User login
Official Newspaper of the American College of Surgeons
Endoscopic mucosal resection new gold standard for esophageal adenocarcinoma
Endoscopic resection for mucosal esophageal adenocarcinoma is safe and highly effective, and should be the new standard of care.
That’s according to Dr. Oliver Pech, whose study in the March issue of Gastroenterology showed a complete remission rate of 93.8% over nearly 5 years of follow-up (doi: 10.1053/j.gastro.2013.11.006).
Dr. Pech, of the University of Regensburg, Germany, and his colleagues looked at 1,000 consecutive patients (mean age, 69 years; 861 men) with mucosal adenocarcinoma of the esophagus, who were referred to a single center between October 1996 and September 2010.
All patients had mucosal Barrett’s carcinoma; lesions judged resectable were first subjected to diagnostic endoscopic resection for staging, even when the macroscopic appearance suggested submucosal disease. Patients with low-grade dysplasia, high-grade dysplasia, and submucosal or more advanced cancer (T1 or greater) were excluded.
In total, 481 patients had short-segment Barrett’s esophagus, and the remainder had long-segment Barrett’s. The majority (n = 493) had intraepithelial adenocarcinoma, according to staging by endoscopic resection, while 240 patients had adenocarcinoma invading the tunica propria, 124 had invasion of the first layer of the muscularis mucosae, and the remaining 143 had disease of the second layer of the muscularis mucosae.
En bloc resection was performed in 508 patients and piecemeal resection in the rest.
The authors found that complete remission, defined as an R0 resection plus one normal surveillance endoscopy, was achieved in 963 (96.3%) of the 1,000 patients in the study.
Among these, recurrence of neoplasia (high-grade dysplasia or adenocarcinoma) was detected in 14.5% of the patients (140 out of the 963) after a median 26.5 months; 115 were successfully retreated with additional endoscopic resection.
That translated to a long-term complete remission rate of 93.8% (mean, 56.6 months) and a 5-year survival rate of 91.5%.
Looking at safety, Dr. Pech reported that 15 patients experienced major complications, including bleeding with a corresponding drop in hemoglobin of at least 2 g/dL (in 14 cases) and perforation (in 1).
He added that the relatively minor complication of stenosis requiring dilation occurred in 13 cases, all of which were managed endoscopically. Finally, in an analysis of which patients were more likely to have successful endoscopic treatment, the authors determined that long-segment Barrett’s as well as poorly differentiated mucosal adenocarcinoma (as opposed to well-differentiated lesions) had a significantly higher risk for failure (P less than .0001 for both).
The authors conceded that referral bias cannot be excluded in this cohort, "because it is possible that only patients with early Barrett’s carcinoma that was endoscopically well treatable may have been referred."
Additionally, over the long course of the study, best practices for Barrett’s esophagus and high grade-dysplasia have evolved considerably, "moving away from multimodal therapy for early Barrett’s carcinoma using a combination of [endoscopic resection], photodynamic therapy, [argon plasma coagulation], and laser toward a strict and purely resectional form of treatment."
Nevertheless, "the data presented here on the largest series published to date on endoscopic therapy for mucosal adenocarcinomas in 1,000 patients confirm the safety of endoscopic resection," the authors wrote.
"Endoscopic therapy for mucosal Barrett’s carcinoma should therefore become the international gold standard for treatment," they added.
The authors stated that they had no conflicts of interest to disclose. They disclosed no funding.
In this month’s issue of Gastroenterology, Oliver Pech, Christian Ell, and colleagues continue their pioneering work on endoscopic treatment of Barrett’s neoplasia with a study of the long-term efficacy and safety of endoscopic resection for T1a esophageal adenocarcinoma. Based on prior work by this German group and several others around the world, endoscopic resection of nodular high-grade dysplasia followed by ablation or resection of all residual Barrett’s has become the standard of care for high-grade dysplasia. There are fewer data available on endoscopic treatment of T1a lesions, which have a small but nonzero incidence of lymph node spread that needs to be considered in treatment algorithms.
While many therapeutic endoscopy programs have embraced endoscopic treatment of T1a esophageal adenocarcinoma with favorable histology, some skepticism remains, and the current study will go a long way toward justifying endoscopic treatment of these tumors. The scope and magnitude of this study are striking – 1,000 consecutive patients with T1a tumors treated by endoscopic resection were followed for an average of nearly 5 years. The results are outstanding, with a long-term remission rate of 94% and only two deaths from Barrett’s cancer. Major complications were rare and were successfully treated endoscopically. Very few patients ultimately required surgery – for lymphatic infiltration, inability to resect the lesion endoscopically due to scarring, poor wound healing, incorrect assessment of the tumor stage during initial treatment, or additional cancer developing during the study. Even those patients ultimately did well.
Given the well-documented mortality risk of esophagectomy even in expert centers (2%-5%), as well as the high morbidity of surgery, it is clear from this study that endoscopic treatment of T1a esophageal adenocarcinoma needs to be the standard of care.
Dr. Shai Friedland is an associate professor of medicine at Stanford (Calif.) University. He is a consultant for C2 Medical.
In this month’s issue of Gastroenterology, Oliver Pech, Christian Ell, and colleagues continue their pioneering work on endoscopic treatment of Barrett’s neoplasia with a study of the long-term efficacy and safety of endoscopic resection for T1a esophageal adenocarcinoma. Based on prior work by this German group and several others around the world, endoscopic resection of nodular high-grade dysplasia followed by ablation or resection of all residual Barrett’s has become the standard of care for high-grade dysplasia. There are fewer data available on endoscopic treatment of T1a lesions, which have a small but nonzero incidence of lymph node spread that needs to be considered in treatment algorithms.
While many therapeutic endoscopy programs have embraced endoscopic treatment of T1a esophageal adenocarcinoma with favorable histology, some skepticism remains, and the current study will go a long way toward justifying endoscopic treatment of these tumors. The scope and magnitude of this study are striking – 1,000 consecutive patients with T1a tumors treated by endoscopic resection were followed for an average of nearly 5 years. The results are outstanding, with a long-term remission rate of 94% and only two deaths from Barrett’s cancer. Major complications were rare and were successfully treated endoscopically. Very few patients ultimately required surgery – for lymphatic infiltration, inability to resect the lesion endoscopically due to scarring, poor wound healing, incorrect assessment of the tumor stage during initial treatment, or additional cancer developing during the study. Even those patients ultimately did well.
Given the well-documented mortality risk of esophagectomy even in expert centers (2%-5%), as well as the high morbidity of surgery, it is clear from this study that endoscopic treatment of T1a esophageal adenocarcinoma needs to be the standard of care.
Dr. Shai Friedland is an associate professor of medicine at Stanford (Calif.) University. He is a consultant for C2 Medical.
In this month’s issue of Gastroenterology, Oliver Pech, Christian Ell, and colleagues continue their pioneering work on endoscopic treatment of Barrett’s neoplasia with a study of the long-term efficacy and safety of endoscopic resection for T1a esophageal adenocarcinoma. Based on prior work by this German group and several others around the world, endoscopic resection of nodular high-grade dysplasia followed by ablation or resection of all residual Barrett’s has become the standard of care for high-grade dysplasia. There are fewer data available on endoscopic treatment of T1a lesions, which have a small but nonzero incidence of lymph node spread that needs to be considered in treatment algorithms.
While many therapeutic endoscopy programs have embraced endoscopic treatment of T1a esophageal adenocarcinoma with favorable histology, some skepticism remains, and the current study will go a long way toward justifying endoscopic treatment of these tumors. The scope and magnitude of this study are striking – 1,000 consecutive patients with T1a tumors treated by endoscopic resection were followed for an average of nearly 5 years. The results are outstanding, with a long-term remission rate of 94% and only two deaths from Barrett’s cancer. Major complications were rare and were successfully treated endoscopically. Very few patients ultimately required surgery – for lymphatic infiltration, inability to resect the lesion endoscopically due to scarring, poor wound healing, incorrect assessment of the tumor stage during initial treatment, or additional cancer developing during the study. Even those patients ultimately did well.
Given the well-documented mortality risk of esophagectomy even in expert centers (2%-5%), as well as the high morbidity of surgery, it is clear from this study that endoscopic treatment of T1a esophageal adenocarcinoma needs to be the standard of care.
Dr. Shai Friedland is an associate professor of medicine at Stanford (Calif.) University. He is a consultant for C2 Medical.
Endoscopic resection for mucosal esophageal adenocarcinoma is safe and highly effective, and should be the new standard of care.
That’s according to Dr. Oliver Pech, whose study in the March issue of Gastroenterology showed a complete remission rate of 93.8% over nearly 5 years of follow-up (doi: 10.1053/j.gastro.2013.11.006).
Dr. Pech, of the University of Regensburg, Germany, and his colleagues looked at 1,000 consecutive patients (mean age, 69 years; 861 men) with mucosal adenocarcinoma of the esophagus, who were referred to a single center between October 1996 and September 2010.
All patients had mucosal Barrett’s carcinoma; lesions judged resectable were first subjected to diagnostic endoscopic resection for staging, even when the macroscopic appearance suggested submucosal disease. Patients with low-grade dysplasia, high-grade dysplasia, and submucosal or more advanced cancer (T1 or greater) were excluded.
In total, 481 patients had short-segment Barrett’s esophagus, and the remainder had long-segment Barrett’s. The majority (n = 493) had intraepithelial adenocarcinoma, according to staging by endoscopic resection, while 240 patients had adenocarcinoma invading the tunica propria, 124 had invasion of the first layer of the muscularis mucosae, and the remaining 143 had disease of the second layer of the muscularis mucosae.
En bloc resection was performed in 508 patients and piecemeal resection in the rest.
The authors found that complete remission, defined as an R0 resection plus one normal surveillance endoscopy, was achieved in 963 (96.3%) of the 1,000 patients in the study.
Among these, recurrence of neoplasia (high-grade dysplasia or adenocarcinoma) was detected in 14.5% of the patients (140 out of the 963) after a median 26.5 months; 115 were successfully retreated with additional endoscopic resection.
That translated to a long-term complete remission rate of 93.8% (mean, 56.6 months) and a 5-year survival rate of 91.5%.
Looking at safety, Dr. Pech reported that 15 patients experienced major complications, including bleeding with a corresponding drop in hemoglobin of at least 2 g/dL (in 14 cases) and perforation (in 1).
He added that the relatively minor complication of stenosis requiring dilation occurred in 13 cases, all of which were managed endoscopically. Finally, in an analysis of which patients were more likely to have successful endoscopic treatment, the authors determined that long-segment Barrett’s as well as poorly differentiated mucosal adenocarcinoma (as opposed to well-differentiated lesions) had a significantly higher risk for failure (P less than .0001 for both).
The authors conceded that referral bias cannot be excluded in this cohort, "because it is possible that only patients with early Barrett’s carcinoma that was endoscopically well treatable may have been referred."
Additionally, over the long course of the study, best practices for Barrett’s esophagus and high grade-dysplasia have evolved considerably, "moving away from multimodal therapy for early Barrett’s carcinoma using a combination of [endoscopic resection], photodynamic therapy, [argon plasma coagulation], and laser toward a strict and purely resectional form of treatment."
Nevertheless, "the data presented here on the largest series published to date on endoscopic therapy for mucosal adenocarcinomas in 1,000 patients confirm the safety of endoscopic resection," the authors wrote.
"Endoscopic therapy for mucosal Barrett’s carcinoma should therefore become the international gold standard for treatment," they added.
The authors stated that they had no conflicts of interest to disclose. They disclosed no funding.
Endoscopic resection for mucosal esophageal adenocarcinoma is safe and highly effective, and should be the new standard of care.
That’s according to Dr. Oliver Pech, whose study in the March issue of Gastroenterology showed a complete remission rate of 93.8% over nearly 5 years of follow-up (doi: 10.1053/j.gastro.2013.11.006).
Dr. Pech, of the University of Regensburg, Germany, and his colleagues looked at 1,000 consecutive patients (mean age, 69 years; 861 men) with mucosal adenocarcinoma of the esophagus, who were referred to a single center between October 1996 and September 2010.
All patients had mucosal Barrett’s carcinoma; lesions judged resectable were first subjected to diagnostic endoscopic resection for staging, even when the macroscopic appearance suggested submucosal disease. Patients with low-grade dysplasia, high-grade dysplasia, and submucosal or more advanced cancer (T1 or greater) were excluded.
In total, 481 patients had short-segment Barrett’s esophagus, and the remainder had long-segment Barrett’s. The majority (n = 493) had intraepithelial adenocarcinoma, according to staging by endoscopic resection, while 240 patients had adenocarcinoma invading the tunica propria, 124 had invasion of the first layer of the muscularis mucosae, and the remaining 143 had disease of the second layer of the muscularis mucosae.
En bloc resection was performed in 508 patients and piecemeal resection in the rest.
The authors found that complete remission, defined as an R0 resection plus one normal surveillance endoscopy, was achieved in 963 (96.3%) of the 1,000 patients in the study.
Among these, recurrence of neoplasia (high-grade dysplasia or adenocarcinoma) was detected in 14.5% of the patients (140 out of the 963) after a median 26.5 months; 115 were successfully retreated with additional endoscopic resection.
That translated to a long-term complete remission rate of 93.8% (mean, 56.6 months) and a 5-year survival rate of 91.5%.
Looking at safety, Dr. Pech reported that 15 patients experienced major complications, including bleeding with a corresponding drop in hemoglobin of at least 2 g/dL (in 14 cases) and perforation (in 1).
He added that the relatively minor complication of stenosis requiring dilation occurred in 13 cases, all of which were managed endoscopically. Finally, in an analysis of which patients were more likely to have successful endoscopic treatment, the authors determined that long-segment Barrett’s as well as poorly differentiated mucosal adenocarcinoma (as opposed to well-differentiated lesions) had a significantly higher risk for failure (P less than .0001 for both).
The authors conceded that referral bias cannot be excluded in this cohort, "because it is possible that only patients with early Barrett’s carcinoma that was endoscopically well treatable may have been referred."
Additionally, over the long course of the study, best practices for Barrett’s esophagus and high grade-dysplasia have evolved considerably, "moving away from multimodal therapy for early Barrett’s carcinoma using a combination of [endoscopic resection], photodynamic therapy, [argon plasma coagulation], and laser toward a strict and purely resectional form of treatment."
Nevertheless, "the data presented here on the largest series published to date on endoscopic therapy for mucosal adenocarcinomas in 1,000 patients confirm the safety of endoscopic resection," the authors wrote.
"Endoscopic therapy for mucosal Barrett’s carcinoma should therefore become the international gold standard for treatment," they added.
The authors stated that they had no conflicts of interest to disclose. They disclosed no funding.
FROM GASTROENTEROLOGY
Major finding: Endoscopic resection of esophageal adenocarcinoma resulted in a long-term complete remission rate of 93.8%.
Data source: Data from 1,000 consecutive patients with mucosal adenocarcinoma of the esophagus.
Disclosures: The authors stated that they had no conflicts of interest to disclose. They disclosed no funding.
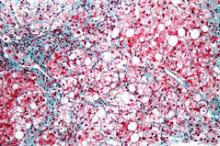
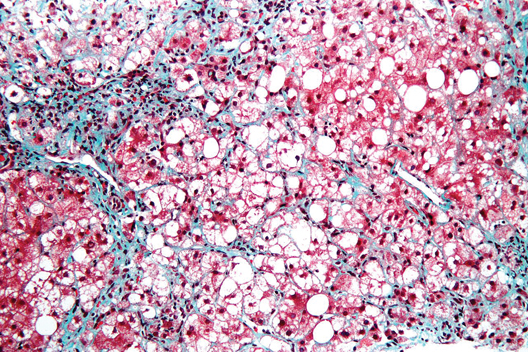
NASH liver transplant mortality differs from non-NASH
Cardiovascular complications and sepsis are two major concerns after liver transplant for nonalcoholic steatohepatitis, more so than after liver transplant for other indications.
The finding comes from what the authors called the first systematic review and meta-analysis to investigate the cumulative clinical experience of liver transplant for NASH, wrote Dr. Xiaofei Wang and colleagues in the March issue of Clinical Gastroenterology and Hepatology (doi: 10.1016/j.cgh.2013.09.023).
In their analysis, Dr. Wang, of the Affiliated Hospital of North Sichuan Medical College, Nanchong, China, and colleagues performed a search of PubMed, Embase, the Cochrane Library, and the Web of Science for studies published through Sept. 1, 2012, that looked at liver transplant for nonalcoholic fatty liver disease (NAFLD) or NASH. Reviews were excluded, as were studies in which NASH patients could not be separated out from non-NASH liver transplant recipients.
Overall, nine studies were analyzed, all of which measured outcomes after liver transplant for NASH. Other indications for transplant included primary biliary cirrhosis/primary sclerosing cholangitis, alcoholic liver disease, hepatitis C, hepatitis B, and cryptogenic cirrhosis.
The researchers found that, on the whole, survival rates at post-transplant years 1, 3, and 5 did not significantly differ between NASH patients and their non-NASH counterparts (1-year odds ratio, 0.77; 95% confidence interval, 0.59-1.00; P = .05; 3-year OR, 0.97; 95% CI, 0.67-1.40; P = .86; 5-year OR, 1.09; 95% CI, 0.77-1.56; P = .63).
Nevertheless, NASH patients registered more deaths due to "cardiovascular events" compared with non-NASH liver transplant recipients, the authors wrote (OR, 1.65; 95% CI, 1.01-2.70; P = .05).
The authors also found that NASH patients were significantly more likely to die of sepsis post transplant than were non-NASH patients (OR, 1.71; 95% CI, 1.17-2.50; P = .006).
On the other hand, NASH patients had fewer deaths caused by graft failure than did patients undergoing liver transplant for other indications (OR, 0.21; 95% CI, 0.05-0.89; P = .03).
In an attempt to explain their findings, the authors pointed out that while cardiovascular events are the top cause of non–graft-related mortality in all liver transplants, NASH patients might be especially susceptible, given that their diagnosis is "frequently associated" with cardiovascular risk factors such as obesity, diabetes, and hypertension. Indeed, these same characteristics might also predispose these patients to postoperative infection and sepsis, they added.
And regarding the finding that graft-related mortality is actually lower among NASH patients, Dr. Wang and colleagues postulated that this may be due to the "lower likelihood of disease recurrence, and this may mean lower rates of graft failure compared with other liver diseases, such as hepatitis C virus and hepatitis B virus infection."
The authors conceded that there were several limitations to their analysis, not the least being heterogeneity between included studies. Moreover, two large population-based studies using national databases were ultimately excluded due to patient duplication and poor accuracy with regard to the cause of death.
In any case, the current analysis shows that "more attention and careful consideration are required in selecting patients with NASH for liver transplant, along with aggressive management of cardiovascular complications and sepsis after transplantation."
The authors disclosed no conflicts of interest related to this study. They wrote that funding was provided by the scientific research development project of North Sichuan Medical College.
Cardiovascular complications and sepsis are two major concerns after liver transplant for nonalcoholic steatohepatitis, more so than after liver transplant for other indications.
The finding comes from what the authors called the first systematic review and meta-analysis to investigate the cumulative clinical experience of liver transplant for NASH, wrote Dr. Xiaofei Wang and colleagues in the March issue of Clinical Gastroenterology and Hepatology (doi: 10.1016/j.cgh.2013.09.023).
In their analysis, Dr. Wang, of the Affiliated Hospital of North Sichuan Medical College, Nanchong, China, and colleagues performed a search of PubMed, Embase, the Cochrane Library, and the Web of Science for studies published through Sept. 1, 2012, that looked at liver transplant for nonalcoholic fatty liver disease (NAFLD) or NASH. Reviews were excluded, as were studies in which NASH patients could not be separated out from non-NASH liver transplant recipients.
Overall, nine studies were analyzed, all of which measured outcomes after liver transplant for NASH. Other indications for transplant included primary biliary cirrhosis/primary sclerosing cholangitis, alcoholic liver disease, hepatitis C, hepatitis B, and cryptogenic cirrhosis.
The researchers found that, on the whole, survival rates at post-transplant years 1, 3, and 5 did not significantly differ between NASH patients and their non-NASH counterparts (1-year odds ratio, 0.77; 95% confidence interval, 0.59-1.00; P = .05; 3-year OR, 0.97; 95% CI, 0.67-1.40; P = .86; 5-year OR, 1.09; 95% CI, 0.77-1.56; P = .63).
Nevertheless, NASH patients registered more deaths due to "cardiovascular events" compared with non-NASH liver transplant recipients, the authors wrote (OR, 1.65; 95% CI, 1.01-2.70; P = .05).
The authors also found that NASH patients were significantly more likely to die of sepsis post transplant than were non-NASH patients (OR, 1.71; 95% CI, 1.17-2.50; P = .006).
On the other hand, NASH patients had fewer deaths caused by graft failure than did patients undergoing liver transplant for other indications (OR, 0.21; 95% CI, 0.05-0.89; P = .03).
In an attempt to explain their findings, the authors pointed out that while cardiovascular events are the top cause of non–graft-related mortality in all liver transplants, NASH patients might be especially susceptible, given that their diagnosis is "frequently associated" with cardiovascular risk factors such as obesity, diabetes, and hypertension. Indeed, these same characteristics might also predispose these patients to postoperative infection and sepsis, they added.
And regarding the finding that graft-related mortality is actually lower among NASH patients, Dr. Wang and colleagues postulated that this may be due to the "lower likelihood of disease recurrence, and this may mean lower rates of graft failure compared with other liver diseases, such as hepatitis C virus and hepatitis B virus infection."
The authors conceded that there were several limitations to their analysis, not the least being heterogeneity between included studies. Moreover, two large population-based studies using national databases were ultimately excluded due to patient duplication and poor accuracy with regard to the cause of death.
In any case, the current analysis shows that "more attention and careful consideration are required in selecting patients with NASH for liver transplant, along with aggressive management of cardiovascular complications and sepsis after transplantation."
The authors disclosed no conflicts of interest related to this study. They wrote that funding was provided by the scientific research development project of North Sichuan Medical College.
Cardiovascular complications and sepsis are two major concerns after liver transplant for nonalcoholic steatohepatitis, more so than after liver transplant for other indications.
The finding comes from what the authors called the first systematic review and meta-analysis to investigate the cumulative clinical experience of liver transplant for NASH, wrote Dr. Xiaofei Wang and colleagues in the March issue of Clinical Gastroenterology and Hepatology (doi: 10.1016/j.cgh.2013.09.023).
In their analysis, Dr. Wang, of the Affiliated Hospital of North Sichuan Medical College, Nanchong, China, and colleagues performed a search of PubMed, Embase, the Cochrane Library, and the Web of Science for studies published through Sept. 1, 2012, that looked at liver transplant for nonalcoholic fatty liver disease (NAFLD) or NASH. Reviews were excluded, as were studies in which NASH patients could not be separated out from non-NASH liver transplant recipients.
Overall, nine studies were analyzed, all of which measured outcomes after liver transplant for NASH. Other indications for transplant included primary biliary cirrhosis/primary sclerosing cholangitis, alcoholic liver disease, hepatitis C, hepatitis B, and cryptogenic cirrhosis.
The researchers found that, on the whole, survival rates at post-transplant years 1, 3, and 5 did not significantly differ between NASH patients and their non-NASH counterparts (1-year odds ratio, 0.77; 95% confidence interval, 0.59-1.00; P = .05; 3-year OR, 0.97; 95% CI, 0.67-1.40; P = .86; 5-year OR, 1.09; 95% CI, 0.77-1.56; P = .63).
Nevertheless, NASH patients registered more deaths due to "cardiovascular events" compared with non-NASH liver transplant recipients, the authors wrote (OR, 1.65; 95% CI, 1.01-2.70; P = .05).
The authors also found that NASH patients were significantly more likely to die of sepsis post transplant than were non-NASH patients (OR, 1.71; 95% CI, 1.17-2.50; P = .006).
On the other hand, NASH patients had fewer deaths caused by graft failure than did patients undergoing liver transplant for other indications (OR, 0.21; 95% CI, 0.05-0.89; P = .03).
In an attempt to explain their findings, the authors pointed out that while cardiovascular events are the top cause of non–graft-related mortality in all liver transplants, NASH patients might be especially susceptible, given that their diagnosis is "frequently associated" with cardiovascular risk factors such as obesity, diabetes, and hypertension. Indeed, these same characteristics might also predispose these patients to postoperative infection and sepsis, they added.
And regarding the finding that graft-related mortality is actually lower among NASH patients, Dr. Wang and colleagues postulated that this may be due to the "lower likelihood of disease recurrence, and this may mean lower rates of graft failure compared with other liver diseases, such as hepatitis C virus and hepatitis B virus infection."
The authors conceded that there were several limitations to their analysis, not the least being heterogeneity between included studies. Moreover, two large population-based studies using national databases were ultimately excluded due to patient duplication and poor accuracy with regard to the cause of death.
In any case, the current analysis shows that "more attention and careful consideration are required in selecting patients with NASH for liver transplant, along with aggressive management of cardiovascular complications and sepsis after transplantation."
The authors disclosed no conflicts of interest related to this study. They wrote that funding was provided by the scientific research development project of North Sichuan Medical College.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Major finding: Patients undergoing liver transplant for nonalcoholic steatohepatitis are at a greater risk for death due to sepsis and cardiovascular complications.
Data source: A meta-analysis of nine studies published through September 2012.
Disclosures: The authors disclosed no conflicts of interest related to this study. They wrote that funding was provided by the scientific research development project of North Sichuan Medical College.
Arthroplasty for rheumatoid arthritis doesn’t boost cardiovascular risk
SNOWMASS, COLO. – During a recent 15-year period in which the annual arthroplasty rate for osteoarthritis and other noninflammatory arthritides doubled, the arthroplasty rate for rheumatoid arthritis actually declined. Moreover, the mean age at the time of arthroplasty for RA rose.
"In a time frame when utilization of total knee and total hip replacement for osteoarthritis is really skyrocketing, with younger and younger patients, I think this speaks to something pretty good going on with our RA patients," Dr. Susan M. Goodman observed at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
She presented data from a soon to be published study of nearly 2.8 million arthroplasties included in 10 state databases. The arthroplasty rate for noninflammatory arthritis – the great majority of which is osteoarthritis (OA) – zoomed from 124.5/100,000 population in 1991 to 247.5/100,000 in 2005.
Meanwhile the rate of arthroplasty for RA fell slightly, albeit statistically significantly, from 4.6 to 4.5 per 100,000. The mean age at the time of arthroplasty for RA rose from 63.4 years in 1991 to 64.9 years in 2005, reported Dr. Goodman, a rheumatologist at the Hospital for Special Surgery in New York.
She turned to data from other sources to address issues related to the morbidity of arthroplasty for RA.
For example, it’s well documented that rheumatoid arthritis is associated with elevated cardiovascular risk, such that the typical RA patient has a cardiovascular morbidity burden comparable to that of someone without RA who’s 5 years older. So what does this mean for the many RA patients who come into the hospital for total hip or knee replacement?
Surprisingly, nothing. That is, data from multiple sources indicate RA patients are at no greater perioperative risk of cardiovascular events than are patients with OA undergoing the same procedures.
The take-away message? "Clearly we’re doing something right in managing our patients with RA," Dr. Goodman commented.
Similarly, an analysis of 7.75 million patients in the Nationwide Inpatient Sample database found that among RA patients undergoing intermediate-risk noncardiac surgery, such as total joint arthroplasty, the perioperative cardiovascular event rate was 0.34%, significantly less than the 1.07% rate in diabetic patients undergoing intermediate-risk surgeries. Moreover, perioperative mortality was 0.30% in the RA patients, less than half the 0.65% figure in diabetics (Arthritis Rheum. 2012;64:2429-37). These findings disproved the study hypothesis, which was that the two groups would have similar cardiovascular event rates, since both diseases are – unlike osteoarthritis – systemic inflammatory conditions associated with increased cardiovascular mortality.
"I think this means that we as rheumatologists are taking better care of our patients than the endocrinologists next door whose patients have a similar atherosclerotic burden," she continued.
Dr. Goodman was a coinvestigator in a population-based study of 351,103 total knee replacements and 157,775 total hip replacements done at 400 hospitals during 2006-2010. This retrospective analysis of an administrative database included 11,755 total knee and 5,400 total hip replacements for RA.
The prevalence of a prior history of MI, peripheral vascular disease, or cerebrovascular disease was closely similar in the RA and OA patients undergoing surgery. However, the prevalence of baseline COPD was significantly greater in the RA patients, at roughly 17.5%, or an absolute 3%-4% more than in the osteoarthritis patients.
The 30-day rates of cardiac events, venous thromboembolism, and cerebrovascular events were closely similar in the RA and OA arthroplasty patients. The RA patients undergoing total hip replacement had significantly higher rates of pulmonary compromise, infections, blood product transfusions, mechanical ventilation, and length of stay than did OA patients (Clin. Exp. Rheumatol. 2013;31:889-95). The RA patients with total knee replacement differed from their OA counterparts only in terms of greater need for transfusions and lengthier hospital stays (J. Arthroplasty 2014;29:308-13).
However, an analysis of the Hospital for Special Surgery experience failed to confirm the increased complication risks found in this study of a large administrative database. This retrospective review of adverse events within 6 months of total knee replacement in 156 RA patients and 318 OA controls showed no differences between the two groups in pneumonia, other infections, or venous thromboembolism. Moreover, the reoperation rate was 2.5% in the RA patients, compared with 8.8% in the OA group.
"The advantage of a smaller study like this is you really know who has RA and you have a lot of very granular information about the drugs they’re taking. The disadvantage, of course, is that you’re really not powered to look at major adverse events. But boy, there wasn’t even a hint of an increase in the complication rate amongst these RA patients," according to Dr. Goodman.
In a study she presented at the 2013 European Congress of Rheumatology, she compared 2-year outcomes post arthroplasty in RA and OA patients in the contemporary era of high use of biologic agents and traditional DMARDs for RA. The 178 RA patients who had total knee replacement had significantly greater comorbidities preoperatively than the 5,206 OA patients. Yet by 2 years postoperatively, they had fully caught up in terms of improved Western Ontario and McMaster Osteoarthritis Index (WOMAC) function and pain scores.
Total hip replacement was a very different story. The 202 RA patients were four times more likely to have poor WOMAC functional outcome and three times more likely to have poor pain outcome scores at 2 years, compared with 5,810 OA patients. In a multivariate analysis, higher expectations for surgery, better preoperative mental health, and more advanced education were associated with better 2-year outcomes.
"I’m not sure why our hip replacement patients with RA aren’t doing as well as the knee replacement patients, but they’re clearly not," according to the rheumatologist.
She reported having no financial disclosures.
SNOWMASS, COLO. – During a recent 15-year period in which the annual arthroplasty rate for osteoarthritis and other noninflammatory arthritides doubled, the arthroplasty rate for rheumatoid arthritis actually declined. Moreover, the mean age at the time of arthroplasty for RA rose.
"In a time frame when utilization of total knee and total hip replacement for osteoarthritis is really skyrocketing, with younger and younger patients, I think this speaks to something pretty good going on with our RA patients," Dr. Susan M. Goodman observed at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
She presented data from a soon to be published study of nearly 2.8 million arthroplasties included in 10 state databases. The arthroplasty rate for noninflammatory arthritis – the great majority of which is osteoarthritis (OA) – zoomed from 124.5/100,000 population in 1991 to 247.5/100,000 in 2005.
Meanwhile the rate of arthroplasty for RA fell slightly, albeit statistically significantly, from 4.6 to 4.5 per 100,000. The mean age at the time of arthroplasty for RA rose from 63.4 years in 1991 to 64.9 years in 2005, reported Dr. Goodman, a rheumatologist at the Hospital for Special Surgery in New York.
She turned to data from other sources to address issues related to the morbidity of arthroplasty for RA.
For example, it’s well documented that rheumatoid arthritis is associated with elevated cardiovascular risk, such that the typical RA patient has a cardiovascular morbidity burden comparable to that of someone without RA who’s 5 years older. So what does this mean for the many RA patients who come into the hospital for total hip or knee replacement?
Surprisingly, nothing. That is, data from multiple sources indicate RA patients are at no greater perioperative risk of cardiovascular events than are patients with OA undergoing the same procedures.
The take-away message? "Clearly we’re doing something right in managing our patients with RA," Dr. Goodman commented.
Similarly, an analysis of 7.75 million patients in the Nationwide Inpatient Sample database found that among RA patients undergoing intermediate-risk noncardiac surgery, such as total joint arthroplasty, the perioperative cardiovascular event rate was 0.34%, significantly less than the 1.07% rate in diabetic patients undergoing intermediate-risk surgeries. Moreover, perioperative mortality was 0.30% in the RA patients, less than half the 0.65% figure in diabetics (Arthritis Rheum. 2012;64:2429-37). These findings disproved the study hypothesis, which was that the two groups would have similar cardiovascular event rates, since both diseases are – unlike osteoarthritis – systemic inflammatory conditions associated with increased cardiovascular mortality.
"I think this means that we as rheumatologists are taking better care of our patients than the endocrinologists next door whose patients have a similar atherosclerotic burden," she continued.
Dr. Goodman was a coinvestigator in a population-based study of 351,103 total knee replacements and 157,775 total hip replacements done at 400 hospitals during 2006-2010. This retrospective analysis of an administrative database included 11,755 total knee and 5,400 total hip replacements for RA.
The prevalence of a prior history of MI, peripheral vascular disease, or cerebrovascular disease was closely similar in the RA and OA patients undergoing surgery. However, the prevalence of baseline COPD was significantly greater in the RA patients, at roughly 17.5%, or an absolute 3%-4% more than in the osteoarthritis patients.
The 30-day rates of cardiac events, venous thromboembolism, and cerebrovascular events were closely similar in the RA and OA arthroplasty patients. The RA patients undergoing total hip replacement had significantly higher rates of pulmonary compromise, infections, blood product transfusions, mechanical ventilation, and length of stay than did OA patients (Clin. Exp. Rheumatol. 2013;31:889-95). The RA patients with total knee replacement differed from their OA counterparts only in terms of greater need for transfusions and lengthier hospital stays (J. Arthroplasty 2014;29:308-13).
However, an analysis of the Hospital for Special Surgery experience failed to confirm the increased complication risks found in this study of a large administrative database. This retrospective review of adverse events within 6 months of total knee replacement in 156 RA patients and 318 OA controls showed no differences between the two groups in pneumonia, other infections, or venous thromboembolism. Moreover, the reoperation rate was 2.5% in the RA patients, compared with 8.8% in the OA group.
"The advantage of a smaller study like this is you really know who has RA and you have a lot of very granular information about the drugs they’re taking. The disadvantage, of course, is that you’re really not powered to look at major adverse events. But boy, there wasn’t even a hint of an increase in the complication rate amongst these RA patients," according to Dr. Goodman.
In a study she presented at the 2013 European Congress of Rheumatology, she compared 2-year outcomes post arthroplasty in RA and OA patients in the contemporary era of high use of biologic agents and traditional DMARDs for RA. The 178 RA patients who had total knee replacement had significantly greater comorbidities preoperatively than the 5,206 OA patients. Yet by 2 years postoperatively, they had fully caught up in terms of improved Western Ontario and McMaster Osteoarthritis Index (WOMAC) function and pain scores.
Total hip replacement was a very different story. The 202 RA patients were four times more likely to have poor WOMAC functional outcome and three times more likely to have poor pain outcome scores at 2 years, compared with 5,810 OA patients. In a multivariate analysis, higher expectations for surgery, better preoperative mental health, and more advanced education were associated with better 2-year outcomes.
"I’m not sure why our hip replacement patients with RA aren’t doing as well as the knee replacement patients, but they’re clearly not," according to the rheumatologist.
She reported having no financial disclosures.
SNOWMASS, COLO. – During a recent 15-year period in which the annual arthroplasty rate for osteoarthritis and other noninflammatory arthritides doubled, the arthroplasty rate for rheumatoid arthritis actually declined. Moreover, the mean age at the time of arthroplasty for RA rose.
"In a time frame when utilization of total knee and total hip replacement for osteoarthritis is really skyrocketing, with younger and younger patients, I think this speaks to something pretty good going on with our RA patients," Dr. Susan M. Goodman observed at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
She presented data from a soon to be published study of nearly 2.8 million arthroplasties included in 10 state databases. The arthroplasty rate for noninflammatory arthritis – the great majority of which is osteoarthritis (OA) – zoomed from 124.5/100,000 population in 1991 to 247.5/100,000 in 2005.
Meanwhile the rate of arthroplasty for RA fell slightly, albeit statistically significantly, from 4.6 to 4.5 per 100,000. The mean age at the time of arthroplasty for RA rose from 63.4 years in 1991 to 64.9 years in 2005, reported Dr. Goodman, a rheumatologist at the Hospital for Special Surgery in New York.
She turned to data from other sources to address issues related to the morbidity of arthroplasty for RA.
For example, it’s well documented that rheumatoid arthritis is associated with elevated cardiovascular risk, such that the typical RA patient has a cardiovascular morbidity burden comparable to that of someone without RA who’s 5 years older. So what does this mean for the many RA patients who come into the hospital for total hip or knee replacement?
Surprisingly, nothing. That is, data from multiple sources indicate RA patients are at no greater perioperative risk of cardiovascular events than are patients with OA undergoing the same procedures.
The take-away message? "Clearly we’re doing something right in managing our patients with RA," Dr. Goodman commented.
Similarly, an analysis of 7.75 million patients in the Nationwide Inpatient Sample database found that among RA patients undergoing intermediate-risk noncardiac surgery, such as total joint arthroplasty, the perioperative cardiovascular event rate was 0.34%, significantly less than the 1.07% rate in diabetic patients undergoing intermediate-risk surgeries. Moreover, perioperative mortality was 0.30% in the RA patients, less than half the 0.65% figure in diabetics (Arthritis Rheum. 2012;64:2429-37). These findings disproved the study hypothesis, which was that the two groups would have similar cardiovascular event rates, since both diseases are – unlike osteoarthritis – systemic inflammatory conditions associated with increased cardiovascular mortality.
"I think this means that we as rheumatologists are taking better care of our patients than the endocrinologists next door whose patients have a similar atherosclerotic burden," she continued.
Dr. Goodman was a coinvestigator in a population-based study of 351,103 total knee replacements and 157,775 total hip replacements done at 400 hospitals during 2006-2010. This retrospective analysis of an administrative database included 11,755 total knee and 5,400 total hip replacements for RA.
The prevalence of a prior history of MI, peripheral vascular disease, or cerebrovascular disease was closely similar in the RA and OA patients undergoing surgery. However, the prevalence of baseline COPD was significantly greater in the RA patients, at roughly 17.5%, or an absolute 3%-4% more than in the osteoarthritis patients.
The 30-day rates of cardiac events, venous thromboembolism, and cerebrovascular events were closely similar in the RA and OA arthroplasty patients. The RA patients undergoing total hip replacement had significantly higher rates of pulmonary compromise, infections, blood product transfusions, mechanical ventilation, and length of stay than did OA patients (Clin. Exp. Rheumatol. 2013;31:889-95). The RA patients with total knee replacement differed from their OA counterparts only in terms of greater need for transfusions and lengthier hospital stays (J. Arthroplasty 2014;29:308-13).
However, an analysis of the Hospital for Special Surgery experience failed to confirm the increased complication risks found in this study of a large administrative database. This retrospective review of adverse events within 6 months of total knee replacement in 156 RA patients and 318 OA controls showed no differences between the two groups in pneumonia, other infections, or venous thromboembolism. Moreover, the reoperation rate was 2.5% in the RA patients, compared with 8.8% in the OA group.
"The advantage of a smaller study like this is you really know who has RA and you have a lot of very granular information about the drugs they’re taking. The disadvantage, of course, is that you’re really not powered to look at major adverse events. But boy, there wasn’t even a hint of an increase in the complication rate amongst these RA patients," according to Dr. Goodman.
In a study she presented at the 2013 European Congress of Rheumatology, she compared 2-year outcomes post arthroplasty in RA and OA patients in the contemporary era of high use of biologic agents and traditional DMARDs for RA. The 178 RA patients who had total knee replacement had significantly greater comorbidities preoperatively than the 5,206 OA patients. Yet by 2 years postoperatively, they had fully caught up in terms of improved Western Ontario and McMaster Osteoarthritis Index (WOMAC) function and pain scores.
Total hip replacement was a very different story. The 202 RA patients were four times more likely to have poor WOMAC functional outcome and three times more likely to have poor pain outcome scores at 2 years, compared with 5,810 OA patients. In a multivariate analysis, higher expectations for surgery, better preoperative mental health, and more advanced education were associated with better 2-year outcomes.
"I’m not sure why our hip replacement patients with RA aren’t doing as well as the knee replacement patients, but they’re clearly not," according to the rheumatologist.
She reported having no financial disclosures.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
Post-LVAD acute kidney injury is predictor of all-cause mortality
Acute kidney injury is not only common after implantation of a left ventricular assist device, it is also an independent predictor of 30-day and 1-year all-cause mortality, according to a retrospective, single-institution study.
In addition, multivariate analysis showed that only diabetes was a significant predictor of post-LVAD implantation acute kidney injury (AKI), the investigators stated in the report published online Feb. 15 in the American Journal of Nephrology.
Dr. Abhijit Naik and Dr. Jay L. Koyner of the section of nephrology at the University of Chicago and their colleagues identified 168 patients who underwent LVAD implantation at the university. They excluded 11 patients because of previous end-stage renal disease and intraoperative mortality. The remaining cohort of 157 patients served as their study population, which was 78% men and 58% white and had a mean age of around 57 years (Am. J. Nephrol. 2014 Feb. 15 [doi:10.1159/000358495]).
The obtained demographic, biochemical, and clinical profiles from the national Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS), the Society of Thoracic Surgeons (STS) database, and the University of Chicago medical record (EMR). Baseline creatinine was defined as the listing of creatinine data obtained from the INTERMACS registry, or failing that, the last serum creatinine in the EMR prior to device implantation.
They defined the primary outcome of AKI as a 50% rise in serum creatinine over the preoperative baseline during the first 7 postoperative days. All-cause mortality was monitored over 1 year after implantation, with data reported at 30 and 365 days.
A total of 44 of 157 (28%) of patients developed AKI based on the study criteria. The only significant baseline differences between the patients with and without AKI were the presence of diabetes and cerebrovascular disease (CVD), each significantly higher in the patients who developed AKI.
Univariate analysis showed CVD and diabetes as significant predictors of developing AKI, but upon multivariate analysis, only diabetes retained significance (odds ratio, 2.27; P = .04).
As for mortality, only AKI (hazard ratio, 3.01; P = .03) and cardiopulmonary bypass time (HR, 1.01; P = .02) were significant predictors of 30-day mortality. Preoperative diabetes mellitus (HR, 1.9; P = .03) or postimplantation AKI (HR, 1.85; P = .03) were significant independent predictors of 1-year mortality. Higher preoperative body mass index was significantly and slightly protective (HR, 0.95; P = .03).
The authors stated that the strength of their study was that it is the largest cohort study of LVAD patients to employ a consensus definition of AKI; limitations were its single-center and retrospective nature and the use of database reviews.
As AKI is a common adverse outcome following traditional cardiac surgery, including coronary artery bypass grafting and/or valve replacement, more research is needed to tease out any differences unique to LVAD implantation, the investigators said.
"Given the high AKI rates and the mounting evidence linking AKI to mortality following VAD implantation, the use of biomarkers to identify patients at risk may have a role. Large prospective multicenter trials are needed to develop a risk stratification system to identify patients at risk for developing post-VAD implantation AKI," the researchers concluded.
Dr. Koyner was supported by a K23 grant from the National Institutes of Health. One of the coauthors reported research funding from Thoratec and another reported consulting fees from Thoratec and HeartWare. The other authors reported no disclosures.
Acute kidney injury is not only common after implantation of a left ventricular assist device, it is also an independent predictor of 30-day and 1-year all-cause mortality, according to a retrospective, single-institution study.
In addition, multivariate analysis showed that only diabetes was a significant predictor of post-LVAD implantation acute kidney injury (AKI), the investigators stated in the report published online Feb. 15 in the American Journal of Nephrology.
Dr. Abhijit Naik and Dr. Jay L. Koyner of the section of nephrology at the University of Chicago and their colleagues identified 168 patients who underwent LVAD implantation at the university. They excluded 11 patients because of previous end-stage renal disease and intraoperative mortality. The remaining cohort of 157 patients served as their study population, which was 78% men and 58% white and had a mean age of around 57 years (Am. J. Nephrol. 2014 Feb. 15 [doi:10.1159/000358495]).
The obtained demographic, biochemical, and clinical profiles from the national Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS), the Society of Thoracic Surgeons (STS) database, and the University of Chicago medical record (EMR). Baseline creatinine was defined as the listing of creatinine data obtained from the INTERMACS registry, or failing that, the last serum creatinine in the EMR prior to device implantation.
They defined the primary outcome of AKI as a 50% rise in serum creatinine over the preoperative baseline during the first 7 postoperative days. All-cause mortality was monitored over 1 year after implantation, with data reported at 30 and 365 days.
A total of 44 of 157 (28%) of patients developed AKI based on the study criteria. The only significant baseline differences between the patients with and without AKI were the presence of diabetes and cerebrovascular disease (CVD), each significantly higher in the patients who developed AKI.
Univariate analysis showed CVD and diabetes as significant predictors of developing AKI, but upon multivariate analysis, only diabetes retained significance (odds ratio, 2.27; P = .04).
As for mortality, only AKI (hazard ratio, 3.01; P = .03) and cardiopulmonary bypass time (HR, 1.01; P = .02) were significant predictors of 30-day mortality. Preoperative diabetes mellitus (HR, 1.9; P = .03) or postimplantation AKI (HR, 1.85; P = .03) were significant independent predictors of 1-year mortality. Higher preoperative body mass index was significantly and slightly protective (HR, 0.95; P = .03).
The authors stated that the strength of their study was that it is the largest cohort study of LVAD patients to employ a consensus definition of AKI; limitations were its single-center and retrospective nature and the use of database reviews.
As AKI is a common adverse outcome following traditional cardiac surgery, including coronary artery bypass grafting and/or valve replacement, more research is needed to tease out any differences unique to LVAD implantation, the investigators said.
"Given the high AKI rates and the mounting evidence linking AKI to mortality following VAD implantation, the use of biomarkers to identify patients at risk may have a role. Large prospective multicenter trials are needed to develop a risk stratification system to identify patients at risk for developing post-VAD implantation AKI," the researchers concluded.
Dr. Koyner was supported by a K23 grant from the National Institutes of Health. One of the coauthors reported research funding from Thoratec and another reported consulting fees from Thoratec and HeartWare. The other authors reported no disclosures.
Acute kidney injury is not only common after implantation of a left ventricular assist device, it is also an independent predictor of 30-day and 1-year all-cause mortality, according to a retrospective, single-institution study.
In addition, multivariate analysis showed that only diabetes was a significant predictor of post-LVAD implantation acute kidney injury (AKI), the investigators stated in the report published online Feb. 15 in the American Journal of Nephrology.
Dr. Abhijit Naik and Dr. Jay L. Koyner of the section of nephrology at the University of Chicago and their colleagues identified 168 patients who underwent LVAD implantation at the university. They excluded 11 patients because of previous end-stage renal disease and intraoperative mortality. The remaining cohort of 157 patients served as their study population, which was 78% men and 58% white and had a mean age of around 57 years (Am. J. Nephrol. 2014 Feb. 15 [doi:10.1159/000358495]).
The obtained demographic, biochemical, and clinical profiles from the national Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS), the Society of Thoracic Surgeons (STS) database, and the University of Chicago medical record (EMR). Baseline creatinine was defined as the listing of creatinine data obtained from the INTERMACS registry, or failing that, the last serum creatinine in the EMR prior to device implantation.
They defined the primary outcome of AKI as a 50% rise in serum creatinine over the preoperative baseline during the first 7 postoperative days. All-cause mortality was monitored over 1 year after implantation, with data reported at 30 and 365 days.
A total of 44 of 157 (28%) of patients developed AKI based on the study criteria. The only significant baseline differences between the patients with and without AKI were the presence of diabetes and cerebrovascular disease (CVD), each significantly higher in the patients who developed AKI.
Univariate analysis showed CVD and diabetes as significant predictors of developing AKI, but upon multivariate analysis, only diabetes retained significance (odds ratio, 2.27; P = .04).
As for mortality, only AKI (hazard ratio, 3.01; P = .03) and cardiopulmonary bypass time (HR, 1.01; P = .02) were significant predictors of 30-day mortality. Preoperative diabetes mellitus (HR, 1.9; P = .03) or postimplantation AKI (HR, 1.85; P = .03) were significant independent predictors of 1-year mortality. Higher preoperative body mass index was significantly and slightly protective (HR, 0.95; P = .03).
The authors stated that the strength of their study was that it is the largest cohort study of LVAD patients to employ a consensus definition of AKI; limitations were its single-center and retrospective nature and the use of database reviews.
As AKI is a common adverse outcome following traditional cardiac surgery, including coronary artery bypass grafting and/or valve replacement, more research is needed to tease out any differences unique to LVAD implantation, the investigators said.
"Given the high AKI rates and the mounting evidence linking AKI to mortality following VAD implantation, the use of biomarkers to identify patients at risk may have a role. Large prospective multicenter trials are needed to develop a risk stratification system to identify patients at risk for developing post-VAD implantation AKI," the researchers concluded.
Dr. Koyner was supported by a K23 grant from the National Institutes of Health. One of the coauthors reported research funding from Thoratec and another reported consulting fees from Thoratec and HeartWare. The other authors reported no disclosures.
FROM THE AMERICAN JOURNAL OF NEPHROLOGY
Major finding: Acute kidney injury was a significant predictor of 30-day mortality (hazard ratio, 3.01; P = .03) in patients who had implantation of a left ventricular assist device.
Data source: A retrospective, single-institution study using INTERMACS and STS databases with a sample of 168 patients who had undergone LVAD implantation.
Disclosures: Dr. Koyner was supported by a K23 grant from the National Institutes of Health. One of the coauthors reported research funding from Thoratec and another reported consulting fees from Thoratec and HeartWare. The other authors reported no disclosures.
Flexibility – but no passes – on meaningful use Stage 2
ORLANDO – The government can be a bit more flexible on physicians meeting Stage 2 of meaningful use of electronic health records, but won’t give blanket permission to slide on deadlines.
That’s according to Marilyn Tavenner, administrator of the Centers for Medicare & Medicaid Services (CMS), who spoke Feb. 27 at the annual meeting of the Healthcare Information and Management Systems Society.
Physicians have been seeking more leeway from CMS on participating in meaningful use this year, in part because they must purchase or upgrade to the 2014 edition of certified EHR (electronic health records) technology, and in part because they must get ready to switch over to the ICD-10 coding set on Oct. 1.
Physicians who participate in meaningful use get incentive payments from Medicare or Medicaid. If they don’t participate this year, they’ll be penalized starting in 2015.
On Feb. 21, 48 physician organizations wrote to the Health and Human Services department, asking for delays in some of the deadlines for meaningful use this year and for more flexibility from the CMS.
Ms. Tavenner said that CMS officials had heard many concerns about moving forward with Stage 2, and "are sensitive to those concerns." She noted that over the past few years, the agency had delayed the start of Stage 1 and Stage 2, and most recently pushed back implementation of Stage 3 to 2017.
"But now is not the time to stop moving forward," she said. Ms. Tavenner said that it was understood that some health care providers and vendors "may legitimately have issues with establishing Stage 2 reporting deadlines."
Because of that, the CMS has "decided to permit flexibility in how hardship exemptions will be granted in the 2014 reporting year," she said.
The agency will look at hardship requests case-by-case, as is required by law. And it is expected to issue further guidance on what qualifies as a hardship very soon.
But Ms. Tavenner said the agency would not give everyone a pass.
"I must stress to you that we do expect all eligible Stage 2 providers to fully meet all requirements in 2015," Ms. Tavenner said. "And I urge all of you to do everything you can to meet the Stage 2 requirements this year."
[email protected]
On Twitter @aliciaault
ORLANDO – The government can be a bit more flexible on physicians meeting Stage 2 of meaningful use of electronic health records, but won’t give blanket permission to slide on deadlines.
That’s according to Marilyn Tavenner, administrator of the Centers for Medicare & Medicaid Services (CMS), who spoke Feb. 27 at the annual meeting of the Healthcare Information and Management Systems Society.
Physicians have been seeking more leeway from CMS on participating in meaningful use this year, in part because they must purchase or upgrade to the 2014 edition of certified EHR (electronic health records) technology, and in part because they must get ready to switch over to the ICD-10 coding set on Oct. 1.
Physicians who participate in meaningful use get incentive payments from Medicare or Medicaid. If they don’t participate this year, they’ll be penalized starting in 2015.
On Feb. 21, 48 physician organizations wrote to the Health and Human Services department, asking for delays in some of the deadlines for meaningful use this year and for more flexibility from the CMS.
Ms. Tavenner said that CMS officials had heard many concerns about moving forward with Stage 2, and "are sensitive to those concerns." She noted that over the past few years, the agency had delayed the start of Stage 1 and Stage 2, and most recently pushed back implementation of Stage 3 to 2017.
"But now is not the time to stop moving forward," she said. Ms. Tavenner said that it was understood that some health care providers and vendors "may legitimately have issues with establishing Stage 2 reporting deadlines."
Because of that, the CMS has "decided to permit flexibility in how hardship exemptions will be granted in the 2014 reporting year," she said.
The agency will look at hardship requests case-by-case, as is required by law. And it is expected to issue further guidance on what qualifies as a hardship very soon.
But Ms. Tavenner said the agency would not give everyone a pass.
"I must stress to you that we do expect all eligible Stage 2 providers to fully meet all requirements in 2015," Ms. Tavenner said. "And I urge all of you to do everything you can to meet the Stage 2 requirements this year."
[email protected]
On Twitter @aliciaault
ORLANDO – The government can be a bit more flexible on physicians meeting Stage 2 of meaningful use of electronic health records, but won’t give blanket permission to slide on deadlines.
That’s according to Marilyn Tavenner, administrator of the Centers for Medicare & Medicaid Services (CMS), who spoke Feb. 27 at the annual meeting of the Healthcare Information and Management Systems Society.
Physicians have been seeking more leeway from CMS on participating in meaningful use this year, in part because they must purchase or upgrade to the 2014 edition of certified EHR (electronic health records) technology, and in part because they must get ready to switch over to the ICD-10 coding set on Oct. 1.
Physicians who participate in meaningful use get incentive payments from Medicare or Medicaid. If they don’t participate this year, they’ll be penalized starting in 2015.
On Feb. 21, 48 physician organizations wrote to the Health and Human Services department, asking for delays in some of the deadlines for meaningful use this year and for more flexibility from the CMS.
Ms. Tavenner said that CMS officials had heard many concerns about moving forward with Stage 2, and "are sensitive to those concerns." She noted that over the past few years, the agency had delayed the start of Stage 1 and Stage 2, and most recently pushed back implementation of Stage 3 to 2017.
"But now is not the time to stop moving forward," she said. Ms. Tavenner said that it was understood that some health care providers and vendors "may legitimately have issues with establishing Stage 2 reporting deadlines."
Because of that, the CMS has "decided to permit flexibility in how hardship exemptions will be granted in the 2014 reporting year," she said.
The agency will look at hardship requests case-by-case, as is required by law. And it is expected to issue further guidance on what qualifies as a hardship very soon.
But Ms. Tavenner said the agency would not give everyone a pass.
"I must stress to you that we do expect all eligible Stage 2 providers to fully meet all requirements in 2015," Ms. Tavenner said. "And I urge all of you to do everything you can to meet the Stage 2 requirements this year."
[email protected]
On Twitter @aliciaault
AT HIMSS14
Sizzle magnets: a worrisome buzz in the emergency department
NAPLES, FLA. – They sizzle, they buzz, they vibrate. And they’re sending some children to the emergency department.
"One of the newest things we’re seeing a fair amount of are magnets, those sizzle magnets," reported Karen Macauley, DHA, R.N., trauma program director, All Children’s Hospital, St. Petersburg, Fla., and secretary of the Society of Trauma Nurses.
So-called "sizzle" magnets make a sound when they connect and are being sold as novelty toys, jewelry, and even for purported health benefits. One website specializing in gifts for the visually impaired notes that the magnetic field of these hematite magnets is "so strong," they’ll stay put if placed on the front and back of hands or ear lobes.
That strong magnetic force, however, can create havoc if children ingest magnets of any kind, Dr. Macauley said at the annual scientific assembly of the Eastern Association for the Surgery of Trauma.
"If the magnets stay in a string, they’re probably okay; but if they swallow one and wait a little while and swallow another, you can start to imagine what happens as it starts to pass through," she said. "One magnet stays, another comes in another part of the bowel, and they sizzle together. Now all of a sudden you have magnets that aren’t moving and cause a lot of necrosis. They’re actually very dangerous, and we don’t think too much about how dangerous they can be."
Between 2009 and 2011, an estimated 1,700 emergency department visits occurred because of magnet ingestion, with more than 70% of those cases involving children between age 4 and 12 years, according to researchers at the U.S. Public Interest Research Group, which highlighted magnets in its most recent annual toy safety survey.
Dr. Macauley also cautioned that button-size batteries, now in everything from remote controls to Grandma’s singing greeting card and hearing aid, are also a concern because they can cause burns or erode through tissue if ingested or put into body orifices.
"These batteries are everywhere, and kids do crazy stuff with them," she said.
The National Capital Poison Center, which operates a 24-hour National Battery Ingestion Hotline (202-625-3333)* for swallowed battery cases, estimates 3,500 Americans of all ages swallow miniature disc or button batteries each year.
The problem has been recognized for some time, but what few parents or providers realize is how quickly burns can occur, Dr. Macauley said.
She described a case involving a 6-year-old girl who picked up a small battery off the playground and amazed her friends by making it disappear in her ear. The child complained of severe pain overnight and presented to the emergency department in the morning, where surgery to remove the battery revealed third-degree burns to the ear canal and 65% perforation of the eardrum.
"It seemed like it should have been nothing; it was in there maybe 12 hours, but she had very severe injuries to her ear," Dr. Macauley said. "She’s been in the operating room probably a total of six different times trying to get that repaired, has a fair amount of hearing loss that stays, and ended up at a specialist hospital, out of network, to get a graft put in that ear."
Greeting card makers such as Hallmark have taken steps to secure button batteries, such as enclosing them underneath metal caps or in modules in which the cap is secured with screws. And the card makers warn consumers to properly dispose of old batteries after they’ve been replaced.
Dr. Macauley reported having no financial disclosures.
Correction, 3/5/2014: An earlier version of this article misstated the phone number for the Battery Ingestion hotline.
NAPLES, FLA. – They sizzle, they buzz, they vibrate. And they’re sending some children to the emergency department.
"One of the newest things we’re seeing a fair amount of are magnets, those sizzle magnets," reported Karen Macauley, DHA, R.N., trauma program director, All Children’s Hospital, St. Petersburg, Fla., and secretary of the Society of Trauma Nurses.
So-called "sizzle" magnets make a sound when they connect and are being sold as novelty toys, jewelry, and even for purported health benefits. One website specializing in gifts for the visually impaired notes that the magnetic field of these hematite magnets is "so strong," they’ll stay put if placed on the front and back of hands or ear lobes.
That strong magnetic force, however, can create havoc if children ingest magnets of any kind, Dr. Macauley said at the annual scientific assembly of the Eastern Association for the Surgery of Trauma.
"If the magnets stay in a string, they’re probably okay; but if they swallow one and wait a little while and swallow another, you can start to imagine what happens as it starts to pass through," she said. "One magnet stays, another comes in another part of the bowel, and they sizzle together. Now all of a sudden you have magnets that aren’t moving and cause a lot of necrosis. They’re actually very dangerous, and we don’t think too much about how dangerous they can be."
Between 2009 and 2011, an estimated 1,700 emergency department visits occurred because of magnet ingestion, with more than 70% of those cases involving children between age 4 and 12 years, according to researchers at the U.S. Public Interest Research Group, which highlighted magnets in its most recent annual toy safety survey.
Dr. Macauley also cautioned that button-size batteries, now in everything from remote controls to Grandma’s singing greeting card and hearing aid, are also a concern because they can cause burns or erode through tissue if ingested or put into body orifices.
"These batteries are everywhere, and kids do crazy stuff with them," she said.
The National Capital Poison Center, which operates a 24-hour National Battery Ingestion Hotline (202-625-3333)* for swallowed battery cases, estimates 3,500 Americans of all ages swallow miniature disc or button batteries each year.
The problem has been recognized for some time, but what few parents or providers realize is how quickly burns can occur, Dr. Macauley said.
She described a case involving a 6-year-old girl who picked up a small battery off the playground and amazed her friends by making it disappear in her ear. The child complained of severe pain overnight and presented to the emergency department in the morning, where surgery to remove the battery revealed third-degree burns to the ear canal and 65% perforation of the eardrum.
"It seemed like it should have been nothing; it was in there maybe 12 hours, but she had very severe injuries to her ear," Dr. Macauley said. "She’s been in the operating room probably a total of six different times trying to get that repaired, has a fair amount of hearing loss that stays, and ended up at a specialist hospital, out of network, to get a graft put in that ear."
Greeting card makers such as Hallmark have taken steps to secure button batteries, such as enclosing them underneath metal caps or in modules in which the cap is secured with screws. And the card makers warn consumers to properly dispose of old batteries after they’ve been replaced.
Dr. Macauley reported having no financial disclosures.
Correction, 3/5/2014: An earlier version of this article misstated the phone number for the Battery Ingestion hotline.
NAPLES, FLA. – They sizzle, they buzz, they vibrate. And they’re sending some children to the emergency department.
"One of the newest things we’re seeing a fair amount of are magnets, those sizzle magnets," reported Karen Macauley, DHA, R.N., trauma program director, All Children’s Hospital, St. Petersburg, Fla., and secretary of the Society of Trauma Nurses.
So-called "sizzle" magnets make a sound when they connect and are being sold as novelty toys, jewelry, and even for purported health benefits. One website specializing in gifts for the visually impaired notes that the magnetic field of these hematite magnets is "so strong," they’ll stay put if placed on the front and back of hands or ear lobes.
That strong magnetic force, however, can create havoc if children ingest magnets of any kind, Dr. Macauley said at the annual scientific assembly of the Eastern Association for the Surgery of Trauma.
"If the magnets stay in a string, they’re probably okay; but if they swallow one and wait a little while and swallow another, you can start to imagine what happens as it starts to pass through," she said. "One magnet stays, another comes in another part of the bowel, and they sizzle together. Now all of a sudden you have magnets that aren’t moving and cause a lot of necrosis. They’re actually very dangerous, and we don’t think too much about how dangerous they can be."
Between 2009 and 2011, an estimated 1,700 emergency department visits occurred because of magnet ingestion, with more than 70% of those cases involving children between age 4 and 12 years, according to researchers at the U.S. Public Interest Research Group, which highlighted magnets in its most recent annual toy safety survey.
Dr. Macauley also cautioned that button-size batteries, now in everything from remote controls to Grandma’s singing greeting card and hearing aid, are also a concern because they can cause burns or erode through tissue if ingested or put into body orifices.
"These batteries are everywhere, and kids do crazy stuff with them," she said.
The National Capital Poison Center, which operates a 24-hour National Battery Ingestion Hotline (202-625-3333)* for swallowed battery cases, estimates 3,500 Americans of all ages swallow miniature disc or button batteries each year.
The problem has been recognized for some time, but what few parents or providers realize is how quickly burns can occur, Dr. Macauley said.
She described a case involving a 6-year-old girl who picked up a small battery off the playground and amazed her friends by making it disappear in her ear. The child complained of severe pain overnight and presented to the emergency department in the morning, where surgery to remove the battery revealed third-degree burns to the ear canal and 65% perforation of the eardrum.
"It seemed like it should have been nothing; it was in there maybe 12 hours, but she had very severe injuries to her ear," Dr. Macauley said. "She’s been in the operating room probably a total of six different times trying to get that repaired, has a fair amount of hearing loss that stays, and ended up at a specialist hospital, out of network, to get a graft put in that ear."
Greeting card makers such as Hallmark have taken steps to secure button batteries, such as enclosing them underneath metal caps or in modules in which the cap is secured with screws. And the card makers warn consumers to properly dispose of old batteries after they’ve been replaced.
Dr. Macauley reported having no financial disclosures.
Correction, 3/5/2014: An earlier version of this article misstated the phone number for the Battery Ingestion hotline.
AT EAST 2014
Stage 2 of meaningful use: Expect tougher objectives, pre-payment audits
ORLANDO – Expect a more rigorous process of certification for Stage 2 of meaningful use and be prepared for audits that will withhold incentive payments until all issues are resolved, federal officials advised.
This year is the first that physicians can work to meet the requirements for Stage 2 of meaningful use. Stage 1 was about data capture, but Stage 2 is about sharing information across care settings and between patients and providers, Robert Anthony, deputy director of the Health IT Initiatives Group at the Centers for Medicare and Medicaid Services’ Office of E-Health Standards and Services, said at the annual meeting of the Healthcare Information and Management Systems Society.
Among the core requirements for Stage 2 are establishing a patient portal and ensuring and using secure messaging between patients and providers. Physicians must be able to link to imaging results, report to a registry, and record electronic progress notes.
Mr. Anthony said that CMS purposely set what he called a "low bar" for patient engagement in Stage 2. To demonstrate meaningful use, 5% of patients must use the practice’s patient portal and 5% must participate in secure messaging beyond appointment booking, he said.
Even so, meeting that target might not be easy. Mr. Anthony said that he had heard from some physicians that they are sitting down with patients at the end of a visit and walking them through use of the portal or the messaging process. Those encounters, he added, can be counted toward the target.
Requirements for documenting care transitions are stricter as well, Mr. Anthony said.
"In Stage 1, ‘transitions of care’ was a menu objective, and virtually none of you selected transitions of care," he said. "We know why everybody didn’t select it – because it’s a difficult objective to achieve."
Care transitions, though, are "the Holy Grail" for showing that systems and clinicians can talk to each other, so the measure was moved into the core objectives for stage 2, he said.
Under Stage 1, physicians could send a summary of care to the next provider by any method, as long as it arrived, said Mr. Anthony. Under Stage 2, summaries must be electronically transmitted 10% or more of the time. At least some have to be sent to clinicians using a different electronic health record system.
Finally, CMS will be performing more audits under Stage 2. The agency must be accountable for the $21 billion spent on incentive payments so far, he said. In stage 1, the audits for Medicare were primarily post payment. Now, payments will be withheld until the audits are resolved. Some 5%-10% of physicians will be subject to an audit. They will be chosen at random or through risk profiling.
And, "no, we’re not going to talk about what raises a red flag, because that’s the point of an oversight program," he added.
So far, documentation has been the primary deficiency found through the audit process, Mr. Anthony said.
"I am shocked by the number of people who do not retain any documentation related to their attestation figures," he said. Physicians need to document the numerators and denominators they use for attestation and then keep that documentation for 6 years.
There are no consultants or companies that have any special knowledge about how to avoid audits or resolve an audit more quickly. They also don’t know anything special about the appeals process. "The process is the same for everybody and is transparent and public for everybody," he said.
"Anybody who is telling you separately that they have some kind of inside line to CMS is drumming up business and nothing more," Mr. Anthony said.
He also said that there is no secret process to getting an appeal. The agency spells out what it is looking for in the appeals documentation available on the CMS ICD-10 website. There’s also a sample audit letter from the CMS contractor, Figliozzi and Co.
On Twitter @aliciaault
ORLANDO – Expect a more rigorous process of certification for Stage 2 of meaningful use and be prepared for audits that will withhold incentive payments until all issues are resolved, federal officials advised.
This year is the first that physicians can work to meet the requirements for Stage 2 of meaningful use. Stage 1 was about data capture, but Stage 2 is about sharing information across care settings and between patients and providers, Robert Anthony, deputy director of the Health IT Initiatives Group at the Centers for Medicare and Medicaid Services’ Office of E-Health Standards and Services, said at the annual meeting of the Healthcare Information and Management Systems Society.
Among the core requirements for Stage 2 are establishing a patient portal and ensuring and using secure messaging between patients and providers. Physicians must be able to link to imaging results, report to a registry, and record electronic progress notes.
Mr. Anthony said that CMS purposely set what he called a "low bar" for patient engagement in Stage 2. To demonstrate meaningful use, 5% of patients must use the practice’s patient portal and 5% must participate in secure messaging beyond appointment booking, he said.
Even so, meeting that target might not be easy. Mr. Anthony said that he had heard from some physicians that they are sitting down with patients at the end of a visit and walking them through use of the portal or the messaging process. Those encounters, he added, can be counted toward the target.
Requirements for documenting care transitions are stricter as well, Mr. Anthony said.
"In Stage 1, ‘transitions of care’ was a menu objective, and virtually none of you selected transitions of care," he said. "We know why everybody didn’t select it – because it’s a difficult objective to achieve."
Care transitions, though, are "the Holy Grail" for showing that systems and clinicians can talk to each other, so the measure was moved into the core objectives for stage 2, he said.
Under Stage 1, physicians could send a summary of care to the next provider by any method, as long as it arrived, said Mr. Anthony. Under Stage 2, summaries must be electronically transmitted 10% or more of the time. At least some have to be sent to clinicians using a different electronic health record system.
Finally, CMS will be performing more audits under Stage 2. The agency must be accountable for the $21 billion spent on incentive payments so far, he said. In stage 1, the audits for Medicare were primarily post payment. Now, payments will be withheld until the audits are resolved. Some 5%-10% of physicians will be subject to an audit. They will be chosen at random or through risk profiling.
And, "no, we’re not going to talk about what raises a red flag, because that’s the point of an oversight program," he added.
So far, documentation has been the primary deficiency found through the audit process, Mr. Anthony said.
"I am shocked by the number of people who do not retain any documentation related to their attestation figures," he said. Physicians need to document the numerators and denominators they use for attestation and then keep that documentation for 6 years.
There are no consultants or companies that have any special knowledge about how to avoid audits or resolve an audit more quickly. They also don’t know anything special about the appeals process. "The process is the same for everybody and is transparent and public for everybody," he said.
"Anybody who is telling you separately that they have some kind of inside line to CMS is drumming up business and nothing more," Mr. Anthony said.
He also said that there is no secret process to getting an appeal. The agency spells out what it is looking for in the appeals documentation available on the CMS ICD-10 website. There’s also a sample audit letter from the CMS contractor, Figliozzi and Co.
On Twitter @aliciaault
ORLANDO – Expect a more rigorous process of certification for Stage 2 of meaningful use and be prepared for audits that will withhold incentive payments until all issues are resolved, federal officials advised.
This year is the first that physicians can work to meet the requirements for Stage 2 of meaningful use. Stage 1 was about data capture, but Stage 2 is about sharing information across care settings and between patients and providers, Robert Anthony, deputy director of the Health IT Initiatives Group at the Centers for Medicare and Medicaid Services’ Office of E-Health Standards and Services, said at the annual meeting of the Healthcare Information and Management Systems Society.
Among the core requirements for Stage 2 are establishing a patient portal and ensuring and using secure messaging between patients and providers. Physicians must be able to link to imaging results, report to a registry, and record electronic progress notes.
Mr. Anthony said that CMS purposely set what he called a "low bar" for patient engagement in Stage 2. To demonstrate meaningful use, 5% of patients must use the practice’s patient portal and 5% must participate in secure messaging beyond appointment booking, he said.
Even so, meeting that target might not be easy. Mr. Anthony said that he had heard from some physicians that they are sitting down with patients at the end of a visit and walking them through use of the portal or the messaging process. Those encounters, he added, can be counted toward the target.
Requirements for documenting care transitions are stricter as well, Mr. Anthony said.
"In Stage 1, ‘transitions of care’ was a menu objective, and virtually none of you selected transitions of care," he said. "We know why everybody didn’t select it – because it’s a difficult objective to achieve."
Care transitions, though, are "the Holy Grail" for showing that systems and clinicians can talk to each other, so the measure was moved into the core objectives for stage 2, he said.
Under Stage 1, physicians could send a summary of care to the next provider by any method, as long as it arrived, said Mr. Anthony. Under Stage 2, summaries must be electronically transmitted 10% or more of the time. At least some have to be sent to clinicians using a different electronic health record system.
Finally, CMS will be performing more audits under Stage 2. The agency must be accountable for the $21 billion spent on incentive payments so far, he said. In stage 1, the audits for Medicare were primarily post payment. Now, payments will be withheld until the audits are resolved. Some 5%-10% of physicians will be subject to an audit. They will be chosen at random or through risk profiling.
And, "no, we’re not going to talk about what raises a red flag, because that’s the point of an oversight program," he added.
So far, documentation has been the primary deficiency found through the audit process, Mr. Anthony said.
"I am shocked by the number of people who do not retain any documentation related to their attestation figures," he said. Physicians need to document the numerators and denominators they use for attestation and then keep that documentation for 6 years.
There are no consultants or companies that have any special knowledge about how to avoid audits or resolve an audit more quickly. They also don’t know anything special about the appeals process. "The process is the same for everybody and is transparent and public for everybody," he said.
"Anybody who is telling you separately that they have some kind of inside line to CMS is drumming up business and nothing more," Mr. Anthony said.
He also said that there is no secret process to getting an appeal. The agency spells out what it is looking for in the appeals documentation available on the CMS ICD-10 website. There’s also a sample audit letter from the CMS contractor, Figliozzi and Co.
On Twitter @aliciaault
AT HIMSS14
Prior resection poses certain risks in lung transplantation
ORLANDO – Prior lung resection is associated with increased early mortality and with a more than twofold increased risk of renal failure requiring dialysis in lung transplant recipients, data from the United Network for Organ Sharing suggest.
Prior resection is not, however, associated with increased long-term mortality, prolonged hospital length of stay, or airway dehiscence, Dr. Asvin M. Ganapathi reported at the annual meeting of the Society of Thoracic Surgeons.
Of 15,300 adult lung transplant recipients in the UNOS database who received lungs between October 1999 and December 2011, 80 had a prior lobectomy and 22 had a prior pneumonectomy. After 3:1 propensity matching based on 17 recipient variables known to affect perioperative morbidity and mortality, 90-day mortality in 306 nonresection patients was 5.8%, compared with 13.9% in the 102 with prior resection. Renal failure requiring dialysis occurred in 6.6% and 13.9% of patients in the groups, respectively, said Dr. Ganapathi of the anesthesiology division of Duke University, Durham, N.C.
Hospital length of stay longer than 25 days was required in 36.9% and 36.4% of the nonresection and resection groups, respectively, and airway dehiscence occurred in 1.3% and 2%, he said.
Survival at 1 and 5 years was 83.6% and 48.4% in the nonresection group, and 78.9% and 45.6% in the prior-resection group.
A subanalysis comparing the prior-lobectomy patients with patients with no prior resection revealed no differences between the two groups in any of the examined outcomes, although there was a trend toward increased 90-day mortality in the lobectomy patient, and a more than twofold increase in renal failure requiring dialysis. A subanalysis comparing those with prior pneumonectomy with those with no prior resection showed a significantly greater need for dialysis in the pneumonectomy patients (8.9% vs. 3.8%), and a trend toward increased 90-day mortality in the pneumonectomy patients, but no differences in the other examined outcomes.
The propensity-matched groups were similar with respect to recipient, donor, and operative characteristics. There were 10 double and 12 single lung transplants after pneumonectomy, and 51 double and 29 single transplants after lobectomy.
Lung transplantation provides a durable, efficacious treatment for end-stage lung disease, but indications for transplant can vary, and as a result, a select group of patients may have had prior lung resection for treatment of their underlying disease, Dr. Ganapathi said.
Both lobectomy and pneumonectomy are known to cause anatomic changes such as mediastinal shift and vascular abnormalities.
"As such, a history of previous lung resection may affect selection of donor organs and increase the difficulty of transplantation," he said.
In fact, historically, prior thoracic surgery was considered a relative contraindication to lung transplantation because of increased risk of poor outcomes, but recent case reports and single-institution case series – though limited by small patient numbers and the inclusion of both major and minor thoracic procedures ranging from chest tube insertion to pneumonectomy – have suggested that transplantation is feasible in these patients, he said.
Studies specifically looking at lung transplant after resection are lacking; the largest series involved pneumonectomy and included only 14 patients, he said.
The current findings suggest that prior resection should not preclude lung transplantation.
Notable limits of the study include its retrospective design, which may have introduced unexpected bias into the analysis; the fact that the analysis is limited to the variable collected for the UNOS database; and the number of patients with prior resection, although this may be secondary to underreporting of prior thoracic procedures in the UNOS candidate registration form or a result of data being collected only from 1999 onward.
Other variables that may have been of interest for the current analysis were knowledge of laterality of prior resection, time from resection to treatment, days of postoperative ventilator use, and operative time, Dr. Ganapathi noted. Specifically, in cases of single lung transplant, the issue of laterality would be of great interest, he said.
"In conclusion, lung transplantation subsequent to previous major lung resection is associated with an increased risk of early mortality, but did not demonstrate any significant long-term survival differences. Additionally, prior major lung resection predisposes to increased morbidity in the form of renal failure requiring dialysis," he said. The increased rate of dialysis may be secondary to longer operative time, the need for cardiopulmonary bypass, or other unquantified factors, he added.
Careful, individualized preoperative recipient evaluation and technical planning are necessary to minimize these risks in the patients, he concluded.
Dr. Ganapathi reported having no relevant disclosures.
ORLANDO – Prior lung resection is associated with increased early mortality and with a more than twofold increased risk of renal failure requiring dialysis in lung transplant recipients, data from the United Network for Organ Sharing suggest.
Prior resection is not, however, associated with increased long-term mortality, prolonged hospital length of stay, or airway dehiscence, Dr. Asvin M. Ganapathi reported at the annual meeting of the Society of Thoracic Surgeons.
Of 15,300 adult lung transplant recipients in the UNOS database who received lungs between October 1999 and December 2011, 80 had a prior lobectomy and 22 had a prior pneumonectomy. After 3:1 propensity matching based on 17 recipient variables known to affect perioperative morbidity and mortality, 90-day mortality in 306 nonresection patients was 5.8%, compared with 13.9% in the 102 with prior resection. Renal failure requiring dialysis occurred in 6.6% and 13.9% of patients in the groups, respectively, said Dr. Ganapathi of the anesthesiology division of Duke University, Durham, N.C.
Hospital length of stay longer than 25 days was required in 36.9% and 36.4% of the nonresection and resection groups, respectively, and airway dehiscence occurred in 1.3% and 2%, he said.
Survival at 1 and 5 years was 83.6% and 48.4% in the nonresection group, and 78.9% and 45.6% in the prior-resection group.
A subanalysis comparing the prior-lobectomy patients with patients with no prior resection revealed no differences between the two groups in any of the examined outcomes, although there was a trend toward increased 90-day mortality in the lobectomy patient, and a more than twofold increase in renal failure requiring dialysis. A subanalysis comparing those with prior pneumonectomy with those with no prior resection showed a significantly greater need for dialysis in the pneumonectomy patients (8.9% vs. 3.8%), and a trend toward increased 90-day mortality in the pneumonectomy patients, but no differences in the other examined outcomes.
The propensity-matched groups were similar with respect to recipient, donor, and operative characteristics. There were 10 double and 12 single lung transplants after pneumonectomy, and 51 double and 29 single transplants after lobectomy.
Lung transplantation provides a durable, efficacious treatment for end-stage lung disease, but indications for transplant can vary, and as a result, a select group of patients may have had prior lung resection for treatment of their underlying disease, Dr. Ganapathi said.
Both lobectomy and pneumonectomy are known to cause anatomic changes such as mediastinal shift and vascular abnormalities.
"As such, a history of previous lung resection may affect selection of donor organs and increase the difficulty of transplantation," he said.
In fact, historically, prior thoracic surgery was considered a relative contraindication to lung transplantation because of increased risk of poor outcomes, but recent case reports and single-institution case series – though limited by small patient numbers and the inclusion of both major and minor thoracic procedures ranging from chest tube insertion to pneumonectomy – have suggested that transplantation is feasible in these patients, he said.
Studies specifically looking at lung transplant after resection are lacking; the largest series involved pneumonectomy and included only 14 patients, he said.
The current findings suggest that prior resection should not preclude lung transplantation.
Notable limits of the study include its retrospective design, which may have introduced unexpected bias into the analysis; the fact that the analysis is limited to the variable collected for the UNOS database; and the number of patients with prior resection, although this may be secondary to underreporting of prior thoracic procedures in the UNOS candidate registration form or a result of data being collected only from 1999 onward.
Other variables that may have been of interest for the current analysis were knowledge of laterality of prior resection, time from resection to treatment, days of postoperative ventilator use, and operative time, Dr. Ganapathi noted. Specifically, in cases of single lung transplant, the issue of laterality would be of great interest, he said.
"In conclusion, lung transplantation subsequent to previous major lung resection is associated with an increased risk of early mortality, but did not demonstrate any significant long-term survival differences. Additionally, prior major lung resection predisposes to increased morbidity in the form of renal failure requiring dialysis," he said. The increased rate of dialysis may be secondary to longer operative time, the need for cardiopulmonary bypass, or other unquantified factors, he added.
Careful, individualized preoperative recipient evaluation and technical planning are necessary to minimize these risks in the patients, he concluded.
Dr. Ganapathi reported having no relevant disclosures.
ORLANDO – Prior lung resection is associated with increased early mortality and with a more than twofold increased risk of renal failure requiring dialysis in lung transplant recipients, data from the United Network for Organ Sharing suggest.
Prior resection is not, however, associated with increased long-term mortality, prolonged hospital length of stay, or airway dehiscence, Dr. Asvin M. Ganapathi reported at the annual meeting of the Society of Thoracic Surgeons.
Of 15,300 adult lung transplant recipients in the UNOS database who received lungs between October 1999 and December 2011, 80 had a prior lobectomy and 22 had a prior pneumonectomy. After 3:1 propensity matching based on 17 recipient variables known to affect perioperative morbidity and mortality, 90-day mortality in 306 nonresection patients was 5.8%, compared with 13.9% in the 102 with prior resection. Renal failure requiring dialysis occurred in 6.6% and 13.9% of patients in the groups, respectively, said Dr. Ganapathi of the anesthesiology division of Duke University, Durham, N.C.
Hospital length of stay longer than 25 days was required in 36.9% and 36.4% of the nonresection and resection groups, respectively, and airway dehiscence occurred in 1.3% and 2%, he said.
Survival at 1 and 5 years was 83.6% and 48.4% in the nonresection group, and 78.9% and 45.6% in the prior-resection group.
A subanalysis comparing the prior-lobectomy patients with patients with no prior resection revealed no differences between the two groups in any of the examined outcomes, although there was a trend toward increased 90-day mortality in the lobectomy patient, and a more than twofold increase in renal failure requiring dialysis. A subanalysis comparing those with prior pneumonectomy with those with no prior resection showed a significantly greater need for dialysis in the pneumonectomy patients (8.9% vs. 3.8%), and a trend toward increased 90-day mortality in the pneumonectomy patients, but no differences in the other examined outcomes.
The propensity-matched groups were similar with respect to recipient, donor, and operative characteristics. There were 10 double and 12 single lung transplants after pneumonectomy, and 51 double and 29 single transplants after lobectomy.
Lung transplantation provides a durable, efficacious treatment for end-stage lung disease, but indications for transplant can vary, and as a result, a select group of patients may have had prior lung resection for treatment of their underlying disease, Dr. Ganapathi said.
Both lobectomy and pneumonectomy are known to cause anatomic changes such as mediastinal shift and vascular abnormalities.
"As such, a history of previous lung resection may affect selection of donor organs and increase the difficulty of transplantation," he said.
In fact, historically, prior thoracic surgery was considered a relative contraindication to lung transplantation because of increased risk of poor outcomes, but recent case reports and single-institution case series – though limited by small patient numbers and the inclusion of both major and minor thoracic procedures ranging from chest tube insertion to pneumonectomy – have suggested that transplantation is feasible in these patients, he said.
Studies specifically looking at lung transplant after resection are lacking; the largest series involved pneumonectomy and included only 14 patients, he said.
The current findings suggest that prior resection should not preclude lung transplantation.
Notable limits of the study include its retrospective design, which may have introduced unexpected bias into the analysis; the fact that the analysis is limited to the variable collected for the UNOS database; and the number of patients with prior resection, although this may be secondary to underreporting of prior thoracic procedures in the UNOS candidate registration form or a result of data being collected only from 1999 onward.
Other variables that may have been of interest for the current analysis were knowledge of laterality of prior resection, time from resection to treatment, days of postoperative ventilator use, and operative time, Dr. Ganapathi noted. Specifically, in cases of single lung transplant, the issue of laterality would be of great interest, he said.
"In conclusion, lung transplantation subsequent to previous major lung resection is associated with an increased risk of early mortality, but did not demonstrate any significant long-term survival differences. Additionally, prior major lung resection predisposes to increased morbidity in the form of renal failure requiring dialysis," he said. The increased rate of dialysis may be secondary to longer operative time, the need for cardiopulmonary bypass, or other unquantified factors, he added.
Careful, individualized preoperative recipient evaluation and technical planning are necessary to minimize these risks in the patients, he concluded.
Dr. Ganapathi reported having no relevant disclosures.
AT THE STS ANNUAL MEETING
Major finding: Survival at 1 and 5 years was 83.6% and 48.4%, respectively, in the nonresection group, and 78.9% and 45.6% in the prior-resection group.
Data source: An analysis of data from more than 15,000 patients in the United Network of Organ Sharing database.
Disclosures: Dr. Ganapathi reported having no relevant disclosures.
VIDEO: Traumatic injury ups stroke risk in people under 50
SAN DIEGO – Eleven of every 100,000 patients younger than 50 years who were seen for traumatic injury to the head or neck developed a stroke within a month, a study of 1.3 million found. Trauma can tear blood vessels that lead to the brain and cause blood clots resulting in ischemic stroke. A total of 10% of patients in the study who developed a stroke were diagnosed with tears in blood vessels leading to the brain, but some of these arterial dissections were diagnosed after the stroke occurred.
Dr. Bruce Ovbiagele spoke with us about the significance of the findings for both young adults and children who experience physical trauma.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – Eleven of every 100,000 patients younger than 50 years who were seen for traumatic injury to the head or neck developed a stroke within a month, a study of 1.3 million found. Trauma can tear blood vessels that lead to the brain and cause blood clots resulting in ischemic stroke. A total of 10% of patients in the study who developed a stroke were diagnosed with tears in blood vessels leading to the brain, but some of these arterial dissections were diagnosed after the stroke occurred.
Dr. Bruce Ovbiagele spoke with us about the significance of the findings for both young adults and children who experience physical trauma.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – Eleven of every 100,000 patients younger than 50 years who were seen for traumatic injury to the head or neck developed a stroke within a month, a study of 1.3 million found. Trauma can tear blood vessels that lead to the brain and cause blood clots resulting in ischemic stroke. A total of 10% of patients in the study who developed a stroke were diagnosed with tears in blood vessels leading to the brain, but some of these arterial dissections were diagnosed after the stroke occurred.
Dr. Bruce Ovbiagele spoke with us about the significance of the findings for both young adults and children who experience physical trauma.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE INTERNATIONAL STROKE CONFERENCE
Doctors seek to delay 2014 meaningful use deadlines
ORLANDO – Several dozen physician organizations are imploring the Department of Health and Human Services to slow down implementation of the meaningful use program for electronic health records, saying that the pace is overwhelming doctors’ ability to keep up.
The request comes as more than 35,500 attendees gather for the annual meeting of the Healthcare Information and Management Systems Society to learn more about government and private efforts to digitize the U.S. health care system.
In a letter to HHS Secretary Kathleen Sebelius, the physician groups said that doctors are struggling to make the investments needed to meet the federal targets. The groups want the government to give physicians through 2015 to transition to new EHR software and to meet stage 1 and 2 meaningful use requirements. They are also seeking more flexibility in how physicians meet those requirements.
Under the meaningful use program, eligible providers – physicians, nurse practitioners, and physician assistants who meet specific criteria – can receive incentive payments from Medicare and Medicaid. In 2014, the Medicare payment can be up to $23,520.
Centers for Medicare & Medicaid Services (CMS) officials at the HIMSS meeting said that 267,029 eligible providers have received Medicare incentive payments and 142,801 have received Medicaid payments since the program began in 2011, amounting to some $21 billion in payouts.
Providers will be penalized in 2015 if they do not sign up to be a meaningful user by March 31, said Elizabeth Holland, director of the HIT initiatives group at the CMS Office of E-Health Standards and Services. That date was recently extended by a month. Doctors who have never participated in the meaningful use program have until Oct. 1 to sign up to avoid penalties next year, and many physicians may be eligible for hardship exemptions that give them even more time to sign up, Ms. Holland said.
The physician groups still want more leeway. A big sticking point: Currently, eligible professionals have to adopt the 2014 Edition of Certified Electronic Health Record Technology (CEHRT) and also meet more stringent meaningful use standards by the end of the year. Most providers are still working with the last edition – the 2011 CEHRT. The Office of the National Coordinator for Health Information Technology (ONC) created the CEHRT program to guide providers to EHRs that contain all the elements necessary to achieve meaningful use. The 2014 edition supports stage 2 of meaningful use, which started this year.
"With only a fraction of 2011 Edition products currently certified to 2014 Edition standards, it is clear the pace and scope of change have outstripped the ability of vendors to support providers," according to the letter from the physician groups. If doctors can’t easily move on to the 2014 edition, they might "either abandon the possibility of meeting meaningful use criteria in 2014 or be forced to implement a system much more rapidly than would otherwise be the case."
While physicians worry about the 2014 edition, the ONC on Feb. 21 proposed to move forward again, spelling out standards for the 2015 edition of the CEHRT.
The ONC said that compliance with the 2015 edition would be voluntary – providers would not have to upgrade to be able to get incentive payments.
"This provides the opportunity for developers and health care providers to move to the 2015 Edition on their own terms and at their own pace," Dr. Karen DeSalvo, national coordinator for health IT, said in a statement.
The rule on the 2015 edition will be published in the Federal Register on Feb. 26, and the ONC will take comments at www.regulations.gov until April 28. The final rule will be published this summer.
On Twitter @aliciaault
ORLANDO – Several dozen physician organizations are imploring the Department of Health and Human Services to slow down implementation of the meaningful use program for electronic health records, saying that the pace is overwhelming doctors’ ability to keep up.
The request comes as more than 35,500 attendees gather for the annual meeting of the Healthcare Information and Management Systems Society to learn more about government and private efforts to digitize the U.S. health care system.
In a letter to HHS Secretary Kathleen Sebelius, the physician groups said that doctors are struggling to make the investments needed to meet the federal targets. The groups want the government to give physicians through 2015 to transition to new EHR software and to meet stage 1 and 2 meaningful use requirements. They are also seeking more flexibility in how physicians meet those requirements.
Under the meaningful use program, eligible providers – physicians, nurse practitioners, and physician assistants who meet specific criteria – can receive incentive payments from Medicare and Medicaid. In 2014, the Medicare payment can be up to $23,520.
Centers for Medicare & Medicaid Services (CMS) officials at the HIMSS meeting said that 267,029 eligible providers have received Medicare incentive payments and 142,801 have received Medicaid payments since the program began in 2011, amounting to some $21 billion in payouts.
Providers will be penalized in 2015 if they do not sign up to be a meaningful user by March 31, said Elizabeth Holland, director of the HIT initiatives group at the CMS Office of E-Health Standards and Services. That date was recently extended by a month. Doctors who have never participated in the meaningful use program have until Oct. 1 to sign up to avoid penalties next year, and many physicians may be eligible for hardship exemptions that give them even more time to sign up, Ms. Holland said.
The physician groups still want more leeway. A big sticking point: Currently, eligible professionals have to adopt the 2014 Edition of Certified Electronic Health Record Technology (CEHRT) and also meet more stringent meaningful use standards by the end of the year. Most providers are still working with the last edition – the 2011 CEHRT. The Office of the National Coordinator for Health Information Technology (ONC) created the CEHRT program to guide providers to EHRs that contain all the elements necessary to achieve meaningful use. The 2014 edition supports stage 2 of meaningful use, which started this year.
"With only a fraction of 2011 Edition products currently certified to 2014 Edition standards, it is clear the pace and scope of change have outstripped the ability of vendors to support providers," according to the letter from the physician groups. If doctors can’t easily move on to the 2014 edition, they might "either abandon the possibility of meeting meaningful use criteria in 2014 or be forced to implement a system much more rapidly than would otherwise be the case."
While physicians worry about the 2014 edition, the ONC on Feb. 21 proposed to move forward again, spelling out standards for the 2015 edition of the CEHRT.
The ONC said that compliance with the 2015 edition would be voluntary – providers would not have to upgrade to be able to get incentive payments.
"This provides the opportunity for developers and health care providers to move to the 2015 Edition on their own terms and at their own pace," Dr. Karen DeSalvo, national coordinator for health IT, said in a statement.
The rule on the 2015 edition will be published in the Federal Register on Feb. 26, and the ONC will take comments at www.regulations.gov until April 28. The final rule will be published this summer.
On Twitter @aliciaault
ORLANDO – Several dozen physician organizations are imploring the Department of Health and Human Services to slow down implementation of the meaningful use program for electronic health records, saying that the pace is overwhelming doctors’ ability to keep up.
The request comes as more than 35,500 attendees gather for the annual meeting of the Healthcare Information and Management Systems Society to learn more about government and private efforts to digitize the U.S. health care system.
In a letter to HHS Secretary Kathleen Sebelius, the physician groups said that doctors are struggling to make the investments needed to meet the federal targets. The groups want the government to give physicians through 2015 to transition to new EHR software and to meet stage 1 and 2 meaningful use requirements. They are also seeking more flexibility in how physicians meet those requirements.
Under the meaningful use program, eligible providers – physicians, nurse practitioners, and physician assistants who meet specific criteria – can receive incentive payments from Medicare and Medicaid. In 2014, the Medicare payment can be up to $23,520.
Centers for Medicare & Medicaid Services (CMS) officials at the HIMSS meeting said that 267,029 eligible providers have received Medicare incentive payments and 142,801 have received Medicaid payments since the program began in 2011, amounting to some $21 billion in payouts.
Providers will be penalized in 2015 if they do not sign up to be a meaningful user by March 31, said Elizabeth Holland, director of the HIT initiatives group at the CMS Office of E-Health Standards and Services. That date was recently extended by a month. Doctors who have never participated in the meaningful use program have until Oct. 1 to sign up to avoid penalties next year, and many physicians may be eligible for hardship exemptions that give them even more time to sign up, Ms. Holland said.
The physician groups still want more leeway. A big sticking point: Currently, eligible professionals have to adopt the 2014 Edition of Certified Electronic Health Record Technology (CEHRT) and also meet more stringent meaningful use standards by the end of the year. Most providers are still working with the last edition – the 2011 CEHRT. The Office of the National Coordinator for Health Information Technology (ONC) created the CEHRT program to guide providers to EHRs that contain all the elements necessary to achieve meaningful use. The 2014 edition supports stage 2 of meaningful use, which started this year.
"With only a fraction of 2011 Edition products currently certified to 2014 Edition standards, it is clear the pace and scope of change have outstripped the ability of vendors to support providers," according to the letter from the physician groups. If doctors can’t easily move on to the 2014 edition, they might "either abandon the possibility of meeting meaningful use criteria in 2014 or be forced to implement a system much more rapidly than would otherwise be the case."
While physicians worry about the 2014 edition, the ONC on Feb. 21 proposed to move forward again, spelling out standards for the 2015 edition of the CEHRT.
The ONC said that compliance with the 2015 edition would be voluntary – providers would not have to upgrade to be able to get incentive payments.
"This provides the opportunity for developers and health care providers to move to the 2015 Edition on their own terms and at their own pace," Dr. Karen DeSalvo, national coordinator for health IT, said in a statement.
The rule on the 2015 edition will be published in the Federal Register on Feb. 26, and the ONC will take comments at www.regulations.gov until April 28. The final rule will be published this summer.
On Twitter @aliciaault
AT HIMSS14