User login
Data Trends 2025: HIV
Data Trends 2025: HIV
Click here to view more from Federal Health Care Data Trends 2025.
- Varley CD, et al. Clin Infect Dis. 2024;78(6):1571-1579. doi:10.1093/cid/
ciae025 Hicks WL, et al. HIV Med. 2025;26(2):218-229. doi:10.1111/hiv.13724
Click here to view more from Federal Health Care Data Trends 2025.
Click here to view more from Federal Health Care Data Trends 2025.
- Varley CD, et al. Clin Infect Dis. 2024;78(6):1571-1579. doi:10.1093/cid/
ciae025 Hicks WL, et al. HIV Med. 2025;26(2):218-229. doi:10.1111/hiv.13724
- Varley CD, et al. Clin Infect Dis. 2024;78(6):1571-1579. doi:10.1093/cid/
ciae025 Hicks WL, et al. HIV Med. 2025;26(2):218-229. doi:10.1111/hiv.13724
Data Trends 2025: HIV
Data Trends 2025: HIV
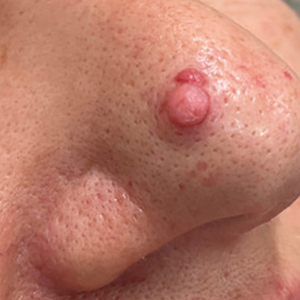
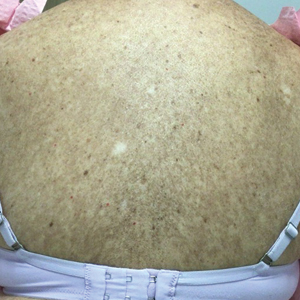
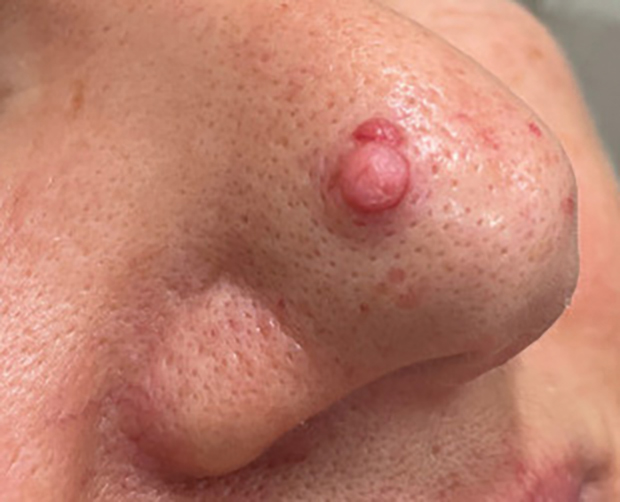
Pedunculated Pink Papule on the Nose
THE DIAGNOSIS: Pedunculated Lipofibroma
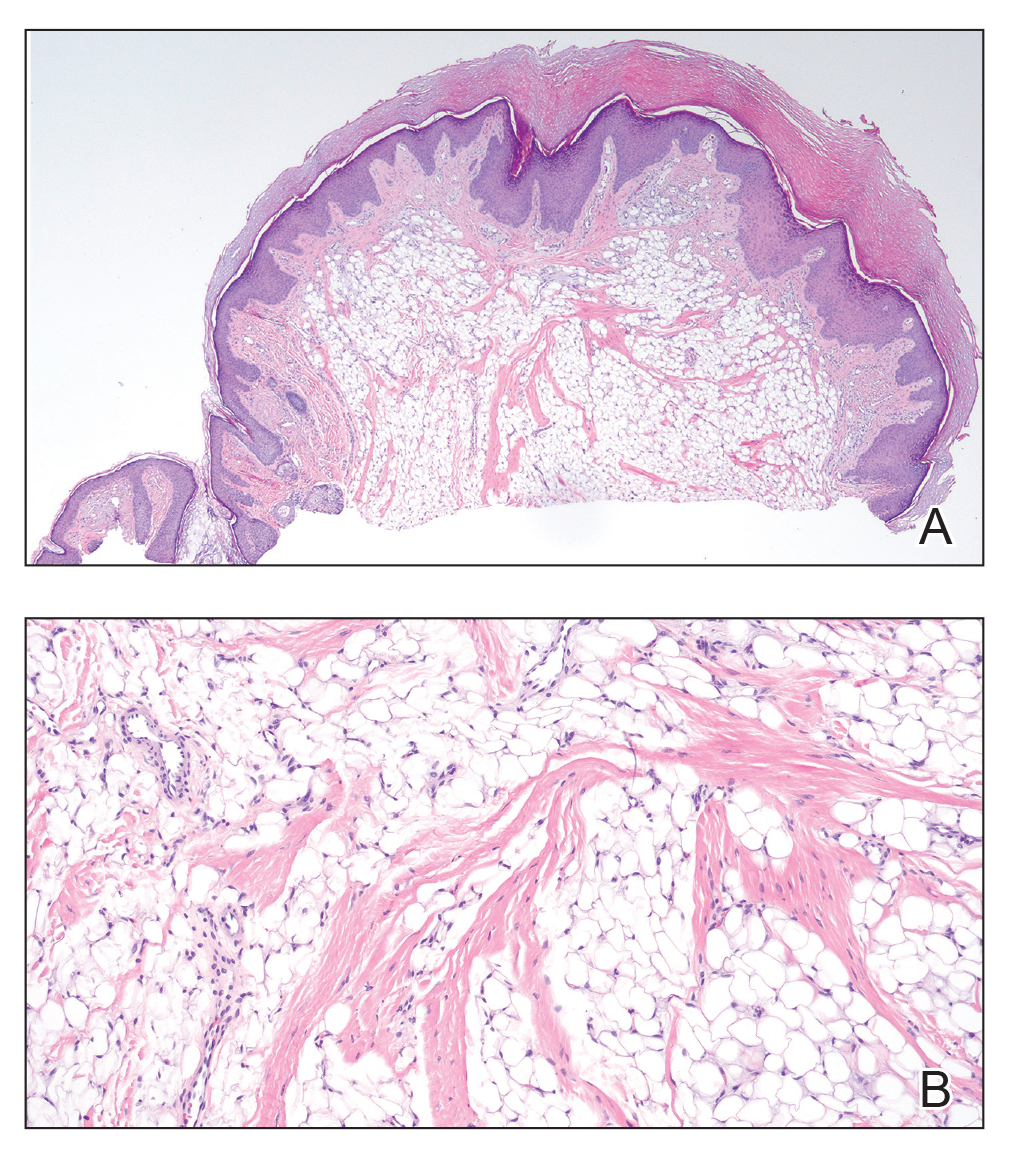
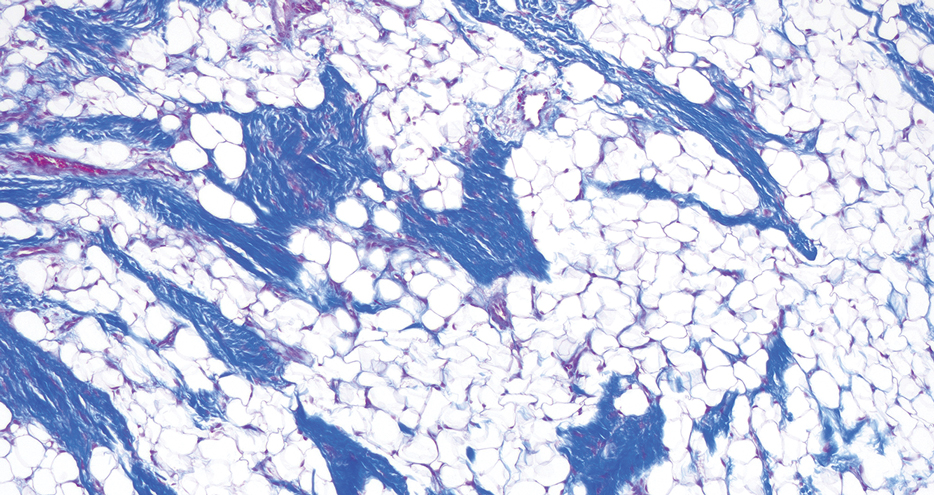
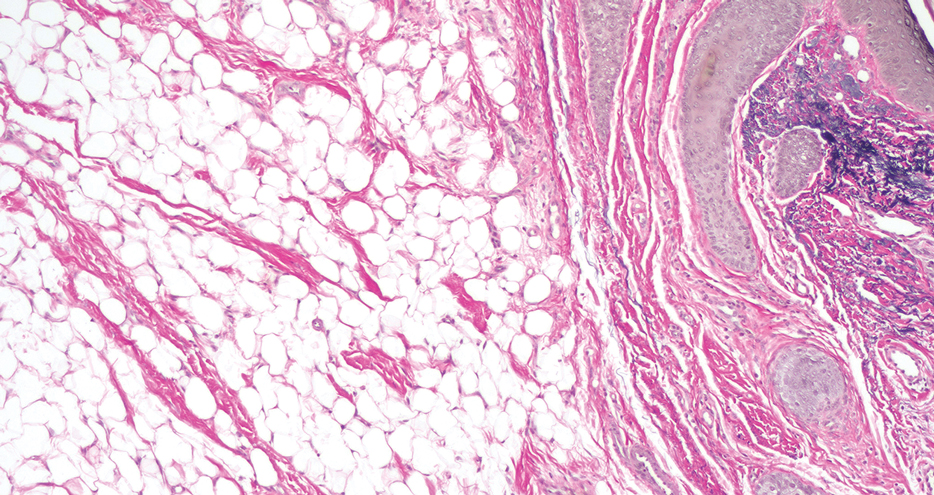
Histopathology confirmed a pedunculated/polypoid lesion with intradermal lobules of adipocytes/mature adipose tissue admixed with connective tissue bundles and vascular ectasias. Overlying epidermal acanthosis with slight papillomatosis and hyperkeratosis was present (Figure 1). Masson trichrome staining highlighted admixed collagen bundles (Figure 2). Verhoeff–van Gieson staining showed marked reduction in elastic fibers (Figure 3). Immunostaining was negative for smooth muscle actin and desmin. A diagnosis of pedunculated lipofibroma on the nose was made based on both clinical and histopathologic findings.
Pedunculated lipofibroma (or solitary lipofibroma) is the solitary form of nevus lipomatosus cutaneous superficialis (NLCS).7 First described by Hoffmann and Zurhelle1 in 1921, NLCS is an uncommon benign hamartomatous cutaneous lesion/connective tissue nevus that also has a classic multiple form.1-13 The etiology of NLCS remains unclear, but several theories have been proposed to explain its pathogenesis, including deposition of adipocytes secondary to degenerative changes in dermal connective tissue, focal/local heterotopic development of adipose tissue, and derivation from differentiating lipoblasts (preadipose tissue) originating from precursor vascular or perivascular cells.2-13
Pedunculated lipofibroma usually develops during the third to sixth decades of life and manifests as a single cutaneous lesion with a smooth surface, often on a non–pelvic girdle location.7-13 No particular predilection sites are noted, with lesions reported on the arm, axilla, back, upper thigh, knee, and sole.5,12 There are rare reports of this type of NLCS on the ear, scalp, forehead, or eyelid.7-11
In the classic form of NLCS, multiple cutaneous lesions are present at birth or develop within the first 2 to 3 decades of life.2-6 Lesions consist of soft, nontender, pedunculated, flesh-colored or yellowish papules and nodules with a verrucoid or cerebriform surface that may later coalesce to form plaques.2-6 Predilection sites include the pelvic girdle, buttocks, sacral and coccygeal regions, and upper posterior thighs, with a linear or zosteriform pattern of distribution.2-6 Rarely, the classic form can arise in elderly patients and/or at an atypical anatomic location (eg, clitoris,3 shoulder,5 thorax,5 abdomen5) and can demonstrate extension of lesions across the midline.4 Rare cases of classic NLCS on the scalp2 and face3-6 have been reported, including lesions localized to the nose3 and chin4 and others extending from the right mandible to the neck5 and right lower lip to the submandibular/posteriorateral cervical region.6 In some cases, lesions clinically resemble plane xanthoma4 and localized scleroderma.6
Adotama et al13 proposed a set of clinical features to differentiate classic NLCS, pedunculated lipofibroma (solitary NLCS), and fibroepithelial polyp with adipocytes (distinguished by their furrowed surface, hyperpigmentation, and anatomic predilection for the neck and axilla). Lesions are asymptomatic in both forms of NLCS.2-13 Family history or predominant sex involvement have not been reported in either clinical type.2-13 Reported associations with NLCS include a number of endocrinologic conditions including diabetes.7 Other coexisting skin findings can include café-au-lait macules, leukodermic (white) spots, overlying hypertrichosis, comedolike alterations, angiokeratoma, hemangioma, and folliculosebaceous cystic hamartoma.4 None of these were evident in our patient.
Lesions from both types of NLCS are indistinguishable on histopathology, characterized by the presence of a central core of ectopic mature adipocytes in the papillary/reticular dermis.2-13 Additional light microscopic features (some seen in our case) have been described, including thickened collagen bundles, reduction of elastic fibers, increased numbers of fibroblasts and/or mast cells, increased (small-vessel) vascularity, focal mucin deposition/myxoid degeneration, a mild perivascular lymphocytic infiltrate, attenuation of adnexal structures, and abnormalities of the epidermis (eg, surface ulceration).2-13
Prior to biopsy, the differential diagnosis in our patient included angiofibroma, pyogenic granuloma, and basal cell carcinoma given the exophytic, pink, papular appearance of the lesion; however, the histopathologic differential diagnosis included angiofibroma, angiomyolipoma, lymphangioma, nevus sebaceus, and spindle cell lipoma (SCL). In angiofibroma, a dermal proliferation of stellate fibroblasts, dilated blood vessels, and collagenous stroma are seen. Cutaneous angiomyolipoma demonstrates smooth muscle bundles in addition to thickened blood vessels and variable proportions of mature adipocytes. Lymphangioma is characterized by dilated lymph channels lined by flat endothelial cells. Nevus sebaceus shows superficial immature and abnormally formed pilosebaceous units, with epidermal papillomatosis.
Rare cases of SCL on the nose have been described.14 Similar to pedunculated lipofibroma, reported examples demonstrate mature univacuolar adipocytes with thick collagen fibers and bland uniform spindle cells. Unlike the lesion seen in our patient, nasal SCL may be clinically mobile and typically is localized to the subcutaneous tissue, although dermal tumors also occur.14 Variably reported histopathologic findings in nasal SCL include circumscription/encapsulation, spindle cells arranged in short fascicles with nuclear palisading, a myxoid/mucinous interstitial matrix, and/or multinucleated giant cells—all light microscopic features that were not identified in our case; however, variable proportions of adipocytic, fibrous, and myxoid components among reported examples of SCL on the nose14 can make distinction from pedunculated lipofibroma difficult, as both are benign lipomatous tumor variants.
Clinically, pedunculated lipofibroma may be confused with more common benign cutaneous lesions and must be distinguished from other fibrolipomatous lesions on the nose. Specifically, the differential diagnosis includes benign cutaneous papillomas such as acrochordon, angiofibroma, melanocytic nevi, neurofibroma, nevus sebaceus, lymphangioma, and eccrine poroma.7-13 These all can be readily excluded on histopathology. Pedunculated lipofibroma on the nose, as in our patient, must be distinguished from fibrolipoma15 and dendritic myxofibrolipoma.16 Fibrolipoma is a subcutaneous proliferation of mature adipose tissue and fibrous tissue and comprises 1.6% of all facial lipomas reported worldwide.15 Dendritic myxofibrolipoma is a recently described benign soft-tissue tumor characterized by an admixture of mature adipose tissue, spindle and stellate cells, and an abundant myxoid stroma with prominent collagenization.16
Treatment of pedunculated lipofibroma on the nose is not indicated except for cosmetic reasons, in which case simple surgical excision would be considered satisfactory. Following biopsy, no further treatment was pursued in our patient.
- Hoffmann E, Zurhelle E. Uber einen naevus lipomatodes cutaneous superficialis der linken Glutaalgegend. Arch Derm Syph. 1921;130:327-333.
- Chanoki M, Isukos S, Suzuki S, et al. Nevus lipomatosus cutaneus superficialis of the scalp. Cutis. 1989;43:143-144.
- Sáez Rodríguez M, Rodríguez-Martin M, Carnerero A, et al. Naevus lipomatosus cutaneous superficialis on the nose. J Eur Acad Dermatol Venereol. 2005;19:751-752.
- Hassab-El-Naby HMM, Rageh MA. Adult-onset nevus lipomatosus cutaneous superficialis mimicking plane xanthoma. J Clin Aesthet Dermatol. 2022;15:10-11.
- Park HJ, Park CJ, Yi JY, et al. Nevus lipomatosus superficialis on the face. Int J Dermatol. 1997;36:435-437.
- Ioannidou DJ, Stefanidou MP, Panayiotides JG, et al. Nevus lipomatosus cutaneous superficialis (Hoffman-Zurhelle) with localized scleroderma like appearance. Int J Dermatol. 2001;40:54-57.
- Nogita T, Wong TY, Hidano A, et al. Pedunculated lipofibroma. a clinicopathologic study of thirty-two cases supporting a simplified nomenclature. J Am Acad Dermatol. 1994;31(2 pt 1):235-240.
- Sawada Y. Solitary nevus lipomatosus superficialis on the forehead. Ann Plast Surg. 1986;16:356-358.
- Knoth W. Uber Naevus lipomatosus cutaneus superficialis Hoffmann-Zurhelle und uber Naevus naevocellularis partim lipomatodes. Dermatologica. 1962;125:161.
- Weitzner S. Solitary naevus lipomatosus cutaneus superficialis of scalp. Arch Dermatol. 1968;97:540-542.
- Kaw P, Carlson A, Meyer DR. Nevus lipomatosus (pedunculated lipofibroma) of the eyelid. Ophthalmic Plast Reconstr Surg. 2005;21:74-76.
- Vano-Galvan S, Moreno C, Vano-Galvan E, et al. Solitary naevus lipomatosus cutaneous superficialis on the sole. Eur J Dermatol. 2008;18:353-354.
- Adotama P, Hutson SD, Rieder EA, et al. Revisiting solitary pedunculated lipofibromas. Am J Clin Pathol. 2021;156:954-957.
- Kubin ME, Lantto U, Lindgren O, et al. A rare, recurrent spindle cell lipoma of the nose. Acta Derm Venereol. 2021;101:adv00571.
- Jung SN, Shin JW, Kwon H, et al. Fibrolipoma of the tip of the nose. J Craniofac Surg. 2009;20:555-556.
- Han XC, Zheng LQ, Shang XL. Dendritic fibromyxolipoma on the nasal tip in an old patient. Int J Clin Exp Pathol. 2014;7:7064-7067.
THE DIAGNOSIS: Pedunculated Lipofibroma
Histopathology confirmed a pedunculated/polypoid lesion with intradermal lobules of adipocytes/mature adipose tissue admixed with connective tissue bundles and vascular ectasias. Overlying epidermal acanthosis with slight papillomatosis and hyperkeratosis was present (Figure 1). Masson trichrome staining highlighted admixed collagen bundles (Figure 2). Verhoeff–van Gieson staining showed marked reduction in elastic fibers (Figure 3). Immunostaining was negative for smooth muscle actin and desmin. A diagnosis of pedunculated lipofibroma on the nose was made based on both clinical and histopathologic findings.



Pedunculated lipofibroma (or solitary lipofibroma) is the solitary form of nevus lipomatosus cutaneous superficialis (NLCS).7 First described by Hoffmann and Zurhelle1 in 1921, NLCS is an uncommon benign hamartomatous cutaneous lesion/connective tissue nevus that also has a classic multiple form.1-13 The etiology of NLCS remains unclear, but several theories have been proposed to explain its pathogenesis, including deposition of adipocytes secondary to degenerative changes in dermal connective tissue, focal/local heterotopic development of adipose tissue, and derivation from differentiating lipoblasts (preadipose tissue) originating from precursor vascular or perivascular cells.2-13
Pedunculated lipofibroma usually develops during the third to sixth decades of life and manifests as a single cutaneous lesion with a smooth surface, often on a non–pelvic girdle location.7-13 No particular predilection sites are noted, with lesions reported on the arm, axilla, back, upper thigh, knee, and sole.5,12 There are rare reports of this type of NLCS on the ear, scalp, forehead, or eyelid.7-11
In the classic form of NLCS, multiple cutaneous lesions are present at birth or develop within the first 2 to 3 decades of life.2-6 Lesions consist of soft, nontender, pedunculated, flesh-colored or yellowish papules and nodules with a verrucoid or cerebriform surface that may later coalesce to form plaques.2-6 Predilection sites include the pelvic girdle, buttocks, sacral and coccygeal regions, and upper posterior thighs, with a linear or zosteriform pattern of distribution.2-6 Rarely, the classic form can arise in elderly patients and/or at an atypical anatomic location (eg, clitoris,3 shoulder,5 thorax,5 abdomen5) and can demonstrate extension of lesions across the midline.4 Rare cases of classic NLCS on the scalp2 and face3-6 have been reported, including lesions localized to the nose3 and chin4 and others extending from the right mandible to the neck5 and right lower lip to the submandibular/posteriorateral cervical region.6 In some cases, lesions clinically resemble plane xanthoma4 and localized scleroderma.6
Adotama et al13 proposed a set of clinical features to differentiate classic NLCS, pedunculated lipofibroma (solitary NLCS), and fibroepithelial polyp with adipocytes (distinguished by their furrowed surface, hyperpigmentation, and anatomic predilection for the neck and axilla). Lesions are asymptomatic in both forms of NLCS.2-13 Family history or predominant sex involvement have not been reported in either clinical type.2-13 Reported associations with NLCS include a number of endocrinologic conditions including diabetes.7 Other coexisting skin findings can include café-au-lait macules, leukodermic (white) spots, overlying hypertrichosis, comedolike alterations, angiokeratoma, hemangioma, and folliculosebaceous cystic hamartoma.4 None of these were evident in our patient.
Lesions from both types of NLCS are indistinguishable on histopathology, characterized by the presence of a central core of ectopic mature adipocytes in the papillary/reticular dermis.2-13 Additional light microscopic features (some seen in our case) have been described, including thickened collagen bundles, reduction of elastic fibers, increased numbers of fibroblasts and/or mast cells, increased (small-vessel) vascularity, focal mucin deposition/myxoid degeneration, a mild perivascular lymphocytic infiltrate, attenuation of adnexal structures, and abnormalities of the epidermis (eg, surface ulceration).2-13
Prior to biopsy, the differential diagnosis in our patient included angiofibroma, pyogenic granuloma, and basal cell carcinoma given the exophytic, pink, papular appearance of the lesion; however, the histopathologic differential diagnosis included angiofibroma, angiomyolipoma, lymphangioma, nevus sebaceus, and spindle cell lipoma (SCL). In angiofibroma, a dermal proliferation of stellate fibroblasts, dilated blood vessels, and collagenous stroma are seen. Cutaneous angiomyolipoma demonstrates smooth muscle bundles in addition to thickened blood vessels and variable proportions of mature adipocytes. Lymphangioma is characterized by dilated lymph channels lined by flat endothelial cells. Nevus sebaceus shows superficial immature and abnormally formed pilosebaceous units, with epidermal papillomatosis.
Rare cases of SCL on the nose have been described.14 Similar to pedunculated lipofibroma, reported examples demonstrate mature univacuolar adipocytes with thick collagen fibers and bland uniform spindle cells. Unlike the lesion seen in our patient, nasal SCL may be clinically mobile and typically is localized to the subcutaneous tissue, although dermal tumors also occur.14 Variably reported histopathologic findings in nasal SCL include circumscription/encapsulation, spindle cells arranged in short fascicles with nuclear palisading, a myxoid/mucinous interstitial matrix, and/or multinucleated giant cells—all light microscopic features that were not identified in our case; however, variable proportions of adipocytic, fibrous, and myxoid components among reported examples of SCL on the nose14 can make distinction from pedunculated lipofibroma difficult, as both are benign lipomatous tumor variants.
Clinically, pedunculated lipofibroma may be confused with more common benign cutaneous lesions and must be distinguished from other fibrolipomatous lesions on the nose. Specifically, the differential diagnosis includes benign cutaneous papillomas such as acrochordon, angiofibroma, melanocytic nevi, neurofibroma, nevus sebaceus, lymphangioma, and eccrine poroma.7-13 These all can be readily excluded on histopathology. Pedunculated lipofibroma on the nose, as in our patient, must be distinguished from fibrolipoma15 and dendritic myxofibrolipoma.16 Fibrolipoma is a subcutaneous proliferation of mature adipose tissue and fibrous tissue and comprises 1.6% of all facial lipomas reported worldwide.15 Dendritic myxofibrolipoma is a recently described benign soft-tissue tumor characterized by an admixture of mature adipose tissue, spindle and stellate cells, and an abundant myxoid stroma with prominent collagenization.16
Treatment of pedunculated lipofibroma on the nose is not indicated except for cosmetic reasons, in which case simple surgical excision would be considered satisfactory. Following biopsy, no further treatment was pursued in our patient.
THE DIAGNOSIS: Pedunculated Lipofibroma
Histopathology confirmed a pedunculated/polypoid lesion with intradermal lobules of adipocytes/mature adipose tissue admixed with connective tissue bundles and vascular ectasias. Overlying epidermal acanthosis with slight papillomatosis and hyperkeratosis was present (Figure 1). Masson trichrome staining highlighted admixed collagen bundles (Figure 2). Verhoeff–van Gieson staining showed marked reduction in elastic fibers (Figure 3). Immunostaining was negative for smooth muscle actin and desmin. A diagnosis of pedunculated lipofibroma on the nose was made based on both clinical and histopathologic findings.



Pedunculated lipofibroma (or solitary lipofibroma) is the solitary form of nevus lipomatosus cutaneous superficialis (NLCS).7 First described by Hoffmann and Zurhelle1 in 1921, NLCS is an uncommon benign hamartomatous cutaneous lesion/connective tissue nevus that also has a classic multiple form.1-13 The etiology of NLCS remains unclear, but several theories have been proposed to explain its pathogenesis, including deposition of adipocytes secondary to degenerative changes in dermal connective tissue, focal/local heterotopic development of adipose tissue, and derivation from differentiating lipoblasts (preadipose tissue) originating from precursor vascular or perivascular cells.2-13
Pedunculated lipofibroma usually develops during the third to sixth decades of life and manifests as a single cutaneous lesion with a smooth surface, often on a non–pelvic girdle location.7-13 No particular predilection sites are noted, with lesions reported on the arm, axilla, back, upper thigh, knee, and sole.5,12 There are rare reports of this type of NLCS on the ear, scalp, forehead, or eyelid.7-11
In the classic form of NLCS, multiple cutaneous lesions are present at birth or develop within the first 2 to 3 decades of life.2-6 Lesions consist of soft, nontender, pedunculated, flesh-colored or yellowish papules and nodules with a verrucoid or cerebriform surface that may later coalesce to form plaques.2-6 Predilection sites include the pelvic girdle, buttocks, sacral and coccygeal regions, and upper posterior thighs, with a linear or zosteriform pattern of distribution.2-6 Rarely, the classic form can arise in elderly patients and/or at an atypical anatomic location (eg, clitoris,3 shoulder,5 thorax,5 abdomen5) and can demonstrate extension of lesions across the midline.4 Rare cases of classic NLCS on the scalp2 and face3-6 have been reported, including lesions localized to the nose3 and chin4 and others extending from the right mandible to the neck5 and right lower lip to the submandibular/posteriorateral cervical region.6 In some cases, lesions clinically resemble plane xanthoma4 and localized scleroderma.6
Adotama et al13 proposed a set of clinical features to differentiate classic NLCS, pedunculated lipofibroma (solitary NLCS), and fibroepithelial polyp with adipocytes (distinguished by their furrowed surface, hyperpigmentation, and anatomic predilection for the neck and axilla). Lesions are asymptomatic in both forms of NLCS.2-13 Family history or predominant sex involvement have not been reported in either clinical type.2-13 Reported associations with NLCS include a number of endocrinologic conditions including diabetes.7 Other coexisting skin findings can include café-au-lait macules, leukodermic (white) spots, overlying hypertrichosis, comedolike alterations, angiokeratoma, hemangioma, and folliculosebaceous cystic hamartoma.4 None of these were evident in our patient.
Lesions from both types of NLCS are indistinguishable on histopathology, characterized by the presence of a central core of ectopic mature adipocytes in the papillary/reticular dermis.2-13 Additional light microscopic features (some seen in our case) have been described, including thickened collagen bundles, reduction of elastic fibers, increased numbers of fibroblasts and/or mast cells, increased (small-vessel) vascularity, focal mucin deposition/myxoid degeneration, a mild perivascular lymphocytic infiltrate, attenuation of adnexal structures, and abnormalities of the epidermis (eg, surface ulceration).2-13
Prior to biopsy, the differential diagnosis in our patient included angiofibroma, pyogenic granuloma, and basal cell carcinoma given the exophytic, pink, papular appearance of the lesion; however, the histopathologic differential diagnosis included angiofibroma, angiomyolipoma, lymphangioma, nevus sebaceus, and spindle cell lipoma (SCL). In angiofibroma, a dermal proliferation of stellate fibroblasts, dilated blood vessels, and collagenous stroma are seen. Cutaneous angiomyolipoma demonstrates smooth muscle bundles in addition to thickened blood vessels and variable proportions of mature adipocytes. Lymphangioma is characterized by dilated lymph channels lined by flat endothelial cells. Nevus sebaceus shows superficial immature and abnormally formed pilosebaceous units, with epidermal papillomatosis.
Rare cases of SCL on the nose have been described.14 Similar to pedunculated lipofibroma, reported examples demonstrate mature univacuolar adipocytes with thick collagen fibers and bland uniform spindle cells. Unlike the lesion seen in our patient, nasal SCL may be clinically mobile and typically is localized to the subcutaneous tissue, although dermal tumors also occur.14 Variably reported histopathologic findings in nasal SCL include circumscription/encapsulation, spindle cells arranged in short fascicles with nuclear palisading, a myxoid/mucinous interstitial matrix, and/or multinucleated giant cells—all light microscopic features that were not identified in our case; however, variable proportions of adipocytic, fibrous, and myxoid components among reported examples of SCL on the nose14 can make distinction from pedunculated lipofibroma difficult, as both are benign lipomatous tumor variants.
Clinically, pedunculated lipofibroma may be confused with more common benign cutaneous lesions and must be distinguished from other fibrolipomatous lesions on the nose. Specifically, the differential diagnosis includes benign cutaneous papillomas such as acrochordon, angiofibroma, melanocytic nevi, neurofibroma, nevus sebaceus, lymphangioma, and eccrine poroma.7-13 These all can be readily excluded on histopathology. Pedunculated lipofibroma on the nose, as in our patient, must be distinguished from fibrolipoma15 and dendritic myxofibrolipoma.16 Fibrolipoma is a subcutaneous proliferation of mature adipose tissue and fibrous tissue and comprises 1.6% of all facial lipomas reported worldwide.15 Dendritic myxofibrolipoma is a recently described benign soft-tissue tumor characterized by an admixture of mature adipose tissue, spindle and stellate cells, and an abundant myxoid stroma with prominent collagenization.16
Treatment of pedunculated lipofibroma on the nose is not indicated except for cosmetic reasons, in which case simple surgical excision would be considered satisfactory. Following biopsy, no further treatment was pursued in our patient.
- Hoffmann E, Zurhelle E. Uber einen naevus lipomatodes cutaneous superficialis der linken Glutaalgegend. Arch Derm Syph. 1921;130:327-333.
- Chanoki M, Isukos S, Suzuki S, et al. Nevus lipomatosus cutaneus superficialis of the scalp. Cutis. 1989;43:143-144.
- Sáez Rodríguez M, Rodríguez-Martin M, Carnerero A, et al. Naevus lipomatosus cutaneous superficialis on the nose. J Eur Acad Dermatol Venereol. 2005;19:751-752.
- Hassab-El-Naby HMM, Rageh MA. Adult-onset nevus lipomatosus cutaneous superficialis mimicking plane xanthoma. J Clin Aesthet Dermatol. 2022;15:10-11.
- Park HJ, Park CJ, Yi JY, et al. Nevus lipomatosus superficialis on the face. Int J Dermatol. 1997;36:435-437.
- Ioannidou DJ, Stefanidou MP, Panayiotides JG, et al. Nevus lipomatosus cutaneous superficialis (Hoffman-Zurhelle) with localized scleroderma like appearance. Int J Dermatol. 2001;40:54-57.
- Nogita T, Wong TY, Hidano A, et al. Pedunculated lipofibroma. a clinicopathologic study of thirty-two cases supporting a simplified nomenclature. J Am Acad Dermatol. 1994;31(2 pt 1):235-240.
- Sawada Y. Solitary nevus lipomatosus superficialis on the forehead. Ann Plast Surg. 1986;16:356-358.
- Knoth W. Uber Naevus lipomatosus cutaneus superficialis Hoffmann-Zurhelle und uber Naevus naevocellularis partim lipomatodes. Dermatologica. 1962;125:161.
- Weitzner S. Solitary naevus lipomatosus cutaneus superficialis of scalp. Arch Dermatol. 1968;97:540-542.
- Kaw P, Carlson A, Meyer DR. Nevus lipomatosus (pedunculated lipofibroma) of the eyelid. Ophthalmic Plast Reconstr Surg. 2005;21:74-76.
- Vano-Galvan S, Moreno C, Vano-Galvan E, et al. Solitary naevus lipomatosus cutaneous superficialis on the sole. Eur J Dermatol. 2008;18:353-354.
- Adotama P, Hutson SD, Rieder EA, et al. Revisiting solitary pedunculated lipofibromas. Am J Clin Pathol. 2021;156:954-957.
- Kubin ME, Lantto U, Lindgren O, et al. A rare, recurrent spindle cell lipoma of the nose. Acta Derm Venereol. 2021;101:adv00571.
- Jung SN, Shin JW, Kwon H, et al. Fibrolipoma of the tip of the nose. J Craniofac Surg. 2009;20:555-556.
- Han XC, Zheng LQ, Shang XL. Dendritic fibromyxolipoma on the nasal tip in an old patient. Int J Clin Exp Pathol. 2014;7:7064-7067.
- Hoffmann E, Zurhelle E. Uber einen naevus lipomatodes cutaneous superficialis der linken Glutaalgegend. Arch Derm Syph. 1921;130:327-333.
- Chanoki M, Isukos S, Suzuki S, et al. Nevus lipomatosus cutaneus superficialis of the scalp. Cutis. 1989;43:143-144.
- Sáez Rodríguez M, Rodríguez-Martin M, Carnerero A, et al. Naevus lipomatosus cutaneous superficialis on the nose. J Eur Acad Dermatol Venereol. 2005;19:751-752.
- Hassab-El-Naby HMM, Rageh MA. Adult-onset nevus lipomatosus cutaneous superficialis mimicking plane xanthoma. J Clin Aesthet Dermatol. 2022;15:10-11.
- Park HJ, Park CJ, Yi JY, et al. Nevus lipomatosus superficialis on the face. Int J Dermatol. 1997;36:435-437.
- Ioannidou DJ, Stefanidou MP, Panayiotides JG, et al. Nevus lipomatosus cutaneous superficialis (Hoffman-Zurhelle) with localized scleroderma like appearance. Int J Dermatol. 2001;40:54-57.
- Nogita T, Wong TY, Hidano A, et al. Pedunculated lipofibroma. a clinicopathologic study of thirty-two cases supporting a simplified nomenclature. J Am Acad Dermatol. 1994;31(2 pt 1):235-240.
- Sawada Y. Solitary nevus lipomatosus superficialis on the forehead. Ann Plast Surg. 1986;16:356-358.
- Knoth W. Uber Naevus lipomatosus cutaneus superficialis Hoffmann-Zurhelle und uber Naevus naevocellularis partim lipomatodes. Dermatologica. 1962;125:161.
- Weitzner S. Solitary naevus lipomatosus cutaneus superficialis of scalp. Arch Dermatol. 1968;97:540-542.
- Kaw P, Carlson A, Meyer DR. Nevus lipomatosus (pedunculated lipofibroma) of the eyelid. Ophthalmic Plast Reconstr Surg. 2005;21:74-76.
- Vano-Galvan S, Moreno C, Vano-Galvan E, et al. Solitary naevus lipomatosus cutaneous superficialis on the sole. Eur J Dermatol. 2008;18:353-354.
- Adotama P, Hutson SD, Rieder EA, et al. Revisiting solitary pedunculated lipofibromas. Am J Clin Pathol. 2021;156:954-957.
- Kubin ME, Lantto U, Lindgren O, et al. A rare, recurrent spindle cell lipoma of the nose. Acta Derm Venereol. 2021;101:adv00571.
- Jung SN, Shin JW, Kwon H, et al. Fibrolipoma of the tip of the nose. J Craniofac Surg. 2009;20:555-556.
- Han XC, Zheng LQ, Shang XL. Dendritic fibromyxolipoma on the nasal tip in an old patient. Int J Clin Exp Pathol. 2014;7:7064-7067.
A 60-year-old woman presented to the dermatology department with a 6-mm, firm, pink, nonulcerated, nonmobile papule on the right nasal side wall of 1 year’s duration. It had grown slowly and was asymptomatic with no tenderness or bleeding. No other skin lesions were noted on physical examination, and her medical history was otherwise unremarkable. A shave biopsy was performed.
Data Trends 2025: Dermatology
Click here to view more from Federal Health Care Data Trends 2025.
- Rezaei SJ, et al. JAMA Dermatol. 2024;160(10):1107-1111. doi:10.1001/jamadermatol. 2024.3043
- Singal A, Lipner SR. Ann Med. 2023;55(2):2267425. doi:10.1080/07853890.2023.2267425
- Reese R, et al. J Dermatolog Treat. 2024;35(1):2402912. doi:10.1080/09546634.2024.2402912
- Wallace MM, et al. Telemed J E Health. 2024;30(5):1411-1417. doi:10.1089/tmj.2022.0528
- Russell A, et al. Mil Med. 2024;189(11-12):e2374-e2381. doi:10.1093/milmed/usae139
Salahuddin T, et al. J Eur Acad Dermatol Venereol. 2023;37(7):e862-e864. doi:10.1111/jdv.18964
Click here to view more from Federal Health Care Data Trends 2025.
Click here to view more from Federal Health Care Data Trends 2025.
- Rezaei SJ, et al. JAMA Dermatol. 2024;160(10):1107-1111. doi:10.1001/jamadermatol. 2024.3043
- Singal A, Lipner SR. Ann Med. 2023;55(2):2267425. doi:10.1080/07853890.2023.2267425
- Reese R, et al. J Dermatolog Treat. 2024;35(1):2402912. doi:10.1080/09546634.2024.2402912
- Wallace MM, et al. Telemed J E Health. 2024;30(5):1411-1417. doi:10.1089/tmj.2022.0528
- Russell A, et al. Mil Med. 2024;189(11-12):e2374-e2381. doi:10.1093/milmed/usae139
Salahuddin T, et al. J Eur Acad Dermatol Venereol. 2023;37(7):e862-e864. doi:10.1111/jdv.18964
- Rezaei SJ, et al. JAMA Dermatol. 2024;160(10):1107-1111. doi:10.1001/jamadermatol. 2024.3043
- Singal A, Lipner SR. Ann Med. 2023;55(2):2267425. doi:10.1080/07853890.2023.2267425
- Reese R, et al. J Dermatolog Treat. 2024;35(1):2402912. doi:10.1080/09546634.2024.2402912
- Wallace MM, et al. Telemed J E Health. 2024;30(5):1411-1417. doi:10.1089/tmj.2022.0528
- Russell A, et al. Mil Med. 2024;189(11-12):e2374-e2381. doi:10.1093/milmed/usae139
Salahuddin T, et al. J Eur Acad Dermatol Venereol. 2023;37(7):e862-e864. doi:10.1111/jdv.18964
Data Trends 2025: Neurology
Data Trends 2025: Neurology
Click to view more from Federal Health Care Data Trends 2025.
- Lin C, et al. Front Neurol. 2024;15:1392721. Published 2024 Mar 12. doi:10.3389/fneur.2024.1392721
- Defense Medical Surveillance System, Theater Medical Data Store provided by the Armed Forces Health Surveillance Division. Prepared by the Traumatic Brain Injury Center of Excellence. Accessed April 2, 2025. https://health.mil/Military-Health-Topics/Centers-of-Excellence/Traumatic-Brain-Injury-Center-of-Excellence/DODTBI-Worldwide-Numbers
- Karr JE, et al. Arch Phys Med Rehabil. 2025;106(4):537-547. doi:10.1016/j.apmr.2024.11.010
- Howard JT, et al. J Racial Ethn Health Disparities. 2025;12(3):1745-1756. doi:10.1007/s40615-024-02004-1
- Gasperi M, et al. JAMA Netw Open. 2024;7(3):e242299. doi:10.1001/jamanetworkopen.2024.2299
- Roghani A, et al. Epilepsia. 2024;65(8):2255-2269. doi:10.1111/epi.18026
- Herbert MS, et al. Headache. 2025;65(3):430-438. doi:10.1111/head.14815
- Fleming NH, et al. Mult Scler Relat Disord. 2024;82:105372. doi:10.1016/j.msard.2023.105372
- Silveira SL, et al. CNS Spectr. 2024;29(6):1-8. doi:10.1017/S1092852924002165
- Whiteneck G, et al. J Head Trauma Rehabil. 2024;39(5):E462-E469. doi:10.1097/HTR.0000000000000952
Seng EK, et al. Headache. 2024;64(10):1273-1284. doi:10.1111/head.14842
Click to view more from Federal Health Care Data Trends 2025.
Click to view more from Federal Health Care Data Trends 2025.
- Lin C, et al. Front Neurol. 2024;15:1392721. Published 2024 Mar 12. doi:10.3389/fneur.2024.1392721
- Defense Medical Surveillance System, Theater Medical Data Store provided by the Armed Forces Health Surveillance Division. Prepared by the Traumatic Brain Injury Center of Excellence. Accessed April 2, 2025. https://health.mil/Military-Health-Topics/Centers-of-Excellence/Traumatic-Brain-Injury-Center-of-Excellence/DODTBI-Worldwide-Numbers
- Karr JE, et al. Arch Phys Med Rehabil. 2025;106(4):537-547. doi:10.1016/j.apmr.2024.11.010
- Howard JT, et al. J Racial Ethn Health Disparities. 2025;12(3):1745-1756. doi:10.1007/s40615-024-02004-1
- Gasperi M, et al. JAMA Netw Open. 2024;7(3):e242299. doi:10.1001/jamanetworkopen.2024.2299
- Roghani A, et al. Epilepsia. 2024;65(8):2255-2269. doi:10.1111/epi.18026
- Herbert MS, et al. Headache. 2025;65(3):430-438. doi:10.1111/head.14815
- Fleming NH, et al. Mult Scler Relat Disord. 2024;82:105372. doi:10.1016/j.msard.2023.105372
- Silveira SL, et al. CNS Spectr. 2024;29(6):1-8. doi:10.1017/S1092852924002165
- Whiteneck G, et al. J Head Trauma Rehabil. 2024;39(5):E462-E469. doi:10.1097/HTR.0000000000000952
Seng EK, et al. Headache. 2024;64(10):1273-1284. doi:10.1111/head.14842
- Lin C, et al. Front Neurol. 2024;15:1392721. Published 2024 Mar 12. doi:10.3389/fneur.2024.1392721
- Defense Medical Surveillance System, Theater Medical Data Store provided by the Armed Forces Health Surveillance Division. Prepared by the Traumatic Brain Injury Center of Excellence. Accessed April 2, 2025. https://health.mil/Military-Health-Topics/Centers-of-Excellence/Traumatic-Brain-Injury-Center-of-Excellence/DODTBI-Worldwide-Numbers
- Karr JE, et al. Arch Phys Med Rehabil. 2025;106(4):537-547. doi:10.1016/j.apmr.2024.11.010
- Howard JT, et al. J Racial Ethn Health Disparities. 2025;12(3):1745-1756. doi:10.1007/s40615-024-02004-1
- Gasperi M, et al. JAMA Netw Open. 2024;7(3):e242299. doi:10.1001/jamanetworkopen.2024.2299
- Roghani A, et al. Epilepsia. 2024;65(8):2255-2269. doi:10.1111/epi.18026
- Herbert MS, et al. Headache. 2025;65(3):430-438. doi:10.1111/head.14815
- Fleming NH, et al. Mult Scler Relat Disord. 2024;82:105372. doi:10.1016/j.msard.2023.105372
- Silveira SL, et al. CNS Spectr. 2024;29(6):1-8. doi:10.1017/S1092852924002165
- Whiteneck G, et al. J Head Trauma Rehabil. 2024;39(5):E462-E469. doi:10.1097/HTR.0000000000000952
Seng EK, et al. Headache. 2024;64(10):1273-1284. doi:10.1111/head.14842
Data Trends 2025: Neurology
Data Trends 2025: Neurology
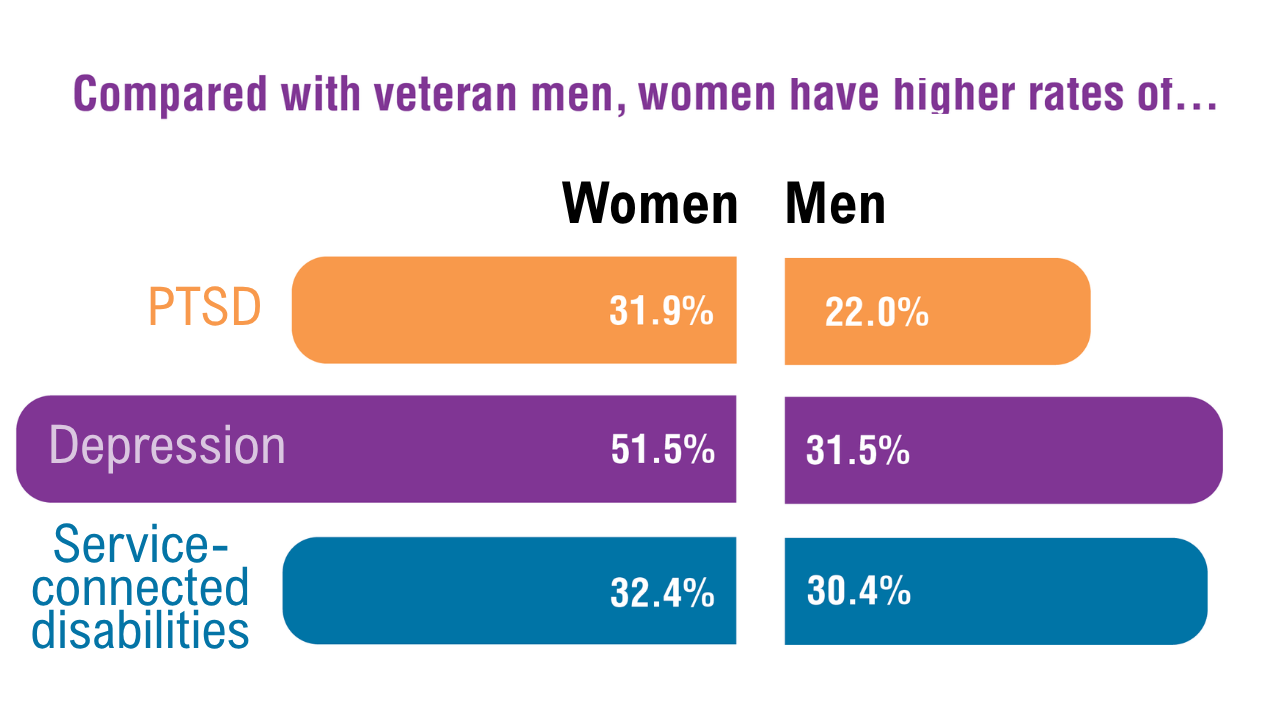
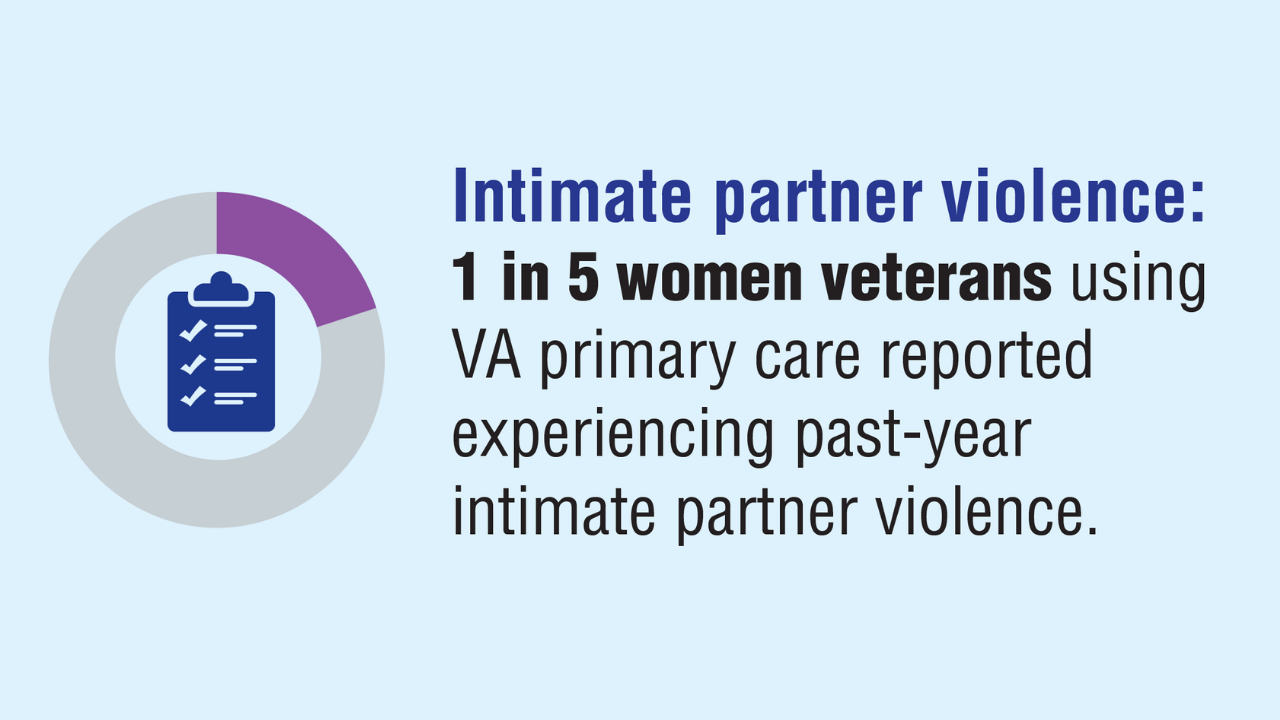
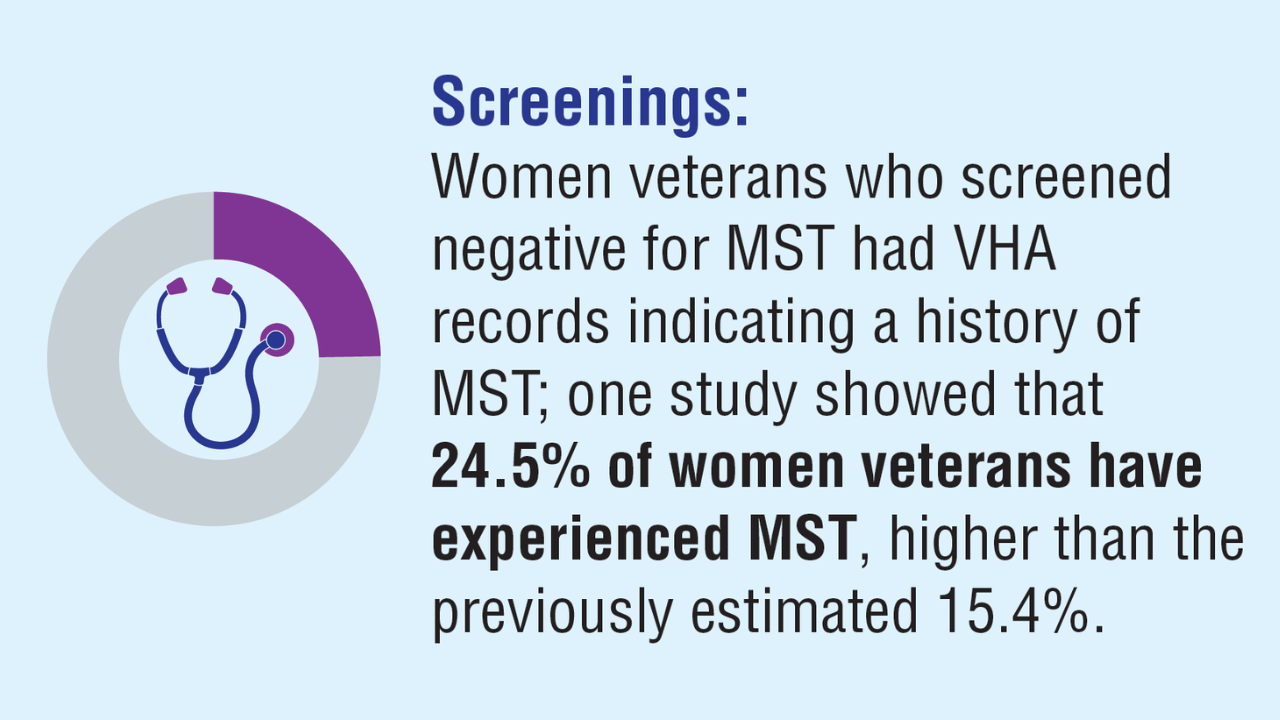
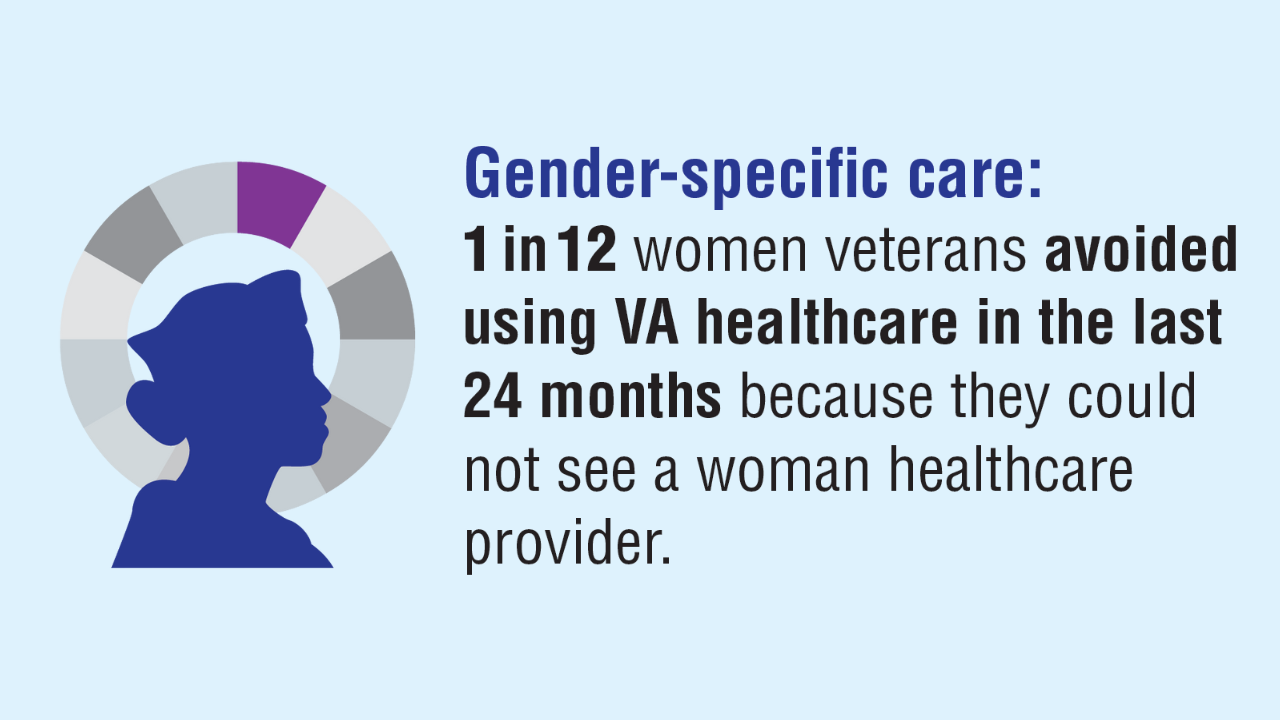
Data Trends 2025: Women's Health
Data Trends 2025: Women's Health
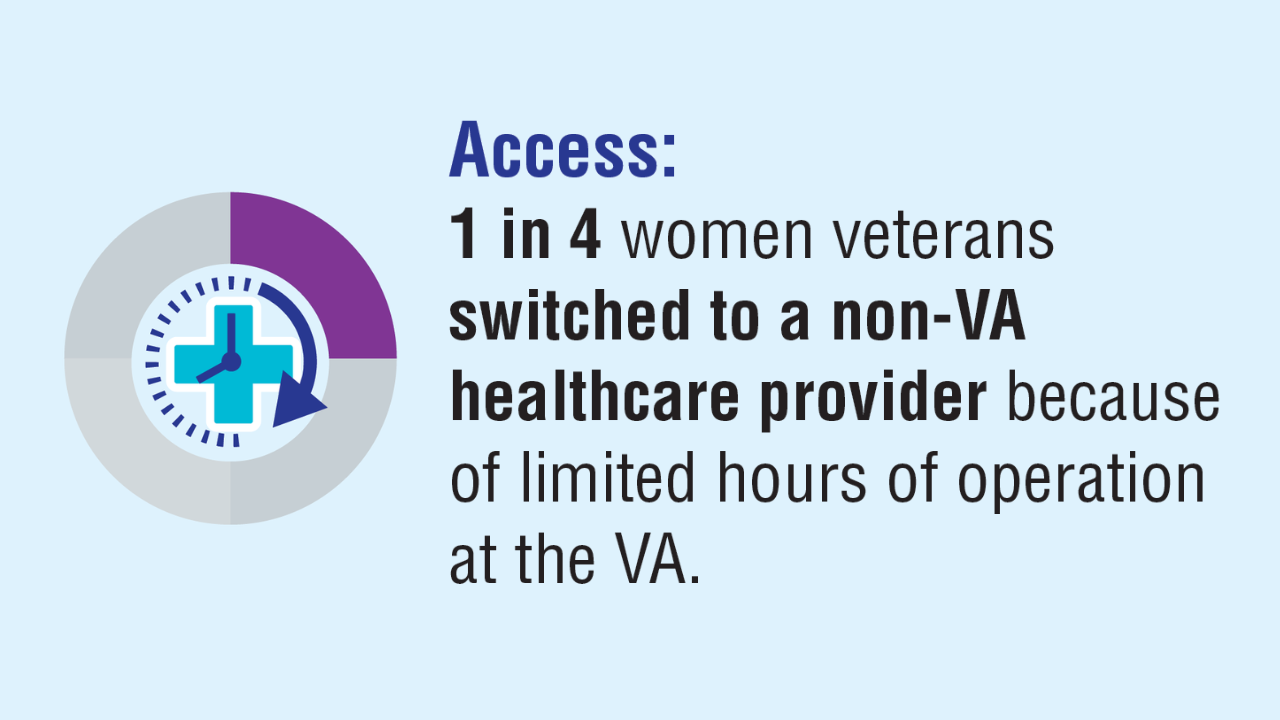
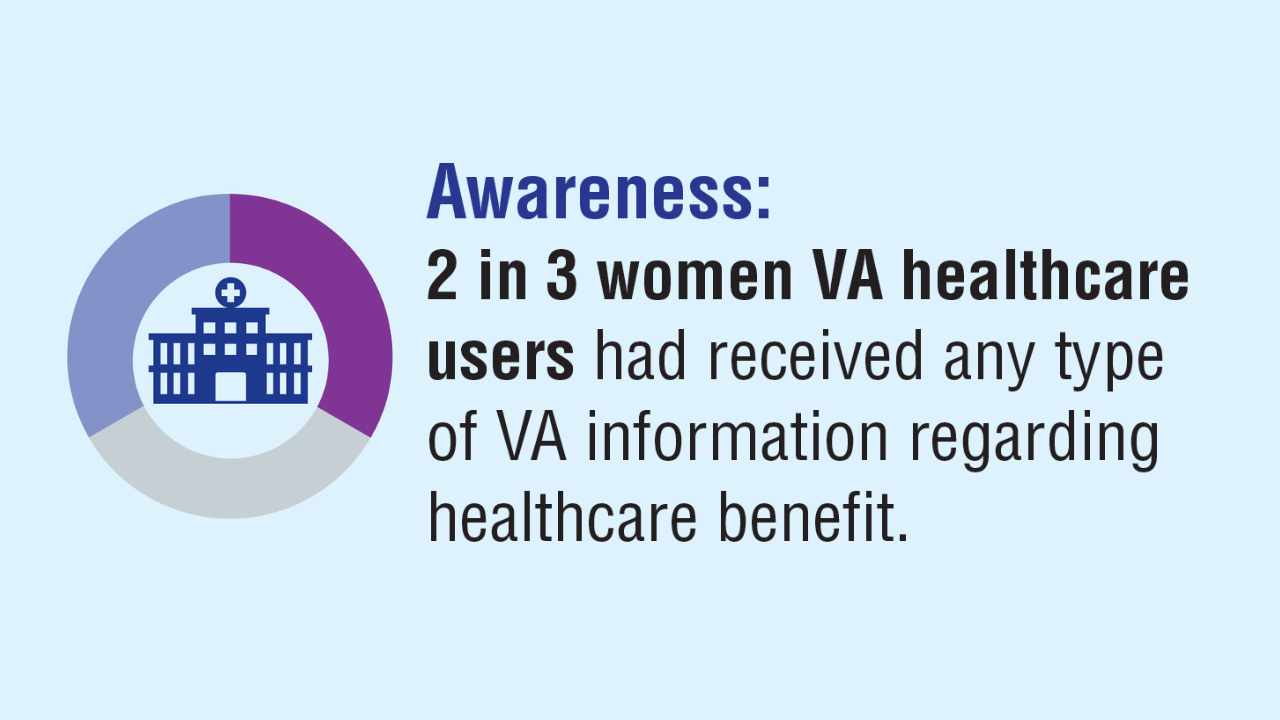
Click here to view more from Federal Health Care Data Trends 2025.
- Women Veterans Health Care: Facts and Statistics. US Department of Veterans Affairs. Published 2022. Accessed May 23, 2025. https://www.womenshealth.va.gov/materials-and-resources/facts-and-statistics.asp
- Sourcebook: Women Veterans in the Veterans Health Administration. Volume 5: Longitudinal Trends in Sociodemographics and Utilization, Including Type, Modality, and Source of Care. US Department of Veterans Affairs; 2024. Accessed May 23, 2025. https://www.womenshealth.va.gov/WOMENSHEALTH/docs/VHA-Source-book-V5-FINAL.pdf
- Goldstein KM, et al. JAMA Netw Open. 2025;8(4):e256372. doi:10.1001/ jamanetworkopen.2025.6372
- Sheahan KL, et al. J Gen Intern Med. 2022;37(Suppl 3):791-798. doi:10.1007/s11606-022-07585-3
- Adams RE, et al. BMC Womens Health. 2021;21(1):1-10. doi:10.1186/s12905-021-01183-z
- Haskell SG, et al. J Womens Health (Larchmt). 2010;19(2):267-271. doi:10.1089/jwh.2008.1262
- VHA Directive 1330.01(1): Health Care Services for Women Veterans. US Department of Veterans Affairs; February 15, 2023. Accessed May 23, 2025. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=10576
- VHA Directive 1115(1): Military Sexual Trauma (MST) Program. US Department of Veterans Affairs; May 8, 2018. Accessed May 23, 2025. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=6432
- Marshall V, et al. Womens Health Issues. 2021;31(2):150-157. doi:10.1016/j.whi.2020.10.005
- Washington DL, et al. J Gen Intern Med. 2011;26(suppl 2):655-661. doi:10.1007/s11606-011-1772-z
- Hadlandsmyth K, et al. Eur J Pain. 2024;28(8):1311-1319. doi:10.1002/ejp.2258
- Military Sexual Trauma Fact Sheet–VA Mental Health. US Department of Veterans Affairs. May 1, 2021. Accessed March 21, 2025. https://www.mentalhealth.va.gov/docs/mst_general_factsheet.pdf
- National Center for Veterans Analysis and Statistics. Population Tables: the nation, age/sex. US Department of Veterans Affairs website. Accessed March 21, 2025. https://www.va.gov/vetdata/Veteran_Population.asp
- Serving Her Country: Exploring the Characteristics of Women Veterans. US Department of Veterans Affairs. Accessed March 21, 2025. https://www.data.va.gov/stories/s/Women-Veterans-in-2023/wci3-yrsv/
- Gasperi M, et al. JAMA Netw Open. 2024;7(3):e242299. doi:10.1001/jamanetworkopen.2024.2299
- US Department of Veterans Affairs. Study of Barriers for Women Veterans to VA Health Care: Final Report. February 2024. Accessed March 21, 2025. https://www.womenshealth.va.gov/materials-and-resources/publications-and-reports.asp
- Iverson KM, et al. J Gen Intern Med. 2019;34(11):2435-2442. doi:10.1007/s11606-019-05240-y
- Spinelli S, et al. J Gen Intern Med. 2022;37(suppl 3):837-841. doi:10.1007/s11606-022-07577-3
- Carlson K, et al. In: StatPearls. StatPearls Publishing; 2025. Updated January 22,2025. Accessed March 21, 2025. https://www.ncbi.nlm.nih.gov/books/NBK519070/
Monteith LL, et al. J Interpers Violence. 2023;38(11-12):7578-7601. doi:10.1177/08862605221145725
Click here to view more from Federal Health Care Data Trends 2025.
Click here to view more from Federal Health Care Data Trends 2025.
- Women Veterans Health Care: Facts and Statistics. US Department of Veterans Affairs. Published 2022. Accessed May 23, 2025. https://www.womenshealth.va.gov/materials-and-resources/facts-and-statistics.asp
- Sourcebook: Women Veterans in the Veterans Health Administration. Volume 5: Longitudinal Trends in Sociodemographics and Utilization, Including Type, Modality, and Source of Care. US Department of Veterans Affairs; 2024. Accessed May 23, 2025. https://www.womenshealth.va.gov/WOMENSHEALTH/docs/VHA-Source-book-V5-FINAL.pdf
- Goldstein KM, et al. JAMA Netw Open. 2025;8(4):e256372. doi:10.1001/ jamanetworkopen.2025.6372
- Sheahan KL, et al. J Gen Intern Med. 2022;37(Suppl 3):791-798. doi:10.1007/s11606-022-07585-3
- Adams RE, et al. BMC Womens Health. 2021;21(1):1-10. doi:10.1186/s12905-021-01183-z
- Haskell SG, et al. J Womens Health (Larchmt). 2010;19(2):267-271. doi:10.1089/jwh.2008.1262
- VHA Directive 1330.01(1): Health Care Services for Women Veterans. US Department of Veterans Affairs; February 15, 2023. Accessed May 23, 2025. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=10576
- VHA Directive 1115(1): Military Sexual Trauma (MST) Program. US Department of Veterans Affairs; May 8, 2018. Accessed May 23, 2025. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=6432
- Marshall V, et al. Womens Health Issues. 2021;31(2):150-157. doi:10.1016/j.whi.2020.10.005
- Washington DL, et al. J Gen Intern Med. 2011;26(suppl 2):655-661. doi:10.1007/s11606-011-1772-z
- Hadlandsmyth K, et al. Eur J Pain. 2024;28(8):1311-1319. doi:10.1002/ejp.2258
- Military Sexual Trauma Fact Sheet–VA Mental Health. US Department of Veterans Affairs. May 1, 2021. Accessed March 21, 2025. https://www.mentalhealth.va.gov/docs/mst_general_factsheet.pdf
- National Center for Veterans Analysis and Statistics. Population Tables: the nation, age/sex. US Department of Veterans Affairs website. Accessed March 21, 2025. https://www.va.gov/vetdata/Veteran_Population.asp
- Serving Her Country: Exploring the Characteristics of Women Veterans. US Department of Veterans Affairs. Accessed March 21, 2025. https://www.data.va.gov/stories/s/Women-Veterans-in-2023/wci3-yrsv/
- Gasperi M, et al. JAMA Netw Open. 2024;7(3):e242299. doi:10.1001/jamanetworkopen.2024.2299
- US Department of Veterans Affairs. Study of Barriers for Women Veterans to VA Health Care: Final Report. February 2024. Accessed March 21, 2025. https://www.womenshealth.va.gov/materials-and-resources/publications-and-reports.asp
- Iverson KM, et al. J Gen Intern Med. 2019;34(11):2435-2442. doi:10.1007/s11606-019-05240-y
- Spinelli S, et al. J Gen Intern Med. 2022;37(suppl 3):837-841. doi:10.1007/s11606-022-07577-3
- Carlson K, et al. In: StatPearls. StatPearls Publishing; 2025. Updated January 22,2025. Accessed March 21, 2025. https://www.ncbi.nlm.nih.gov/books/NBK519070/
Monteith LL, et al. J Interpers Violence. 2023;38(11-12):7578-7601. doi:10.1177/08862605221145725
- Women Veterans Health Care: Facts and Statistics. US Department of Veterans Affairs. Published 2022. Accessed May 23, 2025. https://www.womenshealth.va.gov/materials-and-resources/facts-and-statistics.asp
- Sourcebook: Women Veterans in the Veterans Health Administration. Volume 5: Longitudinal Trends in Sociodemographics and Utilization, Including Type, Modality, and Source of Care. US Department of Veterans Affairs; 2024. Accessed May 23, 2025. https://www.womenshealth.va.gov/WOMENSHEALTH/docs/VHA-Source-book-V5-FINAL.pdf
- Goldstein KM, et al. JAMA Netw Open. 2025;8(4):e256372. doi:10.1001/ jamanetworkopen.2025.6372
- Sheahan KL, et al. J Gen Intern Med. 2022;37(Suppl 3):791-798. doi:10.1007/s11606-022-07585-3
- Adams RE, et al. BMC Womens Health. 2021;21(1):1-10. doi:10.1186/s12905-021-01183-z
- Haskell SG, et al. J Womens Health (Larchmt). 2010;19(2):267-271. doi:10.1089/jwh.2008.1262
- VHA Directive 1330.01(1): Health Care Services for Women Veterans. US Department of Veterans Affairs; February 15, 2023. Accessed May 23, 2025. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=10576
- VHA Directive 1115(1): Military Sexual Trauma (MST) Program. US Department of Veterans Affairs; May 8, 2018. Accessed May 23, 2025. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=6432
- Marshall V, et al. Womens Health Issues. 2021;31(2):150-157. doi:10.1016/j.whi.2020.10.005
- Washington DL, et al. J Gen Intern Med. 2011;26(suppl 2):655-661. doi:10.1007/s11606-011-1772-z
- Hadlandsmyth K, et al. Eur J Pain. 2024;28(8):1311-1319. doi:10.1002/ejp.2258
- Military Sexual Trauma Fact Sheet–VA Mental Health. US Department of Veterans Affairs. May 1, 2021. Accessed March 21, 2025. https://www.mentalhealth.va.gov/docs/mst_general_factsheet.pdf
- National Center for Veterans Analysis and Statistics. Population Tables: the nation, age/sex. US Department of Veterans Affairs website. Accessed March 21, 2025. https://www.va.gov/vetdata/Veteran_Population.asp
- Serving Her Country: Exploring the Characteristics of Women Veterans. US Department of Veterans Affairs. Accessed March 21, 2025. https://www.data.va.gov/stories/s/Women-Veterans-in-2023/wci3-yrsv/
- Gasperi M, et al. JAMA Netw Open. 2024;7(3):e242299. doi:10.1001/jamanetworkopen.2024.2299
- US Department of Veterans Affairs. Study of Barriers for Women Veterans to VA Health Care: Final Report. February 2024. Accessed March 21, 2025. https://www.womenshealth.va.gov/materials-and-resources/publications-and-reports.asp
- Iverson KM, et al. J Gen Intern Med. 2019;34(11):2435-2442. doi:10.1007/s11606-019-05240-y
- Spinelli S, et al. J Gen Intern Med. 2022;37(suppl 3):837-841. doi:10.1007/s11606-022-07577-3
- Carlson K, et al. In: StatPearls. StatPearls Publishing; 2025. Updated January 22,2025. Accessed March 21, 2025. https://www.ncbi.nlm.nih.gov/books/NBK519070/
Monteith LL, et al. J Interpers Violence. 2023;38(11-12):7578-7601. doi:10.1177/08862605221145725
Data Trends 2025: Women's Health
Data Trends 2025: Women's Health
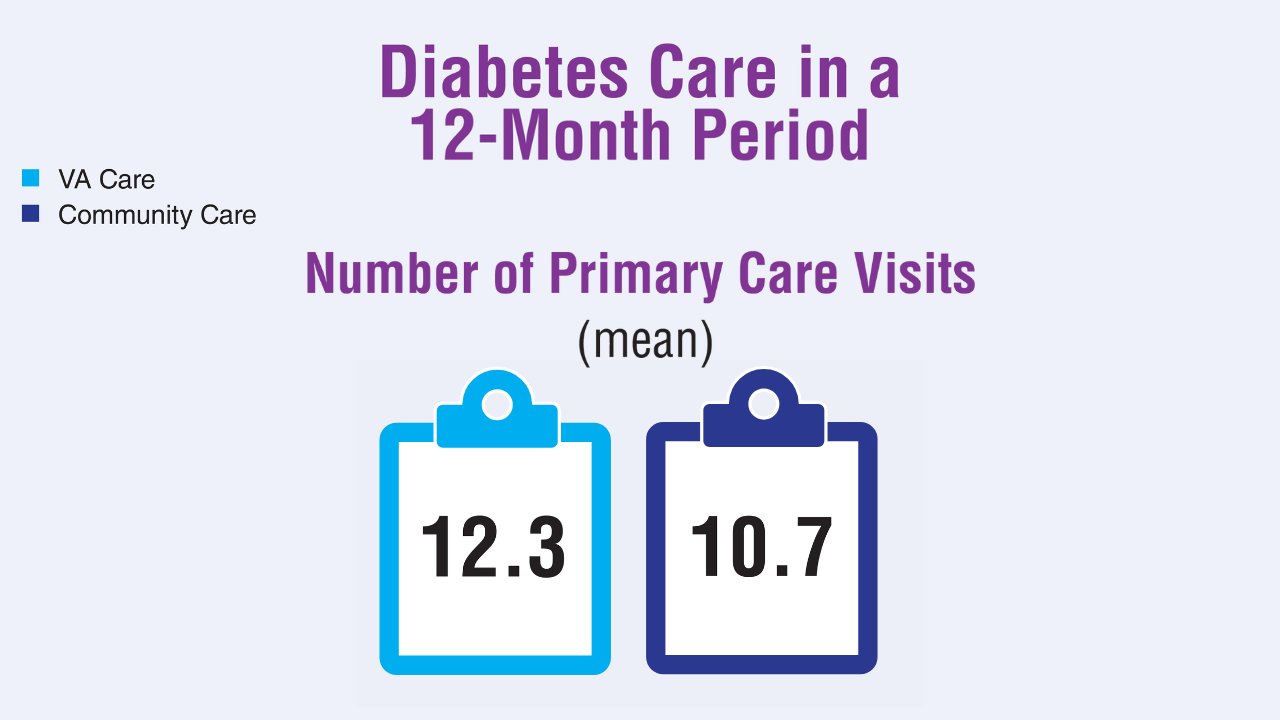
Data Trends 2025: Diabetes
Diabetes
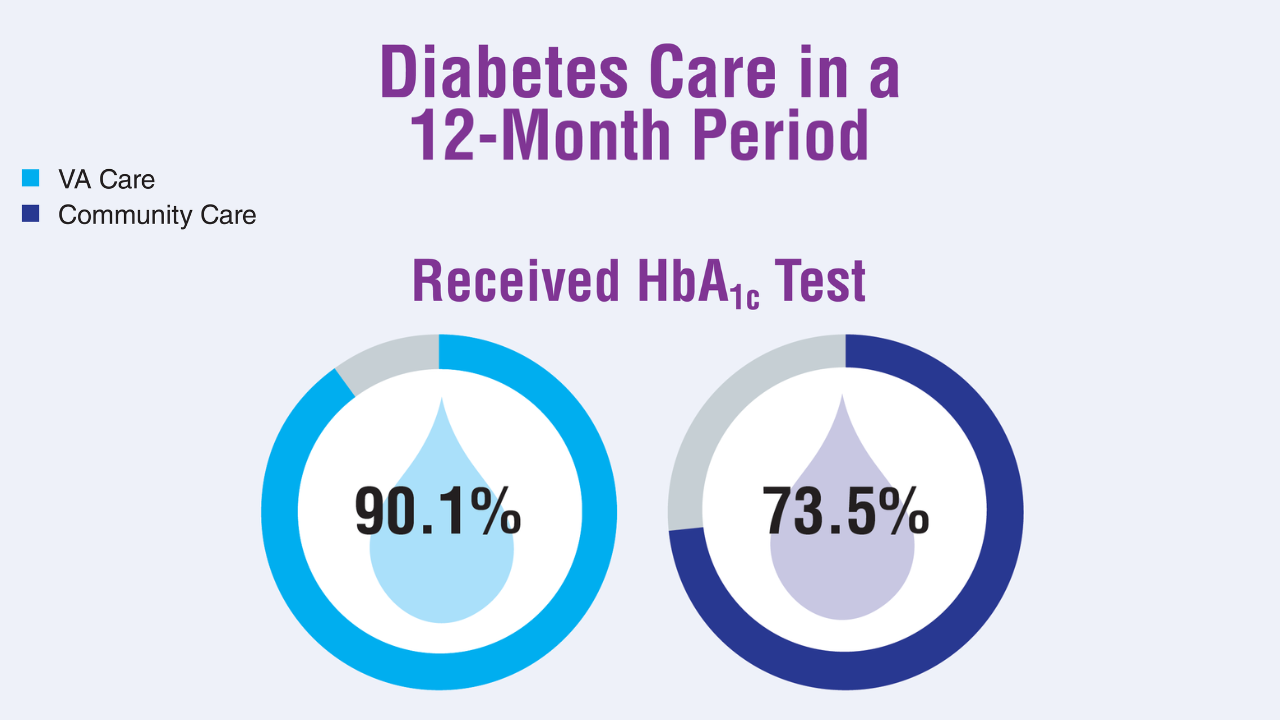
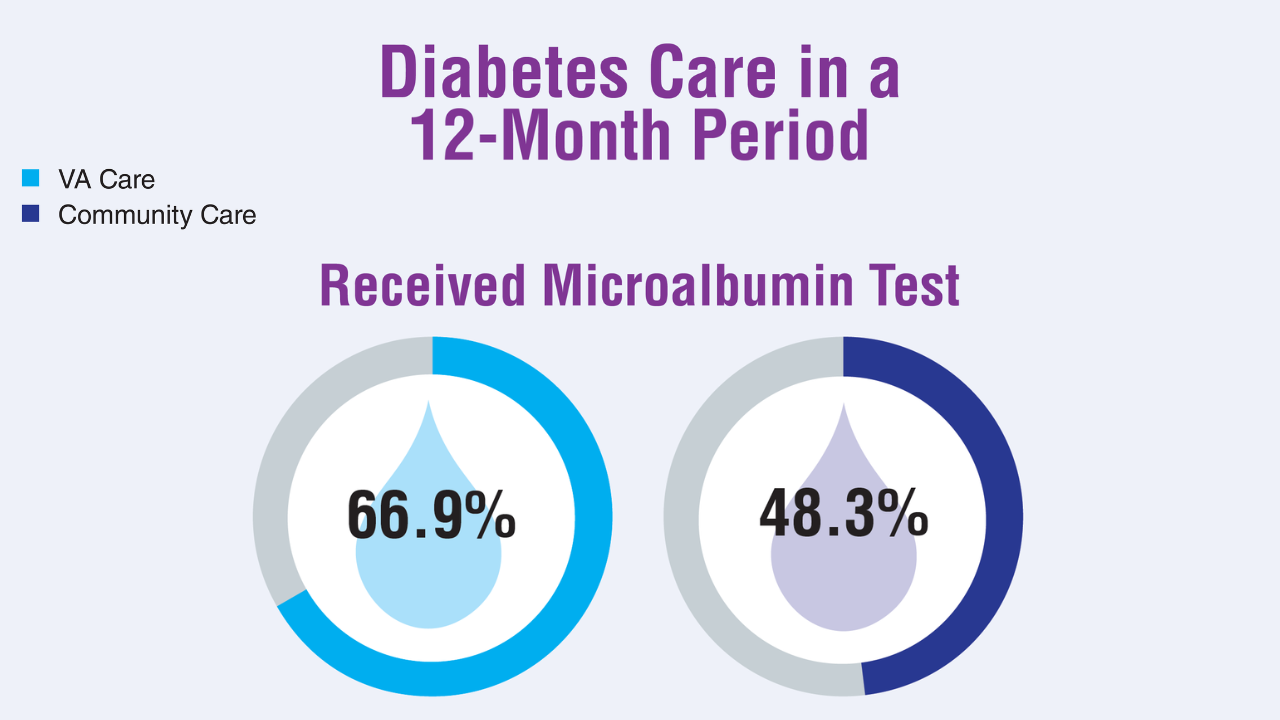
Click here to view more from Federal Health Care Data Trends 2025.
1. US Department of Veterans Affairs. VA improving diabetes care with patient generated health data. VA News. March 12, 2025. Accessed April 24, 2025. https://news.va.gov/138644/va-diabetes-care-with-patient-generated-data/
2. Diabetes basics. Centers for Disease Control and Prevention. May 15, 2024. Accessed April 24, 2025. https://www.cdc.gov/diabetes/about/index.html
3. Hua S, et al. Diabetes Care. 2024;47(11):1978-1984. doi:10.2337/dc24-0892
4. Lipska KJ, et al. Diabetes Technol Ther. 2024;26(12):908-917. doi:10.1089/dia.2024.0152
5. Yoon J, et al. J Gen Intern Med. 2025;40(3):647-653. doi:10.1007/s11606-024-08968-4
Click here to view more from Federal Health Care Data Trends 2025.
Click here to view more from Federal Health Care Data Trends 2025.
1. US Department of Veterans Affairs. VA improving diabetes care with patient generated health data. VA News. March 12, 2025. Accessed April 24, 2025. https://news.va.gov/138644/va-diabetes-care-with-patient-generated-data/
2. Diabetes basics. Centers for Disease Control and Prevention. May 15, 2024. Accessed April 24, 2025. https://www.cdc.gov/diabetes/about/index.html
3. Hua S, et al. Diabetes Care. 2024;47(11):1978-1984. doi:10.2337/dc24-0892
4. Lipska KJ, et al. Diabetes Technol Ther. 2024;26(12):908-917. doi:10.1089/dia.2024.0152
5. Yoon J, et al. J Gen Intern Med. 2025;40(3):647-653. doi:10.1007/s11606-024-08968-4
1. US Department of Veterans Affairs. VA improving diabetes care with patient generated health data. VA News. March 12, 2025. Accessed April 24, 2025. https://news.va.gov/138644/va-diabetes-care-with-patient-generated-data/
2. Diabetes basics. Centers for Disease Control and Prevention. May 15, 2024. Accessed April 24, 2025. https://www.cdc.gov/diabetes/about/index.html
3. Hua S, et al. Diabetes Care. 2024;47(11):1978-1984. doi:10.2337/dc24-0892
4. Lipska KJ, et al. Diabetes Technol Ther. 2024;26(12):908-917. doi:10.1089/dia.2024.0152
5. Yoon J, et al. J Gen Intern Med. 2025;40(3):647-653. doi:10.1007/s11606-024-08968-4
Diabetes
Diabetes
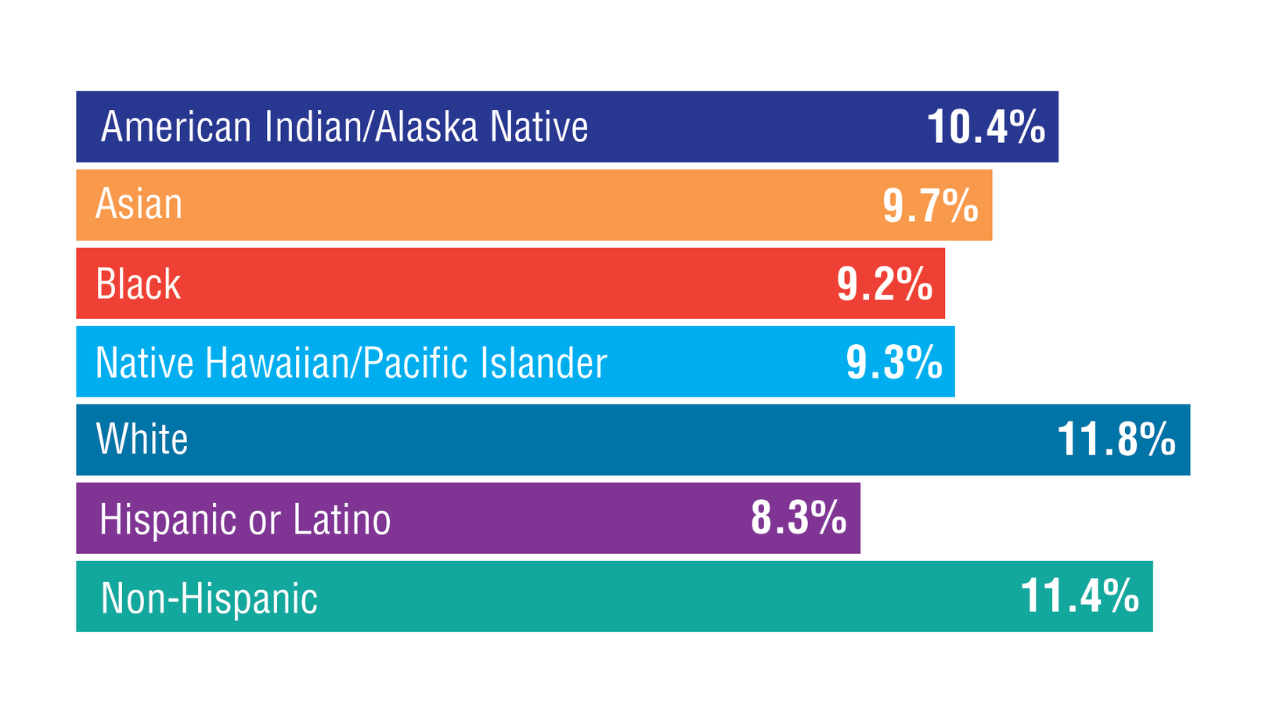
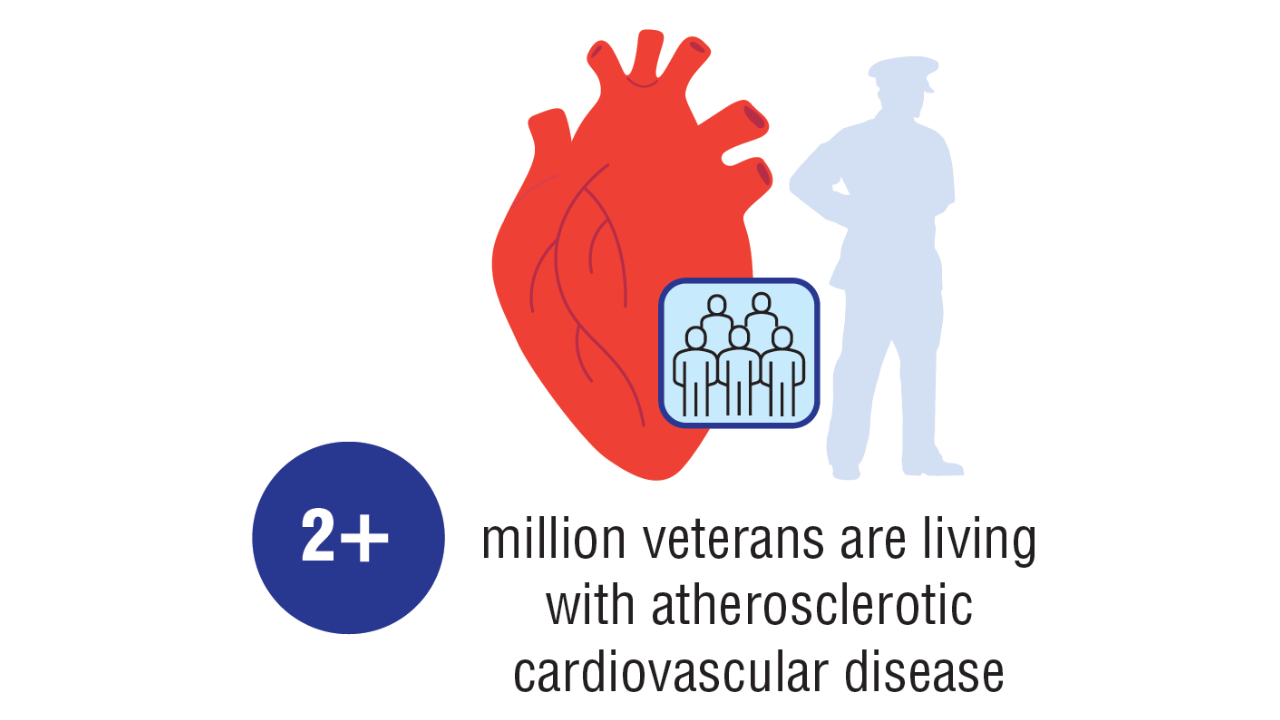
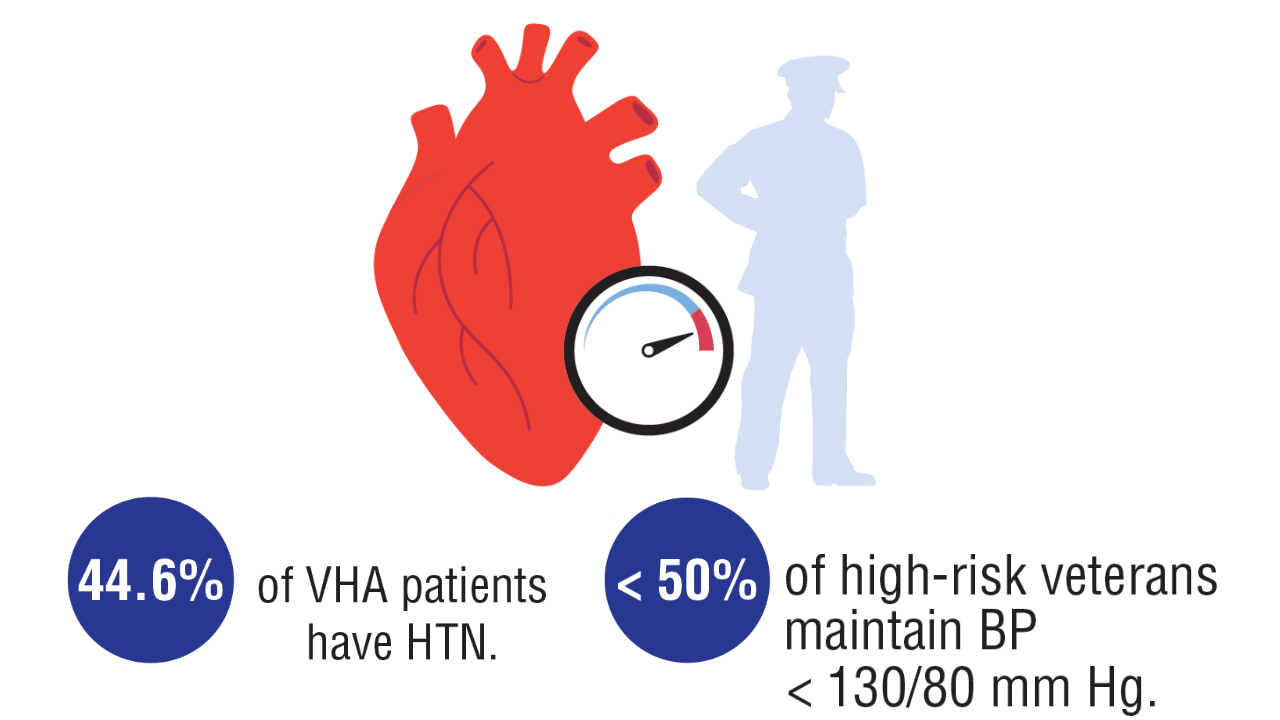
Data Trends 2025: Cardiology
Data Trends 2025: Cardiology
Click here to view more from Federal Health Care Data Trends 2025.
- Almuwaqqat Z, et al. JAMA Netw Open. 2024;7(3):e243062. doi:10.1001/jamanetworkopen.2024.3062
- Carrico M, et al. Telemed J E Health. 2024;30(4):1006-1012. doi:10.1089/tmj.2023.0269
- US Department of Veterans Affairs, Veterans Health Administration, Office of Health Equity. National veteran health equity report 2021. September 2022:177-179. Accessed April 11, 2025. https://www.va.gov/HEALTHEQUITY/docs/NVHER_2021_Report_508_Conformant.pdf
- New program for veterans with cholesterol, associated cardiovascular disease [press release]. American Heart Association. March 21, 2023. Accessed April 11, 2025. https://newsroom.heart.org/news/new-program-for-veterans-with-high-cholesterol-associated-cardiovascular-disease
Washington DL, et al. US Department of Veterans Affairs, Veterans Health Administration, Office of Health Equity. February 2024. Accessed April 11, 2025. https://www.va.gov/HEALTHEQUITY/docs/Rates_of_Hypertension_by_Race_or_Ethnicity.pdf
Click here to view more from Federal Health Care Data Trends 2025.
Click here to view more from Federal Health Care Data Trends 2025.
- Almuwaqqat Z, et al. JAMA Netw Open. 2024;7(3):e243062. doi:10.1001/jamanetworkopen.2024.3062
- Carrico M, et al. Telemed J E Health. 2024;30(4):1006-1012. doi:10.1089/tmj.2023.0269
- US Department of Veterans Affairs, Veterans Health Administration, Office of Health Equity. National veteran health equity report 2021. September 2022:177-179. Accessed April 11, 2025. https://www.va.gov/HEALTHEQUITY/docs/NVHER_2021_Report_508_Conformant.pdf
- New program for veterans with cholesterol, associated cardiovascular disease [press release]. American Heart Association. March 21, 2023. Accessed April 11, 2025. https://newsroom.heart.org/news/new-program-for-veterans-with-high-cholesterol-associated-cardiovascular-disease
Washington DL, et al. US Department of Veterans Affairs, Veterans Health Administration, Office of Health Equity. February 2024. Accessed April 11, 2025. https://www.va.gov/HEALTHEQUITY/docs/Rates_of_Hypertension_by_Race_or_Ethnicity.pdf
- Almuwaqqat Z, et al. JAMA Netw Open. 2024;7(3):e243062. doi:10.1001/jamanetworkopen.2024.3062
- Carrico M, et al. Telemed J E Health. 2024;30(4):1006-1012. doi:10.1089/tmj.2023.0269
- US Department of Veterans Affairs, Veterans Health Administration, Office of Health Equity. National veteran health equity report 2021. September 2022:177-179. Accessed April 11, 2025. https://www.va.gov/HEALTHEQUITY/docs/NVHER_2021_Report_508_Conformant.pdf
- New program for veterans with cholesterol, associated cardiovascular disease [press release]. American Heart Association. March 21, 2023. Accessed April 11, 2025. https://newsroom.heart.org/news/new-program-for-veterans-with-high-cholesterol-associated-cardiovascular-disease
Washington DL, et al. US Department of Veterans Affairs, Veterans Health Administration, Office of Health Equity. February 2024. Accessed April 11, 2025. https://www.va.gov/HEALTHEQUITY/docs/Rates_of_Hypertension_by_Race_or_Ethnicity.pdf
Data Trends 2025: Cardiology
Data Trends 2025: Cardiology
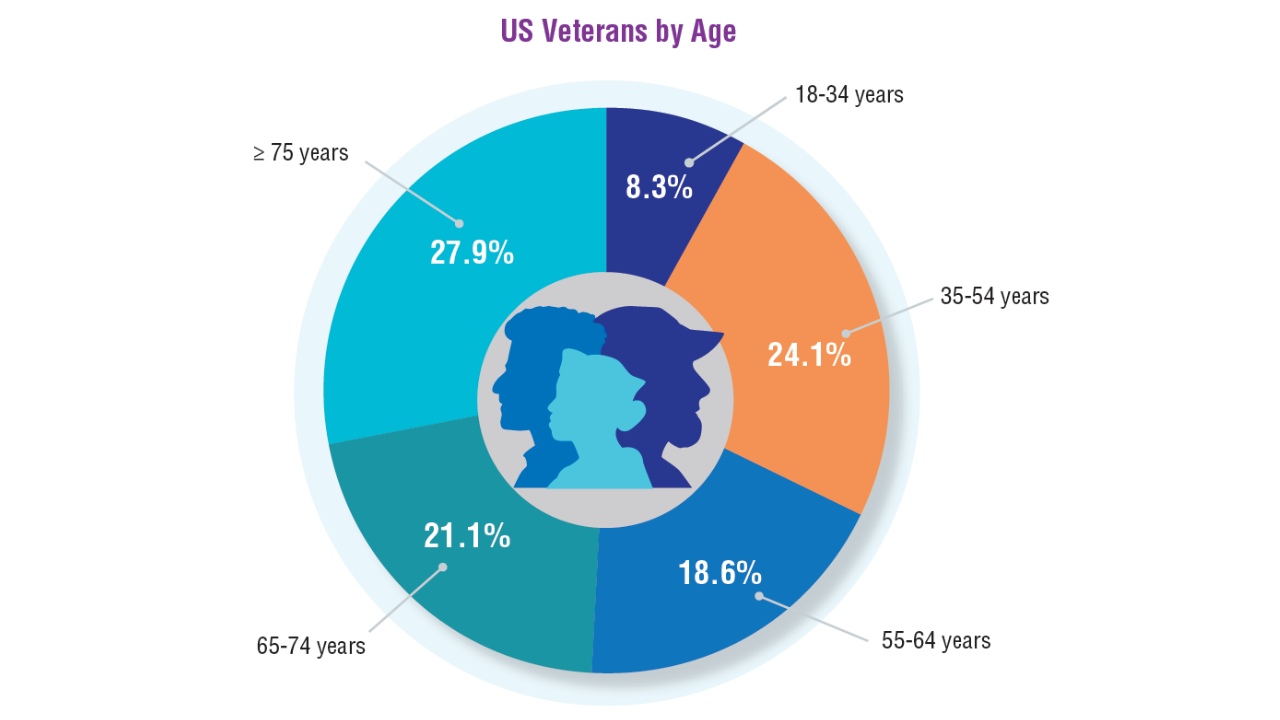
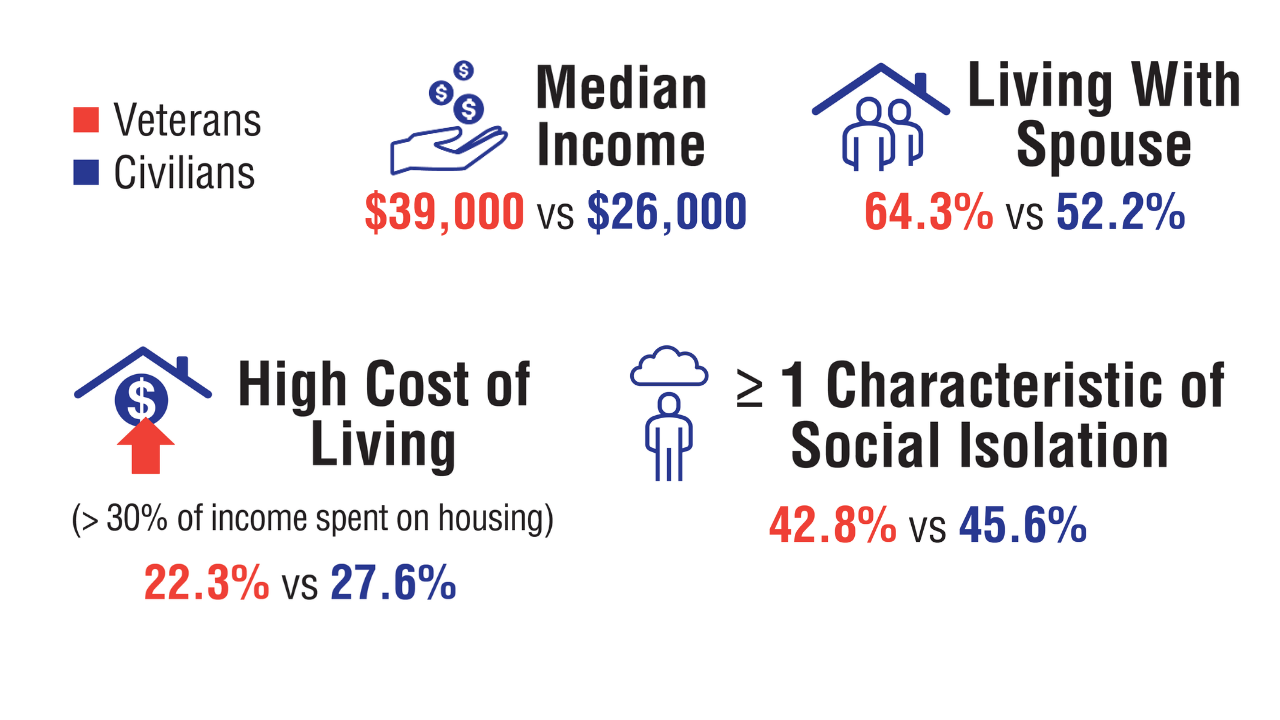
Data Trends 2025: Veteran Health at a Glance
Veteran Health at a Glance
Click here to view more from Federal Health Care Data Trends 2025.
1. United States Census Bureau. Veterans Day 2024: November 11 [press release]. October 16, 2024. Accessed April 8, 2025. https://www.census.gov/newsroom/
facts-for-features/2024/veterans-day.html
2. American Community Survey, 2023: ACS 1-year estimates subject tables:S2101 veteran status. United States Census Bureau. Accessed April 8, 2025. https://data.
census.gov/table/ACSST1Y2023.S2101
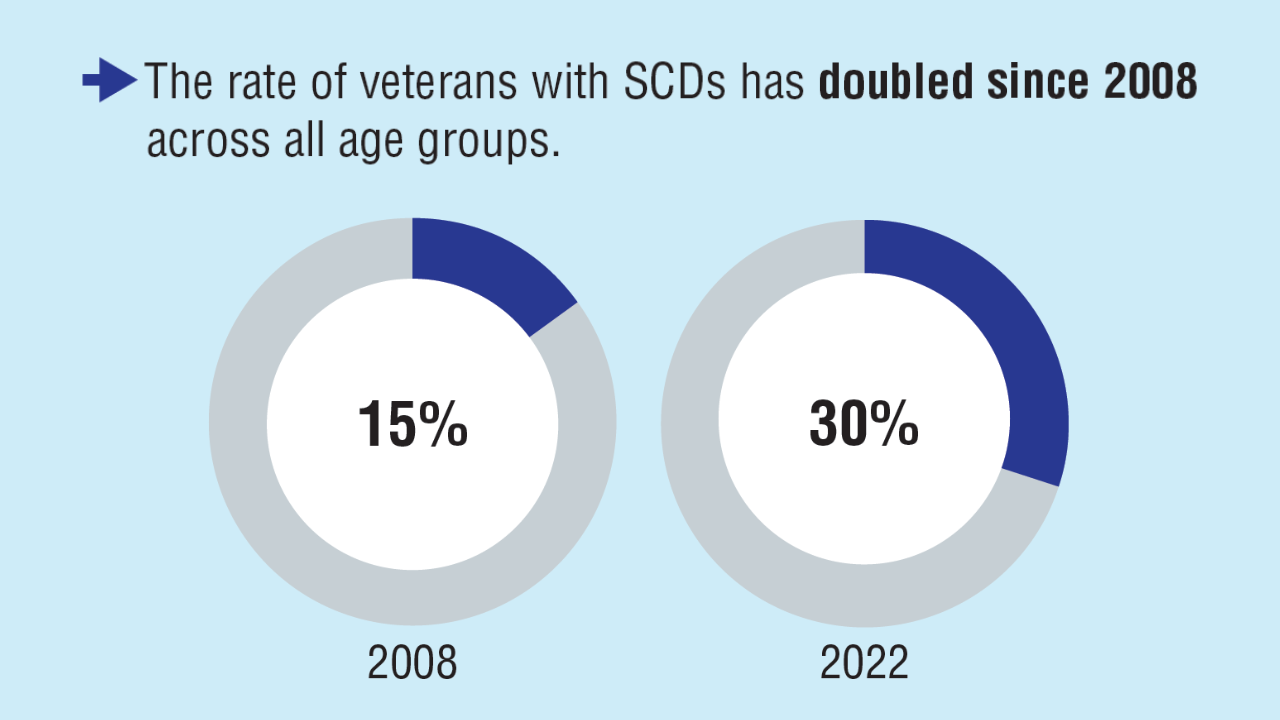
3. Vespa J, Carter C. Trends in veteran disability status and service-connected disability: 2008-2022. ACS-58. United States Census Bureau. November 6, 2024. Accessed
April 8, 2025. https://www.census.gov/library/publications/2024/acs/acs-58.html
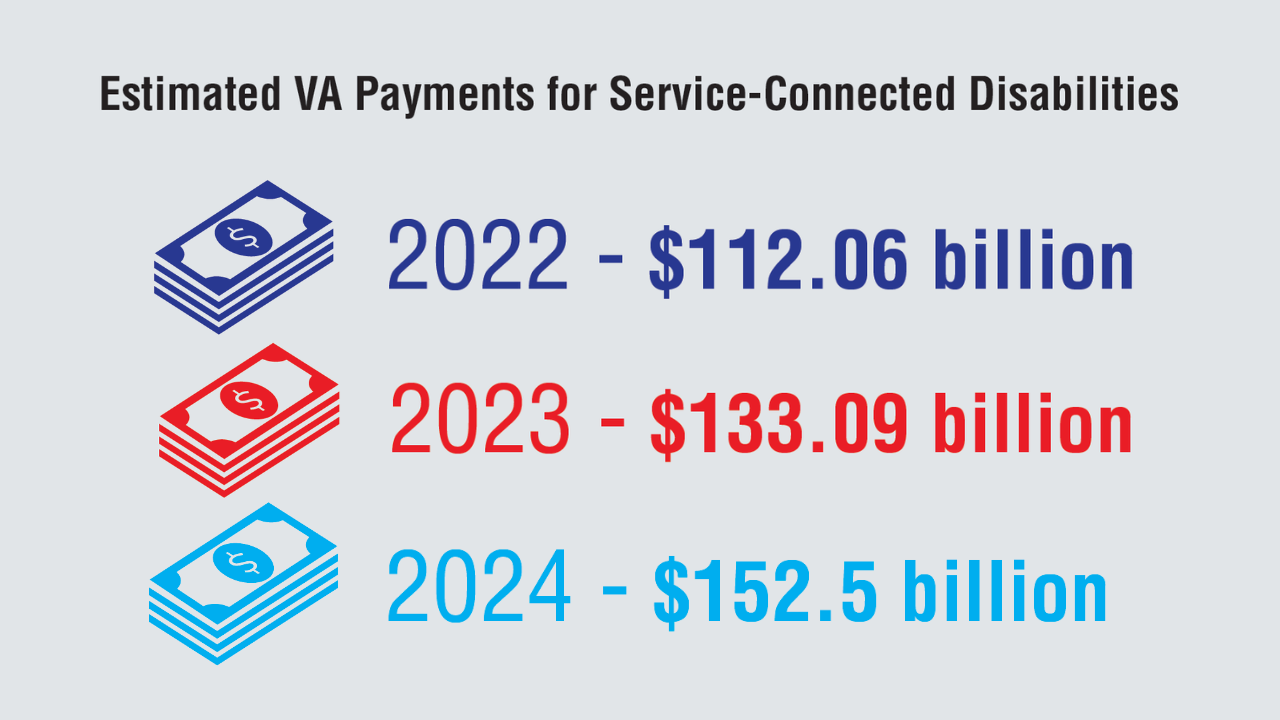
4. US Department of Veterans Affairs, Veterans Benefits Administration. Annual benefits report fiscal year 2024. Accessed June 9, 2025. https://www.benefits.va.gov/
REPORTS/abr/docs/2024-abr.pdf
5. US Department of Veterans Affairs, Veterans Benefits Administration. Annual benefits report fiscal year 2023. Accessed April 8, 2025. https://www.benefits.va.gov/
REPORTS/abr/docs/2023-abr.pdf
6. US Department of Veterans Affairs, Veterans Benefits Administration. Annual benefits report fiscal year 2022. Accessed April 8, 2025. https://www.benefits.va.gov/
REPORTS/abr/docs/2022-abr.pdf
7. Vespa J. Aging veterans: America’s veteran population in later life. ACS-54. United States Census Bureau. July 2023. Accessed April 8, 2025. https://www.census.gov/
content/dam/Census/library/publications/2023/acs/acs-54.pdf
8. A profile of older US veterans. National Council on Aging. November 6, 2019. Accessed April 8, 2025. https://www.ncoa.org/article/a-profile-of-older-us-veterans/
Click here to view more from Federal Health Care Data Trends 2025.
Click here to view more from Federal Health Care Data Trends 2025.
1. United States Census Bureau. Veterans Day 2024: November 11 [press release]. October 16, 2024. Accessed April 8, 2025. https://www.census.gov/newsroom/
facts-for-features/2024/veterans-day.html
2. American Community Survey, 2023: ACS 1-year estimates subject tables:S2101 veteran status. United States Census Bureau. Accessed April 8, 2025. https://data.
census.gov/table/ACSST1Y2023.S2101
3. Vespa J, Carter C. Trends in veteran disability status and service-connected disability: 2008-2022. ACS-58. United States Census Bureau. November 6, 2024. Accessed
April 8, 2025. https://www.census.gov/library/publications/2024/acs/acs-58.html
4. US Department of Veterans Affairs, Veterans Benefits Administration. Annual benefits report fiscal year 2024. Accessed June 9, 2025. https://www.benefits.va.gov/
REPORTS/abr/docs/2024-abr.pdf
5. US Department of Veterans Affairs, Veterans Benefits Administration. Annual benefits report fiscal year 2023. Accessed April 8, 2025. https://www.benefits.va.gov/
REPORTS/abr/docs/2023-abr.pdf
6. US Department of Veterans Affairs, Veterans Benefits Administration. Annual benefits report fiscal year 2022. Accessed April 8, 2025. https://www.benefits.va.gov/
REPORTS/abr/docs/2022-abr.pdf
7. Vespa J. Aging veterans: America’s veteran population in later life. ACS-54. United States Census Bureau. July 2023. Accessed April 8, 2025. https://www.census.gov/
content/dam/Census/library/publications/2023/acs/acs-54.pdf
8. A profile of older US veterans. National Council on Aging. November 6, 2019. Accessed April 8, 2025. https://www.ncoa.org/article/a-profile-of-older-us-veterans/
1. United States Census Bureau. Veterans Day 2024: November 11 [press release]. October 16, 2024. Accessed April 8, 2025. https://www.census.gov/newsroom/
facts-for-features/2024/veterans-day.html
2. American Community Survey, 2023: ACS 1-year estimates subject tables:S2101 veteran status. United States Census Bureau. Accessed April 8, 2025. https://data.
census.gov/table/ACSST1Y2023.S2101
3. Vespa J, Carter C. Trends in veteran disability status and service-connected disability: 2008-2022. ACS-58. United States Census Bureau. November 6, 2024. Accessed
April 8, 2025. https://www.census.gov/library/publications/2024/acs/acs-58.html
4. US Department of Veterans Affairs, Veterans Benefits Administration. Annual benefits report fiscal year 2024. Accessed June 9, 2025. https://www.benefits.va.gov/
REPORTS/abr/docs/2024-abr.pdf
5. US Department of Veterans Affairs, Veterans Benefits Administration. Annual benefits report fiscal year 2023. Accessed April 8, 2025. https://www.benefits.va.gov/
REPORTS/abr/docs/2023-abr.pdf
6. US Department of Veterans Affairs, Veterans Benefits Administration. Annual benefits report fiscal year 2022. Accessed April 8, 2025. https://www.benefits.va.gov/
REPORTS/abr/docs/2022-abr.pdf
7. Vespa J. Aging veterans: America’s veteran population in later life. ACS-54. United States Census Bureau. July 2023. Accessed April 8, 2025. https://www.census.gov/
content/dam/Census/library/publications/2023/acs/acs-54.pdf
8. A profile of older US veterans. National Council on Aging. November 6, 2019. Accessed April 8, 2025. https://www.ncoa.org/article/a-profile-of-older-us-veterans/
Veteran Health at a Glance
Veteran Health at a Glance
Atypical Skin Bronzing in Response to Belumosudil for Graft-vs-Host Disease
Atypical Skin Bronzing in Response to Belumosudil for Graft-vs-Host Disease
To the Editor:
Drug-induced hyperpigmentation is a common cause of an acquired increase in pigmentation. Belumosudil is an oral selective inhibitor of Rho-associated coiled-coil containing protein kinase (ROCK2) that is approved for the treatment of chronic graft-vs-host disease (GVHD). We describe a patient who developed diffuse skin bronzing 3 weeks after initiation of belumosudil treatment.
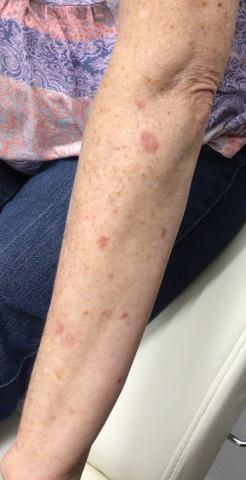
A 64-year-old fair-skinned woman presented to the dermatology clinic with bronzing of the skin and dystrophic nails 3 weeks after starting belumosudil for treatment of chronic GVHD. Six months prior to presentation, the patient had received a bone marrow transplant for chronic lymphoid leukemia. She presented to dermatology 6 months after the transplant with a new-onset rash that was suspicious for GVHD. Physical examination revealed pruritic pink papules diffusely scattered on the legs and forearms (Figure 1). The patient declined biopsy at that time and later followed up with oncology. The patient’s oncologist supported a diagnosis of GVHD, and the patient began treatment with belumosudil 200 mg/d which was intended to be taken until treatment failure due to progression of chronic GVHD.
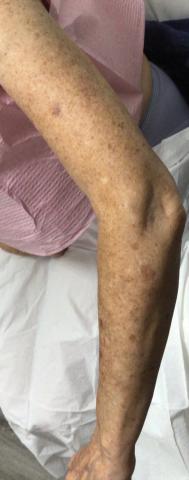
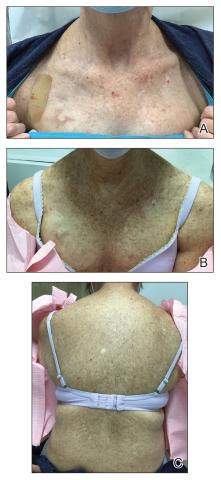
Three weeks after starting belumosudil, the patient developed diffuse bronzing of the skin and brown, evenly colored patches scattered on the trunk, back, and upper and lower extremities on a background of the presumed GVHD rash (Figure 2). The hyperpigmentation was abrupt, starting on the chest and spreading to the abdomen, extremities, and back (Figure 3).
developed on the patient’s chest and back within 3 weeks of initiating treatment with belumosudil.
Again, the patient was offered biopsy for the new-onset pigmentation but declined. During this time, she had no notable sun exposure and primarily stayed indoors despite living in a region with a sunny semi-arid climate. Her medication and supplement list were reviewed and included acalabrutinib, a multivitamin, lutein, biotin, and a fish oil supplement. A compete blood cell count as well as ferritin, transferrin, cortisol, and adrenocorticotropic hormone levels were unremarkable.
The patient continued to take belumosudil for treatment of GVHD. The hyperpigmentation faded slightly by a 2-month follow-up visit but persisted and was stable. She has not tried other treatments for GVHD to manage the hyperpigmentation.
Conditions known to cause diffuse bronzing of the skin include Addison disease, hemochromatosis, Cushing disease, and medication adverse events. Our patient presented with an absence of systemic symptoms, normal laboratory results, and no clinical indicators suggesting alternate causes. Given that the onset of the hyperpigmentation was 3 weeks after she started a new medication, we hypothesized that the bronzing was an adverse effect of the belumosudil—though this correlation cannot be definitively proven by this case.
The most common offending agents for drug-induced skin hyperpigmentation are nonsteroidal anti- inflammatory drugs, antimalarials, amiodarone, cytotoxic drugs, and tetracyclines.1,2 Our patient’s medication list included the cytotoxic agent acalabrutinib, a Bruton tyrosine kinase inhibitor used for the treatment of non-Hodgkin lymphoma. It has been associated with dermatologic findings of ecchymosis, bruising, panniculitis, and cellulitis, but there are no known reports of hyperpigmentation.3 Our patient had been taking acalabrutinib for 6 months when the GVHD rash developed. At the time, she also was taking a multivitamin and lutein, biotin, and fish oil supplements, none of which have been associated with hyperpigmentation.
Polypharmacy adds a layer of difficulty in identifying the inciting cause of pigmentary change. In our case, symptoms began 3 weeks after the initiation of belumosudil. There were no cutaneous reactions observed in the ROCKstar study of belumosudil; the most common adverse events were upper respiratory tract infection, diarrhea, fatigue, nausea, increased liver enzymes, and dyspnea.4,5 Patients on belumosudil have developed aggressive cutaneous squamous cell carcinoma.6 However, a search of PubMed articles indexed for MEDLINE using the search terms acalabrutinib or belumosudil with hyperpigmentation or cutaneous reaction returned no reports of these medications causing hyperpigmentation or cutaneous deposits.
Treatment of drug-induced hyperpigmentation is difficult because discontinuation of the offending agent typically confirms diagnosis, but interruption of treatment is not always possible, as in our patient. The skin changes can fade over time, but effects typically are long lasting.
Dermatologists play a key role in the identification of drug-induced skin hyperpigmentation. After endocrine or metabolic causes of skin hyperpigmentation have been ruled out, a thorough review of the patient’s medication list should be done to assess for a drug-induced cause. Treatment is limited to sun avoidance, as interruption of treatment may not be possible, and lesions typically do fade over time. These chronic skin changes can have a psychosocial effect on patients and regular follow-up is recommended.
- Giménez García RM, Carrasco Molina S. Drug-induced hyperpigmentation: review and case series. J Am Board Fam Med. 2019;32:628-638. doi:10.3122/jabfm.2019.04.180212
- Dereure O. Drug-induced skin pigmentation. epidemiology, diagnosis and treatment. Am J Clin Dermatol. 2001;2:253-62. doi:10.2165/00128071-200102040-00006
- Sibaud V, Beylot-Barry M, Protin C, et al. Dermatological toxicities of Bruton’s tyrosine kinase inhibitors. Am J Clin Dermatol. 2020; 21:799-812. doi:10.1007/s40257-020-00535-x
- Cutler C, Lee SJ, Arai S, et al. Belumosudil for chronic graft-versus-host disease after 2 or more prior lines of therapy: the ROCKstar Study. Blood. 2021;138:2278-2289. doi:10.1182/blood.2021012021
- Jagasia M, Lazaryan A, Bachier CR, et al. ROCK2 inhibition with belumosudil (KD025) for the treatment of chronic graftversus- host disease. J Clin Oncol. 2021;39:1888-1898. doi:10.1200 /JCO.20.02754
- Lee GH, Guzman AK, Divito SJ, et al. Cutaneous squamous-cell carcinoma after treatment with ruxolitinib or belumosudil. N Engl J Med. 2023;389:188-190. doi:10.1056/NEJMc2304157
To the Editor:
Drug-induced hyperpigmentation is a common cause of an acquired increase in pigmentation. Belumosudil is an oral selective inhibitor of Rho-associated coiled-coil containing protein kinase (ROCK2) that is approved for the treatment of chronic graft-vs-host disease (GVHD). We describe a patient who developed diffuse skin bronzing 3 weeks after initiation of belumosudil treatment.
A 64-year-old fair-skinned woman presented to the dermatology clinic with bronzing of the skin and dystrophic nails 3 weeks after starting belumosudil for treatment of chronic GVHD. Six months prior to presentation, the patient had received a bone marrow transplant for chronic lymphoid leukemia. She presented to dermatology 6 months after the transplant with a new-onset rash that was suspicious for GVHD. Physical examination revealed pruritic pink papules diffusely scattered on the legs and forearms (Figure 1). The patient declined biopsy at that time and later followed up with oncology. The patient’s oncologist supported a diagnosis of GVHD, and the patient began treatment with belumosudil 200 mg/d which was intended to be taken until treatment failure due to progression of chronic GVHD.
Three weeks after starting belumosudil, the patient developed diffuse bronzing of the skin and brown, evenly colored patches scattered on the trunk, back, and upper and lower extremities on a background of the presumed GVHD rash (Figure 2). The hyperpigmentation was abrupt, starting on the chest and spreading to the abdomen, extremities, and back (Figure 3).
developed on the patient’s chest and back within 3 weeks of initiating treatment with belumosudil.
Again, the patient was offered biopsy for the new-onset pigmentation but declined. During this time, she had no notable sun exposure and primarily stayed indoors despite living in a region with a sunny semi-arid climate. Her medication and supplement list were reviewed and included acalabrutinib, a multivitamin, lutein, biotin, and a fish oil supplement. A compete blood cell count as well as ferritin, transferrin, cortisol, and adrenocorticotropic hormone levels were unremarkable.
The patient continued to take belumosudil for treatment of GVHD. The hyperpigmentation faded slightly by a 2-month follow-up visit but persisted and was stable. She has not tried other treatments for GVHD to manage the hyperpigmentation.
Conditions known to cause diffuse bronzing of the skin include Addison disease, hemochromatosis, Cushing disease, and medication adverse events. Our patient presented with an absence of systemic symptoms, normal laboratory results, and no clinical indicators suggesting alternate causes. Given that the onset of the hyperpigmentation was 3 weeks after she started a new medication, we hypothesized that the bronzing was an adverse effect of the belumosudil—though this correlation cannot be definitively proven by this case.
The most common offending agents for drug-induced skin hyperpigmentation are nonsteroidal anti- inflammatory drugs, antimalarials, amiodarone, cytotoxic drugs, and tetracyclines.1,2 Our patient’s medication list included the cytotoxic agent acalabrutinib, a Bruton tyrosine kinase inhibitor used for the treatment of non-Hodgkin lymphoma. It has been associated with dermatologic findings of ecchymosis, bruising, panniculitis, and cellulitis, but there are no known reports of hyperpigmentation.3 Our patient had been taking acalabrutinib for 6 months when the GVHD rash developed. At the time, she also was taking a multivitamin and lutein, biotin, and fish oil supplements, none of which have been associated with hyperpigmentation.
Polypharmacy adds a layer of difficulty in identifying the inciting cause of pigmentary change. In our case, symptoms began 3 weeks after the initiation of belumosudil. There were no cutaneous reactions observed in the ROCKstar study of belumosudil; the most common adverse events were upper respiratory tract infection, diarrhea, fatigue, nausea, increased liver enzymes, and dyspnea.4,5 Patients on belumosudil have developed aggressive cutaneous squamous cell carcinoma.6 However, a search of PubMed articles indexed for MEDLINE using the search terms acalabrutinib or belumosudil with hyperpigmentation or cutaneous reaction returned no reports of these medications causing hyperpigmentation or cutaneous deposits.
Treatment of drug-induced hyperpigmentation is difficult because discontinuation of the offending agent typically confirms diagnosis, but interruption of treatment is not always possible, as in our patient. The skin changes can fade over time, but effects typically are long lasting.
Dermatologists play a key role in the identification of drug-induced skin hyperpigmentation. After endocrine or metabolic causes of skin hyperpigmentation have been ruled out, a thorough review of the patient’s medication list should be done to assess for a drug-induced cause. Treatment is limited to sun avoidance, as interruption of treatment may not be possible, and lesions typically do fade over time. These chronic skin changes can have a psychosocial effect on patients and regular follow-up is recommended.
To the Editor:
Drug-induced hyperpigmentation is a common cause of an acquired increase in pigmentation. Belumosudil is an oral selective inhibitor of Rho-associated coiled-coil containing protein kinase (ROCK2) that is approved for the treatment of chronic graft-vs-host disease (GVHD). We describe a patient who developed diffuse skin bronzing 3 weeks after initiation of belumosudil treatment.
A 64-year-old fair-skinned woman presented to the dermatology clinic with bronzing of the skin and dystrophic nails 3 weeks after starting belumosudil for treatment of chronic GVHD. Six months prior to presentation, the patient had received a bone marrow transplant for chronic lymphoid leukemia. She presented to dermatology 6 months after the transplant with a new-onset rash that was suspicious for GVHD. Physical examination revealed pruritic pink papules diffusely scattered on the legs and forearms (Figure 1). The patient declined biopsy at that time and later followed up with oncology. The patient’s oncologist supported a diagnosis of GVHD, and the patient began treatment with belumosudil 200 mg/d which was intended to be taken until treatment failure due to progression of chronic GVHD.
Three weeks after starting belumosudil, the patient developed diffuse bronzing of the skin and brown, evenly colored patches scattered on the trunk, back, and upper and lower extremities on a background of the presumed GVHD rash (Figure 2). The hyperpigmentation was abrupt, starting on the chest and spreading to the abdomen, extremities, and back (Figure 3).
developed on the patient’s chest and back within 3 weeks of initiating treatment with belumosudil.
Again, the patient was offered biopsy for the new-onset pigmentation but declined. During this time, she had no notable sun exposure and primarily stayed indoors despite living in a region with a sunny semi-arid climate. Her medication and supplement list were reviewed and included acalabrutinib, a multivitamin, lutein, biotin, and a fish oil supplement. A compete blood cell count as well as ferritin, transferrin, cortisol, and adrenocorticotropic hormone levels were unremarkable.
The patient continued to take belumosudil for treatment of GVHD. The hyperpigmentation faded slightly by a 2-month follow-up visit but persisted and was stable. She has not tried other treatments for GVHD to manage the hyperpigmentation.
Conditions known to cause diffuse bronzing of the skin include Addison disease, hemochromatosis, Cushing disease, and medication adverse events. Our patient presented with an absence of systemic symptoms, normal laboratory results, and no clinical indicators suggesting alternate causes. Given that the onset of the hyperpigmentation was 3 weeks after she started a new medication, we hypothesized that the bronzing was an adverse effect of the belumosudil—though this correlation cannot be definitively proven by this case.
The most common offending agents for drug-induced skin hyperpigmentation are nonsteroidal anti- inflammatory drugs, antimalarials, amiodarone, cytotoxic drugs, and tetracyclines.1,2 Our patient’s medication list included the cytotoxic agent acalabrutinib, a Bruton tyrosine kinase inhibitor used for the treatment of non-Hodgkin lymphoma. It has been associated with dermatologic findings of ecchymosis, bruising, panniculitis, and cellulitis, but there are no known reports of hyperpigmentation.3 Our patient had been taking acalabrutinib for 6 months when the GVHD rash developed. At the time, she also was taking a multivitamin and lutein, biotin, and fish oil supplements, none of which have been associated with hyperpigmentation.
Polypharmacy adds a layer of difficulty in identifying the inciting cause of pigmentary change. In our case, symptoms began 3 weeks after the initiation of belumosudil. There were no cutaneous reactions observed in the ROCKstar study of belumosudil; the most common adverse events were upper respiratory tract infection, diarrhea, fatigue, nausea, increased liver enzymes, and dyspnea.4,5 Patients on belumosudil have developed aggressive cutaneous squamous cell carcinoma.6 However, a search of PubMed articles indexed for MEDLINE using the search terms acalabrutinib or belumosudil with hyperpigmentation or cutaneous reaction returned no reports of these medications causing hyperpigmentation or cutaneous deposits.
Treatment of drug-induced hyperpigmentation is difficult because discontinuation of the offending agent typically confirms diagnosis, but interruption of treatment is not always possible, as in our patient. The skin changes can fade over time, but effects typically are long lasting.
Dermatologists play a key role in the identification of drug-induced skin hyperpigmentation. After endocrine or metabolic causes of skin hyperpigmentation have been ruled out, a thorough review of the patient’s medication list should be done to assess for a drug-induced cause. Treatment is limited to sun avoidance, as interruption of treatment may not be possible, and lesions typically do fade over time. These chronic skin changes can have a psychosocial effect on patients and regular follow-up is recommended.
- Giménez García RM, Carrasco Molina S. Drug-induced hyperpigmentation: review and case series. J Am Board Fam Med. 2019;32:628-638. doi:10.3122/jabfm.2019.04.180212
- Dereure O. Drug-induced skin pigmentation. epidemiology, diagnosis and treatment. Am J Clin Dermatol. 2001;2:253-62. doi:10.2165/00128071-200102040-00006
- Sibaud V, Beylot-Barry M, Protin C, et al. Dermatological toxicities of Bruton’s tyrosine kinase inhibitors. Am J Clin Dermatol. 2020; 21:799-812. doi:10.1007/s40257-020-00535-x
- Cutler C, Lee SJ, Arai S, et al. Belumosudil for chronic graft-versus-host disease after 2 or more prior lines of therapy: the ROCKstar Study. Blood. 2021;138:2278-2289. doi:10.1182/blood.2021012021
- Jagasia M, Lazaryan A, Bachier CR, et al. ROCK2 inhibition with belumosudil (KD025) for the treatment of chronic graftversus- host disease. J Clin Oncol. 2021;39:1888-1898. doi:10.1200 /JCO.20.02754
- Lee GH, Guzman AK, Divito SJ, et al. Cutaneous squamous-cell carcinoma after treatment with ruxolitinib or belumosudil. N Engl J Med. 2023;389:188-190. doi:10.1056/NEJMc2304157
- Giménez García RM, Carrasco Molina S. Drug-induced hyperpigmentation: review and case series. J Am Board Fam Med. 2019;32:628-638. doi:10.3122/jabfm.2019.04.180212
- Dereure O. Drug-induced skin pigmentation. epidemiology, diagnosis and treatment. Am J Clin Dermatol. 2001;2:253-62. doi:10.2165/00128071-200102040-00006
- Sibaud V, Beylot-Barry M, Protin C, et al. Dermatological toxicities of Bruton’s tyrosine kinase inhibitors. Am J Clin Dermatol. 2020; 21:799-812. doi:10.1007/s40257-020-00535-x
- Cutler C, Lee SJ, Arai S, et al. Belumosudil for chronic graft-versus-host disease after 2 or more prior lines of therapy: the ROCKstar Study. Blood. 2021;138:2278-2289. doi:10.1182/blood.2021012021
- Jagasia M, Lazaryan A, Bachier CR, et al. ROCK2 inhibition with belumosudil (KD025) for the treatment of chronic graftversus- host disease. J Clin Oncol. 2021;39:1888-1898. doi:10.1200 /JCO.20.02754
- Lee GH, Guzman AK, Divito SJ, et al. Cutaneous squamous-cell carcinoma after treatment with ruxolitinib or belumosudil. N Engl J Med. 2023;389:188-190. doi:10.1056/NEJMc2304157
Atypical Skin Bronzing in Response to Belumosudil for Graft-vs-Host Disease
Atypical Skin Bronzing in Response to Belumosudil for Graft-vs-Host Disease
PRACTICE POINTS
- Drug-induced hyperpigmentation is a common cause of acquired hyperpigmentation and should be evaluated after metabolic or endocrine causes are ruled out.
- Belumosudil for chronic graft-vs-host disease can induce rapid-onset diffuse bronzing hyperpigmentation, even in the absence of other systemic or laboratory abnormalities.
- Treatment entails discontinuation of the offending agent and limitation of exacerbating factors such as sun exposure.
Enhancing Patient Satisfaction and Quality of Life With Mohs Micrographic Surgery: A Systematic Review of Patient Education, Communication, and Anxiety Management
Enhancing Patient Satisfaction and Quality of Life With Mohs Micrographic Surgery: A Systematic Review of Patient Education, Communication, and Anxiety Management
Mohs micrographic surgery (MMS)—developed by Dr. Frederic Mohs in the 1930s—is the gold standard for treating various cutaneous malignancies. It provides maximal conservation of uninvolved tissues while producing higher cure rates compared to wide local excision.1,2
We sought to assess the various characteristics that impact patient satisfaction to help Mohs surgeons incorporate relatively simple yet clinically significant practices into their patient encounters. We conducted a systematic literature search of peer-reviewed PubMed articles indexed for MEDLINE from database inception through November 2023 using the terms Mohs micrographic surgery and patient satisfaction. Among the inclusion criteria were studies involving participants having undergone MMS, with objective assessments on patient-reported satisfaction or preferences related to patient education, communication, anxiety-alleviating measures, or QOL in MMS. Studies were excluded if they failed to meet these criteria, were outdated and no longer clinically relevant, or measured unalterable factors with no significant impact on how Mohs surgeons could change clinical practice. Of the 157 nonreplicated studies identified, 34 met inclusion criteria.
Perioperative Patient Communication and Education Techniques
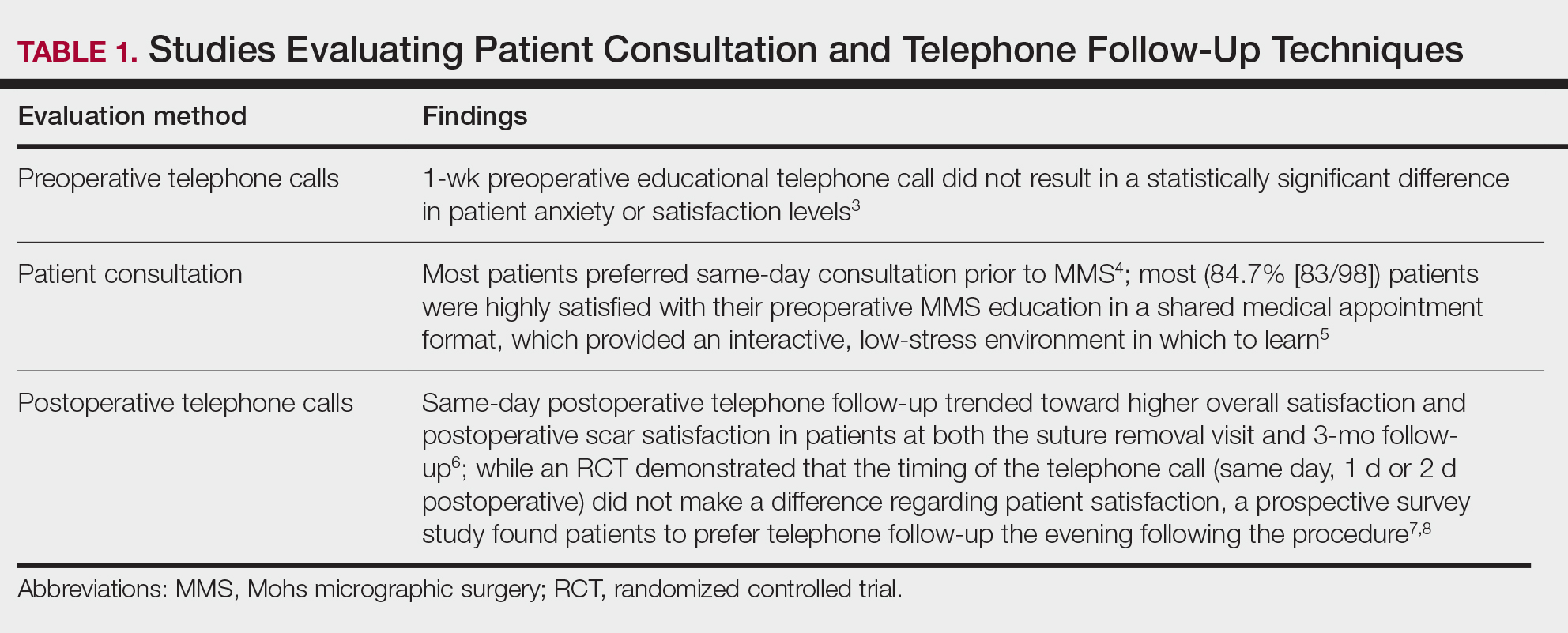
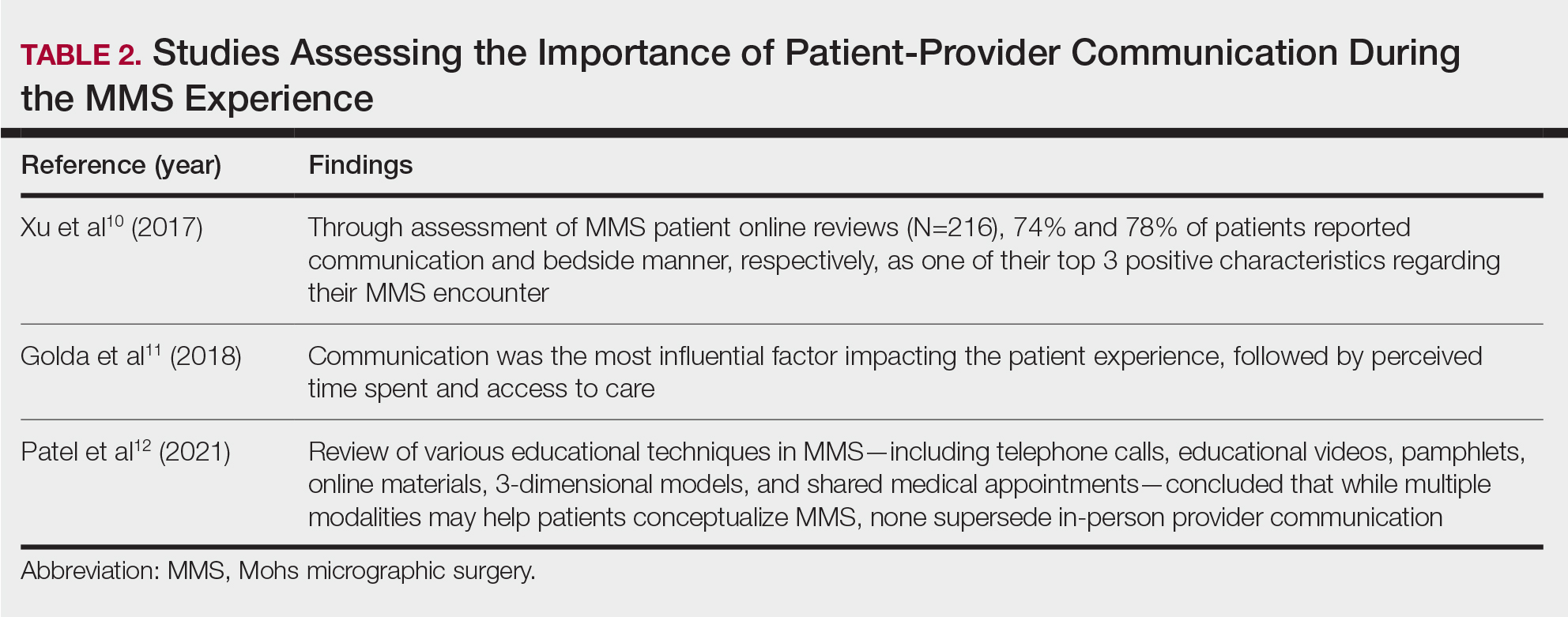
Perioperative Patient Communication—Many studies have evaluated the impact of perioperative patient-provider communication and education on patient satisfaction in those undergoing MMS. Studies focusing on preoperative and postoperative telephone calls, patient consultation formats, and patient-perceived impact of such communication modalities have been well documented (Table 1).3-8 The importance of the patient follow-up after MMS was further supported by a retrospective study concluding that 88.7% (86/97) of patients regarded follow-up visits as important, and 80% (77/97) desired additional follow-up 3 months after MMS.9 Additional studies have highlighted the importance of thorough and open perioperative patient-provider communication during MMS (Table 2).10-12
Patient-Education Techniques—Many studies have assessed the use of visual models to aid in patient education on MMS, specifically the preprocedural consent process (Table 3).13-16 Additionally, 2 randomized controlled trials assessing the use of at-home and same-day in-office preoperative educational videos concluded that these interventions increased patient knowledge and confidence regarding procedural risks and benefits, with no statistically significant differences in patient anxiety or satisfaction.17,18
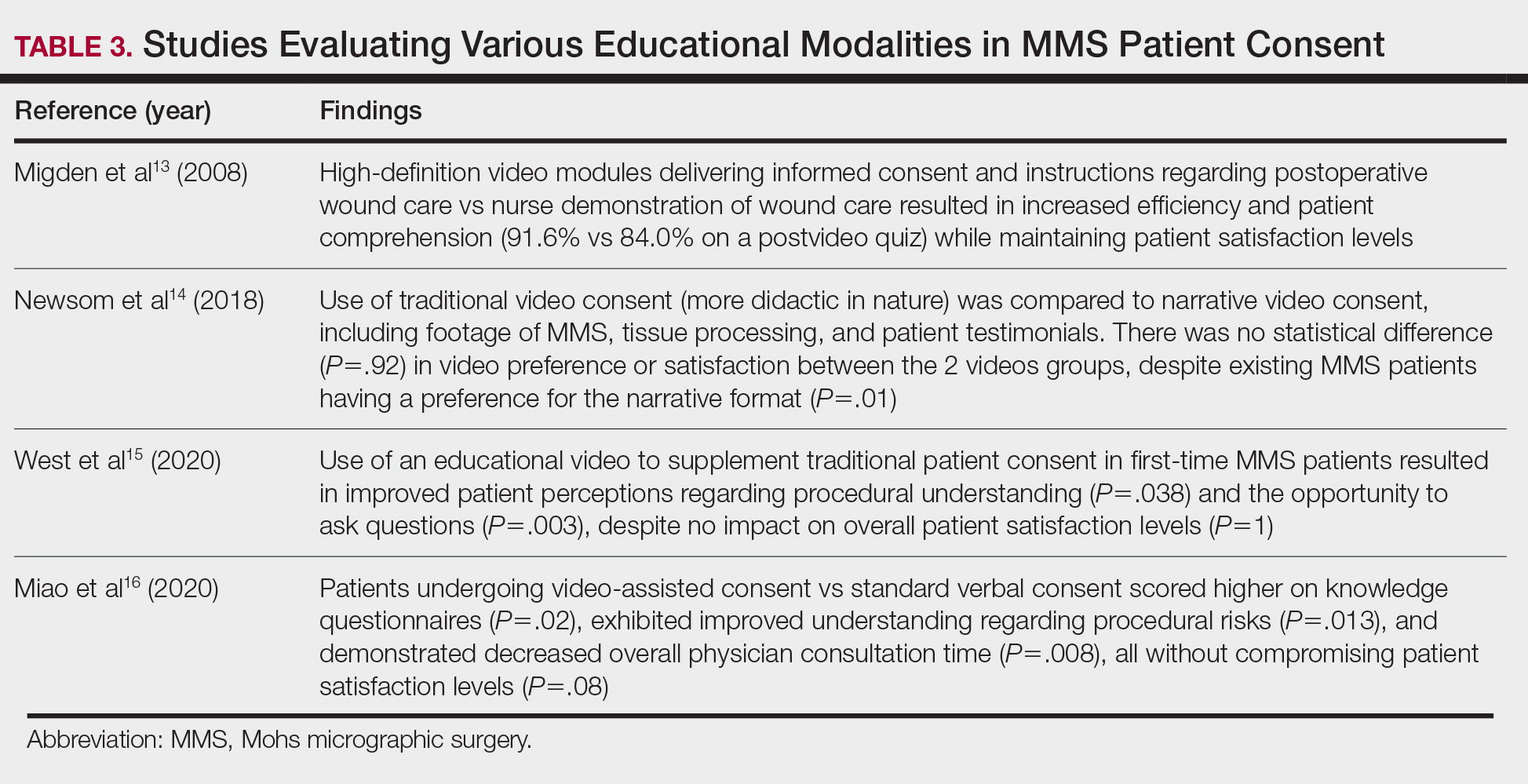
Despite the availability of these educational videos, many patients often turn to online resources for self-education, which is problematic if reader literacy is incongruent with online readability. One study assessing readability of online MMS resources concluded that the most accessed articles exceeded the recommended reading level for adequate patient comprehension.19 A survey studying a wide range of variables related to patient satisfaction (eg, demographics, socioeconomics, health status) in 339 MMS patients found that those who considered themselves more involved in the decision-making process were more satisfied in the short-term, and married patients had even higher long-term satisfaction. Interestingly, this study also concluded that undergoing 3 or more MMS stages was associated with higher short- and long-term satisfaction, likely secondary to perceived effects of increased overall care, medical attention, and time spent with the provider.20
Synthesis of this information with emphasis on the higher evidence-based studies—including systematic reviews, meta-analyses, and randomized controlled trials—yields the following beneficial interventions regarding patient education and communication13-20:
- Preoperative and same-day postoperative telephone follow-up (TFU) do not show statistically significant impacts on patient satisfaction; however, TFU allows for identification of postoperative concerns and inadequate pain management, which may have downstream effects on long-term perception of the overall patient experience.
- The use of video-assisted consent yields improved patient satisfaction and knowledge, while video content—traditional or didactic—has no impact on satisfaction in new MMS patients.
- The use of at-home or same-day in-office preoperative educational videos can improve procedural knowledge and risk-benefit understanding of MMS while having no impact on satisfaction.
- Bedside manner and effective in-person communication by the provider often takes precedence in the patient experience; however, implementation of additional educational modalities should be considered.
Patient Anxiety and QOL
Reducing Patient Anxiety—The use of perioperative distractors to reduce patient anxiety may play an integral role when patients undergo MMS, as there often are prolonged waiting periods between stages when patients may feel increasingly vulnerable or anxious. Table 4 reviews studies on perioperative distractors that showed a statistically significant reduction in MMS patient anxiety.21-24
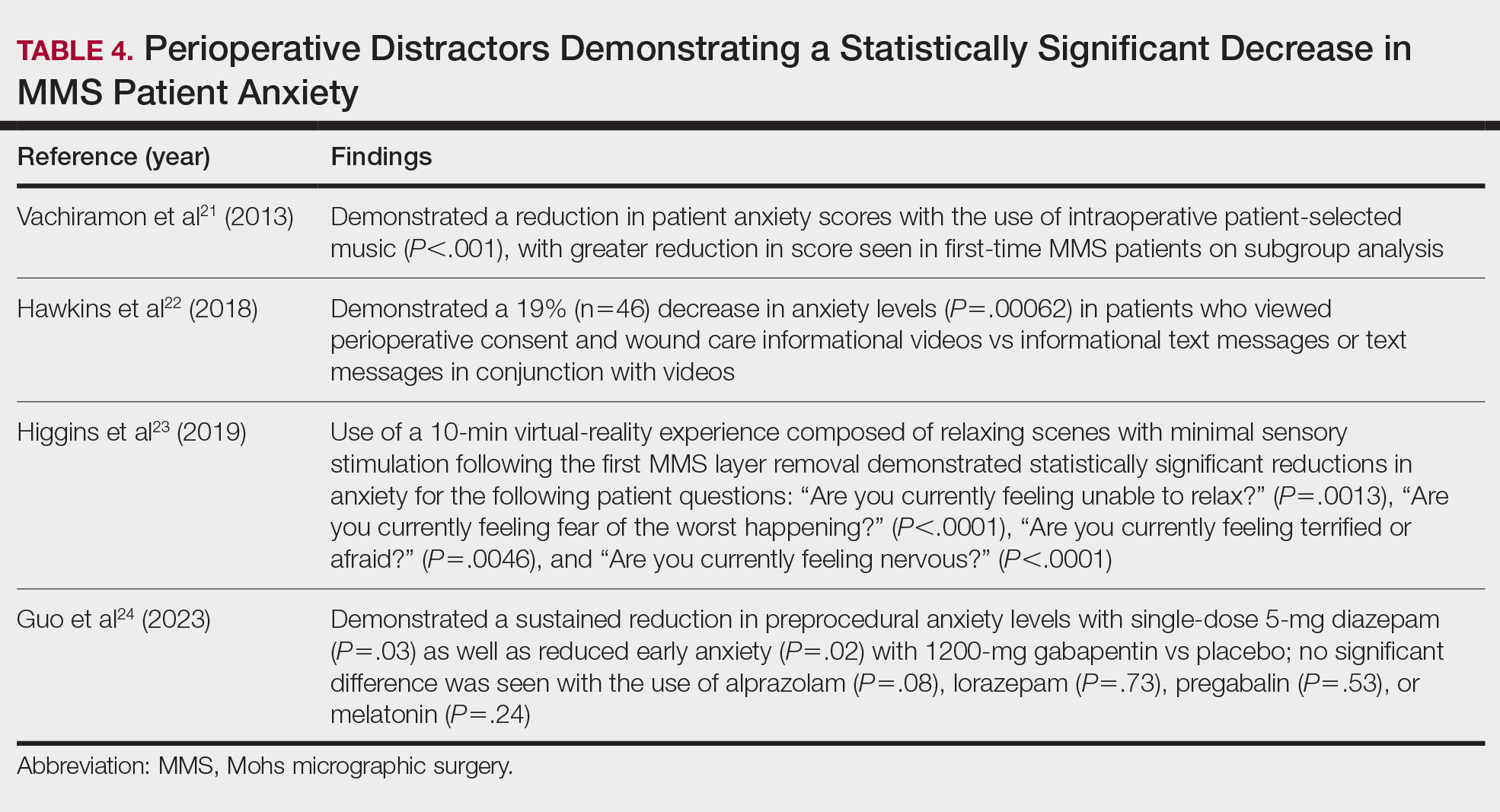
Although not statistically significant, additional studies evaluating the use of intraoperative anxiety-reduction methods in MMS have demonstrated a downtrend in patient anxiety with the following interventions: engaging in small talk with clinic staff, bringing a guest, eating, watching television, communicating surgical expectations with the provider, handholding, use of a stress ball, and use of 3-dimensional educational MMS models.25-27 Similarly, a survey of 73 patients undergoing MMS found that patients tended to enjoy complimentary beverages preprocedurally in the waiting room, reading, speaking with their guest, watching television, or using their telephone during wait times.28 Table 5 lists additional perioperative factors encompassing specific patient and surgical characteristics that help reduce patient anxiety.29-32
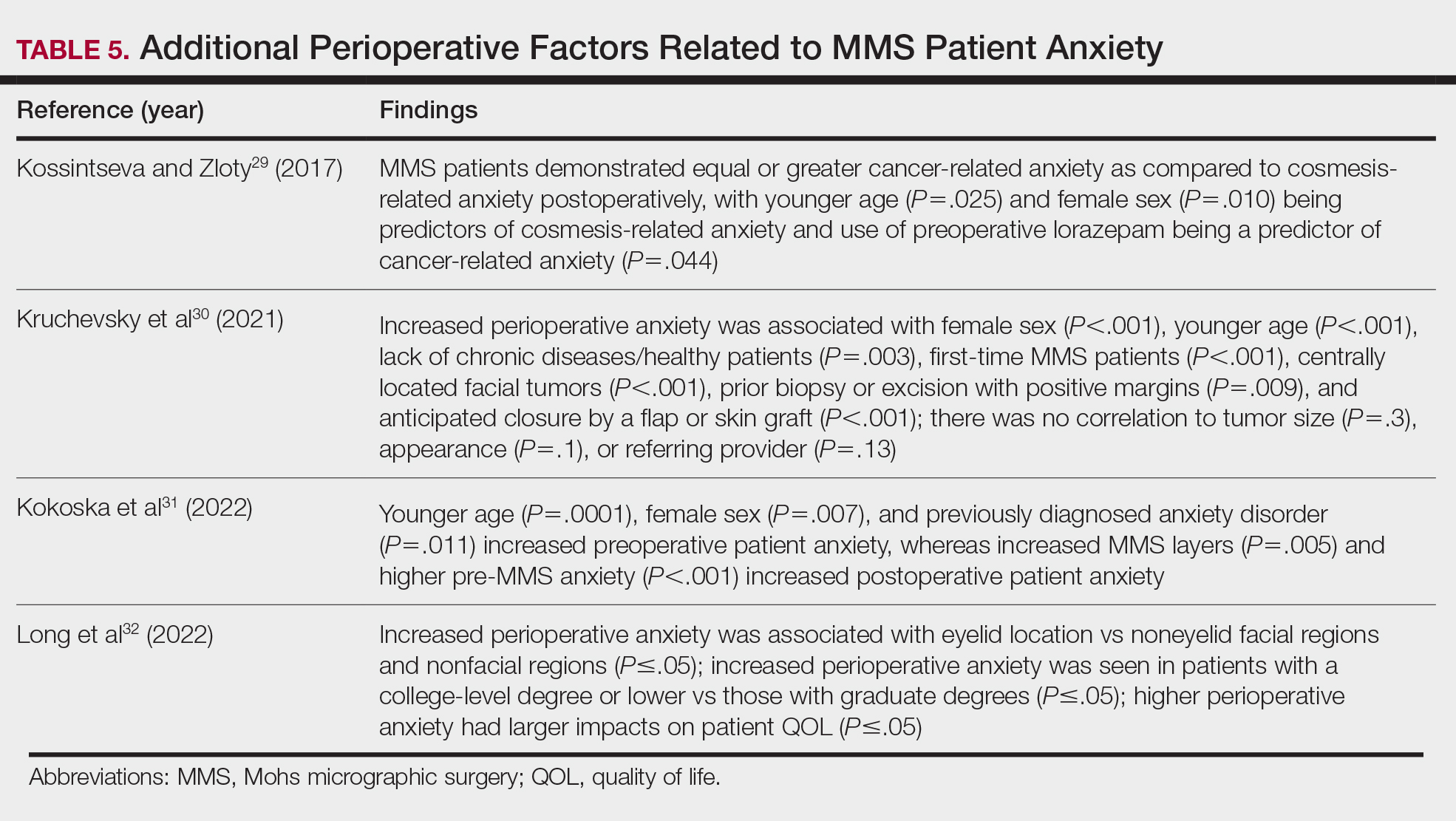
Patient QOL—Many methods aimed at decreasing MMS-related patient anxiety often show no direct impact on patient satisfaction, likely due to the multifactorial nature of the patient-perceived experience. A prospective observational study of MMS patients noted a statistically significant improvement in patient QOL scores 3 months postsurgery (P=.0007), demonstrating that MMS generally results in positive patient outcomes despite preprocedural anxiety.33 An additional prospective study in MMS patients with nonmelanoma skin cancer concluded that sex, age, and closure type—factors often shown to affect anxiety levels—did not significantly impact patient satisfaction.34 Similarly, high satisfaction levels can be expected among MMS patients undergoing treatment of melanoma in situ, with more than 90% of patients rating their treatment experience a 4 (agree) or 5 (strongly agree) out of 5 in short- and long-term satisfaction assessments (38/41 and 40/42, respectively).35 This assessment, conducted 3 months postoperatively, asked patients to score the statement, “I am completely satisfied with the treatment of my skin problem,” on a scale ranging from 1 (strongly disagree) to 5 (strongly agree).
Lastly, patient perception of their surgeon’s skill may contribute to levels of patient satisfaction. Although suture spacing has not been shown to affect surgical outcomes, it has been demonstrated to impact the patient’s perception of surgical skill and is further supported by a study concluding that closures with 2-mm spacing were ranked significantly lower by patients compared with closures with either 4- or 6-mm spacing (P=.005 and P=.012, respectively).36
Synthesis of this information with emphasis on the higher evidence-based studies—including systematic reviews, meta-analyses, and randomized controlled trials—yields the following beneficial interventions regarding anxiety-reducing measures and patient-perceived QOL21-36:
- Factors shown to decrease patient anxiety include patient personalized music, virtual-reality experience, perioperative informational videos, and 3-dimensional–printed MMS models.
- Many methods aimed at decreasing MMS-related patient anxiety show no direct impact on patient satisfaction, likely due to the multifactorial nature of the patient-perceived experience.
- Higher anxiety can be associated with worse QOL scores in MMS patients, and additional factors that may have a negative impact on anxiety include female sex, younger age, and tumor location on the face.
Conclusion
Many factors affect patient satisfaction in MMS. Increased awareness and acknowledgement of these factors can foster improved clinical practice and patient experience, which can have downstream effects on patient compliance and overall psychosocial and medical well-being. With the movement toward value-based health care, patient satisfaction ratings are likely to play an increasingly important role in physician reimbursement. Adapting one’s practice to include high-quality, time-efficient, patient-centered care goes hand in hand with increasing MMS patient satisfaction. Careful evaluation and scrutiny of one’s current practices while remaining cognizant of patient population, resource availability, and clinical limitations often reveal opportunities for small adjustments that can have a great impact on patient satisfaction. This thorough assessment and review of the published literature aims to assist MMS surgeons in understanding the role that certain factors—(1) perioperative patient communication and education techniques and (2) patient anxiety, QOL, and additional considerations—have on overall satisfaction with MMS. Specific consideration should be placed on the fact that patient satisfaction is multifactorial, and many different interventions can have a positive impact on the overall patient experience.
- Trost LB, Bailin PL. History of Mohs surgery. Dermatol Clin. 2011; 29:135-139, vii. doi:10.1016/j.det.2011.01.010
- Leslie DF, Greenway HT. Mohs micrographic surgery for skin cancer. Australas J Dermatol. 1991;32:159-164. doi:10.1111/j.1440 -0960.1991.tb01783.x
- Sobanko JF, Da Silva D, Chiesa Fuxench ZC, et al. Preoperative telephone consultation does not decrease patient anxiety before Mohs micrographic surgery. J Am Acad Dermatol. 2017;76:519-526. doi:10.1016/j.jaad.2016.09.027
- Sharon VR, Armstrong AW, Jim On SC, et al. Separate- versus same-day preoperative consultation in dermatologic surgery: a patient-centered investigation in an academic practice. Dermatol Surg. 2013;39:240-247. doi:10.1111/dsu.12083
- Knackstedt TJ, Samie FH. Shared medical appointments for the preoperative consultation visit of Mohs micrographic surgery. J Am Acad Dermatol. 2015;72:340-344. doi:10.1016/j.jaad.2014.10.022
- Vance S, Fontecilla N, Samie FH, et al. Effect of postoperative telephone calls on patient satisfaction and scar satisfaction after Mohs micrographic surgery. Dermatol Surg. 2019;45:1459-1464. doi:10.1097/DSS.0000000000001913
- Hafiji J, Salmon P, Hussain W. Patient satisfaction with post-operative telephone calls after Mohs micrographic surgery: a New Zealand and U.K. experience. Br J Dermatol. 2012;167:570-574. doi:10.1111 /j.1365-2133.2012.11011.x
- Bednarek R, Jonak C, Golda N. Optimal timing of postoperative patient telephone calls after Mohs micrographic surgery: a randomized controlled trial. J Am Acad Dermatol. 2021;85:220-221. doi:10.1016 /j.jaad.2020.07.106
- Sharon VR, Armstrong AW, Jim-On S, et al. Postoperative preferences in cutaneous surgery: a patient-centered investigation from an academic dermatologic surgery practice. Dermatol Surg. 2013;39:773-778. doi:10.1111/dsu.12136
- Xu S, Atanelov Z, Bhatia AC. Online patient-reported reviews of Mohs micrographic surgery: qualitative analysis of positive and negative experiences. Cutis. 2017;99:E25-E29.
- Golda N, Beeson S, Kohli N, et al. Recommendations for improving the patient experience in specialty encounters. J Am Acad Dermatol. 2018;78:653-659. doi:10.1016/j.jaad.2017.05.040
- Patel P, Malik K, Khachemoune A. Patient education in Mohs surgery: a review and critical evaluation of techniques. Arch Dermatol Res. 2021;313:217-224. doi:10.1007/s00403-020-02119-5
- Migden M, Chavez-Frazier A, Nguyen T. The use of high definition video modules for delivery of informed consent and wound care education in the Mohs surgery unit. Semin Cutan Med Surg. 2008;27:89-93. doi:10.1016/j.sder.2008.02.001
- Newsom E, Lee E, Rossi A, et al. Modernizing the Mohs surgery consultation: instituting a video module for improved patient education and satisfaction. Dermatol Surg. 2018;44:778-784. doi:10.1097/DSS.0000000000001473
- West L, Srivastava D, Goldberg LH, et al. Multimedia technology used to supplement patient consent for Mohs micrographic surgery. Dermatol Surg. 2020;46:586-590. doi:10.1097/DSS.0000000000002134
- Miao Y, Venning VL, Mallitt KA, et al. A randomized controlled trial comparing video-assisted informed consent with standard consent for Mohs micrographic surgery. JAAD Int. 2020;1:13-20. doi:10.1016 /j.jdin.2020.03.005
- Mann J, Li L, Kulakov E, et al. Home viewing of educational video improves patient understanding of Mohs micrographic surgery. Clin Exp Dermatol. 2022;47:93-97. doi:10.1111/ced.14845
- Delcambre M, Haynes D, Hajar T, et al. Using a multimedia tool for informed consent in Mohs surgery: a randomized trial measuring effects on patient anxiety, knowledge, and satisfaction. Dermatol Surg. 2020;46:591-598. doi:10.1097/DSS.0000000000002213
- Vargas CR, DePry J, Lee BT, et al. The readability of online patient information about Mohs micrographic surgery. Dermatol Surg. 2016;42:1135-1141. doi:10.1097/DSS.0000000000000866
- Asgari MM, Warton EM, Neugebauer R, et al. Predictors of patient satisfaction with Mohs surgery: analysis of preoperative, intraoperative, and postoperative factors in a prospective cohort. Arch Dermatol. 2011;147:1387-1394.
- Vachiramon V, Sobanko JF, Rattanaumpawan P, et al. Music reduces patient anxiety during Mohs surgery: an open-label randomized controlled trial. Dermatol Surg. 2013;39:298-305. doi:10.1111/dsu.12047
- Hawkins SD, Koch SB, Williford PM, et al. Web app- and text message-based patient education in Mohs micrographic surgery-a randomized controlled trial. Dermatol Surg. 2018;44:924-932. doi:10.1097/DSS.0000000000001489
- Higgins S, Feinstein S, Hawkins M, et al. Virtual reality to improve the experience of the Mohs patient-a prospective interventional study. Dermatol Surg. 2019;45:1009-1018. doi:10.1097 /DSS.0000000000001854
- Guo D, Zloty DM, Kossintseva I. Efficacy and safety of anxiolytics in Mohs micrographic surgery: a randomized, double-blinded, placebo-controlled trial. Dermatol Surg. 2023;49:989-994. doi:10.1097 /DSS.0000000000003905
- Locke MC, Wilkerson EC, Mistur RL, et al. 2015 Arte Poster Competition first place winner: assessing the correlation between patient anxiety and satisfaction for Mohs surgery. J Drugs Dermatol. 2015;14:1070-1072.
- Yanes AF, Weil A, Furlan KC, et al. Effect of stress ball use or hand-holding on anxiety during skin cancer excision: a randomized clinical trial. JAMA Dermatol. 2018;154:1045-1049. doi:10.1001 /jamadermatol.2018.1783
- Biro M, Kim I, Huynh A, et al. The use of 3-dimensionally printed models to optimize patient education and alleviate perioperative anxiety in Mohs micrographic surgery: a randomized controlled trial. J Am Acad Dermatol. 2019;81:1339-1345. doi:10.1016/j.jaad.2019.05.085
- Ali FR, Al-Niaimi F, Craythorne EE, et al. Patient satisfaction and the waiting room in Mohs surgery: appropriate prewarning may abrogate boredom. J Eur Acad Dermatol Venereol. 2017;31:e337-e338.
- Kossintseva I, Zloty D. Determinants and timeline of perioperative anxiety in Mohs surgery. Dermatol Surg. 2017;43:1029-1035.
- Kruchevsky D, Hirth J, Capucha T, et al. Triggers of preoperative anxiety in patients undergoing Mohs micrographic surgery. Dermatol Surg. 2021;47:1110-1112.
- Kokoska RE, Szeto MD, Steadman L, et al. Analysis of factors contributing to perioperative Mohs micrographic surgery anxiety: patient survey study at an academic center. Dermatol Surg. 2022;48:1279-1282.
- Long J, Rajabi-Estarabadi A, Levin A, et al. Perioperative anxiety associated with Mohs micrographic surgery: a survey-based study. Dermatol Surg. 2022;48:711-715.
- Zhang J, Miller CJ, O’Malley V, et al. Patient quality of life fluctuates before and after Mohs micrographic surgery: a longitudinal assessment of the patient experience. J Am Acad Dermatol. 2018;78:1060-1067.
- Lee EB, Ford A, Clarey D, et al. Patient outcomes and satisfaction after Mohs micrographic surgery in patients with nonmelanoma skin cancer. Dermatol Sur. 2021;47:1190-1194.
- Condie D, West L, Hynan LS, et al. Patient satisfaction with Mohs surgery for melanoma in situ. Dermatol Surg. 2021;47:288-290.
- Arshanapalli A, Tra n JM, Aylward JL, et al. The effect of suture spacing on patient perception of surgical skill. J Am Acad Dermatol. 2021;84:735-736.
Mohs micrographic surgery (MMS)—developed by Dr. Frederic Mohs in the 1930s—is the gold standard for treating various cutaneous malignancies. It provides maximal conservation of uninvolved tissues while producing higher cure rates compared to wide local excision.1,2
We sought to assess the various characteristics that impact patient satisfaction to help Mohs surgeons incorporate relatively simple yet clinically significant practices into their patient encounters. We conducted a systematic literature search of peer-reviewed PubMed articles indexed for MEDLINE from database inception through November 2023 using the terms Mohs micrographic surgery and patient satisfaction. Among the inclusion criteria were studies involving participants having undergone MMS, with objective assessments on patient-reported satisfaction or preferences related to patient education, communication, anxiety-alleviating measures, or QOL in MMS. Studies were excluded if they failed to meet these criteria, were outdated and no longer clinically relevant, or measured unalterable factors with no significant impact on how Mohs surgeons could change clinical practice. Of the 157 nonreplicated studies identified, 34 met inclusion criteria.
Perioperative Patient Communication and Education Techniques
Perioperative Patient Communication—Many studies have evaluated the impact of perioperative patient-provider communication and education on patient satisfaction in those undergoing MMS. Studies focusing on preoperative and postoperative telephone calls, patient consultation formats, and patient-perceived impact of such communication modalities have been well documented (Table 1).3-8 The importance of the patient follow-up after MMS was further supported by a retrospective study concluding that 88.7% (86/97) of patients regarded follow-up visits as important, and 80% (77/97) desired additional follow-up 3 months after MMS.9 Additional studies have highlighted the importance of thorough and open perioperative patient-provider communication during MMS (Table 2).10-12


Patient-Education Techniques—Many studies have assessed the use of visual models to aid in patient education on MMS, specifically the preprocedural consent process (Table 3).13-16 Additionally, 2 randomized controlled trials assessing the use of at-home and same-day in-office preoperative educational videos concluded that these interventions increased patient knowledge and confidence regarding procedural risks and benefits, with no statistically significant differences in patient anxiety or satisfaction.17,18

Despite the availability of these educational videos, many patients often turn to online resources for self-education, which is problematic if reader literacy is incongruent with online readability. One study assessing readability of online MMS resources concluded that the most accessed articles exceeded the recommended reading level for adequate patient comprehension.19 A survey studying a wide range of variables related to patient satisfaction (eg, demographics, socioeconomics, health status) in 339 MMS patients found that those who considered themselves more involved in the decision-making process were more satisfied in the short-term, and married patients had even higher long-term satisfaction. Interestingly, this study also concluded that undergoing 3 or more MMS stages was associated with higher short- and long-term satisfaction, likely secondary to perceived effects of increased overall care, medical attention, and time spent with the provider.20
Synthesis of this information with emphasis on the higher evidence-based studies—including systematic reviews, meta-analyses, and randomized controlled trials—yields the following beneficial interventions regarding patient education and communication13-20:
- Preoperative and same-day postoperative telephone follow-up (TFU) do not show statistically significant impacts on patient satisfaction; however, TFU allows for identification of postoperative concerns and inadequate pain management, which may have downstream effects on long-term perception of the overall patient experience.
- The use of video-assisted consent yields improved patient satisfaction and knowledge, while video content—traditional or didactic—has no impact on satisfaction in new MMS patients.
- The use of at-home or same-day in-office preoperative educational videos can improve procedural knowledge and risk-benefit understanding of MMS while having no impact on satisfaction.
- Bedside manner and effective in-person communication by the provider often takes precedence in the patient experience; however, implementation of additional educational modalities should be considered.
Patient Anxiety and QOL
Reducing Patient Anxiety—The use of perioperative distractors to reduce patient anxiety may play an integral role when patients undergo MMS, as there often are prolonged waiting periods between stages when patients may feel increasingly vulnerable or anxious. Table 4 reviews studies on perioperative distractors that showed a statistically significant reduction in MMS patient anxiety.21-24

Although not statistically significant, additional studies evaluating the use of intraoperative anxiety-reduction methods in MMS have demonstrated a downtrend in patient anxiety with the following interventions: engaging in small talk with clinic staff, bringing a guest, eating, watching television, communicating surgical expectations with the provider, handholding, use of a stress ball, and use of 3-dimensional educational MMS models.25-27 Similarly, a survey of 73 patients undergoing MMS found that patients tended to enjoy complimentary beverages preprocedurally in the waiting room, reading, speaking with their guest, watching television, or using their telephone during wait times.28 Table 5 lists additional perioperative factors encompassing specific patient and surgical characteristics that help reduce patient anxiety.29-32

Patient QOL—Many methods aimed at decreasing MMS-related patient anxiety often show no direct impact on patient satisfaction, likely due to the multifactorial nature of the patient-perceived experience. A prospective observational study of MMS patients noted a statistically significant improvement in patient QOL scores 3 months postsurgery (P=.0007), demonstrating that MMS generally results in positive patient outcomes despite preprocedural anxiety.33 An additional prospective study in MMS patients with nonmelanoma skin cancer concluded that sex, age, and closure type—factors often shown to affect anxiety levels—did not significantly impact patient satisfaction.34 Similarly, high satisfaction levels can be expected among MMS patients undergoing treatment of melanoma in situ, with more than 90% of patients rating their treatment experience a 4 (agree) or 5 (strongly agree) out of 5 in short- and long-term satisfaction assessments (38/41 and 40/42, respectively).35 This assessment, conducted 3 months postoperatively, asked patients to score the statement, “I am completely satisfied with the treatment of my skin problem,” on a scale ranging from 1 (strongly disagree) to 5 (strongly agree).
Lastly, patient perception of their surgeon’s skill may contribute to levels of patient satisfaction. Although suture spacing has not been shown to affect surgical outcomes, it has been demonstrated to impact the patient’s perception of surgical skill and is further supported by a study concluding that closures with 2-mm spacing were ranked significantly lower by patients compared with closures with either 4- or 6-mm spacing (P=.005 and P=.012, respectively).36
Synthesis of this information with emphasis on the higher evidence-based studies—including systematic reviews, meta-analyses, and randomized controlled trials—yields the following beneficial interventions regarding anxiety-reducing measures and patient-perceived QOL21-36:
- Factors shown to decrease patient anxiety include patient personalized music, virtual-reality experience, perioperative informational videos, and 3-dimensional–printed MMS models.
- Many methods aimed at decreasing MMS-related patient anxiety show no direct impact on patient satisfaction, likely due to the multifactorial nature of the patient-perceived experience.
- Higher anxiety can be associated with worse QOL scores in MMS patients, and additional factors that may have a negative impact on anxiety include female sex, younger age, and tumor location on the face.
Conclusion
Many factors affect patient satisfaction in MMS. Increased awareness and acknowledgement of these factors can foster improved clinical practice and patient experience, which can have downstream effects on patient compliance and overall psychosocial and medical well-being. With the movement toward value-based health care, patient satisfaction ratings are likely to play an increasingly important role in physician reimbursement. Adapting one’s practice to include high-quality, time-efficient, patient-centered care goes hand in hand with increasing MMS patient satisfaction. Careful evaluation and scrutiny of one’s current practices while remaining cognizant of patient population, resource availability, and clinical limitations often reveal opportunities for small adjustments that can have a great impact on patient satisfaction. This thorough assessment and review of the published literature aims to assist MMS surgeons in understanding the role that certain factors—(1) perioperative patient communication and education techniques and (2) patient anxiety, QOL, and additional considerations—have on overall satisfaction with MMS. Specific consideration should be placed on the fact that patient satisfaction is multifactorial, and many different interventions can have a positive impact on the overall patient experience.
Mohs micrographic surgery (MMS)—developed by Dr. Frederic Mohs in the 1930s—is the gold standard for treating various cutaneous malignancies. It provides maximal conservation of uninvolved tissues while producing higher cure rates compared to wide local excision.1,2
We sought to assess the various characteristics that impact patient satisfaction to help Mohs surgeons incorporate relatively simple yet clinically significant practices into their patient encounters. We conducted a systematic literature search of peer-reviewed PubMed articles indexed for MEDLINE from database inception through November 2023 using the terms Mohs micrographic surgery and patient satisfaction. Among the inclusion criteria were studies involving participants having undergone MMS, with objective assessments on patient-reported satisfaction or preferences related to patient education, communication, anxiety-alleviating measures, or QOL in MMS. Studies were excluded if they failed to meet these criteria, were outdated and no longer clinically relevant, or measured unalterable factors with no significant impact on how Mohs surgeons could change clinical practice. Of the 157 nonreplicated studies identified, 34 met inclusion criteria.
Perioperative Patient Communication and Education Techniques
Perioperative Patient Communication—Many studies have evaluated the impact of perioperative patient-provider communication and education on patient satisfaction in those undergoing MMS. Studies focusing on preoperative and postoperative telephone calls, patient consultation formats, and patient-perceived impact of such communication modalities have been well documented (Table 1).3-8 The importance of the patient follow-up after MMS was further supported by a retrospective study concluding that 88.7% (86/97) of patients regarded follow-up visits as important, and 80% (77/97) desired additional follow-up 3 months after MMS.9 Additional studies have highlighted the importance of thorough and open perioperative patient-provider communication during MMS (Table 2).10-12


Patient-Education Techniques—Many studies have assessed the use of visual models to aid in patient education on MMS, specifically the preprocedural consent process (Table 3).13-16 Additionally, 2 randomized controlled trials assessing the use of at-home and same-day in-office preoperative educational videos concluded that these interventions increased patient knowledge and confidence regarding procedural risks and benefits, with no statistically significant differences in patient anxiety or satisfaction.17,18

Despite the availability of these educational videos, many patients often turn to online resources for self-education, which is problematic if reader literacy is incongruent with online readability. One study assessing readability of online MMS resources concluded that the most accessed articles exceeded the recommended reading level for adequate patient comprehension.19 A survey studying a wide range of variables related to patient satisfaction (eg, demographics, socioeconomics, health status) in 339 MMS patients found that those who considered themselves more involved in the decision-making process were more satisfied in the short-term, and married patients had even higher long-term satisfaction. Interestingly, this study also concluded that undergoing 3 or more MMS stages was associated with higher short- and long-term satisfaction, likely secondary to perceived effects of increased overall care, medical attention, and time spent with the provider.20
Synthesis of this information with emphasis on the higher evidence-based studies—including systematic reviews, meta-analyses, and randomized controlled trials—yields the following beneficial interventions regarding patient education and communication13-20:
- Preoperative and same-day postoperative telephone follow-up (TFU) do not show statistically significant impacts on patient satisfaction; however, TFU allows for identification of postoperative concerns and inadequate pain management, which may have downstream effects on long-term perception of the overall patient experience.
- The use of video-assisted consent yields improved patient satisfaction and knowledge, while video content—traditional or didactic—has no impact on satisfaction in new MMS patients.
- The use of at-home or same-day in-office preoperative educational videos can improve procedural knowledge and risk-benefit understanding of MMS while having no impact on satisfaction.
- Bedside manner and effective in-person communication by the provider often takes precedence in the patient experience; however, implementation of additional educational modalities should be considered.
Patient Anxiety and QOL
Reducing Patient Anxiety—The use of perioperative distractors to reduce patient anxiety may play an integral role when patients undergo MMS, as there often are prolonged waiting periods between stages when patients may feel increasingly vulnerable or anxious. Table 4 reviews studies on perioperative distractors that showed a statistically significant reduction in MMS patient anxiety.21-24

Although not statistically significant, additional studies evaluating the use of intraoperative anxiety-reduction methods in MMS have demonstrated a downtrend in patient anxiety with the following interventions: engaging in small talk with clinic staff, bringing a guest, eating, watching television, communicating surgical expectations with the provider, handholding, use of a stress ball, and use of 3-dimensional educational MMS models.25-27 Similarly, a survey of 73 patients undergoing MMS found that patients tended to enjoy complimentary beverages preprocedurally in the waiting room, reading, speaking with their guest, watching television, or using their telephone during wait times.28 Table 5 lists additional perioperative factors encompassing specific patient and surgical characteristics that help reduce patient anxiety.29-32

Patient QOL—Many methods aimed at decreasing MMS-related patient anxiety often show no direct impact on patient satisfaction, likely due to the multifactorial nature of the patient-perceived experience. A prospective observational study of MMS patients noted a statistically significant improvement in patient QOL scores 3 months postsurgery (P=.0007), demonstrating that MMS generally results in positive patient outcomes despite preprocedural anxiety.33 An additional prospective study in MMS patients with nonmelanoma skin cancer concluded that sex, age, and closure type—factors often shown to affect anxiety levels—did not significantly impact patient satisfaction.34 Similarly, high satisfaction levels can be expected among MMS patients undergoing treatment of melanoma in situ, with more than 90% of patients rating their treatment experience a 4 (agree) or 5 (strongly agree) out of 5 in short- and long-term satisfaction assessments (38/41 and 40/42, respectively).35 This assessment, conducted 3 months postoperatively, asked patients to score the statement, “I am completely satisfied with the treatment of my skin problem,” on a scale ranging from 1 (strongly disagree) to 5 (strongly agree).
Lastly, patient perception of their surgeon’s skill may contribute to levels of patient satisfaction. Although suture spacing has not been shown to affect surgical outcomes, it has been demonstrated to impact the patient’s perception of surgical skill and is further supported by a study concluding that closures with 2-mm spacing were ranked significantly lower by patients compared with closures with either 4- or 6-mm spacing (P=.005 and P=.012, respectively).36
Synthesis of this information with emphasis on the higher evidence-based studies—including systematic reviews, meta-analyses, and randomized controlled trials—yields the following beneficial interventions regarding anxiety-reducing measures and patient-perceived QOL21-36:
- Factors shown to decrease patient anxiety include patient personalized music, virtual-reality experience, perioperative informational videos, and 3-dimensional–printed MMS models.
- Many methods aimed at decreasing MMS-related patient anxiety show no direct impact on patient satisfaction, likely due to the multifactorial nature of the patient-perceived experience.
- Higher anxiety can be associated with worse QOL scores in MMS patients, and additional factors that may have a negative impact on anxiety include female sex, younger age, and tumor location on the face.
Conclusion
Many factors affect patient satisfaction in MMS. Increased awareness and acknowledgement of these factors can foster improved clinical practice and patient experience, which can have downstream effects on patient compliance and overall psychosocial and medical well-being. With the movement toward value-based health care, patient satisfaction ratings are likely to play an increasingly important role in physician reimbursement. Adapting one’s practice to include high-quality, time-efficient, patient-centered care goes hand in hand with increasing MMS patient satisfaction. Careful evaluation and scrutiny of one’s current practices while remaining cognizant of patient population, resource availability, and clinical limitations often reveal opportunities for small adjustments that can have a great impact on patient satisfaction. This thorough assessment and review of the published literature aims to assist MMS surgeons in understanding the role that certain factors—(1) perioperative patient communication and education techniques and (2) patient anxiety, QOL, and additional considerations—have on overall satisfaction with MMS. Specific consideration should be placed on the fact that patient satisfaction is multifactorial, and many different interventions can have a positive impact on the overall patient experience.
- Trost LB, Bailin PL. History of Mohs surgery. Dermatol Clin. 2011; 29:135-139, vii. doi:10.1016/j.det.2011.01.010
- Leslie DF, Greenway HT. Mohs micrographic surgery for skin cancer. Australas J Dermatol. 1991;32:159-164. doi:10.1111/j.1440 -0960.1991.tb01783.x
- Sobanko JF, Da Silva D, Chiesa Fuxench ZC, et al. Preoperative telephone consultation does not decrease patient anxiety before Mohs micrographic surgery. J Am Acad Dermatol. 2017;76:519-526. doi:10.1016/j.jaad.2016.09.027
- Sharon VR, Armstrong AW, Jim On SC, et al. Separate- versus same-day preoperative consultation in dermatologic surgery: a patient-centered investigation in an academic practice. Dermatol Surg. 2013;39:240-247. doi:10.1111/dsu.12083
- Knackstedt TJ, Samie FH. Shared medical appointments for the preoperative consultation visit of Mohs micrographic surgery. J Am Acad Dermatol. 2015;72:340-344. doi:10.1016/j.jaad.2014.10.022
- Vance S, Fontecilla N, Samie FH, et al. Effect of postoperative telephone calls on patient satisfaction and scar satisfaction after Mohs micrographic surgery. Dermatol Surg. 2019;45:1459-1464. doi:10.1097/DSS.0000000000001913
- Hafiji J, Salmon P, Hussain W. Patient satisfaction with post-operative telephone calls after Mohs micrographic surgery: a New Zealand and U.K. experience. Br J Dermatol. 2012;167:570-574. doi:10.1111 /j.1365-2133.2012.11011.x
- Bednarek R, Jonak C, Golda N. Optimal timing of postoperative patient telephone calls after Mohs micrographic surgery: a randomized controlled trial. J Am Acad Dermatol. 2021;85:220-221. doi:10.1016 /j.jaad.2020.07.106
- Sharon VR, Armstrong AW, Jim-On S, et al. Postoperative preferences in cutaneous surgery: a patient-centered investigation from an academic dermatologic surgery practice. Dermatol Surg. 2013;39:773-778. doi:10.1111/dsu.12136
- Xu S, Atanelov Z, Bhatia AC. Online patient-reported reviews of Mohs micrographic surgery: qualitative analysis of positive and negative experiences. Cutis. 2017;99:E25-E29.
- Golda N, Beeson S, Kohli N, et al. Recommendations for improving the patient experience in specialty encounters. J Am Acad Dermatol. 2018;78:653-659. doi:10.1016/j.jaad.2017.05.040
- Patel P, Malik K, Khachemoune A. Patient education in Mohs surgery: a review and critical evaluation of techniques. Arch Dermatol Res. 2021;313:217-224. doi:10.1007/s00403-020-02119-5
- Migden M, Chavez-Frazier A, Nguyen T. The use of high definition video modules for delivery of informed consent and wound care education in the Mohs surgery unit. Semin Cutan Med Surg. 2008;27:89-93. doi:10.1016/j.sder.2008.02.001
- Newsom E, Lee E, Rossi A, et al. Modernizing the Mohs surgery consultation: instituting a video module for improved patient education and satisfaction. Dermatol Surg. 2018;44:778-784. doi:10.1097/DSS.0000000000001473
- West L, Srivastava D, Goldberg LH, et al. Multimedia technology used to supplement patient consent for Mohs micrographic surgery. Dermatol Surg. 2020;46:586-590. doi:10.1097/DSS.0000000000002134
- Miao Y, Venning VL, Mallitt KA, et al. A randomized controlled trial comparing video-assisted informed consent with standard consent for Mohs micrographic surgery. JAAD Int. 2020;1:13-20. doi:10.1016 /j.jdin.2020.03.005
- Mann J, Li L, Kulakov E, et al. Home viewing of educational video improves patient understanding of Mohs micrographic surgery. Clin Exp Dermatol. 2022;47:93-97. doi:10.1111/ced.14845
- Delcambre M, Haynes D, Hajar T, et al. Using a multimedia tool for informed consent in Mohs surgery: a randomized trial measuring effects on patient anxiety, knowledge, and satisfaction. Dermatol Surg. 2020;46:591-598. doi:10.1097/DSS.0000000000002213
- Vargas CR, DePry J, Lee BT, et al. The readability of online patient information about Mohs micrographic surgery. Dermatol Surg. 2016;42:1135-1141. doi:10.1097/DSS.0000000000000866
- Asgari MM, Warton EM, Neugebauer R, et al. Predictors of patient satisfaction with Mohs surgery: analysis of preoperative, intraoperative, and postoperative factors in a prospective cohort. Arch Dermatol. 2011;147:1387-1394.
- Vachiramon V, Sobanko JF, Rattanaumpawan P, et al. Music reduces patient anxiety during Mohs surgery: an open-label randomized controlled trial. Dermatol Surg. 2013;39:298-305. doi:10.1111/dsu.12047
- Hawkins SD, Koch SB, Williford PM, et al. Web app- and text message-based patient education in Mohs micrographic surgery-a randomized controlled trial. Dermatol Surg. 2018;44:924-932. doi:10.1097/DSS.0000000000001489
- Higgins S, Feinstein S, Hawkins M, et al. Virtual reality to improve the experience of the Mohs patient-a prospective interventional study. Dermatol Surg. 2019;45:1009-1018. doi:10.1097 /DSS.0000000000001854
- Guo D, Zloty DM, Kossintseva I. Efficacy and safety of anxiolytics in Mohs micrographic surgery: a randomized, double-blinded, placebo-controlled trial. Dermatol Surg. 2023;49:989-994. doi:10.1097 /DSS.0000000000003905
- Locke MC, Wilkerson EC, Mistur RL, et al. 2015 Arte Poster Competition first place winner: assessing the correlation between patient anxiety and satisfaction for Mohs surgery. J Drugs Dermatol. 2015;14:1070-1072.
- Yanes AF, Weil A, Furlan KC, et al. Effect of stress ball use or hand-holding on anxiety during skin cancer excision: a randomized clinical trial. JAMA Dermatol. 2018;154:1045-1049. doi:10.1001 /jamadermatol.2018.1783
- Biro M, Kim I, Huynh A, et al. The use of 3-dimensionally printed models to optimize patient education and alleviate perioperative anxiety in Mohs micrographic surgery: a randomized controlled trial. J Am Acad Dermatol. 2019;81:1339-1345. doi:10.1016/j.jaad.2019.05.085
- Ali FR, Al-Niaimi F, Craythorne EE, et al. Patient satisfaction and the waiting room in Mohs surgery: appropriate prewarning may abrogate boredom. J Eur Acad Dermatol Venereol. 2017;31:e337-e338.
- Kossintseva I, Zloty D. Determinants and timeline of perioperative anxiety in Mohs surgery. Dermatol Surg. 2017;43:1029-1035.
- Kruchevsky D, Hirth J, Capucha T, et al. Triggers of preoperative anxiety in patients undergoing Mohs micrographic surgery. Dermatol Surg. 2021;47:1110-1112.
- Kokoska RE, Szeto MD, Steadman L, et al. Analysis of factors contributing to perioperative Mohs micrographic surgery anxiety: patient survey study at an academic center. Dermatol Surg. 2022;48:1279-1282.
- Long J, Rajabi-Estarabadi A, Levin A, et al. Perioperative anxiety associated with Mohs micrographic surgery: a survey-based study. Dermatol Surg. 2022;48:711-715.
- Zhang J, Miller CJ, O’Malley V, et al. Patient quality of life fluctuates before and after Mohs micrographic surgery: a longitudinal assessment of the patient experience. J Am Acad Dermatol. 2018;78:1060-1067.
- Lee EB, Ford A, Clarey D, et al. Patient outcomes and satisfaction after Mohs micrographic surgery in patients with nonmelanoma skin cancer. Dermatol Sur. 2021;47:1190-1194.
- Condie D, West L, Hynan LS, et al. Patient satisfaction with Mohs surgery for melanoma in situ. Dermatol Surg. 2021;47:288-290.
- Arshanapalli A, Tra n JM, Aylward JL, et al. The effect of suture spacing on patient perception of surgical skill. J Am Acad Dermatol. 2021;84:735-736.
- Trost LB, Bailin PL. History of Mohs surgery. Dermatol Clin. 2011; 29:135-139, vii. doi:10.1016/j.det.2011.01.010
- Leslie DF, Greenway HT. Mohs micrographic surgery for skin cancer. Australas J Dermatol. 1991;32:159-164. doi:10.1111/j.1440 -0960.1991.tb01783.x
- Sobanko JF, Da Silva D, Chiesa Fuxench ZC, et al. Preoperative telephone consultation does not decrease patient anxiety before Mohs micrographic surgery. J Am Acad Dermatol. 2017;76:519-526. doi:10.1016/j.jaad.2016.09.027
- Sharon VR, Armstrong AW, Jim On SC, et al. Separate- versus same-day preoperative consultation in dermatologic surgery: a patient-centered investigation in an academic practice. Dermatol Surg. 2013;39:240-247. doi:10.1111/dsu.12083
- Knackstedt TJ, Samie FH. Shared medical appointments for the preoperative consultation visit of Mohs micrographic surgery. J Am Acad Dermatol. 2015;72:340-344. doi:10.1016/j.jaad.2014.10.022
- Vance S, Fontecilla N, Samie FH, et al. Effect of postoperative telephone calls on patient satisfaction and scar satisfaction after Mohs micrographic surgery. Dermatol Surg. 2019;45:1459-1464. doi:10.1097/DSS.0000000000001913
- Hafiji J, Salmon P, Hussain W. Patient satisfaction with post-operative telephone calls after Mohs micrographic surgery: a New Zealand and U.K. experience. Br J Dermatol. 2012;167:570-574. doi:10.1111 /j.1365-2133.2012.11011.x
- Bednarek R, Jonak C, Golda N. Optimal timing of postoperative patient telephone calls after Mohs micrographic surgery: a randomized controlled trial. J Am Acad Dermatol. 2021;85:220-221. doi:10.1016 /j.jaad.2020.07.106
- Sharon VR, Armstrong AW, Jim-On S, et al. Postoperative preferences in cutaneous surgery: a patient-centered investigation from an academic dermatologic surgery practice. Dermatol Surg. 2013;39:773-778. doi:10.1111/dsu.12136
- Xu S, Atanelov Z, Bhatia AC. Online patient-reported reviews of Mohs micrographic surgery: qualitative analysis of positive and negative experiences. Cutis. 2017;99:E25-E29.
- Golda N, Beeson S, Kohli N, et al. Recommendations for improving the patient experience in specialty encounters. J Am Acad Dermatol. 2018;78:653-659. doi:10.1016/j.jaad.2017.05.040
- Patel P, Malik K, Khachemoune A. Patient education in Mohs surgery: a review and critical evaluation of techniques. Arch Dermatol Res. 2021;313:217-224. doi:10.1007/s00403-020-02119-5
- Migden M, Chavez-Frazier A, Nguyen T. The use of high definition video modules for delivery of informed consent and wound care education in the Mohs surgery unit. Semin Cutan Med Surg. 2008;27:89-93. doi:10.1016/j.sder.2008.02.001
- Newsom E, Lee E, Rossi A, et al. Modernizing the Mohs surgery consultation: instituting a video module for improved patient education and satisfaction. Dermatol Surg. 2018;44:778-784. doi:10.1097/DSS.0000000000001473
- West L, Srivastava D, Goldberg LH, et al. Multimedia technology used to supplement patient consent for Mohs micrographic surgery. Dermatol Surg. 2020;46:586-590. doi:10.1097/DSS.0000000000002134
- Miao Y, Venning VL, Mallitt KA, et al. A randomized controlled trial comparing video-assisted informed consent with standard consent for Mohs micrographic surgery. JAAD Int. 2020;1:13-20. doi:10.1016 /j.jdin.2020.03.005
- Mann J, Li L, Kulakov E, et al. Home viewing of educational video improves patient understanding of Mohs micrographic surgery. Clin Exp Dermatol. 2022;47:93-97. doi:10.1111/ced.14845
- Delcambre M, Haynes D, Hajar T, et al. Using a multimedia tool for informed consent in Mohs surgery: a randomized trial measuring effects on patient anxiety, knowledge, and satisfaction. Dermatol Surg. 2020;46:591-598. doi:10.1097/DSS.0000000000002213
- Vargas CR, DePry J, Lee BT, et al. The readability of online patient information about Mohs micrographic surgery. Dermatol Surg. 2016;42:1135-1141. doi:10.1097/DSS.0000000000000866
- Asgari MM, Warton EM, Neugebauer R, et al. Predictors of patient satisfaction with Mohs surgery: analysis of preoperative, intraoperative, and postoperative factors in a prospective cohort. Arch Dermatol. 2011;147:1387-1394.
- Vachiramon V, Sobanko JF, Rattanaumpawan P, et al. Music reduces patient anxiety during Mohs surgery: an open-label randomized controlled trial. Dermatol Surg. 2013;39:298-305. doi:10.1111/dsu.12047
- Hawkins SD, Koch SB, Williford PM, et al. Web app- and text message-based patient education in Mohs micrographic surgery-a randomized controlled trial. Dermatol Surg. 2018;44:924-932. doi:10.1097/DSS.0000000000001489
- Higgins S, Feinstein S, Hawkins M, et al. Virtual reality to improve the experience of the Mohs patient-a prospective interventional study. Dermatol Surg. 2019;45:1009-1018. doi:10.1097 /DSS.0000000000001854
- Guo D, Zloty DM, Kossintseva I. Efficacy and safety of anxiolytics in Mohs micrographic surgery: a randomized, double-blinded, placebo-controlled trial. Dermatol Surg. 2023;49:989-994. doi:10.1097 /DSS.0000000000003905
- Locke MC, Wilkerson EC, Mistur RL, et al. 2015 Arte Poster Competition first place winner: assessing the correlation between patient anxiety and satisfaction for Mohs surgery. J Drugs Dermatol. 2015;14:1070-1072.
- Yanes AF, Weil A, Furlan KC, et al. Effect of stress ball use or hand-holding on anxiety during skin cancer excision: a randomized clinical trial. JAMA Dermatol. 2018;154:1045-1049. doi:10.1001 /jamadermatol.2018.1783
- Biro M, Kim I, Huynh A, et al. The use of 3-dimensionally printed models to optimize patient education and alleviate perioperative anxiety in Mohs micrographic surgery: a randomized controlled trial. J Am Acad Dermatol. 2019;81:1339-1345. doi:10.1016/j.jaad.2019.05.085
- Ali FR, Al-Niaimi F, Craythorne EE, et al. Patient satisfaction and the waiting room in Mohs surgery: appropriate prewarning may abrogate boredom. J Eur Acad Dermatol Venereol. 2017;31:e337-e338.
- Kossintseva I, Zloty D. Determinants and timeline of perioperative anxiety in Mohs surgery. Dermatol Surg. 2017;43:1029-1035.
- Kruchevsky D, Hirth J, Capucha T, et al. Triggers of preoperative anxiety in patients undergoing Mohs micrographic surgery. Dermatol Surg. 2021;47:1110-1112.
- Kokoska RE, Szeto MD, Steadman L, et al. Analysis of factors contributing to perioperative Mohs micrographic surgery anxiety: patient survey study at an academic center. Dermatol Surg. 2022;48:1279-1282.
- Long J, Rajabi-Estarabadi A, Levin A, et al. Perioperative anxiety associated with Mohs micrographic surgery: a survey-based study. Dermatol Surg. 2022;48:711-715.
- Zhang J, Miller CJ, O’Malley V, et al. Patient quality of life fluctuates before and after Mohs micrographic surgery: a longitudinal assessment of the patient experience. J Am Acad Dermatol. 2018;78:1060-1067.
- Lee EB, Ford A, Clarey D, et al. Patient outcomes and satisfaction after Mohs micrographic surgery in patients with nonmelanoma skin cancer. Dermatol Sur. 2021;47:1190-1194.
- Condie D, West L, Hynan LS, et al. Patient satisfaction with Mohs surgery for melanoma in situ. Dermatol Surg. 2021;47:288-290.
- Arshanapalli A, Tra n JM, Aylward JL, et al. The effect of suture spacing on patient perception of surgical skill. J Am Acad Dermatol. 2021;84:735-736.
Enhancing Patient Satisfaction and Quality of Life With Mohs Micrographic Surgery: A Systematic Review of Patient Education, Communication, and Anxiety Management
Enhancing Patient Satisfaction and Quality of Life With Mohs Micrographic Surgery: A Systematic Review of Patient Education, Communication, and Anxiety Management
PRACTICE POINTS
- When patients are treated with Mohs micrographic surgery (MMS), thorough in-person dialogue augmented by pre- and same-day telephone follow-ups can help them feel heard and better supported, even though follow-up calls alone may not drive satisfaction scores.
- Increased awareness and implementation of the various factors influencing patient satisfaction and quality of life in MMS can enhance clinical practice and improve patient experiences, with potential impacts on compliance, psychosocial well-being, medical outcomes, and physician reimbursement.
- Patient satisfaction and procedural understanding can be improved with video and visual-based education. Anxiety-reducing methods help lower perioperative stress.