User login
A 68-year-old man with a blue toe
A 68-year-old man presented with concern about a bluish toe. Several months earlier he had undergone total aortic arch replacement and coronary artery bypass grafting. Since then his renal function had declined and he had been losing weight.
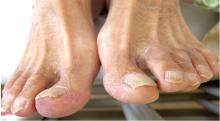
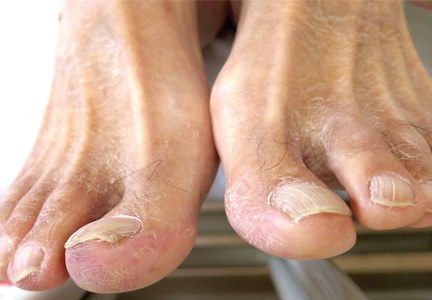
He had hypercholesterolemia, hypertension, and a 20-pack-year smoking history. Physical examination confirmed that his right great toe was indeed bluish (Figure 1). Peripheral, neck, and abdominal vascular examinations were normal. Laboratory testing revealed:
- Serum creatinine concentration 5.15 mg/dL (reference range 0.61–1.04)
- C-reactive protein level 1.5 mg/dL (0–0.3)
- Eosinophil count 0.58 × 109/L (0–0.50)
- Serum complement level normal
- Urine sediment unremarkable.
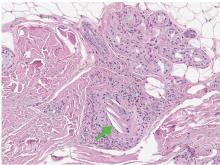
Transthoracic echocardiography revealed no evidence of vegetation, and a series of blood cultures were negative. The right toe was biopsied, and study revealed cholesterol clefts (Figure 2), confirming the diagnosis of cholesterol crystal embolism.
He was treated with prednisolone 20 mg/day, and his weight loss and renal function improved.
CHOLESTEROL CRYSTAL EMBOLISM
Cholesterol embolization typically occurs after arteriography, cardiac catheterization, vascular surgery, or anticoagulant use in men over age 55 with atherosclerosis.1 It presents with renal failure, abdominal pain, systemic symptoms, or, most commonly (in 88% of cases), skin findings.2
“Blue-toe syndrome,” characterized by tissue ischemia, is seen in 65% of patients.2 Lesions can appear anywhere on the body, but most commonly on the lower extremities. Most are painful due to ischemia. The condition can progress to necrosis.
Patients may have elevated C-reactive protein, hypocomplementemia (39%), and eosinophilia (80%).3,4 The diagnosis is confirmed only with histopathologic findings of intravascular cholesterol crystals, seen as cholesterol clefts.
The differential diagnosis includes contrast nephropathy and infectious endocarditis. However, contrast nephropathy begins to recover within several days and is not accompanied by skin lesions. Repeated blood cultures and echocardiography are useful to rule out infectious endocarditis.
Treatment includes managing cardiovascular risk factors and end-organ ischemia and preventing recurrent embolization. Surgical or endovascular treatment has been shown to be effective in decreasing the rate of further embolism.2 Corticosteroid therapy is assumed to control the secondary inflammation associated with cholesterol crystal embolism.1,5
- Paraskevas KI, Koutsias S, Mikhailidis DP, Giannoukas AD. Cholesterol crystal embolization: a possible complication of peripheral endovascular interventions. J Endovasc Ther 2008; 15:614–625.
- Jucgla A, Moreso F, Muniesa C, Moreno A, Vidaller A. Cholesterol embolism: still an unrecognized entity with a high mortality rate. J Am Acad Dermatol 2006; 55:786–793.
- Kronzon I, Saric M. Cholesterol embolization syndrome. Circulation 2010; 122:631–641.
- Lye WC, Cheah JS, Sinniah R. Renal cholesterol embolic disease. Case report and review of the literature. Am J Nephrol 1993; 13:489–493.
- Nakayama M, Izumaru K, Nagata M, et al. The effect of low-dose corticosteroids on short- and long-term renal outcome in patients with cholesterol crystal embolism. Ren Fail 2011; 33:298–306.
A 68-year-old man presented with concern about a bluish toe. Several months earlier he had undergone total aortic arch replacement and coronary artery bypass grafting. Since then his renal function had declined and he had been losing weight.
He had hypercholesterolemia, hypertension, and a 20-pack-year smoking history. Physical examination confirmed that his right great toe was indeed bluish (Figure 1). Peripheral, neck, and abdominal vascular examinations were normal. Laboratory testing revealed:
- Serum creatinine concentration 5.15 mg/dL (reference range 0.61–1.04)
- C-reactive protein level 1.5 mg/dL (0–0.3)
- Eosinophil count 0.58 × 109/L (0–0.50)
- Serum complement level normal
- Urine sediment unremarkable.
Transthoracic echocardiography revealed no evidence of vegetation, and a series of blood cultures were negative. The right toe was biopsied, and study revealed cholesterol clefts (Figure 2), confirming the diagnosis of cholesterol crystal embolism.
He was treated with prednisolone 20 mg/day, and his weight loss and renal function improved.
CHOLESTEROL CRYSTAL EMBOLISM
Cholesterol embolization typically occurs after arteriography, cardiac catheterization, vascular surgery, or anticoagulant use in men over age 55 with atherosclerosis.1 It presents with renal failure, abdominal pain, systemic symptoms, or, most commonly (in 88% of cases), skin findings.2
“Blue-toe syndrome,” characterized by tissue ischemia, is seen in 65% of patients.2 Lesions can appear anywhere on the body, but most commonly on the lower extremities. Most are painful due to ischemia. The condition can progress to necrosis.
Patients may have elevated C-reactive protein, hypocomplementemia (39%), and eosinophilia (80%).3,4 The diagnosis is confirmed only with histopathologic findings of intravascular cholesterol crystals, seen as cholesterol clefts.
The differential diagnosis includes contrast nephropathy and infectious endocarditis. However, contrast nephropathy begins to recover within several days and is not accompanied by skin lesions. Repeated blood cultures and echocardiography are useful to rule out infectious endocarditis.
Treatment includes managing cardiovascular risk factors and end-organ ischemia and preventing recurrent embolization. Surgical or endovascular treatment has been shown to be effective in decreasing the rate of further embolism.2 Corticosteroid therapy is assumed to control the secondary inflammation associated with cholesterol crystal embolism.1,5
A 68-year-old man presented with concern about a bluish toe. Several months earlier he had undergone total aortic arch replacement and coronary artery bypass grafting. Since then his renal function had declined and he had been losing weight.
He had hypercholesterolemia, hypertension, and a 20-pack-year smoking history. Physical examination confirmed that his right great toe was indeed bluish (Figure 1). Peripheral, neck, and abdominal vascular examinations were normal. Laboratory testing revealed:
- Serum creatinine concentration 5.15 mg/dL (reference range 0.61–1.04)
- C-reactive protein level 1.5 mg/dL (0–0.3)
- Eosinophil count 0.58 × 109/L (0–0.50)
- Serum complement level normal
- Urine sediment unremarkable.
Transthoracic echocardiography revealed no evidence of vegetation, and a series of blood cultures were negative. The right toe was biopsied, and study revealed cholesterol clefts (Figure 2), confirming the diagnosis of cholesterol crystal embolism.
He was treated with prednisolone 20 mg/day, and his weight loss and renal function improved.
CHOLESTEROL CRYSTAL EMBOLISM
Cholesterol embolization typically occurs after arteriography, cardiac catheterization, vascular surgery, or anticoagulant use in men over age 55 with atherosclerosis.1 It presents with renal failure, abdominal pain, systemic symptoms, or, most commonly (in 88% of cases), skin findings.2
“Blue-toe syndrome,” characterized by tissue ischemia, is seen in 65% of patients.2 Lesions can appear anywhere on the body, but most commonly on the lower extremities. Most are painful due to ischemia. The condition can progress to necrosis.
Patients may have elevated C-reactive protein, hypocomplementemia (39%), and eosinophilia (80%).3,4 The diagnosis is confirmed only with histopathologic findings of intravascular cholesterol crystals, seen as cholesterol clefts.
The differential diagnosis includes contrast nephropathy and infectious endocarditis. However, contrast nephropathy begins to recover within several days and is not accompanied by skin lesions. Repeated blood cultures and echocardiography are useful to rule out infectious endocarditis.
Treatment includes managing cardiovascular risk factors and end-organ ischemia and preventing recurrent embolization. Surgical or endovascular treatment has been shown to be effective in decreasing the rate of further embolism.2 Corticosteroid therapy is assumed to control the secondary inflammation associated with cholesterol crystal embolism.1,5
- Paraskevas KI, Koutsias S, Mikhailidis DP, Giannoukas AD. Cholesterol crystal embolization: a possible complication of peripheral endovascular interventions. J Endovasc Ther 2008; 15:614–625.
- Jucgla A, Moreso F, Muniesa C, Moreno A, Vidaller A. Cholesterol embolism: still an unrecognized entity with a high mortality rate. J Am Acad Dermatol 2006; 55:786–793.
- Kronzon I, Saric M. Cholesterol embolization syndrome. Circulation 2010; 122:631–641.
- Lye WC, Cheah JS, Sinniah R. Renal cholesterol embolic disease. Case report and review of the literature. Am J Nephrol 1993; 13:489–493.
- Nakayama M, Izumaru K, Nagata M, et al. The effect of low-dose corticosteroids on short- and long-term renal outcome in patients with cholesterol crystal embolism. Ren Fail 2011; 33:298–306.
- Paraskevas KI, Koutsias S, Mikhailidis DP, Giannoukas AD. Cholesterol crystal embolization: a possible complication of peripheral endovascular interventions. J Endovasc Ther 2008; 15:614–625.
- Jucgla A, Moreso F, Muniesa C, Moreno A, Vidaller A. Cholesterol embolism: still an unrecognized entity with a high mortality rate. J Am Acad Dermatol 2006; 55:786–793.
- Kronzon I, Saric M. Cholesterol embolization syndrome. Circulation 2010; 122:631–641.
- Lye WC, Cheah JS, Sinniah R. Renal cholesterol embolic disease. Case report and review of the literature. Am J Nephrol 1993; 13:489–493.
- Nakayama M, Izumaru K, Nagata M, et al. The effect of low-dose corticosteroids on short- and long-term renal outcome in patients with cholesterol crystal embolism. Ren Fail 2011; 33:298–306.
New DES hailed for smallest coronary vessels
Paris – The first multicenter, prospective trial of a drug-eluting stent designed specifically to treat lesions in coronary vessels less than 2.25 mm in diameter showed excellent outcomes, with a 1-year target lesion failure rate of 5% for the Resolute Onyx 2.0 mm diameter zotarolimus-eluting stent.
This result in the pivotal trial easily surpassed the prespecified performance goal of a 19% target lesion failure rate, Matthew J. Price, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Hemodynamically significant lesions in such small vessels are “not uncommon, particularly in diabetic patients,” Dr. Price said in an interview. Indeed, 47% of patients in the clinical trial had diabetes.
At present, the only ways to treat coronary disease in arteries having a reference vessel diameter less than 2.25 mm are off-label placement of an oversized stent, with its attendant risk of complications; standard balloon angioplasty, which entails a particularly high restenosis rate in this setting; or medical management, the cardiologist noted.
He presented a multicenter, prospective, open-label, single-arm trial of 101 patients with documented ischemia-producing obstructions in coronary arteries having a reference vessel diameter less than 2.25 mm, a lesion length less than 27 mm, and evidence of ischemia attributable to the lesion, typically via fractional flow reserve. The mean diameter by quantitative coronary angiography was 1.91 mm.
The primary endpoint was the rate of target lesion failure at 12 months, a composite comprising cardiac death, target vessel MI, or clinically driven target lesion revascularization. This endpoint occurred in 5% of patients. There was a 3% target vessel MI rate and a 2% target lesion revascularization rate. There were no cardiac deaths.
“Importantly, the stent thrombosis rate in these patients with extremely small vessels was zero,” the cardiologist emphasized.
The mean angiographic in-stent late lumen loss at 13 months was 0.26 mm, which Dr. Price characterized as “quite good.” The in-segment binary angiographic restenosis rate was 20%.
“That’s slightly higher than you would expect to see in vessels with larger reference diameters. I think that’s because of the lack of headroom. You have a very small vessel, and, even with a very small stent, even a small amount of late loss will give you a larger percent diameter restenosis over time,” he explained.
The 19% target lesion failure rate selected as a performance goal in the trial was set somewhat arbitrarily. It wasn’t possible to randomize patients to a comparator arm because there are no approved stents for vessels less than 2.25 mm in diameter. The 19% figure was arrived at in discussion with the Food and Drug Administration on the basis of similarity to the performance goal used in clinical trials to gain approval of 2.25-mm, drug-eluting stents. Because the Onyx 2.0-mm-diameter trial was developed in collaboration with the FDA and the stent aced its primary endpoint and showed excellent clinical outcomes, Dr. Price anticipates the device will readily gain regulatory approval. In April 2017, the FDA approved the Resolute Onyx in sizes of 2.25- to 5.0-mm diameter.
The study met with an enthusiastic reception.
“That was terrific. It’s clearly an incredibly important unmet clinical need,” commented session cochair David R. Holmes Jr., MD, of the Mayo Clinic in Rochester, Minn.
Assuming the stent is approved, how should interventionalists put it into practice? he asked.
Dr. Price replied that, first, it’s important to step back and ask if percutaneous coronary intervention of a particular lesion in a very small coronary artery is clinically indicated. The stent itself is readily manipulatable. It is a thin-strut device constructed of a single strand of a cobalt alloy with enhanced radiopacity.
Investigators in the trial used the standard approach to dual antiplatelet therapy – at least 6 months, with 12 months preferable.
The 20% in-segment binary restenosis rate at 13 months provides a clear message for interventionalists, he continued. “What this tells me is that, while this is a very good stent, we can’t forget to treat the patient aggressively with medical therapy to stop the progression of prediabetes, diabetes, and small vessel disease in addition to treating obstructive lesions with a small stent.”
Asked if the lack of headroom in these extra-small arteries warrants liberal use of intraprocedural imaging to make sure the stent is perfectly apposed, Dr. Price replied that he doesn’t think so. He noted that intravascular ultrasound and optical coherence tomography were seldom used in the trial, yet the results were reassuringly excellent.
The study results were published simultaneously with Dr. Price’s presentation (JACC Cardiovasc Interv. 2017 May 17. doi: 10.1016/j.jcin.2017.05.004). The trial was sponsored by Medtronic. Dr. Price reported serving as a consultant and paid speaker on behalf of that company, as well as AstraZeneca, Boston Scientific, St. Jude Medical, and The Medicines Company.
Paris – The first multicenter, prospective trial of a drug-eluting stent designed specifically to treat lesions in coronary vessels less than 2.25 mm in diameter showed excellent outcomes, with a 1-year target lesion failure rate of 5% for the Resolute Onyx 2.0 mm diameter zotarolimus-eluting stent.
This result in the pivotal trial easily surpassed the prespecified performance goal of a 19% target lesion failure rate, Matthew J. Price, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Hemodynamically significant lesions in such small vessels are “not uncommon, particularly in diabetic patients,” Dr. Price said in an interview. Indeed, 47% of patients in the clinical trial had diabetes.
At present, the only ways to treat coronary disease in arteries having a reference vessel diameter less than 2.25 mm are off-label placement of an oversized stent, with its attendant risk of complications; standard balloon angioplasty, which entails a particularly high restenosis rate in this setting; or medical management, the cardiologist noted.
He presented a multicenter, prospective, open-label, single-arm trial of 101 patients with documented ischemia-producing obstructions in coronary arteries having a reference vessel diameter less than 2.25 mm, a lesion length less than 27 mm, and evidence of ischemia attributable to the lesion, typically via fractional flow reserve. The mean diameter by quantitative coronary angiography was 1.91 mm.
The primary endpoint was the rate of target lesion failure at 12 months, a composite comprising cardiac death, target vessel MI, or clinically driven target lesion revascularization. This endpoint occurred in 5% of patients. There was a 3% target vessel MI rate and a 2% target lesion revascularization rate. There were no cardiac deaths.
“Importantly, the stent thrombosis rate in these patients with extremely small vessels was zero,” the cardiologist emphasized.
The mean angiographic in-stent late lumen loss at 13 months was 0.26 mm, which Dr. Price characterized as “quite good.” The in-segment binary angiographic restenosis rate was 20%.
“That’s slightly higher than you would expect to see in vessels with larger reference diameters. I think that’s because of the lack of headroom. You have a very small vessel, and, even with a very small stent, even a small amount of late loss will give you a larger percent diameter restenosis over time,” he explained.
The 19% target lesion failure rate selected as a performance goal in the trial was set somewhat arbitrarily. It wasn’t possible to randomize patients to a comparator arm because there are no approved stents for vessels less than 2.25 mm in diameter. The 19% figure was arrived at in discussion with the Food and Drug Administration on the basis of similarity to the performance goal used in clinical trials to gain approval of 2.25-mm, drug-eluting stents. Because the Onyx 2.0-mm-diameter trial was developed in collaboration with the FDA and the stent aced its primary endpoint and showed excellent clinical outcomes, Dr. Price anticipates the device will readily gain regulatory approval. In April 2017, the FDA approved the Resolute Onyx in sizes of 2.25- to 5.0-mm diameter.
The study met with an enthusiastic reception.
“That was terrific. It’s clearly an incredibly important unmet clinical need,” commented session cochair David R. Holmes Jr., MD, of the Mayo Clinic in Rochester, Minn.
Assuming the stent is approved, how should interventionalists put it into practice? he asked.
Dr. Price replied that, first, it’s important to step back and ask if percutaneous coronary intervention of a particular lesion in a very small coronary artery is clinically indicated. The stent itself is readily manipulatable. It is a thin-strut device constructed of a single strand of a cobalt alloy with enhanced radiopacity.
Investigators in the trial used the standard approach to dual antiplatelet therapy – at least 6 months, with 12 months preferable.
The 20% in-segment binary restenosis rate at 13 months provides a clear message for interventionalists, he continued. “What this tells me is that, while this is a very good stent, we can’t forget to treat the patient aggressively with medical therapy to stop the progression of prediabetes, diabetes, and small vessel disease in addition to treating obstructive lesions with a small stent.”
Asked if the lack of headroom in these extra-small arteries warrants liberal use of intraprocedural imaging to make sure the stent is perfectly apposed, Dr. Price replied that he doesn’t think so. He noted that intravascular ultrasound and optical coherence tomography were seldom used in the trial, yet the results were reassuringly excellent.
The study results were published simultaneously with Dr. Price’s presentation (JACC Cardiovasc Interv. 2017 May 17. doi: 10.1016/j.jcin.2017.05.004). The trial was sponsored by Medtronic. Dr. Price reported serving as a consultant and paid speaker on behalf of that company, as well as AstraZeneca, Boston Scientific, St. Jude Medical, and The Medicines Company.
Paris – The first multicenter, prospective trial of a drug-eluting stent designed specifically to treat lesions in coronary vessels less than 2.25 mm in diameter showed excellent outcomes, with a 1-year target lesion failure rate of 5% for the Resolute Onyx 2.0 mm diameter zotarolimus-eluting stent.
This result in the pivotal trial easily surpassed the prespecified performance goal of a 19% target lesion failure rate, Matthew J. Price, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
Hemodynamically significant lesions in such small vessels are “not uncommon, particularly in diabetic patients,” Dr. Price said in an interview. Indeed, 47% of patients in the clinical trial had diabetes.
At present, the only ways to treat coronary disease in arteries having a reference vessel diameter less than 2.25 mm are off-label placement of an oversized stent, with its attendant risk of complications; standard balloon angioplasty, which entails a particularly high restenosis rate in this setting; or medical management, the cardiologist noted.
He presented a multicenter, prospective, open-label, single-arm trial of 101 patients with documented ischemia-producing obstructions in coronary arteries having a reference vessel diameter less than 2.25 mm, a lesion length less than 27 mm, and evidence of ischemia attributable to the lesion, typically via fractional flow reserve. The mean diameter by quantitative coronary angiography was 1.91 mm.
The primary endpoint was the rate of target lesion failure at 12 months, a composite comprising cardiac death, target vessel MI, or clinically driven target lesion revascularization. This endpoint occurred in 5% of patients. There was a 3% target vessel MI rate and a 2% target lesion revascularization rate. There were no cardiac deaths.
“Importantly, the stent thrombosis rate in these patients with extremely small vessels was zero,” the cardiologist emphasized.
The mean angiographic in-stent late lumen loss at 13 months was 0.26 mm, which Dr. Price characterized as “quite good.” The in-segment binary angiographic restenosis rate was 20%.
“That’s slightly higher than you would expect to see in vessels with larger reference diameters. I think that’s because of the lack of headroom. You have a very small vessel, and, even with a very small stent, even a small amount of late loss will give you a larger percent diameter restenosis over time,” he explained.
The 19% target lesion failure rate selected as a performance goal in the trial was set somewhat arbitrarily. It wasn’t possible to randomize patients to a comparator arm because there are no approved stents for vessels less than 2.25 mm in diameter. The 19% figure was arrived at in discussion with the Food and Drug Administration on the basis of similarity to the performance goal used in clinical trials to gain approval of 2.25-mm, drug-eluting stents. Because the Onyx 2.0-mm-diameter trial was developed in collaboration with the FDA and the stent aced its primary endpoint and showed excellent clinical outcomes, Dr. Price anticipates the device will readily gain regulatory approval. In April 2017, the FDA approved the Resolute Onyx in sizes of 2.25- to 5.0-mm diameter.
The study met with an enthusiastic reception.
“That was terrific. It’s clearly an incredibly important unmet clinical need,” commented session cochair David R. Holmes Jr., MD, of the Mayo Clinic in Rochester, Minn.
Assuming the stent is approved, how should interventionalists put it into practice? he asked.
Dr. Price replied that, first, it’s important to step back and ask if percutaneous coronary intervention of a particular lesion in a very small coronary artery is clinically indicated. The stent itself is readily manipulatable. It is a thin-strut device constructed of a single strand of a cobalt alloy with enhanced radiopacity.
Investigators in the trial used the standard approach to dual antiplatelet therapy – at least 6 months, with 12 months preferable.
The 20% in-segment binary restenosis rate at 13 months provides a clear message for interventionalists, he continued. “What this tells me is that, while this is a very good stent, we can’t forget to treat the patient aggressively with medical therapy to stop the progression of prediabetes, diabetes, and small vessel disease in addition to treating obstructive lesions with a small stent.”
Asked if the lack of headroom in these extra-small arteries warrants liberal use of intraprocedural imaging to make sure the stent is perfectly apposed, Dr. Price replied that he doesn’t think so. He noted that intravascular ultrasound and optical coherence tomography were seldom used in the trial, yet the results were reassuringly excellent.
The study results were published simultaneously with Dr. Price’s presentation (JACC Cardiovasc Interv. 2017 May 17. doi: 10.1016/j.jcin.2017.05.004). The trial was sponsored by Medtronic. Dr. Price reported serving as a consultant and paid speaker on behalf of that company, as well as AstraZeneca, Boston Scientific, St. Jude Medical, and The Medicines Company.
AT EUROPCR
Key clinical point:
Major finding: At 12 months’ follow-up, the key outcomes were a 3% rate of target vessel MI, a 2% rate of clinically driven target lesion revascularization, no stent thrombosis, and no cardiac deaths.
Data source: A prospective, multicenter, open-label trial in 101 patients who underwent percutaneous coronary intervention for coronary lesions with a reference vessel diameter of less than 2.25 mm.
Disclosures: The trial was sponsored by Medtronic. Dr. Price reported serving as a consultant to and paid speaker on behalf of that company as well as AstraZeneca, Boston Scientific, St. Jude Medical, and The Medicines Company.
High readmits after peripheral arterial procedures
WASHINGTON – More than one in six patients who undergo a lower extremity arterial endovascular or surgical procedure are readmitted within 30 days, according to a large national study.
The annual total cost of these early readmissions is high, in excess of $360 million. But because there turned out to be surprisingly little difference in readmission rates between hospitals, 30-day readmissions may not be a rational quality measure on which to base institutional reimbursement or withholding of payment for peripheral arterial interventions, Eric A. Secemsky, MD, said at the annual meeting of the American College of Cardiology.
Forty-seven percent of patients had an endovascular procedure, 42% had surgery, and the remainder had hybrid procedures in which both endovascular and surgical interventions took place during the same admission. Patients with hybrid procedures contributed data to both treatment groups.
In-hospital mortality occurred in 2.5% of patients.
Of the patients who survived to discharge, 21,589, or 17.4%, were readmitted within 30 days. The early readmission rate was higher following endovascular procedures, at 18.7%, than the 16.1% rate in the surgical group. The average cost of a readmission was $15,876. Death during readmission occurred in 4.2% of patients.
The median rate ratio – a measure of the amount of variance in readmission rates between hospitals – was 1.12. That’s a low figure.
“If the median rate ratio is lower, like here, it says there’s not a lot of interhospital variability across the country. So overall this burden seems to be pretty uniform across the institutions included in our analysis,” Dr. Secemsky explained.
This observation drew the attention of session comoderator Naomi M. Hamburg, MD.
“It’s interesting that you didn’t see a lot of heterogeneity across hospitals, because we often think of readmissions as a potentially modifiable quality metric. Do you think it’s modifiable, or is this just the nature of the disease?” asked Dr. Hamburg of Boston Medical Center.
It’s the disease process, Dr. Secemsky replied.
“We were surprised by the lack of hospital variation,” he added. None of the institutional characteristics examined, including teaching hospital status, bed size, and procedural volume, had a significant impact on readmission rates.
But that doesn’t mean there aren’t opportunities to whittle down those readmissions, according to Dr. Secemsky.
He noted that the high readmission rates were driven by procedural complications such as graft or stent failure. Indeed, procedural complications accounted for 29% of all early readmissions. The procedural complication rate was about 20% following endovascular procedures and 39% after surgery. It’s likely that identification and implementation of best practices could trim those high rates. Unfortunately, however, the nationwide database relies upon ICD-9 codes, which don’t provide the granular level of detail required to home in on specific best practices. That will require further studies, according to Dr. Secemsky.
A distant second on the list of causes of early readmission was peripheral atherosclerosis, meaning persistent claudication or rest pain. This accounted for 8.8% of readmissions. Rounding out the top five causes of readmission were sepsis, which was the reason for 6.7% of readmissions; diabetes with complications, at 4.7%; and heart failure, at 4.6%.
The strongest predictors of readmission included having renal disease at baseline, Medicare rather than private insurance, and discharge to a subacute nursing facility or home with home care.
Dr. Hamburg commented that a focus on reducing readmissions for sepsis as well as for skin and soft tissue infections, which accounted for 2.1% of 30-day hospitalizations, could be fruitful.
Dr. Secemsky reported having no financial conflicts regarding his study.
WASHINGTON – More than one in six patients who undergo a lower extremity arterial endovascular or surgical procedure are readmitted within 30 days, according to a large national study.
The annual total cost of these early readmissions is high, in excess of $360 million. But because there turned out to be surprisingly little difference in readmission rates between hospitals, 30-day readmissions may not be a rational quality measure on which to base institutional reimbursement or withholding of payment for peripheral arterial interventions, Eric A. Secemsky, MD, said at the annual meeting of the American College of Cardiology.
Forty-seven percent of patients had an endovascular procedure, 42% had surgery, and the remainder had hybrid procedures in which both endovascular and surgical interventions took place during the same admission. Patients with hybrid procedures contributed data to both treatment groups.
In-hospital mortality occurred in 2.5% of patients.
Of the patients who survived to discharge, 21,589, or 17.4%, were readmitted within 30 days. The early readmission rate was higher following endovascular procedures, at 18.7%, than the 16.1% rate in the surgical group. The average cost of a readmission was $15,876. Death during readmission occurred in 4.2% of patients.
The median rate ratio – a measure of the amount of variance in readmission rates between hospitals – was 1.12. That’s a low figure.
“If the median rate ratio is lower, like here, it says there’s not a lot of interhospital variability across the country. So overall this burden seems to be pretty uniform across the institutions included in our analysis,” Dr. Secemsky explained.
This observation drew the attention of session comoderator Naomi M. Hamburg, MD.
“It’s interesting that you didn’t see a lot of heterogeneity across hospitals, because we often think of readmissions as a potentially modifiable quality metric. Do you think it’s modifiable, or is this just the nature of the disease?” asked Dr. Hamburg of Boston Medical Center.
It’s the disease process, Dr. Secemsky replied.
“We were surprised by the lack of hospital variation,” he added. None of the institutional characteristics examined, including teaching hospital status, bed size, and procedural volume, had a significant impact on readmission rates.
But that doesn’t mean there aren’t opportunities to whittle down those readmissions, according to Dr. Secemsky.
He noted that the high readmission rates were driven by procedural complications such as graft or stent failure. Indeed, procedural complications accounted for 29% of all early readmissions. The procedural complication rate was about 20% following endovascular procedures and 39% after surgery. It’s likely that identification and implementation of best practices could trim those high rates. Unfortunately, however, the nationwide database relies upon ICD-9 codes, which don’t provide the granular level of detail required to home in on specific best practices. That will require further studies, according to Dr. Secemsky.
A distant second on the list of causes of early readmission was peripheral atherosclerosis, meaning persistent claudication or rest pain. This accounted for 8.8% of readmissions. Rounding out the top five causes of readmission were sepsis, which was the reason for 6.7% of readmissions; diabetes with complications, at 4.7%; and heart failure, at 4.6%.
The strongest predictors of readmission included having renal disease at baseline, Medicare rather than private insurance, and discharge to a subacute nursing facility or home with home care.
Dr. Hamburg commented that a focus on reducing readmissions for sepsis as well as for skin and soft tissue infections, which accounted for 2.1% of 30-day hospitalizations, could be fruitful.
Dr. Secemsky reported having no financial conflicts regarding his study.
WASHINGTON – More than one in six patients who undergo a lower extremity arterial endovascular or surgical procedure are readmitted within 30 days, according to a large national study.
The annual total cost of these early readmissions is high, in excess of $360 million. But because there turned out to be surprisingly little difference in readmission rates between hospitals, 30-day readmissions may not be a rational quality measure on which to base institutional reimbursement or withholding of payment for peripheral arterial interventions, Eric A. Secemsky, MD, said at the annual meeting of the American College of Cardiology.
Forty-seven percent of patients had an endovascular procedure, 42% had surgery, and the remainder had hybrid procedures in which both endovascular and surgical interventions took place during the same admission. Patients with hybrid procedures contributed data to both treatment groups.
In-hospital mortality occurred in 2.5% of patients.
Of the patients who survived to discharge, 21,589, or 17.4%, were readmitted within 30 days. The early readmission rate was higher following endovascular procedures, at 18.7%, than the 16.1% rate in the surgical group. The average cost of a readmission was $15,876. Death during readmission occurred in 4.2% of patients.
The median rate ratio – a measure of the amount of variance in readmission rates between hospitals – was 1.12. That’s a low figure.
“If the median rate ratio is lower, like here, it says there’s not a lot of interhospital variability across the country. So overall this burden seems to be pretty uniform across the institutions included in our analysis,” Dr. Secemsky explained.
This observation drew the attention of session comoderator Naomi M. Hamburg, MD.
“It’s interesting that you didn’t see a lot of heterogeneity across hospitals, because we often think of readmissions as a potentially modifiable quality metric. Do you think it’s modifiable, or is this just the nature of the disease?” asked Dr. Hamburg of Boston Medical Center.
It’s the disease process, Dr. Secemsky replied.
“We were surprised by the lack of hospital variation,” he added. None of the institutional characteristics examined, including teaching hospital status, bed size, and procedural volume, had a significant impact on readmission rates.
But that doesn’t mean there aren’t opportunities to whittle down those readmissions, according to Dr. Secemsky.
He noted that the high readmission rates were driven by procedural complications such as graft or stent failure. Indeed, procedural complications accounted for 29% of all early readmissions. The procedural complication rate was about 20% following endovascular procedures and 39% after surgery. It’s likely that identification and implementation of best practices could trim those high rates. Unfortunately, however, the nationwide database relies upon ICD-9 codes, which don’t provide the granular level of detail required to home in on specific best practices. That will require further studies, according to Dr. Secemsky.
A distant second on the list of causes of early readmission was peripheral atherosclerosis, meaning persistent claudication or rest pain. This accounted for 8.8% of readmissions. Rounding out the top five causes of readmission were sepsis, which was the reason for 6.7% of readmissions; diabetes with complications, at 4.7%; and heart failure, at 4.6%.
The strongest predictors of readmission included having renal disease at baseline, Medicare rather than private insurance, and discharge to a subacute nursing facility or home with home care.
Dr. Hamburg commented that a focus on reducing readmissions for sepsis as well as for skin and soft tissue infections, which accounted for 2.1% of 30-day hospitalizations, could be fruitful.
Dr. Secemsky reported having no financial conflicts regarding his study.
AT ACC 2017
Key clinical point:
Major finding: Readmission within 30 days after a peripheral arterial procedure occurred nationally in 17.4% of patients, with little between-hospital variation in rates.
Data source: A retrospective analysis of nearly 124,000 hospital admissions for lower extremity arterial endovascular or surgical procedures.
Disclosures: The study presenter reported having no financial conflicts of interest.
Observation works for most smaller splanchnic artery aneurysms
CHICAGO – Guidelines for the management of splanchnic artery aneurysms have been hard to come by because of their rarity, but investigators at Massachusetts General Hospital and Harvard Medical School, both in Boston, have surveyed their 20-year experience to conclude that surveillance is appropriate for most cases of aneurysms smaller than 25 mm, and selective open or endovascular repair is indicated for larger lesions, depending on their location.
“Most of the small splanchnic artery aneurysms (SAAs) of less than 25 mm did not grow or rupture over time and can be observed with axial imaging every 3 years,” Mark F. Conrad, MD, reported at a symposium on vascular surgery sponsored by Northwestern University.
The predominant sites of aneurysm were the splenic artery (95, 36%) and the celiac artery (78, 30%), followed by the hepatic artery (34, 13%), pancreaticoduodenal artery (PDA; 25, 9.6%), superior mesenteric artery (SMA; 17, 6%), gastroduodenal artery (GDA; 11, 4%), jejunal artery (3, 1%) and inferior mesenteric artery (1, 0.4%).
Surveillance consisted of imaging every 3 years. Of the surveillance cohort, 138 patients had longer-term follow-up. The average aneurysm size was 16.3 mm, “so they’re small,” Dr. Conrad said. Of that whole group, only 12 (9%), of SAAs grew in size, and of those, 8 were 25 mm or smaller when they were identified; 8 of the 12 required repair. “The average time to repair was 2 years,” Dr. Conrad said. “There were no ruptures in the surveillance cohort.”
Among the early repair group, 13 (14.7%) had rupture upon presentation, 3 of which (23%) were pseudoaneurysms. The majority of aneurysms in this group were in either the splenic artery, PDA, or GDA. “Their average size was 31 mm – much larger than the patients that we watched,” he said. A total of 70% of all repairs were endovascular in nature, the remainder open, but endovascular comprised a higher percentage of rupture repairs: 10 (77%) vs. 3 (23%) that underwent open procedures.
The outcomes for endovascular and open repair were similar based on the small number of subjects, Dr. Conrad said: 30-day morbidity of 17% for endovascular repair and 22.2% for open; and 30-day mortality of 3.5% and 4.5%, respectively. However, for ruptured lesions, the outcomes were starkly significant: 54% morbidity and 8% mortality at 30 days.
The researchers performed a univariate analysis of predictors for aneurysm. They were aneurysm size with an odds ratio of 1.04 for every 1 mm of growth; PDA or GDA lesions with an OR of 11.2; and Ehlers-Danlos type IV syndrome with an OR of 32.5. The latter included all the three study patients with Ehlers-Danlos syndrome.
Among patients who had splenic SAAs, 99 (93%) were asymptomatic and 5 (5.3%) had pseudoaneurysm, and almost half (47) went into surveillance. Over a mean observation period of 35 months, six (12.8%) grew in size, comprising half of the growing SAAs in the observation group. Thirty-two had endovascular repair and four open repair, with a 30-day morbidity of 22% and 30-day mortality of 2.7%.
Celiac SAAs proved most problematic in terms of symptomatology; all 78 patients with this variant were asymptomatic, and 12 (15%) had dissection. Sixty patients went into surveillance with a mean time of 43 months, and three (5) had aneurysms that grew in size. Five had intervention, four with open repair, with 30-day morbidity of 20% and no 30-day mortality.
Hepatic SAAs affected 34 study subjects, 29 (85%) of whom were asymptomatic, 4 (15%) who had dissection, and 7 (21%) with pseudoaneurysm. Eleven entered surveillance for an average of 28 months, but none showed any aneurysmal growth. The 16 who had intervention were evenly split between open and endovascular repair with 30-day morbidity of 25% and 30-day morality of 12.5%.
The PDA and GDA aneurysms “are really interesting,” Dr. Conrad said. “I think they’re different in nature than the other aneurysms,” he said, noting that 12 (33%) of these aneurysms were symptomatic and 6 (17%) were pseudoaneurysms. Because of the high rate of rupture of PDA/GDA aneurysms, Dr. Conrad advised repair at diagnosis: “97% of these patients had a celiac stenosis, and of those, two-thirds were atherosclerosis related and one-third related to the median arcuate ligament compression.” The rupture rate was comparatively high – 20%. Twenty cases underwent endovascular repair with a 90% success rate while four cases had open repair. Thirty-day morbidity for intact lesions was 11% with no deaths, and 50% with 14% mortality rate for ruptured lesions.
Of the SMA aneurysms in the study population, only 17% were mycotic with the remainder asymptomatic, Dr. Conrad said. Nine underwent surveillance, with one growing in size over a mean observation period of 28 months, four had open repair, and two endovascular repair. Morbidity was 17% at 30 days with no deaths.
The guidelines Dr. Conrad and his group developed recommend treatment for symptomatic patients and a more nuanced approach for asymptomatic patients, depending on the location and size of SAA. All lesions 25 mm or smaller, except those of the PDA/GDA, can be observed with axial imaging every 3 years, he said; intervention is indicated for all larger lesions. Endovascular repair is in order for all splenic SAAs in pregnancy, liver transplantation, and pseudoaneurysm. For hepatic SAAs, open or endovascular repair is indicated for pseudoaneurysm, but open repair only is indicated for asymptomatic celiac SAAs with pseudoaneurysm. Endovascular intervention can address most SAA aneurysms of the PDA and GDA.
Dr. Conrad disclosed he is a consultant to Medtronic and Volcano and is a member of Bard’s clinical events committee.
CHICAGO – Guidelines for the management of splanchnic artery aneurysms have been hard to come by because of their rarity, but investigators at Massachusetts General Hospital and Harvard Medical School, both in Boston, have surveyed their 20-year experience to conclude that surveillance is appropriate for most cases of aneurysms smaller than 25 mm, and selective open or endovascular repair is indicated for larger lesions, depending on their location.
“Most of the small splanchnic artery aneurysms (SAAs) of less than 25 mm did not grow or rupture over time and can be observed with axial imaging every 3 years,” Mark F. Conrad, MD, reported at a symposium on vascular surgery sponsored by Northwestern University.
The predominant sites of aneurysm were the splenic artery (95, 36%) and the celiac artery (78, 30%), followed by the hepatic artery (34, 13%), pancreaticoduodenal artery (PDA; 25, 9.6%), superior mesenteric artery (SMA; 17, 6%), gastroduodenal artery (GDA; 11, 4%), jejunal artery (3, 1%) and inferior mesenteric artery (1, 0.4%).
Surveillance consisted of imaging every 3 years. Of the surveillance cohort, 138 patients had longer-term follow-up. The average aneurysm size was 16.3 mm, “so they’re small,” Dr. Conrad said. Of that whole group, only 12 (9%), of SAAs grew in size, and of those, 8 were 25 mm or smaller when they were identified; 8 of the 12 required repair. “The average time to repair was 2 years,” Dr. Conrad said. “There were no ruptures in the surveillance cohort.”
Among the early repair group, 13 (14.7%) had rupture upon presentation, 3 of which (23%) were pseudoaneurysms. The majority of aneurysms in this group were in either the splenic artery, PDA, or GDA. “Their average size was 31 mm – much larger than the patients that we watched,” he said. A total of 70% of all repairs were endovascular in nature, the remainder open, but endovascular comprised a higher percentage of rupture repairs: 10 (77%) vs. 3 (23%) that underwent open procedures.
The outcomes for endovascular and open repair were similar based on the small number of subjects, Dr. Conrad said: 30-day morbidity of 17% for endovascular repair and 22.2% for open; and 30-day mortality of 3.5% and 4.5%, respectively. However, for ruptured lesions, the outcomes were starkly significant: 54% morbidity and 8% mortality at 30 days.
The researchers performed a univariate analysis of predictors for aneurysm. They were aneurysm size with an odds ratio of 1.04 for every 1 mm of growth; PDA or GDA lesions with an OR of 11.2; and Ehlers-Danlos type IV syndrome with an OR of 32.5. The latter included all the three study patients with Ehlers-Danlos syndrome.
Among patients who had splenic SAAs, 99 (93%) were asymptomatic and 5 (5.3%) had pseudoaneurysm, and almost half (47) went into surveillance. Over a mean observation period of 35 months, six (12.8%) grew in size, comprising half of the growing SAAs in the observation group. Thirty-two had endovascular repair and four open repair, with a 30-day morbidity of 22% and 30-day mortality of 2.7%.
Celiac SAAs proved most problematic in terms of symptomatology; all 78 patients with this variant were asymptomatic, and 12 (15%) had dissection. Sixty patients went into surveillance with a mean time of 43 months, and three (5) had aneurysms that grew in size. Five had intervention, four with open repair, with 30-day morbidity of 20% and no 30-day mortality.
Hepatic SAAs affected 34 study subjects, 29 (85%) of whom were asymptomatic, 4 (15%) who had dissection, and 7 (21%) with pseudoaneurysm. Eleven entered surveillance for an average of 28 months, but none showed any aneurysmal growth. The 16 who had intervention were evenly split between open and endovascular repair with 30-day morbidity of 25% and 30-day morality of 12.5%.
The PDA and GDA aneurysms “are really interesting,” Dr. Conrad said. “I think they’re different in nature than the other aneurysms,” he said, noting that 12 (33%) of these aneurysms were symptomatic and 6 (17%) were pseudoaneurysms. Because of the high rate of rupture of PDA/GDA aneurysms, Dr. Conrad advised repair at diagnosis: “97% of these patients had a celiac stenosis, and of those, two-thirds were atherosclerosis related and one-third related to the median arcuate ligament compression.” The rupture rate was comparatively high – 20%. Twenty cases underwent endovascular repair with a 90% success rate while four cases had open repair. Thirty-day morbidity for intact lesions was 11% with no deaths, and 50% with 14% mortality rate for ruptured lesions.
Of the SMA aneurysms in the study population, only 17% were mycotic with the remainder asymptomatic, Dr. Conrad said. Nine underwent surveillance, with one growing in size over a mean observation period of 28 months, four had open repair, and two endovascular repair. Morbidity was 17% at 30 days with no deaths.
The guidelines Dr. Conrad and his group developed recommend treatment for symptomatic patients and a more nuanced approach for asymptomatic patients, depending on the location and size of SAA. All lesions 25 mm or smaller, except those of the PDA/GDA, can be observed with axial imaging every 3 years, he said; intervention is indicated for all larger lesions. Endovascular repair is in order for all splenic SAAs in pregnancy, liver transplantation, and pseudoaneurysm. For hepatic SAAs, open or endovascular repair is indicated for pseudoaneurysm, but open repair only is indicated for asymptomatic celiac SAAs with pseudoaneurysm. Endovascular intervention can address most SAA aneurysms of the PDA and GDA.
Dr. Conrad disclosed he is a consultant to Medtronic and Volcano and is a member of Bard’s clinical events committee.
CHICAGO – Guidelines for the management of splanchnic artery aneurysms have been hard to come by because of their rarity, but investigators at Massachusetts General Hospital and Harvard Medical School, both in Boston, have surveyed their 20-year experience to conclude that surveillance is appropriate for most cases of aneurysms smaller than 25 mm, and selective open or endovascular repair is indicated for larger lesions, depending on their location.
“Most of the small splanchnic artery aneurysms (SAAs) of less than 25 mm did not grow or rupture over time and can be observed with axial imaging every 3 years,” Mark F. Conrad, MD, reported at a symposium on vascular surgery sponsored by Northwestern University.
The predominant sites of aneurysm were the splenic artery (95, 36%) and the celiac artery (78, 30%), followed by the hepatic artery (34, 13%), pancreaticoduodenal artery (PDA; 25, 9.6%), superior mesenteric artery (SMA; 17, 6%), gastroduodenal artery (GDA; 11, 4%), jejunal artery (3, 1%) and inferior mesenteric artery (1, 0.4%).
Surveillance consisted of imaging every 3 years. Of the surveillance cohort, 138 patients had longer-term follow-up. The average aneurysm size was 16.3 mm, “so they’re small,” Dr. Conrad said. Of that whole group, only 12 (9%), of SAAs grew in size, and of those, 8 were 25 mm or smaller when they were identified; 8 of the 12 required repair. “The average time to repair was 2 years,” Dr. Conrad said. “There were no ruptures in the surveillance cohort.”
Among the early repair group, 13 (14.7%) had rupture upon presentation, 3 of which (23%) were pseudoaneurysms. The majority of aneurysms in this group were in either the splenic artery, PDA, or GDA. “Their average size was 31 mm – much larger than the patients that we watched,” he said. A total of 70% of all repairs were endovascular in nature, the remainder open, but endovascular comprised a higher percentage of rupture repairs: 10 (77%) vs. 3 (23%) that underwent open procedures.
The outcomes for endovascular and open repair were similar based on the small number of subjects, Dr. Conrad said: 30-day morbidity of 17% for endovascular repair and 22.2% for open; and 30-day mortality of 3.5% and 4.5%, respectively. However, for ruptured lesions, the outcomes were starkly significant: 54% morbidity and 8% mortality at 30 days.
The researchers performed a univariate analysis of predictors for aneurysm. They were aneurysm size with an odds ratio of 1.04 for every 1 mm of growth; PDA or GDA lesions with an OR of 11.2; and Ehlers-Danlos type IV syndrome with an OR of 32.5. The latter included all the three study patients with Ehlers-Danlos syndrome.
Among patients who had splenic SAAs, 99 (93%) were asymptomatic and 5 (5.3%) had pseudoaneurysm, and almost half (47) went into surveillance. Over a mean observation period of 35 months, six (12.8%) grew in size, comprising half of the growing SAAs in the observation group. Thirty-two had endovascular repair and four open repair, with a 30-day morbidity of 22% and 30-day mortality of 2.7%.
Celiac SAAs proved most problematic in terms of symptomatology; all 78 patients with this variant were asymptomatic, and 12 (15%) had dissection. Sixty patients went into surveillance with a mean time of 43 months, and three (5) had aneurysms that grew in size. Five had intervention, four with open repair, with 30-day morbidity of 20% and no 30-day mortality.
Hepatic SAAs affected 34 study subjects, 29 (85%) of whom were asymptomatic, 4 (15%) who had dissection, and 7 (21%) with pseudoaneurysm. Eleven entered surveillance for an average of 28 months, but none showed any aneurysmal growth. The 16 who had intervention were evenly split between open and endovascular repair with 30-day morbidity of 25% and 30-day morality of 12.5%.
The PDA and GDA aneurysms “are really interesting,” Dr. Conrad said. “I think they’re different in nature than the other aneurysms,” he said, noting that 12 (33%) of these aneurysms were symptomatic and 6 (17%) were pseudoaneurysms. Because of the high rate of rupture of PDA/GDA aneurysms, Dr. Conrad advised repair at diagnosis: “97% of these patients had a celiac stenosis, and of those, two-thirds were atherosclerosis related and one-third related to the median arcuate ligament compression.” The rupture rate was comparatively high – 20%. Twenty cases underwent endovascular repair with a 90% success rate while four cases had open repair. Thirty-day morbidity for intact lesions was 11% with no deaths, and 50% with 14% mortality rate for ruptured lesions.
Of the SMA aneurysms in the study population, only 17% were mycotic with the remainder asymptomatic, Dr. Conrad said. Nine underwent surveillance, with one growing in size over a mean observation period of 28 months, four had open repair, and two endovascular repair. Morbidity was 17% at 30 days with no deaths.
The guidelines Dr. Conrad and his group developed recommend treatment for symptomatic patients and a more nuanced approach for asymptomatic patients, depending on the location and size of SAA. All lesions 25 mm or smaller, except those of the PDA/GDA, can be observed with axial imaging every 3 years, he said; intervention is indicated for all larger lesions. Endovascular repair is in order for all splenic SAAs in pregnancy, liver transplantation, and pseudoaneurysm. For hepatic SAAs, open or endovascular repair is indicated for pseudoaneurysm, but open repair only is indicated for asymptomatic celiac SAAs with pseudoaneurysm. Endovascular intervention can address most SAA aneurysms of the PDA and GDA.
Dr. Conrad disclosed he is a consultant to Medtronic and Volcano and is a member of Bard’s clinical events committee.
AT THE NORTHWESTERN VASCULAR SYMPOSIUM
Key clinical point: Surveillance imaging every three years may be adequate to manage splanchnic artery aneurysms (SAA) smaller than 25 mm, because they rarely expand significantly.
Major finding: In the surveillance group that had long-term follow-up, 9% had SAAs that grew in size.
Data source: Analysis of 250 patients with 264 SAAs during 1994-2014 in the Research Patient Data Registry at Massachusetts General Hospital.
Disclosures: Dr. Conrad disclosed he is a consultant to Medtronic and Volcano and is a member of Bard’s clinical events committee.
Point/Counterpoint: Is endograft PAA repair durable?
Endovascular repair is durable
Endovascular repair of popliteal artery aneurysms is vastly superior to all other previous techniques of popliteal aneurysm repair. Half of all popliteal artery aneurysms are bilateral, and 40% are associated with abdominal aortic aneurysm; 1%-2% of patients with abdominal aortic aneurysm have a popliteal aneurysm (ANZ J Surg. 2006 Oct;76[10]:912-5). Less than 0.01% of hospitalized patients have popliteal artery aneurysms, and men are 20 times more prone to them than women are.
Traditional treatment involves either bypass with interval ligation or a direct posterior approach with an interposition graft, but surgery is not without its problems. I think of the retired anesthesiologist who came to me with a popliteal artery aneurysm (PAA) that his primary care doctor diagnosed. “I’m not having any damn femoral popliteal bypass operation,” he told me. “Every single one of those patients dies.”
Endograft repair is a technique that is reaching its prime, as a growing number of reports have shown – although none of these studies has large numbers because the volume just isn’t available. One recent paper compared 52 open and 23 endovascular PAA repairs (Ann Vasc Surg. 2016 Jan;30:253-7) and found both had similarly high rates of reintervention – 50% at 4 years. But it is noteworthy that the endovascular results improved with time.
A University of Pittsburgh study of 186 open and endovascular repairs found that patients with acute presentations of embolization or aneurysm thrombosis did better with open surgery. In addition, while open repair had superior patency initially after surgery, midterm secondary patency and amputation rates of open and endovascular repair were similar (J Vasc Surg. 2016 Jan;63[1]:70-6).
A Netherlands study of 72 PAA treated with endografting showed that 84% had primary patency at 1 year, and 74% had assisted primary patency at 3 years (Eur J Vasc Endovasc Surg. 2016 Jul;52[1]:99-104). Among these patients, 13 had late occlusions, 7 were converted to bypass, and 2 required thrombolysis; but none required limb amputation.
A meta-analysis of 540 patients found no statistically significant difference in outcomes between endovascular and open repair for PAA (Eur J Vasc Endovasc Surg. 2015 Sep;50(3):351-9). Another systematic review and meta-analysis of 14 studies and 514 patients also found no difference in pooled primary and secondary patency at 5 years (J Endovasc Ther. 2015 Jun;22[3]:330-7).
There certainly are contradictory studies, such as one by Dr. Alik Farber’s group in Boston that showed open repair is superior to endovascular surgery (J Vasc Surg. 2015 Mar;61[3]:663-9); but retrospective database mining certainly has its limitations. Their retrospective study queried the Vascular Quality Initiative database and found that 95% of patients who had open elective popliteal aneurysm repair were free from major adverse limb events, vs. 80% for endovascular treatments.
The best outcomes of open repair happen with autologous vein, but there is precious little of that around now. Emergency patients would probably do better with open surgery, but in elective repair there is no clear differential data.
So, if that’s the case, I’m going to take the small incision.
Peter Rossi, MD, FACS, is an associate professor of surgery and radiology, and the clinical director of vascular surgery, at the Medical College of Wisconsin, Milwaukee. He is also on staff at Clement J. Zablocki Veterans Affairs Medical Center in Milwaukee. Dr. Rossi had no financial relationships to disclose.
Endovascular repair may not be durable
Debating the durability of elective endovascular repair of popliteal artery aneurysm raises a question: Who determines durability anyway?
Is it the patients who only want the Band-Aid and no incision? I don’t think so. Is it the interventionalist who only does endovascular repairs? I don’t think so. I’m sure it’s not the insurance companies, who only worry about cost containment, either.
So, who should determine durability of endovascular popliteal artery aneurysm (PAA) repair?
So, the question is, do we have such data?
There are multiple reports looking at how well open repair works. It has been done for decades. In 2008, a Veterans Affairs study of 583 open PAA repairs reported low death rates and excellent rates of limb salvage at 2 years, even in high-risk patients (J Vasc Surg. 2008 Oct;48[4]:845-51). Open surgical repair has excellent documented durability, and that is not the question at hand.
Endovascular repair has some presumed advantages. It’s less invasive and involves less postoperative pain and a quicker recovery. But it is not without problems – graft thrombosis and occlusion, endoleaks, distal limb ischemia, and stent fractures among them.
Surgery, to be clear, is not perfect, either. One of my patients who years ago presented with an occluded PAA underwent open bypass repair – but then went on later to have a pseudoaneurysm of the proximal anastomosis. I repaired this with an endograft, and he has done quite well. So, we all do endograft repairs, walk out, chest bump the Gore rep, and send the patient home that day.
Is it durable, though?
Most of the data on endovascular repair are from single-center studies dating back to 2003. There’s only one prospective trial comparing endovascular vs. open repair (J Vasc Surg. 2005 Aug;42[2]:185-93), but it was a single-center trial with a severe power limitation, because it involved only 30 patients. It found endovascular repair was comparable to open surgery. Also, I suspect a great deal of selection bias is involved in studies of endovascular repair.
A number of studies have found endovascular repair is not inferior to surgical repair. For example, a study by Dr. Audra Duncan, at Mayo Clinic, and her colleagues found that primary and secondary patency rates of elective and emergent stenting were excellent – but the study results only extended out to 2 years (J Vasc Surg. 2013 May;57[5]:1299-305). I don’t think we could hang our hat on that.
A Swedish study that compared open and endovascular surgery in 592 patients reported that endovascular repair has “significantly inferior results compared with open repair,” particularly in those who present with acute ischemia (Eur J Vasc Endovasc Surg. 2015 Sep;50[3]:342-50). A close look at the data shows that primary patency rates were 89% for open repair and 67.4% for stent graft.
Referencing the systematic review and meta-analysis that Dr. Rossi cited, the primary patency of endovascular repair was only 69% and the secondary patency rate was 77% at 5 years (J Endovasc Ther. 2015 Jun;22[3]:330-7). As physicians, I submit that we can do better.
A Netherlands study investigated stent fractures, finding that 17% (13 out of 78 cases) had circumferential fractures (J Vasc Surg. 2010 Jun;51[6]:1413-8). This study only included circumferential stent fractures and excluded localized strut fractures. I think these studies show that endovascular repair is not always durable.
I want to remind you that we are vascular surgeons, so it is appropriate for us to embrace surgical bypass and its known durability, especially when the durability of endovascular repair is still not known.
Patrick Muck, MD, is chief of vascular surgery and director of vascular residency and fellowship at Good Samaritan Hospital, Cincinnati. He is also on staff at Bethesda North Hospital, Cincinnati, and is affiliated with TriHealth Heart Institute in southwestern Ohio. Dr. Muck had no financial relationships to disclose.
Endovascular repair is durable
Endovascular repair of popliteal artery aneurysms is vastly superior to all other previous techniques of popliteal aneurysm repair. Half of all popliteal artery aneurysms are bilateral, and 40% are associated with abdominal aortic aneurysm; 1%-2% of patients with abdominal aortic aneurysm have a popliteal aneurysm (ANZ J Surg. 2006 Oct;76[10]:912-5). Less than 0.01% of hospitalized patients have popliteal artery aneurysms, and men are 20 times more prone to them than women are.
Traditional treatment involves either bypass with interval ligation or a direct posterior approach with an interposition graft, but surgery is not without its problems. I think of the retired anesthesiologist who came to me with a popliteal artery aneurysm (PAA) that his primary care doctor diagnosed. “I’m not having any damn femoral popliteal bypass operation,” he told me. “Every single one of those patients dies.”
Endograft repair is a technique that is reaching its prime, as a growing number of reports have shown – although none of these studies has large numbers because the volume just isn’t available. One recent paper compared 52 open and 23 endovascular PAA repairs (Ann Vasc Surg. 2016 Jan;30:253-7) and found both had similarly high rates of reintervention – 50% at 4 years. But it is noteworthy that the endovascular results improved with time.
A University of Pittsburgh study of 186 open and endovascular repairs found that patients with acute presentations of embolization or aneurysm thrombosis did better with open surgery. In addition, while open repair had superior patency initially after surgery, midterm secondary patency and amputation rates of open and endovascular repair were similar (J Vasc Surg. 2016 Jan;63[1]:70-6).
A Netherlands study of 72 PAA treated with endografting showed that 84% had primary patency at 1 year, and 74% had assisted primary patency at 3 years (Eur J Vasc Endovasc Surg. 2016 Jul;52[1]:99-104). Among these patients, 13 had late occlusions, 7 were converted to bypass, and 2 required thrombolysis; but none required limb amputation.
A meta-analysis of 540 patients found no statistically significant difference in outcomes between endovascular and open repair for PAA (Eur J Vasc Endovasc Surg. 2015 Sep;50(3):351-9). Another systematic review and meta-analysis of 14 studies and 514 patients also found no difference in pooled primary and secondary patency at 5 years (J Endovasc Ther. 2015 Jun;22[3]:330-7).
There certainly are contradictory studies, such as one by Dr. Alik Farber’s group in Boston that showed open repair is superior to endovascular surgery (J Vasc Surg. 2015 Mar;61[3]:663-9); but retrospective database mining certainly has its limitations. Their retrospective study queried the Vascular Quality Initiative database and found that 95% of patients who had open elective popliteal aneurysm repair were free from major adverse limb events, vs. 80% for endovascular treatments.
The best outcomes of open repair happen with autologous vein, but there is precious little of that around now. Emergency patients would probably do better with open surgery, but in elective repair there is no clear differential data.
So, if that’s the case, I’m going to take the small incision.
Peter Rossi, MD, FACS, is an associate professor of surgery and radiology, and the clinical director of vascular surgery, at the Medical College of Wisconsin, Milwaukee. He is also on staff at Clement J. Zablocki Veterans Affairs Medical Center in Milwaukee. Dr. Rossi had no financial relationships to disclose.
Endovascular repair may not be durable
Debating the durability of elective endovascular repair of popliteal artery aneurysm raises a question: Who determines durability anyway?
Is it the patients who only want the Band-Aid and no incision? I don’t think so. Is it the interventionalist who only does endovascular repairs? I don’t think so. I’m sure it’s not the insurance companies, who only worry about cost containment, either.
So, who should determine durability of endovascular popliteal artery aneurysm (PAA) repair?
So, the question is, do we have such data?
There are multiple reports looking at how well open repair works. It has been done for decades. In 2008, a Veterans Affairs study of 583 open PAA repairs reported low death rates and excellent rates of limb salvage at 2 years, even in high-risk patients (J Vasc Surg. 2008 Oct;48[4]:845-51). Open surgical repair has excellent documented durability, and that is not the question at hand.
Endovascular repair has some presumed advantages. It’s less invasive and involves less postoperative pain and a quicker recovery. But it is not without problems – graft thrombosis and occlusion, endoleaks, distal limb ischemia, and stent fractures among them.
Surgery, to be clear, is not perfect, either. One of my patients who years ago presented with an occluded PAA underwent open bypass repair – but then went on later to have a pseudoaneurysm of the proximal anastomosis. I repaired this with an endograft, and he has done quite well. So, we all do endograft repairs, walk out, chest bump the Gore rep, and send the patient home that day.
Is it durable, though?
Most of the data on endovascular repair are from single-center studies dating back to 2003. There’s only one prospective trial comparing endovascular vs. open repair (J Vasc Surg. 2005 Aug;42[2]:185-93), but it was a single-center trial with a severe power limitation, because it involved only 30 patients. It found endovascular repair was comparable to open surgery. Also, I suspect a great deal of selection bias is involved in studies of endovascular repair.
A number of studies have found endovascular repair is not inferior to surgical repair. For example, a study by Dr. Audra Duncan, at Mayo Clinic, and her colleagues found that primary and secondary patency rates of elective and emergent stenting were excellent – but the study results only extended out to 2 years (J Vasc Surg. 2013 May;57[5]:1299-305). I don’t think we could hang our hat on that.
A Swedish study that compared open and endovascular surgery in 592 patients reported that endovascular repair has “significantly inferior results compared with open repair,” particularly in those who present with acute ischemia (Eur J Vasc Endovasc Surg. 2015 Sep;50[3]:342-50). A close look at the data shows that primary patency rates were 89% for open repair and 67.4% for stent graft.
Referencing the systematic review and meta-analysis that Dr. Rossi cited, the primary patency of endovascular repair was only 69% and the secondary patency rate was 77% at 5 years (J Endovasc Ther. 2015 Jun;22[3]:330-7). As physicians, I submit that we can do better.
A Netherlands study investigated stent fractures, finding that 17% (13 out of 78 cases) had circumferential fractures (J Vasc Surg. 2010 Jun;51[6]:1413-8). This study only included circumferential stent fractures and excluded localized strut fractures. I think these studies show that endovascular repair is not always durable.
I want to remind you that we are vascular surgeons, so it is appropriate for us to embrace surgical bypass and its known durability, especially when the durability of endovascular repair is still not known.
Patrick Muck, MD, is chief of vascular surgery and director of vascular residency and fellowship at Good Samaritan Hospital, Cincinnati. He is also on staff at Bethesda North Hospital, Cincinnati, and is affiliated with TriHealth Heart Institute in southwestern Ohio. Dr. Muck had no financial relationships to disclose.
Endovascular repair is durable
Endovascular repair of popliteal artery aneurysms is vastly superior to all other previous techniques of popliteal aneurysm repair. Half of all popliteal artery aneurysms are bilateral, and 40% are associated with abdominal aortic aneurysm; 1%-2% of patients with abdominal aortic aneurysm have a popliteal aneurysm (ANZ J Surg. 2006 Oct;76[10]:912-5). Less than 0.01% of hospitalized patients have popliteal artery aneurysms, and men are 20 times more prone to them than women are.
Traditional treatment involves either bypass with interval ligation or a direct posterior approach with an interposition graft, but surgery is not without its problems. I think of the retired anesthesiologist who came to me with a popliteal artery aneurysm (PAA) that his primary care doctor diagnosed. “I’m not having any damn femoral popliteal bypass operation,” he told me. “Every single one of those patients dies.”
Endograft repair is a technique that is reaching its prime, as a growing number of reports have shown – although none of these studies has large numbers because the volume just isn’t available. One recent paper compared 52 open and 23 endovascular PAA repairs (Ann Vasc Surg. 2016 Jan;30:253-7) and found both had similarly high rates of reintervention – 50% at 4 years. But it is noteworthy that the endovascular results improved with time.
A University of Pittsburgh study of 186 open and endovascular repairs found that patients with acute presentations of embolization or aneurysm thrombosis did better with open surgery. In addition, while open repair had superior patency initially after surgery, midterm secondary patency and amputation rates of open and endovascular repair were similar (J Vasc Surg. 2016 Jan;63[1]:70-6).
A Netherlands study of 72 PAA treated with endografting showed that 84% had primary patency at 1 year, and 74% had assisted primary patency at 3 years (Eur J Vasc Endovasc Surg. 2016 Jul;52[1]:99-104). Among these patients, 13 had late occlusions, 7 were converted to bypass, and 2 required thrombolysis; but none required limb amputation.
A meta-analysis of 540 patients found no statistically significant difference in outcomes between endovascular and open repair for PAA (Eur J Vasc Endovasc Surg. 2015 Sep;50(3):351-9). Another systematic review and meta-analysis of 14 studies and 514 patients also found no difference in pooled primary and secondary patency at 5 years (J Endovasc Ther. 2015 Jun;22[3]:330-7).
There certainly are contradictory studies, such as one by Dr. Alik Farber’s group in Boston that showed open repair is superior to endovascular surgery (J Vasc Surg. 2015 Mar;61[3]:663-9); but retrospective database mining certainly has its limitations. Their retrospective study queried the Vascular Quality Initiative database and found that 95% of patients who had open elective popliteal aneurysm repair were free from major adverse limb events, vs. 80% for endovascular treatments.
The best outcomes of open repair happen with autologous vein, but there is precious little of that around now. Emergency patients would probably do better with open surgery, but in elective repair there is no clear differential data.
So, if that’s the case, I’m going to take the small incision.
Peter Rossi, MD, FACS, is an associate professor of surgery and radiology, and the clinical director of vascular surgery, at the Medical College of Wisconsin, Milwaukee. He is also on staff at Clement J. Zablocki Veterans Affairs Medical Center in Milwaukee. Dr. Rossi had no financial relationships to disclose.
Endovascular repair may not be durable
Debating the durability of elective endovascular repair of popliteal artery aneurysm raises a question: Who determines durability anyway?
Is it the patients who only want the Band-Aid and no incision? I don’t think so. Is it the interventionalist who only does endovascular repairs? I don’t think so. I’m sure it’s not the insurance companies, who only worry about cost containment, either.
So, who should determine durability of endovascular popliteal artery aneurysm (PAA) repair?
So, the question is, do we have such data?
There are multiple reports looking at how well open repair works. It has been done for decades. In 2008, a Veterans Affairs study of 583 open PAA repairs reported low death rates and excellent rates of limb salvage at 2 years, even in high-risk patients (J Vasc Surg. 2008 Oct;48[4]:845-51). Open surgical repair has excellent documented durability, and that is not the question at hand.
Endovascular repair has some presumed advantages. It’s less invasive and involves less postoperative pain and a quicker recovery. But it is not without problems – graft thrombosis and occlusion, endoleaks, distal limb ischemia, and stent fractures among them.
Surgery, to be clear, is not perfect, either. One of my patients who years ago presented with an occluded PAA underwent open bypass repair – but then went on later to have a pseudoaneurysm of the proximal anastomosis. I repaired this with an endograft, and he has done quite well. So, we all do endograft repairs, walk out, chest bump the Gore rep, and send the patient home that day.
Is it durable, though?
Most of the data on endovascular repair are from single-center studies dating back to 2003. There’s only one prospective trial comparing endovascular vs. open repair (J Vasc Surg. 2005 Aug;42[2]:185-93), but it was a single-center trial with a severe power limitation, because it involved only 30 patients. It found endovascular repair was comparable to open surgery. Also, I suspect a great deal of selection bias is involved in studies of endovascular repair.
A number of studies have found endovascular repair is not inferior to surgical repair. For example, a study by Dr. Audra Duncan, at Mayo Clinic, and her colleagues found that primary and secondary patency rates of elective and emergent stenting were excellent – but the study results only extended out to 2 years (J Vasc Surg. 2013 May;57[5]:1299-305). I don’t think we could hang our hat on that.
A Swedish study that compared open and endovascular surgery in 592 patients reported that endovascular repair has “significantly inferior results compared with open repair,” particularly in those who present with acute ischemia (Eur J Vasc Endovasc Surg. 2015 Sep;50[3]:342-50). A close look at the data shows that primary patency rates were 89% for open repair and 67.4% for stent graft.
Referencing the systematic review and meta-analysis that Dr. Rossi cited, the primary patency of endovascular repair was only 69% and the secondary patency rate was 77% at 5 years (J Endovasc Ther. 2015 Jun;22[3]:330-7). As physicians, I submit that we can do better.
A Netherlands study investigated stent fractures, finding that 17% (13 out of 78 cases) had circumferential fractures (J Vasc Surg. 2010 Jun;51[6]:1413-8). This study only included circumferential stent fractures and excluded localized strut fractures. I think these studies show that endovascular repair is not always durable.
I want to remind you that we are vascular surgeons, so it is appropriate for us to embrace surgical bypass and its known durability, especially when the durability of endovascular repair is still not known.
Patrick Muck, MD, is chief of vascular surgery and director of vascular residency and fellowship at Good Samaritan Hospital, Cincinnati. He is also on staff at Bethesda North Hospital, Cincinnati, and is affiliated with TriHealth Heart Institute in southwestern Ohio. Dr. Muck had no financial relationships to disclose.
AT MIDWESTERN VASCULAR 2016
Bleeding esophageal varices: Who should receive a shunt?
A transjugular intrahepatic portosystemic shunt (TIPS) has been shown in randomized controlled trials to be effective for:
- Secondary prevention of variceal bleeding
- Controlling refractory ascites in patients with liver cirrhosis.
In addition, findings from retrospective case series have suggested that it helps in cases of:
- Acute variceal bleeding refractory to endoscopic therapy
- Gastropathy due to portal hypertension
- Bleeding gastric varices
- Refractory hepatic hydrothorax
- Hepatorenal syndrome
- Budd-Chiari syndrome
- Veno-occlusive disease
- Hepatopulmonary syndrome.
Here, we discuss the indications for a TIPS in cirrhotic patients with esophageal variceal bleeding.
CIRRHOSIS CAN LEAD TO PORTAL HYPERTENSION, BLEEDING
Cirrhosis of the liver alters the hepatic architecture. Development of regenerating nodules and deposition of connective tissue between these nodules increase the resistance to portal blood flow, which can lead to portal hypertension.1
Esophageal variceal bleeding is a complication of portal hypertension and a major cause of death in patients with liver cirrhosis. Combined treatment with vasoactive drugs, prophylactic antibiotics, and endoscopic band ligation is the standard of care for patients with acute bleeding. However, this treatment fails in about 10% to 15% of these patients. A TIPS creates a connection between the portal and hepatic veins, resulting in portal decompression and homeostasis.2
PRE-TIPS EVALUATION
Patients being considered for a TIPS should be medically assessed before the procedure. The workup should include the following:
- Routine blood tests, including blood type and screen (indirect Coombs test), complete blood cell count, basic metabolic panel, liver function tests, prothrombin time, and partial thromboplastin time
- Doppler ultrasonography of the liver to ensure that the portal and hepatic veins are patent
- Echocardiography to assess pulmonary arterial pressure and right-side heart function
- The hepatic venous pressure gradient, which is measured at the time of TIPS placement, reflects the degree of portal hypertension. A hepatic vein is catheterized, and the right atrial pressure or the free hepatic venous pressure is subtracted from the wedged hepatic venous pressure. The gradient is normally 1 to 5 mm Hg. A gradient greater than 5 mm Hg indicates portal hypertension, and esophageal varices may start to bleed when the gradient is greater than 12 mm Hg. The goal of TIPS placement is to reduce the gradient to less than 12 mm Hg, or at least by 50%.
Heart failure is a contraindication
Pulmonary hypertension may follow TIPS placement because the shunt increases venous return to the heart. Additionally, systemic vascular resistance decreases in patients who have a shunt. This further worsens the hyperdynamic circulatory state already present in patients with cirrhosis. Cardiac output increases in response to these changes. When the heart’s ability to handle this “volume overload” is exceeded, pulmonary venous pressures rise, with increasing ventilation-perfusion mismatch, hypoxia, and pulmonary vasoconstriction; pulmonary edema may ensue.
Congestive heart failure, severe tricuspid regurgitation, and severe pulmonary hypertension (mean pulmonary pressures > 45 mm Hg) are therefore considered absolute contraindications to TIPS placement.3,4 This is why echocardiography is recommended to assess pulmonary pressure along with the size and function of the right side of the heart before proceeding with TIPS insertion.
Other considerations
TIPS insertion is not recommended in patients with active hepatic encephalopathy, which should be adequately controlled before insertion of a TIPS. This can be achieved with lactulose and rifaximin. Lactulose is a laxative; the recommended target is 3 to 4 bowel movements daily. Rifaximin is a poorly absorbed antibiotic that has a wide spectrum of coverage, affecting gram-negative and gram-positive aerobes and anaerobes. It wipes out the gut bacteria and so decreases the production of ammonia by the gut.
Paracentesis is recommended before TIPS placement if a large volume of ascites is present. Draining the fluid allows the liver to drop down and makes it easier to access the portal vein from the hepatic vein.
WHEN TO CONSIDER A TIPS IN ESOPHAGEAL VARICEAL BLEEDING
Acute bleeding refractory to endoscopic therapy
A TIPS remains the only choice to control acute variceal bleeding refractory to medical and endoscopic therapy (Figure 1), with a success rate of 90% to 100%.5 The urgency of TIPS placement is an independent predictor of early mortality.
Esophageal variceal rebleeding
Once varices bleed, the risk of rebleeding is higher than 50%, and rebleeding is associated with a high mortality rate. TIPS should be considered if nonselective beta-blockers and surveillance with upper endoscopy and banding fail to prevent rebleeding, with many studies showing a TIPS to be superior to pharmacologic and endoscopic therapies.6
A meta-analysis in 1999 by Papatheodoridis et al6 found that variceal rebleeding was significantly more frequent with endoscopic therapies, at 47% vs 19% with a TIPS, but the incidence of hepatic encephalopathy was higher with TIPS (34% vs 19%; P < .001), and there was no difference in mortality rates.
Hepatic encephalopathy occurs in 15% to 25% of patients after TIPS procedures. Risk factors include advanced age, poor renal function, and a history of hepatic encephalopathy. Hepatic encephalopathy can be managed with lactulose or rifaximin, or both (see above). Narcotics, antihistamines, and benzodiazepines should be avoided. In rare cases (5%) when hepatic encephalopathy is refractory to medical therapy, liver transplant should be considered.
A surgical distal splenorenal shunt is another option for patients with refractory or recurrent variceal bleeding. In a large randomized controlled trial,7 140 cirrhotic patients with recurrent variceal bleeding were randomized to receive either a distal splenorenal shunt or a TIPS. At a mean follow-up of 48 months, there was no difference in the rates of rebleeding between the two groups (5.5% with a surgical shunt vs 10.5% with a TIPS, P = .29) or in hepatic encephalopathy (50% in both groups). Survival rates were comparable between the two groups at 2 years (81% with a surgical shunt vs 88% with a TIPS) and 5 years (62% vs 61%).
Early use of TIPS after first variceal bleeding
In a 2010 randomized controlled trial,8 63 patients with cirrhosis (Child-Pugh class B or C) and acute variceal bleeding who had received standard medical and endoscopic therapy were randomized to receive either a TIPS within 72 hours of admission or long-term conservative treatment with nonselective beta-blockers and endoscopic band ligation. The 1-year actuarial probability of remaining free of rebleeding or failure to control bleeding was 50% in the conservative treatment group vs 97% in the early-TIPS group (P < .001). The 1-year actuarial survival rate was 61% in the conservative treatment group vs 86% in the early-TIPS group (P < .001).
The authors8 concluded that early use of TIPS in patients with cirrhosis and Child-Pugh scores of 7 to 13 who were hospitalized for acute variceal bleeding was associated with significant reductions in rates of treatment failure and mortality.
- Brenner D, Rippe RA. Pathogenesis of hepatic fibrosis. In: Yamada T, Alpers DH, Laine L, Kaplowitz N, Owyang C, Powell DW, editors. Textbook of Gastroenterology. 4th edition. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.
- Bhogal HK, Sanyal AJ. Using transjugular intrahepatic portosystemic shunts for complications of cirrhosis. Clin Gastroenterol Hepatol 2011; 9:936–946.
- Garcia-Tsao G, Sanyal AJ, Grace ND, Carey WD; Practice Guidelines Committee of American Association for Study of Liver Diseases; Practice Parameters Committee of American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Am J Gastroenterol 2007; 102:2086–2102.
- Azoulay D, Castaing D, Dennison A, Martino W, Eyraud D, Bismuth H. Transjugular intrahepatic portosystemic shunt worsens the hyperdynamic circulatory state of the cirrhotic patient: preliminary report of a prospective study. Hepatology 1994; 19:129–132.
- Rodríguez-Laiz JM, Bañares R, Echenagusia A, et al. Effects of transjugular intrahepatic portasystemic shunt (TIPS) on splanchnic and systemic hemodynamics, and hepatic function in patients with portal hypertension. Preliminary results. Dig Dis Sci 1995; 40:2121–2127.
- Papatheodoridis GV, Goulis J, Leandro G, Patch D, Burroughs AK. Transjugular intrahepatic portosystemic shunt compared with endoscopic treatment for prevention of variceal rebleeding: a meta-analysis. Hepatology 1999; 30:612–622.
- Henderson JM, Boyer TD, Kutner MH, et al; DIVERT Study Group. Distal splenorenal shunt versus transjugular intrahepatic portal systemic shunt for variceal bleeding: a randomized trial. Gastroenterology 2006; 130:1643–1651.
- García-Pagán JC, Caca K, Bureau C, et al; Early TIPS (Transjugular Intrahepatic Portosystemic Shunt) Cooperative Study Group. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med 2010; 362:2370–2379.
A transjugular intrahepatic portosystemic shunt (TIPS) has been shown in randomized controlled trials to be effective for:
- Secondary prevention of variceal bleeding
- Controlling refractory ascites in patients with liver cirrhosis.
In addition, findings from retrospective case series have suggested that it helps in cases of:
- Acute variceal bleeding refractory to endoscopic therapy
- Gastropathy due to portal hypertension
- Bleeding gastric varices
- Refractory hepatic hydrothorax
- Hepatorenal syndrome
- Budd-Chiari syndrome
- Veno-occlusive disease
- Hepatopulmonary syndrome.
Here, we discuss the indications for a TIPS in cirrhotic patients with esophageal variceal bleeding.
CIRRHOSIS CAN LEAD TO PORTAL HYPERTENSION, BLEEDING
Cirrhosis of the liver alters the hepatic architecture. Development of regenerating nodules and deposition of connective tissue between these nodules increase the resistance to portal blood flow, which can lead to portal hypertension.1
Esophageal variceal bleeding is a complication of portal hypertension and a major cause of death in patients with liver cirrhosis. Combined treatment with vasoactive drugs, prophylactic antibiotics, and endoscopic band ligation is the standard of care for patients with acute bleeding. However, this treatment fails in about 10% to 15% of these patients. A TIPS creates a connection between the portal and hepatic veins, resulting in portal decompression and homeostasis.2
PRE-TIPS EVALUATION
Patients being considered for a TIPS should be medically assessed before the procedure. The workup should include the following:
- Routine blood tests, including blood type and screen (indirect Coombs test), complete blood cell count, basic metabolic panel, liver function tests, prothrombin time, and partial thromboplastin time
- Doppler ultrasonography of the liver to ensure that the portal and hepatic veins are patent
- Echocardiography to assess pulmonary arterial pressure and right-side heart function
- The hepatic venous pressure gradient, which is measured at the time of TIPS placement, reflects the degree of portal hypertension. A hepatic vein is catheterized, and the right atrial pressure or the free hepatic venous pressure is subtracted from the wedged hepatic venous pressure. The gradient is normally 1 to 5 mm Hg. A gradient greater than 5 mm Hg indicates portal hypertension, and esophageal varices may start to bleed when the gradient is greater than 12 mm Hg. The goal of TIPS placement is to reduce the gradient to less than 12 mm Hg, or at least by 50%.
Heart failure is a contraindication
Pulmonary hypertension may follow TIPS placement because the shunt increases venous return to the heart. Additionally, systemic vascular resistance decreases in patients who have a shunt. This further worsens the hyperdynamic circulatory state already present in patients with cirrhosis. Cardiac output increases in response to these changes. When the heart’s ability to handle this “volume overload” is exceeded, pulmonary venous pressures rise, with increasing ventilation-perfusion mismatch, hypoxia, and pulmonary vasoconstriction; pulmonary edema may ensue.
Congestive heart failure, severe tricuspid regurgitation, and severe pulmonary hypertension (mean pulmonary pressures > 45 mm Hg) are therefore considered absolute contraindications to TIPS placement.3,4 This is why echocardiography is recommended to assess pulmonary pressure along with the size and function of the right side of the heart before proceeding with TIPS insertion.
Other considerations
TIPS insertion is not recommended in patients with active hepatic encephalopathy, which should be adequately controlled before insertion of a TIPS. This can be achieved with lactulose and rifaximin. Lactulose is a laxative; the recommended target is 3 to 4 bowel movements daily. Rifaximin is a poorly absorbed antibiotic that has a wide spectrum of coverage, affecting gram-negative and gram-positive aerobes and anaerobes. It wipes out the gut bacteria and so decreases the production of ammonia by the gut.
Paracentesis is recommended before TIPS placement if a large volume of ascites is present. Draining the fluid allows the liver to drop down and makes it easier to access the portal vein from the hepatic vein.
WHEN TO CONSIDER A TIPS IN ESOPHAGEAL VARICEAL BLEEDING
Acute bleeding refractory to endoscopic therapy
A TIPS remains the only choice to control acute variceal bleeding refractory to medical and endoscopic therapy (Figure 1), with a success rate of 90% to 100%.5 The urgency of TIPS placement is an independent predictor of early mortality.
Esophageal variceal rebleeding
Once varices bleed, the risk of rebleeding is higher than 50%, and rebleeding is associated with a high mortality rate. TIPS should be considered if nonselective beta-blockers and surveillance with upper endoscopy and banding fail to prevent rebleeding, with many studies showing a TIPS to be superior to pharmacologic and endoscopic therapies.6
A meta-analysis in 1999 by Papatheodoridis et al6 found that variceal rebleeding was significantly more frequent with endoscopic therapies, at 47% vs 19% with a TIPS, but the incidence of hepatic encephalopathy was higher with TIPS (34% vs 19%; P < .001), and there was no difference in mortality rates.
Hepatic encephalopathy occurs in 15% to 25% of patients after TIPS procedures. Risk factors include advanced age, poor renal function, and a history of hepatic encephalopathy. Hepatic encephalopathy can be managed with lactulose or rifaximin, or both (see above). Narcotics, antihistamines, and benzodiazepines should be avoided. In rare cases (5%) when hepatic encephalopathy is refractory to medical therapy, liver transplant should be considered.
A surgical distal splenorenal shunt is another option for patients with refractory or recurrent variceal bleeding. In a large randomized controlled trial,7 140 cirrhotic patients with recurrent variceal bleeding were randomized to receive either a distal splenorenal shunt or a TIPS. At a mean follow-up of 48 months, there was no difference in the rates of rebleeding between the two groups (5.5% with a surgical shunt vs 10.5% with a TIPS, P = .29) or in hepatic encephalopathy (50% in both groups). Survival rates were comparable between the two groups at 2 years (81% with a surgical shunt vs 88% with a TIPS) and 5 years (62% vs 61%).
Early use of TIPS after first variceal bleeding
In a 2010 randomized controlled trial,8 63 patients with cirrhosis (Child-Pugh class B or C) and acute variceal bleeding who had received standard medical and endoscopic therapy were randomized to receive either a TIPS within 72 hours of admission or long-term conservative treatment with nonselective beta-blockers and endoscopic band ligation. The 1-year actuarial probability of remaining free of rebleeding or failure to control bleeding was 50% in the conservative treatment group vs 97% in the early-TIPS group (P < .001). The 1-year actuarial survival rate was 61% in the conservative treatment group vs 86% in the early-TIPS group (P < .001).
The authors8 concluded that early use of TIPS in patients with cirrhosis and Child-Pugh scores of 7 to 13 who were hospitalized for acute variceal bleeding was associated with significant reductions in rates of treatment failure and mortality.
A transjugular intrahepatic portosystemic shunt (TIPS) has been shown in randomized controlled trials to be effective for:
- Secondary prevention of variceal bleeding
- Controlling refractory ascites in patients with liver cirrhosis.
In addition, findings from retrospective case series have suggested that it helps in cases of:
- Acute variceal bleeding refractory to endoscopic therapy
- Gastropathy due to portal hypertension
- Bleeding gastric varices
- Refractory hepatic hydrothorax
- Hepatorenal syndrome
- Budd-Chiari syndrome
- Veno-occlusive disease
- Hepatopulmonary syndrome.
Here, we discuss the indications for a TIPS in cirrhotic patients with esophageal variceal bleeding.
CIRRHOSIS CAN LEAD TO PORTAL HYPERTENSION, BLEEDING
Cirrhosis of the liver alters the hepatic architecture. Development of regenerating nodules and deposition of connective tissue between these nodules increase the resistance to portal blood flow, which can lead to portal hypertension.1
Esophageal variceal bleeding is a complication of portal hypertension and a major cause of death in patients with liver cirrhosis. Combined treatment with vasoactive drugs, prophylactic antibiotics, and endoscopic band ligation is the standard of care for patients with acute bleeding. However, this treatment fails in about 10% to 15% of these patients. A TIPS creates a connection between the portal and hepatic veins, resulting in portal decompression and homeostasis.2
PRE-TIPS EVALUATION
Patients being considered for a TIPS should be medically assessed before the procedure. The workup should include the following:
- Routine blood tests, including blood type and screen (indirect Coombs test), complete blood cell count, basic metabolic panel, liver function tests, prothrombin time, and partial thromboplastin time
- Doppler ultrasonography of the liver to ensure that the portal and hepatic veins are patent
- Echocardiography to assess pulmonary arterial pressure and right-side heart function
- The hepatic venous pressure gradient, which is measured at the time of TIPS placement, reflects the degree of portal hypertension. A hepatic vein is catheterized, and the right atrial pressure or the free hepatic venous pressure is subtracted from the wedged hepatic venous pressure. The gradient is normally 1 to 5 mm Hg. A gradient greater than 5 mm Hg indicates portal hypertension, and esophageal varices may start to bleed when the gradient is greater than 12 mm Hg. The goal of TIPS placement is to reduce the gradient to less than 12 mm Hg, or at least by 50%.
Heart failure is a contraindication
Pulmonary hypertension may follow TIPS placement because the shunt increases venous return to the heart. Additionally, systemic vascular resistance decreases in patients who have a shunt. This further worsens the hyperdynamic circulatory state already present in patients with cirrhosis. Cardiac output increases in response to these changes. When the heart’s ability to handle this “volume overload” is exceeded, pulmonary venous pressures rise, with increasing ventilation-perfusion mismatch, hypoxia, and pulmonary vasoconstriction; pulmonary edema may ensue.
Congestive heart failure, severe tricuspid regurgitation, and severe pulmonary hypertension (mean pulmonary pressures > 45 mm Hg) are therefore considered absolute contraindications to TIPS placement.3,4 This is why echocardiography is recommended to assess pulmonary pressure along with the size and function of the right side of the heart before proceeding with TIPS insertion.
Other considerations
TIPS insertion is not recommended in patients with active hepatic encephalopathy, which should be adequately controlled before insertion of a TIPS. This can be achieved with lactulose and rifaximin. Lactulose is a laxative; the recommended target is 3 to 4 bowel movements daily. Rifaximin is a poorly absorbed antibiotic that has a wide spectrum of coverage, affecting gram-negative and gram-positive aerobes and anaerobes. It wipes out the gut bacteria and so decreases the production of ammonia by the gut.
Paracentesis is recommended before TIPS placement if a large volume of ascites is present. Draining the fluid allows the liver to drop down and makes it easier to access the portal vein from the hepatic vein.
WHEN TO CONSIDER A TIPS IN ESOPHAGEAL VARICEAL BLEEDING
Acute bleeding refractory to endoscopic therapy
A TIPS remains the only choice to control acute variceal bleeding refractory to medical and endoscopic therapy (Figure 1), with a success rate of 90% to 100%.5 The urgency of TIPS placement is an independent predictor of early mortality.
Esophageal variceal rebleeding
Once varices bleed, the risk of rebleeding is higher than 50%, and rebleeding is associated with a high mortality rate. TIPS should be considered if nonselective beta-blockers and surveillance with upper endoscopy and banding fail to prevent rebleeding, with many studies showing a TIPS to be superior to pharmacologic and endoscopic therapies.6
A meta-analysis in 1999 by Papatheodoridis et al6 found that variceal rebleeding was significantly more frequent with endoscopic therapies, at 47% vs 19% with a TIPS, but the incidence of hepatic encephalopathy was higher with TIPS (34% vs 19%; P < .001), and there was no difference in mortality rates.
Hepatic encephalopathy occurs in 15% to 25% of patients after TIPS procedures. Risk factors include advanced age, poor renal function, and a history of hepatic encephalopathy. Hepatic encephalopathy can be managed with lactulose or rifaximin, or both (see above). Narcotics, antihistamines, and benzodiazepines should be avoided. In rare cases (5%) when hepatic encephalopathy is refractory to medical therapy, liver transplant should be considered.
A surgical distal splenorenal shunt is another option for patients with refractory or recurrent variceal bleeding. In a large randomized controlled trial,7 140 cirrhotic patients with recurrent variceal bleeding were randomized to receive either a distal splenorenal shunt or a TIPS. At a mean follow-up of 48 months, there was no difference in the rates of rebleeding between the two groups (5.5% with a surgical shunt vs 10.5% with a TIPS, P = .29) or in hepatic encephalopathy (50% in both groups). Survival rates were comparable between the two groups at 2 years (81% with a surgical shunt vs 88% with a TIPS) and 5 years (62% vs 61%).
Early use of TIPS after first variceal bleeding
In a 2010 randomized controlled trial,8 63 patients with cirrhosis (Child-Pugh class B or C) and acute variceal bleeding who had received standard medical and endoscopic therapy were randomized to receive either a TIPS within 72 hours of admission or long-term conservative treatment with nonselective beta-blockers and endoscopic band ligation. The 1-year actuarial probability of remaining free of rebleeding or failure to control bleeding was 50% in the conservative treatment group vs 97% in the early-TIPS group (P < .001). The 1-year actuarial survival rate was 61% in the conservative treatment group vs 86% in the early-TIPS group (P < .001).
The authors8 concluded that early use of TIPS in patients with cirrhosis and Child-Pugh scores of 7 to 13 who were hospitalized for acute variceal bleeding was associated with significant reductions in rates of treatment failure and mortality.
- Brenner D, Rippe RA. Pathogenesis of hepatic fibrosis. In: Yamada T, Alpers DH, Laine L, Kaplowitz N, Owyang C, Powell DW, editors. Textbook of Gastroenterology. 4th edition. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.
- Bhogal HK, Sanyal AJ. Using transjugular intrahepatic portosystemic shunts for complications of cirrhosis. Clin Gastroenterol Hepatol 2011; 9:936–946.
- Garcia-Tsao G, Sanyal AJ, Grace ND, Carey WD; Practice Guidelines Committee of American Association for Study of Liver Diseases; Practice Parameters Committee of American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Am J Gastroenterol 2007; 102:2086–2102.
- Azoulay D, Castaing D, Dennison A, Martino W, Eyraud D, Bismuth H. Transjugular intrahepatic portosystemic shunt worsens the hyperdynamic circulatory state of the cirrhotic patient: preliminary report of a prospective study. Hepatology 1994; 19:129–132.
- Rodríguez-Laiz JM, Bañares R, Echenagusia A, et al. Effects of transjugular intrahepatic portasystemic shunt (TIPS) on splanchnic and systemic hemodynamics, and hepatic function in patients with portal hypertension. Preliminary results. Dig Dis Sci 1995; 40:2121–2127.
- Papatheodoridis GV, Goulis J, Leandro G, Patch D, Burroughs AK. Transjugular intrahepatic portosystemic shunt compared with endoscopic treatment for prevention of variceal rebleeding: a meta-analysis. Hepatology 1999; 30:612–622.
- Henderson JM, Boyer TD, Kutner MH, et al; DIVERT Study Group. Distal splenorenal shunt versus transjugular intrahepatic portal systemic shunt for variceal bleeding: a randomized trial. Gastroenterology 2006; 130:1643–1651.
- García-Pagán JC, Caca K, Bureau C, et al; Early TIPS (Transjugular Intrahepatic Portosystemic Shunt) Cooperative Study Group. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med 2010; 362:2370–2379.
- Brenner D, Rippe RA. Pathogenesis of hepatic fibrosis. In: Yamada T, Alpers DH, Laine L, Kaplowitz N, Owyang C, Powell DW, editors. Textbook of Gastroenterology. 4th edition. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.
- Bhogal HK, Sanyal AJ. Using transjugular intrahepatic portosystemic shunts for complications of cirrhosis. Clin Gastroenterol Hepatol 2011; 9:936–946.
- Garcia-Tsao G, Sanyal AJ, Grace ND, Carey WD; Practice Guidelines Committee of American Association for Study of Liver Diseases; Practice Parameters Committee of American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Am J Gastroenterol 2007; 102:2086–2102.
- Azoulay D, Castaing D, Dennison A, Martino W, Eyraud D, Bismuth H. Transjugular intrahepatic portosystemic shunt worsens the hyperdynamic circulatory state of the cirrhotic patient: preliminary report of a prospective study. Hepatology 1994; 19:129–132.
- Rodríguez-Laiz JM, Bañares R, Echenagusia A, et al. Effects of transjugular intrahepatic portasystemic shunt (TIPS) on splanchnic and systemic hemodynamics, and hepatic function in patients with portal hypertension. Preliminary results. Dig Dis Sci 1995; 40:2121–2127.
- Papatheodoridis GV, Goulis J, Leandro G, Patch D, Burroughs AK. Transjugular intrahepatic portosystemic shunt compared with endoscopic treatment for prevention of variceal rebleeding: a meta-analysis. Hepatology 1999; 30:612–622.
- Henderson JM, Boyer TD, Kutner MH, et al; DIVERT Study Group. Distal splenorenal shunt versus transjugular intrahepatic portal systemic shunt for variceal bleeding: a randomized trial. Gastroenterology 2006; 130:1643–1651.
- García-Pagán JC, Caca K, Bureau C, et al; Early TIPS (Transjugular Intrahepatic Portosystemic Shunt) Cooperative Study Group. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med 2010; 362:2370–2379.
Vascular surgeons underutilize palliative care planning
Investment in advanced palliative care planning has the potential to improve the quality of care for vascular surgery patients, according to investigators from Oregon Health and Science University, Portland.
Dale G. Wilson, MD, and his colleagues performed a retrospective review of electronic medical records for 111 patients, who died while on the vascular surgery service at the OHSU Hospital during 2005-2014.
Almost three-quarters (73%) of patients were transitioned to palliative care; of those, 14% presented with an advanced directive, and 28% received a palliative care consultation (JAMA Surg. 2017;152[2]:183-90. doi: 10.1001/jamasurg.2016.3970).
While palliative care services are increasing in hospitals, accounting for 4% of annual hospital admissions in 2012 according to the study, they are not implemented consistently. “Many teams from various specialties care for patients at end of life; however, we still do not know what prompts end-of-life discussions,” Dr. Wilson said. “There is still no consensus on when to involve palliative services in the care of critically ill patients.”
While the decision to advise a consultation is “variable and physician dependent,” the type of treatment required may help identify when consultations are appropriate.
Of the 14 patients who did not choose comfort care, 11 (79%) required CPR. Additionally, all had to be taken to the operating room and required mechanical ventilation.
Of 81 patients who chose palliative care, 31 did so despite potential medical options. These patients were older – average age, 77 years, as compared with 68 years for patients who did not choose comfort care – with 8 of the 31 (26%) presenting an advanced directive, compared with only 7 of 83 patients (8%) for those who did not receive palliative care.
Dr. Wilson and his colleagues found that patients who chose palliative care were more likely to have received a palliative care consultation, as well: 10 of 31 patients who chose comfort care received a consultation, as opposed to 1 of 83 who chose comfort care but did not receive a consultation.
The nature of the vascular surgery service calls for early efforts to gather information regarding patients’ views on end-of-life care, Dr. Wilson said, noting that 73% of patients studied were admitted emergently and 87% underwent surgery, leaving little time for patients to express their wishes.
“Because the events associated with withdrawal of care are often not anticipated, we argue that all vascular surgical patients should have an advance directive, and perhaps, those at particular high risk should have a preoperative palliative care consultation,” Dr. Wilson wrote.
Limitations to the study included the data abstraction, which was performed by a single unblinded physician. Researchers also gathered patients’ reasons for transitioning to comfort care retrospectively.
The low rate of palliative care consultations found in this study mirrors my own experience, as does the feeling of urgency to shed more light on the issue. The biggest hurdle surgeons face when it comes to palliative care consultations is that, in their minds, seeking these meetings is associated with immediate death care. Many surgeons are shy about bringing palliative care specialists on board because approaching families can be daunting.
Family members who do not know enough about comfort care can be upset by the idea. Addressing this misunderstanding is crucial. Consultations are not just conversations about hospice care but can be emotional and spiritual experiences that prepare both the family and the patient for alternative options when surgical intervention cannot guarantee a good quality of life. I would encourage surgeons to be more proactive and less defensive about comfort care . Luckily, understanding the importance of this issue among professionals is growing.
When I approach these situations, it’s important for me to have a full understanding of what families and patients usually expect. Decisions should not be based on how bad things are now but on the future. What was the patient’s last year like? What is the best-case scenario for moving forward on a proposed intervention? What will the patient’s quality of life be? Answering these questions helps the patient understand his or her situation, without diminishing a surgeon’s ability. If you are honest, the family will usually come to the conclusion that they do not want to subject the patient to ultimately unnecessary treatment.
Palliative care services help patients and their families deal with pain beyond the physical symptoms. Dealing with pain, depression, or delirium is only a part of comfort care – coping with a sense of hopelessness, family disruption, or feelings of guilt also can be a part and, significantly, a part that surgeons are not trained to diagnose or treat.
With more than 70 surgeons certified in hospice care and a growing number of fellowships in palliative care, I am extremely optimistic in the progress we have made and will continue to make.
Geoffrey Dunn, MD, FACS, is the medical director of the Palliative Care Consultation Service at UPMC Hamot Medical Center, Erie, Penn. He currently is Community Editor for the Pain and Palliative Care Community for the ACS’s web portal.
The low rate of palliative care consultations found in this study mirrors my own experience, as does the feeling of urgency to shed more light on the issue. The biggest hurdle surgeons face when it comes to palliative care consultations is that, in their minds, seeking these meetings is associated with immediate death care. Many surgeons are shy about bringing palliative care specialists on board because approaching families can be daunting.
Family members who do not know enough about comfort care can be upset by the idea. Addressing this misunderstanding is crucial. Consultations are not just conversations about hospice care but can be emotional and spiritual experiences that prepare both the family and the patient for alternative options when surgical intervention cannot guarantee a good quality of life. I would encourage surgeons to be more proactive and less defensive about comfort care . Luckily, understanding the importance of this issue among professionals is growing.
When I approach these situations, it’s important for me to have a full understanding of what families and patients usually expect. Decisions should not be based on how bad things are now but on the future. What was the patient’s last year like? What is the best-case scenario for moving forward on a proposed intervention? What will the patient’s quality of life be? Answering these questions helps the patient understand his or her situation, without diminishing a surgeon’s ability. If you are honest, the family will usually come to the conclusion that they do not want to subject the patient to ultimately unnecessary treatment.
Palliative care services help patients and their families deal with pain beyond the physical symptoms. Dealing with pain, depression, or delirium is only a part of comfort care – coping with a sense of hopelessness, family disruption, or feelings of guilt also can be a part and, significantly, a part that surgeons are not trained to diagnose or treat.
With more than 70 surgeons certified in hospice care and a growing number of fellowships in palliative care, I am extremely optimistic in the progress we have made and will continue to make.
Geoffrey Dunn, MD, FACS, is the medical director of the Palliative Care Consultation Service at UPMC Hamot Medical Center, Erie, Penn. He currently is Community Editor for the Pain and Palliative Care Community for the ACS’s web portal.
The low rate of palliative care consultations found in this study mirrors my own experience, as does the feeling of urgency to shed more light on the issue. The biggest hurdle surgeons face when it comes to palliative care consultations is that, in their minds, seeking these meetings is associated with immediate death care. Many surgeons are shy about bringing palliative care specialists on board because approaching families can be daunting.
Family members who do not know enough about comfort care can be upset by the idea. Addressing this misunderstanding is crucial. Consultations are not just conversations about hospice care but can be emotional and spiritual experiences that prepare both the family and the patient for alternative options when surgical intervention cannot guarantee a good quality of life. I would encourage surgeons to be more proactive and less defensive about comfort care . Luckily, understanding the importance of this issue among professionals is growing.
When I approach these situations, it’s important for me to have a full understanding of what families and patients usually expect. Decisions should not be based on how bad things are now but on the future. What was the patient’s last year like? What is the best-case scenario for moving forward on a proposed intervention? What will the patient’s quality of life be? Answering these questions helps the patient understand his or her situation, without diminishing a surgeon’s ability. If you are honest, the family will usually come to the conclusion that they do not want to subject the patient to ultimately unnecessary treatment.
Palliative care services help patients and their families deal with pain beyond the physical symptoms. Dealing with pain, depression, or delirium is only a part of comfort care – coping with a sense of hopelessness, family disruption, or feelings of guilt also can be a part and, significantly, a part that surgeons are not trained to diagnose or treat.
With more than 70 surgeons certified in hospice care and a growing number of fellowships in palliative care, I am extremely optimistic in the progress we have made and will continue to make.
Geoffrey Dunn, MD, FACS, is the medical director of the Palliative Care Consultation Service at UPMC Hamot Medical Center, Erie, Penn. He currently is Community Editor for the Pain and Palliative Care Community for the ACS’s web portal.
Investment in advanced palliative care planning has the potential to improve the quality of care for vascular surgery patients, according to investigators from Oregon Health and Science University, Portland.
Dale G. Wilson, MD, and his colleagues performed a retrospective review of electronic medical records for 111 patients, who died while on the vascular surgery service at the OHSU Hospital during 2005-2014.
Almost three-quarters (73%) of patients were transitioned to palliative care; of those, 14% presented with an advanced directive, and 28% received a palliative care consultation (JAMA Surg. 2017;152[2]:183-90. doi: 10.1001/jamasurg.2016.3970).
While palliative care services are increasing in hospitals, accounting for 4% of annual hospital admissions in 2012 according to the study, they are not implemented consistently. “Many teams from various specialties care for patients at end of life; however, we still do not know what prompts end-of-life discussions,” Dr. Wilson said. “There is still no consensus on when to involve palliative services in the care of critically ill patients.”
While the decision to advise a consultation is “variable and physician dependent,” the type of treatment required may help identify when consultations are appropriate.
Of the 14 patients who did not choose comfort care, 11 (79%) required CPR. Additionally, all had to be taken to the operating room and required mechanical ventilation.
Of 81 patients who chose palliative care, 31 did so despite potential medical options. These patients were older – average age, 77 years, as compared with 68 years for patients who did not choose comfort care – with 8 of the 31 (26%) presenting an advanced directive, compared with only 7 of 83 patients (8%) for those who did not receive palliative care.
Dr. Wilson and his colleagues found that patients who chose palliative care were more likely to have received a palliative care consultation, as well: 10 of 31 patients who chose comfort care received a consultation, as opposed to 1 of 83 who chose comfort care but did not receive a consultation.
The nature of the vascular surgery service calls for early efforts to gather information regarding patients’ views on end-of-life care, Dr. Wilson said, noting that 73% of patients studied were admitted emergently and 87% underwent surgery, leaving little time for patients to express their wishes.
“Because the events associated with withdrawal of care are often not anticipated, we argue that all vascular surgical patients should have an advance directive, and perhaps, those at particular high risk should have a preoperative palliative care consultation,” Dr. Wilson wrote.
Limitations to the study included the data abstraction, which was performed by a single unblinded physician. Researchers also gathered patients’ reasons for transitioning to comfort care retrospectively.
Investment in advanced palliative care planning has the potential to improve the quality of care for vascular surgery patients, according to investigators from Oregon Health and Science University, Portland.
Dale G. Wilson, MD, and his colleagues performed a retrospective review of electronic medical records for 111 patients, who died while on the vascular surgery service at the OHSU Hospital during 2005-2014.
Almost three-quarters (73%) of patients were transitioned to palliative care; of those, 14% presented with an advanced directive, and 28% received a palliative care consultation (JAMA Surg. 2017;152[2]:183-90. doi: 10.1001/jamasurg.2016.3970).
While palliative care services are increasing in hospitals, accounting for 4% of annual hospital admissions in 2012 according to the study, they are not implemented consistently. “Many teams from various specialties care for patients at end of life; however, we still do not know what prompts end-of-life discussions,” Dr. Wilson said. “There is still no consensus on when to involve palliative services in the care of critically ill patients.”
While the decision to advise a consultation is “variable and physician dependent,” the type of treatment required may help identify when consultations are appropriate.
Of the 14 patients who did not choose comfort care, 11 (79%) required CPR. Additionally, all had to be taken to the operating room and required mechanical ventilation.
Of 81 patients who chose palliative care, 31 did so despite potential medical options. These patients were older – average age, 77 years, as compared with 68 years for patients who did not choose comfort care – with 8 of the 31 (26%) presenting an advanced directive, compared with only 7 of 83 patients (8%) for those who did not receive palliative care.
Dr. Wilson and his colleagues found that patients who chose palliative care were more likely to have received a palliative care consultation, as well: 10 of 31 patients who chose comfort care received a consultation, as opposed to 1 of 83 who chose comfort care but did not receive a consultation.
The nature of the vascular surgery service calls for early efforts to gather information regarding patients’ views on end-of-life care, Dr. Wilson said, noting that 73% of patients studied were admitted emergently and 87% underwent surgery, leaving little time for patients to express their wishes.
“Because the events associated with withdrawal of care are often not anticipated, we argue that all vascular surgical patients should have an advance directive, and perhaps, those at particular high risk should have a preoperative palliative care consultation,” Dr. Wilson wrote.
Limitations to the study included the data abstraction, which was performed by a single unblinded physician. Researchers also gathered patients’ reasons for transitioning to comfort care retrospectively.
FROM JAMA SURGERY
Key clinical point:
Major finding: Of the 111 patients studied, 81 died on palliative care, but only 15 presented an advanced directive.
Data source: A retrospective cohort study of the records of patients aged 18-99 years who died in the vascular surgery service at Oregon Health and Science University Hospital from 2005-2014.
Disclosures: The authors reported no financial disclosures.
Open vs. endovascular for chronic mesenteric ischemia
Chronic mesenteric ischemia is best treated in an open operation.
Chronic mesenteric ischemia is a rare disorder accounting for about 1 out of 100,000 admissions.1 Because of the rarity of this disease, diagnosis is often delayed. Patients are often evaluated for other gastrointestinal diseases and/or malignancies, which in turn contributes to significant delays in diagnosis. Additionally, there are no prospective, randomized trials on which to base decisions regarding treatment; and it is unlikely that such studies will ever be undertaken.
Chronic mesenteric ischemia develops when two or more of the mesenteric vessels (celiac, superior mesenteric [SMA], or inferior mesenteric [IMA]) become occluded or develop severe stenosis. In my experience, patients most often develop occlusion (as opposed to stenosis) of their mesenteric vessels. The atherosclerotic plaque responsible for the disease originates within the aorta and the stenosis/occlusion develops at the vessel origin.
As a whole, these comorbidities would suggest that a more minimally invasive approach would be preferred. Yet, an open operation for chronic mesenteric ischemia should not be discarded as an initial operation.
Endovascular treatment of mesenteric ischemia is not without risk. As in all endovascular procedures there are complications associated with the access vessels. The orientation of the mesenteric vessels as they arise from the aorta often favors an approach from the left arm. The brachial artery is smaller than the femoral artery, and it is more difficult to apply pressure to the brachial artery to control the puncture site. This leads to a higher rate of access site complications including hemorrhage, pseudoaneurysm and thrombosis of the vessel. Bleeding or hematoma formation within the brachial neurovascular sheath can result in significant neurologic dysfunction of the arm and hand. There is risk of stroke, especially when the access vessel is the brachial artery. There are also complications directly related to the endovascular procedure.
Atheroembolism of plaque can result in occlusion of small mesenteric vessels and focal areas of bowel necrosis. Dissection of the mesenteric vessel can occur. Oderich has advocated for the use of a covered stent.2 A covered stent could cover proximal branches, and thus, in theory, the treatment itself could cause bowel ischemia or infarction. Many series that have compared open and endovascular surgery show no difference in early outcomes, but demonstrate early restenosis, decreased primary patency, and decreased assisted primary patency with endovascular treatment as compared with open operations.3-5
Another concern regarding the treatment of mesenteric ischemia is the status of the end organ, the bowel. Successful treatment of mesenteric ischemia cannot only assess the atherosclerotic lesion, but requires the surgeon to be cognizant of the condition of the bowel.
This concern is especially true for acute mesenteric ischemia,6 but the status of the bowel must also be kept in mind for chronic mesenteric ischemia. Unlike the lower extremity where the results of treatment are easily observed by inspection (color, evidence of atheroembolization), palpation (temperature, pulse), and physiologic testing (ABI), the bowel is not accessible. There are no highly accurate tests to determine if the patient has on-going bowel ischemia or has developed infarction. Should a complication occur, physical examination findings and laboratory changes often become apparent late in the course of the disease. As previously noted, these patients are often physiologically compromised and have little reserve and may not survive such complications. An open operation allows visualization of the bowel, before and after revascularization.
Traditional teaching for the surgical treatment of chronic mesenteric ischemia has emphasized that two mesenteric vessels, usually the celiac and SMA, should be revascularized.7,8 Various approaches to revascularization have been suggested including transaortic endarterectomy and an antegrade bifurcated bypass originating from the supraceliac aorta. These operations are effective in restoring flow to the mesenteric circulation, but both of these approaches involve aortic cross-clamping and are physiologically challenging for patients.
There are alternatives for open revascularization of the mesenteric vessels. The infrarenal aorta, iliac vessels, and even renal arteries can be, and have been used as the inflow source. It has been my experience that a bypass graft using an iliac artery (common or external) as the inflow source is well tolerated by patients. I have preferentially used either great saphenous vein or femoral-popliteal vein as the conduit. In many cases, the distal anastomosis is simply the SMA. However, both the celiac and SMA can be revascularized by creating a side-side anastomosis to the SMA and an end-side anastomosis to the common or proper hepatic artery. Such procedures have been durable, providing relief of symptoms and allowing patients to regain weight.9
In reality, I believe that an endovascular approach for the treatment of chronic mesenteric ischemia has a role. Vascular surgeons are uniquely positioned to carefully evaluate each patient and recommend what they feel is most appropriate. I tend to feel that an endovascular approach is less likely to be successful for patients with occluded vessels, especially those with a flush occlusion, and so will preferentially recommend an open operation for these patients. On the other hand, in my practice, I have used an endovascular approach for patients with severe stenosis, and as Dr. Harris states, know that a bypass is always an option should this be unsuccessful or ultimately fail.
References
1. Ann Vasc Surg. 1991;5:403-6
2. J Vasc Surg. 2013;58:1316-23
3. Ann Vasc Surg. 2015:29;934-40
4. World J Gastroenerol. 2013;19:1333-7
5. J Vasc Surg. 2007;45:1162-71
6. J Vasc Surg. 2015;62:767-72
7. J Vasc Surg. 2002:35:853-9
8. Surgery. 1981;90:940-6
9. J Vasc Surg. 2000;32:37-47
Eric Endean, MD, is the director of the aortic center, Gordon L. Hyde Endowed Professor and Chair, and vascular surgery section head, vascular and endovascular surgery at UK HealthCare, University of Kentucky, Lexington. He had no relevant disclosures.
Presenting the case for endovascular intervention
Chronic mesenteric ischemia (CMI) is an uncommon, but lethal, problem when left untreated. Before the endovascular era, the only option was open revascularization, which is challenging in this chronically ill, malnourished population with diffuse, systemic, atherosclerotic disease. Morbidity and mortality was relatively high because of the comorbid conditions and chronically ill status of the patients. The first mesenteric bypass was performed in 1958 by Maynard and Shaw.1
Options for open repair include transaortic endarterectomy, antegrade bypass from the supraceliac aorta or distal thoracic aorta, or retrograde bypass from the iliac artery, all of which are major abdominal procedures. Endovascular interventions are now the most commonly performed procedures for CMI in the United States based on national studies.2
Technical success with endovascular interventions can be achieved in well over 90% of patients, and multiple vessels can be treated simultaneously from either a femoral or brachial approach. The primary concern with endovascular interventions has been long-term patency, with restenosis from intimal hyperplasia or thrombosis causing recurrent symptoms in up to 30%-50% at 3-5 years.5-7 However, these rates are based on use of bare metal stents, rather than covered stents. Recent studies8 suggest that stent grafts have markedly improved outcomes over bare metal stents for ostial lesions, decreasing recurrence. Oderich found that freedom from recurrence was 92% with stent grafts as compared with 53% for bare metal stents, with primary patency rates of 92%, at 3 years8, comparable to even the best open bypass results. Schoch first reported the use of covered stents in the mesenteric circulation, and found that no patients developed recurrent stenosis at 2 years.9 Other concerns with endovascular intervention include embolization and dissection, which have not been frequently reported.
Mortality from open surgery ranges from 5% to 15%, with morbidity of 30%-40%.4 Mortality from endovascular intervention is markedly lower, in the range of 3.56% vs. 7.23%.5 Long-term survival is not different between endovascular vs. open repair (69% vs. 65%),4 with the majority of deaths related to cardiac, pulmonary, or malignancy issues. Moghadamyeghaneh, in a review of the Nationwide Inpatient Sample database, found that open surgery was one of the major predictors for higher morbidity (odds ratio, 5.07) and mortality (OR, 5.13), despite the fact that endovascular patients were older (another risk factor for adverse outcomes) and had more comorbidities in this nonrandomized, real-world study.2
Further, if one considers cost as a metric for decision making, a Markov clinical decision model by Hogendoorn et al suggests an endovascular first approach is preferred, despite the presumed higher rate of recurrence expected with use of bare metal stents, rather than covered stents, utilized for this analysis.10 Clearly, the financial advantage would be even greater for endovascular with the lower rates of recurrence with covered stents more recently reported.
If a patient develops recurrent stenosis after endovascular intervention, open bypass may be considered as an alternate to repeat endovascular intervention, dependent on the nutritional status, life expectancy, and initial intervention undertaken. Alternately, patients who undergo open repair are not immune to restenosis, with a recurrence rate of 10%-20%.11,12 Oderich found that there was a 22% mortality in those treated with repeat open interventions, with a 47% complication rate. Endovascular interventions, however, had a significantly lower rate of complications, 16%, and mortality. For patients with recurrent disease after open revascularization for CMI, the endovascular approach should also be the preferred approach.
Any lesion which is anatomically suitable for endovascular repair should first have an attempt made via this approach, utilizing covered stents. While there is a role for open revascularization, endovascular interventions can be safely performed, with minimal morbidity and mortality, and good long-term patency, even in the sickest patients. Endovascular intervention should be the procedure of choice for chronic mesenteric ischemia and recurrent chronic mesenteric ischemia.
References
1. NEJM. 1958;258:874-8
2. Am Surg. 2015;81:1149-56
3. Cardiovasc Intervent Radiol. 1980;3:43-4
4. Ann Vasc Surg. 2009;23:700-12
5. Ann Vasc Surg. 2013;27:113-22
6. J Vasc Surg. 2011;54:1422-29
7. J Vasc Surg. 2010;51:140-7
8. J Vasc Surg. 2013;58:1316-24
9. JACS. 2001;212:668-75
10. J Vasc Surg. 2014;60;715-25
11. J Vasc Surg. 200;49:1472-9
12. J Vasc Surg. 2007;45:1162-71
Linda Harris, MD, is professor of surgery; chief, division of vascular surgery; program director, vascular surgery residency & fellowship at the State University of New York at Buffalo; and an associate medical editor for Vascular Specialist. She had no relevant disclosures.
Chronic mesenteric ischemia is best treated in an open operation.
Chronic mesenteric ischemia is a rare disorder accounting for about 1 out of 100,000 admissions.1 Because of the rarity of this disease, diagnosis is often delayed. Patients are often evaluated for other gastrointestinal diseases and/or malignancies, which in turn contributes to significant delays in diagnosis. Additionally, there are no prospective, randomized trials on which to base decisions regarding treatment; and it is unlikely that such studies will ever be undertaken.
Chronic mesenteric ischemia develops when two or more of the mesenteric vessels (celiac, superior mesenteric [SMA], or inferior mesenteric [IMA]) become occluded or develop severe stenosis. In my experience, patients most often develop occlusion (as opposed to stenosis) of their mesenteric vessels. The atherosclerotic plaque responsible for the disease originates within the aorta and the stenosis/occlusion develops at the vessel origin.
As a whole, these comorbidities would suggest that a more minimally invasive approach would be preferred. Yet, an open operation for chronic mesenteric ischemia should not be discarded as an initial operation.
Endovascular treatment of mesenteric ischemia is not without risk. As in all endovascular procedures there are complications associated with the access vessels. The orientation of the mesenteric vessels as they arise from the aorta often favors an approach from the left arm. The brachial artery is smaller than the femoral artery, and it is more difficult to apply pressure to the brachial artery to control the puncture site. This leads to a higher rate of access site complications including hemorrhage, pseudoaneurysm and thrombosis of the vessel. Bleeding or hematoma formation within the brachial neurovascular sheath can result in significant neurologic dysfunction of the arm and hand. There is risk of stroke, especially when the access vessel is the brachial artery. There are also complications directly related to the endovascular procedure.
Atheroembolism of plaque can result in occlusion of small mesenteric vessels and focal areas of bowel necrosis. Dissection of the mesenteric vessel can occur. Oderich has advocated for the use of a covered stent.2 A covered stent could cover proximal branches, and thus, in theory, the treatment itself could cause bowel ischemia or infarction. Many series that have compared open and endovascular surgery show no difference in early outcomes, but demonstrate early restenosis, decreased primary patency, and decreased assisted primary patency with endovascular treatment as compared with open operations.3-5
Another concern regarding the treatment of mesenteric ischemia is the status of the end organ, the bowel. Successful treatment of mesenteric ischemia cannot only assess the atherosclerotic lesion, but requires the surgeon to be cognizant of the condition of the bowel.
This concern is especially true for acute mesenteric ischemia,6 but the status of the bowel must also be kept in mind for chronic mesenteric ischemia. Unlike the lower extremity where the results of treatment are easily observed by inspection (color, evidence of atheroembolization), palpation (temperature, pulse), and physiologic testing (ABI), the bowel is not accessible. There are no highly accurate tests to determine if the patient has on-going bowel ischemia or has developed infarction. Should a complication occur, physical examination findings and laboratory changes often become apparent late in the course of the disease. As previously noted, these patients are often physiologically compromised and have little reserve and may not survive such complications. An open operation allows visualization of the bowel, before and after revascularization.
Traditional teaching for the surgical treatment of chronic mesenteric ischemia has emphasized that two mesenteric vessels, usually the celiac and SMA, should be revascularized.7,8 Various approaches to revascularization have been suggested including transaortic endarterectomy and an antegrade bifurcated bypass originating from the supraceliac aorta. These operations are effective in restoring flow to the mesenteric circulation, but both of these approaches involve aortic cross-clamping and are physiologically challenging for patients.
There are alternatives for open revascularization of the mesenteric vessels. The infrarenal aorta, iliac vessels, and even renal arteries can be, and have been used as the inflow source. It has been my experience that a bypass graft using an iliac artery (common or external) as the inflow source is well tolerated by patients. I have preferentially used either great saphenous vein or femoral-popliteal vein as the conduit. In many cases, the distal anastomosis is simply the SMA. However, both the celiac and SMA can be revascularized by creating a side-side anastomosis to the SMA and an end-side anastomosis to the common or proper hepatic artery. Such procedures have been durable, providing relief of symptoms and allowing patients to regain weight.9
In reality, I believe that an endovascular approach for the treatment of chronic mesenteric ischemia has a role. Vascular surgeons are uniquely positioned to carefully evaluate each patient and recommend what they feel is most appropriate. I tend to feel that an endovascular approach is less likely to be successful for patients with occluded vessels, especially those with a flush occlusion, and so will preferentially recommend an open operation for these patients. On the other hand, in my practice, I have used an endovascular approach for patients with severe stenosis, and as Dr. Harris states, know that a bypass is always an option should this be unsuccessful or ultimately fail.
References
1. Ann Vasc Surg. 1991;5:403-6
2. J Vasc Surg. 2013;58:1316-23
3. Ann Vasc Surg. 2015:29;934-40
4. World J Gastroenerol. 2013;19:1333-7
5. J Vasc Surg. 2007;45:1162-71
6. J Vasc Surg. 2015;62:767-72
7. J Vasc Surg. 2002:35:853-9
8. Surgery. 1981;90:940-6
9. J Vasc Surg. 2000;32:37-47
Eric Endean, MD, is the director of the aortic center, Gordon L. Hyde Endowed Professor and Chair, and vascular surgery section head, vascular and endovascular surgery at UK HealthCare, University of Kentucky, Lexington. He had no relevant disclosures.
Presenting the case for endovascular intervention
Chronic mesenteric ischemia (CMI) is an uncommon, but lethal, problem when left untreated. Before the endovascular era, the only option was open revascularization, which is challenging in this chronically ill, malnourished population with diffuse, systemic, atherosclerotic disease. Morbidity and mortality was relatively high because of the comorbid conditions and chronically ill status of the patients. The first mesenteric bypass was performed in 1958 by Maynard and Shaw.1
Options for open repair include transaortic endarterectomy, antegrade bypass from the supraceliac aorta or distal thoracic aorta, or retrograde bypass from the iliac artery, all of which are major abdominal procedures. Endovascular interventions are now the most commonly performed procedures for CMI in the United States based on national studies.2
Technical success with endovascular interventions can be achieved in well over 90% of patients, and multiple vessels can be treated simultaneously from either a femoral or brachial approach. The primary concern with endovascular interventions has been long-term patency, with restenosis from intimal hyperplasia or thrombosis causing recurrent symptoms in up to 30%-50% at 3-5 years.5-7 However, these rates are based on use of bare metal stents, rather than covered stents. Recent studies8 suggest that stent grafts have markedly improved outcomes over bare metal stents for ostial lesions, decreasing recurrence. Oderich found that freedom from recurrence was 92% with stent grafts as compared with 53% for bare metal stents, with primary patency rates of 92%, at 3 years8, comparable to even the best open bypass results. Schoch first reported the use of covered stents in the mesenteric circulation, and found that no patients developed recurrent stenosis at 2 years.9 Other concerns with endovascular intervention include embolization and dissection, which have not been frequently reported.
Mortality from open surgery ranges from 5% to 15%, with morbidity of 30%-40%.4 Mortality from endovascular intervention is markedly lower, in the range of 3.56% vs. 7.23%.5 Long-term survival is not different between endovascular vs. open repair (69% vs. 65%),4 with the majority of deaths related to cardiac, pulmonary, or malignancy issues. Moghadamyeghaneh, in a review of the Nationwide Inpatient Sample database, found that open surgery was one of the major predictors for higher morbidity (odds ratio, 5.07) and mortality (OR, 5.13), despite the fact that endovascular patients were older (another risk factor for adverse outcomes) and had more comorbidities in this nonrandomized, real-world study.2
Further, if one considers cost as a metric for decision making, a Markov clinical decision model by Hogendoorn et al suggests an endovascular first approach is preferred, despite the presumed higher rate of recurrence expected with use of bare metal stents, rather than covered stents, utilized for this analysis.10 Clearly, the financial advantage would be even greater for endovascular with the lower rates of recurrence with covered stents more recently reported.
If a patient develops recurrent stenosis after endovascular intervention, open bypass may be considered as an alternate to repeat endovascular intervention, dependent on the nutritional status, life expectancy, and initial intervention undertaken. Alternately, patients who undergo open repair are not immune to restenosis, with a recurrence rate of 10%-20%.11,12 Oderich found that there was a 22% mortality in those treated with repeat open interventions, with a 47% complication rate. Endovascular interventions, however, had a significantly lower rate of complications, 16%, and mortality. For patients with recurrent disease after open revascularization for CMI, the endovascular approach should also be the preferred approach.
Any lesion which is anatomically suitable for endovascular repair should first have an attempt made via this approach, utilizing covered stents. While there is a role for open revascularization, endovascular interventions can be safely performed, with minimal morbidity and mortality, and good long-term patency, even in the sickest patients. Endovascular intervention should be the procedure of choice for chronic mesenteric ischemia and recurrent chronic mesenteric ischemia.
References
1. NEJM. 1958;258:874-8
2. Am Surg. 2015;81:1149-56
3. Cardiovasc Intervent Radiol. 1980;3:43-4
4. Ann Vasc Surg. 2009;23:700-12
5. Ann Vasc Surg. 2013;27:113-22
6. J Vasc Surg. 2011;54:1422-29
7. J Vasc Surg. 2010;51:140-7
8. J Vasc Surg. 2013;58:1316-24
9. JACS. 2001;212:668-75
10. J Vasc Surg. 2014;60;715-25
11. J Vasc Surg. 200;49:1472-9
12. J Vasc Surg. 2007;45:1162-71
Linda Harris, MD, is professor of surgery; chief, division of vascular surgery; program director, vascular surgery residency & fellowship at the State University of New York at Buffalo; and an associate medical editor for Vascular Specialist. She had no relevant disclosures.
Chronic mesenteric ischemia is best treated in an open operation.
Chronic mesenteric ischemia is a rare disorder accounting for about 1 out of 100,000 admissions.1 Because of the rarity of this disease, diagnosis is often delayed. Patients are often evaluated for other gastrointestinal diseases and/or malignancies, which in turn contributes to significant delays in diagnosis. Additionally, there are no prospective, randomized trials on which to base decisions regarding treatment; and it is unlikely that such studies will ever be undertaken.
Chronic mesenteric ischemia develops when two or more of the mesenteric vessels (celiac, superior mesenteric [SMA], or inferior mesenteric [IMA]) become occluded or develop severe stenosis. In my experience, patients most often develop occlusion (as opposed to stenosis) of their mesenteric vessels. The atherosclerotic plaque responsible for the disease originates within the aorta and the stenosis/occlusion develops at the vessel origin.
As a whole, these comorbidities would suggest that a more minimally invasive approach would be preferred. Yet, an open operation for chronic mesenteric ischemia should not be discarded as an initial operation.
Endovascular treatment of mesenteric ischemia is not without risk. As in all endovascular procedures there are complications associated with the access vessels. The orientation of the mesenteric vessels as they arise from the aorta often favors an approach from the left arm. The brachial artery is smaller than the femoral artery, and it is more difficult to apply pressure to the brachial artery to control the puncture site. This leads to a higher rate of access site complications including hemorrhage, pseudoaneurysm and thrombosis of the vessel. Bleeding or hematoma formation within the brachial neurovascular sheath can result in significant neurologic dysfunction of the arm and hand. There is risk of stroke, especially when the access vessel is the brachial artery. There are also complications directly related to the endovascular procedure.
Atheroembolism of plaque can result in occlusion of small mesenteric vessels and focal areas of bowel necrosis. Dissection of the mesenteric vessel can occur. Oderich has advocated for the use of a covered stent.2 A covered stent could cover proximal branches, and thus, in theory, the treatment itself could cause bowel ischemia or infarction. Many series that have compared open and endovascular surgery show no difference in early outcomes, but demonstrate early restenosis, decreased primary patency, and decreased assisted primary patency with endovascular treatment as compared with open operations.3-5
Another concern regarding the treatment of mesenteric ischemia is the status of the end organ, the bowel. Successful treatment of mesenteric ischemia cannot only assess the atherosclerotic lesion, but requires the surgeon to be cognizant of the condition of the bowel.
This concern is especially true for acute mesenteric ischemia,6 but the status of the bowel must also be kept in mind for chronic mesenteric ischemia. Unlike the lower extremity where the results of treatment are easily observed by inspection (color, evidence of atheroembolization), palpation (temperature, pulse), and physiologic testing (ABI), the bowel is not accessible. There are no highly accurate tests to determine if the patient has on-going bowel ischemia or has developed infarction. Should a complication occur, physical examination findings and laboratory changes often become apparent late in the course of the disease. As previously noted, these patients are often physiologically compromised and have little reserve and may not survive such complications. An open operation allows visualization of the bowel, before and after revascularization.
Traditional teaching for the surgical treatment of chronic mesenteric ischemia has emphasized that two mesenteric vessels, usually the celiac and SMA, should be revascularized.7,8 Various approaches to revascularization have been suggested including transaortic endarterectomy and an antegrade bifurcated bypass originating from the supraceliac aorta. These operations are effective in restoring flow to the mesenteric circulation, but both of these approaches involve aortic cross-clamping and are physiologically challenging for patients.
There are alternatives for open revascularization of the mesenteric vessels. The infrarenal aorta, iliac vessels, and even renal arteries can be, and have been used as the inflow source. It has been my experience that a bypass graft using an iliac artery (common or external) as the inflow source is well tolerated by patients. I have preferentially used either great saphenous vein or femoral-popliteal vein as the conduit. In many cases, the distal anastomosis is simply the SMA. However, both the celiac and SMA can be revascularized by creating a side-side anastomosis to the SMA and an end-side anastomosis to the common or proper hepatic artery. Such procedures have been durable, providing relief of symptoms and allowing patients to regain weight.9
In reality, I believe that an endovascular approach for the treatment of chronic mesenteric ischemia has a role. Vascular surgeons are uniquely positioned to carefully evaluate each patient and recommend what they feel is most appropriate. I tend to feel that an endovascular approach is less likely to be successful for patients with occluded vessels, especially those with a flush occlusion, and so will preferentially recommend an open operation for these patients. On the other hand, in my practice, I have used an endovascular approach for patients with severe stenosis, and as Dr. Harris states, know that a bypass is always an option should this be unsuccessful or ultimately fail.
References
1. Ann Vasc Surg. 1991;5:403-6
2. J Vasc Surg. 2013;58:1316-23
3. Ann Vasc Surg. 2015:29;934-40
4. World J Gastroenerol. 2013;19:1333-7
5. J Vasc Surg. 2007;45:1162-71
6. J Vasc Surg. 2015;62:767-72
7. J Vasc Surg. 2002:35:853-9
8. Surgery. 1981;90:940-6
9. J Vasc Surg. 2000;32:37-47
Eric Endean, MD, is the director of the aortic center, Gordon L. Hyde Endowed Professor and Chair, and vascular surgery section head, vascular and endovascular surgery at UK HealthCare, University of Kentucky, Lexington. He had no relevant disclosures.
Presenting the case for endovascular intervention
Chronic mesenteric ischemia (CMI) is an uncommon, but lethal, problem when left untreated. Before the endovascular era, the only option was open revascularization, which is challenging in this chronically ill, malnourished population with diffuse, systemic, atherosclerotic disease. Morbidity and mortality was relatively high because of the comorbid conditions and chronically ill status of the patients. The first mesenteric bypass was performed in 1958 by Maynard and Shaw.1
Options for open repair include transaortic endarterectomy, antegrade bypass from the supraceliac aorta or distal thoracic aorta, or retrograde bypass from the iliac artery, all of which are major abdominal procedures. Endovascular interventions are now the most commonly performed procedures for CMI in the United States based on national studies.2
Technical success with endovascular interventions can be achieved in well over 90% of patients, and multiple vessels can be treated simultaneously from either a femoral or brachial approach. The primary concern with endovascular interventions has been long-term patency, with restenosis from intimal hyperplasia or thrombosis causing recurrent symptoms in up to 30%-50% at 3-5 years.5-7 However, these rates are based on use of bare metal stents, rather than covered stents. Recent studies8 suggest that stent grafts have markedly improved outcomes over bare metal stents for ostial lesions, decreasing recurrence. Oderich found that freedom from recurrence was 92% with stent grafts as compared with 53% for bare metal stents, with primary patency rates of 92%, at 3 years8, comparable to even the best open bypass results. Schoch first reported the use of covered stents in the mesenteric circulation, and found that no patients developed recurrent stenosis at 2 years.9 Other concerns with endovascular intervention include embolization and dissection, which have not been frequently reported.
Mortality from open surgery ranges from 5% to 15%, with morbidity of 30%-40%.4 Mortality from endovascular intervention is markedly lower, in the range of 3.56% vs. 7.23%.5 Long-term survival is not different between endovascular vs. open repair (69% vs. 65%),4 with the majority of deaths related to cardiac, pulmonary, or malignancy issues. Moghadamyeghaneh, in a review of the Nationwide Inpatient Sample database, found that open surgery was one of the major predictors for higher morbidity (odds ratio, 5.07) and mortality (OR, 5.13), despite the fact that endovascular patients were older (another risk factor for adverse outcomes) and had more comorbidities in this nonrandomized, real-world study.2
Further, if one considers cost as a metric for decision making, a Markov clinical decision model by Hogendoorn et al suggests an endovascular first approach is preferred, despite the presumed higher rate of recurrence expected with use of bare metal stents, rather than covered stents, utilized for this analysis.10 Clearly, the financial advantage would be even greater for endovascular with the lower rates of recurrence with covered stents more recently reported.
If a patient develops recurrent stenosis after endovascular intervention, open bypass may be considered as an alternate to repeat endovascular intervention, dependent on the nutritional status, life expectancy, and initial intervention undertaken. Alternately, patients who undergo open repair are not immune to restenosis, with a recurrence rate of 10%-20%.11,12 Oderich found that there was a 22% mortality in those treated with repeat open interventions, with a 47% complication rate. Endovascular interventions, however, had a significantly lower rate of complications, 16%, and mortality. For patients with recurrent disease after open revascularization for CMI, the endovascular approach should also be the preferred approach.
Any lesion which is anatomically suitable for endovascular repair should first have an attempt made via this approach, utilizing covered stents. While there is a role for open revascularization, endovascular interventions can be safely performed, with minimal morbidity and mortality, and good long-term patency, even in the sickest patients. Endovascular intervention should be the procedure of choice for chronic mesenteric ischemia and recurrent chronic mesenteric ischemia.
References
1. NEJM. 1958;258:874-8
2. Am Surg. 2015;81:1149-56
3. Cardiovasc Intervent Radiol. 1980;3:43-4
4. Ann Vasc Surg. 2009;23:700-12
5. Ann Vasc Surg. 2013;27:113-22
6. J Vasc Surg. 2011;54:1422-29
7. J Vasc Surg. 2010;51:140-7
8. J Vasc Surg. 2013;58:1316-24
9. JACS. 2001;212:668-75
10. J Vasc Surg. 2014;60;715-25
11. J Vasc Surg. 200;49:1472-9
12. J Vasc Surg. 2007;45:1162-71
Linda Harris, MD, is professor of surgery; chief, division of vascular surgery; program director, vascular surgery residency & fellowship at the State University of New York at Buffalo; and an associate medical editor for Vascular Specialist. She had no relevant disclosures.
New auto-grafting techniques could advance wound healing
MIAMI – Pinch grafting can accelerate the healing of chronic, treatment-resistant wounds such as leg ulcers, while at the same time reducing morbidity to the donor skin site. A new epidermal harvesting device also is showing promise, as is a new tool that minces autologous skin grafts prior to application to promote wound healing.
These and other advances in wound healing were presented at the Orlando Dermatology Aesthetic and Clinical Conference. The pinch grafts and minced grafts each rely on the newly added skin to stimulate cytokines. Interestingly, there is evidence that grafts taken from hair-bearing donor sites could be superior for stimulating cytokines and accelerating wound healing, said Robert Kirsner, MD, PhD, of the University of Miami Health System.
Islands of regrowth
Physicians perform pinch grafting by taking small punches of skin from a donor site on the thigh, abdomen, or elsewhere, and then transferring the grafts to serve as islands of regrowth in a wound. Pinch grafting can be faster and less expensive than techniques typically performed in an operating room, such as meshed auto-grafting. In contrast, pinch grafting can be accomplished in an office setting “and patients can do quite well.” Dr. Kirsner said. In terms of outcomes, “our data is typical,” he added. “About 50% of refractory ulcers heal, 25% improve, and a percentage recur.”
Spreadable skin grafts
Another autologous grafting technique that can be performed at the bedside uses the Xpansion Micro-Autografting Kit, which minces autologous, split thickness skin grafts. “Then you apply them like peanut butter to bread,” Dr. Kirsner said.
The micro-autografts can help heal both acute and chronic wounds, including full thickness wounds from trauma, some burn wounds, diabetic foot ulcers, and venous ulcers, according to the manufacturer’s website.
Epidermal harvesting (without anesthesia)
Epidermal grafting can make sense because the epidermis regenerates. “You can lift off just the epidermis with heat or suction, “ Dr. Kirsner said. For the first time, he added, a new tool allows epidermal grafting without the need for anesthesia (Cellutome Epidermal Harvesting System). The device raises little microdomes of epidermis down to the basal layer, including basal keratinocytes and melanocytes, and a dermatologist can use a sterile dressing to transfer them to the wound. Confocal microscopy shows the dermoepidermal junction healing as early as within 2 days.
The epidermal harvesting was initially developed for pigment problems, such as piebaldism. (Dermatol Surg. 2017 Jan;43[1]:159-60). “We quickly realized it might have applicability for nonhealing wounds,” Dr. Kirsner said.
Deeper wound healing
A novel strategy for triggering deeper wound healing evolved from fractional laser technology, which remove columns of skin to generate healing. Instead, Rox Anderson, MD, of Massachusetts General Hospital, Boston, “envisioned pulling up microcolumns of full thickness epidermis, all the way to the fat, placing them into a wound, and the wound would heal with very little donor site morbidity,” Dr. Kirsner said.
This tool is coming out in spring of this year, he noted. It will resemble a fractional laser, “but now you have the skin available to place in another wound.” Prior animal studies revealed a healing benefit with very little scarring, he added.
Is hairier better?
Does the donor site matter? Dr. Kirsner asked. Although dermatologists typically graft skin from an abdomen or thigh, a hair-bearing site may be a better option because of the presence of pluripotent stem cells, according to a case report (Wounds. 2016 Apr;28[4]:109-11). J.D. Fox of the University of Miami, Dr. Kirsner, and their colleagues treated a large, chronic venous leg ulcer, almost 60 cm2, with punch grafts from a variety of donor sites.
“The side that got scalp punch grafts healed better, suggesting with skin taken from richly hairy area, you’ll get better results,” Dr. Kirsner said.
Another study supports this strategy (J Am Acad Dermatol. 2016 Nov;75[5]:1007-14). These researchers reported greater wound size reduction using grafts containing hair follicles versus nonhairy areas, again suggesting follicular stem cells play a role in better wound healing, Dr. Kirsner said. “This may be a better source of donor skin in the future.”
Dr. Kirsner is a consultant for Cardinal Health, Mölnlycke, Amniox, Organogenesis, Kerecis, Keretec, and KCI, an Acelity company.
MIAMI – Pinch grafting can accelerate the healing of chronic, treatment-resistant wounds such as leg ulcers, while at the same time reducing morbidity to the donor skin site. A new epidermal harvesting device also is showing promise, as is a new tool that minces autologous skin grafts prior to application to promote wound healing.
These and other advances in wound healing were presented at the Orlando Dermatology Aesthetic and Clinical Conference. The pinch grafts and minced grafts each rely on the newly added skin to stimulate cytokines. Interestingly, there is evidence that grafts taken from hair-bearing donor sites could be superior for stimulating cytokines and accelerating wound healing, said Robert Kirsner, MD, PhD, of the University of Miami Health System.
Islands of regrowth
Physicians perform pinch grafting by taking small punches of skin from a donor site on the thigh, abdomen, or elsewhere, and then transferring the grafts to serve as islands of regrowth in a wound. Pinch grafting can be faster and less expensive than techniques typically performed in an operating room, such as meshed auto-grafting. In contrast, pinch grafting can be accomplished in an office setting “and patients can do quite well.” Dr. Kirsner said. In terms of outcomes, “our data is typical,” he added. “About 50% of refractory ulcers heal, 25% improve, and a percentage recur.”
Spreadable skin grafts
Another autologous grafting technique that can be performed at the bedside uses the Xpansion Micro-Autografting Kit, which minces autologous, split thickness skin grafts. “Then you apply them like peanut butter to bread,” Dr. Kirsner said.
The micro-autografts can help heal both acute and chronic wounds, including full thickness wounds from trauma, some burn wounds, diabetic foot ulcers, and venous ulcers, according to the manufacturer’s website.
Epidermal harvesting (without anesthesia)
Epidermal grafting can make sense because the epidermis regenerates. “You can lift off just the epidermis with heat or suction, “ Dr. Kirsner said. For the first time, he added, a new tool allows epidermal grafting without the need for anesthesia (Cellutome Epidermal Harvesting System). The device raises little microdomes of epidermis down to the basal layer, including basal keratinocytes and melanocytes, and a dermatologist can use a sterile dressing to transfer them to the wound. Confocal microscopy shows the dermoepidermal junction healing as early as within 2 days.
The epidermal harvesting was initially developed for pigment problems, such as piebaldism. (Dermatol Surg. 2017 Jan;43[1]:159-60). “We quickly realized it might have applicability for nonhealing wounds,” Dr. Kirsner said.
Deeper wound healing
A novel strategy for triggering deeper wound healing evolved from fractional laser technology, which remove columns of skin to generate healing. Instead, Rox Anderson, MD, of Massachusetts General Hospital, Boston, “envisioned pulling up microcolumns of full thickness epidermis, all the way to the fat, placing them into a wound, and the wound would heal with very little donor site morbidity,” Dr. Kirsner said.
This tool is coming out in spring of this year, he noted. It will resemble a fractional laser, “but now you have the skin available to place in another wound.” Prior animal studies revealed a healing benefit with very little scarring, he added.
Is hairier better?
Does the donor site matter? Dr. Kirsner asked. Although dermatologists typically graft skin from an abdomen or thigh, a hair-bearing site may be a better option because of the presence of pluripotent stem cells, according to a case report (Wounds. 2016 Apr;28[4]:109-11). J.D. Fox of the University of Miami, Dr. Kirsner, and their colleagues treated a large, chronic venous leg ulcer, almost 60 cm2, with punch grafts from a variety of donor sites.
“The side that got scalp punch grafts healed better, suggesting with skin taken from richly hairy area, you’ll get better results,” Dr. Kirsner said.
Another study supports this strategy (J Am Acad Dermatol. 2016 Nov;75[5]:1007-14). These researchers reported greater wound size reduction using grafts containing hair follicles versus nonhairy areas, again suggesting follicular stem cells play a role in better wound healing, Dr. Kirsner said. “This may be a better source of donor skin in the future.”
Dr. Kirsner is a consultant for Cardinal Health, Mölnlycke, Amniox, Organogenesis, Kerecis, Keretec, and KCI, an Acelity company.
MIAMI – Pinch grafting can accelerate the healing of chronic, treatment-resistant wounds such as leg ulcers, while at the same time reducing morbidity to the donor skin site. A new epidermal harvesting device also is showing promise, as is a new tool that minces autologous skin grafts prior to application to promote wound healing.
These and other advances in wound healing were presented at the Orlando Dermatology Aesthetic and Clinical Conference. The pinch grafts and minced grafts each rely on the newly added skin to stimulate cytokines. Interestingly, there is evidence that grafts taken from hair-bearing donor sites could be superior for stimulating cytokines and accelerating wound healing, said Robert Kirsner, MD, PhD, of the University of Miami Health System.
Islands of regrowth
Physicians perform pinch grafting by taking small punches of skin from a donor site on the thigh, abdomen, or elsewhere, and then transferring the grafts to serve as islands of regrowth in a wound. Pinch grafting can be faster and less expensive than techniques typically performed in an operating room, such as meshed auto-grafting. In contrast, pinch grafting can be accomplished in an office setting “and patients can do quite well.” Dr. Kirsner said. In terms of outcomes, “our data is typical,” he added. “About 50% of refractory ulcers heal, 25% improve, and a percentage recur.”
Spreadable skin grafts
Another autologous grafting technique that can be performed at the bedside uses the Xpansion Micro-Autografting Kit, which minces autologous, split thickness skin grafts. “Then you apply them like peanut butter to bread,” Dr. Kirsner said.
The micro-autografts can help heal both acute and chronic wounds, including full thickness wounds from trauma, some burn wounds, diabetic foot ulcers, and venous ulcers, according to the manufacturer’s website.
Epidermal harvesting (without anesthesia)
Epidermal grafting can make sense because the epidermis regenerates. “You can lift off just the epidermis with heat or suction, “ Dr. Kirsner said. For the first time, he added, a new tool allows epidermal grafting without the need for anesthesia (Cellutome Epidermal Harvesting System). The device raises little microdomes of epidermis down to the basal layer, including basal keratinocytes and melanocytes, and a dermatologist can use a sterile dressing to transfer them to the wound. Confocal microscopy shows the dermoepidermal junction healing as early as within 2 days.
The epidermal harvesting was initially developed for pigment problems, such as piebaldism. (Dermatol Surg. 2017 Jan;43[1]:159-60). “We quickly realized it might have applicability for nonhealing wounds,” Dr. Kirsner said.
Deeper wound healing
A novel strategy for triggering deeper wound healing evolved from fractional laser technology, which remove columns of skin to generate healing. Instead, Rox Anderson, MD, of Massachusetts General Hospital, Boston, “envisioned pulling up microcolumns of full thickness epidermis, all the way to the fat, placing them into a wound, and the wound would heal with very little donor site morbidity,” Dr. Kirsner said.
This tool is coming out in spring of this year, he noted. It will resemble a fractional laser, “but now you have the skin available to place in another wound.” Prior animal studies revealed a healing benefit with very little scarring, he added.
Is hairier better?
Does the donor site matter? Dr. Kirsner asked. Although dermatologists typically graft skin from an abdomen or thigh, a hair-bearing site may be a better option because of the presence of pluripotent stem cells, according to a case report (Wounds. 2016 Apr;28[4]:109-11). J.D. Fox of the University of Miami, Dr. Kirsner, and their colleagues treated a large, chronic venous leg ulcer, almost 60 cm2, with punch grafts from a variety of donor sites.
“The side that got scalp punch grafts healed better, suggesting with skin taken from richly hairy area, you’ll get better results,” Dr. Kirsner said.
Another study supports this strategy (J Am Acad Dermatol. 2016 Nov;75[5]:1007-14). These researchers reported greater wound size reduction using grafts containing hair follicles versus nonhairy areas, again suggesting follicular stem cells play a role in better wound healing, Dr. Kirsner said. “This may be a better source of donor skin in the future.”
Dr. Kirsner is a consultant for Cardinal Health, Mölnlycke, Amniox, Organogenesis, Kerecis, Keretec, and KCI, an Acelity company.
Pairing vascular reconstruction, pancreatic cancer resection
CHICAGO – More than 53,000 people will develop pancreatic ductal adenocarcinoma in the United States this year, and upwards of 41,000 will die from the disease, many of them with tumors considered unresectable because they involve adjacent vessels. However, researchers at the University of California, Irvine, have found that careful removal of the tumor around involved veins and arteries, even in borderline cases, can improve outcomes for these patients.
Roy M. Fujitani, MD, updated previously published data on a single-center study he coauthored in 2015 of 270 patients who had undergone a Whipple operation, 183 for pancreatic adenocarcinoma (J Vasc Surg. 2015;61:475-80) at a symposium on vascular surgery sponsored by Northwestern University.
Resection of pancreatic tumors without vascular involvement is fairly straightforward for surgical oncologists to perform, Dr. Fujitani said, but pancreatic tumors enter the borderline resectable category when preoperative CT scan shows portal vein abutment, for which vascular surgery should provide counsel and assist. However, even in some cases when preoperative CT scan shows unresectable, locally advanced pancreatic tumor with celiac artery encasement, neoadjuvant therapy may downstage the disease into the borderline category, he said.
“Patients with borderline resectable or stage II disease are those one should consider for reconstruction,” Dr. Fujitani said. Resectable findings of borderline disease include encasement of the portal vein, superior mesenteric vein and the confluence of the portal venous system (with suitable proximal and distal targets for reconstruction); and less-than-circumferential involvement of the common hepatic artery or right hepatic artery – but without involvement of the superior mesenteric artery or the celiac axis and “certainly not” the aorta. “This would account for about one-fourth of patients in high-volume centers as being able to receive concomitant vascular reconstruction,” Dr. Fujitani said.
In the UCI series, 60 patients with borderline lesions underwent vascular reconstruction. “As it turned out, there was no significant difference in survival between the reconstruction group and the nonreconstruction group,” Dr. Fujitani said, “but it’s important to note that these patients who had the reconstruction would never have been operated on if we were not able to do the reconstruction.” Thirty-day mortality was around 5% and 1-year survival around 70% in both groups, he said. However, at about 1.5 years the Kaplan-Meier survival curves between the two groups diverged, which Dr. Fujitani attributed to more advanced disease in the reconstruction group.
“We found lymph node status and tumor margins were most important in determining survival of these patients,” he said. “Gaining an R0 resection is the most important thing that determines favorable survivability.”
Dr. Fujitani also reviewed different techniques for vascular reconstruction, and while differences in complication rates or 1-, 2-, or 3-year survival were not statistically significant, he did note that mean survival with lateral venorrhaphy exceeded that of primary anastomosis and interposition graft – 21 months vs. 13 months vs. 4 months, suggesting the merits of a more aggressive approach to vascular resection and reconstruction.
“Improvement of survival outcomes may be achieved with concomitant advanced vascular reconstruction in carefully selected patients,” Dr. Fujitani said. “There are multiple options for vascular reconstruction for mesenteric portal venous and visceral arterial involvement using standard vascular surgical techniques.” He added that a dedicated team of experienced surgical oncologists and vascular surgeons for these reconstructions “is essential for successful outcomes.”
Dr. Fujitani had no relevant financial relationships to disclose.
CHICAGO – More than 53,000 people will develop pancreatic ductal adenocarcinoma in the United States this year, and upwards of 41,000 will die from the disease, many of them with tumors considered unresectable because they involve adjacent vessels. However, researchers at the University of California, Irvine, have found that careful removal of the tumor around involved veins and arteries, even in borderline cases, can improve outcomes for these patients.
Roy M. Fujitani, MD, updated previously published data on a single-center study he coauthored in 2015 of 270 patients who had undergone a Whipple operation, 183 for pancreatic adenocarcinoma (J Vasc Surg. 2015;61:475-80) at a symposium on vascular surgery sponsored by Northwestern University.
Resection of pancreatic tumors without vascular involvement is fairly straightforward for surgical oncologists to perform, Dr. Fujitani said, but pancreatic tumors enter the borderline resectable category when preoperative CT scan shows portal vein abutment, for which vascular surgery should provide counsel and assist. However, even in some cases when preoperative CT scan shows unresectable, locally advanced pancreatic tumor with celiac artery encasement, neoadjuvant therapy may downstage the disease into the borderline category, he said.
“Patients with borderline resectable or stage II disease are those one should consider for reconstruction,” Dr. Fujitani said. Resectable findings of borderline disease include encasement of the portal vein, superior mesenteric vein and the confluence of the portal venous system (with suitable proximal and distal targets for reconstruction); and less-than-circumferential involvement of the common hepatic artery or right hepatic artery – but without involvement of the superior mesenteric artery or the celiac axis and “certainly not” the aorta. “This would account for about one-fourth of patients in high-volume centers as being able to receive concomitant vascular reconstruction,” Dr. Fujitani said.
In the UCI series, 60 patients with borderline lesions underwent vascular reconstruction. “As it turned out, there was no significant difference in survival between the reconstruction group and the nonreconstruction group,” Dr. Fujitani said, “but it’s important to note that these patients who had the reconstruction would never have been operated on if we were not able to do the reconstruction.” Thirty-day mortality was around 5% and 1-year survival around 70% in both groups, he said. However, at about 1.5 years the Kaplan-Meier survival curves between the two groups diverged, which Dr. Fujitani attributed to more advanced disease in the reconstruction group.
“We found lymph node status and tumor margins were most important in determining survival of these patients,” he said. “Gaining an R0 resection is the most important thing that determines favorable survivability.”
Dr. Fujitani also reviewed different techniques for vascular reconstruction, and while differences in complication rates or 1-, 2-, or 3-year survival were not statistically significant, he did note that mean survival with lateral venorrhaphy exceeded that of primary anastomosis and interposition graft – 21 months vs. 13 months vs. 4 months, suggesting the merits of a more aggressive approach to vascular resection and reconstruction.
“Improvement of survival outcomes may be achieved with concomitant advanced vascular reconstruction in carefully selected patients,” Dr. Fujitani said. “There are multiple options for vascular reconstruction for mesenteric portal venous and visceral arterial involvement using standard vascular surgical techniques.” He added that a dedicated team of experienced surgical oncologists and vascular surgeons for these reconstructions “is essential for successful outcomes.”
Dr. Fujitani had no relevant financial relationships to disclose.
CHICAGO – More than 53,000 people will develop pancreatic ductal adenocarcinoma in the United States this year, and upwards of 41,000 will die from the disease, many of them with tumors considered unresectable because they involve adjacent vessels. However, researchers at the University of California, Irvine, have found that careful removal of the tumor around involved veins and arteries, even in borderline cases, can improve outcomes for these patients.
Roy M. Fujitani, MD, updated previously published data on a single-center study he coauthored in 2015 of 270 patients who had undergone a Whipple operation, 183 for pancreatic adenocarcinoma (J Vasc Surg. 2015;61:475-80) at a symposium on vascular surgery sponsored by Northwestern University.
Resection of pancreatic tumors without vascular involvement is fairly straightforward for surgical oncologists to perform, Dr. Fujitani said, but pancreatic tumors enter the borderline resectable category when preoperative CT scan shows portal vein abutment, for which vascular surgery should provide counsel and assist. However, even in some cases when preoperative CT scan shows unresectable, locally advanced pancreatic tumor with celiac artery encasement, neoadjuvant therapy may downstage the disease into the borderline category, he said.
“Patients with borderline resectable or stage II disease are those one should consider for reconstruction,” Dr. Fujitani said. Resectable findings of borderline disease include encasement of the portal vein, superior mesenteric vein and the confluence of the portal venous system (with suitable proximal and distal targets for reconstruction); and less-than-circumferential involvement of the common hepatic artery or right hepatic artery – but without involvement of the superior mesenteric artery or the celiac axis and “certainly not” the aorta. “This would account for about one-fourth of patients in high-volume centers as being able to receive concomitant vascular reconstruction,” Dr. Fujitani said.
In the UCI series, 60 patients with borderline lesions underwent vascular reconstruction. “As it turned out, there was no significant difference in survival between the reconstruction group and the nonreconstruction group,” Dr. Fujitani said, “but it’s important to note that these patients who had the reconstruction would never have been operated on if we were not able to do the reconstruction.” Thirty-day mortality was around 5% and 1-year survival around 70% in both groups, he said. However, at about 1.5 years the Kaplan-Meier survival curves between the two groups diverged, which Dr. Fujitani attributed to more advanced disease in the reconstruction group.
“We found lymph node status and tumor margins were most important in determining survival of these patients,” he said. “Gaining an R0 resection is the most important thing that determines favorable survivability.”
Dr. Fujitani also reviewed different techniques for vascular reconstruction, and while differences in complication rates or 1-, 2-, or 3-year survival were not statistically significant, he did note that mean survival with lateral venorrhaphy exceeded that of primary anastomosis and interposition graft – 21 months vs. 13 months vs. 4 months, suggesting the merits of a more aggressive approach to vascular resection and reconstruction.
“Improvement of survival outcomes may be achieved with concomitant advanced vascular reconstruction in carefully selected patients,” Dr. Fujitani said. “There are multiple options for vascular reconstruction for mesenteric portal venous and visceral arterial involvement using standard vascular surgical techniques.” He added that a dedicated team of experienced surgical oncologists and vascular surgeons for these reconstructions “is essential for successful outcomes.”
Dr. Fujitani had no relevant financial relationships to disclose.
AT THE NORTHWESTERN VASCULAR SYMPOSIUM
Key clinical point: A more aggressive vascular resection and reconstruction in pancreatic cancer may improve outcomes and palliation in these patients.
Major finding: Mean survival with lateral venorrhaphy exceeded primary anastomosis and interposition graft (21 months vs. 13 months vs. 4 months).
Data source: Updated data of previously published single-center retrospective review of 183 patients who had Whipple procedure for pancreatic adenocarcinoma.
Disclosures: Dr. Fujitani reported having no financial disclosures.