User login
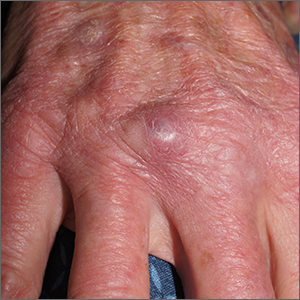
Fatigue and blurred vision
Gestational diabetes is a significant health problem worldwide that is associated with immediate and lifelong consequences for the affected woman and her infant. Gestational diabetes increases the risk for pregnancy-related complications, such as induced labor, cesarean delivery, and preeclampsia. There is also an increased risk for neonatal complications, including large-for-gestational-age birth weight, shoulder dystocia, birth injuries, lung disease, jaundice, and hypoglycemia. Regardless of birth weight, neonates born to mothers with gestational diabetes have greater adiposity than do neonates born to mothers without obesity and with normal glucose tolerance, and they have a predilection toward obesity and obesity-related metabolic disorders, including T2D in childhood and adulthood. Similarly, women who develop gestational diabetes have an increased lifetime risk for T2D as well as an increased risk for cardiovascular disease even if they do not progress to T2D.
According to the International Diabetes Federation, 1 in 6 pregnancies is affected by gestational diabetes. Risk factors include higher age and BMI, previous history of gestational diabetes, a family history of T2D, and polycystic ovarian syndrome. Patients may have few, if any, symptoms of gestational diabetes, or they may mistake their symptoms for the normal side effects of pregnancy. Potential symptoms include blurred vision, tingling or numbness in the hands and/or feet, excessive thirst, frequent urination, sores that heal slowly, and excessive fatigue.
The American Diabetes Association (ADA) states that the treatment of gestational diabetes should include medical nutrition therapy, physical activity, and weight management, depending on pregestational weight. Glucose monitoring is essential: Patients should aim for fasting glucose < 95 mg/dL (5.3 mmol/L) and either 1-hour postprandial glucose < 140 mg/dL (7.8 mmol/L) or 2-hour postprandial glucose < 120 mg/dL (6.7 mmol/L). According to the ADA, insulin should be added to lifestyle modifications if needed to achieve glycemic targets. Metformin and glyburide are not recommended as first-line agents because both cross the placenta to the fetus. Long-term safety data are not available for the use of other oral and noninsulin injectable glucose-lowering medications during pregnancy.
Courtney Whittle, MD, MSW, Diplomate of ABOM, Pediatric Lead, Obesity Champion, TSPMG, Weight A Minute Clinic, Atlanta, Georgia.
Courtney Whittle, MD, MSW, Diplomate of ABOM, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Gestational diabetes is a significant health problem worldwide that is associated with immediate and lifelong consequences for the affected woman and her infant. Gestational diabetes increases the risk for pregnancy-related complications, such as induced labor, cesarean delivery, and preeclampsia. There is also an increased risk for neonatal complications, including large-for-gestational-age birth weight, shoulder dystocia, birth injuries, lung disease, jaundice, and hypoglycemia. Regardless of birth weight, neonates born to mothers with gestational diabetes have greater adiposity than do neonates born to mothers without obesity and with normal glucose tolerance, and they have a predilection toward obesity and obesity-related metabolic disorders, including T2D in childhood and adulthood. Similarly, women who develop gestational diabetes have an increased lifetime risk for T2D as well as an increased risk for cardiovascular disease even if they do not progress to T2D.
According to the International Diabetes Federation, 1 in 6 pregnancies is affected by gestational diabetes. Risk factors include higher age and BMI, previous history of gestational diabetes, a family history of T2D, and polycystic ovarian syndrome. Patients may have few, if any, symptoms of gestational diabetes, or they may mistake their symptoms for the normal side effects of pregnancy. Potential symptoms include blurred vision, tingling or numbness in the hands and/or feet, excessive thirst, frequent urination, sores that heal slowly, and excessive fatigue.
The American Diabetes Association (ADA) states that the treatment of gestational diabetes should include medical nutrition therapy, physical activity, and weight management, depending on pregestational weight. Glucose monitoring is essential: Patients should aim for fasting glucose < 95 mg/dL (5.3 mmol/L) and either 1-hour postprandial glucose < 140 mg/dL (7.8 mmol/L) or 2-hour postprandial glucose < 120 mg/dL (6.7 mmol/L). According to the ADA, insulin should be added to lifestyle modifications if needed to achieve glycemic targets. Metformin and glyburide are not recommended as first-line agents because both cross the placenta to the fetus. Long-term safety data are not available for the use of other oral and noninsulin injectable glucose-lowering medications during pregnancy.
Courtney Whittle, MD, MSW, Diplomate of ABOM, Pediatric Lead, Obesity Champion, TSPMG, Weight A Minute Clinic, Atlanta, Georgia.
Courtney Whittle, MD, MSW, Diplomate of ABOM, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Gestational diabetes is a significant health problem worldwide that is associated with immediate and lifelong consequences for the affected woman and her infant. Gestational diabetes increases the risk for pregnancy-related complications, such as induced labor, cesarean delivery, and preeclampsia. There is also an increased risk for neonatal complications, including large-for-gestational-age birth weight, shoulder dystocia, birth injuries, lung disease, jaundice, and hypoglycemia. Regardless of birth weight, neonates born to mothers with gestational diabetes have greater adiposity than do neonates born to mothers without obesity and with normal glucose tolerance, and they have a predilection toward obesity and obesity-related metabolic disorders, including T2D in childhood and adulthood. Similarly, women who develop gestational diabetes have an increased lifetime risk for T2D as well as an increased risk for cardiovascular disease even if they do not progress to T2D.
According to the International Diabetes Federation, 1 in 6 pregnancies is affected by gestational diabetes. Risk factors include higher age and BMI, previous history of gestational diabetes, a family history of T2D, and polycystic ovarian syndrome. Patients may have few, if any, symptoms of gestational diabetes, or they may mistake their symptoms for the normal side effects of pregnancy. Potential symptoms include blurred vision, tingling or numbness in the hands and/or feet, excessive thirst, frequent urination, sores that heal slowly, and excessive fatigue.
The American Diabetes Association (ADA) states that the treatment of gestational diabetes should include medical nutrition therapy, physical activity, and weight management, depending on pregestational weight. Glucose monitoring is essential: Patients should aim for fasting glucose < 95 mg/dL (5.3 mmol/L) and either 1-hour postprandial glucose < 140 mg/dL (7.8 mmol/L) or 2-hour postprandial glucose < 120 mg/dL (6.7 mmol/L). According to the ADA, insulin should be added to lifestyle modifications if needed to achieve glycemic targets. Metformin and glyburide are not recommended as first-line agents because both cross the placenta to the fetus. Long-term safety data are not available for the use of other oral and noninsulin injectable glucose-lowering medications during pregnancy.
Courtney Whittle, MD, MSW, Diplomate of ABOM, Pediatric Lead, Obesity Champion, TSPMG, Weight A Minute Clinic, Atlanta, Georgia.
Courtney Whittle, MD, MSW, Diplomate of ABOM, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 32-year-old Asian American woman (gravida 2 para 1) presents at 26 weeks' gestation for experiencing fatigue and blurred vision. The patient's previous pregnancy 3 years earlier was an uncomplicated vaginal delivery at 38 weeks' gestation. The baby weighed 7 lb 8 oz at delivery. The patient's maternal family history is notable for hypertension and type 2 diabetes (T2D). At the time of presentation, the patient is 5 ft 4 in, and her prepregnancy body mass index (BMI) was 31.8. Physical examination reveals blood pressure of 130/88 beats/min and fetal heart tones at 148 beats/min. She does not report ocular pain and there is no evidence of ocular redness, swelling, or discharge. The patient reports viral gastroenteritis approximately 10 days earlier, which has since resolved. A 1-hour oral glucose tolerance test (OGTT) at 24 weeks was abnormal; a subsequent 3-hour OGTT showed:
• 109 mg/dL (7:30 AM)
• 205 mg/dL (8:30 AM)
• 164 mg/dL (9:30 AM)
• 166 mg/dL (10:30 AM)
Transition to Tenecteplase From t-PA for Acute Ischemic Stroke at Walter Reed National Military Medical Center
Tissue plasminogen activator (t-PA) has been the standard IV thrombolytic used in acute ischemic stroke treatment since its US Food and Drug Administration (FDA) approval in 1995. Trials have established this drug’s efficacy in the treatment of acute ischemic stroke and the appropriate patient population for therapy.1-3 Published guidelines and experiences have made clear that a written protocol with extensive personnel training is important to deliver this care properly.4
Tenecteplase has been available for use in the treatment of acute myocardial infarction (MI) and studied in acute ischemic strokes since 2000. Recent large multicenter trials have suggested tenecteplase may work better than t-PA in the recanalization of large vessel occlusions (LVOs) and have provided guidance on proper dosing in acute ischemic stroke victims.5-8 Compared with t-PA, tenecteplase has a longer half-life, is more fibrin specific (causing less coagulopathy), and is more resistant to endogenous plasminogen activator inhibitor.9,10 Using tenecteplase for acute ischemic stroke is simpler as a single dose bolus rather than a bolus followed by a 1-hour infusion with t-PA. Immediate mechanical thrombectomy for LVO is less complicated without the 1-hour t-PA infusion.5,6 Tenecteplase use also allows for nonthrombectomy hospitals to accelerate transfer times for patients who need thrombectomy following thrombolysis by eliminating the need for critical care nurse–staffed ambulances for interfacility transfer.11 Tenecteplase also is cheaper: Tenecteplase costs $3748 per vial, whereas t-PA costs $5800 per vial equating to roughly a $2000 savings per patient.12,13 Finally, the pharmacy formulary is simplified by using a single thrombolytic agent for both cardiac and neurologic emergencies.
Tenecteplase does have some drawbacks to consider. Currently, tenecteplase is not approved by the FDA for the indication of acute ischemic stroke, though the drug is endorsed by the American Heart Association stroke guidelines of 2019 as an alternative to t-PA.14 There is no stroke-specific preparation of the drug, leading to potential dosing errors. Therefore, a systematic process to safely transition from t-PA to tenecteplase for acute ischemic stroke was undertaken at Walter Reed National Military Medical Center (WRNMMC) in Bethesda, Maryland. Here, we report the process required in making a complex switch in thrombolytic medication along with the potential benefits of making this transition.
OBSERVATIONS
The process to implement tenecteplase required extensive training and education for staff physicians, nurses, pharmacists, radiologists, trainees, and the rapid response team. Our institution administered IV thrombolytic drugs up to 25 times annually to acute ischemic stroke victims, meaning we had to train personnel extensively and repeatedly.
In preparation for the transition to tenecteplase, hospital leadership gathered staff for multidisciplinary administrative meetings that included neurology, emergency medicine, intensive care, pharmacy, radiology, and nursing departments. The purpose of these meetings was to establish a standard operating procedure (SOP) to ensure a safe transition. This process began in May 2020 and involved regular meetings to draft and revise our SOP. Additionally, several leadership and training sessions were held over a 6-month period. Stroke boxes were developed that contained the required evaluation tools, consent forms, medications (tenecteplase and treatments for known complications), dosing cards, and instructions. Final approval of the updated acute ischemic stroke hospital policy was obtained in November 2020 and signed by the above departments.
All inclusion and exclusion criteria were determined to be the same for tenecteplase as they were for t-PA with the notable exception that the WAKE-UP trial protocol would not be supported until further evidence became available.9 The results of the WAKE-UP trial had previously been used at WRNMMC to justify administration of t-PA in patients who awoke with symptoms of acute ischemic stroke, the last known well was unclear or > 4.5 hours, and for whom a magnetic resonance imaging (MRI) of the brain could be obtained rapidly. Based on the WAKE-UP trial, if the MRI scan of the brain in these patients demonstrated restricted diffusion without fluid attenuated inversion recovery (FLAIR) signal changes (diffusion-weighted [DWI]-FLAIR mismatch sign), this indicated that the stroke had likely occurred recently, and it was safe to administer t-PA. This allowed for administration of t-PA outside the standard treatment window of 4.5 hours from last known well, especially in the cases of patients who awoke with symptoms.
Since safety data are not yet available for the use of tenecteplase in this fashion, the WAKE-UP trial protocol was not used as an inclusion criterion. The informed consent form was modified, and the following scenarios were outlined: (1) If the patient or surrogate is immediately available to consent, paper consent will be documented with the additional note that tenecteplase is being used off-label; and (2) If the patient cannot consent and a surrogate is not immediately available, the medicine will be used emergently as long as the neurology resident and attending physicians agree.15
Risk mitigation was considered carefully. The stroke box described above is stocked and maintained by the pharmacy as we have transitioned to using designated pharmacists for the storage and preparation of tenecteplase. We highly recommend the use of designated pharmacists or emergency department pharmacists in this manner to avoid dosing errors.7,16 Since the current pharmacy-provided tenecteplase bottle contains twice the maximum dose indicated for ischemic stroke, only a 5 mL syringe is included in the stroke box to ensure a maximum dose of 25 mg is drawn up after reconstitution. Dosing card charts were made like existing dosing card charts for t-PA to quickly calculate the 0.25 mg/kg dose. In training, the difference in dosing in ischemic stroke was emphasized. Finally, pharmacy has taken responsibility for dosing the medication during stroke codes.
Any medical personnel at WRNMMC can initiate a stroke code by sending a page to the neurology consult service (Figure).
TRANSITION AND RESULTS
From November 2020 to December 2021, 10 patients have been treated in total at WRNMMC (Table).
CONCLUSIONS
The available evidence supports the transition from t-PA to tenecteplase for acute ischemic stroke. The successful transition required months of preparation involving multidisciplinary meetings between neurology, nursing, pharmacy, radiology, rapid response teams, critical care, and emergency medicine departments. Safeguards must be implemented to avoid a tenecteplase dosing error that can lead to potentially life-threatening adverse effects. The results at WRNMMC thus far are promising for safety and efficacy. Several process improvements are planned: a hospital-wide overhead page will accompany the direct page to neurology; other team members, including radiology and pharmacy, will be included on the acute stroke alert; and a stroke-specific paging application will be implemented to better track real-time stroke metrics and improve flow. These measures mirror processes that are occurring in institutions that treat acute stroke patients.
1. Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375(9727):1695-1703. doi:10.1016/S0140-6736(10)60491-6
2. National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581- 1587. doi:10.1056/NEJM199512143332401
3. Hacke W, Donnan G, Fieschi C, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363(9411):768-774. doi:10.1016/S0140-6736(04)15692-4
4. Jauch EC, Saver JL, Adams HP Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870-947. doi:10.1161/STR.0b013e318284056a
5. Campbell B, Mitchell P, Churilov L, et al. Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Engl J Med. 2018;378(17):1573-1582. doi:10.1056/nejmoa1716405
6. Yang P, Zhang Y, Zhang L, et al. Endovascular thrombectomy with or without intravenous alteplase in acute stroke. N Engl J Med. 2020;382(21):1981-1993. doi:10.1056/NEJMoa2001123
7. Menon BK, Buck BH, Singh N, et al. Intravenous tenecteplase compared with alteplase for acute ischaemic stroke in Canada (AcT): a pragmatic, multicentre, open-label, registry-linked, randomised, controlled, noninferiority trial. Lancet. 2022;400(10347):161-169. doi:10.1016/S0140-6736(22)01054-6
8. Campbell BCV, Mitchell PJ, Churilov L, et al. Effect of intravenous tenecteplase dose on cerebral reperfusion before thrombectomy in patients with large vessel occlusion ischemic stroke: the EXTEND-IA TNK part 2 randomized clinical trial. JAMA. 2020;323(13):1257- 1265. doi:10.1001/jama.2020.1511
9. Warach SJ, Dula AN, Milling TJ Jr. Tenecteplase thrombolysis for acute ischemic stroke. Stroke. 2020;51(11):3440- 3451. doi:10.1161/STROKEAHA.120.029749
10. Huang X, Moreton FC, Kalladka D, et al. Coagulation and fibrinolytic activity of tenecteplase and alteplase in acute ischemic stroke. Stroke. 2015;46(12):3543-3546. doi:10.1161/STROKEAHA.115.011290
11. Burgos AM, Saver JL. Evidence that tenecteplase is noninferior to alteplase for acute ischemic stroke: meta-analysis of 5 randomized trials. Stroke. 2019;50(8):2156-2162. doi:10.1161/STROKEAHA.119.025080
12. Potla N, Ganti L. Tenecteplase vs. alteplase for acute ischemic stroke: a systematic review. Int J Emerg Med. 2022;15(1). doi:10.1186/s12245-021-00399-w
13. Warach SJ, Winegar A, Ottenbacher A, Miller C, Gibson D. Abstract WMP52: reduced hospital costs for ischemic stroke treated with tenecteplase. Stroke. 2022;53(suppl 1):AWMP52. doi:10.1161/str.53.suppl_1.WMP52
14. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi:10.1161/str.0000000000000211
15. Faris H, Dewar B, Dowlatshahi D, et al. Ethical justification for deferral of consent in the AcT trial for acute ischemic stroke. Stroke. 2022;53(7):2420-2423. doi:10.1161/strokeaha.122.038760
16. Kvistad CE, Næss H, Helleberg BH, et al. Tenecteplase versus alteplase for the management of acute ischaemic stroke in Norway (NOR-TEST 2, part A): a phase 3, randomised, open-label, blinded endpoint, non-inferiority trial. Lancet Neurol. 2022;21(6):511-519. doi:10.1016/S1474-4422(22)00124-7
Tissue plasminogen activator (t-PA) has been the standard IV thrombolytic used in acute ischemic stroke treatment since its US Food and Drug Administration (FDA) approval in 1995. Trials have established this drug’s efficacy in the treatment of acute ischemic stroke and the appropriate patient population for therapy.1-3 Published guidelines and experiences have made clear that a written protocol with extensive personnel training is important to deliver this care properly.4
Tenecteplase has been available for use in the treatment of acute myocardial infarction (MI) and studied in acute ischemic strokes since 2000. Recent large multicenter trials have suggested tenecteplase may work better than t-PA in the recanalization of large vessel occlusions (LVOs) and have provided guidance on proper dosing in acute ischemic stroke victims.5-8 Compared with t-PA, tenecteplase has a longer half-life, is more fibrin specific (causing less coagulopathy), and is more resistant to endogenous plasminogen activator inhibitor.9,10 Using tenecteplase for acute ischemic stroke is simpler as a single dose bolus rather than a bolus followed by a 1-hour infusion with t-PA. Immediate mechanical thrombectomy for LVO is less complicated without the 1-hour t-PA infusion.5,6 Tenecteplase use also allows for nonthrombectomy hospitals to accelerate transfer times for patients who need thrombectomy following thrombolysis by eliminating the need for critical care nurse–staffed ambulances for interfacility transfer.11 Tenecteplase also is cheaper: Tenecteplase costs $3748 per vial, whereas t-PA costs $5800 per vial equating to roughly a $2000 savings per patient.12,13 Finally, the pharmacy formulary is simplified by using a single thrombolytic agent for both cardiac and neurologic emergencies.
Tenecteplase does have some drawbacks to consider. Currently, tenecteplase is not approved by the FDA for the indication of acute ischemic stroke, though the drug is endorsed by the American Heart Association stroke guidelines of 2019 as an alternative to t-PA.14 There is no stroke-specific preparation of the drug, leading to potential dosing errors. Therefore, a systematic process to safely transition from t-PA to tenecteplase for acute ischemic stroke was undertaken at Walter Reed National Military Medical Center (WRNMMC) in Bethesda, Maryland. Here, we report the process required in making a complex switch in thrombolytic medication along with the potential benefits of making this transition.
OBSERVATIONS
The process to implement tenecteplase required extensive training and education for staff physicians, nurses, pharmacists, radiologists, trainees, and the rapid response team. Our institution administered IV thrombolytic drugs up to 25 times annually to acute ischemic stroke victims, meaning we had to train personnel extensively and repeatedly.
In preparation for the transition to tenecteplase, hospital leadership gathered staff for multidisciplinary administrative meetings that included neurology, emergency medicine, intensive care, pharmacy, radiology, and nursing departments. The purpose of these meetings was to establish a standard operating procedure (SOP) to ensure a safe transition. This process began in May 2020 and involved regular meetings to draft and revise our SOP. Additionally, several leadership and training sessions were held over a 6-month period. Stroke boxes were developed that contained the required evaluation tools, consent forms, medications (tenecteplase and treatments for known complications), dosing cards, and instructions. Final approval of the updated acute ischemic stroke hospital policy was obtained in November 2020 and signed by the above departments.
All inclusion and exclusion criteria were determined to be the same for tenecteplase as they were for t-PA with the notable exception that the WAKE-UP trial protocol would not be supported until further evidence became available.9 The results of the WAKE-UP trial had previously been used at WRNMMC to justify administration of t-PA in patients who awoke with symptoms of acute ischemic stroke, the last known well was unclear or > 4.5 hours, and for whom a magnetic resonance imaging (MRI) of the brain could be obtained rapidly. Based on the WAKE-UP trial, if the MRI scan of the brain in these patients demonstrated restricted diffusion without fluid attenuated inversion recovery (FLAIR) signal changes (diffusion-weighted [DWI]-FLAIR mismatch sign), this indicated that the stroke had likely occurred recently, and it was safe to administer t-PA. This allowed for administration of t-PA outside the standard treatment window of 4.5 hours from last known well, especially in the cases of patients who awoke with symptoms.
Since safety data are not yet available for the use of tenecteplase in this fashion, the WAKE-UP trial protocol was not used as an inclusion criterion. The informed consent form was modified, and the following scenarios were outlined: (1) If the patient or surrogate is immediately available to consent, paper consent will be documented with the additional note that tenecteplase is being used off-label; and (2) If the patient cannot consent and a surrogate is not immediately available, the medicine will be used emergently as long as the neurology resident and attending physicians agree.15
Risk mitigation was considered carefully. The stroke box described above is stocked and maintained by the pharmacy as we have transitioned to using designated pharmacists for the storage and preparation of tenecteplase. We highly recommend the use of designated pharmacists or emergency department pharmacists in this manner to avoid dosing errors.7,16 Since the current pharmacy-provided tenecteplase bottle contains twice the maximum dose indicated for ischemic stroke, only a 5 mL syringe is included in the stroke box to ensure a maximum dose of 25 mg is drawn up after reconstitution. Dosing card charts were made like existing dosing card charts for t-PA to quickly calculate the 0.25 mg/kg dose. In training, the difference in dosing in ischemic stroke was emphasized. Finally, pharmacy has taken responsibility for dosing the medication during stroke codes.
Any medical personnel at WRNMMC can initiate a stroke code by sending a page to the neurology consult service (Figure).
TRANSITION AND RESULTS
From November 2020 to December 2021, 10 patients have been treated in total at WRNMMC (Table).
CONCLUSIONS
The available evidence supports the transition from t-PA to tenecteplase for acute ischemic stroke. The successful transition required months of preparation involving multidisciplinary meetings between neurology, nursing, pharmacy, radiology, rapid response teams, critical care, and emergency medicine departments. Safeguards must be implemented to avoid a tenecteplase dosing error that can lead to potentially life-threatening adverse effects. The results at WRNMMC thus far are promising for safety and efficacy. Several process improvements are planned: a hospital-wide overhead page will accompany the direct page to neurology; other team members, including radiology and pharmacy, will be included on the acute stroke alert; and a stroke-specific paging application will be implemented to better track real-time stroke metrics and improve flow. These measures mirror processes that are occurring in institutions that treat acute stroke patients.
Tissue plasminogen activator (t-PA) has been the standard IV thrombolytic used in acute ischemic stroke treatment since its US Food and Drug Administration (FDA) approval in 1995. Trials have established this drug’s efficacy in the treatment of acute ischemic stroke and the appropriate patient population for therapy.1-3 Published guidelines and experiences have made clear that a written protocol with extensive personnel training is important to deliver this care properly.4
Tenecteplase has been available for use in the treatment of acute myocardial infarction (MI) and studied in acute ischemic strokes since 2000. Recent large multicenter trials have suggested tenecteplase may work better than t-PA in the recanalization of large vessel occlusions (LVOs) and have provided guidance on proper dosing in acute ischemic stroke victims.5-8 Compared with t-PA, tenecteplase has a longer half-life, is more fibrin specific (causing less coagulopathy), and is more resistant to endogenous plasminogen activator inhibitor.9,10 Using tenecteplase for acute ischemic stroke is simpler as a single dose bolus rather than a bolus followed by a 1-hour infusion with t-PA. Immediate mechanical thrombectomy for LVO is less complicated without the 1-hour t-PA infusion.5,6 Tenecteplase use also allows for nonthrombectomy hospitals to accelerate transfer times for patients who need thrombectomy following thrombolysis by eliminating the need for critical care nurse–staffed ambulances for interfacility transfer.11 Tenecteplase also is cheaper: Tenecteplase costs $3748 per vial, whereas t-PA costs $5800 per vial equating to roughly a $2000 savings per patient.12,13 Finally, the pharmacy formulary is simplified by using a single thrombolytic agent for both cardiac and neurologic emergencies.
Tenecteplase does have some drawbacks to consider. Currently, tenecteplase is not approved by the FDA for the indication of acute ischemic stroke, though the drug is endorsed by the American Heart Association stroke guidelines of 2019 as an alternative to t-PA.14 There is no stroke-specific preparation of the drug, leading to potential dosing errors. Therefore, a systematic process to safely transition from t-PA to tenecteplase for acute ischemic stroke was undertaken at Walter Reed National Military Medical Center (WRNMMC) in Bethesda, Maryland. Here, we report the process required in making a complex switch in thrombolytic medication along with the potential benefits of making this transition.
OBSERVATIONS
The process to implement tenecteplase required extensive training and education for staff physicians, nurses, pharmacists, radiologists, trainees, and the rapid response team. Our institution administered IV thrombolytic drugs up to 25 times annually to acute ischemic stroke victims, meaning we had to train personnel extensively and repeatedly.
In preparation for the transition to tenecteplase, hospital leadership gathered staff for multidisciplinary administrative meetings that included neurology, emergency medicine, intensive care, pharmacy, radiology, and nursing departments. The purpose of these meetings was to establish a standard operating procedure (SOP) to ensure a safe transition. This process began in May 2020 and involved regular meetings to draft and revise our SOP. Additionally, several leadership and training sessions were held over a 6-month period. Stroke boxes were developed that contained the required evaluation tools, consent forms, medications (tenecteplase and treatments for known complications), dosing cards, and instructions. Final approval of the updated acute ischemic stroke hospital policy was obtained in November 2020 and signed by the above departments.
All inclusion and exclusion criteria were determined to be the same for tenecteplase as they were for t-PA with the notable exception that the WAKE-UP trial protocol would not be supported until further evidence became available.9 The results of the WAKE-UP trial had previously been used at WRNMMC to justify administration of t-PA in patients who awoke with symptoms of acute ischemic stroke, the last known well was unclear or > 4.5 hours, and for whom a magnetic resonance imaging (MRI) of the brain could be obtained rapidly. Based on the WAKE-UP trial, if the MRI scan of the brain in these patients demonstrated restricted diffusion without fluid attenuated inversion recovery (FLAIR) signal changes (diffusion-weighted [DWI]-FLAIR mismatch sign), this indicated that the stroke had likely occurred recently, and it was safe to administer t-PA. This allowed for administration of t-PA outside the standard treatment window of 4.5 hours from last known well, especially in the cases of patients who awoke with symptoms.
Since safety data are not yet available for the use of tenecteplase in this fashion, the WAKE-UP trial protocol was not used as an inclusion criterion. The informed consent form was modified, and the following scenarios were outlined: (1) If the patient or surrogate is immediately available to consent, paper consent will be documented with the additional note that tenecteplase is being used off-label; and (2) If the patient cannot consent and a surrogate is not immediately available, the medicine will be used emergently as long as the neurology resident and attending physicians agree.15
Risk mitigation was considered carefully. The stroke box described above is stocked and maintained by the pharmacy as we have transitioned to using designated pharmacists for the storage and preparation of tenecteplase. We highly recommend the use of designated pharmacists or emergency department pharmacists in this manner to avoid dosing errors.7,16 Since the current pharmacy-provided tenecteplase bottle contains twice the maximum dose indicated for ischemic stroke, only a 5 mL syringe is included in the stroke box to ensure a maximum dose of 25 mg is drawn up after reconstitution. Dosing card charts were made like existing dosing card charts for t-PA to quickly calculate the 0.25 mg/kg dose. In training, the difference in dosing in ischemic stroke was emphasized. Finally, pharmacy has taken responsibility for dosing the medication during stroke codes.
Any medical personnel at WRNMMC can initiate a stroke code by sending a page to the neurology consult service (Figure).
TRANSITION AND RESULTS
From November 2020 to December 2021, 10 patients have been treated in total at WRNMMC (Table).
CONCLUSIONS
The available evidence supports the transition from t-PA to tenecteplase for acute ischemic stroke. The successful transition required months of preparation involving multidisciplinary meetings between neurology, nursing, pharmacy, radiology, rapid response teams, critical care, and emergency medicine departments. Safeguards must be implemented to avoid a tenecteplase dosing error that can lead to potentially life-threatening adverse effects. The results at WRNMMC thus far are promising for safety and efficacy. Several process improvements are planned: a hospital-wide overhead page will accompany the direct page to neurology; other team members, including radiology and pharmacy, will be included on the acute stroke alert; and a stroke-specific paging application will be implemented to better track real-time stroke metrics and improve flow. These measures mirror processes that are occurring in institutions that treat acute stroke patients.
1. Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375(9727):1695-1703. doi:10.1016/S0140-6736(10)60491-6
2. National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581- 1587. doi:10.1056/NEJM199512143332401
3. Hacke W, Donnan G, Fieschi C, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363(9411):768-774. doi:10.1016/S0140-6736(04)15692-4
4. Jauch EC, Saver JL, Adams HP Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870-947. doi:10.1161/STR.0b013e318284056a
5. Campbell B, Mitchell P, Churilov L, et al. Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Engl J Med. 2018;378(17):1573-1582. doi:10.1056/nejmoa1716405
6. Yang P, Zhang Y, Zhang L, et al. Endovascular thrombectomy with or without intravenous alteplase in acute stroke. N Engl J Med. 2020;382(21):1981-1993. doi:10.1056/NEJMoa2001123
7. Menon BK, Buck BH, Singh N, et al. Intravenous tenecteplase compared with alteplase for acute ischaemic stroke in Canada (AcT): a pragmatic, multicentre, open-label, registry-linked, randomised, controlled, noninferiority trial. Lancet. 2022;400(10347):161-169. doi:10.1016/S0140-6736(22)01054-6
8. Campbell BCV, Mitchell PJ, Churilov L, et al. Effect of intravenous tenecteplase dose on cerebral reperfusion before thrombectomy in patients with large vessel occlusion ischemic stroke: the EXTEND-IA TNK part 2 randomized clinical trial. JAMA. 2020;323(13):1257- 1265. doi:10.1001/jama.2020.1511
9. Warach SJ, Dula AN, Milling TJ Jr. Tenecteplase thrombolysis for acute ischemic stroke. Stroke. 2020;51(11):3440- 3451. doi:10.1161/STROKEAHA.120.029749
10. Huang X, Moreton FC, Kalladka D, et al. Coagulation and fibrinolytic activity of tenecteplase and alteplase in acute ischemic stroke. Stroke. 2015;46(12):3543-3546. doi:10.1161/STROKEAHA.115.011290
11. Burgos AM, Saver JL. Evidence that tenecteplase is noninferior to alteplase for acute ischemic stroke: meta-analysis of 5 randomized trials. Stroke. 2019;50(8):2156-2162. doi:10.1161/STROKEAHA.119.025080
12. Potla N, Ganti L. Tenecteplase vs. alteplase for acute ischemic stroke: a systematic review. Int J Emerg Med. 2022;15(1). doi:10.1186/s12245-021-00399-w
13. Warach SJ, Winegar A, Ottenbacher A, Miller C, Gibson D. Abstract WMP52: reduced hospital costs for ischemic stroke treated with tenecteplase. Stroke. 2022;53(suppl 1):AWMP52. doi:10.1161/str.53.suppl_1.WMP52
14. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi:10.1161/str.0000000000000211
15. Faris H, Dewar B, Dowlatshahi D, et al. Ethical justification for deferral of consent in the AcT trial for acute ischemic stroke. Stroke. 2022;53(7):2420-2423. doi:10.1161/strokeaha.122.038760
16. Kvistad CE, Næss H, Helleberg BH, et al. Tenecteplase versus alteplase for the management of acute ischaemic stroke in Norway (NOR-TEST 2, part A): a phase 3, randomised, open-label, blinded endpoint, non-inferiority trial. Lancet Neurol. 2022;21(6):511-519. doi:10.1016/S1474-4422(22)00124-7
1. Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375(9727):1695-1703. doi:10.1016/S0140-6736(10)60491-6
2. National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581- 1587. doi:10.1056/NEJM199512143332401
3. Hacke W, Donnan G, Fieschi C, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363(9411):768-774. doi:10.1016/S0140-6736(04)15692-4
4. Jauch EC, Saver JL, Adams HP Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870-947. doi:10.1161/STR.0b013e318284056a
5. Campbell B, Mitchell P, Churilov L, et al. Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Engl J Med. 2018;378(17):1573-1582. doi:10.1056/nejmoa1716405
6. Yang P, Zhang Y, Zhang L, et al. Endovascular thrombectomy with or without intravenous alteplase in acute stroke. N Engl J Med. 2020;382(21):1981-1993. doi:10.1056/NEJMoa2001123
7. Menon BK, Buck BH, Singh N, et al. Intravenous tenecteplase compared with alteplase for acute ischaemic stroke in Canada (AcT): a pragmatic, multicentre, open-label, registry-linked, randomised, controlled, noninferiority trial. Lancet. 2022;400(10347):161-169. doi:10.1016/S0140-6736(22)01054-6
8. Campbell BCV, Mitchell PJ, Churilov L, et al. Effect of intravenous tenecteplase dose on cerebral reperfusion before thrombectomy in patients with large vessel occlusion ischemic stroke: the EXTEND-IA TNK part 2 randomized clinical trial. JAMA. 2020;323(13):1257- 1265. doi:10.1001/jama.2020.1511
9. Warach SJ, Dula AN, Milling TJ Jr. Tenecteplase thrombolysis for acute ischemic stroke. Stroke. 2020;51(11):3440- 3451. doi:10.1161/STROKEAHA.120.029749
10. Huang X, Moreton FC, Kalladka D, et al. Coagulation and fibrinolytic activity of tenecteplase and alteplase in acute ischemic stroke. Stroke. 2015;46(12):3543-3546. doi:10.1161/STROKEAHA.115.011290
11. Burgos AM, Saver JL. Evidence that tenecteplase is noninferior to alteplase for acute ischemic stroke: meta-analysis of 5 randomized trials. Stroke. 2019;50(8):2156-2162. doi:10.1161/STROKEAHA.119.025080
12. Potla N, Ganti L. Tenecteplase vs. alteplase for acute ischemic stroke: a systematic review. Int J Emerg Med. 2022;15(1). doi:10.1186/s12245-021-00399-w
13. Warach SJ, Winegar A, Ottenbacher A, Miller C, Gibson D. Abstract WMP52: reduced hospital costs for ischemic stroke treated with tenecteplase. Stroke. 2022;53(suppl 1):AWMP52. doi:10.1161/str.53.suppl_1.WMP52
14. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi:10.1161/str.0000000000000211
15. Faris H, Dewar B, Dowlatshahi D, et al. Ethical justification for deferral of consent in the AcT trial for acute ischemic stroke. Stroke. 2022;53(7):2420-2423. doi:10.1161/strokeaha.122.038760
16. Kvistad CE, Næss H, Helleberg BH, et al. Tenecteplase versus alteplase for the management of acute ischaemic stroke in Norway (NOR-TEST 2, part A): a phase 3, randomised, open-label, blinded endpoint, non-inferiority trial. Lancet Neurol. 2022;21(6):511-519. doi:10.1016/S1474-4422(22)00124-7
35 years in service to you, our community of reproductive health care clinicians
The mission of OBG
OBG
We wish all our readers a wonderful New Year and the best health possible for our patients.
Arnold P. Advincula, MD
I serve on the executive board that oversees the Fellowships in Minimally Invasive Gynecologic Surgery (FMIGS), and in January 2023 will transition into the role of President. I bring to this leadership role nearly 25 years of surgical experience, both as a clinician educator and inventor. My goal during the next 2 years will be to move toward subspecialty recognition of Complex Gynecology.
Linda D. Bradley, MD
My passion is diagnostic and operative hysteroscopy, simple procedures that can both evaluate and treat intrauterine pathology. Recently, I was thrilled to coauthor an article on office hysteroscopy for Obstetrics & Gynecology (September 2022). I will have a chapter on operative hysteroscopy in the 2023 edition of TeLinde’s Textbook of Gynecology, and I am an author for the topic Office and Operative Hysteroscopy in UpToDate. Locally, I am known as the “foodie gynecologist”—I travel, take cooking classes, and I have more cookbooks than gynecology textbooks. Since Covid, I have embraced biking and just completed a riverboat biking cruise from Salamanca, Spain, to Lisbon, Portugal.
Amy L. Garcia, MD
I am fellowship trained as a minimally invasive gynecologic surgeon (MIGS) and have had a private surgical practice since 2005. I am involved with The American College of Obstetricians and Gynecologists (ACOG), AAGL, and international surgical education for office hysteroscopy and related practice management. I am passionate about working with start-up companies in the gynecologic medical device arena and innovation in gynecologic surgery.
Steven R. Goldstein, MD, NCMP, CCD
I just completed my term as President of the International Menopause Society. This culminated in the society’s 18th World Congress in Lisbon, attended by over 1,700 health care providers from 76 countries. I delivered the Pieter van Keep Memorial Lecture, named for one of the society’s founders who died prematurely of pancreatic cancer. I was further honored by receiving the society’s Distinguished Service Award. I am very proud to have previously received the NAMS Thomas B. Clarkson award for Outstanding Clinical and Basic Science Research in Menopause. I also have one foot in the gynecologic ultrasound world and was given the Joseph H. Holmes Pioneer Award and was the 2023 recipient of the William J. Fry Memorial Lecture Award, both from the American Institute of Ultrasound in Medicine, having written the second book ever on vaginal ultrasonography.
On a personal level, I love to play golf (in spite of my foot drop and 14 orthopedic surgeries). My season tickets show some diversity—the New York City Ballet and St. John’s basketball.
Cheryl B. Iglesia, MD
I am the 49th president of the Society of Gynecologic Surgeons, the 5th woman to hold this position, and the first of Filipino-American descent. I recognize that it is only through extraordinary mentorship and support from other giants in gynecology, like Drs. Andrew Kaunitz (fellow OBG
PS—In the spirit of continually learning, I want to add the Argentine tango to my dancing repertoire and go on an African safari; both are on my bucket list as the pandemic eases.
Andrew M. Kaunitz, MD, NCMP
Since starting with the University of Florida College of Medicine-Jacksonville in 1984, I have enjoyed caring for patients, training residents and medical students, and being involved with publications and research. My areas of focus are menopause, contraception, gyn ultrasound and evaluation/management of women with abnormal uterine bleeding. In 2020, I received the North American Menopause Society/Leon Speroff Outstanding Educator Award. In 2021, I received the ACOG Distinguished Service Award. I enjoy spending time with my family, neighborhood bicycling, and searching for sharks’ teeth at the beach.
Barbara Levy, MD
I have been privileged to serve on the OBG
Continue to: David G. Mutch, MD...
David G. Mutch, MD
I am ending my 6-year term as Chair of the National Cancer Institute’s (NCI) gynecologic cancer steering committee. That is the committee that vets all NCI-sponsored clinical trials in gynecologic oncology. I am on the International Federation of Gynecology and Obstetrics (FIGO) Cancer committee, Co-Chair of the American Joint Committee on Cancer gyn staging committee and on the Reproductive Scientist Development Program selection committee. I also am completing my term as Chair of the Foundation for Women’s Cancer; this is the C3, charitable arm, of the Society of Gynecologic Oncology. We have distributed more than $3.5 million to young investigators to help start their research careers in gynecologic oncology.
Errol R. Norwitz, MD, PhD, MBA
I am a physician-scientist with subspecialty training in high-risk obstetrics (maternal-fetal medicine). I was born and raised in Cape Town, South Africa, and I have trained/practiced in 5 countries on 3 continents. My research interests include the pathophysiology, prediction, prevention, and management of pregnancy complications, primarily preterm birth and preeclampsia. I am a member of the Board of Scientific Counselors of the National Institute of Child Health and Human Development. I am currently President & CEO of Newton-Wellesley Hospital, a comprehensive community-based academic medical center and a member of the Mass General Brigham health care system in Boston, Massachusetts.
Jaimey Pauli, MD
I am the Division Chief and Professor of Maternal-Fetal Medicine (MFM) at the Penn State College of Medicine and Penn State Health Milton S. Hershey Medical Center. I had exceptional mentoring throughout my medical career, particularly by a former member of the Editorial Board, Dr. John T. Repke. One of the biggest perks of my job is that our division provides full-scope MFM care. While I often serve as the more traditional MFM consultant and academic educator, I also provide longitudinal prenatal care and deliver many of my own patients, often through subsequent pregnancies. Serving as a member of the Editorial Board combines my passion for clinical obstetrical care with my talents (as a former English major) of reading, writing, and editing. I believe that the work we do provides accessible, evidence-based, and practical guidance for our colleagues so they can provide excellence in obstetrical care.
JoAnn Pinkerton, MD, NCMP
I am a Professor of Obstetrics and Gynecology and Division Chief of Midlife Health at the University of Virginia (UVA) Health. Passionate about menopause, I am an executive director emeritus of The North American Menopause Society (NAMS) and past-President of NAMS (2008-2009). Within the past few years, I have served as an expert advisor for the recent ACOG Clinical Practice Guidelines on Osteoporosis, the NAMS Position Statements on Hormone Therapy and Osteoporosis, and the Global Consensus on Menopause and Androgen Therapy. I received the 2022 South Atlantic Association of Obstetricians and Gynecologists Lifetime Achievement Award for my expertise and work in menopause and the NAMS 2020 Ann Voda Community Service Award for my biannual community educational symposiums. I remain active in research, currently the lead and UVA principal investigator for the Oasis 2 multicenter clinical trial, which is testing a neurokinin receptor antagonist as a nonhormone therapy for the relief of hot flashes. Serving on the OBG
Joseph S. Sanfilippo, MD, MBA
I feel honored and privileged to have received the Golden Apple Teaching Award from the Universityof Pittsburgh School of Medicine. I am also fortunate to be the recipient of the Faculty Educator of the Month Award for resident teaching. I have been named Top Doctor 20 years in a row. My current academic activities include, since 2007, Program Director for Reproductive Endocrinology & Infertility Fellowship at the University of Pittsburgh and Chair of the Mentor-Mentee Program at University of Pittsburgh Department of Obstetrics, Gynecology & Reproductive Sciences. I am Guest Editor for the medical malpractice section of the journal Clinical Obstetrics and Gynecology. Recently, I completed a patient-focused book, “Experts Guide to Fertility,” which will be published in May 2023 by J Hopkins University Publisher and is designed for patients going through infertility treatment. Regarding outside events, I enjoy climbing steep hills and riding far and wide on my “electric bike.” Highly recommend it!
James Simon, MD, CCD, IF, NCMP
It’s been an honor serving on the OBG
I asked our distinguished Board of Editors to identify the most important changes that they believe will occur over the next 5 years, influencing the practice of obstetrics and gynecology. Their expert predictions are summarized below.
Arnold Advincula, MD
As one of the world’s most experienced gynecologic robotic surgeons, the role of this technology will become even more refined over the next 2-5 years with the introduction of sophisticated image guidance, “smart molecules,” and artificial intelligence. All of this will transform both the patient and surgeon experience as well as impact how we train future surgeons.
Linda Bradley, MD
My hope is that a partnership with industry and hysteroscopy thought leaders will enable new developments/technology in performing hysteroscopic sterilization. Conquering the tubal ostia for sterilization in an office setting would profoundly improve contraceptive options for women. Conquering the tubal ostia is the last frontier in gynecology.
Amy Garcia, MD
I predict that new technologies will allow for a significant increase in the number of gynecologists who perform in-office hysteroscopy and that a paradigm shift will occur to replace blind biopsy with hysteroscopy-directed biopsy and evaluation of the uterine cavity.
Steven Goldstein, MD, NCMP, CCD
Among the most important changes in the next 5 years, in my opinion, will be in the arenas of precision medicine, genetic advancement, and artificial intelligence. In addition, unfortunately, there will be an even greater movement toward guidelines utilizing algorithms and clinical pathways. I leave you with the following quote:
“Neither evidence nor clinical judgement alone is sufficient. Evidence without judgement can be applied by a technician. Judgement without evidence can be applied by a friend. But the integration of evidence and judgement is what the healthcare provider does in order to dispense the best clinical care.” —Hertzel Gerstein, MD
Cheryl Iglesia, MD
Technology related to minimally invasive surgery will continue to change our practice, and I predict that surgery will be more centralized to high volume practices. Reimbursements for these procedures may remain a hot button issue, however. The materials used for pelvic reconstruction will be derived from autologous stem cells and advancements made in regenerative medicine.
Andrew Kaunitz, MD, NCMP
As use of contraceptive implants and intrauterine devices continues to grow, I anticipate the incidence of unintended pregnancies will continue to decline. As the novel gonadotropin-releasing hormone (GnRH) antagonists combined with estrogen-progestin add-back grow in use, I anticipate this will provide our patients with more nonsurgical options for managing abnormal uterine bleeding, including that associated with uterine fibroids.
Barbara Levy, MD
Quality will be redefined by patient-defined outcome measures that assess what matters to the people we serve. Real-world evidence will be incorporated to support those measures and provide data on patient outcomes in populations not studied in the randomized controlled trials on which we have created guidelines. This will help to refine guidelines and support more equitable and accessible care.
David Mutch, MD
Over the next 5 years, our expanding insights into the molecular biology of cancer will lead to targeted therapies that will yield better responses with less toxicity.
Errol R. Norwitz, MD, PhD, MBA
In the near future we will use predictive AI algorithms to: 1) identify patients at risk of adverse pregnancy events; 2) stratify patients into high-, average-, and low-risk; and 3) design a personalized obstetric care journey for each patient based on their individualized risk stratification with a view to improving safety and quality outcome metrics, addressing health care disparity, and lowering the cost of care.
Jaimey Pauli, MD
I predict (and fervently hope) that breakthroughs will occur in the prevention of two of the most devastating diseases to affect obstetric patients and their families—preterm birth and preeclampsia.
JoAnn Pinkerton, MD, NCMP
New nonhormone management therapies will be available to treat hot flashes and the genitourinary syndrome of menopause. These treatments will be especially welcomed by patients who cannot or choose not to take hormone therapy. We should not allow new technology to overshadow the patient. We must remember to treat the patient with the condition, not just the disease. Consider what is important to the individual woman, her quality of life, and her ability to function, and keep that in mind when deciding what therapy to suggest.
Joseph S. Sanfilippo, MD, MBA
Artificial intelligence will change the way we educate and provide patient care. Three-dimensional perspectives will cross a number of horizons, some of which include:
- advances in assisted reproductive technology (IVF), offering the next level of “in vitro maturation” of oocytes for patients heretofore unable to conceive. They can progress to having a baby with decreased ovarian reserve or in association with “life after cancer.”
- biogenic engineering and bioinformatics will allow correction of genetic defects in embryos prior to implantation
- the surgical arena will incorporate direct robotic initiated procedures and bring robotic surgery to the next level
- with regard to medical education, at all levels, virtual reality, computer-generated 3-dimensional imaging will provide innovative tools.
James Simon, MD, CCD, IF, NCMP
Medicine’s near-term future portends the realization of truly personalized medicine based upon one’s genetic predisposition to disease, and intentional genetic manipulation to mitigate it. Such advances are here already, simply pending regulatory and ethical approval. My concern going forward is that such individualization, and an algorithm-driven decision-making process will result in taking the personal out of personalized medicine. We humans are more than the collected downstream impact of our genes. In our quest for advances, let’s not forget the balance between nature (our genes) and nurture (environment). The risk of forgetting this aphorism, like the electronic health record, gives me heartburn, or worse, burnout!
The mission of OBG
OBG
We wish all our readers a wonderful New Year and the best health possible for our patients.
Arnold P. Advincula, MD
I serve on the executive board that oversees the Fellowships in Minimally Invasive Gynecologic Surgery (FMIGS), and in January 2023 will transition into the role of President. I bring to this leadership role nearly 25 years of surgical experience, both as a clinician educator and inventor. My goal during the next 2 years will be to move toward subspecialty recognition of Complex Gynecology.
Linda D. Bradley, MD
My passion is diagnostic and operative hysteroscopy, simple procedures that can both evaluate and treat intrauterine pathology. Recently, I was thrilled to coauthor an article on office hysteroscopy for Obstetrics & Gynecology (September 2022). I will have a chapter on operative hysteroscopy in the 2023 edition of TeLinde’s Textbook of Gynecology, and I am an author for the topic Office and Operative Hysteroscopy in UpToDate. Locally, I am known as the “foodie gynecologist”—I travel, take cooking classes, and I have more cookbooks than gynecology textbooks. Since Covid, I have embraced biking and just completed a riverboat biking cruise from Salamanca, Spain, to Lisbon, Portugal.
Amy L. Garcia, MD
I am fellowship trained as a minimally invasive gynecologic surgeon (MIGS) and have had a private surgical practice since 2005. I am involved with The American College of Obstetricians and Gynecologists (ACOG), AAGL, and international surgical education for office hysteroscopy and related practice management. I am passionate about working with start-up companies in the gynecologic medical device arena and innovation in gynecologic surgery.
Steven R. Goldstein, MD, NCMP, CCD
I just completed my term as President of the International Menopause Society. This culminated in the society’s 18th World Congress in Lisbon, attended by over 1,700 health care providers from 76 countries. I delivered the Pieter van Keep Memorial Lecture, named for one of the society’s founders who died prematurely of pancreatic cancer. I was further honored by receiving the society’s Distinguished Service Award. I am very proud to have previously received the NAMS Thomas B. Clarkson award for Outstanding Clinical and Basic Science Research in Menopause. I also have one foot in the gynecologic ultrasound world and was given the Joseph H. Holmes Pioneer Award and was the 2023 recipient of the William J. Fry Memorial Lecture Award, both from the American Institute of Ultrasound in Medicine, having written the second book ever on vaginal ultrasonography.
On a personal level, I love to play golf (in spite of my foot drop and 14 orthopedic surgeries). My season tickets show some diversity—the New York City Ballet and St. John’s basketball.
Cheryl B. Iglesia, MD
I am the 49th president of the Society of Gynecologic Surgeons, the 5th woman to hold this position, and the first of Filipino-American descent. I recognize that it is only through extraordinary mentorship and support from other giants in gynecology, like Drs. Andrew Kaunitz (fellow OBG
PS—In the spirit of continually learning, I want to add the Argentine tango to my dancing repertoire and go on an African safari; both are on my bucket list as the pandemic eases.
Andrew M. Kaunitz, MD, NCMP
Since starting with the University of Florida College of Medicine-Jacksonville in 1984, I have enjoyed caring for patients, training residents and medical students, and being involved with publications and research. My areas of focus are menopause, contraception, gyn ultrasound and evaluation/management of women with abnormal uterine bleeding. In 2020, I received the North American Menopause Society/Leon Speroff Outstanding Educator Award. In 2021, I received the ACOG Distinguished Service Award. I enjoy spending time with my family, neighborhood bicycling, and searching for sharks’ teeth at the beach.
Barbara Levy, MD
I have been privileged to serve on the OBG
Continue to: David G. Mutch, MD...
David G. Mutch, MD
I am ending my 6-year term as Chair of the National Cancer Institute’s (NCI) gynecologic cancer steering committee. That is the committee that vets all NCI-sponsored clinical trials in gynecologic oncology. I am on the International Federation of Gynecology and Obstetrics (FIGO) Cancer committee, Co-Chair of the American Joint Committee on Cancer gyn staging committee and on the Reproductive Scientist Development Program selection committee. I also am completing my term as Chair of the Foundation for Women’s Cancer; this is the C3, charitable arm, of the Society of Gynecologic Oncology. We have distributed more than $3.5 million to young investigators to help start their research careers in gynecologic oncology.
Errol R. Norwitz, MD, PhD, MBA
I am a physician-scientist with subspecialty training in high-risk obstetrics (maternal-fetal medicine). I was born and raised in Cape Town, South Africa, and I have trained/practiced in 5 countries on 3 continents. My research interests include the pathophysiology, prediction, prevention, and management of pregnancy complications, primarily preterm birth and preeclampsia. I am a member of the Board of Scientific Counselors of the National Institute of Child Health and Human Development. I am currently President & CEO of Newton-Wellesley Hospital, a comprehensive community-based academic medical center and a member of the Mass General Brigham health care system in Boston, Massachusetts.
Jaimey Pauli, MD
I am the Division Chief and Professor of Maternal-Fetal Medicine (MFM) at the Penn State College of Medicine and Penn State Health Milton S. Hershey Medical Center. I had exceptional mentoring throughout my medical career, particularly by a former member of the Editorial Board, Dr. John T. Repke. One of the biggest perks of my job is that our division provides full-scope MFM care. While I often serve as the more traditional MFM consultant and academic educator, I also provide longitudinal prenatal care and deliver many of my own patients, often through subsequent pregnancies. Serving as a member of the Editorial Board combines my passion for clinical obstetrical care with my talents (as a former English major) of reading, writing, and editing. I believe that the work we do provides accessible, evidence-based, and practical guidance for our colleagues so they can provide excellence in obstetrical care.
JoAnn Pinkerton, MD, NCMP
I am a Professor of Obstetrics and Gynecology and Division Chief of Midlife Health at the University of Virginia (UVA) Health. Passionate about menopause, I am an executive director emeritus of The North American Menopause Society (NAMS) and past-President of NAMS (2008-2009). Within the past few years, I have served as an expert advisor for the recent ACOG Clinical Practice Guidelines on Osteoporosis, the NAMS Position Statements on Hormone Therapy and Osteoporosis, and the Global Consensus on Menopause and Androgen Therapy. I received the 2022 South Atlantic Association of Obstetricians and Gynecologists Lifetime Achievement Award for my expertise and work in menopause and the NAMS 2020 Ann Voda Community Service Award for my biannual community educational symposiums. I remain active in research, currently the lead and UVA principal investigator for the Oasis 2 multicenter clinical trial, which is testing a neurokinin receptor antagonist as a nonhormone therapy for the relief of hot flashes. Serving on the OBG
Joseph S. Sanfilippo, MD, MBA
I feel honored and privileged to have received the Golden Apple Teaching Award from the Universityof Pittsburgh School of Medicine. I am also fortunate to be the recipient of the Faculty Educator of the Month Award for resident teaching. I have been named Top Doctor 20 years in a row. My current academic activities include, since 2007, Program Director for Reproductive Endocrinology & Infertility Fellowship at the University of Pittsburgh and Chair of the Mentor-Mentee Program at University of Pittsburgh Department of Obstetrics, Gynecology & Reproductive Sciences. I am Guest Editor for the medical malpractice section of the journal Clinical Obstetrics and Gynecology. Recently, I completed a patient-focused book, “Experts Guide to Fertility,” which will be published in May 2023 by J Hopkins University Publisher and is designed for patients going through infertility treatment. Regarding outside events, I enjoy climbing steep hills and riding far and wide on my “electric bike.” Highly recommend it!
James Simon, MD, CCD, IF, NCMP
It’s been an honor serving on the OBG
I asked our distinguished Board of Editors to identify the most important changes that they believe will occur over the next 5 years, influencing the practice of obstetrics and gynecology. Their expert predictions are summarized below.
Arnold Advincula, MD
As one of the world’s most experienced gynecologic robotic surgeons, the role of this technology will become even more refined over the next 2-5 years with the introduction of sophisticated image guidance, “smart molecules,” and artificial intelligence. All of this will transform both the patient and surgeon experience as well as impact how we train future surgeons.
Linda Bradley, MD
My hope is that a partnership with industry and hysteroscopy thought leaders will enable new developments/technology in performing hysteroscopic sterilization. Conquering the tubal ostia for sterilization in an office setting would profoundly improve contraceptive options for women. Conquering the tubal ostia is the last frontier in gynecology.
Amy Garcia, MD
I predict that new technologies will allow for a significant increase in the number of gynecologists who perform in-office hysteroscopy and that a paradigm shift will occur to replace blind biopsy with hysteroscopy-directed biopsy and evaluation of the uterine cavity.
Steven Goldstein, MD, NCMP, CCD
Among the most important changes in the next 5 years, in my opinion, will be in the arenas of precision medicine, genetic advancement, and artificial intelligence. In addition, unfortunately, there will be an even greater movement toward guidelines utilizing algorithms and clinical pathways. I leave you with the following quote:
“Neither evidence nor clinical judgement alone is sufficient. Evidence without judgement can be applied by a technician. Judgement without evidence can be applied by a friend. But the integration of evidence and judgement is what the healthcare provider does in order to dispense the best clinical care.” —Hertzel Gerstein, MD
Cheryl Iglesia, MD
Technology related to minimally invasive surgery will continue to change our practice, and I predict that surgery will be more centralized to high volume practices. Reimbursements for these procedures may remain a hot button issue, however. The materials used for pelvic reconstruction will be derived from autologous stem cells and advancements made in regenerative medicine.
Andrew Kaunitz, MD, NCMP
As use of contraceptive implants and intrauterine devices continues to grow, I anticipate the incidence of unintended pregnancies will continue to decline. As the novel gonadotropin-releasing hormone (GnRH) antagonists combined with estrogen-progestin add-back grow in use, I anticipate this will provide our patients with more nonsurgical options for managing abnormal uterine bleeding, including that associated with uterine fibroids.
Barbara Levy, MD
Quality will be redefined by patient-defined outcome measures that assess what matters to the people we serve. Real-world evidence will be incorporated to support those measures and provide data on patient outcomes in populations not studied in the randomized controlled trials on which we have created guidelines. This will help to refine guidelines and support more equitable and accessible care.
David Mutch, MD
Over the next 5 years, our expanding insights into the molecular biology of cancer will lead to targeted therapies that will yield better responses with less toxicity.
Errol R. Norwitz, MD, PhD, MBA
In the near future we will use predictive AI algorithms to: 1) identify patients at risk of adverse pregnancy events; 2) stratify patients into high-, average-, and low-risk; and 3) design a personalized obstetric care journey for each patient based on their individualized risk stratification with a view to improving safety and quality outcome metrics, addressing health care disparity, and lowering the cost of care.
Jaimey Pauli, MD
I predict (and fervently hope) that breakthroughs will occur in the prevention of two of the most devastating diseases to affect obstetric patients and their families—preterm birth and preeclampsia.
JoAnn Pinkerton, MD, NCMP
New nonhormone management therapies will be available to treat hot flashes and the genitourinary syndrome of menopause. These treatments will be especially welcomed by patients who cannot or choose not to take hormone therapy. We should not allow new technology to overshadow the patient. We must remember to treat the patient with the condition, not just the disease. Consider what is important to the individual woman, her quality of life, and her ability to function, and keep that in mind when deciding what therapy to suggest.
Joseph S. Sanfilippo, MD, MBA
Artificial intelligence will change the way we educate and provide patient care. Three-dimensional perspectives will cross a number of horizons, some of which include:
- advances in assisted reproductive technology (IVF), offering the next level of “in vitro maturation” of oocytes for patients heretofore unable to conceive. They can progress to having a baby with decreased ovarian reserve or in association with “life after cancer.”
- biogenic engineering and bioinformatics will allow correction of genetic defects in embryos prior to implantation
- the surgical arena will incorporate direct robotic initiated procedures and bring robotic surgery to the next level
- with regard to medical education, at all levels, virtual reality, computer-generated 3-dimensional imaging will provide innovative tools.
James Simon, MD, CCD, IF, NCMP
Medicine’s near-term future portends the realization of truly personalized medicine based upon one’s genetic predisposition to disease, and intentional genetic manipulation to mitigate it. Such advances are here already, simply pending regulatory and ethical approval. My concern going forward is that such individualization, and an algorithm-driven decision-making process will result in taking the personal out of personalized medicine. We humans are more than the collected downstream impact of our genes. In our quest for advances, let’s not forget the balance between nature (our genes) and nurture (environment). The risk of forgetting this aphorism, like the electronic health record, gives me heartburn, or worse, burnout!
The mission of OBG
OBG
We wish all our readers a wonderful New Year and the best health possible for our patients.
Arnold P. Advincula, MD
I serve on the executive board that oversees the Fellowships in Minimally Invasive Gynecologic Surgery (FMIGS), and in January 2023 will transition into the role of President. I bring to this leadership role nearly 25 years of surgical experience, both as a clinician educator and inventor. My goal during the next 2 years will be to move toward subspecialty recognition of Complex Gynecology.
Linda D. Bradley, MD
My passion is diagnostic and operative hysteroscopy, simple procedures that can both evaluate and treat intrauterine pathology. Recently, I was thrilled to coauthor an article on office hysteroscopy for Obstetrics & Gynecology (September 2022). I will have a chapter on operative hysteroscopy in the 2023 edition of TeLinde’s Textbook of Gynecology, and I am an author for the topic Office and Operative Hysteroscopy in UpToDate. Locally, I am known as the “foodie gynecologist”—I travel, take cooking classes, and I have more cookbooks than gynecology textbooks. Since Covid, I have embraced biking and just completed a riverboat biking cruise from Salamanca, Spain, to Lisbon, Portugal.
Amy L. Garcia, MD
I am fellowship trained as a minimally invasive gynecologic surgeon (MIGS) and have had a private surgical practice since 2005. I am involved with The American College of Obstetricians and Gynecologists (ACOG), AAGL, and international surgical education for office hysteroscopy and related practice management. I am passionate about working with start-up companies in the gynecologic medical device arena and innovation in gynecologic surgery.
Steven R. Goldstein, MD, NCMP, CCD
I just completed my term as President of the International Menopause Society. This culminated in the society’s 18th World Congress in Lisbon, attended by over 1,700 health care providers from 76 countries. I delivered the Pieter van Keep Memorial Lecture, named for one of the society’s founders who died prematurely of pancreatic cancer. I was further honored by receiving the society’s Distinguished Service Award. I am very proud to have previously received the NAMS Thomas B. Clarkson award for Outstanding Clinical and Basic Science Research in Menopause. I also have one foot in the gynecologic ultrasound world and was given the Joseph H. Holmes Pioneer Award and was the 2023 recipient of the William J. Fry Memorial Lecture Award, both from the American Institute of Ultrasound in Medicine, having written the second book ever on vaginal ultrasonography.
On a personal level, I love to play golf (in spite of my foot drop and 14 orthopedic surgeries). My season tickets show some diversity—the New York City Ballet and St. John’s basketball.
Cheryl B. Iglesia, MD
I am the 49th president of the Society of Gynecologic Surgeons, the 5th woman to hold this position, and the first of Filipino-American descent. I recognize that it is only through extraordinary mentorship and support from other giants in gynecology, like Drs. Andrew Kaunitz (fellow OBG
PS—In the spirit of continually learning, I want to add the Argentine tango to my dancing repertoire and go on an African safari; both are on my bucket list as the pandemic eases.
Andrew M. Kaunitz, MD, NCMP
Since starting with the University of Florida College of Medicine-Jacksonville in 1984, I have enjoyed caring for patients, training residents and medical students, and being involved with publications and research. My areas of focus are menopause, contraception, gyn ultrasound and evaluation/management of women with abnormal uterine bleeding. In 2020, I received the North American Menopause Society/Leon Speroff Outstanding Educator Award. In 2021, I received the ACOG Distinguished Service Award. I enjoy spending time with my family, neighborhood bicycling, and searching for sharks’ teeth at the beach.
Barbara Levy, MD
I have been privileged to serve on the OBG
Continue to: David G. Mutch, MD...
David G. Mutch, MD
I am ending my 6-year term as Chair of the National Cancer Institute’s (NCI) gynecologic cancer steering committee. That is the committee that vets all NCI-sponsored clinical trials in gynecologic oncology. I am on the International Federation of Gynecology and Obstetrics (FIGO) Cancer committee, Co-Chair of the American Joint Committee on Cancer gyn staging committee and on the Reproductive Scientist Development Program selection committee. I also am completing my term as Chair of the Foundation for Women’s Cancer; this is the C3, charitable arm, of the Society of Gynecologic Oncology. We have distributed more than $3.5 million to young investigators to help start their research careers in gynecologic oncology.
Errol R. Norwitz, MD, PhD, MBA
I am a physician-scientist with subspecialty training in high-risk obstetrics (maternal-fetal medicine). I was born and raised in Cape Town, South Africa, and I have trained/practiced in 5 countries on 3 continents. My research interests include the pathophysiology, prediction, prevention, and management of pregnancy complications, primarily preterm birth and preeclampsia. I am a member of the Board of Scientific Counselors of the National Institute of Child Health and Human Development. I am currently President & CEO of Newton-Wellesley Hospital, a comprehensive community-based academic medical center and a member of the Mass General Brigham health care system in Boston, Massachusetts.
Jaimey Pauli, MD
I am the Division Chief and Professor of Maternal-Fetal Medicine (MFM) at the Penn State College of Medicine and Penn State Health Milton S. Hershey Medical Center. I had exceptional mentoring throughout my medical career, particularly by a former member of the Editorial Board, Dr. John T. Repke. One of the biggest perks of my job is that our division provides full-scope MFM care. While I often serve as the more traditional MFM consultant and academic educator, I also provide longitudinal prenatal care and deliver many of my own patients, often through subsequent pregnancies. Serving as a member of the Editorial Board combines my passion for clinical obstetrical care with my talents (as a former English major) of reading, writing, and editing. I believe that the work we do provides accessible, evidence-based, and practical guidance for our colleagues so they can provide excellence in obstetrical care.
JoAnn Pinkerton, MD, NCMP
I am a Professor of Obstetrics and Gynecology and Division Chief of Midlife Health at the University of Virginia (UVA) Health. Passionate about menopause, I am an executive director emeritus of The North American Menopause Society (NAMS) and past-President of NAMS (2008-2009). Within the past few years, I have served as an expert advisor for the recent ACOG Clinical Practice Guidelines on Osteoporosis, the NAMS Position Statements on Hormone Therapy and Osteoporosis, and the Global Consensus on Menopause and Androgen Therapy. I received the 2022 South Atlantic Association of Obstetricians and Gynecologists Lifetime Achievement Award for my expertise and work in menopause and the NAMS 2020 Ann Voda Community Service Award for my biannual community educational symposiums. I remain active in research, currently the lead and UVA principal investigator for the Oasis 2 multicenter clinical trial, which is testing a neurokinin receptor antagonist as a nonhormone therapy for the relief of hot flashes. Serving on the OBG
Joseph S. Sanfilippo, MD, MBA
I feel honored and privileged to have received the Golden Apple Teaching Award from the Universityof Pittsburgh School of Medicine. I am also fortunate to be the recipient of the Faculty Educator of the Month Award for resident teaching. I have been named Top Doctor 20 years in a row. My current academic activities include, since 2007, Program Director for Reproductive Endocrinology & Infertility Fellowship at the University of Pittsburgh and Chair of the Mentor-Mentee Program at University of Pittsburgh Department of Obstetrics, Gynecology & Reproductive Sciences. I am Guest Editor for the medical malpractice section of the journal Clinical Obstetrics and Gynecology. Recently, I completed a patient-focused book, “Experts Guide to Fertility,” which will be published in May 2023 by J Hopkins University Publisher and is designed for patients going through infertility treatment. Regarding outside events, I enjoy climbing steep hills and riding far and wide on my “electric bike.” Highly recommend it!
James Simon, MD, CCD, IF, NCMP
It’s been an honor serving on the OBG
I asked our distinguished Board of Editors to identify the most important changes that they believe will occur over the next 5 years, influencing the practice of obstetrics and gynecology. Their expert predictions are summarized below.
Arnold Advincula, MD
As one of the world’s most experienced gynecologic robotic surgeons, the role of this technology will become even more refined over the next 2-5 years with the introduction of sophisticated image guidance, “smart molecules,” and artificial intelligence. All of this will transform both the patient and surgeon experience as well as impact how we train future surgeons.
Linda Bradley, MD
My hope is that a partnership with industry and hysteroscopy thought leaders will enable new developments/technology in performing hysteroscopic sterilization. Conquering the tubal ostia for sterilization in an office setting would profoundly improve contraceptive options for women. Conquering the tubal ostia is the last frontier in gynecology.
Amy Garcia, MD
I predict that new technologies will allow for a significant increase in the number of gynecologists who perform in-office hysteroscopy and that a paradigm shift will occur to replace blind biopsy with hysteroscopy-directed biopsy and evaluation of the uterine cavity.
Steven Goldstein, MD, NCMP, CCD
Among the most important changes in the next 5 years, in my opinion, will be in the arenas of precision medicine, genetic advancement, and artificial intelligence. In addition, unfortunately, there will be an even greater movement toward guidelines utilizing algorithms and clinical pathways. I leave you with the following quote:
“Neither evidence nor clinical judgement alone is sufficient. Evidence without judgement can be applied by a technician. Judgement without evidence can be applied by a friend. But the integration of evidence and judgement is what the healthcare provider does in order to dispense the best clinical care.” —Hertzel Gerstein, MD
Cheryl Iglesia, MD
Technology related to minimally invasive surgery will continue to change our practice, and I predict that surgery will be more centralized to high volume practices. Reimbursements for these procedures may remain a hot button issue, however. The materials used for pelvic reconstruction will be derived from autologous stem cells and advancements made in regenerative medicine.
Andrew Kaunitz, MD, NCMP
As use of contraceptive implants and intrauterine devices continues to grow, I anticipate the incidence of unintended pregnancies will continue to decline. As the novel gonadotropin-releasing hormone (GnRH) antagonists combined with estrogen-progestin add-back grow in use, I anticipate this will provide our patients with more nonsurgical options for managing abnormal uterine bleeding, including that associated with uterine fibroids.
Barbara Levy, MD
Quality will be redefined by patient-defined outcome measures that assess what matters to the people we serve. Real-world evidence will be incorporated to support those measures and provide data on patient outcomes in populations not studied in the randomized controlled trials on which we have created guidelines. This will help to refine guidelines and support more equitable and accessible care.
David Mutch, MD
Over the next 5 years, our expanding insights into the molecular biology of cancer will lead to targeted therapies that will yield better responses with less toxicity.
Errol R. Norwitz, MD, PhD, MBA
In the near future we will use predictive AI algorithms to: 1) identify patients at risk of adverse pregnancy events; 2) stratify patients into high-, average-, and low-risk; and 3) design a personalized obstetric care journey for each patient based on their individualized risk stratification with a view to improving safety and quality outcome metrics, addressing health care disparity, and lowering the cost of care.
Jaimey Pauli, MD
I predict (and fervently hope) that breakthroughs will occur in the prevention of two of the most devastating diseases to affect obstetric patients and their families—preterm birth and preeclampsia.
JoAnn Pinkerton, MD, NCMP
New nonhormone management therapies will be available to treat hot flashes and the genitourinary syndrome of menopause. These treatments will be especially welcomed by patients who cannot or choose not to take hormone therapy. We should not allow new technology to overshadow the patient. We must remember to treat the patient with the condition, not just the disease. Consider what is important to the individual woman, her quality of life, and her ability to function, and keep that in mind when deciding what therapy to suggest.
Joseph S. Sanfilippo, MD, MBA
Artificial intelligence will change the way we educate and provide patient care. Three-dimensional perspectives will cross a number of horizons, some of which include:
- advances in assisted reproductive technology (IVF), offering the next level of “in vitro maturation” of oocytes for patients heretofore unable to conceive. They can progress to having a baby with decreased ovarian reserve or in association with “life after cancer.”
- biogenic engineering and bioinformatics will allow correction of genetic defects in embryos prior to implantation
- the surgical arena will incorporate direct robotic initiated procedures and bring robotic surgery to the next level
- with regard to medical education, at all levels, virtual reality, computer-generated 3-dimensional imaging will provide innovative tools.
James Simon, MD, CCD, IF, NCMP
Medicine’s near-term future portends the realization of truly personalized medicine based upon one’s genetic predisposition to disease, and intentional genetic manipulation to mitigate it. Such advances are here already, simply pending regulatory and ethical approval. My concern going forward is that such individualization, and an algorithm-driven decision-making process will result in taking the personal out of personalized medicine. We humans are more than the collected downstream impact of our genes. In our quest for advances, let’s not forget the balance between nature (our genes) and nurture (environment). The risk of forgetting this aphorism, like the electronic health record, gives me heartburn, or worse, burnout!
When Patients Make Unexpected Medical Choices
Due to advances in medicine, people are living longer with the aid of increased options for life-prolonging treatments. These treatment options may improve the quantity but not necessarily the quality of life.1
Kidney failure can be treated with renal replacement therapy (dialysis or renal transplantation) or supportive care.2 In 2017, the global prevalence of kidney failure was about 5.3 to 9.7 million.3 In the United States, about 500,000 patients are receiving maintenance dialysis for end-stage renal disease (ESRD), and about 1 in 4 will stop dialysis before death, coupled with hospice enrollment.4 ESRD is 2 times more prevalent among veterans than in nonveterans, which can be due in part to high rates of comorbid predisposing conditions, such as diabetes mellitus, hypertension, and advanced age, among others.5 The decision to discontinue dialysis and receive hospice care tends to be more difficult than choosing to withhold or forego dialysis.6
A study conducted among patients who were taken off hemodialysis before death reported that the 2 most common reasons for the withdrawal were acute medical complications and frailty.7 A retrospective study among patients with ESRD receiving hemodialysis highlighted the underutilization of hospice care in this patient population.8 The study also found that those patients who were aged > 75 years, had poor functional status, and had dialysis-related complications, such as sepsis and anemia, were more likely to elect withdrawal of hemodialysis. There was no difference in overall survival or quality of life among patients who were aged ≥ 75 years with multiple comorbidities and functional impairment who elected conservative management vs those who started dialysis.8 Long-term continuous dialysis has been associated with a lower quality of life, increased dependence on others, and a variety of symptoms, such as pain, nausea, insomnia, anxiety, or depression.9
Conservative Care vs Medical Paternalism
In the United States, it is unusual for patients with ESRD to choose conservative care, and supportive services are less available for those who do compared with patients with ESRD in Europe, Asia, Australia, and Canada.10 A study looking at a small number of US nephrologists has shown they may have limited experience in caring for patients who forego dialysis and they are not comfortable offering conservative management over dialysis.10 Another small study from Sweden also showed that many nephrologists do not feel prepared for end-of-life care and conversations.11
Patients often rely on knowledgeable recommendations from medical experts. However, medical paternalism occurs when a physician makes decisions deemed to be in the patient’s best interest but are against the patient’s wishes or when the patient is unable to give their consent.12 Hard paternalism occurs when the patient is competent to make their own medical decisions, while soft paternalism occurs when a patient is not competent to make their own medical decisions.13
Patient autonomy is widely recognized as an ethical principle in medicine. It recognizes patients as well-informed decision makers who may act without excessive influence to make intentional determinations on their own behalf.14 Autonomy can be exercised at any point during the health care process.12 Although ethical and legal guidelines encourage physicians to recommend appropriate treatment, medical opinion cannot overrule the wishes of a competent patient who refuses treatment.12
Case Presentation
Mr. S presented to the emergency department at a US Department of Veterans Affairs (VA) medical center with abdominal pain from recurrent pancreatitis. The patient aged > 65 years had a history of depression, ESRD, and was receiving hemodialysis. A computed tomography scan revealed a new pancreatic mass, and he was referred to the palliative care (PC) department nurse practitioner (NP) for a goals-of-care discussion. PC was informed to assist with hospice care initiation: The patient elected a do-not-resuscitate (DNR) code status and hospice care.
At the consultation, Mr. S stated that he had decided to forego life-prolonging treatments, including hemodialysis, and declined further evaluation for his pancreatic mass. He shared a good understanding of concerns for malignancy with his mass but did not wish to pursue further diagnostics as he knew his life expectancy was very limited without dialysis. He had been dependent on hemodialysis for the past 10 years. He had briefly received hospice care 5 years before but changed his mind and decided to pursue standard care, including life-prolonging dialysis treatments. He reported no depression, suicidal ideation, or intentions of hastening his death. He stated that he was just physically tired from his ongoing dialysis, recurrent hospitalizations, and being repeatedly subjected to diagnostic tests. Mr. S added that he had discussed his plan with his family, including his son and sister-in-law who is married to his brother. Mr. S previously identified his brother as his surrogate decision maker.
Mr. S shared that his brother had sustained a traumatic brain injury and was now unable to engage in a meaningful conversation. He shared that his family supported his decision. He also recognized that with his debility, he would need inpatient hospice care. On finding out that Mr. S’s brother was no longer able to act as the surrogate decision maker, the PC NP asked whether he wanted her to contact his son to share the outcome of their visit. The patient declined, adding that he had discussed his care plans with his family and did not feel that his health care team needed to have additional discussions with them.
Mr. S also reported chronic, recurrent right upper quadrant pain. He was prescribed oxycodone 10 mg every 4 hours as needed; however, it did little to control his pain. He also reported generalized pruritus, a complication of his renal failure.
After 1 week, Mr. S was transferred to the inpatient hospice unit. At that time, he allowed the hospice team to contact his son for medical updates and identified him as the primary point of contact for the hospice team if the need arose to reach his family. Due to the restrictions imposed by the pandemic, Mr. S had virtual video visits with his family. Mr. S developed intermittent confusion and worsening fatigue over time. His son was informed of his deteriorating condition and visited his father. Mr. S died peacefully 2 days later with his family present.
Multidisciplinary Inputs on the Case
Medicine. In discussing the case with medicine, the PC NP was informed that the goals the patient had for his care, which included stopping dialysis, having a DNR code status and pursuing hospice care, along with the patient’s pain symptoms prompted the PC consultation. The resident also shared concerns about the patient’s refusal to have his surrogate decision maker and family contacted regarding his decisions for his care.
Palliative care. After meeting with the patient and assisting in identifying goals for care, the PC NP recommended initiation of hospice care in the hospital while the patient awaited transfer to the inpatient hospice unit. The PC NP also recommended a psychiatric evaluation to rule out untreated depression that might influence the patient’s decision making. A follow-up visit with nephrology was also recommended. Optimal management of his distressing physical symptoms was recommended, including prescribing hydromorphone instead of oxycodone for his pain and starting a topical emollient for pruritus.
Nephrology. The patient’s electronic health records (EHR) showed that he informed nephrology of his desire to pursue hospice care and that he decided against further dialysis, including as-needed dialysis for comfort. The records also indicated that the patient understood the consequences of discontinuing dialysis.
Psychiatry. The patient’s EHR also showed that during his psychiatric visits, Mr. S reported he had no thoughts of suicide, and it was against his spiritual beliefs. He said he made his own medical decisions and expressed that his health care team should not attempt to change his mind. He also said he understood that stopping dialysis could lead to early death. He stated he had a close relationship with his family and discussed his medical decisions with them. He was tearful at times when he talked about his family. Mr. S shared his frustration about repeatedly being asked the same questions on succeeding visits.
After evaluation, psychiatry diagnosed Mr. S with mood disorder with depressive features and he was prescribed methylphenidate 5 mg daily and sertraline 25 mg daily. They also recommended continuing to offer dialysis in a supportive manner since the patient had changed his mind about hospice in the past. However, psychiatry followed the patient daily for 5 days and concluded that his medical decisions were not clouded by mood symptoms.
Discussion
Patients who are aged > 65 years and on dialysis are more likely to experience higher rates of hospitalization, intensive care unit admission, procedures, and death in the hospital compared with patients who have cancer or heart failure. They also use hospice services less.15 Often this is not consistent with a patient’s wishes but may occur due to limited discussion of goals, values, and preferences between physician and patient.15 Many nephrologists do not engage in these conversations for fear of upsetting patients, their perceived lack of skill in prognostication and discussing the topic, or the lack of time to have the conversation.15 It is important to have an honest and open communication with patients that allows them to be fully informed as they make their medical decisions and exercise their autonomy.
Medicare hospice guidelines also are used to help determine hospice appropriateness among veterans in the VA. Medicare requires enrollees to discontinue disease-modifying treatment for the medical condition leading to their hospice diagnosis, which can result in late hospice referrals and shorter hospice stays.16 Even though hospice referrals for patients with ESRD have increased over time, they are still happening close to the time of death, and patients’ health care utilization near the end of life remains unchanged.16 According to Medicare, patients qualify for hospice care if they are terminally ill (defined as having a life expectancy of ≤ 6 months), choose comfort care over curative care for their terminal illness, and sign a statement electing hospice care over treatments for their terminal illness.17 A DNR order is not a condition for hospice admission.18
The VA defines hospice care as comfort care provided to patients with a terminal condition, a life expectancy of ≤ 6 months, and who are no longer seeking treatment other than those that are palliative.19 Based on his ESRD, Mr. S was qualified for hospice care, and his goals for care were consistent with the hospice philosophy. Most families of patients who elected to withdraw dialysis reported a good death, using the criteria of the duration of dying, discomfort, and psychosocial circumstances.20
Role of HCPs
Health care practitioners (HCPs) are expected to help patients understand the risks and benefits of their choices and its alternative, align patients’ goals with those risks and benefits, and assist patients in making choices that promote their goals and autonomy.21 Family members are often not involved in medical decision making when patients have the capacity to make their own decisions.22 Patients will also have to give permission for protected health information to be shared with their family members.22 On the other hand, families have been shown to provide valuable emotional support to patients and are considered second patients themselves in the sense that they can be impacted by patients’ clinical situation.22 Families may also need care, time, and attention from HCPs.22
Mr. S was found capable of making his own decisions, and part of that decision was that his family not to be present for the goals-of-care discussion. He added that he would discuss the care decisions with his family. At the time of registering for VA health care services, Mr. S had provided his health care team with his brother and sister-in-law’s emergency contact information as well as named his brother surrogate decision maker. As Mr. S’s condition was expected to rapidly decline wthout dialysis, the HCPs would be able to notify family members once his condition changed, including death.
Neuroplasticity changes can contribute to chronic pain that may also lead to depression.23 Chronic pain and depression may involve the same brain structures, neurotransmitters, and signaling pathway.23 Factors leading to chronic pain and depression include decreased availability of monoamine neurotransmitters, such as serotonin, dopamine, and norepinephrine in the central nervous system, decreased brain-derived neurotrophic factor, inflammatory response, and increased glutamate activity.23 Depression and hopelessness have been associated with the desire to hasten death among patients with a terminal illness.24 Worse mental health has been associated with the desire to hasten death among patients who are older and functionally impaired.25 It was important to optimize Mr. S’s treatment for pain and depression to ensure that these factors were not influencing his medical decisions.
With increasing recognition of the need to improve quality of life, health care utilization, and provide care consistent with patients’ goals in nephrology, the concept of renal PC is emerging but remains limited.26 The need to improve supportive care or PC for patients starting on dialysis for ESRD is high as these patients tend to be older (aged > 75 years), have high rates of cardiovascular comorbidities, can have coexisting cognitive impairment and functional debility, and have an adjusted mortality rate of up to 32.5% within 1 year of starting dialysis.26 Some ways to enhance renal PC programs include incorporating PC skill development and training within nephrology fellowships, educating patients with chronic and ESRD about PC and options for medical management without dialysis, and increasing the collaboration between nephrology and PC.26
Outcomes and Implications
Respect for the ethical principle of autonomy is paramount in health care. Patients should be able to give informed consent for treatment decisions without undue influence from their HCPs and should be able to withdraw that consent at any point during treatment. Factors that may influence patients’ ability to make medical decisions should be considered, including untreated or poorly treated symptoms. The involvement of PC helps optimize symptom management, provide support, and assist in goals-of-care discussions. Advanced practice PC nurses can offer other members of the health care team additional information and support in end-of-life care. Family involvement should be encouraged even for patients who can make their own medical decisions for emotional support and to assist families in what could be a traumatic event, such as the loss of a loved one.
The desire to pursue a comfort-focused approach to terminal illness and stop disease-modifying treatments are criteria for hospice care. An interdisciplinary approach to end-of-life care is beneficial, and every specialty should be equipped to engage in honest communication and skillful prognostication. These conversations should start early in the course of a terminal illness. Multiple factors contribute to poor clinical outcomes among patients with ESRD even with renal replacement therapy, such as dialysis. There is a need to improve PC training in the field of nephrology.
Conclusions
Mr. S was able to choose to withdraw potentially life-prolonging treatments with the support of his family and HCPs. He was able to continue receiving high-quality care and treatment in accordance with his wishes and goals for his care. The provision of interdisciplinary care that focused on supporting him allowed for his peaceful and comfortable death.
1. Carr D, Luth EA. Well-being at the end of life. Annu Rev Sociol. 2019;45:515-534. doi:10.1146/annurev-soc-073018-022524
2. Teno JM, Gozalo P, Trivedi AN, et al. Site of death, place of care, and health care transitions among US Medicare beneficiaries, 2000-2015. JAMA. 2018;320(3):264-271. doi:10.1001/jama.2018.8981
3. Himmelfarb J, Vanholder R, Mehrotra R, Tonelli M. The current and future landscape of dialysis. Nat Rev Nephrol. 2020;16(10):573-585. doi:10.1038/s41581-020-0315-4
4. Richards CA, Hebert PL, Liu CF, et al. Association of family ratings of quality of end-of-life care with stopping dialysis treatment and receipt of hospice services. JAMA Netw Open. 2019;2(10):e1913115. doi:10.1001/jamanetworkopen.2019.13115
5. Fischer MJ, Kourany WM, Sovern K, Forrester K, Griffin C, Lightner N, Loftus S, Murphy K, Roth G, Palevsky PM, Crowley ST. Development, implementation and user experience of the Veterans Health Administration (VHA) dialysis dashboard. BMC Nephrol. 2020 Apr 16;21(1):136. doi:10.1186/s12882-020-01798-6
6. Schwarze ML, Schueller K, Jhagroo RA. Hospice use and end-of-life care for patients with end-stage renal disease: too little, too late. JAMA Intern Med. 2018;178(6):799-801.doi:10.1001/jamainternmed.2018.1078
7. Chen JC, Thorsteinsdottir B, Vaughan LE, et al. End of life, withdrawal, and palliative care utilization among patients receiving maintenance hemodialysis therapy. Clin J Am Soc Nephrol. 2018;13(8):1172-1179. doi:10.2215/CJN.00590118
8. Chen HC, Wu CY, Hsieh HY, He JS, Hwang SJ, Hsieh HM. Predictors and assessment of hospice use for end-stage renal disease patients in Taiwan. Int J Environ Res Public Health. 2021;19(1):85. doi:10.3390/ijerph19010085
9. Rak A, Raina R, Suh TT, et al. Palliative care for patients with end-stage renal disease: approach to treatment that aims to improve quality of life and relieve suffering for patients (and families) with chronic illnesses. Clin Kidney J. 2017;10(1):68-73. doi.10.1093/ckj/sfw10510. Wong SPY, Boyapati S, Engelberg RA, Thorsteinsdottir B, Taylor JS, O’Hare AM. Experiences of US nephrologists in the delivery of conservative care to patients with advanced kidney disease: a national qualitative study. Am J Kidney Dis. 2020;75(2):167-176. doi:10.1053/j.ajkd.2019.07.006
11. Axelsson L, Benzein E, Lindberg J, Persson C. End-of-life and palliative care of patients on maintenance hemodialysis treatment: a focus group study. BMC Palliat Care. 2019;18(1):89. doi:10.1186/s12904-019-0481-y
12. Tweeddale MG. Grasping the nettle—what to do when patients withdraw their consent for treatment: (a clinical perspective on the case of Ms B). J Med Ethics. 2002;28(4):236-237. doi:10.1136/jme.28.4.236
13. Lynøe N, Engström I, Juth N. How to reveal disguised paternalism: version 2.0. BMC Med Ethics. 2021;22(1):170. doi:10.1186/s12910-021-00739-8
14. Murgic L, Hébert PC, Sovic S, Pavlekovic G. Paternalism and autonomy: views of patients and providers in a transitional (post-communist) country. BMC Med Ethics. 2015;16(1):65. doi:10.1186/s12910-015-0059-z
15. Mandel EI, Bernacki RE, Block SD. Serious illness conversations in ESRD. Clin J Am Soc Nephrol. 2017;12(5):854-863. doi:10.2215/CJN.05760516
16. Wachterman MW, Hailpern SM, Keating NL, Kurella Tamura M, O’Hare AM. Association between hospice length of stay, health care utilization, and Medicare costs at the end of life among patients who received maintenance hemodialysis. JAMA Int Med. 2018;178(6):792-799. doi:10.1001/jamainternmed.2018.0256
17. Centers for Medicare and Medicaid Services. Hospice care. Accessed April 2, 2022. https://www.medicare.gov/coverage/hospice-care
18. National Hospice and Palliative Care Organization. Ethical behavior and consumer rights. Standards of Practice for Hospice Programs Professional Development and Resource Series. Accessed December 6, 2022. https://www.nhpco.org/wp-content/uploads/2019/04/Standards_Hospice_2018.pdf
19. US Department of Veterans Affairs. Geriatrics and extended care. Updated October 5, 2022. Accessed August 29, 2022. https://www.va.gov/geriatrics/pages/Hospice_Care.asp
20. Cohen LM, McCue JD, Germain M, Kjellstrand CM. Dialysis discontinuation. A ‘good’ death? Arch Intern Med. 1995;155(1):42-47.
21. Ubel PA, Scherr KA, Fagerlin A. Autonomy: What’s shared decision making have to do with it? Am J Bioeth. 2018;18(2):W11-W12.doi:10.1080/15265161.2017.1409844
22. Laryionava, K, Pfeil TA, Dietrich M. et al. The second patient? Family members of cancer patients and their role in end-of-life decision making. BMC Palliat Care. 2018;17(1):29. doi:10.1186/s12904-018-0288-2
23. Sheng J, Liu S, Wang Y, Cui R, Zhang X. The link between depression and chronic pain: neural mechanisms in the brain. Neural Plast. 2017;2017:9724371. doi:10.1155/2017/9724371
24. Breitbart W, Rosenfeld B, Pessin H, et al. Depression, hopelessness, and desire for hastened death in terminally ill patients with cancer. JAMA. 2000;284(22):2907-2911. doi:10.1001/jama.284.22.2907
25. Sullivan M, Ormel J, Kempen GIJM, Tymstra T. Beliefs concerning death, dying, and hastening death among older, functionally impaired Dutch adults: a one-year longitudinal study. J Am Gec Soc. doi:10.1111/j.1532-5415.1998.tb04541.x26. Gelfand SL, Schell J, Eneanya ND. Palliative care in nephrology: the work and the workforce. Adv Chronic Kidney Dis. 2020;27(4):350-355.e1. doi:10.1053/j.ackd.2020.02.007
Due to advances in medicine, people are living longer with the aid of increased options for life-prolonging treatments. These treatment options may improve the quantity but not necessarily the quality of life.1
Kidney failure can be treated with renal replacement therapy (dialysis or renal transplantation) or supportive care.2 In 2017, the global prevalence of kidney failure was about 5.3 to 9.7 million.3 In the United States, about 500,000 patients are receiving maintenance dialysis for end-stage renal disease (ESRD), and about 1 in 4 will stop dialysis before death, coupled with hospice enrollment.4 ESRD is 2 times more prevalent among veterans than in nonveterans, which can be due in part to high rates of comorbid predisposing conditions, such as diabetes mellitus, hypertension, and advanced age, among others.5 The decision to discontinue dialysis and receive hospice care tends to be more difficult than choosing to withhold or forego dialysis.6
A study conducted among patients who were taken off hemodialysis before death reported that the 2 most common reasons for the withdrawal were acute medical complications and frailty.7 A retrospective study among patients with ESRD receiving hemodialysis highlighted the underutilization of hospice care in this patient population.8 The study also found that those patients who were aged > 75 years, had poor functional status, and had dialysis-related complications, such as sepsis and anemia, were more likely to elect withdrawal of hemodialysis. There was no difference in overall survival or quality of life among patients who were aged ≥ 75 years with multiple comorbidities and functional impairment who elected conservative management vs those who started dialysis.8 Long-term continuous dialysis has been associated with a lower quality of life, increased dependence on others, and a variety of symptoms, such as pain, nausea, insomnia, anxiety, or depression.9
Conservative Care vs Medical Paternalism
In the United States, it is unusual for patients with ESRD to choose conservative care, and supportive services are less available for those who do compared with patients with ESRD in Europe, Asia, Australia, and Canada.10 A study looking at a small number of US nephrologists has shown they may have limited experience in caring for patients who forego dialysis and they are not comfortable offering conservative management over dialysis.10 Another small study from Sweden also showed that many nephrologists do not feel prepared for end-of-life care and conversations.11
Patients often rely on knowledgeable recommendations from medical experts. However, medical paternalism occurs when a physician makes decisions deemed to be in the patient’s best interest but are against the patient’s wishes or when the patient is unable to give their consent.12 Hard paternalism occurs when the patient is competent to make their own medical decisions, while soft paternalism occurs when a patient is not competent to make their own medical decisions.13
Patient autonomy is widely recognized as an ethical principle in medicine. It recognizes patients as well-informed decision makers who may act without excessive influence to make intentional determinations on their own behalf.14 Autonomy can be exercised at any point during the health care process.12 Although ethical and legal guidelines encourage physicians to recommend appropriate treatment, medical opinion cannot overrule the wishes of a competent patient who refuses treatment.12
Case Presentation
Mr. S presented to the emergency department at a US Department of Veterans Affairs (VA) medical center with abdominal pain from recurrent pancreatitis. The patient aged > 65 years had a history of depression, ESRD, and was receiving hemodialysis. A computed tomography scan revealed a new pancreatic mass, and he was referred to the palliative care (PC) department nurse practitioner (NP) for a goals-of-care discussion. PC was informed to assist with hospice care initiation: The patient elected a do-not-resuscitate (DNR) code status and hospice care.
At the consultation, Mr. S stated that he had decided to forego life-prolonging treatments, including hemodialysis, and declined further evaluation for his pancreatic mass. He shared a good understanding of concerns for malignancy with his mass but did not wish to pursue further diagnostics as he knew his life expectancy was very limited without dialysis. He had been dependent on hemodialysis for the past 10 years. He had briefly received hospice care 5 years before but changed his mind and decided to pursue standard care, including life-prolonging dialysis treatments. He reported no depression, suicidal ideation, or intentions of hastening his death. He stated that he was just physically tired from his ongoing dialysis, recurrent hospitalizations, and being repeatedly subjected to diagnostic tests. Mr. S added that he had discussed his plan with his family, including his son and sister-in-law who is married to his brother. Mr. S previously identified his brother as his surrogate decision maker.
Mr. S shared that his brother had sustained a traumatic brain injury and was now unable to engage in a meaningful conversation. He shared that his family supported his decision. He also recognized that with his debility, he would need inpatient hospice care. On finding out that Mr. S’s brother was no longer able to act as the surrogate decision maker, the PC NP asked whether he wanted her to contact his son to share the outcome of their visit. The patient declined, adding that he had discussed his care plans with his family and did not feel that his health care team needed to have additional discussions with them.
Mr. S also reported chronic, recurrent right upper quadrant pain. He was prescribed oxycodone 10 mg every 4 hours as needed; however, it did little to control his pain. He also reported generalized pruritus, a complication of his renal failure.
After 1 week, Mr. S was transferred to the inpatient hospice unit. At that time, he allowed the hospice team to contact his son for medical updates and identified him as the primary point of contact for the hospice team if the need arose to reach his family. Due to the restrictions imposed by the pandemic, Mr. S had virtual video visits with his family. Mr. S developed intermittent confusion and worsening fatigue over time. His son was informed of his deteriorating condition and visited his father. Mr. S died peacefully 2 days later with his family present.
Multidisciplinary Inputs on the Case
Medicine. In discussing the case with medicine, the PC NP was informed that the goals the patient had for his care, which included stopping dialysis, having a DNR code status and pursuing hospice care, along with the patient’s pain symptoms prompted the PC consultation. The resident also shared concerns about the patient’s refusal to have his surrogate decision maker and family contacted regarding his decisions for his care.
Palliative care. After meeting with the patient and assisting in identifying goals for care, the PC NP recommended initiation of hospice care in the hospital while the patient awaited transfer to the inpatient hospice unit. The PC NP also recommended a psychiatric evaluation to rule out untreated depression that might influence the patient’s decision making. A follow-up visit with nephrology was also recommended. Optimal management of his distressing physical symptoms was recommended, including prescribing hydromorphone instead of oxycodone for his pain and starting a topical emollient for pruritus.
Nephrology. The patient’s electronic health records (EHR) showed that he informed nephrology of his desire to pursue hospice care and that he decided against further dialysis, including as-needed dialysis for comfort. The records also indicated that the patient understood the consequences of discontinuing dialysis.
Psychiatry. The patient’s EHR also showed that during his psychiatric visits, Mr. S reported he had no thoughts of suicide, and it was against his spiritual beliefs. He said he made his own medical decisions and expressed that his health care team should not attempt to change his mind. He also said he understood that stopping dialysis could lead to early death. He stated he had a close relationship with his family and discussed his medical decisions with them. He was tearful at times when he talked about his family. Mr. S shared his frustration about repeatedly being asked the same questions on succeeding visits.
After evaluation, psychiatry diagnosed Mr. S with mood disorder with depressive features and he was prescribed methylphenidate 5 mg daily and sertraline 25 mg daily. They also recommended continuing to offer dialysis in a supportive manner since the patient had changed his mind about hospice in the past. However, psychiatry followed the patient daily for 5 days and concluded that his medical decisions were not clouded by mood symptoms.
Discussion
Patients who are aged > 65 years and on dialysis are more likely to experience higher rates of hospitalization, intensive care unit admission, procedures, and death in the hospital compared with patients who have cancer or heart failure. They also use hospice services less.15 Often this is not consistent with a patient’s wishes but may occur due to limited discussion of goals, values, and preferences between physician and patient.15 Many nephrologists do not engage in these conversations for fear of upsetting patients, their perceived lack of skill in prognostication and discussing the topic, or the lack of time to have the conversation.15 It is important to have an honest and open communication with patients that allows them to be fully informed as they make their medical decisions and exercise their autonomy.
Medicare hospice guidelines also are used to help determine hospice appropriateness among veterans in the VA. Medicare requires enrollees to discontinue disease-modifying treatment for the medical condition leading to their hospice diagnosis, which can result in late hospice referrals and shorter hospice stays.16 Even though hospice referrals for patients with ESRD have increased over time, they are still happening close to the time of death, and patients’ health care utilization near the end of life remains unchanged.16 According to Medicare, patients qualify for hospice care if they are terminally ill (defined as having a life expectancy of ≤ 6 months), choose comfort care over curative care for their terminal illness, and sign a statement electing hospice care over treatments for their terminal illness.17 A DNR order is not a condition for hospice admission.18
The VA defines hospice care as comfort care provided to patients with a terminal condition, a life expectancy of ≤ 6 months, and who are no longer seeking treatment other than those that are palliative.19 Based on his ESRD, Mr. S was qualified for hospice care, and his goals for care were consistent with the hospice philosophy. Most families of patients who elected to withdraw dialysis reported a good death, using the criteria of the duration of dying, discomfort, and psychosocial circumstances.20
Role of HCPs
Health care practitioners (HCPs) are expected to help patients understand the risks and benefits of their choices and its alternative, align patients’ goals with those risks and benefits, and assist patients in making choices that promote their goals and autonomy.21 Family members are often not involved in medical decision making when patients have the capacity to make their own decisions.22 Patients will also have to give permission for protected health information to be shared with their family members.22 On the other hand, families have been shown to provide valuable emotional support to patients and are considered second patients themselves in the sense that they can be impacted by patients’ clinical situation.22 Families may also need care, time, and attention from HCPs.22
Mr. S was found capable of making his own decisions, and part of that decision was that his family not to be present for the goals-of-care discussion. He added that he would discuss the care decisions with his family. At the time of registering for VA health care services, Mr. S had provided his health care team with his brother and sister-in-law’s emergency contact information as well as named his brother surrogate decision maker. As Mr. S’s condition was expected to rapidly decline wthout dialysis, the HCPs would be able to notify family members once his condition changed, including death.
Neuroplasticity changes can contribute to chronic pain that may also lead to depression.23 Chronic pain and depression may involve the same brain structures, neurotransmitters, and signaling pathway.23 Factors leading to chronic pain and depression include decreased availability of monoamine neurotransmitters, such as serotonin, dopamine, and norepinephrine in the central nervous system, decreased brain-derived neurotrophic factor, inflammatory response, and increased glutamate activity.23 Depression and hopelessness have been associated with the desire to hasten death among patients with a terminal illness.24 Worse mental health has been associated with the desire to hasten death among patients who are older and functionally impaired.25 It was important to optimize Mr. S’s treatment for pain and depression to ensure that these factors were not influencing his medical decisions.
With increasing recognition of the need to improve quality of life, health care utilization, and provide care consistent with patients’ goals in nephrology, the concept of renal PC is emerging but remains limited.26 The need to improve supportive care or PC for patients starting on dialysis for ESRD is high as these patients tend to be older (aged > 75 years), have high rates of cardiovascular comorbidities, can have coexisting cognitive impairment and functional debility, and have an adjusted mortality rate of up to 32.5% within 1 year of starting dialysis.26 Some ways to enhance renal PC programs include incorporating PC skill development and training within nephrology fellowships, educating patients with chronic and ESRD about PC and options for medical management without dialysis, and increasing the collaboration between nephrology and PC.26
Outcomes and Implications
Respect for the ethical principle of autonomy is paramount in health care. Patients should be able to give informed consent for treatment decisions without undue influence from their HCPs and should be able to withdraw that consent at any point during treatment. Factors that may influence patients’ ability to make medical decisions should be considered, including untreated or poorly treated symptoms. The involvement of PC helps optimize symptom management, provide support, and assist in goals-of-care discussions. Advanced practice PC nurses can offer other members of the health care team additional information and support in end-of-life care. Family involvement should be encouraged even for patients who can make their own medical decisions for emotional support and to assist families in what could be a traumatic event, such as the loss of a loved one.
The desire to pursue a comfort-focused approach to terminal illness and stop disease-modifying treatments are criteria for hospice care. An interdisciplinary approach to end-of-life care is beneficial, and every specialty should be equipped to engage in honest communication and skillful prognostication. These conversations should start early in the course of a terminal illness. Multiple factors contribute to poor clinical outcomes among patients with ESRD even with renal replacement therapy, such as dialysis. There is a need to improve PC training in the field of nephrology.
Conclusions
Mr. S was able to choose to withdraw potentially life-prolonging treatments with the support of his family and HCPs. He was able to continue receiving high-quality care and treatment in accordance with his wishes and goals for his care. The provision of interdisciplinary care that focused on supporting him allowed for his peaceful and comfortable death.
Due to advances in medicine, people are living longer with the aid of increased options for life-prolonging treatments. These treatment options may improve the quantity but not necessarily the quality of life.1
Kidney failure can be treated with renal replacement therapy (dialysis or renal transplantation) or supportive care.2 In 2017, the global prevalence of kidney failure was about 5.3 to 9.7 million.3 In the United States, about 500,000 patients are receiving maintenance dialysis for end-stage renal disease (ESRD), and about 1 in 4 will stop dialysis before death, coupled with hospice enrollment.4 ESRD is 2 times more prevalent among veterans than in nonveterans, which can be due in part to high rates of comorbid predisposing conditions, such as diabetes mellitus, hypertension, and advanced age, among others.5 The decision to discontinue dialysis and receive hospice care tends to be more difficult than choosing to withhold or forego dialysis.6
A study conducted among patients who were taken off hemodialysis before death reported that the 2 most common reasons for the withdrawal were acute medical complications and frailty.7 A retrospective study among patients with ESRD receiving hemodialysis highlighted the underutilization of hospice care in this patient population.8 The study also found that those patients who were aged > 75 years, had poor functional status, and had dialysis-related complications, such as sepsis and anemia, were more likely to elect withdrawal of hemodialysis. There was no difference in overall survival or quality of life among patients who were aged ≥ 75 years with multiple comorbidities and functional impairment who elected conservative management vs those who started dialysis.8 Long-term continuous dialysis has been associated with a lower quality of life, increased dependence on others, and a variety of symptoms, such as pain, nausea, insomnia, anxiety, or depression.9
Conservative Care vs Medical Paternalism
In the United States, it is unusual for patients with ESRD to choose conservative care, and supportive services are less available for those who do compared with patients with ESRD in Europe, Asia, Australia, and Canada.10 A study looking at a small number of US nephrologists has shown they may have limited experience in caring for patients who forego dialysis and they are not comfortable offering conservative management over dialysis.10 Another small study from Sweden also showed that many nephrologists do not feel prepared for end-of-life care and conversations.11
Patients often rely on knowledgeable recommendations from medical experts. However, medical paternalism occurs when a physician makes decisions deemed to be in the patient’s best interest but are against the patient’s wishes or when the patient is unable to give their consent.12 Hard paternalism occurs when the patient is competent to make their own medical decisions, while soft paternalism occurs when a patient is not competent to make their own medical decisions.13
Patient autonomy is widely recognized as an ethical principle in medicine. It recognizes patients as well-informed decision makers who may act without excessive influence to make intentional determinations on their own behalf.14 Autonomy can be exercised at any point during the health care process.12 Although ethical and legal guidelines encourage physicians to recommend appropriate treatment, medical opinion cannot overrule the wishes of a competent patient who refuses treatment.12
Case Presentation
Mr. S presented to the emergency department at a US Department of Veterans Affairs (VA) medical center with abdominal pain from recurrent pancreatitis. The patient aged > 65 years had a history of depression, ESRD, and was receiving hemodialysis. A computed tomography scan revealed a new pancreatic mass, and he was referred to the palliative care (PC) department nurse practitioner (NP) for a goals-of-care discussion. PC was informed to assist with hospice care initiation: The patient elected a do-not-resuscitate (DNR) code status and hospice care.
At the consultation, Mr. S stated that he had decided to forego life-prolonging treatments, including hemodialysis, and declined further evaluation for his pancreatic mass. He shared a good understanding of concerns for malignancy with his mass but did not wish to pursue further diagnostics as he knew his life expectancy was very limited without dialysis. He had been dependent on hemodialysis for the past 10 years. He had briefly received hospice care 5 years before but changed his mind and decided to pursue standard care, including life-prolonging dialysis treatments. He reported no depression, suicidal ideation, or intentions of hastening his death. He stated that he was just physically tired from his ongoing dialysis, recurrent hospitalizations, and being repeatedly subjected to diagnostic tests. Mr. S added that he had discussed his plan with his family, including his son and sister-in-law who is married to his brother. Mr. S previously identified his brother as his surrogate decision maker.
Mr. S shared that his brother had sustained a traumatic brain injury and was now unable to engage in a meaningful conversation. He shared that his family supported his decision. He also recognized that with his debility, he would need inpatient hospice care. On finding out that Mr. S’s brother was no longer able to act as the surrogate decision maker, the PC NP asked whether he wanted her to contact his son to share the outcome of their visit. The patient declined, adding that he had discussed his care plans with his family and did not feel that his health care team needed to have additional discussions with them.
Mr. S also reported chronic, recurrent right upper quadrant pain. He was prescribed oxycodone 10 mg every 4 hours as needed; however, it did little to control his pain. He also reported generalized pruritus, a complication of his renal failure.
After 1 week, Mr. S was transferred to the inpatient hospice unit. At that time, he allowed the hospice team to contact his son for medical updates and identified him as the primary point of contact for the hospice team if the need arose to reach his family. Due to the restrictions imposed by the pandemic, Mr. S had virtual video visits with his family. Mr. S developed intermittent confusion and worsening fatigue over time. His son was informed of his deteriorating condition and visited his father. Mr. S died peacefully 2 days later with his family present.
Multidisciplinary Inputs on the Case
Medicine. In discussing the case with medicine, the PC NP was informed that the goals the patient had for his care, which included stopping dialysis, having a DNR code status and pursuing hospice care, along with the patient’s pain symptoms prompted the PC consultation. The resident also shared concerns about the patient’s refusal to have his surrogate decision maker and family contacted regarding his decisions for his care.
Palliative care. After meeting with the patient and assisting in identifying goals for care, the PC NP recommended initiation of hospice care in the hospital while the patient awaited transfer to the inpatient hospice unit. The PC NP also recommended a psychiatric evaluation to rule out untreated depression that might influence the patient’s decision making. A follow-up visit with nephrology was also recommended. Optimal management of his distressing physical symptoms was recommended, including prescribing hydromorphone instead of oxycodone for his pain and starting a topical emollient for pruritus.
Nephrology. The patient’s electronic health records (EHR) showed that he informed nephrology of his desire to pursue hospice care and that he decided against further dialysis, including as-needed dialysis for comfort. The records also indicated that the patient understood the consequences of discontinuing dialysis.
Psychiatry. The patient’s EHR also showed that during his psychiatric visits, Mr. S reported he had no thoughts of suicide, and it was against his spiritual beliefs. He said he made his own medical decisions and expressed that his health care team should not attempt to change his mind. He also said he understood that stopping dialysis could lead to early death. He stated he had a close relationship with his family and discussed his medical decisions with them. He was tearful at times when he talked about his family. Mr. S shared his frustration about repeatedly being asked the same questions on succeeding visits.
After evaluation, psychiatry diagnosed Mr. S with mood disorder with depressive features and he was prescribed methylphenidate 5 mg daily and sertraline 25 mg daily. They also recommended continuing to offer dialysis in a supportive manner since the patient had changed his mind about hospice in the past. However, psychiatry followed the patient daily for 5 days and concluded that his medical decisions were not clouded by mood symptoms.
Discussion
Patients who are aged > 65 years and on dialysis are more likely to experience higher rates of hospitalization, intensive care unit admission, procedures, and death in the hospital compared with patients who have cancer or heart failure. They also use hospice services less.15 Often this is not consistent with a patient’s wishes but may occur due to limited discussion of goals, values, and preferences between physician and patient.15 Many nephrologists do not engage in these conversations for fear of upsetting patients, their perceived lack of skill in prognostication and discussing the topic, or the lack of time to have the conversation.15 It is important to have an honest and open communication with patients that allows them to be fully informed as they make their medical decisions and exercise their autonomy.
Medicare hospice guidelines also are used to help determine hospice appropriateness among veterans in the VA. Medicare requires enrollees to discontinue disease-modifying treatment for the medical condition leading to their hospice diagnosis, which can result in late hospice referrals and shorter hospice stays.16 Even though hospice referrals for patients with ESRD have increased over time, they are still happening close to the time of death, and patients’ health care utilization near the end of life remains unchanged.16 According to Medicare, patients qualify for hospice care if they are terminally ill (defined as having a life expectancy of ≤ 6 months), choose comfort care over curative care for their terminal illness, and sign a statement electing hospice care over treatments for their terminal illness.17 A DNR order is not a condition for hospice admission.18
The VA defines hospice care as comfort care provided to patients with a terminal condition, a life expectancy of ≤ 6 months, and who are no longer seeking treatment other than those that are palliative.19 Based on his ESRD, Mr. S was qualified for hospice care, and his goals for care were consistent with the hospice philosophy. Most families of patients who elected to withdraw dialysis reported a good death, using the criteria of the duration of dying, discomfort, and psychosocial circumstances.20
Role of HCPs
Health care practitioners (HCPs) are expected to help patients understand the risks and benefits of their choices and its alternative, align patients’ goals with those risks and benefits, and assist patients in making choices that promote their goals and autonomy.21 Family members are often not involved in medical decision making when patients have the capacity to make their own decisions.22 Patients will also have to give permission for protected health information to be shared with their family members.22 On the other hand, families have been shown to provide valuable emotional support to patients and are considered second patients themselves in the sense that they can be impacted by patients’ clinical situation.22 Families may also need care, time, and attention from HCPs.22
Mr. S was found capable of making his own decisions, and part of that decision was that his family not to be present for the goals-of-care discussion. He added that he would discuss the care decisions with his family. At the time of registering for VA health care services, Mr. S had provided his health care team with his brother and sister-in-law’s emergency contact information as well as named his brother surrogate decision maker. As Mr. S’s condition was expected to rapidly decline wthout dialysis, the HCPs would be able to notify family members once his condition changed, including death.
Neuroplasticity changes can contribute to chronic pain that may also lead to depression.23 Chronic pain and depression may involve the same brain structures, neurotransmitters, and signaling pathway.23 Factors leading to chronic pain and depression include decreased availability of monoamine neurotransmitters, such as serotonin, dopamine, and norepinephrine in the central nervous system, decreased brain-derived neurotrophic factor, inflammatory response, and increased glutamate activity.23 Depression and hopelessness have been associated with the desire to hasten death among patients with a terminal illness.24 Worse mental health has been associated with the desire to hasten death among patients who are older and functionally impaired.25 It was important to optimize Mr. S’s treatment for pain and depression to ensure that these factors were not influencing his medical decisions.
With increasing recognition of the need to improve quality of life, health care utilization, and provide care consistent with patients’ goals in nephrology, the concept of renal PC is emerging but remains limited.26 The need to improve supportive care or PC for patients starting on dialysis for ESRD is high as these patients tend to be older (aged > 75 years), have high rates of cardiovascular comorbidities, can have coexisting cognitive impairment and functional debility, and have an adjusted mortality rate of up to 32.5% within 1 year of starting dialysis.26 Some ways to enhance renal PC programs include incorporating PC skill development and training within nephrology fellowships, educating patients with chronic and ESRD about PC and options for medical management without dialysis, and increasing the collaboration between nephrology and PC.26
Outcomes and Implications
Respect for the ethical principle of autonomy is paramount in health care. Patients should be able to give informed consent for treatment decisions without undue influence from their HCPs and should be able to withdraw that consent at any point during treatment. Factors that may influence patients’ ability to make medical decisions should be considered, including untreated or poorly treated symptoms. The involvement of PC helps optimize symptom management, provide support, and assist in goals-of-care discussions. Advanced practice PC nurses can offer other members of the health care team additional information and support in end-of-life care. Family involvement should be encouraged even for patients who can make their own medical decisions for emotional support and to assist families in what could be a traumatic event, such as the loss of a loved one.
The desire to pursue a comfort-focused approach to terminal illness and stop disease-modifying treatments are criteria for hospice care. An interdisciplinary approach to end-of-life care is beneficial, and every specialty should be equipped to engage in honest communication and skillful prognostication. These conversations should start early in the course of a terminal illness. Multiple factors contribute to poor clinical outcomes among patients with ESRD even with renal replacement therapy, such as dialysis. There is a need to improve PC training in the field of nephrology.
Conclusions
Mr. S was able to choose to withdraw potentially life-prolonging treatments with the support of his family and HCPs. He was able to continue receiving high-quality care and treatment in accordance with his wishes and goals for his care. The provision of interdisciplinary care that focused on supporting him allowed for his peaceful and comfortable death.
1. Carr D, Luth EA. Well-being at the end of life. Annu Rev Sociol. 2019;45:515-534. doi:10.1146/annurev-soc-073018-022524
2. Teno JM, Gozalo P, Trivedi AN, et al. Site of death, place of care, and health care transitions among US Medicare beneficiaries, 2000-2015. JAMA. 2018;320(3):264-271. doi:10.1001/jama.2018.8981
3. Himmelfarb J, Vanholder R, Mehrotra R, Tonelli M. The current and future landscape of dialysis. Nat Rev Nephrol. 2020;16(10):573-585. doi:10.1038/s41581-020-0315-4
4. Richards CA, Hebert PL, Liu CF, et al. Association of family ratings of quality of end-of-life care with stopping dialysis treatment and receipt of hospice services. JAMA Netw Open. 2019;2(10):e1913115. doi:10.1001/jamanetworkopen.2019.13115
5. Fischer MJ, Kourany WM, Sovern K, Forrester K, Griffin C, Lightner N, Loftus S, Murphy K, Roth G, Palevsky PM, Crowley ST. Development, implementation and user experience of the Veterans Health Administration (VHA) dialysis dashboard. BMC Nephrol. 2020 Apr 16;21(1):136. doi:10.1186/s12882-020-01798-6
6. Schwarze ML, Schueller K, Jhagroo RA. Hospice use and end-of-life care for patients with end-stage renal disease: too little, too late. JAMA Intern Med. 2018;178(6):799-801.doi:10.1001/jamainternmed.2018.1078
7. Chen JC, Thorsteinsdottir B, Vaughan LE, et al. End of life, withdrawal, and palliative care utilization among patients receiving maintenance hemodialysis therapy. Clin J Am Soc Nephrol. 2018;13(8):1172-1179. doi:10.2215/CJN.00590118
8. Chen HC, Wu CY, Hsieh HY, He JS, Hwang SJ, Hsieh HM. Predictors and assessment of hospice use for end-stage renal disease patients in Taiwan. Int J Environ Res Public Health. 2021;19(1):85. doi:10.3390/ijerph19010085
9. Rak A, Raina R, Suh TT, et al. Palliative care for patients with end-stage renal disease: approach to treatment that aims to improve quality of life and relieve suffering for patients (and families) with chronic illnesses. Clin Kidney J. 2017;10(1):68-73. doi.10.1093/ckj/sfw10510. Wong SPY, Boyapati S, Engelberg RA, Thorsteinsdottir B, Taylor JS, O’Hare AM. Experiences of US nephrologists in the delivery of conservative care to patients with advanced kidney disease: a national qualitative study. Am J Kidney Dis. 2020;75(2):167-176. doi:10.1053/j.ajkd.2019.07.006
11. Axelsson L, Benzein E, Lindberg J, Persson C. End-of-life and palliative care of patients on maintenance hemodialysis treatment: a focus group study. BMC Palliat Care. 2019;18(1):89. doi:10.1186/s12904-019-0481-y
12. Tweeddale MG. Grasping the nettle—what to do when patients withdraw their consent for treatment: (a clinical perspective on the case of Ms B). J Med Ethics. 2002;28(4):236-237. doi:10.1136/jme.28.4.236
13. Lynøe N, Engström I, Juth N. How to reveal disguised paternalism: version 2.0. BMC Med Ethics. 2021;22(1):170. doi:10.1186/s12910-021-00739-8
14. Murgic L, Hébert PC, Sovic S, Pavlekovic G. Paternalism and autonomy: views of patients and providers in a transitional (post-communist) country. BMC Med Ethics. 2015;16(1):65. doi:10.1186/s12910-015-0059-z
15. Mandel EI, Bernacki RE, Block SD. Serious illness conversations in ESRD. Clin J Am Soc Nephrol. 2017;12(5):854-863. doi:10.2215/CJN.05760516
16. Wachterman MW, Hailpern SM, Keating NL, Kurella Tamura M, O’Hare AM. Association between hospice length of stay, health care utilization, and Medicare costs at the end of life among patients who received maintenance hemodialysis. JAMA Int Med. 2018;178(6):792-799. doi:10.1001/jamainternmed.2018.0256
17. Centers for Medicare and Medicaid Services. Hospice care. Accessed April 2, 2022. https://www.medicare.gov/coverage/hospice-care
18. National Hospice and Palliative Care Organization. Ethical behavior and consumer rights. Standards of Practice for Hospice Programs Professional Development and Resource Series. Accessed December 6, 2022. https://www.nhpco.org/wp-content/uploads/2019/04/Standards_Hospice_2018.pdf
19. US Department of Veterans Affairs. Geriatrics and extended care. Updated October 5, 2022. Accessed August 29, 2022. https://www.va.gov/geriatrics/pages/Hospice_Care.asp
20. Cohen LM, McCue JD, Germain M, Kjellstrand CM. Dialysis discontinuation. A ‘good’ death? Arch Intern Med. 1995;155(1):42-47.
21. Ubel PA, Scherr KA, Fagerlin A. Autonomy: What’s shared decision making have to do with it? Am J Bioeth. 2018;18(2):W11-W12.doi:10.1080/15265161.2017.1409844
22. Laryionava, K, Pfeil TA, Dietrich M. et al. The second patient? Family members of cancer patients and their role in end-of-life decision making. BMC Palliat Care. 2018;17(1):29. doi:10.1186/s12904-018-0288-2
23. Sheng J, Liu S, Wang Y, Cui R, Zhang X. The link between depression and chronic pain: neural mechanisms in the brain. Neural Plast. 2017;2017:9724371. doi:10.1155/2017/9724371
24. Breitbart W, Rosenfeld B, Pessin H, et al. Depression, hopelessness, and desire for hastened death in terminally ill patients with cancer. JAMA. 2000;284(22):2907-2911. doi:10.1001/jama.284.22.2907
25. Sullivan M, Ormel J, Kempen GIJM, Tymstra T. Beliefs concerning death, dying, and hastening death among older, functionally impaired Dutch adults: a one-year longitudinal study. J Am Gec Soc. doi:10.1111/j.1532-5415.1998.tb04541.x26. Gelfand SL, Schell J, Eneanya ND. Palliative care in nephrology: the work and the workforce. Adv Chronic Kidney Dis. 2020;27(4):350-355.e1. doi:10.1053/j.ackd.2020.02.007
1. Carr D, Luth EA. Well-being at the end of life. Annu Rev Sociol. 2019;45:515-534. doi:10.1146/annurev-soc-073018-022524
2. Teno JM, Gozalo P, Trivedi AN, et al. Site of death, place of care, and health care transitions among US Medicare beneficiaries, 2000-2015. JAMA. 2018;320(3):264-271. doi:10.1001/jama.2018.8981
3. Himmelfarb J, Vanholder R, Mehrotra R, Tonelli M. The current and future landscape of dialysis. Nat Rev Nephrol. 2020;16(10):573-585. doi:10.1038/s41581-020-0315-4
4. Richards CA, Hebert PL, Liu CF, et al. Association of family ratings of quality of end-of-life care with stopping dialysis treatment and receipt of hospice services. JAMA Netw Open. 2019;2(10):e1913115. doi:10.1001/jamanetworkopen.2019.13115
5. Fischer MJ, Kourany WM, Sovern K, Forrester K, Griffin C, Lightner N, Loftus S, Murphy K, Roth G, Palevsky PM, Crowley ST. Development, implementation and user experience of the Veterans Health Administration (VHA) dialysis dashboard. BMC Nephrol. 2020 Apr 16;21(1):136. doi:10.1186/s12882-020-01798-6
6. Schwarze ML, Schueller K, Jhagroo RA. Hospice use and end-of-life care for patients with end-stage renal disease: too little, too late. JAMA Intern Med. 2018;178(6):799-801.doi:10.1001/jamainternmed.2018.1078
7. Chen JC, Thorsteinsdottir B, Vaughan LE, et al. End of life, withdrawal, and palliative care utilization among patients receiving maintenance hemodialysis therapy. Clin J Am Soc Nephrol. 2018;13(8):1172-1179. doi:10.2215/CJN.00590118
8. Chen HC, Wu CY, Hsieh HY, He JS, Hwang SJ, Hsieh HM. Predictors and assessment of hospice use for end-stage renal disease patients in Taiwan. Int J Environ Res Public Health. 2021;19(1):85. doi:10.3390/ijerph19010085
9. Rak A, Raina R, Suh TT, et al. Palliative care for patients with end-stage renal disease: approach to treatment that aims to improve quality of life and relieve suffering for patients (and families) with chronic illnesses. Clin Kidney J. 2017;10(1):68-73. doi.10.1093/ckj/sfw10510. Wong SPY, Boyapati S, Engelberg RA, Thorsteinsdottir B, Taylor JS, O’Hare AM. Experiences of US nephrologists in the delivery of conservative care to patients with advanced kidney disease: a national qualitative study. Am J Kidney Dis. 2020;75(2):167-176. doi:10.1053/j.ajkd.2019.07.006
11. Axelsson L, Benzein E, Lindberg J, Persson C. End-of-life and palliative care of patients on maintenance hemodialysis treatment: a focus group study. BMC Palliat Care. 2019;18(1):89. doi:10.1186/s12904-019-0481-y
12. Tweeddale MG. Grasping the nettle—what to do when patients withdraw their consent for treatment: (a clinical perspective on the case of Ms B). J Med Ethics. 2002;28(4):236-237. doi:10.1136/jme.28.4.236
13. Lynøe N, Engström I, Juth N. How to reveal disguised paternalism: version 2.0. BMC Med Ethics. 2021;22(1):170. doi:10.1186/s12910-021-00739-8
14. Murgic L, Hébert PC, Sovic S, Pavlekovic G. Paternalism and autonomy: views of patients and providers in a transitional (post-communist) country. BMC Med Ethics. 2015;16(1):65. doi:10.1186/s12910-015-0059-z
15. Mandel EI, Bernacki RE, Block SD. Serious illness conversations in ESRD. Clin J Am Soc Nephrol. 2017;12(5):854-863. doi:10.2215/CJN.05760516
16. Wachterman MW, Hailpern SM, Keating NL, Kurella Tamura M, O’Hare AM. Association between hospice length of stay, health care utilization, and Medicare costs at the end of life among patients who received maintenance hemodialysis. JAMA Int Med. 2018;178(6):792-799. doi:10.1001/jamainternmed.2018.0256
17. Centers for Medicare and Medicaid Services. Hospice care. Accessed April 2, 2022. https://www.medicare.gov/coverage/hospice-care
18. National Hospice and Palliative Care Organization. Ethical behavior and consumer rights. Standards of Practice for Hospice Programs Professional Development and Resource Series. Accessed December 6, 2022. https://www.nhpco.org/wp-content/uploads/2019/04/Standards_Hospice_2018.pdf
19. US Department of Veterans Affairs. Geriatrics and extended care. Updated October 5, 2022. Accessed August 29, 2022. https://www.va.gov/geriatrics/pages/Hospice_Care.asp
20. Cohen LM, McCue JD, Germain M, Kjellstrand CM. Dialysis discontinuation. A ‘good’ death? Arch Intern Med. 1995;155(1):42-47.
21. Ubel PA, Scherr KA, Fagerlin A. Autonomy: What’s shared decision making have to do with it? Am J Bioeth. 2018;18(2):W11-W12.doi:10.1080/15265161.2017.1409844
22. Laryionava, K, Pfeil TA, Dietrich M. et al. The second patient? Family members of cancer patients and their role in end-of-life decision making. BMC Palliat Care. 2018;17(1):29. doi:10.1186/s12904-018-0288-2
23. Sheng J, Liu S, Wang Y, Cui R, Zhang X. The link between depression and chronic pain: neural mechanisms in the brain. Neural Plast. 2017;2017:9724371. doi:10.1155/2017/9724371
24. Breitbart W, Rosenfeld B, Pessin H, et al. Depression, hopelessness, and desire for hastened death in terminally ill patients with cancer. JAMA. 2000;284(22):2907-2911. doi:10.1001/jama.284.22.2907
25. Sullivan M, Ormel J, Kempen GIJM, Tymstra T. Beliefs concerning death, dying, and hastening death among older, functionally impaired Dutch adults: a one-year longitudinal study. J Am Gec Soc. doi:10.1111/j.1532-5415.1998.tb04541.x26. Gelfand SL, Schell J, Eneanya ND. Palliative care in nephrology: the work and the workforce. Adv Chronic Kidney Dis. 2020;27(4):350-355.e1. doi:10.1053/j.ackd.2020.02.007
Chronic Lymphocytic Leukemia Highlights From ASH 2022
Reporting on chronic lymphocytic leukemia (CLL) highlights from the American Society of Hematology meetings, Dr Nicholas Burwick of the Puget Sound Veterans Administration Health Care System discusses studies ranging from first-line treatment to options for relapsed/refractory disease.
Dr Burwick cites a Veterans Health Administration study documenting the increasing movement toward novel agents as frontline treatment in CLL since 2018, and the emerging trend toward use of second-generation Bruton tyrosine kinase (BTK) inhibitors.
Next, he discusses a study examining the frontline combination of ibrutinib and venetoclax, which may help overcome poor prognosis associated with unmutated IGHV CLL.
In another study, the combination of venetoclax and ibrutinib was examined in high-risk patients treated with ibrutinib for 1 year. The results showed the combination deepens responses and even offers the potential for discontinuation of therapy.
Turning to relapsed/refractory disease, Dr Burwick highlights the latest results of pirtobrutinib, which continues to show efficacy among heavily pretreated patients regardless of prior therapy, reason for discontinuation, or mutation status.
Finally, he points to zanubrutinib, which demonstrated superiority to ibrutinib in a long-range study of progression-free survival.
--
Nicholas R. Burwick, MD, Associate Professor, Division of Hematology, Department of Medicine, University of Washington; Attending Physician, Division of Hematology, Department of Medicine, Puget Sound VA Health Care System, Seattle, Washington
Nicholas R. Burwick, MD, has disclosed no relevant financial relationships.
Reporting on chronic lymphocytic leukemia (CLL) highlights from the American Society of Hematology meetings, Dr Nicholas Burwick of the Puget Sound Veterans Administration Health Care System discusses studies ranging from first-line treatment to options for relapsed/refractory disease.
Dr Burwick cites a Veterans Health Administration study documenting the increasing movement toward novel agents as frontline treatment in CLL since 2018, and the emerging trend toward use of second-generation Bruton tyrosine kinase (BTK) inhibitors.
Next, he discusses a study examining the frontline combination of ibrutinib and venetoclax, which may help overcome poor prognosis associated with unmutated IGHV CLL.
In another study, the combination of venetoclax and ibrutinib was examined in high-risk patients treated with ibrutinib for 1 year. The results showed the combination deepens responses and even offers the potential for discontinuation of therapy.
Turning to relapsed/refractory disease, Dr Burwick highlights the latest results of pirtobrutinib, which continues to show efficacy among heavily pretreated patients regardless of prior therapy, reason for discontinuation, or mutation status.
Finally, he points to zanubrutinib, which demonstrated superiority to ibrutinib in a long-range study of progression-free survival.
--
Nicholas R. Burwick, MD, Associate Professor, Division of Hematology, Department of Medicine, University of Washington; Attending Physician, Division of Hematology, Department of Medicine, Puget Sound VA Health Care System, Seattle, Washington
Nicholas R. Burwick, MD, has disclosed no relevant financial relationships.
Reporting on chronic lymphocytic leukemia (CLL) highlights from the American Society of Hematology meetings, Dr Nicholas Burwick of the Puget Sound Veterans Administration Health Care System discusses studies ranging from first-line treatment to options for relapsed/refractory disease.
Dr Burwick cites a Veterans Health Administration study documenting the increasing movement toward novel agents as frontline treatment in CLL since 2018, and the emerging trend toward use of second-generation Bruton tyrosine kinase (BTK) inhibitors.
Next, he discusses a study examining the frontline combination of ibrutinib and venetoclax, which may help overcome poor prognosis associated with unmutated IGHV CLL.
In another study, the combination of venetoclax and ibrutinib was examined in high-risk patients treated with ibrutinib for 1 year. The results showed the combination deepens responses and even offers the potential for discontinuation of therapy.
Turning to relapsed/refractory disease, Dr Burwick highlights the latest results of pirtobrutinib, which continues to show efficacy among heavily pretreated patients regardless of prior therapy, reason for discontinuation, or mutation status.
Finally, he points to zanubrutinib, which demonstrated superiority to ibrutinib in a long-range study of progression-free survival.
--
Nicholas R. Burwick, MD, Associate Professor, Division of Hematology, Department of Medicine, University of Washington; Attending Physician, Division of Hematology, Department of Medicine, Puget Sound VA Health Care System, Seattle, Washington
Nicholas R. Burwick, MD, has disclosed no relevant financial relationships.

Racial disparities in cesarean delivery rates
CASE Patient wants to reduce her risk of cesarean delivery (CD)
A 30-year-old primigravid woman expresses concern about her increased risk for CD as a Black woman. She has been reading in the news about the increased risks of CD and birth complications, and she asks what she can do to decrease her risk of having a CD.
What is the problem?
Recently, attention has been called to the stark racial disparities in severe maternal morbidity and mortality. Cesarean delivery rates illustrate an area in obstetric management in which racial disparities exist. It is well known that morbidity associated with CD is much higher than morbidity associated with vaginal delivery, which begs the question of whether disparities in mode of delivery may play a role in the disparity in maternal morbidity and mortality.
In the United States, 32% of all births between 2018 and 2020 were by CD. However, only 31% of White women delivered via CD as compared with 36% of Black women and 33% of Asian women.1 In 2021, the primary CD rates were 26% for Black women, 24% for Asian women, 21% for Hispanic women, and 22% for White women.2 This racial disparity, particularly between Black and White women, has been seen across nulliparous, term, singleton, vertex (NTSV) groups as well as multiparous women with prior vaginal delivery.3,4 The disparity persists after adjusting for risk factors.
A secondary analysis of groups deemed at low risk for CD within the ARRIVE trial study group reported the adjusted relative risk of CD birth for Black women as 1.21 (95% confidence interval [CI], 1.03–1.42) compared with White women and 1.26 (95% CI, 1.08–1.46) for Hispanic women.5 The investigators estimated that this accounted for 15% of excess maternal morbidity.5 These studies also have shown that a disparity exists in indication for CD, with Black women more likely to have a CD for the diagnosis of nonreassuring fetal tracing while White women are more likely to have a CD for failure to progress.
Patients who undergo CD are less likely to breastfeed, and they have a more difficult recovery, increased risks of infection, thromboembolic events, and increased risks for future pregnancy. Along with increased focus on racial disparities in obstetrics outcomes within the medical community, patients also have become more attuned to these racial disparities in maternal morbidity as this has increasingly become a topic of focus within the mainstream media.
What is behind differences in mode of delivery?
The drivers of racial inequities in mode of delivery remain unclear. One might question whether increased prevalence of morbidities in pregnancy, such as diabetes and hypertension, in minority women might influence the disparity in CD. However, the disparity persists in studies of low-risk women and in studies that statistically adjust for factors that include preeclampsia, obesity, diabetes, and fetal growth restriction, which argues that maternal morbidity alone is not responsible for the differences observed.
Race is a social construct, and as such there is no biologically plausible explanation for the racial disparities in CD rates. Differences in health outcomes should be considered a result of the impact of racism. Disparities can be influenced by patient level, provider level, and systemic level factors.6 Provider biases have a negative impact on care for minority groups and they influence disparities in health care.7 The subjectivity involved in diagnoses of nonreassuring fetal tracing as an indication for CD creates an opportunity for implicit biases and discrimination to enter decision-making for indications for CD. Furthermore, no differences have been seen in Apgar score or admission to the neonatal intensive care unit in studies where indication of nonreassuring fetal heart tracing drove the disparity for CD.5
A study that retrospectively compared labor management strategies intended to reduce CD rates, such as application of guidelines for failed induction of labor, arrest of dilation, arrest of descent, nonreassuring fetus status, or cervical ripening, did not observe differential use of labor management strategies intended to reduce CD rate.8 By contrast, Hamm and colleagues observed that implementation of a standardized induction protocol was associated with a decreased CD rate among Black women but not non-Black women and the standardized protocol was associated with a decrease in the racial disparity in CD.9 A theory behind their findings is that provider bias is less when there is implementation of a standardized protocol, algorithm, or guidelines, which in turn reduces disparity in mode of delivery.
Clearly, more research is needed for the mechanisms behind inequities in mode of delivery and the influence of provider factors. Future studies also are needed to evaluate how patient level factors, including belief systems and culture preferences, and how system level factors, such as access to prenatal care and the health system processes, are associated with CD rates.
Next steps
While the mechanisms that drive the disparities in CD rate and indication may remain unclear, there are potential areas of intervention to decrease CD rates among minority and Black women.
Continuous support from a doula or layperson has been shown to decrease rates of cesarean birth,10,11 and evidence indicates that minority women are interested in doula support but are less likely than White women to have access to doula care.12 Programs that provide doula support for Black women are an intervention that would increase access to support and advocacy during labor for Black women.

Group prenatal care is another strategy that is associated with improved perinatal outcomes among Black women, including decreased rates of preterm birth.13 In women randomly assigned to group prenatal care or individual prenatal visits, there was a trend toward decreased CD rate, although this was not significant. Overall, increased support and engagement during prenatal care and delivery will benefit our Black patients.
Data from a survey of 2,000 members of the Society for Maternal-Fetal Medicine suggest that obstetrics clinicians do recognize that disparities in birth outcomes exist. While clinicians recognize this, these data also identified that there are deficits in clinician knowledge regarding these disparities.14 More than half of surveyed clinicians disagreed that their personal biases affect how they care for patients. Robust data demonstrate broad-reaching differences in the diagnosis and treatment of Black and White patients by physicians across specialties.7 Such surveys illustrate that there is a need for more education regarding disparities, racism in medicine, and implicit bias. As race historically has been used to estimate increased maternal morbidity or likelihood of failure for vaginal birth after CD, we must challenge the idea that race itself confers the increased risks and educate clinicians to recognize that race is a proxy for socioeconomic disadvantages and racism.15
The role of nurses in mode of delivery only recently has been evaluated. An interesting recent cohort study demonstrated a reduction in the NTSV CD rate with dissemination of nurse-specific CD rates, which again may suggest that differing nursing and obstetric clinician management in labor may decrease CD rates.16 Dashboards can serve as a tool within the electronic medical record that can identify unit- or clinician-specific trends and variations in care, and they could serve to identify and potentially reduce group disparities in CDs as well as other obstetric quality metrics.17
Lastly, it is imperative to have evidence-based guidelines and standardized protocols regarding labor management and prenatal care in order to reduce racial disparities. Additional steps to reduce Black-White differences in CD rates and indications should be addressed from multiple levels. These initiatives should include provider training and education, interventions to support minority women through labor and activate patient engagement in their prenatal care, hospital monitoring of racial disparities in CD rates, and standardizing care. Future research should focus on further understanding the mechanisms behind disparities in obstetrics as well as the efficacy of interventions in reducing this gap. ●
- March of Dimes. Peristats: Delivery method. Accessed September 10, 2022. https://www.marchofdimes.org/peristats/data?top=8&lev=1&stop=86&ftop=355®=99&obj=1&slev=1
- Osterman MJK. Changes in primary and repeat cesarean delivery: United States, 2016-2021. Vital Statistics Rapid Release; no. 21. Hyattsville, Maryland: National Center for Health Statistics. July 2022. https://dx.doi.org/10.15620/cdc:117432
- Okwandu IC, Anderson M, Postlethwaite D, et al. Racial and ethnic disparities in cesarean delivery and indications among nulliparous, term, singleton, vertex women. J Racial Ethn Health Disparities. 2022;9:1161-1171. doi:10.1007/s40615-021-01057-w.
- Williams A, Little SE, Bryant AS, et al. Mode of delivery and unplanned cesarean: differences in rates and indication by race, ethnicity, and sociodemographic characteristics. Am J Perinat. June 12, 2022. doi:10.1055/a-1785-8843.
- Debbink MP, Ugwu LG, Grobman WA, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Racial and ethnic inequities in cesarean birth and maternal morbidity in a low-risk, nulliparous cohort. Obstet Gynecol. 2022;139:73-82. doi:10.1097/aog.0000000000004620.
- Kilbourne AM, Switzer G, Hyman K, et al. Advancing health disparities research within the health care system: a conceptual framework. Am J Public Health. 2006;96:2113-2121. doi:10.2105/ajph.2005.077628.
- Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities; Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. National Academies Press; 2003. doi:10.17226/12875.
- Yee LM, Costantine MM, Rice MM, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Racial and ethnic differences in utilization of labor management strategies intended to reduce cesarean delivery rates. Obstet Gynecol. 2017;130:1285-1294. doi:10.1097/aog.0000000000002343.
- Hamm RF, Srinivas SK, Levine LD. A standardized labor induction protocol: impact on racial disparities in obstetrical outcomes. Am J Obstet Gynecol MFM. 2020;2:100148. doi:10.1016/j.ajogmf.2020.100148.
- Kennell J, Klaus M, McGrath S, et al. Continuous emotional support during labor in a US hospital: a randomized controlled trial. JAMA. 1991;265:2197-2201. doi:10.1001/jama.1991.03460170051032.
- Bohren MA, Hofmeyr GJ, Sakala C, et al. Continuous support for women during childbirth. Cochrane Database Syst Rev. 2017;7:CD003766. doi:10.1002/14651858.cd003766.pub6.
- Declercq ER, Sakala C, Corry MP, et al. Listening to Mothers III: Pregnancy and Birth. Childbirth Connection; May 2013. Accessed September 16, 2022. https://www.nationalpartnership.org/our-work/resources/health-care/maternity/listening-to-mothers-iii-pregnancy-and-birth-2013.pdf
- Ickovics JR, Kershaw TS, Westdahl C, et al. Group prenatal care and perinatal outcomes: a randomized controlled trial. Obstet Gynecol. 2007;110(2 pt 1):330-339. doi:10.1097/01.aog.0000275284.24298.23.
- Jain J, Moroz L. Strategies to reduce disparities in maternal morbidity and mortality: patient and provider education. Semin Perinatol. 2017;41:323-328. doi:10.1053/j.semperi.2017.04.010.
- Vyas DA, Jones DS, Meadows AR, et al. Challenging the use of race in the vaginal birth after cesarean section calculator. Womens Health Issues. 2019;29:201-204. doi:10.1016/j.whi.2019.04.007.
- Greene NH, Schwartz N, Gregory KD. Association of primary cesarean delivery rate with dissemination of nurse-specific cesarean delivery rates. Obstet Gynecol. 2022;140:610-612. doi:10.1097/aog.0000000000004919.
- Howell EA, Brown H, Brumley J, et al. Reduction of peripartum racial and ethnic disparities. Obstet Gynecol. 2018;131:770782. doi:10.1097/aog.0000000000002475.
CASE Patient wants to reduce her risk of cesarean delivery (CD)
A 30-year-old primigravid woman expresses concern about her increased risk for CD as a Black woman. She has been reading in the news about the increased risks of CD and birth complications, and she asks what she can do to decrease her risk of having a CD.
What is the problem?
Recently, attention has been called to the stark racial disparities in severe maternal morbidity and mortality. Cesarean delivery rates illustrate an area in obstetric management in which racial disparities exist. It is well known that morbidity associated with CD is much higher than morbidity associated with vaginal delivery, which begs the question of whether disparities in mode of delivery may play a role in the disparity in maternal morbidity and mortality.
In the United States, 32% of all births between 2018 and 2020 were by CD. However, only 31% of White women delivered via CD as compared with 36% of Black women and 33% of Asian women.1 In 2021, the primary CD rates were 26% for Black women, 24% for Asian women, 21% for Hispanic women, and 22% for White women.2 This racial disparity, particularly between Black and White women, has been seen across nulliparous, term, singleton, vertex (NTSV) groups as well as multiparous women with prior vaginal delivery.3,4 The disparity persists after adjusting for risk factors.
A secondary analysis of groups deemed at low risk for CD within the ARRIVE trial study group reported the adjusted relative risk of CD birth for Black women as 1.21 (95% confidence interval [CI], 1.03–1.42) compared with White women and 1.26 (95% CI, 1.08–1.46) for Hispanic women.5 The investigators estimated that this accounted for 15% of excess maternal morbidity.5 These studies also have shown that a disparity exists in indication for CD, with Black women more likely to have a CD for the diagnosis of nonreassuring fetal tracing while White women are more likely to have a CD for failure to progress.
Patients who undergo CD are less likely to breastfeed, and they have a more difficult recovery, increased risks of infection, thromboembolic events, and increased risks for future pregnancy. Along with increased focus on racial disparities in obstetrics outcomes within the medical community, patients also have become more attuned to these racial disparities in maternal morbidity as this has increasingly become a topic of focus within the mainstream media.
What is behind differences in mode of delivery?
The drivers of racial inequities in mode of delivery remain unclear. One might question whether increased prevalence of morbidities in pregnancy, such as diabetes and hypertension, in minority women might influence the disparity in CD. However, the disparity persists in studies of low-risk women and in studies that statistically adjust for factors that include preeclampsia, obesity, diabetes, and fetal growth restriction, which argues that maternal morbidity alone is not responsible for the differences observed.
Race is a social construct, and as such there is no biologically plausible explanation for the racial disparities in CD rates. Differences in health outcomes should be considered a result of the impact of racism. Disparities can be influenced by patient level, provider level, and systemic level factors.6 Provider biases have a negative impact on care for minority groups and they influence disparities in health care.7 The subjectivity involved in diagnoses of nonreassuring fetal tracing as an indication for CD creates an opportunity for implicit biases and discrimination to enter decision-making for indications for CD. Furthermore, no differences have been seen in Apgar score or admission to the neonatal intensive care unit in studies where indication of nonreassuring fetal heart tracing drove the disparity for CD.5
A study that retrospectively compared labor management strategies intended to reduce CD rates, such as application of guidelines for failed induction of labor, arrest of dilation, arrest of descent, nonreassuring fetus status, or cervical ripening, did not observe differential use of labor management strategies intended to reduce CD rate.8 By contrast, Hamm and colleagues observed that implementation of a standardized induction protocol was associated with a decreased CD rate among Black women but not non-Black women and the standardized protocol was associated with a decrease in the racial disparity in CD.9 A theory behind their findings is that provider bias is less when there is implementation of a standardized protocol, algorithm, or guidelines, which in turn reduces disparity in mode of delivery.
Clearly, more research is needed for the mechanisms behind inequities in mode of delivery and the influence of provider factors. Future studies also are needed to evaluate how patient level factors, including belief systems and culture preferences, and how system level factors, such as access to prenatal care and the health system processes, are associated with CD rates.
Next steps
While the mechanisms that drive the disparities in CD rate and indication may remain unclear, there are potential areas of intervention to decrease CD rates among minority and Black women.
Continuous support from a doula or layperson has been shown to decrease rates of cesarean birth,10,11 and evidence indicates that minority women are interested in doula support but are less likely than White women to have access to doula care.12 Programs that provide doula support for Black women are an intervention that would increase access to support and advocacy during labor for Black women.

Group prenatal care is another strategy that is associated with improved perinatal outcomes among Black women, including decreased rates of preterm birth.13 In women randomly assigned to group prenatal care or individual prenatal visits, there was a trend toward decreased CD rate, although this was not significant. Overall, increased support and engagement during prenatal care and delivery will benefit our Black patients.
Data from a survey of 2,000 members of the Society for Maternal-Fetal Medicine suggest that obstetrics clinicians do recognize that disparities in birth outcomes exist. While clinicians recognize this, these data also identified that there are deficits in clinician knowledge regarding these disparities.14 More than half of surveyed clinicians disagreed that their personal biases affect how they care for patients. Robust data demonstrate broad-reaching differences in the diagnosis and treatment of Black and White patients by physicians across specialties.7 Such surveys illustrate that there is a need for more education regarding disparities, racism in medicine, and implicit bias. As race historically has been used to estimate increased maternal morbidity or likelihood of failure for vaginal birth after CD, we must challenge the idea that race itself confers the increased risks and educate clinicians to recognize that race is a proxy for socioeconomic disadvantages and racism.15
The role of nurses in mode of delivery only recently has been evaluated. An interesting recent cohort study demonstrated a reduction in the NTSV CD rate with dissemination of nurse-specific CD rates, which again may suggest that differing nursing and obstetric clinician management in labor may decrease CD rates.16 Dashboards can serve as a tool within the electronic medical record that can identify unit- or clinician-specific trends and variations in care, and they could serve to identify and potentially reduce group disparities in CDs as well as other obstetric quality metrics.17
Lastly, it is imperative to have evidence-based guidelines and standardized protocols regarding labor management and prenatal care in order to reduce racial disparities. Additional steps to reduce Black-White differences in CD rates and indications should be addressed from multiple levels. These initiatives should include provider training and education, interventions to support minority women through labor and activate patient engagement in their prenatal care, hospital monitoring of racial disparities in CD rates, and standardizing care. Future research should focus on further understanding the mechanisms behind disparities in obstetrics as well as the efficacy of interventions in reducing this gap. ●
CASE Patient wants to reduce her risk of cesarean delivery (CD)
A 30-year-old primigravid woman expresses concern about her increased risk for CD as a Black woman. She has been reading in the news about the increased risks of CD and birth complications, and she asks what she can do to decrease her risk of having a CD.
What is the problem?
Recently, attention has been called to the stark racial disparities in severe maternal morbidity and mortality. Cesarean delivery rates illustrate an area in obstetric management in which racial disparities exist. It is well known that morbidity associated with CD is much higher than morbidity associated with vaginal delivery, which begs the question of whether disparities in mode of delivery may play a role in the disparity in maternal morbidity and mortality.
In the United States, 32% of all births between 2018 and 2020 were by CD. However, only 31% of White women delivered via CD as compared with 36% of Black women and 33% of Asian women.1 In 2021, the primary CD rates were 26% for Black women, 24% for Asian women, 21% for Hispanic women, and 22% for White women.2 This racial disparity, particularly between Black and White women, has been seen across nulliparous, term, singleton, vertex (NTSV) groups as well as multiparous women with prior vaginal delivery.3,4 The disparity persists after adjusting for risk factors.
A secondary analysis of groups deemed at low risk for CD within the ARRIVE trial study group reported the adjusted relative risk of CD birth for Black women as 1.21 (95% confidence interval [CI], 1.03–1.42) compared with White women and 1.26 (95% CI, 1.08–1.46) for Hispanic women.5 The investigators estimated that this accounted for 15% of excess maternal morbidity.5 These studies also have shown that a disparity exists in indication for CD, with Black women more likely to have a CD for the diagnosis of nonreassuring fetal tracing while White women are more likely to have a CD for failure to progress.
Patients who undergo CD are less likely to breastfeed, and they have a more difficult recovery, increased risks of infection, thromboembolic events, and increased risks for future pregnancy. Along with increased focus on racial disparities in obstetrics outcomes within the medical community, patients also have become more attuned to these racial disparities in maternal morbidity as this has increasingly become a topic of focus within the mainstream media.
What is behind differences in mode of delivery?
The drivers of racial inequities in mode of delivery remain unclear. One might question whether increased prevalence of morbidities in pregnancy, such as diabetes and hypertension, in minority women might influence the disparity in CD. However, the disparity persists in studies of low-risk women and in studies that statistically adjust for factors that include preeclampsia, obesity, diabetes, and fetal growth restriction, which argues that maternal morbidity alone is not responsible for the differences observed.
Race is a social construct, and as such there is no biologically plausible explanation for the racial disparities in CD rates. Differences in health outcomes should be considered a result of the impact of racism. Disparities can be influenced by patient level, provider level, and systemic level factors.6 Provider biases have a negative impact on care for minority groups and they influence disparities in health care.7 The subjectivity involved in diagnoses of nonreassuring fetal tracing as an indication for CD creates an opportunity for implicit biases and discrimination to enter decision-making for indications for CD. Furthermore, no differences have been seen in Apgar score or admission to the neonatal intensive care unit in studies where indication of nonreassuring fetal heart tracing drove the disparity for CD.5
A study that retrospectively compared labor management strategies intended to reduce CD rates, such as application of guidelines for failed induction of labor, arrest of dilation, arrest of descent, nonreassuring fetus status, or cervical ripening, did not observe differential use of labor management strategies intended to reduce CD rate.8 By contrast, Hamm and colleagues observed that implementation of a standardized induction protocol was associated with a decreased CD rate among Black women but not non-Black women and the standardized protocol was associated with a decrease in the racial disparity in CD.9 A theory behind their findings is that provider bias is less when there is implementation of a standardized protocol, algorithm, or guidelines, which in turn reduces disparity in mode of delivery.
Clearly, more research is needed for the mechanisms behind inequities in mode of delivery and the influence of provider factors. Future studies also are needed to evaluate how patient level factors, including belief systems and culture preferences, and how system level factors, such as access to prenatal care and the health system processes, are associated with CD rates.
Next steps
While the mechanisms that drive the disparities in CD rate and indication may remain unclear, there are potential areas of intervention to decrease CD rates among minority and Black women.
Continuous support from a doula or layperson has been shown to decrease rates of cesarean birth,10,11 and evidence indicates that minority women are interested in doula support but are less likely than White women to have access to doula care.12 Programs that provide doula support for Black women are an intervention that would increase access to support and advocacy during labor for Black women.

Group prenatal care is another strategy that is associated with improved perinatal outcomes among Black women, including decreased rates of preterm birth.13 In women randomly assigned to group prenatal care or individual prenatal visits, there was a trend toward decreased CD rate, although this was not significant. Overall, increased support and engagement during prenatal care and delivery will benefit our Black patients.
Data from a survey of 2,000 members of the Society for Maternal-Fetal Medicine suggest that obstetrics clinicians do recognize that disparities in birth outcomes exist. While clinicians recognize this, these data also identified that there are deficits in clinician knowledge regarding these disparities.14 More than half of surveyed clinicians disagreed that their personal biases affect how they care for patients. Robust data demonstrate broad-reaching differences in the diagnosis and treatment of Black and White patients by physicians across specialties.7 Such surveys illustrate that there is a need for more education regarding disparities, racism in medicine, and implicit bias. As race historically has been used to estimate increased maternal morbidity or likelihood of failure for vaginal birth after CD, we must challenge the idea that race itself confers the increased risks and educate clinicians to recognize that race is a proxy for socioeconomic disadvantages and racism.15
The role of nurses in mode of delivery only recently has been evaluated. An interesting recent cohort study demonstrated a reduction in the NTSV CD rate with dissemination of nurse-specific CD rates, which again may suggest that differing nursing and obstetric clinician management in labor may decrease CD rates.16 Dashboards can serve as a tool within the electronic medical record that can identify unit- or clinician-specific trends and variations in care, and they could serve to identify and potentially reduce group disparities in CDs as well as other obstetric quality metrics.17
Lastly, it is imperative to have evidence-based guidelines and standardized protocols regarding labor management and prenatal care in order to reduce racial disparities. Additional steps to reduce Black-White differences in CD rates and indications should be addressed from multiple levels. These initiatives should include provider training and education, interventions to support minority women through labor and activate patient engagement in their prenatal care, hospital monitoring of racial disparities in CD rates, and standardizing care. Future research should focus on further understanding the mechanisms behind disparities in obstetrics as well as the efficacy of interventions in reducing this gap. ●
- March of Dimes. Peristats: Delivery method. Accessed September 10, 2022. https://www.marchofdimes.org/peristats/data?top=8&lev=1&stop=86&ftop=355®=99&obj=1&slev=1
- Osterman MJK. Changes in primary and repeat cesarean delivery: United States, 2016-2021. Vital Statistics Rapid Release; no. 21. Hyattsville, Maryland: National Center for Health Statistics. July 2022. https://dx.doi.org/10.15620/cdc:117432
- Okwandu IC, Anderson M, Postlethwaite D, et al. Racial and ethnic disparities in cesarean delivery and indications among nulliparous, term, singleton, vertex women. J Racial Ethn Health Disparities. 2022;9:1161-1171. doi:10.1007/s40615-021-01057-w.
- Williams A, Little SE, Bryant AS, et al. Mode of delivery and unplanned cesarean: differences in rates and indication by race, ethnicity, and sociodemographic characteristics. Am J Perinat. June 12, 2022. doi:10.1055/a-1785-8843.
- Debbink MP, Ugwu LG, Grobman WA, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Racial and ethnic inequities in cesarean birth and maternal morbidity in a low-risk, nulliparous cohort. Obstet Gynecol. 2022;139:73-82. doi:10.1097/aog.0000000000004620.
- Kilbourne AM, Switzer G, Hyman K, et al. Advancing health disparities research within the health care system: a conceptual framework. Am J Public Health. 2006;96:2113-2121. doi:10.2105/ajph.2005.077628.
- Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities; Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. National Academies Press; 2003. doi:10.17226/12875.
- Yee LM, Costantine MM, Rice MM, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Racial and ethnic differences in utilization of labor management strategies intended to reduce cesarean delivery rates. Obstet Gynecol. 2017;130:1285-1294. doi:10.1097/aog.0000000000002343.
- Hamm RF, Srinivas SK, Levine LD. A standardized labor induction protocol: impact on racial disparities in obstetrical outcomes. Am J Obstet Gynecol MFM. 2020;2:100148. doi:10.1016/j.ajogmf.2020.100148.
- Kennell J, Klaus M, McGrath S, et al. Continuous emotional support during labor in a US hospital: a randomized controlled trial. JAMA. 1991;265:2197-2201. doi:10.1001/jama.1991.03460170051032.
- Bohren MA, Hofmeyr GJ, Sakala C, et al. Continuous support for women during childbirth. Cochrane Database Syst Rev. 2017;7:CD003766. doi:10.1002/14651858.cd003766.pub6.
- Declercq ER, Sakala C, Corry MP, et al. Listening to Mothers III: Pregnancy and Birth. Childbirth Connection; May 2013. Accessed September 16, 2022. https://www.nationalpartnership.org/our-work/resources/health-care/maternity/listening-to-mothers-iii-pregnancy-and-birth-2013.pdf
- Ickovics JR, Kershaw TS, Westdahl C, et al. Group prenatal care and perinatal outcomes: a randomized controlled trial. Obstet Gynecol. 2007;110(2 pt 1):330-339. doi:10.1097/01.aog.0000275284.24298.23.
- Jain J, Moroz L. Strategies to reduce disparities in maternal morbidity and mortality: patient and provider education. Semin Perinatol. 2017;41:323-328. doi:10.1053/j.semperi.2017.04.010.
- Vyas DA, Jones DS, Meadows AR, et al. Challenging the use of race in the vaginal birth after cesarean section calculator. Womens Health Issues. 2019;29:201-204. doi:10.1016/j.whi.2019.04.007.
- Greene NH, Schwartz N, Gregory KD. Association of primary cesarean delivery rate with dissemination of nurse-specific cesarean delivery rates. Obstet Gynecol. 2022;140:610-612. doi:10.1097/aog.0000000000004919.
- Howell EA, Brown H, Brumley J, et al. Reduction of peripartum racial and ethnic disparities. Obstet Gynecol. 2018;131:770782. doi:10.1097/aog.0000000000002475.
- March of Dimes. Peristats: Delivery method. Accessed September 10, 2022. https://www.marchofdimes.org/peristats/data?top=8&lev=1&stop=86&ftop=355®=99&obj=1&slev=1
- Osterman MJK. Changes in primary and repeat cesarean delivery: United States, 2016-2021. Vital Statistics Rapid Release; no. 21. Hyattsville, Maryland: National Center for Health Statistics. July 2022. https://dx.doi.org/10.15620/cdc:117432
- Okwandu IC, Anderson M, Postlethwaite D, et al. Racial and ethnic disparities in cesarean delivery and indications among nulliparous, term, singleton, vertex women. J Racial Ethn Health Disparities. 2022;9:1161-1171. doi:10.1007/s40615-021-01057-w.
- Williams A, Little SE, Bryant AS, et al. Mode of delivery and unplanned cesarean: differences in rates and indication by race, ethnicity, and sociodemographic characteristics. Am J Perinat. June 12, 2022. doi:10.1055/a-1785-8843.
- Debbink MP, Ugwu LG, Grobman WA, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Racial and ethnic inequities in cesarean birth and maternal morbidity in a low-risk, nulliparous cohort. Obstet Gynecol. 2022;139:73-82. doi:10.1097/aog.0000000000004620.
- Kilbourne AM, Switzer G, Hyman K, et al. Advancing health disparities research within the health care system: a conceptual framework. Am J Public Health. 2006;96:2113-2121. doi:10.2105/ajph.2005.077628.
- Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities; Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. National Academies Press; 2003. doi:10.17226/12875.
- Yee LM, Costantine MM, Rice MM, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Racial and ethnic differences in utilization of labor management strategies intended to reduce cesarean delivery rates. Obstet Gynecol. 2017;130:1285-1294. doi:10.1097/aog.0000000000002343.
- Hamm RF, Srinivas SK, Levine LD. A standardized labor induction protocol: impact on racial disparities in obstetrical outcomes. Am J Obstet Gynecol MFM. 2020;2:100148. doi:10.1016/j.ajogmf.2020.100148.
- Kennell J, Klaus M, McGrath S, et al. Continuous emotional support during labor in a US hospital: a randomized controlled trial. JAMA. 1991;265:2197-2201. doi:10.1001/jama.1991.03460170051032.
- Bohren MA, Hofmeyr GJ, Sakala C, et al. Continuous support for women during childbirth. Cochrane Database Syst Rev. 2017;7:CD003766. doi:10.1002/14651858.cd003766.pub6.
- Declercq ER, Sakala C, Corry MP, et al. Listening to Mothers III: Pregnancy and Birth. Childbirth Connection; May 2013. Accessed September 16, 2022. https://www.nationalpartnership.org/our-work/resources/health-care/maternity/listening-to-mothers-iii-pregnancy-and-birth-2013.pdf
- Ickovics JR, Kershaw TS, Westdahl C, et al. Group prenatal care and perinatal outcomes: a randomized controlled trial. Obstet Gynecol. 2007;110(2 pt 1):330-339. doi:10.1097/01.aog.0000275284.24298.23.
- Jain J, Moroz L. Strategies to reduce disparities in maternal morbidity and mortality: patient and provider education. Semin Perinatol. 2017;41:323-328. doi:10.1053/j.semperi.2017.04.010.
- Vyas DA, Jones DS, Meadows AR, et al. Challenging the use of race in the vaginal birth after cesarean section calculator. Womens Health Issues. 2019;29:201-204. doi:10.1016/j.whi.2019.04.007.
- Greene NH, Schwartz N, Gregory KD. Association of primary cesarean delivery rate with dissemination of nurse-specific cesarean delivery rates. Obstet Gynecol. 2022;140:610-612. doi:10.1097/aog.0000000000004919.
- Howell EA, Brown H, Brumley J, et al. Reduction of peripartum racial and ethnic disparities. Obstet Gynecol. 2018;131:770782. doi:10.1097/aog.0000000000002475.
Insights From the 2020-2021 Dermatology Residency Match
To the Editor:
Data from the program director survey of the National Resident Matching Program offer key insights into the 2021 dermatology application process.1,2 Examination of data from the 2020 (N=12) and 2021 (N=17) program director survey regarding interviewing applicants revealed that specialty-specific letters of recommendation (LORs), personal prior knowledge of an applicant, and personal statement increased in importance by 17%, 7.4%, and 17%, respectively, whereas away rotations within the department decreased in importance by 44.9% (Table).1,2 Interestingly, for ranking applicants, programs decreased their emphasis on specialty-specific LORs by 25.8% and away rotations within the department by 22.7% and increased emphasis on personal statements by 14.7% and personal prior knowledge of an applicant by 0.8% from 2020 to 2021 (Table).1,2 These findings align with the prior recommendation to limit away rotations; data are contradictory—when comparing factors for interviewing as compared to ranking applicants—for specialty-specific LORs.
We further compared data from the otolaryngology cycle, which implemented preference signaling by which an applicant can signal their interest in a particular residency program in the 2021 Match, to data from dermatology with no preference signaling. A 90% probability of matching is estimated to require approximately 8 or 9 interviews for dermatology or 12 interviews for otolaryngology for MD senior students in 2020.4 In prior dermatology application cycles, the most highly qualified candidates constituted 7% to 21% of all applicants but were estimated to receive half of all interviews, causing a maldistribution of interviews.5,6
For the 2021 otolaryngology match, the Society of University Otolaryngologists implemented a novel preference signaling system that allowed candidates to show interest in programs by sending 5 preferences, or tokens.7 Recent data reports from the otolaryngology cycle demonstrated at least a 2-fold increase in the rate of receiving an interview invitation for signaled programs compared to the closest nonsignaled program if applicants were provided an additional token.7 Regarding overall applicant competitiveness (ie, dividing participants into quartiles based on their competitiveness), the highest increase in the overall rate of interview invitations (3.5 [total invitations/total applications]) was demonstrated for fourth-quartile (ie, “lowest quartile”) applicants compared with the increase in the overall rate of interview invitations seen in other quartiles (first quartile, an increase of 2.3; second quartile, an increase of 2.6; and third quartile, an increase of 2.4).7 We look forward to seeing the impact of preference signaling on the results of the 2022 dermatology cycle.
Despite changes in the interviewing process to accommodate COVID-19 pandemic safety recommendations, the overall dermatology postgraduate year (PGY) 2 fill rate remained unchanged from 2018 (98.6%) to 2021 (98.7%). Zero PGY-1 positions and 5 PGY-2 positions were unfilled in the 2021 Main Residency Match compared to 1 unfilled PGY-1 position and 4 unfilled PGY-2 positions in 2018.8 The coordinated interview invitation release, holistic review of applications, increased number of rankings, and virtual interviews might have helped offset potential obstacles imparted by inability to complete away rotations, inability to obtain LORs, and conducting interviews virtually.5
A limitation of our analysis is the low response rate of program directors to National Resident Matching Program surveys.
These strategies—holistic application review and coordinated interview release—may be considered in future cycles given their convenience and negligible impact on the dermatology match rate. For example, virtual interviews relieve the financial and time burdens of in-person interviews—approximately $10,000 for each US senior applicant—thus potentially allowing for a more equitable matching process.3 Inversely, in-person interviews allow participants to effectively network and form more meaningful connections while obtaining a better understanding of facilities and surrounding locales. As such, the medical community should continue to come to a consensus on the optimal format to host interviews.
- Results of the 2021 NRMP Program Director Survey. National Resident Matching Program. August 2021. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/11/2021-PD-Survey-Report-for-WWW.pdf
- Results of the 2020 NRMP Program Director Survey. National Resident Matching Program. August 2020. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2022/01/2020-PD-Survey.pdf
- Rojek NW, Shinkai K, Fett N. Dermatology faculty and residents’ perspectives on the dermatology residency application process: a nationwide survey. J Am Acad Dermatol. 2018;79:157-159. doi:10.1016/j.jaad.2018.01.00
- Charting Outcomes in the Match: Senior Students of U.S. MD Medical Schools. National Resident Matching Program. July 2020. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/08/Charting-Outcomes-in-the-Match-2020_MD-Senior_final.pdf
- Thatiparthi A, Martin A, Liu J, et al. Preliminary outcomes of 2020-2021 dermatology residency application cycle and adverse effects of COVID-19. J Am Acad Dermatol. 2021;84:e263-e264. doi:10.1016/j.jaad.2021.03.034
- Hammoud MM, Standiford T, Carmody JB. Potential implications of COVID-19 for the 2020-2021 residency application cycle. JAMA. 2020;324:29-30. doi:10.1001/jama.2020.8911
- Interview offer rate with/without ENTSignaling. Society of University Otolaryngologists. Updated July 19, 2022. Accessed December 12, 2022. https://opdo-hns.org/mpage/signaling-updates
- Results and Data: 2021 Main Residency Match. National Resident Matching Program. May 2021. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/08/MRM-Results_and-Data_2021.pdf
To the Editor:
Data from the program director survey of the National Resident Matching Program offer key insights into the 2021 dermatology application process.1,2 Examination of data from the 2020 (N=12) and 2021 (N=17) program director survey regarding interviewing applicants revealed that specialty-specific letters of recommendation (LORs), personal prior knowledge of an applicant, and personal statement increased in importance by 17%, 7.4%, and 17%, respectively, whereas away rotations within the department decreased in importance by 44.9% (Table).1,2 Interestingly, for ranking applicants, programs decreased their emphasis on specialty-specific LORs by 25.8% and away rotations within the department by 22.7% and increased emphasis on personal statements by 14.7% and personal prior knowledge of an applicant by 0.8% from 2020 to 2021 (Table).1,2 These findings align with the prior recommendation to limit away rotations; data are contradictory—when comparing factors for interviewing as compared to ranking applicants—for specialty-specific LORs.

We further compared data from the otolaryngology cycle, which implemented preference signaling by which an applicant can signal their interest in a particular residency program in the 2021 Match, to data from dermatology with no preference signaling. A 90% probability of matching is estimated to require approximately 8 or 9 interviews for dermatology or 12 interviews for otolaryngology for MD senior students in 2020.4 In prior dermatology application cycles, the most highly qualified candidates constituted 7% to 21% of all applicants but were estimated to receive half of all interviews, causing a maldistribution of interviews.5,6
For the 2021 otolaryngology match, the Society of University Otolaryngologists implemented a novel preference signaling system that allowed candidates to show interest in programs by sending 5 preferences, or tokens.7 Recent data reports from the otolaryngology cycle demonstrated at least a 2-fold increase in the rate of receiving an interview invitation for signaled programs compared to the closest nonsignaled program if applicants were provided an additional token.7 Regarding overall applicant competitiveness (ie, dividing participants into quartiles based on their competitiveness), the highest increase in the overall rate of interview invitations (3.5 [total invitations/total applications]) was demonstrated for fourth-quartile (ie, “lowest quartile”) applicants compared with the increase in the overall rate of interview invitations seen in other quartiles (first quartile, an increase of 2.3; second quartile, an increase of 2.6; and third quartile, an increase of 2.4).7 We look forward to seeing the impact of preference signaling on the results of the 2022 dermatology cycle.
Despite changes in the interviewing process to accommodate COVID-19 pandemic safety recommendations, the overall dermatology postgraduate year (PGY) 2 fill rate remained unchanged from 2018 (98.6%) to 2021 (98.7%). Zero PGY-1 positions and 5 PGY-2 positions were unfilled in the 2021 Main Residency Match compared to 1 unfilled PGY-1 position and 4 unfilled PGY-2 positions in 2018.8 The coordinated interview invitation release, holistic review of applications, increased number of rankings, and virtual interviews might have helped offset potential obstacles imparted by inability to complete away rotations, inability to obtain LORs, and conducting interviews virtually.5
A limitation of our analysis is the low response rate of program directors to National Resident Matching Program surveys.
These strategies—holistic application review and coordinated interview release—may be considered in future cycles given their convenience and negligible impact on the dermatology match rate. For example, virtual interviews relieve the financial and time burdens of in-person interviews—approximately $10,000 for each US senior applicant—thus potentially allowing for a more equitable matching process.3 Inversely, in-person interviews allow participants to effectively network and form more meaningful connections while obtaining a better understanding of facilities and surrounding locales. As such, the medical community should continue to come to a consensus on the optimal format to host interviews.
To the Editor:
Data from the program director survey of the National Resident Matching Program offer key insights into the 2021 dermatology application process.1,2 Examination of data from the 2020 (N=12) and 2021 (N=17) program director survey regarding interviewing applicants revealed that specialty-specific letters of recommendation (LORs), personal prior knowledge of an applicant, and personal statement increased in importance by 17%, 7.4%, and 17%, respectively, whereas away rotations within the department decreased in importance by 44.9% (Table).1,2 Interestingly, for ranking applicants, programs decreased their emphasis on specialty-specific LORs by 25.8% and away rotations within the department by 22.7% and increased emphasis on personal statements by 14.7% and personal prior knowledge of an applicant by 0.8% from 2020 to 2021 (Table).1,2 These findings align with the prior recommendation to limit away rotations; data are contradictory—when comparing factors for interviewing as compared to ranking applicants—for specialty-specific LORs.

We further compared data from the otolaryngology cycle, which implemented preference signaling by which an applicant can signal their interest in a particular residency program in the 2021 Match, to data from dermatology with no preference signaling. A 90% probability of matching is estimated to require approximately 8 or 9 interviews for dermatology or 12 interviews for otolaryngology for MD senior students in 2020.4 In prior dermatology application cycles, the most highly qualified candidates constituted 7% to 21% of all applicants but were estimated to receive half of all interviews, causing a maldistribution of interviews.5,6
For the 2021 otolaryngology match, the Society of University Otolaryngologists implemented a novel preference signaling system that allowed candidates to show interest in programs by sending 5 preferences, or tokens.7 Recent data reports from the otolaryngology cycle demonstrated at least a 2-fold increase in the rate of receiving an interview invitation for signaled programs compared to the closest nonsignaled program if applicants were provided an additional token.7 Regarding overall applicant competitiveness (ie, dividing participants into quartiles based on their competitiveness), the highest increase in the overall rate of interview invitations (3.5 [total invitations/total applications]) was demonstrated for fourth-quartile (ie, “lowest quartile”) applicants compared with the increase in the overall rate of interview invitations seen in other quartiles (first quartile, an increase of 2.3; second quartile, an increase of 2.6; and third quartile, an increase of 2.4).7 We look forward to seeing the impact of preference signaling on the results of the 2022 dermatology cycle.
Despite changes in the interviewing process to accommodate COVID-19 pandemic safety recommendations, the overall dermatology postgraduate year (PGY) 2 fill rate remained unchanged from 2018 (98.6%) to 2021 (98.7%). Zero PGY-1 positions and 5 PGY-2 positions were unfilled in the 2021 Main Residency Match compared to 1 unfilled PGY-1 position and 4 unfilled PGY-2 positions in 2018.8 The coordinated interview invitation release, holistic review of applications, increased number of rankings, and virtual interviews might have helped offset potential obstacles imparted by inability to complete away rotations, inability to obtain LORs, and conducting interviews virtually.5
A limitation of our analysis is the low response rate of program directors to National Resident Matching Program surveys.
These strategies—holistic application review and coordinated interview release—may be considered in future cycles given their convenience and negligible impact on the dermatology match rate. For example, virtual interviews relieve the financial and time burdens of in-person interviews—approximately $10,000 for each US senior applicant—thus potentially allowing for a more equitable matching process.3 Inversely, in-person interviews allow participants to effectively network and form more meaningful connections while obtaining a better understanding of facilities and surrounding locales. As such, the medical community should continue to come to a consensus on the optimal format to host interviews.
- Results of the 2021 NRMP Program Director Survey. National Resident Matching Program. August 2021. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/11/2021-PD-Survey-Report-for-WWW.pdf
- Results of the 2020 NRMP Program Director Survey. National Resident Matching Program. August 2020. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2022/01/2020-PD-Survey.pdf
- Rojek NW, Shinkai K, Fett N. Dermatology faculty and residents’ perspectives on the dermatology residency application process: a nationwide survey. J Am Acad Dermatol. 2018;79:157-159. doi:10.1016/j.jaad.2018.01.00
- Charting Outcomes in the Match: Senior Students of U.S. MD Medical Schools. National Resident Matching Program. July 2020. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/08/Charting-Outcomes-in-the-Match-2020_MD-Senior_final.pdf
- Thatiparthi A, Martin A, Liu J, et al. Preliminary outcomes of 2020-2021 dermatology residency application cycle and adverse effects of COVID-19. J Am Acad Dermatol. 2021;84:e263-e264. doi:10.1016/j.jaad.2021.03.034
- Hammoud MM, Standiford T, Carmody JB. Potential implications of COVID-19 for the 2020-2021 residency application cycle. JAMA. 2020;324:29-30. doi:10.1001/jama.2020.8911
- Interview offer rate with/without ENTSignaling. Society of University Otolaryngologists. Updated July 19, 2022. Accessed December 12, 2022. https://opdo-hns.org/mpage/signaling-updates
- Results and Data: 2021 Main Residency Match. National Resident Matching Program. May 2021. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/08/MRM-Results_and-Data_2021.pdf
- Results of the 2021 NRMP Program Director Survey. National Resident Matching Program. August 2021. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/11/2021-PD-Survey-Report-for-WWW.pdf
- Results of the 2020 NRMP Program Director Survey. National Resident Matching Program. August 2020. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2022/01/2020-PD-Survey.pdf
- Rojek NW, Shinkai K, Fett N. Dermatology faculty and residents’ perspectives on the dermatology residency application process: a nationwide survey. J Am Acad Dermatol. 2018;79:157-159. doi:10.1016/j.jaad.2018.01.00
- Charting Outcomes in the Match: Senior Students of U.S. MD Medical Schools. National Resident Matching Program. July 2020. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/08/Charting-Outcomes-in-the-Match-2020_MD-Senior_final.pdf
- Thatiparthi A, Martin A, Liu J, et al. Preliminary outcomes of 2020-2021 dermatology residency application cycle and adverse effects of COVID-19. J Am Acad Dermatol. 2021;84:e263-e264. doi:10.1016/j.jaad.2021.03.034
- Hammoud MM, Standiford T, Carmody JB. Potential implications of COVID-19 for the 2020-2021 residency application cycle. JAMA. 2020;324:29-30. doi:10.1001/jama.2020.8911
- Interview offer rate with/without ENTSignaling. Society of University Otolaryngologists. Updated July 19, 2022. Accessed December 12, 2022. https://opdo-hns.org/mpage/signaling-updates
- Results and Data: 2021 Main Residency Match. National Resident Matching Program. May 2021. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/08/MRM-Results_and-Data_2021.pdf
PRACTICE POINTS
- Although there have been numerous changes to the dermatology interview process due to the COVID-19 pandemic, the overall fill rate for postgraduate year 2 positions remained unchanged from 2018 (prepandemic) to 2021 (postpandemic).
- Strategies to accommodate new safety recommendations for interviews may reduce the financial burden (approximately $10,000 for each senior applicant) and time constraints on applicants. These strategies should be considered for implementation in future cycles.
Nodule on gardener’s hand
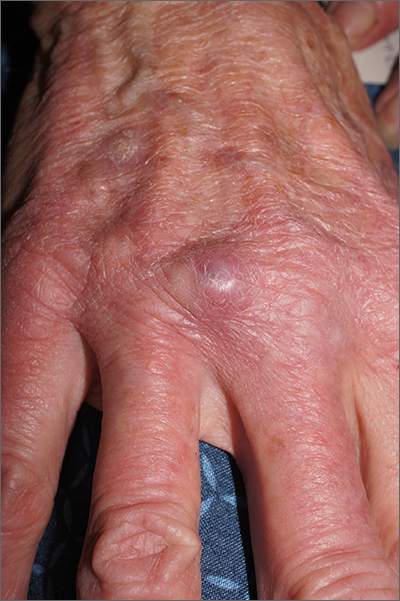
Using an 18-gauge needle, a simple incision and drainage was performed, and copious turbid and bloody material was expressed and cultured for aerobic and acid-fast bacteria, as well as fungus. The patient was started on trimethoprim sulfamethoxazole DS twice daily while cultures and sensitivities were pending. Cultures grew Staphylococcus lugdunensis, a coagulase-negative staph species known to cause a range of infections from simple skin infections to bacteremia and endocarditis.1 If the drainage had been viscous and clear to blood-tinged, that would have been more consistent with a ganglion cyst. Lack of drainage would have prompted a small punch biopsy to exclude a tumor.
Fortunately, S lugdunensis is often broadly sensitive to antibiotics, although treatment choices should follow antibiotic sensitivity testing. Any signs of systemic illness should be worked up with blood cultures and consideration of endocarditis or involvement of an implant. Penicillin is recommended as a first line systemic agent if sensitivities support this, and for abscesses, incision and drainage is recommended. The length of treatment for skin infections is generally 1 to 2 weeks, guided by response to therapy.
This patient’s nodule resolved following the incision and drainage and 7 days of therapy with trimethoprim sulfamethoxazole DS.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Kleiner E, Monk AB, Archer GL, et al. Clinical significance of Staphylococcus lugdunensis isolated from routine cultures. Clin Infect Dis. 2010;51:801-803. doi: 10.1086/656280

Using an 18-gauge needle, a simple incision and drainage was performed, and copious turbid and bloody material was expressed and cultured for aerobic and acid-fast bacteria, as well as fungus. The patient was started on trimethoprim sulfamethoxazole DS twice daily while cultures and sensitivities were pending. Cultures grew Staphylococcus lugdunensis, a coagulase-negative staph species known to cause a range of infections from simple skin infections to bacteremia and endocarditis.1 If the drainage had been viscous and clear to blood-tinged, that would have been more consistent with a ganglion cyst. Lack of drainage would have prompted a small punch biopsy to exclude a tumor.
Fortunately, S lugdunensis is often broadly sensitive to antibiotics, although treatment choices should follow antibiotic sensitivity testing. Any signs of systemic illness should be worked up with blood cultures and consideration of endocarditis or involvement of an implant. Penicillin is recommended as a first line systemic agent if sensitivities support this, and for abscesses, incision and drainage is recommended. The length of treatment for skin infections is generally 1 to 2 weeks, guided by response to therapy.
This patient’s nodule resolved following the incision and drainage and 7 days of therapy with trimethoprim sulfamethoxazole DS.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

Using an 18-gauge needle, a simple incision and drainage was performed, and copious turbid and bloody material was expressed and cultured for aerobic and acid-fast bacteria, as well as fungus. The patient was started on trimethoprim sulfamethoxazole DS twice daily while cultures and sensitivities were pending. Cultures grew Staphylococcus lugdunensis, a coagulase-negative staph species known to cause a range of infections from simple skin infections to bacteremia and endocarditis.1 If the drainage had been viscous and clear to blood-tinged, that would have been more consistent with a ganglion cyst. Lack of drainage would have prompted a small punch biopsy to exclude a tumor.
Fortunately, S lugdunensis is often broadly sensitive to antibiotics, although treatment choices should follow antibiotic sensitivity testing. Any signs of systemic illness should be worked up with blood cultures and consideration of endocarditis or involvement of an implant. Penicillin is recommended as a first line systemic agent if sensitivities support this, and for abscesses, incision and drainage is recommended. The length of treatment for skin infections is generally 1 to 2 weeks, guided by response to therapy.
This patient’s nodule resolved following the incision and drainage and 7 days of therapy with trimethoprim sulfamethoxazole DS.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Kleiner E, Monk AB, Archer GL, et al. Clinical significance of Staphylococcus lugdunensis isolated from routine cultures. Clin Infect Dis. 2010;51:801-803. doi: 10.1086/656280
1. Kleiner E, Monk AB, Archer GL, et al. Clinical significance of Staphylococcus lugdunensis isolated from routine cultures. Clin Infect Dis. 2010;51:801-803. doi: 10.1086/656280
Is the limit of viability shifting again?
Chawla S, Wyckoff MH, Rysavy MA, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Association of antenatal steroid exposure at 21 to 22 weeks of gestation with neonatal survival and survival without morbidities. JAMA Netw Open. 2022;5:e2233331. doi:10.1001/jamanet workopen.2022.33331.
EXPERT COMMENTARY
The single most important intervention available in obstetrics to improve the health outcomes of preterm newborns is the maternal administration of corticosteroids. The 27 randomized controlled trials that formed the basis for this knowledge1 did not include infants delivered at 24 weeks’ gestation or less. This has not dissuaded us, over the last several decades, from using corticosteroids for impending delivery at 24 weeks’ gestation; in the absence of randomized data, this has been based on observational evidence of benefit.
Following the 2011 publication of a retrospective cohort study that analyzed data collected by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network between 1993 and 2009 (the Carlo study),2 ACS started to be used widely even for impending delivery at 23 weeks’ gestation. That study had found that the odds of death and neurodevelopmental impairment at 18 to 22 months of age were significantly lower in cases that received ACS and were born at 23 weeks (n = 1,978). The same benefit could not be verified for infants born at 22 weeks’ gestational age (n = 402).
In a recent study conducted by the same NICHD Neonatal Research Network, antenatal steroid exposure at 21 to 22 weeks of gestation was examined.
Details of the study
Using prospectively collected data from 2016 to 2019, Chawla and colleagues conducted a retrospective cohort study that analyzed data from 431 infants who were born between 22 0/7 and 23 6/7 weeks’ gestation and received neonatal intensive care (179 infants born at 22 weeks’ gestation).3 The infants not exposed to ACS were compared with those who had partial exposure (only 1 dose) and those with complete ACS exposure (2 doses).
Complete ACS exposure proved to be beneficial, increasing survival to discharge from 35.5% in the no-exposure group to 53.9% (adjusted odds ratio [aOR], 1.95; 95% confidence interval [CI], 1.07–3.56). Of the survivors, 26.9% in the complete-exposure group had no major morbidities compared with 10% in the no-exposure group (aOR, 2.74; 95% CI, 1.19–6.30).
Study strengths and limitations
The strengths of this study include the use of a diverse, multicenter cohort, with contemporary delivery data, which increases the generalizability of the findings. The analysis included aspects often overlooked in other similar studies, such as the dose of ACS exposure and the gestational age at the time of exposure.
The observational study design, however, can suggest only associations rather than causal relationships. Observational studies also are apt to be affected by residual confounding. Such limitations can only be overcome by a randomized controlled trial, but such a trial of ACS at periviable gestational ages seems unfeasible due to limited ethical justification.
Another limitation is the reporting on outcomes as a collective group (22–23 weeks’ gestation). It is important to consider each gestational age week separately due to differences in physiology and potential biological limitations. It cannot be assumed that 22 weeks behaves like 23 weeks, just as 21 weeks is not equivalent to 22 weeks.
The study results suggest that the protective effect of ACS was dose dependent. However, the interpretation that only a complete ACS exposure was beneficial should be viewed cautiously because the study had no power to assess the impact of a partial exposure.
A further limitation is the lack of consideration in analysis for maternal comorbidities and fetal growth restriction. In the Carlo study, the beneficial effect of corticosteroids in 23-week gestational age deliveries was not demonstrable in pregnancies affected by fetal growth restriction or maternal hypertension.
Other studies considered
Given all its limitations, can we assume that the study by Chawla and colleagues has reliably refuted the Carlo study’s suggestion of lack of ACS efficacy in infants born at 22 weeks’ gestation? Taken by itself, probably not. In the context of other recent investigations, yes.
A retrospective registry study that used data from the Vermont Oxford Network for the period 2012–2016 on 1,058 infants born at 22 weeks’ gestation found that infants who were exposed to ACS and received postnatal life support were more likely to survive to hospital discharge without major morbidity compared with infants who received postnatal life support alone.4 Overall survival was 38.5% versus 17.7% (adjusted risk ratio [aRR], 2.11; 95% CI, 1.68–2.65), and survival without major morbidity was 4.4% versus 1.0% (aRR, 4.35; 95% CI, 1.84–10.28).
An even larger cohort study that used data from the National Center for Health Statistics concluded that survival at age 1 year for infants born at 22 weeks (n = 2,635) during 2009–2014 was improved in those exposed to ACS followed by postnatal life support compared with postnatal life support alone (45.2% vs 27.8%; aRR, 1.6; 95% CI, 1.2–2.1).5
A meta-analysis of observational studies that reported on infants born between 22 0/7 and 22 6/7 weeks’ gestation (n = 2,226) who received proactive neonatal treatment found that administration of ACS doubled the rate of survival when compared with no ACS administration (39% vs 19.5%; P<.01).6
In September 2021, the recommendations from the American College of Obstetricians and Gynecologists changed, stating that ACS can be considered at 22 weeks’ gestation when active postnatal management is desired.7 This recommendation is largely congruent with those from several other national and international medical organizations, including the World Association of Perinatal Medicine, the Royal Collegeof Obstetricians and Gynaecologists, and the German, Austrian and Swiss societies of gynecology and obstetrics. The implication is that the limit of viability may have shifted again, from 23 to 22 weeks’ gestation, and considering the importance of adequate timing in ACS administration (within 1 week from delivery), Chawla and colleagues posited that ACS administration can be considered as early as 21 weeks’ gestation when birth is anticipated at 22 weeks and active postnatal management is planned (notably, this should be the correct interpretation of the article title, not that ACS may be beneficial in 21-weeks’ gestational age births). ●
In 2001, the Institute of Medicine of the National Academies introduced the concept of shared decision-making as a key component of quality care. In very few other clinical situations is shared decision-making as critical as in the context of planning intervention when delivery is anticipated at 22 weeks’ gestation. The truth remains that even with the coordinated provision of ACS and active postnatal care, survival at this gestational age is still a toss-up, and survivors face a high probability of neurodevelopmental impairment and other long-term adverse health outcomes. In this setting, decision-making is complex, with the need to balance patient autonomy and nonmaleficence. On the other hand, the concept of patient autonomy is blurred because the patient (fetus) is incompetent and the negotiation is conducted between physicians and parents. However, no intervention should be undertaken unless the parents so desire. Since parental wishes are frequently emotional, overwhelmingly driving intervention, thorough and timely interdisciplinary counseling is needed. Evidence indicates that both obstetricians and neonatologists may, at times, underestimate the chance of a favorable health outcome for infants born extremely preterm.8,9 Early involvement of the neonatal and obstetric team is pivotal to put forward a coherent, nonconfusing, nonpaternalistic, balanced message. When outcomes information is shared during prenatal counseling, it should be based on local, not only national, data. Following appropriate consultation with the parents, the physicians will adjust the expectations to the local standards, outcomes data, and availability regarding periviable neonatal support.
Recent data suggest that the rate of cesarean delivery (CD) in the periviable period is increasing.10 There is no clear evidence in favor of CD to improve neonatal outcomes, whereas there is concern that periviable CD is associated with significantly increased maternal risks. Regardless of uterine incision type, periviable CD results in an increased risk of uterine rupture in a subsequent pregnancy.11 Consistent with the principle of nonmaleficence, a discussion of these risks should be included in shared decision-making.
ALEX C. VIDAEFF, MD, MPH, AND NATHAN C. SUNDGREN, MD, PHD
- McGoldrick E, Stewart F, Parker R, et al. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2020;12(12):CD004454.
- Carlo WA, McDonald SA, Fanaroff AA, et al; Eunice Kennedy Schriver National Institute for Child Health and Human Development Neonatal Research Network. Association of antenatal corticosteroids with mortality and neurodevelopmental outcomes among infants born at 22-25 weeks gestation. JAMA. 2011;306:2348-2358.
- Chawla S, Wyckoff MH, Rysavy MA, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Association of antenatal steroid exposure at 21 to 22 weeks of gestation with neonatal survival and survival without morbidities. JAMA Netw Open. 2022;5(9):e2233331. doi:10.1001/ jamanetworkopen.2022.33331.
- Ehret DEY, Edwards EM, Greenberg LT, et al. Association of antenatal steroid exposure with survival among infants receiving postnatal life support at 22 to 25 weeks’ gestation. JAMA Netw Open. 2018;1(6):e183235. doi:10.1001/ jamanetworkopen.2018.3235.
- Rossi RM, DeFranco EA, Hall ES. Association of antenatal corticosteroid exposure and infant survival at 22 and 23 weeks. Am J Perinatol. November 28, 2021. doi:10.1055/s-0041-1740062.
- Backes CH, Rivera BK, Pavlek L, et al. Proactive neonatal treatment at 22 weeks of gestation: a systematic review and meta-analysis. Am J Obstet Gynecol. 2021;224:158-174.
- Cahill AG, Kaimal AJ, Kuller JA, et al; American College of Obstetricians and Gynecologists and Society for Maternal-Fetal Medicine. Practice advisory: Use of antenatal corticosteroids at 22 weeks of gestation. Accessed December 7, 2022. https://www.acog.org/clinical/clinical-guidance /practice-advisory/articles/2021/09/use-of-antenatal -corticosteroids-at-22-weeks-of-gestation#
- Boland RA, Davis PG, Dawson JA, et al. What are we telling the parents of extremely preterm babies? Aust N Z J Obstet Gynaecol. 2016;56:274-281.
- Blanco F, Suresh G, Howard D, et al. Ensuring accurate knowledge of prematurity outcomes for prenatal counseling. Pediatrics. 2005;115:e478-e487.
- Rossi RM, Hall E, DeFranco EA. Contemporary trends in cesarean delivery utilization for live births between 22 0/7 and 23 6/7 weeks of gestation. Obstet Gynecol. 2019;133:451-458.
- Lannon SMR, Guthrie KA, Vanderhoeven JP, et al. Uterine rupture risk after periviable cesarean delivery. Obstet Gynecol. 2015;125:1095-1100.
Chawla S, Wyckoff MH, Rysavy MA, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Association of antenatal steroid exposure at 21 to 22 weeks of gestation with neonatal survival and survival without morbidities. JAMA Netw Open. 2022;5:e2233331. doi:10.1001/jamanet workopen.2022.33331.
EXPERT COMMENTARY
The single most important intervention available in obstetrics to improve the health outcomes of preterm newborns is the maternal administration of corticosteroids. The 27 randomized controlled trials that formed the basis for this knowledge1 did not include infants delivered at 24 weeks’ gestation or less. This has not dissuaded us, over the last several decades, from using corticosteroids for impending delivery at 24 weeks’ gestation; in the absence of randomized data, this has been based on observational evidence of benefit.
Following the 2011 publication of a retrospective cohort study that analyzed data collected by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network between 1993 and 2009 (the Carlo study),2 ACS started to be used widely even for impending delivery at 23 weeks’ gestation. That study had found that the odds of death and neurodevelopmental impairment at 18 to 22 months of age were significantly lower in cases that received ACS and were born at 23 weeks (n = 1,978). The same benefit could not be verified for infants born at 22 weeks’ gestational age (n = 402).
In a recent study conducted by the same NICHD Neonatal Research Network, antenatal steroid exposure at 21 to 22 weeks of gestation was examined.

Details of the study
Using prospectively collected data from 2016 to 2019, Chawla and colleagues conducted a retrospective cohort study that analyzed data from 431 infants who were born between 22 0/7 and 23 6/7 weeks’ gestation and received neonatal intensive care (179 infants born at 22 weeks’ gestation).3 The infants not exposed to ACS were compared with those who had partial exposure (only 1 dose) and those with complete ACS exposure (2 doses).
Complete ACS exposure proved to be beneficial, increasing survival to discharge from 35.5% in the no-exposure group to 53.9% (adjusted odds ratio [aOR], 1.95; 95% confidence interval [CI], 1.07–3.56). Of the survivors, 26.9% in the complete-exposure group had no major morbidities compared with 10% in the no-exposure group (aOR, 2.74; 95% CI, 1.19–6.30).
Study strengths and limitations
The strengths of this study include the use of a diverse, multicenter cohort, with contemporary delivery data, which increases the generalizability of the findings. The analysis included aspects often overlooked in other similar studies, such as the dose of ACS exposure and the gestational age at the time of exposure.
The observational study design, however, can suggest only associations rather than causal relationships. Observational studies also are apt to be affected by residual confounding. Such limitations can only be overcome by a randomized controlled trial, but such a trial of ACS at periviable gestational ages seems unfeasible due to limited ethical justification.
Another limitation is the reporting on outcomes as a collective group (22–23 weeks’ gestation). It is important to consider each gestational age week separately due to differences in physiology and potential biological limitations. It cannot be assumed that 22 weeks behaves like 23 weeks, just as 21 weeks is not equivalent to 22 weeks.
The study results suggest that the protective effect of ACS was dose dependent. However, the interpretation that only a complete ACS exposure was beneficial should be viewed cautiously because the study had no power to assess the impact of a partial exposure.
A further limitation is the lack of consideration in analysis for maternal comorbidities and fetal growth restriction. In the Carlo study, the beneficial effect of corticosteroids in 23-week gestational age deliveries was not demonstrable in pregnancies affected by fetal growth restriction or maternal hypertension.
Other studies considered
Given all its limitations, can we assume that the study by Chawla and colleagues has reliably refuted the Carlo study’s suggestion of lack of ACS efficacy in infants born at 22 weeks’ gestation? Taken by itself, probably not. In the context of other recent investigations, yes.
A retrospective registry study that used data from the Vermont Oxford Network for the period 2012–2016 on 1,058 infants born at 22 weeks’ gestation found that infants who were exposed to ACS and received postnatal life support were more likely to survive to hospital discharge without major morbidity compared with infants who received postnatal life support alone.4 Overall survival was 38.5% versus 17.7% (adjusted risk ratio [aRR], 2.11; 95% CI, 1.68–2.65), and survival without major morbidity was 4.4% versus 1.0% (aRR, 4.35; 95% CI, 1.84–10.28).
An even larger cohort study that used data from the National Center for Health Statistics concluded that survival at age 1 year for infants born at 22 weeks (n = 2,635) during 2009–2014 was improved in those exposed to ACS followed by postnatal life support compared with postnatal life support alone (45.2% vs 27.8%; aRR, 1.6; 95% CI, 1.2–2.1).5
A meta-analysis of observational studies that reported on infants born between 22 0/7 and 22 6/7 weeks’ gestation (n = 2,226) who received proactive neonatal treatment found that administration of ACS doubled the rate of survival when compared with no ACS administration (39% vs 19.5%; P<.01).6
In September 2021, the recommendations from the American College of Obstetricians and Gynecologists changed, stating that ACS can be considered at 22 weeks’ gestation when active postnatal management is desired.7 This recommendation is largely congruent with those from several other national and international medical organizations, including the World Association of Perinatal Medicine, the Royal Collegeof Obstetricians and Gynaecologists, and the German, Austrian and Swiss societies of gynecology and obstetrics. The implication is that the limit of viability may have shifted again, from 23 to 22 weeks’ gestation, and considering the importance of adequate timing in ACS administration (within 1 week from delivery), Chawla and colleagues posited that ACS administration can be considered as early as 21 weeks’ gestation when birth is anticipated at 22 weeks and active postnatal management is planned (notably, this should be the correct interpretation of the article title, not that ACS may be beneficial in 21-weeks’ gestational age births). ●
In 2001, the Institute of Medicine of the National Academies introduced the concept of shared decision-making as a key component of quality care. In very few other clinical situations is shared decision-making as critical as in the context of planning intervention when delivery is anticipated at 22 weeks’ gestation. The truth remains that even with the coordinated provision of ACS and active postnatal care, survival at this gestational age is still a toss-up, and survivors face a high probability of neurodevelopmental impairment and other long-term adverse health outcomes. In this setting, decision-making is complex, with the need to balance patient autonomy and nonmaleficence. On the other hand, the concept of patient autonomy is blurred because the patient (fetus) is incompetent and the negotiation is conducted between physicians and parents. However, no intervention should be undertaken unless the parents so desire. Since parental wishes are frequently emotional, overwhelmingly driving intervention, thorough and timely interdisciplinary counseling is needed. Evidence indicates that both obstetricians and neonatologists may, at times, underestimate the chance of a favorable health outcome for infants born extremely preterm.8,9 Early involvement of the neonatal and obstetric team is pivotal to put forward a coherent, nonconfusing, nonpaternalistic, balanced message. When outcomes information is shared during prenatal counseling, it should be based on local, not only national, data. Following appropriate consultation with the parents, the physicians will adjust the expectations to the local standards, outcomes data, and availability regarding periviable neonatal support.
Recent data suggest that the rate of cesarean delivery (CD) in the periviable period is increasing.10 There is no clear evidence in favor of CD to improve neonatal outcomes, whereas there is concern that periviable CD is associated with significantly increased maternal risks. Regardless of uterine incision type, periviable CD results in an increased risk of uterine rupture in a subsequent pregnancy.11 Consistent with the principle of nonmaleficence, a discussion of these risks should be included in shared decision-making.
ALEX C. VIDAEFF, MD, MPH, AND NATHAN C. SUNDGREN, MD, PHD
Chawla S, Wyckoff MH, Rysavy MA, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Association of antenatal steroid exposure at 21 to 22 weeks of gestation with neonatal survival and survival without morbidities. JAMA Netw Open. 2022;5:e2233331. doi:10.1001/jamanet workopen.2022.33331.
EXPERT COMMENTARY
The single most important intervention available in obstetrics to improve the health outcomes of preterm newborns is the maternal administration of corticosteroids. The 27 randomized controlled trials that formed the basis for this knowledge1 did not include infants delivered at 24 weeks’ gestation or less. This has not dissuaded us, over the last several decades, from using corticosteroids for impending delivery at 24 weeks’ gestation; in the absence of randomized data, this has been based on observational evidence of benefit.
Following the 2011 publication of a retrospective cohort study that analyzed data collected by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network between 1993 and 2009 (the Carlo study),2 ACS started to be used widely even for impending delivery at 23 weeks’ gestation. That study had found that the odds of death and neurodevelopmental impairment at 18 to 22 months of age were significantly lower in cases that received ACS and were born at 23 weeks (n = 1,978). The same benefit could not be verified for infants born at 22 weeks’ gestational age (n = 402).
In a recent study conducted by the same NICHD Neonatal Research Network, antenatal steroid exposure at 21 to 22 weeks of gestation was examined.

Details of the study
Using prospectively collected data from 2016 to 2019, Chawla and colleagues conducted a retrospective cohort study that analyzed data from 431 infants who were born between 22 0/7 and 23 6/7 weeks’ gestation and received neonatal intensive care (179 infants born at 22 weeks’ gestation).3 The infants not exposed to ACS were compared with those who had partial exposure (only 1 dose) and those with complete ACS exposure (2 doses).
Complete ACS exposure proved to be beneficial, increasing survival to discharge from 35.5% in the no-exposure group to 53.9% (adjusted odds ratio [aOR], 1.95; 95% confidence interval [CI], 1.07–3.56). Of the survivors, 26.9% in the complete-exposure group had no major morbidities compared with 10% in the no-exposure group (aOR, 2.74; 95% CI, 1.19–6.30).
Study strengths and limitations
The strengths of this study include the use of a diverse, multicenter cohort, with contemporary delivery data, which increases the generalizability of the findings. The analysis included aspects often overlooked in other similar studies, such as the dose of ACS exposure and the gestational age at the time of exposure.
The observational study design, however, can suggest only associations rather than causal relationships. Observational studies also are apt to be affected by residual confounding. Such limitations can only be overcome by a randomized controlled trial, but such a trial of ACS at periviable gestational ages seems unfeasible due to limited ethical justification.
Another limitation is the reporting on outcomes as a collective group (22–23 weeks’ gestation). It is important to consider each gestational age week separately due to differences in physiology and potential biological limitations. It cannot be assumed that 22 weeks behaves like 23 weeks, just as 21 weeks is not equivalent to 22 weeks.
The study results suggest that the protective effect of ACS was dose dependent. However, the interpretation that only a complete ACS exposure was beneficial should be viewed cautiously because the study had no power to assess the impact of a partial exposure.
A further limitation is the lack of consideration in analysis for maternal comorbidities and fetal growth restriction. In the Carlo study, the beneficial effect of corticosteroids in 23-week gestational age deliveries was not demonstrable in pregnancies affected by fetal growth restriction or maternal hypertension.
Other studies considered
Given all its limitations, can we assume that the study by Chawla and colleagues has reliably refuted the Carlo study’s suggestion of lack of ACS efficacy in infants born at 22 weeks’ gestation? Taken by itself, probably not. In the context of other recent investigations, yes.
A retrospective registry study that used data from the Vermont Oxford Network for the period 2012–2016 on 1,058 infants born at 22 weeks’ gestation found that infants who were exposed to ACS and received postnatal life support were more likely to survive to hospital discharge without major morbidity compared with infants who received postnatal life support alone.4 Overall survival was 38.5% versus 17.7% (adjusted risk ratio [aRR], 2.11; 95% CI, 1.68–2.65), and survival without major morbidity was 4.4% versus 1.0% (aRR, 4.35; 95% CI, 1.84–10.28).
An even larger cohort study that used data from the National Center for Health Statistics concluded that survival at age 1 year for infants born at 22 weeks (n = 2,635) during 2009–2014 was improved in those exposed to ACS followed by postnatal life support compared with postnatal life support alone (45.2% vs 27.8%; aRR, 1.6; 95% CI, 1.2–2.1).5
A meta-analysis of observational studies that reported on infants born between 22 0/7 and 22 6/7 weeks’ gestation (n = 2,226) who received proactive neonatal treatment found that administration of ACS doubled the rate of survival when compared with no ACS administration (39% vs 19.5%; P<.01).6
In September 2021, the recommendations from the American College of Obstetricians and Gynecologists changed, stating that ACS can be considered at 22 weeks’ gestation when active postnatal management is desired.7 This recommendation is largely congruent with those from several other national and international medical organizations, including the World Association of Perinatal Medicine, the Royal Collegeof Obstetricians and Gynaecologists, and the German, Austrian and Swiss societies of gynecology and obstetrics. The implication is that the limit of viability may have shifted again, from 23 to 22 weeks’ gestation, and considering the importance of adequate timing in ACS administration (within 1 week from delivery), Chawla and colleagues posited that ACS administration can be considered as early as 21 weeks’ gestation when birth is anticipated at 22 weeks and active postnatal management is planned (notably, this should be the correct interpretation of the article title, not that ACS may be beneficial in 21-weeks’ gestational age births). ●
In 2001, the Institute of Medicine of the National Academies introduced the concept of shared decision-making as a key component of quality care. In very few other clinical situations is shared decision-making as critical as in the context of planning intervention when delivery is anticipated at 22 weeks’ gestation. The truth remains that even with the coordinated provision of ACS and active postnatal care, survival at this gestational age is still a toss-up, and survivors face a high probability of neurodevelopmental impairment and other long-term adverse health outcomes. In this setting, decision-making is complex, with the need to balance patient autonomy and nonmaleficence. On the other hand, the concept of patient autonomy is blurred because the patient (fetus) is incompetent and the negotiation is conducted between physicians and parents. However, no intervention should be undertaken unless the parents so desire. Since parental wishes are frequently emotional, overwhelmingly driving intervention, thorough and timely interdisciplinary counseling is needed. Evidence indicates that both obstetricians and neonatologists may, at times, underestimate the chance of a favorable health outcome for infants born extremely preterm.8,9 Early involvement of the neonatal and obstetric team is pivotal to put forward a coherent, nonconfusing, nonpaternalistic, balanced message. When outcomes information is shared during prenatal counseling, it should be based on local, not only national, data. Following appropriate consultation with the parents, the physicians will adjust the expectations to the local standards, outcomes data, and availability regarding periviable neonatal support.
Recent data suggest that the rate of cesarean delivery (CD) in the periviable period is increasing.10 There is no clear evidence in favor of CD to improve neonatal outcomes, whereas there is concern that periviable CD is associated with significantly increased maternal risks. Regardless of uterine incision type, periviable CD results in an increased risk of uterine rupture in a subsequent pregnancy.11 Consistent with the principle of nonmaleficence, a discussion of these risks should be included in shared decision-making.
ALEX C. VIDAEFF, MD, MPH, AND NATHAN C. SUNDGREN, MD, PHD
- McGoldrick E, Stewart F, Parker R, et al. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2020;12(12):CD004454.
- Carlo WA, McDonald SA, Fanaroff AA, et al; Eunice Kennedy Schriver National Institute for Child Health and Human Development Neonatal Research Network. Association of antenatal corticosteroids with mortality and neurodevelopmental outcomes among infants born at 22-25 weeks gestation. JAMA. 2011;306:2348-2358.
- Chawla S, Wyckoff MH, Rysavy MA, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Association of antenatal steroid exposure at 21 to 22 weeks of gestation with neonatal survival and survival without morbidities. JAMA Netw Open. 2022;5(9):e2233331. doi:10.1001/ jamanetworkopen.2022.33331.
- Ehret DEY, Edwards EM, Greenberg LT, et al. Association of antenatal steroid exposure with survival among infants receiving postnatal life support at 22 to 25 weeks’ gestation. JAMA Netw Open. 2018;1(6):e183235. doi:10.1001/ jamanetworkopen.2018.3235.
- Rossi RM, DeFranco EA, Hall ES. Association of antenatal corticosteroid exposure and infant survival at 22 and 23 weeks. Am J Perinatol. November 28, 2021. doi:10.1055/s-0041-1740062.
- Backes CH, Rivera BK, Pavlek L, et al. Proactive neonatal treatment at 22 weeks of gestation: a systematic review and meta-analysis. Am J Obstet Gynecol. 2021;224:158-174.
- Cahill AG, Kaimal AJ, Kuller JA, et al; American College of Obstetricians and Gynecologists and Society for Maternal-Fetal Medicine. Practice advisory: Use of antenatal corticosteroids at 22 weeks of gestation. Accessed December 7, 2022. https://www.acog.org/clinical/clinical-guidance /practice-advisory/articles/2021/09/use-of-antenatal -corticosteroids-at-22-weeks-of-gestation#
- Boland RA, Davis PG, Dawson JA, et al. What are we telling the parents of extremely preterm babies? Aust N Z J Obstet Gynaecol. 2016;56:274-281.
- Blanco F, Suresh G, Howard D, et al. Ensuring accurate knowledge of prematurity outcomes for prenatal counseling. Pediatrics. 2005;115:e478-e487.
- Rossi RM, Hall E, DeFranco EA. Contemporary trends in cesarean delivery utilization for live births between 22 0/7 and 23 6/7 weeks of gestation. Obstet Gynecol. 2019;133:451-458.
- Lannon SMR, Guthrie KA, Vanderhoeven JP, et al. Uterine rupture risk after periviable cesarean delivery. Obstet Gynecol. 2015;125:1095-1100.
- McGoldrick E, Stewart F, Parker R, et al. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2020;12(12):CD004454.
- Carlo WA, McDonald SA, Fanaroff AA, et al; Eunice Kennedy Schriver National Institute for Child Health and Human Development Neonatal Research Network. Association of antenatal corticosteroids with mortality and neurodevelopmental outcomes among infants born at 22-25 weeks gestation. JAMA. 2011;306:2348-2358.
- Chawla S, Wyckoff MH, Rysavy MA, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Association of antenatal steroid exposure at 21 to 22 weeks of gestation with neonatal survival and survival without morbidities. JAMA Netw Open. 2022;5(9):e2233331. doi:10.1001/ jamanetworkopen.2022.33331.
- Ehret DEY, Edwards EM, Greenberg LT, et al. Association of antenatal steroid exposure with survival among infants receiving postnatal life support at 22 to 25 weeks’ gestation. JAMA Netw Open. 2018;1(6):e183235. doi:10.1001/ jamanetworkopen.2018.3235.
- Rossi RM, DeFranco EA, Hall ES. Association of antenatal corticosteroid exposure and infant survival at 22 and 23 weeks. Am J Perinatol. November 28, 2021. doi:10.1055/s-0041-1740062.
- Backes CH, Rivera BK, Pavlek L, et al. Proactive neonatal treatment at 22 weeks of gestation: a systematic review and meta-analysis. Am J Obstet Gynecol. 2021;224:158-174.
- Cahill AG, Kaimal AJ, Kuller JA, et al; American College of Obstetricians and Gynecologists and Society for Maternal-Fetal Medicine. Practice advisory: Use of antenatal corticosteroids at 22 weeks of gestation. Accessed December 7, 2022. https://www.acog.org/clinical/clinical-guidance /practice-advisory/articles/2021/09/use-of-antenatal -corticosteroids-at-22-weeks-of-gestation#
- Boland RA, Davis PG, Dawson JA, et al. What are we telling the parents of extremely preterm babies? Aust N Z J Obstet Gynaecol. 2016;56:274-281.
- Blanco F, Suresh G, Howard D, et al. Ensuring accurate knowledge of prematurity outcomes for prenatal counseling. Pediatrics. 2005;115:e478-e487.
- Rossi RM, Hall E, DeFranco EA. Contemporary trends in cesarean delivery utilization for live births between 22 0/7 and 23 6/7 weeks of gestation. Obstet Gynecol. 2019;133:451-458.
- Lannon SMR, Guthrie KA, Vanderhoeven JP, et al. Uterine rupture risk after periviable cesarean delivery. Obstet Gynecol. 2015;125:1095-1100.
Commentary: New Drugs, and Exercise, in Breast Cancer January 2023
This is the longest reported median PFS in HER2+ metastatic BC, highlighting the potential of trastuzumab deruxtecan in treating this disease and confirming this drug as the standard of care in the second-line setting.
A cohort study evaluated 315 postmenopausal BC survivors to estimate the association of physical activity with risk for all-cause mortality. Participants were queried about leisure-time physical activity using the Godin-Shephard Leisure-Time Physical Activity Questionnaire (GSLTPAQ), which provided a composite score that categorized exercise patterns as active, moderately active, or insufficiently active at baseline.
Results showed that participants who were active or moderately active had a 60% decreased risk for death compared with insufficiently active participants (active: HR 0.42 [95% CI 0.21-0.85]; moderately active: HR 0.40 [95% CI 0.17-0.95]). A similar mortality risk was reported among participants who were active and those with moderate physical activity levels.
Prior studies1 have reported similar results, reaffirming the value of exercise in BC survivors and highlighting the need to incorporate physical activity as part of survivorship care plans.
The phase 3 SOPHIA study randomized 536 patients with HER2+ advanced BC who had received two or more prior anti-HER2 regimens to margetuximab plus chemotherapy vs trastuzumab plus chemotherapy. Final OS results after a median follow-up of 20.2 months showed no benefit in OS observed with margetuximab vs trastuzumab (median OS 21.6 months vs 21.9 months; HR 0.95; P = .620). The safety profile of margetuximab was acceptable and comparable to that of trastuzumab. Exploratory analysis of CD16A genotyping suggested a possible improvement in OS for margetuximab in CD16A-158FF patients vs trastuzumab (median OS 23.6 vs 19.2 months; HR 0.72; 95% CI 0.52-1.00) and a possible improvement in OS for trastuzumab in CD16A-158VV patients vs margetuximab (median OS 31.1 vs 22.0 months; HR 1.77; 95% CI 1.01–3.12). The safety profile of margetuximab was acceptable and comparable to that of trastuzumab. Further studies to evaluate the role of margetuximab in patients with HER2+ BC with different CD16A allelic variants are warranted.
Additional References
Cannioto RA, Hutson A, Dighe S, et al. Physical activity before, during, and after chemotherapy for high-risk breast cancer: Relationships with survival. J Natl Cancer Inst. 2021;113:54-63. Doi:10.1093/jnci/djaa046
This is the longest reported median PFS in HER2+ metastatic BC, highlighting the potential of trastuzumab deruxtecan in treating this disease and confirming this drug as the standard of care in the second-line setting.
A cohort study evaluated 315 postmenopausal BC survivors to estimate the association of physical activity with risk for all-cause mortality. Participants were queried about leisure-time physical activity using the Godin-Shephard Leisure-Time Physical Activity Questionnaire (GSLTPAQ), which provided a composite score that categorized exercise patterns as active, moderately active, or insufficiently active at baseline.
Results showed that participants who were active or moderately active had a 60% decreased risk for death compared with insufficiently active participants (active: HR 0.42 [95% CI 0.21-0.85]; moderately active: HR 0.40 [95% CI 0.17-0.95]). A similar mortality risk was reported among participants who were active and those with moderate physical activity levels.
Prior studies1 have reported similar results, reaffirming the value of exercise in BC survivors and highlighting the need to incorporate physical activity as part of survivorship care plans.
The phase 3 SOPHIA study randomized 536 patients with HER2+ advanced BC who had received two or more prior anti-HER2 regimens to margetuximab plus chemotherapy vs trastuzumab plus chemotherapy. Final OS results after a median follow-up of 20.2 months showed no benefit in OS observed with margetuximab vs trastuzumab (median OS 21.6 months vs 21.9 months; HR 0.95; P = .620). The safety profile of margetuximab was acceptable and comparable to that of trastuzumab. Exploratory analysis of CD16A genotyping suggested a possible improvement in OS for margetuximab in CD16A-158FF patients vs trastuzumab (median OS 23.6 vs 19.2 months; HR 0.72; 95% CI 0.52-1.00) and a possible improvement in OS for trastuzumab in CD16A-158VV patients vs margetuximab (median OS 31.1 vs 22.0 months; HR 1.77; 95% CI 1.01–3.12). The safety profile of margetuximab was acceptable and comparable to that of trastuzumab. Further studies to evaluate the role of margetuximab in patients with HER2+ BC with different CD16A allelic variants are warranted.
Additional References
Cannioto RA, Hutson A, Dighe S, et al. Physical activity before, during, and after chemotherapy for high-risk breast cancer: Relationships with survival. J Natl Cancer Inst. 2021;113:54-63. Doi:10.1093/jnci/djaa046
This is the longest reported median PFS in HER2+ metastatic BC, highlighting the potential of trastuzumab deruxtecan in treating this disease and confirming this drug as the standard of care in the second-line setting.
A cohort study evaluated 315 postmenopausal BC survivors to estimate the association of physical activity with risk for all-cause mortality. Participants were queried about leisure-time physical activity using the Godin-Shephard Leisure-Time Physical Activity Questionnaire (GSLTPAQ), which provided a composite score that categorized exercise patterns as active, moderately active, or insufficiently active at baseline.
Results showed that participants who were active or moderately active had a 60% decreased risk for death compared with insufficiently active participants (active: HR 0.42 [95% CI 0.21-0.85]; moderately active: HR 0.40 [95% CI 0.17-0.95]). A similar mortality risk was reported among participants who were active and those with moderate physical activity levels.
Prior studies1 have reported similar results, reaffirming the value of exercise in BC survivors and highlighting the need to incorporate physical activity as part of survivorship care plans.
The phase 3 SOPHIA study randomized 536 patients with HER2+ advanced BC who had received two or more prior anti-HER2 regimens to margetuximab plus chemotherapy vs trastuzumab plus chemotherapy. Final OS results after a median follow-up of 20.2 months showed no benefit in OS observed with margetuximab vs trastuzumab (median OS 21.6 months vs 21.9 months; HR 0.95; P = .620). The safety profile of margetuximab was acceptable and comparable to that of trastuzumab. Exploratory analysis of CD16A genotyping suggested a possible improvement in OS for margetuximab in CD16A-158FF patients vs trastuzumab (median OS 23.6 vs 19.2 months; HR 0.72; 95% CI 0.52-1.00) and a possible improvement in OS for trastuzumab in CD16A-158VV patients vs margetuximab (median OS 31.1 vs 22.0 months; HR 1.77; 95% CI 1.01–3.12). The safety profile of margetuximab was acceptable and comparable to that of trastuzumab. Further studies to evaluate the role of margetuximab in patients with HER2+ BC with different CD16A allelic variants are warranted.
Additional References
Cannioto RA, Hutson A, Dighe S, et al. Physical activity before, during, and after chemotherapy for high-risk breast cancer: Relationships with survival. J Natl Cancer Inst. 2021;113:54-63. Doi:10.1093/jnci/djaa046