User login
Oral Propranolol Used as Adjunct Therapy in Cutaneous Angiosarcoma
To the Editor:
Angiosarcoma is a malignancy of the vascular endothelium that most commonly presents on the skin.1 Patients diagnosed with cutaneous angiosarcoma, which is a rare and aggressive malignancy, have a 5-year survival rate of approximately 30%.2,3 Angiosarcoma can be seen in the setting of chronic lymphedema; radiation therapy; and sporadically in elderly patients, where it is commonly seen on the head and neck. Presentation on the head and neck has been associated with worse outcomes, with a projected overall 10-year survival rate of 13.8%; the survival rate is lower if the tumor is surgically unresectable or larger in size. Metastasis can occur via both lymphatic and hematogenous routes, with pulmonary and hepatic metastases most frequently observed.1 Prognostications of poor outcomes for patients with head and neck cutaneous angiosarcoma via a 5-year survival rate were identified in a meta-analysis and included the following: patient age older than 70 years, larger tumors, tumor location of scalp vs face, nonsurgical treatments, and lack of clear margins on histology.2
Treatment of angiosarcoma historically has encompassed both surgical resection and adjuvant radiation therapy with suboptimal success. Evidence supporting various treatment regimens remains sparse due to the low incidence of the neoplasm. Although surgical resection is the only documented curative treatment, cutaneous angiosarcomas frequently are found to have positive surgical margins and require adjuvant radiation. Use of high-dose radiation (>50 Gy) with application over a wide treatment area such as total scalp irradiation is recommended.4 Although radiation has been found to diminish local recurrence rates, it has not substantially affected rates of distant disease recurrence.1 Cytotoxic chemotherapy has clinical utility in minimizing progression, but standard regimens afford a progression-free survival of only months.3 Adjuvant treatment with paclitaxel has been shown to have improved efficacy in scalp angiosarcoma vs other visceral sites, showing a nonprogression rate of 42% at 4 months after treatment.5 More recently, targeted chemotherapeutics, including the vascular endothelial growth factor inhibitor bevacizumab and tyrosine kinase inhibitor sorafenib, have shown some survival benefit, but it is unclear if these agents are superior to traditional cytotoxic agents.4,6-10 A phase 2 study of paclitaxel administered weekly with or without bevacizumab showed similar progression-free survival and overall survival, albeit at the expense of added toxicity experienced by participants in the combined group.10
The addition of the nonselective β-adrenergic blocker propranolol to the treatment armamentarium, which was pursued due to its utility in the treatment of benign infantile hemangioma and demonstrated ability to limit the expression of adrenergic receptors in angiosarcoma, has gained clinical attention for possible augmentation of cutaneous angiosarcoma therapy.11-14 Propranolol has been shown to reduce metastasis in other neoplasms—both vascular and nonvascular—and may play a role as an adjuvant treatment to current therapies in angiosarcoma.15-20 We report a patient with cutaneous angiosarcoma (T2 classification) with disease-free survival of nearly 6 years without evidence of recurrence in the setting of continuous propranolol use supplementary to chemotherapy and radiation.
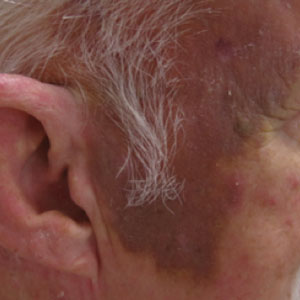
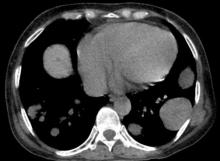
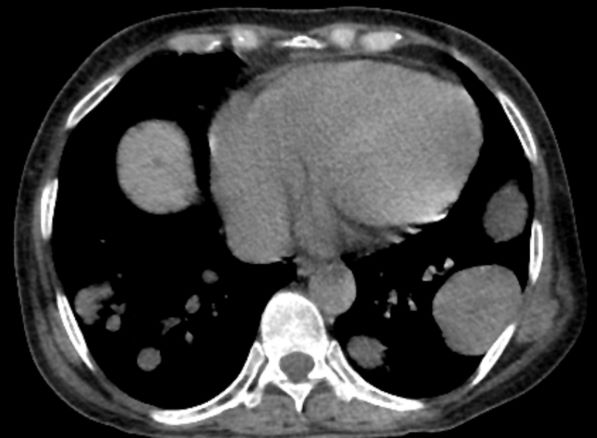
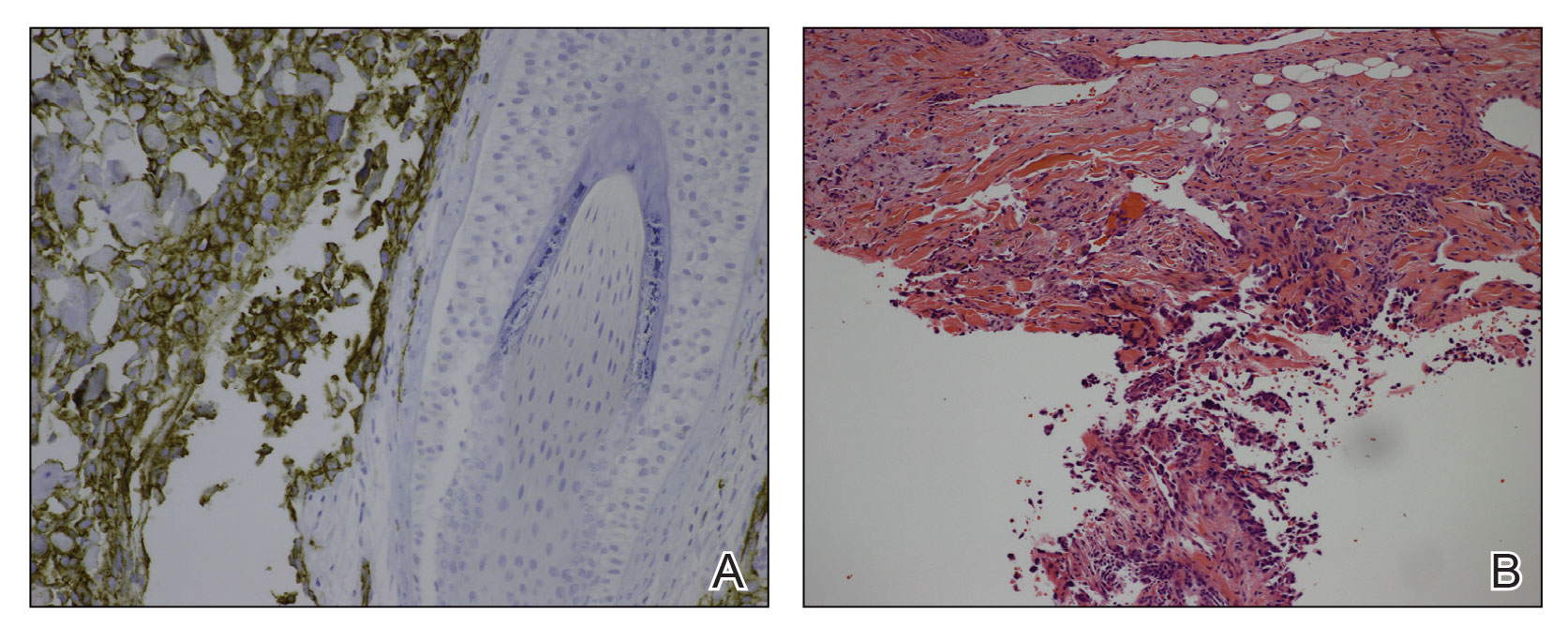
A 78-year-old man with a history of multiple basal cell carcinomas, hypertension, and remote smoking history presented to the dermatology clinic with an enlarging red-brown plaque on the scalp of 2 months’ duration. The lesion had grown rapidly to involve the forehead, right temple, preauricular region, and parietal scalp. At presentation, the tumor measured more than 20 cm in diameter at its greatest point (Figure 1). Physical examination revealed a 6-mm purple nodule within the lesion on the patient’s right parietal scalp. No clinical lymphadenopathy was appreciated at the time of diagnosis. Punch biopsies of the right parietal scalp nodule and right temple patch showed findings consistent with angiosarcoma with diffuse cytoplasmic staining of CD31 in atypical endothelial cells and no staining for human herpesvirus 8 (Figure 2). Concurrent computed tomography of the head showed thickening of the right epidermis, dermis, and deeper scalp tissues, but there was no evidence of skull involvement. Computed tomography of the thorax, abdomen, and pelvis showed no evidence of metastatic disease. After a diagnostic workup, the patient was diagnosed with T2bN0M0 angiosarcoma.
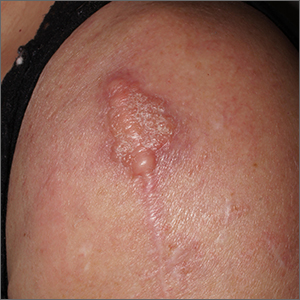
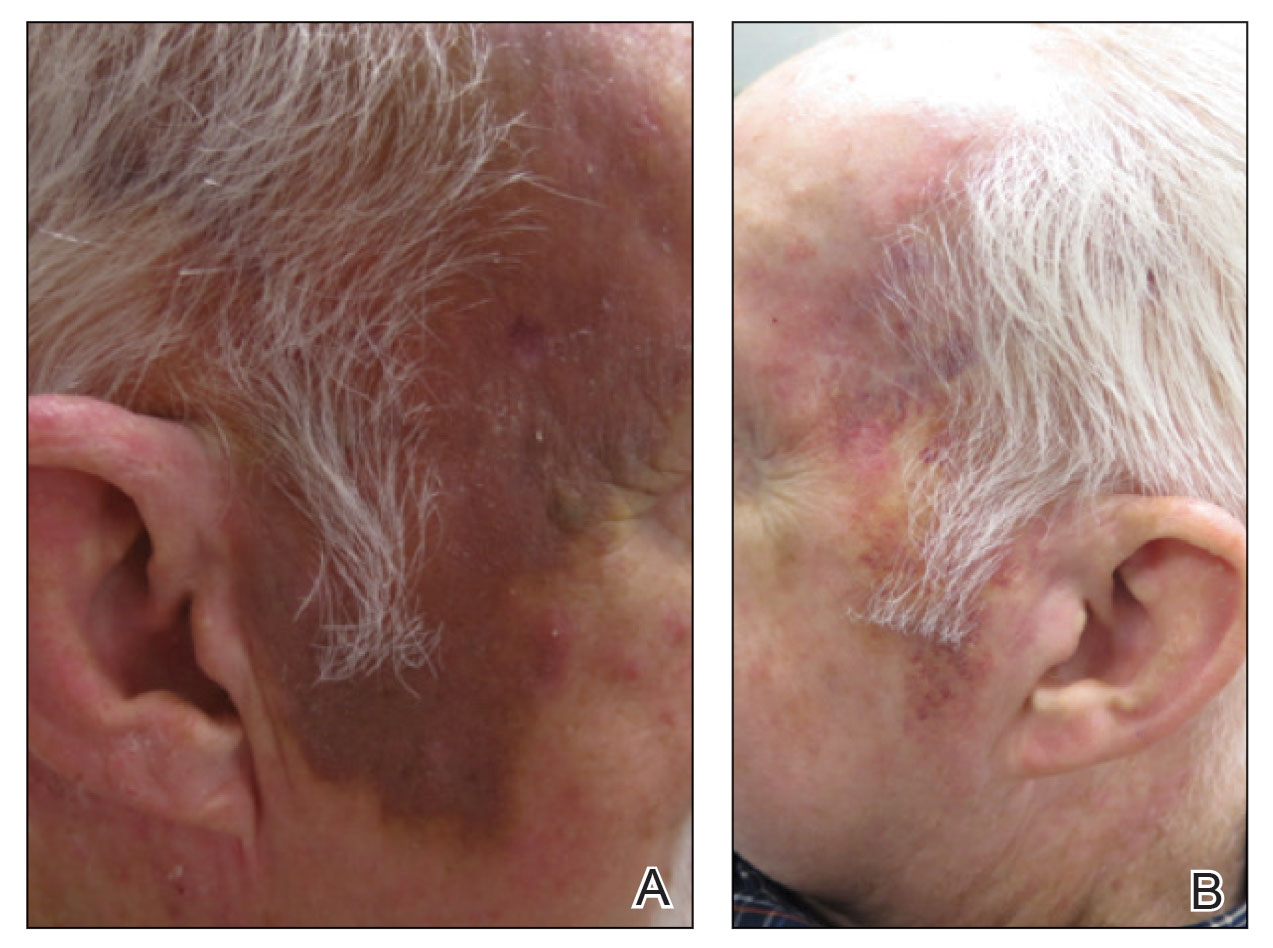
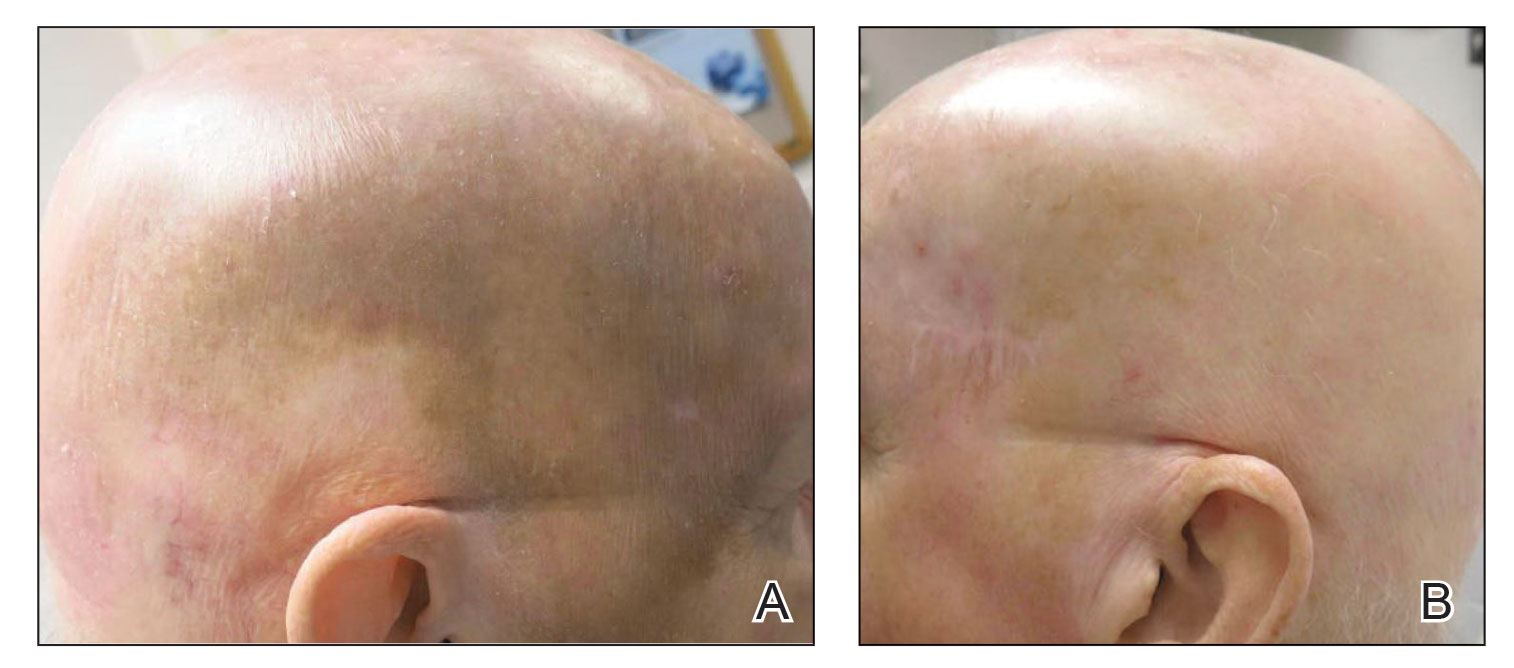
The lesion was determined to be nonresectable due to the extent of the patient’s cutaneous disease. The patient was started on a regimen of paclitaxel, scalp radiation, and oral propranolol. Propranolol 40 mg twice daily was initiated at the time of diagnosis with a plan to continue indefinitely. Starting 1 month after staging, the patient completed 10 weekly cycles of paclitaxel, and he was treated with 60 Gy of scalp radiation in 30 fractions, starting with the second cycle of paclitaxel. He tolerated both well with no reported adverse events. Repeat computed tomography performed 1 month after completion of chemotherapy and radiation showed no evidence of a mass or fluid collection in subcutaneous scalp tissues and no evidence of metastatic disease. This correlated with an observed clinical regression at 1 month and complete clinical response at 5 months with residual hemosiderin and radiation changes. The area of prior disease involvement subsequently evolved from violet to dusky gray in appearance to an eventual complete resolution 26 months after diagnosis, accompanied by atrophic radiation-induced sequelae (Figure 3).
The patient’s postchemotherapy course was complicated by hospitalization for a suspected malignant pleural effusion. Analysis revealed growing ground-glass opacities and nodules in the right lower lung lobe. A thoracentesis with cytology studies was negative for malignancy. Continued monitoring over 19 months demonstrated eventual resolution of those findings. He experienced notable complication from local radiation therapy to the scalp with chronic cutaneous ulceration refractory to wound care and surgical intervention. The patient did not exhibit additional signs or symptoms concerning for recurrence or metastasis and was followed by dermatology and oncology until he died nearly 5 years after initial diagnosis due to complications from acute hypoxic respiratory failure secondary to COVID-19. The last imaging obtained showed no convincing evidence of metastasis, though spinal imaging within a month of his death showed lesions favored to represent benign angiomatous growths. His survival after diagnosis ultimately reached 57 months without confirmed disease recurrence and cause of death unrelated to malignancy history, which is a markedly long documented survival for this extent of disease.
Cutaneous angiosarcoma is an aggressive yet rare malignancy without effective treatments for prolonging survival or eradicating disease. Cutaneous angiosarcoma of the head and neck has a reported 10-year survival rate of 13.8%.1 Although angiosarcoma in any location holds a bleak prognosis, cutaneous angiosarcoma of the scalp with a T2 classification has a 2-year survival rate of 0%. Moreover, even if remission is achieved, disease is highly recurrent, typically within months with the current standard of care.3,21,22
Emerging evidence for the possible role of β-adrenergic receptor blockade in the treatment of malignant vascular neoplasms is promising. Microarrays from a host of vascular growths have demonstrated expression of β-adrenergic receptors in 77% of sampled angiosarcoma specimens in addition to strong expression in infantile hemangiomas, hemangiomas, hemangioendotheliomas, and vascular malformations.19 Research findings have further verified the validity of this approach with the demonstration of b1-, b2-, and b3- adrenergic receptor expression by angiosarcoma cell lines. Propranolol subsequently was shown to effectively target proliferation of these cells and induce apoptosis in a dose-dependent manner and moreover be synergistic in effect with other chemotherapies.15 Several genes have exhibited differential expression between control tumor cells and propranolol-treated cells. Specifically, target genes including AXL (a receptor tyrosine kinase associated with cell adhesion, proliferation, and apoptosis and found to upregulated in melanoma and leukemia) and ERBB receptor feedback inhibitor 1 (receptor tyrosine kinase, with ERBB family members commonly overexpressed or mutated in the setting malignancy) have been posited as possible explanatory factors in the observed angiosarcoma response to propranolol.23
Several cases describing propranolol use as an adjunctive therapy for angiosarcoma suggest a beneficial role in clinical medicine. One case report described propranolol monotherapy for lesion to our patient, with a resultant reduction in Ki-67 as a measure of proliferative index within 1 week of initiating propranolol therapy.13 Propranolol also has been shown to halt or slow progression of metastatic disease in visceral and metastatic angiosarcomas.12-14 In combination with oral etoposide and cyclophosphamide, maintenance propranolol therapy in 7 cases of advanced cutaneous angiosarcoma resulted in 1 complete response and 3 very good partial responses, with a median progression-free survival of 11 months.11 Larger-scale studies have not been published, but the growing number of case reports and case series warrants further investigation of the utility of propranolol as an adjunct to current therapies in advanced angiosarcoma.
- Abraham JA, Hornicek FJ, Kaufman AM, et al. Treatment and outcome of 82 patients with angiosarcoma. Ann Surg Oncol. 2007;14:1953-1967.
- Shin JY, Roh SG, Lee NH, et al. Predisposing factors for poor prognosis of angiosarcoma of the scalp and face: systematic review and meta-analysis. Head Neck. 2017;39:380-386.
- Fury MG, Antonescu CR, Zee KJV, et al. A 14-year retrospective review of angiosarcoma: clinical characteristics, prognostic factors, and treatment outcomes with surgery and chemotherapy. Cancer. 2005;11:241-247.
- Dossett LA, Harrington M, Cruse CW, et al. Cutaneous angiosarcoma. Curr Probl Cancer. 2015;39:258-263.
- Penel N, Bui BN, Bay JO, et al. Phase II trial of weekly paclitaxel for unresectable angiosarcoma: the ANGIOTAX study. J Clin Oncol. 2008;26:5269-5274.
- Agulnik M, Yarber JL, Okuno SH, et al. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol. 2013;24:257-263.
- Maki RG, D’Adamo DR, Keohan ML, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol. 2009;27:3133-3140.
- Ishida Y, Otsuka A, Kabashima K. Cutaneous angiosarcoma: update on biology and latest treatment. Curr Opin Oncol. 2018;30:107-112.
- Ray-Coquard I, Italiano A, Bompas E, et al. Sorafenib for patients with advanced angiosarcoma: a phase II trial from the French Sarcoma Group (GSF/GETO). Oncologist. 2012;17:260-266.
- Ray-Coquard IL, Domont J, Tresch-Bruneel E, et al. Paclitaxel given once per week with or without bevacizumab in patients with advanced angiosarcoma: a randomized phase II trial. J Clin Oncol. 2015;33:2797-2802.
- Pasquier E, Andre N, Street J, et al. Effective management of advanced angiosarcoma by the synergistic combination of propranolol and vinblastine-based metronomic chemotherapy: a bench to bedside study. EBioMedicine. 2016;6:87-95.
- Banavali S, Pasquier E, Andre N. Targeted therapy with propranolol and metronomic chemotherapy combination: sustained complete response of a relapsing metastatic angiosarcoma. Ecancermedicalscience. 2015;9:499.
- Chow W, Amaya CN, Rains S, et al. Growth attenuation of cutaneous angiosarcoma with propranolol-mediated beta-blockade. JAMA Dermatol. 2015;151:1226-1229.
- Daguze J, Saint-Jean M, Peuvrel L, et al. Visceral metastatic angiosarcoma treated effectively with oral cyclophosphamide combined with propranolol. JAAD Case Rep. 2016;2:497-499.
- Stiles JM, Amaya C, Rains S, et al. Targeting of beta adrenergic receptors results in therapeutic efficacy against models of hemangioendothelioma and angiosarcoma. PLoS One. 2013;8:e60021.
- Chang PY, Chung CH, Chang WC, et al. The effect of propranolol on the prognosis of hepatocellular carcinoma: a nationwide population-based study. PLoS One. 2019;14:e0216828.
- De Giorgi V, Grazzini M, Benemei S, et al. Propranolol for off-label treatment of patients with melanoma: results from a cohort study. JAMA Oncol. 2018;4:e172908.
- Rico M, Baglioni M, Bondarenko M, et al. Metformin and propranolol combination prevents cancer progression and metastasis in different breast cancer models. Oncotarget. 2017;8:2874-2889.
- Chisholm KM, Chang KW, Truong MT, et al. β-Adrenergic receptor expression in vascular tumors. Mod Pathol. 2012;25:1446-1451.
- Leaute-Labreze C, Dumas de la Roque E, Hubiche T, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649-2651.
- Maddox JC, Evans HL. Angiosarcoma of skin and soft tissue: a study of forty-four cases. Cancer. 1981;48:1907-1921.
- Morgan MB, Swann M, Somach S, et al. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004;50:867-874.
- Zhou S, Liu P, Jiang W, et al. Identification of potential target genes associated with the effect of propranolol on angiosarcoma via microarray analysis. Oncol Lett. 2017;13:4267-4275.
To the Editor:
Angiosarcoma is a malignancy of the vascular endothelium that most commonly presents on the skin.1 Patients diagnosed with cutaneous angiosarcoma, which is a rare and aggressive malignancy, have a 5-year survival rate of approximately 30%.2,3 Angiosarcoma can be seen in the setting of chronic lymphedema; radiation therapy; and sporadically in elderly patients, where it is commonly seen on the head and neck. Presentation on the head and neck has been associated with worse outcomes, with a projected overall 10-year survival rate of 13.8%; the survival rate is lower if the tumor is surgically unresectable or larger in size. Metastasis can occur via both lymphatic and hematogenous routes, with pulmonary and hepatic metastases most frequently observed.1 Prognostications of poor outcomes for patients with head and neck cutaneous angiosarcoma via a 5-year survival rate were identified in a meta-analysis and included the following: patient age older than 70 years, larger tumors, tumor location of scalp vs face, nonsurgical treatments, and lack of clear margins on histology.2
Treatment of angiosarcoma historically has encompassed both surgical resection and adjuvant radiation therapy with suboptimal success. Evidence supporting various treatment regimens remains sparse due to the low incidence of the neoplasm. Although surgical resection is the only documented curative treatment, cutaneous angiosarcomas frequently are found to have positive surgical margins and require adjuvant radiation. Use of high-dose radiation (>50 Gy) with application over a wide treatment area such as total scalp irradiation is recommended.4 Although radiation has been found to diminish local recurrence rates, it has not substantially affected rates of distant disease recurrence.1 Cytotoxic chemotherapy has clinical utility in minimizing progression, but standard regimens afford a progression-free survival of only months.3 Adjuvant treatment with paclitaxel has been shown to have improved efficacy in scalp angiosarcoma vs other visceral sites, showing a nonprogression rate of 42% at 4 months after treatment.5 More recently, targeted chemotherapeutics, including the vascular endothelial growth factor inhibitor bevacizumab and tyrosine kinase inhibitor sorafenib, have shown some survival benefit, but it is unclear if these agents are superior to traditional cytotoxic agents.4,6-10 A phase 2 study of paclitaxel administered weekly with or without bevacizumab showed similar progression-free survival and overall survival, albeit at the expense of added toxicity experienced by participants in the combined group.10
The addition of the nonselective β-adrenergic blocker propranolol to the treatment armamentarium, which was pursued due to its utility in the treatment of benign infantile hemangioma and demonstrated ability to limit the expression of adrenergic receptors in angiosarcoma, has gained clinical attention for possible augmentation of cutaneous angiosarcoma therapy.11-14 Propranolol has been shown to reduce metastasis in other neoplasms—both vascular and nonvascular—and may play a role as an adjuvant treatment to current therapies in angiosarcoma.15-20 We report a patient with cutaneous angiosarcoma (T2 classification) with disease-free survival of nearly 6 years without evidence of recurrence in the setting of continuous propranolol use supplementary to chemotherapy and radiation.

A 78-year-old man with a history of multiple basal cell carcinomas, hypertension, and remote smoking history presented to the dermatology clinic with an enlarging red-brown plaque on the scalp of 2 months’ duration. The lesion had grown rapidly to involve the forehead, right temple, preauricular region, and parietal scalp. At presentation, the tumor measured more than 20 cm in diameter at its greatest point (Figure 1). Physical examination revealed a 6-mm purple nodule within the lesion on the patient’s right parietal scalp. No clinical lymphadenopathy was appreciated at the time of diagnosis. Punch biopsies of the right parietal scalp nodule and right temple patch showed findings consistent with angiosarcoma with diffuse cytoplasmic staining of CD31 in atypical endothelial cells and no staining for human herpesvirus 8 (Figure 2). Concurrent computed tomography of the head showed thickening of the right epidermis, dermis, and deeper scalp tissues, but there was no evidence of skull involvement. Computed tomography of the thorax, abdomen, and pelvis showed no evidence of metastatic disease. After a diagnostic workup, the patient was diagnosed with T2bN0M0 angiosarcoma.

The lesion was determined to be nonresectable due to the extent of the patient’s cutaneous disease. The patient was started on a regimen of paclitaxel, scalp radiation, and oral propranolol. Propranolol 40 mg twice daily was initiated at the time of diagnosis with a plan to continue indefinitely. Starting 1 month after staging, the patient completed 10 weekly cycles of paclitaxel, and he was treated with 60 Gy of scalp radiation in 30 fractions, starting with the second cycle of paclitaxel. He tolerated both well with no reported adverse events. Repeat computed tomography performed 1 month after completion of chemotherapy and radiation showed no evidence of a mass or fluid collection in subcutaneous scalp tissues and no evidence of metastatic disease. This correlated with an observed clinical regression at 1 month and complete clinical response at 5 months with residual hemosiderin and radiation changes. The area of prior disease involvement subsequently evolved from violet to dusky gray in appearance to an eventual complete resolution 26 months after diagnosis, accompanied by atrophic radiation-induced sequelae (Figure 3).

The patient’s postchemotherapy course was complicated by hospitalization for a suspected malignant pleural effusion. Analysis revealed growing ground-glass opacities and nodules in the right lower lung lobe. A thoracentesis with cytology studies was negative for malignancy. Continued monitoring over 19 months demonstrated eventual resolution of those findings. He experienced notable complication from local radiation therapy to the scalp with chronic cutaneous ulceration refractory to wound care and surgical intervention. The patient did not exhibit additional signs or symptoms concerning for recurrence or metastasis and was followed by dermatology and oncology until he died nearly 5 years after initial diagnosis due to complications from acute hypoxic respiratory failure secondary to COVID-19. The last imaging obtained showed no convincing evidence of metastasis, though spinal imaging within a month of his death showed lesions favored to represent benign angiomatous growths. His survival after diagnosis ultimately reached 57 months without confirmed disease recurrence and cause of death unrelated to malignancy history, which is a markedly long documented survival for this extent of disease.
Cutaneous angiosarcoma is an aggressive yet rare malignancy without effective treatments for prolonging survival or eradicating disease. Cutaneous angiosarcoma of the head and neck has a reported 10-year survival rate of 13.8%.1 Although angiosarcoma in any location holds a bleak prognosis, cutaneous angiosarcoma of the scalp with a T2 classification has a 2-year survival rate of 0%. Moreover, even if remission is achieved, disease is highly recurrent, typically within months with the current standard of care.3,21,22
Emerging evidence for the possible role of β-adrenergic receptor blockade in the treatment of malignant vascular neoplasms is promising. Microarrays from a host of vascular growths have demonstrated expression of β-adrenergic receptors in 77% of sampled angiosarcoma specimens in addition to strong expression in infantile hemangiomas, hemangiomas, hemangioendotheliomas, and vascular malformations.19 Research findings have further verified the validity of this approach with the demonstration of b1-, b2-, and b3- adrenergic receptor expression by angiosarcoma cell lines. Propranolol subsequently was shown to effectively target proliferation of these cells and induce apoptosis in a dose-dependent manner and moreover be synergistic in effect with other chemotherapies.15 Several genes have exhibited differential expression between control tumor cells and propranolol-treated cells. Specifically, target genes including AXL (a receptor tyrosine kinase associated with cell adhesion, proliferation, and apoptosis and found to upregulated in melanoma and leukemia) and ERBB receptor feedback inhibitor 1 (receptor tyrosine kinase, with ERBB family members commonly overexpressed or mutated in the setting malignancy) have been posited as possible explanatory factors in the observed angiosarcoma response to propranolol.23
Several cases describing propranolol use as an adjunctive therapy for angiosarcoma suggest a beneficial role in clinical medicine. One case report described propranolol monotherapy for lesion to our patient, with a resultant reduction in Ki-67 as a measure of proliferative index within 1 week of initiating propranolol therapy.13 Propranolol also has been shown to halt or slow progression of metastatic disease in visceral and metastatic angiosarcomas.12-14 In combination with oral etoposide and cyclophosphamide, maintenance propranolol therapy in 7 cases of advanced cutaneous angiosarcoma resulted in 1 complete response and 3 very good partial responses, with a median progression-free survival of 11 months.11 Larger-scale studies have not been published, but the growing number of case reports and case series warrants further investigation of the utility of propranolol as an adjunct to current therapies in advanced angiosarcoma.
To the Editor:
Angiosarcoma is a malignancy of the vascular endothelium that most commonly presents on the skin.1 Patients diagnosed with cutaneous angiosarcoma, which is a rare and aggressive malignancy, have a 5-year survival rate of approximately 30%.2,3 Angiosarcoma can be seen in the setting of chronic lymphedema; radiation therapy; and sporadically in elderly patients, where it is commonly seen on the head and neck. Presentation on the head and neck has been associated with worse outcomes, with a projected overall 10-year survival rate of 13.8%; the survival rate is lower if the tumor is surgically unresectable or larger in size. Metastasis can occur via both lymphatic and hematogenous routes, with pulmonary and hepatic metastases most frequently observed.1 Prognostications of poor outcomes for patients with head and neck cutaneous angiosarcoma via a 5-year survival rate were identified in a meta-analysis and included the following: patient age older than 70 years, larger tumors, tumor location of scalp vs face, nonsurgical treatments, and lack of clear margins on histology.2
Treatment of angiosarcoma historically has encompassed both surgical resection and adjuvant radiation therapy with suboptimal success. Evidence supporting various treatment regimens remains sparse due to the low incidence of the neoplasm. Although surgical resection is the only documented curative treatment, cutaneous angiosarcomas frequently are found to have positive surgical margins and require adjuvant radiation. Use of high-dose radiation (>50 Gy) with application over a wide treatment area such as total scalp irradiation is recommended.4 Although radiation has been found to diminish local recurrence rates, it has not substantially affected rates of distant disease recurrence.1 Cytotoxic chemotherapy has clinical utility in minimizing progression, but standard regimens afford a progression-free survival of only months.3 Adjuvant treatment with paclitaxel has been shown to have improved efficacy in scalp angiosarcoma vs other visceral sites, showing a nonprogression rate of 42% at 4 months after treatment.5 More recently, targeted chemotherapeutics, including the vascular endothelial growth factor inhibitor bevacizumab and tyrosine kinase inhibitor sorafenib, have shown some survival benefit, but it is unclear if these agents are superior to traditional cytotoxic agents.4,6-10 A phase 2 study of paclitaxel administered weekly with or without bevacizumab showed similar progression-free survival and overall survival, albeit at the expense of added toxicity experienced by participants in the combined group.10
The addition of the nonselective β-adrenergic blocker propranolol to the treatment armamentarium, which was pursued due to its utility in the treatment of benign infantile hemangioma and demonstrated ability to limit the expression of adrenergic receptors in angiosarcoma, has gained clinical attention for possible augmentation of cutaneous angiosarcoma therapy.11-14 Propranolol has been shown to reduce metastasis in other neoplasms—both vascular and nonvascular—and may play a role as an adjuvant treatment to current therapies in angiosarcoma.15-20 We report a patient with cutaneous angiosarcoma (T2 classification) with disease-free survival of nearly 6 years without evidence of recurrence in the setting of continuous propranolol use supplementary to chemotherapy and radiation.

A 78-year-old man with a history of multiple basal cell carcinomas, hypertension, and remote smoking history presented to the dermatology clinic with an enlarging red-brown plaque on the scalp of 2 months’ duration. The lesion had grown rapidly to involve the forehead, right temple, preauricular region, and parietal scalp. At presentation, the tumor measured more than 20 cm in diameter at its greatest point (Figure 1). Physical examination revealed a 6-mm purple nodule within the lesion on the patient’s right parietal scalp. No clinical lymphadenopathy was appreciated at the time of diagnosis. Punch biopsies of the right parietal scalp nodule and right temple patch showed findings consistent with angiosarcoma with diffuse cytoplasmic staining of CD31 in atypical endothelial cells and no staining for human herpesvirus 8 (Figure 2). Concurrent computed tomography of the head showed thickening of the right epidermis, dermis, and deeper scalp tissues, but there was no evidence of skull involvement. Computed tomography of the thorax, abdomen, and pelvis showed no evidence of metastatic disease. After a diagnostic workup, the patient was diagnosed with T2bN0M0 angiosarcoma.

The lesion was determined to be nonresectable due to the extent of the patient’s cutaneous disease. The patient was started on a regimen of paclitaxel, scalp radiation, and oral propranolol. Propranolol 40 mg twice daily was initiated at the time of diagnosis with a plan to continue indefinitely. Starting 1 month after staging, the patient completed 10 weekly cycles of paclitaxel, and he was treated with 60 Gy of scalp radiation in 30 fractions, starting with the second cycle of paclitaxel. He tolerated both well with no reported adverse events. Repeat computed tomography performed 1 month after completion of chemotherapy and radiation showed no evidence of a mass or fluid collection in subcutaneous scalp tissues and no evidence of metastatic disease. This correlated with an observed clinical regression at 1 month and complete clinical response at 5 months with residual hemosiderin and radiation changes. The area of prior disease involvement subsequently evolved from violet to dusky gray in appearance to an eventual complete resolution 26 months after diagnosis, accompanied by atrophic radiation-induced sequelae (Figure 3).

The patient’s postchemotherapy course was complicated by hospitalization for a suspected malignant pleural effusion. Analysis revealed growing ground-glass opacities and nodules in the right lower lung lobe. A thoracentesis with cytology studies was negative for malignancy. Continued monitoring over 19 months demonstrated eventual resolution of those findings. He experienced notable complication from local radiation therapy to the scalp with chronic cutaneous ulceration refractory to wound care and surgical intervention. The patient did not exhibit additional signs or symptoms concerning for recurrence or metastasis and was followed by dermatology and oncology until he died nearly 5 years after initial diagnosis due to complications from acute hypoxic respiratory failure secondary to COVID-19. The last imaging obtained showed no convincing evidence of metastasis, though spinal imaging within a month of his death showed lesions favored to represent benign angiomatous growths. His survival after diagnosis ultimately reached 57 months without confirmed disease recurrence and cause of death unrelated to malignancy history, which is a markedly long documented survival for this extent of disease.
Cutaneous angiosarcoma is an aggressive yet rare malignancy without effective treatments for prolonging survival or eradicating disease. Cutaneous angiosarcoma of the head and neck has a reported 10-year survival rate of 13.8%.1 Although angiosarcoma in any location holds a bleak prognosis, cutaneous angiosarcoma of the scalp with a T2 classification has a 2-year survival rate of 0%. Moreover, even if remission is achieved, disease is highly recurrent, typically within months with the current standard of care.3,21,22
Emerging evidence for the possible role of β-adrenergic receptor blockade in the treatment of malignant vascular neoplasms is promising. Microarrays from a host of vascular growths have demonstrated expression of β-adrenergic receptors in 77% of sampled angiosarcoma specimens in addition to strong expression in infantile hemangiomas, hemangiomas, hemangioendotheliomas, and vascular malformations.19 Research findings have further verified the validity of this approach with the demonstration of b1-, b2-, and b3- adrenergic receptor expression by angiosarcoma cell lines. Propranolol subsequently was shown to effectively target proliferation of these cells and induce apoptosis in a dose-dependent manner and moreover be synergistic in effect with other chemotherapies.15 Several genes have exhibited differential expression between control tumor cells and propranolol-treated cells. Specifically, target genes including AXL (a receptor tyrosine kinase associated with cell adhesion, proliferation, and apoptosis and found to upregulated in melanoma and leukemia) and ERBB receptor feedback inhibitor 1 (receptor tyrosine kinase, with ERBB family members commonly overexpressed or mutated in the setting malignancy) have been posited as possible explanatory factors in the observed angiosarcoma response to propranolol.23
Several cases describing propranolol use as an adjunctive therapy for angiosarcoma suggest a beneficial role in clinical medicine. One case report described propranolol monotherapy for lesion to our patient, with a resultant reduction in Ki-67 as a measure of proliferative index within 1 week of initiating propranolol therapy.13 Propranolol also has been shown to halt or slow progression of metastatic disease in visceral and metastatic angiosarcomas.12-14 In combination with oral etoposide and cyclophosphamide, maintenance propranolol therapy in 7 cases of advanced cutaneous angiosarcoma resulted in 1 complete response and 3 very good partial responses, with a median progression-free survival of 11 months.11 Larger-scale studies have not been published, but the growing number of case reports and case series warrants further investigation of the utility of propranolol as an adjunct to current therapies in advanced angiosarcoma.
- Abraham JA, Hornicek FJ, Kaufman AM, et al. Treatment and outcome of 82 patients with angiosarcoma. Ann Surg Oncol. 2007;14:1953-1967.
- Shin JY, Roh SG, Lee NH, et al. Predisposing factors for poor prognosis of angiosarcoma of the scalp and face: systematic review and meta-analysis. Head Neck. 2017;39:380-386.
- Fury MG, Antonescu CR, Zee KJV, et al. A 14-year retrospective review of angiosarcoma: clinical characteristics, prognostic factors, and treatment outcomes with surgery and chemotherapy. Cancer. 2005;11:241-247.
- Dossett LA, Harrington M, Cruse CW, et al. Cutaneous angiosarcoma. Curr Probl Cancer. 2015;39:258-263.
- Penel N, Bui BN, Bay JO, et al. Phase II trial of weekly paclitaxel for unresectable angiosarcoma: the ANGIOTAX study. J Clin Oncol. 2008;26:5269-5274.
- Agulnik M, Yarber JL, Okuno SH, et al. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol. 2013;24:257-263.
- Maki RG, D’Adamo DR, Keohan ML, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol. 2009;27:3133-3140.
- Ishida Y, Otsuka A, Kabashima K. Cutaneous angiosarcoma: update on biology and latest treatment. Curr Opin Oncol. 2018;30:107-112.
- Ray-Coquard I, Italiano A, Bompas E, et al. Sorafenib for patients with advanced angiosarcoma: a phase II trial from the French Sarcoma Group (GSF/GETO). Oncologist. 2012;17:260-266.
- Ray-Coquard IL, Domont J, Tresch-Bruneel E, et al. Paclitaxel given once per week with or without bevacizumab in patients with advanced angiosarcoma: a randomized phase II trial. J Clin Oncol. 2015;33:2797-2802.
- Pasquier E, Andre N, Street J, et al. Effective management of advanced angiosarcoma by the synergistic combination of propranolol and vinblastine-based metronomic chemotherapy: a bench to bedside study. EBioMedicine. 2016;6:87-95.
- Banavali S, Pasquier E, Andre N. Targeted therapy with propranolol and metronomic chemotherapy combination: sustained complete response of a relapsing metastatic angiosarcoma. Ecancermedicalscience. 2015;9:499.
- Chow W, Amaya CN, Rains S, et al. Growth attenuation of cutaneous angiosarcoma with propranolol-mediated beta-blockade. JAMA Dermatol. 2015;151:1226-1229.
- Daguze J, Saint-Jean M, Peuvrel L, et al. Visceral metastatic angiosarcoma treated effectively with oral cyclophosphamide combined with propranolol. JAAD Case Rep. 2016;2:497-499.
- Stiles JM, Amaya C, Rains S, et al. Targeting of beta adrenergic receptors results in therapeutic efficacy against models of hemangioendothelioma and angiosarcoma. PLoS One. 2013;8:e60021.
- Chang PY, Chung CH, Chang WC, et al. The effect of propranolol on the prognosis of hepatocellular carcinoma: a nationwide population-based study. PLoS One. 2019;14:e0216828.
- De Giorgi V, Grazzini M, Benemei S, et al. Propranolol for off-label treatment of patients with melanoma: results from a cohort study. JAMA Oncol. 2018;4:e172908.
- Rico M, Baglioni M, Bondarenko M, et al. Metformin and propranolol combination prevents cancer progression and metastasis in different breast cancer models. Oncotarget. 2017;8:2874-2889.
- Chisholm KM, Chang KW, Truong MT, et al. β-Adrenergic receptor expression in vascular tumors. Mod Pathol. 2012;25:1446-1451.
- Leaute-Labreze C, Dumas de la Roque E, Hubiche T, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649-2651.
- Maddox JC, Evans HL. Angiosarcoma of skin and soft tissue: a study of forty-four cases. Cancer. 1981;48:1907-1921.
- Morgan MB, Swann M, Somach S, et al. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004;50:867-874.
- Zhou S, Liu P, Jiang W, et al. Identification of potential target genes associated with the effect of propranolol on angiosarcoma via microarray analysis. Oncol Lett. 2017;13:4267-4275.
- Abraham JA, Hornicek FJ, Kaufman AM, et al. Treatment and outcome of 82 patients with angiosarcoma. Ann Surg Oncol. 2007;14:1953-1967.
- Shin JY, Roh SG, Lee NH, et al. Predisposing factors for poor prognosis of angiosarcoma of the scalp and face: systematic review and meta-analysis. Head Neck. 2017;39:380-386.
- Fury MG, Antonescu CR, Zee KJV, et al. A 14-year retrospective review of angiosarcoma: clinical characteristics, prognostic factors, and treatment outcomes with surgery and chemotherapy. Cancer. 2005;11:241-247.
- Dossett LA, Harrington M, Cruse CW, et al. Cutaneous angiosarcoma. Curr Probl Cancer. 2015;39:258-263.
- Penel N, Bui BN, Bay JO, et al. Phase II trial of weekly paclitaxel for unresectable angiosarcoma: the ANGIOTAX study. J Clin Oncol. 2008;26:5269-5274.
- Agulnik M, Yarber JL, Okuno SH, et al. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol. 2013;24:257-263.
- Maki RG, D’Adamo DR, Keohan ML, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol. 2009;27:3133-3140.
- Ishida Y, Otsuka A, Kabashima K. Cutaneous angiosarcoma: update on biology and latest treatment. Curr Opin Oncol. 2018;30:107-112.
- Ray-Coquard I, Italiano A, Bompas E, et al. Sorafenib for patients with advanced angiosarcoma: a phase II trial from the French Sarcoma Group (GSF/GETO). Oncologist. 2012;17:260-266.
- Ray-Coquard IL, Domont J, Tresch-Bruneel E, et al. Paclitaxel given once per week with or without bevacizumab in patients with advanced angiosarcoma: a randomized phase II trial. J Clin Oncol. 2015;33:2797-2802.
- Pasquier E, Andre N, Street J, et al. Effective management of advanced angiosarcoma by the synergistic combination of propranolol and vinblastine-based metronomic chemotherapy: a bench to bedside study. EBioMedicine. 2016;6:87-95.
- Banavali S, Pasquier E, Andre N. Targeted therapy with propranolol and metronomic chemotherapy combination: sustained complete response of a relapsing metastatic angiosarcoma. Ecancermedicalscience. 2015;9:499.
- Chow W, Amaya CN, Rains S, et al. Growth attenuation of cutaneous angiosarcoma with propranolol-mediated beta-blockade. JAMA Dermatol. 2015;151:1226-1229.
- Daguze J, Saint-Jean M, Peuvrel L, et al. Visceral metastatic angiosarcoma treated effectively with oral cyclophosphamide combined with propranolol. JAAD Case Rep. 2016;2:497-499.
- Stiles JM, Amaya C, Rains S, et al. Targeting of beta adrenergic receptors results in therapeutic efficacy against models of hemangioendothelioma and angiosarcoma. PLoS One. 2013;8:e60021.
- Chang PY, Chung CH, Chang WC, et al. The effect of propranolol on the prognosis of hepatocellular carcinoma: a nationwide population-based study. PLoS One. 2019;14:e0216828.
- De Giorgi V, Grazzini M, Benemei S, et al. Propranolol for off-label treatment of patients with melanoma: results from a cohort study. JAMA Oncol. 2018;4:e172908.
- Rico M, Baglioni M, Bondarenko M, et al. Metformin and propranolol combination prevents cancer progression and metastasis in different breast cancer models. Oncotarget. 2017;8:2874-2889.
- Chisholm KM, Chang KW, Truong MT, et al. β-Adrenergic receptor expression in vascular tumors. Mod Pathol. 2012;25:1446-1451.
- Leaute-Labreze C, Dumas de la Roque E, Hubiche T, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649-2651.
- Maddox JC, Evans HL. Angiosarcoma of skin and soft tissue: a study of forty-four cases. Cancer. 1981;48:1907-1921.
- Morgan MB, Swann M, Somach S, et al. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004;50:867-874.
- Zhou S, Liu P, Jiang W, et al. Identification of potential target genes associated with the effect of propranolol on angiosarcoma via microarray analysis. Oncol Lett. 2017;13:4267-4275.
PRACTICE POINTS
- In one classic presentation, cutaneous angiosarcoma characteristically appears as a bruiselike patch on the head and neck of an elderly gentleman.
- Although cutaneous angiosarcoma typically portends a poor prognosis at the time of diagnosis, adjunctive oral propranolol may be a promising and relatively benign therapy, posited to afford benefit in a manner similar to its efficacy in the treatment of infantile hemangiomas.
Use of Dupilumab in Severe, Multifactorial, Chronic Itch for Geriatric Patients
To the Editor:
Today’s geriatric population is the fastest growing in history. The National Institutes of Health predicts there will be over 1.5 billion individuals aged 65 years and older by the year 2050: 17% of the world’s population.1 Pruritus—either acute or chronic (>6 weeks)—is defined as a sensory perception that leads to an intense desire to scratch.2 Chronic pruritus is an increasing health concern that impacts quality of life within the geriatric population. Elderly patients have various risk factors for developing chronic itch, including aging skin, polypharmacy, and increased systemic comorbidities.3-7
Although the therapeutic armamentarium for chronic itch continues to grow, health care providers often are hesitant to prescribe medications for geriatric patients because of comorbidities and potential drug-drug interactions. Novel biologic therapies now provide alternatives for this complex population. Dupilumab is a fully humanized, monoclonal antibody approved for treatment-resistant atopic dermatitis. This biologic prevents helper T-cell (TH2) signaling, IL-4 and IL-13 release, and subsequent effector cell (eg, mast cell, eosinophil) activity.8-10 The combined efficacy and safety of this medication has changed the treatment landscape of resistant atopic dermatitis. We present the use of dupilumab in a geriatric patient with severe and recalcitrant itch resistant to numerous topical and oral medications.
An 81-year-old man presented to the clinic with a long history of generalized pruritic rash. His medical history was significant for insulin-dependent type 2 diabetes mellitus (T2DM), hypertension, and renal cancer following a right nephrectomy. Laboratory results approximately 14 months prior to the visit revealed a blood urea nitrogen level of 31 mg/dL (reference range, 7–20 mg/dL), creatinine level of 2.20 mg/dL (reference range, 0.7–1.3 mg/dL), and glomerular filtration rate of 29 mL/min (reference range, 90–120 mL/min). Physical examination revealed numerous pink excoriated papules on the face, neck, trunk, and extremities. Lichenified plaques were present on both arms and legs. The patient received the diagnosis of severe atopic dermatitis with greater than 10% body surface area involvement. The investigator global assessment score was 4/4, indicating severe disease burden, and biopsy results reported spongiotic dermatitis. He proceeded to trial various topical corticosteroids, including hydrocortisone ointment 2.5%, betamethasone valerate ointment 0.01%, fluocinonide ointment 0.05%, and mupirocin ointment without benefit. Three subsequent courses of oral steroids failed to provide durable relief. At this point, the peak pruritus numerical rating scale (NRS) score was 7/10, indicating severe pruritus, with a negative impact on the patient’s quality of life and sleep.
Therapy was switched to tacrolimus acetonide ointment 0.1%, betamethasone dipropionate ointment 0.05%, and triamcinolone acetonide ointment 0.1%. Eleven days later, the patient denied experiencing any response to the topical regimen and sought alternative therapy for the itch and associated poor sleep; the NRS score was 10/10, indicating very severe pruritus. Prednisone 20 mg and doxepin 10 mg were initiated for symptom management until the intended transition to dupilumab. The patient began dupilumab with a loading dose of 600 mg, then 300 mg every other week thereafter. At 2- and 4-month follow-up, the patient reported notable relief in symptoms. The rash had improved, and the NRS score decreased from 10/10 to 3/10. He endorsed improved sleep and quality of life.
Pruritus may arise from a series of age-related mechanisms such as structural and chemical changes within the epidermis, underlying neuropathy, medication side effects, infection, malignancy, thyroid dysregulation, liver disease, and chronic kidney disease (CKD).5,6,11 Identifying the underlying etiology often is difficult and involves a complete history and physical examination as well as an appropriate contextualized laboratory workup.
Our patient’s comorbid T2DM and renal disease may have contributed to the pruritus. Type 2 diabetes mellitus can cause diabetic neuropathy, a sequela known to lead to various complications, including pruritus. One study identified a 4-fold increase in pruritus in those with diabetic polyneuropathy compared with age-matched nondiabetics.12,13 An additional study found that pruritus was present in 70% of patients with small fiber neuropathy.14 We needed to consider the role of our patient’s insulin-dependent T2DM and potential underlying neuropathy when addressing the pruritic symptoms.
Furthermore, our patient’s stage IV CKD and elevated urea level also may factor into the pruritus. The pathophysiology of CKD-associated pruritus (also referred to as uremic pruritus) remains poorly understood. Suggested mechanisms include immune-mediated neural inflammation and erroneous nociceptive-receptor activity.15,16 Although uremic pruritus is appreciated primarily in late dialysis-dependent disease, research shows that a notable portion of those with lesser disease, similar to our patient, also experience a significant itch burden.17 Diminishing pruritus is difficult and often aided by management of the underlying renal disease.18
In addition to disease management, symptomatic treatment incorporates the use of emollients, corticosteroids, and antihistamines. Unfortunately, the clinical response in the elderly population to such regimens often is poor.19 Dupilumab is an optimistic therapeutic option for chronic pruritus. By inhibiting the IL-4α receptor found on helper T cells, this biologic inhibits TH2 differentiation and subsequent inflammatory activity. One report identified an optimistic response to dupilumab in the management of uremic pruritus.20 The remarkable improvement and absence of adverse effects in our patient confirmed the utility and safety of dupilumab in complex cases such as elderly patients with multiple comorbidities. Such relief may result from inhibition of proinflammatory cytokine activity as well as decreased afferent spinal cord itch stimuli.10 The positive results from this case cast a favorable outlook on the treatment of chronic itch in the complex geriatric population.
- World’s older population grows dramatically. News release. National Institute on Aging. Published March 28, 2016. Accessed December 23, 2022. http://www.nih.gov/news-events/news-releases/worlds-older-population-grows-dramatically
- Grundmann S, Ständer S. Chronic pruritus: clinics and treatment. Ann Dermatol. 2011;23:1-11.
- Berger TG, Shive M, Harper GM. Pruritus in the older patient: a clinical review. JAMA. 2013;310:2443-2450. doi:10.1001/jama.2013.282023
- Valdes-Rodriguez, R, Mollanazar NK, González-Muro J, et al. Itch prevalence and characteristics in a Hispanic geriatric population: a comprehensive study using a standardized itch questionnaire. Acta Derm Venereol. 2015;95:417-421. doi:10.2340/00015555-1968
- Li J, Tang H, Hu X, et al. Aquaporin-3 gene and protein expression in sun-protected human skin decreases with skin ageing. Australas J Dermatol. 2010;51:106-112.
- Choi EH, Man MQ, Xu P, et al. Stratum corneum acidification is impaired in moderately aged human and murine skin. J Invest Dermatol. 2007;127:2847-2856.
- Fenske NA, Lober CW. Structural and functional changes of normal aging skin. J Am Acad Dermatol. 1986;15(4 pt 1):571-585.
- Paller AS, Kabashima K, Bieber T. Therapeutic pipeline for atopic dermatitis: end of the drought? J Allergy Clin Immunol. 2017;140:633-643. doi:10.1016/j.jaci.2017.07.006
- Kabashima K. New concept of the pathogenesis of atopic dermatitis: interplay among the barrier, allergy, and pruritus as a trinity. J Dermatol Sci. 2013;70:3-11.
- Feld M, Garcia R, Buddenkotte J, et al. The pruritus- and TH2-associated cytokine IL-31 promotes growth of sensory nerves. J Allergy Clin Immunol. 2016;138:500-508.
- Valdes-Rodriguez R, Stull C, Yosipovitch G. Chronic pruritus in the elderly: pathophysiology, diagnosis and management. Drugs Aging. 2015;32:201-215. doi:10.1007/s40266-015-0246-0
- Misery L, Brenaut E, Le Garrec R, et al. Neuropathic pruritus. Nat Rev Neurol. 2014;10:408-416.
- Yamaoka H, Sasaki H, Yamasaki H, et al. Truncal pruritus of unknown origin may be a symptom of diabetic polyneuropathy. Diabetes Care. 2010;33:150-155.
- Brenaut E, Marcorelles P, Genestet S, et al. Pruritus: an underrecognized symptom of small-fiber neuropathies. J Am Acad Dermatol. 2015;72:328-332.
- Adigun M, Badu LA, Berner NM, et al. Uremic pruritus review. US Pharm. 2015;40:HS12-HS15.
- Simonsen E, Komenda P, Lerner B, et al. Treatment of uremic pruritus: a systematic review. Am J Kidney Dis. 2017;70:638-655.
- Carstens E, Akiyama T, eds. Itch: Mechanisms and Treatment. CRC Press/Taylor & Francis; 2014.
- Shirazian S, Aina O, Park Y, et al. Chronic kidney disease-associated pruritus: impact on quality of life and current management challenges. Int J Nephrol Renovasc Dis. 2017;10:11-26.
- Brummer GC, Wang LT, Sontheimer RD. A possible role for dupilumab (Dupixent) in the management of idiopathic chronic eczematous eruption of aging. Dermatol Online J. 2018;24:13030/qt55z1f6xh.
- Silverberg JI, Brieva J. A successful case of dupilumab treatment for severe uremic pruritus. JAAD Case Rep. 2019;5:339-341.
To the Editor:
Today’s geriatric population is the fastest growing in history. The National Institutes of Health predicts there will be over 1.5 billion individuals aged 65 years and older by the year 2050: 17% of the world’s population.1 Pruritus—either acute or chronic (>6 weeks)—is defined as a sensory perception that leads to an intense desire to scratch.2 Chronic pruritus is an increasing health concern that impacts quality of life within the geriatric population. Elderly patients have various risk factors for developing chronic itch, including aging skin, polypharmacy, and increased systemic comorbidities.3-7
Although the therapeutic armamentarium for chronic itch continues to grow, health care providers often are hesitant to prescribe medications for geriatric patients because of comorbidities and potential drug-drug interactions. Novel biologic therapies now provide alternatives for this complex population. Dupilumab is a fully humanized, monoclonal antibody approved for treatment-resistant atopic dermatitis. This biologic prevents helper T-cell (TH2) signaling, IL-4 and IL-13 release, and subsequent effector cell (eg, mast cell, eosinophil) activity.8-10 The combined efficacy and safety of this medication has changed the treatment landscape of resistant atopic dermatitis. We present the use of dupilumab in a geriatric patient with severe and recalcitrant itch resistant to numerous topical and oral medications.
An 81-year-old man presented to the clinic with a long history of generalized pruritic rash. His medical history was significant for insulin-dependent type 2 diabetes mellitus (T2DM), hypertension, and renal cancer following a right nephrectomy. Laboratory results approximately 14 months prior to the visit revealed a blood urea nitrogen level of 31 mg/dL (reference range, 7–20 mg/dL), creatinine level of 2.20 mg/dL (reference range, 0.7–1.3 mg/dL), and glomerular filtration rate of 29 mL/min (reference range, 90–120 mL/min). Physical examination revealed numerous pink excoriated papules on the face, neck, trunk, and extremities. Lichenified plaques were present on both arms and legs. The patient received the diagnosis of severe atopic dermatitis with greater than 10% body surface area involvement. The investigator global assessment score was 4/4, indicating severe disease burden, and biopsy results reported spongiotic dermatitis. He proceeded to trial various topical corticosteroids, including hydrocortisone ointment 2.5%, betamethasone valerate ointment 0.01%, fluocinonide ointment 0.05%, and mupirocin ointment without benefit. Three subsequent courses of oral steroids failed to provide durable relief. At this point, the peak pruritus numerical rating scale (NRS) score was 7/10, indicating severe pruritus, with a negative impact on the patient’s quality of life and sleep.
Therapy was switched to tacrolimus acetonide ointment 0.1%, betamethasone dipropionate ointment 0.05%, and triamcinolone acetonide ointment 0.1%. Eleven days later, the patient denied experiencing any response to the topical regimen and sought alternative therapy for the itch and associated poor sleep; the NRS score was 10/10, indicating very severe pruritus. Prednisone 20 mg and doxepin 10 mg were initiated for symptom management until the intended transition to dupilumab. The patient began dupilumab with a loading dose of 600 mg, then 300 mg every other week thereafter. At 2- and 4-month follow-up, the patient reported notable relief in symptoms. The rash had improved, and the NRS score decreased from 10/10 to 3/10. He endorsed improved sleep and quality of life.
Pruritus may arise from a series of age-related mechanisms such as structural and chemical changes within the epidermis, underlying neuropathy, medication side effects, infection, malignancy, thyroid dysregulation, liver disease, and chronic kidney disease (CKD).5,6,11 Identifying the underlying etiology often is difficult and involves a complete history and physical examination as well as an appropriate contextualized laboratory workup.
Our patient’s comorbid T2DM and renal disease may have contributed to the pruritus. Type 2 diabetes mellitus can cause diabetic neuropathy, a sequela known to lead to various complications, including pruritus. One study identified a 4-fold increase in pruritus in those with diabetic polyneuropathy compared with age-matched nondiabetics.12,13 An additional study found that pruritus was present in 70% of patients with small fiber neuropathy.14 We needed to consider the role of our patient’s insulin-dependent T2DM and potential underlying neuropathy when addressing the pruritic symptoms.
Furthermore, our patient’s stage IV CKD and elevated urea level also may factor into the pruritus. The pathophysiology of CKD-associated pruritus (also referred to as uremic pruritus) remains poorly understood. Suggested mechanisms include immune-mediated neural inflammation and erroneous nociceptive-receptor activity.15,16 Although uremic pruritus is appreciated primarily in late dialysis-dependent disease, research shows that a notable portion of those with lesser disease, similar to our patient, also experience a significant itch burden.17 Diminishing pruritus is difficult and often aided by management of the underlying renal disease.18
In addition to disease management, symptomatic treatment incorporates the use of emollients, corticosteroids, and antihistamines. Unfortunately, the clinical response in the elderly population to such regimens often is poor.19 Dupilumab is an optimistic therapeutic option for chronic pruritus. By inhibiting the IL-4α receptor found on helper T cells, this biologic inhibits TH2 differentiation and subsequent inflammatory activity. One report identified an optimistic response to dupilumab in the management of uremic pruritus.20 The remarkable improvement and absence of adverse effects in our patient confirmed the utility and safety of dupilumab in complex cases such as elderly patients with multiple comorbidities. Such relief may result from inhibition of proinflammatory cytokine activity as well as decreased afferent spinal cord itch stimuli.10 The positive results from this case cast a favorable outlook on the treatment of chronic itch in the complex geriatric population.
To the Editor:
Today’s geriatric population is the fastest growing in history. The National Institutes of Health predicts there will be over 1.5 billion individuals aged 65 years and older by the year 2050: 17% of the world’s population.1 Pruritus—either acute or chronic (>6 weeks)—is defined as a sensory perception that leads to an intense desire to scratch.2 Chronic pruritus is an increasing health concern that impacts quality of life within the geriatric population. Elderly patients have various risk factors for developing chronic itch, including aging skin, polypharmacy, and increased systemic comorbidities.3-7
Although the therapeutic armamentarium for chronic itch continues to grow, health care providers often are hesitant to prescribe medications for geriatric patients because of comorbidities and potential drug-drug interactions. Novel biologic therapies now provide alternatives for this complex population. Dupilumab is a fully humanized, monoclonal antibody approved for treatment-resistant atopic dermatitis. This biologic prevents helper T-cell (TH2) signaling, IL-4 and IL-13 release, and subsequent effector cell (eg, mast cell, eosinophil) activity.8-10 The combined efficacy and safety of this medication has changed the treatment landscape of resistant atopic dermatitis. We present the use of dupilumab in a geriatric patient with severe and recalcitrant itch resistant to numerous topical and oral medications.
An 81-year-old man presented to the clinic with a long history of generalized pruritic rash. His medical history was significant for insulin-dependent type 2 diabetes mellitus (T2DM), hypertension, and renal cancer following a right nephrectomy. Laboratory results approximately 14 months prior to the visit revealed a blood urea nitrogen level of 31 mg/dL (reference range, 7–20 mg/dL), creatinine level of 2.20 mg/dL (reference range, 0.7–1.3 mg/dL), and glomerular filtration rate of 29 mL/min (reference range, 90–120 mL/min). Physical examination revealed numerous pink excoriated papules on the face, neck, trunk, and extremities. Lichenified plaques were present on both arms and legs. The patient received the diagnosis of severe atopic dermatitis with greater than 10% body surface area involvement. The investigator global assessment score was 4/4, indicating severe disease burden, and biopsy results reported spongiotic dermatitis. He proceeded to trial various topical corticosteroids, including hydrocortisone ointment 2.5%, betamethasone valerate ointment 0.01%, fluocinonide ointment 0.05%, and mupirocin ointment without benefit. Three subsequent courses of oral steroids failed to provide durable relief. At this point, the peak pruritus numerical rating scale (NRS) score was 7/10, indicating severe pruritus, with a negative impact on the patient’s quality of life and sleep.
Therapy was switched to tacrolimus acetonide ointment 0.1%, betamethasone dipropionate ointment 0.05%, and triamcinolone acetonide ointment 0.1%. Eleven days later, the patient denied experiencing any response to the topical regimen and sought alternative therapy for the itch and associated poor sleep; the NRS score was 10/10, indicating very severe pruritus. Prednisone 20 mg and doxepin 10 mg were initiated for symptom management until the intended transition to dupilumab. The patient began dupilumab with a loading dose of 600 mg, then 300 mg every other week thereafter. At 2- and 4-month follow-up, the patient reported notable relief in symptoms. The rash had improved, and the NRS score decreased from 10/10 to 3/10. He endorsed improved sleep and quality of life.
Pruritus may arise from a series of age-related mechanisms such as structural and chemical changes within the epidermis, underlying neuropathy, medication side effects, infection, malignancy, thyroid dysregulation, liver disease, and chronic kidney disease (CKD).5,6,11 Identifying the underlying etiology often is difficult and involves a complete history and physical examination as well as an appropriate contextualized laboratory workup.
Our patient’s comorbid T2DM and renal disease may have contributed to the pruritus. Type 2 diabetes mellitus can cause diabetic neuropathy, a sequela known to lead to various complications, including pruritus. One study identified a 4-fold increase in pruritus in those with diabetic polyneuropathy compared with age-matched nondiabetics.12,13 An additional study found that pruritus was present in 70% of patients with small fiber neuropathy.14 We needed to consider the role of our patient’s insulin-dependent T2DM and potential underlying neuropathy when addressing the pruritic symptoms.
Furthermore, our patient’s stage IV CKD and elevated urea level also may factor into the pruritus. The pathophysiology of CKD-associated pruritus (also referred to as uremic pruritus) remains poorly understood. Suggested mechanisms include immune-mediated neural inflammation and erroneous nociceptive-receptor activity.15,16 Although uremic pruritus is appreciated primarily in late dialysis-dependent disease, research shows that a notable portion of those with lesser disease, similar to our patient, also experience a significant itch burden.17 Diminishing pruritus is difficult and often aided by management of the underlying renal disease.18
In addition to disease management, symptomatic treatment incorporates the use of emollients, corticosteroids, and antihistamines. Unfortunately, the clinical response in the elderly population to such regimens often is poor.19 Dupilumab is an optimistic therapeutic option for chronic pruritus. By inhibiting the IL-4α receptor found on helper T cells, this biologic inhibits TH2 differentiation and subsequent inflammatory activity. One report identified an optimistic response to dupilumab in the management of uremic pruritus.20 The remarkable improvement and absence of adverse effects in our patient confirmed the utility and safety of dupilumab in complex cases such as elderly patients with multiple comorbidities. Such relief may result from inhibition of proinflammatory cytokine activity as well as decreased afferent spinal cord itch stimuli.10 The positive results from this case cast a favorable outlook on the treatment of chronic itch in the complex geriatric population.
- World’s older population grows dramatically. News release. National Institute on Aging. Published March 28, 2016. Accessed December 23, 2022. http://www.nih.gov/news-events/news-releases/worlds-older-population-grows-dramatically
- Grundmann S, Ständer S. Chronic pruritus: clinics and treatment. Ann Dermatol. 2011;23:1-11.
- Berger TG, Shive M, Harper GM. Pruritus in the older patient: a clinical review. JAMA. 2013;310:2443-2450. doi:10.1001/jama.2013.282023
- Valdes-Rodriguez, R, Mollanazar NK, González-Muro J, et al. Itch prevalence and characteristics in a Hispanic geriatric population: a comprehensive study using a standardized itch questionnaire. Acta Derm Venereol. 2015;95:417-421. doi:10.2340/00015555-1968
- Li J, Tang H, Hu X, et al. Aquaporin-3 gene and protein expression in sun-protected human skin decreases with skin ageing. Australas J Dermatol. 2010;51:106-112.
- Choi EH, Man MQ, Xu P, et al. Stratum corneum acidification is impaired in moderately aged human and murine skin. J Invest Dermatol. 2007;127:2847-2856.
- Fenske NA, Lober CW. Structural and functional changes of normal aging skin. J Am Acad Dermatol. 1986;15(4 pt 1):571-585.
- Paller AS, Kabashima K, Bieber T. Therapeutic pipeline for atopic dermatitis: end of the drought? J Allergy Clin Immunol. 2017;140:633-643. doi:10.1016/j.jaci.2017.07.006
- Kabashima K. New concept of the pathogenesis of atopic dermatitis: interplay among the barrier, allergy, and pruritus as a trinity. J Dermatol Sci. 2013;70:3-11.
- Feld M, Garcia R, Buddenkotte J, et al. The pruritus- and TH2-associated cytokine IL-31 promotes growth of sensory nerves. J Allergy Clin Immunol. 2016;138:500-508.
- Valdes-Rodriguez R, Stull C, Yosipovitch G. Chronic pruritus in the elderly: pathophysiology, diagnosis and management. Drugs Aging. 2015;32:201-215. doi:10.1007/s40266-015-0246-0
- Misery L, Brenaut E, Le Garrec R, et al. Neuropathic pruritus. Nat Rev Neurol. 2014;10:408-416.
- Yamaoka H, Sasaki H, Yamasaki H, et al. Truncal pruritus of unknown origin may be a symptom of diabetic polyneuropathy. Diabetes Care. 2010;33:150-155.
- Brenaut E, Marcorelles P, Genestet S, et al. Pruritus: an underrecognized symptom of small-fiber neuropathies. J Am Acad Dermatol. 2015;72:328-332.
- Adigun M, Badu LA, Berner NM, et al. Uremic pruritus review. US Pharm. 2015;40:HS12-HS15.
- Simonsen E, Komenda P, Lerner B, et al. Treatment of uremic pruritus: a systematic review. Am J Kidney Dis. 2017;70:638-655.
- Carstens E, Akiyama T, eds. Itch: Mechanisms and Treatment. CRC Press/Taylor & Francis; 2014.
- Shirazian S, Aina O, Park Y, et al. Chronic kidney disease-associated pruritus: impact on quality of life and current management challenges. Int J Nephrol Renovasc Dis. 2017;10:11-26.
- Brummer GC, Wang LT, Sontheimer RD. A possible role for dupilumab (Dupixent) in the management of idiopathic chronic eczematous eruption of aging. Dermatol Online J. 2018;24:13030/qt55z1f6xh.
- Silverberg JI, Brieva J. A successful case of dupilumab treatment for severe uremic pruritus. JAAD Case Rep. 2019;5:339-341.
- World’s older population grows dramatically. News release. National Institute on Aging. Published March 28, 2016. Accessed December 23, 2022. http://www.nih.gov/news-events/news-releases/worlds-older-population-grows-dramatically
- Grundmann S, Ständer S. Chronic pruritus: clinics and treatment. Ann Dermatol. 2011;23:1-11.
- Berger TG, Shive M, Harper GM. Pruritus in the older patient: a clinical review. JAMA. 2013;310:2443-2450. doi:10.1001/jama.2013.282023
- Valdes-Rodriguez, R, Mollanazar NK, González-Muro J, et al. Itch prevalence and characteristics in a Hispanic geriatric population: a comprehensive study using a standardized itch questionnaire. Acta Derm Venereol. 2015;95:417-421. doi:10.2340/00015555-1968
- Li J, Tang H, Hu X, et al. Aquaporin-3 gene and protein expression in sun-protected human skin decreases with skin ageing. Australas J Dermatol. 2010;51:106-112.
- Choi EH, Man MQ, Xu P, et al. Stratum corneum acidification is impaired in moderately aged human and murine skin. J Invest Dermatol. 2007;127:2847-2856.
- Fenske NA, Lober CW. Structural and functional changes of normal aging skin. J Am Acad Dermatol. 1986;15(4 pt 1):571-585.
- Paller AS, Kabashima K, Bieber T. Therapeutic pipeline for atopic dermatitis: end of the drought? J Allergy Clin Immunol. 2017;140:633-643. doi:10.1016/j.jaci.2017.07.006
- Kabashima K. New concept of the pathogenesis of atopic dermatitis: interplay among the barrier, allergy, and pruritus as a trinity. J Dermatol Sci. 2013;70:3-11.
- Feld M, Garcia R, Buddenkotte J, et al. The pruritus- and TH2-associated cytokine IL-31 promotes growth of sensory nerves. J Allergy Clin Immunol. 2016;138:500-508.
- Valdes-Rodriguez R, Stull C, Yosipovitch G. Chronic pruritus in the elderly: pathophysiology, diagnosis and management. Drugs Aging. 2015;32:201-215. doi:10.1007/s40266-015-0246-0
- Misery L, Brenaut E, Le Garrec R, et al. Neuropathic pruritus. Nat Rev Neurol. 2014;10:408-416.
- Yamaoka H, Sasaki H, Yamasaki H, et al. Truncal pruritus of unknown origin may be a symptom of diabetic polyneuropathy. Diabetes Care. 2010;33:150-155.
- Brenaut E, Marcorelles P, Genestet S, et al. Pruritus: an underrecognized symptom of small-fiber neuropathies. J Am Acad Dermatol. 2015;72:328-332.
- Adigun M, Badu LA, Berner NM, et al. Uremic pruritus review. US Pharm. 2015;40:HS12-HS15.
- Simonsen E, Komenda P, Lerner B, et al. Treatment of uremic pruritus: a systematic review. Am J Kidney Dis. 2017;70:638-655.
- Carstens E, Akiyama T, eds. Itch: Mechanisms and Treatment. CRC Press/Taylor & Francis; 2014.
- Shirazian S, Aina O, Park Y, et al. Chronic kidney disease-associated pruritus: impact on quality of life and current management challenges. Int J Nephrol Renovasc Dis. 2017;10:11-26.
- Brummer GC, Wang LT, Sontheimer RD. A possible role for dupilumab (Dupixent) in the management of idiopathic chronic eczematous eruption of aging. Dermatol Online J. 2018;24:13030/qt55z1f6xh.
- Silverberg JI, Brieva J. A successful case of dupilumab treatment for severe uremic pruritus. JAAD Case Rep. 2019;5:339-341.
PRACTICE POINTS
- A series of age-related mechanisms within the epidermis, underlying neuropathy, medication side effects, infection, malignancy, thyroid dysregulation, liver disease, and chronic kidney disease may contribute to pruritus in elderly patients.
- Patients with mild kidney disease may still experience a recalcitrant and notable itch burden.
- Dupilumab is efficacious and safe in the management of chronic pruritus, even in complex cases such as elderly patients with multiple comorbidities.
Year in Review: How Targeted Drug Therapies Have Expanded Breast Cancer Treatment Options in 2022
Breast cancer (BC) is the most diagnosed cancer and the second leading cause of cancer deaths in women. In 2022, more than 275,000 women were diagnosed with BC, and at least 43,000 of these cases resulted in death. With targeted drug therapy for treating BC now approved by the US Food and Drug Administration (FDA) and available (or in the late phases of clinical trials and pending availability and FDA approval), clinicians are beginning to be able to move away from a “one-size-fits-all” treatment approach that has been used in the past, enhancing prognosis and survival rates in their patients living with BC.
The new targeted drug therapies available are more precise and individualized. They treat patients more effectively because they are based on the patient’s own biology. These therapies open the possibility of having more valuable treatment options, which can be beneficial for the outcome of many patients diagnosed with BC—especially the highly aggressive forms that were previously difficult to treat.
In March 2022, the FDA approved the drug olaparib to treat HER2-, high-risk, early breast cancer (EBC). The approval was made after the conclusion of the phase 3 OlympiA trial. The clinical trial results showed a statistically significant improvement in overall survival rates (by 32%) with adjuvant olaparib compared with placebo for germline BRCA1/2-mutated EBC.
In August 2022, the FDA approved the antibody drug conjugate fam-trastuzumab deruxtecan-nxki (or T-DXd), which is the first FDA-approved therapy targeted to treat patients who have the HER2-low BC subtype. It is also approved for patients with unresectable or metastatic HER2+ breast cancer who have already been treated with 2 or more prior anti-HER2–based therapies in the metastatic setting. The approval of T-DXd was given on the basis of DESTINY-Breast04, a randomized, multicenter, open-label clinical trial that was published in June 2022. T-DXd had been previously approved in HER2+ metastatic breast cancer.
The results from the phase 3 CAPItello-291 clinical trial of capivasertib in combination with fulvestrant suggests the combination could become a new treatment option for patients with hormone receptor–positive, HER2-low, locally advanced or metastatic BC following recurrence or progression after treatment with endocrine therapy and a CDK4/6 inhibitor. Capivasertib is a novel, selective, ATP-competitive, pan-AKT kinase inhibitor. In clinical trials, the drug was shown to successfully block activity of the cancer-driving protein molecule AKT. This research was presented at the 2022 San Antonio Breast Cancer Symposium, and the findings demonstrated a significant improvement in the overall population, as well as the subgroup of patients with PI3K pathway–altered tumors.
The year 2022 was full of exciting discoveries in the field of targeted drug therapies for treating BC, expanding patients’ treatment options and giving hope to people who have been diagnosed with breast cancer and their loved ones. In addition, emerging technologies such as immunotherapy and new antibody-drug conjugates continue to be evaluated as potential treatment options for treating breast cancer in the near future.
Breast cancer (BC) is the most diagnosed cancer and the second leading cause of cancer deaths in women. In 2022, more than 275,000 women were diagnosed with BC, and at least 43,000 of these cases resulted in death. With targeted drug therapy for treating BC now approved by the US Food and Drug Administration (FDA) and available (or in the late phases of clinical trials and pending availability and FDA approval), clinicians are beginning to be able to move away from a “one-size-fits-all” treatment approach that has been used in the past, enhancing prognosis and survival rates in their patients living with BC.
The new targeted drug therapies available are more precise and individualized. They treat patients more effectively because they are based on the patient’s own biology. These therapies open the possibility of having more valuable treatment options, which can be beneficial for the outcome of many patients diagnosed with BC—especially the highly aggressive forms that were previously difficult to treat.
In March 2022, the FDA approved the drug olaparib to treat HER2-, high-risk, early breast cancer (EBC). The approval was made after the conclusion of the phase 3 OlympiA trial. The clinical trial results showed a statistically significant improvement in overall survival rates (by 32%) with adjuvant olaparib compared with placebo for germline BRCA1/2-mutated EBC.
In August 2022, the FDA approved the antibody drug conjugate fam-trastuzumab deruxtecan-nxki (or T-DXd), which is the first FDA-approved therapy targeted to treat patients who have the HER2-low BC subtype. It is also approved for patients with unresectable or metastatic HER2+ breast cancer who have already been treated with 2 or more prior anti-HER2–based therapies in the metastatic setting. The approval of T-DXd was given on the basis of DESTINY-Breast04, a randomized, multicenter, open-label clinical trial that was published in June 2022. T-DXd had been previously approved in HER2+ metastatic breast cancer.
The results from the phase 3 CAPItello-291 clinical trial of capivasertib in combination with fulvestrant suggests the combination could become a new treatment option for patients with hormone receptor–positive, HER2-low, locally advanced or metastatic BC following recurrence or progression after treatment with endocrine therapy and a CDK4/6 inhibitor. Capivasertib is a novel, selective, ATP-competitive, pan-AKT kinase inhibitor. In clinical trials, the drug was shown to successfully block activity of the cancer-driving protein molecule AKT. This research was presented at the 2022 San Antonio Breast Cancer Symposium, and the findings demonstrated a significant improvement in the overall population, as well as the subgroup of patients with PI3K pathway–altered tumors.
The year 2022 was full of exciting discoveries in the field of targeted drug therapies for treating BC, expanding patients’ treatment options and giving hope to people who have been diagnosed with breast cancer and their loved ones. In addition, emerging technologies such as immunotherapy and new antibody-drug conjugates continue to be evaluated as potential treatment options for treating breast cancer in the near future.
Breast cancer (BC) is the most diagnosed cancer and the second leading cause of cancer deaths in women. In 2022, more than 275,000 women were diagnosed with BC, and at least 43,000 of these cases resulted in death. With targeted drug therapy for treating BC now approved by the US Food and Drug Administration (FDA) and available (or in the late phases of clinical trials and pending availability and FDA approval), clinicians are beginning to be able to move away from a “one-size-fits-all” treatment approach that has been used in the past, enhancing prognosis and survival rates in their patients living with BC.
The new targeted drug therapies available are more precise and individualized. They treat patients more effectively because they are based on the patient’s own biology. These therapies open the possibility of having more valuable treatment options, which can be beneficial for the outcome of many patients diagnosed with BC—especially the highly aggressive forms that were previously difficult to treat.
In March 2022, the FDA approved the drug olaparib to treat HER2-, high-risk, early breast cancer (EBC). The approval was made after the conclusion of the phase 3 OlympiA trial. The clinical trial results showed a statistically significant improvement in overall survival rates (by 32%) with adjuvant olaparib compared with placebo for germline BRCA1/2-mutated EBC.
In August 2022, the FDA approved the antibody drug conjugate fam-trastuzumab deruxtecan-nxki (or T-DXd), which is the first FDA-approved therapy targeted to treat patients who have the HER2-low BC subtype. It is also approved for patients with unresectable or metastatic HER2+ breast cancer who have already been treated with 2 or more prior anti-HER2–based therapies in the metastatic setting. The approval of T-DXd was given on the basis of DESTINY-Breast04, a randomized, multicenter, open-label clinical trial that was published in June 2022. T-DXd had been previously approved in HER2+ metastatic breast cancer.
The results from the phase 3 CAPItello-291 clinical trial of capivasertib in combination with fulvestrant suggests the combination could become a new treatment option for patients with hormone receptor–positive, HER2-low, locally advanced or metastatic BC following recurrence or progression after treatment with endocrine therapy and a CDK4/6 inhibitor. Capivasertib is a novel, selective, ATP-competitive, pan-AKT kinase inhibitor. In clinical trials, the drug was shown to successfully block activity of the cancer-driving protein molecule AKT. This research was presented at the 2022 San Antonio Breast Cancer Symposium, and the findings demonstrated a significant improvement in the overall population, as well as the subgroup of patients with PI3K pathway–altered tumors.
The year 2022 was full of exciting discoveries in the field of targeted drug therapies for treating BC, expanding patients’ treatment options and giving hope to people who have been diagnosed with breast cancer and their loved ones. In addition, emerging technologies such as immunotherapy and new antibody-drug conjugates continue to be evaluated as potential treatment options for treating breast cancer in the near future.
We Have the Tools to Prevent Cervical Cancer— So Why Are Rates Increasing?
Cervical cancer screening is often hailed as the most successful screening program ever implemented. Despite these past successes, cervical cancer incidence is no longer decreasing and is actually rising significantly in younger women within the United States. In a country with a well-established screening program, effective screening tools, and HPV vaccination, it seems unfathomable that we are witnessing an increase in cervical cancers. This alarming trend should be cause for concern among all healthcare professionals.
Cervical cancer screening is often hailed as the most successful screening program ever implemented. Despite these past successes, cervical cancer incidence is no longer decreasing and is actually rising significantly in younger women within the United States. In a country with a well-established screening program, effective screening tools, and HPV vaccination, it seems unfathomable that we are witnessing an increase in cervical cancers. This alarming trend should be cause for concern among all healthcare professionals.
Cervical cancer screening is often hailed as the most successful screening program ever implemented. Despite these past successes, cervical cancer incidence is no longer decreasing and is actually rising significantly in younger women within the United States. In a country with a well-established screening program, effective screening tools, and HPV vaccination, it seems unfathomable that we are witnessing an increase in cervical cancers. This alarming trend should be cause for concern among all healthcare professionals.
Scar overgrowth
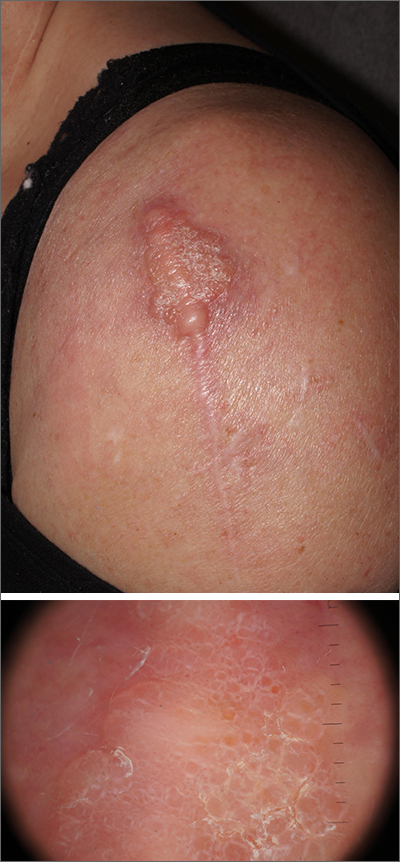
Dermatopathology was consistent with a diagnosis of cutaneous myxoma (CM). There are very few dermoscopic descriptions of CM in the literature, so diagnostic features are not established. However, the absence of more diagnostic features of basal cell carcinoma or squamous cell carcinoma (SCC) increases the likelihood of a rare diagnosis, such as CM.
CMs are rare benign neoplasms that manifest most commonly in young adults as small (< 1 cm) flesh-colored to blue papules on the head, neck, and trunk. The size of this particular CM was an outlier. CMs may be associated with Carney Complex (CNC), a rare inherited syndrome that has been linked to multiple endocrine neoplasias—namely, pituitary adenomas, testicular Sertoli cell tumors, thyroid tumors, and cardiac atrial myxomas.1 Additionally, in CNC, lentigines and multiple blue nevi develop on the skin and mucosal surfaces.
The differential diagnosis for a large, pink to flesh-colored nodule of this size includes benign histiocytoma, SCC, CM, and dermatofibrosarcoma protuberans. Benign histiocytomas and SCCs are much more common than CM. Clinical features only hint at the correct diagnosis, which must be made histologically.
Patients with CMs benefit from ongoing dermatology surveillance to monitor for the development of atypical nevi or new CMs. In this case, a wide excision with generous margins was planned with plastic surgery. (CMs have been reported to recur after surgery, which is why wide margins are essential.)
Additionally, 2 factors prompted an echocardiogram: the association between CMs and possible cardiac tumors and the patient’s need to undergo future orthopedic surgery under general anesthesia. No cardiac tumors were visible on echocardiogram. Thyroid imaging and genetic evaluation were planned but not completed.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Zou Y, Billings SD. Myxoid cutaneous tumors: a review. J Cutan Pathol. 2016;43:903-18. doi: 10.1111/cup.12749.

Dermatopathology was consistent with a diagnosis of cutaneous myxoma (CM). There are very few dermoscopic descriptions of CM in the literature, so diagnostic features are not established. However, the absence of more diagnostic features of basal cell carcinoma or squamous cell carcinoma (SCC) increases the likelihood of a rare diagnosis, such as CM.
CMs are rare benign neoplasms that manifest most commonly in young adults as small (< 1 cm) flesh-colored to blue papules on the head, neck, and trunk. The size of this particular CM was an outlier. CMs may be associated with Carney Complex (CNC), a rare inherited syndrome that has been linked to multiple endocrine neoplasias—namely, pituitary adenomas, testicular Sertoli cell tumors, thyroid tumors, and cardiac atrial myxomas.1 Additionally, in CNC, lentigines and multiple blue nevi develop on the skin and mucosal surfaces.
The differential diagnosis for a large, pink to flesh-colored nodule of this size includes benign histiocytoma, SCC, CM, and dermatofibrosarcoma protuberans. Benign histiocytomas and SCCs are much more common than CM. Clinical features only hint at the correct diagnosis, which must be made histologically.
Patients with CMs benefit from ongoing dermatology surveillance to monitor for the development of atypical nevi or new CMs. In this case, a wide excision with generous margins was planned with plastic surgery. (CMs have been reported to recur after surgery, which is why wide margins are essential.)
Additionally, 2 factors prompted an echocardiogram: the association between CMs and possible cardiac tumors and the patient’s need to undergo future orthopedic surgery under general anesthesia. No cardiac tumors were visible on echocardiogram. Thyroid imaging and genetic evaluation were planned but not completed.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

Dermatopathology was consistent with a diagnosis of cutaneous myxoma (CM). There are very few dermoscopic descriptions of CM in the literature, so diagnostic features are not established. However, the absence of more diagnostic features of basal cell carcinoma or squamous cell carcinoma (SCC) increases the likelihood of a rare diagnosis, such as CM.
CMs are rare benign neoplasms that manifest most commonly in young adults as small (< 1 cm) flesh-colored to blue papules on the head, neck, and trunk. The size of this particular CM was an outlier. CMs may be associated with Carney Complex (CNC), a rare inherited syndrome that has been linked to multiple endocrine neoplasias—namely, pituitary adenomas, testicular Sertoli cell tumors, thyroid tumors, and cardiac atrial myxomas.1 Additionally, in CNC, lentigines and multiple blue nevi develop on the skin and mucosal surfaces.
The differential diagnosis for a large, pink to flesh-colored nodule of this size includes benign histiocytoma, SCC, CM, and dermatofibrosarcoma protuberans. Benign histiocytomas and SCCs are much more common than CM. Clinical features only hint at the correct diagnosis, which must be made histologically.
Patients with CMs benefit from ongoing dermatology surveillance to monitor for the development of atypical nevi or new CMs. In this case, a wide excision with generous margins was planned with plastic surgery. (CMs have been reported to recur after surgery, which is why wide margins are essential.)
Additionally, 2 factors prompted an echocardiogram: the association between CMs and possible cardiac tumors and the patient’s need to undergo future orthopedic surgery under general anesthesia. No cardiac tumors were visible on echocardiogram. Thyroid imaging and genetic evaluation were planned but not completed.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Zou Y, Billings SD. Myxoid cutaneous tumors: a review. J Cutan Pathol. 2016;43:903-18. doi: 10.1111/cup.12749.
1. Zou Y, Billings SD. Myxoid cutaneous tumors: a review. J Cutan Pathol. 2016;43:903-18. doi: 10.1111/cup.12749.
Innovations in Dermatology Fall Abstract Compendium
Fourth Study to Show Consistent Benefit of Highly Purified Eicosapentaenoic Acid on Cardiovascular Outcomes: Results From RESPECT-EPA
In this supplement to Cardiology News, John R. Nelson, MD, FACC, FNLA, FASNC, and Matthew J. Budoff, MD, discuss results from RESPECT-EPA and the existing evidence that purified eicosapentaenoic acid significantly reduces residual CV risk in patients with CVD.
In this supplement to Cardiology News, John R. Nelson, MD, FACC, FNLA, FASNC, and Matthew J. Budoff, MD, discuss results from RESPECT-EPA and the existing evidence that purified eicosapentaenoic acid significantly reduces residual CV risk in patients with CVD.
In this supplement to Cardiology News, John R. Nelson, MD, FACC, FNLA, FASNC, and Matthew J. Budoff, MD, discuss results from RESPECT-EPA and the existing evidence that purified eicosapentaenoic acid significantly reduces residual CV risk in patients with CVD.
“The Nail in the Coffin for Fibrates”: Futility of PROMINENT Trial Definitively Settles Debate on Avoiding Use of Fibrate Class of Medications for Cardiovascular Risk Reduction
In this supplement to Cardiology News, Payal Kohli, MD, FACC, and Nihar Desai, MD, MPH, discuss the PROMINENT trial and the debate on avoiding the use of fibrates for cardiovascular risk reduction.
In this supplement to Cardiology News, Payal Kohli, MD, FACC, and Nihar Desai, MD, MPH, discuss the PROMINENT trial and the debate on avoiding the use of fibrates for cardiovascular risk reduction.
In this supplement to Cardiology News, Payal Kohli, MD, FACC, and Nihar Desai, MD, MPH, discuss the PROMINENT trial and the debate on avoiding the use of fibrates for cardiovascular risk reduction.
Pulling sensation in chest
The history and findings in this case are suggestive of breast cancer with metastatic spread to the chest wall and lungs.
Globally, breast cancer is the most frequently diagnosed life-threatening cancer and the leading cause of cancer death among women. In the United States, an estimated 287,850 new cases of invasive breast cancer will be diagnosed in 2022; in addition, 43,250 deaths because of breast cancer are expected to occur. Despite advances in adjuvant treatment strategies, such as tamoxifen for patients with ER-positive breast cancer, many patients with early breast cancer still experience disease recurrence after primary therapy. Because of its systemic nature and inevitable resistance to therapy, metastatic breast cancer is largely incurable.
Approximately 5%-35% of patients with breast cancer develop locoregional recurrence either alone or with distant metastases. The lung is a frequent site of breast cancer metastasis. In addition, approximately 11% of patients have persistent chest wall progression. Recurrent breast cancer in the chest wall is considered a marker of poor prognosis and is normally accompanied by or a precursor to distant metastases.
Risk factors for chest wall recurrence include primary tumor size, primary stage, and lymph node involvement; in addition, the risk is increased in patients aged 40 years or younger and in those with gross multifocal or multicentric disease. Histopathological risk factors include positive margin status, DCIS, extensive intraductal component, high grade, lymphovascular invasion, tumor oncogene, and tumor suppressor gene expression (eg, p53 and HER2), and ER status.
According to the National Comprehensive Cancer Network (NCCN) 2022 guidelines, the staging evaluation of patients who present with recurrent or stage IV breast cancer should include:
• History and physical exam
• Complete blood count and liver function tests
• Chest diagnostic CT
• Bone scan
• Radiographs of any long or weight-bearing bones that are painful or appear abnormal on bone scan
• Diagnostic CT of the abdomen (with or without diagnostic CT of the pelvis) or MRI of the abdomen
• Biopsy documentation of first recurrence, when possible
The use of sodium fluoride PET or PET-CT for the evaluation of patients with recurrent disease is largely discouraged.
Determination of hormone receptor status (ER and progesterone receptor [PR]) as well as HER2 status should be repeated because ER and PR assays may be falsely negative or falsely positive and there may be discordance between the primary and metastatic tumors.
In the metastatic setting, genetic testing results may have therapeutic implications; specifically, germline mutations in BRCA1/BRCA2 have demonstrated clinical utility and therapeutic impact. Thus, the NCCN panel recommends that germline BRCA1/BRCA2 mutations be evaluated in all patients with recurrent or metastatic breast cancer to identify candidates for appropriate targeted therapies (eg, poly adenosine diphosphate ribose polymerase–inhibitor therapy).
In patients with recurrence of breast cancer to the chest wall, complete chest wall resection and appropriate reconstruction may prolong overall survival, although appropriate patient selection is essential for optimal outcomes. Patients with tumors that display a more aggressive phenotype (eg, triple-negative or HER2-positive disease) may not benefit from this approach and supportive care may be more appropriate.
Avan J. Armaghani, MD, Assistant Member, Department of Breast Oncology, Moffitt Cancer Center, University of South Florida, Tampa, FL.
Avan J. Armaghani, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are suggestive of breast cancer with metastatic spread to the chest wall and lungs.
Globally, breast cancer is the most frequently diagnosed life-threatening cancer and the leading cause of cancer death among women. In the United States, an estimated 287,850 new cases of invasive breast cancer will be diagnosed in 2022; in addition, 43,250 deaths because of breast cancer are expected to occur. Despite advances in adjuvant treatment strategies, such as tamoxifen for patients with ER-positive breast cancer, many patients with early breast cancer still experience disease recurrence after primary therapy. Because of its systemic nature and inevitable resistance to therapy, metastatic breast cancer is largely incurable.
Approximately 5%-35% of patients with breast cancer develop locoregional recurrence either alone or with distant metastases. The lung is a frequent site of breast cancer metastasis. In addition, approximately 11% of patients have persistent chest wall progression. Recurrent breast cancer in the chest wall is considered a marker of poor prognosis and is normally accompanied by or a precursor to distant metastases.
Risk factors for chest wall recurrence include primary tumor size, primary stage, and lymph node involvement; in addition, the risk is increased in patients aged 40 years or younger and in those with gross multifocal or multicentric disease. Histopathological risk factors include positive margin status, DCIS, extensive intraductal component, high grade, lymphovascular invasion, tumor oncogene, and tumor suppressor gene expression (eg, p53 and HER2), and ER status.
According to the National Comprehensive Cancer Network (NCCN) 2022 guidelines, the staging evaluation of patients who present with recurrent or stage IV breast cancer should include:
• History and physical exam
• Complete blood count and liver function tests
• Chest diagnostic CT
• Bone scan
• Radiographs of any long or weight-bearing bones that are painful or appear abnormal on bone scan
• Diagnostic CT of the abdomen (with or without diagnostic CT of the pelvis) or MRI of the abdomen
• Biopsy documentation of first recurrence, when possible
The use of sodium fluoride PET or PET-CT for the evaluation of patients with recurrent disease is largely discouraged.
Determination of hormone receptor status (ER and progesterone receptor [PR]) as well as HER2 status should be repeated because ER and PR assays may be falsely negative or falsely positive and there may be discordance between the primary and metastatic tumors.
In the metastatic setting, genetic testing results may have therapeutic implications; specifically, germline mutations in BRCA1/BRCA2 have demonstrated clinical utility and therapeutic impact. Thus, the NCCN panel recommends that germline BRCA1/BRCA2 mutations be evaluated in all patients with recurrent or metastatic breast cancer to identify candidates for appropriate targeted therapies (eg, poly adenosine diphosphate ribose polymerase–inhibitor therapy).
In patients with recurrence of breast cancer to the chest wall, complete chest wall resection and appropriate reconstruction may prolong overall survival, although appropriate patient selection is essential for optimal outcomes. Patients with tumors that display a more aggressive phenotype (eg, triple-negative or HER2-positive disease) may not benefit from this approach and supportive care may be more appropriate.
Avan J. Armaghani, MD, Assistant Member, Department of Breast Oncology, Moffitt Cancer Center, University of South Florida, Tampa, FL.
Avan J. Armaghani, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are suggestive of breast cancer with metastatic spread to the chest wall and lungs.
Globally, breast cancer is the most frequently diagnosed life-threatening cancer and the leading cause of cancer death among women. In the United States, an estimated 287,850 new cases of invasive breast cancer will be diagnosed in 2022; in addition, 43,250 deaths because of breast cancer are expected to occur. Despite advances in adjuvant treatment strategies, such as tamoxifen for patients with ER-positive breast cancer, many patients with early breast cancer still experience disease recurrence after primary therapy. Because of its systemic nature and inevitable resistance to therapy, metastatic breast cancer is largely incurable.
Approximately 5%-35% of patients with breast cancer develop locoregional recurrence either alone or with distant metastases. The lung is a frequent site of breast cancer metastasis. In addition, approximately 11% of patients have persistent chest wall progression. Recurrent breast cancer in the chest wall is considered a marker of poor prognosis and is normally accompanied by or a precursor to distant metastases.
Risk factors for chest wall recurrence include primary tumor size, primary stage, and lymph node involvement; in addition, the risk is increased in patients aged 40 years or younger and in those with gross multifocal or multicentric disease. Histopathological risk factors include positive margin status, DCIS, extensive intraductal component, high grade, lymphovascular invasion, tumor oncogene, and tumor suppressor gene expression (eg, p53 and HER2), and ER status.
According to the National Comprehensive Cancer Network (NCCN) 2022 guidelines, the staging evaluation of patients who present with recurrent or stage IV breast cancer should include:
• History and physical exam
• Complete blood count and liver function tests
• Chest diagnostic CT
• Bone scan
• Radiographs of any long or weight-bearing bones that are painful or appear abnormal on bone scan
• Diagnostic CT of the abdomen (with or without diagnostic CT of the pelvis) or MRI of the abdomen
• Biopsy documentation of first recurrence, when possible
The use of sodium fluoride PET or PET-CT for the evaluation of patients with recurrent disease is largely discouraged.
Determination of hormone receptor status (ER and progesterone receptor [PR]) as well as HER2 status should be repeated because ER and PR assays may be falsely negative or falsely positive and there may be discordance between the primary and metastatic tumors.
In the metastatic setting, genetic testing results may have therapeutic implications; specifically, germline mutations in BRCA1/BRCA2 have demonstrated clinical utility and therapeutic impact. Thus, the NCCN panel recommends that germline BRCA1/BRCA2 mutations be evaluated in all patients with recurrent or metastatic breast cancer to identify candidates for appropriate targeted therapies (eg, poly adenosine diphosphate ribose polymerase–inhibitor therapy).
In patients with recurrence of breast cancer to the chest wall, complete chest wall resection and appropriate reconstruction may prolong overall survival, although appropriate patient selection is essential for optimal outcomes. Patients with tumors that display a more aggressive phenotype (eg, triple-negative or HER2-positive disease) may not benefit from this approach and supportive care may be more appropriate.
Avan J. Armaghani, MD, Assistant Member, Department of Breast Oncology, Moffitt Cancer Center, University of South Florida, Tampa, FL.
Avan J. Armaghani, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 39-year-old nonsmoking woman in the United States presents with a history of a painful, pulling sensation in her chest that she originally attributed to a change in her exercise regimen. Six years earlier, the patient was diagnosed with estrogen receptor (ER)–positive ductal carcinoma in situ (DCIS) in her left breast. She opted for mastectomy and immediate reconstruction, followed by adjuvant therapy with tamoxifen (20 mg/d for 5 years). Physical examination reveals a palpable mass in the medial half of her left breast with several hard, painful nodules in the left axilla. Mild wheezing throughout the upper lungs is heard on auscultation. Abdominal examination does not reveal any abnormalities. Laboratory findings are all within normal range, apart from C-reactive protein, which is elevated. The patient is 5 ft 7 in and weighs 133 lb.