initiative. The residents were noted to have a higher knowledge of their HTN and colorectal cancer screening rates in comparison to their diabetes metrics. We suspect this is because residents are provided with multiple metrics related to diabetes, including process measures such as A1c testing, as well as outcome measures such as A1c control, so it may be harder for them to elucidate exactly how they are doing with their diabetes patients, whereas in HTN control and colorectal cancer screening, there is only 1 associated metric. Interestingly, even though HTN and colorectal cancer screening were the 2 measures focused on in the study, the residents had a significant improvement in knowledge of the rates of diabetes in their panel as well. This suggests that even just receiving data alone is valuable, hopefully translating to better outcomes with better baseline understanding of panels. We believe that our intervention was successful because it included both a didactic and an experiential component, as well as the use of individual panel performance data.
There were several limitations to our study. It was performed at a single institution, translating to a small sample size. Our data analysis was limited because we were unable to pair our pre- and postintervention survey responses because we used an anonymous survey. We also did not have full participation in postintervention surveys from all residents, which may have biased the study in favor of high performers. Another limitation was that our survey relied on self-reported outcomes for the questions about the residents knowing their patient panels.
This study required a 2-hour workshop every 3 weeks led by a faculty member trained in QI. Given the amount of time needed for the curriculum, this study may be difficult to replicate at other institutions, especially if faculty with an interest or training in QI are not available. Given our finding that residents had increased knowledge of their patient panels after receiving panel metrics, simply providing data with the goal of smaller, focused interventions may be easier to implement. At our institution, we discontinued the longer 2-hour QI workshops designed to teach QI approaches more broadly. We continue to provide individualized panel data to all residents during their primary care rotations and conduct half-hour, small group workshops with the interns that focus on drafting aim statements and planning interventions. All residents are required to submit worksheets to us at the end of their primary care blocks listing their current rates of each predetermined metric and laying out their aim statements and planned interventions. Residents also continue to receive feedback from our faculty with expertise in QI afterward on their plans and evidence of follow-through in the chart, with their preceptors included on the feedback emails. Even without the larger QI workshop, this approach has continued to be successful and appreciated. In fact, it does appear as though improvement in colorectal cancer screening has been sustained over several years. At the end of our study period, the resident patient colorectal cancer screening rate rose from 34% to 43%, and for the 2021-2022 academic year, the rate rose further, from 46% to 50%.
Given that the resident clinic patient population is at higher risk overall, targeted outreach and approaches to improve quality must be continued. Future areas of research include looking at which interventions, whether QI curriculum, provision of panel data, or required panel management interventions, translate to the greatest improvements in patient outcomes in this vulnerable population.
Conclusion
Our study showed that a dedicated QI curriculum for the residents and access to quality metric data improved both resident knowledge and comfort with QI approaches. Beyond resident-centered outcomes, there was also translation to improved patient outcomes, with a significant increase in colon cancer screening rates post intervention.
Corresponding author: Kinjalika Sathi, MD, 800 Washington St., Boston, MA 02111; [email protected]
Disclosures: None reported.
1. Accreditation Council for Graduate Medical Education. ACGME Common Program Requirements (Residency). Approved June 13, 2021. Updated July 1, 2022. Accessed December 29, 2022. https://www.acgme.org/globalassets/pfassets/programrequirements/cprresidency_2022v3.pdf
2. Koh NJ, Wagner R, Newton RC, et al; on behalf of the CLER Evaluation Committee and the CLER Program. CLER National Report of Findings 2021. Accreditation Council for Graduate Medical Education; 2021. Accessed December 29, 2022. https://www.acgme.org/globalassets/pdfs/cler/2021clernationalreportoffindings.pdf
3. Liao JM, Co JP, Kachalia A. Providing educational content and context for training the next generation of physicians in quality improvement. Acad Med. 2015;90(9):1241-1245. doi:10.1097/ACM.0000000000000799
4. Johnson KM, Fiordellisi W, Kuperman E, et al. X + Y = time for QI: meaningful engagement of residents in quality improvement during the ambulatory block. J Grad Med Educ. 2018;10(3):316-324. doi:10.4300/JGME-D-17-00761.1
5. Kesari K, Ali S, Smith S. Integrating residents with institutional quality improvement teams. Med Educ. 2017;51(11):1173. doi:10.1111/medu.13431
6. Ogrinc G, Cohen ES, van Aalst R, et al. Clinical and educational outcomes of an integrated inpatient quality improvement curriculum for internal medicine residents. J Grad Med Educ. 2016;8(4):563-568. doi:10.4300/JGME-D-15-00412.1
7. Malayala SV, Qazi KJ, Samdani AJ, et al. A multidisciplinary performance improvement rotation in an internal medicine training program. Int J Med Educ. 2016;7:212-213. doi:10.5116/ijme.5765.0bda
8. Duello K, Louh I, Greig H, et al. Residents’ knowledge of quality improvement: the impact of using a group project curriculum. Postgrad Med J. 2015;91(1078):431-435. doi:10.1136/postgradmedj-2014-132886
9. Fok MC, Wong RY. Impact of a competency based curriculum on quality improvement among internal medicine residents. BMC Med Educ. 2014;14:252. doi:10.1186/s12909-014-0252-7
10. Wilper AP, Smith CS, Weppner W. Instituting systems-based practice and practice-based learning and improvement: a curriculum of inquiry. Med Educ Online. 2013;18:21612. doi:10.3402/meo.v18i0.21612
11. Weigel C, Suen W, Gupte G. Using lean methodology to teach quality improvement to internal medicine residents at a safety net hospital. Am J Med Qual. 2013;28(5):392-399. doi:10.1177/1062860612474062
12. Tomolo AM, Lawrence RH, Watts B, et al. Pilot study evaluating a practice-based learning and improvement curriculum focusing on the development of system-level quality improvement skills. J Grad Med Educ. 2011;3(1):49-58. doi:10.4300/JGME-D-10-00104.1
13. Djuricich AM, Ciccarelli M, Swigonski NL. A continuous quality improvement curriculum for residents: addressing core competency, improving systems. Acad Med. 2004;79(10 Suppl):S65-S67. doi:10.1097/00001888-200410001-00020
14. Essien UR, He W, Ray A, et al. Disparities in quality of primary care by resident and staff physicians: is there a conflict between training and equity? J Gen Intern Med. 2019;34(7):1184-1191. doi:10.1007/s11606-019-04960-5
15. Amat M, Norian E, Graham KL. Unmasking a vulnerable patient care process: a qualitative study describing the current state of resident continuity clinic in a nationwide cohort of internal medicine residency programs. Am J Med. 2022;135(6):783-786. doi:10.1016/j.amjmed.2022.02.007
16. Wong BM, Etchells EE, Kuper A, et al. Teaching quality improvement and patient safety to trainees: a systematic review. Acad Med. 2010;85(9):1425-1439. doi:10.1097/ACM.0b013e3181e2d0c6
17. Armstrong G, Headrick L, Madigosky W, et al. Designing education to improve care. Jt Comm J Qual Patient Saf. 2012;38:5-14. doi:10.1016/s1553-7250(12)38002-1
18. Hwang AS, Harding AS, Chang Y, et al. An audit and feedback intervention to improve internal medicine residents’ performance on ambulatory quality measures: a randomized controlled trial. Popul Health Manag. 2019;22(6):529-535. doi:10.1089/pop.2018.0217
19. Institute for Healthcare Improvement. Open school. The paper airplane factory. Accessed December 29, 2022. https://www.ihi.org/education/IHIOpenSchool/resources/Pages/Activities/PaperAirplaneFactory.aspx
ABSTRACT
Objective: To teach internal medicine residents quality improvement (QI) principles in an effort to improve resident knowledge and comfort with QI, as well as address quality care gaps in resident clinic primary care patient panels.
Design: A QI curriculum was implemented for all residents rotating through a primary care block over a 6-month period. Residents completed Institute for Healthcare Improvement (IHI) modules, participated in a QI workshop, and received panel data reports, ultimately completing a plan-do-study-act (PDSA) cycle to improve colorectal cancer screening and hypertension control.
Setting and participants: This project was undertaken at Tufts Medical Center Primary Care, Boston, Massachusetts, the primary care teaching practice for all 75 internal medicine residents at Tufts Medical Center. All internal medicine residents were included, with 55 (73%) of the 75 residents completing the presurvey, and 39 (52%) completing the postsurvey.
Measurements: We administered a 10-question pre- and postsurvey looking at resident attitudes toward and comfort with QI and familiarity with their panel data as well as measured rates of colorectal cancer screening and hypertension control in resident panels.
Results: There was an increase in the numbers of residents who performed a PDSA cycle (P = .002), completed outreach based on their panel data (P = .02), and felt comfortable in both creating aim statements and designing and implementing PDSA cycles (P < .0001). The residents’ knowledge of their panel data significantly increased. There was no significant improvement in hypertension control, but there was an increase in colorectal cancer screening rates (P < .0001).
Conclusion: Providing panel data and performing targeted QI interventions can improve resident comfort with QI, translating to improvement in patient outcomes.
Keywords: quality improvement, resident education, medical education, care gaps, quality metrics.
As quality improvement (QI) has become an integral part of clinical practice, residency training programs have continued to evolve in how best to teach QI. The Accreditation Council for Graduate Medical Education (ACGME) Common Program requirements mandate that core competencies in residency programs include practice-based learning and improvement and systems-based practice.1 Residents should receive education in QI, receive data on quality metrics and benchmarks related to their patient population, and participate in QI activities. The Clinical Learning Environment Review (CLER) program was established to provide feedback to institutions on 6 focused areas, including patient safety and health care quality. In visits to institutions across the United States, the CLER committees found that many residents had limited knowledge of QI concepts and limited access to data on quality metrics and benchmarks.2
There are many barriers to implementing a QI curriculum in residency programs, and creating and maintaining successful strategies has proven challenging.3 Many QI curricula for internal medicine residents have been described in the literature, but the results of many of these studies focus on resident self-assessment of QI knowledge and numbers of projects rather than on patient outcomes.4-13 As there is some evidence suggesting that patients treated by residents have worse outcomes on ambulatory quality measures when compared with patients treated by staff physicians,14,15 it is important to also look at patient outcomes when evaluating a QI curriculum. Experts in education recommend the following to optimize learning: exposure to both didactic and experiential opportunities, connection to health system improvement efforts, and assessment of patient outcomes in addition to learner feedback.16,17 A study also found that providing panel data to residents could improve quality metrics.18
In this study, we sought to investigate the effects of a resident QI intervention during an ambulatory block on both residents’ self-assessments of QI knowledge and attitudes as well as on patient quality metrics.
Methods
Curriculum
We implemented this educational initiative at Tufts Medical Center Primary Care, Boston, Massachusetts, the primary care teaching practice for all 75 internal medicine residents at Tufts Medical Center. Co-located with the 415-bed academic medical center in downtown Boston, the practice serves more than 40,000 patients, approximately 7000 of whom are cared for by resident primary care physicians (PCPs). The internal medicine residents rotate through the primary care clinic as part of continuity clinic during ambulatory or elective blocks. In addition to continuity clinic, the residents have 2 dedicated 3-week primary care rotations during the course of an academic year. Primary care rotations consist of 5 clinic sessions a week as well as structured teaching sessions. Each resident inherits a panel of patients from an outgoing senior resident, with an average panel size of 96 patients per resident.
Prior to this study intervention, we did not do any formal QI teaching to our residents as part of their primary care curriculum, and previous panel management had focused more on chart reviews of patients whom residents perceived to be higher risk. Residents from all 3 years were included in the intervention. We taught a QI curriculum to our residents from January 2018 to June 2018 during the 3-week primary care rotation, which consisted of the following components:
- Institute for Healthcare Improvement (IHI) module QI 102 completed independently online.
- A 2-hour QI workshop led by 1 of 2 primary care faculty with backgrounds in QI, during which residents were taught basic principles of QI, including how to craft aim statements and design plan-do-study-act (PDSA) cycles, and participated in a hands-on QI activity designed to model rapid cycle improvement (the Paper Airplane Factory19).
- Distribution of individualized reports of residents’ patient panel data by email at the start of the primary care block that detailed patients’ overall rates of colorectal cancer screening and hypertension (HTN) control, along with the average resident panel rates and the average attending panel rates. The reports also included a list of all residents’ patients who were overdue for colorectal cancer screening or whose last blood pressure (BP) was uncontrolled (systolic BP ≥ 140 mm Hg or diastolic BP ≥ 90 mm Hg). These reports were originally designed by our practice’s QI team and run and exported in Microsoft Excel format monthly by our information technology (IT) administrator.
- Instruction on aim statements as a group, followed by the expectation that each resident create an individualized aim statement tailored to each resident’s patient panel rates, with the PDSA cycle to be implemented during the remainder of the primary care rotation, focusing on improvement of colorectal cancer screening and HTN control (see supplementary eFigure 1 online for the worksheet used for the workshop).
- Residents were held accountable for their interventions by various check-ins. At the end of the primary care block, residents were required to submit their completed worksheets showing the intervention they had undertaken and when it was performed. The 2 primary care attendings primarily responsible for QI education would review the resident’s work approximately 1 to 2 months after they submitted their worksheets describing their intervention. These attendings sent the residents personalized feedback based on whether the intervention had been completed or successful as evidenced by documentation in the chart, including direct patient outreach by phone, letter, or portal; outreach to the resident coordinator; scheduled follow-up appointment; or booking or completion of colorectal cancer screening. Along with this feedback, residents were also sent suggestions for next steps. Resident preceptors were copied on the email to facilitate reinforcement of the goals and plans. Finally, the resident preceptors also helped with accountability by going through the residents’ worksheets and patient panel metrics with the residents during biannual evaluations.

Evaluation
Residents were surveyed with a 10-item questionnaire pre and post intervention regarding their attitudes toward QI, understanding of QI principles, and familiarity with their patient panel data. Surveys were anonymous and distributed via the SurveyMonkey platform (see supplementary eFigure 2 online). Residents were asked if they had ever performed a PDSA cycle, performed patient outreach, or performed an intervention and whether they knew the rates of diabetes, HTN, and colorectal cancer screening in their patient panels. Questions rated on a 5-point Likert scale were used to assess comfort with panel management, developing an aim statement, designing and implementing a PDSA cycle, as well as interest in pursuing QI as a career. For the purposes of analysis, these questions were dichotomized into “somewhat comfortable” and “very comfortable” vs “neutral,” “somewhat uncomfortable,” and “very uncomfortable.” Similarly, we dichotomized the question about interest in QI as a career into “somewhat interested” and “very interested” vs “neutral,” “somewhat disinterested,” and “very disinterested.” As the surveys were anonymous, we were unable to pair the pre- and postintervention surveys and used a chi-square test to evaluate whether there was an association between survey assessments pre intervention vs post intervention and a positive or negative response to the question.

We also examined rates of HTN control and colorectal cancer screening in all 75 resident panels pre and post intervention. The paired t-test was used to determine whether the mean change from pre to post intervention was significant. SAS 9.4 (SAS Institute Inc.) was used for all analyses. Institutional Review Board exemption was obtained from the Tufts Medical Center IRB. There was no funding received for this study.
Results
Respondents
Of the 75 residents, 55 (73%) completed the survey prior to the intervention, and 39 (52%) completed the survey after the intervention.
Panel Knowledge and Intervention
Prior to the intervention, 45% of residents had performed a PDSA cycle, compared with 77% post intervention, which was a significant increase (P = .002) (Table 1). Sixty-two percent of residents had performed outreach or an intervention based on their patient panel reports prior to the intervention, compared with 85% of residents post intervention, which was also a significant increase (P = .02). The increase post intervention was not 100%, as there were residents who either missed the initial workshop or who did not follow through with their planned intervention. Common interventions included the residents giving their coordinators a list of patients to call to schedule appointments, utilizing fellow team members (eg, pharmacists, social workers) for targeted patient outreach, or calling patients themselves to reestablish a connection.

In terms of knowledge of their patient panels, prior to the intervention, 55%, 62%, and 62% of residents knew the rates of patients in their panel with diabetes, HTN, and colorectal cancer screening, respectively. After the intervention, the residents’ knowledge of these rates increased significantly, to 85% for diabetes (P = .002), 97% for HTN (P < .0001), and 97% for colorectal cancer screening (P < .0001).
Comfort With QI Approaches
Prior to the intervention, 82% of residents were comfortable managing their primary care panel, which did not change significantly post intervention (Table 2). The residents’ comfort with designing an aim statement did significantly increase, from 55% to 95% (P < .0001). The residents also had a significant increase in comfort with both designing and implementing a PDSA cycle. Prior to the intervention, 22% felt comfortable designing a PDSA cycle, which increased to 79% (P < .0001) post intervention, and 24% felt comfortable implementing a PDSA cycle, which increased to 77% (P < .0001) post intervention.

Patient Outcome Measures
The rate of HTN control in the residents' patient panels did not change significantly pre and post intervention (Table 3). The rate of resident patients who were up to date with colorectal cancer screening increased by 6.5% post intervention (P < .0001).

Interest in QI as a Career
As part of the survey, residents were asked how interested they were in making QI a part of their career. Fifty percent of residents indicated an interest in QI pre intervention, and 54% indicated an interest post intervention, which was not a significant difference (P = .72).
Discussion
In this study, we found that integration of a QI curriculum into a primary care rotation improved both residents’ knowledge of their patient panels and comfort with QI approaches, which translated to improvement in patient outcomes. Several previous studies have found improvements in resident self-assessment or knowledge after implementation of a QI curriculum.4-13 Liao et al implemented a longitudinal curriculum including both didactic and experiential components and found an improvement in both QI confidence and knowledge.3 Similarly, Duello et al8 found that a curriculum including both didactic lectures and QI projects improved subjective QI knowledge and comfort. Interestingly, Fok and Wong9 found that resident knowledge could be sustained post curriculum after completion of a QI project, suggesting that experiential learning may be helpful in maintaining knowledge.
Studies also have looked at providing performance data to residents. Hwang et al18 found that providing audit and feedback in the form of individual panel performance data to residents compared with practice targets led to statistically significant improvement in cancer screening rates and composite quality score, indicating that there is tremendous potential in providing residents with their data. While the ACGME mandates that residents should receive data on their quality metrics, on CLER visits, many residents interviewed noted limited access to data on their metrics and benchmarks.1,2
Though previous studies have individually looked at teaching QI concepts, providing panel data, or targeting select metrics, our study was unique in that it reviewed both self-reported resident outcomes data as well as actual patient outcomes. In addition to finding increased knowledge of patient panels and comfort with QI approaches, we found a significant increase in colorectal cancer screening rates post intervention. We thought this finding was particularly important given some data that residents' patients have been found to have worse outcomes on quality metrics compared with patients cared for by staff physicians.14,15 Given that having a resident physician as a PCP has been associated with failing to meet quality measures, it is especially important to focus targeted quality improvement initiatives in this patient population to reduce disparities in care.
We found that residents had improved knowledge on their patient panels as a result of this initiative. The residents were noted to have a higher knowledge of their HTN and colorectal cancer screening rates in comparison to their diabetes metrics. We suspect this is because residents are provided with multiple metrics related to diabetes, including process measures such as A1c testing, as well as outcome measures such as A1c control, so it may be harder for them to elucidate exactly how they are doing with their diabetes patients, whereas in HTN control and colorectal cancer screening, there is only 1 associated metric. Interestingly, even though HTN and colorectal cancer screening were the 2 measures focused on in the study, the residents had a significant improvement in knowledge of the rates of diabetes in their panel as well. This suggests that even just receiving data alone is valuable, hopefully translating to better outcomes with better baseline understanding of panels. We believe that our intervention was successful because it included both a didactic and an experiential component, as well as the use of individual panel performance data.
There were several limitations to our study. It was performed at a single institution, translating to a small sample size. Our data analysis was limited because we were unable to pair our pre- and postintervention survey responses because we used an anonymous survey. We also did not have full participation in postintervention surveys from all residents, which may have biased the study in favor of high performers. Another limitation was that our survey relied on self-reported outcomes for the questions about the residents knowing their patient panels.
This study required a 2-hour workshop every 3 weeks led by a faculty member trained in QI. Given the amount of time needed for the curriculum, this study may be difficult to replicate at other institutions, especially if faculty with an interest or training in QI are not available. Given our finding that residents had increased knowledge of their patient panels after receiving panel metrics, simply providing data with the goal of smaller, focused interventions may be easier to implement. At our institution, we discontinued the longer 2-hour QI workshops designed to teach QI approaches more broadly. We continue to provide individualized panel data to all residents during their primary care rotations and conduct half-hour, small group workshops with the interns that focus on drafting aim statements and planning interventions. All residents are required to submit worksheets to us at the end of their primary care blocks listing their current rates of each predetermined metric and laying out their aim statements and planned interventions. Residents also continue to receive feedback from our faculty with expertise in QI afterward on their plans and evidence of follow-through in the chart, with their preceptors included on the feedback emails. Even without the larger QI workshop, this approach has continued to be successful and appreciated. In fact, it does appear as though improvement in colorectal cancer screening has been sustained over several years. At the end of our study period, the resident patient colorectal cancer screening rate rose from 34% to 43%, and for the 2021-2022 academic year, the rate rose further, from 46% to 50%.
Given that the resident clinic patient population is at higher risk overall, targeted outreach and approaches to improve quality must be continued. Future areas of research include looking at which interventions, whether QI curriculum, provision of panel data, or required panel management interventions, translate to the greatest improvements in patient outcomes in this vulnerable population.
Conclusion
Our study showed that a dedicated QI curriculum for the residents and access to quality metric data improved both resident knowledge and comfort with QI approaches. Beyond resident-centered outcomes, there was also translation to improved patient outcomes, with a significant increase in colon cancer screening rates post intervention.
Corresponding author: Kinjalika Sathi, MD, 800 Washington St., Boston, MA 02111; [email protected]
Disclosures: None reported.
ABSTRACT
Objective: To teach internal medicine residents quality improvement (QI) principles in an effort to improve resident knowledge and comfort with QI, as well as address quality care gaps in resident clinic primary care patient panels.
Design: A QI curriculum was implemented for all residents rotating through a primary care block over a 6-month period. Residents completed Institute for Healthcare Improvement (IHI) modules, participated in a QI workshop, and received panel data reports, ultimately completing a plan-do-study-act (PDSA) cycle to improve colorectal cancer screening and hypertension control.
Setting and participants: This project was undertaken at Tufts Medical Center Primary Care, Boston, Massachusetts, the primary care teaching practice for all 75 internal medicine residents at Tufts Medical Center. All internal medicine residents were included, with 55 (73%) of the 75 residents completing the presurvey, and 39 (52%) completing the postsurvey.
Measurements: We administered a 10-question pre- and postsurvey looking at resident attitudes toward and comfort with QI and familiarity with their panel data as well as measured rates of colorectal cancer screening and hypertension control in resident panels.
Results: There was an increase in the numbers of residents who performed a PDSA cycle (P = .002), completed outreach based on their panel data (P = .02), and felt comfortable in both creating aim statements and designing and implementing PDSA cycles (P < .0001). The residents’ knowledge of their panel data significantly increased. There was no significant improvement in hypertension control, but there was an increase in colorectal cancer screening rates (P < .0001).
Conclusion: Providing panel data and performing targeted QI interventions can improve resident comfort with QI, translating to improvement in patient outcomes.
Keywords: quality improvement, resident education, medical education, care gaps, quality metrics.
As quality improvement (QI) has become an integral part of clinical practice, residency training programs have continued to evolve in how best to teach QI. The Accreditation Council for Graduate Medical Education (ACGME) Common Program requirements mandate that core competencies in residency programs include practice-based learning and improvement and systems-based practice.1 Residents should receive education in QI, receive data on quality metrics and benchmarks related to their patient population, and participate in QI activities. The Clinical Learning Environment Review (CLER) program was established to provide feedback to institutions on 6 focused areas, including patient safety and health care quality. In visits to institutions across the United States, the CLER committees found that many residents had limited knowledge of QI concepts and limited access to data on quality metrics and benchmarks.2
There are many barriers to implementing a QI curriculum in residency programs, and creating and maintaining successful strategies has proven challenging.3 Many QI curricula for internal medicine residents have been described in the literature, but the results of many of these studies focus on resident self-assessment of QI knowledge and numbers of projects rather than on patient outcomes.4-13 As there is some evidence suggesting that patients treated by residents have worse outcomes on ambulatory quality measures when compared with patients treated by staff physicians,14,15 it is important to also look at patient outcomes when evaluating a QI curriculum. Experts in education recommend the following to optimize learning: exposure to both didactic and experiential opportunities, connection to health system improvement efforts, and assessment of patient outcomes in addition to learner feedback.16,17 A study also found that providing panel data to residents could improve quality metrics.18
In this study, we sought to investigate the effects of a resident QI intervention during an ambulatory block on both residents’ self-assessments of QI knowledge and attitudes as well as on patient quality metrics.
Methods
Curriculum
We implemented this educational initiative at Tufts Medical Center Primary Care, Boston, Massachusetts, the primary care teaching practice for all 75 internal medicine residents at Tufts Medical Center. Co-located with the 415-bed academic medical center in downtown Boston, the practice serves more than 40,000 patients, approximately 7000 of whom are cared for by resident primary care physicians (PCPs). The internal medicine residents rotate through the primary care clinic as part of continuity clinic during ambulatory or elective blocks. In addition to continuity clinic, the residents have 2 dedicated 3-week primary care rotations during the course of an academic year. Primary care rotations consist of 5 clinic sessions a week as well as structured teaching sessions. Each resident inherits a panel of patients from an outgoing senior resident, with an average panel size of 96 patients per resident.
Prior to this study intervention, we did not do any formal QI teaching to our residents as part of their primary care curriculum, and previous panel management had focused more on chart reviews of patients whom residents perceived to be higher risk. Residents from all 3 years were included in the intervention. We taught a QI curriculum to our residents from January 2018 to June 2018 during the 3-week primary care rotation, which consisted of the following components:
- Institute for Healthcare Improvement (IHI) module QI 102 completed independently online.
- A 2-hour QI workshop led by 1 of 2 primary care faculty with backgrounds in QI, during which residents were taught basic principles of QI, including how to craft aim statements and design plan-do-study-act (PDSA) cycles, and participated in a hands-on QI activity designed to model rapid cycle improvement (the Paper Airplane Factory19).
- Distribution of individualized reports of residents’ patient panel data by email at the start of the primary care block that detailed patients’ overall rates of colorectal cancer screening and hypertension (HTN) control, along with the average resident panel rates and the average attending panel rates. The reports also included a list of all residents’ patients who were overdue for colorectal cancer screening or whose last blood pressure (BP) was uncontrolled (systolic BP ≥ 140 mm Hg or diastolic BP ≥ 90 mm Hg). These reports were originally designed by our practice’s QI team and run and exported in Microsoft Excel format monthly by our information technology (IT) administrator.
- Instruction on aim statements as a group, followed by the expectation that each resident create an individualized aim statement tailored to each resident’s patient panel rates, with the PDSA cycle to be implemented during the remainder of the primary care rotation, focusing on improvement of colorectal cancer screening and HTN control (see supplementary eFigure 1 online for the worksheet used for the workshop).
- Residents were held accountable for their interventions by various check-ins. At the end of the primary care block, residents were required to submit their completed worksheets showing the intervention they had undertaken and when it was performed. The 2 primary care attendings primarily responsible for QI education would review the resident’s work approximately 1 to 2 months after they submitted their worksheets describing their intervention. These attendings sent the residents personalized feedback based on whether the intervention had been completed or successful as evidenced by documentation in the chart, including direct patient outreach by phone, letter, or portal; outreach to the resident coordinator; scheduled follow-up appointment; or booking or completion of colorectal cancer screening. Along with this feedback, residents were also sent suggestions for next steps. Resident preceptors were copied on the email to facilitate reinforcement of the goals and plans. Finally, the resident preceptors also helped with accountability by going through the residents’ worksheets and patient panel metrics with the residents during biannual evaluations.

Evaluation
Residents were surveyed with a 10-item questionnaire pre and post intervention regarding their attitudes toward QI, understanding of QI principles, and familiarity with their patient panel data. Surveys were anonymous and distributed via the SurveyMonkey platform (see supplementary eFigure 2 online). Residents were asked if they had ever performed a PDSA cycle, performed patient outreach, or performed an intervention and whether they knew the rates of diabetes, HTN, and colorectal cancer screening in their patient panels. Questions rated on a 5-point Likert scale were used to assess comfort with panel management, developing an aim statement, designing and implementing a PDSA cycle, as well as interest in pursuing QI as a career. For the purposes of analysis, these questions were dichotomized into “somewhat comfortable” and “very comfortable” vs “neutral,” “somewhat uncomfortable,” and “very uncomfortable.” Similarly, we dichotomized the question about interest in QI as a career into “somewhat interested” and “very interested” vs “neutral,” “somewhat disinterested,” and “very disinterested.” As the surveys were anonymous, we were unable to pair the pre- and postintervention surveys and used a chi-square test to evaluate whether there was an association between survey assessments pre intervention vs post intervention and a positive or negative response to the question.

We also examined rates of HTN control and colorectal cancer screening in all 75 resident panels pre and post intervention. The paired t-test was used to determine whether the mean change from pre to post intervention was significant. SAS 9.4 (SAS Institute Inc.) was used for all analyses. Institutional Review Board exemption was obtained from the Tufts Medical Center IRB. There was no funding received for this study.
Results
Respondents
Of the 75 residents, 55 (73%) completed the survey prior to the intervention, and 39 (52%) completed the survey after the intervention.
Panel Knowledge and Intervention
Prior to the intervention, 45% of residents had performed a PDSA cycle, compared with 77% post intervention, which was a significant increase (P = .002) (Table 1). Sixty-two percent of residents had performed outreach or an intervention based on their patient panel reports prior to the intervention, compared with 85% of residents post intervention, which was also a significant increase (P = .02). The increase post intervention was not 100%, as there were residents who either missed the initial workshop or who did not follow through with their planned intervention. Common interventions included the residents giving their coordinators a list of patients to call to schedule appointments, utilizing fellow team members (eg, pharmacists, social workers) for targeted patient outreach, or calling patients themselves to reestablish a connection.

In terms of knowledge of their patient panels, prior to the intervention, 55%, 62%, and 62% of residents knew the rates of patients in their panel with diabetes, HTN, and colorectal cancer screening, respectively. After the intervention, the residents’ knowledge of these rates increased significantly, to 85% for diabetes (P = .002), 97% for HTN (P < .0001), and 97% for colorectal cancer screening (P < .0001).
Comfort With QI Approaches
Prior to the intervention, 82% of residents were comfortable managing their primary care panel, which did not change significantly post intervention (Table 2). The residents’ comfort with designing an aim statement did significantly increase, from 55% to 95% (P < .0001). The residents also had a significant increase in comfort with both designing and implementing a PDSA cycle. Prior to the intervention, 22% felt comfortable designing a PDSA cycle, which increased to 79% (P < .0001) post intervention, and 24% felt comfortable implementing a PDSA cycle, which increased to 77% (P < .0001) post intervention.

Patient Outcome Measures
The rate of HTN control in the residents' patient panels did not change significantly pre and post intervention (Table 3). The rate of resident patients who were up to date with colorectal cancer screening increased by 6.5% post intervention (P < .0001).

Interest in QI as a Career
As part of the survey, residents were asked how interested they were in making QI a part of their career. Fifty percent of residents indicated an interest in QI pre intervention, and 54% indicated an interest post intervention, which was not a significant difference (P = .72).
Discussion
In this study, we found that integration of a QI curriculum into a primary care rotation improved both residents’ knowledge of their patient panels and comfort with QI approaches, which translated to improvement in patient outcomes. Several previous studies have found improvements in resident self-assessment or knowledge after implementation of a QI curriculum.4-13 Liao et al implemented a longitudinal curriculum including both didactic and experiential components and found an improvement in both QI confidence and knowledge.3 Similarly, Duello et al8 found that a curriculum including both didactic lectures and QI projects improved subjective QI knowledge and comfort. Interestingly, Fok and Wong9 found that resident knowledge could be sustained post curriculum after completion of a QI project, suggesting that experiential learning may be helpful in maintaining knowledge.
Studies also have looked at providing performance data to residents. Hwang et al18 found that providing audit and feedback in the form of individual panel performance data to residents compared with practice targets led to statistically significant improvement in cancer screening rates and composite quality score, indicating that there is tremendous potential in providing residents with their data. While the ACGME mandates that residents should receive data on their quality metrics, on CLER visits, many residents interviewed noted limited access to data on their metrics and benchmarks.1,2
Though previous studies have individually looked at teaching QI concepts, providing panel data, or targeting select metrics, our study was unique in that it reviewed both self-reported resident outcomes data as well as actual patient outcomes. In addition to finding increased knowledge of patient panels and comfort with QI approaches, we found a significant increase in colorectal cancer screening rates post intervention. We thought this finding was particularly important given some data that residents' patients have been found to have worse outcomes on quality metrics compared with patients cared for by staff physicians.14,15 Given that having a resident physician as a PCP has been associated with failing to meet quality measures, it is especially important to focus targeted quality improvement initiatives in this patient population to reduce disparities in care.
We found that residents had improved knowledge on their patient panels as a result of this initiative. The residents were noted to have a higher knowledge of their HTN and colorectal cancer screening rates in comparison to their diabetes metrics. We suspect this is because residents are provided with multiple metrics related to diabetes, including process measures such as A1c testing, as well as outcome measures such as A1c control, so it may be harder for them to elucidate exactly how they are doing with their diabetes patients, whereas in HTN control and colorectal cancer screening, there is only 1 associated metric. Interestingly, even though HTN and colorectal cancer screening were the 2 measures focused on in the study, the residents had a significant improvement in knowledge of the rates of diabetes in their panel as well. This suggests that even just receiving data alone is valuable, hopefully translating to better outcomes with better baseline understanding of panels. We believe that our intervention was successful because it included both a didactic and an experiential component, as well as the use of individual panel performance data.
There were several limitations to our study. It was performed at a single institution, translating to a small sample size. Our data analysis was limited because we were unable to pair our pre- and postintervention survey responses because we used an anonymous survey. We also did not have full participation in postintervention surveys from all residents, which may have biased the study in favor of high performers. Another limitation was that our survey relied on self-reported outcomes for the questions about the residents knowing their patient panels.
This study required a 2-hour workshop every 3 weeks led by a faculty member trained in QI. Given the amount of time needed for the curriculum, this study may be difficult to replicate at other institutions, especially if faculty with an interest or training in QI are not available. Given our finding that residents had increased knowledge of their patient panels after receiving panel metrics, simply providing data with the goal of smaller, focused interventions may be easier to implement. At our institution, we discontinued the longer 2-hour QI workshops designed to teach QI approaches more broadly. We continue to provide individualized panel data to all residents during their primary care rotations and conduct half-hour, small group workshops with the interns that focus on drafting aim statements and planning interventions. All residents are required to submit worksheets to us at the end of their primary care blocks listing their current rates of each predetermined metric and laying out their aim statements and planned interventions. Residents also continue to receive feedback from our faculty with expertise in QI afterward on their plans and evidence of follow-through in the chart, with their preceptors included on the feedback emails. Even without the larger QI workshop, this approach has continued to be successful and appreciated. In fact, it does appear as though improvement in colorectal cancer screening has been sustained over several years. At the end of our study period, the resident patient colorectal cancer screening rate rose from 34% to 43%, and for the 2021-2022 academic year, the rate rose further, from 46% to 50%.
Given that the resident clinic patient population is at higher risk overall, targeted outreach and approaches to improve quality must be continued. Future areas of research include looking at which interventions, whether QI curriculum, provision of panel data, or required panel management interventions, translate to the greatest improvements in patient outcomes in this vulnerable population.
Conclusion
Our study showed that a dedicated QI curriculum for the residents and access to quality metric data improved both resident knowledge and comfort with QI approaches. Beyond resident-centered outcomes, there was also translation to improved patient outcomes, with a significant increase in colon cancer screening rates post intervention.
Corresponding author: Kinjalika Sathi, MD, 800 Washington St., Boston, MA 02111; [email protected]
Disclosures: None reported.
1. Accreditation Council for Graduate Medical Education. ACGME Common Program Requirements (Residency). Approved June 13, 2021. Updated July 1, 2022. Accessed December 29, 2022. https://www.acgme.org/globalassets/pfassets/programrequirements/cprresidency_2022v3.pdf
2. Koh NJ, Wagner R, Newton RC, et al; on behalf of the CLER Evaluation Committee and the CLER Program. CLER National Report of Findings 2021. Accreditation Council for Graduate Medical Education; 2021. Accessed December 29, 2022. https://www.acgme.org/globalassets/pdfs/cler/2021clernationalreportoffindings.pdf
3. Liao JM, Co JP, Kachalia A. Providing educational content and context for training the next generation of physicians in quality improvement. Acad Med. 2015;90(9):1241-1245. doi:10.1097/ACM.0000000000000799
4. Johnson KM, Fiordellisi W, Kuperman E, et al. X + Y = time for QI: meaningful engagement of residents in quality improvement during the ambulatory block. J Grad Med Educ. 2018;10(3):316-324. doi:10.4300/JGME-D-17-00761.1
5. Kesari K, Ali S, Smith S. Integrating residents with institutional quality improvement teams. Med Educ. 2017;51(11):1173. doi:10.1111/medu.13431
6. Ogrinc G, Cohen ES, van Aalst R, et al. Clinical and educational outcomes of an integrated inpatient quality improvement curriculum for internal medicine residents. J Grad Med Educ. 2016;8(4):563-568. doi:10.4300/JGME-D-15-00412.1
7. Malayala SV, Qazi KJ, Samdani AJ, et al. A multidisciplinary performance improvement rotation in an internal medicine training program. Int J Med Educ. 2016;7:212-213. doi:10.5116/ijme.5765.0bda
8. Duello K, Louh I, Greig H, et al. Residents’ knowledge of quality improvement: the impact of using a group project curriculum. Postgrad Med J. 2015;91(1078):431-435. doi:10.1136/postgradmedj-2014-132886
9. Fok MC, Wong RY. Impact of a competency based curriculum on quality improvement among internal medicine residents. BMC Med Educ. 2014;14:252. doi:10.1186/s12909-014-0252-7
10. Wilper AP, Smith CS, Weppner W. Instituting systems-based practice and practice-based learning and improvement: a curriculum of inquiry. Med Educ Online. 2013;18:21612. doi:10.3402/meo.v18i0.21612
11. Weigel C, Suen W, Gupte G. Using lean methodology to teach quality improvement to internal medicine residents at a safety net hospital. Am J Med Qual. 2013;28(5):392-399. doi:10.1177/1062860612474062
12. Tomolo AM, Lawrence RH, Watts B, et al. Pilot study evaluating a practice-based learning and improvement curriculum focusing on the development of system-level quality improvement skills. J Grad Med Educ. 2011;3(1):49-58. doi:10.4300/JGME-D-10-00104.1
13. Djuricich AM, Ciccarelli M, Swigonski NL. A continuous quality improvement curriculum for residents: addressing core competency, improving systems. Acad Med. 2004;79(10 Suppl):S65-S67. doi:10.1097/00001888-200410001-00020
14. Essien UR, He W, Ray A, et al. Disparities in quality of primary care by resident and staff physicians: is there a conflict between training and equity? J Gen Intern Med. 2019;34(7):1184-1191. doi:10.1007/s11606-019-04960-5
15. Amat M, Norian E, Graham KL. Unmasking a vulnerable patient care process: a qualitative study describing the current state of resident continuity clinic in a nationwide cohort of internal medicine residency programs. Am J Med. 2022;135(6):783-786. doi:10.1016/j.amjmed.2022.02.007
16. Wong BM, Etchells EE, Kuper A, et al. Teaching quality improvement and patient safety to trainees: a systematic review. Acad Med. 2010;85(9):1425-1439. doi:10.1097/ACM.0b013e3181e2d0c6
17. Armstrong G, Headrick L, Madigosky W, et al. Designing education to improve care. Jt Comm J Qual Patient Saf. 2012;38:5-14. doi:10.1016/s1553-7250(12)38002-1
18. Hwang AS, Harding AS, Chang Y, et al. An audit and feedback intervention to improve internal medicine residents’ performance on ambulatory quality measures: a randomized controlled trial. Popul Health Manag. 2019;22(6):529-535. doi:10.1089/pop.2018.0217
19. Institute for Healthcare Improvement. Open school. The paper airplane factory. Accessed December 29, 2022. https://www.ihi.org/education/IHIOpenSchool/resources/Pages/Activities/PaperAirplaneFactory.aspx
1. Accreditation Council for Graduate Medical Education. ACGME Common Program Requirements (Residency). Approved June 13, 2021. Updated July 1, 2022. Accessed December 29, 2022. https://www.acgme.org/globalassets/pfassets/programrequirements/cprresidency_2022v3.pdf
2. Koh NJ, Wagner R, Newton RC, et al; on behalf of the CLER Evaluation Committee and the CLER Program. CLER National Report of Findings 2021. Accreditation Council for Graduate Medical Education; 2021. Accessed December 29, 2022. https://www.acgme.org/globalassets/pdfs/cler/2021clernationalreportoffindings.pdf
3. Liao JM, Co JP, Kachalia A. Providing educational content and context for training the next generation of physicians in quality improvement. Acad Med. 2015;90(9):1241-1245. doi:10.1097/ACM.0000000000000799
4. Johnson KM, Fiordellisi W, Kuperman E, et al. X + Y = time for QI: meaningful engagement of residents in quality improvement during the ambulatory block. J Grad Med Educ. 2018;10(3):316-324. doi:10.4300/JGME-D-17-00761.1
5. Kesari K, Ali S, Smith S. Integrating residents with institutional quality improvement teams. Med Educ. 2017;51(11):1173. doi:10.1111/medu.13431
6. Ogrinc G, Cohen ES, van Aalst R, et al. Clinical and educational outcomes of an integrated inpatient quality improvement curriculum for internal medicine residents. J Grad Med Educ. 2016;8(4):563-568. doi:10.4300/JGME-D-15-00412.1
7. Malayala SV, Qazi KJ, Samdani AJ, et al. A multidisciplinary performance improvement rotation in an internal medicine training program. Int J Med Educ. 2016;7:212-213. doi:10.5116/ijme.5765.0bda
8. Duello K, Louh I, Greig H, et al. Residents’ knowledge of quality improvement: the impact of using a group project curriculum. Postgrad Med J. 2015;91(1078):431-435. doi:10.1136/postgradmedj-2014-132886
9. Fok MC, Wong RY. Impact of a competency based curriculum on quality improvement among internal medicine residents. BMC Med Educ. 2014;14:252. doi:10.1186/s12909-014-0252-7
10. Wilper AP, Smith CS, Weppner W. Instituting systems-based practice and practice-based learning and improvement: a curriculum of inquiry. Med Educ Online. 2013;18:21612. doi:10.3402/meo.v18i0.21612
11. Weigel C, Suen W, Gupte G. Using lean methodology to teach quality improvement to internal medicine residents at a safety net hospital. Am J Med Qual. 2013;28(5):392-399. doi:10.1177/1062860612474062
12. Tomolo AM, Lawrence RH, Watts B, et al. Pilot study evaluating a practice-based learning and improvement curriculum focusing on the development of system-level quality improvement skills. J Grad Med Educ. 2011;3(1):49-58. doi:10.4300/JGME-D-10-00104.1
13. Djuricich AM, Ciccarelli M, Swigonski NL. A continuous quality improvement curriculum for residents: addressing core competency, improving systems. Acad Med. 2004;79(10 Suppl):S65-S67. doi:10.1097/00001888-200410001-00020
14. Essien UR, He W, Ray A, et al. Disparities in quality of primary care by resident and staff physicians: is there a conflict between training and equity? J Gen Intern Med. 2019;34(7):1184-1191. doi:10.1007/s11606-019-04960-5
15. Amat M, Norian E, Graham KL. Unmasking a vulnerable patient care process: a qualitative study describing the current state of resident continuity clinic in a nationwide cohort of internal medicine residency programs. Am J Med. 2022;135(6):783-786. doi:10.1016/j.amjmed.2022.02.007
16. Wong BM, Etchells EE, Kuper A, et al. Teaching quality improvement and patient safety to trainees: a systematic review. Acad Med. 2010;85(9):1425-1439. doi:10.1097/ACM.0b013e3181e2d0c6
17. Armstrong G, Headrick L, Madigosky W, et al. Designing education to improve care. Jt Comm J Qual Patient Saf. 2012;38:5-14. doi:10.1016/s1553-7250(12)38002-1
18. Hwang AS, Harding AS, Chang Y, et al. An audit and feedback intervention to improve internal medicine residents’ performance on ambulatory quality measures: a randomized controlled trial. Popul Health Manag. 2019;22(6):529-535. doi:10.1089/pop.2018.0217
19. Institute for Healthcare Improvement. Open school. The paper airplane factory. Accessed December 29, 2022. https://www.ihi.org/education/IHIOpenSchool/resources/Pages/Activities/PaperAirplaneFactory.aspx
Diagnostic Errors in Hospitalized Patients
Abstract
Diagnostic errors in hospitalized patients are a leading cause of preventable morbidity and mortality. Significant challenges in defining and measuring diagnostic errors and underlying process failure points have led to considerable variability in reported rates of diagnostic errors and adverse outcomes. In this article, we explore the diagnostic process and its discrete components, emphasizing the centrality of the patient in decision-making as well as the continuous nature of the process. We review the incidence of diagnostic errors in hospitalized patients and different methodological approaches that have been used to arrive at these estimates. We discuss different but interdependent provider- and system-related process-failure points that lead to diagnostic errors. We examine specific challenges related to measurement of diagnostic errors and describe traditional and novel approaches that are being used to obtain the most precise estimates. Finally, we examine various patient-, provider-, and organizational-level interventions that have been proposed to improve diagnostic safety in hospitalized patients.
Keywords: diagnostic error, hospital medicine, patient safety.
Diagnosis is defined as a “pre-existing set of categories agreed upon by the medical profession to designate a specific condition.”1 The diagnostic process involves obtaining a clinical history, performing a physical examination, conducting diagnostic testing, and consulting with other clinical providers to gather data that are relevant to understanding the underlying disease processes. This exercise involves generating hypotheses and updating prior probabilities as more information and evidence become available. Throughout this process of information gathering, integration, and interpretation, there is an ongoing assessment of whether sufficient and necessary knowledge has been obtained to make an accurate diagnosis and provide appropriate treatment.2
Diagnostic error is defined as a missed opportunity to make a timely diagnosis as part of this iterative process, including the failure of communicating the diagnosis to the patient in a timely manner.3 It can be categorized as a missed, delayed, or incorrect diagnosis based on available evidence at the time. Establishing the correct diagnosis has important implications. A timely and precise diagnosis ensures the patient the highest probability of having a positive health outcome that reflects an appropriate understanding of underlying disease processes and is consistent with their overall goals of care.3 When diagnostic errors occur, they can cause patient harm. Adverse events due to medical errors, including diagnostic errors, are estimated to be the third leading cause of death in the United States.4 Most people will experience at least 1 diagnostic error in their lifetime. In the 2015 National Academy of Medicine report Improving Diagnosis in Healthcare, diagnostic errors were identified as a major hazard as well as an opportunity to improve patient outcomes.2
Diagnostic errors during hospitalizations are especially concerning, as they are more likely to be implicated in a wider spectrum of harm, including permanent disability and death. This has become even more relevant for hospital medicine physicians and other clinical providers as they encounter increasing cognitive and administrative workloads, rising dissatisfaction and burnout, and unique obstacles such as night-time scheduling.5
Incidence of Diagnostic Errors in Hospitalized Patients
Several methodological approaches have been used to estimate the incidence of diagnostic errors in hospitalized patients. These include retrospective reviews of a sample of all hospital admissions, evaluations of selected adverse outcomes including autopsy studies, patient and provider surveys, and malpractice claims. Laboratory testing audits and secondary reviews in other diagnostic subspecialities (eg, radiology, pathology, and microbiology) are also essential to improving diagnostic performance in these specialized fields, which in turn affects overall hospital diagnostic error rates.6-8 These diverse approaches provide unique insights regarding our ability to assess the degree to which potential harms, ranging from temporary impairment to permanent disability, to death, are attributable to different failure points in the diagnostic process.
Large retrospective chart reviews of random hospital admissions remain the most accurate way to determine the overall incidence of diagnostic errors in hospitalized patients.9 The Harvard Medical Practice Study, published in 1991, laid the groundwork for measuring the incidence of adverse events in hospitalized patients and assessing their relation to medical error, negligence, and disability. Reviewing 30,121 randomly selected records from 51 randomly selected acute care hospitals in New York State, the study found that adverse events occurred in 3.7% of hospitalizations, diagnostic errors accounted for 13.8% of these events, and these errors were likely attributable to negligence in 74.7% of cases. The study not only outlined individual-level process failures, but also focused attention on some of the systemic causes, setting the agenda for quality improvement research in hospital-based care for years to come.10-12 A recent systematic review and meta-analysis of 22 hospital admission studies found a pooled rate of 0.7% (95% CI, 0.5%-1.1%) for harmful diagnostic errors.9 It found significant variations in the rates of adverse events, diagnostic errors, and range of diagnoses that were missed. This was primarily because of variabilities in pre-test probabilities in detecting diagnostic errors in these specific cohorts, as well as due to heterogeneity in study definitions and methodologies, especially regarding how they defined and measured “diagnostic error.” The analysis, however, did not account for diagnostic errors that were not related to patient harm (missed opportunities); therefore, it likely significantly underestimated the true incidence of diagnostic errors in these study populations. Table 1 summarizes some of key studies that have examined the incidence of harmful diagnostic errors in hospitalized patients.9-21
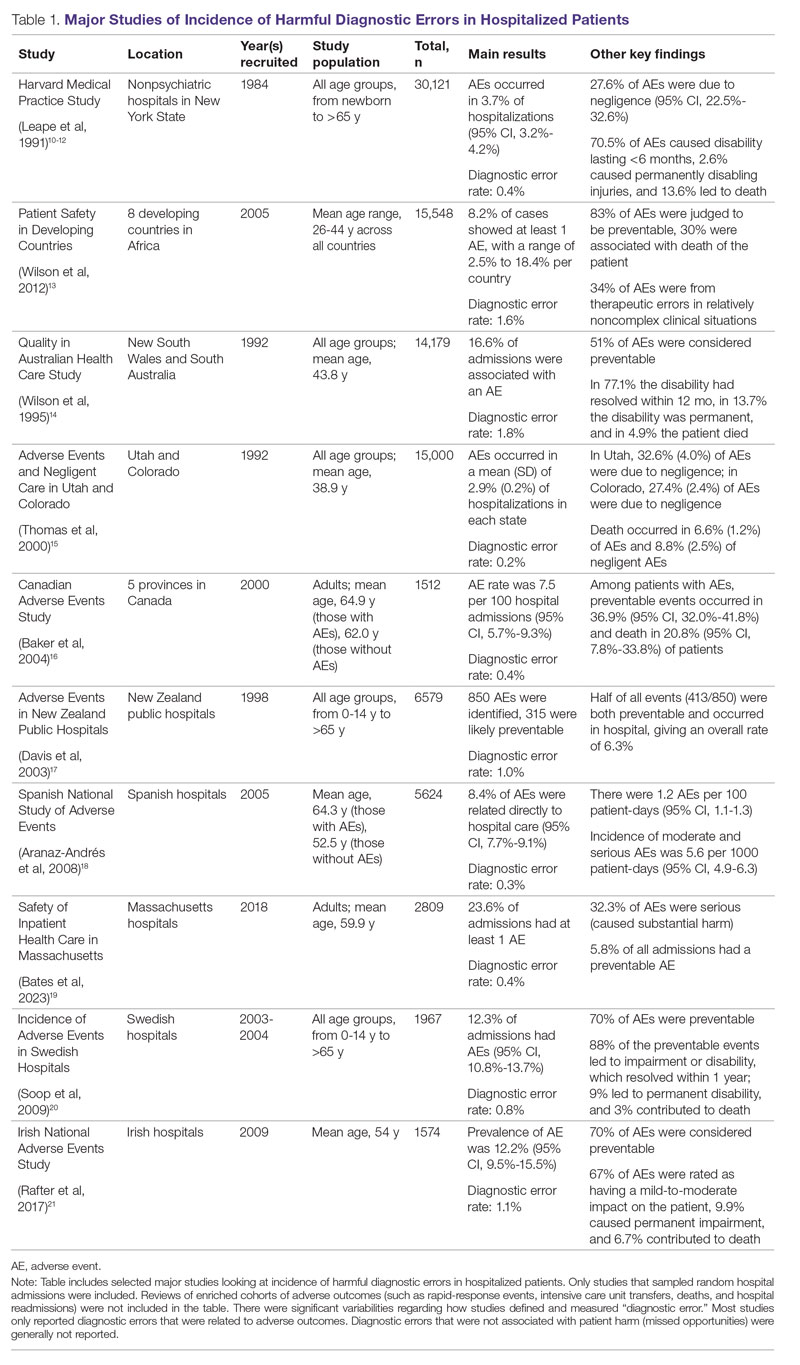
The chief limitation of reviewing random hospital admissions is that, since overall rates of diagnostic errors are still relatively low, a large number of case reviews are required to identify a sufficient sample of adverse outcomes to gain a meaningful understanding of the underlying process failure points and develop tools for remediation. Patient and provider surveys or data from malpractice claims can be high-yield starting points for research on process errors.22,23 Reviews of enriched cohorts of adverse outcomes, such as rapid-response events, intensive care unit (ICU) transfers, deaths, and hospital readmissions, can be an efficient way to identify process failures that lead to greatest harm. Depending on the research approach and the types of underlying patient populations sampled, rates of diagnostic errors in these high-risk groups have been estimated to be approximately 5% to 20%, or even higher.6,24-31 For example, a retrospective study of 391 cases of unplanned 7-day readmissions found that 5.6% of cases contained at least 1 diagnostic error during the index admission.32 In a study conducted at 6 Belgian acute-care hospitals, 56% of patients requiring an unplanned transfer to a higher level of care were determined to have had an adverse event, and of these adverse events, 12.4% of cases were associated with errors in diagnosis.29 A systematic review of 16 hospital-based studies estimated that 3.1% of all inpatient deaths were likely preventable, which corresponded to 22,165 deaths annually in the United States.30 Another such review of 31 autopsy studies reported that 28% of autopsied ICU patients had at least 1 misdiagnosis; of these diagnostic errors, 8% were classified as potentially lethal, and 15% were considered major but not lethal.31 Significant drawbacks of such enriched cohort studies, however, are their poor generalizability and inability to detect failure points that do not lead to patient harm (near-miss events).33
Causes of Diagnostic Errors in Hospitalized Patients
All aspects of the diagnostic process are susceptible to errors. These errors stem from a variety of faulty processes, including failure of the patient to engage with the health care system (eg, due to lack of insurance or transportation, or delay in seeking care); failure in information gathering (eg, missed history or exam findings, ordering wrong tests, laboratory errors); failure in information interpretation (eg, exam finding or test result misinterpretation); inaccurate hypothesis generation (eg, due to suboptimal prioritization or weighing of supporting evidence); and failure in communication (eg, with other team members or with the patient).2,34 Reasons for diagnostic process failures vary widely across different health care settings. While clinician assessment errors (eg, failure to consider or alternatively overweigh competing diagnoses) and errors in testing and the monitoring phase (eg, failure to order or follow up diagnostic tests) can lead to a majority of diagnostic errors in some patient populations, in other settings, social (eg, poor health literacy, punitive cultural practices) and economic factors (eg, lack of access to appropriate diagnostic tests or to specialty expertise) play a more prominent role.34,35
The Figure describes the relationship between components of the diagnostic process and subsequent outcomes, including diagnostic process failures, diagnostic errors, and absence or presence of patient harm.2,36,37 It reemphasizes the centrality of the patient in decision-making and the continuous nature of the process. The Figure also illustrates that only a minority of process failures result in diagnostic errors, and a smaller proportion of diagnostic errors actually lead to patient harm. Conversely, it also shows that diagnostic errors can happen without any obvious process-failure points, and, similarly, patient harm can take place in the absence of any evident diagnostic errors.36-38 Finally, it highlights the need to incorporate feedback from process failures, diagnostic errors, and favorable and unfavorable patient outcomes in order to inform future quality improvement efforts and research.
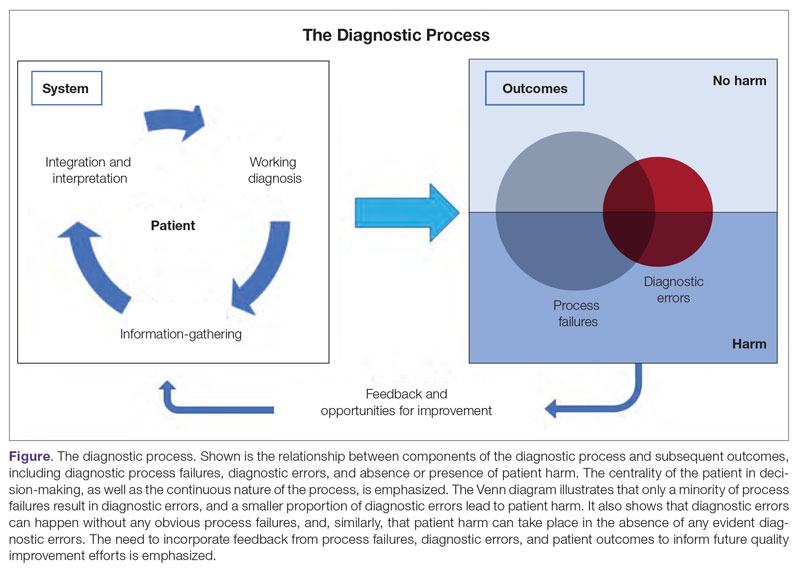
A significant proportion of diagnostic errors are due to system-related vulnerabilities, such as limitations in availability, adoption or quality of work force training, health informatics resources, and diagnostic capabilities. Lack of institutional culture that promotes safety and transparency also predisposes to diagnostic errors.39,40 The other major domain of process failures is related to cognitive errors in clinician decision-making. Anchoring, confirmation bias, availability bias, and base-rate neglect are some of the common cognitive biases that, along with personality traits (aversion to risk or ambiguity, overconfidence) and affective biases (influence of emotion on decision-making), often determine the degree of utilization of resources and the possibility of suboptimal diagnostic performance.41,42 Further, implicit biases related to age, race, gender, and sexual orientation contribute to disparities in access to health care and outcomes.43 In a large number of cases of preventable adverse outcomes, however, there are multiple interdependent individual and system-related failure points that lead to diagnostic error and patient harm.6,32
Challenges in Defining and Measuring Diagnostic Errors
In order to develop effective, evidence-based interventions to reduce diagnostic errors in hospitalized patients, it is essential to be able to first operationally define, and then accurately measure, diagnostic errors and the process failures that contribute to these errors in a standardized way that is reproducible across different settings.6,44 There are a number of obstacles in this endeavor.
A fundamental problem is that establishing a diagnosis is not a single act but a process. Patterns of symptoms and clinical presentations often differ for the same disease. Information required to make a diagnosis is usually gathered in stages, where the clinician obtains additional data, while considering many possibilities, of which 1 may be ultimately correct. Diagnoses evolve over time and in different care settings. “The most likely diagnosis” is not always the same as “the final correct diagnosis.” Moreover, the diagnostic process is influenced by patients’ individual clinical courses and preferences over time. This makes determination of missed, delayed, or incorrect diagnoses challenging.45,46
For hospitalized patients, generally the goal is to first rule out more serious and acute conditions (eg, pulmonary embolism or stroke), even if their probability is rather low. Conversely, a diagnosis that appears less consequential if delayed (eg, chronic anemia of unclear etiology) might not be pursued on an urgent basis, and is often left to outpatient providers to examine, but still may manifest in downstream harm (eg, delayed diagnosis of gastrointestinal malignancy or recurrent admissions for heart failure due to missed iron-deficiency anemia). Therefore, coming up with disease diagnosis likelihoods in hindsight may turn out to be highly subjective and not always accurate. This can be particularly difficult when clinician and other team deliberations are not recorded in their entirety.47
Another hurdle in the practice of diagnostic medicine is to preserve the balance between underdiagnosing versus pursuing overly aggressive diagnostic approaches. Conducting laboratory, imaging, or other diagnostic studies without a clear shared understanding of how they would affect clinical decision-making (eg, use of prostate-specific antigen to detect prostate cancer) not only leads to increased costs but can also delay appropriate care. Worse, subsequent unnecessary diagnostic tests and treatments can sometimes lead to serious harm.48,49
Finally, retrospective reviews by clinicians are subject to multiple potential limitations that include failure to create well-defined research questions, poorly developed inclusion and exclusion criteria, and issues related to inter- and intra-rater reliability.50 These methodological deficiencies can occur despite following "best practice" guidelines during the study planning, execution, and analysis phases. They further add to the challenge of defining and measuring diagnostic errors.47
Strategies to Improve Measurement of Diagnostic Errors
Development of new methodologies to reliably measure diagnostic errors is an area of active research. The advancement of uniform and universally agreed-upon frameworks to define and identify process failure points and diagnostic errors would help reduce measurement error and support development and testing of interventions that could be generalizable across different health care settings. To more accurately define and measure diagnostic errors, several novel approaches have been proposed (Table 2).
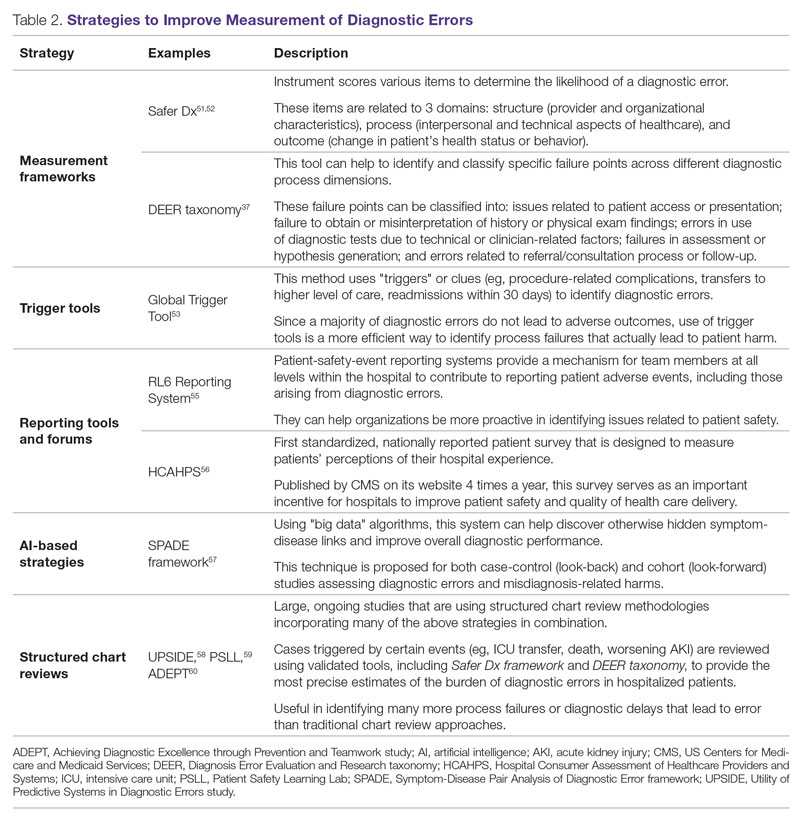
The Safer Dx framework is an all-round tool developed to advance the discipline of measuring diagnostic errors. For an episode of care under review, the instrument scores various items to determine the likelihood of a diagnostic error. These items evaluate multiple dimensions affecting diagnostic performance and measurements across 3 broad domains: structure (provider and organizational characteristics—from everyone involved with patient care, to computing infrastructure, to policies and regulations), process (elements of the patient-provider encounter, diagnostic test performance and follow-up, and subspecialty- and referral-specific factors), and outcome (establishing accurate and timely diagnosis as opposed to missed, delayed, or incorrect diagnosis). This instrument has been revised and can be further modified by a variety of stakeholders, including clinicians, health care organizations, and policymakers, to identify potential diagnostic errors in a standardized way for patient safety and quality improvement research.51,52
Use of standardized tools, such as the Diagnosis Error Evaluation and Research (DEER) taxonomy, can help to identify and classify specific failure points across different diagnostic process dimensions.37 These failure points can be classified into: issues related to patient presentation or access to health care; failure to obtain or misinterpretation of history or physical exam findings; errors in use of diagnostics tests due to technical or clinician-related factors; failures in appropriate weighing of evidence and hypothesis generation; errors associated with referral or consultation process; and failure to monitor the patient or obtain timely follow-up.34 The DEER taxonomy can also be modified based on specific research questions and study populations. Further, it can be recategorized to correspond to Safer Dx framework diagnostic process dimensions to provide insights into reasons for specific process failures and to develop new interventions to mitigate errors and patient harm.6
Since a majority of diagnostic errors do not lead to actual harm, use of “triggers” or clues (eg, procedure-related complications, patient falls, transfers to a higher level of care, readmissions within 30 days) can be a more efficient method to identify diagnostic errors and adverse events that do cause harm. The Global Trigger Tool, developed by the Institute for Healthcare Improvement, uses this strategy. This tool has been shown to identify a significantly higher number of serious adverse events than comparable methods.53 This facilitates selection and development of strategies at the institutional level that are most likely to improve patient outcomes.24
Encouraging and facilitating voluntary or prompted reporting from patients and clinicians can also play an important role in capturing diagnostic errors. Patients and clinicians are not only the key stakeholders but are also uniquely placed within the diagnostic process to detect and report potential errors.25,54 Patient-safety-event reporting systems, such as RL6, play a vital role in reporting near-misses and adverse events. These systems provide a mechanism for team members at all levels within the hospital to contribute toward reporting patient adverse events, including those arising from diagnostic errors.55 The Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey is the first standardized, nationally reported patient survey designed to measure patients’ perceptions of their hospital experience. The US Centers for Medicare and Medicaid Services (CMS) publishes HCAHPS results on its website 4 times a year, which serves as an important incentive for hospitals to improve patient safety and quality of health care delivery.56
Another novel approach links multiple symptoms to a range of target diseases using the Symptom-Disease Pair Analysis of Diagnostic Error (SPADE) framework. Using “big data” technologies, this technique can help discover otherwise hidden symptom-disease links and improve overall diagnostic performance. This approach is proposed for both case-control (look-back) and cohort (look-forward) studies assessing diagnostic errors and misdiagnosis-related harms. For example, starting with a known diagnosis with high potential for harm (eg, stroke), the “look-back” approach can be used to identify high-risk symptoms (eg, dizziness, vertigo). In the “look-forward” approach, a single symptom or exposure risk factor known to be frequently misdiagnosed (eg, dizziness) can be analyzed to identify potential adverse disease outcomes (eg, stroke, migraine).57
Many large ongoing studies looking at diagnostic errors among hospitalized patients, such as Utility of Predictive Systems to identify Inpatient Diagnostic Errors (UPSIDE),58 Patient Safety Learning Lab (PSLL),59 and Achieving Diagnostic Excellence through Prevention and Teamwork (ADEPT),60 are using structured chart review methodologies incorporating many of the above strategies in combination. Cases triggered by certain events (eg, ICU transfer, death, rapid response event, new or worsening acute kidney injury) are reviewed using validated tools, including Safer Dx framework and DEER taxonomy, to provide the most precise estimates of the burden of diagnostic errors in hospitalized patients. These estimates may be much higher than previously predicted using traditional chart review approaches.6,24 For example, a recently published study of 2809 random admissions in 11 Massachusetts hospitals identified 978 adverse events but only 10 diagnostic errors (diagnostic error rate, 0.4%).19 This was likely because the trigger method used in the study did not specifically examine the diagnostic process as critically as done by the Safer Dx framework and DEER taxonomy tools, thereby underestimating the total number of diagnostic errors. Further, these ongoing studies (eg, UPSIDE, ADEPT) aim to employ new and upcoming advanced machine-learning methods to create models that can improve overall diagnostic performance. This would pave the way to test and build novel, efficient, and scalable interventions to reduce diagnostic errors and improve patient outcomes.
Strategies to Improve Diagnostic Safety in Hospitalized Patients
Disease-specific biomedical research, as well as advances in laboratory, imaging, and other technologies, play a critical role in improving diagnostic accuracy. However, these technical approaches do not address many of the broader clinician- and system-level failure points and opportunities for improvement. Various patient-, provider-, and organizational-level interventions that could make diagnostic processes more resilient and reduce the risk of error and patient harm have been proposed.61
Among these strategies are approaches to empower patients and their families. Fostering therapeutic relationships between patients and members of the care team is essential to reducing diagnostic errors.62 Facilitating timely access to health records, ensuring transparency in decision making, and tailoring communication strategies to patients’ cultural and educational backgrounds can reduce harm.63 Similarly, at the system level, enhancing communication among different providers by use of tools such as structured handoffs can prevent communication breakdowns and facilitate positive outcomes.64
Interventions targeted at individual health care providers, such as educational programs to improve content-specific knowledge, can enhance diagnostic performance. Regular feedback, strategies to enhance equity, and fostering an environment where all providers are actively encouraged to think critically and participate in the diagnostic process (training programs to use “diagnostic time-outs” and making it a “team sport”) can improve clinical reasoning.65,66 Use of standardized patients can help identify individual-level cognitive failure points and facilitate creation of new interventions to improve clinical decision-making processes.67
Novel health information technologies can further augment these efforts. These include effective documentation by maintaining dynamic and accurate patient histories, problem lists, and medication lists68-70; use of electronic health record–based algorithms to identify potential diagnostic delays for serious conditions71,72; use of telemedicine technologies to improve accessibility and coordination73; application of mobile health and wearable technologies to facilitate data-gathering and care delivery74,75; and use of computerized decision-support tools, including applications to interpret electrocardiograms, imaging studies, and other diagnostic tests.76
Use of precision medicine, powered by new artificial intelligence (AI) tools, is becoming more widespread. Algorithms powered by AI can augment and sometimes even outperform clinician decision-making in areas such as oncology, radiology, and primary care.77 Creation of large biobanks like the All of Us research program can be used to study thousands of environmental and genetic risk factors and health conditions simultaneously, and help identify specific treatments that work best for people of different backgrounds.78 Active research in these areas holds great promise in terms of how and when we diagnose diseases and make appropriate preventative and treatment decisions. Significant scientific, ethical, and regulatory challenges will need to be overcome before these technologies can address some of the most complex problems in health care.79
Finally, diagnostic performance is affected by the external environment, including the functioning of the medical liability system. Diagnostic errors that lead to patient harm are a leading cause of malpractice claims.80 Developing a legal environment, in collaboration with patient advocacy groups and health care organizations, that promotes and facilitates timely disclosure of diagnostic errors could decrease the incentive to hide errors, advance care processes, and improve outcomes.81,82
Conclusion
The burden of diagnostic errors in hospitalized patients is unacceptably high and remains an underemphasized cause of preventable morbidity and mortality. Diagnostic errors often result from a breakdown in multiple interdependent processes that involve patient-, provider-, and system-level factors. Significant challenges remain in defining and identifying diagnostic errors as well as underlying process-failure points. The most effective interventions to reduce diagnostic errors will require greater patient participation in the diagnostic process and a mix of evidence-based interventions that promote individual-provider excellence as well as system-level changes. Further research and collaboration among various stakeholders should help improve diagnostic safety for hospitalized patients.
Corresponding author: Abhishek Goyal, MD, MPH; [email protected]
Disclosures: Dr. Dalal disclosed receiving income ≥ $250 from MayaMD.
1. Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch Intern Med. 2005;165(13):1493-1499. doi:10.1001/archinte.165.13.1493
2. National Academies of Sciences, Engineering, and Medicine. 2015. Improving Diagnosis in Health Care. The National Academies Press. doi:10.17226/21794
3. Singh H, Graber ML. Improving diagnosis in health care—the next imperative for patient safety. N Engl J Med. 2015;373(26):2493-2495. doi:10.1056/NEJMp1512241
4. Makary MA, Daniel M. Medical error—the third leading cause of death in the US. BMJ. 2016;353:i2139. doi:10.1136/bmj.i2139
5. Flanders SA, Centor B, Weber V, McGinn T, Desalvo K, Auerbach A. Challenges and opportunities in academic hospital medicine: report from the academic hospital medicine summit. J Gen Intern Med. 2009;24(5):636-641. doi:10.1007/s11606-009-0944-6
6. Griffin JA, Carr K, Bersani K, et al. Analyzing diagnostic errors in the acute setting: a process-driven approach. Diagnosis (Berl). 2021;9(1):77-88. doi:10.1515/dx-2021-0033
7. Itri JN, Tappouni RR, McEachern RO, Pesch AJ, Patel SH. Fundamentals of diagnostic error in imaging. RadioGraphics. 2018;38(6):1845-1865. doi:10.1148/rg.2018180021
8. Hammerling JA. A Review of medical errors in laboratory diagnostics and where we are today. Lab Med. 2012;43(2):41-44. doi:10.1309/LM6ER9WJR1IHQAUY
9. Gunderson CG, Bilan VP, Holleck JL, et al. Prevalence of harmful diagnostic errors in hospitalised adults: a systematic review and meta-analysis. BMJ Qual Saf. 2020;29(12):1008-1018. doi:10.1136/bmjqs-2019-010822
10. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370-376. doi:10.1056/NEJM199102073240604
11. Leape LL, Brennan TA, Laird N, et al. The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II. N Engl J Med. 1991;324(6):377-384. doi:10.1056/NEJM199102073240605
12. Localio AR, Lawthers AG, Brennan TA, et al. Relation between malpractice claims and adverse events due to negligence. Results of the Harvard Medical Practice Study III. N Engl J Med. 1991;325(4):245-251. doi:10.1056/NEJM199107253250405
13. Wilson RM, Michel P, Olsen S, et al. Patient safety in developing countries: retrospective estimation of scale and nature of harm to patients in hospital. BMJ. 2012;344:e832. doi:10.1136/bmj.e832
14. Wilson RM, Runciman WB, Gibberd RW, Harrison BT, Newby L, Hamilton JD. The Quality in Australian Health Care Study. Med J Aust. 1995;163(9):458-471. doi:10.5694/j.1326-5377.1995.tb124691.x
15. Thomas EJ, Studdert DM, Burstin HR, et al. Incidence and types of adverse events and negligent care in Utah and Colorado. Med Care. 2000;38(3):261-271. doi:10.1097/00005650-200003000-00003
16. Baker GR, Norton PG, Flintoft V, et al. The Canadian Adverse Events Study: the incidence of adverse events among hospital patients in Canada. CMAJ. 2004;170(11):1678-1686. doi:10.1503/cmaj.1040498
17. Davis P, Lay-Yee R, Briant R, Ali W, Scott A, Schug S. Adverse events in New Zealand public hospitals II: preventability and clinical context. N Z Med J. 2003;116(1183):U624.
18. Aranaz-Andrés JM, Aibar-Remón C, Vitaller-Murillo J, et al. Incidence of adverse events related to health care in Spain: results of the Spanish National Study of Adverse Events. J Epidemiol Community Health. 2008;62(12):1022-1029. doi:10.1136/jech.2007.065227
19. Bates DW, Levine DM, Salmasian H, et al. The safety of inpatient health care. N Engl J Med. 2023;388(2):142-153. doi:10.1056/NEJMsa2206117
20. Soop M, Fryksmark U, Köster M, Haglund B. The incidence of adverse events in Swedish hospitals: a retrospective medical record review study. Int J Qual Health Care. 2009;21(4):285-291. doi:10.1093/intqhc/mzp025
21. Rafter N, Hickey A, Conroy RM, et al. The Irish National Adverse Events Study (INAES): the frequency and nature of adverse events in Irish hospitals—a retrospective record review study. BMJ Qual Saf. 2017;26(2):111-119. doi:10.1136/bmjqs-2015-004828
22. Blendon RJ, DesRoches CM, Brodie M, et al. Views of practicing physicians and the public on medical errors. N Engl J Med. 2002;347(24):1933-1940. doi:10.1056/NEJMsa022151
23. Saber Tehrani AS, Lee H, Mathews SC, et al. 25-year summary of US malpractice claims for diagnostic errors 1986-2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672-680. doi:10.1136/bmjqs-2012-001550
24. Malik MA, Motta-Calderon D, Piniella N, et al. A structured approach to EHR surveillance of diagnostic error in acute care: an exploratory analysis of two institutionally-defined case cohorts. Diagnosis (Berl). 2022;9(4):446-457. doi:10.1515/dx-2022-0032
25. Graber ML. The incidence of diagnostic error in medicine. BMJ Qual Saf. 2013;22(suppl 2):ii21-ii27. doi:10.1136/bmjqs-2012-001615
26. Bergl PA, Taneja A, El-Kareh R, Singh H, Nanchal RS. Frequency, risk factors, causes, and consequences of diagnostic errors in critically ill medical patients: a retrospective cohort study. Crit Care Med. 2019;47(11):e902-e910. doi:10.1097/CCM.0000000000003976
27. Hogan H, Healey F, Neale G, Thomson R, Vincent C, Black N. Preventable deaths due to problems in care in English acute hospitals: a retrospective case record review study. BMJ Qual Saf. 2012;21(9):737-745. doi:10.1136/bmjqs-2011-001159
28. Bergl PA, Nanchal RS, Singh H. Diagnostic error in the critically ill: defining the problem and exploring next steps to advance intensive care unit safety. Ann Am Thorac Soc. 2018;15(8):903-907. doi:10.1513/AnnalsATS.201801-068PS
29. Marquet K, Claes N, De Troy E, et al. One fourth of unplanned transfers to a higher level of care are associated with a highly preventable adverse event: a patient record review in six Belgian hospitals. Crit Care Med. 2015;43(5):1053-1061. doi:10.1097/CCM.0000000000000932
30. Rodwin BA, Bilan VP, Merchant NB, et al. Rate of preventable mortality in hospitalized patients: a systematic review and meta-analysis. J Gen Intern Med. 2020;35(7):2099-2106. doi:10.1007/s11606-019-05592-5
31. Winters B, Custer J, Galvagno SM, et al. Diagnostic errors in the intensive care unit: a systematic review of autopsy studies. BMJ Qual Saf. 2012;21(11):894-902. doi:10.1136/bmjqs-2012-000803
32. Raffel KE, Kantor MA, Barish P, et al. Prevalence and characterisation of diagnostic error among 7-day all-cause hospital medicine readmissions: a retrospective cohort study. BMJ Qual Saf. 2020;29(12):971-979. doi:10.1136/bmjqs-2020-010896
33. Weingart SN, Pagovich O, Sands DZ, et al. What can hospitalized patients tell us about adverse events? learning from patient-reported incidents. J Gen Intern Med. 2005;20(9):830-836. doi:10.1111/j.1525-1497.2005.0180.x
34. Schiff GD, Hasan O, Kim S, et al. Diagnostic error in medicine: analysis of 583 physician-reported errors. Arch Intern Med. 2009;169(20):1881-1887. doi:10.1001/archinternmed.2009.333
35. Singh H, Schiff GD, Graber ML, Onakpoya I, Thompson MJ. The global burden of diagnostic errors in primary care. BMJ Qual Saf. 2017;26(6):484-494. doi:10.1136/bmjqs-2016-005401
36. Schiff GD, Leape LL. Commentary: how can we make diagnosis safer? Acad Med J Assoc Am Med Coll. 2012;87(2):135-138. doi:10.1097/ACM.0b013e31823f711c
37. Schiff GD, Kim S, Abrams R, et al. Diagnosing diagnosis errors: lessons from a multi-institutional collaborative project. In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds. Advances in Patient Safety: From Research to Implementation. Volume 2: Concepts and Methodology. AHRQ Publication No. 05-0021-2. Agency for Healthcare Research and Quality (US); 2005. Accessed January 16, 2023. http://www.ncbi.nlm.nih.gov/books/NBK20492/
38. Newman-Toker DE. A unified conceptual model for diagnostic errors: underdiagnosis, overdiagnosis, and misdiagnosis. Diagnosis (Berl). 2014;1(1):43-48. doi:10.1515/dx-2013-0027
39. Abimanyi-Ochom J, Bohingamu Mudiyanselage S, Catchpool M, Firipis M, Wanni Arachchige Dona S, Watts JJ. Strategies to reduce diagnostic errors: a systematic review. BMC Med Inform Decis Mak. 2019;19(1):174. doi:10.1186/s12911-019-0901-1
40. Gupta A, Harrod M, Quinn M, et al. Mind the overlap: how system problems contribute to cognitive failure and diagnostic errors. Diagnosis (Berl). 2018;5(3):151-156. doi:10.1515/dx-2018-0014
41. Saposnik G, Redelmeier D, Ruff CC, Tobler PN. Cognitive biases associated with medical decisions: a systematic review. BMC Med Inform Decis Mak. 2016;16:138. doi:10.1186/s12911-016-0377-1
42. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78(8):775-780. doi: 10.1097/00001888-200308000-00003
43. Chapman EN, Kaatz A, Carnes M. Physicians and implicit bias: how doctors may unwittingly perpetuate health care disparities. J Gen Intern Med. 2013;28(11):1504-1510. doi:10.1007/s11606-013-2441-1
44. Zwaan L, Singh H. The challenges in defining and measuring diagnostic error. Diagnosis (Ber). 2015;2(2):97-103. doi:10.1515/dx-2014-0069
45. Arkes HR, Wortmann RL, Saville PD, Harkness AR. Hindsight bias among physicians weighing the likelihood of diagnoses. J Appl Psychol. 1981;66(2):252-254.
46. Singh H. Editorial: Helping health care organizations to define diagnostic errors as missed opportunities in diagnosis. Jt Comm J Qual Patient Saf. 2014;40(3):99-101. doi:10.1016/s1553-7250(14)40012-6
47. Vassar M, Holzmann M. The retrospective chart review: important methodological considerations. J Educ Eval Health Prof. 2013;10:12. doi:10.3352/jeehp.2013.10.12
48. Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102(9):605-613. doi:10.1093/jnci/djq099
49. Moynihan R, Doust J, Henry D. Preventing overdiagnosis: how to stop harming the healthy. BMJ. 2012;344:e3502. doi:10.1136/bmj.e3502
50. Hayward RA, Hofer TP. Estimating hospital deaths due to medical errors: preventability is in the eye of the reviewer. JAMA. 2001;286(4):415-420. doi:10.1001/jama.286.4.415
51. Singh H, Sittig DF. Advancing the science of measurement of diagnostic errors in healthcare: the Safer Dx framework. BMJ Qual Saf. 2015;24(2):103-110. doi:10.1136/bmjqs-2014-003675
52. Singh H, Khanna A, Spitzmueller C, Meyer AND. Recommendations for using the Revised Safer Dx Instrument to help measure and improve diagnostic safety. Diagnosis (Berl). 2019;6(4):315-323. doi:10.1515/dx-2019-0012
53. Classen DC, Resar R, Griffin F, et al. “Global trigger tool” shows that adverse events in hospitals may be ten times greater than previously measured. Health Aff (Millwood). 2011;30(4):581-589. doi:10.1377/hlthaff.2011.0190
54. Schiff GD. Minimizing diagnostic error: the importance of follow-up and feedback. Am J Med. 2008;121(5 suppl):S38-S42. doi:10.1016/j.amjmed.2008.02.004
55. Mitchell I, Schuster A, Smith K, Pronovost P, Wu A. Patient safety incident reporting: a qualitative study of thoughts and perceptions of experts 15 years after “To Err is Human.” BMJ Qual Saf. 2016;25(2):92-99. doi:10.1136/bmjqs-2015-004405
56. Mazurenko O, Collum T, Ferdinand A, Menachemi N. Predictors of hospital patient satisfaction as measured by HCAHPS: a systematic review. J Healthc Manag. 2017;62(4):272-283. doi:10.1097/JHM-D-15-00050
57. Liberman AL, Newman-Toker DE. Symptom-Disease Pair Analysis of Diagnostic Error (SPADE): a conceptual framework and methodological approach for unearthing misdiagnosis-related harms using big data. BMJ Qual Saf. 2018;27(7):557-566. doi:10.1136/bmjqs-2017-007032
58. Utility of Predictive Systems to Identify Inpatient Diagnostic Errors: the UPSIDE study. NIH RePort/RePORTER. Accessed January 14, 2023. https://reporter.nih.gov/search/rpoHXlEAcEudQV3B9ld8iw/project-details/10020962
59. Overview of Patient Safety Learning Laboratory (PSLL) Projects. Agency for Healthcare Research and Quality. Accessed January 14, 2023. https://www.ahrq.gov/patient-safety/resources/learning-lab/index.html
60. Achieving Diagnostic Excellence through Prevention and Teamwork (ADEPT). NIH RePort/RePORTER. Accessed January 14, 2023. https://reporter.nih.gov/project-details/10642576
61. Zwaan L, Singh H. Diagnostic error in hospitals: finding forests not just the big trees. BMJ Qual Saf. 2020;29(12):961-964. doi:10.1136/bmjqs-2020-011099
62. Longtin Y, Sax H, Leape LL, Sheridan SE, Donaldson L, Pittet D. Patient participation: current knowledge and applicability to patient safety. Mayo Clin Proc. 2010;85(1):53-62. doi:10.4065/mcp.2009.0248
63. Murphy DR, Singh H, Berlin L. Communication breakdowns and diagnostic errors: a radiology perspective. Diagnosis (Berl). 2014;1(4):253-261. doi:10.1515/dx-2014-0035
64. Singh H, Naik AD, Rao R, Petersen LA. Reducing diagnostic errors through effective communication: harnessing the power of information technology. J Gen Intern Med. 2008;23(4):489-494. doi:10.1007/s11606-007-0393-z
65. Singh H, Connor DM, Dhaliwal G. Five strategies for clinicians to advance diagnostic excellence. BMJ. 2022;376:e068044. doi:10.1136/bmj-2021-068044
66. Yale S, Cohen S, Bordini BJ. Diagnostic time-outs to improve diagnosis. Crit Care Clin. 2022;38(2):185-194. doi:10.1016/j.ccc.2021.11.008
67. Schwartz A, Peskin S, Spiro A, Weiner SJ. Impact of unannounced standardized patient audit and feedback on care, documentation, and costs: an experiment and claims analysis. J Gen Intern Med. 2021;36(1):27-34. doi:10.1007/s11606-020-05965-1
68. Carpenter JD, Gorman PN. Using medication list—problem list mismatches as markers of potential error. Proc AMIA Symp. 2002:106-110.
69. Hron JD, Manzi S, Dionne R, et al. Electronic medication reconciliation and medication errors. Int J Qual Health Care. 2015;27(4):314-319. doi:10.1093/intqhc/mzv046
70. Graber ML, Siegal D, Riah H, Johnston D, Kenyon K. Electronic health record–related events in medical malpractice claims. J Patient Saf. 2019;15(2):77-85. doi:10.1097/PTS.0000000000000240
71. Murphy DR, Wu L, Thomas EJ, Forjuoh SN, Meyer AND, Singh H. Electronic trigger-based intervention to reduce delays in diagnostic evaluation for cancer: a cluster randomized controlled trial. J Clin Oncol. 2015;33(31):3560-3567. doi:10.1200/JCO.2015.61.1301
72. Singh H, Giardina TD, Forjuoh SN, et al. Electronic health record-based surveillance of diagnostic errors in primary care. BMJ Qual Saf. 2012;21(2):93-100. doi:10.1136/bmjqs-2011-000304
73. Armaignac DL, Saxena A, Rubens M, et al. Impact of telemedicine on mortality, length of stay, and cost among patients in progressive care units: experience from a large healthcare system. Crit Care Med. 2018;46(5):728-735. doi:10.1097/CCM.0000000000002994
74. MacKinnon GE, Brittain EL. Mobile health technologies in cardiopulmonary disease. Chest. 2020;157(3):654-664. doi:10.1016/j.chest.2019.10.015
75. DeVore AD, Wosik J, Hernandez AF. The future of wearables in heart failure patients. JACC Heart Fail. 2019;7(11):922-932. doi:10.1016/j.jchf.2019.08.008
76. Tsai TL, Fridsma DB, Gatti G. Computer decision support as a source of interpretation error: the case of electrocardiograms. J Am Med Inform Assoc. 2003;10(5):478-483. doi:10.1197/jamia.M1279
77. Lin SY, Mahoney MR, Sinsky CA. Ten ways artificial intelligence will transform primary care. J Gen Intern Med. 2019;34(8):1626-1630. doi:10.1007/s11606-019-05035-1
78. Ramirez AH, Gebo KA, Harris PA. Progress with the All Of Us research program: opening access for researchers. JAMA. 2021;325(24):2441-2442. doi:10.1001/jama.2021.7702
79. Johnson KB, Wei W, Weeraratne D, et al. Precision medicine, AI, and the future of personalized health care. Clin Transl Sci. 2021;14(1):86-93. doi:10.1111/cts.12884
80. Gupta A, Snyder A, Kachalia A, Flanders S, Saint S, Chopra V. Malpractice claims related to diagnostic errors in the hospital. BMJ Qual Saf. 2017;27(1):bmjqs-2017-006774. doi:10.1136/bmjqs-2017-006774
81. Renkema E, Broekhuis M, Ahaus K. Conditions that influence the impact of malpractice litigation risk on physicians’ behavior regarding patient safety. BMC Health Serv Res. 2014;14(1):38. doi:10.1186/1472-6963-14-38
82. Kachalia A, Mello MM, Nallamothu BK, Studdert DM. Legal and policy interventions to improve patient safety. Circulation. 2016;133(7):661-671. doi:10.1161/CIRCULATIONAHA.115.015880
Abstract
Diagnostic errors in hospitalized patients are a leading cause of preventable morbidity and mortality. Significant challenges in defining and measuring diagnostic errors and underlying process failure points have led to considerable variability in reported rates of diagnostic errors and adverse outcomes. In this article, we explore the diagnostic process and its discrete components, emphasizing the centrality of the patient in decision-making as well as the continuous nature of the process. We review the incidence of diagnostic errors in hospitalized patients and different methodological approaches that have been used to arrive at these estimates. We discuss different but interdependent provider- and system-related process-failure points that lead to diagnostic errors. We examine specific challenges related to measurement of diagnostic errors and describe traditional and novel approaches that are being used to obtain the most precise estimates. Finally, we examine various patient-, provider-, and organizational-level interventions that have been proposed to improve diagnostic safety in hospitalized patients.
Keywords: diagnostic error, hospital medicine, patient safety.
Diagnosis is defined as a “pre-existing set of categories agreed upon by the medical profession to designate a specific condition.”1 The diagnostic process involves obtaining a clinical history, performing a physical examination, conducting diagnostic testing, and consulting with other clinical providers to gather data that are relevant to understanding the underlying disease processes. This exercise involves generating hypotheses and updating prior probabilities as more information and evidence become available. Throughout this process of information gathering, integration, and interpretation, there is an ongoing assessment of whether sufficient and necessary knowledge has been obtained to make an accurate diagnosis and provide appropriate treatment.2
Diagnostic error is defined as a missed opportunity to make a timely diagnosis as part of this iterative process, including the failure of communicating the diagnosis to the patient in a timely manner.3 It can be categorized as a missed, delayed, or incorrect diagnosis based on available evidence at the time. Establishing the correct diagnosis has important implications. A timely and precise diagnosis ensures the patient the highest probability of having a positive health outcome that reflects an appropriate understanding of underlying disease processes and is consistent with their overall goals of care.3 When diagnostic errors occur, they can cause patient harm. Adverse events due to medical errors, including diagnostic errors, are estimated to be the third leading cause of death in the United States.4 Most people will experience at least 1 diagnostic error in their lifetime. In the 2015 National Academy of Medicine report Improving Diagnosis in Healthcare, diagnostic errors were identified as a major hazard as well as an opportunity to improve patient outcomes.2
Diagnostic errors during hospitalizations are especially concerning, as they are more likely to be implicated in a wider spectrum of harm, including permanent disability and death. This has become even more relevant for hospital medicine physicians and other clinical providers as they encounter increasing cognitive and administrative workloads, rising dissatisfaction and burnout, and unique obstacles such as night-time scheduling.5
Incidence of Diagnostic Errors in Hospitalized Patients
Several methodological approaches have been used to estimate the incidence of diagnostic errors in hospitalized patients. These include retrospective reviews of a sample of all hospital admissions, evaluations of selected adverse outcomes including autopsy studies, patient and provider surveys, and malpractice claims. Laboratory testing audits and secondary reviews in other diagnostic subspecialities (eg, radiology, pathology, and microbiology) are also essential to improving diagnostic performance in these specialized fields, which in turn affects overall hospital diagnostic error rates.6-8 These diverse approaches provide unique insights regarding our ability to assess the degree to which potential harms, ranging from temporary impairment to permanent disability, to death, are attributable to different failure points in the diagnostic process.
Large retrospective chart reviews of random hospital admissions remain the most accurate way to determine the overall incidence of diagnostic errors in hospitalized patients.9 The Harvard Medical Practice Study, published in 1991, laid the groundwork for measuring the incidence of adverse events in hospitalized patients and assessing their relation to medical error, negligence, and disability. Reviewing 30,121 randomly selected records from 51 randomly selected acute care hospitals in New York State, the study found that adverse events occurred in 3.7% of hospitalizations, diagnostic errors accounted for 13.8% of these events, and these errors were likely attributable to negligence in 74.7% of cases. The study not only outlined individual-level process failures, but also focused attention on some of the systemic causes, setting the agenda for quality improvement research in hospital-based care for years to come.10-12 A recent systematic review and meta-analysis of 22 hospital admission studies found a pooled rate of 0.7% (95% CI, 0.5%-1.1%) for harmful diagnostic errors.9 It found significant variations in the rates of adverse events, diagnostic errors, and range of diagnoses that were missed. This was primarily because of variabilities in pre-test probabilities in detecting diagnostic errors in these specific cohorts, as well as due to heterogeneity in study definitions and methodologies, especially regarding how they defined and measured “diagnostic error.” The analysis, however, did not account for diagnostic errors that were not related to patient harm (missed opportunities); therefore, it likely significantly underestimated the true incidence of diagnostic errors in these study populations. Table 1 summarizes some of key studies that have examined the incidence of harmful diagnostic errors in hospitalized patients.9-21

The chief limitation of reviewing random hospital admissions is that, since overall rates of diagnostic errors are still relatively low, a large number of case reviews are required to identify a sufficient sample of adverse outcomes to gain a meaningful understanding of the underlying process failure points and develop tools for remediation. Patient and provider surveys or data from malpractice claims can be high-yield starting points for research on process errors.22,23 Reviews of enriched cohorts of adverse outcomes, such as rapid-response events, intensive care unit (ICU) transfers, deaths, and hospital readmissions, can be an efficient way to identify process failures that lead to greatest harm. Depending on the research approach and the types of underlying patient populations sampled, rates of diagnostic errors in these high-risk groups have been estimated to be approximately 5% to 20%, or even higher.6,24-31 For example, a retrospective study of 391 cases of unplanned 7-day readmissions found that 5.6% of cases contained at least 1 diagnostic error during the index admission.32 In a study conducted at 6 Belgian acute-care hospitals, 56% of patients requiring an unplanned transfer to a higher level of care were determined to have had an adverse event, and of these adverse events, 12.4% of cases were associated with errors in diagnosis.29 A systematic review of 16 hospital-based studies estimated that 3.1% of all inpatient deaths were likely preventable, which corresponded to 22,165 deaths annually in the United States.30 Another such review of 31 autopsy studies reported that 28% of autopsied ICU patients had at least 1 misdiagnosis; of these diagnostic errors, 8% were classified as potentially lethal, and 15% were considered major but not lethal.31 Significant drawbacks of such enriched cohort studies, however, are their poor generalizability and inability to detect failure points that do not lead to patient harm (near-miss events).33
Causes of Diagnostic Errors in Hospitalized Patients
All aspects of the diagnostic process are susceptible to errors. These errors stem from a variety of faulty processes, including failure of the patient to engage with the health care system (eg, due to lack of insurance or transportation, or delay in seeking care); failure in information gathering (eg, missed history or exam findings, ordering wrong tests, laboratory errors); failure in information interpretation (eg, exam finding or test result misinterpretation); inaccurate hypothesis generation (eg, due to suboptimal prioritization or weighing of supporting evidence); and failure in communication (eg, with other team members or with the patient).2,34 Reasons for diagnostic process failures vary widely across different health care settings. While clinician assessment errors (eg, failure to consider or alternatively overweigh competing diagnoses) and errors in testing and the monitoring phase (eg, failure to order or follow up diagnostic tests) can lead to a majority of diagnostic errors in some patient populations, in other settings, social (eg, poor health literacy, punitive cultural practices) and economic factors (eg, lack of access to appropriate diagnostic tests or to specialty expertise) play a more prominent role.34,35
The Figure describes the relationship between components of the diagnostic process and subsequent outcomes, including diagnostic process failures, diagnostic errors, and absence or presence of patient harm.2,36,37 It reemphasizes the centrality of the patient in decision-making and the continuous nature of the process. The Figure also illustrates that only a minority of process failures result in diagnostic errors, and a smaller proportion of diagnostic errors actually lead to patient harm. Conversely, it also shows that diagnostic errors can happen without any obvious process-failure points, and, similarly, patient harm can take place in the absence of any evident diagnostic errors.36-38 Finally, it highlights the need to incorporate feedback from process failures, diagnostic errors, and favorable and unfavorable patient outcomes in order to inform future quality improvement efforts and research.

A significant proportion of diagnostic errors are due to system-related vulnerabilities, such as limitations in availability, adoption or quality of work force training, health informatics resources, and diagnostic capabilities. Lack of institutional culture that promotes safety and transparency also predisposes to diagnostic errors.39,40 The other major domain of process failures is related to cognitive errors in clinician decision-making. Anchoring, confirmation bias, availability bias, and base-rate neglect are some of the common cognitive biases that, along with personality traits (aversion to risk or ambiguity, overconfidence) and affective biases (influence of emotion on decision-making), often determine the degree of utilization of resources and the possibility of suboptimal diagnostic performance.41,42 Further, implicit biases related to age, race, gender, and sexual orientation contribute to disparities in access to health care and outcomes.43 In a large number of cases of preventable adverse outcomes, however, there are multiple interdependent individual and system-related failure points that lead to diagnostic error and patient harm.6,32
Challenges in Defining and Measuring Diagnostic Errors
In order to develop effective, evidence-based interventions to reduce diagnostic errors in hospitalized patients, it is essential to be able to first operationally define, and then accurately measure, diagnostic errors and the process failures that contribute to these errors in a standardized way that is reproducible across different settings.6,44 There are a number of obstacles in this endeavor.
A fundamental problem is that establishing a diagnosis is not a single act but a process. Patterns of symptoms and clinical presentations often differ for the same disease. Information required to make a diagnosis is usually gathered in stages, where the clinician obtains additional data, while considering many possibilities, of which 1 may be ultimately correct. Diagnoses evolve over time and in different care settings. “The most likely diagnosis” is not always the same as “the final correct diagnosis.” Moreover, the diagnostic process is influenced by patients’ individual clinical courses and preferences over time. This makes determination of missed, delayed, or incorrect diagnoses challenging.45,46
For hospitalized patients, generally the goal is to first rule out more serious and acute conditions (eg, pulmonary embolism or stroke), even if their probability is rather low. Conversely, a diagnosis that appears less consequential if delayed (eg, chronic anemia of unclear etiology) might not be pursued on an urgent basis, and is often left to outpatient providers to examine, but still may manifest in downstream harm (eg, delayed diagnosis of gastrointestinal malignancy or recurrent admissions for heart failure due to missed iron-deficiency anemia). Therefore, coming up with disease diagnosis likelihoods in hindsight may turn out to be highly subjective and not always accurate. This can be particularly difficult when clinician and other team deliberations are not recorded in their entirety.47
Another hurdle in the practice of diagnostic medicine is to preserve the balance between underdiagnosing versus pursuing overly aggressive diagnostic approaches. Conducting laboratory, imaging, or other diagnostic studies without a clear shared understanding of how they would affect clinical decision-making (eg, use of prostate-specific antigen to detect prostate cancer) not only leads to increased costs but can also delay appropriate care. Worse, subsequent unnecessary diagnostic tests and treatments can sometimes lead to serious harm.48,49
Finally, retrospective reviews by clinicians are subject to multiple potential limitations that include failure to create well-defined research questions, poorly developed inclusion and exclusion criteria, and issues related to inter- and intra-rater reliability.50 These methodological deficiencies can occur despite following "best practice" guidelines during the study planning, execution, and analysis phases. They further add to the challenge of defining and measuring diagnostic errors.47
Strategies to Improve Measurement of Diagnostic Errors
Development of new methodologies to reliably measure diagnostic errors is an area of active research. The advancement of uniform and universally agreed-upon frameworks to define and identify process failure points and diagnostic errors would help reduce measurement error and support development and testing of interventions that could be generalizable across different health care settings. To more accurately define and measure diagnostic errors, several novel approaches have been proposed (Table 2).

The Safer Dx framework is an all-round tool developed to advance the discipline of measuring diagnostic errors. For an episode of care under review, the instrument scores various items to determine the likelihood of a diagnostic error. These items evaluate multiple dimensions affecting diagnostic performance and measurements across 3 broad domains: structure (provider and organizational characteristics—from everyone involved with patient care, to computing infrastructure, to policies and regulations), process (elements of the patient-provider encounter, diagnostic test performance and follow-up, and subspecialty- and referral-specific factors), and outcome (establishing accurate and timely diagnosis as opposed to missed, delayed, or incorrect diagnosis). This instrument has been revised and can be further modified by a variety of stakeholders, including clinicians, health care organizations, and policymakers, to identify potential diagnostic errors in a standardized way for patient safety and quality improvement research.51,52
Use of standardized tools, such as the Diagnosis Error Evaluation and Research (DEER) taxonomy, can help to identify and classify specific failure points across different diagnostic process dimensions.37 These failure points can be classified into: issues related to patient presentation or access to health care; failure to obtain or misinterpretation of history or physical exam findings; errors in use of diagnostics tests due to technical or clinician-related factors; failures in appropriate weighing of evidence and hypothesis generation; errors associated with referral or consultation process; and failure to monitor the patient or obtain timely follow-up.34 The DEER taxonomy can also be modified based on specific research questions and study populations. Further, it can be recategorized to correspond to Safer Dx framework diagnostic process dimensions to provide insights into reasons for specific process failures and to develop new interventions to mitigate errors and patient harm.6
Since a majority of diagnostic errors do not lead to actual harm, use of “triggers” or clues (eg, procedure-related complications, patient falls, transfers to a higher level of care, readmissions within 30 days) can be a more efficient method to identify diagnostic errors and adverse events that do cause harm. The Global Trigger Tool, developed by the Institute for Healthcare Improvement, uses this strategy. This tool has been shown to identify a significantly higher number of serious adverse events than comparable methods.53 This facilitates selection and development of strategies at the institutional level that are most likely to improve patient outcomes.24
Encouraging and facilitating voluntary or prompted reporting from patients and clinicians can also play an important role in capturing diagnostic errors. Patients and clinicians are not only the key stakeholders but are also uniquely placed within the diagnostic process to detect and report potential errors.25,54 Patient-safety-event reporting systems, such as RL6, play a vital role in reporting near-misses and adverse events. These systems provide a mechanism for team members at all levels within the hospital to contribute toward reporting patient adverse events, including those arising from diagnostic errors.55 The Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey is the first standardized, nationally reported patient survey designed to measure patients’ perceptions of their hospital experience. The US Centers for Medicare and Medicaid Services (CMS) publishes HCAHPS results on its website 4 times a year, which serves as an important incentive for hospitals to improve patient safety and quality of health care delivery.56
Another novel approach links multiple symptoms to a range of target diseases using the Symptom-Disease Pair Analysis of Diagnostic Error (SPADE) framework. Using “big data” technologies, this technique can help discover otherwise hidden symptom-disease links and improve overall diagnostic performance. This approach is proposed for both case-control (look-back) and cohort (look-forward) studies assessing diagnostic errors and misdiagnosis-related harms. For example, starting with a known diagnosis with high potential for harm (eg, stroke), the “look-back” approach can be used to identify high-risk symptoms (eg, dizziness, vertigo). In the “look-forward” approach, a single symptom or exposure risk factor known to be frequently misdiagnosed (eg, dizziness) can be analyzed to identify potential adverse disease outcomes (eg, stroke, migraine).57
Many large ongoing studies looking at diagnostic errors among hospitalized patients, such as Utility of Predictive Systems to identify Inpatient Diagnostic Errors (UPSIDE),58 Patient Safety Learning Lab (PSLL),59 and Achieving Diagnostic Excellence through Prevention and Teamwork (ADEPT),60 are using structured chart review methodologies incorporating many of the above strategies in combination. Cases triggered by certain events (eg, ICU transfer, death, rapid response event, new or worsening acute kidney injury) are reviewed using validated tools, including Safer Dx framework and DEER taxonomy, to provide the most precise estimates of the burden of diagnostic errors in hospitalized patients. These estimates may be much higher than previously predicted using traditional chart review approaches.6,24 For example, a recently published study of 2809 random admissions in 11 Massachusetts hospitals identified 978 adverse events but only 10 diagnostic errors (diagnostic error rate, 0.4%).19 This was likely because the trigger method used in the study did not specifically examine the diagnostic process as critically as done by the Safer Dx framework and DEER taxonomy tools, thereby underestimating the total number of diagnostic errors. Further, these ongoing studies (eg, UPSIDE, ADEPT) aim to employ new and upcoming advanced machine-learning methods to create models that can improve overall diagnostic performance. This would pave the way to test and build novel, efficient, and scalable interventions to reduce diagnostic errors and improve patient outcomes.
Strategies to Improve Diagnostic Safety in Hospitalized Patients
Disease-specific biomedical research, as well as advances in laboratory, imaging, and other technologies, play a critical role in improving diagnostic accuracy. However, these technical approaches do not address many of the broader clinician- and system-level failure points and opportunities for improvement. Various patient-, provider-, and organizational-level interventions that could make diagnostic processes more resilient and reduce the risk of error and patient harm have been proposed.61
Among these strategies are approaches to empower patients and their families. Fostering therapeutic relationships between patients and members of the care team is essential to reducing diagnostic errors.62 Facilitating timely access to health records, ensuring transparency in decision making, and tailoring communication strategies to patients’ cultural and educational backgrounds can reduce harm.63 Similarly, at the system level, enhancing communication among different providers by use of tools such as structured handoffs can prevent communication breakdowns and facilitate positive outcomes.64
Interventions targeted at individual health care providers, such as educational programs to improve content-specific knowledge, can enhance diagnostic performance. Regular feedback, strategies to enhance equity, and fostering an environment where all providers are actively encouraged to think critically and participate in the diagnostic process (training programs to use “diagnostic time-outs” and making it a “team sport”) can improve clinical reasoning.65,66 Use of standardized patients can help identify individual-level cognitive failure points and facilitate creation of new interventions to improve clinical decision-making processes.67
Novel health information technologies can further augment these efforts. These include effective documentation by maintaining dynamic and accurate patient histories, problem lists, and medication lists68-70; use of electronic health record–based algorithms to identify potential diagnostic delays for serious conditions71,72; use of telemedicine technologies to improve accessibility and coordination73; application of mobile health and wearable technologies to facilitate data-gathering and care delivery74,75; and use of computerized decision-support tools, including applications to interpret electrocardiograms, imaging studies, and other diagnostic tests.76
Use of precision medicine, powered by new artificial intelligence (AI) tools, is becoming more widespread. Algorithms powered by AI can augment and sometimes even outperform clinician decision-making in areas such as oncology, radiology, and primary care.77 Creation of large biobanks like the All of Us research program can be used to study thousands of environmental and genetic risk factors and health conditions simultaneously, and help identify specific treatments that work best for people of different backgrounds.78 Active research in these areas holds great promise in terms of how and when we diagnose diseases and make appropriate preventative and treatment decisions. Significant scientific, ethical, and regulatory challenges will need to be overcome before these technologies can address some of the most complex problems in health care.79
Finally, diagnostic performance is affected by the external environment, including the functioning of the medical liability system. Diagnostic errors that lead to patient harm are a leading cause of malpractice claims.80 Developing a legal environment, in collaboration with patient advocacy groups and health care organizations, that promotes and facilitates timely disclosure of diagnostic errors could decrease the incentive to hide errors, advance care processes, and improve outcomes.81,82
Conclusion
The burden of diagnostic errors in hospitalized patients is unacceptably high and remains an underemphasized cause of preventable morbidity and mortality. Diagnostic errors often result from a breakdown in multiple interdependent processes that involve patient-, provider-, and system-level factors. Significant challenges remain in defining and identifying diagnostic errors as well as underlying process-failure points. The most effective interventions to reduce diagnostic errors will require greater patient participation in the diagnostic process and a mix of evidence-based interventions that promote individual-provider excellence as well as system-level changes. Further research and collaboration among various stakeholders should help improve diagnostic safety for hospitalized patients.
Corresponding author: Abhishek Goyal, MD, MPH; [email protected]
Disclosures: Dr. Dalal disclosed receiving income ≥ $250 from MayaMD.
Abstract
Diagnostic errors in hospitalized patients are a leading cause of preventable morbidity and mortality. Significant challenges in defining and measuring diagnostic errors and underlying process failure points have led to considerable variability in reported rates of diagnostic errors and adverse outcomes. In this article, we explore the diagnostic process and its discrete components, emphasizing the centrality of the patient in decision-making as well as the continuous nature of the process. We review the incidence of diagnostic errors in hospitalized patients and different methodological approaches that have been used to arrive at these estimates. We discuss different but interdependent provider- and system-related process-failure points that lead to diagnostic errors. We examine specific challenges related to measurement of diagnostic errors and describe traditional and novel approaches that are being used to obtain the most precise estimates. Finally, we examine various patient-, provider-, and organizational-level interventions that have been proposed to improve diagnostic safety in hospitalized patients.
Keywords: diagnostic error, hospital medicine, patient safety.
Diagnosis is defined as a “pre-existing set of categories agreed upon by the medical profession to designate a specific condition.”1 The diagnostic process involves obtaining a clinical history, performing a physical examination, conducting diagnostic testing, and consulting with other clinical providers to gather data that are relevant to understanding the underlying disease processes. This exercise involves generating hypotheses and updating prior probabilities as more information and evidence become available. Throughout this process of information gathering, integration, and interpretation, there is an ongoing assessment of whether sufficient and necessary knowledge has been obtained to make an accurate diagnosis and provide appropriate treatment.2
Diagnostic error is defined as a missed opportunity to make a timely diagnosis as part of this iterative process, including the failure of communicating the diagnosis to the patient in a timely manner.3 It can be categorized as a missed, delayed, or incorrect diagnosis based on available evidence at the time. Establishing the correct diagnosis has important implications. A timely and precise diagnosis ensures the patient the highest probability of having a positive health outcome that reflects an appropriate understanding of underlying disease processes and is consistent with their overall goals of care.3 When diagnostic errors occur, they can cause patient harm. Adverse events due to medical errors, including diagnostic errors, are estimated to be the third leading cause of death in the United States.4 Most people will experience at least 1 diagnostic error in their lifetime. In the 2015 National Academy of Medicine report Improving Diagnosis in Healthcare, diagnostic errors were identified as a major hazard as well as an opportunity to improve patient outcomes.2
Diagnostic errors during hospitalizations are especially concerning, as they are more likely to be implicated in a wider spectrum of harm, including permanent disability and death. This has become even more relevant for hospital medicine physicians and other clinical providers as they encounter increasing cognitive and administrative workloads, rising dissatisfaction and burnout, and unique obstacles such as night-time scheduling.5
Incidence of Diagnostic Errors in Hospitalized Patients
Several methodological approaches have been used to estimate the incidence of diagnostic errors in hospitalized patients. These include retrospective reviews of a sample of all hospital admissions, evaluations of selected adverse outcomes including autopsy studies, patient and provider surveys, and malpractice claims. Laboratory testing audits and secondary reviews in other diagnostic subspecialities (eg, radiology, pathology, and microbiology) are also essential to improving diagnostic performance in these specialized fields, which in turn affects overall hospital diagnostic error rates.6-8 These diverse approaches provide unique insights regarding our ability to assess the degree to which potential harms, ranging from temporary impairment to permanent disability, to death, are attributable to different failure points in the diagnostic process.
Large retrospective chart reviews of random hospital admissions remain the most accurate way to determine the overall incidence of diagnostic errors in hospitalized patients.9 The Harvard Medical Practice Study, published in 1991, laid the groundwork for measuring the incidence of adverse events in hospitalized patients and assessing their relation to medical error, negligence, and disability. Reviewing 30,121 randomly selected records from 51 randomly selected acute care hospitals in New York State, the study found that adverse events occurred in 3.7% of hospitalizations, diagnostic errors accounted for 13.8% of these events, and these errors were likely attributable to negligence in 74.7% of cases. The study not only outlined individual-level process failures, but also focused attention on some of the systemic causes, setting the agenda for quality improvement research in hospital-based care for years to come.10-12 A recent systematic review and meta-analysis of 22 hospital admission studies found a pooled rate of 0.7% (95% CI, 0.5%-1.1%) for harmful diagnostic errors.9 It found significant variations in the rates of adverse events, diagnostic errors, and range of diagnoses that were missed. This was primarily because of variabilities in pre-test probabilities in detecting diagnostic errors in these specific cohorts, as well as due to heterogeneity in study definitions and methodologies, especially regarding how they defined and measured “diagnostic error.” The analysis, however, did not account for diagnostic errors that were not related to patient harm (missed opportunities); therefore, it likely significantly underestimated the true incidence of diagnostic errors in these study populations. Table 1 summarizes some of key studies that have examined the incidence of harmful diagnostic errors in hospitalized patients.9-21

The chief limitation of reviewing random hospital admissions is that, since overall rates of diagnostic errors are still relatively low, a large number of case reviews are required to identify a sufficient sample of adverse outcomes to gain a meaningful understanding of the underlying process failure points and develop tools for remediation. Patient and provider surveys or data from malpractice claims can be high-yield starting points for research on process errors.22,23 Reviews of enriched cohorts of adverse outcomes, such as rapid-response events, intensive care unit (ICU) transfers, deaths, and hospital readmissions, can be an efficient way to identify process failures that lead to greatest harm. Depending on the research approach and the types of underlying patient populations sampled, rates of diagnostic errors in these high-risk groups have been estimated to be approximately 5% to 20%, or even higher.6,24-31 For example, a retrospective study of 391 cases of unplanned 7-day readmissions found that 5.6% of cases contained at least 1 diagnostic error during the index admission.32 In a study conducted at 6 Belgian acute-care hospitals, 56% of patients requiring an unplanned transfer to a higher level of care were determined to have had an adverse event, and of these adverse events, 12.4% of cases were associated with errors in diagnosis.29 A systematic review of 16 hospital-based studies estimated that 3.1% of all inpatient deaths were likely preventable, which corresponded to 22,165 deaths annually in the United States.30 Another such review of 31 autopsy studies reported that 28% of autopsied ICU patients had at least 1 misdiagnosis; of these diagnostic errors, 8% were classified as potentially lethal, and 15% were considered major but not lethal.31 Significant drawbacks of such enriched cohort studies, however, are their poor generalizability and inability to detect failure points that do not lead to patient harm (near-miss events).33
Causes of Diagnostic Errors in Hospitalized Patients
All aspects of the diagnostic process are susceptible to errors. These errors stem from a variety of faulty processes, including failure of the patient to engage with the health care system (eg, due to lack of insurance or transportation, or delay in seeking care); failure in information gathering (eg, missed history or exam findings, ordering wrong tests, laboratory errors); failure in information interpretation (eg, exam finding or test result misinterpretation); inaccurate hypothesis generation (eg, due to suboptimal prioritization or weighing of supporting evidence); and failure in communication (eg, with other team members or with the patient).2,34 Reasons for diagnostic process failures vary widely across different health care settings. While clinician assessment errors (eg, failure to consider or alternatively overweigh competing diagnoses) and errors in testing and the monitoring phase (eg, failure to order or follow up diagnostic tests) can lead to a majority of diagnostic errors in some patient populations, in other settings, social (eg, poor health literacy, punitive cultural practices) and economic factors (eg, lack of access to appropriate diagnostic tests or to specialty expertise) play a more prominent role.34,35
The Figure describes the relationship between components of the diagnostic process and subsequent outcomes, including diagnostic process failures, diagnostic errors, and absence or presence of patient harm.2,36,37 It reemphasizes the centrality of the patient in decision-making and the continuous nature of the process. The Figure also illustrates that only a minority of process failures result in diagnostic errors, and a smaller proportion of diagnostic errors actually lead to patient harm. Conversely, it also shows that diagnostic errors can happen without any obvious process-failure points, and, similarly, patient harm can take place in the absence of any evident diagnostic errors.36-38 Finally, it highlights the need to incorporate feedback from process failures, diagnostic errors, and favorable and unfavorable patient outcomes in order to inform future quality improvement efforts and research.

A significant proportion of diagnostic errors are due to system-related vulnerabilities, such as limitations in availability, adoption or quality of work force training, health informatics resources, and diagnostic capabilities. Lack of institutional culture that promotes safety and transparency also predisposes to diagnostic errors.39,40 The other major domain of process failures is related to cognitive errors in clinician decision-making. Anchoring, confirmation bias, availability bias, and base-rate neglect are some of the common cognitive biases that, along with personality traits (aversion to risk or ambiguity, overconfidence) and affective biases (influence of emotion on decision-making), often determine the degree of utilization of resources and the possibility of suboptimal diagnostic performance.41,42 Further, implicit biases related to age, race, gender, and sexual orientation contribute to disparities in access to health care and outcomes.43 In a large number of cases of preventable adverse outcomes, however, there are multiple interdependent individual and system-related failure points that lead to diagnostic error and patient harm.6,32
Challenges in Defining and Measuring Diagnostic Errors
In order to develop effective, evidence-based interventions to reduce diagnostic errors in hospitalized patients, it is essential to be able to first operationally define, and then accurately measure, diagnostic errors and the process failures that contribute to these errors in a standardized way that is reproducible across different settings.6,44 There are a number of obstacles in this endeavor.
A fundamental problem is that establishing a diagnosis is not a single act but a process. Patterns of symptoms and clinical presentations often differ for the same disease. Information required to make a diagnosis is usually gathered in stages, where the clinician obtains additional data, while considering many possibilities, of which 1 may be ultimately correct. Diagnoses evolve over time and in different care settings. “The most likely diagnosis” is not always the same as “the final correct diagnosis.” Moreover, the diagnostic process is influenced by patients’ individual clinical courses and preferences over time. This makes determination of missed, delayed, or incorrect diagnoses challenging.45,46
For hospitalized patients, generally the goal is to first rule out more serious and acute conditions (eg, pulmonary embolism or stroke), even if their probability is rather low. Conversely, a diagnosis that appears less consequential if delayed (eg, chronic anemia of unclear etiology) might not be pursued on an urgent basis, and is often left to outpatient providers to examine, but still may manifest in downstream harm (eg, delayed diagnosis of gastrointestinal malignancy or recurrent admissions for heart failure due to missed iron-deficiency anemia). Therefore, coming up with disease diagnosis likelihoods in hindsight may turn out to be highly subjective and not always accurate. This can be particularly difficult when clinician and other team deliberations are not recorded in their entirety.47
Another hurdle in the practice of diagnostic medicine is to preserve the balance between underdiagnosing versus pursuing overly aggressive diagnostic approaches. Conducting laboratory, imaging, or other diagnostic studies without a clear shared understanding of how they would affect clinical decision-making (eg, use of prostate-specific antigen to detect prostate cancer) not only leads to increased costs but can also delay appropriate care. Worse, subsequent unnecessary diagnostic tests and treatments can sometimes lead to serious harm.48,49
Finally, retrospective reviews by clinicians are subject to multiple potential limitations that include failure to create well-defined research questions, poorly developed inclusion and exclusion criteria, and issues related to inter- and intra-rater reliability.50 These methodological deficiencies can occur despite following "best practice" guidelines during the study planning, execution, and analysis phases. They further add to the challenge of defining and measuring diagnostic errors.47
Strategies to Improve Measurement of Diagnostic Errors
Development of new methodologies to reliably measure diagnostic errors is an area of active research. The advancement of uniform and universally agreed-upon frameworks to define and identify process failure points and diagnostic errors would help reduce measurement error and support development and testing of interventions that could be generalizable across different health care settings. To more accurately define and measure diagnostic errors, several novel approaches have been proposed (Table 2).

The Safer Dx framework is an all-round tool developed to advance the discipline of measuring diagnostic errors. For an episode of care under review, the instrument scores various items to determine the likelihood of a diagnostic error. These items evaluate multiple dimensions affecting diagnostic performance and measurements across 3 broad domains: structure (provider and organizational characteristics—from everyone involved with patient care, to computing infrastructure, to policies and regulations), process (elements of the patient-provider encounter, diagnostic test performance and follow-up, and subspecialty- and referral-specific factors), and outcome (establishing accurate and timely diagnosis as opposed to missed, delayed, or incorrect diagnosis). This instrument has been revised and can be further modified by a variety of stakeholders, including clinicians, health care organizations, and policymakers, to identify potential diagnostic errors in a standardized way for patient safety and quality improvement research.51,52
Use of standardized tools, such as the Diagnosis Error Evaluation and Research (DEER) taxonomy, can help to identify and classify specific failure points across different diagnostic process dimensions.37 These failure points can be classified into: issues related to patient presentation or access to health care; failure to obtain or misinterpretation of history or physical exam findings; errors in use of diagnostics tests due to technical or clinician-related factors; failures in appropriate weighing of evidence and hypothesis generation; errors associated with referral or consultation process; and failure to monitor the patient or obtain timely follow-up.34 The DEER taxonomy can also be modified based on specific research questions and study populations. Further, it can be recategorized to correspond to Safer Dx framework diagnostic process dimensions to provide insights into reasons for specific process failures and to develop new interventions to mitigate errors and patient harm.6
Since a majority of diagnostic errors do not lead to actual harm, use of “triggers” or clues (eg, procedure-related complications, patient falls, transfers to a higher level of care, readmissions within 30 days) can be a more efficient method to identify diagnostic errors and adverse events that do cause harm. The Global Trigger Tool, developed by the Institute for Healthcare Improvement, uses this strategy. This tool has been shown to identify a significantly higher number of serious adverse events than comparable methods.53 This facilitates selection and development of strategies at the institutional level that are most likely to improve patient outcomes.24
Encouraging and facilitating voluntary or prompted reporting from patients and clinicians can also play an important role in capturing diagnostic errors. Patients and clinicians are not only the key stakeholders but are also uniquely placed within the diagnostic process to detect and report potential errors.25,54 Patient-safety-event reporting systems, such as RL6, play a vital role in reporting near-misses and adverse events. These systems provide a mechanism for team members at all levels within the hospital to contribute toward reporting patient adverse events, including those arising from diagnostic errors.55 The Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey is the first standardized, nationally reported patient survey designed to measure patients’ perceptions of their hospital experience. The US Centers for Medicare and Medicaid Services (CMS) publishes HCAHPS results on its website 4 times a year, which serves as an important incentive for hospitals to improve patient safety and quality of health care delivery.56
Another novel approach links multiple symptoms to a range of target diseases using the Symptom-Disease Pair Analysis of Diagnostic Error (SPADE) framework. Using “big data” technologies, this technique can help discover otherwise hidden symptom-disease links and improve overall diagnostic performance. This approach is proposed for both case-control (look-back) and cohort (look-forward) studies assessing diagnostic errors and misdiagnosis-related harms. For example, starting with a known diagnosis with high potential for harm (eg, stroke), the “look-back” approach can be used to identify high-risk symptoms (eg, dizziness, vertigo). In the “look-forward” approach, a single symptom or exposure risk factor known to be frequently misdiagnosed (eg, dizziness) can be analyzed to identify potential adverse disease outcomes (eg, stroke, migraine).57
Many large ongoing studies looking at diagnostic errors among hospitalized patients, such as Utility of Predictive Systems to identify Inpatient Diagnostic Errors (UPSIDE),58 Patient Safety Learning Lab (PSLL),59 and Achieving Diagnostic Excellence through Prevention and Teamwork (ADEPT),60 are using structured chart review methodologies incorporating many of the above strategies in combination. Cases triggered by certain events (eg, ICU transfer, death, rapid response event, new or worsening acute kidney injury) are reviewed using validated tools, including Safer Dx framework and DEER taxonomy, to provide the most precise estimates of the burden of diagnostic errors in hospitalized patients. These estimates may be much higher than previously predicted using traditional chart review approaches.6,24 For example, a recently published study of 2809 random admissions in 11 Massachusetts hospitals identified 978 adverse events but only 10 diagnostic errors (diagnostic error rate, 0.4%).19 This was likely because the trigger method used in the study did not specifically examine the diagnostic process as critically as done by the Safer Dx framework and DEER taxonomy tools, thereby underestimating the total number of diagnostic errors. Further, these ongoing studies (eg, UPSIDE, ADEPT) aim to employ new and upcoming advanced machine-learning methods to create models that can improve overall diagnostic performance. This would pave the way to test and build novel, efficient, and scalable interventions to reduce diagnostic errors and improve patient outcomes.
Strategies to Improve Diagnostic Safety in Hospitalized Patients
Disease-specific biomedical research, as well as advances in laboratory, imaging, and other technologies, play a critical role in improving diagnostic accuracy. However, these technical approaches do not address many of the broader clinician- and system-level failure points and opportunities for improvement. Various patient-, provider-, and organizational-level interventions that could make diagnostic processes more resilient and reduce the risk of error and patient harm have been proposed.61
Among these strategies are approaches to empower patients and their families. Fostering therapeutic relationships between patients and members of the care team is essential to reducing diagnostic errors.62 Facilitating timely access to health records, ensuring transparency in decision making, and tailoring communication strategies to patients’ cultural and educational backgrounds can reduce harm.63 Similarly, at the system level, enhancing communication among different providers by use of tools such as structured handoffs can prevent communication breakdowns and facilitate positive outcomes.64
Interventions targeted at individual health care providers, such as educational programs to improve content-specific knowledge, can enhance diagnostic performance. Regular feedback, strategies to enhance equity, and fostering an environment where all providers are actively encouraged to think critically and participate in the diagnostic process (training programs to use “diagnostic time-outs” and making it a “team sport”) can improve clinical reasoning.65,66 Use of standardized patients can help identify individual-level cognitive failure points and facilitate creation of new interventions to improve clinical decision-making processes.67
Novel health information technologies can further augment these efforts. These include effective documentation by maintaining dynamic and accurate patient histories, problem lists, and medication lists68-70; use of electronic health record–based algorithms to identify potential diagnostic delays for serious conditions71,72; use of telemedicine technologies to improve accessibility and coordination73; application of mobile health and wearable technologies to facilitate data-gathering and care delivery74,75; and use of computerized decision-support tools, including applications to interpret electrocardiograms, imaging studies, and other diagnostic tests.76
Use of precision medicine, powered by new artificial intelligence (AI) tools, is becoming more widespread. Algorithms powered by AI can augment and sometimes even outperform clinician decision-making in areas such as oncology, radiology, and primary care.77 Creation of large biobanks like the All of Us research program can be used to study thousands of environmental and genetic risk factors and health conditions simultaneously, and help identify specific treatments that work best for people of different backgrounds.78 Active research in these areas holds great promise in terms of how and when we diagnose diseases and make appropriate preventative and treatment decisions. Significant scientific, ethical, and regulatory challenges will need to be overcome before these technologies can address some of the most complex problems in health care.79
Finally, diagnostic performance is affected by the external environment, including the functioning of the medical liability system. Diagnostic errors that lead to patient harm are a leading cause of malpractice claims.80 Developing a legal environment, in collaboration with patient advocacy groups and health care organizations, that promotes and facilitates timely disclosure of diagnostic errors could decrease the incentive to hide errors, advance care processes, and improve outcomes.81,82
Conclusion
The burden of diagnostic errors in hospitalized patients is unacceptably high and remains an underemphasized cause of preventable morbidity and mortality. Diagnostic errors often result from a breakdown in multiple interdependent processes that involve patient-, provider-, and system-level factors. Significant challenges remain in defining and identifying diagnostic errors as well as underlying process-failure points. The most effective interventions to reduce diagnostic errors will require greater patient participation in the diagnostic process and a mix of evidence-based interventions that promote individual-provider excellence as well as system-level changes. Further research and collaboration among various stakeholders should help improve diagnostic safety for hospitalized patients.
Corresponding author: Abhishek Goyal, MD, MPH; [email protected]
Disclosures: Dr. Dalal disclosed receiving income ≥ $250 from MayaMD.
1. Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch Intern Med. 2005;165(13):1493-1499. doi:10.1001/archinte.165.13.1493
2. National Academies of Sciences, Engineering, and Medicine. 2015. Improving Diagnosis in Health Care. The National Academies Press. doi:10.17226/21794
3. Singh H, Graber ML. Improving diagnosis in health care—the next imperative for patient safety. N Engl J Med. 2015;373(26):2493-2495. doi:10.1056/NEJMp1512241
4. Makary MA, Daniel M. Medical error—the third leading cause of death in the US. BMJ. 2016;353:i2139. doi:10.1136/bmj.i2139
5. Flanders SA, Centor B, Weber V, McGinn T, Desalvo K, Auerbach A. Challenges and opportunities in academic hospital medicine: report from the academic hospital medicine summit. J Gen Intern Med. 2009;24(5):636-641. doi:10.1007/s11606-009-0944-6
6. Griffin JA, Carr K, Bersani K, et al. Analyzing diagnostic errors in the acute setting: a process-driven approach. Diagnosis (Berl). 2021;9(1):77-88. doi:10.1515/dx-2021-0033
7. Itri JN, Tappouni RR, McEachern RO, Pesch AJ, Patel SH. Fundamentals of diagnostic error in imaging. RadioGraphics. 2018;38(6):1845-1865. doi:10.1148/rg.2018180021
8. Hammerling JA. A Review of medical errors in laboratory diagnostics and where we are today. Lab Med. 2012;43(2):41-44. doi:10.1309/LM6ER9WJR1IHQAUY
9. Gunderson CG, Bilan VP, Holleck JL, et al. Prevalence of harmful diagnostic errors in hospitalised adults: a systematic review and meta-analysis. BMJ Qual Saf. 2020;29(12):1008-1018. doi:10.1136/bmjqs-2019-010822
10. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370-376. doi:10.1056/NEJM199102073240604
11. Leape LL, Brennan TA, Laird N, et al. The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II. N Engl J Med. 1991;324(6):377-384. doi:10.1056/NEJM199102073240605
12. Localio AR, Lawthers AG, Brennan TA, et al. Relation between malpractice claims and adverse events due to negligence. Results of the Harvard Medical Practice Study III. N Engl J Med. 1991;325(4):245-251. doi:10.1056/NEJM199107253250405
13. Wilson RM, Michel P, Olsen S, et al. Patient safety in developing countries: retrospective estimation of scale and nature of harm to patients in hospital. BMJ. 2012;344:e832. doi:10.1136/bmj.e832
14. Wilson RM, Runciman WB, Gibberd RW, Harrison BT, Newby L, Hamilton JD. The Quality in Australian Health Care Study. Med J Aust. 1995;163(9):458-471. doi:10.5694/j.1326-5377.1995.tb124691.x
15. Thomas EJ, Studdert DM, Burstin HR, et al. Incidence and types of adverse events and negligent care in Utah and Colorado. Med Care. 2000;38(3):261-271. doi:10.1097/00005650-200003000-00003
16. Baker GR, Norton PG, Flintoft V, et al. The Canadian Adverse Events Study: the incidence of adverse events among hospital patients in Canada. CMAJ. 2004;170(11):1678-1686. doi:10.1503/cmaj.1040498
17. Davis P, Lay-Yee R, Briant R, Ali W, Scott A, Schug S. Adverse events in New Zealand public hospitals II: preventability and clinical context. N Z Med J. 2003;116(1183):U624.
18. Aranaz-Andrés JM, Aibar-Remón C, Vitaller-Murillo J, et al. Incidence of adverse events related to health care in Spain: results of the Spanish National Study of Adverse Events. J Epidemiol Community Health. 2008;62(12):1022-1029. doi:10.1136/jech.2007.065227
19. Bates DW, Levine DM, Salmasian H, et al. The safety of inpatient health care. N Engl J Med. 2023;388(2):142-153. doi:10.1056/NEJMsa2206117
20. Soop M, Fryksmark U, Köster M, Haglund B. The incidence of adverse events in Swedish hospitals: a retrospective medical record review study. Int J Qual Health Care. 2009;21(4):285-291. doi:10.1093/intqhc/mzp025
21. Rafter N, Hickey A, Conroy RM, et al. The Irish National Adverse Events Study (INAES): the frequency and nature of adverse events in Irish hospitals—a retrospective record review study. BMJ Qual Saf. 2017;26(2):111-119. doi:10.1136/bmjqs-2015-004828
22. Blendon RJ, DesRoches CM, Brodie M, et al. Views of practicing physicians and the public on medical errors. N Engl J Med. 2002;347(24):1933-1940. doi:10.1056/NEJMsa022151
23. Saber Tehrani AS, Lee H, Mathews SC, et al. 25-year summary of US malpractice claims for diagnostic errors 1986-2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672-680. doi:10.1136/bmjqs-2012-001550
24. Malik MA, Motta-Calderon D, Piniella N, et al. A structured approach to EHR surveillance of diagnostic error in acute care: an exploratory analysis of two institutionally-defined case cohorts. Diagnosis (Berl). 2022;9(4):446-457. doi:10.1515/dx-2022-0032
25. Graber ML. The incidence of diagnostic error in medicine. BMJ Qual Saf. 2013;22(suppl 2):ii21-ii27. doi:10.1136/bmjqs-2012-001615
26. Bergl PA, Taneja A, El-Kareh R, Singh H, Nanchal RS. Frequency, risk factors, causes, and consequences of diagnostic errors in critically ill medical patients: a retrospective cohort study. Crit Care Med. 2019;47(11):e902-e910. doi:10.1097/CCM.0000000000003976
27. Hogan H, Healey F, Neale G, Thomson R, Vincent C, Black N. Preventable deaths due to problems in care in English acute hospitals: a retrospective case record review study. BMJ Qual Saf. 2012;21(9):737-745. doi:10.1136/bmjqs-2011-001159
28. Bergl PA, Nanchal RS, Singh H. Diagnostic error in the critically ill: defining the problem and exploring next steps to advance intensive care unit safety. Ann Am Thorac Soc. 2018;15(8):903-907. doi:10.1513/AnnalsATS.201801-068PS
29. Marquet K, Claes N, De Troy E, et al. One fourth of unplanned transfers to a higher level of care are associated with a highly preventable adverse event: a patient record review in six Belgian hospitals. Crit Care Med. 2015;43(5):1053-1061. doi:10.1097/CCM.0000000000000932
30. Rodwin BA, Bilan VP, Merchant NB, et al. Rate of preventable mortality in hospitalized patients: a systematic review and meta-analysis. J Gen Intern Med. 2020;35(7):2099-2106. doi:10.1007/s11606-019-05592-5
31. Winters B, Custer J, Galvagno SM, et al. Diagnostic errors in the intensive care unit: a systematic review of autopsy studies. BMJ Qual Saf. 2012;21(11):894-902. doi:10.1136/bmjqs-2012-000803
32. Raffel KE, Kantor MA, Barish P, et al. Prevalence and characterisation of diagnostic error among 7-day all-cause hospital medicine readmissions: a retrospective cohort study. BMJ Qual Saf. 2020;29(12):971-979. doi:10.1136/bmjqs-2020-010896
33. Weingart SN, Pagovich O, Sands DZ, et al. What can hospitalized patients tell us about adverse events? learning from patient-reported incidents. J Gen Intern Med. 2005;20(9):830-836. doi:10.1111/j.1525-1497.2005.0180.x
34. Schiff GD, Hasan O, Kim S, et al. Diagnostic error in medicine: analysis of 583 physician-reported errors. Arch Intern Med. 2009;169(20):1881-1887. doi:10.1001/archinternmed.2009.333
35. Singh H, Schiff GD, Graber ML, Onakpoya I, Thompson MJ. The global burden of diagnostic errors in primary care. BMJ Qual Saf. 2017;26(6):484-494. doi:10.1136/bmjqs-2016-005401
36. Schiff GD, Leape LL. Commentary: how can we make diagnosis safer? Acad Med J Assoc Am Med Coll. 2012;87(2):135-138. doi:10.1097/ACM.0b013e31823f711c
37. Schiff GD, Kim S, Abrams R, et al. Diagnosing diagnosis errors: lessons from a multi-institutional collaborative project. In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds. Advances in Patient Safety: From Research to Implementation. Volume 2: Concepts and Methodology. AHRQ Publication No. 05-0021-2. Agency for Healthcare Research and Quality (US); 2005. Accessed January 16, 2023. http://www.ncbi.nlm.nih.gov/books/NBK20492/
38. Newman-Toker DE. A unified conceptual model for diagnostic errors: underdiagnosis, overdiagnosis, and misdiagnosis. Diagnosis (Berl). 2014;1(1):43-48. doi:10.1515/dx-2013-0027
39. Abimanyi-Ochom J, Bohingamu Mudiyanselage S, Catchpool M, Firipis M, Wanni Arachchige Dona S, Watts JJ. Strategies to reduce diagnostic errors: a systematic review. BMC Med Inform Decis Mak. 2019;19(1):174. doi:10.1186/s12911-019-0901-1
40. Gupta A, Harrod M, Quinn M, et al. Mind the overlap: how system problems contribute to cognitive failure and diagnostic errors. Diagnosis (Berl). 2018;5(3):151-156. doi:10.1515/dx-2018-0014
41. Saposnik G, Redelmeier D, Ruff CC, Tobler PN. Cognitive biases associated with medical decisions: a systematic review. BMC Med Inform Decis Mak. 2016;16:138. doi:10.1186/s12911-016-0377-1
42. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78(8):775-780. doi: 10.1097/00001888-200308000-00003
43. Chapman EN, Kaatz A, Carnes M. Physicians and implicit bias: how doctors may unwittingly perpetuate health care disparities. J Gen Intern Med. 2013;28(11):1504-1510. doi:10.1007/s11606-013-2441-1
44. Zwaan L, Singh H. The challenges in defining and measuring diagnostic error. Diagnosis (Ber). 2015;2(2):97-103. doi:10.1515/dx-2014-0069
45. Arkes HR, Wortmann RL, Saville PD, Harkness AR. Hindsight bias among physicians weighing the likelihood of diagnoses. J Appl Psychol. 1981;66(2):252-254.
46. Singh H. Editorial: Helping health care organizations to define diagnostic errors as missed opportunities in diagnosis. Jt Comm J Qual Patient Saf. 2014;40(3):99-101. doi:10.1016/s1553-7250(14)40012-6
47. Vassar M, Holzmann M. The retrospective chart review: important methodological considerations. J Educ Eval Health Prof. 2013;10:12. doi:10.3352/jeehp.2013.10.12
48. Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102(9):605-613. doi:10.1093/jnci/djq099
49. Moynihan R, Doust J, Henry D. Preventing overdiagnosis: how to stop harming the healthy. BMJ. 2012;344:e3502. doi:10.1136/bmj.e3502
50. Hayward RA, Hofer TP. Estimating hospital deaths due to medical errors: preventability is in the eye of the reviewer. JAMA. 2001;286(4):415-420. doi:10.1001/jama.286.4.415
51. Singh H, Sittig DF. Advancing the science of measurement of diagnostic errors in healthcare: the Safer Dx framework. BMJ Qual Saf. 2015;24(2):103-110. doi:10.1136/bmjqs-2014-003675
52. Singh H, Khanna A, Spitzmueller C, Meyer AND. Recommendations for using the Revised Safer Dx Instrument to help measure and improve diagnostic safety. Diagnosis (Berl). 2019;6(4):315-323. doi:10.1515/dx-2019-0012
53. Classen DC, Resar R, Griffin F, et al. “Global trigger tool” shows that adverse events in hospitals may be ten times greater than previously measured. Health Aff (Millwood). 2011;30(4):581-589. doi:10.1377/hlthaff.2011.0190
54. Schiff GD. Minimizing diagnostic error: the importance of follow-up and feedback. Am J Med. 2008;121(5 suppl):S38-S42. doi:10.1016/j.amjmed.2008.02.004
55. Mitchell I, Schuster A, Smith K, Pronovost P, Wu A. Patient safety incident reporting: a qualitative study of thoughts and perceptions of experts 15 years after “To Err is Human.” BMJ Qual Saf. 2016;25(2):92-99. doi:10.1136/bmjqs-2015-004405
56. Mazurenko O, Collum T, Ferdinand A, Menachemi N. Predictors of hospital patient satisfaction as measured by HCAHPS: a systematic review. J Healthc Manag. 2017;62(4):272-283. doi:10.1097/JHM-D-15-00050
57. Liberman AL, Newman-Toker DE. Symptom-Disease Pair Analysis of Diagnostic Error (SPADE): a conceptual framework and methodological approach for unearthing misdiagnosis-related harms using big data. BMJ Qual Saf. 2018;27(7):557-566. doi:10.1136/bmjqs-2017-007032
58. Utility of Predictive Systems to Identify Inpatient Diagnostic Errors: the UPSIDE study. NIH RePort/RePORTER. Accessed January 14, 2023. https://reporter.nih.gov/search/rpoHXlEAcEudQV3B9ld8iw/project-details/10020962
59. Overview of Patient Safety Learning Laboratory (PSLL) Projects. Agency for Healthcare Research and Quality. Accessed January 14, 2023. https://www.ahrq.gov/patient-safety/resources/learning-lab/index.html
60. Achieving Diagnostic Excellence through Prevention and Teamwork (ADEPT). NIH RePort/RePORTER. Accessed January 14, 2023. https://reporter.nih.gov/project-details/10642576
61. Zwaan L, Singh H. Diagnostic error in hospitals: finding forests not just the big trees. BMJ Qual Saf. 2020;29(12):961-964. doi:10.1136/bmjqs-2020-011099
62. Longtin Y, Sax H, Leape LL, Sheridan SE, Donaldson L, Pittet D. Patient participation: current knowledge and applicability to patient safety. Mayo Clin Proc. 2010;85(1):53-62. doi:10.4065/mcp.2009.0248
63. Murphy DR, Singh H, Berlin L. Communication breakdowns and diagnostic errors: a radiology perspective. Diagnosis (Berl). 2014;1(4):253-261. doi:10.1515/dx-2014-0035
64. Singh H, Naik AD, Rao R, Petersen LA. Reducing diagnostic errors through effective communication: harnessing the power of information technology. J Gen Intern Med. 2008;23(4):489-494. doi:10.1007/s11606-007-0393-z
65. Singh H, Connor DM, Dhaliwal G. Five strategies for clinicians to advance diagnostic excellence. BMJ. 2022;376:e068044. doi:10.1136/bmj-2021-068044
66. Yale S, Cohen S, Bordini BJ. Diagnostic time-outs to improve diagnosis. Crit Care Clin. 2022;38(2):185-194. doi:10.1016/j.ccc.2021.11.008
67. Schwartz A, Peskin S, Spiro A, Weiner SJ. Impact of unannounced standardized patient audit and feedback on care, documentation, and costs: an experiment and claims analysis. J Gen Intern Med. 2021;36(1):27-34. doi:10.1007/s11606-020-05965-1
68. Carpenter JD, Gorman PN. Using medication list—problem list mismatches as markers of potential error. Proc AMIA Symp. 2002:106-110.
69. Hron JD, Manzi S, Dionne R, et al. Electronic medication reconciliation and medication errors. Int J Qual Health Care. 2015;27(4):314-319. doi:10.1093/intqhc/mzv046
70. Graber ML, Siegal D, Riah H, Johnston D, Kenyon K. Electronic health record–related events in medical malpractice claims. J Patient Saf. 2019;15(2):77-85. doi:10.1097/PTS.0000000000000240
71. Murphy DR, Wu L, Thomas EJ, Forjuoh SN, Meyer AND, Singh H. Electronic trigger-based intervention to reduce delays in diagnostic evaluation for cancer: a cluster randomized controlled trial. J Clin Oncol. 2015;33(31):3560-3567. doi:10.1200/JCO.2015.61.1301
72. Singh H, Giardina TD, Forjuoh SN, et al. Electronic health record-based surveillance of diagnostic errors in primary care. BMJ Qual Saf. 2012;21(2):93-100. doi:10.1136/bmjqs-2011-000304
73. Armaignac DL, Saxena A, Rubens M, et al. Impact of telemedicine on mortality, length of stay, and cost among patients in progressive care units: experience from a large healthcare system. Crit Care Med. 2018;46(5):728-735. doi:10.1097/CCM.0000000000002994
74. MacKinnon GE, Brittain EL. Mobile health technologies in cardiopulmonary disease. Chest. 2020;157(3):654-664. doi:10.1016/j.chest.2019.10.015
75. DeVore AD, Wosik J, Hernandez AF. The future of wearables in heart failure patients. JACC Heart Fail. 2019;7(11):922-932. doi:10.1016/j.jchf.2019.08.008
76. Tsai TL, Fridsma DB, Gatti G. Computer decision support as a source of interpretation error: the case of electrocardiograms. J Am Med Inform Assoc. 2003;10(5):478-483. doi:10.1197/jamia.M1279
77. Lin SY, Mahoney MR, Sinsky CA. Ten ways artificial intelligence will transform primary care. J Gen Intern Med. 2019;34(8):1626-1630. doi:10.1007/s11606-019-05035-1
78. Ramirez AH, Gebo KA, Harris PA. Progress with the All Of Us research program: opening access for researchers. JAMA. 2021;325(24):2441-2442. doi:10.1001/jama.2021.7702
79. Johnson KB, Wei W, Weeraratne D, et al. Precision medicine, AI, and the future of personalized health care. Clin Transl Sci. 2021;14(1):86-93. doi:10.1111/cts.12884
80. Gupta A, Snyder A, Kachalia A, Flanders S, Saint S, Chopra V. Malpractice claims related to diagnostic errors in the hospital. BMJ Qual Saf. 2017;27(1):bmjqs-2017-006774. doi:10.1136/bmjqs-2017-006774
81. Renkema E, Broekhuis M, Ahaus K. Conditions that influence the impact of malpractice litigation risk on physicians’ behavior regarding patient safety. BMC Health Serv Res. 2014;14(1):38. doi:10.1186/1472-6963-14-38
82. Kachalia A, Mello MM, Nallamothu BK, Studdert DM. Legal and policy interventions to improve patient safety. Circulation. 2016;133(7):661-671. doi:10.1161/CIRCULATIONAHA.115.015880
1. Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch Intern Med. 2005;165(13):1493-1499. doi:10.1001/archinte.165.13.1493
2. National Academies of Sciences, Engineering, and Medicine. 2015. Improving Diagnosis in Health Care. The National Academies Press. doi:10.17226/21794
3. Singh H, Graber ML. Improving diagnosis in health care—the next imperative for patient safety. N Engl J Med. 2015;373(26):2493-2495. doi:10.1056/NEJMp1512241
4. Makary MA, Daniel M. Medical error—the third leading cause of death in the US. BMJ. 2016;353:i2139. doi:10.1136/bmj.i2139
5. Flanders SA, Centor B, Weber V, McGinn T, Desalvo K, Auerbach A. Challenges and opportunities in academic hospital medicine: report from the academic hospital medicine summit. J Gen Intern Med. 2009;24(5):636-641. doi:10.1007/s11606-009-0944-6
6. Griffin JA, Carr K, Bersani K, et al. Analyzing diagnostic errors in the acute setting: a process-driven approach. Diagnosis (Berl). 2021;9(1):77-88. doi:10.1515/dx-2021-0033
7. Itri JN, Tappouni RR, McEachern RO, Pesch AJ, Patel SH. Fundamentals of diagnostic error in imaging. RadioGraphics. 2018;38(6):1845-1865. doi:10.1148/rg.2018180021
8. Hammerling JA. A Review of medical errors in laboratory diagnostics and where we are today. Lab Med. 2012;43(2):41-44. doi:10.1309/LM6ER9WJR1IHQAUY
9. Gunderson CG, Bilan VP, Holleck JL, et al. Prevalence of harmful diagnostic errors in hospitalised adults: a systematic review and meta-analysis. BMJ Qual Saf. 2020;29(12):1008-1018. doi:10.1136/bmjqs-2019-010822
10. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370-376. doi:10.1056/NEJM199102073240604
11. Leape LL, Brennan TA, Laird N, et al. The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II. N Engl J Med. 1991;324(6):377-384. doi:10.1056/NEJM199102073240605
12. Localio AR, Lawthers AG, Brennan TA, et al. Relation between malpractice claims and adverse events due to negligence. Results of the Harvard Medical Practice Study III. N Engl J Med. 1991;325(4):245-251. doi:10.1056/NEJM199107253250405
13. Wilson RM, Michel P, Olsen S, et al. Patient safety in developing countries: retrospective estimation of scale and nature of harm to patients in hospital. BMJ. 2012;344:e832. doi:10.1136/bmj.e832
14. Wilson RM, Runciman WB, Gibberd RW, Harrison BT, Newby L, Hamilton JD. The Quality in Australian Health Care Study. Med J Aust. 1995;163(9):458-471. doi:10.5694/j.1326-5377.1995.tb124691.x
15. Thomas EJ, Studdert DM, Burstin HR, et al. Incidence and types of adverse events and negligent care in Utah and Colorado. Med Care. 2000;38(3):261-271. doi:10.1097/00005650-200003000-00003
16. Baker GR, Norton PG, Flintoft V, et al. The Canadian Adverse Events Study: the incidence of adverse events among hospital patients in Canada. CMAJ. 2004;170(11):1678-1686. doi:10.1503/cmaj.1040498
17. Davis P, Lay-Yee R, Briant R, Ali W, Scott A, Schug S. Adverse events in New Zealand public hospitals II: preventability and clinical context. N Z Med J. 2003;116(1183):U624.
18. Aranaz-Andrés JM, Aibar-Remón C, Vitaller-Murillo J, et al. Incidence of adverse events related to health care in Spain: results of the Spanish National Study of Adverse Events. J Epidemiol Community Health. 2008;62(12):1022-1029. doi:10.1136/jech.2007.065227
19. Bates DW, Levine DM, Salmasian H, et al. The safety of inpatient health care. N Engl J Med. 2023;388(2):142-153. doi:10.1056/NEJMsa2206117
20. Soop M, Fryksmark U, Köster M, Haglund B. The incidence of adverse events in Swedish hospitals: a retrospective medical record review study. Int J Qual Health Care. 2009;21(4):285-291. doi:10.1093/intqhc/mzp025
21. Rafter N, Hickey A, Conroy RM, et al. The Irish National Adverse Events Study (INAES): the frequency and nature of adverse events in Irish hospitals—a retrospective record review study. BMJ Qual Saf. 2017;26(2):111-119. doi:10.1136/bmjqs-2015-004828
22. Blendon RJ, DesRoches CM, Brodie M, et al. Views of practicing physicians and the public on medical errors. N Engl J Med. 2002;347(24):1933-1940. doi:10.1056/NEJMsa022151
23. Saber Tehrani AS, Lee H, Mathews SC, et al. 25-year summary of US malpractice claims for diagnostic errors 1986-2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672-680. doi:10.1136/bmjqs-2012-001550
24. Malik MA, Motta-Calderon D, Piniella N, et al. A structured approach to EHR surveillance of diagnostic error in acute care: an exploratory analysis of two institutionally-defined case cohorts. Diagnosis (Berl). 2022;9(4):446-457. doi:10.1515/dx-2022-0032
25. Graber ML. The incidence of diagnostic error in medicine. BMJ Qual Saf. 2013;22(suppl 2):ii21-ii27. doi:10.1136/bmjqs-2012-001615
26. Bergl PA, Taneja A, El-Kareh R, Singh H, Nanchal RS. Frequency, risk factors, causes, and consequences of diagnostic errors in critically ill medical patients: a retrospective cohort study. Crit Care Med. 2019;47(11):e902-e910. doi:10.1097/CCM.0000000000003976
27. Hogan H, Healey F, Neale G, Thomson R, Vincent C, Black N. Preventable deaths due to problems in care in English acute hospitals: a retrospective case record review study. BMJ Qual Saf. 2012;21(9):737-745. doi:10.1136/bmjqs-2011-001159
28. Bergl PA, Nanchal RS, Singh H. Diagnostic error in the critically ill: defining the problem and exploring next steps to advance intensive care unit safety. Ann Am Thorac Soc. 2018;15(8):903-907. doi:10.1513/AnnalsATS.201801-068PS
29. Marquet K, Claes N, De Troy E, et al. One fourth of unplanned transfers to a higher level of care are associated with a highly preventable adverse event: a patient record review in six Belgian hospitals. Crit Care Med. 2015;43(5):1053-1061. doi:10.1097/CCM.0000000000000932
30. Rodwin BA, Bilan VP, Merchant NB, et al. Rate of preventable mortality in hospitalized patients: a systematic review and meta-analysis. J Gen Intern Med. 2020;35(7):2099-2106. doi:10.1007/s11606-019-05592-5
31. Winters B, Custer J, Galvagno SM, et al. Diagnostic errors in the intensive care unit: a systematic review of autopsy studies. BMJ Qual Saf. 2012;21(11):894-902. doi:10.1136/bmjqs-2012-000803
32. Raffel KE, Kantor MA, Barish P, et al. Prevalence and characterisation of diagnostic error among 7-day all-cause hospital medicine readmissions: a retrospective cohort study. BMJ Qual Saf. 2020;29(12):971-979. doi:10.1136/bmjqs-2020-010896
33. Weingart SN, Pagovich O, Sands DZ, et al. What can hospitalized patients tell us about adverse events? learning from patient-reported incidents. J Gen Intern Med. 2005;20(9):830-836. doi:10.1111/j.1525-1497.2005.0180.x
34. Schiff GD, Hasan O, Kim S, et al. Diagnostic error in medicine: analysis of 583 physician-reported errors. Arch Intern Med. 2009;169(20):1881-1887. doi:10.1001/archinternmed.2009.333
35. Singh H, Schiff GD, Graber ML, Onakpoya I, Thompson MJ. The global burden of diagnostic errors in primary care. BMJ Qual Saf. 2017;26(6):484-494. doi:10.1136/bmjqs-2016-005401
36. Schiff GD, Leape LL. Commentary: how can we make diagnosis safer? Acad Med J Assoc Am Med Coll. 2012;87(2):135-138. doi:10.1097/ACM.0b013e31823f711c
37. Schiff GD, Kim S, Abrams R, et al. Diagnosing diagnosis errors: lessons from a multi-institutional collaborative project. In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds. Advances in Patient Safety: From Research to Implementation. Volume 2: Concepts and Methodology. AHRQ Publication No. 05-0021-2. Agency for Healthcare Research and Quality (US); 2005. Accessed January 16, 2023. http://www.ncbi.nlm.nih.gov/books/NBK20492/
38. Newman-Toker DE. A unified conceptual model for diagnostic errors: underdiagnosis, overdiagnosis, and misdiagnosis. Diagnosis (Berl). 2014;1(1):43-48. doi:10.1515/dx-2013-0027
39. Abimanyi-Ochom J, Bohingamu Mudiyanselage S, Catchpool M, Firipis M, Wanni Arachchige Dona S, Watts JJ. Strategies to reduce diagnostic errors: a systematic review. BMC Med Inform Decis Mak. 2019;19(1):174. doi:10.1186/s12911-019-0901-1
40. Gupta A, Harrod M, Quinn M, et al. Mind the overlap: how system problems contribute to cognitive failure and diagnostic errors. Diagnosis (Berl). 2018;5(3):151-156. doi:10.1515/dx-2018-0014
41. Saposnik G, Redelmeier D, Ruff CC, Tobler PN. Cognitive biases associated with medical decisions: a systematic review. BMC Med Inform Decis Mak. 2016;16:138. doi:10.1186/s12911-016-0377-1
42. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78(8):775-780. doi: 10.1097/00001888-200308000-00003
43. Chapman EN, Kaatz A, Carnes M. Physicians and implicit bias: how doctors may unwittingly perpetuate health care disparities. J Gen Intern Med. 2013;28(11):1504-1510. doi:10.1007/s11606-013-2441-1
44. Zwaan L, Singh H. The challenges in defining and measuring diagnostic error. Diagnosis (Ber). 2015;2(2):97-103. doi:10.1515/dx-2014-0069
45. Arkes HR, Wortmann RL, Saville PD, Harkness AR. Hindsight bias among physicians weighing the likelihood of diagnoses. J Appl Psychol. 1981;66(2):252-254.
46. Singh H. Editorial: Helping health care organizations to define diagnostic errors as missed opportunities in diagnosis. Jt Comm J Qual Patient Saf. 2014;40(3):99-101. doi:10.1016/s1553-7250(14)40012-6
47. Vassar M, Holzmann M. The retrospective chart review: important methodological considerations. J Educ Eval Health Prof. 2013;10:12. doi:10.3352/jeehp.2013.10.12
48. Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102(9):605-613. doi:10.1093/jnci/djq099
49. Moynihan R, Doust J, Henry D. Preventing overdiagnosis: how to stop harming the healthy. BMJ. 2012;344:e3502. doi:10.1136/bmj.e3502
50. Hayward RA, Hofer TP. Estimating hospital deaths due to medical errors: preventability is in the eye of the reviewer. JAMA. 2001;286(4):415-420. doi:10.1001/jama.286.4.415
51. Singh H, Sittig DF. Advancing the science of measurement of diagnostic errors in healthcare: the Safer Dx framework. BMJ Qual Saf. 2015;24(2):103-110. doi:10.1136/bmjqs-2014-003675
52. Singh H, Khanna A, Spitzmueller C, Meyer AND. Recommendations for using the Revised Safer Dx Instrument to help measure and improve diagnostic safety. Diagnosis (Berl). 2019;6(4):315-323. doi:10.1515/dx-2019-0012
53. Classen DC, Resar R, Griffin F, et al. “Global trigger tool” shows that adverse events in hospitals may be ten times greater than previously measured. Health Aff (Millwood). 2011;30(4):581-589. doi:10.1377/hlthaff.2011.0190
54. Schiff GD. Minimizing diagnostic error: the importance of follow-up and feedback. Am J Med. 2008;121(5 suppl):S38-S42. doi:10.1016/j.amjmed.2008.02.004
55. Mitchell I, Schuster A, Smith K, Pronovost P, Wu A. Patient safety incident reporting: a qualitative study of thoughts and perceptions of experts 15 years after “To Err is Human.” BMJ Qual Saf. 2016;25(2):92-99. doi:10.1136/bmjqs-2015-004405
56. Mazurenko O, Collum T, Ferdinand A, Menachemi N. Predictors of hospital patient satisfaction as measured by HCAHPS: a systematic review. J Healthc Manag. 2017;62(4):272-283. doi:10.1097/JHM-D-15-00050
57. Liberman AL, Newman-Toker DE. Symptom-Disease Pair Analysis of Diagnostic Error (SPADE): a conceptual framework and methodological approach for unearthing misdiagnosis-related harms using big data. BMJ Qual Saf. 2018;27(7):557-566. doi:10.1136/bmjqs-2017-007032
58. Utility of Predictive Systems to Identify Inpatient Diagnostic Errors: the UPSIDE study. NIH RePort/RePORTER. Accessed January 14, 2023. https://reporter.nih.gov/search/rpoHXlEAcEudQV3B9ld8iw/project-details/10020962
59. Overview of Patient Safety Learning Laboratory (PSLL) Projects. Agency for Healthcare Research and Quality. Accessed January 14, 2023. https://www.ahrq.gov/patient-safety/resources/learning-lab/index.html
60. Achieving Diagnostic Excellence through Prevention and Teamwork (ADEPT). NIH RePort/RePORTER. Accessed January 14, 2023. https://reporter.nih.gov/project-details/10642576
61. Zwaan L, Singh H. Diagnostic error in hospitals: finding forests not just the big trees. BMJ Qual Saf. 2020;29(12):961-964. doi:10.1136/bmjqs-2020-011099
62. Longtin Y, Sax H, Leape LL, Sheridan SE, Donaldson L, Pittet D. Patient participation: current knowledge and applicability to patient safety. Mayo Clin Proc. 2010;85(1):53-62. doi:10.4065/mcp.2009.0248
63. Murphy DR, Singh H, Berlin L. Communication breakdowns and diagnostic errors: a radiology perspective. Diagnosis (Berl). 2014;1(4):253-261. doi:10.1515/dx-2014-0035
64. Singh H, Naik AD, Rao R, Petersen LA. Reducing diagnostic errors through effective communication: harnessing the power of information technology. J Gen Intern Med. 2008;23(4):489-494. doi:10.1007/s11606-007-0393-z
65. Singh H, Connor DM, Dhaliwal G. Five strategies for clinicians to advance diagnostic excellence. BMJ. 2022;376:e068044. doi:10.1136/bmj-2021-068044
66. Yale S, Cohen S, Bordini BJ. Diagnostic time-outs to improve diagnosis. Crit Care Clin. 2022;38(2):185-194. doi:10.1016/j.ccc.2021.11.008
67. Schwartz A, Peskin S, Spiro A, Weiner SJ. Impact of unannounced standardized patient audit and feedback on care, documentation, and costs: an experiment and claims analysis. J Gen Intern Med. 2021;36(1):27-34. doi:10.1007/s11606-020-05965-1
68. Carpenter JD, Gorman PN. Using medication list—problem list mismatches as markers of potential error. Proc AMIA Symp. 2002:106-110.
69. Hron JD, Manzi S, Dionne R, et al. Electronic medication reconciliation and medication errors. Int J Qual Health Care. 2015;27(4):314-319. doi:10.1093/intqhc/mzv046
70. Graber ML, Siegal D, Riah H, Johnston D, Kenyon K. Electronic health record–related events in medical malpractice claims. J Patient Saf. 2019;15(2):77-85. doi:10.1097/PTS.0000000000000240
71. Murphy DR, Wu L, Thomas EJ, Forjuoh SN, Meyer AND, Singh H. Electronic trigger-based intervention to reduce delays in diagnostic evaluation for cancer: a cluster randomized controlled trial. J Clin Oncol. 2015;33(31):3560-3567. doi:10.1200/JCO.2015.61.1301
72. Singh H, Giardina TD, Forjuoh SN, et al. Electronic health record-based surveillance of diagnostic errors in primary care. BMJ Qual Saf. 2012;21(2):93-100. doi:10.1136/bmjqs-2011-000304
73. Armaignac DL, Saxena A, Rubens M, et al. Impact of telemedicine on mortality, length of stay, and cost among patients in progressive care units: experience from a large healthcare system. Crit Care Med. 2018;46(5):728-735. doi:10.1097/CCM.0000000000002994
74. MacKinnon GE, Brittain EL. Mobile health technologies in cardiopulmonary disease. Chest. 2020;157(3):654-664. doi:10.1016/j.chest.2019.10.015
75. DeVore AD, Wosik J, Hernandez AF. The future of wearables in heart failure patients. JACC Heart Fail. 2019;7(11):922-932. doi:10.1016/j.jchf.2019.08.008
76. Tsai TL, Fridsma DB, Gatti G. Computer decision support as a source of interpretation error: the case of electrocardiograms. J Am Med Inform Assoc. 2003;10(5):478-483. doi:10.1197/jamia.M1279
77. Lin SY, Mahoney MR, Sinsky CA. Ten ways artificial intelligence will transform primary care. J Gen Intern Med. 2019;34(8):1626-1630. doi:10.1007/s11606-019-05035-1
78. Ramirez AH, Gebo KA, Harris PA. Progress with the All Of Us research program: opening access for researchers. JAMA. 2021;325(24):2441-2442. doi:10.1001/jama.2021.7702
79. Johnson KB, Wei W, Weeraratne D, et al. Precision medicine, AI, and the future of personalized health care. Clin Transl Sci. 2021;14(1):86-93. doi:10.1111/cts.12884
80. Gupta A, Snyder A, Kachalia A, Flanders S, Saint S, Chopra V. Malpractice claims related to diagnostic errors in the hospital. BMJ Qual Saf. 2017;27(1):bmjqs-2017-006774. doi:10.1136/bmjqs-2017-006774
81. Renkema E, Broekhuis M, Ahaus K. Conditions that influence the impact of malpractice litigation risk on physicians’ behavior regarding patient safety. BMC Health Serv Res. 2014;14(1):38. doi:10.1186/1472-6963-14-38
82. Kachalia A, Mello MM, Nallamothu BK, Studdert DM. Legal and policy interventions to improve patient safety. Circulation. 2016;133(7):661-671. doi:10.1161/CIRCULATIONAHA.115.015880
Safety in Health Care: An Essential Pillar of Quality
Each year, 40,000 to 98,000 deaths occur due to medical errors.1 The Harvard Medical Practice Study (HMPS), published in 1991, found that 3.7% of hospitalized patients were harmed by adverse events and 1% were harmed by adverse events due to negligence.2 The latest HMPS showed that, despite significant improvements in patient safety over the past 3 decades, patient safety challenges persist. This study found that inpatient care leads to harm in nearly a quarter of patients, and that 1 in 4 of these adverse events are preventable.3
Since the first HMPS study was published, efforts to improve patient safety have focused on identifying causes of medical error and the design and implementation of interventions to mitigate errors. Factors contributing to medical errors have been well documented: the complexity of care delivery from inpatient to outpatient settings, with transitions of care and extensive use of medications; multiple comorbidities; and the fragmentation of care across multiple systems and specialties. Although most errors are related to process or system failure, accountability of each practitioner and clinician is essential to promoting a culture of safety. Many medical errors are preventable through multifaceted approaches employed throughout the phases of the care,4 with medication errors, both prescribing and administration, and diagnostic and treatment errors encompassing most risk prevention areas. Broadly, safety efforts should emphasize building a culture of safety where all safety events are reported, including near-miss events.
Two articles in this issue of JCOM address key elements of patient safety: building a safety culture and diagnostic error. Merchant et al5 report on an initiative designed to promote a safety culture by recognizing and rewarding staff who identify and report near misses. The tiered awards program they designed led to significantly increased staff participation in the safety awards nomination process and was associated with increased reporting of actual and close-call events and greater attendance at monthly safety forums. Goyal et al,6 noting that diagnostic error rates in hospitalized patients remain unacceptably high, provide a concise update on diagnostic error among inpatients, focusing on issues related to defining and measuring diagnostic errors and current strategies to improve diagnostic safety in hospitalized patients. In a third article, Sathi et al report on efforts to teach quality improvement (QI) methods to internal medicine trainees; their project increased residents’ knowledge of their patient panels and comfort with QI approaches and led to improved patient outcomes.
Major progress has been made to improve health care safety since the first HMPS was published. However, the latest HMPS shows that patient safety efforts must continue, given the persistent risk for patient harm in the current health care delivery system. Safety, along with clear accountability for identifying, reporting, and addressing errors, should be a top priority for health care systems throughout the preventive, diagnostic, and therapeutic phases of care.
Corresponding author: Ebrahim Barkoudah, MD, MPH; [email protected]
1. Clancy C, Munier W, Brady J. National healthcare quality report. Agency for Healthcare Research and Quality; 2013.
2. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370-376. doi:10.1056/NEJM199102073240604
3. Bates DW, Levine DM, Salmasian H, et al. The safety of inpatient health care. N Engl J Med. 2023;388(2):142-153. doi:10.1056/NEJMsa2206117
4. Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events: implications for prevention. JAMA. 1995;274(1):29-34.
5. Merchant NB, O’Neal J, Murray JS. Development of a safety awards program at a Veterans Affairs health care system: a quality improvement initiative. J Clin Outcome Manag. 2023;30(1):9-16. doi:10.12788/jcom.0120
6. Goyal A, Martin-Doyle W, Dalal AK. Diagnostic errors in hospitalized patients. J Clin Outcome Manag. 2023;30(1):17-27. doi:10.12788/jcom.0121
7. Sathi K, Huang KTL, Chandler DM, et al. Teaching quality improvement to internal medicine residents to address patient care gaps in ambulatory quality metrics. J Clin Outcome Manag. 2023;30(1):1-6.doi:10.12788/jcom.0119
Each year, 40,000 to 98,000 deaths occur due to medical errors.1 The Harvard Medical Practice Study (HMPS), published in 1991, found that 3.7% of hospitalized patients were harmed by adverse events and 1% were harmed by adverse events due to negligence.2 The latest HMPS showed that, despite significant improvements in patient safety over the past 3 decades, patient safety challenges persist. This study found that inpatient care leads to harm in nearly a quarter of patients, and that 1 in 4 of these adverse events are preventable.3
Since the first HMPS study was published, efforts to improve patient safety have focused on identifying causes of medical error and the design and implementation of interventions to mitigate errors. Factors contributing to medical errors have been well documented: the complexity of care delivery from inpatient to outpatient settings, with transitions of care and extensive use of medications; multiple comorbidities; and the fragmentation of care across multiple systems and specialties. Although most errors are related to process or system failure, accountability of each practitioner and clinician is essential to promoting a culture of safety. Many medical errors are preventable through multifaceted approaches employed throughout the phases of the care,4 with medication errors, both prescribing and administration, and diagnostic and treatment errors encompassing most risk prevention areas. Broadly, safety efforts should emphasize building a culture of safety where all safety events are reported, including near-miss events.
Two articles in this issue of JCOM address key elements of patient safety: building a safety culture and diagnostic error. Merchant et al5 report on an initiative designed to promote a safety culture by recognizing and rewarding staff who identify and report near misses. The tiered awards program they designed led to significantly increased staff participation in the safety awards nomination process and was associated with increased reporting of actual and close-call events and greater attendance at monthly safety forums. Goyal et al,6 noting that diagnostic error rates in hospitalized patients remain unacceptably high, provide a concise update on diagnostic error among inpatients, focusing on issues related to defining and measuring diagnostic errors and current strategies to improve diagnostic safety in hospitalized patients. In a third article, Sathi et al report on efforts to teach quality improvement (QI) methods to internal medicine trainees; their project increased residents’ knowledge of their patient panels and comfort with QI approaches and led to improved patient outcomes.
Major progress has been made to improve health care safety since the first HMPS was published. However, the latest HMPS shows that patient safety efforts must continue, given the persistent risk for patient harm in the current health care delivery system. Safety, along with clear accountability for identifying, reporting, and addressing errors, should be a top priority for health care systems throughout the preventive, diagnostic, and therapeutic phases of care.
Corresponding author: Ebrahim Barkoudah, MD, MPH; [email protected]
Each year, 40,000 to 98,000 deaths occur due to medical errors.1 The Harvard Medical Practice Study (HMPS), published in 1991, found that 3.7% of hospitalized patients were harmed by adverse events and 1% were harmed by adverse events due to negligence.2 The latest HMPS showed that, despite significant improvements in patient safety over the past 3 decades, patient safety challenges persist. This study found that inpatient care leads to harm in nearly a quarter of patients, and that 1 in 4 of these adverse events are preventable.3
Since the first HMPS study was published, efforts to improve patient safety have focused on identifying causes of medical error and the design and implementation of interventions to mitigate errors. Factors contributing to medical errors have been well documented: the complexity of care delivery from inpatient to outpatient settings, with transitions of care and extensive use of medications; multiple comorbidities; and the fragmentation of care across multiple systems and specialties. Although most errors are related to process or system failure, accountability of each practitioner and clinician is essential to promoting a culture of safety. Many medical errors are preventable through multifaceted approaches employed throughout the phases of the care,4 with medication errors, both prescribing and administration, and diagnostic and treatment errors encompassing most risk prevention areas. Broadly, safety efforts should emphasize building a culture of safety where all safety events are reported, including near-miss events.
Two articles in this issue of JCOM address key elements of patient safety: building a safety culture and diagnostic error. Merchant et al5 report on an initiative designed to promote a safety culture by recognizing and rewarding staff who identify and report near misses. The tiered awards program they designed led to significantly increased staff participation in the safety awards nomination process and was associated with increased reporting of actual and close-call events and greater attendance at monthly safety forums. Goyal et al,6 noting that diagnostic error rates in hospitalized patients remain unacceptably high, provide a concise update on diagnostic error among inpatients, focusing on issues related to defining and measuring diagnostic errors and current strategies to improve diagnostic safety in hospitalized patients. In a third article, Sathi et al report on efforts to teach quality improvement (QI) methods to internal medicine trainees; their project increased residents’ knowledge of their patient panels and comfort with QI approaches and led to improved patient outcomes.
Major progress has been made to improve health care safety since the first HMPS was published. However, the latest HMPS shows that patient safety efforts must continue, given the persistent risk for patient harm in the current health care delivery system. Safety, along with clear accountability for identifying, reporting, and addressing errors, should be a top priority for health care systems throughout the preventive, diagnostic, and therapeutic phases of care.
Corresponding author: Ebrahim Barkoudah, MD, MPH; [email protected]
1. Clancy C, Munier W, Brady J. National healthcare quality report. Agency for Healthcare Research and Quality; 2013.
2. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370-376. doi:10.1056/NEJM199102073240604
3. Bates DW, Levine DM, Salmasian H, et al. The safety of inpatient health care. N Engl J Med. 2023;388(2):142-153. doi:10.1056/NEJMsa2206117
4. Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events: implications for prevention. JAMA. 1995;274(1):29-34.
5. Merchant NB, O’Neal J, Murray JS. Development of a safety awards program at a Veterans Affairs health care system: a quality improvement initiative. J Clin Outcome Manag. 2023;30(1):9-16. doi:10.12788/jcom.0120
6. Goyal A, Martin-Doyle W, Dalal AK. Diagnostic errors in hospitalized patients. J Clin Outcome Manag. 2023;30(1):17-27. doi:10.12788/jcom.0121
7. Sathi K, Huang KTL, Chandler DM, et al. Teaching quality improvement to internal medicine residents to address patient care gaps in ambulatory quality metrics. J Clin Outcome Manag. 2023;30(1):1-6.doi:10.12788/jcom.0119
1. Clancy C, Munier W, Brady J. National healthcare quality report. Agency for Healthcare Research and Quality; 2013.
2. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370-376. doi:10.1056/NEJM199102073240604
3. Bates DW, Levine DM, Salmasian H, et al. The safety of inpatient health care. N Engl J Med. 2023;388(2):142-153. doi:10.1056/NEJMsa2206117
4. Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events: implications for prevention. JAMA. 1995;274(1):29-34.
5. Merchant NB, O’Neal J, Murray JS. Development of a safety awards program at a Veterans Affairs health care system: a quality improvement initiative. J Clin Outcome Manag. 2023;30(1):9-16. doi:10.12788/jcom.0120
6. Goyal A, Martin-Doyle W, Dalal AK. Diagnostic errors in hospitalized patients. J Clin Outcome Manag. 2023;30(1):17-27. doi:10.12788/jcom.0121
7. Sathi K, Huang KTL, Chandler DM, et al. Teaching quality improvement to internal medicine residents to address patient care gaps in ambulatory quality metrics. J Clin Outcome Manag. 2023;30(1):1-6.doi:10.12788/jcom.0119