User login
Clinical Outcomes of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis Based on Hospital Admission Type
Clinical Outcomes of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis Based on Hospital Admission Type
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are rare, life-threatening conditions that involve widespread necrosis of the skin and mucous membranes.1 Guidelines for SJS and TEN recommend management in hospitals with access to inpatient dermatology to provide immediate interventions that are necessary for achieving optimal patient outcomes.2 A delay in admission of 5 days or more after onset of symptoms has been associated with increases in overall mortality, bacteremia, intensive care unit (ICU) admission, and length of stay.3 Patients who are not directly admitted to specialized facilities and require transfer from other hospitals may experience delays in receiving critical interventions, further increasing the risk for mortality and complications. In this study, we analyzed the clinical outcomes of patients with SJS/TEN in relation to their admission pathway.
Methods
A single-center retrospective chart review was performed at Atrium Health Wake Forest Baptist Medical Center (AHWFBMC) in Winston-Salem, North Carolina. Participants were identified using i2b2, an informatics tool compliant with the Health Insurance Portability and Accountability Act for integrating biology and the bedside. Inclusion criteria were having a diagnosis of SJS (International Classification of Diseases, Tenth Revision, code L51.1; International Classification of Diseases, Ninth Revision, code 695.13), TEN (International Classification of Diseases, Tenth Revision, code L51.2; International Classification of Diseases, Ninth Revision, code 695.15) or Lyell syndrome from January 2012 to December 2024. Patients with erythema multiforme or bullous drug eruption were excluded, as these conditions initially were misdiagnosed as SJS or TEN. Patients with only a reported history of prior SJS or TEN also were excluded.
The following clinical outcomes were assessed: demographics, comorbidities, age at disease onset, outside hospital transfer status, complications during admission, inpatient length of stay in days, age of mortality (if applicable), culprit medications, interventions received, Severity-of-Illness Score for Toxic Epidermal Necrolysis (SCORTEN) upon admission, site of admission (eg, floor bed, ICU, medical ICU, burn unit), and length of disease process prior to hospital admission. Patients then were categorized as either direct or transfer admissions based on the initial point of care and admission process. Direct admissions included patients who presented to the AHWFBMC emergency department and were subsequently admitted. Transfer patients included patients who initially presented to an outside hospital and were transferred to AHWFBMC. Data regarding the wait time for Physician Access Line requests and the time elapsed from the initial transfer call to arrival at the tertiary hospital also were collected—this is a method that outside hospitals can use to contact physicians at the tertiary hospital for a possible transfer. Statistical analysis was performed using unpaired t tests and X2 tests as necessary using GraphPad By Dotmatics Prism.
Results
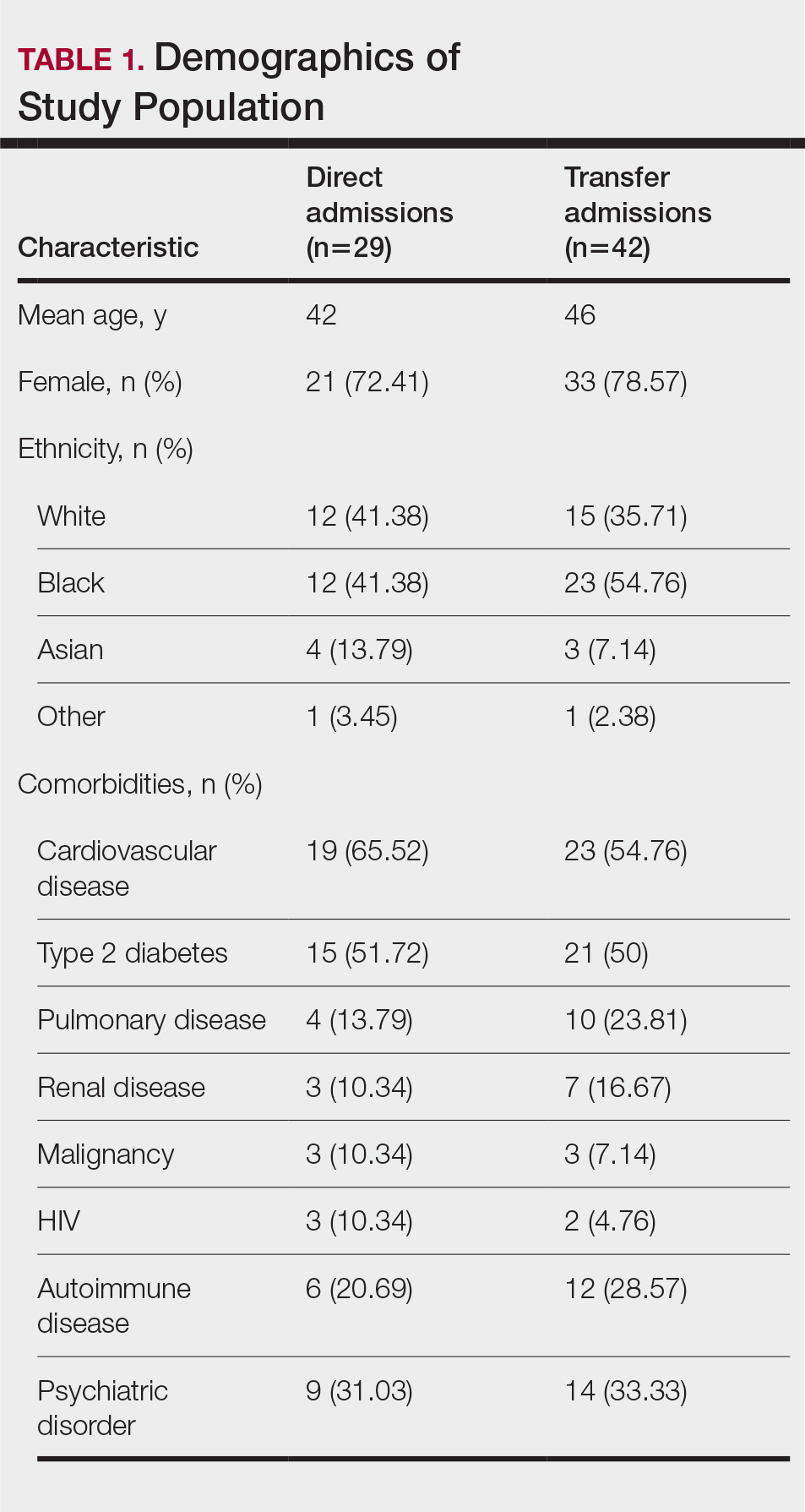
A total of 112 patients were included in the analysis; of these, 71 had a diagnosis with biopsy confirmation of SJS, SJS/TEN overlap, or TEN (Table 1). Forty-one patients were excluded due to having a diagnosis of erythema multiforme or bullous drug eruption or a reported history of prior SJS or TEN without hospitalization. All biopsies were performed at AHWFBMC. Of the 71 confirmed patients with SJS/TEN, 54 (76%) were female with a mean age of 44 years. The majority of patients identified as Black (35 [49%]) or White (27 [38%]), along with Asian (7 [10%]) and other (2 [3%]). The most common comorbidity was cardiovascular disease in 42 (59%) patients, followed by type 2 diabetes in 36 (51%) patients. Among these 71 patients with SJS/TEN, 29 (41%) were directly admitted to the tertiary hospital, while 42 (59%) were transferred from outside hospitals.
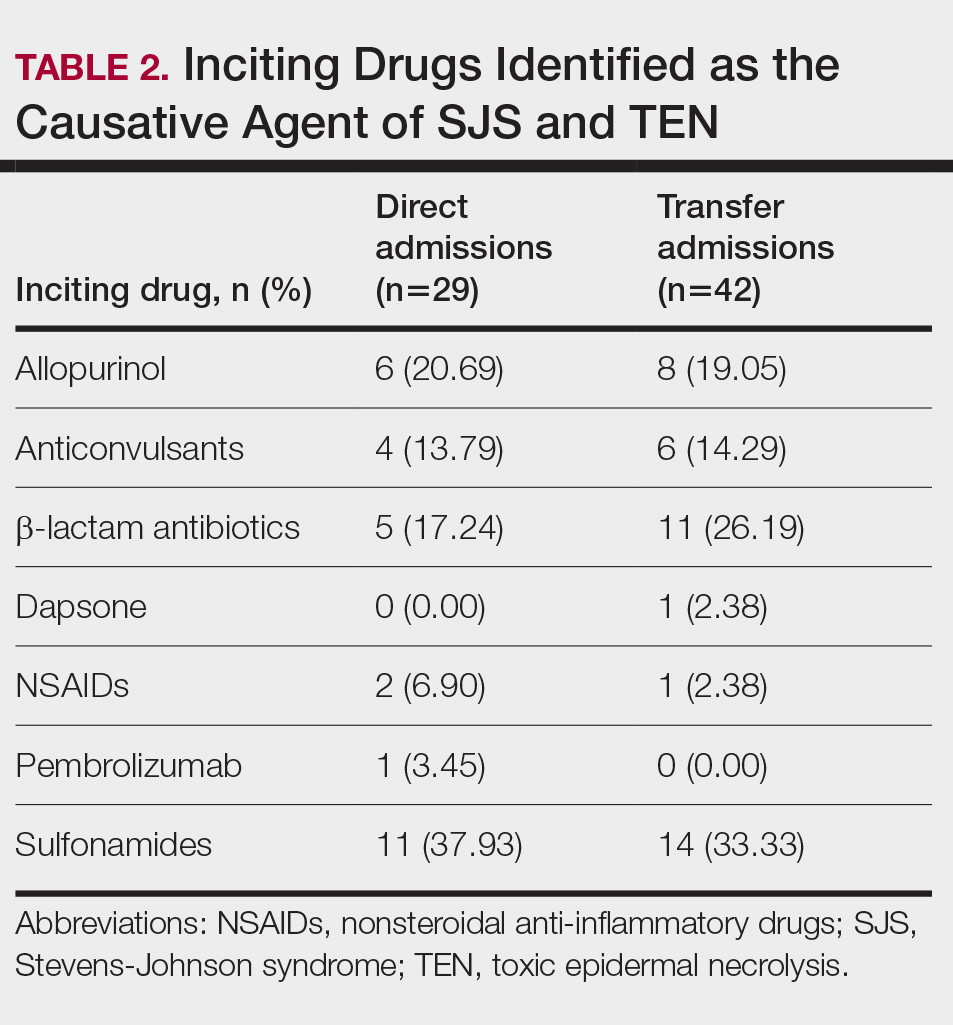
Of the 71 confirmed patients with SJS/TEN, sulfonamides were identified as the most common inciting drug in 25 (41%) patients, followed by beta-lactam antibiotics in 16 (23%) patients (Table 2). This is consistent with previous literature of sulfamethoxazole with trimethoprim as the primary causative drug for SJS and TEN in the United States.1
Clinical Outcomes—Of the 71 patients, there were 23 (32%) cases of SJS, 29 (41%) cases of SJS/TEN overlap, and 19 (27%) cases of TEN (eTable). The initial and maximum affected body surface area (BSA) was higher in transfer admissions, with a mean maximum BSA of 38.55% in the transfer group compared to 19.14% in the direct admissions. The mean SCORTEN (range, 0-5) was 1.6 overall, with a higher mean score of 1.92 in the transfer group compared to 1.07 in the direct admissions.
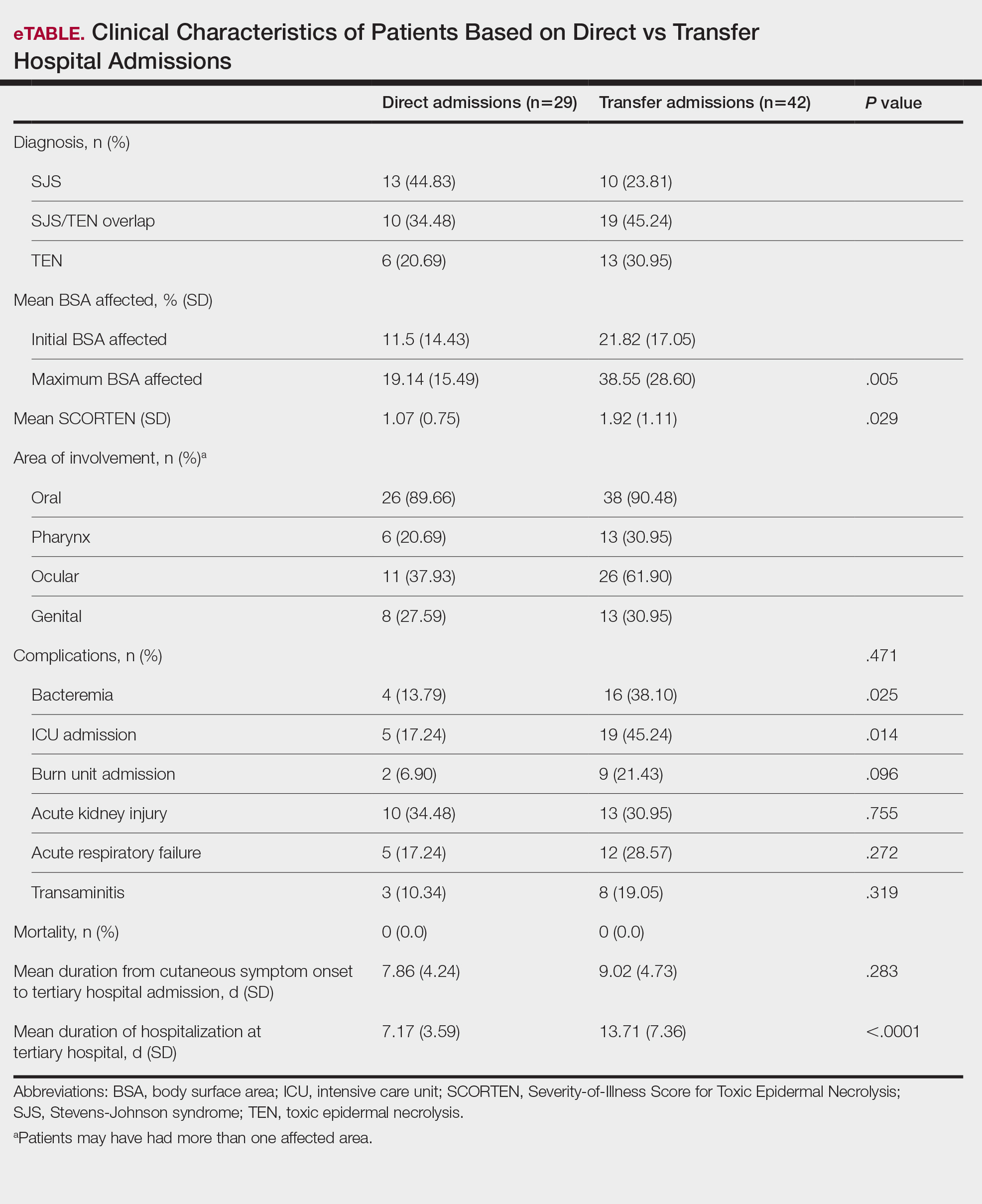
Transfer patients had a longer mean stay at the tertiary hospital (13.71 d) compared to direct admissions (7.17 d). The mean time from symptom onset until tertiary hospital admission was 8.5 days; transfer and direct admission patients had similar mean time from symptom onset of 9.02 days and 7.86 days, respectively. Although the duration of cutaneous symptoms from onset until tertiary hospital admission was similar (P=.283) between direct admissions (7.86 d) and transfer patients (9.02 d), the transfer group presented with greater disease severity at the time of admission. Transfer patients had a higher mean maximum BSA involvement (38.55% vs 19.14% [P=.005]), elevated SCORTEN (1.92 vs 1.07 [P=.029]), and longer mean hospital stays (13.71 d vs 7.17 d [P<.0001]) compared to direct admissions.
Despite the absence of mortality in both groups, transfer patients showed a higher number of ICU admissions (19 vs 5 [P=.014]) and burn unit admissions (9 vs 2 [P=.096]), bacteremia (16 vs 4 [P=.025]), acute kidney injury (13 vs 10 [P=.755]), acute respiratory failure (12 vs 5 [P=.272]), and transaminitis (8 vs 3 [P=.319]).
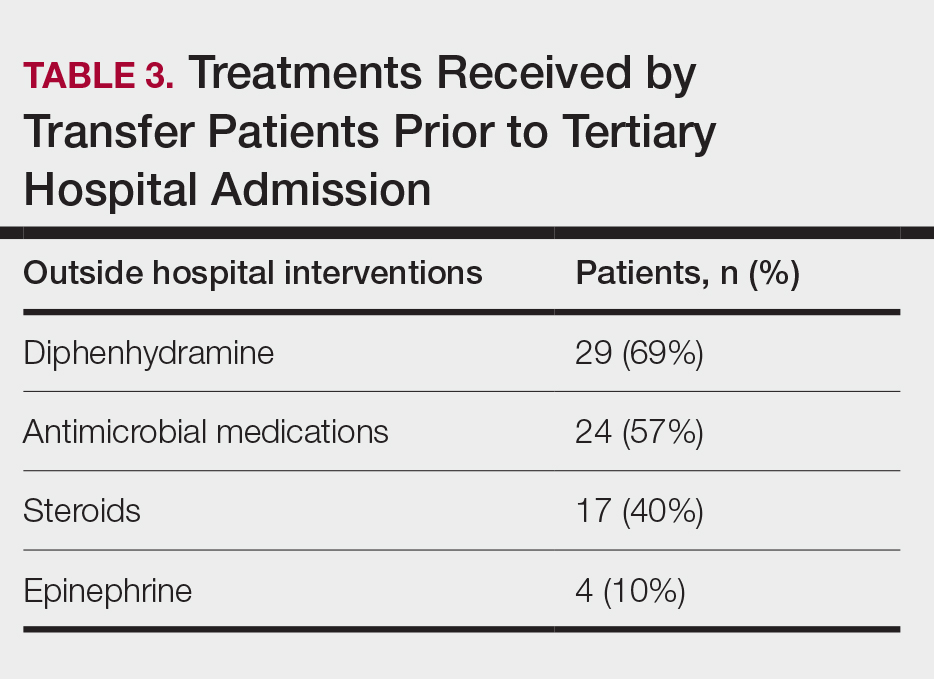
Outside Hospital Treatments—All outside hospitals provided supportive care with intravenous fluids and acetaminophen; however, further care provided at outside hospitals varied (Table 3), with transfer patients most frequently being treated with diphenhydramine (69% [29/42]), antimicrobial medications (57% [24/42]), steroids (40%), and epinephrine (10% [4/42]). Some patients may have received more than one of these treatments. Based on outside hospital treatments, the primary care teams’ main clinical concerns were allergic reactions and infection, as 33 (79%) patients received diphenhydramine (29 [89%]) or epinephrine (4 [12%]) and 24 (52%) received antimicrobial medications. Of the 42 transfer patients, 24 (57%) received or continued these medications before transfer; the medications were promptly discontinued upon tertiary hospital admission.
Once the outside hospitals contacted the tertiary hospital for a referral, the mean length of time between the transfer request and Physician Access Line call was 17.13 minutes (Table 4). Following the transfer request, the mean length of time for arrival at the tertiary hospital was 6.22 hours. The mean length of stay at the outside hospital prior to the patient being transferred was 3.84 days.

Comment
This retrospective study examined 71 patients with biopsy-confirmed SJS, SJS/TEN overlap, or TEN to evaluate differences in clinical outcomes between direct and transfer admissions. Transfer patients had a higher mean maximum affected BSA (38.55% vs 19.14% [P=.005]) and elevated SCORTEN (1.92 vs 1.07 [P=.029]); a higher number of transfer patients were admitted to the ICU (19 vs 5 [P=.014]) and burn unit (9 vs 2 [P=.096]), and this group also demonstrated longer hospitalization stays (13.71 vs 7.17 [P<.0001]). There were more complications among transfer patients, including bacteremia (16 vs 4 [P=.025]), which is consistent with findings from the existing literature.3
Once the decision for transfer of the patients included in our study was initiated and accepted, there was a prompt response and transfer of care; the mean length of time for Physician Access Line request was 17.13 minutes, and the mean transfer time to arrive at the tertiary hospital was 6.22 hours; however, patients spent an average of 3.84 days at outside hospitals, reflecting that transfer calls frequently were initiated due to urgent clinical decline of the patient rather than as an early intervention strategy. The management at outside hospitals often included the continuation of antimicrobial medications, which were discontinued upon transfer to AHWFBMC. Causative agents were either previously prescribed for a new medical condition or initiated for the management of suspected infections at outside hospitals. This may reflect the difficulty in correctly diagnosing SJS/TEN and initiating appropriate management at hospital facilities without an inpatient dermatologist.
The presence of inpatient dermatologists can improve the diagnostic accuracy and treatment of various conditions.4,5 Dermatology consultations added or changed 77% of treatment plans for 271 hospitalized patients.4 The impact of this intervention is reflected by the success of early dermatology consultations in reducing the length of hospitalization and use of inappropriate treatments in the care of skin diseases.6-8
Access to dermatologic care has been an identified need in inpatient hospitals that may limit the ability of hospitals to promptly treat serious conditions such as SJS/TEN.9 From an inpatient dermatology study from 2013 through 2019, 98.2% of 782 inpatient dermatologists reside in metropolitan areas, limiting the availability of care for rural patients; this study also found a decreasing number of facilities with inpatient dermatologists.10
The limitations of our study include a small sample size of 71 patients, which restricted the generalizability of our results. Our study also was based at a single tertiary center, which thereby limited the findings to this geographic area. It also was difficult to match patients by their demographic and comorbid conditions. The retrospective study design depended on the accuracy and completeness of medical records, which can introduce information bias. Future studies should compare the clinical outcomes of SJS/TEN based on burn unit and ICU admissions.
Conclusion
Prompt identification of SJS/TEN and rapid transfer to hospitals with inpatient dermatology are essential to optimize patient outcomes. Developing and validating SJS/TEN diagnosis and transfer protocols across multiple institutions may be helpful.
- Kridin K, Brüggen MC, Chua SL, et al. Assessment of treatment approaches and outcomes in Stevens-Johnson syndrome and toxic epidermal necrolysis: insights from a pan-European multicenter study. JAMA Dermatol. 2021;157:1182-1190. doi:10.1001/jamadermatol.2021.3154
- Seminario-Vidal L, Kroshinsky D, Malachowski SJ, et al. Society of Dermatology Hospitalists supportive care guidelines for the management of Stevens-Johnson syndrome/toxic epidermal necrolysis in adults. J Am Acad Dermatol. 2020;82:1553-1567. doi:10.1016 /j.jaad.2020.02.066
- Clark AE, Fook-Chong S, Choo K, et al. Delayed admission to a specialist referral center for Stevens-Johnson syndrome and toxic epidermal necrolysis is associated with increased mortality: a retrospective cohort study. JAAD Int. 2021;4:10-12. doi:10.1016/j.jdin.2021.03.008
- Davila M, Christenson LJ, Sontheimer RD. Epidemiology and outcomes of dermatology in-patient consultations in a Midwestern U.S. university hospital. Dermatol Online J. 2010;16:12.
- Hu L, Haynes H, Ferrazza D, et al. Impact of specialist consultations on inpatient admissions for dermatology-specific and related DRGs. J Gen Intern Med. 2013;28:1477-1482. doi:10.1007/s11606-013-2440-2
- Harr T, French LE. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis. 2010;5:39. doi:10.1186/1750-1172-5-39
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543. doi:10.1001/jamadermatol.2017.6197
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528. doi:10.1001/jamadermatol.2016.6130
- Messenger E, Kovarik CL, Lipoff JB. Access to inpatient dermatology care in Pennsylvania hospitals. Cutis. 2016;97:49-51.
- Hydol-Smith JA, Gallardo MA, Korman A, et al. The United States dermatology inpatient workforce between 2013 and 2019: a Medicare analysis reveals contraction of the workforce and vast access desertsa cross-sectional analysis. Arch Dermatol Res. 2024;316:103. doi:10.1007 /s00403-024-02845-0
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are rare, life-threatening conditions that involve widespread necrosis of the skin and mucous membranes.1 Guidelines for SJS and TEN recommend management in hospitals with access to inpatient dermatology to provide immediate interventions that are necessary for achieving optimal patient outcomes.2 A delay in admission of 5 days or more after onset of symptoms has been associated with increases in overall mortality, bacteremia, intensive care unit (ICU) admission, and length of stay.3 Patients who are not directly admitted to specialized facilities and require transfer from other hospitals may experience delays in receiving critical interventions, further increasing the risk for mortality and complications. In this study, we analyzed the clinical outcomes of patients with SJS/TEN in relation to their admission pathway.
Methods
A single-center retrospective chart review was performed at Atrium Health Wake Forest Baptist Medical Center (AHWFBMC) in Winston-Salem, North Carolina. Participants were identified using i2b2, an informatics tool compliant with the Health Insurance Portability and Accountability Act for integrating biology and the bedside. Inclusion criteria were having a diagnosis of SJS (International Classification of Diseases, Tenth Revision, code L51.1; International Classification of Diseases, Ninth Revision, code 695.13), TEN (International Classification of Diseases, Tenth Revision, code L51.2; International Classification of Diseases, Ninth Revision, code 695.15) or Lyell syndrome from January 2012 to December 2024. Patients with erythema multiforme or bullous drug eruption were excluded, as these conditions initially were misdiagnosed as SJS or TEN. Patients with only a reported history of prior SJS or TEN also were excluded.
The following clinical outcomes were assessed: demographics, comorbidities, age at disease onset, outside hospital transfer status, complications during admission, inpatient length of stay in days, age of mortality (if applicable), culprit medications, interventions received, Severity-of-Illness Score for Toxic Epidermal Necrolysis (SCORTEN) upon admission, site of admission (eg, floor bed, ICU, medical ICU, burn unit), and length of disease process prior to hospital admission. Patients then were categorized as either direct or transfer admissions based on the initial point of care and admission process. Direct admissions included patients who presented to the AHWFBMC emergency department and were subsequently admitted. Transfer patients included patients who initially presented to an outside hospital and were transferred to AHWFBMC. Data regarding the wait time for Physician Access Line requests and the time elapsed from the initial transfer call to arrival at the tertiary hospital also were collected—this is a method that outside hospitals can use to contact physicians at the tertiary hospital for a possible transfer. Statistical analysis was performed using unpaired t tests and X2 tests as necessary using GraphPad By Dotmatics Prism.
Results
A total of 112 patients were included in the analysis; of these, 71 had a diagnosis with biopsy confirmation of SJS, SJS/TEN overlap, or TEN (Table 1). Forty-one patients were excluded due to having a diagnosis of erythema multiforme or bullous drug eruption or a reported history of prior SJS or TEN without hospitalization. All biopsies were performed at AHWFBMC. Of the 71 confirmed patients with SJS/TEN, 54 (76%) were female with a mean age of 44 years. The majority of patients identified as Black (35 [49%]) or White (27 [38%]), along with Asian (7 [10%]) and other (2 [3%]). The most common comorbidity was cardiovascular disease in 42 (59%) patients, followed by type 2 diabetes in 36 (51%) patients. Among these 71 patients with SJS/TEN, 29 (41%) were directly admitted to the tertiary hospital, while 42 (59%) were transferred from outside hospitals.

Of the 71 confirmed patients with SJS/TEN, sulfonamides were identified as the most common inciting drug in 25 (41%) patients, followed by beta-lactam antibiotics in 16 (23%) patients (Table 2). This is consistent with previous literature of sulfamethoxazole with trimethoprim as the primary causative drug for SJS and TEN in the United States.1

Clinical Outcomes—Of the 71 patients, there were 23 (32%) cases of SJS, 29 (41%) cases of SJS/TEN overlap, and 19 (27%) cases of TEN (eTable). The initial and maximum affected body surface area (BSA) was higher in transfer admissions, with a mean maximum BSA of 38.55% in the transfer group compared to 19.14% in the direct admissions. The mean SCORTEN (range, 0-5) was 1.6 overall, with a higher mean score of 1.92 in the transfer group compared to 1.07 in the direct admissions.

Transfer patients had a longer mean stay at the tertiary hospital (13.71 d) compared to direct admissions (7.17 d). The mean time from symptom onset until tertiary hospital admission was 8.5 days; transfer and direct admission patients had similar mean time from symptom onset of 9.02 days and 7.86 days, respectively. Although the duration of cutaneous symptoms from onset until tertiary hospital admission was similar (P=.283) between direct admissions (7.86 d) and transfer patients (9.02 d), the transfer group presented with greater disease severity at the time of admission. Transfer patients had a higher mean maximum BSA involvement (38.55% vs 19.14% [P=.005]), elevated SCORTEN (1.92 vs 1.07 [P=.029]), and longer mean hospital stays (13.71 d vs 7.17 d [P<.0001]) compared to direct admissions.
Despite the absence of mortality in both groups, transfer patients showed a higher number of ICU admissions (19 vs 5 [P=.014]) and burn unit admissions (9 vs 2 [P=.096]), bacteremia (16 vs 4 [P=.025]), acute kidney injury (13 vs 10 [P=.755]), acute respiratory failure (12 vs 5 [P=.272]), and transaminitis (8 vs 3 [P=.319]).
Outside Hospital Treatments—All outside hospitals provided supportive care with intravenous fluids and acetaminophen; however, further care provided at outside hospitals varied (Table 3), with transfer patients most frequently being treated with diphenhydramine (69% [29/42]), antimicrobial medications (57% [24/42]), steroids (40%), and epinephrine (10% [4/42]). Some patients may have received more than one of these treatments. Based on outside hospital treatments, the primary care teams’ main clinical concerns were allergic reactions and infection, as 33 (79%) patients received diphenhydramine (29 [89%]) or epinephrine (4 [12%]) and 24 (52%) received antimicrobial medications. Of the 42 transfer patients, 24 (57%) received or continued these medications before transfer; the medications were promptly discontinued upon tertiary hospital admission.

Once the outside hospitals contacted the tertiary hospital for a referral, the mean length of time between the transfer request and Physician Access Line call was 17.13 minutes (Table 4). Following the transfer request, the mean length of time for arrival at the tertiary hospital was 6.22 hours. The mean length of stay at the outside hospital prior to the patient being transferred was 3.84 days.

Comment
This retrospective study examined 71 patients with biopsy-confirmed SJS, SJS/TEN overlap, or TEN to evaluate differences in clinical outcomes between direct and transfer admissions. Transfer patients had a higher mean maximum affected BSA (38.55% vs 19.14% [P=.005]) and elevated SCORTEN (1.92 vs 1.07 [P=.029]); a higher number of transfer patients were admitted to the ICU (19 vs 5 [P=.014]) and burn unit (9 vs 2 [P=.096]), and this group also demonstrated longer hospitalization stays (13.71 vs 7.17 [P<.0001]). There were more complications among transfer patients, including bacteremia (16 vs 4 [P=.025]), which is consistent with findings from the existing literature.3
Once the decision for transfer of the patients included in our study was initiated and accepted, there was a prompt response and transfer of care; the mean length of time for Physician Access Line request was 17.13 minutes, and the mean transfer time to arrive at the tertiary hospital was 6.22 hours; however, patients spent an average of 3.84 days at outside hospitals, reflecting that transfer calls frequently were initiated due to urgent clinical decline of the patient rather than as an early intervention strategy. The management at outside hospitals often included the continuation of antimicrobial medications, which were discontinued upon transfer to AHWFBMC. Causative agents were either previously prescribed for a new medical condition or initiated for the management of suspected infections at outside hospitals. This may reflect the difficulty in correctly diagnosing SJS/TEN and initiating appropriate management at hospital facilities without an inpatient dermatologist.
The presence of inpatient dermatologists can improve the diagnostic accuracy and treatment of various conditions.4,5 Dermatology consultations added or changed 77% of treatment plans for 271 hospitalized patients.4 The impact of this intervention is reflected by the success of early dermatology consultations in reducing the length of hospitalization and use of inappropriate treatments in the care of skin diseases.6-8
Access to dermatologic care has been an identified need in inpatient hospitals that may limit the ability of hospitals to promptly treat serious conditions such as SJS/TEN.9 From an inpatient dermatology study from 2013 through 2019, 98.2% of 782 inpatient dermatologists reside in metropolitan areas, limiting the availability of care for rural patients; this study also found a decreasing number of facilities with inpatient dermatologists.10
The limitations of our study include a small sample size of 71 patients, which restricted the generalizability of our results. Our study also was based at a single tertiary center, which thereby limited the findings to this geographic area. It also was difficult to match patients by their demographic and comorbid conditions. The retrospective study design depended on the accuracy and completeness of medical records, which can introduce information bias. Future studies should compare the clinical outcomes of SJS/TEN based on burn unit and ICU admissions.
Conclusion
Prompt identification of SJS/TEN and rapid transfer to hospitals with inpatient dermatology are essential to optimize patient outcomes. Developing and validating SJS/TEN diagnosis and transfer protocols across multiple institutions may be helpful.
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are rare, life-threatening conditions that involve widespread necrosis of the skin and mucous membranes.1 Guidelines for SJS and TEN recommend management in hospitals with access to inpatient dermatology to provide immediate interventions that are necessary for achieving optimal patient outcomes.2 A delay in admission of 5 days or more after onset of symptoms has been associated with increases in overall mortality, bacteremia, intensive care unit (ICU) admission, and length of stay.3 Patients who are not directly admitted to specialized facilities and require transfer from other hospitals may experience delays in receiving critical interventions, further increasing the risk for mortality and complications. In this study, we analyzed the clinical outcomes of patients with SJS/TEN in relation to their admission pathway.
Methods
A single-center retrospective chart review was performed at Atrium Health Wake Forest Baptist Medical Center (AHWFBMC) in Winston-Salem, North Carolina. Participants were identified using i2b2, an informatics tool compliant with the Health Insurance Portability and Accountability Act for integrating biology and the bedside. Inclusion criteria were having a diagnosis of SJS (International Classification of Diseases, Tenth Revision, code L51.1; International Classification of Diseases, Ninth Revision, code 695.13), TEN (International Classification of Diseases, Tenth Revision, code L51.2; International Classification of Diseases, Ninth Revision, code 695.15) or Lyell syndrome from January 2012 to December 2024. Patients with erythema multiforme or bullous drug eruption were excluded, as these conditions initially were misdiagnosed as SJS or TEN. Patients with only a reported history of prior SJS or TEN also were excluded.
The following clinical outcomes were assessed: demographics, comorbidities, age at disease onset, outside hospital transfer status, complications during admission, inpatient length of stay in days, age of mortality (if applicable), culprit medications, interventions received, Severity-of-Illness Score for Toxic Epidermal Necrolysis (SCORTEN) upon admission, site of admission (eg, floor bed, ICU, medical ICU, burn unit), and length of disease process prior to hospital admission. Patients then were categorized as either direct or transfer admissions based on the initial point of care and admission process. Direct admissions included patients who presented to the AHWFBMC emergency department and were subsequently admitted. Transfer patients included patients who initially presented to an outside hospital and were transferred to AHWFBMC. Data regarding the wait time for Physician Access Line requests and the time elapsed from the initial transfer call to arrival at the tertiary hospital also were collected—this is a method that outside hospitals can use to contact physicians at the tertiary hospital for a possible transfer. Statistical analysis was performed using unpaired t tests and X2 tests as necessary using GraphPad By Dotmatics Prism.
Results
A total of 112 patients were included in the analysis; of these, 71 had a diagnosis with biopsy confirmation of SJS, SJS/TEN overlap, or TEN (Table 1). Forty-one patients were excluded due to having a diagnosis of erythema multiforme or bullous drug eruption or a reported history of prior SJS or TEN without hospitalization. All biopsies were performed at AHWFBMC. Of the 71 confirmed patients with SJS/TEN, 54 (76%) were female with a mean age of 44 years. The majority of patients identified as Black (35 [49%]) or White (27 [38%]), along with Asian (7 [10%]) and other (2 [3%]). The most common comorbidity was cardiovascular disease in 42 (59%) patients, followed by type 2 diabetes in 36 (51%) patients. Among these 71 patients with SJS/TEN, 29 (41%) were directly admitted to the tertiary hospital, while 42 (59%) were transferred from outside hospitals.

Of the 71 confirmed patients with SJS/TEN, sulfonamides were identified as the most common inciting drug in 25 (41%) patients, followed by beta-lactam antibiotics in 16 (23%) patients (Table 2). This is consistent with previous literature of sulfamethoxazole with trimethoprim as the primary causative drug for SJS and TEN in the United States.1

Clinical Outcomes—Of the 71 patients, there were 23 (32%) cases of SJS, 29 (41%) cases of SJS/TEN overlap, and 19 (27%) cases of TEN (eTable). The initial and maximum affected body surface area (BSA) was higher in transfer admissions, with a mean maximum BSA of 38.55% in the transfer group compared to 19.14% in the direct admissions. The mean SCORTEN (range, 0-5) was 1.6 overall, with a higher mean score of 1.92 in the transfer group compared to 1.07 in the direct admissions.

Transfer patients had a longer mean stay at the tertiary hospital (13.71 d) compared to direct admissions (7.17 d). The mean time from symptom onset until tertiary hospital admission was 8.5 days; transfer and direct admission patients had similar mean time from symptom onset of 9.02 days and 7.86 days, respectively. Although the duration of cutaneous symptoms from onset until tertiary hospital admission was similar (P=.283) between direct admissions (7.86 d) and transfer patients (9.02 d), the transfer group presented with greater disease severity at the time of admission. Transfer patients had a higher mean maximum BSA involvement (38.55% vs 19.14% [P=.005]), elevated SCORTEN (1.92 vs 1.07 [P=.029]), and longer mean hospital stays (13.71 d vs 7.17 d [P<.0001]) compared to direct admissions.
Despite the absence of mortality in both groups, transfer patients showed a higher number of ICU admissions (19 vs 5 [P=.014]) and burn unit admissions (9 vs 2 [P=.096]), bacteremia (16 vs 4 [P=.025]), acute kidney injury (13 vs 10 [P=.755]), acute respiratory failure (12 vs 5 [P=.272]), and transaminitis (8 vs 3 [P=.319]).
Outside Hospital Treatments—All outside hospitals provided supportive care with intravenous fluids and acetaminophen; however, further care provided at outside hospitals varied (Table 3), with transfer patients most frequently being treated with diphenhydramine (69% [29/42]), antimicrobial medications (57% [24/42]), steroids (40%), and epinephrine (10% [4/42]). Some patients may have received more than one of these treatments. Based on outside hospital treatments, the primary care teams’ main clinical concerns were allergic reactions and infection, as 33 (79%) patients received diphenhydramine (29 [89%]) or epinephrine (4 [12%]) and 24 (52%) received antimicrobial medications. Of the 42 transfer patients, 24 (57%) received or continued these medications before transfer; the medications were promptly discontinued upon tertiary hospital admission.

Once the outside hospitals contacted the tertiary hospital for a referral, the mean length of time between the transfer request and Physician Access Line call was 17.13 minutes (Table 4). Following the transfer request, the mean length of time for arrival at the tertiary hospital was 6.22 hours. The mean length of stay at the outside hospital prior to the patient being transferred was 3.84 days.

Comment
This retrospective study examined 71 patients with biopsy-confirmed SJS, SJS/TEN overlap, or TEN to evaluate differences in clinical outcomes between direct and transfer admissions. Transfer patients had a higher mean maximum affected BSA (38.55% vs 19.14% [P=.005]) and elevated SCORTEN (1.92 vs 1.07 [P=.029]); a higher number of transfer patients were admitted to the ICU (19 vs 5 [P=.014]) and burn unit (9 vs 2 [P=.096]), and this group also demonstrated longer hospitalization stays (13.71 vs 7.17 [P<.0001]). There were more complications among transfer patients, including bacteremia (16 vs 4 [P=.025]), which is consistent with findings from the existing literature.3
Once the decision for transfer of the patients included in our study was initiated and accepted, there was a prompt response and transfer of care; the mean length of time for Physician Access Line request was 17.13 minutes, and the mean transfer time to arrive at the tertiary hospital was 6.22 hours; however, patients spent an average of 3.84 days at outside hospitals, reflecting that transfer calls frequently were initiated due to urgent clinical decline of the patient rather than as an early intervention strategy. The management at outside hospitals often included the continuation of antimicrobial medications, which were discontinued upon transfer to AHWFBMC. Causative agents were either previously prescribed for a new medical condition or initiated for the management of suspected infections at outside hospitals. This may reflect the difficulty in correctly diagnosing SJS/TEN and initiating appropriate management at hospital facilities without an inpatient dermatologist.
The presence of inpatient dermatologists can improve the diagnostic accuracy and treatment of various conditions.4,5 Dermatology consultations added or changed 77% of treatment plans for 271 hospitalized patients.4 The impact of this intervention is reflected by the success of early dermatology consultations in reducing the length of hospitalization and use of inappropriate treatments in the care of skin diseases.6-8
Access to dermatologic care has been an identified need in inpatient hospitals that may limit the ability of hospitals to promptly treat serious conditions such as SJS/TEN.9 From an inpatient dermatology study from 2013 through 2019, 98.2% of 782 inpatient dermatologists reside in metropolitan areas, limiting the availability of care for rural patients; this study also found a decreasing number of facilities with inpatient dermatologists.10
The limitations of our study include a small sample size of 71 patients, which restricted the generalizability of our results. Our study also was based at a single tertiary center, which thereby limited the findings to this geographic area. It also was difficult to match patients by their demographic and comorbid conditions. The retrospective study design depended on the accuracy and completeness of medical records, which can introduce information bias. Future studies should compare the clinical outcomes of SJS/TEN based on burn unit and ICU admissions.
Conclusion
Prompt identification of SJS/TEN and rapid transfer to hospitals with inpatient dermatology are essential to optimize patient outcomes. Developing and validating SJS/TEN diagnosis and transfer protocols across multiple institutions may be helpful.
- Kridin K, Brüggen MC, Chua SL, et al. Assessment of treatment approaches and outcomes in Stevens-Johnson syndrome and toxic epidermal necrolysis: insights from a pan-European multicenter study. JAMA Dermatol. 2021;157:1182-1190. doi:10.1001/jamadermatol.2021.3154
- Seminario-Vidal L, Kroshinsky D, Malachowski SJ, et al. Society of Dermatology Hospitalists supportive care guidelines for the management of Stevens-Johnson syndrome/toxic epidermal necrolysis in adults. J Am Acad Dermatol. 2020;82:1553-1567. doi:10.1016 /j.jaad.2020.02.066
- Clark AE, Fook-Chong S, Choo K, et al. Delayed admission to a specialist referral center for Stevens-Johnson syndrome and toxic epidermal necrolysis is associated with increased mortality: a retrospective cohort study. JAAD Int. 2021;4:10-12. doi:10.1016/j.jdin.2021.03.008
- Davila M, Christenson LJ, Sontheimer RD. Epidemiology and outcomes of dermatology in-patient consultations in a Midwestern U.S. university hospital. Dermatol Online J. 2010;16:12.
- Hu L, Haynes H, Ferrazza D, et al. Impact of specialist consultations on inpatient admissions for dermatology-specific and related DRGs. J Gen Intern Med. 2013;28:1477-1482. doi:10.1007/s11606-013-2440-2
- Harr T, French LE. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis. 2010;5:39. doi:10.1186/1750-1172-5-39
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543. doi:10.1001/jamadermatol.2017.6197
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528. doi:10.1001/jamadermatol.2016.6130
- Messenger E, Kovarik CL, Lipoff JB. Access to inpatient dermatology care in Pennsylvania hospitals. Cutis. 2016;97:49-51.
- Hydol-Smith JA, Gallardo MA, Korman A, et al. The United States dermatology inpatient workforce between 2013 and 2019: a Medicare analysis reveals contraction of the workforce and vast access desertsa cross-sectional analysis. Arch Dermatol Res. 2024;316:103. doi:10.1007 /s00403-024-02845-0
- Kridin K, Brüggen MC, Chua SL, et al. Assessment of treatment approaches and outcomes in Stevens-Johnson syndrome and toxic epidermal necrolysis: insights from a pan-European multicenter study. JAMA Dermatol. 2021;157:1182-1190. doi:10.1001/jamadermatol.2021.3154
- Seminario-Vidal L, Kroshinsky D, Malachowski SJ, et al. Society of Dermatology Hospitalists supportive care guidelines for the management of Stevens-Johnson syndrome/toxic epidermal necrolysis in adults. J Am Acad Dermatol. 2020;82:1553-1567. doi:10.1016 /j.jaad.2020.02.066
- Clark AE, Fook-Chong S, Choo K, et al. Delayed admission to a specialist referral center for Stevens-Johnson syndrome and toxic epidermal necrolysis is associated with increased mortality: a retrospective cohort study. JAAD Int. 2021;4:10-12. doi:10.1016/j.jdin.2021.03.008
- Davila M, Christenson LJ, Sontheimer RD. Epidemiology and outcomes of dermatology in-patient consultations in a Midwestern U.S. university hospital. Dermatol Online J. 2010;16:12.
- Hu L, Haynes H, Ferrazza D, et al. Impact of specialist consultations on inpatient admissions for dermatology-specific and related DRGs. J Gen Intern Med. 2013;28:1477-1482. doi:10.1007/s11606-013-2440-2
- Harr T, French LE. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis. 2010;5:39. doi:10.1186/1750-1172-5-39
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543. doi:10.1001/jamadermatol.2017.6197
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528. doi:10.1001/jamadermatol.2016.6130
- Messenger E, Kovarik CL, Lipoff JB. Access to inpatient dermatology care in Pennsylvania hospitals. Cutis. 2016;97:49-51.
- Hydol-Smith JA, Gallardo MA, Korman A, et al. The United States dermatology inpatient workforce between 2013 and 2019: a Medicare analysis reveals contraction of the workforce and vast access desertsa cross-sectional analysis. Arch Dermatol Res. 2024;316:103. doi:10.1007 /s00403-024-02845-0
Clinical Outcomes of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis Based on Hospital Admission Type
Clinical Outcomes of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis Based on Hospital Admission Type
PRACTICE POINTS
- Early identification and diagnosis of Stevens-Johnson syndrome and toxic epidermal necrolysis are essential to improving patient outcomes.
- Patients transferred from outside hospitals often present with more severe disease due to delays in diagnosis and initiation of appropriate treatment.
- Inpatient dermatology consultation plays a vital role in accurately diagnosing and managing life-threatening dermatologic conditions.
- Establishing timely interhospital transfer protocols may help expedite access to specialized treatment and improve patient outcomes.
Don’t Miss These Signs of Rosacea in Darker Skin Types
Don’t Miss These Signs of Rosacea in Darker Skin Types
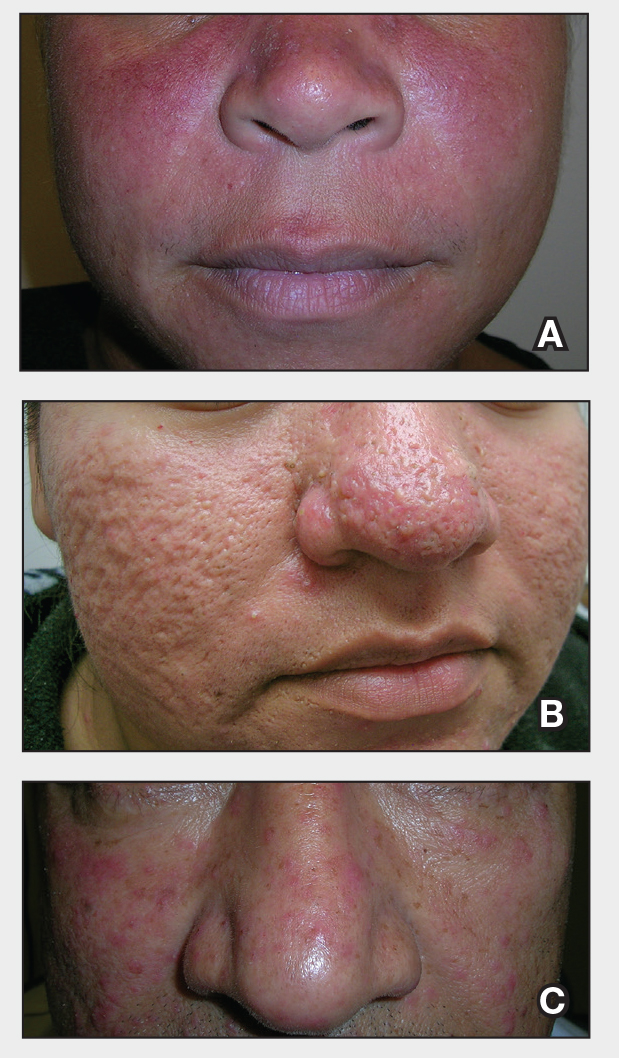
THE COMPARISON:
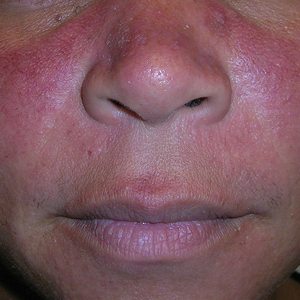
- A. Erythematotelangiectatic rosacea in a polygonal vascular pattern on the cheeks in a Black woman who also has eyelid hypopigmentation due to vitiligo.
- B. Rhinophymatous rosacea in a Hispanic woman who also has papules and pustules on the chin and upper lip region as well as facial scarring from severe inflammatory acne during her teen years.
- C. Papulopustular rosacea in a Hispanic man.
Rosacea is a chronic inflammatory condition characterized by facial flushing and persistent erythema of the central face, typically affecting the cheeks and nose. It also may manifest with papules, pustules, and telangiectasias. The 4 main subtypes of rosacea are erythematotelangiectatic, papulopustular, phymatous (involving thickening of the skin, often of the nose), and ocular (dry, itchy, or irritated eyes).1 Patients also may report stinging, burning, dryness, and edema.2 The etiology of rosacea is unclear but is believed to involve immune dysfunction, neurovascular dysregulation, certain microorganisms, and genetic predisposition.1,2
Epidemiology
Rosacea often is associated with fair skin and more frequently is reported in individuals of Northern European descent.1,2 While it may be less common in darker skin types, rosacea is not rare in patients with skin of color (SOC). A review of US outpatient data from 1993 to 2010 found that 2% of patients with rosacea were Black, 2.3% were Asian or Pacific Islander, and 3.9% were Hispanic or Latino.3 Global estimates suggest that up to 40 million individuals with SOC may be affected by rosacea,4 with the reported prevalence as high as 10%.2 Although early research linked rosacea primarily to adults older than 30 years, newer data show peak prevalence between ages 25 to 39 years, suggesting that younger adults may be affected more than previously recognized.5
Key Clinical Features
In addition to the traditional subtypes, updated guidelines recommend a phenotype- based approach to diagnosing rosacea focusing on observable features such as persistent redness in the central face and thickened skin rather than classifying patients into broad categories. A diagnosis can be made when at least one diagnostic feature is present (eg, fixed facial erythema or phymatous changes) or when 2 or more major features are observed (eg, papules, pustules, flushing, visible blood vessels, or ocular findings).6
In individuals with darker skin types, erythema may not be bright red; rather, the skin may appear pink, reddish-brown, violaceous, or dusky brown.7 Postinflammatory hyperpigmentation, which is common in darker skin tones, can further mask erythema.2 Pressing a microscope slide or magnifying glass against the skin can help assess for blanching, which is indicative of erythema. Telangiectasias also may be more challenging to appreciate in patients with SOC and typically require bright, shadow-free lighting or dermoscopy for detection.2
Skin thickening across the cheeks and nose with overlying acneform papules can be diagnostic clues of rosacea in darker skin types and help distinguish it from acne.2 It also is important to distinguish rosacea from systemic lupus erythematosus, which typically manifests as a malar rash that spares the nasolabial folds and is nonpustular. If uncertain, consider serologic testing for antinuclear antibodies, patch testing, or biopsy.8
Worth Noting
Treatment of rosacea is focused on managing symptoms and reducing flares. First-line strategies include behavioral modifications and trigger avoidance, such as minimizing sun exposure and avoiding consumption of alcohol and spicy foods.9 Gentle skin care practices are essential, including the use of light, fragrance-free, nonirritating cleansers and moisturizers at least once daily. Application of sunscreen with an SPF of at least 30 also is routinely recommended.9,10 Additionally, patients should be counseled to avoid harsh cleansers, such as exfoliants, astringents, and chemicals that may further diminish the skin barrier.10
Treatment options approved by the US Food and Drug Administration for rosacea include oral doxycycline, oral minocycline, topical brimonidine, oxymetazoline, ivermectin, metronidazole, azelaic acid, sodium sulfacetamide/sulfur, encapsulated benzoyl peroxide cream, and minocycline.11-13
Topical treatment options commonly used off-label for rosacea include topical clindamycin, topical retinoids, and azithromycin. Oral tetracyclines should be avoided in children and pregnant women; instead, oral erythromycin and topical metronidazole commonly are used.14
Laser or intense pulsed light therapy may be considered, although results have been mixed, and the long-term benefits are uncertain. Given the higher risk for postinflammatory hyperpigmentation in patients with SOC, these modalities should be used cautiously.15 Among the available options, the Nd:YAG laser is preferred in darker skin types due to its safety profile.16 A small case series reported successful CO2 laser treatment for rhinophyma in patients with melanated skin; however, some patients developed localized scarring, suggesting that conservative depth settings should be used to reduce risk for this adverse event.17
Health Disparity Highlight
Rosacea may be underdiagnosed in individuals with darker skin types,2,15,18 likely due in part to reduced contrast between erythema and background skin tone, which can make features such as flushing and telangiectasias harder to appreciate.1,10,15
Although tools to assess erythema exist, they rarely are used in everyday clinical practice.10 In patients with deeply pigmented skin, ensuring adequate examination room lighting and using dermoscopy can help identify any subtle vascular or textural changes localized across the central face. While various imaging techniques are used in clinical trials to monitor treatment response, few have been studied and optimized across a wide range of skin tones.10 There is a need for dermatologic assessment tools that better capture the degree of erythema, inflammation, and vascular features of rosacea in pigmented skin. Emerging research is focused on developing more equitable imaging technologies.19
- Rainer BM, Kang S, Chien AL. Rosacea: epidemiology, pathogenesis, and treatment. Dermatoendocrinol. 2017;9:E1361574.
- Alexis AF, Callender VD, Baldwin HE, et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience. J Am Acad Dermatol. 2019;80:1722-1729.e7.
- Al-Dabagh A, Davis SA, McMichael AJ, el al. Rosacea in skin of color: not a rare diagnosis. Dermatol Online J. 2014;20:13030/qt1mv9r0ss.
- Tan J, Berg M. Rosacea: current state of epidemiology. J Am Acad Dermatol. 2013;69(6 suppl 1):S27-S35.
- Saurat JH, Halioua B, Baissac C, et al. Epidemiology of acne and rosacea: a worldwide global study. J Am Acad Dermatol. 2024;90:1016-1018.
- Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78:148-155.
- Finlay AY, Griffiths TW, Belmo S, et al. Why we should abandon the misused descriptor ‘erythema’. Br J Dermatol. 2021;185:1240-1241.
- Callender VD, Barbosa V, Burgess CM, et al. Approach to treatment of medical and cosmetic facial concerns in skin of color patients. Cutis. 2017;100:375-380.
- Baldwin H, Alexis A, Andriessen A, et al. Supplement article: skin barrier deficiency in rosacea: an algorithm integrating OTC skincare products into treatment regimens. J Drugs Dermatol. 2022;21:SF3595563-SF35955610.
- Ohanenye C, Taliaferro S, Callender VD. Diagnosing disorders of facial erythema. Dermatol Clin. 2023;41:377-392.
- Thiboutot D, Anderson R, Cook-Bolden F, et al. Standard management options for rosacea: the 2019 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2020;82:1501-1510.
- Del Rosso JQ, Schlessinger J, Werschler P. Comparison of anti-inflammatory dose doxycycline versus doxycycline 100 mg in the treatment of rosacea. J Drugs Dermatol. 2008;7:573-576.
- van der Linden MMD, van Ratingen AR, van Rappard DC, et al. DOMINO, doxycycline 40 mg vs. minocycline 100 mg in the treatment of rosacea: a randomized, single-blinded, noninferiority trial, comparing efficacy and safety. Br J Dermatol. 2017;176:1465-1474.
- Geng R, Bourkas A, Sibbald RG, et al. Efficacy of treatments for rosacea in the pediatric population: a systematic review. JEADV Clinical Practice. 2024;3:17-48.
- Sarkar R, Podder I, Jagadeesan S. Rosacea in skin of color: a comprehensive review. Indian J Dermatol Venereol Leprol. 2020;86:611-621.
- Chen A, Choi J, Balazic E, et al. Review of laser and energy-based devices to treat rosacea in skin of color. J Cosmet Laser Ther. 2024;26:43-53.
- Nganzeu CG, Lopez A, Brennan TE. Ablative CO2 laser treatment of rhinophyma in people of color: a case series. Plast Reconstr Surg Glob Open. 2025;13:E6616.
- Kulthanan K, Andriessen A, Jiang X, et al. A review of the challenges and nuances in treating rosacea in Asian skin types using cleansers and moisturizers as adjuncts. J Drugs Dermatol. 2023;22:45-53.
- Jarang A, McGrath Q, Harunani M, et al. Multispectral SWIR imaging for equitable pigmentation-insensitive assessment of inflammatory acne in darkly pigmented skin. Presented at Photonics in Dermatology and Plastic Surgery 2025; January 25-27, 2025; San Francisco, California.
THE COMPARISON:
- A. Erythematotelangiectatic rosacea in a polygonal vascular pattern on the cheeks in a Black woman who also has eyelid hypopigmentation due to vitiligo.
- B. Rhinophymatous rosacea in a Hispanic woman who also has papules and pustules on the chin and upper lip region as well as facial scarring from severe inflammatory acne during her teen years.
- C. Papulopustular rosacea in a Hispanic man.
Rosacea is a chronic inflammatory condition characterized by facial flushing and persistent erythema of the central face, typically affecting the cheeks and nose. It also may manifest with papules, pustules, and telangiectasias. The 4 main subtypes of rosacea are erythematotelangiectatic, papulopustular, phymatous (involving thickening of the skin, often of the nose), and ocular (dry, itchy, or irritated eyes).1 Patients also may report stinging, burning, dryness, and edema.2 The etiology of rosacea is unclear but is believed to involve immune dysfunction, neurovascular dysregulation, certain microorganisms, and genetic predisposition.1,2

Epidemiology
Rosacea often is associated with fair skin and more frequently is reported in individuals of Northern European descent.1,2 While it may be less common in darker skin types, rosacea is not rare in patients with skin of color (SOC). A review of US outpatient data from 1993 to 2010 found that 2% of patients with rosacea were Black, 2.3% were Asian or Pacific Islander, and 3.9% were Hispanic or Latino.3 Global estimates suggest that up to 40 million individuals with SOC may be affected by rosacea,4 with the reported prevalence as high as 10%.2 Although early research linked rosacea primarily to adults older than 30 years, newer data show peak prevalence between ages 25 to 39 years, suggesting that younger adults may be affected more than previously recognized.5
Key Clinical Features
In addition to the traditional subtypes, updated guidelines recommend a phenotype- based approach to diagnosing rosacea focusing on observable features such as persistent redness in the central face and thickened skin rather than classifying patients into broad categories. A diagnosis can be made when at least one diagnostic feature is present (eg, fixed facial erythema or phymatous changes) or when 2 or more major features are observed (eg, papules, pustules, flushing, visible blood vessels, or ocular findings).6
In individuals with darker skin types, erythema may not be bright red; rather, the skin may appear pink, reddish-brown, violaceous, or dusky brown.7 Postinflammatory hyperpigmentation, which is common in darker skin tones, can further mask erythema.2 Pressing a microscope slide or magnifying glass against the skin can help assess for blanching, which is indicative of erythema. Telangiectasias also may be more challenging to appreciate in patients with SOC and typically require bright, shadow-free lighting or dermoscopy for detection.2
Skin thickening across the cheeks and nose with overlying acneform papules can be diagnostic clues of rosacea in darker skin types and help distinguish it from acne.2 It also is important to distinguish rosacea from systemic lupus erythematosus, which typically manifests as a malar rash that spares the nasolabial folds and is nonpustular. If uncertain, consider serologic testing for antinuclear antibodies, patch testing, or biopsy.8
Worth Noting
Treatment of rosacea is focused on managing symptoms and reducing flares. First-line strategies include behavioral modifications and trigger avoidance, such as minimizing sun exposure and avoiding consumption of alcohol and spicy foods.9 Gentle skin care practices are essential, including the use of light, fragrance-free, nonirritating cleansers and moisturizers at least once daily. Application of sunscreen with an SPF of at least 30 also is routinely recommended.9,10 Additionally, patients should be counseled to avoid harsh cleansers, such as exfoliants, astringents, and chemicals that may further diminish the skin barrier.10
Treatment options approved by the US Food and Drug Administration for rosacea include oral doxycycline, oral minocycline, topical brimonidine, oxymetazoline, ivermectin, metronidazole, azelaic acid, sodium sulfacetamide/sulfur, encapsulated benzoyl peroxide cream, and minocycline.11-13
Topical treatment options commonly used off-label for rosacea include topical clindamycin, topical retinoids, and azithromycin. Oral tetracyclines should be avoided in children and pregnant women; instead, oral erythromycin and topical metronidazole commonly are used.14
Laser or intense pulsed light therapy may be considered, although results have been mixed, and the long-term benefits are uncertain. Given the higher risk for postinflammatory hyperpigmentation in patients with SOC, these modalities should be used cautiously.15 Among the available options, the Nd:YAG laser is preferred in darker skin types due to its safety profile.16 A small case series reported successful CO2 laser treatment for rhinophyma in patients with melanated skin; however, some patients developed localized scarring, suggesting that conservative depth settings should be used to reduce risk for this adverse event.17
Health Disparity Highlight
Rosacea may be underdiagnosed in individuals with darker skin types,2,15,18 likely due in part to reduced contrast between erythema and background skin tone, which can make features such as flushing and telangiectasias harder to appreciate.1,10,15
Although tools to assess erythema exist, they rarely are used in everyday clinical practice.10 In patients with deeply pigmented skin, ensuring adequate examination room lighting and using dermoscopy can help identify any subtle vascular or textural changes localized across the central face. While various imaging techniques are used in clinical trials to monitor treatment response, few have been studied and optimized across a wide range of skin tones.10 There is a need for dermatologic assessment tools that better capture the degree of erythema, inflammation, and vascular features of rosacea in pigmented skin. Emerging research is focused on developing more equitable imaging technologies.19
THE COMPARISON:
- A. Erythematotelangiectatic rosacea in a polygonal vascular pattern on the cheeks in a Black woman who also has eyelid hypopigmentation due to vitiligo.
- B. Rhinophymatous rosacea in a Hispanic woman who also has papules and pustules on the chin and upper lip region as well as facial scarring from severe inflammatory acne during her teen years.
- C. Papulopustular rosacea in a Hispanic man.
Rosacea is a chronic inflammatory condition characterized by facial flushing and persistent erythema of the central face, typically affecting the cheeks and nose. It also may manifest with papules, pustules, and telangiectasias. The 4 main subtypes of rosacea are erythematotelangiectatic, papulopustular, phymatous (involving thickening of the skin, often of the nose), and ocular (dry, itchy, or irritated eyes).1 Patients also may report stinging, burning, dryness, and edema.2 The etiology of rosacea is unclear but is believed to involve immune dysfunction, neurovascular dysregulation, certain microorganisms, and genetic predisposition.1,2

Epidemiology
Rosacea often is associated with fair skin and more frequently is reported in individuals of Northern European descent.1,2 While it may be less common in darker skin types, rosacea is not rare in patients with skin of color (SOC). A review of US outpatient data from 1993 to 2010 found that 2% of patients with rosacea were Black, 2.3% were Asian or Pacific Islander, and 3.9% were Hispanic or Latino.3 Global estimates suggest that up to 40 million individuals with SOC may be affected by rosacea,4 with the reported prevalence as high as 10%.2 Although early research linked rosacea primarily to adults older than 30 years, newer data show peak prevalence between ages 25 to 39 years, suggesting that younger adults may be affected more than previously recognized.5
Key Clinical Features
In addition to the traditional subtypes, updated guidelines recommend a phenotype- based approach to diagnosing rosacea focusing on observable features such as persistent redness in the central face and thickened skin rather than classifying patients into broad categories. A diagnosis can be made when at least one diagnostic feature is present (eg, fixed facial erythema or phymatous changes) or when 2 or more major features are observed (eg, papules, pustules, flushing, visible blood vessels, or ocular findings).6
In individuals with darker skin types, erythema may not be bright red; rather, the skin may appear pink, reddish-brown, violaceous, or dusky brown.7 Postinflammatory hyperpigmentation, which is common in darker skin tones, can further mask erythema.2 Pressing a microscope slide or magnifying glass against the skin can help assess for blanching, which is indicative of erythema. Telangiectasias also may be more challenging to appreciate in patients with SOC and typically require bright, shadow-free lighting or dermoscopy for detection.2
Skin thickening across the cheeks and nose with overlying acneform papules can be diagnostic clues of rosacea in darker skin types and help distinguish it from acne.2 It also is important to distinguish rosacea from systemic lupus erythematosus, which typically manifests as a malar rash that spares the nasolabial folds and is nonpustular. If uncertain, consider serologic testing for antinuclear antibodies, patch testing, or biopsy.8
Worth Noting
Treatment of rosacea is focused on managing symptoms and reducing flares. First-line strategies include behavioral modifications and trigger avoidance, such as minimizing sun exposure and avoiding consumption of alcohol and spicy foods.9 Gentle skin care practices are essential, including the use of light, fragrance-free, nonirritating cleansers and moisturizers at least once daily. Application of sunscreen with an SPF of at least 30 also is routinely recommended.9,10 Additionally, patients should be counseled to avoid harsh cleansers, such as exfoliants, astringents, and chemicals that may further diminish the skin barrier.10
Treatment options approved by the US Food and Drug Administration for rosacea include oral doxycycline, oral minocycline, topical brimonidine, oxymetazoline, ivermectin, metronidazole, azelaic acid, sodium sulfacetamide/sulfur, encapsulated benzoyl peroxide cream, and minocycline.11-13
Topical treatment options commonly used off-label for rosacea include topical clindamycin, topical retinoids, and azithromycin. Oral tetracyclines should be avoided in children and pregnant women; instead, oral erythromycin and topical metronidazole commonly are used.14
Laser or intense pulsed light therapy may be considered, although results have been mixed, and the long-term benefits are uncertain. Given the higher risk for postinflammatory hyperpigmentation in patients with SOC, these modalities should be used cautiously.15 Among the available options, the Nd:YAG laser is preferred in darker skin types due to its safety profile.16 A small case series reported successful CO2 laser treatment for rhinophyma in patients with melanated skin; however, some patients developed localized scarring, suggesting that conservative depth settings should be used to reduce risk for this adverse event.17
Health Disparity Highlight
Rosacea may be underdiagnosed in individuals with darker skin types,2,15,18 likely due in part to reduced contrast between erythema and background skin tone, which can make features such as flushing and telangiectasias harder to appreciate.1,10,15
Although tools to assess erythema exist, they rarely are used in everyday clinical practice.10 In patients with deeply pigmented skin, ensuring adequate examination room lighting and using dermoscopy can help identify any subtle vascular or textural changes localized across the central face. While various imaging techniques are used in clinical trials to monitor treatment response, few have been studied and optimized across a wide range of skin tones.10 There is a need for dermatologic assessment tools that better capture the degree of erythema, inflammation, and vascular features of rosacea in pigmented skin. Emerging research is focused on developing more equitable imaging technologies.19
- Rainer BM, Kang S, Chien AL. Rosacea: epidemiology, pathogenesis, and treatment. Dermatoendocrinol. 2017;9:E1361574.
- Alexis AF, Callender VD, Baldwin HE, et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience. J Am Acad Dermatol. 2019;80:1722-1729.e7.
- Al-Dabagh A, Davis SA, McMichael AJ, el al. Rosacea in skin of color: not a rare diagnosis. Dermatol Online J. 2014;20:13030/qt1mv9r0ss.
- Tan J, Berg M. Rosacea: current state of epidemiology. J Am Acad Dermatol. 2013;69(6 suppl 1):S27-S35.
- Saurat JH, Halioua B, Baissac C, et al. Epidemiology of acne and rosacea: a worldwide global study. J Am Acad Dermatol. 2024;90:1016-1018.
- Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78:148-155.
- Finlay AY, Griffiths TW, Belmo S, et al. Why we should abandon the misused descriptor ‘erythema’. Br J Dermatol. 2021;185:1240-1241.
- Callender VD, Barbosa V, Burgess CM, et al. Approach to treatment of medical and cosmetic facial concerns in skin of color patients. Cutis. 2017;100:375-380.
- Baldwin H, Alexis A, Andriessen A, et al. Supplement article: skin barrier deficiency in rosacea: an algorithm integrating OTC skincare products into treatment regimens. J Drugs Dermatol. 2022;21:SF3595563-SF35955610.
- Ohanenye C, Taliaferro S, Callender VD. Diagnosing disorders of facial erythema. Dermatol Clin. 2023;41:377-392.
- Thiboutot D, Anderson R, Cook-Bolden F, et al. Standard management options for rosacea: the 2019 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2020;82:1501-1510.
- Del Rosso JQ, Schlessinger J, Werschler P. Comparison of anti-inflammatory dose doxycycline versus doxycycline 100 mg in the treatment of rosacea. J Drugs Dermatol. 2008;7:573-576.
- van der Linden MMD, van Ratingen AR, van Rappard DC, et al. DOMINO, doxycycline 40 mg vs. minocycline 100 mg in the treatment of rosacea: a randomized, single-blinded, noninferiority trial, comparing efficacy and safety. Br J Dermatol. 2017;176:1465-1474.
- Geng R, Bourkas A, Sibbald RG, et al. Efficacy of treatments for rosacea in the pediatric population: a systematic review. JEADV Clinical Practice. 2024;3:17-48.
- Sarkar R, Podder I, Jagadeesan S. Rosacea in skin of color: a comprehensive review. Indian J Dermatol Venereol Leprol. 2020;86:611-621.
- Chen A, Choi J, Balazic E, et al. Review of laser and energy-based devices to treat rosacea in skin of color. J Cosmet Laser Ther. 2024;26:43-53.
- Nganzeu CG, Lopez A, Brennan TE. Ablative CO2 laser treatment of rhinophyma in people of color: a case series. Plast Reconstr Surg Glob Open. 2025;13:E6616.
- Kulthanan K, Andriessen A, Jiang X, et al. A review of the challenges and nuances in treating rosacea in Asian skin types using cleansers and moisturizers as adjuncts. J Drugs Dermatol. 2023;22:45-53.
- Jarang A, McGrath Q, Harunani M, et al. Multispectral SWIR imaging for equitable pigmentation-insensitive assessment of inflammatory acne in darkly pigmented skin. Presented at Photonics in Dermatology and Plastic Surgery 2025; January 25-27, 2025; San Francisco, California.
- Rainer BM, Kang S, Chien AL. Rosacea: epidemiology, pathogenesis, and treatment. Dermatoendocrinol. 2017;9:E1361574.
- Alexis AF, Callender VD, Baldwin HE, et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience. J Am Acad Dermatol. 2019;80:1722-1729.e7.
- Al-Dabagh A, Davis SA, McMichael AJ, el al. Rosacea in skin of color: not a rare diagnosis. Dermatol Online J. 2014;20:13030/qt1mv9r0ss.
- Tan J, Berg M. Rosacea: current state of epidemiology. J Am Acad Dermatol. 2013;69(6 suppl 1):S27-S35.
- Saurat JH, Halioua B, Baissac C, et al. Epidemiology of acne and rosacea: a worldwide global study. J Am Acad Dermatol. 2024;90:1016-1018.
- Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78:148-155.
- Finlay AY, Griffiths TW, Belmo S, et al. Why we should abandon the misused descriptor ‘erythema’. Br J Dermatol. 2021;185:1240-1241.
- Callender VD, Barbosa V, Burgess CM, et al. Approach to treatment of medical and cosmetic facial concerns in skin of color patients. Cutis. 2017;100:375-380.
- Baldwin H, Alexis A, Andriessen A, et al. Supplement article: skin barrier deficiency in rosacea: an algorithm integrating OTC skincare products into treatment regimens. J Drugs Dermatol. 2022;21:SF3595563-SF35955610.
- Ohanenye C, Taliaferro S, Callender VD. Diagnosing disorders of facial erythema. Dermatol Clin. 2023;41:377-392.
- Thiboutot D, Anderson R, Cook-Bolden F, et al. Standard management options for rosacea: the 2019 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2020;82:1501-1510.
- Del Rosso JQ, Schlessinger J, Werschler P. Comparison of anti-inflammatory dose doxycycline versus doxycycline 100 mg in the treatment of rosacea. J Drugs Dermatol. 2008;7:573-576.
- van der Linden MMD, van Ratingen AR, van Rappard DC, et al. DOMINO, doxycycline 40 mg vs. minocycline 100 mg in the treatment of rosacea: a randomized, single-blinded, noninferiority trial, comparing efficacy and safety. Br J Dermatol. 2017;176:1465-1474.
- Geng R, Bourkas A, Sibbald RG, et al. Efficacy of treatments for rosacea in the pediatric population: a systematic review. JEADV Clinical Practice. 2024;3:17-48.
- Sarkar R, Podder I, Jagadeesan S. Rosacea in skin of color: a comprehensive review. Indian J Dermatol Venereol Leprol. 2020;86:611-621.
- Chen A, Choi J, Balazic E, et al. Review of laser and energy-based devices to treat rosacea in skin of color. J Cosmet Laser Ther. 2024;26:43-53.
- Nganzeu CG, Lopez A, Brennan TE. Ablative CO2 laser treatment of rhinophyma in people of color: a case series. Plast Reconstr Surg Glob Open. 2025;13:E6616.
- Kulthanan K, Andriessen A, Jiang X, et al. A review of the challenges and nuances in treating rosacea in Asian skin types using cleansers and moisturizers as adjuncts. J Drugs Dermatol. 2023;22:45-53.
- Jarang A, McGrath Q, Harunani M, et al. Multispectral SWIR imaging for equitable pigmentation-insensitive assessment of inflammatory acne in darkly pigmented skin. Presented at Photonics in Dermatology and Plastic Surgery 2025; January 25-27, 2025; San Francisco, California.
Don’t Miss These Signs of Rosacea in Darker Skin Types
Don’t Miss These Signs of Rosacea in Darker Skin Types
Reddish Nodule on the Left Shoulder
Reddish Nodule on the Left Shoulder
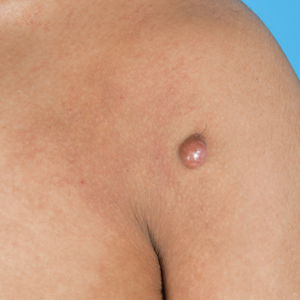
THE DIAGNOSIS: Atypical Fibroxanthoma
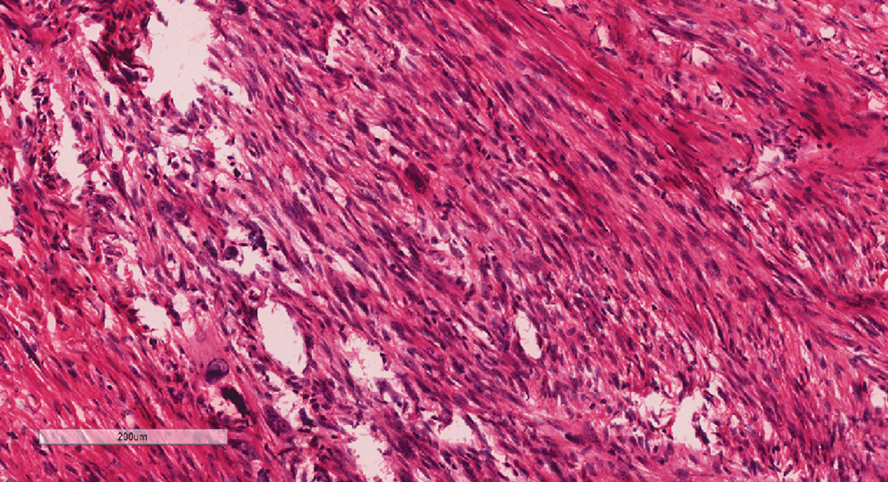
Given the appearance of the nodule and the absence of features of a keloid scar, a soft-tissue or adnexal tumor was suspected. Histology revealed a thin epidermis with loss of rete ridges and a Grenz zone. There was a nodular uncircumscribed dermal proliferation of spindle cells forming interweaving fascicles with elongated ovoid nuclei and prominent nucleoli (Figure). There was moderate cellular and nuclear atypia, and no necrosis was observed. The spindle cells stained positive for CD10 and negative for AE1/AE3, cytokeratin 5/6, S100, melanoma triple marker, Factor XIII 1, ERG, CD31, CD34, desmin, and smooth muscle actin; ERG, CD31, CD34, and SMA highlighted small vessels within the tumor. The histologic diagnosis was an atypical spindle cell tumor favoring atypical fibroxanthoma (AFX). The excisional biopsy margins were clear.
The patient was referred to surgical oncology to consider re-excision of margins after the diagnosis was made. A chest radiograph was clear, and magnetic resonance imaging showed mild skin thickening and image enhancement at the left shoulder—possibly a postsurgical change—with no nodularity suggesting a residual or recurrent tumor. Surgical oncology determined that the patient did not require further excision and placed him on regular follow-up every 2 to 3 months for the next 2 years.
uncertain origin that is considered to be on a spectrum with the more aggressive pleomorphic dermal sarcoma (PDS); it can be distinguished from PDS by histologic features such as nerve or vessel invasion.1 Both entities share oncogenes (eg, tumor protein 53 gene mutations) and are histologically and immunohistochemically similar. Atypical fibroxanthoma largely is viewed as an intermediate-risk tumor that is locally aggressive but rarely metastasizes, with a reported local recurrence rate of 5% to 11% and metastasis risk of 1% to 2%. Conversely, PDS is a more aggressive diagnosis with a high risk for local recurrence and metastasis (7%-69% and 4%-20%, respectively).1
Atypical fibroxanthomas may mimic other entities, both clinically and histologically. It commonly manifests as a flesh-colored to erythematous, sometimes ulcerated nodule on sun-exposed skin in elderly patients, leading to a broad range of clinical differential diagnoses, including other primary cutaneous malignancies (eg, squamous cell carcinoma, amelanotic melanoma), cutaneous sarcomas (eg, dermatofibrosarcoma protuberans), adnexal and other tumors (eg, pleomorphic fibroma, pilomatricoma), cutaneous metastases, and even keloid scars. As the differentials can look clinically similar, a skin biopsy may be necessary to confirm the diagnosis.
Histologically, AFX tends to show an undifferentiated pleomorphic spindle cell morphology. Notably, histology can be highly variable, with other reported histologic patterns including keloidlike, pleomorphic, epithelioid, rhabdoid, clear-cell, foamy cell, granular cell, bizarre cell, pseudoangiomatous, inflammatory, and osteoclast-rich patterns.2 Thus, the histologic differential diagnosis also is broad, and AFX primarily is a diagnosis of exclusion without specific immunohistochemical markers that serve to exclude other diagnoses. For example, AFX tends to stain positive for CD10 and CD68, though these are not specific markers for AFX. Furthermore, although certain histologic markers may commonly be more positive in AFX than PDS (eg, CD74 stains positive in 20% of AFXs and only 1% of PDSs), this is not reliable enough to be diagnostic.3 As such, AFX is distinguished from PDS primarily by histologic features such as subcutaneous tissue invasion, vascular or perineural invasion, necrosis, or local invasion/ metastases.1 Given the rarity of both tumors, no established management guidelines exist, although excision (wide local excision or Mohs micrographic surgery) usually is recommended, with some authors suggesting margins of 1 cm for AFX and 2 cm to 3 cm for PDS.1
This atypical case of AFX arising in non–sun-exposed skin in a young man raises questions about whether unknown genetic factors or possibly prior immunosuppression could have contributed to the development of the tumor. A thorough history and physical examination can provide valuable clues for biopsy, including ongoing growth, absence of known prior trauma or acne at the site, and clinical appearance, such as the reddish, solitary, dome-shaped lesion in our patient.
- Ørholt M, Abebe K, Rasmussen LE, et al. Atypical fibroxanthoma and pleomorphic dermal sarcoma: local recurrence and metastasis in a nationwide population-based cohort of 1118 patients. J Am Acad Dermatol. 2023;89:1177-1184. doi:10.1016/j.jaad.2023.08.050
- Agaimy A. The many faces of atypical fibroxanthoma. Semin Diagn Pathol. 2023;40:306-312. doi:10.1053/j.semdp.2023.06.001
- Rapini RP. Practical Dermatopathology. 3rd ed. Elsevier Health Sciences; 2021.
THE DIAGNOSIS: Atypical Fibroxanthoma
Given the appearance of the nodule and the absence of features of a keloid scar, a soft-tissue or adnexal tumor was suspected. Histology revealed a thin epidermis with loss of rete ridges and a Grenz zone. There was a nodular uncircumscribed dermal proliferation of spindle cells forming interweaving fascicles with elongated ovoid nuclei and prominent nucleoli (Figure). There was moderate cellular and nuclear atypia, and no necrosis was observed. The spindle cells stained positive for CD10 and negative for AE1/AE3, cytokeratin 5/6, S100, melanoma triple marker, Factor XIII 1, ERG, CD31, CD34, desmin, and smooth muscle actin; ERG, CD31, CD34, and SMA highlighted small vessels within the tumor. The histologic diagnosis was an atypical spindle cell tumor favoring atypical fibroxanthoma (AFX). The excisional biopsy margins were clear.

The patient was referred to surgical oncology to consider re-excision of margins after the diagnosis was made. A chest radiograph was clear, and magnetic resonance imaging showed mild skin thickening and image enhancement at the left shoulder—possibly a postsurgical change—with no nodularity suggesting a residual or recurrent tumor. Surgical oncology determined that the patient did not require further excision and placed him on regular follow-up every 2 to 3 months for the next 2 years.
uncertain origin that is considered to be on a spectrum with the more aggressive pleomorphic dermal sarcoma (PDS); it can be distinguished from PDS by histologic features such as nerve or vessel invasion.1 Both entities share oncogenes (eg, tumor protein 53 gene mutations) and are histologically and immunohistochemically similar. Atypical fibroxanthoma largely is viewed as an intermediate-risk tumor that is locally aggressive but rarely metastasizes, with a reported local recurrence rate of 5% to 11% and metastasis risk of 1% to 2%. Conversely, PDS is a more aggressive diagnosis with a high risk for local recurrence and metastasis (7%-69% and 4%-20%, respectively).1
Atypical fibroxanthomas may mimic other entities, both clinically and histologically. It commonly manifests as a flesh-colored to erythematous, sometimes ulcerated nodule on sun-exposed skin in elderly patients, leading to a broad range of clinical differential diagnoses, including other primary cutaneous malignancies (eg, squamous cell carcinoma, amelanotic melanoma), cutaneous sarcomas (eg, dermatofibrosarcoma protuberans), adnexal and other tumors (eg, pleomorphic fibroma, pilomatricoma), cutaneous metastases, and even keloid scars. As the differentials can look clinically similar, a skin biopsy may be necessary to confirm the diagnosis.
Histologically, AFX tends to show an undifferentiated pleomorphic spindle cell morphology. Notably, histology can be highly variable, with other reported histologic patterns including keloidlike, pleomorphic, epithelioid, rhabdoid, clear-cell, foamy cell, granular cell, bizarre cell, pseudoangiomatous, inflammatory, and osteoclast-rich patterns.2 Thus, the histologic differential diagnosis also is broad, and AFX primarily is a diagnosis of exclusion without specific immunohistochemical markers that serve to exclude other diagnoses. For example, AFX tends to stain positive for CD10 and CD68, though these are not specific markers for AFX. Furthermore, although certain histologic markers may commonly be more positive in AFX than PDS (eg, CD74 stains positive in 20% of AFXs and only 1% of PDSs), this is not reliable enough to be diagnostic.3 As such, AFX is distinguished from PDS primarily by histologic features such as subcutaneous tissue invasion, vascular or perineural invasion, necrosis, or local invasion/ metastases.1 Given the rarity of both tumors, no established management guidelines exist, although excision (wide local excision or Mohs micrographic surgery) usually is recommended, with some authors suggesting margins of 1 cm for AFX and 2 cm to 3 cm for PDS.1
This atypical case of AFX arising in non–sun-exposed skin in a young man raises questions about whether unknown genetic factors or possibly prior immunosuppression could have contributed to the development of the tumor. A thorough history and physical examination can provide valuable clues for biopsy, including ongoing growth, absence of known prior trauma or acne at the site, and clinical appearance, such as the reddish, solitary, dome-shaped lesion in our patient.
THE DIAGNOSIS: Atypical Fibroxanthoma
Given the appearance of the nodule and the absence of features of a keloid scar, a soft-tissue or adnexal tumor was suspected. Histology revealed a thin epidermis with loss of rete ridges and a Grenz zone. There was a nodular uncircumscribed dermal proliferation of spindle cells forming interweaving fascicles with elongated ovoid nuclei and prominent nucleoli (Figure). There was moderate cellular and nuclear atypia, and no necrosis was observed. The spindle cells stained positive for CD10 and negative for AE1/AE3, cytokeratin 5/6, S100, melanoma triple marker, Factor XIII 1, ERG, CD31, CD34, desmin, and smooth muscle actin; ERG, CD31, CD34, and SMA highlighted small vessels within the tumor. The histologic diagnosis was an atypical spindle cell tumor favoring atypical fibroxanthoma (AFX). The excisional biopsy margins were clear.

The patient was referred to surgical oncology to consider re-excision of margins after the diagnosis was made. A chest radiograph was clear, and magnetic resonance imaging showed mild skin thickening and image enhancement at the left shoulder—possibly a postsurgical change—with no nodularity suggesting a residual or recurrent tumor. Surgical oncology determined that the patient did not require further excision and placed him on regular follow-up every 2 to 3 months for the next 2 years.
uncertain origin that is considered to be on a spectrum with the more aggressive pleomorphic dermal sarcoma (PDS); it can be distinguished from PDS by histologic features such as nerve or vessel invasion.1 Both entities share oncogenes (eg, tumor protein 53 gene mutations) and are histologically and immunohistochemically similar. Atypical fibroxanthoma largely is viewed as an intermediate-risk tumor that is locally aggressive but rarely metastasizes, with a reported local recurrence rate of 5% to 11% and metastasis risk of 1% to 2%. Conversely, PDS is a more aggressive diagnosis with a high risk for local recurrence and metastasis (7%-69% and 4%-20%, respectively).1
Atypical fibroxanthomas may mimic other entities, both clinically and histologically. It commonly manifests as a flesh-colored to erythematous, sometimes ulcerated nodule on sun-exposed skin in elderly patients, leading to a broad range of clinical differential diagnoses, including other primary cutaneous malignancies (eg, squamous cell carcinoma, amelanotic melanoma), cutaneous sarcomas (eg, dermatofibrosarcoma protuberans), adnexal and other tumors (eg, pleomorphic fibroma, pilomatricoma), cutaneous metastases, and even keloid scars. As the differentials can look clinically similar, a skin biopsy may be necessary to confirm the diagnosis.
Histologically, AFX tends to show an undifferentiated pleomorphic spindle cell morphology. Notably, histology can be highly variable, with other reported histologic patterns including keloidlike, pleomorphic, epithelioid, rhabdoid, clear-cell, foamy cell, granular cell, bizarre cell, pseudoangiomatous, inflammatory, and osteoclast-rich patterns.2 Thus, the histologic differential diagnosis also is broad, and AFX primarily is a diagnosis of exclusion without specific immunohistochemical markers that serve to exclude other diagnoses. For example, AFX tends to stain positive for CD10 and CD68, though these are not specific markers for AFX. Furthermore, although certain histologic markers may commonly be more positive in AFX than PDS (eg, CD74 stains positive in 20% of AFXs and only 1% of PDSs), this is not reliable enough to be diagnostic.3 As such, AFX is distinguished from PDS primarily by histologic features such as subcutaneous tissue invasion, vascular or perineural invasion, necrosis, or local invasion/ metastases.1 Given the rarity of both tumors, no established management guidelines exist, although excision (wide local excision or Mohs micrographic surgery) usually is recommended, with some authors suggesting margins of 1 cm for AFX and 2 cm to 3 cm for PDS.1
This atypical case of AFX arising in non–sun-exposed skin in a young man raises questions about whether unknown genetic factors or possibly prior immunosuppression could have contributed to the development of the tumor. A thorough history and physical examination can provide valuable clues for biopsy, including ongoing growth, absence of known prior trauma or acne at the site, and clinical appearance, such as the reddish, solitary, dome-shaped lesion in our patient.
- Ørholt M, Abebe K, Rasmussen LE, et al. Atypical fibroxanthoma and pleomorphic dermal sarcoma: local recurrence and metastasis in a nationwide population-based cohort of 1118 patients. J Am Acad Dermatol. 2023;89:1177-1184. doi:10.1016/j.jaad.2023.08.050
- Agaimy A. The many faces of atypical fibroxanthoma. Semin Diagn Pathol. 2023;40:306-312. doi:10.1053/j.semdp.2023.06.001
- Rapini RP. Practical Dermatopathology. 3rd ed. Elsevier Health Sciences; 2021.
- Ørholt M, Abebe K, Rasmussen LE, et al. Atypical fibroxanthoma and pleomorphic dermal sarcoma: local recurrence and metastasis in a nationwide population-based cohort of 1118 patients. J Am Acad Dermatol. 2023;89:1177-1184. doi:10.1016/j.jaad.2023.08.050
- Agaimy A. The many faces of atypical fibroxanthoma. Semin Diagn Pathol. 2023;40:306-312. doi:10.1053/j.semdp.2023.06.001
- Rapini RP. Practical Dermatopathology. 3rd ed. Elsevier Health Sciences; 2021.
Reddish Nodule on the Left Shoulder
Reddish Nodule on the Left Shoulder
A 20-year-old man presented to the dermatology clinic for evaluation of a slow-growing nodule on the left shoulder of 1 year’s duration. The patient reported a history of eczema since childhood, which had been treated by an external physician with cyclosporine and methotrexate; however, exact treatment records were unavailable as the patient had been treated at another institution. The eczema had been well controlled over the past year on topical steroids alone. The nodule was asymptomatic, and the patient denied any history of trauma or acne at the affected site. He also denied any family history of similar nodules or other notable skin findings. Physical examination revealed a well circumscribed, 15×12-mm, firm, flesh-colored to reddish nodule on the left shoulder with a slightly whitish center. An excisional biopsy was performed.
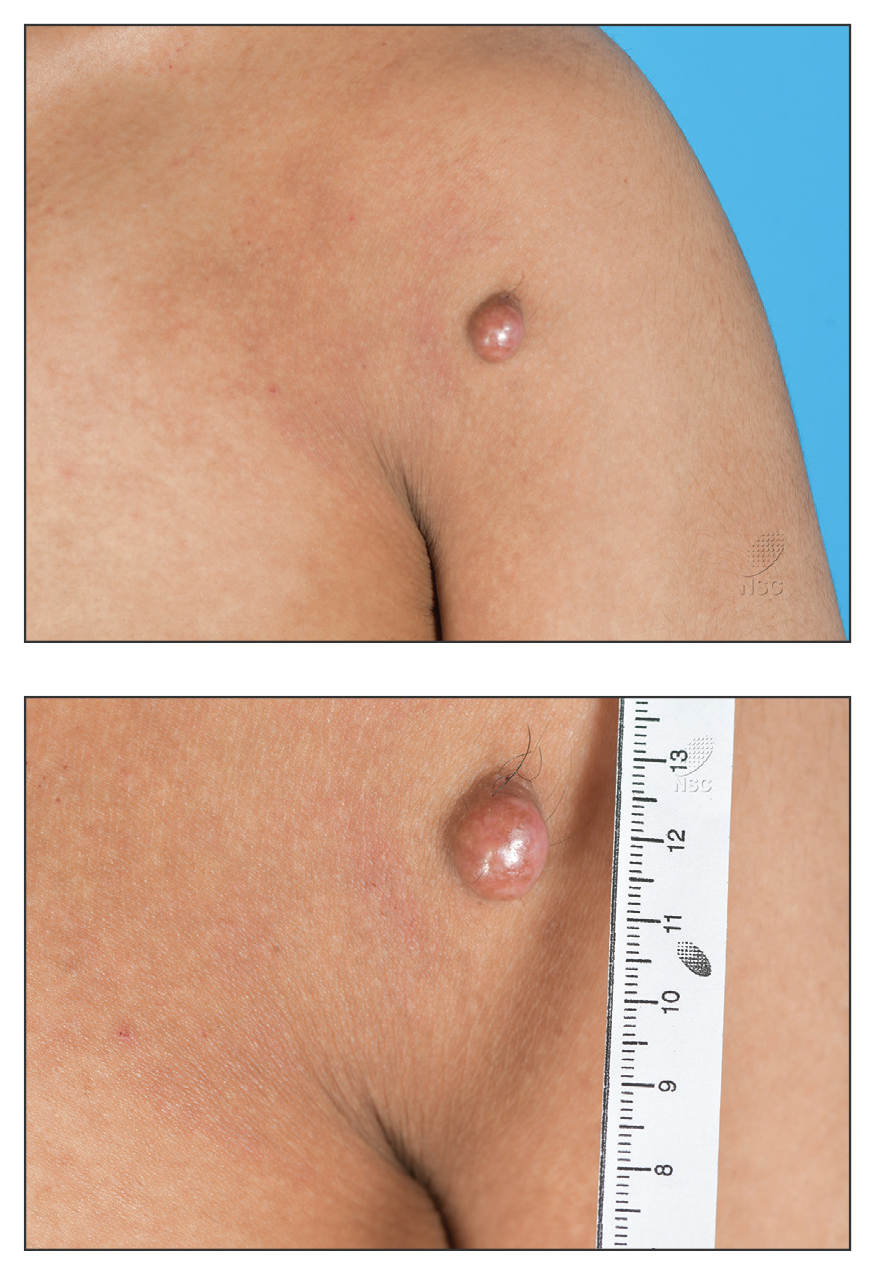
Upadacitinib for Treatment of Severe Atopic Dermatitis in a Child
Upadacitinib for Treatment of Severe Atopic Dermatitis in a Child
To the Editor:
Atopic dermatitis (AD) is one of the most common chronic inflammatory skin diseases and is characterized by age-related morphology and distribution of lesions. Although AD can manifest at any age, it often develops during childhood, with an estimated worldwide prevalence of 15% to 25% in children and 1% to 10% in adults.1 Clinical manifestation includes chronic or recurrent xerosis, pruritic eczematous lesions involving the flexural and extensor areas, and cutaneous infections. Immediate skin test reactivity and elevated total IgE levels can be found in up to 80% of patients.2
Although the pathogenesis of AD is complex, multifactorial, and not completely understood, some studies have highlighted the central role of a type 2 immune response, resulting in skin barrier dysfunction, cutaneous inflammation, and neuroimmune dysregulation.3,4 The primary goals of treatment are to mitigate these factors through improvement of symptoms and long-term disease control. Topical emollients are used to repair the epidermal barrier, and topical anti-inflammatory therapy with corticosteroids or calcineurin inhibitors might be applied during flares; however, systemic treatment is essential for patients with moderate to severe AD that is not controlled with topical treatment or phototherapy.5
Until recently, systemic immunosuppressant agents such as corticosteroids, cyclosporine, and methotrexate were the only systemic treatment options for severe AD; however, their effectiveness is limited and they may cause serious long-term adverse events, limiting their regular usage, especially in children.6
Therapies that target type 2 immune responses include anti–IL-4/IL-13, anti–IL-13, and anti–IL-31 biologics. Dupilumab is a fully human monoclonal antibody targeting the type 2 immune response. This biologic directly binds to IL-4Rα,which prevents signaling by both the IL-4 and IL-13 pathways. Dupilumab was the first biologic approved by the US Food and Drug Administration (FDA) for the treatment of moderate to severe AD, with demonstrated efficacy and a favorable safety profile.5
In addition to biologics, Janus kinase (JAK) inhibitors belong to the small-molecule class. These drugs block the JAK/STAT intracellular signaling pathway, leading to inhibition of downstream effects triggered by several cytokines related to AD pathogenesis. Upadacitinib is an oral JAK inhibitor that was approved by the FDA in 2022 for treatment of severe AD in adults and children aged 12 years and older. This drug promotes a selective and reversible JAK-1 inhibition and has demonstrated rapid onset of action and a sustained reduction in the signs and symptoms of AD.7 We report the case of a child with recalcitrant severe AD that showed significant clinical improvement following off-label treatment with upadacitinib after showing a poor clinical response to dupilumab.
A 9-year-old girl presented to our pediatrics department with progressive worsening of severe AD over the previous 2 years. The patient had been diagnosed with AD at 6 months old, at which time she was treated with several prescribed moisturizers, topical and systemic corticosteroids, and calcineurin inhibitors with no clinical improvement.
The patient initially presented to us for evaluation of severe pruritus and associated sleep loss at age 7 years; physical examination revealed severe xerosis and disseminated pruritic eczematous lesions. Her SCORAD (SCORing Atopic Dermatitis) score was 70 (range, 0-103), and laboratory testing showed a high eosinophil count (1.5×103/μL [range, 0-0.6×103], 13%) and IgE level (1686 κU/L [range, 0-90]); a skin prick test on the forearm was positive for Blomia tropicalis.
Following her presentation with severe AD at 7 years old, the patient was prescribed systemic treatments including methotrexate and cyclosporine. During treatment with these agents, she presented to our department with several bacterial skin infections that required oral and intravenous antibiotics for treatment. These agents ultimately were discontinued after 12 months due to the adverse effects and poor clinical improvement. At age 8 years, the patient received an initial 600-mg dose of dupilumab followed by 300 mg subcutaneously every 4 weeks for 6 months along with topical corticosteroids and emollients. During treatment with dupilumab, the patient showed no clinical improvement (SCORAD score, 62). Therefore, we decided to change the dose to 200 mg every 2 weeks. The patient still showed no improvement and presented at age 9 years with moderate conjunctivitis and oculocutaneous infection caused by herpes simplex virus, which required treatment with oral acyclovir (Figure 1).
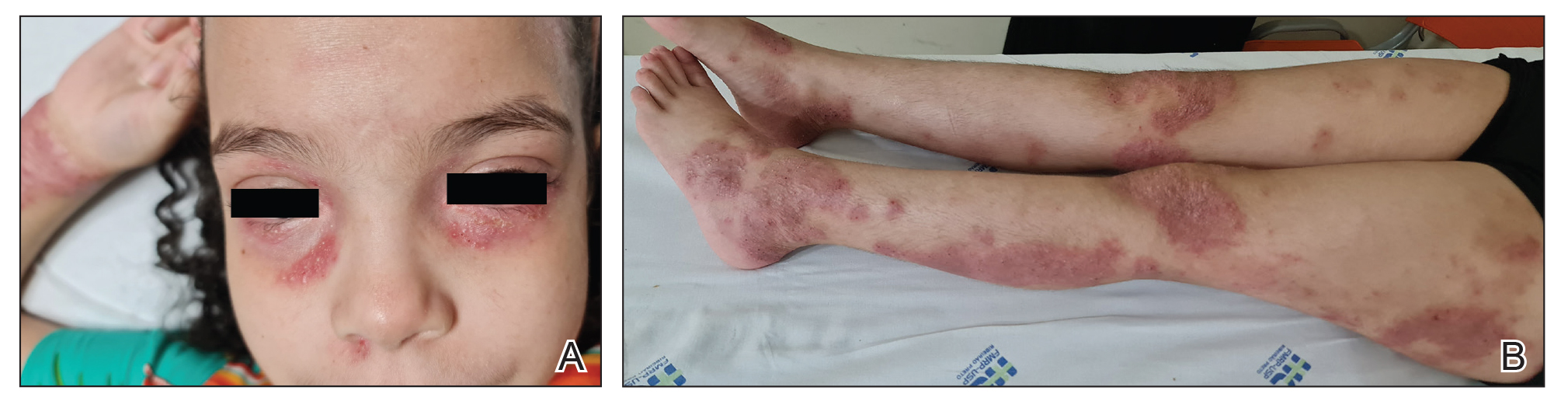
Considering the severe and refractory clinical course and the poor response to the recommended treatments for the patient’s age, oral upadacitinib was administered off label at a dose of 15 mg once daily after informed consent was obtained from her parents. She returned for follow-up once weekly for 1 month. Three days after starting treatment with upadacitinib, she showed considerable improvement in itch, and her SCORAD score decreased from 62 to 31 after 15 days. After 2 months of treatment, she reported no pruritus or sleep loss, and her SCORAD score was 4.5 (Figure 2). The results of a complete blood count, coagulation function test, and liver and kidney function tests were normal at 6-month and 12-month follow-up during upadacitinib therapy. No adverse effects were observed. The patient currently has completed 18 months of treatment, and the disease remains in complete remission.
Atopic dermatitis is highly prevalent in children. According to the International Study of Asthma and Allergies in Childhood, the prevalence of eczema in 2009 was 8.2% among children aged 6 to 7 years and 5% among adolescents aged between 13 and 14 years in Brazil; severe AD was present in 1.5% of children in both age groups.8
The main systemic therapies currently available for patients with severe AD are immunosuppressants, biologics, and small-molecule drugs. The considerable adverse effects of immunosuppressants limit their application. Dupilumab is considered the first-line treatment for children with severe AD. Clinical trials and case reports have demonstrated that dupilumab is effective in patients with AD, promoting notable improvement of pruritic eczematous lesions and quality-of-life scores.9 Dupilumab has been approved by the FDA for children older than 6 months, and some studies have shown up to a 49% reduction of pruritus in this age group.9 The main reported adverse effects were mild conjunctivitis and oral herpes simplex virus infection.9,10
Upadacitinib is a reversible and selective JAK-1 inhibitor approved by the FDA for treatment of severe AD in patients aged 12 years and older. A multicenter, randomized, double-blind, placebo-controlled trial evaluated adolescents (12-17 years) and adults (18-75 years) with moderate to severe AD who were randomly assigned (1:1:1) to receive upadacitinib 15 mg, upadacitinib 30 mg, or placebo once daily for 16 weeks.11 A higher proportion of patients achieved an Eczema Area and Severity Index score of 75 at week 16 with both upadacitinib 15 mg daily (70%) and 30 mg daily (80%) compared to placebo. Improvements also were observed in both SCORAD and pruritus scores. The most commonly reported adverse events were acne, lipid profile abnormalities, and herpes zoster infection.11
Our patient was a child with severe refractory AD that demonstrated a poor treatment response to dupilumab. When switched to off-label upadacitinib, her disease was effectively controlled; the treatment also was well tolerated with no adverse effects. Reports of upadacitinib used to treat AD in patients younger than 12 years are limited in the literature. One case report described a 9-year-old child with concurrent alopecia areata and severe AD who was successfully treated off label with upadacitinib.12 A clinical trial also has evaluated the pharmacokinetics, safety, and tolerability of upadacitinib in children aged 2 to 12 years with severe AD (ClinicalTrials.gov Identifier: NCT03646604); although the trial was completed in 2024, at the time of this review (July 2025), the results have not been published.
Interestingly, there have been a few reports of adults with severe AD that failed to respond to treatment with immunosuppressants and dupilumab but showed notable clinical improvement when therapy was switched to upadacitinib,13,14 as we noticed with our patient. These findings suggest that the JAK-STAT intracellular signaling pathway plays an important role in the pathogenesis of AD.
Continued development of safe and efficient targeted treatment for children with severe AD is critical. Upadacitinib was a safe and effective option for treatment of refractory and severe AD in our patient; however, further studies are needed to confirm both the efficacy and safety of JAK inhibitors in this age group.
- Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387:1109-1122.
- Wollenberg A, Christen-Zäch S, Taieb A, et al. ETFAD/EADV Eczema Task Force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J Eur Acad Dermatol Venereol. 2020;34 :2717-2744.
- Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venererol. 1980;92:44-47.
- Nakahara T, Kido-Nakahara M, Tsuji G, et al. Basics and recent advances in the pathophysiology of atopic dermatitis. J Dermatol. 2021;48:130-139.
- Wollenberg A, Kinberger M, Arents B, et al. European guideline (EuroGuiDerm) on atopic eczema: part I—systemic therapy. J Eur Acad Dermatol Venereol. 2022;36:1409-1431.
- Chu DK, Schneider L, Asiniwasis RN, et al. Atopic dermatitis (eczema) guidelines: 2023 American Academy of Allergy, Asthma and Immunology/American College of Allergy, Asthma and Immunology Joint Task Force on Practice Parameters GRADE– and Institute of Medicine–based recommendations. Ann Allergy Asthma Immunol. 2024;132:274-312.
- Rick JW, Lio P, Atluri S, et al. Atopic dermatitis: a guide to transitioning to janus kinase inhibitors. Dermatitis. 2023;34:297-300.
- Prado E, Pastorino AC, Harari DK, et al. Severe atopic dermatitis: a practical treatment guide from the Brazilian Association of Allergy and Immunology and the Brazilian Society of Pediatrics. Arq Asma Alerg Imunol. 2022;6:432-467.
- Paller AS, Simpson EL, Siegfried EC, et al. Dupilumab in children aged 6 months to younger than 6 years with uncontrolled atopic dermatitis: a randomized, double-blind, placebo-controlled, phase 3 trial. Lancet. 2022;400:908-919.
- Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389:2287-2303.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021 ;397:2151-2168.
- Yu D, Ren Y. Upadacitinib for successful treatment of alopecia universalis in a child: a case report and literature review. Acta Derm Venererol. 2023;103:adv5578.
- Cantelli M, Martora F, Patruno C, et al. Upadacitinib improved alopecia areata in a patient with atopic dermatitis: a case report. Dermatol Ther. 2022;35:E15346.
- Gambardella A, Licata G, Calabrese G, et al. Dual efficacy of upadacitinib in 2 patients with concomitant severe atopic dermatitis and alopecia areata. Dermatitis. 2021;32:E85-E86.
To the Editor:
Atopic dermatitis (AD) is one of the most common chronic inflammatory skin diseases and is characterized by age-related morphology and distribution of lesions. Although AD can manifest at any age, it often develops during childhood, with an estimated worldwide prevalence of 15% to 25% in children and 1% to 10% in adults.1 Clinical manifestation includes chronic or recurrent xerosis, pruritic eczematous lesions involving the flexural and extensor areas, and cutaneous infections. Immediate skin test reactivity and elevated total IgE levels can be found in up to 80% of patients.2
Although the pathogenesis of AD is complex, multifactorial, and not completely understood, some studies have highlighted the central role of a type 2 immune response, resulting in skin barrier dysfunction, cutaneous inflammation, and neuroimmune dysregulation.3,4 The primary goals of treatment are to mitigate these factors through improvement of symptoms and long-term disease control. Topical emollients are used to repair the epidermal barrier, and topical anti-inflammatory therapy with corticosteroids or calcineurin inhibitors might be applied during flares; however, systemic treatment is essential for patients with moderate to severe AD that is not controlled with topical treatment or phototherapy.5
Until recently, systemic immunosuppressant agents such as corticosteroids, cyclosporine, and methotrexate were the only systemic treatment options for severe AD; however, their effectiveness is limited and they may cause serious long-term adverse events, limiting their regular usage, especially in children.6
Therapies that target type 2 immune responses include anti–IL-4/IL-13, anti–IL-13, and anti–IL-31 biologics. Dupilumab is a fully human monoclonal antibody targeting the type 2 immune response. This biologic directly binds to IL-4Rα,which prevents signaling by both the IL-4 and IL-13 pathways. Dupilumab was the first biologic approved by the US Food and Drug Administration (FDA) for the treatment of moderate to severe AD, with demonstrated efficacy and a favorable safety profile.5
In addition to biologics, Janus kinase (JAK) inhibitors belong to the small-molecule class. These drugs block the JAK/STAT intracellular signaling pathway, leading to inhibition of downstream effects triggered by several cytokines related to AD pathogenesis. Upadacitinib is an oral JAK inhibitor that was approved by the FDA in 2022 for treatment of severe AD in adults and children aged 12 years and older. This drug promotes a selective and reversible JAK-1 inhibition and has demonstrated rapid onset of action and a sustained reduction in the signs and symptoms of AD.7 We report the case of a child with recalcitrant severe AD that showed significant clinical improvement following off-label treatment with upadacitinib after showing a poor clinical response to dupilumab.
A 9-year-old girl presented to our pediatrics department with progressive worsening of severe AD over the previous 2 years. The patient had been diagnosed with AD at 6 months old, at which time she was treated with several prescribed moisturizers, topical and systemic corticosteroids, and calcineurin inhibitors with no clinical improvement.
The patient initially presented to us for evaluation of severe pruritus and associated sleep loss at age 7 years; physical examination revealed severe xerosis and disseminated pruritic eczematous lesions. Her SCORAD (SCORing Atopic Dermatitis) score was 70 (range, 0-103), and laboratory testing showed a high eosinophil count (1.5×103/μL [range, 0-0.6×103], 13%) and IgE level (1686 κU/L [range, 0-90]); a skin prick test on the forearm was positive for Blomia tropicalis.
Following her presentation with severe AD at 7 years old, the patient was prescribed systemic treatments including methotrexate and cyclosporine. During treatment with these agents, she presented to our department with several bacterial skin infections that required oral and intravenous antibiotics for treatment. These agents ultimately were discontinued after 12 months due to the adverse effects and poor clinical improvement. At age 8 years, the patient received an initial 600-mg dose of dupilumab followed by 300 mg subcutaneously every 4 weeks for 6 months along with topical corticosteroids and emollients. During treatment with dupilumab, the patient showed no clinical improvement (SCORAD score, 62). Therefore, we decided to change the dose to 200 mg every 2 weeks. The patient still showed no improvement and presented at age 9 years with moderate conjunctivitis and oculocutaneous infection caused by herpes simplex virus, which required treatment with oral acyclovir (Figure 1).

Considering the severe and refractory clinical course and the poor response to the recommended treatments for the patient’s age, oral upadacitinib was administered off label at a dose of 15 mg once daily after informed consent was obtained from her parents. She returned for follow-up once weekly for 1 month. Three days after starting treatment with upadacitinib, she showed considerable improvement in itch, and her SCORAD score decreased from 62 to 31 after 15 days. After 2 months of treatment, she reported no pruritus or sleep loss, and her SCORAD score was 4.5 (Figure 2). The results of a complete blood count, coagulation function test, and liver and kidney function tests were normal at 6-month and 12-month follow-up during upadacitinib therapy. No adverse effects were observed. The patient currently has completed 18 months of treatment, and the disease remains in complete remission.

Atopic dermatitis is highly prevalent in children. According to the International Study of Asthma and Allergies in Childhood, the prevalence of eczema in 2009 was 8.2% among children aged 6 to 7 years and 5% among adolescents aged between 13 and 14 years in Brazil; severe AD was present in 1.5% of children in both age groups.8
The main systemic therapies currently available for patients with severe AD are immunosuppressants, biologics, and small-molecule drugs. The considerable adverse effects of immunosuppressants limit their application. Dupilumab is considered the first-line treatment for children with severe AD. Clinical trials and case reports have demonstrated that dupilumab is effective in patients with AD, promoting notable improvement of pruritic eczematous lesions and quality-of-life scores.9 Dupilumab has been approved by the FDA for children older than 6 months, and some studies have shown up to a 49% reduction of pruritus in this age group.9 The main reported adverse effects were mild conjunctivitis and oral herpes simplex virus infection.9,10
Upadacitinib is a reversible and selective JAK-1 inhibitor approved by the FDA for treatment of severe AD in patients aged 12 years and older. A multicenter, randomized, double-blind, placebo-controlled trial evaluated adolescents (12-17 years) and adults (18-75 years) with moderate to severe AD who were randomly assigned (1:1:1) to receive upadacitinib 15 mg, upadacitinib 30 mg, or placebo once daily for 16 weeks.11 A higher proportion of patients achieved an Eczema Area and Severity Index score of 75 at week 16 with both upadacitinib 15 mg daily (70%) and 30 mg daily (80%) compared to placebo. Improvements also were observed in both SCORAD and pruritus scores. The most commonly reported adverse events were acne, lipid profile abnormalities, and herpes zoster infection.11
Our patient was a child with severe refractory AD that demonstrated a poor treatment response to dupilumab. When switched to off-label upadacitinib, her disease was effectively controlled; the treatment also was well tolerated with no adverse effects. Reports of upadacitinib used to treat AD in patients younger than 12 years are limited in the literature. One case report described a 9-year-old child with concurrent alopecia areata and severe AD who was successfully treated off label with upadacitinib.12 A clinical trial also has evaluated the pharmacokinetics, safety, and tolerability of upadacitinib in children aged 2 to 12 years with severe AD (ClinicalTrials.gov Identifier: NCT03646604); although the trial was completed in 2024, at the time of this review (July 2025), the results have not been published.
Interestingly, there have been a few reports of adults with severe AD that failed to respond to treatment with immunosuppressants and dupilumab but showed notable clinical improvement when therapy was switched to upadacitinib,13,14 as we noticed with our patient. These findings suggest that the JAK-STAT intracellular signaling pathway plays an important role in the pathogenesis of AD.
Continued development of safe and efficient targeted treatment for children with severe AD is critical. Upadacitinib was a safe and effective option for treatment of refractory and severe AD in our patient; however, further studies are needed to confirm both the efficacy and safety of JAK inhibitors in this age group.
To the Editor:
Atopic dermatitis (AD) is one of the most common chronic inflammatory skin diseases and is characterized by age-related morphology and distribution of lesions. Although AD can manifest at any age, it often develops during childhood, with an estimated worldwide prevalence of 15% to 25% in children and 1% to 10% in adults.1 Clinical manifestation includes chronic or recurrent xerosis, pruritic eczematous lesions involving the flexural and extensor areas, and cutaneous infections. Immediate skin test reactivity and elevated total IgE levels can be found in up to 80% of patients.2
Although the pathogenesis of AD is complex, multifactorial, and not completely understood, some studies have highlighted the central role of a type 2 immune response, resulting in skin barrier dysfunction, cutaneous inflammation, and neuroimmune dysregulation.3,4 The primary goals of treatment are to mitigate these factors through improvement of symptoms and long-term disease control. Topical emollients are used to repair the epidermal barrier, and topical anti-inflammatory therapy with corticosteroids or calcineurin inhibitors might be applied during flares; however, systemic treatment is essential for patients with moderate to severe AD that is not controlled with topical treatment or phototherapy.5
Until recently, systemic immunosuppressant agents such as corticosteroids, cyclosporine, and methotrexate were the only systemic treatment options for severe AD; however, their effectiveness is limited and they may cause serious long-term adverse events, limiting their regular usage, especially in children.6
Therapies that target type 2 immune responses include anti–IL-4/IL-13, anti–IL-13, and anti–IL-31 biologics. Dupilumab is a fully human monoclonal antibody targeting the type 2 immune response. This biologic directly binds to IL-4Rα,which prevents signaling by both the IL-4 and IL-13 pathways. Dupilumab was the first biologic approved by the US Food and Drug Administration (FDA) for the treatment of moderate to severe AD, with demonstrated efficacy and a favorable safety profile.5
In addition to biologics, Janus kinase (JAK) inhibitors belong to the small-molecule class. These drugs block the JAK/STAT intracellular signaling pathway, leading to inhibition of downstream effects triggered by several cytokines related to AD pathogenesis. Upadacitinib is an oral JAK inhibitor that was approved by the FDA in 2022 for treatment of severe AD in adults and children aged 12 years and older. This drug promotes a selective and reversible JAK-1 inhibition and has demonstrated rapid onset of action and a sustained reduction in the signs and symptoms of AD.7 We report the case of a child with recalcitrant severe AD that showed significant clinical improvement following off-label treatment with upadacitinib after showing a poor clinical response to dupilumab.
A 9-year-old girl presented to our pediatrics department with progressive worsening of severe AD over the previous 2 years. The patient had been diagnosed with AD at 6 months old, at which time she was treated with several prescribed moisturizers, topical and systemic corticosteroids, and calcineurin inhibitors with no clinical improvement.
The patient initially presented to us for evaluation of severe pruritus and associated sleep loss at age 7 years; physical examination revealed severe xerosis and disseminated pruritic eczematous lesions. Her SCORAD (SCORing Atopic Dermatitis) score was 70 (range, 0-103), and laboratory testing showed a high eosinophil count (1.5×103/μL [range, 0-0.6×103], 13%) and IgE level (1686 κU/L [range, 0-90]); a skin prick test on the forearm was positive for Blomia tropicalis.
Following her presentation with severe AD at 7 years old, the patient was prescribed systemic treatments including methotrexate and cyclosporine. During treatment with these agents, she presented to our department with several bacterial skin infections that required oral and intravenous antibiotics for treatment. These agents ultimately were discontinued after 12 months due to the adverse effects and poor clinical improvement. At age 8 years, the patient received an initial 600-mg dose of dupilumab followed by 300 mg subcutaneously every 4 weeks for 6 months along with topical corticosteroids and emollients. During treatment with dupilumab, the patient showed no clinical improvement (SCORAD score, 62). Therefore, we decided to change the dose to 200 mg every 2 weeks. The patient still showed no improvement and presented at age 9 years with moderate conjunctivitis and oculocutaneous infection caused by herpes simplex virus, which required treatment with oral acyclovir (Figure 1).

Considering the severe and refractory clinical course and the poor response to the recommended treatments for the patient’s age, oral upadacitinib was administered off label at a dose of 15 mg once daily after informed consent was obtained from her parents. She returned for follow-up once weekly for 1 month. Three days after starting treatment with upadacitinib, she showed considerable improvement in itch, and her SCORAD score decreased from 62 to 31 after 15 days. After 2 months of treatment, she reported no pruritus or sleep loss, and her SCORAD score was 4.5 (Figure 2). The results of a complete blood count, coagulation function test, and liver and kidney function tests were normal at 6-month and 12-month follow-up during upadacitinib therapy. No adverse effects were observed. The patient currently has completed 18 months of treatment, and the disease remains in complete remission.

Atopic dermatitis is highly prevalent in children. According to the International Study of Asthma and Allergies in Childhood, the prevalence of eczema in 2009 was 8.2% among children aged 6 to 7 years and 5% among adolescents aged between 13 and 14 years in Brazil; severe AD was present in 1.5% of children in both age groups.8
The main systemic therapies currently available for patients with severe AD are immunosuppressants, biologics, and small-molecule drugs. The considerable adverse effects of immunosuppressants limit their application. Dupilumab is considered the first-line treatment for children with severe AD. Clinical trials and case reports have demonstrated that dupilumab is effective in patients with AD, promoting notable improvement of pruritic eczematous lesions and quality-of-life scores.9 Dupilumab has been approved by the FDA for children older than 6 months, and some studies have shown up to a 49% reduction of pruritus in this age group.9 The main reported adverse effects were mild conjunctivitis and oral herpes simplex virus infection.9,10
Upadacitinib is a reversible and selective JAK-1 inhibitor approved by the FDA for treatment of severe AD in patients aged 12 years and older. A multicenter, randomized, double-blind, placebo-controlled trial evaluated adolescents (12-17 years) and adults (18-75 years) with moderate to severe AD who were randomly assigned (1:1:1) to receive upadacitinib 15 mg, upadacitinib 30 mg, or placebo once daily for 16 weeks.11 A higher proportion of patients achieved an Eczema Area and Severity Index score of 75 at week 16 with both upadacitinib 15 mg daily (70%) and 30 mg daily (80%) compared to placebo. Improvements also were observed in both SCORAD and pruritus scores. The most commonly reported adverse events were acne, lipid profile abnormalities, and herpes zoster infection.11
Our patient was a child with severe refractory AD that demonstrated a poor treatment response to dupilumab. When switched to off-label upadacitinib, her disease was effectively controlled; the treatment also was well tolerated with no adverse effects. Reports of upadacitinib used to treat AD in patients younger than 12 years are limited in the literature. One case report described a 9-year-old child with concurrent alopecia areata and severe AD who was successfully treated off label with upadacitinib.12 A clinical trial also has evaluated the pharmacokinetics, safety, and tolerability of upadacitinib in children aged 2 to 12 years with severe AD (ClinicalTrials.gov Identifier: NCT03646604); although the trial was completed in 2024, at the time of this review (July 2025), the results have not been published.
Interestingly, there have been a few reports of adults with severe AD that failed to respond to treatment with immunosuppressants and dupilumab but showed notable clinical improvement when therapy was switched to upadacitinib,13,14 as we noticed with our patient. These findings suggest that the JAK-STAT intracellular signaling pathway plays an important role in the pathogenesis of AD.
Continued development of safe and efficient targeted treatment for children with severe AD is critical. Upadacitinib was a safe and effective option for treatment of refractory and severe AD in our patient; however, further studies are needed to confirm both the efficacy and safety of JAK inhibitors in this age group.
- Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387:1109-1122.
- Wollenberg A, Christen-Zäch S, Taieb A, et al. ETFAD/EADV Eczema Task Force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J Eur Acad Dermatol Venereol. 2020;34 :2717-2744.
- Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venererol. 1980;92:44-47.
- Nakahara T, Kido-Nakahara M, Tsuji G, et al. Basics and recent advances in the pathophysiology of atopic dermatitis. J Dermatol. 2021;48:130-139.
- Wollenberg A, Kinberger M, Arents B, et al. European guideline (EuroGuiDerm) on atopic eczema: part I—systemic therapy. J Eur Acad Dermatol Venereol. 2022;36:1409-1431.
- Chu DK, Schneider L, Asiniwasis RN, et al. Atopic dermatitis (eczema) guidelines: 2023 American Academy of Allergy, Asthma and Immunology/American College of Allergy, Asthma and Immunology Joint Task Force on Practice Parameters GRADE– and Institute of Medicine–based recommendations. Ann Allergy Asthma Immunol. 2024;132:274-312.
- Rick JW, Lio P, Atluri S, et al. Atopic dermatitis: a guide to transitioning to janus kinase inhibitors. Dermatitis. 2023;34:297-300.
- Prado E, Pastorino AC, Harari DK, et al. Severe atopic dermatitis: a practical treatment guide from the Brazilian Association of Allergy and Immunology and the Brazilian Society of Pediatrics. Arq Asma Alerg Imunol. 2022;6:432-467.
- Paller AS, Simpson EL, Siegfried EC, et al. Dupilumab in children aged 6 months to younger than 6 years with uncontrolled atopic dermatitis: a randomized, double-blind, placebo-controlled, phase 3 trial. Lancet. 2022;400:908-919.
- Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389:2287-2303.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021 ;397:2151-2168.
- Yu D, Ren Y. Upadacitinib for successful treatment of alopecia universalis in a child: a case report and literature review. Acta Derm Venererol. 2023;103:adv5578.
- Cantelli M, Martora F, Patruno C, et al. Upadacitinib improved alopecia areata in a patient with atopic dermatitis: a case report. Dermatol Ther. 2022;35:E15346.
- Gambardella A, Licata G, Calabrese G, et al. Dual efficacy of upadacitinib in 2 patients with concomitant severe atopic dermatitis and alopecia areata. Dermatitis. 2021;32:E85-E86.
- Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387:1109-1122.
- Wollenberg A, Christen-Zäch S, Taieb A, et al. ETFAD/EADV Eczema Task Force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J Eur Acad Dermatol Venereol. 2020;34 :2717-2744.
- Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venererol. 1980;92:44-47.
- Nakahara T, Kido-Nakahara M, Tsuji G, et al. Basics and recent advances in the pathophysiology of atopic dermatitis. J Dermatol. 2021;48:130-139.
- Wollenberg A, Kinberger M, Arents B, et al. European guideline (EuroGuiDerm) on atopic eczema: part I—systemic therapy. J Eur Acad Dermatol Venereol. 2022;36:1409-1431.
- Chu DK, Schneider L, Asiniwasis RN, et al. Atopic dermatitis (eczema) guidelines: 2023 American Academy of Allergy, Asthma and Immunology/American College of Allergy, Asthma and Immunology Joint Task Force on Practice Parameters GRADE– and Institute of Medicine–based recommendations. Ann Allergy Asthma Immunol. 2024;132:274-312.
- Rick JW, Lio P, Atluri S, et al. Atopic dermatitis: a guide to transitioning to janus kinase inhibitors. Dermatitis. 2023;34:297-300.
- Prado E, Pastorino AC, Harari DK, et al. Severe atopic dermatitis: a practical treatment guide from the Brazilian Association of Allergy and Immunology and the Brazilian Society of Pediatrics. Arq Asma Alerg Imunol. 2022;6:432-467.
- Paller AS, Simpson EL, Siegfried EC, et al. Dupilumab in children aged 6 months to younger than 6 years with uncontrolled atopic dermatitis: a randomized, double-blind, placebo-controlled, phase 3 trial. Lancet. 2022;400:908-919.
- Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389:2287-2303.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021 ;397:2151-2168.
- Yu D, Ren Y. Upadacitinib for successful treatment of alopecia universalis in a child: a case report and literature review. Acta Derm Venererol. 2023;103:adv5578.
- Cantelli M, Martora F, Patruno C, et al. Upadacitinib improved alopecia areata in a patient with atopic dermatitis: a case report. Dermatol Ther. 2022;35:E15346.
- Gambardella A, Licata G, Calabrese G, et al. Dual efficacy of upadacitinib in 2 patients with concomitant severe atopic dermatitis and alopecia areata. Dermatitis. 2021;32:E85-E86.
Upadacitinib for Treatment of Severe Atopic Dermatitis in a Child
Upadacitinib for Treatment of Severe Atopic Dermatitis in a Child
PRACTICE POINTS
- Atopic dermatitis (AD) is one of the most common chronic inflammatory skin diseases in pediatric patients.
- Dupilumab is the first-line treatment for severe AD in children and is approved for use in patients aged 6 months and older. Janus kinase inhibitors are approved only for patients aged 12 years and older.
- Upadacitinib may be a safe treatment option for severe AD in children, even those younger than 12 years.
Data Trends 2025: Pulmonology
Data Trends 2025: Pulmonology
Click to view more from Federal Health Care Data Trends 2025.
- Bozick R, Neil R. Respiratory health among US veterans across age and over time. RAND Corporation;2024. Accessed April 10, 2025. https://www.rand.org/pubs/research_reports/RRA1363-13.html
- Kaul B, et al. Am J Respir Crit Care Med. 2022;206(6):750-757. doi:10.1164/rccm.202112-2724OC
- Garshick E, Blanc PD. Curr Opin Pulm Med. 2023;29(2):83-89. doi:10.1097/MCP.0000000000000946
- Bamonti PM, et al. J Psychiatr Res. 2024;176:140-147. doi:10.1016/j.jpsychires.2024.05.053
- Bamonti PM, et al. Int J Chron Obstruct Pulmon Dis. 2022;17:1269-1283. doi:10.2147/COPD.S339323
- Goldstein LA, et al. Am J Health Promot. 2025;39(2):215-223. doi:10.1177/08901171241273443
- Leng Y, et al. Neurology. 2021;96(13):e1792-e1799. doi:10.1212/WNL.0000000000011656
- Rau A, et al. Ann Am Thorac Soc. 2025;22(2):200-207. doi:10.1513/AnnalATS.202312-1089OC
Click to view more from Federal Health Care Data Trends 2025.
Click to view more from Federal Health Care Data Trends 2025.
- Bozick R, Neil R. Respiratory health among US veterans across age and over time. RAND Corporation;2024. Accessed April 10, 2025. https://www.rand.org/pubs/research_reports/RRA1363-13.html
- Kaul B, et al. Am J Respir Crit Care Med. 2022;206(6):750-757. doi:10.1164/rccm.202112-2724OC
- Garshick E, Blanc PD. Curr Opin Pulm Med. 2023;29(2):83-89. doi:10.1097/MCP.0000000000000946
- Bamonti PM, et al. J Psychiatr Res. 2024;176:140-147. doi:10.1016/j.jpsychires.2024.05.053
- Bamonti PM, et al. Int J Chron Obstruct Pulmon Dis. 2022;17:1269-1283. doi:10.2147/COPD.S339323
- Goldstein LA, et al. Am J Health Promot. 2025;39(2):215-223. doi:10.1177/08901171241273443
- Leng Y, et al. Neurology. 2021;96(13):e1792-e1799. doi:10.1212/WNL.0000000000011656
- Rau A, et al. Ann Am Thorac Soc. 2025;22(2):200-207. doi:10.1513/AnnalATS.202312-1089OC
- Bozick R, Neil R. Respiratory health among US veterans across age and over time. RAND Corporation;2024. Accessed April 10, 2025. https://www.rand.org/pubs/research_reports/RRA1363-13.html
- Kaul B, et al. Am J Respir Crit Care Med. 2022;206(6):750-757. doi:10.1164/rccm.202112-2724OC
- Garshick E, Blanc PD. Curr Opin Pulm Med. 2023;29(2):83-89. doi:10.1097/MCP.0000000000000946
- Bamonti PM, et al. J Psychiatr Res. 2024;176:140-147. doi:10.1016/j.jpsychires.2024.05.053
- Bamonti PM, et al. Int J Chron Obstruct Pulmon Dis. 2022;17:1269-1283. doi:10.2147/COPD.S339323
- Goldstein LA, et al. Am J Health Promot. 2025;39(2):215-223. doi:10.1177/08901171241273443
- Leng Y, et al. Neurology. 2021;96(13):e1792-e1799. doi:10.1212/WNL.0000000000011656
- Rau A, et al. Ann Am Thorac Soc. 2025;22(2):200-207. doi:10.1513/AnnalATS.202312-1089OC
Data Trends 2025: Pulmonology
Data Trends 2025: Pulmonology
Data Trends 2025: Acute Pain
Data Trends 2025: Acute Pain
Click to view more from Federal Health Care Data Trends 2025.
- Baumann L, et al. Curr Pain Headache Rep. 2023;27(9):437-444. doi:10.1007/s11916-023-01127-0
- Reif S, et al. Mil Med. 2018;183(9-10):e330-e337. doi:10.1093/milmed/usx200
- Sharp LK, e t a l . Pain. 2023;164( 4 ) : 749-757. doi:10.1097/j .pain.0000000000002759
- Dalton MK, et al. J Trauma Acute Care Surg. 2021;91(2S Suppl 2):S213-S220. doi:10.1097/TA.0000000000003133
- Mahyar L, et al. Reg Anesth Pain Med. 2024;49(2):117-121. doi:10.1136/rapm-2023-104610
- Gupta K, et al. Eur J Trauma Emerg Surg. 2025;51(1):103. doi:10.1007/s00068-025-02778-x
- Mariano ER, et al. Reg Anesth Pain Med. 2022;47(2):118-127. doi:10.1136/rapm-2021-103083
Click to view more from Federal Health Care Data Trends 2025.
Click to view more from Federal Health Care Data Trends 2025.
- Baumann L, et al. Curr Pain Headache Rep. 2023;27(9):437-444. doi:10.1007/s11916-023-01127-0
- Reif S, et al. Mil Med. 2018;183(9-10):e330-e337. doi:10.1093/milmed/usx200
- Sharp LK, e t a l . Pain. 2023;164( 4 ) : 749-757. doi:10.1097/j .pain.0000000000002759
- Dalton MK, et al. J Trauma Acute Care Surg. 2021;91(2S Suppl 2):S213-S220. doi:10.1097/TA.0000000000003133
- Mahyar L, et al. Reg Anesth Pain Med. 2024;49(2):117-121. doi:10.1136/rapm-2023-104610
- Gupta K, et al. Eur J Trauma Emerg Surg. 2025;51(1):103. doi:10.1007/s00068-025-02778-x
- Mariano ER, et al. Reg Anesth Pain Med. 2022;47(2):118-127. doi:10.1136/rapm-2021-103083
- Baumann L, et al. Curr Pain Headache Rep. 2023;27(9):437-444. doi:10.1007/s11916-023-01127-0
- Reif S, et al. Mil Med. 2018;183(9-10):e330-e337. doi:10.1093/milmed/usx200
- Sharp LK, e t a l . Pain. 2023;164( 4 ) : 749-757. doi:10.1097/j .pain.0000000000002759
- Dalton MK, et al. J Trauma Acute Care Surg. 2021;91(2S Suppl 2):S213-S220. doi:10.1097/TA.0000000000003133
- Mahyar L, et al. Reg Anesth Pain Med. 2024;49(2):117-121. doi:10.1136/rapm-2023-104610
- Gupta K, et al. Eur J Trauma Emerg Surg. 2025;51(1):103. doi:10.1007/s00068-025-02778-x
- Mariano ER, et al. Reg Anesth Pain Med. 2022;47(2):118-127. doi:10.1136/rapm-2021-103083
Data Trends 2025: Acute Pain
Data Trends 2025: Acute Pain
Data Trends 2025: Mental Health
Data Trends 2025: Mental Health
Click to view more from Federal Health Care Data Trends 2025.
- US Department of Veterans Affairs, Office of Suicide Prevention. 2024 National Veteran Suicide Prevention Annual Report. 2024. https://www.mentalhealth.va.gov/suicide_prevention/data.asp.
- Tenso K, et al. JAMA Netw Open. 2024;7(11):e2443054. doi:10.1001/jamanetworkopen.2024.43054
- Saulnier KG, et al. JAMA Netw Open. 2024;7(12):e2452144. doi:10.1001/jamanetworkopen.2024.52144
- Elser H, et al. Am J Epidemiol. 2025;194(2):123-132. doi:10.1093/aje/kwaf002
Click to view more from Federal Health Care Data Trends 2025.
Click to view more from Federal Health Care Data Trends 2025.
- US Department of Veterans Affairs, Office of Suicide Prevention. 2024 National Veteran Suicide Prevention Annual Report. 2024. https://www.mentalhealth.va.gov/suicide_prevention/data.asp.
- Tenso K, et al. JAMA Netw Open. 2024;7(11):e2443054. doi:10.1001/jamanetworkopen.2024.43054
- Saulnier KG, et al. JAMA Netw Open. 2024;7(12):e2452144. doi:10.1001/jamanetworkopen.2024.52144
- Elser H, et al. Am J Epidemiol. 2025;194(2):123-132. doi:10.1093/aje/kwaf002
- US Department of Veterans Affairs, Office of Suicide Prevention. 2024 National Veteran Suicide Prevention Annual Report. 2024. https://www.mentalhealth.va.gov/suicide_prevention/data.asp.
- Tenso K, et al. JAMA Netw Open. 2024;7(11):e2443054. doi:10.1001/jamanetworkopen.2024.43054
- Saulnier KG, et al. JAMA Netw Open. 2024;7(12):e2452144. doi:10.1001/jamanetworkopen.2024.52144
- Elser H, et al. Am J Epidemiol. 2025;194(2):123-132. doi:10.1093/aje/kwaf002
Data Trends 2025: Mental Health
Data Trends 2025: Mental Health
Data Trends 2025: Obesity
Obesity
Click here to view more from Federal Health Care Data Trends 2025.
1. GBD 2021 US Obesity Forecasting Collaborators. National-level and state-level prevalence of overweight and obesity among children, adolescents, and adults in the USA, 1990-2021, and forecasts up to 2050. Lancet. 2024;404(10469):2278-2298. doi:10.1016/S0140-6736(24)01548-4
2. Breland JY, et al. J Gen Intern Med. 2017;32(Suppl 1):11-17. doi:10.1007/s11606-016-3962-1
3. American Security Project. Costs and consequences: obesity’s compounding impact on the Military Health System. September 2024. Accessed April 21, 2025. https://www.americansecurityproject.org/wp-content/uploads/2024/09/Ref-0295-Costs-and-Consequences-Obesitys-Compounding-Impact-on-the-Military-Health-System.pdf
4. Baser O, et al. Healthcare (Basel). 2023;11(11):1529. doi:10.3390/healthcare11111529
5. Maclin-Akinyemi C, et al. Mil Med. 2017;182(9):e1816-e1823. doi:10.7205/MILMED-D-16-00380.
6. Yang D, et al. Mil Med. 2022;187(7-8):e948-e954. doi:10.1093/milmed/usab292
7. American Security Project. Ready the Reserve: obesity’s impacts on National Guard and Reserve readiness. April 2025. Accessed April 21, 2025. https://www.americansecurityproject.org/white-paper-ready-the-reserve-obesitys-impacts-onnational-guard-and-reserve-readiness/
8. Betancourt JA, et al. Healthcare (Basel). 2020;8(3):191. doi:10.3390/healthcare8030191
9. Breland JY, et al. Psychiatr Serv. 2020;1;71(5):506-509. doi:10.1176/appi.ps.201900078
Click here to view more from Federal Health Care Data Trends 2025.
Click here to view more from Federal Health Care Data Trends 2025.
1. GBD 2021 US Obesity Forecasting Collaborators. National-level and state-level prevalence of overweight and obesity among children, adolescents, and adults in the USA, 1990-2021, and forecasts up to 2050. Lancet. 2024;404(10469):2278-2298. doi:10.1016/S0140-6736(24)01548-4
2. Breland JY, et al. J Gen Intern Med. 2017;32(Suppl 1):11-17. doi:10.1007/s11606-016-3962-1
3. American Security Project. Costs and consequences: obesity’s compounding impact on the Military Health System. September 2024. Accessed April 21, 2025. https://www.americansecurityproject.org/wp-content/uploads/2024/09/Ref-0295-Costs-and-Consequences-Obesitys-Compounding-Impact-on-the-Military-Health-System.pdf
4. Baser O, et al. Healthcare (Basel). 2023;11(11):1529. doi:10.3390/healthcare11111529
5. Maclin-Akinyemi C, et al. Mil Med. 2017;182(9):e1816-e1823. doi:10.7205/MILMED-D-16-00380.
6. Yang D, et al. Mil Med. 2022;187(7-8):e948-e954. doi:10.1093/milmed/usab292
7. American Security Project. Ready the Reserve: obesity’s impacts on National Guard and Reserve readiness. April 2025. Accessed April 21, 2025. https://www.americansecurityproject.org/white-paper-ready-the-reserve-obesitys-impacts-onnational-guard-and-reserve-readiness/
8. Betancourt JA, et al. Healthcare (Basel). 2020;8(3):191. doi:10.3390/healthcare8030191
9. Breland JY, et al. Psychiatr Serv. 2020;1;71(5):506-509. doi:10.1176/appi.ps.201900078
1. GBD 2021 US Obesity Forecasting Collaborators. National-level and state-level prevalence of overweight and obesity among children, adolescents, and adults in the USA, 1990-2021, and forecasts up to 2050. Lancet. 2024;404(10469):2278-2298. doi:10.1016/S0140-6736(24)01548-4
2. Breland JY, et al. J Gen Intern Med. 2017;32(Suppl 1):11-17. doi:10.1007/s11606-016-3962-1
3. American Security Project. Costs and consequences: obesity’s compounding impact on the Military Health System. September 2024. Accessed April 21, 2025. https://www.americansecurityproject.org/wp-content/uploads/2024/09/Ref-0295-Costs-and-Consequences-Obesitys-Compounding-Impact-on-the-Military-Health-System.pdf
4. Baser O, et al. Healthcare (Basel). 2023;11(11):1529. doi:10.3390/healthcare11111529
5. Maclin-Akinyemi C, et al. Mil Med. 2017;182(9):e1816-e1823. doi:10.7205/MILMED-D-16-00380.
6. Yang D, et al. Mil Med. 2022;187(7-8):e948-e954. doi:10.1093/milmed/usab292
7. American Security Project. Ready the Reserve: obesity’s impacts on National Guard and Reserve readiness. April 2025. Accessed April 21, 2025. https://www.americansecurityproject.org/white-paper-ready-the-reserve-obesitys-impacts-onnational-guard-and-reserve-readiness/
8. Betancourt JA, et al. Healthcare (Basel). 2020;8(3):191. doi:10.3390/healthcare8030191
9. Breland JY, et al. Psychiatr Serv. 2020;1;71(5):506-509. doi:10.1176/appi.ps.201900078
Obesity
Obesity
Data Trends 2025: Hepatology
Data Trends 2025: Hepatology
Click here to view more from Federal Health Care Data Trends 2025.
- Niezen S, et al. Am J Gastroenterol. Published online January 7, 2025. doi:10.14309/ajg.0000000000003312
- Beydoun HA, Tsai J. J Viral Hepat. 2024;31(10):601-613. doi:10.1111/jvh.13981
- Yeoh A, et al. J Clin Gastroenterol. 2024;58(7):718-725. doi:10.1097/MCG.0000000000001921
- Varley CD, et al. Clin Infect Dis. 2024;78(6):1571-1579. doi:10.1093/cid/ciae025
- Njei B, et al. Dig Dis Sci. 2025;70(2):802-813. doi:10.1007/s10620-024-08764-4
Click here to view more from Federal Health Care Data Trends 2025.
Click here to view more from Federal Health Care Data Trends 2025.
- Niezen S, et al. Am J Gastroenterol. Published online January 7, 2025. doi:10.14309/ajg.0000000000003312
- Beydoun HA, Tsai J. J Viral Hepat. 2024;31(10):601-613. doi:10.1111/jvh.13981
- Yeoh A, et al. J Clin Gastroenterol. 2024;58(7):718-725. doi:10.1097/MCG.0000000000001921
- Varley CD, et al. Clin Infect Dis. 2024;78(6):1571-1579. doi:10.1093/cid/ciae025
- Njei B, et al. Dig Dis Sci. 2025;70(2):802-813. doi:10.1007/s10620-024-08764-4
- Niezen S, et al. Am J Gastroenterol. Published online January 7, 2025. doi:10.14309/ajg.0000000000003312
- Beydoun HA, Tsai J. J Viral Hepat. 2024;31(10):601-613. doi:10.1111/jvh.13981
- Yeoh A, et al. J Clin Gastroenterol. 2024;58(7):718-725. doi:10.1097/MCG.0000000000001921
- Varley CD, et al. Clin Infect Dis. 2024;78(6):1571-1579. doi:10.1093/cid/ciae025
- Njei B, et al. Dig Dis Sci. 2025;70(2):802-813. doi:10.1007/s10620-024-08764-4
Data Trends 2025: Hepatology
Data Trends 2025: Hepatology
Federal Health Care Data Trends 2025
Federal Health Care Data Trends 2025
Federal Health Care Data Trends is a special supplement to Federal Practitioner, showcasing the latest research in health care for veterans and active-duty military members via compelling infographics.
Topics include:
Federal Health Care Data Trends is a special supplement to Federal Practitioner, showcasing the latest research in health care for veterans and active-duty military members via compelling infographics.
Topics include:
Federal Health Care Data Trends is a special supplement to Federal Practitioner, showcasing the latest research in health care for veterans and active-duty military members via compelling infographics.
Topics include:
Federal Health Care Data Trends 2025
Federal Health Care Data Trends 2025