User login
Renewed Concern for Navy Conditions Following Suicides
Eight Navy sailors have died by suicide in less than a year. The most recent death was on January 23. Three sailors who died in the past 2 months have more than suicide in common: They were all stationed aboard Navy aircraft carriers undergoing refits: the USS George Washington and the USS Theodore Roosevelt.
These deaths come only a month after the Navy released a report on 3 deaths by suicide on the George Washington, all of which happened in a single week last April. Military.com reported that the ship’s commander, Capt. Brent Gaut, had said 10 sailors had died by suicide in under a year.
In November and December 2022, at least 4 sailors assigned to the Mid-Atlantic Regional Maintenance Center (MARMC) in Virginia died by suicide, multiplying concerns about a fleetwide mental health crisis. “I was inundated with the amount of hopelessness at that command,” Kayla Arestivo, a counselor brought in to help, told nbcnews.com. Sailors spoke of being overworked, undervalued, and not getting the mental health help they needed. “Part of it is toxic leadership. The sailors immediately pointed that out,” Arestivo said.
She noted that many of the people assigned to MARMC are on limited duty due to mental or physical disabilities or have personal stressors that prevent them from full unrestricted duty. Electronics technician Kody Lee Decker, for instance, was on limited duty due to mental health issues when he died by suicide on October 29, 2022, according to a friend. Those people, Arestivo suggested, should have been provided help earlier.
Disabilities are not the only potential risk factors, though. Sailors living aboard the George Washington from April 2021 until April 2022 reported difficult and noisy living conditions with shortages of power, running water, and heat, and poor ventilation. Sailors would sleep in their cars or rent rooms in town rather than stay on board.
The George Washington has been docked at Newport News [Virginia] Shipbuilding for a major overhaul and repairs since 2017 (expected to extend into 2023, nearly 2 years later than the original deadline). The Navy investigation acknowledged “overwhelming” stress and noted that the living conditions created by an “intense and complex” maintenance process were posing hardships for the sailors, including sleep deprivation. (The Theodore Roosevelt has been at the Puget Sound shipyard since August 2021, although none of the sailors live onboard.)
However, the Navy investigation concluded that the 3 April suicide deaths were not directly connected to living conditions. According to the US Fleet Forces Command, “each Sailor was experiencing unique and individualized life stressors, which were contributing factors leading to their deaths.” The 3 suicide deaths were deemed independent events, with no direct correlation among them.
But the report also charged that leaders were oblivious to the problems, and the mental health care the Navy offered was insufficient: “Multiple command members knew or should have known that MASR Mitchell-Sandor [who died by suicide] was experiencing displeasure with Navy life and could have intervened to help him better cope or seek out available support services.”
In the official response to the Navy report, Rear Adm. John Meier, Commander, Naval Air Force Atlantic, noted that he had convened a “second and broader investigation” to assess quality of life issues and other systemic issues for aircraft carriers undergoing extensive maintenance or construction in the Newport News shipyard. “It is safe to say,” he wrote, that “generations of Navy leaders had become accustomed to the reduced quality of life in the shipyard, and accepted the status quo as par for the course…”
He agreed that the general stress of the environment was not the root cause of the deaths but was “certainly a contributing factor” in at least one case. The report, he said, placed too much emphasis on the sailor’s personal decisions to not improve his own living conditions (he was offered the opportunity to change berthing) and thus placed “too much burden on him for his situation.” Senior enlisted leadership knew that the sailor was sleeping in his car and counseled him, but Meier found no evidence of follow-through. More senior sailors or an assigned mentor should have been there to support the sailor, Meier said, and help him make decisions that were in his best interests. “This was a time for intrusive leadership.”
Adm. Daryl Caudle, Commander, US Fleet Forces Command, advised revising the wording in the report to say “No one at the command knew, or had a reason to know, of MASR [Xavier] Mitchell-Sandor’s previous suicidal ideations.” He also advised modifying the wording with: “Had the Navy been aware of MASR Mitchell-Sandor’s previous suicidal ideations, existing programs and procedures were in place that make it likely that he would have been placed in a ‘do not arm’ status and received necessary care.”
Vice Admiral Kenneth Whitesell, Commander, Naval Air Force, US Pacific Fleet, also endorsed the report findings with some revisions, saying, “We cannot assume these issues are isolated to a single ship, or to shipyards alone. Rather, these 3 tragic losses brought to light the ultimate need to remain laser-focused on providing care and guidance to our sailors.”
The Navy is providing mental health support to sailors, including an embedded mental health team and 2 civilian resiliency counselors who work on the George Washington. According to an action update in the report, Commander, Naval Air Force directed all CVNs and Naval Aviation Units to have a minimum of 1 safeTALK (Suicide Alertness For Everyone; Tell, Ask, Listen and KeepSafe) trained member onboard, and 2 to 3 safeTALK trained personnel in each division no later than December 31, 2022.
At MARMC, Arestivo was brought in for several mandatory suicide prevention sessions but without systemic changes, she said, “We’re putting Band-Aids on bullet holes.”
She said she told MARMC’s commanding officer, “You will have another one.” The fourth sailor died by suicide 10 days later.
If you or someone you know is having thoughts of suicide, call or text 988 to reach the National Suicide Prevention Lifeline or contact the Veterans Crisis Line.
Eight Navy sailors have died by suicide in less than a year. The most recent death was on January 23. Three sailors who died in the past 2 months have more than suicide in common: They were all stationed aboard Navy aircraft carriers undergoing refits: the USS George Washington and the USS Theodore Roosevelt.
These deaths come only a month after the Navy released a report on 3 deaths by suicide on the George Washington, all of which happened in a single week last April. Military.com reported that the ship’s commander, Capt. Brent Gaut, had said 10 sailors had died by suicide in under a year.
In November and December 2022, at least 4 sailors assigned to the Mid-Atlantic Regional Maintenance Center (MARMC) in Virginia died by suicide, multiplying concerns about a fleetwide mental health crisis. “I was inundated with the amount of hopelessness at that command,” Kayla Arestivo, a counselor brought in to help, told nbcnews.com. Sailors spoke of being overworked, undervalued, and not getting the mental health help they needed. “Part of it is toxic leadership. The sailors immediately pointed that out,” Arestivo said.
She noted that many of the people assigned to MARMC are on limited duty due to mental or physical disabilities or have personal stressors that prevent them from full unrestricted duty. Electronics technician Kody Lee Decker, for instance, was on limited duty due to mental health issues when he died by suicide on October 29, 2022, according to a friend. Those people, Arestivo suggested, should have been provided help earlier.
Disabilities are not the only potential risk factors, though. Sailors living aboard the George Washington from April 2021 until April 2022 reported difficult and noisy living conditions with shortages of power, running water, and heat, and poor ventilation. Sailors would sleep in their cars or rent rooms in town rather than stay on board.
The George Washington has been docked at Newport News [Virginia] Shipbuilding for a major overhaul and repairs since 2017 (expected to extend into 2023, nearly 2 years later than the original deadline). The Navy investigation acknowledged “overwhelming” stress and noted that the living conditions created by an “intense and complex” maintenance process were posing hardships for the sailors, including sleep deprivation. (The Theodore Roosevelt has been at the Puget Sound shipyard since August 2021, although none of the sailors live onboard.)
However, the Navy investigation concluded that the 3 April suicide deaths were not directly connected to living conditions. According to the US Fleet Forces Command, “each Sailor was experiencing unique and individualized life stressors, which were contributing factors leading to their deaths.” The 3 suicide deaths were deemed independent events, with no direct correlation among them.
But the report also charged that leaders were oblivious to the problems, and the mental health care the Navy offered was insufficient: “Multiple command members knew or should have known that MASR Mitchell-Sandor [who died by suicide] was experiencing displeasure with Navy life and could have intervened to help him better cope or seek out available support services.”
In the official response to the Navy report, Rear Adm. John Meier, Commander, Naval Air Force Atlantic, noted that he had convened a “second and broader investigation” to assess quality of life issues and other systemic issues for aircraft carriers undergoing extensive maintenance or construction in the Newport News shipyard. “It is safe to say,” he wrote, that “generations of Navy leaders had become accustomed to the reduced quality of life in the shipyard, and accepted the status quo as par for the course…”
He agreed that the general stress of the environment was not the root cause of the deaths but was “certainly a contributing factor” in at least one case. The report, he said, placed too much emphasis on the sailor’s personal decisions to not improve his own living conditions (he was offered the opportunity to change berthing) and thus placed “too much burden on him for his situation.” Senior enlisted leadership knew that the sailor was sleeping in his car and counseled him, but Meier found no evidence of follow-through. More senior sailors or an assigned mentor should have been there to support the sailor, Meier said, and help him make decisions that were in his best interests. “This was a time for intrusive leadership.”
Adm. Daryl Caudle, Commander, US Fleet Forces Command, advised revising the wording in the report to say “No one at the command knew, or had a reason to know, of MASR [Xavier] Mitchell-Sandor’s previous suicidal ideations.” He also advised modifying the wording with: “Had the Navy been aware of MASR Mitchell-Sandor’s previous suicidal ideations, existing programs and procedures were in place that make it likely that he would have been placed in a ‘do not arm’ status and received necessary care.”
Vice Admiral Kenneth Whitesell, Commander, Naval Air Force, US Pacific Fleet, also endorsed the report findings with some revisions, saying, “We cannot assume these issues are isolated to a single ship, or to shipyards alone. Rather, these 3 tragic losses brought to light the ultimate need to remain laser-focused on providing care and guidance to our sailors.”
The Navy is providing mental health support to sailors, including an embedded mental health team and 2 civilian resiliency counselors who work on the George Washington. According to an action update in the report, Commander, Naval Air Force directed all CVNs and Naval Aviation Units to have a minimum of 1 safeTALK (Suicide Alertness For Everyone; Tell, Ask, Listen and KeepSafe) trained member onboard, and 2 to 3 safeTALK trained personnel in each division no later than December 31, 2022.
At MARMC, Arestivo was brought in for several mandatory suicide prevention sessions but without systemic changes, she said, “We’re putting Band-Aids on bullet holes.”
She said she told MARMC’s commanding officer, “You will have another one.” The fourth sailor died by suicide 10 days later.
If you or someone you know is having thoughts of suicide, call or text 988 to reach the National Suicide Prevention Lifeline or contact the Veterans Crisis Line.
Eight Navy sailors have died by suicide in less than a year. The most recent death was on January 23. Three sailors who died in the past 2 months have more than suicide in common: They were all stationed aboard Navy aircraft carriers undergoing refits: the USS George Washington and the USS Theodore Roosevelt.
These deaths come only a month after the Navy released a report on 3 deaths by suicide on the George Washington, all of which happened in a single week last April. Military.com reported that the ship’s commander, Capt. Brent Gaut, had said 10 sailors had died by suicide in under a year.
In November and December 2022, at least 4 sailors assigned to the Mid-Atlantic Regional Maintenance Center (MARMC) in Virginia died by suicide, multiplying concerns about a fleetwide mental health crisis. “I was inundated with the amount of hopelessness at that command,” Kayla Arestivo, a counselor brought in to help, told nbcnews.com. Sailors spoke of being overworked, undervalued, and not getting the mental health help they needed. “Part of it is toxic leadership. The sailors immediately pointed that out,” Arestivo said.
She noted that many of the people assigned to MARMC are on limited duty due to mental or physical disabilities or have personal stressors that prevent them from full unrestricted duty. Electronics technician Kody Lee Decker, for instance, was on limited duty due to mental health issues when he died by suicide on October 29, 2022, according to a friend. Those people, Arestivo suggested, should have been provided help earlier.
Disabilities are not the only potential risk factors, though. Sailors living aboard the George Washington from April 2021 until April 2022 reported difficult and noisy living conditions with shortages of power, running water, and heat, and poor ventilation. Sailors would sleep in their cars or rent rooms in town rather than stay on board.
The George Washington has been docked at Newport News [Virginia] Shipbuilding for a major overhaul and repairs since 2017 (expected to extend into 2023, nearly 2 years later than the original deadline). The Navy investigation acknowledged “overwhelming” stress and noted that the living conditions created by an “intense and complex” maintenance process were posing hardships for the sailors, including sleep deprivation. (The Theodore Roosevelt has been at the Puget Sound shipyard since August 2021, although none of the sailors live onboard.)
However, the Navy investigation concluded that the 3 April suicide deaths were not directly connected to living conditions. According to the US Fleet Forces Command, “each Sailor was experiencing unique and individualized life stressors, which were contributing factors leading to their deaths.” The 3 suicide deaths were deemed independent events, with no direct correlation among them.
But the report also charged that leaders were oblivious to the problems, and the mental health care the Navy offered was insufficient: “Multiple command members knew or should have known that MASR Mitchell-Sandor [who died by suicide] was experiencing displeasure with Navy life and could have intervened to help him better cope or seek out available support services.”
In the official response to the Navy report, Rear Adm. John Meier, Commander, Naval Air Force Atlantic, noted that he had convened a “second and broader investigation” to assess quality of life issues and other systemic issues for aircraft carriers undergoing extensive maintenance or construction in the Newport News shipyard. “It is safe to say,” he wrote, that “generations of Navy leaders had become accustomed to the reduced quality of life in the shipyard, and accepted the status quo as par for the course…”
He agreed that the general stress of the environment was not the root cause of the deaths but was “certainly a contributing factor” in at least one case. The report, he said, placed too much emphasis on the sailor’s personal decisions to not improve his own living conditions (he was offered the opportunity to change berthing) and thus placed “too much burden on him for his situation.” Senior enlisted leadership knew that the sailor was sleeping in his car and counseled him, but Meier found no evidence of follow-through. More senior sailors or an assigned mentor should have been there to support the sailor, Meier said, and help him make decisions that were in his best interests. “This was a time for intrusive leadership.”
Adm. Daryl Caudle, Commander, US Fleet Forces Command, advised revising the wording in the report to say “No one at the command knew, or had a reason to know, of MASR [Xavier] Mitchell-Sandor’s previous suicidal ideations.” He also advised modifying the wording with: “Had the Navy been aware of MASR Mitchell-Sandor’s previous suicidal ideations, existing programs and procedures were in place that make it likely that he would have been placed in a ‘do not arm’ status and received necessary care.”
Vice Admiral Kenneth Whitesell, Commander, Naval Air Force, US Pacific Fleet, also endorsed the report findings with some revisions, saying, “We cannot assume these issues are isolated to a single ship, or to shipyards alone. Rather, these 3 tragic losses brought to light the ultimate need to remain laser-focused on providing care and guidance to our sailors.”
The Navy is providing mental health support to sailors, including an embedded mental health team and 2 civilian resiliency counselors who work on the George Washington. According to an action update in the report, Commander, Naval Air Force directed all CVNs and Naval Aviation Units to have a minimum of 1 safeTALK (Suicide Alertness For Everyone; Tell, Ask, Listen and KeepSafe) trained member onboard, and 2 to 3 safeTALK trained personnel in each division no later than December 31, 2022.
At MARMC, Arestivo was brought in for several mandatory suicide prevention sessions but without systemic changes, she said, “We’re putting Band-Aids on bullet holes.”
She said she told MARMC’s commanding officer, “You will have another one.” The fourth sailor died by suicide 10 days later.
If you or someone you know is having thoughts of suicide, call or text 988 to reach the National Suicide Prevention Lifeline or contact the Veterans Crisis Line.
Why Did Nonventilator-Associated HAP Peak During the Pandemic?
Cases of nonventilator-associated hospital-acquired pneumonia (NV-HAP) declined by 32% between 2015 and 2020. Then, of course, COVID-19 changed the trajectory and rates began to rise. After February 2020, the incidence rate rose by 25% among veterans without COVID-19—but by 108% among those who had COVID-19.
Those are findings from a study by researchers at Rocky Mountain Regional VA Medical Center, Aurora, Colorado. They studied data on 1,567,275 veterans admitted to 135 VA facilities in acute care settings between October 2015 and March 2021, with a stay of at least 48 hours.
They say, to their knowledge, this is the first published report of changes in NV-HAP risk associated with the onset of COVID-19 among all hospitalized veterans in a national health care system.
The questions for the researchers were: What drove the increase in NV-HAP rates? Was it the elevated risk among veterans with COVID-19, reduced NV-HAP prevention measures during the extreme pandemic-related stress on the system, and/or increased patient acuity among hospitalized veterans?
They concluded that the observed increase in NV-HAP risk among all patients during the COVID-19 pandemic is “likely multifactorial.” The stresses on clinical workload may have hampered fundamental preventive nursing care, such as early mobility programs, consistent oral care, and aspiration precautions. The researchers also cite barriers including wearing personal protective equipment, which affected communication and the ability to get needed supplies to the bedside without cross-contamination.
Among patients with COVID-19 infections, the greater NV-HAP risk could be due to changes in the lower respiratory tract microbiome, disruption of the immune response, and synergism seen with COVID-19 infection. Moreover, they note, placing patients in a prone position to improve oxygenation might have raised the risk of NV-HAP.
The hospitalized veterans in the study also had a high burden of clinical comorbidities. Those with COVID-19 were more likely to have documented diagnosis of dementia in the previous year, compared with COVID-19-negative veterans or those hospitalized before the pandemic began. The researchers point out that dementia increased the risk of microaspiration, which can lead to secondary bacterial pneumonia.
In addition to reinforcing prevention efforts, the researchers suggest that NV-HAP monitoring via automated electronic surveillance could “serve as a cornerstone of a strong infection prevention program.” A system like that, installed before the pandemic, they say, might have identified the NV-HAP risk sooner.
Most importantly, they add, strategies to reduce NV-HAP risk “should be designed with resilience to significant system stress such as the COVID-19 pandemic.”
Cases of nonventilator-associated hospital-acquired pneumonia (NV-HAP) declined by 32% between 2015 and 2020. Then, of course, COVID-19 changed the trajectory and rates began to rise. After February 2020, the incidence rate rose by 25% among veterans without COVID-19—but by 108% among those who had COVID-19.
Those are findings from a study by researchers at Rocky Mountain Regional VA Medical Center, Aurora, Colorado. They studied data on 1,567,275 veterans admitted to 135 VA facilities in acute care settings between October 2015 and March 2021, with a stay of at least 48 hours.
They say, to their knowledge, this is the first published report of changes in NV-HAP risk associated with the onset of COVID-19 among all hospitalized veterans in a national health care system.
The questions for the researchers were: What drove the increase in NV-HAP rates? Was it the elevated risk among veterans with COVID-19, reduced NV-HAP prevention measures during the extreme pandemic-related stress on the system, and/or increased patient acuity among hospitalized veterans?
They concluded that the observed increase in NV-HAP risk among all patients during the COVID-19 pandemic is “likely multifactorial.” The stresses on clinical workload may have hampered fundamental preventive nursing care, such as early mobility programs, consistent oral care, and aspiration precautions. The researchers also cite barriers including wearing personal protective equipment, which affected communication and the ability to get needed supplies to the bedside without cross-contamination.
Among patients with COVID-19 infections, the greater NV-HAP risk could be due to changes in the lower respiratory tract microbiome, disruption of the immune response, and synergism seen with COVID-19 infection. Moreover, they note, placing patients in a prone position to improve oxygenation might have raised the risk of NV-HAP.
The hospitalized veterans in the study also had a high burden of clinical comorbidities. Those with COVID-19 were more likely to have documented diagnosis of dementia in the previous year, compared with COVID-19-negative veterans or those hospitalized before the pandemic began. The researchers point out that dementia increased the risk of microaspiration, which can lead to secondary bacterial pneumonia.
In addition to reinforcing prevention efforts, the researchers suggest that NV-HAP monitoring via automated electronic surveillance could “serve as a cornerstone of a strong infection prevention program.” A system like that, installed before the pandemic, they say, might have identified the NV-HAP risk sooner.
Most importantly, they add, strategies to reduce NV-HAP risk “should be designed with resilience to significant system stress such as the COVID-19 pandemic.”
Cases of nonventilator-associated hospital-acquired pneumonia (NV-HAP) declined by 32% between 2015 and 2020. Then, of course, COVID-19 changed the trajectory and rates began to rise. After February 2020, the incidence rate rose by 25% among veterans without COVID-19—but by 108% among those who had COVID-19.
Those are findings from a study by researchers at Rocky Mountain Regional VA Medical Center, Aurora, Colorado. They studied data on 1,567,275 veterans admitted to 135 VA facilities in acute care settings between October 2015 and March 2021, with a stay of at least 48 hours.
They say, to their knowledge, this is the first published report of changes in NV-HAP risk associated with the onset of COVID-19 among all hospitalized veterans in a national health care system.
The questions for the researchers were: What drove the increase in NV-HAP rates? Was it the elevated risk among veterans with COVID-19, reduced NV-HAP prevention measures during the extreme pandemic-related stress on the system, and/or increased patient acuity among hospitalized veterans?
They concluded that the observed increase in NV-HAP risk among all patients during the COVID-19 pandemic is “likely multifactorial.” The stresses on clinical workload may have hampered fundamental preventive nursing care, such as early mobility programs, consistent oral care, and aspiration precautions. The researchers also cite barriers including wearing personal protective equipment, which affected communication and the ability to get needed supplies to the bedside without cross-contamination.
Among patients with COVID-19 infections, the greater NV-HAP risk could be due to changes in the lower respiratory tract microbiome, disruption of the immune response, and synergism seen with COVID-19 infection. Moreover, they note, placing patients in a prone position to improve oxygenation might have raised the risk of NV-HAP.
The hospitalized veterans in the study also had a high burden of clinical comorbidities. Those with COVID-19 were more likely to have documented diagnosis of dementia in the previous year, compared with COVID-19-negative veterans or those hospitalized before the pandemic began. The researchers point out that dementia increased the risk of microaspiration, which can lead to secondary bacterial pneumonia.
In addition to reinforcing prevention efforts, the researchers suggest that NV-HAP monitoring via automated electronic surveillance could “serve as a cornerstone of a strong infection prevention program.” A system like that, installed before the pandemic, they say, might have identified the NV-HAP risk sooner.
Most importantly, they add, strategies to reduce NV-HAP risk “should be designed with resilience to significant system stress such as the COVID-19 pandemic.”
Periorbital Orange Spots
The Diagnosis: Orange Palpebral Spots
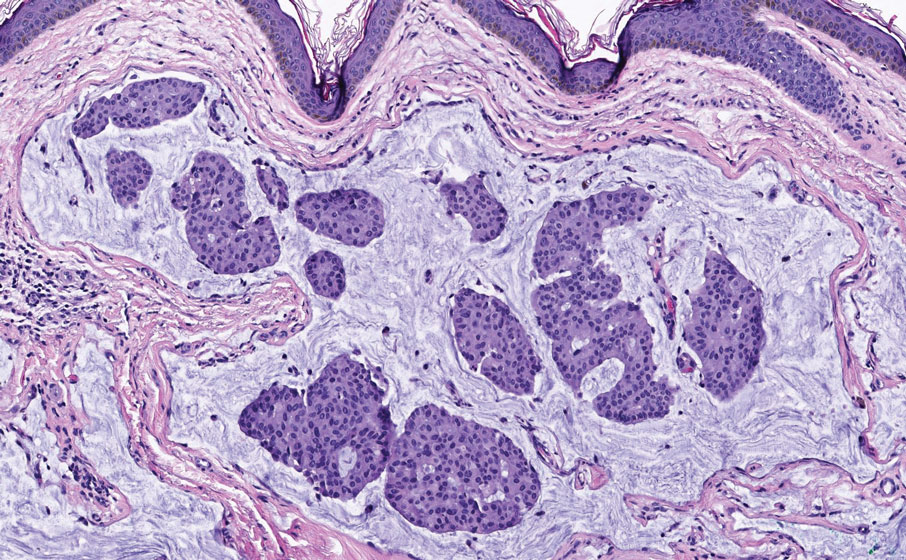
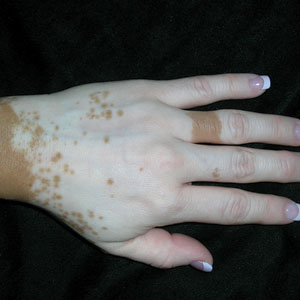
The clinical presentation of our patient was consistent with a diagnosis of orange palpebral spots (OPSs), an uncommon discoloration that most often appears in White patients in the fifth or sixth decades of life. Orange palpebral spots were first described in 2008 by Assouly et al1 in 27 patients (23 females and 4 males). In 2015, Belliveau et al2 expanded the designation to yellow-orange palpebral spots because they felt the term more fully expressed the color variations depicted in their patients; however, this term more frequently is used in ophthalmology.
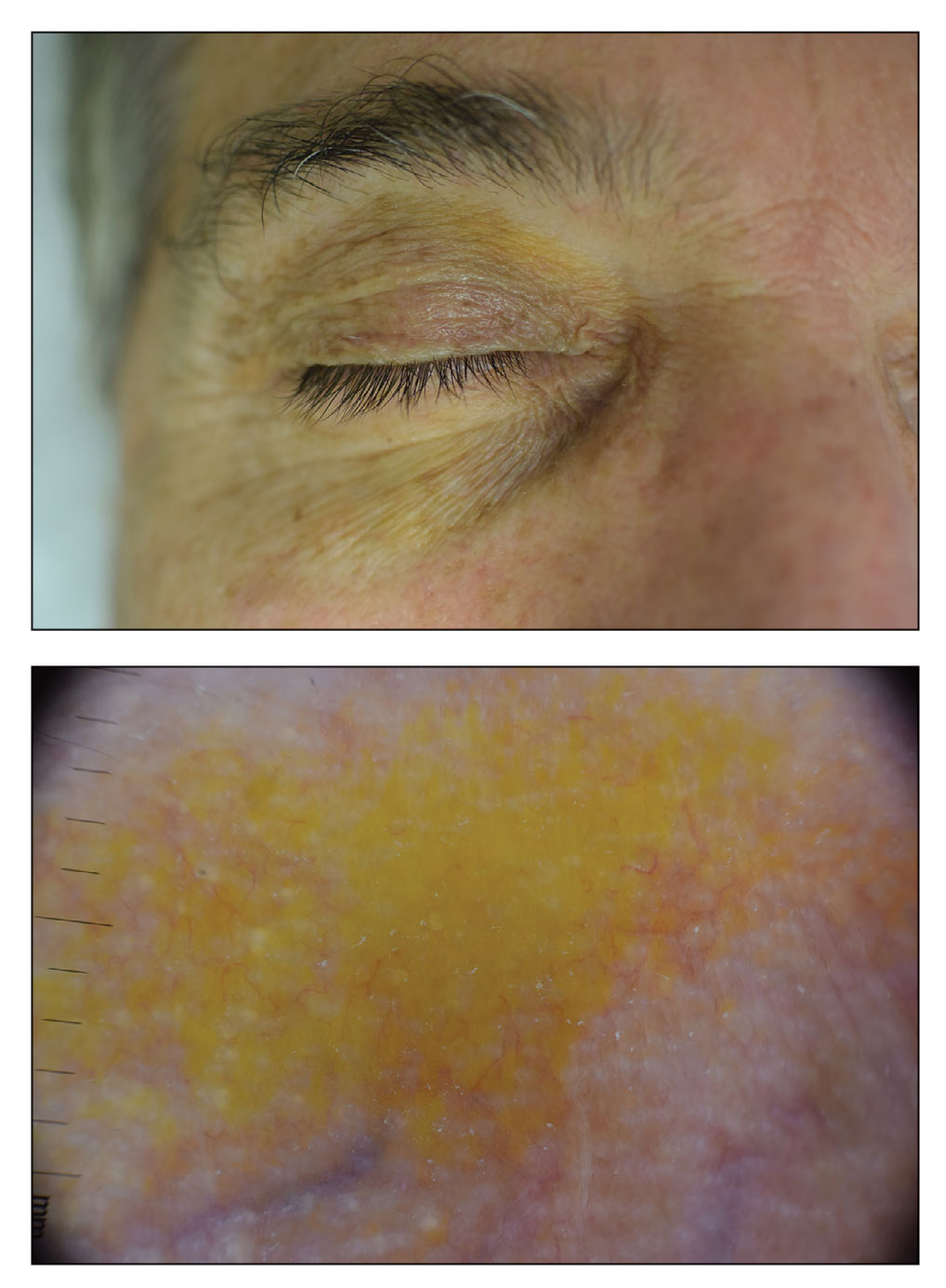
Orange palpebral spots commonly appear as asymptomatic, yellow-orange, symmetric lesions with a predilection for the recessed areas of the superior eyelids but also can present on the canthi and inferior eyelids. The discolorations are more easily visible on fair skin and have been reported to measure from 10 to 15 mm in the long axis.3 Assouly et al1 described the orange spots as having indistinct margins, with borders similar to “sand on a sea shore.” Orange palpebral spots can be a persistent discoloration, and there are no reports of spontaneous regression. No known association with malignancy or systemic illness has been reported.
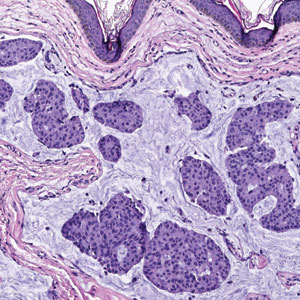
Case reports of OPSs describe histologic similarities between specimens, including increased adipose tissue and pigment-laden macrophages in the superficial dermis.2 The pigmented deposits sometimes may be found in the basal keratinocytes of the epidermis and turn black with Fontana-Masson stain.1 No inflammatory infiltrates, necrosis, or xanthomization are characteristically found. Stains for iron, mucin, and amyloid also have been negative.2
The cause of pigmentation in OPSs is unknown; however, lipofuscin deposits and high-situated adipocytes in the reticular dermis colored by carotenoids have been proposed as possible mechanisms.1 No unifying cause for pigmentation in the serum (eg, cholesterol, triglycerides, thyroid-stimulating hormone, free retinol, vitamin E, carotenoids) was found in 11 of 27 patients with OPSs assessed by Assouly et al.1 In one case, lipofuscin, a degradation product of lysosomes, was detected by microscopic autofluorescence in the superficial dermis. However, lipofuscin typically is a breakdown product associated with aging, and OPSs have been present in patients as young as 28 years.1 Local trauma related to eye rubbing is another theory that has been proposed due to the finding of melanin in the superficial dermis. However, the absence of hemosiderin deposits as well as the extensive duration of the discolorations makes local trauma a less likely explanation for the etiology of OPSs.2
The clinical differential diagnosis for OPSs includes xanthelasma, jaundice, and carotenoderma. Xanthelasma presents as elevated yellow plaques usually found over the medial aspect of the eyes. In contrast, OPSs are nonelevated with both orange and yellow hues typically present. Histologic samples of xanthelasma are characterized by lipid-laden macrophages (foam cells) in the dermis in contrast to the adipose tissue seen in OPSs that has not been phagocytized.1,2 The lack of scleral icterus made jaundice an unlikely diagnosis in our patient. Bilirubin elevations substantial enough to cause skin discoloration also would be expected to discolor the conjunctiva. In carotenoderma, carotenoids are deposited in the sweat and sebum of the stratum corneum with the orange pigmentation most prominent in regions of increased sweating such as the palms, soles, and nasolabial folds.4 Our patient’s lack of discoloration in places other than the periorbital region made carotenoderma less likely.
In the study by Assouly et al,1 10 of 11 patients who underwent laboratory analysis self-reported eating a diet rich in fruit and vegetables, though no standardized questionnaire was given. One patient was found to have an elevated vitamin E level, and in 5 cases there was an elevated level of β-cryptoxanthin. The significance of these elevations in such a small minority is unknown, and increased β-cryptoxanthin has been attributed to increased consumption of citrus fruits during the winter season. Our patient reported ingesting a daily oral supplement rich in carotenoids that constituted 60% of the daily value of vitamin E including mixed tocopherols as well as 90% of the daily value of vitamin A with many sources of carotenoids including beta-carotenes, lutein/zeaxanthin, lycopene, and astaxanthin. An invasive biopsy was not taken in this case, as OPSs largely are diagnosed clinically. Greater awareness and recognition of OPSs may help to identify common underlying causes for this unique diagnosis.
- Assouly P, Cavelier-Balloy B, Dupré T. Orange palpebral spots. Dermatology. 2008;216:166-170.
- Belliveau MJ, Odashiro AN, Harvey JT. Yellow-orange palpebral spots. Ophthalmology. 2015;122:2139-2140.
- Kluger N, Guillot B. Bilateral orange discoloration of the upper eyelids: a quiz. Acta Derm Venereol. 2011;91:211-212.
- Maharshak N, Shapiro J, Trau H. Carotenoderma—a review of the current literature. Int J Dermatol. 2003;42:178-181.
The Diagnosis: Orange Palpebral Spots
The clinical presentation of our patient was consistent with a diagnosis of orange palpebral spots (OPSs), an uncommon discoloration that most often appears in White patients in the fifth or sixth decades of life. Orange palpebral spots were first described in 2008 by Assouly et al1 in 27 patients (23 females and 4 males). In 2015, Belliveau et al2 expanded the designation to yellow-orange palpebral spots because they felt the term more fully expressed the color variations depicted in their patients; however, this term more frequently is used in ophthalmology.
Orange palpebral spots commonly appear as asymptomatic, yellow-orange, symmetric lesions with a predilection for the recessed areas of the superior eyelids but also can present on the canthi and inferior eyelids. The discolorations are more easily visible on fair skin and have been reported to measure from 10 to 15 mm in the long axis.3 Assouly et al1 described the orange spots as having indistinct margins, with borders similar to “sand on a sea shore.” Orange palpebral spots can be a persistent discoloration, and there are no reports of spontaneous regression. No known association with malignancy or systemic illness has been reported.
Case reports of OPSs describe histologic similarities between specimens, including increased adipose tissue and pigment-laden macrophages in the superficial dermis.2 The pigmented deposits sometimes may be found in the basal keratinocytes of the epidermis and turn black with Fontana-Masson stain.1 No inflammatory infiltrates, necrosis, or xanthomization are characteristically found. Stains for iron, mucin, and amyloid also have been negative.2
The cause of pigmentation in OPSs is unknown; however, lipofuscin deposits and high-situated adipocytes in the reticular dermis colored by carotenoids have been proposed as possible mechanisms.1 No unifying cause for pigmentation in the serum (eg, cholesterol, triglycerides, thyroid-stimulating hormone, free retinol, vitamin E, carotenoids) was found in 11 of 27 patients with OPSs assessed by Assouly et al.1 In one case, lipofuscin, a degradation product of lysosomes, was detected by microscopic autofluorescence in the superficial dermis. However, lipofuscin typically is a breakdown product associated with aging, and OPSs have been present in patients as young as 28 years.1 Local trauma related to eye rubbing is another theory that has been proposed due to the finding of melanin in the superficial dermis. However, the absence of hemosiderin deposits as well as the extensive duration of the discolorations makes local trauma a less likely explanation for the etiology of OPSs.2
The clinical differential diagnosis for OPSs includes xanthelasma, jaundice, and carotenoderma. Xanthelasma presents as elevated yellow plaques usually found over the medial aspect of the eyes. In contrast, OPSs are nonelevated with both orange and yellow hues typically present. Histologic samples of xanthelasma are characterized by lipid-laden macrophages (foam cells) in the dermis in contrast to the adipose tissue seen in OPSs that has not been phagocytized.1,2 The lack of scleral icterus made jaundice an unlikely diagnosis in our patient. Bilirubin elevations substantial enough to cause skin discoloration also would be expected to discolor the conjunctiva. In carotenoderma, carotenoids are deposited in the sweat and sebum of the stratum corneum with the orange pigmentation most prominent in regions of increased sweating such as the palms, soles, and nasolabial folds.4 Our patient’s lack of discoloration in places other than the periorbital region made carotenoderma less likely.
In the study by Assouly et al,1 10 of 11 patients who underwent laboratory analysis self-reported eating a diet rich in fruit and vegetables, though no standardized questionnaire was given. One patient was found to have an elevated vitamin E level, and in 5 cases there was an elevated level of β-cryptoxanthin. The significance of these elevations in such a small minority is unknown, and increased β-cryptoxanthin has been attributed to increased consumption of citrus fruits during the winter season. Our patient reported ingesting a daily oral supplement rich in carotenoids that constituted 60% of the daily value of vitamin E including mixed tocopherols as well as 90% of the daily value of vitamin A with many sources of carotenoids including beta-carotenes, lutein/zeaxanthin, lycopene, and astaxanthin. An invasive biopsy was not taken in this case, as OPSs largely are diagnosed clinically. Greater awareness and recognition of OPSs may help to identify common underlying causes for this unique diagnosis.
The Diagnosis: Orange Palpebral Spots
The clinical presentation of our patient was consistent with a diagnosis of orange palpebral spots (OPSs), an uncommon discoloration that most often appears in White patients in the fifth or sixth decades of life. Orange palpebral spots were first described in 2008 by Assouly et al1 in 27 patients (23 females and 4 males). In 2015, Belliveau et al2 expanded the designation to yellow-orange palpebral spots because they felt the term more fully expressed the color variations depicted in their patients; however, this term more frequently is used in ophthalmology.
Orange palpebral spots commonly appear as asymptomatic, yellow-orange, symmetric lesions with a predilection for the recessed areas of the superior eyelids but also can present on the canthi and inferior eyelids. The discolorations are more easily visible on fair skin and have been reported to measure from 10 to 15 mm in the long axis.3 Assouly et al1 described the orange spots as having indistinct margins, with borders similar to “sand on a sea shore.” Orange palpebral spots can be a persistent discoloration, and there are no reports of spontaneous regression. No known association with malignancy or systemic illness has been reported.
Case reports of OPSs describe histologic similarities between specimens, including increased adipose tissue and pigment-laden macrophages in the superficial dermis.2 The pigmented deposits sometimes may be found in the basal keratinocytes of the epidermis and turn black with Fontana-Masson stain.1 No inflammatory infiltrates, necrosis, or xanthomization are characteristically found. Stains for iron, mucin, and amyloid also have been negative.2
The cause of pigmentation in OPSs is unknown; however, lipofuscin deposits and high-situated adipocytes in the reticular dermis colored by carotenoids have been proposed as possible mechanisms.1 No unifying cause for pigmentation in the serum (eg, cholesterol, triglycerides, thyroid-stimulating hormone, free retinol, vitamin E, carotenoids) was found in 11 of 27 patients with OPSs assessed by Assouly et al.1 In one case, lipofuscin, a degradation product of lysosomes, was detected by microscopic autofluorescence in the superficial dermis. However, lipofuscin typically is a breakdown product associated with aging, and OPSs have been present in patients as young as 28 years.1 Local trauma related to eye rubbing is another theory that has been proposed due to the finding of melanin in the superficial dermis. However, the absence of hemosiderin deposits as well as the extensive duration of the discolorations makes local trauma a less likely explanation for the etiology of OPSs.2
The clinical differential diagnosis for OPSs includes xanthelasma, jaundice, and carotenoderma. Xanthelasma presents as elevated yellow plaques usually found over the medial aspect of the eyes. In contrast, OPSs are nonelevated with both orange and yellow hues typically present. Histologic samples of xanthelasma are characterized by lipid-laden macrophages (foam cells) in the dermis in contrast to the adipose tissue seen in OPSs that has not been phagocytized.1,2 The lack of scleral icterus made jaundice an unlikely diagnosis in our patient. Bilirubin elevations substantial enough to cause skin discoloration also would be expected to discolor the conjunctiva. In carotenoderma, carotenoids are deposited in the sweat and sebum of the stratum corneum with the orange pigmentation most prominent in regions of increased sweating such as the palms, soles, and nasolabial folds.4 Our patient’s lack of discoloration in places other than the periorbital region made carotenoderma less likely.
In the study by Assouly et al,1 10 of 11 patients who underwent laboratory analysis self-reported eating a diet rich in fruit and vegetables, though no standardized questionnaire was given. One patient was found to have an elevated vitamin E level, and in 5 cases there was an elevated level of β-cryptoxanthin. The significance of these elevations in such a small minority is unknown, and increased β-cryptoxanthin has been attributed to increased consumption of citrus fruits during the winter season. Our patient reported ingesting a daily oral supplement rich in carotenoids that constituted 60% of the daily value of vitamin E including mixed tocopherols as well as 90% of the daily value of vitamin A with many sources of carotenoids including beta-carotenes, lutein/zeaxanthin, lycopene, and astaxanthin. An invasive biopsy was not taken in this case, as OPSs largely are diagnosed clinically. Greater awareness and recognition of OPSs may help to identify common underlying causes for this unique diagnosis.
- Assouly P, Cavelier-Balloy B, Dupré T. Orange palpebral spots. Dermatology. 2008;216:166-170.
- Belliveau MJ, Odashiro AN, Harvey JT. Yellow-orange palpebral spots. Ophthalmology. 2015;122:2139-2140.
- Kluger N, Guillot B. Bilateral orange discoloration of the upper eyelids: a quiz. Acta Derm Venereol. 2011;91:211-212.
- Maharshak N, Shapiro J, Trau H. Carotenoderma—a review of the current literature. Int J Dermatol. 2003;42:178-181.
- Assouly P, Cavelier-Balloy B, Dupré T. Orange palpebral spots. Dermatology. 2008;216:166-170.
- Belliveau MJ, Odashiro AN, Harvey JT. Yellow-orange palpebral spots. Ophthalmology. 2015;122:2139-2140.
- Kluger N, Guillot B. Bilateral orange discoloration of the upper eyelids: a quiz. Acta Derm Venereol. 2011;91:211-212.
- Maharshak N, Shapiro J, Trau H. Carotenoderma—a review of the current literature. Int J Dermatol. 2003;42:178-181.
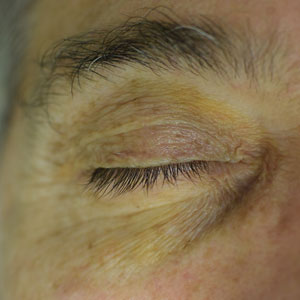
A 63-year-old White man with a history of melanoma presented to our dermatology clinic for evaluation of gradually worsening yellow discoloration around the eyes of 2 years’ duration. Physical examination revealed periorbital yellow-orange patches (top). The discolorations were nonelevated and nonpalpable. Dermoscopy revealed yellow blotches with sparing of the hair follicles (bottom). The remainder of the skin examination was unremarkable.
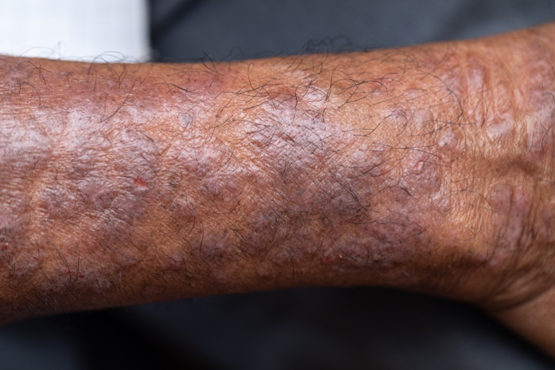
Pruritic rash on arms and legs
Atopic dermatitis (AD) is one of the most common chronic, inflammatory skin diseases encountered by dermatologists. AD is characterized by pruritus and a chronic course of exacerbations and remissions. AD is thought to involve the interplay of genetic predisposition, immune dysregulation, and environmental factors. It is also associated with other allergic conditions, including asthma.
Although AD typically presents with pruritus as the hallmark symptom in all patients, the appearance of skin lesions may vary among different skin types. In individuals with light-colored skin, AD often appears as erythematous patches and plaques. It also more commonly affects the flexor surfaces of the skin. In individuals with darker skin tones, AD may more often result in follicularly centered papules, lichenification, and pigmentary changes. Lesions may also present on extensor surfaces rather than the typical flexure surfaces. Erythema in darker skin types may appear reddish-brown, have a violaceous hue, or be an ashen gray or darker brown color rather than bright red. Because erythema is more difficult to detect in darker skin types, clinicians may mistakenly minimize the severity of AD.
Clinical severity may also differ between ethnicities. Black patients have an increased tendency toward hyperlinearity of the palms, periorbital dark circles, Dennie-Morgan lines, and diffuse xerosis. Compared with White patients, Black patients with AD are also more likely to develop prurigo nodularis and lichenification. In contrast, Asian patients with AD often experience psoriasiform features, with lesions having more well-defined borders and increased scaling and lichenification.
Beyond differences in clinical appearance, AD may appear molecularly and histologically distinct in ethnic skin. One study suggests that Black patients with AD may have decreased Th1 and Th17 but share similar upregulation of Th2 and Th22 as seen in White patients. Another study showed that Asian patients may have higher Th17 and Th22 and lower Th1/interferon compared with White patients.
Regardless of skin type, treatment goals remain the same. Treatment goals aim to repair and improve the function of the skin barrier while preventing and managing flares. Clinical studies have shown that skincare regimens incorporating ceramide-containing moisturizers may improve AD by increasing the lipid content in the skin. This may offer clinical benefit in patients with skin of color. However, some treatments often used for AD may lead to other skin issues in skin in color. For example, long-term use of topical steroids may worsen hypopigmentation in darker skin types. Management strategies should take into account the unique clinical and genetic features of AD among different patient demographic groups.
William D. James, MD, Professor, Department of Dermatology, University of Pennsylvania, Philadelphia.
Disclosure: William D. James, MD, has disclosed the following relevant financial relationships:
Received income in an amount equal to or greater than $250 from: Elsevier.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Atopic dermatitis (AD) is one of the most common chronic, inflammatory skin diseases encountered by dermatologists. AD is characterized by pruritus and a chronic course of exacerbations and remissions. AD is thought to involve the interplay of genetic predisposition, immune dysregulation, and environmental factors. It is also associated with other allergic conditions, including asthma.
Although AD typically presents with pruritus as the hallmark symptom in all patients, the appearance of skin lesions may vary among different skin types. In individuals with light-colored skin, AD often appears as erythematous patches and plaques. It also more commonly affects the flexor surfaces of the skin. In individuals with darker skin tones, AD may more often result in follicularly centered papules, lichenification, and pigmentary changes. Lesions may also present on extensor surfaces rather than the typical flexure surfaces. Erythema in darker skin types may appear reddish-brown, have a violaceous hue, or be an ashen gray or darker brown color rather than bright red. Because erythema is more difficult to detect in darker skin types, clinicians may mistakenly minimize the severity of AD.
Clinical severity may also differ between ethnicities. Black patients have an increased tendency toward hyperlinearity of the palms, periorbital dark circles, Dennie-Morgan lines, and diffuse xerosis. Compared with White patients, Black patients with AD are also more likely to develop prurigo nodularis and lichenification. In contrast, Asian patients with AD often experience psoriasiform features, with lesions having more well-defined borders and increased scaling and lichenification.
Beyond differences in clinical appearance, AD may appear molecularly and histologically distinct in ethnic skin. One study suggests that Black patients with AD may have decreased Th1 and Th17 but share similar upregulation of Th2 and Th22 as seen in White patients. Another study showed that Asian patients may have higher Th17 and Th22 and lower Th1/interferon compared with White patients.
Regardless of skin type, treatment goals remain the same. Treatment goals aim to repair and improve the function of the skin barrier while preventing and managing flares. Clinical studies have shown that skincare regimens incorporating ceramide-containing moisturizers may improve AD by increasing the lipid content in the skin. This may offer clinical benefit in patients with skin of color. However, some treatments often used for AD may lead to other skin issues in skin in color. For example, long-term use of topical steroids may worsen hypopigmentation in darker skin types. Management strategies should take into account the unique clinical and genetic features of AD among different patient demographic groups.
William D. James, MD, Professor, Department of Dermatology, University of Pennsylvania, Philadelphia.
Disclosure: William D. James, MD, has disclosed the following relevant financial relationships:
Received income in an amount equal to or greater than $250 from: Elsevier.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Atopic dermatitis (AD) is one of the most common chronic, inflammatory skin diseases encountered by dermatologists. AD is characterized by pruritus and a chronic course of exacerbations and remissions. AD is thought to involve the interplay of genetic predisposition, immune dysregulation, and environmental factors. It is also associated with other allergic conditions, including asthma.
Although AD typically presents with pruritus as the hallmark symptom in all patients, the appearance of skin lesions may vary among different skin types. In individuals with light-colored skin, AD often appears as erythematous patches and plaques. It also more commonly affects the flexor surfaces of the skin. In individuals with darker skin tones, AD may more often result in follicularly centered papules, lichenification, and pigmentary changes. Lesions may also present on extensor surfaces rather than the typical flexure surfaces. Erythema in darker skin types may appear reddish-brown, have a violaceous hue, or be an ashen gray or darker brown color rather than bright red. Because erythema is more difficult to detect in darker skin types, clinicians may mistakenly minimize the severity of AD.
Clinical severity may also differ between ethnicities. Black patients have an increased tendency toward hyperlinearity of the palms, periorbital dark circles, Dennie-Morgan lines, and diffuse xerosis. Compared with White patients, Black patients with AD are also more likely to develop prurigo nodularis and lichenification. In contrast, Asian patients with AD often experience psoriasiform features, with lesions having more well-defined borders and increased scaling and lichenification.
Beyond differences in clinical appearance, AD may appear molecularly and histologically distinct in ethnic skin. One study suggests that Black patients with AD may have decreased Th1 and Th17 but share similar upregulation of Th2 and Th22 as seen in White patients. Another study showed that Asian patients may have higher Th17 and Th22 and lower Th1/interferon compared with White patients.
Regardless of skin type, treatment goals remain the same. Treatment goals aim to repair and improve the function of the skin barrier while preventing and managing flares. Clinical studies have shown that skincare regimens incorporating ceramide-containing moisturizers may improve AD by increasing the lipid content in the skin. This may offer clinical benefit in patients with skin of color. However, some treatments often used for AD may lead to other skin issues in skin in color. For example, long-term use of topical steroids may worsen hypopigmentation in darker skin types. Management strategies should take into account the unique clinical and genetic features of AD among different patient demographic groups.
William D. James, MD, Professor, Department of Dermatology, University of Pennsylvania, Philadelphia.
Disclosure: William D. James, MD, has disclosed the following relevant financial relationships:
Received income in an amount equal to or greater than $250 from: Elsevier.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
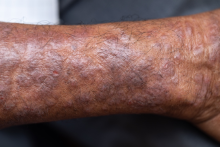
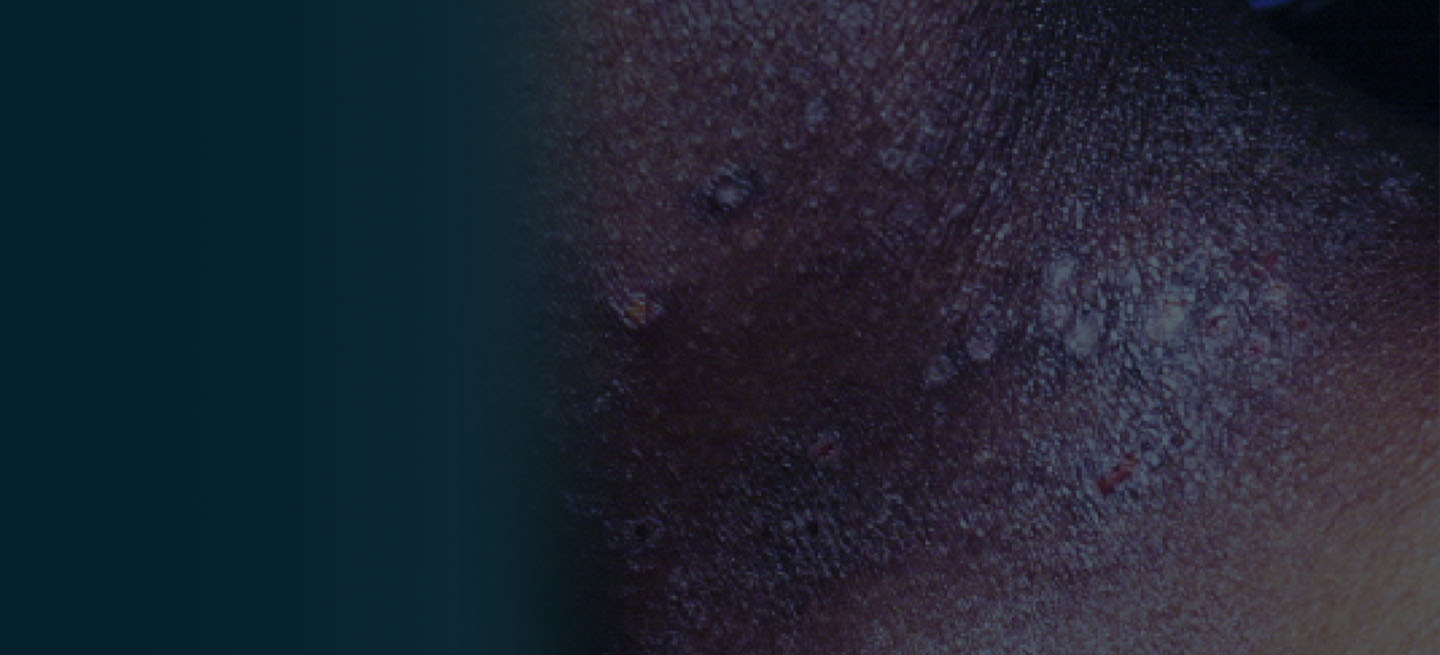
A 27-year-old student presents with a pruritic rash on his hands and in the bends of his arms and legs. He recently started clinical rotations in a nursing facility and has been using hand sanitizer multiple times per day, which has exacerbated the rash on his hands, causing them to ooze and sting. He describes the rash as itchy, especially at night. At times he reports that the itching causes difficulty sleeping. In addition, his skin has little cracks that frequently bleed. He notes that he has experienced similar symptoms in the past, which resolved with moisturizers and topical cream from the drugstore. He has tried over-the-counter hydrocortisone during this episode, with minimal improvement in symptoms. He denies any change in laundry detergents or use of new household products.
Physical examination reveals large erythematous plaques on the hands and flexure surfaces of his neck, antecubital fossa, and behind the knees with scattered excoriations. Erythematous, slightly lichenified coalescing papules are noted on the proximal arms. His face is clear. General skin pigmentation is brown and free of masses and lumps.
Product updates and reviews
HEGENBERGER RETRACTOR: IS IT HELPFUL FOR PERINEAL REPAIR?
The Hegenberger Retractor, manufactured by Hegenberger Medical (Abingdon, United Kingdom) is available for purchase in the United States through Rocket Medical. A video that I find particularly useful for explaining its use is available here: https://www.youtube.com /watch?v=p-jilXgXZLY
Background. About 85% of women having a vaginal birth experience some form of perineal trauma, and 60% to 70% receive stitches for those spontaneous tears or intentional incisions. As such, repairing perineal lacerations is a requisite skill for all obstetricians and midwives, and every provider has developed exposure techniques to perform their suturing with the goals of good tissue re-approximation, efficiency, minimized patient discomfort, reduced blood loss, and safety from needle sticks. For several millennia, the most commonly used tissue retractor for these repairs has been one’s own fingers, or those of a colleague. While cost-effective and readily available, fingers do have drawbacks as a vaginal retractor. First, their use as a retractor precludes their use for other tasks. Second, their frequent need to be inserted and replaced (see drawback #1) can be uncomfortable for patients. Third, their limited surface area is often insufficient to appropriately provide adequate tissue retraction for optimal surgical site visualization. Finally, they get tired and typically do not appreciate being stuck with needles. Given all this, it is surprising that so many centuries have passed with so little innovation for this ubiquitous procedure. Fortunately, Danish midwife Malene Hegenberger thought now was a good time to change the status quo.
Design/Functionality. The Hegenberger Retractor is brilliant in its simplicity. Its unique molded plastic design is smooth, ergonomic, nonconductive, and packaged as a single-use sterile device. Amazingly, it has a near-perfect pliability balance, making it simultaneously easy to compress for insertion while providing enough retraction tension for good visualization once it has been reexpanded. The subtle ridges on the compression points are just enough to allow for a good grip, and the notches on the sides are a convenient addition for holding extra suture if needed. The device has been cleared by the US Food and Drug Administration (FDA) as a Class 1 device and is approved for sale in the United States. In my experience with its use, I thought it was easy to place and provided excellent exposure for the repairs I was doing. In fact, I thought it provided as good if not better exposure than what I would expect from a Gelpi retractor without any of the trauma the Gelpi adds with its pointed ends. Smile emoji!
Innovation. In the early 1800s, French midwifery pioneer Marie Boivin introduced a novel pelvimeter and a revolutionary 2-part speculum to the technology of the day. Why it took more than 200 years for the ideas of another cutting-edge midwife to breach the walls of the obstetric technological establishment remains a mystery, but fortunately it has been done. While seemingly obvious, the Hegenberger Retractor is the culmination of years of work and 88 prototypes. It looks simple, but the secret to its functionality is the precision with which each dimension and every curve was designed. The device has been cleared by the FDA as a Class 1 device and is approved for sale in the United States.
Summary. There are a lot of reasons to like the Hegenberger Retractor. I like it for its simplicity; I like it for its functionality; I like it for its ability to fill a real need. On the downside, I do not like that it is a single-use plastic device, and I am not happy about adding cost to obstetric care. Most of all, I hate that I did not invent it.
Is the Hegenberger Retractor going to be needed to repair every obstetric laceration? No. Will it provide perfect exposure to repair every obstetric laceration? Of course not. But it is an incredibly clever device that will be very helpful in many situations, and I suspect it will soon become a mainstay on most maternity units as it gains recognition.
FOR MORE INFORMATION, VISIT www.rocketmedical.com
- McCandlish R, Bowler U, van Asten H, et al. A randomised controlled trial of care of the perineum during second stage of normal labour. Br J Obstet Gynaecol. 1998;105:1262-1272.
- Ferry G. Marie Boivin: from midwife to gynaecologist. Lancet. 2019;393:2192-2193. doi: 10.1016/S0140-6736(19)31188-2.
HEGENBERGER RETRACTOR: IS IT HELPFUL FOR PERINEAL REPAIR?
The Hegenberger Retractor, manufactured by Hegenberger Medical (Abingdon, United Kingdom) is available for purchase in the United States through Rocket Medical. A video that I find particularly useful for explaining its use is available here: https://www.youtube.com /watch?v=p-jilXgXZLY
Background. About 85% of women having a vaginal birth experience some form of perineal trauma, and 60% to 70% receive stitches for those spontaneous tears or intentional incisions. As such, repairing perineal lacerations is a requisite skill for all obstetricians and midwives, and every provider has developed exposure techniques to perform their suturing with the goals of good tissue re-approximation, efficiency, minimized patient discomfort, reduced blood loss, and safety from needle sticks. For several millennia, the most commonly used tissue retractor for these repairs has been one’s own fingers, or those of a colleague. While cost-effective and readily available, fingers do have drawbacks as a vaginal retractor. First, their use as a retractor precludes their use for other tasks. Second, their frequent need to be inserted and replaced (see drawback #1) can be uncomfortable for patients. Third, their limited surface area is often insufficient to appropriately provide adequate tissue retraction for optimal surgical site visualization. Finally, they get tired and typically do not appreciate being stuck with needles. Given all this, it is surprising that so many centuries have passed with so little innovation for this ubiquitous procedure. Fortunately, Danish midwife Malene Hegenberger thought now was a good time to change the status quo.
Design/Functionality. The Hegenberger Retractor is brilliant in its simplicity. Its unique molded plastic design is smooth, ergonomic, nonconductive, and packaged as a single-use sterile device. Amazingly, it has a near-perfect pliability balance, making it simultaneously easy to compress for insertion while providing enough retraction tension for good visualization once it has been reexpanded. The subtle ridges on the compression points are just enough to allow for a good grip, and the notches on the sides are a convenient addition for holding extra suture if needed. The device has been cleared by the US Food and Drug Administration (FDA) as a Class 1 device and is approved for sale in the United States. In my experience with its use, I thought it was easy to place and provided excellent exposure for the repairs I was doing. In fact, I thought it provided as good if not better exposure than what I would expect from a Gelpi retractor without any of the trauma the Gelpi adds with its pointed ends. Smile emoji!
Innovation. In the early 1800s, French midwifery pioneer Marie Boivin introduced a novel pelvimeter and a revolutionary 2-part speculum to the technology of the day. Why it took more than 200 years for the ideas of another cutting-edge midwife to breach the walls of the obstetric technological establishment remains a mystery, but fortunately it has been done. While seemingly obvious, the Hegenberger Retractor is the culmination of years of work and 88 prototypes. It looks simple, but the secret to its functionality is the precision with which each dimension and every curve was designed. The device has been cleared by the FDA as a Class 1 device and is approved for sale in the United States.
Summary. There are a lot of reasons to like the Hegenberger Retractor. I like it for its simplicity; I like it for its functionality; I like it for its ability to fill a real need. On the downside, I do not like that it is a single-use plastic device, and I am not happy about adding cost to obstetric care. Most of all, I hate that I did not invent it.
Is the Hegenberger Retractor going to be needed to repair every obstetric laceration? No. Will it provide perfect exposure to repair every obstetric laceration? Of course not. But it is an incredibly clever device that will be very helpful in many situations, and I suspect it will soon become a mainstay on most maternity units as it gains recognition.
FOR MORE INFORMATION, VISIT www.rocketmedical.com
HEGENBERGER RETRACTOR: IS IT HELPFUL FOR PERINEAL REPAIR?
The Hegenberger Retractor, manufactured by Hegenberger Medical (Abingdon, United Kingdom) is available for purchase in the United States through Rocket Medical. A video that I find particularly useful for explaining its use is available here: https://www.youtube.com /watch?v=p-jilXgXZLY
Background. About 85% of women having a vaginal birth experience some form of perineal trauma, and 60% to 70% receive stitches for those spontaneous tears or intentional incisions. As such, repairing perineal lacerations is a requisite skill for all obstetricians and midwives, and every provider has developed exposure techniques to perform their suturing with the goals of good tissue re-approximation, efficiency, minimized patient discomfort, reduced blood loss, and safety from needle sticks. For several millennia, the most commonly used tissue retractor for these repairs has been one’s own fingers, or those of a colleague. While cost-effective and readily available, fingers do have drawbacks as a vaginal retractor. First, their use as a retractor precludes their use for other tasks. Second, their frequent need to be inserted and replaced (see drawback #1) can be uncomfortable for patients. Third, their limited surface area is often insufficient to appropriately provide adequate tissue retraction for optimal surgical site visualization. Finally, they get tired and typically do not appreciate being stuck with needles. Given all this, it is surprising that so many centuries have passed with so little innovation for this ubiquitous procedure. Fortunately, Danish midwife Malene Hegenberger thought now was a good time to change the status quo.
Design/Functionality. The Hegenberger Retractor is brilliant in its simplicity. Its unique molded plastic design is smooth, ergonomic, nonconductive, and packaged as a single-use sterile device. Amazingly, it has a near-perfect pliability balance, making it simultaneously easy to compress for insertion while providing enough retraction tension for good visualization once it has been reexpanded. The subtle ridges on the compression points are just enough to allow for a good grip, and the notches on the sides are a convenient addition for holding extra suture if needed. The device has been cleared by the US Food and Drug Administration (FDA) as a Class 1 device and is approved for sale in the United States. In my experience with its use, I thought it was easy to place and provided excellent exposure for the repairs I was doing. In fact, I thought it provided as good if not better exposure than what I would expect from a Gelpi retractor without any of the trauma the Gelpi adds with its pointed ends. Smile emoji!
Innovation. In the early 1800s, French midwifery pioneer Marie Boivin introduced a novel pelvimeter and a revolutionary 2-part speculum to the technology of the day. Why it took more than 200 years for the ideas of another cutting-edge midwife to breach the walls of the obstetric technological establishment remains a mystery, but fortunately it has been done. While seemingly obvious, the Hegenberger Retractor is the culmination of years of work and 88 prototypes. It looks simple, but the secret to its functionality is the precision with which each dimension and every curve was designed. The device has been cleared by the FDA as a Class 1 device and is approved for sale in the United States.
Summary. There are a lot of reasons to like the Hegenberger Retractor. I like it for its simplicity; I like it for its functionality; I like it for its ability to fill a real need. On the downside, I do not like that it is a single-use plastic device, and I am not happy about adding cost to obstetric care. Most of all, I hate that I did not invent it.
Is the Hegenberger Retractor going to be needed to repair every obstetric laceration? No. Will it provide perfect exposure to repair every obstetric laceration? Of course not. But it is an incredibly clever device that will be very helpful in many situations, and I suspect it will soon become a mainstay on most maternity units as it gains recognition.
FOR MORE INFORMATION, VISIT www.rocketmedical.com
- McCandlish R, Bowler U, van Asten H, et al. A randomised controlled trial of care of the perineum during second stage of normal labour. Br J Obstet Gynaecol. 1998;105:1262-1272.
- Ferry G. Marie Boivin: from midwife to gynaecologist. Lancet. 2019;393:2192-2193. doi: 10.1016/S0140-6736(19)31188-2.
- McCandlish R, Bowler U, van Asten H, et al. A randomised controlled trial of care of the perineum during second stage of normal labour. Br J Obstet Gynaecol. 1998;105:1262-1272.
- Ferry G. Marie Boivin: from midwife to gynaecologist. Lancet. 2019;393:2192-2193. doi: 10.1016/S0140-6736(19)31188-2.
COMMENT & CONTROVERSY
Should treatment be initiated for mild chronic hypertension in pregnancy to improve outcomes?
JAIMEY M. PAULI, MD (JUNE 2022)
Consider this, when it comes to treating chronic hypertension
I welcome the article by Dr. Jaimey Pauli, which focuses on initiating treatment for mild chronic hypertension in pregnancy to reach a goal blood pressure (BP) of <140/90 mm Hg to prevent adverse maternal and fetal outcomes.1 I would like to offer 3 additional thoughts for your consideration. First, it is known that there is a physiological decrease in BP during the second trimester, which results in a normotensive presentation. Thus, it would be beneficial to see if pregnant women with high-normal BP levels before the third trimester be administered a lower dose of antihypertensives. However, there is also a concern that decreased maternal BP may compromise uteroplacental perfusion and fetal circulation, which also could be evaluated.2
Second, I would like to see how comorbidities affect the initiation of antihypertensives for mild chronic hypertension in pregnancy. Research incorporating pregnant women with borderline hypertension and comorbidities such as obesity, hyperlipidemia, and diabetes mellitus type 2 (DM) is likely to yield informative results. This is especially beneficial since, for example, chronic hypertension and DM are independent risk factors for adverse maternal and fetal outcomes; therefore, a mother with both these conditions may have additive effects on obstetric outcomes.3
Lastly, I would suggest you include a brief conversation about prepregnancy ways to manage women with chronic hypertension. Because many women who enter pregnancy with chronic hypertension have hypertension of unknown origin, it would be beneficial to optimize antihypertensive regimens before conception.4 Also, it should be further evaluated whether initiation of lifestyle modifications, such as weight reduction and the DASH diet before pregnancy, for women with chronic hypertension improves pregnancy outcomes.
Cassandra Maafoh, MD
Macon, Georgia
References
- Pauli JM. Should treatment be initiated for mild chronic hypertension in pregnancy to improve outcomes? OBG Manag. 2022;34:14-15.
- Brown CM, Garovic VD. Drug treatment of hypertension in pregnancy. Drugs. 2014;74:283-296. https://doi.org/10.1007/s40265-014-0187-7.
- Yanit KE, Snowden JM, Cheng YW, et al. The impact of chronic hypertension and pregestational diabetes on pregnancy outcomes. Am J Obstet Gynecol. 2012;207. https://doi. org/10.1016/j.ajog.2012.06.066.
- Seely EW, Ecker J. Chronic hypertension in pregnancy. Circulation. 2014;129:1254-1261. https:// doi.org/10.1161/circulationaha.113.003904.
BARBARA LEVY, MD (AUGUST 2022)
Are these new and rare syndromes’ pathophysiological mechanisms related?
I read with great interest Dr. Barbara Levy’s UPDATE in the August 2022 issue on testosterone therapy for women with hypoactive sexual desire disorder (HSDD), as well as her comments on persistent genital arousal disorder/genito-pelvic dysesthesia (PGAD/GPD) that was recently so coined by the International Society for the Study of Women’s Sexual Health (ISSWSH) as a 2-component syndrome.1 The new syndrome, explains Dr. Levy, presents with “the perception of genital arousal that is involuntary, unrelated to sexual desire, without any identified cause, not relieved with orgasm, and distressing to the patient (the PGAD component),” combined with “itching, burning, tingling, or pain” (the GPD component).
Although agreeing with ISSWSH that diagnosis and management require a multidisciplinary biopsychosocial approach, in her practical advice, Dr. Levy mentioned: “neuropathic signaling” with “aberrant sensory processing” as the syndrome’s possible main pathophysiology. Interestingly, there are 2 other rare, chronic, and “poorly recognized source(s) of major distress to a small but significant group of patients.” Persistent idiopathic oro-facial pain (PIFP) disorder2 after dental interventions and burning mouth syndrome (BMS),3 defined by the absence of any local or systemic contributing etiology, also present with continuous local burning and pain (as in GPD). Consequently, PGAD/GPD may indeed have the same pathophysiological explanation—as Dr. Levy suggested—of being a (genital) peripheral chronic neuropathic pain condition.
A potentially promising new therapeutic approach for PGAD/GPD would then be to use the same, or similar, antineuropathic medications (Clonazepam, Nortriptyline, Pregabalin, etc.) in the form of topical vaginal swishing solutions similar to the presently recommended antiepileptic and/or antidepressant oral swishing treatment for PIFP and BMS. As the topical approach works well for oral neuropathic pain, vaginal swishing could potentially be the answer for PGAD/GPD peripheral neuropathic pain. Moreover, such a novel topical approach would significantly increase patient motivation for treatment by avoiding the adverse effects of ingested antiepileptic or antidepressant drugs.
This is the first time that anticonvulsant and/or antidepressant vaginal swishing is proposed as topical therapy for GPD peripheral neuropathic pain, still pending scientific/clinical validation. ●
Zwi Hoch, MD
Newton, Massachusetts
- Goldstein I, Komisaruk BR, Pukall CF, et al. International Society for the Study of Women’s Sexual Health (ISSWSH) Review of Epidemiology and Pathophysiology, and a Consensus Nomenclature and Process of Care for the Management of Persistent Genital Arousal Disorder/Genito-Pelvic Dysesthesia (PGAD/GPD). J Sex Med. 2021;18:665-697.
- Baad-Hansen L, Benoliel R. Neuropathic orofacial pain: facts and fiction. Cephalgia. 2017;37:670-679.
- Kuten-Shorer M, Treister NS, Stock S, et al. Safety and tolerability of topical clonazepam solution for management of oral dysesthesia. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;124: 146-151.
Should treatment be initiated for mild chronic hypertension in pregnancy to improve outcomes?
JAIMEY M. PAULI, MD (JUNE 2022)
Consider this, when it comes to treating chronic hypertension
I welcome the article by Dr. Jaimey Pauli, which focuses on initiating treatment for mild chronic hypertension in pregnancy to reach a goal blood pressure (BP) of <140/90 mm Hg to prevent adverse maternal and fetal outcomes.1 I would like to offer 3 additional thoughts for your consideration. First, it is known that there is a physiological decrease in BP during the second trimester, which results in a normotensive presentation. Thus, it would be beneficial to see if pregnant women with high-normal BP levels before the third trimester be administered a lower dose of antihypertensives. However, there is also a concern that decreased maternal BP may compromise uteroplacental perfusion and fetal circulation, which also could be evaluated.2
Second, I would like to see how comorbidities affect the initiation of antihypertensives for mild chronic hypertension in pregnancy. Research incorporating pregnant women with borderline hypertension and comorbidities such as obesity, hyperlipidemia, and diabetes mellitus type 2 (DM) is likely to yield informative results. This is especially beneficial since, for example, chronic hypertension and DM are independent risk factors for adverse maternal and fetal outcomes; therefore, a mother with both these conditions may have additive effects on obstetric outcomes.3
Lastly, I would suggest you include a brief conversation about prepregnancy ways to manage women with chronic hypertension. Because many women who enter pregnancy with chronic hypertension have hypertension of unknown origin, it would be beneficial to optimize antihypertensive regimens before conception.4 Also, it should be further evaluated whether initiation of lifestyle modifications, such as weight reduction and the DASH diet before pregnancy, for women with chronic hypertension improves pregnancy outcomes.
Cassandra Maafoh, MD
Macon, Georgia
References
- Pauli JM. Should treatment be initiated for mild chronic hypertension in pregnancy to improve outcomes? OBG Manag. 2022;34:14-15.
- Brown CM, Garovic VD. Drug treatment of hypertension in pregnancy. Drugs. 2014;74:283-296. https://doi.org/10.1007/s40265-014-0187-7.
- Yanit KE, Snowden JM, Cheng YW, et al. The impact of chronic hypertension and pregestational diabetes on pregnancy outcomes. Am J Obstet Gynecol. 2012;207. https://doi. org/10.1016/j.ajog.2012.06.066.
- Seely EW, Ecker J. Chronic hypertension in pregnancy. Circulation. 2014;129:1254-1261. https:// doi.org/10.1161/circulationaha.113.003904.
BARBARA LEVY, MD (AUGUST 2022)
Are these new and rare syndromes’ pathophysiological mechanisms related?
I read with great interest Dr. Barbara Levy’s UPDATE in the August 2022 issue on testosterone therapy for women with hypoactive sexual desire disorder (HSDD), as well as her comments on persistent genital arousal disorder/genito-pelvic dysesthesia (PGAD/GPD) that was recently so coined by the International Society for the Study of Women’s Sexual Health (ISSWSH) as a 2-component syndrome.1 The new syndrome, explains Dr. Levy, presents with “the perception of genital arousal that is involuntary, unrelated to sexual desire, without any identified cause, not relieved with orgasm, and distressing to the patient (the PGAD component),” combined with “itching, burning, tingling, or pain” (the GPD component).
Although agreeing with ISSWSH that diagnosis and management require a multidisciplinary biopsychosocial approach, in her practical advice, Dr. Levy mentioned: “neuropathic signaling” with “aberrant sensory processing” as the syndrome’s possible main pathophysiology. Interestingly, there are 2 other rare, chronic, and “poorly recognized source(s) of major distress to a small but significant group of patients.” Persistent idiopathic oro-facial pain (PIFP) disorder2 after dental interventions and burning mouth syndrome (BMS),3 defined by the absence of any local or systemic contributing etiology, also present with continuous local burning and pain (as in GPD). Consequently, PGAD/GPD may indeed have the same pathophysiological explanation—as Dr. Levy suggested—of being a (genital) peripheral chronic neuropathic pain condition.
A potentially promising new therapeutic approach for PGAD/GPD would then be to use the same, or similar, antineuropathic medications (Clonazepam, Nortriptyline, Pregabalin, etc.) in the form of topical vaginal swishing solutions similar to the presently recommended antiepileptic and/or antidepressant oral swishing treatment for PIFP and BMS. As the topical approach works well for oral neuropathic pain, vaginal swishing could potentially be the answer for PGAD/GPD peripheral neuropathic pain. Moreover, such a novel topical approach would significantly increase patient motivation for treatment by avoiding the adverse effects of ingested antiepileptic or antidepressant drugs.
This is the first time that anticonvulsant and/or antidepressant vaginal swishing is proposed as topical therapy for GPD peripheral neuropathic pain, still pending scientific/clinical validation. ●
Zwi Hoch, MD
Newton, Massachusetts
- Goldstein I, Komisaruk BR, Pukall CF, et al. International Society for the Study of Women’s Sexual Health (ISSWSH) Review of Epidemiology and Pathophysiology, and a Consensus Nomenclature and Process of Care for the Management of Persistent Genital Arousal Disorder/Genito-Pelvic Dysesthesia (PGAD/GPD). J Sex Med. 2021;18:665-697.
- Baad-Hansen L, Benoliel R. Neuropathic orofacial pain: facts and fiction. Cephalgia. 2017;37:670-679.
- Kuten-Shorer M, Treister NS, Stock S, et al. Safety and tolerability of topical clonazepam solution for management of oral dysesthesia. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;124: 146-151.
Should treatment be initiated for mild chronic hypertension in pregnancy to improve outcomes?
JAIMEY M. PAULI, MD (JUNE 2022)
Consider this, when it comes to treating chronic hypertension
I welcome the article by Dr. Jaimey Pauli, which focuses on initiating treatment for mild chronic hypertension in pregnancy to reach a goal blood pressure (BP) of <140/90 mm Hg to prevent adverse maternal and fetal outcomes.1 I would like to offer 3 additional thoughts for your consideration. First, it is known that there is a physiological decrease in BP during the second trimester, which results in a normotensive presentation. Thus, it would be beneficial to see if pregnant women with high-normal BP levels before the third trimester be administered a lower dose of antihypertensives. However, there is also a concern that decreased maternal BP may compromise uteroplacental perfusion and fetal circulation, which also could be evaluated.2
Second, I would like to see how comorbidities affect the initiation of antihypertensives for mild chronic hypertension in pregnancy. Research incorporating pregnant women with borderline hypertension and comorbidities such as obesity, hyperlipidemia, and diabetes mellitus type 2 (DM) is likely to yield informative results. This is especially beneficial since, for example, chronic hypertension and DM are independent risk factors for adverse maternal and fetal outcomes; therefore, a mother with both these conditions may have additive effects on obstetric outcomes.3
Lastly, I would suggest you include a brief conversation about prepregnancy ways to manage women with chronic hypertension. Because many women who enter pregnancy with chronic hypertension have hypertension of unknown origin, it would be beneficial to optimize antihypertensive regimens before conception.4 Also, it should be further evaluated whether initiation of lifestyle modifications, such as weight reduction and the DASH diet before pregnancy, for women with chronic hypertension improves pregnancy outcomes.
Cassandra Maafoh, MD
Macon, Georgia
References
- Pauli JM. Should treatment be initiated for mild chronic hypertension in pregnancy to improve outcomes? OBG Manag. 2022;34:14-15.
- Brown CM, Garovic VD. Drug treatment of hypertension in pregnancy. Drugs. 2014;74:283-296. https://doi.org/10.1007/s40265-014-0187-7.
- Yanit KE, Snowden JM, Cheng YW, et al. The impact of chronic hypertension and pregestational diabetes on pregnancy outcomes. Am J Obstet Gynecol. 2012;207. https://doi. org/10.1016/j.ajog.2012.06.066.
- Seely EW, Ecker J. Chronic hypertension in pregnancy. Circulation. 2014;129:1254-1261. https:// doi.org/10.1161/circulationaha.113.003904.
BARBARA LEVY, MD (AUGUST 2022)
Are these new and rare syndromes’ pathophysiological mechanisms related?
I read with great interest Dr. Barbara Levy’s UPDATE in the August 2022 issue on testosterone therapy for women with hypoactive sexual desire disorder (HSDD), as well as her comments on persistent genital arousal disorder/genito-pelvic dysesthesia (PGAD/GPD) that was recently so coined by the International Society for the Study of Women’s Sexual Health (ISSWSH) as a 2-component syndrome.1 The new syndrome, explains Dr. Levy, presents with “the perception of genital arousal that is involuntary, unrelated to sexual desire, without any identified cause, not relieved with orgasm, and distressing to the patient (the PGAD component),” combined with “itching, burning, tingling, or pain” (the GPD component).
Although agreeing with ISSWSH that diagnosis and management require a multidisciplinary biopsychosocial approach, in her practical advice, Dr. Levy mentioned: “neuropathic signaling” with “aberrant sensory processing” as the syndrome’s possible main pathophysiology. Interestingly, there are 2 other rare, chronic, and “poorly recognized source(s) of major distress to a small but significant group of patients.” Persistent idiopathic oro-facial pain (PIFP) disorder2 after dental interventions and burning mouth syndrome (BMS),3 defined by the absence of any local or systemic contributing etiology, also present with continuous local burning and pain (as in GPD). Consequently, PGAD/GPD may indeed have the same pathophysiological explanation—as Dr. Levy suggested—of being a (genital) peripheral chronic neuropathic pain condition.
A potentially promising new therapeutic approach for PGAD/GPD would then be to use the same, or similar, antineuropathic medications (Clonazepam, Nortriptyline, Pregabalin, etc.) in the form of topical vaginal swishing solutions similar to the presently recommended antiepileptic and/or antidepressant oral swishing treatment for PIFP and BMS. As the topical approach works well for oral neuropathic pain, vaginal swishing could potentially be the answer for PGAD/GPD peripheral neuropathic pain. Moreover, such a novel topical approach would significantly increase patient motivation for treatment by avoiding the adverse effects of ingested antiepileptic or antidepressant drugs.
This is the first time that anticonvulsant and/or antidepressant vaginal swishing is proposed as topical therapy for GPD peripheral neuropathic pain, still pending scientific/clinical validation. ●
Zwi Hoch, MD
Newton, Massachusetts
- Goldstein I, Komisaruk BR, Pukall CF, et al. International Society for the Study of Women’s Sexual Health (ISSWSH) Review of Epidemiology and Pathophysiology, and a Consensus Nomenclature and Process of Care for the Management of Persistent Genital Arousal Disorder/Genito-Pelvic Dysesthesia (PGAD/GPD). J Sex Med. 2021;18:665-697.
- Baad-Hansen L, Benoliel R. Neuropathic orofacial pain: facts and fiction. Cephalgia. 2017;37:670-679.
- Kuten-Shorer M, Treister NS, Stock S, et al. Safety and tolerability of topical clonazepam solution for management of oral dysesthesia. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;124: 146-151.
A Dermatology Hospitalist Team’s Response to the Inpatient Consult Flowchart
To the Editor:
We read with interest the Cutis article by Dobkin et al1 (Cutis. 2022;109:218-220) regarding guidelines for inpatient and emergency department dermatology consultations. We agree with the authors that dermatology training is lacking in other medical specialties, which makes it challenging for teams to assess the appropriateness of a dermatology consultation in the inpatient setting. Inpatient dermatology consultation can be utilized in a hospital system to aid in rapid and accurate diagnosis, avoid inappropriate therapies, and decrease length of stay2 and readmission rates3 while providing education to the primary teams. This is precisely why in many instances the availability of inpatient dermatology consultation is so important because nondermatologists often are unable to determine whether a rash is life-threatening, benign, or something in between. From the perspective of dermatology hospitalists, there is room for improvement in the flowchart Dobkin et al1 presented to guide inpatient dermatology consultation.
To have a productive relationship with our internal medicine, surgery, pediatrics, psychiatry, and other hospital-based colleagues, we must keep an open mind when a consultation is received. We feel that the flowchart proposed by Dobkin et al1 presents too narrow a viewpoint on the utility of inpatient dermatology. It rests on assertions that other teams will be able to determine the appropriate dermatologic diagnosis without involving a dermatologist, which often is not the case.
We disagree with several recommendations in the flowchart, the first being the assertion that patients who are “hemodynamically unstable due to [a] nondermatologic problem (eg, intubated on pressors, febrile, and hypotensive)” are not appropriate for inpatient dermatology consultation.1 Although dermatologists do not commonly encounter patients with critical illness in the outpatient clinic, dermatology consultation can be extremely helpful and even lifesaving in the inpatient setting. It is unrealistic to expect the primary teams to know whether cutaneous manifestations potentially could be related to the patient’s overall clinical picture. On the contrary, we would encourage the primary team in charge of a hemodynamically unstable patient to consult dermatology at the first sign of an unexplained rash. Take for example an acutely ill patient who develops retiform purpura. There are well-established dermatology guidelines for the workup of retiform purpura,4 including prompt biopsy and assessment of broad, potentially life-threatening differential diagnoses from calciphylaxis to angioinvasive fungal infection. In this scenario, the dermatology consultant may render the correct diagnosis and recommend immediate treatment that could be lifesaving.
Secondly, we do not agree with the recommendation that a patient in hospice care is not appropriate for inpatient dermatology consultation. Patients receiving hospice or palliative care have high rates of potentially symptomatic cutaneous diseases,5 including intertrigo and dermatitis—comprising stasis, seborrheic, and contact dermatitis.6 Although aggressive intervention for asymptomatic benign or malignant skin conditions may not be in line with their goals of care, an inpatient dermatology consultation can reduce symptoms and improve quality of life. This population also is one that is unlikely to be able to attend an outpatient dermatology clinic appointment and therefore are good candidates for inpatient consultation.
Lastly, we want to highlight the difference between a stable chronic dermatologic disease and an acute flare that may occur while a patient is hospitalized, regardless of whether it is the reason for admission. For example, a patient with psoriasis affecting limited body surface area who is hospitalized for a myocardial infarction is not appropriate for a dermatology consultation. However, if that same patient develops erythroderma while they are hospitalized for cardiac monitoring, it would certainly be appropriate for dermatology to be consulted. Additionally, there are times when a chronic skin disease is the reason for hospitalization; dermatology, although technically a consulting service, would be the primary decision-maker for the patient’s care in this situation. In these scenarios, it is important for the patient to be able to establish care for long-term outpatient management of their condition; however, it is prudent to involve dermatology while the patient is acutely hospitalized to guide their treatment plan until they are able to see a dermatologist after discharge.
In conclusion, we believe that hospital dermatology is a valuable tool that can be utilized in many different scenarios. Although there are certainly situations more appropriate for outpatient dermatology referral, we would caution against overly simplified algorithms that could discourage valuable inpatient dermatology consultations. It often is worth a conversation with your dermatology consultant (when available at an institution) to determine the best course of action for each patient. Additionally, we recognize the need for more formalized guidelines on when to pursue inpatient dermatology consultation. We are members of the Society of Dermatology Hospitalists and encourage readers to reference their website, which provides additional resources on inpatient dermatology (https://societydermatologyhospitalists.com/inpatient-dermatology-literature/).
Authors’ Response
We appreciate the letter in response to our commentary on the appropriateness of inpatient dermatology consultations. It is the continued refining and re-evaluation of concepts such as these that allow our field to grow and improve knowledge and patient care.
We sought to provide a nonpatronizing yet simple consultation flowchart that would help guide triage of patients in need or not in need of dermatologic evaluation by the inpatient teams. Understandably, the impressions of our flowchart have been variable based on different readers’ medical backgrounds and experiences. It is certainly possible that our flowchart lacked certain exceptions and oversimplified certain concepts, and we welcome further refining of this flowchart to better guide inpatient dermatology consultations.
We do, however, disagree that the primary team would not know whether a patient is intubated in the intensive care unit for a dermatology reason. If the patient is in such a status, it would be pertinent for the primary team to conduct a timely workup that could include consultations until a diagnosis is made. We were not implying that every dermatology consultation in the intensive care unit is unwarranted, especially if it can lead to a primary dermatologic diagnosis. We do believe that a thorough history could elicit an allergy or other chronic skin condition that could save resources and spending within a hospital. Likewise, psoriasis comes in many different presentations, and although we do not believe a consultation for chronic psoriatic plaques is appropriate in the hospital, it is absolutely appropriate for a patient who is erythrodermic from any cause.
Our flowchart was intended to be the first step to providing education on when consultations are appropriate, and further refinement will be necessary.
Hershel Dobkin, MD; Timothy Blackwell, BS; Robin Ashinoff, MD
Drs. Dobkin and Ashinoff are from Hackensack University Medical Center, New Jersey. Mr. Blackwell is from the Rowan University School of Osteopathic Medicine, Stratford, New Jersey.
The authors report no conflict of interest.
Correspondence: Hershel Dobkin, MD, Hackensack University Medical Center, 30 Prospect Ave, Hackensack, NJ 07601 ([email protected]).
- Dobkin H, Blackwell T, Ashinoff R. When are inpatient and emergency dermatologic consultations appropriate? Cutis. 2022;109:218-220. doi:10.12788/cutis.0492
- Ko LN, Garza-Mayers AC, St John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis: a randomized clinical trial. JAMA Dermatol. 2018;154:529-536. doi:10.1001/jamadermatol.2017.6196
- Hu L, Haynes H, Ferrazza D, et al. Impact of specialist consultations on inpatient admissions for dermatology-specific and related DRGs. J Gen Intern Med. 2013;28:1477-1482. doi:10.1007/s11606-013-2440-2
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796. doi:10.1016/j.jaad.2019.07.112
- Pisano C, Paladichuk H, Keeling B. Dermatology in palliative medicine [published online October 14, 2021]. BMJ Support Palliat Care. doi:10.1136/bmjspcare-2021-003342
- Barnabé C, Daeninck P. “Beauty is only skin deep”: prevalence of dermatologic disease on a palliative care unit. J Pain Symptom Manage. 2005;29:419-422. doi:10.1016/j.jpainsymman.2004.08.009
To the Editor:
We read with interest the Cutis article by Dobkin et al1 (Cutis. 2022;109:218-220) regarding guidelines for inpatient and emergency department dermatology consultations. We agree with the authors that dermatology training is lacking in other medical specialties, which makes it challenging for teams to assess the appropriateness of a dermatology consultation in the inpatient setting. Inpatient dermatology consultation can be utilized in a hospital system to aid in rapid and accurate diagnosis, avoid inappropriate therapies, and decrease length of stay2 and readmission rates3 while providing education to the primary teams. This is precisely why in many instances the availability of inpatient dermatology consultation is so important because nondermatologists often are unable to determine whether a rash is life-threatening, benign, or something in between. From the perspective of dermatology hospitalists, there is room for improvement in the flowchart Dobkin et al1 presented to guide inpatient dermatology consultation.
To have a productive relationship with our internal medicine, surgery, pediatrics, psychiatry, and other hospital-based colleagues, we must keep an open mind when a consultation is received. We feel that the flowchart proposed by Dobkin et al1 presents too narrow a viewpoint on the utility of inpatient dermatology. It rests on assertions that other teams will be able to determine the appropriate dermatologic diagnosis without involving a dermatologist, which often is not the case.
We disagree with several recommendations in the flowchart, the first being the assertion that patients who are “hemodynamically unstable due to [a] nondermatologic problem (eg, intubated on pressors, febrile, and hypotensive)” are not appropriate for inpatient dermatology consultation.1 Although dermatologists do not commonly encounter patients with critical illness in the outpatient clinic, dermatology consultation can be extremely helpful and even lifesaving in the inpatient setting. It is unrealistic to expect the primary teams to know whether cutaneous manifestations potentially could be related to the patient’s overall clinical picture. On the contrary, we would encourage the primary team in charge of a hemodynamically unstable patient to consult dermatology at the first sign of an unexplained rash. Take for example an acutely ill patient who develops retiform purpura. There are well-established dermatology guidelines for the workup of retiform purpura,4 including prompt biopsy and assessment of broad, potentially life-threatening differential diagnoses from calciphylaxis to angioinvasive fungal infection. In this scenario, the dermatology consultant may render the correct diagnosis and recommend immediate treatment that could be lifesaving.
Secondly, we do not agree with the recommendation that a patient in hospice care is not appropriate for inpatient dermatology consultation. Patients receiving hospice or palliative care have high rates of potentially symptomatic cutaneous diseases,5 including intertrigo and dermatitis—comprising stasis, seborrheic, and contact dermatitis.6 Although aggressive intervention for asymptomatic benign or malignant skin conditions may not be in line with their goals of care, an inpatient dermatology consultation can reduce symptoms and improve quality of life. This population also is one that is unlikely to be able to attend an outpatient dermatology clinic appointment and therefore are good candidates for inpatient consultation.
Lastly, we want to highlight the difference between a stable chronic dermatologic disease and an acute flare that may occur while a patient is hospitalized, regardless of whether it is the reason for admission. For example, a patient with psoriasis affecting limited body surface area who is hospitalized for a myocardial infarction is not appropriate for a dermatology consultation. However, if that same patient develops erythroderma while they are hospitalized for cardiac monitoring, it would certainly be appropriate for dermatology to be consulted. Additionally, there are times when a chronic skin disease is the reason for hospitalization; dermatology, although technically a consulting service, would be the primary decision-maker for the patient’s care in this situation. In these scenarios, it is important for the patient to be able to establish care for long-term outpatient management of their condition; however, it is prudent to involve dermatology while the patient is acutely hospitalized to guide their treatment plan until they are able to see a dermatologist after discharge.
In conclusion, we believe that hospital dermatology is a valuable tool that can be utilized in many different scenarios. Although there are certainly situations more appropriate for outpatient dermatology referral, we would caution against overly simplified algorithms that could discourage valuable inpatient dermatology consultations. It often is worth a conversation with your dermatology consultant (when available at an institution) to determine the best course of action for each patient. Additionally, we recognize the need for more formalized guidelines on when to pursue inpatient dermatology consultation. We are members of the Society of Dermatology Hospitalists and encourage readers to reference their website, which provides additional resources on inpatient dermatology (https://societydermatologyhospitalists.com/inpatient-dermatology-literature/).
Authors’ Response
We appreciate the letter in response to our commentary on the appropriateness of inpatient dermatology consultations. It is the continued refining and re-evaluation of concepts such as these that allow our field to grow and improve knowledge and patient care.
We sought to provide a nonpatronizing yet simple consultation flowchart that would help guide triage of patients in need or not in need of dermatologic evaluation by the inpatient teams. Understandably, the impressions of our flowchart have been variable based on different readers’ medical backgrounds and experiences. It is certainly possible that our flowchart lacked certain exceptions and oversimplified certain concepts, and we welcome further refining of this flowchart to better guide inpatient dermatology consultations.
We do, however, disagree that the primary team would not know whether a patient is intubated in the intensive care unit for a dermatology reason. If the patient is in such a status, it would be pertinent for the primary team to conduct a timely workup that could include consultations until a diagnosis is made. We were not implying that every dermatology consultation in the intensive care unit is unwarranted, especially if it can lead to a primary dermatologic diagnosis. We do believe that a thorough history could elicit an allergy or other chronic skin condition that could save resources and spending within a hospital. Likewise, psoriasis comes in many different presentations, and although we do not believe a consultation for chronic psoriatic plaques is appropriate in the hospital, it is absolutely appropriate for a patient who is erythrodermic from any cause.
Our flowchart was intended to be the first step to providing education on when consultations are appropriate, and further refinement will be necessary.
Hershel Dobkin, MD; Timothy Blackwell, BS; Robin Ashinoff, MD
Drs. Dobkin and Ashinoff are from Hackensack University Medical Center, New Jersey. Mr. Blackwell is from the Rowan University School of Osteopathic Medicine, Stratford, New Jersey.
The authors report no conflict of interest.
Correspondence: Hershel Dobkin, MD, Hackensack University Medical Center, 30 Prospect Ave, Hackensack, NJ 07601 ([email protected]).
To the Editor:
We read with interest the Cutis article by Dobkin et al1 (Cutis. 2022;109:218-220) regarding guidelines for inpatient and emergency department dermatology consultations. We agree with the authors that dermatology training is lacking in other medical specialties, which makes it challenging for teams to assess the appropriateness of a dermatology consultation in the inpatient setting. Inpatient dermatology consultation can be utilized in a hospital system to aid in rapid and accurate diagnosis, avoid inappropriate therapies, and decrease length of stay2 and readmission rates3 while providing education to the primary teams. This is precisely why in many instances the availability of inpatient dermatology consultation is so important because nondermatologists often are unable to determine whether a rash is life-threatening, benign, or something in between. From the perspective of dermatology hospitalists, there is room for improvement in the flowchart Dobkin et al1 presented to guide inpatient dermatology consultation.
To have a productive relationship with our internal medicine, surgery, pediatrics, psychiatry, and other hospital-based colleagues, we must keep an open mind when a consultation is received. We feel that the flowchart proposed by Dobkin et al1 presents too narrow a viewpoint on the utility of inpatient dermatology. It rests on assertions that other teams will be able to determine the appropriate dermatologic diagnosis without involving a dermatologist, which often is not the case.
We disagree with several recommendations in the flowchart, the first being the assertion that patients who are “hemodynamically unstable due to [a] nondermatologic problem (eg, intubated on pressors, febrile, and hypotensive)” are not appropriate for inpatient dermatology consultation.1 Although dermatologists do not commonly encounter patients with critical illness in the outpatient clinic, dermatology consultation can be extremely helpful and even lifesaving in the inpatient setting. It is unrealistic to expect the primary teams to know whether cutaneous manifestations potentially could be related to the patient’s overall clinical picture. On the contrary, we would encourage the primary team in charge of a hemodynamically unstable patient to consult dermatology at the first sign of an unexplained rash. Take for example an acutely ill patient who develops retiform purpura. There are well-established dermatology guidelines for the workup of retiform purpura,4 including prompt biopsy and assessment of broad, potentially life-threatening differential diagnoses from calciphylaxis to angioinvasive fungal infection. In this scenario, the dermatology consultant may render the correct diagnosis and recommend immediate treatment that could be lifesaving.
Secondly, we do not agree with the recommendation that a patient in hospice care is not appropriate for inpatient dermatology consultation. Patients receiving hospice or palliative care have high rates of potentially symptomatic cutaneous diseases,5 including intertrigo and dermatitis—comprising stasis, seborrheic, and contact dermatitis.6 Although aggressive intervention for asymptomatic benign or malignant skin conditions may not be in line with their goals of care, an inpatient dermatology consultation can reduce symptoms and improve quality of life. This population also is one that is unlikely to be able to attend an outpatient dermatology clinic appointment and therefore are good candidates for inpatient consultation.
Lastly, we want to highlight the difference between a stable chronic dermatologic disease and an acute flare that may occur while a patient is hospitalized, regardless of whether it is the reason for admission. For example, a patient with psoriasis affecting limited body surface area who is hospitalized for a myocardial infarction is not appropriate for a dermatology consultation. However, if that same patient develops erythroderma while they are hospitalized for cardiac monitoring, it would certainly be appropriate for dermatology to be consulted. Additionally, there are times when a chronic skin disease is the reason for hospitalization; dermatology, although technically a consulting service, would be the primary decision-maker for the patient’s care in this situation. In these scenarios, it is important for the patient to be able to establish care for long-term outpatient management of their condition; however, it is prudent to involve dermatology while the patient is acutely hospitalized to guide their treatment plan until they are able to see a dermatologist after discharge.
In conclusion, we believe that hospital dermatology is a valuable tool that can be utilized in many different scenarios. Although there are certainly situations more appropriate for outpatient dermatology referral, we would caution against overly simplified algorithms that could discourage valuable inpatient dermatology consultations. It often is worth a conversation with your dermatology consultant (when available at an institution) to determine the best course of action for each patient. Additionally, we recognize the need for more formalized guidelines on when to pursue inpatient dermatology consultation. We are members of the Society of Dermatology Hospitalists and encourage readers to reference their website, which provides additional resources on inpatient dermatology (https://societydermatologyhospitalists.com/inpatient-dermatology-literature/).
Authors’ Response
We appreciate the letter in response to our commentary on the appropriateness of inpatient dermatology consultations. It is the continued refining and re-evaluation of concepts such as these that allow our field to grow and improve knowledge and patient care.
We sought to provide a nonpatronizing yet simple consultation flowchart that would help guide triage of patients in need or not in need of dermatologic evaluation by the inpatient teams. Understandably, the impressions of our flowchart have been variable based on different readers’ medical backgrounds and experiences. It is certainly possible that our flowchart lacked certain exceptions and oversimplified certain concepts, and we welcome further refining of this flowchart to better guide inpatient dermatology consultations.
We do, however, disagree that the primary team would not know whether a patient is intubated in the intensive care unit for a dermatology reason. If the patient is in such a status, it would be pertinent for the primary team to conduct a timely workup that could include consultations until a diagnosis is made. We were not implying that every dermatology consultation in the intensive care unit is unwarranted, especially if it can lead to a primary dermatologic diagnosis. We do believe that a thorough history could elicit an allergy or other chronic skin condition that could save resources and spending within a hospital. Likewise, psoriasis comes in many different presentations, and although we do not believe a consultation for chronic psoriatic plaques is appropriate in the hospital, it is absolutely appropriate for a patient who is erythrodermic from any cause.
Our flowchart was intended to be the first step to providing education on when consultations are appropriate, and further refinement will be necessary.
Hershel Dobkin, MD; Timothy Blackwell, BS; Robin Ashinoff, MD
Drs. Dobkin and Ashinoff are from Hackensack University Medical Center, New Jersey. Mr. Blackwell is from the Rowan University School of Osteopathic Medicine, Stratford, New Jersey.
The authors report no conflict of interest.
Correspondence: Hershel Dobkin, MD, Hackensack University Medical Center, 30 Prospect Ave, Hackensack, NJ 07601 ([email protected]).
- Dobkin H, Blackwell T, Ashinoff R. When are inpatient and emergency dermatologic consultations appropriate? Cutis. 2022;109:218-220. doi:10.12788/cutis.0492
- Ko LN, Garza-Mayers AC, St John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis: a randomized clinical trial. JAMA Dermatol. 2018;154:529-536. doi:10.1001/jamadermatol.2017.6196
- Hu L, Haynes H, Ferrazza D, et al. Impact of specialist consultations on inpatient admissions for dermatology-specific and related DRGs. J Gen Intern Med. 2013;28:1477-1482. doi:10.1007/s11606-013-2440-2
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796. doi:10.1016/j.jaad.2019.07.112
- Pisano C, Paladichuk H, Keeling B. Dermatology in palliative medicine [published online October 14, 2021]. BMJ Support Palliat Care. doi:10.1136/bmjspcare-2021-003342
- Barnabé C, Daeninck P. “Beauty is only skin deep”: prevalence of dermatologic disease on a palliative care unit. J Pain Symptom Manage. 2005;29:419-422. doi:10.1016/j.jpainsymman.2004.08.009
- Dobkin H, Blackwell T, Ashinoff R. When are inpatient and emergency dermatologic consultations appropriate? Cutis. 2022;109:218-220. doi:10.12788/cutis.0492
- Ko LN, Garza-Mayers AC, St John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis: a randomized clinical trial. JAMA Dermatol. 2018;154:529-536. doi:10.1001/jamadermatol.2017.6196
- Hu L, Haynes H, Ferrazza D, et al. Impact of specialist consultations on inpatient admissions for dermatology-specific and related DRGs. J Gen Intern Med. 2013;28:1477-1482. doi:10.1007/s11606-013-2440-2
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796. doi:10.1016/j.jaad.2019.07.112
- Pisano C, Paladichuk H, Keeling B. Dermatology in palliative medicine [published online October 14, 2021]. BMJ Support Palliat Care. doi:10.1136/bmjspcare-2021-003342
- Barnabé C, Daeninck P. “Beauty is only skin deep”: prevalence of dermatologic disease on a palliative care unit. J Pain Symptom Manage. 2005;29:419-422. doi:10.1016/j.jpainsymman.2004.08.009
Vitiligo
THE COMPARISON
A Vitiligo in a young Hispanic female, which spared the area under a ring. The patient has spotty return of pigment on the hand after narrowband UVB treatment.
B Vitiligo on the hand in a young Hispanic male.

Vitiligo is a chronic autoimmune disorder characterized by areas of depigmented white patches on the skin due to the loss of melanocytes in the epidermis. Various theories on the pathogenesis of vitiligo exist; however, autoimmune destruction of melanocytes remains the leading hypothesis, followed by intrinsic defects in melanocytes.1 Vitiligo is associated with various autoimmune diseases but is most frequently reported in conjunction with thyroid disorders.2
Epidemiology
Vitiligo affects approximately 1% of the US population and up to 8% worldwide.2 There is no difference in prevalence between races or genders. Females typically acquire the disease earlier than males. Onset may occur at any age, although about half of patients will have vitiligo by 20 years of age.1
Key clinical features in people with darker skin tones
Bright white patches are characteristic of vitiligo. The patches typically are asymptomatic and often affect the hands (Figures A and B), perioral skin, feet, and scalp, as well as areas more vulnerable to friction and trauma, such as the elbows and knees.2 Trichrome lesions—consisting of varying zones of white (depigmented), lighter brown (hypopigmented), and normal skin—are most commonly seen in individuals with darker skin. Trichrome vitiligo is considered an actively progressing variant of vitiligo.2
An important distinction when diagnosing vitiligo is evaluating for segmental vs nonsegmental vitiligo. Although nonsegmental vitiligo—the more common subtype—is characterized by symmetric distribution and a less predictable course, segmental vitiligo manifests in a localized and unilateral distribution, often avoiding extension past the midline. Segmental vitiligo typically manifests at a younger age and follows a more rapidly stabilizing course.3
Worth noting
Given that stark contrasts between pigmented and depigmented lesions are more prominent in darker skin tones, vitiligo can be more socially stigmatizing and psychologically devastating in these patients.4,5
Treatment of vitiligo includes narrowband UVB (NB-UVB) light phototherapy, excimer laser, topical corticosteroids, topical calcineurin inhibitors such as tacrolimus and pimecrolimus, and surgical melanocyte transplantation.1 In July 2022, ruxolitinib cream 1.5% was approved by the US Food and Drug Administration (FDA) for nonsegmental vitiligo in patients 12 years and older.6,7 It is the only FDA-approved therapy for vitiligo. It is thought to work by inhibiting the Janus kinase– signal transducers and activators of the transcription pathway.6 However, topical ruxolitinib is expensive, costing more than $2000 for 60 g.8
Health disparity highlight
A 2021 study reviewing the coverage policies of 15 commercial health care insurance companies, 50 BlueCross BlueShield plans, Medicaid, Medicare, and Veterans Affairs plans found inequities in the insurance coverage patterns for therapies used to treat vitiligo. There were 2 commonly cited reasons for denying coverage for therapies: vitiligo was considered cosmetic and therapies were not FDA approved.7 In comparison, NB-UVB light phototherapy for psoriasis is not considered cosmetic and has a much higher insurance coverage rate.9,10 The out-of-pocket cost for a patient to purchase their own NB-UVB light phototherapy is more than $5000.11 Not all patients of color are economically disadvantaged, but in the United States, Black and Hispanic populations experience disproportionately higher rates of poverty (19% and 17%, respectively) compared to their White counterparts (8%).12
Final thoughts
US Food and Drug Administration approval of new drugs or new treatment indications comes after years of research discovery and large-scale trials. This pursuit of new discovery, however, is uneven. Vitiligo has historically been understudied and underfunded for research; this is common among several conditions adversely affecting people of color in the United States.13
- Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi:10.1016/j.det.2016.11.014
- Alikhan A, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview part I. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473-491. doi:10.1016/j.jaad.2010.11.061
- van Geel N, Speeckaert R. Segmental vitiligo. Dermatol Clin. 2017; 35:145-150. doi:10.1016/j.det.2016.11.005
- Grimes PE, Miller MM. Vitiligo: patient stories, self-esteem, and the psychological burden of disease. Int J Womens Dermatol. 2018;4:32-37. doi:10.1016/j.ijwd.2017.11.005
- Ezzedine K, Eleftheriadou V, Jones H, et al. Psychosocial effects of vitiligo: a systematic literature review [published online September 23, 2021]. Am J Clin Dermatol. 2021;22:757-774. doi:10.1007/s40257 -021-00631-6
- FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. News release. US Food and Drug Administration; July 19, 2022. Accessed December 27, 2022. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical-treatment-addressing-repigmentation-vitiligo-patients -aged-12-and-older
- Blundell A, Sachar M, Gabel CK, et al. The scope of health insurance coverage of vitiligo treatments in the United States: implications for health care outcomes and disparities in children of color [published online July 16, 2021]. Pediatr Dermatol. 2021; 38(suppl 2):79-85. doi:10.1111/pde.14714
- Opzelura prices, coupons, and patient assistance programs. Drugs.com. Accessed January 10, 2023. https://www.drugs.com /price-guide/opzelura#:~:text=Opzelura%20Prices%2C%20 Coupons%20and%20Patient,on%20the%20pharmacy%20you%20visit
- Bhutani T, Liao W. A practical approach to home UVB phototherapy for the treatment of generalized psoriasis. Pract Dermatol. 2010;7:31-35.
- Castro Porto Silva Lopes F, Ahmed A. Insurance coverage for phototherapy for vitiligo in comparison to psoriasis and atopic dermatitis. SKIN The Journal of Cutaneous Medicine. 2022;6:217-224. https://doi.org/10.25251/skin.6.3.6
- Smith MP, Ly K, Thibodeaux Q, et al. Home phototherapy for patients with vitiligo: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:451-459. doi:10.2147/CCID.S185798
- Shrider EA, Kollar M, Chen F, et al. Income and poverty in the United States: 2020. US Census Bureau. September 14, 2021. Accessed December 27, 2022. https://www.census.gov/library/publications/2021/demo/p60-273.html
- Whitton ME, Pinart M, Batchelor J, et al. Interventions for vitiligo. Cochrane Database Syst Rev. 2010;(1):CD003263. doi:10.1002/14651858.CD003263.pub4
THE COMPARISON
A Vitiligo in a young Hispanic female, which spared the area under a ring. The patient has spotty return of pigment on the hand after narrowband UVB treatment.
B Vitiligo on the hand in a young Hispanic male.

Vitiligo is a chronic autoimmune disorder characterized by areas of depigmented white patches on the skin due to the loss of melanocytes in the epidermis. Various theories on the pathogenesis of vitiligo exist; however, autoimmune destruction of melanocytes remains the leading hypothesis, followed by intrinsic defects in melanocytes.1 Vitiligo is associated with various autoimmune diseases but is most frequently reported in conjunction with thyroid disorders.2
Epidemiology
Vitiligo affects approximately 1% of the US population and up to 8% worldwide.2 There is no difference in prevalence between races or genders. Females typically acquire the disease earlier than males. Onset may occur at any age, although about half of patients will have vitiligo by 20 years of age.1
Key clinical features in people with darker skin tones
Bright white patches are characteristic of vitiligo. The patches typically are asymptomatic and often affect the hands (Figures A and B), perioral skin, feet, and scalp, as well as areas more vulnerable to friction and trauma, such as the elbows and knees.2 Trichrome lesions—consisting of varying zones of white (depigmented), lighter brown (hypopigmented), and normal skin—are most commonly seen in individuals with darker skin. Trichrome vitiligo is considered an actively progressing variant of vitiligo.2
An important distinction when diagnosing vitiligo is evaluating for segmental vs nonsegmental vitiligo. Although nonsegmental vitiligo—the more common subtype—is characterized by symmetric distribution and a less predictable course, segmental vitiligo manifests in a localized and unilateral distribution, often avoiding extension past the midline. Segmental vitiligo typically manifests at a younger age and follows a more rapidly stabilizing course.3
Worth noting
Given that stark contrasts between pigmented and depigmented lesions are more prominent in darker skin tones, vitiligo can be more socially stigmatizing and psychologically devastating in these patients.4,5
Treatment of vitiligo includes narrowband UVB (NB-UVB) light phototherapy, excimer laser, topical corticosteroids, topical calcineurin inhibitors such as tacrolimus and pimecrolimus, and surgical melanocyte transplantation.1 In July 2022, ruxolitinib cream 1.5% was approved by the US Food and Drug Administration (FDA) for nonsegmental vitiligo in patients 12 years and older.6,7 It is the only FDA-approved therapy for vitiligo. It is thought to work by inhibiting the Janus kinase– signal transducers and activators of the transcription pathway.6 However, topical ruxolitinib is expensive, costing more than $2000 for 60 g.8
Health disparity highlight
A 2021 study reviewing the coverage policies of 15 commercial health care insurance companies, 50 BlueCross BlueShield plans, Medicaid, Medicare, and Veterans Affairs plans found inequities in the insurance coverage patterns for therapies used to treat vitiligo. There were 2 commonly cited reasons for denying coverage for therapies: vitiligo was considered cosmetic and therapies were not FDA approved.7 In comparison, NB-UVB light phototherapy for psoriasis is not considered cosmetic and has a much higher insurance coverage rate.9,10 The out-of-pocket cost for a patient to purchase their own NB-UVB light phototherapy is more than $5000.11 Not all patients of color are economically disadvantaged, but in the United States, Black and Hispanic populations experience disproportionately higher rates of poverty (19% and 17%, respectively) compared to their White counterparts (8%).12
Final thoughts
US Food and Drug Administration approval of new drugs or new treatment indications comes after years of research discovery and large-scale trials. This pursuit of new discovery, however, is uneven. Vitiligo has historically been understudied and underfunded for research; this is common among several conditions adversely affecting people of color in the United States.13
THE COMPARISON
A Vitiligo in a young Hispanic female, which spared the area under a ring. The patient has spotty return of pigment on the hand after narrowband UVB treatment.
B Vitiligo on the hand in a young Hispanic male.

Vitiligo is a chronic autoimmune disorder characterized by areas of depigmented white patches on the skin due to the loss of melanocytes in the epidermis. Various theories on the pathogenesis of vitiligo exist; however, autoimmune destruction of melanocytes remains the leading hypothesis, followed by intrinsic defects in melanocytes.1 Vitiligo is associated with various autoimmune diseases but is most frequently reported in conjunction with thyroid disorders.2
Epidemiology
Vitiligo affects approximately 1% of the US population and up to 8% worldwide.2 There is no difference in prevalence between races or genders. Females typically acquire the disease earlier than males. Onset may occur at any age, although about half of patients will have vitiligo by 20 years of age.1
Key clinical features in people with darker skin tones
Bright white patches are characteristic of vitiligo. The patches typically are asymptomatic and often affect the hands (Figures A and B), perioral skin, feet, and scalp, as well as areas more vulnerable to friction and trauma, such as the elbows and knees.2 Trichrome lesions—consisting of varying zones of white (depigmented), lighter brown (hypopigmented), and normal skin—are most commonly seen in individuals with darker skin. Trichrome vitiligo is considered an actively progressing variant of vitiligo.2
An important distinction when diagnosing vitiligo is evaluating for segmental vs nonsegmental vitiligo. Although nonsegmental vitiligo—the more common subtype—is characterized by symmetric distribution and a less predictable course, segmental vitiligo manifests in a localized and unilateral distribution, often avoiding extension past the midline. Segmental vitiligo typically manifests at a younger age and follows a more rapidly stabilizing course.3
Worth noting
Given that stark contrasts between pigmented and depigmented lesions are more prominent in darker skin tones, vitiligo can be more socially stigmatizing and psychologically devastating in these patients.4,5
Treatment of vitiligo includes narrowband UVB (NB-UVB) light phototherapy, excimer laser, topical corticosteroids, topical calcineurin inhibitors such as tacrolimus and pimecrolimus, and surgical melanocyte transplantation.1 In July 2022, ruxolitinib cream 1.5% was approved by the US Food and Drug Administration (FDA) for nonsegmental vitiligo in patients 12 years and older.6,7 It is the only FDA-approved therapy for vitiligo. It is thought to work by inhibiting the Janus kinase– signal transducers and activators of the transcription pathway.6 However, topical ruxolitinib is expensive, costing more than $2000 for 60 g.8
Health disparity highlight
A 2021 study reviewing the coverage policies of 15 commercial health care insurance companies, 50 BlueCross BlueShield plans, Medicaid, Medicare, and Veterans Affairs plans found inequities in the insurance coverage patterns for therapies used to treat vitiligo. There were 2 commonly cited reasons for denying coverage for therapies: vitiligo was considered cosmetic and therapies were not FDA approved.7 In comparison, NB-UVB light phototherapy for psoriasis is not considered cosmetic and has a much higher insurance coverage rate.9,10 The out-of-pocket cost for a patient to purchase their own NB-UVB light phototherapy is more than $5000.11 Not all patients of color are economically disadvantaged, but in the United States, Black and Hispanic populations experience disproportionately higher rates of poverty (19% and 17%, respectively) compared to their White counterparts (8%).12
Final thoughts
US Food and Drug Administration approval of new drugs or new treatment indications comes after years of research discovery and large-scale trials. This pursuit of new discovery, however, is uneven. Vitiligo has historically been understudied and underfunded for research; this is common among several conditions adversely affecting people of color in the United States.13
- Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi:10.1016/j.det.2016.11.014
- Alikhan A, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview part I. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473-491. doi:10.1016/j.jaad.2010.11.061
- van Geel N, Speeckaert R. Segmental vitiligo. Dermatol Clin. 2017; 35:145-150. doi:10.1016/j.det.2016.11.005
- Grimes PE, Miller MM. Vitiligo: patient stories, self-esteem, and the psychological burden of disease. Int J Womens Dermatol. 2018;4:32-37. doi:10.1016/j.ijwd.2017.11.005
- Ezzedine K, Eleftheriadou V, Jones H, et al. Psychosocial effects of vitiligo: a systematic literature review [published online September 23, 2021]. Am J Clin Dermatol. 2021;22:757-774. doi:10.1007/s40257 -021-00631-6
- FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. News release. US Food and Drug Administration; July 19, 2022. Accessed December 27, 2022. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical-treatment-addressing-repigmentation-vitiligo-patients -aged-12-and-older
- Blundell A, Sachar M, Gabel CK, et al. The scope of health insurance coverage of vitiligo treatments in the United States: implications for health care outcomes and disparities in children of color [published online July 16, 2021]. Pediatr Dermatol. 2021; 38(suppl 2):79-85. doi:10.1111/pde.14714
- Opzelura prices, coupons, and patient assistance programs. Drugs.com. Accessed January 10, 2023. https://www.drugs.com /price-guide/opzelura#:~:text=Opzelura%20Prices%2C%20 Coupons%20and%20Patient,on%20the%20pharmacy%20you%20visit
- Bhutani T, Liao W. A practical approach to home UVB phototherapy for the treatment of generalized psoriasis. Pract Dermatol. 2010;7:31-35.
- Castro Porto Silva Lopes F, Ahmed A. Insurance coverage for phototherapy for vitiligo in comparison to psoriasis and atopic dermatitis. SKIN The Journal of Cutaneous Medicine. 2022;6:217-224. https://doi.org/10.25251/skin.6.3.6
- Smith MP, Ly K, Thibodeaux Q, et al. Home phototherapy for patients with vitiligo: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:451-459. doi:10.2147/CCID.S185798
- Shrider EA, Kollar M, Chen F, et al. Income and poverty in the United States: 2020. US Census Bureau. September 14, 2021. Accessed December 27, 2022. https://www.census.gov/library/publications/2021/demo/p60-273.html
- Whitton ME, Pinart M, Batchelor J, et al. Interventions for vitiligo. Cochrane Database Syst Rev. 2010;(1):CD003263. doi:10.1002/14651858.CD003263.pub4
- Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi:10.1016/j.det.2016.11.014
- Alikhan A, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview part I. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473-491. doi:10.1016/j.jaad.2010.11.061
- van Geel N, Speeckaert R. Segmental vitiligo. Dermatol Clin. 2017; 35:145-150. doi:10.1016/j.det.2016.11.005
- Grimes PE, Miller MM. Vitiligo: patient stories, self-esteem, and the psychological burden of disease. Int J Womens Dermatol. 2018;4:32-37. doi:10.1016/j.ijwd.2017.11.005
- Ezzedine K, Eleftheriadou V, Jones H, et al. Psychosocial effects of vitiligo: a systematic literature review [published online September 23, 2021]. Am J Clin Dermatol. 2021;22:757-774. doi:10.1007/s40257 -021-00631-6
- FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. News release. US Food and Drug Administration; July 19, 2022. Accessed December 27, 2022. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical-treatment-addressing-repigmentation-vitiligo-patients -aged-12-and-older
- Blundell A, Sachar M, Gabel CK, et al. The scope of health insurance coverage of vitiligo treatments in the United States: implications for health care outcomes and disparities in children of color [published online July 16, 2021]. Pediatr Dermatol. 2021; 38(suppl 2):79-85. doi:10.1111/pde.14714
- Opzelura prices, coupons, and patient assistance programs. Drugs.com. Accessed January 10, 2023. https://www.drugs.com /price-guide/opzelura#:~:text=Opzelura%20Prices%2C%20 Coupons%20and%20Patient,on%20the%20pharmacy%20you%20visit
- Bhutani T, Liao W. A practical approach to home UVB phototherapy for the treatment of generalized psoriasis. Pract Dermatol. 2010;7:31-35.
- Castro Porto Silva Lopes F, Ahmed A. Insurance coverage for phototherapy for vitiligo in comparison to psoriasis and atopic dermatitis. SKIN The Journal of Cutaneous Medicine. 2022;6:217-224. https://doi.org/10.25251/skin.6.3.6
- Smith MP, Ly K, Thibodeaux Q, et al. Home phototherapy for patients with vitiligo: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:451-459. doi:10.2147/CCID.S185798
- Shrider EA, Kollar M, Chen F, et al. Income and poverty in the United States: 2020. US Census Bureau. September 14, 2021. Accessed December 27, 2022. https://www.census.gov/library/publications/2021/demo/p60-273.html
- Whitton ME, Pinart M, Batchelor J, et al. Interventions for vitiligo. Cochrane Database Syst Rev. 2010;(1):CD003263. doi:10.1002/14651858.CD003263.pub4
Janus Kinase Inhibitors: A Promising Therapeutic Option for Allergic Contact Dermatitis
Allergic contact dermatitis (ACD) is a delayed type IV hypersensitivity reaction that usually manifests with eczematous lesions within hours to days after exposure to a contact allergen. The primary treatment of ACD consists of allergen avoidance, but medications also may be necessary to manage symptoms, particularly in cases where avoidance alone does not lead to resolution of dermatitis. At present, no medical therapies are explicitly approved for use in the management of ACD. Janus kinase (JAK) inhibitors are a class of small molecule inhibitors that are used for the treatment of a range of inflammatory diseases, such as rheumatoid arthritis and psoriatic arthritis. Several oral and topical JAK inhibitors also have recently been approved by the US Food and Drug Administration (FDA) for atopic dermatitis (AD). In this article, we discuss this important class of medications and the role that they may play in the off-label management of refractory ACD.
JAK/STAT Signaling Pathway
The JAK/signal transducer and activator of transcription (STAT) pathway plays a crucial role in many biologic processes. Notably, JAK/STAT signaling is involved in the development and regulation of the immune system.1 The cascade begins when a particular transmembrane receptor binds a ligand, such as an interferon or interleukin.2 Upon ligand binding, the receptor dimerizes or oligomerizes, bringing the relevant JAK proteins into close approximation to each other.3 This allows the JAK proteins to autophosphorylate or transphosphorylate.2-4 Phosphorylation activates the JAK proteins and increases their kinase activity.3 In humans, there are 4 JAK proteins: JAK1, JAK2, JAK3, and tyrosine kinase 2.4 When activated, the JAK proteins phosphorylate specific tyrosine residues on the receptor, which creates a docking site for STAT proteins. After binding, the STAT proteins then are phosphorylated, leading to their dimerization and translocation to the nucleus.2,3 Once in the nucleus, the STAT proteins act as transcription factors for target genes.3
JAK Inhibitors
Janus kinase inhibitors are immunomodulatory medications that work through inhibition of 1 or more of the JAK proteins in the JAK/STAT pathway. Through this mechanism, JAK inhibitors can impede the activity of proinflammatory cytokines and T cells.4 A brief overview of the commercially available JAK inhibitors in Europe, Japan, and the United States is provided in the Table.5-29
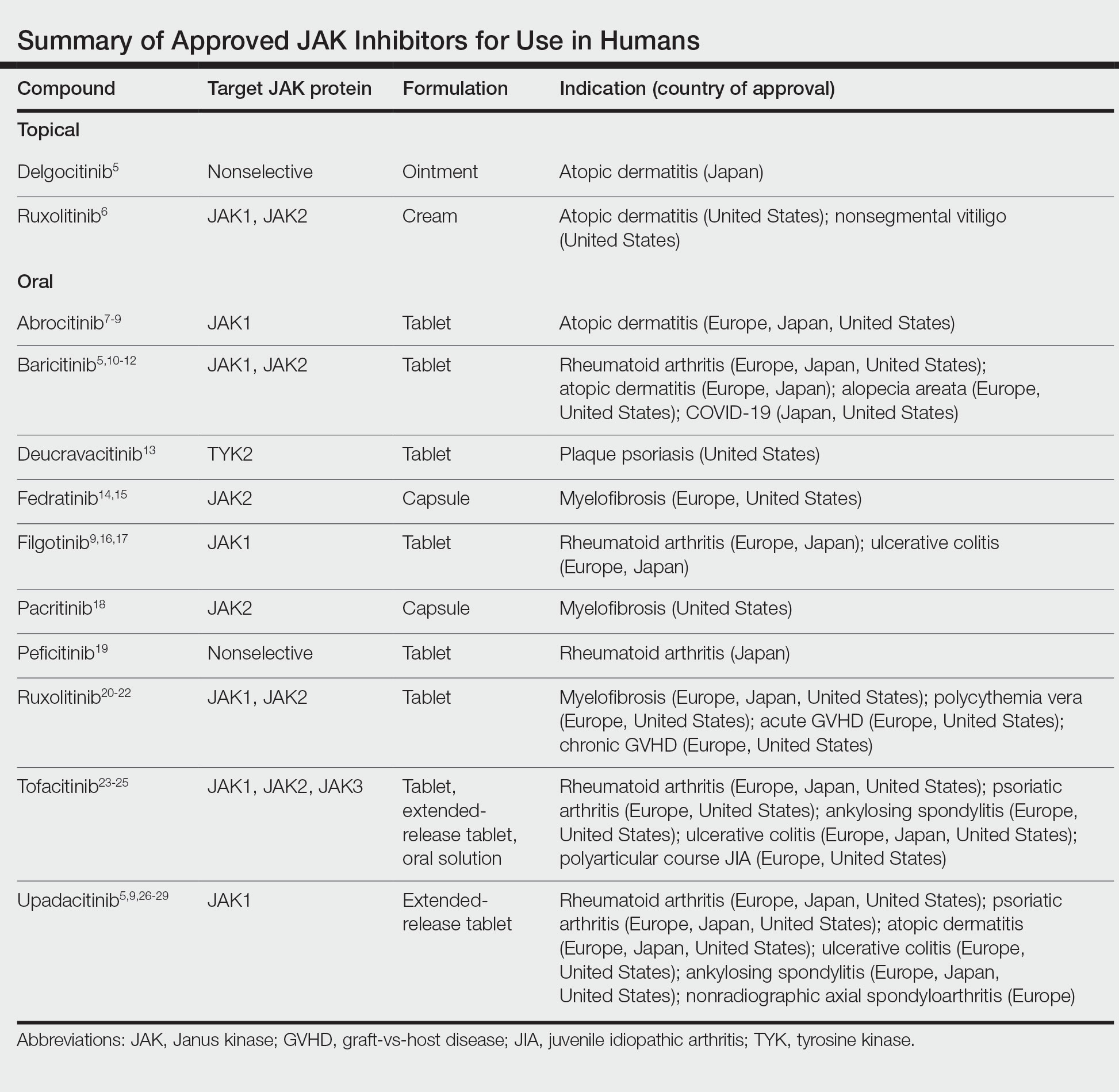
Of the approved JAK inhibitors, more than 40% are indicated for AD. The first JAK inhibitor to be approved in the topical form was delgocitinib in 2020 in Japan.5 In a phase 3 trial, delgocitinib demonstrated significant reductions in modified Eczema Area and Severity Index (EASI) score (P<.001) as well as Peak Pruritus Numerical Rating Scale (P<.001) when compared with vehicle.30 Topical ruxolitinib soon followed when its approval for AD was announced by the FDA in 2021.31 Results from 2 phase 3 trials found that significantly more patients achieved investigator global assessment (IGA) treatment success (P<.0001) and a significant reduction in itch as measured by the Peak Pruritus Numerical Rating Scale (P<.001) with topical ruxolitinib vs vehicle.32
The first oral JAK inhibitor to attain approval for AD was baricitinib in Europe and Japan, but it is not currently approved for this indication in the United States by the FDA.11,12,33 Consistent findings across phase 3 trials revealed that baricitinib was more effective at achieving IGA treatment success and improved EASI scores compared with placebo.33
Upadacitinib, another oral JAK inhibitor, was subsequently approved for AD in Europe and Japan in 2021 and in the United States in early 2022.5,9,26,27 Two replicate phase 3 trials demonstrated significant improvement in EASI score, itch, and quality of life with upadacitinib compared with placebo (P<.0001).34 Abrocitinib was granted FDA approval for AD in the same time period, with phase 3 trials exhibiting greater responses in IGA and EASI scores vs placebo.35
Potential for Use in ACD
Given the successful use of JAK inhibitors in the management of AD, there is optimism that these medications also may have potential application in ACD. Recent literature suggests that the 2 conditions may be more closely related mechanistically than previously understood. As a result, AD and ACD often are managed with the same therapeutic agents.36
Although the exact etiology of ACD is still being elucidated, activation of T cells and cytokines plays an important role.37 Notably, more than 40 cytokines exert their effects through the JAK/STAT signaling pathway, including IL-2, IL-6, IL-17, IL-22, and IFN-γ.37,38 A study on nickel contact allergy revealed that JAK/STAT activation may regulate the balance between IL-12 and IL-23 and increase type 1 T-helper (TH1) polarization.39 Skin inflammation and chronic pruritus, which are major components of ACD, also are thought to be mediated in part by JAK signaling.34,40
Animal studies have suggested that JAK inhibitors may show benefit in the management of ACD. Rats with oxazolone-induced ACD were found to have less swelling and epidermal thickening in the area of induced dermatitis after treatment with oral tofacitinib, comparable to the effects of cyclosporine. Tofacitinib was presumed to exert its effects through cytokine suppression, particularly that of IFN-γ, IL-22, and tumor necrosis factor α.41 In a separate study on mice with toluene-2,4-diisocyanate–induced ACD, both tofacitinib and another JAK inhibitor, oclacitinib, demonstrated inhibition of cytokine production, migration, and maturation of bone marrow–derived dendritic cells. Both topical and oral formulations of these 2 JAK inhibitors also were found to decrease scratching behavior; only the topicals improved ear thickness (used as a marker of skin inflammation), suggesting potential benefits to local application.42 In a murine model, oral delgocitinib also attenuated contact hypersensitivity via inhibition of antigen-specific T-cell proliferation and cytokine production.37 Finally, in a randomized clinical trial conducted on dogs with allergic dermatitis (of which 10% were presumed to be from contact allergy), oral oclacitinib significantly reduced pruritus and clinical severity scores vs placebo (P<.0001).43
There also are early clinical studies and case reports highlighting the effective use of JAK inhibitors in the management of ACD in humans. A 37-year-old man with occupational airborne ACD to Compositae saw full clearance of his dermatitis with daily oral abrocitinib after topical corticosteroids and dupilumab failed.44 Another patient, a 57-year-old woman, had near-complete resolution of chronic Parthenium-induced airborne ACD after starting twice-daily oral tofacitinib. Allergen avoidance, as well as multiple medications, including topical and oral corticosteroids, topical calcineurin inhibitors, and azathioprine, previously failed in this patient.45 Finally, a phase 2 study on patients with irritant and nonirritant chronic hand eczema found that significantly more patients achieved treatment success (as measured by the physician global assessment) with topical delgocitinib vs vehicle (P=.009).46 Chronic hand eczema may be due to a variety of causes, including AD, irritant contact dermatitis, and ACD. Thus, these studies begin to highlight the potential role for JAK inhibitors in the management of refractory ACD.
Side Effects of JAK Inhibitors
The safety profile of JAK inhibitors must be taken into consideration. In general, topical JAK inhibitors are safe and well tolerated, with the majority of adverse events (AEs) seen in clinical trials considered mild or unrelated to the medication.30,32 Nasopharyngitis, local skin infection, and acne were reported; a systematic review found no increased risk of AEs with topical JAK inhibitors compared with placebo.30,32,47 Application-site reactions, a common concern among the existing topical calcineurin and phosphodiesterase 4 inhibitors, were rare (approximately 2% of patients).47 The most frequent AEs seen in clinical trials of oral JAK inhibitors included acne, nasopharyngitis/upper respiratory tract infections, nausea, and headache.33-35 Herpes simplex virus infection and worsening of AD also were seen. Although elevations in creatine phosphokinase levels were reported, patients often were asymptomatic and elevations were related to exercise or resolved without treatment interruption.33-35
As a class, JAK inhibitors carry a boxed warning for serious infections, malignancy, major adverse cardiovascular events, thrombosis, and mortality. The FDA placed this label on JAK inhibitors because of the results of a randomized controlled trial of oral tofacitinib vs tumor necrosis factor α inhibitors in RA.48,49 Notably, participants in the trial had to be 50 years or older and have at least 1 additional cardiovascular risk factor. Postmarket safety data are still being collected for patients with AD and other dermatologic conditions, but the findings of safety analyses have been reassuring to date.50,51 Regular follow-up and routine laboratory monitoring are recommended for any patient started on an oral JAK inhibitor, which often includes monitoring of the complete blood cell count, comprehensive metabolic panel, and lipids, as well as baseline screening for tuberculosis and hepatitis.52,53 For topical JAK inhibitors, no specific laboratory monitoring is recommended.
Finally, it must be considered that the challenges of off-label prescribing combined with high costs may limit access to JAK inhibitors for use in ACD.
Final Interpretation
Early investigations, including studies on animals and humans, suggest that JAK inhibitors are a promising option in the management of treatment-refractory ACD. Patients and providers should be aware of both the benefits and known side effects of JAK inhibitors prior to treatment initiation.
- Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228:273-287.
- Bousoik E, Montazeri Aliabadi H. “Do we know Jack” about JAK? a closer look at JAK/STAT signaling pathway. Front Oncol. 2018;8:287.
- Jatiani SS, Baker SJ, Silverman LR, et al. Jak/STAT pathways in cytokine signaling and myeloproliferative disorders: approaches for targeted therapies. Genes Cancer. 2010;1:979-993.
- Seif F, Khoshmirsafa M, Aazami H, et al. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun Signal. 2017;15:23.
- Traidl S, Freimooser S, Werfel T. Janus kinase inhibitors for the therapy of atopic dermatitis. Allergol Select. 2021;5:293-304.
- Opzelura (ruxolitinib) cream. Prescribing information. Incyte Corporation; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215309s001lbl.pdf
- Cibinqo (abrocitinib) tablets. Prescribing information. Pfizer Labs; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/213871s000lbl.pdf
- Cibinqo. Product information. European Medicines Agency. Published December 17, 2021. Updated November 10, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/cibinqo
- New drugs approved in FY 2021. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000246734.pdf
- Olumiant (baricitinib) tablets. Prescribing information. Eli Lilly and Company; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/207924s007lbl.pdf
- Olumiant. Product information. European Medicines Agency. Published March 16, 2017. Updated June 29, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/olumiant
- Review report: Olumiant. Pharmaceuticals and Medical Devices Agency. April 21, 2021. Accessed January 20, 2023. https://www.pmda.go.jp/files/000243207.pdf
- Sotyktu (deucravacitinib) tablets. Prescribing information. Bristol-Myers Squibb Company; 2022. Accessed January 20, 2023.https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/214958s000lbl.pdf
- Inrebic (fedratinib) capsules. Prescribing information. Celgene Corporation; 2019. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212327s000lbl.pdf
- Inrebic. Product information. European Medicines Agency. Published March 3, 2021. Updated December 8, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/inrebic
- Jyseleca. Product information. European Medicines Agency. Published September 28, 2020. Updated November 9, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/documents/product-information/jyseleca-epar-product-information_en.pdf
- Review report: Jyseleca. Pharmaceuticals and Medical Devices Agency. September 8, 2020. Accessed January 20, 2023. https://www.pmda.go.jp/files/000247830.pdf
- Vonjo (pacritinib) capsules. Prescribing information. CTI BioPharma Corp; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/208712s000lbl.pdf
- Review report: Smyraf. Pharmaceuticals and Medical Devices Agency. February 28, 2019. Accessed January 20, 2023. https://www.pmda.go.jp/files/000233074.pdf
- Jakafi (ruxolitinib) tablets. Prescribing information. Incyte Corporation; 2021. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/202192s023lbl.pdf
- Jakavi. Product information. European Medicines Agency. Published October 4, 2012. Updated May 18, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/jakavi
- New drugs approved in FY 2014. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000229076.pdf
- Xeljanz (tofacitinib). Prescribing information. Pfizer Labs; 2021. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/203214s028,208246s013,213082s003lbl.pdf
- Xeljanz. Product information. European Medicines Agency. Accessed January 20, 2023. https://www.ema.europa.eu/en/documents/product-information/xeljanz-epar-product-information_en.pdf
- Review report: Xeljanz. Pharmaceuticals and Medical Devices Agency. January 20, 2023. https://www.pmda.go.jp/files/000237584.pdf
- Rinvoq (upadacitinib) extended-release tablets. Prescribing information. AbbVie Inc; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/211675s003lbl.pdf
- Rinvoq. Product information. European Medicines Agency. Published December 18, 2019. Updated December 7, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/rinvoq
- New drugs approved in FY 2019. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000235289.pdfs
- New drugs approved in May 2022. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000248626.pdf
- Nakagawa H, Nemoto O, Igarashi A, et al. Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: a phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. J Am Acad Dermatol. 2020;82:823-831. Erratum appears in J Am Acad Dermatol. 2021;85:1069.
- Sideris N, Paschou E, Bakirtzi K, et al. New and upcoming topical treatments for atopic dermatitis: a review of the literature. J Clin Med. 2022;11:4974.
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85:863-872.
- Radi G, Simonetti O, Rizzetto G, et al. Baricitinib: the first Jak inhibitor approved in Europe for the treatment of moderate to severe atopic dermatitis in adult patients. Healthcare (Basel). 2021;9:1575.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168. Erratum appears in Lancet. 2021;397:2150.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Johnson H, Novack DE, Adler BL, et al. Can atopic dermatitis and allergic contact dermatitis coexist? Cutis. 2022;110:139-142.
- Amano W, Nakajima S, Yamamoto Y, et al. JAK inhibitor JTE-052 regulates contact hypersensitivity by downmodulating T cell activation and differentiation. J Dermatol Sci. 2016;84:258-265.
- O’Shea JJ, Schwartz DM, Villarino AV, et al. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311-328.
- Bechara R, Antonios D, Azouri H, et al. Nickel sulfate promotes IL-17A producing CD4+ T cells by an IL-23-dependent mechanism regulated by TLR4 and JAK-STAT pathways. J Invest Dermatol. 2017;137:2140-2148.
- Oetjen LK, Mack MR, Feng J, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171:217-228.e13.
- Fujii Y, Sengoku T. Effects of the Janus kinase inhibitor CP-690550 (tofacitinib) in a rat model of oxazolone-induced chronic dermatitis. Pharmacology. 2013;91:207-213.
- Fukuyama T, Ehling S, Cook E, et al. Topically administered Janus-kinase inhibitors tofacitinib and oclacitinib display impressive antipruritic and anti-inflammatory responses in a model of allergic dermatitis. J Pharmacol Exp Ther. 2015;354:394-405.
- Cosgrove SB, Wren JA, Cleaver DM, et al. Efficacy and safety of oclacitinib for the control of pruritus and associated skin lesions in dogs with canine allergic dermatitis. Vet Dermatol. 2013;24:479, E114.
- Baltazar D, Shinamoto SR, Hamann CP, et al. Occupational airborne allergic contact dermatitis to invasive Compositae species treated with abrocitinib: a case report. Contact Dermatitis. 2022;87:542-544.
- Muddebihal A, Sardana K, Sinha S, et al. Tofacitinib in refractory Parthenium-induced airborne allergic contact dermatitis [published online October 12, 2022]. Contact Dermatitis. doi:10.1111/cod.14234
- Worm M, Bauer A, Elsner P, et al. Efficacy and safety of topical delgocitinib in patients with chronic hand eczema: data from a randomized, double-blind, vehicle-controlled phase IIa study. Br J Dermatol. 2020;182:1103-1110.
- Chen J, Cheng J, Yang H, et al. The efficacy and safety of Janus kinase inhibitors in patients with atopic dermatitis: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:495-496.
- Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316-326.
- US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. Updated December 7, 2021. Accessed January 20, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Chen TL, Lee LL, Huang HK, et al. Association of risk of incident venous thromboembolism with atopic dermatitis and treatment with Janus kinase inhibitors: a systematic review and meta-analysis. JAMA Dermatol. 2022;158:1254-1261.
- King B, Maari C, Lain E, et al. Extended safety analysis of baricitinib 2 mg in adult patients with atopic dermatitis: an integrated analysis from eight randomized clinical trials. Am J Clin Dermatol. 2021;22:395-405.
- Nash P, Kerschbaumer A, Dörner T, et al. Points to consider for the treatment of immune-mediated inflammatory diseases with Janus kinase inhibitors: a consensus statement. Ann Rheum Dis. 2021;80:71-87.
- Narla S, Silverberg JI. The suitability of treating atopic dermatitis with Janus kinase inhibitors. Exp Rev Clin Immunol. 2022;18:439-459.
Allergic contact dermatitis (ACD) is a delayed type IV hypersensitivity reaction that usually manifests with eczematous lesions within hours to days after exposure to a contact allergen. The primary treatment of ACD consists of allergen avoidance, but medications also may be necessary to manage symptoms, particularly in cases where avoidance alone does not lead to resolution of dermatitis. At present, no medical therapies are explicitly approved for use in the management of ACD. Janus kinase (JAK) inhibitors are a class of small molecule inhibitors that are used for the treatment of a range of inflammatory diseases, such as rheumatoid arthritis and psoriatic arthritis. Several oral and topical JAK inhibitors also have recently been approved by the US Food and Drug Administration (FDA) for atopic dermatitis (AD). In this article, we discuss this important class of medications and the role that they may play in the off-label management of refractory ACD.
JAK/STAT Signaling Pathway
The JAK/signal transducer and activator of transcription (STAT) pathway plays a crucial role in many biologic processes. Notably, JAK/STAT signaling is involved in the development and regulation of the immune system.1 The cascade begins when a particular transmembrane receptor binds a ligand, such as an interferon or interleukin.2 Upon ligand binding, the receptor dimerizes or oligomerizes, bringing the relevant JAK proteins into close approximation to each other.3 This allows the JAK proteins to autophosphorylate or transphosphorylate.2-4 Phosphorylation activates the JAK proteins and increases their kinase activity.3 In humans, there are 4 JAK proteins: JAK1, JAK2, JAK3, and tyrosine kinase 2.4 When activated, the JAK proteins phosphorylate specific tyrosine residues on the receptor, which creates a docking site for STAT proteins. After binding, the STAT proteins then are phosphorylated, leading to their dimerization and translocation to the nucleus.2,3 Once in the nucleus, the STAT proteins act as transcription factors for target genes.3
JAK Inhibitors
Janus kinase inhibitors are immunomodulatory medications that work through inhibition of 1 or more of the JAK proteins in the JAK/STAT pathway. Through this mechanism, JAK inhibitors can impede the activity of proinflammatory cytokines and T cells.4 A brief overview of the commercially available JAK inhibitors in Europe, Japan, and the United States is provided in the Table.5-29

Of the approved JAK inhibitors, more than 40% are indicated for AD. The first JAK inhibitor to be approved in the topical form was delgocitinib in 2020 in Japan.5 In a phase 3 trial, delgocitinib demonstrated significant reductions in modified Eczema Area and Severity Index (EASI) score (P<.001) as well as Peak Pruritus Numerical Rating Scale (P<.001) when compared with vehicle.30 Topical ruxolitinib soon followed when its approval for AD was announced by the FDA in 2021.31 Results from 2 phase 3 trials found that significantly more patients achieved investigator global assessment (IGA) treatment success (P<.0001) and a significant reduction in itch as measured by the Peak Pruritus Numerical Rating Scale (P<.001) with topical ruxolitinib vs vehicle.32
The first oral JAK inhibitor to attain approval for AD was baricitinib in Europe and Japan, but it is not currently approved for this indication in the United States by the FDA.11,12,33 Consistent findings across phase 3 trials revealed that baricitinib was more effective at achieving IGA treatment success and improved EASI scores compared with placebo.33
Upadacitinib, another oral JAK inhibitor, was subsequently approved for AD in Europe and Japan in 2021 and in the United States in early 2022.5,9,26,27 Two replicate phase 3 trials demonstrated significant improvement in EASI score, itch, and quality of life with upadacitinib compared with placebo (P<.0001).34 Abrocitinib was granted FDA approval for AD in the same time period, with phase 3 trials exhibiting greater responses in IGA and EASI scores vs placebo.35
Potential for Use in ACD
Given the successful use of JAK inhibitors in the management of AD, there is optimism that these medications also may have potential application in ACD. Recent literature suggests that the 2 conditions may be more closely related mechanistically than previously understood. As a result, AD and ACD often are managed with the same therapeutic agents.36
Although the exact etiology of ACD is still being elucidated, activation of T cells and cytokines plays an important role.37 Notably, more than 40 cytokines exert their effects through the JAK/STAT signaling pathway, including IL-2, IL-6, IL-17, IL-22, and IFN-γ.37,38 A study on nickel contact allergy revealed that JAK/STAT activation may regulate the balance between IL-12 and IL-23 and increase type 1 T-helper (TH1) polarization.39 Skin inflammation and chronic pruritus, which are major components of ACD, also are thought to be mediated in part by JAK signaling.34,40
Animal studies have suggested that JAK inhibitors may show benefit in the management of ACD. Rats with oxazolone-induced ACD were found to have less swelling and epidermal thickening in the area of induced dermatitis after treatment with oral tofacitinib, comparable to the effects of cyclosporine. Tofacitinib was presumed to exert its effects through cytokine suppression, particularly that of IFN-γ, IL-22, and tumor necrosis factor α.41 In a separate study on mice with toluene-2,4-diisocyanate–induced ACD, both tofacitinib and another JAK inhibitor, oclacitinib, demonstrated inhibition of cytokine production, migration, and maturation of bone marrow–derived dendritic cells. Both topical and oral formulations of these 2 JAK inhibitors also were found to decrease scratching behavior; only the topicals improved ear thickness (used as a marker of skin inflammation), suggesting potential benefits to local application.42 In a murine model, oral delgocitinib also attenuated contact hypersensitivity via inhibition of antigen-specific T-cell proliferation and cytokine production.37 Finally, in a randomized clinical trial conducted on dogs with allergic dermatitis (of which 10% were presumed to be from contact allergy), oral oclacitinib significantly reduced pruritus and clinical severity scores vs placebo (P<.0001).43
There also are early clinical studies and case reports highlighting the effective use of JAK inhibitors in the management of ACD in humans. A 37-year-old man with occupational airborne ACD to Compositae saw full clearance of his dermatitis with daily oral abrocitinib after topical corticosteroids and dupilumab failed.44 Another patient, a 57-year-old woman, had near-complete resolution of chronic Parthenium-induced airborne ACD after starting twice-daily oral tofacitinib. Allergen avoidance, as well as multiple medications, including topical and oral corticosteroids, topical calcineurin inhibitors, and azathioprine, previously failed in this patient.45 Finally, a phase 2 study on patients with irritant and nonirritant chronic hand eczema found that significantly more patients achieved treatment success (as measured by the physician global assessment) with topical delgocitinib vs vehicle (P=.009).46 Chronic hand eczema may be due to a variety of causes, including AD, irritant contact dermatitis, and ACD. Thus, these studies begin to highlight the potential role for JAK inhibitors in the management of refractory ACD.
Side Effects of JAK Inhibitors
The safety profile of JAK inhibitors must be taken into consideration. In general, topical JAK inhibitors are safe and well tolerated, with the majority of adverse events (AEs) seen in clinical trials considered mild or unrelated to the medication.30,32 Nasopharyngitis, local skin infection, and acne were reported; a systematic review found no increased risk of AEs with topical JAK inhibitors compared with placebo.30,32,47 Application-site reactions, a common concern among the existing topical calcineurin and phosphodiesterase 4 inhibitors, were rare (approximately 2% of patients).47 The most frequent AEs seen in clinical trials of oral JAK inhibitors included acne, nasopharyngitis/upper respiratory tract infections, nausea, and headache.33-35 Herpes simplex virus infection and worsening of AD also were seen. Although elevations in creatine phosphokinase levels were reported, patients often were asymptomatic and elevations were related to exercise or resolved without treatment interruption.33-35
As a class, JAK inhibitors carry a boxed warning for serious infections, malignancy, major adverse cardiovascular events, thrombosis, and mortality. The FDA placed this label on JAK inhibitors because of the results of a randomized controlled trial of oral tofacitinib vs tumor necrosis factor α inhibitors in RA.48,49 Notably, participants in the trial had to be 50 years or older and have at least 1 additional cardiovascular risk factor. Postmarket safety data are still being collected for patients with AD and other dermatologic conditions, but the findings of safety analyses have been reassuring to date.50,51 Regular follow-up and routine laboratory monitoring are recommended for any patient started on an oral JAK inhibitor, which often includes monitoring of the complete blood cell count, comprehensive metabolic panel, and lipids, as well as baseline screening for tuberculosis and hepatitis.52,53 For topical JAK inhibitors, no specific laboratory monitoring is recommended.
Finally, it must be considered that the challenges of off-label prescribing combined with high costs may limit access to JAK inhibitors for use in ACD.
Final Interpretation
Early investigations, including studies on animals and humans, suggest that JAK inhibitors are a promising option in the management of treatment-refractory ACD. Patients and providers should be aware of both the benefits and known side effects of JAK inhibitors prior to treatment initiation.
Allergic contact dermatitis (ACD) is a delayed type IV hypersensitivity reaction that usually manifests with eczematous lesions within hours to days after exposure to a contact allergen. The primary treatment of ACD consists of allergen avoidance, but medications also may be necessary to manage symptoms, particularly in cases where avoidance alone does not lead to resolution of dermatitis. At present, no medical therapies are explicitly approved for use in the management of ACD. Janus kinase (JAK) inhibitors are a class of small molecule inhibitors that are used for the treatment of a range of inflammatory diseases, such as rheumatoid arthritis and psoriatic arthritis. Several oral and topical JAK inhibitors also have recently been approved by the US Food and Drug Administration (FDA) for atopic dermatitis (AD). In this article, we discuss this important class of medications and the role that they may play in the off-label management of refractory ACD.
JAK/STAT Signaling Pathway
The JAK/signal transducer and activator of transcription (STAT) pathway plays a crucial role in many biologic processes. Notably, JAK/STAT signaling is involved in the development and regulation of the immune system.1 The cascade begins when a particular transmembrane receptor binds a ligand, such as an interferon or interleukin.2 Upon ligand binding, the receptor dimerizes or oligomerizes, bringing the relevant JAK proteins into close approximation to each other.3 This allows the JAK proteins to autophosphorylate or transphosphorylate.2-4 Phosphorylation activates the JAK proteins and increases their kinase activity.3 In humans, there are 4 JAK proteins: JAK1, JAK2, JAK3, and tyrosine kinase 2.4 When activated, the JAK proteins phosphorylate specific tyrosine residues on the receptor, which creates a docking site for STAT proteins. After binding, the STAT proteins then are phosphorylated, leading to their dimerization and translocation to the nucleus.2,3 Once in the nucleus, the STAT proteins act as transcription factors for target genes.3
JAK Inhibitors
Janus kinase inhibitors are immunomodulatory medications that work through inhibition of 1 or more of the JAK proteins in the JAK/STAT pathway. Through this mechanism, JAK inhibitors can impede the activity of proinflammatory cytokines and T cells.4 A brief overview of the commercially available JAK inhibitors in Europe, Japan, and the United States is provided in the Table.5-29

Of the approved JAK inhibitors, more than 40% are indicated for AD. The first JAK inhibitor to be approved in the topical form was delgocitinib in 2020 in Japan.5 In a phase 3 trial, delgocitinib demonstrated significant reductions in modified Eczema Area and Severity Index (EASI) score (P<.001) as well as Peak Pruritus Numerical Rating Scale (P<.001) when compared with vehicle.30 Topical ruxolitinib soon followed when its approval for AD was announced by the FDA in 2021.31 Results from 2 phase 3 trials found that significantly more patients achieved investigator global assessment (IGA) treatment success (P<.0001) and a significant reduction in itch as measured by the Peak Pruritus Numerical Rating Scale (P<.001) with topical ruxolitinib vs vehicle.32
The first oral JAK inhibitor to attain approval for AD was baricitinib in Europe and Japan, but it is not currently approved for this indication in the United States by the FDA.11,12,33 Consistent findings across phase 3 trials revealed that baricitinib was more effective at achieving IGA treatment success and improved EASI scores compared with placebo.33
Upadacitinib, another oral JAK inhibitor, was subsequently approved for AD in Europe and Japan in 2021 and in the United States in early 2022.5,9,26,27 Two replicate phase 3 trials demonstrated significant improvement in EASI score, itch, and quality of life with upadacitinib compared with placebo (P<.0001).34 Abrocitinib was granted FDA approval for AD in the same time period, with phase 3 trials exhibiting greater responses in IGA and EASI scores vs placebo.35
Potential for Use in ACD
Given the successful use of JAK inhibitors in the management of AD, there is optimism that these medications also may have potential application in ACD. Recent literature suggests that the 2 conditions may be more closely related mechanistically than previously understood. As a result, AD and ACD often are managed with the same therapeutic agents.36
Although the exact etiology of ACD is still being elucidated, activation of T cells and cytokines plays an important role.37 Notably, more than 40 cytokines exert their effects through the JAK/STAT signaling pathway, including IL-2, IL-6, IL-17, IL-22, and IFN-γ.37,38 A study on nickel contact allergy revealed that JAK/STAT activation may regulate the balance between IL-12 and IL-23 and increase type 1 T-helper (TH1) polarization.39 Skin inflammation and chronic pruritus, which are major components of ACD, also are thought to be mediated in part by JAK signaling.34,40
Animal studies have suggested that JAK inhibitors may show benefit in the management of ACD. Rats with oxazolone-induced ACD were found to have less swelling and epidermal thickening in the area of induced dermatitis after treatment with oral tofacitinib, comparable to the effects of cyclosporine. Tofacitinib was presumed to exert its effects through cytokine suppression, particularly that of IFN-γ, IL-22, and tumor necrosis factor α.41 In a separate study on mice with toluene-2,4-diisocyanate–induced ACD, both tofacitinib and another JAK inhibitor, oclacitinib, demonstrated inhibition of cytokine production, migration, and maturation of bone marrow–derived dendritic cells. Both topical and oral formulations of these 2 JAK inhibitors also were found to decrease scratching behavior; only the topicals improved ear thickness (used as a marker of skin inflammation), suggesting potential benefits to local application.42 In a murine model, oral delgocitinib also attenuated contact hypersensitivity via inhibition of antigen-specific T-cell proliferation and cytokine production.37 Finally, in a randomized clinical trial conducted on dogs with allergic dermatitis (of which 10% were presumed to be from contact allergy), oral oclacitinib significantly reduced pruritus and clinical severity scores vs placebo (P<.0001).43
There also are early clinical studies and case reports highlighting the effective use of JAK inhibitors in the management of ACD in humans. A 37-year-old man with occupational airborne ACD to Compositae saw full clearance of his dermatitis with daily oral abrocitinib after topical corticosteroids and dupilumab failed.44 Another patient, a 57-year-old woman, had near-complete resolution of chronic Parthenium-induced airborne ACD after starting twice-daily oral tofacitinib. Allergen avoidance, as well as multiple medications, including topical and oral corticosteroids, topical calcineurin inhibitors, and azathioprine, previously failed in this patient.45 Finally, a phase 2 study on patients with irritant and nonirritant chronic hand eczema found that significantly more patients achieved treatment success (as measured by the physician global assessment) with topical delgocitinib vs vehicle (P=.009).46 Chronic hand eczema may be due to a variety of causes, including AD, irritant contact dermatitis, and ACD. Thus, these studies begin to highlight the potential role for JAK inhibitors in the management of refractory ACD.
Side Effects of JAK Inhibitors
The safety profile of JAK inhibitors must be taken into consideration. In general, topical JAK inhibitors are safe and well tolerated, with the majority of adverse events (AEs) seen in clinical trials considered mild or unrelated to the medication.30,32 Nasopharyngitis, local skin infection, and acne were reported; a systematic review found no increased risk of AEs with topical JAK inhibitors compared with placebo.30,32,47 Application-site reactions, a common concern among the existing topical calcineurin and phosphodiesterase 4 inhibitors, were rare (approximately 2% of patients).47 The most frequent AEs seen in clinical trials of oral JAK inhibitors included acne, nasopharyngitis/upper respiratory tract infections, nausea, and headache.33-35 Herpes simplex virus infection and worsening of AD also were seen. Although elevations in creatine phosphokinase levels were reported, patients often were asymptomatic and elevations were related to exercise or resolved without treatment interruption.33-35
As a class, JAK inhibitors carry a boxed warning for serious infections, malignancy, major adverse cardiovascular events, thrombosis, and mortality. The FDA placed this label on JAK inhibitors because of the results of a randomized controlled trial of oral tofacitinib vs tumor necrosis factor α inhibitors in RA.48,49 Notably, participants in the trial had to be 50 years or older and have at least 1 additional cardiovascular risk factor. Postmarket safety data are still being collected for patients with AD and other dermatologic conditions, but the findings of safety analyses have been reassuring to date.50,51 Regular follow-up and routine laboratory monitoring are recommended for any patient started on an oral JAK inhibitor, which often includes monitoring of the complete blood cell count, comprehensive metabolic panel, and lipids, as well as baseline screening for tuberculosis and hepatitis.52,53 For topical JAK inhibitors, no specific laboratory monitoring is recommended.
Finally, it must be considered that the challenges of off-label prescribing combined with high costs may limit access to JAK inhibitors for use in ACD.
Final Interpretation
Early investigations, including studies on animals and humans, suggest that JAK inhibitors are a promising option in the management of treatment-refractory ACD. Patients and providers should be aware of both the benefits and known side effects of JAK inhibitors prior to treatment initiation.
- Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228:273-287.
- Bousoik E, Montazeri Aliabadi H. “Do we know Jack” about JAK? a closer look at JAK/STAT signaling pathway. Front Oncol. 2018;8:287.
- Jatiani SS, Baker SJ, Silverman LR, et al. Jak/STAT pathways in cytokine signaling and myeloproliferative disorders: approaches for targeted therapies. Genes Cancer. 2010;1:979-993.
- Seif F, Khoshmirsafa M, Aazami H, et al. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun Signal. 2017;15:23.
- Traidl S, Freimooser S, Werfel T. Janus kinase inhibitors for the therapy of atopic dermatitis. Allergol Select. 2021;5:293-304.
- Opzelura (ruxolitinib) cream. Prescribing information. Incyte Corporation; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215309s001lbl.pdf
- Cibinqo (abrocitinib) tablets. Prescribing information. Pfizer Labs; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/213871s000lbl.pdf
- Cibinqo. Product information. European Medicines Agency. Published December 17, 2021. Updated November 10, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/cibinqo
- New drugs approved in FY 2021. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000246734.pdf
- Olumiant (baricitinib) tablets. Prescribing information. Eli Lilly and Company; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/207924s007lbl.pdf
- Olumiant. Product information. European Medicines Agency. Published March 16, 2017. Updated June 29, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/olumiant
- Review report: Olumiant. Pharmaceuticals and Medical Devices Agency. April 21, 2021. Accessed January 20, 2023. https://www.pmda.go.jp/files/000243207.pdf
- Sotyktu (deucravacitinib) tablets. Prescribing information. Bristol-Myers Squibb Company; 2022. Accessed January 20, 2023.https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/214958s000lbl.pdf
- Inrebic (fedratinib) capsules. Prescribing information. Celgene Corporation; 2019. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212327s000lbl.pdf
- Inrebic. Product information. European Medicines Agency. Published March 3, 2021. Updated December 8, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/inrebic
- Jyseleca. Product information. European Medicines Agency. Published September 28, 2020. Updated November 9, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/documents/product-information/jyseleca-epar-product-information_en.pdf
- Review report: Jyseleca. Pharmaceuticals and Medical Devices Agency. September 8, 2020. Accessed January 20, 2023. https://www.pmda.go.jp/files/000247830.pdf
- Vonjo (pacritinib) capsules. Prescribing information. CTI BioPharma Corp; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/208712s000lbl.pdf
- Review report: Smyraf. Pharmaceuticals and Medical Devices Agency. February 28, 2019. Accessed January 20, 2023. https://www.pmda.go.jp/files/000233074.pdf
- Jakafi (ruxolitinib) tablets. Prescribing information. Incyte Corporation; 2021. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/202192s023lbl.pdf
- Jakavi. Product information. European Medicines Agency. Published October 4, 2012. Updated May 18, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/jakavi
- New drugs approved in FY 2014. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000229076.pdf
- Xeljanz (tofacitinib). Prescribing information. Pfizer Labs; 2021. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/203214s028,208246s013,213082s003lbl.pdf
- Xeljanz. Product information. European Medicines Agency. Accessed January 20, 2023. https://www.ema.europa.eu/en/documents/product-information/xeljanz-epar-product-information_en.pdf
- Review report: Xeljanz. Pharmaceuticals and Medical Devices Agency. January 20, 2023. https://www.pmda.go.jp/files/000237584.pdf
- Rinvoq (upadacitinib) extended-release tablets. Prescribing information. AbbVie Inc; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/211675s003lbl.pdf
- Rinvoq. Product information. European Medicines Agency. Published December 18, 2019. Updated December 7, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/rinvoq
- New drugs approved in FY 2019. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000235289.pdfs
- New drugs approved in May 2022. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000248626.pdf
- Nakagawa H, Nemoto O, Igarashi A, et al. Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: a phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. J Am Acad Dermatol. 2020;82:823-831. Erratum appears in J Am Acad Dermatol. 2021;85:1069.
- Sideris N, Paschou E, Bakirtzi K, et al. New and upcoming topical treatments for atopic dermatitis: a review of the literature. J Clin Med. 2022;11:4974.
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85:863-872.
- Radi G, Simonetti O, Rizzetto G, et al. Baricitinib: the first Jak inhibitor approved in Europe for the treatment of moderate to severe atopic dermatitis in adult patients. Healthcare (Basel). 2021;9:1575.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168. Erratum appears in Lancet. 2021;397:2150.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Johnson H, Novack DE, Adler BL, et al. Can atopic dermatitis and allergic contact dermatitis coexist? Cutis. 2022;110:139-142.
- Amano W, Nakajima S, Yamamoto Y, et al. JAK inhibitor JTE-052 regulates contact hypersensitivity by downmodulating T cell activation and differentiation. J Dermatol Sci. 2016;84:258-265.
- O’Shea JJ, Schwartz DM, Villarino AV, et al. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311-328.
- Bechara R, Antonios D, Azouri H, et al. Nickel sulfate promotes IL-17A producing CD4+ T cells by an IL-23-dependent mechanism regulated by TLR4 and JAK-STAT pathways. J Invest Dermatol. 2017;137:2140-2148.
- Oetjen LK, Mack MR, Feng J, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171:217-228.e13.
- Fujii Y, Sengoku T. Effects of the Janus kinase inhibitor CP-690550 (tofacitinib) in a rat model of oxazolone-induced chronic dermatitis. Pharmacology. 2013;91:207-213.
- Fukuyama T, Ehling S, Cook E, et al. Topically administered Janus-kinase inhibitors tofacitinib and oclacitinib display impressive antipruritic and anti-inflammatory responses in a model of allergic dermatitis. J Pharmacol Exp Ther. 2015;354:394-405.
- Cosgrove SB, Wren JA, Cleaver DM, et al. Efficacy and safety of oclacitinib for the control of pruritus and associated skin lesions in dogs with canine allergic dermatitis. Vet Dermatol. 2013;24:479, E114.
- Baltazar D, Shinamoto SR, Hamann CP, et al. Occupational airborne allergic contact dermatitis to invasive Compositae species treated with abrocitinib: a case report. Contact Dermatitis. 2022;87:542-544.
- Muddebihal A, Sardana K, Sinha S, et al. Tofacitinib in refractory Parthenium-induced airborne allergic contact dermatitis [published online October 12, 2022]. Contact Dermatitis. doi:10.1111/cod.14234
- Worm M, Bauer A, Elsner P, et al. Efficacy and safety of topical delgocitinib in patients with chronic hand eczema: data from a randomized, double-blind, vehicle-controlled phase IIa study. Br J Dermatol. 2020;182:1103-1110.
- Chen J, Cheng J, Yang H, et al. The efficacy and safety of Janus kinase inhibitors in patients with atopic dermatitis: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:495-496.
- Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316-326.
- US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. Updated December 7, 2021. Accessed January 20, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Chen TL, Lee LL, Huang HK, et al. Association of risk of incident venous thromboembolism with atopic dermatitis and treatment with Janus kinase inhibitors: a systematic review and meta-analysis. JAMA Dermatol. 2022;158:1254-1261.
- King B, Maari C, Lain E, et al. Extended safety analysis of baricitinib 2 mg in adult patients with atopic dermatitis: an integrated analysis from eight randomized clinical trials. Am J Clin Dermatol. 2021;22:395-405.
- Nash P, Kerschbaumer A, Dörner T, et al. Points to consider for the treatment of immune-mediated inflammatory diseases with Janus kinase inhibitors: a consensus statement. Ann Rheum Dis. 2021;80:71-87.
- Narla S, Silverberg JI. The suitability of treating atopic dermatitis with Janus kinase inhibitors. Exp Rev Clin Immunol. 2022;18:439-459.
- Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228:273-287.
- Bousoik E, Montazeri Aliabadi H. “Do we know Jack” about JAK? a closer look at JAK/STAT signaling pathway. Front Oncol. 2018;8:287.
- Jatiani SS, Baker SJ, Silverman LR, et al. Jak/STAT pathways in cytokine signaling and myeloproliferative disorders: approaches for targeted therapies. Genes Cancer. 2010;1:979-993.
- Seif F, Khoshmirsafa M, Aazami H, et al. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun Signal. 2017;15:23.
- Traidl S, Freimooser S, Werfel T. Janus kinase inhibitors for the therapy of atopic dermatitis. Allergol Select. 2021;5:293-304.
- Opzelura (ruxolitinib) cream. Prescribing information. Incyte Corporation; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215309s001lbl.pdf
- Cibinqo (abrocitinib) tablets. Prescribing information. Pfizer Labs; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/213871s000lbl.pdf
- Cibinqo. Product information. European Medicines Agency. Published December 17, 2021. Updated November 10, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/cibinqo
- New drugs approved in FY 2021. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000246734.pdf
- Olumiant (baricitinib) tablets. Prescribing information. Eli Lilly and Company; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/207924s007lbl.pdf
- Olumiant. Product information. European Medicines Agency. Published March 16, 2017. Updated June 29, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/olumiant
- Review report: Olumiant. Pharmaceuticals and Medical Devices Agency. April 21, 2021. Accessed January 20, 2023. https://www.pmda.go.jp/files/000243207.pdf
- Sotyktu (deucravacitinib) tablets. Prescribing information. Bristol-Myers Squibb Company; 2022. Accessed January 20, 2023.https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/214958s000lbl.pdf
- Inrebic (fedratinib) capsules. Prescribing information. Celgene Corporation; 2019. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212327s000lbl.pdf
- Inrebic. Product information. European Medicines Agency. Published March 3, 2021. Updated December 8, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/inrebic
- Jyseleca. Product information. European Medicines Agency. Published September 28, 2020. Updated November 9, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/documents/product-information/jyseleca-epar-product-information_en.pdf
- Review report: Jyseleca. Pharmaceuticals and Medical Devices Agency. September 8, 2020. Accessed January 20, 2023. https://www.pmda.go.jp/files/000247830.pdf
- Vonjo (pacritinib) capsules. Prescribing information. CTI BioPharma Corp; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/208712s000lbl.pdf
- Review report: Smyraf. Pharmaceuticals and Medical Devices Agency. February 28, 2019. Accessed January 20, 2023. https://www.pmda.go.jp/files/000233074.pdf
- Jakafi (ruxolitinib) tablets. Prescribing information. Incyte Corporation; 2021. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/202192s023lbl.pdf
- Jakavi. Product information. European Medicines Agency. Published October 4, 2012. Updated May 18, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/jakavi
- New drugs approved in FY 2014. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000229076.pdf
- Xeljanz (tofacitinib). Prescribing information. Pfizer Labs; 2021. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/203214s028,208246s013,213082s003lbl.pdf
- Xeljanz. Product information. European Medicines Agency. Accessed January 20, 2023. https://www.ema.europa.eu/en/documents/product-information/xeljanz-epar-product-information_en.pdf
- Review report: Xeljanz. Pharmaceuticals and Medical Devices Agency. January 20, 2023. https://www.pmda.go.jp/files/000237584.pdf
- Rinvoq (upadacitinib) extended-release tablets. Prescribing information. AbbVie Inc; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/211675s003lbl.pdf
- Rinvoq. Product information. European Medicines Agency. Published December 18, 2019. Updated December 7, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/rinvoq
- New drugs approved in FY 2019. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000235289.pdfs
- New drugs approved in May 2022. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000248626.pdf
- Nakagawa H, Nemoto O, Igarashi A, et al. Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: a phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. J Am Acad Dermatol. 2020;82:823-831. Erratum appears in J Am Acad Dermatol. 2021;85:1069.
- Sideris N, Paschou E, Bakirtzi K, et al. New and upcoming topical treatments for atopic dermatitis: a review of the literature. J Clin Med. 2022;11:4974.
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85:863-872.
- Radi G, Simonetti O, Rizzetto G, et al. Baricitinib: the first Jak inhibitor approved in Europe for the treatment of moderate to severe atopic dermatitis in adult patients. Healthcare (Basel). 2021;9:1575.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168. Erratum appears in Lancet. 2021;397:2150.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Johnson H, Novack DE, Adler BL, et al. Can atopic dermatitis and allergic contact dermatitis coexist? Cutis. 2022;110:139-142.
- Amano W, Nakajima S, Yamamoto Y, et al. JAK inhibitor JTE-052 regulates contact hypersensitivity by downmodulating T cell activation and differentiation. J Dermatol Sci. 2016;84:258-265.
- O’Shea JJ, Schwartz DM, Villarino AV, et al. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311-328.
- Bechara R, Antonios D, Azouri H, et al. Nickel sulfate promotes IL-17A producing CD4+ T cells by an IL-23-dependent mechanism regulated by TLR4 and JAK-STAT pathways. J Invest Dermatol. 2017;137:2140-2148.
- Oetjen LK, Mack MR, Feng J, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171:217-228.e13.
- Fujii Y, Sengoku T. Effects of the Janus kinase inhibitor CP-690550 (tofacitinib) in a rat model of oxazolone-induced chronic dermatitis. Pharmacology. 2013;91:207-213.
- Fukuyama T, Ehling S, Cook E, et al. Topically administered Janus-kinase inhibitors tofacitinib and oclacitinib display impressive antipruritic and anti-inflammatory responses in a model of allergic dermatitis. J Pharmacol Exp Ther. 2015;354:394-405.
- Cosgrove SB, Wren JA, Cleaver DM, et al. Efficacy and safety of oclacitinib for the control of pruritus and associated skin lesions in dogs with canine allergic dermatitis. Vet Dermatol. 2013;24:479, E114.
- Baltazar D, Shinamoto SR, Hamann CP, et al. Occupational airborne allergic contact dermatitis to invasive Compositae species treated with abrocitinib: a case report. Contact Dermatitis. 2022;87:542-544.
- Muddebihal A, Sardana K, Sinha S, et al. Tofacitinib in refractory Parthenium-induced airborne allergic contact dermatitis [published online October 12, 2022]. Contact Dermatitis. doi:10.1111/cod.14234
- Worm M, Bauer A, Elsner P, et al. Efficacy and safety of topical delgocitinib in patients with chronic hand eczema: data from a randomized, double-blind, vehicle-controlled phase IIa study. Br J Dermatol. 2020;182:1103-1110.
- Chen J, Cheng J, Yang H, et al. The efficacy and safety of Janus kinase inhibitors in patients with atopic dermatitis: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:495-496.
- Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316-326.
- US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. Updated December 7, 2021. Accessed January 20, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Chen TL, Lee LL, Huang HK, et al. Association of risk of incident venous thromboembolism with atopic dermatitis and treatment with Janus kinase inhibitors: a systematic review and meta-analysis. JAMA Dermatol. 2022;158:1254-1261.
- King B, Maari C, Lain E, et al. Extended safety analysis of baricitinib 2 mg in adult patients with atopic dermatitis: an integrated analysis from eight randomized clinical trials. Am J Clin Dermatol. 2021;22:395-405.
- Nash P, Kerschbaumer A, Dörner T, et al. Points to consider for the treatment of immune-mediated inflammatory diseases with Janus kinase inhibitors: a consensus statement. Ann Rheum Dis. 2021;80:71-87.
- Narla S, Silverberg JI. The suitability of treating atopic dermatitis with Janus kinase inhibitors. Exp Rev Clin Immunol. 2022;18:439-459.
PRACTICE POINTS
- Janus kinase (JAK) inhibitors are a novel class of small molecule inhibitors that modulate the JAK/signal transducer and activator of transcription signaling pathway.
- Select JAK inhibitors have been approved by the US Food and Drug Administration for the management of atopic dermatitis. Their use in allergic contact dermatitis is under active investigation.
- Regular follow-up and laboratory monitoring for patients on oral JAK inhibitors is recommended, given the potential for treatment-related adverse effects.
Dome-Shaped Periorbital Papule
The Diagnosis: Endocrine Mucin-Producing Sweat Gland Carcinoma
Endocrine mucin-producing sweat gland carcinoma (EMPSGC) is a rare cutaneous adnexal tumor that characteristically presents as slowgrowing, flesh-colored papules, nodules, or cystic lesions around the periorbital skin in elderly female patients.1 Histopathology of EMPSGCs reveals well-circumscribed multinodular dermal lesions that can be either cystic or solid and often are arranged in papillary and cribriform patterns (quiz image). Nests of uniform tumor cells are composed of small- to medium-sized epithelial cells with monomorphic nuclei showing fine to stippled chromatin.2 Histologically, EMPSGC resembles a solid papillary carcinoma of the breast, which is attributed to their common embryologic origin.3 Intracytoplasmic and extracellular mucin often are seen on hematoxylin and eosin staining.2 Variable immunohistochemical stain expression has been reported, including positive staining with synaptophysin and chromogranin. Other markers include cytokeratin CAM 5.2, epithelial membrane antigen, estrogen or progesterone receptors, and cytokeratin 7.4 Endocrine mucin-producing sweat gland carcinoma is thought to be a precursor to invasive neuroendocrine-type primary cutaneous mucinous carcinoma. Primary cutaneous mucinous carcinoma has been associated with EMPSGC in approximately 35.7% of cases. Histologically, primary cutaneous mucinous carcinoma that has transformed from EMPSGC would show an infiltration of tumor nests with desmoplastic stroma or mucin pools with clusters of tumor cells.2
Primary cutaneous adenoid cystic carcinoma is a rare malignant tumor that often presents on the head and neck. It usually appears as a single, slowly growing subcutaneous nodule or multinodular plaque.5,6 Histologic features include basaloid cells in alternating tubular and cribriform patterns. The cribriform areas are composed of pseudoglandular adenoid spaces that contain mucin, basement membrane zone material, and cellular debris from necrotic neoplastic cells (Figure 1).7 Primary cutaneous adenoid cystic carcinoma predominantly is dermal with extension to the subcutaneous tissue. True ductal structures that demonstrate decapitation secretion also may be present.7

Basal cell carcinoma (adenoid type) presents as a pigmented or nonpigmented nodule or ulcer on sunexposed areas of the head and neck. Histopathology reveals basaloid cells surrounding islands of connective tissue resulting in a lacelike pattern (Figure 2). The lumina may contain a colloidal substance or amorphous granular material.8 The characteristic features of basal cell carcinomas, such as nests of basaloid cells with peripheral palisading cells, retraction of adjacent stroma, increased apoptosis and mitotic figures, and connection to the epidermis, can be helpful to distinguish basal cell carcinoma histologically from EMPSGC.2

Apocrine hidrocystomas clinically present as round, flesh-colored, shiny or translucent, dome-shaped papules or nodules near the eyelid margin or lateral canthus.9 Histologically, they are composed of proliferating apocrine secretory coils with an epithelial side of cuboidal or columnar cells and a luminal side exhibiting decapitation secretion (Figure 3).2 An epidermal connection is absent.9 Apocrine hidrocystomas may exhibit complex architecture and papillary ductal hyperplasia that are difficult to distinguish from EMPSGC, especially if EMPSGC presents with cystic morphology. Apocrine cytomorphology and the lack of neuroendocrine marker expression and mucin production distinguish apocrine hidrocystomas. Furthermore, hidrocystomas infrequently demonstrate the nodular, solid, cribriform areas appreciated in EMPSGC.2

Microcystic adnexal carcinoma is a rare, slowly growing, locally aggressive sweat gland tumor that commonly presents as a flesh-colored to yellow papule, nodule, or plaque on the central face.10 Histopathologic examination reveals both eccrine and follicular differentiation. Keratin cysts, bland keratinocyte cords, and epithelium with ductal differentiation is observed in the superficial layers (Figure 4). Deep invasion into the subcutis and perineural invasion frequently is observed.

- Mulay K, Menon V, Lahane S, et al. Endocrine mucinproducing sweat gland carcinoma (EMPSGC) of the eyelid: clinicopathologic features, immunohistochemical findings and review of literature. Indian J Ophthalmol. 2019;67:1374-1377. doi:10.4103/ijo.IJO_1745_18
- Au RTM, Bundele MM. Endocrine mucin-producing sweat gland carcinoma and associated primary cutaneous mucinous carcinoma: review of the literature. J Cutan Pathol. 2021;48:1156-1165. doi:10.1111/cup.13983
- Flieder A, Koerner FC, Pilch BZ, et al. Endocrine mucin-producing sweat gland carcinoma: a cutaneous neoplasm analogous to solid papillary carcinoma of breast. Am J Surg Pathol. 1997;21:1501-1506. doi:10.1097/00000478-199712000-00014
- Shimizu I, Dufresne R, Robinson-Bostom L. Endocrine mucinproducing sweat gland carcinoma. Cutis. 2014;93:47-49.
- Ahn CS, Sangüeza OP. Malignant sweat gland tumors. Hematol Oncol Clin North Am. 2019;33:53-71. doi:10.1016/j.hoc.2018.09.002
- Tonev ID, Pirgova YS, Conev NV. Primary adenoid cystic carcinoma of the skin with multiple local recurrences. Case Rep Oncol. 2015;8:251-255. doi:10.1159/000431082
- Coca-Pelaz A, Rodrigo JP, Bradley PJ, et al. Adenoid cystic carcinoma of the head and neck—an update. Oral Oncol. 2015;51:652-661. doi:10.1016/j.oraloncology.2015.04.005
- Tambe SA, Ghate SS, Jerajani HR. Adenoid type of basal cell carcinoma: rare histopathological variant at an unusual location. Indian J Dermatol. 2013;58:159. doi:10.4103/0019-5154.108080
- Kikuchi K, Fukunaga S, Inoue H, et al. Apocrine hidrocystoma of the lower lip: a case report and literature review. Head Neck Pathol. 2014;8:117-121. doi:10.1007/s12105-013-0451-2
- Zito PM, Mazzoni T. Microcystic adnexal carcinoma. StatPearls. StatPearls Publishing; 2021.
The Diagnosis: Endocrine Mucin-Producing Sweat Gland Carcinoma
Endocrine mucin-producing sweat gland carcinoma (EMPSGC) is a rare cutaneous adnexal tumor that characteristically presents as slowgrowing, flesh-colored papules, nodules, or cystic lesions around the periorbital skin in elderly female patients.1 Histopathology of EMPSGCs reveals well-circumscribed multinodular dermal lesions that can be either cystic or solid and often are arranged in papillary and cribriform patterns (quiz image). Nests of uniform tumor cells are composed of small- to medium-sized epithelial cells with monomorphic nuclei showing fine to stippled chromatin.2 Histologically, EMPSGC resembles a solid papillary carcinoma of the breast, which is attributed to their common embryologic origin.3 Intracytoplasmic and extracellular mucin often are seen on hematoxylin and eosin staining.2 Variable immunohistochemical stain expression has been reported, including positive staining with synaptophysin and chromogranin. Other markers include cytokeratin CAM 5.2, epithelial membrane antigen, estrogen or progesterone receptors, and cytokeratin 7.4 Endocrine mucin-producing sweat gland carcinoma is thought to be a precursor to invasive neuroendocrine-type primary cutaneous mucinous carcinoma. Primary cutaneous mucinous carcinoma has been associated with EMPSGC in approximately 35.7% of cases. Histologically, primary cutaneous mucinous carcinoma that has transformed from EMPSGC would show an infiltration of tumor nests with desmoplastic stroma or mucin pools with clusters of tumor cells.2
Primary cutaneous adenoid cystic carcinoma is a rare malignant tumor that often presents on the head and neck. It usually appears as a single, slowly growing subcutaneous nodule or multinodular plaque.5,6 Histologic features include basaloid cells in alternating tubular and cribriform patterns. The cribriform areas are composed of pseudoglandular adenoid spaces that contain mucin, basement membrane zone material, and cellular debris from necrotic neoplastic cells (Figure 1).7 Primary cutaneous adenoid cystic carcinoma predominantly is dermal with extension to the subcutaneous tissue. True ductal structures that demonstrate decapitation secretion also may be present.7

Basal cell carcinoma (adenoid type) presents as a pigmented or nonpigmented nodule or ulcer on sunexposed areas of the head and neck. Histopathology reveals basaloid cells surrounding islands of connective tissue resulting in a lacelike pattern (Figure 2). The lumina may contain a colloidal substance or amorphous granular material.8 The characteristic features of basal cell carcinomas, such as nests of basaloid cells with peripheral palisading cells, retraction of adjacent stroma, increased apoptosis and mitotic figures, and connection to the epidermis, can be helpful to distinguish basal cell carcinoma histologically from EMPSGC.2

Apocrine hidrocystomas clinically present as round, flesh-colored, shiny or translucent, dome-shaped papules or nodules near the eyelid margin or lateral canthus.9 Histologically, they are composed of proliferating apocrine secretory coils with an epithelial side of cuboidal or columnar cells and a luminal side exhibiting decapitation secretion (Figure 3).2 An epidermal connection is absent.9 Apocrine hidrocystomas may exhibit complex architecture and papillary ductal hyperplasia that are difficult to distinguish from EMPSGC, especially if EMPSGC presents with cystic morphology. Apocrine cytomorphology and the lack of neuroendocrine marker expression and mucin production distinguish apocrine hidrocystomas. Furthermore, hidrocystomas infrequently demonstrate the nodular, solid, cribriform areas appreciated in EMPSGC.2

Microcystic adnexal carcinoma is a rare, slowly growing, locally aggressive sweat gland tumor that commonly presents as a flesh-colored to yellow papule, nodule, or plaque on the central face.10 Histopathologic examination reveals both eccrine and follicular differentiation. Keratin cysts, bland keratinocyte cords, and epithelium with ductal differentiation is observed in the superficial layers (Figure 4). Deep invasion into the subcutis and perineural invasion frequently is observed.

The Diagnosis: Endocrine Mucin-Producing Sweat Gland Carcinoma
Endocrine mucin-producing sweat gland carcinoma (EMPSGC) is a rare cutaneous adnexal tumor that characteristically presents as slowgrowing, flesh-colored papules, nodules, or cystic lesions around the periorbital skin in elderly female patients.1 Histopathology of EMPSGCs reveals well-circumscribed multinodular dermal lesions that can be either cystic or solid and often are arranged in papillary and cribriform patterns (quiz image). Nests of uniform tumor cells are composed of small- to medium-sized epithelial cells with monomorphic nuclei showing fine to stippled chromatin.2 Histologically, EMPSGC resembles a solid papillary carcinoma of the breast, which is attributed to their common embryologic origin.3 Intracytoplasmic and extracellular mucin often are seen on hematoxylin and eosin staining.2 Variable immunohistochemical stain expression has been reported, including positive staining with synaptophysin and chromogranin. Other markers include cytokeratin CAM 5.2, epithelial membrane antigen, estrogen or progesterone receptors, and cytokeratin 7.4 Endocrine mucin-producing sweat gland carcinoma is thought to be a precursor to invasive neuroendocrine-type primary cutaneous mucinous carcinoma. Primary cutaneous mucinous carcinoma has been associated with EMPSGC in approximately 35.7% of cases. Histologically, primary cutaneous mucinous carcinoma that has transformed from EMPSGC would show an infiltration of tumor nests with desmoplastic stroma or mucin pools with clusters of tumor cells.2
Primary cutaneous adenoid cystic carcinoma is a rare malignant tumor that often presents on the head and neck. It usually appears as a single, slowly growing subcutaneous nodule or multinodular plaque.5,6 Histologic features include basaloid cells in alternating tubular and cribriform patterns. The cribriform areas are composed of pseudoglandular adenoid spaces that contain mucin, basement membrane zone material, and cellular debris from necrotic neoplastic cells (Figure 1).7 Primary cutaneous adenoid cystic carcinoma predominantly is dermal with extension to the subcutaneous tissue. True ductal structures that demonstrate decapitation secretion also may be present.7

Basal cell carcinoma (adenoid type) presents as a pigmented or nonpigmented nodule or ulcer on sunexposed areas of the head and neck. Histopathology reveals basaloid cells surrounding islands of connective tissue resulting in a lacelike pattern (Figure 2). The lumina may contain a colloidal substance or amorphous granular material.8 The characteristic features of basal cell carcinomas, such as nests of basaloid cells with peripheral palisading cells, retraction of adjacent stroma, increased apoptosis and mitotic figures, and connection to the epidermis, can be helpful to distinguish basal cell carcinoma histologically from EMPSGC.2

Apocrine hidrocystomas clinically present as round, flesh-colored, shiny or translucent, dome-shaped papules or nodules near the eyelid margin or lateral canthus.9 Histologically, they are composed of proliferating apocrine secretory coils with an epithelial side of cuboidal or columnar cells and a luminal side exhibiting decapitation secretion (Figure 3).2 An epidermal connection is absent.9 Apocrine hidrocystomas may exhibit complex architecture and papillary ductal hyperplasia that are difficult to distinguish from EMPSGC, especially if EMPSGC presents with cystic morphology. Apocrine cytomorphology and the lack of neuroendocrine marker expression and mucin production distinguish apocrine hidrocystomas. Furthermore, hidrocystomas infrequently demonstrate the nodular, solid, cribriform areas appreciated in EMPSGC.2

Microcystic adnexal carcinoma is a rare, slowly growing, locally aggressive sweat gland tumor that commonly presents as a flesh-colored to yellow papule, nodule, or plaque on the central face.10 Histopathologic examination reveals both eccrine and follicular differentiation. Keratin cysts, bland keratinocyte cords, and epithelium with ductal differentiation is observed in the superficial layers (Figure 4). Deep invasion into the subcutis and perineural invasion frequently is observed.

- Mulay K, Menon V, Lahane S, et al. Endocrine mucinproducing sweat gland carcinoma (EMPSGC) of the eyelid: clinicopathologic features, immunohistochemical findings and review of literature. Indian J Ophthalmol. 2019;67:1374-1377. doi:10.4103/ijo.IJO_1745_18
- Au RTM, Bundele MM. Endocrine mucin-producing sweat gland carcinoma and associated primary cutaneous mucinous carcinoma: review of the literature. J Cutan Pathol. 2021;48:1156-1165. doi:10.1111/cup.13983
- Flieder A, Koerner FC, Pilch BZ, et al. Endocrine mucin-producing sweat gland carcinoma: a cutaneous neoplasm analogous to solid papillary carcinoma of breast. Am J Surg Pathol. 1997;21:1501-1506. doi:10.1097/00000478-199712000-00014
- Shimizu I, Dufresne R, Robinson-Bostom L. Endocrine mucinproducing sweat gland carcinoma. Cutis. 2014;93:47-49.
- Ahn CS, Sangüeza OP. Malignant sweat gland tumors. Hematol Oncol Clin North Am. 2019;33:53-71. doi:10.1016/j.hoc.2018.09.002
- Tonev ID, Pirgova YS, Conev NV. Primary adenoid cystic carcinoma of the skin with multiple local recurrences. Case Rep Oncol. 2015;8:251-255. doi:10.1159/000431082
- Coca-Pelaz A, Rodrigo JP, Bradley PJ, et al. Adenoid cystic carcinoma of the head and neck—an update. Oral Oncol. 2015;51:652-661. doi:10.1016/j.oraloncology.2015.04.005
- Tambe SA, Ghate SS, Jerajani HR. Adenoid type of basal cell carcinoma: rare histopathological variant at an unusual location. Indian J Dermatol. 2013;58:159. doi:10.4103/0019-5154.108080
- Kikuchi K, Fukunaga S, Inoue H, et al. Apocrine hidrocystoma of the lower lip: a case report and literature review. Head Neck Pathol. 2014;8:117-121. doi:10.1007/s12105-013-0451-2
- Zito PM, Mazzoni T. Microcystic adnexal carcinoma. StatPearls. StatPearls Publishing; 2021.
- Mulay K, Menon V, Lahane S, et al. Endocrine mucinproducing sweat gland carcinoma (EMPSGC) of the eyelid: clinicopathologic features, immunohistochemical findings and review of literature. Indian J Ophthalmol. 2019;67:1374-1377. doi:10.4103/ijo.IJO_1745_18
- Au RTM, Bundele MM. Endocrine mucin-producing sweat gland carcinoma and associated primary cutaneous mucinous carcinoma: review of the literature. J Cutan Pathol. 2021;48:1156-1165. doi:10.1111/cup.13983
- Flieder A, Koerner FC, Pilch BZ, et al. Endocrine mucin-producing sweat gland carcinoma: a cutaneous neoplasm analogous to solid papillary carcinoma of breast. Am J Surg Pathol. 1997;21:1501-1506. doi:10.1097/00000478-199712000-00014
- Shimizu I, Dufresne R, Robinson-Bostom L. Endocrine mucinproducing sweat gland carcinoma. Cutis. 2014;93:47-49.
- Ahn CS, Sangüeza OP. Malignant sweat gland tumors. Hematol Oncol Clin North Am. 2019;33:53-71. doi:10.1016/j.hoc.2018.09.002
- Tonev ID, Pirgova YS, Conev NV. Primary adenoid cystic carcinoma of the skin with multiple local recurrences. Case Rep Oncol. 2015;8:251-255. doi:10.1159/000431082
- Coca-Pelaz A, Rodrigo JP, Bradley PJ, et al. Adenoid cystic carcinoma of the head and neck—an update. Oral Oncol. 2015;51:652-661. doi:10.1016/j.oraloncology.2015.04.005
- Tambe SA, Ghate SS, Jerajani HR. Adenoid type of basal cell carcinoma: rare histopathological variant at an unusual location. Indian J Dermatol. 2013;58:159. doi:10.4103/0019-5154.108080
- Kikuchi K, Fukunaga S, Inoue H, et al. Apocrine hidrocystoma of the lower lip: a case report and literature review. Head Neck Pathol. 2014;8:117-121. doi:10.1007/s12105-013-0451-2
- Zito PM, Mazzoni T. Microcystic adnexal carcinoma. StatPearls. StatPearls Publishing; 2021.
A 76-year-old woman presented with a slowly growing, asymptomatic, 5-mm, pink-brown, dome-shaped papule adjacent to the left lateral canthus of several years’ duration. Dermoscopic examination revealed fine linear peripheral blood vessels. The lesional cells were positive with cytokeratin 7, estrogen receptor, progesterone receptor, chromogranin, synaptophysin, and neuron-specific enolase. Cytokeratin 20 and p63 were negative, and the Ki-67 proliferative index was less than 5%.