User login
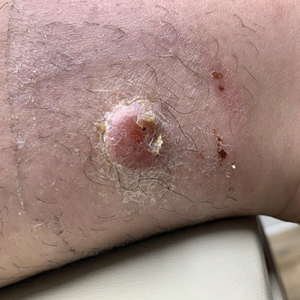
Alzheimer's Disease Signs and Symptoms
Editor's Note: This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Editor's Note: This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Editor's Note: This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Development of a VA Clinician Resource to Facilitate Care Among Veterans Experiencing Homelessness
Development of a VA Clinician Resource to Facilitate Care Among Veterans Experiencing Homelessness
Veterans experiencing homelessness are at an elevated risk for adverse health outcomes, including suicide. This population also experiences chronic health conditions (eg, cardiovascular disease and sexually transmitted infections) and psychiatric conditions (eg, substance use disorders and posttraumatic stress disorder) with a greater propensity than veterans without history of homelessness.1,2 Similarly, veterans experiencing homelessness often report concurrent stressors, such as justice involvement and unemployment, which further impact social functioning.3
The US Department of Veterans Affairs (VA) offers a range of health and social services to veterans experiencing homelessness. These programs are designed to respond to the multifactorial challenges faced by this population and are aimed at achieving sustained, permanent housing.4 To facilitate this effort, these programs provide targeted and tailored health (eg, primary care) and social (eg, case management and vocational rehabilitation) services to address barriers to housing stability (eg, substance use, serious mental illness, interacting with the criminal legal system, and unemployment).
Despite the availability of these programs, engaging veterans in VA services—whether in general or tailored for those experiencing or at risk for homelessness—remains challenging. Many veterans at risk for or experiencing homelessness overuse service settings that provide immediate care, such as urgent care or emergency departments (EDs).5,6 These individuals often visit an ED to augment or complement medical care they received in an outpatient setting, which can result in an elevated health care burden as well as impacted provision of treatment, especially surrounding care for chronic conditions (eg, cardiovascular health or serious mental illness).7-9
VA EDs offer urgent care and emergency services and often serve as a point of entry for veterans experiencing homelessness.10 They offer veterans expedient access to care that can address immediate needs (eg, substance use withdrawal, pain management, and suicide risk). EDs may be easier to access given they have longer hours of operation and patients can present without a scheduled appointment. VA EDs are an important point to identify homelessness and connect individuals to social service resources and outpatient health care referrals (eg, primary care and mental health).4,11
Some clinicians experience uncertainty in navigating or providing care for veterans experiencing or at risk for homelessness. A qualitative study conducted outside the VA found many clinicians did not know how to approach clinical conversations among unstably housed individuals, particularly when they discussed how to manage care for complex health conditions in the context of ongoing case management challenges, such as discharge planning.12 Another study found that clinicians working with individuals experiencing homelessness may have limited prior training or experience treating these patients.13 As a result, these clinicians may be unaware of available social services or unknowingly have biases that negatively impact care. Research remains limited surrounding beliefs about and methods of enhancing care among VA clinicians working with veterans experiencing homelessness in the ED.
This multiphase pilot study sought to understand service delivery processes and gaps in VA ED settings. Phase 1 examined ED clinician perceptions of care, facilitators, and barriers to providing care (including suicide risk assessments) and making postdischarge outpatient referrals among VA ED clinicians who regularly work with veterans experiencing homelessness. Phase 2 used this information to develop a clinical psychoeducational resource to enhance post-ED access to care for veterans experiencing or at risk for homelessness.
QUALITATIVE INTERVIEWS
Semistructured qualitative interviews were conducted with 11 VA ED clinicians from 6 Veteran Integrated Service Networks between August 2022 and February 2023. Clinicians were eligible if they currently worked within a VA ED setting (including urgent care) and indicated that some of their patients were veterans experiencing homelessness. All health care practitioners (HCPs) participated in an interview and a postinterview self-report survey that assessed demographic and job-related characteristics. Eight HCPs identified as female and 3 identified as male. All clinicians identified as White and 3 as Hispanic or Latino. Eight clinicians were licensed clinical social workers, 2 were ED nurses, and 1 was an ED physician.
After each clinician provided informed consent, they were invited to complete a telephone or Microsoft Teams interview. All interviews were recorded and subsequently transcribed. Interviews explored clinicians’ experiences caring for veterans experiencing homelessness, with a focus on services provided within the ED, as well as mandated ED screenings such as a suicide risk assessment. Interview questions also addressed postdischarge knowledge and experiences with referrals to VA health services (eg, primary care, mental health) and social services (eg, housing programs). Interviews lasted 30 to 90 minutes.
Recruitment ended after attaining sufficient thematic data, accomplished via an information power approach to sampling. This occurred when the study aims, sample characteristics, existing theory, and depth and quality of interviews dynamically informed the decision to cease recruitment of additional participants.14,15 Given the scope of study (examining service delivery and knowledge gaps), the specificity of the targeted sample (VA ED clinicians providing care to veterans experiencing homelessness), the level of pre-existing theoretical background informing the study aims, and depth and quality of interview dialogue, this information power approach provides justification for attaining small sample sizes. Following the interview, HCPs completed a demographic questionnaire. Participants were not compensated.
Data Analysis
Directed content analysis was used to analyze qualitative data, with the framework method employed as an analytic instrument to facilitate analysis.16-18 Analysts engaged in bracketing and discussed reflexivity before data analysis to reflect on personal subjectivities and reduce potential bias.19,20
A prototype coding framework was developed that enabled coders to meaningfully summarize and condense data within transcripts into varying domains, categories, or topics found within the interview guide. Domain examples included clinical backgrounds, suicide risk and assessment protocols among veterans experiencing homelessness, beliefs about service delivery for veterans experiencing homelessness, and barriers and facilitators that may impact their ability to provide post-ED discharge care. Coders discussed the findings and if there was a need to modify templates. All transcripts were double coded. Once complete, individual templates were merged into a unified Microsoft Excel sheet, which allowed for more discrete analyses, enabling analysts to examine trends across content areas within the dataset.
Clinical Resource Development
HCPs were queried regarding available outpatient resources for post-ED care (eg, printed discharge paperwork and best practice alerts or automated workflows within the electronic health record). Resources used by participants were examined, as well as which resources clinicians thought would help them care for veterans experiencing homelessness. Noted gaps were used to develop a tailored resource for clinicians who treat veterans experiencing homelessness in the ED. This resource was created with the intention it could inform all ED clinicians, with the option for personalization to align with the needs of local services, based on needed content areas identified (eg, emergency shelters and suicide prevention resources).
Resource development followed an information systems research (ISR) framework that used a 3-pronged process of identifying circumstances for how a tool is developed, the problems it aims to address, and the knowledge that informs its development, implementation, and evaluation.21,22 Initial wireframes of the resource were provided via email to 10 subject matter experts (SMEs) in veteran suicide prevention, emergency medicine, and homeless programs. SMEs were identified via professional listservs, VA program office leadership, literature searches of similar research, and snowball sampling. Solicited feedback on the resource from the SMEs included its design, language, tone, flow, format, and content (ideation and prototyping). The feedback was collated and used to revise the resource. SMEs then reviewed and provided feedback on the revised resource. This iterative cycle (prototype review, commentary, ideation, prototype review) continued until the SMEs offered no additional edits to the resource. In total, 7 iterations of the resource were developed, critiqued, and revised.
INTERVIEW RESULTS
Compassion Fatigue
Many participants expressed concerns about compassion fatigue among VA ED clinicians. Those interviewed indicated that treating veterans experiencing homelessness sometimes led to the development of what they described as a “callus,” a “sixth sense,” or an inherent sense of “suspicion” or distrust. These feelings resulted from concerns about an individual’s secondary gain or potential hidden agenda (eg, a veteran reporting suicidal ideation to attain shelter on a cold night), with clinicians not wanting to feel as if they were taken advantage of or deceived.
Many clinicians noted that compassion fatigue resulted from witnessing the same veterans experiencing homelessness routinely use emergency services for nonemergent or nonmedical needs. Some also expressed that over time this may result in them becoming less empathetic when caring for veterans experiencing homelessness. They hypothesized that clinicians may experience burnout, which could potentially result in a lack of curiosity and concern about a veteran’s risk for suicide or need for social services. Others may “take things for granted,” leading them to discount stressors that are “very real to the patient, this person.”
Clinicians indicated that such sentiments may impact overall care. Potential negative consequences included stigmatization of veterans experiencing homelessness, incomplete or partial suicide risk screenings with this population, inattentive or impersonal care, and expedited discharge from the ED without appropriate safety planning or social service referrals. Clinicians interviewed intended to find ways to combat compassion fatigue and maintain a commitment to provide comprehensive care to all veterans, including those experiencing homelessness. They felt conflict between a lack of empathy for individuals experiencing homelessness and becoming numb to the problem due to overexposure. However, these clinicians remained committed to providing care to these veterans and fighting to maintain the purpose of recovery-focused care.
Knowledge Gaps on Available Services
While many clinicians knew of general resources available to veterans experiencing homelessness, few had detailed information on where to seek consults for other homeless programs, who to contact regarding these services, when they were available, or how to refer to them. Many reported feeling uneasy when discharging veterans experiencing homelessness from care, often being unable to provide local, comprehensive referrals to support their needs and ensure their well-being. These sentiments were compounded when the veteran reported suicidal thoughts or recent suicidal behavior; clinicians felt concerned about the methods to engage these individuals into evidence-based mental health care within the context of unstable housing arrangements.
Some clinicians appeared to lack awareness of the wide array of VA homeless programming. Most could acknowledge at least some aspects of available programming (eg, the US Department of Housing and Urban Development– VA Supportive Housing program), while others were unaware of services tailored to the needs of those experiencing homelessness (eg, homeless patient aligned care teams), or of services targeting concurrent psychosocial stressors (eg, Veterans Justice Programs). Interviewees hypothesized this as being particularly notable among clinicians who are new to the VA or those who work in VA settings as part of their graduate or medical school training. Those aware of the services were uncertain of the referral process, relying on a single social worker or nurse to connect individuals experiencing homelessness to health and social services.
Interviewed clinicians noted that suicide risk screening of veterans experiencing homelessness was only performed by a limited number of individuals within the ED. Some did not feel sufficiently trained, comfortable, or knowledgeable about how to navigate care for veterans experiencing homelessness and at risk of suicide. Clinicians described “an uncomfortableness about suicidal ideation, where people just freeze up” and “don’t know what to do and don’t know what to say.”
Lack of Tangible Resources, Trainings, and Referrals
HCPs reported occasionally lacking the necessary clinical resources and information in the ED to properly support veterans experiencing homelessness and suicidal ideation. Common concerns included case management and discharge planning, as well as navigating health factors, such as elevated suicide risk. Some HCPs felt the local resources they do have access to—discharge packets or other forms of patient information—were not always tailored for the needs (eg, transportation) or abilities of veterans experiencing homelessness. One noted: “We give them a sheet of paper with some resources, which they don’t have the skills to follow up [with] anyway.”
Many interviewees wished for additional training in working with veterans experiencing homelessness. They reported that prior training from the VA Talent Management System or through unit-based programming could assist in educating clinicians on homeless services and suicide risk assessment. When queried on what training they had received, many noted there was “no formal training on what the VA offers homeless vets,” leading many to describe it as on-the-job training. This appeared especially among newer clinicians, who reported they were reliant upon learning from other, more senior staff within the ED.
The absence of training further illustrates the issue of institutional knowledge on these services and referrals, which was often confined to a single individual or team. Not having readily accessible resources, training, or information appropriate for all skill levels and positions within the ED hindered the ability of HCPs to connect veterans experiencing homelessness with social services to ensure their health and safety postdischarge: “If we had a better knowledge base of what the VA offers and the steps to go through in order to get the veteran set up for those things, it would be helpful.”
CLINICAL RESOURCE
A psychoeducational resource was developed for HCPs treating veterans experiencing homelessness (Figure). The resource was designed to mitigate compassion fatigue and recenter attention on the VA commitment to care while emphasizing the need to be responsive to the concerns of these individuals. Initial wireframes of the resource were developed by a small group of authors in review and appraisal of qualitative findings (EP, RH). These wireframes were developed to broadly illustrate the arrangement/structure of content, range of resources to potentially include (eg, available VA homeless programs or consultation resources), and to draft initial wording and phrasing. Subject matter expert feedback refined these wireframes, providing commentary on specific programs to include or exclude, changes and alterations to the design and flow of the resource, and edits to language, word choice, and tone over numerous iterations.
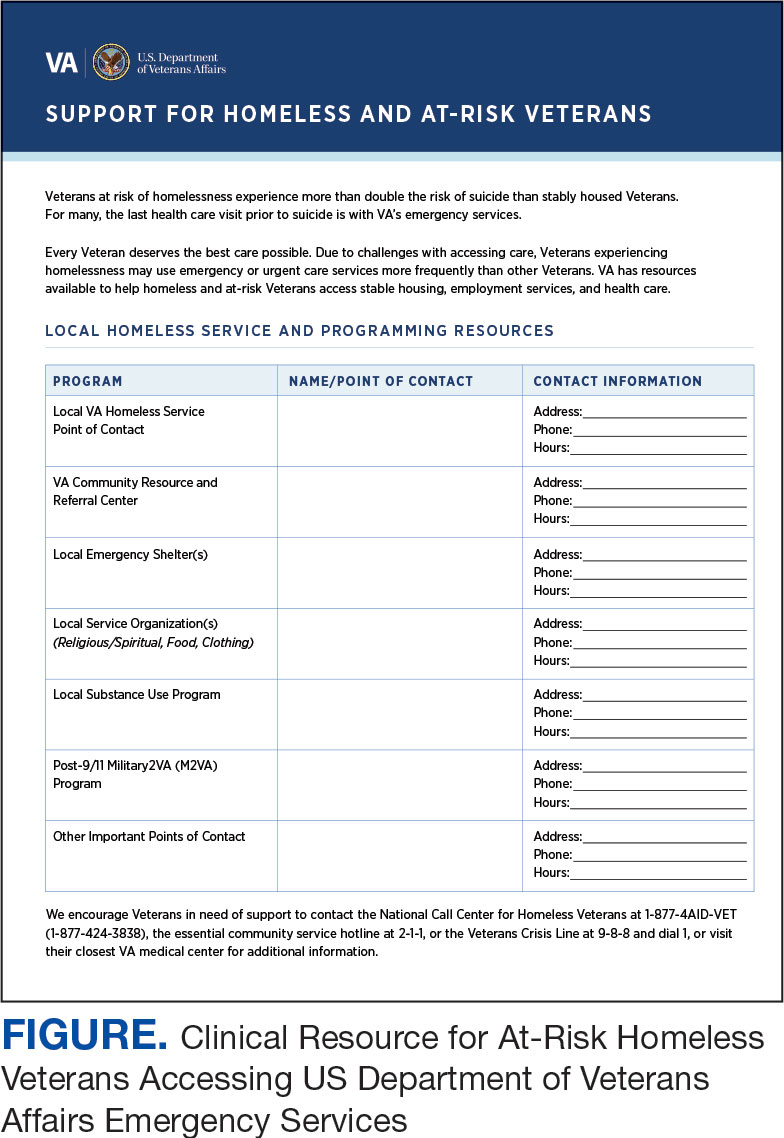
Given that many ED HCPs presented concerns surrounding secondary gain in the context of suicide risk, this resource focused on suicide risk. At the top of the resource, it states “Veterans at risk for homelessness experience more than double the risk for suicide than stably housed veterans.”23 Also at the top, the resource states: “For many, the last health care visit prior to suicide is often with VA emergency services."24 The goal of these statements was to educate users on the elevated risk for suicide in veterans experiencing homelessness and their role in preventing such deaths.
Text in this section emphasizes that every veteran deserves the best care possible and recenters HCP attention on providing quality, comprehensive care regardless of housing status. The inclusion of this material was prioritized given the concerns expressed regarding compassion fatigue and suspicions of secondary gain (eg, a veteran reporting suicidal ideation to attain shelter or respite from outside conditions).
The resource also attempts to address high rates of emergency service by veterans experiencing homelessness: “Due to challenges with accessing care, Veterans experiencing homelessness may use emergency or urgent care services more frequently than other Veterans.”25 The resource also indicates that VA resources are available to help homeless and at-risk veterans to acquire stable housing, employment, and engage in healthcare, which are outlined with specific contact information. Given the breadth of local and VA services, a portion of the resource is dedicated to local health and social services available for veterans experiencing homelessness. HCPs complete the first page, which is devoted to local homeless service and program resources.
Following SME consultation, the list of programs provided underwent a series of iterations. The program types listed are deemed to be of greatest benefit to veterans experiencing homelessness and most consulted by HCPs. Including VA and non-VA emergency shelters allows clinicians flexible options if a particular shelter is full, closed, or would not meet the veteran’s needs or preference (eg, lack of childcare or does not allow pets). The second column of this section is left intentionally blank; here, the HCP is to list a local point-of- contact at each program. This encourages clinical teams to seek out and make direct contact with these programs and establish (in)formal relationships with them. The HCP then completes the third column with contact information.
Once completed, the resource acts as a living document. Clinicians and SMEs consulted for this study expressed the desire to have an easily accessible resource that can be updated based on necessary changes (eg, emergency shelter address or hours of operation). The resource can be housed within each local VA emergency or urgent care service setting alongside other available clinical tools.
While local resources are the primary focus, interviewees also suggested that some HCPs are not aware of the available VA services . This material, found on the back of the resource, provides a general overview of services available through VA homeless programs. SME consultation and discussion led to selecting the 5 listed categories: housing services, health care services, case management, employment services, and justice-related programming, each with a brief description.
Information for the National Call Center for Homeless Veterans, community service hotline, and Veterans Crisis Line are included on the front page. These hotlines and phone numbers are always available for veterans experiencing homelessness, enabling them to make these connections themselves, if desired. Additionally, given the challenges noted by some HCPs in performing suicide risk screening, evaluation, and intervention, a prompt for the VA Suicide Risk Management Consultation service was also included on the back page.
Creating a Shared and Local Resource
This clinical resource was developed to establish a centralized, shared, local resource available to VA ED HCPs who lacked knowledge of available services or reported discomfort conducting suicide risk screening for veterans experiencing homelessness. In many cases, ED referrals to homeless programs and suicide prevention care was assigned to a single individual, often a nurse or social worker. As a result, an undue amount of work and strain was placed on these individuals, as this forced them to act as the sole bridge between care in the ED and postdischarge social (eg, homeless programs) and mental health (eg, suicide prevention) services. The creation of a unified, easily accessible document aimed to distribute this responsibility more equitably across ED staff.
DISCUSSION
This project intended to develop a clinician resource to support VA ED clinicians caring for veterans experiencing homelessness and their access to services postdischarge. Qualitative interviews provided insights into the burnout and compassion fatigue present in these settings, as well as the challenges and needs regarding knowledge of local and VA services. Emphasis was placed on leveraging extant resources and subject matter expertise to develop a resource capable of providing brief and informative guidance.
This resource is particularly relevant for HCPs new to the VA, including trainees and new hires, who may be less aware of VA and local social services. It has the potential to reduce the burden on VA ED staff to provide guidance and recommendations surrounding postdischarge social services. The resource acknowledges homeless programming focused on social determinants of health that can destabilize housing (eg, legal or occupational challenges). This can incentivize clinicians to discuss these programs with veterans to facilitate their ability to navigate complex health and psychosocial challenges.
HCPs interviewed for this study indicated their apprehension regarding suicide risk screening and evaluation, a process currently mandated within VA ED settings.26 This may be compounded among HCPs with minimal mental health training or those who have worked in community-based settings where such screening and evaluation efforts are not required. The resource reminds clinicians of available VA consultation services, which can provide additional training, clinical guidance, and review of existing local ED processes.
While the resource was directly informed by qualitative interviews conducted with VA emergency service HCPs and developed through an iterative process with SMEs, further research is necessary to determine its effectiveness at increasing access to health and social services among veterans experiencing homelessness. The resource has not been used by HCPs working in these settings to examine uptake or sustained use, nor clinicians’ perceptions of its utility, including acceptability and feasibility; these are important next steps to understand if the resource is functioning as intended.
Compassion fatigue, as well as associated sequelae (eg, burnout, distress, and psychiatric symptoms), is well-documented among individuals working with individuals experiencing homelessness, including VA HCPs.27-30 Such experiences are likely driven by several factors, including the clinical complexity and service needs of this veteran population. Although compassion fatigue was noted by many clinicians interviewed for this study, it is unclear if the resource alone would address factors driving compassion fatigue, or if additional programming or services may be necessary.
Limitations
The resource requires local HCPs to routinely update its content (eg, establishment of a new emergency shelter in the community or change in hours or contact information of an existing one), which may be challenging. This is especially true as it relates to community resources, which may be more likely to change than national VA programming.
This resource was initially developed following qualitative interviews with a small sample of VA HCPs (explicitly those working within ED settings) and may not be representative of all HCPs engaged in VA care with veterans experiencing homelessness. The perspectives and experiences of those interviewed do not represent the views of all VA ED HCPs and may differ from the perspectives of those in regions with unique cultural and regional considerations.31
Given that most of the interviewees were social workers in EDs engaged in care for veterans experiencing homelessness, these findings and informational needs may differ among other types of HCPs who provide services for veterans experiencing homelessness in other settings. Content in the resource was included based on clinician input, and may not reflect the perspectives of veterans, who may perceive some resources as more important (eg, access to primary care or dental services).28
CONCLUSIONS
This project represents the culmination of qualitative interviews and SME input to develop a free-to-use clinician resource to facilitate service delivery and connection to services following discharge from VA EDs for veterans experiencing homelessness. Serving as a template, this resource can be customized to increase knowledge of local VA and community resources to support these individuals. Continued refinement and piloting of this resource to evaluate acceptability, implementation barriers, and use remains warranted.
- Holliday R, Kinney AR, Smith AA, et al. A latent class analysis to identify subgroups of VHA using homeless veterans at greater risk for suicide mortality. J Affect Disord. 2022;315:162-167. doi:10.1016/j.jad.2022.07.062
- Weber J, Lee RC, Martsolf D. Understanding the health of veterans who are homeless: a review of the literature. Public Health Nurs. 2017;34(5):505-511. doi:10.1111/phn.12338
- Holliday R, Desai A, Stimmel M, Liu S, Monteith LL, Stewart KE. Meeting the health and social service needs of veterans who interact with the criminal justice system and experience homelessness: a holistic conceptualization and recommendations for tailoring care. Curr Treat Options Psychiatry. 2022;9(3):174-185. doi:10.1007/s40501-022-00275-1
- Holliday R, Desai A, Gerard G, Liu S, Stimmel M. Understanding the intersection of homelessness and justice involvement: enhancing veteran suicide prevention through VA programming. Fed Pract. 2022;39(1):8-11. doi:10.12788/fp.0216
- Kushel MB, Perry S, Bangsberg D, Clark R, Moss AR. Emergency department use among the homeless and marginally housed: results from a community-based study. Am J Public Health. 2002;92(5):778-784. doi:10.2105/ajph.92.5.778
- Tsai J, Doran KM, Rosenheck RA. When health insurance is not a factor: national comparison of homeless and nonhomeless US veterans who use Veterans Affairs emergency departments. Am J Public Health. 2013;103(Suppl 2):S225-S231. doi:10.2105/AJPH.2013.301307
- Doran KM, Raven MC, Rosenheck RA. What drives frequent emergency department use in an integrated health system? National data from the Veterans Health Administration. Ann Emerg Med. 2013;62(2):151-159. doi:10.1016/j.annemergmed.2013.02.016
- Tsai J, Rosenheck RA. Risk factors for ED use among homeless veterans. Am J Emerg Med. 2013;31(5):855-858. doi:10.1016/j.ajem.2013.02.046
- Nelson RE, Suo Y, Pettey W, et al. Costs associated with health care services accessed through VA and in the community through Medicare for veterans experiencing homelessness. Health Serv Res. 2018;53(Suppl 3):5352-5374. doi:10.1111/1475-6773.13054
- Gabrielian S, Yuan AH, Andersen RM, Rubenstein LV, Gelberg L. VA health service utilization for homeless and low-income veterans: a spotlight on the VA Supportive Housing (VASH) program in greater Los Angeles. Med Care. 2014;52(5):454-461. doi:10.1097/MLR.0000000000000112
- Larkin GL, Beautrais AL. Emergency departments are underutilized sites for suicide prevention. Crisis. 2010;31(1):1- 6. doi:10.1027/0227-5910/a000001
- Decker H, Raguram M, Kanzaria HK, Duke M, Wick E. Provider perceptions of challenges and facilitators to surgical care in unhoused patients: a qualitative analysis. Surgery. 2024;175(4):1095-1102. doi:10.1016/j.surg.2023.11.009
- Panushka KA, Kozlowski Z, Dalessandro C, Sanders JN, Millar MM, Gawron LM. “It’s not a top priority”: a qualitative analysis of provider views on barriers to reproductive healthcare provision for homeless women in the United States. Soc Work Public Health. 2023;38(5 -8):428-436. doi:10.1080/19371918.2024.2315180
- Saunders B, Sim J, Kingstone T, et al. Saturation in qualitative research: exploring its conceptualization and operationalization. Qual Quant. 2018;52:1893-1907. doi:10.1007/s11135-017-0574-8
- Malterud K, Siersma VD, Guassora AD. Sample size in qualitative interview studies: guided by information power. Qual Health Res. 2016;26(13):1753-1760. doi:10.1177/1049732315617444
- Assarroudi A, Heshmati Nabavi F, Armat MR, Ebadi A, Vaismoradi M. Directed qualitative content analysis: the description and elaboration of its underpinning methods and data analysis process. J Res Nurs. 2018;23(1):42-55. doi:10.1177/1744987117741667
- Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277-1288.
- Goldsmith LJ. Using Framework Analysis in Applied Qualitative Research. Qual Rep. 2021;26(6):2061-2076. doi:10.46743/2160-3715/2021.5011
- Tufford L, Newman P. Bracketing in qualitative research. Qual Soc Work. 2012;11(1):80-96.
- Dodgson JE. Reflexivity in Qualitative Research. J Hum Lact. 2019;35(2):220-222. doi:10.1177/0890334419830990
- Hevner AR. A three cycle view of design science research. Scand J Inf Syst. 2007;19(2):4.
- Farao J, Malila B, Conrad N, Mutsvangwa T, Rangaka MX, Douglas TS. A user-centred design frame work for mHealth. PLOS ONE. 2020;15(8):e0237910. doi:10.1371/journal.pone.0237910
- Hoffberg AS, Spitzer E, Mackelprang JL, Farro SA, Brenner LA. Suicidal Self-Directed Violence Among Homeless US Veterans: A Systematic Review. Suicide Life Threat Behav. 2018;48(4):481-498. doi:10.1111/sltb.12369
- Larkin GL, Beautrais AL. Emergency departments are underutilized sites for suicide prevention. Crisis. 2010;31(1):1- 6. doi:10.1027/0227-5910/a000001
- Gabrielian S, Yuan AH, Andersen RM, Rubenstein LV, Gelberg L. VA health service utilization for homeless and lowincome Veterans: a spotlight on the VA Supportive Housing (VASH) program in greater Los Angeles. Med Care. 2014;52(5):454-461. doi:10.1097/MLR.0000000000000112
- Holliday R, Hostetter T, Brenner LA, Bahraini N, Tsai J. Suicide risk screening and evaluation among patients accessing VHA services and identified as being newly homeless. Health Serv Res. 2024;59(5):e14301. doi:10.1111/1475-6773.14301
- Waegemakers Schiff J, Lane AM. PTSD symptoms, vicarious traumatization, and burnout in front line workers in the homeless sector. Community Ment Health J. 2019;55(3):454-462. doi:10.1007/s10597-018-00364-7
- Steenekamp BL, Barker SL. Exploring the experiences of compassion fatigue amongst peer support workers in homelessness services. Community Ment Health J. 2024;60(4):772-783. doi:10.1007/s10597-024-01234-1
- Perez S, Kerman N, Dej E, et al. When I can’t help, I suffer: a scoping review of moral distress in service providers working with persons experiencing homelessness. J Ment Health. Published online 2024:1-16. doi:10.1080/09638237.2024.2426986
- Monteith LL, Holliday R, Christe’An DI, Sherrill A, Brenner LA, Hoffmire CA. Suicide risk and prevention in Guam: clinical and research considerations and a call to action. Asian J Psychiatry. 2023;83:103546. doi:10.1016/j.ajp.2023.103546
- Surís A, Holliday R, Hooshyar D, et al. Development and implementation of a homeless mobile medical/mental veteran intervention. Fed Pract. 2017;34(9):18.
Veterans experiencing homelessness are at an elevated risk for adverse health outcomes, including suicide. This population also experiences chronic health conditions (eg, cardiovascular disease and sexually transmitted infections) and psychiatric conditions (eg, substance use disorders and posttraumatic stress disorder) with a greater propensity than veterans without history of homelessness.1,2 Similarly, veterans experiencing homelessness often report concurrent stressors, such as justice involvement and unemployment, which further impact social functioning.3
The US Department of Veterans Affairs (VA) offers a range of health and social services to veterans experiencing homelessness. These programs are designed to respond to the multifactorial challenges faced by this population and are aimed at achieving sustained, permanent housing.4 To facilitate this effort, these programs provide targeted and tailored health (eg, primary care) and social (eg, case management and vocational rehabilitation) services to address barriers to housing stability (eg, substance use, serious mental illness, interacting with the criminal legal system, and unemployment).
Despite the availability of these programs, engaging veterans in VA services—whether in general or tailored for those experiencing or at risk for homelessness—remains challenging. Many veterans at risk for or experiencing homelessness overuse service settings that provide immediate care, such as urgent care or emergency departments (EDs).5,6 These individuals often visit an ED to augment or complement medical care they received in an outpatient setting, which can result in an elevated health care burden as well as impacted provision of treatment, especially surrounding care for chronic conditions (eg, cardiovascular health or serious mental illness).7-9
VA EDs offer urgent care and emergency services and often serve as a point of entry for veterans experiencing homelessness.10 They offer veterans expedient access to care that can address immediate needs (eg, substance use withdrawal, pain management, and suicide risk). EDs may be easier to access given they have longer hours of operation and patients can present without a scheduled appointment. VA EDs are an important point to identify homelessness and connect individuals to social service resources and outpatient health care referrals (eg, primary care and mental health).4,11
Some clinicians experience uncertainty in navigating or providing care for veterans experiencing or at risk for homelessness. A qualitative study conducted outside the VA found many clinicians did not know how to approach clinical conversations among unstably housed individuals, particularly when they discussed how to manage care for complex health conditions in the context of ongoing case management challenges, such as discharge planning.12 Another study found that clinicians working with individuals experiencing homelessness may have limited prior training or experience treating these patients.13 As a result, these clinicians may be unaware of available social services or unknowingly have biases that negatively impact care. Research remains limited surrounding beliefs about and methods of enhancing care among VA clinicians working with veterans experiencing homelessness in the ED.
This multiphase pilot study sought to understand service delivery processes and gaps in VA ED settings. Phase 1 examined ED clinician perceptions of care, facilitators, and barriers to providing care (including suicide risk assessments) and making postdischarge outpatient referrals among VA ED clinicians who regularly work with veterans experiencing homelessness. Phase 2 used this information to develop a clinical psychoeducational resource to enhance post-ED access to care for veterans experiencing or at risk for homelessness.
QUALITATIVE INTERVIEWS
Semistructured qualitative interviews were conducted with 11 VA ED clinicians from 6 Veteran Integrated Service Networks between August 2022 and February 2023. Clinicians were eligible if they currently worked within a VA ED setting (including urgent care) and indicated that some of their patients were veterans experiencing homelessness. All health care practitioners (HCPs) participated in an interview and a postinterview self-report survey that assessed demographic and job-related characteristics. Eight HCPs identified as female and 3 identified as male. All clinicians identified as White and 3 as Hispanic or Latino. Eight clinicians were licensed clinical social workers, 2 were ED nurses, and 1 was an ED physician.
After each clinician provided informed consent, they were invited to complete a telephone or Microsoft Teams interview. All interviews were recorded and subsequently transcribed. Interviews explored clinicians’ experiences caring for veterans experiencing homelessness, with a focus on services provided within the ED, as well as mandated ED screenings such as a suicide risk assessment. Interview questions also addressed postdischarge knowledge and experiences with referrals to VA health services (eg, primary care, mental health) and social services (eg, housing programs). Interviews lasted 30 to 90 minutes.
Recruitment ended after attaining sufficient thematic data, accomplished via an information power approach to sampling. This occurred when the study aims, sample characteristics, existing theory, and depth and quality of interviews dynamically informed the decision to cease recruitment of additional participants.14,15 Given the scope of study (examining service delivery and knowledge gaps), the specificity of the targeted sample (VA ED clinicians providing care to veterans experiencing homelessness), the level of pre-existing theoretical background informing the study aims, and depth and quality of interview dialogue, this information power approach provides justification for attaining small sample sizes. Following the interview, HCPs completed a demographic questionnaire. Participants were not compensated.
Data Analysis
Directed content analysis was used to analyze qualitative data, with the framework method employed as an analytic instrument to facilitate analysis.16-18 Analysts engaged in bracketing and discussed reflexivity before data analysis to reflect on personal subjectivities and reduce potential bias.19,20
A prototype coding framework was developed that enabled coders to meaningfully summarize and condense data within transcripts into varying domains, categories, or topics found within the interview guide. Domain examples included clinical backgrounds, suicide risk and assessment protocols among veterans experiencing homelessness, beliefs about service delivery for veterans experiencing homelessness, and barriers and facilitators that may impact their ability to provide post-ED discharge care. Coders discussed the findings and if there was a need to modify templates. All transcripts were double coded. Once complete, individual templates were merged into a unified Microsoft Excel sheet, which allowed for more discrete analyses, enabling analysts to examine trends across content areas within the dataset.
Clinical Resource Development
HCPs were queried regarding available outpatient resources for post-ED care (eg, printed discharge paperwork and best practice alerts or automated workflows within the electronic health record). Resources used by participants were examined, as well as which resources clinicians thought would help them care for veterans experiencing homelessness. Noted gaps were used to develop a tailored resource for clinicians who treat veterans experiencing homelessness in the ED. This resource was created with the intention it could inform all ED clinicians, with the option for personalization to align with the needs of local services, based on needed content areas identified (eg, emergency shelters and suicide prevention resources).
Resource development followed an information systems research (ISR) framework that used a 3-pronged process of identifying circumstances for how a tool is developed, the problems it aims to address, and the knowledge that informs its development, implementation, and evaluation.21,22 Initial wireframes of the resource were provided via email to 10 subject matter experts (SMEs) in veteran suicide prevention, emergency medicine, and homeless programs. SMEs were identified via professional listservs, VA program office leadership, literature searches of similar research, and snowball sampling. Solicited feedback on the resource from the SMEs included its design, language, tone, flow, format, and content (ideation and prototyping). The feedback was collated and used to revise the resource. SMEs then reviewed and provided feedback on the revised resource. This iterative cycle (prototype review, commentary, ideation, prototype review) continued until the SMEs offered no additional edits to the resource. In total, 7 iterations of the resource were developed, critiqued, and revised.
INTERVIEW RESULTS
Compassion Fatigue
Many participants expressed concerns about compassion fatigue among VA ED clinicians. Those interviewed indicated that treating veterans experiencing homelessness sometimes led to the development of what they described as a “callus,” a “sixth sense,” or an inherent sense of “suspicion” or distrust. These feelings resulted from concerns about an individual’s secondary gain or potential hidden agenda (eg, a veteran reporting suicidal ideation to attain shelter on a cold night), with clinicians not wanting to feel as if they were taken advantage of or deceived.
Many clinicians noted that compassion fatigue resulted from witnessing the same veterans experiencing homelessness routinely use emergency services for nonemergent or nonmedical needs. Some also expressed that over time this may result in them becoming less empathetic when caring for veterans experiencing homelessness. They hypothesized that clinicians may experience burnout, which could potentially result in a lack of curiosity and concern about a veteran’s risk for suicide or need for social services. Others may “take things for granted,” leading them to discount stressors that are “very real to the patient, this person.”
Clinicians indicated that such sentiments may impact overall care. Potential negative consequences included stigmatization of veterans experiencing homelessness, incomplete or partial suicide risk screenings with this population, inattentive or impersonal care, and expedited discharge from the ED without appropriate safety planning or social service referrals. Clinicians interviewed intended to find ways to combat compassion fatigue and maintain a commitment to provide comprehensive care to all veterans, including those experiencing homelessness. They felt conflict between a lack of empathy for individuals experiencing homelessness and becoming numb to the problem due to overexposure. However, these clinicians remained committed to providing care to these veterans and fighting to maintain the purpose of recovery-focused care.
Knowledge Gaps on Available Services
While many clinicians knew of general resources available to veterans experiencing homelessness, few had detailed information on where to seek consults for other homeless programs, who to contact regarding these services, when they were available, or how to refer to them. Many reported feeling uneasy when discharging veterans experiencing homelessness from care, often being unable to provide local, comprehensive referrals to support their needs and ensure their well-being. These sentiments were compounded when the veteran reported suicidal thoughts or recent suicidal behavior; clinicians felt concerned about the methods to engage these individuals into evidence-based mental health care within the context of unstable housing arrangements.
Some clinicians appeared to lack awareness of the wide array of VA homeless programming. Most could acknowledge at least some aspects of available programming (eg, the US Department of Housing and Urban Development– VA Supportive Housing program), while others were unaware of services tailored to the needs of those experiencing homelessness (eg, homeless patient aligned care teams), or of services targeting concurrent psychosocial stressors (eg, Veterans Justice Programs). Interviewees hypothesized this as being particularly notable among clinicians who are new to the VA or those who work in VA settings as part of their graduate or medical school training. Those aware of the services were uncertain of the referral process, relying on a single social worker or nurse to connect individuals experiencing homelessness to health and social services.
Interviewed clinicians noted that suicide risk screening of veterans experiencing homelessness was only performed by a limited number of individuals within the ED. Some did not feel sufficiently trained, comfortable, or knowledgeable about how to navigate care for veterans experiencing homelessness and at risk of suicide. Clinicians described “an uncomfortableness about suicidal ideation, where people just freeze up” and “don’t know what to do and don’t know what to say.”
Lack of Tangible Resources, Trainings, and Referrals
HCPs reported occasionally lacking the necessary clinical resources and information in the ED to properly support veterans experiencing homelessness and suicidal ideation. Common concerns included case management and discharge planning, as well as navigating health factors, such as elevated suicide risk. Some HCPs felt the local resources they do have access to—discharge packets or other forms of patient information—were not always tailored for the needs (eg, transportation) or abilities of veterans experiencing homelessness. One noted: “We give them a sheet of paper with some resources, which they don’t have the skills to follow up [with] anyway.”
Many interviewees wished for additional training in working with veterans experiencing homelessness. They reported that prior training from the VA Talent Management System or through unit-based programming could assist in educating clinicians on homeless services and suicide risk assessment. When queried on what training they had received, many noted there was “no formal training on what the VA offers homeless vets,” leading many to describe it as on-the-job training. This appeared especially among newer clinicians, who reported they were reliant upon learning from other, more senior staff within the ED.
The absence of training further illustrates the issue of institutional knowledge on these services and referrals, which was often confined to a single individual or team. Not having readily accessible resources, training, or information appropriate for all skill levels and positions within the ED hindered the ability of HCPs to connect veterans experiencing homelessness with social services to ensure their health and safety postdischarge: “If we had a better knowledge base of what the VA offers and the steps to go through in order to get the veteran set up for those things, it would be helpful.”
CLINICAL RESOURCE
A psychoeducational resource was developed for HCPs treating veterans experiencing homelessness (Figure). The resource was designed to mitigate compassion fatigue and recenter attention on the VA commitment to care while emphasizing the need to be responsive to the concerns of these individuals. Initial wireframes of the resource were developed by a small group of authors in review and appraisal of qualitative findings (EP, RH). These wireframes were developed to broadly illustrate the arrangement/structure of content, range of resources to potentially include (eg, available VA homeless programs or consultation resources), and to draft initial wording and phrasing. Subject matter expert feedback refined these wireframes, providing commentary on specific programs to include or exclude, changes and alterations to the design and flow of the resource, and edits to language, word choice, and tone over numerous iterations.

Given that many ED HCPs presented concerns surrounding secondary gain in the context of suicide risk, this resource focused on suicide risk. At the top of the resource, it states “Veterans at risk for homelessness experience more than double the risk for suicide than stably housed veterans.”23 Also at the top, the resource states: “For many, the last health care visit prior to suicide is often with VA emergency services."24 The goal of these statements was to educate users on the elevated risk for suicide in veterans experiencing homelessness and their role in preventing such deaths.
Text in this section emphasizes that every veteran deserves the best care possible and recenters HCP attention on providing quality, comprehensive care regardless of housing status. The inclusion of this material was prioritized given the concerns expressed regarding compassion fatigue and suspicions of secondary gain (eg, a veteran reporting suicidal ideation to attain shelter or respite from outside conditions).
The resource also attempts to address high rates of emergency service by veterans experiencing homelessness: “Due to challenges with accessing care, Veterans experiencing homelessness may use emergency or urgent care services more frequently than other Veterans.”25 The resource also indicates that VA resources are available to help homeless and at-risk veterans to acquire stable housing, employment, and engage in healthcare, which are outlined with specific contact information. Given the breadth of local and VA services, a portion of the resource is dedicated to local health and social services available for veterans experiencing homelessness. HCPs complete the first page, which is devoted to local homeless service and program resources.
Following SME consultation, the list of programs provided underwent a series of iterations. The program types listed are deemed to be of greatest benefit to veterans experiencing homelessness and most consulted by HCPs. Including VA and non-VA emergency shelters allows clinicians flexible options if a particular shelter is full, closed, or would not meet the veteran’s needs or preference (eg, lack of childcare or does not allow pets). The second column of this section is left intentionally blank; here, the HCP is to list a local point-of- contact at each program. This encourages clinical teams to seek out and make direct contact with these programs and establish (in)formal relationships with them. The HCP then completes the third column with contact information.
Once completed, the resource acts as a living document. Clinicians and SMEs consulted for this study expressed the desire to have an easily accessible resource that can be updated based on necessary changes (eg, emergency shelter address or hours of operation). The resource can be housed within each local VA emergency or urgent care service setting alongside other available clinical tools.
While local resources are the primary focus, interviewees also suggested that some HCPs are not aware of the available VA services . This material, found on the back of the resource, provides a general overview of services available through VA homeless programs. SME consultation and discussion led to selecting the 5 listed categories: housing services, health care services, case management, employment services, and justice-related programming, each with a brief description.
Information for the National Call Center for Homeless Veterans, community service hotline, and Veterans Crisis Line are included on the front page. These hotlines and phone numbers are always available for veterans experiencing homelessness, enabling them to make these connections themselves, if desired. Additionally, given the challenges noted by some HCPs in performing suicide risk screening, evaluation, and intervention, a prompt for the VA Suicide Risk Management Consultation service was also included on the back page.
Creating a Shared and Local Resource
This clinical resource was developed to establish a centralized, shared, local resource available to VA ED HCPs who lacked knowledge of available services or reported discomfort conducting suicide risk screening for veterans experiencing homelessness. In many cases, ED referrals to homeless programs and suicide prevention care was assigned to a single individual, often a nurse or social worker. As a result, an undue amount of work and strain was placed on these individuals, as this forced them to act as the sole bridge between care in the ED and postdischarge social (eg, homeless programs) and mental health (eg, suicide prevention) services. The creation of a unified, easily accessible document aimed to distribute this responsibility more equitably across ED staff.
DISCUSSION
This project intended to develop a clinician resource to support VA ED clinicians caring for veterans experiencing homelessness and their access to services postdischarge. Qualitative interviews provided insights into the burnout and compassion fatigue present in these settings, as well as the challenges and needs regarding knowledge of local and VA services. Emphasis was placed on leveraging extant resources and subject matter expertise to develop a resource capable of providing brief and informative guidance.
This resource is particularly relevant for HCPs new to the VA, including trainees and new hires, who may be less aware of VA and local social services. It has the potential to reduce the burden on VA ED staff to provide guidance and recommendations surrounding postdischarge social services. The resource acknowledges homeless programming focused on social determinants of health that can destabilize housing (eg, legal or occupational challenges). This can incentivize clinicians to discuss these programs with veterans to facilitate their ability to navigate complex health and psychosocial challenges.
HCPs interviewed for this study indicated their apprehension regarding suicide risk screening and evaluation, a process currently mandated within VA ED settings.26 This may be compounded among HCPs with minimal mental health training or those who have worked in community-based settings where such screening and evaluation efforts are not required. The resource reminds clinicians of available VA consultation services, which can provide additional training, clinical guidance, and review of existing local ED processes.
While the resource was directly informed by qualitative interviews conducted with VA emergency service HCPs and developed through an iterative process with SMEs, further research is necessary to determine its effectiveness at increasing access to health and social services among veterans experiencing homelessness. The resource has not been used by HCPs working in these settings to examine uptake or sustained use, nor clinicians’ perceptions of its utility, including acceptability and feasibility; these are important next steps to understand if the resource is functioning as intended.
Compassion fatigue, as well as associated sequelae (eg, burnout, distress, and psychiatric symptoms), is well-documented among individuals working with individuals experiencing homelessness, including VA HCPs.27-30 Such experiences are likely driven by several factors, including the clinical complexity and service needs of this veteran population. Although compassion fatigue was noted by many clinicians interviewed for this study, it is unclear if the resource alone would address factors driving compassion fatigue, or if additional programming or services may be necessary.
Limitations
The resource requires local HCPs to routinely update its content (eg, establishment of a new emergency shelter in the community or change in hours or contact information of an existing one), which may be challenging. This is especially true as it relates to community resources, which may be more likely to change than national VA programming.
This resource was initially developed following qualitative interviews with a small sample of VA HCPs (explicitly those working within ED settings) and may not be representative of all HCPs engaged in VA care with veterans experiencing homelessness. The perspectives and experiences of those interviewed do not represent the views of all VA ED HCPs and may differ from the perspectives of those in regions with unique cultural and regional considerations.31
Given that most of the interviewees were social workers in EDs engaged in care for veterans experiencing homelessness, these findings and informational needs may differ among other types of HCPs who provide services for veterans experiencing homelessness in other settings. Content in the resource was included based on clinician input, and may not reflect the perspectives of veterans, who may perceive some resources as more important (eg, access to primary care or dental services).28
CONCLUSIONS
This project represents the culmination of qualitative interviews and SME input to develop a free-to-use clinician resource to facilitate service delivery and connection to services following discharge from VA EDs for veterans experiencing homelessness. Serving as a template, this resource can be customized to increase knowledge of local VA and community resources to support these individuals. Continued refinement and piloting of this resource to evaluate acceptability, implementation barriers, and use remains warranted.
Veterans experiencing homelessness are at an elevated risk for adverse health outcomes, including suicide. This population also experiences chronic health conditions (eg, cardiovascular disease and sexually transmitted infections) and psychiatric conditions (eg, substance use disorders and posttraumatic stress disorder) with a greater propensity than veterans without history of homelessness.1,2 Similarly, veterans experiencing homelessness often report concurrent stressors, such as justice involvement and unemployment, which further impact social functioning.3
The US Department of Veterans Affairs (VA) offers a range of health and social services to veterans experiencing homelessness. These programs are designed to respond to the multifactorial challenges faced by this population and are aimed at achieving sustained, permanent housing.4 To facilitate this effort, these programs provide targeted and tailored health (eg, primary care) and social (eg, case management and vocational rehabilitation) services to address barriers to housing stability (eg, substance use, serious mental illness, interacting with the criminal legal system, and unemployment).
Despite the availability of these programs, engaging veterans in VA services—whether in general or tailored for those experiencing or at risk for homelessness—remains challenging. Many veterans at risk for or experiencing homelessness overuse service settings that provide immediate care, such as urgent care or emergency departments (EDs).5,6 These individuals often visit an ED to augment or complement medical care they received in an outpatient setting, which can result in an elevated health care burden as well as impacted provision of treatment, especially surrounding care for chronic conditions (eg, cardiovascular health or serious mental illness).7-9
VA EDs offer urgent care and emergency services and often serve as a point of entry for veterans experiencing homelessness.10 They offer veterans expedient access to care that can address immediate needs (eg, substance use withdrawal, pain management, and suicide risk). EDs may be easier to access given they have longer hours of operation and patients can present without a scheduled appointment. VA EDs are an important point to identify homelessness and connect individuals to social service resources and outpatient health care referrals (eg, primary care and mental health).4,11
Some clinicians experience uncertainty in navigating or providing care for veterans experiencing or at risk for homelessness. A qualitative study conducted outside the VA found many clinicians did not know how to approach clinical conversations among unstably housed individuals, particularly when they discussed how to manage care for complex health conditions in the context of ongoing case management challenges, such as discharge planning.12 Another study found that clinicians working with individuals experiencing homelessness may have limited prior training or experience treating these patients.13 As a result, these clinicians may be unaware of available social services or unknowingly have biases that negatively impact care. Research remains limited surrounding beliefs about and methods of enhancing care among VA clinicians working with veterans experiencing homelessness in the ED.
This multiphase pilot study sought to understand service delivery processes and gaps in VA ED settings. Phase 1 examined ED clinician perceptions of care, facilitators, and barriers to providing care (including suicide risk assessments) and making postdischarge outpatient referrals among VA ED clinicians who regularly work with veterans experiencing homelessness. Phase 2 used this information to develop a clinical psychoeducational resource to enhance post-ED access to care for veterans experiencing or at risk for homelessness.
QUALITATIVE INTERVIEWS
Semistructured qualitative interviews were conducted with 11 VA ED clinicians from 6 Veteran Integrated Service Networks between August 2022 and February 2023. Clinicians were eligible if they currently worked within a VA ED setting (including urgent care) and indicated that some of their patients were veterans experiencing homelessness. All health care practitioners (HCPs) participated in an interview and a postinterview self-report survey that assessed demographic and job-related characteristics. Eight HCPs identified as female and 3 identified as male. All clinicians identified as White and 3 as Hispanic or Latino. Eight clinicians were licensed clinical social workers, 2 were ED nurses, and 1 was an ED physician.
After each clinician provided informed consent, they were invited to complete a telephone or Microsoft Teams interview. All interviews were recorded and subsequently transcribed. Interviews explored clinicians’ experiences caring for veterans experiencing homelessness, with a focus on services provided within the ED, as well as mandated ED screenings such as a suicide risk assessment. Interview questions also addressed postdischarge knowledge and experiences with referrals to VA health services (eg, primary care, mental health) and social services (eg, housing programs). Interviews lasted 30 to 90 minutes.
Recruitment ended after attaining sufficient thematic data, accomplished via an information power approach to sampling. This occurred when the study aims, sample characteristics, existing theory, and depth and quality of interviews dynamically informed the decision to cease recruitment of additional participants.14,15 Given the scope of study (examining service delivery and knowledge gaps), the specificity of the targeted sample (VA ED clinicians providing care to veterans experiencing homelessness), the level of pre-existing theoretical background informing the study aims, and depth and quality of interview dialogue, this information power approach provides justification for attaining small sample sizes. Following the interview, HCPs completed a demographic questionnaire. Participants were not compensated.
Data Analysis
Directed content analysis was used to analyze qualitative data, with the framework method employed as an analytic instrument to facilitate analysis.16-18 Analysts engaged in bracketing and discussed reflexivity before data analysis to reflect on personal subjectivities and reduce potential bias.19,20
A prototype coding framework was developed that enabled coders to meaningfully summarize and condense data within transcripts into varying domains, categories, or topics found within the interview guide. Domain examples included clinical backgrounds, suicide risk and assessment protocols among veterans experiencing homelessness, beliefs about service delivery for veterans experiencing homelessness, and barriers and facilitators that may impact their ability to provide post-ED discharge care. Coders discussed the findings and if there was a need to modify templates. All transcripts were double coded. Once complete, individual templates were merged into a unified Microsoft Excel sheet, which allowed for more discrete analyses, enabling analysts to examine trends across content areas within the dataset.
Clinical Resource Development
HCPs were queried regarding available outpatient resources for post-ED care (eg, printed discharge paperwork and best practice alerts or automated workflows within the electronic health record). Resources used by participants were examined, as well as which resources clinicians thought would help them care for veterans experiencing homelessness. Noted gaps were used to develop a tailored resource for clinicians who treat veterans experiencing homelessness in the ED. This resource was created with the intention it could inform all ED clinicians, with the option for personalization to align with the needs of local services, based on needed content areas identified (eg, emergency shelters and suicide prevention resources).
Resource development followed an information systems research (ISR) framework that used a 3-pronged process of identifying circumstances for how a tool is developed, the problems it aims to address, and the knowledge that informs its development, implementation, and evaluation.21,22 Initial wireframes of the resource were provided via email to 10 subject matter experts (SMEs) in veteran suicide prevention, emergency medicine, and homeless programs. SMEs were identified via professional listservs, VA program office leadership, literature searches of similar research, and snowball sampling. Solicited feedback on the resource from the SMEs included its design, language, tone, flow, format, and content (ideation and prototyping). The feedback was collated and used to revise the resource. SMEs then reviewed and provided feedback on the revised resource. This iterative cycle (prototype review, commentary, ideation, prototype review) continued until the SMEs offered no additional edits to the resource. In total, 7 iterations of the resource were developed, critiqued, and revised.
INTERVIEW RESULTS
Compassion Fatigue
Many participants expressed concerns about compassion fatigue among VA ED clinicians. Those interviewed indicated that treating veterans experiencing homelessness sometimes led to the development of what they described as a “callus,” a “sixth sense,” or an inherent sense of “suspicion” or distrust. These feelings resulted from concerns about an individual’s secondary gain or potential hidden agenda (eg, a veteran reporting suicidal ideation to attain shelter on a cold night), with clinicians not wanting to feel as if they were taken advantage of or deceived.
Many clinicians noted that compassion fatigue resulted from witnessing the same veterans experiencing homelessness routinely use emergency services for nonemergent or nonmedical needs. Some also expressed that over time this may result in them becoming less empathetic when caring for veterans experiencing homelessness. They hypothesized that clinicians may experience burnout, which could potentially result in a lack of curiosity and concern about a veteran’s risk for suicide or need for social services. Others may “take things for granted,” leading them to discount stressors that are “very real to the patient, this person.”
Clinicians indicated that such sentiments may impact overall care. Potential negative consequences included stigmatization of veterans experiencing homelessness, incomplete or partial suicide risk screenings with this population, inattentive or impersonal care, and expedited discharge from the ED without appropriate safety planning or social service referrals. Clinicians interviewed intended to find ways to combat compassion fatigue and maintain a commitment to provide comprehensive care to all veterans, including those experiencing homelessness. They felt conflict between a lack of empathy for individuals experiencing homelessness and becoming numb to the problem due to overexposure. However, these clinicians remained committed to providing care to these veterans and fighting to maintain the purpose of recovery-focused care.
Knowledge Gaps on Available Services
While many clinicians knew of general resources available to veterans experiencing homelessness, few had detailed information on where to seek consults for other homeless programs, who to contact regarding these services, when they were available, or how to refer to them. Many reported feeling uneasy when discharging veterans experiencing homelessness from care, often being unable to provide local, comprehensive referrals to support their needs and ensure their well-being. These sentiments were compounded when the veteran reported suicidal thoughts or recent suicidal behavior; clinicians felt concerned about the methods to engage these individuals into evidence-based mental health care within the context of unstable housing arrangements.
Some clinicians appeared to lack awareness of the wide array of VA homeless programming. Most could acknowledge at least some aspects of available programming (eg, the US Department of Housing and Urban Development– VA Supportive Housing program), while others were unaware of services tailored to the needs of those experiencing homelessness (eg, homeless patient aligned care teams), or of services targeting concurrent psychosocial stressors (eg, Veterans Justice Programs). Interviewees hypothesized this as being particularly notable among clinicians who are new to the VA or those who work in VA settings as part of their graduate or medical school training. Those aware of the services were uncertain of the referral process, relying on a single social worker or nurse to connect individuals experiencing homelessness to health and social services.
Interviewed clinicians noted that suicide risk screening of veterans experiencing homelessness was only performed by a limited number of individuals within the ED. Some did not feel sufficiently trained, comfortable, or knowledgeable about how to navigate care for veterans experiencing homelessness and at risk of suicide. Clinicians described “an uncomfortableness about suicidal ideation, where people just freeze up” and “don’t know what to do and don’t know what to say.”
Lack of Tangible Resources, Trainings, and Referrals
HCPs reported occasionally lacking the necessary clinical resources and information in the ED to properly support veterans experiencing homelessness and suicidal ideation. Common concerns included case management and discharge planning, as well as navigating health factors, such as elevated suicide risk. Some HCPs felt the local resources they do have access to—discharge packets or other forms of patient information—were not always tailored for the needs (eg, transportation) or abilities of veterans experiencing homelessness. One noted: “We give them a sheet of paper with some resources, which they don’t have the skills to follow up [with] anyway.”
Many interviewees wished for additional training in working with veterans experiencing homelessness. They reported that prior training from the VA Talent Management System or through unit-based programming could assist in educating clinicians on homeless services and suicide risk assessment. When queried on what training they had received, many noted there was “no formal training on what the VA offers homeless vets,” leading many to describe it as on-the-job training. This appeared especially among newer clinicians, who reported they were reliant upon learning from other, more senior staff within the ED.
The absence of training further illustrates the issue of institutional knowledge on these services and referrals, which was often confined to a single individual or team. Not having readily accessible resources, training, or information appropriate for all skill levels and positions within the ED hindered the ability of HCPs to connect veterans experiencing homelessness with social services to ensure their health and safety postdischarge: “If we had a better knowledge base of what the VA offers and the steps to go through in order to get the veteran set up for those things, it would be helpful.”
CLINICAL RESOURCE
A psychoeducational resource was developed for HCPs treating veterans experiencing homelessness (Figure). The resource was designed to mitigate compassion fatigue and recenter attention on the VA commitment to care while emphasizing the need to be responsive to the concerns of these individuals. Initial wireframes of the resource were developed by a small group of authors in review and appraisal of qualitative findings (EP, RH). These wireframes were developed to broadly illustrate the arrangement/structure of content, range of resources to potentially include (eg, available VA homeless programs or consultation resources), and to draft initial wording and phrasing. Subject matter expert feedback refined these wireframes, providing commentary on specific programs to include or exclude, changes and alterations to the design and flow of the resource, and edits to language, word choice, and tone over numerous iterations.

Given that many ED HCPs presented concerns surrounding secondary gain in the context of suicide risk, this resource focused on suicide risk. At the top of the resource, it states “Veterans at risk for homelessness experience more than double the risk for suicide than stably housed veterans.”23 Also at the top, the resource states: “For many, the last health care visit prior to suicide is often with VA emergency services."24 The goal of these statements was to educate users on the elevated risk for suicide in veterans experiencing homelessness and their role in preventing such deaths.
Text in this section emphasizes that every veteran deserves the best care possible and recenters HCP attention on providing quality, comprehensive care regardless of housing status. The inclusion of this material was prioritized given the concerns expressed regarding compassion fatigue and suspicions of secondary gain (eg, a veteran reporting suicidal ideation to attain shelter or respite from outside conditions).
The resource also attempts to address high rates of emergency service by veterans experiencing homelessness: “Due to challenges with accessing care, Veterans experiencing homelessness may use emergency or urgent care services more frequently than other Veterans.”25 The resource also indicates that VA resources are available to help homeless and at-risk veterans to acquire stable housing, employment, and engage in healthcare, which are outlined with specific contact information. Given the breadth of local and VA services, a portion of the resource is dedicated to local health and social services available for veterans experiencing homelessness. HCPs complete the first page, which is devoted to local homeless service and program resources.
Following SME consultation, the list of programs provided underwent a series of iterations. The program types listed are deemed to be of greatest benefit to veterans experiencing homelessness and most consulted by HCPs. Including VA and non-VA emergency shelters allows clinicians flexible options if a particular shelter is full, closed, or would not meet the veteran’s needs or preference (eg, lack of childcare or does not allow pets). The second column of this section is left intentionally blank; here, the HCP is to list a local point-of- contact at each program. This encourages clinical teams to seek out and make direct contact with these programs and establish (in)formal relationships with them. The HCP then completes the third column with contact information.
Once completed, the resource acts as a living document. Clinicians and SMEs consulted for this study expressed the desire to have an easily accessible resource that can be updated based on necessary changes (eg, emergency shelter address or hours of operation). The resource can be housed within each local VA emergency or urgent care service setting alongside other available clinical tools.
While local resources are the primary focus, interviewees also suggested that some HCPs are not aware of the available VA services . This material, found on the back of the resource, provides a general overview of services available through VA homeless programs. SME consultation and discussion led to selecting the 5 listed categories: housing services, health care services, case management, employment services, and justice-related programming, each with a brief description.
Information for the National Call Center for Homeless Veterans, community service hotline, and Veterans Crisis Line are included on the front page. These hotlines and phone numbers are always available for veterans experiencing homelessness, enabling them to make these connections themselves, if desired. Additionally, given the challenges noted by some HCPs in performing suicide risk screening, evaluation, and intervention, a prompt for the VA Suicide Risk Management Consultation service was also included on the back page.
Creating a Shared and Local Resource
This clinical resource was developed to establish a centralized, shared, local resource available to VA ED HCPs who lacked knowledge of available services or reported discomfort conducting suicide risk screening for veterans experiencing homelessness. In many cases, ED referrals to homeless programs and suicide prevention care was assigned to a single individual, often a nurse or social worker. As a result, an undue amount of work and strain was placed on these individuals, as this forced them to act as the sole bridge between care in the ED and postdischarge social (eg, homeless programs) and mental health (eg, suicide prevention) services. The creation of a unified, easily accessible document aimed to distribute this responsibility more equitably across ED staff.
DISCUSSION
This project intended to develop a clinician resource to support VA ED clinicians caring for veterans experiencing homelessness and their access to services postdischarge. Qualitative interviews provided insights into the burnout and compassion fatigue present in these settings, as well as the challenges and needs regarding knowledge of local and VA services. Emphasis was placed on leveraging extant resources and subject matter expertise to develop a resource capable of providing brief and informative guidance.
This resource is particularly relevant for HCPs new to the VA, including trainees and new hires, who may be less aware of VA and local social services. It has the potential to reduce the burden on VA ED staff to provide guidance and recommendations surrounding postdischarge social services. The resource acknowledges homeless programming focused on social determinants of health that can destabilize housing (eg, legal or occupational challenges). This can incentivize clinicians to discuss these programs with veterans to facilitate their ability to navigate complex health and psychosocial challenges.
HCPs interviewed for this study indicated their apprehension regarding suicide risk screening and evaluation, a process currently mandated within VA ED settings.26 This may be compounded among HCPs with minimal mental health training or those who have worked in community-based settings where such screening and evaluation efforts are not required. The resource reminds clinicians of available VA consultation services, which can provide additional training, clinical guidance, and review of existing local ED processes.
While the resource was directly informed by qualitative interviews conducted with VA emergency service HCPs and developed through an iterative process with SMEs, further research is necessary to determine its effectiveness at increasing access to health and social services among veterans experiencing homelessness. The resource has not been used by HCPs working in these settings to examine uptake or sustained use, nor clinicians’ perceptions of its utility, including acceptability and feasibility; these are important next steps to understand if the resource is functioning as intended.
Compassion fatigue, as well as associated sequelae (eg, burnout, distress, and psychiatric symptoms), is well-documented among individuals working with individuals experiencing homelessness, including VA HCPs.27-30 Such experiences are likely driven by several factors, including the clinical complexity and service needs of this veteran population. Although compassion fatigue was noted by many clinicians interviewed for this study, it is unclear if the resource alone would address factors driving compassion fatigue, or if additional programming or services may be necessary.
Limitations
The resource requires local HCPs to routinely update its content (eg, establishment of a new emergency shelter in the community or change in hours or contact information of an existing one), which may be challenging. This is especially true as it relates to community resources, which may be more likely to change than national VA programming.
This resource was initially developed following qualitative interviews with a small sample of VA HCPs (explicitly those working within ED settings) and may not be representative of all HCPs engaged in VA care with veterans experiencing homelessness. The perspectives and experiences of those interviewed do not represent the views of all VA ED HCPs and may differ from the perspectives of those in regions with unique cultural and regional considerations.31
Given that most of the interviewees were social workers in EDs engaged in care for veterans experiencing homelessness, these findings and informational needs may differ among other types of HCPs who provide services for veterans experiencing homelessness in other settings. Content in the resource was included based on clinician input, and may not reflect the perspectives of veterans, who may perceive some resources as more important (eg, access to primary care or dental services).28
CONCLUSIONS
This project represents the culmination of qualitative interviews and SME input to develop a free-to-use clinician resource to facilitate service delivery and connection to services following discharge from VA EDs for veterans experiencing homelessness. Serving as a template, this resource can be customized to increase knowledge of local VA and community resources to support these individuals. Continued refinement and piloting of this resource to evaluate acceptability, implementation barriers, and use remains warranted.
- Holliday R, Kinney AR, Smith AA, et al. A latent class analysis to identify subgroups of VHA using homeless veterans at greater risk for suicide mortality. J Affect Disord. 2022;315:162-167. doi:10.1016/j.jad.2022.07.062
- Weber J, Lee RC, Martsolf D. Understanding the health of veterans who are homeless: a review of the literature. Public Health Nurs. 2017;34(5):505-511. doi:10.1111/phn.12338
- Holliday R, Desai A, Stimmel M, Liu S, Monteith LL, Stewart KE. Meeting the health and social service needs of veterans who interact with the criminal justice system and experience homelessness: a holistic conceptualization and recommendations for tailoring care. Curr Treat Options Psychiatry. 2022;9(3):174-185. doi:10.1007/s40501-022-00275-1
- Holliday R, Desai A, Gerard G, Liu S, Stimmel M. Understanding the intersection of homelessness and justice involvement: enhancing veteran suicide prevention through VA programming. Fed Pract. 2022;39(1):8-11. doi:10.12788/fp.0216
- Kushel MB, Perry S, Bangsberg D, Clark R, Moss AR. Emergency department use among the homeless and marginally housed: results from a community-based study. Am J Public Health. 2002;92(5):778-784. doi:10.2105/ajph.92.5.778
- Tsai J, Doran KM, Rosenheck RA. When health insurance is not a factor: national comparison of homeless and nonhomeless US veterans who use Veterans Affairs emergency departments. Am J Public Health. 2013;103(Suppl 2):S225-S231. doi:10.2105/AJPH.2013.301307
- Doran KM, Raven MC, Rosenheck RA. What drives frequent emergency department use in an integrated health system? National data from the Veterans Health Administration. Ann Emerg Med. 2013;62(2):151-159. doi:10.1016/j.annemergmed.2013.02.016
- Tsai J, Rosenheck RA. Risk factors for ED use among homeless veterans. Am J Emerg Med. 2013;31(5):855-858. doi:10.1016/j.ajem.2013.02.046
- Nelson RE, Suo Y, Pettey W, et al. Costs associated with health care services accessed through VA and in the community through Medicare for veterans experiencing homelessness. Health Serv Res. 2018;53(Suppl 3):5352-5374. doi:10.1111/1475-6773.13054
- Gabrielian S, Yuan AH, Andersen RM, Rubenstein LV, Gelberg L. VA health service utilization for homeless and low-income veterans: a spotlight on the VA Supportive Housing (VASH) program in greater Los Angeles. Med Care. 2014;52(5):454-461. doi:10.1097/MLR.0000000000000112
- Larkin GL, Beautrais AL. Emergency departments are underutilized sites for suicide prevention. Crisis. 2010;31(1):1- 6. doi:10.1027/0227-5910/a000001
- Decker H, Raguram M, Kanzaria HK, Duke M, Wick E. Provider perceptions of challenges and facilitators to surgical care in unhoused patients: a qualitative analysis. Surgery. 2024;175(4):1095-1102. doi:10.1016/j.surg.2023.11.009
- Panushka KA, Kozlowski Z, Dalessandro C, Sanders JN, Millar MM, Gawron LM. “It’s not a top priority”: a qualitative analysis of provider views on barriers to reproductive healthcare provision for homeless women in the United States. Soc Work Public Health. 2023;38(5 -8):428-436. doi:10.1080/19371918.2024.2315180
- Saunders B, Sim J, Kingstone T, et al. Saturation in qualitative research: exploring its conceptualization and operationalization. Qual Quant. 2018;52:1893-1907. doi:10.1007/s11135-017-0574-8
- Malterud K, Siersma VD, Guassora AD. Sample size in qualitative interview studies: guided by information power. Qual Health Res. 2016;26(13):1753-1760. doi:10.1177/1049732315617444
- Assarroudi A, Heshmati Nabavi F, Armat MR, Ebadi A, Vaismoradi M. Directed qualitative content analysis: the description and elaboration of its underpinning methods and data analysis process. J Res Nurs. 2018;23(1):42-55. doi:10.1177/1744987117741667
- Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277-1288.
- Goldsmith LJ. Using Framework Analysis in Applied Qualitative Research. Qual Rep. 2021;26(6):2061-2076. doi:10.46743/2160-3715/2021.5011
- Tufford L, Newman P. Bracketing in qualitative research. Qual Soc Work. 2012;11(1):80-96.
- Dodgson JE. Reflexivity in Qualitative Research. J Hum Lact. 2019;35(2):220-222. doi:10.1177/0890334419830990
- Hevner AR. A three cycle view of design science research. Scand J Inf Syst. 2007;19(2):4.
- Farao J, Malila B, Conrad N, Mutsvangwa T, Rangaka MX, Douglas TS. A user-centred design frame work for mHealth. PLOS ONE. 2020;15(8):e0237910. doi:10.1371/journal.pone.0237910
- Hoffberg AS, Spitzer E, Mackelprang JL, Farro SA, Brenner LA. Suicidal Self-Directed Violence Among Homeless US Veterans: A Systematic Review. Suicide Life Threat Behav. 2018;48(4):481-498. doi:10.1111/sltb.12369
- Larkin GL, Beautrais AL. Emergency departments are underutilized sites for suicide prevention. Crisis. 2010;31(1):1- 6. doi:10.1027/0227-5910/a000001
- Gabrielian S, Yuan AH, Andersen RM, Rubenstein LV, Gelberg L. VA health service utilization for homeless and lowincome Veterans: a spotlight on the VA Supportive Housing (VASH) program in greater Los Angeles. Med Care. 2014;52(5):454-461. doi:10.1097/MLR.0000000000000112
- Holliday R, Hostetter T, Brenner LA, Bahraini N, Tsai J. Suicide risk screening and evaluation among patients accessing VHA services and identified as being newly homeless. Health Serv Res. 2024;59(5):e14301. doi:10.1111/1475-6773.14301
- Waegemakers Schiff J, Lane AM. PTSD symptoms, vicarious traumatization, and burnout in front line workers in the homeless sector. Community Ment Health J. 2019;55(3):454-462. doi:10.1007/s10597-018-00364-7
- Steenekamp BL, Barker SL. Exploring the experiences of compassion fatigue amongst peer support workers in homelessness services. Community Ment Health J. 2024;60(4):772-783. doi:10.1007/s10597-024-01234-1
- Perez S, Kerman N, Dej E, et al. When I can’t help, I suffer: a scoping review of moral distress in service providers working with persons experiencing homelessness. J Ment Health. Published online 2024:1-16. doi:10.1080/09638237.2024.2426986
- Monteith LL, Holliday R, Christe’An DI, Sherrill A, Brenner LA, Hoffmire CA. Suicide risk and prevention in Guam: clinical and research considerations and a call to action. Asian J Psychiatry. 2023;83:103546. doi:10.1016/j.ajp.2023.103546
- Surís A, Holliday R, Hooshyar D, et al. Development and implementation of a homeless mobile medical/mental veteran intervention. Fed Pract. 2017;34(9):18.
- Holliday R, Kinney AR, Smith AA, et al. A latent class analysis to identify subgroups of VHA using homeless veterans at greater risk for suicide mortality. J Affect Disord. 2022;315:162-167. doi:10.1016/j.jad.2022.07.062
- Weber J, Lee RC, Martsolf D. Understanding the health of veterans who are homeless: a review of the literature. Public Health Nurs. 2017;34(5):505-511. doi:10.1111/phn.12338
- Holliday R, Desai A, Stimmel M, Liu S, Monteith LL, Stewart KE. Meeting the health and social service needs of veterans who interact with the criminal justice system and experience homelessness: a holistic conceptualization and recommendations for tailoring care. Curr Treat Options Psychiatry. 2022;9(3):174-185. doi:10.1007/s40501-022-00275-1
- Holliday R, Desai A, Gerard G, Liu S, Stimmel M. Understanding the intersection of homelessness and justice involvement: enhancing veteran suicide prevention through VA programming. Fed Pract. 2022;39(1):8-11. doi:10.12788/fp.0216
- Kushel MB, Perry S, Bangsberg D, Clark R, Moss AR. Emergency department use among the homeless and marginally housed: results from a community-based study. Am J Public Health. 2002;92(5):778-784. doi:10.2105/ajph.92.5.778
- Tsai J, Doran KM, Rosenheck RA. When health insurance is not a factor: national comparison of homeless and nonhomeless US veterans who use Veterans Affairs emergency departments. Am J Public Health. 2013;103(Suppl 2):S225-S231. doi:10.2105/AJPH.2013.301307
- Doran KM, Raven MC, Rosenheck RA. What drives frequent emergency department use in an integrated health system? National data from the Veterans Health Administration. Ann Emerg Med. 2013;62(2):151-159. doi:10.1016/j.annemergmed.2013.02.016
- Tsai J, Rosenheck RA. Risk factors for ED use among homeless veterans. Am J Emerg Med. 2013;31(5):855-858. doi:10.1016/j.ajem.2013.02.046
- Nelson RE, Suo Y, Pettey W, et al. Costs associated with health care services accessed through VA and in the community through Medicare for veterans experiencing homelessness. Health Serv Res. 2018;53(Suppl 3):5352-5374. doi:10.1111/1475-6773.13054
- Gabrielian S, Yuan AH, Andersen RM, Rubenstein LV, Gelberg L. VA health service utilization for homeless and low-income veterans: a spotlight on the VA Supportive Housing (VASH) program in greater Los Angeles. Med Care. 2014;52(5):454-461. doi:10.1097/MLR.0000000000000112
- Larkin GL, Beautrais AL. Emergency departments are underutilized sites for suicide prevention. Crisis. 2010;31(1):1- 6. doi:10.1027/0227-5910/a000001
- Decker H, Raguram M, Kanzaria HK, Duke M, Wick E. Provider perceptions of challenges and facilitators to surgical care in unhoused patients: a qualitative analysis. Surgery. 2024;175(4):1095-1102. doi:10.1016/j.surg.2023.11.009
- Panushka KA, Kozlowski Z, Dalessandro C, Sanders JN, Millar MM, Gawron LM. “It’s not a top priority”: a qualitative analysis of provider views on barriers to reproductive healthcare provision for homeless women in the United States. Soc Work Public Health. 2023;38(5 -8):428-436. doi:10.1080/19371918.2024.2315180
- Saunders B, Sim J, Kingstone T, et al. Saturation in qualitative research: exploring its conceptualization and operationalization. Qual Quant. 2018;52:1893-1907. doi:10.1007/s11135-017-0574-8
- Malterud K, Siersma VD, Guassora AD. Sample size in qualitative interview studies: guided by information power. Qual Health Res. 2016;26(13):1753-1760. doi:10.1177/1049732315617444
- Assarroudi A, Heshmati Nabavi F, Armat MR, Ebadi A, Vaismoradi M. Directed qualitative content analysis: the description and elaboration of its underpinning methods and data analysis process. J Res Nurs. 2018;23(1):42-55. doi:10.1177/1744987117741667
- Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277-1288.
- Goldsmith LJ. Using Framework Analysis in Applied Qualitative Research. Qual Rep. 2021;26(6):2061-2076. doi:10.46743/2160-3715/2021.5011
- Tufford L, Newman P. Bracketing in qualitative research. Qual Soc Work. 2012;11(1):80-96.
- Dodgson JE. Reflexivity in Qualitative Research. J Hum Lact. 2019;35(2):220-222. doi:10.1177/0890334419830990
- Hevner AR. A three cycle view of design science research. Scand J Inf Syst. 2007;19(2):4.
- Farao J, Malila B, Conrad N, Mutsvangwa T, Rangaka MX, Douglas TS. A user-centred design frame work for mHealth. PLOS ONE. 2020;15(8):e0237910. doi:10.1371/journal.pone.0237910
- Hoffberg AS, Spitzer E, Mackelprang JL, Farro SA, Brenner LA. Suicidal Self-Directed Violence Among Homeless US Veterans: A Systematic Review. Suicide Life Threat Behav. 2018;48(4):481-498. doi:10.1111/sltb.12369
- Larkin GL, Beautrais AL. Emergency departments are underutilized sites for suicide prevention. Crisis. 2010;31(1):1- 6. doi:10.1027/0227-5910/a000001
- Gabrielian S, Yuan AH, Andersen RM, Rubenstein LV, Gelberg L. VA health service utilization for homeless and lowincome Veterans: a spotlight on the VA Supportive Housing (VASH) program in greater Los Angeles. Med Care. 2014;52(5):454-461. doi:10.1097/MLR.0000000000000112
- Holliday R, Hostetter T, Brenner LA, Bahraini N, Tsai J. Suicide risk screening and evaluation among patients accessing VHA services and identified as being newly homeless. Health Serv Res. 2024;59(5):e14301. doi:10.1111/1475-6773.14301
- Waegemakers Schiff J, Lane AM. PTSD symptoms, vicarious traumatization, and burnout in front line workers in the homeless sector. Community Ment Health J. 2019;55(3):454-462. doi:10.1007/s10597-018-00364-7
- Steenekamp BL, Barker SL. Exploring the experiences of compassion fatigue amongst peer support workers in homelessness services. Community Ment Health J. 2024;60(4):772-783. doi:10.1007/s10597-024-01234-1
- Perez S, Kerman N, Dej E, et al. When I can’t help, I suffer: a scoping review of moral distress in service providers working with persons experiencing homelessness. J Ment Health. Published online 2024:1-16. doi:10.1080/09638237.2024.2426986
- Monteith LL, Holliday R, Christe’An DI, Sherrill A, Brenner LA, Hoffmire CA. Suicide risk and prevention in Guam: clinical and research considerations and a call to action. Asian J Psychiatry. 2023;83:103546. doi:10.1016/j.ajp.2023.103546
- Surís A, Holliday R, Hooshyar D, et al. Development and implementation of a homeless mobile medical/mental veteran intervention. Fed Pract. 2017;34(9):18.
Development of a VA Clinician Resource to Facilitate Care Among Veterans Experiencing Homelessness
Development of a VA Clinician Resource to Facilitate Care Among Veterans Experiencing Homelessness
Impact of Rapid Blood Culture Identification on Antibiotic De-escalation at a Veterans Affairs Medical Center
Impact of Rapid Blood Culture Identification on Antibiotic De-escalation at a Veterans Affairs Medical Center
About 530,000 to 628,000 episodes of bloodstream infections (BSI) occur annually in the US.1 Early identification and treatment of bacteremia are essential to improve patient outcomes because it allows for more timely targeted antibiotic therapy.2 Organism identification and susceptibility testing can take 2 to 5 days, prolonging the use of broad-spectrum empiric antibiotics and increasing the risk of adverse events.3,4 The Infectious Disease Society of America recommends the use of rapid diagnostic testing and antimicrobial stewardship programs (ASPs) to improve rates of antibiotic susceptibilities to targeted antibiotics and optimize resource utilization.3 Rapid blood culture identification (BCID) technologies reduce the duration of empiric antibiotics in patients with contaminated blood cultures, resulting in shorter hospital stays and saving money per each patient tested.4
In March 2023, Veteran Health Indiana (VHI) implemented the BioFire FilmArray Blood Culture Identification (BCID2), a BSI panel test that identifies select gram-negative bacteria, gram-positive bacteria, yeast, and antimicrobial resistance genes with an aggregate sensitivity of 99% and a specificity of 99.8%. The BCID2 presents clinically relevant information faster than traditional culture methods, allowing clinicians to make more efficient and educated antibiotic regimen decisions than with previous methods.5
It takes 24 to 48 hours from blood collection for culture incubation, positivity, and gram staining to occur at VHI. If the gram stain is positive, the blood culture is placed on the BioFire BCID2 in addition to traditional culture medium. BioFire BCID2 results are ready in 45 to 60 minutes. Results are uploaded into the electronic health record (EHR) ≤ 2 hours after they are obtained and the primary team is notified if the test is positive for certain critical results. Susceptibility testing of an identified organism typically requires an additional 24 to 48 hours for finalization. VHI Infectious Disease created an evidence-based antibiotic recommendation chart for certain medication(s) and alternate therapies based on the reported organism and its interpreted presence of resistance markers (eg, ceftriaxone for Escherichia coli when extended-spectrum beta lactamases are not detected vs meropenem if extended-spectrum beta lactamases marker are present). These charts optimize the antibiotic regimen while awaiting susceptibility finalizations.
Two previous studies describe the impact of rapid diagnostic testing technology at US Department of Veterans Affairs (VA) medical centers.6,7 In Texas, the ASP reviewed BCID panel results via clinical decision support software for about 1 hour per day.6 A Los Angeles study analyzed the impact of Biofire BCID with an interpretation guide centered on unnecessary vancomycin use and determined that shorter duration of the medication may have been the result of more frequent infectious disease consultation.7
This study assessed the time to optimal antibiotic de-escalation before and after the implementation of BioFire BCID2 with results reviewed by the ASP without active notification or assistance of any clinical decision support technology. The primary objective was to evaluate difference in time to optimal antibiotics from blood culture draw pre- vs postintervention. Secondary objectives included differences in time to organism identification, difference in time on broad-spectrum antibiotics, and difference in time to appropriate antibiotics.
Methods
This quasi-experimental retrospective chart review assessed the impact of BioFire BCID2 use on timely antibiotic de-escalation for patients who experienced a BSI at VHI between March 1, 2022, and October 1, 2023. Microbiology laboratory records identified eligible patients with positive blood cultures within the study time frame. Data were collected from the VHI EHR.
Patients were included if they had a positive bacterial blood culture and received ≥ 1 antibiotic indicated for bacteremia while receiving inpatient care. Patients were excluded if they died prior to blood culture results, transferred out of VHI, left against medical advice, or had untreated contaminants in blood culture results (ie, never received antibiotics aimed at the contaminated culture).
Patient lists were generated for before and after implementation of BioFire BCID2 (pre- and postintervention) using the VHI EHR and microbiology laboratory record system. The pre- and postinterventions groups were different sizes. As a result, a random sampling of the preintervention group was selected and included patients from March 1, 2022, through March 26, 2023. The postintervention group was smaller due to time constraints between initiation of BioFire BCID2 for data collection and included all patients from March 27, 2023, through October 1, 2023.
Optimal antibiotics were defined as escalation from inappropriate therapy to broader agent(s), de-escalation from broad-spectrum therapy to targeted agent(s), discontinuation of therapy due to an organism being identified as a contaminant, or optimization of a regimen to the preferred antimicrobial agent based on evidence-based consensus guidelines. Broad-spectrum antibiotics included: piperacillin/tazobactam, cefepime, ceftazidime, ceftazidime-avibactam, cefiderocol, carbapenems, fluroquinolones, vancomycin, daptomycin, ceftaroline, linezolid, or aztreonam. Appropriate antibiotics were defined as those with activity toward the final identified organism(s).
Deidentified participant data were entered into Microsoft Excel and kept on a secure VA server to complete statistical analyses. Parametric continuous data, such as age, were analyzed using the t-test, while nonparametric continuous data, such as time to optimal antibiotics, were analyzed using the Mann-Whitney U test. Categorical data, like sex and race, were analyzed using either Fisher exact test for small sample sizes or X2 test for a larger sample size. Statistical significance levels was defined as P < .05.
Results
Using patient lists drawn from the EHR and the microbiology laboratory records, 110 electronic charts were randomly selected for review. Fifteen patients were excluded: 8 had untreated contaminants, 4 died, and 3 were transferred out of VHI. Of the 95 patients included, 48 were in the preintervention group and 47 were in the postintervention group (Figure 1).
Baseline characteristics were similar between the 2 groups (Table 1). Most patients were White males aged > 70 years in the EHR. The urinary tract was the most common source of infection, impacting 12 patients in each group (Figure 2). Escherichia coli, Klebsiella, Staphylococcus, and Streptococcus were the most common bloodstream isolates identified.
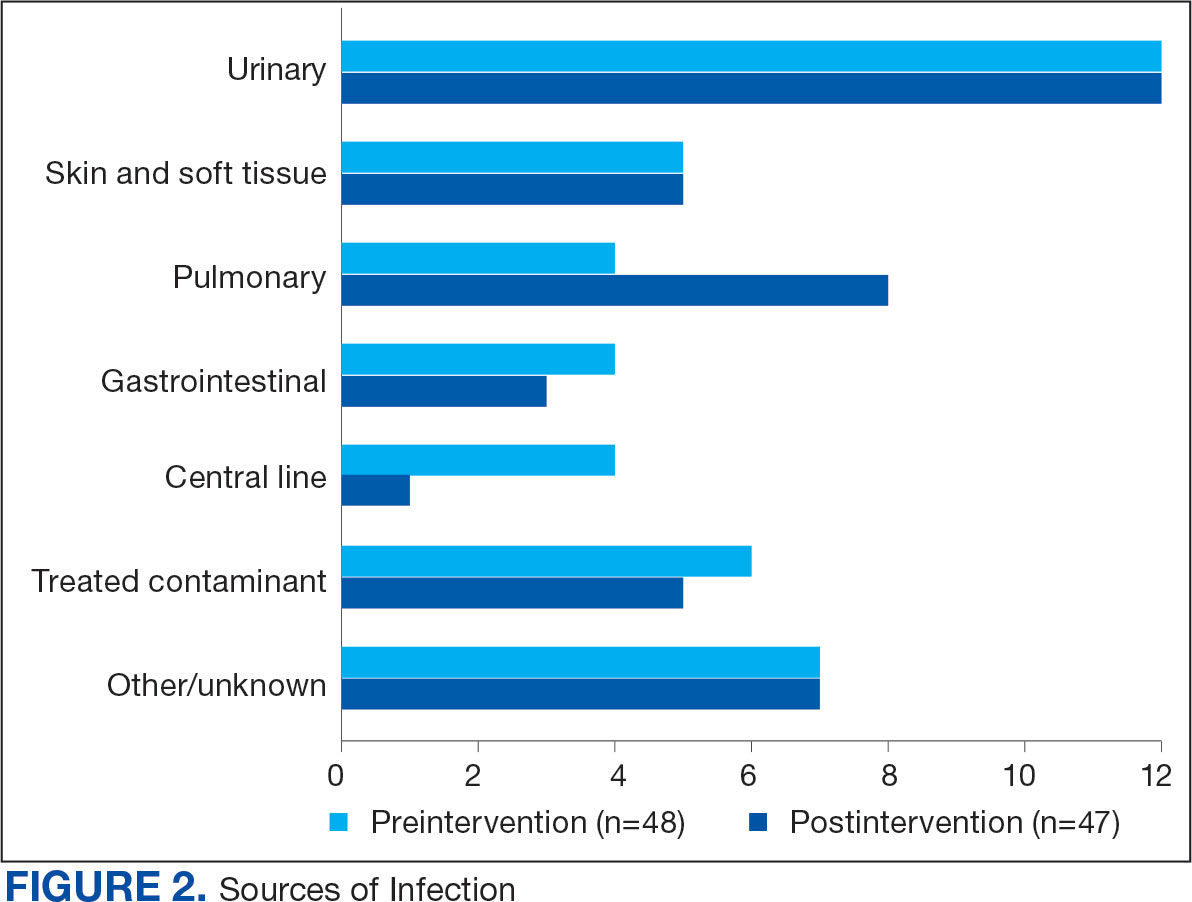
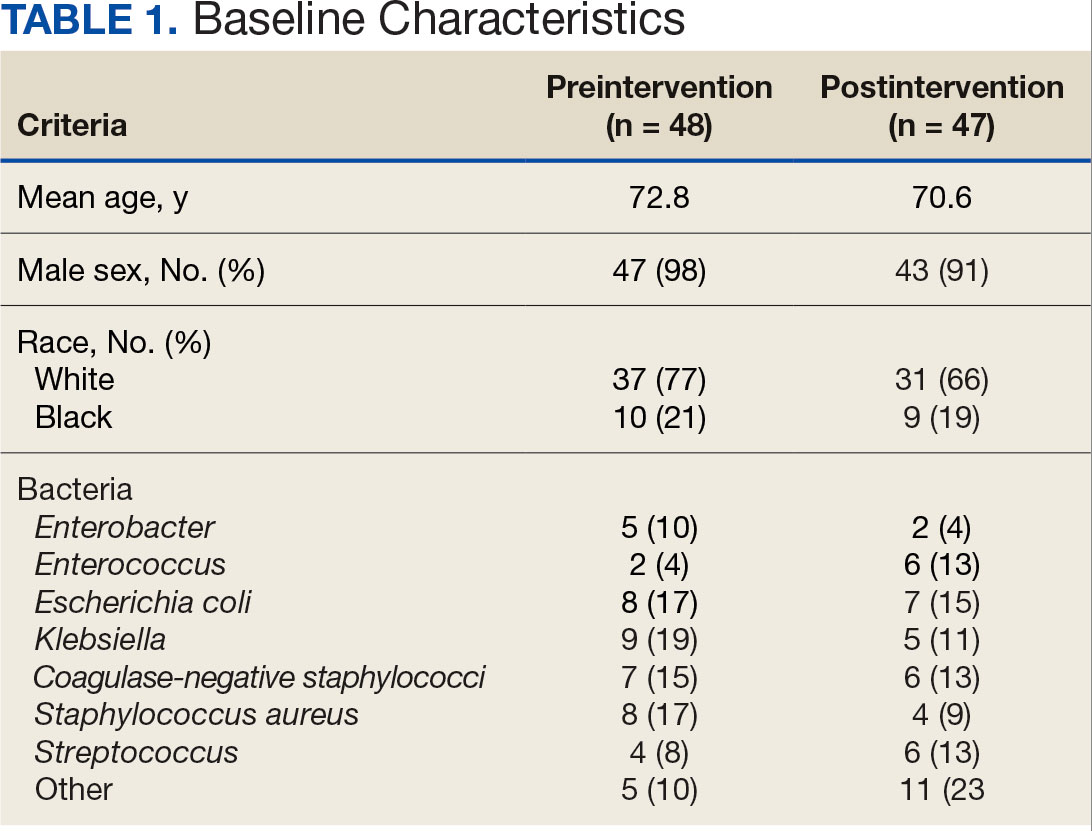
The median time to optimal antibiotics in the preintervention group was 58.5 hours vs 43.4 hours in the postintervention group (P = .11). The median time to organism identification was 37.8 hours in the preintervention group vs 16.9 hours in the postintervention group (P < .001). The median time on broad-spectrum antibiotics was 45.2 hours in the preintervention group vs 46.6 hours in the postintervention group (P = .99). The median time on appropriate antibiotics in the preintervention group was 2.3 hours vs 1.9 hours in the postintervention group (P = .79). Differences in other measured outcomes between the groups were not statistically significant (Table 2).
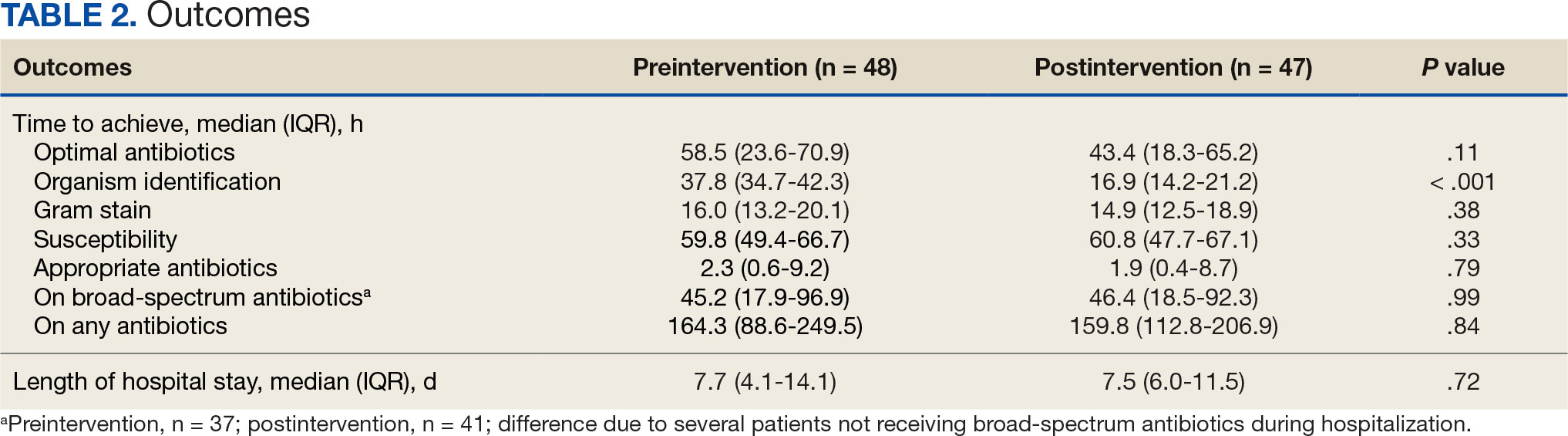
Although implementation of rapid diagnostic technology reduced the median time to optimal antibiotics, the results were not statistically significant. Shorter time to organism identification in the postintervention group compared to the preintervention group was the lone statistically significant metric (P < .001).
Discussion
A lack of statistical significance in the primary outcome may have been due to nonadherence to facility de-escalation protocols or a suboptimal BioFire BCID2 result notification system. Additionally, use of rapid BCID at VHI may improve over time as clinicians become more familiar with the technology. Gaps in clinical pharmacy coverage during the night shift may have also contributed to delays in antibiotic optimization, particularly if other clinicians are not equipped with the knowledge or training to appropriately deescalate antibiotics based on microorganisms identified. A 2017 study by Donner et al concluded that physician interpretation of BCID results is suboptimal and should be augmented with clinical decision support tools as new technology becomes available.8 Despite the statistically insignificant results of this study, it did highlight potential areas of improvement which can lead to improved patient care.
Previous research has evaluated the impact of rapid BCID technology on antibiotic treatment and clinical outcomes. Chiasson et al found that median time to optimal therapy was 73.8 hours in the pre-BCID arm compared to 34.7 hours in the post- BCID arm (P ≤ .001), emphasizing the importance of combining rapid BCID with clinical decision support tools and pharmacy input.6 Senok et al found that BCID2 implementation led to a significant decrease in median time to culture result, which informed optimal antibiotic therapy and decreased 30-day mortality in the intensive care setting.9 In contrast, the current study did not stratify patients according to medical ward or illness severity even though clinicians may be less likely to de-escalate antibiotic therapy in critically ill patients.
Bae et al reported findings consistent with the current study and concluded that BCID did not affect the clinical outcomes of overall BSIs; however, it contributed to early administration of effective antibiotics in cases of BSIs caused by multidrug-resistant organisms.10 Results of this study were not stratified according to multidrug-resistant organisms because the sample size was too small. The current study also included patients with polymicrobial infections, which may have impacted the results due to a less streamlined approach to antibiotic optimization.
Limitations
This single-center, retrospective study had a small sample size, short time frame, and lacked patient diversity, and therefore may not be generalizable to other health care systems. The sample size was limited by shorter date range and smaller patient list between BioFire BCID2 implementation and data collection, which was used to determine the number of charts selected in each group. Some patients received antibiotics prior to blood cultures being drawn, which may falsely decrease time to optimal/ appropriate antibiotics and falsely increase time on broad spectrum/any antibiotics due to early antibiotic administration. The total number of patients on broad-spectrum antibiotics differed from the total number of patients for other outcomes because several patients never received the defined broad spectrum antibiotics.
Conclusions
When combined with a pre-existing ASP without active notification, the implementation of BioFire BCID2 did not return statistically significant data showing a decrease in time to optimal antibiotics, time to appropriate antibiotics, or time on broad-spectrum antibiotics at VHI. To make this program more successful, pharmacist intervention and clinical decision support tools may be needed.
Additional research is required to determine the optimal integration of antimicrobial stewardship, rapid diagnostic technology, and pharmacy services for maximum benefit. Even though the primary outcome was not statistically significant, the results may be clinically significant from a stewardship perspective. Realigning microbiology workflows to mimic other research, which emphasizes the importance of funneling rapid BCID results through the ASP, may improve outcomes. Future studies may be warranted following the implementation of clinical decision support tools to assess their impact on stewardship practices and patient outcomes.
- Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect. 2013;19(6):501- 509. doi:10.1111/1469-0691.12195
- Pardo J, Klinker KP, Borgert SJ, Butler BM, Giglio PG, Rand KH. Clinical and economic impact of antimicrobial stewardship interventions with the FilmArray blood culture identification panel. Diagn Microbiol Infect Dis. 2016;84(2):159-164. doi:10.1016/j.diagmicrobio.2015.10.023.
- Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51-e77. doi:10.1093/cid/ciw118
- BIOFIRE® Blood Culture Identification 2 (BCID2) Panel. Biomerierux. Updated 2025. Accessed May 10, 2025. https://www.biofiredx.com/products/the-filmarray-panels/filmarraybcid/
- Huang AM, Newton D, Kunapuli A, et al. Impact of rapid organism identification via matrix-assisted laser desorption/ionization time-of-flight combined with antimicrobial stewardship team intervention in adult patients with bacteremia and candidemia. Clin Infect Dis. 2013;57(9):1237-1245. doi:10.1093/cid/cit498
- Chiasson JM, Smith WJ, Jodlowski TZ, Kouma MA, Cutrell JB. Impact of a rapid blood culture diagnostic panel on time to optimal antimicrobial therapy at a veterans affairs medical center. J Pharm Pract. 2022;35(5):722-729. doi:10.1177/08971900211000686
- Wu S, Watson RL, Graber CJ. 2007. Impact of combining rapid diagnostics with an interpretation guide on vancomycin usage for contaminant blood cultures growing coagulase- negative staphylococci (CoNS). Open Forum Infect Dis. 2019;6(Suppl 2):S674. doi:10.1093/ofid/ofz360.1687
- Donner LM, Campbell WS, Lyden E, Van Schooneveld TC. Assessment of rapid-blood-culture-identification result interpretation and antibiotic prescribing practices. J Clin Microbiol. 2017;55(5):1496-1507. doi:10.1128/JCM.02395-16
- Senok A, Dabal LA, Alfaresi M, et al. Clinical impact of the BIOFIRE blood culture identification 2 panel in adult patients with bloodstream infection: a multicentre observational study in the United Arab Emirates. Diagnostics (Basel). 2023;13(14):2433. doi:10.3390/diagnostics13142433
- Bae JY, Bae J, So MK, Choi HJ, Lee M. The impact of the rapid blood culture identification panel on antibiotic treatment and clinical outcomes in bloodstream infections, particularly those associated with multidrug-resistant micro-organisms. Diagnostics (Basel). 2023;13(23):3504. doi:10.3390/diagnostics13233504
About 530,000 to 628,000 episodes of bloodstream infections (BSI) occur annually in the US.1 Early identification and treatment of bacteremia are essential to improve patient outcomes because it allows for more timely targeted antibiotic therapy.2 Organism identification and susceptibility testing can take 2 to 5 days, prolonging the use of broad-spectrum empiric antibiotics and increasing the risk of adverse events.3,4 The Infectious Disease Society of America recommends the use of rapid diagnostic testing and antimicrobial stewardship programs (ASPs) to improve rates of antibiotic susceptibilities to targeted antibiotics and optimize resource utilization.3 Rapid blood culture identification (BCID) technologies reduce the duration of empiric antibiotics in patients with contaminated blood cultures, resulting in shorter hospital stays and saving money per each patient tested.4
In March 2023, Veteran Health Indiana (VHI) implemented the BioFire FilmArray Blood Culture Identification (BCID2), a BSI panel test that identifies select gram-negative bacteria, gram-positive bacteria, yeast, and antimicrobial resistance genes with an aggregate sensitivity of 99% and a specificity of 99.8%. The BCID2 presents clinically relevant information faster than traditional culture methods, allowing clinicians to make more efficient and educated antibiotic regimen decisions than with previous methods.5
It takes 24 to 48 hours from blood collection for culture incubation, positivity, and gram staining to occur at VHI. If the gram stain is positive, the blood culture is placed on the BioFire BCID2 in addition to traditional culture medium. BioFire BCID2 results are ready in 45 to 60 minutes. Results are uploaded into the electronic health record (EHR) ≤ 2 hours after they are obtained and the primary team is notified if the test is positive for certain critical results. Susceptibility testing of an identified organism typically requires an additional 24 to 48 hours for finalization. VHI Infectious Disease created an evidence-based antibiotic recommendation chart for certain medication(s) and alternate therapies based on the reported organism and its interpreted presence of resistance markers (eg, ceftriaxone for Escherichia coli when extended-spectrum beta lactamases are not detected vs meropenem if extended-spectrum beta lactamases marker are present). These charts optimize the antibiotic regimen while awaiting susceptibility finalizations.
Two previous studies describe the impact of rapid diagnostic testing technology at US Department of Veterans Affairs (VA) medical centers.6,7 In Texas, the ASP reviewed BCID panel results via clinical decision support software for about 1 hour per day.6 A Los Angeles study analyzed the impact of Biofire BCID with an interpretation guide centered on unnecessary vancomycin use and determined that shorter duration of the medication may have been the result of more frequent infectious disease consultation.7
This study assessed the time to optimal antibiotic de-escalation before and after the implementation of BioFire BCID2 with results reviewed by the ASP without active notification or assistance of any clinical decision support technology. The primary objective was to evaluate difference in time to optimal antibiotics from blood culture draw pre- vs postintervention. Secondary objectives included differences in time to organism identification, difference in time on broad-spectrum antibiotics, and difference in time to appropriate antibiotics.
Methods
This quasi-experimental retrospective chart review assessed the impact of BioFire BCID2 use on timely antibiotic de-escalation for patients who experienced a BSI at VHI between March 1, 2022, and October 1, 2023. Microbiology laboratory records identified eligible patients with positive blood cultures within the study time frame. Data were collected from the VHI EHR.
Patients were included if they had a positive bacterial blood culture and received ≥ 1 antibiotic indicated for bacteremia while receiving inpatient care. Patients were excluded if they died prior to blood culture results, transferred out of VHI, left against medical advice, or had untreated contaminants in blood culture results (ie, never received antibiotics aimed at the contaminated culture).
Patient lists were generated for before and after implementation of BioFire BCID2 (pre- and postintervention) using the VHI EHR and microbiology laboratory record system. The pre- and postinterventions groups were different sizes. As a result, a random sampling of the preintervention group was selected and included patients from March 1, 2022, through March 26, 2023. The postintervention group was smaller due to time constraints between initiation of BioFire BCID2 for data collection and included all patients from March 27, 2023, through October 1, 2023.
Optimal antibiotics were defined as escalation from inappropriate therapy to broader agent(s), de-escalation from broad-spectrum therapy to targeted agent(s), discontinuation of therapy due to an organism being identified as a contaminant, or optimization of a regimen to the preferred antimicrobial agent based on evidence-based consensus guidelines. Broad-spectrum antibiotics included: piperacillin/tazobactam, cefepime, ceftazidime, ceftazidime-avibactam, cefiderocol, carbapenems, fluroquinolones, vancomycin, daptomycin, ceftaroline, linezolid, or aztreonam. Appropriate antibiotics were defined as those with activity toward the final identified organism(s).
Deidentified participant data were entered into Microsoft Excel and kept on a secure VA server to complete statistical analyses. Parametric continuous data, such as age, were analyzed using the t-test, while nonparametric continuous data, such as time to optimal antibiotics, were analyzed using the Mann-Whitney U test. Categorical data, like sex and race, were analyzed using either Fisher exact test for small sample sizes or X2 test for a larger sample size. Statistical significance levels was defined as P < .05.
Results
Using patient lists drawn from the EHR and the microbiology laboratory records, 110 electronic charts were randomly selected for review. Fifteen patients were excluded: 8 had untreated contaminants, 4 died, and 3 were transferred out of VHI. Of the 95 patients included, 48 were in the preintervention group and 47 were in the postintervention group (Figure 1).

Baseline characteristics were similar between the 2 groups (Table 1). Most patients were White males aged > 70 years in the EHR. The urinary tract was the most common source of infection, impacting 12 patients in each group (Figure 2). Escherichia coli, Klebsiella, Staphylococcus, and Streptococcus were the most common bloodstream isolates identified.


The median time to optimal antibiotics in the preintervention group was 58.5 hours vs 43.4 hours in the postintervention group (P = .11). The median time to organism identification was 37.8 hours in the preintervention group vs 16.9 hours in the postintervention group (P < .001). The median time on broad-spectrum antibiotics was 45.2 hours in the preintervention group vs 46.6 hours in the postintervention group (P = .99). The median time on appropriate antibiotics in the preintervention group was 2.3 hours vs 1.9 hours in the postintervention group (P = .79). Differences in other measured outcomes between the groups were not statistically significant (Table 2).

Although implementation of rapid diagnostic technology reduced the median time to optimal antibiotics, the results were not statistically significant. Shorter time to organism identification in the postintervention group compared to the preintervention group was the lone statistically significant metric (P < .001).
Discussion
A lack of statistical significance in the primary outcome may have been due to nonadherence to facility de-escalation protocols or a suboptimal BioFire BCID2 result notification system. Additionally, use of rapid BCID at VHI may improve over time as clinicians become more familiar with the technology. Gaps in clinical pharmacy coverage during the night shift may have also contributed to delays in antibiotic optimization, particularly if other clinicians are not equipped with the knowledge or training to appropriately deescalate antibiotics based on microorganisms identified. A 2017 study by Donner et al concluded that physician interpretation of BCID results is suboptimal and should be augmented with clinical decision support tools as new technology becomes available.8 Despite the statistically insignificant results of this study, it did highlight potential areas of improvement which can lead to improved patient care.
Previous research has evaluated the impact of rapid BCID technology on antibiotic treatment and clinical outcomes. Chiasson et al found that median time to optimal therapy was 73.8 hours in the pre-BCID arm compared to 34.7 hours in the post- BCID arm (P ≤ .001), emphasizing the importance of combining rapid BCID with clinical decision support tools and pharmacy input.6 Senok et al found that BCID2 implementation led to a significant decrease in median time to culture result, which informed optimal antibiotic therapy and decreased 30-day mortality in the intensive care setting.9 In contrast, the current study did not stratify patients according to medical ward or illness severity even though clinicians may be less likely to de-escalate antibiotic therapy in critically ill patients.
Bae et al reported findings consistent with the current study and concluded that BCID did not affect the clinical outcomes of overall BSIs; however, it contributed to early administration of effective antibiotics in cases of BSIs caused by multidrug-resistant organisms.10 Results of this study were not stratified according to multidrug-resistant organisms because the sample size was too small. The current study also included patients with polymicrobial infections, which may have impacted the results due to a less streamlined approach to antibiotic optimization.
Limitations
This single-center, retrospective study had a small sample size, short time frame, and lacked patient diversity, and therefore may not be generalizable to other health care systems. The sample size was limited by shorter date range and smaller patient list between BioFire BCID2 implementation and data collection, which was used to determine the number of charts selected in each group. Some patients received antibiotics prior to blood cultures being drawn, which may falsely decrease time to optimal/ appropriate antibiotics and falsely increase time on broad spectrum/any antibiotics due to early antibiotic administration. The total number of patients on broad-spectrum antibiotics differed from the total number of patients for other outcomes because several patients never received the defined broad spectrum antibiotics.
Conclusions
When combined with a pre-existing ASP without active notification, the implementation of BioFire BCID2 did not return statistically significant data showing a decrease in time to optimal antibiotics, time to appropriate antibiotics, or time on broad-spectrum antibiotics at VHI. To make this program more successful, pharmacist intervention and clinical decision support tools may be needed.
Additional research is required to determine the optimal integration of antimicrobial stewardship, rapid diagnostic technology, and pharmacy services for maximum benefit. Even though the primary outcome was not statistically significant, the results may be clinically significant from a stewardship perspective. Realigning microbiology workflows to mimic other research, which emphasizes the importance of funneling rapid BCID results through the ASP, may improve outcomes. Future studies may be warranted following the implementation of clinical decision support tools to assess their impact on stewardship practices and patient outcomes.
About 530,000 to 628,000 episodes of bloodstream infections (BSI) occur annually in the US.1 Early identification and treatment of bacteremia are essential to improve patient outcomes because it allows for more timely targeted antibiotic therapy.2 Organism identification and susceptibility testing can take 2 to 5 days, prolonging the use of broad-spectrum empiric antibiotics and increasing the risk of adverse events.3,4 The Infectious Disease Society of America recommends the use of rapid diagnostic testing and antimicrobial stewardship programs (ASPs) to improve rates of antibiotic susceptibilities to targeted antibiotics and optimize resource utilization.3 Rapid blood culture identification (BCID) technologies reduce the duration of empiric antibiotics in patients with contaminated blood cultures, resulting in shorter hospital stays and saving money per each patient tested.4
In March 2023, Veteran Health Indiana (VHI) implemented the BioFire FilmArray Blood Culture Identification (BCID2), a BSI panel test that identifies select gram-negative bacteria, gram-positive bacteria, yeast, and antimicrobial resistance genes with an aggregate sensitivity of 99% and a specificity of 99.8%. The BCID2 presents clinically relevant information faster than traditional culture methods, allowing clinicians to make more efficient and educated antibiotic regimen decisions than with previous methods.5
It takes 24 to 48 hours from blood collection for culture incubation, positivity, and gram staining to occur at VHI. If the gram stain is positive, the blood culture is placed on the BioFire BCID2 in addition to traditional culture medium. BioFire BCID2 results are ready in 45 to 60 minutes. Results are uploaded into the electronic health record (EHR) ≤ 2 hours after they are obtained and the primary team is notified if the test is positive for certain critical results. Susceptibility testing of an identified organism typically requires an additional 24 to 48 hours for finalization. VHI Infectious Disease created an evidence-based antibiotic recommendation chart for certain medication(s) and alternate therapies based on the reported organism and its interpreted presence of resistance markers (eg, ceftriaxone for Escherichia coli when extended-spectrum beta lactamases are not detected vs meropenem if extended-spectrum beta lactamases marker are present). These charts optimize the antibiotic regimen while awaiting susceptibility finalizations.
Two previous studies describe the impact of rapid diagnostic testing technology at US Department of Veterans Affairs (VA) medical centers.6,7 In Texas, the ASP reviewed BCID panel results via clinical decision support software for about 1 hour per day.6 A Los Angeles study analyzed the impact of Biofire BCID with an interpretation guide centered on unnecessary vancomycin use and determined that shorter duration of the medication may have been the result of more frequent infectious disease consultation.7
This study assessed the time to optimal antibiotic de-escalation before and after the implementation of BioFire BCID2 with results reviewed by the ASP without active notification or assistance of any clinical decision support technology. The primary objective was to evaluate difference in time to optimal antibiotics from blood culture draw pre- vs postintervention. Secondary objectives included differences in time to organism identification, difference in time on broad-spectrum antibiotics, and difference in time to appropriate antibiotics.
Methods
This quasi-experimental retrospective chart review assessed the impact of BioFire BCID2 use on timely antibiotic de-escalation for patients who experienced a BSI at VHI between March 1, 2022, and October 1, 2023. Microbiology laboratory records identified eligible patients with positive blood cultures within the study time frame. Data were collected from the VHI EHR.
Patients were included if they had a positive bacterial blood culture and received ≥ 1 antibiotic indicated for bacteremia while receiving inpatient care. Patients were excluded if they died prior to blood culture results, transferred out of VHI, left against medical advice, or had untreated contaminants in blood culture results (ie, never received antibiotics aimed at the contaminated culture).
Patient lists were generated for before and after implementation of BioFire BCID2 (pre- and postintervention) using the VHI EHR and microbiology laboratory record system. The pre- and postinterventions groups were different sizes. As a result, a random sampling of the preintervention group was selected and included patients from March 1, 2022, through March 26, 2023. The postintervention group was smaller due to time constraints between initiation of BioFire BCID2 for data collection and included all patients from March 27, 2023, through October 1, 2023.
Optimal antibiotics were defined as escalation from inappropriate therapy to broader agent(s), de-escalation from broad-spectrum therapy to targeted agent(s), discontinuation of therapy due to an organism being identified as a contaminant, or optimization of a regimen to the preferred antimicrobial agent based on evidence-based consensus guidelines. Broad-spectrum antibiotics included: piperacillin/tazobactam, cefepime, ceftazidime, ceftazidime-avibactam, cefiderocol, carbapenems, fluroquinolones, vancomycin, daptomycin, ceftaroline, linezolid, or aztreonam. Appropriate antibiotics were defined as those with activity toward the final identified organism(s).
Deidentified participant data were entered into Microsoft Excel and kept on a secure VA server to complete statistical analyses. Parametric continuous data, such as age, were analyzed using the t-test, while nonparametric continuous data, such as time to optimal antibiotics, were analyzed using the Mann-Whitney U test. Categorical data, like sex and race, were analyzed using either Fisher exact test for small sample sizes or X2 test for a larger sample size. Statistical significance levels was defined as P < .05.
Results
Using patient lists drawn from the EHR and the microbiology laboratory records, 110 electronic charts were randomly selected for review. Fifteen patients were excluded: 8 had untreated contaminants, 4 died, and 3 were transferred out of VHI. Of the 95 patients included, 48 were in the preintervention group and 47 were in the postintervention group (Figure 1).

Baseline characteristics were similar between the 2 groups (Table 1). Most patients were White males aged > 70 years in the EHR. The urinary tract was the most common source of infection, impacting 12 patients in each group (Figure 2). Escherichia coli, Klebsiella, Staphylococcus, and Streptococcus were the most common bloodstream isolates identified.


The median time to optimal antibiotics in the preintervention group was 58.5 hours vs 43.4 hours in the postintervention group (P = .11). The median time to organism identification was 37.8 hours in the preintervention group vs 16.9 hours in the postintervention group (P < .001). The median time on broad-spectrum antibiotics was 45.2 hours in the preintervention group vs 46.6 hours in the postintervention group (P = .99). The median time on appropriate antibiotics in the preintervention group was 2.3 hours vs 1.9 hours in the postintervention group (P = .79). Differences in other measured outcomes between the groups were not statistically significant (Table 2).

Although implementation of rapid diagnostic technology reduced the median time to optimal antibiotics, the results were not statistically significant. Shorter time to organism identification in the postintervention group compared to the preintervention group was the lone statistically significant metric (P < .001).
Discussion
A lack of statistical significance in the primary outcome may have been due to nonadherence to facility de-escalation protocols or a suboptimal BioFire BCID2 result notification system. Additionally, use of rapid BCID at VHI may improve over time as clinicians become more familiar with the technology. Gaps in clinical pharmacy coverage during the night shift may have also contributed to delays in antibiotic optimization, particularly if other clinicians are not equipped with the knowledge or training to appropriately deescalate antibiotics based on microorganisms identified. A 2017 study by Donner et al concluded that physician interpretation of BCID results is suboptimal and should be augmented with clinical decision support tools as new technology becomes available.8 Despite the statistically insignificant results of this study, it did highlight potential areas of improvement which can lead to improved patient care.
Previous research has evaluated the impact of rapid BCID technology on antibiotic treatment and clinical outcomes. Chiasson et al found that median time to optimal therapy was 73.8 hours in the pre-BCID arm compared to 34.7 hours in the post- BCID arm (P ≤ .001), emphasizing the importance of combining rapid BCID with clinical decision support tools and pharmacy input.6 Senok et al found that BCID2 implementation led to a significant decrease in median time to culture result, which informed optimal antibiotic therapy and decreased 30-day mortality in the intensive care setting.9 In contrast, the current study did not stratify patients according to medical ward or illness severity even though clinicians may be less likely to de-escalate antibiotic therapy in critically ill patients.
Bae et al reported findings consistent with the current study and concluded that BCID did not affect the clinical outcomes of overall BSIs; however, it contributed to early administration of effective antibiotics in cases of BSIs caused by multidrug-resistant organisms.10 Results of this study were not stratified according to multidrug-resistant organisms because the sample size was too small. The current study also included patients with polymicrobial infections, which may have impacted the results due to a less streamlined approach to antibiotic optimization.
Limitations
This single-center, retrospective study had a small sample size, short time frame, and lacked patient diversity, and therefore may not be generalizable to other health care systems. The sample size was limited by shorter date range and smaller patient list between BioFire BCID2 implementation and data collection, which was used to determine the number of charts selected in each group. Some patients received antibiotics prior to blood cultures being drawn, which may falsely decrease time to optimal/ appropriate antibiotics and falsely increase time on broad spectrum/any antibiotics due to early antibiotic administration. The total number of patients on broad-spectrum antibiotics differed from the total number of patients for other outcomes because several patients never received the defined broad spectrum antibiotics.
Conclusions
When combined with a pre-existing ASP without active notification, the implementation of BioFire BCID2 did not return statistically significant data showing a decrease in time to optimal antibiotics, time to appropriate antibiotics, or time on broad-spectrum antibiotics at VHI. To make this program more successful, pharmacist intervention and clinical decision support tools may be needed.
Additional research is required to determine the optimal integration of antimicrobial stewardship, rapid diagnostic technology, and pharmacy services for maximum benefit. Even though the primary outcome was not statistically significant, the results may be clinically significant from a stewardship perspective. Realigning microbiology workflows to mimic other research, which emphasizes the importance of funneling rapid BCID results through the ASP, may improve outcomes. Future studies may be warranted following the implementation of clinical decision support tools to assess their impact on stewardship practices and patient outcomes.
- Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect. 2013;19(6):501- 509. doi:10.1111/1469-0691.12195
- Pardo J, Klinker KP, Borgert SJ, Butler BM, Giglio PG, Rand KH. Clinical and economic impact of antimicrobial stewardship interventions with the FilmArray blood culture identification panel. Diagn Microbiol Infect Dis. 2016;84(2):159-164. doi:10.1016/j.diagmicrobio.2015.10.023.
- Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51-e77. doi:10.1093/cid/ciw118
- BIOFIRE® Blood Culture Identification 2 (BCID2) Panel. Biomerierux. Updated 2025. Accessed May 10, 2025. https://www.biofiredx.com/products/the-filmarray-panels/filmarraybcid/
- Huang AM, Newton D, Kunapuli A, et al. Impact of rapid organism identification via matrix-assisted laser desorption/ionization time-of-flight combined with antimicrobial stewardship team intervention in adult patients with bacteremia and candidemia. Clin Infect Dis. 2013;57(9):1237-1245. doi:10.1093/cid/cit498
- Chiasson JM, Smith WJ, Jodlowski TZ, Kouma MA, Cutrell JB. Impact of a rapid blood culture diagnostic panel on time to optimal antimicrobial therapy at a veterans affairs medical center. J Pharm Pract. 2022;35(5):722-729. doi:10.1177/08971900211000686
- Wu S, Watson RL, Graber CJ. 2007. Impact of combining rapid diagnostics with an interpretation guide on vancomycin usage for contaminant blood cultures growing coagulase- negative staphylococci (CoNS). Open Forum Infect Dis. 2019;6(Suppl 2):S674. doi:10.1093/ofid/ofz360.1687
- Donner LM, Campbell WS, Lyden E, Van Schooneveld TC. Assessment of rapid-blood-culture-identification result interpretation and antibiotic prescribing practices. J Clin Microbiol. 2017;55(5):1496-1507. doi:10.1128/JCM.02395-16
- Senok A, Dabal LA, Alfaresi M, et al. Clinical impact of the BIOFIRE blood culture identification 2 panel in adult patients with bloodstream infection: a multicentre observational study in the United Arab Emirates. Diagnostics (Basel). 2023;13(14):2433. doi:10.3390/diagnostics13142433
- Bae JY, Bae J, So MK, Choi HJ, Lee M. The impact of the rapid blood culture identification panel on antibiotic treatment and clinical outcomes in bloodstream infections, particularly those associated with multidrug-resistant micro-organisms. Diagnostics (Basel). 2023;13(23):3504. doi:10.3390/diagnostics13233504
- Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect. 2013;19(6):501- 509. doi:10.1111/1469-0691.12195
- Pardo J, Klinker KP, Borgert SJ, Butler BM, Giglio PG, Rand KH. Clinical and economic impact of antimicrobial stewardship interventions with the FilmArray blood culture identification panel. Diagn Microbiol Infect Dis. 2016;84(2):159-164. doi:10.1016/j.diagmicrobio.2015.10.023.
- Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51-e77. doi:10.1093/cid/ciw118
- BIOFIRE® Blood Culture Identification 2 (BCID2) Panel. Biomerierux. Updated 2025. Accessed May 10, 2025. https://www.biofiredx.com/products/the-filmarray-panels/filmarraybcid/
- Huang AM, Newton D, Kunapuli A, et al. Impact of rapid organism identification via matrix-assisted laser desorption/ionization time-of-flight combined with antimicrobial stewardship team intervention in adult patients with bacteremia and candidemia. Clin Infect Dis. 2013;57(9):1237-1245. doi:10.1093/cid/cit498
- Chiasson JM, Smith WJ, Jodlowski TZ, Kouma MA, Cutrell JB. Impact of a rapid blood culture diagnostic panel on time to optimal antimicrobial therapy at a veterans affairs medical center. J Pharm Pract. 2022;35(5):722-729. doi:10.1177/08971900211000686
- Wu S, Watson RL, Graber CJ. 2007. Impact of combining rapid diagnostics with an interpretation guide on vancomycin usage for contaminant blood cultures growing coagulase- negative staphylococci (CoNS). Open Forum Infect Dis. 2019;6(Suppl 2):S674. doi:10.1093/ofid/ofz360.1687
- Donner LM, Campbell WS, Lyden E, Van Schooneveld TC. Assessment of rapid-blood-culture-identification result interpretation and antibiotic prescribing practices. J Clin Microbiol. 2017;55(5):1496-1507. doi:10.1128/JCM.02395-16
- Senok A, Dabal LA, Alfaresi M, et al. Clinical impact of the BIOFIRE blood culture identification 2 panel in adult patients with bloodstream infection: a multicentre observational study in the United Arab Emirates. Diagnostics (Basel). 2023;13(14):2433. doi:10.3390/diagnostics13142433
- Bae JY, Bae J, So MK, Choi HJ, Lee M. The impact of the rapid blood culture identification panel on antibiotic treatment and clinical outcomes in bloodstream infections, particularly those associated with multidrug-resistant micro-organisms. Diagnostics (Basel). 2023;13(23):3504. doi:10.3390/diagnostics13233504
Impact of Rapid Blood Culture Identification on Antibiotic De-escalation at a Veterans Affairs Medical Center
Impact of Rapid Blood Culture Identification on Antibiotic De-escalation at a Veterans Affairs Medical Center
Disparate Prednisone Starting Dosages for Systemic Corticosteroid-Naïve Veterans With Active Sarcoidosis
Disparate Prednisone Starting Dosages for Systemic Corticosteroid-Naïve Veterans With Active Sarcoidosis
Sarcoidosis is a multiorgan granulomatous disorder of unknown etiology that impacts many US veterans.1 At diagnosis, clinical manifestations vary and partially depend on the extent and severity of organ involvement, particularly of the lungs, heart, and eyes.2,3 Sarcoidosis may lead to progressive organ dysfunction, long-term disability, and death.1-3 Clinical practice guidelines recommend prednisone 20 to 40 mg daily or equivalent-prednisone dose followed by a slow tapering, as first-line pharmacotherapy for patients with active sarcoidosis who are naïve to systemic corticosteroids.2-4
Use of prolonged, high-dosage prednisone (> 40 mg daily) is discouraged due to a high risk of corticosteroid-related adverse events and associated health care costs.5,6 Research suggests that initial lower prednisone dosage (< 20 mg daily) may be effective in systemic corticosteroid-naïve patients with active sarcoidosis.3
Adherence to this regimen by specialists (eg, pulmonologists, dermatologists, ophthalmologists, rheumatologists, and cardiologists) has not been established. This study sought to determine the starting dosages for prednisone prescribed at the Jesse Brown Department of Veterans Affairs Medical Center (JBVAMC) to patients with active sarcoidosis who were systemic corticosteroid-naïve.
Methods
Patient data were reviewed from the Computerized Patient Record System (CPRS) for individuals diagnosed with sarcoidosis who were corticosteroid-naïve and prescribed initial prednisone dosages by health care practitioners (HCPs) from several specialties between 2014 and 2023 at JBVAMC. This 200-bed acute care facility serves about 62,000 veterans who live in Illinois or Indiana. JBVAMC is affiliated with the University of Illinois College of Medicine at Chicago, Northwestern University Feinberg School of Medicine, and the University of Chicago Pritzker School of Medicine; many JBVAMC HCPs hold academic appointments with these medical schools.
Patient demographics, prescriber specialty, and daily starting dosage were recorded. The decision to initiate prednisone therapy and its dosage were at the discretion of HCPs who diagnosed active sarcoidosis based on compatible clinical and ancillary test findings as documented in CPRS.2-4,6-10 Statistical analyses were conducted using a t test, and a threshold of P < .05 was considered statistically significant. This study was reviewed and determined to be exempt by the JBVAMC institutional review board.
Results
Sixty-eight patients who were systemic corticosteroid- naïve and had sarcoidosis were prescribed prednisone by HCPs at JBVAMC. Fifty-two were Black (76%), 62 were male (91%), and 53 were current or former smokers (78%). The mean (SD) age was 63 (11) years (Table 1). Forty patients (59%) had lung involvement, 6 had eye (9%), 6 had skin (9%), and 5 had musculoskeletal system (7%) involvement.
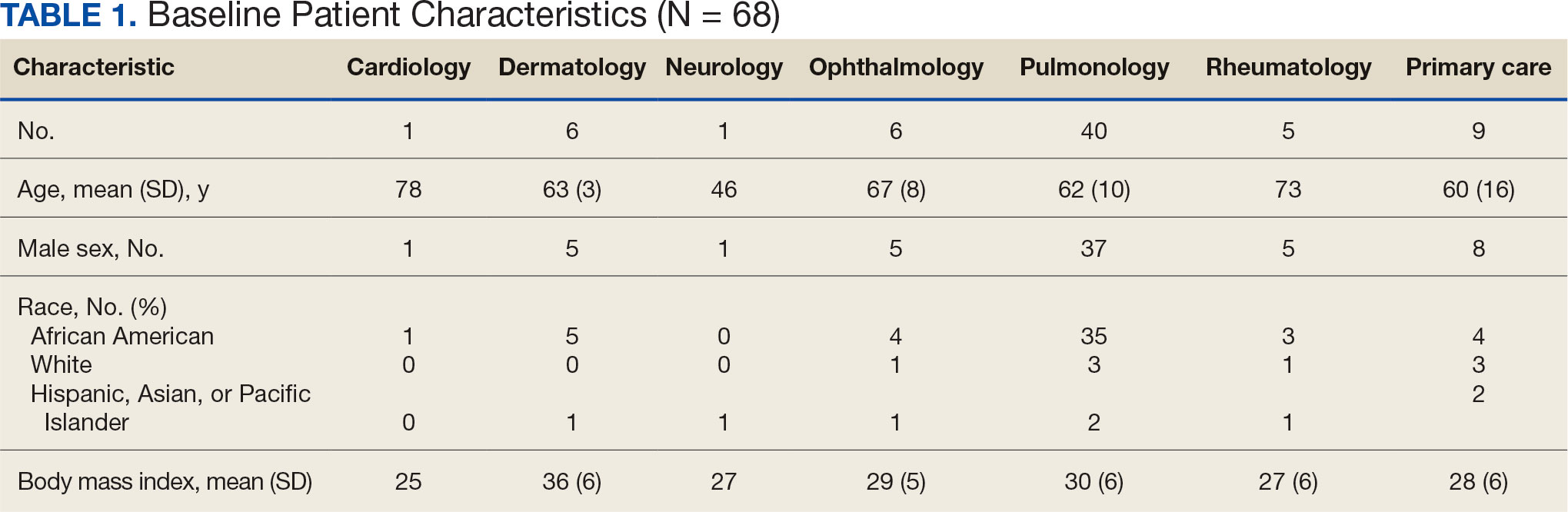
Pulmonologists predominantly prescribed initial dosage of 20 mg to 40 mg (median, 35 mg daily) (Figure). Other HCPs, including primary care, tended to prescribe prednisone < 20 mg (median, 17.5 mg; P < .05) (Table 2). The highest initial prednisone dosage was 80 mg daily, prescribed by a neurologist for a patient with neurosarcoidosis. Voortman et al recommend 20 to 40 mg prednisone daily for neurosarcoidosis.7 Both groups, pulmonologists and nonpulmonologists, had no significant differences in patient characteristics.
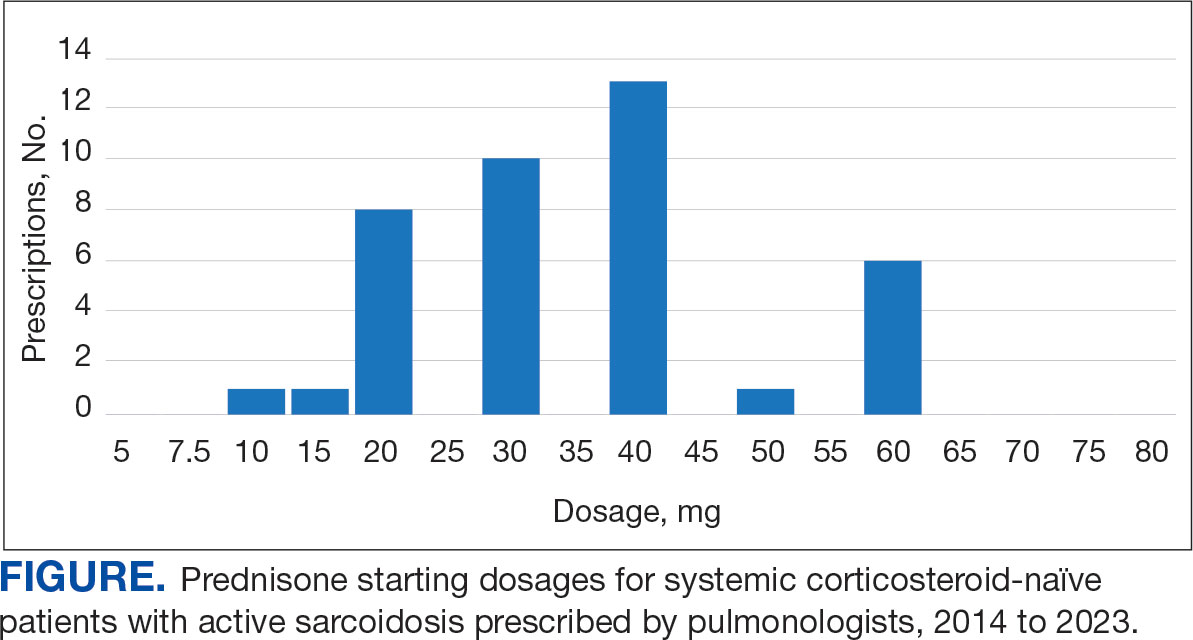
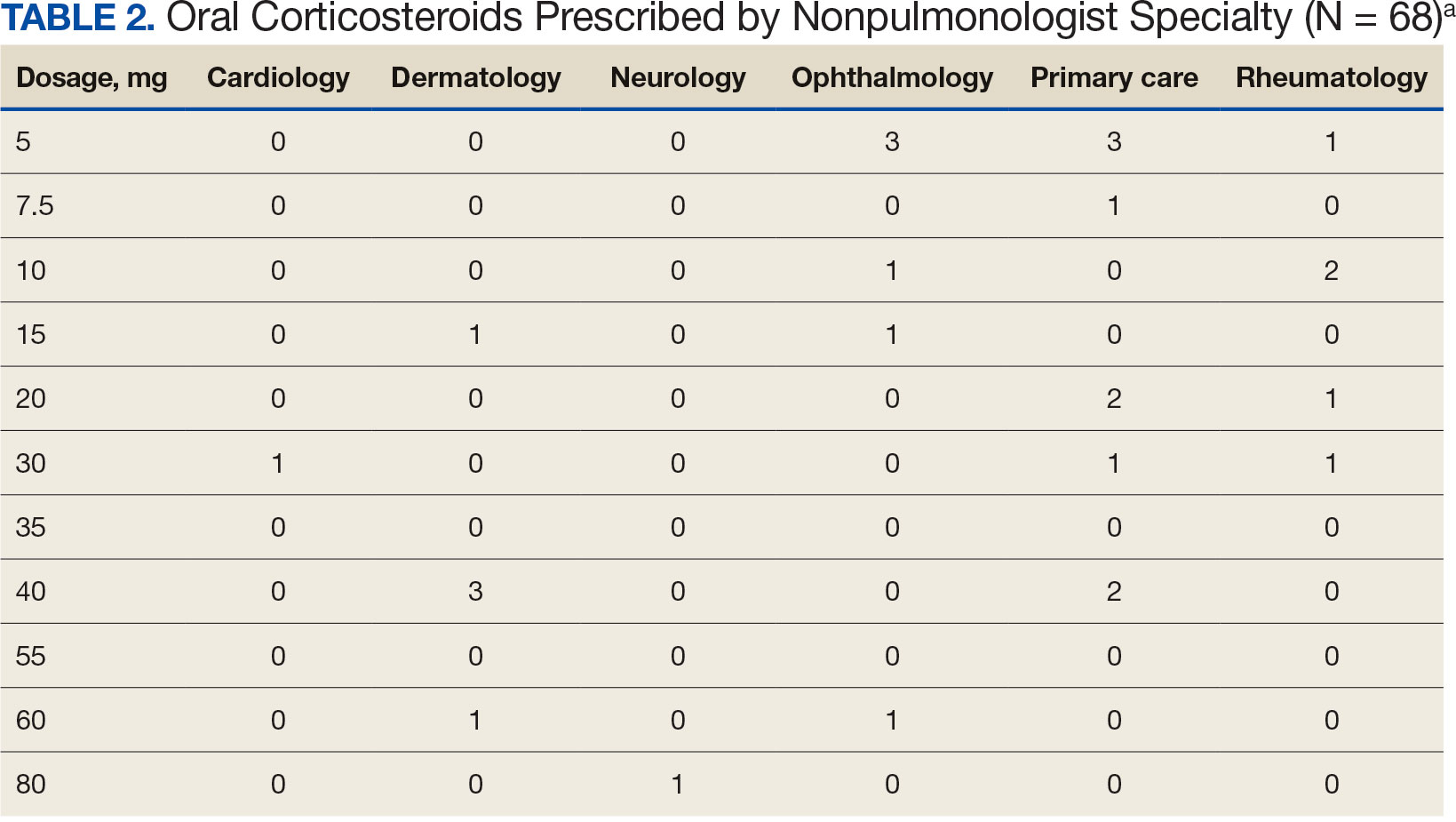
Discussion
Disparate prescription patterns of initial prednisone dosages were observed between pulmonologists and nonpulmonologists treating systemic corticosteroid-naïve patients with active sarcoidosis at JBVAMC. This study did not determine the underlying reasons for this phenomenon, nor its impact on patient outcomes.
Clinical practice guidelines have not been independently validated for each organ affected by sarcoidosis.2-4,6-10 Variations in clinical practice for other specialties may account for the variable prednisone starting dosage selection. For example, among 6 patients with active ocular sarcoidosis treated by ophthalmologists, 4 were prescribed an initial prednisone dosage of ≥ 10 mg daily. The American Academy of Ophthalmology recommends an initial short-term course of prednisone at 1 to 1.5 mg/kg daily, tapered down to the lowest effective dosage.10
Limitations
This study used a small, single-center predominantly older Black male patient cohort. The generalizability of these observations is unknown. A larger, multicenter prospective study is warranted to further evaluate these initial observations.
Conclusions
HCPs treating patients who are systemic corticosteroid-naïve with active sarcoidosis for whom prednisone is indicated should adhere to current clinical practice guidelines by prescribing prednisone in the 20 to 40 mg daily range.
- Seedahmed MI, Baugh AD, Albirair MT, et al. Epidemiology of sarcoidosis in U.S. veterans from 2003 to 2019. Ann Am Thorac Soc. 2023;20(6):797-806. doi:10.1513/AnnalsATS.202206-515OC
- Baughman RP, Valeyre D, Korsten P, et al. ERS clinical practice guidelines on treatment of sarcoidosis. Eur Respir J. 2021;58(6):2004079. doi:10.1183/13993003.04079-2020
- Rahaghi FF, Baughman RP, Saketkoo LA, et al. Delphi consensus recommendations for a treatment algorithm in pulmonary sarcoidosis. Eur Respir Rev. 2020;29(155):190146. doi:10.1183/16000617.0146-2019
- Kwon S, Judson MA. Clinical pharmacology in sarcoidosis: how to use and monitor sarcoidosis medications. J Clin Med. 2024;13(5):1250. doi:10.3390/jcm13051250
- Rice JB, White AG, Johnson M, et al. Quantitative characterization of the relationship between levels of extended corticosteroid use and related adverse events in a US population. Curr Med Res Opin. 2018;34(8):1519-1527. doi:10.1080/03007995.2018.1474090
- Rice JB, White AG, Johnson M, Wagh A, Qin Y, Bartels-Peculis L, et al. Healthcare resource use and cost associated with varying dosages of extended corticosteroid exposure in a US population. J Med Econ. 2018;21(9):846-852. doi:10.1080/13696998.2018.1474750
- Voortman M, Drent M, Baughman RP. Management of neurosarcoidosis: a clinical challenge. Curr Opin Neurol. 2019;32(3):475-483. doi:10.1097/WCO.0000000000000684
- Cheng RK, Kittleson MM, Beavers CJ, et al. Diagnosis and management of cardiac sarcoidosis: a scientific statement from the American Heart Association. Circulation. 2024;149(21):e1197-e1216. doi:10.1161/CIR.0000000000001240
- Cohen E, Lheure C, Ingen-Housz-Oro S, et al. Which firstline treatment for cutaneous sarcoidosis? A retrospective study of 120 patients. Eur J Dermatol. 2023;33(6):680-685. doi:10.1684/ejd.2023.4584
- American Academy of Ophthalmology. Ocular manifestations of sarcoidosis. EyeWiki. Accessed June 3, 2025. https://eyewiki.org/Ocular_Manifestations_of_Sarcoidosis
Sarcoidosis is a multiorgan granulomatous disorder of unknown etiology that impacts many US veterans.1 At diagnosis, clinical manifestations vary and partially depend on the extent and severity of organ involvement, particularly of the lungs, heart, and eyes.2,3 Sarcoidosis may lead to progressive organ dysfunction, long-term disability, and death.1-3 Clinical practice guidelines recommend prednisone 20 to 40 mg daily or equivalent-prednisone dose followed by a slow tapering, as first-line pharmacotherapy for patients with active sarcoidosis who are naïve to systemic corticosteroids.2-4
Use of prolonged, high-dosage prednisone (> 40 mg daily) is discouraged due to a high risk of corticosteroid-related adverse events and associated health care costs.5,6 Research suggests that initial lower prednisone dosage (< 20 mg daily) may be effective in systemic corticosteroid-naïve patients with active sarcoidosis.3
Adherence to this regimen by specialists (eg, pulmonologists, dermatologists, ophthalmologists, rheumatologists, and cardiologists) has not been established. This study sought to determine the starting dosages for prednisone prescribed at the Jesse Brown Department of Veterans Affairs Medical Center (JBVAMC) to patients with active sarcoidosis who were systemic corticosteroid-naïve.
Methods
Patient data were reviewed from the Computerized Patient Record System (CPRS) for individuals diagnosed with sarcoidosis who were corticosteroid-naïve and prescribed initial prednisone dosages by health care practitioners (HCPs) from several specialties between 2014 and 2023 at JBVAMC. This 200-bed acute care facility serves about 62,000 veterans who live in Illinois or Indiana. JBVAMC is affiliated with the University of Illinois College of Medicine at Chicago, Northwestern University Feinberg School of Medicine, and the University of Chicago Pritzker School of Medicine; many JBVAMC HCPs hold academic appointments with these medical schools.
Patient demographics, prescriber specialty, and daily starting dosage were recorded. The decision to initiate prednisone therapy and its dosage were at the discretion of HCPs who diagnosed active sarcoidosis based on compatible clinical and ancillary test findings as documented in CPRS.2-4,6-10 Statistical analyses were conducted using a t test, and a threshold of P < .05 was considered statistically significant. This study was reviewed and determined to be exempt by the JBVAMC institutional review board.
Results
Sixty-eight patients who were systemic corticosteroid- naïve and had sarcoidosis were prescribed prednisone by HCPs at JBVAMC. Fifty-two were Black (76%), 62 were male (91%), and 53 were current or former smokers (78%). The mean (SD) age was 63 (11) years (Table 1). Forty patients (59%) had lung involvement, 6 had eye (9%), 6 had skin (9%), and 5 had musculoskeletal system (7%) involvement.

Pulmonologists predominantly prescribed initial dosage of 20 mg to 40 mg (median, 35 mg daily) (Figure). Other HCPs, including primary care, tended to prescribe prednisone < 20 mg (median, 17.5 mg; P < .05) (Table 2). The highest initial prednisone dosage was 80 mg daily, prescribed by a neurologist for a patient with neurosarcoidosis. Voortman et al recommend 20 to 40 mg prednisone daily for neurosarcoidosis.7 Both groups, pulmonologists and nonpulmonologists, had no significant differences in patient characteristics.


Discussion
Disparate prescription patterns of initial prednisone dosages were observed between pulmonologists and nonpulmonologists treating systemic corticosteroid-naïve patients with active sarcoidosis at JBVAMC. This study did not determine the underlying reasons for this phenomenon, nor its impact on patient outcomes.
Clinical practice guidelines have not been independently validated for each organ affected by sarcoidosis.2-4,6-10 Variations in clinical practice for other specialties may account for the variable prednisone starting dosage selection. For example, among 6 patients with active ocular sarcoidosis treated by ophthalmologists, 4 were prescribed an initial prednisone dosage of ≥ 10 mg daily. The American Academy of Ophthalmology recommends an initial short-term course of prednisone at 1 to 1.5 mg/kg daily, tapered down to the lowest effective dosage.10
Limitations
This study used a small, single-center predominantly older Black male patient cohort. The generalizability of these observations is unknown. A larger, multicenter prospective study is warranted to further evaluate these initial observations.
Conclusions
HCPs treating patients who are systemic corticosteroid-naïve with active sarcoidosis for whom prednisone is indicated should adhere to current clinical practice guidelines by prescribing prednisone in the 20 to 40 mg daily range.
Sarcoidosis is a multiorgan granulomatous disorder of unknown etiology that impacts many US veterans.1 At diagnosis, clinical manifestations vary and partially depend on the extent and severity of organ involvement, particularly of the lungs, heart, and eyes.2,3 Sarcoidosis may lead to progressive organ dysfunction, long-term disability, and death.1-3 Clinical practice guidelines recommend prednisone 20 to 40 mg daily or equivalent-prednisone dose followed by a slow tapering, as first-line pharmacotherapy for patients with active sarcoidosis who are naïve to systemic corticosteroids.2-4
Use of prolonged, high-dosage prednisone (> 40 mg daily) is discouraged due to a high risk of corticosteroid-related adverse events and associated health care costs.5,6 Research suggests that initial lower prednisone dosage (< 20 mg daily) may be effective in systemic corticosteroid-naïve patients with active sarcoidosis.3
Adherence to this regimen by specialists (eg, pulmonologists, dermatologists, ophthalmologists, rheumatologists, and cardiologists) has not been established. This study sought to determine the starting dosages for prednisone prescribed at the Jesse Brown Department of Veterans Affairs Medical Center (JBVAMC) to patients with active sarcoidosis who were systemic corticosteroid-naïve.
Methods
Patient data were reviewed from the Computerized Patient Record System (CPRS) for individuals diagnosed with sarcoidosis who were corticosteroid-naïve and prescribed initial prednisone dosages by health care practitioners (HCPs) from several specialties between 2014 and 2023 at JBVAMC. This 200-bed acute care facility serves about 62,000 veterans who live in Illinois or Indiana. JBVAMC is affiliated with the University of Illinois College of Medicine at Chicago, Northwestern University Feinberg School of Medicine, and the University of Chicago Pritzker School of Medicine; many JBVAMC HCPs hold academic appointments with these medical schools.
Patient demographics, prescriber specialty, and daily starting dosage were recorded. The decision to initiate prednisone therapy and its dosage were at the discretion of HCPs who diagnosed active sarcoidosis based on compatible clinical and ancillary test findings as documented in CPRS.2-4,6-10 Statistical analyses were conducted using a t test, and a threshold of P < .05 was considered statistically significant. This study was reviewed and determined to be exempt by the JBVAMC institutional review board.
Results
Sixty-eight patients who were systemic corticosteroid- naïve and had sarcoidosis were prescribed prednisone by HCPs at JBVAMC. Fifty-two were Black (76%), 62 were male (91%), and 53 were current or former smokers (78%). The mean (SD) age was 63 (11) years (Table 1). Forty patients (59%) had lung involvement, 6 had eye (9%), 6 had skin (9%), and 5 had musculoskeletal system (7%) involvement.

Pulmonologists predominantly prescribed initial dosage of 20 mg to 40 mg (median, 35 mg daily) (Figure). Other HCPs, including primary care, tended to prescribe prednisone < 20 mg (median, 17.5 mg; P < .05) (Table 2). The highest initial prednisone dosage was 80 mg daily, prescribed by a neurologist for a patient with neurosarcoidosis. Voortman et al recommend 20 to 40 mg prednisone daily for neurosarcoidosis.7 Both groups, pulmonologists and nonpulmonologists, had no significant differences in patient characteristics.


Discussion
Disparate prescription patterns of initial prednisone dosages were observed between pulmonologists and nonpulmonologists treating systemic corticosteroid-naïve patients with active sarcoidosis at JBVAMC. This study did not determine the underlying reasons for this phenomenon, nor its impact on patient outcomes.
Clinical practice guidelines have not been independently validated for each organ affected by sarcoidosis.2-4,6-10 Variations in clinical practice for other specialties may account for the variable prednisone starting dosage selection. For example, among 6 patients with active ocular sarcoidosis treated by ophthalmologists, 4 were prescribed an initial prednisone dosage of ≥ 10 mg daily. The American Academy of Ophthalmology recommends an initial short-term course of prednisone at 1 to 1.5 mg/kg daily, tapered down to the lowest effective dosage.10
Limitations
This study used a small, single-center predominantly older Black male patient cohort. The generalizability of these observations is unknown. A larger, multicenter prospective study is warranted to further evaluate these initial observations.
Conclusions
HCPs treating patients who are systemic corticosteroid-naïve with active sarcoidosis for whom prednisone is indicated should adhere to current clinical practice guidelines by prescribing prednisone in the 20 to 40 mg daily range.
- Seedahmed MI, Baugh AD, Albirair MT, et al. Epidemiology of sarcoidosis in U.S. veterans from 2003 to 2019. Ann Am Thorac Soc. 2023;20(6):797-806. doi:10.1513/AnnalsATS.202206-515OC
- Baughman RP, Valeyre D, Korsten P, et al. ERS clinical practice guidelines on treatment of sarcoidosis. Eur Respir J. 2021;58(6):2004079. doi:10.1183/13993003.04079-2020
- Rahaghi FF, Baughman RP, Saketkoo LA, et al. Delphi consensus recommendations for a treatment algorithm in pulmonary sarcoidosis. Eur Respir Rev. 2020;29(155):190146. doi:10.1183/16000617.0146-2019
- Kwon S, Judson MA. Clinical pharmacology in sarcoidosis: how to use and monitor sarcoidosis medications. J Clin Med. 2024;13(5):1250. doi:10.3390/jcm13051250
- Rice JB, White AG, Johnson M, et al. Quantitative characterization of the relationship between levels of extended corticosteroid use and related adverse events in a US population. Curr Med Res Opin. 2018;34(8):1519-1527. doi:10.1080/03007995.2018.1474090
- Rice JB, White AG, Johnson M, Wagh A, Qin Y, Bartels-Peculis L, et al. Healthcare resource use and cost associated with varying dosages of extended corticosteroid exposure in a US population. J Med Econ. 2018;21(9):846-852. doi:10.1080/13696998.2018.1474750
- Voortman M, Drent M, Baughman RP. Management of neurosarcoidosis: a clinical challenge. Curr Opin Neurol. 2019;32(3):475-483. doi:10.1097/WCO.0000000000000684
- Cheng RK, Kittleson MM, Beavers CJ, et al. Diagnosis and management of cardiac sarcoidosis: a scientific statement from the American Heart Association. Circulation. 2024;149(21):e1197-e1216. doi:10.1161/CIR.0000000000001240
- Cohen E, Lheure C, Ingen-Housz-Oro S, et al. Which firstline treatment for cutaneous sarcoidosis? A retrospective study of 120 patients. Eur J Dermatol. 2023;33(6):680-685. doi:10.1684/ejd.2023.4584
- American Academy of Ophthalmology. Ocular manifestations of sarcoidosis. EyeWiki. Accessed June 3, 2025. https://eyewiki.org/Ocular_Manifestations_of_Sarcoidosis
- Seedahmed MI, Baugh AD, Albirair MT, et al. Epidemiology of sarcoidosis in U.S. veterans from 2003 to 2019. Ann Am Thorac Soc. 2023;20(6):797-806. doi:10.1513/AnnalsATS.202206-515OC
- Baughman RP, Valeyre D, Korsten P, et al. ERS clinical practice guidelines on treatment of sarcoidosis. Eur Respir J. 2021;58(6):2004079. doi:10.1183/13993003.04079-2020
- Rahaghi FF, Baughman RP, Saketkoo LA, et al. Delphi consensus recommendations for a treatment algorithm in pulmonary sarcoidosis. Eur Respir Rev. 2020;29(155):190146. doi:10.1183/16000617.0146-2019
- Kwon S, Judson MA. Clinical pharmacology in sarcoidosis: how to use and monitor sarcoidosis medications. J Clin Med. 2024;13(5):1250. doi:10.3390/jcm13051250
- Rice JB, White AG, Johnson M, et al. Quantitative characterization of the relationship between levels of extended corticosteroid use and related adverse events in a US population. Curr Med Res Opin. 2018;34(8):1519-1527. doi:10.1080/03007995.2018.1474090
- Rice JB, White AG, Johnson M, Wagh A, Qin Y, Bartels-Peculis L, et al. Healthcare resource use and cost associated with varying dosages of extended corticosteroid exposure in a US population. J Med Econ. 2018;21(9):846-852. doi:10.1080/13696998.2018.1474750
- Voortman M, Drent M, Baughman RP. Management of neurosarcoidosis: a clinical challenge. Curr Opin Neurol. 2019;32(3):475-483. doi:10.1097/WCO.0000000000000684
- Cheng RK, Kittleson MM, Beavers CJ, et al. Diagnosis and management of cardiac sarcoidosis: a scientific statement from the American Heart Association. Circulation. 2024;149(21):e1197-e1216. doi:10.1161/CIR.0000000000001240
- Cohen E, Lheure C, Ingen-Housz-Oro S, et al. Which firstline treatment for cutaneous sarcoidosis? A retrospective study of 120 patients. Eur J Dermatol. 2023;33(6):680-685. doi:10.1684/ejd.2023.4584
- American Academy of Ophthalmology. Ocular manifestations of sarcoidosis. EyeWiki. Accessed June 3, 2025. https://eyewiki.org/Ocular_Manifestations_of_Sarcoidosis
Disparate Prednisone Starting Dosages for Systemic Corticosteroid-Naïve Veterans With Active Sarcoidosis
Disparate Prednisone Starting Dosages for Systemic Corticosteroid-Naïve Veterans With Active Sarcoidosis
Successful Treatment of Tinea Versicolor With Salicylic Acid 30% Peel
Successful Treatment of Tinea Versicolor With Salicylic Acid 30% Peel
Tinea versicolor (TV) is a common, chronic, and recurrent superficial fungal infection caused by Malassezia species, most commonly Malassezia furfur (M. furfur)—a dimorphic fungus that is a part of the normal skin flora and resides in the stratum corneum.1 TV manifests as hypopigmented, hyperpigmented, or erythematous macules and patches with scaling, typically found on the trunk and proximal upper extremities. The condition is most common among young to middle-aged individuals exposed to high temperatures and humidity.1
While many cases respond to topical antifungal treatment, application can be cumbersome, particularly in large areas that are difficult to reach. An efficient and cost effective in-office treatment option could alleviate patient burden and improve satisfaction. This article presents a case of TV successfully treated with an in-office salicylic acid (SA) 30% peel, an uncommon application of this medication.
Case Presentation
An 18-year-old female active-duty US Army service member with a history of acne vulgaris presented to a dermatology clinic with a mildly pruritic rash that had been present for several weeks. An examination revealed hyperpigmented macules and patches with overlying fine scales across the patient’s back and bilateral arms (Figures 1 and 2). She reported no history of similar lesions. The patient had recently completed a military basic training course during which she wore a uniform jacket and trousers daily in hot and humid conditions. A skin scraping was obtained. Microscopic examination with potassium hydroxide preparation revealed hyphae and spores, consistent with TV.
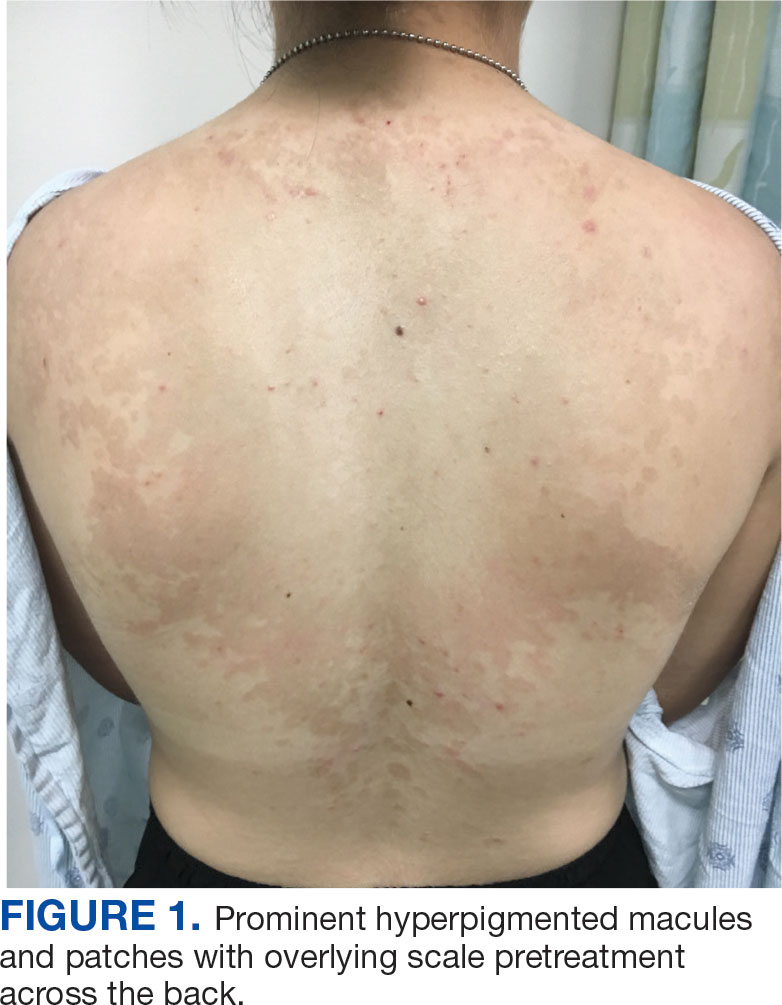
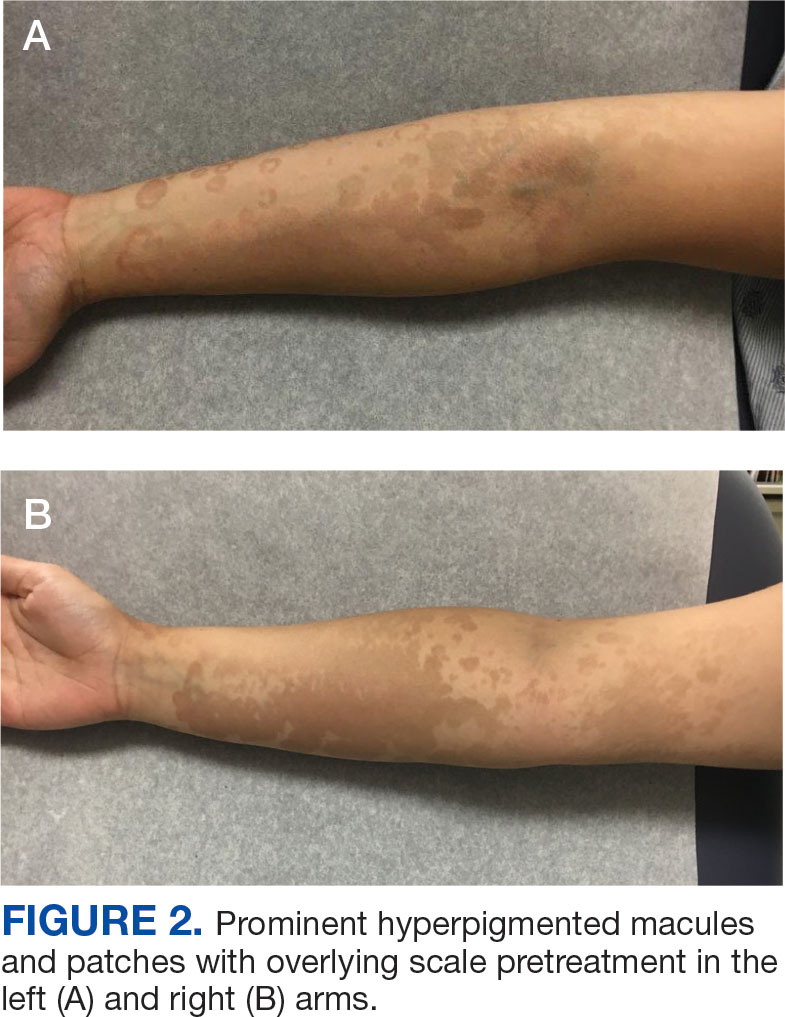
The diagnosis of TV and treatment options (topical ketoconazole 2% shampoo, topical terbinafine, or oral fluconazole) were discussed with the patient. Due to military training-related constraints, residence in the barracks, and personal preference, the patient felt unable to regularly apply topical medications to the entirety of the affected area and preferred to avoid oral medication. The decision was made to pursue in-clinic treatment with a SA 30% peel. The affected areas (back and bilateral arms) were thoroughly cleansed and prepped with alcohol. SA 30% in hydroethanolic solution was applied evenly to the affected area. The patient was observed for pseudofrosting, a precipitation of SA crystals that indicates peel completion (Figure 3). The peel was left in place, as it is self-neutralizing, and the patient was instructed to shower that same day with a gentle cleanser. This procedure was repeated 10 days later. Both treatments were well tolerated, with only a transient burning sensation reported during the application. At 3-week follow-up, the patient presented with complete resolution of her arm lesions and significant improvement of the back lesions (Figures 4 and 5). She also reported improvement in the acne vulgaris on her back.
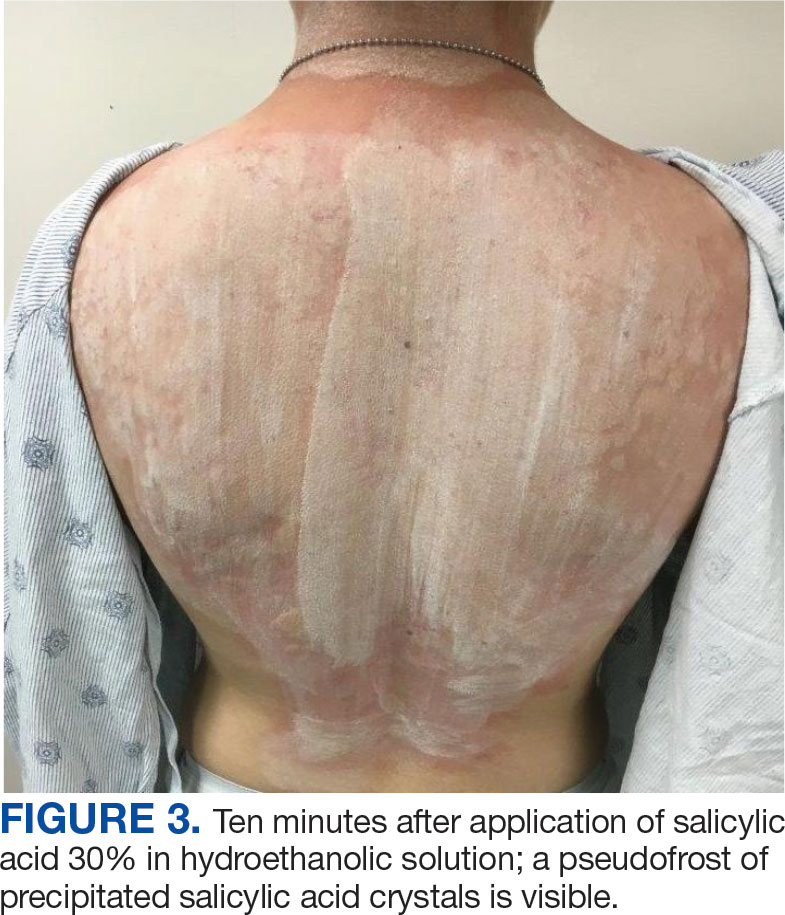
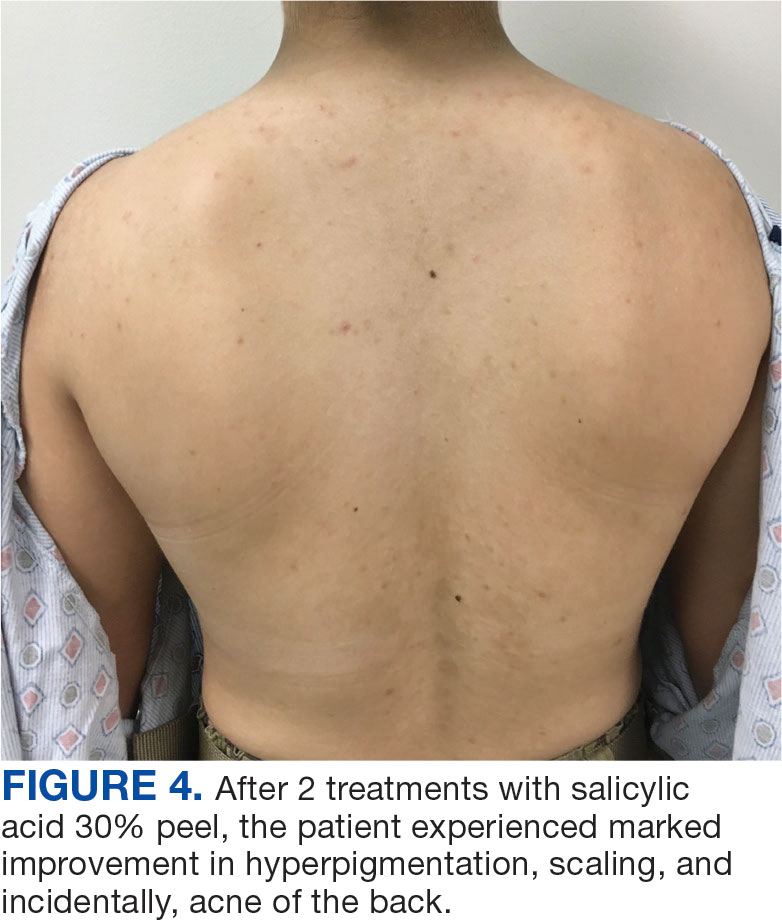
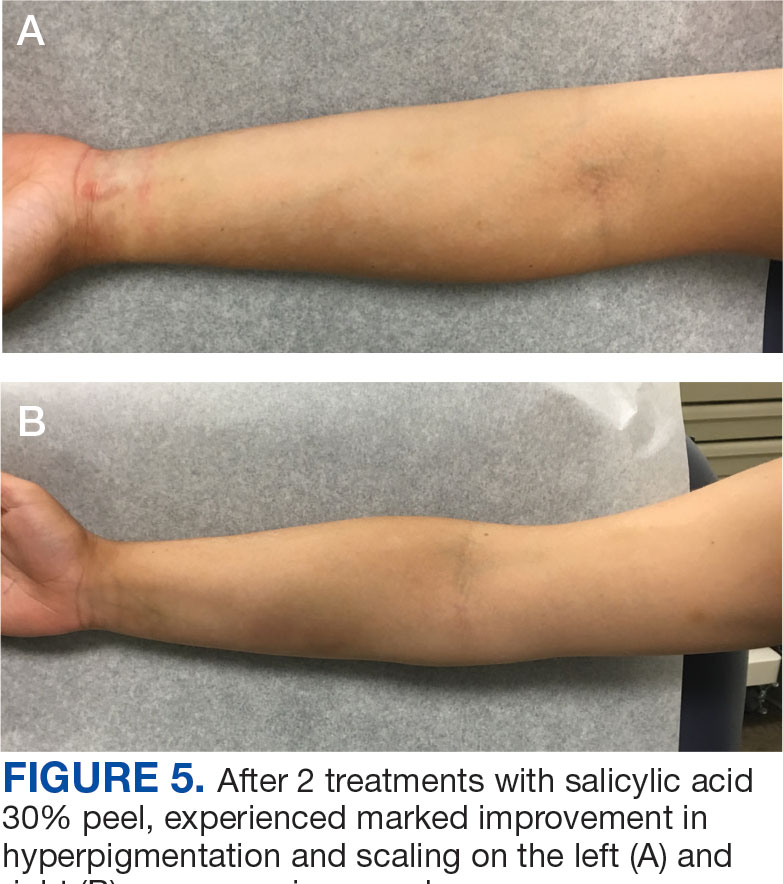
Discussion
SA 30% is a lipid-soluble hydroxybenzoic acid with comedolytic and desmolytic qualities. This results in the disruption of epidermal cell cohesion and promotes exfoliation.2 Lipophilic properties allow SA to penetrate sebaceous glands and disrupt sebum production, making it particularly effective in seborrheic conditions such as acne. This mechanism may have increased therapeutic effect in this case.3 Additionally, as a salicylate, SA possesses anti-inflammatory properties, though this effect is most pronounced at lower concentrations. SA 30% is considered a superficial peel, as the depth of chemexfoliation is limited to the epidermis.3 A modified SA preparation is a safe and effective treatment for moderate-to-severe acne vulgaris. The apparent pseudofrost during application is due to precipitated SA, rather than the precipitation of dermal proteins seen in deeper peels, such as trichloroacetic acid.2 Unlike glycolic or pyruvic acid peels, SA does not require neutralization.
SA is cost-effective and has been used safely in all skin types to treat various epidermal conditions, including acne vulgaris, melasma, photodamage, freckles, lentigines and postinflammatory hyperpigmentation (PIH).2 Mild adverse effects occur in about 15% to 30% of patients and include prolonged erythema, intense exfoliation, dryness, crusting, and pigmentary dyschromias. Rare adverse effects include systemic toxicity (salicylism) and hypoglycemia. Contraindications to SA 30% peels include history of allergy to salicylates, active bacterial or viral infection, dermatitis in the treatment area, pregnancy, and skin malignancy.2
Chemical peels are typically used with caution in patients with skin of color due to a higher risk of PIH. However, SA 30% has been shown to be safe and effective in these populations.4 A study by Grimes found that 88% of patients with Fitzpatrick skin types V and VI experienced significant improvement in PIH, melasma, or enlarged pores with minimal to no adverse effects.4 Subsequent larger studies have reinforced these findings. In a study involving 250 patients with Fitzpatrick skin types IV and V, no patients experienced PIH, confirming the safety of SA in darker skin tones. This is likely due to the superficial nature of the peel, which does not affect the basal layer of the epidermis where melanocytes reside, reducing the risk of pigmentary complications. Additionally, SA peels are self-neutralizing, unlike glycolic or trichloroacetic acid peels, which require manual neutralization and carry a higher risk of PIH if not neutralized properly.5
SA has been as shown to be a moderately successful treatment for PIH. The Grimes study found that 4 of 5 patients with Fitzpatrick skin types IV and V saw a 75% improvement in PIH after SA peels.4 Davis et al found a nonsignificant trend toward skin lightening in Korean adults treated for acne and PIH, with significant decreases in erythema and improvements in greasiness, dryness, and scaliness.6 Importantly, the risk of PIH following TV is higher in patients with skin of color.7 SA may be effective in treating TV and PIH, offering a multifactorial approach by addressing both conditions while posing a low risk for causing PIH.8
TV and other Malassezia spp infections are common concerns in dermatology and primary care, with Malassezia-associated superficial mycoses (eg, dandruff, pityriasis versicolor, and folliculitis) affecting up to 50% of the population worldwide.9 Despite this, there has been little recent advancement in antifungal treatments. Ketoconazole, terbinafine, and fluconazole have been in use since the 1980s and 1990s.8 Most antifungal drugs target ergosterol, a component of the fungal cell wall.10 Additionally, Malassezia spp have been increasingly reported to cause invasive infections in immunocompromised patients.11 Given the rise in antifungal resistance, the judicious use of antifungals and implementation of novel treatment strategies is essential.
While SA lacks intrinsic antifungal properties, different combinations (Whitfield ointment consisting of 3% SA and 6% benzoic acid; 2% sulfur and 2% SA) have been effective in the treatment of TV.1 It is theorized that the effectiveness of SA against TV is due to its ability to exfoliate and acidify the stratum corneum, the natural habitat of M. furfur.
SA also reduces sebum production by downregulating sebocyte lipogenesis via the sterol regulatory element-binding protein-1 pathway and suppressing the nuclear factor κB (NF-κB) pathway, a key pathway in inflammation.12 These mechanisms make SA an effective acne treatment. Additionally, M. furfur is a lipid-dependent yeast, thus the decreased lipogenesis by sebocytes may be beneficial in treating TV as well.12 A study of 25 patients with TV in India found that 88% achieved clinical and microbiological cure after 4 once-weekly treatments of a SA 30% peel.8
In a study of deployed military personnel, fungal infections affected about 11% of participants.13 Contributing factors to the development of fungal infections included excessive sweating, humid conditions, and limited access to hygiene facilities. In such settings, traditional antifungal therapies may be less effective or challenging to adhere to, making alternative treatments more desirable. SA peels could offer a practical solution in these circumstances, as they are easily applied in the clinic, require no neutralization or downtime, and do not require the patient to apply medications between visits.
In this case, the patient demonstrated significant improvement with 2 SA peels, with noted improvement in her acne. SA 30% peel was highlighted as a useful treatment option for patients with TV who struggle with topical medication adherence; furthermore, it may be particularly beneficial for patients with concomitant acne.
Conclusions
This case demonstrates the successful use of in-office SA 30% peel as a treatment for TV. The rapid improvement and resolution of lesions with minimal adverse effects suggest that SA peel may serve as a valuable alternative for patients with extensive disease in difficult-to-reach affected areas, or those who are dissatisfied with traditional therapies. Additionally, the concurrent improvement of the patient’s back acne underscores the dual therapeutic potential of this treatment. Given the ease of application, cost effectiveness, and favorable safety profile, SA 30% peel is a viable option in the management of TV, especially in cases where topical or oral antifungals are impractical. Further studies could help establish standardized protocols and assess long-term outcomes of this treatment modality.
- Leung AK, Barankin B, Lam JM, et al. Tinea versicolor: an updated review. Drugs Context. 2022;11:2022-9-2. doi:10.7573/dic.2022-9-2
- Arif T. Salicylic acid as a peeling agent: a comprehensive review. Clin Cosmet Investig Dermatol. 2015;8:455-461. doi:10.2147/CCID.S84765
- Shao X, Chen Y, Zhang L, et al. Effect of 30% supramolecular salicylic acid peel on skin microbiota and inflammation in patients with moderate-to-severe acne vulgaris. Dermatol Ther. 2022;13(1):155-168. doi:10.1007/s13555-022-00844-5
- Grimes PE. The safety and efficacy of salicylic acid chemical peels in darker racial-ethnic groups. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 1999;25(1). doi:10.1046/j.1524-4725.1999.08145.x
- Kang HY, Choi Y, Cho HJ. Salicylic acid peels for the treatment of acne vulgaris in Fitzpatrick skin types IV-V: a multicenter study. Dermatol Surg. Published online 2006. doi:10.1111/j.1524-4725.2006.32146.x.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation. J Clin Aesthetic Dermatol. 2010;3(7):20-31.
- Kallini JR, Riaz F, Khachemoune A. Tinea versicolor in dark-skinned individuals. Int J Dermatol. 2014;53(2):137- 141. doi:10.1111/ijd.12345
- Saoji V, Madke B. Efficacy of salicylic acid peel in dermatophytosis. Indian J Dermatol Venereol Leprol. 2021;87(5). doi:10.4103/ijdvl.IJDVL_853_18
- Arce M, Gutiérrez-Mendoza D. Pityriasis versicolor: treatment update. Curr Fungal Infect Rep 2018;12(11):195–200. https://doi.org/10.1007/s12281-018-0328-7
- Leong C, Kit JCW, Lee SM, et al. Azole resistance mechanisms in pathogenic M. furfur. Antimicrob Agents Chemother. 2021;65(5):e01975-20. doi:10.1128/AAC.01975-20
- Chang HJ, Miller HL, Watkins N, et al. An epidemic of Malassezia pachydermatis in an intensive care nursery associated with colonization of health care workers’ pet dogs. N Engl J Med. 1998;338(11):706-711. doi:10.1056/NEJM199803123381102
- Lu J, Cong T, Wen X, et al. Salicylic acid treats acne vulgaris by suppressing AMPK/SREBP1 pathway in sebocytes. Exp Dermatol. 2019;28(7):786-794. doi:10.1111/exd.13934
- Singal A, Lipner SR. A review of skin disease in military soldiers: challenges and potential solutions. Ann Med. 2023;55(2):2267425. doi:10.1080/07853890.2023.2267425
Tinea versicolor (TV) is a common, chronic, and recurrent superficial fungal infection caused by Malassezia species, most commonly Malassezia furfur (M. furfur)—a dimorphic fungus that is a part of the normal skin flora and resides in the stratum corneum.1 TV manifests as hypopigmented, hyperpigmented, or erythematous macules and patches with scaling, typically found on the trunk and proximal upper extremities. The condition is most common among young to middle-aged individuals exposed to high temperatures and humidity.1
While many cases respond to topical antifungal treatment, application can be cumbersome, particularly in large areas that are difficult to reach. An efficient and cost effective in-office treatment option could alleviate patient burden and improve satisfaction. This article presents a case of TV successfully treated with an in-office salicylic acid (SA) 30% peel, an uncommon application of this medication.
Case Presentation
An 18-year-old female active-duty US Army service member with a history of acne vulgaris presented to a dermatology clinic with a mildly pruritic rash that had been present for several weeks. An examination revealed hyperpigmented macules and patches with overlying fine scales across the patient’s back and bilateral arms (Figures 1 and 2). She reported no history of similar lesions. The patient had recently completed a military basic training course during which she wore a uniform jacket and trousers daily in hot and humid conditions. A skin scraping was obtained. Microscopic examination with potassium hydroxide preparation revealed hyphae and spores, consistent with TV.


The diagnosis of TV and treatment options (topical ketoconazole 2% shampoo, topical terbinafine, or oral fluconazole) were discussed with the patient. Due to military training-related constraints, residence in the barracks, and personal preference, the patient felt unable to regularly apply topical medications to the entirety of the affected area and preferred to avoid oral medication. The decision was made to pursue in-clinic treatment with a SA 30% peel. The affected areas (back and bilateral arms) were thoroughly cleansed and prepped with alcohol. SA 30% in hydroethanolic solution was applied evenly to the affected area. The patient was observed for pseudofrosting, a precipitation of SA crystals that indicates peel completion (Figure 3). The peel was left in place, as it is self-neutralizing, and the patient was instructed to shower that same day with a gentle cleanser. This procedure was repeated 10 days later. Both treatments were well tolerated, with only a transient burning sensation reported during the application. At 3-week follow-up, the patient presented with complete resolution of her arm lesions and significant improvement of the back lesions (Figures 4 and 5). She also reported improvement in the acne vulgaris on her back.



Discussion
SA 30% is a lipid-soluble hydroxybenzoic acid with comedolytic and desmolytic qualities. This results in the disruption of epidermal cell cohesion and promotes exfoliation.2 Lipophilic properties allow SA to penetrate sebaceous glands and disrupt sebum production, making it particularly effective in seborrheic conditions such as acne. This mechanism may have increased therapeutic effect in this case.3 Additionally, as a salicylate, SA possesses anti-inflammatory properties, though this effect is most pronounced at lower concentrations. SA 30% is considered a superficial peel, as the depth of chemexfoliation is limited to the epidermis.3 A modified SA preparation is a safe and effective treatment for moderate-to-severe acne vulgaris. The apparent pseudofrost during application is due to precipitated SA, rather than the precipitation of dermal proteins seen in deeper peels, such as trichloroacetic acid.2 Unlike glycolic or pyruvic acid peels, SA does not require neutralization.
SA is cost-effective and has been used safely in all skin types to treat various epidermal conditions, including acne vulgaris, melasma, photodamage, freckles, lentigines and postinflammatory hyperpigmentation (PIH).2 Mild adverse effects occur in about 15% to 30% of patients and include prolonged erythema, intense exfoliation, dryness, crusting, and pigmentary dyschromias. Rare adverse effects include systemic toxicity (salicylism) and hypoglycemia. Contraindications to SA 30% peels include history of allergy to salicylates, active bacterial or viral infection, dermatitis in the treatment area, pregnancy, and skin malignancy.2
Chemical peels are typically used with caution in patients with skin of color due to a higher risk of PIH. However, SA 30% has been shown to be safe and effective in these populations.4 A study by Grimes found that 88% of patients with Fitzpatrick skin types V and VI experienced significant improvement in PIH, melasma, or enlarged pores with minimal to no adverse effects.4 Subsequent larger studies have reinforced these findings. In a study involving 250 patients with Fitzpatrick skin types IV and V, no patients experienced PIH, confirming the safety of SA in darker skin tones. This is likely due to the superficial nature of the peel, which does not affect the basal layer of the epidermis where melanocytes reside, reducing the risk of pigmentary complications. Additionally, SA peels are self-neutralizing, unlike glycolic or trichloroacetic acid peels, which require manual neutralization and carry a higher risk of PIH if not neutralized properly.5
SA has been as shown to be a moderately successful treatment for PIH. The Grimes study found that 4 of 5 patients with Fitzpatrick skin types IV and V saw a 75% improvement in PIH after SA peels.4 Davis et al found a nonsignificant trend toward skin lightening in Korean adults treated for acne and PIH, with significant decreases in erythema and improvements in greasiness, dryness, and scaliness.6 Importantly, the risk of PIH following TV is higher in patients with skin of color.7 SA may be effective in treating TV and PIH, offering a multifactorial approach by addressing both conditions while posing a low risk for causing PIH.8
TV and other Malassezia spp infections are common concerns in dermatology and primary care, with Malassezia-associated superficial mycoses (eg, dandruff, pityriasis versicolor, and folliculitis) affecting up to 50% of the population worldwide.9 Despite this, there has been little recent advancement in antifungal treatments. Ketoconazole, terbinafine, and fluconazole have been in use since the 1980s and 1990s.8 Most antifungal drugs target ergosterol, a component of the fungal cell wall.10 Additionally, Malassezia spp have been increasingly reported to cause invasive infections in immunocompromised patients.11 Given the rise in antifungal resistance, the judicious use of antifungals and implementation of novel treatment strategies is essential.
While SA lacks intrinsic antifungal properties, different combinations (Whitfield ointment consisting of 3% SA and 6% benzoic acid; 2% sulfur and 2% SA) have been effective in the treatment of TV.1 It is theorized that the effectiveness of SA against TV is due to its ability to exfoliate and acidify the stratum corneum, the natural habitat of M. furfur.
SA also reduces sebum production by downregulating sebocyte lipogenesis via the sterol regulatory element-binding protein-1 pathway and suppressing the nuclear factor κB (NF-κB) pathway, a key pathway in inflammation.12 These mechanisms make SA an effective acne treatment. Additionally, M. furfur is a lipid-dependent yeast, thus the decreased lipogenesis by sebocytes may be beneficial in treating TV as well.12 A study of 25 patients with TV in India found that 88% achieved clinical and microbiological cure after 4 once-weekly treatments of a SA 30% peel.8
In a study of deployed military personnel, fungal infections affected about 11% of participants.13 Contributing factors to the development of fungal infections included excessive sweating, humid conditions, and limited access to hygiene facilities. In such settings, traditional antifungal therapies may be less effective or challenging to adhere to, making alternative treatments more desirable. SA peels could offer a practical solution in these circumstances, as they are easily applied in the clinic, require no neutralization or downtime, and do not require the patient to apply medications between visits.
In this case, the patient demonstrated significant improvement with 2 SA peels, with noted improvement in her acne. SA 30% peel was highlighted as a useful treatment option for patients with TV who struggle with topical medication adherence; furthermore, it may be particularly beneficial for patients with concomitant acne.
Conclusions
This case demonstrates the successful use of in-office SA 30% peel as a treatment for TV. The rapid improvement and resolution of lesions with minimal adverse effects suggest that SA peel may serve as a valuable alternative for patients with extensive disease in difficult-to-reach affected areas, or those who are dissatisfied with traditional therapies. Additionally, the concurrent improvement of the patient’s back acne underscores the dual therapeutic potential of this treatment. Given the ease of application, cost effectiveness, and favorable safety profile, SA 30% peel is a viable option in the management of TV, especially in cases where topical or oral antifungals are impractical. Further studies could help establish standardized protocols and assess long-term outcomes of this treatment modality.
Tinea versicolor (TV) is a common, chronic, and recurrent superficial fungal infection caused by Malassezia species, most commonly Malassezia furfur (M. furfur)—a dimorphic fungus that is a part of the normal skin flora and resides in the stratum corneum.1 TV manifests as hypopigmented, hyperpigmented, or erythematous macules and patches with scaling, typically found on the trunk and proximal upper extremities. The condition is most common among young to middle-aged individuals exposed to high temperatures and humidity.1
While many cases respond to topical antifungal treatment, application can be cumbersome, particularly in large areas that are difficult to reach. An efficient and cost effective in-office treatment option could alleviate patient burden and improve satisfaction. This article presents a case of TV successfully treated with an in-office salicylic acid (SA) 30% peel, an uncommon application of this medication.
Case Presentation
An 18-year-old female active-duty US Army service member with a history of acne vulgaris presented to a dermatology clinic with a mildly pruritic rash that had been present for several weeks. An examination revealed hyperpigmented macules and patches with overlying fine scales across the patient’s back and bilateral arms (Figures 1 and 2). She reported no history of similar lesions. The patient had recently completed a military basic training course during which she wore a uniform jacket and trousers daily in hot and humid conditions. A skin scraping was obtained. Microscopic examination with potassium hydroxide preparation revealed hyphae and spores, consistent with TV.


The diagnosis of TV and treatment options (topical ketoconazole 2% shampoo, topical terbinafine, or oral fluconazole) were discussed with the patient. Due to military training-related constraints, residence in the barracks, and personal preference, the patient felt unable to regularly apply topical medications to the entirety of the affected area and preferred to avoid oral medication. The decision was made to pursue in-clinic treatment with a SA 30% peel. The affected areas (back and bilateral arms) were thoroughly cleansed and prepped with alcohol. SA 30% in hydroethanolic solution was applied evenly to the affected area. The patient was observed for pseudofrosting, a precipitation of SA crystals that indicates peel completion (Figure 3). The peel was left in place, as it is self-neutralizing, and the patient was instructed to shower that same day with a gentle cleanser. This procedure was repeated 10 days later. Both treatments were well tolerated, with only a transient burning sensation reported during the application. At 3-week follow-up, the patient presented with complete resolution of her arm lesions and significant improvement of the back lesions (Figures 4 and 5). She also reported improvement in the acne vulgaris on her back.



Discussion
SA 30% is a lipid-soluble hydroxybenzoic acid with comedolytic and desmolytic qualities. This results in the disruption of epidermal cell cohesion and promotes exfoliation.2 Lipophilic properties allow SA to penetrate sebaceous glands and disrupt sebum production, making it particularly effective in seborrheic conditions such as acne. This mechanism may have increased therapeutic effect in this case.3 Additionally, as a salicylate, SA possesses anti-inflammatory properties, though this effect is most pronounced at lower concentrations. SA 30% is considered a superficial peel, as the depth of chemexfoliation is limited to the epidermis.3 A modified SA preparation is a safe and effective treatment for moderate-to-severe acne vulgaris. The apparent pseudofrost during application is due to precipitated SA, rather than the precipitation of dermal proteins seen in deeper peels, such as trichloroacetic acid.2 Unlike glycolic or pyruvic acid peels, SA does not require neutralization.
SA is cost-effective and has been used safely in all skin types to treat various epidermal conditions, including acne vulgaris, melasma, photodamage, freckles, lentigines and postinflammatory hyperpigmentation (PIH).2 Mild adverse effects occur in about 15% to 30% of patients and include prolonged erythema, intense exfoliation, dryness, crusting, and pigmentary dyschromias. Rare adverse effects include systemic toxicity (salicylism) and hypoglycemia. Contraindications to SA 30% peels include history of allergy to salicylates, active bacterial or viral infection, dermatitis in the treatment area, pregnancy, and skin malignancy.2
Chemical peels are typically used with caution in patients with skin of color due to a higher risk of PIH. However, SA 30% has been shown to be safe and effective in these populations.4 A study by Grimes found that 88% of patients with Fitzpatrick skin types V and VI experienced significant improvement in PIH, melasma, or enlarged pores with minimal to no adverse effects.4 Subsequent larger studies have reinforced these findings. In a study involving 250 patients with Fitzpatrick skin types IV and V, no patients experienced PIH, confirming the safety of SA in darker skin tones. This is likely due to the superficial nature of the peel, which does not affect the basal layer of the epidermis where melanocytes reside, reducing the risk of pigmentary complications. Additionally, SA peels are self-neutralizing, unlike glycolic or trichloroacetic acid peels, which require manual neutralization and carry a higher risk of PIH if not neutralized properly.5
SA has been as shown to be a moderately successful treatment for PIH. The Grimes study found that 4 of 5 patients with Fitzpatrick skin types IV and V saw a 75% improvement in PIH after SA peels.4 Davis et al found a nonsignificant trend toward skin lightening in Korean adults treated for acne and PIH, with significant decreases in erythema and improvements in greasiness, dryness, and scaliness.6 Importantly, the risk of PIH following TV is higher in patients with skin of color.7 SA may be effective in treating TV and PIH, offering a multifactorial approach by addressing both conditions while posing a low risk for causing PIH.8
TV and other Malassezia spp infections are common concerns in dermatology and primary care, with Malassezia-associated superficial mycoses (eg, dandruff, pityriasis versicolor, and folliculitis) affecting up to 50% of the population worldwide.9 Despite this, there has been little recent advancement in antifungal treatments. Ketoconazole, terbinafine, and fluconazole have been in use since the 1980s and 1990s.8 Most antifungal drugs target ergosterol, a component of the fungal cell wall.10 Additionally, Malassezia spp have been increasingly reported to cause invasive infections in immunocompromised patients.11 Given the rise in antifungal resistance, the judicious use of antifungals and implementation of novel treatment strategies is essential.
While SA lacks intrinsic antifungal properties, different combinations (Whitfield ointment consisting of 3% SA and 6% benzoic acid; 2% sulfur and 2% SA) have been effective in the treatment of TV.1 It is theorized that the effectiveness of SA against TV is due to its ability to exfoliate and acidify the stratum corneum, the natural habitat of M. furfur.
SA also reduces sebum production by downregulating sebocyte lipogenesis via the sterol regulatory element-binding protein-1 pathway and suppressing the nuclear factor κB (NF-κB) pathway, a key pathway in inflammation.12 These mechanisms make SA an effective acne treatment. Additionally, M. furfur is a lipid-dependent yeast, thus the decreased lipogenesis by sebocytes may be beneficial in treating TV as well.12 A study of 25 patients with TV in India found that 88% achieved clinical and microbiological cure after 4 once-weekly treatments of a SA 30% peel.8
In a study of deployed military personnel, fungal infections affected about 11% of participants.13 Contributing factors to the development of fungal infections included excessive sweating, humid conditions, and limited access to hygiene facilities. In such settings, traditional antifungal therapies may be less effective or challenging to adhere to, making alternative treatments more desirable. SA peels could offer a practical solution in these circumstances, as they are easily applied in the clinic, require no neutralization or downtime, and do not require the patient to apply medications between visits.
In this case, the patient demonstrated significant improvement with 2 SA peels, with noted improvement in her acne. SA 30% peel was highlighted as a useful treatment option for patients with TV who struggle with topical medication adherence; furthermore, it may be particularly beneficial for patients with concomitant acne.
Conclusions
This case demonstrates the successful use of in-office SA 30% peel as a treatment for TV. The rapid improvement and resolution of lesions with minimal adverse effects suggest that SA peel may serve as a valuable alternative for patients with extensive disease in difficult-to-reach affected areas, or those who are dissatisfied with traditional therapies. Additionally, the concurrent improvement of the patient’s back acne underscores the dual therapeutic potential of this treatment. Given the ease of application, cost effectiveness, and favorable safety profile, SA 30% peel is a viable option in the management of TV, especially in cases where topical or oral antifungals are impractical. Further studies could help establish standardized protocols and assess long-term outcomes of this treatment modality.
- Leung AK, Barankin B, Lam JM, et al. Tinea versicolor: an updated review. Drugs Context. 2022;11:2022-9-2. doi:10.7573/dic.2022-9-2
- Arif T. Salicylic acid as a peeling agent: a comprehensive review. Clin Cosmet Investig Dermatol. 2015;8:455-461. doi:10.2147/CCID.S84765
- Shao X, Chen Y, Zhang L, et al. Effect of 30% supramolecular salicylic acid peel on skin microbiota and inflammation in patients with moderate-to-severe acne vulgaris. Dermatol Ther. 2022;13(1):155-168. doi:10.1007/s13555-022-00844-5
- Grimes PE. The safety and efficacy of salicylic acid chemical peels in darker racial-ethnic groups. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 1999;25(1). doi:10.1046/j.1524-4725.1999.08145.x
- Kang HY, Choi Y, Cho HJ. Salicylic acid peels for the treatment of acne vulgaris in Fitzpatrick skin types IV-V: a multicenter study. Dermatol Surg. Published online 2006. doi:10.1111/j.1524-4725.2006.32146.x.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation. J Clin Aesthetic Dermatol. 2010;3(7):20-31.
- Kallini JR, Riaz F, Khachemoune A. Tinea versicolor in dark-skinned individuals. Int J Dermatol. 2014;53(2):137- 141. doi:10.1111/ijd.12345
- Saoji V, Madke B. Efficacy of salicylic acid peel in dermatophytosis. Indian J Dermatol Venereol Leprol. 2021;87(5). doi:10.4103/ijdvl.IJDVL_853_18
- Arce M, Gutiérrez-Mendoza D. Pityriasis versicolor: treatment update. Curr Fungal Infect Rep 2018;12(11):195–200. https://doi.org/10.1007/s12281-018-0328-7
- Leong C, Kit JCW, Lee SM, et al. Azole resistance mechanisms in pathogenic M. furfur. Antimicrob Agents Chemother. 2021;65(5):e01975-20. doi:10.1128/AAC.01975-20
- Chang HJ, Miller HL, Watkins N, et al. An epidemic of Malassezia pachydermatis in an intensive care nursery associated with colonization of health care workers’ pet dogs. N Engl J Med. 1998;338(11):706-711. doi:10.1056/NEJM199803123381102
- Lu J, Cong T, Wen X, et al. Salicylic acid treats acne vulgaris by suppressing AMPK/SREBP1 pathway in sebocytes. Exp Dermatol. 2019;28(7):786-794. doi:10.1111/exd.13934
- Singal A, Lipner SR. A review of skin disease in military soldiers: challenges and potential solutions. Ann Med. 2023;55(2):2267425. doi:10.1080/07853890.2023.2267425
- Leung AK, Barankin B, Lam JM, et al. Tinea versicolor: an updated review. Drugs Context. 2022;11:2022-9-2. doi:10.7573/dic.2022-9-2
- Arif T. Salicylic acid as a peeling agent: a comprehensive review. Clin Cosmet Investig Dermatol. 2015;8:455-461. doi:10.2147/CCID.S84765
- Shao X, Chen Y, Zhang L, et al. Effect of 30% supramolecular salicylic acid peel on skin microbiota and inflammation in patients with moderate-to-severe acne vulgaris. Dermatol Ther. 2022;13(1):155-168. doi:10.1007/s13555-022-00844-5
- Grimes PE. The safety and efficacy of salicylic acid chemical peels in darker racial-ethnic groups. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 1999;25(1). doi:10.1046/j.1524-4725.1999.08145.x
- Kang HY, Choi Y, Cho HJ. Salicylic acid peels for the treatment of acne vulgaris in Fitzpatrick skin types IV-V: a multicenter study. Dermatol Surg. Published online 2006. doi:10.1111/j.1524-4725.2006.32146.x.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation. J Clin Aesthetic Dermatol. 2010;3(7):20-31.
- Kallini JR, Riaz F, Khachemoune A. Tinea versicolor in dark-skinned individuals. Int J Dermatol. 2014;53(2):137- 141. doi:10.1111/ijd.12345
- Saoji V, Madke B. Efficacy of salicylic acid peel in dermatophytosis. Indian J Dermatol Venereol Leprol. 2021;87(5). doi:10.4103/ijdvl.IJDVL_853_18
- Arce M, Gutiérrez-Mendoza D. Pityriasis versicolor: treatment update. Curr Fungal Infect Rep 2018;12(11):195–200. https://doi.org/10.1007/s12281-018-0328-7
- Leong C, Kit JCW, Lee SM, et al. Azole resistance mechanisms in pathogenic M. furfur. Antimicrob Agents Chemother. 2021;65(5):e01975-20. doi:10.1128/AAC.01975-20
- Chang HJ, Miller HL, Watkins N, et al. An epidemic of Malassezia pachydermatis in an intensive care nursery associated with colonization of health care workers’ pet dogs. N Engl J Med. 1998;338(11):706-711. doi:10.1056/NEJM199803123381102
- Lu J, Cong T, Wen X, et al. Salicylic acid treats acne vulgaris by suppressing AMPK/SREBP1 pathway in sebocytes. Exp Dermatol. 2019;28(7):786-794. doi:10.1111/exd.13934
- Singal A, Lipner SR. A review of skin disease in military soldiers: challenges and potential solutions. Ann Med. 2023;55(2):2267425. doi:10.1080/07853890.2023.2267425
Successful Treatment of Tinea Versicolor With Salicylic Acid 30% Peel
Successful Treatment of Tinea Versicolor With Salicylic Acid 30% Peel
The Gut Microbiome and Cardiac Arrhythmias
The Gut Microbiome and Cardiac Arrhythmias
The extensive surface of the gastrointestinal tract presents an interface between the human body and its environment. Residing within the intestinal lumen, ingested food and various microorganisms are an essential aspect of this relationship. The trillions of microorganisms, primarily commensal bacteria hosted by the human gut, constitute the human gut microbiome.
There is growing evidence that the human gut microbiome plays a role in maintaining normal body function and homeostasis.1 Research, such as the National Institute of Health Microbiome Project, is helping to show the impact of gut microorganisms and their negative influence on metabolic diseases and chronic inflammatory disorders.2-5 An imbalance in the microbiota, known as dysbiosis, has been associated with metabolic and cardiovascular diseases (CVD), including hypertension, diabetes mellitus, obesity, and coronary artery disease (CAD). Gut dysbiosis has also been associated with cardiac arrhythmias, including atrial fibrillation (AF) and ventricular arrhythmias (Figure).6-12
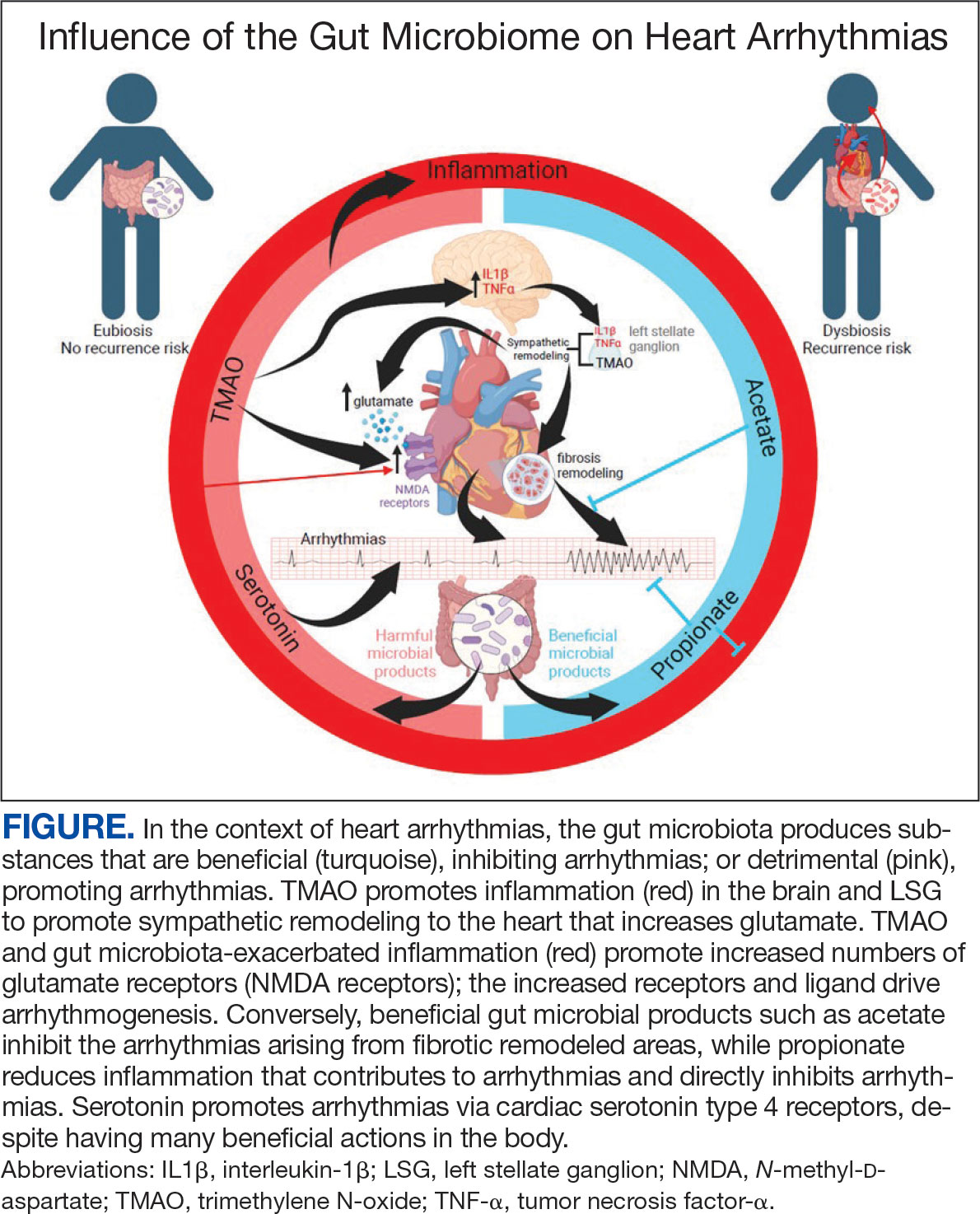
Whether gut dysbiosis is a cause or effect of the human disease process is unclear. While further research is warranted, some evidence of causation has been found. In 2018, Yoshida et al demonstrated an association between patients with CAD who had a significantly lower burden of the gut bacteria species Bacteroides vulgatus and Bacteroides dorei compared to that of patients without CAD. The study found that administration of these Bacteroides species reduced atherosclerotic lesion formation in atherosclerosis-prone mice.13 If altering gut microbial composition can affect the disease process, it may indicate a causative role for gut dysbiosis in disease pathogenesis. Furthermore, this finding also suggests agents may be used to alter the gut microbiome and potentially prevent and treat diseases. An altered gut microbiome may serve as an early marker for human disease, aiding in timely diagnosis and institution of disease-modifying treatments.
This review outlines the broad relationship of the pathways and intermediaries that may be involved in mediating the interaction between the gut microbiome and cardiac arrhythmias based on rapidly increasing evidence. A comprehensive search among PubMed and Google Scholar databases was conducted to find articles relevant to the topic.
Potential Intermediaries
Potential pathways for how the gut microbiome and cardiovascular system interact are subjects of active research. However, recent research may point to potential mechanisms of the association between the systems. The gut microbiome may influence human physiology through 3 principal routes: the autonomic nervous system, inflammatory pathways, and metabolic processes.
Autonomic Nervous System
The concept of bidirectional communication between the gut and central nervous system, known as the microbiota-gut-brain axis, is widely accepted.14 Proposed mediators of this interaction include the vagus nerve, the sympathetic nervous system, and the hypothalamic-pituitary-adrenal axis; cytokines produced by the immune system, tryptophan metabolism, and the production of short-chain fatty acids (SCFAs).15,16
The gut microbiome appears to have a direct impact on the autonomic nervous system, through which it can influence cardiovascular function. Muller et al described how the gut microbiome modulated gut-extrinsic sympathetic neurons and that the depletion of gut microbiota led to activation of both brainstem sensory nuclei and efferent sympathetic premotor glutamatergic neurons.16 Meng et al found that systemic injection of the gut microbiota-derived metabolite trimethylamine N-oxide (TMAO) led to significantly increased activity in the paraventricular nucleus, a hypothalamic structure essential to the central autonomic network. Their study demonstrated that systemic TMAO also led to increased left stellate ganglion (LSG) activity, a known contributor to cardiac sympathetic tone.12
Inflammatory Pathways
Inflammatory responses are another pathway for the gut microbiome to influence the cardiovascular system. SCFAs are a set of gut microbial metabolites produced in the colon by bacterial fermentation and decomposition of resistant starches and dietary fibers.17 These metabolites are increasingly recognized for their role in modulating disease processes, including cardiac disease. Aguilar et al found that the progression of atherosclerosis was slowed in apolipoprotein E (Apo-E) knockout mice by a chow diet supplemented with butyrate, a SCFA, suggesting it is an atheroprotective therapeutic agent. Less adhesion and migration of macrophages, reduced inflammation, improved plaque stability, and lowered atherosclerosis progression.18 Wei et al demonstrated in animal models that direct microinjection of the proinflammatory factors interleukin (IL)-1Β and tumor necrosis factor (TNF)-αdirectly into the subfornical organ increased heart rate, mean blood pressure, and renal sympathetic nerve activity.19
Metabolic Processes
Serotonin (5-HT), a metabolite of tryptophan, is a neurotransmitter that regulates many bodily functions and plays a significant role in the microbiota-brain gut axis.20 Oral ingestion of the bacterial species Bifidobacterium infantis increased plasma tryptophan in rat models.21 Additionally, many other microorganisms, including species of Candida, Streptococcus, Escherichia, and Enterococcus are known to produce 5-HT.22 While a relationship between the gut microbiome and plasma 5-HT has been established, interactions between 5-HT and the cardiovascular system are complex. Research has shown that stimulation of 5-HT1A receptors produces bradycardic and vasopressor effects, while stimulation of the 5-HT2 receptor induces vasoconstriction and tachycardia.23
A high-fiber diet can lower the incidence of hypertension, although the mechanisms are not clear. One potential reason could be alteration in gut bacteria, as a diet high in fiber has been shown to increase the prevalence of acetate-producing bacteria.24
Atherosclerosis
Research investigating the relationship of the gut microbiome with arrhythmias is in its early stages; however, the connection of the gut microbiome and atherosclerosis is more established.25 Contemporary studies have shown various gut microorganisms associated with atherosclerosis.26 Jie et al reported that patients with atherosclerotic cardiovascular disease had increased Enterobacteriaceae loads and oral cavity-associated bacteria with lower levels of butyrate producing bacteria when compared with healthy controls.27 In addition, microbial metabolites such as TMAO appear to promote atherosclerosis by increasing vascular inflammation and platelet reactivity.26 Researchers are investigating the modulation of these associations to help reduce atherosclerotic burden. Kasahara et al found that Roseburia intestinalis could reduce atherosclerotic disease in mice through the production of butyrate.28 Roberts et al established that administration of TMAO inhibitors reduced TMAO levels while reducing thrombus formation without observable toxicity or increased bleeding risk.29
Atrial Arrhythmias
The gut microbiome can also specifically affect cardiac arrhythmogenesis, and multiple studies suggest possible mediators of this interaction. Certain gut microbiome derived metabolites like TMAO may have a role in promoting AF.30 Other gut microbial metabolites like lipopolysaccharides and indoxyl sulfate are implicated in atrial electrical instability.31,32 Microbe-derived free fatty acids such as palmitic acid and adrenic acid can precipitate arrhythmogenesis. 33,34 Preponderances of certain gut bacteria like Ruminococcus, Streptococcus, and Enterococcus, as well as reductions of Faecalibacterium, Alistipes, Oscillibacter, and Bilophila have been detected in patients with AF.8 Tabata et al found that certain clusters of bacterial groups led by Ruminococcus species seem to show higher prevalence in patients with AF, whereas the genus Enterobacter was significantly lower compared with control subjects. That study also noted that gut microbial composition is affected by diet and antacid use.35 Gut microbiome-derived serotonin may be another mediator for AF, which may be related to the fact that 5-HT4 receptors are present in atrial tissue.36
Ventricular Arrhythmias
A critical component to the development of malignant ventricular arrhythmias is an imbalance in autonomic tone; in particular, the overactivation of the sympathetic nervous system.37 Animal models have shown that augmentation of the sympathetic nervous system plays an essential role in the subsequent development of ventricular arrhythmias. 38 Several studies have established the LSG as an important component of the cardiac sympathetic nervous system pathway. 38,39 Ablation of the LSG has been shown to effectively reduce the burden of malignant arrhythmias, further pointing toward the role of excess sympathetic activity.37,39 Stellate ganglion denervation has become an established method for managing life-threatening ventricular arrhythmias.40
Gut metabolites may have significant effects on cardiac sympathetic activity. Meng et al investigated the effect of TMAO on the LSG in animals and its overall effect on the incidence of ventricular arrhythmias under ischemic conditions. To fully explore this interaction, they examined the effect of TMAO on LSG function though 2 mechanisms: local administration of TMAO within the LSG and systemic administration of TMAO leading to activation of the central sympathetic nervous system. In both protocols, left anterior descending coronary artery occlusion was performed after TMAO administration. Injection of TMAO directly into the LSG was found to significantly increase the cardiac sympathetic tone and incidence of ventricular arrhythmias. In the systemic administration control arm, ventricular arrhythmias were also significantly increased.12
Increased inflammatory states appear to correlate with an increase in sympathetic tone and ventricular arrhythmias.12 In an animal study, direct injection of the proinflammatory factor IL-1Β into the LSG not only resulted in increased inflammation, but aggravated cardiac sympathetic remodeling. This led to a decreased effective refractory period and action potential duration, leading to an increased maximal slope of the restitution curve and higher occurrence of ventricular arrhythmias.41 Shi et al demonstrated that paraventricular nucleus microinjection with TNF-α and IL-1Β also enhanced the cardiac sympathetic afferent reflex, showing that these proinflammatory cytokines not only upregulate the inflammatory response, but can also have excitatory effects that stimulate sympathetic activity and have the potential to be proarrhythmic.19,42 Local and systemic administration of the gut microbe-derived TMAO increased the expression of IL-1Β and TNF-α, thus implicating the microbiome as a potential mediator of the inflammatory response and as another potential pathway for increased ventricular arrhythmias.12
The N-methyl-d-aspartate receptor (NMDAR) is found in multiple organs—including the heart—but more specifically in the conducting system and myocardium.43,44 Research has discovered an upregulation of NMDARs in the setting of cardiac sympathetic hyperinnervation in rat models both with healed myocardial necrotic injury and without. The infusion of their ligand, NMDA, provoked ventricular tachycardia and ventricular fibrillation in rat models with sympathetic hyperinnervation and healed myocardial necrotic injury.45 Another study found that NMDAR activation provoked ventricular arrhythmias, but also prolonged repolarization and induced electrical instability.46 Proinflammatory markers have been shown to upregulate the expression of NMDARs; more importantly, NMDAR expression has been shown to be significantly increased in the setting of TMAO administration.12,47,48
5-HT also appears to have a substantial association with ventricular arrhythmias in addition to atrial arrhythmias. el-Mahdy demonstrated in anesthetized rats with acute coronary ligation that systemic doses of 5-HT represented a significant dose-dependent increase in the duration of ventricular tachycardia and ventricular fibrillation, while also increasing the number of ventricular ectopic beats.49 Certain gut microorganisms are known to produce 5-HT, including those in the genera Streptococcus, Escherichia, and Enterococcus.22 Additionally, oral ingestion of the Bifidobacterium infantis increased plasma levels of tryptophan in rat models.21 The gut microbiome may have significant effects on plasma serotonin levels, and thus have the potential to alter the risk for ventricular arrhythmias.
The deleterious effects of the gut microbiome have been documented. However, it appears to have potential protective effects, and several studies point to the possible mechanisms of this beneficial interaction. Propionate is a SCFA microorganism produced by gut microbial fermentation.50 In a rat model study, Zhou et al found that infusion of sodium propionate significantly reduced ventricular arrhythmias during acute myocardial ischemia or burst stimulation, thus confirming cardioprotective effects.50,51
Proposed mechanisms for reduced susceptibility to ventricular arrhythmias with propionate infusion include parasympathetic activation via the gut-brain axis, anti-inflammatory pathways, and improved cardiac electrophysiology instability.50 In addition butyrate has been found to reduce inflammation and myocardial hypertrophy. Jiang et al demonstrated in rats postmyocardial infarction that butyrate promoted expression of anti-inflammatory M2 macrophage markers, decreased expressions of nerve growth factor and norepinephrine, and decreased the density of nerve fibers for growth-associated protein-43 and tyrosine hydroxylase. The cumulative impact of butyrate led to suppression of inflammation and the inhibition of sympathetic neural remodeling, ultimately resulting in improved cardiac function and reduction in ventricular arrhythmias after myocardial infarction.52
Gut bacteria-derived acetate-mediated reduction in cardiac fibrosis may be another mechanism for the effects on ventricular arrhythmias. Cardiac fibrosis and scar are established as the primary substrate for reentrant ventricular arrhythmias seen in various cardiomyopathies.
Future Directions
The microbiome residing in the human gut has a significant impact on cardiac arrhythmias, the details of which remain unknown. A likely bidirectional relationship exists in which the gut microbiome may affect arrhythmogenesis and in turn be affected by cardiac arrhythmias. The mechanisms of action are not well understood, but likely involve the autonomic nervous system, inflammation, and metabolic pathways.
The gut microbiome is a complex collection of heterogenous microorganisms that have dramatic effects on the human body. Additional research is necessary to identify further associations and causations of gut microorganisms with various human body processes, as well as cardiovascular disease. The microbiome has been shown to directly and indirectly influence the development of different disease states, including the cardiovascular system and cardiac arrhythmias. Several pathways have been proposed through which the gut microbiome can potentially affect cardiac arrhythmogenesis. There are likely several mechanisms simultaneously in operation. Understanding the role of human gut microbiome in the genesis of cardiac arrhythmias not only may improve our understanding of arrhythmias, but also may result in novel treatment options. This could potentially lead to the development of therapeutic options and strategies to modulate the gut microbiome to help detect, prevent, and treat cardiac arrhythmias.
- Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The central nervous system and the gut microbiome. Cell. 2016;167(4):915-932. doi:10.1016/j.cell.2016.10.027
- Karlsson F, Tremaroli V, Nielsen J, Bäckhed F. Assessing the human gut microbiota in metabolic diseases. Diabetes. 2013;62(10):3341-3349. doi:10.2337/db13-0844
- Danneskiold-Samsøe NB, Dias de Freitas Queiroz Barros H, Santos R, et al. Interplay between food and gut microbiota in health and disease. Food Res Int. 2019;115:23-31. doi:10.1016/j.foodres.2018.07.043
- Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe- derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446-450. doi:10.1038/nature12721
- Integrative HMP (iHMP) Research Network Consortium. The integrative human microbiome project. Nature. 2019;569(7758):641-648. doi:10.1038/s41586-019-1238-8
- Zubcevic J, Richards EM, Yang T, et al. Impaired autonomic nervous system-microbiome circuit in hypertension. Circ Res. 2019;125(1):104-116. doi:10.1161/CIRCRESAHA.119.313965
- Emoto T, Yamashita T, Sasaki N, et al. Analysis of gut microbiota in coronary artery disease patients: a possible link between gut microbiota and coronary artery disease. J Atheroscler Thromb. 2016;23(8):908-921. doi:10.5551/jat.32672
- Zuo K, Li J, Li K, et al. Disordered gut microbiota and alterations in metabolic patterns are associated with atrial fibrillation. Gigascience. 2019;8(6):giz058. doi:10.1093/gigascience/giz058
- Li J, Zhao F, Wang Y, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5(1):14. doi:10.1186/s40168-016-0222-x
- Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55-60. doi:10.1038/nature11450
- Chang CJ, Lin CS, Lu CC, et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat Commun. 2015;6:7489. doi:10.1038/ncomms8489
- Meng G, Zhou X, Wang M, et al. Gut microbederived metabolite trimethylamine N-oxide activates the cardiac autonomic nervous system and facilitates ischemia-induced ventricular arrhythmia via two different pathways. EBioMedicine. 2019;44:656-664. doi:10.1016/j.ebiom.2019.03.066
- Yoshida N, Emoto T, Yamashita T, et al. Bacteroides vulgatus and Bacteroides dorei reduce gut microbial lipopolysaccharide production and inhibit atherosclerosis. Circulation. 2018;138(22):2486-2498. doi:10.1161/CIRCULATIONAHA.118.033714
- Cussotto S, Sandhu KV, Dinan TG, Cryan JF. The neuroendocrinology of the microbiota-gut-brain axis: a behavioural perspective. Front Neuroendocrinol. 2018;51:80-101. doi:10.1016/j.yfrne.2018.04.002
- Dinan TG, Stilling RM, Stanton C, Cryan JF. Collective unconscious: how gut microbes shape human behavior. J Psychiatr Res. 2015;63:1-9. doi:10.1016/j.jpsychires.2015.02.021
- Muller PA, Schneeberger M, Matheis F, et al. Microbiota modulate sympathetic neurons via a gutbrain circuit. Nature. 2020;583(7816):441-446. doi:10.1038/s41586-020-2474-7
- Ohira H, Tsutsui W, Fujioka Y. Are short chain fatty acids in gut microbiota defensive players for inflammation and atherosclerosis? J Atheroscler Thromb. 2017;24(7):660-672. doi:10.5551/jat.RV17006
- Aguilar EC, Leonel AJ, Teixeira LG, et al. Butyrate impairs atherogenesis by reducing plaque inflammation and vulnerability and decreasing NFêB activation. Nutr Metab Cardiovasc Dis. 2014;24(6):606-613. doi:10.1016/j.numecd.2014.01.002
- Wei SG, Yu Y, Zhang ZH, Felder RB. Proinflammatory cytokines upregulate sympathoexcit - atory mechanisms in the subfornical organ of the rat. Hypertension. 2015;65(5):1126-1133. doi:10.1161/HYPERTENSIONAHA.114.05112
- Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry. 2013;74(10):720- 726. doi:10.1016/j.biopsych.2013.05.001
- Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res. 2008;43(2):164-174. doi:10.1016/j.jpsychires.2008.03.009
- Lyte M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: microbial endocrinology in the design and use of probiotics. Bioessays. 2011;33(8):574-581. doi:10.1002/bies.201100024
- Yusuf S, Al-Saady N, Camm AJ. 5-hydroxytryptamine and atrial fibrillation: how significant is this piece in the puzzle? J Cardiovasc Electrophysiol. 2003;14(2):209-214. doi:10.1046/j.1540-8167.2003.02381.x
- Marques FZ, Nelson E, Chu PY, et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation. 2017;135(10):964-977. doi:10.1161/CIRCULATIONAHA.116.024545
- Björkegren JLM, Lusis AJ. Atherosclerosis: recent developments. Cell. 2022;185(10):1630-1645. doi:10.1016/j.cell.2022.04.004
- Tang WHW, Bäckhed F, Landmesser U, Hazen SL. Intestinal microbiota in cardiovascular health and disease: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(16):2089-2105. doi:10.1016/j.jacc.2019.03.024
- Jie Z, Xia H, Zhong SL, et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun. 2017;8(1):845. doi:10.1038/s41467-017-00900-1
- Kasahara K, Krautkramer KA, Org E, et al. Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nat Microbiol. 2018;3(12):1461- 1471. doi:10.1038/s41564-018-0272-x
- Roberts AB, Gu X, Buffa JA, et al. Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential. Nat Med. 2018;24(9):1407-1417. doi:10.1038/s41591-018-0128-1
- Yu L, Meng G, Huang B, et al. A potential relationship between gut microbes and atrial fibrillation: trimethylamine N-oxide, a gut microbe-derived metabolite, facilitates the progression of atrial fibrillation. Int J Cardiol. 2018;255:92- 98. doi:10.1016/j.ijcard.2017.11.071
- Okazaki R, Iwasaki YK, Miyauchi Y, et al. Lipopolysaccharide induces atrial arrhythmogenesis via down-regulation of L-type Ca2+ channel genes in rats. Int Heart J. 2009;50(3):353-363. doi:10.1536/ihj.50.353
- Chen WT, Chen YC, Hsieh MH, et al. The uremic toxin indoxyl sulfate increases pulmonary vein and atrial arrhythmogenesis. J Cardiovasc Electrophysiol. 2015;26(2):203- 210. doi:10.1111/jce.12554
- Fretts AM, Mozaffarian D, Siscovick DS, et al. Plasma phospholipid saturated fatty acids and incident atrial fibrillation: the Cardiovascular Health Study. J Am Heart Assoc. 2014;3(3):e000889. doi:10.1161/JAHA.114.000889
- Horas HNS, Nishiumi S, Kawano Y, Kobayashi T, Yoshida M, Azuma T. Adrenic acid as an inflammation enhancer in non-alcoholic fatty liver disease. Arch Biochem Biophys. 2017;623-624:64-75. doi:10.1016/j.abb.2017.04.009
- Tabata T, Yamashita T, Hosomi K, et al. Gut microbial composition in patients with atrial fibrillation: effects of diet and drugs. Heart Vessels. 2021;36(1):105-114. doi:10.1007/s00380-020-01669-y
- López-Rodriguez ML, Benhamú B, Morcillo MJ, et al. 5-HT(4) receptor antagonists: structure-affinity relationships and ligand-receptor interactions. Curr Top Med Chem. 2002;2(6):625-641. doi:10.2174/1568026023393769
- Yu L, Zhou L, Cao G, et al. Optogenetic modulation of cardiac sympathetic nerve activity to prevent ventricular arrhythmias. J Am Coll Cardiol. 2017;70(22):2778-2790. doi:10.1016/j.jacc.2017.09.1107
- Schwartz PJ, Vanoli E. Cardiac arrhythmias elicited by interaction between acute myocardial ischemia and sympathetic hyperactivity: a new experimental model for the study of antiarrhythmic drugs. J Cardiovasc Pharmacol. 1981;3(6):1251-1259. doi:10.1097/00005344-198111000-00012
- Puddu PE, Jouve R, Langlet F, Guillen JC, Lanti M, Reale A. Prevention of postischemic ventricular fibrillation late after right or left stellate ganglionectomy in dogs. Circulation. 1988;77(4):935-946. doi:10.1161/01.cir.77.4.935
- Vaseghi M, Gima J, Kanaan C, et al. Cardiac sympathetic denervation in patients with refractory ventricular arrhythmias or electrical storm: intermediate and longterm follow-up. Heart Rhythm. 2014;11(3):360-366. doi:10.1016/j.hrthm.2013.11.028
- Wang M, Li S, Zhou X, et al. Increased inflammation promotes ventricular arrhythmia through aggravating left stellate ganglion remodeling in a canine ischemia model. Int J Cardiol. 2017;248:286-293. doi:10.1016/j.ijcard.2017.08.011
- Shi Z, Gan XB, Fan ZD, et al. Inflammatory cytokines in paraventricular nucleus modulate sympathetic activity and cardiac sympathetic afferent reflex in rats. Acta Physiol (Oxf). 2011;203(2):289-297. doi:10.1111/j.1748-1716.2011.02313.x
- Gill S, Veinot J, Kavanagh M, Pulido O. Human heart glutamate receptors - implications for toxicology, food safety, and drug discovery. Toxicol Pathol. 2007;35(3):411-417. doi:10.1080/01926230701230361
- Govoruskina N, Jakovljevic V, Zivkovic V, et al. The role of cardiac N-methyl-D-aspartate receptors in heart conditioning— effects on heart function and oxidative stress. Biomolecules. 2020;10(7):1065. doi:10.3390/biom10071065
- Lü J, Gao X, Gu J, et al. Nerve sprouting contributes to increased severity of ventricular tachyarrhythmias by upregulating iGluRs in rats with healed myocardial necrotic injury. J Mol Neurosci. 2012;48(2):448-455. doi:10.1007/s12031-012-9720-x
- Shi S, Liu T, Li Y, et al. Chronic N-methyl-D-aspartate receptor activation induces cardiac electrical remodeling and increases susceptibility to ventricular arrhythmias. Pacing Clin Electrophysiol. 2014;37(10):1367-1377. doi:10.1111/pace.12430
- Zhang Z, Bassam B, Thomas AG, et al. Maternal inflammation leads to impaired glutamate homeostasis and upregulation of glutamate carboxypeptidase II in activated microglia in the fetal/newborn rabbit brain. Neurobiol Dis. 2016;94:116-128. doi:10.1016/j.nbd.2016.06.010
- Wu LJ, Toyoda H, Zhao MG, et al. Upregulation of forebrain NMDA NR2B receptors contributes to behavioral sensitization after inflammation. J Neurosci. 2005;25(48):11107-11116. doi:10.1523/JNEUROSCI.1678-05.2005
- el-Mahdy SA. 5-hydroxytryptamine (serotonin) enhances ventricular arrhythmias induced by acute coronary artery ligation in rats. Res Commun Chem Pathol Pharmacol. 1990;68(3):383-386.
- Zhou M, Li D, Xie K, et al. The short-chain fatty acid propionate improved ventricular electrical remodeling in a rat model with myocardial infarction. Food Funct. 2021;12(24):12580-12593. doi:10.1039/d1fo02040d
- Bartolomaeus H, Balogh A, Yakoub M, et al. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation. 2019;139(11):1407-1421. doi:10.1161/CIRCULATIONAHA.118.036652
- Jiang X, Huang X, Tong Y, Gao H. Butyrate improves cardiac function and sympathetic neural remodeling following myocardial infarction in rats. Can J Physiol Pharmacol. 2020;98(6):391-399. doi:10.1139/cjpp-2019-0531
The extensive surface of the gastrointestinal tract presents an interface between the human body and its environment. Residing within the intestinal lumen, ingested food and various microorganisms are an essential aspect of this relationship. The trillions of microorganisms, primarily commensal bacteria hosted by the human gut, constitute the human gut microbiome.
There is growing evidence that the human gut microbiome plays a role in maintaining normal body function and homeostasis.1 Research, such as the National Institute of Health Microbiome Project, is helping to show the impact of gut microorganisms and their negative influence on metabolic diseases and chronic inflammatory disorders.2-5 An imbalance in the microbiota, known as dysbiosis, has been associated with metabolic and cardiovascular diseases (CVD), including hypertension, diabetes mellitus, obesity, and coronary artery disease (CAD). Gut dysbiosis has also been associated with cardiac arrhythmias, including atrial fibrillation (AF) and ventricular arrhythmias (Figure).6-12

Whether gut dysbiosis is a cause or effect of the human disease process is unclear. While further research is warranted, some evidence of causation has been found. In 2018, Yoshida et al demonstrated an association between patients with CAD who had a significantly lower burden of the gut bacteria species Bacteroides vulgatus and Bacteroides dorei compared to that of patients without CAD. The study found that administration of these Bacteroides species reduced atherosclerotic lesion formation in atherosclerosis-prone mice.13 If altering gut microbial composition can affect the disease process, it may indicate a causative role for gut dysbiosis in disease pathogenesis. Furthermore, this finding also suggests agents may be used to alter the gut microbiome and potentially prevent and treat diseases. An altered gut microbiome may serve as an early marker for human disease, aiding in timely diagnosis and institution of disease-modifying treatments.
This review outlines the broad relationship of the pathways and intermediaries that may be involved in mediating the interaction between the gut microbiome and cardiac arrhythmias based on rapidly increasing evidence. A comprehensive search among PubMed and Google Scholar databases was conducted to find articles relevant to the topic.
Potential Intermediaries
Potential pathways for how the gut microbiome and cardiovascular system interact are subjects of active research. However, recent research may point to potential mechanisms of the association between the systems. The gut microbiome may influence human physiology through 3 principal routes: the autonomic nervous system, inflammatory pathways, and metabolic processes.
Autonomic Nervous System
The concept of bidirectional communication between the gut and central nervous system, known as the microbiota-gut-brain axis, is widely accepted.14 Proposed mediators of this interaction include the vagus nerve, the sympathetic nervous system, and the hypothalamic-pituitary-adrenal axis; cytokines produced by the immune system, tryptophan metabolism, and the production of short-chain fatty acids (SCFAs).15,16
The gut microbiome appears to have a direct impact on the autonomic nervous system, through which it can influence cardiovascular function. Muller et al described how the gut microbiome modulated gut-extrinsic sympathetic neurons and that the depletion of gut microbiota led to activation of both brainstem sensory nuclei and efferent sympathetic premotor glutamatergic neurons.16 Meng et al found that systemic injection of the gut microbiota-derived metabolite trimethylamine N-oxide (TMAO) led to significantly increased activity in the paraventricular nucleus, a hypothalamic structure essential to the central autonomic network. Their study demonstrated that systemic TMAO also led to increased left stellate ganglion (LSG) activity, a known contributor to cardiac sympathetic tone.12
Inflammatory Pathways
Inflammatory responses are another pathway for the gut microbiome to influence the cardiovascular system. SCFAs are a set of gut microbial metabolites produced in the colon by bacterial fermentation and decomposition of resistant starches and dietary fibers.17 These metabolites are increasingly recognized for their role in modulating disease processes, including cardiac disease. Aguilar et al found that the progression of atherosclerosis was slowed in apolipoprotein E (Apo-E) knockout mice by a chow diet supplemented with butyrate, a SCFA, suggesting it is an atheroprotective therapeutic agent. Less adhesion and migration of macrophages, reduced inflammation, improved plaque stability, and lowered atherosclerosis progression.18 Wei et al demonstrated in animal models that direct microinjection of the proinflammatory factors interleukin (IL)-1Β and tumor necrosis factor (TNF)-αdirectly into the subfornical organ increased heart rate, mean blood pressure, and renal sympathetic nerve activity.19
Metabolic Processes
Serotonin (5-HT), a metabolite of tryptophan, is a neurotransmitter that regulates many bodily functions and plays a significant role in the microbiota-brain gut axis.20 Oral ingestion of the bacterial species Bifidobacterium infantis increased plasma tryptophan in rat models.21 Additionally, many other microorganisms, including species of Candida, Streptococcus, Escherichia, and Enterococcus are known to produce 5-HT.22 While a relationship between the gut microbiome and plasma 5-HT has been established, interactions between 5-HT and the cardiovascular system are complex. Research has shown that stimulation of 5-HT1A receptors produces bradycardic and vasopressor effects, while stimulation of the 5-HT2 receptor induces vasoconstriction and tachycardia.23
A high-fiber diet can lower the incidence of hypertension, although the mechanisms are not clear. One potential reason could be alteration in gut bacteria, as a diet high in fiber has been shown to increase the prevalence of acetate-producing bacteria.24
Atherosclerosis
Research investigating the relationship of the gut microbiome with arrhythmias is in its early stages; however, the connection of the gut microbiome and atherosclerosis is more established.25 Contemporary studies have shown various gut microorganisms associated with atherosclerosis.26 Jie et al reported that patients with atherosclerotic cardiovascular disease had increased Enterobacteriaceae loads and oral cavity-associated bacteria with lower levels of butyrate producing bacteria when compared with healthy controls.27 In addition, microbial metabolites such as TMAO appear to promote atherosclerosis by increasing vascular inflammation and platelet reactivity.26 Researchers are investigating the modulation of these associations to help reduce atherosclerotic burden. Kasahara et al found that Roseburia intestinalis could reduce atherosclerotic disease in mice through the production of butyrate.28 Roberts et al established that administration of TMAO inhibitors reduced TMAO levels while reducing thrombus formation without observable toxicity or increased bleeding risk.29
Atrial Arrhythmias
The gut microbiome can also specifically affect cardiac arrhythmogenesis, and multiple studies suggest possible mediators of this interaction. Certain gut microbiome derived metabolites like TMAO may have a role in promoting AF.30 Other gut microbial metabolites like lipopolysaccharides and indoxyl sulfate are implicated in atrial electrical instability.31,32 Microbe-derived free fatty acids such as palmitic acid and adrenic acid can precipitate arrhythmogenesis. 33,34 Preponderances of certain gut bacteria like Ruminococcus, Streptococcus, and Enterococcus, as well as reductions of Faecalibacterium, Alistipes, Oscillibacter, and Bilophila have been detected in patients with AF.8 Tabata et al found that certain clusters of bacterial groups led by Ruminococcus species seem to show higher prevalence in patients with AF, whereas the genus Enterobacter was significantly lower compared with control subjects. That study also noted that gut microbial composition is affected by diet and antacid use.35 Gut microbiome-derived serotonin may be another mediator for AF, which may be related to the fact that 5-HT4 receptors are present in atrial tissue.36
Ventricular Arrhythmias
A critical component to the development of malignant ventricular arrhythmias is an imbalance in autonomic tone; in particular, the overactivation of the sympathetic nervous system.37 Animal models have shown that augmentation of the sympathetic nervous system plays an essential role in the subsequent development of ventricular arrhythmias. 38 Several studies have established the LSG as an important component of the cardiac sympathetic nervous system pathway. 38,39 Ablation of the LSG has been shown to effectively reduce the burden of malignant arrhythmias, further pointing toward the role of excess sympathetic activity.37,39 Stellate ganglion denervation has become an established method for managing life-threatening ventricular arrhythmias.40
Gut metabolites may have significant effects on cardiac sympathetic activity. Meng et al investigated the effect of TMAO on the LSG in animals and its overall effect on the incidence of ventricular arrhythmias under ischemic conditions. To fully explore this interaction, they examined the effect of TMAO on LSG function though 2 mechanisms: local administration of TMAO within the LSG and systemic administration of TMAO leading to activation of the central sympathetic nervous system. In both protocols, left anterior descending coronary artery occlusion was performed after TMAO administration. Injection of TMAO directly into the LSG was found to significantly increase the cardiac sympathetic tone and incidence of ventricular arrhythmias. In the systemic administration control arm, ventricular arrhythmias were also significantly increased.12
Increased inflammatory states appear to correlate with an increase in sympathetic tone and ventricular arrhythmias.12 In an animal study, direct injection of the proinflammatory factor IL-1Β into the LSG not only resulted in increased inflammation, but aggravated cardiac sympathetic remodeling. This led to a decreased effective refractory period and action potential duration, leading to an increased maximal slope of the restitution curve and higher occurrence of ventricular arrhythmias.41 Shi et al demonstrated that paraventricular nucleus microinjection with TNF-α and IL-1Β also enhanced the cardiac sympathetic afferent reflex, showing that these proinflammatory cytokines not only upregulate the inflammatory response, but can also have excitatory effects that stimulate sympathetic activity and have the potential to be proarrhythmic.19,42 Local and systemic administration of the gut microbe-derived TMAO increased the expression of IL-1Β and TNF-α, thus implicating the microbiome as a potential mediator of the inflammatory response and as another potential pathway for increased ventricular arrhythmias.12
The N-methyl-d-aspartate receptor (NMDAR) is found in multiple organs—including the heart—but more specifically in the conducting system and myocardium.43,44 Research has discovered an upregulation of NMDARs in the setting of cardiac sympathetic hyperinnervation in rat models both with healed myocardial necrotic injury and without. The infusion of their ligand, NMDA, provoked ventricular tachycardia and ventricular fibrillation in rat models with sympathetic hyperinnervation and healed myocardial necrotic injury.45 Another study found that NMDAR activation provoked ventricular arrhythmias, but also prolonged repolarization and induced electrical instability.46 Proinflammatory markers have been shown to upregulate the expression of NMDARs; more importantly, NMDAR expression has been shown to be significantly increased in the setting of TMAO administration.12,47,48
5-HT also appears to have a substantial association with ventricular arrhythmias in addition to atrial arrhythmias. el-Mahdy demonstrated in anesthetized rats with acute coronary ligation that systemic doses of 5-HT represented a significant dose-dependent increase in the duration of ventricular tachycardia and ventricular fibrillation, while also increasing the number of ventricular ectopic beats.49 Certain gut microorganisms are known to produce 5-HT, including those in the genera Streptococcus, Escherichia, and Enterococcus.22 Additionally, oral ingestion of the Bifidobacterium infantis increased plasma levels of tryptophan in rat models.21 The gut microbiome may have significant effects on plasma serotonin levels, and thus have the potential to alter the risk for ventricular arrhythmias.
The deleterious effects of the gut microbiome have been documented. However, it appears to have potential protective effects, and several studies point to the possible mechanisms of this beneficial interaction. Propionate is a SCFA microorganism produced by gut microbial fermentation.50 In a rat model study, Zhou et al found that infusion of sodium propionate significantly reduced ventricular arrhythmias during acute myocardial ischemia or burst stimulation, thus confirming cardioprotective effects.50,51
Proposed mechanisms for reduced susceptibility to ventricular arrhythmias with propionate infusion include parasympathetic activation via the gut-brain axis, anti-inflammatory pathways, and improved cardiac electrophysiology instability.50 In addition butyrate has been found to reduce inflammation and myocardial hypertrophy. Jiang et al demonstrated in rats postmyocardial infarction that butyrate promoted expression of anti-inflammatory M2 macrophage markers, decreased expressions of nerve growth factor and norepinephrine, and decreased the density of nerve fibers for growth-associated protein-43 and tyrosine hydroxylase. The cumulative impact of butyrate led to suppression of inflammation and the inhibition of sympathetic neural remodeling, ultimately resulting in improved cardiac function and reduction in ventricular arrhythmias after myocardial infarction.52
Gut bacteria-derived acetate-mediated reduction in cardiac fibrosis may be another mechanism for the effects on ventricular arrhythmias. Cardiac fibrosis and scar are established as the primary substrate for reentrant ventricular arrhythmias seen in various cardiomyopathies.
Future Directions
The microbiome residing in the human gut has a significant impact on cardiac arrhythmias, the details of which remain unknown. A likely bidirectional relationship exists in which the gut microbiome may affect arrhythmogenesis and in turn be affected by cardiac arrhythmias. The mechanisms of action are not well understood, but likely involve the autonomic nervous system, inflammation, and metabolic pathways.
The gut microbiome is a complex collection of heterogenous microorganisms that have dramatic effects on the human body. Additional research is necessary to identify further associations and causations of gut microorganisms with various human body processes, as well as cardiovascular disease. The microbiome has been shown to directly and indirectly influence the development of different disease states, including the cardiovascular system and cardiac arrhythmias. Several pathways have been proposed through which the gut microbiome can potentially affect cardiac arrhythmogenesis. There are likely several mechanisms simultaneously in operation. Understanding the role of human gut microbiome in the genesis of cardiac arrhythmias not only may improve our understanding of arrhythmias, but also may result in novel treatment options. This could potentially lead to the development of therapeutic options and strategies to modulate the gut microbiome to help detect, prevent, and treat cardiac arrhythmias.
The extensive surface of the gastrointestinal tract presents an interface between the human body and its environment. Residing within the intestinal lumen, ingested food and various microorganisms are an essential aspect of this relationship. The trillions of microorganisms, primarily commensal bacteria hosted by the human gut, constitute the human gut microbiome.
There is growing evidence that the human gut microbiome plays a role in maintaining normal body function and homeostasis.1 Research, such as the National Institute of Health Microbiome Project, is helping to show the impact of gut microorganisms and their negative influence on metabolic diseases and chronic inflammatory disorders.2-5 An imbalance in the microbiota, known as dysbiosis, has been associated with metabolic and cardiovascular diseases (CVD), including hypertension, diabetes mellitus, obesity, and coronary artery disease (CAD). Gut dysbiosis has also been associated with cardiac arrhythmias, including atrial fibrillation (AF) and ventricular arrhythmias (Figure).6-12

Whether gut dysbiosis is a cause or effect of the human disease process is unclear. While further research is warranted, some evidence of causation has been found. In 2018, Yoshida et al demonstrated an association between patients with CAD who had a significantly lower burden of the gut bacteria species Bacteroides vulgatus and Bacteroides dorei compared to that of patients without CAD. The study found that administration of these Bacteroides species reduced atherosclerotic lesion formation in atherosclerosis-prone mice.13 If altering gut microbial composition can affect the disease process, it may indicate a causative role for gut dysbiosis in disease pathogenesis. Furthermore, this finding also suggests agents may be used to alter the gut microbiome and potentially prevent and treat diseases. An altered gut microbiome may serve as an early marker for human disease, aiding in timely diagnosis and institution of disease-modifying treatments.
This review outlines the broad relationship of the pathways and intermediaries that may be involved in mediating the interaction between the gut microbiome and cardiac arrhythmias based on rapidly increasing evidence. A comprehensive search among PubMed and Google Scholar databases was conducted to find articles relevant to the topic.
Potential Intermediaries
Potential pathways for how the gut microbiome and cardiovascular system interact are subjects of active research. However, recent research may point to potential mechanisms of the association between the systems. The gut microbiome may influence human physiology through 3 principal routes: the autonomic nervous system, inflammatory pathways, and metabolic processes.
Autonomic Nervous System
The concept of bidirectional communication between the gut and central nervous system, known as the microbiota-gut-brain axis, is widely accepted.14 Proposed mediators of this interaction include the vagus nerve, the sympathetic nervous system, and the hypothalamic-pituitary-adrenal axis; cytokines produced by the immune system, tryptophan metabolism, and the production of short-chain fatty acids (SCFAs).15,16
The gut microbiome appears to have a direct impact on the autonomic nervous system, through which it can influence cardiovascular function. Muller et al described how the gut microbiome modulated gut-extrinsic sympathetic neurons and that the depletion of gut microbiota led to activation of both brainstem sensory nuclei and efferent sympathetic premotor glutamatergic neurons.16 Meng et al found that systemic injection of the gut microbiota-derived metabolite trimethylamine N-oxide (TMAO) led to significantly increased activity in the paraventricular nucleus, a hypothalamic structure essential to the central autonomic network. Their study demonstrated that systemic TMAO also led to increased left stellate ganglion (LSG) activity, a known contributor to cardiac sympathetic tone.12
Inflammatory Pathways
Inflammatory responses are another pathway for the gut microbiome to influence the cardiovascular system. SCFAs are a set of gut microbial metabolites produced in the colon by bacterial fermentation and decomposition of resistant starches and dietary fibers.17 These metabolites are increasingly recognized for their role in modulating disease processes, including cardiac disease. Aguilar et al found that the progression of atherosclerosis was slowed in apolipoprotein E (Apo-E) knockout mice by a chow diet supplemented with butyrate, a SCFA, suggesting it is an atheroprotective therapeutic agent. Less adhesion and migration of macrophages, reduced inflammation, improved plaque stability, and lowered atherosclerosis progression.18 Wei et al demonstrated in animal models that direct microinjection of the proinflammatory factors interleukin (IL)-1Β and tumor necrosis factor (TNF)-αdirectly into the subfornical organ increased heart rate, mean blood pressure, and renal sympathetic nerve activity.19
Metabolic Processes
Serotonin (5-HT), a metabolite of tryptophan, is a neurotransmitter that regulates many bodily functions and plays a significant role in the microbiota-brain gut axis.20 Oral ingestion of the bacterial species Bifidobacterium infantis increased plasma tryptophan in rat models.21 Additionally, many other microorganisms, including species of Candida, Streptococcus, Escherichia, and Enterococcus are known to produce 5-HT.22 While a relationship between the gut microbiome and plasma 5-HT has been established, interactions between 5-HT and the cardiovascular system are complex. Research has shown that stimulation of 5-HT1A receptors produces bradycardic and vasopressor effects, while stimulation of the 5-HT2 receptor induces vasoconstriction and tachycardia.23
A high-fiber diet can lower the incidence of hypertension, although the mechanisms are not clear. One potential reason could be alteration in gut bacteria, as a diet high in fiber has been shown to increase the prevalence of acetate-producing bacteria.24
Atherosclerosis
Research investigating the relationship of the gut microbiome with arrhythmias is in its early stages; however, the connection of the gut microbiome and atherosclerosis is more established.25 Contemporary studies have shown various gut microorganisms associated with atherosclerosis.26 Jie et al reported that patients with atherosclerotic cardiovascular disease had increased Enterobacteriaceae loads and oral cavity-associated bacteria with lower levels of butyrate producing bacteria when compared with healthy controls.27 In addition, microbial metabolites such as TMAO appear to promote atherosclerosis by increasing vascular inflammation and platelet reactivity.26 Researchers are investigating the modulation of these associations to help reduce atherosclerotic burden. Kasahara et al found that Roseburia intestinalis could reduce atherosclerotic disease in mice through the production of butyrate.28 Roberts et al established that administration of TMAO inhibitors reduced TMAO levels while reducing thrombus formation without observable toxicity or increased bleeding risk.29
Atrial Arrhythmias
The gut microbiome can also specifically affect cardiac arrhythmogenesis, and multiple studies suggest possible mediators of this interaction. Certain gut microbiome derived metabolites like TMAO may have a role in promoting AF.30 Other gut microbial metabolites like lipopolysaccharides and indoxyl sulfate are implicated in atrial electrical instability.31,32 Microbe-derived free fatty acids such as palmitic acid and adrenic acid can precipitate arrhythmogenesis. 33,34 Preponderances of certain gut bacteria like Ruminococcus, Streptococcus, and Enterococcus, as well as reductions of Faecalibacterium, Alistipes, Oscillibacter, and Bilophila have been detected in patients with AF.8 Tabata et al found that certain clusters of bacterial groups led by Ruminococcus species seem to show higher prevalence in patients with AF, whereas the genus Enterobacter was significantly lower compared with control subjects. That study also noted that gut microbial composition is affected by diet and antacid use.35 Gut microbiome-derived serotonin may be another mediator for AF, which may be related to the fact that 5-HT4 receptors are present in atrial tissue.36
Ventricular Arrhythmias
A critical component to the development of malignant ventricular arrhythmias is an imbalance in autonomic tone; in particular, the overactivation of the sympathetic nervous system.37 Animal models have shown that augmentation of the sympathetic nervous system plays an essential role in the subsequent development of ventricular arrhythmias. 38 Several studies have established the LSG as an important component of the cardiac sympathetic nervous system pathway. 38,39 Ablation of the LSG has been shown to effectively reduce the burden of malignant arrhythmias, further pointing toward the role of excess sympathetic activity.37,39 Stellate ganglion denervation has become an established method for managing life-threatening ventricular arrhythmias.40
Gut metabolites may have significant effects on cardiac sympathetic activity. Meng et al investigated the effect of TMAO on the LSG in animals and its overall effect on the incidence of ventricular arrhythmias under ischemic conditions. To fully explore this interaction, they examined the effect of TMAO on LSG function though 2 mechanisms: local administration of TMAO within the LSG and systemic administration of TMAO leading to activation of the central sympathetic nervous system. In both protocols, left anterior descending coronary artery occlusion was performed after TMAO administration. Injection of TMAO directly into the LSG was found to significantly increase the cardiac sympathetic tone and incidence of ventricular arrhythmias. In the systemic administration control arm, ventricular arrhythmias were also significantly increased.12
Increased inflammatory states appear to correlate with an increase in sympathetic tone and ventricular arrhythmias.12 In an animal study, direct injection of the proinflammatory factor IL-1Β into the LSG not only resulted in increased inflammation, but aggravated cardiac sympathetic remodeling. This led to a decreased effective refractory period and action potential duration, leading to an increased maximal slope of the restitution curve and higher occurrence of ventricular arrhythmias.41 Shi et al demonstrated that paraventricular nucleus microinjection with TNF-α and IL-1Β also enhanced the cardiac sympathetic afferent reflex, showing that these proinflammatory cytokines not only upregulate the inflammatory response, but can also have excitatory effects that stimulate sympathetic activity and have the potential to be proarrhythmic.19,42 Local and systemic administration of the gut microbe-derived TMAO increased the expression of IL-1Β and TNF-α, thus implicating the microbiome as a potential mediator of the inflammatory response and as another potential pathway for increased ventricular arrhythmias.12
The N-methyl-d-aspartate receptor (NMDAR) is found in multiple organs—including the heart—but more specifically in the conducting system and myocardium.43,44 Research has discovered an upregulation of NMDARs in the setting of cardiac sympathetic hyperinnervation in rat models both with healed myocardial necrotic injury and without. The infusion of their ligand, NMDA, provoked ventricular tachycardia and ventricular fibrillation in rat models with sympathetic hyperinnervation and healed myocardial necrotic injury.45 Another study found that NMDAR activation provoked ventricular arrhythmias, but also prolonged repolarization and induced electrical instability.46 Proinflammatory markers have been shown to upregulate the expression of NMDARs; more importantly, NMDAR expression has been shown to be significantly increased in the setting of TMAO administration.12,47,48
5-HT also appears to have a substantial association with ventricular arrhythmias in addition to atrial arrhythmias. el-Mahdy demonstrated in anesthetized rats with acute coronary ligation that systemic doses of 5-HT represented a significant dose-dependent increase in the duration of ventricular tachycardia and ventricular fibrillation, while also increasing the number of ventricular ectopic beats.49 Certain gut microorganisms are known to produce 5-HT, including those in the genera Streptococcus, Escherichia, and Enterococcus.22 Additionally, oral ingestion of the Bifidobacterium infantis increased plasma levels of tryptophan in rat models.21 The gut microbiome may have significant effects on plasma serotonin levels, and thus have the potential to alter the risk for ventricular arrhythmias.
The deleterious effects of the gut microbiome have been documented. However, it appears to have potential protective effects, and several studies point to the possible mechanisms of this beneficial interaction. Propionate is a SCFA microorganism produced by gut microbial fermentation.50 In a rat model study, Zhou et al found that infusion of sodium propionate significantly reduced ventricular arrhythmias during acute myocardial ischemia or burst stimulation, thus confirming cardioprotective effects.50,51
Proposed mechanisms for reduced susceptibility to ventricular arrhythmias with propionate infusion include parasympathetic activation via the gut-brain axis, anti-inflammatory pathways, and improved cardiac electrophysiology instability.50 In addition butyrate has been found to reduce inflammation and myocardial hypertrophy. Jiang et al demonstrated in rats postmyocardial infarction that butyrate promoted expression of anti-inflammatory M2 macrophage markers, decreased expressions of nerve growth factor and norepinephrine, and decreased the density of nerve fibers for growth-associated protein-43 and tyrosine hydroxylase. The cumulative impact of butyrate led to suppression of inflammation and the inhibition of sympathetic neural remodeling, ultimately resulting in improved cardiac function and reduction in ventricular arrhythmias after myocardial infarction.52
Gut bacteria-derived acetate-mediated reduction in cardiac fibrosis may be another mechanism for the effects on ventricular arrhythmias. Cardiac fibrosis and scar are established as the primary substrate for reentrant ventricular arrhythmias seen in various cardiomyopathies.
Future Directions
The microbiome residing in the human gut has a significant impact on cardiac arrhythmias, the details of which remain unknown. A likely bidirectional relationship exists in which the gut microbiome may affect arrhythmogenesis and in turn be affected by cardiac arrhythmias. The mechanisms of action are not well understood, but likely involve the autonomic nervous system, inflammation, and metabolic pathways.
The gut microbiome is a complex collection of heterogenous microorganisms that have dramatic effects on the human body. Additional research is necessary to identify further associations and causations of gut microorganisms with various human body processes, as well as cardiovascular disease. The microbiome has been shown to directly and indirectly influence the development of different disease states, including the cardiovascular system and cardiac arrhythmias. Several pathways have been proposed through which the gut microbiome can potentially affect cardiac arrhythmogenesis. There are likely several mechanisms simultaneously in operation. Understanding the role of human gut microbiome in the genesis of cardiac arrhythmias not only may improve our understanding of arrhythmias, but also may result in novel treatment options. This could potentially lead to the development of therapeutic options and strategies to modulate the gut microbiome to help detect, prevent, and treat cardiac arrhythmias.
- Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The central nervous system and the gut microbiome. Cell. 2016;167(4):915-932. doi:10.1016/j.cell.2016.10.027
- Karlsson F, Tremaroli V, Nielsen J, Bäckhed F. Assessing the human gut microbiota in metabolic diseases. Diabetes. 2013;62(10):3341-3349. doi:10.2337/db13-0844
- Danneskiold-Samsøe NB, Dias de Freitas Queiroz Barros H, Santos R, et al. Interplay between food and gut microbiota in health and disease. Food Res Int. 2019;115:23-31. doi:10.1016/j.foodres.2018.07.043
- Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe- derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446-450. doi:10.1038/nature12721
- Integrative HMP (iHMP) Research Network Consortium. The integrative human microbiome project. Nature. 2019;569(7758):641-648. doi:10.1038/s41586-019-1238-8
- Zubcevic J, Richards EM, Yang T, et al. Impaired autonomic nervous system-microbiome circuit in hypertension. Circ Res. 2019;125(1):104-116. doi:10.1161/CIRCRESAHA.119.313965
- Emoto T, Yamashita T, Sasaki N, et al. Analysis of gut microbiota in coronary artery disease patients: a possible link between gut microbiota and coronary artery disease. J Atheroscler Thromb. 2016;23(8):908-921. doi:10.5551/jat.32672
- Zuo K, Li J, Li K, et al. Disordered gut microbiota and alterations in metabolic patterns are associated with atrial fibrillation. Gigascience. 2019;8(6):giz058. doi:10.1093/gigascience/giz058
- Li J, Zhao F, Wang Y, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5(1):14. doi:10.1186/s40168-016-0222-x
- Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55-60. doi:10.1038/nature11450
- Chang CJ, Lin CS, Lu CC, et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat Commun. 2015;6:7489. doi:10.1038/ncomms8489
- Meng G, Zhou X, Wang M, et al. Gut microbederived metabolite trimethylamine N-oxide activates the cardiac autonomic nervous system and facilitates ischemia-induced ventricular arrhythmia via two different pathways. EBioMedicine. 2019;44:656-664. doi:10.1016/j.ebiom.2019.03.066
- Yoshida N, Emoto T, Yamashita T, et al. Bacteroides vulgatus and Bacteroides dorei reduce gut microbial lipopolysaccharide production and inhibit atherosclerosis. Circulation. 2018;138(22):2486-2498. doi:10.1161/CIRCULATIONAHA.118.033714
- Cussotto S, Sandhu KV, Dinan TG, Cryan JF. The neuroendocrinology of the microbiota-gut-brain axis: a behavioural perspective. Front Neuroendocrinol. 2018;51:80-101. doi:10.1016/j.yfrne.2018.04.002
- Dinan TG, Stilling RM, Stanton C, Cryan JF. Collective unconscious: how gut microbes shape human behavior. J Psychiatr Res. 2015;63:1-9. doi:10.1016/j.jpsychires.2015.02.021
- Muller PA, Schneeberger M, Matheis F, et al. Microbiota modulate sympathetic neurons via a gutbrain circuit. Nature. 2020;583(7816):441-446. doi:10.1038/s41586-020-2474-7
- Ohira H, Tsutsui W, Fujioka Y. Are short chain fatty acids in gut microbiota defensive players for inflammation and atherosclerosis? J Atheroscler Thromb. 2017;24(7):660-672. doi:10.5551/jat.RV17006
- Aguilar EC, Leonel AJ, Teixeira LG, et al. Butyrate impairs atherogenesis by reducing plaque inflammation and vulnerability and decreasing NFêB activation. Nutr Metab Cardiovasc Dis. 2014;24(6):606-613. doi:10.1016/j.numecd.2014.01.002
- Wei SG, Yu Y, Zhang ZH, Felder RB. Proinflammatory cytokines upregulate sympathoexcit - atory mechanisms in the subfornical organ of the rat. Hypertension. 2015;65(5):1126-1133. doi:10.1161/HYPERTENSIONAHA.114.05112
- Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry. 2013;74(10):720- 726. doi:10.1016/j.biopsych.2013.05.001
- Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res. 2008;43(2):164-174. doi:10.1016/j.jpsychires.2008.03.009
- Lyte M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: microbial endocrinology in the design and use of probiotics. Bioessays. 2011;33(8):574-581. doi:10.1002/bies.201100024
- Yusuf S, Al-Saady N, Camm AJ. 5-hydroxytryptamine and atrial fibrillation: how significant is this piece in the puzzle? J Cardiovasc Electrophysiol. 2003;14(2):209-214. doi:10.1046/j.1540-8167.2003.02381.x
- Marques FZ, Nelson E, Chu PY, et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation. 2017;135(10):964-977. doi:10.1161/CIRCULATIONAHA.116.024545
- Björkegren JLM, Lusis AJ. Atherosclerosis: recent developments. Cell. 2022;185(10):1630-1645. doi:10.1016/j.cell.2022.04.004
- Tang WHW, Bäckhed F, Landmesser U, Hazen SL. Intestinal microbiota in cardiovascular health and disease: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(16):2089-2105. doi:10.1016/j.jacc.2019.03.024
- Jie Z, Xia H, Zhong SL, et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun. 2017;8(1):845. doi:10.1038/s41467-017-00900-1
- Kasahara K, Krautkramer KA, Org E, et al. Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nat Microbiol. 2018;3(12):1461- 1471. doi:10.1038/s41564-018-0272-x
- Roberts AB, Gu X, Buffa JA, et al. Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential. Nat Med. 2018;24(9):1407-1417. doi:10.1038/s41591-018-0128-1
- Yu L, Meng G, Huang B, et al. A potential relationship between gut microbes and atrial fibrillation: trimethylamine N-oxide, a gut microbe-derived metabolite, facilitates the progression of atrial fibrillation. Int J Cardiol. 2018;255:92- 98. doi:10.1016/j.ijcard.2017.11.071
- Okazaki R, Iwasaki YK, Miyauchi Y, et al. Lipopolysaccharide induces atrial arrhythmogenesis via down-regulation of L-type Ca2+ channel genes in rats. Int Heart J. 2009;50(3):353-363. doi:10.1536/ihj.50.353
- Chen WT, Chen YC, Hsieh MH, et al. The uremic toxin indoxyl sulfate increases pulmonary vein and atrial arrhythmogenesis. J Cardiovasc Electrophysiol. 2015;26(2):203- 210. doi:10.1111/jce.12554
- Fretts AM, Mozaffarian D, Siscovick DS, et al. Plasma phospholipid saturated fatty acids and incident atrial fibrillation: the Cardiovascular Health Study. J Am Heart Assoc. 2014;3(3):e000889. doi:10.1161/JAHA.114.000889
- Horas HNS, Nishiumi S, Kawano Y, Kobayashi T, Yoshida M, Azuma T. Adrenic acid as an inflammation enhancer in non-alcoholic fatty liver disease. Arch Biochem Biophys. 2017;623-624:64-75. doi:10.1016/j.abb.2017.04.009
- Tabata T, Yamashita T, Hosomi K, et al. Gut microbial composition in patients with atrial fibrillation: effects of diet and drugs. Heart Vessels. 2021;36(1):105-114. doi:10.1007/s00380-020-01669-y
- López-Rodriguez ML, Benhamú B, Morcillo MJ, et al. 5-HT(4) receptor antagonists: structure-affinity relationships and ligand-receptor interactions. Curr Top Med Chem. 2002;2(6):625-641. doi:10.2174/1568026023393769
- Yu L, Zhou L, Cao G, et al. Optogenetic modulation of cardiac sympathetic nerve activity to prevent ventricular arrhythmias. J Am Coll Cardiol. 2017;70(22):2778-2790. doi:10.1016/j.jacc.2017.09.1107
- Schwartz PJ, Vanoli E. Cardiac arrhythmias elicited by interaction between acute myocardial ischemia and sympathetic hyperactivity: a new experimental model for the study of antiarrhythmic drugs. J Cardiovasc Pharmacol. 1981;3(6):1251-1259. doi:10.1097/00005344-198111000-00012
- Puddu PE, Jouve R, Langlet F, Guillen JC, Lanti M, Reale A. Prevention of postischemic ventricular fibrillation late after right or left stellate ganglionectomy in dogs. Circulation. 1988;77(4):935-946. doi:10.1161/01.cir.77.4.935
- Vaseghi M, Gima J, Kanaan C, et al. Cardiac sympathetic denervation in patients with refractory ventricular arrhythmias or electrical storm: intermediate and longterm follow-up. Heart Rhythm. 2014;11(3):360-366. doi:10.1016/j.hrthm.2013.11.028
- Wang M, Li S, Zhou X, et al. Increased inflammation promotes ventricular arrhythmia through aggravating left stellate ganglion remodeling in a canine ischemia model. Int J Cardiol. 2017;248:286-293. doi:10.1016/j.ijcard.2017.08.011
- Shi Z, Gan XB, Fan ZD, et al. Inflammatory cytokines in paraventricular nucleus modulate sympathetic activity and cardiac sympathetic afferent reflex in rats. Acta Physiol (Oxf). 2011;203(2):289-297. doi:10.1111/j.1748-1716.2011.02313.x
- Gill S, Veinot J, Kavanagh M, Pulido O. Human heart glutamate receptors - implications for toxicology, food safety, and drug discovery. Toxicol Pathol. 2007;35(3):411-417. doi:10.1080/01926230701230361
- Govoruskina N, Jakovljevic V, Zivkovic V, et al. The role of cardiac N-methyl-D-aspartate receptors in heart conditioning— effects on heart function and oxidative stress. Biomolecules. 2020;10(7):1065. doi:10.3390/biom10071065
- Lü J, Gao X, Gu J, et al. Nerve sprouting contributes to increased severity of ventricular tachyarrhythmias by upregulating iGluRs in rats with healed myocardial necrotic injury. J Mol Neurosci. 2012;48(2):448-455. doi:10.1007/s12031-012-9720-x
- Shi S, Liu T, Li Y, et al. Chronic N-methyl-D-aspartate receptor activation induces cardiac electrical remodeling and increases susceptibility to ventricular arrhythmias. Pacing Clin Electrophysiol. 2014;37(10):1367-1377. doi:10.1111/pace.12430
- Zhang Z, Bassam B, Thomas AG, et al. Maternal inflammation leads to impaired glutamate homeostasis and upregulation of glutamate carboxypeptidase II in activated microglia in the fetal/newborn rabbit brain. Neurobiol Dis. 2016;94:116-128. doi:10.1016/j.nbd.2016.06.010
- Wu LJ, Toyoda H, Zhao MG, et al. Upregulation of forebrain NMDA NR2B receptors contributes to behavioral sensitization after inflammation. J Neurosci. 2005;25(48):11107-11116. doi:10.1523/JNEUROSCI.1678-05.2005
- el-Mahdy SA. 5-hydroxytryptamine (serotonin) enhances ventricular arrhythmias induced by acute coronary artery ligation in rats. Res Commun Chem Pathol Pharmacol. 1990;68(3):383-386.
- Zhou M, Li D, Xie K, et al. The short-chain fatty acid propionate improved ventricular electrical remodeling in a rat model with myocardial infarction. Food Funct. 2021;12(24):12580-12593. doi:10.1039/d1fo02040d
- Bartolomaeus H, Balogh A, Yakoub M, et al. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation. 2019;139(11):1407-1421. doi:10.1161/CIRCULATIONAHA.118.036652
- Jiang X, Huang X, Tong Y, Gao H. Butyrate improves cardiac function and sympathetic neural remodeling following myocardial infarction in rats. Can J Physiol Pharmacol. 2020;98(6):391-399. doi:10.1139/cjpp-2019-0531
- Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The central nervous system and the gut microbiome. Cell. 2016;167(4):915-932. doi:10.1016/j.cell.2016.10.027
- Karlsson F, Tremaroli V, Nielsen J, Bäckhed F. Assessing the human gut microbiota in metabolic diseases. Diabetes. 2013;62(10):3341-3349. doi:10.2337/db13-0844
- Danneskiold-Samsøe NB, Dias de Freitas Queiroz Barros H, Santos R, et al. Interplay between food and gut microbiota in health and disease. Food Res Int. 2019;115:23-31. doi:10.1016/j.foodres.2018.07.043
- Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe- derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446-450. doi:10.1038/nature12721
- Integrative HMP (iHMP) Research Network Consortium. The integrative human microbiome project. Nature. 2019;569(7758):641-648. doi:10.1038/s41586-019-1238-8
- Zubcevic J, Richards EM, Yang T, et al. Impaired autonomic nervous system-microbiome circuit in hypertension. Circ Res. 2019;125(1):104-116. doi:10.1161/CIRCRESAHA.119.313965
- Emoto T, Yamashita T, Sasaki N, et al. Analysis of gut microbiota in coronary artery disease patients: a possible link between gut microbiota and coronary artery disease. J Atheroscler Thromb. 2016;23(8):908-921. doi:10.5551/jat.32672
- Zuo K, Li J, Li K, et al. Disordered gut microbiota and alterations in metabolic patterns are associated with atrial fibrillation. Gigascience. 2019;8(6):giz058. doi:10.1093/gigascience/giz058
- Li J, Zhao F, Wang Y, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5(1):14. doi:10.1186/s40168-016-0222-x
- Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55-60. doi:10.1038/nature11450
- Chang CJ, Lin CS, Lu CC, et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat Commun. 2015;6:7489. doi:10.1038/ncomms8489
- Meng G, Zhou X, Wang M, et al. Gut microbederived metabolite trimethylamine N-oxide activates the cardiac autonomic nervous system and facilitates ischemia-induced ventricular arrhythmia via two different pathways. EBioMedicine. 2019;44:656-664. doi:10.1016/j.ebiom.2019.03.066
- Yoshida N, Emoto T, Yamashita T, et al. Bacteroides vulgatus and Bacteroides dorei reduce gut microbial lipopolysaccharide production and inhibit atherosclerosis. Circulation. 2018;138(22):2486-2498. doi:10.1161/CIRCULATIONAHA.118.033714
- Cussotto S, Sandhu KV, Dinan TG, Cryan JF. The neuroendocrinology of the microbiota-gut-brain axis: a behavioural perspective. Front Neuroendocrinol. 2018;51:80-101. doi:10.1016/j.yfrne.2018.04.002
- Dinan TG, Stilling RM, Stanton C, Cryan JF. Collective unconscious: how gut microbes shape human behavior. J Psychiatr Res. 2015;63:1-9. doi:10.1016/j.jpsychires.2015.02.021
- Muller PA, Schneeberger M, Matheis F, et al. Microbiota modulate sympathetic neurons via a gutbrain circuit. Nature. 2020;583(7816):441-446. doi:10.1038/s41586-020-2474-7
- Ohira H, Tsutsui W, Fujioka Y. Are short chain fatty acids in gut microbiota defensive players for inflammation and atherosclerosis? J Atheroscler Thromb. 2017;24(7):660-672. doi:10.5551/jat.RV17006
- Aguilar EC, Leonel AJ, Teixeira LG, et al. Butyrate impairs atherogenesis by reducing plaque inflammation and vulnerability and decreasing NFêB activation. Nutr Metab Cardiovasc Dis. 2014;24(6):606-613. doi:10.1016/j.numecd.2014.01.002
- Wei SG, Yu Y, Zhang ZH, Felder RB. Proinflammatory cytokines upregulate sympathoexcit - atory mechanisms in the subfornical organ of the rat. Hypertension. 2015;65(5):1126-1133. doi:10.1161/HYPERTENSIONAHA.114.05112
- Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry. 2013;74(10):720- 726. doi:10.1016/j.biopsych.2013.05.001
- Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res. 2008;43(2):164-174. doi:10.1016/j.jpsychires.2008.03.009
- Lyte M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: microbial endocrinology in the design and use of probiotics. Bioessays. 2011;33(8):574-581. doi:10.1002/bies.201100024
- Yusuf S, Al-Saady N, Camm AJ. 5-hydroxytryptamine and atrial fibrillation: how significant is this piece in the puzzle? J Cardiovasc Electrophysiol. 2003;14(2):209-214. doi:10.1046/j.1540-8167.2003.02381.x
- Marques FZ, Nelson E, Chu PY, et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation. 2017;135(10):964-977. doi:10.1161/CIRCULATIONAHA.116.024545
- Björkegren JLM, Lusis AJ. Atherosclerosis: recent developments. Cell. 2022;185(10):1630-1645. doi:10.1016/j.cell.2022.04.004
- Tang WHW, Bäckhed F, Landmesser U, Hazen SL. Intestinal microbiota in cardiovascular health and disease: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(16):2089-2105. doi:10.1016/j.jacc.2019.03.024
- Jie Z, Xia H, Zhong SL, et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun. 2017;8(1):845. doi:10.1038/s41467-017-00900-1
- Kasahara K, Krautkramer KA, Org E, et al. Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nat Microbiol. 2018;3(12):1461- 1471. doi:10.1038/s41564-018-0272-x
- Roberts AB, Gu X, Buffa JA, et al. Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential. Nat Med. 2018;24(9):1407-1417. doi:10.1038/s41591-018-0128-1
- Yu L, Meng G, Huang B, et al. A potential relationship between gut microbes and atrial fibrillation: trimethylamine N-oxide, a gut microbe-derived metabolite, facilitates the progression of atrial fibrillation. Int J Cardiol. 2018;255:92- 98. doi:10.1016/j.ijcard.2017.11.071
- Okazaki R, Iwasaki YK, Miyauchi Y, et al. Lipopolysaccharide induces atrial arrhythmogenesis via down-regulation of L-type Ca2+ channel genes in rats. Int Heart J. 2009;50(3):353-363. doi:10.1536/ihj.50.353
- Chen WT, Chen YC, Hsieh MH, et al. The uremic toxin indoxyl sulfate increases pulmonary vein and atrial arrhythmogenesis. J Cardiovasc Electrophysiol. 2015;26(2):203- 210. doi:10.1111/jce.12554
- Fretts AM, Mozaffarian D, Siscovick DS, et al. Plasma phospholipid saturated fatty acids and incident atrial fibrillation: the Cardiovascular Health Study. J Am Heart Assoc. 2014;3(3):e000889. doi:10.1161/JAHA.114.000889
- Horas HNS, Nishiumi S, Kawano Y, Kobayashi T, Yoshida M, Azuma T. Adrenic acid as an inflammation enhancer in non-alcoholic fatty liver disease. Arch Biochem Biophys. 2017;623-624:64-75. doi:10.1016/j.abb.2017.04.009
- Tabata T, Yamashita T, Hosomi K, et al. Gut microbial composition in patients with atrial fibrillation: effects of diet and drugs. Heart Vessels. 2021;36(1):105-114. doi:10.1007/s00380-020-01669-y
- López-Rodriguez ML, Benhamú B, Morcillo MJ, et al. 5-HT(4) receptor antagonists: structure-affinity relationships and ligand-receptor interactions. Curr Top Med Chem. 2002;2(6):625-641. doi:10.2174/1568026023393769
- Yu L, Zhou L, Cao G, et al. Optogenetic modulation of cardiac sympathetic nerve activity to prevent ventricular arrhythmias. J Am Coll Cardiol. 2017;70(22):2778-2790. doi:10.1016/j.jacc.2017.09.1107
- Schwartz PJ, Vanoli E. Cardiac arrhythmias elicited by interaction between acute myocardial ischemia and sympathetic hyperactivity: a new experimental model for the study of antiarrhythmic drugs. J Cardiovasc Pharmacol. 1981;3(6):1251-1259. doi:10.1097/00005344-198111000-00012
- Puddu PE, Jouve R, Langlet F, Guillen JC, Lanti M, Reale A. Prevention of postischemic ventricular fibrillation late after right or left stellate ganglionectomy in dogs. Circulation. 1988;77(4):935-946. doi:10.1161/01.cir.77.4.935
- Vaseghi M, Gima J, Kanaan C, et al. Cardiac sympathetic denervation in patients with refractory ventricular arrhythmias or electrical storm: intermediate and longterm follow-up. Heart Rhythm. 2014;11(3):360-366. doi:10.1016/j.hrthm.2013.11.028
- Wang M, Li S, Zhou X, et al. Increased inflammation promotes ventricular arrhythmia through aggravating left stellate ganglion remodeling in a canine ischemia model. Int J Cardiol. 2017;248:286-293. doi:10.1016/j.ijcard.2017.08.011
- Shi Z, Gan XB, Fan ZD, et al. Inflammatory cytokines in paraventricular nucleus modulate sympathetic activity and cardiac sympathetic afferent reflex in rats. Acta Physiol (Oxf). 2011;203(2):289-297. doi:10.1111/j.1748-1716.2011.02313.x
- Gill S, Veinot J, Kavanagh M, Pulido O. Human heart glutamate receptors - implications for toxicology, food safety, and drug discovery. Toxicol Pathol. 2007;35(3):411-417. doi:10.1080/01926230701230361
- Govoruskina N, Jakovljevic V, Zivkovic V, et al. The role of cardiac N-methyl-D-aspartate receptors in heart conditioning— effects on heart function and oxidative stress. Biomolecules. 2020;10(7):1065. doi:10.3390/biom10071065
- Lü J, Gao X, Gu J, et al. Nerve sprouting contributes to increased severity of ventricular tachyarrhythmias by upregulating iGluRs in rats with healed myocardial necrotic injury. J Mol Neurosci. 2012;48(2):448-455. doi:10.1007/s12031-012-9720-x
- Shi S, Liu T, Li Y, et al. Chronic N-methyl-D-aspartate receptor activation induces cardiac electrical remodeling and increases susceptibility to ventricular arrhythmias. Pacing Clin Electrophysiol. 2014;37(10):1367-1377. doi:10.1111/pace.12430
- Zhang Z, Bassam B, Thomas AG, et al. Maternal inflammation leads to impaired glutamate homeostasis and upregulation of glutamate carboxypeptidase II in activated microglia in the fetal/newborn rabbit brain. Neurobiol Dis. 2016;94:116-128. doi:10.1016/j.nbd.2016.06.010
- Wu LJ, Toyoda H, Zhao MG, et al. Upregulation of forebrain NMDA NR2B receptors contributes to behavioral sensitization after inflammation. J Neurosci. 2005;25(48):11107-11116. doi:10.1523/JNEUROSCI.1678-05.2005
- el-Mahdy SA. 5-hydroxytryptamine (serotonin) enhances ventricular arrhythmias induced by acute coronary artery ligation in rats. Res Commun Chem Pathol Pharmacol. 1990;68(3):383-386.
- Zhou M, Li D, Xie K, et al. The short-chain fatty acid propionate improved ventricular electrical remodeling in a rat model with myocardial infarction. Food Funct. 2021;12(24):12580-12593. doi:10.1039/d1fo02040d
- Bartolomaeus H, Balogh A, Yakoub M, et al. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation. 2019;139(11):1407-1421. doi:10.1161/CIRCULATIONAHA.118.036652
- Jiang X, Huang X, Tong Y, Gao H. Butyrate improves cardiac function and sympathetic neural remodeling following myocardial infarction in rats. Can J Physiol Pharmacol. 2020;98(6):391-399. doi:10.1139/cjpp-2019-0531
The Gut Microbiome and Cardiac Arrhythmias
The Gut Microbiome and Cardiac Arrhythmias
A Systemic Lupus Erythematosus Incidence Surveillance Report Among DoD Beneficiaries During the COVID-19 Pandemic
A Systemic Lupus Erythematosus Incidence Surveillance Report Among DoD Beneficiaries During the COVID-19 Pandemic
Systemic lupus erythematosus (SLE), or lupus, is a rare autoimmune disease estimated to occur in about 5.1 cases per 100,000 person-years in the United States in 2018.1 The disease predominantly affects females, with an incidence of 8.7 cases per 100,000 person-years vs 1.2 cases per 100,000 person-years in males, and is most common in patients aged 15 to 44 years.1,2
Lupus presents with a constellation of clinical signs and symptoms that evolve, along with hallmark laboratory findings indicative of immune dysregulation and polyclonal B-cell activation. Consequently, a wide array of autoantibodies may be produced, although the combination of epitope specificity can vary from patient to patient.3 Nevertheless, > 98% of individuals diagnosed with lupus produce antinuclear antibodies (ANA), making ANA positivity a near-universal serologic feature at the time of diagnosis.
The pathogenesis of lupus is complex. Research from the past 5 decades supports the role of certain viral infections—such as Epstein-Barr virus (EBV) and cytomegalovirus—as potential triggers.4 These viruses are thought to initiate disease through mechanisms including activation of interferon pathways, exposure of cryptic intracellular antigens, molecular mimicry, and epitope spreading. Subsequent clonal expansion and autoantibody production occur to varying degrees, influenced by viral load and host susceptibility factors.
During the COVID-19 pandemic, it became evident that SARS-CoV-2 exerts profound effects on immune regulation, influencing infection outcomes through mechanisms such as hyperactivation of innate immunity, especially in the lungs, leading to acute respiratory distress syndrome. Additionally, SARS-CoV-2 has been associated with polyclonal B-cell activation and the generation of autoantibodies. This association gained attention after Bastard et al identified anti–type I interferon antibodies in patients with severe COVID-19, predominantly among males with a genetic predisposition. These autoantibodies were shown to impair antiviral defenses and contribute to life-threatening pneumonia.5
Subsequent studies demonstrated the production of a wide spectrum of functional autoantibodies, including ANA, in patients with COVID-19.6,7 These findings were attributed to the acute expansion of autoreactive clones among naïve-derived immunoglobulin G1 antibody-secreting cells during the early stages of infection, with the degree of expansion correlating with disease severity.8,9 Although longitudinal data up to 15 months postinfection suggest this serologic abnormality resolves in more than two-thirds of patients, the number of individuals infected globally has raised serious public health concerns regarding the potential long-term sequelae, including the onset of lupus or other autoimmune diseases in COVID-19 survivors.6-9 A limited number of case reports describing the onset of lupus following SARS-CoV-2 infection support this hypothesis.10
This surveillance analysis investigates lupus incidence among patients within the Military Health System (MHS), encompassing all TRICARE beneficiaries, from January 2018 to December 2022. The objective of this analysis was to examine lupus incidence trends throughout the COVID-19 pandemic, stratified by sex, age, and active-duty status.
Methods
The MHS provides health care services to about 9.5 million US Department of Defense (DoD) beneficiaries. Outpatient health records and laboratory results for individuals receiving care at military treatment facilities (MTFs) between January 1, 2018, and December 31, 2022, were obtained from the Comprehensive Ambulatory/ Professional Encounter Record and MHS GENESIS. For beneficiaries receiving care in the private sector, data were sourced from the TRICARE Encounter Data—Non-Institutional database.
Laboratory test results, including ANA testing, were available only for individuals receiving care at MTFs. These laboratory data were extracted from the Composite Health Care System Chemistry database and MHS GENESIS laboratory systems for the same time frame. Inpatient data were not included in this analysis. Data from 2017 were used solely as a look-back (or washout) period to identify and exclude prevalent lupus cases diagnosed before 2018 and were not included in the final results.
Lupus cases were identified by the presence of a positive ANA test and appropriate International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) codes. A positive ANA result was defined as either a qualitative result marked positive or a titer ≥ 1:80. The ICD-10-CM codes considered indicative of lupus included variations of M32, L93, or H01.12.
M32, L93, or H01.12. For cases with a positive ANA test, a lupus diagnosis required the presence of ≥ 2 lupus related ICD-10-CM codes. In the absence of ANA test results, a stricter criterion was applied: ≥ 4 lupus ICD-10-CM diagnosis codes recorded on separate days were required for inclusion.
Beneficiaries were excluded if they had a negative ANA result, only 1 lupus ICD- 10-CM diagnosis code, 1 positive ANA with only 1 corresponding ICD-10-CM code, or if their diagnosis occurred outside the defined study period. Patients and members of the public were not involved in the design, conduct, reporting, or dissemination of this study.
Results
Between January 1, 2017, and December 31, 2022, 99,946 TRICARE beneficiaries had some indication of lupus testing or diagnosis in their health records (Figure 1). Of these beneficiaries, 5335 had a positive ANA result and ≥ 2 ICD-10-CM lupus diagnosis codes. An additional 28,275 beneficiaries had ≥ 4 ICD-10-CM lupus diagnosis codes but no ANA test results. From these groups, the final sample included 10,760 beneficiaries who met the incident case definitions for SLE during the study period (2018 through 2022).
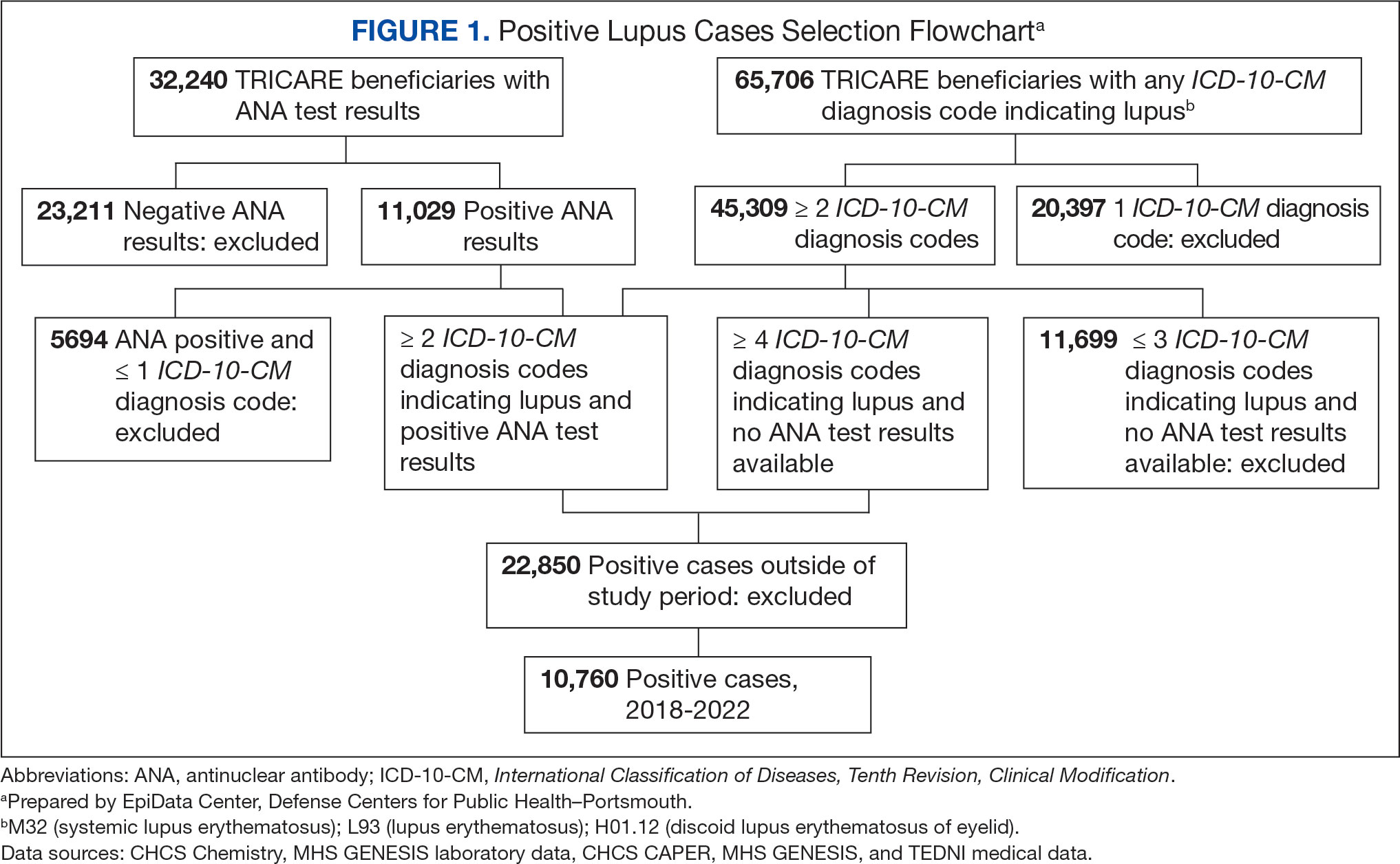
Most cases (85.1%, n = 9157) were diagnosed through TRICARE claims, while 1205 (11.2%) were diagnosed within the MHS. Another 398 (3.7%) had documentation of care both within and outside the MHS. Incident SLE cases declined by an average of 16% annually during the study period (Figure 2). This trend amounted to an overall reduction of 48.2%, from 2866 cases in 2018 to 1399 cases in 2022. This decline occurred despite total medical encounters among DoD beneficiaries remaining relatively stable during the pandemic years, with only a 3.5% change between 2018 and 2022.
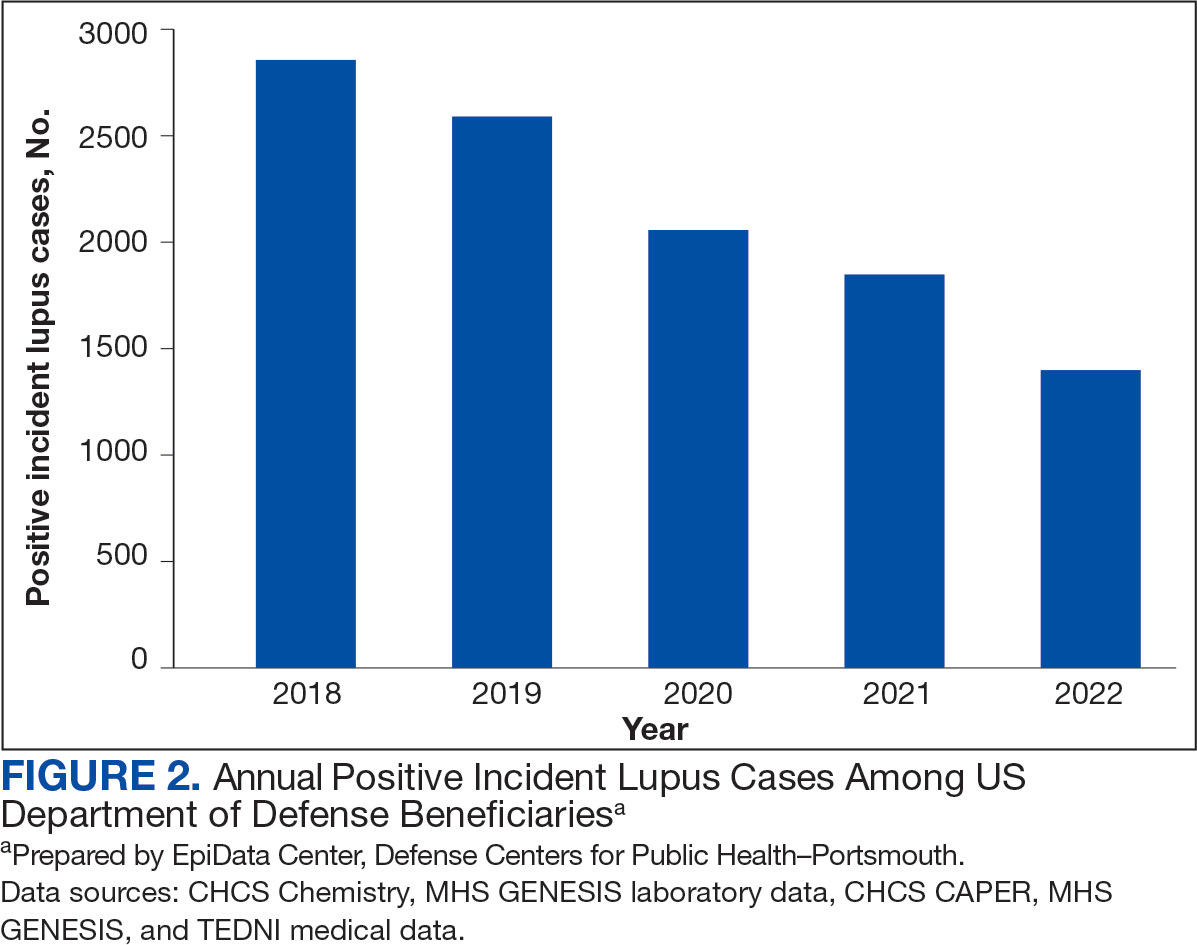
The disease was more prevalent among female beneficiaries, with a female to- male ratio of 7:1 (Table 1). Among women, the number of new cases declined from 2519 in 2018 to 1223 in 2022, while the number of cases among men remained consistently < 350 annually. Similar trends were observed across other strata. Incident SLE cases were more common among nonactive-duty beneficiaries than active-duty service members, with a ratio of 18:1. New cases among active-duty members remained < 155 per year. Age-stratified data revealed that SLE was diagnosed predominantly in individuals aged ≥ 18 years, with a ratio of 37:1 compared with individuals aged < 18 years. Among children, the number of new cases remained < 75 per year throughout the study period.
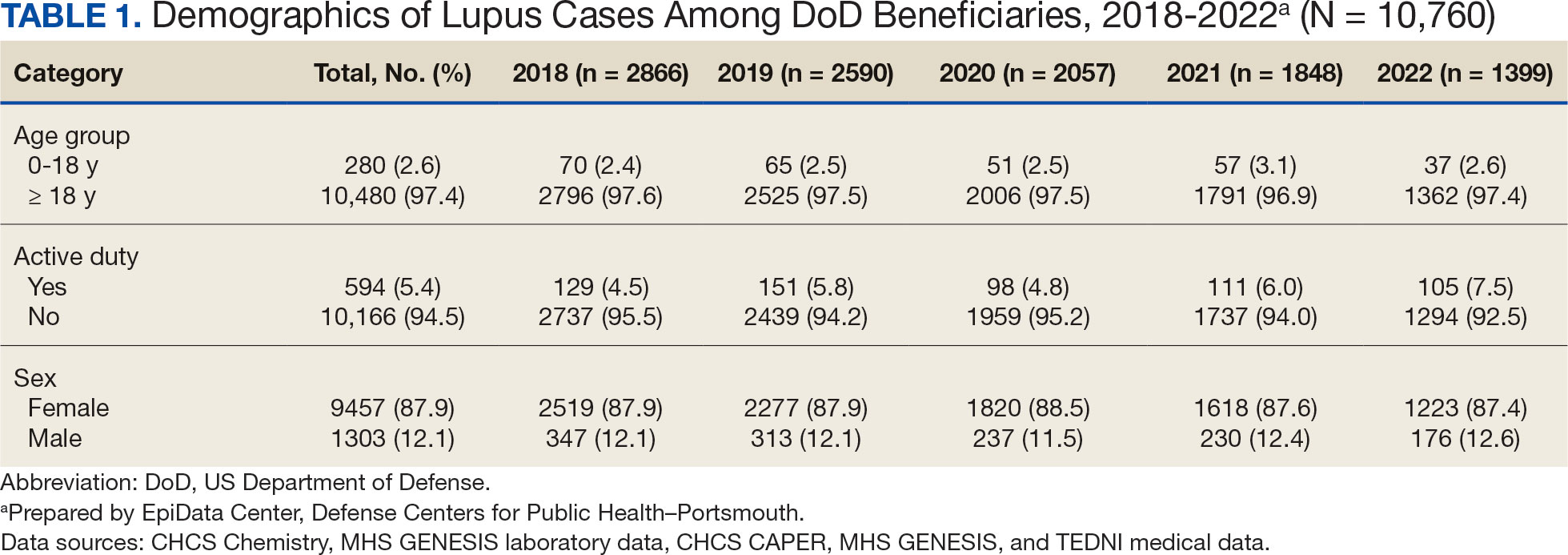
A mean 56,850 ANA tests were conducted annually in centralized laboratories using standardized protocols (Table 2). The mean ANA positivity rate was 17.3%, which remained relatively stable from 2018 through 2022.
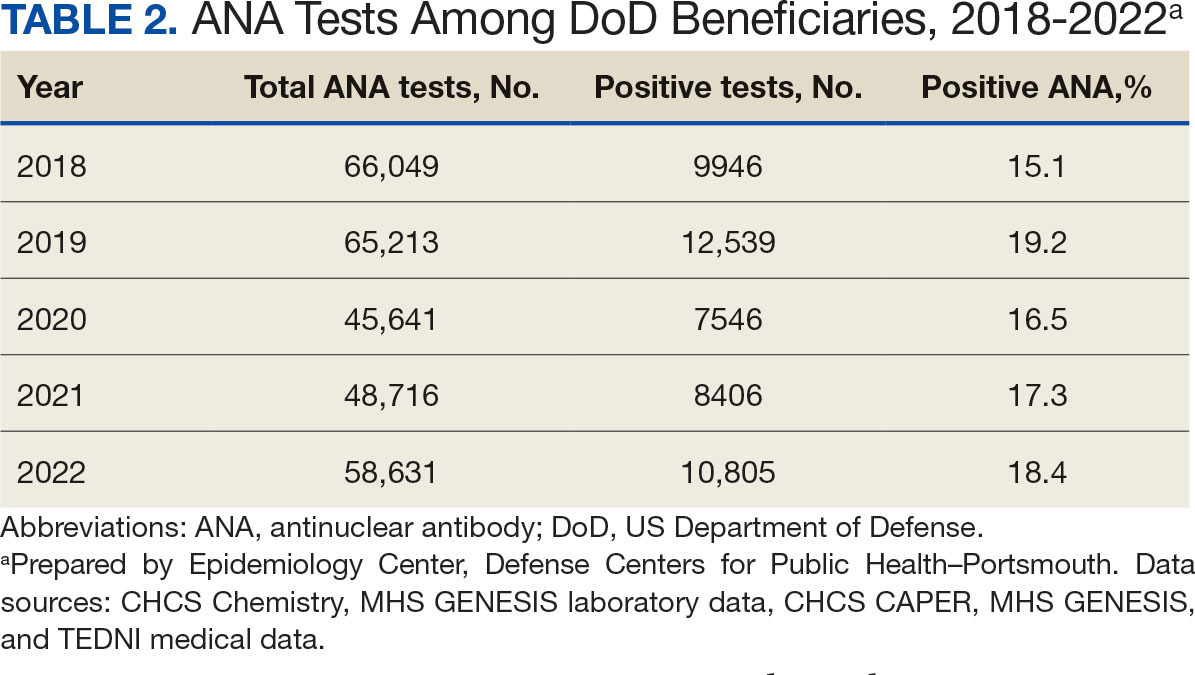
Discussion
This study examined the annual incidence of newly diagnosed SLE cases among all TRICARE beneficiaries from January 1, 2018, through December 31, 2022, covering both before and during the peak years of the COVID-19 pandemic. This analysis revealed a steady decline in SLE cases during this period. The reliability of these findings is reinforced by the comprehensiveness of the MHS, one of the largest US health care delivery systems, which maintains near-complete medical data capture for about 9.5 million DoD TRICARE beneficiaries across domestic and international settings.
SLE is a rare autoimmune disorder that presents a diagnostic challenge due to its wide range of nonspecific symptoms, many of which resemble other conditions. To reduce the likelihood of false-positive results and ensure diagnostic accuracy, this study adopted a stringent case definition. Incident cases were identified by the presence of ANA testing in conjunction with lupus-specific ICD-10-CM codes and required ≥ 4 lupus related diagnostic entries. This criterion was necessary due to the absence of ANA test results in data from private sector care settings. Our case definition aligns with established literature. For example, a Vanderbilt University chart review study demonstrated that combining ANA positivity with ≥ 4 lupus related ICD-10-CM codes achieves a positive predictive value of 100%, albeit with a sensitivity of 45%.11 Other studies similarly affirm the diagnostic validity of using recurrent ICD-10-CM codes to improve specificity in identifying lupus cases.12,13
The primary objective of this study was to examine the temporal trend in newly diagnosed lupus cases, rather than derive precise incidence rates. Although the TRICARE system includes about 9.5 million beneficiaries, this number represents a dynamic population with continual inflow and outflow. Accurate incidence rate calculation would require access to detailed denominator data, which were not readily available. In comparison with our findings, a study limited to active-duty service members reported fewer lupus cases. This discrepancy likely reflects differences in case definitions—specifically, the absence of laboratory data, the restricted range of diagnostic codes, and the requirement that diagnoses be rendered by specialists.14 Despite these differences, demographic patterns were consistent, with higher incidence observed in females and individuals aged ≥ 20 years.
A Centers for Disease Control and Prevention (CDC) study of lupus incidence in the general population also reported lower case counts.1 However, the CDC estimates were based on 5 state-level registries, which rely on clinician-reported cases and therefore may underestimate true disease burden. Moreover, the DoD beneficiary population differs markedly from the general population: it includes a large cohort of retirees, ensuring an older demographic; all members have comprehensive health care access; and active-duty personnel are subject to pre-enlistment medical screening. Taken together, these factors suggest this study may offer a more complete and systematically captured profile of lupus incidence.
We observed a marked decline of newly diagnosed SLE cases during the study period, which coincided with the widespread circulation of COVID-19. This decrease is unlikely to be attributable to reduced access to care during the pandemic. The MHS operates under a single-payer model, and the total number of patient encounters remained relatively stable throughout the pandemic.
To our knowledge, this is the only study to monitor lupus incidence in a large US population over the 5-year period encompassing before and during the COVID-19 pandemic. To date, only 4 large-scale surveillance studies have addressed similar questions. 14-17 Our findings are consistent with the most recent of these reports: an analysis limited to active-duty members of the US Armed Forces identified 1127 patients with newly diagnosed lupus between 2000 and 2022 and reported stable incidence trends throughout the pandemic.14 The other 3 studies adopted a different approach, comparing the emergence of autoimmune diseases, including lupus, between individuals with confirmed SARS-CoV-2 infection and those without. Each of these trials concluded that COVID-19 increases the risk of various autoimmune conditions, although the findings specific to lupus were inconsistent.15-17
Chang et al reported a significant increase in new lupus diagnoses (n = 2,926,016), with an adjusted hazard ratio (aHR) of 2.99 (95% CI, 2.68-3.34), spanning all ages and both sexes. The highest incidence was observed in individuals of Asian descent.15 Using German routine health care data from 2020, Tesch et al identified a heightened risk of autoimmune diseases, including lupus, among patients with a history of SARS-CoV-2 infection (n = 641,407; 9.4% children, 57.3% female, 6.4% hospitalized), compared with matched infection-naïve controls (n = 1,560,357).16 Both studies excluded vaccinated individuals.
These 2 studies diverged in their assessment of the relationship between COVID-19 severity and subsequent autoimmune risk. Chang et al found a higher incidence among nonhospitalized ambulatory patients, while Tesch et al reported that increased risk was associated with patients requiring intensive care unit admission.15,16
In contrast, based on a cohort of 4,197,188 individuals, Peng et al found no significant difference in lupus incidence among patients with SARS-CoV-2 infection (aHR, 1.05; 95% CI, 0.79-1.39).17 Notably, within the infected group, the incidence of SLE was significantly lower among vaccinated individuals compared with the unvaccinated group (aHR, 0.29; 95% CI, 0.18-0.47). Similar protective associations were observed for other antibody-mediated autoimmune disorders, including pemphigoid, Graves’ disease, and antiphospholipid antibody syndrome.
Limitations
Due to fundamental differences in study design, we were unable to directly reconcile our findings with those reported in the literature. This study lacked access to reliable documentation of COVID-19 infection status, primarily due to the widespread use of home testing among TRICARE beneficiaries. Additionally, the dataset did not include inpatient records and therefore did not permit evaluation of disease severity. Despite these constraints, it is plausible that the overall burden of COVID-19 infection within the study population was lower than that observed in the general US population.
As of December 2022, the DoD had reported about 750,000 confirmed COVID-19 cases among military personnel, civilian employees, dependents, and DoD contractors.18 Given that TRICARE beneficiaries represent about 2.8% of the total US population—and that > 90 million US individuals were infected between 2020 and 2022—the implied infection rate in our cohort appears to be about one-third of what might be expected.19 This discrepancy may be due to higher adherence to mitigation measures, such as social distancing and mask usage, among DoD-affiliated populations. COVID-19 vaccination was mandated for all active-duty service members, who constitute 5.4% of the study population. The remaining TRICARE beneficiaries also had access to guaranteed health care and vaccination coverage, likely contributing to high overall vaccination rates.
Because > 80% of the study population was composed of individuals from diverse civilian backgrounds, we expect the distribution of infection severity within the DoD beneficiary population to approximate that of the general US population.
Future Directions
The findings of this study offer circumstantial yet real-time evidence of the complexity underlying immune dysregulation at the intersection of host susceptibility and environmental exposures. The stability in ANA positivity rates during the study period mitigates concerns regarding undiagnosed subclinical lupus and may suggest that, overall, immune homeostasis was preserved among DoD beneficiaries.
It is noteworthy that during the COVID-19 pandemic, the incidence of several common infections—such as influenza and EBV—declined markedly, likely as a result of widespread social distancing and other public health interventions.20 Mitigation strategies implemented within the military may have conferred protection not only against COVID-19 but also against other community-acquired pathogens.
In light of these observations, we hypothesize that for COVID-19 to act as a trigger for SLE, a prolonged or repeated disruption of immune equilibrium may be required—potentially mediated by recurrent infections or sustained inflammatory states. The association between viral infections and autoimmunity is well established. Immune dysregulation leading to autoantibody production has been observed not only in the context of SARS-CoV-2 but also following infections with EBV, cytomegalovirus, enteroviruses, hepatitis B and C viruses, HIV, and parvovirus B19.21
This dysregulation is often transient, accompanied by compensatory immune regulatory responses. However, in individuals subjected to successive or overlapping infections, these regulatory mechanisms may become compromised or overwhelmed, due to emergent patterns of immune interference of varying severity. In such cases, a transient immune perturbation may progress into a bona fide autoimmune disease, contingent upon individual risk factors such as genetic predisposition, preexisting immune memory, and regenerative capacity.21
Therefore, we believe the significance of this study is 2-fold. First, lupus is known to develop gradually and may require 3 to 5 years to clinically manifest after the initial break in immunological tolerance.3 Continued public health surveillance represents a more pragmatic strategy than retrospective cohort construction, especially as histories of COVID-19 infection become increasingly complete and definitive. Our findings provide a valuable baseline reference point for future longitudinal studies.
The interpretation of surveillance outcomes—whether indicating an upward trend, a stable baseline, or a downward trend—offers distinct analytical value. Within this study population, we observed neither an upward trajectory that might suggest a direct causal link, nor a flat trend that would imply absence of association between COVID-19 and lupus pathogenesis. Instead, the observation of a downward trend invites consideration of nonlinear or protective influences. From this perspective, we recommend that future investigations adopt a holistic framework when assessing environmental contributions to immune dysregulation—particularly when evaluating the long-term immunopathological consequences of the COVID-19 pandemic on lupus and related autoimmune conditions.
Conclusions
This study identified a declining trend in incident lupus cases during the COVID-19 pandemic among the DoD beneficiary population. Further investigation is warranted to elucidate the underlying factors contributing to this decline. Conducting longitudinal epidemiologic studies and applying multivariable regression analyses will be essential to determine whether incidence rates revert to prepandemic baselines and how these trends may be influenced by evolving environmental factors within the general population.
Systemic lupus erythematosus (SLE), or lupus, is a rare autoimmune disease estimated to occur in about 5.1 cases per 100,000 person-years in the United States in 2018.1 The disease predominantly affects females, with an incidence of 8.7 cases per 100,000 person-years vs 1.2 cases per 100,000 person-years in males, and is most common in patients aged 15 to 44 years.1,2
Lupus presents with a constellation of clinical signs and symptoms that evolve, along with hallmark laboratory findings indicative of immune dysregulation and polyclonal B-cell activation. Consequently, a wide array of autoantibodies may be produced, although the combination of epitope specificity can vary from patient to patient.3 Nevertheless, > 98% of individuals diagnosed with lupus produce antinuclear antibodies (ANA), making ANA positivity a near-universal serologic feature at the time of diagnosis.
The pathogenesis of lupus is complex. Research from the past 5 decades supports the role of certain viral infections—such as Epstein-Barr virus (EBV) and cytomegalovirus—as potential triggers.4 These viruses are thought to initiate disease through mechanisms including activation of interferon pathways, exposure of cryptic intracellular antigens, molecular mimicry, and epitope spreading. Subsequent clonal expansion and autoantibody production occur to varying degrees, influenced by viral load and host susceptibility factors.
During the COVID-19 pandemic, it became evident that SARS-CoV-2 exerts profound effects on immune regulation, influencing infection outcomes through mechanisms such as hyperactivation of innate immunity, especially in the lungs, leading to acute respiratory distress syndrome. Additionally, SARS-CoV-2 has been associated with polyclonal B-cell activation and the generation of autoantibodies. This association gained attention after Bastard et al identified anti–type I interferon antibodies in patients with severe COVID-19, predominantly among males with a genetic predisposition. These autoantibodies were shown to impair antiviral defenses and contribute to life-threatening pneumonia.5
Subsequent studies demonstrated the production of a wide spectrum of functional autoantibodies, including ANA, in patients with COVID-19.6,7 These findings were attributed to the acute expansion of autoreactive clones among naïve-derived immunoglobulin G1 antibody-secreting cells during the early stages of infection, with the degree of expansion correlating with disease severity.8,9 Although longitudinal data up to 15 months postinfection suggest this serologic abnormality resolves in more than two-thirds of patients, the number of individuals infected globally has raised serious public health concerns regarding the potential long-term sequelae, including the onset of lupus or other autoimmune diseases in COVID-19 survivors.6-9 A limited number of case reports describing the onset of lupus following SARS-CoV-2 infection support this hypothesis.10
This surveillance analysis investigates lupus incidence among patients within the Military Health System (MHS), encompassing all TRICARE beneficiaries, from January 2018 to December 2022. The objective of this analysis was to examine lupus incidence trends throughout the COVID-19 pandemic, stratified by sex, age, and active-duty status.
Methods
The MHS provides health care services to about 9.5 million US Department of Defense (DoD) beneficiaries. Outpatient health records and laboratory results for individuals receiving care at military treatment facilities (MTFs) between January 1, 2018, and December 31, 2022, were obtained from the Comprehensive Ambulatory/ Professional Encounter Record and MHS GENESIS. For beneficiaries receiving care in the private sector, data were sourced from the TRICARE Encounter Data—Non-Institutional database.
Laboratory test results, including ANA testing, were available only for individuals receiving care at MTFs. These laboratory data were extracted from the Composite Health Care System Chemistry database and MHS GENESIS laboratory systems for the same time frame. Inpatient data were not included in this analysis. Data from 2017 were used solely as a look-back (or washout) period to identify and exclude prevalent lupus cases diagnosed before 2018 and were not included in the final results.
Lupus cases were identified by the presence of a positive ANA test and appropriate International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) codes. A positive ANA result was defined as either a qualitative result marked positive or a titer ≥ 1:80. The ICD-10-CM codes considered indicative of lupus included variations of M32, L93, or H01.12.
M32, L93, or H01.12. For cases with a positive ANA test, a lupus diagnosis required the presence of ≥ 2 lupus related ICD-10-CM codes. In the absence of ANA test results, a stricter criterion was applied: ≥ 4 lupus ICD-10-CM diagnosis codes recorded on separate days were required for inclusion.
Beneficiaries were excluded if they had a negative ANA result, only 1 lupus ICD- 10-CM diagnosis code, 1 positive ANA with only 1 corresponding ICD-10-CM code, or if their diagnosis occurred outside the defined study period. Patients and members of the public were not involved in the design, conduct, reporting, or dissemination of this study.
Results
Between January 1, 2017, and December 31, 2022, 99,946 TRICARE beneficiaries had some indication of lupus testing or diagnosis in their health records (Figure 1). Of these beneficiaries, 5335 had a positive ANA result and ≥ 2 ICD-10-CM lupus diagnosis codes. An additional 28,275 beneficiaries had ≥ 4 ICD-10-CM lupus diagnosis codes but no ANA test results. From these groups, the final sample included 10,760 beneficiaries who met the incident case definitions for SLE during the study period (2018 through 2022).

Most cases (85.1%, n = 9157) were diagnosed through TRICARE claims, while 1205 (11.2%) were diagnosed within the MHS. Another 398 (3.7%) had documentation of care both within and outside the MHS. Incident SLE cases declined by an average of 16% annually during the study period (Figure 2). This trend amounted to an overall reduction of 48.2%, from 2866 cases in 2018 to 1399 cases in 2022. This decline occurred despite total medical encounters among DoD beneficiaries remaining relatively stable during the pandemic years, with only a 3.5% change between 2018 and 2022.

The disease was more prevalent among female beneficiaries, with a female to- male ratio of 7:1 (Table 1). Among women, the number of new cases declined from 2519 in 2018 to 1223 in 2022, while the number of cases among men remained consistently < 350 annually. Similar trends were observed across other strata. Incident SLE cases were more common among nonactive-duty beneficiaries than active-duty service members, with a ratio of 18:1. New cases among active-duty members remained < 155 per year. Age-stratified data revealed that SLE was diagnosed predominantly in individuals aged ≥ 18 years, with a ratio of 37:1 compared with individuals aged < 18 years. Among children, the number of new cases remained < 75 per year throughout the study period.

A mean 56,850 ANA tests were conducted annually in centralized laboratories using standardized protocols (Table 2). The mean ANA positivity rate was 17.3%, which remained relatively stable from 2018 through 2022.

Discussion
This study examined the annual incidence of newly diagnosed SLE cases among all TRICARE beneficiaries from January 1, 2018, through December 31, 2022, covering both before and during the peak years of the COVID-19 pandemic. This analysis revealed a steady decline in SLE cases during this period. The reliability of these findings is reinforced by the comprehensiveness of the MHS, one of the largest US health care delivery systems, which maintains near-complete medical data capture for about 9.5 million DoD TRICARE beneficiaries across domestic and international settings.
SLE is a rare autoimmune disorder that presents a diagnostic challenge due to its wide range of nonspecific symptoms, many of which resemble other conditions. To reduce the likelihood of false-positive results and ensure diagnostic accuracy, this study adopted a stringent case definition. Incident cases were identified by the presence of ANA testing in conjunction with lupus-specific ICD-10-CM codes and required ≥ 4 lupus related diagnostic entries. This criterion was necessary due to the absence of ANA test results in data from private sector care settings. Our case definition aligns with established literature. For example, a Vanderbilt University chart review study demonstrated that combining ANA positivity with ≥ 4 lupus related ICD-10-CM codes achieves a positive predictive value of 100%, albeit with a sensitivity of 45%.11 Other studies similarly affirm the diagnostic validity of using recurrent ICD-10-CM codes to improve specificity in identifying lupus cases.12,13
The primary objective of this study was to examine the temporal trend in newly diagnosed lupus cases, rather than derive precise incidence rates. Although the TRICARE system includes about 9.5 million beneficiaries, this number represents a dynamic population with continual inflow and outflow. Accurate incidence rate calculation would require access to detailed denominator data, which were not readily available. In comparison with our findings, a study limited to active-duty service members reported fewer lupus cases. This discrepancy likely reflects differences in case definitions—specifically, the absence of laboratory data, the restricted range of diagnostic codes, and the requirement that diagnoses be rendered by specialists.14 Despite these differences, demographic patterns were consistent, with higher incidence observed in females and individuals aged ≥ 20 years.
A Centers for Disease Control and Prevention (CDC) study of lupus incidence in the general population also reported lower case counts.1 However, the CDC estimates were based on 5 state-level registries, which rely on clinician-reported cases and therefore may underestimate true disease burden. Moreover, the DoD beneficiary population differs markedly from the general population: it includes a large cohort of retirees, ensuring an older demographic; all members have comprehensive health care access; and active-duty personnel are subject to pre-enlistment medical screening. Taken together, these factors suggest this study may offer a more complete and systematically captured profile of lupus incidence.
We observed a marked decline of newly diagnosed SLE cases during the study period, which coincided with the widespread circulation of COVID-19. This decrease is unlikely to be attributable to reduced access to care during the pandemic. The MHS operates under a single-payer model, and the total number of patient encounters remained relatively stable throughout the pandemic.
To our knowledge, this is the only study to monitor lupus incidence in a large US population over the 5-year period encompassing before and during the COVID-19 pandemic. To date, only 4 large-scale surveillance studies have addressed similar questions. 14-17 Our findings are consistent with the most recent of these reports: an analysis limited to active-duty members of the US Armed Forces identified 1127 patients with newly diagnosed lupus between 2000 and 2022 and reported stable incidence trends throughout the pandemic.14 The other 3 studies adopted a different approach, comparing the emergence of autoimmune diseases, including lupus, between individuals with confirmed SARS-CoV-2 infection and those without. Each of these trials concluded that COVID-19 increases the risk of various autoimmune conditions, although the findings specific to lupus were inconsistent.15-17
Chang et al reported a significant increase in new lupus diagnoses (n = 2,926,016), with an adjusted hazard ratio (aHR) of 2.99 (95% CI, 2.68-3.34), spanning all ages and both sexes. The highest incidence was observed in individuals of Asian descent.15 Using German routine health care data from 2020, Tesch et al identified a heightened risk of autoimmune diseases, including lupus, among patients with a history of SARS-CoV-2 infection (n = 641,407; 9.4% children, 57.3% female, 6.4% hospitalized), compared with matched infection-naïve controls (n = 1,560,357).16 Both studies excluded vaccinated individuals.
These 2 studies diverged in their assessment of the relationship between COVID-19 severity and subsequent autoimmune risk. Chang et al found a higher incidence among nonhospitalized ambulatory patients, while Tesch et al reported that increased risk was associated with patients requiring intensive care unit admission.15,16
In contrast, based on a cohort of 4,197,188 individuals, Peng et al found no significant difference in lupus incidence among patients with SARS-CoV-2 infection (aHR, 1.05; 95% CI, 0.79-1.39).17 Notably, within the infected group, the incidence of SLE was significantly lower among vaccinated individuals compared with the unvaccinated group (aHR, 0.29; 95% CI, 0.18-0.47). Similar protective associations were observed for other antibody-mediated autoimmune disorders, including pemphigoid, Graves’ disease, and antiphospholipid antibody syndrome.
Limitations
Due to fundamental differences in study design, we were unable to directly reconcile our findings with those reported in the literature. This study lacked access to reliable documentation of COVID-19 infection status, primarily due to the widespread use of home testing among TRICARE beneficiaries. Additionally, the dataset did not include inpatient records and therefore did not permit evaluation of disease severity. Despite these constraints, it is plausible that the overall burden of COVID-19 infection within the study population was lower than that observed in the general US population.
As of December 2022, the DoD had reported about 750,000 confirmed COVID-19 cases among military personnel, civilian employees, dependents, and DoD contractors.18 Given that TRICARE beneficiaries represent about 2.8% of the total US population—and that > 90 million US individuals were infected between 2020 and 2022—the implied infection rate in our cohort appears to be about one-third of what might be expected.19 This discrepancy may be due to higher adherence to mitigation measures, such as social distancing and mask usage, among DoD-affiliated populations. COVID-19 vaccination was mandated for all active-duty service members, who constitute 5.4% of the study population. The remaining TRICARE beneficiaries also had access to guaranteed health care and vaccination coverage, likely contributing to high overall vaccination rates.
Because > 80% of the study population was composed of individuals from diverse civilian backgrounds, we expect the distribution of infection severity within the DoD beneficiary population to approximate that of the general US population.
Future Directions
The findings of this study offer circumstantial yet real-time evidence of the complexity underlying immune dysregulation at the intersection of host susceptibility and environmental exposures. The stability in ANA positivity rates during the study period mitigates concerns regarding undiagnosed subclinical lupus and may suggest that, overall, immune homeostasis was preserved among DoD beneficiaries.
It is noteworthy that during the COVID-19 pandemic, the incidence of several common infections—such as influenza and EBV—declined markedly, likely as a result of widespread social distancing and other public health interventions.20 Mitigation strategies implemented within the military may have conferred protection not only against COVID-19 but also against other community-acquired pathogens.
In light of these observations, we hypothesize that for COVID-19 to act as a trigger for SLE, a prolonged or repeated disruption of immune equilibrium may be required—potentially mediated by recurrent infections or sustained inflammatory states. The association between viral infections and autoimmunity is well established. Immune dysregulation leading to autoantibody production has been observed not only in the context of SARS-CoV-2 but also following infections with EBV, cytomegalovirus, enteroviruses, hepatitis B and C viruses, HIV, and parvovirus B19.21
This dysregulation is often transient, accompanied by compensatory immune regulatory responses. However, in individuals subjected to successive or overlapping infections, these regulatory mechanisms may become compromised or overwhelmed, due to emergent patterns of immune interference of varying severity. In such cases, a transient immune perturbation may progress into a bona fide autoimmune disease, contingent upon individual risk factors such as genetic predisposition, preexisting immune memory, and regenerative capacity.21
Therefore, we believe the significance of this study is 2-fold. First, lupus is known to develop gradually and may require 3 to 5 years to clinically manifest after the initial break in immunological tolerance.3 Continued public health surveillance represents a more pragmatic strategy than retrospective cohort construction, especially as histories of COVID-19 infection become increasingly complete and definitive. Our findings provide a valuable baseline reference point for future longitudinal studies.
The interpretation of surveillance outcomes—whether indicating an upward trend, a stable baseline, or a downward trend—offers distinct analytical value. Within this study population, we observed neither an upward trajectory that might suggest a direct causal link, nor a flat trend that would imply absence of association between COVID-19 and lupus pathogenesis. Instead, the observation of a downward trend invites consideration of nonlinear or protective influences. From this perspective, we recommend that future investigations adopt a holistic framework when assessing environmental contributions to immune dysregulation—particularly when evaluating the long-term immunopathological consequences of the COVID-19 pandemic on lupus and related autoimmune conditions.
Conclusions
This study identified a declining trend in incident lupus cases during the COVID-19 pandemic among the DoD beneficiary population. Further investigation is warranted to elucidate the underlying factors contributing to this decline. Conducting longitudinal epidemiologic studies and applying multivariable regression analyses will be essential to determine whether incidence rates revert to prepandemic baselines and how these trends may be influenced by evolving environmental factors within the general population.
Systemic lupus erythematosus (SLE), or lupus, is a rare autoimmune disease estimated to occur in about 5.1 cases per 100,000 person-years in the United States in 2018.1 The disease predominantly affects females, with an incidence of 8.7 cases per 100,000 person-years vs 1.2 cases per 100,000 person-years in males, and is most common in patients aged 15 to 44 years.1,2
Lupus presents with a constellation of clinical signs and symptoms that evolve, along with hallmark laboratory findings indicative of immune dysregulation and polyclonal B-cell activation. Consequently, a wide array of autoantibodies may be produced, although the combination of epitope specificity can vary from patient to patient.3 Nevertheless, > 98% of individuals diagnosed with lupus produce antinuclear antibodies (ANA), making ANA positivity a near-universal serologic feature at the time of diagnosis.
The pathogenesis of lupus is complex. Research from the past 5 decades supports the role of certain viral infections—such as Epstein-Barr virus (EBV) and cytomegalovirus—as potential triggers.4 These viruses are thought to initiate disease through mechanisms including activation of interferon pathways, exposure of cryptic intracellular antigens, molecular mimicry, and epitope spreading. Subsequent clonal expansion and autoantibody production occur to varying degrees, influenced by viral load and host susceptibility factors.
During the COVID-19 pandemic, it became evident that SARS-CoV-2 exerts profound effects on immune regulation, influencing infection outcomes through mechanisms such as hyperactivation of innate immunity, especially in the lungs, leading to acute respiratory distress syndrome. Additionally, SARS-CoV-2 has been associated with polyclonal B-cell activation and the generation of autoantibodies. This association gained attention after Bastard et al identified anti–type I interferon antibodies in patients with severe COVID-19, predominantly among males with a genetic predisposition. These autoantibodies were shown to impair antiviral defenses and contribute to life-threatening pneumonia.5
Subsequent studies demonstrated the production of a wide spectrum of functional autoantibodies, including ANA, in patients with COVID-19.6,7 These findings were attributed to the acute expansion of autoreactive clones among naïve-derived immunoglobulin G1 antibody-secreting cells during the early stages of infection, with the degree of expansion correlating with disease severity.8,9 Although longitudinal data up to 15 months postinfection suggest this serologic abnormality resolves in more than two-thirds of patients, the number of individuals infected globally has raised serious public health concerns regarding the potential long-term sequelae, including the onset of lupus or other autoimmune diseases in COVID-19 survivors.6-9 A limited number of case reports describing the onset of lupus following SARS-CoV-2 infection support this hypothesis.10
This surveillance analysis investigates lupus incidence among patients within the Military Health System (MHS), encompassing all TRICARE beneficiaries, from January 2018 to December 2022. The objective of this analysis was to examine lupus incidence trends throughout the COVID-19 pandemic, stratified by sex, age, and active-duty status.
Methods
The MHS provides health care services to about 9.5 million US Department of Defense (DoD) beneficiaries. Outpatient health records and laboratory results for individuals receiving care at military treatment facilities (MTFs) between January 1, 2018, and December 31, 2022, were obtained from the Comprehensive Ambulatory/ Professional Encounter Record and MHS GENESIS. For beneficiaries receiving care in the private sector, data were sourced from the TRICARE Encounter Data—Non-Institutional database.
Laboratory test results, including ANA testing, were available only for individuals receiving care at MTFs. These laboratory data were extracted from the Composite Health Care System Chemistry database and MHS GENESIS laboratory systems for the same time frame. Inpatient data were not included in this analysis. Data from 2017 were used solely as a look-back (or washout) period to identify and exclude prevalent lupus cases diagnosed before 2018 and were not included in the final results.
Lupus cases were identified by the presence of a positive ANA test and appropriate International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) codes. A positive ANA result was defined as either a qualitative result marked positive or a titer ≥ 1:80. The ICD-10-CM codes considered indicative of lupus included variations of M32, L93, or H01.12.
M32, L93, or H01.12. For cases with a positive ANA test, a lupus diagnosis required the presence of ≥ 2 lupus related ICD-10-CM codes. In the absence of ANA test results, a stricter criterion was applied: ≥ 4 lupus ICD-10-CM diagnosis codes recorded on separate days were required for inclusion.
Beneficiaries were excluded if they had a negative ANA result, only 1 lupus ICD- 10-CM diagnosis code, 1 positive ANA with only 1 corresponding ICD-10-CM code, or if their diagnosis occurred outside the defined study period. Patients and members of the public were not involved in the design, conduct, reporting, or dissemination of this study.
Results
Between January 1, 2017, and December 31, 2022, 99,946 TRICARE beneficiaries had some indication of lupus testing or diagnosis in their health records (Figure 1). Of these beneficiaries, 5335 had a positive ANA result and ≥ 2 ICD-10-CM lupus diagnosis codes. An additional 28,275 beneficiaries had ≥ 4 ICD-10-CM lupus diagnosis codes but no ANA test results. From these groups, the final sample included 10,760 beneficiaries who met the incident case definitions for SLE during the study period (2018 through 2022).

Most cases (85.1%, n = 9157) were diagnosed through TRICARE claims, while 1205 (11.2%) were diagnosed within the MHS. Another 398 (3.7%) had documentation of care both within and outside the MHS. Incident SLE cases declined by an average of 16% annually during the study period (Figure 2). This trend amounted to an overall reduction of 48.2%, from 2866 cases in 2018 to 1399 cases in 2022. This decline occurred despite total medical encounters among DoD beneficiaries remaining relatively stable during the pandemic years, with only a 3.5% change between 2018 and 2022.

The disease was more prevalent among female beneficiaries, with a female to- male ratio of 7:1 (Table 1). Among women, the number of new cases declined from 2519 in 2018 to 1223 in 2022, while the number of cases among men remained consistently < 350 annually. Similar trends were observed across other strata. Incident SLE cases were more common among nonactive-duty beneficiaries than active-duty service members, with a ratio of 18:1. New cases among active-duty members remained < 155 per year. Age-stratified data revealed that SLE was diagnosed predominantly in individuals aged ≥ 18 years, with a ratio of 37:1 compared with individuals aged < 18 years. Among children, the number of new cases remained < 75 per year throughout the study period.

A mean 56,850 ANA tests were conducted annually in centralized laboratories using standardized protocols (Table 2). The mean ANA positivity rate was 17.3%, which remained relatively stable from 2018 through 2022.

Discussion
This study examined the annual incidence of newly diagnosed SLE cases among all TRICARE beneficiaries from January 1, 2018, through December 31, 2022, covering both before and during the peak years of the COVID-19 pandemic. This analysis revealed a steady decline in SLE cases during this period. The reliability of these findings is reinforced by the comprehensiveness of the MHS, one of the largest US health care delivery systems, which maintains near-complete medical data capture for about 9.5 million DoD TRICARE beneficiaries across domestic and international settings.
SLE is a rare autoimmune disorder that presents a diagnostic challenge due to its wide range of nonspecific symptoms, many of which resemble other conditions. To reduce the likelihood of false-positive results and ensure diagnostic accuracy, this study adopted a stringent case definition. Incident cases were identified by the presence of ANA testing in conjunction with lupus-specific ICD-10-CM codes and required ≥ 4 lupus related diagnostic entries. This criterion was necessary due to the absence of ANA test results in data from private sector care settings. Our case definition aligns with established literature. For example, a Vanderbilt University chart review study demonstrated that combining ANA positivity with ≥ 4 lupus related ICD-10-CM codes achieves a positive predictive value of 100%, albeit with a sensitivity of 45%.11 Other studies similarly affirm the diagnostic validity of using recurrent ICD-10-CM codes to improve specificity in identifying lupus cases.12,13
The primary objective of this study was to examine the temporal trend in newly diagnosed lupus cases, rather than derive precise incidence rates. Although the TRICARE system includes about 9.5 million beneficiaries, this number represents a dynamic population with continual inflow and outflow. Accurate incidence rate calculation would require access to detailed denominator data, which were not readily available. In comparison with our findings, a study limited to active-duty service members reported fewer lupus cases. This discrepancy likely reflects differences in case definitions—specifically, the absence of laboratory data, the restricted range of diagnostic codes, and the requirement that diagnoses be rendered by specialists.14 Despite these differences, demographic patterns were consistent, with higher incidence observed in females and individuals aged ≥ 20 years.
A Centers for Disease Control and Prevention (CDC) study of lupus incidence in the general population also reported lower case counts.1 However, the CDC estimates were based on 5 state-level registries, which rely on clinician-reported cases and therefore may underestimate true disease burden. Moreover, the DoD beneficiary population differs markedly from the general population: it includes a large cohort of retirees, ensuring an older demographic; all members have comprehensive health care access; and active-duty personnel are subject to pre-enlistment medical screening. Taken together, these factors suggest this study may offer a more complete and systematically captured profile of lupus incidence.
We observed a marked decline of newly diagnosed SLE cases during the study period, which coincided with the widespread circulation of COVID-19. This decrease is unlikely to be attributable to reduced access to care during the pandemic. The MHS operates under a single-payer model, and the total number of patient encounters remained relatively stable throughout the pandemic.
To our knowledge, this is the only study to monitor lupus incidence in a large US population over the 5-year period encompassing before and during the COVID-19 pandemic. To date, only 4 large-scale surveillance studies have addressed similar questions. 14-17 Our findings are consistent with the most recent of these reports: an analysis limited to active-duty members of the US Armed Forces identified 1127 patients with newly diagnosed lupus between 2000 and 2022 and reported stable incidence trends throughout the pandemic.14 The other 3 studies adopted a different approach, comparing the emergence of autoimmune diseases, including lupus, between individuals with confirmed SARS-CoV-2 infection and those without. Each of these trials concluded that COVID-19 increases the risk of various autoimmune conditions, although the findings specific to lupus were inconsistent.15-17
Chang et al reported a significant increase in new lupus diagnoses (n = 2,926,016), with an adjusted hazard ratio (aHR) of 2.99 (95% CI, 2.68-3.34), spanning all ages and both sexes. The highest incidence was observed in individuals of Asian descent.15 Using German routine health care data from 2020, Tesch et al identified a heightened risk of autoimmune diseases, including lupus, among patients with a history of SARS-CoV-2 infection (n = 641,407; 9.4% children, 57.3% female, 6.4% hospitalized), compared with matched infection-naïve controls (n = 1,560,357).16 Both studies excluded vaccinated individuals.
These 2 studies diverged in their assessment of the relationship between COVID-19 severity and subsequent autoimmune risk. Chang et al found a higher incidence among nonhospitalized ambulatory patients, while Tesch et al reported that increased risk was associated with patients requiring intensive care unit admission.15,16
In contrast, based on a cohort of 4,197,188 individuals, Peng et al found no significant difference in lupus incidence among patients with SARS-CoV-2 infection (aHR, 1.05; 95% CI, 0.79-1.39).17 Notably, within the infected group, the incidence of SLE was significantly lower among vaccinated individuals compared with the unvaccinated group (aHR, 0.29; 95% CI, 0.18-0.47). Similar protective associations were observed for other antibody-mediated autoimmune disorders, including pemphigoid, Graves’ disease, and antiphospholipid antibody syndrome.
Limitations
Due to fundamental differences in study design, we were unable to directly reconcile our findings with those reported in the literature. This study lacked access to reliable documentation of COVID-19 infection status, primarily due to the widespread use of home testing among TRICARE beneficiaries. Additionally, the dataset did not include inpatient records and therefore did not permit evaluation of disease severity. Despite these constraints, it is plausible that the overall burden of COVID-19 infection within the study population was lower than that observed in the general US population.
As of December 2022, the DoD had reported about 750,000 confirmed COVID-19 cases among military personnel, civilian employees, dependents, and DoD contractors.18 Given that TRICARE beneficiaries represent about 2.8% of the total US population—and that > 90 million US individuals were infected between 2020 and 2022—the implied infection rate in our cohort appears to be about one-third of what might be expected.19 This discrepancy may be due to higher adherence to mitigation measures, such as social distancing and mask usage, among DoD-affiliated populations. COVID-19 vaccination was mandated for all active-duty service members, who constitute 5.4% of the study population. The remaining TRICARE beneficiaries also had access to guaranteed health care and vaccination coverage, likely contributing to high overall vaccination rates.
Because > 80% of the study population was composed of individuals from diverse civilian backgrounds, we expect the distribution of infection severity within the DoD beneficiary population to approximate that of the general US population.
Future Directions
The findings of this study offer circumstantial yet real-time evidence of the complexity underlying immune dysregulation at the intersection of host susceptibility and environmental exposures. The stability in ANA positivity rates during the study period mitigates concerns regarding undiagnosed subclinical lupus and may suggest that, overall, immune homeostasis was preserved among DoD beneficiaries.
It is noteworthy that during the COVID-19 pandemic, the incidence of several common infections—such as influenza and EBV—declined markedly, likely as a result of widespread social distancing and other public health interventions.20 Mitigation strategies implemented within the military may have conferred protection not only against COVID-19 but also against other community-acquired pathogens.
In light of these observations, we hypothesize that for COVID-19 to act as a trigger for SLE, a prolonged or repeated disruption of immune equilibrium may be required—potentially mediated by recurrent infections or sustained inflammatory states. The association between viral infections and autoimmunity is well established. Immune dysregulation leading to autoantibody production has been observed not only in the context of SARS-CoV-2 but also following infections with EBV, cytomegalovirus, enteroviruses, hepatitis B and C viruses, HIV, and parvovirus B19.21
This dysregulation is often transient, accompanied by compensatory immune regulatory responses. However, in individuals subjected to successive or overlapping infections, these regulatory mechanisms may become compromised or overwhelmed, due to emergent patterns of immune interference of varying severity. In such cases, a transient immune perturbation may progress into a bona fide autoimmune disease, contingent upon individual risk factors such as genetic predisposition, preexisting immune memory, and regenerative capacity.21
Therefore, we believe the significance of this study is 2-fold. First, lupus is known to develop gradually and may require 3 to 5 years to clinically manifest after the initial break in immunological tolerance.3 Continued public health surveillance represents a more pragmatic strategy than retrospective cohort construction, especially as histories of COVID-19 infection become increasingly complete and definitive. Our findings provide a valuable baseline reference point for future longitudinal studies.
The interpretation of surveillance outcomes—whether indicating an upward trend, a stable baseline, or a downward trend—offers distinct analytical value. Within this study population, we observed neither an upward trajectory that might suggest a direct causal link, nor a flat trend that would imply absence of association between COVID-19 and lupus pathogenesis. Instead, the observation of a downward trend invites consideration of nonlinear or protective influences. From this perspective, we recommend that future investigations adopt a holistic framework when assessing environmental contributions to immune dysregulation—particularly when evaluating the long-term immunopathological consequences of the COVID-19 pandemic on lupus and related autoimmune conditions.
Conclusions
This study identified a declining trend in incident lupus cases during the COVID-19 pandemic among the DoD beneficiary population. Further investigation is warranted to elucidate the underlying factors contributing to this decline. Conducting longitudinal epidemiologic studies and applying multivariable regression analyses will be essential to determine whether incidence rates revert to prepandemic baselines and how these trends may be influenced by evolving environmental factors within the general population.
A Systemic Lupus Erythematosus Incidence Surveillance Report Among DoD Beneficiaries During the COVID-19 Pandemic
A Systemic Lupus Erythematosus Incidence Surveillance Report Among DoD Beneficiaries During the COVID-19 Pandemic
- Izmirly PM, Ferucci ED, Somers EC, et al. Incidence rates of systemic lupus erythematosus in the USA: estimates from a meta-analysis of the Centers for Disease Control and Prevention national lupus registries. Lupus Sci Med. 2021;8(1):e000614. doi:10.1136/lupus-2021-000614
- Centers for Disease Control and Prevention. People with lupus. May 15, 2024. Accessed May 10, 2025. https:// www.cdc.gov/lupus/data-research/index.html
- Arbuckle MR, McClain MT, Rubertone MV, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349(16):1526-1533. doi:10.1056/nejmoa021933
- Li ZX, Zeng S, Wu HX, Zhou Y. The risk of systemic lupus erythematosus associated with Epstein–Barr virus infection: a systematic review and meta-analysis. Clin Exp Med. 2019;19(1):23-36. doi:10.1007/s10238-018-0535-0
- Bastard P, Rosen LB, Zhang Q, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370(6515):eabd4585. doi:10.1126/science.abd4585
- Chang SE, Feng A, Meng W, et al. New-onset IgG autoantibodies in hospitalized patients with COVID-19. Nat Commun. 2021;12(1):5417. doi:10.1038/s41467-021-25509-3
- Lee SJ, Yoon T, Ha JW, et al. Prevalence, clinical significance, and persistence of autoantibodies in COVID-19. Virol J. 2023;20(1):236. doi:10.1186/s12985-023-02191-z
- Woodruff MC, Ramonell RP, Haddad NS, et al. Dysregulated naive B cells and de novo autoreactivity in severe COVID-19. Nature. 2022;611(7934):139-147. doi:10.1038/s41586-022-05273-0
- Taeschler P, Cervia C, Zurbuchen Y, et al. Autoantibodies in COVID-19 correlate with antiviral humoral responses and distinct immune signatures. Allergy. 2022;77(8):2415-2430. doi:10.1111/all.15302
- Gracia-Ramos AE, Martin-Nares E, Hernández-Molina G. New onset of autoimmune diseases following COVID-19 diagnosis. Cells. 2021;10(12):3592 doi:10.3390/cells10123592
- Barnado A, Carroll R, Denny JC, Crofford L. Using IC-10-CM codes to identify patients with systemic lupus erythematosus in the electronic health record [abstract]. Arthritis Rheumatol. 2018;70(suppl 9):abstract 1692. Accessed May 10, 2025. https://acrabstracts.org/abstract/using-icd-10-cm-codes-to-identify-patients-with-systemic-lupus-erythematosus-in-the-electronic-health-record
- Feldman C, Curtis JR, Oates JC, Yazdany J, Izmirly P. Validating claims-based algorithms for a systemic lupus erythematosus diagnosis in Medicare data for informed use of the Lupus Index: a tool for geospatial research. Lupus Sci Med. 2024;11(2):e001329. doi:10.1136/lupus-2024-001329
- Moe SR, Haukeland H, Brunborg C, et al. POS1472: Accuracy of disease-specific ICD-10 code for incident systemic lupus erythematosus; results from a population-based cohort study set in Norway [abstract]. Ann Rheum Dis. 2023;82(suppl 1):1090-1091. doi:10.1136/annrheumdis-2023-eular.1189
- Denagamage P, Mabila SL, McQuistan AA. Trends and disparities in systemic lupus erythematosus incidence among U.S. active component service members, 2000–2022. MSMR. 2023;30(12):2-5.
- Chang R, Yen-Ting Chen T, Wang SI, Hung YM, Chen HY, Wei CJ. Risk of autoimmune diseases in patients with COVID-19: a retrospective cohort study. EClinicalMedicine. 2023;56:101783. doi:10.1016/j.eclinm.2022.101783
- Tesch F, Ehm F, Vivirito A, et al. Incident autoimmune diseases in association with SARS-CoV-2 infection: a matched cohort study. Clin Rheumatol. 2023;42(10):2905- 2914. doi:10.1007/s10067-023-06670-0
- Peng K, Li X, Yang D, et al. Risk of autoimmune diseases following COVID-19 and the potential protective effect from vaccination: a population-based cohort study. EClinicalMedicine. 2023;63:102154. doi:10.1016/j.eclinm.2023.102154
- US Department of Defense. Coronavirus: DOD response. Updated December 20, 2022. Accessed May 10, 2025. https://www.defense.gov/Spotlights/Coronavirus-DOD-Response/
- Elflein J. Number of cumulative cases of COVID-19 in the United States from January 20, 2020 to November 11, 2022, by week. Statista. https://www.statista.com/statistics/1103185/cumulative-coronavirus-covid19-cases-number-us-by-day
- Ye Z, Chen L, Zhong H, Cao L, Fu P, Xu J. Epidemiology and clinical characteristics of Epstein-Barr virus infection among children in Shanghai, China, 2017- 2022. Front Cell Infect Microbiol. 2023;13:1139068. doi:10.3389/fcimb.2023.1139068
- Johnson D, Jiang W. Infectious diseases, autoantibodies, and autoimmunity. J Autoimmun. 2023;137:102962. doi:10.1016/j.jaut.2022.102962
A Nationwide Survey of Dermatology Faculty and Mentors on Their Advice for the Dermatology Match Process
A Nationwide Survey of Dermatology Faculty and Mentors on Their Advice for the Dermatology Match Process
While strong relationships with mentors and advisers are critical to navigating the competitive dermatology match process, the advice medical students receive from different individuals can be contradictory. Unaccredited information online—particularly on social media—as well as data reported by applicants can add to potential confusion.1 Published research has elicited comments and observations from successfully matched medical students about highly discussed topics such as presentations and publications, letters of recommendation, away rotations, and interviews.2,3 However, there currently are no published data about advice that dermatology mentors actually offer medical students. In this study, we aimed to investigate this gap in the current literature and examine the advice dermatology faculty, program directors, and other mentors at institutions accredited by the Accreditation Council for Graduate Medical Education across the United States give to medical students applying to dermatology residency.
Methods
A 14-question Johns Hopkins Qualtrics survey was sent via the Association of Professors of Dermatology (APD) listserve in June 2024 soliciting responses from members who consider themselves to be mentors to dermatology applicants across the United States. The survey included multiple-choice questions with the option to select multiple answers and a space for open-ended responses. The questions first gathered information on the respondents, including the capacity in which the mentors advised medical students (eg, program director, department chair, clinical faculty). Mentors were asked for the number of years they had been advising mentees and if they were advising students with a home dermatology program. In addition, mentors were asked what advice they give their mentees about aspects of the application process, including gap years, dual applications, research involvement, couples matching, program signaling, away rotations, internship year, letters of recommendation, geographic signaling, interviewing advice, and volunteering during medical school.
On August 18, 2024, survey results from 115 respondents were aggregated. The responses for each question were quantitatively assessed to determine whether there was consensus on specific advice offered. The open-ended responses also were qualitatively assessed to determine the most common responses.
Results
The respondents included program directors (30% [35/115]), clinical faculty (22% [25/115]), department chairs (18% [21/115]), assistant program directors (15% [17/115]), medical school clerkship directors (8% [9/115]), primary mentors (ie, faculty who did not fall into any of the aforementioned categories but still advised medical students interested in dermatology)(5% [6/115]), division chiefs (1% [1/115]), and deans (1% [1/115]). Respondents had been advising students for a median of 10 years (range, 1-40 years [25th percentile, 5.00 years; 75th percentile, 13.75 years]). The majority (90% [103/115]) of mentors surveyed were advising students with a home dermatology program.
Areas of Consensus
In some areas, there was broad consensus among the advice offered by the mentors that were surveyed (eTable).
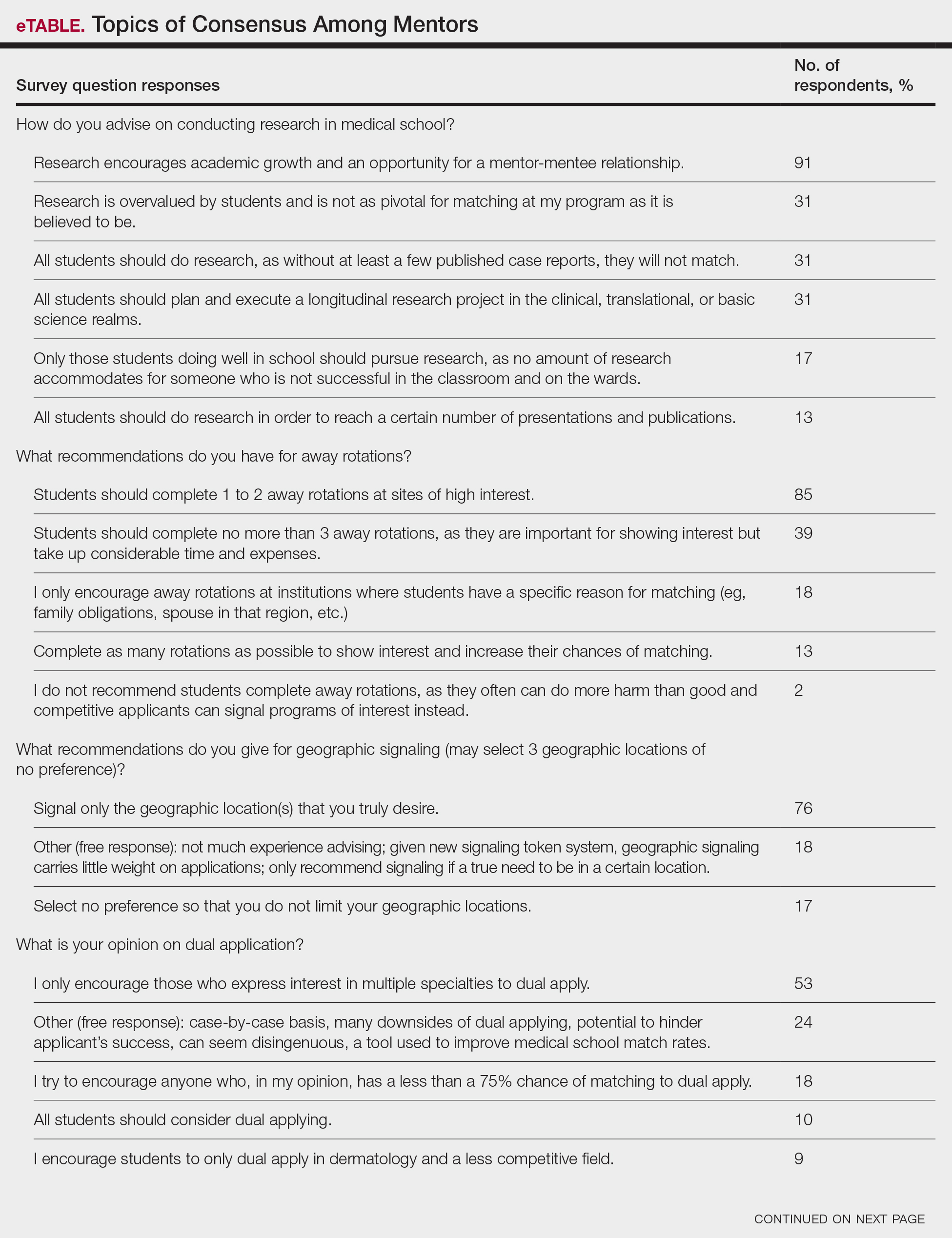

Research During Medical School—More than 91% (105/115) of the respondents recommended research to encourage academic growth and indicated that the most important reason for conducting research during medical school is to foster mentor-mentee relationships; however, more than one-third of respondents believed research is overvalued by students and research productivity is not as critical for matching as they perceive it to be. When these responses were categorized by respondent positions, 29% (15/52) of program or assistant directors indicated agreement with the statement that research is overvalued.
Away Rotations—There also was a consensus about the importance of away rotations, with 85% (98/115) of respondents advising students to complete 1 to 2 away rotations at sites of high interest, and 13% (15/115) suggesting that students complete as many away rotations as possible. It is worth noting, however, that the official APD Residency Program Directors Section’s statement on away rotations recommends no more than 2 away rotations (or no more than 3 for students with no home program).4
Reapplication Advice—Additionally, in a situation where students do not match into a dermatology residency program, the vast majority (71% [82/115]) of respondents advised students to rank competitive intern years to foster connections and improve the chance of matching on the second attempt.
Volunteering During Medical School—Seventy-seven percent (89/115) of mentors encouraged students to engage in volunteerism and advocacy during medical school to create a well-rounded application, and 69% (79/115) of mentors encouraged students to display leadership in their volunteer efforts.
Areas Without Consensus
Letters of Recommendation—Most respondents recommended submitting letters of recommendation only from dermatology professionals (55% [63/115]), with the remainder recommending students request a letter from anyone who could provide a strong recommendation regardless of specialty mix (42% [48/115]).
Dermatologic Subspecialties—For students interested in dermatologic subspecialties, 73% (84/115) of mentors advised that students be honest during interviews but keep an open mind that interests during residencies may change. Forty-three percent (49/115) of respondents encouraged students to promote a subspecialty interest during their interview only if they can demonstrate effort within that subspecialty on their application.
Couples Matching—Most respondents approach couples matching on a case-by-case basis and assess individual priorities when they do advise on this topic. Respondents often advise applicants to identify a few cities/regions and focus strongly on the programs within those regions to avoid spreading themselves too thin; however, one-third (38/115) of respondents indicated that they do not personally offer advice regarding the couples match.
Areas With Diverse Opinions
Gap Years—Nearly one-quarter (24% [28/115]) of mentors reported that they rarely recommend students take a year off and only support those who are adamant about doing so, or that they never support taking a gap year at all. A slight majority (58% [67/115]) recommend a gap year for students strongly interested in dermatologic research, and 38% (44/115) recommend a gap year for students with weaker applications (Figure 1). We received many open-ended responses to this question, with mentors frequently indicating that they advise students to take a gap year on a case-by-case basis, with 44% (51/115) of commenters recommending that students only take paid gap-year research positions.
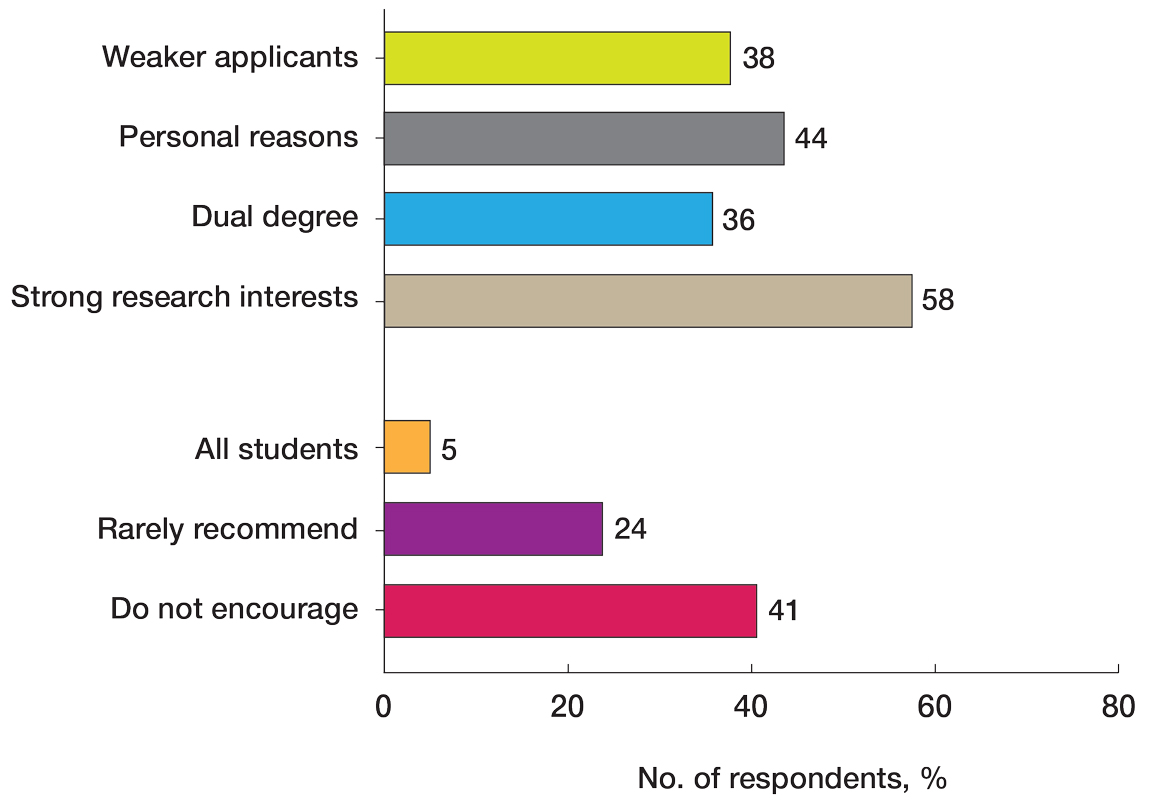
Program Signaling—The dermatology residency application process implemented a system of preference signaling tokens (PSTs) starting with the 2021-2022 cycle. Not quite half (46% [53/115]) of respondents recommend students apply only to places that they signaled, while 20% (23/115) advise responding to 10 to 15 additional programs. Very few (8% [9/115]) advise students to signal only in their stated region of interest. Approximately half (49% [56/115]) of mentors recommend students only signal based on the programs they feel would be the best fit for them without regard for perceived competitiveness—which aligns with the APD Residency Program Directors Section’s recommendation4—while 37% (43/115) recommend students distribute their signals to a wide range of programs. Sixty-three percent (72/115) of respondents recommend gold signaling to the student’s 3 most desired programs regardless of home and away rotation considerations, while 19% (22/115) recommend students give silver signals to their home and away rotation programs, as a rotation is already a signal of a strong desire to be there (Figure 2).
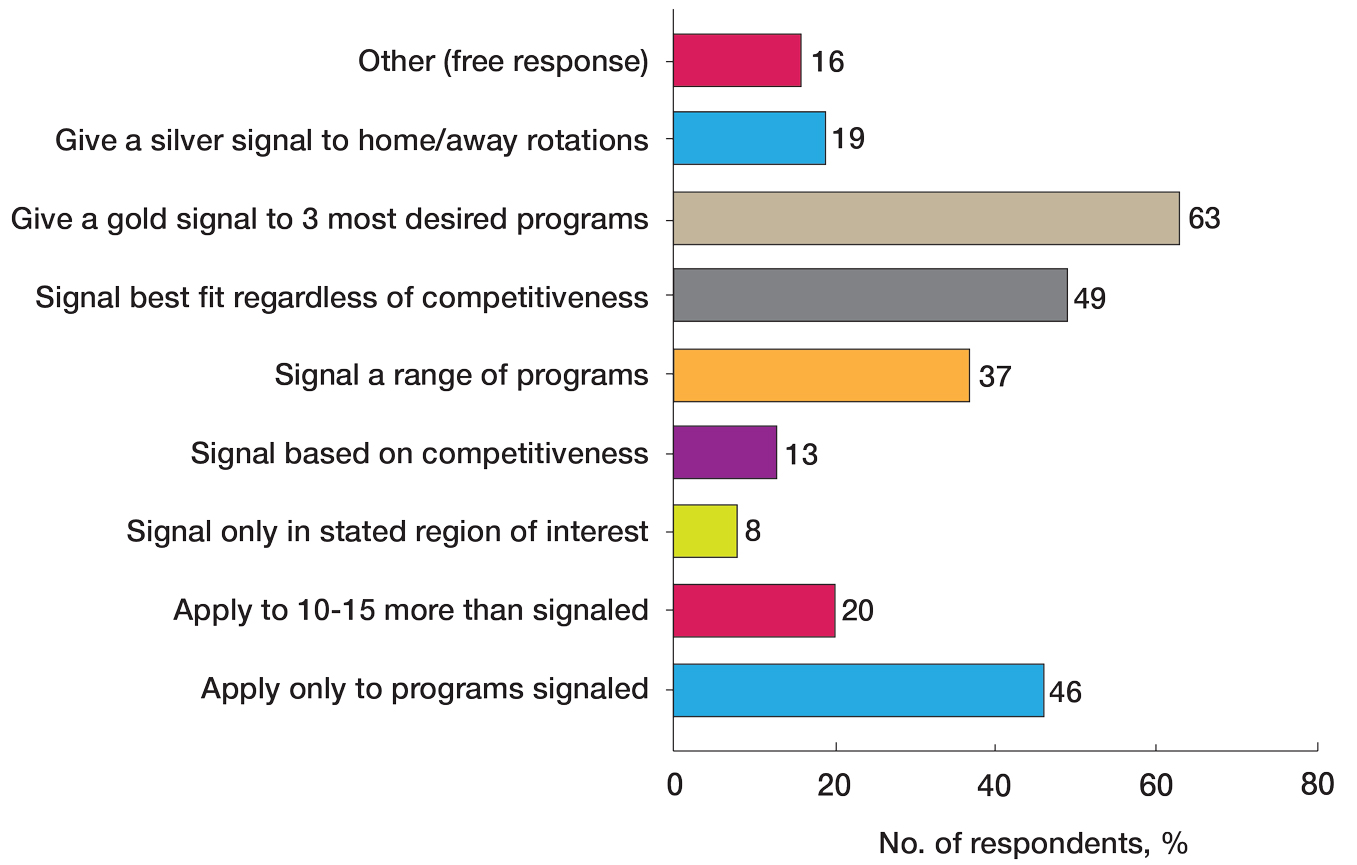
Dual Application—Fifty-three percent (61/115) of mentors recommended dual applying only for those truly interested in multiple specialties. Eighteen percent (21/115) of respondents advised dual applying for those with less than a 75% chance of matching. Twenty-five percent (29/115) of respondents free-wrote comments about approaching dual applying on a case-by-case basis, with many discussing the downsides of dual application and raising concerns that dual applications can hinder applicants’ success, can seem disingenuous, and seem to be a tool used to improve medical school match rates without benefit for the student.
We also stratified the data to compare overall responses from the total cohort with those from only program and assistant program directors. Across the 14 questions, responses from program and assistant program directors alone were similar to the overall cohort results
Comment
This study evaluated nationwide data on mentorship advising in dermatology, detailing mentors’ advice regarding research, gap years, dual applications, away rotations, intern year, couples matching, program signaling, and volunteering during medical school. Based on our results, most respondents agree on the importance of research during medical school, the utility of away rotations, and the value of volunteering during medical school. Similarly, respondents agreed on the importance of having strong letters of recommendation; while some advised asking only dermatology faculty to write letters, others did not have a specialty preference for the letter writers. Respondents also had varying views about sharing interest in subspecialties during residency interviews. Many of the respondents do not provide recommendations regarding geographic signaling and couples matching, expressing that these are parts of an application that are important to approach on a case-by-case basis. Lastly, respondents had diverse opinions regarding the utility of gap years, whether to encourage or discourage dual applications, and how to advise regarding program signaling.
Our results also showed that one-third of respondents believed that research is not as important as it is perceived to be by dermatology applicants. While engaging in research during medical school was almost unanimously encouraged to foster mentor-mentee relationships, respondents expressed that the number of research experiences and publications was not critical. This is an important topic of discussion, as taking a dedicated year away from medical school to complete a research fellowship is becoming a trend among dermatology applicants.5 There has been discussion both on unofficial online platforms as well as in the published literature regarding the pressure for medical students interested in dermatology to publish, which may result in a gap year for research.6 The literature on the utility of a gap year in match rates is sparse, with one study showing no difference in match rates among Mayo Clinic dermatology residents who took research years vs those who did not.7 However, this contrasts with match rates at top dermatology residency programs where 41% of applicants who took a gap year matched vs 19% who did not.7,8 These conflicting data are reflected in our study results, with respondents expressing different opinions on the utility of gap years.
There also are important equity concerns regarding the role of research years in the dermatology residency match process. Dermatology is one of the least racially diverse specialties, although there have been efforts to increase representation among residents and attending physicians.9-11 Research years can be important contributors to this lack of representation, as these often are unpaid and can discourage economically disadvantaged students from applying.9-11 Additionally, applicants may not have the flexibility to defer future salary for a year to match into dermatology; therefore, mentors should offer multiple options to individual applicants instead of solely encouraging gap years, given the conflicting feelings regarding their productivity.
Another topic of disagreement was dual application. Approximately one-third of respondents said they encourage either all students or those with less than a 75% chance of matching to dual apply, while about half only encourage students who are truly interested in multiple specialties to do so. Additionally, a large subset of respondents said they do not encourage dual applications due to concerns that they make applicants a worse candidate for each specialty and overall have negative effects on matching. Twenty-five percent of respondents opted to leave an open-ended response to this question: some offered the perspective that, if applicants feel a need to dual apply due to a weaker application, they do not advise the applicant to apply to dermatology. Many open ended responses underscored that the respondent does not encourage dual applications because they are inherently more time consuming, could hinder the applicant’s success, can seem disingenuous, and are a tool used to improve medical school match rates without being beneficial for the student. Some respondents also favored reapplying to dermatology the following year instead of dual applying. Finally, a subset of mentors indicated that they approach dual applications on a case-by-case basis, and others reported they do not have much experience advising on this topic. Currently, there are no known data in the literature on the efficacy and utility of dual applications in the dermatology match process; therefore, our study provides valuable insight for applicants interested in the impacts of the dual application. Overall, students should approach this option with mentors on an individual basis but ultimately should be aware of the concerns and mixed perceptions of the dual application process.
With regard to program signaling, previous research has shown that PSTs have a large impact on the chance of being granted an interview.12 In our study, we provide a comprehensive overview of advising regarding these signals. While mentors often responded that they did not have much experience advising in this domain—and it is too soon to tell the impact of this program signaling—many offered differing opinions. Many said they recommend that students give a gold signal to their 3 most desired programs regardless of home and away rotations and perceived competitiveness, which follows the guidelines issued by the APD; however, 19% recommend only giving silver signals to home and away rotation programs, as participation in those programs is considered a sufficient signal of interest. Additionally, about half of mentors recommended that students only apply where they signal, whereas 20% recommended applying to 10 to 15 programs beyond those signaled. Future studies should investigate the impact of PSTs on interview invitations once sufficient application cycles have occurred.
Study Limitations
This study was conducted via email to the APD listserve. The total number of faculty on this listserve is unknown; therefore, we do not know the total response rate of the survey. Additionally, we surveyed mentors in this listserve, who therefore receive more emails and overall correspondence about the dermatology match and may be more involved in these conversations. The mentors who responded to our survey may have a different approach and response to our various survey questions than a given mentor across the United States who did not respond to this survey. A final limitation of our study is that the survey responses a mentor gives may not fully match the advice that they give their students privately.
Conclusion
Our survey of dermatology mentors across the United States provides valuable insight into how mentors advise for a strong dermatology residency application. Mentors agreed on the importance of research during medical school, away rotations, strong letters of recommendation, and volunteerism and advocacy to promote a strong residency application. Important topics of disagreement include the decision for dermatology applicants to take a dedicated gap year in medical school, how to use tokens/signals effectively, and the dual application process. Our findings also underscore important application components that applicants and mentors should approach on an individual basis. Future studies should investigate the impact of signals/tokens on the match process as well as the utility of gap years and dual applications, working to standardize the advice applicants receive.
- Ramachandran V, Nguyen HY, Dao H Jr. Does it match? analyzing self-reported online dermatology match data to charting outcomes in the match. Dermatol Online J. 2020;26:13030 /qt4604h1w4.
- Kolli SS, Feldman SR, Huang WW. The dermatology residency application process. Dermatol Online J. 2021;26:13030/qt4k1570vj.
- Stratman EJ, Ness RM. Factors associated with successful matching to dermatology residency programs by reapplicants and other applicants who previously graduated from medical school. Arch Dermatol. 2011;147:196-202. doi:10.1001/archdermatol.2010.303
- Association of Professors of Dermatology Residency Program Directors Section Information Regarding the 2023-2024 Application Cycle. Published 2023. Accessed June 1, 2024. https://students-residents.aamc.org/media/12386/download
- Alikhan A, Sivamani RK, Mutizwa MM, et al. Advice for medical students interested in dermatology: perspectives from fourth year students who matched. Dermatol Online J. 2009;15:4.
- Wang JV, Keller M. Pressure to publish for residency applicants in dermatology. Dermatol Online J. 2016;22:13030/qt56x1t7ww.
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role research gap years play in a successful dermatology match. Int J Dermatol. 2022;61:226-230. doi:10.1111/ijd.15964
- Yeh C, Desai AD, Wassef C, et al. The importance of mentorship during research gap years for the dermatology residency match. Int J Dermatol. 2023;62:E209-E210. doi:10.1111/ijd.16084
- Zheng DX, Gallo Marin B, Mulligan KM, et al. Inequity concerns surrounding research years and the dermatology residency match. Int J Dermatol. 2022;61:E247-E248. doi:10.1111/ijd.16179
- Vasquez R, Jeong H, Florez-Pollack S, et al. What are the barriers faced by under-represented minorities applying to dermatology? a qualitative cross-sectional study of applicants applying to a large dermatology residency program. J Am Acad Dermatol. 2020;83:1770-1773. doi:10.1016/j.jaad.2020.03.067
- Jones VA, Clark KA, Cordova A, et al. Challenging the status quo: increasing diversity in dermatology. J Am Acad Dermatol. 2020;83:E421. doi:10.1016/j.jaad.2020.04.185
- Dirr MA, Brownstone N, Zakria D, et al. Dermatology match preference signaling tokens: impact and implications. Dermatol Surg. 2022;48:1367-1368. doi:10.1097/DSS.0000000000003645
While strong relationships with mentors and advisers are critical to navigating the competitive dermatology match process, the advice medical students receive from different individuals can be contradictory. Unaccredited information online—particularly on social media—as well as data reported by applicants can add to potential confusion.1 Published research has elicited comments and observations from successfully matched medical students about highly discussed topics such as presentations and publications, letters of recommendation, away rotations, and interviews.2,3 However, there currently are no published data about advice that dermatology mentors actually offer medical students. In this study, we aimed to investigate this gap in the current literature and examine the advice dermatology faculty, program directors, and other mentors at institutions accredited by the Accreditation Council for Graduate Medical Education across the United States give to medical students applying to dermatology residency.
Methods
A 14-question Johns Hopkins Qualtrics survey was sent via the Association of Professors of Dermatology (APD) listserve in June 2024 soliciting responses from members who consider themselves to be mentors to dermatology applicants across the United States. The survey included multiple-choice questions with the option to select multiple answers and a space for open-ended responses. The questions first gathered information on the respondents, including the capacity in which the mentors advised medical students (eg, program director, department chair, clinical faculty). Mentors were asked for the number of years they had been advising mentees and if they were advising students with a home dermatology program. In addition, mentors were asked what advice they give their mentees about aspects of the application process, including gap years, dual applications, research involvement, couples matching, program signaling, away rotations, internship year, letters of recommendation, geographic signaling, interviewing advice, and volunteering during medical school.
On August 18, 2024, survey results from 115 respondents were aggregated. The responses for each question were quantitatively assessed to determine whether there was consensus on specific advice offered. The open-ended responses also were qualitatively assessed to determine the most common responses.
Results
The respondents included program directors (30% [35/115]), clinical faculty (22% [25/115]), department chairs (18% [21/115]), assistant program directors (15% [17/115]), medical school clerkship directors (8% [9/115]), primary mentors (ie, faculty who did not fall into any of the aforementioned categories but still advised medical students interested in dermatology)(5% [6/115]), division chiefs (1% [1/115]), and deans (1% [1/115]). Respondents had been advising students for a median of 10 years (range, 1-40 years [25th percentile, 5.00 years; 75th percentile, 13.75 years]). The majority (90% [103/115]) of mentors surveyed were advising students with a home dermatology program.
Areas of Consensus
In some areas, there was broad consensus among the advice offered by the mentors that were surveyed (eTable).


Research During Medical School—More than 91% (105/115) of the respondents recommended research to encourage academic growth and indicated that the most important reason for conducting research during medical school is to foster mentor-mentee relationships; however, more than one-third of respondents believed research is overvalued by students and research productivity is not as critical for matching as they perceive it to be. When these responses were categorized by respondent positions, 29% (15/52) of program or assistant directors indicated agreement with the statement that research is overvalued.
Away Rotations—There also was a consensus about the importance of away rotations, with 85% (98/115) of respondents advising students to complete 1 to 2 away rotations at sites of high interest, and 13% (15/115) suggesting that students complete as many away rotations as possible. It is worth noting, however, that the official APD Residency Program Directors Section’s statement on away rotations recommends no more than 2 away rotations (or no more than 3 for students with no home program).4
Reapplication Advice—Additionally, in a situation where students do not match into a dermatology residency program, the vast majority (71% [82/115]) of respondents advised students to rank competitive intern years to foster connections and improve the chance of matching on the second attempt.
Volunteering During Medical School—Seventy-seven percent (89/115) of mentors encouraged students to engage in volunteerism and advocacy during medical school to create a well-rounded application, and 69% (79/115) of mentors encouraged students to display leadership in their volunteer efforts.
Areas Without Consensus
Letters of Recommendation—Most respondents recommended submitting letters of recommendation only from dermatology professionals (55% [63/115]), with the remainder recommending students request a letter from anyone who could provide a strong recommendation regardless of specialty mix (42% [48/115]).
Dermatologic Subspecialties—For students interested in dermatologic subspecialties, 73% (84/115) of mentors advised that students be honest during interviews but keep an open mind that interests during residencies may change. Forty-three percent (49/115) of respondents encouraged students to promote a subspecialty interest during their interview only if they can demonstrate effort within that subspecialty on their application.
Couples Matching—Most respondents approach couples matching on a case-by-case basis and assess individual priorities when they do advise on this topic. Respondents often advise applicants to identify a few cities/regions and focus strongly on the programs within those regions to avoid spreading themselves too thin; however, one-third (38/115) of respondents indicated that they do not personally offer advice regarding the couples match.
Areas With Diverse Opinions
Gap Years—Nearly one-quarter (24% [28/115]) of mentors reported that they rarely recommend students take a year off and only support those who are adamant about doing so, or that they never support taking a gap year at all. A slight majority (58% [67/115]) recommend a gap year for students strongly interested in dermatologic research, and 38% (44/115) recommend a gap year for students with weaker applications (Figure 1). We received many open-ended responses to this question, with mentors frequently indicating that they advise students to take a gap year on a case-by-case basis, with 44% (51/115) of commenters recommending that students only take paid gap-year research positions.

Program Signaling—The dermatology residency application process implemented a system of preference signaling tokens (PSTs) starting with the 2021-2022 cycle. Not quite half (46% [53/115]) of respondents recommend students apply only to places that they signaled, while 20% (23/115) advise responding to 10 to 15 additional programs. Very few (8% [9/115]) advise students to signal only in their stated region of interest. Approximately half (49% [56/115]) of mentors recommend students only signal based on the programs they feel would be the best fit for them without regard for perceived competitiveness—which aligns with the APD Residency Program Directors Section’s recommendation4—while 37% (43/115) recommend students distribute their signals to a wide range of programs. Sixty-three percent (72/115) of respondents recommend gold signaling to the student’s 3 most desired programs regardless of home and away rotation considerations, while 19% (22/115) recommend students give silver signals to their home and away rotation programs, as a rotation is already a signal of a strong desire to be there (Figure 2).

Dual Application—Fifty-three percent (61/115) of mentors recommended dual applying only for those truly interested in multiple specialties. Eighteen percent (21/115) of respondents advised dual applying for those with less than a 75% chance of matching. Twenty-five percent (29/115) of respondents free-wrote comments about approaching dual applying on a case-by-case basis, with many discussing the downsides of dual application and raising concerns that dual applications can hinder applicants’ success, can seem disingenuous, and seem to be a tool used to improve medical school match rates without benefit for the student.
We also stratified the data to compare overall responses from the total cohort with those from only program and assistant program directors. Across the 14 questions, responses from program and assistant program directors alone were similar to the overall cohort results
Comment
This study evaluated nationwide data on mentorship advising in dermatology, detailing mentors’ advice regarding research, gap years, dual applications, away rotations, intern year, couples matching, program signaling, and volunteering during medical school. Based on our results, most respondents agree on the importance of research during medical school, the utility of away rotations, and the value of volunteering during medical school. Similarly, respondents agreed on the importance of having strong letters of recommendation; while some advised asking only dermatology faculty to write letters, others did not have a specialty preference for the letter writers. Respondents also had varying views about sharing interest in subspecialties during residency interviews. Many of the respondents do not provide recommendations regarding geographic signaling and couples matching, expressing that these are parts of an application that are important to approach on a case-by-case basis. Lastly, respondents had diverse opinions regarding the utility of gap years, whether to encourage or discourage dual applications, and how to advise regarding program signaling.
Our results also showed that one-third of respondents believed that research is not as important as it is perceived to be by dermatology applicants. While engaging in research during medical school was almost unanimously encouraged to foster mentor-mentee relationships, respondents expressed that the number of research experiences and publications was not critical. This is an important topic of discussion, as taking a dedicated year away from medical school to complete a research fellowship is becoming a trend among dermatology applicants.5 There has been discussion both on unofficial online platforms as well as in the published literature regarding the pressure for medical students interested in dermatology to publish, which may result in a gap year for research.6 The literature on the utility of a gap year in match rates is sparse, with one study showing no difference in match rates among Mayo Clinic dermatology residents who took research years vs those who did not.7 However, this contrasts with match rates at top dermatology residency programs where 41% of applicants who took a gap year matched vs 19% who did not.7,8 These conflicting data are reflected in our study results, with respondents expressing different opinions on the utility of gap years.
There also are important equity concerns regarding the role of research years in the dermatology residency match process. Dermatology is one of the least racially diverse specialties, although there have been efforts to increase representation among residents and attending physicians.9-11 Research years can be important contributors to this lack of representation, as these often are unpaid and can discourage economically disadvantaged students from applying.9-11 Additionally, applicants may not have the flexibility to defer future salary for a year to match into dermatology; therefore, mentors should offer multiple options to individual applicants instead of solely encouraging gap years, given the conflicting feelings regarding their productivity.
Another topic of disagreement was dual application. Approximately one-third of respondents said they encourage either all students or those with less than a 75% chance of matching to dual apply, while about half only encourage students who are truly interested in multiple specialties to do so. Additionally, a large subset of respondents said they do not encourage dual applications due to concerns that they make applicants a worse candidate for each specialty and overall have negative effects on matching. Twenty-five percent of respondents opted to leave an open-ended response to this question: some offered the perspective that, if applicants feel a need to dual apply due to a weaker application, they do not advise the applicant to apply to dermatology. Many open ended responses underscored that the respondent does not encourage dual applications because they are inherently more time consuming, could hinder the applicant’s success, can seem disingenuous, and are a tool used to improve medical school match rates without being beneficial for the student. Some respondents also favored reapplying to dermatology the following year instead of dual applying. Finally, a subset of mentors indicated that they approach dual applications on a case-by-case basis, and others reported they do not have much experience advising on this topic. Currently, there are no known data in the literature on the efficacy and utility of dual applications in the dermatology match process; therefore, our study provides valuable insight for applicants interested in the impacts of the dual application. Overall, students should approach this option with mentors on an individual basis but ultimately should be aware of the concerns and mixed perceptions of the dual application process.
With regard to program signaling, previous research has shown that PSTs have a large impact on the chance of being granted an interview.12 In our study, we provide a comprehensive overview of advising regarding these signals. While mentors often responded that they did not have much experience advising in this domain—and it is too soon to tell the impact of this program signaling—many offered differing opinions. Many said they recommend that students give a gold signal to their 3 most desired programs regardless of home and away rotations and perceived competitiveness, which follows the guidelines issued by the APD; however, 19% recommend only giving silver signals to home and away rotation programs, as participation in those programs is considered a sufficient signal of interest. Additionally, about half of mentors recommended that students only apply where they signal, whereas 20% recommended applying to 10 to 15 programs beyond those signaled. Future studies should investigate the impact of PSTs on interview invitations once sufficient application cycles have occurred.
Study Limitations
This study was conducted via email to the APD listserve. The total number of faculty on this listserve is unknown; therefore, we do not know the total response rate of the survey. Additionally, we surveyed mentors in this listserve, who therefore receive more emails and overall correspondence about the dermatology match and may be more involved in these conversations. The mentors who responded to our survey may have a different approach and response to our various survey questions than a given mentor across the United States who did not respond to this survey. A final limitation of our study is that the survey responses a mentor gives may not fully match the advice that they give their students privately.
Conclusion
Our survey of dermatology mentors across the United States provides valuable insight into how mentors advise for a strong dermatology residency application. Mentors agreed on the importance of research during medical school, away rotations, strong letters of recommendation, and volunteerism and advocacy to promote a strong residency application. Important topics of disagreement include the decision for dermatology applicants to take a dedicated gap year in medical school, how to use tokens/signals effectively, and the dual application process. Our findings also underscore important application components that applicants and mentors should approach on an individual basis. Future studies should investigate the impact of signals/tokens on the match process as well as the utility of gap years and dual applications, working to standardize the advice applicants receive.
While strong relationships with mentors and advisers are critical to navigating the competitive dermatology match process, the advice medical students receive from different individuals can be contradictory. Unaccredited information online—particularly on social media—as well as data reported by applicants can add to potential confusion.1 Published research has elicited comments and observations from successfully matched medical students about highly discussed topics such as presentations and publications, letters of recommendation, away rotations, and interviews.2,3 However, there currently are no published data about advice that dermatology mentors actually offer medical students. In this study, we aimed to investigate this gap in the current literature and examine the advice dermatology faculty, program directors, and other mentors at institutions accredited by the Accreditation Council for Graduate Medical Education across the United States give to medical students applying to dermatology residency.
Methods
A 14-question Johns Hopkins Qualtrics survey was sent via the Association of Professors of Dermatology (APD) listserve in June 2024 soliciting responses from members who consider themselves to be mentors to dermatology applicants across the United States. The survey included multiple-choice questions with the option to select multiple answers and a space for open-ended responses. The questions first gathered information on the respondents, including the capacity in which the mentors advised medical students (eg, program director, department chair, clinical faculty). Mentors were asked for the number of years they had been advising mentees and if they were advising students with a home dermatology program. In addition, mentors were asked what advice they give their mentees about aspects of the application process, including gap years, dual applications, research involvement, couples matching, program signaling, away rotations, internship year, letters of recommendation, geographic signaling, interviewing advice, and volunteering during medical school.
On August 18, 2024, survey results from 115 respondents were aggregated. The responses for each question were quantitatively assessed to determine whether there was consensus on specific advice offered. The open-ended responses also were qualitatively assessed to determine the most common responses.
Results
The respondents included program directors (30% [35/115]), clinical faculty (22% [25/115]), department chairs (18% [21/115]), assistant program directors (15% [17/115]), medical school clerkship directors (8% [9/115]), primary mentors (ie, faculty who did not fall into any of the aforementioned categories but still advised medical students interested in dermatology)(5% [6/115]), division chiefs (1% [1/115]), and deans (1% [1/115]). Respondents had been advising students for a median of 10 years (range, 1-40 years [25th percentile, 5.00 years; 75th percentile, 13.75 years]). The majority (90% [103/115]) of mentors surveyed were advising students with a home dermatology program.
Areas of Consensus
In some areas, there was broad consensus among the advice offered by the mentors that were surveyed (eTable).


Research During Medical School—More than 91% (105/115) of the respondents recommended research to encourage academic growth and indicated that the most important reason for conducting research during medical school is to foster mentor-mentee relationships; however, more than one-third of respondents believed research is overvalued by students and research productivity is not as critical for matching as they perceive it to be. When these responses were categorized by respondent positions, 29% (15/52) of program or assistant directors indicated agreement with the statement that research is overvalued.
Away Rotations—There also was a consensus about the importance of away rotations, with 85% (98/115) of respondents advising students to complete 1 to 2 away rotations at sites of high interest, and 13% (15/115) suggesting that students complete as many away rotations as possible. It is worth noting, however, that the official APD Residency Program Directors Section’s statement on away rotations recommends no more than 2 away rotations (or no more than 3 for students with no home program).4
Reapplication Advice—Additionally, in a situation where students do not match into a dermatology residency program, the vast majority (71% [82/115]) of respondents advised students to rank competitive intern years to foster connections and improve the chance of matching on the second attempt.
Volunteering During Medical School—Seventy-seven percent (89/115) of mentors encouraged students to engage in volunteerism and advocacy during medical school to create a well-rounded application, and 69% (79/115) of mentors encouraged students to display leadership in their volunteer efforts.
Areas Without Consensus
Letters of Recommendation—Most respondents recommended submitting letters of recommendation only from dermatology professionals (55% [63/115]), with the remainder recommending students request a letter from anyone who could provide a strong recommendation regardless of specialty mix (42% [48/115]).
Dermatologic Subspecialties—For students interested in dermatologic subspecialties, 73% (84/115) of mentors advised that students be honest during interviews but keep an open mind that interests during residencies may change. Forty-three percent (49/115) of respondents encouraged students to promote a subspecialty interest during their interview only if they can demonstrate effort within that subspecialty on their application.
Couples Matching—Most respondents approach couples matching on a case-by-case basis and assess individual priorities when they do advise on this topic. Respondents often advise applicants to identify a few cities/regions and focus strongly on the programs within those regions to avoid spreading themselves too thin; however, one-third (38/115) of respondents indicated that they do not personally offer advice regarding the couples match.
Areas With Diverse Opinions
Gap Years—Nearly one-quarter (24% [28/115]) of mentors reported that they rarely recommend students take a year off and only support those who are adamant about doing so, or that they never support taking a gap year at all. A slight majority (58% [67/115]) recommend a gap year for students strongly interested in dermatologic research, and 38% (44/115) recommend a gap year for students with weaker applications (Figure 1). We received many open-ended responses to this question, with mentors frequently indicating that they advise students to take a gap year on a case-by-case basis, with 44% (51/115) of commenters recommending that students only take paid gap-year research positions.

Program Signaling—The dermatology residency application process implemented a system of preference signaling tokens (PSTs) starting with the 2021-2022 cycle. Not quite half (46% [53/115]) of respondents recommend students apply only to places that they signaled, while 20% (23/115) advise responding to 10 to 15 additional programs. Very few (8% [9/115]) advise students to signal only in their stated region of interest. Approximately half (49% [56/115]) of mentors recommend students only signal based on the programs they feel would be the best fit for them without regard for perceived competitiveness—which aligns with the APD Residency Program Directors Section’s recommendation4—while 37% (43/115) recommend students distribute their signals to a wide range of programs. Sixty-three percent (72/115) of respondents recommend gold signaling to the student’s 3 most desired programs regardless of home and away rotation considerations, while 19% (22/115) recommend students give silver signals to their home and away rotation programs, as a rotation is already a signal of a strong desire to be there (Figure 2).

Dual Application—Fifty-three percent (61/115) of mentors recommended dual applying only for those truly interested in multiple specialties. Eighteen percent (21/115) of respondents advised dual applying for those with less than a 75% chance of matching. Twenty-five percent (29/115) of respondents free-wrote comments about approaching dual applying on a case-by-case basis, with many discussing the downsides of dual application and raising concerns that dual applications can hinder applicants’ success, can seem disingenuous, and seem to be a tool used to improve medical school match rates without benefit for the student.
We also stratified the data to compare overall responses from the total cohort with those from only program and assistant program directors. Across the 14 questions, responses from program and assistant program directors alone were similar to the overall cohort results
Comment
This study evaluated nationwide data on mentorship advising in dermatology, detailing mentors’ advice regarding research, gap years, dual applications, away rotations, intern year, couples matching, program signaling, and volunteering during medical school. Based on our results, most respondents agree on the importance of research during medical school, the utility of away rotations, and the value of volunteering during medical school. Similarly, respondents agreed on the importance of having strong letters of recommendation; while some advised asking only dermatology faculty to write letters, others did not have a specialty preference for the letter writers. Respondents also had varying views about sharing interest in subspecialties during residency interviews. Many of the respondents do not provide recommendations regarding geographic signaling and couples matching, expressing that these are parts of an application that are important to approach on a case-by-case basis. Lastly, respondents had diverse opinions regarding the utility of gap years, whether to encourage or discourage dual applications, and how to advise regarding program signaling.
Our results also showed that one-third of respondents believed that research is not as important as it is perceived to be by dermatology applicants. While engaging in research during medical school was almost unanimously encouraged to foster mentor-mentee relationships, respondents expressed that the number of research experiences and publications was not critical. This is an important topic of discussion, as taking a dedicated year away from medical school to complete a research fellowship is becoming a trend among dermatology applicants.5 There has been discussion both on unofficial online platforms as well as in the published literature regarding the pressure for medical students interested in dermatology to publish, which may result in a gap year for research.6 The literature on the utility of a gap year in match rates is sparse, with one study showing no difference in match rates among Mayo Clinic dermatology residents who took research years vs those who did not.7 However, this contrasts with match rates at top dermatology residency programs where 41% of applicants who took a gap year matched vs 19% who did not.7,8 These conflicting data are reflected in our study results, with respondents expressing different opinions on the utility of gap years.
There also are important equity concerns regarding the role of research years in the dermatology residency match process. Dermatology is one of the least racially diverse specialties, although there have been efforts to increase representation among residents and attending physicians.9-11 Research years can be important contributors to this lack of representation, as these often are unpaid and can discourage economically disadvantaged students from applying.9-11 Additionally, applicants may not have the flexibility to defer future salary for a year to match into dermatology; therefore, mentors should offer multiple options to individual applicants instead of solely encouraging gap years, given the conflicting feelings regarding their productivity.
Another topic of disagreement was dual application. Approximately one-third of respondents said they encourage either all students or those with less than a 75% chance of matching to dual apply, while about half only encourage students who are truly interested in multiple specialties to do so. Additionally, a large subset of respondents said they do not encourage dual applications due to concerns that they make applicants a worse candidate for each specialty and overall have negative effects on matching. Twenty-five percent of respondents opted to leave an open-ended response to this question: some offered the perspective that, if applicants feel a need to dual apply due to a weaker application, they do not advise the applicant to apply to dermatology. Many open ended responses underscored that the respondent does not encourage dual applications because they are inherently more time consuming, could hinder the applicant’s success, can seem disingenuous, and are a tool used to improve medical school match rates without being beneficial for the student. Some respondents also favored reapplying to dermatology the following year instead of dual applying. Finally, a subset of mentors indicated that they approach dual applications on a case-by-case basis, and others reported they do not have much experience advising on this topic. Currently, there are no known data in the literature on the efficacy and utility of dual applications in the dermatology match process; therefore, our study provides valuable insight for applicants interested in the impacts of the dual application. Overall, students should approach this option with mentors on an individual basis but ultimately should be aware of the concerns and mixed perceptions of the dual application process.
With regard to program signaling, previous research has shown that PSTs have a large impact on the chance of being granted an interview.12 In our study, we provide a comprehensive overview of advising regarding these signals. While mentors often responded that they did not have much experience advising in this domain—and it is too soon to tell the impact of this program signaling—many offered differing opinions. Many said they recommend that students give a gold signal to their 3 most desired programs regardless of home and away rotations and perceived competitiveness, which follows the guidelines issued by the APD; however, 19% recommend only giving silver signals to home and away rotation programs, as participation in those programs is considered a sufficient signal of interest. Additionally, about half of mentors recommended that students only apply where they signal, whereas 20% recommended applying to 10 to 15 programs beyond those signaled. Future studies should investigate the impact of PSTs on interview invitations once sufficient application cycles have occurred.
Study Limitations
This study was conducted via email to the APD listserve. The total number of faculty on this listserve is unknown; therefore, we do not know the total response rate of the survey. Additionally, we surveyed mentors in this listserve, who therefore receive more emails and overall correspondence about the dermatology match and may be more involved in these conversations. The mentors who responded to our survey may have a different approach and response to our various survey questions than a given mentor across the United States who did not respond to this survey. A final limitation of our study is that the survey responses a mentor gives may not fully match the advice that they give their students privately.
Conclusion
Our survey of dermatology mentors across the United States provides valuable insight into how mentors advise for a strong dermatology residency application. Mentors agreed on the importance of research during medical school, away rotations, strong letters of recommendation, and volunteerism and advocacy to promote a strong residency application. Important topics of disagreement include the decision for dermatology applicants to take a dedicated gap year in medical school, how to use tokens/signals effectively, and the dual application process. Our findings also underscore important application components that applicants and mentors should approach on an individual basis. Future studies should investigate the impact of signals/tokens on the match process as well as the utility of gap years and dual applications, working to standardize the advice applicants receive.
- Ramachandran V, Nguyen HY, Dao H Jr. Does it match? analyzing self-reported online dermatology match data to charting outcomes in the match. Dermatol Online J. 2020;26:13030 /qt4604h1w4.
- Kolli SS, Feldman SR, Huang WW. The dermatology residency application process. Dermatol Online J. 2021;26:13030/qt4k1570vj.
- Stratman EJ, Ness RM. Factors associated with successful matching to dermatology residency programs by reapplicants and other applicants who previously graduated from medical school. Arch Dermatol. 2011;147:196-202. doi:10.1001/archdermatol.2010.303
- Association of Professors of Dermatology Residency Program Directors Section Information Regarding the 2023-2024 Application Cycle. Published 2023. Accessed June 1, 2024. https://students-residents.aamc.org/media/12386/download
- Alikhan A, Sivamani RK, Mutizwa MM, et al. Advice for medical students interested in dermatology: perspectives from fourth year students who matched. Dermatol Online J. 2009;15:4.
- Wang JV, Keller M. Pressure to publish for residency applicants in dermatology. Dermatol Online J. 2016;22:13030/qt56x1t7ww.
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role research gap years play in a successful dermatology match. Int J Dermatol. 2022;61:226-230. doi:10.1111/ijd.15964
- Yeh C, Desai AD, Wassef C, et al. The importance of mentorship during research gap years for the dermatology residency match. Int J Dermatol. 2023;62:E209-E210. doi:10.1111/ijd.16084
- Zheng DX, Gallo Marin B, Mulligan KM, et al. Inequity concerns surrounding research years and the dermatology residency match. Int J Dermatol. 2022;61:E247-E248. doi:10.1111/ijd.16179
- Vasquez R, Jeong H, Florez-Pollack S, et al. What are the barriers faced by under-represented minorities applying to dermatology? a qualitative cross-sectional study of applicants applying to a large dermatology residency program. J Am Acad Dermatol. 2020;83:1770-1773. doi:10.1016/j.jaad.2020.03.067
- Jones VA, Clark KA, Cordova A, et al. Challenging the status quo: increasing diversity in dermatology. J Am Acad Dermatol. 2020;83:E421. doi:10.1016/j.jaad.2020.04.185
- Dirr MA, Brownstone N, Zakria D, et al. Dermatology match preference signaling tokens: impact and implications. Dermatol Surg. 2022;48:1367-1368. doi:10.1097/DSS.0000000000003645
- Ramachandran V, Nguyen HY, Dao H Jr. Does it match? analyzing self-reported online dermatology match data to charting outcomes in the match. Dermatol Online J. 2020;26:13030 /qt4604h1w4.
- Kolli SS, Feldman SR, Huang WW. The dermatology residency application process. Dermatol Online J. 2021;26:13030/qt4k1570vj.
- Stratman EJ, Ness RM. Factors associated with successful matching to dermatology residency programs by reapplicants and other applicants who previously graduated from medical school. Arch Dermatol. 2011;147:196-202. doi:10.1001/archdermatol.2010.303
- Association of Professors of Dermatology Residency Program Directors Section Information Regarding the 2023-2024 Application Cycle. Published 2023. Accessed June 1, 2024. https://students-residents.aamc.org/media/12386/download
- Alikhan A, Sivamani RK, Mutizwa MM, et al. Advice for medical students interested in dermatology: perspectives from fourth year students who matched. Dermatol Online J. 2009;15:4.
- Wang JV, Keller M. Pressure to publish for residency applicants in dermatology. Dermatol Online J. 2016;22:13030/qt56x1t7ww.
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role research gap years play in a successful dermatology match. Int J Dermatol. 2022;61:226-230. doi:10.1111/ijd.15964
- Yeh C, Desai AD, Wassef C, et al. The importance of mentorship during research gap years for the dermatology residency match. Int J Dermatol. 2023;62:E209-E210. doi:10.1111/ijd.16084
- Zheng DX, Gallo Marin B, Mulligan KM, et al. Inequity concerns surrounding research years and the dermatology residency match. Int J Dermatol. 2022;61:E247-E248. doi:10.1111/ijd.16179
- Vasquez R, Jeong H, Florez-Pollack S, et al. What are the barriers faced by under-represented minorities applying to dermatology? a qualitative cross-sectional study of applicants applying to a large dermatology residency program. J Am Acad Dermatol. 2020;83:1770-1773. doi:10.1016/j.jaad.2020.03.067
- Jones VA, Clark KA, Cordova A, et al. Challenging the status quo: increasing diversity in dermatology. J Am Acad Dermatol. 2020;83:E421. doi:10.1016/j.jaad.2020.04.185
- Dirr MA, Brownstone N, Zakria D, et al. Dermatology match preference signaling tokens: impact and implications. Dermatol Surg. 2022;48:1367-1368. doi:10.1097/DSS.0000000000003645
A Nationwide Survey of Dermatology Faculty and Mentors on Their Advice for the Dermatology Match Process
A Nationwide Survey of Dermatology Faculty and Mentors on Their Advice for the Dermatology Match Process
PRACTICE POINTS
- Dermatology mentors should abide by Association of Professors of Dermatology guidelines when advising regarding signals and away rotations.
- Mentors agree with the utility of research during medical school, completing away rotations, and volunteering during medical school.
- There are differing opinions regarding the utility of a research year, program signaling, couples matching, and dual applying.
Growing Pink Nodule on the Ankle
Growing Pink Nodule on the Ankle
THE DIAGNOSIS: Epithelioid Fibrous Histiocytoma
In our patient, immunohistochemical stains for Factor XIIIa, CD68, and anaplastic lymphoma kinase (ALK) 1 confirmed the diagnosis of epithelioid fibrous histiocytoma (EFH). The location and relatively large size of the lesion led to a joint decision by the patient and physician to perform a complete excision, which was done with no complications.
Once considered a rare variant of dermatofibroma, EFH most commonly manifests as a solitary, vascular-appearing or flesh-colored papule or nodule on the legs. It often develops in the fifth decade of life with greater prevalence in men.1-5 Our patient is one of the few known cases of EFH in children that have been reported in the literature.3,6 Although EFH is benign, complete excision typically is performed due to the rarity of the lesion.3
The overexpression of ALK distinguishes EFH from other fibrohistiocytic lesions (Figure 1).5 The most common fusion partners are sequestosome 1 and vinculin (VCL), which account for more than 70% of cases.3,5,7 Interestingly, VCL-ALK fusions have been reported to occur in a subset of pediatric renal cell carcinomas and recently in an ovoid spindle cell neoplasm considered to be a low-grade sarcoma.3,7-9 Further studies have identified less common fusion partners, including the dynactin subunit 1, ETS variant transcription factor 6, protein-tyrosine phosphatase, receptor-type, F polypeptide-interacting protein-binding protein 1, sperm antigen with calponin homology and coiled-coil domains 1, tropomyosin 3, protein kinase cAMP-dependent type II regulatory subunit alpha, melanophilin, and Echinoderm microtubule-associated protein-like 4 genes.3,8
In contrast to benign fibrous histiocytomas, EFHs primarily consist of epithelioid cells, have well-defined borders, exhibit prominent vascularity, usually are situated close to the epidermis, and lack multinucleated cells or histiocytes laden with lipids or hemosiderin.2 The characteristic histopathologic finding is rounded or angulated epithelioid cells, with eosinophilic cytoplasm accounting for more than 50% of the tumor cell population.1-3,5 The nuclei of the epithelioid cells are rounded and vesicular with small eosinophilic nucleoli and low mitotic activity. Common clinical features include an exophytic nodule with a classic epidermal collarette and an epidermis that exhibits variable degrees of hyperplasia.1-3,5 Epithelioid fibrous histiocytomas often are confined to the superficial dermis and rarely extend to the subcutaneous layer. The stroma is collagenous with prominent vascularity, although older lesions can become more hyalinized and sclerotic.3 Histopathologically, these tumors can be a diagnostic challenge, as they often are mistaken for other fibrohistiocytic or melanocytic lesions.
Atypical fibroxanthoma (AFX) manifests as a dome-shaped exophytic nodule that can rapidly grow to 1 to 2 cm. Historically, it was thought to be a pseudomalignancy, but most investigators consider it within the spectrum of pleomorphic dermal sarcoma and undifferentiated pleomorphic sarcoma. Atypical fibroxanthoma usually occurs on the head and neck in elderly patients with sun-damaged skin. Histopathologically, the neoplastic cells of AFX range from atypical spindle cells and pleomorphic round to polygonal epithelioid cells to large, irregularly shaped multinucleated cells, some with foamy cytoplasm (Figure 2). The atypical spindle cells stain diffusely positive for CD10 and vimentin, while small subpopulations stain positively for CD68 or CD163 and procollagen 1. Smooth muscle actin inconsistently stains the tumor, and when it does, the staining typically is faint and patchy. Atypical fibroxanthomas usually do not stain positively for melanocytic, skeletal muscle, or keratinocytic markers.
Cellular dermatofibroma typically manifests as small, dome-shaped papules on the arms and legs that normally range from a few millimeters to 1 cm but occasionally measure up to 2 cm. Histopathologically, there are interweaving fascicles of spindle cells with hyperchromatic nuclei and peripheral splaying of the plump spindle cells that wrap around collagen bundles, known as collagen trapping (Figure 3). Unlike EFH, multinucleated cells and histiocytes with abundant lipids and hemosiderin often accompany the spindle cells in cellular dermatofibromas, which stain strongly positive for CD10 and vimentin, similar to AFX and EFH. The smooth muscle actin–staining pattern usually is faint and patchy, and in some cases, cellular dermatofibroma may not stain at all. Factor XIIIa and CD68 highlight the 2 populations of cells—fibroblasts and histiocytes—that make up the lesion.4
Epithelioid sarcoma comprises 2 types: distal (or conventional) type occurring on the distal arms and legs, particularly the hands and fingers of young adults, and proximal type occurring on the trunk and proximal extremities, including the upper arms and thighs.10 Epithelioid sarcoma is a rare aggressive malignancy that usually manifests as a firm nodule, sometimes with ulceration depending on the size. Histopathologically, diffuse dermal proliferation of ovoid to polygonal epithelioid cells arranged in short fascicles and nodular aggregations is observed (Figure 4). Spindle cells may be observed at the periphery of the lesion. Areas of necrosis are a frequent finding and a helpful diagnostic clue. Nearly all cases stain positively for pancytokeratin, CAM5.2, epithelial membrane antigen, and vimentin, and approximately half stain positively for CD34; there are variable expressions of ERG and smooth muscle actin.10 In most cases, epithelioid sarcoma does not stain positively for S100 or CD68. The majority (90%) of cases harbor a mutation in the SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily B member 1 gene, resulting in the loss of INI1 protein expression, which can be demonstrated by immunohistochemistry. 10 As the cytologic atypia usually is minimal, epithelioid sarcoma may be misdiagnosed as a necrotizing granuloma and benign fibrous lesions, particularly when superficial or small partial biopsies are performed.
Intradermal Spitz nevi can measure from a few millimeters to more than 2 cm and can range from pink to brown to black. The most common locations are the lower extremities as well as the head and neck. Histopathologically, intradermal Spitz nevi have nests of large epithelioid melanocytes with large nuclei and abundant cytoplasm (eFigure). Nuclear pseudo-inclusions, which are cytoplasmic invaginations into the nucleus, are frequent. Unlike the other conditions in the differential, these entities stain positively for melanocytic markers—S100, SOX10, and Melan-A—but not CD68 or CD163.11 A variety of kinase fusions are observed in Spitz nevi, including the ALK gene, neurotrophic tyrosine receptor kinase, ROS proto-oncogene 1, megakaryocyte-erythroid progenitor, and v-raf murine sarcoma viral oncogene homolog B1 genes.12
- Jones EW, Cerio R, Smith NP. Epithelioid cell histiocytoma: a new entity. Br J Dermatol. 1989;120:185-195.
- Glusac EJ, McNiff JM. Epithelioid cell histiocytoma: a simulant of vascular and melanocytic neoplasms. Am J Dermatopathol. 1999;21:1-7.
- Felty CC, Linos K. Epithelioid fibrous histiocytoma: a concise review [published correction appears in Am J Dermatopathol. 2020 Aug;42(8):628]. Am J Dermatopathol. 2019;41:879-883.
- Luzar B, Calonje E. Cutaneous fibrohistiocytic tumours—an update. Histopathology. 2010;56:148-165. doi:10.1111/j.1365-2559.2009.03447.x
- Doyle LA, Mariño-Enriquez A, Fletcher CD, et al. ALK rearrangement and overexpression in epithelioid fibrous histiocytoma. Mod Pathol. 2015;28:904-912.
- Singh Gomez C, Calonje E, Fletcher CD. Epithelioid benign fibrous histiocytoma of skin: clinico-pathological analysis of 20 cases of a poorly known variant. Histopathology. 1994;24:123-129.
- Jedrych J, Nikiforova M, Kennedy TF, et al. Epithelioid cell histiocytoma of the skin with clonal ALK gene rearrangement resulting in VCL- and SQSTM1-ALK gene fusions. Br J Dermatol. 2015;172: 1427-1429.
- Dickson BC, Swanson D, Charames GS, et al. Epithelioid fibrous histiocytoma: molecular characterization of ALK fusion partners in 23 cases. Mod Pathol. 2018;31:753-762.
- Helm M, Chang A, Fanburg-Smith JC, et al. Cutaneous VCL::ALK fusion ovoid-spindle cell neoplasm. J Cutan Pathol. 2023;50:405-409.
- Thway K, Jones RL, Noujaim J, et al. Epithelioid sarcoma: diagnostic features and genetics. Adv Anat Pathol. 2016;23:41-49.
- Bolognia JL, Jorizzo JJ, Schaffer JV et al. Dermatology, 4th ed. Philadelphia: Elsevier; 2018.
- Wiesner T, He J, Yelensky R, et al. Kinase fusions are frequent in Spitz tumours and spitzoid melanomas. Nat Commun. 2014;5:3116.
THE DIAGNOSIS: Epithelioid Fibrous Histiocytoma
In our patient, immunohistochemical stains for Factor XIIIa, CD68, and anaplastic lymphoma kinase (ALK) 1 confirmed the diagnosis of epithelioid fibrous histiocytoma (EFH). The location and relatively large size of the lesion led to a joint decision by the patient and physician to perform a complete excision, which was done with no complications.
Once considered a rare variant of dermatofibroma, EFH most commonly manifests as a solitary, vascular-appearing or flesh-colored papule or nodule on the legs. It often develops in the fifth decade of life with greater prevalence in men.1-5 Our patient is one of the few known cases of EFH in children that have been reported in the literature.3,6 Although EFH is benign, complete excision typically is performed due to the rarity of the lesion.3
The overexpression of ALK distinguishes EFH from other fibrohistiocytic lesions (Figure 1).5 The most common fusion partners are sequestosome 1 and vinculin (VCL), which account for more than 70% of cases.3,5,7 Interestingly, VCL-ALK fusions have been reported to occur in a subset of pediatric renal cell carcinomas and recently in an ovoid spindle cell neoplasm considered to be a low-grade sarcoma.3,7-9 Further studies have identified less common fusion partners, including the dynactin subunit 1, ETS variant transcription factor 6, protein-tyrosine phosphatase, receptor-type, F polypeptide-interacting protein-binding protein 1, sperm antigen with calponin homology and coiled-coil domains 1, tropomyosin 3, protein kinase cAMP-dependent type II regulatory subunit alpha, melanophilin, and Echinoderm microtubule-associated protein-like 4 genes.3,8

In contrast to benign fibrous histiocytomas, EFHs primarily consist of epithelioid cells, have well-defined borders, exhibit prominent vascularity, usually are situated close to the epidermis, and lack multinucleated cells or histiocytes laden with lipids or hemosiderin.2 The characteristic histopathologic finding is rounded or angulated epithelioid cells, with eosinophilic cytoplasm accounting for more than 50% of the tumor cell population.1-3,5 The nuclei of the epithelioid cells are rounded and vesicular with small eosinophilic nucleoli and low mitotic activity. Common clinical features include an exophytic nodule with a classic epidermal collarette and an epidermis that exhibits variable degrees of hyperplasia.1-3,5 Epithelioid fibrous histiocytomas often are confined to the superficial dermis and rarely extend to the subcutaneous layer. The stroma is collagenous with prominent vascularity, although older lesions can become more hyalinized and sclerotic.3 Histopathologically, these tumors can be a diagnostic challenge, as they often are mistaken for other fibrohistiocytic or melanocytic lesions.
Atypical fibroxanthoma (AFX) manifests as a dome-shaped exophytic nodule that can rapidly grow to 1 to 2 cm. Historically, it was thought to be a pseudomalignancy, but most investigators consider it within the spectrum of pleomorphic dermal sarcoma and undifferentiated pleomorphic sarcoma. Atypical fibroxanthoma usually occurs on the head and neck in elderly patients with sun-damaged skin. Histopathologically, the neoplastic cells of AFX range from atypical spindle cells and pleomorphic round to polygonal epithelioid cells to large, irregularly shaped multinucleated cells, some with foamy cytoplasm (Figure 2). The atypical spindle cells stain diffusely positive for CD10 and vimentin, while small subpopulations stain positively for CD68 or CD163 and procollagen 1. Smooth muscle actin inconsistently stains the tumor, and when it does, the staining typically is faint and patchy. Atypical fibroxanthomas usually do not stain positively for melanocytic, skeletal muscle, or keratinocytic markers.

Cellular dermatofibroma typically manifests as small, dome-shaped papules on the arms and legs that normally range from a few millimeters to 1 cm but occasionally measure up to 2 cm. Histopathologically, there are interweaving fascicles of spindle cells with hyperchromatic nuclei and peripheral splaying of the plump spindle cells that wrap around collagen bundles, known as collagen trapping (Figure 3). Unlike EFH, multinucleated cells and histiocytes with abundant lipids and hemosiderin often accompany the spindle cells in cellular dermatofibromas, which stain strongly positive for CD10 and vimentin, similar to AFX and EFH. The smooth muscle actin–staining pattern usually is faint and patchy, and in some cases, cellular dermatofibroma may not stain at all. Factor XIIIa and CD68 highlight the 2 populations of cells—fibroblasts and histiocytes—that make up the lesion.4

Epithelioid sarcoma comprises 2 types: distal (or conventional) type occurring on the distal arms and legs, particularly the hands and fingers of young adults, and proximal type occurring on the trunk and proximal extremities, including the upper arms and thighs.10 Epithelioid sarcoma is a rare aggressive malignancy that usually manifests as a firm nodule, sometimes with ulceration depending on the size. Histopathologically, diffuse dermal proliferation of ovoid to polygonal epithelioid cells arranged in short fascicles and nodular aggregations is observed (Figure 4). Spindle cells may be observed at the periphery of the lesion. Areas of necrosis are a frequent finding and a helpful diagnostic clue. Nearly all cases stain positively for pancytokeratin, CAM5.2, epithelial membrane antigen, and vimentin, and approximately half stain positively for CD34; there are variable expressions of ERG and smooth muscle actin.10 In most cases, epithelioid sarcoma does not stain positively for S100 or CD68. The majority (90%) of cases harbor a mutation in the SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily B member 1 gene, resulting in the loss of INI1 protein expression, which can be demonstrated by immunohistochemistry. 10 As the cytologic atypia usually is minimal, epithelioid sarcoma may be misdiagnosed as a necrotizing granuloma and benign fibrous lesions, particularly when superficial or small partial biopsies are performed.

Intradermal Spitz nevi can measure from a few millimeters to more than 2 cm and can range from pink to brown to black. The most common locations are the lower extremities as well as the head and neck. Histopathologically, intradermal Spitz nevi have nests of large epithelioid melanocytes with large nuclei and abundant cytoplasm (eFigure). Nuclear pseudo-inclusions, which are cytoplasmic invaginations into the nucleus, are frequent. Unlike the other conditions in the differential, these entities stain positively for melanocytic markers—S100, SOX10, and Melan-A—but not CD68 or CD163.11 A variety of kinase fusions are observed in Spitz nevi, including the ALK gene, neurotrophic tyrosine receptor kinase, ROS proto-oncogene 1, megakaryocyte-erythroid progenitor, and v-raf murine sarcoma viral oncogene homolog B1 genes.12

THE DIAGNOSIS: Epithelioid Fibrous Histiocytoma
In our patient, immunohistochemical stains for Factor XIIIa, CD68, and anaplastic lymphoma kinase (ALK) 1 confirmed the diagnosis of epithelioid fibrous histiocytoma (EFH). The location and relatively large size of the lesion led to a joint decision by the patient and physician to perform a complete excision, which was done with no complications.
Once considered a rare variant of dermatofibroma, EFH most commonly manifests as a solitary, vascular-appearing or flesh-colored papule or nodule on the legs. It often develops in the fifth decade of life with greater prevalence in men.1-5 Our patient is one of the few known cases of EFH in children that have been reported in the literature.3,6 Although EFH is benign, complete excision typically is performed due to the rarity of the lesion.3
The overexpression of ALK distinguishes EFH from other fibrohistiocytic lesions (Figure 1).5 The most common fusion partners are sequestosome 1 and vinculin (VCL), which account for more than 70% of cases.3,5,7 Interestingly, VCL-ALK fusions have been reported to occur in a subset of pediatric renal cell carcinomas and recently in an ovoid spindle cell neoplasm considered to be a low-grade sarcoma.3,7-9 Further studies have identified less common fusion partners, including the dynactin subunit 1, ETS variant transcription factor 6, protein-tyrosine phosphatase, receptor-type, F polypeptide-interacting protein-binding protein 1, sperm antigen with calponin homology and coiled-coil domains 1, tropomyosin 3, protein kinase cAMP-dependent type II regulatory subunit alpha, melanophilin, and Echinoderm microtubule-associated protein-like 4 genes.3,8

In contrast to benign fibrous histiocytomas, EFHs primarily consist of epithelioid cells, have well-defined borders, exhibit prominent vascularity, usually are situated close to the epidermis, and lack multinucleated cells or histiocytes laden with lipids or hemosiderin.2 The characteristic histopathologic finding is rounded or angulated epithelioid cells, with eosinophilic cytoplasm accounting for more than 50% of the tumor cell population.1-3,5 The nuclei of the epithelioid cells are rounded and vesicular with small eosinophilic nucleoli and low mitotic activity. Common clinical features include an exophytic nodule with a classic epidermal collarette and an epidermis that exhibits variable degrees of hyperplasia.1-3,5 Epithelioid fibrous histiocytomas often are confined to the superficial dermis and rarely extend to the subcutaneous layer. The stroma is collagenous with prominent vascularity, although older lesions can become more hyalinized and sclerotic.3 Histopathologically, these tumors can be a diagnostic challenge, as they often are mistaken for other fibrohistiocytic or melanocytic lesions.
Atypical fibroxanthoma (AFX) manifests as a dome-shaped exophytic nodule that can rapidly grow to 1 to 2 cm. Historically, it was thought to be a pseudomalignancy, but most investigators consider it within the spectrum of pleomorphic dermal sarcoma and undifferentiated pleomorphic sarcoma. Atypical fibroxanthoma usually occurs on the head and neck in elderly patients with sun-damaged skin. Histopathologically, the neoplastic cells of AFX range from atypical spindle cells and pleomorphic round to polygonal epithelioid cells to large, irregularly shaped multinucleated cells, some with foamy cytoplasm (Figure 2). The atypical spindle cells stain diffusely positive for CD10 and vimentin, while small subpopulations stain positively for CD68 or CD163 and procollagen 1. Smooth muscle actin inconsistently stains the tumor, and when it does, the staining typically is faint and patchy. Atypical fibroxanthomas usually do not stain positively for melanocytic, skeletal muscle, or keratinocytic markers.

Cellular dermatofibroma typically manifests as small, dome-shaped papules on the arms and legs that normally range from a few millimeters to 1 cm but occasionally measure up to 2 cm. Histopathologically, there are interweaving fascicles of spindle cells with hyperchromatic nuclei and peripheral splaying of the plump spindle cells that wrap around collagen bundles, known as collagen trapping (Figure 3). Unlike EFH, multinucleated cells and histiocytes with abundant lipids and hemosiderin often accompany the spindle cells in cellular dermatofibromas, which stain strongly positive for CD10 and vimentin, similar to AFX and EFH. The smooth muscle actin–staining pattern usually is faint and patchy, and in some cases, cellular dermatofibroma may not stain at all. Factor XIIIa and CD68 highlight the 2 populations of cells—fibroblasts and histiocytes—that make up the lesion.4

Epithelioid sarcoma comprises 2 types: distal (or conventional) type occurring on the distal arms and legs, particularly the hands and fingers of young adults, and proximal type occurring on the trunk and proximal extremities, including the upper arms and thighs.10 Epithelioid sarcoma is a rare aggressive malignancy that usually manifests as a firm nodule, sometimes with ulceration depending on the size. Histopathologically, diffuse dermal proliferation of ovoid to polygonal epithelioid cells arranged in short fascicles and nodular aggregations is observed (Figure 4). Spindle cells may be observed at the periphery of the lesion. Areas of necrosis are a frequent finding and a helpful diagnostic clue. Nearly all cases stain positively for pancytokeratin, CAM5.2, epithelial membrane antigen, and vimentin, and approximately half stain positively for CD34; there are variable expressions of ERG and smooth muscle actin.10 In most cases, epithelioid sarcoma does not stain positively for S100 or CD68. The majority (90%) of cases harbor a mutation in the SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily B member 1 gene, resulting in the loss of INI1 protein expression, which can be demonstrated by immunohistochemistry. 10 As the cytologic atypia usually is minimal, epithelioid sarcoma may be misdiagnosed as a necrotizing granuloma and benign fibrous lesions, particularly when superficial or small partial biopsies are performed.

Intradermal Spitz nevi can measure from a few millimeters to more than 2 cm and can range from pink to brown to black. The most common locations are the lower extremities as well as the head and neck. Histopathologically, intradermal Spitz nevi have nests of large epithelioid melanocytes with large nuclei and abundant cytoplasm (eFigure). Nuclear pseudo-inclusions, which are cytoplasmic invaginations into the nucleus, are frequent. Unlike the other conditions in the differential, these entities stain positively for melanocytic markers—S100, SOX10, and Melan-A—but not CD68 or CD163.11 A variety of kinase fusions are observed in Spitz nevi, including the ALK gene, neurotrophic tyrosine receptor kinase, ROS proto-oncogene 1, megakaryocyte-erythroid progenitor, and v-raf murine sarcoma viral oncogene homolog B1 genes.12

- Jones EW, Cerio R, Smith NP. Epithelioid cell histiocytoma: a new entity. Br J Dermatol. 1989;120:185-195.
- Glusac EJ, McNiff JM. Epithelioid cell histiocytoma: a simulant of vascular and melanocytic neoplasms. Am J Dermatopathol. 1999;21:1-7.
- Felty CC, Linos K. Epithelioid fibrous histiocytoma: a concise review [published correction appears in Am J Dermatopathol. 2020 Aug;42(8):628]. Am J Dermatopathol. 2019;41:879-883.
- Luzar B, Calonje E. Cutaneous fibrohistiocytic tumours—an update. Histopathology. 2010;56:148-165. doi:10.1111/j.1365-2559.2009.03447.x
- Doyle LA, Mariño-Enriquez A, Fletcher CD, et al. ALK rearrangement and overexpression in epithelioid fibrous histiocytoma. Mod Pathol. 2015;28:904-912.
- Singh Gomez C, Calonje E, Fletcher CD. Epithelioid benign fibrous histiocytoma of skin: clinico-pathological analysis of 20 cases of a poorly known variant. Histopathology. 1994;24:123-129.
- Jedrych J, Nikiforova M, Kennedy TF, et al. Epithelioid cell histiocytoma of the skin with clonal ALK gene rearrangement resulting in VCL- and SQSTM1-ALK gene fusions. Br J Dermatol. 2015;172: 1427-1429.
- Dickson BC, Swanson D, Charames GS, et al. Epithelioid fibrous histiocytoma: molecular characterization of ALK fusion partners in 23 cases. Mod Pathol. 2018;31:753-762.
- Helm M, Chang A, Fanburg-Smith JC, et al. Cutaneous VCL::ALK fusion ovoid-spindle cell neoplasm. J Cutan Pathol. 2023;50:405-409.
- Thway K, Jones RL, Noujaim J, et al. Epithelioid sarcoma: diagnostic features and genetics. Adv Anat Pathol. 2016;23:41-49.
- Bolognia JL, Jorizzo JJ, Schaffer JV et al. Dermatology, 4th ed. Philadelphia: Elsevier; 2018.
- Wiesner T, He J, Yelensky R, et al. Kinase fusions are frequent in Spitz tumours and spitzoid melanomas. Nat Commun. 2014;5:3116.
- Jones EW, Cerio R, Smith NP. Epithelioid cell histiocytoma: a new entity. Br J Dermatol. 1989;120:185-195.
- Glusac EJ, McNiff JM. Epithelioid cell histiocytoma: a simulant of vascular and melanocytic neoplasms. Am J Dermatopathol. 1999;21:1-7.
- Felty CC, Linos K. Epithelioid fibrous histiocytoma: a concise review [published correction appears in Am J Dermatopathol. 2020 Aug;42(8):628]. Am J Dermatopathol. 2019;41:879-883.
- Luzar B, Calonje E. Cutaneous fibrohistiocytic tumours—an update. Histopathology. 2010;56:148-165. doi:10.1111/j.1365-2559.2009.03447.x
- Doyle LA, Mariño-Enriquez A, Fletcher CD, et al. ALK rearrangement and overexpression in epithelioid fibrous histiocytoma. Mod Pathol. 2015;28:904-912.
- Singh Gomez C, Calonje E, Fletcher CD. Epithelioid benign fibrous histiocytoma of skin: clinico-pathological analysis of 20 cases of a poorly known variant. Histopathology. 1994;24:123-129.
- Jedrych J, Nikiforova M, Kennedy TF, et al. Epithelioid cell histiocytoma of the skin with clonal ALK gene rearrangement resulting in VCL- and SQSTM1-ALK gene fusions. Br J Dermatol. 2015;172: 1427-1429.
- Dickson BC, Swanson D, Charames GS, et al. Epithelioid fibrous histiocytoma: molecular characterization of ALK fusion partners in 23 cases. Mod Pathol. 2018;31:753-762.
- Helm M, Chang A, Fanburg-Smith JC, et al. Cutaneous VCL::ALK fusion ovoid-spindle cell neoplasm. J Cutan Pathol. 2023;50:405-409.
- Thway K, Jones RL, Noujaim J, et al. Epithelioid sarcoma: diagnostic features and genetics. Adv Anat Pathol. 2016;23:41-49.
- Bolognia JL, Jorizzo JJ, Schaffer JV et al. Dermatology, 4th ed. Philadelphia: Elsevier; 2018.
- Wiesner T, He J, Yelensky R, et al. Kinase fusions are frequent in Spitz tumours and spitzoid melanomas. Nat Commun. 2014;5:3116.
Growing Pink Nodule on the Ankle
Growing Pink Nodule on the Ankle
A 17-year-old girl presented to the dermatology department with a slow-growing lesion on the right lower leg that progressed in size over 1 year. The patient reported that the lesion occasionally bled but denied any other associated symptoms or a personal or family history of skin cancer. Physical examination revealed a solitary, well-circumscribed, circular, pink nodule on the right lateral upper ankle. Dermoscopy showed an amorphous mixture of pale and pink areas. A shave biopsy revealed a proliferation of epithelioid cells that diffusely stained positive for Factor XIIIa and anaplastic lymphoma kinase 1 and stained negatively for pancytokeratin, Melan A, CD34, ERG, CD31, SOX10, smooth muscle actin, desmin, and CD30. Next-generation sequencing revealed a vinculin and anaplastic lymphoma kinase gene fusion.
Continuous Testing Method for Contact Allergy to Topical Therapies in the Management of Chronic and Postoperative Wounds
Patients who undergo cutaneous surgery and chronic wound care often are exposed to various topical
Practice Gap
Contact allergies are common in patients with postoperative or chronic wounds. When patch tested, approximately 80% of patients with chronic venous ulcers demonstrated at least 1 positive allergic reaction based on a Canadian study.3 Similarly, postoperative ACD in dermatologic surgery occurs in more than 1.6% of cases in North America and Europe, a rate that is similar to or higher than the rate of postoperative infection, approximately 1% to 2%.4 Postoperative patients and those with chronic wounds have multiple risk factors for ACD. Firstly, applying topical therapies to inflamed or compromised skin increases the risk for contact sensitization.5 Additionally, multiple topical therapies containing known allergenic components may be recommended for wound care, including impregnated or organic dressings, antibiotic ointments, adhesives, antiseptic washes, and topical therapies containing inactive ingredients such as lanolin derivatives.6 Contact with numerous compounds at the same time increases the risk for a contact allergy as well as co-sensitization.7 Similarly, the longer topical agents are applied, the greater the risk for a contact allergy, with sensitization liable to occur at any point during treatment.
Preventive topical antibiotics have garnered a negative reputation among dermatologists, often due to varying data on their efficacy and the overuse of highly allergenic over-the-counter topical antibiotics such as neomycin.8 However, data also have suggested that topical antibiotics can reduce postoperative infections in higher risk surgical cases, specifically certain head and neck surgeries.9 Likewise, topical antibiotics are useful for wound colonization with Pseudomonas, which can remain superficial and slow down healing without progressing to a systemic infection.10 Such cases can be successfully treated or prevented with topical therapies, thereby bypassing the more concerning adverse effects of systemic antibiotics. In particular, systemic fluoroquinolones often are used to treat Pseudomonas and can have many serious adverse effects, including tendon rupture, drug interactions, and arrhythmias.11 Therefore, it is worth implementing topical treatments for wounds colonized with Pseudomonas to spare patients these potential complications.
When a postoperative patient develops a rash at the surgical site, it is critical to differentiate between wound infection and contact allergy, as the treatments for these two conditions may be mutually exclusive and treating the wrong condition may exacerbate the other, such as mistakenly using topical corticosteroids for a wound infection.7 Prompt treatment is necessary for wound infections, as time is limited for patch testing when a rash is already present and the diagnosis is questionable. Allergic contact dermatitis typically erupts 48 to 96 hours following exposure to a contact allergen, often manifesting as intensely pruritic erythematous patches or vesicles.6 Wound infections are characterized by pain and warmth, with erythema and edema present in both conditions. Postoperative infections manifest usually 4 to 7 days following surgery.12 Despite these differences, pruritus and pain are common in the wound healing process; thus, differentiating an infection from ACD on a clinical basis alone is not always possible. Furthermore, presentation of a contact allergy may be delayed beyond the typical 96-hour timeframe if a patient is newly sensitized to an allergen, causing the timeline of rash development to appear similar to that of a wound infection. In such cases, systemic antibiotics often are prescribed empirically; hence, clearer and timelier differentiation between contact allergy and wound infection reduces unnecessary antibiotic prescriptions, thereby avoiding systemic adverse effects and promoting responsible antibiotic stewardship.12
The Technique
Since potentially allergenic topical therapies often are indicated in wound management, we propose that patients serve as internal controls to test continuously for contact allergy sensitization. We recommend that patients apply a small amount of the topical agent, product, or dressing to the inner forearm each time they apply it to the wound. If the patient is sensitized to the product initially or becomes sensitized during treatment, evidence of ACD will be visible not only at the site of the wound but also in the area of secondary application. The inner forearm is recommended for convenience and reproducibility, but a patient may choose a different site as long as it remains consistent. Although certain contact allergens rarely may react solely at a site of inflamed skin, our team has quickly identified ACD and avoided misdiagnosis of chronic or postsurgical wound infection using this approach.13 Subsequent patch testing is indicated when a contact allergy is detected.
Practice Implications
Topical therapies including ointments, washes, and dressing components have the potential to cause sensitization and contact allergy. Despite the concern for development of ACD, topical antibiotics play a useful role in cutaneous surgery.7 Synchronous testing for contact allergy when managing wounds with topical therapies could improve diagnostic accuracy when an allergic reaction occurs. This technique provides a means of harnessing the benefits of topical agents while monitoring the risk for ACD in postoperative and chronic wound care settings.
Butler L, Mowad C. Allergic contact dermatitis in dermatologic surgery: review of common allergens. Dermatitis. 2013;24:215-221. doi:10.1097/DER.0b013e3182a0d3a9
So SP, Yoon JY, Kim JW. Postoperative contact dermatitis caused by skin adhesives used in orthopedic surgery: incidence, characteristics, and difference from surgical site infection. Medicine (Baltimore). 2021;100:e26053. doi:10.1097/md.0000000000026053
Alavi A, Sibbald RG, Ladizinski B, et al. Wound-related allergic/irritant contact dermatitis. Adv Skin Wound Care. 2016;29:278-286. doi:10.1097/01.ASW.0000482834.94375.1e
Sheth VM, Weitzul S. Postoperative topical antimicrobial use. Dermatitis. 2008;19:181-189.
Kohli N, Nedorost S. Inflamed skin predisposes to sensitization to less potent allergens. J Am Acad Dermatol. 2016;75:312-317.e1. doi:10.1016/j.jaad.2016.03.010
Cook KA, Kelso JM. Surgery-related contact dermatitis: a review of potential irritants and allergens. J Allergy Clin Immunol Pract. 2017;5:1234-1240. doi:10.1016/j.jaip.2017.03.001
Kreft B, Wohlrab J. Contact allergies to topical antibiotic applications. Allergol Select. 2022;6:18-26. doi:10.5414/alx02253e
Scherrer MAR, Abreu ÉP, Rocha VB. Neomycin: sources of contact and sensitization evaluation in 1162 patients treated at a tertiary service. An Bras Dermatol. 2023;98:487-492. doi:10.1016/j.abd.2022.07.008
Ashraf DC, Idowu OO, Wang Q, et al. The role of topical antibiotic prophylaxis in oculofacial plastic surgery: a randomized controlled study. Ophthalmology. 2020;127:1747-1754. doi:10.1016/j.ophtha.2020.07.032
Zielin´ska M, Pawłowska A, Orzeł A, et al. Wound microbiota and its impact on wound healing. Int J Mol Sci. 2023;24:17318. doi:10.3390/ijms242417318
Baggio D, Ananda-Rajah MR. Fluoroquinolone antibiotics and adverse events. Aust Prescr. 2021;44:161-164. doi:10.18773/austprescr.2021.035
Ken KM, Johnson MM, Leitenberger JJ, et al. Postoperative infections in dermatologic surgery: the role of wound cultures. Dermatol Surg. 2020;46:1294-1299. doi:10.1097/dss.0000000000002317
Wolf R. The lanolin paradox. Dermatology. 1996;192:198-202. doi:10.1159/000246365
Patients who undergo cutaneous surgery and chronic wound care often are exposed to various topical
Practice Gap
Contact allergies are common in patients with postoperative or chronic wounds. When patch tested, approximately 80% of patients with chronic venous ulcers demonstrated at least 1 positive allergic reaction based on a Canadian study.3 Similarly, postoperative ACD in dermatologic surgery occurs in more than 1.6% of cases in North America and Europe, a rate that is similar to or higher than the rate of postoperative infection, approximately 1% to 2%.4 Postoperative patients and those with chronic wounds have multiple risk factors for ACD. Firstly, applying topical therapies to inflamed or compromised skin increases the risk for contact sensitization.5 Additionally, multiple topical therapies containing known allergenic components may be recommended for wound care, including impregnated or organic dressings, antibiotic ointments, adhesives, antiseptic washes, and topical therapies containing inactive ingredients such as lanolin derivatives.6 Contact with numerous compounds at the same time increases the risk for a contact allergy as well as co-sensitization.7 Similarly, the longer topical agents are applied, the greater the risk for a contact allergy, with sensitization liable to occur at any point during treatment.
Preventive topical antibiotics have garnered a negative reputation among dermatologists, often due to varying data on their efficacy and the overuse of highly allergenic over-the-counter topical antibiotics such as neomycin.8 However, data also have suggested that topical antibiotics can reduce postoperative infections in higher risk surgical cases, specifically certain head and neck surgeries.9 Likewise, topical antibiotics are useful for wound colonization with Pseudomonas, which can remain superficial and slow down healing without progressing to a systemic infection.10 Such cases can be successfully treated or prevented with topical therapies, thereby bypassing the more concerning adverse effects of systemic antibiotics. In particular, systemic fluoroquinolones often are used to treat Pseudomonas and can have many serious adverse effects, including tendon rupture, drug interactions, and arrhythmias.11 Therefore, it is worth implementing topical treatments for wounds colonized with Pseudomonas to spare patients these potential complications.
When a postoperative patient develops a rash at the surgical site, it is critical to differentiate between wound infection and contact allergy, as the treatments for these two conditions may be mutually exclusive and treating the wrong condition may exacerbate the other, such as mistakenly using topical corticosteroids for a wound infection.7 Prompt treatment is necessary for wound infections, as time is limited for patch testing when a rash is already present and the diagnosis is questionable. Allergic contact dermatitis typically erupts 48 to 96 hours following exposure to a contact allergen, often manifesting as intensely pruritic erythematous patches or vesicles.6 Wound infections are characterized by pain and warmth, with erythema and edema present in both conditions. Postoperative infections manifest usually 4 to 7 days following surgery.12 Despite these differences, pruritus and pain are common in the wound healing process; thus, differentiating an infection from ACD on a clinical basis alone is not always possible. Furthermore, presentation of a contact allergy may be delayed beyond the typical 96-hour timeframe if a patient is newly sensitized to an allergen, causing the timeline of rash development to appear similar to that of a wound infection. In such cases, systemic antibiotics often are prescribed empirically; hence, clearer and timelier differentiation between contact allergy and wound infection reduces unnecessary antibiotic prescriptions, thereby avoiding systemic adverse effects and promoting responsible antibiotic stewardship.12
The Technique
Since potentially allergenic topical therapies often are indicated in wound management, we propose that patients serve as internal controls to test continuously for contact allergy sensitization. We recommend that patients apply a small amount of the topical agent, product, or dressing to the inner forearm each time they apply it to the wound. If the patient is sensitized to the product initially or becomes sensitized during treatment, evidence of ACD will be visible not only at the site of the wound but also in the area of secondary application. The inner forearm is recommended for convenience and reproducibility, but a patient may choose a different site as long as it remains consistent. Although certain contact allergens rarely may react solely at a site of inflamed skin, our team has quickly identified ACD and avoided misdiagnosis of chronic or postsurgical wound infection using this approach.13 Subsequent patch testing is indicated when a contact allergy is detected.
Practice Implications
Topical therapies including ointments, washes, and dressing components have the potential to cause sensitization and contact allergy. Despite the concern for development of ACD, topical antibiotics play a useful role in cutaneous surgery.7 Synchronous testing for contact allergy when managing wounds with topical therapies could improve diagnostic accuracy when an allergic reaction occurs. This technique provides a means of harnessing the benefits of topical agents while monitoring the risk for ACD in postoperative and chronic wound care settings.
Patients who undergo cutaneous surgery and chronic wound care often are exposed to various topical
Practice Gap
Contact allergies are common in patients with postoperative or chronic wounds. When patch tested, approximately 80% of patients with chronic venous ulcers demonstrated at least 1 positive allergic reaction based on a Canadian study.3 Similarly, postoperative ACD in dermatologic surgery occurs in more than 1.6% of cases in North America and Europe, a rate that is similar to or higher than the rate of postoperative infection, approximately 1% to 2%.4 Postoperative patients and those with chronic wounds have multiple risk factors for ACD. Firstly, applying topical therapies to inflamed or compromised skin increases the risk for contact sensitization.5 Additionally, multiple topical therapies containing known allergenic components may be recommended for wound care, including impregnated or organic dressings, antibiotic ointments, adhesives, antiseptic washes, and topical therapies containing inactive ingredients such as lanolin derivatives.6 Contact with numerous compounds at the same time increases the risk for a contact allergy as well as co-sensitization.7 Similarly, the longer topical agents are applied, the greater the risk for a contact allergy, with sensitization liable to occur at any point during treatment.
Preventive topical antibiotics have garnered a negative reputation among dermatologists, often due to varying data on their efficacy and the overuse of highly allergenic over-the-counter topical antibiotics such as neomycin.8 However, data also have suggested that topical antibiotics can reduce postoperative infections in higher risk surgical cases, specifically certain head and neck surgeries.9 Likewise, topical antibiotics are useful for wound colonization with Pseudomonas, which can remain superficial and slow down healing without progressing to a systemic infection.10 Such cases can be successfully treated or prevented with topical therapies, thereby bypassing the more concerning adverse effects of systemic antibiotics. In particular, systemic fluoroquinolones often are used to treat Pseudomonas and can have many serious adverse effects, including tendon rupture, drug interactions, and arrhythmias.11 Therefore, it is worth implementing topical treatments for wounds colonized with Pseudomonas to spare patients these potential complications.
When a postoperative patient develops a rash at the surgical site, it is critical to differentiate between wound infection and contact allergy, as the treatments for these two conditions may be mutually exclusive and treating the wrong condition may exacerbate the other, such as mistakenly using topical corticosteroids for a wound infection.7 Prompt treatment is necessary for wound infections, as time is limited for patch testing when a rash is already present and the diagnosis is questionable. Allergic contact dermatitis typically erupts 48 to 96 hours following exposure to a contact allergen, often manifesting as intensely pruritic erythematous patches or vesicles.6 Wound infections are characterized by pain and warmth, with erythema and edema present in both conditions. Postoperative infections manifest usually 4 to 7 days following surgery.12 Despite these differences, pruritus and pain are common in the wound healing process; thus, differentiating an infection from ACD on a clinical basis alone is not always possible. Furthermore, presentation of a contact allergy may be delayed beyond the typical 96-hour timeframe if a patient is newly sensitized to an allergen, causing the timeline of rash development to appear similar to that of a wound infection. In such cases, systemic antibiotics often are prescribed empirically; hence, clearer and timelier differentiation between contact allergy and wound infection reduces unnecessary antibiotic prescriptions, thereby avoiding systemic adverse effects and promoting responsible antibiotic stewardship.12
The Technique
Since potentially allergenic topical therapies often are indicated in wound management, we propose that patients serve as internal controls to test continuously for contact allergy sensitization. We recommend that patients apply a small amount of the topical agent, product, or dressing to the inner forearm each time they apply it to the wound. If the patient is sensitized to the product initially or becomes sensitized during treatment, evidence of ACD will be visible not only at the site of the wound but also in the area of secondary application. The inner forearm is recommended for convenience and reproducibility, but a patient may choose a different site as long as it remains consistent. Although certain contact allergens rarely may react solely at a site of inflamed skin, our team has quickly identified ACD and avoided misdiagnosis of chronic or postsurgical wound infection using this approach.13 Subsequent patch testing is indicated when a contact allergy is detected.
Practice Implications
Topical therapies including ointments, washes, and dressing components have the potential to cause sensitization and contact allergy. Despite the concern for development of ACD, topical antibiotics play a useful role in cutaneous surgery.7 Synchronous testing for contact allergy when managing wounds with topical therapies could improve diagnostic accuracy when an allergic reaction occurs. This technique provides a means of harnessing the benefits of topical agents while monitoring the risk for ACD in postoperative and chronic wound care settings.
Butler L, Mowad C. Allergic contact dermatitis in dermatologic surgery: review of common allergens. Dermatitis. 2013;24:215-221. doi:10.1097/DER.0b013e3182a0d3a9
So SP, Yoon JY, Kim JW. Postoperative contact dermatitis caused by skin adhesives used in orthopedic surgery: incidence, characteristics, and difference from surgical site infection. Medicine (Baltimore). 2021;100:e26053. doi:10.1097/md.0000000000026053
Alavi A, Sibbald RG, Ladizinski B, et al. Wound-related allergic/irritant contact dermatitis. Adv Skin Wound Care. 2016;29:278-286. doi:10.1097/01.ASW.0000482834.94375.1e
Sheth VM, Weitzul S. Postoperative topical antimicrobial use. Dermatitis. 2008;19:181-189.
Kohli N, Nedorost S. Inflamed skin predisposes to sensitization to less potent allergens. J Am Acad Dermatol. 2016;75:312-317.e1. doi:10.1016/j.jaad.2016.03.010
Cook KA, Kelso JM. Surgery-related contact dermatitis: a review of potential irritants and allergens. J Allergy Clin Immunol Pract. 2017;5:1234-1240. doi:10.1016/j.jaip.2017.03.001
Kreft B, Wohlrab J. Contact allergies to topical antibiotic applications. Allergol Select. 2022;6:18-26. doi:10.5414/alx02253e
Scherrer MAR, Abreu ÉP, Rocha VB. Neomycin: sources of contact and sensitization evaluation in 1162 patients treated at a tertiary service. An Bras Dermatol. 2023;98:487-492. doi:10.1016/j.abd.2022.07.008
Ashraf DC, Idowu OO, Wang Q, et al. The role of topical antibiotic prophylaxis in oculofacial plastic surgery: a randomized controlled study. Ophthalmology. 2020;127:1747-1754. doi:10.1016/j.ophtha.2020.07.032
Zielin´ska M, Pawłowska A, Orzeł A, et al. Wound microbiota and its impact on wound healing. Int J Mol Sci. 2023;24:17318. doi:10.3390/ijms242417318
Baggio D, Ananda-Rajah MR. Fluoroquinolone antibiotics and adverse events. Aust Prescr. 2021;44:161-164. doi:10.18773/austprescr.2021.035
Ken KM, Johnson MM, Leitenberger JJ, et al. Postoperative infections in dermatologic surgery: the role of wound cultures. Dermatol Surg. 2020;46:1294-1299. doi:10.1097/dss.0000000000002317
Wolf R. The lanolin paradox. Dermatology. 1996;192:198-202. doi:10.1159/000246365
Butler L, Mowad C. Allergic contact dermatitis in dermatologic surgery: review of common allergens. Dermatitis. 2013;24:215-221. doi:10.1097/DER.0b013e3182a0d3a9
So SP, Yoon JY, Kim JW. Postoperative contact dermatitis caused by skin adhesives used in orthopedic surgery: incidence, characteristics, and difference from surgical site infection. Medicine (Baltimore). 2021;100:e26053. doi:10.1097/md.0000000000026053
Alavi A, Sibbald RG, Ladizinski B, et al. Wound-related allergic/irritant contact dermatitis. Adv Skin Wound Care. 2016;29:278-286. doi:10.1097/01.ASW.0000482834.94375.1e
Sheth VM, Weitzul S. Postoperative topical antimicrobial use. Dermatitis. 2008;19:181-189.
Kohli N, Nedorost S. Inflamed skin predisposes to sensitization to less potent allergens. J Am Acad Dermatol. 2016;75:312-317.e1. doi:10.1016/j.jaad.2016.03.010
Cook KA, Kelso JM. Surgery-related contact dermatitis: a review of potential irritants and allergens. J Allergy Clin Immunol Pract. 2017;5:1234-1240. doi:10.1016/j.jaip.2017.03.001
Kreft B, Wohlrab J. Contact allergies to topical antibiotic applications. Allergol Select. 2022;6:18-26. doi:10.5414/alx02253e
Scherrer MAR, Abreu ÉP, Rocha VB. Neomycin: sources of contact and sensitization evaluation in 1162 patients treated at a tertiary service. An Bras Dermatol. 2023;98:487-492. doi:10.1016/j.abd.2022.07.008
Ashraf DC, Idowu OO, Wang Q, et al. The role of topical antibiotic prophylaxis in oculofacial plastic surgery: a randomized controlled study. Ophthalmology. 2020;127:1747-1754. doi:10.1016/j.ophtha.2020.07.032
Zielin´ska M, Pawłowska A, Orzeł A, et al. Wound microbiota and its impact on wound healing. Int J Mol Sci. 2023;24:17318. doi:10.3390/ijms242417318
Baggio D, Ananda-Rajah MR. Fluoroquinolone antibiotics and adverse events. Aust Prescr. 2021;44:161-164. doi:10.18773/austprescr.2021.035
Ken KM, Johnson MM, Leitenberger JJ, et al. Postoperative infections in dermatologic surgery: the role of wound cultures. Dermatol Surg. 2020;46:1294-1299. doi:10.1097/dss.0000000000002317
Wolf R. The lanolin paradox. Dermatology. 1996;192:198-202. doi:10.1159/000246365