User login
Hair loss affects more than half of postmenopausal women
Female-pattern hair loss (FPHL) was identified in 52% of postmenopausal women, and 4% of these cases involved extensive baldness, based on data from 178 individuals.
FPHL can develop at any time from teenage years through and beyond menopause, wrote Sukanya Chaikittisilpa, MD, of Chulalongkorn University, Bangkok, and colleagues.
The cause of FPHL remains uncertain, but the presence of estrogen receptors in hair follicles suggests that the hormone changes of menopause may affect hair growth, the researchers said.
In a study published in Menopause, the researchers evaluated 178 postmenopausal women aged 50-65 years for FPHL. FPLH was determined based on photographs and on measures of hormone levels, hair density, and hair diameter.
The overall prevalence of FPHL was 52.2%. The hair loss was divided into three categories indicating mild, moderate, and severe (Ludwig grades I, II, and III) with prevalence of 73.2%, 22.6%, and 4.3%, respectively. The prevalence of FPHL also increased with age and time since menopause. In a simple logistic regression analysis, age 56 years and older and more than 6 years since menopause were significantly associated with FPHL (odds ratios, 3.41 and 1.98, respectively).
However, after adjustment for multiple variables, only a body mass index of 25 kg/m2 or higher also was associated with increased prevalence of FPHL (adjusted OR, 2.65).
A total of 60% of the study participants met criteria for low self-esteem, including all the women in the severe hair loss category.
“The postmenopausal women with FPHL in our cohort had lower total hair density, terminal hair density, hair thickness, hair unit density, and average hair per unit than those with normal hair patterns,” although vellus hair density was higher in women with FPHL, the researchers wrote in their discussion of the findings. This distinction may be caused in part by the shortened hair cycle and reduced anagen phase of velluslike follicles, they said.
The study findings were limited by several factors, including the cross-sectional design and the inclusion of only women from a single menopause clinic, which may not reflect FPHL in the general population, as well as the reliance on patients’ recall, the researchers noted. Another limitation was the inability to assess postmenopausal hormone levels, they added.
However, “This study may be the first FPHL study conducted in a menopause clinic that targeted only healthy postmenopausal women,” they wrote. More research is needed to determine the potential role of estrogen and testosterone on FPHL in postmenopausal women, and whether a history of polycystic ovarian syndrome has an effect, they said. Meanwhile, current study results may help clinicians and patients determine the most appropriate menopausal hormone therapies for postmenopausal women with FPHL, they concluded.
Consider lifestyle and self-esteem issues
The current study is important at this time because a larger proportion of women are either reaching menopause or are menopausal, said Constance Bohon, MD, a gynecologist in private practice in Washington, in an interview.
“Whatever we in the medical community can do to help women transition into the menopausal years with the least anxiety is important,” including helping women feel comfortable about their appearance, she said.
“For women in the peri- and postmenopausal years, hair loss is a relatively common concern,” Dr. Bohon said. However, in the current study, “I was surprised that it was associated with low self-esteem and obesity,” she noted. “For these women, it would be interesting to know whether they also had concerns about the appearance of their bodies, or just their hair loss,” she said. The question is whether the hair loss in and of itself caused low self-esteem in the study population, or whether it exacerbated their already poor self-assessment, Dr. Bohon said. “Another consideration is that perhaps these women were already feeling the effects of aging and were trying to change their appearance by using hair dyes, and now they find themselves losing hair as well,” she noted.
The takeaway message for clinicians is that discussions with perimenopausal and postmenopausal women should include the topic of hair loss along with hot flashes and night sweats, said Dr. Bohon.
Women who are experiencing hair loss or concerned about the possibility of hair loss should ask their doctors about possible interventions that may mitigate or prevent further hair loss, she said.
As for additional research, “the most important issue is to determine the factors that are associated with hair loss in the perimenopausal and postmenopausal years,” Dr. Bohon said. Research questions should include impact of dyeing or straightening hair on the likelihood of hair loss, and whether women with more severe hot flashes/night sweats and/or sleeplessness have more hair loss than women who do not experience any of the symptoms as they go through menopause, she emphasized.
Other considerations are whether certain diets or foods are more common among women who have more hair loss, and whether weight loss into a normal range or weight gain into a body mass index greater than 25 kg/m2 affects hair loss, said Dr. Bohon. Also, don’t discount the impact of stress, and whether women who have lost hair identify certain stressful times that preceded their hair loss, as well as what medications could be associated with hair loss, and whether hormone therapy might prevent hair loss, she said.
The study was supported by the Ratchadapiseksompotch Fund, Faculty of Medicine, Chulalongkorn University. The researchers had no financial conflicts to disclose. Dr. Bohon had no financial conflicts to disclose and serves on the Editorial Advisory Board of Ob.Gyn. News.
Female-pattern hair loss (FPHL) was identified in 52% of postmenopausal women, and 4% of these cases involved extensive baldness, based on data from 178 individuals.
FPHL can develop at any time from teenage years through and beyond menopause, wrote Sukanya Chaikittisilpa, MD, of Chulalongkorn University, Bangkok, and colleagues.
The cause of FPHL remains uncertain, but the presence of estrogen receptors in hair follicles suggests that the hormone changes of menopause may affect hair growth, the researchers said.
In a study published in Menopause, the researchers evaluated 178 postmenopausal women aged 50-65 years for FPHL. FPLH was determined based on photographs and on measures of hormone levels, hair density, and hair diameter.
The overall prevalence of FPHL was 52.2%. The hair loss was divided into three categories indicating mild, moderate, and severe (Ludwig grades I, II, and III) with prevalence of 73.2%, 22.6%, and 4.3%, respectively. The prevalence of FPHL also increased with age and time since menopause. In a simple logistic regression analysis, age 56 years and older and more than 6 years since menopause were significantly associated with FPHL (odds ratios, 3.41 and 1.98, respectively).
However, after adjustment for multiple variables, only a body mass index of 25 kg/m2 or higher also was associated with increased prevalence of FPHL (adjusted OR, 2.65).
A total of 60% of the study participants met criteria for low self-esteem, including all the women in the severe hair loss category.
“The postmenopausal women with FPHL in our cohort had lower total hair density, terminal hair density, hair thickness, hair unit density, and average hair per unit than those with normal hair patterns,” although vellus hair density was higher in women with FPHL, the researchers wrote in their discussion of the findings. This distinction may be caused in part by the shortened hair cycle and reduced anagen phase of velluslike follicles, they said.
The study findings were limited by several factors, including the cross-sectional design and the inclusion of only women from a single menopause clinic, which may not reflect FPHL in the general population, as well as the reliance on patients’ recall, the researchers noted. Another limitation was the inability to assess postmenopausal hormone levels, they added.
However, “This study may be the first FPHL study conducted in a menopause clinic that targeted only healthy postmenopausal women,” they wrote. More research is needed to determine the potential role of estrogen and testosterone on FPHL in postmenopausal women, and whether a history of polycystic ovarian syndrome has an effect, they said. Meanwhile, current study results may help clinicians and patients determine the most appropriate menopausal hormone therapies for postmenopausal women with FPHL, they concluded.
Consider lifestyle and self-esteem issues
The current study is important at this time because a larger proportion of women are either reaching menopause or are menopausal, said Constance Bohon, MD, a gynecologist in private practice in Washington, in an interview.
“Whatever we in the medical community can do to help women transition into the menopausal years with the least anxiety is important,” including helping women feel comfortable about their appearance, she said.
“For women in the peri- and postmenopausal years, hair loss is a relatively common concern,” Dr. Bohon said. However, in the current study, “I was surprised that it was associated with low self-esteem and obesity,” she noted. “For these women, it would be interesting to know whether they also had concerns about the appearance of their bodies, or just their hair loss,” she said. The question is whether the hair loss in and of itself caused low self-esteem in the study population, or whether it exacerbated their already poor self-assessment, Dr. Bohon said. “Another consideration is that perhaps these women were already feeling the effects of aging and were trying to change their appearance by using hair dyes, and now they find themselves losing hair as well,” she noted.
The takeaway message for clinicians is that discussions with perimenopausal and postmenopausal women should include the topic of hair loss along with hot flashes and night sweats, said Dr. Bohon.
Women who are experiencing hair loss or concerned about the possibility of hair loss should ask their doctors about possible interventions that may mitigate or prevent further hair loss, she said.
As for additional research, “the most important issue is to determine the factors that are associated with hair loss in the perimenopausal and postmenopausal years,” Dr. Bohon said. Research questions should include impact of dyeing or straightening hair on the likelihood of hair loss, and whether women with more severe hot flashes/night sweats and/or sleeplessness have more hair loss than women who do not experience any of the symptoms as they go through menopause, she emphasized.
Other considerations are whether certain diets or foods are more common among women who have more hair loss, and whether weight loss into a normal range or weight gain into a body mass index greater than 25 kg/m2 affects hair loss, said Dr. Bohon. Also, don’t discount the impact of stress, and whether women who have lost hair identify certain stressful times that preceded their hair loss, as well as what medications could be associated with hair loss, and whether hormone therapy might prevent hair loss, she said.
The study was supported by the Ratchadapiseksompotch Fund, Faculty of Medicine, Chulalongkorn University. The researchers had no financial conflicts to disclose. Dr. Bohon had no financial conflicts to disclose and serves on the Editorial Advisory Board of Ob.Gyn. News.
Female-pattern hair loss (FPHL) was identified in 52% of postmenopausal women, and 4% of these cases involved extensive baldness, based on data from 178 individuals.
FPHL can develop at any time from teenage years through and beyond menopause, wrote Sukanya Chaikittisilpa, MD, of Chulalongkorn University, Bangkok, and colleagues.
The cause of FPHL remains uncertain, but the presence of estrogen receptors in hair follicles suggests that the hormone changes of menopause may affect hair growth, the researchers said.
In a study published in Menopause, the researchers evaluated 178 postmenopausal women aged 50-65 years for FPHL. FPLH was determined based on photographs and on measures of hormone levels, hair density, and hair diameter.
The overall prevalence of FPHL was 52.2%. The hair loss was divided into three categories indicating mild, moderate, and severe (Ludwig grades I, II, and III) with prevalence of 73.2%, 22.6%, and 4.3%, respectively. The prevalence of FPHL also increased with age and time since menopause. In a simple logistic regression analysis, age 56 years and older and more than 6 years since menopause were significantly associated with FPHL (odds ratios, 3.41 and 1.98, respectively).
However, after adjustment for multiple variables, only a body mass index of 25 kg/m2 or higher also was associated with increased prevalence of FPHL (adjusted OR, 2.65).
A total of 60% of the study participants met criteria for low self-esteem, including all the women in the severe hair loss category.
“The postmenopausal women with FPHL in our cohort had lower total hair density, terminal hair density, hair thickness, hair unit density, and average hair per unit than those with normal hair patterns,” although vellus hair density was higher in women with FPHL, the researchers wrote in their discussion of the findings. This distinction may be caused in part by the shortened hair cycle and reduced anagen phase of velluslike follicles, they said.
The study findings were limited by several factors, including the cross-sectional design and the inclusion of only women from a single menopause clinic, which may not reflect FPHL in the general population, as well as the reliance on patients’ recall, the researchers noted. Another limitation was the inability to assess postmenopausal hormone levels, they added.
However, “This study may be the first FPHL study conducted in a menopause clinic that targeted only healthy postmenopausal women,” they wrote. More research is needed to determine the potential role of estrogen and testosterone on FPHL in postmenopausal women, and whether a history of polycystic ovarian syndrome has an effect, they said. Meanwhile, current study results may help clinicians and patients determine the most appropriate menopausal hormone therapies for postmenopausal women with FPHL, they concluded.
Consider lifestyle and self-esteem issues
The current study is important at this time because a larger proportion of women are either reaching menopause or are menopausal, said Constance Bohon, MD, a gynecologist in private practice in Washington, in an interview.
“Whatever we in the medical community can do to help women transition into the menopausal years with the least anxiety is important,” including helping women feel comfortable about their appearance, she said.
“For women in the peri- and postmenopausal years, hair loss is a relatively common concern,” Dr. Bohon said. However, in the current study, “I was surprised that it was associated with low self-esteem and obesity,” she noted. “For these women, it would be interesting to know whether they also had concerns about the appearance of their bodies, or just their hair loss,” she said. The question is whether the hair loss in and of itself caused low self-esteem in the study population, or whether it exacerbated their already poor self-assessment, Dr. Bohon said. “Another consideration is that perhaps these women were already feeling the effects of aging and were trying to change their appearance by using hair dyes, and now they find themselves losing hair as well,” she noted.
The takeaway message for clinicians is that discussions with perimenopausal and postmenopausal women should include the topic of hair loss along with hot flashes and night sweats, said Dr. Bohon.
Women who are experiencing hair loss or concerned about the possibility of hair loss should ask their doctors about possible interventions that may mitigate or prevent further hair loss, she said.
As for additional research, “the most important issue is to determine the factors that are associated with hair loss in the perimenopausal and postmenopausal years,” Dr. Bohon said. Research questions should include impact of dyeing or straightening hair on the likelihood of hair loss, and whether women with more severe hot flashes/night sweats and/or sleeplessness have more hair loss than women who do not experience any of the symptoms as they go through menopause, she emphasized.
Other considerations are whether certain diets or foods are more common among women who have more hair loss, and whether weight loss into a normal range or weight gain into a body mass index greater than 25 kg/m2 affects hair loss, said Dr. Bohon. Also, don’t discount the impact of stress, and whether women who have lost hair identify certain stressful times that preceded their hair loss, as well as what medications could be associated with hair loss, and whether hormone therapy might prevent hair loss, she said.
The study was supported by the Ratchadapiseksompotch Fund, Faculty of Medicine, Chulalongkorn University. The researchers had no financial conflicts to disclose. Dr. Bohon had no financial conflicts to disclose and serves on the Editorial Advisory Board of Ob.Gyn. News.
FROM MENOPAUSE
Past spontaneous abortion raises risk for gestational diabetes
Pregnant women with a history of spontaneous abortion had a significantly increased risk of gestational diabetes in subsequent pregnancies, based on data from more than 100,000 women.
Gestational diabetes is associated not only with adverse perinatal outcomes, but also with an increased risk of long-term cardiovascular and metabolic health issues in mothers and children, wrote Yan Zhao, PhD, of Tongji University, Shanghai, and colleagues.
Previous studies also have shown that spontaneous abortion (SAB) is associated with later maternal risk of cardiovascular disease and venous thromboembolism, the researchers said. The same mechanisms might contribute to the development of gestational diabetes, but the association between abortion history and gestational diabetes risk in subsequent pregnancies remains unclear, they added.
In a study published in JAMA Network Open, the researchers identified 102,259 pregnant women seen for routine prenatal care at a single hospital in Shanghai between January 2014 and December 2019. The mean age of the women was 29.8 years.
During the study period, 14,579 women experienced SAB (14.3%), 17,935 experienced induced abortion (17.5%), and 4,017 experienced both (11.9%).
In all, 12,153 cases of gestational diabetes were identified, for a prevalence of 11.9%. The relative risk of gestational diabetes was 1.25 for women who experienced SAB and 1.15 for those who experienced both SAB and induced abortion, and the association between SAB and gestational diabetes increased in a number-dependent manner, the researchers said. The increase in relative risk for gestational diabetes in pregnant women with one SAB, two SABs, and three or more SABs was 18%, 41%, and 43%, compared to pregnant women with no SAB history.
However, no association appeared between a history of induced abortion and gestational diabetes, the researchers said. “To date, no study has reported the association of prior induced abortion with gestational diabetes,” they wrote.
The study findings were limited by several factors including the reliance on self-reports for history of SAB and therefore possible underreporting, the researchers noted. Other limitations included the lack of data on the timing of SABs; therefore, the time between SAB and gestational diabetes diagnosis could not be included in the analysis, they said. Unknown variables and the inclusion only of women from a single city in China might limit the generalizability of the results, they added.
More research is needed to understand the biological mechanisms behind the association between SAB and gestational diabetes, an association that has potential public health implications, they noted. However, the results suggest that “pregnant women with a history of SAB, especially those with a history of recurrent SAB, should attend more antenatal visits to monitor their blood glucose and implement early prevention and intervention,” such as healthful eating and regular exercise, they wrote.
Findings confirm, not surprise
The diagnosis of gestational diabetes in the current study “was made with a slightly different test than we typically use in the United States – a 1-hour nonfasting glucola followed by a confirmatory 3-hour fasting glucola,” Sarah W. Prager, MD, of the University of Washington, Seattle, said in an interview. The current study of both SAB and gestational diabetes is important because both conditions are very common and have been the focus of increased attention in the popular media and in scientific study, she said.
Dr. Prager said she was not surprised by the findings of a link between a history of gestational diabetes and a history of SAB, “but the association is likely that people at risk for gestational diabetes or who have undiagnosed diabetes/glucose intolerance are more likely to experience SAB,” she noted. “I would be surprised if the direction of the association is that SAB puts people at risk for gestational diabetes; more likely undiagnosed diabetes is a risk factor for SAB,” she added. “Perhaps we should be screening for glucose intolerance and other metabolic disorders more frequently in people who have especially recurrent SAB, as the more miscarriages someone had, the more likely they were in this study to be diagnosed with gestational diabetes;” or perhaps those with a history of SAB/recurrent SAB should be screened closer to 24 weeks’ than 28 weeks’ gestation to enable earlier intervention in those more likely to have gestational diabetes, Dr. Prager said.
The study was supported by the Key Program of the National Natural Science Foundation of China, the National Natural Science Foundation of China, the National Key Research and Development Program of China, the Shanghai Municipal Medical and Health Discipline Construction Projects, and the Shanghai Rising-Star Program. The researchers and Dr. Prager had no financial conflicts to disclose. Dr. Prager serves on the editorial advisory board of Ob.Gyn. News.
Pregnant women with a history of spontaneous abortion had a significantly increased risk of gestational diabetes in subsequent pregnancies, based on data from more than 100,000 women.
Gestational diabetes is associated not only with adverse perinatal outcomes, but also with an increased risk of long-term cardiovascular and metabolic health issues in mothers and children, wrote Yan Zhao, PhD, of Tongji University, Shanghai, and colleagues.
Previous studies also have shown that spontaneous abortion (SAB) is associated with later maternal risk of cardiovascular disease and venous thromboembolism, the researchers said. The same mechanisms might contribute to the development of gestational diabetes, but the association between abortion history and gestational diabetes risk in subsequent pregnancies remains unclear, they added.
In a study published in JAMA Network Open, the researchers identified 102,259 pregnant women seen for routine prenatal care at a single hospital in Shanghai between January 2014 and December 2019. The mean age of the women was 29.8 years.
During the study period, 14,579 women experienced SAB (14.3%), 17,935 experienced induced abortion (17.5%), and 4,017 experienced both (11.9%).
In all, 12,153 cases of gestational diabetes were identified, for a prevalence of 11.9%. The relative risk of gestational diabetes was 1.25 for women who experienced SAB and 1.15 for those who experienced both SAB and induced abortion, and the association between SAB and gestational diabetes increased in a number-dependent manner, the researchers said. The increase in relative risk for gestational diabetes in pregnant women with one SAB, two SABs, and three or more SABs was 18%, 41%, and 43%, compared to pregnant women with no SAB history.
However, no association appeared between a history of induced abortion and gestational diabetes, the researchers said. “To date, no study has reported the association of prior induced abortion with gestational diabetes,” they wrote.
The study findings were limited by several factors including the reliance on self-reports for history of SAB and therefore possible underreporting, the researchers noted. Other limitations included the lack of data on the timing of SABs; therefore, the time between SAB and gestational diabetes diagnosis could not be included in the analysis, they said. Unknown variables and the inclusion only of women from a single city in China might limit the generalizability of the results, they added.
More research is needed to understand the biological mechanisms behind the association between SAB and gestational diabetes, an association that has potential public health implications, they noted. However, the results suggest that “pregnant women with a history of SAB, especially those with a history of recurrent SAB, should attend more antenatal visits to monitor their blood glucose and implement early prevention and intervention,” such as healthful eating and regular exercise, they wrote.
Findings confirm, not surprise
The diagnosis of gestational diabetes in the current study “was made with a slightly different test than we typically use in the United States – a 1-hour nonfasting glucola followed by a confirmatory 3-hour fasting glucola,” Sarah W. Prager, MD, of the University of Washington, Seattle, said in an interview. The current study of both SAB and gestational diabetes is important because both conditions are very common and have been the focus of increased attention in the popular media and in scientific study, she said.
Dr. Prager said she was not surprised by the findings of a link between a history of gestational diabetes and a history of SAB, “but the association is likely that people at risk for gestational diabetes or who have undiagnosed diabetes/glucose intolerance are more likely to experience SAB,” she noted. “I would be surprised if the direction of the association is that SAB puts people at risk for gestational diabetes; more likely undiagnosed diabetes is a risk factor for SAB,” she added. “Perhaps we should be screening for glucose intolerance and other metabolic disorders more frequently in people who have especially recurrent SAB, as the more miscarriages someone had, the more likely they were in this study to be diagnosed with gestational diabetes;” or perhaps those with a history of SAB/recurrent SAB should be screened closer to 24 weeks’ than 28 weeks’ gestation to enable earlier intervention in those more likely to have gestational diabetes, Dr. Prager said.
The study was supported by the Key Program of the National Natural Science Foundation of China, the National Natural Science Foundation of China, the National Key Research and Development Program of China, the Shanghai Municipal Medical and Health Discipline Construction Projects, and the Shanghai Rising-Star Program. The researchers and Dr. Prager had no financial conflicts to disclose. Dr. Prager serves on the editorial advisory board of Ob.Gyn. News.
Pregnant women with a history of spontaneous abortion had a significantly increased risk of gestational diabetes in subsequent pregnancies, based on data from more than 100,000 women.
Gestational diabetes is associated not only with adverse perinatal outcomes, but also with an increased risk of long-term cardiovascular and metabolic health issues in mothers and children, wrote Yan Zhao, PhD, of Tongji University, Shanghai, and colleagues.
Previous studies also have shown that spontaneous abortion (SAB) is associated with later maternal risk of cardiovascular disease and venous thromboembolism, the researchers said. The same mechanisms might contribute to the development of gestational diabetes, but the association between abortion history and gestational diabetes risk in subsequent pregnancies remains unclear, they added.
In a study published in JAMA Network Open, the researchers identified 102,259 pregnant women seen for routine prenatal care at a single hospital in Shanghai between January 2014 and December 2019. The mean age of the women was 29.8 years.
During the study period, 14,579 women experienced SAB (14.3%), 17,935 experienced induced abortion (17.5%), and 4,017 experienced both (11.9%).
In all, 12,153 cases of gestational diabetes were identified, for a prevalence of 11.9%. The relative risk of gestational diabetes was 1.25 for women who experienced SAB and 1.15 for those who experienced both SAB and induced abortion, and the association between SAB and gestational diabetes increased in a number-dependent manner, the researchers said. The increase in relative risk for gestational diabetes in pregnant women with one SAB, two SABs, and three or more SABs was 18%, 41%, and 43%, compared to pregnant women with no SAB history.
However, no association appeared between a history of induced abortion and gestational diabetes, the researchers said. “To date, no study has reported the association of prior induced abortion with gestational diabetes,” they wrote.
The study findings were limited by several factors including the reliance on self-reports for history of SAB and therefore possible underreporting, the researchers noted. Other limitations included the lack of data on the timing of SABs; therefore, the time between SAB and gestational diabetes diagnosis could not be included in the analysis, they said. Unknown variables and the inclusion only of women from a single city in China might limit the generalizability of the results, they added.
More research is needed to understand the biological mechanisms behind the association between SAB and gestational diabetes, an association that has potential public health implications, they noted. However, the results suggest that “pregnant women with a history of SAB, especially those with a history of recurrent SAB, should attend more antenatal visits to monitor their blood glucose and implement early prevention and intervention,” such as healthful eating and regular exercise, they wrote.
Findings confirm, not surprise
The diagnosis of gestational diabetes in the current study “was made with a slightly different test than we typically use in the United States – a 1-hour nonfasting glucola followed by a confirmatory 3-hour fasting glucola,” Sarah W. Prager, MD, of the University of Washington, Seattle, said in an interview. The current study of both SAB and gestational diabetes is important because both conditions are very common and have been the focus of increased attention in the popular media and in scientific study, she said.
Dr. Prager said she was not surprised by the findings of a link between a history of gestational diabetes and a history of SAB, “but the association is likely that people at risk for gestational diabetes or who have undiagnosed diabetes/glucose intolerance are more likely to experience SAB,” she noted. “I would be surprised if the direction of the association is that SAB puts people at risk for gestational diabetes; more likely undiagnosed diabetes is a risk factor for SAB,” she added. “Perhaps we should be screening for glucose intolerance and other metabolic disorders more frequently in people who have especially recurrent SAB, as the more miscarriages someone had, the more likely they were in this study to be diagnosed with gestational diabetes;” or perhaps those with a history of SAB/recurrent SAB should be screened closer to 24 weeks’ than 28 weeks’ gestation to enable earlier intervention in those more likely to have gestational diabetes, Dr. Prager said.
The study was supported by the Key Program of the National Natural Science Foundation of China, the National Natural Science Foundation of China, the National Key Research and Development Program of China, the Shanghai Municipal Medical and Health Discipline Construction Projects, and the Shanghai Rising-Star Program. The researchers and Dr. Prager had no financial conflicts to disclose. Dr. Prager serves on the editorial advisory board of Ob.Gyn. News.
FROM JAMA NETWORK OPEN
15th Report on Carcinogens Adds to Its List
From environmental tobacco smoke to ultraviolet (UV) radiation, diesel exhaust particulates, lead, and now, chronic infection with Helicobacter pylori (H pylori)—the Report on Carcinogens has regularly updated the list of substances known or “reasonably anticipated” to cause cancer.
The 15th report, which is prepared by the National Toxicology Program (NTP) for the Department of Health and Human Services, has 8 new entries, bringing the number of human carcinogens (eg, metals, pesticides, and drugs) on the list to 256. (The first report, released in 1980, listed 26.) In addition to H pylori infection, this edition adds the flame-retardant chemical antimony trioxide, and 6 haloacetic acids found as water disinfection byproducts.
In 1971, then President Nixon declared “war on cancer” (the second leading cause of death in the US) and signed the National Cancer Act. In 1978, Congress ordered the Report on Carcinogens, to educate the public and health professionals on potential environmental carcinogenic hazards.
Perhaps disheartening to know that even with 256 entries, the list probably understates the number of carcinogens humans and other creatures are exposed to. But things can change with time. Each list goes through a rigorous round of reviews. Sometimes substances are “delisted” after, for instance, litigation or new research. Saccharin, for example, was removed from the ninth edition. It was listed as “reasonably anticipated” in 1981, based on “sufficient evidence of carcinogenicity in experimental animals.” It was removed, however, after extensive review of decades of saccharin use determined that the data were not sufficient to meet current criteria. Further research had revealed, also, that the observed bladder tumors in rats arose from a mechanism not relevant to humans.
Other entries, such as the controversial listing of the cancer drug tamoxifen, walk a fine line between risk and benefit. Tamoxifen, first listed in the ninth report (and still in the 15th report), was included because studies revealed that it could increase the risk of uterine cancer in women. But there also was conclusive evidence that it may prevent or delay breast cancer in women who are at high risk.
Ultimately, the report’s authors make it clear that it is for informative value and guidance, not necessarily a dictate. As one report put it: “Personal decisions concerning voluntary exposures to carcinogenic agents need to be based on additional information that is beyond the scope” of the report.
“As the identification of carcinogens is a key step in cancer prevention,” said Rick Woychik, PhD, director of the National Institute of Environmental Health Sciences and NTP, “publication of the report represents an important government activity towards improving public health.”
From environmental tobacco smoke to ultraviolet (UV) radiation, diesel exhaust particulates, lead, and now, chronic infection with Helicobacter pylori (H pylori)—the Report on Carcinogens has regularly updated the list of substances known or “reasonably anticipated” to cause cancer.
The 15th report, which is prepared by the National Toxicology Program (NTP) for the Department of Health and Human Services, has 8 new entries, bringing the number of human carcinogens (eg, metals, pesticides, and drugs) on the list to 256. (The first report, released in 1980, listed 26.) In addition to H pylori infection, this edition adds the flame-retardant chemical antimony trioxide, and 6 haloacetic acids found as water disinfection byproducts.
In 1971, then President Nixon declared “war on cancer” (the second leading cause of death in the US) and signed the National Cancer Act. In 1978, Congress ordered the Report on Carcinogens, to educate the public and health professionals on potential environmental carcinogenic hazards.
Perhaps disheartening to know that even with 256 entries, the list probably understates the number of carcinogens humans and other creatures are exposed to. But things can change with time. Each list goes through a rigorous round of reviews. Sometimes substances are “delisted” after, for instance, litigation or new research. Saccharin, for example, was removed from the ninth edition. It was listed as “reasonably anticipated” in 1981, based on “sufficient evidence of carcinogenicity in experimental animals.” It was removed, however, after extensive review of decades of saccharin use determined that the data were not sufficient to meet current criteria. Further research had revealed, also, that the observed bladder tumors in rats arose from a mechanism not relevant to humans.
Other entries, such as the controversial listing of the cancer drug tamoxifen, walk a fine line between risk and benefit. Tamoxifen, first listed in the ninth report (and still in the 15th report), was included because studies revealed that it could increase the risk of uterine cancer in women. But there also was conclusive evidence that it may prevent or delay breast cancer in women who are at high risk.
Ultimately, the report’s authors make it clear that it is for informative value and guidance, not necessarily a dictate. As one report put it: “Personal decisions concerning voluntary exposures to carcinogenic agents need to be based on additional information that is beyond the scope” of the report.
“As the identification of carcinogens is a key step in cancer prevention,” said Rick Woychik, PhD, director of the National Institute of Environmental Health Sciences and NTP, “publication of the report represents an important government activity towards improving public health.”
From environmental tobacco smoke to ultraviolet (UV) radiation, diesel exhaust particulates, lead, and now, chronic infection with Helicobacter pylori (H pylori)—the Report on Carcinogens has regularly updated the list of substances known or “reasonably anticipated” to cause cancer.
The 15th report, which is prepared by the National Toxicology Program (NTP) for the Department of Health and Human Services, has 8 new entries, bringing the number of human carcinogens (eg, metals, pesticides, and drugs) on the list to 256. (The first report, released in 1980, listed 26.) In addition to H pylori infection, this edition adds the flame-retardant chemical antimony trioxide, and 6 haloacetic acids found as water disinfection byproducts.
In 1971, then President Nixon declared “war on cancer” (the second leading cause of death in the US) and signed the National Cancer Act. In 1978, Congress ordered the Report on Carcinogens, to educate the public and health professionals on potential environmental carcinogenic hazards.
Perhaps disheartening to know that even with 256 entries, the list probably understates the number of carcinogens humans and other creatures are exposed to. But things can change with time. Each list goes through a rigorous round of reviews. Sometimes substances are “delisted” after, for instance, litigation or new research. Saccharin, for example, was removed from the ninth edition. It was listed as “reasonably anticipated” in 1981, based on “sufficient evidence of carcinogenicity in experimental animals.” It was removed, however, after extensive review of decades of saccharin use determined that the data were not sufficient to meet current criteria. Further research had revealed, also, that the observed bladder tumors in rats arose from a mechanism not relevant to humans.
Other entries, such as the controversial listing of the cancer drug tamoxifen, walk a fine line between risk and benefit. Tamoxifen, first listed in the ninth report (and still in the 15th report), was included because studies revealed that it could increase the risk of uterine cancer in women. But there also was conclusive evidence that it may prevent or delay breast cancer in women who are at high risk.
Ultimately, the report’s authors make it clear that it is for informative value and guidance, not necessarily a dictate. As one report put it: “Personal decisions concerning voluntary exposures to carcinogenic agents need to be based on additional information that is beyond the scope” of the report.
“As the identification of carcinogens is a key step in cancer prevention,” said Rick Woychik, PhD, director of the National Institute of Environmental Health Sciences and NTP, “publication of the report represents an important government activity towards improving public health.”
Early menopause, early dementia risk, study suggests
Earlier menopause appears to be associated with a higher risk of dementia, and earlier onset of dementia, compared with menopause at normal age or later, according to a large study.
“Being aware of this increased risk can help women practice strategies to prevent dementia and to work with their physicians to closely monitor their cognitive status as they age,” study investigator Wenting Hao, MD, with Shandong University, Jinan, China, says in a news release.
The findings were presented in an e-poster March 1 at the Epidemiology, Prevention, Lifestyle & Cardiometabolic Health (EPI|Lifestyle) 2022 conference sponsored by the American Heart Association.
UK Biobank data
Dr. Hao and colleagues examined health data for 153,291 women who were 60 years old on average when they became participants in the UK Biobank.
Age at menopause was categorized as premature (younger than age 40), early (40 to 44 years), reference (45 to 51), 52 to 55 years, and 55+ years.
Compared with women who entered menopause around age 50 years (reference), women who experienced premature menopause were 35% more likely to develop some type of dementia later in life (hazard ratio, 1.35; 95% confidence interval, 1.22 to 1.91).
Women with early menopause were also more likely to develop early-onset dementia, that is, before age 65 (HR, 1.31; 95% confidence interval, 1.07 to 1.72).
Women who entered menopause later (at age 52+) had dementia risk similar to women who entered menopause at the average age of 50 to 51 years.
The results were adjusted for relevant cofactors, including age at last exam, race, educational level, cigarette and alcohol use, body mass index, cardiovascular disease, diabetes, income, and leisure and physical activities.
Blame it on estrogen?
Reduced estrogen levels may be a factor in the possible connection between early menopause and dementia, Dr. Hao and her colleagues say.
Estradiol plays a key role in a range of neurological functions, so the reduction of endogenous estrogen at menopause may aggravate brain changes related to neurodegenerative disease and speed up progression of dementia, they explain.
“We know that the lack of estrogen over the long term enhances oxidative stress, which may increase brain aging and lead to cognitive impairment,” Dr. Hao adds.
Limitations of the study include reliance on self-reported information about age at menopause onset.
Also, the researchers did not evaluate dementia rates in women who had a naturally occurring early menopause separate from the women with surgery-induced menopause, which may affect the results.
Finally, the data used for this study included mostly White women living in the U.K. and may not generalize to other populations.
Supportive evidence, critical area of research
The U.K. study supports results of a previously reported Kaiser Permanente study, which showed women who entered menopause at age 45 or younger were at 28% greater dementia risk, compared with women who experienced menopause after age 45.
Reached for comment, Heather Snyder, PhD, Alzheimer’s Association vice president of medical and scientific relations, noted that nearly two-thirds of Americans with Alzheimer’s are women.
“We know Alzheimer’s and other dementias impact a greater number of women than men, but we don’t know why,” she told this news organization.
“Lifelong differences in women may affect their risk or affect what is contributing to their underlying biology of the disease, and we need more research to better understand what may be these contributing factors,” said Dr. Snyder.
“Reproductive history is one critical area being studied. The physical and hormonal changes that occur during menopause – as well as other hormonal changes throughout life – are considerable, and it’s important to understand what impact, if any, these changes may have on the brain,” Dr. Snyder added.
“The potential link between reproduction history and brain health is intriguing, but much more research in this area is needed to understand these links,” she said.
The study was funded by the Start-up Foundation for Scientific Research at Shandong University. Dr. Hao and Dr. Snyder have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Earlier menopause appears to be associated with a higher risk of dementia, and earlier onset of dementia, compared with menopause at normal age or later, according to a large study.
“Being aware of this increased risk can help women practice strategies to prevent dementia and to work with their physicians to closely monitor their cognitive status as they age,” study investigator Wenting Hao, MD, with Shandong University, Jinan, China, says in a news release.
The findings were presented in an e-poster March 1 at the Epidemiology, Prevention, Lifestyle & Cardiometabolic Health (EPI|Lifestyle) 2022 conference sponsored by the American Heart Association.
UK Biobank data
Dr. Hao and colleagues examined health data for 153,291 women who were 60 years old on average when they became participants in the UK Biobank.
Age at menopause was categorized as premature (younger than age 40), early (40 to 44 years), reference (45 to 51), 52 to 55 years, and 55+ years.
Compared with women who entered menopause around age 50 years (reference), women who experienced premature menopause were 35% more likely to develop some type of dementia later in life (hazard ratio, 1.35; 95% confidence interval, 1.22 to 1.91).
Women with early menopause were also more likely to develop early-onset dementia, that is, before age 65 (HR, 1.31; 95% confidence interval, 1.07 to 1.72).
Women who entered menopause later (at age 52+) had dementia risk similar to women who entered menopause at the average age of 50 to 51 years.
The results were adjusted for relevant cofactors, including age at last exam, race, educational level, cigarette and alcohol use, body mass index, cardiovascular disease, diabetes, income, and leisure and physical activities.
Blame it on estrogen?
Reduced estrogen levels may be a factor in the possible connection between early menopause and dementia, Dr. Hao and her colleagues say.
Estradiol plays a key role in a range of neurological functions, so the reduction of endogenous estrogen at menopause may aggravate brain changes related to neurodegenerative disease and speed up progression of dementia, they explain.
“We know that the lack of estrogen over the long term enhances oxidative stress, which may increase brain aging and lead to cognitive impairment,” Dr. Hao adds.
Limitations of the study include reliance on self-reported information about age at menopause onset.
Also, the researchers did not evaluate dementia rates in women who had a naturally occurring early menopause separate from the women with surgery-induced menopause, which may affect the results.
Finally, the data used for this study included mostly White women living in the U.K. and may not generalize to other populations.
Supportive evidence, critical area of research
The U.K. study supports results of a previously reported Kaiser Permanente study, which showed women who entered menopause at age 45 or younger were at 28% greater dementia risk, compared with women who experienced menopause after age 45.
Reached for comment, Heather Snyder, PhD, Alzheimer’s Association vice president of medical and scientific relations, noted that nearly two-thirds of Americans with Alzheimer’s are women.
“We know Alzheimer’s and other dementias impact a greater number of women than men, but we don’t know why,” she told this news organization.
“Lifelong differences in women may affect their risk or affect what is contributing to their underlying biology of the disease, and we need more research to better understand what may be these contributing factors,” said Dr. Snyder.
“Reproductive history is one critical area being studied. The physical and hormonal changes that occur during menopause – as well as other hormonal changes throughout life – are considerable, and it’s important to understand what impact, if any, these changes may have on the brain,” Dr. Snyder added.
“The potential link between reproduction history and brain health is intriguing, but much more research in this area is needed to understand these links,” she said.
The study was funded by the Start-up Foundation for Scientific Research at Shandong University. Dr. Hao and Dr. Snyder have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Earlier menopause appears to be associated with a higher risk of dementia, and earlier onset of dementia, compared with menopause at normal age or later, according to a large study.
“Being aware of this increased risk can help women practice strategies to prevent dementia and to work with their physicians to closely monitor their cognitive status as they age,” study investigator Wenting Hao, MD, with Shandong University, Jinan, China, says in a news release.
The findings were presented in an e-poster March 1 at the Epidemiology, Prevention, Lifestyle & Cardiometabolic Health (EPI|Lifestyle) 2022 conference sponsored by the American Heart Association.
UK Biobank data
Dr. Hao and colleagues examined health data for 153,291 women who were 60 years old on average when they became participants in the UK Biobank.
Age at menopause was categorized as premature (younger than age 40), early (40 to 44 years), reference (45 to 51), 52 to 55 years, and 55+ years.
Compared with women who entered menopause around age 50 years (reference), women who experienced premature menopause were 35% more likely to develop some type of dementia later in life (hazard ratio, 1.35; 95% confidence interval, 1.22 to 1.91).
Women with early menopause were also more likely to develop early-onset dementia, that is, before age 65 (HR, 1.31; 95% confidence interval, 1.07 to 1.72).
Women who entered menopause later (at age 52+) had dementia risk similar to women who entered menopause at the average age of 50 to 51 years.
The results were adjusted for relevant cofactors, including age at last exam, race, educational level, cigarette and alcohol use, body mass index, cardiovascular disease, diabetes, income, and leisure and physical activities.
Blame it on estrogen?
Reduced estrogen levels may be a factor in the possible connection between early menopause and dementia, Dr. Hao and her colleagues say.
Estradiol plays a key role in a range of neurological functions, so the reduction of endogenous estrogen at menopause may aggravate brain changes related to neurodegenerative disease and speed up progression of dementia, they explain.
“We know that the lack of estrogen over the long term enhances oxidative stress, which may increase brain aging and lead to cognitive impairment,” Dr. Hao adds.
Limitations of the study include reliance on self-reported information about age at menopause onset.
Also, the researchers did not evaluate dementia rates in women who had a naturally occurring early menopause separate from the women with surgery-induced menopause, which may affect the results.
Finally, the data used for this study included mostly White women living in the U.K. and may not generalize to other populations.
Supportive evidence, critical area of research
The U.K. study supports results of a previously reported Kaiser Permanente study, which showed women who entered menopause at age 45 or younger were at 28% greater dementia risk, compared with women who experienced menopause after age 45.
Reached for comment, Heather Snyder, PhD, Alzheimer’s Association vice president of medical and scientific relations, noted that nearly two-thirds of Americans with Alzheimer’s are women.
“We know Alzheimer’s and other dementias impact a greater number of women than men, but we don’t know why,” she told this news organization.
“Lifelong differences in women may affect their risk or affect what is contributing to their underlying biology of the disease, and we need more research to better understand what may be these contributing factors,” said Dr. Snyder.
“Reproductive history is one critical area being studied. The physical and hormonal changes that occur during menopause – as well as other hormonal changes throughout life – are considerable, and it’s important to understand what impact, if any, these changes may have on the brain,” Dr. Snyder added.
“The potential link between reproduction history and brain health is intriguing, but much more research in this area is needed to understand these links,” she said.
The study was funded by the Start-up Foundation for Scientific Research at Shandong University. Dr. Hao and Dr. Snyder have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Gestational diabetes: Optimizing Dx and management in primary care
Gestational diabetes mellitus (GDM), defined as new-onset hyperglycemia detected in a pregnant woman after 24 weeks of gestation, affects 4% to 10% of pregnancies in the United States annually1 and is a major challenge for health care professionals.2 During pregnancy, the body’s physiologic responses are altered to support the growing fetus. One of these changes is an increase in insulin resistance, which suggests that pregnancy alone increases the patient’s risk for type 2 diabetes (T2D). However, several other factors also increase this risk, including maternal age, social barriers to care, obesity, poor weight control, and family history.

If not controlled, GDM results in poor health outcomes for the mother, such as preeclampsia, preterm labor, and maternal T2D.3-5 For the infant, intrauterine exposure to persistent hyperglycemia is correlated with neonatal macrosomia, hypoglycemia, perinatal complications (eg, preterm delivery, fetal demise), and obesity and insulin resistance later in life.4
Primary care physicians (PCPs) are the patient’s main point of contact prior to pregnancy. This relationship makes PCPs a resource for the patient and specialists during and after pregnancy. In this article, we discuss risk factors and how to screen for GDM, provide an update on practice recommendations for treatment and management of GDM in primary care, and describe the effects of uncontrolled GDM.
Know the key risk factors
Prevention begins with identifying the major risk factors that contribute to the development of GDM. These include maternal age, social barriers to care, family history of prediabetes, and obesity and poor weight control.
Older age. A meta-analysis of 24 studies noted strong positive correlation between GDM risk and maternal age.6 One of the population-based cohort studies in the meta-analysis examined relationships between maternal age and pregnancy outcomes in women living in British Columbia, Canada (n = 203,414). Data suggested that the relative risk of GDM increased linearly with maternal age to 3.2, 4.2, and 4.4 among women ages ≥ 35, ≥ 40, and ≥ 45 years, respectively.7
Social barriers to care. Although the prevalence of GDM has increased over the past few decades,1 from 2011 to 2019 the increase in GDM in individuals at first live birth was significantly higher in non-Hispanic Asian and Hispanic/Latina women than in non-Hispanic White women.8 Data from the Centers for Disease Control and Prevention further suggest that diabetes was more prevalent among individuals with a lower socioeconomic status as indicated by their level of education.9 Ogunwole et al10 suggest that racism is the root cause of these disparities and leads to long-term barriers to care (eg, socioeconomic deprivation, lack of health insurance, limited access to care, and poor health literacy), which ultimately contribute to the development of GDM and progression of diabetes. It is important for PCPs and all health professionals to be aware of these barriers so that they may practice mindfulness and deliver culturally sensitive care to patients from marginalized communities.
Family history of prediabetes. In a population-based cohort study (n = 7020), women with prediabetes (A1C, 5.7%-6.4%) were 2.8 times more likely to develop GDM compared with women with normal A1C (< 5.7%).11 Similar results were seen in a retrospective cohort study (n = 2812), in which women with prediabetes were more likely than women with a normal first trimester A1C to have GDM (29.1% vs 13.7%, respectively; adjusted relative risk = 1.48; 95% CI, 1.15-1.89).12 In both studies, prediabetes was not associated with a higher risk for adverse maternal or neonatal outcomes.11,12
Continue to: While there are no current...
While there are no current guidelines for treating prediabetes in pregnancy, women diagnosed with prediabetes in 1 study were found to have significantly less weight gain during pregnancy compared with patients with normal A1C,12 suggesting there may be a benefit in early identification and intervention, although further research is needed.11 In a separate case-control study (n = 345 women with GDM; n = 800 control), high rates of gestational weight gain (> 0.41 kg/wk) were associated with an increased risk of GDM (odds ratio [OR] = 1.74; 95% CI, 1.16-2.60) compared with women with the lowest rate of gestational weight gain (0.27-0.4 kg/wk [OR = 1.43; 95% CI, 0.96-2.14]).13 Thus, it is helpful to have proactive conversations about family planning and adequate weight and glycemic control with high-risk patients to prepare for a healthy pregnancy.
Obesity and weight management. Patients who are overweight (body mass index [BMI], 25-29.9) or obese (BMI > 30) have a substantially increased risk of GDM (adjusted OR = 1.44; 95% CI, 1.04-1.81), as seen in a retrospective cohort study of 1951 pregnant Malaysian women.14 Several factors have been found to contribute to successful weight control, including calorie prescription, a structured meal plan, high physical activity goals (60-90 min/d), daily weighing and monitoring of food intake, behavior therapy, and continued patient–provider contact.15
The safety, efficacy, and sustainability of weight loss with various dietary plans have been studied in individuals who are overweight and obese.16 Ultimately, energy expenditure must be greater than energy intake to promote weight loss. Conventional diets with continuous energy restriction (ie, low-fat, low-carbohydrate, and high-protein diets) have proven to be effective for short-term weight loss but data on long-term weight maintenance are limited.16 The Mediterranean diet, which is comprised mostly of vegetables, fruits, legumes, fish, and grains—with a lower intake of meat and dairy—may reduce gestational weight gain and risk of GDM as suggested by a randomized controlled trial (RCT; n = 1252).17 Although the choice of diet is up to the patient, it is important to be aware of different diets or refer the patient to a registered dietician who can help the patient if needed.
Reduce risk with adequate weight and glycemic control
Prevention of GDM during pregnancy should focus on weight maintenance and optimal glycemic control. Two systematic reviews, one with 8 RCTs (n = 1792) and another with 5 studies (n = 539), assessed the efficacy and safety of energy-restricted dietary intervention on GDM prevention.18 The first review found a significant reduction in gestational weight gain and improved glycemic control without increased risk of adverse maternal and fetal outcomes.18 The second review showed no clear difference between energy-restricted and non–energy-restricted diets on outcomes such as preeclampsia, gestational weight gain, large for gestational age, and macrosomia.18 These data suggest that while energy-restricted dietary interventions made no difference on maternal and fetal complications, they may still be safely used in pregnancy to reduce gestational weight gain and improve glycemic control.18
Once a woman is pregnant, it becomes difficult to lose weight because additional calories are needed to support a growing fetus. It is recommended that patients with healthy pregestational BMI consume an extra 200 to 300 calories/d after the first trimester. However, extra caloric intake in a woman with obesity who is pregnant leads to metabolic impairment and increased risk of diabetes for both the mother and fetus.19 Therefore, it is recommended that patients with obese pregestational BMI not consume additional calories because excess maternal fat is sufficient to support the energy needs of the growing fetus.19
Continue to: Ultimately, earlier intervention...
Ultimately, earlier intervention—prior to conception—helps patients prepare for a healthier pregnancy, resulting in better long-term outcomes. It is helpful to be familiar with the advantages and disadvantages of common approaches to weight management and to be able to refer patients to nutritionists for optimal planning. When establishing a dietary plan, consider patient-specific factors, such as cultural diets, financial and time constraints, and the patient’s readiness to make and maintain these changes. Consistent follow-up and behavioral therapy are necessary to maintain successful weight control.
There are many screening tools, but 1 is preferred in pregnancy
There are several ways to diagnose diabetes in patients who are not pregnant, including A1C, a fasting glucose test, an oral glucose tolerance test (OGTT), or random glucose testing (plus symptoms). However, the preferred method for diagnosing GDM is OGTT because it has a higher sensitivity.20 A1C, while a good measure of hyperglycemic stability, does not register hyperglycemia early enough to diagnose GDM and fasting glucose testing is less sensitive because for most women with GDM, that abnormal postprandial glucose level is the first glycemic abnormality.21
When to screen. Blood glucose levels should be checked in all pregnant women as part of their metabolic panel at the first prenatal visit. A reflex A1C for high glucose levels can be ordered based on the physician’s preference. This may help you to identify patients with prediabetes who are at risk for GDM and implement early behavioral and lifestyle changes. However, further research is needed to determine if intervention early in pregnancy can truly reduce the risk of GDM.11
Screening for GDM should be completed at 24 to 28 weeks of gestation20 because it is likely that this is when the hormonal effects of the placenta that contribute to insulin resistance set the woman up for postprandial hyperglycemia. Currently, there are no evidence-based guidelines for the use of continuous glucose monitoring prior to 24 weeks of gestation to identify GDM.20 If persistent hyperglycemia is present before 24 weeks of gestation, it is considered evidence of a pre-existing metabolic abnormality and is diagnosed as “pregestational diabetes.” Treatment should follow guidelines established for women who had diabetes prior to pregnancy.
How to screen? There is ongoing discussion about what is the optimal screening method for GDM: a 1-step strategy with a fasting 75-g OGTT only, or a 2-step strategy with a 50-g non-fasting glucose load test followed by a fasting 100-g OGTT in women who do not meet the plasma glucose cutoff (TABLE 1).22-24 Hillier et al25 compared the effectiveness of these strategies in diagnosing GDM and identifying pregnancy complications for the mother and infant. They found that while the 1-step strategy resulted in a 2-fold increase in the diagnosis of GDM, it did not lead to better outcomes for mothers and infants when compared with the 2-step method.25 Currently, the majority of obstetricians (95%) prefer to use the 2-step method.24
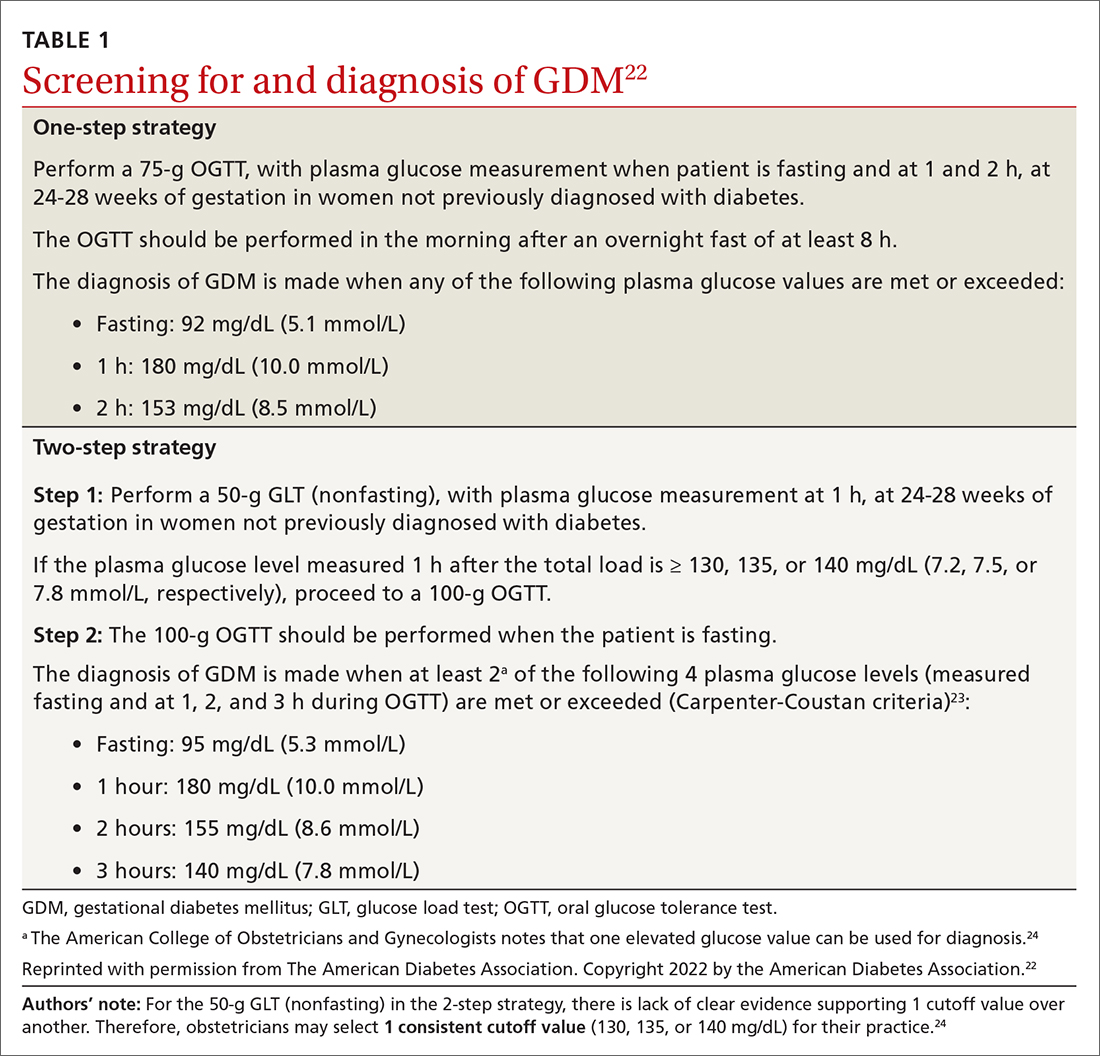
Continue to: Manage lifestyle, monitor glucose
Manage lifestyle, monitor glucose
Management of GDM in most women starts with diabetes self-management education and support for therapeutic lifestyle changes, such as nutritional interventions that reduce hyperglycemia and contribute to healthy weight gain during pregnancy.20 This may include medical nutrition therapy that focuses on adequate nutrition for the mother and fetus. Currently, the recommended dietary intake for women who are pregnant (regardless of diabetes) includes a minimum of 175 g of carbohydrates, 71 g of daily protein, and at least 28 g of fiber. Further refinement of dietary intake, including carbohydrate restriction, should be done with guidance from a registered dietitian.20 If the obstetrics team does not include a registered dietitian, a referral to one may be necessary. Regular physical activity should be continued throughout pregnancy as tolerated. Social support, stress reduction, and good sleep hygiene should be encouraged as much as possible.
For successful outcomes, therapeutic lifestyle changes should be coupled with glucose monitoring. The Fifth International Workshop-Conference on Gestational Diabetes Mellitus recommends that women with GDM monitor fasting blood glucose and typically 1-hour postprandial glucose. The glucose goals in GDM are as follows26:
- Fasting glucose < 95 mg/dL (5.3 mmol/L), and either
- 1-hour postprandial glucose < 140 mg/dL (7.8 mmol/L), or
- 2-hour postprandial glucose < 120 mg/dL (6.7 mmol/L).
Importantly, in the second and third trimester, the A1C goal for women with GDM is 6.0%. This is lower than the more traditional A1C goal for 2 reasons: (1) increases in A1C, even within the normal range, increase adverse outcomes; and (2) pregnant women will have an increased red blood cell count turnover, which can lower the A1C.27 In a historical cohort study (n = 27,213), Abell et al28 found that women who have an A1C < 6.0% in the second and third trimester have the lowest risk of giving birth to large-for-gestational-age infants and for having preeclampsia.
Add insulin if glucose targets are not met
Most women who engage in therapeutic lifestyle change (70%-85%) can achieve an A1C < 6% and will not need to take medication to manage GDM.29 If pharmacotherapy is needed to manage glucose, insulin is the preferred treatment for all women with GDM.20 Treatment should be individualized based on the glucose trends the woman is experiencing. Common treatments include bedtime NPH if fasting hyperglycemia is most prominent and analogue insulin at mealtimes for women with prominent postprandial hyperglycemia.
Noninsulin agents such as metformin and sulfonylureas are not currently recommended by the American College of Obstetricians and Gynecologists or the American Diabetes Association for use in GDM.20,24 Despite being used for years in women with pregestational diabetes, metabolic syndrome, and polycystic ovary syndrome, there is evidence that metformin crosses the placenta and fetal safety has not yet been established in RCTs. The Metformin in Gestational Diabetes: The Offspring Follow-Up (MiG TOFU) study was a longitudinal follow-up study that evaluated body composition and metabolic outcomes in children (ages 7-9 years) of women with GDM who had received metformin or insulin while pregnant.30 At age 9 years, children who were exposed to metformin weighed more and had a higher waist-to-height ratio and waist circumference than those exposed to insulin.30
Continue to: Sulfonylureas are no longer recommended...
Sulfonylureas are no longer recommended because of the risk of maternal and fetal hypoglycemia and concerns about this medication crossing the placenta.24,31,32 Specifically, in a 2015 meta-analysis and systematic review of 15 articles (n = 2509), glyburide had a higher risk of neonatal hypoglycemia and macrosomia than insulin or metformin.33 For women who cannot manage their glucose with therapeutic lifestyle changes and cannot take insulin, oral therapies may be considered if the risk-benefit ratio is balanced for that person.34
Watch for effects of poor glycemic control on mother, infant
Preeclampsia is defined as new-onset hypertension and proteinuria after 20 weeks of gestation. The correlation between GDM and preeclampsia has partly been explained by their shared overlapping risk factors, including maternal obesity, excessive gestational weight gain, and persistent hyperglycemia.35 On a biochemical level, these risk factors contribute to oxidative stress and systemic vascular dysfunction, which have been hypothesized as the underlying pathophysiology for the development of preeclampsia.35
Neonatal macrosomia, defined as a birth weight ≥ 4000 g, is a common complication that develops in 15% to 45% of infants of mothers with GDM.36 Placental transfer of glucose in mothers with hyperglycemia stimulates the secretion of neonatal insulin and the ultimate storage of the excess glucose as body fat. After delivery, the abrupt discontinuation of placental transfer of glucose to an infant who is actively secreting insulin leads to neonatal hypoglycemia, which if not detected or managed, can lead to long-term neurologic deficits, including recurrent seizures and developmental delays.37 Therefore, it is essential to screen for neonatal hypoglycemia immediately after birth and serially up to 12 hours.38
Postpartum T2D. Poor glycemic control increases the risk of increasing insulin resistance developing into T2D postpartum for mothers.39 It also increases the risk of obesity and insulin resistance later in life for the infant.40 A retrospective cohort study (n = 461) found a positive correlation between exposure to maternal GDM and elevated BMI in children ages 6 to 13 years.41 Kamana et al36 further discussed this correlation and suggested that exposure to maternal hyperglycemia in utero contributes to fetal programming of later adipose deposition. Children may develop without a notable increase in BMI until after puberty.42
Partner with specialists to improve outcomes
Although most women with GDM are managed by specialists (obstetricians, endocrinologists, and maternal-fetal medicine specialists),43 these patients are still seeking care from their family physicians for other complaints. These visits provide key touchpoints during pregnancy and are opportunities for PCPs to identify a pregnancy-related complication or provide additional education or referral to the obstetrician.
Continue to: Also, if you work in an area...
Also, if you work in an area where specialists are less accessible, you may be the clinician providing the majority of care to a patient with GDM. If this is the case, you’ll want to watch for the following risk factors, which should prompt a referral to specialty care:
- a previous pregnancy with GDM20
- a previous birth of an infant weighing > 4000 g44
- baseline history of hypertension45
- evidence of insulin resistance or polycystic ovary syndrome46,47
- a history of cardiovascular disease20
- a need to treat GDM with pharmacotherapy.48
Ensuring a smooth transition after the birth
Optimal communication and hand-offs throughout pregnancy and after delivery will benefit everyone. When the pregnant patient’s care has been managed by an obstetrician, it is important to address the following issues during the hand-off:
- baseline medical problems
- medical screenings and treatments in pregnancy (retinopathy and nephropathy screening)
- aspirin initiation, if indicated
- management of thyroid abnormalities
- management of mental health conditions
- postpartum glucose management and T2D screening postpartum
- management of complications identified during pregnancy (retinopathy and nephropathy).
Timing and other elements of postpartum care. The first postpartum screen should occur at 4 to 12 weeks postpartum. OGTT is recommended instead of A1C at this time because A1C may still be lowered by the increased red blood cell turnover related to pregnancy and blood loss at delivery. Because women with GDM have a 50% to 75% lifetime risk of T2D,20 patients with normal test results should be re-tested every 1 to 3 years using any of the standard screening methods (A1C, fasting glucose, or OGTT).20
After delivery it may be difficult for women to follow-up with their own personal health care because they are focused on the care of their baby. The increased use of telehealth may make postpartum follow-up visits easier to attend.
Visits present opportunities. Postpartum visits present another opportunity for PCPs to screen for diabetes and other postpartum complications, including depression and thyroid abnormalities. Visits are also an opportunity to discuss timely contraception so as to prevent an early, unplanned pregnancy. Other important aspects of postpartum care are outlined in TABLE 2.20,49

CORRESPONDENCE
Connie L. Ha, BS, OMS IV, Department of Primary Care, 1310 Club Drive, Touro University California, Vallejo, CA 94592; [email protected]
1. Sheiner E. Gestational diabetes mellitus: long-term consequences for the mother and child grand challenge: how to move on towards secondary prevention? Front Clin Diabetes Healthc. 2020. doi: 10.3389/fcdhc.2020.546256
2. Angueira AR, Ludvik AE, Reddy TE, et al. New insights into gestational glucose metabolism: lessons learned from 21st century approaches. Diabetes. 2015;64:327-334. doi: 10.2337/db14-0877
3. Shou C, Wei Y-M, Wang C, et al. Updates in long-term maternal and fetal adverse effects of gestational diabetes mellitus. Maternal-Fetal Med. 2019;1:91-94. doi: 10.1097/FM9.0000000000000019
4. Plows JF, Stanley JL, Baker PN, et al. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. 2018;19:3342. doi: 10.3390/ijms19113342
5. Kulshrestha V, Agarwal N. Maternal complications in pregnancy with diabetes. J Pak Med Assoc. 2016;66(9 suppl 1):S74-S77.
6. Li Y, Ren X, He L, et al. Maternal age and the risk of gestational diabetes mellitus: a systematic review and meta-analysis of over 120 million participants. Diabetes Res Clin Pract. 2020;162:108044. doi: 10.1016/j.diabres.2020.108044
7. Schummers L, Hutcheon JA, Hacker MR, et al. Absolute risks of obstetric outcomes by maternal age at first birth: a population-based cohort. Epidemiology. 2018;29:379-387. doi: 10.1097/EDE.0000000000000818
8. Shah NS, Wang MC, Freaney PM, et al. Trends in gestational diabetes at first live birth by race and ethnicity in the US, 2011-2019. JAMA. 2021;326:660-669. doi: 10.1001/jama.2021.7217
9. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2020. Accessed February 2, 2022. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
10. Ogunwole SM, Golden SH. Social determinants of health and structural inequities—root causes of diabetes disparities. Diabetes Care. 2021;44:11-13. doi: 10.2337/dci20-0060
11. Chen L, Pocobelli G, Yu O, et al. Early pregnancy hemoglobin A1C and pregnancy outcomes: a population-based study. Am J Perinatol. 2019;36:1045-1053. doi: 10.1055/s-0038-1675619
12. Osmundson S, Zhao BS, Kunz L, et al. First trimester hemoglobin A1C prediction of gestational diabetes. Am J Perinatol. 2016;33:977-982. doi: 10.1055/s-0036-1581055
13. Hedderson MM, Gunderson EP, Ferrara A. Gestational weight gain and risk of gestational diabetes mellitus [published correction appears in Obstet Gynecol. 2010;115:1092]. Obstet Gynecol. 2010;115:597-604. doi: 10.1097/AOG.0b013e3181cfce4f
14. Yong HY, Mohd Shariff Z, Mohd Yusof BN, et al. Independent and combined effects of age, body mass index and gestational weight gain on the risk of gestational diabetes mellitus. Sci Rep. 2020;10:8486. doi: 10.1038/s41598-020-65251-2
15. Phelan S. Windows of opportunity for lifestyle interventions to prevent gestational diabetes mellitus. Am J Perinatol. 2016;33:1291-1299. doi: 10.1055/s-0036-1586504
16. Koliaki C, Spinos T, Spinou M, et al. Defining the optimal dietary approach for safe, effective and sustainable weight loss in overweight and obese adults. Healthcare (Basel). 2018;6:73. doi: 10.3390/healthcare6030073
17. Al Wattar BH, Dodds J, Placzek A, et al. Mediterranean-style diet in pregnant women with metabolic risk factors (ESTEEM): a pragmatic multicentre randomised trial. PLOS Med. 2019;16:e1002857. doi: 10.1371/journal.pmed.1002857
18. Zarogiannis S. Are novel lifestyle approaches to management of type 2 diabetes applicable to prevention and treatment of women with gestational diabetes mellitus? Global Diabetes Open Access J. 2019;1:1-14.
19. Most J, Amant MS, Hsia DS, et al. Evidence-based recommendations for energy intake in pregnant women with obesity. J Clin Invest. 2019;129:4682-4690. doi: 10.1172/JCI130341
20. American Diabetes Association. 14. Management of diabetes in pregnancy: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S200-S210. doi: 10.2337/dc21-S014
21. McIntyre HD, Sacks DA, Barbour LA, et al. Issues with the diagnosis and classification of hyperglycemia in early pregnancy. Diabetes Care. 2016;39:53-54. doi: 10.2337/dc15-1887
22. American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S17-S38. doi: 10.2337/dc22-S002
23. Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768-773. doi: 10.1016/0002-9378(82)90349-0
24. ACOG Practice Bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131:e49-e64. doi: 10.1097/AOG.0000000000002501
25. Hillier TA, Pedula KL, Ogasawara KK, et al. A pragmatic, randomized clinical trial of gestational diabetes screening. N Engl J Med. 2021;384:895-904. doi: 10.1056/NEJMoa2026028
26. Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(suppl 2):S251-S260. doi: 10.2337/dc07-s225
27. Nielsen LR, Ekbom P, Damm P, et al. HbA1c levels are significantly lower in early and late pregnancy. Diabetes Care. 2004;27:1200-1201. doi: 10.2337/diacare.27.5.1200
28. Abell SK, Boyle JA, de Courten B, et al. Impact of type 2 diabetes, obesity and glycaemic control on pregnancy outcomes. Aust N Z J Obstet Gynaecol. 2017;57:308-314. doi: 10.1111/ajo.12521
29. Viana LV, Gross JL, Azevedo MJ. Dietary intervention in patients with gestational diabetes mellitus: a systematic review and meta-analysis of randomized clinical trials on maternal and newborn outcomes. Diabetes Care. 2014;37:3345-3355. doi: 10.2337/dc14-1530
30. Rowan JA, Rush EC, Plank LD, et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition and metabolic outcomes at 7-9 years of age. BMJ Open Diabetes Res Care. 2018;6:e000456. doi: 10.1136/bmjdrc-2017-000456
31. Hebert MF, Ma X, Naraharisetti SB, et al. Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clin Pharmacol Ther. 2009;85:607-614. doi: 10.1038/clpt.2009.5
32. Malek R, Davis SN. Pharmacokinetics, efficacy and safety of glyburide for treatment of gestational diabetes mellitus. Expert Opin Drug Metab Toxicol. 2016;12:691-699. doi: 10.1080/17425255.2016.1187131
33. Balsells M, García-Patterson A, Solà I, et al. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ. 2015;350:h102. doi: 10.1136/bmj.h102
34. Kavitha N, De S, Kanagasabai S. Oral hypoglycemic agents in pregnancy: an update. J Obstet Gynaecol India. 2013;63:82-87. doi: 10.1007/s13224-012-0312-z
35. Weissgerber TL, Mudd LM. Preeclampsia and diabetes. Curr Diab Rep. 2015;15:9. doi: 10.1007/s11892-015-0579-4
36. Kamana KC, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metab. 2015;66(suppl 2):14-20. doi: 10.1159/000371628
37. Mitanchez D, Yzydorczyk C, Simeoni U. What neonatal complications should the pediatrician be aware of in case of maternal gestational diabetes? World J Diabetes. 2015;6:734-743. doi: 10.4239/wjd.v6.i5.734
38. Stanescu A, Stoicescu SM. Neonatal hypoglycemia screening in newborns from diabetic mothers—arguments and controversies. J Med Life. 2014;7(spec iss 3):51-52.
39. Kim C. Maternal outcomes and follow-up after gestational diabetes mellitus. Diabet Med. 2014;31:292-301. doi: 10.1111/dme.12382
40. Stewart A, Malhotra A. Gestational diabetes and the neonate: challenges and solutions. Res Rep Neonatol. 2015;5:31-39. doi: 10.2147/RRN.S30971
41. Crume TL, Ogden L, West NA, et al. Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: the Exploring Perinatal Outcomes among Children (EPOCH) Study. Diabetologia. 2011;54:87-92. doi: 10.1007/s00125-010-1925-3
42. Crume TL, Ogden L, Daniels S, et al. The impact of in utero exposure to diabetes on childhood body mass index growth trajectories: the EPOCH study. J Pediatr. 2011;158:941-946. doi: 10.1016/j.jpeds.2010.12.007
43. Levels of maternal care. Obstetric Care Consensus No. 9. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2019;134:e41-e55. doi: 10.1097/AOG.0000000000003383
44. Caughey AB, Cheng YW, Stotland NE, et al. Maternal and paternal race/ethnicity are both associated with gestational diabetes. Am J Obstet Gynecol. 2010;202:616.e1-e5. doi: 10.1016/j.ajog.2010.01.082
45. Yogev Y, Xenakis EM, Langer O. The association between preeclampsia and severity of gestational diabetes: the impact of glycemic control. Am J Obstet Gynecol. 2004;191:1655-1660. doi: 10.1016/j.ajog.2004.03.074
46. Brown J, Alwan NA, West J, et al. Lifestyle interventions for the treatment of women with gestational diabetes. Cochrane Database Syst Rev. 2017;5:CD011970. doi: 10.1002/14651858.CD011970.pub2
47. Ceysens G, Rouiller D, Boulvain M. Exercise for the diabetic pregnant woman. Cochrane Database Syst Rev. 2006;3:CD004225. doi: 10.1002/14651858.CD004225.pub2
48. Chawla R, Mukherjee JJ, Chawla M, et al. Expert group recommendations on the effective use of bolus insulin in the management of type 2 diabetes mellitus. Med Sci (Basel). 2021;9:38. doi: 10.3390/medsci9020038
49. American Diabetes Association. Introduction: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S1-S2. doi: 10.2337/dc22-Sint
Gestational diabetes mellitus (GDM), defined as new-onset hyperglycemia detected in a pregnant woman after 24 weeks of gestation, affects 4% to 10% of pregnancies in the United States annually1 and is a major challenge for health care professionals.2 During pregnancy, the body’s physiologic responses are altered to support the growing fetus. One of these changes is an increase in insulin resistance, which suggests that pregnancy alone increases the patient’s risk for type 2 diabetes (T2D). However, several other factors also increase this risk, including maternal age, social barriers to care, obesity, poor weight control, and family history.

If not controlled, GDM results in poor health outcomes for the mother, such as preeclampsia, preterm labor, and maternal T2D.3-5 For the infant, intrauterine exposure to persistent hyperglycemia is correlated with neonatal macrosomia, hypoglycemia, perinatal complications (eg, preterm delivery, fetal demise), and obesity and insulin resistance later in life.4
Primary care physicians (PCPs) are the patient’s main point of contact prior to pregnancy. This relationship makes PCPs a resource for the patient and specialists during and after pregnancy. In this article, we discuss risk factors and how to screen for GDM, provide an update on practice recommendations for treatment and management of GDM in primary care, and describe the effects of uncontrolled GDM.
Know the key risk factors
Prevention begins with identifying the major risk factors that contribute to the development of GDM. These include maternal age, social barriers to care, family history of prediabetes, and obesity and poor weight control.
Older age. A meta-analysis of 24 studies noted strong positive correlation between GDM risk and maternal age.6 One of the population-based cohort studies in the meta-analysis examined relationships between maternal age and pregnancy outcomes in women living in British Columbia, Canada (n = 203,414). Data suggested that the relative risk of GDM increased linearly with maternal age to 3.2, 4.2, and 4.4 among women ages ≥ 35, ≥ 40, and ≥ 45 years, respectively.7
Social barriers to care. Although the prevalence of GDM has increased over the past few decades,1 from 2011 to 2019 the increase in GDM in individuals at first live birth was significantly higher in non-Hispanic Asian and Hispanic/Latina women than in non-Hispanic White women.8 Data from the Centers for Disease Control and Prevention further suggest that diabetes was more prevalent among individuals with a lower socioeconomic status as indicated by their level of education.9 Ogunwole et al10 suggest that racism is the root cause of these disparities and leads to long-term barriers to care (eg, socioeconomic deprivation, lack of health insurance, limited access to care, and poor health literacy), which ultimately contribute to the development of GDM and progression of diabetes. It is important for PCPs and all health professionals to be aware of these barriers so that they may practice mindfulness and deliver culturally sensitive care to patients from marginalized communities.
Family history of prediabetes. In a population-based cohort study (n = 7020), women with prediabetes (A1C, 5.7%-6.4%) were 2.8 times more likely to develop GDM compared with women with normal A1C (< 5.7%).11 Similar results were seen in a retrospective cohort study (n = 2812), in which women with prediabetes were more likely than women with a normal first trimester A1C to have GDM (29.1% vs 13.7%, respectively; adjusted relative risk = 1.48; 95% CI, 1.15-1.89).12 In both studies, prediabetes was not associated with a higher risk for adverse maternal or neonatal outcomes.11,12
Continue to: While there are no current...
While there are no current guidelines for treating prediabetes in pregnancy, women diagnosed with prediabetes in 1 study were found to have significantly less weight gain during pregnancy compared with patients with normal A1C,12 suggesting there may be a benefit in early identification and intervention, although further research is needed.11 In a separate case-control study (n = 345 women with GDM; n = 800 control), high rates of gestational weight gain (> 0.41 kg/wk) were associated with an increased risk of GDM (odds ratio [OR] = 1.74; 95% CI, 1.16-2.60) compared with women with the lowest rate of gestational weight gain (0.27-0.4 kg/wk [OR = 1.43; 95% CI, 0.96-2.14]).13 Thus, it is helpful to have proactive conversations about family planning and adequate weight and glycemic control with high-risk patients to prepare for a healthy pregnancy.
Obesity and weight management. Patients who are overweight (body mass index [BMI], 25-29.9) or obese (BMI > 30) have a substantially increased risk of GDM (adjusted OR = 1.44; 95% CI, 1.04-1.81), as seen in a retrospective cohort study of 1951 pregnant Malaysian women.14 Several factors have been found to contribute to successful weight control, including calorie prescription, a structured meal plan, high physical activity goals (60-90 min/d), daily weighing and monitoring of food intake, behavior therapy, and continued patient–provider contact.15
The safety, efficacy, and sustainability of weight loss with various dietary plans have been studied in individuals who are overweight and obese.16 Ultimately, energy expenditure must be greater than energy intake to promote weight loss. Conventional diets with continuous energy restriction (ie, low-fat, low-carbohydrate, and high-protein diets) have proven to be effective for short-term weight loss but data on long-term weight maintenance are limited.16 The Mediterranean diet, which is comprised mostly of vegetables, fruits, legumes, fish, and grains—with a lower intake of meat and dairy—may reduce gestational weight gain and risk of GDM as suggested by a randomized controlled trial (RCT; n = 1252).17 Although the choice of diet is up to the patient, it is important to be aware of different diets or refer the patient to a registered dietician who can help the patient if needed.
Reduce risk with adequate weight and glycemic control
Prevention of GDM during pregnancy should focus on weight maintenance and optimal glycemic control. Two systematic reviews, one with 8 RCTs (n = 1792) and another with 5 studies (n = 539), assessed the efficacy and safety of energy-restricted dietary intervention on GDM prevention.18 The first review found a significant reduction in gestational weight gain and improved glycemic control without increased risk of adverse maternal and fetal outcomes.18 The second review showed no clear difference between energy-restricted and non–energy-restricted diets on outcomes such as preeclampsia, gestational weight gain, large for gestational age, and macrosomia.18 These data suggest that while energy-restricted dietary interventions made no difference on maternal and fetal complications, they may still be safely used in pregnancy to reduce gestational weight gain and improve glycemic control.18
Once a woman is pregnant, it becomes difficult to lose weight because additional calories are needed to support a growing fetus. It is recommended that patients with healthy pregestational BMI consume an extra 200 to 300 calories/d after the first trimester. However, extra caloric intake in a woman with obesity who is pregnant leads to metabolic impairment and increased risk of diabetes for both the mother and fetus.19 Therefore, it is recommended that patients with obese pregestational BMI not consume additional calories because excess maternal fat is sufficient to support the energy needs of the growing fetus.19
Continue to: Ultimately, earlier intervention...
Ultimately, earlier intervention—prior to conception—helps patients prepare for a healthier pregnancy, resulting in better long-term outcomes. It is helpful to be familiar with the advantages and disadvantages of common approaches to weight management and to be able to refer patients to nutritionists for optimal planning. When establishing a dietary plan, consider patient-specific factors, such as cultural diets, financial and time constraints, and the patient’s readiness to make and maintain these changes. Consistent follow-up and behavioral therapy are necessary to maintain successful weight control.
There are many screening tools, but 1 is preferred in pregnancy
There are several ways to diagnose diabetes in patients who are not pregnant, including A1C, a fasting glucose test, an oral glucose tolerance test (OGTT), or random glucose testing (plus symptoms). However, the preferred method for diagnosing GDM is OGTT because it has a higher sensitivity.20 A1C, while a good measure of hyperglycemic stability, does not register hyperglycemia early enough to diagnose GDM and fasting glucose testing is less sensitive because for most women with GDM, that abnormal postprandial glucose level is the first glycemic abnormality.21
When to screen. Blood glucose levels should be checked in all pregnant women as part of their metabolic panel at the first prenatal visit. A reflex A1C for high glucose levels can be ordered based on the physician’s preference. This may help you to identify patients with prediabetes who are at risk for GDM and implement early behavioral and lifestyle changes. However, further research is needed to determine if intervention early in pregnancy can truly reduce the risk of GDM.11
Screening for GDM should be completed at 24 to 28 weeks of gestation20 because it is likely that this is when the hormonal effects of the placenta that contribute to insulin resistance set the woman up for postprandial hyperglycemia. Currently, there are no evidence-based guidelines for the use of continuous glucose monitoring prior to 24 weeks of gestation to identify GDM.20 If persistent hyperglycemia is present before 24 weeks of gestation, it is considered evidence of a pre-existing metabolic abnormality and is diagnosed as “pregestational diabetes.” Treatment should follow guidelines established for women who had diabetes prior to pregnancy.
How to screen? There is ongoing discussion about what is the optimal screening method for GDM: a 1-step strategy with a fasting 75-g OGTT only, or a 2-step strategy with a 50-g non-fasting glucose load test followed by a fasting 100-g OGTT in women who do not meet the plasma glucose cutoff (TABLE 1).22-24 Hillier et al25 compared the effectiveness of these strategies in diagnosing GDM and identifying pregnancy complications for the mother and infant. They found that while the 1-step strategy resulted in a 2-fold increase in the diagnosis of GDM, it did not lead to better outcomes for mothers and infants when compared with the 2-step method.25 Currently, the majority of obstetricians (95%) prefer to use the 2-step method.24

Continue to: Manage lifestyle, monitor glucose
Manage lifestyle, monitor glucose
Management of GDM in most women starts with diabetes self-management education and support for therapeutic lifestyle changes, such as nutritional interventions that reduce hyperglycemia and contribute to healthy weight gain during pregnancy.20 This may include medical nutrition therapy that focuses on adequate nutrition for the mother and fetus. Currently, the recommended dietary intake for women who are pregnant (regardless of diabetes) includes a minimum of 175 g of carbohydrates, 71 g of daily protein, and at least 28 g of fiber. Further refinement of dietary intake, including carbohydrate restriction, should be done with guidance from a registered dietitian.20 If the obstetrics team does not include a registered dietitian, a referral to one may be necessary. Regular physical activity should be continued throughout pregnancy as tolerated. Social support, stress reduction, and good sleep hygiene should be encouraged as much as possible.
For successful outcomes, therapeutic lifestyle changes should be coupled with glucose monitoring. The Fifth International Workshop-Conference on Gestational Diabetes Mellitus recommends that women with GDM monitor fasting blood glucose and typically 1-hour postprandial glucose. The glucose goals in GDM are as follows26:
- Fasting glucose < 95 mg/dL (5.3 mmol/L), and either
- 1-hour postprandial glucose < 140 mg/dL (7.8 mmol/L), or
- 2-hour postprandial glucose < 120 mg/dL (6.7 mmol/L).
Importantly, in the second and third trimester, the A1C goal for women with GDM is 6.0%. This is lower than the more traditional A1C goal for 2 reasons: (1) increases in A1C, even within the normal range, increase adverse outcomes; and (2) pregnant women will have an increased red blood cell count turnover, which can lower the A1C.27 In a historical cohort study (n = 27,213), Abell et al28 found that women who have an A1C < 6.0% in the second and third trimester have the lowest risk of giving birth to large-for-gestational-age infants and for having preeclampsia.
Add insulin if glucose targets are not met
Most women who engage in therapeutic lifestyle change (70%-85%) can achieve an A1C < 6% and will not need to take medication to manage GDM.29 If pharmacotherapy is needed to manage glucose, insulin is the preferred treatment for all women with GDM.20 Treatment should be individualized based on the glucose trends the woman is experiencing. Common treatments include bedtime NPH if fasting hyperglycemia is most prominent and analogue insulin at mealtimes for women with prominent postprandial hyperglycemia.
Noninsulin agents such as metformin and sulfonylureas are not currently recommended by the American College of Obstetricians and Gynecologists or the American Diabetes Association for use in GDM.20,24 Despite being used for years in women with pregestational diabetes, metabolic syndrome, and polycystic ovary syndrome, there is evidence that metformin crosses the placenta and fetal safety has not yet been established in RCTs. The Metformin in Gestational Diabetes: The Offspring Follow-Up (MiG TOFU) study was a longitudinal follow-up study that evaluated body composition and metabolic outcomes in children (ages 7-9 years) of women with GDM who had received metformin or insulin while pregnant.30 At age 9 years, children who were exposed to metformin weighed more and had a higher waist-to-height ratio and waist circumference than those exposed to insulin.30
Continue to: Sulfonylureas are no longer recommended...
Sulfonylureas are no longer recommended because of the risk of maternal and fetal hypoglycemia and concerns about this medication crossing the placenta.24,31,32 Specifically, in a 2015 meta-analysis and systematic review of 15 articles (n = 2509), glyburide had a higher risk of neonatal hypoglycemia and macrosomia than insulin or metformin.33 For women who cannot manage their glucose with therapeutic lifestyle changes and cannot take insulin, oral therapies may be considered if the risk-benefit ratio is balanced for that person.34
Watch for effects of poor glycemic control on mother, infant
Preeclampsia is defined as new-onset hypertension and proteinuria after 20 weeks of gestation. The correlation between GDM and preeclampsia has partly been explained by their shared overlapping risk factors, including maternal obesity, excessive gestational weight gain, and persistent hyperglycemia.35 On a biochemical level, these risk factors contribute to oxidative stress and systemic vascular dysfunction, which have been hypothesized as the underlying pathophysiology for the development of preeclampsia.35
Neonatal macrosomia, defined as a birth weight ≥ 4000 g, is a common complication that develops in 15% to 45% of infants of mothers with GDM.36 Placental transfer of glucose in mothers with hyperglycemia stimulates the secretion of neonatal insulin and the ultimate storage of the excess glucose as body fat. After delivery, the abrupt discontinuation of placental transfer of glucose to an infant who is actively secreting insulin leads to neonatal hypoglycemia, which if not detected or managed, can lead to long-term neurologic deficits, including recurrent seizures and developmental delays.37 Therefore, it is essential to screen for neonatal hypoglycemia immediately after birth and serially up to 12 hours.38
Postpartum T2D. Poor glycemic control increases the risk of increasing insulin resistance developing into T2D postpartum for mothers.39 It also increases the risk of obesity and insulin resistance later in life for the infant.40 A retrospective cohort study (n = 461) found a positive correlation between exposure to maternal GDM and elevated BMI in children ages 6 to 13 years.41 Kamana et al36 further discussed this correlation and suggested that exposure to maternal hyperglycemia in utero contributes to fetal programming of later adipose deposition. Children may develop without a notable increase in BMI until after puberty.42
Partner with specialists to improve outcomes
Although most women with GDM are managed by specialists (obstetricians, endocrinologists, and maternal-fetal medicine specialists),43 these patients are still seeking care from their family physicians for other complaints. These visits provide key touchpoints during pregnancy and are opportunities for PCPs to identify a pregnancy-related complication or provide additional education or referral to the obstetrician.
Continue to: Also, if you work in an area...
Also, if you work in an area where specialists are less accessible, you may be the clinician providing the majority of care to a patient with GDM. If this is the case, you’ll want to watch for the following risk factors, which should prompt a referral to specialty care:
- a previous pregnancy with GDM20
- a previous birth of an infant weighing > 4000 g44
- baseline history of hypertension45
- evidence of insulin resistance or polycystic ovary syndrome46,47
- a history of cardiovascular disease20
- a need to treat GDM with pharmacotherapy.48
Ensuring a smooth transition after the birth
Optimal communication and hand-offs throughout pregnancy and after delivery will benefit everyone. When the pregnant patient’s care has been managed by an obstetrician, it is important to address the following issues during the hand-off:
- baseline medical problems
- medical screenings and treatments in pregnancy (retinopathy and nephropathy screening)
- aspirin initiation, if indicated
- management of thyroid abnormalities
- management of mental health conditions
- postpartum glucose management and T2D screening postpartum
- management of complications identified during pregnancy (retinopathy and nephropathy).
Timing and other elements of postpartum care. The first postpartum screen should occur at 4 to 12 weeks postpartum. OGTT is recommended instead of A1C at this time because A1C may still be lowered by the increased red blood cell turnover related to pregnancy and blood loss at delivery. Because women with GDM have a 50% to 75% lifetime risk of T2D,20 patients with normal test results should be re-tested every 1 to 3 years using any of the standard screening methods (A1C, fasting glucose, or OGTT).20
After delivery it may be difficult for women to follow-up with their own personal health care because they are focused on the care of their baby. The increased use of telehealth may make postpartum follow-up visits easier to attend.
Visits present opportunities. Postpartum visits present another opportunity for PCPs to screen for diabetes and other postpartum complications, including depression and thyroid abnormalities. Visits are also an opportunity to discuss timely contraception so as to prevent an early, unplanned pregnancy. Other important aspects of postpartum care are outlined in TABLE 2.20,49

CORRESPONDENCE
Connie L. Ha, BS, OMS IV, Department of Primary Care, 1310 Club Drive, Touro University California, Vallejo, CA 94592; [email protected]
Gestational diabetes mellitus (GDM), defined as new-onset hyperglycemia detected in a pregnant woman after 24 weeks of gestation, affects 4% to 10% of pregnancies in the United States annually1 and is a major challenge for health care professionals.2 During pregnancy, the body’s physiologic responses are altered to support the growing fetus. One of these changes is an increase in insulin resistance, which suggests that pregnancy alone increases the patient’s risk for type 2 diabetes (T2D). However, several other factors also increase this risk, including maternal age, social barriers to care, obesity, poor weight control, and family history.

If not controlled, GDM results in poor health outcomes for the mother, such as preeclampsia, preterm labor, and maternal T2D.3-5 For the infant, intrauterine exposure to persistent hyperglycemia is correlated with neonatal macrosomia, hypoglycemia, perinatal complications (eg, preterm delivery, fetal demise), and obesity and insulin resistance later in life.4
Primary care physicians (PCPs) are the patient’s main point of contact prior to pregnancy. This relationship makes PCPs a resource for the patient and specialists during and after pregnancy. In this article, we discuss risk factors and how to screen for GDM, provide an update on practice recommendations for treatment and management of GDM in primary care, and describe the effects of uncontrolled GDM.
Know the key risk factors
Prevention begins with identifying the major risk factors that contribute to the development of GDM. These include maternal age, social barriers to care, family history of prediabetes, and obesity and poor weight control.
Older age. A meta-analysis of 24 studies noted strong positive correlation between GDM risk and maternal age.6 One of the population-based cohort studies in the meta-analysis examined relationships between maternal age and pregnancy outcomes in women living in British Columbia, Canada (n = 203,414). Data suggested that the relative risk of GDM increased linearly with maternal age to 3.2, 4.2, and 4.4 among women ages ≥ 35, ≥ 40, and ≥ 45 years, respectively.7
Social barriers to care. Although the prevalence of GDM has increased over the past few decades,1 from 2011 to 2019 the increase in GDM in individuals at first live birth was significantly higher in non-Hispanic Asian and Hispanic/Latina women than in non-Hispanic White women.8 Data from the Centers for Disease Control and Prevention further suggest that diabetes was more prevalent among individuals with a lower socioeconomic status as indicated by their level of education.9 Ogunwole et al10 suggest that racism is the root cause of these disparities and leads to long-term barriers to care (eg, socioeconomic deprivation, lack of health insurance, limited access to care, and poor health literacy), which ultimately contribute to the development of GDM and progression of diabetes. It is important for PCPs and all health professionals to be aware of these barriers so that they may practice mindfulness and deliver culturally sensitive care to patients from marginalized communities.
Family history of prediabetes. In a population-based cohort study (n = 7020), women with prediabetes (A1C, 5.7%-6.4%) were 2.8 times more likely to develop GDM compared with women with normal A1C (< 5.7%).11 Similar results were seen in a retrospective cohort study (n = 2812), in which women with prediabetes were more likely than women with a normal first trimester A1C to have GDM (29.1% vs 13.7%, respectively; adjusted relative risk = 1.48; 95% CI, 1.15-1.89).12 In both studies, prediabetes was not associated with a higher risk for adverse maternal or neonatal outcomes.11,12
Continue to: While there are no current...
While there are no current guidelines for treating prediabetes in pregnancy, women diagnosed with prediabetes in 1 study were found to have significantly less weight gain during pregnancy compared with patients with normal A1C,12 suggesting there may be a benefit in early identification and intervention, although further research is needed.11 In a separate case-control study (n = 345 women with GDM; n = 800 control), high rates of gestational weight gain (> 0.41 kg/wk) were associated with an increased risk of GDM (odds ratio [OR] = 1.74; 95% CI, 1.16-2.60) compared with women with the lowest rate of gestational weight gain (0.27-0.4 kg/wk [OR = 1.43; 95% CI, 0.96-2.14]).13 Thus, it is helpful to have proactive conversations about family planning and adequate weight and glycemic control with high-risk patients to prepare for a healthy pregnancy.
Obesity and weight management. Patients who are overweight (body mass index [BMI], 25-29.9) or obese (BMI > 30) have a substantially increased risk of GDM (adjusted OR = 1.44; 95% CI, 1.04-1.81), as seen in a retrospective cohort study of 1951 pregnant Malaysian women.14 Several factors have been found to contribute to successful weight control, including calorie prescription, a structured meal plan, high physical activity goals (60-90 min/d), daily weighing and monitoring of food intake, behavior therapy, and continued patient–provider contact.15
The safety, efficacy, and sustainability of weight loss with various dietary plans have been studied in individuals who are overweight and obese.16 Ultimately, energy expenditure must be greater than energy intake to promote weight loss. Conventional diets with continuous energy restriction (ie, low-fat, low-carbohydrate, and high-protein diets) have proven to be effective for short-term weight loss but data on long-term weight maintenance are limited.16 The Mediterranean diet, which is comprised mostly of vegetables, fruits, legumes, fish, and grains—with a lower intake of meat and dairy—may reduce gestational weight gain and risk of GDM as suggested by a randomized controlled trial (RCT; n = 1252).17 Although the choice of diet is up to the patient, it is important to be aware of different diets or refer the patient to a registered dietician who can help the patient if needed.
Reduce risk with adequate weight and glycemic control
Prevention of GDM during pregnancy should focus on weight maintenance and optimal glycemic control. Two systematic reviews, one with 8 RCTs (n = 1792) and another with 5 studies (n = 539), assessed the efficacy and safety of energy-restricted dietary intervention on GDM prevention.18 The first review found a significant reduction in gestational weight gain and improved glycemic control without increased risk of adverse maternal and fetal outcomes.18 The second review showed no clear difference between energy-restricted and non–energy-restricted diets on outcomes such as preeclampsia, gestational weight gain, large for gestational age, and macrosomia.18 These data suggest that while energy-restricted dietary interventions made no difference on maternal and fetal complications, they may still be safely used in pregnancy to reduce gestational weight gain and improve glycemic control.18
Once a woman is pregnant, it becomes difficult to lose weight because additional calories are needed to support a growing fetus. It is recommended that patients with healthy pregestational BMI consume an extra 200 to 300 calories/d after the first trimester. However, extra caloric intake in a woman with obesity who is pregnant leads to metabolic impairment and increased risk of diabetes for both the mother and fetus.19 Therefore, it is recommended that patients with obese pregestational BMI not consume additional calories because excess maternal fat is sufficient to support the energy needs of the growing fetus.19
Continue to: Ultimately, earlier intervention...
Ultimately, earlier intervention—prior to conception—helps patients prepare for a healthier pregnancy, resulting in better long-term outcomes. It is helpful to be familiar with the advantages and disadvantages of common approaches to weight management and to be able to refer patients to nutritionists for optimal planning. When establishing a dietary plan, consider patient-specific factors, such as cultural diets, financial and time constraints, and the patient’s readiness to make and maintain these changes. Consistent follow-up and behavioral therapy are necessary to maintain successful weight control.
There are many screening tools, but 1 is preferred in pregnancy
There are several ways to diagnose diabetes in patients who are not pregnant, including A1C, a fasting glucose test, an oral glucose tolerance test (OGTT), or random glucose testing (plus symptoms). However, the preferred method for diagnosing GDM is OGTT because it has a higher sensitivity.20 A1C, while a good measure of hyperglycemic stability, does not register hyperglycemia early enough to diagnose GDM and fasting glucose testing is less sensitive because for most women with GDM, that abnormal postprandial glucose level is the first glycemic abnormality.21
When to screen. Blood glucose levels should be checked in all pregnant women as part of their metabolic panel at the first prenatal visit. A reflex A1C for high glucose levels can be ordered based on the physician’s preference. This may help you to identify patients with prediabetes who are at risk for GDM and implement early behavioral and lifestyle changes. However, further research is needed to determine if intervention early in pregnancy can truly reduce the risk of GDM.11
Screening for GDM should be completed at 24 to 28 weeks of gestation20 because it is likely that this is when the hormonal effects of the placenta that contribute to insulin resistance set the woman up for postprandial hyperglycemia. Currently, there are no evidence-based guidelines for the use of continuous glucose monitoring prior to 24 weeks of gestation to identify GDM.20 If persistent hyperglycemia is present before 24 weeks of gestation, it is considered evidence of a pre-existing metabolic abnormality and is diagnosed as “pregestational diabetes.” Treatment should follow guidelines established for women who had diabetes prior to pregnancy.
How to screen? There is ongoing discussion about what is the optimal screening method for GDM: a 1-step strategy with a fasting 75-g OGTT only, or a 2-step strategy with a 50-g non-fasting glucose load test followed by a fasting 100-g OGTT in women who do not meet the plasma glucose cutoff (TABLE 1).22-24 Hillier et al25 compared the effectiveness of these strategies in diagnosing GDM and identifying pregnancy complications for the mother and infant. They found that while the 1-step strategy resulted in a 2-fold increase in the diagnosis of GDM, it did not lead to better outcomes for mothers and infants when compared with the 2-step method.25 Currently, the majority of obstetricians (95%) prefer to use the 2-step method.24

Continue to: Manage lifestyle, monitor glucose
Manage lifestyle, monitor glucose
Management of GDM in most women starts with diabetes self-management education and support for therapeutic lifestyle changes, such as nutritional interventions that reduce hyperglycemia and contribute to healthy weight gain during pregnancy.20 This may include medical nutrition therapy that focuses on adequate nutrition for the mother and fetus. Currently, the recommended dietary intake for women who are pregnant (regardless of diabetes) includes a minimum of 175 g of carbohydrates, 71 g of daily protein, and at least 28 g of fiber. Further refinement of dietary intake, including carbohydrate restriction, should be done with guidance from a registered dietitian.20 If the obstetrics team does not include a registered dietitian, a referral to one may be necessary. Regular physical activity should be continued throughout pregnancy as tolerated. Social support, stress reduction, and good sleep hygiene should be encouraged as much as possible.
For successful outcomes, therapeutic lifestyle changes should be coupled with glucose monitoring. The Fifth International Workshop-Conference on Gestational Diabetes Mellitus recommends that women with GDM monitor fasting blood glucose and typically 1-hour postprandial glucose. The glucose goals in GDM are as follows26:
- Fasting glucose < 95 mg/dL (5.3 mmol/L), and either
- 1-hour postprandial glucose < 140 mg/dL (7.8 mmol/L), or
- 2-hour postprandial glucose < 120 mg/dL (6.7 mmol/L).
Importantly, in the second and third trimester, the A1C goal for women with GDM is 6.0%. This is lower than the more traditional A1C goal for 2 reasons: (1) increases in A1C, even within the normal range, increase adverse outcomes; and (2) pregnant women will have an increased red blood cell count turnover, which can lower the A1C.27 In a historical cohort study (n = 27,213), Abell et al28 found that women who have an A1C < 6.0% in the second and third trimester have the lowest risk of giving birth to large-for-gestational-age infants and for having preeclampsia.
Add insulin if glucose targets are not met
Most women who engage in therapeutic lifestyle change (70%-85%) can achieve an A1C < 6% and will not need to take medication to manage GDM.29 If pharmacotherapy is needed to manage glucose, insulin is the preferred treatment for all women with GDM.20 Treatment should be individualized based on the glucose trends the woman is experiencing. Common treatments include bedtime NPH if fasting hyperglycemia is most prominent and analogue insulin at mealtimes for women with prominent postprandial hyperglycemia.
Noninsulin agents such as metformin and sulfonylureas are not currently recommended by the American College of Obstetricians and Gynecologists or the American Diabetes Association for use in GDM.20,24 Despite being used for years in women with pregestational diabetes, metabolic syndrome, and polycystic ovary syndrome, there is evidence that metformin crosses the placenta and fetal safety has not yet been established in RCTs. The Metformin in Gestational Diabetes: The Offspring Follow-Up (MiG TOFU) study was a longitudinal follow-up study that evaluated body composition and metabolic outcomes in children (ages 7-9 years) of women with GDM who had received metformin or insulin while pregnant.30 At age 9 years, children who were exposed to metformin weighed more and had a higher waist-to-height ratio and waist circumference than those exposed to insulin.30
Continue to: Sulfonylureas are no longer recommended...
Sulfonylureas are no longer recommended because of the risk of maternal and fetal hypoglycemia and concerns about this medication crossing the placenta.24,31,32 Specifically, in a 2015 meta-analysis and systematic review of 15 articles (n = 2509), glyburide had a higher risk of neonatal hypoglycemia and macrosomia than insulin or metformin.33 For women who cannot manage their glucose with therapeutic lifestyle changes and cannot take insulin, oral therapies may be considered if the risk-benefit ratio is balanced for that person.34
Watch for effects of poor glycemic control on mother, infant
Preeclampsia is defined as new-onset hypertension and proteinuria after 20 weeks of gestation. The correlation between GDM and preeclampsia has partly been explained by their shared overlapping risk factors, including maternal obesity, excessive gestational weight gain, and persistent hyperglycemia.35 On a biochemical level, these risk factors contribute to oxidative stress and systemic vascular dysfunction, which have been hypothesized as the underlying pathophysiology for the development of preeclampsia.35
Neonatal macrosomia, defined as a birth weight ≥ 4000 g, is a common complication that develops in 15% to 45% of infants of mothers with GDM.36 Placental transfer of glucose in mothers with hyperglycemia stimulates the secretion of neonatal insulin and the ultimate storage of the excess glucose as body fat. After delivery, the abrupt discontinuation of placental transfer of glucose to an infant who is actively secreting insulin leads to neonatal hypoglycemia, which if not detected or managed, can lead to long-term neurologic deficits, including recurrent seizures and developmental delays.37 Therefore, it is essential to screen for neonatal hypoglycemia immediately after birth and serially up to 12 hours.38
Postpartum T2D. Poor glycemic control increases the risk of increasing insulin resistance developing into T2D postpartum for mothers.39 It also increases the risk of obesity and insulin resistance later in life for the infant.40 A retrospective cohort study (n = 461) found a positive correlation between exposure to maternal GDM and elevated BMI in children ages 6 to 13 years.41 Kamana et al36 further discussed this correlation and suggested that exposure to maternal hyperglycemia in utero contributes to fetal programming of later adipose deposition. Children may develop without a notable increase in BMI until after puberty.42
Partner with specialists to improve outcomes
Although most women with GDM are managed by specialists (obstetricians, endocrinologists, and maternal-fetal medicine specialists),43 these patients are still seeking care from their family physicians for other complaints. These visits provide key touchpoints during pregnancy and are opportunities for PCPs to identify a pregnancy-related complication or provide additional education or referral to the obstetrician.
Continue to: Also, if you work in an area...
Also, if you work in an area where specialists are less accessible, you may be the clinician providing the majority of care to a patient with GDM. If this is the case, you’ll want to watch for the following risk factors, which should prompt a referral to specialty care:
- a previous pregnancy with GDM20
- a previous birth of an infant weighing > 4000 g44
- baseline history of hypertension45
- evidence of insulin resistance or polycystic ovary syndrome46,47
- a history of cardiovascular disease20
- a need to treat GDM with pharmacotherapy.48
Ensuring a smooth transition after the birth
Optimal communication and hand-offs throughout pregnancy and after delivery will benefit everyone. When the pregnant patient’s care has been managed by an obstetrician, it is important to address the following issues during the hand-off:
- baseline medical problems
- medical screenings and treatments in pregnancy (retinopathy and nephropathy screening)
- aspirin initiation, if indicated
- management of thyroid abnormalities
- management of mental health conditions
- postpartum glucose management and T2D screening postpartum
- management of complications identified during pregnancy (retinopathy and nephropathy).
Timing and other elements of postpartum care. The first postpartum screen should occur at 4 to 12 weeks postpartum. OGTT is recommended instead of A1C at this time because A1C may still be lowered by the increased red blood cell turnover related to pregnancy and blood loss at delivery. Because women with GDM have a 50% to 75% lifetime risk of T2D,20 patients with normal test results should be re-tested every 1 to 3 years using any of the standard screening methods (A1C, fasting glucose, or OGTT).20
After delivery it may be difficult for women to follow-up with their own personal health care because they are focused on the care of their baby. The increased use of telehealth may make postpartum follow-up visits easier to attend.
Visits present opportunities. Postpartum visits present another opportunity for PCPs to screen for diabetes and other postpartum complications, including depression and thyroid abnormalities. Visits are also an opportunity to discuss timely contraception so as to prevent an early, unplanned pregnancy. Other important aspects of postpartum care are outlined in TABLE 2.20,49

CORRESPONDENCE
Connie L. Ha, BS, OMS IV, Department of Primary Care, 1310 Club Drive, Touro University California, Vallejo, CA 94592; [email protected]
1. Sheiner E. Gestational diabetes mellitus: long-term consequences for the mother and child grand challenge: how to move on towards secondary prevention? Front Clin Diabetes Healthc. 2020. doi: 10.3389/fcdhc.2020.546256
2. Angueira AR, Ludvik AE, Reddy TE, et al. New insights into gestational glucose metabolism: lessons learned from 21st century approaches. Diabetes. 2015;64:327-334. doi: 10.2337/db14-0877
3. Shou C, Wei Y-M, Wang C, et al. Updates in long-term maternal and fetal adverse effects of gestational diabetes mellitus. Maternal-Fetal Med. 2019;1:91-94. doi: 10.1097/FM9.0000000000000019
4. Plows JF, Stanley JL, Baker PN, et al. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. 2018;19:3342. doi: 10.3390/ijms19113342
5. Kulshrestha V, Agarwal N. Maternal complications in pregnancy with diabetes. J Pak Med Assoc. 2016;66(9 suppl 1):S74-S77.
6. Li Y, Ren X, He L, et al. Maternal age and the risk of gestational diabetes mellitus: a systematic review and meta-analysis of over 120 million participants. Diabetes Res Clin Pract. 2020;162:108044. doi: 10.1016/j.diabres.2020.108044
7. Schummers L, Hutcheon JA, Hacker MR, et al. Absolute risks of obstetric outcomes by maternal age at first birth: a population-based cohort. Epidemiology. 2018;29:379-387. doi: 10.1097/EDE.0000000000000818
8. Shah NS, Wang MC, Freaney PM, et al. Trends in gestational diabetes at first live birth by race and ethnicity in the US, 2011-2019. JAMA. 2021;326:660-669. doi: 10.1001/jama.2021.7217
9. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2020. Accessed February 2, 2022. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
10. Ogunwole SM, Golden SH. Social determinants of health and structural inequities—root causes of diabetes disparities. Diabetes Care. 2021;44:11-13. doi: 10.2337/dci20-0060
11. Chen L, Pocobelli G, Yu O, et al. Early pregnancy hemoglobin A1C and pregnancy outcomes: a population-based study. Am J Perinatol. 2019;36:1045-1053. doi: 10.1055/s-0038-1675619
12. Osmundson S, Zhao BS, Kunz L, et al. First trimester hemoglobin A1C prediction of gestational diabetes. Am J Perinatol. 2016;33:977-982. doi: 10.1055/s-0036-1581055
13. Hedderson MM, Gunderson EP, Ferrara A. Gestational weight gain and risk of gestational diabetes mellitus [published correction appears in Obstet Gynecol. 2010;115:1092]. Obstet Gynecol. 2010;115:597-604. doi: 10.1097/AOG.0b013e3181cfce4f
14. Yong HY, Mohd Shariff Z, Mohd Yusof BN, et al. Independent and combined effects of age, body mass index and gestational weight gain on the risk of gestational diabetes mellitus. Sci Rep. 2020;10:8486. doi: 10.1038/s41598-020-65251-2
15. Phelan S. Windows of opportunity for lifestyle interventions to prevent gestational diabetes mellitus. Am J Perinatol. 2016;33:1291-1299. doi: 10.1055/s-0036-1586504
16. Koliaki C, Spinos T, Spinou M, et al. Defining the optimal dietary approach for safe, effective and sustainable weight loss in overweight and obese adults. Healthcare (Basel). 2018;6:73. doi: 10.3390/healthcare6030073
17. Al Wattar BH, Dodds J, Placzek A, et al. Mediterranean-style diet in pregnant women with metabolic risk factors (ESTEEM): a pragmatic multicentre randomised trial. PLOS Med. 2019;16:e1002857. doi: 10.1371/journal.pmed.1002857
18. Zarogiannis S. Are novel lifestyle approaches to management of type 2 diabetes applicable to prevention and treatment of women with gestational diabetes mellitus? Global Diabetes Open Access J. 2019;1:1-14.
19. Most J, Amant MS, Hsia DS, et al. Evidence-based recommendations for energy intake in pregnant women with obesity. J Clin Invest. 2019;129:4682-4690. doi: 10.1172/JCI130341
20. American Diabetes Association. 14. Management of diabetes in pregnancy: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S200-S210. doi: 10.2337/dc21-S014
21. McIntyre HD, Sacks DA, Barbour LA, et al. Issues with the diagnosis and classification of hyperglycemia in early pregnancy. Diabetes Care. 2016;39:53-54. doi: 10.2337/dc15-1887
22. American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S17-S38. doi: 10.2337/dc22-S002
23. Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768-773. doi: 10.1016/0002-9378(82)90349-0
24. ACOG Practice Bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131:e49-e64. doi: 10.1097/AOG.0000000000002501
25. Hillier TA, Pedula KL, Ogasawara KK, et al. A pragmatic, randomized clinical trial of gestational diabetes screening. N Engl J Med. 2021;384:895-904. doi: 10.1056/NEJMoa2026028
26. Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(suppl 2):S251-S260. doi: 10.2337/dc07-s225
27. Nielsen LR, Ekbom P, Damm P, et al. HbA1c levels are significantly lower in early and late pregnancy. Diabetes Care. 2004;27:1200-1201. doi: 10.2337/diacare.27.5.1200
28. Abell SK, Boyle JA, de Courten B, et al. Impact of type 2 diabetes, obesity and glycaemic control on pregnancy outcomes. Aust N Z J Obstet Gynaecol. 2017;57:308-314. doi: 10.1111/ajo.12521
29. Viana LV, Gross JL, Azevedo MJ. Dietary intervention in patients with gestational diabetes mellitus: a systematic review and meta-analysis of randomized clinical trials on maternal and newborn outcomes. Diabetes Care. 2014;37:3345-3355. doi: 10.2337/dc14-1530
30. Rowan JA, Rush EC, Plank LD, et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition and metabolic outcomes at 7-9 years of age. BMJ Open Diabetes Res Care. 2018;6:e000456. doi: 10.1136/bmjdrc-2017-000456
31. Hebert MF, Ma X, Naraharisetti SB, et al. Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clin Pharmacol Ther. 2009;85:607-614. doi: 10.1038/clpt.2009.5
32. Malek R, Davis SN. Pharmacokinetics, efficacy and safety of glyburide for treatment of gestational diabetes mellitus. Expert Opin Drug Metab Toxicol. 2016;12:691-699. doi: 10.1080/17425255.2016.1187131
33. Balsells M, García-Patterson A, Solà I, et al. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ. 2015;350:h102. doi: 10.1136/bmj.h102
34. Kavitha N, De S, Kanagasabai S. Oral hypoglycemic agents in pregnancy: an update. J Obstet Gynaecol India. 2013;63:82-87. doi: 10.1007/s13224-012-0312-z
35. Weissgerber TL, Mudd LM. Preeclampsia and diabetes. Curr Diab Rep. 2015;15:9. doi: 10.1007/s11892-015-0579-4
36. Kamana KC, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metab. 2015;66(suppl 2):14-20. doi: 10.1159/000371628
37. Mitanchez D, Yzydorczyk C, Simeoni U. What neonatal complications should the pediatrician be aware of in case of maternal gestational diabetes? World J Diabetes. 2015;6:734-743. doi: 10.4239/wjd.v6.i5.734
38. Stanescu A, Stoicescu SM. Neonatal hypoglycemia screening in newborns from diabetic mothers—arguments and controversies. J Med Life. 2014;7(spec iss 3):51-52.
39. Kim C. Maternal outcomes and follow-up after gestational diabetes mellitus. Diabet Med. 2014;31:292-301. doi: 10.1111/dme.12382
40. Stewart A, Malhotra A. Gestational diabetes and the neonate: challenges and solutions. Res Rep Neonatol. 2015;5:31-39. doi: 10.2147/RRN.S30971
41. Crume TL, Ogden L, West NA, et al. Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: the Exploring Perinatal Outcomes among Children (EPOCH) Study. Diabetologia. 2011;54:87-92. doi: 10.1007/s00125-010-1925-3
42. Crume TL, Ogden L, Daniels S, et al. The impact of in utero exposure to diabetes on childhood body mass index growth trajectories: the EPOCH study. J Pediatr. 2011;158:941-946. doi: 10.1016/j.jpeds.2010.12.007
43. Levels of maternal care. Obstetric Care Consensus No. 9. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2019;134:e41-e55. doi: 10.1097/AOG.0000000000003383
44. Caughey AB, Cheng YW, Stotland NE, et al. Maternal and paternal race/ethnicity are both associated with gestational diabetes. Am J Obstet Gynecol. 2010;202:616.e1-e5. doi: 10.1016/j.ajog.2010.01.082
45. Yogev Y, Xenakis EM, Langer O. The association between preeclampsia and severity of gestational diabetes: the impact of glycemic control. Am J Obstet Gynecol. 2004;191:1655-1660. doi: 10.1016/j.ajog.2004.03.074
46. Brown J, Alwan NA, West J, et al. Lifestyle interventions for the treatment of women with gestational diabetes. Cochrane Database Syst Rev. 2017;5:CD011970. doi: 10.1002/14651858.CD011970.pub2
47. Ceysens G, Rouiller D, Boulvain M. Exercise for the diabetic pregnant woman. Cochrane Database Syst Rev. 2006;3:CD004225. doi: 10.1002/14651858.CD004225.pub2
48. Chawla R, Mukherjee JJ, Chawla M, et al. Expert group recommendations on the effective use of bolus insulin in the management of type 2 diabetes mellitus. Med Sci (Basel). 2021;9:38. doi: 10.3390/medsci9020038
49. American Diabetes Association. Introduction: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S1-S2. doi: 10.2337/dc22-Sint
1. Sheiner E. Gestational diabetes mellitus: long-term consequences for the mother and child grand challenge: how to move on towards secondary prevention? Front Clin Diabetes Healthc. 2020. doi: 10.3389/fcdhc.2020.546256
2. Angueira AR, Ludvik AE, Reddy TE, et al. New insights into gestational glucose metabolism: lessons learned from 21st century approaches. Diabetes. 2015;64:327-334. doi: 10.2337/db14-0877
3. Shou C, Wei Y-M, Wang C, et al. Updates in long-term maternal and fetal adverse effects of gestational diabetes mellitus. Maternal-Fetal Med. 2019;1:91-94. doi: 10.1097/FM9.0000000000000019
4. Plows JF, Stanley JL, Baker PN, et al. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. 2018;19:3342. doi: 10.3390/ijms19113342
5. Kulshrestha V, Agarwal N. Maternal complications in pregnancy with diabetes. J Pak Med Assoc. 2016;66(9 suppl 1):S74-S77.
6. Li Y, Ren X, He L, et al. Maternal age and the risk of gestational diabetes mellitus: a systematic review and meta-analysis of over 120 million participants. Diabetes Res Clin Pract. 2020;162:108044. doi: 10.1016/j.diabres.2020.108044
7. Schummers L, Hutcheon JA, Hacker MR, et al. Absolute risks of obstetric outcomes by maternal age at first birth: a population-based cohort. Epidemiology. 2018;29:379-387. doi: 10.1097/EDE.0000000000000818
8. Shah NS, Wang MC, Freaney PM, et al. Trends in gestational diabetes at first live birth by race and ethnicity in the US, 2011-2019. JAMA. 2021;326:660-669. doi: 10.1001/jama.2021.7217
9. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2020. Accessed February 2, 2022. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
10. Ogunwole SM, Golden SH. Social determinants of health and structural inequities—root causes of diabetes disparities. Diabetes Care. 2021;44:11-13. doi: 10.2337/dci20-0060
11. Chen L, Pocobelli G, Yu O, et al. Early pregnancy hemoglobin A1C and pregnancy outcomes: a population-based study. Am J Perinatol. 2019;36:1045-1053. doi: 10.1055/s-0038-1675619
12. Osmundson S, Zhao BS, Kunz L, et al. First trimester hemoglobin A1C prediction of gestational diabetes. Am J Perinatol. 2016;33:977-982. doi: 10.1055/s-0036-1581055
13. Hedderson MM, Gunderson EP, Ferrara A. Gestational weight gain and risk of gestational diabetes mellitus [published correction appears in Obstet Gynecol. 2010;115:1092]. Obstet Gynecol. 2010;115:597-604. doi: 10.1097/AOG.0b013e3181cfce4f
14. Yong HY, Mohd Shariff Z, Mohd Yusof BN, et al. Independent and combined effects of age, body mass index and gestational weight gain on the risk of gestational diabetes mellitus. Sci Rep. 2020;10:8486. doi: 10.1038/s41598-020-65251-2
15. Phelan S. Windows of opportunity for lifestyle interventions to prevent gestational diabetes mellitus. Am J Perinatol. 2016;33:1291-1299. doi: 10.1055/s-0036-1586504
16. Koliaki C, Spinos T, Spinou M, et al. Defining the optimal dietary approach for safe, effective and sustainable weight loss in overweight and obese adults. Healthcare (Basel). 2018;6:73. doi: 10.3390/healthcare6030073
17. Al Wattar BH, Dodds J, Placzek A, et al. Mediterranean-style diet in pregnant women with metabolic risk factors (ESTEEM): a pragmatic multicentre randomised trial. PLOS Med. 2019;16:e1002857. doi: 10.1371/journal.pmed.1002857
18. Zarogiannis S. Are novel lifestyle approaches to management of type 2 diabetes applicable to prevention and treatment of women with gestational diabetes mellitus? Global Diabetes Open Access J. 2019;1:1-14.
19. Most J, Amant MS, Hsia DS, et al. Evidence-based recommendations for energy intake in pregnant women with obesity. J Clin Invest. 2019;129:4682-4690. doi: 10.1172/JCI130341
20. American Diabetes Association. 14. Management of diabetes in pregnancy: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S200-S210. doi: 10.2337/dc21-S014
21. McIntyre HD, Sacks DA, Barbour LA, et al. Issues with the diagnosis and classification of hyperglycemia in early pregnancy. Diabetes Care. 2016;39:53-54. doi: 10.2337/dc15-1887
22. American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S17-S38. doi: 10.2337/dc22-S002
23. Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768-773. doi: 10.1016/0002-9378(82)90349-0
24. ACOG Practice Bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131:e49-e64. doi: 10.1097/AOG.0000000000002501
25. Hillier TA, Pedula KL, Ogasawara KK, et al. A pragmatic, randomized clinical trial of gestational diabetes screening. N Engl J Med. 2021;384:895-904. doi: 10.1056/NEJMoa2026028
26. Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(suppl 2):S251-S260. doi: 10.2337/dc07-s225
27. Nielsen LR, Ekbom P, Damm P, et al. HbA1c levels are significantly lower in early and late pregnancy. Diabetes Care. 2004;27:1200-1201. doi: 10.2337/diacare.27.5.1200
28. Abell SK, Boyle JA, de Courten B, et al. Impact of type 2 diabetes, obesity and glycaemic control on pregnancy outcomes. Aust N Z J Obstet Gynaecol. 2017;57:308-314. doi: 10.1111/ajo.12521
29. Viana LV, Gross JL, Azevedo MJ. Dietary intervention in patients with gestational diabetes mellitus: a systematic review and meta-analysis of randomized clinical trials on maternal and newborn outcomes. Diabetes Care. 2014;37:3345-3355. doi: 10.2337/dc14-1530
30. Rowan JA, Rush EC, Plank LD, et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition and metabolic outcomes at 7-9 years of age. BMJ Open Diabetes Res Care. 2018;6:e000456. doi: 10.1136/bmjdrc-2017-000456
31. Hebert MF, Ma X, Naraharisetti SB, et al. Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clin Pharmacol Ther. 2009;85:607-614. doi: 10.1038/clpt.2009.5
32. Malek R, Davis SN. Pharmacokinetics, efficacy and safety of glyburide for treatment of gestational diabetes mellitus. Expert Opin Drug Metab Toxicol. 2016;12:691-699. doi: 10.1080/17425255.2016.1187131
33. Balsells M, García-Patterson A, Solà I, et al. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ. 2015;350:h102. doi: 10.1136/bmj.h102
34. Kavitha N, De S, Kanagasabai S. Oral hypoglycemic agents in pregnancy: an update. J Obstet Gynaecol India. 2013;63:82-87. doi: 10.1007/s13224-012-0312-z
35. Weissgerber TL, Mudd LM. Preeclampsia and diabetes. Curr Diab Rep. 2015;15:9. doi: 10.1007/s11892-015-0579-4
36. Kamana KC, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metab. 2015;66(suppl 2):14-20. doi: 10.1159/000371628
37. Mitanchez D, Yzydorczyk C, Simeoni U. What neonatal complications should the pediatrician be aware of in case of maternal gestational diabetes? World J Diabetes. 2015;6:734-743. doi: 10.4239/wjd.v6.i5.734
38. Stanescu A, Stoicescu SM. Neonatal hypoglycemia screening in newborns from diabetic mothers—arguments and controversies. J Med Life. 2014;7(spec iss 3):51-52.
39. Kim C. Maternal outcomes and follow-up after gestational diabetes mellitus. Diabet Med. 2014;31:292-301. doi: 10.1111/dme.12382
40. Stewart A, Malhotra A. Gestational diabetes and the neonate: challenges and solutions. Res Rep Neonatol. 2015;5:31-39. doi: 10.2147/RRN.S30971
41. Crume TL, Ogden L, West NA, et al. Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: the Exploring Perinatal Outcomes among Children (EPOCH) Study. Diabetologia. 2011;54:87-92. doi: 10.1007/s00125-010-1925-3
42. Crume TL, Ogden L, Daniels S, et al. The impact of in utero exposure to diabetes on childhood body mass index growth trajectories: the EPOCH study. J Pediatr. 2011;158:941-946. doi: 10.1016/j.jpeds.2010.12.007
43. Levels of maternal care. Obstetric Care Consensus No. 9. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2019;134:e41-e55. doi: 10.1097/AOG.0000000000003383
44. Caughey AB, Cheng YW, Stotland NE, et al. Maternal and paternal race/ethnicity are both associated with gestational diabetes. Am J Obstet Gynecol. 2010;202:616.e1-e5. doi: 10.1016/j.ajog.2010.01.082
45. Yogev Y, Xenakis EM, Langer O. The association between preeclampsia and severity of gestational diabetes: the impact of glycemic control. Am J Obstet Gynecol. 2004;191:1655-1660. doi: 10.1016/j.ajog.2004.03.074
46. Brown J, Alwan NA, West J, et al. Lifestyle interventions for the treatment of women with gestational diabetes. Cochrane Database Syst Rev. 2017;5:CD011970. doi: 10.1002/14651858.CD011970.pub2
47. Ceysens G, Rouiller D, Boulvain M. Exercise for the diabetic pregnant woman. Cochrane Database Syst Rev. 2006;3:CD004225. doi: 10.1002/14651858.CD004225.pub2
48. Chawla R, Mukherjee JJ, Chawla M, et al. Expert group recommendations on the effective use of bolus insulin in the management of type 2 diabetes mellitus. Med Sci (Basel). 2021;9:38. doi: 10.3390/medsci9020038
49. American Diabetes Association. Introduction: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S1-S2. doi: 10.2337/dc22-Sint
PRACTICE RECOMMENDATIONS
› Manage gestational diabetes mellitus (GDM) with lifestyle behavior changes first and add insulin as a secondary treatment only if glycemic targets are not being met. A
› Treat hyperglycemia in GDM with insulin, not metformin or glyburide; these agents cross the placenta to the fetus. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
COVID-19 vaccine does not affect in vitro fertilization outcomes
Getting a COVID-19 mRNA vaccine did not affect pregnancy rates for women trying to conceive with in vitro fertilization or ovarian response to treatment, findings of a new study indicate.
The study was led by Sarit Avraham, MD, with the IVF unit, department of obstetrics and gynecology, Shamir Medical Center in Tzrifi, Israel. The findings were published online in Fertility and Sterility in a preproof version.
“Women should be vaccinated for COVID-19 prior to attempting to conceive via IVF treatments, given the higher risk of severe illness in pregnant women,” the authors wrote.
Doubts arose from “the theoretical concept of the supposed similarity between the SARS-CoV-2 spike protein and the syncytin protein that is speculated to take part in the fertilization process and the formation of the placenta,” the authors wrote.
Some then assumed that the COVID vaccine might kick off an immune response that could affect implantation and pregnancy. But this study and others before it found otherwise.
Researchers included 200 vaccinated women trying to conceive with IVF treatments in the retrospective study, and compared them with 200 unvaccinated patients of similar age (average age in both groups, 36 years) who were not previously infected with COVID-19. All the women were undergoing IVF from January to April 2021 and all the vaccinated women completed two doses of the BNT162b2 (Pfizer/BioNTech) vaccine at least 2 weeks before ovarian stimulation.
Researchers compared the average number of oocytes retrieved and clinical pregnancy rates between the two groups.
No difference between groups
Two hundred patients underwent oocyte retrieval 14-68 days after receiving a COVID shot; there was no significant difference by vaccination status in the number retrieved per cycle (10.63 in the vaccinated group vs. 10.72 in the unvaccinated group; P = .93).
There was also no difference in the clinical pregnancy rates after fresh embryo transfers. The rate among 128 vaccinated patients was 32.8% versus 33.1% in the 133 unvaccinated patients (P = .96), with 42 and 44 clinical pregnancies, respectively.
A total of 113 patients (66 in the study group and 47 in the controls) underwent freeze-all cycles to preserve fertility and fertilization rates were similar between vaccinated and unvaccinated (55.43% vaccinated vs. 54.29% unvaccinated; P = .73). The average number of cryopreserved embryos was 3.59 (vaccinated) versus 3.28 (unvaccinated) (P = .80).
In a subanalysis of outcomes by age, researchers found vaccination status had no effect on number of oocytes or pregnancy rates in the 39-and-older group. That’s important because it shows the vaccine did not affect outcomes even in a population with reduced ovarian reserves, the authors wrote.
The authors noted one of the study’s limitations is that it didn’t include information about vaccination or past infection status of the male partners.
Question should be put to rest
Sarah Cross, MD, a maternal-fetal medicine specialist at the University of Minnesota, Minneapolis, said the study is the biggest she’s seen that concludes COVID vaccinations are safe and highly encouraged for women before trying to conceive, but other smaller studies have come to the same conclusion.
She pointed to research including a study from 2021 with similar findings that concluded: “Physicians and public health personnel can counsel women of reproductive age that neither previous illness with COVID-19 nor antibodies produced from vaccination to COVID-19 will cause sterility.”
She said she thinks the question of whether COVID shots are safe with IVF has been answered and the results of the latest study add proof to counter misinformation around the issue.
“The COVID-19 vaccine does not affect fertility,” she said. “I don’t know how many more [studies] we need.”
The harm is in not getting vaccinated, she said. Pregnancy significantly increases a woman’s chance of getting severe COVID, the need for hospitalization, mechanical ventilation, and risk of death.
“I personally have never had a hospitalized patient who’s been vaccinated,” Dr. Cross said. “The worst thing for the fetus is to have a critically ill mother.”
Dr. Cross, whose high-risk patients include those seeking counseling before IVF, added: “I would counsel all of them that they should absolutely get vaccinated prior to pregnancy, when they’re pregnant, whenever it is, as soon as they possibly can.”
The study authors and Dr. Cross report no relevant financial relationships.
Getting a COVID-19 mRNA vaccine did not affect pregnancy rates for women trying to conceive with in vitro fertilization or ovarian response to treatment, findings of a new study indicate.
The study was led by Sarit Avraham, MD, with the IVF unit, department of obstetrics and gynecology, Shamir Medical Center in Tzrifi, Israel. The findings were published online in Fertility and Sterility in a preproof version.
“Women should be vaccinated for COVID-19 prior to attempting to conceive via IVF treatments, given the higher risk of severe illness in pregnant women,” the authors wrote.
Doubts arose from “the theoretical concept of the supposed similarity between the SARS-CoV-2 spike protein and the syncytin protein that is speculated to take part in the fertilization process and the formation of the placenta,” the authors wrote.
Some then assumed that the COVID vaccine might kick off an immune response that could affect implantation and pregnancy. But this study and others before it found otherwise.
Researchers included 200 vaccinated women trying to conceive with IVF treatments in the retrospective study, and compared them with 200 unvaccinated patients of similar age (average age in both groups, 36 years) who were not previously infected with COVID-19. All the women were undergoing IVF from January to April 2021 and all the vaccinated women completed two doses of the BNT162b2 (Pfizer/BioNTech) vaccine at least 2 weeks before ovarian stimulation.
Researchers compared the average number of oocytes retrieved and clinical pregnancy rates between the two groups.
No difference between groups
Two hundred patients underwent oocyte retrieval 14-68 days after receiving a COVID shot; there was no significant difference by vaccination status in the number retrieved per cycle (10.63 in the vaccinated group vs. 10.72 in the unvaccinated group; P = .93).
There was also no difference in the clinical pregnancy rates after fresh embryo transfers. The rate among 128 vaccinated patients was 32.8% versus 33.1% in the 133 unvaccinated patients (P = .96), with 42 and 44 clinical pregnancies, respectively.
A total of 113 patients (66 in the study group and 47 in the controls) underwent freeze-all cycles to preserve fertility and fertilization rates were similar between vaccinated and unvaccinated (55.43% vaccinated vs. 54.29% unvaccinated; P = .73). The average number of cryopreserved embryos was 3.59 (vaccinated) versus 3.28 (unvaccinated) (P = .80).
In a subanalysis of outcomes by age, researchers found vaccination status had no effect on number of oocytes or pregnancy rates in the 39-and-older group. That’s important because it shows the vaccine did not affect outcomes even in a population with reduced ovarian reserves, the authors wrote.
The authors noted one of the study’s limitations is that it didn’t include information about vaccination or past infection status of the male partners.
Question should be put to rest
Sarah Cross, MD, a maternal-fetal medicine specialist at the University of Minnesota, Minneapolis, said the study is the biggest she’s seen that concludes COVID vaccinations are safe and highly encouraged for women before trying to conceive, but other smaller studies have come to the same conclusion.
She pointed to research including a study from 2021 with similar findings that concluded: “Physicians and public health personnel can counsel women of reproductive age that neither previous illness with COVID-19 nor antibodies produced from vaccination to COVID-19 will cause sterility.”
She said she thinks the question of whether COVID shots are safe with IVF has been answered and the results of the latest study add proof to counter misinformation around the issue.
“The COVID-19 vaccine does not affect fertility,” she said. “I don’t know how many more [studies] we need.”
The harm is in not getting vaccinated, she said. Pregnancy significantly increases a woman’s chance of getting severe COVID, the need for hospitalization, mechanical ventilation, and risk of death.
“I personally have never had a hospitalized patient who’s been vaccinated,” Dr. Cross said. “The worst thing for the fetus is to have a critically ill mother.”
Dr. Cross, whose high-risk patients include those seeking counseling before IVF, added: “I would counsel all of them that they should absolutely get vaccinated prior to pregnancy, when they’re pregnant, whenever it is, as soon as they possibly can.”
The study authors and Dr. Cross report no relevant financial relationships.
Getting a COVID-19 mRNA vaccine did not affect pregnancy rates for women trying to conceive with in vitro fertilization or ovarian response to treatment, findings of a new study indicate.
The study was led by Sarit Avraham, MD, with the IVF unit, department of obstetrics and gynecology, Shamir Medical Center in Tzrifi, Israel. The findings were published online in Fertility and Sterility in a preproof version.
“Women should be vaccinated for COVID-19 prior to attempting to conceive via IVF treatments, given the higher risk of severe illness in pregnant women,” the authors wrote.
Doubts arose from “the theoretical concept of the supposed similarity between the SARS-CoV-2 spike protein and the syncytin protein that is speculated to take part in the fertilization process and the formation of the placenta,” the authors wrote.
Some then assumed that the COVID vaccine might kick off an immune response that could affect implantation and pregnancy. But this study and others before it found otherwise.
Researchers included 200 vaccinated women trying to conceive with IVF treatments in the retrospective study, and compared them with 200 unvaccinated patients of similar age (average age in both groups, 36 years) who were not previously infected with COVID-19. All the women were undergoing IVF from January to April 2021 and all the vaccinated women completed two doses of the BNT162b2 (Pfizer/BioNTech) vaccine at least 2 weeks before ovarian stimulation.
Researchers compared the average number of oocytes retrieved and clinical pregnancy rates between the two groups.
No difference between groups
Two hundred patients underwent oocyte retrieval 14-68 days after receiving a COVID shot; there was no significant difference by vaccination status in the number retrieved per cycle (10.63 in the vaccinated group vs. 10.72 in the unvaccinated group; P = .93).
There was also no difference in the clinical pregnancy rates after fresh embryo transfers. The rate among 128 vaccinated patients was 32.8% versus 33.1% in the 133 unvaccinated patients (P = .96), with 42 and 44 clinical pregnancies, respectively.
A total of 113 patients (66 in the study group and 47 in the controls) underwent freeze-all cycles to preserve fertility and fertilization rates were similar between vaccinated and unvaccinated (55.43% vaccinated vs. 54.29% unvaccinated; P = .73). The average number of cryopreserved embryos was 3.59 (vaccinated) versus 3.28 (unvaccinated) (P = .80).
In a subanalysis of outcomes by age, researchers found vaccination status had no effect on number of oocytes or pregnancy rates in the 39-and-older group. That’s important because it shows the vaccine did not affect outcomes even in a population with reduced ovarian reserves, the authors wrote.
The authors noted one of the study’s limitations is that it didn’t include information about vaccination or past infection status of the male partners.
Question should be put to rest
Sarah Cross, MD, a maternal-fetal medicine specialist at the University of Minnesota, Minneapolis, said the study is the biggest she’s seen that concludes COVID vaccinations are safe and highly encouraged for women before trying to conceive, but other smaller studies have come to the same conclusion.
She pointed to research including a study from 2021 with similar findings that concluded: “Physicians and public health personnel can counsel women of reproductive age that neither previous illness with COVID-19 nor antibodies produced from vaccination to COVID-19 will cause sterility.”
She said she thinks the question of whether COVID shots are safe with IVF has been answered and the results of the latest study add proof to counter misinformation around the issue.
“The COVID-19 vaccine does not affect fertility,” she said. “I don’t know how many more [studies] we need.”
The harm is in not getting vaccinated, she said. Pregnancy significantly increases a woman’s chance of getting severe COVID, the need for hospitalization, mechanical ventilation, and risk of death.
“I personally have never had a hospitalized patient who’s been vaccinated,” Dr. Cross said. “The worst thing for the fetus is to have a critically ill mother.”
Dr. Cross, whose high-risk patients include those seeking counseling before IVF, added: “I would counsel all of them that they should absolutely get vaccinated prior to pregnancy, when they’re pregnant, whenever it is, as soon as they possibly can.”
The study authors and Dr. Cross report no relevant financial relationships.
FROM FERTILITY AND STERILITY
A Pioneer in Women’s Federal Practice
March is Women’s History Month. Many women have served in all branches of government health care over centuries and are worthy of celebrating. These nurses, physicians, pharmacists, and other allied health professionals devoted their time and talents, compassion, and competence to deliver and improve the care of wounded service members, disabled veterans, and the underresourced in our communities. To honor the collective contribution of women to federal practice in the Indian Health Service, Public Health Service Core, US Department of Veterans Affairs (VA) and the US Department of Defense, this column examines one pioneer in women’s federal practice—Margaret D. Craighill, MD—who epitomizes the spirit of the selfless dedication that generations of women have given to public service. Craighill is an ideal choice to represent this noble cadre of women as her career spanned active military duty, public health, and the Veterans Health Administration.
Craighill was a graduate of several of the finest institutions of medical training in the United States. Born in Southport, North Carolina, in 1898, she earned her undergraduate degree Phi Beta Kappa and master’s degree from the University of Wisconsin.2 She set her sights on becoming a physician at a period in American history when many prominent medical schools accepted few women. A marked exception—due to the fund raising and lobbying of influential women—was the prestigious Johns Hopkins University School of Medicine.3 She graduated in 1924 and held a postgraduate position at Yale Medical School. She then worked as a physiologist at a military arsenal, a pathologist, a general surgeon, and completed a residency in obstetrics and gynecology. This broad training gave her the diverse expertise she would need for her future work.4
Craighill came from a military family: Her father was a colonel in the engineering corps, and her grandfather rose to become chief engineer of the Army.5 Along with many of America’s best and brightest, Craighill left her successful medical career as dean of the Women’s Medical College of Pennsylvania to join the war effort. Author Alan G. Knight points out, more than in civilian medicine, gender stereotypes kept women from entering the military: Women were expected and accepted as nurses, not doctors.5 But in 1943 Congress passed and President Roosevelt signed the Sparkman-Johnson Bill, enabling women to enter the then all-male Army and Navy Medical Corps. Craighill took advantage of this opportunity and accepted an appointment to the Women’s Army Corps (WAC) as a major in 1943 at age 45 years, becoming the first woman physician to be commissioned an officer in the Army.
Major Craighill’s initial assignment was to the Office of the Surgeon General in the Preventive Medicine Division as the consultant for health and welfare of women. Here, she served as liaison to another innovation in women’s history in military medicine—the WAC. Journeying 56,000 miles to war zones in multiple countries, she assessed the health of 160,000 Army nurses and other staff whose focus was public health and infectious disease and hygiene. The history of women in medicine in and out of federal service is marked by overcoming innumerable biases and barriers. Craighill faced the prevailing presumption that women were unfit for military duty. In an early example of evidence-based medicine, she disproved this theory, showing that women were faring well doing hard jobs in tough environments.4
Their fortitude is more remarkable considering induction examinations for women during World War II were cursory and not tailored to address women’s health care needs. Based on her visits to WACs in theater and at home, Craighill observed recruits suffering from previously undiagnosed gynecologic and psychiatric conditions that adversely affected their health and function. She advocated for comprehensive standardized examinations that would detect many of these disorders.5
Craighill promoted other prejudices of her era. WAC command wanted to win public approval of women in the service and was concerned that lesbian relationships and “heterosexual promiscuity” would damage their public relations aims. They pressured Craighill to develop induction examinations that would screen lesbians and women with behavioral problems. She urged tolerance of homosexual behavior until it was proven.
Though clearly discriminatory and personally offensive to gay persons in federal service, we must recognize that only last year did the Pentagon move to overturn the prior administration’s prohibition against transgender persons serving in uniform.6 In this light Craighill, as the first female physician-leader in a 1940s military, adopted a relatively progressive stance.
Craighill rose to the rank of lieutenant colonel and received the Legion of Merit award for her exemplary wartime service. In 1945, she earned another first when she was appointed to be a consultant on the medical care of women veterans. For women veterans, gaining access to newly earned benefits and receiving appropriate care were serious problems that Craighill worked to solve. For many women veterans, those challenges remain, and Craighill’s legacy summons us to take up the charge to empower women in federal health professions to enhance the quality of care women veterans receive in all sectors of US medicine.
Critics and advocates agree that the VA still has a long way to go to achieve equity and excellence in our care for women veterans.7,8 Craighill’s position stands as a landmark in this effort. During her VA tenure, Craighill entered a residency in the first class of the Menninger School of Psychiatry in Topeka, Kansas, and completed psychoanalytic training. Her wartime experiences had convinced her of the need to provide high-quality mental health care to women veterans. She put her new psychosomatic knowledge and skills to use, serving as the chief of a women’s health clinic at the VA Hospital in Topeka and published several important scholarly papers.5,9Craighill went on to have a distinguished career in academic medicine, underscoring the long and valuable relationship of US medicine and the scholarly medical community. Once her psychiatric training was finished, she returned to private practice, ending her career as chief psychiatrist at Connecticut College for Women.
Craighill made a significant contribution to the role of women in federal practice. She was a visionary in her conviction that women, whether physicians, nurses, or other health care professionals, had the gifts and the grit to serve with distinction and valor and that their military service entitled them in war and peace to gender-sensitive health care. As the epigraph for this editorial shows, Craighill knew the path for women in federal practice or service while not easy is well worth treading. Her pioneering career can inspire all those women who today and in the future choose to follow in her footsteps.
1. Bellafaire J, Graf MH. Women Doctors in War. Texas A&M University Press; 2009:61.
2. Nuland SB. Doctors: The Biography of Medicine. Alfred A. Knopf; 1988:399-405.
3. Dr. Margaret D. Craighill, at 78, former dean of medical college. Obituary. New York Times, July 26, 1977. Accessed February 24, 2022. https://www.nytimes.com/1977/07/26/archives/dr-margaret-d-craighill-at-78-former-dean-of-medical-college.html
4. US Library of Medicine. Changing the face of medicine: Dr. Margaret D. Craighill. Updated June 03, 2015. Accessed February 23, 2022. https://cfmedicine.nlm.nih.gov/physicians/biography_72.html
5. Knight AG. Dr. Margaret D. Craighill, M.D. On Point. 2018;23(4):19-22. Accessed February 24, 2022. https://www.jstor.org/stable/26478427.
6. Wamsley L. Pentagon releases new policies enabling transgender people to serve in the military. Updated March 31, 2021. Accessed February 23, 2022. https://www.npr.org/2021/03/31/983118029/pentagon-releases-new-policies-enabling-transgender-people-to-serve-in-the-milit
7. Shane L. Is VA shortchanging women’s health programs. Military Times. Published February 28, 2019. Accessed February 24, 2022. https://www.militarytimes.com/news/pentagon-congress/2019/02/28/is-va-spending-enough-on-womens-health-programs
8. Marshall V, Stryczek KC, Haverhals L, et al. The focus they deserve: improving women veterans’ health care access. Womens Health Issues. 2021;31(4):399-407. doi:10.1016/j.whi.2020.12.011
9. Craighill MD. Psychiatric aspects of women serving in the Army. Am J Psychiatry. 1947;104(4):226-230. doi:10.1176/ajp.104.4.226
March is Women’s History Month. Many women have served in all branches of government health care over centuries and are worthy of celebrating. These nurses, physicians, pharmacists, and other allied health professionals devoted their time and talents, compassion, and competence to deliver and improve the care of wounded service members, disabled veterans, and the underresourced in our communities. To honor the collective contribution of women to federal practice in the Indian Health Service, Public Health Service Core, US Department of Veterans Affairs (VA) and the US Department of Defense, this column examines one pioneer in women’s federal practice—Margaret D. Craighill, MD—who epitomizes the spirit of the selfless dedication that generations of women have given to public service. Craighill is an ideal choice to represent this noble cadre of women as her career spanned active military duty, public health, and the Veterans Health Administration.
Craighill was a graduate of several of the finest institutions of medical training in the United States. Born in Southport, North Carolina, in 1898, she earned her undergraduate degree Phi Beta Kappa and master’s degree from the University of Wisconsin.2 She set her sights on becoming a physician at a period in American history when many prominent medical schools accepted few women. A marked exception—due to the fund raising and lobbying of influential women—was the prestigious Johns Hopkins University School of Medicine.3 She graduated in 1924 and held a postgraduate position at Yale Medical School. She then worked as a physiologist at a military arsenal, a pathologist, a general surgeon, and completed a residency in obstetrics and gynecology. This broad training gave her the diverse expertise she would need for her future work.4
Craighill came from a military family: Her father was a colonel in the engineering corps, and her grandfather rose to become chief engineer of the Army.5 Along with many of America’s best and brightest, Craighill left her successful medical career as dean of the Women’s Medical College of Pennsylvania to join the war effort. Author Alan G. Knight points out, more than in civilian medicine, gender stereotypes kept women from entering the military: Women were expected and accepted as nurses, not doctors.5 But in 1943 Congress passed and President Roosevelt signed the Sparkman-Johnson Bill, enabling women to enter the then all-male Army and Navy Medical Corps. Craighill took advantage of this opportunity and accepted an appointment to the Women’s Army Corps (WAC) as a major in 1943 at age 45 years, becoming the first woman physician to be commissioned an officer in the Army.
Major Craighill’s initial assignment was to the Office of the Surgeon General in the Preventive Medicine Division as the consultant for health and welfare of women. Here, she served as liaison to another innovation in women’s history in military medicine—the WAC. Journeying 56,000 miles to war zones in multiple countries, she assessed the health of 160,000 Army nurses and other staff whose focus was public health and infectious disease and hygiene. The history of women in medicine in and out of federal service is marked by overcoming innumerable biases and barriers. Craighill faced the prevailing presumption that women were unfit for military duty. In an early example of evidence-based medicine, she disproved this theory, showing that women were faring well doing hard jobs in tough environments.4
Their fortitude is more remarkable considering induction examinations for women during World War II were cursory and not tailored to address women’s health care needs. Based on her visits to WACs in theater and at home, Craighill observed recruits suffering from previously undiagnosed gynecologic and psychiatric conditions that adversely affected their health and function. She advocated for comprehensive standardized examinations that would detect many of these disorders.5
Craighill promoted other prejudices of her era. WAC command wanted to win public approval of women in the service and was concerned that lesbian relationships and “heterosexual promiscuity” would damage their public relations aims. They pressured Craighill to develop induction examinations that would screen lesbians and women with behavioral problems. She urged tolerance of homosexual behavior until it was proven.
Though clearly discriminatory and personally offensive to gay persons in federal service, we must recognize that only last year did the Pentagon move to overturn the prior administration’s prohibition against transgender persons serving in uniform.6 In this light Craighill, as the first female physician-leader in a 1940s military, adopted a relatively progressive stance.
Craighill rose to the rank of lieutenant colonel and received the Legion of Merit award for her exemplary wartime service. In 1945, she earned another first when she was appointed to be a consultant on the medical care of women veterans. For women veterans, gaining access to newly earned benefits and receiving appropriate care were serious problems that Craighill worked to solve. For many women veterans, those challenges remain, and Craighill’s legacy summons us to take up the charge to empower women in federal health professions to enhance the quality of care women veterans receive in all sectors of US medicine.
Critics and advocates agree that the VA still has a long way to go to achieve equity and excellence in our care for women veterans.7,8 Craighill’s position stands as a landmark in this effort. During her VA tenure, Craighill entered a residency in the first class of the Menninger School of Psychiatry in Topeka, Kansas, and completed psychoanalytic training. Her wartime experiences had convinced her of the need to provide high-quality mental health care to women veterans. She put her new psychosomatic knowledge and skills to use, serving as the chief of a women’s health clinic at the VA Hospital in Topeka and published several important scholarly papers.5,9Craighill went on to have a distinguished career in academic medicine, underscoring the long and valuable relationship of US medicine and the scholarly medical community. Once her psychiatric training was finished, she returned to private practice, ending her career as chief psychiatrist at Connecticut College for Women.
Craighill made a significant contribution to the role of women in federal practice. She was a visionary in her conviction that women, whether physicians, nurses, or other health care professionals, had the gifts and the grit to serve with distinction and valor and that their military service entitled them in war and peace to gender-sensitive health care. As the epigraph for this editorial shows, Craighill knew the path for women in federal practice or service while not easy is well worth treading. Her pioneering career can inspire all those women who today and in the future choose to follow in her footsteps.
March is Women’s History Month. Many women have served in all branches of government health care over centuries and are worthy of celebrating. These nurses, physicians, pharmacists, and other allied health professionals devoted their time and talents, compassion, and competence to deliver and improve the care of wounded service members, disabled veterans, and the underresourced in our communities. To honor the collective contribution of women to federal practice in the Indian Health Service, Public Health Service Core, US Department of Veterans Affairs (VA) and the US Department of Defense, this column examines one pioneer in women’s federal practice—Margaret D. Craighill, MD—who epitomizes the spirit of the selfless dedication that generations of women have given to public service. Craighill is an ideal choice to represent this noble cadre of women as her career spanned active military duty, public health, and the Veterans Health Administration.
Craighill was a graduate of several of the finest institutions of medical training in the United States. Born in Southport, North Carolina, in 1898, she earned her undergraduate degree Phi Beta Kappa and master’s degree from the University of Wisconsin.2 She set her sights on becoming a physician at a period in American history when many prominent medical schools accepted few women. A marked exception—due to the fund raising and lobbying of influential women—was the prestigious Johns Hopkins University School of Medicine.3 She graduated in 1924 and held a postgraduate position at Yale Medical School. She then worked as a physiologist at a military arsenal, a pathologist, a general surgeon, and completed a residency in obstetrics and gynecology. This broad training gave her the diverse expertise she would need for her future work.4
Craighill came from a military family: Her father was a colonel in the engineering corps, and her grandfather rose to become chief engineer of the Army.5 Along with many of America’s best and brightest, Craighill left her successful medical career as dean of the Women’s Medical College of Pennsylvania to join the war effort. Author Alan G. Knight points out, more than in civilian medicine, gender stereotypes kept women from entering the military: Women were expected and accepted as nurses, not doctors.5 But in 1943 Congress passed and President Roosevelt signed the Sparkman-Johnson Bill, enabling women to enter the then all-male Army and Navy Medical Corps. Craighill took advantage of this opportunity and accepted an appointment to the Women’s Army Corps (WAC) as a major in 1943 at age 45 years, becoming the first woman physician to be commissioned an officer in the Army.
Major Craighill’s initial assignment was to the Office of the Surgeon General in the Preventive Medicine Division as the consultant for health and welfare of women. Here, she served as liaison to another innovation in women’s history in military medicine—the WAC. Journeying 56,000 miles to war zones in multiple countries, she assessed the health of 160,000 Army nurses and other staff whose focus was public health and infectious disease and hygiene. The history of women in medicine in and out of federal service is marked by overcoming innumerable biases and barriers. Craighill faced the prevailing presumption that women were unfit for military duty. In an early example of evidence-based medicine, she disproved this theory, showing that women were faring well doing hard jobs in tough environments.4
Their fortitude is more remarkable considering induction examinations for women during World War II were cursory and not tailored to address women’s health care needs. Based on her visits to WACs in theater and at home, Craighill observed recruits suffering from previously undiagnosed gynecologic and psychiatric conditions that adversely affected their health and function. She advocated for comprehensive standardized examinations that would detect many of these disorders.5
Craighill promoted other prejudices of her era. WAC command wanted to win public approval of women in the service and was concerned that lesbian relationships and “heterosexual promiscuity” would damage their public relations aims. They pressured Craighill to develop induction examinations that would screen lesbians and women with behavioral problems. She urged tolerance of homosexual behavior until it was proven.
Though clearly discriminatory and personally offensive to gay persons in federal service, we must recognize that only last year did the Pentagon move to overturn the prior administration’s prohibition against transgender persons serving in uniform.6 In this light Craighill, as the first female physician-leader in a 1940s military, adopted a relatively progressive stance.
Craighill rose to the rank of lieutenant colonel and received the Legion of Merit award for her exemplary wartime service. In 1945, she earned another first when she was appointed to be a consultant on the medical care of women veterans. For women veterans, gaining access to newly earned benefits and receiving appropriate care were serious problems that Craighill worked to solve. For many women veterans, those challenges remain, and Craighill’s legacy summons us to take up the charge to empower women in federal health professions to enhance the quality of care women veterans receive in all sectors of US medicine.
Critics and advocates agree that the VA still has a long way to go to achieve equity and excellence in our care for women veterans.7,8 Craighill’s position stands as a landmark in this effort. During her VA tenure, Craighill entered a residency in the first class of the Menninger School of Psychiatry in Topeka, Kansas, and completed psychoanalytic training. Her wartime experiences had convinced her of the need to provide high-quality mental health care to women veterans. She put her new psychosomatic knowledge and skills to use, serving as the chief of a women’s health clinic at the VA Hospital in Topeka and published several important scholarly papers.5,9Craighill went on to have a distinguished career in academic medicine, underscoring the long and valuable relationship of US medicine and the scholarly medical community. Once her psychiatric training was finished, she returned to private practice, ending her career as chief psychiatrist at Connecticut College for Women.
Craighill made a significant contribution to the role of women in federal practice. She was a visionary in her conviction that women, whether physicians, nurses, or other health care professionals, had the gifts and the grit to serve with distinction and valor and that their military service entitled them in war and peace to gender-sensitive health care. As the epigraph for this editorial shows, Craighill knew the path for women in federal practice or service while not easy is well worth treading. Her pioneering career can inspire all those women who today and in the future choose to follow in her footsteps.
1. Bellafaire J, Graf MH. Women Doctors in War. Texas A&M University Press; 2009:61.
2. Nuland SB. Doctors: The Biography of Medicine. Alfred A. Knopf; 1988:399-405.
3. Dr. Margaret D. Craighill, at 78, former dean of medical college. Obituary. New York Times, July 26, 1977. Accessed February 24, 2022. https://www.nytimes.com/1977/07/26/archives/dr-margaret-d-craighill-at-78-former-dean-of-medical-college.html
4. US Library of Medicine. Changing the face of medicine: Dr. Margaret D. Craighill. Updated June 03, 2015. Accessed February 23, 2022. https://cfmedicine.nlm.nih.gov/physicians/biography_72.html
5. Knight AG. Dr. Margaret D. Craighill, M.D. On Point. 2018;23(4):19-22. Accessed February 24, 2022. https://www.jstor.org/stable/26478427.
6. Wamsley L. Pentagon releases new policies enabling transgender people to serve in the military. Updated March 31, 2021. Accessed February 23, 2022. https://www.npr.org/2021/03/31/983118029/pentagon-releases-new-policies-enabling-transgender-people-to-serve-in-the-milit
7. Shane L. Is VA shortchanging women’s health programs. Military Times. Published February 28, 2019. Accessed February 24, 2022. https://www.militarytimes.com/news/pentagon-congress/2019/02/28/is-va-spending-enough-on-womens-health-programs
8. Marshall V, Stryczek KC, Haverhals L, et al. The focus they deserve: improving women veterans’ health care access. Womens Health Issues. 2021;31(4):399-407. doi:10.1016/j.whi.2020.12.011
9. Craighill MD. Psychiatric aspects of women serving in the Army. Am J Psychiatry. 1947;104(4):226-230. doi:10.1176/ajp.104.4.226
1. Bellafaire J, Graf MH. Women Doctors in War. Texas A&M University Press; 2009:61.
2. Nuland SB. Doctors: The Biography of Medicine. Alfred A. Knopf; 1988:399-405.
3. Dr. Margaret D. Craighill, at 78, former dean of medical college. Obituary. New York Times, July 26, 1977. Accessed February 24, 2022. https://www.nytimes.com/1977/07/26/archives/dr-margaret-d-craighill-at-78-former-dean-of-medical-college.html
4. US Library of Medicine. Changing the face of medicine: Dr. Margaret D. Craighill. Updated June 03, 2015. Accessed February 23, 2022. https://cfmedicine.nlm.nih.gov/physicians/biography_72.html
5. Knight AG. Dr. Margaret D. Craighill, M.D. On Point. 2018;23(4):19-22. Accessed February 24, 2022. https://www.jstor.org/stable/26478427.
6. Wamsley L. Pentagon releases new policies enabling transgender people to serve in the military. Updated March 31, 2021. Accessed February 23, 2022. https://www.npr.org/2021/03/31/983118029/pentagon-releases-new-policies-enabling-transgender-people-to-serve-in-the-milit
7. Shane L. Is VA shortchanging women’s health programs. Military Times. Published February 28, 2019. Accessed February 24, 2022. https://www.militarytimes.com/news/pentagon-congress/2019/02/28/is-va-spending-enough-on-womens-health-programs
8. Marshall V, Stryczek KC, Haverhals L, et al. The focus they deserve: improving women veterans’ health care access. Womens Health Issues. 2021;31(4):399-407. doi:10.1016/j.whi.2020.12.011
9. Craighill MD. Psychiatric aspects of women serving in the Army. Am J Psychiatry. 1947;104(4):226-230. doi:10.1176/ajp.104.4.226
Irregular and long periods linked to NAFLD
Long or irregular menstrual cycles in relatively young women are linked an increased risk of both prevalent and incident nonalcoholic fatty liver disease (NAFLD), according to a cross-sectional study that included data on more than 70,000 women.
“Our results indicate that menstrual irregularity, which is easier to diagnose and usually presented earlier than PCOS [polycystic ovary syndrome] highlights the possibility of identifying premenopausal women at risk of developing NAFLD,” reported a team of authors primarily from Sungkyunkwan University, Seoul, South Korea.
The study evaluated women aged younger than 40 years who were participating in the Kangbuk Samsung Health Study, which involves a comprehensive biennial health examination at health centers in South Korea. Of the 135,090 women enrolled over a 6-year period who had at least one follow-up examination, 72,092 were available for analysis after excluding for a sizable list of confounding factors such as liver disease and infections; exposure to steatogenic medications, such as corticosteroids; hysterectomy; and pregnancy.
NAFLD prevalence climbs with longer menses
Of these women, 36.378 (27.7%) had menstrual cycles of 26-30 days and were identified as the index group. The prevalence of NAFLD in this group was 5.8%. For those with a menstrual cycle of 31-39 days, the prevalence rate climbed to 7.2%. For those with a menstrual cycle of at least 40 days or too irregular to estimate, the prevalence was 9.7%. The prevalence was 7.1% for those with a menstrual cycle less than 21 days.
The results of this study were published in the Journal of Clinical Endocrinology & Metabolism.
In those without NAFLD at baseline who were then followed for a mean of 4.4 years, there were 4,524 incident cases of NAFLD. Incidence density was calculated per 103 patient-years. In the index group, the rate was 18.4. It climbed to 20.2 for those with a menstrual cycle of 31-39 days and then to 22.9 for those with a menstrual cycle of at least 40 days. For those with a cycle of fewer than 21 days, the rate was 26.8.
After adjusting for age, body mass index, insulin resistance, and other confounders, the hazard ratio for incident NAFLD for those with long or irregular menstrual cycles compared with the incident group corresponded with a 22% increased risk (HR, 1.22; 95% confidence interval, 1.14-1.31). When calculated in a time-dependent analysis, the risk of NAFLD was increased by almost 50% (HR, 1.49; 95% CI, 1.38-1.60).
Risk persists with PCOS exclusion
PCOS has previously been associated with increased risk of NAFLD, but the association between long or irregular menstrual cycles and NAFLD persisted after women with PCOS were excluded.
The mechanism that links menstrual irregularity with NAFLD is unclear, but the investigators said that estrogen exposure is implicated. In addition to a previously reported associated between low estradiol levels and antiestrogens such as tamoxifen with increased risk of NAFLD, they cited studies associating estrogen replacement therapy with a reduced risk of NAFLD. The role of estrogen in suppressing inflammation, oxidative stress, and insulin resistance are all activities that might link more regular menses with a reduced risk of NAFLD, the authors contended.
Women older than 40 years were excluded from this analysis to reduce the possibility of perimenopausal changes as a confounding factor.
Of study limitations acknowledged by the investigators, the presence of NAFLD was diagnosed on ultrasonography rather than histology. Information on sex hormone or prolactin levels was not captured in relation to NAFLD incidence, and the lack of exposure to estrogen replacement therapy and oral contraceptives was based on self-reports from the participants.
Still, the large study size and the consistency of results after adjustment for multiple risk factors argue that long and irregular menstrual cycles do identify women at risk for NAFLD. One implication is that irregular menses can be a marker for NAFLD risk.
“Our findings do not prove a causal relationship, but they show that long or irregular menstrual cycles were significantly associated with an increased risk of developing NAFLD,” said Seungho Ryu, MD, PhD, a professor at the Sungkyunkwan University. Senior author of this study, Dr. Ryu emphasized in an interview that the association “was not explained by obesity or any other risk factor for NAFLD.”
Lifestyle changes may lower risk
The message is that “young women with long or irregular menstrual cycles may benefit from lifestyle changes to reduce the risk of NAFLD,” Dr. Ryu stated.
The Study of Women’s Health Across the Nation, which was started in 1994, has not evaluated NAFLD, but it did show a relationship between longer menstrual cycles and more cardiometabolic risk factors, according to Nanette Santoro MD, professor and chair, department of obstetrics & gynecology, University of Colorado at Denver, Aurora.
This suggests that others are “thinking along the same lines,” but in discussing this study with this news organization, she characterized some of the design elements as well as some of the findings in this study as “peculiar.”
In addition to a “very, very narrow definition of regular cycles,” she questioned the consistent hazard ratio for NAFLD for those with long cycles relative to other types of irregular menses. Presuming that the group with longer cycles would have included at least some patients with undiagnosed PCOS, she was would have expected that the risk would have been highest in this group. While conceding that differences in body composition of Korean women is a potential explanation for this apparent discrepancy, “I would like to see confirmed in other samples of women with more detailed metabolic assessments to understand who is at risk,” she said.
Not least problematic for the strength of the conclusions, the hazard ratio for NAFLD among women with long or irregular menstrual cycles was “pretty low.” She described this as a level at which the risk “is very susceptible to confounding and unlikely to influence clinical practice.”
Anuja Dokras, MD, PHD, a professor of obstetrics and gynecology and director of the PCOS Center at the University of Pennsylvania, Philadelphia, also questioned whether undiagnosed PCOS might have skewed the data.
“There is increasing data on the association between PCOS and NAFLD. Irregular menses is a key criterion for PCOS, and PCOS is the commonest reason for anovulation,” she said. Dr. Dokras therefore considered it possible that patients with unrecognized PCOS were included in the study, weakening the claim that risk of NAFLD and long menstrual cycles remains significant after controlling for PCOS.
Dr. Ryu and coinvestigators, Dr. Santoro, and Dr. Dokras reported no potential conflicts of interest.
Long or irregular menstrual cycles in relatively young women are linked an increased risk of both prevalent and incident nonalcoholic fatty liver disease (NAFLD), according to a cross-sectional study that included data on more than 70,000 women.
“Our results indicate that menstrual irregularity, which is easier to diagnose and usually presented earlier than PCOS [polycystic ovary syndrome] highlights the possibility of identifying premenopausal women at risk of developing NAFLD,” reported a team of authors primarily from Sungkyunkwan University, Seoul, South Korea.
The study evaluated women aged younger than 40 years who were participating in the Kangbuk Samsung Health Study, which involves a comprehensive biennial health examination at health centers in South Korea. Of the 135,090 women enrolled over a 6-year period who had at least one follow-up examination, 72,092 were available for analysis after excluding for a sizable list of confounding factors such as liver disease and infections; exposure to steatogenic medications, such as corticosteroids; hysterectomy; and pregnancy.
NAFLD prevalence climbs with longer menses
Of these women, 36.378 (27.7%) had menstrual cycles of 26-30 days and were identified as the index group. The prevalence of NAFLD in this group was 5.8%. For those with a menstrual cycle of 31-39 days, the prevalence rate climbed to 7.2%. For those with a menstrual cycle of at least 40 days or too irregular to estimate, the prevalence was 9.7%. The prevalence was 7.1% for those with a menstrual cycle less than 21 days.
The results of this study were published in the Journal of Clinical Endocrinology & Metabolism.
In those without NAFLD at baseline who were then followed for a mean of 4.4 years, there were 4,524 incident cases of NAFLD. Incidence density was calculated per 103 patient-years. In the index group, the rate was 18.4. It climbed to 20.2 for those with a menstrual cycle of 31-39 days and then to 22.9 for those with a menstrual cycle of at least 40 days. For those with a cycle of fewer than 21 days, the rate was 26.8.
After adjusting for age, body mass index, insulin resistance, and other confounders, the hazard ratio for incident NAFLD for those with long or irregular menstrual cycles compared with the incident group corresponded with a 22% increased risk (HR, 1.22; 95% confidence interval, 1.14-1.31). When calculated in a time-dependent analysis, the risk of NAFLD was increased by almost 50% (HR, 1.49; 95% CI, 1.38-1.60).
Risk persists with PCOS exclusion
PCOS has previously been associated with increased risk of NAFLD, but the association between long or irregular menstrual cycles and NAFLD persisted after women with PCOS were excluded.
The mechanism that links menstrual irregularity with NAFLD is unclear, but the investigators said that estrogen exposure is implicated. In addition to a previously reported associated between low estradiol levels and antiestrogens such as tamoxifen with increased risk of NAFLD, they cited studies associating estrogen replacement therapy with a reduced risk of NAFLD. The role of estrogen in suppressing inflammation, oxidative stress, and insulin resistance are all activities that might link more regular menses with a reduced risk of NAFLD, the authors contended.
Women older than 40 years were excluded from this analysis to reduce the possibility of perimenopausal changes as a confounding factor.
Of study limitations acknowledged by the investigators, the presence of NAFLD was diagnosed on ultrasonography rather than histology. Information on sex hormone or prolactin levels was not captured in relation to NAFLD incidence, and the lack of exposure to estrogen replacement therapy and oral contraceptives was based on self-reports from the participants.
Still, the large study size and the consistency of results after adjustment for multiple risk factors argue that long and irregular menstrual cycles do identify women at risk for NAFLD. One implication is that irregular menses can be a marker for NAFLD risk.
“Our findings do not prove a causal relationship, but they show that long or irregular menstrual cycles were significantly associated with an increased risk of developing NAFLD,” said Seungho Ryu, MD, PhD, a professor at the Sungkyunkwan University. Senior author of this study, Dr. Ryu emphasized in an interview that the association “was not explained by obesity or any other risk factor for NAFLD.”
Lifestyle changes may lower risk
The message is that “young women with long or irregular menstrual cycles may benefit from lifestyle changes to reduce the risk of NAFLD,” Dr. Ryu stated.
The Study of Women’s Health Across the Nation, which was started in 1994, has not evaluated NAFLD, but it did show a relationship between longer menstrual cycles and more cardiometabolic risk factors, according to Nanette Santoro MD, professor and chair, department of obstetrics & gynecology, University of Colorado at Denver, Aurora.
This suggests that others are “thinking along the same lines,” but in discussing this study with this news organization, she characterized some of the design elements as well as some of the findings in this study as “peculiar.”
In addition to a “very, very narrow definition of regular cycles,” she questioned the consistent hazard ratio for NAFLD for those with long cycles relative to other types of irregular menses. Presuming that the group with longer cycles would have included at least some patients with undiagnosed PCOS, she was would have expected that the risk would have been highest in this group. While conceding that differences in body composition of Korean women is a potential explanation for this apparent discrepancy, “I would like to see confirmed in other samples of women with more detailed metabolic assessments to understand who is at risk,” she said.
Not least problematic for the strength of the conclusions, the hazard ratio for NAFLD among women with long or irregular menstrual cycles was “pretty low.” She described this as a level at which the risk “is very susceptible to confounding and unlikely to influence clinical practice.”
Anuja Dokras, MD, PHD, a professor of obstetrics and gynecology and director of the PCOS Center at the University of Pennsylvania, Philadelphia, also questioned whether undiagnosed PCOS might have skewed the data.
“There is increasing data on the association between PCOS and NAFLD. Irregular menses is a key criterion for PCOS, and PCOS is the commonest reason for anovulation,” she said. Dr. Dokras therefore considered it possible that patients with unrecognized PCOS were included in the study, weakening the claim that risk of NAFLD and long menstrual cycles remains significant after controlling for PCOS.
Dr. Ryu and coinvestigators, Dr. Santoro, and Dr. Dokras reported no potential conflicts of interest.
Long or irregular menstrual cycles in relatively young women are linked an increased risk of both prevalent and incident nonalcoholic fatty liver disease (NAFLD), according to a cross-sectional study that included data on more than 70,000 women.
“Our results indicate that menstrual irregularity, which is easier to diagnose and usually presented earlier than PCOS [polycystic ovary syndrome] highlights the possibility of identifying premenopausal women at risk of developing NAFLD,” reported a team of authors primarily from Sungkyunkwan University, Seoul, South Korea.
The study evaluated women aged younger than 40 years who were participating in the Kangbuk Samsung Health Study, which involves a comprehensive biennial health examination at health centers in South Korea. Of the 135,090 women enrolled over a 6-year period who had at least one follow-up examination, 72,092 were available for analysis after excluding for a sizable list of confounding factors such as liver disease and infections; exposure to steatogenic medications, such as corticosteroids; hysterectomy; and pregnancy.
NAFLD prevalence climbs with longer menses
Of these women, 36.378 (27.7%) had menstrual cycles of 26-30 days and were identified as the index group. The prevalence of NAFLD in this group was 5.8%. For those with a menstrual cycle of 31-39 days, the prevalence rate climbed to 7.2%. For those with a menstrual cycle of at least 40 days or too irregular to estimate, the prevalence was 9.7%. The prevalence was 7.1% for those with a menstrual cycle less than 21 days.
The results of this study were published in the Journal of Clinical Endocrinology & Metabolism.
In those without NAFLD at baseline who were then followed for a mean of 4.4 years, there were 4,524 incident cases of NAFLD. Incidence density was calculated per 103 patient-years. In the index group, the rate was 18.4. It climbed to 20.2 for those with a menstrual cycle of 31-39 days and then to 22.9 for those with a menstrual cycle of at least 40 days. For those with a cycle of fewer than 21 days, the rate was 26.8.
After adjusting for age, body mass index, insulin resistance, and other confounders, the hazard ratio for incident NAFLD for those with long or irregular menstrual cycles compared with the incident group corresponded with a 22% increased risk (HR, 1.22; 95% confidence interval, 1.14-1.31). When calculated in a time-dependent analysis, the risk of NAFLD was increased by almost 50% (HR, 1.49; 95% CI, 1.38-1.60).
Risk persists with PCOS exclusion
PCOS has previously been associated with increased risk of NAFLD, but the association between long or irregular menstrual cycles and NAFLD persisted after women with PCOS were excluded.
The mechanism that links menstrual irregularity with NAFLD is unclear, but the investigators said that estrogen exposure is implicated. In addition to a previously reported associated between low estradiol levels and antiestrogens such as tamoxifen with increased risk of NAFLD, they cited studies associating estrogen replacement therapy with a reduced risk of NAFLD. The role of estrogen in suppressing inflammation, oxidative stress, and insulin resistance are all activities that might link more regular menses with a reduced risk of NAFLD, the authors contended.
Women older than 40 years were excluded from this analysis to reduce the possibility of perimenopausal changes as a confounding factor.
Of study limitations acknowledged by the investigators, the presence of NAFLD was diagnosed on ultrasonography rather than histology. Information on sex hormone or prolactin levels was not captured in relation to NAFLD incidence, and the lack of exposure to estrogen replacement therapy and oral contraceptives was based on self-reports from the participants.
Still, the large study size and the consistency of results after adjustment for multiple risk factors argue that long and irregular menstrual cycles do identify women at risk for NAFLD. One implication is that irregular menses can be a marker for NAFLD risk.
“Our findings do not prove a causal relationship, but they show that long or irregular menstrual cycles were significantly associated with an increased risk of developing NAFLD,” said Seungho Ryu, MD, PhD, a professor at the Sungkyunkwan University. Senior author of this study, Dr. Ryu emphasized in an interview that the association “was not explained by obesity or any other risk factor for NAFLD.”
Lifestyle changes may lower risk
The message is that “young women with long or irregular menstrual cycles may benefit from lifestyle changes to reduce the risk of NAFLD,” Dr. Ryu stated.
The Study of Women’s Health Across the Nation, which was started in 1994, has not evaluated NAFLD, but it did show a relationship between longer menstrual cycles and more cardiometabolic risk factors, according to Nanette Santoro MD, professor and chair, department of obstetrics & gynecology, University of Colorado at Denver, Aurora.
This suggests that others are “thinking along the same lines,” but in discussing this study with this news organization, she characterized some of the design elements as well as some of the findings in this study as “peculiar.”
In addition to a “very, very narrow definition of regular cycles,” she questioned the consistent hazard ratio for NAFLD for those with long cycles relative to other types of irregular menses. Presuming that the group with longer cycles would have included at least some patients with undiagnosed PCOS, she was would have expected that the risk would have been highest in this group. While conceding that differences in body composition of Korean women is a potential explanation for this apparent discrepancy, “I would like to see confirmed in other samples of women with more detailed metabolic assessments to understand who is at risk,” she said.
Not least problematic for the strength of the conclusions, the hazard ratio for NAFLD among women with long or irregular menstrual cycles was “pretty low.” She described this as a level at which the risk “is very susceptible to confounding and unlikely to influence clinical practice.”
Anuja Dokras, MD, PHD, a professor of obstetrics and gynecology and director of the PCOS Center at the University of Pennsylvania, Philadelphia, also questioned whether undiagnosed PCOS might have skewed the data.
“There is increasing data on the association between PCOS and NAFLD. Irregular menses is a key criterion for PCOS, and PCOS is the commonest reason for anovulation,” she said. Dr. Dokras therefore considered it possible that patients with unrecognized PCOS were included in the study, weakening the claim that risk of NAFLD and long menstrual cycles remains significant after controlling for PCOS.
Dr. Ryu and coinvestigators, Dr. Santoro, and Dr. Dokras reported no potential conflicts of interest.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
Immediate postpartum IUD insertion increases expulsion risk
Expulsion of intrauterine devices was significantly more likely when the devices were inserted within the first 3 days after delivery compared with later insertions, based on data from more than 300,000 women.
Intrauterine devices are effective contraception, and current guidelines support immediate postpartum IUD insertion as a safe, effective, and convenient option, Mary Anne Armstrong, MA, of Kaiser Permanente Northern California, Oakland, and colleagues wrote. Although IUD expulsion rates are low overall, data from previous studies suggest that timing of insertion may affect expulsion rates, and that breastfeeding may play a role.
In the Association of Perforation and Expulsion of Intrauterine Devices (APEX-IUD) cohort study published in JAMA Network Open, the researchers reviewed data from the electronic health records at four sites; the study population included women aged 50 years and younger who underwent IUD insertion between 2001 and 2018.
The women were grouped by postpartum status and timing of IUD placement: 0-3 days, 4 days to 6 weeks, 6-14 weeks, 14-52 weeks, and nonpostpartum (defined as more than 52 weeks or no evidence of delivery).
The researchers also compared expulsion rates in postpartum women who were and were not breastfeeding at the time of IUD insertion based on clinical records, diagnostic codes, or questionnaires at well-baby visits.
The total study population included 326,658 women with a mean age of 32.0 years; 42% were non-Hispanic White, 17.2% were Hispanic other, 13.0% were Hispanic White, 11.9% were Asian or Pacific Islander, 8.7% were non-Hispanic Black, and 0.2% were Hispanic Black. Approximately 80% of the IUDs were levonorgestrel releasing.
A total of 8,943 expulsions were reported, for an overall expulsion rate of 13.94 per 1,000 person-years.
The adjusted hazard ratios for IUD expulsion were 5.34, 1.22, 1.06, and 1.43 for women with insertion times, respectively, of 0-3 days, 4 days to 6 or fewer weeks, 6-14 weeks, and 14-52 weeks. Women with nonpostpartum IUD insertion served as the referent.
The 5-year cumulative incidence of IUD expulsion was highest with placement between 0 and 3 days post partum and lowest with placement at 6-14 weeks postpartum (10.73% and 3.18%, respectively).
“Within the group with IUD insertions 0-3 days postpartum, the highest expulsion rates were discovered within 12 weeks of insertion, with the highest incidence rate occurring at week 6 (844 per 1,000 person-years), a time women are commonly seen post delivery,” the researchers noted.
In a subcohort of 94,817 women with known breastfeeding status, the 5-year cumulative incidence of expulsion was 3.49% for breastfeeding women and 4.57% for nonbreastfeeding women, with an adjusted HR of 0.71 for breastfeeding versus not breastfeeding.
“While women who accept immediate postpartum IUD placement report high satisfaction rates, information on women’s preferences and satisfaction associated with different timing of postpartum placement would also be helpful to understand the benefit-risk profile,” the researchers wrote in their discussion of the findings. “The fact that most expulsions in the immediate postpartum group occurred early presents an opportunity to mitigate risk of unrecognized expulsion and unintended pregnancy via counseling on signs of expulsion and follow-up examination.”
The study findings were limited by several factors including the potential misclassification of exposures and the primary outcome of expulsion, especially since some postpartum women may be lactating whether or not they are breastfeeding, the researchers noted. Other limitations included the combination of complete and partial expulsions, and the dating of IUD expulsion based on when it came to medical attention, which was not necessarily when it occurred. More data are needed on the potential association between lactational amenorrhea and lower expulsion risk among postpartum women who are breastfeeding.
However, the results were strengthened by the large and diverse study population, the use of linked mother-infant records to identify exposures, and the use electronic health records to identify outcomes, and the data can inform patient counseling for postpartum IUDs, the researchers concluded.
Study reflects findings from Europe
“The FDA mandated this study in response to a European study, EURAS-IUD1, a European prospective observational study that enrolled 61,448 participants between 2006 and 2012,” Ms. Armstrong said in an interview. In the European study “women breastfeeding at the time of device insertion or with the device inserted at 36 weeks’ postpartum or less had higher risk of uterine perforation. The FDA wanted to know if the risks were similar in the United States population”
The APEX-IUD study was designed to reflect current United States clinical practice. “The aims of APEX-IUD are to evaluate risk of IUD-related uterine perforation and device expulsion among women who are breastfeeding or within 12 months postpartum at insertion. The perforation outcome is addressed in a separate paper,” Ms. Armstrong noted.
“We were not surprised by the findings; they aligned with previous findings and confirm the overall safety of intrauterine devices,” said Ms. Armstrong. “Data from this study provides IUD expulsion risk estimates that can be used to inform clinical practice and preinsertion counseling. IUD insertions 0-3 days postpartum might decrease the risk of unintended pregnancy and provide more convenience and efficiency for new mothers. This has proven to be especially important during the pandemic. The higher risk of expulsion at 0-3 days post partum must be balanced with the low IUD-related uterine perforation risk to provide a comprehensive picture that aids in clinical decision-making.
“Potential barriers to postpartum IUD placement include lack of provision of education on the range of contraceptive options available during prenatal care and failure or inability of hospital inpatient units to stock the intrauterine devices for use when needed,” said Ms. Armstrong.
Looking ahead, “future research could evaluate risk factors for partial versus complete expulsions, the association of preinsertion counseling with recognition of potential expulsions and corresponding IUD failure rates, and whether ultrasound verification of IUD position in the uterus after insertion is associated with expulsion risk,” she said.
Identifying risk factors informs patient counseling
“The current study examines breastfeeding at time of IUD insertion as a risk factor for expulsion,” Iris Krishna, MD, of Emory University, Atlanta, said in an interview. “There is biologic plausibility that breastfeeding may be a risk factor of IUD expulsion. Breastfeeding stimulates secretion of oxytocin, a hormone which plays a key role in the contraction of the uterus during labor and uterine involution postpartum. It also plays a key role in the contraction of milk ducts to allow for milk letdown. Because of its dual role some mothers may occasionally report uterine cramping with breastfeeding. Prior studies have suggested that breastfeeding may be associated with an increased risk of uterine perforation with postpartum IUD placement, but how breastfeeding may contribute to risk of IUD expulsion has not been studied extensively.”
The current data are consistent with previous studies suggesting the highest risk of IUD expulsion is with placement in the immediate postpartum period (0-3 days). “In a subcohort analysis by breastfeeding status, the risk of IUD expulsion was lower for women who were breastfeeding versus not breastfeeding;” however, “these findings may be due to amenorrhea that can also be seen with breastfeeding,” Dr. Krishna said. “Menstrual bleeding is an independent risk factor for IUD expulsion and not having menstrual bleeding while breastfeeding may lower risk of expulsion.
“Patients should be counseled on the benefits of immediate postpartum IUD placement, the risk of IUD expulsion, and alternative contraception options to be able to make an informed decision about the right contraception for them,” Dr. Krishna emphasized. “Clinicians can reassure patients that the uterine cramping they may feel while breastfeeding does not appear to increase the risk of IUD expulsion and that the amenorrhea that may result from breastfeeding also may lower the risk of IUD expulsion.”
The study was supported by Bayer through support to RTI Health Solutions, Kaiser Permanente Northern California, Kaiser Permanente Southern California, Kaiser Permanente Washington, and the Regenstrief Institute. Ms. Armstrong and several coauthors disclosed support from Bayer during the study. Dr. Krishna had no relevant disclosures.
Expulsion of intrauterine devices was significantly more likely when the devices were inserted within the first 3 days after delivery compared with later insertions, based on data from more than 300,000 women.
Intrauterine devices are effective contraception, and current guidelines support immediate postpartum IUD insertion as a safe, effective, and convenient option, Mary Anne Armstrong, MA, of Kaiser Permanente Northern California, Oakland, and colleagues wrote. Although IUD expulsion rates are low overall, data from previous studies suggest that timing of insertion may affect expulsion rates, and that breastfeeding may play a role.
In the Association of Perforation and Expulsion of Intrauterine Devices (APEX-IUD) cohort study published in JAMA Network Open, the researchers reviewed data from the electronic health records at four sites; the study population included women aged 50 years and younger who underwent IUD insertion between 2001 and 2018.
The women were grouped by postpartum status and timing of IUD placement: 0-3 days, 4 days to 6 weeks, 6-14 weeks, 14-52 weeks, and nonpostpartum (defined as more than 52 weeks or no evidence of delivery).
The researchers also compared expulsion rates in postpartum women who were and were not breastfeeding at the time of IUD insertion based on clinical records, diagnostic codes, or questionnaires at well-baby visits.
The total study population included 326,658 women with a mean age of 32.0 years; 42% were non-Hispanic White, 17.2% were Hispanic other, 13.0% were Hispanic White, 11.9% were Asian or Pacific Islander, 8.7% were non-Hispanic Black, and 0.2% were Hispanic Black. Approximately 80% of the IUDs were levonorgestrel releasing.
A total of 8,943 expulsions were reported, for an overall expulsion rate of 13.94 per 1,000 person-years.
The adjusted hazard ratios for IUD expulsion were 5.34, 1.22, 1.06, and 1.43 for women with insertion times, respectively, of 0-3 days, 4 days to 6 or fewer weeks, 6-14 weeks, and 14-52 weeks. Women with nonpostpartum IUD insertion served as the referent.
The 5-year cumulative incidence of IUD expulsion was highest with placement between 0 and 3 days post partum and lowest with placement at 6-14 weeks postpartum (10.73% and 3.18%, respectively).
“Within the group with IUD insertions 0-3 days postpartum, the highest expulsion rates were discovered within 12 weeks of insertion, with the highest incidence rate occurring at week 6 (844 per 1,000 person-years), a time women are commonly seen post delivery,” the researchers noted.
In a subcohort of 94,817 women with known breastfeeding status, the 5-year cumulative incidence of expulsion was 3.49% for breastfeeding women and 4.57% for nonbreastfeeding women, with an adjusted HR of 0.71 for breastfeeding versus not breastfeeding.
“While women who accept immediate postpartum IUD placement report high satisfaction rates, information on women’s preferences and satisfaction associated with different timing of postpartum placement would also be helpful to understand the benefit-risk profile,” the researchers wrote in their discussion of the findings. “The fact that most expulsions in the immediate postpartum group occurred early presents an opportunity to mitigate risk of unrecognized expulsion and unintended pregnancy via counseling on signs of expulsion and follow-up examination.”
The study findings were limited by several factors including the potential misclassification of exposures and the primary outcome of expulsion, especially since some postpartum women may be lactating whether or not they are breastfeeding, the researchers noted. Other limitations included the combination of complete and partial expulsions, and the dating of IUD expulsion based on when it came to medical attention, which was not necessarily when it occurred. More data are needed on the potential association between lactational amenorrhea and lower expulsion risk among postpartum women who are breastfeeding.
However, the results were strengthened by the large and diverse study population, the use of linked mother-infant records to identify exposures, and the use electronic health records to identify outcomes, and the data can inform patient counseling for postpartum IUDs, the researchers concluded.
Study reflects findings from Europe
“The FDA mandated this study in response to a European study, EURAS-IUD1, a European prospective observational study that enrolled 61,448 participants between 2006 and 2012,” Ms. Armstrong said in an interview. In the European study “women breastfeeding at the time of device insertion or with the device inserted at 36 weeks’ postpartum or less had higher risk of uterine perforation. The FDA wanted to know if the risks were similar in the United States population”
The APEX-IUD study was designed to reflect current United States clinical practice. “The aims of APEX-IUD are to evaluate risk of IUD-related uterine perforation and device expulsion among women who are breastfeeding or within 12 months postpartum at insertion. The perforation outcome is addressed in a separate paper,” Ms. Armstrong noted.
“We were not surprised by the findings; they aligned with previous findings and confirm the overall safety of intrauterine devices,” said Ms. Armstrong. “Data from this study provides IUD expulsion risk estimates that can be used to inform clinical practice and preinsertion counseling. IUD insertions 0-3 days postpartum might decrease the risk of unintended pregnancy and provide more convenience and efficiency for new mothers. This has proven to be especially important during the pandemic. The higher risk of expulsion at 0-3 days post partum must be balanced with the low IUD-related uterine perforation risk to provide a comprehensive picture that aids in clinical decision-making.
“Potential barriers to postpartum IUD placement include lack of provision of education on the range of contraceptive options available during prenatal care and failure or inability of hospital inpatient units to stock the intrauterine devices for use when needed,” said Ms. Armstrong.
Looking ahead, “future research could evaluate risk factors for partial versus complete expulsions, the association of preinsertion counseling with recognition of potential expulsions and corresponding IUD failure rates, and whether ultrasound verification of IUD position in the uterus after insertion is associated with expulsion risk,” she said.
Identifying risk factors informs patient counseling
“The current study examines breastfeeding at time of IUD insertion as a risk factor for expulsion,” Iris Krishna, MD, of Emory University, Atlanta, said in an interview. “There is biologic plausibility that breastfeeding may be a risk factor of IUD expulsion. Breastfeeding stimulates secretion of oxytocin, a hormone which plays a key role in the contraction of the uterus during labor and uterine involution postpartum. It also plays a key role in the contraction of milk ducts to allow for milk letdown. Because of its dual role some mothers may occasionally report uterine cramping with breastfeeding. Prior studies have suggested that breastfeeding may be associated with an increased risk of uterine perforation with postpartum IUD placement, but how breastfeeding may contribute to risk of IUD expulsion has not been studied extensively.”
The current data are consistent with previous studies suggesting the highest risk of IUD expulsion is with placement in the immediate postpartum period (0-3 days). “In a subcohort analysis by breastfeeding status, the risk of IUD expulsion was lower for women who were breastfeeding versus not breastfeeding;” however, “these findings may be due to amenorrhea that can also be seen with breastfeeding,” Dr. Krishna said. “Menstrual bleeding is an independent risk factor for IUD expulsion and not having menstrual bleeding while breastfeeding may lower risk of expulsion.
“Patients should be counseled on the benefits of immediate postpartum IUD placement, the risk of IUD expulsion, and alternative contraception options to be able to make an informed decision about the right contraception for them,” Dr. Krishna emphasized. “Clinicians can reassure patients that the uterine cramping they may feel while breastfeeding does not appear to increase the risk of IUD expulsion and that the amenorrhea that may result from breastfeeding also may lower the risk of IUD expulsion.”
The study was supported by Bayer through support to RTI Health Solutions, Kaiser Permanente Northern California, Kaiser Permanente Southern California, Kaiser Permanente Washington, and the Regenstrief Institute. Ms. Armstrong and several coauthors disclosed support from Bayer during the study. Dr. Krishna had no relevant disclosures.
Expulsion of intrauterine devices was significantly more likely when the devices were inserted within the first 3 days after delivery compared with later insertions, based on data from more than 300,000 women.
Intrauterine devices are effective contraception, and current guidelines support immediate postpartum IUD insertion as a safe, effective, and convenient option, Mary Anne Armstrong, MA, of Kaiser Permanente Northern California, Oakland, and colleagues wrote. Although IUD expulsion rates are low overall, data from previous studies suggest that timing of insertion may affect expulsion rates, and that breastfeeding may play a role.
In the Association of Perforation and Expulsion of Intrauterine Devices (APEX-IUD) cohort study published in JAMA Network Open, the researchers reviewed data from the electronic health records at four sites; the study population included women aged 50 years and younger who underwent IUD insertion between 2001 and 2018.
The women were grouped by postpartum status and timing of IUD placement: 0-3 days, 4 days to 6 weeks, 6-14 weeks, 14-52 weeks, and nonpostpartum (defined as more than 52 weeks or no evidence of delivery).
The researchers also compared expulsion rates in postpartum women who were and were not breastfeeding at the time of IUD insertion based on clinical records, diagnostic codes, or questionnaires at well-baby visits.
The total study population included 326,658 women with a mean age of 32.0 years; 42% were non-Hispanic White, 17.2% were Hispanic other, 13.0% were Hispanic White, 11.9% were Asian or Pacific Islander, 8.7% were non-Hispanic Black, and 0.2% were Hispanic Black. Approximately 80% of the IUDs were levonorgestrel releasing.
A total of 8,943 expulsions were reported, for an overall expulsion rate of 13.94 per 1,000 person-years.
The adjusted hazard ratios for IUD expulsion were 5.34, 1.22, 1.06, and 1.43 for women with insertion times, respectively, of 0-3 days, 4 days to 6 or fewer weeks, 6-14 weeks, and 14-52 weeks. Women with nonpostpartum IUD insertion served as the referent.
The 5-year cumulative incidence of IUD expulsion was highest with placement between 0 and 3 days post partum and lowest with placement at 6-14 weeks postpartum (10.73% and 3.18%, respectively).
“Within the group with IUD insertions 0-3 days postpartum, the highest expulsion rates were discovered within 12 weeks of insertion, with the highest incidence rate occurring at week 6 (844 per 1,000 person-years), a time women are commonly seen post delivery,” the researchers noted.
In a subcohort of 94,817 women with known breastfeeding status, the 5-year cumulative incidence of expulsion was 3.49% for breastfeeding women and 4.57% for nonbreastfeeding women, with an adjusted HR of 0.71 for breastfeeding versus not breastfeeding.
“While women who accept immediate postpartum IUD placement report high satisfaction rates, information on women’s preferences and satisfaction associated with different timing of postpartum placement would also be helpful to understand the benefit-risk profile,” the researchers wrote in their discussion of the findings. “The fact that most expulsions in the immediate postpartum group occurred early presents an opportunity to mitigate risk of unrecognized expulsion and unintended pregnancy via counseling on signs of expulsion and follow-up examination.”
The study findings were limited by several factors including the potential misclassification of exposures and the primary outcome of expulsion, especially since some postpartum women may be lactating whether or not they are breastfeeding, the researchers noted. Other limitations included the combination of complete and partial expulsions, and the dating of IUD expulsion based on when it came to medical attention, which was not necessarily when it occurred. More data are needed on the potential association between lactational amenorrhea and lower expulsion risk among postpartum women who are breastfeeding.
However, the results were strengthened by the large and diverse study population, the use of linked mother-infant records to identify exposures, and the use electronic health records to identify outcomes, and the data can inform patient counseling for postpartum IUDs, the researchers concluded.
Study reflects findings from Europe
“The FDA mandated this study in response to a European study, EURAS-IUD1, a European prospective observational study that enrolled 61,448 participants between 2006 and 2012,” Ms. Armstrong said in an interview. In the European study “women breastfeeding at the time of device insertion or with the device inserted at 36 weeks’ postpartum or less had higher risk of uterine perforation. The FDA wanted to know if the risks were similar in the United States population”
The APEX-IUD study was designed to reflect current United States clinical practice. “The aims of APEX-IUD are to evaluate risk of IUD-related uterine perforation and device expulsion among women who are breastfeeding or within 12 months postpartum at insertion. The perforation outcome is addressed in a separate paper,” Ms. Armstrong noted.
“We were not surprised by the findings; they aligned with previous findings and confirm the overall safety of intrauterine devices,” said Ms. Armstrong. “Data from this study provides IUD expulsion risk estimates that can be used to inform clinical practice and preinsertion counseling. IUD insertions 0-3 days postpartum might decrease the risk of unintended pregnancy and provide more convenience and efficiency for new mothers. This has proven to be especially important during the pandemic. The higher risk of expulsion at 0-3 days post partum must be balanced with the low IUD-related uterine perforation risk to provide a comprehensive picture that aids in clinical decision-making.
“Potential barriers to postpartum IUD placement include lack of provision of education on the range of contraceptive options available during prenatal care and failure or inability of hospital inpatient units to stock the intrauterine devices for use when needed,” said Ms. Armstrong.
Looking ahead, “future research could evaluate risk factors for partial versus complete expulsions, the association of preinsertion counseling with recognition of potential expulsions and corresponding IUD failure rates, and whether ultrasound verification of IUD position in the uterus after insertion is associated with expulsion risk,” she said.
Identifying risk factors informs patient counseling
“The current study examines breastfeeding at time of IUD insertion as a risk factor for expulsion,” Iris Krishna, MD, of Emory University, Atlanta, said in an interview. “There is biologic plausibility that breastfeeding may be a risk factor of IUD expulsion. Breastfeeding stimulates secretion of oxytocin, a hormone which plays a key role in the contraction of the uterus during labor and uterine involution postpartum. It also plays a key role in the contraction of milk ducts to allow for milk letdown. Because of its dual role some mothers may occasionally report uterine cramping with breastfeeding. Prior studies have suggested that breastfeeding may be associated with an increased risk of uterine perforation with postpartum IUD placement, but how breastfeeding may contribute to risk of IUD expulsion has not been studied extensively.”
The current data are consistent with previous studies suggesting the highest risk of IUD expulsion is with placement in the immediate postpartum period (0-3 days). “In a subcohort analysis by breastfeeding status, the risk of IUD expulsion was lower for women who were breastfeeding versus not breastfeeding;” however, “these findings may be due to amenorrhea that can also be seen with breastfeeding,” Dr. Krishna said. “Menstrual bleeding is an independent risk factor for IUD expulsion and not having menstrual bleeding while breastfeeding may lower risk of expulsion.
“Patients should be counseled on the benefits of immediate postpartum IUD placement, the risk of IUD expulsion, and alternative contraception options to be able to make an informed decision about the right contraception for them,” Dr. Krishna emphasized. “Clinicians can reassure patients that the uterine cramping they may feel while breastfeeding does not appear to increase the risk of IUD expulsion and that the amenorrhea that may result from breastfeeding also may lower the risk of IUD expulsion.”
The study was supported by Bayer through support to RTI Health Solutions, Kaiser Permanente Northern California, Kaiser Permanente Southern California, Kaiser Permanente Washington, and the Regenstrief Institute. Ms. Armstrong and several coauthors disclosed support from Bayer during the study. Dr. Krishna had no relevant disclosures.
FROM JAMA NETWORK OPEN
Older age for menopause raises risk for lung cancer
This study was published on Medrxiv.org as a preprint and has not yet been peer reviewed.
Key takeaways
- in analyses of more than 100,000 women that used Mendelian randomization (MR) as a tool to reduce residual confounding.
- The MR analyses showed no significant association between ANM and breast cancer, endometrial cancer, ovarian cancer, coronary heart disease, ischemic stroke, and Alzheimer’s disease.
- The clear lack of a causal effect of ANM on the outcomes of coronary heart disease and ischemic stroke in the MR analyses despite a strong inverse association seen in the observational data of this study (without MR) suggests residual confounding plays a substantial role in driving the observed outcomes.
Why this matters
- The authors said that, to their knowledge, this is the first study that has shown a causal association between older ANM and higher risk of postmenopausal lung cancer.
- This finding was directionally opposite to the significant protective effect of increased ANM documented in an observational analysis of roughly the same data as well as prior reports that did not use MR. This “notable inconsistency” suggests very substantial residual confounding without MR that could be driven by factors such as smoking, diet, and exercise.
- If these results are replicated in additional datasets, it would highlight a need for randomized, controlled trials of antiestrogen therapies in postmenopausal women for the prevention or treatment of lung cancer.
Study design
- The study included data from 106,853 postmenopausal women enrolled in the Women’s Health Initiative (WHI) and 95,464 women who were 37-73 years old included in the UK Biobank (UKB). Analyses for each outcome also included data from smaller numbers of women obtained from several additional datasets.
- The MR analysis used up to 55 single-nucleotide polymorphisms previously discovered through a genome-wide association study of about 70,000 women of European ancestry and independent of all datasets analyzed in the current study. The authors included all single-nucleotide polymorphisms with a consistent direction of effect on ANM.
- The MR analysis for lung cancer included 113,371 women from the two primary datasets and an additional 3012 women from six additional datasets.
- The MR analysis for bone fracture involved 113,239 women from the WHI and UKB only. The MR analysis for osteoporosis involved 137,080 women from the WHI, UKB, and one additional external dataset.
Key results
- Results from a meta-analysis of the MR results using data from the WHI, UKB, and the additional datasets showed ANM was causally associated with an increased risk of lung cancer by an odds ratio of 1.35 for each 5-year increase in ANM. In contrast, the adjusted observational analysis of data just from the WHI and UKB showed a significant 11% relative risk reduction in the incidence of lung cancer for each 5-year increase in ANM.
- The MR results also showed causally protective effects for fracture, with a 24% relative risk reduction, and for osteoporosis, with a 19% relative risk reduction for each 5-year increase in ANM.
- The MR analyses showed no significant association between AMN and outcome for breast cancer, endometrial cancer, ovarian cancer, coronary heart disease, ischemic stroke, and Alzheimer’s disease.
Limitations
The main limitation of the MR study was the potential for inadequate power for assessing some outcomes despite the large overall size of the study cohort. Lack of adequate power may be responsible for some of the nonsignificant associations seen in the study, such as for breast and endometrial cancers, where substantial prior evidence has implicated increased risk through the effects of prolonged exposure to endogenous or exogenous estrogens.
The healthy cohort effect in the UKB is a known weakness of this dataset that may have limited the number of cases and generalizability of findings.
Osteoporosis and Alzheimer’s disease were self-reported.
The study only included participants of European ancestry because most subjects in most of the cohorts examined were White women and the applied MR instruments were found by genome-wide association studies run predominantly in White women. The authors said the causal effects of ANM need study in more diverse populations.
Disclosures
- The study received no commercial funding.
- None of the authors had disclosures.
This is a summary of a preprint research study, “Genetic evidence for causal relationships between age at natural menopause and the risk of aging-associated adverse health outcomes,” written by authors primarily based at Stanford University School of Medicine i
A version of this article first appeared on Medscape.com.
This study was published on Medrxiv.org as a preprint and has not yet been peer reviewed.
Key takeaways
- in analyses of more than 100,000 women that used Mendelian randomization (MR) as a tool to reduce residual confounding.
- The MR analyses showed no significant association between ANM and breast cancer, endometrial cancer, ovarian cancer, coronary heart disease, ischemic stroke, and Alzheimer’s disease.
- The clear lack of a causal effect of ANM on the outcomes of coronary heart disease and ischemic stroke in the MR analyses despite a strong inverse association seen in the observational data of this study (without MR) suggests residual confounding plays a substantial role in driving the observed outcomes.
Why this matters
- The authors said that, to their knowledge, this is the first study that has shown a causal association between older ANM and higher risk of postmenopausal lung cancer.
- This finding was directionally opposite to the significant protective effect of increased ANM documented in an observational analysis of roughly the same data as well as prior reports that did not use MR. This “notable inconsistency” suggests very substantial residual confounding without MR that could be driven by factors such as smoking, diet, and exercise.
- If these results are replicated in additional datasets, it would highlight a need for randomized, controlled trials of antiestrogen therapies in postmenopausal women for the prevention or treatment of lung cancer.
Study design
- The study included data from 106,853 postmenopausal women enrolled in the Women’s Health Initiative (WHI) and 95,464 women who were 37-73 years old included in the UK Biobank (UKB). Analyses for each outcome also included data from smaller numbers of women obtained from several additional datasets.
- The MR analysis used up to 55 single-nucleotide polymorphisms previously discovered through a genome-wide association study of about 70,000 women of European ancestry and independent of all datasets analyzed in the current study. The authors included all single-nucleotide polymorphisms with a consistent direction of effect on ANM.
- The MR analysis for lung cancer included 113,371 women from the two primary datasets and an additional 3012 women from six additional datasets.
- The MR analysis for bone fracture involved 113,239 women from the WHI and UKB only. The MR analysis for osteoporosis involved 137,080 women from the WHI, UKB, and one additional external dataset.
Key results
- Results from a meta-analysis of the MR results using data from the WHI, UKB, and the additional datasets showed ANM was causally associated with an increased risk of lung cancer by an odds ratio of 1.35 for each 5-year increase in ANM. In contrast, the adjusted observational analysis of data just from the WHI and UKB showed a significant 11% relative risk reduction in the incidence of lung cancer for each 5-year increase in ANM.
- The MR results also showed causally protective effects for fracture, with a 24% relative risk reduction, and for osteoporosis, with a 19% relative risk reduction for each 5-year increase in ANM.
- The MR analyses showed no significant association between AMN and outcome for breast cancer, endometrial cancer, ovarian cancer, coronary heart disease, ischemic stroke, and Alzheimer’s disease.
Limitations
The main limitation of the MR study was the potential for inadequate power for assessing some outcomes despite the large overall size of the study cohort. Lack of adequate power may be responsible for some of the nonsignificant associations seen in the study, such as for breast and endometrial cancers, where substantial prior evidence has implicated increased risk through the effects of prolonged exposure to endogenous or exogenous estrogens.
The healthy cohort effect in the UKB is a known weakness of this dataset that may have limited the number of cases and generalizability of findings.
Osteoporosis and Alzheimer’s disease were self-reported.
The study only included participants of European ancestry because most subjects in most of the cohorts examined were White women and the applied MR instruments were found by genome-wide association studies run predominantly in White women. The authors said the causal effects of ANM need study in more diverse populations.
Disclosures
- The study received no commercial funding.
- None of the authors had disclosures.
This is a summary of a preprint research study, “Genetic evidence for causal relationships between age at natural menopause and the risk of aging-associated adverse health outcomes,” written by authors primarily based at Stanford University School of Medicine i
A version of this article first appeared on Medscape.com.
This study was published on Medrxiv.org as a preprint and has not yet been peer reviewed.
Key takeaways
- in analyses of more than 100,000 women that used Mendelian randomization (MR) as a tool to reduce residual confounding.
- The MR analyses showed no significant association between ANM and breast cancer, endometrial cancer, ovarian cancer, coronary heart disease, ischemic stroke, and Alzheimer’s disease.
- The clear lack of a causal effect of ANM on the outcomes of coronary heart disease and ischemic stroke in the MR analyses despite a strong inverse association seen in the observational data of this study (without MR) suggests residual confounding plays a substantial role in driving the observed outcomes.
Why this matters
- The authors said that, to their knowledge, this is the first study that has shown a causal association between older ANM and higher risk of postmenopausal lung cancer.
- This finding was directionally opposite to the significant protective effect of increased ANM documented in an observational analysis of roughly the same data as well as prior reports that did not use MR. This “notable inconsistency” suggests very substantial residual confounding without MR that could be driven by factors such as smoking, diet, and exercise.
- If these results are replicated in additional datasets, it would highlight a need for randomized, controlled trials of antiestrogen therapies in postmenopausal women for the prevention or treatment of lung cancer.
Study design
- The study included data from 106,853 postmenopausal women enrolled in the Women’s Health Initiative (WHI) and 95,464 women who were 37-73 years old included in the UK Biobank (UKB). Analyses for each outcome also included data from smaller numbers of women obtained from several additional datasets.
- The MR analysis used up to 55 single-nucleotide polymorphisms previously discovered through a genome-wide association study of about 70,000 women of European ancestry and independent of all datasets analyzed in the current study. The authors included all single-nucleotide polymorphisms with a consistent direction of effect on ANM.
- The MR analysis for lung cancer included 113,371 women from the two primary datasets and an additional 3012 women from six additional datasets.
- The MR analysis for bone fracture involved 113,239 women from the WHI and UKB only. The MR analysis for osteoporosis involved 137,080 women from the WHI, UKB, and one additional external dataset.
Key results
- Results from a meta-analysis of the MR results using data from the WHI, UKB, and the additional datasets showed ANM was causally associated with an increased risk of lung cancer by an odds ratio of 1.35 for each 5-year increase in ANM. In contrast, the adjusted observational analysis of data just from the WHI and UKB showed a significant 11% relative risk reduction in the incidence of lung cancer for each 5-year increase in ANM.
- The MR results also showed causally protective effects for fracture, with a 24% relative risk reduction, and for osteoporosis, with a 19% relative risk reduction for each 5-year increase in ANM.
- The MR analyses showed no significant association between AMN and outcome for breast cancer, endometrial cancer, ovarian cancer, coronary heart disease, ischemic stroke, and Alzheimer’s disease.
Limitations
The main limitation of the MR study was the potential for inadequate power for assessing some outcomes despite the large overall size of the study cohort. Lack of adequate power may be responsible for some of the nonsignificant associations seen in the study, such as for breast and endometrial cancers, where substantial prior evidence has implicated increased risk through the effects of prolonged exposure to endogenous or exogenous estrogens.
The healthy cohort effect in the UKB is a known weakness of this dataset that may have limited the number of cases and generalizability of findings.
Osteoporosis and Alzheimer’s disease were self-reported.
The study only included participants of European ancestry because most subjects in most of the cohorts examined were White women and the applied MR instruments were found by genome-wide association studies run predominantly in White women. The authors said the causal effects of ANM need study in more diverse populations.
Disclosures
- The study received no commercial funding.
- None of the authors had disclosures.
This is a summary of a preprint research study, “Genetic evidence for causal relationships between age at natural menopause and the risk of aging-associated adverse health outcomes,” written by authors primarily based at Stanford University School of Medicine i
A version of this article first appeared on Medscape.com.




