User login
Commentary: New Research on BC Chemotherapies, March 2024
The phase 3 KEYNOTE-355 trial established the role of chemotherapy in combination with pembrolizumab in the first-line setting for programmed death-ligand 1 (PD-L1)–positive advanced triple-negative breast cancer (TNBC). Patients unselected for PD-L1 status in this trial who received platinum- or taxane-based chemotherapy with placebo had a median progression-free survival of 5.6 months.[3] Strategies to improve upon efficacy and tolerability are desired in this space, and various trials have evaluated "switch maintenance" that involves receipt of an intensive induction regimen followed by a switch to an alternative/more tolerable regimen after response is achieved.[4] The phase II DORA trial randomized 45 patients with advanced TNBC and ongoing stable disease or complete or partial response from first- or second-line platinum-based chemotherapy to a maintenance regimen of olaparib (300 mg orally twice daily) with or without durvalumab (1500 mg on day 1 and every 4 weeks) (Tan et al). At a median follow-up of 9.8 months, median progression-free survival was 4.0 months (95% CI 2.6-6.1) with olaparib and 6.1 months (95% CI 3.7-10.1) with the combination; both were significantly longer than the historical control of continued platinum-based therapy (P = .0023 and P < .0001, respectively). Durable disease control appeared more pronounced in patients with complete or partial response to prior platinum therapy, and no new safety signals were observed. Future efforts to study this approach include the phase 2/3 KEYLYNK-009 trial, which is evaluating olaparib plus pembrolizumab maintenance therapy after first-line chemotherapy plus pembrolizumab for TNBC.[5]
TNBC is a heterogenous subtype, characterized by aggressive biology, and it benefits from chemotherapy and immunotherapy treatment approaches. Presently, the management of early-stage TNBC often involves neoadjuvant systemic therapy; however, a proportion of patients receive treatment in the postoperative setting, highlighting the relevance of time to initiation of adjuvant therapy as well.[6] Various prior studies have showed that delayed administration of adjuvant chemotherapy for EBC can lead to adverse survival outcomes. Furthermore, this effect is subtype-dependent, with more aggressive tumors (luminal B, triple-negative, human epidermal growth factor receptor 2 [HER2]-positive) exhibiting inferior outcomes with delayed chemotherapy.[7] A retrospective cohort study that included 245 patients with early TNBC who received adjuvant chemotherapy after surgery evaluated the impact of time to initiation of adjuvant therapy in this population (Hatzipanagiotou et al). Superior survival outcomes were observed for the group receiving systemic therapy 22-28 days after surgery (median overall survival 10.2 years) compared with those receiving adjuvant chemotherapy at later time points (29-35 days, 36-42 days, and >6 weeks after surgery; median overall survival 8.3 years, 7.8 years, and 6.9 years, respectively). Patients receiving chemotherapy 22-28 days after surgery had significantly better survival than those receiving chemotherapy 29-35 days (P = .043) and >6 weeks (P = 0.033) postoperatively. This study emphasizes the importance of timely administration of adjuvant chemotherapy for early TNBC, and efforts aimed to identify potential challenges and propose solutions to optimize outcomes in this space are valuable.
Additional References
- Gnant M, Frantal S, Pfeiler G, et al, for the Austrian Breast & Colorectal Cancer Study Group. Long-term outcomes of adjuvant denosumab in breast cancer. NEJM Evid. 2022;1:EVIDoa2200162. doi: 10.1056/EVIDoa2200162 Source
- Fassio A, Idolazzi L, Rossini M, et al. The obesity paradox and osteoporosis. Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity. 2018;23:293-30 doi: 10.1007/s40519-018-0505-2 Source
- Cortes J, Cescon DW, Rugo HS, et al, for the KEYNOTE-355 Investigators. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396:1817-1828. doi: 10.1016/S0140-6736(20)32531-9 Source
- Bachelot T, Filleron T, Bieche I, et al. Durvalumab compared to maintenance chemotherapy in metastatic breast cancer: The randomized phase II SAFIR02-BREAST IMMUNO trial. Nat Med. 2021;27:250-255. doi: 10.1038/s41591-020-01189-2 Source
- Saji S, Cussac AL, Andre F, et al. 68TiP KEYLYNK-009: a phase II/III, open-label, randomized study of pembrolizumab (pembro) + olaparib (ola) vs pembro + chemotherapy after induction with first-line (1L) pembro + chemo in patients (pts) with locally recurrent inoperable or metastatic TNBC (abstract). Ann Oncol. 2020;31(Suppl 6):S1268. doi: 10.1016/j.annonc.2020.10.088 Source
- Ortmann O, Blohmer JU, Sibert NT, et al for 55 breast cancer centers certified by the German Cancer Society. Current clinical practice and outcome of neoadjuvant chemotherapy for early breast cancer: Analysis of individual data from 94,638 patients treated in 55 breast cancer centers. J Cancer Res Clin Oncol. 2023;149:1195-1209. doi: 10.1007/s00432-022-03938-x Source
- Yu KD, Fan L, Qiu LX, et al. Influence of delayed initiation of adjuvant chemotherapy on breast cancer survival is subtype-dependent. Oncotarget. 2017;8:46549-46556. doi: 10.18632/oncotarget.10551 Source
The phase 3 KEYNOTE-355 trial established the role of chemotherapy in combination with pembrolizumab in the first-line setting for programmed death-ligand 1 (PD-L1)–positive advanced triple-negative breast cancer (TNBC). Patients unselected for PD-L1 status in this trial who received platinum- or taxane-based chemotherapy with placebo had a median progression-free survival of 5.6 months.[3] Strategies to improve upon efficacy and tolerability are desired in this space, and various trials have evaluated "switch maintenance" that involves receipt of an intensive induction regimen followed by a switch to an alternative/more tolerable regimen after response is achieved.[4] The phase II DORA trial randomized 45 patients with advanced TNBC and ongoing stable disease or complete or partial response from first- or second-line platinum-based chemotherapy to a maintenance regimen of olaparib (300 mg orally twice daily) with or without durvalumab (1500 mg on day 1 and every 4 weeks) (Tan et al). At a median follow-up of 9.8 months, median progression-free survival was 4.0 months (95% CI 2.6-6.1) with olaparib and 6.1 months (95% CI 3.7-10.1) with the combination; both were significantly longer than the historical control of continued platinum-based therapy (P = .0023 and P < .0001, respectively). Durable disease control appeared more pronounced in patients with complete or partial response to prior platinum therapy, and no new safety signals were observed. Future efforts to study this approach include the phase 2/3 KEYLYNK-009 trial, which is evaluating olaparib plus pembrolizumab maintenance therapy after first-line chemotherapy plus pembrolizumab for TNBC.[5]
TNBC is a heterogenous subtype, characterized by aggressive biology, and it benefits from chemotherapy and immunotherapy treatment approaches. Presently, the management of early-stage TNBC often involves neoadjuvant systemic therapy; however, a proportion of patients receive treatment in the postoperative setting, highlighting the relevance of time to initiation of adjuvant therapy as well.[6] Various prior studies have showed that delayed administration of adjuvant chemotherapy for EBC can lead to adverse survival outcomes. Furthermore, this effect is subtype-dependent, with more aggressive tumors (luminal B, triple-negative, human epidermal growth factor receptor 2 [HER2]-positive) exhibiting inferior outcomes with delayed chemotherapy.[7] A retrospective cohort study that included 245 patients with early TNBC who received adjuvant chemotherapy after surgery evaluated the impact of time to initiation of adjuvant therapy in this population (Hatzipanagiotou et al). Superior survival outcomes were observed for the group receiving systemic therapy 22-28 days after surgery (median overall survival 10.2 years) compared with those receiving adjuvant chemotherapy at later time points (29-35 days, 36-42 days, and >6 weeks after surgery; median overall survival 8.3 years, 7.8 years, and 6.9 years, respectively). Patients receiving chemotherapy 22-28 days after surgery had significantly better survival than those receiving chemotherapy 29-35 days (P = .043) and >6 weeks (P = 0.033) postoperatively. This study emphasizes the importance of timely administration of adjuvant chemotherapy for early TNBC, and efforts aimed to identify potential challenges and propose solutions to optimize outcomes in this space are valuable.
Additional References
- Gnant M, Frantal S, Pfeiler G, et al, for the Austrian Breast & Colorectal Cancer Study Group. Long-term outcomes of adjuvant denosumab in breast cancer. NEJM Evid. 2022;1:EVIDoa2200162. doi: 10.1056/EVIDoa2200162 Source
- Fassio A, Idolazzi L, Rossini M, et al. The obesity paradox and osteoporosis. Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity. 2018;23:293-30 doi: 10.1007/s40519-018-0505-2 Source
- Cortes J, Cescon DW, Rugo HS, et al, for the KEYNOTE-355 Investigators. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396:1817-1828. doi: 10.1016/S0140-6736(20)32531-9 Source
- Bachelot T, Filleron T, Bieche I, et al. Durvalumab compared to maintenance chemotherapy in metastatic breast cancer: The randomized phase II SAFIR02-BREAST IMMUNO trial. Nat Med. 2021;27:250-255. doi: 10.1038/s41591-020-01189-2 Source
- Saji S, Cussac AL, Andre F, et al. 68TiP KEYLYNK-009: a phase II/III, open-label, randomized study of pembrolizumab (pembro) + olaparib (ola) vs pembro + chemotherapy after induction with first-line (1L) pembro + chemo in patients (pts) with locally recurrent inoperable or metastatic TNBC (abstract). Ann Oncol. 2020;31(Suppl 6):S1268. doi: 10.1016/j.annonc.2020.10.088 Source
- Ortmann O, Blohmer JU, Sibert NT, et al for 55 breast cancer centers certified by the German Cancer Society. Current clinical practice and outcome of neoadjuvant chemotherapy for early breast cancer: Analysis of individual data from 94,638 patients treated in 55 breast cancer centers. J Cancer Res Clin Oncol. 2023;149:1195-1209. doi: 10.1007/s00432-022-03938-x Source
- Yu KD, Fan L, Qiu LX, et al. Influence of delayed initiation of adjuvant chemotherapy on breast cancer survival is subtype-dependent. Oncotarget. 2017;8:46549-46556. doi: 10.18632/oncotarget.10551 Source
The phase 3 KEYNOTE-355 trial established the role of chemotherapy in combination with pembrolizumab in the first-line setting for programmed death-ligand 1 (PD-L1)–positive advanced triple-negative breast cancer (TNBC). Patients unselected for PD-L1 status in this trial who received platinum- or taxane-based chemotherapy with placebo had a median progression-free survival of 5.6 months.[3] Strategies to improve upon efficacy and tolerability are desired in this space, and various trials have evaluated "switch maintenance" that involves receipt of an intensive induction regimen followed by a switch to an alternative/more tolerable regimen after response is achieved.[4] The phase II DORA trial randomized 45 patients with advanced TNBC and ongoing stable disease or complete or partial response from first- or second-line platinum-based chemotherapy to a maintenance regimen of olaparib (300 mg orally twice daily) with or without durvalumab (1500 mg on day 1 and every 4 weeks) (Tan et al). At a median follow-up of 9.8 months, median progression-free survival was 4.0 months (95% CI 2.6-6.1) with olaparib and 6.1 months (95% CI 3.7-10.1) with the combination; both were significantly longer than the historical control of continued platinum-based therapy (P = .0023 and P < .0001, respectively). Durable disease control appeared more pronounced in patients with complete or partial response to prior platinum therapy, and no new safety signals were observed. Future efforts to study this approach include the phase 2/3 KEYLYNK-009 trial, which is evaluating olaparib plus pembrolizumab maintenance therapy after first-line chemotherapy plus pembrolizumab for TNBC.[5]
TNBC is a heterogenous subtype, characterized by aggressive biology, and it benefits from chemotherapy and immunotherapy treatment approaches. Presently, the management of early-stage TNBC often involves neoadjuvant systemic therapy; however, a proportion of patients receive treatment in the postoperative setting, highlighting the relevance of time to initiation of adjuvant therapy as well.[6] Various prior studies have showed that delayed administration of adjuvant chemotherapy for EBC can lead to adverse survival outcomes. Furthermore, this effect is subtype-dependent, with more aggressive tumors (luminal B, triple-negative, human epidermal growth factor receptor 2 [HER2]-positive) exhibiting inferior outcomes with delayed chemotherapy.[7] A retrospective cohort study that included 245 patients with early TNBC who received adjuvant chemotherapy after surgery evaluated the impact of time to initiation of adjuvant therapy in this population (Hatzipanagiotou et al). Superior survival outcomes were observed for the group receiving systemic therapy 22-28 days after surgery (median overall survival 10.2 years) compared with those receiving adjuvant chemotherapy at later time points (29-35 days, 36-42 days, and >6 weeks after surgery; median overall survival 8.3 years, 7.8 years, and 6.9 years, respectively). Patients receiving chemotherapy 22-28 days after surgery had significantly better survival than those receiving chemotherapy 29-35 days (P = .043) and >6 weeks (P = 0.033) postoperatively. This study emphasizes the importance of timely administration of adjuvant chemotherapy for early TNBC, and efforts aimed to identify potential challenges and propose solutions to optimize outcomes in this space are valuable.
Additional References
- Gnant M, Frantal S, Pfeiler G, et al, for the Austrian Breast & Colorectal Cancer Study Group. Long-term outcomes of adjuvant denosumab in breast cancer. NEJM Evid. 2022;1:EVIDoa2200162. doi: 10.1056/EVIDoa2200162 Source
- Fassio A, Idolazzi L, Rossini M, et al. The obesity paradox and osteoporosis. Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity. 2018;23:293-30 doi: 10.1007/s40519-018-0505-2 Source
- Cortes J, Cescon DW, Rugo HS, et al, for the KEYNOTE-355 Investigators. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396:1817-1828. doi: 10.1016/S0140-6736(20)32531-9 Source
- Bachelot T, Filleron T, Bieche I, et al. Durvalumab compared to maintenance chemotherapy in metastatic breast cancer: The randomized phase II SAFIR02-BREAST IMMUNO trial. Nat Med. 2021;27:250-255. doi: 10.1038/s41591-020-01189-2 Source
- Saji S, Cussac AL, Andre F, et al. 68TiP KEYLYNK-009: a phase II/III, open-label, randomized study of pembrolizumab (pembro) + olaparib (ola) vs pembro + chemotherapy after induction with first-line (1L) pembro + chemo in patients (pts) with locally recurrent inoperable or metastatic TNBC (abstract). Ann Oncol. 2020;31(Suppl 6):S1268. doi: 10.1016/j.annonc.2020.10.088 Source
- Ortmann O, Blohmer JU, Sibert NT, et al for 55 breast cancer centers certified by the German Cancer Society. Current clinical practice and outcome of neoadjuvant chemotherapy for early breast cancer: Analysis of individual data from 94,638 patients treated in 55 breast cancer centers. J Cancer Res Clin Oncol. 2023;149:1195-1209. doi: 10.1007/s00432-022-03938-x Source
- Yu KD, Fan L, Qiu LX, et al. Influence of delayed initiation of adjuvant chemotherapy on breast cancer survival is subtype-dependent. Oncotarget. 2017;8:46549-46556. doi: 10.18632/oncotarget.10551 Source
Commentary: Medication Timing and Other Dupilumab Concerns, March 2024
When skin diseases affect the palm or sole, they can have a disproportionately large negative effect on patients' lives. Hand and foot dermatitis can be disabling. Simpson and colleagues find that dupilumab is an effective treatment for AD of the hands and feet. Having safe and effective treatment for hand and foot dermatitis will be life-changing for many of our patients.
Patients often do very well with biologic treatment. When they do, they often wonder, Do I need to continue taking the medication? Lasheras-Pérez and colleagues found that the great majority of patients doing well taking dupilumab for AD could stretch out their dosing interval. I suspect a lot of our patients are doing this already. I used to worry that stretching out the dosing interval might lead to antidrug antibodies and loss of activity. Such loss of activity doesn't appear common. Because we also have multiple alternative treatments for severe AD, I think it may be quite reasonable for patients to try spreading out their doses after their disease has been well controlled for a good long time.
Superficial skin infections aren't rare in children, particularly children with AD. Paller and colleagues' study is informative about the safety of dupilumab in children. The drug, which blocks a pathway of the immune system, was associated with fewer infections. This is good news. The reduction in infections could be through restoring "immune balance" (whatever that means) or by improving skin barrier function. Perhaps the low rate of infection explains why dupilumab is not considered immunosuppressive.
I love studies of drug survival because I think that knowing the percentage of patients who stay with drug treatment is a good measure of overall safety and efficacy. Pezzolo and colleagues found — perhaps not surprisingly given the extraordinary efficacy of upadacitinib for AD — that almost no one discontinued the drug over 1.5 years due to lack of efficacy. There were patients who discontinued due to adverse events (and additional patients lost to follow-up who perhaps also discontinued the drug), but 80% of patients were still in the study at the end of 1.5 years. Three patients who weren't vaccinated for shingles developed shingles; encouraging patients to get the shingles vaccine may be a prudent measure when starting patients taking upadacitinib.
When skin diseases affect the palm or sole, they can have a disproportionately large negative effect on patients' lives. Hand and foot dermatitis can be disabling. Simpson and colleagues find that dupilumab is an effective treatment for AD of the hands and feet. Having safe and effective treatment for hand and foot dermatitis will be life-changing for many of our patients.
Patients often do very well with biologic treatment. When they do, they often wonder, Do I need to continue taking the medication? Lasheras-Pérez and colleagues found that the great majority of patients doing well taking dupilumab for AD could stretch out their dosing interval. I suspect a lot of our patients are doing this already. I used to worry that stretching out the dosing interval might lead to antidrug antibodies and loss of activity. Such loss of activity doesn't appear common. Because we also have multiple alternative treatments for severe AD, I think it may be quite reasonable for patients to try spreading out their doses after their disease has been well controlled for a good long time.
Superficial skin infections aren't rare in children, particularly children with AD. Paller and colleagues' study is informative about the safety of dupilumab in children. The drug, which blocks a pathway of the immune system, was associated with fewer infections. This is good news. The reduction in infections could be through restoring "immune balance" (whatever that means) or by improving skin barrier function. Perhaps the low rate of infection explains why dupilumab is not considered immunosuppressive.
I love studies of drug survival because I think that knowing the percentage of patients who stay with drug treatment is a good measure of overall safety and efficacy. Pezzolo and colleagues found — perhaps not surprisingly given the extraordinary efficacy of upadacitinib for AD — that almost no one discontinued the drug over 1.5 years due to lack of efficacy. There were patients who discontinued due to adverse events (and additional patients lost to follow-up who perhaps also discontinued the drug), but 80% of patients were still in the study at the end of 1.5 years. Three patients who weren't vaccinated for shingles developed shingles; encouraging patients to get the shingles vaccine may be a prudent measure when starting patients taking upadacitinib.
When skin diseases affect the palm or sole, they can have a disproportionately large negative effect on patients' lives. Hand and foot dermatitis can be disabling. Simpson and colleagues find that dupilumab is an effective treatment for AD of the hands and feet. Having safe and effective treatment for hand and foot dermatitis will be life-changing for many of our patients.
Patients often do very well with biologic treatment. When they do, they often wonder, Do I need to continue taking the medication? Lasheras-Pérez and colleagues found that the great majority of patients doing well taking dupilumab for AD could stretch out their dosing interval. I suspect a lot of our patients are doing this already. I used to worry that stretching out the dosing interval might lead to antidrug antibodies and loss of activity. Such loss of activity doesn't appear common. Because we also have multiple alternative treatments for severe AD, I think it may be quite reasonable for patients to try spreading out their doses after their disease has been well controlled for a good long time.
Superficial skin infections aren't rare in children, particularly children with AD. Paller and colleagues' study is informative about the safety of dupilumab in children. The drug, which blocks a pathway of the immune system, was associated with fewer infections. This is good news. The reduction in infections could be through restoring "immune balance" (whatever that means) or by improving skin barrier function. Perhaps the low rate of infection explains why dupilumab is not considered immunosuppressive.
I love studies of drug survival because I think that knowing the percentage of patients who stay with drug treatment is a good measure of overall safety and efficacy. Pezzolo and colleagues found — perhaps not surprisingly given the extraordinary efficacy of upadacitinib for AD — that almost no one discontinued the drug over 1.5 years due to lack of efficacy. There were patients who discontinued due to adverse events (and additional patients lost to follow-up who perhaps also discontinued the drug), but 80% of patients were still in the study at the end of 1.5 years. Three patients who weren't vaccinated for shingles developed shingles; encouraging patients to get the shingles vaccine may be a prudent measure when starting patients taking upadacitinib.
Improved Communication Center Stage in Multiple Sclerosis
This transcript has been edited for clarity.
Joseph R. Berger, MD: Hi. I'm Dr Joseph Berger, and I'm joined for this Care Cues conversation with my patient, Michelle Biloon, who has had multiple sclerosis (MS) for the past 6 years. Hello, Michelle. Welcome.
Michelle Biloon: Thank you, Dr Berger.
Berger: Can you tell us a little bit about yourself, how you came to understand you had MS, and how you've done since the diagnosis was rendered?
Biloon: Yeah. It was a very short diagnosis period for me. In the winter of 2017, I started experiencing dizzy spells, and I didn't really know why. I eventually went to my primary care clinic where my doctor is, and they did blood work. Then, they did a CT and didn't see anything, and I just kind of kept feeling worse.
Then, finally, I went to an ENT just to see if it was maybe related to my ears. The ENT actually said, "You need to go to the ER and get an MRI." And while I was in the MRI, I could feel the dizzy spells. And I thought, Well, something is happening. I don't know what it is. And then a resident came in and said that they saw lesions on my brain, and they knew that it was going to be MS or something like it.
Berger: How did you feel about that?
Biloon: At the time, I was kind of glad to hear it was something. And I just asked her if, like, you die from it. That was the first thing I asked. It was like falling off a cliff.
It was making it hard for me to function in what I was doing, which was stand-up comedy, because of the cognitive issues I was having, the cognitive fog. That was how I ended up with you. Right away, you talked to me and were actually able to introduce to me some new medications that are out and are phenomenally better for MS plus were not pills or shots every day. It's made my MS over the years a lot more manageable.
Berger: I'd like to pick up on a couple of things you said.
Biloon: Sure.
Berger: One is, because most people envision MS as this terrible, crippling illness that's going to leave them wheelchair-bound, deprived of their profession, finding it difficult to stay in a marriage it's vested with what has been termed "lamentable results." And one of the first things that we as physicians have to do is to calm people down and say, "You know what. You have MS. You're going to be just fine. Trust me. We have wonderful medications for what you have, and we'll take care of it." In fact, I've made a habit of telling people quit worrying. You hired me to worry for you.
Biloon: Yep.
Berger: And I think that's helpful.
Biloon: I've been just so appreciative of that. There's a balance of being condescended to — do you know what I mean — and also being given information. I'm very sensitive to that balance because I consider myself an intelligent person. And you're being put in a position where someone knows more than you, and you have to listen.
Berger: One of the other challenges we face is getting somebody on a treatment. And we elected to put you on an intravenous therapy every 6 months.
Biloon: Especially because as a stand-up comedian, I was traveling a lot, doing these every-6-months infusion, especially with the high efficacy rate that it had been reported from what we had read and the low amount of side effects. I mean, just those things together was just something that seemed the easiest for me.
Berger: So did you encounter any challenges when we first got you started on the infusion therapy?
Biloon: The first infusion I got was at the hospital. But then after that, I had to go to the suburbs, to a center out there for the infusion. That was difficult because to get a ride out there and a ride back — it was a long trip for someone to wait with me. Taking an Uber is expensive, so was it for me to drive. You don't feel good for a couple of days after. So that was how it was, and I complained about it. Probably at every appointment we had, I complained about it.
Berger: Yeah. So some of the challenges you talked about are very, very common. As a physician on medications myself, I can tell you that I am not particularly compliant. And what I love about infusion therapies is that I know that the patient is getting their medicine. Because when they don't show up for a scheduled appointment, I'm called, and I know.
Biloon: I do have a bit of an allergic reaction to the drug. But that's been easily managed over time. Now, the drug infusions are actually being done at my home, which makes the whole process twice-a-year–world's better.
Berger: But there are other barriers that people confront other than the initiation of drugs. Had you encountered any?
Biloon: I think the problem that I had more so was finding the drugs that would manage some of my symptoms. It took a couple of years to sort of figure out what that would be, both with figuring them out and both dealing with insurance on certain medications.
Berger: That's one sort of problem that we confront. The other, of course, are those individuals who, for a variety of reasons, have difficulty with the diagnosis because of their backgrounds. And they may be sociocultural in nature. Every time you go to the physical therapist, it's some degree of money.
Now for some people, it's trivial. But for others, it's a considerable amount of money, relative to what it is that they earn. And you simply have to work within those confines as best you can.
We do have various programs that help people. So we try to employ them. There are, in addition to the sociocultural barriers, language barriers that we often confront. We, in our situation here in a large city, have a very large migrant population.
Fortunately, most of the people speak languages that either you speak as well, or there's somebody in the next room that speaks pretty well. But that's not always the case. So we do have an interpreter service that has to be employed.
Biloon: I cannot imagine the nuance in speaking to people from different ages and different backgrounds, who have different types of lifestyles, for them to understand.
Berger: I don't write at a computer. I think that really degrades the patient-physician relationship. What I do is I obtain a history. I do it on a piece of paper with a pen or a pencil.
I recapitulate them to the patient in paraphrasing it, to make sure that I have gotten it right and that they understand what I think I heard. That, I think, has been enormously helpful in helping people understand what may happen in the absence of treatment and why the treatment is important. That you can do, regardless of what the person's background is. So that's how I approach it.
Biloon: How do you deal with patients when they're not on the same page with you?
Berger: One important thing is that you have to be patient. That is something that it took me 50 years in medicine to learn. And then accepting the patient's opinion and saying, "All right, go home and think about it," because you often don't convince them when they're in the office with you.
Biloon: I did have a little bit of a cushion between my diagnosis and when we actually saw each other, where I was able to really sit in my thoughts on the different treatments and stuff. By the time that we were able to talk, it reassured me on that was the right plan.
Berger: I'm curious what your experience has been with our MS center.
Biloon: Through the portal, every time I need something, I'm usually reaching out, keeping you up-to-date on my primary care or whether it's trying to get a refill on one of my medications that I have to reach out. I really do feel that having that team there, being able to reach out, that's been extremely helpful to have and keeps me very secure because that's all I really need, especially during the pandemic, right? Because then I was very isolated and dealing with going through MS. So it was great to at least — and I did — shoot off emails or texts in the portal, and that's usually primarily how I communicated.
Berger: I will tell you, in my opinion, maybe nine out of 10 messages in the portal or calls that we get simply require reassurance.
Biloon: Yes.
Berger: You just either pick up the phone or shoot back a note, say, "This is not your MS. Don't worry about it." I mean, the most important thing for me is to keep people from worrying because that doesn't solve any problem.
Biloon: No, and it causes stress, which causes fatigue. I mean, it's a bad cycle.
Berger: In the past year, you've actually felt better, and you've gone back to performing. It sounds like the volume of performances has gotten back to what it was pre-illness. What do you see for the future?
Biloon: What I see is traveling more for stand-up and doing the sort of clubs and cities that I had kind of stopped doing from before I was diagnosed, so 2017 and prior to that. And then also even working on other things, writing and maybe even doing sort of books or one-person shows that even talk about sort of my struggles with MS and kind of coming back to where I am. I'm looking forward to the future, and I hope that that's the track I can keep going on.
Berger: I see no reason why you shouldn't.
Biloon: Thank you.
Berger: Michelle, thank you very much for joining me today in this conversation.
Biloon: Thank you so much for having me. It's been really wonderful to be able to sit down here with you.
Joseph R. Berger, MD, has disclosed the following relevant financial relationships: Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Celgene/Bristol-Myers Squibb; Cellevolve; EMD Serono/Merck/Genentech; Genzyme; Janssen/Johnson & Johnson; Morphic; Novartis; Roche; Sanofi; Takeda; TG Therapeutics; MAPI; Excision Bio
Received research grant from: Genentech/Roche
Michelle Biloon has disclosed no relevant financial relationships
This transcript has been edited for clarity.
Joseph R. Berger, MD: Hi. I'm Dr Joseph Berger, and I'm joined for this Care Cues conversation with my patient, Michelle Biloon, who has had multiple sclerosis (MS) for the past 6 years. Hello, Michelle. Welcome.
Michelle Biloon: Thank you, Dr Berger.
Berger: Can you tell us a little bit about yourself, how you came to understand you had MS, and how you've done since the diagnosis was rendered?
Biloon: Yeah. It was a very short diagnosis period for me. In the winter of 2017, I started experiencing dizzy spells, and I didn't really know why. I eventually went to my primary care clinic where my doctor is, and they did blood work. Then, they did a CT and didn't see anything, and I just kind of kept feeling worse.
Then, finally, I went to an ENT just to see if it was maybe related to my ears. The ENT actually said, "You need to go to the ER and get an MRI." And while I was in the MRI, I could feel the dizzy spells. And I thought, Well, something is happening. I don't know what it is. And then a resident came in and said that they saw lesions on my brain, and they knew that it was going to be MS or something like it.
Berger: How did you feel about that?
Biloon: At the time, I was kind of glad to hear it was something. And I just asked her if, like, you die from it. That was the first thing I asked. It was like falling off a cliff.
It was making it hard for me to function in what I was doing, which was stand-up comedy, because of the cognitive issues I was having, the cognitive fog. That was how I ended up with you. Right away, you talked to me and were actually able to introduce to me some new medications that are out and are phenomenally better for MS plus were not pills or shots every day. It's made my MS over the years a lot more manageable.
Berger: I'd like to pick up on a couple of things you said.
Biloon: Sure.
Berger: One is, because most people envision MS as this terrible, crippling illness that's going to leave them wheelchair-bound, deprived of their profession, finding it difficult to stay in a marriage it's vested with what has been termed "lamentable results." And one of the first things that we as physicians have to do is to calm people down and say, "You know what. You have MS. You're going to be just fine. Trust me. We have wonderful medications for what you have, and we'll take care of it." In fact, I've made a habit of telling people quit worrying. You hired me to worry for you.
Biloon: Yep.
Berger: And I think that's helpful.
Biloon: I've been just so appreciative of that. There's a balance of being condescended to — do you know what I mean — and also being given information. I'm very sensitive to that balance because I consider myself an intelligent person. And you're being put in a position where someone knows more than you, and you have to listen.
Berger: One of the other challenges we face is getting somebody on a treatment. And we elected to put you on an intravenous therapy every 6 months.
Biloon: Especially because as a stand-up comedian, I was traveling a lot, doing these every-6-months infusion, especially with the high efficacy rate that it had been reported from what we had read and the low amount of side effects. I mean, just those things together was just something that seemed the easiest for me.
Berger: So did you encounter any challenges when we first got you started on the infusion therapy?
Biloon: The first infusion I got was at the hospital. But then after that, I had to go to the suburbs, to a center out there for the infusion. That was difficult because to get a ride out there and a ride back — it was a long trip for someone to wait with me. Taking an Uber is expensive, so was it for me to drive. You don't feel good for a couple of days after. So that was how it was, and I complained about it. Probably at every appointment we had, I complained about it.
Berger: Yeah. So some of the challenges you talked about are very, very common. As a physician on medications myself, I can tell you that I am not particularly compliant. And what I love about infusion therapies is that I know that the patient is getting their medicine. Because when they don't show up for a scheduled appointment, I'm called, and I know.
Biloon: I do have a bit of an allergic reaction to the drug. But that's been easily managed over time. Now, the drug infusions are actually being done at my home, which makes the whole process twice-a-year–world's better.
Berger: But there are other barriers that people confront other than the initiation of drugs. Had you encountered any?
Biloon: I think the problem that I had more so was finding the drugs that would manage some of my symptoms. It took a couple of years to sort of figure out what that would be, both with figuring them out and both dealing with insurance on certain medications.
Berger: That's one sort of problem that we confront. The other, of course, are those individuals who, for a variety of reasons, have difficulty with the diagnosis because of their backgrounds. And they may be sociocultural in nature. Every time you go to the physical therapist, it's some degree of money.
Now for some people, it's trivial. But for others, it's a considerable amount of money, relative to what it is that they earn. And you simply have to work within those confines as best you can.
We do have various programs that help people. So we try to employ them. There are, in addition to the sociocultural barriers, language barriers that we often confront. We, in our situation here in a large city, have a very large migrant population.
Fortunately, most of the people speak languages that either you speak as well, or there's somebody in the next room that speaks pretty well. But that's not always the case. So we do have an interpreter service that has to be employed.
Biloon: I cannot imagine the nuance in speaking to people from different ages and different backgrounds, who have different types of lifestyles, for them to understand.
Berger: I don't write at a computer. I think that really degrades the patient-physician relationship. What I do is I obtain a history. I do it on a piece of paper with a pen or a pencil.
I recapitulate them to the patient in paraphrasing it, to make sure that I have gotten it right and that they understand what I think I heard. That, I think, has been enormously helpful in helping people understand what may happen in the absence of treatment and why the treatment is important. That you can do, regardless of what the person's background is. So that's how I approach it.
Biloon: How do you deal with patients when they're not on the same page with you?
Berger: One important thing is that you have to be patient. That is something that it took me 50 years in medicine to learn. And then accepting the patient's opinion and saying, "All right, go home and think about it," because you often don't convince them when they're in the office with you.
Biloon: I did have a little bit of a cushion between my diagnosis and when we actually saw each other, where I was able to really sit in my thoughts on the different treatments and stuff. By the time that we were able to talk, it reassured me on that was the right plan.
Berger: I'm curious what your experience has been with our MS center.
Biloon: Through the portal, every time I need something, I'm usually reaching out, keeping you up-to-date on my primary care or whether it's trying to get a refill on one of my medications that I have to reach out. I really do feel that having that team there, being able to reach out, that's been extremely helpful to have and keeps me very secure because that's all I really need, especially during the pandemic, right? Because then I was very isolated and dealing with going through MS. So it was great to at least — and I did — shoot off emails or texts in the portal, and that's usually primarily how I communicated.
Berger: I will tell you, in my opinion, maybe nine out of 10 messages in the portal or calls that we get simply require reassurance.
Biloon: Yes.
Berger: You just either pick up the phone or shoot back a note, say, "This is not your MS. Don't worry about it." I mean, the most important thing for me is to keep people from worrying because that doesn't solve any problem.
Biloon: No, and it causes stress, which causes fatigue. I mean, it's a bad cycle.
Berger: In the past year, you've actually felt better, and you've gone back to performing. It sounds like the volume of performances has gotten back to what it was pre-illness. What do you see for the future?
Biloon: What I see is traveling more for stand-up and doing the sort of clubs and cities that I had kind of stopped doing from before I was diagnosed, so 2017 and prior to that. And then also even working on other things, writing and maybe even doing sort of books or one-person shows that even talk about sort of my struggles with MS and kind of coming back to where I am. I'm looking forward to the future, and I hope that that's the track I can keep going on.
Berger: I see no reason why you shouldn't.
Biloon: Thank you.
Berger: Michelle, thank you very much for joining me today in this conversation.
Biloon: Thank you so much for having me. It's been really wonderful to be able to sit down here with you.
Joseph R. Berger, MD, has disclosed the following relevant financial relationships: Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Celgene/Bristol-Myers Squibb; Cellevolve; EMD Serono/Merck/Genentech; Genzyme; Janssen/Johnson & Johnson; Morphic; Novartis; Roche; Sanofi; Takeda; TG Therapeutics; MAPI; Excision Bio
Received research grant from: Genentech/Roche
Michelle Biloon has disclosed no relevant financial relationships
This transcript has been edited for clarity.
Joseph R. Berger, MD: Hi. I'm Dr Joseph Berger, and I'm joined for this Care Cues conversation with my patient, Michelle Biloon, who has had multiple sclerosis (MS) for the past 6 years. Hello, Michelle. Welcome.
Michelle Biloon: Thank you, Dr Berger.
Berger: Can you tell us a little bit about yourself, how you came to understand you had MS, and how you've done since the diagnosis was rendered?
Biloon: Yeah. It was a very short diagnosis period for me. In the winter of 2017, I started experiencing dizzy spells, and I didn't really know why. I eventually went to my primary care clinic where my doctor is, and they did blood work. Then, they did a CT and didn't see anything, and I just kind of kept feeling worse.
Then, finally, I went to an ENT just to see if it was maybe related to my ears. The ENT actually said, "You need to go to the ER and get an MRI." And while I was in the MRI, I could feel the dizzy spells. And I thought, Well, something is happening. I don't know what it is. And then a resident came in and said that they saw lesions on my brain, and they knew that it was going to be MS or something like it.
Berger: How did you feel about that?
Biloon: At the time, I was kind of glad to hear it was something. And I just asked her if, like, you die from it. That was the first thing I asked. It was like falling off a cliff.
It was making it hard for me to function in what I was doing, which was stand-up comedy, because of the cognitive issues I was having, the cognitive fog. That was how I ended up with you. Right away, you talked to me and were actually able to introduce to me some new medications that are out and are phenomenally better for MS plus were not pills or shots every day. It's made my MS over the years a lot more manageable.
Berger: I'd like to pick up on a couple of things you said.
Biloon: Sure.
Berger: One is, because most people envision MS as this terrible, crippling illness that's going to leave them wheelchair-bound, deprived of their profession, finding it difficult to stay in a marriage it's vested with what has been termed "lamentable results." And one of the first things that we as physicians have to do is to calm people down and say, "You know what. You have MS. You're going to be just fine. Trust me. We have wonderful medications for what you have, and we'll take care of it." In fact, I've made a habit of telling people quit worrying. You hired me to worry for you.
Biloon: Yep.
Berger: And I think that's helpful.
Biloon: I've been just so appreciative of that. There's a balance of being condescended to — do you know what I mean — and also being given information. I'm very sensitive to that balance because I consider myself an intelligent person. And you're being put in a position where someone knows more than you, and you have to listen.
Berger: One of the other challenges we face is getting somebody on a treatment. And we elected to put you on an intravenous therapy every 6 months.
Biloon: Especially because as a stand-up comedian, I was traveling a lot, doing these every-6-months infusion, especially with the high efficacy rate that it had been reported from what we had read and the low amount of side effects. I mean, just those things together was just something that seemed the easiest for me.
Berger: So did you encounter any challenges when we first got you started on the infusion therapy?
Biloon: The first infusion I got was at the hospital. But then after that, I had to go to the suburbs, to a center out there for the infusion. That was difficult because to get a ride out there and a ride back — it was a long trip for someone to wait with me. Taking an Uber is expensive, so was it for me to drive. You don't feel good for a couple of days after. So that was how it was, and I complained about it. Probably at every appointment we had, I complained about it.
Berger: Yeah. So some of the challenges you talked about are very, very common. As a physician on medications myself, I can tell you that I am not particularly compliant. And what I love about infusion therapies is that I know that the patient is getting their medicine. Because when they don't show up for a scheduled appointment, I'm called, and I know.
Biloon: I do have a bit of an allergic reaction to the drug. But that's been easily managed over time. Now, the drug infusions are actually being done at my home, which makes the whole process twice-a-year–world's better.
Berger: But there are other barriers that people confront other than the initiation of drugs. Had you encountered any?
Biloon: I think the problem that I had more so was finding the drugs that would manage some of my symptoms. It took a couple of years to sort of figure out what that would be, both with figuring them out and both dealing with insurance on certain medications.
Berger: That's one sort of problem that we confront. The other, of course, are those individuals who, for a variety of reasons, have difficulty with the diagnosis because of their backgrounds. And they may be sociocultural in nature. Every time you go to the physical therapist, it's some degree of money.
Now for some people, it's trivial. But for others, it's a considerable amount of money, relative to what it is that they earn. And you simply have to work within those confines as best you can.
We do have various programs that help people. So we try to employ them. There are, in addition to the sociocultural barriers, language barriers that we often confront. We, in our situation here in a large city, have a very large migrant population.
Fortunately, most of the people speak languages that either you speak as well, or there's somebody in the next room that speaks pretty well. But that's not always the case. So we do have an interpreter service that has to be employed.
Biloon: I cannot imagine the nuance in speaking to people from different ages and different backgrounds, who have different types of lifestyles, for them to understand.
Berger: I don't write at a computer. I think that really degrades the patient-physician relationship. What I do is I obtain a history. I do it on a piece of paper with a pen or a pencil.
I recapitulate them to the patient in paraphrasing it, to make sure that I have gotten it right and that they understand what I think I heard. That, I think, has been enormously helpful in helping people understand what may happen in the absence of treatment and why the treatment is important. That you can do, regardless of what the person's background is. So that's how I approach it.
Biloon: How do you deal with patients when they're not on the same page with you?
Berger: One important thing is that you have to be patient. That is something that it took me 50 years in medicine to learn. And then accepting the patient's opinion and saying, "All right, go home and think about it," because you often don't convince them when they're in the office with you.
Biloon: I did have a little bit of a cushion between my diagnosis and when we actually saw each other, where I was able to really sit in my thoughts on the different treatments and stuff. By the time that we were able to talk, it reassured me on that was the right plan.
Berger: I'm curious what your experience has been with our MS center.
Biloon: Through the portal, every time I need something, I'm usually reaching out, keeping you up-to-date on my primary care or whether it's trying to get a refill on one of my medications that I have to reach out. I really do feel that having that team there, being able to reach out, that's been extremely helpful to have and keeps me very secure because that's all I really need, especially during the pandemic, right? Because then I was very isolated and dealing with going through MS. So it was great to at least — and I did — shoot off emails or texts in the portal, and that's usually primarily how I communicated.
Berger: I will tell you, in my opinion, maybe nine out of 10 messages in the portal or calls that we get simply require reassurance.
Biloon: Yes.
Berger: You just either pick up the phone or shoot back a note, say, "This is not your MS. Don't worry about it." I mean, the most important thing for me is to keep people from worrying because that doesn't solve any problem.
Biloon: No, and it causes stress, which causes fatigue. I mean, it's a bad cycle.
Berger: In the past year, you've actually felt better, and you've gone back to performing. It sounds like the volume of performances has gotten back to what it was pre-illness. What do you see for the future?
Biloon: What I see is traveling more for stand-up and doing the sort of clubs and cities that I had kind of stopped doing from before I was diagnosed, so 2017 and prior to that. And then also even working on other things, writing and maybe even doing sort of books or one-person shows that even talk about sort of my struggles with MS and kind of coming back to where I am. I'm looking forward to the future, and I hope that that's the track I can keep going on.
Berger: I see no reason why you shouldn't.
Biloon: Thank you.
Berger: Michelle, thank you very much for joining me today in this conversation.
Biloon: Thank you so much for having me. It's been really wonderful to be able to sit down here with you.
Joseph R. Berger, MD, has disclosed the following relevant financial relationships: Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Celgene/Bristol-Myers Squibb; Cellevolve; EMD Serono/Merck/Genentech; Genzyme; Janssen/Johnson & Johnson; Morphic; Novartis; Roche; Sanofi; Takeda; TG Therapeutics; MAPI; Excision Bio
Received research grant from: Genentech/Roche
Michelle Biloon has disclosed no relevant financial relationships
Herpes Zoster and Varicella Encephalitis Following the Recombinant Zoster Vaccine
To the Editor:
Reported adverse effects following the recombinant zoster vaccine (RZV) include pyrexia, myalgia, and fatigue.1 We report the case of a patient who developed herpes zoster and subsequent varicella encephalitis within 8 days of receiving the second dose of the RZV.
A 75-year-old man presented to the emergency department with burning pain and pruritus involving the left hip and calf 2 days after receiving the second dose of the RZV. He had a history of chronic lymphocytic leukemia (CLL) and was being clinically monitored. He received the first dose of the RZV without complication 3 months prior. In the emergency department, he was diagnosed with “nerve pain,” given acetaminophen, and discharged home; however, he continued to have worsening pain 8 days later followed by a vesicular eruption that wrapped around the left leg and was concentrated on the inner thigh/groin area in a dermatomal distribution. His primary care physician diagnosed him with herpes zoster and prescribed valacyclovir 1000 mg every 8 hours for 7 days. Two days later, the patient developed weakness and confusion and returned to the emergency department. Upon admission, computed tomography and magnetic resonance imaging/magnetic resonance angiography of the brain was normal. A lumbar puncture confirmed varicella encephalitis via a polymerase chain reaction assay. He was treated with intravenous acyclovir and discharged to a rehabilitation facility. His course was further complicated by a subarachnoid hemorrhage and normal pressure hydrocephalus. He did not require a shunt but continues to have memory impairment, weakness, and cognitive impairment. He is steadily improving with rehabilitative services.
The RZV is an inactivated vaccine composed of the varicella-zoster virus (VZV) glycoprotein E antigen and an adjuvant, AS01B, that boosts both innate and adaptive immunity.2 It was approved by the US Food and Drug Administration in 2017 for prevention of herpes zoster in adults aged 50 years or older. It requires 2 separate injections administered 2 to 6 months apart. Its efficacy for the prevention of cutaneous herpes zoster and postherpetic neuralgia is 97% and 80% to 91%, respectively. It was developed to improve on the existing zoster vaccine live, which contains a live attenuated virus, with efficacy ranging from 38% to 70%.3
The Centers for Disease Control and Prevention initially recommended the RZV for immunocompetent individuals or those taking low-dose immunosuppressant medications as well those who have recovered from an immunocompromising illness. In immunocompetent patients, reported adverse effects include injection site pain and redness, headache, myalgia, fatigue, shivering, fever, and gastrointestinal tract symptoms; however, when the vaccine first came out, many of the studies excluded patients with CLL.4 Our patient’s herpes zoster and varicella encephalitis occurred following administration of the second dose of the RZV. Herpes zoster occurs from declining VZV-specific cell-mediated immunity. Given that the vaccine contains inactive virus, it is unlikely that our patient’s infection was the direct result of dissemination of the virus contained within the vaccine. The RZV specifically generates T-cell responses to the glycoprotein E subunit of VZV, which is thought to be responsible for the high levels of VZV-specific memory T cells with the RZV compared to the zoster vaccine live.5 However, this response does not occur until after the second dose of RZV. Although our patient already had 1 dose of RZV, it was unlikely that he had a substantial number of glycoprotein E and VZV-specific memory T cells to combat virus reactivation. Additionally, his CLL, though mild, may have resulted in an aberrant T-cell response in the presence of already low VZV-specific lymphocytes, allowing for reactivation and dissemination of the virus. Since then, there has been more of an emphasis on looking at the immunogenicity elicited by the vaccine in patients with CLL—both those who are treatment naive and those treated with Bruton tyrosine kinase inhibitors. Both groups of patients have demonstrated reduced immunogenicity in response to RZV, leaving the opportunity for viral reactivation in this patient population.6,7
The safety of the RZV has now been demonstrated in patients with CLL.7 However, even after RZV vaccination, patients with CLL are still at risk for herpes zoster reactivation and may have an aberrant response due to immune cell dysregulation. Our case demonstrates the need to increase monitoring of CLL patients for signs of viral reactivation and shift our focus to providing antiviral therapy quickly after symptom occurrence.
- Centers for Disease Control and Prevention. Shingles: about the vaccine. Updated January 24, 2022. Accessed February 7, 2024. https://www.cdc.gov/vaccines/vpd/shingles/hcp/shingrix/about-vaccine.html
- Dooling KL, Guo A, Patel M, et al. Recommendations of the advisory committee on immunization practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103-108. doi:10.15585/mmwr.mm6703a5external icon
- Hunter P, Fryhofer SA, Szilagyi PG. Vaccination of adults in general medical practice. Mayo Clin Proc. 2020;95:169-183. doi:10.1016/j.mayocp.2019.02.024
- Dagnew AF, Ilhan O, Lee WS, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haematological malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis [published correction appears in Lancet Infect Dis. 2020;20:E1]. Lancet Infect Dis. 2019;19:988-1000. doi:10.1016/S1473-3099(19)30163-X
- Levin MJ, Kroehl ME, Johnson MJ, et al. Th1 memory differentiates recombinant from live herpes zoster vaccines. J Clin Invest. 2018;128:4429-4440.
- Pleyer C, Laing KJ, Ali MA, et al. BTK inhibitors impair humoral and cellular responses to recombinant zoster vaccine in CLL. Blood Adv. 2022;6:1732-1740. doi:10.1182/bloodadvances.2021006574
- Pleyer C, Cohen J, Soto S, et al. Response to the Shingrix varicella zoster virus (VZV) vaccine in patients with chronic lymphocytic leukemia (CLL) that are treatment naive or treated with a Bruton’s tyrosine kinase inhibitor (BTK-I). Blood. 2019;134(suppl 1):3053. doi:10.1182/blood-2019-121675
To the Editor:
Reported adverse effects following the recombinant zoster vaccine (RZV) include pyrexia, myalgia, and fatigue.1 We report the case of a patient who developed herpes zoster and subsequent varicella encephalitis within 8 days of receiving the second dose of the RZV.
A 75-year-old man presented to the emergency department with burning pain and pruritus involving the left hip and calf 2 days after receiving the second dose of the RZV. He had a history of chronic lymphocytic leukemia (CLL) and was being clinically monitored. He received the first dose of the RZV without complication 3 months prior. In the emergency department, he was diagnosed with “nerve pain,” given acetaminophen, and discharged home; however, he continued to have worsening pain 8 days later followed by a vesicular eruption that wrapped around the left leg and was concentrated on the inner thigh/groin area in a dermatomal distribution. His primary care physician diagnosed him with herpes zoster and prescribed valacyclovir 1000 mg every 8 hours for 7 days. Two days later, the patient developed weakness and confusion and returned to the emergency department. Upon admission, computed tomography and magnetic resonance imaging/magnetic resonance angiography of the brain was normal. A lumbar puncture confirmed varicella encephalitis via a polymerase chain reaction assay. He was treated with intravenous acyclovir and discharged to a rehabilitation facility. His course was further complicated by a subarachnoid hemorrhage and normal pressure hydrocephalus. He did not require a shunt but continues to have memory impairment, weakness, and cognitive impairment. He is steadily improving with rehabilitative services.
The RZV is an inactivated vaccine composed of the varicella-zoster virus (VZV) glycoprotein E antigen and an adjuvant, AS01B, that boosts both innate and adaptive immunity.2 It was approved by the US Food and Drug Administration in 2017 for prevention of herpes zoster in adults aged 50 years or older. It requires 2 separate injections administered 2 to 6 months apart. Its efficacy for the prevention of cutaneous herpes zoster and postherpetic neuralgia is 97% and 80% to 91%, respectively. It was developed to improve on the existing zoster vaccine live, which contains a live attenuated virus, with efficacy ranging from 38% to 70%.3
The Centers for Disease Control and Prevention initially recommended the RZV for immunocompetent individuals or those taking low-dose immunosuppressant medications as well those who have recovered from an immunocompromising illness. In immunocompetent patients, reported adverse effects include injection site pain and redness, headache, myalgia, fatigue, shivering, fever, and gastrointestinal tract symptoms; however, when the vaccine first came out, many of the studies excluded patients with CLL.4 Our patient’s herpes zoster and varicella encephalitis occurred following administration of the second dose of the RZV. Herpes zoster occurs from declining VZV-specific cell-mediated immunity. Given that the vaccine contains inactive virus, it is unlikely that our patient’s infection was the direct result of dissemination of the virus contained within the vaccine. The RZV specifically generates T-cell responses to the glycoprotein E subunit of VZV, which is thought to be responsible for the high levels of VZV-specific memory T cells with the RZV compared to the zoster vaccine live.5 However, this response does not occur until after the second dose of RZV. Although our patient already had 1 dose of RZV, it was unlikely that he had a substantial number of glycoprotein E and VZV-specific memory T cells to combat virus reactivation. Additionally, his CLL, though mild, may have resulted in an aberrant T-cell response in the presence of already low VZV-specific lymphocytes, allowing for reactivation and dissemination of the virus. Since then, there has been more of an emphasis on looking at the immunogenicity elicited by the vaccine in patients with CLL—both those who are treatment naive and those treated with Bruton tyrosine kinase inhibitors. Both groups of patients have demonstrated reduced immunogenicity in response to RZV, leaving the opportunity for viral reactivation in this patient population.6,7
The safety of the RZV has now been demonstrated in patients with CLL.7 However, even after RZV vaccination, patients with CLL are still at risk for herpes zoster reactivation and may have an aberrant response due to immune cell dysregulation. Our case demonstrates the need to increase monitoring of CLL patients for signs of viral reactivation and shift our focus to providing antiviral therapy quickly after symptom occurrence.
To the Editor:
Reported adverse effects following the recombinant zoster vaccine (RZV) include pyrexia, myalgia, and fatigue.1 We report the case of a patient who developed herpes zoster and subsequent varicella encephalitis within 8 days of receiving the second dose of the RZV.
A 75-year-old man presented to the emergency department with burning pain and pruritus involving the left hip and calf 2 days after receiving the second dose of the RZV. He had a history of chronic lymphocytic leukemia (CLL) and was being clinically monitored. He received the first dose of the RZV without complication 3 months prior. In the emergency department, he was diagnosed with “nerve pain,” given acetaminophen, and discharged home; however, he continued to have worsening pain 8 days later followed by a vesicular eruption that wrapped around the left leg and was concentrated on the inner thigh/groin area in a dermatomal distribution. His primary care physician diagnosed him with herpes zoster and prescribed valacyclovir 1000 mg every 8 hours for 7 days. Two days later, the patient developed weakness and confusion and returned to the emergency department. Upon admission, computed tomography and magnetic resonance imaging/magnetic resonance angiography of the brain was normal. A lumbar puncture confirmed varicella encephalitis via a polymerase chain reaction assay. He was treated with intravenous acyclovir and discharged to a rehabilitation facility. His course was further complicated by a subarachnoid hemorrhage and normal pressure hydrocephalus. He did not require a shunt but continues to have memory impairment, weakness, and cognitive impairment. He is steadily improving with rehabilitative services.
The RZV is an inactivated vaccine composed of the varicella-zoster virus (VZV) glycoprotein E antigen and an adjuvant, AS01B, that boosts both innate and adaptive immunity.2 It was approved by the US Food and Drug Administration in 2017 for prevention of herpes zoster in adults aged 50 years or older. It requires 2 separate injections administered 2 to 6 months apart. Its efficacy for the prevention of cutaneous herpes zoster and postherpetic neuralgia is 97% and 80% to 91%, respectively. It was developed to improve on the existing zoster vaccine live, which contains a live attenuated virus, with efficacy ranging from 38% to 70%.3
The Centers for Disease Control and Prevention initially recommended the RZV for immunocompetent individuals or those taking low-dose immunosuppressant medications as well those who have recovered from an immunocompromising illness. In immunocompetent patients, reported adverse effects include injection site pain and redness, headache, myalgia, fatigue, shivering, fever, and gastrointestinal tract symptoms; however, when the vaccine first came out, many of the studies excluded patients with CLL.4 Our patient’s herpes zoster and varicella encephalitis occurred following administration of the second dose of the RZV. Herpes zoster occurs from declining VZV-specific cell-mediated immunity. Given that the vaccine contains inactive virus, it is unlikely that our patient’s infection was the direct result of dissemination of the virus contained within the vaccine. The RZV specifically generates T-cell responses to the glycoprotein E subunit of VZV, which is thought to be responsible for the high levels of VZV-specific memory T cells with the RZV compared to the zoster vaccine live.5 However, this response does not occur until after the second dose of RZV. Although our patient already had 1 dose of RZV, it was unlikely that he had a substantial number of glycoprotein E and VZV-specific memory T cells to combat virus reactivation. Additionally, his CLL, though mild, may have resulted in an aberrant T-cell response in the presence of already low VZV-specific lymphocytes, allowing for reactivation and dissemination of the virus. Since then, there has been more of an emphasis on looking at the immunogenicity elicited by the vaccine in patients with CLL—both those who are treatment naive and those treated with Bruton tyrosine kinase inhibitors. Both groups of patients have demonstrated reduced immunogenicity in response to RZV, leaving the opportunity for viral reactivation in this patient population.6,7
The safety of the RZV has now been demonstrated in patients with CLL.7 However, even after RZV vaccination, patients with CLL are still at risk for herpes zoster reactivation and may have an aberrant response due to immune cell dysregulation. Our case demonstrates the need to increase monitoring of CLL patients for signs of viral reactivation and shift our focus to providing antiviral therapy quickly after symptom occurrence.
- Centers for Disease Control and Prevention. Shingles: about the vaccine. Updated January 24, 2022. Accessed February 7, 2024. https://www.cdc.gov/vaccines/vpd/shingles/hcp/shingrix/about-vaccine.html
- Dooling KL, Guo A, Patel M, et al. Recommendations of the advisory committee on immunization practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103-108. doi:10.15585/mmwr.mm6703a5external icon
- Hunter P, Fryhofer SA, Szilagyi PG. Vaccination of adults in general medical practice. Mayo Clin Proc. 2020;95:169-183. doi:10.1016/j.mayocp.2019.02.024
- Dagnew AF, Ilhan O, Lee WS, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haematological malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis [published correction appears in Lancet Infect Dis. 2020;20:E1]. Lancet Infect Dis. 2019;19:988-1000. doi:10.1016/S1473-3099(19)30163-X
- Levin MJ, Kroehl ME, Johnson MJ, et al. Th1 memory differentiates recombinant from live herpes zoster vaccines. J Clin Invest. 2018;128:4429-4440.
- Pleyer C, Laing KJ, Ali MA, et al. BTK inhibitors impair humoral and cellular responses to recombinant zoster vaccine in CLL. Blood Adv. 2022;6:1732-1740. doi:10.1182/bloodadvances.2021006574
- Pleyer C, Cohen J, Soto S, et al. Response to the Shingrix varicella zoster virus (VZV) vaccine in patients with chronic lymphocytic leukemia (CLL) that are treatment naive or treated with a Bruton’s tyrosine kinase inhibitor (BTK-I). Blood. 2019;134(suppl 1):3053. doi:10.1182/blood-2019-121675
- Centers for Disease Control and Prevention. Shingles: about the vaccine. Updated January 24, 2022. Accessed February 7, 2024. https://www.cdc.gov/vaccines/vpd/shingles/hcp/shingrix/about-vaccine.html
- Dooling KL, Guo A, Patel M, et al. Recommendations of the advisory committee on immunization practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103-108. doi:10.15585/mmwr.mm6703a5external icon
- Hunter P, Fryhofer SA, Szilagyi PG. Vaccination of adults in general medical practice. Mayo Clin Proc. 2020;95:169-183. doi:10.1016/j.mayocp.2019.02.024
- Dagnew AF, Ilhan O, Lee WS, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haematological malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis [published correction appears in Lancet Infect Dis. 2020;20:E1]. Lancet Infect Dis. 2019;19:988-1000. doi:10.1016/S1473-3099(19)30163-X
- Levin MJ, Kroehl ME, Johnson MJ, et al. Th1 memory differentiates recombinant from live herpes zoster vaccines. J Clin Invest. 2018;128:4429-4440.
- Pleyer C, Laing KJ, Ali MA, et al. BTK inhibitors impair humoral and cellular responses to recombinant zoster vaccine in CLL. Blood Adv. 2022;6:1732-1740. doi:10.1182/bloodadvances.2021006574
- Pleyer C, Cohen J, Soto S, et al. Response to the Shingrix varicella zoster virus (VZV) vaccine in patients with chronic lymphocytic leukemia (CLL) that are treatment naive or treated with a Bruton’s tyrosine kinase inhibitor (BTK-I). Blood. 2019;134(suppl 1):3053. doi:10.1182/blood-2019-121675
Practice Points
- Patients with chronic lymphocytic leukemia (CLL) are at risk for herpes zoster reactivation even with vaccination due to a decreased immune response. These patients may have an aberrant response due to immune cell dysregulation.
- It is important to increase monitoring of CLL patients for signs of viral reactivation and shift the focus to providing antiviral therapy quickly if herpes zoster symptoms occur.
Rapidly Progressive Necrotizing Myositis Mimicking Pyoderma Gangrenosum
To the Editor:
Necrotizing myositis (NM) is an exceedingly rare necrotizing soft-tissue infection (NSTI) that is characterized by skeletal muscle involvement. β -Hemolytic streptococci, such as Streptococcus pyogenes , are the most common causative organisms. The overall prevalence and incidence of NM is unknown. A review of the literature by Adams et al 2 identified only 21 cases between 1900 and 1985.
Timely treatment of this infection leads to improved outcomes, but diagnosis can be challenging due to the ambiguous presentation of NM and lack of specific cutaneous changes.3 Clinical manifestations including bullae, blisters, vesicles, and petechiae become more prominent as infection progresses.4 If NM is suspected due to cutaneous manifestations, it is imperative that the underlying cause be identified; for example, NM must be distinguished from the overlapping presentation of pyoderma gangrenosum (PG). Because NM has nearly 100% mortality without prompt surgical intervention, early identification is critical.5 Herein, we report a case of NM that illustrates the correlation of clinical, histological, and imaging findings required to diagnose this potentially fatal infection.
An 80-year-old man presented to the emergency department with worsening pain, edema, and spreading redness of the right wrist over the last 5 weeks. He had a history of atopic dermatitis that was refractory to topical steroids and methotrexate; he was dependent on an oral steroid (prednisone 30 mg/d) for symptom control. The patient reported minor trauma to the area after performing home renovations. He received numerous rounds of oral antibiotics as an outpatient for presumed cellulitis and reported he was “getting better” but that the signs and symptoms of the condition grew worse after outpatient arthrocentesis. Dermatology was consulted to evaluate for a necrotizing neutrophilic dermatosis such as PG.
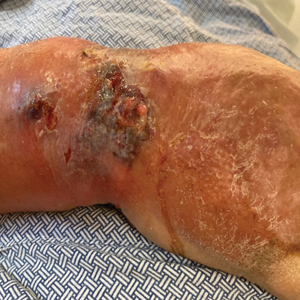
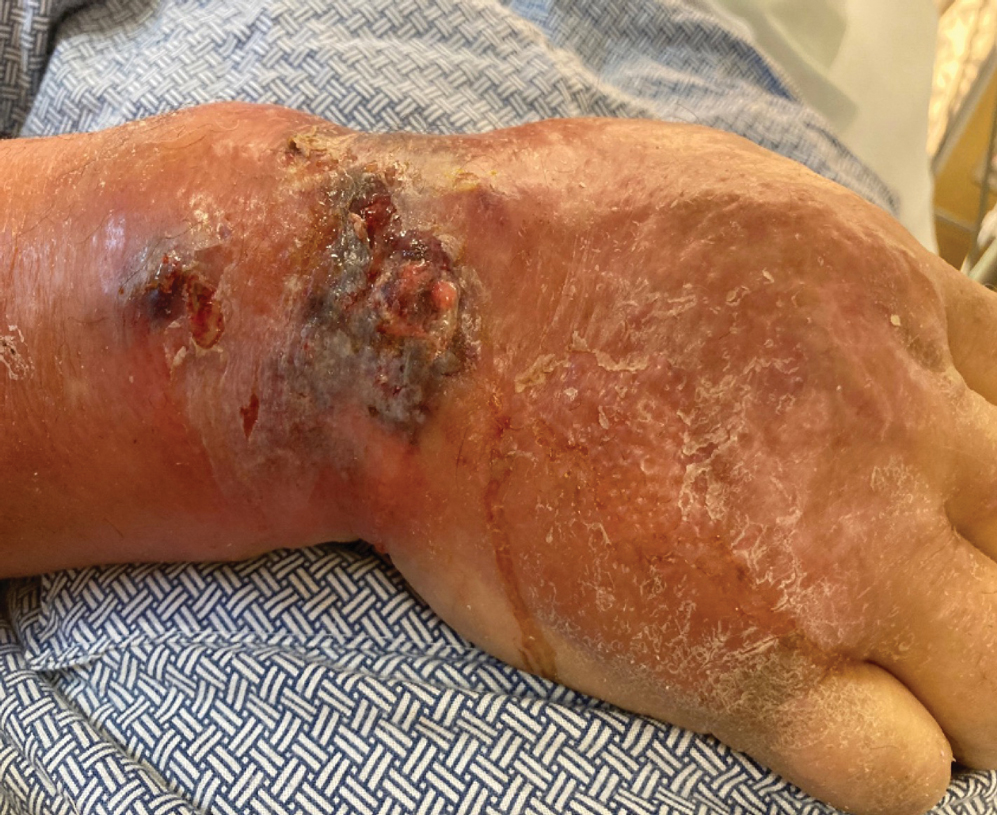
At the current presentation, the patient was tachycardic and afebrile (temperature, 98.2 °F [36.8 °C]). Physical examination revealed large, exquisitely tender, ill-defined necrotic ulceration of the right wrist with purulent debris and diffuse edema (Figure 1). Sequential evaluation at 6-hour intervals revealed notably increasing purulence, edema, and tenderness. Interconnected sinus tracts that extended to the fascial plane were observed.
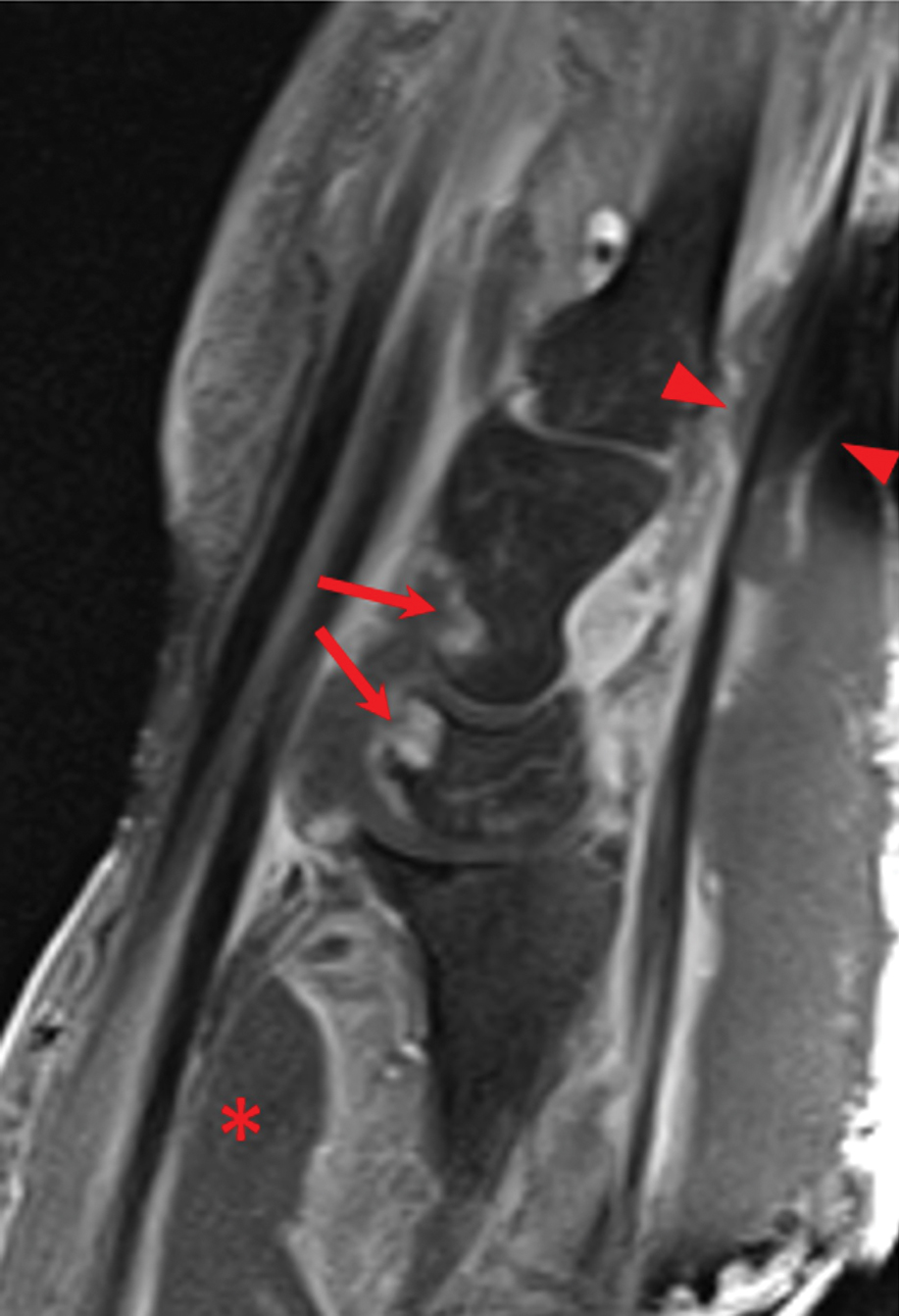
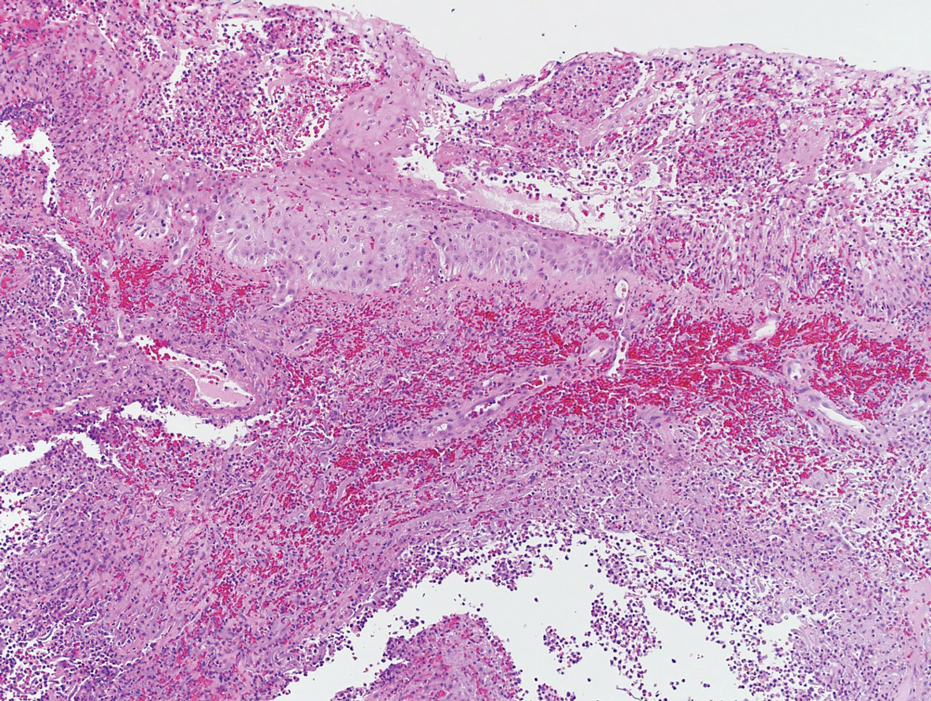
Laboratory workup was notable for a markedly elevated C-reactive protein level of 18.9 mg/dL (reference range, 0–0.8 mg/dL) and an elevated white blood cell count of 19.92×109/L (reference range, 4.5–11.0×109/L). Blood and tissue cultures were positive for methicillin-sensitive Staphylococcus aureus. Computed tomography and magnetic resonance imaging (MRI) prior to biopsy demonstrated findings consistent with extensive subcutaneous and intramuscular areas of loculation and foci of gas (Figure 2). These findings were consistent with intramuscular involvement. A punch biopsy revealed a necrotic epidermis filled with neutrophilic pustules and a dense dermal infiltrate of neutrophilic inflammation consistent with infection (Figure 3).
Emergency surgery was performed with debridement of necrotic tissue and muscle. Postoperatively, he became more clinically stable after being placed on cefazolin through a peripherally inserted central catheter. He underwent 4 additional washouts over the ensuing month, as well as tendon reconstructions, a radial forearm flap, and reverse radial forearm flap reconstruction of the forearm. At the time of publication, there has been no recurrence. The patient’s atopic dermatitis is well controlled on dupilumab and topical fluocinonide alone, with a recent IgA level of 1 g/L and a body surface area measurement of 2%. Dupilumab was started 3 months after surgery.
Necrotizing myositis is a rare, rapidly progressive infection involving muscle that can manifest as superficial cutaneous involvement. The clinical manifestation of NM is harder to recognize than other NSTIs such as necrotizing fasciitis, likely due to the initial prodromal phase of NM, which consists of nonspecific constitutional symptoms.3 Systemic findings such as tachycardia, fever, hypotension, and shock occur in only 10% to 40% of NM patients.4,5
In our patient, clues of NM included fulfillment of criteria for systemic inflammatory response syndrome at admission and a presumed source of infection; taken together, these findings should lead to a diagnosis of sepsis until otherwise proven. The patient also reported pain that was not proportional to the skin findings, which suggested an NSTI. His lack of constitutional symptoms may have been due to the effects of prednisone, which was changed to dupilumab during hospitalization.
The clinical and histological findings of NM are nonspecific. Clinical findings include skin discoloration with bullae, blisters, vesicles, or petechiae.4 Our case adds to the descriptive morphology by including marked edema with ulceration, progressive purulence, and interconnected sinuses tracking to the fascial plane. Histologic findings can include confluent necrosis extending from the epidermis to the underlying muscle with dense neutrophilic inflammation. Notably, these findings can mirror necrotizing neutrophilic dermatoses in the absence of an infectious cause. Failure to recognize simple systemic inflammatory response syndrome criteria in NM patients due to slow treatment response or incorrect treatment can can lead to loss of a limb or death.
Workup reveals overlap with necrotizing neutrophilic dermatoses including PG, which is the prototypical neutrophilic dermatosis. Morphologically, PG presents as an ulcer with a purple and undermined border, often having developed from an initial papule, vesicle, or pustule. A neutrophilic infiltrate of the ulcer edge is the major criterion required to diagnose PG6; minor criteria include a positive pathergy test, history of inflammatory arthritis or inflammatory bowel disease, and exclusion of infection.6 When compared directly to an NSTI such as NM, the most important variable that sets PG apart is the absence of bacterial growth on blood and tissue cultures.7
Imaging studies can aid in the clinical diagnosis of NM and help distinguish the disease from PG. Computed tomography and MRI may demonstrate hallmarks of extensive necrotizing infection, such as gas formation and consequent fascial swelling, thickening and edema of involved muscle, and subfascial fluid collection.3,4 Distinct from NM, imaging findings in PG are more subtle, suggesting cellulitic inflammation with edema.8 A defining radiographic feature of NM can be foci of gas within muscle or fascia, though absence of this finding does not exclude NM.1,4
In conclusion, NM is a rare intramuscular infection that can be difficult to diagnose due to its nonspecific presentation and lack of constitutional symptoms. Dermatologists should maintain a high level of suspicion for NM in the setting of rapidly progressive clinical findings; accurate diagnosis requires a multimodal approach with complete correlation of clinical, histological, and imaging findings. Computed tomography and MRI can heighten the approach, even when necrotizing neutrophilic dermatoses and NM have similar clinical and histological appearances. Once a diagnosis of NM is established, prompt surgical and medical intervention improves the prognosis.
- Stevens DL, Baddour LM. Necrotizing soft tissue infections. UpToDate. Updated October 7, 2022. Accessed February 13, 2024. https://www.uptodate.com/contents/necrotizing-soft-tissue-infections?search=Necrotizing%20soft%20tissue%20infections&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
- Adams EM, Gudmundsson S, Yocum DE, et al. Streptococcal myositis. Arch Intern Med . 1985;145:1020-1023.
- Khanna A, Gurusinghe D, Taylor D. Necrotizing myositis: highlighting the hidden depths—case series and review of the literature. ANZ J Surg . 2020;90:130-134. doi:10.1111/ans.15429
- Boinpally H, Howell RS, Ram B, et al. Necrotizing myositis: a rare necrotizing soft tissue infection involving muscle. Wounds . 2018;30:E116-E120.
- Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis . 2007;44:705-710. doi:10.1086/511638
- Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a Delphi consensus of international experts. JAMA Dermatol . 2018;154:461-466. doi:10.1001/jamadermatol.2017.5980
- Sanchez IM, Lowenstein S, Johnson KA, et al. Clinical features of neutrophilic dermatosis variants resembling necrotizing fasciitis. JAMA Dermatol . 2019;155:79-84. doi:10.1001/jamadermatol.2018.3890
- Demirdover C, Geyik A, Vayvada H. Necrotising fasciitis or pyoderma gangrenosum: a fatal dilemma. Int Wound J . 2019;16:1347-1353. doi:10.1111/iwj.13196
To the Editor:
Necrotizing myositis (NM) is an exceedingly rare necrotizing soft-tissue infection (NSTI) that is characterized by skeletal muscle involvement. β -Hemolytic streptococci, such as Streptococcus pyogenes , are the most common causative organisms. The overall prevalence and incidence of NM is unknown. A review of the literature by Adams et al 2 identified only 21 cases between 1900 and 1985.
Timely treatment of this infection leads to improved outcomes, but diagnosis can be challenging due to the ambiguous presentation of NM and lack of specific cutaneous changes.3 Clinical manifestations including bullae, blisters, vesicles, and petechiae become more prominent as infection progresses.4 If NM is suspected due to cutaneous manifestations, it is imperative that the underlying cause be identified; for example, NM must be distinguished from the overlapping presentation of pyoderma gangrenosum (PG). Because NM has nearly 100% mortality without prompt surgical intervention, early identification is critical.5 Herein, we report a case of NM that illustrates the correlation of clinical, histological, and imaging findings required to diagnose this potentially fatal infection.
An 80-year-old man presented to the emergency department with worsening pain, edema, and spreading redness of the right wrist over the last 5 weeks. He had a history of atopic dermatitis that was refractory to topical steroids and methotrexate; he was dependent on an oral steroid (prednisone 30 mg/d) for symptom control. The patient reported minor trauma to the area after performing home renovations. He received numerous rounds of oral antibiotics as an outpatient for presumed cellulitis and reported he was “getting better” but that the signs and symptoms of the condition grew worse after outpatient arthrocentesis. Dermatology was consulted to evaluate for a necrotizing neutrophilic dermatosis such as PG.
At the current presentation, the patient was tachycardic and afebrile (temperature, 98.2 °F [36.8 °C]). Physical examination revealed large, exquisitely tender, ill-defined necrotic ulceration of the right wrist with purulent debris and diffuse edema (Figure 1). Sequential evaluation at 6-hour intervals revealed notably increasing purulence, edema, and tenderness. Interconnected sinus tracts that extended to the fascial plane were observed.

Laboratory workup was notable for a markedly elevated C-reactive protein level of 18.9 mg/dL (reference range, 0–0.8 mg/dL) and an elevated white blood cell count of 19.92×109/L (reference range, 4.5–11.0×109/L). Blood and tissue cultures were positive for methicillin-sensitive Staphylococcus aureus. Computed tomography and magnetic resonance imaging (MRI) prior to biopsy demonstrated findings consistent with extensive subcutaneous and intramuscular areas of loculation and foci of gas (Figure 2). These findings were consistent with intramuscular involvement. A punch biopsy revealed a necrotic epidermis filled with neutrophilic pustules and a dense dermal infiltrate of neutrophilic inflammation consistent with infection (Figure 3).

Emergency surgery was performed with debridement of necrotic tissue and muscle. Postoperatively, he became more clinically stable after being placed on cefazolin through a peripherally inserted central catheter. He underwent 4 additional washouts over the ensuing month, as well as tendon reconstructions, a radial forearm flap, and reverse radial forearm flap reconstruction of the forearm. At the time of publication, there has been no recurrence. The patient’s atopic dermatitis is well controlled on dupilumab and topical fluocinonide alone, with a recent IgA level of 1 g/L and a body surface area measurement of 2%. Dupilumab was started 3 months after surgery.

Necrotizing myositis is a rare, rapidly progressive infection involving muscle that can manifest as superficial cutaneous involvement. The clinical manifestation of NM is harder to recognize than other NSTIs such as necrotizing fasciitis, likely due to the initial prodromal phase of NM, which consists of nonspecific constitutional symptoms.3 Systemic findings such as tachycardia, fever, hypotension, and shock occur in only 10% to 40% of NM patients.4,5
In our patient, clues of NM included fulfillment of criteria for systemic inflammatory response syndrome at admission and a presumed source of infection; taken together, these findings should lead to a diagnosis of sepsis until otherwise proven. The patient also reported pain that was not proportional to the skin findings, which suggested an NSTI. His lack of constitutional symptoms may have been due to the effects of prednisone, which was changed to dupilumab during hospitalization.
The clinical and histological findings of NM are nonspecific. Clinical findings include skin discoloration with bullae, blisters, vesicles, or petechiae.4 Our case adds to the descriptive morphology by including marked edema with ulceration, progressive purulence, and interconnected sinuses tracking to the fascial plane. Histologic findings can include confluent necrosis extending from the epidermis to the underlying muscle with dense neutrophilic inflammation. Notably, these findings can mirror necrotizing neutrophilic dermatoses in the absence of an infectious cause. Failure to recognize simple systemic inflammatory response syndrome criteria in NM patients due to slow treatment response or incorrect treatment can can lead to loss of a limb or death.
Workup reveals overlap with necrotizing neutrophilic dermatoses including PG, which is the prototypical neutrophilic dermatosis. Morphologically, PG presents as an ulcer with a purple and undermined border, often having developed from an initial papule, vesicle, or pustule. A neutrophilic infiltrate of the ulcer edge is the major criterion required to diagnose PG6; minor criteria include a positive pathergy test, history of inflammatory arthritis or inflammatory bowel disease, and exclusion of infection.6 When compared directly to an NSTI such as NM, the most important variable that sets PG apart is the absence of bacterial growth on blood and tissue cultures.7
Imaging studies can aid in the clinical diagnosis of NM and help distinguish the disease from PG. Computed tomography and MRI may demonstrate hallmarks of extensive necrotizing infection, such as gas formation and consequent fascial swelling, thickening and edema of involved muscle, and subfascial fluid collection.3,4 Distinct from NM, imaging findings in PG are more subtle, suggesting cellulitic inflammation with edema.8 A defining radiographic feature of NM can be foci of gas within muscle or fascia, though absence of this finding does not exclude NM.1,4
In conclusion, NM is a rare intramuscular infection that can be difficult to diagnose due to its nonspecific presentation and lack of constitutional symptoms. Dermatologists should maintain a high level of suspicion for NM in the setting of rapidly progressive clinical findings; accurate diagnosis requires a multimodal approach with complete correlation of clinical, histological, and imaging findings. Computed tomography and MRI can heighten the approach, even when necrotizing neutrophilic dermatoses and NM have similar clinical and histological appearances. Once a diagnosis of NM is established, prompt surgical and medical intervention improves the prognosis.
To the Editor:
Necrotizing myositis (NM) is an exceedingly rare necrotizing soft-tissue infection (NSTI) that is characterized by skeletal muscle involvement. β -Hemolytic streptococci, such as Streptococcus pyogenes , are the most common causative organisms. The overall prevalence and incidence of NM is unknown. A review of the literature by Adams et al 2 identified only 21 cases between 1900 and 1985.
Timely treatment of this infection leads to improved outcomes, but diagnosis can be challenging due to the ambiguous presentation of NM and lack of specific cutaneous changes.3 Clinical manifestations including bullae, blisters, vesicles, and petechiae become more prominent as infection progresses.4 If NM is suspected due to cutaneous manifestations, it is imperative that the underlying cause be identified; for example, NM must be distinguished from the overlapping presentation of pyoderma gangrenosum (PG). Because NM has nearly 100% mortality without prompt surgical intervention, early identification is critical.5 Herein, we report a case of NM that illustrates the correlation of clinical, histological, and imaging findings required to diagnose this potentially fatal infection.
An 80-year-old man presented to the emergency department with worsening pain, edema, and spreading redness of the right wrist over the last 5 weeks. He had a history of atopic dermatitis that was refractory to topical steroids and methotrexate; he was dependent on an oral steroid (prednisone 30 mg/d) for symptom control. The patient reported minor trauma to the area after performing home renovations. He received numerous rounds of oral antibiotics as an outpatient for presumed cellulitis and reported he was “getting better” but that the signs and symptoms of the condition grew worse after outpatient arthrocentesis. Dermatology was consulted to evaluate for a necrotizing neutrophilic dermatosis such as PG.
At the current presentation, the patient was tachycardic and afebrile (temperature, 98.2 °F [36.8 °C]). Physical examination revealed large, exquisitely tender, ill-defined necrotic ulceration of the right wrist with purulent debris and diffuse edema (Figure 1). Sequential evaluation at 6-hour intervals revealed notably increasing purulence, edema, and tenderness. Interconnected sinus tracts that extended to the fascial plane were observed.

Laboratory workup was notable for a markedly elevated C-reactive protein level of 18.9 mg/dL (reference range, 0–0.8 mg/dL) and an elevated white blood cell count of 19.92×109/L (reference range, 4.5–11.0×109/L). Blood and tissue cultures were positive for methicillin-sensitive Staphylococcus aureus. Computed tomography and magnetic resonance imaging (MRI) prior to biopsy demonstrated findings consistent with extensive subcutaneous and intramuscular areas of loculation and foci of gas (Figure 2). These findings were consistent with intramuscular involvement. A punch biopsy revealed a necrotic epidermis filled with neutrophilic pustules and a dense dermal infiltrate of neutrophilic inflammation consistent with infection (Figure 3).

Emergency surgery was performed with debridement of necrotic tissue and muscle. Postoperatively, he became more clinically stable after being placed on cefazolin through a peripherally inserted central catheter. He underwent 4 additional washouts over the ensuing month, as well as tendon reconstructions, a radial forearm flap, and reverse radial forearm flap reconstruction of the forearm. At the time of publication, there has been no recurrence. The patient’s atopic dermatitis is well controlled on dupilumab and topical fluocinonide alone, with a recent IgA level of 1 g/L and a body surface area measurement of 2%. Dupilumab was started 3 months after surgery.

Necrotizing myositis is a rare, rapidly progressive infection involving muscle that can manifest as superficial cutaneous involvement. The clinical manifestation of NM is harder to recognize than other NSTIs such as necrotizing fasciitis, likely due to the initial prodromal phase of NM, which consists of nonspecific constitutional symptoms.3 Systemic findings such as tachycardia, fever, hypotension, and shock occur in only 10% to 40% of NM patients.4,5
In our patient, clues of NM included fulfillment of criteria for systemic inflammatory response syndrome at admission and a presumed source of infection; taken together, these findings should lead to a diagnosis of sepsis until otherwise proven. The patient also reported pain that was not proportional to the skin findings, which suggested an NSTI. His lack of constitutional symptoms may have been due to the effects of prednisone, which was changed to dupilumab during hospitalization.
The clinical and histological findings of NM are nonspecific. Clinical findings include skin discoloration with bullae, blisters, vesicles, or petechiae.4 Our case adds to the descriptive morphology by including marked edema with ulceration, progressive purulence, and interconnected sinuses tracking to the fascial plane. Histologic findings can include confluent necrosis extending from the epidermis to the underlying muscle with dense neutrophilic inflammation. Notably, these findings can mirror necrotizing neutrophilic dermatoses in the absence of an infectious cause. Failure to recognize simple systemic inflammatory response syndrome criteria in NM patients due to slow treatment response or incorrect treatment can can lead to loss of a limb or death.
Workup reveals overlap with necrotizing neutrophilic dermatoses including PG, which is the prototypical neutrophilic dermatosis. Morphologically, PG presents as an ulcer with a purple and undermined border, often having developed from an initial papule, vesicle, or pustule. A neutrophilic infiltrate of the ulcer edge is the major criterion required to diagnose PG6; minor criteria include a positive pathergy test, history of inflammatory arthritis or inflammatory bowel disease, and exclusion of infection.6 When compared directly to an NSTI such as NM, the most important variable that sets PG apart is the absence of bacterial growth on blood and tissue cultures.7
Imaging studies can aid in the clinical diagnosis of NM and help distinguish the disease from PG. Computed tomography and MRI may demonstrate hallmarks of extensive necrotizing infection, such as gas formation and consequent fascial swelling, thickening and edema of involved muscle, and subfascial fluid collection.3,4 Distinct from NM, imaging findings in PG are more subtle, suggesting cellulitic inflammation with edema.8 A defining radiographic feature of NM can be foci of gas within muscle or fascia, though absence of this finding does not exclude NM.1,4
In conclusion, NM is a rare intramuscular infection that can be difficult to diagnose due to its nonspecific presentation and lack of constitutional symptoms. Dermatologists should maintain a high level of suspicion for NM in the setting of rapidly progressive clinical findings; accurate diagnosis requires a multimodal approach with complete correlation of clinical, histological, and imaging findings. Computed tomography and MRI can heighten the approach, even when necrotizing neutrophilic dermatoses and NM have similar clinical and histological appearances. Once a diagnosis of NM is established, prompt surgical and medical intervention improves the prognosis.
- Stevens DL, Baddour LM. Necrotizing soft tissue infections. UpToDate. Updated October 7, 2022. Accessed February 13, 2024. https://www.uptodate.com/contents/necrotizing-soft-tissue-infections?search=Necrotizing%20soft%20tissue%20infections&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
- Adams EM, Gudmundsson S, Yocum DE, et al. Streptococcal myositis. Arch Intern Med . 1985;145:1020-1023.
- Khanna A, Gurusinghe D, Taylor D. Necrotizing myositis: highlighting the hidden depths—case series and review of the literature. ANZ J Surg . 2020;90:130-134. doi:10.1111/ans.15429
- Boinpally H, Howell RS, Ram B, et al. Necrotizing myositis: a rare necrotizing soft tissue infection involving muscle. Wounds . 2018;30:E116-E120.
- Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis . 2007;44:705-710. doi:10.1086/511638
- Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a Delphi consensus of international experts. JAMA Dermatol . 2018;154:461-466. doi:10.1001/jamadermatol.2017.5980
- Sanchez IM, Lowenstein S, Johnson KA, et al. Clinical features of neutrophilic dermatosis variants resembling necrotizing fasciitis. JAMA Dermatol . 2019;155:79-84. doi:10.1001/jamadermatol.2018.3890
- Demirdover C, Geyik A, Vayvada H. Necrotising fasciitis or pyoderma gangrenosum: a fatal dilemma. Int Wound J . 2019;16:1347-1353. doi:10.1111/iwj.13196
- Stevens DL, Baddour LM. Necrotizing soft tissue infections. UpToDate. Updated October 7, 2022. Accessed February 13, 2024. https://www.uptodate.com/contents/necrotizing-soft-tissue-infections?search=Necrotizing%20soft%20tissue%20infections&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
- Adams EM, Gudmundsson S, Yocum DE, et al. Streptococcal myositis. Arch Intern Med . 1985;145:1020-1023.
- Khanna A, Gurusinghe D, Taylor D. Necrotizing myositis: highlighting the hidden depths—case series and review of the literature. ANZ J Surg . 2020;90:130-134. doi:10.1111/ans.15429
- Boinpally H, Howell RS, Ram B, et al. Necrotizing myositis: a rare necrotizing soft tissue infection involving muscle. Wounds . 2018;30:E116-E120.
- Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis . 2007;44:705-710. doi:10.1086/511638
- Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a Delphi consensus of international experts. JAMA Dermatol . 2018;154:461-466. doi:10.1001/jamadermatol.2017.5980
- Sanchez IM, Lowenstein S, Johnson KA, et al. Clinical features of neutrophilic dermatosis variants resembling necrotizing fasciitis. JAMA Dermatol . 2019;155:79-84. doi:10.1001/jamadermatol.2018.3890
- Demirdover C, Geyik A, Vayvada H. Necrotising fasciitis or pyoderma gangrenosum: a fatal dilemma. Int Wound J . 2019;16:1347-1353. doi:10.1111/iwj.13196
Practice Points
- The accurate diagnosis of necrotizing myositis (NM) requires a multimodal approach with complete clinical, histological, and radiographic correlation.
- Necrotizing myositis can manifest as violaceous erythematous plaques, bullae, blisters, or vesicles with petechiae, marked edema with ulceration, progressive purulence, and interconnected sinuses tracking to the fascial plane.
- The differential diagnosis of NM includes pyoderma gangrenosum.
Dermatologic Reactions Following COVID-19 Vaccination: A Case Series
Cutaneous reactions associated with the Pfizer-BioNTech COVID-19 vaccine have been reported worldwide since December 2020. Local injection site reactions (<1%) such as erythema, swelling, delayed local reactions (1%–10%), morbilliform rash, urticarial reactions, pityriasis rosea, Rowell syndrome, and lichen planus have been reported following the Pfizer-BioNTech COVID-19 vaccine.1 Cutaneous reactions reported in association with the Sinovac-Coronavac COVID-19 vaccine include swelling, redness, itching, discoloration, induration (1%–10%), urticaria, petechial rash, and exacerbation of psoriasis at the local injection site (<1%).2
We describe 7 patients from Turkey who presented with various dermatologic problems 5 to 28 days after COVID-19 vaccination, highlighting the possibility of early and late cutaneous reactions related to the vaccine (Table).
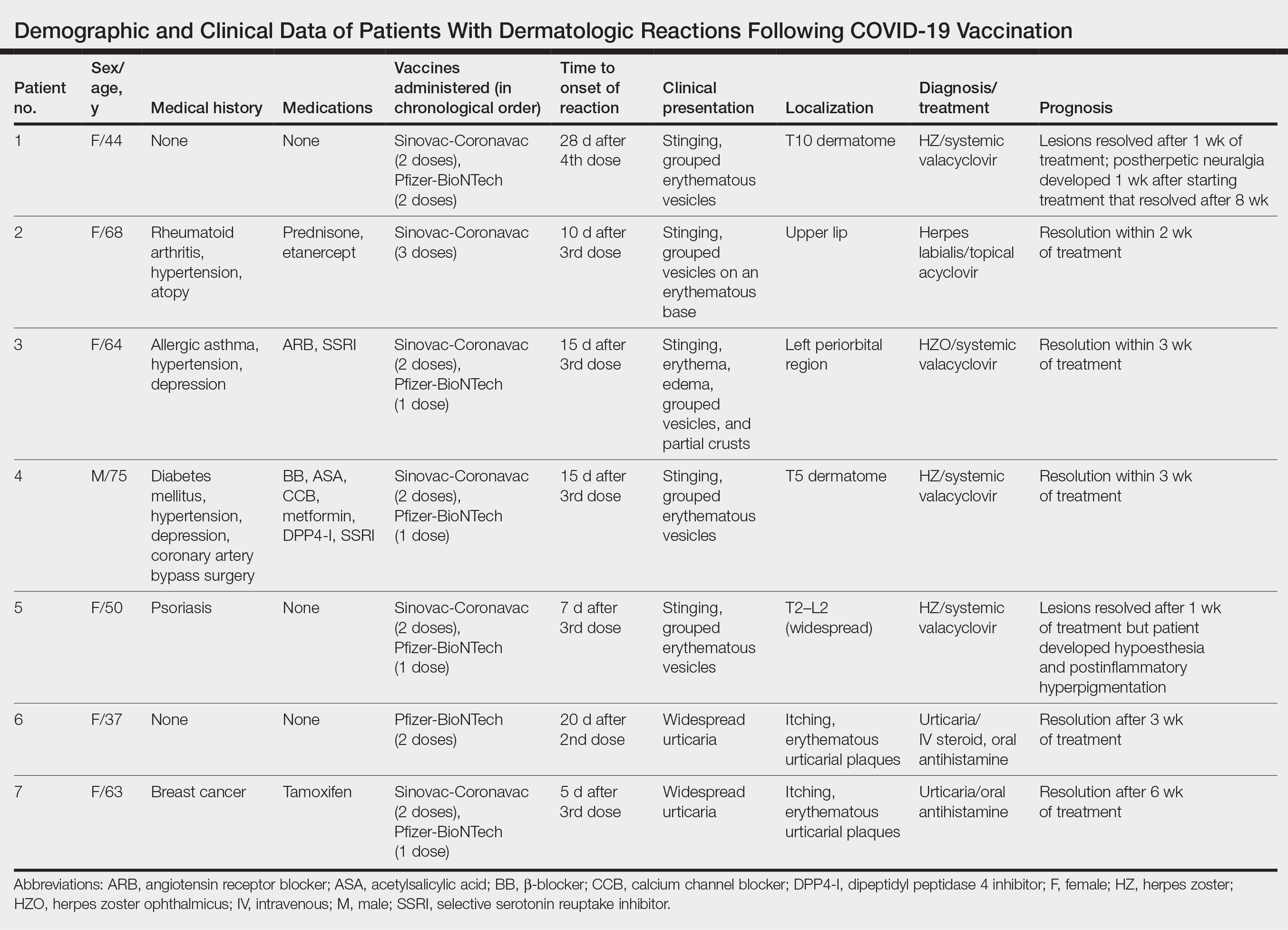
Case Reports
Patient 1—A 44-year-old woman was admitted to the dermatology clinic with painful lesions on the trunk of 3 days’ duration. Dermatologic examination revealed grouped erythematous vesicles showing dermatomal spread in the right thoracolumbar (dermatome T10) region. The patient reported that she had received 2 doses of the Sinovac-Coronavac vaccine (doses 1 and 2) and 2 doses of the BioNTech COVID-19 vaccine (doses 3 and 4); the rash had developed 28 days after she received the 4th dose. Her medical history was unremarkable. The lesions regressed after 1 week of treatment with oral valacyclovir 1000 mg 3 times daily, but she developed postherpetic neuralgia 1 week after starting treatment, which resolved after 8 weeks.
Patient 2—A 68-year-old woman presented to the dermatology clinic for evaluation of painful sores on the upper lip of 1 day’s duration. She had a history of rheumatoid arthritis, hypertension, and atopy and was currently taking prednisone and etanercept. Dermatologic examination revealed grouped vesicles on an erythematous base on the upper lip. A diagnosis of herpes labialis was made. The patient reported that she had received a third dose of the Sinovac-Coronavac vaccine 10 days prior to the appearance of the lesions. Her symptoms resolved completely within 2 weeks of treatment with topical acyclovir.
Patient 3—A 64-year-old woman was admitted to the hospital with pain, redness, and watery sores on and around the left eyelid of 2 days’ duration. Dermatologic evaluation revealed the erythematous surface of the left eyelid and periorbital area showed partial crusts, clustered vesicles, erythema, and edema. Additionally, the conjunctiva was purulent and erythematous. The patient’s medical history was notable for allergic asthma, hypertension, anxiety, and depression. For this reason, the patient was prescribed an angiotensin receptor blocker and a selective serotonin reuptake inhibitor. She noted that a similar rash had developed around the left eye 6 years prior that was diagnosed as herpes zoster (HZ). She also reported that she had received 2 doses of the Sinovac-Coronavac COVID-19 vaccine followed by 1 dose of the BioNTech COVID-19 vaccine, which she had received 2 weeks before the rash developed. The patient was treated at the eye clinic and was found to have ocular involvement. Ophthalmology was consulted and a diagnosis of herpes zoster ophthalmicus (HZO) was made. Systemic valacyclovir treatment was initiated, resulting in clinical improvement within 3 weeks.
Patient 4—A 75-year-old man was admitted to the hospital with chest and back pain and widespread muscle pain of several days’ duration. His medical history was remarkable for diabetes mellitus, hypertension, depression, and coronary artery bypass surgery. A medication history revealed treatment with a β-blocker, acetylsalicylic acid, a calcium channel blocker, a dipeptidyl peptidase 4 inhibitor, and a selective serotonin reuptake inhibitor. Dermatologic examination revealed grouped vesicles on an erythematous background in dermatome T5 on the right chest and back. A diagnosis of HZ was made. The patient reported that he had received 2 doses of the Sinovac-Coronavac vaccine followed by 1 dose of the Pfizer-BioNTech vaccine 2 weeks prior to the current presentation. He was treated with valacyclovir for 1 week, and his symptoms resolved entirely within 3 weeks.
Patient 5—A 50-year-old woman presented to the hospital for evaluation of painful sores on the back, chest, groin, and abdomen of 10 days’ duration. The lesions initially had developed 7 days after receiving the BioNTech COVID-19 vaccine; she previously had received 2 doses of the Sinovac-Coronavac vaccine. The patient had a history of untreated psoriasis. Dermatologic examination revealed grouped vesicles on an erythematous background in the T2–L2 dermatomes on the left side of the trunk. A diagnosis of HZ was made. The lesions resolved after 1 week of treatment with systemic valacyclovir; however, she subsequently developed postherpetic neuralgia, hypoesthesia, and postinflammatory hyperpigmentation in the affected regions.
Patient 6—A 37-year-old woman presented to the hospital with redness, swelling, and itching all over the body of 3 days’ duration. The patient noted that the rash would subside and reappear throughout the day. Her medical history was unremarkable, except for COVID-19 infection 6 months prior. She had received a second dose of the BioNTech vaccine 20 days prior to development of symptoms. Dermatologic examination revealed widespread erythematous urticarial plaques. A diagnosis of acute urticaria was made. The patient recovered completely after 1 week of treatment with a systemic steroid and 3 weeks of antihistamine treatment.
Patient 7—A 63-year-old woman presented to the hospital with widespread itching and rash that appeared 5 days after the first dose of the BioNTech COVID-19 vaccine. The patient reported that the rash resolved spontaneously within a few hours but then reappeared. Her medical history revealed that she was taking tamoxifen for breast cancer and that she previously had received 2 doses of the Sinovac-Coronavac vaccine. Dermatologic examination revealed erythematous urticarial plaques on the trunk and arms. A diagnosis of urticaria was made, and her symptoms resolved after 6 weeks of antihistamine treatment.
Comment
Skin lesions associated with COVID-19 infection have been reported worldwide3,4 as well as dermatologic reactions following COVID-19 vaccination. In one case from Turkey, HZ infection was reported in a 68-year-old man 5 days after he received a second dose of the COVID-19 vaccine.5 In another case, HZ infection developed in a 78-year-old man 5 days after COVID-19 vaccination.6 Numerous cases of HZ infection developing within 1 to 26 days of COVID-19 vaccination have been reported worldwide.7-9
In a study conducted in the United States, 40 skin reactions associated with the COVID-19 vaccine were investigated; of these cases, 87.5% (35/40) were reported as varicella-zoster virus, and 12.5% (5/40) were reported as herpes simplex reactivation; 54% (19/35) and 80% (4/5) of these cases, respectively, were associated with the Pfizer-BioNTech vaccine.10 The average age of patients who developed a skin reaction was 46 years, and 70% (28/40) were women. The time to onset of the reaction was 2 to 13 days after vaccination, and symptoms were reported to improve within 7 days on average.10
Another study from Spain examined 405 vaccine-related skin reactions, 40.2% of which were related to the Pfizer-BioNTech vaccine. Among them, 80.2% occurred in women; 13.8% of cases were diagnosed as varicella-zoster virus or HZ virus reactivation, and 14.6% were urticaria. Eighty reactions (21%) were classified as severe/very severe and 81% required treatment.11 One study reported 414 skin reactions from the COVID-19 vaccine from December 2020 to February 2021; of these cases, 83% occurred after the Moderna vaccine, which is not available in Turkey, and 17% occurred after the Pfizer-BioNTech vaccine.12A systematic review of 91 patients who developed HZ infection after COVID-19 vaccination reported that 10% (9/91) of cases were receiving immunosuppressive therapy and 13% (12/91) had an autoimmune disease.7 In our case series, it is known that at least 2 of the patients (patients 2 and 5), including 1 patient with rheumatoid arthritis (patient 2) who was on immunosuppressive treatment, had autoimmune disorders. However, reports in the literature indicate that most patients with autoimmune inflammatory rheumatic diseases remain stable after vaccination.13
Herpes zoster ophthalmicus is a rare form of HZ caused by involvement of the ophthalmic branch of the trigeminal nerve that manifests as vesicular lesions and retinitis, uveitis, keratitis, conjunctivitis, and pain on an erythematous background. Two cases of women who developed HZO infection after Pfizer-BioNTech vaccination were reported in the literature.14 Although patient 3 in our case series had a history of HZO 6 years prior, the possibility of the Pfizer-BioNTech vaccine triggering HZO should be taken into consideration.
Although cutaneous reactions after the Sinovac-Coronavac vaccine were observed in only 1 of 7 patients in our case series, skin reactions after Sinovac-Coronavac (an inactivated viral vaccine) have been reported in the literature. In one study, after a total of 35,229 injections, the incidence of cutaneous adverse events due to Sinovac-Coronavac was reported to be 0.94% and 0.70% after the first and second doses, respectively.15 Therefore, further study results are needed to directly attribute the reactions to COVID-19 vaccination.
Conclusion
Our case series highlights that clinicians should be vigilant in diagnosing cutaneous reactions following COVID-19 vaccination early to prevent potential complications. Early recognition of reactions is crucial, and the prognosis can be improved with appropriate treatment. Despite the potential dermatologic adverse effects of the COVID-19 vaccine, the most effective way to protect against serious COVID-19 infection is to continue to be vaccinated.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603-2615.
- Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181-192.
- Tan SW, Tam YC, Oh CC. Skin manifestations of COVID-19: a worldwide review. JAAD Int. 2021;2:119-133.
- Singh H, Kaur H, Singh K, et al. Cutaneous manifestations of COVID-19: a systematic review. advances in wound care. 2021;10:51-80.
- Aksu SB, Öztürk GZ. A rare case of shingles after COVID-19 vaccine: is it a possible adverse effect? clinical and experimental vaccine research. 2021;10:198-201.
- Bostan E, Yalici-Armagan B. Herpes zoster following inactivated COVID-19 vaccine: a coexistence or coincidence? J Cosmet Dermatol. 2021;20:1566-1567.
- Katsikas Triantafyllidis K, Giannos P, Mian IT, et al. Varicella zoster virus reactivation following COVID-19 vaccination: a systematic review of case reports. Vaccines (Basel). 2021;9:1013. doi:10.3390/vaccines9091013
- Rodríguez-Jiménez P, Chicharro P, Cabrera LM, et al. Varicella-zoster virus reactivation after SARS-CoV-2 BNT162b2 mRNA vaccination: report of 5 cases. JAAD Case Rep. 2021;12:58-59. doi:10.1016/j.jdcr.2021.04.014
- Lee C, Cotter D, Basa J, et al. 20 Post-COVID-19 vaccine-related shingles cases seen at the Las Vegas Dermatology clinic and sent to us via social media. J Cosmet Dermatol. 2021;20:1960-1964.
- Fathy RA, McMahon DE, Lee C, et al. Varicella-zoster and herpes simplex virus reactivation post-COVID-19 vaccination: a review of 40 cases in an International Dermatology Registry. J Eur Acad Dermatol Venerol. 2022;36:E6-E9.
- Català A, Muñoz-Santos C, Galván-Casas C, et al. Cutaneous reactions after SARS-CoV-2 vaccination: a cross-sectional Spanish nationwide study of 405 cases. Br J Dermatol. 2022;186:142-152.
- McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021;85:46-55.
- Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80:1330-1338.
- Bernardini N, Skroza N, Mambrin A, et al. Herpes zoster ophthalmicus in two women after Pfizer-BioNTech (BNT162b2) vaccine. J Med Virol. 2022;94:817-818.
- Rerknimitr P, Puaratanaarunkon T, Wongtada C, et al. Cutaneous adverse reactions from 35,229 doses of Sinovac and AstraZeneca COVID-19 vaccination: a prospective cohort study in healthcare workers. J Eur Acad Dermatol Venereol. 2022;36:E158-E161.
Cutaneous reactions associated with the Pfizer-BioNTech COVID-19 vaccine have been reported worldwide since December 2020. Local injection site reactions (<1%) such as erythema, swelling, delayed local reactions (1%–10%), morbilliform rash, urticarial reactions, pityriasis rosea, Rowell syndrome, and lichen planus have been reported following the Pfizer-BioNTech COVID-19 vaccine.1 Cutaneous reactions reported in association with the Sinovac-Coronavac COVID-19 vaccine include swelling, redness, itching, discoloration, induration (1%–10%), urticaria, petechial rash, and exacerbation of psoriasis at the local injection site (<1%).2
We describe 7 patients from Turkey who presented with various dermatologic problems 5 to 28 days after COVID-19 vaccination, highlighting the possibility of early and late cutaneous reactions related to the vaccine (Table).

Case Reports
Patient 1—A 44-year-old woman was admitted to the dermatology clinic with painful lesions on the trunk of 3 days’ duration. Dermatologic examination revealed grouped erythematous vesicles showing dermatomal spread in the right thoracolumbar (dermatome T10) region. The patient reported that she had received 2 doses of the Sinovac-Coronavac vaccine (doses 1 and 2) and 2 doses of the BioNTech COVID-19 vaccine (doses 3 and 4); the rash had developed 28 days after she received the 4th dose. Her medical history was unremarkable. The lesions regressed after 1 week of treatment with oral valacyclovir 1000 mg 3 times daily, but she developed postherpetic neuralgia 1 week after starting treatment, which resolved after 8 weeks.
Patient 2—A 68-year-old woman presented to the dermatology clinic for evaluation of painful sores on the upper lip of 1 day’s duration. She had a history of rheumatoid arthritis, hypertension, and atopy and was currently taking prednisone and etanercept. Dermatologic examination revealed grouped vesicles on an erythematous base on the upper lip. A diagnosis of herpes labialis was made. The patient reported that she had received a third dose of the Sinovac-Coronavac vaccine 10 days prior to the appearance of the lesions. Her symptoms resolved completely within 2 weeks of treatment with topical acyclovir.
Patient 3—A 64-year-old woman was admitted to the hospital with pain, redness, and watery sores on and around the left eyelid of 2 days’ duration. Dermatologic evaluation revealed the erythematous surface of the left eyelid and periorbital area showed partial crusts, clustered vesicles, erythema, and edema. Additionally, the conjunctiva was purulent and erythematous. The patient’s medical history was notable for allergic asthma, hypertension, anxiety, and depression. For this reason, the patient was prescribed an angiotensin receptor blocker and a selective serotonin reuptake inhibitor. She noted that a similar rash had developed around the left eye 6 years prior that was diagnosed as herpes zoster (HZ). She also reported that she had received 2 doses of the Sinovac-Coronavac COVID-19 vaccine followed by 1 dose of the BioNTech COVID-19 vaccine, which she had received 2 weeks before the rash developed. The patient was treated at the eye clinic and was found to have ocular involvement. Ophthalmology was consulted and a diagnosis of herpes zoster ophthalmicus (HZO) was made. Systemic valacyclovir treatment was initiated, resulting in clinical improvement within 3 weeks.
Patient 4—A 75-year-old man was admitted to the hospital with chest and back pain and widespread muscle pain of several days’ duration. His medical history was remarkable for diabetes mellitus, hypertension, depression, and coronary artery bypass surgery. A medication history revealed treatment with a β-blocker, acetylsalicylic acid, a calcium channel blocker, a dipeptidyl peptidase 4 inhibitor, and a selective serotonin reuptake inhibitor. Dermatologic examination revealed grouped vesicles on an erythematous background in dermatome T5 on the right chest and back. A diagnosis of HZ was made. The patient reported that he had received 2 doses of the Sinovac-Coronavac vaccine followed by 1 dose of the Pfizer-BioNTech vaccine 2 weeks prior to the current presentation. He was treated with valacyclovir for 1 week, and his symptoms resolved entirely within 3 weeks.
Patient 5—A 50-year-old woman presented to the hospital for evaluation of painful sores on the back, chest, groin, and abdomen of 10 days’ duration. The lesions initially had developed 7 days after receiving the BioNTech COVID-19 vaccine; she previously had received 2 doses of the Sinovac-Coronavac vaccine. The patient had a history of untreated psoriasis. Dermatologic examination revealed grouped vesicles on an erythematous background in the T2–L2 dermatomes on the left side of the trunk. A diagnosis of HZ was made. The lesions resolved after 1 week of treatment with systemic valacyclovir; however, she subsequently developed postherpetic neuralgia, hypoesthesia, and postinflammatory hyperpigmentation in the affected regions.
Patient 6—A 37-year-old woman presented to the hospital with redness, swelling, and itching all over the body of 3 days’ duration. The patient noted that the rash would subside and reappear throughout the day. Her medical history was unremarkable, except for COVID-19 infection 6 months prior. She had received a second dose of the BioNTech vaccine 20 days prior to development of symptoms. Dermatologic examination revealed widespread erythematous urticarial plaques. A diagnosis of acute urticaria was made. The patient recovered completely after 1 week of treatment with a systemic steroid and 3 weeks of antihistamine treatment.
Patient 7—A 63-year-old woman presented to the hospital with widespread itching and rash that appeared 5 days after the first dose of the BioNTech COVID-19 vaccine. The patient reported that the rash resolved spontaneously within a few hours but then reappeared. Her medical history revealed that she was taking tamoxifen for breast cancer and that she previously had received 2 doses of the Sinovac-Coronavac vaccine. Dermatologic examination revealed erythematous urticarial plaques on the trunk and arms. A diagnosis of urticaria was made, and her symptoms resolved after 6 weeks of antihistamine treatment.
Comment
Skin lesions associated with COVID-19 infection have been reported worldwide3,4 as well as dermatologic reactions following COVID-19 vaccination. In one case from Turkey, HZ infection was reported in a 68-year-old man 5 days after he received a second dose of the COVID-19 vaccine.5 In another case, HZ infection developed in a 78-year-old man 5 days after COVID-19 vaccination.6 Numerous cases of HZ infection developing within 1 to 26 days of COVID-19 vaccination have been reported worldwide.7-9
In a study conducted in the United States, 40 skin reactions associated with the COVID-19 vaccine were investigated; of these cases, 87.5% (35/40) were reported as varicella-zoster virus, and 12.5% (5/40) were reported as herpes simplex reactivation; 54% (19/35) and 80% (4/5) of these cases, respectively, were associated with the Pfizer-BioNTech vaccine.10 The average age of patients who developed a skin reaction was 46 years, and 70% (28/40) were women. The time to onset of the reaction was 2 to 13 days after vaccination, and symptoms were reported to improve within 7 days on average.10
Another study from Spain examined 405 vaccine-related skin reactions, 40.2% of which were related to the Pfizer-BioNTech vaccine. Among them, 80.2% occurred in women; 13.8% of cases were diagnosed as varicella-zoster virus or HZ virus reactivation, and 14.6% were urticaria. Eighty reactions (21%) were classified as severe/very severe and 81% required treatment.11 One study reported 414 skin reactions from the COVID-19 vaccine from December 2020 to February 2021; of these cases, 83% occurred after the Moderna vaccine, which is not available in Turkey, and 17% occurred after the Pfizer-BioNTech vaccine.12A systematic review of 91 patients who developed HZ infection after COVID-19 vaccination reported that 10% (9/91) of cases were receiving immunosuppressive therapy and 13% (12/91) had an autoimmune disease.7 In our case series, it is known that at least 2 of the patients (patients 2 and 5), including 1 patient with rheumatoid arthritis (patient 2) who was on immunosuppressive treatment, had autoimmune disorders. However, reports in the literature indicate that most patients with autoimmune inflammatory rheumatic diseases remain stable after vaccination.13
Herpes zoster ophthalmicus is a rare form of HZ caused by involvement of the ophthalmic branch of the trigeminal nerve that manifests as vesicular lesions and retinitis, uveitis, keratitis, conjunctivitis, and pain on an erythematous background. Two cases of women who developed HZO infection after Pfizer-BioNTech vaccination were reported in the literature.14 Although patient 3 in our case series had a history of HZO 6 years prior, the possibility of the Pfizer-BioNTech vaccine triggering HZO should be taken into consideration.
Although cutaneous reactions after the Sinovac-Coronavac vaccine were observed in only 1 of 7 patients in our case series, skin reactions after Sinovac-Coronavac (an inactivated viral vaccine) have been reported in the literature. In one study, after a total of 35,229 injections, the incidence of cutaneous adverse events due to Sinovac-Coronavac was reported to be 0.94% and 0.70% after the first and second doses, respectively.15 Therefore, further study results are needed to directly attribute the reactions to COVID-19 vaccination.
Conclusion
Our case series highlights that clinicians should be vigilant in diagnosing cutaneous reactions following COVID-19 vaccination early to prevent potential complications. Early recognition of reactions is crucial, and the prognosis can be improved with appropriate treatment. Despite the potential dermatologic adverse effects of the COVID-19 vaccine, the most effective way to protect against serious COVID-19 infection is to continue to be vaccinated.
Cutaneous reactions associated with the Pfizer-BioNTech COVID-19 vaccine have been reported worldwide since December 2020. Local injection site reactions (<1%) such as erythema, swelling, delayed local reactions (1%–10%), morbilliform rash, urticarial reactions, pityriasis rosea, Rowell syndrome, and lichen planus have been reported following the Pfizer-BioNTech COVID-19 vaccine.1 Cutaneous reactions reported in association with the Sinovac-Coronavac COVID-19 vaccine include swelling, redness, itching, discoloration, induration (1%–10%), urticaria, petechial rash, and exacerbation of psoriasis at the local injection site (<1%).2
We describe 7 patients from Turkey who presented with various dermatologic problems 5 to 28 days after COVID-19 vaccination, highlighting the possibility of early and late cutaneous reactions related to the vaccine (Table).

Case Reports
Patient 1—A 44-year-old woman was admitted to the dermatology clinic with painful lesions on the trunk of 3 days’ duration. Dermatologic examination revealed grouped erythematous vesicles showing dermatomal spread in the right thoracolumbar (dermatome T10) region. The patient reported that she had received 2 doses of the Sinovac-Coronavac vaccine (doses 1 and 2) and 2 doses of the BioNTech COVID-19 vaccine (doses 3 and 4); the rash had developed 28 days after she received the 4th dose. Her medical history was unremarkable. The lesions regressed after 1 week of treatment with oral valacyclovir 1000 mg 3 times daily, but she developed postherpetic neuralgia 1 week after starting treatment, which resolved after 8 weeks.
Patient 2—A 68-year-old woman presented to the dermatology clinic for evaluation of painful sores on the upper lip of 1 day’s duration. She had a history of rheumatoid arthritis, hypertension, and atopy and was currently taking prednisone and etanercept. Dermatologic examination revealed grouped vesicles on an erythematous base on the upper lip. A diagnosis of herpes labialis was made. The patient reported that she had received a third dose of the Sinovac-Coronavac vaccine 10 days prior to the appearance of the lesions. Her symptoms resolved completely within 2 weeks of treatment with topical acyclovir.
Patient 3—A 64-year-old woman was admitted to the hospital with pain, redness, and watery sores on and around the left eyelid of 2 days’ duration. Dermatologic evaluation revealed the erythematous surface of the left eyelid and periorbital area showed partial crusts, clustered vesicles, erythema, and edema. Additionally, the conjunctiva was purulent and erythematous. The patient’s medical history was notable for allergic asthma, hypertension, anxiety, and depression. For this reason, the patient was prescribed an angiotensin receptor blocker and a selective serotonin reuptake inhibitor. She noted that a similar rash had developed around the left eye 6 years prior that was diagnosed as herpes zoster (HZ). She also reported that she had received 2 doses of the Sinovac-Coronavac COVID-19 vaccine followed by 1 dose of the BioNTech COVID-19 vaccine, which she had received 2 weeks before the rash developed. The patient was treated at the eye clinic and was found to have ocular involvement. Ophthalmology was consulted and a diagnosis of herpes zoster ophthalmicus (HZO) was made. Systemic valacyclovir treatment was initiated, resulting in clinical improvement within 3 weeks.
Patient 4—A 75-year-old man was admitted to the hospital with chest and back pain and widespread muscle pain of several days’ duration. His medical history was remarkable for diabetes mellitus, hypertension, depression, and coronary artery bypass surgery. A medication history revealed treatment with a β-blocker, acetylsalicylic acid, a calcium channel blocker, a dipeptidyl peptidase 4 inhibitor, and a selective serotonin reuptake inhibitor. Dermatologic examination revealed grouped vesicles on an erythematous background in dermatome T5 on the right chest and back. A diagnosis of HZ was made. The patient reported that he had received 2 doses of the Sinovac-Coronavac vaccine followed by 1 dose of the Pfizer-BioNTech vaccine 2 weeks prior to the current presentation. He was treated with valacyclovir for 1 week, and his symptoms resolved entirely within 3 weeks.
Patient 5—A 50-year-old woman presented to the hospital for evaluation of painful sores on the back, chest, groin, and abdomen of 10 days’ duration. The lesions initially had developed 7 days after receiving the BioNTech COVID-19 vaccine; she previously had received 2 doses of the Sinovac-Coronavac vaccine. The patient had a history of untreated psoriasis. Dermatologic examination revealed grouped vesicles on an erythematous background in the T2–L2 dermatomes on the left side of the trunk. A diagnosis of HZ was made. The lesions resolved after 1 week of treatment with systemic valacyclovir; however, she subsequently developed postherpetic neuralgia, hypoesthesia, and postinflammatory hyperpigmentation in the affected regions.
Patient 6—A 37-year-old woman presented to the hospital with redness, swelling, and itching all over the body of 3 days’ duration. The patient noted that the rash would subside and reappear throughout the day. Her medical history was unremarkable, except for COVID-19 infection 6 months prior. She had received a second dose of the BioNTech vaccine 20 days prior to development of symptoms. Dermatologic examination revealed widespread erythematous urticarial plaques. A diagnosis of acute urticaria was made. The patient recovered completely after 1 week of treatment with a systemic steroid and 3 weeks of antihistamine treatment.
Patient 7—A 63-year-old woman presented to the hospital with widespread itching and rash that appeared 5 days after the first dose of the BioNTech COVID-19 vaccine. The patient reported that the rash resolved spontaneously within a few hours but then reappeared. Her medical history revealed that she was taking tamoxifen for breast cancer and that she previously had received 2 doses of the Sinovac-Coronavac vaccine. Dermatologic examination revealed erythematous urticarial plaques on the trunk and arms. A diagnosis of urticaria was made, and her symptoms resolved after 6 weeks of antihistamine treatment.
Comment
Skin lesions associated with COVID-19 infection have been reported worldwide3,4 as well as dermatologic reactions following COVID-19 vaccination. In one case from Turkey, HZ infection was reported in a 68-year-old man 5 days after he received a second dose of the COVID-19 vaccine.5 In another case, HZ infection developed in a 78-year-old man 5 days after COVID-19 vaccination.6 Numerous cases of HZ infection developing within 1 to 26 days of COVID-19 vaccination have been reported worldwide.7-9
In a study conducted in the United States, 40 skin reactions associated with the COVID-19 vaccine were investigated; of these cases, 87.5% (35/40) were reported as varicella-zoster virus, and 12.5% (5/40) were reported as herpes simplex reactivation; 54% (19/35) and 80% (4/5) of these cases, respectively, were associated with the Pfizer-BioNTech vaccine.10 The average age of patients who developed a skin reaction was 46 years, and 70% (28/40) were women. The time to onset of the reaction was 2 to 13 days after vaccination, and symptoms were reported to improve within 7 days on average.10
Another study from Spain examined 405 vaccine-related skin reactions, 40.2% of which were related to the Pfizer-BioNTech vaccine. Among them, 80.2% occurred in women; 13.8% of cases were diagnosed as varicella-zoster virus or HZ virus reactivation, and 14.6% were urticaria. Eighty reactions (21%) were classified as severe/very severe and 81% required treatment.11 One study reported 414 skin reactions from the COVID-19 vaccine from December 2020 to February 2021; of these cases, 83% occurred after the Moderna vaccine, which is not available in Turkey, and 17% occurred after the Pfizer-BioNTech vaccine.12A systematic review of 91 patients who developed HZ infection after COVID-19 vaccination reported that 10% (9/91) of cases were receiving immunosuppressive therapy and 13% (12/91) had an autoimmune disease.7 In our case series, it is known that at least 2 of the patients (patients 2 and 5), including 1 patient with rheumatoid arthritis (patient 2) who was on immunosuppressive treatment, had autoimmune disorders. However, reports in the literature indicate that most patients with autoimmune inflammatory rheumatic diseases remain stable after vaccination.13
Herpes zoster ophthalmicus is a rare form of HZ caused by involvement of the ophthalmic branch of the trigeminal nerve that manifests as vesicular lesions and retinitis, uveitis, keratitis, conjunctivitis, and pain on an erythematous background. Two cases of women who developed HZO infection after Pfizer-BioNTech vaccination were reported in the literature.14 Although patient 3 in our case series had a history of HZO 6 years prior, the possibility of the Pfizer-BioNTech vaccine triggering HZO should be taken into consideration.
Although cutaneous reactions after the Sinovac-Coronavac vaccine were observed in only 1 of 7 patients in our case series, skin reactions after Sinovac-Coronavac (an inactivated viral vaccine) have been reported in the literature. In one study, after a total of 35,229 injections, the incidence of cutaneous adverse events due to Sinovac-Coronavac was reported to be 0.94% and 0.70% after the first and second doses, respectively.15 Therefore, further study results are needed to directly attribute the reactions to COVID-19 vaccination.
Conclusion
Our case series highlights that clinicians should be vigilant in diagnosing cutaneous reactions following COVID-19 vaccination early to prevent potential complications. Early recognition of reactions is crucial, and the prognosis can be improved with appropriate treatment. Despite the potential dermatologic adverse effects of the COVID-19 vaccine, the most effective way to protect against serious COVID-19 infection is to continue to be vaccinated.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603-2615.
- Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181-192.
- Tan SW, Tam YC, Oh CC. Skin manifestations of COVID-19: a worldwide review. JAAD Int. 2021;2:119-133.
- Singh H, Kaur H, Singh K, et al. Cutaneous manifestations of COVID-19: a systematic review. advances in wound care. 2021;10:51-80.
- Aksu SB, Öztürk GZ. A rare case of shingles after COVID-19 vaccine: is it a possible adverse effect? clinical and experimental vaccine research. 2021;10:198-201.
- Bostan E, Yalici-Armagan B. Herpes zoster following inactivated COVID-19 vaccine: a coexistence or coincidence? J Cosmet Dermatol. 2021;20:1566-1567.
- Katsikas Triantafyllidis K, Giannos P, Mian IT, et al. Varicella zoster virus reactivation following COVID-19 vaccination: a systematic review of case reports. Vaccines (Basel). 2021;9:1013. doi:10.3390/vaccines9091013
- Rodríguez-Jiménez P, Chicharro P, Cabrera LM, et al. Varicella-zoster virus reactivation after SARS-CoV-2 BNT162b2 mRNA vaccination: report of 5 cases. JAAD Case Rep. 2021;12:58-59. doi:10.1016/j.jdcr.2021.04.014
- Lee C, Cotter D, Basa J, et al. 20 Post-COVID-19 vaccine-related shingles cases seen at the Las Vegas Dermatology clinic and sent to us via social media. J Cosmet Dermatol. 2021;20:1960-1964.
- Fathy RA, McMahon DE, Lee C, et al. Varicella-zoster and herpes simplex virus reactivation post-COVID-19 vaccination: a review of 40 cases in an International Dermatology Registry. J Eur Acad Dermatol Venerol. 2022;36:E6-E9.
- Català A, Muñoz-Santos C, Galván-Casas C, et al. Cutaneous reactions after SARS-CoV-2 vaccination: a cross-sectional Spanish nationwide study of 405 cases. Br J Dermatol. 2022;186:142-152.
- McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021;85:46-55.
- Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80:1330-1338.
- Bernardini N, Skroza N, Mambrin A, et al. Herpes zoster ophthalmicus in two women after Pfizer-BioNTech (BNT162b2) vaccine. J Med Virol. 2022;94:817-818.
- Rerknimitr P, Puaratanaarunkon T, Wongtada C, et al. Cutaneous adverse reactions from 35,229 doses of Sinovac and AstraZeneca COVID-19 vaccination: a prospective cohort study in healthcare workers. J Eur Acad Dermatol Venereol. 2022;36:E158-E161.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603-2615.
- Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181-192.
- Tan SW, Tam YC, Oh CC. Skin manifestations of COVID-19: a worldwide review. JAAD Int. 2021;2:119-133.
- Singh H, Kaur H, Singh K, et al. Cutaneous manifestations of COVID-19: a systematic review. advances in wound care. 2021;10:51-80.
- Aksu SB, Öztürk GZ. A rare case of shingles after COVID-19 vaccine: is it a possible adverse effect? clinical and experimental vaccine research. 2021;10:198-201.
- Bostan E, Yalici-Armagan B. Herpes zoster following inactivated COVID-19 vaccine: a coexistence or coincidence? J Cosmet Dermatol. 2021;20:1566-1567.
- Katsikas Triantafyllidis K, Giannos P, Mian IT, et al. Varicella zoster virus reactivation following COVID-19 vaccination: a systematic review of case reports. Vaccines (Basel). 2021;9:1013. doi:10.3390/vaccines9091013
- Rodríguez-Jiménez P, Chicharro P, Cabrera LM, et al. Varicella-zoster virus reactivation after SARS-CoV-2 BNT162b2 mRNA vaccination: report of 5 cases. JAAD Case Rep. 2021;12:58-59. doi:10.1016/j.jdcr.2021.04.014
- Lee C, Cotter D, Basa J, et al. 20 Post-COVID-19 vaccine-related shingles cases seen at the Las Vegas Dermatology clinic and sent to us via social media. J Cosmet Dermatol. 2021;20:1960-1964.
- Fathy RA, McMahon DE, Lee C, et al. Varicella-zoster and herpes simplex virus reactivation post-COVID-19 vaccination: a review of 40 cases in an International Dermatology Registry. J Eur Acad Dermatol Venerol. 2022;36:E6-E9.
- Català A, Muñoz-Santos C, Galván-Casas C, et al. Cutaneous reactions after SARS-CoV-2 vaccination: a cross-sectional Spanish nationwide study of 405 cases. Br J Dermatol. 2022;186:142-152.
- McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021;85:46-55.
- Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80:1330-1338.
- Bernardini N, Skroza N, Mambrin A, et al. Herpes zoster ophthalmicus in two women after Pfizer-BioNTech (BNT162b2) vaccine. J Med Virol. 2022;94:817-818.
- Rerknimitr P, Puaratanaarunkon T, Wongtada C, et al. Cutaneous adverse reactions from 35,229 doses of Sinovac and AstraZeneca COVID-19 vaccination: a prospective cohort study in healthcare workers. J Eur Acad Dermatol Venereol. 2022;36:E158-E161.
Practice Points
- Cutaneous reactions have been reported following COVID-19 vaccination.
- Herpes infections and urticarial reactions can be associated with COVID-19 vaccination, regardless of the delay in onset between the injection and symptom development.
Determinants of Topical Corticosteroid Effectiveness in Eosinophilic Esophagitis in Real‐World Practice
Key clinical point: Reduced symptom severity and use of budesonide orodispersible tablets and high topical corticosteroid (tC) doses (eg, fluticasone propionate metered dose ≥ 1 mg/day from inhalation devices) are all independent predictors of tC effectiveness in patients with eosinophilic esophagitis (EoE) in the real‐world setting.
Major finding: Corticosteroid treatment proved to be the most important determining factor in achieving clinico-histological remission, with budesonide orodispersible tablets presenting the highest efficacy (adjusted odds ratio [aOR], 18.9; P < .001). High tC doses (aOR, 4.3; P = .03) and lower symptom scores (aOR, 0.9; P = .01) were also significant predictors of tC effectiveness.
Study details: This real-world cross‐sectional analysis of the multicenter EoE CONNECT registry assessed the data on 1456 prescriptions of tC monotherapy used in 866 patients with EoE.
Disclosures: This study did not receive any specific funding. The authors declared no conflicts of interest.
Source: Laserna‐Mendieta EJ, Navarro P, Casabona-Francés S, et al. Swallowed topical corticosteroids for eosinophilic esophagitis: Utilization and real‐world efficacy from the EoE CONNECT registry. United European Gastroenterol J. Published online January 29, 2024. Source
Key clinical point: Reduced symptom severity and use of budesonide orodispersible tablets and high topical corticosteroid (tC) doses (eg, fluticasone propionate metered dose ≥ 1 mg/day from inhalation devices) are all independent predictors of tC effectiveness in patients with eosinophilic esophagitis (EoE) in the real‐world setting.
Major finding: Corticosteroid treatment proved to be the most important determining factor in achieving clinico-histological remission, with budesonide orodispersible tablets presenting the highest efficacy (adjusted odds ratio [aOR], 18.9; P < .001). High tC doses (aOR, 4.3; P = .03) and lower symptom scores (aOR, 0.9; P = .01) were also significant predictors of tC effectiveness.
Study details: This real-world cross‐sectional analysis of the multicenter EoE CONNECT registry assessed the data on 1456 prescriptions of tC monotherapy used in 866 patients with EoE.
Disclosures: This study did not receive any specific funding. The authors declared no conflicts of interest.
Source: Laserna‐Mendieta EJ, Navarro P, Casabona-Francés S, et al. Swallowed topical corticosteroids for eosinophilic esophagitis: Utilization and real‐world efficacy from the EoE CONNECT registry. United European Gastroenterol J. Published online January 29, 2024. Source
Key clinical point: Reduced symptom severity and use of budesonide orodispersible tablets and high topical corticosteroid (tC) doses (eg, fluticasone propionate metered dose ≥ 1 mg/day from inhalation devices) are all independent predictors of tC effectiveness in patients with eosinophilic esophagitis (EoE) in the real‐world setting.
Major finding: Corticosteroid treatment proved to be the most important determining factor in achieving clinico-histological remission, with budesonide orodispersible tablets presenting the highest efficacy (adjusted odds ratio [aOR], 18.9; P < .001). High tC doses (aOR, 4.3; P = .03) and lower symptom scores (aOR, 0.9; P = .01) were also significant predictors of tC effectiveness.
Study details: This real-world cross‐sectional analysis of the multicenter EoE CONNECT registry assessed the data on 1456 prescriptions of tC monotherapy used in 866 patients with EoE.
Disclosures: This study did not receive any specific funding. The authors declared no conflicts of interest.
Source: Laserna‐Mendieta EJ, Navarro P, Casabona-Francés S, et al. Swallowed topical corticosteroids for eosinophilic esophagitis: Utilization and real‐world efficacy from the EoE CONNECT registry. United European Gastroenterol J. Published online January 29, 2024. Source
Salivary Proteins May Have a Diagnostic Potential in Pediatric Eosinophilic Esophagitis
Key clinical point: Children with eosinophilic esophagitis (EoE) have distinct salivary immunoinflammatory protein profiles compared with those without EoE, highlighting the diagnostic potential of salivary proteins in pediatric EoE.
Major finding: Children with EoE were distinguished from those without EoE through a panel of 10 proteins (higher expression levels of C-C motif chemokine-3, macrophage metalloelastase, granulocyte colony-stimulating factor-3, interleukin [IL]-15, pro-transforming growth factor alpha, and oncostatin-M and lower expression levels of IL-18, C-C motif chemokine-2, interstitial collagenase, and macrophage colony-stimulating factor-1), with an accuracy of 0.95 validated through the Least Absolute Shrinkage and Selection Operator regression.
Study details: This cross-sectional study analyzed the saliva samples collected from 40 children aged 6-18 years with (n = 23) or without EoE (n = 17) immediately before their scheduled esophagogastroduodenoscopy with biopsies.
Disclosures: Girish Hiremath and Seesandra V Rajagopala were supported by grants from the US National Institutes of Health. The former declared serving as a consultant for and receiving speaker fees from various organizations.
Source: Hiremath G, Wang Y, Correa H, Sheng Q, Rajagopala SV. Salivary immunoinflammatory proteins identify children with eosinophilic esophagitis. Allergy. 2024 (Jan 29). doi: 10.1111/all.16040 Source
Key clinical point: Children with eosinophilic esophagitis (EoE) have distinct salivary immunoinflammatory protein profiles compared with those without EoE, highlighting the diagnostic potential of salivary proteins in pediatric EoE.
Major finding: Children with EoE were distinguished from those without EoE through a panel of 10 proteins (higher expression levels of C-C motif chemokine-3, macrophage metalloelastase, granulocyte colony-stimulating factor-3, interleukin [IL]-15, pro-transforming growth factor alpha, and oncostatin-M and lower expression levels of IL-18, C-C motif chemokine-2, interstitial collagenase, and macrophage colony-stimulating factor-1), with an accuracy of 0.95 validated through the Least Absolute Shrinkage and Selection Operator regression.
Study details: This cross-sectional study analyzed the saliva samples collected from 40 children aged 6-18 years with (n = 23) or without EoE (n = 17) immediately before their scheduled esophagogastroduodenoscopy with biopsies.
Disclosures: Girish Hiremath and Seesandra V Rajagopala were supported by grants from the US National Institutes of Health. The former declared serving as a consultant for and receiving speaker fees from various organizations.
Source: Hiremath G, Wang Y, Correa H, Sheng Q, Rajagopala SV. Salivary immunoinflammatory proteins identify children with eosinophilic esophagitis. Allergy. 2024 (Jan 29). doi: 10.1111/all.16040 Source
Key clinical point: Children with eosinophilic esophagitis (EoE) have distinct salivary immunoinflammatory protein profiles compared with those without EoE, highlighting the diagnostic potential of salivary proteins in pediatric EoE.
Major finding: Children with EoE were distinguished from those without EoE through a panel of 10 proteins (higher expression levels of C-C motif chemokine-3, macrophage metalloelastase, granulocyte colony-stimulating factor-3, interleukin [IL]-15, pro-transforming growth factor alpha, and oncostatin-M and lower expression levels of IL-18, C-C motif chemokine-2, interstitial collagenase, and macrophage colony-stimulating factor-1), with an accuracy of 0.95 validated through the Least Absolute Shrinkage and Selection Operator regression.
Study details: This cross-sectional study analyzed the saliva samples collected from 40 children aged 6-18 years with (n = 23) or without EoE (n = 17) immediately before their scheduled esophagogastroduodenoscopy with biopsies.
Disclosures: Girish Hiremath and Seesandra V Rajagopala were supported by grants from the US National Institutes of Health. The former declared serving as a consultant for and receiving speaker fees from various organizations.
Source: Hiremath G, Wang Y, Correa H, Sheng Q, Rajagopala SV. Salivary immunoinflammatory proteins identify children with eosinophilic esophagitis. Allergy. 2024 (Jan 29). doi: 10.1111/all.16040 Source
Eosinophilic Esophagitis Alters Eating Behaviors in Children
Key clinical point: Altered eating behaviors, including increased chewing and increased consumption time, observed in children with eosinophilic esophagitis (EoE) correlated with patient-reported symptoms, endoscopic findings, and histologic features.
Major finding: Children with vs without EoE demonstrated more chews per bite and increased consumption time with soft solid (P = .031 and P = .002, respectively), chewable (P = .047 and P = .005, respectively), and hard solid (P = .037 and P = .034, respectively) foods. Increased consumption time significantly correlated with a peak eosinophil count (r 0.42; P = .050) and decreased esophageal distensibility (r −0.82; P < .0001).
Study details: This prospective observational study included 27 children with EoE (age 5-17 years) and 25 control children without EoE who consumed four food items, each representing a different texture (puree, soft solid, chewable, and hard solid).
Disclosures: This study was supported by the 2021 American College of Gastroenterology Clinical Research Award and other sources. The authors declared no conflicts of interest.
Source: Kennedy KV, Umeweni CN, Alston M, et al. Esophageal remodeling correlates with eating behaviors in pediatric eosinophilic esophagitis. Am J Gastroenterol. 2024 (Jan 18). doi: 10.14309/ajg.0000000000002661 Source
Key clinical point: Altered eating behaviors, including increased chewing and increased consumption time, observed in children with eosinophilic esophagitis (EoE) correlated with patient-reported symptoms, endoscopic findings, and histologic features.
Major finding: Children with vs without EoE demonstrated more chews per bite and increased consumption time with soft solid (P = .031 and P = .002, respectively), chewable (P = .047 and P = .005, respectively), and hard solid (P = .037 and P = .034, respectively) foods. Increased consumption time significantly correlated with a peak eosinophil count (r 0.42; P = .050) and decreased esophageal distensibility (r −0.82; P < .0001).
Study details: This prospective observational study included 27 children with EoE (age 5-17 years) and 25 control children without EoE who consumed four food items, each representing a different texture (puree, soft solid, chewable, and hard solid).
Disclosures: This study was supported by the 2021 American College of Gastroenterology Clinical Research Award and other sources. The authors declared no conflicts of interest.
Source: Kennedy KV, Umeweni CN, Alston M, et al. Esophageal remodeling correlates with eating behaviors in pediatric eosinophilic esophagitis. Am J Gastroenterol. 2024 (Jan 18). doi: 10.14309/ajg.0000000000002661 Source
Key clinical point: Altered eating behaviors, including increased chewing and increased consumption time, observed in children with eosinophilic esophagitis (EoE) correlated with patient-reported symptoms, endoscopic findings, and histologic features.
Major finding: Children with vs without EoE demonstrated more chews per bite and increased consumption time with soft solid (P = .031 and P = .002, respectively), chewable (P = .047 and P = .005, respectively), and hard solid (P = .037 and P = .034, respectively) foods. Increased consumption time significantly correlated with a peak eosinophil count (r 0.42; P = .050) and decreased esophageal distensibility (r −0.82; P < .0001).
Study details: This prospective observational study included 27 children with EoE (age 5-17 years) and 25 control children without EoE who consumed four food items, each representing a different texture (puree, soft solid, chewable, and hard solid).
Disclosures: This study was supported by the 2021 American College of Gastroenterology Clinical Research Award and other sources. The authors declared no conflicts of interest.
Source: Kennedy KV, Umeweni CN, Alston M, et al. Esophageal remodeling correlates with eating behaviors in pediatric eosinophilic esophagitis. Am J Gastroenterol. 2024 (Jan 18). doi: 10.14309/ajg.0000000000002661 Source
Major Basic Protein Can Distinguish Eosinophilic Esophagitis From IBD-Associated Eosinophilia
Key clinical point: Eosinophilic esophagitis (EoE) can be distinguished from eosinophilia caused by inflammatory bowel diseases (IBD) by measuring the expression levels of the major basic protein (MBP) biomarker.
Major finding: The median MBP staining levels were significantly higher in patients with EoE vs those with IBD-associated eosinophilia (52.8 vs 0.2; P < .001). Based on the MBP cutoff point of 3.49 units that distinguished EoE from non-EoE cases, 100% of patients with EoE were MBP positive compared with 3% of patients with IBD-associated eosinophilia (P < .05).
Study details: This retrospective study included 29 patients with EoE, 27 patients with both EoE and IBD, 29 patients with IBD-associated eosinophilia, 30 patients with IBD, and 30 control individuals without either EoE or IBD.
Disclosures: This study did not report any source of funding. The authors declared no conflicts of interest.
Source: Butzke S, Nasiri-Blomgren S, Corao-Uribe D, He Z, Molle-Rios Z. Major basic protein is a useful marker to distinguish eosinophilic esophagitis from IBD-associated eosinophilia in children. J Pediatr Gastroenterol Nutr. 2024 (Feb 5). doi: 10.1002/jpn3.12143 Source
Key clinical point: Eosinophilic esophagitis (EoE) can be distinguished from eosinophilia caused by inflammatory bowel diseases (IBD) by measuring the expression levels of the major basic protein (MBP) biomarker.
Major finding: The median MBP staining levels were significantly higher in patients with EoE vs those with IBD-associated eosinophilia (52.8 vs 0.2; P < .001). Based on the MBP cutoff point of 3.49 units that distinguished EoE from non-EoE cases, 100% of patients with EoE were MBP positive compared with 3% of patients with IBD-associated eosinophilia (P < .05).
Study details: This retrospective study included 29 patients with EoE, 27 patients with both EoE and IBD, 29 patients with IBD-associated eosinophilia, 30 patients with IBD, and 30 control individuals without either EoE or IBD.
Disclosures: This study did not report any source of funding. The authors declared no conflicts of interest.
Source: Butzke S, Nasiri-Blomgren S, Corao-Uribe D, He Z, Molle-Rios Z. Major basic protein is a useful marker to distinguish eosinophilic esophagitis from IBD-associated eosinophilia in children. J Pediatr Gastroenterol Nutr. 2024 (Feb 5). doi: 10.1002/jpn3.12143 Source
Key clinical point: Eosinophilic esophagitis (EoE) can be distinguished from eosinophilia caused by inflammatory bowel diseases (IBD) by measuring the expression levels of the major basic protein (MBP) biomarker.
Major finding: The median MBP staining levels were significantly higher in patients with EoE vs those with IBD-associated eosinophilia (52.8 vs 0.2; P < .001). Based on the MBP cutoff point of 3.49 units that distinguished EoE from non-EoE cases, 100% of patients with EoE were MBP positive compared with 3% of patients with IBD-associated eosinophilia (P < .05).
Study details: This retrospective study included 29 patients with EoE, 27 patients with both EoE and IBD, 29 patients with IBD-associated eosinophilia, 30 patients with IBD, and 30 control individuals without either EoE or IBD.
Disclosures: This study did not report any source of funding. The authors declared no conflicts of interest.
Source: Butzke S, Nasiri-Blomgren S, Corao-Uribe D, He Z, Molle-Rios Z. Major basic protein is a useful marker to distinguish eosinophilic esophagitis from IBD-associated eosinophilia in children. J Pediatr Gastroenterol Nutr. 2024 (Feb 5). doi: 10.1002/jpn3.12143 Source