User login
New nonhormonal hot flash treatments on the way
researchers told attendees at the virtual North American Menopause Society 2020 Annual Meeting.
“The KNDy [kisspeptin/neurokinin B/dynorphin] neuron manipulation is really exciting and holds great promise for rapid and highly effective amelioration of hot flashes, up to 80%, and improvement in other menopausal symptoms, though we’re still looking at the safety in phase 3 trials,” reported Susan D. Reed, MD, MPH, director of the Women’s Reproductive Health Research Program at the University of Washington, Seattle.
“If we continue to see good safety data, these are going to be the greatest things since sliced bread,” Dr. Reed said in an interview. “I don’t think we’ve seen anything like this in menopause therapeutics in a long time.”
While several nonhormonal drugs are already used to treat vasomotor symptoms in menopausal women with and without breast cancer, none are as effective as hormone treatments.
“For now, the SSRIs, SNRIs [serotonin norepinephrine reuptake inhibitors], and GABAergics are the best frontline nonhormonal options with a moderate effect, and clonidine and oxybutynin are effective, but we see more side effects with these,” Dr. Reed said. She noted the importance of considering patients’ mood, sleep, pain, sexual function, weight gain, overactive bladder, blood pressure, and individual quality of life (QOL) goals in tailoring those therapies.
But women still need more nonhormonal options that are at least as effective as hormonal options, Dr. Reed said. Some women are unable to take hormonal options because they are at risk for blood clots or breast cancer.
“Then there’s preference,” she said. “Sometimes people don’t like the way they feel when they take hormones, or they just don’t want hormones in their body. It’s absolutely critical to have these options available for women.”
Nanette F. Santoro, MD, a professor of ob.gyn. at the University of Colorado at Denver, Aurora, who was not involved in the presentation, said in an interview that physicians may not always realize the extent to which vasomotor symptoms interfere with women’s daily lives.
“They have an eroding effect on QOL that is not appreciated sometimes,” she said. Though hot flashes eventually subside in most women, others may continue to experience them into their 70s, when hormonal therapies can begin causing more harm than benefit.
“It goes underappreciated that, for a proportion of women, hot flashes will never go away, and they’re just as bad [as] when they were in their 50s,” Dr. Santoro said. “They need to be treated, and the nonhormonal treatments do not work for everybody.”
Promising KNDy therapeutics
Autopsy studies of postmenopausal women revealed that a complex of neurons in the hypothalamus was “massively hypertrophied” and sits right next to the thermoregulatory center of the brain, Dr. Reed explained.
The complex produces three types of molecules: kisspeptin (a neuropeptide), neurokinin B (a neuropeptide), and dynorphin (a kappa opioid), collectively referred to as the KNDy. The KNDy neural complex is located in the same place as the majority of hormone receptors in the arcuate nucleus, a collection of nerve cells in the hypothalamus.
The current hypothesis is that the KNDy neurons, which communicate with each other, become hyperactivated and cause hot flashes by spilling over to and triggering the thermoregulatory center next door. NKB (kisspeptin and neurokinin B) agonists activate KNDy neurons and dynorphin agonists inactivate KNDy, so the expectation is that NKB antagonists or dynorphin agonists would stop hot flashes.
Indeed, research published in 2015 showed that women taking kappa agonists experienced fewer hot flashes than women in the placebo group. However, no peripherally restricted kappa agonists are currently in clinical trials, so their future as therapeutics is unclear.
Right now, three different NK antagonists are in the pipeline for reducing vasomotor symptoms: MLE 4901 (pavinetant) and ESN364 (fezolinetant) are both NK3R antagonists, and NT-814 is a dual NK1R/NK3R antagonist. All three of these drugs were originally developed to treat schizophrenia.
Phase 2 clinical trials of pavinetant were discontinued in November 2017 by Millendo Therapeutics because 3 of 28 women experienced abnormal liver function, which normalized within 90 days. However, the study had shown an 80% decrease in hot flashes in women taking pavinetant, compared with a 30% decrease in the placebo group.
Fezolinetant, currently in phase 3 trials with Astellas, showed a dose response effect on reproductive hormones in phase 1 studies and a short half-life (4-6 hours) in women. It also showed no concerning side effects.
“There was, in fact, a decrease in the endometrial thickness, a delayed or impeded ovulation and a prolonged cycle duration,” Reed said.
The subsequent phase 2a study showed a reduction of five hot flashes a day (93% decrease), compared with placebo (54% decrease, P <.001) “with an abrupt return to baseline hot flash frequency after cessation,” she said. Improvements also occurred in sleep quality, quality of life, disability, and interference of hot flashes in daily life.
The phase 2b study found no difference in effects between once-daily versus twice-daily doses. However, two severe adverse events occurred: a drug-induced liver injury in one woman and cholelithiasis in another, both on the 60-mg, once-daily dose. Additionally, five women on varying doses had transient increases (above 1000 U/L) in creatinine kinase, though apparently without dose response.
A 52-week, three-arm, phase 3 trial of fezolinetant is currently under way with a goal of enrolling 1,740 participants, and plans to be completed by December 2021. Participants will undergo regular adverse event screening first biweekly, then monthly, with vital signs, blood, and urine monitoring.
Meanwhile, NT-814 from KaNDy Therapeutics, has completed phase 2a and phase 2b trials with phase 3 slated to begin in 2021. Adverse events in phase 1 included sleepiness and headache, and it had a long half-life (about 26 hours) and rapid absorption (an hour).
The phase 2a trial found a reduction of five hot flashes a day, compared with placebo, with main side effects again being sleepiness and headache. No events of abnormal liver function occurred. Phase 2b results have not been published.
So far, existing research suggests that KNDy interventions will involve a single daily oral dose that begins taking effect within 3 days and is fully in effect within 1-2 weeks. The reduction in hot flashes, about five fewer a day, is more effective than any other currently used nonhormonal medications for vasomotor symptoms. SSRIs and SNRIs tend to result in 1.5-2 fewer hot flashes a day, and gabapentin results in about 3 fewer per day. It will take longer-term studies, however, and paying attention to liver concerns for the NK3R antagonists to move into clinic.
“We want to keep our eye on the [luteinizing hormone] because if it decreases too much, it could adversely affect sexual function, and this does appear to be a dose-response finding,” Dr. Reed said. It would also be ideal, she said, to target only the KNDy neurons with NK3 antagonists without effects on the NK3 receptors in the liver.
Other nonhormonal options
Oxybutynin is another a nonhormonal agent under investigation for vasomotor symptoms. It’s an anticholinergic that resulted in 80% fewer hot flashes, compared with 30% with placebo in a 2016 trial, but 52% of women complained of dry mouth. A more recent study similarly found high efficacy – a 60%-80% drop in hot flashes, compared with 30% with placebo – but also side effects of dry mouth, difficulty urinating, and abdominal pain.
Finally, Dr. Reed mentioned three other agents under investigation as possible nonhormonal therapeutics, though she has little information about them. They include MT-8554 by Mitsubishi Tanabe; FP-101 by Fervent Pharmaceuticals; and Q-122 by QUE Oncology with Emory University, Atlanta, and the University of Queensland, Brisbane, Australia.
None of the currently available nonhormonal options provide as high efficacy as hormones, but they do reduce symptoms:
Clonidine is an off-label option some physicians already use as a nonhormonal treatment for vasomotor symptoms, but again, the side effects are problematic: dry mouth, constipation, drowsiness, postural hypotension, and poor sleep.
Paroxetine, at 7.5-10 mg, is the only FDA-approved nonhormonal treatment for vasomotor symptoms, but she listed other off-label options found effective in evidence reviews: gabapentin (100-2,400 mg), venlafaxine (37.5-75 mg), citalopram (10 mg), desvenlafaxine (150 mg), and escitalopram (10 mg).
“I want you to take note of the lower doses in all of these products that are efficacious above those doses that might be used for mood,” Dr. Reed added.
Dr. Reed receives royalties from UpToDate and research funding from Bayer. Dr. Santoro owns stock in MenoGeniX and serves as a consultant or advisor to Ansh Labs, MenoGeniX, and Ogeda/Astellas.
A version of this article originally appeared on Medscape.com.
researchers told attendees at the virtual North American Menopause Society 2020 Annual Meeting.
“The KNDy [kisspeptin/neurokinin B/dynorphin] neuron manipulation is really exciting and holds great promise for rapid and highly effective amelioration of hot flashes, up to 80%, and improvement in other menopausal symptoms, though we’re still looking at the safety in phase 3 trials,” reported Susan D. Reed, MD, MPH, director of the Women’s Reproductive Health Research Program at the University of Washington, Seattle.
“If we continue to see good safety data, these are going to be the greatest things since sliced bread,” Dr. Reed said in an interview. “I don’t think we’ve seen anything like this in menopause therapeutics in a long time.”
While several nonhormonal drugs are already used to treat vasomotor symptoms in menopausal women with and without breast cancer, none are as effective as hormone treatments.
“For now, the SSRIs, SNRIs [serotonin norepinephrine reuptake inhibitors], and GABAergics are the best frontline nonhormonal options with a moderate effect, and clonidine and oxybutynin are effective, but we see more side effects with these,” Dr. Reed said. She noted the importance of considering patients’ mood, sleep, pain, sexual function, weight gain, overactive bladder, blood pressure, and individual quality of life (QOL) goals in tailoring those therapies.
But women still need more nonhormonal options that are at least as effective as hormonal options, Dr. Reed said. Some women are unable to take hormonal options because they are at risk for blood clots or breast cancer.
“Then there’s preference,” she said. “Sometimes people don’t like the way they feel when they take hormones, or they just don’t want hormones in their body. It’s absolutely critical to have these options available for women.”
Nanette F. Santoro, MD, a professor of ob.gyn. at the University of Colorado at Denver, Aurora, who was not involved in the presentation, said in an interview that physicians may not always realize the extent to which vasomotor symptoms interfere with women’s daily lives.
“They have an eroding effect on QOL that is not appreciated sometimes,” she said. Though hot flashes eventually subside in most women, others may continue to experience them into their 70s, when hormonal therapies can begin causing more harm than benefit.
“It goes underappreciated that, for a proportion of women, hot flashes will never go away, and they’re just as bad [as] when they were in their 50s,” Dr. Santoro said. “They need to be treated, and the nonhormonal treatments do not work for everybody.”
Promising KNDy therapeutics
Autopsy studies of postmenopausal women revealed that a complex of neurons in the hypothalamus was “massively hypertrophied” and sits right next to the thermoregulatory center of the brain, Dr. Reed explained.
The complex produces three types of molecules: kisspeptin (a neuropeptide), neurokinin B (a neuropeptide), and dynorphin (a kappa opioid), collectively referred to as the KNDy. The KNDy neural complex is located in the same place as the majority of hormone receptors in the arcuate nucleus, a collection of nerve cells in the hypothalamus.
The current hypothesis is that the KNDy neurons, which communicate with each other, become hyperactivated and cause hot flashes by spilling over to and triggering the thermoregulatory center next door. NKB (kisspeptin and neurokinin B) agonists activate KNDy neurons and dynorphin agonists inactivate KNDy, so the expectation is that NKB antagonists or dynorphin agonists would stop hot flashes.
Indeed, research published in 2015 showed that women taking kappa agonists experienced fewer hot flashes than women in the placebo group. However, no peripherally restricted kappa agonists are currently in clinical trials, so their future as therapeutics is unclear.
Right now, three different NK antagonists are in the pipeline for reducing vasomotor symptoms: MLE 4901 (pavinetant) and ESN364 (fezolinetant) are both NK3R antagonists, and NT-814 is a dual NK1R/NK3R antagonist. All three of these drugs were originally developed to treat schizophrenia.
Phase 2 clinical trials of pavinetant were discontinued in November 2017 by Millendo Therapeutics because 3 of 28 women experienced abnormal liver function, which normalized within 90 days. However, the study had shown an 80% decrease in hot flashes in women taking pavinetant, compared with a 30% decrease in the placebo group.
Fezolinetant, currently in phase 3 trials with Astellas, showed a dose response effect on reproductive hormones in phase 1 studies and a short half-life (4-6 hours) in women. It also showed no concerning side effects.
“There was, in fact, a decrease in the endometrial thickness, a delayed or impeded ovulation and a prolonged cycle duration,” Reed said.
The subsequent phase 2a study showed a reduction of five hot flashes a day (93% decrease), compared with placebo (54% decrease, P <.001) “with an abrupt return to baseline hot flash frequency after cessation,” she said. Improvements also occurred in sleep quality, quality of life, disability, and interference of hot flashes in daily life.
The phase 2b study found no difference in effects between once-daily versus twice-daily doses. However, two severe adverse events occurred: a drug-induced liver injury in one woman and cholelithiasis in another, both on the 60-mg, once-daily dose. Additionally, five women on varying doses had transient increases (above 1000 U/L) in creatinine kinase, though apparently without dose response.
A 52-week, three-arm, phase 3 trial of fezolinetant is currently under way with a goal of enrolling 1,740 participants, and plans to be completed by December 2021. Participants will undergo regular adverse event screening first biweekly, then monthly, with vital signs, blood, and urine monitoring.
Meanwhile, NT-814 from KaNDy Therapeutics, has completed phase 2a and phase 2b trials with phase 3 slated to begin in 2021. Adverse events in phase 1 included sleepiness and headache, and it had a long half-life (about 26 hours) and rapid absorption (an hour).
The phase 2a trial found a reduction of five hot flashes a day, compared with placebo, with main side effects again being sleepiness and headache. No events of abnormal liver function occurred. Phase 2b results have not been published.
So far, existing research suggests that KNDy interventions will involve a single daily oral dose that begins taking effect within 3 days and is fully in effect within 1-2 weeks. The reduction in hot flashes, about five fewer a day, is more effective than any other currently used nonhormonal medications for vasomotor symptoms. SSRIs and SNRIs tend to result in 1.5-2 fewer hot flashes a day, and gabapentin results in about 3 fewer per day. It will take longer-term studies, however, and paying attention to liver concerns for the NK3R antagonists to move into clinic.
“We want to keep our eye on the [luteinizing hormone] because if it decreases too much, it could adversely affect sexual function, and this does appear to be a dose-response finding,” Dr. Reed said. It would also be ideal, she said, to target only the KNDy neurons with NK3 antagonists without effects on the NK3 receptors in the liver.
Other nonhormonal options
Oxybutynin is another a nonhormonal agent under investigation for vasomotor symptoms. It’s an anticholinergic that resulted in 80% fewer hot flashes, compared with 30% with placebo in a 2016 trial, but 52% of women complained of dry mouth. A more recent study similarly found high efficacy – a 60%-80% drop in hot flashes, compared with 30% with placebo – but also side effects of dry mouth, difficulty urinating, and abdominal pain.
Finally, Dr. Reed mentioned three other agents under investigation as possible nonhormonal therapeutics, though she has little information about them. They include MT-8554 by Mitsubishi Tanabe; FP-101 by Fervent Pharmaceuticals; and Q-122 by QUE Oncology with Emory University, Atlanta, and the University of Queensland, Brisbane, Australia.
None of the currently available nonhormonal options provide as high efficacy as hormones, but they do reduce symptoms:
Clonidine is an off-label option some physicians already use as a nonhormonal treatment for vasomotor symptoms, but again, the side effects are problematic: dry mouth, constipation, drowsiness, postural hypotension, and poor sleep.
Paroxetine, at 7.5-10 mg, is the only FDA-approved nonhormonal treatment for vasomotor symptoms, but she listed other off-label options found effective in evidence reviews: gabapentin (100-2,400 mg), venlafaxine (37.5-75 mg), citalopram (10 mg), desvenlafaxine (150 mg), and escitalopram (10 mg).
“I want you to take note of the lower doses in all of these products that are efficacious above those doses that might be used for mood,” Dr. Reed added.
Dr. Reed receives royalties from UpToDate and research funding from Bayer. Dr. Santoro owns stock in MenoGeniX and serves as a consultant or advisor to Ansh Labs, MenoGeniX, and Ogeda/Astellas.
A version of this article originally appeared on Medscape.com.
researchers told attendees at the virtual North American Menopause Society 2020 Annual Meeting.
“The KNDy [kisspeptin/neurokinin B/dynorphin] neuron manipulation is really exciting and holds great promise for rapid and highly effective amelioration of hot flashes, up to 80%, and improvement in other menopausal symptoms, though we’re still looking at the safety in phase 3 trials,” reported Susan D. Reed, MD, MPH, director of the Women’s Reproductive Health Research Program at the University of Washington, Seattle.
“If we continue to see good safety data, these are going to be the greatest things since sliced bread,” Dr. Reed said in an interview. “I don’t think we’ve seen anything like this in menopause therapeutics in a long time.”
While several nonhormonal drugs are already used to treat vasomotor symptoms in menopausal women with and without breast cancer, none are as effective as hormone treatments.
“For now, the SSRIs, SNRIs [serotonin norepinephrine reuptake inhibitors], and GABAergics are the best frontline nonhormonal options with a moderate effect, and clonidine and oxybutynin are effective, but we see more side effects with these,” Dr. Reed said. She noted the importance of considering patients’ mood, sleep, pain, sexual function, weight gain, overactive bladder, blood pressure, and individual quality of life (QOL) goals in tailoring those therapies.
But women still need more nonhormonal options that are at least as effective as hormonal options, Dr. Reed said. Some women are unable to take hormonal options because they are at risk for blood clots or breast cancer.
“Then there’s preference,” she said. “Sometimes people don’t like the way they feel when they take hormones, or they just don’t want hormones in their body. It’s absolutely critical to have these options available for women.”
Nanette F. Santoro, MD, a professor of ob.gyn. at the University of Colorado at Denver, Aurora, who was not involved in the presentation, said in an interview that physicians may not always realize the extent to which vasomotor symptoms interfere with women’s daily lives.
“They have an eroding effect on QOL that is not appreciated sometimes,” she said. Though hot flashes eventually subside in most women, others may continue to experience them into their 70s, when hormonal therapies can begin causing more harm than benefit.
“It goes underappreciated that, for a proportion of women, hot flashes will never go away, and they’re just as bad [as] when they were in their 50s,” Dr. Santoro said. “They need to be treated, and the nonhormonal treatments do not work for everybody.”
Promising KNDy therapeutics
Autopsy studies of postmenopausal women revealed that a complex of neurons in the hypothalamus was “massively hypertrophied” and sits right next to the thermoregulatory center of the brain, Dr. Reed explained.
The complex produces three types of molecules: kisspeptin (a neuropeptide), neurokinin B (a neuropeptide), and dynorphin (a kappa opioid), collectively referred to as the KNDy. The KNDy neural complex is located in the same place as the majority of hormone receptors in the arcuate nucleus, a collection of nerve cells in the hypothalamus.
The current hypothesis is that the KNDy neurons, which communicate with each other, become hyperactivated and cause hot flashes by spilling over to and triggering the thermoregulatory center next door. NKB (kisspeptin and neurokinin B) agonists activate KNDy neurons and dynorphin agonists inactivate KNDy, so the expectation is that NKB antagonists or dynorphin agonists would stop hot flashes.
Indeed, research published in 2015 showed that women taking kappa agonists experienced fewer hot flashes than women in the placebo group. However, no peripherally restricted kappa agonists are currently in clinical trials, so their future as therapeutics is unclear.
Right now, three different NK antagonists are in the pipeline for reducing vasomotor symptoms: MLE 4901 (pavinetant) and ESN364 (fezolinetant) are both NK3R antagonists, and NT-814 is a dual NK1R/NK3R antagonist. All three of these drugs were originally developed to treat schizophrenia.
Phase 2 clinical trials of pavinetant were discontinued in November 2017 by Millendo Therapeutics because 3 of 28 women experienced abnormal liver function, which normalized within 90 days. However, the study had shown an 80% decrease in hot flashes in women taking pavinetant, compared with a 30% decrease in the placebo group.
Fezolinetant, currently in phase 3 trials with Astellas, showed a dose response effect on reproductive hormones in phase 1 studies and a short half-life (4-6 hours) in women. It also showed no concerning side effects.
“There was, in fact, a decrease in the endometrial thickness, a delayed or impeded ovulation and a prolonged cycle duration,” Reed said.
The subsequent phase 2a study showed a reduction of five hot flashes a day (93% decrease), compared with placebo (54% decrease, P <.001) “with an abrupt return to baseline hot flash frequency after cessation,” she said. Improvements also occurred in sleep quality, quality of life, disability, and interference of hot flashes in daily life.
The phase 2b study found no difference in effects between once-daily versus twice-daily doses. However, two severe adverse events occurred: a drug-induced liver injury in one woman and cholelithiasis in another, both on the 60-mg, once-daily dose. Additionally, five women on varying doses had transient increases (above 1000 U/L) in creatinine kinase, though apparently without dose response.
A 52-week, three-arm, phase 3 trial of fezolinetant is currently under way with a goal of enrolling 1,740 participants, and plans to be completed by December 2021. Participants will undergo regular adverse event screening first biweekly, then monthly, with vital signs, blood, and urine monitoring.
Meanwhile, NT-814 from KaNDy Therapeutics, has completed phase 2a and phase 2b trials with phase 3 slated to begin in 2021. Adverse events in phase 1 included sleepiness and headache, and it had a long half-life (about 26 hours) and rapid absorption (an hour).
The phase 2a trial found a reduction of five hot flashes a day, compared with placebo, with main side effects again being sleepiness and headache. No events of abnormal liver function occurred. Phase 2b results have not been published.
So far, existing research suggests that KNDy interventions will involve a single daily oral dose that begins taking effect within 3 days and is fully in effect within 1-2 weeks. The reduction in hot flashes, about five fewer a day, is more effective than any other currently used nonhormonal medications for vasomotor symptoms. SSRIs and SNRIs tend to result in 1.5-2 fewer hot flashes a day, and gabapentin results in about 3 fewer per day. It will take longer-term studies, however, and paying attention to liver concerns for the NK3R antagonists to move into clinic.
“We want to keep our eye on the [luteinizing hormone] because if it decreases too much, it could adversely affect sexual function, and this does appear to be a dose-response finding,” Dr. Reed said. It would also be ideal, she said, to target only the KNDy neurons with NK3 antagonists without effects on the NK3 receptors in the liver.
Other nonhormonal options
Oxybutynin is another a nonhormonal agent under investigation for vasomotor symptoms. It’s an anticholinergic that resulted in 80% fewer hot flashes, compared with 30% with placebo in a 2016 trial, but 52% of women complained of dry mouth. A more recent study similarly found high efficacy – a 60%-80% drop in hot flashes, compared with 30% with placebo – but also side effects of dry mouth, difficulty urinating, and abdominal pain.
Finally, Dr. Reed mentioned three other agents under investigation as possible nonhormonal therapeutics, though she has little information about them. They include MT-8554 by Mitsubishi Tanabe; FP-101 by Fervent Pharmaceuticals; and Q-122 by QUE Oncology with Emory University, Atlanta, and the University of Queensland, Brisbane, Australia.
None of the currently available nonhormonal options provide as high efficacy as hormones, but they do reduce symptoms:
Clonidine is an off-label option some physicians already use as a nonhormonal treatment for vasomotor symptoms, but again, the side effects are problematic: dry mouth, constipation, drowsiness, postural hypotension, and poor sleep.
Paroxetine, at 7.5-10 mg, is the only FDA-approved nonhormonal treatment for vasomotor symptoms, but she listed other off-label options found effective in evidence reviews: gabapentin (100-2,400 mg), venlafaxine (37.5-75 mg), citalopram (10 mg), desvenlafaxine (150 mg), and escitalopram (10 mg).
“I want you to take note of the lower doses in all of these products that are efficacious above those doses that might be used for mood,” Dr. Reed added.
Dr. Reed receives royalties from UpToDate and research funding from Bayer. Dr. Santoro owns stock in MenoGeniX and serves as a consultant or advisor to Ansh Labs, MenoGeniX, and Ogeda/Astellas.
A version of this article originally appeared on Medscape.com.
FDA proposes withdrawing Makena’s approval
Makena should be withdrawn from the market because a postmarketing study did not show clinical benefit, according to a statement released today from the Center for Drug Evaluation and Research at the Food and Drug Administration.
The drug, hydroxyprogesterone caproate injection, was approved in 2011 to reduce the risk of preterm birth in women who with previous spontaneous preterm birth. The FDA approved the medication under an accelerated pathway that required another trial to confirm clinical benefit.
The required postmarketing study “not only failed to demonstrate Makena’s benefit to the neonate, but also failed to substantiate any effect of Makena on the surrogate endpoint of gestational age at delivery that was the basis of the initial approval,” Patrizia Cavazzoni, MD, acting director of the CDER, wrote in a letter to AMAG Pharma USA, which markets Makena. The letter also was sent to other companies developing products that use the drug.
Beyond the lack of efficacy, risks associated with the drug include thromboembolic disorders, allergic reactions, decreased glucose tolerance, and fluid retention. “The risk of exposing treated pregnant women to these harms, in addition to false hopes, costs, and additional healthcare utilization outweighs Makena’s unproven benefit,” Dr. Cavazzoni said.
The letter notifies companies about the opportunity for a hearing on the proposed withdrawal of marketing approval. Makena and its generic equivalents will remain on the market until the manufacturers remove the drugs or the FDA commissioner mandates their removal, the CDER said.
The FDA commissioner ultimately will decide whether to withdraw approval of the drug. An FDA panel previously voted to withdraw the drug from the market in October 2019, and the drug has remained in limbo since.
Health care professionals should discuss “Makena’s benefits, risks, and uncertainties with their patients to decide whether to use Makena while a final decision is being made about the drug’s marketing status,” the CDER announcement said.
A version of this article originally appeared on Medscape.com.
Makena should be withdrawn from the market because a postmarketing study did not show clinical benefit, according to a statement released today from the Center for Drug Evaluation and Research at the Food and Drug Administration.
The drug, hydroxyprogesterone caproate injection, was approved in 2011 to reduce the risk of preterm birth in women who with previous spontaneous preterm birth. The FDA approved the medication under an accelerated pathway that required another trial to confirm clinical benefit.
The required postmarketing study “not only failed to demonstrate Makena’s benefit to the neonate, but also failed to substantiate any effect of Makena on the surrogate endpoint of gestational age at delivery that was the basis of the initial approval,” Patrizia Cavazzoni, MD, acting director of the CDER, wrote in a letter to AMAG Pharma USA, which markets Makena. The letter also was sent to other companies developing products that use the drug.
Beyond the lack of efficacy, risks associated with the drug include thromboembolic disorders, allergic reactions, decreased glucose tolerance, and fluid retention. “The risk of exposing treated pregnant women to these harms, in addition to false hopes, costs, and additional healthcare utilization outweighs Makena’s unproven benefit,” Dr. Cavazzoni said.
The letter notifies companies about the opportunity for a hearing on the proposed withdrawal of marketing approval. Makena and its generic equivalents will remain on the market until the manufacturers remove the drugs or the FDA commissioner mandates their removal, the CDER said.
The FDA commissioner ultimately will decide whether to withdraw approval of the drug. An FDA panel previously voted to withdraw the drug from the market in October 2019, and the drug has remained in limbo since.
Health care professionals should discuss “Makena’s benefits, risks, and uncertainties with their patients to decide whether to use Makena while a final decision is being made about the drug’s marketing status,” the CDER announcement said.
A version of this article originally appeared on Medscape.com.
Makena should be withdrawn from the market because a postmarketing study did not show clinical benefit, according to a statement released today from the Center for Drug Evaluation and Research at the Food and Drug Administration.
The drug, hydroxyprogesterone caproate injection, was approved in 2011 to reduce the risk of preterm birth in women who with previous spontaneous preterm birth. The FDA approved the medication under an accelerated pathway that required another trial to confirm clinical benefit.
The required postmarketing study “not only failed to demonstrate Makena’s benefit to the neonate, but also failed to substantiate any effect of Makena on the surrogate endpoint of gestational age at delivery that was the basis of the initial approval,” Patrizia Cavazzoni, MD, acting director of the CDER, wrote in a letter to AMAG Pharma USA, which markets Makena. The letter also was sent to other companies developing products that use the drug.
Beyond the lack of efficacy, risks associated with the drug include thromboembolic disorders, allergic reactions, decreased glucose tolerance, and fluid retention. “The risk of exposing treated pregnant women to these harms, in addition to false hopes, costs, and additional healthcare utilization outweighs Makena’s unproven benefit,” Dr. Cavazzoni said.
The letter notifies companies about the opportunity for a hearing on the proposed withdrawal of marketing approval. Makena and its generic equivalents will remain on the market until the manufacturers remove the drugs or the FDA commissioner mandates their removal, the CDER said.
The FDA commissioner ultimately will decide whether to withdraw approval of the drug. An FDA panel previously voted to withdraw the drug from the market in October 2019, and the drug has remained in limbo since.
Health care professionals should discuss “Makena’s benefits, risks, and uncertainties with their patients to decide whether to use Makena while a final decision is being made about the drug’s marketing status,” the CDER announcement said.
A version of this article originally appeared on Medscape.com.
FDA updates info on postmarketing surveillance study of Essure
The Food and Drug Administration has updated its page on Essure information for patients and health care providers to add additional information on adverse events reported by its manufacturer.
Essure was a permanent implantable birth control device approved by the FDA in 2002. FDA ordered Bayer in 2016 to conduct a postmarket surveillance study of Essure following reports of safety concerns, and expanded the study from 3 years to 5 years in 2018. Bayer voluntarily removed Essure from the market at the end of 2018, citing low sales after a “black box” warning was placed on the device. All devices were returned to the company by the end of 2019.
Bayer is required to report variances in Medical Device Reporting (MDR) requirements of Essure related to litigation to the FDA, which includes adverse events such death, serious injury, and “malfunction that would be likely to cause or contribute to a death or serious injury if the malfunction were to recur.” The reports are limited to events Bayer becomes aware of between November 2016 and November 2020. Bayer will continue to provide these reports until April 2021.
The FDA emphasized that the collected data are based on social media reports and already may be reported to the FDA, rather than being a collection of new events. “The limited information provided in the reports prevents the ability to draw any conclusions as to whether the device, or its removal, caused or contributed to any of the events in the reports,” Benjamin Fisher, PhD, director of the Reproductive, Gastro-Renal, Urological, General Hospital Device and Human Factors Office in the Center for Devices and Radiological Health, said in an FDA In Brief statement on Aug. 11.
The FDA first uploaded an Essure MDR variance spreadsheet in August 2020, listing 1,453 events, consisting of 53 reports of deaths, 1,376 reports of serious injury, and 24 reports of device malfunction that occurred as of June 2020. In September 2020, FDA uploaded a second variance spreadsheet, which added another 1,934 events that occurred as of July.
Interim analysis of postmarketing surveillance study
An interim analysis of 1,128 patients from 67 centers in the Essure postmarket surveillance study, which compared women who received Essure with those who received laparoscopic tubal sterilization, revealed that 94.6% (265 of 280 patients) in the Essure group had a successful implantation of the device, compared with 99.6% of women who achieved bilateral tubal occlusion from laparoscopic tubal sterilization.
Regarding safety, 9.1% of women in the Essure group and 4.5% in the laparoscopic tubal sterilization group reported chronic lower abdominal and/or pelvic pain, and 16.3% in the Essure group and 10.2% in the laparoscopic tubal sterilization group reported new or worsening abnormal uterine bleeding. In the Essure group, 22.3% of women said they experienced hypersensitivity, an allergic reaction, and new “autoimmune-like reactions” compared with 12.5% of women in the laparoscopic tubal sterilization group.
The interim analysis also showed 19.7% of women in the Essure group and 3.0% in the laparoscopic tubal sterilization group underwent gynecologic surgical procedures, which were “driven primarily by Essure removal and endometrial ablation procedures in Essure patients.” Device removal occurred in 6.8% of women with the Essure device.
Consistent data on Essure
An FDA search of the Manufacturer and User Facility Device Experience (MAUDE) database in January of 2020 revealed 47,856 medical device reports of Essure between November 2002 and December 2019. The most common adverse events observed during this period were:
- Pain or abdominal pain (32,901 cases).
- Heavy or irregular menses (14,573 cases). Headache (8,570 cases).
- Device fragment or foreign body in a patient (8,501 cases).
- Perforation (7,825 cases).
- Fatigue (7,083 cases).
- Gain or loss in weight (5,980 cases).
- Anxiety and/or depression (5,366 cases).
- Rash and/or hypersensitivity (5,077 cases)
- Hair loss (4,999 cases).
Problems with the device itself included reports of:
- Device incompatibility such as an allergy (7,515 cases).
- The device migrating (4,535 cases).
- The device breaking or fracturing (2,297 cases).
- The device dislodging or dislocating (1,797 cases).
- Improper operation including implant failure and pregnancy (1,058 cases).
In 2019, Essure received 15,083 medical device reports, an increase from 6,000 reports in 2018 and 11,854 reports in 2017.
To date, nearly 39,000 women in the United States have made claims to injuries related to the Essure device. In August, Bayer announced it would pay approximately $1.6 billion U.S. dollars to settle 90% of these cases in exchange for claimants to “dismiss their cases or not file.” Bayer also said in a press release that the settlement is not an admission of wrongdoing or liability on the part of the company.
In an interview, Catherine Cansino, MD, MPH, of the department of obstetrics and gynecology at the University of California, Davis, said the latest adverse event reports show “consistent info from [the] MAUDE database when comparing 2019 to previous years, highlighting most common problems related to pain and heavy or irregular bleeding.”
She emphasized ob.gyns with patients who have an Essure device should “consider Essure-related etiology that may necessitate device removal when evaluating patients with gynecological problems, especially with regard to abdominal/pelvic pain and heavy/irregular bleeding.”
Dr. Cansino reported no relevant financial disclosures. She is a member of the Ob.Gyn. News Editorial Advisory Board.
The Food and Drug Administration has updated its page on Essure information for patients and health care providers to add additional information on adverse events reported by its manufacturer.
Essure was a permanent implantable birth control device approved by the FDA in 2002. FDA ordered Bayer in 2016 to conduct a postmarket surveillance study of Essure following reports of safety concerns, and expanded the study from 3 years to 5 years in 2018. Bayer voluntarily removed Essure from the market at the end of 2018, citing low sales after a “black box” warning was placed on the device. All devices were returned to the company by the end of 2019.
Bayer is required to report variances in Medical Device Reporting (MDR) requirements of Essure related to litigation to the FDA, which includes adverse events such death, serious injury, and “malfunction that would be likely to cause or contribute to a death or serious injury if the malfunction were to recur.” The reports are limited to events Bayer becomes aware of between November 2016 and November 2020. Bayer will continue to provide these reports until April 2021.
The FDA emphasized that the collected data are based on social media reports and already may be reported to the FDA, rather than being a collection of new events. “The limited information provided in the reports prevents the ability to draw any conclusions as to whether the device, or its removal, caused or contributed to any of the events in the reports,” Benjamin Fisher, PhD, director of the Reproductive, Gastro-Renal, Urological, General Hospital Device and Human Factors Office in the Center for Devices and Radiological Health, said in an FDA In Brief statement on Aug. 11.
The FDA first uploaded an Essure MDR variance spreadsheet in August 2020, listing 1,453 events, consisting of 53 reports of deaths, 1,376 reports of serious injury, and 24 reports of device malfunction that occurred as of June 2020. In September 2020, FDA uploaded a second variance spreadsheet, which added another 1,934 events that occurred as of July.
Interim analysis of postmarketing surveillance study
An interim analysis of 1,128 patients from 67 centers in the Essure postmarket surveillance study, which compared women who received Essure with those who received laparoscopic tubal sterilization, revealed that 94.6% (265 of 280 patients) in the Essure group had a successful implantation of the device, compared with 99.6% of women who achieved bilateral tubal occlusion from laparoscopic tubal sterilization.
Regarding safety, 9.1% of women in the Essure group and 4.5% in the laparoscopic tubal sterilization group reported chronic lower abdominal and/or pelvic pain, and 16.3% in the Essure group and 10.2% in the laparoscopic tubal sterilization group reported new or worsening abnormal uterine bleeding. In the Essure group, 22.3% of women said they experienced hypersensitivity, an allergic reaction, and new “autoimmune-like reactions” compared with 12.5% of women in the laparoscopic tubal sterilization group.
The interim analysis also showed 19.7% of women in the Essure group and 3.0% in the laparoscopic tubal sterilization group underwent gynecologic surgical procedures, which were “driven primarily by Essure removal and endometrial ablation procedures in Essure patients.” Device removal occurred in 6.8% of women with the Essure device.
Consistent data on Essure
An FDA search of the Manufacturer and User Facility Device Experience (MAUDE) database in January of 2020 revealed 47,856 medical device reports of Essure between November 2002 and December 2019. The most common adverse events observed during this period were:
- Pain or abdominal pain (32,901 cases).
- Heavy or irregular menses (14,573 cases). Headache (8,570 cases).
- Device fragment or foreign body in a patient (8,501 cases).
- Perforation (7,825 cases).
- Fatigue (7,083 cases).
- Gain or loss in weight (5,980 cases).
- Anxiety and/or depression (5,366 cases).
- Rash and/or hypersensitivity (5,077 cases)
- Hair loss (4,999 cases).
Problems with the device itself included reports of:
- Device incompatibility such as an allergy (7,515 cases).
- The device migrating (4,535 cases).
- The device breaking or fracturing (2,297 cases).
- The device dislodging or dislocating (1,797 cases).
- Improper operation including implant failure and pregnancy (1,058 cases).
In 2019, Essure received 15,083 medical device reports, an increase from 6,000 reports in 2018 and 11,854 reports in 2017.
To date, nearly 39,000 women in the United States have made claims to injuries related to the Essure device. In August, Bayer announced it would pay approximately $1.6 billion U.S. dollars to settle 90% of these cases in exchange for claimants to “dismiss their cases or not file.” Bayer also said in a press release that the settlement is not an admission of wrongdoing or liability on the part of the company.
In an interview, Catherine Cansino, MD, MPH, of the department of obstetrics and gynecology at the University of California, Davis, said the latest adverse event reports show “consistent info from [the] MAUDE database when comparing 2019 to previous years, highlighting most common problems related to pain and heavy or irregular bleeding.”
She emphasized ob.gyns with patients who have an Essure device should “consider Essure-related etiology that may necessitate device removal when evaluating patients with gynecological problems, especially with regard to abdominal/pelvic pain and heavy/irregular bleeding.”
Dr. Cansino reported no relevant financial disclosures. She is a member of the Ob.Gyn. News Editorial Advisory Board.
The Food and Drug Administration has updated its page on Essure information for patients and health care providers to add additional information on adverse events reported by its manufacturer.
Essure was a permanent implantable birth control device approved by the FDA in 2002. FDA ordered Bayer in 2016 to conduct a postmarket surveillance study of Essure following reports of safety concerns, and expanded the study from 3 years to 5 years in 2018. Bayer voluntarily removed Essure from the market at the end of 2018, citing low sales after a “black box” warning was placed on the device. All devices were returned to the company by the end of 2019.
Bayer is required to report variances in Medical Device Reporting (MDR) requirements of Essure related to litigation to the FDA, which includes adverse events such death, serious injury, and “malfunction that would be likely to cause or contribute to a death or serious injury if the malfunction were to recur.” The reports are limited to events Bayer becomes aware of between November 2016 and November 2020. Bayer will continue to provide these reports until April 2021.
The FDA emphasized that the collected data are based on social media reports and already may be reported to the FDA, rather than being a collection of new events. “The limited information provided in the reports prevents the ability to draw any conclusions as to whether the device, or its removal, caused or contributed to any of the events in the reports,” Benjamin Fisher, PhD, director of the Reproductive, Gastro-Renal, Urological, General Hospital Device and Human Factors Office in the Center for Devices and Radiological Health, said in an FDA In Brief statement on Aug. 11.
The FDA first uploaded an Essure MDR variance spreadsheet in August 2020, listing 1,453 events, consisting of 53 reports of deaths, 1,376 reports of serious injury, and 24 reports of device malfunction that occurred as of June 2020. In September 2020, FDA uploaded a second variance spreadsheet, which added another 1,934 events that occurred as of July.
Interim analysis of postmarketing surveillance study
An interim analysis of 1,128 patients from 67 centers in the Essure postmarket surveillance study, which compared women who received Essure with those who received laparoscopic tubal sterilization, revealed that 94.6% (265 of 280 patients) in the Essure group had a successful implantation of the device, compared with 99.6% of women who achieved bilateral tubal occlusion from laparoscopic tubal sterilization.
Regarding safety, 9.1% of women in the Essure group and 4.5% in the laparoscopic tubal sterilization group reported chronic lower abdominal and/or pelvic pain, and 16.3% in the Essure group and 10.2% in the laparoscopic tubal sterilization group reported new or worsening abnormal uterine bleeding. In the Essure group, 22.3% of women said they experienced hypersensitivity, an allergic reaction, and new “autoimmune-like reactions” compared with 12.5% of women in the laparoscopic tubal sterilization group.
The interim analysis also showed 19.7% of women in the Essure group and 3.0% in the laparoscopic tubal sterilization group underwent gynecologic surgical procedures, which were “driven primarily by Essure removal and endometrial ablation procedures in Essure patients.” Device removal occurred in 6.8% of women with the Essure device.
Consistent data on Essure
An FDA search of the Manufacturer and User Facility Device Experience (MAUDE) database in January of 2020 revealed 47,856 medical device reports of Essure between November 2002 and December 2019. The most common adverse events observed during this period were:
- Pain or abdominal pain (32,901 cases).
- Heavy or irregular menses (14,573 cases). Headache (8,570 cases).
- Device fragment or foreign body in a patient (8,501 cases).
- Perforation (7,825 cases).
- Fatigue (7,083 cases).
- Gain or loss in weight (5,980 cases).
- Anxiety and/or depression (5,366 cases).
- Rash and/or hypersensitivity (5,077 cases)
- Hair loss (4,999 cases).
Problems with the device itself included reports of:
- Device incompatibility such as an allergy (7,515 cases).
- The device migrating (4,535 cases).
- The device breaking or fracturing (2,297 cases).
- The device dislodging or dislocating (1,797 cases).
- Improper operation including implant failure and pregnancy (1,058 cases).
In 2019, Essure received 15,083 medical device reports, an increase from 6,000 reports in 2018 and 11,854 reports in 2017.
To date, nearly 39,000 women in the United States have made claims to injuries related to the Essure device. In August, Bayer announced it would pay approximately $1.6 billion U.S. dollars to settle 90% of these cases in exchange for claimants to “dismiss their cases or not file.” Bayer also said in a press release that the settlement is not an admission of wrongdoing or liability on the part of the company.
In an interview, Catherine Cansino, MD, MPH, of the department of obstetrics and gynecology at the University of California, Davis, said the latest adverse event reports show “consistent info from [the] MAUDE database when comparing 2019 to previous years, highlighting most common problems related to pain and heavy or irregular bleeding.”
She emphasized ob.gyns with patients who have an Essure device should “consider Essure-related etiology that may necessitate device removal when evaluating patients with gynecological problems, especially with regard to abdominal/pelvic pain and heavy/irregular bleeding.”
Dr. Cansino reported no relevant financial disclosures. She is a member of the Ob.Gyn. News Editorial Advisory Board.
HPV vaccine shown to substantially reduce cervical cancer risk
It’s been shown that the vaccine (Gardasil) helps prevent genital warts and high-grade cervical lesions, but until now, data on the ability of the vaccine to prevent cervical cancer, although widely assumed, had been lacking.
“Our results extend [the] knowledge base by showing that quadrivalent HPV vaccination is also associated with a substantially reduced risk of invasive cervical cancer, which is the ultimate intent of HPV vaccination programs,” said investigators led by Jiayao Lei, PhD, a researcher in the department of medical epidemiology and biostatistics at the Karolinska Institute, Stockholm.
The study was published online Oct. 1 in the New England Journal of Medicine.
“This work provides evidence of actual cancer prevention,” commented Diane Harper, MD, an HPV expert and professor in the departments of family medicine and obstetrics & gynecology at the University of Michigan, Ann Arbor. She was the principal investigator on the original Gardasil trial.
This study “shows that the quadrivalent HPV vaccine provides prevention from the sexually transmitted HPV infection that actually reduces the incidence of cervical cancer in young women up to 30 years of age,” she said when approached for comment.
However, she also added a note of caution. These new results show “that vaccinated women still develop cervical cancer, but at a slower rate. This makes the connection between early-age vaccination and continued adult life screening incredibly important,” Dr. Harper said in an interview
Cervical cancer was diagnosed in 19 of the 527,871 women (0.004%) who had received at least one dose of the vaccine versus 538 among the 1,145,112 women (0.05%) who had not.
The cumulative incidence was 47 cases per 100,000 vaccinated women and 94 cases per 100,000 unvaccinated women. The cervical cancer incidence rate ratio for the comparison of vaccinated versus unvaccinated women was 0.37 (95% confidence interval, 0.21-0.57).
The risk reduction was even greater among women who had been vaccinated before the age of 17, with a cumulative incidence of 4 versus 54 cases per 100,000 for women vaccinated after age 17. The incidence rate ratio was 0.12 (95% CI, 0.00-0.34) for women who had been vaccinated before age 17 versus 0.47 (95% CI, 0.27-0.75) among those vaccinated from age 17 to 30 years.
Overall, “the risk of cervical cancer among participants who had initiated vaccination before the age of 17 years was 88% lower than among those who had never been vaccinated,” the investigators noted.
These results “support the recommendation to administer quadrivalent HPV vaccine before exposure to HPV infection to achieve the most substantial benefit,” the investigators wrote.
Details of the Swedish review
For their review, Dr. Lei and colleagues used several Swedish demographic and health registries to connect vaccination status to incident cervical cancers, using the personal identification numbers Sweden issues to residents.
Participants were followed starting either on their 10th birthday or on Jan. 1, 2006, whichever came later. They were followed until, among other things, diagnosis of invasive cervical cancer; their 31st birthday; or until Dec. 31, 2017, whichever came first.
The quadrivalent HPV vaccine, approved in Sweden in 2006, was used almost exclusively during the study period. Participants were considered vaccinated if they had received only one shot, but the investigators set out to analyze a relationship between the incidence of invasive cervical cancer and the number of shots given.
Among other things, the team controlled for age at follow-up, calendar year, county of residence, maternal disease history, and parental characteristics, including education and household income.
The investigators commented that it’s possible that HPV-vaccinated women could have been generally healthier than unvaccinated women and so would have been at lower risk for cervical cancer.
“Confounding by lifestyle and health factors in the women (such as smoking status, sexual activity, oral contraceptive use, and obesity) cannot be excluded; these factors are known to be associated with a risk of cervical cancer,” the investigators wrote.
HPV is also associated with other types of cancer, including anal and oropharyngeal cancers. But these cancers develop over a longer period than cervical cancer.
Dr. Harper noted that the “probability of HPV 16 cancer by time since infection peaks at 40 years after infection for anal cancers and nearly 50 years after infection for oropharyngeal cancers. This means that registries, such as in Sweden, for the next 40 years will record the evidence to say whether HPV vaccination lasts long enough to prevent [these] other HPV 16–associated cancers occurring at a much later time in life.”
The work was funded by the Swedish Foundation for Strategic Research, the Swedish Cancer Society, and the Swedish Research Council and by the China Scholarship Council. Dr. Lei and two other investigators reported HPV vaccine research funding from Merck, the maker of Gardasil. Harper disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
It’s been shown that the vaccine (Gardasil) helps prevent genital warts and high-grade cervical lesions, but until now, data on the ability of the vaccine to prevent cervical cancer, although widely assumed, had been lacking.
“Our results extend [the] knowledge base by showing that quadrivalent HPV vaccination is also associated with a substantially reduced risk of invasive cervical cancer, which is the ultimate intent of HPV vaccination programs,” said investigators led by Jiayao Lei, PhD, a researcher in the department of medical epidemiology and biostatistics at the Karolinska Institute, Stockholm.
The study was published online Oct. 1 in the New England Journal of Medicine.
“This work provides evidence of actual cancer prevention,” commented Diane Harper, MD, an HPV expert and professor in the departments of family medicine and obstetrics & gynecology at the University of Michigan, Ann Arbor. She was the principal investigator on the original Gardasil trial.
This study “shows that the quadrivalent HPV vaccine provides prevention from the sexually transmitted HPV infection that actually reduces the incidence of cervical cancer in young women up to 30 years of age,” she said when approached for comment.
However, she also added a note of caution. These new results show “that vaccinated women still develop cervical cancer, but at a slower rate. This makes the connection between early-age vaccination and continued adult life screening incredibly important,” Dr. Harper said in an interview
Cervical cancer was diagnosed in 19 of the 527,871 women (0.004%) who had received at least one dose of the vaccine versus 538 among the 1,145,112 women (0.05%) who had not.
The cumulative incidence was 47 cases per 100,000 vaccinated women and 94 cases per 100,000 unvaccinated women. The cervical cancer incidence rate ratio for the comparison of vaccinated versus unvaccinated women was 0.37 (95% confidence interval, 0.21-0.57).
The risk reduction was even greater among women who had been vaccinated before the age of 17, with a cumulative incidence of 4 versus 54 cases per 100,000 for women vaccinated after age 17. The incidence rate ratio was 0.12 (95% CI, 0.00-0.34) for women who had been vaccinated before age 17 versus 0.47 (95% CI, 0.27-0.75) among those vaccinated from age 17 to 30 years.
Overall, “the risk of cervical cancer among participants who had initiated vaccination before the age of 17 years was 88% lower than among those who had never been vaccinated,” the investigators noted.
These results “support the recommendation to administer quadrivalent HPV vaccine before exposure to HPV infection to achieve the most substantial benefit,” the investigators wrote.
Details of the Swedish review
For their review, Dr. Lei and colleagues used several Swedish demographic and health registries to connect vaccination status to incident cervical cancers, using the personal identification numbers Sweden issues to residents.
Participants were followed starting either on their 10th birthday or on Jan. 1, 2006, whichever came later. They were followed until, among other things, diagnosis of invasive cervical cancer; their 31st birthday; or until Dec. 31, 2017, whichever came first.
The quadrivalent HPV vaccine, approved in Sweden in 2006, was used almost exclusively during the study period. Participants were considered vaccinated if they had received only one shot, but the investigators set out to analyze a relationship between the incidence of invasive cervical cancer and the number of shots given.
Among other things, the team controlled for age at follow-up, calendar year, county of residence, maternal disease history, and parental characteristics, including education and household income.
The investigators commented that it’s possible that HPV-vaccinated women could have been generally healthier than unvaccinated women and so would have been at lower risk for cervical cancer.
“Confounding by lifestyle and health factors in the women (such as smoking status, sexual activity, oral contraceptive use, and obesity) cannot be excluded; these factors are known to be associated with a risk of cervical cancer,” the investigators wrote.
HPV is also associated with other types of cancer, including anal and oropharyngeal cancers. But these cancers develop over a longer period than cervical cancer.
Dr. Harper noted that the “probability of HPV 16 cancer by time since infection peaks at 40 years after infection for anal cancers and nearly 50 years after infection for oropharyngeal cancers. This means that registries, such as in Sweden, for the next 40 years will record the evidence to say whether HPV vaccination lasts long enough to prevent [these] other HPV 16–associated cancers occurring at a much later time in life.”
The work was funded by the Swedish Foundation for Strategic Research, the Swedish Cancer Society, and the Swedish Research Council and by the China Scholarship Council. Dr. Lei and two other investigators reported HPV vaccine research funding from Merck, the maker of Gardasil. Harper disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
It’s been shown that the vaccine (Gardasil) helps prevent genital warts and high-grade cervical lesions, but until now, data on the ability of the vaccine to prevent cervical cancer, although widely assumed, had been lacking.
“Our results extend [the] knowledge base by showing that quadrivalent HPV vaccination is also associated with a substantially reduced risk of invasive cervical cancer, which is the ultimate intent of HPV vaccination programs,” said investigators led by Jiayao Lei, PhD, a researcher in the department of medical epidemiology and biostatistics at the Karolinska Institute, Stockholm.
The study was published online Oct. 1 in the New England Journal of Medicine.
“This work provides evidence of actual cancer prevention,” commented Diane Harper, MD, an HPV expert and professor in the departments of family medicine and obstetrics & gynecology at the University of Michigan, Ann Arbor. She was the principal investigator on the original Gardasil trial.
This study “shows that the quadrivalent HPV vaccine provides prevention from the sexually transmitted HPV infection that actually reduces the incidence of cervical cancer in young women up to 30 years of age,” she said when approached for comment.
However, she also added a note of caution. These new results show “that vaccinated women still develop cervical cancer, but at a slower rate. This makes the connection between early-age vaccination and continued adult life screening incredibly important,” Dr. Harper said in an interview
Cervical cancer was diagnosed in 19 of the 527,871 women (0.004%) who had received at least one dose of the vaccine versus 538 among the 1,145,112 women (0.05%) who had not.
The cumulative incidence was 47 cases per 100,000 vaccinated women and 94 cases per 100,000 unvaccinated women. The cervical cancer incidence rate ratio for the comparison of vaccinated versus unvaccinated women was 0.37 (95% confidence interval, 0.21-0.57).
The risk reduction was even greater among women who had been vaccinated before the age of 17, with a cumulative incidence of 4 versus 54 cases per 100,000 for women vaccinated after age 17. The incidence rate ratio was 0.12 (95% CI, 0.00-0.34) for women who had been vaccinated before age 17 versus 0.47 (95% CI, 0.27-0.75) among those vaccinated from age 17 to 30 years.
Overall, “the risk of cervical cancer among participants who had initiated vaccination before the age of 17 years was 88% lower than among those who had never been vaccinated,” the investigators noted.
These results “support the recommendation to administer quadrivalent HPV vaccine before exposure to HPV infection to achieve the most substantial benefit,” the investigators wrote.
Details of the Swedish review
For their review, Dr. Lei and colleagues used several Swedish demographic and health registries to connect vaccination status to incident cervical cancers, using the personal identification numbers Sweden issues to residents.
Participants were followed starting either on their 10th birthday or on Jan. 1, 2006, whichever came later. They were followed until, among other things, diagnosis of invasive cervical cancer; their 31st birthday; or until Dec. 31, 2017, whichever came first.
The quadrivalent HPV vaccine, approved in Sweden in 2006, was used almost exclusively during the study period. Participants were considered vaccinated if they had received only one shot, but the investigators set out to analyze a relationship between the incidence of invasive cervical cancer and the number of shots given.
Among other things, the team controlled for age at follow-up, calendar year, county of residence, maternal disease history, and parental characteristics, including education and household income.
The investigators commented that it’s possible that HPV-vaccinated women could have been generally healthier than unvaccinated women and so would have been at lower risk for cervical cancer.
“Confounding by lifestyle and health factors in the women (such as smoking status, sexual activity, oral contraceptive use, and obesity) cannot be excluded; these factors are known to be associated with a risk of cervical cancer,” the investigators wrote.
HPV is also associated with other types of cancer, including anal and oropharyngeal cancers. But these cancers develop over a longer period than cervical cancer.
Dr. Harper noted that the “probability of HPV 16 cancer by time since infection peaks at 40 years after infection for anal cancers and nearly 50 years after infection for oropharyngeal cancers. This means that registries, such as in Sweden, for the next 40 years will record the evidence to say whether HPV vaccination lasts long enough to prevent [these] other HPV 16–associated cancers occurring at a much later time in life.”
The work was funded by the Swedish Foundation for Strategic Research, the Swedish Cancer Society, and the Swedish Research Council and by the China Scholarship Council. Dr. Lei and two other investigators reported HPV vaccine research funding from Merck, the maker of Gardasil. Harper disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Breast cancer screening complexities
Breast cancer in women remains one of the most common types of cancer in the United States, affecting about one in eight women1 over the course of their lifetime. Despite its pervasiveness, the 5-year survival rate for women with breast cancer remains high, estimated at around 90%2 based on data from 2010-2016, in large part because of early detection and treatment through screening. However, many organizations disagree on when to start and how often to screen women at average risk.
Important to discussions about breast cancer screening is the trend that many women delay childbirth until their 30s and 40s. In 2018 the birth rate increased for women ages 35-44, and the mean age of first birth increased from the prior year across all racial and ethnic groups.3 Therefore, ob.gyns. may need to consider that their patients not only may have increased risk of developing breast cancer based on age alone – women aged 35-44 have four times greater risk of disease than women aged 20-342 – but that the pregnancy itself may further exacerbate risk in older women. A 2019 pooled analysis found that women who were older at first birth had a greater chance of developing breast cancer compared with women with no children.4
In addition, ob.gyns. should consider that their patients may have received a breast cancer diagnosis prior to initiation or completion of their family plans or that their patients are cancer survivors – in 2013-2017, breast cancer was the most common form of cancer in adolescents and young adults.5 Thus, practitioners should be prepared to discuss not only options for fertility preservation but the evidence regarding cancer recurrence after pregnancy.
We have invited Dr. Katherine Tkaczuk, professor of medicine at the University of Maryland School of Medicine* and director of the breast evaluation and treatment program at the Marlene and Stewart Greenebaum Comprehensive Cancer Center, to discuss the vital role of screening in the shared decision-making process of breast cancer prevention.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore,* as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
Correction, 1/8/21: *An earlier version of this article misstated the university affiliations for Dr. Tkaczuk and Dr. Reece.
References
1. U.S. Breast Cancer Statistics. breastcancer.org.
2. “Cancer Stat Facts: Female Breast Cancer,” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
3. Martin JA et al. “Births: Final Data for 2018.” National Vital Statistics Reports. 2019 Nov 27;68(13):1-46.
4. Nichols HB et al. Ann Intern Med. 2019 Jan;170(1):22-30.
5. “Cancer Stat Facts: Cancer Among Adolescents and Young Adults (AYAs) (Ages 15-39),” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
Breast cancer in women remains one of the most common types of cancer in the United States, affecting about one in eight women1 over the course of their lifetime. Despite its pervasiveness, the 5-year survival rate for women with breast cancer remains high, estimated at around 90%2 based on data from 2010-2016, in large part because of early detection and treatment through screening. However, many organizations disagree on when to start and how often to screen women at average risk.
Important to discussions about breast cancer screening is the trend that many women delay childbirth until their 30s and 40s. In 2018 the birth rate increased for women ages 35-44, and the mean age of first birth increased from the prior year across all racial and ethnic groups.3 Therefore, ob.gyns. may need to consider that their patients not only may have increased risk of developing breast cancer based on age alone – women aged 35-44 have four times greater risk of disease than women aged 20-342 – but that the pregnancy itself may further exacerbate risk in older women. A 2019 pooled analysis found that women who were older at first birth had a greater chance of developing breast cancer compared with women with no children.4
In addition, ob.gyns. should consider that their patients may have received a breast cancer diagnosis prior to initiation or completion of their family plans or that their patients are cancer survivors – in 2013-2017, breast cancer was the most common form of cancer in adolescents and young adults.5 Thus, practitioners should be prepared to discuss not only options for fertility preservation but the evidence regarding cancer recurrence after pregnancy.
We have invited Dr. Katherine Tkaczuk, professor of medicine at the University of Maryland School of Medicine* and director of the breast evaluation and treatment program at the Marlene and Stewart Greenebaum Comprehensive Cancer Center, to discuss the vital role of screening in the shared decision-making process of breast cancer prevention.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore,* as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
Correction, 1/8/21: *An earlier version of this article misstated the university affiliations for Dr. Tkaczuk and Dr. Reece.
References
1. U.S. Breast Cancer Statistics. breastcancer.org.
2. “Cancer Stat Facts: Female Breast Cancer,” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
3. Martin JA et al. “Births: Final Data for 2018.” National Vital Statistics Reports. 2019 Nov 27;68(13):1-46.
4. Nichols HB et al. Ann Intern Med. 2019 Jan;170(1):22-30.
5. “Cancer Stat Facts: Cancer Among Adolescents and Young Adults (AYAs) (Ages 15-39),” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
Breast cancer in women remains one of the most common types of cancer in the United States, affecting about one in eight women1 over the course of their lifetime. Despite its pervasiveness, the 5-year survival rate for women with breast cancer remains high, estimated at around 90%2 based on data from 2010-2016, in large part because of early detection and treatment through screening. However, many organizations disagree on when to start and how often to screen women at average risk.
Important to discussions about breast cancer screening is the trend that many women delay childbirth until their 30s and 40s. In 2018 the birth rate increased for women ages 35-44, and the mean age of first birth increased from the prior year across all racial and ethnic groups.3 Therefore, ob.gyns. may need to consider that their patients not only may have increased risk of developing breast cancer based on age alone – women aged 35-44 have four times greater risk of disease than women aged 20-342 – but that the pregnancy itself may further exacerbate risk in older women. A 2019 pooled analysis found that women who were older at first birth had a greater chance of developing breast cancer compared with women with no children.4
In addition, ob.gyns. should consider that their patients may have received a breast cancer diagnosis prior to initiation or completion of their family plans or that their patients are cancer survivors – in 2013-2017, breast cancer was the most common form of cancer in adolescents and young adults.5 Thus, practitioners should be prepared to discuss not only options for fertility preservation but the evidence regarding cancer recurrence after pregnancy.
We have invited Dr. Katherine Tkaczuk, professor of medicine at the University of Maryland School of Medicine* and director of the breast evaluation and treatment program at the Marlene and Stewart Greenebaum Comprehensive Cancer Center, to discuss the vital role of screening in the shared decision-making process of breast cancer prevention.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore,* as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
Correction, 1/8/21: *An earlier version of this article misstated the university affiliations for Dr. Tkaczuk and Dr. Reece.
References
1. U.S. Breast Cancer Statistics. breastcancer.org.
2. “Cancer Stat Facts: Female Breast Cancer,” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
3. Martin JA et al. “Births: Final Data for 2018.” National Vital Statistics Reports. 2019 Nov 27;68(13):1-46.
4. Nichols HB et al. Ann Intern Med. 2019 Jan;170(1):22-30.
5. “Cancer Stat Facts: Cancer Among Adolescents and Young Adults (AYAs) (Ages 15-39),” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
An oncologist’s view on screening mammography
Screening mammography has contributed to the lowering of mortality from breast cancer by facilitating earlier diagnosis and a lower stage at diagnosis. With more effective treatment options for women who are diagnosed with lower-stage breast cancer, the current 5-year survival rate has risen to 90% – significantly higher than the 5-year survival rate of 75% in 1975.1
Women who are at much higher risk for developing breast cancer – mainly because of family history, certain genetic mutations, or a history of radiation therapy to the chest – will benefit the most from earlier and more frequent screening mammography as well as enhanced screening with non-x-ray methods of breast imaging. It is important that ob.gyns. help to identify these women.
However, the majority of women who are screened with mammography are at “average risk,” with a lifetime risk for developing breast cancer of 12.9%, based on 2015-2017 data from the National Cancer Institute’s (NCI) Surveillance, Epidemiology, and End Results Program (SEER).1 The median age at diagnosis of breast cancer in the U.S. is 62 years,1 and advancing age is the most important risk factor for these women.
A 20% relative risk reduction in breast cancer mortality with screening mammography has been demonstrated both in systematic reviews of randomized and observational studies2 and in a meta-analysis of 11 randomized trials comparing screening and no screening.3 Even though the majority of randomized trials were done in the age of film mammography, experts believe that we still see at least a 20% reduction today.
Among average-risk women, those aged 50-74 with a life expectancy of at least 10 years will benefit the most from regular screening. According to the 2016 screening guideline of the United States Preventive Services Task Force (USPSTF), relative risk reductions in breast cancer mortality from mammography screening, by age group, are 0.88 (confidence interval, 0.73-1.003) for ages 39-49; 0.86 (CI, 0.68-0.97) for ages 50-59; 0.67 (CI, 0.55-0.91) for ages 60-69; and 0.80 (CI, 0.51 to 1.28) for ages 70-74.2
For women aged 40-49 years, most of the guidelines in the United States recommend individualized screening every 1 or 2 years – screening that is guided by shared decision-making that takes into account each woman’s values regarding relative harms and benefits. This is because their risk of developing breast cancer is relatively low while the risk of false-positive results can be higher.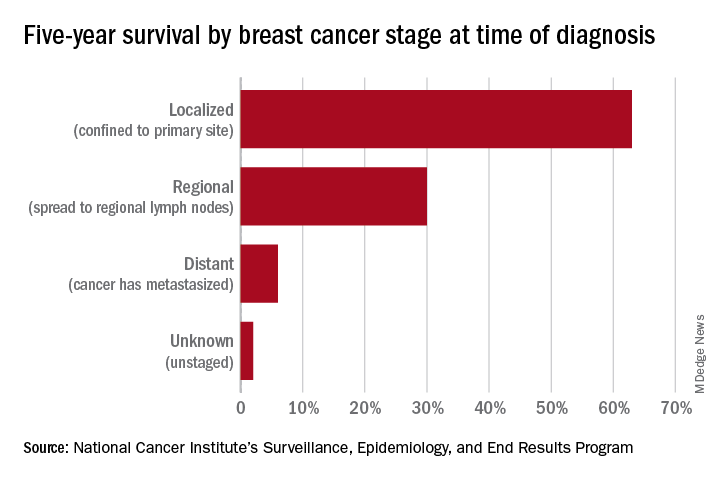
A few exceptions include guidelines by the National Comprehensive Cancer Network (NCCN) and the American College of Radiology, which recommend annual screening mammography starting at age 40 years for all average-risk women. In our program, we adhere to these latter recommendations and advise annual digital 3-D mammograms starting at age 40 and continuing until age 74, or longer if the woman is otherwise healthy with a life expectancy greater than 10 years.
Screening and overdiagnosis
Overdiagnosis – the diagnosis of cancers that may not actually cause mortality or may not even have become apparent without screening – is a concern for all women undergoing routine screening for breast cancer. There is significant uncertainty about its frequency, however.
Research cited by the USPSTF suggests that as many as one in five women diagnosed with breast cancer over approximately 10 years will be overdiagnosed. Other modeling studies have estimated one in eight overdiagnoses, for women aged 50-75 years specifically. By the more conservative estimate, according to the USPSTF, one breast cancer death will be prevented for every 2-3 cases of unnecessary treatment.2
Ductal carcinoma in situ is confined to the mammary ductal-lobular system and lacks the classic characteristics of cancer. Technically, it should not metastasize. But we do not know with certainty which cases of DCIS will or will not progress to invasive cancer. Therefore these women often are offered surgical approaches mirroring invasive cancer treatments (lumpectomy with radiation or even mastectomy in some cases), while for some, such treatments may be unnecessary.
Screening younger women (40-49)
Shared decision-making is always important for breast cancer screening, but in our program we routinely recommend annual screening in average-risk women starting at age 40 for several reasons. For one, younger women may present with more aggressive types of breast cancer such as triple-negative breast cancer. These are much less common than hormone-receptor positive breast cancers – they represent 15%-20% of all breast cancers – but they are faster growing and may develop in the interim if women are screened less often (at 2-year intervals).
In addition, finding an invasive breast cancer early is almost always beneficial. Earlier diagnosis (lower stage at diagnosis) is associated with increased breast cancer-specific and overall survival, as well as less-aggressive treatment approaches.
As a medical oncologist who treats women with breast cancer, I see these benefits firsthand. With earlier diagnosis, we are more likely to offer less aggressive surgical approaches such as partial mastectomy (lumpectomy) and sentinel lymph node biopsy as opposed to total mastectomy with axillary lymph node dissection, the latter of which is more likely to be associated with lymphedema and which can lead to postmastectomy chest wall pain syndromes.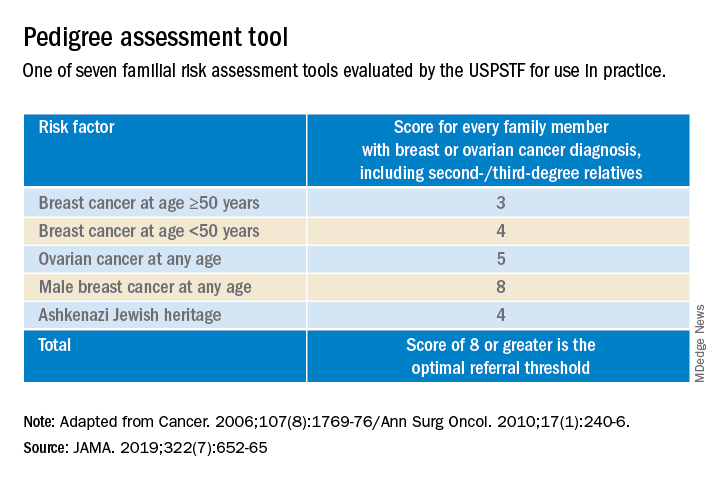
We also are able to use less aggressive radiation therapy approaches such as partial breast radiation, and less aggressive breast cancer–specific systemic treatments for women with a lower stage of breast cancer at diagnosis. In some cases, adjuvant or neoadjuvant chemotherapy may not be needed – and when it is necessary, shorter courses of chemotherapy or targeted chemotherapeutic regimens may be offered. This means lower systemic toxicities, both early and late, such as less cytopenias, risk of infections, mucositis, hair loss, cardiotoxicity, secondary malignancies/leukemia, and peripheral sensory neuropathy.
It is important to note that Black women in the United States have the highest death rate from breast cancer – 27.3 per 100,000 per year, versus 19.6 per 100,000 per year for White women1 – and that younger Black women appear to have a higher risk of developing triple-negative breast cancer, a more aggressive type of breast cancer. The higher breast cancer mortality in Black women is likely multifactorial and may be attributed partly to disparities in health care and partly to tumor biology. The case for annual screening in this population thus seems especially strong.
Screening modalities
Digital 3-D mammography, or digital breast tomosynthesis (DBT), is widely considered to be a more sensitive screening tool than conventional digital mammography alone. The NCCN recommends DBT for women with an average risk of developing breast cancer starting at age 40,4,5 and the USPSTF, while offering no recommendation on DBT as a primary screening method (“insufficient evidence”), says that DBT appears to increase cancer detection rates.2 So, I do routinely recommend it.
DBT may be especially beneficial for women with dense breast tissue (determined mammographically), who are most often premenopausal women – particularly non-Hispanic White women. Dense breast tissue itself can contribute to an increased risk of breast cancer – an approximately 20% higher relative risk in an average-risk woman with heterogeneously dense breast tissue, and an approximately 100% higher relative risk in a woman with extremely dense breasts6 – but unfortunately it affects the sensitivity and specificity of screening mammography.
I do not recommend routine supplemental screening with other methods (breast ultrasonography or MRI) for women at average risk of breast cancer who have dense breasts. MRI with gadolinium contrast is recommended as an adjunct to mammography for women who have a lifetime risk of developing breast cancer of more than 20%-25% (e.g., women with known BRCA1/2 mutations or radiation to breast tissue), and can be done annually at the same time as the screening mammogram is done. Some clinicians and patients prefer to alternate these two tests – one every 6 months.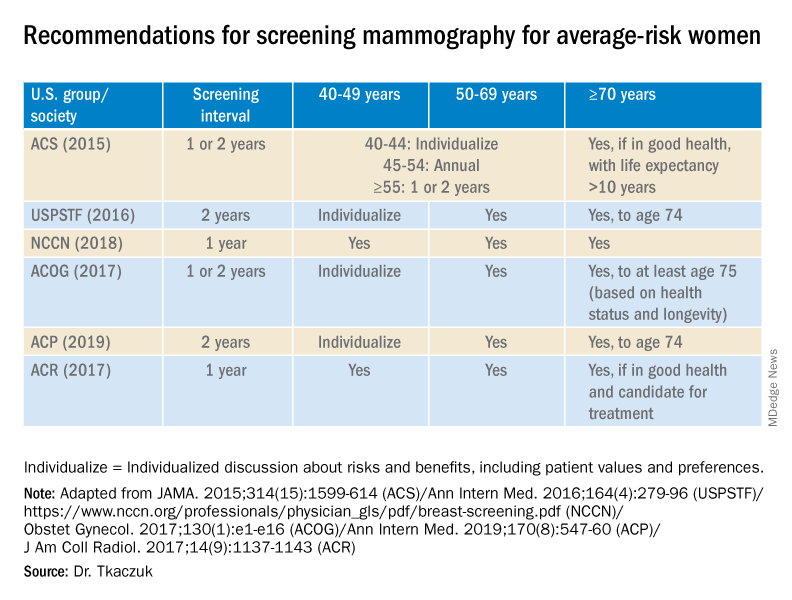
Screening breast MRI is more sensitive but less specific than mammography; combining the two screening modalities leads to overall increased sensitivity and specificity in high-risk populations.
Risk assessment
Identifying higher-risk women who need to be sent to a genetic counselor is critically important. The USPSTF recommends that women who have family members with breast, ovarian, tubal or peritoneal cancer, or who have an ancestry associated with BRCA1/2 gene mutations, be assessed with a brief familial risk assessment tool such as the Pedigree Assessment Tool. This and other validated tools have been evaluated by the USPSTF and can be used to guide referrals to genetic counseling for more definitive risk assessment.7
These tools are different from general breast cancer risk assessment models, such as the NCI’s Breast Cancer Risk Assessment Tool,8 which are designed to calculate the 5-year and lifetime risk of developing invasive breast cancer for an average-risk woman but not to identify BRCA-related cancer risk. (The NCI’s tool is based on the Gail model, which has been widely used over the years.)
The general risk assessment models use a women’s personal medical and reproductive history as well as the history of breast cancer among her first-degree relatives to estimate her risk.
Dr. Tkaczuk reported that she has no disclosures.
References
1. “Cancer Stat Facts: Female Breast Cancer.” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
2. Siu AL et al. Ann Intern Med. 2016 Feb 16. doi: 10.7326/M15-2886.
3. Independent UK Panel on Breast Cancer Screening. Lancet. 2012 Nov 17;380(9855):1778-86.
4. NCCN guidelines for Detection, Prevention, & Risk Reduction: Breast Cancer Screening and Diagnosis. National Comprehensive Cancer Network.
5. NCCN guidelines for Detection, Prevention, & Risk Reduction: Breast Cancer Risk Reduction. National Comprehensive Cancer Network.
6. Ziv E et al. Cancer Epidemiol Biomarkers Prev. 2004;13(12):2090-5.
7. USPSTF. JAMA. 2019;322(7):652-65.
8. The Breast Cancer Risk Assessment Tool. National Cancer Institute.
Screening mammography has contributed to the lowering of mortality from breast cancer by facilitating earlier diagnosis and a lower stage at diagnosis. With more effective treatment options for women who are diagnosed with lower-stage breast cancer, the current 5-year survival rate has risen to 90% – significantly higher than the 5-year survival rate of 75% in 1975.1
Women who are at much higher risk for developing breast cancer – mainly because of family history, certain genetic mutations, or a history of radiation therapy to the chest – will benefit the most from earlier and more frequent screening mammography as well as enhanced screening with non-x-ray methods of breast imaging. It is important that ob.gyns. help to identify these women.
However, the majority of women who are screened with mammography are at “average risk,” with a lifetime risk for developing breast cancer of 12.9%, based on 2015-2017 data from the National Cancer Institute’s (NCI) Surveillance, Epidemiology, and End Results Program (SEER).1 The median age at diagnosis of breast cancer in the U.S. is 62 years,1 and advancing age is the most important risk factor for these women.
A 20% relative risk reduction in breast cancer mortality with screening mammography has been demonstrated both in systematic reviews of randomized and observational studies2 and in a meta-analysis of 11 randomized trials comparing screening and no screening.3 Even though the majority of randomized trials were done in the age of film mammography, experts believe that we still see at least a 20% reduction today.
Among average-risk women, those aged 50-74 with a life expectancy of at least 10 years will benefit the most from regular screening. According to the 2016 screening guideline of the United States Preventive Services Task Force (USPSTF), relative risk reductions in breast cancer mortality from mammography screening, by age group, are 0.88 (confidence interval, 0.73-1.003) for ages 39-49; 0.86 (CI, 0.68-0.97) for ages 50-59; 0.67 (CI, 0.55-0.91) for ages 60-69; and 0.80 (CI, 0.51 to 1.28) for ages 70-74.2
For women aged 40-49 years, most of the guidelines in the United States recommend individualized screening every 1 or 2 years – screening that is guided by shared decision-making that takes into account each woman’s values regarding relative harms and benefits. This is because their risk of developing breast cancer is relatively low while the risk of false-positive results can be higher.
A few exceptions include guidelines by the National Comprehensive Cancer Network (NCCN) and the American College of Radiology, which recommend annual screening mammography starting at age 40 years for all average-risk women. In our program, we adhere to these latter recommendations and advise annual digital 3-D mammograms starting at age 40 and continuing until age 74, or longer if the woman is otherwise healthy with a life expectancy greater than 10 years.
Screening and overdiagnosis
Overdiagnosis – the diagnosis of cancers that may not actually cause mortality or may not even have become apparent without screening – is a concern for all women undergoing routine screening for breast cancer. There is significant uncertainty about its frequency, however.
Research cited by the USPSTF suggests that as many as one in five women diagnosed with breast cancer over approximately 10 years will be overdiagnosed. Other modeling studies have estimated one in eight overdiagnoses, for women aged 50-75 years specifically. By the more conservative estimate, according to the USPSTF, one breast cancer death will be prevented for every 2-3 cases of unnecessary treatment.2
Ductal carcinoma in situ is confined to the mammary ductal-lobular system and lacks the classic characteristics of cancer. Technically, it should not metastasize. But we do not know with certainty which cases of DCIS will or will not progress to invasive cancer. Therefore these women often are offered surgical approaches mirroring invasive cancer treatments (lumpectomy with radiation or even mastectomy in some cases), while for some, such treatments may be unnecessary.
Screening younger women (40-49)
Shared decision-making is always important for breast cancer screening, but in our program we routinely recommend annual screening in average-risk women starting at age 40 for several reasons. For one, younger women may present with more aggressive types of breast cancer such as triple-negative breast cancer. These are much less common than hormone-receptor positive breast cancers – they represent 15%-20% of all breast cancers – but they are faster growing and may develop in the interim if women are screened less often (at 2-year intervals).
In addition, finding an invasive breast cancer early is almost always beneficial. Earlier diagnosis (lower stage at diagnosis) is associated with increased breast cancer-specific and overall survival, as well as less-aggressive treatment approaches.
As a medical oncologist who treats women with breast cancer, I see these benefits firsthand. With earlier diagnosis, we are more likely to offer less aggressive surgical approaches such as partial mastectomy (lumpectomy) and sentinel lymph node biopsy as opposed to total mastectomy with axillary lymph node dissection, the latter of which is more likely to be associated with lymphedema and which can lead to postmastectomy chest wall pain syndromes.
We also are able to use less aggressive radiation therapy approaches such as partial breast radiation, and less aggressive breast cancer–specific systemic treatments for women with a lower stage of breast cancer at diagnosis. In some cases, adjuvant or neoadjuvant chemotherapy may not be needed – and when it is necessary, shorter courses of chemotherapy or targeted chemotherapeutic regimens may be offered. This means lower systemic toxicities, both early and late, such as less cytopenias, risk of infections, mucositis, hair loss, cardiotoxicity, secondary malignancies/leukemia, and peripheral sensory neuropathy.
It is important to note that Black women in the United States have the highest death rate from breast cancer – 27.3 per 100,000 per year, versus 19.6 per 100,000 per year for White women1 – and that younger Black women appear to have a higher risk of developing triple-negative breast cancer, a more aggressive type of breast cancer. The higher breast cancer mortality in Black women is likely multifactorial and may be attributed partly to disparities in health care and partly to tumor biology. The case for annual screening in this population thus seems especially strong.
Screening modalities
Digital 3-D mammography, or digital breast tomosynthesis (DBT), is widely considered to be a more sensitive screening tool than conventional digital mammography alone. The NCCN recommends DBT for women with an average risk of developing breast cancer starting at age 40,4,5 and the USPSTF, while offering no recommendation on DBT as a primary screening method (“insufficient evidence”), says that DBT appears to increase cancer detection rates.2 So, I do routinely recommend it.
DBT may be especially beneficial for women with dense breast tissue (determined mammographically), who are most often premenopausal women – particularly non-Hispanic White women. Dense breast tissue itself can contribute to an increased risk of breast cancer – an approximately 20% higher relative risk in an average-risk woman with heterogeneously dense breast tissue, and an approximately 100% higher relative risk in a woman with extremely dense breasts6 – but unfortunately it affects the sensitivity and specificity of screening mammography.
I do not recommend routine supplemental screening with other methods (breast ultrasonography or MRI) for women at average risk of breast cancer who have dense breasts. MRI with gadolinium contrast is recommended as an adjunct to mammography for women who have a lifetime risk of developing breast cancer of more than 20%-25% (e.g., women with known BRCA1/2 mutations or radiation to breast tissue), and can be done annually at the same time as the screening mammogram is done. Some clinicians and patients prefer to alternate these two tests – one every 6 months.
Screening breast MRI is more sensitive but less specific than mammography; combining the two screening modalities leads to overall increased sensitivity and specificity in high-risk populations.
Risk assessment
Identifying higher-risk women who need to be sent to a genetic counselor is critically important. The USPSTF recommends that women who have family members with breast, ovarian, tubal or peritoneal cancer, or who have an ancestry associated with BRCA1/2 gene mutations, be assessed with a brief familial risk assessment tool such as the Pedigree Assessment Tool. This and other validated tools have been evaluated by the USPSTF and can be used to guide referrals to genetic counseling for more definitive risk assessment.7
These tools are different from general breast cancer risk assessment models, such as the NCI’s Breast Cancer Risk Assessment Tool,8 which are designed to calculate the 5-year and lifetime risk of developing invasive breast cancer for an average-risk woman but not to identify BRCA-related cancer risk. (The NCI’s tool is based on the Gail model, which has been widely used over the years.)
The general risk assessment models use a women’s personal medical and reproductive history as well as the history of breast cancer among her first-degree relatives to estimate her risk.
Dr. Tkaczuk reported that she has no disclosures.
References
1. “Cancer Stat Facts: Female Breast Cancer.” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
2. Siu AL et al. Ann Intern Med. 2016 Feb 16. doi: 10.7326/M15-2886.
3. Independent UK Panel on Breast Cancer Screening. Lancet. 2012 Nov 17;380(9855):1778-86.
4. NCCN guidelines for Detection, Prevention, & Risk Reduction: Breast Cancer Screening and Diagnosis. National Comprehensive Cancer Network.
5. NCCN guidelines for Detection, Prevention, & Risk Reduction: Breast Cancer Risk Reduction. National Comprehensive Cancer Network.
6. Ziv E et al. Cancer Epidemiol Biomarkers Prev. 2004;13(12):2090-5.
7. USPSTF. JAMA. 2019;322(7):652-65.
8. The Breast Cancer Risk Assessment Tool. National Cancer Institute.
Screening mammography has contributed to the lowering of mortality from breast cancer by facilitating earlier diagnosis and a lower stage at diagnosis. With more effective treatment options for women who are diagnosed with lower-stage breast cancer, the current 5-year survival rate has risen to 90% – significantly higher than the 5-year survival rate of 75% in 1975.1
Women who are at much higher risk for developing breast cancer – mainly because of family history, certain genetic mutations, or a history of radiation therapy to the chest – will benefit the most from earlier and more frequent screening mammography as well as enhanced screening with non-x-ray methods of breast imaging. It is important that ob.gyns. help to identify these women.
However, the majority of women who are screened with mammography are at “average risk,” with a lifetime risk for developing breast cancer of 12.9%, based on 2015-2017 data from the National Cancer Institute’s (NCI) Surveillance, Epidemiology, and End Results Program (SEER).1 The median age at diagnosis of breast cancer in the U.S. is 62 years,1 and advancing age is the most important risk factor for these women.
A 20% relative risk reduction in breast cancer mortality with screening mammography has been demonstrated both in systematic reviews of randomized and observational studies2 and in a meta-analysis of 11 randomized trials comparing screening and no screening.3 Even though the majority of randomized trials were done in the age of film mammography, experts believe that we still see at least a 20% reduction today.
Among average-risk women, those aged 50-74 with a life expectancy of at least 10 years will benefit the most from regular screening. According to the 2016 screening guideline of the United States Preventive Services Task Force (USPSTF), relative risk reductions in breast cancer mortality from mammography screening, by age group, are 0.88 (confidence interval, 0.73-1.003) for ages 39-49; 0.86 (CI, 0.68-0.97) for ages 50-59; 0.67 (CI, 0.55-0.91) for ages 60-69; and 0.80 (CI, 0.51 to 1.28) for ages 70-74.2
For women aged 40-49 years, most of the guidelines in the United States recommend individualized screening every 1 or 2 years – screening that is guided by shared decision-making that takes into account each woman’s values regarding relative harms and benefits. This is because their risk of developing breast cancer is relatively low while the risk of false-positive results can be higher.
A few exceptions include guidelines by the National Comprehensive Cancer Network (NCCN) and the American College of Radiology, which recommend annual screening mammography starting at age 40 years for all average-risk women. In our program, we adhere to these latter recommendations and advise annual digital 3-D mammograms starting at age 40 and continuing until age 74, or longer if the woman is otherwise healthy with a life expectancy greater than 10 years.
Screening and overdiagnosis
Overdiagnosis – the diagnosis of cancers that may not actually cause mortality or may not even have become apparent without screening – is a concern for all women undergoing routine screening for breast cancer. There is significant uncertainty about its frequency, however.
Research cited by the USPSTF suggests that as many as one in five women diagnosed with breast cancer over approximately 10 years will be overdiagnosed. Other modeling studies have estimated one in eight overdiagnoses, for women aged 50-75 years specifically. By the more conservative estimate, according to the USPSTF, one breast cancer death will be prevented for every 2-3 cases of unnecessary treatment.2
Ductal carcinoma in situ is confined to the mammary ductal-lobular system and lacks the classic characteristics of cancer. Technically, it should not metastasize. But we do not know with certainty which cases of DCIS will or will not progress to invasive cancer. Therefore these women often are offered surgical approaches mirroring invasive cancer treatments (lumpectomy with radiation or even mastectomy in some cases), while for some, such treatments may be unnecessary.
Screening younger women (40-49)
Shared decision-making is always important for breast cancer screening, but in our program we routinely recommend annual screening in average-risk women starting at age 40 for several reasons. For one, younger women may present with more aggressive types of breast cancer such as triple-negative breast cancer. These are much less common than hormone-receptor positive breast cancers – they represent 15%-20% of all breast cancers – but they are faster growing and may develop in the interim if women are screened less often (at 2-year intervals).
In addition, finding an invasive breast cancer early is almost always beneficial. Earlier diagnosis (lower stage at diagnosis) is associated with increased breast cancer-specific and overall survival, as well as less-aggressive treatment approaches.
As a medical oncologist who treats women with breast cancer, I see these benefits firsthand. With earlier diagnosis, we are more likely to offer less aggressive surgical approaches such as partial mastectomy (lumpectomy) and sentinel lymph node biopsy as opposed to total mastectomy with axillary lymph node dissection, the latter of which is more likely to be associated with lymphedema and which can lead to postmastectomy chest wall pain syndromes.
We also are able to use less aggressive radiation therapy approaches such as partial breast radiation, and less aggressive breast cancer–specific systemic treatments for women with a lower stage of breast cancer at diagnosis. In some cases, adjuvant or neoadjuvant chemotherapy may not be needed – and when it is necessary, shorter courses of chemotherapy or targeted chemotherapeutic regimens may be offered. This means lower systemic toxicities, both early and late, such as less cytopenias, risk of infections, mucositis, hair loss, cardiotoxicity, secondary malignancies/leukemia, and peripheral sensory neuropathy.
It is important to note that Black women in the United States have the highest death rate from breast cancer – 27.3 per 100,000 per year, versus 19.6 per 100,000 per year for White women1 – and that younger Black women appear to have a higher risk of developing triple-negative breast cancer, a more aggressive type of breast cancer. The higher breast cancer mortality in Black women is likely multifactorial and may be attributed partly to disparities in health care and partly to tumor biology. The case for annual screening in this population thus seems especially strong.
Screening modalities
Digital 3-D mammography, or digital breast tomosynthesis (DBT), is widely considered to be a more sensitive screening tool than conventional digital mammography alone. The NCCN recommends DBT for women with an average risk of developing breast cancer starting at age 40,4,5 and the USPSTF, while offering no recommendation on DBT as a primary screening method (“insufficient evidence”), says that DBT appears to increase cancer detection rates.2 So, I do routinely recommend it.
DBT may be especially beneficial for women with dense breast tissue (determined mammographically), who are most often premenopausal women – particularly non-Hispanic White women. Dense breast tissue itself can contribute to an increased risk of breast cancer – an approximately 20% higher relative risk in an average-risk woman with heterogeneously dense breast tissue, and an approximately 100% higher relative risk in a woman with extremely dense breasts6 – but unfortunately it affects the sensitivity and specificity of screening mammography.
I do not recommend routine supplemental screening with other methods (breast ultrasonography or MRI) for women at average risk of breast cancer who have dense breasts. MRI with gadolinium contrast is recommended as an adjunct to mammography for women who have a lifetime risk of developing breast cancer of more than 20%-25% (e.g., women with known BRCA1/2 mutations or radiation to breast tissue), and can be done annually at the same time as the screening mammogram is done. Some clinicians and patients prefer to alternate these two tests – one every 6 months.
Screening breast MRI is more sensitive but less specific than mammography; combining the two screening modalities leads to overall increased sensitivity and specificity in high-risk populations.
Risk assessment
Identifying higher-risk women who need to be sent to a genetic counselor is critically important. The USPSTF recommends that women who have family members with breast, ovarian, tubal or peritoneal cancer, or who have an ancestry associated with BRCA1/2 gene mutations, be assessed with a brief familial risk assessment tool such as the Pedigree Assessment Tool. This and other validated tools have been evaluated by the USPSTF and can be used to guide referrals to genetic counseling for more definitive risk assessment.7
These tools are different from general breast cancer risk assessment models, such as the NCI’s Breast Cancer Risk Assessment Tool,8 which are designed to calculate the 5-year and lifetime risk of developing invasive breast cancer for an average-risk woman but not to identify BRCA-related cancer risk. (The NCI’s tool is based on the Gail model, which has been widely used over the years.)
The general risk assessment models use a women’s personal medical and reproductive history as well as the history of breast cancer among her first-degree relatives to estimate her risk.
Dr. Tkaczuk reported that she has no disclosures.
References
1. “Cancer Stat Facts: Female Breast Cancer.” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
2. Siu AL et al. Ann Intern Med. 2016 Feb 16. doi: 10.7326/M15-2886.
3. Independent UK Panel on Breast Cancer Screening. Lancet. 2012 Nov 17;380(9855):1778-86.
4. NCCN guidelines for Detection, Prevention, & Risk Reduction: Breast Cancer Screening and Diagnosis. National Comprehensive Cancer Network.
5. NCCN guidelines for Detection, Prevention, & Risk Reduction: Breast Cancer Risk Reduction. National Comprehensive Cancer Network.
6. Ziv E et al. Cancer Epidemiol Biomarkers Prev. 2004;13(12):2090-5.
7. USPSTF. JAMA. 2019;322(7):652-65.
8. The Breast Cancer Risk Assessment Tool. National Cancer Institute.
Pregnancy studies on psoriasis, PsA medications pick up
Christina Chambers, PhD, MPH, who runs the MotherToBaby Pregnancy Studies research center at the University of California, San Diego, has found most pregnant women to be “entirely altruistic” about sharing their experiences with drug treatment during pregnancy.
– but dermatologists, rheumatologists, and their female patients with psoriasis and psoriatic arthritis (PsA) want much more.
And women’s participation in the MotherToBaby studies conducted by the nonprofit Organization of Teratology Information Specialists (OTIS) is key, say physicians who are treating women of reproductive age. OTIS is now listed in drug labeling as the “pregnancy registry” contact for many of the medications they may be discussing with patients.
Dr. Chambers said that most women appreciate “that participating in a study may not help her with her pregnancy, but it can help her sister or her friend or someone else who has these same questions in planning a pregnancy of ‘Can I stay on my treatment?’ or, in the case of an unplanned pregnancy, ‘Should I be concerned?’ ”
OTIS has enrolled women with psoriasis and/or PsA in studies of nine medications, most of them biologics (both TNF-alpha blockers and newer anti-interleukin agents).
Four of the studies – those evaluating etanercept (Enbrel), adalimumab (Humira), abatacept (Orencia), and ustekinumab (Stelara) – are now closed to enrollment with analyses either underway or completed. The other five are currently enrolling patients and involve treatment with certolizumab pegol (Cimzia), tildrakizumab (Ilumya), apremilast (Otezla), guselkumab (Tremfya), and tofacitinib (Xeljanz).
Lisa R. Sammaritano, MD, a rheumatologist at the Hospital for Special Surgery, New York, who led the development of the American College of Rheumatology’s first guideline for the management of reproductive health in rheumatic and musculoskeletal diseases, recommends to some of her patients that they contact OTIS. “Their pregnancy registry studies have added important information to the field over the years,” she said.
Most recently, a study of the anti–TNF-alpha medication adalimumab that began in 2004 in pregnant patients with RA and Crohn’s disease culminated in a 2019 PLOS ONE paper reporting no associations between exposure to the medication and an increased risk of adverse outcomes. The outcomes studied were major structural birth defects, minor defects, spontaneous abortion, preterm delivery, prenatal and postnatal growth deficiency, serious or opportunistic infections, and malignancies.
An analysis is underway of adalimumab exposure in women with PsA – a patient subset that was added after the study started. But in the meantime, Dr. Chambers said, the 2019 research article is relevant to questions of drug safety across indications.
OTIS’s MothertoBaby studies are structured as prospective cohort studies. Dr. Chambers, a perinatal epidemiologist, is president of OTIS, which recruits women who have an exposure to the medication under study – at least one dose, for any length of time. And in most cases, it also recruits women with the underlying condition but no exposure and healthy women without the condition to represent the general population.
It’s the disease-matched comparison group that makes OTIS’s studies different from traditional pregnancy registries involving “a simple exposure series and outcomes that are described in the context of what you’d expect in the general population,” said Dr. Chambers, professor in the department of pediatrics, as well as family and preventative medicine, at UCSD and codirector of the Center for Better Beginnings at that university. “Many maternal conditions themselves [or their comorbidities] carry some risk of adverse outcomes in pregnancy.”
The OTIS studies typically involve at least 100 exposed pregnancies and a similar number of unexposed pregnancies; some have cohorts of 200-300.
The recently published study of adalimumab, for instance, included 257 women with exposure to the drug and 120 women in a disease comparison group with no exposure. In addition to finding no associations between drug exposure and adverse outcomes, the study found that women with RA or Crohn’s were at increased risk of preterm delivery, irrespective of adalimumab exposure.
“There’s insufficient [power with any of these numbers] to come to the conclusion that a drug is safe,” she said. “But what we have been able to say [through our studies] is that we’ve looked carefully at the whole array of outcomes ... and we don’t see anything unusual. That early view can be reassuring” until large population-based studies or claims analyses become possible.
Dr. Sammaritano, also with Weill Cornell Medicine, New York, said that she does not recommend registry participation for patients who stop biologics at the diagnosis of pregnancy. Since “the start of IgG antibody transfer during pregnancy is about 16 weeks,” she worries that including these patients might lead to falsely reassuring findings. “We are most interested in [knowing the outcomes of] patients who must continue the drugs through pregnancy,” she said.
Dr. Chambers, however, said that in her view, placental transfer is not a requirement for a medication to have some effect on the outcome of pregnancy. “The outcome could be influenced by an effect of the medication that doesn’t require placental transfer or require placental transfer in large amounts,” she said. “So it’s relevant to examine exposures that have occurred only in the first trimester, and this is especially true for the outcome of major birth defects, most of which are initiated in the first trimester.”
The MotherToBaby studies typically include both early, short exposures and longer exposures, she said. “And certainly, duration of use is a factor that we do consider in looking at specific outcomes such as growth, preterm delivery, and risk of serious or opportunistic infections.”
(In the published study of adalimumab, 65.3% of women in the medication-exposed cohort used the medication in all three trimesters, 10.5% in the first and second trimesters, and 22.4% in the first trimester only.)
Women participating in the MotherToBaby studies complete two to four interviews during pregnancy and may be interviewed again after delivery. They are asked for their permission to share a copy of their medical records – and their baby’s medical records – and their babies receive a follow-up pediatric exam by a pediatrician with expertise in dysmorphology/genetics (who is blinded to exposure status), most commonly in the participant’s home. Providers are not asked to enter any data.
Eliza Chakravarty, MD, a rheumatologist with the Oklahoma Medical Research Foundation in Oklahoma City who treats patients with PsA who are pregnant or considering pregnancy, said that her referrals for research participation “have been mostly to MothertoBaby.”
“Most drug companies [in the autoimmune space] are now contracting with them [for their pregnancy exposure research],” she said. “I really like that it’s become so centralized.”
She tells patients that many questions can be answered through research, that their experience matters, and that “there are benefits” to the extra pediatric examination. “I give them the information and let them decide whether or not they want to call [MotherToBaby],” she said. “I don’t want to impose. I want to make them aware.”
Dr. Chambers emphasizes to patients and physicians that the studies are strictly observational and do not require any changes in personal or medical regimens. “When people hear the word ‘research’ they think of clinical trials. We’re saying, you and your provider do everything you normally would do, just let us observe what happens during your pregnancy.”
Physicians should assure patients, moreover, that “just because the drug is being studied doesn’t mean there’s a known risk or even a suspected risk,” she said.
The MotherToBaby studies receive funding from the pharmaceutical companies, which are required by the Food and Drug Administration to conduct pregnancy exposure registries for medications used during pregnancy or in women of reproductive age. OTIS has an independent advisory board, however, and independently analyzes and publishes its findings. Progress reports are shared with the pharmaceutical companies, and in turn, the FDA, Dr. Chambers said.
To refer patients for MotherToBaby studies, physicians can use an online referral form found on the MothertoBaby web site, a service of OTIS, or call the pregnancy studies team at 877-311-8972 to provide them with the patient’s name or number. Patients may also be given the number and advised to consider calling. MotherToBaby offers medication fact sheets that answer questions about exposures during pregnancy and breastfeeding, and runs a free and confidential teratogen counseling service: 866-626-6847.
Christina Chambers, PhD, MPH, who runs the MotherToBaby Pregnancy Studies research center at the University of California, San Diego, has found most pregnant women to be “entirely altruistic” about sharing their experiences with drug treatment during pregnancy.
– but dermatologists, rheumatologists, and their female patients with psoriasis and psoriatic arthritis (PsA) want much more.
And women’s participation in the MotherToBaby studies conducted by the nonprofit Organization of Teratology Information Specialists (OTIS) is key, say physicians who are treating women of reproductive age. OTIS is now listed in drug labeling as the “pregnancy registry” contact for many of the medications they may be discussing with patients.
Dr. Chambers said that most women appreciate “that participating in a study may not help her with her pregnancy, but it can help her sister or her friend or someone else who has these same questions in planning a pregnancy of ‘Can I stay on my treatment?’ or, in the case of an unplanned pregnancy, ‘Should I be concerned?’ ”
OTIS has enrolled women with psoriasis and/or PsA in studies of nine medications, most of them biologics (both TNF-alpha blockers and newer anti-interleukin agents).
Four of the studies – those evaluating etanercept (Enbrel), adalimumab (Humira), abatacept (Orencia), and ustekinumab (Stelara) – are now closed to enrollment with analyses either underway or completed. The other five are currently enrolling patients and involve treatment with certolizumab pegol (Cimzia), tildrakizumab (Ilumya), apremilast (Otezla), guselkumab (Tremfya), and tofacitinib (Xeljanz).
Lisa R. Sammaritano, MD, a rheumatologist at the Hospital for Special Surgery, New York, who led the development of the American College of Rheumatology’s first guideline for the management of reproductive health in rheumatic and musculoskeletal diseases, recommends to some of her patients that they contact OTIS. “Their pregnancy registry studies have added important information to the field over the years,” she said.
Most recently, a study of the anti–TNF-alpha medication adalimumab that began in 2004 in pregnant patients with RA and Crohn’s disease culminated in a 2019 PLOS ONE paper reporting no associations between exposure to the medication and an increased risk of adverse outcomes. The outcomes studied were major structural birth defects, minor defects, spontaneous abortion, preterm delivery, prenatal and postnatal growth deficiency, serious or opportunistic infections, and malignancies.
An analysis is underway of adalimumab exposure in women with PsA – a patient subset that was added after the study started. But in the meantime, Dr. Chambers said, the 2019 research article is relevant to questions of drug safety across indications.
OTIS’s MothertoBaby studies are structured as prospective cohort studies. Dr. Chambers, a perinatal epidemiologist, is president of OTIS, which recruits women who have an exposure to the medication under study – at least one dose, for any length of time. And in most cases, it also recruits women with the underlying condition but no exposure and healthy women without the condition to represent the general population.
It’s the disease-matched comparison group that makes OTIS’s studies different from traditional pregnancy registries involving “a simple exposure series and outcomes that are described in the context of what you’d expect in the general population,” said Dr. Chambers, professor in the department of pediatrics, as well as family and preventative medicine, at UCSD and codirector of the Center for Better Beginnings at that university. “Many maternal conditions themselves [or their comorbidities] carry some risk of adverse outcomes in pregnancy.”
The OTIS studies typically involve at least 100 exposed pregnancies and a similar number of unexposed pregnancies; some have cohorts of 200-300.
The recently published study of adalimumab, for instance, included 257 women with exposure to the drug and 120 women in a disease comparison group with no exposure. In addition to finding no associations between drug exposure and adverse outcomes, the study found that women with RA or Crohn’s were at increased risk of preterm delivery, irrespective of adalimumab exposure.
“There’s insufficient [power with any of these numbers] to come to the conclusion that a drug is safe,” she said. “But what we have been able to say [through our studies] is that we’ve looked carefully at the whole array of outcomes ... and we don’t see anything unusual. That early view can be reassuring” until large population-based studies or claims analyses become possible.
Dr. Sammaritano, also with Weill Cornell Medicine, New York, said that she does not recommend registry participation for patients who stop biologics at the diagnosis of pregnancy. Since “the start of IgG antibody transfer during pregnancy is about 16 weeks,” she worries that including these patients might lead to falsely reassuring findings. “We are most interested in [knowing the outcomes of] patients who must continue the drugs through pregnancy,” she said.
Dr. Chambers, however, said that in her view, placental transfer is not a requirement for a medication to have some effect on the outcome of pregnancy. “The outcome could be influenced by an effect of the medication that doesn’t require placental transfer or require placental transfer in large amounts,” she said. “So it’s relevant to examine exposures that have occurred only in the first trimester, and this is especially true for the outcome of major birth defects, most of which are initiated in the first trimester.”
The MotherToBaby studies typically include both early, short exposures and longer exposures, she said. “And certainly, duration of use is a factor that we do consider in looking at specific outcomes such as growth, preterm delivery, and risk of serious or opportunistic infections.”
(In the published study of adalimumab, 65.3% of women in the medication-exposed cohort used the medication in all three trimesters, 10.5% in the first and second trimesters, and 22.4% in the first trimester only.)
Women participating in the MotherToBaby studies complete two to four interviews during pregnancy and may be interviewed again after delivery. They are asked for their permission to share a copy of their medical records – and their baby’s medical records – and their babies receive a follow-up pediatric exam by a pediatrician with expertise in dysmorphology/genetics (who is blinded to exposure status), most commonly in the participant’s home. Providers are not asked to enter any data.
Eliza Chakravarty, MD, a rheumatologist with the Oklahoma Medical Research Foundation in Oklahoma City who treats patients with PsA who are pregnant or considering pregnancy, said that her referrals for research participation “have been mostly to MothertoBaby.”
“Most drug companies [in the autoimmune space] are now contracting with them [for their pregnancy exposure research],” she said. “I really like that it’s become so centralized.”
She tells patients that many questions can be answered through research, that their experience matters, and that “there are benefits” to the extra pediatric examination. “I give them the information and let them decide whether or not they want to call [MotherToBaby],” she said. “I don’t want to impose. I want to make them aware.”
Dr. Chambers emphasizes to patients and physicians that the studies are strictly observational and do not require any changes in personal or medical regimens. “When people hear the word ‘research’ they think of clinical trials. We’re saying, you and your provider do everything you normally would do, just let us observe what happens during your pregnancy.”
Physicians should assure patients, moreover, that “just because the drug is being studied doesn’t mean there’s a known risk or even a suspected risk,” she said.
The MotherToBaby studies receive funding from the pharmaceutical companies, which are required by the Food and Drug Administration to conduct pregnancy exposure registries for medications used during pregnancy or in women of reproductive age. OTIS has an independent advisory board, however, and independently analyzes and publishes its findings. Progress reports are shared with the pharmaceutical companies, and in turn, the FDA, Dr. Chambers said.
To refer patients for MotherToBaby studies, physicians can use an online referral form found on the MothertoBaby web site, a service of OTIS, or call the pregnancy studies team at 877-311-8972 to provide them with the patient’s name or number. Patients may also be given the number and advised to consider calling. MotherToBaby offers medication fact sheets that answer questions about exposures during pregnancy and breastfeeding, and runs a free and confidential teratogen counseling service: 866-626-6847.
Christina Chambers, PhD, MPH, who runs the MotherToBaby Pregnancy Studies research center at the University of California, San Diego, has found most pregnant women to be “entirely altruistic” about sharing their experiences with drug treatment during pregnancy.
– but dermatologists, rheumatologists, and their female patients with psoriasis and psoriatic arthritis (PsA) want much more.
And women’s participation in the MotherToBaby studies conducted by the nonprofit Organization of Teratology Information Specialists (OTIS) is key, say physicians who are treating women of reproductive age. OTIS is now listed in drug labeling as the “pregnancy registry” contact for many of the medications they may be discussing with patients.
Dr. Chambers said that most women appreciate “that participating in a study may not help her with her pregnancy, but it can help her sister or her friend or someone else who has these same questions in planning a pregnancy of ‘Can I stay on my treatment?’ or, in the case of an unplanned pregnancy, ‘Should I be concerned?’ ”
OTIS has enrolled women with psoriasis and/or PsA in studies of nine medications, most of them biologics (both TNF-alpha blockers and newer anti-interleukin agents).
Four of the studies – those evaluating etanercept (Enbrel), adalimumab (Humira), abatacept (Orencia), and ustekinumab (Stelara) – are now closed to enrollment with analyses either underway or completed. The other five are currently enrolling patients and involve treatment with certolizumab pegol (Cimzia), tildrakizumab (Ilumya), apremilast (Otezla), guselkumab (Tremfya), and tofacitinib (Xeljanz).
Lisa R. Sammaritano, MD, a rheumatologist at the Hospital for Special Surgery, New York, who led the development of the American College of Rheumatology’s first guideline for the management of reproductive health in rheumatic and musculoskeletal diseases, recommends to some of her patients that they contact OTIS. “Their pregnancy registry studies have added important information to the field over the years,” she said.
Most recently, a study of the anti–TNF-alpha medication adalimumab that began in 2004 in pregnant patients with RA and Crohn’s disease culminated in a 2019 PLOS ONE paper reporting no associations between exposure to the medication and an increased risk of adverse outcomes. The outcomes studied were major structural birth defects, minor defects, spontaneous abortion, preterm delivery, prenatal and postnatal growth deficiency, serious or opportunistic infections, and malignancies.
An analysis is underway of adalimumab exposure in women with PsA – a patient subset that was added after the study started. But in the meantime, Dr. Chambers said, the 2019 research article is relevant to questions of drug safety across indications.
OTIS’s MothertoBaby studies are structured as prospective cohort studies. Dr. Chambers, a perinatal epidemiologist, is president of OTIS, which recruits women who have an exposure to the medication under study – at least one dose, for any length of time. And in most cases, it also recruits women with the underlying condition but no exposure and healthy women without the condition to represent the general population.
It’s the disease-matched comparison group that makes OTIS’s studies different from traditional pregnancy registries involving “a simple exposure series and outcomes that are described in the context of what you’d expect in the general population,” said Dr. Chambers, professor in the department of pediatrics, as well as family and preventative medicine, at UCSD and codirector of the Center for Better Beginnings at that university. “Many maternal conditions themselves [or their comorbidities] carry some risk of adverse outcomes in pregnancy.”
The OTIS studies typically involve at least 100 exposed pregnancies and a similar number of unexposed pregnancies; some have cohorts of 200-300.
The recently published study of adalimumab, for instance, included 257 women with exposure to the drug and 120 women in a disease comparison group with no exposure. In addition to finding no associations between drug exposure and adverse outcomes, the study found that women with RA or Crohn’s were at increased risk of preterm delivery, irrespective of adalimumab exposure.
“There’s insufficient [power with any of these numbers] to come to the conclusion that a drug is safe,” she said. “But what we have been able to say [through our studies] is that we’ve looked carefully at the whole array of outcomes ... and we don’t see anything unusual. That early view can be reassuring” until large population-based studies or claims analyses become possible.
Dr. Sammaritano, also with Weill Cornell Medicine, New York, said that she does not recommend registry participation for patients who stop biologics at the diagnosis of pregnancy. Since “the start of IgG antibody transfer during pregnancy is about 16 weeks,” she worries that including these patients might lead to falsely reassuring findings. “We are most interested in [knowing the outcomes of] patients who must continue the drugs through pregnancy,” she said.
Dr. Chambers, however, said that in her view, placental transfer is not a requirement for a medication to have some effect on the outcome of pregnancy. “The outcome could be influenced by an effect of the medication that doesn’t require placental transfer or require placental transfer in large amounts,” she said. “So it’s relevant to examine exposures that have occurred only in the first trimester, and this is especially true for the outcome of major birth defects, most of which are initiated in the first trimester.”
The MotherToBaby studies typically include both early, short exposures and longer exposures, she said. “And certainly, duration of use is a factor that we do consider in looking at specific outcomes such as growth, preterm delivery, and risk of serious or opportunistic infections.”
(In the published study of adalimumab, 65.3% of women in the medication-exposed cohort used the medication in all three trimesters, 10.5% in the first and second trimesters, and 22.4% in the first trimester only.)
Women participating in the MotherToBaby studies complete two to four interviews during pregnancy and may be interviewed again after delivery. They are asked for their permission to share a copy of their medical records – and their baby’s medical records – and their babies receive a follow-up pediatric exam by a pediatrician with expertise in dysmorphology/genetics (who is blinded to exposure status), most commonly in the participant’s home. Providers are not asked to enter any data.
Eliza Chakravarty, MD, a rheumatologist with the Oklahoma Medical Research Foundation in Oklahoma City who treats patients with PsA who are pregnant or considering pregnancy, said that her referrals for research participation “have been mostly to MothertoBaby.”
“Most drug companies [in the autoimmune space] are now contracting with them [for their pregnancy exposure research],” she said. “I really like that it’s become so centralized.”
She tells patients that many questions can be answered through research, that their experience matters, and that “there are benefits” to the extra pediatric examination. “I give them the information and let them decide whether or not they want to call [MotherToBaby],” she said. “I don’t want to impose. I want to make them aware.”
Dr. Chambers emphasizes to patients and physicians that the studies are strictly observational and do not require any changes in personal or medical regimens. “When people hear the word ‘research’ they think of clinical trials. We’re saying, you and your provider do everything you normally would do, just let us observe what happens during your pregnancy.”
Physicians should assure patients, moreover, that “just because the drug is being studied doesn’t mean there’s a known risk or even a suspected risk,” she said.
The MotherToBaby studies receive funding from the pharmaceutical companies, which are required by the Food and Drug Administration to conduct pregnancy exposure registries for medications used during pregnancy or in women of reproductive age. OTIS has an independent advisory board, however, and independently analyzes and publishes its findings. Progress reports are shared with the pharmaceutical companies, and in turn, the FDA, Dr. Chambers said.
To refer patients for MotherToBaby studies, physicians can use an online referral form found on the MothertoBaby web site, a service of OTIS, or call the pregnancy studies team at 877-311-8972 to provide them with the patient’s name or number. Patients may also be given the number and advised to consider calling. MotherToBaby offers medication fact sheets that answer questions about exposures during pregnancy and breastfeeding, and runs a free and confidential teratogen counseling service: 866-626-6847.
Recurrent leg lesions
Tender erythematous nodules or plaques on the extensor surfaces—usually on the legs and occasionally on the arms—are the hallmarks for erythema nodosum, which was diagnosed in this case. It typically occurs in young women, ages 15 to 30, and the nodules or plaques are often accompanied by prodromal fever and malaise. The lesions often are painful and tender to pressure or palpation; they are thought to be caused by a reaction to a stimulus, leading to inflammation of the septa in the subcutaneous fat. While the trigger is often unknown, in some cases, an underlying infection, particularly Streptococcus or tuberculosis (TB), is identified. Sarcoidosis, malignancy, or an increase in estrogen (exogenous or endogenous) also can provoke the disorder.
Due to the risk of underlying disease or triggers, it is prudent to perform radiography of the chest, as well as obtain a complete blood count, sedimentation rate or C reactive protein, and an antistreptolysin O titer when you suspect erythema nodosum. TB testing is also advised. Biopsy typically is not performed because the diagnosis usually is made clinically. If the diagnosis is in doubt, a biopsy can offer confirmation or lead to a different diagnosis such as vasculitis—especially if the lesions are eroded. Since erythema nodosum is an inflammation of the subcutaneous fat, it is important to sample skin lesions deeper than the usual punch biopsy; an incisional biopsy may be required to get an adequate sample.
Erythema nodosum typically resolves spontaneously over a period of weeks, even if there is underlying disease. Therefore, it may be possible to defer treatment if minimal symptoms are present. Otherwise, first-line treatment for the pain and malaise is a nonsteroidal anti-inflammatory drug (NSAID). Oral potassium iodide (360-900 mg/d) is considered second-line treatment and systemic corticosteroids are a third-line option.
For this patient, biopsy was deferred and diagnostic tests were all negative. She had notable pain and a history of good resolution of symptoms with prednisone (5 mg/d), so this drug was prescribed for a 7-day course. She was counseled to avoid taking the NSAIDs and prednisone together due to increased risk of gastritis and ulceration. Recurrent disease can be treated with dapsone (100 mg/d) or hydroxychloroquine (200 mg bid).
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Blake T, Manahan M, Rodins K. Erythema nodosum - a review of an uncommon panniculitis. Dermatol Online J. 2014;20:22376.

Tender erythematous nodules or plaques on the extensor surfaces—usually on the legs and occasionally on the arms—are the hallmarks for erythema nodosum, which was diagnosed in this case. It typically occurs in young women, ages 15 to 30, and the nodules or plaques are often accompanied by prodromal fever and malaise. The lesions often are painful and tender to pressure or palpation; they are thought to be caused by a reaction to a stimulus, leading to inflammation of the septa in the subcutaneous fat. While the trigger is often unknown, in some cases, an underlying infection, particularly Streptococcus or tuberculosis (TB), is identified. Sarcoidosis, malignancy, or an increase in estrogen (exogenous or endogenous) also can provoke the disorder.
Due to the risk of underlying disease or triggers, it is prudent to perform radiography of the chest, as well as obtain a complete blood count, sedimentation rate or C reactive protein, and an antistreptolysin O titer when you suspect erythema nodosum. TB testing is also advised. Biopsy typically is not performed because the diagnosis usually is made clinically. If the diagnosis is in doubt, a biopsy can offer confirmation or lead to a different diagnosis such as vasculitis—especially if the lesions are eroded. Since erythema nodosum is an inflammation of the subcutaneous fat, it is important to sample skin lesions deeper than the usual punch biopsy; an incisional biopsy may be required to get an adequate sample.
Erythema nodosum typically resolves spontaneously over a period of weeks, even if there is underlying disease. Therefore, it may be possible to defer treatment if minimal symptoms are present. Otherwise, first-line treatment for the pain and malaise is a nonsteroidal anti-inflammatory drug (NSAID). Oral potassium iodide (360-900 mg/d) is considered second-line treatment and systemic corticosteroids are a third-line option.
For this patient, biopsy was deferred and diagnostic tests were all negative. She had notable pain and a history of good resolution of symptoms with prednisone (5 mg/d), so this drug was prescribed for a 7-day course. She was counseled to avoid taking the NSAIDs and prednisone together due to increased risk of gastritis and ulceration. Recurrent disease can be treated with dapsone (100 mg/d) or hydroxychloroquine (200 mg bid).
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

Tender erythematous nodules or plaques on the extensor surfaces—usually on the legs and occasionally on the arms—are the hallmarks for erythema nodosum, which was diagnosed in this case. It typically occurs in young women, ages 15 to 30, and the nodules or plaques are often accompanied by prodromal fever and malaise. The lesions often are painful and tender to pressure or palpation; they are thought to be caused by a reaction to a stimulus, leading to inflammation of the septa in the subcutaneous fat. While the trigger is often unknown, in some cases, an underlying infection, particularly Streptococcus or tuberculosis (TB), is identified. Sarcoidosis, malignancy, or an increase in estrogen (exogenous or endogenous) also can provoke the disorder.
Due to the risk of underlying disease or triggers, it is prudent to perform radiography of the chest, as well as obtain a complete blood count, sedimentation rate or C reactive protein, and an antistreptolysin O titer when you suspect erythema nodosum. TB testing is also advised. Biopsy typically is not performed because the diagnosis usually is made clinically. If the diagnosis is in doubt, a biopsy can offer confirmation or lead to a different diagnosis such as vasculitis—especially if the lesions are eroded. Since erythema nodosum is an inflammation of the subcutaneous fat, it is important to sample skin lesions deeper than the usual punch biopsy; an incisional biopsy may be required to get an adequate sample.
Erythema nodosum typically resolves spontaneously over a period of weeks, even if there is underlying disease. Therefore, it may be possible to defer treatment if minimal symptoms are present. Otherwise, first-line treatment for the pain and malaise is a nonsteroidal anti-inflammatory drug (NSAID). Oral potassium iodide (360-900 mg/d) is considered second-line treatment and systemic corticosteroids are a third-line option.
For this patient, biopsy was deferred and diagnostic tests were all negative. She had notable pain and a history of good resolution of symptoms with prednisone (5 mg/d), so this drug was prescribed for a 7-day course. She was counseled to avoid taking the NSAIDs and prednisone together due to increased risk of gastritis and ulceration. Recurrent disease can be treated with dapsone (100 mg/d) or hydroxychloroquine (200 mg bid).
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Blake T, Manahan M, Rodins K. Erythema nodosum - a review of an uncommon panniculitis. Dermatol Online J. 2014;20:22376.
Blake T, Manahan M, Rodins K. Erythema nodosum - a review of an uncommon panniculitis. Dermatol Online J. 2014;20:22376.
Nearly half of brachial plexus injury cases occur without shoulder dystocia
according to research published in Obstetrics & Gynecology.
Grace J. Johnson, MD, and colleagues at Baylor College of Medicine in Houston performed a medical review of 41,525 deliveries at Texas Children’s Hospital between March 2012 and July 2019, identifying cases of brachial plexus injury, with and without shoulder dystocia, occurring and persisting. The researchers also evaluated whether clinical experience (5 years or fewer, 6-15 years, or more than 15 years since training) and education impacted the risk of children developing shoulder dystocia or brachial plexus injury.
There were 547 cases of shoulder dystocia in 26,163 vaginal births (2.1%) and 9 cases in 15,362 cesarean births (0.06%), while 33 cases of brachial plexus injury occurred overall. Nearly all brachial plexus injuries were in vaginal deliveries (30 cases; 0.1%), while 3 cases occurred in cesarean deliveries (0.02%). Of these, 14 cases (42%) of brachial plexus injury did not co-occur with shoulder dystocia. Brachial plexus injury that persisted to discharge was similar for children with shoulder dystocia (17 of 19 cases; 89%) and without shoulder dystocia (10 of 14 cases; 71%). In the 27 children with persistent brachial plexus injury, 2 of 23 children who received follow-up care continued to experience persistent brachial plexus injury at 9 months (1 case with shoulder dystocia) and 12 months (1 case without shoulder dystocia).
“The frequent co-occurrence of shoulder dystocia and brachial plexus injury coupled with the equally frequent occurrence of isolated brachial plexus injury suggests that both brachial plexus injury and shoulder dystocia often reflect two causally unrelated complications of uterine forces driving a fetus through the birth canal in the presence of disproportion between the passage and the shoulder girdle of the passenger,” Dr. Johnson and colleagues wrote.
Results unchanged by clinician experience
Factors that impacted the risk of brachial plexus injury in children without shoulder dystocia were lack of maternal diabetes (0 women vs. 6 women; P = .03) and second-stage labor length (mean 103 minutes vs. 53 minutes; P = .08). Dr. Johnson and colleagues found no significant between-group differences regarding operative delivery, maternal age, or gestational age.
The researchers also examined the experience of the clinician who delivered children with brachial plexus injuries, and discovered there were no significant differences in children who had transient as opposed to persistent brachial plexus injury based on the number of years a clinician had been in practice (P = .97). There also were no significant changes in the “ratios of brachial plexus injury per total deliveries, brachial plexus injury per vaginal deliveries, and brachial plexus injury per shoulder dystocia” despite the presence of education and training for shoulder dystocia.
Questions require further study
Torri Metz, MD, MS, a maternal-fetal medicine subspecialist and associate professor of obstetrics and gynecology at University of Utah Health in Salt Lake City, said in an interview that the review by Johnson and colleagues was able to address limitations in previous studies by looking at the medical records of shoulder dystocia cases at a single tertiary care center.
“Brachial plexus injury occurs both with and without a diagnosis of shoulder dystocia. The finding that the non–shoulder dystocia brachial plexus injuries were associated with a longer second stage of labor suggests that these injuries can occur even prior to delivery of the fetal head and are often not related to maneuvers employed by an obstetrician during delivery,” Dr. Metz said.
The findings that brachial plexus injury severity was unrelated to clinician experience suggests “the occurrence, severity, and persistence of brachial plexus injury may be unrelated to maneuvers by the practitioner at the time of delivery,” she said.
Although Johnson et al. found education and training initiatives did not significantly impact the ratio of brachial plexus injury cases, “importantly, there are likely many other benefits to shoulder dystocia simulation including team communication and comfort of the practitioner in an obstetrical emergency. Thus, the conclusion should not be that simulation training should be abandoned,” Dr. Metz explained.
The results of the study should be confirmed in future research, she noted. “Despite looking at all cases of shoulder dystocia at a tertiary center over a 7-year period, the incidence of brachial plexus injury is low enough that only 33 cases were evaluated. As such, many questions about obstetrical management and the risk of brachial plexus injury still require further study,” said Dr. Metz, who was asked to comment on the study.
The authors reported no relevant financial disclosures. Dr. Metz is an editorial board member for Obstetrics and Gynecology. She was not involved in the review of this manuscript or the decision to publish it.
SOURCE: Johnson GJ et al. Obstet Gynecol. 2020 Oct. doi: 10.1097/AOG.0000000000004013.
according to research published in Obstetrics & Gynecology.
Grace J. Johnson, MD, and colleagues at Baylor College of Medicine in Houston performed a medical review of 41,525 deliveries at Texas Children’s Hospital between March 2012 and July 2019, identifying cases of brachial plexus injury, with and without shoulder dystocia, occurring and persisting. The researchers also evaluated whether clinical experience (5 years or fewer, 6-15 years, or more than 15 years since training) and education impacted the risk of children developing shoulder dystocia or brachial plexus injury.
There were 547 cases of shoulder dystocia in 26,163 vaginal births (2.1%) and 9 cases in 15,362 cesarean births (0.06%), while 33 cases of brachial plexus injury occurred overall. Nearly all brachial plexus injuries were in vaginal deliveries (30 cases; 0.1%), while 3 cases occurred in cesarean deliveries (0.02%). Of these, 14 cases (42%) of brachial plexus injury did not co-occur with shoulder dystocia. Brachial plexus injury that persisted to discharge was similar for children with shoulder dystocia (17 of 19 cases; 89%) and without shoulder dystocia (10 of 14 cases; 71%). In the 27 children with persistent brachial plexus injury, 2 of 23 children who received follow-up care continued to experience persistent brachial plexus injury at 9 months (1 case with shoulder dystocia) and 12 months (1 case without shoulder dystocia).
“The frequent co-occurrence of shoulder dystocia and brachial plexus injury coupled with the equally frequent occurrence of isolated brachial plexus injury suggests that both brachial plexus injury and shoulder dystocia often reflect two causally unrelated complications of uterine forces driving a fetus through the birth canal in the presence of disproportion between the passage and the shoulder girdle of the passenger,” Dr. Johnson and colleagues wrote.
Results unchanged by clinician experience
Factors that impacted the risk of brachial plexus injury in children without shoulder dystocia were lack of maternal diabetes (0 women vs. 6 women; P = .03) and second-stage labor length (mean 103 minutes vs. 53 minutes; P = .08). Dr. Johnson and colleagues found no significant between-group differences regarding operative delivery, maternal age, or gestational age.
The researchers also examined the experience of the clinician who delivered children with brachial plexus injuries, and discovered there were no significant differences in children who had transient as opposed to persistent brachial plexus injury based on the number of years a clinician had been in practice (P = .97). There also were no significant changes in the “ratios of brachial plexus injury per total deliveries, brachial plexus injury per vaginal deliveries, and brachial plexus injury per shoulder dystocia” despite the presence of education and training for shoulder dystocia.
Questions require further study
Torri Metz, MD, MS, a maternal-fetal medicine subspecialist and associate professor of obstetrics and gynecology at University of Utah Health in Salt Lake City, said in an interview that the review by Johnson and colleagues was able to address limitations in previous studies by looking at the medical records of shoulder dystocia cases at a single tertiary care center.
“Brachial plexus injury occurs both with and without a diagnosis of shoulder dystocia. The finding that the non–shoulder dystocia brachial plexus injuries were associated with a longer second stage of labor suggests that these injuries can occur even prior to delivery of the fetal head and are often not related to maneuvers employed by an obstetrician during delivery,” Dr. Metz said.
The findings that brachial plexus injury severity was unrelated to clinician experience suggests “the occurrence, severity, and persistence of brachial plexus injury may be unrelated to maneuvers by the practitioner at the time of delivery,” she said.
Although Johnson et al. found education and training initiatives did not significantly impact the ratio of brachial plexus injury cases, “importantly, there are likely many other benefits to shoulder dystocia simulation including team communication and comfort of the practitioner in an obstetrical emergency. Thus, the conclusion should not be that simulation training should be abandoned,” Dr. Metz explained.
The results of the study should be confirmed in future research, she noted. “Despite looking at all cases of shoulder dystocia at a tertiary center over a 7-year period, the incidence of brachial plexus injury is low enough that only 33 cases were evaluated. As such, many questions about obstetrical management and the risk of brachial plexus injury still require further study,” said Dr. Metz, who was asked to comment on the study.
The authors reported no relevant financial disclosures. Dr. Metz is an editorial board member for Obstetrics and Gynecology. She was not involved in the review of this manuscript or the decision to publish it.
SOURCE: Johnson GJ et al. Obstet Gynecol. 2020 Oct. doi: 10.1097/AOG.0000000000004013.
according to research published in Obstetrics & Gynecology.
Grace J. Johnson, MD, and colleagues at Baylor College of Medicine in Houston performed a medical review of 41,525 deliveries at Texas Children’s Hospital between March 2012 and July 2019, identifying cases of brachial plexus injury, with and without shoulder dystocia, occurring and persisting. The researchers also evaluated whether clinical experience (5 years or fewer, 6-15 years, or more than 15 years since training) and education impacted the risk of children developing shoulder dystocia or brachial plexus injury.
There were 547 cases of shoulder dystocia in 26,163 vaginal births (2.1%) and 9 cases in 15,362 cesarean births (0.06%), while 33 cases of brachial plexus injury occurred overall. Nearly all brachial plexus injuries were in vaginal deliveries (30 cases; 0.1%), while 3 cases occurred in cesarean deliveries (0.02%). Of these, 14 cases (42%) of brachial plexus injury did not co-occur with shoulder dystocia. Brachial plexus injury that persisted to discharge was similar for children with shoulder dystocia (17 of 19 cases; 89%) and without shoulder dystocia (10 of 14 cases; 71%). In the 27 children with persistent brachial plexus injury, 2 of 23 children who received follow-up care continued to experience persistent brachial plexus injury at 9 months (1 case with shoulder dystocia) and 12 months (1 case without shoulder dystocia).
“The frequent co-occurrence of shoulder dystocia and brachial plexus injury coupled with the equally frequent occurrence of isolated brachial plexus injury suggests that both brachial plexus injury and shoulder dystocia often reflect two causally unrelated complications of uterine forces driving a fetus through the birth canal in the presence of disproportion between the passage and the shoulder girdle of the passenger,” Dr. Johnson and colleagues wrote.
Results unchanged by clinician experience
Factors that impacted the risk of brachial plexus injury in children without shoulder dystocia were lack of maternal diabetes (0 women vs. 6 women; P = .03) and second-stage labor length (mean 103 minutes vs. 53 minutes; P = .08). Dr. Johnson and colleagues found no significant between-group differences regarding operative delivery, maternal age, or gestational age.
The researchers also examined the experience of the clinician who delivered children with brachial plexus injuries, and discovered there were no significant differences in children who had transient as opposed to persistent brachial plexus injury based on the number of years a clinician had been in practice (P = .97). There also were no significant changes in the “ratios of brachial plexus injury per total deliveries, brachial plexus injury per vaginal deliveries, and brachial plexus injury per shoulder dystocia” despite the presence of education and training for shoulder dystocia.
Questions require further study
Torri Metz, MD, MS, a maternal-fetal medicine subspecialist and associate professor of obstetrics and gynecology at University of Utah Health in Salt Lake City, said in an interview that the review by Johnson and colleagues was able to address limitations in previous studies by looking at the medical records of shoulder dystocia cases at a single tertiary care center.
“Brachial plexus injury occurs both with and without a diagnosis of shoulder dystocia. The finding that the non–shoulder dystocia brachial plexus injuries were associated with a longer second stage of labor suggests that these injuries can occur even prior to delivery of the fetal head and are often not related to maneuvers employed by an obstetrician during delivery,” Dr. Metz said.
The findings that brachial plexus injury severity was unrelated to clinician experience suggests “the occurrence, severity, and persistence of brachial plexus injury may be unrelated to maneuvers by the practitioner at the time of delivery,” she said.
Although Johnson et al. found education and training initiatives did not significantly impact the ratio of brachial plexus injury cases, “importantly, there are likely many other benefits to shoulder dystocia simulation including team communication and comfort of the practitioner in an obstetrical emergency. Thus, the conclusion should not be that simulation training should be abandoned,” Dr. Metz explained.
The results of the study should be confirmed in future research, she noted. “Despite looking at all cases of shoulder dystocia at a tertiary center over a 7-year period, the incidence of brachial plexus injury is low enough that only 33 cases were evaluated. As such, many questions about obstetrical management and the risk of brachial plexus injury still require further study,” said Dr. Metz, who was asked to comment on the study.
The authors reported no relevant financial disclosures. Dr. Metz is an editorial board member for Obstetrics and Gynecology. She was not involved in the review of this manuscript or the decision to publish it.
SOURCE: Johnson GJ et al. Obstet Gynecol. 2020 Oct. doi: 10.1097/AOG.0000000000004013.
FROM OBSTETRICS & GYNECOLOGY
Pandemic drives demand for self-managed abortions
Requests for self-managed abortion via a telemedicine service increased by 27% from March 20, 2020, to April 11, 2020, in the United States in the wake of widespread lockdowns and shelter-in-place directives because of the COVID-19 pandemic, based on data from a provider of such services.
Access to abortion care is challenging in many areas under ordinary circumstances, but the disruption of the COVID-19 pandemic led to many states suspending or limiting in-clinic services, wrote Abigail R.A. Aiken, MD, PhD, of the University of Texas at Austin and colleagues.
“As a result, people may increasingly be seeking self-managed abortion outside the formal health care system,” they said.
In a research letter published in Obstetrics & Gynecology, the investigators reviewed request data from Aid Access, a telemedicine service that provides medication for abortion at up to 10 weeks’ gestation for users who complete an online consultation form. They also collected data on the implementation and scope of COVID-19–related abortion restrictions by state.
The analysis included all 49,935 requests made between January 1, 2019, and April 11, 2020.
Overall, the rate of requests for self-managed medical abortions increased significantly, by 27%, during the period from March 20, 2020, to April 11, 2020, which reflected the average period after clinic restrictions or closures at the state level. A total of 11 states showed individually significant increases in requests for self-managed medical abortions, with the highest of 94% in Texas and the lowest of 22% in Ohio. In these 11 states, the median time spent at home was 5% higher than in states without significant increases in requests for self-managed medical abortions during the same period. These states also had “particularly high COVID-19 rates or more severe COVID-19–related restrictions on in-clinic abortion access,” the researchers noted.
Patients want alternatives to in-person care
“Our results may reflect two distinct phenomena,” Dr. Aiken and associates wrote. “First, more people may be seeking abortion through all channels, whether due to COVID-19 risks during pregnancy, reduced access to prenatal care, or the pandemic-related economic downturn. Second, there may be shift in demand from in-clinic to self-managed abortion during the pandemic, possibly owing to fear of infection during in-person care or inability to get to a clinic because of childcare and transit disruptions,” they explained.
The study findings were limited by the inability to measure all options for women to achieve self-managed abortions and a lack of power to detect changes in states with low request numbers or where restrictions were implemented at later dates, the researchers noted. However, the results suggest that telemedicine services for medication abortion should be a policy priority because patients may continue to seek alternatives while in-clinic services remain restricted, they said.
In fact, “the World Health Organization recommends telemedicine and self-management abortion-care models during the pandemic, and the United Kingdom has temporarily implemented fully remote provision of abortion medications,” the researchers wrote. However, similar strategies in the United States “would depend on sustained changes to the U.S. Food and Drug Administration’s Risk Evaluation and Mitigation Strategy, which requires patients to collect mifepristone at a hospital or medical facility, as well as changes to state-specific laws that prohibit remote provider consultation,” Dr. Aiken and associates concluded.
Lift barriers to protect patients
Eve Espey, MD, of the University of New Mexico, Albuquerque, said in an interview.
“As background, state abortion restrictions have increased exponentially over the last decade, while research over the same time period has demonstrated the safety of telemedicine abortion – a form of self-managed abortion – with no in-person visit for appropriate candidates,” she said.
“Enter the coronavirus pandemic with safety concerns related to in-person medical visits and certain states leveraging the opportunity to enact even more stringent abortion restrictions. Unsurprisingly, the result, as documented in this excellent research report, is a significant increase in requests for telemedicine abortion in many states, particularly the most restrictive, from the single online service in the United States, Aid Access,” said Dr. Espey.
“Barriers to self-managed abortion include the [FDA] Risk Evaluation and Mitigation Strategy for mifepristone, a set of unnecessary restrictions requiring that providers meet certain qualifications and dispense the medication only in a clinic, office, or hospital,” she said. “The REMS precludes the use of telemedicine abortion; Aid Access and the FDA are in legal proceedings,” she noted.
“Most recently, the [American Civil Liberties Union] sued the FDA on behalf of a coalition of medical experts led by [American College of Obstetricians and Gynecologists] to suspend the REMS for mifepristone during the COVID public health emergency, to allow patients to receive the medications for early abortion without a visit to a health care provider,” Dr. Espey said. “Fortunately, a federal district court required the temporary suspension of the in-person dispensing restriction. Although this is a great step to improve abortion access during the pandemic, a permanent removal of the REMS would pave the way for ongoing safe, effective, and patient-centered early abortion care,” noted Dr. Espey, who was asked to comment on the research letter.
Dr. Aiken disclosed serving as a consultant for Agile Therapeutics, and a coauthor is the founder and director of Aid Access. Dr. Espey had no financial conflicts to disclose. She is a member of the Ob.Gyn. News Editorial Advisory Board.
SOURCE: Aiken ARA et al. Obstet Gynecol. 2020 Jul 21. doi: 10.1097/AOG.0000000000004081.
Requests for self-managed abortion via a telemedicine service increased by 27% from March 20, 2020, to April 11, 2020, in the United States in the wake of widespread lockdowns and shelter-in-place directives because of the COVID-19 pandemic, based on data from a provider of such services.
Access to abortion care is challenging in many areas under ordinary circumstances, but the disruption of the COVID-19 pandemic led to many states suspending or limiting in-clinic services, wrote Abigail R.A. Aiken, MD, PhD, of the University of Texas at Austin and colleagues.
“As a result, people may increasingly be seeking self-managed abortion outside the formal health care system,” they said.
In a research letter published in Obstetrics & Gynecology, the investigators reviewed request data from Aid Access, a telemedicine service that provides medication for abortion at up to 10 weeks’ gestation for users who complete an online consultation form. They also collected data on the implementation and scope of COVID-19–related abortion restrictions by state.
The analysis included all 49,935 requests made between January 1, 2019, and April 11, 2020.
Overall, the rate of requests for self-managed medical abortions increased significantly, by 27%, during the period from March 20, 2020, to April 11, 2020, which reflected the average period after clinic restrictions or closures at the state level. A total of 11 states showed individually significant increases in requests for self-managed medical abortions, with the highest of 94% in Texas and the lowest of 22% in Ohio. In these 11 states, the median time spent at home was 5% higher than in states without significant increases in requests for self-managed medical abortions during the same period. These states also had “particularly high COVID-19 rates or more severe COVID-19–related restrictions on in-clinic abortion access,” the researchers noted.
Patients want alternatives to in-person care
“Our results may reflect two distinct phenomena,” Dr. Aiken and associates wrote. “First, more people may be seeking abortion through all channels, whether due to COVID-19 risks during pregnancy, reduced access to prenatal care, or the pandemic-related economic downturn. Second, there may be shift in demand from in-clinic to self-managed abortion during the pandemic, possibly owing to fear of infection during in-person care or inability to get to a clinic because of childcare and transit disruptions,” they explained.
The study findings were limited by the inability to measure all options for women to achieve self-managed abortions and a lack of power to detect changes in states with low request numbers or where restrictions were implemented at later dates, the researchers noted. However, the results suggest that telemedicine services for medication abortion should be a policy priority because patients may continue to seek alternatives while in-clinic services remain restricted, they said.
In fact, “the World Health Organization recommends telemedicine and self-management abortion-care models during the pandemic, and the United Kingdom has temporarily implemented fully remote provision of abortion medications,” the researchers wrote. However, similar strategies in the United States “would depend on sustained changes to the U.S. Food and Drug Administration’s Risk Evaluation and Mitigation Strategy, which requires patients to collect mifepristone at a hospital or medical facility, as well as changes to state-specific laws that prohibit remote provider consultation,” Dr. Aiken and associates concluded.
Lift barriers to protect patients
Eve Espey, MD, of the University of New Mexico, Albuquerque, said in an interview.
“As background, state abortion restrictions have increased exponentially over the last decade, while research over the same time period has demonstrated the safety of telemedicine abortion – a form of self-managed abortion – with no in-person visit for appropriate candidates,” she said.
“Enter the coronavirus pandemic with safety concerns related to in-person medical visits and certain states leveraging the opportunity to enact even more stringent abortion restrictions. Unsurprisingly, the result, as documented in this excellent research report, is a significant increase in requests for telemedicine abortion in many states, particularly the most restrictive, from the single online service in the United States, Aid Access,” said Dr. Espey.
“Barriers to self-managed abortion include the [FDA] Risk Evaluation and Mitigation Strategy for mifepristone, a set of unnecessary restrictions requiring that providers meet certain qualifications and dispense the medication only in a clinic, office, or hospital,” she said. “The REMS precludes the use of telemedicine abortion; Aid Access and the FDA are in legal proceedings,” she noted.
“Most recently, the [American Civil Liberties Union] sued the FDA on behalf of a coalition of medical experts led by [American College of Obstetricians and Gynecologists] to suspend the REMS for mifepristone during the COVID public health emergency, to allow patients to receive the medications for early abortion without a visit to a health care provider,” Dr. Espey said. “Fortunately, a federal district court required the temporary suspension of the in-person dispensing restriction. Although this is a great step to improve abortion access during the pandemic, a permanent removal of the REMS would pave the way for ongoing safe, effective, and patient-centered early abortion care,” noted Dr. Espey, who was asked to comment on the research letter.
Dr. Aiken disclosed serving as a consultant for Agile Therapeutics, and a coauthor is the founder and director of Aid Access. Dr. Espey had no financial conflicts to disclose. She is a member of the Ob.Gyn. News Editorial Advisory Board.
SOURCE: Aiken ARA et al. Obstet Gynecol. 2020 Jul 21. doi: 10.1097/AOG.0000000000004081.
Requests for self-managed abortion via a telemedicine service increased by 27% from March 20, 2020, to April 11, 2020, in the United States in the wake of widespread lockdowns and shelter-in-place directives because of the COVID-19 pandemic, based on data from a provider of such services.
Access to abortion care is challenging in many areas under ordinary circumstances, but the disruption of the COVID-19 pandemic led to many states suspending or limiting in-clinic services, wrote Abigail R.A. Aiken, MD, PhD, of the University of Texas at Austin and colleagues.
“As a result, people may increasingly be seeking self-managed abortion outside the formal health care system,” they said.
In a research letter published in Obstetrics & Gynecology, the investigators reviewed request data from Aid Access, a telemedicine service that provides medication for abortion at up to 10 weeks’ gestation for users who complete an online consultation form. They also collected data on the implementation and scope of COVID-19–related abortion restrictions by state.
The analysis included all 49,935 requests made between January 1, 2019, and April 11, 2020.
Overall, the rate of requests for self-managed medical abortions increased significantly, by 27%, during the period from March 20, 2020, to April 11, 2020, which reflected the average period after clinic restrictions or closures at the state level. A total of 11 states showed individually significant increases in requests for self-managed medical abortions, with the highest of 94% in Texas and the lowest of 22% in Ohio. In these 11 states, the median time spent at home was 5% higher than in states without significant increases in requests for self-managed medical abortions during the same period. These states also had “particularly high COVID-19 rates or more severe COVID-19–related restrictions on in-clinic abortion access,” the researchers noted.
Patients want alternatives to in-person care
“Our results may reflect two distinct phenomena,” Dr. Aiken and associates wrote. “First, more people may be seeking abortion through all channels, whether due to COVID-19 risks during pregnancy, reduced access to prenatal care, or the pandemic-related economic downturn. Second, there may be shift in demand from in-clinic to self-managed abortion during the pandemic, possibly owing to fear of infection during in-person care or inability to get to a clinic because of childcare and transit disruptions,” they explained.
The study findings were limited by the inability to measure all options for women to achieve self-managed abortions and a lack of power to detect changes in states with low request numbers or where restrictions were implemented at later dates, the researchers noted. However, the results suggest that telemedicine services for medication abortion should be a policy priority because patients may continue to seek alternatives while in-clinic services remain restricted, they said.
In fact, “the World Health Organization recommends telemedicine and self-management abortion-care models during the pandemic, and the United Kingdom has temporarily implemented fully remote provision of abortion medications,” the researchers wrote. However, similar strategies in the United States “would depend on sustained changes to the U.S. Food and Drug Administration’s Risk Evaluation and Mitigation Strategy, which requires patients to collect mifepristone at a hospital or medical facility, as well as changes to state-specific laws that prohibit remote provider consultation,” Dr. Aiken and associates concluded.
Lift barriers to protect patients
Eve Espey, MD, of the University of New Mexico, Albuquerque, said in an interview.
“As background, state abortion restrictions have increased exponentially over the last decade, while research over the same time period has demonstrated the safety of telemedicine abortion – a form of self-managed abortion – with no in-person visit for appropriate candidates,” she said.
“Enter the coronavirus pandemic with safety concerns related to in-person medical visits and certain states leveraging the opportunity to enact even more stringent abortion restrictions. Unsurprisingly, the result, as documented in this excellent research report, is a significant increase in requests for telemedicine abortion in many states, particularly the most restrictive, from the single online service in the United States, Aid Access,” said Dr. Espey.
“Barriers to self-managed abortion include the [FDA] Risk Evaluation and Mitigation Strategy for mifepristone, a set of unnecessary restrictions requiring that providers meet certain qualifications and dispense the medication only in a clinic, office, or hospital,” she said. “The REMS precludes the use of telemedicine abortion; Aid Access and the FDA are in legal proceedings,” she noted.
“Most recently, the [American Civil Liberties Union] sued the FDA on behalf of a coalition of medical experts led by [American College of Obstetricians and Gynecologists] to suspend the REMS for mifepristone during the COVID public health emergency, to allow patients to receive the medications for early abortion without a visit to a health care provider,” Dr. Espey said. “Fortunately, a federal district court required the temporary suspension of the in-person dispensing restriction. Although this is a great step to improve abortion access during the pandemic, a permanent removal of the REMS would pave the way for ongoing safe, effective, and patient-centered early abortion care,” noted Dr. Espey, who was asked to comment on the research letter.
Dr. Aiken disclosed serving as a consultant for Agile Therapeutics, and a coauthor is the founder and director of Aid Access. Dr. Espey had no financial conflicts to disclose. She is a member of the Ob.Gyn. News Editorial Advisory Board.
SOURCE: Aiken ARA et al. Obstet Gynecol. 2020 Jul 21. doi: 10.1097/AOG.0000000000004081.
FROM OBSTETRICS & GYNECOLOGY