User login
Official Newspaper of the American College of Surgeons
Point-Counterpoint: Is surgery the best option for acute appendicitis?
YES – Surgery remains the best option for acute appendicitis
The vast majority of surgeons the world over use operative therapy as the prime mode of treatment for appendicitis. As Dr. Liu argues below, acute appendicitis can be treated successfully nonoperatively with antibiotics. That it can be done so successfully does not mean that this is the correct or best way to treat this disease, however. Appendicitis is a spectrum disease: It can present anywhere from simple and uncomplicated, to retrocecal, to perforated with abscess, and even with free perforation with diffuse purulence and generalized peritonitis.
There continues to be a small, but definite mortality due to appendicitis, mostly in cases of delayed diagnosis, as well as very significant morbidity. In the pediatric population, perforation of the appendix, which usually occurs 36-72 hours after symptoms begin, has a postoperative abscess rate requiring further therapy (drainage and/or further antibiotic treatment) in excess of 15% (Ann. Surg. 2012;256:581-5).
One of the arguments for nonoperative treatment of appendicitis is that the appendix is simply another diverticulum of the colon, and acute diverticulitis can frequently be treated successfully with antibiotics, leaving operative treatment to be delayed for subsequent bouts or for complications of the disease. However, the appendix differs from many colonic diverticula in that it frequently lies free in the abdomen whereas many colonic diverticula are surrounded by mesentery. Thus, perforation of a colonic diverticulum is immediately contained, whereas the appendix often perforates freely into the abdomen, leading to widespread contamination. The result is generalized sepsis versus controlled, localized sepsis that may be treated successfully with powerful antibiotics.
In the case of simple, controlled appendicitis, an argument can certainly be made for early treatment with antibiotics and careful clinical follow-up. Undoubtedly, many of these patients will improve. However, the difficulty lies in diagnosing the simple case without knowing who is going to progress to complication, especially in the pediatric age group. Prompt, not emergent, operation when the diagnosis is made is probably the more prudent course.
Finally, a few brief words should be said about laparoscopic treatment of acute appendicitis as well as drainage of well-established abscesses followed by interval appendectomy. There is little question now that laparoscopic appendectomy treats the disease as well as or better than open operation, even in cases of perforation. A low incidence of complications secondary to laparoscopy notwithstanding, this approach leads to shorter hospitalizations and faster recovery, and compares favorably with nonoperative treatment. This is a clear improvement in care over the past 20 years.
There are cases, however, where a well-established abscess exists. Immediate open operation in these cases can make a stable patient extremely septic. Such cases are probably better managed with percutaneous abscess drainage (a necessity) followed a number of weeks later by interval appendectomy (which some may consider elective). Not removing the appendix later in these cases creates some risk for recurrent appendicitis, however.
Thus, despite data that would argue for a nonoperative approach to acute appendicitis, this author firmly believes that the overwhelming long-term experience favors operative treatment of acute appendicitis once the diagnosis is made. Treatment algorithms for timing of surgery and duration of perioperative antibiotics continue to be the topic of much clinical research. However, the basic principle of operative treatment remains the safest and most expeditious approach for patients. This, after all, should be the surgeon’s goal.
Dr. Lund is an ACS Fellow and surgeon-in-chief, Phoenix Children’s Hospital, and professor of child health and surgery at the University of Arizona College of Medicine–Phoenix.
NO – There is a strong case for antibiotics to treat acute appendicitis
Most surgeons who treat acute appendicitis have had to resort to antibiotic treatment occasionally, either because of significant patient comorbidities or due to the presence of phlegmon/abscess. The first time I did so was nearly 10 years ago in a 70-year-old man with severe COPD, whose surgical mortality was estimated to be greater than 50% by my medical colleagues. After some discussion, the patient elected to try antibiotics, while I stood ready to operate at the first sign of trouble. Within 48 hours, his symptoms resolved, his laboratory findings normalized, and he was headed home.
Despite similar experiences, treating appendicitis with antibiotics has been controversial. Appendectomy has been the treatment since Fitz advocated it in 1886 and Wangensteen proposed obstruction as the cause in 1940. Wangensteen observed histological changes consistent with appendicitis in 5 out of 22 ligated appendices brought out through abdominal incisions in patients undergoing colostomy operations (Ann. Surg. 1939;110:629-47). He later concluded that "obstruction of the lumen, if maintained for a sufficient number of hours, would result in acute gangrenous appendicitis with rupture" (Surg. Gynecol. Obstet. 1940;70:799-808). Since then, generations have accepted this without question, even though many observations suggested otherwise.
One such observation was cited by Fitz himself (Am. J. Med. Sci. 1886;92:321-46) that 110 of 300 postmortem examinations showed diseased appendix incidentally, and spontaneous resolution was observed in 20% of appendicitis in the era of CT (Abdom. Imaging 2003;28:276-9). Other observations include finding appendiceal dilation only in most advanced cases (Am. J. Surg. 1971;122:378-80), and normal intraluminal pressures in 70% of phlegmonous or gangrenous appendices (Am. J. Surg. 1984;147:390-2). Recent studies describing different epidemiologic trends for nonperforated and perforated appendicitis (Ann. Surg. 2007;245:886-92), and dissimilar variables affecting their incidence (BMJ 1994;308:107-10) suggest that appendicitis is probably not a single disease entity.
In addition, antibiotics have become far more effective in recent years, thus making them a viable treatment option in appendicitis. A morbidity rate of 35.6% with immediate appendectomy and 13.5% with antibiotic treatment was demonstrated for complicated appendicitis with phlegmon or abscess (Ann. Surg. 2007;246:741-8), and a similar lower morbidity rate for antibiotics (6.7%-8.0%) compared to appendectomy (13%-18%) was observed in patients with appendicitis without phlegmon or abscess (Surgery 2011;150:673-83, Surg. Infect. 2012;13:74-84).
The strong suit of appendectomy has always been source control. The advantage of antibiotics is their limited complications, such as diarrhea or drug reaction. Some appendectomy complications can be quite major, and are not rendered less likely or less serious by the finding of a normal appendix in 3%-15% of patients.
The limitations of antibiotics are the 7%-10% failure rate and 5%-14% recurrences. However, with a 3%-15% negative appendectomy rate and up to 20% spontaneous resolution, antibiotic treatment may be less harmful and potentially less expensive. Since no increase in morbidity and mortality was observed with a delay of appendectomy for a mean of 26 hours (Arch. Surg. 2010;145:886-92), it should be safe to administer antibiotics for 24 hours and forego appendectomy in patients with significant improvement.
In summary, antibiotic treatment merits consideration in patients with acute appendicitis, not because it could replace surgery, but because, as a first-line treatment, it is safe with less serious and less frequent complications than appendectomy. For patients who have failed antibiotics, the risk of surgical complications becomes necessary. A randomized study comparing the two approaches is being conducted in Finland, and hopefully will provide new information to further our understanding of this common but complex disease entity.
Dr. Liu is a general surgeon at FHN Memorial Hospital in Freeport, Ill., who has studied the role of antibiotics in appendicitis for several years.
YES – Surgery remains the best option for acute appendicitis
The vast majority of surgeons the world over use operative therapy as the prime mode of treatment for appendicitis. As Dr. Liu argues below, acute appendicitis can be treated successfully nonoperatively with antibiotics. That it can be done so successfully does not mean that this is the correct or best way to treat this disease, however. Appendicitis is a spectrum disease: It can present anywhere from simple and uncomplicated, to retrocecal, to perforated with abscess, and even with free perforation with diffuse purulence and generalized peritonitis.
There continues to be a small, but definite mortality due to appendicitis, mostly in cases of delayed diagnosis, as well as very significant morbidity. In the pediatric population, perforation of the appendix, which usually occurs 36-72 hours after symptoms begin, has a postoperative abscess rate requiring further therapy (drainage and/or further antibiotic treatment) in excess of 15% (Ann. Surg. 2012;256:581-5).
One of the arguments for nonoperative treatment of appendicitis is that the appendix is simply another diverticulum of the colon, and acute diverticulitis can frequently be treated successfully with antibiotics, leaving operative treatment to be delayed for subsequent bouts or for complications of the disease. However, the appendix differs from many colonic diverticula in that it frequently lies free in the abdomen whereas many colonic diverticula are surrounded by mesentery. Thus, perforation of a colonic diverticulum is immediately contained, whereas the appendix often perforates freely into the abdomen, leading to widespread contamination. The result is generalized sepsis versus controlled, localized sepsis that may be treated successfully with powerful antibiotics.
In the case of simple, controlled appendicitis, an argument can certainly be made for early treatment with antibiotics and careful clinical follow-up. Undoubtedly, many of these patients will improve. However, the difficulty lies in diagnosing the simple case without knowing who is going to progress to complication, especially in the pediatric age group. Prompt, not emergent, operation when the diagnosis is made is probably the more prudent course.
Finally, a few brief words should be said about laparoscopic treatment of acute appendicitis as well as drainage of well-established abscesses followed by interval appendectomy. There is little question now that laparoscopic appendectomy treats the disease as well as or better than open operation, even in cases of perforation. A low incidence of complications secondary to laparoscopy notwithstanding, this approach leads to shorter hospitalizations and faster recovery, and compares favorably with nonoperative treatment. This is a clear improvement in care over the past 20 years.
There are cases, however, where a well-established abscess exists. Immediate open operation in these cases can make a stable patient extremely septic. Such cases are probably better managed with percutaneous abscess drainage (a necessity) followed a number of weeks later by interval appendectomy (which some may consider elective). Not removing the appendix later in these cases creates some risk for recurrent appendicitis, however.
Thus, despite data that would argue for a nonoperative approach to acute appendicitis, this author firmly believes that the overwhelming long-term experience favors operative treatment of acute appendicitis once the diagnosis is made. Treatment algorithms for timing of surgery and duration of perioperative antibiotics continue to be the topic of much clinical research. However, the basic principle of operative treatment remains the safest and most expeditious approach for patients. This, after all, should be the surgeon’s goal.
Dr. Lund is an ACS Fellow and surgeon-in-chief, Phoenix Children’s Hospital, and professor of child health and surgery at the University of Arizona College of Medicine–Phoenix.
NO – There is a strong case for antibiotics to treat acute appendicitis
Most surgeons who treat acute appendicitis have had to resort to antibiotic treatment occasionally, either because of significant patient comorbidities or due to the presence of phlegmon/abscess. The first time I did so was nearly 10 years ago in a 70-year-old man with severe COPD, whose surgical mortality was estimated to be greater than 50% by my medical colleagues. After some discussion, the patient elected to try antibiotics, while I stood ready to operate at the first sign of trouble. Within 48 hours, his symptoms resolved, his laboratory findings normalized, and he was headed home.
Despite similar experiences, treating appendicitis with antibiotics has been controversial. Appendectomy has been the treatment since Fitz advocated it in 1886 and Wangensteen proposed obstruction as the cause in 1940. Wangensteen observed histological changes consistent with appendicitis in 5 out of 22 ligated appendices brought out through abdominal incisions in patients undergoing colostomy operations (Ann. Surg. 1939;110:629-47). He later concluded that "obstruction of the lumen, if maintained for a sufficient number of hours, would result in acute gangrenous appendicitis with rupture" (Surg. Gynecol. Obstet. 1940;70:799-808). Since then, generations have accepted this without question, even though many observations suggested otherwise.
One such observation was cited by Fitz himself (Am. J. Med. Sci. 1886;92:321-46) that 110 of 300 postmortem examinations showed diseased appendix incidentally, and spontaneous resolution was observed in 20% of appendicitis in the era of CT (Abdom. Imaging 2003;28:276-9). Other observations include finding appendiceal dilation only in most advanced cases (Am. J. Surg. 1971;122:378-80), and normal intraluminal pressures in 70% of phlegmonous or gangrenous appendices (Am. J. Surg. 1984;147:390-2). Recent studies describing different epidemiologic trends for nonperforated and perforated appendicitis (Ann. Surg. 2007;245:886-92), and dissimilar variables affecting their incidence (BMJ 1994;308:107-10) suggest that appendicitis is probably not a single disease entity.
In addition, antibiotics have become far more effective in recent years, thus making them a viable treatment option in appendicitis. A morbidity rate of 35.6% with immediate appendectomy and 13.5% with antibiotic treatment was demonstrated for complicated appendicitis with phlegmon or abscess (Ann. Surg. 2007;246:741-8), and a similar lower morbidity rate for antibiotics (6.7%-8.0%) compared to appendectomy (13%-18%) was observed in patients with appendicitis without phlegmon or abscess (Surgery 2011;150:673-83, Surg. Infect. 2012;13:74-84).
The strong suit of appendectomy has always been source control. The advantage of antibiotics is their limited complications, such as diarrhea or drug reaction. Some appendectomy complications can be quite major, and are not rendered less likely or less serious by the finding of a normal appendix in 3%-15% of patients.
The limitations of antibiotics are the 7%-10% failure rate and 5%-14% recurrences. However, with a 3%-15% negative appendectomy rate and up to 20% spontaneous resolution, antibiotic treatment may be less harmful and potentially less expensive. Since no increase in morbidity and mortality was observed with a delay of appendectomy for a mean of 26 hours (Arch. Surg. 2010;145:886-92), it should be safe to administer antibiotics for 24 hours and forego appendectomy in patients with significant improvement.
In summary, antibiotic treatment merits consideration in patients with acute appendicitis, not because it could replace surgery, but because, as a first-line treatment, it is safe with less serious and less frequent complications than appendectomy. For patients who have failed antibiotics, the risk of surgical complications becomes necessary. A randomized study comparing the two approaches is being conducted in Finland, and hopefully will provide new information to further our understanding of this common but complex disease entity.
Dr. Liu is a general surgeon at FHN Memorial Hospital in Freeport, Ill., who has studied the role of antibiotics in appendicitis for several years.
YES – Surgery remains the best option for acute appendicitis
The vast majority of surgeons the world over use operative therapy as the prime mode of treatment for appendicitis. As Dr. Liu argues below, acute appendicitis can be treated successfully nonoperatively with antibiotics. That it can be done so successfully does not mean that this is the correct or best way to treat this disease, however. Appendicitis is a spectrum disease: It can present anywhere from simple and uncomplicated, to retrocecal, to perforated with abscess, and even with free perforation with diffuse purulence and generalized peritonitis.
There continues to be a small, but definite mortality due to appendicitis, mostly in cases of delayed diagnosis, as well as very significant morbidity. In the pediatric population, perforation of the appendix, which usually occurs 36-72 hours after symptoms begin, has a postoperative abscess rate requiring further therapy (drainage and/or further antibiotic treatment) in excess of 15% (Ann. Surg. 2012;256:581-5).
One of the arguments for nonoperative treatment of appendicitis is that the appendix is simply another diverticulum of the colon, and acute diverticulitis can frequently be treated successfully with antibiotics, leaving operative treatment to be delayed for subsequent bouts or for complications of the disease. However, the appendix differs from many colonic diverticula in that it frequently lies free in the abdomen whereas many colonic diverticula are surrounded by mesentery. Thus, perforation of a colonic diverticulum is immediately contained, whereas the appendix often perforates freely into the abdomen, leading to widespread contamination. The result is generalized sepsis versus controlled, localized sepsis that may be treated successfully with powerful antibiotics.
In the case of simple, controlled appendicitis, an argument can certainly be made for early treatment with antibiotics and careful clinical follow-up. Undoubtedly, many of these patients will improve. However, the difficulty lies in diagnosing the simple case without knowing who is going to progress to complication, especially in the pediatric age group. Prompt, not emergent, operation when the diagnosis is made is probably the more prudent course.
Finally, a few brief words should be said about laparoscopic treatment of acute appendicitis as well as drainage of well-established abscesses followed by interval appendectomy. There is little question now that laparoscopic appendectomy treats the disease as well as or better than open operation, even in cases of perforation. A low incidence of complications secondary to laparoscopy notwithstanding, this approach leads to shorter hospitalizations and faster recovery, and compares favorably with nonoperative treatment. This is a clear improvement in care over the past 20 years.
There are cases, however, where a well-established abscess exists. Immediate open operation in these cases can make a stable patient extremely septic. Such cases are probably better managed with percutaneous abscess drainage (a necessity) followed a number of weeks later by interval appendectomy (which some may consider elective). Not removing the appendix later in these cases creates some risk for recurrent appendicitis, however.
Thus, despite data that would argue for a nonoperative approach to acute appendicitis, this author firmly believes that the overwhelming long-term experience favors operative treatment of acute appendicitis once the diagnosis is made. Treatment algorithms for timing of surgery and duration of perioperative antibiotics continue to be the topic of much clinical research. However, the basic principle of operative treatment remains the safest and most expeditious approach for patients. This, after all, should be the surgeon’s goal.
Dr. Lund is an ACS Fellow and surgeon-in-chief, Phoenix Children’s Hospital, and professor of child health and surgery at the University of Arizona College of Medicine–Phoenix.
NO – There is a strong case for antibiotics to treat acute appendicitis
Most surgeons who treat acute appendicitis have had to resort to antibiotic treatment occasionally, either because of significant patient comorbidities or due to the presence of phlegmon/abscess. The first time I did so was nearly 10 years ago in a 70-year-old man with severe COPD, whose surgical mortality was estimated to be greater than 50% by my medical colleagues. After some discussion, the patient elected to try antibiotics, while I stood ready to operate at the first sign of trouble. Within 48 hours, his symptoms resolved, his laboratory findings normalized, and he was headed home.
Despite similar experiences, treating appendicitis with antibiotics has been controversial. Appendectomy has been the treatment since Fitz advocated it in 1886 and Wangensteen proposed obstruction as the cause in 1940. Wangensteen observed histological changes consistent with appendicitis in 5 out of 22 ligated appendices brought out through abdominal incisions in patients undergoing colostomy operations (Ann. Surg. 1939;110:629-47). He later concluded that "obstruction of the lumen, if maintained for a sufficient number of hours, would result in acute gangrenous appendicitis with rupture" (Surg. Gynecol. Obstet. 1940;70:799-808). Since then, generations have accepted this without question, even though many observations suggested otherwise.
One such observation was cited by Fitz himself (Am. J. Med. Sci. 1886;92:321-46) that 110 of 300 postmortem examinations showed diseased appendix incidentally, and spontaneous resolution was observed in 20% of appendicitis in the era of CT (Abdom. Imaging 2003;28:276-9). Other observations include finding appendiceal dilation only in most advanced cases (Am. J. Surg. 1971;122:378-80), and normal intraluminal pressures in 70% of phlegmonous or gangrenous appendices (Am. J. Surg. 1984;147:390-2). Recent studies describing different epidemiologic trends for nonperforated and perforated appendicitis (Ann. Surg. 2007;245:886-92), and dissimilar variables affecting their incidence (BMJ 1994;308:107-10) suggest that appendicitis is probably not a single disease entity.
In addition, antibiotics have become far more effective in recent years, thus making them a viable treatment option in appendicitis. A morbidity rate of 35.6% with immediate appendectomy and 13.5% with antibiotic treatment was demonstrated for complicated appendicitis with phlegmon or abscess (Ann. Surg. 2007;246:741-8), and a similar lower morbidity rate for antibiotics (6.7%-8.0%) compared to appendectomy (13%-18%) was observed in patients with appendicitis without phlegmon or abscess (Surgery 2011;150:673-83, Surg. Infect. 2012;13:74-84).
The strong suit of appendectomy has always been source control. The advantage of antibiotics is their limited complications, such as diarrhea or drug reaction. Some appendectomy complications can be quite major, and are not rendered less likely or less serious by the finding of a normal appendix in 3%-15% of patients.
The limitations of antibiotics are the 7%-10% failure rate and 5%-14% recurrences. However, with a 3%-15% negative appendectomy rate and up to 20% spontaneous resolution, antibiotic treatment may be less harmful and potentially less expensive. Since no increase in morbidity and mortality was observed with a delay of appendectomy for a mean of 26 hours (Arch. Surg. 2010;145:886-92), it should be safe to administer antibiotics for 24 hours and forego appendectomy in patients with significant improvement.
In summary, antibiotic treatment merits consideration in patients with acute appendicitis, not because it could replace surgery, but because, as a first-line treatment, it is safe with less serious and less frequent complications than appendectomy. For patients who have failed antibiotics, the risk of surgical complications becomes necessary. A randomized study comparing the two approaches is being conducted in Finland, and hopefully will provide new information to further our understanding of this common but complex disease entity.
Dr. Liu is a general surgeon at FHN Memorial Hospital in Freeport, Ill., who has studied the role of antibiotics in appendicitis for several years.
Adhesiolysis linked to high morbidity, higher hospitalization costs
Adhesiolysis was associated with an increased risk for a variety of morbidities during repeat abdominal surgery including inadvertent bowel defects, seromuscular injuries, and postoperative sepsis, according to the results of a prospective study.
"All physicians treating patients with disorders of the abdominal cavity that might require surgery should be aware of the adverse effects of adhesiolysis," reported Dr. Richard P.G. ten Broek and his associates in the department of surgery at Radboud University Nijmegen (the Netherlands) Medical Center. The study was published in Annals of Surgery (2013;258:98-106).
The investigators conducted the prospective cohort study to evaluate the direct effects of adhesiolysis on unintentional organ damage, morbidity, and costs during repeat operations, to address the lack of information in this area. They collected data from a total of 755 elective laparotomy or laparoscopy procedures in 715 patients performed at the medical center between June 2008 and June 2010.
The removal of adhesions was undertaken in almost 63% (475) of these procedures. Median adhesiolysis time was 20 minutes, ranging from 1 minute to almost 13 hours. Previous intra-abdominal surgery and peritonitis were among the most common causes of adhesions.
"All physicians treating patients with disorders of the abdominal cavity that might require surgery should be aware of the adverse effects of adhesiolysis,"
In the adhesiolysis group, the rates of perioperative complications were statistically significantly higher, compared with those in patients who did not require adhesiolysis, including full-thickness bowel defect (10.5% in the adhesiolysis group, versus no cases in the nonadhesiolysis group), injury to the seromuscular layer (27.6% v. 3.9%), and injuries to other organs (8.6% vs 2.5%).
Overall, the rate of surgical complications was 23.4% among those who had adhesiolysis, compared with 17.5% of those in the nonadhesiolysis group, also a statistically significant difference.
Adhesiolysis was associated with more than a fivefold increased rate of sepsis (odds ratio, 5.12), almost a fourfold increased risk of intra-abdominal complications (OR, 3.46), and more than a twofold increased risk of incisional wound infection (OR, 2.45), all statistically significant differences. The differences in mortality, urinary tract infections, pneumonia, or hemorrhage were not significantly different.
Adhesiolysis also resulted in longer surgery time (a mean of 22.5 minutes longer), recovery time (a mean of about 2 hours longer), and total hospital stay (a mean of about 3 days longer), as well as 29% greater operative blood loss. Among the cases reviewed, the mean hospital cost when adhesiolysis was performed was $18,579, vs. $14,063 when it was not performed.
This is the first study "showing adhesiolysis as a risk factor for postoperative surgical complications, longer hospital stays, more readmissions, and increased costs," the authors pointed out. These data can be helpful "when counseling patients before surgery, when physicians and health care providers make decisions on implementing antiadhesive strategies, and for the reimbursement policy of insurance companies," they added, noting that fewer than 10% of surgeons counsel patients about the risk of adhesions.
"With the projected increase in more repeat abdominal surgeries because of a longer life expectancy and newer technologies, prevention of adhesiolysis-related morbidity might be even more cost effective," they added.
The study was sponsored by Radboud University Nijmegen Medical Center, with no external funding, and the authors said they had no relevant financial conflicts.
Adhesiolysis was associated with an increased risk for a variety of morbidities during repeat abdominal surgery including inadvertent bowel defects, seromuscular injuries, and postoperative sepsis, according to the results of a prospective study.
"All physicians treating patients with disorders of the abdominal cavity that might require surgery should be aware of the adverse effects of adhesiolysis," reported Dr. Richard P.G. ten Broek and his associates in the department of surgery at Radboud University Nijmegen (the Netherlands) Medical Center. The study was published in Annals of Surgery (2013;258:98-106).
The investigators conducted the prospective cohort study to evaluate the direct effects of adhesiolysis on unintentional organ damage, morbidity, and costs during repeat operations, to address the lack of information in this area. They collected data from a total of 755 elective laparotomy or laparoscopy procedures in 715 patients performed at the medical center between June 2008 and June 2010.
The removal of adhesions was undertaken in almost 63% (475) of these procedures. Median adhesiolysis time was 20 minutes, ranging from 1 minute to almost 13 hours. Previous intra-abdominal surgery and peritonitis were among the most common causes of adhesions.
"All physicians treating patients with disorders of the abdominal cavity that might require surgery should be aware of the adverse effects of adhesiolysis,"
In the adhesiolysis group, the rates of perioperative complications were statistically significantly higher, compared with those in patients who did not require adhesiolysis, including full-thickness bowel defect (10.5% in the adhesiolysis group, versus no cases in the nonadhesiolysis group), injury to the seromuscular layer (27.6% v. 3.9%), and injuries to other organs (8.6% vs 2.5%).
Overall, the rate of surgical complications was 23.4% among those who had adhesiolysis, compared with 17.5% of those in the nonadhesiolysis group, also a statistically significant difference.
Adhesiolysis was associated with more than a fivefold increased rate of sepsis (odds ratio, 5.12), almost a fourfold increased risk of intra-abdominal complications (OR, 3.46), and more than a twofold increased risk of incisional wound infection (OR, 2.45), all statistically significant differences. The differences in mortality, urinary tract infections, pneumonia, or hemorrhage were not significantly different.
Adhesiolysis also resulted in longer surgery time (a mean of 22.5 minutes longer), recovery time (a mean of about 2 hours longer), and total hospital stay (a mean of about 3 days longer), as well as 29% greater operative blood loss. Among the cases reviewed, the mean hospital cost when adhesiolysis was performed was $18,579, vs. $14,063 when it was not performed.
This is the first study "showing adhesiolysis as a risk factor for postoperative surgical complications, longer hospital stays, more readmissions, and increased costs," the authors pointed out. These data can be helpful "when counseling patients before surgery, when physicians and health care providers make decisions on implementing antiadhesive strategies, and for the reimbursement policy of insurance companies," they added, noting that fewer than 10% of surgeons counsel patients about the risk of adhesions.
"With the projected increase in more repeat abdominal surgeries because of a longer life expectancy and newer technologies, prevention of adhesiolysis-related morbidity might be even more cost effective," they added.
The study was sponsored by Radboud University Nijmegen Medical Center, with no external funding, and the authors said they had no relevant financial conflicts.
Adhesiolysis was associated with an increased risk for a variety of morbidities during repeat abdominal surgery including inadvertent bowel defects, seromuscular injuries, and postoperative sepsis, according to the results of a prospective study.
"All physicians treating patients with disorders of the abdominal cavity that might require surgery should be aware of the adverse effects of adhesiolysis," reported Dr. Richard P.G. ten Broek and his associates in the department of surgery at Radboud University Nijmegen (the Netherlands) Medical Center. The study was published in Annals of Surgery (2013;258:98-106).
The investigators conducted the prospective cohort study to evaluate the direct effects of adhesiolysis on unintentional organ damage, morbidity, and costs during repeat operations, to address the lack of information in this area. They collected data from a total of 755 elective laparotomy or laparoscopy procedures in 715 patients performed at the medical center between June 2008 and June 2010.
The removal of adhesions was undertaken in almost 63% (475) of these procedures. Median adhesiolysis time was 20 minutes, ranging from 1 minute to almost 13 hours. Previous intra-abdominal surgery and peritonitis were among the most common causes of adhesions.
"All physicians treating patients with disorders of the abdominal cavity that might require surgery should be aware of the adverse effects of adhesiolysis,"
In the adhesiolysis group, the rates of perioperative complications were statistically significantly higher, compared with those in patients who did not require adhesiolysis, including full-thickness bowel defect (10.5% in the adhesiolysis group, versus no cases in the nonadhesiolysis group), injury to the seromuscular layer (27.6% v. 3.9%), and injuries to other organs (8.6% vs 2.5%).
Overall, the rate of surgical complications was 23.4% among those who had adhesiolysis, compared with 17.5% of those in the nonadhesiolysis group, also a statistically significant difference.
Adhesiolysis was associated with more than a fivefold increased rate of sepsis (odds ratio, 5.12), almost a fourfold increased risk of intra-abdominal complications (OR, 3.46), and more than a twofold increased risk of incisional wound infection (OR, 2.45), all statistically significant differences. The differences in mortality, urinary tract infections, pneumonia, or hemorrhage were not significantly different.
Adhesiolysis also resulted in longer surgery time (a mean of 22.5 minutes longer), recovery time (a mean of about 2 hours longer), and total hospital stay (a mean of about 3 days longer), as well as 29% greater operative blood loss. Among the cases reviewed, the mean hospital cost when adhesiolysis was performed was $18,579, vs. $14,063 when it was not performed.
This is the first study "showing adhesiolysis as a risk factor for postoperative surgical complications, longer hospital stays, more readmissions, and increased costs," the authors pointed out. These data can be helpful "when counseling patients before surgery, when physicians and health care providers make decisions on implementing antiadhesive strategies, and for the reimbursement policy of insurance companies," they added, noting that fewer than 10% of surgeons counsel patients about the risk of adhesions.
"With the projected increase in more repeat abdominal surgeries because of a longer life expectancy and newer technologies, prevention of adhesiolysis-related morbidity might be even more cost effective," they added.
The study was sponsored by Radboud University Nijmegen Medical Center, with no external funding, and the authors said they had no relevant financial conflicts.
FROM ANNALS OF SURGERY
Major finding: The adverse effects of adhesiolysis during abdominal surgery included inadvertently incurred bowel defects, as well as a fivefold greater risk of sepsis, and significantly longer hospital stays and higher hospital costs.
Data source: A prospective cohort study of 755 elective abdominal surgeries in 715 patients, evaluating perioperative and postoperative outcomes associated with those that required adhesiolysis and those that did not.
Disclosures: The study was sponsored by Radboud University Nijmegen Medical Center, with no external funding. The authors said they had no relevant financial conflicts.
Cost of health insurance moderates, but workers pay more
The rise in the cost of employer-sponsored health insurance has ameliorated in the past year, continuing a flattening trend, but patients continue to be asked to pay a bigger share.
That’s the conclusion of the 13th annual survey of nonfederal private and public employers with three or more workers conducted by the Kaiser Family Foundation and the Health Research & Educational Trust, and published Aug. 20 in the journal Health Affairs (doi: 10.1377/hlthaff.2013.0644).
Kaiser and HRET estimate that in 2013, 149 million nonelderly people receive coverage through employer-sponsored insurance, with 57% of U.S. firms offering health benefits – a statistically insignificant change from 61% in 2012 and 60% in 2011. But smaller companies and those with many low-wage workers do not offer benefits as frequently.
In 2013, the average annual premiums for employer-sponsored health insurance rose 5% to $5,884 for single coverage and 4% to $16,351 for family coverage from 2012. This continues the pattern seen over the past few years, with relatively small premium increases.
Workers on average pay 18% of the premium for single coverage and 29% for family coverage, again, similar to the two previous years. "We are in a prolonged period of moderation in premiums, which should create some breathing room for the private sector to try to reduce costs without cutting back benefits for workers," said Kaiser President and CEO Drew Altman, in a statement.
But workers are still struggling to cover health costs. During the same period (2012-2013), wages increased 1.8% and inflation increased 1.1%. And since 2003, the average premium for family coverage has increased 80%, and the average worker contribution has risen 89%.
Employers continue to cost-shift. Seventy-eight percent of covered workers have a deductible, with the average for single coverage running about $1,100 (largely unchanged from 2012). But big deductibles are getting to be more common. Firms with less than 200 employees generally charged more than $1,000, and the number doing that rose from 49% of all such firms in 2012 to 58% in 2013.
Three-quarters of workers have a fixed copayment for office visits with primary care physicians and specialists. That copay averages $23 for primary care and $35 for specialty care for in-network physicians. Out-of-network amounts were not covered in the survey, but Kaiser assumes that patients bear a greater cost for those visits. About 20% of workers pay a percent of the visit (coinsurance) for primary care and the same amount for specialty care. They pay an average 18% of the visit.
The survey found that preferred provider organization (PPO) plans are the most common, enrolling 57% of covered workers in 2013. High-deductible health plans, also known as catastrophic plans, are the next most popular, with 20% of covered workers. Deductibles in those plans range from around $2,000 for single coverage to $4,000 for family coverage, with some much higher.
Only 14% of workers are enrolled in health maintenance organizations (HMOs), 9% in a point-of-service (POS) plan, and less than 1% in a conventional fee-for-service plan. High-deductible plans have grown exponentially since first coming on the scene – going from 8% in 2009 to 17% in 2011 – but have plateaued in the last few years, according to the report.
Also of interest to physicians:
• 23% of employers offering benefits have high performance or tiered networks in their largest health plan. The programs help steer patients to providers who have been identified as being more efficient or giving higher-quality care.
• 56% cover services provided by retail health clinics. Among firms covering services in these settings, 17% provide a financial incentive to receive services in a retail clinic instead of a physician’s office.
• Almost all employers with more than 200 workers and most smaller employers offer at least one wellness program. The majority offer at least one of the following in 2013: weight loss programs, gym membership discounts or on-site exercise facilities, biometric screening, smoking cessation, personal health coaching, health and nutrition education, web-based resources, flu shots or vaccinations, employee assistance programs (EAPs), or a wellness newsletter. Thirty-six percent of large firms and 8% of smaller firms offer employees a financial incentive to participate.
The growth in wellness programs "will be an important issue to watch next year, as employers will have more flexibility and could ask workers to pay more because of their lifestyles and health conditions," said Kaiser Vice President Gary Claxton, the study’s lead investigator and director of the Foundation’s Health Care Marketplace Project.
Mr. Claxton and his colleagues found that an increasing number of employees are likely to be subject to the Affordable Care Act’s various provisions. Thirty-six percent of covered workers are in "grandfathered" plans – that is, they are exempt from the law – down from 48% in 2012 and 56% in 2011.
The authors reported no disclosures.
[email protected]
On Twitter @aliciaault
The rise in the cost of employer-sponsored health insurance has ameliorated in the past year, continuing a flattening trend, but patients continue to be asked to pay a bigger share.
That’s the conclusion of the 13th annual survey of nonfederal private and public employers with three or more workers conducted by the Kaiser Family Foundation and the Health Research & Educational Trust, and published Aug. 20 in the journal Health Affairs (doi: 10.1377/hlthaff.2013.0644).
Kaiser and HRET estimate that in 2013, 149 million nonelderly people receive coverage through employer-sponsored insurance, with 57% of U.S. firms offering health benefits – a statistically insignificant change from 61% in 2012 and 60% in 2011. But smaller companies and those with many low-wage workers do not offer benefits as frequently.
In 2013, the average annual premiums for employer-sponsored health insurance rose 5% to $5,884 for single coverage and 4% to $16,351 for family coverage from 2012. This continues the pattern seen over the past few years, with relatively small premium increases.
Workers on average pay 18% of the premium for single coverage and 29% for family coverage, again, similar to the two previous years. "We are in a prolonged period of moderation in premiums, which should create some breathing room for the private sector to try to reduce costs without cutting back benefits for workers," said Kaiser President and CEO Drew Altman, in a statement.
But workers are still struggling to cover health costs. During the same period (2012-2013), wages increased 1.8% and inflation increased 1.1%. And since 2003, the average premium for family coverage has increased 80%, and the average worker contribution has risen 89%.
Employers continue to cost-shift. Seventy-eight percent of covered workers have a deductible, with the average for single coverage running about $1,100 (largely unchanged from 2012). But big deductibles are getting to be more common. Firms with less than 200 employees generally charged more than $1,000, and the number doing that rose from 49% of all such firms in 2012 to 58% in 2013.
Three-quarters of workers have a fixed copayment for office visits with primary care physicians and specialists. That copay averages $23 for primary care and $35 for specialty care for in-network physicians. Out-of-network amounts were not covered in the survey, but Kaiser assumes that patients bear a greater cost for those visits. About 20% of workers pay a percent of the visit (coinsurance) for primary care and the same amount for specialty care. They pay an average 18% of the visit.
The survey found that preferred provider organization (PPO) plans are the most common, enrolling 57% of covered workers in 2013. High-deductible health plans, also known as catastrophic plans, are the next most popular, with 20% of covered workers. Deductibles in those plans range from around $2,000 for single coverage to $4,000 for family coverage, with some much higher.
Only 14% of workers are enrolled in health maintenance organizations (HMOs), 9% in a point-of-service (POS) plan, and less than 1% in a conventional fee-for-service plan. High-deductible plans have grown exponentially since first coming on the scene – going from 8% in 2009 to 17% in 2011 – but have plateaued in the last few years, according to the report.
Also of interest to physicians:
• 23% of employers offering benefits have high performance or tiered networks in their largest health plan. The programs help steer patients to providers who have been identified as being more efficient or giving higher-quality care.
• 56% cover services provided by retail health clinics. Among firms covering services in these settings, 17% provide a financial incentive to receive services in a retail clinic instead of a physician’s office.
• Almost all employers with more than 200 workers and most smaller employers offer at least one wellness program. The majority offer at least one of the following in 2013: weight loss programs, gym membership discounts or on-site exercise facilities, biometric screening, smoking cessation, personal health coaching, health and nutrition education, web-based resources, flu shots or vaccinations, employee assistance programs (EAPs), or a wellness newsletter. Thirty-six percent of large firms and 8% of smaller firms offer employees a financial incentive to participate.
The growth in wellness programs "will be an important issue to watch next year, as employers will have more flexibility and could ask workers to pay more because of their lifestyles and health conditions," said Kaiser Vice President Gary Claxton, the study’s lead investigator and director of the Foundation’s Health Care Marketplace Project.
Mr. Claxton and his colleagues found that an increasing number of employees are likely to be subject to the Affordable Care Act’s various provisions. Thirty-six percent of covered workers are in "grandfathered" plans – that is, they are exempt from the law – down from 48% in 2012 and 56% in 2011.
The authors reported no disclosures.
[email protected]
On Twitter @aliciaault
The rise in the cost of employer-sponsored health insurance has ameliorated in the past year, continuing a flattening trend, but patients continue to be asked to pay a bigger share.
That’s the conclusion of the 13th annual survey of nonfederal private and public employers with three or more workers conducted by the Kaiser Family Foundation and the Health Research & Educational Trust, and published Aug. 20 in the journal Health Affairs (doi: 10.1377/hlthaff.2013.0644).
Kaiser and HRET estimate that in 2013, 149 million nonelderly people receive coverage through employer-sponsored insurance, with 57% of U.S. firms offering health benefits – a statistically insignificant change from 61% in 2012 and 60% in 2011. But smaller companies and those with many low-wage workers do not offer benefits as frequently.
In 2013, the average annual premiums for employer-sponsored health insurance rose 5% to $5,884 for single coverage and 4% to $16,351 for family coverage from 2012. This continues the pattern seen over the past few years, with relatively small premium increases.
Workers on average pay 18% of the premium for single coverage and 29% for family coverage, again, similar to the two previous years. "We are in a prolonged period of moderation in premiums, which should create some breathing room for the private sector to try to reduce costs without cutting back benefits for workers," said Kaiser President and CEO Drew Altman, in a statement.
But workers are still struggling to cover health costs. During the same period (2012-2013), wages increased 1.8% and inflation increased 1.1%. And since 2003, the average premium for family coverage has increased 80%, and the average worker contribution has risen 89%.
Employers continue to cost-shift. Seventy-eight percent of covered workers have a deductible, with the average for single coverage running about $1,100 (largely unchanged from 2012). But big deductibles are getting to be more common. Firms with less than 200 employees generally charged more than $1,000, and the number doing that rose from 49% of all such firms in 2012 to 58% in 2013.
Three-quarters of workers have a fixed copayment for office visits with primary care physicians and specialists. That copay averages $23 for primary care and $35 for specialty care for in-network physicians. Out-of-network amounts were not covered in the survey, but Kaiser assumes that patients bear a greater cost for those visits. About 20% of workers pay a percent of the visit (coinsurance) for primary care and the same amount for specialty care. They pay an average 18% of the visit.
The survey found that preferred provider organization (PPO) plans are the most common, enrolling 57% of covered workers in 2013. High-deductible health plans, also known as catastrophic plans, are the next most popular, with 20% of covered workers. Deductibles in those plans range from around $2,000 for single coverage to $4,000 for family coverage, with some much higher.
Only 14% of workers are enrolled in health maintenance organizations (HMOs), 9% in a point-of-service (POS) plan, and less than 1% in a conventional fee-for-service plan. High-deductible plans have grown exponentially since first coming on the scene – going from 8% in 2009 to 17% in 2011 – but have plateaued in the last few years, according to the report.
Also of interest to physicians:
• 23% of employers offering benefits have high performance or tiered networks in their largest health plan. The programs help steer patients to providers who have been identified as being more efficient or giving higher-quality care.
• 56% cover services provided by retail health clinics. Among firms covering services in these settings, 17% provide a financial incentive to receive services in a retail clinic instead of a physician’s office.
• Almost all employers with more than 200 workers and most smaller employers offer at least one wellness program. The majority offer at least one of the following in 2013: weight loss programs, gym membership discounts or on-site exercise facilities, biometric screening, smoking cessation, personal health coaching, health and nutrition education, web-based resources, flu shots or vaccinations, employee assistance programs (EAPs), or a wellness newsletter. Thirty-six percent of large firms and 8% of smaller firms offer employees a financial incentive to participate.
The growth in wellness programs "will be an important issue to watch next year, as employers will have more flexibility and could ask workers to pay more because of their lifestyles and health conditions," said Kaiser Vice President Gary Claxton, the study’s lead investigator and director of the Foundation’s Health Care Marketplace Project.
Mr. Claxton and his colleagues found that an increasing number of employees are likely to be subject to the Affordable Care Act’s various provisions. Thirty-six percent of covered workers are in "grandfathered" plans – that is, they are exempt from the law – down from 48% in 2012 and 56% in 2011.
The authors reported no disclosures.
[email protected]
On Twitter @aliciaault
FROM HEALTH AFFAIRS
Major finding: Employer-sponsored health insurance premiums rose 4% for family coverage and 5% for single coverage in 2013; deductibles, coinsurance, and other out-of-pocket costs also continued to rise for workers.
Data source: Survey of more than 2,000 nonfederal private and public employers with three or more workers.
Disclosures: The authors reported no disclosures.
Colorectal cancer risk increased with bariatric surgery
The risk of colorectal cancer was significantly increased among people who had undergone obesity surgery in a retrospective cohort study of more than 77,000 obese patients enrolled in a Swedish registry.
The increased risk for colorectal cancer was associated with all three bariatric procedures – vertical banded gastroplasty, adjustable gastric banding, and Roux-en-Y gastric bypass – and increased further over time, reported Dr. Maryam Derogar, of the Karolinska Institutet, Stockholm, and her associates. No such pattern over time was seen among the obese patients who did not have surgery.
"Our data suggest that increased colorectal cancer risk may be a long-term consequence of such surgery," they concluded. If the association is confirmed, they added, "it should stimulate research addressing colonoscopic evaluation of the incidence of colorectal adenomatous polyps after obesity surgery with a view to defining an optimum colonoscopy surveillance strategy for the increasing number of patients who undergo obesity surgery. The study was published online in the Annals of Surgery (2013 [doi:10.1097/SLA.0b013e318288463a]).
To address their "unexpected" finding in an earlier study of an apparent increase in the risk of colorectal cancer after obesity surgery, but no increase in the risk of other cancers related to obesity, they conducted a retrospective cohort study using national registry data between 1980 and 2009, of 15,095 obese patients who had undergone obesity surgery and 62,016 patients who had been diagnosed with obesity but did not undergo surgery. They calculated the colorectal cancer risk using the standardized incidence ratio (SIR), the observed number of cases divided by the number of expected cases in that group.
Over a median of 10 years, there were 70 colorectal cancers in the obesity surgery group; and over a median of 7 years, 373 among those who had no surgery. The SIR for colorectal cancer among those who had surgery was 1.60, which was statistically significant. Among those who had no surgery, there was a small, insignificant increase in risk group (a SIR of 1.26). In the surgery group, the risk increased over time in men and women, up to a twofold increased risk among those patients followed for at least 10 years, a pattern than was not observed in the obese patients who had no surgery.
The "substantial increase in colorectal cancer risk, above that associated with excess body weight alone, more than 10 years after surgery is compatible with the long natural history of colorectal carcinogenesis from normal mucosa to a malignant colorectal cancer," the authors wrote. Why the risk was increased is not clear, but one possible explanation could be that the malabsorption effects of the gastric bypass procedure results in local mucosal changes, the authors speculated. Previously, they had identified rectal mucosal hyperproliferation in patients who had undergone obesity surgery, present at least 3 years after the procedure, a finding that was "associated with increased mucosal expression of the protumorigenic cytokine macrophage migration inhibitory factor," they wrote.
The study’s strengths included the size of the sample, long follow-up, and the validity of Swedish national registry data, while the limitations included the retrospective design and the lack of data on body weight over time.
As in the United States and other countries, obesity has been increasing in Sweden, with a corresponding increase in bariatric surgery. Over the last 20 years, the prevalence of obesity in Sweden has doubled, and the annual number of obesity operations performed has increased from 1,500 in 2006 to almost 4,000 in 2009, according to the authors.
The study was supported by the Swedish Research Council. The authors had no conflicts of interest to declare.
It is well established that overweight and obese individuals have a higher incidence of certain types of cancer; such as breast, colorectal, and endometrial to name a few. The exact mechanism is not known but it is generally linked with chronic inflammation associated with adipocyte release of inflammatory cytokines, an increase in sex steroid hormones, and an increase in insulin resistance. Therefore, it would seem logical to suggest that with significant and sustained weight loss (with or without bariatric surgery) the risk of cancer development may be reduced. Unfortunately, the data on weight loss and subsequent reduction in cancer risk are not solid. This is in part attributed to the fact that significant and sustained weight loss is extremely difficult to achieve without bariatric surgery.
There are several reports that show a protective effect of bariatric surgery on future cancer risk. Christou and colleagues (Surg. Obese Relat. Dis. 2008;4:691-5) reported that obese adults who undergo bariatric surgery may reduce their risk of developing some cancers by as much as 80%. Adams and colleagues (Obesity 2009;17:796-802) compared 6,596 patients who had gastric bypass with 9,442 severely obese individuals who had not, and found a significant decrease in the incidence of cancer and cancer-related deaths after bariatric surgery.
This report by Derogar and colleagues, published in Annals of Surgery, is the first to suggest that bariatric surgery is associated with an increased risk of colorectal cancer over time. It is difficult to interpret the results, however, as they directly contradict other reports and our general understanding of obesity and cancer. There are clearly limitations with the retrospective design of the study and the omission of any body weight or weight loss data. In addition, certain colorectal cancer risk factors, such as family history of cancer and prior adenomatous polyps, are not controlled for between the two groups.
Furthermore, there is a notion that individuals who undergo bariatric surgery tend to be more proactive about their health and take actions to prevent cancer. The authors suggest a possible mechanism that may be related to an increase in putative mucosal biomarkers of colorectal cancer risk and mucosal proinflammatory gene expression following Roux-en-Y gastric bypass. Also, a high-protein diet can promote detrimental metabolic profiles promoting carcinogenesis in the colon and rectum.
The exact mechanism remains elusive and as in most biologic systems, the answer in the end will be complex. Further research is needed to help answer some of these questions. This paper is important because it again highlights the increased risk of cancer associated with obesity and it is important for bariatric surgery programs to implement the proper screening protocols for cancer detection and prevention. Patients who undergo bariatric surgery should be screened both pre- and postoperatively as currently recommended depending on whether they are at average or high risk for certain cancers, such as breast or colon cancer, where screening has been shown to be effective.
Dr. Alex Nagle, FACS, is director of bariatric surgery, Northwestern Memorial Hospital, and associate professor of surgery, Northwestern University Feinberg School of Medicine, Chicago. He disclosed no conflicts of interest.
It is well established that overweight and obese individuals have a higher incidence of certain types of cancer; such as breast, colorectal, and endometrial to name a few. The exact mechanism is not known but it is generally linked with chronic inflammation associated with adipocyte release of inflammatory cytokines, an increase in sex steroid hormones, and an increase in insulin resistance. Therefore, it would seem logical to suggest that with significant and sustained weight loss (with or without bariatric surgery) the risk of cancer development may be reduced. Unfortunately, the data on weight loss and subsequent reduction in cancer risk are not solid. This is in part attributed to the fact that significant and sustained weight loss is extremely difficult to achieve without bariatric surgery.
There are several reports that show a protective effect of bariatric surgery on future cancer risk. Christou and colleagues (Surg. Obese Relat. Dis. 2008;4:691-5) reported that obese adults who undergo bariatric surgery may reduce their risk of developing some cancers by as much as 80%. Adams and colleagues (Obesity 2009;17:796-802) compared 6,596 patients who had gastric bypass with 9,442 severely obese individuals who had not, and found a significant decrease in the incidence of cancer and cancer-related deaths after bariatric surgery.
This report by Derogar and colleagues, published in Annals of Surgery, is the first to suggest that bariatric surgery is associated with an increased risk of colorectal cancer over time. It is difficult to interpret the results, however, as they directly contradict other reports and our general understanding of obesity and cancer. There are clearly limitations with the retrospective design of the study and the omission of any body weight or weight loss data. In addition, certain colorectal cancer risk factors, such as family history of cancer and prior adenomatous polyps, are not controlled for between the two groups.
Furthermore, there is a notion that individuals who undergo bariatric surgery tend to be more proactive about their health and take actions to prevent cancer. The authors suggest a possible mechanism that may be related to an increase in putative mucosal biomarkers of colorectal cancer risk and mucosal proinflammatory gene expression following Roux-en-Y gastric bypass. Also, a high-protein diet can promote detrimental metabolic profiles promoting carcinogenesis in the colon and rectum.
The exact mechanism remains elusive and as in most biologic systems, the answer in the end will be complex. Further research is needed to help answer some of these questions. This paper is important because it again highlights the increased risk of cancer associated with obesity and it is important for bariatric surgery programs to implement the proper screening protocols for cancer detection and prevention. Patients who undergo bariatric surgery should be screened both pre- and postoperatively as currently recommended depending on whether they are at average or high risk for certain cancers, such as breast or colon cancer, where screening has been shown to be effective.
Dr. Alex Nagle, FACS, is director of bariatric surgery, Northwestern Memorial Hospital, and associate professor of surgery, Northwestern University Feinberg School of Medicine, Chicago. He disclosed no conflicts of interest.
It is well established that overweight and obese individuals have a higher incidence of certain types of cancer; such as breast, colorectal, and endometrial to name a few. The exact mechanism is not known but it is generally linked with chronic inflammation associated with adipocyte release of inflammatory cytokines, an increase in sex steroid hormones, and an increase in insulin resistance. Therefore, it would seem logical to suggest that with significant and sustained weight loss (with or without bariatric surgery) the risk of cancer development may be reduced. Unfortunately, the data on weight loss and subsequent reduction in cancer risk are not solid. This is in part attributed to the fact that significant and sustained weight loss is extremely difficult to achieve without bariatric surgery.
There are several reports that show a protective effect of bariatric surgery on future cancer risk. Christou and colleagues (Surg. Obese Relat. Dis. 2008;4:691-5) reported that obese adults who undergo bariatric surgery may reduce their risk of developing some cancers by as much as 80%. Adams and colleagues (Obesity 2009;17:796-802) compared 6,596 patients who had gastric bypass with 9,442 severely obese individuals who had not, and found a significant decrease in the incidence of cancer and cancer-related deaths after bariatric surgery.
This report by Derogar and colleagues, published in Annals of Surgery, is the first to suggest that bariatric surgery is associated with an increased risk of colorectal cancer over time. It is difficult to interpret the results, however, as they directly contradict other reports and our general understanding of obesity and cancer. There are clearly limitations with the retrospective design of the study and the omission of any body weight or weight loss data. In addition, certain colorectal cancer risk factors, such as family history of cancer and prior adenomatous polyps, are not controlled for between the two groups.
Furthermore, there is a notion that individuals who undergo bariatric surgery tend to be more proactive about their health and take actions to prevent cancer. The authors suggest a possible mechanism that may be related to an increase in putative mucosal biomarkers of colorectal cancer risk and mucosal proinflammatory gene expression following Roux-en-Y gastric bypass. Also, a high-protein diet can promote detrimental metabolic profiles promoting carcinogenesis in the colon and rectum.
The exact mechanism remains elusive and as in most biologic systems, the answer in the end will be complex. Further research is needed to help answer some of these questions. This paper is important because it again highlights the increased risk of cancer associated with obesity and it is important for bariatric surgery programs to implement the proper screening protocols for cancer detection and prevention. Patients who undergo bariatric surgery should be screened both pre- and postoperatively as currently recommended depending on whether they are at average or high risk for certain cancers, such as breast or colon cancer, where screening has been shown to be effective.
Dr. Alex Nagle, FACS, is director of bariatric surgery, Northwestern Memorial Hospital, and associate professor of surgery, Northwestern University Feinberg School of Medicine, Chicago. He disclosed no conflicts of interest.
The risk of colorectal cancer was significantly increased among people who had undergone obesity surgery in a retrospective cohort study of more than 77,000 obese patients enrolled in a Swedish registry.
The increased risk for colorectal cancer was associated with all three bariatric procedures – vertical banded gastroplasty, adjustable gastric banding, and Roux-en-Y gastric bypass – and increased further over time, reported Dr. Maryam Derogar, of the Karolinska Institutet, Stockholm, and her associates. No such pattern over time was seen among the obese patients who did not have surgery.
"Our data suggest that increased colorectal cancer risk may be a long-term consequence of such surgery," they concluded. If the association is confirmed, they added, "it should stimulate research addressing colonoscopic evaluation of the incidence of colorectal adenomatous polyps after obesity surgery with a view to defining an optimum colonoscopy surveillance strategy for the increasing number of patients who undergo obesity surgery. The study was published online in the Annals of Surgery (2013 [doi:10.1097/SLA.0b013e318288463a]).
To address their "unexpected" finding in an earlier study of an apparent increase in the risk of colorectal cancer after obesity surgery, but no increase in the risk of other cancers related to obesity, they conducted a retrospective cohort study using national registry data between 1980 and 2009, of 15,095 obese patients who had undergone obesity surgery and 62,016 patients who had been diagnosed with obesity but did not undergo surgery. They calculated the colorectal cancer risk using the standardized incidence ratio (SIR), the observed number of cases divided by the number of expected cases in that group.
Over a median of 10 years, there were 70 colorectal cancers in the obesity surgery group; and over a median of 7 years, 373 among those who had no surgery. The SIR for colorectal cancer among those who had surgery was 1.60, which was statistically significant. Among those who had no surgery, there was a small, insignificant increase in risk group (a SIR of 1.26). In the surgery group, the risk increased over time in men and women, up to a twofold increased risk among those patients followed for at least 10 years, a pattern than was not observed in the obese patients who had no surgery.
The "substantial increase in colorectal cancer risk, above that associated with excess body weight alone, more than 10 years after surgery is compatible with the long natural history of colorectal carcinogenesis from normal mucosa to a malignant colorectal cancer," the authors wrote. Why the risk was increased is not clear, but one possible explanation could be that the malabsorption effects of the gastric bypass procedure results in local mucosal changes, the authors speculated. Previously, they had identified rectal mucosal hyperproliferation in patients who had undergone obesity surgery, present at least 3 years after the procedure, a finding that was "associated with increased mucosal expression of the protumorigenic cytokine macrophage migration inhibitory factor," they wrote.
The study’s strengths included the size of the sample, long follow-up, and the validity of Swedish national registry data, while the limitations included the retrospective design and the lack of data on body weight over time.
As in the United States and other countries, obesity has been increasing in Sweden, with a corresponding increase in bariatric surgery. Over the last 20 years, the prevalence of obesity in Sweden has doubled, and the annual number of obesity operations performed has increased from 1,500 in 2006 to almost 4,000 in 2009, according to the authors.
The study was supported by the Swedish Research Council. The authors had no conflicts of interest to declare.
The risk of colorectal cancer was significantly increased among people who had undergone obesity surgery in a retrospective cohort study of more than 77,000 obese patients enrolled in a Swedish registry.
The increased risk for colorectal cancer was associated with all three bariatric procedures – vertical banded gastroplasty, adjustable gastric banding, and Roux-en-Y gastric bypass – and increased further over time, reported Dr. Maryam Derogar, of the Karolinska Institutet, Stockholm, and her associates. No such pattern over time was seen among the obese patients who did not have surgery.
"Our data suggest that increased colorectal cancer risk may be a long-term consequence of such surgery," they concluded. If the association is confirmed, they added, "it should stimulate research addressing colonoscopic evaluation of the incidence of colorectal adenomatous polyps after obesity surgery with a view to defining an optimum colonoscopy surveillance strategy for the increasing number of patients who undergo obesity surgery. The study was published online in the Annals of Surgery (2013 [doi:10.1097/SLA.0b013e318288463a]).
To address their "unexpected" finding in an earlier study of an apparent increase in the risk of colorectal cancer after obesity surgery, but no increase in the risk of other cancers related to obesity, they conducted a retrospective cohort study using national registry data between 1980 and 2009, of 15,095 obese patients who had undergone obesity surgery and 62,016 patients who had been diagnosed with obesity but did not undergo surgery. They calculated the colorectal cancer risk using the standardized incidence ratio (SIR), the observed number of cases divided by the number of expected cases in that group.
Over a median of 10 years, there were 70 colorectal cancers in the obesity surgery group; and over a median of 7 years, 373 among those who had no surgery. The SIR for colorectal cancer among those who had surgery was 1.60, which was statistically significant. Among those who had no surgery, there was a small, insignificant increase in risk group (a SIR of 1.26). In the surgery group, the risk increased over time in men and women, up to a twofold increased risk among those patients followed for at least 10 years, a pattern than was not observed in the obese patients who had no surgery.
The "substantial increase in colorectal cancer risk, above that associated with excess body weight alone, more than 10 years after surgery is compatible with the long natural history of colorectal carcinogenesis from normal mucosa to a malignant colorectal cancer," the authors wrote. Why the risk was increased is not clear, but one possible explanation could be that the malabsorption effects of the gastric bypass procedure results in local mucosal changes, the authors speculated. Previously, they had identified rectal mucosal hyperproliferation in patients who had undergone obesity surgery, present at least 3 years after the procedure, a finding that was "associated with increased mucosal expression of the protumorigenic cytokine macrophage migration inhibitory factor," they wrote.
The study’s strengths included the size of the sample, long follow-up, and the validity of Swedish national registry data, while the limitations included the retrospective design and the lack of data on body weight over time.
As in the United States and other countries, obesity has been increasing in Sweden, with a corresponding increase in bariatric surgery. Over the last 20 years, the prevalence of obesity in Sweden has doubled, and the annual number of obesity operations performed has increased from 1,500 in 2006 to almost 4,000 in 2009, according to the authors.
The study was supported by the Swedish Research Council. The authors had no conflicts of interest to declare.
FROM THE ANNALS OF SURGERY
Major finding: The risk of colorectal cancer increased by 60% among obese patients, a mean of 7 years after bariatric surgery but was only slightly increased among obese patients who did not undergo surgery, over the expected rate.
Data source: A retrospective cohort study using national patient registry data in Sweden compared the risk of colorectal cancer in about 15,000 obese patients who had undergone bariatric surgery and among about 62,000 obese patients who did not have surgery, compared to matched controls.
Disclosures: The study was supported by the Swedish Research Council. The authors had no conflicts of interest to declare.
Medicolegal Lessons: A question of duty
Story
ML was an 83-year-old woman who presented from her assisted-living facility to her local emergency room with abdominal pain.
She described acute-onset epigastric pain within minutes of her evening meal. The pain was rated 5 out of 10 and was associated with some nausea but no emesis. ML had a past medical history of irritable bowel symptoms along with diverticulosis, but she was otherwise healthy and took no regular medications except for occasional loperamide. Her CBC, amylase, lipase, and chemistry panels were normal. CT scan of the abdomen showed mildly dilated loops of small bowel.
ML was admitted to the hospital by her family physician, who consulted a gastroenterologist the following morning. The gastroenterologist concluded that ML was most likely suffering from food intolerance and recommended bowel rest and observation.
ML continued to have the pain and was unable to advance her diet. On hospital day 2, the GI consultant noted that ML’s abdomen was soft and nondistended, but he ordered an acute abdominal series and requested to be called with the results. The study was not completed until 5:30 p.m. Later that evening during a routine chart check, ML’s nurse noted that the acute abdominal series had been completed, but not read. She paged the hospitalist on call to review the film so that she could contact the GI consultant pursuant to his order.
Dr. Hospitalist reviewed the film and called the nurse back to report that the film showed no free air but the colon was dilated. The nurse subsequently called the GI consultant and relayed the information. No new orders were received.
At 7 a.m. on hospital day 3, ML developed mental status changes. Her abdomen was now noted to be distended with rigidity. ML was evaluated by her family physician and the GI consultant.
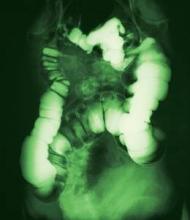
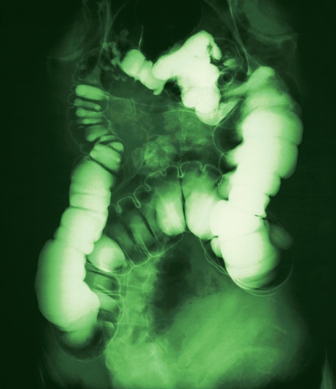
A surgical consult was obtained along with further imaging, which confirmed a small bowel obstruction (SBO) with massively dilated small bowel. Morning labs also showed acute kidney injury. Formal radiology review of the abdominal series looked at by Dr. Hospitalist established the presence of significant small bowel dilatation highly concerning for SBO. ML was transferred to a larger hospital where she eventually underwent an exploratory laparotomy for perforated bowel. Following a tumultuous postoperative course including dialysis, ML expired 1 month later.
Complaint
ML’s daughter was a pediatrician at a major teaching institution nearby. She was frustrated that the original CT showed dilated small bowel and that the conclusion of her treating doctors was that her mother was suffering from "food intolerance." Together with her father, they filed suit against the hospital, the GI consultant, and Dr. Hospitalist.
ML’s family alleged that ML had small bowel obstruction from the start and should have had surgical involvement soon enough to intervene before she ultimately perforated her bowel. Surgical repair prior to perforation would have significantly changed ML’s outcome.
They further alleged that Dr. Hospitalist was negligent in her review of the abdominal radiographs and she had a duty to see and examine ML, communicate directly with the GI consultant, and obtain a STAT surgical consult.
Scientific principles
Small bowel obstruction occurs when the normal flow of intestinal contents is interrupted, and it is usually confirmed by plain abdominal radiography.
The most frequent causes are postoperative adhesions and hernias, which cause extrinsic compression of the intestine. Obstruction leads to dilation of the stomach and small intestine proximal to the blockage, while distal to the blockage the bowel will decompress as luminal contents are passed. Symptoms include obstipation, nausea, vomiting, and abdominal pain. As the small bowel dilates, its blood flow can be compromised, leading to strangulation and sepsis.
Unfortunately, there is no reliable sign or symptom differentiating patients with strangulation or impending strangulation from those in whom surgery will not be necessary.
Complaint rebuttal and discussion
For the night in question, Dr. Hospitalist asserted that she had two roles and only one of them involved ML.
First, Dr. Hospitalist was responsible for admissions and cross-coverage for her own group’s patients at night. ML was not a patient of Dr. Hospitalist or her group. ML was being cared for by her own family physician and his practice group 24/7.
Second, Dr. Hospitalist was the hospital’s overnight "house doctor" for codes, IV access, radiology "wet reads," and other emergencies. It was in this capacity that Dr. Hospitalist was contacted. Dr. Hospitalist was not a radiologist. As a house doctor, Dr. Hospitalist would be expected to look for serious and life-threatening findings and to rule out the presence of free air. Dr. Hospitalist asserted that she was never asked to see ML by the attending physician or even by the nurse.
The family argued that Dr. Hospitalist had a duty to question the nurse regarding ML’s condition and, because of the evidence for obstruction on the film, see ML for an assessment. The family argued that Dr. Hospitalist’s misinterpretation of the film (i.e., colon dilatation in the face of obvious small bowel dilatation) represented gross incompetence.
Dr. Hospitalist testified that she had no memory of ML, this case, or what she may or may not have told the nurse that night. ML’s medical record confirms that Dr. Hospitalist wrote no orders or charted any notes on her. The only documentation of Dr. Hospitalist’s "wet read" was in ML’s nursing notes.
The GI consultant testified that he had no memory of his call from the nurse with the radiograph results. The nurse testified that she wouldn’t have written "colon dilatation" if Dr. Hospitalist had told her it was small bowel.
Conclusion
The "house doc" role typically encompasses a limited scope of responsibility. But all physicians carry a professional duty to the patients that we become involved with.
By performing a "wet read" on a film of ML, Dr. Hospitalist established a doctor-patient relationship. It would have been prudent for Dr. Hospitalist to record her film interpretation herself (thus creating an opportunity for brief chart review), and to call the GI consultant with the information instead of relying on the nurse. A 1-minute conversation between Dr. Hospitalist and the GI consultant may have led to further intervention by either party.
Ultimately this case was resolved for an undisclosed amount. In the "house doc" role, Dr. Hospitalist was functioning as an employee of the hospital (despite being an independent contractor), and there were hospital care issues independent of Dr. Hospitalist. However, Dr. Hospitalist could have avoided her role in this suit with better documentation and communication.
Dr. Michota is director of academic affairs in the hospital medicine department at the Cleveland Clinic and medical editor of Hospitalist News. Read earlier columns online at ehospitalistnews.com/Lessons.
Medicolegal Review has the opportunity to become the morbidity and mortality conference of the modern era. Each month, this column presents a case vignette that explores some aspect of medicine and the applicable standard of care.
The more we share in our collective failures, the less likely we are to repeat those same mistakes.
Medicolegal Review has the opportunity to become the morbidity and mortality conference of the modern era. Each month, this column presents a case vignette that explores some aspect of medicine and the applicable standard of care.
The more we share in our collective failures, the less likely we are to repeat those same mistakes.
Medicolegal Review has the opportunity to become the morbidity and mortality conference of the modern era. Each month, this column presents a case vignette that explores some aspect of medicine and the applicable standard of care.
The more we share in our collective failures, the less likely we are to repeat those same mistakes.
Story
ML was an 83-year-old woman who presented from her assisted-living facility to her local emergency room with abdominal pain.
She described acute-onset epigastric pain within minutes of her evening meal. The pain was rated 5 out of 10 and was associated with some nausea but no emesis. ML had a past medical history of irritable bowel symptoms along with diverticulosis, but she was otherwise healthy and took no regular medications except for occasional loperamide. Her CBC, amylase, lipase, and chemistry panels were normal. CT scan of the abdomen showed mildly dilated loops of small bowel.
ML was admitted to the hospital by her family physician, who consulted a gastroenterologist the following morning. The gastroenterologist concluded that ML was most likely suffering from food intolerance and recommended bowel rest and observation.
ML continued to have the pain and was unable to advance her diet. On hospital day 2, the GI consultant noted that ML’s abdomen was soft and nondistended, but he ordered an acute abdominal series and requested to be called with the results. The study was not completed until 5:30 p.m. Later that evening during a routine chart check, ML’s nurse noted that the acute abdominal series had been completed, but not read. She paged the hospitalist on call to review the film so that she could contact the GI consultant pursuant to his order.
Dr. Hospitalist reviewed the film and called the nurse back to report that the film showed no free air but the colon was dilated. The nurse subsequently called the GI consultant and relayed the information. No new orders were received.
At 7 a.m. on hospital day 3, ML developed mental status changes. Her abdomen was now noted to be distended with rigidity. ML was evaluated by her family physician and the GI consultant.
A surgical consult was obtained along with further imaging, which confirmed a small bowel obstruction (SBO) with massively dilated small bowel. Morning labs also showed acute kidney injury. Formal radiology review of the abdominal series looked at by Dr. Hospitalist established the presence of significant small bowel dilatation highly concerning for SBO. ML was transferred to a larger hospital where she eventually underwent an exploratory laparotomy for perforated bowel. Following a tumultuous postoperative course including dialysis, ML expired 1 month later.
Complaint
ML’s daughter was a pediatrician at a major teaching institution nearby. She was frustrated that the original CT showed dilated small bowel and that the conclusion of her treating doctors was that her mother was suffering from "food intolerance." Together with her father, they filed suit against the hospital, the GI consultant, and Dr. Hospitalist.
ML’s family alleged that ML had small bowel obstruction from the start and should have had surgical involvement soon enough to intervene before she ultimately perforated her bowel. Surgical repair prior to perforation would have significantly changed ML’s outcome.
They further alleged that Dr. Hospitalist was negligent in her review of the abdominal radiographs and she had a duty to see and examine ML, communicate directly with the GI consultant, and obtain a STAT surgical consult.
Scientific principles
Small bowel obstruction occurs when the normal flow of intestinal contents is interrupted, and it is usually confirmed by plain abdominal radiography.
The most frequent causes are postoperative adhesions and hernias, which cause extrinsic compression of the intestine. Obstruction leads to dilation of the stomach and small intestine proximal to the blockage, while distal to the blockage the bowel will decompress as luminal contents are passed. Symptoms include obstipation, nausea, vomiting, and abdominal pain. As the small bowel dilates, its blood flow can be compromised, leading to strangulation and sepsis.
Unfortunately, there is no reliable sign or symptom differentiating patients with strangulation or impending strangulation from those in whom surgery will not be necessary.
Complaint rebuttal and discussion
For the night in question, Dr. Hospitalist asserted that she had two roles and only one of them involved ML.
First, Dr. Hospitalist was responsible for admissions and cross-coverage for her own group’s patients at night. ML was not a patient of Dr. Hospitalist or her group. ML was being cared for by her own family physician and his practice group 24/7.
Second, Dr. Hospitalist was the hospital’s overnight "house doctor" for codes, IV access, radiology "wet reads," and other emergencies. It was in this capacity that Dr. Hospitalist was contacted. Dr. Hospitalist was not a radiologist. As a house doctor, Dr. Hospitalist would be expected to look for serious and life-threatening findings and to rule out the presence of free air. Dr. Hospitalist asserted that she was never asked to see ML by the attending physician or even by the nurse.
The family argued that Dr. Hospitalist had a duty to question the nurse regarding ML’s condition and, because of the evidence for obstruction on the film, see ML for an assessment. The family argued that Dr. Hospitalist’s misinterpretation of the film (i.e., colon dilatation in the face of obvious small bowel dilatation) represented gross incompetence.
Dr. Hospitalist testified that she had no memory of ML, this case, or what she may or may not have told the nurse that night. ML’s medical record confirms that Dr. Hospitalist wrote no orders or charted any notes on her. The only documentation of Dr. Hospitalist’s "wet read" was in ML’s nursing notes.
The GI consultant testified that he had no memory of his call from the nurse with the radiograph results. The nurse testified that she wouldn’t have written "colon dilatation" if Dr. Hospitalist had told her it was small bowel.
Conclusion
The "house doc" role typically encompasses a limited scope of responsibility. But all physicians carry a professional duty to the patients that we become involved with.
By performing a "wet read" on a film of ML, Dr. Hospitalist established a doctor-patient relationship. It would have been prudent for Dr. Hospitalist to record her film interpretation herself (thus creating an opportunity for brief chart review), and to call the GI consultant with the information instead of relying on the nurse. A 1-minute conversation between Dr. Hospitalist and the GI consultant may have led to further intervention by either party.
Ultimately this case was resolved for an undisclosed amount. In the "house doc" role, Dr. Hospitalist was functioning as an employee of the hospital (despite being an independent contractor), and there were hospital care issues independent of Dr. Hospitalist. However, Dr. Hospitalist could have avoided her role in this suit with better documentation and communication.
Dr. Michota is director of academic affairs in the hospital medicine department at the Cleveland Clinic and medical editor of Hospitalist News. Read earlier columns online at ehospitalistnews.com/Lessons.
Story
ML was an 83-year-old woman who presented from her assisted-living facility to her local emergency room with abdominal pain.
She described acute-onset epigastric pain within minutes of her evening meal. The pain was rated 5 out of 10 and was associated with some nausea but no emesis. ML had a past medical history of irritable bowel symptoms along with diverticulosis, but she was otherwise healthy and took no regular medications except for occasional loperamide. Her CBC, amylase, lipase, and chemistry panels were normal. CT scan of the abdomen showed mildly dilated loops of small bowel.
ML was admitted to the hospital by her family physician, who consulted a gastroenterologist the following morning. The gastroenterologist concluded that ML was most likely suffering from food intolerance and recommended bowel rest and observation.
ML continued to have the pain and was unable to advance her diet. On hospital day 2, the GI consultant noted that ML’s abdomen was soft and nondistended, but he ordered an acute abdominal series and requested to be called with the results. The study was not completed until 5:30 p.m. Later that evening during a routine chart check, ML’s nurse noted that the acute abdominal series had been completed, but not read. She paged the hospitalist on call to review the film so that she could contact the GI consultant pursuant to his order.
Dr. Hospitalist reviewed the film and called the nurse back to report that the film showed no free air but the colon was dilated. The nurse subsequently called the GI consultant and relayed the information. No new orders were received.
At 7 a.m. on hospital day 3, ML developed mental status changes. Her abdomen was now noted to be distended with rigidity. ML was evaluated by her family physician and the GI consultant.
A surgical consult was obtained along with further imaging, which confirmed a small bowel obstruction (SBO) with massively dilated small bowel. Morning labs also showed acute kidney injury. Formal radiology review of the abdominal series looked at by Dr. Hospitalist established the presence of significant small bowel dilatation highly concerning for SBO. ML was transferred to a larger hospital where she eventually underwent an exploratory laparotomy for perforated bowel. Following a tumultuous postoperative course including dialysis, ML expired 1 month later.
Complaint
ML’s daughter was a pediatrician at a major teaching institution nearby. She was frustrated that the original CT showed dilated small bowel and that the conclusion of her treating doctors was that her mother was suffering from "food intolerance." Together with her father, they filed suit against the hospital, the GI consultant, and Dr. Hospitalist.
ML’s family alleged that ML had small bowel obstruction from the start and should have had surgical involvement soon enough to intervene before she ultimately perforated her bowel. Surgical repair prior to perforation would have significantly changed ML’s outcome.
They further alleged that Dr. Hospitalist was negligent in her review of the abdominal radiographs and she had a duty to see and examine ML, communicate directly with the GI consultant, and obtain a STAT surgical consult.
Scientific principles
Small bowel obstruction occurs when the normal flow of intestinal contents is interrupted, and it is usually confirmed by plain abdominal radiography.
The most frequent causes are postoperative adhesions and hernias, which cause extrinsic compression of the intestine. Obstruction leads to dilation of the stomach and small intestine proximal to the blockage, while distal to the blockage the bowel will decompress as luminal contents are passed. Symptoms include obstipation, nausea, vomiting, and abdominal pain. As the small bowel dilates, its blood flow can be compromised, leading to strangulation and sepsis.
Unfortunately, there is no reliable sign or symptom differentiating patients with strangulation or impending strangulation from those in whom surgery will not be necessary.
Complaint rebuttal and discussion
For the night in question, Dr. Hospitalist asserted that she had two roles and only one of them involved ML.
First, Dr. Hospitalist was responsible for admissions and cross-coverage for her own group’s patients at night. ML was not a patient of Dr. Hospitalist or her group. ML was being cared for by her own family physician and his practice group 24/7.
Second, Dr. Hospitalist was the hospital’s overnight "house doctor" for codes, IV access, radiology "wet reads," and other emergencies. It was in this capacity that Dr. Hospitalist was contacted. Dr. Hospitalist was not a radiologist. As a house doctor, Dr. Hospitalist would be expected to look for serious and life-threatening findings and to rule out the presence of free air. Dr. Hospitalist asserted that she was never asked to see ML by the attending physician or even by the nurse.
The family argued that Dr. Hospitalist had a duty to question the nurse regarding ML’s condition and, because of the evidence for obstruction on the film, see ML for an assessment. The family argued that Dr. Hospitalist’s misinterpretation of the film (i.e., colon dilatation in the face of obvious small bowel dilatation) represented gross incompetence.
Dr. Hospitalist testified that she had no memory of ML, this case, or what she may or may not have told the nurse that night. ML’s medical record confirms that Dr. Hospitalist wrote no orders or charted any notes on her. The only documentation of Dr. Hospitalist’s "wet read" was in ML’s nursing notes.
The GI consultant testified that he had no memory of his call from the nurse with the radiograph results. The nurse testified that she wouldn’t have written "colon dilatation" if Dr. Hospitalist had told her it was small bowel.
Conclusion
The "house doc" role typically encompasses a limited scope of responsibility. But all physicians carry a professional duty to the patients that we become involved with.
By performing a "wet read" on a film of ML, Dr. Hospitalist established a doctor-patient relationship. It would have been prudent for Dr. Hospitalist to record her film interpretation herself (thus creating an opportunity for brief chart review), and to call the GI consultant with the information instead of relying on the nurse. A 1-minute conversation between Dr. Hospitalist and the GI consultant may have led to further intervention by either party.
Ultimately this case was resolved for an undisclosed amount. In the "house doc" role, Dr. Hospitalist was functioning as an employee of the hospital (despite being an independent contractor), and there were hospital care issues independent of Dr. Hospitalist. However, Dr. Hospitalist could have avoided her role in this suit with better documentation and communication.
Dr. Michota is director of academic affairs in the hospital medicine department at the Cleveland Clinic and medical editor of Hospitalist News. Read earlier columns online at ehospitalistnews.com/Lessons.
Apps for your smart phone
The number of health apps continues to grow at a rapid pace, and if you’re in search of more apps to download and experiment with, Dr. Craig Burkhart has a list for you.
To give a sense of how fast health apps are arriving in the market, Dr. Burkhart of the University of North Carolina at Chapel Hill, broke down the number of health applications for Apple devices at the times of American Academy of Dermatology’s meetings: At the 2012 AAD annual meeting, there were 5,000 iOS health apps. That number went up to 13,000 during the 2012 Summer AAD, and 40,000 at the 2013 AAD annual meeting.
He listed some of his favorites during the 2013 AAD summer academy meeting:
1password – to remember passwords
Byword – a simple writing app
Drafts – to automate text actions, also good for transcriptions
Dropbox – to store and share documents, large or small
Epocrates – for drug reference
Evernote – for note-taking
Flipboard – popular news reader
Google Drive – for documents and spreadsheets
Launch Center Pro – to get quick shortcuts for specific features buried in apps
Mind Node – for mind mapping
Omnifocus – for task management, based on GDT system
PDF Pen and Good Reader – PDF readers with annotating capabilities
PubMed Mobile – to search PubMed for journal articles
Read by QXMD – to keep up with medical and scientific research
Scanner Pro – to capture documents and receipts as PDF
Text Expander Touch – for those who write
Tweetbot – if you use twitter for news
What health apps would you recommend to your colleagues? Write to [email protected] and let us know, or post your favorites on the Skin & Allergy News Facebook page.
Dr. Burkhart had no disclosures relevant to mobile apps.
[email protected] On Twitter @NaseemSMiller
The number of health apps continues to grow at a rapid pace, and if you’re in search of more apps to download and experiment with, Dr. Craig Burkhart has a list for you.
To give a sense of how fast health apps are arriving in the market, Dr. Burkhart of the University of North Carolina at Chapel Hill, broke down the number of health applications for Apple devices at the times of American Academy of Dermatology’s meetings: At the 2012 AAD annual meeting, there were 5,000 iOS health apps. That number went up to 13,000 during the 2012 Summer AAD, and 40,000 at the 2013 AAD annual meeting.
He listed some of his favorites during the 2013 AAD summer academy meeting:
1password – to remember passwords
Byword – a simple writing app
Drafts – to automate text actions, also good for transcriptions
Dropbox – to store and share documents, large or small
Epocrates – for drug reference
Evernote – for note-taking
Flipboard – popular news reader
Google Drive – for documents and spreadsheets
Launch Center Pro – to get quick shortcuts for specific features buried in apps
Mind Node – for mind mapping
Omnifocus – for task management, based on GDT system
PDF Pen and Good Reader – PDF readers with annotating capabilities
PubMed Mobile – to search PubMed for journal articles
Read by QXMD – to keep up with medical and scientific research
Scanner Pro – to capture documents and receipts as PDF
Text Expander Touch – for those who write
Tweetbot – if you use twitter for news
What health apps would you recommend to your colleagues? Write to [email protected] and let us know, or post your favorites on the Skin & Allergy News Facebook page.
Dr. Burkhart had no disclosures relevant to mobile apps.
[email protected] On Twitter @NaseemSMiller
The number of health apps continues to grow at a rapid pace, and if you’re in search of more apps to download and experiment with, Dr. Craig Burkhart has a list for you.
To give a sense of how fast health apps are arriving in the market, Dr. Burkhart of the University of North Carolina at Chapel Hill, broke down the number of health applications for Apple devices at the times of American Academy of Dermatology’s meetings: At the 2012 AAD annual meeting, there were 5,000 iOS health apps. That number went up to 13,000 during the 2012 Summer AAD, and 40,000 at the 2013 AAD annual meeting.
He listed some of his favorites during the 2013 AAD summer academy meeting:
1password – to remember passwords
Byword – a simple writing app
Drafts – to automate text actions, also good for transcriptions
Dropbox – to store and share documents, large or small
Epocrates – for drug reference
Evernote – for note-taking
Flipboard – popular news reader
Google Drive – for documents and spreadsheets
Launch Center Pro – to get quick shortcuts for specific features buried in apps
Mind Node – for mind mapping
Omnifocus – for task management, based on GDT system
PDF Pen and Good Reader – PDF readers with annotating capabilities
PubMed Mobile – to search PubMed for journal articles
Read by QXMD – to keep up with medical and scientific research
Scanner Pro – to capture documents and receipts as PDF
Text Expander Touch – for those who write
Tweetbot – if you use twitter for news
What health apps would you recommend to your colleagues? Write to [email protected] and let us know, or post your favorites on the Skin & Allergy News Facebook page.
Dr. Burkhart had no disclosures relevant to mobile apps.
[email protected] On Twitter @NaseemSMiller
FDA requires stronger peripheral neuropathy warning for quinolones
The Food and Drug Administration is requiring a stronger warning about the potential for peripheral neuropathy with fluoroquinolone antibiotics that are taken orally or by injection.
The warning does not apply to topical formulations, which have not been associated with neuropathy. Drug labels and patient medication guides must be updated, said the agency.
The potential for neuropathy was first added to the labels of all drugs in the class in 2004. The new warning was necessary because "the potential rapid onset and risk of permanence were not adequately described" in the 2004 label iteration, the FDA noted.
Before requiring new warnings, the FDA reviewed its Adverse Event Reporting System (FAERS, formerly known as AERS) database. The agency found that cases of fluoroquinolone-associated peripheral neuropathy with an outcome of "disability" had been reported to the AERS database between January 2003 and August 2012, although the FDA did not say how many cases it found. The reports indicated a rapid onset of peripheral neuropathy, often within a few days of starting the quinolone. Some cases reported neuropathy that continued for a year, even though the medication had been stopped.
The database was not able to show whether neuropathy was permanent, however, because it is designed to collect spontaneous reports.
The FDA said it had not been able to identify any risk factors for the development of peripheral neuropathy. But the onset of the condition seemed to have no correlation with the patient’s age or how long they took the antibiotic.
The updated warnings apply to all approved fluoroquinolones: levofloxacin (Levaquin), ciprofloxacin (Cipro), moxifloxacin (Avelox), norfloxacin (Noroxin), ofloxacin (Floxin), and gemifloxacin (Factive).
According to the FDA, 23 million outpatients were prescribed an oral quinolone in 2011. A total of 70% received ciprofloxacin; 28% were prescribed levofloxacin; and 9% were given moxifloxacin. Gemifloxacin, ofloxacin, and norfloxacin each accounted for less than 1% of those patients in 2011.
There were 3.8 million inpatients who received an injectable quinolone in 2011. The most-prescribed was levofloxacin, accounting for 63% of prescriptions, followed by ciprofloxacin (28%) and moxifloxacin (13%).
The agency recommended that patients who develop neuropathy symptoms stop taking the quinolone and be treated with a different antibiotic, unless the benefit outweighs the risk. Patients taking the medications who develop symptoms are urged to tell their physician immediately.
On Twitter @aliciaault
The Food and Drug Administration is requiring a stronger warning about the potential for peripheral neuropathy with fluoroquinolone antibiotics that are taken orally or by injection.
The warning does not apply to topical formulations, which have not been associated with neuropathy. Drug labels and patient medication guides must be updated, said the agency.
The potential for neuropathy was first added to the labels of all drugs in the class in 2004. The new warning was necessary because "the potential rapid onset and risk of permanence were not adequately described" in the 2004 label iteration, the FDA noted.
Before requiring new warnings, the FDA reviewed its Adverse Event Reporting System (FAERS, formerly known as AERS) database. The agency found that cases of fluoroquinolone-associated peripheral neuropathy with an outcome of "disability" had been reported to the AERS database between January 2003 and August 2012, although the FDA did not say how many cases it found. The reports indicated a rapid onset of peripheral neuropathy, often within a few days of starting the quinolone. Some cases reported neuropathy that continued for a year, even though the medication had been stopped.
The database was not able to show whether neuropathy was permanent, however, because it is designed to collect spontaneous reports.
The FDA said it had not been able to identify any risk factors for the development of peripheral neuropathy. But the onset of the condition seemed to have no correlation with the patient’s age or how long they took the antibiotic.
The updated warnings apply to all approved fluoroquinolones: levofloxacin (Levaquin), ciprofloxacin (Cipro), moxifloxacin (Avelox), norfloxacin (Noroxin), ofloxacin (Floxin), and gemifloxacin (Factive).
According to the FDA, 23 million outpatients were prescribed an oral quinolone in 2011. A total of 70% received ciprofloxacin; 28% were prescribed levofloxacin; and 9% were given moxifloxacin. Gemifloxacin, ofloxacin, and norfloxacin each accounted for less than 1% of those patients in 2011.
There were 3.8 million inpatients who received an injectable quinolone in 2011. The most-prescribed was levofloxacin, accounting for 63% of prescriptions, followed by ciprofloxacin (28%) and moxifloxacin (13%).
The agency recommended that patients who develop neuropathy symptoms stop taking the quinolone and be treated with a different antibiotic, unless the benefit outweighs the risk. Patients taking the medications who develop symptoms are urged to tell their physician immediately.
On Twitter @aliciaault
The Food and Drug Administration is requiring a stronger warning about the potential for peripheral neuropathy with fluoroquinolone antibiotics that are taken orally or by injection.
The warning does not apply to topical formulations, which have not been associated with neuropathy. Drug labels and patient medication guides must be updated, said the agency.
The potential for neuropathy was first added to the labels of all drugs in the class in 2004. The new warning was necessary because "the potential rapid onset and risk of permanence were not adequately described" in the 2004 label iteration, the FDA noted.
Before requiring new warnings, the FDA reviewed its Adverse Event Reporting System (FAERS, formerly known as AERS) database. The agency found that cases of fluoroquinolone-associated peripheral neuropathy with an outcome of "disability" had been reported to the AERS database between January 2003 and August 2012, although the FDA did not say how many cases it found. The reports indicated a rapid onset of peripheral neuropathy, often within a few days of starting the quinolone. Some cases reported neuropathy that continued for a year, even though the medication had been stopped.
The database was not able to show whether neuropathy was permanent, however, because it is designed to collect spontaneous reports.
The FDA said it had not been able to identify any risk factors for the development of peripheral neuropathy. But the onset of the condition seemed to have no correlation with the patient’s age or how long they took the antibiotic.
The updated warnings apply to all approved fluoroquinolones: levofloxacin (Levaquin), ciprofloxacin (Cipro), moxifloxacin (Avelox), norfloxacin (Noroxin), ofloxacin (Floxin), and gemifloxacin (Factive).
According to the FDA, 23 million outpatients were prescribed an oral quinolone in 2011. A total of 70% received ciprofloxacin; 28% were prescribed levofloxacin; and 9% were given moxifloxacin. Gemifloxacin, ofloxacin, and norfloxacin each accounted for less than 1% of those patients in 2011.
There were 3.8 million inpatients who received an injectable quinolone in 2011. The most-prescribed was levofloxacin, accounting for 63% of prescriptions, followed by ciprofloxacin (28%) and moxifloxacin (13%).
The agency recommended that patients who develop neuropathy symptoms stop taking the quinolone and be treated with a different antibiotic, unless the benefit outweighs the risk. Patients taking the medications who develop symptoms are urged to tell their physician immediately.
On Twitter @aliciaault
Managing blunt abdominal trauma in children tricky business
SAN DIEGO – In the clinical experience of Dr. Julia Grabowski, managing blunt abdominal trauma injuries in children can be tricky business because of the wide variation in development between infants and adolescents.
Such differences "affect both the care of the injured child and injury prevention efforts," she said at the University of California San Diego Critical Care Summer Session. Anatomic considerations in the management of pediatric abdominal trauma include the close proximity of multiple organs, "which can affect their overall injury patterns," said Dr. Grabowski, a pediatric surgeon at Rady Children’s Hospital in San Diego. "In addition, their solid organs are larger compared with the rest of their abdomen. They generally have less body fat, less connective tissue, and less muscle mass, and their bony skeleton is incompletely ossified."
Compared with adults, the rib cage in children "is higher and much more pliable, so rib fractures are quite uncommon in the pediatric population," she said. "If you do see a child who has a rib fracture, that’s a trigger to think they had a much worse trauma than you originally expected."
Blunt injuries account for about 90% of all injuries and deaths in children, Dr. Grabowski said. In blunt abdominal trauma, the most common mechanism of action is a fall, followed by motor vehicle collisions, pedestrian versus auto accidents, bicycle accidents, and assaults. The most commonly injured organs are the spleen and liver, followed distantly by the kidney, small bowel, and pancreas.
Diagnostic evaluation of blunt abdominal trauma includes C-spine imaging for those in whom you suspect C-spine trauma, chest x-rays, anterior-posterior x-ray of the pelvis as necessary, and a computed tomography scan, "which is really the workhorse of evaluation for blunt abdominal trauma," she said. Lab studies may include CBC, liver function tests, amylase, lipase, and blood type and cross.
Another option is Focused Assessment With Sonography for Trauma (the FAST scan). According to Dr. Grabowski, recent research has demonstrated that FAST has a low sensitivity and is inappropriate for use in hemodynamically stable children, but that it may be useful in unstable patients.
For splenic and hepatic injuries, grade and clinical exam dictates the need for PICU admission, frequency of vital signs, hematocrit and hemoglobin testing, diet, and activity. The American Pediatric Surgical Association published guidelines for the management of hemodynamically stable children with isolated spleen or liver injury (J. Pediatr. Surg. 2000; 35:164-9).
"Most children are in the hospital 1 day longer than their grade of injury, and they’re out of any activity for 2 weeks longer than their grade of injury," said Dr. Grabowski. Splenic injuries from to sports competition "are quite common, especially around football and hockey seasons," as are those caused by motor vehicle accidents and accidents from all-terrain vehicles. Common complaints include abdominal pain/tenderness, shoulder pain, nausea and vomiting, and anemia. CT scan is 98% sensitive in identifying the injury.
"Over time we have found that splenic salvage can be achieved in greater than 90% of children with blunt splenic injury, even up to a grade IV or V injury," she said. "Management of splenic injury should be based on physiologic parameters, rather than on a grading of the spleen injury or the presence of ‘blush’ on a CT scan. When an operation is required it’s usually for hemodynamic stability or for ongoing transfusion requirements."
For patients who undergo splenectomy, incidence of overwhelming post-splenectomy sepsis is thought to be about 0.8%, and the risk is greatest in the first 2 years. "Even though it’s such a low incidence, overwhelming post-splenectomy sepsis has a very high mortality, up to 50%," she said. "It’s important to educate the patients and the parents that if they develop a fever, it’s important to come to the hospital as soon as possible for evaluation."
Next, Dr. Grabowski discussed hepatic injuries, which involve the right lobe of the liver in 60%-78% of cases. Children with hepatic injuries commonly present with abdominal pain/tenderness and 56%-100% have associated injuries, most commonly involving the brain. Shock occurs in fewer than 10% of patients who present with a liver injury, while an aspartate aminotransferase/alanine aminotransferase (AST/ALT) level of greater than 250 units/L suggests liver injury.
Nonoperative treatment is successful about 90% of the time. This requires hemodynamic stability and absence of peritoneal signs. "Head injury is not a contraindication for nonoperative management," she said. "We have less experience with, and less studies looking at, angioembolization for hepatic injuries, but since we’ve had such good success with splenic injuries, we think it’s going to be helpful for hepatic injuries as well."
Operative treatment is indicated in cases of persistent bleeding, hemodynamic instability, or to rule out a missed injury.
Dr. Grabowski pointed out that there is little value in routine follow-up imaging studies after splenic or hepatic injury. The American Pediatric Surgical Association guidelines recommend a return to normal activities after a period of 2 weeks plus the grade of injury. "Normal activity is considered returning to school and walking," she said. "It’s not return to sports competition like football or wrestling. We usually say if your spleen gets injured during the football season, you can return to play the following season."
Bowel injuries comprise just 15% of intra-abdominal injuries in children, "but there is a high mortality, about 25%, and they’re easily missed on initial exam," Dr. Grabowski said. Clinical examination remains the most important diagnostic tool in the awake patient because only 60% of radiographic studies will be diagnostic. "It’s a difficult diagnosis to make, and delays occur in about 10% of cases," she said. "But many good studies have shown that children who have a delayed diagnosis of bowel injury did not have a worse outcome."
Seat belt injures also are common because most children are too large for car seats and too small for an adult seat belt system. "So they either don’t wear the cross-chest harness or they wear it inappropriately," Dr. Grabowski said. "Children also have a higher center of gravity, an immaturity and lack of structural integrity of their bony pelvis, and in most cases they have a relative paucity of abdominal musculature. Because they’re wearing their seat belt wrong they have a tendency to get injured by their seat belt more often than adults do."
An estimated 50%-70% of seat belt injuries are associated with a chance fracture, or a rupture of the posterior spinal ligament, or wedge, most commonly at L1 and L3. Those particular injuries "are very often associated with a bowel injury, so there’s a high index of suspicion in those children," she said. Indications for exploration in children who present with seat belt injuries include hemodynamic instability, pneumoperitoneum, peritonitis, bladder rupture, abdominal tenderness with free fluid in pelvis on CT without solid organ injury, if they worsen on exam, if they spike a fever, or if their labs become abnormal.
Dr. Grabowski advises clinicians to think nonaccidental trauma if children present with no history or explanation for injury, if the history is incompatible with the type or degree of injury, if a sibling is blamed for the injury, if caregivers give conflicting histories when interviewed separately, or if the history is not credible. "Health care providers are mandated reporters of nonaccidental trauma," she said, noting than an estimated 1 million children are victims of abuse each year.
Dr. Grabowski said that she had no relevant financial conflicts to make.
SAN DIEGO – In the clinical experience of Dr. Julia Grabowski, managing blunt abdominal trauma injuries in children can be tricky business because of the wide variation in development between infants and adolescents.
Such differences "affect both the care of the injured child and injury prevention efforts," she said at the University of California San Diego Critical Care Summer Session. Anatomic considerations in the management of pediatric abdominal trauma include the close proximity of multiple organs, "which can affect their overall injury patterns," said Dr. Grabowski, a pediatric surgeon at Rady Children’s Hospital in San Diego. "In addition, their solid organs are larger compared with the rest of their abdomen. They generally have less body fat, less connective tissue, and less muscle mass, and their bony skeleton is incompletely ossified."
Compared with adults, the rib cage in children "is higher and much more pliable, so rib fractures are quite uncommon in the pediatric population," she said. "If you do see a child who has a rib fracture, that’s a trigger to think they had a much worse trauma than you originally expected."
Blunt injuries account for about 90% of all injuries and deaths in children, Dr. Grabowski said. In blunt abdominal trauma, the most common mechanism of action is a fall, followed by motor vehicle collisions, pedestrian versus auto accidents, bicycle accidents, and assaults. The most commonly injured organs are the spleen and liver, followed distantly by the kidney, small bowel, and pancreas.
Diagnostic evaluation of blunt abdominal trauma includes C-spine imaging for those in whom you suspect C-spine trauma, chest x-rays, anterior-posterior x-ray of the pelvis as necessary, and a computed tomography scan, "which is really the workhorse of evaluation for blunt abdominal trauma," she said. Lab studies may include CBC, liver function tests, amylase, lipase, and blood type and cross.
Another option is Focused Assessment With Sonography for Trauma (the FAST scan). According to Dr. Grabowski, recent research has demonstrated that FAST has a low sensitivity and is inappropriate for use in hemodynamically stable children, but that it may be useful in unstable patients.
For splenic and hepatic injuries, grade and clinical exam dictates the need for PICU admission, frequency of vital signs, hematocrit and hemoglobin testing, diet, and activity. The American Pediatric Surgical Association published guidelines for the management of hemodynamically stable children with isolated spleen or liver injury (J. Pediatr. Surg. 2000; 35:164-9).
"Most children are in the hospital 1 day longer than their grade of injury, and they’re out of any activity for 2 weeks longer than their grade of injury," said Dr. Grabowski. Splenic injuries from to sports competition "are quite common, especially around football and hockey seasons," as are those caused by motor vehicle accidents and accidents from all-terrain vehicles. Common complaints include abdominal pain/tenderness, shoulder pain, nausea and vomiting, and anemia. CT scan is 98% sensitive in identifying the injury.
"Over time we have found that splenic salvage can be achieved in greater than 90% of children with blunt splenic injury, even up to a grade IV or V injury," she said. "Management of splenic injury should be based on physiologic parameters, rather than on a grading of the spleen injury or the presence of ‘blush’ on a CT scan. When an operation is required it’s usually for hemodynamic stability or for ongoing transfusion requirements."
For patients who undergo splenectomy, incidence of overwhelming post-splenectomy sepsis is thought to be about 0.8%, and the risk is greatest in the first 2 years. "Even though it’s such a low incidence, overwhelming post-splenectomy sepsis has a very high mortality, up to 50%," she said. "It’s important to educate the patients and the parents that if they develop a fever, it’s important to come to the hospital as soon as possible for evaluation."
Next, Dr. Grabowski discussed hepatic injuries, which involve the right lobe of the liver in 60%-78% of cases. Children with hepatic injuries commonly present with abdominal pain/tenderness and 56%-100% have associated injuries, most commonly involving the brain. Shock occurs in fewer than 10% of patients who present with a liver injury, while an aspartate aminotransferase/alanine aminotransferase (AST/ALT) level of greater than 250 units/L suggests liver injury.
Nonoperative treatment is successful about 90% of the time. This requires hemodynamic stability and absence of peritoneal signs. "Head injury is not a contraindication for nonoperative management," she said. "We have less experience with, and less studies looking at, angioembolization for hepatic injuries, but since we’ve had such good success with splenic injuries, we think it’s going to be helpful for hepatic injuries as well."
Operative treatment is indicated in cases of persistent bleeding, hemodynamic instability, or to rule out a missed injury.
Dr. Grabowski pointed out that there is little value in routine follow-up imaging studies after splenic or hepatic injury. The American Pediatric Surgical Association guidelines recommend a return to normal activities after a period of 2 weeks plus the grade of injury. "Normal activity is considered returning to school and walking," she said. "It’s not return to sports competition like football or wrestling. We usually say if your spleen gets injured during the football season, you can return to play the following season."
Bowel injuries comprise just 15% of intra-abdominal injuries in children, "but there is a high mortality, about 25%, and they’re easily missed on initial exam," Dr. Grabowski said. Clinical examination remains the most important diagnostic tool in the awake patient because only 60% of radiographic studies will be diagnostic. "It’s a difficult diagnosis to make, and delays occur in about 10% of cases," she said. "But many good studies have shown that children who have a delayed diagnosis of bowel injury did not have a worse outcome."
Seat belt injures also are common because most children are too large for car seats and too small for an adult seat belt system. "So they either don’t wear the cross-chest harness or they wear it inappropriately," Dr. Grabowski said. "Children also have a higher center of gravity, an immaturity and lack of structural integrity of their bony pelvis, and in most cases they have a relative paucity of abdominal musculature. Because they’re wearing their seat belt wrong they have a tendency to get injured by their seat belt more often than adults do."
An estimated 50%-70% of seat belt injuries are associated with a chance fracture, or a rupture of the posterior spinal ligament, or wedge, most commonly at L1 and L3. Those particular injuries "are very often associated with a bowel injury, so there’s a high index of suspicion in those children," she said. Indications for exploration in children who present with seat belt injuries include hemodynamic instability, pneumoperitoneum, peritonitis, bladder rupture, abdominal tenderness with free fluid in pelvis on CT without solid organ injury, if they worsen on exam, if they spike a fever, or if their labs become abnormal.
Dr. Grabowski advises clinicians to think nonaccidental trauma if children present with no history or explanation for injury, if the history is incompatible with the type or degree of injury, if a sibling is blamed for the injury, if caregivers give conflicting histories when interviewed separately, or if the history is not credible. "Health care providers are mandated reporters of nonaccidental trauma," she said, noting than an estimated 1 million children are victims of abuse each year.
Dr. Grabowski said that she had no relevant financial conflicts to make.
SAN DIEGO – In the clinical experience of Dr. Julia Grabowski, managing blunt abdominal trauma injuries in children can be tricky business because of the wide variation in development between infants and adolescents.
Such differences "affect both the care of the injured child and injury prevention efforts," she said at the University of California San Diego Critical Care Summer Session. Anatomic considerations in the management of pediatric abdominal trauma include the close proximity of multiple organs, "which can affect their overall injury patterns," said Dr. Grabowski, a pediatric surgeon at Rady Children’s Hospital in San Diego. "In addition, their solid organs are larger compared with the rest of their abdomen. They generally have less body fat, less connective tissue, and less muscle mass, and their bony skeleton is incompletely ossified."
Compared with adults, the rib cage in children "is higher and much more pliable, so rib fractures are quite uncommon in the pediatric population," she said. "If you do see a child who has a rib fracture, that’s a trigger to think they had a much worse trauma than you originally expected."
Blunt injuries account for about 90% of all injuries and deaths in children, Dr. Grabowski said. In blunt abdominal trauma, the most common mechanism of action is a fall, followed by motor vehicle collisions, pedestrian versus auto accidents, bicycle accidents, and assaults. The most commonly injured organs are the spleen and liver, followed distantly by the kidney, small bowel, and pancreas.
Diagnostic evaluation of blunt abdominal trauma includes C-spine imaging for those in whom you suspect C-spine trauma, chest x-rays, anterior-posterior x-ray of the pelvis as necessary, and a computed tomography scan, "which is really the workhorse of evaluation for blunt abdominal trauma," she said. Lab studies may include CBC, liver function tests, amylase, lipase, and blood type and cross.
Another option is Focused Assessment With Sonography for Trauma (the FAST scan). According to Dr. Grabowski, recent research has demonstrated that FAST has a low sensitivity and is inappropriate for use in hemodynamically stable children, but that it may be useful in unstable patients.
For splenic and hepatic injuries, grade and clinical exam dictates the need for PICU admission, frequency of vital signs, hematocrit and hemoglobin testing, diet, and activity. The American Pediatric Surgical Association published guidelines for the management of hemodynamically stable children with isolated spleen or liver injury (J. Pediatr. Surg. 2000; 35:164-9).
"Most children are in the hospital 1 day longer than their grade of injury, and they’re out of any activity for 2 weeks longer than their grade of injury," said Dr. Grabowski. Splenic injuries from to sports competition "are quite common, especially around football and hockey seasons," as are those caused by motor vehicle accidents and accidents from all-terrain vehicles. Common complaints include abdominal pain/tenderness, shoulder pain, nausea and vomiting, and anemia. CT scan is 98% sensitive in identifying the injury.
"Over time we have found that splenic salvage can be achieved in greater than 90% of children with blunt splenic injury, even up to a grade IV or V injury," she said. "Management of splenic injury should be based on physiologic parameters, rather than on a grading of the spleen injury or the presence of ‘blush’ on a CT scan. When an operation is required it’s usually for hemodynamic stability or for ongoing transfusion requirements."
For patients who undergo splenectomy, incidence of overwhelming post-splenectomy sepsis is thought to be about 0.8%, and the risk is greatest in the first 2 years. "Even though it’s such a low incidence, overwhelming post-splenectomy sepsis has a very high mortality, up to 50%," she said. "It’s important to educate the patients and the parents that if they develop a fever, it’s important to come to the hospital as soon as possible for evaluation."
Next, Dr. Grabowski discussed hepatic injuries, which involve the right lobe of the liver in 60%-78% of cases. Children with hepatic injuries commonly present with abdominal pain/tenderness and 56%-100% have associated injuries, most commonly involving the brain. Shock occurs in fewer than 10% of patients who present with a liver injury, while an aspartate aminotransferase/alanine aminotransferase (AST/ALT) level of greater than 250 units/L suggests liver injury.
Nonoperative treatment is successful about 90% of the time. This requires hemodynamic stability and absence of peritoneal signs. "Head injury is not a contraindication for nonoperative management," she said. "We have less experience with, and less studies looking at, angioembolization for hepatic injuries, but since we’ve had such good success with splenic injuries, we think it’s going to be helpful for hepatic injuries as well."
Operative treatment is indicated in cases of persistent bleeding, hemodynamic instability, or to rule out a missed injury.
Dr. Grabowski pointed out that there is little value in routine follow-up imaging studies after splenic or hepatic injury. The American Pediatric Surgical Association guidelines recommend a return to normal activities after a period of 2 weeks plus the grade of injury. "Normal activity is considered returning to school and walking," she said. "It’s not return to sports competition like football or wrestling. We usually say if your spleen gets injured during the football season, you can return to play the following season."
Bowel injuries comprise just 15% of intra-abdominal injuries in children, "but there is a high mortality, about 25%, and they’re easily missed on initial exam," Dr. Grabowski said. Clinical examination remains the most important diagnostic tool in the awake patient because only 60% of radiographic studies will be diagnostic. "It’s a difficult diagnosis to make, and delays occur in about 10% of cases," she said. "But many good studies have shown that children who have a delayed diagnosis of bowel injury did not have a worse outcome."
Seat belt injures also are common because most children are too large for car seats and too small for an adult seat belt system. "So they either don’t wear the cross-chest harness or they wear it inappropriately," Dr. Grabowski said. "Children also have a higher center of gravity, an immaturity and lack of structural integrity of their bony pelvis, and in most cases they have a relative paucity of abdominal musculature. Because they’re wearing their seat belt wrong they have a tendency to get injured by their seat belt more often than adults do."
An estimated 50%-70% of seat belt injuries are associated with a chance fracture, or a rupture of the posterior spinal ligament, or wedge, most commonly at L1 and L3. Those particular injuries "are very often associated with a bowel injury, so there’s a high index of suspicion in those children," she said. Indications for exploration in children who present with seat belt injuries include hemodynamic instability, pneumoperitoneum, peritonitis, bladder rupture, abdominal tenderness with free fluid in pelvis on CT without solid organ injury, if they worsen on exam, if they spike a fever, or if their labs become abnormal.
Dr. Grabowski advises clinicians to think nonaccidental trauma if children present with no history or explanation for injury, if the history is incompatible with the type or degree of injury, if a sibling is blamed for the injury, if caregivers give conflicting histories when interviewed separately, or if the history is not credible. "Health care providers are mandated reporters of nonaccidental trauma," she said, noting than an estimated 1 million children are victims of abuse each year.
Dr. Grabowski said that she had no relevant financial conflicts to make.
EXPERT ANALYSIS AT THE UCSD CRITICAL CARE SUMMER SESSION
Prostate cancer stage has declined more than Gleason score
The proportion of advanced prostate cancers has declined by more than sixfold over the last 2 decades; however, the proportion of high Gleason grade tumors has not followed suit.
The findings from a 22-year review of two large databases suggest that most low-grade prostate tumors do not progress over time. They further suggest that men can be successfully managed with active watchful waiting – repeated, close follow-up that could spare many from unnecessary and possibly harmful interventions, reported Kathryn Penney, Sc.D. The study was published in the Aug. 14 issue of Cancer Research (Cancer Res. 2013;73:5163-8).
"Men with low-grade disease are being encouraged more and more to do this sort of active surveillance, which isn’t just watching and waiting, but returning for regular visits, additional blood tests, or additional biopsies," Dr. Penney said in an interview. "This is an encouraging trend, because men who are candidates for this kind of follow-up may not end up being candidates for radiation or surgery, which can have long-term side effects."
Dr. Penney, an associate epidemiologist at Brigham and Women’s Hospital, Boston, examined prostate tumor characteristics among 1,207 men who were included in two longitudinal studies: 420 in the Physicians’ Health Study (PHS) and 787 in the ongoing Health Professionals Follow-Up Study (HPFS). All of the men had undergone radical prostatectomy. Dr. Penney and her colleagues analyzed tissue from each tumor and categorized the results from four epochs: 1982-1993, 1993-1996, 1996-2000, and 2000-2004.
Mean age at diagnosis was 66 years in both studies. Information about prostate specific antigen was not available for the PHS, since it was an earlier cohort examined from 1982 to 2004. For the HPFS data, PSA results were available from 1994 onward. In 1994, 42% of the entire study group had been tested in the previous 2 years; that increased to 81% by 2000.
From 1982 to 1993, the earliest epoch, 20% of tumors were stage T3 or higher. This proportion decreased over all four epochs, declining to 3% by the last period – an 85% drop. There were no T4 tumors in that epoch.
There was a 30% decrease in tumors with a Gleason score of 8 or more, dropping from 25% in the first epoch to 17.6% in the last.
"When restricting [the analysis] to men with stage T1/T2, the proportion of Gleason score 8-10 decreased even less across time periods, from 19.5% to 16%," the researchers wrote.
While there was a significant age/grade relationship, with older men having higher Gleason scores, it primarily occurred during the pre-PSA screening epochs. "This suggests that the change over time we observe for Gleason score is not due to a change in age at diagnosis and may represent an increase in screening of younger men detecting more indolent, lower grade tumors," they wrote. "Widespread PSA screening not only advanced the time of diagnosis of prostate cancer, but also has increased the prevalence of detected indolent tumors that would otherwise never have been diagnosed ... Although we cannot rule out the possibility that Gleason grade progresses within an individual, we conclude that it is not a major feature of prostate cancer."
The study speaks to the biology of prostate cancer as well, Dr. Penney and her coinvestigators noted. If Gleason score "seems set early in the disease," then later, potentially modifiable risk factors might be a trigger for progression in low-grade disease. "If we suppose that a Gleason score 3+3 will remain 3+3 for the entire course of the disease, active surveillance could be considered a definitive treatment for selected patients with [10 or fewer years life expectancy] and could significantly delay (potentially forever) the treatment of selected patients with [more than] 10 years life expectancy."
"Alternatively, earlier influences, such as genetics, may drive the development of a subtype of cancer that is more aggressive in a way that is not related to differentiation status," they said.
The study was funded by the Dana-Farber/Harvard Cancer Center, the National Cancer Institute, and the National Heart, Lung, and Blood Institute. Dr. Penney had no financial disclosures.
On Twitter @Alz_Gal
The proportion of advanced prostate cancers has declined by more than sixfold over the last 2 decades; however, the proportion of high Gleason grade tumors has not followed suit.
The findings from a 22-year review of two large databases suggest that most low-grade prostate tumors do not progress over time. They further suggest that men can be successfully managed with active watchful waiting – repeated, close follow-up that could spare many from unnecessary and possibly harmful interventions, reported Kathryn Penney, Sc.D. The study was published in the Aug. 14 issue of Cancer Research (Cancer Res. 2013;73:5163-8).
"Men with low-grade disease are being encouraged more and more to do this sort of active surveillance, which isn’t just watching and waiting, but returning for regular visits, additional blood tests, or additional biopsies," Dr. Penney said in an interview. "This is an encouraging trend, because men who are candidates for this kind of follow-up may not end up being candidates for radiation or surgery, which can have long-term side effects."
Dr. Penney, an associate epidemiologist at Brigham and Women’s Hospital, Boston, examined prostate tumor characteristics among 1,207 men who were included in two longitudinal studies: 420 in the Physicians’ Health Study (PHS) and 787 in the ongoing Health Professionals Follow-Up Study (HPFS). All of the men had undergone radical prostatectomy. Dr. Penney and her colleagues analyzed tissue from each tumor and categorized the results from four epochs: 1982-1993, 1993-1996, 1996-2000, and 2000-2004.
Mean age at diagnosis was 66 years in both studies. Information about prostate specific antigen was not available for the PHS, since it was an earlier cohort examined from 1982 to 2004. For the HPFS data, PSA results were available from 1994 onward. In 1994, 42% of the entire study group had been tested in the previous 2 years; that increased to 81% by 2000.
From 1982 to 1993, the earliest epoch, 20% of tumors were stage T3 or higher. This proportion decreased over all four epochs, declining to 3% by the last period – an 85% drop. There were no T4 tumors in that epoch.
There was a 30% decrease in tumors with a Gleason score of 8 or more, dropping from 25% in the first epoch to 17.6% in the last.
"When restricting [the analysis] to men with stage T1/T2, the proportion of Gleason score 8-10 decreased even less across time periods, from 19.5% to 16%," the researchers wrote.
While there was a significant age/grade relationship, with older men having higher Gleason scores, it primarily occurred during the pre-PSA screening epochs. "This suggests that the change over time we observe for Gleason score is not due to a change in age at diagnosis and may represent an increase in screening of younger men detecting more indolent, lower grade tumors," they wrote. "Widespread PSA screening not only advanced the time of diagnosis of prostate cancer, but also has increased the prevalence of detected indolent tumors that would otherwise never have been diagnosed ... Although we cannot rule out the possibility that Gleason grade progresses within an individual, we conclude that it is not a major feature of prostate cancer."
The study speaks to the biology of prostate cancer as well, Dr. Penney and her coinvestigators noted. If Gleason score "seems set early in the disease," then later, potentially modifiable risk factors might be a trigger for progression in low-grade disease. "If we suppose that a Gleason score 3+3 will remain 3+3 for the entire course of the disease, active surveillance could be considered a definitive treatment for selected patients with [10 or fewer years life expectancy] and could significantly delay (potentially forever) the treatment of selected patients with [more than] 10 years life expectancy."
"Alternatively, earlier influences, such as genetics, may drive the development of a subtype of cancer that is more aggressive in a way that is not related to differentiation status," they said.
The study was funded by the Dana-Farber/Harvard Cancer Center, the National Cancer Institute, and the National Heart, Lung, and Blood Institute. Dr. Penney had no financial disclosures.
On Twitter @Alz_Gal
The proportion of advanced prostate cancers has declined by more than sixfold over the last 2 decades; however, the proportion of high Gleason grade tumors has not followed suit.
The findings from a 22-year review of two large databases suggest that most low-grade prostate tumors do not progress over time. They further suggest that men can be successfully managed with active watchful waiting – repeated, close follow-up that could spare many from unnecessary and possibly harmful interventions, reported Kathryn Penney, Sc.D. The study was published in the Aug. 14 issue of Cancer Research (Cancer Res. 2013;73:5163-8).
"Men with low-grade disease are being encouraged more and more to do this sort of active surveillance, which isn’t just watching and waiting, but returning for regular visits, additional blood tests, or additional biopsies," Dr. Penney said in an interview. "This is an encouraging trend, because men who are candidates for this kind of follow-up may not end up being candidates for radiation or surgery, which can have long-term side effects."
Dr. Penney, an associate epidemiologist at Brigham and Women’s Hospital, Boston, examined prostate tumor characteristics among 1,207 men who were included in two longitudinal studies: 420 in the Physicians’ Health Study (PHS) and 787 in the ongoing Health Professionals Follow-Up Study (HPFS). All of the men had undergone radical prostatectomy. Dr. Penney and her colleagues analyzed tissue from each tumor and categorized the results from four epochs: 1982-1993, 1993-1996, 1996-2000, and 2000-2004.
Mean age at diagnosis was 66 years in both studies. Information about prostate specific antigen was not available for the PHS, since it was an earlier cohort examined from 1982 to 2004. For the HPFS data, PSA results were available from 1994 onward. In 1994, 42% of the entire study group had been tested in the previous 2 years; that increased to 81% by 2000.
From 1982 to 1993, the earliest epoch, 20% of tumors were stage T3 or higher. This proportion decreased over all four epochs, declining to 3% by the last period – an 85% drop. There were no T4 tumors in that epoch.
There was a 30% decrease in tumors with a Gleason score of 8 or more, dropping from 25% in the first epoch to 17.6% in the last.
"When restricting [the analysis] to men with stage T1/T2, the proportion of Gleason score 8-10 decreased even less across time periods, from 19.5% to 16%," the researchers wrote.
While there was a significant age/grade relationship, with older men having higher Gleason scores, it primarily occurred during the pre-PSA screening epochs. "This suggests that the change over time we observe for Gleason score is not due to a change in age at diagnosis and may represent an increase in screening of younger men detecting more indolent, lower grade tumors," they wrote. "Widespread PSA screening not only advanced the time of diagnosis of prostate cancer, but also has increased the prevalence of detected indolent tumors that would otherwise never have been diagnosed ... Although we cannot rule out the possibility that Gleason grade progresses within an individual, we conclude that it is not a major feature of prostate cancer."
The study speaks to the biology of prostate cancer as well, Dr. Penney and her coinvestigators noted. If Gleason score "seems set early in the disease," then later, potentially modifiable risk factors might be a trigger for progression in low-grade disease. "If we suppose that a Gleason score 3+3 will remain 3+3 for the entire course of the disease, active surveillance could be considered a definitive treatment for selected patients with [10 or fewer years life expectancy] and could significantly delay (potentially forever) the treatment of selected patients with [more than] 10 years life expectancy."
"Alternatively, earlier influences, such as genetics, may drive the development of a subtype of cancer that is more aggressive in a way that is not related to differentiation status," they said.
The study was funded by the Dana-Farber/Harvard Cancer Center, the National Cancer Institute, and the National Heart, Lung, and Blood Institute. Dr. Penney had no financial disclosures.
On Twitter @Alz_Gal
Major finding: Over 22 years, the number of prostate cancers stage T3 or higher fell by 85%, while the number with a Gleason score of 8 or higher fell by 30% – a significant difference.
Data source: Study of 1,207 men who underwent radical prostatectomy from 1982 to 2004.
Disclosures: The Dana-Farber/Harvard Cancer Center, the National Cancer Institute, and the National Heart, Lung, and Blood Institute provided funding. Dr. Penney had no disclosures.
Best CRT outcomes with LBBB and prolonged QRS
In a large, real-world population of patients who underwent cardiac resynchronization therapy-defibrillator implantation, those who had left bundle-branch block and a QRS duration of 150 ms or more had the best outcomes, according to a report published online August 13 in JAMA.
Patients with left bundle-branch block (LBBB) and a long QRS duration had the lowest mortality risk and the lowest rates of all-cause, cardiovascular, and heart failure readmissions, while patients without LBBB and with a QRS duration of 120-149 ms "consistently had the greatest risks of adverse outcomes," said Dr. Pamela N. Peterson of the Denver Health Medical Center and her associates.
These findings "are particularly notable, given that both LBBB and prolonged QRS duration have been shown to be independent predictors of mortality among patients with left ventricular systolic dysfunction without CRT [cardiac resynchronization therapy]," the investigators said.
The results support the use of QRS morphology and duration to identify which patients can expect the greatest benefit from CRT-D implantation, they noted.
Even though current guidelines recommend selecting patients for CRT based primarily on their QRS morphology and duration, these recommendations are based primarily on meta-analyses and subgroup analyses of clinical trials that only considered these two factors separately. The only study to evaluate the combination of QRS morphology and duration "did not assess meaningful patient outcomes," so the utility of these recommendations in real-world practice hasn’t been clear, Dr. Peterson and her colleagues wrote.
They examined the issue using information from the National Cardiovascular Data Registry’s ICD database. They assessed outcomes in 24,169 Medicare fee-for-service patients who underwent CRT-D implantation in a 3-year period, between 2006 and 2009.
Only patients with a QRS interval of 120 ms or longer were included. The mean age of these study subjects was 75 years. Most (90%) were white and 68% were men.
Comorbid conditions were common, including hypertension (78% of patients), ischemic heart disease (65%), diabetes (38%), atrial fibrillation or flutter (32%), and chronic lung disease (23%). The majority of patients (61%) had not had coronary artery bypass graft surgery.
Most patients (83%) had New York Heart Association class III heart failure symptoms. A total of 67% had LBBB, and 55% had a QRS duration of 150 ms or longer.
Overall mortality was 0.8% at 30 days, 9.2% at 1 year, and 25.9% at 3 years. Overall rates of all-cause readmission were 10.2% at 30 days and 43.3% at 1 year. Rates of heart failure readmission were 2.2% at 30 days and 12.3% at 1 year.
In an unadjusted analysis of the data, rates of all adverse outcomes were significantly lower among patients who had LBBB and a QRS duration of 150 ms or more, the investigators said (JAMA 2013; 310:617-26; [doi:10.1001/jama.2013.8641]).
After the data were adjusted to account for numerous demographic and clinical factors, this difference remained robust. Patients who had LBBB and a QRS duration of 150 ms or greater had a 3-year mortality risk of 21%, compared with 27% for those with LBBB and QRS duration of 120-149 ms. Those with no LBBB and QRS duration of 150 ms or greater had an adjusted 3-year mortality risk of 31%), and patients with no LBBB and QRS durations of 120-149 ms had a 3-year risk of 32%. All differences were significant.
Patients with no LBBB and a long QRS duration, those with LBBB and a short QRS duration, and those with no LBBB and a short QRS duration consistently had higher risks of all adverse outcomes.
"Our real-world data add to the increasing body of evidence that patients with LBBB have better outcomes after CRT," Dr. Peterson and her associates said.
This study was supported by the U.S. Agency for Healthcare Research and Quality and the American College of Cardiology Foundation. Dr. Peterson reported serving as a consultant for Merck, and her associates reported numerous ties to industry sources.
In a large, real-world population of patients who underwent cardiac resynchronization therapy-defibrillator implantation, those who had left bundle-branch block and a QRS duration of 150 ms or more had the best outcomes, according to a report published online August 13 in JAMA.
Patients with left bundle-branch block (LBBB) and a long QRS duration had the lowest mortality risk and the lowest rates of all-cause, cardiovascular, and heart failure readmissions, while patients without LBBB and with a QRS duration of 120-149 ms "consistently had the greatest risks of adverse outcomes," said Dr. Pamela N. Peterson of the Denver Health Medical Center and her associates.
These findings "are particularly notable, given that both LBBB and prolonged QRS duration have been shown to be independent predictors of mortality among patients with left ventricular systolic dysfunction without CRT [cardiac resynchronization therapy]," the investigators said.
The results support the use of QRS morphology and duration to identify which patients can expect the greatest benefit from CRT-D implantation, they noted.
Even though current guidelines recommend selecting patients for CRT based primarily on their QRS morphology and duration, these recommendations are based primarily on meta-analyses and subgroup analyses of clinical trials that only considered these two factors separately. The only study to evaluate the combination of QRS morphology and duration "did not assess meaningful patient outcomes," so the utility of these recommendations in real-world practice hasn’t been clear, Dr. Peterson and her colleagues wrote.
They examined the issue using information from the National Cardiovascular Data Registry’s ICD database. They assessed outcomes in 24,169 Medicare fee-for-service patients who underwent CRT-D implantation in a 3-year period, between 2006 and 2009.
Only patients with a QRS interval of 120 ms or longer were included. The mean age of these study subjects was 75 years. Most (90%) were white and 68% were men.
Comorbid conditions were common, including hypertension (78% of patients), ischemic heart disease (65%), diabetes (38%), atrial fibrillation or flutter (32%), and chronic lung disease (23%). The majority of patients (61%) had not had coronary artery bypass graft surgery.
Most patients (83%) had New York Heart Association class III heart failure symptoms. A total of 67% had LBBB, and 55% had a QRS duration of 150 ms or longer.
Overall mortality was 0.8% at 30 days, 9.2% at 1 year, and 25.9% at 3 years. Overall rates of all-cause readmission were 10.2% at 30 days and 43.3% at 1 year. Rates of heart failure readmission were 2.2% at 30 days and 12.3% at 1 year.
In an unadjusted analysis of the data, rates of all adverse outcomes were significantly lower among patients who had LBBB and a QRS duration of 150 ms or more, the investigators said (JAMA 2013; 310:617-26; [doi:10.1001/jama.2013.8641]).
After the data were adjusted to account for numerous demographic and clinical factors, this difference remained robust. Patients who had LBBB and a QRS duration of 150 ms or greater had a 3-year mortality risk of 21%, compared with 27% for those with LBBB and QRS duration of 120-149 ms. Those with no LBBB and QRS duration of 150 ms or greater had an adjusted 3-year mortality risk of 31%), and patients with no LBBB and QRS durations of 120-149 ms had a 3-year risk of 32%. All differences were significant.
Patients with no LBBB and a long QRS duration, those with LBBB and a short QRS duration, and those with no LBBB and a short QRS duration consistently had higher risks of all adverse outcomes.
"Our real-world data add to the increasing body of evidence that patients with LBBB have better outcomes after CRT," Dr. Peterson and her associates said.
This study was supported by the U.S. Agency for Healthcare Research and Quality and the American College of Cardiology Foundation. Dr. Peterson reported serving as a consultant for Merck, and her associates reported numerous ties to industry sources.
In a large, real-world population of patients who underwent cardiac resynchronization therapy-defibrillator implantation, those who had left bundle-branch block and a QRS duration of 150 ms or more had the best outcomes, according to a report published online August 13 in JAMA.
Patients with left bundle-branch block (LBBB) and a long QRS duration had the lowest mortality risk and the lowest rates of all-cause, cardiovascular, and heart failure readmissions, while patients without LBBB and with a QRS duration of 120-149 ms "consistently had the greatest risks of adverse outcomes," said Dr. Pamela N. Peterson of the Denver Health Medical Center and her associates.
These findings "are particularly notable, given that both LBBB and prolonged QRS duration have been shown to be independent predictors of mortality among patients with left ventricular systolic dysfunction without CRT [cardiac resynchronization therapy]," the investigators said.
The results support the use of QRS morphology and duration to identify which patients can expect the greatest benefit from CRT-D implantation, they noted.
Even though current guidelines recommend selecting patients for CRT based primarily on their QRS morphology and duration, these recommendations are based primarily on meta-analyses and subgroup analyses of clinical trials that only considered these two factors separately. The only study to evaluate the combination of QRS morphology and duration "did not assess meaningful patient outcomes," so the utility of these recommendations in real-world practice hasn’t been clear, Dr. Peterson and her colleagues wrote.
They examined the issue using information from the National Cardiovascular Data Registry’s ICD database. They assessed outcomes in 24,169 Medicare fee-for-service patients who underwent CRT-D implantation in a 3-year period, between 2006 and 2009.
Only patients with a QRS interval of 120 ms or longer were included. The mean age of these study subjects was 75 years. Most (90%) were white and 68% were men.
Comorbid conditions were common, including hypertension (78% of patients), ischemic heart disease (65%), diabetes (38%), atrial fibrillation or flutter (32%), and chronic lung disease (23%). The majority of patients (61%) had not had coronary artery bypass graft surgery.
Most patients (83%) had New York Heart Association class III heart failure symptoms. A total of 67% had LBBB, and 55% had a QRS duration of 150 ms or longer.
Overall mortality was 0.8% at 30 days, 9.2% at 1 year, and 25.9% at 3 years. Overall rates of all-cause readmission were 10.2% at 30 days and 43.3% at 1 year. Rates of heart failure readmission were 2.2% at 30 days and 12.3% at 1 year.
In an unadjusted analysis of the data, rates of all adverse outcomes were significantly lower among patients who had LBBB and a QRS duration of 150 ms or more, the investigators said (JAMA 2013; 310:617-26; [doi:10.1001/jama.2013.8641]).
After the data were adjusted to account for numerous demographic and clinical factors, this difference remained robust. Patients who had LBBB and a QRS duration of 150 ms or greater had a 3-year mortality risk of 21%, compared with 27% for those with LBBB and QRS duration of 120-149 ms. Those with no LBBB and QRS duration of 150 ms or greater had an adjusted 3-year mortality risk of 31%), and patients with no LBBB and QRS durations of 120-149 ms had a 3-year risk of 32%. All differences were significant.
Patients with no LBBB and a long QRS duration, those with LBBB and a short QRS duration, and those with no LBBB and a short QRS duration consistently had higher risks of all adverse outcomes.
"Our real-world data add to the increasing body of evidence that patients with LBBB have better outcomes after CRT," Dr. Peterson and her associates said.
This study was supported by the U.S. Agency for Healthcare Research and Quality and the American College of Cardiology Foundation. Dr. Peterson reported serving as a consultant for Merck, and her associates reported numerous ties to industry sources.
FROM JAMA
Major finding: Patients with LBBB and a prolonged QRS interval (150 ms or more) had the lowest mortality at 1 month, 1 year, and 3 years, as well as the lowest rates of hospital readmission for all causes, cardiovascular causes, and heart failure.
Data source: A retrospective cohort study of outcomes in 24,169 Medicare fee-for-service beneficiaries in a national ICD registry who underwent CRT-D implantation in a 3-year period.
Disclosures: This study was supported by the U.S. Agency for Healthcare Research and Quality and the American College of Cardiology Foundation. Dr. Peterson reported serving as a consultant for Merck, and her associates reported numerous ties to industry sources.