User login
A judgment call
A 22-year-old African American man with sickle cell disease comes to the in his joints and chest—a presentation similar to those of his previous sickle cell crises. He is given intravenous fluids for dehydration and morphine sulfate for pain via a peripheral line, and he is admitted to the hospital.
Shortly afterward, the peripheral line becomes infiltrated. After failed attempts at peripheral cannulation, central venous cannulation via an internal jugular site is considered.
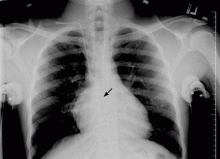
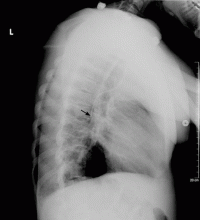
WHERE IS THE CATHETER TIP?
HAZARDS OF ABERRANT LINE PLACEMENT
Central venous catheters are commonly used to give various infusions (eg, parenteral nutrition), to draw blood, and to monitor the central venous pressure.1 The internal jugular vein is one of the preferred veins for central venous access.1,2 Normally, the anatomy of the jugular venous system and the design of the catheter facilitate proper insertion. Occasionally, however, despite proper technique, the tip of the catheter may not terminate at the desired level, resulting in aberrant placement in the internal thoracic vein, superior vena cava, azygos vein, accessory hemiazygos vein, or axillary vein.1–8
The use of chest radiographs to establish the correct placement of internal jugular and subclavian lines has been advocated and is routinely practiced.6,7 Obtaining a chest x-ray to confirm line placement is particularly important in patients with prior multiple and difficult catheterizations. The lateral view is seldom obtained when confirming central neck line placement, but when the anteroposterior view is not reassuring, it may be prudent to obtain this alternate view.
In a large retrospective analysis,9 cannulation of the azygos arch occurred in about 1.2% of insertions, and the rate was about seven times higher when the left jugular vein was used than when the right one was used. A smaller study gave the frequency of azygos arch cannulation as 0.7%.10
All procedure-related complications of central line insertion in the neck can also occur with aberrant azygos vein cannulation. These include infection, bacteremia, pneumothorax, hemothorax, arterial puncture, and various other mechanical complications. It should be noted that aberrant cannulation of the azygos arch is particularly hazardous, and that complication rates are typically higher. These complications are mainly due to the smaller vascular lumen and to the direction of blood flow in the azygos venous system.
KNOWING THE ANATOMY IS CRUCIAL
Knowledge of venous anatomy and its variants is crucial both for insertion and for ascertaining the correct placement of central venous lines.
The azygos vein has a much smaller lumen than the superior vena cava. Although the lumen size may vary significantly, the maximum diameter of the anterior arch of the azygos vein is about 6 to 7 mm,11 whereas the superior vena cava lumen is typically 1.5 to 2 cm in diameter.12 In addition, when a catheter is inserted into the superior vena cava, the direction of blood flow and the direction of the infusion are the same, but when the catheter is inserted into the azygos system, the directions of blood flow and infusion are opposite, contributing to local turbulence.
Both these factors increase the chance of puncturing the vein when the azygos arch is aberrantly cannulated for central venous access.9 Venous perforation has been reported in as many as 19% of cases in which the azygos arch was inadvertently cannulated. Venous perforation can lead to hemopericardium, hemomediastinum, and hemorrhagic pleural effusions, which can lead to significant morbidity and even death. Perforation, thrombosis, stenosis, and complete occlusion have been reported subsequent to catheter malposition in the azygos vein.13
Patients in whom the azygos vein is inadvertently cannulated may tolerate infusions and blood draws, but this does not mean that inadvertent azygos vein cannulation is acceptable, especially given the late complications of vascular perforation that can occur.9
The cannulation of the azygos vein in our patient was completely unintentional; nevertheless, the line was kept in and used for a short period for the initial rehydration and pain control and was subsequently removed without any complications.
WHEN IS CANNULATION OF THE AZYGOS VEIN AN OPTION?
In patients with previous multiple central vein cannulations, the rates of thrombosis and of fibrotic changes in these veins are high. In patients with thrombosis of both the superior vena cava and the inferior vena cava, direct insertion of a catheter into the azygos vein has been suggested as an alternate route to obtain access for dialysis.8 This approach has also been used successfully to administer total parenteral nutrition for a prolonged time in pediatric patients.14 In short, the azygos vein is never preferred for central venous access, but it can occasionally serve as an alternate route.5–9
TAKE-HOME POINTS
The radiographic assessment of an internal jugular or subclavian line may occasionally be deceptive if based solely on the anteroposterior view; confirmation may require a lateral view. We found no guidelines for using the azygos vein for central venous access. The options in cases of aberrant cannulation include leaving the line in, removing and reinserting it at the same or another site under fluoroscopy, and attempting to reposition it after changing the catheter over a guidewire.
The use of central lines found to be in an aberrant position should be driven by clinical judgment based on the urgency of the need of access, the availability or feasibility of other access options, and the intended use. The use of the azygos vein is fraught with procedural complications, as well as postprocedural complications related to vascular perforation. If the position of the catheter tip on the anteroposterior radiographic view is not satisfactory, obtaining a lateral view should be considered.
- McGee DC, Goud MK. Preventing complications of central venous catheterization. N Engl J Med. 2003; 348:1123–1133.
- Pittiruti M, Malerba M, Carriero C, Tazza L, Gui D. Which is the easiest and safest technique for central venous access? A retrospective survey of more than 5,400 cases. J Vasc Access. 2000; 1:100–107.
- Towers MJ. Preventing complications of central venous catheterization. N Engl J Med 2003; 348:2684–2686; author reply 2684–2686.
- Langston CS. The aberrant central venous catheter and its complications. Radiology. 1971; 100:55–59.
- Smith DC, Pop PM. Malposition of a total parenteral nutrition catheter in the accessory hemiazygos vein. JPEN J Parenter Enteral Nutr. 1983; 7:289–292.
- Abood GJ, Davis KA, Esposito TJ, Luchette FA, Gamelli RL. Comparison of routine chest radiograph versus clinician judgment to determine adequate central line placement in critically ill patients. J Trauma. 2007; 63:50–56.
- Gladwin MT, Slonim A, Landucci DL, Gutierrez DC, Cunnion RE. Cannulation of the internal jugular vein: is postprocedural chest radiography always necessary? Crit Care Med 1999; 27:1819–1823.
- Meranze SG, McLean GK, Stein EJ, Jordan HA. Catheter placement in the azygos system: an unusual approach to venous access. Am J Roentgenol. 1985; 144:1075–1076.
- Bankier AA, Mallek R, Wiesmayr MN, et al. Azygos arch cannulation by central venous catheters: radiographic detection of malposition and subsequent complications. J Thorac Imaging. 1997; 12:64–69.
- Langston CT. The aberrant central venous catheter and its complications. Radiology. 1971; 100:55–59.
- Heitzman ER. Radiologic appearance of the azygos vein in cardiovascular disease. Circulation. 1973; 47:628–634.
- McGowan AR, Pugatch RD. Partial obstruction of the superior vena cava. BrighamRAD. Available at: http://brighamrad.harvard.edu/Cases/bwh/hcache/58/full.html. Accessed 9/4/2008.
- Granata A, Figuera M, Castellino S, Logias F, Basile A. Azygos arch cannulation by central venous catheters for hemodialysis. J Vasc Access. 2006; 7:43–45.
- Malt RA, Kempster M. Direct azygos vein and superior vena cava cannulation for parenteral nutrition. JPEN J Parenter Enteral Nutr. 1983; 7:580–581.
A 22-year-old African American man with sickle cell disease comes to the in his joints and chest—a presentation similar to those of his previous sickle cell crises. He is given intravenous fluids for dehydration and morphine sulfate for pain via a peripheral line, and he is admitted to the hospital.
Shortly afterward, the peripheral line becomes infiltrated. After failed attempts at peripheral cannulation, central venous cannulation via an internal jugular site is considered.
WHERE IS THE CATHETER TIP?
HAZARDS OF ABERRANT LINE PLACEMENT
Central venous catheters are commonly used to give various infusions (eg, parenteral nutrition), to draw blood, and to monitor the central venous pressure.1 The internal jugular vein is one of the preferred veins for central venous access.1,2 Normally, the anatomy of the jugular venous system and the design of the catheter facilitate proper insertion. Occasionally, however, despite proper technique, the tip of the catheter may not terminate at the desired level, resulting in aberrant placement in the internal thoracic vein, superior vena cava, azygos vein, accessory hemiazygos vein, or axillary vein.1–8
The use of chest radiographs to establish the correct placement of internal jugular and subclavian lines has been advocated and is routinely practiced.6,7 Obtaining a chest x-ray to confirm line placement is particularly important in patients with prior multiple and difficult catheterizations. The lateral view is seldom obtained when confirming central neck line placement, but when the anteroposterior view is not reassuring, it may be prudent to obtain this alternate view.
In a large retrospective analysis,9 cannulation of the azygos arch occurred in about 1.2% of insertions, and the rate was about seven times higher when the left jugular vein was used than when the right one was used. A smaller study gave the frequency of azygos arch cannulation as 0.7%.10
All procedure-related complications of central line insertion in the neck can also occur with aberrant azygos vein cannulation. These include infection, bacteremia, pneumothorax, hemothorax, arterial puncture, and various other mechanical complications. It should be noted that aberrant cannulation of the azygos arch is particularly hazardous, and that complication rates are typically higher. These complications are mainly due to the smaller vascular lumen and to the direction of blood flow in the azygos venous system.
KNOWING THE ANATOMY IS CRUCIAL
Knowledge of venous anatomy and its variants is crucial both for insertion and for ascertaining the correct placement of central venous lines.
The azygos vein has a much smaller lumen than the superior vena cava. Although the lumen size may vary significantly, the maximum diameter of the anterior arch of the azygos vein is about 6 to 7 mm,11 whereas the superior vena cava lumen is typically 1.5 to 2 cm in diameter.12 In addition, when a catheter is inserted into the superior vena cava, the direction of blood flow and the direction of the infusion are the same, but when the catheter is inserted into the azygos system, the directions of blood flow and infusion are opposite, contributing to local turbulence.
Both these factors increase the chance of puncturing the vein when the azygos arch is aberrantly cannulated for central venous access.9 Venous perforation has been reported in as many as 19% of cases in which the azygos arch was inadvertently cannulated. Venous perforation can lead to hemopericardium, hemomediastinum, and hemorrhagic pleural effusions, which can lead to significant morbidity and even death. Perforation, thrombosis, stenosis, and complete occlusion have been reported subsequent to catheter malposition in the azygos vein.13
Patients in whom the azygos vein is inadvertently cannulated may tolerate infusions and blood draws, but this does not mean that inadvertent azygos vein cannulation is acceptable, especially given the late complications of vascular perforation that can occur.9
The cannulation of the azygos vein in our patient was completely unintentional; nevertheless, the line was kept in and used for a short period for the initial rehydration and pain control and was subsequently removed without any complications.
WHEN IS CANNULATION OF THE AZYGOS VEIN AN OPTION?
In patients with previous multiple central vein cannulations, the rates of thrombosis and of fibrotic changes in these veins are high. In patients with thrombosis of both the superior vena cava and the inferior vena cava, direct insertion of a catheter into the azygos vein has been suggested as an alternate route to obtain access for dialysis.8 This approach has also been used successfully to administer total parenteral nutrition for a prolonged time in pediatric patients.14 In short, the azygos vein is never preferred for central venous access, but it can occasionally serve as an alternate route.5–9
TAKE-HOME POINTS
The radiographic assessment of an internal jugular or subclavian line may occasionally be deceptive if based solely on the anteroposterior view; confirmation may require a lateral view. We found no guidelines for using the azygos vein for central venous access. The options in cases of aberrant cannulation include leaving the line in, removing and reinserting it at the same or another site under fluoroscopy, and attempting to reposition it after changing the catheter over a guidewire.
The use of central lines found to be in an aberrant position should be driven by clinical judgment based on the urgency of the need of access, the availability or feasibility of other access options, and the intended use. The use of the azygos vein is fraught with procedural complications, as well as postprocedural complications related to vascular perforation. If the position of the catheter tip on the anteroposterior radiographic view is not satisfactory, obtaining a lateral view should be considered.
A 22-year-old African American man with sickle cell disease comes to the in his joints and chest—a presentation similar to those of his previous sickle cell crises. He is given intravenous fluids for dehydration and morphine sulfate for pain via a peripheral line, and he is admitted to the hospital.
Shortly afterward, the peripheral line becomes infiltrated. After failed attempts at peripheral cannulation, central venous cannulation via an internal jugular site is considered.
WHERE IS THE CATHETER TIP?
HAZARDS OF ABERRANT LINE PLACEMENT
Central venous catheters are commonly used to give various infusions (eg, parenteral nutrition), to draw blood, and to monitor the central venous pressure.1 The internal jugular vein is one of the preferred veins for central venous access.1,2 Normally, the anatomy of the jugular venous system and the design of the catheter facilitate proper insertion. Occasionally, however, despite proper technique, the tip of the catheter may not terminate at the desired level, resulting in aberrant placement in the internal thoracic vein, superior vena cava, azygos vein, accessory hemiazygos vein, or axillary vein.1–8
The use of chest radiographs to establish the correct placement of internal jugular and subclavian lines has been advocated and is routinely practiced.6,7 Obtaining a chest x-ray to confirm line placement is particularly important in patients with prior multiple and difficult catheterizations. The lateral view is seldom obtained when confirming central neck line placement, but when the anteroposterior view is not reassuring, it may be prudent to obtain this alternate view.
In a large retrospective analysis,9 cannulation of the azygos arch occurred in about 1.2% of insertions, and the rate was about seven times higher when the left jugular vein was used than when the right one was used. A smaller study gave the frequency of azygos arch cannulation as 0.7%.10
All procedure-related complications of central line insertion in the neck can also occur with aberrant azygos vein cannulation. These include infection, bacteremia, pneumothorax, hemothorax, arterial puncture, and various other mechanical complications. It should be noted that aberrant cannulation of the azygos arch is particularly hazardous, and that complication rates are typically higher. These complications are mainly due to the smaller vascular lumen and to the direction of blood flow in the azygos venous system.
KNOWING THE ANATOMY IS CRUCIAL
Knowledge of venous anatomy and its variants is crucial both for insertion and for ascertaining the correct placement of central venous lines.
The azygos vein has a much smaller lumen than the superior vena cava. Although the lumen size may vary significantly, the maximum diameter of the anterior arch of the azygos vein is about 6 to 7 mm,11 whereas the superior vena cava lumen is typically 1.5 to 2 cm in diameter.12 In addition, when a catheter is inserted into the superior vena cava, the direction of blood flow and the direction of the infusion are the same, but when the catheter is inserted into the azygos system, the directions of blood flow and infusion are opposite, contributing to local turbulence.
Both these factors increase the chance of puncturing the vein when the azygos arch is aberrantly cannulated for central venous access.9 Venous perforation has been reported in as many as 19% of cases in which the azygos arch was inadvertently cannulated. Venous perforation can lead to hemopericardium, hemomediastinum, and hemorrhagic pleural effusions, which can lead to significant morbidity and even death. Perforation, thrombosis, stenosis, and complete occlusion have been reported subsequent to catheter malposition in the azygos vein.13
Patients in whom the azygos vein is inadvertently cannulated may tolerate infusions and blood draws, but this does not mean that inadvertent azygos vein cannulation is acceptable, especially given the late complications of vascular perforation that can occur.9
The cannulation of the azygos vein in our patient was completely unintentional; nevertheless, the line was kept in and used for a short period for the initial rehydration and pain control and was subsequently removed without any complications.
WHEN IS CANNULATION OF THE AZYGOS VEIN AN OPTION?
In patients with previous multiple central vein cannulations, the rates of thrombosis and of fibrotic changes in these veins are high. In patients with thrombosis of both the superior vena cava and the inferior vena cava, direct insertion of a catheter into the azygos vein has been suggested as an alternate route to obtain access for dialysis.8 This approach has also been used successfully to administer total parenteral nutrition for a prolonged time in pediatric patients.14 In short, the azygos vein is never preferred for central venous access, but it can occasionally serve as an alternate route.5–9
TAKE-HOME POINTS
The radiographic assessment of an internal jugular or subclavian line may occasionally be deceptive if based solely on the anteroposterior view; confirmation may require a lateral view. We found no guidelines for using the azygos vein for central venous access. The options in cases of aberrant cannulation include leaving the line in, removing and reinserting it at the same or another site under fluoroscopy, and attempting to reposition it after changing the catheter over a guidewire.
The use of central lines found to be in an aberrant position should be driven by clinical judgment based on the urgency of the need of access, the availability or feasibility of other access options, and the intended use. The use of the azygos vein is fraught with procedural complications, as well as postprocedural complications related to vascular perforation. If the position of the catheter tip on the anteroposterior radiographic view is not satisfactory, obtaining a lateral view should be considered.
- McGee DC, Goud MK. Preventing complications of central venous catheterization. N Engl J Med. 2003; 348:1123–1133.
- Pittiruti M, Malerba M, Carriero C, Tazza L, Gui D. Which is the easiest and safest technique for central venous access? A retrospective survey of more than 5,400 cases. J Vasc Access. 2000; 1:100–107.
- Towers MJ. Preventing complications of central venous catheterization. N Engl J Med 2003; 348:2684–2686; author reply 2684–2686.
- Langston CS. The aberrant central venous catheter and its complications. Radiology. 1971; 100:55–59.
- Smith DC, Pop PM. Malposition of a total parenteral nutrition catheter in the accessory hemiazygos vein. JPEN J Parenter Enteral Nutr. 1983; 7:289–292.
- Abood GJ, Davis KA, Esposito TJ, Luchette FA, Gamelli RL. Comparison of routine chest radiograph versus clinician judgment to determine adequate central line placement in critically ill patients. J Trauma. 2007; 63:50–56.
- Gladwin MT, Slonim A, Landucci DL, Gutierrez DC, Cunnion RE. Cannulation of the internal jugular vein: is postprocedural chest radiography always necessary? Crit Care Med 1999; 27:1819–1823.
- Meranze SG, McLean GK, Stein EJ, Jordan HA. Catheter placement in the azygos system: an unusual approach to venous access. Am J Roentgenol. 1985; 144:1075–1076.
- Bankier AA, Mallek R, Wiesmayr MN, et al. Azygos arch cannulation by central venous catheters: radiographic detection of malposition and subsequent complications. J Thorac Imaging. 1997; 12:64–69.
- Langston CT. The aberrant central venous catheter and its complications. Radiology. 1971; 100:55–59.
- Heitzman ER. Radiologic appearance of the azygos vein in cardiovascular disease. Circulation. 1973; 47:628–634.
- McGowan AR, Pugatch RD. Partial obstruction of the superior vena cava. BrighamRAD. Available at: http://brighamrad.harvard.edu/Cases/bwh/hcache/58/full.html. Accessed 9/4/2008.
- Granata A, Figuera M, Castellino S, Logias F, Basile A. Azygos arch cannulation by central venous catheters for hemodialysis. J Vasc Access. 2006; 7:43–45.
- Malt RA, Kempster M. Direct azygos vein and superior vena cava cannulation for parenteral nutrition. JPEN J Parenter Enteral Nutr. 1983; 7:580–581.
- McGee DC, Goud MK. Preventing complications of central venous catheterization. N Engl J Med. 2003; 348:1123–1133.
- Pittiruti M, Malerba M, Carriero C, Tazza L, Gui D. Which is the easiest and safest technique for central venous access? A retrospective survey of more than 5,400 cases. J Vasc Access. 2000; 1:100–107.
- Towers MJ. Preventing complications of central venous catheterization. N Engl J Med 2003; 348:2684–2686; author reply 2684–2686.
- Langston CS. The aberrant central venous catheter and its complications. Radiology. 1971; 100:55–59.
- Smith DC, Pop PM. Malposition of a total parenteral nutrition catheter in the accessory hemiazygos vein. JPEN J Parenter Enteral Nutr. 1983; 7:289–292.
- Abood GJ, Davis KA, Esposito TJ, Luchette FA, Gamelli RL. Comparison of routine chest radiograph versus clinician judgment to determine adequate central line placement in critically ill patients. J Trauma. 2007; 63:50–56.
- Gladwin MT, Slonim A, Landucci DL, Gutierrez DC, Cunnion RE. Cannulation of the internal jugular vein: is postprocedural chest radiography always necessary? Crit Care Med 1999; 27:1819–1823.
- Meranze SG, McLean GK, Stein EJ, Jordan HA. Catheter placement in the azygos system: an unusual approach to venous access. Am J Roentgenol. 1985; 144:1075–1076.
- Bankier AA, Mallek R, Wiesmayr MN, et al. Azygos arch cannulation by central venous catheters: radiographic detection of malposition and subsequent complications. J Thorac Imaging. 1997; 12:64–69.
- Langston CT. The aberrant central venous catheter and its complications. Radiology. 1971; 100:55–59.
- Heitzman ER. Radiologic appearance of the azygos vein in cardiovascular disease. Circulation. 1973; 47:628–634.
- McGowan AR, Pugatch RD. Partial obstruction of the superior vena cava. BrighamRAD. Available at: http://brighamrad.harvard.edu/Cases/bwh/hcache/58/full.html. Accessed 9/4/2008.
- Granata A, Figuera M, Castellino S, Logias F, Basile A. Azygos arch cannulation by central venous catheters for hemodialysis. J Vasc Access. 2006; 7:43–45.
- Malt RA, Kempster M. Direct azygos vein and superior vena cava cannulation for parenteral nutrition. JPEN J Parenter Enteral Nutr. 1983; 7:580–581.
When a quick sound bite won’t do
The sound bites about these trials in the news have confused physicians and patients alike. But, as we have all experienced during this election year, to understand complex problems requires an in-depth analysis instead of a sound bite.
I was troubled by the results of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial,2 in which more patients who were treated with an intense hemoglobin A1c-lowering strategy died (mostly of macrovascular events) than those treated with a standard strategy. Older data showing a beneficial effect of glucose-lowering on the microvascular complications of diabetes are solid. I did not understand the mechanistic basis of the ACCORD results, unless the very aggressive therapy caused many hypoglycemic events with catecholamine surges, resulting in stroke or myocardial infarction, or whether a problem with a specific drug arose more often in the intensive-treatment group. There has been similar dialogue surrounding intensity of glucose control in critically ill inpatients3; here, the data suggest that hypoglycemic episodes may limit other benefits of aggressive treatment in the intensive care unit, such as reduced infection rates.
Not to be ignored is that the patients in all arms of the ACCORD trial fared far better than historical diabetic controls. The meticulous attention to management of blood pressure and LDL-C that all patients in the ACCORD trial received paid off. (If only we could do as well in our practices!) But what do we do about the sugar?
This large, well-done, ongoing trial deserves a detailed analysis for those of us who need to translate the conclusions regarding glucose control to our patients. This month in the Journal, I have invited Byron Hoogwerf, a clinical diabetologist, former internal medicine program director, well-published clinical trialist, and ACCORD investigator, to provide this analysis.4 His discussion is more detailed than what we often print purposefully, and it is well worth reading. Some issues simply can’t be understood as a sound bite.
- Kastelein JJ, Akdim F, Stroes ES, et alENHANCE Investigators. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med. 2008; 358:1431–1443.
- Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008; 358:2545–2559.
- Soylemez Wiener R, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. JAMA. 2008; 300:933–944.
- Hoogwerf BF. A clinician and clinical trialist’s perspective: does intensive therapy of type 2 diabetes help or harm? Seeking accord on ACCORD. Cleve Clin J Med. 2008; 75:729–737.
The sound bites about these trials in the news have confused physicians and patients alike. But, as we have all experienced during this election year, to understand complex problems requires an in-depth analysis instead of a sound bite.
I was troubled by the results of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial,2 in which more patients who were treated with an intense hemoglobin A1c-lowering strategy died (mostly of macrovascular events) than those treated with a standard strategy. Older data showing a beneficial effect of glucose-lowering on the microvascular complications of diabetes are solid. I did not understand the mechanistic basis of the ACCORD results, unless the very aggressive therapy caused many hypoglycemic events with catecholamine surges, resulting in stroke or myocardial infarction, or whether a problem with a specific drug arose more often in the intensive-treatment group. There has been similar dialogue surrounding intensity of glucose control in critically ill inpatients3; here, the data suggest that hypoglycemic episodes may limit other benefits of aggressive treatment in the intensive care unit, such as reduced infection rates.
Not to be ignored is that the patients in all arms of the ACCORD trial fared far better than historical diabetic controls. The meticulous attention to management of blood pressure and LDL-C that all patients in the ACCORD trial received paid off. (If only we could do as well in our practices!) But what do we do about the sugar?
This large, well-done, ongoing trial deserves a detailed analysis for those of us who need to translate the conclusions regarding glucose control to our patients. This month in the Journal, I have invited Byron Hoogwerf, a clinical diabetologist, former internal medicine program director, well-published clinical trialist, and ACCORD investigator, to provide this analysis.4 His discussion is more detailed than what we often print purposefully, and it is well worth reading. Some issues simply can’t be understood as a sound bite.
The sound bites about these trials in the news have confused physicians and patients alike. But, as we have all experienced during this election year, to understand complex problems requires an in-depth analysis instead of a sound bite.
I was troubled by the results of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial,2 in which more patients who were treated with an intense hemoglobin A1c-lowering strategy died (mostly of macrovascular events) than those treated with a standard strategy. Older data showing a beneficial effect of glucose-lowering on the microvascular complications of diabetes are solid. I did not understand the mechanistic basis of the ACCORD results, unless the very aggressive therapy caused many hypoglycemic events with catecholamine surges, resulting in stroke or myocardial infarction, or whether a problem with a specific drug arose more often in the intensive-treatment group. There has been similar dialogue surrounding intensity of glucose control in critically ill inpatients3; here, the data suggest that hypoglycemic episodes may limit other benefits of aggressive treatment in the intensive care unit, such as reduced infection rates.
Not to be ignored is that the patients in all arms of the ACCORD trial fared far better than historical diabetic controls. The meticulous attention to management of blood pressure and LDL-C that all patients in the ACCORD trial received paid off. (If only we could do as well in our practices!) But what do we do about the sugar?
This large, well-done, ongoing trial deserves a detailed analysis for those of us who need to translate the conclusions regarding glucose control to our patients. This month in the Journal, I have invited Byron Hoogwerf, a clinical diabetologist, former internal medicine program director, well-published clinical trialist, and ACCORD investigator, to provide this analysis.4 His discussion is more detailed than what we often print purposefully, and it is well worth reading. Some issues simply can’t be understood as a sound bite.
- Kastelein JJ, Akdim F, Stroes ES, et alENHANCE Investigators. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med. 2008; 358:1431–1443.
- Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008; 358:2545–2559.
- Soylemez Wiener R, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. JAMA. 2008; 300:933–944.
- Hoogwerf BF. A clinician and clinical trialist’s perspective: does intensive therapy of type 2 diabetes help or harm? Seeking accord on ACCORD. Cleve Clin J Med. 2008; 75:729–737.
- Kastelein JJ, Akdim F, Stroes ES, et alENHANCE Investigators. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med. 2008; 358:1431–1443.
- Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008; 358:2545–2559.
- Soylemez Wiener R, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. JAMA. 2008; 300:933–944.
- Hoogwerf BF. A clinician and clinical trialist’s perspective: does intensive therapy of type 2 diabetes help or harm? Seeking accord on ACCORD. Cleve Clin J Med. 2008; 75:729–737.
What is the proper workup of a patient with hypertension?
How extensive a workup does a patient with high blood pressure need?
On one hand, we would not want to start therapy on the basis of a single elevated reading, as blood pressure fluctuates considerably during the day, and even experienced physicians often make errors in taking blood pressure that tend to falsely elevate the patient’s readings. Similarly, we would not want to miss the diagnosis of a potentially curable cause of hypertension or of a condition that increases a patient’s risk of cardiovascular disease. But considering that nearly one-third of adults in the United States have hypertension and that another one-fourth have prehypertension (formerly called high-normal blood pressure),1 if we were to launch an intensive workup for every patient with high blood pressure, the cost and effort would be enormous.
Fortunately, for most patients, it is enough to measure blood pressure accurately and repeatedly, perform a focused history and physical examination, and obtain the results of a few basic laboratory tests and an electrocardiogram, with additional tests in special cases.
In this review we address four fundamental questions in the evaluation of patients with a high blood pressure reading, and how to answer them.
ANSWERING FOUR QUESTIONS
The goal of the hypertension evaluation is to answer four questions:
- Does the patient have sustained hypertension? And if so—
- Is the hypertension primary or secondary?
- Does the patient have other cardiovascular risk factors?
- Does he or she have evidence of target organ damage?
DOES THE PATIENT HAVE SUSTAINED HYPERTENSION?
It is important to measure blood pressure accurately, for several reasons. A diagnosis of hypertension has a measurable impact on the patient’s quality of life.2 Furthermore, we want to avoid undertaking a full evaluation of hypertension if the patient doesn’t actually have high blood pressure, ie, systolic blood pressure greater than 140 mm Hg or diastolic pressure greater than 90 mm Hg. However, many people have blood pressures in the prehypertensive range (ie, 120–139 mm Hg systolic; 80–89 mm Hg diastolic). Many people in this latter group can expect to develop hypertension in time, as the prevalence of hypertension increases steadily with age unless effective preventive measures are implemented, such as losing weight, exercising regularly, and avoiding excessive consumption of sodium and alcohol.
The best position to use is sitting, as the Framingham Heart Study and most randomized clinical trials that established the value of treating hypertension used this position for diagnosis and follow-up.6
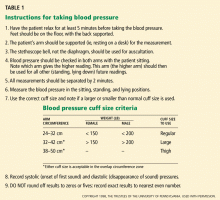
Proper patient positioning, the correct cuff size, calibrated equipment, and good inflation and deflation technique will yield the best assessment of blood pressure levels. But even if your technique is perfect, blood pressure is a dynamic vital sign, so it is necessary to repeat the measurement, average the values for any particular day, and keep in mind that the pressure is higher (or lower) on some days than on others, so that the running average is more important than individual readings. This leads to two final points about blood pressure measurement:
- Take it right, at least two times on any occasion
- Take it on at least two (preferably three) separate days.
Following up on blood pressure
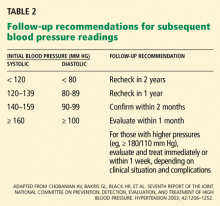
After measuring the blood pressure, it is necessary to plan for follow-up readings, guided by both the blood pressure levels (Table 2) and your clinical judgment.
If the systolic and diastolic blood pressures fall into different categories, you should follow the recommendations for the shorter follow-up time.
IS THE HYPERTENSION PRIMARY OR SECONDARY?
Most patients with hypertension have primary (“essential”) hypertension and are likely to remain hypertensive for life. However, some have secondary hypertension, ie, high blood pressure due to an identifiable cause. Some of these conditions (and the hypertension that they cause) can be cured. For example, pheochromocytoma can be cured if found and removed. Other causes of secondary hypertension, such as parenchymal renal disease, are infrequently cured, and the goal is usually to control the blood pressure with drugs.
The sudden onset of severe hypertension in a patient previously known to have had normal blood pressure raises the suspicion of a secondary form of hypertension, as does the onset of hypertension in a young person (< 25 years) or an older person (> 55 years). However, these ages are arbitrary; with the increasing body mass index in young people, essential hypertension is now more commonly diagnosed in the third decade. And since systolic pressure increases throughout life, we can expect many older patients to develop essential hypertension.7 Indeed, current guidelines are urging us to pay more attention to systolic pressure than in the past.
WHAT IS THE PATIENT’S CARDIOVASCULAR RISK?
The relationship between blood pressure and risk of cardiovascular disease is linear, continuous, and independent of (though additive to) other risk factors.1 For people 40 to 70 years old, each increment of either 20 mm Hg in systolic blood pressure or 10 mm Hg in diastolic blood pressure doubles the risk of cardiovascular disease across the entire range from 115/75 to 185/115 mm Hg.1 If the patient smokes or has elevated cholesterol, other cardiovascular risk factors, or the metabolic syndrome, the risk is even higher.8
The usual goal of antihypertensive treatment is systolic pressure less than 140 mm Hg and diastolic pressure less than 90 mm Hg. However, the target is lower—less than 130/80 mm Hg—for those with diabetes9 or target organ damage such as heart failure or renal disease.1,10 Thus, it is important to try to detect these conditions in the evaluation of the hypertensive patient.
Another reason it is important is that reducing such risk sometimes calls for using (or avoiding) antihypertensive drugs that are likely to alter these factors. For example, the use of beta-blockers in patients with a low level of high-density lipoprotein cholesterol (HDL-C) can lower HDL-C further.11
DOES THE PATIENT HAVE TARGET ORGAN DAMAGE?
Target organ damage is very important to detect because it changes the goal of treatment from primary prevention of adverse target organ outcomes into the more challenging realm of secondary prevention. For example, if a patient has had a stroke, his or her chance of having another stroke in the next 5 years is about 20%. This is much higher than the risk in an average hypertensive patient without such a history. For such patients, the current guidelines1 recommend the combination of a diuretic and an angiotensin-converting enzyme inhibitor, a combination shown to reduce the risk of a second stroke.12 Thus, we need to discover whether the patient had a stroke in the first place.
HISTORY
- The duration (if known) and severity of the hypertension
- The degree of blood pressure fluctuation
- Concomitant medical conditions, especially cardiovascular or renal problems
- Dietary habits
- Alcohol consumption
- Tobacco use
- Level of physical activity
- A family history of hypertension, renal disease, cardiovascular problems, or diabetes mellitus
- Past medications, with particular attention to their side effects and their efficacy in controlling blood pressure
- Current medications, including over-the-counter preparations. One reason: non-steroidal anti-inflammatory drugs other than aspirin can decrease the efficacy of antihypertensive drugs, presumably through mechanisms that inhibit the effects of vasodilatory and natriuretic prostaglandins and potentiate those of angiotensin II.13
PHYSICAL EXAMINATION
The physical examination starts with measurement of height, weight, waist circumference, and blood pressure—in both arms and the leg if coarctation of the aorta is suspected. Measurements with the patient supine, sitting, and standing are usually taken at the first visit, though such an approach is more suited to a hypertension specialty clinic than a primary care setting, in which time constraints usually limit the blood pressure readings to two or three seated values. Most prospective data on the benefits of hypertension treatment are based on a seated blood pressure, so we favor that measurement for follow-up.
Special attention in the physical examination is directed to:
The retina (to assess the vascular impact of the high blood pressure). Look for arteriolar narrowing (grade 1), arteriovenous compression (grade 2), hemorrhages or exudates (grade 3), and papilledema.2 Such findings not only relate to severity (higher grade = more severe blood pressure) but also predict future cardiovascular disease.14
The blood vessels. Bruits in the neck may indicate carotid stenosis, bruits in the abdomen may indicate renovascular disease, and femoral bruits are a sign of general atherosclerosis. Bruits also signal vascular stenosis and irregularity and may be a clue to vascular damage or future loss of target organ function. However, bruits may simply result from vascular tortuosity, particularly with significant flow in the vessel.
Also check the femoral pulses: poor or delayed femoral pulses are a sign of aortic coarctation. The radial artery is about as far away from the heart as the femoral artery; consequently, when palpating both sites simultaneously the pulse should arrive at about the same moment. In aortic coarctation, a palpable delay in the arrival of the femoral pulse may occur, and an interscapular murmur may be heard during auscultation of the back. In these instances, a low leg blood pressure (usually measured by placing a thigh-sized adult cuff on the patient’s thigh and listening over the popliteal area with the patient prone) may confirm the presence of aortic obstruction. When taking a leg blood pressure, the large cuff and the amount of pressure necessary to occlude the artery may be uncomfortable, and one should warn the patient about the discomfort before taking the measurement.
Poor or absent pedal pulses are a sign of peripheral arterial disease.
The heart (to detect gallops, enlargement, or both). Palpation may reveal a displaced apical impulse, which can indicate left ventricular enlargement. A sustained apical impulse may indicate left ventricular hypertrophy. Listen for a fourth heart sound (S4), one of the earliest physical findings of hypertension when physical findings are present. An S4 indicates that the left atrium is working hard to overcome the stiffness of the left ventricle. An S3 indicates an impairment in left ventricular function and is usually a harbinger of underlying heart disease. In some cases, lung rales can also be heard, though the combination of an S3 gallop and rales is an unusual office presentation in the early management of the hypertensive patient.
The lungs. Listen for rales (see above).
The lower extremities should be examined for peripheral arterial pulsations and edema. The loss of pedal pulses is a common finding, particularly in smokers, and is a clue to increased cardiovascular risk.
Strength, gait, and cognition. Perform a brief neurologic examination for evidence of remote stroke. We usually observe our patients’ gait as they enter or leave the examination room, test their bilateral grip strength, and assess their judgment, speech, and memory during the history and physical examination.
A great deal of research has linked high blood pressure to future loss of cognitive function,15 and it is useful to know that impairment is present before beginning treatment, since some patients will complain of memory loss after starting antihypertensive drug treatment.
LABORATORY EVALUATION
Routine tests
The routine evaluation of hypertensive patients should include, at a minimum:
- A hemoglobin or hematocrit measurement
- Urinalysis with microscopic examination
- Serum electrolyte concentrations
- Serum glucose concentration
- A fasting lipid profile
- A 12-lead electrocardiogram (Table 5).
Nonroutine tests
In some cases, other studies may be appropriate, depending on the clinical situation, eg:
- Serum uric acid in those with a history of gout, since some antihypertensive drugs (eg, diuretics) may increase serum uric acid and predispose to further episodes of gout
- Serum calcium in those with a personal or family history of kidney stones, to detect subtle parathyroid excess
- Thyroid-stimulating hormone or other thyroid studies if the history suggests thyroid excess, or if a thyroid nodule is discovered
- Limited echocardiography, which is more sensitive than electrocardiography for detecting left ventricular hypertrophy.
We sometimes use echocardiography if the patient is overweight but seems motivated to lose weight. In these cases we might not start drug therapy right away, choosing rather to wait and see if the patient can lose some weight (which might lower the blood pressure and make drug therapy unnecessary)—but only if the echocardiogram shows that he or she does not have left ventricular hypertrophy.
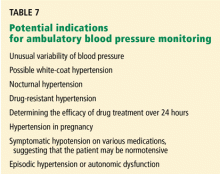
We also use echocardiography in patients with white-coat hypertension (see below), in whom office pressures are consistently high but whom we have elected to either not treat or not alter treatment. In these cases the echocardiogram serves as a “second opinion” about the merits of not altering therapy and supports this decision when the left ventricular wall thicknesses are normal (and remain so during long-term follow-up). In cases of suspected white-coat hypertension, home or ambulatory blood pressure monitoring is valuable to establish or exclude this diagnosis.1
Urinary albumin excretion. Microalbuminuria is an early manifestation of diabetic nephropathy and hypertension. Although routine urine screening for microalbuminuria is typically done in the management of diabetes, it is still not considered a standard of care, though the growing literature on its role as a cardiovascular risk predictor16–18 and its value as a therapeutic target in diabetes19,20 make it an attractive aid in the overall assessment of patients with hypertension.
Plasma renin activity and serum aldosterone concentrations are useful in screening for aldosterone excess, but are usually reserved as follow-up tests in patients with either hypokalemia or failure to achieve blood pressure control on a three-drug regimen in which at least one drug is a diuretic.1,21
Of note, primary aldosteronism is not as rare as previously thought. In a study of patients referred to hypertension centers, 11% had primary aldosteronism according to prospective diagnostic criteria, almost 5% had curable aldosterone-producing adenomas, and 6% had idiopathic hyperaldosteronism.22
If secondary hypertension is suspected
A search for secondary forms of hypertension is usually considered in patients with moderate or severe hypertension that does not respond to antihypertensive agents. Another situation is in hypertensive patients younger than 25 years, since curable forms of hypertension are more common in this age group. In older patients, the prevalence of secondary hypertension is lower and does not justify the costs and effort of routine elaborate workups unless there is evidence from the history, physical examination, or routine laboratory work for suspecting its presence. An exception to this rule is the need to exclude atherosclerotic renovascular hypertension in an elderly patient. This cause of secondary hypertension is common in the elderly and may be amenable to therapeutic intervention.26
WHEN TO CONSIDER HOME OR AMBULATORY MONITORING
Suspected white-coat hypertension
Blood pressure can be influenced by an environment such as an office or hospital clinic. This has led to the development of ambulatory blood pressure monitors and more use of self-measurement of blood pressure in the home. Blood pressure readings with these techniques are generally lower than those measured in an office or hospital clinic. These methods make it possible to screen for white-coat hypertension. In 10% to 20% of people with hypertensive readings, the blood pressure may be elevated persistently only in the presence of a physician.28 When measured elsewhere, including at work, the blood pressure is not elevated in those with the white-coat effect. Although this response may become less prominent with repeated measurements, it occasionally persists in the office setting, sometimes for years in our experience.
Suspected nocturnal hypertension (’nondipping’ status)
Ambulatory blood pressure is also helpful to screen for nocturnal hypertension. Evidence is accumulating to suggest that hypertensive patients whose pressure remains relatively high at night (“nondippers,” ie, those with less than a 10% reduction at night compared with daytime blood pressure readings) are at greater risk of cardiovascular morbidity than “dippers” (those whose blood pressure is at least 10% lower at night than during the day).29
An early morning surge
Ambulatory monitoring can also detect morning surges in systolic blood pressure,30 a marker of cerebrovascular risk. Generally, these patients have an increase of more than 55 mm Hg in systolic pressure between their sleeping and early-hour waking values, and we may wish to start or alter treatment specifically to address these high morning systolic values.31
‘PIPESTEM’ VESSELS AND PSEUDOHYPERTENSION
Occasionally, one encounters patients with vessels that are stiff and difficult to compress. If the pressure required to compress the brachial artery and stop audible blood flow with a standard blood pressure cuff is greater than the actual blood pressure within the artery as measured invasively, the condition is called pseudohypertension. The stiffness is thought to be due to calcification of the arterial wall.
A way to check for this condition is to inflate the cuff to at least 30 mm Hg above the palpable systolic pressure and then try to “roll” the brachial or radial artery underneath your fingertips, a procedure known as Osler’s maneuver.32 If you feel something that resembles a stiff tube reminiscent of the stem of a tobacco smoker’s pipe (healthy arteries are not palpable when empty), the patient may have pseudohypertension. However, the specificity of Osler’s maneuver has been questioned, particularly in hospitalized elderly patients.33
Pseudohypertension is important because the patients in whom it occurs, usually the elderly or the chronically ill (with diabetes or chronic kidney disease), are prone to orthostatic or postural hypotension, which may be aggravated by increasing their antihypertensive treatment on the basis of a cuff pressure that is actually much higher than the real blood pressure.33
- Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- Wenger NK. Quality of life issues in hypertension: consequences of diagnosis and considerations in management. Am Heart J 1988; 116:628–632.
- McFadden CB, Townsend RR. Blood pressure measurement: common pitfalls and how to avoid them. Consultant 2003; 43:161–165.
- Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 2005; 111:697–716.
- Myers MG. Automated blood pressure measurement in routine clinical practice. Blood Press Monit 2006; 11:59–62.
- Mosenkis A, Townsend RR. Sitting on the evidence: what is the proper patient position for the office measurement of blood pressure? J Clin Hypertens (Greenwich) 2005; 7:365–366.
- Vasan RS, Beiser A, Seshadri S, et al. Residual lifetime risk for developing hypertension in middle-aged women and men: The Framingham Heart Study. JAMA 2002; 287:1003–1010.
- Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol 2004; 44:720–732.
- American Diabetes Association. Treatment of hypertension in adults with diabetes. Diabetes Care 2002; 25:199–201.
- Rosendorff C, Black HR, Cannon CP, et al. Treatment of hypertension in the prevention and management of ischemic heart disease: a scientific statement from the American Heart Association Council for High Blood Pressure Research and the Councils on Clinical Cardiology and Epidemiology and Prevention. Circulation 2007; 115:2761–2788.
- Papadakis JA, Mikhailidis DP, Vrentzos GE, Kalikaki A, Kazakou I, Ganotakis ES. Effect of antihypertensive treatment on plasma fibrinogen and serum HDL levels in patients with essential hypertension. Clin Appl Thromb Hemost 2005; 11:139–146.
- PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001; 358:1033–1041.
- Fierro-Carrion GA, Ram CV. Nonsteroidal anti-inflammatory drugs (NSAIDs) and blood pressure. Am J Cardiol 1997; 80:775–776.
- Wong TY, McIntosh R. Hypertensive retinopathy signs as risk indicators of cardiovascular morbidity and mortality. Br Med Bull 2005; 73–74:57–70.
- Forette F, Boller F. Hypertension and the risk of dementia in the elderly. Am J Med 1991; 90:14S–19S.
- Schrader J, Luders S, Kulschewski A, et al. Microalbuminuria and tubular proteinuria as risk predictors of cardiovascular morbidity and mortality in essential hypertension: final results of a prospective long-term study (MARPLE Study). J Hypertens 2006; 24:541–548.
- Luque M, de Rivas B, Alvarez B, Garcia G, Fernandez C, Martell N. Influence of target organ lesion detection (assessment of microalbuminuria and echocardiogram) in cardiovascular risk stratification and treatment of untreated hypertensive patients. J Hum Hypertens 2006; 20:187–192.
- Pontremoli R, Leoncini G, Viazzi F, et al. Role of microalbuminuria in the assessment of cardiovascular risk in essential hypertension. J Am Soc Nephrol 2005; 16 suppl 1:S39–S41.
- Erdmann E. Microalbuminuria as a marker of cardiovascular risk in patients with type 2 diabetes. Int J Cardiol 2006; 107:147–153.
- Bakris GL, Sowers JR. Microalbuminuria in diabetes: focus on cardiovascular and renal risk reduction. Curr Diab Rep 2002; 2:258–262.
- Gallay BJ, Ahmad S, Xu L, Toivola B, Davidson RC. Screening for primary aldosteronism without discontinuing hypertensive medications: plasma aldosteronerenin ratio. Am J Kidney Dis 2001; 37:699–705.
- Rossi GP, Bernini G, Caliumi C, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol 2006; 48:2293–2300.
- Onusko E. Diagnosing secondary hypertension. Am Fam Physician 2003; 67:67–74.
- Aurell M. Screening for secondary hypertension. Curr Hypertens Rep 1999; 1:461.
- Garovic VD, Kane GC, Schwartz GL. Renovascular hypertension: balancing the controversies in diagnosis and treatment. Cleve Clin J Med 2005; 72:1135–1137.
- Textor SC. Renovascular hypertension in 2007: where are we now? Curr Cardiol Rep 2007; 9:453–461.
- Pickering TG, Shimbo D, Haas D. Ambulatory blood-pressure monitoring. N Engl J Med 2006; 354:2368–2374.
- Angeli F, Verdecchia P, Gattobigio R, Sardone M, Reboldi G. White-coat hypertension in adults. Blood Press Monit 2005; 10:301–305.
- Cicconetti P, Morelli S, De Serra C, et al. Left ventricular mass in dippers and nondippers with newly diagnosed hypertension. Angiology 2003; 54:661–669.
- Kario K, Pickering TG, Umeda Y, et al. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation 2003; 107:1401–1406.
- Katakam R, Townsend RR. Morning surges in blood pressure. J Clin Hypertens 2006; 8:450–451.
- Messerli FH. Osler’s maneuver, pseudohypertension, and true hypertension in the elderly. Am J Med 1986; 80:906–910.
- Belmin J, Visintin JM, Salvatore R, Sebban C, Moulias R. Osler’s maneuver: absence of usefulness for the detection of pseudohypertension in an elderly population. Am J Med 1995; 98:42–49.
- Messerli FH, Ventura HO, Amodeo C. Osler’s maneuver and pseudohypertension. N Engl J Med 1985; 312:1548–1551.
How extensive a workup does a patient with high blood pressure need?
On one hand, we would not want to start therapy on the basis of a single elevated reading, as blood pressure fluctuates considerably during the day, and even experienced physicians often make errors in taking blood pressure that tend to falsely elevate the patient’s readings. Similarly, we would not want to miss the diagnosis of a potentially curable cause of hypertension or of a condition that increases a patient’s risk of cardiovascular disease. But considering that nearly one-third of adults in the United States have hypertension and that another one-fourth have prehypertension (formerly called high-normal blood pressure),1 if we were to launch an intensive workup for every patient with high blood pressure, the cost and effort would be enormous.
Fortunately, for most patients, it is enough to measure blood pressure accurately and repeatedly, perform a focused history and physical examination, and obtain the results of a few basic laboratory tests and an electrocardiogram, with additional tests in special cases.
In this review we address four fundamental questions in the evaluation of patients with a high blood pressure reading, and how to answer them.
ANSWERING FOUR QUESTIONS
The goal of the hypertension evaluation is to answer four questions:
- Does the patient have sustained hypertension? And if so—
- Is the hypertension primary or secondary?
- Does the patient have other cardiovascular risk factors?
- Does he or she have evidence of target organ damage?
DOES THE PATIENT HAVE SUSTAINED HYPERTENSION?
It is important to measure blood pressure accurately, for several reasons. A diagnosis of hypertension has a measurable impact on the patient’s quality of life.2 Furthermore, we want to avoid undertaking a full evaluation of hypertension if the patient doesn’t actually have high blood pressure, ie, systolic blood pressure greater than 140 mm Hg or diastolic pressure greater than 90 mm Hg. However, many people have blood pressures in the prehypertensive range (ie, 120–139 mm Hg systolic; 80–89 mm Hg diastolic). Many people in this latter group can expect to develop hypertension in time, as the prevalence of hypertension increases steadily with age unless effective preventive measures are implemented, such as losing weight, exercising regularly, and avoiding excessive consumption of sodium and alcohol.
The best position to use is sitting, as the Framingham Heart Study and most randomized clinical trials that established the value of treating hypertension used this position for diagnosis and follow-up.6
Proper patient positioning, the correct cuff size, calibrated equipment, and good inflation and deflation technique will yield the best assessment of blood pressure levels. But even if your technique is perfect, blood pressure is a dynamic vital sign, so it is necessary to repeat the measurement, average the values for any particular day, and keep in mind that the pressure is higher (or lower) on some days than on others, so that the running average is more important than individual readings. This leads to two final points about blood pressure measurement:
- Take it right, at least two times on any occasion
- Take it on at least two (preferably three) separate days.
Following up on blood pressure
After measuring the blood pressure, it is necessary to plan for follow-up readings, guided by both the blood pressure levels (Table 2) and your clinical judgment.
If the systolic and diastolic blood pressures fall into different categories, you should follow the recommendations for the shorter follow-up time.
IS THE HYPERTENSION PRIMARY OR SECONDARY?
Most patients with hypertension have primary (“essential”) hypertension and are likely to remain hypertensive for life. However, some have secondary hypertension, ie, high blood pressure due to an identifiable cause. Some of these conditions (and the hypertension that they cause) can be cured. For example, pheochromocytoma can be cured if found and removed. Other causes of secondary hypertension, such as parenchymal renal disease, are infrequently cured, and the goal is usually to control the blood pressure with drugs.
The sudden onset of severe hypertension in a patient previously known to have had normal blood pressure raises the suspicion of a secondary form of hypertension, as does the onset of hypertension in a young person (< 25 years) or an older person (> 55 years). However, these ages are arbitrary; with the increasing body mass index in young people, essential hypertension is now more commonly diagnosed in the third decade. And since systolic pressure increases throughout life, we can expect many older patients to develop essential hypertension.7 Indeed, current guidelines are urging us to pay more attention to systolic pressure than in the past.
WHAT IS THE PATIENT’S CARDIOVASCULAR RISK?
The relationship between blood pressure and risk of cardiovascular disease is linear, continuous, and independent of (though additive to) other risk factors.1 For people 40 to 70 years old, each increment of either 20 mm Hg in systolic blood pressure or 10 mm Hg in diastolic blood pressure doubles the risk of cardiovascular disease across the entire range from 115/75 to 185/115 mm Hg.1 If the patient smokes or has elevated cholesterol, other cardiovascular risk factors, or the metabolic syndrome, the risk is even higher.8
The usual goal of antihypertensive treatment is systolic pressure less than 140 mm Hg and diastolic pressure less than 90 mm Hg. However, the target is lower—less than 130/80 mm Hg—for those with diabetes9 or target organ damage such as heart failure or renal disease.1,10 Thus, it is important to try to detect these conditions in the evaluation of the hypertensive patient.
Another reason it is important is that reducing such risk sometimes calls for using (or avoiding) antihypertensive drugs that are likely to alter these factors. For example, the use of beta-blockers in patients with a low level of high-density lipoprotein cholesterol (HDL-C) can lower HDL-C further.11
DOES THE PATIENT HAVE TARGET ORGAN DAMAGE?
Target organ damage is very important to detect because it changes the goal of treatment from primary prevention of adverse target organ outcomes into the more challenging realm of secondary prevention. For example, if a patient has had a stroke, his or her chance of having another stroke in the next 5 years is about 20%. This is much higher than the risk in an average hypertensive patient without such a history. For such patients, the current guidelines1 recommend the combination of a diuretic and an angiotensin-converting enzyme inhibitor, a combination shown to reduce the risk of a second stroke.12 Thus, we need to discover whether the patient had a stroke in the first place.
HISTORY
- The duration (if known) and severity of the hypertension
- The degree of blood pressure fluctuation
- Concomitant medical conditions, especially cardiovascular or renal problems
- Dietary habits
- Alcohol consumption
- Tobacco use
- Level of physical activity
- A family history of hypertension, renal disease, cardiovascular problems, or diabetes mellitus
- Past medications, with particular attention to their side effects and their efficacy in controlling blood pressure
- Current medications, including over-the-counter preparations. One reason: non-steroidal anti-inflammatory drugs other than aspirin can decrease the efficacy of antihypertensive drugs, presumably through mechanisms that inhibit the effects of vasodilatory and natriuretic prostaglandins and potentiate those of angiotensin II.13
PHYSICAL EXAMINATION
The physical examination starts with measurement of height, weight, waist circumference, and blood pressure—in both arms and the leg if coarctation of the aorta is suspected. Measurements with the patient supine, sitting, and standing are usually taken at the first visit, though such an approach is more suited to a hypertension specialty clinic than a primary care setting, in which time constraints usually limit the blood pressure readings to two or three seated values. Most prospective data on the benefits of hypertension treatment are based on a seated blood pressure, so we favor that measurement for follow-up.
Special attention in the physical examination is directed to:
The retina (to assess the vascular impact of the high blood pressure). Look for arteriolar narrowing (grade 1), arteriovenous compression (grade 2), hemorrhages or exudates (grade 3), and papilledema.2 Such findings not only relate to severity (higher grade = more severe blood pressure) but also predict future cardiovascular disease.14
The blood vessels. Bruits in the neck may indicate carotid stenosis, bruits in the abdomen may indicate renovascular disease, and femoral bruits are a sign of general atherosclerosis. Bruits also signal vascular stenosis and irregularity and may be a clue to vascular damage or future loss of target organ function. However, bruits may simply result from vascular tortuosity, particularly with significant flow in the vessel.
Also check the femoral pulses: poor or delayed femoral pulses are a sign of aortic coarctation. The radial artery is about as far away from the heart as the femoral artery; consequently, when palpating both sites simultaneously the pulse should arrive at about the same moment. In aortic coarctation, a palpable delay in the arrival of the femoral pulse may occur, and an interscapular murmur may be heard during auscultation of the back. In these instances, a low leg blood pressure (usually measured by placing a thigh-sized adult cuff on the patient’s thigh and listening over the popliteal area with the patient prone) may confirm the presence of aortic obstruction. When taking a leg blood pressure, the large cuff and the amount of pressure necessary to occlude the artery may be uncomfortable, and one should warn the patient about the discomfort before taking the measurement.
Poor or absent pedal pulses are a sign of peripheral arterial disease.
The heart (to detect gallops, enlargement, or both). Palpation may reveal a displaced apical impulse, which can indicate left ventricular enlargement. A sustained apical impulse may indicate left ventricular hypertrophy. Listen for a fourth heart sound (S4), one of the earliest physical findings of hypertension when physical findings are present. An S4 indicates that the left atrium is working hard to overcome the stiffness of the left ventricle. An S3 indicates an impairment in left ventricular function and is usually a harbinger of underlying heart disease. In some cases, lung rales can also be heard, though the combination of an S3 gallop and rales is an unusual office presentation in the early management of the hypertensive patient.
The lungs. Listen for rales (see above).
The lower extremities should be examined for peripheral arterial pulsations and edema. The loss of pedal pulses is a common finding, particularly in smokers, and is a clue to increased cardiovascular risk.
Strength, gait, and cognition. Perform a brief neurologic examination for evidence of remote stroke. We usually observe our patients’ gait as they enter or leave the examination room, test their bilateral grip strength, and assess their judgment, speech, and memory during the history and physical examination.
A great deal of research has linked high blood pressure to future loss of cognitive function,15 and it is useful to know that impairment is present before beginning treatment, since some patients will complain of memory loss after starting antihypertensive drug treatment.
LABORATORY EVALUATION
Routine tests
The routine evaluation of hypertensive patients should include, at a minimum:
- A hemoglobin or hematocrit measurement
- Urinalysis with microscopic examination
- Serum electrolyte concentrations
- Serum glucose concentration
- A fasting lipid profile
- A 12-lead electrocardiogram (Table 5).
Nonroutine tests
In some cases, other studies may be appropriate, depending on the clinical situation, eg:
- Serum uric acid in those with a history of gout, since some antihypertensive drugs (eg, diuretics) may increase serum uric acid and predispose to further episodes of gout
- Serum calcium in those with a personal or family history of kidney stones, to detect subtle parathyroid excess
- Thyroid-stimulating hormone or other thyroid studies if the history suggests thyroid excess, or if a thyroid nodule is discovered
- Limited echocardiography, which is more sensitive than electrocardiography for detecting left ventricular hypertrophy.
We sometimes use echocardiography if the patient is overweight but seems motivated to lose weight. In these cases we might not start drug therapy right away, choosing rather to wait and see if the patient can lose some weight (which might lower the blood pressure and make drug therapy unnecessary)—but only if the echocardiogram shows that he or she does not have left ventricular hypertrophy.
We also use echocardiography in patients with white-coat hypertension (see below), in whom office pressures are consistently high but whom we have elected to either not treat or not alter treatment. In these cases the echocardiogram serves as a “second opinion” about the merits of not altering therapy and supports this decision when the left ventricular wall thicknesses are normal (and remain so during long-term follow-up). In cases of suspected white-coat hypertension, home or ambulatory blood pressure monitoring is valuable to establish or exclude this diagnosis.1
Urinary albumin excretion. Microalbuminuria is an early manifestation of diabetic nephropathy and hypertension. Although routine urine screening for microalbuminuria is typically done in the management of diabetes, it is still not considered a standard of care, though the growing literature on its role as a cardiovascular risk predictor16–18 and its value as a therapeutic target in diabetes19,20 make it an attractive aid in the overall assessment of patients with hypertension.
Plasma renin activity and serum aldosterone concentrations are useful in screening for aldosterone excess, but are usually reserved as follow-up tests in patients with either hypokalemia or failure to achieve blood pressure control on a three-drug regimen in which at least one drug is a diuretic.1,21
Of note, primary aldosteronism is not as rare as previously thought. In a study of patients referred to hypertension centers, 11% had primary aldosteronism according to prospective diagnostic criteria, almost 5% had curable aldosterone-producing adenomas, and 6% had idiopathic hyperaldosteronism.22
If secondary hypertension is suspected
A search for secondary forms of hypertension is usually considered in patients with moderate or severe hypertension that does not respond to antihypertensive agents. Another situation is in hypertensive patients younger than 25 years, since curable forms of hypertension are more common in this age group. In older patients, the prevalence of secondary hypertension is lower and does not justify the costs and effort of routine elaborate workups unless there is evidence from the history, physical examination, or routine laboratory work for suspecting its presence. An exception to this rule is the need to exclude atherosclerotic renovascular hypertension in an elderly patient. This cause of secondary hypertension is common in the elderly and may be amenable to therapeutic intervention.26
WHEN TO CONSIDER HOME OR AMBULATORY MONITORING
Suspected white-coat hypertension
Blood pressure can be influenced by an environment such as an office or hospital clinic. This has led to the development of ambulatory blood pressure monitors and more use of self-measurement of blood pressure in the home. Blood pressure readings with these techniques are generally lower than those measured in an office or hospital clinic. These methods make it possible to screen for white-coat hypertension. In 10% to 20% of people with hypertensive readings, the blood pressure may be elevated persistently only in the presence of a physician.28 When measured elsewhere, including at work, the blood pressure is not elevated in those with the white-coat effect. Although this response may become less prominent with repeated measurements, it occasionally persists in the office setting, sometimes for years in our experience.
Suspected nocturnal hypertension (’nondipping’ status)
Ambulatory blood pressure is also helpful to screen for nocturnal hypertension. Evidence is accumulating to suggest that hypertensive patients whose pressure remains relatively high at night (“nondippers,” ie, those with less than a 10% reduction at night compared with daytime blood pressure readings) are at greater risk of cardiovascular morbidity than “dippers” (those whose blood pressure is at least 10% lower at night than during the day).29
An early morning surge
Ambulatory monitoring can also detect morning surges in systolic blood pressure,30 a marker of cerebrovascular risk. Generally, these patients have an increase of more than 55 mm Hg in systolic pressure between their sleeping and early-hour waking values, and we may wish to start or alter treatment specifically to address these high morning systolic values.31
‘PIPESTEM’ VESSELS AND PSEUDOHYPERTENSION
Occasionally, one encounters patients with vessels that are stiff and difficult to compress. If the pressure required to compress the brachial artery and stop audible blood flow with a standard blood pressure cuff is greater than the actual blood pressure within the artery as measured invasively, the condition is called pseudohypertension. The stiffness is thought to be due to calcification of the arterial wall.
A way to check for this condition is to inflate the cuff to at least 30 mm Hg above the palpable systolic pressure and then try to “roll” the brachial or radial artery underneath your fingertips, a procedure known as Osler’s maneuver.32 If you feel something that resembles a stiff tube reminiscent of the stem of a tobacco smoker’s pipe (healthy arteries are not palpable when empty), the patient may have pseudohypertension. However, the specificity of Osler’s maneuver has been questioned, particularly in hospitalized elderly patients.33
Pseudohypertension is important because the patients in whom it occurs, usually the elderly or the chronically ill (with diabetes or chronic kidney disease), are prone to orthostatic or postural hypotension, which may be aggravated by increasing their antihypertensive treatment on the basis of a cuff pressure that is actually much higher than the real blood pressure.33
How extensive a workup does a patient with high blood pressure need?
On one hand, we would not want to start therapy on the basis of a single elevated reading, as blood pressure fluctuates considerably during the day, and even experienced physicians often make errors in taking blood pressure that tend to falsely elevate the patient’s readings. Similarly, we would not want to miss the diagnosis of a potentially curable cause of hypertension or of a condition that increases a patient’s risk of cardiovascular disease. But considering that nearly one-third of adults in the United States have hypertension and that another one-fourth have prehypertension (formerly called high-normal blood pressure),1 if we were to launch an intensive workup for every patient with high blood pressure, the cost and effort would be enormous.
Fortunately, for most patients, it is enough to measure blood pressure accurately and repeatedly, perform a focused history and physical examination, and obtain the results of a few basic laboratory tests and an electrocardiogram, with additional tests in special cases.
In this review we address four fundamental questions in the evaluation of patients with a high blood pressure reading, and how to answer them.
ANSWERING FOUR QUESTIONS
The goal of the hypertension evaluation is to answer four questions:
- Does the patient have sustained hypertension? And if so—
- Is the hypertension primary or secondary?
- Does the patient have other cardiovascular risk factors?
- Does he or she have evidence of target organ damage?
DOES THE PATIENT HAVE SUSTAINED HYPERTENSION?
It is important to measure blood pressure accurately, for several reasons. A diagnosis of hypertension has a measurable impact on the patient’s quality of life.2 Furthermore, we want to avoid undertaking a full evaluation of hypertension if the patient doesn’t actually have high blood pressure, ie, systolic blood pressure greater than 140 mm Hg or diastolic pressure greater than 90 mm Hg. However, many people have blood pressures in the prehypertensive range (ie, 120–139 mm Hg systolic; 80–89 mm Hg diastolic). Many people in this latter group can expect to develop hypertension in time, as the prevalence of hypertension increases steadily with age unless effective preventive measures are implemented, such as losing weight, exercising regularly, and avoiding excessive consumption of sodium and alcohol.
The best position to use is sitting, as the Framingham Heart Study and most randomized clinical trials that established the value of treating hypertension used this position for diagnosis and follow-up.6
Proper patient positioning, the correct cuff size, calibrated equipment, and good inflation and deflation technique will yield the best assessment of blood pressure levels. But even if your technique is perfect, blood pressure is a dynamic vital sign, so it is necessary to repeat the measurement, average the values for any particular day, and keep in mind that the pressure is higher (or lower) on some days than on others, so that the running average is more important than individual readings. This leads to two final points about blood pressure measurement:
- Take it right, at least two times on any occasion
- Take it on at least two (preferably three) separate days.
Following up on blood pressure
After measuring the blood pressure, it is necessary to plan for follow-up readings, guided by both the blood pressure levels (Table 2) and your clinical judgment.
If the systolic and diastolic blood pressures fall into different categories, you should follow the recommendations for the shorter follow-up time.
IS THE HYPERTENSION PRIMARY OR SECONDARY?
Most patients with hypertension have primary (“essential”) hypertension and are likely to remain hypertensive for life. However, some have secondary hypertension, ie, high blood pressure due to an identifiable cause. Some of these conditions (and the hypertension that they cause) can be cured. For example, pheochromocytoma can be cured if found and removed. Other causes of secondary hypertension, such as parenchymal renal disease, are infrequently cured, and the goal is usually to control the blood pressure with drugs.
The sudden onset of severe hypertension in a patient previously known to have had normal blood pressure raises the suspicion of a secondary form of hypertension, as does the onset of hypertension in a young person (< 25 years) or an older person (> 55 years). However, these ages are arbitrary; with the increasing body mass index in young people, essential hypertension is now more commonly diagnosed in the third decade. And since systolic pressure increases throughout life, we can expect many older patients to develop essential hypertension.7 Indeed, current guidelines are urging us to pay more attention to systolic pressure than in the past.
WHAT IS THE PATIENT’S CARDIOVASCULAR RISK?
The relationship between blood pressure and risk of cardiovascular disease is linear, continuous, and independent of (though additive to) other risk factors.1 For people 40 to 70 years old, each increment of either 20 mm Hg in systolic blood pressure or 10 mm Hg in diastolic blood pressure doubles the risk of cardiovascular disease across the entire range from 115/75 to 185/115 mm Hg.1 If the patient smokes or has elevated cholesterol, other cardiovascular risk factors, or the metabolic syndrome, the risk is even higher.8
The usual goal of antihypertensive treatment is systolic pressure less than 140 mm Hg and diastolic pressure less than 90 mm Hg. However, the target is lower—less than 130/80 mm Hg—for those with diabetes9 or target organ damage such as heart failure or renal disease.1,10 Thus, it is important to try to detect these conditions in the evaluation of the hypertensive patient.
Another reason it is important is that reducing such risk sometimes calls for using (or avoiding) antihypertensive drugs that are likely to alter these factors. For example, the use of beta-blockers in patients with a low level of high-density lipoprotein cholesterol (HDL-C) can lower HDL-C further.11
DOES THE PATIENT HAVE TARGET ORGAN DAMAGE?
Target organ damage is very important to detect because it changes the goal of treatment from primary prevention of adverse target organ outcomes into the more challenging realm of secondary prevention. For example, if a patient has had a stroke, his or her chance of having another stroke in the next 5 years is about 20%. This is much higher than the risk in an average hypertensive patient without such a history. For such patients, the current guidelines1 recommend the combination of a diuretic and an angiotensin-converting enzyme inhibitor, a combination shown to reduce the risk of a second stroke.12 Thus, we need to discover whether the patient had a stroke in the first place.
HISTORY
- The duration (if known) and severity of the hypertension
- The degree of blood pressure fluctuation
- Concomitant medical conditions, especially cardiovascular or renal problems
- Dietary habits
- Alcohol consumption
- Tobacco use
- Level of physical activity
- A family history of hypertension, renal disease, cardiovascular problems, or diabetes mellitus
- Past medications, with particular attention to their side effects and their efficacy in controlling blood pressure
- Current medications, including over-the-counter preparations. One reason: non-steroidal anti-inflammatory drugs other than aspirin can decrease the efficacy of antihypertensive drugs, presumably through mechanisms that inhibit the effects of vasodilatory and natriuretic prostaglandins and potentiate those of angiotensin II.13
PHYSICAL EXAMINATION
The physical examination starts with measurement of height, weight, waist circumference, and blood pressure—in both arms and the leg if coarctation of the aorta is suspected. Measurements with the patient supine, sitting, and standing are usually taken at the first visit, though such an approach is more suited to a hypertension specialty clinic than a primary care setting, in which time constraints usually limit the blood pressure readings to two or three seated values. Most prospective data on the benefits of hypertension treatment are based on a seated blood pressure, so we favor that measurement for follow-up.
Special attention in the physical examination is directed to:
The retina (to assess the vascular impact of the high blood pressure). Look for arteriolar narrowing (grade 1), arteriovenous compression (grade 2), hemorrhages or exudates (grade 3), and papilledema.2 Such findings not only relate to severity (higher grade = more severe blood pressure) but also predict future cardiovascular disease.14
The blood vessels. Bruits in the neck may indicate carotid stenosis, bruits in the abdomen may indicate renovascular disease, and femoral bruits are a sign of general atherosclerosis. Bruits also signal vascular stenosis and irregularity and may be a clue to vascular damage or future loss of target organ function. However, bruits may simply result from vascular tortuosity, particularly with significant flow in the vessel.
Also check the femoral pulses: poor or delayed femoral pulses are a sign of aortic coarctation. The radial artery is about as far away from the heart as the femoral artery; consequently, when palpating both sites simultaneously the pulse should arrive at about the same moment. In aortic coarctation, a palpable delay in the arrival of the femoral pulse may occur, and an interscapular murmur may be heard during auscultation of the back. In these instances, a low leg blood pressure (usually measured by placing a thigh-sized adult cuff on the patient’s thigh and listening over the popliteal area with the patient prone) may confirm the presence of aortic obstruction. When taking a leg blood pressure, the large cuff and the amount of pressure necessary to occlude the artery may be uncomfortable, and one should warn the patient about the discomfort before taking the measurement.
Poor or absent pedal pulses are a sign of peripheral arterial disease.
The heart (to detect gallops, enlargement, or both). Palpation may reveal a displaced apical impulse, which can indicate left ventricular enlargement. A sustained apical impulse may indicate left ventricular hypertrophy. Listen for a fourth heart sound (S4), one of the earliest physical findings of hypertension when physical findings are present. An S4 indicates that the left atrium is working hard to overcome the stiffness of the left ventricle. An S3 indicates an impairment in left ventricular function and is usually a harbinger of underlying heart disease. In some cases, lung rales can also be heard, though the combination of an S3 gallop and rales is an unusual office presentation in the early management of the hypertensive patient.
The lungs. Listen for rales (see above).
The lower extremities should be examined for peripheral arterial pulsations and edema. The loss of pedal pulses is a common finding, particularly in smokers, and is a clue to increased cardiovascular risk.
Strength, gait, and cognition. Perform a brief neurologic examination for evidence of remote stroke. We usually observe our patients’ gait as they enter or leave the examination room, test their bilateral grip strength, and assess their judgment, speech, and memory during the history and physical examination.
A great deal of research has linked high blood pressure to future loss of cognitive function,15 and it is useful to know that impairment is present before beginning treatment, since some patients will complain of memory loss after starting antihypertensive drug treatment.
LABORATORY EVALUATION
Routine tests
The routine evaluation of hypertensive patients should include, at a minimum:
- A hemoglobin or hematocrit measurement
- Urinalysis with microscopic examination
- Serum electrolyte concentrations
- Serum glucose concentration
- A fasting lipid profile
- A 12-lead electrocardiogram (Table 5).
Nonroutine tests
In some cases, other studies may be appropriate, depending on the clinical situation, eg:
- Serum uric acid in those with a history of gout, since some antihypertensive drugs (eg, diuretics) may increase serum uric acid and predispose to further episodes of gout
- Serum calcium in those with a personal or family history of kidney stones, to detect subtle parathyroid excess
- Thyroid-stimulating hormone or other thyroid studies if the history suggests thyroid excess, or if a thyroid nodule is discovered
- Limited echocardiography, which is more sensitive than electrocardiography for detecting left ventricular hypertrophy.
We sometimes use echocardiography if the patient is overweight but seems motivated to lose weight. In these cases we might not start drug therapy right away, choosing rather to wait and see if the patient can lose some weight (which might lower the blood pressure and make drug therapy unnecessary)—but only if the echocardiogram shows that he or she does not have left ventricular hypertrophy.
We also use echocardiography in patients with white-coat hypertension (see below), in whom office pressures are consistently high but whom we have elected to either not treat or not alter treatment. In these cases the echocardiogram serves as a “second opinion” about the merits of not altering therapy and supports this decision when the left ventricular wall thicknesses are normal (and remain so during long-term follow-up). In cases of suspected white-coat hypertension, home or ambulatory blood pressure monitoring is valuable to establish or exclude this diagnosis.1
Urinary albumin excretion. Microalbuminuria is an early manifestation of diabetic nephropathy and hypertension. Although routine urine screening for microalbuminuria is typically done in the management of diabetes, it is still not considered a standard of care, though the growing literature on its role as a cardiovascular risk predictor16–18 and its value as a therapeutic target in diabetes19,20 make it an attractive aid in the overall assessment of patients with hypertension.
Plasma renin activity and serum aldosterone concentrations are useful in screening for aldosterone excess, but are usually reserved as follow-up tests in patients with either hypokalemia or failure to achieve blood pressure control on a three-drug regimen in which at least one drug is a diuretic.1,21
Of note, primary aldosteronism is not as rare as previously thought. In a study of patients referred to hypertension centers, 11% had primary aldosteronism according to prospective diagnostic criteria, almost 5% had curable aldosterone-producing adenomas, and 6% had idiopathic hyperaldosteronism.22
If secondary hypertension is suspected
A search for secondary forms of hypertension is usually considered in patients with moderate or severe hypertension that does not respond to antihypertensive agents. Another situation is in hypertensive patients younger than 25 years, since curable forms of hypertension are more common in this age group. In older patients, the prevalence of secondary hypertension is lower and does not justify the costs and effort of routine elaborate workups unless there is evidence from the history, physical examination, or routine laboratory work for suspecting its presence. An exception to this rule is the need to exclude atherosclerotic renovascular hypertension in an elderly patient. This cause of secondary hypertension is common in the elderly and may be amenable to therapeutic intervention.26
WHEN TO CONSIDER HOME OR AMBULATORY MONITORING
Suspected white-coat hypertension
Blood pressure can be influenced by an environment such as an office or hospital clinic. This has led to the development of ambulatory blood pressure monitors and more use of self-measurement of blood pressure in the home. Blood pressure readings with these techniques are generally lower than those measured in an office or hospital clinic. These methods make it possible to screen for white-coat hypertension. In 10% to 20% of people with hypertensive readings, the blood pressure may be elevated persistently only in the presence of a physician.28 When measured elsewhere, including at work, the blood pressure is not elevated in those with the white-coat effect. Although this response may become less prominent with repeated measurements, it occasionally persists in the office setting, sometimes for years in our experience.
Suspected nocturnal hypertension (’nondipping’ status)
Ambulatory blood pressure is also helpful to screen for nocturnal hypertension. Evidence is accumulating to suggest that hypertensive patients whose pressure remains relatively high at night (“nondippers,” ie, those with less than a 10% reduction at night compared with daytime blood pressure readings) are at greater risk of cardiovascular morbidity than “dippers” (those whose blood pressure is at least 10% lower at night than during the day).29
An early morning surge
Ambulatory monitoring can also detect morning surges in systolic blood pressure,30 a marker of cerebrovascular risk. Generally, these patients have an increase of more than 55 mm Hg in systolic pressure between their sleeping and early-hour waking values, and we may wish to start or alter treatment specifically to address these high morning systolic values.31
‘PIPESTEM’ VESSELS AND PSEUDOHYPERTENSION
Occasionally, one encounters patients with vessels that are stiff and difficult to compress. If the pressure required to compress the brachial artery and stop audible blood flow with a standard blood pressure cuff is greater than the actual blood pressure within the artery as measured invasively, the condition is called pseudohypertension. The stiffness is thought to be due to calcification of the arterial wall.
A way to check for this condition is to inflate the cuff to at least 30 mm Hg above the palpable systolic pressure and then try to “roll” the brachial or radial artery underneath your fingertips, a procedure known as Osler’s maneuver.32 If you feel something that resembles a stiff tube reminiscent of the stem of a tobacco smoker’s pipe (healthy arteries are not palpable when empty), the patient may have pseudohypertension. However, the specificity of Osler’s maneuver has been questioned, particularly in hospitalized elderly patients.33
Pseudohypertension is important because the patients in whom it occurs, usually the elderly or the chronically ill (with diabetes or chronic kidney disease), are prone to orthostatic or postural hypotension, which may be aggravated by increasing their antihypertensive treatment on the basis of a cuff pressure that is actually much higher than the real blood pressure.33
- Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- Wenger NK. Quality of life issues in hypertension: consequences of diagnosis and considerations in management. Am Heart J 1988; 116:628–632.
- McFadden CB, Townsend RR. Blood pressure measurement: common pitfalls and how to avoid them. Consultant 2003; 43:161–165.
- Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 2005; 111:697–716.
- Myers MG. Automated blood pressure measurement in routine clinical practice. Blood Press Monit 2006; 11:59–62.
- Mosenkis A, Townsend RR. Sitting on the evidence: what is the proper patient position for the office measurement of blood pressure? J Clin Hypertens (Greenwich) 2005; 7:365–366.
- Vasan RS, Beiser A, Seshadri S, et al. Residual lifetime risk for developing hypertension in middle-aged women and men: The Framingham Heart Study. JAMA 2002; 287:1003–1010.
- Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol 2004; 44:720–732.
- American Diabetes Association. Treatment of hypertension in adults with diabetes. Diabetes Care 2002; 25:199–201.
- Rosendorff C, Black HR, Cannon CP, et al. Treatment of hypertension in the prevention and management of ischemic heart disease: a scientific statement from the American Heart Association Council for High Blood Pressure Research and the Councils on Clinical Cardiology and Epidemiology and Prevention. Circulation 2007; 115:2761–2788.
- Papadakis JA, Mikhailidis DP, Vrentzos GE, Kalikaki A, Kazakou I, Ganotakis ES. Effect of antihypertensive treatment on plasma fibrinogen and serum HDL levels in patients with essential hypertension. Clin Appl Thromb Hemost 2005; 11:139–146.
- PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001; 358:1033–1041.
- Fierro-Carrion GA, Ram CV. Nonsteroidal anti-inflammatory drugs (NSAIDs) and blood pressure. Am J Cardiol 1997; 80:775–776.
- Wong TY, McIntosh R. Hypertensive retinopathy signs as risk indicators of cardiovascular morbidity and mortality. Br Med Bull 2005; 73–74:57–70.
- Forette F, Boller F. Hypertension and the risk of dementia in the elderly. Am J Med 1991; 90:14S–19S.
- Schrader J, Luders S, Kulschewski A, et al. Microalbuminuria and tubular proteinuria as risk predictors of cardiovascular morbidity and mortality in essential hypertension: final results of a prospective long-term study (MARPLE Study). J Hypertens 2006; 24:541–548.
- Luque M, de Rivas B, Alvarez B, Garcia G, Fernandez C, Martell N. Influence of target organ lesion detection (assessment of microalbuminuria and echocardiogram) in cardiovascular risk stratification and treatment of untreated hypertensive patients. J Hum Hypertens 2006; 20:187–192.
- Pontremoli R, Leoncini G, Viazzi F, et al. Role of microalbuminuria in the assessment of cardiovascular risk in essential hypertension. J Am Soc Nephrol 2005; 16 suppl 1:S39–S41.
- Erdmann E. Microalbuminuria as a marker of cardiovascular risk in patients with type 2 diabetes. Int J Cardiol 2006; 107:147–153.
- Bakris GL, Sowers JR. Microalbuminuria in diabetes: focus on cardiovascular and renal risk reduction. Curr Diab Rep 2002; 2:258–262.
- Gallay BJ, Ahmad S, Xu L, Toivola B, Davidson RC. Screening for primary aldosteronism without discontinuing hypertensive medications: plasma aldosteronerenin ratio. Am J Kidney Dis 2001; 37:699–705.
- Rossi GP, Bernini G, Caliumi C, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol 2006; 48:2293–2300.
- Onusko E. Diagnosing secondary hypertension. Am Fam Physician 2003; 67:67–74.
- Aurell M. Screening for secondary hypertension. Curr Hypertens Rep 1999; 1:461.
- Garovic VD, Kane GC, Schwartz GL. Renovascular hypertension: balancing the controversies in diagnosis and treatment. Cleve Clin J Med 2005; 72:1135–1137.
- Textor SC. Renovascular hypertension in 2007: where are we now? Curr Cardiol Rep 2007; 9:453–461.
- Pickering TG, Shimbo D, Haas D. Ambulatory blood-pressure monitoring. N Engl J Med 2006; 354:2368–2374.
- Angeli F, Verdecchia P, Gattobigio R, Sardone M, Reboldi G. White-coat hypertension in adults. Blood Press Monit 2005; 10:301–305.
- Cicconetti P, Morelli S, De Serra C, et al. Left ventricular mass in dippers and nondippers with newly diagnosed hypertension. Angiology 2003; 54:661–669.
- Kario K, Pickering TG, Umeda Y, et al. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation 2003; 107:1401–1406.
- Katakam R, Townsend RR. Morning surges in blood pressure. J Clin Hypertens 2006; 8:450–451.
- Messerli FH. Osler’s maneuver, pseudohypertension, and true hypertension in the elderly. Am J Med 1986; 80:906–910.
- Belmin J, Visintin JM, Salvatore R, Sebban C, Moulias R. Osler’s maneuver: absence of usefulness for the detection of pseudohypertension in an elderly population. Am J Med 1995; 98:42–49.
- Messerli FH, Ventura HO, Amodeo C. Osler’s maneuver and pseudohypertension. N Engl J Med 1985; 312:1548–1551.
- Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- Wenger NK. Quality of life issues in hypertension: consequences of diagnosis and considerations in management. Am Heart J 1988; 116:628–632.
- McFadden CB, Townsend RR. Blood pressure measurement: common pitfalls and how to avoid them. Consultant 2003; 43:161–165.
- Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 2005; 111:697–716.
- Myers MG. Automated blood pressure measurement in routine clinical practice. Blood Press Monit 2006; 11:59–62.
- Mosenkis A, Townsend RR. Sitting on the evidence: what is the proper patient position for the office measurement of blood pressure? J Clin Hypertens (Greenwich) 2005; 7:365–366.
- Vasan RS, Beiser A, Seshadri S, et al. Residual lifetime risk for developing hypertension in middle-aged women and men: The Framingham Heart Study. JAMA 2002; 287:1003–1010.
- Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol 2004; 44:720–732.
- American Diabetes Association. Treatment of hypertension in adults with diabetes. Diabetes Care 2002; 25:199–201.
- Rosendorff C, Black HR, Cannon CP, et al. Treatment of hypertension in the prevention and management of ischemic heart disease: a scientific statement from the American Heart Association Council for High Blood Pressure Research and the Councils on Clinical Cardiology and Epidemiology and Prevention. Circulation 2007; 115:2761–2788.
- Papadakis JA, Mikhailidis DP, Vrentzos GE, Kalikaki A, Kazakou I, Ganotakis ES. Effect of antihypertensive treatment on plasma fibrinogen and serum HDL levels in patients with essential hypertension. Clin Appl Thromb Hemost 2005; 11:139–146.
- PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001; 358:1033–1041.
- Fierro-Carrion GA, Ram CV. Nonsteroidal anti-inflammatory drugs (NSAIDs) and blood pressure. Am J Cardiol 1997; 80:775–776.
- Wong TY, McIntosh R. Hypertensive retinopathy signs as risk indicators of cardiovascular morbidity and mortality. Br Med Bull 2005; 73–74:57–70.
- Forette F, Boller F. Hypertension and the risk of dementia in the elderly. Am J Med 1991; 90:14S–19S.
- Schrader J, Luders S, Kulschewski A, et al. Microalbuminuria and tubular proteinuria as risk predictors of cardiovascular morbidity and mortality in essential hypertension: final results of a prospective long-term study (MARPLE Study). J Hypertens 2006; 24:541–548.
- Luque M, de Rivas B, Alvarez B, Garcia G, Fernandez C, Martell N. Influence of target organ lesion detection (assessment of microalbuminuria and echocardiogram) in cardiovascular risk stratification and treatment of untreated hypertensive patients. J Hum Hypertens 2006; 20:187–192.
- Pontremoli R, Leoncini G, Viazzi F, et al. Role of microalbuminuria in the assessment of cardiovascular risk in essential hypertension. J Am Soc Nephrol 2005; 16 suppl 1:S39–S41.
- Erdmann E. Microalbuminuria as a marker of cardiovascular risk in patients with type 2 diabetes. Int J Cardiol 2006; 107:147–153.
- Bakris GL, Sowers JR. Microalbuminuria in diabetes: focus on cardiovascular and renal risk reduction. Curr Diab Rep 2002; 2:258–262.
- Gallay BJ, Ahmad S, Xu L, Toivola B, Davidson RC. Screening for primary aldosteronism without discontinuing hypertensive medications: plasma aldosteronerenin ratio. Am J Kidney Dis 2001; 37:699–705.
- Rossi GP, Bernini G, Caliumi C, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol 2006; 48:2293–2300.
- Onusko E. Diagnosing secondary hypertension. Am Fam Physician 2003; 67:67–74.
- Aurell M. Screening for secondary hypertension. Curr Hypertens Rep 1999; 1:461.
- Garovic VD, Kane GC, Schwartz GL. Renovascular hypertension: balancing the controversies in diagnosis and treatment. Cleve Clin J Med 2005; 72:1135–1137.
- Textor SC. Renovascular hypertension in 2007: where are we now? Curr Cardiol Rep 2007; 9:453–461.
- Pickering TG, Shimbo D, Haas D. Ambulatory blood-pressure monitoring. N Engl J Med 2006; 354:2368–2374.
- Angeli F, Verdecchia P, Gattobigio R, Sardone M, Reboldi G. White-coat hypertension in adults. Blood Press Monit 2005; 10:301–305.
- Cicconetti P, Morelli S, De Serra C, et al. Left ventricular mass in dippers and nondippers with newly diagnosed hypertension. Angiology 2003; 54:661–669.
- Kario K, Pickering TG, Umeda Y, et al. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation 2003; 107:1401–1406.
- Katakam R, Townsend RR. Morning surges in blood pressure. J Clin Hypertens 2006; 8:450–451.
- Messerli FH. Osler’s maneuver, pseudohypertension, and true hypertension in the elderly. Am J Med 1986; 80:906–910.
- Belmin J, Visintin JM, Salvatore R, Sebban C, Moulias R. Osler’s maneuver: absence of usefulness for the detection of pseudohypertension in an elderly population. Am J Med 1995; 98:42–49.
- Messerli FH, Ventura HO, Amodeo C. Osler’s maneuver and pseudohypertension. N Engl J Med 1985; 312:1548–1551.
KEY POINTS
- To confirm the diagnosis of hypertension, multiple readings should be taken at various times.
- Proper technique is important in measuring blood pressure, including using the correct cuff size, having the patient sit quietly for 5 minutes before taking the pressure, and supporting the arm at the level of the heart.
- If white-coat hypertension is suspected, one can consider ambulatory or home blood pressure measurements to confirm that the hypertension is sustained.
Perioperative statins: More than lipid-lowering?
Soon, the checklist for internists seeing patients about to undergo surgery may include prescribing one of the lipid-lowering hydroxymethylglutaryl-CoA reductase inhibitors, also called statins.
Statins? Not long ago, we were debating whether patients who take statins should stop taking them before surgery, based on the manufacturers’ recommendations.1 The discussion, however, has changed to whether patients who have never received a statin should be started on one before surgery to provide immediate prophylaxis against cardiac morbidity, and how much harm long-term statin users face if these drugs are withheld perioperatively.
The evidence is still very preliminary and based mostly on studies in animals and retrospective studies in people. However, an expanding body of indirect evidence suggests that these drugs are beneficial in this situation.
In this review, we discuss the mechanisms by which statins may protect the heart in the short term, drawing on data from animal and human studies of acute myocardial infarction, and we review the current (albeit limited) data from the perioperative setting.
FEW INTERVENTIONS DECREASE RISK
Each year, approximately 50,000 patients suffer a perioperative cardiovascular event; the incidence of myocardial infarction during or after noncardiac surgery is 2% to 3%.2 The primary goal of preoperative cardiovascular risk assessment is to predict and avert these events.
But short of canceling surgery, few interventions have been found to reduce a patient’s risk. For example, a landmark study in 2004 cast doubt on the efficacy of preoperative coronary revascularization.3 Similarly, although early studies of beta-blockers were promising4,5 and although most internists prescribe these drugs before surgery, more recent studies have cast doubt on their efficacy, particularly in patients at low risk undergoing intermediate-risk (rather than vascular) surgery.6–8
This changing clinical landscape has prompted a search for new strategies for perioperative risk-reduction. Several recent studies have placed statins in the spotlight.
POTENTIAL MECHANISMS OF SHORT-TERM BENEFIT
Statins have been proven to save lives when used long-term, but how could this class of drugs, designed to prevent the accumulation of arterial plaques by lowering low-density lipoprotein cholesterol (LDL-C) levels, have any short-term impact on operative outcomes? Although LDL-C reduction is the principal mechanism of action of statins, not all of the benefit can be ascribed to this mechanism.9 The answer may lie in their “pleiotropic” effects—ie, actions other than LDL-C reduction.
The more immediate pleiotropic effects of statins in the proinflammatory and prothrombotic environment of the perioperative period are thought to include improved endothelial function (both antithrombotic function and vasomotor function in response to ischemic stress), enhanced stability of atherosclerotic plaques, decreased oxidative stress, and decreased vascular inflammation.10–12
EVIDENCE FROM ANIMAL STUDIES
Experiments in animals suggest that statins, given shortly before or after a cardiovascular event, confer benefit before any changes in LDL-C are measurable.
Lefer et al13 found that simvastatin (Zocor), given 18 hours before an ischemic episode in rats, blunted the inflammatory response in cardiac reperfusion injury. Not only was reperfusion injury significantly less in the hearts of the rats that received simvastatin than in the saline control group, but the simvastatin-treated hearts also expressed fewer neutrophil adhesion molecules such as P-selectin, and they had more basal release of nitric oxide, the potent endothelial-derived vasodilator with antithrombotic, anti-inflammatory, and antiproliferative effects.14 These results suggest that statins may improve endothelial function acutely, particularly during ischemic stress.
Osborne et al15 fed rabbits a cholesterol-rich diet plus either lovastatin (Mevacor) or placebo. After 2 weeks, the rabbits underwent either surgery to induce a myocardial infarction or a sham procedure. Regardless of the pretreatment, biopsies of the aorta did not reveal any atherosclerosis; yet the lovastatin-treated rabbits sustained less myocardial ischemic damage and they had more endothelium-mediated vasodilatation.
Statin therapy also may improve cerebral ischemia outcomes in animal models.14,16
Sironi et al16 induced strokes in rats by occluding the middle cerebral artery. The rats received either simvastatin or vehicle for 3 days before the stroke or immediately afterwards. Even though simvastatin did not have enough time to affect the total cholesterol level, rats treated with simvastatin had smaller infarcts (as measured by magnetic resonance imaging) and produced more nitric oxide.
Comment. Taken together, these studies offer tantalizing evidence that statins have short-term, beneficial nonlipid effects and may reduce not only the likelihood of an ischemic event, but—should one occur—the degree of tissue damage that ensues.
EFFECTS OF STATINS IN ACUTE CORONARY SYNDROME
The National Registry of Myocardial Infarction17 is a prospective, observational database of all patients with acute myocardial infarction admitted to 1,230 participating hospitals throughout the United States. In an analysis from this cohort, patients were divided into four groups: those receiving statins before and after admission, those receiving statins only before admission, those receiving statins only after admission, and those who never received statins.
Compared with those who never received statins, fewer patients who received them both before and after admission died while in the hospital (unadjusted odds ratio 0.23, 95% confidence interval [CI] 0.22–0.25), and the odds ratio for those who received statins for the first time was 0.31 (95% CI 0.29–0.33). Patients who stopped receiving a statin on admission were more likely to die than were patients who never received statins (odds ratio 1.09, 95% CI 1.03–1.15). These trends held true even when adjustments were made for potential confounding factors.
Comment. Unmeasured confounding factors (such as the inability to take pills due to altered mental status or the different practice styles of the providers who chose to discontinue statins) might have affected the results. Nevertheless, these results suggest that the protective effects of statins stop almost immediately when these drugs are discontinued, and that there may even be an adverse “rebound” effect when patients who have been taking these drugs for a long time stop taking them temporarily.
The Platelet Receptor Inhibition in Ischemic Syndrome Management trial,18 in a subgroup analysis, had nearly identical findings. In the main part of this trial, patients with coronary artery disease and chest pain at rest or accelerating pain in the last 24 hours were randomized to receive tirofiban (Aggrastat) or heparin. Complete data on statin use were available for 1,616 (50%) of the 3,232 patients in this trial, and the rate of the primary end point (death, myocardial infarction, or recurrent ischemia) was analyzed on the basis of statin therapy in this subgroup.
Comment. Together, these data lead to the conclusion that, when admitted for either acute myocardial infarction or acute coronary syndrome, patients already receiving statins should not have them stopped, and those who had not been receiving statins should receive them immediately. The safety of these medications in the acute setting appears excellent: in the Myocardial Ischemia Reduction With Acute Cholesterol Lowering (MIRACL)12 and the Pravastatin or Atorvastatin Evaluation and Infection Therapy (PROVE-IT)11 trials, fewer than 5% of statin-treated patients had transient elevations in transaminase levels, and no cases of rhabdomyolysis were reported.
PERIOPERATIVE STATIN STUDIES
The data on perioperative statin use are mostly observational and retrospective and fall into essentially four surgical categories: coronary artery bypass grafting (CABG), carotid endarterectomy,19,20 noncardiac vascular surgery, and major noncardiac surgery. Two meta-analyses have also evaluated the data.21,22 The only randomized controlled trial (performed by Durazzo et al23) was small and was carried out at a single center in vascular surgery patients, and the event rate was low.
Current recommendations from the National Cholesterol Education Program (NCEP)24 say that patients who need CABG, have peripheral arterial disease, have an abdominal aortic aneurysm, or have cerebrovascular disease should already be on a statin to achieve an LDL-C goal level of less than 100 mg/dL, with an optional goal of less than 70 mg/dL, independent of surgery.
Since not all patients who should be on statins are actually on them, questions arise:
- Is it important (and safe) to start statin treatment preoperatively?
- Will patients with cardiovascular risk factors but without known cardiovascular disease benefit from statins perioperatively?
Noncardiac vascular surgery
Multiple retrospective studies have evaluated the effect of statins in patients undergoing major noncardiac vascular surgery.25–32
Kertai et al25 evaluated 570 patients in Holland who underwent elective open surgery for infrarenal abdominal aortic aneurysms between 1991 and 2001, looking for an association between statin use and the incidence of perioperative death from myocardial infarction. Only 162 of the 570 patients had been on long-term statin therapy before the surgery. The use of statins was only one of many known baseline characteristics that were significantly different between the two groups, including age, body mass index, known coronary artery disease, and use of angiotensin-converting enzyme inhibitors and beta-blockers. In univariate analysis, statins appeared to be protective: 6 (3.7%) of the patients in the statin group died of a myocardial infarction, compared with 45 (11%) of those in the nostatin group. A multivariate analysis yielded similar findings, with an odds ratio of 0.24 (95% CI 0.11–0.54).
Ward et al27 performed a very similar retrospective study, with similar findings. In 446 patients who underwent surgery for infrarenal abdominal aortic aneurysm, statin therapy was associated with a significantly lower incidence of the combined end point of death, myocardial infarction, stroke, and major peripheral vascular complications, with an adjusted odds ratio of 0.36 (95% CI 0.14–0.93).
Poldermans et al26 noted similar findings in a case-control study of noncardiac vascular surgery patients. Statin users had a much lower perioperative risk of death than did nonusers, with an adjusted odds ratio of 0.22 (95% CI 0.10–0.47).
O’Neil-Callahan et al,28 in a cohort study, found that statin users had fewer perioperative cardiac complications, with an adjusted odds ratio of 0.49 (95% CI 0.28–0.84, P = .009).
Dogma of withdrawing statins before major surgery is challenged
Le Manach et al33 reviewed the outcomes for all patients of a single hospital in Paris who underwent nonemergency infrarenal aortic procedures between January 2001 and December 2004. In January 2004, the hospital instituted guidelines to ensure that patients on statins continue taking them up to the evening before surgery and that statins be restarted on the first postoperative day (via nasogastric tube if necessary). Before 2004, there had been no specific guidelines, and patients on statins did not receive them for a median of 4 days postoperatively. Types of procedures were similar during the two time periods, as were the rates of beta-blocker use, preoperative revascularization, venous thromboembolism prophylaxis, and perioperative blood pressure control. After surgery, topononin I levels were measured in all patients as surveillance for cardiac events, and were defined as elevated when greater than 0.2 ng/mL.
Compared with patients not on statins at all, those treated with statins continuously throughout the perioperative period (after January 2004) had a lower rate of elevated troponin (relative risk 0.38). In contrast, those who had their statins transiently discontinued perioperatively (prior to 2004) had troponin elevations more often than those who had never been treated (relative risk 2.1). This suggested an over fivefold risk reduction (P < .001) conferred by not discontinuing statins in the immediate postoperative period. This finding was maintained after multivariate adjustment: statin withdrawal was associated with a 2.9-fold (95% CI 1.6–5.5) increase in the risk of cardiac enzyme elevations postoperatively. No fewer deaths were noted, but the study was not powered to detect a mortality difference.
Comment. Although secular trends cannot be entirely discounted as contributing to these findings, the prompt increase in cardiac events after just 4 days of statin withdrawal adds to the growing body of evidence suggesting that statin discontinuation can have harmful acute effects. It also brings up the question: Can starting statins benefit patients in the same time period?
Should statins be started before vascular surgery?
Schouten et al32 evaluated the effects of newly started or continued statin treatment in patients undergoing major elective vascular surgery. Patients were screened before surgery and started on statins if they were not already receiving them and their total cholesterol levels were elevated; new users received the medication for about 40 days before surgery. Of the 981 screened patients, 44 (5%) were newly started on statins and 182 (19%) were continued on their therapy. Perioperative death or myocardial infarction occurred in 22 (8.8%) of the statin users and 111 (14.7%) of the nonusers, a statistically significant difference. Temporary discontinuation (median 1 day) of statins in this study due to the inability to take an oral medication did not appear to affect the likelihood of a myocardial infarction.
Durazzo et al23 performed a single-center, randomized, prospective, placebo-controlled, double-blind clinical trial of atorvastatin (Lipitor) 20 mg daily vs placebo in 100 patients undergoing noncardiac arterial vascular surgery. Patients were excluded if they had previously used medications to treat dyslipidemia, recently had a cardiovascular event, or had contraindications to statin treatment such as a baseline creatinine level greater than 2.0 mg/dL or severe hepatic disease. The intervention group received atorvastatin starting at least 2 weeks before surgery for a total of 45 days. Patients were then continued or started on a statin after surgery if their LDL-C level was greater than 100 mg/dL. Beta-blocker use was recommended “on the basis of current guidelines.”
One month after surgery, the LDL-C level was statistically significantly lower in the atorvastatin group. Since most patients did not continue or start statin therapy after the 45-day treatment period, the LDL-C levels were not statistically different at 3 and 6 months after surgery.
At 6 months, the rate of the primary end point (death from cardiovascular causes, nonfatal acute myocardial infarction, ischemic stroke, or unstable angina) was 26.0% in the placebo group and 8.0% in the atorvastatin group, a statistically significant difference. Three patients in the atorvastatin group had cardiac events in the first 10 days after surgery, compared with 11 patients in the placebo group. Thirteen of the 17 total cardiac events took place within 10 days after surgery.
One of the atorvastatin patients developed rhabdomyolysis and elevated aminotransferase levels.
Major noncardiac surgery
Lindenauer et al2 performed a retrospective cohort study of surgical patients who were at least 18 years old and survived beyond the second hospital day. Patients were divided into a group receiving any form of lipid-lowering treatment (of whom more than 90% were taking statins) and a group that had never never received a lipid-lowering drug or only started one on the third day of the hospitalization or later. The period of study was from January 1, 2000, to December 31, 2001.
In all, 780,591 patients from 329 hospitals throughout the United States were included, of whom only 77,082 (9.9%) received lipid-lowering therapy. Eight percent of the patients underwent vascular surgery. Not surprisingly, the treated patients were more likely to have a history of hypertension, diabetes, ischemic heart disease, or hyperlipidemia. They also were more likely to have a vascular procedure performed, to have two or more cardiac risk factors (high-risk surgery, ischemic heart disease, congestive heart failure, cerebrovascular disease, renal insufficiency, or diabetes mellitus), and to be treated with beta-blockers and angiotensin-converting enzyme inhibitors, but they were less likely to have high-risk and emergency surgery performed.
The primary end point, perioperative death, occurred in 2.13% of the treated patients and 3.05% of the nontreated group. Compared with the rate in a propensity-matched cohort, the odds ratio adjusted for unbalanced covariates was 0.62 (95% CI 0.58–0.67) in favor of lipid treatment. Stratification by cardiac risk index revealed a number needed to treat of 186 for those with no risk factors, 60 for those with two risk factors, and 30 for those with four or more risk factors.
Unfortunately, this analysis was not able to take into account whether and for how long patients were receiving lipid-lowering therapy before hospitalization. It therefore does not answer the questions of whether starting lipid-lowering therapy before surgery is beneficial or whether stopping it is harmful. It also does not shed light on whether perioperative lipid-lowering increases the risk of rhabdomyolysis or liver disease.
Carotid endarterectomy
Two recent retrospective cohort studies evaluated the outcomes in patients undergoing carotid endarterectomy.19,20
Kennedy et al19 found that patients on a statin at the time of admission who had symptomatic carotid disease had lower rates of inhospital death (adjusted odds ratio 0.24, 95% CI 0.06–0.91) and ischemic stroke or death (adjusted odds ratio 0.55, 95% CI 0.31–0.97). However, cardiac outcomes among these symptomatic patients were not significantly improved (odds ratio 0.82, 95% CI 0.45–1.50), nor was there benefit for asymptomatic patients, raising the possibility that the positive findings were due to chance or that patients at lower baseline risk for vascular events may have less benefit.
McGirt et al20 performed a similar study; they did not, however, distinguish whether patients had symptomatic vs asymptomatic carotid disease. The 30-day risk of perioperative stroke was lower in patients treated with a statin, with an odds ratio of 0.41 (95% CI 0.18–0.93); the odds ratio for death was 0.21 (95% CI 0.05–0.96). Cardiac outcomes were not significantly affected.
Coronary artery bypass graft surgery
According to the NCEP recommendations, nearly all patients undergoing CABG should already be on a statin before surgery since they all have known coronary artery disease. Multiple observational studies have offered confirmatory evidence that statins are beneficial in this setting.34–38
Liakopoulos et al39 evaluated whether the anti-inflammatory effects of statins may, in part, account for their beneficial effect in the perioperative period. The authors prospectively matched 18 patients who were taking statins and were referred for elective CABG with 18 patients who were not prescribed statins previously. The only major measured baseline characteristic that differed between the two groups was a statistically significantly lower LDL-C level in the statin group. The operative characteristics did not differ, and cytokine levels at baseline were similar.
Tumor necrosis factor alpha levels increased significantly in the control group but did not change significantly in the statin group. Interleukin 8 increased in both groups by a similar amount. Interleukin 6 (the major inducer of C-reactive protein) increased from baseline in both groups but did not increase nearly as much in the statin group as in the control group; the intergroup difference was statistically significant. The anti-inflammatory cytokine interleukin 10 increased minimally from baseline in the control group, while the statin group’s levels increased significantly above baseline and those of the control group.
Christenson40 also found that inflammatory markers were improved with pre-CABG statin treatment in a small randomized trial in which patients received simvastatin 20 mg 4 weeks prior to CABG surgery vs no statin. Interestingly, far fewer statin-treated patients developed thrombocytosis (platelet count > 400 × 109/L) than did control patients (3% vs 81%, P < .0001).
RISKS OF PERIOPERATIVE STATINS
The risks associated with statin therapy in general appear low, but specific perioperative risks have not been well studied.
Baigent et al,41 in a meta-analysis of randomized trials of nonperioperative statin therapy, found that rhabdomyolysis occurred in 9 (0.023%) of 39,884 patients receiving statins vs 6 (0.015%) of the 39,817 controls, with a number needed to harm of 12,500. Moreover, the rates of nonvascular death and cancer did not increase. It is plausible that the risk is somewhat greater in the perioperative setting but is likely not enough to outweigh the potential benefits, especially since the risk of ischemic vascular events is particularly high then.
Some of the perioperative studies cited above specifically addressed potential risks. For example, in the study by Schouten et al,32 mild creatine kinase elevations were more common in the statin-treated group, but the incidence of moderate and severe creatine kinase elevations did not differ significantly. No case of rhabdomyolysis occurred, and length of surgery was the only predictor of myopathy. MIRACL and PROVE-IT revealed similar safety profiles; aminotransferase levels normalized when statins were stopped, and no cases of rhabdomyolysis occurred.11,12 In the vascular surgery study by Durazzo et al,23 1 (2%) of the 50 atorvastatin-treated patients developed both rhabdomyolysis and elevated aminotransferase levels that prompted discontinuation of the statin.
Overall, the observational studies do not indicate that statin continuation or treatment is harmful in perioperative patients. However, these studies did not specifically evaluate patients with acute insults from surgery such as sepsis, renal failure, or hepatitis. It is unknown what effect statin therapy would have in those patients and whether statins should be selectively discontinued in patients who develop major hepatic, musculoskeletal, or renal complications after surgery.
OUR RECOMMENDATIONS
Before CABG or vascular surgery
Given the NCEP recommendations, existing primary and secondary prevention studies, observational studies of CABG and noncardiac vascular surgery patients, and the one randomized trial of vascular surgery patients, data support the use of statins in nearly all patients undergoing cardiac or vascular surgery. We advocate starting statins in the perioperative period to take advantage of their rapid-acting pleiotropic effects, and continuing them long-term to take advantage of their lipid-lowering effects. This recommendation is in line with the recently released American College of Cardiology/American Heart Association (ACC/AHA) 2007 perioperative guidelines that state “for patients undergoing vascular surgery with or without clinical risk factors, statin use is reasonable.”42
Although the ideal time to start statins is not certain, the study by Durazzo et al23 suggests that they should be started at least 2 weeks before surgery if possible. Moreover, patients already taking statins should definitely not have their statins discontinued if at all possible.
Before major nonvascular surgery
For patients undergoing major nonvascular (intermediate-risk) surgery, physicians should first ascertain if the patient has an indication for statin therapy based on current nonsurgical lipid level recommendations. However, even if there is no clear indication for statin therapy based on NCEP guidelines, we endorse the recently released ACC/AHA perioperative guidelines that state that statin therapy can be considered in patients with a risk factor who are undergoing intermediate-risk procedures. Moreover, we wholeheartedly support the ACC/AHA’s strongest recommendation that patients who are already receiving statins and are undergoing noncardiac surgery should not have their statins discontinued.
When to discontinue statins?
The risk of harm overall appears to be minimal and certainly less than the likelihood of benefit. It is reasonable to observe patients postoperatively for adverse clinical events that may increase the risk of perioperative statin treatment, such as acute renal failure, hepatic failure, or sepsis, but whether statins should be stopped in patients with these complications remains unknown; we advocate individualizing the decision.
More studies needed
We need more data on whether moderate-risk patients undergoing moderate-risk surgery benefit from perioperative statin therapy, when therapy should be started, whether therapy should be started on the day of surgery if it was not started earlier, which statin and what doses are optimal, how long therapy should be continued, and what degree of risk is associated with perioperative statin therapy.
Fortunately, important data should be forthcoming in the next few years: the Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography (DECREASE-IV) study43 is a 4-year two-by-two factorial placebo-controlled study evaluating the use of fluvastatin (Lescol) and bisoprolol (Zebeta, a beta-blocker) separately and together in patients who are older than 40 years, are undergoing elective noncardiac surgery, have an estimated risk of cardiovascular death of more than 1%, have not used statins previously, and do not have elevated cholesterol.
- Grant PJ, Kedia N. Should statins be discontinued preoperatively? IMPACT consults. Proceedings of the 2nd Annual Cleveland Clinic Perioperative Medicine Summit. Cleve Clin J Med 2006; 73 Electronic suppl 1:S9–S10.
- Lindenauer PK, Pekow P, Wang K, Gutierrez B, Benjamin EM. Lipid-lowering therapy and in-hospital mortality following major noncardiac surgery. JAMA 2004; 291:2092–2099.
- McFalls EO, Ward HB, Moritz TE, et al. Coronary-artery revascularization before elective major vascular surgery. N Engl J Med 2004; 351:2795–2804.
- Mangano DT, Layug EL, Wallace A, Tateo I. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter Study of Perioperative Ischemia Research Group. N Engl J Med 1996; 335:1713–1720.
- Poldermans D, Boersma E, Bax JJ, et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med 1999; 341:1789–1794.
- Brady AR, Gibbs JS, Greenhalgh RM, Powell JT, Sydes MR. Perioperative beta-blockade (POBBLE) for patients undergoing infrarenal vascular surgery: results of a randomized double-blind controlled trial. J Vasc Surg 2005; 41:602–609.
- Juul AB, Wetterslev J, Gluud C, et al. Effect of perioperative beta blockade in patients with diabetes undergoing major non-cardiac surgery: randomised placebo controlled, blinded multicentre trial. BMJ 2006; 332:1482.
- Yang H, Raymer K, Butler R, Parlow J, Roberts R. The effects of perioperative beta-blockade: results of the Metoprolol after Vascular Surgery (MaVS) study, a randomized controlled trial. Am Heart J 2006; 152:983–990.
- Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med 2005; 352:20–28.
- Ito MK, Talbert RL, Tsimikas S. Statin-associated pleiotropy: possible beneficial effects beyond cholesterol reduction. Pharmacotherapy 2006; 26:85S–97S.
- Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350:1495–1504.
- Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 2001; 285:1711–1718.
- Lefer AM, Campbell B, Shin YK, Scalia R, Hayward R, Lefer DJ. Simvastatin preserves the ischemic-reperfused myocardium in normocholesterolemic rat hearts. Circulation 1999; 100:178–184.
- Endres M, Laufs U, Liao JK, Moskowitz MA. Targeting eNOS for stroke protection. Trends Neurosci 2004; 27:283–289.
- Osborne JA, Lento PH, Siegfried MR, Stahl GL, Fusman B, Lefer AM. Cardiovascular effects of acute hypercholesterolemia in rabbits. Reversal with lovastatin treatment. J Clin Invest 1989; 83:465–473.
- Sironi L, Cimino M, Guerrini U, et al. Treatment with statins after induction of focal ischemia in rats reduces the extent of brain damage. Arterioscler Thromb Vasc Biol 2003; 23:322–327.
- Fonarow GC, Wright RS, Spencer FA, et al. Effect of statin use within the first 24 hours of admission for acute myocardial infarction on early morbidity and mortality. Am J Cardiol 2005; 96:611–616.
- Heeschen C, Hamm CW, Laufs U, Snapinn S, Bohm M, White HD. Withdrawal of statins increases event rates in patients with acute coronary syndromes. Circulation 2002; 105:1446–1452.
- Kennedy J, Quan H, Buchan AM, Ghali WA, Feasby TE. Statins are associated with better outcomes after carotid endarterectomy in symptomatic patients. Stroke 2005; 36:2072–2076.
- McGirt MJ, Perler BA, Brooke BS, et al. 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors reduce the risk of perioperative stroke and mortality after carotid endarterectomy. J Vasc Surg 2005; 42:829–836.
- Hindler K, Shaw AD, Samuels J, Fulton S, Collard CD, Riedel B. Improved postoperative outcomes associated with preoperative statin therapy. Anesthesiology 2006; 105:1260–1272.
- Kapoor AS, Kanji H, Buckingham J, Devereaux PJ, McAlister FA. Strength of evidence for perioperative use of statins to reduce cardiovascular risk: systematic review of controlled studies. BMJ 2006; 333:1149.
- Durazzo AE, Machado FS, Ikeoka DT, et al. Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial. J Vasc Surg 2004; 39:967–975.
- Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004; 110:227–239.
- Kertai MD, Boersma E, Westerhout CM, et al. A combination of statins and beta-blockers is independently associated with a reduction in the incidence of perioperative mortality and nonfatal myocardial infarction in patients undergoing abdominal aortic aneurysm surgery. Eur J Vasc Endovasc Surg 2004; 28:343–352.
- Poldermans D, Bax JJ, Kertai MD, et al. Statins are associated with a reduced incidence of perioperative mortality in patients undergoing major noncardiac vascular surgery. Circulation 2003; 107:1848–1851.
- Ward RP, Leeper NJ, Kirkpatrick JN, Lang RM, Sorrentino MJ, Williams KA. The effect of preoperative statin therapy on cardiovascular outcomes in patients undergoing infrainguinal vascular surgery. Int J Cardiol 2005; 104:264–268.
- O’Neil-Callahan K, Katsimaglis G, Tepper MR, et al. Statins decrease perioperative cardiac complications in patients undergoing non-cardiac vascular surgery: the Statins for Risk Reduction in Surgery (StaRRS) study. J Am Coll Cardiol 2005; 45:336–342.
- Abbruzzese TA, Havens J, Belkin M, et al. Statin therapy is associated with improved patency of autogenous infrainguinal bypass grafts. J Vasc Surg 2004; 39:1178–1185.
- Boersma E, Poldermans D, Bax JJ, et al. Predictors of cardiac events after major vascular surgery: role of clinical characteristics, dobutamine echocardiography, and beta-blocker therapy. JAMA 2001; 285:1865–1873.
- Landesberg G, Mosseri M, Wolf YG, et al. Preoperative thallium scanning, selective coronary revascularization, and long-term survival after major vascular surgery. Circulation 2003; 108:177–183.
- Schouten O, Kertai MD, Bax JJ, et al. Safety of perioperative statin use in high-risk patients undergoing major vascular surgery. Am J Cardiol 2005; 95:658–660.
- Le Manach Y, Godet G, Coriat P, et al. The impact of postoperative discontinuation or continuation of chronic statin therapy on cardiac outcome after major vascular surgery. Anesth Analg 2007; 104:1326–1333.
- Ali IS, Buth KJ. Preoperative statin use and outcomes following cardiac surgery. Int J Cardiol 2005; 103:12–18.
- Clark LL, Ikonomidis JS, Crawford FA, et al. Preoperative statin treatment is associated with reduced postoperative mortality and morbidity in patients undergoing cardiac surgery: an 8-year retrospective cohort study. J Thorac Cardiovasc Surg 2006; 131:679–685.
- Pan W, Pintar T, Anton J, Lee VV, Vaughn WK, Collard CD. Statins are associated with a reduced incidence of perioperative mortality after coronary artery bypass graft surgery. Circulation 2004; 110(suppl 2):II45–II49.
- Pascual DA, Arribas JM, Tornel PL, et al. Preoperative statin therapy and troponin T predict early complications of coronary artery surgery. Ann Thorac Surg 2006; 81:78–83.
- Dotani MI, Elnicki DM, Jain AC, Gibson CM. Effect of preoperative statin therapy and cardiac outcomes after coronary artery bypass grafting. Am J Cardiol 2000; 86:1128–1130.
- Liakopoulos OJ, Dorge H, Schmitto JD, Nagorsnik U, Grabedunkel J, Schoendube FA. Effects of preoperative statin therapy on cytokines after cardiac surgery. Thorac Cardiovasc Surg 2006; 54:250–254.
- Christenson JT. Preoperative lipid-control with simvastatin reduces the risk of postoperative thrombocytosis and thrombotic complications following CABG. Eur J Cardiothorac Surg 1999; 15:394–399.
- Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366:1267–1278.
- Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 Guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). Circulation 2007; 116:e418–e499.
- Schouten O, Poldermans D, Visser L, et al. Fluvastatin and bisoprolol for the reduction of perioperative cardiac mortality and morbidity in high-risk patients undergoing non-cardiac surgery: rationale and design of the DECREASE-IV study. Am Heart J 2004; 148:1047–1052.
- Amar D, Zhang H, Heerdt PM, Park B, Fleisher M, Thaler HT. Statin use is associated with a reduction in atrial fibrillation after noncardiac thoracic surgery independent of C-reactive protein. Chest 2005; 128:3421–3427.
Soon, the checklist for internists seeing patients about to undergo surgery may include prescribing one of the lipid-lowering hydroxymethylglutaryl-CoA reductase inhibitors, also called statins.
Statins? Not long ago, we were debating whether patients who take statins should stop taking them before surgery, based on the manufacturers’ recommendations.1 The discussion, however, has changed to whether patients who have never received a statin should be started on one before surgery to provide immediate prophylaxis against cardiac morbidity, and how much harm long-term statin users face if these drugs are withheld perioperatively.
The evidence is still very preliminary and based mostly on studies in animals and retrospective studies in people. However, an expanding body of indirect evidence suggests that these drugs are beneficial in this situation.
In this review, we discuss the mechanisms by which statins may protect the heart in the short term, drawing on data from animal and human studies of acute myocardial infarction, and we review the current (albeit limited) data from the perioperative setting.
FEW INTERVENTIONS DECREASE RISK
Each year, approximately 50,000 patients suffer a perioperative cardiovascular event; the incidence of myocardial infarction during or after noncardiac surgery is 2% to 3%.2 The primary goal of preoperative cardiovascular risk assessment is to predict and avert these events.
But short of canceling surgery, few interventions have been found to reduce a patient’s risk. For example, a landmark study in 2004 cast doubt on the efficacy of preoperative coronary revascularization.3 Similarly, although early studies of beta-blockers were promising4,5 and although most internists prescribe these drugs before surgery, more recent studies have cast doubt on their efficacy, particularly in patients at low risk undergoing intermediate-risk (rather than vascular) surgery.6–8
This changing clinical landscape has prompted a search for new strategies for perioperative risk-reduction. Several recent studies have placed statins in the spotlight.
POTENTIAL MECHANISMS OF SHORT-TERM BENEFIT
Statins have been proven to save lives when used long-term, but how could this class of drugs, designed to prevent the accumulation of arterial plaques by lowering low-density lipoprotein cholesterol (LDL-C) levels, have any short-term impact on operative outcomes? Although LDL-C reduction is the principal mechanism of action of statins, not all of the benefit can be ascribed to this mechanism.9 The answer may lie in their “pleiotropic” effects—ie, actions other than LDL-C reduction.
The more immediate pleiotropic effects of statins in the proinflammatory and prothrombotic environment of the perioperative period are thought to include improved endothelial function (both antithrombotic function and vasomotor function in response to ischemic stress), enhanced stability of atherosclerotic plaques, decreased oxidative stress, and decreased vascular inflammation.10–12
EVIDENCE FROM ANIMAL STUDIES
Experiments in animals suggest that statins, given shortly before or after a cardiovascular event, confer benefit before any changes in LDL-C are measurable.
Lefer et al13 found that simvastatin (Zocor), given 18 hours before an ischemic episode in rats, blunted the inflammatory response in cardiac reperfusion injury. Not only was reperfusion injury significantly less in the hearts of the rats that received simvastatin than in the saline control group, but the simvastatin-treated hearts also expressed fewer neutrophil adhesion molecules such as P-selectin, and they had more basal release of nitric oxide, the potent endothelial-derived vasodilator with antithrombotic, anti-inflammatory, and antiproliferative effects.14 These results suggest that statins may improve endothelial function acutely, particularly during ischemic stress.
Osborne et al15 fed rabbits a cholesterol-rich diet plus either lovastatin (Mevacor) or placebo. After 2 weeks, the rabbits underwent either surgery to induce a myocardial infarction or a sham procedure. Regardless of the pretreatment, biopsies of the aorta did not reveal any atherosclerosis; yet the lovastatin-treated rabbits sustained less myocardial ischemic damage and they had more endothelium-mediated vasodilatation.
Statin therapy also may improve cerebral ischemia outcomes in animal models.14,16
Sironi et al16 induced strokes in rats by occluding the middle cerebral artery. The rats received either simvastatin or vehicle for 3 days before the stroke or immediately afterwards. Even though simvastatin did not have enough time to affect the total cholesterol level, rats treated with simvastatin had smaller infarcts (as measured by magnetic resonance imaging) and produced more nitric oxide.
Comment. Taken together, these studies offer tantalizing evidence that statins have short-term, beneficial nonlipid effects and may reduce not only the likelihood of an ischemic event, but—should one occur—the degree of tissue damage that ensues.
EFFECTS OF STATINS IN ACUTE CORONARY SYNDROME
The National Registry of Myocardial Infarction17 is a prospective, observational database of all patients with acute myocardial infarction admitted to 1,230 participating hospitals throughout the United States. In an analysis from this cohort, patients were divided into four groups: those receiving statins before and after admission, those receiving statins only before admission, those receiving statins only after admission, and those who never received statins.
Compared with those who never received statins, fewer patients who received them both before and after admission died while in the hospital (unadjusted odds ratio 0.23, 95% confidence interval [CI] 0.22–0.25), and the odds ratio for those who received statins for the first time was 0.31 (95% CI 0.29–0.33). Patients who stopped receiving a statin on admission were more likely to die than were patients who never received statins (odds ratio 1.09, 95% CI 1.03–1.15). These trends held true even when adjustments were made for potential confounding factors.
Comment. Unmeasured confounding factors (such as the inability to take pills due to altered mental status or the different practice styles of the providers who chose to discontinue statins) might have affected the results. Nevertheless, these results suggest that the protective effects of statins stop almost immediately when these drugs are discontinued, and that there may even be an adverse “rebound” effect when patients who have been taking these drugs for a long time stop taking them temporarily.
The Platelet Receptor Inhibition in Ischemic Syndrome Management trial,18 in a subgroup analysis, had nearly identical findings. In the main part of this trial, patients with coronary artery disease and chest pain at rest or accelerating pain in the last 24 hours were randomized to receive tirofiban (Aggrastat) or heparin. Complete data on statin use were available for 1,616 (50%) of the 3,232 patients in this trial, and the rate of the primary end point (death, myocardial infarction, or recurrent ischemia) was analyzed on the basis of statin therapy in this subgroup.
Comment. Together, these data lead to the conclusion that, when admitted for either acute myocardial infarction or acute coronary syndrome, patients already receiving statins should not have them stopped, and those who had not been receiving statins should receive them immediately. The safety of these medications in the acute setting appears excellent: in the Myocardial Ischemia Reduction With Acute Cholesterol Lowering (MIRACL)12 and the Pravastatin or Atorvastatin Evaluation and Infection Therapy (PROVE-IT)11 trials, fewer than 5% of statin-treated patients had transient elevations in transaminase levels, and no cases of rhabdomyolysis were reported.
PERIOPERATIVE STATIN STUDIES
The data on perioperative statin use are mostly observational and retrospective and fall into essentially four surgical categories: coronary artery bypass grafting (CABG), carotid endarterectomy,19,20 noncardiac vascular surgery, and major noncardiac surgery. Two meta-analyses have also evaluated the data.21,22 The only randomized controlled trial (performed by Durazzo et al23) was small and was carried out at a single center in vascular surgery patients, and the event rate was low.
Current recommendations from the National Cholesterol Education Program (NCEP)24 say that patients who need CABG, have peripheral arterial disease, have an abdominal aortic aneurysm, or have cerebrovascular disease should already be on a statin to achieve an LDL-C goal level of less than 100 mg/dL, with an optional goal of less than 70 mg/dL, independent of surgery.
Since not all patients who should be on statins are actually on them, questions arise:
- Is it important (and safe) to start statin treatment preoperatively?
- Will patients with cardiovascular risk factors but without known cardiovascular disease benefit from statins perioperatively?
Noncardiac vascular surgery
Multiple retrospective studies have evaluated the effect of statins in patients undergoing major noncardiac vascular surgery.25–32
Kertai et al25 evaluated 570 patients in Holland who underwent elective open surgery for infrarenal abdominal aortic aneurysms between 1991 and 2001, looking for an association between statin use and the incidence of perioperative death from myocardial infarction. Only 162 of the 570 patients had been on long-term statin therapy before the surgery. The use of statins was only one of many known baseline characteristics that were significantly different between the two groups, including age, body mass index, known coronary artery disease, and use of angiotensin-converting enzyme inhibitors and beta-blockers. In univariate analysis, statins appeared to be protective: 6 (3.7%) of the patients in the statin group died of a myocardial infarction, compared with 45 (11%) of those in the nostatin group. A multivariate analysis yielded similar findings, with an odds ratio of 0.24 (95% CI 0.11–0.54).
Ward et al27 performed a very similar retrospective study, with similar findings. In 446 patients who underwent surgery for infrarenal abdominal aortic aneurysm, statin therapy was associated with a significantly lower incidence of the combined end point of death, myocardial infarction, stroke, and major peripheral vascular complications, with an adjusted odds ratio of 0.36 (95% CI 0.14–0.93).
Poldermans et al26 noted similar findings in a case-control study of noncardiac vascular surgery patients. Statin users had a much lower perioperative risk of death than did nonusers, with an adjusted odds ratio of 0.22 (95% CI 0.10–0.47).
O’Neil-Callahan et al,28 in a cohort study, found that statin users had fewer perioperative cardiac complications, with an adjusted odds ratio of 0.49 (95% CI 0.28–0.84, P = .009).
Dogma of withdrawing statins before major surgery is challenged
Le Manach et al33 reviewed the outcomes for all patients of a single hospital in Paris who underwent nonemergency infrarenal aortic procedures between January 2001 and December 2004. In January 2004, the hospital instituted guidelines to ensure that patients on statins continue taking them up to the evening before surgery and that statins be restarted on the first postoperative day (via nasogastric tube if necessary). Before 2004, there had been no specific guidelines, and patients on statins did not receive them for a median of 4 days postoperatively. Types of procedures were similar during the two time periods, as were the rates of beta-blocker use, preoperative revascularization, venous thromboembolism prophylaxis, and perioperative blood pressure control. After surgery, topononin I levels were measured in all patients as surveillance for cardiac events, and were defined as elevated when greater than 0.2 ng/mL.
Compared with patients not on statins at all, those treated with statins continuously throughout the perioperative period (after January 2004) had a lower rate of elevated troponin (relative risk 0.38). In contrast, those who had their statins transiently discontinued perioperatively (prior to 2004) had troponin elevations more often than those who had never been treated (relative risk 2.1). This suggested an over fivefold risk reduction (P < .001) conferred by not discontinuing statins in the immediate postoperative period. This finding was maintained after multivariate adjustment: statin withdrawal was associated with a 2.9-fold (95% CI 1.6–5.5) increase in the risk of cardiac enzyme elevations postoperatively. No fewer deaths were noted, but the study was not powered to detect a mortality difference.
Comment. Although secular trends cannot be entirely discounted as contributing to these findings, the prompt increase in cardiac events after just 4 days of statin withdrawal adds to the growing body of evidence suggesting that statin discontinuation can have harmful acute effects. It also brings up the question: Can starting statins benefit patients in the same time period?
Should statins be started before vascular surgery?
Schouten et al32 evaluated the effects of newly started or continued statin treatment in patients undergoing major elective vascular surgery. Patients were screened before surgery and started on statins if they were not already receiving them and their total cholesterol levels were elevated; new users received the medication for about 40 days before surgery. Of the 981 screened patients, 44 (5%) were newly started on statins and 182 (19%) were continued on their therapy. Perioperative death or myocardial infarction occurred in 22 (8.8%) of the statin users and 111 (14.7%) of the nonusers, a statistically significant difference. Temporary discontinuation (median 1 day) of statins in this study due to the inability to take an oral medication did not appear to affect the likelihood of a myocardial infarction.
Durazzo et al23 performed a single-center, randomized, prospective, placebo-controlled, double-blind clinical trial of atorvastatin (Lipitor) 20 mg daily vs placebo in 100 patients undergoing noncardiac arterial vascular surgery. Patients were excluded if they had previously used medications to treat dyslipidemia, recently had a cardiovascular event, or had contraindications to statin treatment such as a baseline creatinine level greater than 2.0 mg/dL or severe hepatic disease. The intervention group received atorvastatin starting at least 2 weeks before surgery for a total of 45 days. Patients were then continued or started on a statin after surgery if their LDL-C level was greater than 100 mg/dL. Beta-blocker use was recommended “on the basis of current guidelines.”
One month after surgery, the LDL-C level was statistically significantly lower in the atorvastatin group. Since most patients did not continue or start statin therapy after the 45-day treatment period, the LDL-C levels were not statistically different at 3 and 6 months after surgery.
At 6 months, the rate of the primary end point (death from cardiovascular causes, nonfatal acute myocardial infarction, ischemic stroke, or unstable angina) was 26.0% in the placebo group and 8.0% in the atorvastatin group, a statistically significant difference. Three patients in the atorvastatin group had cardiac events in the first 10 days after surgery, compared with 11 patients in the placebo group. Thirteen of the 17 total cardiac events took place within 10 days after surgery.
One of the atorvastatin patients developed rhabdomyolysis and elevated aminotransferase levels.
Major noncardiac surgery
Lindenauer et al2 performed a retrospective cohort study of surgical patients who were at least 18 years old and survived beyond the second hospital day. Patients were divided into a group receiving any form of lipid-lowering treatment (of whom more than 90% were taking statins) and a group that had never never received a lipid-lowering drug or only started one on the third day of the hospitalization or later. The period of study was from January 1, 2000, to December 31, 2001.
In all, 780,591 patients from 329 hospitals throughout the United States were included, of whom only 77,082 (9.9%) received lipid-lowering therapy. Eight percent of the patients underwent vascular surgery. Not surprisingly, the treated patients were more likely to have a history of hypertension, diabetes, ischemic heart disease, or hyperlipidemia. They also were more likely to have a vascular procedure performed, to have two or more cardiac risk factors (high-risk surgery, ischemic heart disease, congestive heart failure, cerebrovascular disease, renal insufficiency, or diabetes mellitus), and to be treated with beta-blockers and angiotensin-converting enzyme inhibitors, but they were less likely to have high-risk and emergency surgery performed.
The primary end point, perioperative death, occurred in 2.13% of the treated patients and 3.05% of the nontreated group. Compared with the rate in a propensity-matched cohort, the odds ratio adjusted for unbalanced covariates was 0.62 (95% CI 0.58–0.67) in favor of lipid treatment. Stratification by cardiac risk index revealed a number needed to treat of 186 for those with no risk factors, 60 for those with two risk factors, and 30 for those with four or more risk factors.
Unfortunately, this analysis was not able to take into account whether and for how long patients were receiving lipid-lowering therapy before hospitalization. It therefore does not answer the questions of whether starting lipid-lowering therapy before surgery is beneficial or whether stopping it is harmful. It also does not shed light on whether perioperative lipid-lowering increases the risk of rhabdomyolysis or liver disease.
Carotid endarterectomy
Two recent retrospective cohort studies evaluated the outcomes in patients undergoing carotid endarterectomy.19,20
Kennedy et al19 found that patients on a statin at the time of admission who had symptomatic carotid disease had lower rates of inhospital death (adjusted odds ratio 0.24, 95% CI 0.06–0.91) and ischemic stroke or death (adjusted odds ratio 0.55, 95% CI 0.31–0.97). However, cardiac outcomes among these symptomatic patients were not significantly improved (odds ratio 0.82, 95% CI 0.45–1.50), nor was there benefit for asymptomatic patients, raising the possibility that the positive findings were due to chance or that patients at lower baseline risk for vascular events may have less benefit.
McGirt et al20 performed a similar study; they did not, however, distinguish whether patients had symptomatic vs asymptomatic carotid disease. The 30-day risk of perioperative stroke was lower in patients treated with a statin, with an odds ratio of 0.41 (95% CI 0.18–0.93); the odds ratio for death was 0.21 (95% CI 0.05–0.96). Cardiac outcomes were not significantly affected.
Coronary artery bypass graft surgery
According to the NCEP recommendations, nearly all patients undergoing CABG should already be on a statin before surgery since they all have known coronary artery disease. Multiple observational studies have offered confirmatory evidence that statins are beneficial in this setting.34–38
Liakopoulos et al39 evaluated whether the anti-inflammatory effects of statins may, in part, account for their beneficial effect in the perioperative period. The authors prospectively matched 18 patients who were taking statins and were referred for elective CABG with 18 patients who were not prescribed statins previously. The only major measured baseline characteristic that differed between the two groups was a statistically significantly lower LDL-C level in the statin group. The operative characteristics did not differ, and cytokine levels at baseline were similar.
Tumor necrosis factor alpha levels increased significantly in the control group but did not change significantly in the statin group. Interleukin 8 increased in both groups by a similar amount. Interleukin 6 (the major inducer of C-reactive protein) increased from baseline in both groups but did not increase nearly as much in the statin group as in the control group; the intergroup difference was statistically significant. The anti-inflammatory cytokine interleukin 10 increased minimally from baseline in the control group, while the statin group’s levels increased significantly above baseline and those of the control group.
Christenson40 also found that inflammatory markers were improved with pre-CABG statin treatment in a small randomized trial in which patients received simvastatin 20 mg 4 weeks prior to CABG surgery vs no statin. Interestingly, far fewer statin-treated patients developed thrombocytosis (platelet count > 400 × 109/L) than did control patients (3% vs 81%, P < .0001).
RISKS OF PERIOPERATIVE STATINS
The risks associated with statin therapy in general appear low, but specific perioperative risks have not been well studied.
Baigent et al,41 in a meta-analysis of randomized trials of nonperioperative statin therapy, found that rhabdomyolysis occurred in 9 (0.023%) of 39,884 patients receiving statins vs 6 (0.015%) of the 39,817 controls, with a number needed to harm of 12,500. Moreover, the rates of nonvascular death and cancer did not increase. It is plausible that the risk is somewhat greater in the perioperative setting but is likely not enough to outweigh the potential benefits, especially since the risk of ischemic vascular events is particularly high then.
Some of the perioperative studies cited above specifically addressed potential risks. For example, in the study by Schouten et al,32 mild creatine kinase elevations were more common in the statin-treated group, but the incidence of moderate and severe creatine kinase elevations did not differ significantly. No case of rhabdomyolysis occurred, and length of surgery was the only predictor of myopathy. MIRACL and PROVE-IT revealed similar safety profiles; aminotransferase levels normalized when statins were stopped, and no cases of rhabdomyolysis occurred.11,12 In the vascular surgery study by Durazzo et al,23 1 (2%) of the 50 atorvastatin-treated patients developed both rhabdomyolysis and elevated aminotransferase levels that prompted discontinuation of the statin.
Overall, the observational studies do not indicate that statin continuation or treatment is harmful in perioperative patients. However, these studies did not specifically evaluate patients with acute insults from surgery such as sepsis, renal failure, or hepatitis. It is unknown what effect statin therapy would have in those patients and whether statins should be selectively discontinued in patients who develop major hepatic, musculoskeletal, or renal complications after surgery.
OUR RECOMMENDATIONS
Before CABG or vascular surgery
Given the NCEP recommendations, existing primary and secondary prevention studies, observational studies of CABG and noncardiac vascular surgery patients, and the one randomized trial of vascular surgery patients, data support the use of statins in nearly all patients undergoing cardiac or vascular surgery. We advocate starting statins in the perioperative period to take advantage of their rapid-acting pleiotropic effects, and continuing them long-term to take advantage of their lipid-lowering effects. This recommendation is in line with the recently released American College of Cardiology/American Heart Association (ACC/AHA) 2007 perioperative guidelines that state “for patients undergoing vascular surgery with or without clinical risk factors, statin use is reasonable.”42
Although the ideal time to start statins is not certain, the study by Durazzo et al23 suggests that they should be started at least 2 weeks before surgery if possible. Moreover, patients already taking statins should definitely not have their statins discontinued if at all possible.
Before major nonvascular surgery
For patients undergoing major nonvascular (intermediate-risk) surgery, physicians should first ascertain if the patient has an indication for statin therapy based on current nonsurgical lipid level recommendations. However, even if there is no clear indication for statin therapy based on NCEP guidelines, we endorse the recently released ACC/AHA perioperative guidelines that state that statin therapy can be considered in patients with a risk factor who are undergoing intermediate-risk procedures. Moreover, we wholeheartedly support the ACC/AHA’s strongest recommendation that patients who are already receiving statins and are undergoing noncardiac surgery should not have their statins discontinued.
When to discontinue statins?
The risk of harm overall appears to be minimal and certainly less than the likelihood of benefit. It is reasonable to observe patients postoperatively for adverse clinical events that may increase the risk of perioperative statin treatment, such as acute renal failure, hepatic failure, or sepsis, but whether statins should be stopped in patients with these complications remains unknown; we advocate individualizing the decision.
More studies needed
We need more data on whether moderate-risk patients undergoing moderate-risk surgery benefit from perioperative statin therapy, when therapy should be started, whether therapy should be started on the day of surgery if it was not started earlier, which statin and what doses are optimal, how long therapy should be continued, and what degree of risk is associated with perioperative statin therapy.
Fortunately, important data should be forthcoming in the next few years: the Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography (DECREASE-IV) study43 is a 4-year two-by-two factorial placebo-controlled study evaluating the use of fluvastatin (Lescol) and bisoprolol (Zebeta, a beta-blocker) separately and together in patients who are older than 40 years, are undergoing elective noncardiac surgery, have an estimated risk of cardiovascular death of more than 1%, have not used statins previously, and do not have elevated cholesterol.
Soon, the checklist for internists seeing patients about to undergo surgery may include prescribing one of the lipid-lowering hydroxymethylglutaryl-CoA reductase inhibitors, also called statins.
Statins? Not long ago, we were debating whether patients who take statins should stop taking them before surgery, based on the manufacturers’ recommendations.1 The discussion, however, has changed to whether patients who have never received a statin should be started on one before surgery to provide immediate prophylaxis against cardiac morbidity, and how much harm long-term statin users face if these drugs are withheld perioperatively.
The evidence is still very preliminary and based mostly on studies in animals and retrospective studies in people. However, an expanding body of indirect evidence suggests that these drugs are beneficial in this situation.
In this review, we discuss the mechanisms by which statins may protect the heart in the short term, drawing on data from animal and human studies of acute myocardial infarction, and we review the current (albeit limited) data from the perioperative setting.
FEW INTERVENTIONS DECREASE RISK
Each year, approximately 50,000 patients suffer a perioperative cardiovascular event; the incidence of myocardial infarction during or after noncardiac surgery is 2% to 3%.2 The primary goal of preoperative cardiovascular risk assessment is to predict and avert these events.
But short of canceling surgery, few interventions have been found to reduce a patient’s risk. For example, a landmark study in 2004 cast doubt on the efficacy of preoperative coronary revascularization.3 Similarly, although early studies of beta-blockers were promising4,5 and although most internists prescribe these drugs before surgery, more recent studies have cast doubt on their efficacy, particularly in patients at low risk undergoing intermediate-risk (rather than vascular) surgery.6–8
This changing clinical landscape has prompted a search for new strategies for perioperative risk-reduction. Several recent studies have placed statins in the spotlight.
POTENTIAL MECHANISMS OF SHORT-TERM BENEFIT
Statins have been proven to save lives when used long-term, but how could this class of drugs, designed to prevent the accumulation of arterial plaques by lowering low-density lipoprotein cholesterol (LDL-C) levels, have any short-term impact on operative outcomes? Although LDL-C reduction is the principal mechanism of action of statins, not all of the benefit can be ascribed to this mechanism.9 The answer may lie in their “pleiotropic” effects—ie, actions other than LDL-C reduction.
The more immediate pleiotropic effects of statins in the proinflammatory and prothrombotic environment of the perioperative period are thought to include improved endothelial function (both antithrombotic function and vasomotor function in response to ischemic stress), enhanced stability of atherosclerotic plaques, decreased oxidative stress, and decreased vascular inflammation.10–12
EVIDENCE FROM ANIMAL STUDIES
Experiments in animals suggest that statins, given shortly before or after a cardiovascular event, confer benefit before any changes in LDL-C are measurable.
Lefer et al13 found that simvastatin (Zocor), given 18 hours before an ischemic episode in rats, blunted the inflammatory response in cardiac reperfusion injury. Not only was reperfusion injury significantly less in the hearts of the rats that received simvastatin than in the saline control group, but the simvastatin-treated hearts also expressed fewer neutrophil adhesion molecules such as P-selectin, and they had more basal release of nitric oxide, the potent endothelial-derived vasodilator with antithrombotic, anti-inflammatory, and antiproliferative effects.14 These results suggest that statins may improve endothelial function acutely, particularly during ischemic stress.
Osborne et al15 fed rabbits a cholesterol-rich diet plus either lovastatin (Mevacor) or placebo. After 2 weeks, the rabbits underwent either surgery to induce a myocardial infarction or a sham procedure. Regardless of the pretreatment, biopsies of the aorta did not reveal any atherosclerosis; yet the lovastatin-treated rabbits sustained less myocardial ischemic damage and they had more endothelium-mediated vasodilatation.
Statin therapy also may improve cerebral ischemia outcomes in animal models.14,16
Sironi et al16 induced strokes in rats by occluding the middle cerebral artery. The rats received either simvastatin or vehicle for 3 days before the stroke or immediately afterwards. Even though simvastatin did not have enough time to affect the total cholesterol level, rats treated with simvastatin had smaller infarcts (as measured by magnetic resonance imaging) and produced more nitric oxide.
Comment. Taken together, these studies offer tantalizing evidence that statins have short-term, beneficial nonlipid effects and may reduce not only the likelihood of an ischemic event, but—should one occur—the degree of tissue damage that ensues.
EFFECTS OF STATINS IN ACUTE CORONARY SYNDROME
The National Registry of Myocardial Infarction17 is a prospective, observational database of all patients with acute myocardial infarction admitted to 1,230 participating hospitals throughout the United States. In an analysis from this cohort, patients were divided into four groups: those receiving statins before and after admission, those receiving statins only before admission, those receiving statins only after admission, and those who never received statins.
Compared with those who never received statins, fewer patients who received them both before and after admission died while in the hospital (unadjusted odds ratio 0.23, 95% confidence interval [CI] 0.22–0.25), and the odds ratio for those who received statins for the first time was 0.31 (95% CI 0.29–0.33). Patients who stopped receiving a statin on admission were more likely to die than were patients who never received statins (odds ratio 1.09, 95% CI 1.03–1.15). These trends held true even when adjustments were made for potential confounding factors.
Comment. Unmeasured confounding factors (such as the inability to take pills due to altered mental status or the different practice styles of the providers who chose to discontinue statins) might have affected the results. Nevertheless, these results suggest that the protective effects of statins stop almost immediately when these drugs are discontinued, and that there may even be an adverse “rebound” effect when patients who have been taking these drugs for a long time stop taking them temporarily.
The Platelet Receptor Inhibition in Ischemic Syndrome Management trial,18 in a subgroup analysis, had nearly identical findings. In the main part of this trial, patients with coronary artery disease and chest pain at rest or accelerating pain in the last 24 hours were randomized to receive tirofiban (Aggrastat) or heparin. Complete data on statin use were available for 1,616 (50%) of the 3,232 patients in this trial, and the rate of the primary end point (death, myocardial infarction, or recurrent ischemia) was analyzed on the basis of statin therapy in this subgroup.
Comment. Together, these data lead to the conclusion that, when admitted for either acute myocardial infarction or acute coronary syndrome, patients already receiving statins should not have them stopped, and those who had not been receiving statins should receive them immediately. The safety of these medications in the acute setting appears excellent: in the Myocardial Ischemia Reduction With Acute Cholesterol Lowering (MIRACL)12 and the Pravastatin or Atorvastatin Evaluation and Infection Therapy (PROVE-IT)11 trials, fewer than 5% of statin-treated patients had transient elevations in transaminase levels, and no cases of rhabdomyolysis were reported.
PERIOPERATIVE STATIN STUDIES
The data on perioperative statin use are mostly observational and retrospective and fall into essentially four surgical categories: coronary artery bypass grafting (CABG), carotid endarterectomy,19,20 noncardiac vascular surgery, and major noncardiac surgery. Two meta-analyses have also evaluated the data.21,22 The only randomized controlled trial (performed by Durazzo et al23) was small and was carried out at a single center in vascular surgery patients, and the event rate was low.
Current recommendations from the National Cholesterol Education Program (NCEP)24 say that patients who need CABG, have peripheral arterial disease, have an abdominal aortic aneurysm, or have cerebrovascular disease should already be on a statin to achieve an LDL-C goal level of less than 100 mg/dL, with an optional goal of less than 70 mg/dL, independent of surgery.
Since not all patients who should be on statins are actually on them, questions arise:
- Is it important (and safe) to start statin treatment preoperatively?
- Will patients with cardiovascular risk factors but without known cardiovascular disease benefit from statins perioperatively?
Noncardiac vascular surgery
Multiple retrospective studies have evaluated the effect of statins in patients undergoing major noncardiac vascular surgery.25–32
Kertai et al25 evaluated 570 patients in Holland who underwent elective open surgery for infrarenal abdominal aortic aneurysms between 1991 and 2001, looking for an association between statin use and the incidence of perioperative death from myocardial infarction. Only 162 of the 570 patients had been on long-term statin therapy before the surgery. The use of statins was only one of many known baseline characteristics that were significantly different between the two groups, including age, body mass index, known coronary artery disease, and use of angiotensin-converting enzyme inhibitors and beta-blockers. In univariate analysis, statins appeared to be protective: 6 (3.7%) of the patients in the statin group died of a myocardial infarction, compared with 45 (11%) of those in the nostatin group. A multivariate analysis yielded similar findings, with an odds ratio of 0.24 (95% CI 0.11–0.54).
Ward et al27 performed a very similar retrospective study, with similar findings. In 446 patients who underwent surgery for infrarenal abdominal aortic aneurysm, statin therapy was associated with a significantly lower incidence of the combined end point of death, myocardial infarction, stroke, and major peripheral vascular complications, with an adjusted odds ratio of 0.36 (95% CI 0.14–0.93).
Poldermans et al26 noted similar findings in a case-control study of noncardiac vascular surgery patients. Statin users had a much lower perioperative risk of death than did nonusers, with an adjusted odds ratio of 0.22 (95% CI 0.10–0.47).
O’Neil-Callahan et al,28 in a cohort study, found that statin users had fewer perioperative cardiac complications, with an adjusted odds ratio of 0.49 (95% CI 0.28–0.84, P = .009).
Dogma of withdrawing statins before major surgery is challenged
Le Manach et al33 reviewed the outcomes for all patients of a single hospital in Paris who underwent nonemergency infrarenal aortic procedures between January 2001 and December 2004. In January 2004, the hospital instituted guidelines to ensure that patients on statins continue taking them up to the evening before surgery and that statins be restarted on the first postoperative day (via nasogastric tube if necessary). Before 2004, there had been no specific guidelines, and patients on statins did not receive them for a median of 4 days postoperatively. Types of procedures were similar during the two time periods, as were the rates of beta-blocker use, preoperative revascularization, venous thromboembolism prophylaxis, and perioperative blood pressure control. After surgery, topononin I levels were measured in all patients as surveillance for cardiac events, and were defined as elevated when greater than 0.2 ng/mL.
Compared with patients not on statins at all, those treated with statins continuously throughout the perioperative period (after January 2004) had a lower rate of elevated troponin (relative risk 0.38). In contrast, those who had their statins transiently discontinued perioperatively (prior to 2004) had troponin elevations more often than those who had never been treated (relative risk 2.1). This suggested an over fivefold risk reduction (P < .001) conferred by not discontinuing statins in the immediate postoperative period. This finding was maintained after multivariate adjustment: statin withdrawal was associated with a 2.9-fold (95% CI 1.6–5.5) increase in the risk of cardiac enzyme elevations postoperatively. No fewer deaths were noted, but the study was not powered to detect a mortality difference.
Comment. Although secular trends cannot be entirely discounted as contributing to these findings, the prompt increase in cardiac events after just 4 days of statin withdrawal adds to the growing body of evidence suggesting that statin discontinuation can have harmful acute effects. It also brings up the question: Can starting statins benefit patients in the same time period?
Should statins be started before vascular surgery?
Schouten et al32 evaluated the effects of newly started or continued statin treatment in patients undergoing major elective vascular surgery. Patients were screened before surgery and started on statins if they were not already receiving them and their total cholesterol levels were elevated; new users received the medication for about 40 days before surgery. Of the 981 screened patients, 44 (5%) were newly started on statins and 182 (19%) were continued on their therapy. Perioperative death or myocardial infarction occurred in 22 (8.8%) of the statin users and 111 (14.7%) of the nonusers, a statistically significant difference. Temporary discontinuation (median 1 day) of statins in this study due to the inability to take an oral medication did not appear to affect the likelihood of a myocardial infarction.
Durazzo et al23 performed a single-center, randomized, prospective, placebo-controlled, double-blind clinical trial of atorvastatin (Lipitor) 20 mg daily vs placebo in 100 patients undergoing noncardiac arterial vascular surgery. Patients were excluded if they had previously used medications to treat dyslipidemia, recently had a cardiovascular event, or had contraindications to statin treatment such as a baseline creatinine level greater than 2.0 mg/dL or severe hepatic disease. The intervention group received atorvastatin starting at least 2 weeks before surgery for a total of 45 days. Patients were then continued or started on a statin after surgery if their LDL-C level was greater than 100 mg/dL. Beta-blocker use was recommended “on the basis of current guidelines.”
One month after surgery, the LDL-C level was statistically significantly lower in the atorvastatin group. Since most patients did not continue or start statin therapy after the 45-day treatment period, the LDL-C levels were not statistically different at 3 and 6 months after surgery.
At 6 months, the rate of the primary end point (death from cardiovascular causes, nonfatal acute myocardial infarction, ischemic stroke, or unstable angina) was 26.0% in the placebo group and 8.0% in the atorvastatin group, a statistically significant difference. Three patients in the atorvastatin group had cardiac events in the first 10 days after surgery, compared with 11 patients in the placebo group. Thirteen of the 17 total cardiac events took place within 10 days after surgery.
One of the atorvastatin patients developed rhabdomyolysis and elevated aminotransferase levels.
Major noncardiac surgery
Lindenauer et al2 performed a retrospective cohort study of surgical patients who were at least 18 years old and survived beyond the second hospital day. Patients were divided into a group receiving any form of lipid-lowering treatment (of whom more than 90% were taking statins) and a group that had never never received a lipid-lowering drug or only started one on the third day of the hospitalization or later. The period of study was from January 1, 2000, to December 31, 2001.
In all, 780,591 patients from 329 hospitals throughout the United States were included, of whom only 77,082 (9.9%) received lipid-lowering therapy. Eight percent of the patients underwent vascular surgery. Not surprisingly, the treated patients were more likely to have a history of hypertension, diabetes, ischemic heart disease, or hyperlipidemia. They also were more likely to have a vascular procedure performed, to have two or more cardiac risk factors (high-risk surgery, ischemic heart disease, congestive heart failure, cerebrovascular disease, renal insufficiency, or diabetes mellitus), and to be treated with beta-blockers and angiotensin-converting enzyme inhibitors, but they were less likely to have high-risk and emergency surgery performed.
The primary end point, perioperative death, occurred in 2.13% of the treated patients and 3.05% of the nontreated group. Compared with the rate in a propensity-matched cohort, the odds ratio adjusted for unbalanced covariates was 0.62 (95% CI 0.58–0.67) in favor of lipid treatment. Stratification by cardiac risk index revealed a number needed to treat of 186 for those with no risk factors, 60 for those with two risk factors, and 30 for those with four or more risk factors.
Unfortunately, this analysis was not able to take into account whether and for how long patients were receiving lipid-lowering therapy before hospitalization. It therefore does not answer the questions of whether starting lipid-lowering therapy before surgery is beneficial or whether stopping it is harmful. It also does not shed light on whether perioperative lipid-lowering increases the risk of rhabdomyolysis or liver disease.
Carotid endarterectomy
Two recent retrospective cohort studies evaluated the outcomes in patients undergoing carotid endarterectomy.19,20
Kennedy et al19 found that patients on a statin at the time of admission who had symptomatic carotid disease had lower rates of inhospital death (adjusted odds ratio 0.24, 95% CI 0.06–0.91) and ischemic stroke or death (adjusted odds ratio 0.55, 95% CI 0.31–0.97). However, cardiac outcomes among these symptomatic patients were not significantly improved (odds ratio 0.82, 95% CI 0.45–1.50), nor was there benefit for asymptomatic patients, raising the possibility that the positive findings were due to chance or that patients at lower baseline risk for vascular events may have less benefit.
McGirt et al20 performed a similar study; they did not, however, distinguish whether patients had symptomatic vs asymptomatic carotid disease. The 30-day risk of perioperative stroke was lower in patients treated with a statin, with an odds ratio of 0.41 (95% CI 0.18–0.93); the odds ratio for death was 0.21 (95% CI 0.05–0.96). Cardiac outcomes were not significantly affected.
Coronary artery bypass graft surgery
According to the NCEP recommendations, nearly all patients undergoing CABG should already be on a statin before surgery since they all have known coronary artery disease. Multiple observational studies have offered confirmatory evidence that statins are beneficial in this setting.34–38
Liakopoulos et al39 evaluated whether the anti-inflammatory effects of statins may, in part, account for their beneficial effect in the perioperative period. The authors prospectively matched 18 patients who were taking statins and were referred for elective CABG with 18 patients who were not prescribed statins previously. The only major measured baseline characteristic that differed between the two groups was a statistically significantly lower LDL-C level in the statin group. The operative characteristics did not differ, and cytokine levels at baseline were similar.
Tumor necrosis factor alpha levels increased significantly in the control group but did not change significantly in the statin group. Interleukin 8 increased in both groups by a similar amount. Interleukin 6 (the major inducer of C-reactive protein) increased from baseline in both groups but did not increase nearly as much in the statin group as in the control group; the intergroup difference was statistically significant. The anti-inflammatory cytokine interleukin 10 increased minimally from baseline in the control group, while the statin group’s levels increased significantly above baseline and those of the control group.
Christenson40 also found that inflammatory markers were improved with pre-CABG statin treatment in a small randomized trial in which patients received simvastatin 20 mg 4 weeks prior to CABG surgery vs no statin. Interestingly, far fewer statin-treated patients developed thrombocytosis (platelet count > 400 × 109/L) than did control patients (3% vs 81%, P < .0001).
RISKS OF PERIOPERATIVE STATINS
The risks associated with statin therapy in general appear low, but specific perioperative risks have not been well studied.
Baigent et al,41 in a meta-analysis of randomized trials of nonperioperative statin therapy, found that rhabdomyolysis occurred in 9 (0.023%) of 39,884 patients receiving statins vs 6 (0.015%) of the 39,817 controls, with a number needed to harm of 12,500. Moreover, the rates of nonvascular death and cancer did not increase. It is plausible that the risk is somewhat greater in the perioperative setting but is likely not enough to outweigh the potential benefits, especially since the risk of ischemic vascular events is particularly high then.
Some of the perioperative studies cited above specifically addressed potential risks. For example, in the study by Schouten et al,32 mild creatine kinase elevations were more common in the statin-treated group, but the incidence of moderate and severe creatine kinase elevations did not differ significantly. No case of rhabdomyolysis occurred, and length of surgery was the only predictor of myopathy. MIRACL and PROVE-IT revealed similar safety profiles; aminotransferase levels normalized when statins were stopped, and no cases of rhabdomyolysis occurred.11,12 In the vascular surgery study by Durazzo et al,23 1 (2%) of the 50 atorvastatin-treated patients developed both rhabdomyolysis and elevated aminotransferase levels that prompted discontinuation of the statin.
Overall, the observational studies do not indicate that statin continuation or treatment is harmful in perioperative patients. However, these studies did not specifically evaluate patients with acute insults from surgery such as sepsis, renal failure, or hepatitis. It is unknown what effect statin therapy would have in those patients and whether statins should be selectively discontinued in patients who develop major hepatic, musculoskeletal, or renal complications after surgery.
OUR RECOMMENDATIONS
Before CABG or vascular surgery
Given the NCEP recommendations, existing primary and secondary prevention studies, observational studies of CABG and noncardiac vascular surgery patients, and the one randomized trial of vascular surgery patients, data support the use of statins in nearly all patients undergoing cardiac or vascular surgery. We advocate starting statins in the perioperative period to take advantage of their rapid-acting pleiotropic effects, and continuing them long-term to take advantage of their lipid-lowering effects. This recommendation is in line with the recently released American College of Cardiology/American Heart Association (ACC/AHA) 2007 perioperative guidelines that state “for patients undergoing vascular surgery with or without clinical risk factors, statin use is reasonable.”42
Although the ideal time to start statins is not certain, the study by Durazzo et al23 suggests that they should be started at least 2 weeks before surgery if possible. Moreover, patients already taking statins should definitely not have their statins discontinued if at all possible.
Before major nonvascular surgery
For patients undergoing major nonvascular (intermediate-risk) surgery, physicians should first ascertain if the patient has an indication for statin therapy based on current nonsurgical lipid level recommendations. However, even if there is no clear indication for statin therapy based on NCEP guidelines, we endorse the recently released ACC/AHA perioperative guidelines that state that statin therapy can be considered in patients with a risk factor who are undergoing intermediate-risk procedures. Moreover, we wholeheartedly support the ACC/AHA’s strongest recommendation that patients who are already receiving statins and are undergoing noncardiac surgery should not have their statins discontinued.
When to discontinue statins?
The risk of harm overall appears to be minimal and certainly less than the likelihood of benefit. It is reasonable to observe patients postoperatively for adverse clinical events that may increase the risk of perioperative statin treatment, such as acute renal failure, hepatic failure, or sepsis, but whether statins should be stopped in patients with these complications remains unknown; we advocate individualizing the decision.
More studies needed
We need more data on whether moderate-risk patients undergoing moderate-risk surgery benefit from perioperative statin therapy, when therapy should be started, whether therapy should be started on the day of surgery if it was not started earlier, which statin and what doses are optimal, how long therapy should be continued, and what degree of risk is associated with perioperative statin therapy.
Fortunately, important data should be forthcoming in the next few years: the Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography (DECREASE-IV) study43 is a 4-year two-by-two factorial placebo-controlled study evaluating the use of fluvastatin (Lescol) and bisoprolol (Zebeta, a beta-blocker) separately and together in patients who are older than 40 years, are undergoing elective noncardiac surgery, have an estimated risk of cardiovascular death of more than 1%, have not used statins previously, and do not have elevated cholesterol.
- Grant PJ, Kedia N. Should statins be discontinued preoperatively? IMPACT consults. Proceedings of the 2nd Annual Cleveland Clinic Perioperative Medicine Summit. Cleve Clin J Med 2006; 73 Electronic suppl 1:S9–S10.
- Lindenauer PK, Pekow P, Wang K, Gutierrez B, Benjamin EM. Lipid-lowering therapy and in-hospital mortality following major noncardiac surgery. JAMA 2004; 291:2092–2099.
- McFalls EO, Ward HB, Moritz TE, et al. Coronary-artery revascularization before elective major vascular surgery. N Engl J Med 2004; 351:2795–2804.
- Mangano DT, Layug EL, Wallace A, Tateo I. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter Study of Perioperative Ischemia Research Group. N Engl J Med 1996; 335:1713–1720.
- Poldermans D, Boersma E, Bax JJ, et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med 1999; 341:1789–1794.
- Brady AR, Gibbs JS, Greenhalgh RM, Powell JT, Sydes MR. Perioperative beta-blockade (POBBLE) for patients undergoing infrarenal vascular surgery: results of a randomized double-blind controlled trial. J Vasc Surg 2005; 41:602–609.
- Juul AB, Wetterslev J, Gluud C, et al. Effect of perioperative beta blockade in patients with diabetes undergoing major non-cardiac surgery: randomised placebo controlled, blinded multicentre trial. BMJ 2006; 332:1482.
- Yang H, Raymer K, Butler R, Parlow J, Roberts R. The effects of perioperative beta-blockade: results of the Metoprolol after Vascular Surgery (MaVS) study, a randomized controlled trial. Am Heart J 2006; 152:983–990.
- Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med 2005; 352:20–28.
- Ito MK, Talbert RL, Tsimikas S. Statin-associated pleiotropy: possible beneficial effects beyond cholesterol reduction. Pharmacotherapy 2006; 26:85S–97S.
- Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350:1495–1504.
- Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 2001; 285:1711–1718.
- Lefer AM, Campbell B, Shin YK, Scalia R, Hayward R, Lefer DJ. Simvastatin preserves the ischemic-reperfused myocardium in normocholesterolemic rat hearts. Circulation 1999; 100:178–184.
- Endres M, Laufs U, Liao JK, Moskowitz MA. Targeting eNOS for stroke protection. Trends Neurosci 2004; 27:283–289.
- Osborne JA, Lento PH, Siegfried MR, Stahl GL, Fusman B, Lefer AM. Cardiovascular effects of acute hypercholesterolemia in rabbits. Reversal with lovastatin treatment. J Clin Invest 1989; 83:465–473.
- Sironi L, Cimino M, Guerrini U, et al. Treatment with statins after induction of focal ischemia in rats reduces the extent of brain damage. Arterioscler Thromb Vasc Biol 2003; 23:322–327.
- Fonarow GC, Wright RS, Spencer FA, et al. Effect of statin use within the first 24 hours of admission for acute myocardial infarction on early morbidity and mortality. Am J Cardiol 2005; 96:611–616.
- Heeschen C, Hamm CW, Laufs U, Snapinn S, Bohm M, White HD. Withdrawal of statins increases event rates in patients with acute coronary syndromes. Circulation 2002; 105:1446–1452.
- Kennedy J, Quan H, Buchan AM, Ghali WA, Feasby TE. Statins are associated with better outcomes after carotid endarterectomy in symptomatic patients. Stroke 2005; 36:2072–2076.
- McGirt MJ, Perler BA, Brooke BS, et al. 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors reduce the risk of perioperative stroke and mortality after carotid endarterectomy. J Vasc Surg 2005; 42:829–836.
- Hindler K, Shaw AD, Samuels J, Fulton S, Collard CD, Riedel B. Improved postoperative outcomes associated with preoperative statin therapy. Anesthesiology 2006; 105:1260–1272.
- Kapoor AS, Kanji H, Buckingham J, Devereaux PJ, McAlister FA. Strength of evidence for perioperative use of statins to reduce cardiovascular risk: systematic review of controlled studies. BMJ 2006; 333:1149.
- Durazzo AE, Machado FS, Ikeoka DT, et al. Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial. J Vasc Surg 2004; 39:967–975.
- Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004; 110:227–239.
- Kertai MD, Boersma E, Westerhout CM, et al. A combination of statins and beta-blockers is independently associated with a reduction in the incidence of perioperative mortality and nonfatal myocardial infarction in patients undergoing abdominal aortic aneurysm surgery. Eur J Vasc Endovasc Surg 2004; 28:343–352.
- Poldermans D, Bax JJ, Kertai MD, et al. Statins are associated with a reduced incidence of perioperative mortality in patients undergoing major noncardiac vascular surgery. Circulation 2003; 107:1848–1851.
- Ward RP, Leeper NJ, Kirkpatrick JN, Lang RM, Sorrentino MJ, Williams KA. The effect of preoperative statin therapy on cardiovascular outcomes in patients undergoing infrainguinal vascular surgery. Int J Cardiol 2005; 104:264–268.
- O’Neil-Callahan K, Katsimaglis G, Tepper MR, et al. Statins decrease perioperative cardiac complications in patients undergoing non-cardiac vascular surgery: the Statins for Risk Reduction in Surgery (StaRRS) study. J Am Coll Cardiol 2005; 45:336–342.
- Abbruzzese TA, Havens J, Belkin M, et al. Statin therapy is associated with improved patency of autogenous infrainguinal bypass grafts. J Vasc Surg 2004; 39:1178–1185.
- Boersma E, Poldermans D, Bax JJ, et al. Predictors of cardiac events after major vascular surgery: role of clinical characteristics, dobutamine echocardiography, and beta-blocker therapy. JAMA 2001; 285:1865–1873.
- Landesberg G, Mosseri M, Wolf YG, et al. Preoperative thallium scanning, selective coronary revascularization, and long-term survival after major vascular surgery. Circulation 2003; 108:177–183.
- Schouten O, Kertai MD, Bax JJ, et al. Safety of perioperative statin use in high-risk patients undergoing major vascular surgery. Am J Cardiol 2005; 95:658–660.
- Le Manach Y, Godet G, Coriat P, et al. The impact of postoperative discontinuation or continuation of chronic statin therapy on cardiac outcome after major vascular surgery. Anesth Analg 2007; 104:1326–1333.
- Ali IS, Buth KJ. Preoperative statin use and outcomes following cardiac surgery. Int J Cardiol 2005; 103:12–18.
- Clark LL, Ikonomidis JS, Crawford FA, et al. Preoperative statin treatment is associated with reduced postoperative mortality and morbidity in patients undergoing cardiac surgery: an 8-year retrospective cohort study. J Thorac Cardiovasc Surg 2006; 131:679–685.
- Pan W, Pintar T, Anton J, Lee VV, Vaughn WK, Collard CD. Statins are associated with a reduced incidence of perioperative mortality after coronary artery bypass graft surgery. Circulation 2004; 110(suppl 2):II45–II49.
- Pascual DA, Arribas JM, Tornel PL, et al. Preoperative statin therapy and troponin T predict early complications of coronary artery surgery. Ann Thorac Surg 2006; 81:78–83.
- Dotani MI, Elnicki DM, Jain AC, Gibson CM. Effect of preoperative statin therapy and cardiac outcomes after coronary artery bypass grafting. Am J Cardiol 2000; 86:1128–1130.
- Liakopoulos OJ, Dorge H, Schmitto JD, Nagorsnik U, Grabedunkel J, Schoendube FA. Effects of preoperative statin therapy on cytokines after cardiac surgery. Thorac Cardiovasc Surg 2006; 54:250–254.
- Christenson JT. Preoperative lipid-control with simvastatin reduces the risk of postoperative thrombocytosis and thrombotic complications following CABG. Eur J Cardiothorac Surg 1999; 15:394–399.
- Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366:1267–1278.
- Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 Guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). Circulation 2007; 116:e418–e499.
- Schouten O, Poldermans D, Visser L, et al. Fluvastatin and bisoprolol for the reduction of perioperative cardiac mortality and morbidity in high-risk patients undergoing non-cardiac surgery: rationale and design of the DECREASE-IV study. Am Heart J 2004; 148:1047–1052.
- Amar D, Zhang H, Heerdt PM, Park B, Fleisher M, Thaler HT. Statin use is associated with a reduction in atrial fibrillation after noncardiac thoracic surgery independent of C-reactive protein. Chest 2005; 128:3421–3427.
- Grant PJ, Kedia N. Should statins be discontinued preoperatively? IMPACT consults. Proceedings of the 2nd Annual Cleveland Clinic Perioperative Medicine Summit. Cleve Clin J Med 2006; 73 Electronic suppl 1:S9–S10.
- Lindenauer PK, Pekow P, Wang K, Gutierrez B, Benjamin EM. Lipid-lowering therapy and in-hospital mortality following major noncardiac surgery. JAMA 2004; 291:2092–2099.
- McFalls EO, Ward HB, Moritz TE, et al. Coronary-artery revascularization before elective major vascular surgery. N Engl J Med 2004; 351:2795–2804.
- Mangano DT, Layug EL, Wallace A, Tateo I. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter Study of Perioperative Ischemia Research Group. N Engl J Med 1996; 335:1713–1720.
- Poldermans D, Boersma E, Bax JJ, et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med 1999; 341:1789–1794.
- Brady AR, Gibbs JS, Greenhalgh RM, Powell JT, Sydes MR. Perioperative beta-blockade (POBBLE) for patients undergoing infrarenal vascular surgery: results of a randomized double-blind controlled trial. J Vasc Surg 2005; 41:602–609.
- Juul AB, Wetterslev J, Gluud C, et al. Effect of perioperative beta blockade in patients with diabetes undergoing major non-cardiac surgery: randomised placebo controlled, blinded multicentre trial. BMJ 2006; 332:1482.
- Yang H, Raymer K, Butler R, Parlow J, Roberts R. The effects of perioperative beta-blockade: results of the Metoprolol after Vascular Surgery (MaVS) study, a randomized controlled trial. Am Heart J 2006; 152:983–990.
- Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med 2005; 352:20–28.
- Ito MK, Talbert RL, Tsimikas S. Statin-associated pleiotropy: possible beneficial effects beyond cholesterol reduction. Pharmacotherapy 2006; 26:85S–97S.
- Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350:1495–1504.
- Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 2001; 285:1711–1718.
- Lefer AM, Campbell B, Shin YK, Scalia R, Hayward R, Lefer DJ. Simvastatin preserves the ischemic-reperfused myocardium in normocholesterolemic rat hearts. Circulation 1999; 100:178–184.
- Endres M, Laufs U, Liao JK, Moskowitz MA. Targeting eNOS for stroke protection. Trends Neurosci 2004; 27:283–289.
- Osborne JA, Lento PH, Siegfried MR, Stahl GL, Fusman B, Lefer AM. Cardiovascular effects of acute hypercholesterolemia in rabbits. Reversal with lovastatin treatment. J Clin Invest 1989; 83:465–473.
- Sironi L, Cimino M, Guerrini U, et al. Treatment with statins after induction of focal ischemia in rats reduces the extent of brain damage. Arterioscler Thromb Vasc Biol 2003; 23:322–327.
- Fonarow GC, Wright RS, Spencer FA, et al. Effect of statin use within the first 24 hours of admission for acute myocardial infarction on early morbidity and mortality. Am J Cardiol 2005; 96:611–616.
- Heeschen C, Hamm CW, Laufs U, Snapinn S, Bohm M, White HD. Withdrawal of statins increases event rates in patients with acute coronary syndromes. Circulation 2002; 105:1446–1452.
- Kennedy J, Quan H, Buchan AM, Ghali WA, Feasby TE. Statins are associated with better outcomes after carotid endarterectomy in symptomatic patients. Stroke 2005; 36:2072–2076.
- McGirt MJ, Perler BA, Brooke BS, et al. 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors reduce the risk of perioperative stroke and mortality after carotid endarterectomy. J Vasc Surg 2005; 42:829–836.
- Hindler K, Shaw AD, Samuels J, Fulton S, Collard CD, Riedel B. Improved postoperative outcomes associated with preoperative statin therapy. Anesthesiology 2006; 105:1260–1272.
- Kapoor AS, Kanji H, Buckingham J, Devereaux PJ, McAlister FA. Strength of evidence for perioperative use of statins to reduce cardiovascular risk: systematic review of controlled studies. BMJ 2006; 333:1149.
- Durazzo AE, Machado FS, Ikeoka DT, et al. Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial. J Vasc Surg 2004; 39:967–975.
- Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004; 110:227–239.
- Kertai MD, Boersma E, Westerhout CM, et al. A combination of statins and beta-blockers is independently associated with a reduction in the incidence of perioperative mortality and nonfatal myocardial infarction in patients undergoing abdominal aortic aneurysm surgery. Eur J Vasc Endovasc Surg 2004; 28:343–352.
- Poldermans D, Bax JJ, Kertai MD, et al. Statins are associated with a reduced incidence of perioperative mortality in patients undergoing major noncardiac vascular surgery. Circulation 2003; 107:1848–1851.
- Ward RP, Leeper NJ, Kirkpatrick JN, Lang RM, Sorrentino MJ, Williams KA. The effect of preoperative statin therapy on cardiovascular outcomes in patients undergoing infrainguinal vascular surgery. Int J Cardiol 2005; 104:264–268.
- O’Neil-Callahan K, Katsimaglis G, Tepper MR, et al. Statins decrease perioperative cardiac complications in patients undergoing non-cardiac vascular surgery: the Statins for Risk Reduction in Surgery (StaRRS) study. J Am Coll Cardiol 2005; 45:336–342.
- Abbruzzese TA, Havens J, Belkin M, et al. Statin therapy is associated with improved patency of autogenous infrainguinal bypass grafts. J Vasc Surg 2004; 39:1178–1185.
- Boersma E, Poldermans D, Bax JJ, et al. Predictors of cardiac events after major vascular surgery: role of clinical characteristics, dobutamine echocardiography, and beta-blocker therapy. JAMA 2001; 285:1865–1873.
- Landesberg G, Mosseri M, Wolf YG, et al. Preoperative thallium scanning, selective coronary revascularization, and long-term survival after major vascular surgery. Circulation 2003; 108:177–183.
- Schouten O, Kertai MD, Bax JJ, et al. Safety of perioperative statin use in high-risk patients undergoing major vascular surgery. Am J Cardiol 2005; 95:658–660.
- Le Manach Y, Godet G, Coriat P, et al. The impact of postoperative discontinuation or continuation of chronic statin therapy on cardiac outcome after major vascular surgery. Anesth Analg 2007; 104:1326–1333.
- Ali IS, Buth KJ. Preoperative statin use and outcomes following cardiac surgery. Int J Cardiol 2005; 103:12–18.
- Clark LL, Ikonomidis JS, Crawford FA, et al. Preoperative statin treatment is associated with reduced postoperative mortality and morbidity in patients undergoing cardiac surgery: an 8-year retrospective cohort study. J Thorac Cardiovasc Surg 2006; 131:679–685.
- Pan W, Pintar T, Anton J, Lee VV, Vaughn WK, Collard CD. Statins are associated with a reduced incidence of perioperative mortality after coronary artery bypass graft surgery. Circulation 2004; 110(suppl 2):II45–II49.
- Pascual DA, Arribas JM, Tornel PL, et al. Preoperative statin therapy and troponin T predict early complications of coronary artery surgery. Ann Thorac Surg 2006; 81:78–83.
- Dotani MI, Elnicki DM, Jain AC, Gibson CM. Effect of preoperative statin therapy and cardiac outcomes after coronary artery bypass grafting. Am J Cardiol 2000; 86:1128–1130.
- Liakopoulos OJ, Dorge H, Schmitto JD, Nagorsnik U, Grabedunkel J, Schoendube FA. Effects of preoperative statin therapy on cytokines after cardiac surgery. Thorac Cardiovasc Surg 2006; 54:250–254.
- Christenson JT. Preoperative lipid-control with simvastatin reduces the risk of postoperative thrombocytosis and thrombotic complications following CABG. Eur J Cardiothorac Surg 1999; 15:394–399.
- Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366:1267–1278.
- Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 Guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). Circulation 2007; 116:e418–e499.
- Schouten O, Poldermans D, Visser L, et al. Fluvastatin and bisoprolol for the reduction of perioperative cardiac mortality and morbidity in high-risk patients undergoing non-cardiac surgery: rationale and design of the DECREASE-IV study. Am Heart J 2004; 148:1047–1052.
- Amar D, Zhang H, Heerdt PM, Park B, Fleisher M, Thaler HT. Statin use is associated with a reduction in atrial fibrillation after noncardiac thoracic surgery independent of C-reactive protein. Chest 2005; 128:3421–3427.
KEY POINTS
- Experiments in animals suggest that statins, given shortly before or after a cardiovascular event, confer benefit before any changes in lipids are measurable.
- Retrospective and prospective studies indicate that patients with either acute myocardial infarction or acute coronary syndrome who are already receiving statins should not have them stopped, and those who had not been receiving statins should receive them immediately.
- Most patients undergoing coronary artery bypass grafting or noncardiac vascular surgery should already be receiving a statin. These drugs can also be considered in patients undergoing intermediate-risk nonvascular surgery. Patients who have been receiving statins prior to surgery should not have them stopped for surgery.
Perioperative beta-blockers in noncardiac surgery: Evolution of the evidence
The pendulum of expert opinion is swinging away from routinely recommending beta-blockers to prevent cardiac events in non-cardiac surgery patients. We won’t be abandoning the perioperative use of beta-blockers altogether, but we will probably be using them more selectively than in the past.
The latest factor driving the trend is the online publication in May 2008 of the results of the Perioperative Ischemic Evaluation (POISE) trial,1 the largest placebo-controlled trial of perioperative beta-blocker use to date. In brief, in a cohort of patients with atherosclerotic disease or at risk for it who were undergoing noncardiac surgery, fewer patients who received extended-release metoprolol succinate had a myocardial infarction, but more of them died or had a stroke compared with those receiving placebo. (Extended-release metoprolol succinate is available in the United States as Toprol-XL and generically.)
Not so long ago, the pendulum was going the other way. After two small trials in the 1990s concluded that beta-blockers reduced the risk of perioperative cardiac events in selected patients with known or suspected coronary disease,2,3 their perioperative use was subsequently endorsed by the Leapfrog Group and the Agency for Healthcare Research and Quality. The National Quality Forum included perioperative beta-blockade in its “Safe Practices for Better Healthcare 2006 update,”4,5 and the Physician Consortium for Performance Improvement and the Surgical Care Improvement Project both listed it as a quality measure.
Since then, this practice has been closely studied, especially as concomitant research has failed to demonstrate that pre-operative coronary revascularization improves outcomes, even in the presence of ischemic disease. But evidence has been accumulating that routine use of beta-blockers may not benefit as many patients as was hoped, and may actually cause harm. The 2007 joint American College of Cardiology (ACC) and American Heart Association (AHA) guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery gives its strongest recommendation (class I: benefit clearly outweighs risk) for perioperative beta-blocker use only for patients at high risk: those with known ischemic heart disease undergoing vascular surgery and those who are already on these drugs before surgery.6
However, there are still gaps in our knowledge. Perhaps, with proper implementation, we may be able to use beta-blockers to improve outcomes in patients at intermediate risk as well. In this paper, we review the rationale and the evidence for and against perioperative use of beta-blockers and provide practical guidance for internists and hospitalists.
WHY CARDIAC EVENTS OCCUR AFTER SURGERY
Adverse cardiovascular events such as myocardial infarction and unstable angina are the leading causes of death after surgery.7 Such events occur in approximately 1% of patients older than 50 years undergoing elective inpatient surgery, but this number may be higher (approximately 5%) in those with known or suspected coronary disease.8,9 Perioperative cardiac events can also be harbingers of further complications, dramatically increasing hospital length of stay.10
Some ischemic events are caused by physiologic derangements involving the balance between inflammatory mediators, sympathetic tone, and oxygen supply and demand that occur under the stress of surgery. Others are more “traditional” in etiology, involving acute plaque rupture, thrombosis, and occlusion. Studies have consistently found a correlation between perioperative ischemia and cardiac events (both in-hospital and long-term) and death.11–17 Other studies suggest that most perioperative cardiac infarcts are non-Q-wave events,18 and most events occur within the first few days after surgery, particularly the first 48 hours, when the effects of anesthetics, pain, fluid shifts, and physiologic derangements are greatest.
Factors that may trigger acute occlusion in the perioperative period include abrupt changes in sympathetic tone, increased levels of cortisol and catecholamines, and tissue hypoxia. Other potential triggers activated or increased by the stress of surgery include coagulation factors such as alterations in platelet function; inflammatory factors such as tumor necrosis factor alpha, interleukin 1, interleukin 6, and C-reactive protein; and metabolism of free fatty acids (which contribute to increased oxygen demand as well as endothelial dysfunction).9,19,20
A 1996 autopsy study found that 38 (90%) of 42 patients who died of a perioperative infarct had evidence of acute plaque rupture or plaque hemorrhage on coronary sectioning, findings corroborated in another, similar study.21,22 These studies suggest that multiple causes contribute to perioperative myocardial infarction, and a single strategy may not suffice for prevention.
IF BETA-BLOCKERS PROTECT, HOW DO THEY DO IT?
Beta-blockers have several effects that should, in theory, protect against cardiac events during and after surgery.23 They reduce cardiac oxygen demand by reducing the force of contraction and the heart rate, and they increase the duration of diastole, when the heart muscle is perfused. They are also antiarrhythmic, and they may limit free radical production, metalloproteinase activity, and myocardial plaque inflammation.24
Some researchers have speculated that using beta-blockers long-term may alter intra-cellular signaling processes, for example decreasing the expression of receptors that receive signals for cell death, which in turn may affect the response to reperfusion cell injury and death. If this is true, there may be an advantage to starting beta-blockers well in advance of surgery.25
EARLY CLINICAL EVIDENCE IN FAVOR OF PERIOPERATIVE BETA-BLOCKER USE
Evidence in patients at high risk
Mangano et al,2 in a study published in 1996, randomized 200 patients with known coronary disease or established risk factors for it who were undergoing noncardiac surgery to receive the beta-blocker atenolol orally and intravenously or placebo in the immediate perioperative period. Fewer patients in the atenolol group died in the first 6 months after hospital discharge (0 vs 8%, P < .001), the first year (3% vs 14%, P = .005), and the first 2 years (10% vs 21%, P = .019). However, there was no difference in short-term outcomes, and the study excluded patients who died in the immediate postoperative period. If these patients had been included in the analysis, the difference in the death rate at 2 years would not have been statistically significant.26 Other critical findings: more patients in the atenolol group were using angiotensin-converting enzyme inhibitors and beta-blockers when they were discharged, and the placebo group had slightly more patients with prior myocardial infarction or diabetes.27 (Atenolol is available in the United Sates as Tenormin and generically.)
Poldermans et al,3 in a study published in 1999, randomized 112 vascular surgery patients to receive either oral bisoprolol or placebo. These patients were selected from a larger cohort of 1,351 patients on the basis of high-risk clinical features and abnormal results on dobutamine echocardiography. Bisoprolol was started at least 1 week before surgery (range 7–89 days, mean 37 days), and patients were reevaluated before surgery so that the dose could be titrated to a goal heart rate of less than 60 beats per minute. After surgery, the drug was continued for another 30 days. The study was stopped early because the bisoprolol group had a 90% lower rate of non-fatal myocardial infarction and cardiac death at 30 days. Despite the study’s limitation (eg, enrolling selected patients and using an unblinded protocol), these compelling findings made a strong case for the use of beta-blockers perioperatively in patients at high risk, ie, those with ischemic heart disease who are undergoing major vascular surgery. (Bisoprolol is available in the United States as Zebeta and generically)
Evidence in patients at intermediate risk
Boersma et al28 performed a follow-up to the study by Poldermans et al, published in 2001, in which they analyzed characteristics of all 1,351 patients who had been originally considered for enrollment. Using regression analysis, they identified seven clinical risk factors that predicted adverse cardiac events: angina, prior myocardial infarction, congestive heart failure, prior stroke, diabetes, renal failure, and age 70 years or older. Furthermore, for the entire cohort, patients receiving beta-blockers had a lower risk of cardiac complications (0.8%) than those not receiving beta-blockers (2.3%). In particular, the patients at intermediate risk (defined as having one or two risk factors) had a very low event rate regardless of stress test results, provided they were on beta-blockers: their risk of death or myocardial infarction was 0.9%, compared with 3.0% for those not on beta-blockers.
The authors concluded that dobutamine stress testing may not be necessary in patients at intermediate risk if beta-blockers are appropriately prescribed. However, others took issue with their data and conclusions, arguing that there have been so few trials that the data are still inconclusive and inadequate to ascertain the benefit of perioperative beta-blockade, particularly in patients not at high risk.26,29
The Revised Cardiac Risk Index. Although the Boersma risk-factor index is not used in general practice, numerous experts27,20–32 recommend a similar one, the Revised Cardiac Risk Index, devised by Lee et al.8 This index consists of six risk factors, each of which is worth one point:
- Congestive heart failure, based on history or examination
- Myocardial infarction, symptomatic ischemic heart disease, or a positive stress test
- Renal insufficiency (ie, serum creatinine level > 2 mg/dL)
- History of stroke or transient ischemic attack
- Diabetes requiring insulin
- High-risk surgery (defined as intrathoracic, intra-abdominal, or suprainguinal vascular surgery).
Patients with three or more points are considered to be at high risk, and those with one or two points are considered to be at intermediate risk. The ACC/AHA 2007 guidelines6 use a modified version of this index that considers the issue of surgical risk separately from the other five clinical conditions.
Devereaux et al33 performed a meta-analysis, published in 2005, of 22 studies of perioperative beta-blockade. They concluded that beta-blockers had no discernable benefit in any outcome measured, including deaths from any cause, deaths from cardiovascular causes, other cardiac events, hypotension, bradycardia, and bronchospasm. However, they based this conclusion on the use of a 99% confidence interval for each relative risk, which they believed was justified because the trials were small and the numbers of events were only moderate. When the outcomes are assessed using the more common 95% confidence interval, benefit was detected in the combined end point of cardiovascular death, nonfatal myocardial infarction, and nonfatal cardiac arrest.
Yang et al,34 Brady et al,35 and Juul et al36 performed three subsequent randomized trials that added to the controversy. Most of the patients in these trials were at intermediate or low risk, and none of the trials found a significant benefit with perioperative beta-blocker use. However, the protocols in these studies were different from the one in the study by Poldermans et al,3 which had found perioperative beta-blockade to be beneficial. Whereas patients in that earlier study started taking a beta-blocker at least 1 week before surgery (and on average much earlier), had their dose aggressively titrated to a target heart rate, and continued taking it for 30 days afterward, the protocols in the later trials called for the drug to be started within 24 hours before surgery and continued for only a short time afterward.
Lindenauer et al,37 in a retrospective study published in 2005, found that fewer surgical patients who received beta-blockers in the hospital died in the hospital. The researchers used an administrative database of more than 780,000 patients who underwent noncardiac surgery, and they used propensity-score matching to compare the postoperative mortality rates of patients who received beta-blockers and a matched group in the same large cohort who did not. Beta-blockers were associated with a lower morality rate in patients in whom the Revised Cardiac Risk Index score was 3 or greater. However, although there was a trend toward a lower rate with beta-blocker use in patients whose score was 2 (ie, at intermediate risk), the difference was not statistically significant, and patients with a score of 0 or 1 saw no benefit and were possibly harmed.
The authors admitted that their study had a number of limitations, including a retrospective design and the use of an administrative database for information regarding risk index conditions and comorbidities. In addition, because they assumed that any patient who received a beta-blocker on the first 2 hospital days was receiving appropriate perioperative treatment, they may have incorrectly estimated the number of patients who actually received these drugs as a risk-reduction strategy. For instance, some patients at low risk could have received beta-blockers for treatment of a specific event, which would be reflected as an increase in event rates for this group. They also had no data on what medications the patients received before they were hospitalized or whether the dose was titrated effectively. The study excluded all patients with congestive heart failure and chronic obstructive pulmonary disease, who may be candidates for beta-blockers in actual practice. In fact, a recent observational study in patients with severe left ventricular dysfunction suggested that these drugs substantially reduced the incidence of death in the short term and the long term.38 Finally, half the surgeries were nonelective, which makes extrapolation of their risk profile by the Revised Cardiac Risk Index difficult, since Lee et al excluded patients undergoing emergency surgery from the cohorts from which they derived and validated their index criteria.
Nevertheless, the authors concluded that patients at intermediate risk derive no benefit from perioperative beta-blocker use, and that the odds ratio for death was actually higher in patients with no risk factors who received a beta-blocker.
DOES PERIOPERATIVE BETA-BLOCKER USE CAUSE HARM?
The published data on whether perioperative beta-blocker use harms patients are conflicting and up to now have been limited.
Stone et al39 reported a substantial incidence of bradycardia requiring atropine in patients treated with a single dose of a beta-blocker preoperatively, but the complications were not clearly characterized.
The Perioperative Beta-Blockade trial.35 Significantly more patients given short-acting metoprolol had intraoperative falls in blood pressure and heart rate, and more required inotropic support during surgery, although the treating anesthesiologists refused to be blinded in that study. (Short-acting metoprolol is available in the United States as Lopressor and generically.)
Devereaux et al,33 in their meta-analysis, found a higher risk of bradycardia requiring treatment (but not a higher risk of hypotension) in beta-blocker users in nine studies, including the study by Stone et al and the Perioperative Beta-Blockade trial (relative risk 2.27, 95% confidence interval 1.36–3.80).
Conversely, at least three other studies found no difference in rates of intraoperative events.36,40,41 There are few data on the incidence of other complications such as perioperative pulmonary edema and bronchospasm.
POISE: THE FIRST LARGE RANDOMIZED TRIAL
In May 2008, results were published from POISE, the first large randomized controlled trial of perioperative beta-blockade.1 An impressive 8,351 patients—most of them at intermediate risk—were randomized to receive extended-release metoprolol succinate or placebo starting just before surgery and continuing for 30 days afterward.
Although the incidence of the primary composite end point (cardiovascular death, nonfatal myocardial infarction, and nonfatal cardiac arrest) was lower at 30 days in the metoprolol group than in the placebo group (5.8% vs 6.9%, hazard ratio 0.83, P = .04), other findings were worrisome: more metoprolol recipients died of any cause (3.1% vs 2.3%, P = .03) or had a stroke (1.0% vs 0.5%, P = .005). The major contributor to the higher mortality rate in this group appears to have been sepsis.
How beta-blockers might promote death by sepsis is unclear. The authors offered two possible explanations: perhaps beta-blocker-induced hypotension predisposes patients to infection and sepsis, or perhaps the slower heart rate and lower force of contraction induced by beta-blockers could mask normal responses to systemic infection, which in turn could delay recognition and treatment or impede the normal immune response. These mechanisms, like others, are speculative.
The risks of other adverse outcomes such as bradycardia and hypotension were substantially higher in the metoprolol group. The authors also pointed out that most of the patients who suffered nonfatal strokes were subsequently disabled or incapacitated, while most of those who suffered nonfatal cardiac events did not progress to further cardiac problems.
This new study has not yet been rigorously debated, but it will likely come under scrutiny for its dosing regimen (extended-release metoprolol succinate 100 mg or placebo 2–4 hours before surgery; another 100 mg or placebo 6 hours after surgery or sooner if the heart rate was 80 beats per minute or more and the systolic blood pressure 100 mm Hg or higher; and then 200 mg or placebo 12 hours after the second dose and every 24 hours thereafter for 30 days). This was fairly aggressive, especially for patients who have never received a beta-blocker before. In contrast, the protocol for the Perioperative Beta-Blockade trial called for only 25 to 50 mg of short-acting metoprolol twice a day. Another criticism is that the medication was started only a few hours before surgery, although there is no current standard practice for either the dose or when the treatment should be started. The population had a fairly high rate of cerebrovascular disease (perhaps predisposing to stroke whenever blood pressure dropped), and 10% of patients were undergoing urgent or emergency surgery, which carries a higher risk of morbidity.
ANY ROLE FOR BETA-BLOCKERS IN THOSE AT INTERMEDIATE RISK?
Thus, in the past decade, the appropriate perioperative use of beta-blockers, which, after the findings by Mangano et al and Poldermans et al, were seen as potentially beneficial for any patient at risk of coronary disease, with little suggestion of harm, has become more clearly defined, and the risks are more evident. The most compelling evidence in favor of using them comes from patients with ischemic heart disease undergoing vascular surgery; the 2007 ACC/AHA guidelines recommend that this group be offered beta-blockers in the absence of a contraindication (class I recommendation: benefit clearly outweighs risk).6 The guidelines also point out that these drugs should be continued in patients already taking them for cardiac indications before surgery, because ischemia may be precipitated if a beta-blocker is abruptly discontinued.42,43
Additionally, the guidelines recommend considering beta-blockers for vascular surgery patients at high cardiac risk (with a Revised Cardiac Risk Index score of 3 or more), even if they are not known to have ischemic heart disease. This is a class IIa recommendation (the benefit outweighs the risk, but more studies are required).
The guidelines also recommend that beta-blockers be considered for patients who have a score of 0 if they are undergoing vascular surgery (class IIb recommendation) or a score of 1 if they are undergoing vascular surgery (class IIa recommendation) or intermediate-risk surgery (class IIb recommendation). However, in view of the POISE results, these recommendations need to be carefully scrutinized.
These data notwithstanding, beta-blockers still might be beneficial in perioperative patients at intermediate risk.
Start beta-blockers sooner?
To help patients at intermediate risk (such as those with diabetes without known heart disease), we may need to do what Poldermans et al did3: instead of seeing patients only once a day or two before surgery, we may need to do the preoperative assessment as much as a month before and, if necessary, start a beta-blocker at a low dose, titrate it to a goal heart rate, and follow the patient closely up until surgery and afterward.
The importance of heart-rate control was illustrated in a recent cohort study of perioperative beta-blockers in vascular surgery patients,44 in which higher beta-blocker doses, carefully monitored, were associated with less ischemia and cardiac enzyme release. In addition, long-term mortality rates were lower in patients with lower heart rates. And Poldermans et al45 recently performed a study in more than 700 intermediate-risk patients who were divided into two groups, one that underwent preoperative stress testing and one that did not. Beta-blockers were given to both groups, and doses were titrated to a goal heart rate of less than 65. The patients with optimally controlled heart rates had the lowest event rates.
However, the logistics of such a program would be challenging. For the most part, internists and hospitalists involved in perioperative assessment do not control the timing of referral or surgery, and adjustments cannot be made for patients whose preoperative clinic visit falls only a few days before surgery. Instituting a second or third visit to assess the efficacy of beta-blockade burdens the patient and may not be practical.
Are all beta-blockers equivalent?
An additional factor is the choice of agent. While the most significant studies of perioperative beta-blockade have used beta-1 receptor-selective agents (ie, metoprolol, atenolol, and bisoprolol), there is no prospective evidence that any particular agent is superior. However, a recent retrospective analysis of elderly surgical patients did suggest that longer-acting beta-blockers may be preferable: patients who had been on atenolol in the year before surgery had a 20% lower risk of postoperative myocardial infarction or death than those who had been on short-acting metoprolol, with no difference in noncardiac outcomes.46
- POISE Study Group. Effect of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008; published online May 13. DOI: 10.1016/S0140-6736(08)60601-7.
- Mangano DT, Layug EL, Wallace A, Tateo I. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter Study of Perioperative Ischemia Research Group. N Engl J Med 1996; 335:1713–1720.
- Poldermans D, Boersma E, Bax JJ, et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med 1999; 341:1789–1794.
- Shojania KG, Duncan BW, McDonald KM, Wachter RM, Markowitz AJ. Making health care safer: a critical analysis of patient safety practices. Evid Rep Technol Assess (Summ) 2001; ( 43):i–x,1–668.
- National Quality Forum. Safe Practices for Better Healthcare—2006 update. Washington, DC: National Quality Forum, 2006.
- Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). Circulation 2007; 116:1971–1996.
- Mangano DT. Perioperative cardiac morbidity. Anesthesiology 1990; 72:153–184.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Devereaux PJ, Goldman L, Cook DJ, Gilbert K, Leslie K, Guyatt GH. Perioperative cardiac events in patients undergoing noncardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. Can Med Assoc J 2005; 173:627–634.
- Fleischmann KE, Goldman L, Young B, Lee TH. Association between cardiac and noncardiac complications in patients undergoing noncardiac surgery: outcomes and effects on length of stay. Am J Med 2003; 115:515–520.
- Landesberg G, Luria MH, Cotev S, et al. Importance of long-duration postoperative ST-segment depression in cardiac morbidity after vascular surgery. Lancet 1993; 341:715–719.
- Mangano DT, Browner WS, Hollenberg M, London MJ, Tubau JF, Tateo IM. Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery. The Study of Perioperative Ischemia Research Group. N Engl J Med 1990; 323:1781–1788.
- Raby KE, Goldman L, Creager MA, et al. Correlation between preoperative ischemia and major cardiac events after peripheral vascular surgery. N Engl J Med 1989; 321:1296–1300.
- Landesberg G, Mosseri M, Zahger D, et al. Myocardial infarction after vascular surgery: the role of prolonged stress-induced, ST depression-type ischemia. J Am Coll Cardiol 2001; 37:1839–1845.
- Mangano DT, Browner WS, Hollenberg M, Li J, Tateo IM. Long-term cardiac prognosis following noncardiac surgery. The Study of Perioperative Ischemia Research Group. JAMA 1992; 268:233–239.
- Kim LJ, Martinez EA, Faraday N, et al. Cardiac troponin I predicts short-term mortality in vascular surgery patients. Circulation 2002; 106:2366–2371.
- Landesberg G, Shatz V, Akopnik I, et al. Association of cardiac troponin, CK-MB, and postoperative myocardial ischemia with long-term survival after major vascular surgery. J Am Coll Cardiol 2003; 42:1547–1554.
- Badner NH, Knill RL, Brown JE, Novick TV, Gelb AW. Myocardial infarction after noncardiac surgery. Anesthesiology 1998; 88:572–578.
- Priebe HJ. Triggers of perioperative myocardial ischaemia and infarction. Br J Anaesth 2004; 93:9–20.
- Zaugg M, Schaub MC, Foex P. Myocardial injury and its prevention in the perioperative setting. Br J Anaesth 2004; 93:21–33.
- Dawood MM, Gutpa DK, Southern J, Walia A, Atkinson JB, Eagle KA. Pathology of fatal perioperative myocardial infarction: implications regarding pathophysiology and prevention. Int J Cardiol 1996; 57:37–44.
- Cohen MC, Aretz TH. Histological analysis of coronary artery lesions in fatal postoperative myocardial infarction. Cardiovasc Pathol 1999; 8:133–139.
- London MJ, Zaugg M, Schaub MC, Spahn DR. Perioperative beta-adrenergic receptor blockade: physiologic foundations and clinical controversies. Anesthesiology 2004; 100:170–175.
- Yeager MP, Fillinger MP, Hettleman BD, Hartman GS. Perioperative beta-blockade and late cardiac outcomes: a complementary hypothesis. J Cardiothorac Vasc Anesth 2005; 19:237–241.
- Zaugg M, Schaub MC, Pasch T, Spahn DR. Modulation of beta-adrenergic receptor subtype activities in perioperative medicine: mechanisms and sites of action. Br J Anaesth 2002; 88:101–123.
- Devereaux PJ, Yusuf S, Yang H, Choi PT, Guyatt GH. Are the recommendations to use perioperative beta-blocker therapy in patients undergoing non-cardiac surgery based on reliable evidence? Can Med Assoc J 2004; 171:245–247.
- Eagle KA, Froehlich JB. Reducing cardiovascular risk in patients undergoing noncardiac surgery. N Engl J Med 1996; 335:1761–1763.
- Boersma E, Poldermans D, Bax JJ, et al. Predictors of cardiac events after major vascular surgery: role of clinical characteristics, dobutamine echocardiography, and beta-blocker therapy. JAMA 2001; 285:1865–1873.
- Stevens RD, Burri H, Tramer MR. Pharmacologic myocardial protection in patients undergoing noncardiac surgery: a quantitative systematic review. Anesth Analg 2003; 97:623–633.
- Goldman L. Assessing and reducing cardiac risks of noncardiac surgery. Am J Med 2001; 110:320–323.
- Wesorick DH, Eagle KA. The preoperative cardiovascular evaluation of the intermediate-risk patient: new data, changing strategies. Am J Med 2005; 118:1413.
- Auerbach AD, Goldman L. Beta-blockers and reduction of cardiac events in noncardiac surgery: scientific review. JAMA 2002; 287:1435–1444.
- Devereaux PJ, Beattie WS, Choi PT, et al. How strong is the evidence for the use of perioperative beta blockers in non-cardiac surgery? Systematic review and meta–analysis of randomized controlled trials. BMJ 2005; 331:313–321.
- Yang H, Raymer K, Butler R, Parlow JL, Roberts RS. Metoprolol after vascular surgery (MaVS). Can J Anaesth 2004; 51( suppl 1):A7.
- Brady AR, Gibbs JS, Greenhalgh RM, Powell JT, Sydes MR. Perioperative beta-blockade (POBBLE) for patients undergoing infrarenal vascular surgery: results of a randomized double-blind controlled trial. J Vasc Surg 2005; 41:602–609.
- Juul AB, Wetterslev J, Gluud C, et al. Effect of perioperative βblockade in patients with diabetes undergoing major non-cardiac surgery: randomised placebo controlled, blinded multicentre trial. BMJ 2006; 332:1482.
- Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med 2005; 353:349–361.
- Feringa HH, Bax JJ, Schouten O, et al. Beta-blockers improve in-hospital and long-term survival in patients with severe left ventricular dysfunction undergoing major vascular surgery. Eur J Vasc Endovasc Surg 2005; 31:351–358.
- Stone JG, Foex P, Sear JW, Johnson LL, Khambatta HJ, Triner L. Myocardial ischemia in untreated hypertensive patients: effect of a single small oral dose of a beta-adrenergic blocking agent. Anesthesiology 1988; 68:495–500.
- Zaugg M, Tagliente T, Lucchinetti E, et al. Beneficial effects from beta-adrenergic blockade in elderly patients undergoing noncardiac surgery. Anesthesiology 1999; 91:1674–1686.
- Wallace A, Layug B, Tateo I, et al. Prophylactic atenolol reduces postoperative myocardial ischemia. McSPI Research Group. Anesthesiology 1998; 88:7–17.
- Psaty BM, Koepsell TD, Wagner EH, LoGerfo JP, Inui TS. The relative risk of incident coronary heart disease associated with recently stopping the use of beta-blockers. JAMA 1990; 263:1653–1657.
- Shammash JB, Trost JC, Gold JM, Berlin JA, Golden MA, Kimmel SE. Perioperative beta-blocker withdrawal and mortality in vascular surgical patients. Am Heart J 2001; 141:148–153.
- Feringa HHH, Bax JJ, Boersma E, et al. High-dose beta-blockers and tight heart rate control reduce myocardial ischemia and troponin T release in vascular surgery patients. Circulation 2006; 114( suppl 1):I-344–I-349.
- Poldermans D, Bax JJ, Schouten O, et al. Should major vascular surgery be delayed because of preoperative cardiac testing in intermediate-risk patients receiving beta-blocker therapy with tight heart rate control? J Am Coll Cardiol 2006; 48:964–969.
- Redelmeier D, Scales D, Kopp A. Beta blockers for elective surgery in elderly patients: population based, retrospective cohort study. BMJ 2005; 331:932.
The pendulum of expert opinion is swinging away from routinely recommending beta-blockers to prevent cardiac events in non-cardiac surgery patients. We won’t be abandoning the perioperative use of beta-blockers altogether, but we will probably be using them more selectively than in the past.
The latest factor driving the trend is the online publication in May 2008 of the results of the Perioperative Ischemic Evaluation (POISE) trial,1 the largest placebo-controlled trial of perioperative beta-blocker use to date. In brief, in a cohort of patients with atherosclerotic disease or at risk for it who were undergoing noncardiac surgery, fewer patients who received extended-release metoprolol succinate had a myocardial infarction, but more of them died or had a stroke compared with those receiving placebo. (Extended-release metoprolol succinate is available in the United States as Toprol-XL and generically.)
Not so long ago, the pendulum was going the other way. After two small trials in the 1990s concluded that beta-blockers reduced the risk of perioperative cardiac events in selected patients with known or suspected coronary disease,2,3 their perioperative use was subsequently endorsed by the Leapfrog Group and the Agency for Healthcare Research and Quality. The National Quality Forum included perioperative beta-blockade in its “Safe Practices for Better Healthcare 2006 update,”4,5 and the Physician Consortium for Performance Improvement and the Surgical Care Improvement Project both listed it as a quality measure.
Since then, this practice has been closely studied, especially as concomitant research has failed to demonstrate that pre-operative coronary revascularization improves outcomes, even in the presence of ischemic disease. But evidence has been accumulating that routine use of beta-blockers may not benefit as many patients as was hoped, and may actually cause harm. The 2007 joint American College of Cardiology (ACC) and American Heart Association (AHA) guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery gives its strongest recommendation (class I: benefit clearly outweighs risk) for perioperative beta-blocker use only for patients at high risk: those with known ischemic heart disease undergoing vascular surgery and those who are already on these drugs before surgery.6
However, there are still gaps in our knowledge. Perhaps, with proper implementation, we may be able to use beta-blockers to improve outcomes in patients at intermediate risk as well. In this paper, we review the rationale and the evidence for and against perioperative use of beta-blockers and provide practical guidance for internists and hospitalists.
WHY CARDIAC EVENTS OCCUR AFTER SURGERY
Adverse cardiovascular events such as myocardial infarction and unstable angina are the leading causes of death after surgery.7 Such events occur in approximately 1% of patients older than 50 years undergoing elective inpatient surgery, but this number may be higher (approximately 5%) in those with known or suspected coronary disease.8,9 Perioperative cardiac events can also be harbingers of further complications, dramatically increasing hospital length of stay.10
Some ischemic events are caused by physiologic derangements involving the balance between inflammatory mediators, sympathetic tone, and oxygen supply and demand that occur under the stress of surgery. Others are more “traditional” in etiology, involving acute plaque rupture, thrombosis, and occlusion. Studies have consistently found a correlation between perioperative ischemia and cardiac events (both in-hospital and long-term) and death.11–17 Other studies suggest that most perioperative cardiac infarcts are non-Q-wave events,18 and most events occur within the first few days after surgery, particularly the first 48 hours, when the effects of anesthetics, pain, fluid shifts, and physiologic derangements are greatest.
Factors that may trigger acute occlusion in the perioperative period include abrupt changes in sympathetic tone, increased levels of cortisol and catecholamines, and tissue hypoxia. Other potential triggers activated or increased by the stress of surgery include coagulation factors such as alterations in platelet function; inflammatory factors such as tumor necrosis factor alpha, interleukin 1, interleukin 6, and C-reactive protein; and metabolism of free fatty acids (which contribute to increased oxygen demand as well as endothelial dysfunction).9,19,20
A 1996 autopsy study found that 38 (90%) of 42 patients who died of a perioperative infarct had evidence of acute plaque rupture or plaque hemorrhage on coronary sectioning, findings corroborated in another, similar study.21,22 These studies suggest that multiple causes contribute to perioperative myocardial infarction, and a single strategy may not suffice for prevention.
IF BETA-BLOCKERS PROTECT, HOW DO THEY DO IT?
Beta-blockers have several effects that should, in theory, protect against cardiac events during and after surgery.23 They reduce cardiac oxygen demand by reducing the force of contraction and the heart rate, and they increase the duration of diastole, when the heart muscle is perfused. They are also antiarrhythmic, and they may limit free radical production, metalloproteinase activity, and myocardial plaque inflammation.24
Some researchers have speculated that using beta-blockers long-term may alter intra-cellular signaling processes, for example decreasing the expression of receptors that receive signals for cell death, which in turn may affect the response to reperfusion cell injury and death. If this is true, there may be an advantage to starting beta-blockers well in advance of surgery.25
EARLY CLINICAL EVIDENCE IN FAVOR OF PERIOPERATIVE BETA-BLOCKER USE
Evidence in patients at high risk
Mangano et al,2 in a study published in 1996, randomized 200 patients with known coronary disease or established risk factors for it who were undergoing noncardiac surgery to receive the beta-blocker atenolol orally and intravenously or placebo in the immediate perioperative period. Fewer patients in the atenolol group died in the first 6 months after hospital discharge (0 vs 8%, P < .001), the first year (3% vs 14%, P = .005), and the first 2 years (10% vs 21%, P = .019). However, there was no difference in short-term outcomes, and the study excluded patients who died in the immediate postoperative period. If these patients had been included in the analysis, the difference in the death rate at 2 years would not have been statistically significant.26 Other critical findings: more patients in the atenolol group were using angiotensin-converting enzyme inhibitors and beta-blockers when they were discharged, and the placebo group had slightly more patients with prior myocardial infarction or diabetes.27 (Atenolol is available in the United Sates as Tenormin and generically.)
Poldermans et al,3 in a study published in 1999, randomized 112 vascular surgery patients to receive either oral bisoprolol or placebo. These patients were selected from a larger cohort of 1,351 patients on the basis of high-risk clinical features and abnormal results on dobutamine echocardiography. Bisoprolol was started at least 1 week before surgery (range 7–89 days, mean 37 days), and patients were reevaluated before surgery so that the dose could be titrated to a goal heart rate of less than 60 beats per minute. After surgery, the drug was continued for another 30 days. The study was stopped early because the bisoprolol group had a 90% lower rate of non-fatal myocardial infarction and cardiac death at 30 days. Despite the study’s limitation (eg, enrolling selected patients and using an unblinded protocol), these compelling findings made a strong case for the use of beta-blockers perioperatively in patients at high risk, ie, those with ischemic heart disease who are undergoing major vascular surgery. (Bisoprolol is available in the United States as Zebeta and generically)
Evidence in patients at intermediate risk
Boersma et al28 performed a follow-up to the study by Poldermans et al, published in 2001, in which they analyzed characteristics of all 1,351 patients who had been originally considered for enrollment. Using regression analysis, they identified seven clinical risk factors that predicted adverse cardiac events: angina, prior myocardial infarction, congestive heart failure, prior stroke, diabetes, renal failure, and age 70 years or older. Furthermore, for the entire cohort, patients receiving beta-blockers had a lower risk of cardiac complications (0.8%) than those not receiving beta-blockers (2.3%). In particular, the patients at intermediate risk (defined as having one or two risk factors) had a very low event rate regardless of stress test results, provided they were on beta-blockers: their risk of death or myocardial infarction was 0.9%, compared with 3.0% for those not on beta-blockers.
The authors concluded that dobutamine stress testing may not be necessary in patients at intermediate risk if beta-blockers are appropriately prescribed. However, others took issue with their data and conclusions, arguing that there have been so few trials that the data are still inconclusive and inadequate to ascertain the benefit of perioperative beta-blockade, particularly in patients not at high risk.26,29
The Revised Cardiac Risk Index. Although the Boersma risk-factor index is not used in general practice, numerous experts27,20–32 recommend a similar one, the Revised Cardiac Risk Index, devised by Lee et al.8 This index consists of six risk factors, each of which is worth one point:
- Congestive heart failure, based on history or examination
- Myocardial infarction, symptomatic ischemic heart disease, or a positive stress test
- Renal insufficiency (ie, serum creatinine level > 2 mg/dL)
- History of stroke or transient ischemic attack
- Diabetes requiring insulin
- High-risk surgery (defined as intrathoracic, intra-abdominal, or suprainguinal vascular surgery).
Patients with three or more points are considered to be at high risk, and those with one or two points are considered to be at intermediate risk. The ACC/AHA 2007 guidelines6 use a modified version of this index that considers the issue of surgical risk separately from the other five clinical conditions.
Devereaux et al33 performed a meta-analysis, published in 2005, of 22 studies of perioperative beta-blockade. They concluded that beta-blockers had no discernable benefit in any outcome measured, including deaths from any cause, deaths from cardiovascular causes, other cardiac events, hypotension, bradycardia, and bronchospasm. However, they based this conclusion on the use of a 99% confidence interval for each relative risk, which they believed was justified because the trials were small and the numbers of events were only moderate. When the outcomes are assessed using the more common 95% confidence interval, benefit was detected in the combined end point of cardiovascular death, nonfatal myocardial infarction, and nonfatal cardiac arrest.
Yang et al,34 Brady et al,35 and Juul et al36 performed three subsequent randomized trials that added to the controversy. Most of the patients in these trials were at intermediate or low risk, and none of the trials found a significant benefit with perioperative beta-blocker use. However, the protocols in these studies were different from the one in the study by Poldermans et al,3 which had found perioperative beta-blockade to be beneficial. Whereas patients in that earlier study started taking a beta-blocker at least 1 week before surgery (and on average much earlier), had their dose aggressively titrated to a target heart rate, and continued taking it for 30 days afterward, the protocols in the later trials called for the drug to be started within 24 hours before surgery and continued for only a short time afterward.
Lindenauer et al,37 in a retrospective study published in 2005, found that fewer surgical patients who received beta-blockers in the hospital died in the hospital. The researchers used an administrative database of more than 780,000 patients who underwent noncardiac surgery, and they used propensity-score matching to compare the postoperative mortality rates of patients who received beta-blockers and a matched group in the same large cohort who did not. Beta-blockers were associated with a lower morality rate in patients in whom the Revised Cardiac Risk Index score was 3 or greater. However, although there was a trend toward a lower rate with beta-blocker use in patients whose score was 2 (ie, at intermediate risk), the difference was not statistically significant, and patients with a score of 0 or 1 saw no benefit and were possibly harmed.
The authors admitted that their study had a number of limitations, including a retrospective design and the use of an administrative database for information regarding risk index conditions and comorbidities. In addition, because they assumed that any patient who received a beta-blocker on the first 2 hospital days was receiving appropriate perioperative treatment, they may have incorrectly estimated the number of patients who actually received these drugs as a risk-reduction strategy. For instance, some patients at low risk could have received beta-blockers for treatment of a specific event, which would be reflected as an increase in event rates for this group. They also had no data on what medications the patients received before they were hospitalized or whether the dose was titrated effectively. The study excluded all patients with congestive heart failure and chronic obstructive pulmonary disease, who may be candidates for beta-blockers in actual practice. In fact, a recent observational study in patients with severe left ventricular dysfunction suggested that these drugs substantially reduced the incidence of death in the short term and the long term.38 Finally, half the surgeries were nonelective, which makes extrapolation of their risk profile by the Revised Cardiac Risk Index difficult, since Lee et al excluded patients undergoing emergency surgery from the cohorts from which they derived and validated their index criteria.
Nevertheless, the authors concluded that patients at intermediate risk derive no benefit from perioperative beta-blocker use, and that the odds ratio for death was actually higher in patients with no risk factors who received a beta-blocker.
DOES PERIOPERATIVE BETA-BLOCKER USE CAUSE HARM?
The published data on whether perioperative beta-blocker use harms patients are conflicting and up to now have been limited.
Stone et al39 reported a substantial incidence of bradycardia requiring atropine in patients treated with a single dose of a beta-blocker preoperatively, but the complications were not clearly characterized.
The Perioperative Beta-Blockade trial.35 Significantly more patients given short-acting metoprolol had intraoperative falls in blood pressure and heart rate, and more required inotropic support during surgery, although the treating anesthesiologists refused to be blinded in that study. (Short-acting metoprolol is available in the United States as Lopressor and generically.)
Devereaux et al,33 in their meta-analysis, found a higher risk of bradycardia requiring treatment (but not a higher risk of hypotension) in beta-blocker users in nine studies, including the study by Stone et al and the Perioperative Beta-Blockade trial (relative risk 2.27, 95% confidence interval 1.36–3.80).
Conversely, at least three other studies found no difference in rates of intraoperative events.36,40,41 There are few data on the incidence of other complications such as perioperative pulmonary edema and bronchospasm.
POISE: THE FIRST LARGE RANDOMIZED TRIAL
In May 2008, results were published from POISE, the first large randomized controlled trial of perioperative beta-blockade.1 An impressive 8,351 patients—most of them at intermediate risk—were randomized to receive extended-release metoprolol succinate or placebo starting just before surgery and continuing for 30 days afterward.
Although the incidence of the primary composite end point (cardiovascular death, nonfatal myocardial infarction, and nonfatal cardiac arrest) was lower at 30 days in the metoprolol group than in the placebo group (5.8% vs 6.9%, hazard ratio 0.83, P = .04), other findings were worrisome: more metoprolol recipients died of any cause (3.1% vs 2.3%, P = .03) or had a stroke (1.0% vs 0.5%, P = .005). The major contributor to the higher mortality rate in this group appears to have been sepsis.
How beta-blockers might promote death by sepsis is unclear. The authors offered two possible explanations: perhaps beta-blocker-induced hypotension predisposes patients to infection and sepsis, or perhaps the slower heart rate and lower force of contraction induced by beta-blockers could mask normal responses to systemic infection, which in turn could delay recognition and treatment or impede the normal immune response. These mechanisms, like others, are speculative.
The risks of other adverse outcomes such as bradycardia and hypotension were substantially higher in the metoprolol group. The authors also pointed out that most of the patients who suffered nonfatal strokes were subsequently disabled or incapacitated, while most of those who suffered nonfatal cardiac events did not progress to further cardiac problems.
This new study has not yet been rigorously debated, but it will likely come under scrutiny for its dosing regimen (extended-release metoprolol succinate 100 mg or placebo 2–4 hours before surgery; another 100 mg or placebo 6 hours after surgery or sooner if the heart rate was 80 beats per minute or more and the systolic blood pressure 100 mm Hg or higher; and then 200 mg or placebo 12 hours after the second dose and every 24 hours thereafter for 30 days). This was fairly aggressive, especially for patients who have never received a beta-blocker before. In contrast, the protocol for the Perioperative Beta-Blockade trial called for only 25 to 50 mg of short-acting metoprolol twice a day. Another criticism is that the medication was started only a few hours before surgery, although there is no current standard practice for either the dose or when the treatment should be started. The population had a fairly high rate of cerebrovascular disease (perhaps predisposing to stroke whenever blood pressure dropped), and 10% of patients were undergoing urgent or emergency surgery, which carries a higher risk of morbidity.
ANY ROLE FOR BETA-BLOCKERS IN THOSE AT INTERMEDIATE RISK?
Thus, in the past decade, the appropriate perioperative use of beta-blockers, which, after the findings by Mangano et al and Poldermans et al, were seen as potentially beneficial for any patient at risk of coronary disease, with little suggestion of harm, has become more clearly defined, and the risks are more evident. The most compelling evidence in favor of using them comes from patients with ischemic heart disease undergoing vascular surgery; the 2007 ACC/AHA guidelines recommend that this group be offered beta-blockers in the absence of a contraindication (class I recommendation: benefit clearly outweighs risk).6 The guidelines also point out that these drugs should be continued in patients already taking them for cardiac indications before surgery, because ischemia may be precipitated if a beta-blocker is abruptly discontinued.42,43
Additionally, the guidelines recommend considering beta-blockers for vascular surgery patients at high cardiac risk (with a Revised Cardiac Risk Index score of 3 or more), even if they are not known to have ischemic heart disease. This is a class IIa recommendation (the benefit outweighs the risk, but more studies are required).
The guidelines also recommend that beta-blockers be considered for patients who have a score of 0 if they are undergoing vascular surgery (class IIb recommendation) or a score of 1 if they are undergoing vascular surgery (class IIa recommendation) or intermediate-risk surgery (class IIb recommendation). However, in view of the POISE results, these recommendations need to be carefully scrutinized.
These data notwithstanding, beta-blockers still might be beneficial in perioperative patients at intermediate risk.
Start beta-blockers sooner?
To help patients at intermediate risk (such as those with diabetes without known heart disease), we may need to do what Poldermans et al did3: instead of seeing patients only once a day or two before surgery, we may need to do the preoperative assessment as much as a month before and, if necessary, start a beta-blocker at a low dose, titrate it to a goal heart rate, and follow the patient closely up until surgery and afterward.
The importance of heart-rate control was illustrated in a recent cohort study of perioperative beta-blockers in vascular surgery patients,44 in which higher beta-blocker doses, carefully monitored, were associated with less ischemia and cardiac enzyme release. In addition, long-term mortality rates were lower in patients with lower heart rates. And Poldermans et al45 recently performed a study in more than 700 intermediate-risk patients who were divided into two groups, one that underwent preoperative stress testing and one that did not. Beta-blockers were given to both groups, and doses were titrated to a goal heart rate of less than 65. The patients with optimally controlled heart rates had the lowest event rates.
However, the logistics of such a program would be challenging. For the most part, internists and hospitalists involved in perioperative assessment do not control the timing of referral or surgery, and adjustments cannot be made for patients whose preoperative clinic visit falls only a few days before surgery. Instituting a second or third visit to assess the efficacy of beta-blockade burdens the patient and may not be practical.
Are all beta-blockers equivalent?
An additional factor is the choice of agent. While the most significant studies of perioperative beta-blockade have used beta-1 receptor-selective agents (ie, metoprolol, atenolol, and bisoprolol), there is no prospective evidence that any particular agent is superior. However, a recent retrospective analysis of elderly surgical patients did suggest that longer-acting beta-blockers may be preferable: patients who had been on atenolol in the year before surgery had a 20% lower risk of postoperative myocardial infarction or death than those who had been on short-acting metoprolol, with no difference in noncardiac outcomes.46
The pendulum of expert opinion is swinging away from routinely recommending beta-blockers to prevent cardiac events in non-cardiac surgery patients. We won’t be abandoning the perioperative use of beta-blockers altogether, but we will probably be using them more selectively than in the past.
The latest factor driving the trend is the online publication in May 2008 of the results of the Perioperative Ischemic Evaluation (POISE) trial,1 the largest placebo-controlled trial of perioperative beta-blocker use to date. In brief, in a cohort of patients with atherosclerotic disease or at risk for it who were undergoing noncardiac surgery, fewer patients who received extended-release metoprolol succinate had a myocardial infarction, but more of them died or had a stroke compared with those receiving placebo. (Extended-release metoprolol succinate is available in the United States as Toprol-XL and generically.)
Not so long ago, the pendulum was going the other way. After two small trials in the 1990s concluded that beta-blockers reduced the risk of perioperative cardiac events in selected patients with known or suspected coronary disease,2,3 their perioperative use was subsequently endorsed by the Leapfrog Group and the Agency for Healthcare Research and Quality. The National Quality Forum included perioperative beta-blockade in its “Safe Practices for Better Healthcare 2006 update,”4,5 and the Physician Consortium for Performance Improvement and the Surgical Care Improvement Project both listed it as a quality measure.
Since then, this practice has been closely studied, especially as concomitant research has failed to demonstrate that pre-operative coronary revascularization improves outcomes, even in the presence of ischemic disease. But evidence has been accumulating that routine use of beta-blockers may not benefit as many patients as was hoped, and may actually cause harm. The 2007 joint American College of Cardiology (ACC) and American Heart Association (AHA) guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery gives its strongest recommendation (class I: benefit clearly outweighs risk) for perioperative beta-blocker use only for patients at high risk: those with known ischemic heart disease undergoing vascular surgery and those who are already on these drugs before surgery.6
However, there are still gaps in our knowledge. Perhaps, with proper implementation, we may be able to use beta-blockers to improve outcomes in patients at intermediate risk as well. In this paper, we review the rationale and the evidence for and against perioperative use of beta-blockers and provide practical guidance for internists and hospitalists.
WHY CARDIAC EVENTS OCCUR AFTER SURGERY
Adverse cardiovascular events such as myocardial infarction and unstable angina are the leading causes of death after surgery.7 Such events occur in approximately 1% of patients older than 50 years undergoing elective inpatient surgery, but this number may be higher (approximately 5%) in those with known or suspected coronary disease.8,9 Perioperative cardiac events can also be harbingers of further complications, dramatically increasing hospital length of stay.10
Some ischemic events are caused by physiologic derangements involving the balance between inflammatory mediators, sympathetic tone, and oxygen supply and demand that occur under the stress of surgery. Others are more “traditional” in etiology, involving acute plaque rupture, thrombosis, and occlusion. Studies have consistently found a correlation between perioperative ischemia and cardiac events (both in-hospital and long-term) and death.11–17 Other studies suggest that most perioperative cardiac infarcts are non-Q-wave events,18 and most events occur within the first few days after surgery, particularly the first 48 hours, when the effects of anesthetics, pain, fluid shifts, and physiologic derangements are greatest.
Factors that may trigger acute occlusion in the perioperative period include abrupt changes in sympathetic tone, increased levels of cortisol and catecholamines, and tissue hypoxia. Other potential triggers activated or increased by the stress of surgery include coagulation factors such as alterations in platelet function; inflammatory factors such as tumor necrosis factor alpha, interleukin 1, interleukin 6, and C-reactive protein; and metabolism of free fatty acids (which contribute to increased oxygen demand as well as endothelial dysfunction).9,19,20
A 1996 autopsy study found that 38 (90%) of 42 patients who died of a perioperative infarct had evidence of acute plaque rupture or plaque hemorrhage on coronary sectioning, findings corroborated in another, similar study.21,22 These studies suggest that multiple causes contribute to perioperative myocardial infarction, and a single strategy may not suffice for prevention.
IF BETA-BLOCKERS PROTECT, HOW DO THEY DO IT?
Beta-blockers have several effects that should, in theory, protect against cardiac events during and after surgery.23 They reduce cardiac oxygen demand by reducing the force of contraction and the heart rate, and they increase the duration of diastole, when the heart muscle is perfused. They are also antiarrhythmic, and they may limit free radical production, metalloproteinase activity, and myocardial plaque inflammation.24
Some researchers have speculated that using beta-blockers long-term may alter intra-cellular signaling processes, for example decreasing the expression of receptors that receive signals for cell death, which in turn may affect the response to reperfusion cell injury and death. If this is true, there may be an advantage to starting beta-blockers well in advance of surgery.25
EARLY CLINICAL EVIDENCE IN FAVOR OF PERIOPERATIVE BETA-BLOCKER USE
Evidence in patients at high risk
Mangano et al,2 in a study published in 1996, randomized 200 patients with known coronary disease or established risk factors for it who were undergoing noncardiac surgery to receive the beta-blocker atenolol orally and intravenously or placebo in the immediate perioperative period. Fewer patients in the atenolol group died in the first 6 months after hospital discharge (0 vs 8%, P < .001), the first year (3% vs 14%, P = .005), and the first 2 years (10% vs 21%, P = .019). However, there was no difference in short-term outcomes, and the study excluded patients who died in the immediate postoperative period. If these patients had been included in the analysis, the difference in the death rate at 2 years would not have been statistically significant.26 Other critical findings: more patients in the atenolol group were using angiotensin-converting enzyme inhibitors and beta-blockers when they were discharged, and the placebo group had slightly more patients with prior myocardial infarction or diabetes.27 (Atenolol is available in the United Sates as Tenormin and generically.)
Poldermans et al,3 in a study published in 1999, randomized 112 vascular surgery patients to receive either oral bisoprolol or placebo. These patients were selected from a larger cohort of 1,351 patients on the basis of high-risk clinical features and abnormal results on dobutamine echocardiography. Bisoprolol was started at least 1 week before surgery (range 7–89 days, mean 37 days), and patients were reevaluated before surgery so that the dose could be titrated to a goal heart rate of less than 60 beats per minute. After surgery, the drug was continued for another 30 days. The study was stopped early because the bisoprolol group had a 90% lower rate of non-fatal myocardial infarction and cardiac death at 30 days. Despite the study’s limitation (eg, enrolling selected patients and using an unblinded protocol), these compelling findings made a strong case for the use of beta-blockers perioperatively in patients at high risk, ie, those with ischemic heart disease who are undergoing major vascular surgery. (Bisoprolol is available in the United States as Zebeta and generically)
Evidence in patients at intermediate risk
Boersma et al28 performed a follow-up to the study by Poldermans et al, published in 2001, in which they analyzed characteristics of all 1,351 patients who had been originally considered for enrollment. Using regression analysis, they identified seven clinical risk factors that predicted adverse cardiac events: angina, prior myocardial infarction, congestive heart failure, prior stroke, diabetes, renal failure, and age 70 years or older. Furthermore, for the entire cohort, patients receiving beta-blockers had a lower risk of cardiac complications (0.8%) than those not receiving beta-blockers (2.3%). In particular, the patients at intermediate risk (defined as having one or two risk factors) had a very low event rate regardless of stress test results, provided they were on beta-blockers: their risk of death or myocardial infarction was 0.9%, compared with 3.0% for those not on beta-blockers.
The authors concluded that dobutamine stress testing may not be necessary in patients at intermediate risk if beta-blockers are appropriately prescribed. However, others took issue with their data and conclusions, arguing that there have been so few trials that the data are still inconclusive and inadequate to ascertain the benefit of perioperative beta-blockade, particularly in patients not at high risk.26,29
The Revised Cardiac Risk Index. Although the Boersma risk-factor index is not used in general practice, numerous experts27,20–32 recommend a similar one, the Revised Cardiac Risk Index, devised by Lee et al.8 This index consists of six risk factors, each of which is worth one point:
- Congestive heart failure, based on history or examination
- Myocardial infarction, symptomatic ischemic heart disease, or a positive stress test
- Renal insufficiency (ie, serum creatinine level > 2 mg/dL)
- History of stroke or transient ischemic attack
- Diabetes requiring insulin
- High-risk surgery (defined as intrathoracic, intra-abdominal, or suprainguinal vascular surgery).
Patients with three or more points are considered to be at high risk, and those with one or two points are considered to be at intermediate risk. The ACC/AHA 2007 guidelines6 use a modified version of this index that considers the issue of surgical risk separately from the other five clinical conditions.
Devereaux et al33 performed a meta-analysis, published in 2005, of 22 studies of perioperative beta-blockade. They concluded that beta-blockers had no discernable benefit in any outcome measured, including deaths from any cause, deaths from cardiovascular causes, other cardiac events, hypotension, bradycardia, and bronchospasm. However, they based this conclusion on the use of a 99% confidence interval for each relative risk, which they believed was justified because the trials were small and the numbers of events were only moderate. When the outcomes are assessed using the more common 95% confidence interval, benefit was detected in the combined end point of cardiovascular death, nonfatal myocardial infarction, and nonfatal cardiac arrest.
Yang et al,34 Brady et al,35 and Juul et al36 performed three subsequent randomized trials that added to the controversy. Most of the patients in these trials were at intermediate or low risk, and none of the trials found a significant benefit with perioperative beta-blocker use. However, the protocols in these studies were different from the one in the study by Poldermans et al,3 which had found perioperative beta-blockade to be beneficial. Whereas patients in that earlier study started taking a beta-blocker at least 1 week before surgery (and on average much earlier), had their dose aggressively titrated to a target heart rate, and continued taking it for 30 days afterward, the protocols in the later trials called for the drug to be started within 24 hours before surgery and continued for only a short time afterward.
Lindenauer et al,37 in a retrospective study published in 2005, found that fewer surgical patients who received beta-blockers in the hospital died in the hospital. The researchers used an administrative database of more than 780,000 patients who underwent noncardiac surgery, and they used propensity-score matching to compare the postoperative mortality rates of patients who received beta-blockers and a matched group in the same large cohort who did not. Beta-blockers were associated with a lower morality rate in patients in whom the Revised Cardiac Risk Index score was 3 or greater. However, although there was a trend toward a lower rate with beta-blocker use in patients whose score was 2 (ie, at intermediate risk), the difference was not statistically significant, and patients with a score of 0 or 1 saw no benefit and were possibly harmed.
The authors admitted that their study had a number of limitations, including a retrospective design and the use of an administrative database for information regarding risk index conditions and comorbidities. In addition, because they assumed that any patient who received a beta-blocker on the first 2 hospital days was receiving appropriate perioperative treatment, they may have incorrectly estimated the number of patients who actually received these drugs as a risk-reduction strategy. For instance, some patients at low risk could have received beta-blockers for treatment of a specific event, which would be reflected as an increase in event rates for this group. They also had no data on what medications the patients received before they were hospitalized or whether the dose was titrated effectively. The study excluded all patients with congestive heart failure and chronic obstructive pulmonary disease, who may be candidates for beta-blockers in actual practice. In fact, a recent observational study in patients with severe left ventricular dysfunction suggested that these drugs substantially reduced the incidence of death in the short term and the long term.38 Finally, half the surgeries were nonelective, which makes extrapolation of their risk profile by the Revised Cardiac Risk Index difficult, since Lee et al excluded patients undergoing emergency surgery from the cohorts from which they derived and validated their index criteria.
Nevertheless, the authors concluded that patients at intermediate risk derive no benefit from perioperative beta-blocker use, and that the odds ratio for death was actually higher in patients with no risk factors who received a beta-blocker.
DOES PERIOPERATIVE BETA-BLOCKER USE CAUSE HARM?
The published data on whether perioperative beta-blocker use harms patients are conflicting and up to now have been limited.
Stone et al39 reported a substantial incidence of bradycardia requiring atropine in patients treated with a single dose of a beta-blocker preoperatively, but the complications were not clearly characterized.
The Perioperative Beta-Blockade trial.35 Significantly more patients given short-acting metoprolol had intraoperative falls in blood pressure and heart rate, and more required inotropic support during surgery, although the treating anesthesiologists refused to be blinded in that study. (Short-acting metoprolol is available in the United States as Lopressor and generically.)
Devereaux et al,33 in their meta-analysis, found a higher risk of bradycardia requiring treatment (but not a higher risk of hypotension) in beta-blocker users in nine studies, including the study by Stone et al and the Perioperative Beta-Blockade trial (relative risk 2.27, 95% confidence interval 1.36–3.80).
Conversely, at least three other studies found no difference in rates of intraoperative events.36,40,41 There are few data on the incidence of other complications such as perioperative pulmonary edema and bronchospasm.
POISE: THE FIRST LARGE RANDOMIZED TRIAL
In May 2008, results were published from POISE, the first large randomized controlled trial of perioperative beta-blockade.1 An impressive 8,351 patients—most of them at intermediate risk—were randomized to receive extended-release metoprolol succinate or placebo starting just before surgery and continuing for 30 days afterward.
Although the incidence of the primary composite end point (cardiovascular death, nonfatal myocardial infarction, and nonfatal cardiac arrest) was lower at 30 days in the metoprolol group than in the placebo group (5.8% vs 6.9%, hazard ratio 0.83, P = .04), other findings were worrisome: more metoprolol recipients died of any cause (3.1% vs 2.3%, P = .03) or had a stroke (1.0% vs 0.5%, P = .005). The major contributor to the higher mortality rate in this group appears to have been sepsis.
How beta-blockers might promote death by sepsis is unclear. The authors offered two possible explanations: perhaps beta-blocker-induced hypotension predisposes patients to infection and sepsis, or perhaps the slower heart rate and lower force of contraction induced by beta-blockers could mask normal responses to systemic infection, which in turn could delay recognition and treatment or impede the normal immune response. These mechanisms, like others, are speculative.
The risks of other adverse outcomes such as bradycardia and hypotension were substantially higher in the metoprolol group. The authors also pointed out that most of the patients who suffered nonfatal strokes were subsequently disabled or incapacitated, while most of those who suffered nonfatal cardiac events did not progress to further cardiac problems.
This new study has not yet been rigorously debated, but it will likely come under scrutiny for its dosing regimen (extended-release metoprolol succinate 100 mg or placebo 2–4 hours before surgery; another 100 mg or placebo 6 hours after surgery or sooner if the heart rate was 80 beats per minute or more and the systolic blood pressure 100 mm Hg or higher; and then 200 mg or placebo 12 hours after the second dose and every 24 hours thereafter for 30 days). This was fairly aggressive, especially for patients who have never received a beta-blocker before. In contrast, the protocol for the Perioperative Beta-Blockade trial called for only 25 to 50 mg of short-acting metoprolol twice a day. Another criticism is that the medication was started only a few hours before surgery, although there is no current standard practice for either the dose or when the treatment should be started. The population had a fairly high rate of cerebrovascular disease (perhaps predisposing to stroke whenever blood pressure dropped), and 10% of patients were undergoing urgent or emergency surgery, which carries a higher risk of morbidity.
ANY ROLE FOR BETA-BLOCKERS IN THOSE AT INTERMEDIATE RISK?
Thus, in the past decade, the appropriate perioperative use of beta-blockers, which, after the findings by Mangano et al and Poldermans et al, were seen as potentially beneficial for any patient at risk of coronary disease, with little suggestion of harm, has become more clearly defined, and the risks are more evident. The most compelling evidence in favor of using them comes from patients with ischemic heart disease undergoing vascular surgery; the 2007 ACC/AHA guidelines recommend that this group be offered beta-blockers in the absence of a contraindication (class I recommendation: benefit clearly outweighs risk).6 The guidelines also point out that these drugs should be continued in patients already taking them for cardiac indications before surgery, because ischemia may be precipitated if a beta-blocker is abruptly discontinued.42,43
Additionally, the guidelines recommend considering beta-blockers for vascular surgery patients at high cardiac risk (with a Revised Cardiac Risk Index score of 3 or more), even if they are not known to have ischemic heart disease. This is a class IIa recommendation (the benefit outweighs the risk, but more studies are required).
The guidelines also recommend that beta-blockers be considered for patients who have a score of 0 if they are undergoing vascular surgery (class IIb recommendation) or a score of 1 if they are undergoing vascular surgery (class IIa recommendation) or intermediate-risk surgery (class IIb recommendation). However, in view of the POISE results, these recommendations need to be carefully scrutinized.
These data notwithstanding, beta-blockers still might be beneficial in perioperative patients at intermediate risk.
Start beta-blockers sooner?
To help patients at intermediate risk (such as those with diabetes without known heart disease), we may need to do what Poldermans et al did3: instead of seeing patients only once a day or two before surgery, we may need to do the preoperative assessment as much as a month before and, if necessary, start a beta-blocker at a low dose, titrate it to a goal heart rate, and follow the patient closely up until surgery and afterward.
The importance of heart-rate control was illustrated in a recent cohort study of perioperative beta-blockers in vascular surgery patients,44 in which higher beta-blocker doses, carefully monitored, were associated with less ischemia and cardiac enzyme release. In addition, long-term mortality rates were lower in patients with lower heart rates. And Poldermans et al45 recently performed a study in more than 700 intermediate-risk patients who were divided into two groups, one that underwent preoperative stress testing and one that did not. Beta-blockers were given to both groups, and doses were titrated to a goal heart rate of less than 65. The patients with optimally controlled heart rates had the lowest event rates.
However, the logistics of such a program would be challenging. For the most part, internists and hospitalists involved in perioperative assessment do not control the timing of referral or surgery, and adjustments cannot be made for patients whose preoperative clinic visit falls only a few days before surgery. Instituting a second or third visit to assess the efficacy of beta-blockade burdens the patient and may not be practical.
Are all beta-blockers equivalent?
An additional factor is the choice of agent. While the most significant studies of perioperative beta-blockade have used beta-1 receptor-selective agents (ie, metoprolol, atenolol, and bisoprolol), there is no prospective evidence that any particular agent is superior. However, a recent retrospective analysis of elderly surgical patients did suggest that longer-acting beta-blockers may be preferable: patients who had been on atenolol in the year before surgery had a 20% lower risk of postoperative myocardial infarction or death than those who had been on short-acting metoprolol, with no difference in noncardiac outcomes.46
- POISE Study Group. Effect of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008; published online May 13. DOI: 10.1016/S0140-6736(08)60601-7.
- Mangano DT, Layug EL, Wallace A, Tateo I. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter Study of Perioperative Ischemia Research Group. N Engl J Med 1996; 335:1713–1720.
- Poldermans D, Boersma E, Bax JJ, et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med 1999; 341:1789–1794.
- Shojania KG, Duncan BW, McDonald KM, Wachter RM, Markowitz AJ. Making health care safer: a critical analysis of patient safety practices. Evid Rep Technol Assess (Summ) 2001; ( 43):i–x,1–668.
- National Quality Forum. Safe Practices for Better Healthcare—2006 update. Washington, DC: National Quality Forum, 2006.
- Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). Circulation 2007; 116:1971–1996.
- Mangano DT. Perioperative cardiac morbidity. Anesthesiology 1990; 72:153–184.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Devereaux PJ, Goldman L, Cook DJ, Gilbert K, Leslie K, Guyatt GH. Perioperative cardiac events in patients undergoing noncardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. Can Med Assoc J 2005; 173:627–634.
- Fleischmann KE, Goldman L, Young B, Lee TH. Association between cardiac and noncardiac complications in patients undergoing noncardiac surgery: outcomes and effects on length of stay. Am J Med 2003; 115:515–520.
- Landesberg G, Luria MH, Cotev S, et al. Importance of long-duration postoperative ST-segment depression in cardiac morbidity after vascular surgery. Lancet 1993; 341:715–719.
- Mangano DT, Browner WS, Hollenberg M, London MJ, Tubau JF, Tateo IM. Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery. The Study of Perioperative Ischemia Research Group. N Engl J Med 1990; 323:1781–1788.
- Raby KE, Goldman L, Creager MA, et al. Correlation between preoperative ischemia and major cardiac events after peripheral vascular surgery. N Engl J Med 1989; 321:1296–1300.
- Landesberg G, Mosseri M, Zahger D, et al. Myocardial infarction after vascular surgery: the role of prolonged stress-induced, ST depression-type ischemia. J Am Coll Cardiol 2001; 37:1839–1845.
- Mangano DT, Browner WS, Hollenberg M, Li J, Tateo IM. Long-term cardiac prognosis following noncardiac surgery. The Study of Perioperative Ischemia Research Group. JAMA 1992; 268:233–239.
- Kim LJ, Martinez EA, Faraday N, et al. Cardiac troponin I predicts short-term mortality in vascular surgery patients. Circulation 2002; 106:2366–2371.
- Landesberg G, Shatz V, Akopnik I, et al. Association of cardiac troponin, CK-MB, and postoperative myocardial ischemia with long-term survival after major vascular surgery. J Am Coll Cardiol 2003; 42:1547–1554.
- Badner NH, Knill RL, Brown JE, Novick TV, Gelb AW. Myocardial infarction after noncardiac surgery. Anesthesiology 1998; 88:572–578.
- Priebe HJ. Triggers of perioperative myocardial ischaemia and infarction. Br J Anaesth 2004; 93:9–20.
- Zaugg M, Schaub MC, Foex P. Myocardial injury and its prevention in the perioperative setting. Br J Anaesth 2004; 93:21–33.
- Dawood MM, Gutpa DK, Southern J, Walia A, Atkinson JB, Eagle KA. Pathology of fatal perioperative myocardial infarction: implications regarding pathophysiology and prevention. Int J Cardiol 1996; 57:37–44.
- Cohen MC, Aretz TH. Histological analysis of coronary artery lesions in fatal postoperative myocardial infarction. Cardiovasc Pathol 1999; 8:133–139.
- London MJ, Zaugg M, Schaub MC, Spahn DR. Perioperative beta-adrenergic receptor blockade: physiologic foundations and clinical controversies. Anesthesiology 2004; 100:170–175.
- Yeager MP, Fillinger MP, Hettleman BD, Hartman GS. Perioperative beta-blockade and late cardiac outcomes: a complementary hypothesis. J Cardiothorac Vasc Anesth 2005; 19:237–241.
- Zaugg M, Schaub MC, Pasch T, Spahn DR. Modulation of beta-adrenergic receptor subtype activities in perioperative medicine: mechanisms and sites of action. Br J Anaesth 2002; 88:101–123.
- Devereaux PJ, Yusuf S, Yang H, Choi PT, Guyatt GH. Are the recommendations to use perioperative beta-blocker therapy in patients undergoing non-cardiac surgery based on reliable evidence? Can Med Assoc J 2004; 171:245–247.
- Eagle KA, Froehlich JB. Reducing cardiovascular risk in patients undergoing noncardiac surgery. N Engl J Med 1996; 335:1761–1763.
- Boersma E, Poldermans D, Bax JJ, et al. Predictors of cardiac events after major vascular surgery: role of clinical characteristics, dobutamine echocardiography, and beta-blocker therapy. JAMA 2001; 285:1865–1873.
- Stevens RD, Burri H, Tramer MR. Pharmacologic myocardial protection in patients undergoing noncardiac surgery: a quantitative systematic review. Anesth Analg 2003; 97:623–633.
- Goldman L. Assessing and reducing cardiac risks of noncardiac surgery. Am J Med 2001; 110:320–323.
- Wesorick DH, Eagle KA. The preoperative cardiovascular evaluation of the intermediate-risk patient: new data, changing strategies. Am J Med 2005; 118:1413.
- Auerbach AD, Goldman L. Beta-blockers and reduction of cardiac events in noncardiac surgery: scientific review. JAMA 2002; 287:1435–1444.
- Devereaux PJ, Beattie WS, Choi PT, et al. How strong is the evidence for the use of perioperative beta blockers in non-cardiac surgery? Systematic review and meta–analysis of randomized controlled trials. BMJ 2005; 331:313–321.
- Yang H, Raymer K, Butler R, Parlow JL, Roberts RS. Metoprolol after vascular surgery (MaVS). Can J Anaesth 2004; 51( suppl 1):A7.
- Brady AR, Gibbs JS, Greenhalgh RM, Powell JT, Sydes MR. Perioperative beta-blockade (POBBLE) for patients undergoing infrarenal vascular surgery: results of a randomized double-blind controlled trial. J Vasc Surg 2005; 41:602–609.
- Juul AB, Wetterslev J, Gluud C, et al. Effect of perioperative βblockade in patients with diabetes undergoing major non-cardiac surgery: randomised placebo controlled, blinded multicentre trial. BMJ 2006; 332:1482.
- Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med 2005; 353:349–361.
- Feringa HH, Bax JJ, Schouten O, et al. Beta-blockers improve in-hospital and long-term survival in patients with severe left ventricular dysfunction undergoing major vascular surgery. Eur J Vasc Endovasc Surg 2005; 31:351–358.
- Stone JG, Foex P, Sear JW, Johnson LL, Khambatta HJ, Triner L. Myocardial ischemia in untreated hypertensive patients: effect of a single small oral dose of a beta-adrenergic blocking agent. Anesthesiology 1988; 68:495–500.
- Zaugg M, Tagliente T, Lucchinetti E, et al. Beneficial effects from beta-adrenergic blockade in elderly patients undergoing noncardiac surgery. Anesthesiology 1999; 91:1674–1686.
- Wallace A, Layug B, Tateo I, et al. Prophylactic atenolol reduces postoperative myocardial ischemia. McSPI Research Group. Anesthesiology 1998; 88:7–17.
- Psaty BM, Koepsell TD, Wagner EH, LoGerfo JP, Inui TS. The relative risk of incident coronary heart disease associated with recently stopping the use of beta-blockers. JAMA 1990; 263:1653–1657.
- Shammash JB, Trost JC, Gold JM, Berlin JA, Golden MA, Kimmel SE. Perioperative beta-blocker withdrawal and mortality in vascular surgical patients. Am Heart J 2001; 141:148–153.
- Feringa HHH, Bax JJ, Boersma E, et al. High-dose beta-blockers and tight heart rate control reduce myocardial ischemia and troponin T release in vascular surgery patients. Circulation 2006; 114( suppl 1):I-344–I-349.
- Poldermans D, Bax JJ, Schouten O, et al. Should major vascular surgery be delayed because of preoperative cardiac testing in intermediate-risk patients receiving beta-blocker therapy with tight heart rate control? J Am Coll Cardiol 2006; 48:964–969.
- Redelmeier D, Scales D, Kopp A. Beta blockers for elective surgery in elderly patients: population based, retrospective cohort study. BMJ 2005; 331:932.
- POISE Study Group. Effect of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008; published online May 13. DOI: 10.1016/S0140-6736(08)60601-7.
- Mangano DT, Layug EL, Wallace A, Tateo I. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter Study of Perioperative Ischemia Research Group. N Engl J Med 1996; 335:1713–1720.
- Poldermans D, Boersma E, Bax JJ, et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med 1999; 341:1789–1794.
- Shojania KG, Duncan BW, McDonald KM, Wachter RM, Markowitz AJ. Making health care safer: a critical analysis of patient safety practices. Evid Rep Technol Assess (Summ) 2001; ( 43):i–x,1–668.
- National Quality Forum. Safe Practices for Better Healthcare—2006 update. Washington, DC: National Quality Forum, 2006.
- Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). Circulation 2007; 116:1971–1996.
- Mangano DT. Perioperative cardiac morbidity. Anesthesiology 1990; 72:153–184.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Devereaux PJ, Goldman L, Cook DJ, Gilbert K, Leslie K, Guyatt GH. Perioperative cardiac events in patients undergoing noncardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. Can Med Assoc J 2005; 173:627–634.
- Fleischmann KE, Goldman L, Young B, Lee TH. Association between cardiac and noncardiac complications in patients undergoing noncardiac surgery: outcomes and effects on length of stay. Am J Med 2003; 115:515–520.
- Landesberg G, Luria MH, Cotev S, et al. Importance of long-duration postoperative ST-segment depression in cardiac morbidity after vascular surgery. Lancet 1993; 341:715–719.
- Mangano DT, Browner WS, Hollenberg M, London MJ, Tubau JF, Tateo IM. Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery. The Study of Perioperative Ischemia Research Group. N Engl J Med 1990; 323:1781–1788.
- Raby KE, Goldman L, Creager MA, et al. Correlation between preoperative ischemia and major cardiac events after peripheral vascular surgery. N Engl J Med 1989; 321:1296–1300.
- Landesberg G, Mosseri M, Zahger D, et al. Myocardial infarction after vascular surgery: the role of prolonged stress-induced, ST depression-type ischemia. J Am Coll Cardiol 2001; 37:1839–1845.
- Mangano DT, Browner WS, Hollenberg M, Li J, Tateo IM. Long-term cardiac prognosis following noncardiac surgery. The Study of Perioperative Ischemia Research Group. JAMA 1992; 268:233–239.
- Kim LJ, Martinez EA, Faraday N, et al. Cardiac troponin I predicts short-term mortality in vascular surgery patients. Circulation 2002; 106:2366–2371.
- Landesberg G, Shatz V, Akopnik I, et al. Association of cardiac troponin, CK-MB, and postoperative myocardial ischemia with long-term survival after major vascular surgery. J Am Coll Cardiol 2003; 42:1547–1554.
- Badner NH, Knill RL, Brown JE, Novick TV, Gelb AW. Myocardial infarction after noncardiac surgery. Anesthesiology 1998; 88:572–578.
- Priebe HJ. Triggers of perioperative myocardial ischaemia and infarction. Br J Anaesth 2004; 93:9–20.
- Zaugg M, Schaub MC, Foex P. Myocardial injury and its prevention in the perioperative setting. Br J Anaesth 2004; 93:21–33.
- Dawood MM, Gutpa DK, Southern J, Walia A, Atkinson JB, Eagle KA. Pathology of fatal perioperative myocardial infarction: implications regarding pathophysiology and prevention. Int J Cardiol 1996; 57:37–44.
- Cohen MC, Aretz TH. Histological analysis of coronary artery lesions in fatal postoperative myocardial infarction. Cardiovasc Pathol 1999; 8:133–139.
- London MJ, Zaugg M, Schaub MC, Spahn DR. Perioperative beta-adrenergic receptor blockade: physiologic foundations and clinical controversies. Anesthesiology 2004; 100:170–175.
- Yeager MP, Fillinger MP, Hettleman BD, Hartman GS. Perioperative beta-blockade and late cardiac outcomes: a complementary hypothesis. J Cardiothorac Vasc Anesth 2005; 19:237–241.
- Zaugg M, Schaub MC, Pasch T, Spahn DR. Modulation of beta-adrenergic receptor subtype activities in perioperative medicine: mechanisms and sites of action. Br J Anaesth 2002; 88:101–123.
- Devereaux PJ, Yusuf S, Yang H, Choi PT, Guyatt GH. Are the recommendations to use perioperative beta-blocker therapy in patients undergoing non-cardiac surgery based on reliable evidence? Can Med Assoc J 2004; 171:245–247.
- Eagle KA, Froehlich JB. Reducing cardiovascular risk in patients undergoing noncardiac surgery. N Engl J Med 1996; 335:1761–1763.
- Boersma E, Poldermans D, Bax JJ, et al. Predictors of cardiac events after major vascular surgery: role of clinical characteristics, dobutamine echocardiography, and beta-blocker therapy. JAMA 2001; 285:1865–1873.
- Stevens RD, Burri H, Tramer MR. Pharmacologic myocardial protection in patients undergoing noncardiac surgery: a quantitative systematic review. Anesth Analg 2003; 97:623–633.
- Goldman L. Assessing and reducing cardiac risks of noncardiac surgery. Am J Med 2001; 110:320–323.
- Wesorick DH, Eagle KA. The preoperative cardiovascular evaluation of the intermediate-risk patient: new data, changing strategies. Am J Med 2005; 118:1413.
- Auerbach AD, Goldman L. Beta-blockers and reduction of cardiac events in noncardiac surgery: scientific review. JAMA 2002; 287:1435–1444.
- Devereaux PJ, Beattie WS, Choi PT, et al. How strong is the evidence for the use of perioperative beta blockers in non-cardiac surgery? Systematic review and meta–analysis of randomized controlled trials. BMJ 2005; 331:313–321.
- Yang H, Raymer K, Butler R, Parlow JL, Roberts RS. Metoprolol after vascular surgery (MaVS). Can J Anaesth 2004; 51( suppl 1):A7.
- Brady AR, Gibbs JS, Greenhalgh RM, Powell JT, Sydes MR. Perioperative beta-blockade (POBBLE) for patients undergoing infrarenal vascular surgery: results of a randomized double-blind controlled trial. J Vasc Surg 2005; 41:602–609.
- Juul AB, Wetterslev J, Gluud C, et al. Effect of perioperative βblockade in patients with diabetes undergoing major non-cardiac surgery: randomised placebo controlled, blinded multicentre trial. BMJ 2006; 332:1482.
- Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med 2005; 353:349–361.
- Feringa HH, Bax JJ, Schouten O, et al. Beta-blockers improve in-hospital and long-term survival in patients with severe left ventricular dysfunction undergoing major vascular surgery. Eur J Vasc Endovasc Surg 2005; 31:351–358.
- Stone JG, Foex P, Sear JW, Johnson LL, Khambatta HJ, Triner L. Myocardial ischemia in untreated hypertensive patients: effect of a single small oral dose of a beta-adrenergic blocking agent. Anesthesiology 1988; 68:495–500.
- Zaugg M, Tagliente T, Lucchinetti E, et al. Beneficial effects from beta-adrenergic blockade in elderly patients undergoing noncardiac surgery. Anesthesiology 1999; 91:1674–1686.
- Wallace A, Layug B, Tateo I, et al. Prophylactic atenolol reduces postoperative myocardial ischemia. McSPI Research Group. Anesthesiology 1998; 88:7–17.
- Psaty BM, Koepsell TD, Wagner EH, LoGerfo JP, Inui TS. The relative risk of incident coronary heart disease associated with recently stopping the use of beta-blockers. JAMA 1990; 263:1653–1657.
- Shammash JB, Trost JC, Gold JM, Berlin JA, Golden MA, Kimmel SE. Perioperative beta-blocker withdrawal and mortality in vascular surgical patients. Am Heart J 2001; 141:148–153.
- Feringa HHH, Bax JJ, Boersma E, et al. High-dose beta-blockers and tight heart rate control reduce myocardial ischemia and troponin T release in vascular surgery patients. Circulation 2006; 114( suppl 1):I-344–I-349.
- Poldermans D, Bax JJ, Schouten O, et al. Should major vascular surgery be delayed because of preoperative cardiac testing in intermediate-risk patients receiving beta-blocker therapy with tight heart rate control? J Am Coll Cardiol 2006; 48:964–969.
- Redelmeier D, Scales D, Kopp A. Beta blockers for elective surgery in elderly patients: population based, retrospective cohort study. BMJ 2005; 331:932.
KEY POINTS
- Beta-blockers reduce perioperative ischemia, but the benefit may be only in high-risk patients undergoing high-risk surgery. Currently, the best evidence supports their use in two groups: patients undergoing vascular surgery who have known ischemic heart disease or multiple risk factors for it, and patients who are already on beta-blockers.
- The Perioperative Ischemic Evaluation (POISE) findings suggest that beta-blockers should be used in the immediate preoperative period only with great caution, after ensuring that the patient is clinically stable and without evidence of infection, hypovolemia, anemia, or other conditions that could make heart-rate titration misleading or use of the drug dangerous.
- When feasible, beta-blockers should be started a month before surgery, titrated to a heart rate of 60 beats per minute, and continued for approximately a month. If the drug is then to be discontinued, it should be tapered slowly.
Given the ENHANCE trial results, ezetimibe is still unproven
Ezetimibe (Zetia) was licensed by the US Food and Drug Administration in 2002 on the basis of its ability to reduce low-density lipoprotein cholesterol (LDL-C) levels. The reductions are mild, approximately 15%,1 which is comparable to the effects of a stringent diet and exercise or of a statin in titrated doses.
However, there was no evidence that ezetimbe, which has a unique mechanism of action, delivers a benefit in terms of clinical outcomes. Despite this, the use of ezetimibe (alone or in fixed-dose combination with simvastatin, a preparation sold as Vytorin) grew rapidly, generating annual sales of $5.2 billion. Clinicians and the manufacturer (Merck/Schering-Plough) broadly assumed that LDL-C reduction would carry ezetimibe’s day as clinical trials emerged.
The assumption seemed reasonable, since evidence from the past 3 decades has established a clear link between lowering LDL-C levels via diverse mechanisms and positive clinical outcomes, particularly lower rates of cardiovascular disease and death. Indeed, LDL-C measurement is now a focus of cardiovascular risk assessment and management, as reflected in national treatment guidelines.
THE ENHANCE TRIAL: EZETIMIBE FAILS A KEY TEST
Unexpectedly, ezetimibe failed its first step in clinical trial validation, the Ezetimibe and Simvastatin in Hypercholesterolemia Enhances Atherosclerosis Regression (ENHANCE) trial.2 Apart from the scientifically irrelevant political regulatory intrigue generated by the sponsor’s conduct in this trial, ENHANCE’s findings challenge us to confront issues of what we assume vs what we really know, and how to interpret the complex results of clinical trials.
To be fair to the trial’s investigators, ENHANCE achieved its objective of enrolling a population with a very high LDL-C level, which is ezetimibe’s target and has been widely used in the study of atherosclerosis progression as a marker of potential drug benefit. Nevertheless, and even though the LDL-C level 2 years later was 52 mg/dL lower in the group receiving ezetimibe/simvastatin than in the group receiving simvastatin alone (Zocor), at LDL-C levels that are typically associated with atherosclerosis progression (140–190 mg/dL), ezetimibe failed to reduce the progression of atherosclerosis.
These trends are particularly worrisome, given that the ezetimibe/simvastatin group achieved a greater reduction in C-reactive protein levels, which typically has resulted in superior outcomes in atherosclerosis3 and clinical effects4 in combination with LDL-C reduction.
In view of these findings, should clinicians stand firm and continue to use ezetimibe? Or should we reevaluate our position and await more data about this unique, first-in-class compound?
WISHFUL POST HOC HYPOTHESES
In this issue of the Cleveland Clinic Journal of Medicine, Dr. Michael Davidson,5 a respected lipid expert but one invested in ezetimibe’s development, assures us that all is in order and that the results of ENHANCE can be explained away by several arguments, most notably that most of the trial’s participants had previously received lipid-lowering treatment, which obscured the effects of ezetimibe. Moreover, he argues that ezetimibe’s mechanism of action is well understood and that the drug is safe and well tolerated and thus should remain a first-line treatment for hyperlipidemia.
These arguments may eventually prove to be correct, but as of now they are merely wishful post hoc hypotheses awaiting more data apart from ENHANCE. Negative clinical trials do occur as a matter of chance, but we should be cautious in any attempts to explain away a trial that was designed, executed, and reported as conceived simply because the results do not match our expectations.
Confronted with ENHANCE, the astute clinician should ask three questions: Do we really understand ezetimibe’s mechanism of action? Do other lines of evidence indicate the drug is beneficial? And how reliable is the arterial thickness as a surrogate end point?
DO WE UNDERSTAND EZETIMIBE’S MECHANISM OF ACTION?
Do we understand ezetimibe’s full mechanism of action? Not really.
True, ezetimibe inhibits cholesterol transport, a process that is integral both to cholesterol’s enteric absorption and to its systemic clearance. But although Dr. Davidson asserts that ezetimibe has cellular effects similar to those of statins, in fact it has the opposite effect on HMG-coA reductase, and no effects on LDL receptors.6
Furthermore, although initial studies suggested that ezetimibe inhibits enteric cholesterol absorption by inhibiting the Niemann-Pick C1L1 (NPC1L1) receptor, more recent investigations call this into serious question and point more definitively at a receptor known as scavenger receptor-B1 (SR-B1). As stated in a recent editorial, “SR-B1 in the apical site of enterocytes is the primary high-affinity site of cholesterol uptake and ezetimibe can inhibit this process. Moreover, the [possibility is ruled out] of NPC1L1 being a major player in this cholesterol uptake. This is at variance with the view of the colleagues from Schering-Plough who claim the same for NPC1L1.”7
SR-B1 is also a high-affinity receptor for high-density lipoprotein8 and thus is active in the antiatherosclerotic process of reverse cholesterol transport, inhibition of which significantly accelerates the development of atherosclerosis.9
Additionally, in vitro and thus unrelated to the effects of changing cholesterol concentration, ezetimibe down-regulates SR-B1 and another key cholesterol transporter protein called ABCA1.10 Further, ezetimibe induces down-regulation of raft protein domains, including CD36,11 another effect opposite to that of statins.
These little-recognized effects of ezetimibe are among many that are completely unrelated to enteric cholesterol absorption. Yet, they are likely to be active within the liver and systemically where these proteins reside, and they are putatively proatherosclerotic. Contrary to often-cited opinion, ezetimibe is systemically absorbed, with 11% of the compound excreted in the urine.12 Thus, the compound is systemically available to exert these same actions in the liver and elsewhere. Moreover, the absorbed drug is glucuronidated and is extensively recirculated in the liver in a form (its glucuronide) that is more potent than the parent compound.
In sum, present opinion is that ezetimibe inhibits lipid transport and interacts with a variety of receptors, not only in the gut but also systemically at the cell membrane and also inside the cell, focally disrupting several tightly regulated biologic processes.7 Thus, although ezetimibe reduces serum LDL-C levels via its effect in the gut, this effect may well be offset or even overridden systemically by other, unmeasurable effects, leading to counterintuitive results in terms of atherosclerosis or clinical events.
This would not be the first time a lipid-lowering drug has disappointed us: torcetrapib, another transport inhibitor, dramatically raises serum high-density lipoprotein cholesterol levels and reduces LDL-C but was found not only to have no effect on atherosclerosis, but also to potentiate adverse clinical outcomes.
The net impact of these other actions of ezetimibe is not known. We will discover its true clinical effects only through studies of endothelial function, atherosclerosis, and clinical cardiovascular outcomes. ENHANCE, which looked at atherosclerosis, is thus our strongest signal to date on the net effect of ezetimibe.
DO OTHER LINES OF EVIDENCE INDICATE EZETIMIBE IS BENEFICIAL?
Can we be reassured that ENHANCE’s results are spurious on the basis of other lines of evidence? Again, not really.
Experiments in animals, particularly in mice,13 have shown that ezetimibe may be antiatherosclerotic, although mice are considered the “worst model”7 for the study of ezetimibe, and notably, LDL-C levels were lowered far more in these experiments than they are clinically. Enthusiasm for these animal models should be tempered by interspecies variability in ezetimibe’s “off-target” effects and in the recent failure of other lipid transport drugs in human trials (torcetrapib and ACAT inhibitors) that had shown initial success in animals. No animal model is established for evaluating drugs of ezetimibe’s class, given its complex mechanism of action.
In human studies, the only other surrogate of the net effect of ezetimibe is endothelial function. Among several randomized clinical trials of ezetimibe,14–18 only one was designed to compare the effects of ezetimibe alone, ezetimibe plus a statin, and a statin by itself in titrated or in maximum doses.15 After 4 weeks of therapy, all groups had lower LDL-C levels. However, ezetimibe monotherapy and ezetimibe/simvastatin combination therapy had no detectable effect on the arterial response to acetylcholine, but atorvastatin (Lipitor) monotherapy did. To be fair, the other (very small) trials showed mixed results, thus keeping the hypothesis of ezetimibe’s benefit alive, but with nothing close to a clear signal of benefit.
IS ARTERIAL THICKNESS RELIABLE AS A SURROGATE END POINT?
Was the principal problem in ENHANCE the use of carotid intima-media thickness as the primary end point? No.
This issue has received a lot of attention, much of which I believe is misinformed. No trial end point is infallible, including carotid intima-media thickness, and one must remain open to the possibility of chance findings. However, it has been a relatively reasonable end point in trials of diverse cardiovascular preventive strategies, including lipid-lowering, blood-pressure-lowering, and lifestyle interventions and as a directional biomarker of clinical atherosclerotic events.
We should be cautious about comparing data on carotid intima-media thickness from different trials, as Dr. Davidson attempts to do, in view of methodologic and population differences: each trial must be considered independently. Of greatest concern in ENHANCE is the consistency among intima-media thickness end points, including strong trends toward adverse effects in the most diseased carotid and femoral segments.
Moreover, ENHANCE’s detractors contend that the carotid intima-media thickness of the studied population was normal, citing this as evidence of delipidation from prior treatment. Although not impossible (as shown by the work of Zhao and colleagues in the setting of prolonged, intense lipid-lowering therapy19), at the moment this hypothesis is a matter of conjecture in the ENHANCE participants, particularly because their LDL-C levels were still quite elevated during the trial and conceivably even before randomization.
But these patients were not normal: they were typical patients with familial hypercholesterolemia with extremely elevated LDL-C levels and abnormally thick arteries for their age. Population screening estimates show that, for age and sex, the carotid intima-media thickness values in ENHANCE would lie in the upper quartile of those in the general population.20 Moreover, their mean value is consistent with that in similar-aged groups of patients with familial hypercholesterolemia, even with lower rates of prior statin pretreatment.21
The most convincing evidence for the validity of the ENHANCE findings comes from the published subgroup data (Figure 1). In participants whose baseline carotid intima-media thickness was above the median at baseline, the thickness increased more with ezetimibe/simvastatin than with simvastatin alone. The same was true in the subgroup with above-average LDL-C levels at baseline. The subgroups with no prior statin treatment, low-dose prior statin treatment, and high-dose prior statin showed no heterogeneity of response: their carotid intima-media thickness increased more with ezetimibe/simvastatin than with simvastatin alone. None of these differences was statistically significant; however, these prespecified subgroup data seemingly invalidate arguments against the ENHANCE results based on carotid intima-media thickness findings.
In this context, ENHANCE can only be interpreted as a strong initial negative signal, a “red flag” about ezetimibe’s net health benefits.
WHAT NEXT?
The proper present focus of this debate is not on LDL-C but rather on ezetimibe, its unique mechanism of action, and on the need for more evidence about this complex compound.
At present, ezetimibe’s mechanism of action is not fully understood, and its benefit—for now, only mild LDL-C reduction—is too uncertain for us to be spending $5.2 billion a year for it. Its manufacturer is fortunate that the drug is even licensed, given the current and seemingly appropriate regulatory changes under which drugs introducing new therapeutic classes are scrutinized more closely for benefits and risks. “Safe and well tolerated,” as contended by Dr. Davidson, is not nearly enough: drugs must show clinically important benefits. We still know too little about this drug, the manufacturer of which has invested far more in marketing than in science, a point on which Dr. Davidson and I agree.
In 2008, ezetimibe is an appropriate candidate for testing in clinical trials, and in years to come it may be worthy of clinical attention—if rigorous and objectively conducted clinical trials prove its worth. At present, clinical equipoise dictates that ezetimibe is not an appropriate alternative to a statin in titrated doses, to the addition of other lipid-lowering drugs to a statin, to greater attention to drug adherence, or to lifestyle modification.
For the moment, given the ENHANCE results, the clinical usefulness of ezetimibe still remains to be proven. Much more evidence is needed before we can confidently reembrace the clinical use of ezetimibe.
- Ballantyne CM, Houri J, Notarbartolo A, et al. Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Circulation 2003; 107:2409–2415.
- Kastelein JJ, Akdim F, Stroes ES, et al. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med 2008; 358:1431–1443.
- Kent SM, Taylor AJ. Usefulness of lowering low-density lipoprotein cholesterol to < 70 mg/dL and usefulness of C-reactive protein in patient selection. Am J Cardiol 2003; 92:1224–1227.
- Nissen SE, Tuzcu EM, Schoenhagen P, et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med 2005; 352:29–38.
- Davidson MH. Interpreting the ENHANCE trial. Is ezetimibe/simvastatin no better than simvastatin alone? Leessons learned and clinical implications. Cleve Clin J Med 2008; 75:479–491.
- Gouni-Berthold I, Berthold HK, Gylling H, et al. Effects of ezetimibe and/or simvastatin on LDL receptor protein expression and on LDL receptor and HMG-CoA reductase gene expression: a randomized trial in healthy men. Atherosclerosis 2008; 198:198–207.
- Spener F. Ezetimibe in search of receptor(s)—still a never-ending challenge in cholesterol absorption and transport. Biochim Biophys Acta 2007; 1771:1113–1116.
- Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science 1996; 271:518–520.
- Kitayama K, Nishizawa T, Abe K, et al. Blockade of scavenger receptor class B type I raises high density lipoprotein cholesterol levels but exacerbates atherosclerotic lesion formation in apolipoprotein E deficient mice. J Pharm Pharmacol 2006; 58:1629–1638.
- During A, Dawson HD, Harrison EH. Carotenoid transport is decreased and expression of the lipid transporters SR-BI, NPC1L1, and ABCA1 is downregulated in Caco-2 cells treated with ezetimibe. J Nutr 2005; 135:2305–2312.
- Orso E, Werner T, Wolf Z, Bandulik S, Kramer W, Schmitz G. Ezetimib influences the expression of raft-associated antigens in human monocytes. Cytometry A 2006; 69:206–208.
- Patrick JE, Kosoglou T, Stauber KL, et al. Disposition of the selective cholesterol absorption inhibitor ezetimibe in healthy male subjects. Drug Metab Dispos 2002; 30:430–437.
- Kuhlencordt PJ, Padmapriya P, Rutzel S, et al. Ezetimibe potently reduces vascular inflammation and arteriosclerosis in eNOS-deficient ApoE ko mice. Atherosclerosis 2008; April 6.
- Bulut D, Hanefeld C, Bulut-Streich N, Graf C, Mugge A, Spiecker M. Endothelial function in the forearm circulation of patients with the metabolic syndrome—effect of different lipid-lowering regimens. Cardiology 2005; 104:176–180.
- Fichtlscherer S, Schmidt-Lucke C, Bojunga S, et al. Differential effects of short-term lipid lowering with ezetimibe and statins on endothelial function in patients with CAD: clinical evidence for ‘pleiotropic’ functions of statin therapy. Eur Heart J 2006; 27:1182–1190.
- Landmesser U, Bahlmann F, Mueller M, et al. Simvastatin versus ezetimibe: pleiotropic and lipid-lowering effects on endothelial function in humans. Circulation 2005; 111:2356–2363.
- Maki-Petaja KM, Booth AD, Hall FC, et al. Ezetimibe and simvastatin reduce inflammation, disease activity, and aortic stiffness and improve endothelial function in rheumatoid arthritis. J Am Coll Cardiol 2007; 50:852–858.
- Settergren M, Bohm F, Ryden L, Pernow J. Cholesterol lowering is more important than pleiotropic effects of statins for endothelial function in patients with dysglycaemia and coronary artery disease. Eur Heart J 2008 April 25.
- Zhao XQ, Yuan C, Hatsukami TS, et al. Effects of prolonged intensive lipid-lowering therapy on the characteristics of carotid atherosclerotic plaques in vivo by MRI: a case-control study. Arterioscler Thromb Vasc Biol 2001; 21:1623–1629.
- Stein JH, Korcarz CE, Hurst RT, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr 2008; 21:93–111.
- Junyent M, Cofan M, Nunez I, Gilabert R, Zambon D, Ros E. Influence of HDL cholesterol on preclinical carotid atherosclerosis in familial hypercholesterolemia. Arterioscler Thromb Vasc Biol 2006; 26:1107–1113.
Ezetimibe (Zetia) was licensed by the US Food and Drug Administration in 2002 on the basis of its ability to reduce low-density lipoprotein cholesterol (LDL-C) levels. The reductions are mild, approximately 15%,1 which is comparable to the effects of a stringent diet and exercise or of a statin in titrated doses.
However, there was no evidence that ezetimbe, which has a unique mechanism of action, delivers a benefit in terms of clinical outcomes. Despite this, the use of ezetimibe (alone or in fixed-dose combination with simvastatin, a preparation sold as Vytorin) grew rapidly, generating annual sales of $5.2 billion. Clinicians and the manufacturer (Merck/Schering-Plough) broadly assumed that LDL-C reduction would carry ezetimibe’s day as clinical trials emerged.
The assumption seemed reasonable, since evidence from the past 3 decades has established a clear link between lowering LDL-C levels via diverse mechanisms and positive clinical outcomes, particularly lower rates of cardiovascular disease and death. Indeed, LDL-C measurement is now a focus of cardiovascular risk assessment and management, as reflected in national treatment guidelines.
THE ENHANCE TRIAL: EZETIMIBE FAILS A KEY TEST
Unexpectedly, ezetimibe failed its first step in clinical trial validation, the Ezetimibe and Simvastatin in Hypercholesterolemia Enhances Atherosclerosis Regression (ENHANCE) trial.2 Apart from the scientifically irrelevant political regulatory intrigue generated by the sponsor’s conduct in this trial, ENHANCE’s findings challenge us to confront issues of what we assume vs what we really know, and how to interpret the complex results of clinical trials.
To be fair to the trial’s investigators, ENHANCE achieved its objective of enrolling a population with a very high LDL-C level, which is ezetimibe’s target and has been widely used in the study of atherosclerosis progression as a marker of potential drug benefit. Nevertheless, and even though the LDL-C level 2 years later was 52 mg/dL lower in the group receiving ezetimibe/simvastatin than in the group receiving simvastatin alone (Zocor), at LDL-C levels that are typically associated with atherosclerosis progression (140–190 mg/dL), ezetimibe failed to reduce the progression of atherosclerosis.
These trends are particularly worrisome, given that the ezetimibe/simvastatin group achieved a greater reduction in C-reactive protein levels, which typically has resulted in superior outcomes in atherosclerosis3 and clinical effects4 in combination with LDL-C reduction.
In view of these findings, should clinicians stand firm and continue to use ezetimibe? Or should we reevaluate our position and await more data about this unique, first-in-class compound?
WISHFUL POST HOC HYPOTHESES
In this issue of the Cleveland Clinic Journal of Medicine, Dr. Michael Davidson,5 a respected lipid expert but one invested in ezetimibe’s development, assures us that all is in order and that the results of ENHANCE can be explained away by several arguments, most notably that most of the trial’s participants had previously received lipid-lowering treatment, which obscured the effects of ezetimibe. Moreover, he argues that ezetimibe’s mechanism of action is well understood and that the drug is safe and well tolerated and thus should remain a first-line treatment for hyperlipidemia.
These arguments may eventually prove to be correct, but as of now they are merely wishful post hoc hypotheses awaiting more data apart from ENHANCE. Negative clinical trials do occur as a matter of chance, but we should be cautious in any attempts to explain away a trial that was designed, executed, and reported as conceived simply because the results do not match our expectations.
Confronted with ENHANCE, the astute clinician should ask three questions: Do we really understand ezetimibe’s mechanism of action? Do other lines of evidence indicate the drug is beneficial? And how reliable is the arterial thickness as a surrogate end point?
DO WE UNDERSTAND EZETIMIBE’S MECHANISM OF ACTION?
Do we understand ezetimibe’s full mechanism of action? Not really.
True, ezetimibe inhibits cholesterol transport, a process that is integral both to cholesterol’s enteric absorption and to its systemic clearance. But although Dr. Davidson asserts that ezetimibe has cellular effects similar to those of statins, in fact it has the opposite effect on HMG-coA reductase, and no effects on LDL receptors.6
Furthermore, although initial studies suggested that ezetimibe inhibits enteric cholesterol absorption by inhibiting the Niemann-Pick C1L1 (NPC1L1) receptor, more recent investigations call this into serious question and point more definitively at a receptor known as scavenger receptor-B1 (SR-B1). As stated in a recent editorial, “SR-B1 in the apical site of enterocytes is the primary high-affinity site of cholesterol uptake and ezetimibe can inhibit this process. Moreover, the [possibility is ruled out] of NPC1L1 being a major player in this cholesterol uptake. This is at variance with the view of the colleagues from Schering-Plough who claim the same for NPC1L1.”7
SR-B1 is also a high-affinity receptor for high-density lipoprotein8 and thus is active in the antiatherosclerotic process of reverse cholesterol transport, inhibition of which significantly accelerates the development of atherosclerosis.9
Additionally, in vitro and thus unrelated to the effects of changing cholesterol concentration, ezetimibe down-regulates SR-B1 and another key cholesterol transporter protein called ABCA1.10 Further, ezetimibe induces down-regulation of raft protein domains, including CD36,11 another effect opposite to that of statins.
These little-recognized effects of ezetimibe are among many that are completely unrelated to enteric cholesterol absorption. Yet, they are likely to be active within the liver and systemically where these proteins reside, and they are putatively proatherosclerotic. Contrary to often-cited opinion, ezetimibe is systemically absorbed, with 11% of the compound excreted in the urine.12 Thus, the compound is systemically available to exert these same actions in the liver and elsewhere. Moreover, the absorbed drug is glucuronidated and is extensively recirculated in the liver in a form (its glucuronide) that is more potent than the parent compound.
In sum, present opinion is that ezetimibe inhibits lipid transport and interacts with a variety of receptors, not only in the gut but also systemically at the cell membrane and also inside the cell, focally disrupting several tightly regulated biologic processes.7 Thus, although ezetimibe reduces serum LDL-C levels via its effect in the gut, this effect may well be offset or even overridden systemically by other, unmeasurable effects, leading to counterintuitive results in terms of atherosclerosis or clinical events.
This would not be the first time a lipid-lowering drug has disappointed us: torcetrapib, another transport inhibitor, dramatically raises serum high-density lipoprotein cholesterol levels and reduces LDL-C but was found not only to have no effect on atherosclerosis, but also to potentiate adverse clinical outcomes.
The net impact of these other actions of ezetimibe is not known. We will discover its true clinical effects only through studies of endothelial function, atherosclerosis, and clinical cardiovascular outcomes. ENHANCE, which looked at atherosclerosis, is thus our strongest signal to date on the net effect of ezetimibe.
DO OTHER LINES OF EVIDENCE INDICATE EZETIMIBE IS BENEFICIAL?
Can we be reassured that ENHANCE’s results are spurious on the basis of other lines of evidence? Again, not really.
Experiments in animals, particularly in mice,13 have shown that ezetimibe may be antiatherosclerotic, although mice are considered the “worst model”7 for the study of ezetimibe, and notably, LDL-C levels were lowered far more in these experiments than they are clinically. Enthusiasm for these animal models should be tempered by interspecies variability in ezetimibe’s “off-target” effects and in the recent failure of other lipid transport drugs in human trials (torcetrapib and ACAT inhibitors) that had shown initial success in animals. No animal model is established for evaluating drugs of ezetimibe’s class, given its complex mechanism of action.
In human studies, the only other surrogate of the net effect of ezetimibe is endothelial function. Among several randomized clinical trials of ezetimibe,14–18 only one was designed to compare the effects of ezetimibe alone, ezetimibe plus a statin, and a statin by itself in titrated or in maximum doses.15 After 4 weeks of therapy, all groups had lower LDL-C levels. However, ezetimibe monotherapy and ezetimibe/simvastatin combination therapy had no detectable effect on the arterial response to acetylcholine, but atorvastatin (Lipitor) monotherapy did. To be fair, the other (very small) trials showed mixed results, thus keeping the hypothesis of ezetimibe’s benefit alive, but with nothing close to a clear signal of benefit.
IS ARTERIAL THICKNESS RELIABLE AS A SURROGATE END POINT?
Was the principal problem in ENHANCE the use of carotid intima-media thickness as the primary end point? No.
This issue has received a lot of attention, much of which I believe is misinformed. No trial end point is infallible, including carotid intima-media thickness, and one must remain open to the possibility of chance findings. However, it has been a relatively reasonable end point in trials of diverse cardiovascular preventive strategies, including lipid-lowering, blood-pressure-lowering, and lifestyle interventions and as a directional biomarker of clinical atherosclerotic events.
We should be cautious about comparing data on carotid intima-media thickness from different trials, as Dr. Davidson attempts to do, in view of methodologic and population differences: each trial must be considered independently. Of greatest concern in ENHANCE is the consistency among intima-media thickness end points, including strong trends toward adverse effects in the most diseased carotid and femoral segments.
Moreover, ENHANCE’s detractors contend that the carotid intima-media thickness of the studied population was normal, citing this as evidence of delipidation from prior treatment. Although not impossible (as shown by the work of Zhao and colleagues in the setting of prolonged, intense lipid-lowering therapy19), at the moment this hypothesis is a matter of conjecture in the ENHANCE participants, particularly because their LDL-C levels were still quite elevated during the trial and conceivably even before randomization.
But these patients were not normal: they were typical patients with familial hypercholesterolemia with extremely elevated LDL-C levels and abnormally thick arteries for their age. Population screening estimates show that, for age and sex, the carotid intima-media thickness values in ENHANCE would lie in the upper quartile of those in the general population.20 Moreover, their mean value is consistent with that in similar-aged groups of patients with familial hypercholesterolemia, even with lower rates of prior statin pretreatment.21
The most convincing evidence for the validity of the ENHANCE findings comes from the published subgroup data (Figure 1). In participants whose baseline carotid intima-media thickness was above the median at baseline, the thickness increased more with ezetimibe/simvastatin than with simvastatin alone. The same was true in the subgroup with above-average LDL-C levels at baseline. The subgroups with no prior statin treatment, low-dose prior statin treatment, and high-dose prior statin showed no heterogeneity of response: their carotid intima-media thickness increased more with ezetimibe/simvastatin than with simvastatin alone. None of these differences was statistically significant; however, these prespecified subgroup data seemingly invalidate arguments against the ENHANCE results based on carotid intima-media thickness findings.
In this context, ENHANCE can only be interpreted as a strong initial negative signal, a “red flag” about ezetimibe’s net health benefits.
WHAT NEXT?
The proper present focus of this debate is not on LDL-C but rather on ezetimibe, its unique mechanism of action, and on the need for more evidence about this complex compound.
At present, ezetimibe’s mechanism of action is not fully understood, and its benefit—for now, only mild LDL-C reduction—is too uncertain for us to be spending $5.2 billion a year for it. Its manufacturer is fortunate that the drug is even licensed, given the current and seemingly appropriate regulatory changes under which drugs introducing new therapeutic classes are scrutinized more closely for benefits and risks. “Safe and well tolerated,” as contended by Dr. Davidson, is not nearly enough: drugs must show clinically important benefits. We still know too little about this drug, the manufacturer of which has invested far more in marketing than in science, a point on which Dr. Davidson and I agree.
In 2008, ezetimibe is an appropriate candidate for testing in clinical trials, and in years to come it may be worthy of clinical attention—if rigorous and objectively conducted clinical trials prove its worth. At present, clinical equipoise dictates that ezetimibe is not an appropriate alternative to a statin in titrated doses, to the addition of other lipid-lowering drugs to a statin, to greater attention to drug adherence, or to lifestyle modification.
For the moment, given the ENHANCE results, the clinical usefulness of ezetimibe still remains to be proven. Much more evidence is needed before we can confidently reembrace the clinical use of ezetimibe.
Ezetimibe (Zetia) was licensed by the US Food and Drug Administration in 2002 on the basis of its ability to reduce low-density lipoprotein cholesterol (LDL-C) levels. The reductions are mild, approximately 15%,1 which is comparable to the effects of a stringent diet and exercise or of a statin in titrated doses.
However, there was no evidence that ezetimbe, which has a unique mechanism of action, delivers a benefit in terms of clinical outcomes. Despite this, the use of ezetimibe (alone or in fixed-dose combination with simvastatin, a preparation sold as Vytorin) grew rapidly, generating annual sales of $5.2 billion. Clinicians and the manufacturer (Merck/Schering-Plough) broadly assumed that LDL-C reduction would carry ezetimibe’s day as clinical trials emerged.
The assumption seemed reasonable, since evidence from the past 3 decades has established a clear link between lowering LDL-C levels via diverse mechanisms and positive clinical outcomes, particularly lower rates of cardiovascular disease and death. Indeed, LDL-C measurement is now a focus of cardiovascular risk assessment and management, as reflected in national treatment guidelines.
THE ENHANCE TRIAL: EZETIMIBE FAILS A KEY TEST
Unexpectedly, ezetimibe failed its first step in clinical trial validation, the Ezetimibe and Simvastatin in Hypercholesterolemia Enhances Atherosclerosis Regression (ENHANCE) trial.2 Apart from the scientifically irrelevant political regulatory intrigue generated by the sponsor’s conduct in this trial, ENHANCE’s findings challenge us to confront issues of what we assume vs what we really know, and how to interpret the complex results of clinical trials.
To be fair to the trial’s investigators, ENHANCE achieved its objective of enrolling a population with a very high LDL-C level, which is ezetimibe’s target and has been widely used in the study of atherosclerosis progression as a marker of potential drug benefit. Nevertheless, and even though the LDL-C level 2 years later was 52 mg/dL lower in the group receiving ezetimibe/simvastatin than in the group receiving simvastatin alone (Zocor), at LDL-C levels that are typically associated with atherosclerosis progression (140–190 mg/dL), ezetimibe failed to reduce the progression of atherosclerosis.
These trends are particularly worrisome, given that the ezetimibe/simvastatin group achieved a greater reduction in C-reactive protein levels, which typically has resulted in superior outcomes in atherosclerosis3 and clinical effects4 in combination with LDL-C reduction.
In view of these findings, should clinicians stand firm and continue to use ezetimibe? Or should we reevaluate our position and await more data about this unique, first-in-class compound?
WISHFUL POST HOC HYPOTHESES
In this issue of the Cleveland Clinic Journal of Medicine, Dr. Michael Davidson,5 a respected lipid expert but one invested in ezetimibe’s development, assures us that all is in order and that the results of ENHANCE can be explained away by several arguments, most notably that most of the trial’s participants had previously received lipid-lowering treatment, which obscured the effects of ezetimibe. Moreover, he argues that ezetimibe’s mechanism of action is well understood and that the drug is safe and well tolerated and thus should remain a first-line treatment for hyperlipidemia.
These arguments may eventually prove to be correct, but as of now they are merely wishful post hoc hypotheses awaiting more data apart from ENHANCE. Negative clinical trials do occur as a matter of chance, but we should be cautious in any attempts to explain away a trial that was designed, executed, and reported as conceived simply because the results do not match our expectations.
Confronted with ENHANCE, the astute clinician should ask three questions: Do we really understand ezetimibe’s mechanism of action? Do other lines of evidence indicate the drug is beneficial? And how reliable is the arterial thickness as a surrogate end point?
DO WE UNDERSTAND EZETIMIBE’S MECHANISM OF ACTION?
Do we understand ezetimibe’s full mechanism of action? Not really.
True, ezetimibe inhibits cholesterol transport, a process that is integral both to cholesterol’s enteric absorption and to its systemic clearance. But although Dr. Davidson asserts that ezetimibe has cellular effects similar to those of statins, in fact it has the opposite effect on HMG-coA reductase, and no effects on LDL receptors.6
Furthermore, although initial studies suggested that ezetimibe inhibits enteric cholesterol absorption by inhibiting the Niemann-Pick C1L1 (NPC1L1) receptor, more recent investigations call this into serious question and point more definitively at a receptor known as scavenger receptor-B1 (SR-B1). As stated in a recent editorial, “SR-B1 in the apical site of enterocytes is the primary high-affinity site of cholesterol uptake and ezetimibe can inhibit this process. Moreover, the [possibility is ruled out] of NPC1L1 being a major player in this cholesterol uptake. This is at variance with the view of the colleagues from Schering-Plough who claim the same for NPC1L1.”7
SR-B1 is also a high-affinity receptor for high-density lipoprotein8 and thus is active in the antiatherosclerotic process of reverse cholesterol transport, inhibition of which significantly accelerates the development of atherosclerosis.9
Additionally, in vitro and thus unrelated to the effects of changing cholesterol concentration, ezetimibe down-regulates SR-B1 and another key cholesterol transporter protein called ABCA1.10 Further, ezetimibe induces down-regulation of raft protein domains, including CD36,11 another effect opposite to that of statins.
These little-recognized effects of ezetimibe are among many that are completely unrelated to enteric cholesterol absorption. Yet, they are likely to be active within the liver and systemically where these proteins reside, and they are putatively proatherosclerotic. Contrary to often-cited opinion, ezetimibe is systemically absorbed, with 11% of the compound excreted in the urine.12 Thus, the compound is systemically available to exert these same actions in the liver and elsewhere. Moreover, the absorbed drug is glucuronidated and is extensively recirculated in the liver in a form (its glucuronide) that is more potent than the parent compound.
In sum, present opinion is that ezetimibe inhibits lipid transport and interacts with a variety of receptors, not only in the gut but also systemically at the cell membrane and also inside the cell, focally disrupting several tightly regulated biologic processes.7 Thus, although ezetimibe reduces serum LDL-C levels via its effect in the gut, this effect may well be offset or even overridden systemically by other, unmeasurable effects, leading to counterintuitive results in terms of atherosclerosis or clinical events.
This would not be the first time a lipid-lowering drug has disappointed us: torcetrapib, another transport inhibitor, dramatically raises serum high-density lipoprotein cholesterol levels and reduces LDL-C but was found not only to have no effect on atherosclerosis, but also to potentiate adverse clinical outcomes.
The net impact of these other actions of ezetimibe is not known. We will discover its true clinical effects only through studies of endothelial function, atherosclerosis, and clinical cardiovascular outcomes. ENHANCE, which looked at atherosclerosis, is thus our strongest signal to date on the net effect of ezetimibe.
DO OTHER LINES OF EVIDENCE INDICATE EZETIMIBE IS BENEFICIAL?
Can we be reassured that ENHANCE’s results are spurious on the basis of other lines of evidence? Again, not really.
Experiments in animals, particularly in mice,13 have shown that ezetimibe may be antiatherosclerotic, although mice are considered the “worst model”7 for the study of ezetimibe, and notably, LDL-C levels were lowered far more in these experiments than they are clinically. Enthusiasm for these animal models should be tempered by interspecies variability in ezetimibe’s “off-target” effects and in the recent failure of other lipid transport drugs in human trials (torcetrapib and ACAT inhibitors) that had shown initial success in animals. No animal model is established for evaluating drugs of ezetimibe’s class, given its complex mechanism of action.
In human studies, the only other surrogate of the net effect of ezetimibe is endothelial function. Among several randomized clinical trials of ezetimibe,14–18 only one was designed to compare the effects of ezetimibe alone, ezetimibe plus a statin, and a statin by itself in titrated or in maximum doses.15 After 4 weeks of therapy, all groups had lower LDL-C levels. However, ezetimibe monotherapy and ezetimibe/simvastatin combination therapy had no detectable effect on the arterial response to acetylcholine, but atorvastatin (Lipitor) monotherapy did. To be fair, the other (very small) trials showed mixed results, thus keeping the hypothesis of ezetimibe’s benefit alive, but with nothing close to a clear signal of benefit.
IS ARTERIAL THICKNESS RELIABLE AS A SURROGATE END POINT?
Was the principal problem in ENHANCE the use of carotid intima-media thickness as the primary end point? No.
This issue has received a lot of attention, much of which I believe is misinformed. No trial end point is infallible, including carotid intima-media thickness, and one must remain open to the possibility of chance findings. However, it has been a relatively reasonable end point in trials of diverse cardiovascular preventive strategies, including lipid-lowering, blood-pressure-lowering, and lifestyle interventions and as a directional biomarker of clinical atherosclerotic events.
We should be cautious about comparing data on carotid intima-media thickness from different trials, as Dr. Davidson attempts to do, in view of methodologic and population differences: each trial must be considered independently. Of greatest concern in ENHANCE is the consistency among intima-media thickness end points, including strong trends toward adverse effects in the most diseased carotid and femoral segments.
Moreover, ENHANCE’s detractors contend that the carotid intima-media thickness of the studied population was normal, citing this as evidence of delipidation from prior treatment. Although not impossible (as shown by the work of Zhao and colleagues in the setting of prolonged, intense lipid-lowering therapy19), at the moment this hypothesis is a matter of conjecture in the ENHANCE participants, particularly because their LDL-C levels were still quite elevated during the trial and conceivably even before randomization.
But these patients were not normal: they were typical patients with familial hypercholesterolemia with extremely elevated LDL-C levels and abnormally thick arteries for their age. Population screening estimates show that, for age and sex, the carotid intima-media thickness values in ENHANCE would lie in the upper quartile of those in the general population.20 Moreover, their mean value is consistent with that in similar-aged groups of patients with familial hypercholesterolemia, even with lower rates of prior statin pretreatment.21
The most convincing evidence for the validity of the ENHANCE findings comes from the published subgroup data (Figure 1). In participants whose baseline carotid intima-media thickness was above the median at baseline, the thickness increased more with ezetimibe/simvastatin than with simvastatin alone. The same was true in the subgroup with above-average LDL-C levels at baseline. The subgroups with no prior statin treatment, low-dose prior statin treatment, and high-dose prior statin showed no heterogeneity of response: their carotid intima-media thickness increased more with ezetimibe/simvastatin than with simvastatin alone. None of these differences was statistically significant; however, these prespecified subgroup data seemingly invalidate arguments against the ENHANCE results based on carotid intima-media thickness findings.
In this context, ENHANCE can only be interpreted as a strong initial negative signal, a “red flag” about ezetimibe’s net health benefits.
WHAT NEXT?
The proper present focus of this debate is not on LDL-C but rather on ezetimibe, its unique mechanism of action, and on the need for more evidence about this complex compound.
At present, ezetimibe’s mechanism of action is not fully understood, and its benefit—for now, only mild LDL-C reduction—is too uncertain for us to be spending $5.2 billion a year for it. Its manufacturer is fortunate that the drug is even licensed, given the current and seemingly appropriate regulatory changes under which drugs introducing new therapeutic classes are scrutinized more closely for benefits and risks. “Safe and well tolerated,” as contended by Dr. Davidson, is not nearly enough: drugs must show clinically important benefits. We still know too little about this drug, the manufacturer of which has invested far more in marketing than in science, a point on which Dr. Davidson and I agree.
In 2008, ezetimibe is an appropriate candidate for testing in clinical trials, and in years to come it may be worthy of clinical attention—if rigorous and objectively conducted clinical trials prove its worth. At present, clinical equipoise dictates that ezetimibe is not an appropriate alternative to a statin in titrated doses, to the addition of other lipid-lowering drugs to a statin, to greater attention to drug adherence, or to lifestyle modification.
For the moment, given the ENHANCE results, the clinical usefulness of ezetimibe still remains to be proven. Much more evidence is needed before we can confidently reembrace the clinical use of ezetimibe.
- Ballantyne CM, Houri J, Notarbartolo A, et al. Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Circulation 2003; 107:2409–2415.
- Kastelein JJ, Akdim F, Stroes ES, et al. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med 2008; 358:1431–1443.
- Kent SM, Taylor AJ. Usefulness of lowering low-density lipoprotein cholesterol to < 70 mg/dL and usefulness of C-reactive protein in patient selection. Am J Cardiol 2003; 92:1224–1227.
- Nissen SE, Tuzcu EM, Schoenhagen P, et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med 2005; 352:29–38.
- Davidson MH. Interpreting the ENHANCE trial. Is ezetimibe/simvastatin no better than simvastatin alone? Leessons learned and clinical implications. Cleve Clin J Med 2008; 75:479–491.
- Gouni-Berthold I, Berthold HK, Gylling H, et al. Effects of ezetimibe and/or simvastatin on LDL receptor protein expression and on LDL receptor and HMG-CoA reductase gene expression: a randomized trial in healthy men. Atherosclerosis 2008; 198:198–207.
- Spener F. Ezetimibe in search of receptor(s)—still a never-ending challenge in cholesterol absorption and transport. Biochim Biophys Acta 2007; 1771:1113–1116.
- Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science 1996; 271:518–520.
- Kitayama K, Nishizawa T, Abe K, et al. Blockade of scavenger receptor class B type I raises high density lipoprotein cholesterol levels but exacerbates atherosclerotic lesion formation in apolipoprotein E deficient mice. J Pharm Pharmacol 2006; 58:1629–1638.
- During A, Dawson HD, Harrison EH. Carotenoid transport is decreased and expression of the lipid transporters SR-BI, NPC1L1, and ABCA1 is downregulated in Caco-2 cells treated with ezetimibe. J Nutr 2005; 135:2305–2312.
- Orso E, Werner T, Wolf Z, Bandulik S, Kramer W, Schmitz G. Ezetimib influences the expression of raft-associated antigens in human monocytes. Cytometry A 2006; 69:206–208.
- Patrick JE, Kosoglou T, Stauber KL, et al. Disposition of the selective cholesterol absorption inhibitor ezetimibe in healthy male subjects. Drug Metab Dispos 2002; 30:430–437.
- Kuhlencordt PJ, Padmapriya P, Rutzel S, et al. Ezetimibe potently reduces vascular inflammation and arteriosclerosis in eNOS-deficient ApoE ko mice. Atherosclerosis 2008; April 6.
- Bulut D, Hanefeld C, Bulut-Streich N, Graf C, Mugge A, Spiecker M. Endothelial function in the forearm circulation of patients with the metabolic syndrome—effect of different lipid-lowering regimens. Cardiology 2005; 104:176–180.
- Fichtlscherer S, Schmidt-Lucke C, Bojunga S, et al. Differential effects of short-term lipid lowering with ezetimibe and statins on endothelial function in patients with CAD: clinical evidence for ‘pleiotropic’ functions of statin therapy. Eur Heart J 2006; 27:1182–1190.
- Landmesser U, Bahlmann F, Mueller M, et al. Simvastatin versus ezetimibe: pleiotropic and lipid-lowering effects on endothelial function in humans. Circulation 2005; 111:2356–2363.
- Maki-Petaja KM, Booth AD, Hall FC, et al. Ezetimibe and simvastatin reduce inflammation, disease activity, and aortic stiffness and improve endothelial function in rheumatoid arthritis. J Am Coll Cardiol 2007; 50:852–858.
- Settergren M, Bohm F, Ryden L, Pernow J. Cholesterol lowering is more important than pleiotropic effects of statins for endothelial function in patients with dysglycaemia and coronary artery disease. Eur Heart J 2008 April 25.
- Zhao XQ, Yuan C, Hatsukami TS, et al. Effects of prolonged intensive lipid-lowering therapy on the characteristics of carotid atherosclerotic plaques in vivo by MRI: a case-control study. Arterioscler Thromb Vasc Biol 2001; 21:1623–1629.
- Stein JH, Korcarz CE, Hurst RT, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr 2008; 21:93–111.
- Junyent M, Cofan M, Nunez I, Gilabert R, Zambon D, Ros E. Influence of HDL cholesterol on preclinical carotid atherosclerosis in familial hypercholesterolemia. Arterioscler Thromb Vasc Biol 2006; 26:1107–1113.
- Ballantyne CM, Houri J, Notarbartolo A, et al. Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Circulation 2003; 107:2409–2415.
- Kastelein JJ, Akdim F, Stroes ES, et al. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med 2008; 358:1431–1443.
- Kent SM, Taylor AJ. Usefulness of lowering low-density lipoprotein cholesterol to < 70 mg/dL and usefulness of C-reactive protein in patient selection. Am J Cardiol 2003; 92:1224–1227.
- Nissen SE, Tuzcu EM, Schoenhagen P, et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med 2005; 352:29–38.
- Davidson MH. Interpreting the ENHANCE trial. Is ezetimibe/simvastatin no better than simvastatin alone? Leessons learned and clinical implications. Cleve Clin J Med 2008; 75:479–491.
- Gouni-Berthold I, Berthold HK, Gylling H, et al. Effects of ezetimibe and/or simvastatin on LDL receptor protein expression and on LDL receptor and HMG-CoA reductase gene expression: a randomized trial in healthy men. Atherosclerosis 2008; 198:198–207.
- Spener F. Ezetimibe in search of receptor(s)—still a never-ending challenge in cholesterol absorption and transport. Biochim Biophys Acta 2007; 1771:1113–1116.
- Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science 1996; 271:518–520.
- Kitayama K, Nishizawa T, Abe K, et al. Blockade of scavenger receptor class B type I raises high density lipoprotein cholesterol levels but exacerbates atherosclerotic lesion formation in apolipoprotein E deficient mice. J Pharm Pharmacol 2006; 58:1629–1638.
- During A, Dawson HD, Harrison EH. Carotenoid transport is decreased and expression of the lipid transporters SR-BI, NPC1L1, and ABCA1 is downregulated in Caco-2 cells treated with ezetimibe. J Nutr 2005; 135:2305–2312.
- Orso E, Werner T, Wolf Z, Bandulik S, Kramer W, Schmitz G. Ezetimib influences the expression of raft-associated antigens in human monocytes. Cytometry A 2006; 69:206–208.
- Patrick JE, Kosoglou T, Stauber KL, et al. Disposition of the selective cholesterol absorption inhibitor ezetimibe in healthy male subjects. Drug Metab Dispos 2002; 30:430–437.
- Kuhlencordt PJ, Padmapriya P, Rutzel S, et al. Ezetimibe potently reduces vascular inflammation and arteriosclerosis in eNOS-deficient ApoE ko mice. Atherosclerosis 2008; April 6.
- Bulut D, Hanefeld C, Bulut-Streich N, Graf C, Mugge A, Spiecker M. Endothelial function in the forearm circulation of patients with the metabolic syndrome—effect of different lipid-lowering regimens. Cardiology 2005; 104:176–180.
- Fichtlscherer S, Schmidt-Lucke C, Bojunga S, et al. Differential effects of short-term lipid lowering with ezetimibe and statins on endothelial function in patients with CAD: clinical evidence for ‘pleiotropic’ functions of statin therapy. Eur Heart J 2006; 27:1182–1190.
- Landmesser U, Bahlmann F, Mueller M, et al. Simvastatin versus ezetimibe: pleiotropic and lipid-lowering effects on endothelial function in humans. Circulation 2005; 111:2356–2363.
- Maki-Petaja KM, Booth AD, Hall FC, et al. Ezetimibe and simvastatin reduce inflammation, disease activity, and aortic stiffness and improve endothelial function in rheumatoid arthritis. J Am Coll Cardiol 2007; 50:852–858.
- Settergren M, Bohm F, Ryden L, Pernow J. Cholesterol lowering is more important than pleiotropic effects of statins for endothelial function in patients with dysglycaemia and coronary artery disease. Eur Heart J 2008 April 25.
- Zhao XQ, Yuan C, Hatsukami TS, et al. Effects of prolonged intensive lipid-lowering therapy on the characteristics of carotid atherosclerotic plaques in vivo by MRI: a case-control study. Arterioscler Thromb Vasc Biol 2001; 21:1623–1629.
- Stein JH, Korcarz CE, Hurst RT, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr 2008; 21:93–111.
- Junyent M, Cofan M, Nunez I, Gilabert R, Zambon D, Ros E. Influence of HDL cholesterol on preclinical carotid atherosclerosis in familial hypercholesterolemia. Arterioscler Thromb Vasc Biol 2006; 26:1107–1113.
Is ezetimibe/simvastatin no better than simvastatin alone? Lessons learned and clinical implications
The Ezetimibe and Simvastatin in Hypercholesterolemia Enhances Atherosclerosis Regression (ENHANCE) trial1 was probably the most widely publicized clinical study of the past decade. How did a 720-patient imaging trial with a neutral result in patients with severe hypercholesterolemia rise to a level warranting massive media attention, a congressional investigation, and a recommendation to curtail the use of a drug widely used to reduce levels of low-density-lipoprotein cholesterol (LDL-C)?
The reaction to the ENHANCE trial reveals more about the political climate and the relationship between the pharmaceutical industry and the American public than it does about the effects of ezetimibe (available combined with simvastatin as Vytorin and by itself as Zetia) on the progression of atherosclerosis.
SOME SELF-DISCLOSURE
Before I discuss the clinical implications of the ENHANCE trial, I must describe both my financial conflicts and intellectual biases. I am a paid consultant, speaker, and researcher on behalf of Merck/Schering-Plough, the sponsor of the ENHANCE trial. I was a principal investigator in the first phase II trial of ezetimibe and have conducted more than 10 clinical trials of either ezetimibe or ezetimibe/simvastatin. I also have been a strong advocate for imaging trials to assist in the clinical development of novel therapeutic agents and to support regulatory approval.
Therefore, I believe that the thickness of the intima and media layers of the carotid arteries is a useful surrogate to evaluate the potential antiatherosclerotic effects of drugs (more on this topic below). Also, I believe that the LDL-C-lowering hypothesis has been proven: ie, that all drugs that lower LDL-C safely, without off-target adverse effects, should reduce cardiovascular events. I support the goal levels of LDL-C and non-high-density-lipoprotein cholesterol set by the National Cholesterol Education Program’s third Adult Treatment Panel (ATP III) guidelines,2,3 which specify LDL-C targets rather than the use of specific drugs. In spite of these conflicts and potential biases, I believe I have always served the best interests of patient care.
HISTORY OF THE ENHANCE TRIAL
The end point defined as the mean of six measurements
The primary end point was the change in the thickness of the intima and media layers of the carotid arteries over a 2-year period, measured by ultrasonography. A composite measure was used: the mean of the thicknesses in the far walls of the right and left common carotid arteries, the right and left carotid bulbs, and the right and left internal carotid arteries. Secondary end points included the change in the mean maximal carotid artery intima-media thickness (ie, the thickest of the six baseline measurements), the proportion of participants who developed new carotid artery plaque (defined arbitrarily as an intima-media thickness > 1.3 mm), and changes in the mean of the intima-media thickness of the six carotid sites plus the common femoral arteries.
The last participant completed the trial in April 2006. Reading of the almost 30,000 scans was not started until the last participant was finished, so that all scans for each participant could be read in a blinded, randomized order by five separate readers. A significant proportion of the images that the protocol called for could not be obtained or analyzed, particularly in the internal carotid artery and the carotid bulb, which are often difficult to visualize. As a result, 17% of the internal carotid or carotid bulb measurements were discarded.
To change the end point post hoc, or not to change the end point?
The sponsor of the trial was concerned about the missing data points and convened a special advisory board to review the blinded data. This group suggested a solution: changing the primary end point from the six-site composite value to the mean value in just the common carotid arteries. They based this suggestion on the greater success rate in measuring the common carotids (97%) than in measuring all six sites (88%), as well as on recent trials that indicated that the common carotid artery measurement correlates better with clinical outcomes (because the internal carotid and the bulb measurements vary more). On November 26, 2007, Merck/Schering-Plough announced the primary end point would be changed to the mean change in the common carotid arteries.
However, during a separate meeting on November 30, 2007, some members of the Merck/Schering-Plough advisory board objected to the change. On December 11, 2007, the company announced that the original primary end point would not be changed.
Neutral results, negative publicity
On December 31, 2007, the ENHANCE study was unblinded, and on January 14, 2008, Merck/Schering-Plough issued a press release announcing the results. The press release stated that there were no statistically significant differences between the treatment groups in the primary end point or in any of the secondary end points, despite a 16.5% greater reduction in LDL-C (about 50 mg/dL) in the group receiving the ezetimibe/simvastatin combination. The composite intima-media thickness had increased by an average of 0.0111 mm in the combined-therapy group vs 0.0058 mm in the simvastatin-only group (P = .29) over the 24-month treatment period.5
The press release received unprecedented international media attention. One leading cardiologist commented to the media that ENHANCE showed “millions of patients may be taking a drug [ezetimibe] that does not benefit them, raising their risk of heart attacks and exposing them to potential side effects.”6 The perceived message that ezetimibe/simvastatin is harmful resulted in thousands of phone calls from concerned patients to their physicians throughout the United States. The American Heart Association (AHA) and the American College of Cardiology (ACC) issued a joint statement the next day saying that ezetimibe/simvastatin does not appear to be unsafe and that patients should not stop taking the drug on their own. In the following days, Merck/Schering-Plough placed advertisements in newspapers reaffirming the safety of ezetimibe and quoting the AHA/ACC statement.
But the full results of the study were not available at that point. In fact, Senator Charles Grassley (R-Iowa) had launched a congressional investigation into the delays in releasing the results of the ENHANCE trial in December 2007. A focus of the investigation was whether the sponsor was delaying the release either because the data reflected negatively on its product or because it was legitimately concerned about the quality of the measurements of the carotid intima-media thickness. After Merck/Schering-Plough placed the advertisements quoting the AHA/ACC statement, these organizations were criticized for touting the safety of ezetimibe while receiving educational grants and other funds from Merck/Schering-Plough. Senator Grassley sent a letter to the ACC in late March requesting information about the amount of funds the ACC had received.
Full results are published, and the ACC is misquoted
The ENHANCE study was selected for a special presentation at the ACC annual scientific session on March 30, 2008. The full ENHANCE results were presented by Dr. Kastelein, after which an expert panel led by Harlan M. Krumholz, MD, discussed the trial’s implications. The ENHANCE results were simultaneously published in the New England Journal of Medicine,1 accompanied by an editorial by B. Greg Brown, MD, and Allen J. Taylor, MD,7 and another editorial by the editors of that journal, Jeffrey M. Drazen, MD, and colleagues.8 The expert panel and the editorialists concluded that the ENHANCE trial data raised concerns about the cardiovascular benefits of ezetimibe; that statins should be used as initial therapy for hyperlipidemia and titrated to the goal LDL-C level or to the maximally tolerated dose; and that other drugs such as bile acid sequestrants, fibrates, and niacin should be used in combination with statins before considering ezetimibe.9
The next day, stories appeared in the media mistakenly stating that the ACC had recommended that ezetimibe/simvastatin be discontinued. This view was fueled by an article in the ACC’s Scientific Session News, penned by a contract writer and editor, with the headline, “ACC on Vytorin: Go Back to Statins” that said, “After waiting for 18 months for the results of the ENHANCE study, an ACC panel on Sunday encouraged physicians to use statins as a first line and prescribe Vytorin only as a last resort for patients unable to tolerate other cholesterol-lowering agents.”10
The ACC later clarified that this was the opinion of the panelists and not that of the ACC, and they reiterated statements from the AHA/ACC Secondary Prevention Guidelines11 recommending statins in maximally tolerated doses or titrated to a goal LDL-C level for first-line drug treatment of coronary artery disease, and recommending that patients speak with their physicians before discontinuing any therapy.
WHY WERE THE ENHANCE STUDY RESULTS NEUTRAL?
The ACC expert panel concluded that the most likely reason for the neutral ENHANCE results was that ezetimibe lowers LDL-C but does not confer a cardiovascular benefit. In the words of Dr. Krumholz (as quoted by Shannon Pettypiece and Michelle Fay Cortez on bloomberg.com), ezetimibe is “just an expensive placebo.”12
There are at least three potential explanations for the lack of benefit with ezetimibe in the ENHANCE trial. I list them below in order of lowest to highest probability, in my opinion:
Theory 1: Ezetimibe lowers LDL-C but is not antiatherogenic
Since almost all experts agree that lowering LDL-C confers cardiovascular benefits, if ezetimibe does not inhibit atherosclerosis it must have some “off-target” effect that negates its LDL-C-lowering benefit. Critics of ezetimibe point out that oral estrogen and torcetrapib also lower LDL-C but do not improve cardiovascular outcomes.13,14
The lack of benefit with these two other agents can be explained. Oral estrogen does not lower apolipoprotein B (an indication of the number of atherogenic particles), but rather it increases the levels of both triglycerides and C-reactive protein, and it is prothrombotic in some people.15 Torcetrapib increases aldosterone production and substantially raises blood pressure.16 Therefore, both drugs have true off-target effects that could explain their failure to reduce cardiovascular risk despite reductions in LDL-C. (Interestingly, though, oral estrogen has been shown to slow the progression of carotid intima-media thickness in newly postmenopausal women.17
Ezetimibe, however, lowers LDL-C by an ultimate mechanism similar to that of statins and bile acid sequestrants, ie, by up-regulating LDL receptors, although these drugs reach this mechanism via different pathways. Statins inhibit cholesterol synthesis, thereby lowering hepatic intracellular cholesterol and thus up-regulating LDL-receptors and enhancing LDL-C clearance from the plasma. Bile acid sequestrants interrupt bile acid reabsorption in the ileum, thereby decreasing intracellular hepatic cholesterol and up-regulating LDL receptors. Ezetimibe, like bile acid sequestrants, also decreases cholesterol return to the liver, lowering hepatic intracellular levels and thus up-regulating LDL receptors.18
Ezetimibe is unlikely to have an off-target effect because it is only fractionally absorbed systemically, and a recent animal study showed that it enhances macrophage efflux of cholesterol, thereby potentially increasing reverse cholesterol transport.19 Ezetimibe has also been shown to reduce atherosclerosis in animal models.20
In their editorial, Drs. Brown and Taylor7 noted that ezetimibe reduces the expression of adenosine triphosphate binding cassette A1 (ABCA1) in Caco-2 (an intestinal cell line), and this may be an example of an off-target effect. However, statins also reduce ABCA1 expression in macrophages.21 ABCA1 is sensitive to intracellular cholesterol, and when cholesterol levels are decreased, whether by statins or by ezetimibe, ABCA1 expression is down-regulated.22
Theory 2: Intima-media thickness does not reflect the true benefits of lowering LDL-C
The carotid intima-media thickness is a surrogate end point that predicts coronary events and the rate of progression of coronary atherosclerosis.23 In trials of lovastatin (Mevacor),24 pravastatin (Pravachol),25 and rosuvastatin (Crestor),26 the carotid intima-media was thinner at 24 months with the active drug than with placebo. In two relatively small trials—ARBITER 1 (n = 161),27 which was open-label, and ASAP (n = 325)28,29—aggressive lipid-lowering reduced the progression of intima-media thickness better than less-aggressive therapy. However, this measure has been used to evaluate the effects of differing degrees of LDL-C reduction between active treatments in fewer than 500 research participants.
Furthermore, what part or parts of the carotid system are we talking about? In recent trials led by Dr. Kastelein, the intima-media thickness of the common carotid arteries increased with pactimibe (an acyl-coenzyme A:cholesterol O-acyltransferase, or ACAT, inhibitor)30 and torcetrapib,31 but the six-site composite measure (which was the primary end point in these trials, as in ENHANCE) did not increase more than in the control groups. Pactimibe was also shown to increase atheroma volume as measured by intravascular ultrasonography in the ACTIVATE trial.32 Therefore, the thickness of the common carotid arteries has been shown to be a better predictor of harm from a therapy than the composite measurement.
The advantage of measuring the common carotid artery is that it is easier to visualize and measure, and therefore the measurements vary less. In the METEOR trial,26 the six-site measurement increased significantly less with rosuvastatin than with placebo, but the common carotid measurement alone was more strongly associated with a difference in progression. In the ENHANCE trial, the thickness of the common carotid arteries increased by 0.0024 mm with simvastatin alone vs 0.0019 mm with simvastatin/ezetimibe, a difference of 0.005 mm that was not statistically significant (P = .93).1
Although the six-site measurement appears to be good for predicting coronary events and evaluating therapies, the measurement in the common carotid arteries appears to be a more reliable surrogate end point for predicting both benefit and harm from antiatherogenic agents. However, trials of statins and other lipid-lowering therapies that assessed clinical events have shown that the reduction in risk associated with a given reduction in cholesterol is similar regardless of the mechanism by which cholesterol is lowered.33 Therefore, the LDL-C level is far superior as a marker of clinical benefit.
Theory 3: Previous statin treatment affected the ENHANCE results
By far the most likely explanation for the neutral findings in ENHANCE is that the patients were so well treated before entry that it was impossible to detect a difference between the two treatment groups in carotid intima-media thickness at the end of the study. Eighty percent of the patients had received statins previously, and at baseline the mean intima-media thickness of the common carotid arteries was only 0.68 mm.1 In contrast, most other trials required a thickness greater than 0.7 mm for entry.
The two main reasons for selecting a population with familial hypercholesterolemia were the assumptions that these participants would have a greater-than-average carotid intima-media thickness at baseline and that they would show an above-average progression rate, even on high-dose statin therapy.4 Both of these assumptions were incorrect: the baseline thickness was normal and the progression rate was negligible in both groups.
Accordingly, the high prevalence of statin pretreatment and the near-normal carotid intima-media thickness at baseline may have prevented the 16.5% greater reduction in LDL-C due to ezetimibe from producing a difference in progression over 24 months of treatment. This conclusion is supported by the long-term follow-up results from ASAP, RADIANCE 1, and CAPTIVATE, all of which showed that in patients with familial hypercholesterolemia well treated with statins, progression of carotid intima-media thickness is negligible.30,31
Further supporting this view, in a previous trial by Dr. Kastelein’s group in patients with familial hypercholesterolemia,34 giving simvastatin 80 mg for 2 years decreased the intima-medial thickness by .081 mm (P < .001), compared with 0.0058 mm in ENHANCE (a 14-fold difference). In the previous trial, the baseline measurement was 1.07 mm (vs 0.68 mm in ENHANCE), and the extent of the change was significantly associated with the baseline measurement (r = .53, P < .001) but not with the change in LDL-C levels.
This is powerful evidence that, in two similar studies that used the same methodology and the same drug, the thinner arteries in the ENHANCE trial are by far the most likely explanation for the lack of change with the addition of ezetimibe to high-dose simvastatin. The METEOR trial enrolled only patients who had never received statins and whose carotid intima-media was thicker than 1.2 mm. In retrospect, a similar design would have been preferable for ENHANCE.35
LESSONS LEARNED AND CLINICAL IMPLICATIONS
For Merck/Schering-Plough, missed opportunities
Although Dr. Krumholz (the spokesman for the ACC panel discussion) and I disagree on the clinical implications of the ENHANCE trial, we do agree on an important point. Dr. Krumholz posed the question that if the LDL-C-lowering hypothesis was already proven for ezetimibe, why was the ENHANCE trial conducted? After 6 years on the market, the efficacy of ezetimibe on cardiovascular outcomes should already have been established. It should not take this long to determine the clinical outcome benefit for a drug.
Merck/Schering-Plough’s outcome program for ezetimibe was inadequately designed to demonstrate the clinical value of this novel compound. Rather than assuming the LDL-C-lowering hypothesis was already established, they conducted another “lower-is-better” trial with the carotid intima-media thickness as the end point, and they succeeded only in raising doubt about the benefits of ezetimibe rather than showing that dual therapy is at least equivalent to high-dose statin therapy.
A preferable approach would have been to compare the effects of a statin in low doses plus ezetimibe vs high-dose statin monotherapy on either surrogate or hard outcomes. If the low-dose statin/ezetimibe combination, which should lower the LDL-C level as much as high-dose statin monotherapy, could provide similar or better outcomes with fewer side effects, this trial would change our practice.
One had hoped that dual therapy, by reducing both intestinal cholesterol absorption and hepatic synthesis of cholesterol, would improve outcomes by modifying postprandial chylomicron composition or by reducing plant sterol absorption.36 Unfortunately, other outcome trials of ezetimibe/simvastatin will not provide an answer regarding the potential advantages of dual therapy. The SEAS study is comparing the number of clinical events in patients with aortic stenosis who receive ezetimibe/simvastatin or placebo; SHARP is being conducted in patients with chronic kidney disease. Although both groups of patients have high rates of coronary events, these trials will not address whether adding ezetimibe provides additional benefits. In fact, if the results of these trials turn out neutral, as in ENHANCE, then ezetimibe will be blamed for potentially offsetting the benefits of simvastatin, and if the trials show a benefit, the simvastatin component of ezetimibe/simvastatin will be given the credit.
The answer may come in 3 to 4 years with the results of IMPROVE-IT, a study of 18,000 patients with acute coronary syndrome treated with ezetimibe/simvastatin or simvastatin. The simvastatin monotherapy group will have a target LDL-C level of less than 80 mg/dL and the ezetimibe/simvastatin group will have an LDL-C target about 15% less. Although this trial is testing the lower-is-better hypothesis with ezetimibe, if the study does not show a benefit, it may not be because ezetimibe lacks clinical efficacy but rather because the LDL-C effect is curvilinear, and there is minimal further benefit of lowering the LDL-C level past 70 mg/dL. If the results of the IMPROVE-IT trial are negative, it may mean the end of ezetimibe as an LDL-C-lowering drug.
Merck/Schering-Plough has lost valuable time in not demonstrating the benefits of ezetimibe on clinical events. In contrast, consider rosuvastatin, an AstraZeneca product. Rosuvastatin was approved about the same time as ezetimibe/simvastatin, and 6 years later it has already received a label change for the reduction of progression of atherosclerosis, based on positive outcomes in the METEOR trial,35 the ASTEROID intravascular ultrasonography trial,37 and the CORONA trial (an important trial that examined hard clinical end points).38 More importantly, the JUPITER trial was recently stopped early owing to a reduction in cardiovascular deaths. Initially, rosuvastatin received an unfair media portrayal as an unsafe drug. Now, because of its proven benefits in outcome trials, it will receive more widespread consideration for clinical use.
For preventive cardiologists, a painful reminder to focus on LDL-C
For the preventive cardiologist or lipidologist, the ENHANCE trial has been a painful reminder that despite overwhelming evidence, the mantra of “the lower the LDL-C the better” is still not universally accepted. We acknowledge the great benefits of statins, but the lure of “pleiotropic effects” distracts many of us from the necessity of more aggressive LDL-C reduction.
The pleiotropic benefits of statins were first raised as a means of supporting increased clinical use of pravastatin vis-a-vis other, more efficacious statins. It was not until the PROVE-IT study that pravastatin’s pleiotropic effects were found not to translate into a benefit equivalent to that of the more efficacious statin, atorvastatin.39
The success of ezetimibe was its ability to safely and easily lower LDL-C in combination with statins to achieve treatment goals. For many patients, a lower-dose statin and ezetimibe together provide a well-tolerated and efficacious approach to treating hyperlipidemia. The fallout from the ENHANCE trial is that many patients who were well treated or who could be better treated with ezetimibe in combination with a statin will not receive the best tolerated regimen. In fact, preliminary prescription data after the release of the ENHANCE study support our worse fear, ie, that patients at high risk will receive less aggressive LDL-C reduction. Since the ENHANCE data were released, more than 300,000 patients have stopped taking either ezetimibe/simvastatin or ezetimibe, and nearly all have continued on generic simvastatin or on a dose of statin with less overall efficacy.
An example is Senator John McCain, who, according to his recently released medical records, has a Framingham 10-year risk of more than 20% and was on ezetimibe/simvastatin to treat an elevated cholesterol level. After release of the ENHANCE trial, he was switched to generic simvastatin, and his LDL-C increased from 82 mg/dL to 122 mg/dL. He most likely has an LDL-C goal of less than 100 mg/dL according to the ATP III guidelines, and he is therefore no longer at his target.
For physicians in the community, questions from concerned patients
For the physicians who have received hundreds of phone calls and e-mails from concerned patients, the ENHANCE trial results must have been both discouraging and confusing. At present, I think we should remember the following:
- Ezetimibe’s mechanism of action is well understood
- It is safe and well-tolerated
- It still has a role as an add-on to statin therapy (or as monotherapy or combined with other agents in those who cannot tolerate statins) for patients who have not yet achieved their LDL-C target.
For the pharmaceutical industry, enormous challenges
The neutral ENHANCE trial results created an uncomfortable situation for the trial sponsor. A heavily marketed drug failed to achieve its expected result after the study results were delayed for a few months. The pharmaceutical industry ranks 14th out of 17 industries in public trust among the American public, and this study provided an opportunity for its critics to attack what is, in their opinion, an overly marketed drug.
Enormous challenges are on the horizon for the pharmaceutical industry, with a shrinking pipeline of potential new drugs, increasing regulatory hurdles, greater liability risk, political pressure for price controls, enhanced scrutiny of sales practices, and a growing media bias. As a cardiologist and clinical researcher whose father died at age 47 of a myocardial infarction, I am concerned that, unless change occurs, a vibrant pharmaceutical industry with the financial and intellectual capital to find and develop new, more effective treatments will cease to exist.
- Kastelein JJ, Akdim F, Stroes ES, et al ENHANCE Investigators. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med 2008; 358:1431–1443.
- Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106:3143–3421.
- Grundy SM, Cleeman JI, Bairey Merz N, et al for the Coordinating Committee of the National Cholesterol Education Program. Circulation 2004; 110:227–239.
- Kastelein JJ, Sager PT, de Groot E, Veltri E. Comparison of ezetimibe plus simvastatin versus simvastatin monotherapy on atherosclerosis progression in familial hypercholesterolemia. Design and rationale of the Ezetimibe and Simvastatin in Hypercholesterolemia Enhances Atherosclerosis Regression (ENHANCE) trial. Am Heart J 2005; 49:234–239.
- Merck/Schering-Plough Pharmaceutical Press Release, January 14, 2008.
- Berenson A. Study reveals doubt on drug for cholesterol. New York Times January 15, 2008.
- Brown BG, Taylor AJ. Does ENHANCE diminish confidence in lowering LDL or in ezetimibe? N Engl J Med 2008; 358:1504–1507.
- Drazen JM, Jarcho JA, Morrissey S, Curfman GD. Cholesterol lowering and ezetimibe. N Engl J Med 2008; 358:1507–1508.
- American College of Cardiology. ENHANCED analysis of ezetimibe. ACC News, April 2, 2008. www.acc.org/emails/myacc/accnews%5Fapril%5F02%5F08.htm. Accessed 6/2/2008.
- American College of Cardiology. ACC panel on Vytorin: Go back to statins. Scientific Session News 3/31/2008. http://www.acc08.acc.org/SSN/Documents/ACC%20Monday%20v2.pdf. Accessed 6/2/2008.
- Smith SC, Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update. Endorsed by the National Heart, Lung, and Blood Institute. J Am Coll Cardiol 2006; 47:2130–2139.
- Pettypiece S, Cortez MF. Merck, Schering plunge as doctors discourage Vytorin. www.bloomberg.com/apps/news?pid=20601103&refer=news&sid=aV_T9WirgAkI. Accessed 6/2/2008.
- Barter PJ, Caulfield M, Eriksson M, et al ILLUMINATE Investigators. . Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 2007; 357:2109–2122.
- Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in post-menopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA 1998; 280:605–613.
- Rader DJ. Illuminating HDL—is it still a viable therapeutic target? N Engl J Med 2007; 357:2180–2183.
- Davidson MH, Maki KC, Marx P, et al. Effects of continuous estrogen and estrogen-progestin replacement regimens on cardiovascular risk markers in postmenopausal women. Arch Intern Med 2000; 160:3315–3325.
- Hodis HN, Mack WJ, Lobo RA, et al Estrogen in the Prevention of Atherosclerosis Trial Research Group. . Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 2001; 135:939–953.
- Turley SD. Cholesterol metabolism and therapeutic targets: rationale for targeting multiple metabolic pathways. Clin Cardiol 2004; 27( suppl 3):III16–III21.
- Sehayek E, Hazen SL. Cholesterol absorption from the intestine is a major determinant of reverse cholesterol transport from peripheral tissue macrophages. Arterioscler Thromb Vasc Biol 2008;27 (Epub ahead of print].
- Davis HR, Compton DS, Hoos L, Tetzloff G. Ezetimibe, a potent cholesterol absorption inhibitor, inhibits the development of atherosclerosis in ApoE knockout mice. Arterioscler Thromb Vasc Biol 2001; 21:2032–2038.
- Wong J, Quinn CM, Gelissen IC, Jessup W, Brown AJ. The effect of statins on ABCA1 and ABCG1 expression in human macrophages is influenced by cellular cholesterol levels and extent of differentiation. Atherosclerosis 2008; 196:180–189.
- Wang N, Tall AR. Regulation and mechanisms of ATP-binding cassette transporter A1-mediated cellular cholesterol efflux. Arterioscler Thromb Vasc Biol 2003; 23:1178–1184.
- Bots ML. Carotid intima-media thickness as a surrogate marker for cardiovascular disease in intervention studies. Curr Med Res Opin 2006; 22:2181–2190.
- Byington RP, Evans GW, Espeland MA, et al. Effects of lovastatin and warfarin on early carotid atherosclerosis: sex-specific analyses. Asymptomatic Carotid Artery Progression Study (ACAPS) Research Group. Circulation 1999; 100:e14–e17.
- Byington RP, Furberg CD, Crouse JR, Espeland MA, Bond MG. Pravastatin, Lipids, and Atherosclerosis in the Carotid Arteries (PLAC-II). Am J Cardiol 1995; 76:54C–59C.
- Crouse JR, Raichlen JS, Riley WA, et al METEOR Study Group. . Effect of rosuvastatin on progression of carotid intima-media thickness in low-risk individuals with subclinical atherosclerosis: the METEOR trial. JAMA 2007; 297:1344–1353.
- Taylor AJ, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation 2004; 110:3512–3517.
- Smilde TJ, van Wissen S, Wollersheim H, Trip MD, Kastelein JJ, Stalenhoef AF. Effect of aggressive versus conventional lipid lowering on atherosclerosis progression in familial hypercholesterolaemia (ASAP): a prospective, randomised, double-blind trial. Lancet 2001; 357:577–581.
- van Wissen S, Smilde TJ, Trip MD, Stalenhoef AFH, Kastelein JJP. Long-term safety and efficacy of high-dose atorvastatin treatment in patients with familial hypercholesterolemia. Am J Cardiol 2005; 95:264–266.
- Meuwese MC, Franssen R, Stroes ES, Kastelein JJ. And then there were acyl coenzyme A:cholesterol acyl transferase inhibitors. Curr Opin Lipidol 2006; 17:426–430.
- Kastelein JJ, van Leuven SI, Burgess L, et al RADIANCE 1 Investigators. . Effect of torcetrapib on carotid atherosclerosis in familial hypercholesterolemia. N Engl J Med 2007; 356:1620–1630.
- Nissen SE, Tuzcu EM, Brewer HB, et al ACAT Intravascular Atherosclerosis Treatment Evaluation (ACTIVATE) Investigators. Effect of ACAT inhibition on the progression of coronary atherosclerosis. N Engl J Med 2006; 354:1253–1263.
- Davidson MH. Clinical significance of statin pleiotropic effects: hypotheses versus evidence. Circulation 2005; 111:2280–2281.
- Nolting PR, de Groot E, Zwinderman AH, Buirma RJ, Trip MD, Kastelein JJ. Regression of carotid and femoral artery intima-media thickness in familial hypercholesterolemia. Arch Intern Med 2003; 163:1837–1841.
- Crouse JR, Grobbee DE, O’Leary DH, et al Measuring Effects on intima media Thickness: an Evaluation Of Rosuvastatin Study Group. . Measuring effects on intima media thickness: an evaluation of rosuvastatin in subclinical atherosclerosis—the rationale and methodology of the METEOR study. Cardiovasc Drugs Ther 2004; 18:231–238.
- Toth PP, Davidson MH. Cholesterol absorption blockade with ezetimibe. Curr Drug Targets Cardiovasc Haematol Disord 2005; 5:455–462.
- Nissen SE, Nicholls SJ, Sipahi I, et al ASTEROID Investigators. . Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA 2006; 295:1556–1565.
- Kjekshus J, Apetrei E, Barrios V, et al CORONA Group. . Rosuvastatin in older patients with systolic heart failure. N Engl J Med 2007; 357:2248–2261.
- Cannon CP, Braunwald E, McCabe CH, et al Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. . Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350:1495–1504.
The Ezetimibe and Simvastatin in Hypercholesterolemia Enhances Atherosclerosis Regression (ENHANCE) trial1 was probably the most widely publicized clinical study of the past decade. How did a 720-patient imaging trial with a neutral result in patients with severe hypercholesterolemia rise to a level warranting massive media attention, a congressional investigation, and a recommendation to curtail the use of a drug widely used to reduce levels of low-density-lipoprotein cholesterol (LDL-C)?
The reaction to the ENHANCE trial reveals more about the political climate and the relationship between the pharmaceutical industry and the American public than it does about the effects of ezetimibe (available combined with simvastatin as Vytorin and by itself as Zetia) on the progression of atherosclerosis.
SOME SELF-DISCLOSURE
Before I discuss the clinical implications of the ENHANCE trial, I must describe both my financial conflicts and intellectual biases. I am a paid consultant, speaker, and researcher on behalf of Merck/Schering-Plough, the sponsor of the ENHANCE trial. I was a principal investigator in the first phase II trial of ezetimibe and have conducted more than 10 clinical trials of either ezetimibe or ezetimibe/simvastatin. I also have been a strong advocate for imaging trials to assist in the clinical development of novel therapeutic agents and to support regulatory approval.
Therefore, I believe that the thickness of the intima and media layers of the carotid arteries is a useful surrogate to evaluate the potential antiatherosclerotic effects of drugs (more on this topic below). Also, I believe that the LDL-C-lowering hypothesis has been proven: ie, that all drugs that lower LDL-C safely, without off-target adverse effects, should reduce cardiovascular events. I support the goal levels of LDL-C and non-high-density-lipoprotein cholesterol set by the National Cholesterol Education Program’s third Adult Treatment Panel (ATP III) guidelines,2,3 which specify LDL-C targets rather than the use of specific drugs. In spite of these conflicts and potential biases, I believe I have always served the best interests of patient care.
HISTORY OF THE ENHANCE TRIAL
The end point defined as the mean of six measurements
The primary end point was the change in the thickness of the intima and media layers of the carotid arteries over a 2-year period, measured by ultrasonography. A composite measure was used: the mean of the thicknesses in the far walls of the right and left common carotid arteries, the right and left carotid bulbs, and the right and left internal carotid arteries. Secondary end points included the change in the mean maximal carotid artery intima-media thickness (ie, the thickest of the six baseline measurements), the proportion of participants who developed new carotid artery plaque (defined arbitrarily as an intima-media thickness > 1.3 mm), and changes in the mean of the intima-media thickness of the six carotid sites plus the common femoral arteries.
The last participant completed the trial in April 2006. Reading of the almost 30,000 scans was not started until the last participant was finished, so that all scans for each participant could be read in a blinded, randomized order by five separate readers. A significant proportion of the images that the protocol called for could not be obtained or analyzed, particularly in the internal carotid artery and the carotid bulb, which are often difficult to visualize. As a result, 17% of the internal carotid or carotid bulb measurements were discarded.
To change the end point post hoc, or not to change the end point?
The sponsor of the trial was concerned about the missing data points and convened a special advisory board to review the blinded data. This group suggested a solution: changing the primary end point from the six-site composite value to the mean value in just the common carotid arteries. They based this suggestion on the greater success rate in measuring the common carotids (97%) than in measuring all six sites (88%), as well as on recent trials that indicated that the common carotid artery measurement correlates better with clinical outcomes (because the internal carotid and the bulb measurements vary more). On November 26, 2007, Merck/Schering-Plough announced the primary end point would be changed to the mean change in the common carotid arteries.
However, during a separate meeting on November 30, 2007, some members of the Merck/Schering-Plough advisory board objected to the change. On December 11, 2007, the company announced that the original primary end point would not be changed.
Neutral results, negative publicity
On December 31, 2007, the ENHANCE study was unblinded, and on January 14, 2008, Merck/Schering-Plough issued a press release announcing the results. The press release stated that there were no statistically significant differences between the treatment groups in the primary end point or in any of the secondary end points, despite a 16.5% greater reduction in LDL-C (about 50 mg/dL) in the group receiving the ezetimibe/simvastatin combination. The composite intima-media thickness had increased by an average of 0.0111 mm in the combined-therapy group vs 0.0058 mm in the simvastatin-only group (P = .29) over the 24-month treatment period.5
The press release received unprecedented international media attention. One leading cardiologist commented to the media that ENHANCE showed “millions of patients may be taking a drug [ezetimibe] that does not benefit them, raising their risk of heart attacks and exposing them to potential side effects.”6 The perceived message that ezetimibe/simvastatin is harmful resulted in thousands of phone calls from concerned patients to their physicians throughout the United States. The American Heart Association (AHA) and the American College of Cardiology (ACC) issued a joint statement the next day saying that ezetimibe/simvastatin does not appear to be unsafe and that patients should not stop taking the drug on their own. In the following days, Merck/Schering-Plough placed advertisements in newspapers reaffirming the safety of ezetimibe and quoting the AHA/ACC statement.
But the full results of the study were not available at that point. In fact, Senator Charles Grassley (R-Iowa) had launched a congressional investigation into the delays in releasing the results of the ENHANCE trial in December 2007. A focus of the investigation was whether the sponsor was delaying the release either because the data reflected negatively on its product or because it was legitimately concerned about the quality of the measurements of the carotid intima-media thickness. After Merck/Schering-Plough placed the advertisements quoting the AHA/ACC statement, these organizations were criticized for touting the safety of ezetimibe while receiving educational grants and other funds from Merck/Schering-Plough. Senator Grassley sent a letter to the ACC in late March requesting information about the amount of funds the ACC had received.
Full results are published, and the ACC is misquoted
The ENHANCE study was selected for a special presentation at the ACC annual scientific session on March 30, 2008. The full ENHANCE results were presented by Dr. Kastelein, after which an expert panel led by Harlan M. Krumholz, MD, discussed the trial’s implications. The ENHANCE results were simultaneously published in the New England Journal of Medicine,1 accompanied by an editorial by B. Greg Brown, MD, and Allen J. Taylor, MD,7 and another editorial by the editors of that journal, Jeffrey M. Drazen, MD, and colleagues.8 The expert panel and the editorialists concluded that the ENHANCE trial data raised concerns about the cardiovascular benefits of ezetimibe; that statins should be used as initial therapy for hyperlipidemia and titrated to the goal LDL-C level or to the maximally tolerated dose; and that other drugs such as bile acid sequestrants, fibrates, and niacin should be used in combination with statins before considering ezetimibe.9
The next day, stories appeared in the media mistakenly stating that the ACC had recommended that ezetimibe/simvastatin be discontinued. This view was fueled by an article in the ACC’s Scientific Session News, penned by a contract writer and editor, with the headline, “ACC on Vytorin: Go Back to Statins” that said, “After waiting for 18 months for the results of the ENHANCE study, an ACC panel on Sunday encouraged physicians to use statins as a first line and prescribe Vytorin only as a last resort for patients unable to tolerate other cholesterol-lowering agents.”10
The ACC later clarified that this was the opinion of the panelists and not that of the ACC, and they reiterated statements from the AHA/ACC Secondary Prevention Guidelines11 recommending statins in maximally tolerated doses or titrated to a goal LDL-C level for first-line drug treatment of coronary artery disease, and recommending that patients speak with their physicians before discontinuing any therapy.
WHY WERE THE ENHANCE STUDY RESULTS NEUTRAL?
The ACC expert panel concluded that the most likely reason for the neutral ENHANCE results was that ezetimibe lowers LDL-C but does not confer a cardiovascular benefit. In the words of Dr. Krumholz (as quoted by Shannon Pettypiece and Michelle Fay Cortez on bloomberg.com), ezetimibe is “just an expensive placebo.”12
There are at least three potential explanations for the lack of benefit with ezetimibe in the ENHANCE trial. I list them below in order of lowest to highest probability, in my opinion:
Theory 1: Ezetimibe lowers LDL-C but is not antiatherogenic
Since almost all experts agree that lowering LDL-C confers cardiovascular benefits, if ezetimibe does not inhibit atherosclerosis it must have some “off-target” effect that negates its LDL-C-lowering benefit. Critics of ezetimibe point out that oral estrogen and torcetrapib also lower LDL-C but do not improve cardiovascular outcomes.13,14
The lack of benefit with these two other agents can be explained. Oral estrogen does not lower apolipoprotein B (an indication of the number of atherogenic particles), but rather it increases the levels of both triglycerides and C-reactive protein, and it is prothrombotic in some people.15 Torcetrapib increases aldosterone production and substantially raises blood pressure.16 Therefore, both drugs have true off-target effects that could explain their failure to reduce cardiovascular risk despite reductions in LDL-C. (Interestingly, though, oral estrogen has been shown to slow the progression of carotid intima-media thickness in newly postmenopausal women.17
Ezetimibe, however, lowers LDL-C by an ultimate mechanism similar to that of statins and bile acid sequestrants, ie, by up-regulating LDL receptors, although these drugs reach this mechanism via different pathways. Statins inhibit cholesterol synthesis, thereby lowering hepatic intracellular cholesterol and thus up-regulating LDL-receptors and enhancing LDL-C clearance from the plasma. Bile acid sequestrants interrupt bile acid reabsorption in the ileum, thereby decreasing intracellular hepatic cholesterol and up-regulating LDL receptors. Ezetimibe, like bile acid sequestrants, also decreases cholesterol return to the liver, lowering hepatic intracellular levels and thus up-regulating LDL receptors.18
Ezetimibe is unlikely to have an off-target effect because it is only fractionally absorbed systemically, and a recent animal study showed that it enhances macrophage efflux of cholesterol, thereby potentially increasing reverse cholesterol transport.19 Ezetimibe has also been shown to reduce atherosclerosis in animal models.20
In their editorial, Drs. Brown and Taylor7 noted that ezetimibe reduces the expression of adenosine triphosphate binding cassette A1 (ABCA1) in Caco-2 (an intestinal cell line), and this may be an example of an off-target effect. However, statins also reduce ABCA1 expression in macrophages.21 ABCA1 is sensitive to intracellular cholesterol, and when cholesterol levels are decreased, whether by statins or by ezetimibe, ABCA1 expression is down-regulated.22
Theory 2: Intima-media thickness does not reflect the true benefits of lowering LDL-C
The carotid intima-media thickness is a surrogate end point that predicts coronary events and the rate of progression of coronary atherosclerosis.23 In trials of lovastatin (Mevacor),24 pravastatin (Pravachol),25 and rosuvastatin (Crestor),26 the carotid intima-media was thinner at 24 months with the active drug than with placebo. In two relatively small trials—ARBITER 1 (n = 161),27 which was open-label, and ASAP (n = 325)28,29—aggressive lipid-lowering reduced the progression of intima-media thickness better than less-aggressive therapy. However, this measure has been used to evaluate the effects of differing degrees of LDL-C reduction between active treatments in fewer than 500 research participants.
Furthermore, what part or parts of the carotid system are we talking about? In recent trials led by Dr. Kastelein, the intima-media thickness of the common carotid arteries increased with pactimibe (an acyl-coenzyme A:cholesterol O-acyltransferase, or ACAT, inhibitor)30 and torcetrapib,31 but the six-site composite measure (which was the primary end point in these trials, as in ENHANCE) did not increase more than in the control groups. Pactimibe was also shown to increase atheroma volume as measured by intravascular ultrasonography in the ACTIVATE trial.32 Therefore, the thickness of the common carotid arteries has been shown to be a better predictor of harm from a therapy than the composite measurement.
The advantage of measuring the common carotid artery is that it is easier to visualize and measure, and therefore the measurements vary less. In the METEOR trial,26 the six-site measurement increased significantly less with rosuvastatin than with placebo, but the common carotid measurement alone was more strongly associated with a difference in progression. In the ENHANCE trial, the thickness of the common carotid arteries increased by 0.0024 mm with simvastatin alone vs 0.0019 mm with simvastatin/ezetimibe, a difference of 0.005 mm that was not statistically significant (P = .93).1
Although the six-site measurement appears to be good for predicting coronary events and evaluating therapies, the measurement in the common carotid arteries appears to be a more reliable surrogate end point for predicting both benefit and harm from antiatherogenic agents. However, trials of statins and other lipid-lowering therapies that assessed clinical events have shown that the reduction in risk associated with a given reduction in cholesterol is similar regardless of the mechanism by which cholesterol is lowered.33 Therefore, the LDL-C level is far superior as a marker of clinical benefit.
Theory 3: Previous statin treatment affected the ENHANCE results
By far the most likely explanation for the neutral findings in ENHANCE is that the patients were so well treated before entry that it was impossible to detect a difference between the two treatment groups in carotid intima-media thickness at the end of the study. Eighty percent of the patients had received statins previously, and at baseline the mean intima-media thickness of the common carotid arteries was only 0.68 mm.1 In contrast, most other trials required a thickness greater than 0.7 mm for entry.
The two main reasons for selecting a population with familial hypercholesterolemia were the assumptions that these participants would have a greater-than-average carotid intima-media thickness at baseline and that they would show an above-average progression rate, even on high-dose statin therapy.4 Both of these assumptions were incorrect: the baseline thickness was normal and the progression rate was negligible in both groups.
Accordingly, the high prevalence of statin pretreatment and the near-normal carotid intima-media thickness at baseline may have prevented the 16.5% greater reduction in LDL-C due to ezetimibe from producing a difference in progression over 24 months of treatment. This conclusion is supported by the long-term follow-up results from ASAP, RADIANCE 1, and CAPTIVATE, all of which showed that in patients with familial hypercholesterolemia well treated with statins, progression of carotid intima-media thickness is negligible.30,31
Further supporting this view, in a previous trial by Dr. Kastelein’s group in patients with familial hypercholesterolemia,34 giving simvastatin 80 mg for 2 years decreased the intima-medial thickness by .081 mm (P < .001), compared with 0.0058 mm in ENHANCE (a 14-fold difference). In the previous trial, the baseline measurement was 1.07 mm (vs 0.68 mm in ENHANCE), and the extent of the change was significantly associated with the baseline measurement (r = .53, P < .001) but not with the change in LDL-C levels.
This is powerful evidence that, in two similar studies that used the same methodology and the same drug, the thinner arteries in the ENHANCE trial are by far the most likely explanation for the lack of change with the addition of ezetimibe to high-dose simvastatin. The METEOR trial enrolled only patients who had never received statins and whose carotid intima-media was thicker than 1.2 mm. In retrospect, a similar design would have been preferable for ENHANCE.35
LESSONS LEARNED AND CLINICAL IMPLICATIONS
For Merck/Schering-Plough, missed opportunities
Although Dr. Krumholz (the spokesman for the ACC panel discussion) and I disagree on the clinical implications of the ENHANCE trial, we do agree on an important point. Dr. Krumholz posed the question that if the LDL-C-lowering hypothesis was already proven for ezetimibe, why was the ENHANCE trial conducted? After 6 years on the market, the efficacy of ezetimibe on cardiovascular outcomes should already have been established. It should not take this long to determine the clinical outcome benefit for a drug.
Merck/Schering-Plough’s outcome program for ezetimibe was inadequately designed to demonstrate the clinical value of this novel compound. Rather than assuming the LDL-C-lowering hypothesis was already established, they conducted another “lower-is-better” trial with the carotid intima-media thickness as the end point, and they succeeded only in raising doubt about the benefits of ezetimibe rather than showing that dual therapy is at least equivalent to high-dose statin therapy.
A preferable approach would have been to compare the effects of a statin in low doses plus ezetimibe vs high-dose statin monotherapy on either surrogate or hard outcomes. If the low-dose statin/ezetimibe combination, which should lower the LDL-C level as much as high-dose statin monotherapy, could provide similar or better outcomes with fewer side effects, this trial would change our practice.
One had hoped that dual therapy, by reducing both intestinal cholesterol absorption and hepatic synthesis of cholesterol, would improve outcomes by modifying postprandial chylomicron composition or by reducing plant sterol absorption.36 Unfortunately, other outcome trials of ezetimibe/simvastatin will not provide an answer regarding the potential advantages of dual therapy. The SEAS study is comparing the number of clinical events in patients with aortic stenosis who receive ezetimibe/simvastatin or placebo; SHARP is being conducted in patients with chronic kidney disease. Although both groups of patients have high rates of coronary events, these trials will not address whether adding ezetimibe provides additional benefits. In fact, if the results of these trials turn out neutral, as in ENHANCE, then ezetimibe will be blamed for potentially offsetting the benefits of simvastatin, and if the trials show a benefit, the simvastatin component of ezetimibe/simvastatin will be given the credit.
The answer may come in 3 to 4 years with the results of IMPROVE-IT, a study of 18,000 patients with acute coronary syndrome treated with ezetimibe/simvastatin or simvastatin. The simvastatin monotherapy group will have a target LDL-C level of less than 80 mg/dL and the ezetimibe/simvastatin group will have an LDL-C target about 15% less. Although this trial is testing the lower-is-better hypothesis with ezetimibe, if the study does not show a benefit, it may not be because ezetimibe lacks clinical efficacy but rather because the LDL-C effect is curvilinear, and there is minimal further benefit of lowering the LDL-C level past 70 mg/dL. If the results of the IMPROVE-IT trial are negative, it may mean the end of ezetimibe as an LDL-C-lowering drug.
Merck/Schering-Plough has lost valuable time in not demonstrating the benefits of ezetimibe on clinical events. In contrast, consider rosuvastatin, an AstraZeneca product. Rosuvastatin was approved about the same time as ezetimibe/simvastatin, and 6 years later it has already received a label change for the reduction of progression of atherosclerosis, based on positive outcomes in the METEOR trial,35 the ASTEROID intravascular ultrasonography trial,37 and the CORONA trial (an important trial that examined hard clinical end points).38 More importantly, the JUPITER trial was recently stopped early owing to a reduction in cardiovascular deaths. Initially, rosuvastatin received an unfair media portrayal as an unsafe drug. Now, because of its proven benefits in outcome trials, it will receive more widespread consideration for clinical use.
For preventive cardiologists, a painful reminder to focus on LDL-C
For the preventive cardiologist or lipidologist, the ENHANCE trial has been a painful reminder that despite overwhelming evidence, the mantra of “the lower the LDL-C the better” is still not universally accepted. We acknowledge the great benefits of statins, but the lure of “pleiotropic effects” distracts many of us from the necessity of more aggressive LDL-C reduction.
The pleiotropic benefits of statins were first raised as a means of supporting increased clinical use of pravastatin vis-a-vis other, more efficacious statins. It was not until the PROVE-IT study that pravastatin’s pleiotropic effects were found not to translate into a benefit equivalent to that of the more efficacious statin, atorvastatin.39
The success of ezetimibe was its ability to safely and easily lower LDL-C in combination with statins to achieve treatment goals. For many patients, a lower-dose statin and ezetimibe together provide a well-tolerated and efficacious approach to treating hyperlipidemia. The fallout from the ENHANCE trial is that many patients who were well treated or who could be better treated with ezetimibe in combination with a statin will not receive the best tolerated regimen. In fact, preliminary prescription data after the release of the ENHANCE study support our worse fear, ie, that patients at high risk will receive less aggressive LDL-C reduction. Since the ENHANCE data were released, more than 300,000 patients have stopped taking either ezetimibe/simvastatin or ezetimibe, and nearly all have continued on generic simvastatin or on a dose of statin with less overall efficacy.
An example is Senator John McCain, who, according to his recently released medical records, has a Framingham 10-year risk of more than 20% and was on ezetimibe/simvastatin to treat an elevated cholesterol level. After release of the ENHANCE trial, he was switched to generic simvastatin, and his LDL-C increased from 82 mg/dL to 122 mg/dL. He most likely has an LDL-C goal of less than 100 mg/dL according to the ATP III guidelines, and he is therefore no longer at his target.
For physicians in the community, questions from concerned patients
For the physicians who have received hundreds of phone calls and e-mails from concerned patients, the ENHANCE trial results must have been both discouraging and confusing. At present, I think we should remember the following:
- Ezetimibe’s mechanism of action is well understood
- It is safe and well-tolerated
- It still has a role as an add-on to statin therapy (or as monotherapy or combined with other agents in those who cannot tolerate statins) for patients who have not yet achieved their LDL-C target.
For the pharmaceutical industry, enormous challenges
The neutral ENHANCE trial results created an uncomfortable situation for the trial sponsor. A heavily marketed drug failed to achieve its expected result after the study results were delayed for a few months. The pharmaceutical industry ranks 14th out of 17 industries in public trust among the American public, and this study provided an opportunity for its critics to attack what is, in their opinion, an overly marketed drug.
Enormous challenges are on the horizon for the pharmaceutical industry, with a shrinking pipeline of potential new drugs, increasing regulatory hurdles, greater liability risk, political pressure for price controls, enhanced scrutiny of sales practices, and a growing media bias. As a cardiologist and clinical researcher whose father died at age 47 of a myocardial infarction, I am concerned that, unless change occurs, a vibrant pharmaceutical industry with the financial and intellectual capital to find and develop new, more effective treatments will cease to exist.
The Ezetimibe and Simvastatin in Hypercholesterolemia Enhances Atherosclerosis Regression (ENHANCE) trial1 was probably the most widely publicized clinical study of the past decade. How did a 720-patient imaging trial with a neutral result in patients with severe hypercholesterolemia rise to a level warranting massive media attention, a congressional investigation, and a recommendation to curtail the use of a drug widely used to reduce levels of low-density-lipoprotein cholesterol (LDL-C)?
The reaction to the ENHANCE trial reveals more about the political climate and the relationship between the pharmaceutical industry and the American public than it does about the effects of ezetimibe (available combined with simvastatin as Vytorin and by itself as Zetia) on the progression of atherosclerosis.
SOME SELF-DISCLOSURE
Before I discuss the clinical implications of the ENHANCE trial, I must describe both my financial conflicts and intellectual biases. I am a paid consultant, speaker, and researcher on behalf of Merck/Schering-Plough, the sponsor of the ENHANCE trial. I was a principal investigator in the first phase II trial of ezetimibe and have conducted more than 10 clinical trials of either ezetimibe or ezetimibe/simvastatin. I also have been a strong advocate for imaging trials to assist in the clinical development of novel therapeutic agents and to support regulatory approval.
Therefore, I believe that the thickness of the intima and media layers of the carotid arteries is a useful surrogate to evaluate the potential antiatherosclerotic effects of drugs (more on this topic below). Also, I believe that the LDL-C-lowering hypothesis has been proven: ie, that all drugs that lower LDL-C safely, without off-target adverse effects, should reduce cardiovascular events. I support the goal levels of LDL-C and non-high-density-lipoprotein cholesterol set by the National Cholesterol Education Program’s third Adult Treatment Panel (ATP III) guidelines,2,3 which specify LDL-C targets rather than the use of specific drugs. In spite of these conflicts and potential biases, I believe I have always served the best interests of patient care.
HISTORY OF THE ENHANCE TRIAL
The end point defined as the mean of six measurements
The primary end point was the change in the thickness of the intima and media layers of the carotid arteries over a 2-year period, measured by ultrasonography. A composite measure was used: the mean of the thicknesses in the far walls of the right and left common carotid arteries, the right and left carotid bulbs, and the right and left internal carotid arteries. Secondary end points included the change in the mean maximal carotid artery intima-media thickness (ie, the thickest of the six baseline measurements), the proportion of participants who developed new carotid artery plaque (defined arbitrarily as an intima-media thickness > 1.3 mm), and changes in the mean of the intima-media thickness of the six carotid sites plus the common femoral arteries.
The last participant completed the trial in April 2006. Reading of the almost 30,000 scans was not started until the last participant was finished, so that all scans for each participant could be read in a blinded, randomized order by five separate readers. A significant proportion of the images that the protocol called for could not be obtained or analyzed, particularly in the internal carotid artery and the carotid bulb, which are often difficult to visualize. As a result, 17% of the internal carotid or carotid bulb measurements were discarded.
To change the end point post hoc, or not to change the end point?
The sponsor of the trial was concerned about the missing data points and convened a special advisory board to review the blinded data. This group suggested a solution: changing the primary end point from the six-site composite value to the mean value in just the common carotid arteries. They based this suggestion on the greater success rate in measuring the common carotids (97%) than in measuring all six sites (88%), as well as on recent trials that indicated that the common carotid artery measurement correlates better with clinical outcomes (because the internal carotid and the bulb measurements vary more). On November 26, 2007, Merck/Schering-Plough announced the primary end point would be changed to the mean change in the common carotid arteries.
However, during a separate meeting on November 30, 2007, some members of the Merck/Schering-Plough advisory board objected to the change. On December 11, 2007, the company announced that the original primary end point would not be changed.
Neutral results, negative publicity
On December 31, 2007, the ENHANCE study was unblinded, and on January 14, 2008, Merck/Schering-Plough issued a press release announcing the results. The press release stated that there were no statistically significant differences between the treatment groups in the primary end point or in any of the secondary end points, despite a 16.5% greater reduction in LDL-C (about 50 mg/dL) in the group receiving the ezetimibe/simvastatin combination. The composite intima-media thickness had increased by an average of 0.0111 mm in the combined-therapy group vs 0.0058 mm in the simvastatin-only group (P = .29) over the 24-month treatment period.5
The press release received unprecedented international media attention. One leading cardiologist commented to the media that ENHANCE showed “millions of patients may be taking a drug [ezetimibe] that does not benefit them, raising their risk of heart attacks and exposing them to potential side effects.”6 The perceived message that ezetimibe/simvastatin is harmful resulted in thousands of phone calls from concerned patients to their physicians throughout the United States. The American Heart Association (AHA) and the American College of Cardiology (ACC) issued a joint statement the next day saying that ezetimibe/simvastatin does not appear to be unsafe and that patients should not stop taking the drug on their own. In the following days, Merck/Schering-Plough placed advertisements in newspapers reaffirming the safety of ezetimibe and quoting the AHA/ACC statement.
But the full results of the study were not available at that point. In fact, Senator Charles Grassley (R-Iowa) had launched a congressional investigation into the delays in releasing the results of the ENHANCE trial in December 2007. A focus of the investigation was whether the sponsor was delaying the release either because the data reflected negatively on its product or because it was legitimately concerned about the quality of the measurements of the carotid intima-media thickness. After Merck/Schering-Plough placed the advertisements quoting the AHA/ACC statement, these organizations were criticized for touting the safety of ezetimibe while receiving educational grants and other funds from Merck/Schering-Plough. Senator Grassley sent a letter to the ACC in late March requesting information about the amount of funds the ACC had received.
Full results are published, and the ACC is misquoted
The ENHANCE study was selected for a special presentation at the ACC annual scientific session on March 30, 2008. The full ENHANCE results were presented by Dr. Kastelein, after which an expert panel led by Harlan M. Krumholz, MD, discussed the trial’s implications. The ENHANCE results were simultaneously published in the New England Journal of Medicine,1 accompanied by an editorial by B. Greg Brown, MD, and Allen J. Taylor, MD,7 and another editorial by the editors of that journal, Jeffrey M. Drazen, MD, and colleagues.8 The expert panel and the editorialists concluded that the ENHANCE trial data raised concerns about the cardiovascular benefits of ezetimibe; that statins should be used as initial therapy for hyperlipidemia and titrated to the goal LDL-C level or to the maximally tolerated dose; and that other drugs such as bile acid sequestrants, fibrates, and niacin should be used in combination with statins before considering ezetimibe.9
The next day, stories appeared in the media mistakenly stating that the ACC had recommended that ezetimibe/simvastatin be discontinued. This view was fueled by an article in the ACC’s Scientific Session News, penned by a contract writer and editor, with the headline, “ACC on Vytorin: Go Back to Statins” that said, “After waiting for 18 months for the results of the ENHANCE study, an ACC panel on Sunday encouraged physicians to use statins as a first line and prescribe Vytorin only as a last resort for patients unable to tolerate other cholesterol-lowering agents.”10
The ACC later clarified that this was the opinion of the panelists and not that of the ACC, and they reiterated statements from the AHA/ACC Secondary Prevention Guidelines11 recommending statins in maximally tolerated doses or titrated to a goal LDL-C level for first-line drug treatment of coronary artery disease, and recommending that patients speak with their physicians before discontinuing any therapy.
WHY WERE THE ENHANCE STUDY RESULTS NEUTRAL?
The ACC expert panel concluded that the most likely reason for the neutral ENHANCE results was that ezetimibe lowers LDL-C but does not confer a cardiovascular benefit. In the words of Dr. Krumholz (as quoted by Shannon Pettypiece and Michelle Fay Cortez on bloomberg.com), ezetimibe is “just an expensive placebo.”12
There are at least three potential explanations for the lack of benefit with ezetimibe in the ENHANCE trial. I list them below in order of lowest to highest probability, in my opinion:
Theory 1: Ezetimibe lowers LDL-C but is not antiatherogenic
Since almost all experts agree that lowering LDL-C confers cardiovascular benefits, if ezetimibe does not inhibit atherosclerosis it must have some “off-target” effect that negates its LDL-C-lowering benefit. Critics of ezetimibe point out that oral estrogen and torcetrapib also lower LDL-C but do not improve cardiovascular outcomes.13,14
The lack of benefit with these two other agents can be explained. Oral estrogen does not lower apolipoprotein B (an indication of the number of atherogenic particles), but rather it increases the levels of both triglycerides and C-reactive protein, and it is prothrombotic in some people.15 Torcetrapib increases aldosterone production and substantially raises blood pressure.16 Therefore, both drugs have true off-target effects that could explain their failure to reduce cardiovascular risk despite reductions in LDL-C. (Interestingly, though, oral estrogen has been shown to slow the progression of carotid intima-media thickness in newly postmenopausal women.17
Ezetimibe, however, lowers LDL-C by an ultimate mechanism similar to that of statins and bile acid sequestrants, ie, by up-regulating LDL receptors, although these drugs reach this mechanism via different pathways. Statins inhibit cholesterol synthesis, thereby lowering hepatic intracellular cholesterol and thus up-regulating LDL-receptors and enhancing LDL-C clearance from the plasma. Bile acid sequestrants interrupt bile acid reabsorption in the ileum, thereby decreasing intracellular hepatic cholesterol and up-regulating LDL receptors. Ezetimibe, like bile acid sequestrants, also decreases cholesterol return to the liver, lowering hepatic intracellular levels and thus up-regulating LDL receptors.18
Ezetimibe is unlikely to have an off-target effect because it is only fractionally absorbed systemically, and a recent animal study showed that it enhances macrophage efflux of cholesterol, thereby potentially increasing reverse cholesterol transport.19 Ezetimibe has also been shown to reduce atherosclerosis in animal models.20
In their editorial, Drs. Brown and Taylor7 noted that ezetimibe reduces the expression of adenosine triphosphate binding cassette A1 (ABCA1) in Caco-2 (an intestinal cell line), and this may be an example of an off-target effect. However, statins also reduce ABCA1 expression in macrophages.21 ABCA1 is sensitive to intracellular cholesterol, and when cholesterol levels are decreased, whether by statins or by ezetimibe, ABCA1 expression is down-regulated.22
Theory 2: Intima-media thickness does not reflect the true benefits of lowering LDL-C
The carotid intima-media thickness is a surrogate end point that predicts coronary events and the rate of progression of coronary atherosclerosis.23 In trials of lovastatin (Mevacor),24 pravastatin (Pravachol),25 and rosuvastatin (Crestor),26 the carotid intima-media was thinner at 24 months with the active drug than with placebo. In two relatively small trials—ARBITER 1 (n = 161),27 which was open-label, and ASAP (n = 325)28,29—aggressive lipid-lowering reduced the progression of intima-media thickness better than less-aggressive therapy. However, this measure has been used to evaluate the effects of differing degrees of LDL-C reduction between active treatments in fewer than 500 research participants.
Furthermore, what part or parts of the carotid system are we talking about? In recent trials led by Dr. Kastelein, the intima-media thickness of the common carotid arteries increased with pactimibe (an acyl-coenzyme A:cholesterol O-acyltransferase, or ACAT, inhibitor)30 and torcetrapib,31 but the six-site composite measure (which was the primary end point in these trials, as in ENHANCE) did not increase more than in the control groups. Pactimibe was also shown to increase atheroma volume as measured by intravascular ultrasonography in the ACTIVATE trial.32 Therefore, the thickness of the common carotid arteries has been shown to be a better predictor of harm from a therapy than the composite measurement.
The advantage of measuring the common carotid artery is that it is easier to visualize and measure, and therefore the measurements vary less. In the METEOR trial,26 the six-site measurement increased significantly less with rosuvastatin than with placebo, but the common carotid measurement alone was more strongly associated with a difference in progression. In the ENHANCE trial, the thickness of the common carotid arteries increased by 0.0024 mm with simvastatin alone vs 0.0019 mm with simvastatin/ezetimibe, a difference of 0.005 mm that was not statistically significant (P = .93).1
Although the six-site measurement appears to be good for predicting coronary events and evaluating therapies, the measurement in the common carotid arteries appears to be a more reliable surrogate end point for predicting both benefit and harm from antiatherogenic agents. However, trials of statins and other lipid-lowering therapies that assessed clinical events have shown that the reduction in risk associated with a given reduction in cholesterol is similar regardless of the mechanism by which cholesterol is lowered.33 Therefore, the LDL-C level is far superior as a marker of clinical benefit.
Theory 3: Previous statin treatment affected the ENHANCE results
By far the most likely explanation for the neutral findings in ENHANCE is that the patients were so well treated before entry that it was impossible to detect a difference between the two treatment groups in carotid intima-media thickness at the end of the study. Eighty percent of the patients had received statins previously, and at baseline the mean intima-media thickness of the common carotid arteries was only 0.68 mm.1 In contrast, most other trials required a thickness greater than 0.7 mm for entry.
The two main reasons for selecting a population with familial hypercholesterolemia were the assumptions that these participants would have a greater-than-average carotid intima-media thickness at baseline and that they would show an above-average progression rate, even on high-dose statin therapy.4 Both of these assumptions were incorrect: the baseline thickness was normal and the progression rate was negligible in both groups.
Accordingly, the high prevalence of statin pretreatment and the near-normal carotid intima-media thickness at baseline may have prevented the 16.5% greater reduction in LDL-C due to ezetimibe from producing a difference in progression over 24 months of treatment. This conclusion is supported by the long-term follow-up results from ASAP, RADIANCE 1, and CAPTIVATE, all of which showed that in patients with familial hypercholesterolemia well treated with statins, progression of carotid intima-media thickness is negligible.30,31
Further supporting this view, in a previous trial by Dr. Kastelein’s group in patients with familial hypercholesterolemia,34 giving simvastatin 80 mg for 2 years decreased the intima-medial thickness by .081 mm (P < .001), compared with 0.0058 mm in ENHANCE (a 14-fold difference). In the previous trial, the baseline measurement was 1.07 mm (vs 0.68 mm in ENHANCE), and the extent of the change was significantly associated with the baseline measurement (r = .53, P < .001) but not with the change in LDL-C levels.
This is powerful evidence that, in two similar studies that used the same methodology and the same drug, the thinner arteries in the ENHANCE trial are by far the most likely explanation for the lack of change with the addition of ezetimibe to high-dose simvastatin. The METEOR trial enrolled only patients who had never received statins and whose carotid intima-media was thicker than 1.2 mm. In retrospect, a similar design would have been preferable for ENHANCE.35
LESSONS LEARNED AND CLINICAL IMPLICATIONS
For Merck/Schering-Plough, missed opportunities
Although Dr. Krumholz (the spokesman for the ACC panel discussion) and I disagree on the clinical implications of the ENHANCE trial, we do agree on an important point. Dr. Krumholz posed the question that if the LDL-C-lowering hypothesis was already proven for ezetimibe, why was the ENHANCE trial conducted? After 6 years on the market, the efficacy of ezetimibe on cardiovascular outcomes should already have been established. It should not take this long to determine the clinical outcome benefit for a drug.
Merck/Schering-Plough’s outcome program for ezetimibe was inadequately designed to demonstrate the clinical value of this novel compound. Rather than assuming the LDL-C-lowering hypothesis was already established, they conducted another “lower-is-better” trial with the carotid intima-media thickness as the end point, and they succeeded only in raising doubt about the benefits of ezetimibe rather than showing that dual therapy is at least equivalent to high-dose statin therapy.
A preferable approach would have been to compare the effects of a statin in low doses plus ezetimibe vs high-dose statin monotherapy on either surrogate or hard outcomes. If the low-dose statin/ezetimibe combination, which should lower the LDL-C level as much as high-dose statin monotherapy, could provide similar or better outcomes with fewer side effects, this trial would change our practice.
One had hoped that dual therapy, by reducing both intestinal cholesterol absorption and hepatic synthesis of cholesterol, would improve outcomes by modifying postprandial chylomicron composition or by reducing plant sterol absorption.36 Unfortunately, other outcome trials of ezetimibe/simvastatin will not provide an answer regarding the potential advantages of dual therapy. The SEAS study is comparing the number of clinical events in patients with aortic stenosis who receive ezetimibe/simvastatin or placebo; SHARP is being conducted in patients with chronic kidney disease. Although both groups of patients have high rates of coronary events, these trials will not address whether adding ezetimibe provides additional benefits. In fact, if the results of these trials turn out neutral, as in ENHANCE, then ezetimibe will be blamed for potentially offsetting the benefits of simvastatin, and if the trials show a benefit, the simvastatin component of ezetimibe/simvastatin will be given the credit.
The answer may come in 3 to 4 years with the results of IMPROVE-IT, a study of 18,000 patients with acute coronary syndrome treated with ezetimibe/simvastatin or simvastatin. The simvastatin monotherapy group will have a target LDL-C level of less than 80 mg/dL and the ezetimibe/simvastatin group will have an LDL-C target about 15% less. Although this trial is testing the lower-is-better hypothesis with ezetimibe, if the study does not show a benefit, it may not be because ezetimibe lacks clinical efficacy but rather because the LDL-C effect is curvilinear, and there is minimal further benefit of lowering the LDL-C level past 70 mg/dL. If the results of the IMPROVE-IT trial are negative, it may mean the end of ezetimibe as an LDL-C-lowering drug.
Merck/Schering-Plough has lost valuable time in not demonstrating the benefits of ezetimibe on clinical events. In contrast, consider rosuvastatin, an AstraZeneca product. Rosuvastatin was approved about the same time as ezetimibe/simvastatin, and 6 years later it has already received a label change for the reduction of progression of atherosclerosis, based on positive outcomes in the METEOR trial,35 the ASTEROID intravascular ultrasonography trial,37 and the CORONA trial (an important trial that examined hard clinical end points).38 More importantly, the JUPITER trial was recently stopped early owing to a reduction in cardiovascular deaths. Initially, rosuvastatin received an unfair media portrayal as an unsafe drug. Now, because of its proven benefits in outcome trials, it will receive more widespread consideration for clinical use.
For preventive cardiologists, a painful reminder to focus on LDL-C
For the preventive cardiologist or lipidologist, the ENHANCE trial has been a painful reminder that despite overwhelming evidence, the mantra of “the lower the LDL-C the better” is still not universally accepted. We acknowledge the great benefits of statins, but the lure of “pleiotropic effects” distracts many of us from the necessity of more aggressive LDL-C reduction.
The pleiotropic benefits of statins were first raised as a means of supporting increased clinical use of pravastatin vis-a-vis other, more efficacious statins. It was not until the PROVE-IT study that pravastatin’s pleiotropic effects were found not to translate into a benefit equivalent to that of the more efficacious statin, atorvastatin.39
The success of ezetimibe was its ability to safely and easily lower LDL-C in combination with statins to achieve treatment goals. For many patients, a lower-dose statin and ezetimibe together provide a well-tolerated and efficacious approach to treating hyperlipidemia. The fallout from the ENHANCE trial is that many patients who were well treated or who could be better treated with ezetimibe in combination with a statin will not receive the best tolerated regimen. In fact, preliminary prescription data after the release of the ENHANCE study support our worse fear, ie, that patients at high risk will receive less aggressive LDL-C reduction. Since the ENHANCE data were released, more than 300,000 patients have stopped taking either ezetimibe/simvastatin or ezetimibe, and nearly all have continued on generic simvastatin or on a dose of statin with less overall efficacy.
An example is Senator John McCain, who, according to his recently released medical records, has a Framingham 10-year risk of more than 20% and was on ezetimibe/simvastatin to treat an elevated cholesterol level. After release of the ENHANCE trial, he was switched to generic simvastatin, and his LDL-C increased from 82 mg/dL to 122 mg/dL. He most likely has an LDL-C goal of less than 100 mg/dL according to the ATP III guidelines, and he is therefore no longer at his target.
For physicians in the community, questions from concerned patients
For the physicians who have received hundreds of phone calls and e-mails from concerned patients, the ENHANCE trial results must have been both discouraging and confusing. At present, I think we should remember the following:
- Ezetimibe’s mechanism of action is well understood
- It is safe and well-tolerated
- It still has a role as an add-on to statin therapy (or as monotherapy or combined with other agents in those who cannot tolerate statins) for patients who have not yet achieved their LDL-C target.
For the pharmaceutical industry, enormous challenges
The neutral ENHANCE trial results created an uncomfortable situation for the trial sponsor. A heavily marketed drug failed to achieve its expected result after the study results were delayed for a few months. The pharmaceutical industry ranks 14th out of 17 industries in public trust among the American public, and this study provided an opportunity for its critics to attack what is, in their opinion, an overly marketed drug.
Enormous challenges are on the horizon for the pharmaceutical industry, with a shrinking pipeline of potential new drugs, increasing regulatory hurdles, greater liability risk, political pressure for price controls, enhanced scrutiny of sales practices, and a growing media bias. As a cardiologist and clinical researcher whose father died at age 47 of a myocardial infarction, I am concerned that, unless change occurs, a vibrant pharmaceutical industry with the financial and intellectual capital to find and develop new, more effective treatments will cease to exist.
- Kastelein JJ, Akdim F, Stroes ES, et al ENHANCE Investigators. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med 2008; 358:1431–1443.
- Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106:3143–3421.
- Grundy SM, Cleeman JI, Bairey Merz N, et al for the Coordinating Committee of the National Cholesterol Education Program. Circulation 2004; 110:227–239.
- Kastelein JJ, Sager PT, de Groot E, Veltri E. Comparison of ezetimibe plus simvastatin versus simvastatin monotherapy on atherosclerosis progression in familial hypercholesterolemia. Design and rationale of the Ezetimibe and Simvastatin in Hypercholesterolemia Enhances Atherosclerosis Regression (ENHANCE) trial. Am Heart J 2005; 49:234–239.
- Merck/Schering-Plough Pharmaceutical Press Release, January 14, 2008.
- Berenson A. Study reveals doubt on drug for cholesterol. New York Times January 15, 2008.
- Brown BG, Taylor AJ. Does ENHANCE diminish confidence in lowering LDL or in ezetimibe? N Engl J Med 2008; 358:1504–1507.
- Drazen JM, Jarcho JA, Morrissey S, Curfman GD. Cholesterol lowering and ezetimibe. N Engl J Med 2008; 358:1507–1508.
- American College of Cardiology. ENHANCED analysis of ezetimibe. ACC News, April 2, 2008. www.acc.org/emails/myacc/accnews%5Fapril%5F02%5F08.htm. Accessed 6/2/2008.
- American College of Cardiology. ACC panel on Vytorin: Go back to statins. Scientific Session News 3/31/2008. http://www.acc08.acc.org/SSN/Documents/ACC%20Monday%20v2.pdf. Accessed 6/2/2008.
- Smith SC, Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update. Endorsed by the National Heart, Lung, and Blood Institute. J Am Coll Cardiol 2006; 47:2130–2139.
- Pettypiece S, Cortez MF. Merck, Schering plunge as doctors discourage Vytorin. www.bloomberg.com/apps/news?pid=20601103&refer=news&sid=aV_T9WirgAkI. Accessed 6/2/2008.
- Barter PJ, Caulfield M, Eriksson M, et al ILLUMINATE Investigators. . Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 2007; 357:2109–2122.
- Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in post-menopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA 1998; 280:605–613.
- Rader DJ. Illuminating HDL—is it still a viable therapeutic target? N Engl J Med 2007; 357:2180–2183.
- Davidson MH, Maki KC, Marx P, et al. Effects of continuous estrogen and estrogen-progestin replacement regimens on cardiovascular risk markers in postmenopausal women. Arch Intern Med 2000; 160:3315–3325.
- Hodis HN, Mack WJ, Lobo RA, et al Estrogen in the Prevention of Atherosclerosis Trial Research Group. . Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 2001; 135:939–953.
- Turley SD. Cholesterol metabolism and therapeutic targets: rationale for targeting multiple metabolic pathways. Clin Cardiol 2004; 27( suppl 3):III16–III21.
- Sehayek E, Hazen SL. Cholesterol absorption from the intestine is a major determinant of reverse cholesterol transport from peripheral tissue macrophages. Arterioscler Thromb Vasc Biol 2008;27 (Epub ahead of print].
- Davis HR, Compton DS, Hoos L, Tetzloff G. Ezetimibe, a potent cholesterol absorption inhibitor, inhibits the development of atherosclerosis in ApoE knockout mice. Arterioscler Thromb Vasc Biol 2001; 21:2032–2038.
- Wong J, Quinn CM, Gelissen IC, Jessup W, Brown AJ. The effect of statins on ABCA1 and ABCG1 expression in human macrophages is influenced by cellular cholesterol levels and extent of differentiation. Atherosclerosis 2008; 196:180–189.
- Wang N, Tall AR. Regulation and mechanisms of ATP-binding cassette transporter A1-mediated cellular cholesterol efflux. Arterioscler Thromb Vasc Biol 2003; 23:1178–1184.
- Bots ML. Carotid intima-media thickness as a surrogate marker for cardiovascular disease in intervention studies. Curr Med Res Opin 2006; 22:2181–2190.
- Byington RP, Evans GW, Espeland MA, et al. Effects of lovastatin and warfarin on early carotid atherosclerosis: sex-specific analyses. Asymptomatic Carotid Artery Progression Study (ACAPS) Research Group. Circulation 1999; 100:e14–e17.
- Byington RP, Furberg CD, Crouse JR, Espeland MA, Bond MG. Pravastatin, Lipids, and Atherosclerosis in the Carotid Arteries (PLAC-II). Am J Cardiol 1995; 76:54C–59C.
- Crouse JR, Raichlen JS, Riley WA, et al METEOR Study Group. . Effect of rosuvastatin on progression of carotid intima-media thickness in low-risk individuals with subclinical atherosclerosis: the METEOR trial. JAMA 2007; 297:1344–1353.
- Taylor AJ, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation 2004; 110:3512–3517.
- Smilde TJ, van Wissen S, Wollersheim H, Trip MD, Kastelein JJ, Stalenhoef AF. Effect of aggressive versus conventional lipid lowering on atherosclerosis progression in familial hypercholesterolaemia (ASAP): a prospective, randomised, double-blind trial. Lancet 2001; 357:577–581.
- van Wissen S, Smilde TJ, Trip MD, Stalenhoef AFH, Kastelein JJP. Long-term safety and efficacy of high-dose atorvastatin treatment in patients with familial hypercholesterolemia. Am J Cardiol 2005; 95:264–266.
- Meuwese MC, Franssen R, Stroes ES, Kastelein JJ. And then there were acyl coenzyme A:cholesterol acyl transferase inhibitors. Curr Opin Lipidol 2006; 17:426–430.
- Kastelein JJ, van Leuven SI, Burgess L, et al RADIANCE 1 Investigators. . Effect of torcetrapib on carotid atherosclerosis in familial hypercholesterolemia. N Engl J Med 2007; 356:1620–1630.
- Nissen SE, Tuzcu EM, Brewer HB, et al ACAT Intravascular Atherosclerosis Treatment Evaluation (ACTIVATE) Investigators. Effect of ACAT inhibition on the progression of coronary atherosclerosis. N Engl J Med 2006; 354:1253–1263.
- Davidson MH. Clinical significance of statin pleiotropic effects: hypotheses versus evidence. Circulation 2005; 111:2280–2281.
- Nolting PR, de Groot E, Zwinderman AH, Buirma RJ, Trip MD, Kastelein JJ. Regression of carotid and femoral artery intima-media thickness in familial hypercholesterolemia. Arch Intern Med 2003; 163:1837–1841.
- Crouse JR, Grobbee DE, O’Leary DH, et al Measuring Effects on intima media Thickness: an Evaluation Of Rosuvastatin Study Group. . Measuring effects on intima media thickness: an evaluation of rosuvastatin in subclinical atherosclerosis—the rationale and methodology of the METEOR study. Cardiovasc Drugs Ther 2004; 18:231–238.
- Toth PP, Davidson MH. Cholesterol absorption blockade with ezetimibe. Curr Drug Targets Cardiovasc Haematol Disord 2005; 5:455–462.
- Nissen SE, Nicholls SJ, Sipahi I, et al ASTEROID Investigators. . Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA 2006; 295:1556–1565.
- Kjekshus J, Apetrei E, Barrios V, et al CORONA Group. . Rosuvastatin in older patients with systolic heart failure. N Engl J Med 2007; 357:2248–2261.
- Cannon CP, Braunwald E, McCabe CH, et al Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. . Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350:1495–1504.
- Kastelein JJ, Akdim F, Stroes ES, et al ENHANCE Investigators. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med 2008; 358:1431–1443.
- Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106:3143–3421.
- Grundy SM, Cleeman JI, Bairey Merz N, et al for the Coordinating Committee of the National Cholesterol Education Program. Circulation 2004; 110:227–239.
- Kastelein JJ, Sager PT, de Groot E, Veltri E. Comparison of ezetimibe plus simvastatin versus simvastatin monotherapy on atherosclerosis progression in familial hypercholesterolemia. Design and rationale of the Ezetimibe and Simvastatin in Hypercholesterolemia Enhances Atherosclerosis Regression (ENHANCE) trial. Am Heart J 2005; 49:234–239.
- Merck/Schering-Plough Pharmaceutical Press Release, January 14, 2008.
- Berenson A. Study reveals doubt on drug for cholesterol. New York Times January 15, 2008.
- Brown BG, Taylor AJ. Does ENHANCE diminish confidence in lowering LDL or in ezetimibe? N Engl J Med 2008; 358:1504–1507.
- Drazen JM, Jarcho JA, Morrissey S, Curfman GD. Cholesterol lowering and ezetimibe. N Engl J Med 2008; 358:1507–1508.
- American College of Cardiology. ENHANCED analysis of ezetimibe. ACC News, April 2, 2008. www.acc.org/emails/myacc/accnews%5Fapril%5F02%5F08.htm. Accessed 6/2/2008.
- American College of Cardiology. ACC panel on Vytorin: Go back to statins. Scientific Session News 3/31/2008. http://www.acc08.acc.org/SSN/Documents/ACC%20Monday%20v2.pdf. Accessed 6/2/2008.
- Smith SC, Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update. Endorsed by the National Heart, Lung, and Blood Institute. J Am Coll Cardiol 2006; 47:2130–2139.
- Pettypiece S, Cortez MF. Merck, Schering plunge as doctors discourage Vytorin. www.bloomberg.com/apps/news?pid=20601103&refer=news&sid=aV_T9WirgAkI. Accessed 6/2/2008.
- Barter PJ, Caulfield M, Eriksson M, et al ILLUMINATE Investigators. . Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 2007; 357:2109–2122.
- Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in post-menopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA 1998; 280:605–613.
- Rader DJ. Illuminating HDL—is it still a viable therapeutic target? N Engl J Med 2007; 357:2180–2183.
- Davidson MH, Maki KC, Marx P, et al. Effects of continuous estrogen and estrogen-progestin replacement regimens on cardiovascular risk markers in postmenopausal women. Arch Intern Med 2000; 160:3315–3325.
- Hodis HN, Mack WJ, Lobo RA, et al Estrogen in the Prevention of Atherosclerosis Trial Research Group. . Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 2001; 135:939–953.
- Turley SD. Cholesterol metabolism and therapeutic targets: rationale for targeting multiple metabolic pathways. Clin Cardiol 2004; 27( suppl 3):III16–III21.
- Sehayek E, Hazen SL. Cholesterol absorption from the intestine is a major determinant of reverse cholesterol transport from peripheral tissue macrophages. Arterioscler Thromb Vasc Biol 2008;27 (Epub ahead of print].
- Davis HR, Compton DS, Hoos L, Tetzloff G. Ezetimibe, a potent cholesterol absorption inhibitor, inhibits the development of atherosclerosis in ApoE knockout mice. Arterioscler Thromb Vasc Biol 2001; 21:2032–2038.
- Wong J, Quinn CM, Gelissen IC, Jessup W, Brown AJ. The effect of statins on ABCA1 and ABCG1 expression in human macrophages is influenced by cellular cholesterol levels and extent of differentiation. Atherosclerosis 2008; 196:180–189.
- Wang N, Tall AR. Regulation and mechanisms of ATP-binding cassette transporter A1-mediated cellular cholesterol efflux. Arterioscler Thromb Vasc Biol 2003; 23:1178–1184.
- Bots ML. Carotid intima-media thickness as a surrogate marker for cardiovascular disease in intervention studies. Curr Med Res Opin 2006; 22:2181–2190.
- Byington RP, Evans GW, Espeland MA, et al. Effects of lovastatin and warfarin on early carotid atherosclerosis: sex-specific analyses. Asymptomatic Carotid Artery Progression Study (ACAPS) Research Group. Circulation 1999; 100:e14–e17.
- Byington RP, Furberg CD, Crouse JR, Espeland MA, Bond MG. Pravastatin, Lipids, and Atherosclerosis in the Carotid Arteries (PLAC-II). Am J Cardiol 1995; 76:54C–59C.
- Crouse JR, Raichlen JS, Riley WA, et al METEOR Study Group. . Effect of rosuvastatin on progression of carotid intima-media thickness in low-risk individuals with subclinical atherosclerosis: the METEOR trial. JAMA 2007; 297:1344–1353.
- Taylor AJ, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation 2004; 110:3512–3517.
- Smilde TJ, van Wissen S, Wollersheim H, Trip MD, Kastelein JJ, Stalenhoef AF. Effect of aggressive versus conventional lipid lowering on atherosclerosis progression in familial hypercholesterolaemia (ASAP): a prospective, randomised, double-blind trial. Lancet 2001; 357:577–581.
- van Wissen S, Smilde TJ, Trip MD, Stalenhoef AFH, Kastelein JJP. Long-term safety and efficacy of high-dose atorvastatin treatment in patients with familial hypercholesterolemia. Am J Cardiol 2005; 95:264–266.
- Meuwese MC, Franssen R, Stroes ES, Kastelein JJ. And then there were acyl coenzyme A:cholesterol acyl transferase inhibitors. Curr Opin Lipidol 2006; 17:426–430.
- Kastelein JJ, van Leuven SI, Burgess L, et al RADIANCE 1 Investigators. . Effect of torcetrapib on carotid atherosclerosis in familial hypercholesterolemia. N Engl J Med 2007; 356:1620–1630.
- Nissen SE, Tuzcu EM, Brewer HB, et al ACAT Intravascular Atherosclerosis Treatment Evaluation (ACTIVATE) Investigators. Effect of ACAT inhibition on the progression of coronary atherosclerosis. N Engl J Med 2006; 354:1253–1263.
- Davidson MH. Clinical significance of statin pleiotropic effects: hypotheses versus evidence. Circulation 2005; 111:2280–2281.
- Nolting PR, de Groot E, Zwinderman AH, Buirma RJ, Trip MD, Kastelein JJ. Regression of carotid and femoral artery intima-media thickness in familial hypercholesterolemia. Arch Intern Med 2003; 163:1837–1841.
- Crouse JR, Grobbee DE, O’Leary DH, et al Measuring Effects on intima media Thickness: an Evaluation Of Rosuvastatin Study Group. . Measuring effects on intima media thickness: an evaluation of rosuvastatin in subclinical atherosclerosis—the rationale and methodology of the METEOR study. Cardiovasc Drugs Ther 2004; 18:231–238.
- Toth PP, Davidson MH. Cholesterol absorption blockade with ezetimibe. Curr Drug Targets Cardiovasc Haematol Disord 2005; 5:455–462.
- Nissen SE, Nicholls SJ, Sipahi I, et al ASTEROID Investigators. . Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA 2006; 295:1556–1565.
- Kjekshus J, Apetrei E, Barrios V, et al CORONA Group. . Rosuvastatin in older patients with systolic heart failure. N Engl J Med 2007; 357:2248–2261.
- Cannon CP, Braunwald E, McCabe CH, et al Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. . Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350:1495–1504.
The exercise treadmill test: Estimating cardiovascular prognosis
- Patient B is more likely than patient A to develop coronary artery disease.
- Patient B has a worse cardiovascular prognosis than patient A.
- Patient A’s exercise ECG results are falsely positive, whereas patient B’s results are truly positive.
- On the basis of their blood pressures during exercise, patient A has a higher risk of stroke than patient B.
EXERCISE TESTING FOR DIAGNOSIS AND PROGNOSIS
When we perform a stress test such as the treadmill test, we are asking two questions: does the patient have coronary artery disease (ie, what is the patient’s diagnosis) and is he or she likely to die or suffer a coronary event soon (ie, what is the patient’s prognosis).1,2
A stress test used diagnostically is considered to have a positive result if the patient develops signs and symptoms of ischemia during stress, ie, ST-segment depression and angina.1 The diagnostic accuracy of exercise testing is commonly assessed separately from its prognostic accuracy. Unfortunately, diagnostic accuracy can be assessed only in the minority of patients who subsequently undergo coronary angiography—the gold standard for comparison.
In contrast, the prognostic accuracy of a stress test can be assessed in a much larger group of patients, using clinical outcomes as the comparison standard; only those who undergo early revascularization and those who are lost to follow-up are excluded from this group.
Although the stress-induced markers of ischemia used in diagnosis—ST-segment depression and angina—have prognostic value as well, other variables are more powerful predictors of outcome. In this article I will discuss those other prognostic variables and how to interpret them.
PROGNOSTIC VARIABLES
Variables measured during exercise treadmill testing that predict outcome are actually indicators of general fitness and function of the autonomic nervous system:
- Exercise duration
- Exercise hypotension
- Exercise hypertension
- Chronotropic incompetence
- Heart rate recovery
- Ventricular ectopy.
Exercise duration
In the Bruce protocol used in exercise stress testing, the test begins with the treadmill set to a low speed (1.7 miles per hour) and a 10% incline, and every 3 minutes the speed and angle of incline are increased. Other protocols are similar. The test continues for a maximum of 27 minutes (usually attainable only by well-trained individuals) or until the patient quits or develops signs or symptoms of ischemia or an arrhythmia. Average time for a middle-aged adult is 8 to 10 minutes.
Because the longer the patient goes, the harder he or she must work, exercise duration—the number of minutes the patient can continue in the protocol—is a good measure of his or her functional capacity. Another way to measure functional capacity is to measure oxygen uptake during exercise, which can be converted to metabolic equivalents (METs): 1 MET = 3.5 mL O2/kg/min. However, most laboratories estimate functional capacity from exercise duration in a specific exercise protocol (eg, the Bruce protocol) based on published nomograms.
Remarkably, the longer the patient can keep going on the treadmill, the less likely he or she is to die soon of coronary artery disease—or of any cause. In fact, of the prognostic variables measured during exercise treadmill testing, exercise duration is the strongest.1,2 Its prognostic value has been demonstrated in healthy subjects being screened for coronary artery disease (Figure 1)3–6 and in patients being evaluated for suspected or known coronary artery disease (Figure 2).7–10 The independent prognostic value of exercise duration has been demonstrated in men,3,4,7,8 women,4–7,9 and the elderly.11 Although functional capacity decreases with age and generally is lower in women than men, exercise duration retains its prognostic value after adjusting for age and sex.
Exercise duration is such a good prognostic indicator that it is included in risk scores for exercise treadmill testing.13,14
Blood pressure during and after exercise
During exercise testing, blood pressure is usually measured by cuff sphygmomanometry. However, motion during exercise and background noise from the treadmill machine can reduce the accuracy of this measurement.
Several studies have compared blood pressures measured by cuff sphygmomanometry vs intra-arterial measurements,15 and most have found that systolic pressures are lower as measured by cuff sphygmomanometry, with smaller differences between methods at higher exercise intensity. The diastolic pressure is significantly lower as measured by cuff sphygmomanometry than by intra-arterial measurements at rest and during exercise; error increases with exercise intensity.
Hypotensive and hypertensive blood pressure responses to exercise have been defined in various ways.
Exercise hypotension is best defined as systolic blood pressure that is lower during exercise than while standing at rest before exercise.16 It reflects a failure of cardiac output to increase during exercise and is associated with severe coronary artery disease (eg, left main coronary artery or three-vessel involvement), left ventricular systolic dysfunction, or both.17,18
Dubach et al,16 in a study of 2,036 patients who underwent exercise treadmill testing to evaluate chronic coronary artery disease, found that exercise hypotension was associated with a threefold higher risk of cardiac events over 2 years.
In a large meta-analysis of exercise testing following myocardial infarction, the only independent predictors of risk were limited exercise workload and exercise hypotension.19
Exercise hypertension is defined as a rise in systolic blood pressure during exercise above a threshold, usually between 190 and 220 mm Hg.20 Some studies suggest that exercise hypertension predicts future arterial hypertension in people with normal resting blood pressure.21,22
Whether exercise hypertension predicts future cardiovascular events has not been extensively investigated. A Mayo Clinic study reported that exercise hypertension was significantly associated (P = .03) with cardiovascular events in people without symptoms or clinically evident cardiovascular disease during a mean follow-up of 7.7 years.23 On the other hand, a study from Cleveland Clinic showed that patients being evaluated for coronary artery disease who had a hypertensive response to exercise had a lower prevalence of severe angiographic coronary disease (P = .004) and a lower risk of death over the next 2 years (P = .03) compared with the rest of the study population.24
An abnormal systolic blood pressure recovery ratio, defined as an increase (rather than the expected decrease) in systolic blood pressure in the early postexercise recovery period has been shown to be a marker of underlying coronary artery disease,25 but has not consistently been associated with an adverse prognosis.26
Chronotropic incompetence
The heart rate normally increases with exercise and decreases as soon as exercise stops. Failure of the heart rate to increase as expected during exercise is termed chronotropic incompetence. Chronotropic incompetence predicts all-cause and cardiovascular death.27–30
Different criteria for defining chronotropic incompetence were used in different studies, based on resting heart rate, exercise protocol, patient age, and medications (especially beta-blockers).
The predicted chronotropic response can be calculated by a suggested formula31: (peak heart rate minus resting heart rate) ÷ (220 minus age minus resting heart rate). The difference between peak heart rate and resting heart rate is known as the heart rate reserve.
Chronotropic incompetence is defined as less than 80% of the predicted value and as less than 62% for patients taking beta-blockers.31,32
Heart rate recovery
Several variables influence heart rate recovery, including activity (eg, complete cessation of exercise or cool-down) and position (supine, sitting, standing). Suggested thresholds for abnormal responses are31:
- Upright: the heart rate should slow down by at least 12 beats/minute at 1 minute
- Supine: at least 18 beats/minute at 1 minute
- Sitting: at least 22 beats/minute at 2 minutes.
Heart rate variability
Heart rate variability, ie, differences in the beat-to-beat interval among successive heart cycles, can be quantified by spectral analysis, although this is not routinely available clinically. Dewey et al37 measured heart rate variability during the first and last 2 minutes of exercise and during the first 2 minutes of recovery in 1,335 subjects (95% men, mean age 58 years). Markers of impaired heart rate variability measured during exercise and in recovery were independent predictors of all-cause and cardiovascular death during a mean follow-up of 5 years.
Ventricular ectopy
Uncommon types of ventricular arrhythmias can occur during exercise testing:
- Sustained ventricular tachycardia or ventricular fibrillation due to coronary artery disease or left ventricular dysfunction occurs rarely but is life-threatening.
- Ventricular tachycardia in healthy young adults without structural heart disease may arise from the right ventricular outflow tract. It is benign.38
- Arrhythmogenic right ventricular dysplasia, a cardiomyopathy involving the right ventricle, can also occur in healthy young adults and has a poor prognosis. It must be distinguished from the benign form.
Short ventricular ectopies: Significance uncertain
Single ventricular premature contractions, couplets, or short episodes of nonsustained ventricular tachycardia occur during or soon after exercise treadmill testing more commonly than the sustained ventricular arrhythmias mentioned above. The prognostic significance of these ectopies is controversial. A recent review found that ventricular ectopy during exercise testing or recovery was associated with an increased death rate in 13 out of 22 studies.39 Fifteen of these studies included patient populations with symptomatic or known coronary artery disease; the other 7 studies were in healthy people without symptoms (eg, being screened for employment).
Jouven et al40 found that among 6,101 asymptomatic male French civil servants without clinically evident cardiovascular disease who underwent exercise testing, 2.3% had frequent premature ventricular contractions (defined as > 10% of all ventricular beats) and 4.4% had ECG changes during exercise that indicated ischemia. Having frequent premature ventricular contractions was associated with a higher risk (RR = 2.67) of cardiovascular death over 23 years of follow-up, independent of ischemia (Figure 4).
Frolkis et al41 evaluated 29,244 patients referred to Cleveland Clinic for exercise treadmill testing and found a low prevalence of frequent ventricular ectopy (3% during exercise, 2% after exercise, and 2% both during and after exercise). The 5-year mortality rate was higher in patients with frequent ventricular ectopy during exercise vs those without (9% vs 5%, P < .001) and was even higher in those with frequent ventricular ectopy in recovery vs those without (11% vs 5%, P < .001). After adjusting for confounding variables, only frequent ventricular ectopy in recovery, but not during exercise, was associated with an increased death rate (adjusted hazard ratio 1.5; 95% CI 1.1–1.9; P = .003).
The associations between exercise-induced ventricular ectopy and ischemia and left ventricular function are unclear.
CASE STUDIES REVISITED
As for the two men described at the beginning of this article, patient B has a worse cardiovascular prognosis than patient A.
Both men have the same pretest probability of coronary artery disease (about 50%), based on identical age, sex, and chest pain characteristics. The ST-segment response during exercise—the traditional marker of ischemia used to diagnose coronary disease—is also the same for each patient.
However, hemodynamic variables are markedly different between the two patients: patient B has several adverse prognostic indicators, including lower functional capacity, a hypotensive blood pressure response, and abnormal heart rate recovery.
The most widely used treadmill risk score, the Duke treadmill score,13 can be calculated as:
Exercise time (in minutes, Bruce protocol) minus 5 times the magnitude of ST-segment depression (in millimeters) minus 4 times the treadmill angina index (ie, 0 = no angina, 1 = nonlimiting angina, 2 = angina that is the reason for terminating exercise).
Applying this formula yields a Duke score of 4.5 (estimated annual cardiovascular mortality risk 0.25%) for patient A and a score of –3.5 (estimated annual cardiovascular mortality risk 2%) for patient B.
Because patient A exercised to a high workload, he is more likely to have a false-positive exercise ECG result than patient B. But whether an exercise ECG test is falsely positive or falsely negative can only be determined after coronary angiography.
Exercise hypotension, as seen in patient B, can indicate left ventricular systolic dysfunction with exercise but has not been shown to predict stroke risk.
MANAGEMENT CONSIDERATIONS
How to manage patients with an abnormal hemodynamic response in the absence of ischemia is uncertain. Given the excellent prognosis of patients with well-preserved exercise capacity, it is unlikely that revascularization procedures in these patients would improve outcome.
On the other hand, patients with an abnormal hemodynamic response due to poor general health or autonomic nervous system dysfunction may be able to achieve a better prognosis with interventions that improve some of the abnormal responses. Increased functional capacity through exercise training is associated with a lower mortality rate,42 and coronary artery bypass surgery can abolish exercise-induced hypotension.43
Strategies to further evaluate and treat patients with an isolated finding of chronotropic incompetence, abnormal heart rate recovery, or frequent exercise-induced ventricular ectopy are not clear and require future study.
- Arena R, Myers J, Williams MA, et al American Heart Association Committee on Exercise, Rehabilitation, and Prevention of the Council on Clinical Cardiology; American Heart Association Council on Cardiovascular Nursing. Assessment of functional capacity in clinical and research settings: a scientific statement from the American Heart Association Committee on Exercise, Rehabilitation, and Prevention of the Council on Clinical Cardiology and the Council on Cardiovascular Nursing. Circulation 2007; 116:329–343.
- Gibbons RJ, Balady GJ, Bricker JT, et al American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). Circulation 2002; 106:1883–1892.
- Ekelund LG, Haskell WL, Johnson JL, Whaley FS, Criqui MH, Sheps DS. Physical fitness as a predictor of cardiovascular mortality in asymptomatic North American men. The Lipid Research Clinics Mortality Follow-up Study. N Engl J Med 1988; 319:1379–1384.
- Blair SN, Kohl HW, Paffenbarger RS, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA 1989; 262:2395–2401.
- Mora S, Redberg RF, Cui Y, et al. Ability of exercise testing to predict cardiovascular and all-cause death in asymptomatic women: a 20-year follow-up of the Lipid Research Clinics prevalence study. JAMA 2003; 290:1600–1607.
- Gulati M, Pandey DK, Arnsdorf MF, et al. Exercise capacity and the risk of death in women: the St. James Women Take Heart Project. Circulation 2003; 108:1554–1559.
- Roger VL, Jacobsen SJ, Pellikka PA, Miller TD, Bailey KR, Gersh BJ. Prognostic value of treadmill exercise testing: a population-based study in Olmsted County, Minnesota. Circulation 1998; 98:2836–2841.
- Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 2002; 346:793–801.
- Gulati M, Black HR, Shaw LJ, et al. The prognostic value of a nomogram for exercise capacity in women. N Engl J Med 2005; 353:468–475.
- Snader CE, Marwick TH, Pashkow FJ, Harvey SA, Thomas JD, Lauer MS. Importance of estimated functional capacity as a predictor of all-cause mortality among patients referred for exercise thallium single-photon emission computed tomography: report of 3,400 patients from a single center. J Am Coll Cardiol 1997; 30:641–648.
- Goraya TY, Jacobsen SJ, Pellikka PA, et al. Prognostic value of treadmill exercise testing in elderly persons. Ann Intern Med 2000; 132:862–870.
- Weiner DA, Ryan TJ, McCabe CH, et al. Prognostic importance of a clinical profile and exercise test in medically treated patients with coronary artery disease. J Am Coll Cardiol 1984; 3:772–779.
- Mark DB, Hlatky MA, Harrell FE, Lee KL, Califf RM, Pryor DB. Exercise treadmill score for predicting prognosis in coronary artery disease. Ann Intern Med 1987; 106:793–800.
- Prakash M, Myers J, Froelicher VF, et al. Clinical and exercise test predictors of all-cause mortality: results from > 6,000 consecutive referred male patients. Chest 2001; 120:1003–1013.
- Griffin SE, Robergs RA, Heyward VH. Blood pressure measurement during exercise: a review. Med Sci Sports Exerc 1997; 29:149–159.
- Dubach P, Froelicher VF, Klein J, Oakes D, Grover-McKay M, Friis R. Exercise-induced hypotension in a male population. Criteria, causes, and prognosis. Circulation 1988; 78:1380–1387.
- Hammermeister KE, DeRouen TA, Dodge HT, Zia M. Prognostic and predictive value of exertional hypotension in suspected coronary artery disease. Am J Cardiol 1983; 51:1261–1266.
- Hakki AH, Munley BM, Hadjimiltiades S, Meissner MD, Iskandrian AS. Determinants of abnormal blood pressure response to exercise in coronary artery disease. Am J Cardiol 1986; 57:71–75.
- Froelicher VF, Perdue S, Pewen W, Risch M. Application of meta-analysis using an electronic spread sheet to exercise testing in patients after myocardial infarction. Am J Med 1987; 83:1045–1054.
- Tzemos N, Lim PO, MacDonald TM. Is exercise blood pressure a marker of vascular endothelial function? QJM 2002; 95:423–429.
- Wilson NV, Meyer BM. Early prediction of hypertension using exercise blood pressure. Prev Med 1981; 10:62–68.
- Dlin RA, Hanne N, Silverberg DS, Bar-Or O. Follow-up of normotensive men with exaggerated blood pressure response to exercise. Am Heart J 1983; 106:316–320.
- Allison TG, Cordeiro MA, Miller TD, Daida H, Squires RW, Gau GT. Prognostic significance of exercise-induced systemic hypertension in healthy subjects. Am J Cardiol 1999; 83:371–375.
- Lauer MS, Pashkow FJ, Harvey SA, Marwick TH, Thomas JD. Angiographic and prognostic implications of an exaggerated exercise systolic blood pressure response and rest systolic blood pressure in adults undergoing evaluation for suspected coronary artery disease. J Am Coll Cardiol 1995; 26:1630–1636.
- Amon KW, Richards KL, Crawford MH. Usefulness of the postexercise response of systolic blood pressure in the diagnosis of coronary artery disease. Circulation 1984; 70:951–956.
- Ellis K, Pothier CE, Blackstone EH, Lauer MS. Is systolic blood pressure recovery after exercise a predictor of mortality? Am Heart J 2004; 147:287–292.
- Lauer MS, Okin PM, Larson MG, Evans JC, Levy D. Impaired heart rate response to graded exercise. Prognostic implications of chronotropic incompetence in the Framingham Heart Study. Circulation 1996; 93:1520–1526.
- Lauer MS, Francis GS, Okin PM, Pashkow FJ, Snader CE, Marwick TH. Impaired chronotropic response to exercise stress testing as a predictor of mortality. JAMA 1999; 281:524–529.
- Azarbal B, Hayes SW, Lewin HC, Hachamovitch R, Cohen I, Berman DS. The incremental prognostic value of percentage of heart rate reserve achieved over myocardial perfusion single-photon emission computed tomography in the prediction of cardiac death and all-cause mortality: superiority over 85% of maximal age-predicted heart rate. J Am Coll Cardiol 2004; 44:423–430.
- Myers J, Tan SY, Abella J, Aleti V, Froelicher VF. Comparison of the chronotropic response to exercise and heart rate recovery in predicting cardiovascular mortality. Eur J Cardiovasc Prev Rehab 2007; 14:215–221.
- Kligfield P, Lauer MS. Exercise electrocardiogram testing: beyond the ST segment. Circulation 2006; 114:2070–2082.
- Khan MN, Pothier CE, Lauer MS. Chronotropic incompetence as a predictor of death among patients with normal electrograms taking beta blockers (metoprolol or atenolol). Am J Cardiol 2005; 96:1328–1333.
- Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med 1999; 341:1351–1357.
- Cole CR, Foody JM, Blackstone EH, Lauer MS. Heart rate recovery after submaximal exercise testing as a predictor of mortality in a cardiovascularly healthy cohort. Ann Intern Med 2000; 132:552–555.
- Vivekananthan DP, Blackstone EH, Pothier CE, Lauer MS. Heart rate recovery after exercise is a predictor of mortality, independent of the angiographic severity of coronary disease. J Am Coll Cardiol 2003; 42:831–838.
- Jouven X, Empana JP, Schwartz PJ, Desnos M, Courbon D, Ducimetiere P. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med 2005; 352:1951–1958.
- Dewey FE, Freeman JV, Engel G, et al. Novel predictor of prognosis from exercise stress testing: heart rate variability response to the exercise treadmill test. Am Heart J 2007; 153:281–288.
- Lerman BB, Stein KM, Markowitz SM, Mittal S, Slotwiner DJ. Right ventricular outflow tract tachycardia: an update. Card Electrophysiol Rev 2002; 6:68–71.
- Beckerman J, Wu T, Jones S, Froelicher VF. Exercise test-induced arrhythmias. Prog Cardiovasc Dis 2005; 47:285–305.
- Jouven X, Zureik M, Desnos M, Courbon D, Ducimetiere P. Long-term outcome in asymptomatic men with exercise-induced premature ventricular depolarizations. N Engl J Med 2000; 343:826–833.
- Frolkis JP, Pothier CE, Blackstone EH, Lauer MS. Frequent ventricular ectopy after exercise as a predictor of death. N Engl J Med 2003; 348:781–790.
- Blair SN, Kohl HW, Barlow CE, Paffenbarger RS, Gibbons LW, Macera CA. Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. JAMA 1995; 273:1093–1098.
- Thomson PD, Kelemen MH. Hypotension accompanying the onset of exertional angina. A sign of severe compromise of left ventricular blood supply. Circulation 1975; 52:28–32.
- Patient B is more likely than patient A to develop coronary artery disease.
- Patient B has a worse cardiovascular prognosis than patient A.
- Patient A’s exercise ECG results are falsely positive, whereas patient B’s results are truly positive.
- On the basis of their blood pressures during exercise, patient A has a higher risk of stroke than patient B.
EXERCISE TESTING FOR DIAGNOSIS AND PROGNOSIS
When we perform a stress test such as the treadmill test, we are asking two questions: does the patient have coronary artery disease (ie, what is the patient’s diagnosis) and is he or she likely to die or suffer a coronary event soon (ie, what is the patient’s prognosis).1,2
A stress test used diagnostically is considered to have a positive result if the patient develops signs and symptoms of ischemia during stress, ie, ST-segment depression and angina.1 The diagnostic accuracy of exercise testing is commonly assessed separately from its prognostic accuracy. Unfortunately, diagnostic accuracy can be assessed only in the minority of patients who subsequently undergo coronary angiography—the gold standard for comparison.
In contrast, the prognostic accuracy of a stress test can be assessed in a much larger group of patients, using clinical outcomes as the comparison standard; only those who undergo early revascularization and those who are lost to follow-up are excluded from this group.
Although the stress-induced markers of ischemia used in diagnosis—ST-segment depression and angina—have prognostic value as well, other variables are more powerful predictors of outcome. In this article I will discuss those other prognostic variables and how to interpret them.
PROGNOSTIC VARIABLES
Variables measured during exercise treadmill testing that predict outcome are actually indicators of general fitness and function of the autonomic nervous system:
- Exercise duration
- Exercise hypotension
- Exercise hypertension
- Chronotropic incompetence
- Heart rate recovery
- Ventricular ectopy.
Exercise duration
In the Bruce protocol used in exercise stress testing, the test begins with the treadmill set to a low speed (1.7 miles per hour) and a 10% incline, and every 3 minutes the speed and angle of incline are increased. Other protocols are similar. The test continues for a maximum of 27 minutes (usually attainable only by well-trained individuals) or until the patient quits or develops signs or symptoms of ischemia or an arrhythmia. Average time for a middle-aged adult is 8 to 10 minutes.
Because the longer the patient goes, the harder he or she must work, exercise duration—the number of minutes the patient can continue in the protocol—is a good measure of his or her functional capacity. Another way to measure functional capacity is to measure oxygen uptake during exercise, which can be converted to metabolic equivalents (METs): 1 MET = 3.5 mL O2/kg/min. However, most laboratories estimate functional capacity from exercise duration in a specific exercise protocol (eg, the Bruce protocol) based on published nomograms.
Remarkably, the longer the patient can keep going on the treadmill, the less likely he or she is to die soon of coronary artery disease—or of any cause. In fact, of the prognostic variables measured during exercise treadmill testing, exercise duration is the strongest.1,2 Its prognostic value has been demonstrated in healthy subjects being screened for coronary artery disease (Figure 1)3–6 and in patients being evaluated for suspected or known coronary artery disease (Figure 2).7–10 The independent prognostic value of exercise duration has been demonstrated in men,3,4,7,8 women,4–7,9 and the elderly.11 Although functional capacity decreases with age and generally is lower in women than men, exercise duration retains its prognostic value after adjusting for age and sex.
Exercise duration is such a good prognostic indicator that it is included in risk scores for exercise treadmill testing.13,14
Blood pressure during and after exercise
During exercise testing, blood pressure is usually measured by cuff sphygmomanometry. However, motion during exercise and background noise from the treadmill machine can reduce the accuracy of this measurement.
Several studies have compared blood pressures measured by cuff sphygmomanometry vs intra-arterial measurements,15 and most have found that systolic pressures are lower as measured by cuff sphygmomanometry, with smaller differences between methods at higher exercise intensity. The diastolic pressure is significantly lower as measured by cuff sphygmomanometry than by intra-arterial measurements at rest and during exercise; error increases with exercise intensity.
Hypotensive and hypertensive blood pressure responses to exercise have been defined in various ways.
Exercise hypotension is best defined as systolic blood pressure that is lower during exercise than while standing at rest before exercise.16 It reflects a failure of cardiac output to increase during exercise and is associated with severe coronary artery disease (eg, left main coronary artery or three-vessel involvement), left ventricular systolic dysfunction, or both.17,18
Dubach et al,16 in a study of 2,036 patients who underwent exercise treadmill testing to evaluate chronic coronary artery disease, found that exercise hypotension was associated with a threefold higher risk of cardiac events over 2 years.
In a large meta-analysis of exercise testing following myocardial infarction, the only independent predictors of risk were limited exercise workload and exercise hypotension.19
Exercise hypertension is defined as a rise in systolic blood pressure during exercise above a threshold, usually between 190 and 220 mm Hg.20 Some studies suggest that exercise hypertension predicts future arterial hypertension in people with normal resting blood pressure.21,22
Whether exercise hypertension predicts future cardiovascular events has not been extensively investigated. A Mayo Clinic study reported that exercise hypertension was significantly associated (P = .03) with cardiovascular events in people without symptoms or clinically evident cardiovascular disease during a mean follow-up of 7.7 years.23 On the other hand, a study from Cleveland Clinic showed that patients being evaluated for coronary artery disease who had a hypertensive response to exercise had a lower prevalence of severe angiographic coronary disease (P = .004) and a lower risk of death over the next 2 years (P = .03) compared with the rest of the study population.24
An abnormal systolic blood pressure recovery ratio, defined as an increase (rather than the expected decrease) in systolic blood pressure in the early postexercise recovery period has been shown to be a marker of underlying coronary artery disease,25 but has not consistently been associated with an adverse prognosis.26
Chronotropic incompetence
The heart rate normally increases with exercise and decreases as soon as exercise stops. Failure of the heart rate to increase as expected during exercise is termed chronotropic incompetence. Chronotropic incompetence predicts all-cause and cardiovascular death.27–30
Different criteria for defining chronotropic incompetence were used in different studies, based on resting heart rate, exercise protocol, patient age, and medications (especially beta-blockers).
The predicted chronotropic response can be calculated by a suggested formula31: (peak heart rate minus resting heart rate) ÷ (220 minus age minus resting heart rate). The difference between peak heart rate and resting heart rate is known as the heart rate reserve.
Chronotropic incompetence is defined as less than 80% of the predicted value and as less than 62% for patients taking beta-blockers.31,32
Heart rate recovery
Several variables influence heart rate recovery, including activity (eg, complete cessation of exercise or cool-down) and position (supine, sitting, standing). Suggested thresholds for abnormal responses are31:
- Upright: the heart rate should slow down by at least 12 beats/minute at 1 minute
- Supine: at least 18 beats/minute at 1 minute
- Sitting: at least 22 beats/minute at 2 minutes.
Heart rate variability
Heart rate variability, ie, differences in the beat-to-beat interval among successive heart cycles, can be quantified by spectral analysis, although this is not routinely available clinically. Dewey et al37 measured heart rate variability during the first and last 2 minutes of exercise and during the first 2 minutes of recovery in 1,335 subjects (95% men, mean age 58 years). Markers of impaired heart rate variability measured during exercise and in recovery were independent predictors of all-cause and cardiovascular death during a mean follow-up of 5 years.
Ventricular ectopy
Uncommon types of ventricular arrhythmias can occur during exercise testing:
- Sustained ventricular tachycardia or ventricular fibrillation due to coronary artery disease or left ventricular dysfunction occurs rarely but is life-threatening.
- Ventricular tachycardia in healthy young adults without structural heart disease may arise from the right ventricular outflow tract. It is benign.38
- Arrhythmogenic right ventricular dysplasia, a cardiomyopathy involving the right ventricle, can also occur in healthy young adults and has a poor prognosis. It must be distinguished from the benign form.
Short ventricular ectopies: Significance uncertain
Single ventricular premature contractions, couplets, or short episodes of nonsustained ventricular tachycardia occur during or soon after exercise treadmill testing more commonly than the sustained ventricular arrhythmias mentioned above. The prognostic significance of these ectopies is controversial. A recent review found that ventricular ectopy during exercise testing or recovery was associated with an increased death rate in 13 out of 22 studies.39 Fifteen of these studies included patient populations with symptomatic or known coronary artery disease; the other 7 studies were in healthy people without symptoms (eg, being screened for employment).
Jouven et al40 found that among 6,101 asymptomatic male French civil servants without clinically evident cardiovascular disease who underwent exercise testing, 2.3% had frequent premature ventricular contractions (defined as > 10% of all ventricular beats) and 4.4% had ECG changes during exercise that indicated ischemia. Having frequent premature ventricular contractions was associated with a higher risk (RR = 2.67) of cardiovascular death over 23 years of follow-up, independent of ischemia (Figure 4).
Frolkis et al41 evaluated 29,244 patients referred to Cleveland Clinic for exercise treadmill testing and found a low prevalence of frequent ventricular ectopy (3% during exercise, 2% after exercise, and 2% both during and after exercise). The 5-year mortality rate was higher in patients with frequent ventricular ectopy during exercise vs those without (9% vs 5%, P < .001) and was even higher in those with frequent ventricular ectopy in recovery vs those without (11% vs 5%, P < .001). After adjusting for confounding variables, only frequent ventricular ectopy in recovery, but not during exercise, was associated with an increased death rate (adjusted hazard ratio 1.5; 95% CI 1.1–1.9; P = .003).
The associations between exercise-induced ventricular ectopy and ischemia and left ventricular function are unclear.
CASE STUDIES REVISITED
As for the two men described at the beginning of this article, patient B has a worse cardiovascular prognosis than patient A.
Both men have the same pretest probability of coronary artery disease (about 50%), based on identical age, sex, and chest pain characteristics. The ST-segment response during exercise—the traditional marker of ischemia used to diagnose coronary disease—is also the same for each patient.
However, hemodynamic variables are markedly different between the two patients: patient B has several adverse prognostic indicators, including lower functional capacity, a hypotensive blood pressure response, and abnormal heart rate recovery.
The most widely used treadmill risk score, the Duke treadmill score,13 can be calculated as:
Exercise time (in minutes, Bruce protocol) minus 5 times the magnitude of ST-segment depression (in millimeters) minus 4 times the treadmill angina index (ie, 0 = no angina, 1 = nonlimiting angina, 2 = angina that is the reason for terminating exercise).
Applying this formula yields a Duke score of 4.5 (estimated annual cardiovascular mortality risk 0.25%) for patient A and a score of –3.5 (estimated annual cardiovascular mortality risk 2%) for patient B.
Because patient A exercised to a high workload, he is more likely to have a false-positive exercise ECG result than patient B. But whether an exercise ECG test is falsely positive or falsely negative can only be determined after coronary angiography.
Exercise hypotension, as seen in patient B, can indicate left ventricular systolic dysfunction with exercise but has not been shown to predict stroke risk.
MANAGEMENT CONSIDERATIONS
How to manage patients with an abnormal hemodynamic response in the absence of ischemia is uncertain. Given the excellent prognosis of patients with well-preserved exercise capacity, it is unlikely that revascularization procedures in these patients would improve outcome.
On the other hand, patients with an abnormal hemodynamic response due to poor general health or autonomic nervous system dysfunction may be able to achieve a better prognosis with interventions that improve some of the abnormal responses. Increased functional capacity through exercise training is associated with a lower mortality rate,42 and coronary artery bypass surgery can abolish exercise-induced hypotension.43
Strategies to further evaluate and treat patients with an isolated finding of chronotropic incompetence, abnormal heart rate recovery, or frequent exercise-induced ventricular ectopy are not clear and require future study.
- Patient B is more likely than patient A to develop coronary artery disease.
- Patient B has a worse cardiovascular prognosis than patient A.
- Patient A’s exercise ECG results are falsely positive, whereas patient B’s results are truly positive.
- On the basis of their blood pressures during exercise, patient A has a higher risk of stroke than patient B.
EXERCISE TESTING FOR DIAGNOSIS AND PROGNOSIS
When we perform a stress test such as the treadmill test, we are asking two questions: does the patient have coronary artery disease (ie, what is the patient’s diagnosis) and is he or she likely to die or suffer a coronary event soon (ie, what is the patient’s prognosis).1,2
A stress test used diagnostically is considered to have a positive result if the patient develops signs and symptoms of ischemia during stress, ie, ST-segment depression and angina.1 The diagnostic accuracy of exercise testing is commonly assessed separately from its prognostic accuracy. Unfortunately, diagnostic accuracy can be assessed only in the minority of patients who subsequently undergo coronary angiography—the gold standard for comparison.
In contrast, the prognostic accuracy of a stress test can be assessed in a much larger group of patients, using clinical outcomes as the comparison standard; only those who undergo early revascularization and those who are lost to follow-up are excluded from this group.
Although the stress-induced markers of ischemia used in diagnosis—ST-segment depression and angina—have prognostic value as well, other variables are more powerful predictors of outcome. In this article I will discuss those other prognostic variables and how to interpret them.
PROGNOSTIC VARIABLES
Variables measured during exercise treadmill testing that predict outcome are actually indicators of general fitness and function of the autonomic nervous system:
- Exercise duration
- Exercise hypotension
- Exercise hypertension
- Chronotropic incompetence
- Heart rate recovery
- Ventricular ectopy.
Exercise duration
In the Bruce protocol used in exercise stress testing, the test begins with the treadmill set to a low speed (1.7 miles per hour) and a 10% incline, and every 3 minutes the speed and angle of incline are increased. Other protocols are similar. The test continues for a maximum of 27 minutes (usually attainable only by well-trained individuals) or until the patient quits or develops signs or symptoms of ischemia or an arrhythmia. Average time for a middle-aged adult is 8 to 10 minutes.
Because the longer the patient goes, the harder he or she must work, exercise duration—the number of minutes the patient can continue in the protocol—is a good measure of his or her functional capacity. Another way to measure functional capacity is to measure oxygen uptake during exercise, which can be converted to metabolic equivalents (METs): 1 MET = 3.5 mL O2/kg/min. However, most laboratories estimate functional capacity from exercise duration in a specific exercise protocol (eg, the Bruce protocol) based on published nomograms.
Remarkably, the longer the patient can keep going on the treadmill, the less likely he or she is to die soon of coronary artery disease—or of any cause. In fact, of the prognostic variables measured during exercise treadmill testing, exercise duration is the strongest.1,2 Its prognostic value has been demonstrated in healthy subjects being screened for coronary artery disease (Figure 1)3–6 and in patients being evaluated for suspected or known coronary artery disease (Figure 2).7–10 The independent prognostic value of exercise duration has been demonstrated in men,3,4,7,8 women,4–7,9 and the elderly.11 Although functional capacity decreases with age and generally is lower in women than men, exercise duration retains its prognostic value after adjusting for age and sex.
Exercise duration is such a good prognostic indicator that it is included in risk scores for exercise treadmill testing.13,14
Blood pressure during and after exercise
During exercise testing, blood pressure is usually measured by cuff sphygmomanometry. However, motion during exercise and background noise from the treadmill machine can reduce the accuracy of this measurement.
Several studies have compared blood pressures measured by cuff sphygmomanometry vs intra-arterial measurements,15 and most have found that systolic pressures are lower as measured by cuff sphygmomanometry, with smaller differences between methods at higher exercise intensity. The diastolic pressure is significantly lower as measured by cuff sphygmomanometry than by intra-arterial measurements at rest and during exercise; error increases with exercise intensity.
Hypotensive and hypertensive blood pressure responses to exercise have been defined in various ways.
Exercise hypotension is best defined as systolic blood pressure that is lower during exercise than while standing at rest before exercise.16 It reflects a failure of cardiac output to increase during exercise and is associated with severe coronary artery disease (eg, left main coronary artery or three-vessel involvement), left ventricular systolic dysfunction, or both.17,18
Dubach et al,16 in a study of 2,036 patients who underwent exercise treadmill testing to evaluate chronic coronary artery disease, found that exercise hypotension was associated with a threefold higher risk of cardiac events over 2 years.
In a large meta-analysis of exercise testing following myocardial infarction, the only independent predictors of risk were limited exercise workload and exercise hypotension.19
Exercise hypertension is defined as a rise in systolic blood pressure during exercise above a threshold, usually between 190 and 220 mm Hg.20 Some studies suggest that exercise hypertension predicts future arterial hypertension in people with normal resting blood pressure.21,22
Whether exercise hypertension predicts future cardiovascular events has not been extensively investigated. A Mayo Clinic study reported that exercise hypertension was significantly associated (P = .03) with cardiovascular events in people without symptoms or clinically evident cardiovascular disease during a mean follow-up of 7.7 years.23 On the other hand, a study from Cleveland Clinic showed that patients being evaluated for coronary artery disease who had a hypertensive response to exercise had a lower prevalence of severe angiographic coronary disease (P = .004) and a lower risk of death over the next 2 years (P = .03) compared with the rest of the study population.24
An abnormal systolic blood pressure recovery ratio, defined as an increase (rather than the expected decrease) in systolic blood pressure in the early postexercise recovery period has been shown to be a marker of underlying coronary artery disease,25 but has not consistently been associated with an adverse prognosis.26
Chronotropic incompetence
The heart rate normally increases with exercise and decreases as soon as exercise stops. Failure of the heart rate to increase as expected during exercise is termed chronotropic incompetence. Chronotropic incompetence predicts all-cause and cardiovascular death.27–30
Different criteria for defining chronotropic incompetence were used in different studies, based on resting heart rate, exercise protocol, patient age, and medications (especially beta-blockers).
The predicted chronotropic response can be calculated by a suggested formula31: (peak heart rate minus resting heart rate) ÷ (220 minus age minus resting heart rate). The difference between peak heart rate and resting heart rate is known as the heart rate reserve.
Chronotropic incompetence is defined as less than 80% of the predicted value and as less than 62% for patients taking beta-blockers.31,32
Heart rate recovery
Several variables influence heart rate recovery, including activity (eg, complete cessation of exercise or cool-down) and position (supine, sitting, standing). Suggested thresholds for abnormal responses are31:
- Upright: the heart rate should slow down by at least 12 beats/minute at 1 minute
- Supine: at least 18 beats/minute at 1 minute
- Sitting: at least 22 beats/minute at 2 minutes.
Heart rate variability
Heart rate variability, ie, differences in the beat-to-beat interval among successive heart cycles, can be quantified by spectral analysis, although this is not routinely available clinically. Dewey et al37 measured heart rate variability during the first and last 2 minutes of exercise and during the first 2 minutes of recovery in 1,335 subjects (95% men, mean age 58 years). Markers of impaired heart rate variability measured during exercise and in recovery were independent predictors of all-cause and cardiovascular death during a mean follow-up of 5 years.
Ventricular ectopy
Uncommon types of ventricular arrhythmias can occur during exercise testing:
- Sustained ventricular tachycardia or ventricular fibrillation due to coronary artery disease or left ventricular dysfunction occurs rarely but is life-threatening.
- Ventricular tachycardia in healthy young adults without structural heart disease may arise from the right ventricular outflow tract. It is benign.38
- Arrhythmogenic right ventricular dysplasia, a cardiomyopathy involving the right ventricle, can also occur in healthy young adults and has a poor prognosis. It must be distinguished from the benign form.
Short ventricular ectopies: Significance uncertain
Single ventricular premature contractions, couplets, or short episodes of nonsustained ventricular tachycardia occur during or soon after exercise treadmill testing more commonly than the sustained ventricular arrhythmias mentioned above. The prognostic significance of these ectopies is controversial. A recent review found that ventricular ectopy during exercise testing or recovery was associated with an increased death rate in 13 out of 22 studies.39 Fifteen of these studies included patient populations with symptomatic or known coronary artery disease; the other 7 studies were in healthy people without symptoms (eg, being screened for employment).
Jouven et al40 found that among 6,101 asymptomatic male French civil servants without clinically evident cardiovascular disease who underwent exercise testing, 2.3% had frequent premature ventricular contractions (defined as > 10% of all ventricular beats) and 4.4% had ECG changes during exercise that indicated ischemia. Having frequent premature ventricular contractions was associated with a higher risk (RR = 2.67) of cardiovascular death over 23 years of follow-up, independent of ischemia (Figure 4).
Frolkis et al41 evaluated 29,244 patients referred to Cleveland Clinic for exercise treadmill testing and found a low prevalence of frequent ventricular ectopy (3% during exercise, 2% after exercise, and 2% both during and after exercise). The 5-year mortality rate was higher in patients with frequent ventricular ectopy during exercise vs those without (9% vs 5%, P < .001) and was even higher in those with frequent ventricular ectopy in recovery vs those without (11% vs 5%, P < .001). After adjusting for confounding variables, only frequent ventricular ectopy in recovery, but not during exercise, was associated with an increased death rate (adjusted hazard ratio 1.5; 95% CI 1.1–1.9; P = .003).
The associations between exercise-induced ventricular ectopy and ischemia and left ventricular function are unclear.
CASE STUDIES REVISITED
As for the two men described at the beginning of this article, patient B has a worse cardiovascular prognosis than patient A.
Both men have the same pretest probability of coronary artery disease (about 50%), based on identical age, sex, and chest pain characteristics. The ST-segment response during exercise—the traditional marker of ischemia used to diagnose coronary disease—is also the same for each patient.
However, hemodynamic variables are markedly different between the two patients: patient B has several adverse prognostic indicators, including lower functional capacity, a hypotensive blood pressure response, and abnormal heart rate recovery.
The most widely used treadmill risk score, the Duke treadmill score,13 can be calculated as:
Exercise time (in minutes, Bruce protocol) minus 5 times the magnitude of ST-segment depression (in millimeters) minus 4 times the treadmill angina index (ie, 0 = no angina, 1 = nonlimiting angina, 2 = angina that is the reason for terminating exercise).
Applying this formula yields a Duke score of 4.5 (estimated annual cardiovascular mortality risk 0.25%) for patient A and a score of –3.5 (estimated annual cardiovascular mortality risk 2%) for patient B.
Because patient A exercised to a high workload, he is more likely to have a false-positive exercise ECG result than patient B. But whether an exercise ECG test is falsely positive or falsely negative can only be determined after coronary angiography.
Exercise hypotension, as seen in patient B, can indicate left ventricular systolic dysfunction with exercise but has not been shown to predict stroke risk.
MANAGEMENT CONSIDERATIONS
How to manage patients with an abnormal hemodynamic response in the absence of ischemia is uncertain. Given the excellent prognosis of patients with well-preserved exercise capacity, it is unlikely that revascularization procedures in these patients would improve outcome.
On the other hand, patients with an abnormal hemodynamic response due to poor general health or autonomic nervous system dysfunction may be able to achieve a better prognosis with interventions that improve some of the abnormal responses. Increased functional capacity through exercise training is associated with a lower mortality rate,42 and coronary artery bypass surgery can abolish exercise-induced hypotension.43
Strategies to further evaluate and treat patients with an isolated finding of chronotropic incompetence, abnormal heart rate recovery, or frequent exercise-induced ventricular ectopy are not clear and require future study.
- Arena R, Myers J, Williams MA, et al American Heart Association Committee on Exercise, Rehabilitation, and Prevention of the Council on Clinical Cardiology; American Heart Association Council on Cardiovascular Nursing. Assessment of functional capacity in clinical and research settings: a scientific statement from the American Heart Association Committee on Exercise, Rehabilitation, and Prevention of the Council on Clinical Cardiology and the Council on Cardiovascular Nursing. Circulation 2007; 116:329–343.
- Gibbons RJ, Balady GJ, Bricker JT, et al American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). Circulation 2002; 106:1883–1892.
- Ekelund LG, Haskell WL, Johnson JL, Whaley FS, Criqui MH, Sheps DS. Physical fitness as a predictor of cardiovascular mortality in asymptomatic North American men. The Lipid Research Clinics Mortality Follow-up Study. N Engl J Med 1988; 319:1379–1384.
- Blair SN, Kohl HW, Paffenbarger RS, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA 1989; 262:2395–2401.
- Mora S, Redberg RF, Cui Y, et al. Ability of exercise testing to predict cardiovascular and all-cause death in asymptomatic women: a 20-year follow-up of the Lipid Research Clinics prevalence study. JAMA 2003; 290:1600–1607.
- Gulati M, Pandey DK, Arnsdorf MF, et al. Exercise capacity and the risk of death in women: the St. James Women Take Heart Project. Circulation 2003; 108:1554–1559.
- Roger VL, Jacobsen SJ, Pellikka PA, Miller TD, Bailey KR, Gersh BJ. Prognostic value of treadmill exercise testing: a population-based study in Olmsted County, Minnesota. Circulation 1998; 98:2836–2841.
- Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 2002; 346:793–801.
- Gulati M, Black HR, Shaw LJ, et al. The prognostic value of a nomogram for exercise capacity in women. N Engl J Med 2005; 353:468–475.
- Snader CE, Marwick TH, Pashkow FJ, Harvey SA, Thomas JD, Lauer MS. Importance of estimated functional capacity as a predictor of all-cause mortality among patients referred for exercise thallium single-photon emission computed tomography: report of 3,400 patients from a single center. J Am Coll Cardiol 1997; 30:641–648.
- Goraya TY, Jacobsen SJ, Pellikka PA, et al. Prognostic value of treadmill exercise testing in elderly persons. Ann Intern Med 2000; 132:862–870.
- Weiner DA, Ryan TJ, McCabe CH, et al. Prognostic importance of a clinical profile and exercise test in medically treated patients with coronary artery disease. J Am Coll Cardiol 1984; 3:772–779.
- Mark DB, Hlatky MA, Harrell FE, Lee KL, Califf RM, Pryor DB. Exercise treadmill score for predicting prognosis in coronary artery disease. Ann Intern Med 1987; 106:793–800.
- Prakash M, Myers J, Froelicher VF, et al. Clinical and exercise test predictors of all-cause mortality: results from > 6,000 consecutive referred male patients. Chest 2001; 120:1003–1013.
- Griffin SE, Robergs RA, Heyward VH. Blood pressure measurement during exercise: a review. Med Sci Sports Exerc 1997; 29:149–159.
- Dubach P, Froelicher VF, Klein J, Oakes D, Grover-McKay M, Friis R. Exercise-induced hypotension in a male population. Criteria, causes, and prognosis. Circulation 1988; 78:1380–1387.
- Hammermeister KE, DeRouen TA, Dodge HT, Zia M. Prognostic and predictive value of exertional hypotension in suspected coronary artery disease. Am J Cardiol 1983; 51:1261–1266.
- Hakki AH, Munley BM, Hadjimiltiades S, Meissner MD, Iskandrian AS. Determinants of abnormal blood pressure response to exercise in coronary artery disease. Am J Cardiol 1986; 57:71–75.
- Froelicher VF, Perdue S, Pewen W, Risch M. Application of meta-analysis using an electronic spread sheet to exercise testing in patients after myocardial infarction. Am J Med 1987; 83:1045–1054.
- Tzemos N, Lim PO, MacDonald TM. Is exercise blood pressure a marker of vascular endothelial function? QJM 2002; 95:423–429.
- Wilson NV, Meyer BM. Early prediction of hypertension using exercise blood pressure. Prev Med 1981; 10:62–68.
- Dlin RA, Hanne N, Silverberg DS, Bar-Or O. Follow-up of normotensive men with exaggerated blood pressure response to exercise. Am Heart J 1983; 106:316–320.
- Allison TG, Cordeiro MA, Miller TD, Daida H, Squires RW, Gau GT. Prognostic significance of exercise-induced systemic hypertension in healthy subjects. Am J Cardiol 1999; 83:371–375.
- Lauer MS, Pashkow FJ, Harvey SA, Marwick TH, Thomas JD. Angiographic and prognostic implications of an exaggerated exercise systolic blood pressure response and rest systolic blood pressure in adults undergoing evaluation for suspected coronary artery disease. J Am Coll Cardiol 1995; 26:1630–1636.
- Amon KW, Richards KL, Crawford MH. Usefulness of the postexercise response of systolic blood pressure in the diagnosis of coronary artery disease. Circulation 1984; 70:951–956.
- Ellis K, Pothier CE, Blackstone EH, Lauer MS. Is systolic blood pressure recovery after exercise a predictor of mortality? Am Heart J 2004; 147:287–292.
- Lauer MS, Okin PM, Larson MG, Evans JC, Levy D. Impaired heart rate response to graded exercise. Prognostic implications of chronotropic incompetence in the Framingham Heart Study. Circulation 1996; 93:1520–1526.
- Lauer MS, Francis GS, Okin PM, Pashkow FJ, Snader CE, Marwick TH. Impaired chronotropic response to exercise stress testing as a predictor of mortality. JAMA 1999; 281:524–529.
- Azarbal B, Hayes SW, Lewin HC, Hachamovitch R, Cohen I, Berman DS. The incremental prognostic value of percentage of heart rate reserve achieved over myocardial perfusion single-photon emission computed tomography in the prediction of cardiac death and all-cause mortality: superiority over 85% of maximal age-predicted heart rate. J Am Coll Cardiol 2004; 44:423–430.
- Myers J, Tan SY, Abella J, Aleti V, Froelicher VF. Comparison of the chronotropic response to exercise and heart rate recovery in predicting cardiovascular mortality. Eur J Cardiovasc Prev Rehab 2007; 14:215–221.
- Kligfield P, Lauer MS. Exercise electrocardiogram testing: beyond the ST segment. Circulation 2006; 114:2070–2082.
- Khan MN, Pothier CE, Lauer MS. Chronotropic incompetence as a predictor of death among patients with normal electrograms taking beta blockers (metoprolol or atenolol). Am J Cardiol 2005; 96:1328–1333.
- Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med 1999; 341:1351–1357.
- Cole CR, Foody JM, Blackstone EH, Lauer MS. Heart rate recovery after submaximal exercise testing as a predictor of mortality in a cardiovascularly healthy cohort. Ann Intern Med 2000; 132:552–555.
- Vivekananthan DP, Blackstone EH, Pothier CE, Lauer MS. Heart rate recovery after exercise is a predictor of mortality, independent of the angiographic severity of coronary disease. J Am Coll Cardiol 2003; 42:831–838.
- Jouven X, Empana JP, Schwartz PJ, Desnos M, Courbon D, Ducimetiere P. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med 2005; 352:1951–1958.
- Dewey FE, Freeman JV, Engel G, et al. Novel predictor of prognosis from exercise stress testing: heart rate variability response to the exercise treadmill test. Am Heart J 2007; 153:281–288.
- Lerman BB, Stein KM, Markowitz SM, Mittal S, Slotwiner DJ. Right ventricular outflow tract tachycardia: an update. Card Electrophysiol Rev 2002; 6:68–71.
- Beckerman J, Wu T, Jones S, Froelicher VF. Exercise test-induced arrhythmias. Prog Cardiovasc Dis 2005; 47:285–305.
- Jouven X, Zureik M, Desnos M, Courbon D, Ducimetiere P. Long-term outcome in asymptomatic men with exercise-induced premature ventricular depolarizations. N Engl J Med 2000; 343:826–833.
- Frolkis JP, Pothier CE, Blackstone EH, Lauer MS. Frequent ventricular ectopy after exercise as a predictor of death. N Engl J Med 2003; 348:781–790.
- Blair SN, Kohl HW, Barlow CE, Paffenbarger RS, Gibbons LW, Macera CA. Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. JAMA 1995; 273:1093–1098.
- Thomson PD, Kelemen MH. Hypotension accompanying the onset of exertional angina. A sign of severe compromise of left ventricular blood supply. Circulation 1975; 52:28–32.
- Arena R, Myers J, Williams MA, et al American Heart Association Committee on Exercise, Rehabilitation, and Prevention of the Council on Clinical Cardiology; American Heart Association Council on Cardiovascular Nursing. Assessment of functional capacity in clinical and research settings: a scientific statement from the American Heart Association Committee on Exercise, Rehabilitation, and Prevention of the Council on Clinical Cardiology and the Council on Cardiovascular Nursing. Circulation 2007; 116:329–343.
- Gibbons RJ, Balady GJ, Bricker JT, et al American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). Circulation 2002; 106:1883–1892.
- Ekelund LG, Haskell WL, Johnson JL, Whaley FS, Criqui MH, Sheps DS. Physical fitness as a predictor of cardiovascular mortality in asymptomatic North American men. The Lipid Research Clinics Mortality Follow-up Study. N Engl J Med 1988; 319:1379–1384.
- Blair SN, Kohl HW, Paffenbarger RS, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA 1989; 262:2395–2401.
- Mora S, Redberg RF, Cui Y, et al. Ability of exercise testing to predict cardiovascular and all-cause death in asymptomatic women: a 20-year follow-up of the Lipid Research Clinics prevalence study. JAMA 2003; 290:1600–1607.
- Gulati M, Pandey DK, Arnsdorf MF, et al. Exercise capacity and the risk of death in women: the St. James Women Take Heart Project. Circulation 2003; 108:1554–1559.
- Roger VL, Jacobsen SJ, Pellikka PA, Miller TD, Bailey KR, Gersh BJ. Prognostic value of treadmill exercise testing: a population-based study in Olmsted County, Minnesota. Circulation 1998; 98:2836–2841.
- Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 2002; 346:793–801.
- Gulati M, Black HR, Shaw LJ, et al. The prognostic value of a nomogram for exercise capacity in women. N Engl J Med 2005; 353:468–475.
- Snader CE, Marwick TH, Pashkow FJ, Harvey SA, Thomas JD, Lauer MS. Importance of estimated functional capacity as a predictor of all-cause mortality among patients referred for exercise thallium single-photon emission computed tomography: report of 3,400 patients from a single center. J Am Coll Cardiol 1997; 30:641–648.
- Goraya TY, Jacobsen SJ, Pellikka PA, et al. Prognostic value of treadmill exercise testing in elderly persons. Ann Intern Med 2000; 132:862–870.
- Weiner DA, Ryan TJ, McCabe CH, et al. Prognostic importance of a clinical profile and exercise test in medically treated patients with coronary artery disease. J Am Coll Cardiol 1984; 3:772–779.
- Mark DB, Hlatky MA, Harrell FE, Lee KL, Califf RM, Pryor DB. Exercise treadmill score for predicting prognosis in coronary artery disease. Ann Intern Med 1987; 106:793–800.
- Prakash M, Myers J, Froelicher VF, et al. Clinical and exercise test predictors of all-cause mortality: results from > 6,000 consecutive referred male patients. Chest 2001; 120:1003–1013.
- Griffin SE, Robergs RA, Heyward VH. Blood pressure measurement during exercise: a review. Med Sci Sports Exerc 1997; 29:149–159.
- Dubach P, Froelicher VF, Klein J, Oakes D, Grover-McKay M, Friis R. Exercise-induced hypotension in a male population. Criteria, causes, and prognosis. Circulation 1988; 78:1380–1387.
- Hammermeister KE, DeRouen TA, Dodge HT, Zia M. Prognostic and predictive value of exertional hypotension in suspected coronary artery disease. Am J Cardiol 1983; 51:1261–1266.
- Hakki AH, Munley BM, Hadjimiltiades S, Meissner MD, Iskandrian AS. Determinants of abnormal blood pressure response to exercise in coronary artery disease. Am J Cardiol 1986; 57:71–75.
- Froelicher VF, Perdue S, Pewen W, Risch M. Application of meta-analysis using an electronic spread sheet to exercise testing in patients after myocardial infarction. Am J Med 1987; 83:1045–1054.
- Tzemos N, Lim PO, MacDonald TM. Is exercise blood pressure a marker of vascular endothelial function? QJM 2002; 95:423–429.
- Wilson NV, Meyer BM. Early prediction of hypertension using exercise blood pressure. Prev Med 1981; 10:62–68.
- Dlin RA, Hanne N, Silverberg DS, Bar-Or O. Follow-up of normotensive men with exaggerated blood pressure response to exercise. Am Heart J 1983; 106:316–320.
- Allison TG, Cordeiro MA, Miller TD, Daida H, Squires RW, Gau GT. Prognostic significance of exercise-induced systemic hypertension in healthy subjects. Am J Cardiol 1999; 83:371–375.
- Lauer MS, Pashkow FJ, Harvey SA, Marwick TH, Thomas JD. Angiographic and prognostic implications of an exaggerated exercise systolic blood pressure response and rest systolic blood pressure in adults undergoing evaluation for suspected coronary artery disease. J Am Coll Cardiol 1995; 26:1630–1636.
- Amon KW, Richards KL, Crawford MH. Usefulness of the postexercise response of systolic blood pressure in the diagnosis of coronary artery disease. Circulation 1984; 70:951–956.
- Ellis K, Pothier CE, Blackstone EH, Lauer MS. Is systolic blood pressure recovery after exercise a predictor of mortality? Am Heart J 2004; 147:287–292.
- Lauer MS, Okin PM, Larson MG, Evans JC, Levy D. Impaired heart rate response to graded exercise. Prognostic implications of chronotropic incompetence in the Framingham Heart Study. Circulation 1996; 93:1520–1526.
- Lauer MS, Francis GS, Okin PM, Pashkow FJ, Snader CE, Marwick TH. Impaired chronotropic response to exercise stress testing as a predictor of mortality. JAMA 1999; 281:524–529.
- Azarbal B, Hayes SW, Lewin HC, Hachamovitch R, Cohen I, Berman DS. The incremental prognostic value of percentage of heart rate reserve achieved over myocardial perfusion single-photon emission computed tomography in the prediction of cardiac death and all-cause mortality: superiority over 85% of maximal age-predicted heart rate. J Am Coll Cardiol 2004; 44:423–430.
- Myers J, Tan SY, Abella J, Aleti V, Froelicher VF. Comparison of the chronotropic response to exercise and heart rate recovery in predicting cardiovascular mortality. Eur J Cardiovasc Prev Rehab 2007; 14:215–221.
- Kligfield P, Lauer MS. Exercise electrocardiogram testing: beyond the ST segment. Circulation 2006; 114:2070–2082.
- Khan MN, Pothier CE, Lauer MS. Chronotropic incompetence as a predictor of death among patients with normal electrograms taking beta blockers (metoprolol or atenolol). Am J Cardiol 2005; 96:1328–1333.
- Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med 1999; 341:1351–1357.
- Cole CR, Foody JM, Blackstone EH, Lauer MS. Heart rate recovery after submaximal exercise testing as a predictor of mortality in a cardiovascularly healthy cohort. Ann Intern Med 2000; 132:552–555.
- Vivekananthan DP, Blackstone EH, Pothier CE, Lauer MS. Heart rate recovery after exercise is a predictor of mortality, independent of the angiographic severity of coronary disease. J Am Coll Cardiol 2003; 42:831–838.
- Jouven X, Empana JP, Schwartz PJ, Desnos M, Courbon D, Ducimetiere P. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med 2005; 352:1951–1958.
- Dewey FE, Freeman JV, Engel G, et al. Novel predictor of prognosis from exercise stress testing: heart rate variability response to the exercise treadmill test. Am Heart J 2007; 153:281–288.
- Lerman BB, Stein KM, Markowitz SM, Mittal S, Slotwiner DJ. Right ventricular outflow tract tachycardia: an update. Card Electrophysiol Rev 2002; 6:68–71.
- Beckerman J, Wu T, Jones S, Froelicher VF. Exercise test-induced arrhythmias. Prog Cardiovasc Dis 2005; 47:285–305.
- Jouven X, Zureik M, Desnos M, Courbon D, Ducimetiere P. Long-term outcome in asymptomatic men with exercise-induced premature ventricular depolarizations. N Engl J Med 2000; 343:826–833.
- Frolkis JP, Pothier CE, Blackstone EH, Lauer MS. Frequent ventricular ectopy after exercise as a predictor of death. N Engl J Med 2003; 348:781–790.
- Blair SN, Kohl HW, Barlow CE, Paffenbarger RS, Gibbons LW, Macera CA. Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. JAMA 1995; 273:1093–1098.
- Thomson PD, Kelemen MH. Hypotension accompanying the onset of exertional angina. A sign of severe compromise of left ventricular blood supply. Circulation 1975; 52:28–32.
KEY POINTS
- Of the prognostic factors, exercise duration is the one most strongly associated with risk of coronary events and death, independent of age, sex, or known presence and severity of coronary artery disease.
- A decrease in blood pressure with exercise can reflect severe coronary artery disease or left ventricular systolic dysfunction.
- A heart rate that does not increase adequately during exercise or does not recover rapidly after exercise is associated with an increased risk of death.
- Exercise training may help to improve the prognosis of patients with an abnormal hemodynamic response to exercise caused by poor general health.
Preventing Venous Thromboembolism Throughout the Continuum of Care
Supplement Editor:
Amir K. Jaffer, MD
Contents
An overview of venous thromboembolism: Impact, risks, and issues in prophylaxis
A.K. Jaffer
Prevention of venous thromboembolism in the hospitalized medical patient
A.K. Jaffer, A.N. Amin, D.J. Brotman, S.B. Deitelzweig, S.C. McKean, A.C. Spyropoulos
Prevention of venous thromboembolism in the cancer surgery patient
A.C. Spyropoulos, D.J. Brotman, A.N. Amin, S.B. Deitelzweig, A.K. Jaffer, S.C. McKean
Prevention of venous thromboembolism in the orthopedic surgery patient
S.B. Deitelzweig, S.C. McKean, A.N. Amin, D.J. Brotman, A.K. Jaffer, A.C. Spyropoulos
Supplement Editor:
Amir K. Jaffer, MD
Contents
An overview of venous thromboembolism: Impact, risks, and issues in prophylaxis
A.K. Jaffer
Prevention of venous thromboembolism in the hospitalized medical patient
A.K. Jaffer, A.N. Amin, D.J. Brotman, S.B. Deitelzweig, S.C. McKean, A.C. Spyropoulos
Prevention of venous thromboembolism in the cancer surgery patient
A.C. Spyropoulos, D.J. Brotman, A.N. Amin, S.B. Deitelzweig, A.K. Jaffer, S.C. McKean
Prevention of venous thromboembolism in the orthopedic surgery patient
S.B. Deitelzweig, S.C. McKean, A.N. Amin, D.J. Brotman, A.K. Jaffer, A.C. Spyropoulos
Supplement Editor:
Amir K. Jaffer, MD
Contents
An overview of venous thromboembolism: Impact, risks, and issues in prophylaxis
A.K. Jaffer
Prevention of venous thromboembolism in the hospitalized medical patient
A.K. Jaffer, A.N. Amin, D.J. Brotman, S.B. Deitelzweig, S.C. McKean, A.C. Spyropoulos
Prevention of venous thromboembolism in the cancer surgery patient
A.C. Spyropoulos, D.J. Brotman, A.N. Amin, S.B. Deitelzweig, A.K. Jaffer, S.C. McKean
Prevention of venous thromboembolism in the orthopedic surgery patient
S.B. Deitelzweig, S.C. McKean, A.N. Amin, D.J. Brotman, A.K. Jaffer, A.C. Spyropoulos
What is the role of dual antiplatelet therapy with clopidogrel and aspirin?
In patients at risk of myocardial infarction or stroke, two antiplatelet drugs are not always better than one. In a large recent trial,1,2 adding clopidogrel (Plavix) to aspirin therapy did not offer much benefit to a cohort of patients at risk of cardiovascular events, although a subgroup did appear to benefit: those at even higher risk because they already had a history of myocardial infarction, ischemic stroke, or peripheral arterial disease.
These were the principal findings in the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) study,1,2 in which one of us (D.L.B.) was principal investigator.
These findings further our understanding of who should receive dual antiplatelet therapy, and who would be better served with aspirin therapy alone. In this article, we discuss important studies that led up to the CHARISMA trial, review CHARISMA’s purpose and study design, and interpret its results.
PREVENTING ATHEROTHROMBOSIS BY BLOCKING PLATELETS
Platelets are key players in the atherothrom-botic process.3–5 The Antiplatelet Trialists’ Collaboration,6 in a meta-analysis of trials performed up to 1997, calculated that antiplatelet therapy (mostly with aspirin) reduced the vascular mortality rate by 15% in patients with acute or previous vascular disease or some other predisposing condition. Thus, aspirin has already been shown to be effective as primary prevention (ie, in patients at risk but without established vascular disease) and as secondary prevention (ie, in those with established disease).7,8
Yet many patients have significant vascular events in spite of taking aspirin.6 Aspirin failure is thought to be multifactorial, with causes that include weak platelet inhibition, noncompliance, discontinuation due to adverse effects (including severe bleeding), and drug interactions. In addition, aspirin resistance has been linked to worse prognosis and may prove to be another cause of aspirin failure.9–11
Clopidogrel, an adenosine diphosphate (ADP) receptor antagonist, has also been studied extensively as an antiplatelet agent.5,12 Several studies have indicated that clopidogrel and ticlopidine (Ticlid, a related drug) may be more potent than aspirin, both in the test tube and in real patients.13–15
KEY TRIALS LEADING TO CHARISMA
- Clopidogrel is more effective and slightly safer than aspirin as secondary prevention, as shown in the Clopidogrel Versus Aspirin in Patients at Risk of Ischemic Events (CAPRIE) trial.16–21
- The combination of clopidogrel plus aspirin is more beneficial than placebo plus aspirin in patients with acute coronary syndromes, as shown in the Clopidogrel in Unstable Angina to Prevent Recurrent Ischemic Events (CURE) trial,22–24 the Clopidogrel as Adjunctive Reperfusion Therapy-Thrombolysis in Myo-car-dial Infarction (CLARITY-TIMI 28) trial,25 and the Clopidogrel and Metoprolol in Myocardial Infarction Trial (COMMIT).26
- The combination of clopidogrel plus aspirin is beneficial in patients undergoing percutaneous coronary interventions, with or without drug-eluting stent placement,27–30 as shown in the Clopidogrel for the Reduction of Events During Observation (CREDO) trial,28 the Effect of Clopidogrel Pretreatment Before Percutaneous Coronary Intervention in Patients With ST-Elevation Myocardial Infarction With Fibrinolytics (PCI-CLARITY) study,29 and the Effects of Pre-treatment With Clopidogrel and Aspirin Followed by Long-term Therapy in Patients Undergoing Percutaneous Coronary Intervention (PCI-CURE) study.30 In fact, most patients undergoing percutaneous interventions now receive a loading dose of clopidogrel before the procedure and continue to take it for up to 1 year afterward. However, the ideal long-term duration of clopidogrel treatment is still under debate.
In view of these previous studies, we wanted to test dual antiplatelet therapy in a broader population at high risk of atherothrombosis, ie, in patients with either established vascular disease or with multiple risk factors for it.
CHARISMA STUDY DESIGN
CHARISMA was a prospective, randomized, double-blind, placebo-controlled study of the efficacy and safety of clopidogrel plus aspirin vs placebo plus aspirin in patients at high risk of cardiovascular events.
A total of 15,603 patients, all older than 45 years, were randomly assigned to receive clopidogrel 75 mg/day plus aspirin 75 to 162 mg/day or placebo plus aspirin, in addition to standard therapy as directed by individual clinicians (eg, statins, beta-blockers). Patients were followed up at 1, 3, and 6 months and every 6 months thereafter until study completion, which occurred after 1,040 primary efficacy end points. The median duration of follow-up was 28 months.1
Patients had to have one of the following to be included: multiple atherothrombotic risk factors, documented coronary disease, documented cerebrovascular disease, or documented peripheral arterial disease (Table 2). Specific exclusion criteria included the use of oral antithrombotic or chronic nonsteroidal anti-inflammatory medications.1
End points
The primary end point was the combined incidence of the first episode of myocardial infarction or stroke, or death from cardiovascular causes.
The secondary end point was the combined incidence of myocardial infarction, stroke, death from cardiovascular causes, or hospitalization for unstable angina, a transient ischemic attack, or revascularization procedure.
The primary safety end point was severe bleeding, as defined in the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO) study31 as intracranial hemorrhage, fatal bleeding, or bleeding leading to hemody-namic compromise. Moderate bleeding was defined as bleeding that required transfusion but did not meet the GUSTO definition of severe bleeding.
OVERALL, NO BENEFIT
The rates of the secondary end point were 16.7% vs 17.9% (absolute risk reduction 1.2%; relative risk reduction 8%; P = .04).
Possible benefit in symptomatic patients
In a prespecified analysis, patients were classified as being “symptomatic” (having documented cardiovascular disease, ie, coronary, cerebrovascular, or symptomatic peripheral arterial disease) or “asymptomatic” (having multiple risk factors without established cardiovascular disease).1
In the symptomatic group (n = 12,153), the primary end point was reached in 6.9% of patients treated with clopidogrel vs 7.9% with placebo (absolute risk reduction 1.0%; relative risk reduction 13%; P = .046). The 3,284 asymptomatic patients showed no benefit; the rate of the primary end point for the clopido-grel group was 6.6% vs 5.5% in the placebo group (P = .20).
In a post hoc analysis, we examined the data from 9,478 patients who were similar to those in the CAPRIE study (ie, with documented prior myocardial infarction, prior ischemic stroke, or symptomatic peripheral arterial disease). The rate of cardiovascular death, myocardial infarction, or stroke was 8.8% in the placebo-plus-aspirin group and 7.3% in the clopidogrel-plus-aspirin group (absolute risk reduction 1.5%; relative risk reduction 17%; P = .01; Figure 1).2
HOW SHOULD WE INTERPRET THESE FINDINGS?
CHARISMA was the first trial to evaluate whether adding clopidogrel to aspirin therapy would reduce the rates of vascular events and death from cardiovascular causes in stable patients at risk of ischemic events. As in other trials, the benefit of clopidogrel-plus-aspirin therapy was weighed against the risk of bleeding with this regimen. How are we to interpret the findings?
- In the group with multiple risk factors but without clearly documented cardiovascular disease, there was no benefit—and there was an increase in moderate bleeding. Given these findings, physicians should not prescribe dual antiplatelet therapy for primary prevention in patients without known vascular disease.
- A potential benefit was seen in a prespecified subgroup who had documented cardiovascular disease. Given the limitations of subgroup analysis, however, and given the increased risk of moderate bleeding, this positive result should be interpreted with some degree of caution.
- CHARISMA suggests that there may be benefit of protracted dual antiplatelet therapy in stable patients with documented prior ischemic events.
A possible reason for the observed lack of benefit in the overall cohort but the positive results in the subgroups with established vascular disease is that plaque rupture and thrombosis may be a precondition for dual antiplatelet therapy to work.
Another possibility is that, although we have been saying that diabetes mellitus (one of the possible entry criteria in CHARISMA) is a “coronary risk equivalent,” this may not be absolutely true. Although it had been demonstrated that patients with certain risk factors, such as diabetes, have an incidence of ischemic events similar to that in patients with prior MI and should be considered for antiplatelet therapy to prevent vascular events,32 more recent data have shown that patients with prior ischemic events are at much higher risk than patients without ischemic events, even if the latter have diabetes.33,34
- The observation in CHARISMA that the incremental bleeding risk of dual antiplatelet therapy vs aspirin does not persist beyond a year in patients who have tolerated therapy for a year without a bleeding event may affect the decision to continue clopidogrel beyond 1 year, such as in patients with acute coronary syndromes or patients who have received drug-eluting stents.35,36
- Another important consideration is cost-effectiveness. Several studies have analyzed the impact of cost and found clopidogrel to be cost-effective by preventing ischemic events and adding years of life.37,38 A recent analysis from CHARISMA also shows cost-effectiveness in the subgroup of patients enrolled with established cardiovascular disease.39 Once clopidogrel becomes generic, the cost-effectiveness will become even better.
Further studies should better define which stable patients with cardiovascular disease should be on more than aspirin alone.
- Bhatt DL, Fox KA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006; 354:1706–1717.
- Bhatt DL, Flather MD, Hacke W, et al. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol 2007; 49:1982–1988.
- Ruggeri ZM. Platelets in atherothrombosis. Nat Med 2002; 8:1227–1234.
- Fuster V, Moreno PR, Fayad ZA, Corti R, Badimon JJ. Atherothrombosis and high-risk plaque: part I: evolving concepts. J Am Coll Cardiol 2005; 46:937–954.
- Meadows TA, Bhatt DL. Clinical aspects of platelet inhibitors and thrombus formation. Circ Res 2007; 100:1261–1275.
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002; 324:71–86.
- Sanmuganathan PS, Ghahramani P, Jackson PR, Wallis EJ, Ramsay LE. Aspirin for primary prevention of coronary heart disease: safety and absolute benefit related to coronary risk derived from meta-analysis of randomised trials. Heart 2001; 85:265–271.
- Hayden M, Pignone M, Phillips C, Mulrow C. Aspirin for the primary prevention of cardiovascular events: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002; 136:161–172.
- Helgason CM, Bolin KM, Hoff JA, et al. Development of aspirin resistance in persons with previous ischemic stroke. Stroke 1994; 25:2331–2336.
- Helgason CM, Tortorice KL, Winkler SR, et al. Aspirin response and failure in cerebral infarction. Stroke 1993; 24:345–350.
- Gum PA, Kottke-Marchant K, Poggio ED, et al. Profile and prevalence of aspirin resistance in patients with cardiovascular disease. Am J Cardiol 2001; 88:230–235.
- Coukell AJ, Markham A. Clopidogrel. Drugs 1997; 54:745–750.
- Humbert M, Nurden P, Bihour C, et al. Ultrastructural studies of platelet aggregates from human subjects receiving clopidogrel and from a patient with an inherited defect of an ADP-dependent pathway of platelet activation. Arterioscler Thromb Vasc Biol 1996; 16:1532–1543.
- Hass WK, Easton JD, Adams HP, et al. A randomized trial comparing ticlopidine hydrochloride with aspirin for the prevention of stroke in high-risk patients. Ticlopidine Aspirin Stroke Study Group. N Engl J Med 1989; 321:501–507.
- Savi P, Bernat A, Dumas A, Ait-Chek L, Herbert JM. Effect of aspirin and clopidogrel on platelet-dependent tissue factor expression in endothelial cells. Thromb Res 1994; 73:117–124.
- CAPRIE Steering Committee. A randomised, blinded, trial of clopido-grel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996; 348:1329–1339.
- Bhatt DL, Marso SP, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Amplified benefit of clopidogrel versus aspirin in patients with diabetes mellitus. Am J Cardiol 2002; 90:625–628.
- Bhatt DL, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Reduction in the need for hospitalization for recurrent ischemic events and bleeding with clopidogrel instead of aspirin. CAPRIE investigators. Am Heart J 2000; 140:67–73.
- Bhatt DL, Topol EJ. Antiplatelet and anticoagulant therapy in the secondary prevention of ischemic heart disease. Med Clin North Am 2000; 84 1:163–179.
- Ringleb PA, Bhatt DL, Hirsch AT, Topol EJ, Hacke W Clopidogrel Versus Aspirin in Patients at Risk of Ischemic Events Investigators. Benefit of clopidogrel over aspirin is amplified in patients with a history of ischemic events. Stroke 2004; 35:528–532.
- Bhatt DL, Chew DP, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Superiority of clopidogrel versus aspirin in patients with prior cardiac surgery. Circulation 2001; 103:363–368.
- Yusuf S, Zhao F, Mehta SR, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001; 345:494–502.
- Budaj A, Yusuf S, Mehta SR, et al. Benefit of clopidogrel in patients with acute coronary syndromes without ST-segment elevation in various risk groups. Circulation 2002; 106:1622–1626.
- Fox KA, Mehta SR, Peters R, et al. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non–ST-elevation acute coronary syndrome: the Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) Trial. Circulation 2004; 110:1202–1208.
- Sabatine MS, Cannon CP, Gibson CM, et al. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N Engl J Med 2005; 352:1179–1189.
- Chen ZM, Jiang LX, Chen YP, et al. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005; 366:1607–1621.
- Bhatt DL, Kapadia SR, Bajzer CT, et al. Dual antiplatelet therapy with clopidogrel and aspirin after carotid artery stenting. J Invasive Cardiol 2001; 13:767–771.
- Steinhubl SR, Berger PB, Mann JT, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 2002; 288:2411–2420.
- Sabatine MS, Cannon CP, Gibson CM, et al. Effect of clopidogrel pre-treatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated with fibrinolytics: the PCI-CLARITY study. JAMA 2005; 294:1224–1232.
- Mehta SR, Yusuf S, Peters RJ, et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet 2001; 358:527–533.
- The GUSTO Investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med 1993; 329:673–682.
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Bhatt DL, Steg PG, Ohman EM, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA 2006; 295:180–189.
- Steg PG, Bhatt DL, Wilson PW, et al. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA 2007; 297:1197–1206.
- Bavry AA, Kumbhani DJ, Helton TJ, Borek PP, Mood GR, Bhatt DL. Late thrombosis of drug-eluting stents: a meta-analysis of randomized clinical trials. Am J Med 2006; 119:1056–1061.
- Rabbat MG, Bavry AA, Bhatt DL, Ellis SG. Understanding and minimizing late thrombosis of drug-eluting stents. Cleve Clin J Med 2007; 74:129–136.
- Gaspoz JM, Coxson PG, Goldman PA, et al. Cost effectiveness of aspirin, clopidogrel, or both for secondary prevention of coronary heart disease. N Engl J Med 2002; 346:1800–1806.
- Beinart SC, Kolm P, Veledar E, et al. Longterm cost effectiveness of early and sustained dual oral antiplatelet therapy with clopidogrel given for up to one year after percutaneous coronary intervention results: from the Clopidogrel for the Reduction of Events During Observation (CREDO) trial. J Am Coll Cardiol 2005; 46:761–769.
- Chen J, Bhatt DL, Schneider E, et al. Cost-effectiveness of clopidogrel + aspirin vs. aspirin alone for secondary prevention of cardiovascular events: results from the CHARISMA Trial Session; APS.96.1; Presentation 3855; American Heart Association Scientific Sessions; Nov 12–15, 2006; Chicago IL.
In patients at risk of myocardial infarction or stroke, two antiplatelet drugs are not always better than one. In a large recent trial,1,2 adding clopidogrel (Plavix) to aspirin therapy did not offer much benefit to a cohort of patients at risk of cardiovascular events, although a subgroup did appear to benefit: those at even higher risk because they already had a history of myocardial infarction, ischemic stroke, or peripheral arterial disease.
These were the principal findings in the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) study,1,2 in which one of us (D.L.B.) was principal investigator.
These findings further our understanding of who should receive dual antiplatelet therapy, and who would be better served with aspirin therapy alone. In this article, we discuss important studies that led up to the CHARISMA trial, review CHARISMA’s purpose and study design, and interpret its results.
PREVENTING ATHEROTHROMBOSIS BY BLOCKING PLATELETS
Platelets are key players in the atherothrom-botic process.3–5 The Antiplatelet Trialists’ Collaboration,6 in a meta-analysis of trials performed up to 1997, calculated that antiplatelet therapy (mostly with aspirin) reduced the vascular mortality rate by 15% in patients with acute or previous vascular disease or some other predisposing condition. Thus, aspirin has already been shown to be effective as primary prevention (ie, in patients at risk but without established vascular disease) and as secondary prevention (ie, in those with established disease).7,8
Yet many patients have significant vascular events in spite of taking aspirin.6 Aspirin failure is thought to be multifactorial, with causes that include weak platelet inhibition, noncompliance, discontinuation due to adverse effects (including severe bleeding), and drug interactions. In addition, aspirin resistance has been linked to worse prognosis and may prove to be another cause of aspirin failure.9–11
Clopidogrel, an adenosine diphosphate (ADP) receptor antagonist, has also been studied extensively as an antiplatelet agent.5,12 Several studies have indicated that clopidogrel and ticlopidine (Ticlid, a related drug) may be more potent than aspirin, both in the test tube and in real patients.13–15
KEY TRIALS LEADING TO CHARISMA
- Clopidogrel is more effective and slightly safer than aspirin as secondary prevention, as shown in the Clopidogrel Versus Aspirin in Patients at Risk of Ischemic Events (CAPRIE) trial.16–21
- The combination of clopidogrel plus aspirin is more beneficial than placebo plus aspirin in patients with acute coronary syndromes, as shown in the Clopidogrel in Unstable Angina to Prevent Recurrent Ischemic Events (CURE) trial,22–24 the Clopidogrel as Adjunctive Reperfusion Therapy-Thrombolysis in Myo-car-dial Infarction (CLARITY-TIMI 28) trial,25 and the Clopidogrel and Metoprolol in Myocardial Infarction Trial (COMMIT).26
- The combination of clopidogrel plus aspirin is beneficial in patients undergoing percutaneous coronary interventions, with or without drug-eluting stent placement,27–30 as shown in the Clopidogrel for the Reduction of Events During Observation (CREDO) trial,28 the Effect of Clopidogrel Pretreatment Before Percutaneous Coronary Intervention in Patients With ST-Elevation Myocardial Infarction With Fibrinolytics (PCI-CLARITY) study,29 and the Effects of Pre-treatment With Clopidogrel and Aspirin Followed by Long-term Therapy in Patients Undergoing Percutaneous Coronary Intervention (PCI-CURE) study.30 In fact, most patients undergoing percutaneous interventions now receive a loading dose of clopidogrel before the procedure and continue to take it for up to 1 year afterward. However, the ideal long-term duration of clopidogrel treatment is still under debate.
In view of these previous studies, we wanted to test dual antiplatelet therapy in a broader population at high risk of atherothrombosis, ie, in patients with either established vascular disease or with multiple risk factors for it.
CHARISMA STUDY DESIGN
CHARISMA was a prospective, randomized, double-blind, placebo-controlled study of the efficacy and safety of clopidogrel plus aspirin vs placebo plus aspirin in patients at high risk of cardiovascular events.
A total of 15,603 patients, all older than 45 years, were randomly assigned to receive clopidogrel 75 mg/day plus aspirin 75 to 162 mg/day or placebo plus aspirin, in addition to standard therapy as directed by individual clinicians (eg, statins, beta-blockers). Patients were followed up at 1, 3, and 6 months and every 6 months thereafter until study completion, which occurred after 1,040 primary efficacy end points. The median duration of follow-up was 28 months.1
Patients had to have one of the following to be included: multiple atherothrombotic risk factors, documented coronary disease, documented cerebrovascular disease, or documented peripheral arterial disease (Table 2). Specific exclusion criteria included the use of oral antithrombotic or chronic nonsteroidal anti-inflammatory medications.1
End points
The primary end point was the combined incidence of the first episode of myocardial infarction or stroke, or death from cardiovascular causes.
The secondary end point was the combined incidence of myocardial infarction, stroke, death from cardiovascular causes, or hospitalization for unstable angina, a transient ischemic attack, or revascularization procedure.
The primary safety end point was severe bleeding, as defined in the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO) study31 as intracranial hemorrhage, fatal bleeding, or bleeding leading to hemody-namic compromise. Moderate bleeding was defined as bleeding that required transfusion but did not meet the GUSTO definition of severe bleeding.
OVERALL, NO BENEFIT
The rates of the secondary end point were 16.7% vs 17.9% (absolute risk reduction 1.2%; relative risk reduction 8%; P = .04).
Possible benefit in symptomatic patients
In a prespecified analysis, patients were classified as being “symptomatic” (having documented cardiovascular disease, ie, coronary, cerebrovascular, or symptomatic peripheral arterial disease) or “asymptomatic” (having multiple risk factors without established cardiovascular disease).1
In the symptomatic group (n = 12,153), the primary end point was reached in 6.9% of patients treated with clopidogrel vs 7.9% with placebo (absolute risk reduction 1.0%; relative risk reduction 13%; P = .046). The 3,284 asymptomatic patients showed no benefit; the rate of the primary end point for the clopido-grel group was 6.6% vs 5.5% in the placebo group (P = .20).
In a post hoc analysis, we examined the data from 9,478 patients who were similar to those in the CAPRIE study (ie, with documented prior myocardial infarction, prior ischemic stroke, or symptomatic peripheral arterial disease). The rate of cardiovascular death, myocardial infarction, or stroke was 8.8% in the placebo-plus-aspirin group and 7.3% in the clopidogrel-plus-aspirin group (absolute risk reduction 1.5%; relative risk reduction 17%; P = .01; Figure 1).2
HOW SHOULD WE INTERPRET THESE FINDINGS?
CHARISMA was the first trial to evaluate whether adding clopidogrel to aspirin therapy would reduce the rates of vascular events and death from cardiovascular causes in stable patients at risk of ischemic events. As in other trials, the benefit of clopidogrel-plus-aspirin therapy was weighed against the risk of bleeding with this regimen. How are we to interpret the findings?
- In the group with multiple risk factors but without clearly documented cardiovascular disease, there was no benefit—and there was an increase in moderate bleeding. Given these findings, physicians should not prescribe dual antiplatelet therapy for primary prevention in patients without known vascular disease.
- A potential benefit was seen in a prespecified subgroup who had documented cardiovascular disease. Given the limitations of subgroup analysis, however, and given the increased risk of moderate bleeding, this positive result should be interpreted with some degree of caution.
- CHARISMA suggests that there may be benefit of protracted dual antiplatelet therapy in stable patients with documented prior ischemic events.
A possible reason for the observed lack of benefit in the overall cohort but the positive results in the subgroups with established vascular disease is that plaque rupture and thrombosis may be a precondition for dual antiplatelet therapy to work.
Another possibility is that, although we have been saying that diabetes mellitus (one of the possible entry criteria in CHARISMA) is a “coronary risk equivalent,” this may not be absolutely true. Although it had been demonstrated that patients with certain risk factors, such as diabetes, have an incidence of ischemic events similar to that in patients with prior MI and should be considered for antiplatelet therapy to prevent vascular events,32 more recent data have shown that patients with prior ischemic events are at much higher risk than patients without ischemic events, even if the latter have diabetes.33,34
- The observation in CHARISMA that the incremental bleeding risk of dual antiplatelet therapy vs aspirin does not persist beyond a year in patients who have tolerated therapy for a year without a bleeding event may affect the decision to continue clopidogrel beyond 1 year, such as in patients with acute coronary syndromes or patients who have received drug-eluting stents.35,36
- Another important consideration is cost-effectiveness. Several studies have analyzed the impact of cost and found clopidogrel to be cost-effective by preventing ischemic events and adding years of life.37,38 A recent analysis from CHARISMA also shows cost-effectiveness in the subgroup of patients enrolled with established cardiovascular disease.39 Once clopidogrel becomes generic, the cost-effectiveness will become even better.
Further studies should better define which stable patients with cardiovascular disease should be on more than aspirin alone.
In patients at risk of myocardial infarction or stroke, two antiplatelet drugs are not always better than one. In a large recent trial,1,2 adding clopidogrel (Plavix) to aspirin therapy did not offer much benefit to a cohort of patients at risk of cardiovascular events, although a subgroup did appear to benefit: those at even higher risk because they already had a history of myocardial infarction, ischemic stroke, or peripheral arterial disease.
These were the principal findings in the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) study,1,2 in which one of us (D.L.B.) was principal investigator.
These findings further our understanding of who should receive dual antiplatelet therapy, and who would be better served with aspirin therapy alone. In this article, we discuss important studies that led up to the CHARISMA trial, review CHARISMA’s purpose and study design, and interpret its results.
PREVENTING ATHEROTHROMBOSIS BY BLOCKING PLATELETS
Platelets are key players in the atherothrom-botic process.3–5 The Antiplatelet Trialists’ Collaboration,6 in a meta-analysis of trials performed up to 1997, calculated that antiplatelet therapy (mostly with aspirin) reduced the vascular mortality rate by 15% in patients with acute or previous vascular disease or some other predisposing condition. Thus, aspirin has already been shown to be effective as primary prevention (ie, in patients at risk but without established vascular disease) and as secondary prevention (ie, in those with established disease).7,8
Yet many patients have significant vascular events in spite of taking aspirin.6 Aspirin failure is thought to be multifactorial, with causes that include weak platelet inhibition, noncompliance, discontinuation due to adverse effects (including severe bleeding), and drug interactions. In addition, aspirin resistance has been linked to worse prognosis and may prove to be another cause of aspirin failure.9–11
Clopidogrel, an adenosine diphosphate (ADP) receptor antagonist, has also been studied extensively as an antiplatelet agent.5,12 Several studies have indicated that clopidogrel and ticlopidine (Ticlid, a related drug) may be more potent than aspirin, both in the test tube and in real patients.13–15
KEY TRIALS LEADING TO CHARISMA
- Clopidogrel is more effective and slightly safer than aspirin as secondary prevention, as shown in the Clopidogrel Versus Aspirin in Patients at Risk of Ischemic Events (CAPRIE) trial.16–21
- The combination of clopidogrel plus aspirin is more beneficial than placebo plus aspirin in patients with acute coronary syndromes, as shown in the Clopidogrel in Unstable Angina to Prevent Recurrent Ischemic Events (CURE) trial,22–24 the Clopidogrel as Adjunctive Reperfusion Therapy-Thrombolysis in Myo-car-dial Infarction (CLARITY-TIMI 28) trial,25 and the Clopidogrel and Metoprolol in Myocardial Infarction Trial (COMMIT).26
- The combination of clopidogrel plus aspirin is beneficial in patients undergoing percutaneous coronary interventions, with or without drug-eluting stent placement,27–30 as shown in the Clopidogrel for the Reduction of Events During Observation (CREDO) trial,28 the Effect of Clopidogrel Pretreatment Before Percutaneous Coronary Intervention in Patients With ST-Elevation Myocardial Infarction With Fibrinolytics (PCI-CLARITY) study,29 and the Effects of Pre-treatment With Clopidogrel and Aspirin Followed by Long-term Therapy in Patients Undergoing Percutaneous Coronary Intervention (PCI-CURE) study.30 In fact, most patients undergoing percutaneous interventions now receive a loading dose of clopidogrel before the procedure and continue to take it for up to 1 year afterward. However, the ideal long-term duration of clopidogrel treatment is still under debate.
In view of these previous studies, we wanted to test dual antiplatelet therapy in a broader population at high risk of atherothrombosis, ie, in patients with either established vascular disease or with multiple risk factors for it.
CHARISMA STUDY DESIGN
CHARISMA was a prospective, randomized, double-blind, placebo-controlled study of the efficacy and safety of clopidogrel plus aspirin vs placebo plus aspirin in patients at high risk of cardiovascular events.
A total of 15,603 patients, all older than 45 years, were randomly assigned to receive clopidogrel 75 mg/day plus aspirin 75 to 162 mg/day or placebo plus aspirin, in addition to standard therapy as directed by individual clinicians (eg, statins, beta-blockers). Patients were followed up at 1, 3, and 6 months and every 6 months thereafter until study completion, which occurred after 1,040 primary efficacy end points. The median duration of follow-up was 28 months.1
Patients had to have one of the following to be included: multiple atherothrombotic risk factors, documented coronary disease, documented cerebrovascular disease, or documented peripheral arterial disease (Table 2). Specific exclusion criteria included the use of oral antithrombotic or chronic nonsteroidal anti-inflammatory medications.1
End points
The primary end point was the combined incidence of the first episode of myocardial infarction or stroke, or death from cardiovascular causes.
The secondary end point was the combined incidence of myocardial infarction, stroke, death from cardiovascular causes, or hospitalization for unstable angina, a transient ischemic attack, or revascularization procedure.
The primary safety end point was severe bleeding, as defined in the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO) study31 as intracranial hemorrhage, fatal bleeding, or bleeding leading to hemody-namic compromise. Moderate bleeding was defined as bleeding that required transfusion but did not meet the GUSTO definition of severe bleeding.
OVERALL, NO BENEFIT
The rates of the secondary end point were 16.7% vs 17.9% (absolute risk reduction 1.2%; relative risk reduction 8%; P = .04).
Possible benefit in symptomatic patients
In a prespecified analysis, patients were classified as being “symptomatic” (having documented cardiovascular disease, ie, coronary, cerebrovascular, or symptomatic peripheral arterial disease) or “asymptomatic” (having multiple risk factors without established cardiovascular disease).1
In the symptomatic group (n = 12,153), the primary end point was reached in 6.9% of patients treated with clopidogrel vs 7.9% with placebo (absolute risk reduction 1.0%; relative risk reduction 13%; P = .046). The 3,284 asymptomatic patients showed no benefit; the rate of the primary end point for the clopido-grel group was 6.6% vs 5.5% in the placebo group (P = .20).
In a post hoc analysis, we examined the data from 9,478 patients who were similar to those in the CAPRIE study (ie, with documented prior myocardial infarction, prior ischemic stroke, or symptomatic peripheral arterial disease). The rate of cardiovascular death, myocardial infarction, or stroke was 8.8% in the placebo-plus-aspirin group and 7.3% in the clopidogrel-plus-aspirin group (absolute risk reduction 1.5%; relative risk reduction 17%; P = .01; Figure 1).2
HOW SHOULD WE INTERPRET THESE FINDINGS?
CHARISMA was the first trial to evaluate whether adding clopidogrel to aspirin therapy would reduce the rates of vascular events and death from cardiovascular causes in stable patients at risk of ischemic events. As in other trials, the benefit of clopidogrel-plus-aspirin therapy was weighed against the risk of bleeding with this regimen. How are we to interpret the findings?
- In the group with multiple risk factors but without clearly documented cardiovascular disease, there was no benefit—and there was an increase in moderate bleeding. Given these findings, physicians should not prescribe dual antiplatelet therapy for primary prevention in patients without known vascular disease.
- A potential benefit was seen in a prespecified subgroup who had documented cardiovascular disease. Given the limitations of subgroup analysis, however, and given the increased risk of moderate bleeding, this positive result should be interpreted with some degree of caution.
- CHARISMA suggests that there may be benefit of protracted dual antiplatelet therapy in stable patients with documented prior ischemic events.
A possible reason for the observed lack of benefit in the overall cohort but the positive results in the subgroups with established vascular disease is that plaque rupture and thrombosis may be a precondition for dual antiplatelet therapy to work.
Another possibility is that, although we have been saying that diabetes mellitus (one of the possible entry criteria in CHARISMA) is a “coronary risk equivalent,” this may not be absolutely true. Although it had been demonstrated that patients with certain risk factors, such as diabetes, have an incidence of ischemic events similar to that in patients with prior MI and should be considered for antiplatelet therapy to prevent vascular events,32 more recent data have shown that patients with prior ischemic events are at much higher risk than patients without ischemic events, even if the latter have diabetes.33,34
- The observation in CHARISMA that the incremental bleeding risk of dual antiplatelet therapy vs aspirin does not persist beyond a year in patients who have tolerated therapy for a year without a bleeding event may affect the decision to continue clopidogrel beyond 1 year, such as in patients with acute coronary syndromes or patients who have received drug-eluting stents.35,36
- Another important consideration is cost-effectiveness. Several studies have analyzed the impact of cost and found clopidogrel to be cost-effective by preventing ischemic events and adding years of life.37,38 A recent analysis from CHARISMA also shows cost-effectiveness in the subgroup of patients enrolled with established cardiovascular disease.39 Once clopidogrel becomes generic, the cost-effectiveness will become even better.
Further studies should better define which stable patients with cardiovascular disease should be on more than aspirin alone.
- Bhatt DL, Fox KA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006; 354:1706–1717.
- Bhatt DL, Flather MD, Hacke W, et al. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol 2007; 49:1982–1988.
- Ruggeri ZM. Platelets in atherothrombosis. Nat Med 2002; 8:1227–1234.
- Fuster V, Moreno PR, Fayad ZA, Corti R, Badimon JJ. Atherothrombosis and high-risk plaque: part I: evolving concepts. J Am Coll Cardiol 2005; 46:937–954.
- Meadows TA, Bhatt DL. Clinical aspects of platelet inhibitors and thrombus formation. Circ Res 2007; 100:1261–1275.
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002; 324:71–86.
- Sanmuganathan PS, Ghahramani P, Jackson PR, Wallis EJ, Ramsay LE. Aspirin for primary prevention of coronary heart disease: safety and absolute benefit related to coronary risk derived from meta-analysis of randomised trials. Heart 2001; 85:265–271.
- Hayden M, Pignone M, Phillips C, Mulrow C. Aspirin for the primary prevention of cardiovascular events: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002; 136:161–172.
- Helgason CM, Bolin KM, Hoff JA, et al. Development of aspirin resistance in persons with previous ischemic stroke. Stroke 1994; 25:2331–2336.
- Helgason CM, Tortorice KL, Winkler SR, et al. Aspirin response and failure in cerebral infarction. Stroke 1993; 24:345–350.
- Gum PA, Kottke-Marchant K, Poggio ED, et al. Profile and prevalence of aspirin resistance in patients with cardiovascular disease. Am J Cardiol 2001; 88:230–235.
- Coukell AJ, Markham A. Clopidogrel. Drugs 1997; 54:745–750.
- Humbert M, Nurden P, Bihour C, et al. Ultrastructural studies of platelet aggregates from human subjects receiving clopidogrel and from a patient with an inherited defect of an ADP-dependent pathway of platelet activation. Arterioscler Thromb Vasc Biol 1996; 16:1532–1543.
- Hass WK, Easton JD, Adams HP, et al. A randomized trial comparing ticlopidine hydrochloride with aspirin for the prevention of stroke in high-risk patients. Ticlopidine Aspirin Stroke Study Group. N Engl J Med 1989; 321:501–507.
- Savi P, Bernat A, Dumas A, Ait-Chek L, Herbert JM. Effect of aspirin and clopidogrel on platelet-dependent tissue factor expression in endothelial cells. Thromb Res 1994; 73:117–124.
- CAPRIE Steering Committee. A randomised, blinded, trial of clopido-grel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996; 348:1329–1339.
- Bhatt DL, Marso SP, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Amplified benefit of clopidogrel versus aspirin in patients with diabetes mellitus. Am J Cardiol 2002; 90:625–628.
- Bhatt DL, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Reduction in the need for hospitalization for recurrent ischemic events and bleeding with clopidogrel instead of aspirin. CAPRIE investigators. Am Heart J 2000; 140:67–73.
- Bhatt DL, Topol EJ. Antiplatelet and anticoagulant therapy in the secondary prevention of ischemic heart disease. Med Clin North Am 2000; 84 1:163–179.
- Ringleb PA, Bhatt DL, Hirsch AT, Topol EJ, Hacke W Clopidogrel Versus Aspirin in Patients at Risk of Ischemic Events Investigators. Benefit of clopidogrel over aspirin is amplified in patients with a history of ischemic events. Stroke 2004; 35:528–532.
- Bhatt DL, Chew DP, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Superiority of clopidogrel versus aspirin in patients with prior cardiac surgery. Circulation 2001; 103:363–368.
- Yusuf S, Zhao F, Mehta SR, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001; 345:494–502.
- Budaj A, Yusuf S, Mehta SR, et al. Benefit of clopidogrel in patients with acute coronary syndromes without ST-segment elevation in various risk groups. Circulation 2002; 106:1622–1626.
- Fox KA, Mehta SR, Peters R, et al. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non–ST-elevation acute coronary syndrome: the Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) Trial. Circulation 2004; 110:1202–1208.
- Sabatine MS, Cannon CP, Gibson CM, et al. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N Engl J Med 2005; 352:1179–1189.
- Chen ZM, Jiang LX, Chen YP, et al. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005; 366:1607–1621.
- Bhatt DL, Kapadia SR, Bajzer CT, et al. Dual antiplatelet therapy with clopidogrel and aspirin after carotid artery stenting. J Invasive Cardiol 2001; 13:767–771.
- Steinhubl SR, Berger PB, Mann JT, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 2002; 288:2411–2420.
- Sabatine MS, Cannon CP, Gibson CM, et al. Effect of clopidogrel pre-treatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated with fibrinolytics: the PCI-CLARITY study. JAMA 2005; 294:1224–1232.
- Mehta SR, Yusuf S, Peters RJ, et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet 2001; 358:527–533.
- The GUSTO Investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med 1993; 329:673–682.
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Bhatt DL, Steg PG, Ohman EM, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA 2006; 295:180–189.
- Steg PG, Bhatt DL, Wilson PW, et al. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA 2007; 297:1197–1206.
- Bavry AA, Kumbhani DJ, Helton TJ, Borek PP, Mood GR, Bhatt DL. Late thrombosis of drug-eluting stents: a meta-analysis of randomized clinical trials. Am J Med 2006; 119:1056–1061.
- Rabbat MG, Bavry AA, Bhatt DL, Ellis SG. Understanding and minimizing late thrombosis of drug-eluting stents. Cleve Clin J Med 2007; 74:129–136.
- Gaspoz JM, Coxson PG, Goldman PA, et al. Cost effectiveness of aspirin, clopidogrel, or both for secondary prevention of coronary heart disease. N Engl J Med 2002; 346:1800–1806.
- Beinart SC, Kolm P, Veledar E, et al. Longterm cost effectiveness of early and sustained dual oral antiplatelet therapy with clopidogrel given for up to one year after percutaneous coronary intervention results: from the Clopidogrel for the Reduction of Events During Observation (CREDO) trial. J Am Coll Cardiol 2005; 46:761–769.
- Chen J, Bhatt DL, Schneider E, et al. Cost-effectiveness of clopidogrel + aspirin vs. aspirin alone for secondary prevention of cardiovascular events: results from the CHARISMA Trial Session; APS.96.1; Presentation 3855; American Heart Association Scientific Sessions; Nov 12–15, 2006; Chicago IL.
- Bhatt DL, Fox KA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006; 354:1706–1717.
- Bhatt DL, Flather MD, Hacke W, et al. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol 2007; 49:1982–1988.
- Ruggeri ZM. Platelets in atherothrombosis. Nat Med 2002; 8:1227–1234.
- Fuster V, Moreno PR, Fayad ZA, Corti R, Badimon JJ. Atherothrombosis and high-risk plaque: part I: evolving concepts. J Am Coll Cardiol 2005; 46:937–954.
- Meadows TA, Bhatt DL. Clinical aspects of platelet inhibitors and thrombus formation. Circ Res 2007; 100:1261–1275.
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002; 324:71–86.
- Sanmuganathan PS, Ghahramani P, Jackson PR, Wallis EJ, Ramsay LE. Aspirin for primary prevention of coronary heart disease: safety and absolute benefit related to coronary risk derived from meta-analysis of randomised trials. Heart 2001; 85:265–271.
- Hayden M, Pignone M, Phillips C, Mulrow C. Aspirin for the primary prevention of cardiovascular events: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002; 136:161–172.
- Helgason CM, Bolin KM, Hoff JA, et al. Development of aspirin resistance in persons with previous ischemic stroke. Stroke 1994; 25:2331–2336.
- Helgason CM, Tortorice KL, Winkler SR, et al. Aspirin response and failure in cerebral infarction. Stroke 1993; 24:345–350.
- Gum PA, Kottke-Marchant K, Poggio ED, et al. Profile and prevalence of aspirin resistance in patients with cardiovascular disease. Am J Cardiol 2001; 88:230–235.
- Coukell AJ, Markham A. Clopidogrel. Drugs 1997; 54:745–750.
- Humbert M, Nurden P, Bihour C, et al. Ultrastructural studies of platelet aggregates from human subjects receiving clopidogrel and from a patient with an inherited defect of an ADP-dependent pathway of platelet activation. Arterioscler Thromb Vasc Biol 1996; 16:1532–1543.
- Hass WK, Easton JD, Adams HP, et al. A randomized trial comparing ticlopidine hydrochloride with aspirin for the prevention of stroke in high-risk patients. Ticlopidine Aspirin Stroke Study Group. N Engl J Med 1989; 321:501–507.
- Savi P, Bernat A, Dumas A, Ait-Chek L, Herbert JM. Effect of aspirin and clopidogrel on platelet-dependent tissue factor expression in endothelial cells. Thromb Res 1994; 73:117–124.
- CAPRIE Steering Committee. A randomised, blinded, trial of clopido-grel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996; 348:1329–1339.
- Bhatt DL, Marso SP, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Amplified benefit of clopidogrel versus aspirin in patients with diabetes mellitus. Am J Cardiol 2002; 90:625–628.
- Bhatt DL, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Reduction in the need for hospitalization for recurrent ischemic events and bleeding with clopidogrel instead of aspirin. CAPRIE investigators. Am Heart J 2000; 140:67–73.
- Bhatt DL, Topol EJ. Antiplatelet and anticoagulant therapy in the secondary prevention of ischemic heart disease. Med Clin North Am 2000; 84 1:163–179.
- Ringleb PA, Bhatt DL, Hirsch AT, Topol EJ, Hacke W Clopidogrel Versus Aspirin in Patients at Risk of Ischemic Events Investigators. Benefit of clopidogrel over aspirin is amplified in patients with a history of ischemic events. Stroke 2004; 35:528–532.
- Bhatt DL, Chew DP, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Superiority of clopidogrel versus aspirin in patients with prior cardiac surgery. Circulation 2001; 103:363–368.
- Yusuf S, Zhao F, Mehta SR, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001; 345:494–502.
- Budaj A, Yusuf S, Mehta SR, et al. Benefit of clopidogrel in patients with acute coronary syndromes without ST-segment elevation in various risk groups. Circulation 2002; 106:1622–1626.
- Fox KA, Mehta SR, Peters R, et al. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non–ST-elevation acute coronary syndrome: the Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) Trial. Circulation 2004; 110:1202–1208.
- Sabatine MS, Cannon CP, Gibson CM, et al. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N Engl J Med 2005; 352:1179–1189.
- Chen ZM, Jiang LX, Chen YP, et al. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005; 366:1607–1621.
- Bhatt DL, Kapadia SR, Bajzer CT, et al. Dual antiplatelet therapy with clopidogrel and aspirin after carotid artery stenting. J Invasive Cardiol 2001; 13:767–771.
- Steinhubl SR, Berger PB, Mann JT, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 2002; 288:2411–2420.
- Sabatine MS, Cannon CP, Gibson CM, et al. Effect of clopidogrel pre-treatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated with fibrinolytics: the PCI-CLARITY study. JAMA 2005; 294:1224–1232.
- Mehta SR, Yusuf S, Peters RJ, et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet 2001; 358:527–533.
- The GUSTO Investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med 1993; 329:673–682.
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Bhatt DL, Steg PG, Ohman EM, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA 2006; 295:180–189.
- Steg PG, Bhatt DL, Wilson PW, et al. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA 2007; 297:1197–1206.
- Bavry AA, Kumbhani DJ, Helton TJ, Borek PP, Mood GR, Bhatt DL. Late thrombosis of drug-eluting stents: a meta-analysis of randomized clinical trials. Am J Med 2006; 119:1056–1061.
- Rabbat MG, Bavry AA, Bhatt DL, Ellis SG. Understanding and minimizing late thrombosis of drug-eluting stents. Cleve Clin J Med 2007; 74:129–136.
- Gaspoz JM, Coxson PG, Goldman PA, et al. Cost effectiveness of aspirin, clopidogrel, or both for secondary prevention of coronary heart disease. N Engl J Med 2002; 346:1800–1806.
- Beinart SC, Kolm P, Veledar E, et al. Longterm cost effectiveness of early and sustained dual oral antiplatelet therapy with clopidogrel given for up to one year after percutaneous coronary intervention results: from the Clopidogrel for the Reduction of Events During Observation (CREDO) trial. J Am Coll Cardiol 2005; 46:761–769.
- Chen J, Bhatt DL, Schneider E, et al. Cost-effectiveness of clopidogrel + aspirin vs. aspirin alone for secondary prevention of cardiovascular events: results from the CHARISMA Trial Session; APS.96.1; Presentation 3855; American Heart Association Scientific Sessions; Nov 12–15, 2006; Chicago IL.
KEY POINTS
- Platelets are key players in atherothrombosis, and antiplatelet drugs such as aspirin and clopidogrel prevent events in patients at risk.
- In studies leading up to CHARISMA, the combination of clopidogrel and aspirin was found to be beneficial in patients with acute coronary syndromes and in those undergoing percutaneous coronary interventions.
- Clopidogrel should not be combined with aspirin as a primary preventive therapy (ie, for people without established vascular disease). How dual antiplatelet therapy should be used as secondary prevention in stable patients needs further study.