User login
FDA warns of endoleaks associated with endovascular grafts for AAA
The Food and Drug Administration has reported an apparent increase in device-related adverse events from the use of endovascular graft repair (EVAR) to treat abdominal aortic aneurysms (AAA).
In a Letter to Health Care Providers issued on Sept. 28, the FDA indicated that “recent information from several sources, including FDA’s Medical Device Reporting system and Annual Clinical Updates to Physicians by the manufacturers, suggests an increase in the occurrence of Type III endoleaks.”
A Type III endoleak is defined by the failure to completely exclude the AAA from blood flow, thereby allowing a systematic arterial pressurization of the aneurysm sac, increasing the risk of rupture, which is a life-threatening event.
The FDA stated that predictors of Type III endoleaks included treatment with early-generation graft materials, the presence of calcified plaque, and inadequate overlap between graft components.
Secondary interventions to treat Type III endoleaks carry their own risk of adverse events.
It is recommended that health care providers should do the following:
- Consider lifelong surveillance of patients who have been treated with EVAR.
- Consider type III endoleaks in the differential diagnosis of patients who present with symptoms of potential aneurysm expansion or rupture.
- Discuss all treatment options in depth with patients before deciding on the best treatment for Type III endoleaks.
- Report any early or late device-related adverse events, including Type IIIa and Type IIIb endoleaks, associated with EVAR, as well as any device-related adverse events that occur as the result of a secondary intervention to treat Type III endoleaks.
These events should be reported to MedWatch, using the FDA’s Safety Information and Adverse Event Reporting Program Online Voluntary Reporting Form. A form also can be requested by calling 800-332-1088.
The FDA stated that it “continues to work with all manufacturers of endovascular graft systems to better understand this issue,” and that the agency would keep the public informed when significant new information becomes available.
The Food and Drug Administration has reported an apparent increase in device-related adverse events from the use of endovascular graft repair (EVAR) to treat abdominal aortic aneurysms (AAA).
In a Letter to Health Care Providers issued on Sept. 28, the FDA indicated that “recent information from several sources, including FDA’s Medical Device Reporting system and Annual Clinical Updates to Physicians by the manufacturers, suggests an increase in the occurrence of Type III endoleaks.”
A Type III endoleak is defined by the failure to completely exclude the AAA from blood flow, thereby allowing a systematic arterial pressurization of the aneurysm sac, increasing the risk of rupture, which is a life-threatening event.
The FDA stated that predictors of Type III endoleaks included treatment with early-generation graft materials, the presence of calcified plaque, and inadequate overlap between graft components.
Secondary interventions to treat Type III endoleaks carry their own risk of adverse events.
It is recommended that health care providers should do the following:
- Consider lifelong surveillance of patients who have been treated with EVAR.
- Consider type III endoleaks in the differential diagnosis of patients who present with symptoms of potential aneurysm expansion or rupture.
- Discuss all treatment options in depth with patients before deciding on the best treatment for Type III endoleaks.
- Report any early or late device-related adverse events, including Type IIIa and Type IIIb endoleaks, associated with EVAR, as well as any device-related adverse events that occur as the result of a secondary intervention to treat Type III endoleaks.
These events should be reported to MedWatch, using the FDA’s Safety Information and Adverse Event Reporting Program Online Voluntary Reporting Form. A form also can be requested by calling 800-332-1088.
The FDA stated that it “continues to work with all manufacturers of endovascular graft systems to better understand this issue,” and that the agency would keep the public informed when significant new information becomes available.
The Food and Drug Administration has reported an apparent increase in device-related adverse events from the use of endovascular graft repair (EVAR) to treat abdominal aortic aneurysms (AAA).
In a Letter to Health Care Providers issued on Sept. 28, the FDA indicated that “recent information from several sources, including FDA’s Medical Device Reporting system and Annual Clinical Updates to Physicians by the manufacturers, suggests an increase in the occurrence of Type III endoleaks.”
A Type III endoleak is defined by the failure to completely exclude the AAA from blood flow, thereby allowing a systematic arterial pressurization of the aneurysm sac, increasing the risk of rupture, which is a life-threatening event.
The FDA stated that predictors of Type III endoleaks included treatment with early-generation graft materials, the presence of calcified plaque, and inadequate overlap between graft components.
Secondary interventions to treat Type III endoleaks carry their own risk of adverse events.
It is recommended that health care providers should do the following:
- Consider lifelong surveillance of patients who have been treated with EVAR.
- Consider type III endoleaks in the differential diagnosis of patients who present with symptoms of potential aneurysm expansion or rupture.
- Discuss all treatment options in depth with patients before deciding on the best treatment for Type III endoleaks.
- Report any early or late device-related adverse events, including Type IIIa and Type IIIb endoleaks, associated with EVAR, as well as any device-related adverse events that occur as the result of a secondary intervention to treat Type III endoleaks.
These events should be reported to MedWatch, using the FDA’s Safety Information and Adverse Event Reporting Program Online Voluntary Reporting Form. A form also can be requested by calling 800-332-1088.
The FDA stated that it “continues to work with all manufacturers of endovascular graft systems to better understand this issue,” and that the agency would keep the public informed when significant new information becomes available.
Key clinical point:
Major finding: FDA monitoring sources have detected an apparent increase in the occurrence of Type III endoleaks following EVAR.
Data source: FDA’s Medical Device Reporting System and the Annual Clinical Updates to Physicians by the manufacturers.
Disclosures: None.
Another complication of cirrhosis
A 53-year-old Native American woman with a history of liver cirrhosis secondary to alcohol abuse presents to the emergency department after 2 days of diffuse abdominal pain and weakness. The pain was sudden in onset and has progressed relentlessly over the last day, reaching 9 on a scale of 10 in severity. Family members say that her oral intake has been decreased for the last 2 days, but she has had no fever, vomiting, change in bowel habit, blood in stool, or black stool. She has never undergone surgery, and has had one uncomplicated pregnancy.
Physical examination
Vital signs:
- Blood pressure 82/57 mm Hg
- Heart rate 96 beats per minute
- Temperature 37.3°C (99.1°F)
- Respiratory rate 16 per minute
- Oxygen saturation 92% while receiving oxygen at 2 L/minute.
The patient is somnolent and has scleral icterus. Her cardiopulmonary examination is normal. Her abdomen is tense, distended, and diffusely tender. She has bilateral +2 pitting edema in her lower extremities. She is oriented to person only and is noted to have asterixis. Her baseline Model for End-stage Liver Disease score is 18 points on a scale of 6 (less ill) to 40 (gravely ill).
Laboratory studies:
- Hemoglobin 9.8 g/dL (reference range 11.5–15.5)
- Platelet count 100 × 109/L (150–400)
- White blood cell count 9.9 × 109/L (3.7–11.0)
- Serum creatinine 1.06 mg/dL (0.58–0.96)
- Bilirubin 6.3 mg/dL (0.2–1.3)
- International normalized ratio of the prothrombin time 2.15 (0.8–1.2)
- Blood urea nitrogen 13 mg/dL (7–21)
- Serum albumin 2.7 g/dL (3.9–4.9).
Intravenous fluid resuscitation is initiated but the patient remains hypotensive, and on repeat laboratory testing 4 hours later her hemoglobin level has dropped to 7.3 mg/dL.
DIFFERENTIAL DIAGNOSIS
1. Which of the following are likely causes of this patient’s presentation?
- Splenic arterial aneurysm rupture
- Spontaneous bacterial peritonitis
- Variceal hemorrhage
- Portal vein thrombosis
- Abdominal aortic aneurysm rupture
Ruptured splenic artery aneurysm
Splenic artery aneurysms are the third most common intra-abdominal aneurysm, after those of the abdominal aorta and iliac artery.1 They are often asymptomatic and are being detected more frequently because of increased use of computed tomography (CT).2 Symptomatic splenic artery aneurysms may present with abdominal pain and have the potential to rupture, which can be life-threatening.3,4
This patient may have a ruptured splenic artery aneurysm, given her hemodynamic shock.
Spontaneous bacterial peritonitis
Ten percent to 20% of hospitalized patients with cirrhosis and ascites develop spontaneous bacterial peritonitis. Patients may present with ascites and abdominal pain, tenderness to palpation, fever, encephalopathy, or worsening liver and renal function.
Diagnostic paracentesis is paramount to delineate the cause of ascites; one should calculate the serum-ascites albumin gradient and obtain a cell count and culture of the ascitic fluid. The diagnosis of spontaneous bacterial peritonitis can be made if the ascitic fluid polymorphonuclear cell count is 0.25 × 109/L or higher, even if the ascitic fluid culture is negative.5,6 Simultaneous blood cultures should also be collected, as 50% of cases are associated with bacteremia.
The in-hospital mortality rate of an episode of spontaneous bacterial peritonitis has been reduced to 10% to 20% thanks to prompt diagnosis and empiric treatment with third-generation cephalosporins.7
Five percent of cases of infected ascites fluid are due to secondary bacterial peritonitis from a perforated viscus or a loculated abscess, which cannot be differentiated clinically from spontaneous bacterial peritonitis but can be diagnosed with CT.8
This patient may be presenting with septic shock secondary to either of these causes.
Variceal hemorrhage
Half of patients with cirrhosis have gastroesophageal varices due to portal hypertension. Endoscopic surveillance is warranted, as the risk of hemorrhage is 12% to 15% per year, and the mortality rate approaches 15% to 20% with each episode. Prompt resuscitation, diagnosis, and control of bleeding is paramount.
Esophagogastroduodenoscopy is used for both diagnosis and intervention. Short-term prophylactic use of antibiotics improves survival by preventing infections in the event bleeding recurs.9–11
Our patient may be presenting with hemodynamic shock from bleeding esophageal varices.
Portal vein thrombosis
Portal vein thrombosis is a common complication of cirrhosis, occurring in 5% to 28% of patients. The risk increases with the severity of liver disease and in association with hepatocellular carcinoma.12 Forty-three percent of cases are discovered incidentally in asymptomatic patients during ultrasonography, 39% present with upper gastrointestinal bleeding, and 18% present with abdominal pain.13,14
Portal vein thrombosis is the complete or partial obstruction of blood flow due to a thrombus in the lumen of the portal vein. Contrast ultrasonography and CT can be used to establish the diagnosis.15
Anticoagulation is recommended in cases of complete thrombosis in candidates for living-donor liver transplant and for those at risk of mesenteric ischemia because of the thrombus extending into the mesenteric veins. In symptomatic patients, the decision to initiate anticoagulation should be made on a case-by-case basis after appropriate screening and management of varices.16–18
Our patient’s thrombocytopenia reflects the severity of portal hypertension and increases her risk of portal vein thrombosis, but this is unlikely to be the sole cause of the hemodynamic compromise in this patient.
Ruptured abdominal aortic aneurysm
Rupture of an abdominal aortic aneurysm is a medical emergency, with a mortality rate approaching 90%. Risk factors for abdominal aortic aneurysms are smoking, male sex, age over 65, history of cardiovascular disease, hypertension, and a family history of abdominal aortic aneurysm, especially if a first-degree relative is affected.19 Endovascular repair is associated with lower rates of death and complications compared with open repair.20
The patient does not have any of those risk factors, making this diagnosis less likely.
CASE CONTINUED: RUPTURED SPLENIC ARTERY ANEURYSM
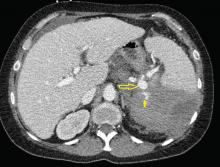
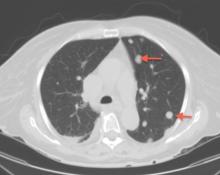
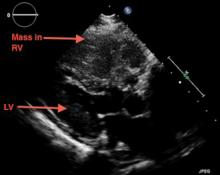
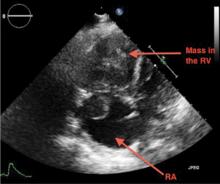
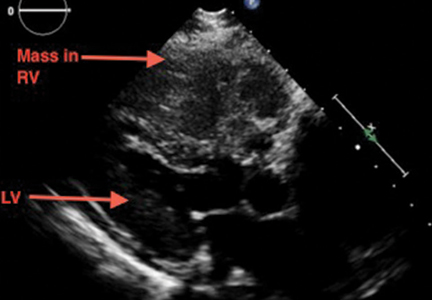
Emergency CT of the abdomen and pelvis with contrast enhancement shows a large left intraperitoneal hematoma with active extravasation from a ruptured splenic artery aneurysm (Figure 1). The patient receives packed red blood cells and fresh-frozen plasma before being transferred to our hospital.
2. Which of the following is false regarding splenic artery aneurysms?
- They are the most common type of splanchnic arterial aneurysm
- True aneurysms are more common than pseudoaneurysms
- Asymptomatic aneurysms are discovered incidentally during assessment for other radiographic indications
- Splenic artery aneurysm in portal hypertension is the result of athero-sclerotic changes to the vascular intima
Splenic artery aneurysm in portal hypertension is not the result of atherosclerotic change to the vascular intima.
Splenic artery aneurysms are the most common type of splanchnic artery aneurysm.1 True aneurysms involve all 3 layers of the arterial wall, ie, intima, media, and adventitia. Cirrhosis and portal hypertension are associated with true aneurysm formation. The proposed mechanism of aneurysm formation is increased splenic blood flow in response to portal congestion with resultant hemodynamic stress that disrupts arterial wall structure, leading to aneurysmal dilation.21
In earlier reports, the incidence of true splenic artery aneurysm in portal hypertension varied from 2.9% to 50%, the latter representing autopsy findings of small aneurysms that were found in the splenic hilum of patients with cirrhosis.22–25 The incidence of clinically significant aneurysms in cirrhosis is unknown but incidental asymptomatic aneurysm is being detected more frequently on imaging studies pursued for screening purposes.26
The risk of rupture is low, only 2% to 10% in older studies and likely even lower now due to increased incidental detection in asymptomatic patients.27 However, emergent management of rupture at a tertiary care facility is paramount, as the mortality rate of ruptured splenic artery aneurysm is 29% to 36%.1,26,28
Splenic artery pseudoaneurysm is rarer and has a different pathophysiologic process than true aneurysm. It usually arises in the setting of trauma, pancreatitis, or postsurgery.29,30 Pseudoaneurysm is more likely to rupture, owing to compromise in the vascular wall integrity.4,21,28 As a result, treatment is indicated for every pseudoaneurysm regardless of size.
RISK FACTORS FOR SPLENIC ARTERY ANEURYSM
3. Which of the following is true regarding our patient’s risk of splenic artery aneurysm?
- Liver cirrhosis and portal hypertension are her greatest risk factors for it
- Female sex and prior pregnancy are her greatest risk factors for it
- Being Native American makes it more likely that the patient has splenic artery aneurysm secondary to collagen vascular disease
- Her risk of rupture would diminish after receiving a liver transplant
Liver cirrhosis and portal hypertension are her greatest risk factors for splenic artery aneurysm.
Risk factors for true aneurysm include hypertension, atherosclerosis, portal hypertension with or without liver cirrhosis, liver transplant, third trimester of pregnancy, and multiparity.1,4,26,28,31 Splenic artery aneurysm is usually diagnosed in the sixth decade. It may be 4 times as common in women, given a hormonal influence.32 Cirrhosis is also associated with massive splenic artery aneurysm (≥ 5 cm). Although rare, massive splenic artery aneurysm is more frequent in men (the male-to-female ratio is 1.78:1) and has a heightened risk of rupture.28 The incidence of rupture increases to around 3% to 4% after liver transplant.33 Rare causes of true aneurysm include fibrodysplasia, collagen vascular disease (eg, Loeys-Dietz and type IV Ehler-Danlos syndromes), vasculitis (eg, polyarteritis nodosa due to amphetamine abuse), and mycotic aneurysms.24,25,28,29
This patient’s age, sex, and history of cirrhosis puts her at increased risk of splenic artery aneurysm. The risk of rupture is highest in the peripartum period and in patients with cirrhosis who become pregnant. Although being Native American portends an increased risk for collagen vascular disease, the latter is unlikely to be a contributing factor.
TREATMENT OF SPLENIC ARTERY ANEURYSM
4. Which of the following is false regarding treatment of splenic artery aneurysms?
- Aneurysms larger than 2 cm and those that are expanding require repair
- Treatment should be offered if the patient has symptoms attributable to the aneurysm
- Asymptomatic aneurysms in pregnant women can be followed with watchful waiting
- Minimally invasive therapies such as percutaneous embolization may be a good option in poor operative candidates
Asymptomatic aneurysms in pregnant women should not be followed with watchful waiting—they should be repaired, as rupture carries a maternal mortality rate of 75% and a fetal mortality rate of 95%.34
Complications of splenic artery aneurysm depend on the type of aneurysm and its predisposing factors. Indications for treatment of true aneurysms include:
- Symptoms attributable to the aneurysm (hence, the second answer choice above is true)
- Diameter 2 cm or greater or enlarging diameter (hence, the first answer choice is true)
- Women of childbearing age in anticipation of pregnancy
- Need for surgical intervention such as portocaval shunt and liver transplant.
Conservative management is associated with a late mortality risk of 4.9%.2 Interventional options include percutaneous embolization or stenting; or laparotomy with splenic artery ligation or excision with or without splenectomy.1,28,35–37
Endovascular and open surgical repair have both been used to treat splenic artery aneurysms. The method used depends on the patient’s surgical history and aneurysm anatomy such as splenic artery tortuosity hindering passage of a catheter. Open surgery is associated with longer intraoperative time and length of hospital stay and higher rates of 30-day mortality and perioperative morbidity.38–41 With endovascular repair, the complication of persistent or recurrent flow occurs in 3% to 5% of cases by 30 days; hence, postprocedural surveillance is recommended.42–44 Endovascular repair has a higher reintervention rate but may still be more cost-effective than open surgical repair.
Because patients with cirrhosis have a higher risk of surgical complications,45 elective endovascular treatment may be an option for patients with aneurysms at high risk of rupturing. Endovascular treatment of visceral aneurysms is associated with complications such as postembolization syndrome (fever, abdominal pain, pleural effusion, and pancreatitis), access site hematoma, splenic infarction, and persistent abdominal pain.42
Patients with cirrhosis as the cause of splenic artery aneurysm tend to need longer hospitalization after endovascular treatment, but there is insufficient evidence to suggest that they are at higher risk of other complications.37
CASE CONTINUED: SPLENIC ARTERY EMBOLIZATION
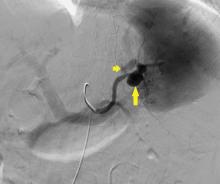
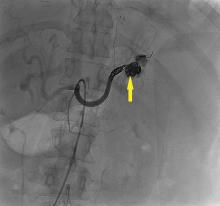
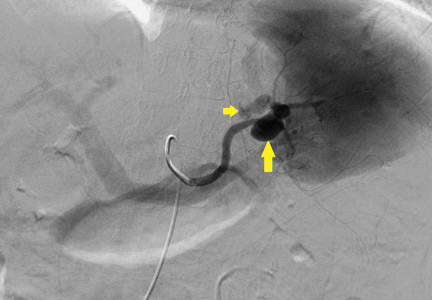
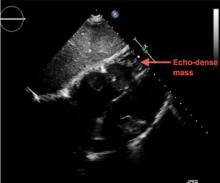
The patient undergoes emergency splenic artery embolization, performed by an interventional radiology team (Figure 2 and Figure 3). Over the next few days, her mental status improves and her abdominal pain resolves. Her hemoglobin level remains stable after the procedure.
The surgical and interventional radiology teams discuss the risk of repeat intervention with the patient and her family, who prefer a nonoperative approach. She is managed supportively in the intensive care unit and is finally discharged home in stable condition and is scheduled for outpatient follow-up.
SUSPECT THIS FATAL CONDITION
The low prevalence of ruptured splenic artery aneurysm may lead physicians to attribute septic shock to spontaneous bacterial peritonitis or hemorrhagic shock from gastroesophageal varices in patients with cirrhosis, but a high index of suspicion and early recognition of this rare disease can lead to timely diagnosis and treatment of this highly fatal complication.
KEY POINTS
- Splenic artery aneurysm is a common complication of cirrhosis, often diagnosed incidentally.
- Elective embolization should be considered for asymptomatic splenic artery aneurysms larger than 2 cm in diameter, clinically symptomatic aneurysms, women of childbearing age, and patients who are candidates for liver transplant.
- Although splenic artery aneurysm rupture is rare, it has a high mortality rate and warrants a high index of suspicion to institute prompt specialized intervention.
- We recommend that physicians consider splenic artery aneurysm rupture in their differential diagnoses in patients with liver cirrhosis presenting with abdominal pain, altered mental status, and hemodynamic shock.
- Bakhos CT, McIntosh BC, Nukta FA, et al. Staged arterial embolization and surgical resection of a giant splenic artery aneurysm. Ann Vasc Surg 2007; 21:208–210.
- Hogendoorn W, Lavida A, Hunink MG, et al. Open repair, endovascular repair, and conservative management of true splenic artery aneurysms. J Vasc Surg 2014; 60:1667–1676.e1.
- Algudkar A. Unruptured splenic artery aneurysm presenting as epigastric pain. JRSM Short Rep 2010; 1:24.
- Abbas MA, Stone WM, Fowl RJ, et al. Splenic artery aneurysms: two decades experience at Mayo Clinic. Ann Vasc Surg 2002; 16:442–449.
- Hoefs JC, Canawati HN, Sapico FL, Hopkins RR, Weiner J, Montgomerie JZ. Spontaneous bacterial peritonitis. Hepatology 1982; 2:399–407.
- Runyon BA, Hoefs JC. Culture-negative neutrocytic ascites: a variant of spontaneous bacterial peritonitis. Hepatology 1984; 4:1209–1211.
- Garcia-Tsao G. Spontaneous bacterial peritonitis: a historical perspective. J Hepatol 2004; 41:522–527.
- Soriano G, Castellote J, Alvarez C, et al. Secondary bacterial peritonitis in cirrhosis: a retrospective study of clinical and analytical characteristics, diagnosis and management. J Hepatol 2010; 52:39–44.
- D’Amico G, De Franchis R; Cooperative Study Group. Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology 2003; 38:599–612.
- Garcia-Tsao G, Sanyal AJ, Grace ND, Carey WD; Practice Guidelines Committee of American Association for Study of Liver Diseases; Practice Parameters Committee of American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Am J Gastroenterol 2007; 102:2086–2102.
- Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W; Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007; 46:922–938.
- Tsochatzis EA, Senzolo M, Germani G, Gatt A, Burroughs AK. Systematic review: portal vein thrombosis in cirrhosis. Aliment Pharmacol Ther 2010; 31:366–374.
- Kobori L, van der Kolk MJ, de Jong KP, et al. Splenic artery aneurysms in liver transplant patients. Liver Transplant Group. J Hepatol 1997; 27:890–893.
- Manzano-Robleda Mdel C, Barranco-Fragoso B, Uribe M, Mendez-Sanchez N. Portal vein thrombosis: what is new? Ann Hepatol 2015; 14:20–27.
- Sarin SK, Philips CA, Kamath PS, et al. Toward a comprehensive new classification of portal vein thrombosis in patients with cirrhosis. Gastroenterology 2016; 151:574–577.e3.
- DeLeve LD, Valla DC, Garcia-Tsao G; American Association for the Study of Liver Diseases. Vascular disorders of the liver. Hepatology 2009; 49:1729–1764.
- Manzanet G, Sanjuan F, Orbis P, et al. Liver transplantation in patients with portal vein thrombosis. Liver Transpl 2001; 7:125–131.
- John BV, Konjeti R, Aggarwal A, et al. Impact of untreated portal vein thrombosis on pre and post liver transplant outcomes in cirrhosis. Ann Hepatol 2013; 12:952–958.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al; American Association for Vascular Surgery/Society for Vascular Surgery; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology; ACC/AHA Task Force on Practice Guidelines. ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease)—summary of recommendations. J Vasc Interv Radiol 2006; 17:1383–1397.
- Schermerhorn ML, O’Malley AJ, Jhaveri A, Cotterill P, Pomposelli F, Landon BE. Endovascular vs open repair of abdominal aortic aneurysms in the Medicare population. N Engl J Med 2008; 358:464–474.
- Ohta M, Hashizume M, Ueno K, Tanoue K, Sugimachi K, Hasuo K. Hemodynamic study of splenic artery aneurysm in portal hypertension. Hepatogastroenterology 1994; 41:181–184.
- Sunagozaka H, Tsuji H, Mizukoshi E, et al. The development and clinical features of splenic aneurysm associated with liver cirrhosis. Liver Int 2006; 26:291–297.
- Manenti F, Williams R. Injection studies of the splenic vasculature in portal hypertension. Gut 1966; 7:175–180.
- Stanley JC, Fry WJ. Pathogenesis and clinical significance of splenic artery aneurysms. Surgery 1974; 76:898–909.
- Lee PC, Rhee RY, Gordon RY, Fung JJ, Webster MW. Management of splenic artery aneurysms: the significance of portal and essential hypertension. J Am Coll Surg 1999; 189:483–490.
- Al-Habbal Y, Christophi C, Muralidharan V. Aneurysms of the splenic artery—a review. Surgeon 2010; 8:223–231.
- Mattar SG, Lumsden AB. The management of splenic artery aneurysms: experience with 23 cases. Am J Surg 1995; 169:580–584.
- Akbulut S, Otan E. Management of giant splenic artery aneurysm: comprehensive literature review. Medicine (Baltimore) 2015; 94:e1016.
- Agrawal GA, Johnson PT, Fishman EK. Splenic artery aneurysms and pseudoaneurysms: clinical distinctions and CT appearances. AJR Am J Roentgenol 2007; 188:992–999.
- Tessier DJ, Stone WM, Fowl RJ, et al. Clinical features and management of splenic artery pseudoaneurysm: case series and cumulative review of literature. J Vasc Surg 2003; 38:969–974.
- Dave SP, Reis ED, Hossain A, Taub PJ, Kerstein MD, Hollier LH. Splenic artery aneurysm in the 1990s. Ann Vasc Surg 2000; 14:223–229.
- Parrish J, Maxwell C, Beecroft JR. Splenic artery aneurysm in pregnancy. J Obstet Gynaecol Can 2015; 37:816–818.
- Moon DB, Lee SG, Hwang S, et al. Characteristics and management of splenic artery aneurysms in adult living donor liver transplant recipients. Liver Transpl 2009; 15:1535–1541.
- Sadat U, Dar O, Walsh S, Varty K. Splenic artery aneurysms in pregnancy—a systematic review. Int J Surg 2008; 6:261–265.
- Geoghegan T, McAuley G, Snow A, Torreggiani WC. Emergency embolization of multiple splenic artery pseudoaneurysms associated with portal hypertension complicating cystic fibrosis. Australas Radiol 2007; 51(suppl):B337–B339.
- Jiang R, Ding X, Jian W, Jiang J, Hu S, Zhang Z. Combined endovascular embolization and open surgery for splenic artery aneurysm with arteriovenous fistula. Ann Vasc Surg 2016; 30:311.e1–311.e4.
- Naganuma M, Matsui H, Koizumi J, Fushimi K, Yasunaga H. Short-term outcomes following elective transcatheter arterial embolization for splenic artery aneurysms: data from a nationwide administrative database. Acta Radiol Open 2015; 4:2047981615574354.
- Batagini NC, El-Arousy H, Clair DG, Kirksey L. Open versus endovascular treatment of visceral artery aneurysms and pseudoaneurysms. Ann Vasc Surg 2016; 35:1–8.
- Marone EM, Mascia D, Kahlberg A, Brioschi C, Tshomba Y, Chiesa R. Is open repair still the gold standard in visceral artery aneurysm management? Ann Vasc Surg 2011; 25:936–946.
- Sticco A, Aggarwal A, Shapiro M, Pratt A, Rissuci D, D'Ayala M. A comparison of open and endovascular treatment strategies for the management of splenic artery aneurysms. Vascular 2016; 24:487–491.
- Hogendoorn W, Lavida A, Hunink MG, et al. Cost-effectiveness of endovascular repair, open repair, and conservative management of splenic artery aneurysms. J Vasc Surg 2015; 61:1432–1440.
- Fankhauser GT, Stone WM, Naidu SG, et al; Mayo Vascular Research Center Consortium. The minimally invasive management of visceral artery aneurysms and pseudoaneurysms. J Vasc Surg 2011; 53:966–970.
- Lagana D, Carrafiello G, Mangini M, et al. Multimodal approach to endovascular treatment of visceral artery aneurysms and pseudoaneurysms. Eur J Radiol 2006; 59:104–111.
- Guillon R, Garcier JM, Abergel A, et al. Management of splenic artery aneurysms and false aneurysms with endovascular treatment in 12 patients. Cardiovasc Intervent Radiol 2003; 26:256–260.
- Northup PG, Wanamaker RC, Lee VD, Adams RB, Berg CL. Model for end-stage liver disease (MELD) predicts nontransplant surgical mortality in patients with cirrhosis. Ann Surg 2005; 242:244–251.
A 53-year-old Native American woman with a history of liver cirrhosis secondary to alcohol abuse presents to the emergency department after 2 days of diffuse abdominal pain and weakness. The pain was sudden in onset and has progressed relentlessly over the last day, reaching 9 on a scale of 10 in severity. Family members say that her oral intake has been decreased for the last 2 days, but she has had no fever, vomiting, change in bowel habit, blood in stool, or black stool. She has never undergone surgery, and has had one uncomplicated pregnancy.
Physical examination
Vital signs:
- Blood pressure 82/57 mm Hg
- Heart rate 96 beats per minute
- Temperature 37.3°C (99.1°F)
- Respiratory rate 16 per minute
- Oxygen saturation 92% while receiving oxygen at 2 L/minute.
The patient is somnolent and has scleral icterus. Her cardiopulmonary examination is normal. Her abdomen is tense, distended, and diffusely tender. She has bilateral +2 pitting edema in her lower extremities. She is oriented to person only and is noted to have asterixis. Her baseline Model for End-stage Liver Disease score is 18 points on a scale of 6 (less ill) to 40 (gravely ill).
Laboratory studies:
- Hemoglobin 9.8 g/dL (reference range 11.5–15.5)
- Platelet count 100 × 109/L (150–400)
- White blood cell count 9.9 × 109/L (3.7–11.0)
- Serum creatinine 1.06 mg/dL (0.58–0.96)
- Bilirubin 6.3 mg/dL (0.2–1.3)
- International normalized ratio of the prothrombin time 2.15 (0.8–1.2)
- Blood urea nitrogen 13 mg/dL (7–21)
- Serum albumin 2.7 g/dL (3.9–4.9).
Intravenous fluid resuscitation is initiated but the patient remains hypotensive, and on repeat laboratory testing 4 hours later her hemoglobin level has dropped to 7.3 mg/dL.
DIFFERENTIAL DIAGNOSIS
1. Which of the following are likely causes of this patient’s presentation?
- Splenic arterial aneurysm rupture
- Spontaneous bacterial peritonitis
- Variceal hemorrhage
- Portal vein thrombosis
- Abdominal aortic aneurysm rupture
Ruptured splenic artery aneurysm
Splenic artery aneurysms are the third most common intra-abdominal aneurysm, after those of the abdominal aorta and iliac artery.1 They are often asymptomatic and are being detected more frequently because of increased use of computed tomography (CT).2 Symptomatic splenic artery aneurysms may present with abdominal pain and have the potential to rupture, which can be life-threatening.3,4
This patient may have a ruptured splenic artery aneurysm, given her hemodynamic shock.
Spontaneous bacterial peritonitis
Ten percent to 20% of hospitalized patients with cirrhosis and ascites develop spontaneous bacterial peritonitis. Patients may present with ascites and abdominal pain, tenderness to palpation, fever, encephalopathy, or worsening liver and renal function.
Diagnostic paracentesis is paramount to delineate the cause of ascites; one should calculate the serum-ascites albumin gradient and obtain a cell count and culture of the ascitic fluid. The diagnosis of spontaneous bacterial peritonitis can be made if the ascitic fluid polymorphonuclear cell count is 0.25 × 109/L or higher, even if the ascitic fluid culture is negative.5,6 Simultaneous blood cultures should also be collected, as 50% of cases are associated with bacteremia.
The in-hospital mortality rate of an episode of spontaneous bacterial peritonitis has been reduced to 10% to 20% thanks to prompt diagnosis and empiric treatment with third-generation cephalosporins.7
Five percent of cases of infected ascites fluid are due to secondary bacterial peritonitis from a perforated viscus or a loculated abscess, which cannot be differentiated clinically from spontaneous bacterial peritonitis but can be diagnosed with CT.8
This patient may be presenting with septic shock secondary to either of these causes.
Variceal hemorrhage
Half of patients with cirrhosis have gastroesophageal varices due to portal hypertension. Endoscopic surveillance is warranted, as the risk of hemorrhage is 12% to 15% per year, and the mortality rate approaches 15% to 20% with each episode. Prompt resuscitation, diagnosis, and control of bleeding is paramount.
Esophagogastroduodenoscopy is used for both diagnosis and intervention. Short-term prophylactic use of antibiotics improves survival by preventing infections in the event bleeding recurs.9–11
Our patient may be presenting with hemodynamic shock from bleeding esophageal varices.
Portal vein thrombosis
Portal vein thrombosis is a common complication of cirrhosis, occurring in 5% to 28% of patients. The risk increases with the severity of liver disease and in association with hepatocellular carcinoma.12 Forty-three percent of cases are discovered incidentally in asymptomatic patients during ultrasonography, 39% present with upper gastrointestinal bleeding, and 18% present with abdominal pain.13,14
Portal vein thrombosis is the complete or partial obstruction of blood flow due to a thrombus in the lumen of the portal vein. Contrast ultrasonography and CT can be used to establish the diagnosis.15
Anticoagulation is recommended in cases of complete thrombosis in candidates for living-donor liver transplant and for those at risk of mesenteric ischemia because of the thrombus extending into the mesenteric veins. In symptomatic patients, the decision to initiate anticoagulation should be made on a case-by-case basis after appropriate screening and management of varices.16–18
Our patient’s thrombocytopenia reflects the severity of portal hypertension and increases her risk of portal vein thrombosis, but this is unlikely to be the sole cause of the hemodynamic compromise in this patient.
Ruptured abdominal aortic aneurysm
Rupture of an abdominal aortic aneurysm is a medical emergency, with a mortality rate approaching 90%. Risk factors for abdominal aortic aneurysms are smoking, male sex, age over 65, history of cardiovascular disease, hypertension, and a family history of abdominal aortic aneurysm, especially if a first-degree relative is affected.19 Endovascular repair is associated with lower rates of death and complications compared with open repair.20
The patient does not have any of those risk factors, making this diagnosis less likely.
CASE CONTINUED: RUPTURED SPLENIC ARTERY ANEURYSM
Emergency CT of the abdomen and pelvis with contrast enhancement shows a large left intraperitoneal hematoma with active extravasation from a ruptured splenic artery aneurysm (Figure 1). The patient receives packed red blood cells and fresh-frozen plasma before being transferred to our hospital.
2. Which of the following is false regarding splenic artery aneurysms?
- They are the most common type of splanchnic arterial aneurysm
- True aneurysms are more common than pseudoaneurysms
- Asymptomatic aneurysms are discovered incidentally during assessment for other radiographic indications
- Splenic artery aneurysm in portal hypertension is the result of athero-sclerotic changes to the vascular intima
Splenic artery aneurysm in portal hypertension is not the result of atherosclerotic change to the vascular intima.
Splenic artery aneurysms are the most common type of splanchnic artery aneurysm.1 True aneurysms involve all 3 layers of the arterial wall, ie, intima, media, and adventitia. Cirrhosis and portal hypertension are associated with true aneurysm formation. The proposed mechanism of aneurysm formation is increased splenic blood flow in response to portal congestion with resultant hemodynamic stress that disrupts arterial wall structure, leading to aneurysmal dilation.21
In earlier reports, the incidence of true splenic artery aneurysm in portal hypertension varied from 2.9% to 50%, the latter representing autopsy findings of small aneurysms that were found in the splenic hilum of patients with cirrhosis.22–25 The incidence of clinically significant aneurysms in cirrhosis is unknown but incidental asymptomatic aneurysm is being detected more frequently on imaging studies pursued for screening purposes.26
The risk of rupture is low, only 2% to 10% in older studies and likely even lower now due to increased incidental detection in asymptomatic patients.27 However, emergent management of rupture at a tertiary care facility is paramount, as the mortality rate of ruptured splenic artery aneurysm is 29% to 36%.1,26,28
Splenic artery pseudoaneurysm is rarer and has a different pathophysiologic process than true aneurysm. It usually arises in the setting of trauma, pancreatitis, or postsurgery.29,30 Pseudoaneurysm is more likely to rupture, owing to compromise in the vascular wall integrity.4,21,28 As a result, treatment is indicated for every pseudoaneurysm regardless of size.
RISK FACTORS FOR SPLENIC ARTERY ANEURYSM
3. Which of the following is true regarding our patient’s risk of splenic artery aneurysm?
- Liver cirrhosis and portal hypertension are her greatest risk factors for it
- Female sex and prior pregnancy are her greatest risk factors for it
- Being Native American makes it more likely that the patient has splenic artery aneurysm secondary to collagen vascular disease
- Her risk of rupture would diminish after receiving a liver transplant
Liver cirrhosis and portal hypertension are her greatest risk factors for splenic artery aneurysm.
Risk factors for true aneurysm include hypertension, atherosclerosis, portal hypertension with or without liver cirrhosis, liver transplant, third trimester of pregnancy, and multiparity.1,4,26,28,31 Splenic artery aneurysm is usually diagnosed in the sixth decade. It may be 4 times as common in women, given a hormonal influence.32 Cirrhosis is also associated with massive splenic artery aneurysm (≥ 5 cm). Although rare, massive splenic artery aneurysm is more frequent in men (the male-to-female ratio is 1.78:1) and has a heightened risk of rupture.28 The incidence of rupture increases to around 3% to 4% after liver transplant.33 Rare causes of true aneurysm include fibrodysplasia, collagen vascular disease (eg, Loeys-Dietz and type IV Ehler-Danlos syndromes), vasculitis (eg, polyarteritis nodosa due to amphetamine abuse), and mycotic aneurysms.24,25,28,29
This patient’s age, sex, and history of cirrhosis puts her at increased risk of splenic artery aneurysm. The risk of rupture is highest in the peripartum period and in patients with cirrhosis who become pregnant. Although being Native American portends an increased risk for collagen vascular disease, the latter is unlikely to be a contributing factor.
TREATMENT OF SPLENIC ARTERY ANEURYSM
4. Which of the following is false regarding treatment of splenic artery aneurysms?
- Aneurysms larger than 2 cm and those that are expanding require repair
- Treatment should be offered if the patient has symptoms attributable to the aneurysm
- Asymptomatic aneurysms in pregnant women can be followed with watchful waiting
- Minimally invasive therapies such as percutaneous embolization may be a good option in poor operative candidates
Asymptomatic aneurysms in pregnant women should not be followed with watchful waiting—they should be repaired, as rupture carries a maternal mortality rate of 75% and a fetal mortality rate of 95%.34
Complications of splenic artery aneurysm depend on the type of aneurysm and its predisposing factors. Indications for treatment of true aneurysms include:
- Symptoms attributable to the aneurysm (hence, the second answer choice above is true)
- Diameter 2 cm or greater or enlarging diameter (hence, the first answer choice is true)
- Women of childbearing age in anticipation of pregnancy
- Need for surgical intervention such as portocaval shunt and liver transplant.
Conservative management is associated with a late mortality risk of 4.9%.2 Interventional options include percutaneous embolization or stenting; or laparotomy with splenic artery ligation or excision with or without splenectomy.1,28,35–37
Endovascular and open surgical repair have both been used to treat splenic artery aneurysms. The method used depends on the patient’s surgical history and aneurysm anatomy such as splenic artery tortuosity hindering passage of a catheter. Open surgery is associated with longer intraoperative time and length of hospital stay and higher rates of 30-day mortality and perioperative morbidity.38–41 With endovascular repair, the complication of persistent or recurrent flow occurs in 3% to 5% of cases by 30 days; hence, postprocedural surveillance is recommended.42–44 Endovascular repair has a higher reintervention rate but may still be more cost-effective than open surgical repair.
Because patients with cirrhosis have a higher risk of surgical complications,45 elective endovascular treatment may be an option for patients with aneurysms at high risk of rupturing. Endovascular treatment of visceral aneurysms is associated with complications such as postembolization syndrome (fever, abdominal pain, pleural effusion, and pancreatitis), access site hematoma, splenic infarction, and persistent abdominal pain.42
Patients with cirrhosis as the cause of splenic artery aneurysm tend to need longer hospitalization after endovascular treatment, but there is insufficient evidence to suggest that they are at higher risk of other complications.37
CASE CONTINUED: SPLENIC ARTERY EMBOLIZATION
The patient undergoes emergency splenic artery embolization, performed by an interventional radiology team (Figure 2 and Figure 3). Over the next few days, her mental status improves and her abdominal pain resolves. Her hemoglobin level remains stable after the procedure.
The surgical and interventional radiology teams discuss the risk of repeat intervention with the patient and her family, who prefer a nonoperative approach. She is managed supportively in the intensive care unit and is finally discharged home in stable condition and is scheduled for outpatient follow-up.
SUSPECT THIS FATAL CONDITION
The low prevalence of ruptured splenic artery aneurysm may lead physicians to attribute septic shock to spontaneous bacterial peritonitis or hemorrhagic shock from gastroesophageal varices in patients with cirrhosis, but a high index of suspicion and early recognition of this rare disease can lead to timely diagnosis and treatment of this highly fatal complication.
KEY POINTS
- Splenic artery aneurysm is a common complication of cirrhosis, often diagnosed incidentally.
- Elective embolization should be considered for asymptomatic splenic artery aneurysms larger than 2 cm in diameter, clinically symptomatic aneurysms, women of childbearing age, and patients who are candidates for liver transplant.
- Although splenic artery aneurysm rupture is rare, it has a high mortality rate and warrants a high index of suspicion to institute prompt specialized intervention.
- We recommend that physicians consider splenic artery aneurysm rupture in their differential diagnoses in patients with liver cirrhosis presenting with abdominal pain, altered mental status, and hemodynamic shock.
A 53-year-old Native American woman with a history of liver cirrhosis secondary to alcohol abuse presents to the emergency department after 2 days of diffuse abdominal pain and weakness. The pain was sudden in onset and has progressed relentlessly over the last day, reaching 9 on a scale of 10 in severity. Family members say that her oral intake has been decreased for the last 2 days, but she has had no fever, vomiting, change in bowel habit, blood in stool, or black stool. She has never undergone surgery, and has had one uncomplicated pregnancy.
Physical examination
Vital signs:
- Blood pressure 82/57 mm Hg
- Heart rate 96 beats per minute
- Temperature 37.3°C (99.1°F)
- Respiratory rate 16 per minute
- Oxygen saturation 92% while receiving oxygen at 2 L/minute.
The patient is somnolent and has scleral icterus. Her cardiopulmonary examination is normal. Her abdomen is tense, distended, and diffusely tender. She has bilateral +2 pitting edema in her lower extremities. She is oriented to person only and is noted to have asterixis. Her baseline Model for End-stage Liver Disease score is 18 points on a scale of 6 (less ill) to 40 (gravely ill).
Laboratory studies:
- Hemoglobin 9.8 g/dL (reference range 11.5–15.5)
- Platelet count 100 × 109/L (150–400)
- White blood cell count 9.9 × 109/L (3.7–11.0)
- Serum creatinine 1.06 mg/dL (0.58–0.96)
- Bilirubin 6.3 mg/dL (0.2–1.3)
- International normalized ratio of the prothrombin time 2.15 (0.8–1.2)
- Blood urea nitrogen 13 mg/dL (7–21)
- Serum albumin 2.7 g/dL (3.9–4.9).
Intravenous fluid resuscitation is initiated but the patient remains hypotensive, and on repeat laboratory testing 4 hours later her hemoglobin level has dropped to 7.3 mg/dL.
DIFFERENTIAL DIAGNOSIS
1. Which of the following are likely causes of this patient’s presentation?
- Splenic arterial aneurysm rupture
- Spontaneous bacterial peritonitis
- Variceal hemorrhage
- Portal vein thrombosis
- Abdominal aortic aneurysm rupture
Ruptured splenic artery aneurysm
Splenic artery aneurysms are the third most common intra-abdominal aneurysm, after those of the abdominal aorta and iliac artery.1 They are often asymptomatic and are being detected more frequently because of increased use of computed tomography (CT).2 Symptomatic splenic artery aneurysms may present with abdominal pain and have the potential to rupture, which can be life-threatening.3,4
This patient may have a ruptured splenic artery aneurysm, given her hemodynamic shock.
Spontaneous bacterial peritonitis
Ten percent to 20% of hospitalized patients with cirrhosis and ascites develop spontaneous bacterial peritonitis. Patients may present with ascites and abdominal pain, tenderness to palpation, fever, encephalopathy, or worsening liver and renal function.
Diagnostic paracentesis is paramount to delineate the cause of ascites; one should calculate the serum-ascites albumin gradient and obtain a cell count and culture of the ascitic fluid. The diagnosis of spontaneous bacterial peritonitis can be made if the ascitic fluid polymorphonuclear cell count is 0.25 × 109/L or higher, even if the ascitic fluid culture is negative.5,6 Simultaneous blood cultures should also be collected, as 50% of cases are associated with bacteremia.
The in-hospital mortality rate of an episode of spontaneous bacterial peritonitis has been reduced to 10% to 20% thanks to prompt diagnosis and empiric treatment with third-generation cephalosporins.7
Five percent of cases of infected ascites fluid are due to secondary bacterial peritonitis from a perforated viscus or a loculated abscess, which cannot be differentiated clinically from spontaneous bacterial peritonitis but can be diagnosed with CT.8
This patient may be presenting with septic shock secondary to either of these causes.
Variceal hemorrhage
Half of patients with cirrhosis have gastroesophageal varices due to portal hypertension. Endoscopic surveillance is warranted, as the risk of hemorrhage is 12% to 15% per year, and the mortality rate approaches 15% to 20% with each episode. Prompt resuscitation, diagnosis, and control of bleeding is paramount.
Esophagogastroduodenoscopy is used for both diagnosis and intervention. Short-term prophylactic use of antibiotics improves survival by preventing infections in the event bleeding recurs.9–11
Our patient may be presenting with hemodynamic shock from bleeding esophageal varices.
Portal vein thrombosis
Portal vein thrombosis is a common complication of cirrhosis, occurring in 5% to 28% of patients. The risk increases with the severity of liver disease and in association with hepatocellular carcinoma.12 Forty-three percent of cases are discovered incidentally in asymptomatic patients during ultrasonography, 39% present with upper gastrointestinal bleeding, and 18% present with abdominal pain.13,14
Portal vein thrombosis is the complete or partial obstruction of blood flow due to a thrombus in the lumen of the portal vein. Contrast ultrasonography and CT can be used to establish the diagnosis.15
Anticoagulation is recommended in cases of complete thrombosis in candidates for living-donor liver transplant and for those at risk of mesenteric ischemia because of the thrombus extending into the mesenteric veins. In symptomatic patients, the decision to initiate anticoagulation should be made on a case-by-case basis after appropriate screening and management of varices.16–18
Our patient’s thrombocytopenia reflects the severity of portal hypertension and increases her risk of portal vein thrombosis, but this is unlikely to be the sole cause of the hemodynamic compromise in this patient.
Ruptured abdominal aortic aneurysm
Rupture of an abdominal aortic aneurysm is a medical emergency, with a mortality rate approaching 90%. Risk factors for abdominal aortic aneurysms are smoking, male sex, age over 65, history of cardiovascular disease, hypertension, and a family history of abdominal aortic aneurysm, especially if a first-degree relative is affected.19 Endovascular repair is associated with lower rates of death and complications compared with open repair.20
The patient does not have any of those risk factors, making this diagnosis less likely.
CASE CONTINUED: RUPTURED SPLENIC ARTERY ANEURYSM
Emergency CT of the abdomen and pelvis with contrast enhancement shows a large left intraperitoneal hematoma with active extravasation from a ruptured splenic artery aneurysm (Figure 1). The patient receives packed red blood cells and fresh-frozen plasma before being transferred to our hospital.
2. Which of the following is false regarding splenic artery aneurysms?
- They are the most common type of splanchnic arterial aneurysm
- True aneurysms are more common than pseudoaneurysms
- Asymptomatic aneurysms are discovered incidentally during assessment for other radiographic indications
- Splenic artery aneurysm in portal hypertension is the result of athero-sclerotic changes to the vascular intima
Splenic artery aneurysm in portal hypertension is not the result of atherosclerotic change to the vascular intima.
Splenic artery aneurysms are the most common type of splanchnic artery aneurysm.1 True aneurysms involve all 3 layers of the arterial wall, ie, intima, media, and adventitia. Cirrhosis and portal hypertension are associated with true aneurysm formation. The proposed mechanism of aneurysm formation is increased splenic blood flow in response to portal congestion with resultant hemodynamic stress that disrupts arterial wall structure, leading to aneurysmal dilation.21
In earlier reports, the incidence of true splenic artery aneurysm in portal hypertension varied from 2.9% to 50%, the latter representing autopsy findings of small aneurysms that were found in the splenic hilum of patients with cirrhosis.22–25 The incidence of clinically significant aneurysms in cirrhosis is unknown but incidental asymptomatic aneurysm is being detected more frequently on imaging studies pursued for screening purposes.26
The risk of rupture is low, only 2% to 10% in older studies and likely even lower now due to increased incidental detection in asymptomatic patients.27 However, emergent management of rupture at a tertiary care facility is paramount, as the mortality rate of ruptured splenic artery aneurysm is 29% to 36%.1,26,28
Splenic artery pseudoaneurysm is rarer and has a different pathophysiologic process than true aneurysm. It usually arises in the setting of trauma, pancreatitis, or postsurgery.29,30 Pseudoaneurysm is more likely to rupture, owing to compromise in the vascular wall integrity.4,21,28 As a result, treatment is indicated for every pseudoaneurysm regardless of size.
RISK FACTORS FOR SPLENIC ARTERY ANEURYSM
3. Which of the following is true regarding our patient’s risk of splenic artery aneurysm?
- Liver cirrhosis and portal hypertension are her greatest risk factors for it
- Female sex and prior pregnancy are her greatest risk factors for it
- Being Native American makes it more likely that the patient has splenic artery aneurysm secondary to collagen vascular disease
- Her risk of rupture would diminish after receiving a liver transplant
Liver cirrhosis and portal hypertension are her greatest risk factors for splenic artery aneurysm.
Risk factors for true aneurysm include hypertension, atherosclerosis, portal hypertension with or without liver cirrhosis, liver transplant, third trimester of pregnancy, and multiparity.1,4,26,28,31 Splenic artery aneurysm is usually diagnosed in the sixth decade. It may be 4 times as common in women, given a hormonal influence.32 Cirrhosis is also associated with massive splenic artery aneurysm (≥ 5 cm). Although rare, massive splenic artery aneurysm is more frequent in men (the male-to-female ratio is 1.78:1) and has a heightened risk of rupture.28 The incidence of rupture increases to around 3% to 4% after liver transplant.33 Rare causes of true aneurysm include fibrodysplasia, collagen vascular disease (eg, Loeys-Dietz and type IV Ehler-Danlos syndromes), vasculitis (eg, polyarteritis nodosa due to amphetamine abuse), and mycotic aneurysms.24,25,28,29
This patient’s age, sex, and history of cirrhosis puts her at increased risk of splenic artery aneurysm. The risk of rupture is highest in the peripartum period and in patients with cirrhosis who become pregnant. Although being Native American portends an increased risk for collagen vascular disease, the latter is unlikely to be a contributing factor.
TREATMENT OF SPLENIC ARTERY ANEURYSM
4. Which of the following is false regarding treatment of splenic artery aneurysms?
- Aneurysms larger than 2 cm and those that are expanding require repair
- Treatment should be offered if the patient has symptoms attributable to the aneurysm
- Asymptomatic aneurysms in pregnant women can be followed with watchful waiting
- Minimally invasive therapies such as percutaneous embolization may be a good option in poor operative candidates
Asymptomatic aneurysms in pregnant women should not be followed with watchful waiting—they should be repaired, as rupture carries a maternal mortality rate of 75% and a fetal mortality rate of 95%.34
Complications of splenic artery aneurysm depend on the type of aneurysm and its predisposing factors. Indications for treatment of true aneurysms include:
- Symptoms attributable to the aneurysm (hence, the second answer choice above is true)
- Diameter 2 cm or greater or enlarging diameter (hence, the first answer choice is true)
- Women of childbearing age in anticipation of pregnancy
- Need for surgical intervention such as portocaval shunt and liver transplant.
Conservative management is associated with a late mortality risk of 4.9%.2 Interventional options include percutaneous embolization or stenting; or laparotomy with splenic artery ligation or excision with or without splenectomy.1,28,35–37
Endovascular and open surgical repair have both been used to treat splenic artery aneurysms. The method used depends on the patient’s surgical history and aneurysm anatomy such as splenic artery tortuosity hindering passage of a catheter. Open surgery is associated with longer intraoperative time and length of hospital stay and higher rates of 30-day mortality and perioperative morbidity.38–41 With endovascular repair, the complication of persistent or recurrent flow occurs in 3% to 5% of cases by 30 days; hence, postprocedural surveillance is recommended.42–44 Endovascular repair has a higher reintervention rate but may still be more cost-effective than open surgical repair.
Because patients with cirrhosis have a higher risk of surgical complications,45 elective endovascular treatment may be an option for patients with aneurysms at high risk of rupturing. Endovascular treatment of visceral aneurysms is associated with complications such as postembolization syndrome (fever, abdominal pain, pleural effusion, and pancreatitis), access site hematoma, splenic infarction, and persistent abdominal pain.42
Patients with cirrhosis as the cause of splenic artery aneurysm tend to need longer hospitalization after endovascular treatment, but there is insufficient evidence to suggest that they are at higher risk of other complications.37
CASE CONTINUED: SPLENIC ARTERY EMBOLIZATION
The patient undergoes emergency splenic artery embolization, performed by an interventional radiology team (Figure 2 and Figure 3). Over the next few days, her mental status improves and her abdominal pain resolves. Her hemoglobin level remains stable after the procedure.
The surgical and interventional radiology teams discuss the risk of repeat intervention with the patient and her family, who prefer a nonoperative approach. She is managed supportively in the intensive care unit and is finally discharged home in stable condition and is scheduled for outpatient follow-up.
SUSPECT THIS FATAL CONDITION
The low prevalence of ruptured splenic artery aneurysm may lead physicians to attribute septic shock to spontaneous bacterial peritonitis or hemorrhagic shock from gastroesophageal varices in patients with cirrhosis, but a high index of suspicion and early recognition of this rare disease can lead to timely diagnosis and treatment of this highly fatal complication.
KEY POINTS
- Splenic artery aneurysm is a common complication of cirrhosis, often diagnosed incidentally.
- Elective embolization should be considered for asymptomatic splenic artery aneurysms larger than 2 cm in diameter, clinically symptomatic aneurysms, women of childbearing age, and patients who are candidates for liver transplant.
- Although splenic artery aneurysm rupture is rare, it has a high mortality rate and warrants a high index of suspicion to institute prompt specialized intervention.
- We recommend that physicians consider splenic artery aneurysm rupture in their differential diagnoses in patients with liver cirrhosis presenting with abdominal pain, altered mental status, and hemodynamic shock.
- Bakhos CT, McIntosh BC, Nukta FA, et al. Staged arterial embolization and surgical resection of a giant splenic artery aneurysm. Ann Vasc Surg 2007; 21:208–210.
- Hogendoorn W, Lavida A, Hunink MG, et al. Open repair, endovascular repair, and conservative management of true splenic artery aneurysms. J Vasc Surg 2014; 60:1667–1676.e1.
- Algudkar A. Unruptured splenic artery aneurysm presenting as epigastric pain. JRSM Short Rep 2010; 1:24.
- Abbas MA, Stone WM, Fowl RJ, et al. Splenic artery aneurysms: two decades experience at Mayo Clinic. Ann Vasc Surg 2002; 16:442–449.
- Hoefs JC, Canawati HN, Sapico FL, Hopkins RR, Weiner J, Montgomerie JZ. Spontaneous bacterial peritonitis. Hepatology 1982; 2:399–407.
- Runyon BA, Hoefs JC. Culture-negative neutrocytic ascites: a variant of spontaneous bacterial peritonitis. Hepatology 1984; 4:1209–1211.
- Garcia-Tsao G. Spontaneous bacterial peritonitis: a historical perspective. J Hepatol 2004; 41:522–527.
- Soriano G, Castellote J, Alvarez C, et al. Secondary bacterial peritonitis in cirrhosis: a retrospective study of clinical and analytical characteristics, diagnosis and management. J Hepatol 2010; 52:39–44.
- D’Amico G, De Franchis R; Cooperative Study Group. Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology 2003; 38:599–612.
- Garcia-Tsao G, Sanyal AJ, Grace ND, Carey WD; Practice Guidelines Committee of American Association for Study of Liver Diseases; Practice Parameters Committee of American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Am J Gastroenterol 2007; 102:2086–2102.
- Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W; Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007; 46:922–938.
- Tsochatzis EA, Senzolo M, Germani G, Gatt A, Burroughs AK. Systematic review: portal vein thrombosis in cirrhosis. Aliment Pharmacol Ther 2010; 31:366–374.
- Kobori L, van der Kolk MJ, de Jong KP, et al. Splenic artery aneurysms in liver transplant patients. Liver Transplant Group. J Hepatol 1997; 27:890–893.
- Manzano-Robleda Mdel C, Barranco-Fragoso B, Uribe M, Mendez-Sanchez N. Portal vein thrombosis: what is new? Ann Hepatol 2015; 14:20–27.
- Sarin SK, Philips CA, Kamath PS, et al. Toward a comprehensive new classification of portal vein thrombosis in patients with cirrhosis. Gastroenterology 2016; 151:574–577.e3.
- DeLeve LD, Valla DC, Garcia-Tsao G; American Association for the Study of Liver Diseases. Vascular disorders of the liver. Hepatology 2009; 49:1729–1764.
- Manzanet G, Sanjuan F, Orbis P, et al. Liver transplantation in patients with portal vein thrombosis. Liver Transpl 2001; 7:125–131.
- John BV, Konjeti R, Aggarwal A, et al. Impact of untreated portal vein thrombosis on pre and post liver transplant outcomes in cirrhosis. Ann Hepatol 2013; 12:952–958.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al; American Association for Vascular Surgery/Society for Vascular Surgery; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology; ACC/AHA Task Force on Practice Guidelines. ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease)—summary of recommendations. J Vasc Interv Radiol 2006; 17:1383–1397.
- Schermerhorn ML, O’Malley AJ, Jhaveri A, Cotterill P, Pomposelli F, Landon BE. Endovascular vs open repair of abdominal aortic aneurysms in the Medicare population. N Engl J Med 2008; 358:464–474.
- Ohta M, Hashizume M, Ueno K, Tanoue K, Sugimachi K, Hasuo K. Hemodynamic study of splenic artery aneurysm in portal hypertension. Hepatogastroenterology 1994; 41:181–184.
- Sunagozaka H, Tsuji H, Mizukoshi E, et al. The development and clinical features of splenic aneurysm associated with liver cirrhosis. Liver Int 2006; 26:291–297.
- Manenti F, Williams R. Injection studies of the splenic vasculature in portal hypertension. Gut 1966; 7:175–180.
- Stanley JC, Fry WJ. Pathogenesis and clinical significance of splenic artery aneurysms. Surgery 1974; 76:898–909.
- Lee PC, Rhee RY, Gordon RY, Fung JJ, Webster MW. Management of splenic artery aneurysms: the significance of portal and essential hypertension. J Am Coll Surg 1999; 189:483–490.
- Al-Habbal Y, Christophi C, Muralidharan V. Aneurysms of the splenic artery—a review. Surgeon 2010; 8:223–231.
- Mattar SG, Lumsden AB. The management of splenic artery aneurysms: experience with 23 cases. Am J Surg 1995; 169:580–584.
- Akbulut S, Otan E. Management of giant splenic artery aneurysm: comprehensive literature review. Medicine (Baltimore) 2015; 94:e1016.
- Agrawal GA, Johnson PT, Fishman EK. Splenic artery aneurysms and pseudoaneurysms: clinical distinctions and CT appearances. AJR Am J Roentgenol 2007; 188:992–999.
- Tessier DJ, Stone WM, Fowl RJ, et al. Clinical features and management of splenic artery pseudoaneurysm: case series and cumulative review of literature. J Vasc Surg 2003; 38:969–974.
- Dave SP, Reis ED, Hossain A, Taub PJ, Kerstein MD, Hollier LH. Splenic artery aneurysm in the 1990s. Ann Vasc Surg 2000; 14:223–229.
- Parrish J, Maxwell C, Beecroft JR. Splenic artery aneurysm in pregnancy. J Obstet Gynaecol Can 2015; 37:816–818.
- Moon DB, Lee SG, Hwang S, et al. Characteristics and management of splenic artery aneurysms in adult living donor liver transplant recipients. Liver Transpl 2009; 15:1535–1541.
- Sadat U, Dar O, Walsh S, Varty K. Splenic artery aneurysms in pregnancy—a systematic review. Int J Surg 2008; 6:261–265.
- Geoghegan T, McAuley G, Snow A, Torreggiani WC. Emergency embolization of multiple splenic artery pseudoaneurysms associated with portal hypertension complicating cystic fibrosis. Australas Radiol 2007; 51(suppl):B337–B339.
- Jiang R, Ding X, Jian W, Jiang J, Hu S, Zhang Z. Combined endovascular embolization and open surgery for splenic artery aneurysm with arteriovenous fistula. Ann Vasc Surg 2016; 30:311.e1–311.e4.
- Naganuma M, Matsui H, Koizumi J, Fushimi K, Yasunaga H. Short-term outcomes following elective transcatheter arterial embolization for splenic artery aneurysms: data from a nationwide administrative database. Acta Radiol Open 2015; 4:2047981615574354.
- Batagini NC, El-Arousy H, Clair DG, Kirksey L. Open versus endovascular treatment of visceral artery aneurysms and pseudoaneurysms. Ann Vasc Surg 2016; 35:1–8.
- Marone EM, Mascia D, Kahlberg A, Brioschi C, Tshomba Y, Chiesa R. Is open repair still the gold standard in visceral artery aneurysm management? Ann Vasc Surg 2011; 25:936–946.
- Sticco A, Aggarwal A, Shapiro M, Pratt A, Rissuci D, D'Ayala M. A comparison of open and endovascular treatment strategies for the management of splenic artery aneurysms. Vascular 2016; 24:487–491.
- Hogendoorn W, Lavida A, Hunink MG, et al. Cost-effectiveness of endovascular repair, open repair, and conservative management of splenic artery aneurysms. J Vasc Surg 2015; 61:1432–1440.
- Fankhauser GT, Stone WM, Naidu SG, et al; Mayo Vascular Research Center Consortium. The minimally invasive management of visceral artery aneurysms and pseudoaneurysms. J Vasc Surg 2011; 53:966–970.
- Lagana D, Carrafiello G, Mangini M, et al. Multimodal approach to endovascular treatment of visceral artery aneurysms and pseudoaneurysms. Eur J Radiol 2006; 59:104–111.
- Guillon R, Garcier JM, Abergel A, et al. Management of splenic artery aneurysms and false aneurysms with endovascular treatment in 12 patients. Cardiovasc Intervent Radiol 2003; 26:256–260.
- Northup PG, Wanamaker RC, Lee VD, Adams RB, Berg CL. Model for end-stage liver disease (MELD) predicts nontransplant surgical mortality in patients with cirrhosis. Ann Surg 2005; 242:244–251.
- Bakhos CT, McIntosh BC, Nukta FA, et al. Staged arterial embolization and surgical resection of a giant splenic artery aneurysm. Ann Vasc Surg 2007; 21:208–210.
- Hogendoorn W, Lavida A, Hunink MG, et al. Open repair, endovascular repair, and conservative management of true splenic artery aneurysms. J Vasc Surg 2014; 60:1667–1676.e1.
- Algudkar A. Unruptured splenic artery aneurysm presenting as epigastric pain. JRSM Short Rep 2010; 1:24.
- Abbas MA, Stone WM, Fowl RJ, et al. Splenic artery aneurysms: two decades experience at Mayo Clinic. Ann Vasc Surg 2002; 16:442–449.
- Hoefs JC, Canawati HN, Sapico FL, Hopkins RR, Weiner J, Montgomerie JZ. Spontaneous bacterial peritonitis. Hepatology 1982; 2:399–407.
- Runyon BA, Hoefs JC. Culture-negative neutrocytic ascites: a variant of spontaneous bacterial peritonitis. Hepatology 1984; 4:1209–1211.
- Garcia-Tsao G. Spontaneous bacterial peritonitis: a historical perspective. J Hepatol 2004; 41:522–527.
- Soriano G, Castellote J, Alvarez C, et al. Secondary bacterial peritonitis in cirrhosis: a retrospective study of clinical and analytical characteristics, diagnosis and management. J Hepatol 2010; 52:39–44.
- D’Amico G, De Franchis R; Cooperative Study Group. Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology 2003; 38:599–612.
- Garcia-Tsao G, Sanyal AJ, Grace ND, Carey WD; Practice Guidelines Committee of American Association for Study of Liver Diseases; Practice Parameters Committee of American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Am J Gastroenterol 2007; 102:2086–2102.
- Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W; Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007; 46:922–938.
- Tsochatzis EA, Senzolo M, Germani G, Gatt A, Burroughs AK. Systematic review: portal vein thrombosis in cirrhosis. Aliment Pharmacol Ther 2010; 31:366–374.
- Kobori L, van der Kolk MJ, de Jong KP, et al. Splenic artery aneurysms in liver transplant patients. Liver Transplant Group. J Hepatol 1997; 27:890–893.
- Manzano-Robleda Mdel C, Barranco-Fragoso B, Uribe M, Mendez-Sanchez N. Portal vein thrombosis: what is new? Ann Hepatol 2015; 14:20–27.
- Sarin SK, Philips CA, Kamath PS, et al. Toward a comprehensive new classification of portal vein thrombosis in patients with cirrhosis. Gastroenterology 2016; 151:574–577.e3.
- DeLeve LD, Valla DC, Garcia-Tsao G; American Association for the Study of Liver Diseases. Vascular disorders of the liver. Hepatology 2009; 49:1729–1764.
- Manzanet G, Sanjuan F, Orbis P, et al. Liver transplantation in patients with portal vein thrombosis. Liver Transpl 2001; 7:125–131.
- John BV, Konjeti R, Aggarwal A, et al. Impact of untreated portal vein thrombosis on pre and post liver transplant outcomes in cirrhosis. Ann Hepatol 2013; 12:952–958.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al; American Association for Vascular Surgery/Society for Vascular Surgery; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology; ACC/AHA Task Force on Practice Guidelines. ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease)—summary of recommendations. J Vasc Interv Radiol 2006; 17:1383–1397.
- Schermerhorn ML, O’Malley AJ, Jhaveri A, Cotterill P, Pomposelli F, Landon BE. Endovascular vs open repair of abdominal aortic aneurysms in the Medicare population. N Engl J Med 2008; 358:464–474.
- Ohta M, Hashizume M, Ueno K, Tanoue K, Sugimachi K, Hasuo K. Hemodynamic study of splenic artery aneurysm in portal hypertension. Hepatogastroenterology 1994; 41:181–184.
- Sunagozaka H, Tsuji H, Mizukoshi E, et al. The development and clinical features of splenic aneurysm associated with liver cirrhosis. Liver Int 2006; 26:291–297.
- Manenti F, Williams R. Injection studies of the splenic vasculature in portal hypertension. Gut 1966; 7:175–180.
- Stanley JC, Fry WJ. Pathogenesis and clinical significance of splenic artery aneurysms. Surgery 1974; 76:898–909.
- Lee PC, Rhee RY, Gordon RY, Fung JJ, Webster MW. Management of splenic artery aneurysms: the significance of portal and essential hypertension. J Am Coll Surg 1999; 189:483–490.
- Al-Habbal Y, Christophi C, Muralidharan V. Aneurysms of the splenic artery—a review. Surgeon 2010; 8:223–231.
- Mattar SG, Lumsden AB. The management of splenic artery aneurysms: experience with 23 cases. Am J Surg 1995; 169:580–584.
- Akbulut S, Otan E. Management of giant splenic artery aneurysm: comprehensive literature review. Medicine (Baltimore) 2015; 94:e1016.
- Agrawal GA, Johnson PT, Fishman EK. Splenic artery aneurysms and pseudoaneurysms: clinical distinctions and CT appearances. AJR Am J Roentgenol 2007; 188:992–999.
- Tessier DJ, Stone WM, Fowl RJ, et al. Clinical features and management of splenic artery pseudoaneurysm: case series and cumulative review of literature. J Vasc Surg 2003; 38:969–974.
- Dave SP, Reis ED, Hossain A, Taub PJ, Kerstein MD, Hollier LH. Splenic artery aneurysm in the 1990s. Ann Vasc Surg 2000; 14:223–229.
- Parrish J, Maxwell C, Beecroft JR. Splenic artery aneurysm in pregnancy. J Obstet Gynaecol Can 2015; 37:816–818.
- Moon DB, Lee SG, Hwang S, et al. Characteristics and management of splenic artery aneurysms in adult living donor liver transplant recipients. Liver Transpl 2009; 15:1535–1541.
- Sadat U, Dar O, Walsh S, Varty K. Splenic artery aneurysms in pregnancy—a systematic review. Int J Surg 2008; 6:261–265.
- Geoghegan T, McAuley G, Snow A, Torreggiani WC. Emergency embolization of multiple splenic artery pseudoaneurysms associated with portal hypertension complicating cystic fibrosis. Australas Radiol 2007; 51(suppl):B337–B339.
- Jiang R, Ding X, Jian W, Jiang J, Hu S, Zhang Z. Combined endovascular embolization and open surgery for splenic artery aneurysm with arteriovenous fistula. Ann Vasc Surg 2016; 30:311.e1–311.e4.
- Naganuma M, Matsui H, Koizumi J, Fushimi K, Yasunaga H. Short-term outcomes following elective transcatheter arterial embolization for splenic artery aneurysms: data from a nationwide administrative database. Acta Radiol Open 2015; 4:2047981615574354.
- Batagini NC, El-Arousy H, Clair DG, Kirksey L. Open versus endovascular treatment of visceral artery aneurysms and pseudoaneurysms. Ann Vasc Surg 2016; 35:1–8.
- Marone EM, Mascia D, Kahlberg A, Brioschi C, Tshomba Y, Chiesa R. Is open repair still the gold standard in visceral artery aneurysm management? Ann Vasc Surg 2011; 25:936–946.
- Sticco A, Aggarwal A, Shapiro M, Pratt A, Rissuci D, D'Ayala M. A comparison of open and endovascular treatment strategies for the management of splenic artery aneurysms. Vascular 2016; 24:487–491.
- Hogendoorn W, Lavida A, Hunink MG, et al. Cost-effectiveness of endovascular repair, open repair, and conservative management of splenic artery aneurysms. J Vasc Surg 2015; 61:1432–1440.
- Fankhauser GT, Stone WM, Naidu SG, et al; Mayo Vascular Research Center Consortium. The minimally invasive management of visceral artery aneurysms and pseudoaneurysms. J Vasc Surg 2011; 53:966–970.
- Lagana D, Carrafiello G, Mangini M, et al. Multimodal approach to endovascular treatment of visceral artery aneurysms and pseudoaneurysms. Eur J Radiol 2006; 59:104–111.
- Guillon R, Garcier JM, Abergel A, et al. Management of splenic artery aneurysms and false aneurysms with endovascular treatment in 12 patients. Cardiovasc Intervent Radiol 2003; 26:256–260.
- Northup PG, Wanamaker RC, Lee VD, Adams RB, Berg CL. Model for end-stage liver disease (MELD) predicts nontransplant surgical mortality in patients with cirrhosis. Ann Surg 2005; 242:244–251.
Renal denervation: What happened, and why?
Many patients, clinicians, and researchers had hoped that renal denervation would help control resistant hypertension. However, in the SYMPLICITY HTN-3 trial,1 named for the catheter-based system used in the study (Symplicity RDN, Medtronic, Dublin, Ireland), this endovascular procedure failed to meet its primary and secondary efficacy end points, although it was found to be safe. These results were surprising, especially given the results of an earlier randomized trial (SYMPLICITY HTN-2),2 which showed larger reductions in blood pressures 6 months after denervation than in the current trial.
Here, we discuss the results of the SYMPLICITY HTN-3 trial and offer possible explanations for its negative outcomes.
LEAD-UP TO SYMPLICITY HTN-3
Renal denervation consists of passing a catheter through the femoral artery into the renal arteries and ablating their sympathetic nerves using radiofrequency energy. In theory, this should interrupt efferent sympathetic communication between the brain and renal arteries, reducing muscular contraction of these arteries, increasing renal blood flow, reducing activation of the renin-angiotensin-adosterone system, thus reducing sodium retention, reducing afferent sympathetic communication between the kidneys and brain, and in turn reducing further sympathetic activity elsewhere in the body, such as in the heart. Blood pressure should fall.3
The results of the SYMPLICITY HTN-1 and 2 trials were discussed in an earlier article in this Journal,3 and the Medtronic-Ardian renal denervation system has been available in Europe and Australia for clinical use for over 2 years.4 Indeed, after the SYMPLICITY HTN-2 results were published in 2010, Boston Scientific’s Vessix, St. Jude Medical’s EnligHTN, and Covidien’s OneShot radiofrequency renal denervation devices—albeit each with some modifications—received a Conformité Européene (CE) mark and became available in Europe and Australia for clinical use. These devices are not available for clinical use or research in the United States.3,5
Therefore, SYMPLICITY HTN-3, sponsored by Medtronic, was designed to obtain US Food and Drug Administration approval in the United States.6
SYMPLICITY HTN-3 DESIGN
Inclusion criteria were similar to those in the earlier SYMPLICITY trials. Patients had to have resistant hypertension, defined as a systolic blood pressure ≥ 160 mm Hg despite taking at least 3 blood pressure medications at maximum tolerated doses. Patients were excluded if they had a glomerular filtration rate of less than 45 mL/min/1.73 m2, renal artery stenosis, or known secondary hypertension.
A total of 1,441 patients were enrolled, of whom 364 were eventually randomized to undergo renal denervation, and 171 were randomized to undergo a sham procedure. The mean systolic blood pressure at baseline was 188 mm Hg in each group. Most patients were taking maximum doses of blood pressure medications, and almost one-fourth were taking an aldosterone antagonist. Patients in both groups were taking an average of 5 medications.
The 2 groups were well matched for important covariates, including obstructive sleep apnea, diabetes mellitus, and renal insufficiency. Most of the patients were white; 25% of the renal denervation group and 29% of the sham procedure group were black.
The physicians conducting the follow-up appointments did not know which procedure the patients underwent, and neither did the patients. Medications were closely monitored, and patients had close follow-up. The catheter (Symplicity RDS, Medtronic) was of the same design that was used in the earlier SYMPLICITY trials and in clinical practice in countries where renal denervation was available.
Researchers expected that the systolic blood pressure, as measured in the office, would fall in both groups, but they hoped it would fall farther in the denervation group—at least 5 mm Hg farther, the primary end point of the trial. The secondary effectiveness end point was a 2-mm Hg greater reduction in 24-hour ambulatory systolic blood pressure.
SYMPLICITY HTN-3 RESULTS
No statistically significant difference in safety was observed between the denervation and control groups. However, the procedure was associated with 1 embolic event and 1 case of renal artery stenosis.
Blood pressure fell in both groups. However, at 6 months, office systolic pressure had fallen by a mean of 14.13 mm Hg in the denervation group and 11.74 mm Hg in the sham procedure group, a difference of only 2.39 mm Hg. The mean ambulatory systolic blood pressure had fallen by 6.75 vs 4.79 mm Hg, a difference of only 1.96 mm Hg. Neither difference was statistically significant.
A number of prespecified subgroup analyses were conducted, but the benefit of the procedure was statistically significant in only 3 subgroups: patients who were not black (P = .01), patients who were less than 65 years old (P = .04), and patients who had an estimated glomerular filtration rate of 60 mL/min/1.73 m2 or higher (P = .05).
WHAT WENT WRONG?
The results of SYMPLICITY HTN-3 were disappointing and led companies that were developing renal denervation devices to discontinue or reevaluate their programs.
Although the results were surprising, many observers (including our group) raised concerns about the initial enthusiasm surrounding renal denervation.3–7 Indeed, in 2010, we had concerns about the discrepancy between office-based blood pressure measurements (the primary end point of all renal denervation trials) and ambulatory blood pressure measurements in SYMPLICITY HTN-2.7
The enthusiasm surrounding this procedure led to the publication of 2 consensus documents on this novel therapy based on only 1 small randomized controlled study (SYMPLICITY HTN-2).8,9 Renal denervation was even reported to be useful in other conditions involving the sympathorenal axis, including diabetes mellitus, metabolic syndrome, and obstructive sleep apnea, and also as a potential treatment adjunct in atrial fibrillation and other arrhythmias.5
What went wrong?
Shortcomings in trial design?
The trial was well designed. Both patients and operators were blinded to the procedure, and 24-hour ambulatory blood pressure monitoring was used. We presume that appropriate patients with resistant hypertension were enrolled—the mean baseline systolic blood pressure was 188 mm Hg, and patients in each group were taking an average of 5 medications.
On the other hand, true medication adherence is difficult to ascertain. Further, the term maximal “tolerated” doses of medications is vague, and we cannot rule out the possibility that some patients were enrolled who did not truly have resistant hypertension—they simply did not want to take medications.
Patients were required to be on a stable medication regimen before enrollment and, ideally, to not have any medication changes during the course of the study, but at least 40% of patients did require medication changes during the study. Additionally, it is unclear whether all patients underwent specific testing to rule out secondary hypertension, as this was done at the discretion of the treating physician.
First-generation catheters?
The same type of catheter was used as in the earlier SYMPLICITY trials, and it had been used in many patients in clinical practice in countries where the catheter is routinely available. It is unknown, however, whether newer multisite denervation devices would yield better results than the first-generation devices used in SYMPLICITY HTN-3. But even this would not explain the discrepancies in data between earlier trials and this trial.
Operator inexperience?
It has been suggested that operator inexperience may have played a role, but an analysis of operator volume did not find any association between this variable and the outcomes. Each procedure was supervised by at least 1 and in most cases 2 certified Medtronic representatives, who made certain that meticulous attention was paid to procedure details and that no shortcuts were taken during the procedure.
Inadequate ablation?
While we can assume that the correct technique was followed in most cases, renal denervation is still a “blind” procedure, and there is no nerve mapping to ascertain the degree of ablation achieved. Notably, patients who had the most ablations reportedly had a greater average drop in systolic ambulatory blood pressure than those who received fewer ablations. Sympathetic nervous system activity is a potential marker of adequacy of ablation, but it was not routinely assessed in the SYMPLICITY HTN-3 trial. Techniques to assess sympathetic nerve activity such as norepinephrine spillover and muscle sympathetic nerve activity are highly specialized and available only at a few research centers, and are not available for routine clinical use.
While these points may explain the negative findings of this trial, they fail to account for the discrepant results between this study and previous trials that used exactly the same definitions and techniques.
Patient demographics?
Is it possible that renal denervation has a differential effect according to race? All previous renal denervation studies were conducted in Europe or Australia; therefore, few data are available on the efficacy of the procedure in other racial groups, such as black Americans. Most of the patients in this trial were white, but approximately 25% were black—a good representation. There was a statistically significant benefit favoring renal denervation in nonblack (mostly white) patients, but not in black patients. This may be related to racial differences in the pathophysiology of hypertension or possibly due to chance alone.
A Hawthorne effect?
A Hawthorne effect (patients being more compliant because physicians are paying more attention to them) is unlikely, since the renal denervation arm did not have any reduction in blood pressure medications. At 6 months, both the sham group and the procedure group were still on an average of 5 medications.
Additionally, while the blood pressure reduction in both treatment groups was significant, the systolic blood pressure at 6 months was still 166 mm Hg in the denervation group and 168 mm Hg in the sham group. If denervation was effective, one would have expected a greater reduction in blood pressure or at least a decrease in the number of medications needed, eg, 1 to 2 fewer medications in the denervation group compared with the sham procedure group.
Regression to the mean?
It is unknown whether the results represent a statistical error such as regression to the mean. But given the run-in period and the confirmatory data from 24-hour ambulatory blood pressure, this would be unlikely.
WHAT NOW?
Is renal denervation dead? SYMPLICITY HTN-3 is only a single trial with multiple shortcomings and lessons to learn from. Since its publication, there have been updates from 2 prospective, randomized, open-label trials concerning the efficacy of catheter-based renal denervation in lowering blood pressure.10,11
DENERHTN (Renal Denervation for Hypertension)10 studied patients with ambulatory systolic blood pressure higher than 135 mm Hg, diastolic blood pressure higher than 80 mm Hg, or both (after excluding secondary etiologies), despite 4 weeks of standardized triple-drug treatment including a diuretic. Patients were randomized to standardized stepped-care antihypertensive treatment alone (control group) or standard care plus renal denervation. The latter resulted in a significant further reduction in ambulatory blood pressure at 6 months.
The Prague-15 trial11 studied patients with resistant hypertension. Secondary etiologies were excluded and adherence to therapy was confirmed by measuring plasma medication levels. It showed that renal denervation along with optimal antihypertensive medical therapy (unchanged after randomization) resulted in a significant reduction in ambulatory blood pressure that was comparable to the effect of intensified antihypertensive medical therapy including spironolactone. (Studies have shown that spironolactone is effective when added on as a fourth-line medication in resistant hypertension.12) At 6 months, patients in the intensive medical therapy group were using an average of 0.3 more antihypertensive medications than those in the procedure group.
These two trials addressed some of the drawbacks of the SYMPLICITY HTN-3 trial. However, both have many limitations including and not limited to being open-label and nonblinded, lacking a sham procedure, using a lower blood pressure threshold than SYMPLICITY HTN-3 did to define resistant hypertension, and using the same catheter as in the SYMPLICITY trials.
Better technology is coming
Advanced renal denervation catheters are needed that are multielectrode, smaller, easier to manipulate, and capable of providing simultaneous, circumferential, more-intense, and deeper ablations. The ongoing Investigator-Steered Project on Intravascular Renal Denervation for Management of Drug-Resistant Hypertension (INSPIRED)16 and Renal Denervation Using the Vessix Renal Denervation System for the Treatment of Hypertension (REDUCE-HTN: REINFORCE)17 trials are using contemporary innovative ablation catheters to address the limitations of the first-generation Symplicity catheter.
Further, Fischell et al18 reported encouraging results of renal denervation performed by injecting ethanol into the adventitial space of the renal arteries. This is still an invasive procedure; however, ethanol can spread out in all directions and reach all targeted nerves, potentially resulting in a more complete renal artery sympathetic ablation.
As technology advances, the WAVE IV trial19 is examining renal denervation performed from the outside through the skin using high-intensity focused ultrasound, which eliminates the need for femoral arterial catheterization, a promising noninvasive approach.
Proposals for future trials
The European Clinical Consensus Conference for Renal Denervation20 proposed that future trials of renal denervation include patients with moderate rather than resistant hypertension, reflecting the pathogenic importance of sympathetic activity in earlier stages of hypertension. The conference also proposed excluding patients with stiff large arteries, a cause of isolated systolic hypertension. Other proposals included standardizing concomitant antihypertensive therapy, preferably treating all patients with the combination of a renin-angiotensin system blocker, calcium channel blocker, and diuretic in the run-in period; monitoring drug adherence through the use of pill counts, electronic pill dispensers, and drug blood tests; and using change in ambulatory blood pressure as the primary efficacy end point and change in office blood pressure as a secondary end point.
Trials ongoing
To possibly address the limitations posed by the SYMPLICITY HTN-3 trial and to answer other important questions, several sham-controlled clinical trials of renal denervation are currently being conducted:
- INSPiRED16
- REDUCE-HTN: REINFORCE17
- Spyral HTN-Off Med21
- Spyral HTN-On Med21
- Study of the ReCor Medical Paradise System in Clinical Hypertension (RADIANCE-HTN).22
We hope these new studies can more clearly identify subsets of patients who would benefit from this technology, determine predictors of blood pressure reduction in such patients, and lead to newer devices that may provide more complete ablation.
Obviously, we also need better ways to identify the exact location of these sympathetic nerves within the renal artery and have a clearer sense of procedural success.
Until then, our colleagues in Europe and Australia continue to treat patients with this technology as we appropriately and patiently wait for level 1 clinical evidence of its efficacy.
Acknowledgments: We thank Kathryn Brock, BA, Editorial Services Manager, Heart and Vascular Institute, Cleveland Clinic, for her assistance in the preparation of this paper.
- Bhatt DL, Kandzari DE, O’Neill WW, et al, for the SYMPLICITY HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med 2014; 370:1393–1401.
- Symplicity HTN-2 Investigators, Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Bohm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (the Symplicity HTN-2 trial): a randomised controlled trial. Lancet 2010; 376:1903–1909.
- Bunte MC, Infante de Oliveira E, Shishehbor MH. Endovascular treatment of resistant and uncontrolled hypertension: therapies on the horizon. JACC Cardiovasc Interv 2013; 6:1–9.
- Thomas G, Shishehbor MH, Bravo EL, Nally JV. Renal denervation to treat resistant hypertension: guarded optimism. Cleve Clin J Med 2012; 79:501–510.
- Shishehbor MH, Bunte MC. Anatomical exclusion for renal denervation: are we putting the cart before the horse? JACC Cardiovasc Interv 2014; 7:193–194.
- Bhatt DL, Bakris GL. The promise of renal denervation. Cleve Clin J Med 2012; 79:498–500.
- Bunte MC. Renal sympathetic denervation for refractory hypertension. Lancet 2011; 377:1074; author reply 1075.
- Mahfoud F, Luscher TF, Andersson B, et al; European Society of Cardiology. Expert consensus document from the European Society of Cardiology on catheter-based renal denervation. Eur Heart J 2013; 34:2149–2157.
- Schlaich MP, Schmieder RE, Bakris G, et al. International expert consensus statement: percutaneous transluminal renal denervation for the treatment of resistant hypertension. J Am Coll Cardiol 2013; 62:2031–2045.
- Azizi M, Sapoval M, Gosse P, et al; Renal Denervation for Hypertension (DENERHTN) investigators. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet 2015; 385:1957–1965.
- Rosa J, Widimsky P, Tousek P, et al. Randomized comparison of renal denervation versus intensified pharmacotherapy including spironolactone in true-resistant hypertension: six-month results from the Prague-15 study. Hypertension 2015; 65:407–413.
- Williams B, MacDonald TM, Morant S, et al; British Hypertension Society’s PATHWAY Studies Group. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet 2015; 386:2059–2068.
- Sakakura K, Ladich E, Cheng Q, et al. Anatomic assessment of sympathetic peri-arterial renal nerves in man. J Am Coll Cardiol 2014; 64:635–643.
- Mahfoud F, Edelman ER, Bohm M. Catheter-based renal denervation is no simple matter: lessons to be learned from our anatomy? J Am Coll Cardiol 2014; 64:644–646.
- Id D, Kaltenbach B, Bertog SC, et al. Does the presence of accessory renal arteries affect the efficacy of renal denervation? JACC Cardiovasc Interv 2013; 6:1085–1091.
- Jin Y, Jacobs L, Baelen M, et al; Investigator-Steered Project on Intravascular Renal Denervation for Management of Drug-Resistant Hypertension (Inspired) Investigators. Rationale and design of the Investigator-Steered Project on Intravascular Renal Denervation for Management of Drug-Resistant Hypertension (INSPiRED) trial. Blood Press 2014; 23:138–146.
- ClinicalTrialsgov. Renal Denervation Using the Vessix Renal Denervation System for the Treatment of Hypertension (REDUCE HTN: REINFORCE). https://clinicaltrials.gov/ct2/show/NCT02392351?term=REDUCE-HTN%3A+REINFORCE&rank=1. Accessed August 3, 2017.
- Fischell TA, Ebner A, Gallo S, et al. Transcatheter alcohol-mediated perivascular renal denervation with the peregrine system: first-in-human experience. JACC Cardiovasc Interv 2016; 9:589–598.
- ClinicalTrialsgov. Sham controlled study of renal denervation for subjects with uncontrolled hypertension (WAVE_IV) (NCT02029885). https://clinicaltrials.gov/ct2/show/results/NCT02029885. Accessed August 3, 2017.
- Mahfoud F, Bohm M, Azizi M, et al. Proceedings from the European clinical consensus conference for renal denervation: considerations on future clinical trial design. Eur Heart J 2015; 36:2219–2227.
- Kandzari DE, Kario K, Mahfoud F, et al. The SPYRAL HTN Global Clinical Trial Program: rationale and design for studies of renal denervation in the absence (SPYRAL HTN OFF-MED) and presence (SPYRAL HTN ON-MED) of antihypertensive medications. Am Heart J 2016; 171:82–91.
- ClinicalTrialsgov. A Study of the ReCor Medical Paradise System in Clinical Hypertension (RADIANCE-HTN). https://clinicaltrials.gov/ct2/show/NCT02649426?term=RADIANCE&rank=3. Accessed August 3, 2017.
Many patients, clinicians, and researchers had hoped that renal denervation would help control resistant hypertension. However, in the SYMPLICITY HTN-3 trial,1 named for the catheter-based system used in the study (Symplicity RDN, Medtronic, Dublin, Ireland), this endovascular procedure failed to meet its primary and secondary efficacy end points, although it was found to be safe. These results were surprising, especially given the results of an earlier randomized trial (SYMPLICITY HTN-2),2 which showed larger reductions in blood pressures 6 months after denervation than in the current trial.
Here, we discuss the results of the SYMPLICITY HTN-3 trial and offer possible explanations for its negative outcomes.
LEAD-UP TO SYMPLICITY HTN-3
Renal denervation consists of passing a catheter through the femoral artery into the renal arteries and ablating their sympathetic nerves using radiofrequency energy. In theory, this should interrupt efferent sympathetic communication between the brain and renal arteries, reducing muscular contraction of these arteries, increasing renal blood flow, reducing activation of the renin-angiotensin-adosterone system, thus reducing sodium retention, reducing afferent sympathetic communication between the kidneys and brain, and in turn reducing further sympathetic activity elsewhere in the body, such as in the heart. Blood pressure should fall.3
The results of the SYMPLICITY HTN-1 and 2 trials were discussed in an earlier article in this Journal,3 and the Medtronic-Ardian renal denervation system has been available in Europe and Australia for clinical use for over 2 years.4 Indeed, after the SYMPLICITY HTN-2 results were published in 2010, Boston Scientific’s Vessix, St. Jude Medical’s EnligHTN, and Covidien’s OneShot radiofrequency renal denervation devices—albeit each with some modifications—received a Conformité Européene (CE) mark and became available in Europe and Australia for clinical use. These devices are not available for clinical use or research in the United States.3,5
Therefore, SYMPLICITY HTN-3, sponsored by Medtronic, was designed to obtain US Food and Drug Administration approval in the United States.6
SYMPLICITY HTN-3 DESIGN
Inclusion criteria were similar to those in the earlier SYMPLICITY trials. Patients had to have resistant hypertension, defined as a systolic blood pressure ≥ 160 mm Hg despite taking at least 3 blood pressure medications at maximum tolerated doses. Patients were excluded if they had a glomerular filtration rate of less than 45 mL/min/1.73 m2, renal artery stenosis, or known secondary hypertension.
A total of 1,441 patients were enrolled, of whom 364 were eventually randomized to undergo renal denervation, and 171 were randomized to undergo a sham procedure. The mean systolic blood pressure at baseline was 188 mm Hg in each group. Most patients were taking maximum doses of blood pressure medications, and almost one-fourth were taking an aldosterone antagonist. Patients in both groups were taking an average of 5 medications.
The 2 groups were well matched for important covariates, including obstructive sleep apnea, diabetes mellitus, and renal insufficiency. Most of the patients were white; 25% of the renal denervation group and 29% of the sham procedure group were black.
The physicians conducting the follow-up appointments did not know which procedure the patients underwent, and neither did the patients. Medications were closely monitored, and patients had close follow-up. The catheter (Symplicity RDS, Medtronic) was of the same design that was used in the earlier SYMPLICITY trials and in clinical practice in countries where renal denervation was available.
Researchers expected that the systolic blood pressure, as measured in the office, would fall in both groups, but they hoped it would fall farther in the denervation group—at least 5 mm Hg farther, the primary end point of the trial. The secondary effectiveness end point was a 2-mm Hg greater reduction in 24-hour ambulatory systolic blood pressure.
SYMPLICITY HTN-3 RESULTS
No statistically significant difference in safety was observed between the denervation and control groups. However, the procedure was associated with 1 embolic event and 1 case of renal artery stenosis.
Blood pressure fell in both groups. However, at 6 months, office systolic pressure had fallen by a mean of 14.13 mm Hg in the denervation group and 11.74 mm Hg in the sham procedure group, a difference of only 2.39 mm Hg. The mean ambulatory systolic blood pressure had fallen by 6.75 vs 4.79 mm Hg, a difference of only 1.96 mm Hg. Neither difference was statistically significant.
A number of prespecified subgroup analyses were conducted, but the benefit of the procedure was statistically significant in only 3 subgroups: patients who were not black (P = .01), patients who were less than 65 years old (P = .04), and patients who had an estimated glomerular filtration rate of 60 mL/min/1.73 m2 or higher (P = .05).
WHAT WENT WRONG?
The results of SYMPLICITY HTN-3 were disappointing and led companies that were developing renal denervation devices to discontinue or reevaluate their programs.
Although the results were surprising, many observers (including our group) raised concerns about the initial enthusiasm surrounding renal denervation.3–7 Indeed, in 2010, we had concerns about the discrepancy between office-based blood pressure measurements (the primary end point of all renal denervation trials) and ambulatory blood pressure measurements in SYMPLICITY HTN-2.7
The enthusiasm surrounding this procedure led to the publication of 2 consensus documents on this novel therapy based on only 1 small randomized controlled study (SYMPLICITY HTN-2).8,9 Renal denervation was even reported to be useful in other conditions involving the sympathorenal axis, including diabetes mellitus, metabolic syndrome, and obstructive sleep apnea, and also as a potential treatment adjunct in atrial fibrillation and other arrhythmias.5
What went wrong?
Shortcomings in trial design?
The trial was well designed. Both patients and operators were blinded to the procedure, and 24-hour ambulatory blood pressure monitoring was used. We presume that appropriate patients with resistant hypertension were enrolled—the mean baseline systolic blood pressure was 188 mm Hg, and patients in each group were taking an average of 5 medications.
On the other hand, true medication adherence is difficult to ascertain. Further, the term maximal “tolerated” doses of medications is vague, and we cannot rule out the possibility that some patients were enrolled who did not truly have resistant hypertension—they simply did not want to take medications.
Patients were required to be on a stable medication regimen before enrollment and, ideally, to not have any medication changes during the course of the study, but at least 40% of patients did require medication changes during the study. Additionally, it is unclear whether all patients underwent specific testing to rule out secondary hypertension, as this was done at the discretion of the treating physician.
First-generation catheters?
The same type of catheter was used as in the earlier SYMPLICITY trials, and it had been used in many patients in clinical practice in countries where the catheter is routinely available. It is unknown, however, whether newer multisite denervation devices would yield better results than the first-generation devices used in SYMPLICITY HTN-3. But even this would not explain the discrepancies in data between earlier trials and this trial.
Operator inexperience?
It has been suggested that operator inexperience may have played a role, but an analysis of operator volume did not find any association between this variable and the outcomes. Each procedure was supervised by at least 1 and in most cases 2 certified Medtronic representatives, who made certain that meticulous attention was paid to procedure details and that no shortcuts were taken during the procedure.
Inadequate ablation?
While we can assume that the correct technique was followed in most cases, renal denervation is still a “blind” procedure, and there is no nerve mapping to ascertain the degree of ablation achieved. Notably, patients who had the most ablations reportedly had a greater average drop in systolic ambulatory blood pressure than those who received fewer ablations. Sympathetic nervous system activity is a potential marker of adequacy of ablation, but it was not routinely assessed in the SYMPLICITY HTN-3 trial. Techniques to assess sympathetic nerve activity such as norepinephrine spillover and muscle sympathetic nerve activity are highly specialized and available only at a few research centers, and are not available for routine clinical use.
While these points may explain the negative findings of this trial, they fail to account for the discrepant results between this study and previous trials that used exactly the same definitions and techniques.
Patient demographics?
Is it possible that renal denervation has a differential effect according to race? All previous renal denervation studies were conducted in Europe or Australia; therefore, few data are available on the efficacy of the procedure in other racial groups, such as black Americans. Most of the patients in this trial were white, but approximately 25% were black—a good representation. There was a statistically significant benefit favoring renal denervation in nonblack (mostly white) patients, but not in black patients. This may be related to racial differences in the pathophysiology of hypertension or possibly due to chance alone.
A Hawthorne effect?
A Hawthorne effect (patients being more compliant because physicians are paying more attention to them) is unlikely, since the renal denervation arm did not have any reduction in blood pressure medications. At 6 months, both the sham group and the procedure group were still on an average of 5 medications.
Additionally, while the blood pressure reduction in both treatment groups was significant, the systolic blood pressure at 6 months was still 166 mm Hg in the denervation group and 168 mm Hg in the sham group. If denervation was effective, one would have expected a greater reduction in blood pressure or at least a decrease in the number of medications needed, eg, 1 to 2 fewer medications in the denervation group compared with the sham procedure group.
Regression to the mean?
It is unknown whether the results represent a statistical error such as regression to the mean. But given the run-in period and the confirmatory data from 24-hour ambulatory blood pressure, this would be unlikely.
WHAT NOW?
Is renal denervation dead? SYMPLICITY HTN-3 is only a single trial with multiple shortcomings and lessons to learn from. Since its publication, there have been updates from 2 prospective, randomized, open-label trials concerning the efficacy of catheter-based renal denervation in lowering blood pressure.10,11
DENERHTN (Renal Denervation for Hypertension)10 studied patients with ambulatory systolic blood pressure higher than 135 mm Hg, diastolic blood pressure higher than 80 mm Hg, or both (after excluding secondary etiologies), despite 4 weeks of standardized triple-drug treatment including a diuretic. Patients were randomized to standardized stepped-care antihypertensive treatment alone (control group) or standard care plus renal denervation. The latter resulted in a significant further reduction in ambulatory blood pressure at 6 months.
The Prague-15 trial11 studied patients with resistant hypertension. Secondary etiologies were excluded and adherence to therapy was confirmed by measuring plasma medication levels. It showed that renal denervation along with optimal antihypertensive medical therapy (unchanged after randomization) resulted in a significant reduction in ambulatory blood pressure that was comparable to the effect of intensified antihypertensive medical therapy including spironolactone. (Studies have shown that spironolactone is effective when added on as a fourth-line medication in resistant hypertension.12) At 6 months, patients in the intensive medical therapy group were using an average of 0.3 more antihypertensive medications than those in the procedure group.
These two trials addressed some of the drawbacks of the SYMPLICITY HTN-3 trial. However, both have many limitations including and not limited to being open-label and nonblinded, lacking a sham procedure, using a lower blood pressure threshold than SYMPLICITY HTN-3 did to define resistant hypertension, and using the same catheter as in the SYMPLICITY trials.
Better technology is coming
Advanced renal denervation catheters are needed that are multielectrode, smaller, easier to manipulate, and capable of providing simultaneous, circumferential, more-intense, and deeper ablations. The ongoing Investigator-Steered Project on Intravascular Renal Denervation for Management of Drug-Resistant Hypertension (INSPIRED)16 and Renal Denervation Using the Vessix Renal Denervation System for the Treatment of Hypertension (REDUCE-HTN: REINFORCE)17 trials are using contemporary innovative ablation catheters to address the limitations of the first-generation Symplicity catheter.
Further, Fischell et al18 reported encouraging results of renal denervation performed by injecting ethanol into the adventitial space of the renal arteries. This is still an invasive procedure; however, ethanol can spread out in all directions and reach all targeted nerves, potentially resulting in a more complete renal artery sympathetic ablation.
As technology advances, the WAVE IV trial19 is examining renal denervation performed from the outside through the skin using high-intensity focused ultrasound, which eliminates the need for femoral arterial catheterization, a promising noninvasive approach.
Proposals for future trials
The European Clinical Consensus Conference for Renal Denervation20 proposed that future trials of renal denervation include patients with moderate rather than resistant hypertension, reflecting the pathogenic importance of sympathetic activity in earlier stages of hypertension. The conference also proposed excluding patients with stiff large arteries, a cause of isolated systolic hypertension. Other proposals included standardizing concomitant antihypertensive therapy, preferably treating all patients with the combination of a renin-angiotensin system blocker, calcium channel blocker, and diuretic in the run-in period; monitoring drug adherence through the use of pill counts, electronic pill dispensers, and drug blood tests; and using change in ambulatory blood pressure as the primary efficacy end point and change in office blood pressure as a secondary end point.
Trials ongoing
To possibly address the limitations posed by the SYMPLICITY HTN-3 trial and to answer other important questions, several sham-controlled clinical trials of renal denervation are currently being conducted:
- INSPiRED16
- REDUCE-HTN: REINFORCE17
- Spyral HTN-Off Med21
- Spyral HTN-On Med21
- Study of the ReCor Medical Paradise System in Clinical Hypertension (RADIANCE-HTN).22
We hope these new studies can more clearly identify subsets of patients who would benefit from this technology, determine predictors of blood pressure reduction in such patients, and lead to newer devices that may provide more complete ablation.
Obviously, we also need better ways to identify the exact location of these sympathetic nerves within the renal artery and have a clearer sense of procedural success.
Until then, our colleagues in Europe and Australia continue to treat patients with this technology as we appropriately and patiently wait for level 1 clinical evidence of its efficacy.
Acknowledgments: We thank Kathryn Brock, BA, Editorial Services Manager, Heart and Vascular Institute, Cleveland Clinic, for her assistance in the preparation of this paper.
Many patients, clinicians, and researchers had hoped that renal denervation would help control resistant hypertension. However, in the SYMPLICITY HTN-3 trial,1 named for the catheter-based system used in the study (Symplicity RDN, Medtronic, Dublin, Ireland), this endovascular procedure failed to meet its primary and secondary efficacy end points, although it was found to be safe. These results were surprising, especially given the results of an earlier randomized trial (SYMPLICITY HTN-2),2 which showed larger reductions in blood pressures 6 months after denervation than in the current trial.
Here, we discuss the results of the SYMPLICITY HTN-3 trial and offer possible explanations for its negative outcomes.
LEAD-UP TO SYMPLICITY HTN-3
Renal denervation consists of passing a catheter through the femoral artery into the renal arteries and ablating their sympathetic nerves using radiofrequency energy. In theory, this should interrupt efferent sympathetic communication between the brain and renal arteries, reducing muscular contraction of these arteries, increasing renal blood flow, reducing activation of the renin-angiotensin-adosterone system, thus reducing sodium retention, reducing afferent sympathetic communication between the kidneys and brain, and in turn reducing further sympathetic activity elsewhere in the body, such as in the heart. Blood pressure should fall.3
The results of the SYMPLICITY HTN-1 and 2 trials were discussed in an earlier article in this Journal,3 and the Medtronic-Ardian renal denervation system has been available in Europe and Australia for clinical use for over 2 years.4 Indeed, after the SYMPLICITY HTN-2 results were published in 2010, Boston Scientific’s Vessix, St. Jude Medical’s EnligHTN, and Covidien’s OneShot radiofrequency renal denervation devices—albeit each with some modifications—received a Conformité Européene (CE) mark and became available in Europe and Australia for clinical use. These devices are not available for clinical use or research in the United States.3,5
Therefore, SYMPLICITY HTN-3, sponsored by Medtronic, was designed to obtain US Food and Drug Administration approval in the United States.6
SYMPLICITY HTN-3 DESIGN
Inclusion criteria were similar to those in the earlier SYMPLICITY trials. Patients had to have resistant hypertension, defined as a systolic blood pressure ≥ 160 mm Hg despite taking at least 3 blood pressure medications at maximum tolerated doses. Patients were excluded if they had a glomerular filtration rate of less than 45 mL/min/1.73 m2, renal artery stenosis, or known secondary hypertension.
A total of 1,441 patients were enrolled, of whom 364 were eventually randomized to undergo renal denervation, and 171 were randomized to undergo a sham procedure. The mean systolic blood pressure at baseline was 188 mm Hg in each group. Most patients were taking maximum doses of blood pressure medications, and almost one-fourth were taking an aldosterone antagonist. Patients in both groups were taking an average of 5 medications.
The 2 groups were well matched for important covariates, including obstructive sleep apnea, diabetes mellitus, and renal insufficiency. Most of the patients were white; 25% of the renal denervation group and 29% of the sham procedure group were black.
The physicians conducting the follow-up appointments did not know which procedure the patients underwent, and neither did the patients. Medications were closely monitored, and patients had close follow-up. The catheter (Symplicity RDS, Medtronic) was of the same design that was used in the earlier SYMPLICITY trials and in clinical practice in countries where renal denervation was available.
Researchers expected that the systolic blood pressure, as measured in the office, would fall in both groups, but they hoped it would fall farther in the denervation group—at least 5 mm Hg farther, the primary end point of the trial. The secondary effectiveness end point was a 2-mm Hg greater reduction in 24-hour ambulatory systolic blood pressure.
SYMPLICITY HTN-3 RESULTS
No statistically significant difference in safety was observed between the denervation and control groups. However, the procedure was associated with 1 embolic event and 1 case of renal artery stenosis.
Blood pressure fell in both groups. However, at 6 months, office systolic pressure had fallen by a mean of 14.13 mm Hg in the denervation group and 11.74 mm Hg in the sham procedure group, a difference of only 2.39 mm Hg. The mean ambulatory systolic blood pressure had fallen by 6.75 vs 4.79 mm Hg, a difference of only 1.96 mm Hg. Neither difference was statistically significant.
A number of prespecified subgroup analyses were conducted, but the benefit of the procedure was statistically significant in only 3 subgroups: patients who were not black (P = .01), patients who were less than 65 years old (P = .04), and patients who had an estimated glomerular filtration rate of 60 mL/min/1.73 m2 or higher (P = .05).
WHAT WENT WRONG?
The results of SYMPLICITY HTN-3 were disappointing and led companies that were developing renal denervation devices to discontinue or reevaluate their programs.
Although the results were surprising, many observers (including our group) raised concerns about the initial enthusiasm surrounding renal denervation.3–7 Indeed, in 2010, we had concerns about the discrepancy between office-based blood pressure measurements (the primary end point of all renal denervation trials) and ambulatory blood pressure measurements in SYMPLICITY HTN-2.7
The enthusiasm surrounding this procedure led to the publication of 2 consensus documents on this novel therapy based on only 1 small randomized controlled study (SYMPLICITY HTN-2).8,9 Renal denervation was even reported to be useful in other conditions involving the sympathorenal axis, including diabetes mellitus, metabolic syndrome, and obstructive sleep apnea, and also as a potential treatment adjunct in atrial fibrillation and other arrhythmias.5
What went wrong?
Shortcomings in trial design?
The trial was well designed. Both patients and operators were blinded to the procedure, and 24-hour ambulatory blood pressure monitoring was used. We presume that appropriate patients with resistant hypertension were enrolled—the mean baseline systolic blood pressure was 188 mm Hg, and patients in each group were taking an average of 5 medications.
On the other hand, true medication adherence is difficult to ascertain. Further, the term maximal “tolerated” doses of medications is vague, and we cannot rule out the possibility that some patients were enrolled who did not truly have resistant hypertension—they simply did not want to take medications.
Patients were required to be on a stable medication regimen before enrollment and, ideally, to not have any medication changes during the course of the study, but at least 40% of patients did require medication changes during the study. Additionally, it is unclear whether all patients underwent specific testing to rule out secondary hypertension, as this was done at the discretion of the treating physician.
First-generation catheters?
The same type of catheter was used as in the earlier SYMPLICITY trials, and it had been used in many patients in clinical practice in countries where the catheter is routinely available. It is unknown, however, whether newer multisite denervation devices would yield better results than the first-generation devices used in SYMPLICITY HTN-3. But even this would not explain the discrepancies in data between earlier trials and this trial.
Operator inexperience?
It has been suggested that operator inexperience may have played a role, but an analysis of operator volume did not find any association between this variable and the outcomes. Each procedure was supervised by at least 1 and in most cases 2 certified Medtronic representatives, who made certain that meticulous attention was paid to procedure details and that no shortcuts were taken during the procedure.
Inadequate ablation?
While we can assume that the correct technique was followed in most cases, renal denervation is still a “blind” procedure, and there is no nerve mapping to ascertain the degree of ablation achieved. Notably, patients who had the most ablations reportedly had a greater average drop in systolic ambulatory blood pressure than those who received fewer ablations. Sympathetic nervous system activity is a potential marker of adequacy of ablation, but it was not routinely assessed in the SYMPLICITY HTN-3 trial. Techniques to assess sympathetic nerve activity such as norepinephrine spillover and muscle sympathetic nerve activity are highly specialized and available only at a few research centers, and are not available for routine clinical use.
While these points may explain the negative findings of this trial, they fail to account for the discrepant results between this study and previous trials that used exactly the same definitions and techniques.
Patient demographics?
Is it possible that renal denervation has a differential effect according to race? All previous renal denervation studies were conducted in Europe or Australia; therefore, few data are available on the efficacy of the procedure in other racial groups, such as black Americans. Most of the patients in this trial were white, but approximately 25% were black—a good representation. There was a statistically significant benefit favoring renal denervation in nonblack (mostly white) patients, but not in black patients. This may be related to racial differences in the pathophysiology of hypertension or possibly due to chance alone.
A Hawthorne effect?
A Hawthorne effect (patients being more compliant because physicians are paying more attention to them) is unlikely, since the renal denervation arm did not have any reduction in blood pressure medications. At 6 months, both the sham group and the procedure group were still on an average of 5 medications.
Additionally, while the blood pressure reduction in both treatment groups was significant, the systolic blood pressure at 6 months was still 166 mm Hg in the denervation group and 168 mm Hg in the sham group. If denervation was effective, one would have expected a greater reduction in blood pressure or at least a decrease in the number of medications needed, eg, 1 to 2 fewer medications in the denervation group compared with the sham procedure group.
Regression to the mean?
It is unknown whether the results represent a statistical error such as regression to the mean. But given the run-in period and the confirmatory data from 24-hour ambulatory blood pressure, this would be unlikely.
WHAT NOW?
Is renal denervation dead? SYMPLICITY HTN-3 is only a single trial with multiple shortcomings and lessons to learn from. Since its publication, there have been updates from 2 prospective, randomized, open-label trials concerning the efficacy of catheter-based renal denervation in lowering blood pressure.10,11
DENERHTN (Renal Denervation for Hypertension)10 studied patients with ambulatory systolic blood pressure higher than 135 mm Hg, diastolic blood pressure higher than 80 mm Hg, or both (after excluding secondary etiologies), despite 4 weeks of standardized triple-drug treatment including a diuretic. Patients were randomized to standardized stepped-care antihypertensive treatment alone (control group) or standard care plus renal denervation. The latter resulted in a significant further reduction in ambulatory blood pressure at 6 months.
The Prague-15 trial11 studied patients with resistant hypertension. Secondary etiologies were excluded and adherence to therapy was confirmed by measuring plasma medication levels. It showed that renal denervation along with optimal antihypertensive medical therapy (unchanged after randomization) resulted in a significant reduction in ambulatory blood pressure that was comparable to the effect of intensified antihypertensive medical therapy including spironolactone. (Studies have shown that spironolactone is effective when added on as a fourth-line medication in resistant hypertension.12) At 6 months, patients in the intensive medical therapy group were using an average of 0.3 more antihypertensive medications than those in the procedure group.
These two trials addressed some of the drawbacks of the SYMPLICITY HTN-3 trial. However, both have many limitations including and not limited to being open-label and nonblinded, lacking a sham procedure, using a lower blood pressure threshold than SYMPLICITY HTN-3 did to define resistant hypertension, and using the same catheter as in the SYMPLICITY trials.
Better technology is coming
Advanced renal denervation catheters are needed that are multielectrode, smaller, easier to manipulate, and capable of providing simultaneous, circumferential, more-intense, and deeper ablations. The ongoing Investigator-Steered Project on Intravascular Renal Denervation for Management of Drug-Resistant Hypertension (INSPIRED)16 and Renal Denervation Using the Vessix Renal Denervation System for the Treatment of Hypertension (REDUCE-HTN: REINFORCE)17 trials are using contemporary innovative ablation catheters to address the limitations of the first-generation Symplicity catheter.
Further, Fischell et al18 reported encouraging results of renal denervation performed by injecting ethanol into the adventitial space of the renal arteries. This is still an invasive procedure; however, ethanol can spread out in all directions and reach all targeted nerves, potentially resulting in a more complete renal artery sympathetic ablation.
As technology advances, the WAVE IV trial19 is examining renal denervation performed from the outside through the skin using high-intensity focused ultrasound, which eliminates the need for femoral arterial catheterization, a promising noninvasive approach.
Proposals for future trials
The European Clinical Consensus Conference for Renal Denervation20 proposed that future trials of renal denervation include patients with moderate rather than resistant hypertension, reflecting the pathogenic importance of sympathetic activity in earlier stages of hypertension. The conference also proposed excluding patients with stiff large arteries, a cause of isolated systolic hypertension. Other proposals included standardizing concomitant antihypertensive therapy, preferably treating all patients with the combination of a renin-angiotensin system blocker, calcium channel blocker, and diuretic in the run-in period; monitoring drug adherence through the use of pill counts, electronic pill dispensers, and drug blood tests; and using change in ambulatory blood pressure as the primary efficacy end point and change in office blood pressure as a secondary end point.
Trials ongoing
To possibly address the limitations posed by the SYMPLICITY HTN-3 trial and to answer other important questions, several sham-controlled clinical trials of renal denervation are currently being conducted:
- INSPiRED16
- REDUCE-HTN: REINFORCE17
- Spyral HTN-Off Med21
- Spyral HTN-On Med21
- Study of the ReCor Medical Paradise System in Clinical Hypertension (RADIANCE-HTN).22
We hope these new studies can more clearly identify subsets of patients who would benefit from this technology, determine predictors of blood pressure reduction in such patients, and lead to newer devices that may provide more complete ablation.
Obviously, we also need better ways to identify the exact location of these sympathetic nerves within the renal artery and have a clearer sense of procedural success.
Until then, our colleagues in Europe and Australia continue to treat patients with this technology as we appropriately and patiently wait for level 1 clinical evidence of its efficacy.
Acknowledgments: We thank Kathryn Brock, BA, Editorial Services Manager, Heart and Vascular Institute, Cleveland Clinic, for her assistance in the preparation of this paper.
- Bhatt DL, Kandzari DE, O’Neill WW, et al, for the SYMPLICITY HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med 2014; 370:1393–1401.
- Symplicity HTN-2 Investigators, Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Bohm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (the Symplicity HTN-2 trial): a randomised controlled trial. Lancet 2010; 376:1903–1909.
- Bunte MC, Infante de Oliveira E, Shishehbor MH. Endovascular treatment of resistant and uncontrolled hypertension: therapies on the horizon. JACC Cardiovasc Interv 2013; 6:1–9.
- Thomas G, Shishehbor MH, Bravo EL, Nally JV. Renal denervation to treat resistant hypertension: guarded optimism. Cleve Clin J Med 2012; 79:501–510.
- Shishehbor MH, Bunte MC. Anatomical exclusion for renal denervation: are we putting the cart before the horse? JACC Cardiovasc Interv 2014; 7:193–194.
- Bhatt DL, Bakris GL. The promise of renal denervation. Cleve Clin J Med 2012; 79:498–500.
- Bunte MC. Renal sympathetic denervation for refractory hypertension. Lancet 2011; 377:1074; author reply 1075.
- Mahfoud F, Luscher TF, Andersson B, et al; European Society of Cardiology. Expert consensus document from the European Society of Cardiology on catheter-based renal denervation. Eur Heart J 2013; 34:2149–2157.
- Schlaich MP, Schmieder RE, Bakris G, et al. International expert consensus statement: percutaneous transluminal renal denervation for the treatment of resistant hypertension. J Am Coll Cardiol 2013; 62:2031–2045.
- Azizi M, Sapoval M, Gosse P, et al; Renal Denervation for Hypertension (DENERHTN) investigators. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet 2015; 385:1957–1965.
- Rosa J, Widimsky P, Tousek P, et al. Randomized comparison of renal denervation versus intensified pharmacotherapy including spironolactone in true-resistant hypertension: six-month results from the Prague-15 study. Hypertension 2015; 65:407–413.
- Williams B, MacDonald TM, Morant S, et al; British Hypertension Society’s PATHWAY Studies Group. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet 2015; 386:2059–2068.
- Sakakura K, Ladich E, Cheng Q, et al. Anatomic assessment of sympathetic peri-arterial renal nerves in man. J Am Coll Cardiol 2014; 64:635–643.
- Mahfoud F, Edelman ER, Bohm M. Catheter-based renal denervation is no simple matter: lessons to be learned from our anatomy? J Am Coll Cardiol 2014; 64:644–646.
- Id D, Kaltenbach B, Bertog SC, et al. Does the presence of accessory renal arteries affect the efficacy of renal denervation? JACC Cardiovasc Interv 2013; 6:1085–1091.
- Jin Y, Jacobs L, Baelen M, et al; Investigator-Steered Project on Intravascular Renal Denervation for Management of Drug-Resistant Hypertension (Inspired) Investigators. Rationale and design of the Investigator-Steered Project on Intravascular Renal Denervation for Management of Drug-Resistant Hypertension (INSPiRED) trial. Blood Press 2014; 23:138–146.
- ClinicalTrialsgov. Renal Denervation Using the Vessix Renal Denervation System for the Treatment of Hypertension (REDUCE HTN: REINFORCE). https://clinicaltrials.gov/ct2/show/NCT02392351?term=REDUCE-HTN%3A+REINFORCE&rank=1. Accessed August 3, 2017.
- Fischell TA, Ebner A, Gallo S, et al. Transcatheter alcohol-mediated perivascular renal denervation with the peregrine system: first-in-human experience. JACC Cardiovasc Interv 2016; 9:589–598.
- ClinicalTrialsgov. Sham controlled study of renal denervation for subjects with uncontrolled hypertension (WAVE_IV) (NCT02029885). https://clinicaltrials.gov/ct2/show/results/NCT02029885. Accessed August 3, 2017.
- Mahfoud F, Bohm M, Azizi M, et al. Proceedings from the European clinical consensus conference for renal denervation: considerations on future clinical trial design. Eur Heart J 2015; 36:2219–2227.
- Kandzari DE, Kario K, Mahfoud F, et al. The SPYRAL HTN Global Clinical Trial Program: rationale and design for studies of renal denervation in the absence (SPYRAL HTN OFF-MED) and presence (SPYRAL HTN ON-MED) of antihypertensive medications. Am Heart J 2016; 171:82–91.
- ClinicalTrialsgov. A Study of the ReCor Medical Paradise System in Clinical Hypertension (RADIANCE-HTN). https://clinicaltrials.gov/ct2/show/NCT02649426?term=RADIANCE&rank=3. Accessed August 3, 2017.
- Bhatt DL, Kandzari DE, O’Neill WW, et al, for the SYMPLICITY HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med 2014; 370:1393–1401.
- Symplicity HTN-2 Investigators, Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Bohm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (the Symplicity HTN-2 trial): a randomised controlled trial. Lancet 2010; 376:1903–1909.
- Bunte MC, Infante de Oliveira E, Shishehbor MH. Endovascular treatment of resistant and uncontrolled hypertension: therapies on the horizon. JACC Cardiovasc Interv 2013; 6:1–9.
- Thomas G, Shishehbor MH, Bravo EL, Nally JV. Renal denervation to treat resistant hypertension: guarded optimism. Cleve Clin J Med 2012; 79:501–510.
- Shishehbor MH, Bunte MC. Anatomical exclusion for renal denervation: are we putting the cart before the horse? JACC Cardiovasc Interv 2014; 7:193–194.
- Bhatt DL, Bakris GL. The promise of renal denervation. Cleve Clin J Med 2012; 79:498–500.
- Bunte MC. Renal sympathetic denervation for refractory hypertension. Lancet 2011; 377:1074; author reply 1075.
- Mahfoud F, Luscher TF, Andersson B, et al; European Society of Cardiology. Expert consensus document from the European Society of Cardiology on catheter-based renal denervation. Eur Heart J 2013; 34:2149–2157.
- Schlaich MP, Schmieder RE, Bakris G, et al. International expert consensus statement: percutaneous transluminal renal denervation for the treatment of resistant hypertension. J Am Coll Cardiol 2013; 62:2031–2045.
- Azizi M, Sapoval M, Gosse P, et al; Renal Denervation for Hypertension (DENERHTN) investigators. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet 2015; 385:1957–1965.
- Rosa J, Widimsky P, Tousek P, et al. Randomized comparison of renal denervation versus intensified pharmacotherapy including spironolactone in true-resistant hypertension: six-month results from the Prague-15 study. Hypertension 2015; 65:407–413.
- Williams B, MacDonald TM, Morant S, et al; British Hypertension Society’s PATHWAY Studies Group. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet 2015; 386:2059–2068.
- Sakakura K, Ladich E, Cheng Q, et al. Anatomic assessment of sympathetic peri-arterial renal nerves in man. J Am Coll Cardiol 2014; 64:635–643.
- Mahfoud F, Edelman ER, Bohm M. Catheter-based renal denervation is no simple matter: lessons to be learned from our anatomy? J Am Coll Cardiol 2014; 64:644–646.
- Id D, Kaltenbach B, Bertog SC, et al. Does the presence of accessory renal arteries affect the efficacy of renal denervation? JACC Cardiovasc Interv 2013; 6:1085–1091.
- Jin Y, Jacobs L, Baelen M, et al; Investigator-Steered Project on Intravascular Renal Denervation for Management of Drug-Resistant Hypertension (Inspired) Investigators. Rationale and design of the Investigator-Steered Project on Intravascular Renal Denervation for Management of Drug-Resistant Hypertension (INSPiRED) trial. Blood Press 2014; 23:138–146.
- ClinicalTrialsgov. Renal Denervation Using the Vessix Renal Denervation System for the Treatment of Hypertension (REDUCE HTN: REINFORCE). https://clinicaltrials.gov/ct2/show/NCT02392351?term=REDUCE-HTN%3A+REINFORCE&rank=1. Accessed August 3, 2017.
- Fischell TA, Ebner A, Gallo S, et al. Transcatheter alcohol-mediated perivascular renal denervation with the peregrine system: first-in-human experience. JACC Cardiovasc Interv 2016; 9:589–598.
- ClinicalTrialsgov. Sham controlled study of renal denervation for subjects with uncontrolled hypertension (WAVE_IV) (NCT02029885). https://clinicaltrials.gov/ct2/show/results/NCT02029885. Accessed August 3, 2017.
- Mahfoud F, Bohm M, Azizi M, et al. Proceedings from the European clinical consensus conference for renal denervation: considerations on future clinical trial design. Eur Heart J 2015; 36:2219–2227.
- Kandzari DE, Kario K, Mahfoud F, et al. The SPYRAL HTN Global Clinical Trial Program: rationale and design for studies of renal denervation in the absence (SPYRAL HTN OFF-MED) and presence (SPYRAL HTN ON-MED) of antihypertensive medications. Am Heart J 2016; 171:82–91.
- ClinicalTrialsgov. A Study of the ReCor Medical Paradise System in Clinical Hypertension (RADIANCE-HTN). https://clinicaltrials.gov/ct2/show/NCT02649426?term=RADIANCE&rank=3. Accessed August 3, 2017.
KEY POINTS
- Renal denervation consists of passing a catheter into the renal arteries and ablating their sympathetic nerves using radiofrequency energy. In theory, it should lower blood pressure and be an attractive option for treating resistant hypertension.
- SYMPLICITY HTN-3 was a blinded trial in which patients with resistant hypertension were randomized to undergo real or sham renal denervation.
- At 6 months, office systolic blood pressure had failed to fall more in the renal denervation group than in the sham denervation group by a margin of at least 5 mm Hg, the primary efficacy end point of the trial.
- Methodologic and technical shortcomings may explain the negative results of the SYMPLICITY HTN-3 trial, but most device manufacturers have put the brakes on future research into this novel therapy.
- Today, renal denervation is not available in the United States but is available for routine care in Europe and Australia.
Renal denervation: Are we on the right path?
When renal sympathetic denervation, an endovascular procedure designed to treat resistant hypertension, failed to meet its efficacy goal in the SYMPLICITY HTN-3 trial,1 the news was disappointing.
In this issue of the Cleveland Clinic Journal of Medicine, Shishehbor et al2 provide a critical review of the findings of that trial and summarize its intricacies, as well as the results of other important trials of renal denervation therapy for hypertension. To their excellent observations, we would like to add some of our own.
HYPERTENSION: COMMON, OFTEN RESISTANT
The worldwide prevalence of hypertension is increasing. In the year 2000, about 26% of the adult world population had hypertension; by the year 2025, the number is projected to rise to 29%—1.56 billion people.3
Only about 50% of patients with hypertension are treated for it and, of those, about half have it adequately controlled. In one report, about 30% of US patients with hypertension had adequate blood pressure control.4
Patients who have uncontrolled hypertension are usually older and more obese, have higher baseline blood pressure and excessive salt intake, and are more likely to have chronic kidney disease, diabetes, obstructive sleep apnea, and aldosterone excess.5 Many of these conditions are also associated with increased sympathetic nervous system activity.6
Resistance and pseudoresistance
But lack of control of blood pressure is not the same as resistant hypertension. It is important to differentiate resistant hypertension from pseudoresistant hypertension, ie, hypertension that only seems to be resistant.7 Resistant hypertension affects 12.8% of all drug-treated hypertensive patients in the United States, according to data from the National Health and Nutrition Examination Survey.8
Factors that can cause pseudoresistant hypertension include:
Suboptimal antihypertensive regimens (truly resistant hypertension means blood pressure that remains high despite concurrent treatment with 3 antihypertensive drugs of different classes, 1 of which is a diuretic, in maximal doses)
The white coat effect (higher blood pressure in the office than at home, presumably due to the stress of an office visit)
- Suboptimal blood pressure measurement techniques (eg, use of a cuff that is too small, causing falsely high readings)
- Physician inertia (eg, failure to change a regimen that is not working)
- Lifestyle factors (eg, excessive sodium intake)
- Medications that interfere with blood pressure control (eg, nonsteroidal anti-inflammatory drugs)
- Poor adherence to prescribed medications.
Causes of secondary hypertension such as obstructive sleep apnea, primary aldosteronism, and renal artery stenosis should also be ruled out before concluding that a patient has resistant hypertension.
Treatment prevents complications
Hypertension causes a myriad of medical diseases, including accelerated atherosclerosis, myocardial ischemia and infarction, both systolic and diastolic heart failure, rhythm problems (eg, atrial fibrillation), and stroke.
Most patients with resistant hypertension have no identifiable reversible causes of it, exhibit increased sympathetic nervous system activity, and have increased risk of cardiovascular events. The risk can be reduced by treatment.9,10
Adequate and sustained treatment of hypertension prevents and mitigates its complications. The classic Veterans Administration Cooperative Study in the 1960s demonstrated a 96% reduction in cardiovascular events over 18 months with the use of 3 antihypertensive medications in patients with severe hypertension.11 A reduction of as little as 2 mm Hg in the mean blood pressure has been associated with a 10% reduction in the risk of stroke mortality and a 7% decrease in ischemic heart disease mortality.12 This is an important consideration when evaluating the clinical end points of hypertension trials.
SYMPLICITY HTN-3 TRIAL: WHAT DID WE LEARN?
As controlling blood pressure is paramount in reducing cardiovascular complications, it is only natural to look for innovative strategies to supplement the medical treatments of hypertension.
The multicenter SYMPLICITY HTN-3 trial1 was undertaken to establish the efficacy of renal-artery denervation using radiofrequency energy delivered by a catheter-based system (Symplicity RDN, Medtronic, Dublin, Ireland). This randomized, sham-controlled, blinded study did not show a benefit from this procedure with respect to either of its efficacy end points—at 6 months, a reduction in office systolic blood pressure of at least 5 mm Hg more than with medical therapy alone, or a reduction in mean ambulatory systolic pressure of at least 2 mm Hg more than with medical therapy alone.
Despite the negative results, this medium-size (N = 535) randomized clinical trial still represents the highest-level evidence in the field, and we ought to learn something from it.
Limitations of SYMPLICITY HTN-3
Several factors may have contributed to the negative results of the trial.
Patient selection. For the most part, patients enrolled in renal denervation trials, including SYMPLICITY HTN-3, were not selected on the basis of heightened sympathetic nervous system activity. Assessment of sympathetic nervous system activity may identify the population most likely to achieve an adequate response.
Of note, the baseline blood pressure readings of patients in this trial were higher in the office than on ambulatory monitoring. Patients with white coat hypertension have increased sympathetic nervous system activity and thus might actually be good candidates for renal denervation therapy.
Adequacy of ablation was not measured. Many argue that an objective measure of the adequacy of the denervation procedure (qualitative or quantitative) should have been implemented and, if it had been, the results might have been different. For example, when ablation is performed in the main renal artery as well as the branches, the efficacy in reducing levels of norepinephrine is improved.13
Blood pressure fell in both groups. In SYMPLICITY HTN-3 and many other renal denervation trials, patients were assessed using both office and ambulatory blood pressure measurements. The primary end point was the office blood pressure measurement, with a 5-mm Hg difference in reduction chosen to define the superiority margin. This margin was chosen because even small reductions in blood pressure are known to decrease adverse events caused by hypertension. Notably, blood pressure fell significantly in both the control and intervention groups, with an intergroup difference of 2.39 mm Hg (not statistically significant) in favor of denervation.
Medication questions. The SYMPLICITY HTN-3 patients were supposed to be on stable medical regimens with maximal tolerated doses before the procedure. However, it was difficult to assess patients’ adherence to and tolerance of medical therapies. Many (about 40%) of the patients had their medications changed during the study.1
Therefore, a critical look at the study enrollment criteria may shed more light on the reasons for the negative findings. Did these patients truly have resistant hypertension? Before they underwent the treatment, was their prestudy pharmacologic regimen adequately intensified?
ONGOING STUDIES
After the findings of the SYMPLICITY HTN-3 study were released, several other trials—such as the Renal Denervation for Hypertension (DENERHTN)14 and Prague-15 trials15—reported conflicting results. Notably, these were not sham-controlled trials.
Newer studies with robust trial designs are ongoing. A quick search of www.clinicaltrials.gov reveals that at least 89 active clinical trials of renal denervation are registered as of the date of this writing. Excluding those with unknown status, there are 63 trials open or ongoing.
Clinical trials are also ongoing to determine the effects of renal denervation in patients with heart failure, atrial fibrillation, sleep apnea, and chronic kidney disease, all of which are known to involve heightened sympathetic nervous system activity.
NOT READY FOR CLINICAL USE
Although nonpharmacologic treatments of hypertension continue to be studied and are supported by an avalanche of trials in animals and small, mostly nonrandomized trials in humans, one should not forget that the SYMPLICITY HTN-3 trial simply did not meet its primary efficacy end points. We need definitive clinical evidence showing that renal denervation reduces either blood pressure or clinical events before it becomes a mainstream therapy in humans.
Additional trials are being conducted that were designed in accordance with the recommendations of the European Clinical Consensus Conference for Renal Denervation16 in terms of study population, design, and end points. Well-designed studies that conform to those recommendations are critical.
Finally, although our enthusiasm for renal denervation as a treatment of hypertension is tempered, there have been no noteworthy safety concerns related to the procedure, which certainly helps maintain the research momentum in this field.
- Bhatt DL, Kandzari DE, O’Neill WW, et al; SYMPLICITY HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med 2014; 370:1393–1401.
- Shishehbor MH, Hammad TA, Thomas G. Renal denervation: what happened, and why? Cleve Clin J Med 2017; 84:681–686.
- Kearney PM, Whelton M, Reynolds K, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet 2005; 365:217–223.
- Kearney PM, Whelton M, Reynolds K, Whelton PK, He J. Worldwide prevalence of hypertension: a systematic review. J Hypertens 2004; 22:11–19.
- Calhoun DA, Jones D, Textor S, et al; American Heart Association Professional Education Committee. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 2008; 117:e510–e526.
- Tsioufis C, Papademetriou V, Thomopoulos C, Stefanadis C. Renal denervation for sleep apnea and resistant hypertension: alternative or complementary to effective continuous positive airway pressure treatment? Hypertension 2011; 58:e191–e192.
- Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research.Hypertension 2008; 51:1403–1419.
- Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension 2011; 57:1076–1080.
- Papademetriou V, Doumas M, Tsioufis K. Renal sympathetic denervation for the treatment of difficult-to-control or resistant hypertension. Int J Hypertens 2011; 2011:196518.
- Doumas M, Faselis C, Papademetriou V. Renal sympathetic denervation in hypertension. Curr Opin Nephrol Hypertens 2011; 20:647–653.
- Veterans Administration Cooperative Study Group on Antihypertensive Agents. Effect of treatment on morbidity in hypertension: results in patients with diastolic blood pressures averaging 115 through 129 mm Hg. JAMA 1967; 202:1028–1034.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913.
- Henegar JR, Zhang Y, Hata C, Narciso I, Hall ME, Hall JE. Catheter-based radiofrequency renal denervation: location effects on renal norepinephrine. Am J Hypertens 2015; 28:909–914.
- Azizi M, Sapoval M, Gosse P, et al; Renal Denervation for Hypertension (DENERHTN) investigators. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet 2015; 385:1957–1965.
- Rosa J, Widimsky P, Waldauf P, et al. Role of adding spironolactone and renal denervation in true resistant hypertension: one-year outcomes of randomized PRAGUE-15 study. Hypertension 2016; 67:397–403.
- Mahfoud F, Bohm M, Azizi M, et al. Proceedings from the European Clinical Consensus Conference for Renal Denervation: Considerations on Future Clinical Trial Design. Eur Heart J 2015; 6:2219–2227.
When renal sympathetic denervation, an endovascular procedure designed to treat resistant hypertension, failed to meet its efficacy goal in the SYMPLICITY HTN-3 trial,1 the news was disappointing.
In this issue of the Cleveland Clinic Journal of Medicine, Shishehbor et al2 provide a critical review of the findings of that trial and summarize its intricacies, as well as the results of other important trials of renal denervation therapy for hypertension. To their excellent observations, we would like to add some of our own.
HYPERTENSION: COMMON, OFTEN RESISTANT
The worldwide prevalence of hypertension is increasing. In the year 2000, about 26% of the adult world population had hypertension; by the year 2025, the number is projected to rise to 29%—1.56 billion people.3
Only about 50% of patients with hypertension are treated for it and, of those, about half have it adequately controlled. In one report, about 30% of US patients with hypertension had adequate blood pressure control.4
Patients who have uncontrolled hypertension are usually older and more obese, have higher baseline blood pressure and excessive salt intake, and are more likely to have chronic kidney disease, diabetes, obstructive sleep apnea, and aldosterone excess.5 Many of these conditions are also associated with increased sympathetic nervous system activity.6
Resistance and pseudoresistance
But lack of control of blood pressure is not the same as resistant hypertension. It is important to differentiate resistant hypertension from pseudoresistant hypertension, ie, hypertension that only seems to be resistant.7 Resistant hypertension affects 12.8% of all drug-treated hypertensive patients in the United States, according to data from the National Health and Nutrition Examination Survey.8
Factors that can cause pseudoresistant hypertension include:
Suboptimal antihypertensive regimens (truly resistant hypertension means blood pressure that remains high despite concurrent treatment with 3 antihypertensive drugs of different classes, 1 of which is a diuretic, in maximal doses)
The white coat effect (higher blood pressure in the office than at home, presumably due to the stress of an office visit)
- Suboptimal blood pressure measurement techniques (eg, use of a cuff that is too small, causing falsely high readings)
- Physician inertia (eg, failure to change a regimen that is not working)
- Lifestyle factors (eg, excessive sodium intake)
- Medications that interfere with blood pressure control (eg, nonsteroidal anti-inflammatory drugs)
- Poor adherence to prescribed medications.
Causes of secondary hypertension such as obstructive sleep apnea, primary aldosteronism, and renal artery stenosis should also be ruled out before concluding that a patient has resistant hypertension.
Treatment prevents complications
Hypertension causes a myriad of medical diseases, including accelerated atherosclerosis, myocardial ischemia and infarction, both systolic and diastolic heart failure, rhythm problems (eg, atrial fibrillation), and stroke.
Most patients with resistant hypertension have no identifiable reversible causes of it, exhibit increased sympathetic nervous system activity, and have increased risk of cardiovascular events. The risk can be reduced by treatment.9,10
Adequate and sustained treatment of hypertension prevents and mitigates its complications. The classic Veterans Administration Cooperative Study in the 1960s demonstrated a 96% reduction in cardiovascular events over 18 months with the use of 3 antihypertensive medications in patients with severe hypertension.11 A reduction of as little as 2 mm Hg in the mean blood pressure has been associated with a 10% reduction in the risk of stroke mortality and a 7% decrease in ischemic heart disease mortality.12 This is an important consideration when evaluating the clinical end points of hypertension trials.
SYMPLICITY HTN-3 TRIAL: WHAT DID WE LEARN?
As controlling blood pressure is paramount in reducing cardiovascular complications, it is only natural to look for innovative strategies to supplement the medical treatments of hypertension.
The multicenter SYMPLICITY HTN-3 trial1 was undertaken to establish the efficacy of renal-artery denervation using radiofrequency energy delivered by a catheter-based system (Symplicity RDN, Medtronic, Dublin, Ireland). This randomized, sham-controlled, blinded study did not show a benefit from this procedure with respect to either of its efficacy end points—at 6 months, a reduction in office systolic blood pressure of at least 5 mm Hg more than with medical therapy alone, or a reduction in mean ambulatory systolic pressure of at least 2 mm Hg more than with medical therapy alone.
Despite the negative results, this medium-size (N = 535) randomized clinical trial still represents the highest-level evidence in the field, and we ought to learn something from it.
Limitations of SYMPLICITY HTN-3
Several factors may have contributed to the negative results of the trial.
Patient selection. For the most part, patients enrolled in renal denervation trials, including SYMPLICITY HTN-3, were not selected on the basis of heightened sympathetic nervous system activity. Assessment of sympathetic nervous system activity may identify the population most likely to achieve an adequate response.
Of note, the baseline blood pressure readings of patients in this trial were higher in the office than on ambulatory monitoring. Patients with white coat hypertension have increased sympathetic nervous system activity and thus might actually be good candidates for renal denervation therapy.
Adequacy of ablation was not measured. Many argue that an objective measure of the adequacy of the denervation procedure (qualitative or quantitative) should have been implemented and, if it had been, the results might have been different. For example, when ablation is performed in the main renal artery as well as the branches, the efficacy in reducing levels of norepinephrine is improved.13
Blood pressure fell in both groups. In SYMPLICITY HTN-3 and many other renal denervation trials, patients were assessed using both office and ambulatory blood pressure measurements. The primary end point was the office blood pressure measurement, with a 5-mm Hg difference in reduction chosen to define the superiority margin. This margin was chosen because even small reductions in blood pressure are known to decrease adverse events caused by hypertension. Notably, blood pressure fell significantly in both the control and intervention groups, with an intergroup difference of 2.39 mm Hg (not statistically significant) in favor of denervation.
Medication questions. The SYMPLICITY HTN-3 patients were supposed to be on stable medical regimens with maximal tolerated doses before the procedure. However, it was difficult to assess patients’ adherence to and tolerance of medical therapies. Many (about 40%) of the patients had their medications changed during the study.1
Therefore, a critical look at the study enrollment criteria may shed more light on the reasons for the negative findings. Did these patients truly have resistant hypertension? Before they underwent the treatment, was their prestudy pharmacologic regimen adequately intensified?
ONGOING STUDIES
After the findings of the SYMPLICITY HTN-3 study were released, several other trials—such as the Renal Denervation for Hypertension (DENERHTN)14 and Prague-15 trials15—reported conflicting results. Notably, these were not sham-controlled trials.
Newer studies with robust trial designs are ongoing. A quick search of www.clinicaltrials.gov reveals that at least 89 active clinical trials of renal denervation are registered as of the date of this writing. Excluding those with unknown status, there are 63 trials open or ongoing.
Clinical trials are also ongoing to determine the effects of renal denervation in patients with heart failure, atrial fibrillation, sleep apnea, and chronic kidney disease, all of which are known to involve heightened sympathetic nervous system activity.
NOT READY FOR CLINICAL USE
Although nonpharmacologic treatments of hypertension continue to be studied and are supported by an avalanche of trials in animals and small, mostly nonrandomized trials in humans, one should not forget that the SYMPLICITY HTN-3 trial simply did not meet its primary efficacy end points. We need definitive clinical evidence showing that renal denervation reduces either blood pressure or clinical events before it becomes a mainstream therapy in humans.
Additional trials are being conducted that were designed in accordance with the recommendations of the European Clinical Consensus Conference for Renal Denervation16 in terms of study population, design, and end points. Well-designed studies that conform to those recommendations are critical.
Finally, although our enthusiasm for renal denervation as a treatment of hypertension is tempered, there have been no noteworthy safety concerns related to the procedure, which certainly helps maintain the research momentum in this field.
When renal sympathetic denervation, an endovascular procedure designed to treat resistant hypertension, failed to meet its efficacy goal in the SYMPLICITY HTN-3 trial,1 the news was disappointing.
In this issue of the Cleveland Clinic Journal of Medicine, Shishehbor et al2 provide a critical review of the findings of that trial and summarize its intricacies, as well as the results of other important trials of renal denervation therapy for hypertension. To their excellent observations, we would like to add some of our own.
HYPERTENSION: COMMON, OFTEN RESISTANT
The worldwide prevalence of hypertension is increasing. In the year 2000, about 26% of the adult world population had hypertension; by the year 2025, the number is projected to rise to 29%—1.56 billion people.3
Only about 50% of patients with hypertension are treated for it and, of those, about half have it adequately controlled. In one report, about 30% of US patients with hypertension had adequate blood pressure control.4
Patients who have uncontrolled hypertension are usually older and more obese, have higher baseline blood pressure and excessive salt intake, and are more likely to have chronic kidney disease, diabetes, obstructive sleep apnea, and aldosterone excess.5 Many of these conditions are also associated with increased sympathetic nervous system activity.6
Resistance and pseudoresistance
But lack of control of blood pressure is not the same as resistant hypertension. It is important to differentiate resistant hypertension from pseudoresistant hypertension, ie, hypertension that only seems to be resistant.7 Resistant hypertension affects 12.8% of all drug-treated hypertensive patients in the United States, according to data from the National Health and Nutrition Examination Survey.8
Factors that can cause pseudoresistant hypertension include:
Suboptimal antihypertensive regimens (truly resistant hypertension means blood pressure that remains high despite concurrent treatment with 3 antihypertensive drugs of different classes, 1 of which is a diuretic, in maximal doses)
The white coat effect (higher blood pressure in the office than at home, presumably due to the stress of an office visit)
- Suboptimal blood pressure measurement techniques (eg, use of a cuff that is too small, causing falsely high readings)
- Physician inertia (eg, failure to change a regimen that is not working)
- Lifestyle factors (eg, excessive sodium intake)
- Medications that interfere with blood pressure control (eg, nonsteroidal anti-inflammatory drugs)
- Poor adherence to prescribed medications.
Causes of secondary hypertension such as obstructive sleep apnea, primary aldosteronism, and renal artery stenosis should also be ruled out before concluding that a patient has resistant hypertension.
Treatment prevents complications
Hypertension causes a myriad of medical diseases, including accelerated atherosclerosis, myocardial ischemia and infarction, both systolic and diastolic heart failure, rhythm problems (eg, atrial fibrillation), and stroke.
Most patients with resistant hypertension have no identifiable reversible causes of it, exhibit increased sympathetic nervous system activity, and have increased risk of cardiovascular events. The risk can be reduced by treatment.9,10
Adequate and sustained treatment of hypertension prevents and mitigates its complications. The classic Veterans Administration Cooperative Study in the 1960s demonstrated a 96% reduction in cardiovascular events over 18 months with the use of 3 antihypertensive medications in patients with severe hypertension.11 A reduction of as little as 2 mm Hg in the mean blood pressure has been associated with a 10% reduction in the risk of stroke mortality and a 7% decrease in ischemic heart disease mortality.12 This is an important consideration when evaluating the clinical end points of hypertension trials.
SYMPLICITY HTN-3 TRIAL: WHAT DID WE LEARN?
As controlling blood pressure is paramount in reducing cardiovascular complications, it is only natural to look for innovative strategies to supplement the medical treatments of hypertension.
The multicenter SYMPLICITY HTN-3 trial1 was undertaken to establish the efficacy of renal-artery denervation using radiofrequency energy delivered by a catheter-based system (Symplicity RDN, Medtronic, Dublin, Ireland). This randomized, sham-controlled, blinded study did not show a benefit from this procedure with respect to either of its efficacy end points—at 6 months, a reduction in office systolic blood pressure of at least 5 mm Hg more than with medical therapy alone, or a reduction in mean ambulatory systolic pressure of at least 2 mm Hg more than with medical therapy alone.
Despite the negative results, this medium-size (N = 535) randomized clinical trial still represents the highest-level evidence in the field, and we ought to learn something from it.
Limitations of SYMPLICITY HTN-3
Several factors may have contributed to the negative results of the trial.
Patient selection. For the most part, patients enrolled in renal denervation trials, including SYMPLICITY HTN-3, were not selected on the basis of heightened sympathetic nervous system activity. Assessment of sympathetic nervous system activity may identify the population most likely to achieve an adequate response.
Of note, the baseline blood pressure readings of patients in this trial were higher in the office than on ambulatory monitoring. Patients with white coat hypertension have increased sympathetic nervous system activity and thus might actually be good candidates for renal denervation therapy.
Adequacy of ablation was not measured. Many argue that an objective measure of the adequacy of the denervation procedure (qualitative or quantitative) should have been implemented and, if it had been, the results might have been different. For example, when ablation is performed in the main renal artery as well as the branches, the efficacy in reducing levels of norepinephrine is improved.13
Blood pressure fell in both groups. In SYMPLICITY HTN-3 and many other renal denervation trials, patients were assessed using both office and ambulatory blood pressure measurements. The primary end point was the office blood pressure measurement, with a 5-mm Hg difference in reduction chosen to define the superiority margin. This margin was chosen because even small reductions in blood pressure are known to decrease adverse events caused by hypertension. Notably, blood pressure fell significantly in both the control and intervention groups, with an intergroup difference of 2.39 mm Hg (not statistically significant) in favor of denervation.
Medication questions. The SYMPLICITY HTN-3 patients were supposed to be on stable medical regimens with maximal tolerated doses before the procedure. However, it was difficult to assess patients’ adherence to and tolerance of medical therapies. Many (about 40%) of the patients had their medications changed during the study.1
Therefore, a critical look at the study enrollment criteria may shed more light on the reasons for the negative findings. Did these patients truly have resistant hypertension? Before they underwent the treatment, was their prestudy pharmacologic regimen adequately intensified?
ONGOING STUDIES
After the findings of the SYMPLICITY HTN-3 study were released, several other trials—such as the Renal Denervation for Hypertension (DENERHTN)14 and Prague-15 trials15—reported conflicting results. Notably, these were not sham-controlled trials.
Newer studies with robust trial designs are ongoing. A quick search of www.clinicaltrials.gov reveals that at least 89 active clinical trials of renal denervation are registered as of the date of this writing. Excluding those with unknown status, there are 63 trials open or ongoing.
Clinical trials are also ongoing to determine the effects of renal denervation in patients with heart failure, atrial fibrillation, sleep apnea, and chronic kidney disease, all of which are known to involve heightened sympathetic nervous system activity.
NOT READY FOR CLINICAL USE
Although nonpharmacologic treatments of hypertension continue to be studied and are supported by an avalanche of trials in animals and small, mostly nonrandomized trials in humans, one should not forget that the SYMPLICITY HTN-3 trial simply did not meet its primary efficacy end points. We need definitive clinical evidence showing that renal denervation reduces either blood pressure or clinical events before it becomes a mainstream therapy in humans.
Additional trials are being conducted that were designed in accordance with the recommendations of the European Clinical Consensus Conference for Renal Denervation16 in terms of study population, design, and end points. Well-designed studies that conform to those recommendations are critical.
Finally, although our enthusiasm for renal denervation as a treatment of hypertension is tempered, there have been no noteworthy safety concerns related to the procedure, which certainly helps maintain the research momentum in this field.
- Bhatt DL, Kandzari DE, O’Neill WW, et al; SYMPLICITY HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med 2014; 370:1393–1401.
- Shishehbor MH, Hammad TA, Thomas G. Renal denervation: what happened, and why? Cleve Clin J Med 2017; 84:681–686.
- Kearney PM, Whelton M, Reynolds K, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet 2005; 365:217–223.
- Kearney PM, Whelton M, Reynolds K, Whelton PK, He J. Worldwide prevalence of hypertension: a systematic review. J Hypertens 2004; 22:11–19.
- Calhoun DA, Jones D, Textor S, et al; American Heart Association Professional Education Committee. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 2008; 117:e510–e526.
- Tsioufis C, Papademetriou V, Thomopoulos C, Stefanadis C. Renal denervation for sleep apnea and resistant hypertension: alternative or complementary to effective continuous positive airway pressure treatment? Hypertension 2011; 58:e191–e192.
- Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research.Hypertension 2008; 51:1403–1419.
- Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension 2011; 57:1076–1080.
- Papademetriou V, Doumas M, Tsioufis K. Renal sympathetic denervation for the treatment of difficult-to-control or resistant hypertension. Int J Hypertens 2011; 2011:196518.
- Doumas M, Faselis C, Papademetriou V. Renal sympathetic denervation in hypertension. Curr Opin Nephrol Hypertens 2011; 20:647–653.
- Veterans Administration Cooperative Study Group on Antihypertensive Agents. Effect of treatment on morbidity in hypertension: results in patients with diastolic blood pressures averaging 115 through 129 mm Hg. JAMA 1967; 202:1028–1034.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913.
- Henegar JR, Zhang Y, Hata C, Narciso I, Hall ME, Hall JE. Catheter-based radiofrequency renal denervation: location effects on renal norepinephrine. Am J Hypertens 2015; 28:909–914.
- Azizi M, Sapoval M, Gosse P, et al; Renal Denervation for Hypertension (DENERHTN) investigators. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet 2015; 385:1957–1965.
- Rosa J, Widimsky P, Waldauf P, et al. Role of adding spironolactone and renal denervation in true resistant hypertension: one-year outcomes of randomized PRAGUE-15 study. Hypertension 2016; 67:397–403.
- Mahfoud F, Bohm M, Azizi M, et al. Proceedings from the European Clinical Consensus Conference for Renal Denervation: Considerations on Future Clinical Trial Design. Eur Heart J 2015; 6:2219–2227.
- Bhatt DL, Kandzari DE, O’Neill WW, et al; SYMPLICITY HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med 2014; 370:1393–1401.
- Shishehbor MH, Hammad TA, Thomas G. Renal denervation: what happened, and why? Cleve Clin J Med 2017; 84:681–686.
- Kearney PM, Whelton M, Reynolds K, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet 2005; 365:217–223.
- Kearney PM, Whelton M, Reynolds K, Whelton PK, He J. Worldwide prevalence of hypertension: a systematic review. J Hypertens 2004; 22:11–19.
- Calhoun DA, Jones D, Textor S, et al; American Heart Association Professional Education Committee. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 2008; 117:e510–e526.
- Tsioufis C, Papademetriou V, Thomopoulos C, Stefanadis C. Renal denervation for sleep apnea and resistant hypertension: alternative or complementary to effective continuous positive airway pressure treatment? Hypertension 2011; 58:e191–e192.
- Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research.Hypertension 2008; 51:1403–1419.
- Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension 2011; 57:1076–1080.
- Papademetriou V, Doumas M, Tsioufis K. Renal sympathetic denervation for the treatment of difficult-to-control or resistant hypertension. Int J Hypertens 2011; 2011:196518.
- Doumas M, Faselis C, Papademetriou V. Renal sympathetic denervation in hypertension. Curr Opin Nephrol Hypertens 2011; 20:647–653.
- Veterans Administration Cooperative Study Group on Antihypertensive Agents. Effect of treatment on morbidity in hypertension: results in patients with diastolic blood pressures averaging 115 through 129 mm Hg. JAMA 1967; 202:1028–1034.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913.
- Henegar JR, Zhang Y, Hata C, Narciso I, Hall ME, Hall JE. Catheter-based radiofrequency renal denervation: location effects on renal norepinephrine. Am J Hypertens 2015; 28:909–914.
- Azizi M, Sapoval M, Gosse P, et al; Renal Denervation for Hypertension (DENERHTN) investigators. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet 2015; 385:1957–1965.
- Rosa J, Widimsky P, Waldauf P, et al. Role of adding spironolactone and renal denervation in true resistant hypertension: one-year outcomes of randomized PRAGUE-15 study. Hypertension 2016; 67:397–403.
- Mahfoud F, Bohm M, Azizi M, et al. Proceedings from the European Clinical Consensus Conference for Renal Denervation: Considerations on Future Clinical Trial Design. Eur Heart J 2015; 6:2219–2227.
Elective open conversion after failed EVAR safer than emergent
Emergency open conversion after failed endovascular aortic aneurysm repair shows significantly higher mortality and morbidity, compared with elective conversion, according to the results of a retrospective, observational study of 31 patients at a single institution.
The primary endpoints of the study were 30-day and in-hospital mortality. Secondary endpoints included moderate to severe complications, secondary interventions, length of ICU stay, and length of hospital stay (LOS), according to I. Ben Abdallah, MD, of the Hôpital Européen Georges Pompidou and his colleagues.
During the study period, a total of 338 patients received EVAR at the institution. Of these, 31 patients underwent open conversion (19 elective, 12 emergent) after EVAR between August 2008 and September 2016. The median time from the index EVAR to the open conversion was 35 months, with the most common indications for intervention being endoleaks (24 patients, 77%), stent graft infection (3, 10%), thrombosis (3, 10%) and kinking (1, 3%). Stents removed were manufactured by various device makers, according to the report (Eur J Vasc Endovasc Surg. 2017;53:831-6).
The patient population had a mean age of 73 years and comprised 84% men. The two groups, elective and emergent, were highly similar in numerous comorbidities, with the only significant difference between them being a greater incidence of chronic renal disease among the emergent group, as compared with the elective (42% vs. 5%).
Overall in-hospital mortality was 10%, and significantly greater in emergent vs. elective conversion (25% vs. 0%). Renal and pulmonary complications were significantly higher in the emergency group (42% vs. 5% and 42% vs. 0%, respectively). There was no significant difference between elective and emergent hospital stay (14 days vs. 20 days), but ICU stay was significantly shorter for elective conversion (2 days vs. 7 days).
There were no late complications or death seen in either group after a mean follow-up of 18 months.
“In this series, open conversion seems to be significantly safer and more effective when performed electively with no mortality, a lower incidence of morbidity (renal and pulmonary), and shorter ICU stay. These results underline that close surveillance, allowing planned elective open conversion, is the key to better outcomes after failed EVAR,” the researchers concluded.
The authors reported that they had no conflicts of interest, and the study received no outside funding.
Emergency open conversion after failed endovascular aortic aneurysm repair shows significantly higher mortality and morbidity, compared with elective conversion, according to the results of a retrospective, observational study of 31 patients at a single institution.
The primary endpoints of the study were 30-day and in-hospital mortality. Secondary endpoints included moderate to severe complications, secondary interventions, length of ICU stay, and length of hospital stay (LOS), according to I. Ben Abdallah, MD, of the Hôpital Européen Georges Pompidou and his colleagues.
During the study period, a total of 338 patients received EVAR at the institution. Of these, 31 patients underwent open conversion (19 elective, 12 emergent) after EVAR between August 2008 and September 2016. The median time from the index EVAR to the open conversion was 35 months, with the most common indications for intervention being endoleaks (24 patients, 77%), stent graft infection (3, 10%), thrombosis (3, 10%) and kinking (1, 3%). Stents removed were manufactured by various device makers, according to the report (Eur J Vasc Endovasc Surg. 2017;53:831-6).
The patient population had a mean age of 73 years and comprised 84% men. The two groups, elective and emergent, were highly similar in numerous comorbidities, with the only significant difference between them being a greater incidence of chronic renal disease among the emergent group, as compared with the elective (42% vs. 5%).
Overall in-hospital mortality was 10%, and significantly greater in emergent vs. elective conversion (25% vs. 0%). Renal and pulmonary complications were significantly higher in the emergency group (42% vs. 5% and 42% vs. 0%, respectively). There was no significant difference between elective and emergent hospital stay (14 days vs. 20 days), but ICU stay was significantly shorter for elective conversion (2 days vs. 7 days).
There were no late complications or death seen in either group after a mean follow-up of 18 months.
“In this series, open conversion seems to be significantly safer and more effective when performed electively with no mortality, a lower incidence of morbidity (renal and pulmonary), and shorter ICU stay. These results underline that close surveillance, allowing planned elective open conversion, is the key to better outcomes after failed EVAR,” the researchers concluded.
The authors reported that they had no conflicts of interest, and the study received no outside funding.
Emergency open conversion after failed endovascular aortic aneurysm repair shows significantly higher mortality and morbidity, compared with elective conversion, according to the results of a retrospective, observational study of 31 patients at a single institution.
The primary endpoints of the study were 30-day and in-hospital mortality. Secondary endpoints included moderate to severe complications, secondary interventions, length of ICU stay, and length of hospital stay (LOS), according to I. Ben Abdallah, MD, of the Hôpital Européen Georges Pompidou and his colleagues.
During the study period, a total of 338 patients received EVAR at the institution. Of these, 31 patients underwent open conversion (19 elective, 12 emergent) after EVAR between August 2008 and September 2016. The median time from the index EVAR to the open conversion was 35 months, with the most common indications for intervention being endoleaks (24 patients, 77%), stent graft infection (3, 10%), thrombosis (3, 10%) and kinking (1, 3%). Stents removed were manufactured by various device makers, according to the report (Eur J Vasc Endovasc Surg. 2017;53:831-6).
The patient population had a mean age of 73 years and comprised 84% men. The two groups, elective and emergent, were highly similar in numerous comorbidities, with the only significant difference between them being a greater incidence of chronic renal disease among the emergent group, as compared with the elective (42% vs. 5%).
Overall in-hospital mortality was 10%, and significantly greater in emergent vs. elective conversion (25% vs. 0%). Renal and pulmonary complications were significantly higher in the emergency group (42% vs. 5% and 42% vs. 0%, respectively). There was no significant difference between elective and emergent hospital stay (14 days vs. 20 days), but ICU stay was significantly shorter for elective conversion (2 days vs. 7 days).
There were no late complications or death seen in either group after a mean follow-up of 18 months.
“In this series, open conversion seems to be significantly safer and more effective when performed electively with no mortality, a lower incidence of morbidity (renal and pulmonary), and shorter ICU stay. These results underline that close surveillance, allowing planned elective open conversion, is the key to better outcomes after failed EVAR,” the researchers concluded.
The authors reported that they had no conflicts of interest, and the study received no outside funding.
FROM THE EUROPEAN JOURNAL OF VASCULAR AND ENDOVASCULAR SURGERY
Key clinical point:
Major finding: Overall in-hospital mortality was 10% and significantly greater in emergent vs. elective conversion (25% vs. 0%).
Data source: A retrospective database analysis of 31 patients undergoing EVAR open conversion at a single institution.
Disclosures: The authors reported that they had no conflicts of interest, and the study received no outside funding.
VA cohort study: Individualize SSI prophylaxis based on patient factors
The combined use of vancomycin and a beta-lactam antibiotic for prophylaxis against surgical site infections is associated with both benefits and harms, according to findings from a national propensity-score–adjusted retrospective cohort study.
For example, the combination treatment reduced surgical site infections (SSIs) 30 days after cardiac surgical procedures but increased the risk of postoperative acute kidney injury (AKI) in some patients, Westyn Branch-Elliman, MD, of the VA Boston Healthcare System and her colleagues reported online July 10 in PLOS Medicine.
Among cardiac surgery patients, the incidence of surgical site infections was significantly lower for the 6,953 patients treated with both drugs vs. the 12,834 treated with a single agent (0.95% vs. 1.48%), the investigators found (PLOS Med. 2017 Jul 10. doi: 10.1371/journal.pmed.1002340).
SSI benefit with combination therapy
“After controlling for age, diabetes, ASA [American Society of Anesthesiologists] score, mupirocin administration, current smoking status, and preoperative MRSA [methicillin-resistant Staphylococcus aureus] colonization status, receipt of combination antimicrobial prophylaxis was associated with reduced SSI risk following cardiac surgical procedures (adjusted risk ratio, 0.61),” they wrote, noting that, when combination therapy was compared with either of the agents alone, the associations were similar and that no association between SSI reduction and the combination regimen was seen for the other types of surgical procedures assessed.
Secondary analyses showed that, among the cardiac patients, differences in the rates of SSIs were seen based on MRSA status in patients undergoing cardiac surgery. Among MRSA-colonized patients, SSIs occurred in 8 of 346 patients (2.3%) who received combination prophylaxis vs. 4 of 100 patients (4%) who received vancomycin alone (aRR, 0.53), and, among MRSA-negative and MRSA-unknown cardiac surgery patients, SSIs occurred in 58 of 6,607 patients (0.88%) receiving combination prophylaxis and 146 of 10,215 patients (1.4%) receiving a beta-lactam alone (aRR, 0.60).
“Among MRSA-colonized patients undergoing cardiac surgery, the associated absolute risk reduction for SSI was approximately triple that of the absolute risk reduction in MRSA-negative or -unknown patients, with a [number needed to treat] to prevent 1 SSI of 53 for the MRSA-colonized group, compared with 176 for the MRSA-negative or -unknown groups,” they wrote.
The incidence of Clostridium difficile infection was similar in both exposure groups (0.72% and 0.81% with combination and single agent prophylaxis, respectively).
Higher AKI risk with combination therapy
“In contrast, combination versus single prophylaxis was associated with higher relative risk of AKI in the 7-day postoperative period after adjusting for prophylaxis regimen duration, age, diabetes, ASA score, and smoking,” they said.
The rate of AKI was 23.75% among patients receiving combination prophylaxis, compared with 20.79% and 13.93% among those receiving vancomycin alone and a beta-lactam alone, respectively.
Significant associations between absolute risk of AKI and receipt of combination regimens were seen across all types of procedures, the investigators said.
“Overall, the NNH [number needed to harm] to cause one episode of AKI in cardiac surgery patients receiving combination therapy was 22, and, for stage 3 AKI, 167. The NNH associated with one additional episode of any postoperative AKI after receipt of combination therapy was 76 following orthopedic procedures and 25 following vascular surgical procedures,” they said.
The optimal approach for preventing SSIs is unclear. Although the multidisciplinary Clinical Practice Guidelines for Antimicrobial Prophylaxis in Surgery recommend single agent prophylaxis most often, with a beta-lactam antibiotic, for most surgical procedures, the use of vancomycin alone is a consideration in MRSA-colonized patients and in centers with a high MRSA incidence, and combination prophylaxis with a beta-lactam plus vancomycin is increasing. However, the relative risks and benefit of this strategy have not been carefully studied, the investigators said.
Thus, the investigators used a propensity-adjusted, log-binomial regression model stratified by type of surgical procedure among the cases identified in the Veterans Affairs cohort to assess the association between SSIs and receipt of combination prophylaxis versus single agent prophylaxis.
Though limited by the observational study design and by factors such as a predominantly male and slightly older and more rural population, the findings suggest that “clinicians may need to individualize prophylaxis strategy based on patient-specific factors that influence the risk-versus-benefit equation,” they said, concluding that “future studies are needed to evaluate the utility of MRSA screening protocols for optimizing and individualizing surgical prophylaxis regimen.”
This study was funded by Veterans Affairs Health Services Research and Development. Dr. Branch-Elliman reported having no disclosures. One other author, Eli Perencevich, MD, received an investigator initiated Grant from Merck Pharmaceuticals in 2013.
The combined use of vancomycin and a beta-lactam antibiotic for prophylaxis against surgical site infections is associated with both benefits and harms, according to findings from a national propensity-score–adjusted retrospective cohort study.
For example, the combination treatment reduced surgical site infections (SSIs) 30 days after cardiac surgical procedures but increased the risk of postoperative acute kidney injury (AKI) in some patients, Westyn Branch-Elliman, MD, of the VA Boston Healthcare System and her colleagues reported online July 10 in PLOS Medicine.
Among cardiac surgery patients, the incidence of surgical site infections was significantly lower for the 6,953 patients treated with both drugs vs. the 12,834 treated with a single agent (0.95% vs. 1.48%), the investigators found (PLOS Med. 2017 Jul 10. doi: 10.1371/journal.pmed.1002340).
SSI benefit with combination therapy
“After controlling for age, diabetes, ASA [American Society of Anesthesiologists] score, mupirocin administration, current smoking status, and preoperative MRSA [methicillin-resistant Staphylococcus aureus] colonization status, receipt of combination antimicrobial prophylaxis was associated with reduced SSI risk following cardiac surgical procedures (adjusted risk ratio, 0.61),” they wrote, noting that, when combination therapy was compared with either of the agents alone, the associations were similar and that no association between SSI reduction and the combination regimen was seen for the other types of surgical procedures assessed.
Secondary analyses showed that, among the cardiac patients, differences in the rates of SSIs were seen based on MRSA status in patients undergoing cardiac surgery. Among MRSA-colonized patients, SSIs occurred in 8 of 346 patients (2.3%) who received combination prophylaxis vs. 4 of 100 patients (4%) who received vancomycin alone (aRR, 0.53), and, among MRSA-negative and MRSA-unknown cardiac surgery patients, SSIs occurred in 58 of 6,607 patients (0.88%) receiving combination prophylaxis and 146 of 10,215 patients (1.4%) receiving a beta-lactam alone (aRR, 0.60).
“Among MRSA-colonized patients undergoing cardiac surgery, the associated absolute risk reduction for SSI was approximately triple that of the absolute risk reduction in MRSA-negative or -unknown patients, with a [number needed to treat] to prevent 1 SSI of 53 for the MRSA-colonized group, compared with 176 for the MRSA-negative or -unknown groups,” they wrote.
The incidence of Clostridium difficile infection was similar in both exposure groups (0.72% and 0.81% with combination and single agent prophylaxis, respectively).
Higher AKI risk with combination therapy
“In contrast, combination versus single prophylaxis was associated with higher relative risk of AKI in the 7-day postoperative period after adjusting for prophylaxis regimen duration, age, diabetes, ASA score, and smoking,” they said.
The rate of AKI was 23.75% among patients receiving combination prophylaxis, compared with 20.79% and 13.93% among those receiving vancomycin alone and a beta-lactam alone, respectively.
Significant associations between absolute risk of AKI and receipt of combination regimens were seen across all types of procedures, the investigators said.
“Overall, the NNH [number needed to harm] to cause one episode of AKI in cardiac surgery patients receiving combination therapy was 22, and, for stage 3 AKI, 167. The NNH associated with one additional episode of any postoperative AKI after receipt of combination therapy was 76 following orthopedic procedures and 25 following vascular surgical procedures,” they said.
The optimal approach for preventing SSIs is unclear. Although the multidisciplinary Clinical Practice Guidelines for Antimicrobial Prophylaxis in Surgery recommend single agent prophylaxis most often, with a beta-lactam antibiotic, for most surgical procedures, the use of vancomycin alone is a consideration in MRSA-colonized patients and in centers with a high MRSA incidence, and combination prophylaxis with a beta-lactam plus vancomycin is increasing. However, the relative risks and benefit of this strategy have not been carefully studied, the investigators said.
Thus, the investigators used a propensity-adjusted, log-binomial regression model stratified by type of surgical procedure among the cases identified in the Veterans Affairs cohort to assess the association between SSIs and receipt of combination prophylaxis versus single agent prophylaxis.
Though limited by the observational study design and by factors such as a predominantly male and slightly older and more rural population, the findings suggest that “clinicians may need to individualize prophylaxis strategy based on patient-specific factors that influence the risk-versus-benefit equation,” they said, concluding that “future studies are needed to evaluate the utility of MRSA screening protocols for optimizing and individualizing surgical prophylaxis regimen.”
This study was funded by Veterans Affairs Health Services Research and Development. Dr. Branch-Elliman reported having no disclosures. One other author, Eli Perencevich, MD, received an investigator initiated Grant from Merck Pharmaceuticals in 2013.
The combined use of vancomycin and a beta-lactam antibiotic for prophylaxis against surgical site infections is associated with both benefits and harms, according to findings from a national propensity-score–adjusted retrospective cohort study.
For example, the combination treatment reduced surgical site infections (SSIs) 30 days after cardiac surgical procedures but increased the risk of postoperative acute kidney injury (AKI) in some patients, Westyn Branch-Elliman, MD, of the VA Boston Healthcare System and her colleagues reported online July 10 in PLOS Medicine.
Among cardiac surgery patients, the incidence of surgical site infections was significantly lower for the 6,953 patients treated with both drugs vs. the 12,834 treated with a single agent (0.95% vs. 1.48%), the investigators found (PLOS Med. 2017 Jul 10. doi: 10.1371/journal.pmed.1002340).
SSI benefit with combination therapy
“After controlling for age, diabetes, ASA [American Society of Anesthesiologists] score, mupirocin administration, current smoking status, and preoperative MRSA [methicillin-resistant Staphylococcus aureus] colonization status, receipt of combination antimicrobial prophylaxis was associated with reduced SSI risk following cardiac surgical procedures (adjusted risk ratio, 0.61),” they wrote, noting that, when combination therapy was compared with either of the agents alone, the associations were similar and that no association between SSI reduction and the combination regimen was seen for the other types of surgical procedures assessed.
Secondary analyses showed that, among the cardiac patients, differences in the rates of SSIs were seen based on MRSA status in patients undergoing cardiac surgery. Among MRSA-colonized patients, SSIs occurred in 8 of 346 patients (2.3%) who received combination prophylaxis vs. 4 of 100 patients (4%) who received vancomycin alone (aRR, 0.53), and, among MRSA-negative and MRSA-unknown cardiac surgery patients, SSIs occurred in 58 of 6,607 patients (0.88%) receiving combination prophylaxis and 146 of 10,215 patients (1.4%) receiving a beta-lactam alone (aRR, 0.60).
“Among MRSA-colonized patients undergoing cardiac surgery, the associated absolute risk reduction for SSI was approximately triple that of the absolute risk reduction in MRSA-negative or -unknown patients, with a [number needed to treat] to prevent 1 SSI of 53 for the MRSA-colonized group, compared with 176 for the MRSA-negative or -unknown groups,” they wrote.
The incidence of Clostridium difficile infection was similar in both exposure groups (0.72% and 0.81% with combination and single agent prophylaxis, respectively).
Higher AKI risk with combination therapy
“In contrast, combination versus single prophylaxis was associated with higher relative risk of AKI in the 7-day postoperative period after adjusting for prophylaxis regimen duration, age, diabetes, ASA score, and smoking,” they said.
The rate of AKI was 23.75% among patients receiving combination prophylaxis, compared with 20.79% and 13.93% among those receiving vancomycin alone and a beta-lactam alone, respectively.
Significant associations between absolute risk of AKI and receipt of combination regimens were seen across all types of procedures, the investigators said.
“Overall, the NNH [number needed to harm] to cause one episode of AKI in cardiac surgery patients receiving combination therapy was 22, and, for stage 3 AKI, 167. The NNH associated with one additional episode of any postoperative AKI after receipt of combination therapy was 76 following orthopedic procedures and 25 following vascular surgical procedures,” they said.
The optimal approach for preventing SSIs is unclear. Although the multidisciplinary Clinical Practice Guidelines for Antimicrobial Prophylaxis in Surgery recommend single agent prophylaxis most often, with a beta-lactam antibiotic, for most surgical procedures, the use of vancomycin alone is a consideration in MRSA-colonized patients and in centers with a high MRSA incidence, and combination prophylaxis with a beta-lactam plus vancomycin is increasing. However, the relative risks and benefit of this strategy have not been carefully studied, the investigators said.
Thus, the investigators used a propensity-adjusted, log-binomial regression model stratified by type of surgical procedure among the cases identified in the Veterans Affairs cohort to assess the association between SSIs and receipt of combination prophylaxis versus single agent prophylaxis.
Though limited by the observational study design and by factors such as a predominantly male and slightly older and more rural population, the findings suggest that “clinicians may need to individualize prophylaxis strategy based on patient-specific factors that influence the risk-versus-benefit equation,” they said, concluding that “future studies are needed to evaluate the utility of MRSA screening protocols for optimizing and individualizing surgical prophylaxis regimen.”
This study was funded by Veterans Affairs Health Services Research and Development. Dr. Branch-Elliman reported having no disclosures. One other author, Eli Perencevich, MD, received an investigator initiated Grant from Merck Pharmaceuticals in 2013.
FROM PLOS MEDICINE
Key clinical point:
Major finding: The SSI incidence was 0.95% vs. 1.48% with combination vs. single agent–therapy in cardiac surgery patients. Acute kidney injuries occurred in 23.75% of all surgery patients receiving combination prophylaxis, compared with 20.79% and 13.93% with vancomycin or a beta-lactam, respectively.
Data source: A retrospective cohort study of more than 70,000 surgical procedures.
Disclosures: This study was funded by Veterans Affairs Health Services Research and Development. Dr. Branch-Elliman reported having no disclosures. One other author, Eli Perencevich, MD, received an investigator initiated grant from Merck Pharmaceuticals in 2013.
Multiple factors predict surgical site infection risk after lower extremity revascularization
Patient, operative, and hospital factors were found to be significant predictors of the risk of surgical site infection in patients who underwent open lower extremity bypass procedures, according to the results of a retrospective data analysis.
The study assessed the outcomes of 3,033 patients who underwent elective or urgent open LEB procedures between January 2012 and June 2015 using data from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium Vascular Intervention Collaborative (BMC2 VIC), a statewide cardiovascular consortium of 35 hospitals, according to Frank M. Davis, MD, and his colleagues at the University of Michigan, Ann Arbor.
Demographic information, medical history, laboratory test results before and after the procedure, procedural indication, procedural urgency, technical details of procedures, and associated complications were assessed for each patient. Women comprised 31% of patients, the average patient age was 66 years, and 83% of the population was white (J Vasc Surg. 2017 Jun;65[6]:1769-78).
Among all of the patients treated, 320 developed SSIs and 2,713 did not. The procedural indications included one or more of the following: claudication (72%), rest pain (50.5%), ulcer/gangrene (32.4%), or acute limb ischemia (15.1%). Antibiotics were appropriately administered to 97% of the patients, according to the researchers, “demonstrating high compliance across the BMC2 VIC.”
- Patient factors: As indicated by previous studies, obesity (odds ratio, 1.78), dialysis dependence (OR, 4.33), and hypertension (OR, 4.29) conferred a significant increased risk of SSI after LEB, according to Dr. Davis and his colleagues. In addition, however, they found that previous vascular surgery (OR, 1.57), previous percutaneous coronary intervention (OR, 1.47), use of antiplatelet medication (OR, 4.29), and low Peripheral Artery Questionnaire symptom severity (OR, 1.48) were significant independent predictors of SSI.
- Operative factors: Prolonged procedural length (OR, 2.95), iodine-only antiseptic skin preparation (OR, 1.73), and high peak intraoperative glucose (defined as a peak glucose greater than 180 mg/dL; OR, 1.99) were significant independent predictors of SSI. However, concomitant stent placement was found to be significantly predictive (OR, .38), “perhaps due to improvement in regional and subcutaneous vascular flow after the intervention,” the researchers suggested.
- Hospital factors: Larger overall hospital size (OR, 2.22) and major teaching center (OR, 1.66) were associated with increased risk of SSI. “Interestingly, we did not find an association with SSI and the hospital annual volume or the hospital urgent/emergent procedure rate,” the researchers added.
SSIs were not found to be significantly associated with a difference in 30-day mortality. However, they were significantly associated with an increased rate of several postoperative morbidities, including transfusion, lymph leak, major amputation, and open surgical bypass revision at or within 30 days of the index operation, according to Dr. Davis and his colleagues.
“Although some factors, such as patients comorbidities, are not modifiable, others represent areas for quality improvement in at-risk patients,” the researchers indicated. “Diligence should be devoted to decreasing operative length, controlling intraoperative glucose levels, and avoiding iodine-only skin preparation to decrease the rate of SSIs and its numerous associate morbidities in vascular surgery patients.”
In discussing the issue of antiplatelet medication being an indicator of increased risk, the authors pointed out that it was a hitherto unreported factor in the vascular literature, and of concern because, “as expected, a high percentage of patients (78.7%) were taking antiplatelet medication at the time of their LEB.”
Because the association of antiplatelet medication with SSIs was independent of the need for operative transfusion or the need for repeat intervention, the researchers speculated that “all antiplatelet agents have the theoretical potential to diminish activation-dependent platelet immune functions.” They referred to previous studies showing that clopidogrel was associated with significantly higher clinical rates of infection, particularly pneumonia.
Limitations cited for the study were the retrospective nature of the database analysis, the possibility of confounders not assessed in the data, and the fact that outcomes were limited to 30-day events, which would not take into account longer-term graft failure or mortality.
The authors reported having no conflicts of interest with regard to the study.
Patient, operative, and hospital factors were found to be significant predictors of the risk of surgical site infection in patients who underwent open lower extremity bypass procedures, according to the results of a retrospective data analysis.
The study assessed the outcomes of 3,033 patients who underwent elective or urgent open LEB procedures between January 2012 and June 2015 using data from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium Vascular Intervention Collaborative (BMC2 VIC), a statewide cardiovascular consortium of 35 hospitals, according to Frank M. Davis, MD, and his colleagues at the University of Michigan, Ann Arbor.
Demographic information, medical history, laboratory test results before and after the procedure, procedural indication, procedural urgency, technical details of procedures, and associated complications were assessed for each patient. Women comprised 31% of patients, the average patient age was 66 years, and 83% of the population was white (J Vasc Surg. 2017 Jun;65[6]:1769-78).
Among all of the patients treated, 320 developed SSIs and 2,713 did not. The procedural indications included one or more of the following: claudication (72%), rest pain (50.5%), ulcer/gangrene (32.4%), or acute limb ischemia (15.1%). Antibiotics were appropriately administered to 97% of the patients, according to the researchers, “demonstrating high compliance across the BMC2 VIC.”
- Patient factors: As indicated by previous studies, obesity (odds ratio, 1.78), dialysis dependence (OR, 4.33), and hypertension (OR, 4.29) conferred a significant increased risk of SSI after LEB, according to Dr. Davis and his colleagues. In addition, however, they found that previous vascular surgery (OR, 1.57), previous percutaneous coronary intervention (OR, 1.47), use of antiplatelet medication (OR, 4.29), and low Peripheral Artery Questionnaire symptom severity (OR, 1.48) were significant independent predictors of SSI.
- Operative factors: Prolonged procedural length (OR, 2.95), iodine-only antiseptic skin preparation (OR, 1.73), and high peak intraoperative glucose (defined as a peak glucose greater than 180 mg/dL; OR, 1.99) were significant independent predictors of SSI. However, concomitant stent placement was found to be significantly predictive (OR, .38), “perhaps due to improvement in regional and subcutaneous vascular flow after the intervention,” the researchers suggested.
- Hospital factors: Larger overall hospital size (OR, 2.22) and major teaching center (OR, 1.66) were associated with increased risk of SSI. “Interestingly, we did not find an association with SSI and the hospital annual volume or the hospital urgent/emergent procedure rate,” the researchers added.
SSIs were not found to be significantly associated with a difference in 30-day mortality. However, they were significantly associated with an increased rate of several postoperative morbidities, including transfusion, lymph leak, major amputation, and open surgical bypass revision at or within 30 days of the index operation, according to Dr. Davis and his colleagues.
“Although some factors, such as patients comorbidities, are not modifiable, others represent areas for quality improvement in at-risk patients,” the researchers indicated. “Diligence should be devoted to decreasing operative length, controlling intraoperative glucose levels, and avoiding iodine-only skin preparation to decrease the rate of SSIs and its numerous associate morbidities in vascular surgery patients.”
In discussing the issue of antiplatelet medication being an indicator of increased risk, the authors pointed out that it was a hitherto unreported factor in the vascular literature, and of concern because, “as expected, a high percentage of patients (78.7%) were taking antiplatelet medication at the time of their LEB.”
Because the association of antiplatelet medication with SSIs was independent of the need for operative transfusion or the need for repeat intervention, the researchers speculated that “all antiplatelet agents have the theoretical potential to diminish activation-dependent platelet immune functions.” They referred to previous studies showing that clopidogrel was associated with significantly higher clinical rates of infection, particularly pneumonia.
Limitations cited for the study were the retrospective nature of the database analysis, the possibility of confounders not assessed in the data, and the fact that outcomes were limited to 30-day events, which would not take into account longer-term graft failure or mortality.
The authors reported having no conflicts of interest with regard to the study.
Patient, operative, and hospital factors were found to be significant predictors of the risk of surgical site infection in patients who underwent open lower extremity bypass procedures, according to the results of a retrospective data analysis.
The study assessed the outcomes of 3,033 patients who underwent elective or urgent open LEB procedures between January 2012 and June 2015 using data from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium Vascular Intervention Collaborative (BMC2 VIC), a statewide cardiovascular consortium of 35 hospitals, according to Frank M. Davis, MD, and his colleagues at the University of Michigan, Ann Arbor.
Demographic information, medical history, laboratory test results before and after the procedure, procedural indication, procedural urgency, technical details of procedures, and associated complications were assessed for each patient. Women comprised 31% of patients, the average patient age was 66 years, and 83% of the population was white (J Vasc Surg. 2017 Jun;65[6]:1769-78).
Among all of the patients treated, 320 developed SSIs and 2,713 did not. The procedural indications included one or more of the following: claudication (72%), rest pain (50.5%), ulcer/gangrene (32.4%), or acute limb ischemia (15.1%). Antibiotics were appropriately administered to 97% of the patients, according to the researchers, “demonstrating high compliance across the BMC2 VIC.”
- Patient factors: As indicated by previous studies, obesity (odds ratio, 1.78), dialysis dependence (OR, 4.33), and hypertension (OR, 4.29) conferred a significant increased risk of SSI after LEB, according to Dr. Davis and his colleagues. In addition, however, they found that previous vascular surgery (OR, 1.57), previous percutaneous coronary intervention (OR, 1.47), use of antiplatelet medication (OR, 4.29), and low Peripheral Artery Questionnaire symptom severity (OR, 1.48) were significant independent predictors of SSI.
- Operative factors: Prolonged procedural length (OR, 2.95), iodine-only antiseptic skin preparation (OR, 1.73), and high peak intraoperative glucose (defined as a peak glucose greater than 180 mg/dL; OR, 1.99) were significant independent predictors of SSI. However, concomitant stent placement was found to be significantly predictive (OR, .38), “perhaps due to improvement in regional and subcutaneous vascular flow after the intervention,” the researchers suggested.
- Hospital factors: Larger overall hospital size (OR, 2.22) and major teaching center (OR, 1.66) were associated with increased risk of SSI. “Interestingly, we did not find an association with SSI and the hospital annual volume or the hospital urgent/emergent procedure rate,” the researchers added.
SSIs were not found to be significantly associated with a difference in 30-day mortality. However, they were significantly associated with an increased rate of several postoperative morbidities, including transfusion, lymph leak, major amputation, and open surgical bypass revision at or within 30 days of the index operation, according to Dr. Davis and his colleagues.
“Although some factors, such as patients comorbidities, are not modifiable, others represent areas for quality improvement in at-risk patients,” the researchers indicated. “Diligence should be devoted to decreasing operative length, controlling intraoperative glucose levels, and avoiding iodine-only skin preparation to decrease the rate of SSIs and its numerous associate morbidities in vascular surgery patients.”
In discussing the issue of antiplatelet medication being an indicator of increased risk, the authors pointed out that it was a hitherto unreported factor in the vascular literature, and of concern because, “as expected, a high percentage of patients (78.7%) were taking antiplatelet medication at the time of their LEB.”
Because the association of antiplatelet medication with SSIs was independent of the need for operative transfusion or the need for repeat intervention, the researchers speculated that “all antiplatelet agents have the theoretical potential to diminish activation-dependent platelet immune functions.” They referred to previous studies showing that clopidogrel was associated with significantly higher clinical rates of infection, particularly pneumonia.
Limitations cited for the study were the retrospective nature of the database analysis, the possibility of confounders not assessed in the data, and the fact that outcomes were limited to 30-day events, which would not take into account longer-term graft failure or mortality.
The authors reported having no conflicts of interest with regard to the study.
FROM THE JOURNAL OF VASCULAR SURGERY
Key clinical point:
Major finding: SSIs occurred in 10.6% of lower extremity bypasses.
Data source: A retrospective analysis of 3,033 elective or urgent open LEB procedures performed over a 3.5-year period in a statewide cardiovascular consortium of 35 hospitals.
Disclosures: The authors reported having no conflicts of interest with regard to the study.
Optimizing diagnostic testing for venous thromboembolism
When a patient presents with suspected venous thromboembolism, ie, deep vein thrombosis or pulmonary embolism, what diagnostic tests are needed to confirm the diagnosis? The clinical signs and symptoms of venous thromboembolism are nonspecific and often difficult to interpret. Therefore, it is essential for clinicians to use a standardized, structured approach to diagnosis that incorporates clinical findings and laboratory testing, as well as judicious use of diagnostic imaging. But while information is important, clinicians must also strive to avoid unnecessary testing, not only to decrease costs, but also to avoid potential harm.
If the diagnosis is confirmed, does the patient need testing for an underlying thrombophilic disorder? Such screening is often considered after a thromboembolic event occurs. However, a growing body of evidence indicates that the results of thrombophilia testing can be misinterpreted and potentially harmful.1 We need to understand the utility of this testing as well as when and how it should be used. Patients and thrombosis specialists should be involved in deciding whether to perform these tests.
In this article, we provide practical information about how to diagnose venous thromboembolism, including strategies to optimize testing in suspected cases. We also offer guidance on how to decide whether further thrombophilia testing is warranted.
COMMON AND SERIOUS
Venous thromboembolism is a major cause of morbidity and death. Approximately 900,000 cases of pulmonary embolism and deep vein thrombosis occur in the United States each year, causing 60,000 to 300,000 deaths,2 with the number of cases projected to double over the next 40 years.3
INITIAL APPROACH: PRETEST PROBABILITY
Given the morbidity and mortality associated with venous thromboembolism, prompt recognition and diagnosis are imperative. Clinical diagnosis alone is insufficient, with confirmed disease found in only 15% to 25% of patients suspected of having venous thromboembolism.4–8 Therefore, the pretest probability should be coupled with objective testing.
The Wells score shows good discrimination in the outpatient and emergency department settings, but it has been invalidated in the inpatient setting, and thus it should not be used in inpatients.10
LABORATORY TESTS FOR SUSPECTED VENOUS THROMBOEMBOLISM
Employing an understanding of diagnostic testing is fundamental to identifying patients with venous thromboembolism.
D-dimer is a byproduct of fibrinolysis.
D-dimer testing has very high sensitivity for venous thromboembolism (> 90%) but low specificity (about 50%), and levels can be elevated in a variety of situations such as advanced age, acute inflammation, and cancer.15 The standard threshold is 500 μg/L, but because the D-dimer level increases with age, some clinicians advocate using an age-adjusted threshold for patients age 50 or older (age in years × 10 μg/L) to increase the diagnostic yield.16
Of the laboratory tests for D-dimer, the enzyme-linked immunosorbent assay has the highest sensitivity and highest negative predictive value (100%) and may be preferred over the other test methodologies.17
With its high sensitivity, D-dimer testing is clinically useful for ruling out venous thromboembolism, particularly when the pretest probability is low, but it lacks the specificity required for diagnosing and treating the disease if positive. Thus, it is not useful for ruling in venous thromboembolism. If the patient has a high pretest probability, we can omit D-dimer testing in favor of imaging studies.
Other laboratory tests such as arterial blood gas and brain natriuretic peptide levels have been proposed as markers of pulmonary embolism, but studies suggest they have limited utility in predicting the presence of disease.18,19
DIAGNOSTIC TESTS FOR DEEP VEIN THROMBOSIS
Ultrasonography
If the pretest probability of deep vein thrombosis is high or a D-dimer test is found to be positive, the next step in evaluation is compression ultrasonography.
While some guidelines recommend scanning only the proximal leg, many facilities in the United States scan the whole leg, which may reveal distal deep vein thrombosis.20 The clinical significance of isolated distal deep vein thrombosis is unknown, and a selective anticoagulation approach may be used if this condition is discovered. The 2012 and 2016 American College of Chest Physicians (ACCP) guidelines on diagnosis and management of venous thromboembolism address this topic.20,21
Deep vein thrombosis in the arm should be evaluated in the same manner as in the lower extremities.
Venography
Invasive and therefore no longer often used, venography is considered the gold standard for diagnosing deep vein thrombosis. Computed tomographic (CT) or magnetic resonance (MR) venography is most useful if the patient has aberrant anatomy such as a deformity of the leg, or in situations where the use of ultrasonography is difficult or unreliable, such as in the setting of severe obesity. CT or MR venography may be considered when looking for thrombosis in noncompressible veins of the thorax and abdomen (eg, the subclavian vein, iliac vein, and inferior vena cava) if ultrasonography is negative but clinical suspicion is high. Venous-phase CT angiography is particularly useful in diagnosing deep vein thrombosis in the inferior vena cava and iliac vein when deep vein thrombosis is clinically suspected but cannot be visualized on duplex ultrasonography.
DIAGNOSTIC TESTS FOR PULMONARY EMBOLISM
Computed tomography
Imaging is warranted in patients who have a high pretest probability of pulmonary embolism, or in whom the D-dimer assay was positive but the pretest probability was low or moderate.
Once the gold standard, pulmonary angiography is no longer recommended for the initial diagnosis of pulmonary embolism because it is invasive, often unavailable, less sophisticated, and more expensive than noninvasive imaging techniques such as CT angiography. It is still used, however, in catheter-directed thrombolysis.
Thus, multiphasic CT angiography, as guided by pretest probability and the D-dimer level, is the imaging test of choice in the evaluation of pulmonary embolism. It can also offer insight into thrombotic burden and can reveal concurrent or alternative diagnoses (eg, pneumonia).
Ventilation-perfusion scanning
When CT angiography is unavailable or the patient should not be exposed to contrast medium (eg, due to concern for contrast-induced nephropathy or contrast allergy), ventilation-perfusion (V/Q) scanning remains an option for ruling out pulmonary embolism.22
Anderson et al23 compared CT angiography and V/Q scanning in a study in 1,417 patients considered likely to have acute pulmonary embolism. Rates of symptomatic pulmonary embolism during 3-month follow-up were similar in patients who initially had negative results on V/Q scanning compared with those who initially had negative results on CT angiography. However, this study used single-detector CT scanners for one-third of the patients. Therefore, the results may have been different if current technology had been used.
Limitations of V/Q scanning include length of time to perform (30–45 minutes), cost, inability to identify other causes of symptoms, and difficulty with interpretation when other pulmonary pathology is present (eg, lung infiltrate). V/Q scanning is helpful when negative but is often reported based on probability (low, intermediate, or high) and may not provide adequate guidance. Therefore, CT angiography should be used whenever possible for diagnosing pulmonary embolism.
Other tests for pulmonary embolism
Electrocardiography, transthoracic echocardiography, and chest radiography may aid in the search for alternative diagnoses and assess the degree of right heart strain as a sequela of pulmonary embolism, but they do not confirm the diagnosis.
ORDER IMAGING ONLY IF NEEDED
Diagnostic imaging can be optimized by avoiding unnecessary tests that carry both costs and clinical risks.
Most patients in whom acute pulmonary embolism is discovered will not need testing for deep vein thrombosis, as they will receive anticoagulation regardless. Similarly, many patients with acute symptomatic deep vein thrombosis do not need testing for pulmonary embolism with chest CT imaging, as they too will receive anticoagulation regardless.
Therefore, clinicians are encouraged to use diagnostic reasoning while practicing high-value care (including estimating pretest probability and measuring D-dimer when appropriate), ordering additional tests judiciously and only if indicated.
THROMBOEMBOLISM IS CONFIRMED—IS FURTHER TESTING WARRANTED?
Once acute venous thromboembolism is confirmed, key considerations include whether the event was provoked or unprovoked (ie, idiopathic) and whether the patient needs indefinite anticoagulation (eg, after 2 or more unprovoked events).
Was the event provoked or unprovoked?
Even in cases of unprovoked venous thromboembolism, no clear consensus exists as to which patients should be tested for thrombophilia. Experts do advocate, however, that it be done only in highly selected patients and that it be coordinated with the patient, family members, and an expert in this testing. Patients for whom further testing may be considered include those with venous thromboembolism in unusual sites (eg, the cavernous sinus), with warfarin-induced skin necrosis, or with recurrent pregnancy loss.
While screening for malignancy may seem prudent in the case of unexplained venous thromboembolism, the use of CT imaging for this purpose has been found to be of low yield. In one study,24 it was not found to detect additional neoplasms, and it can lead to additional cost and no added benefit for patients.
The American Board of Internal Medicine’s Choosing Wisely campaign strongly recommends consultation with an expert in thrombophilia (eg, a hematologist) before testing.25 Ordering multiple tests in bundles (hypercoagulability panels) is unlikely to alter management, could have a negative clinical impact on patients, and is generally not recommended.
The ‘4 Ps’ approach to testing
- Patient selection
- Pretest counseling
- Proper laboratory interpretation
- Provision of education and advice.
Importantly, testing should be reserved for patients in whom the pretest probability of the thrombophilic disease is moderate to high, such as testing for antiphospholipid antibody syndrome in patients with systemic lupus erythematosus or recurrent miscarriage.
Venous thromboembolism in a patient who is known to have a malignant disease does not typically warrant further thrombophilia testing, as the event was likely a sequela of the malignancy. The evaluation and management of venous thromboembolism with concurrent neoplasm is covered elsewhere.21
WHAT IF VENOUS THROMBOEMBOLISM IS DISCOVERED INCIDENTALLY?
Thrombophilia testing should be approached the same regardless of whether the venous thromboembolism was diagnosed intentionally or incidentally. First, determine whether the thrombosis was provoked or unprovoked, then order additional tests only if indicated, as recommended. Alternative approaches such as forgoing anticoagulation (but performing serial imaging, if indicated) may be reasonable if the thrombus is deemed clinically irrelevant (eg, nonocclusive, asymptomatic, subsegmental pulmonary embolism in the absence of proximal deep vein thrombosis; isolated distal deep vein thrombosis).25,27
It is still debatable whether the increasing incidence of asymptomatic pulmonary embolism due to enhanced sensitivity of noninvasive diagnostic imaging warrants a change in diagnostic approach.28
FACTORS TO CONSIDER BEFORE THROMBOPHILIA TESTING
Important factors to consider before testing for thrombophilia are29:
- How will the results affect the anticoagulation plan?
- How may the patient’s clinical status and medications influence the results?
- Has the patient expressed a desire to understand why venous thromboembolism occurred?
- Will the results have a potential impact on the patient’s family members?
How will the results of thrombophilia testing affect anticoagulation management?
Because the goal of any diagnostic test is to find out what type of care the patient needs, clinicians must determine whether knowledge of an underlying thrombophilia will alter the short-term or long-term anticoagulation therapy the patient is receiving for an acute venous thromboembolic event.
As most acute episodes of venous thromboembolism require an initial 3 months of anticoagulation (with the exception of some nonclinically relevant events such as isolated distal deep vein thrombosis without extension on reimaging), testing in the acute setting does not change the short-term management of anticoagulation. Many hospitals have advocated for outpatient-only thrombophilia testing (if testing does occur), as testing in the acute setting may render test results uninterpretable (see What factors can influence thrombophilia testing? below) and can inappropriately affect the long-term management of anticoagulation. We recommend against testing in the inpatient setting.
To determine the duration of anticoagulation, clinicians must balance the risk of recurrent venous thromboembolism and the risk of bleeding. If a patient is at significant risk of bleeding or does not tolerate anticoagulation, clinicians may consider stopping therapy instead of evaluating for thrombophilia. For patients with provoked venous thromboembolism, anticoagulation should generally be limited to 3 months, as the risk of recurrence does not outweigh the risk of bleeding with continued anticoagulation therapy.
Patients with unprovoked venous thromboembolism have a risk of recurrence twice as high as those with provoked venous thromboembolism and generally need a longer duration of anticoagulation.30,31 Once a patient with an unprovoked venous thromboembolic event has completed the initial 3 months of anticoagulation, a formal risk-benefit evaluation should be performed to determine whether to continue it.
Up to 42% of patients with unprovoked venous thromboembolism may have 1 or more thrombotic disorders, and some clinicians believe that detecting an underlying thrombophilia will aid in decisions regarding duration of therapy.32 However, the risk of recurrent venous thromboembolism in these patients does not differ significantly from that in patients without an underlying thrombophilia.33–35 As such, it has been suggested that the unprovoked character of the thrombotic event, rather than an underlying thrombophilia, determines the risk of future recurrence and should be used instead of testing to guide the duration of anticoagulation therapy.32
For more information, see the 2016 ACCP guideline update on antithrombotic therapy for venous thromboembolism.27
What factors can influence the results of thrombophilia testing?
For example, antithrombin is consumed during thrombus formation; therefore, antithrombin levels may be transiently suppressed in acute venous thromboembolism. Moreover, since antithrombin binds to unfractionated heparin, low-molecular-weight heparin, and fondaparinux and mediates their activity as anticoagulants, antithrombin levels may be decreased by heparin therapy.
Similarly, vitamin K antagonists (eg, warfarin) suppress protein C and S activity levels by inhibiting vitamin K epoxide reductase and may falsely indicate a protein C or S deficiency.
Direct oral anticoagulants can cause false-positive results on lupus anticoagulant assays (dilute Russell viper venom time, augmented partial thromboplastin time), raise protein C, protein S, and antithrombin activity levels, and normalize activated protein C resistance assays, leading to missed diagnoses.41
Since estrogen therapy and pregnancy lead to increases in C4b binding protein, resulting in decreased free protein S, these situations can result in clinicians falsely labeling patients as having congenital protein S deficiency when in fact the patient had a transient reduction in protein S levels.33
Therefore, to optimize accuracy and interpretation of results, thrombophilia testing should ideally be performed when the patient:
- Is past the acute event and out of the hospital
- Is not pregnant
- Has received the required 3 months of anticoagulation and is off this therapy.
For warfarin, most recommendations say that testing should be performed after the patient has been off therapy for 2 to 6 weeks.42 Low-molecular-weight heparins and direct oral anticoagulants should be discontinued for at least 48 to 72 hours, or longer if the patient has kidney impairment, as these medications are renally eliminated.
Genetic tests such as factor V Leiden and prothrombin gene mutation are not affected by these factors and do not require repeat or confirmatory testing.
What if the patient or family wants to understand why an event occurred?
Some experts advocate thrombophilia testing of asymptomatic family members to identify carriers who may need prophylaxis against venous thromboembolism in high-risk situations such as pregnancy, oral contraceptive use, hospitalization, and surgery.29 Asymptomatic family members of a first-degree relative with a history of venous thromboembolism have a 2 times higher risk of an index event.43 Thus, it may be argued that these asymptomatic individuals should receive prophylactic measures in any high-risk situation, based on the family history itself rather than results of thrombophilia testing.
Occasionally, patients and family members want to know the cause of the thrombotic event and want to be tested. In these instances, pretest counseling for the patient and family about the potential implications of testing and shared decision-making between the provider and patient are of utmost importance.29
What is the impact on family members if thrombophilia is diagnosed?
While positive test results can give patients some satisfaction, this knowledge may also cause unnecessary worry, as the patient knows he or she has a hematologic disorder and could possible die of venous thromboembolism.
Thrombophilia testing can have other adverse consequences. For example, while the Genetic Information Nondiscrimination Act of 2008 protects against denial of health insurance benefits based on genetic information, known carriers of thrombophilia may have trouble obtaining life or disability insurance.44
Unfortunately, it is not uncommon for thrombophilia testing to be inappropriately performed, interpreted, or followed up. These suboptimal approaches can lead to unnecessary exposure to high-risk therapeutic anticoagulation, excessive durations of therapy, and labeling with an unconfirmed or incorrect diagnosis. Additionally, there are significant costs associated with thrombophilia testing, including the cost of the tests and anticoagulant medications and management of adverse events such as bleeding.
WHAT ARE THE ALTERNATIVES TO THROMBOPHILIA TESTING?
Because discovered thrombophilias (eg, factor V Leiden mutation, prothrombin gene mutation) have not consistently shown a strong correlation with increased recurrence of venous thromboembolism, alternative approaches are emerging to determine the duration of therapy for unprovoked events.
Clinical prediction tools based on patient characteristics and laboratory markers that are more consistently associated with recurrent venous thromboembolism (eg, male sex, persistently elevated D-dimer) have been developed to aid clinicians dealing with this challenging question. Several prediction tools are available:
The “Men Continue and HERDOO2” rule (HERDOO2 = hyperpigmentation, edema, or redness in either leg; D-dimer level ≥ 250 μg/L; obesity with body mass index ≥ 30 kg/m2; or older age, ≥ 65)45
The DASH score (D-dimer, age, sex, and hormonal therapy)46
The Vienna score,47,48 at http://cemsiis.meduniwien.ac.at/en/kb/science-research/software/clinical-software/recurrent-vte/.
SUMMARY OF THROMBOPHILIA TESTING RECOMMENDATIONS
Test for thrombophilia only when…
- Discussing with a specialist (eg, hematologist) who has an understanding of thrombophilia
- Using the 4 Ps approach
- A patient requests testing to understand why a thrombotic event occurred, and the patient understands the implications of testing (ie, received counseling) for self and for family
- An expert deems identification of asymptomatic family members important for those who may be carriers of a detected thrombophilia
- The patient with a venous thromboembolic event has completed 3 months of anticoagulation and has been off anticoagulation for the appropriate length of time
- The results will change management.
Forgo thrombophilia testing when…
- A patient has a provoked venous thromboembolic event
- You do not intend to discontinue anticoagulation (ie, anticoagulation is indefinite)
- The patient is in the acute (eg, inpatient) setting
- The patient is on anticoagulants that may render test results uninterpretable
- The patient is pregnant or on oral contraceptives
- Use of alternative patient characteristics and laboratory markers to predict venous thromboembolism recurrence may be an option.
OPTIMIZING THE DIAGNOSIS
With the incidence of venous thromboembolism rapidly increasing, optimizing its diagnosis from both a financial and clinical perspective is becoming increasingly important. Clinicians should be familiar with the use of pretest probability scoring for venous thromboembolism, as well as which diagnostic tests are preferred if further workup is indicated. They should strive to minimize or avoid indiscriminate thrombophilia testing, which may lead to increased healthcare costs and patient exposure to potentially harmful anticoagulation.
Testing for thrombophilia should be based on whether a venous thromboembolic event was provoked or unprovoked. Patients with provoked venous thromboembolism or those receiving indefinite anticoagulation therapy should not be tested for thrombophilia. If testing is being considered in a patient with unprovoked venous thromboembolism, a specialist who is able to implement the 4 Ps approach should be consulted to ensure well-informed, shared decision-making with patients and family members.
- National Institute for Health and Care Excellence (NICE). Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. https://www.nice.org.uk/guidance/cg144. Accessed June 13, 2017.
- Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol 2008; 28:370–372.
- Deitelzweig SB, Johnson BH, Lin J, Schulman KL. Prevalence of clinical venous thromboembolism in the USA: current trends and future projections. Am J Hematol 2011; 86:217–220.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):e419S–e494S.
- Pengo V, Lensing AW, Prins MH, et al; Thromboembolic Pulmonary Hypertension Study Group. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004; 350:2257–2264.
- Kahn SR, Hirsch A, Shrier I. Effect of postthrombotic syndrome on health-related quality of life after deep venous thrombosis. Arch Intern Med 2002; 162:1144–1148.
- Wells PS, Owen C, Doucette S, Fergusson D, Tran H. Does this patient have deep vein thrombosis? JAMA 2006; 295:199–207.
- Ljungqvist M, Söderberg M, Moritz P, Ahlgren A, Lärfars G. Evaluation of Wells score and repeated D-dimer in diagnosing venous thromboembolism. Eur J Intern Med 2008; 19:285–288.
- Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med 2001; 135:98–107.
- Silveira PC, Ip IK, Goldhaber SZ, Piazza G, Benson CB, Khorasani R. Performance of Wells score for deep vein thrombosis in the inpatient setting. JAMA Intern Med 2015; 175:1112–1117.
- Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet 1997; 350:1795–1798.
- Wells PS, Anderson DR, Rodger M, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med 2003; 349:1227–1235.
- van Belle A, Büller HR, Huisman MV, et al. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. JAMA 2006; 295:172–179.
- Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemos 2000; 83:416–420.
- Schrecengost JE, LeGallo RD, Boyd JC, et al. Comparison of diagnostic accuracies in outpatients and hospitalized patients of D-dimer testing for the evaluation of suspected pulmonary embolism. Clin Chem 2003; 49:1483–1490.
- Righini M, Van Es J, Den Exter PL, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA 2014; 311:1117–1124.
- Pulivarthi S, Gurram MK. Effectiveness of D-dimer as a screening test for venous thromboembolism: an update. N Am J Med Sci 2014; 6:491–499.
- Söhne M, Ten Wolde M, Boomsma F, Reitsma JB, Douketis JD, Büller HR. Brain natriuretic peptide in hemodynamically stable acute pulmonary embolism. J Thromb Haemost 2006; 4:552–556.
- Stein PD, Goldhaber SZ, Henry JW, Miller AC. Arterial blood gas analysis in the assessment of suspected acute pulmonary embolism. Chest 1996; 109:78–81.
- Bates SM, Jaeschke R, Stevens SM, et al; American College of Chest Physicians. Diagnosis of DVT: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):e351S–e418S.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest 2016; 149:315–352.
- PIOPED Investigators. Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). JAMA 1990; 263:2753–2759.
- Anderson DR, Kahn SR, Rodger MA, et al. Computed tomographic pulmonary angiography vs ventilation-perfusion lung scanning in patients with suspected pulmonary embolism: a randomized controlled trial. JAMA 2007; 298:2743–2753.
- Carrier M. Cancer screening in unprovoked venous thromboembolism. N Engl J Med 2015; 373:2475.
- American Society of Hematology. Don’t test for thrombophilia in adult patients with venous thromboembolism (VTE) occurring in the setting of major transient risk factors (surgery, trauma or prolonged immobility). www.choosingwisely.org/clinician-lists/american-society-hematology-testing-for-thrombophilia-in-adults/. Accessed June 13, 2017.
- Cushman M. Thrombophilia testing in women with venous thrombosis: the 4 Ps approach. Clin Chem 2014; 60:134–137.
- Bates SM, Greer IA, Middeldorp S, Veenstra DL, Prabulos AM, Vandvik PO; American College of Chest Physicians. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl): e691S–e736S.
- Ritchie G, McGurk S, McCreath C, Graham C, Murchison JT. Prospective evaluation of unsuspected pulmonary embolism on contrast enhanced multidetector CT (MDCT) scanning. Thorax 2007; 62:536–540.
- Moll S. Thrombophilia: clinical-practical aspects. J Thromb Thrombolysis 2015; 39:367–378.
- Prandoni P, Noventa F, Ghirarduzzi A, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica 2007; 92:199–205.
- Boutitie F, Pinede L, Schulman S, et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants’ data from seven trials. BMJ 2011; 342:d3036.
- Kearon C, Julian JA, Kovacs MJ, et al; ELATE Investigators. Influence of thrombophilia on risk of recurrent venous thromboembolism while on warfarin: results from a randomized trial. Blood 2008; 112:4432–4436.
- Lijfering WM, Middeldorp S, Veeger NJ, et al. Risk of recurrent venous thrombosis in homozygous carriers and double heterozygous carriers of factor V Leiden and prothrombin G20210A. Circulation 2010; 121:1706–1712.
- Hron G, Eichinger S, Weltermann A, et al. Family history for venous thromboembolism and the risk for recurrence. Am J Med 2006; 119:50–53.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA 2005; 293:2352–2361.
- Lijfering WM. Selective testing for thrombophilia in patients with first venous thrombosis: results from a retrospective family cohort study on absolute thrombotic risk for currently known thrombophilic defects in 2479 relatives. Blood 2009; 113:5314–5322.
- Segal JB. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation. JAMA 2009; 301:2472–2485.
- Juul K. Factor V Leiden and the risk for venous thromboembolism in the adult Danish population. Ann Intern Med 2004; 140: 330–337.
- Emmerich J. Combined effect of factor V Leiden and prothrombin 20210A on the risk of venous thromboembolism: pooled analysis of 8 case-control studies including 2310 cases and 3204 controls. Thromb Haemost 2001; 86: 809–816.
- Garcia D. Antiphospholipid antibodies and the risk of recurrence after a first episode of venous thromboembolism: a systematic review. Blood 2013; 122:817–824.
- Gosselin R, Adcock DM. The laboratory’s 2015 perspective on direct oral anticoagulant testing. J Thromb Haemost 2016; 14:886–893.
- Marlar RA, Gausman JN. Protein S abnormalities: a diagnostic nightmare. Am J Hematol 2011; 86:418–421.
- Bezemer ID, van der Meer FJ, Eikenboom JC, Rosendaal FR, Doggen CJ. The value of family history as a risk indicator for venous thrombosis. Arch Intern Med 2009; 169:610–615.
- Middeldorp S. Evidence-based approach to thrombophilia testing. J Thromb Thrombolysis 2011; 31:275–281.
- Rodger MA, Le Gal G, Anderson DR, et al, for the REVERSE II Study Investigators. Validating the HERDOO2 rule to guide treatment duration for women with unprovoked venous thrombosis: multinational prospective cohort management study. BMJ 2017; 356:j1065.
- Tosetto A, Iorio A, Marcucci M, et al. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH). J Thromb Haemost 2012; 10:1019–1025.
- Eichinger S, Heinze G, Jandeck LM, Kyrle PA. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna prediction model. Circulation 2010; 121:1630–1636.
- Rodger MA, Kahn SR, Wells PS, et al. Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. CMAJ 2008; 179:417–426.
When a patient presents with suspected venous thromboembolism, ie, deep vein thrombosis or pulmonary embolism, what diagnostic tests are needed to confirm the diagnosis? The clinical signs and symptoms of venous thromboembolism are nonspecific and often difficult to interpret. Therefore, it is essential for clinicians to use a standardized, structured approach to diagnosis that incorporates clinical findings and laboratory testing, as well as judicious use of diagnostic imaging. But while information is important, clinicians must also strive to avoid unnecessary testing, not only to decrease costs, but also to avoid potential harm.
If the diagnosis is confirmed, does the patient need testing for an underlying thrombophilic disorder? Such screening is often considered after a thromboembolic event occurs. However, a growing body of evidence indicates that the results of thrombophilia testing can be misinterpreted and potentially harmful.1 We need to understand the utility of this testing as well as when and how it should be used. Patients and thrombosis specialists should be involved in deciding whether to perform these tests.
In this article, we provide practical information about how to diagnose venous thromboembolism, including strategies to optimize testing in suspected cases. We also offer guidance on how to decide whether further thrombophilia testing is warranted.
COMMON AND SERIOUS
Venous thromboembolism is a major cause of morbidity and death. Approximately 900,000 cases of pulmonary embolism and deep vein thrombosis occur in the United States each year, causing 60,000 to 300,000 deaths,2 with the number of cases projected to double over the next 40 years.3
INITIAL APPROACH: PRETEST PROBABILITY
Given the morbidity and mortality associated with venous thromboembolism, prompt recognition and diagnosis are imperative. Clinical diagnosis alone is insufficient, with confirmed disease found in only 15% to 25% of patients suspected of having venous thromboembolism.4–8 Therefore, the pretest probability should be coupled with objective testing.
The Wells score shows good discrimination in the outpatient and emergency department settings, but it has been invalidated in the inpatient setting, and thus it should not be used in inpatients.10
LABORATORY TESTS FOR SUSPECTED VENOUS THROMBOEMBOLISM
Employing an understanding of diagnostic testing is fundamental to identifying patients with venous thromboembolism.
D-dimer is a byproduct of fibrinolysis.
D-dimer testing has very high sensitivity for venous thromboembolism (> 90%) but low specificity (about 50%), and levels can be elevated in a variety of situations such as advanced age, acute inflammation, and cancer.15 The standard threshold is 500 μg/L, but because the D-dimer level increases with age, some clinicians advocate using an age-adjusted threshold for patients age 50 or older (age in years × 10 μg/L) to increase the diagnostic yield.16
Of the laboratory tests for D-dimer, the enzyme-linked immunosorbent assay has the highest sensitivity and highest negative predictive value (100%) and may be preferred over the other test methodologies.17
With its high sensitivity, D-dimer testing is clinically useful for ruling out venous thromboembolism, particularly when the pretest probability is low, but it lacks the specificity required for diagnosing and treating the disease if positive. Thus, it is not useful for ruling in venous thromboembolism. If the patient has a high pretest probability, we can omit D-dimer testing in favor of imaging studies.
Other laboratory tests such as arterial blood gas and brain natriuretic peptide levels have been proposed as markers of pulmonary embolism, but studies suggest they have limited utility in predicting the presence of disease.18,19
DIAGNOSTIC TESTS FOR DEEP VEIN THROMBOSIS
Ultrasonography
If the pretest probability of deep vein thrombosis is high or a D-dimer test is found to be positive, the next step in evaluation is compression ultrasonography.
While some guidelines recommend scanning only the proximal leg, many facilities in the United States scan the whole leg, which may reveal distal deep vein thrombosis.20 The clinical significance of isolated distal deep vein thrombosis is unknown, and a selective anticoagulation approach may be used if this condition is discovered. The 2012 and 2016 American College of Chest Physicians (ACCP) guidelines on diagnosis and management of venous thromboembolism address this topic.20,21
Deep vein thrombosis in the arm should be evaluated in the same manner as in the lower extremities.
Venography
Invasive and therefore no longer often used, venography is considered the gold standard for diagnosing deep vein thrombosis. Computed tomographic (CT) or magnetic resonance (MR) venography is most useful if the patient has aberrant anatomy such as a deformity of the leg, or in situations where the use of ultrasonography is difficult or unreliable, such as in the setting of severe obesity. CT or MR venography may be considered when looking for thrombosis in noncompressible veins of the thorax and abdomen (eg, the subclavian vein, iliac vein, and inferior vena cava) if ultrasonography is negative but clinical suspicion is high. Venous-phase CT angiography is particularly useful in diagnosing deep vein thrombosis in the inferior vena cava and iliac vein when deep vein thrombosis is clinically suspected but cannot be visualized on duplex ultrasonography.
DIAGNOSTIC TESTS FOR PULMONARY EMBOLISM
Computed tomography
Imaging is warranted in patients who have a high pretest probability of pulmonary embolism, or in whom the D-dimer assay was positive but the pretest probability was low or moderate.
Once the gold standard, pulmonary angiography is no longer recommended for the initial diagnosis of pulmonary embolism because it is invasive, often unavailable, less sophisticated, and more expensive than noninvasive imaging techniques such as CT angiography. It is still used, however, in catheter-directed thrombolysis.
Thus, multiphasic CT angiography, as guided by pretest probability and the D-dimer level, is the imaging test of choice in the evaluation of pulmonary embolism. It can also offer insight into thrombotic burden and can reveal concurrent or alternative diagnoses (eg, pneumonia).
Ventilation-perfusion scanning
When CT angiography is unavailable or the patient should not be exposed to contrast medium (eg, due to concern for contrast-induced nephropathy or contrast allergy), ventilation-perfusion (V/Q) scanning remains an option for ruling out pulmonary embolism.22
Anderson et al23 compared CT angiography and V/Q scanning in a study in 1,417 patients considered likely to have acute pulmonary embolism. Rates of symptomatic pulmonary embolism during 3-month follow-up were similar in patients who initially had negative results on V/Q scanning compared with those who initially had negative results on CT angiography. However, this study used single-detector CT scanners for one-third of the patients. Therefore, the results may have been different if current technology had been used.
Limitations of V/Q scanning include length of time to perform (30–45 minutes), cost, inability to identify other causes of symptoms, and difficulty with interpretation when other pulmonary pathology is present (eg, lung infiltrate). V/Q scanning is helpful when negative but is often reported based on probability (low, intermediate, or high) and may not provide adequate guidance. Therefore, CT angiography should be used whenever possible for diagnosing pulmonary embolism.
Other tests for pulmonary embolism
Electrocardiography, transthoracic echocardiography, and chest radiography may aid in the search for alternative diagnoses and assess the degree of right heart strain as a sequela of pulmonary embolism, but they do not confirm the diagnosis.
ORDER IMAGING ONLY IF NEEDED
Diagnostic imaging can be optimized by avoiding unnecessary tests that carry both costs and clinical risks.
Most patients in whom acute pulmonary embolism is discovered will not need testing for deep vein thrombosis, as they will receive anticoagulation regardless. Similarly, many patients with acute symptomatic deep vein thrombosis do not need testing for pulmonary embolism with chest CT imaging, as they too will receive anticoagulation regardless.
Therefore, clinicians are encouraged to use diagnostic reasoning while practicing high-value care (including estimating pretest probability and measuring D-dimer when appropriate), ordering additional tests judiciously and only if indicated.
THROMBOEMBOLISM IS CONFIRMED—IS FURTHER TESTING WARRANTED?
Once acute venous thromboembolism is confirmed, key considerations include whether the event was provoked or unprovoked (ie, idiopathic) and whether the patient needs indefinite anticoagulation (eg, after 2 or more unprovoked events).
Was the event provoked or unprovoked?
Even in cases of unprovoked venous thromboembolism, no clear consensus exists as to which patients should be tested for thrombophilia. Experts do advocate, however, that it be done only in highly selected patients and that it be coordinated with the patient, family members, and an expert in this testing. Patients for whom further testing may be considered include those with venous thromboembolism in unusual sites (eg, the cavernous sinus), with warfarin-induced skin necrosis, or with recurrent pregnancy loss.
While screening for malignancy may seem prudent in the case of unexplained venous thromboembolism, the use of CT imaging for this purpose has been found to be of low yield. In one study,24 it was not found to detect additional neoplasms, and it can lead to additional cost and no added benefit for patients.
The American Board of Internal Medicine’s Choosing Wisely campaign strongly recommends consultation with an expert in thrombophilia (eg, a hematologist) before testing.25 Ordering multiple tests in bundles (hypercoagulability panels) is unlikely to alter management, could have a negative clinical impact on patients, and is generally not recommended.
The ‘4 Ps’ approach to testing
- Patient selection
- Pretest counseling
- Proper laboratory interpretation
- Provision of education and advice.
Importantly, testing should be reserved for patients in whom the pretest probability of the thrombophilic disease is moderate to high, such as testing for antiphospholipid antibody syndrome in patients with systemic lupus erythematosus or recurrent miscarriage.
Venous thromboembolism in a patient who is known to have a malignant disease does not typically warrant further thrombophilia testing, as the event was likely a sequela of the malignancy. The evaluation and management of venous thromboembolism with concurrent neoplasm is covered elsewhere.21
WHAT IF VENOUS THROMBOEMBOLISM IS DISCOVERED INCIDENTALLY?
Thrombophilia testing should be approached the same regardless of whether the venous thromboembolism was diagnosed intentionally or incidentally. First, determine whether the thrombosis was provoked or unprovoked, then order additional tests only if indicated, as recommended. Alternative approaches such as forgoing anticoagulation (but performing serial imaging, if indicated) may be reasonable if the thrombus is deemed clinically irrelevant (eg, nonocclusive, asymptomatic, subsegmental pulmonary embolism in the absence of proximal deep vein thrombosis; isolated distal deep vein thrombosis).25,27
It is still debatable whether the increasing incidence of asymptomatic pulmonary embolism due to enhanced sensitivity of noninvasive diagnostic imaging warrants a change in diagnostic approach.28
FACTORS TO CONSIDER BEFORE THROMBOPHILIA TESTING
Important factors to consider before testing for thrombophilia are29:
- How will the results affect the anticoagulation plan?
- How may the patient’s clinical status and medications influence the results?
- Has the patient expressed a desire to understand why venous thromboembolism occurred?
- Will the results have a potential impact on the patient’s family members?
How will the results of thrombophilia testing affect anticoagulation management?
Because the goal of any diagnostic test is to find out what type of care the patient needs, clinicians must determine whether knowledge of an underlying thrombophilia will alter the short-term or long-term anticoagulation therapy the patient is receiving for an acute venous thromboembolic event.
As most acute episodes of venous thromboembolism require an initial 3 months of anticoagulation (with the exception of some nonclinically relevant events such as isolated distal deep vein thrombosis without extension on reimaging), testing in the acute setting does not change the short-term management of anticoagulation. Many hospitals have advocated for outpatient-only thrombophilia testing (if testing does occur), as testing in the acute setting may render test results uninterpretable (see What factors can influence thrombophilia testing? below) and can inappropriately affect the long-term management of anticoagulation. We recommend against testing in the inpatient setting.
To determine the duration of anticoagulation, clinicians must balance the risk of recurrent venous thromboembolism and the risk of bleeding. If a patient is at significant risk of bleeding or does not tolerate anticoagulation, clinicians may consider stopping therapy instead of evaluating for thrombophilia. For patients with provoked venous thromboembolism, anticoagulation should generally be limited to 3 months, as the risk of recurrence does not outweigh the risk of bleeding with continued anticoagulation therapy.
Patients with unprovoked venous thromboembolism have a risk of recurrence twice as high as those with provoked venous thromboembolism and generally need a longer duration of anticoagulation.30,31 Once a patient with an unprovoked venous thromboembolic event has completed the initial 3 months of anticoagulation, a formal risk-benefit evaluation should be performed to determine whether to continue it.
Up to 42% of patients with unprovoked venous thromboembolism may have 1 or more thrombotic disorders, and some clinicians believe that detecting an underlying thrombophilia will aid in decisions regarding duration of therapy.32 However, the risk of recurrent venous thromboembolism in these patients does not differ significantly from that in patients without an underlying thrombophilia.33–35 As such, it has been suggested that the unprovoked character of the thrombotic event, rather than an underlying thrombophilia, determines the risk of future recurrence and should be used instead of testing to guide the duration of anticoagulation therapy.32
For more information, see the 2016 ACCP guideline update on antithrombotic therapy for venous thromboembolism.27
What factors can influence the results of thrombophilia testing?
For example, antithrombin is consumed during thrombus formation; therefore, antithrombin levels may be transiently suppressed in acute venous thromboembolism. Moreover, since antithrombin binds to unfractionated heparin, low-molecular-weight heparin, and fondaparinux and mediates their activity as anticoagulants, antithrombin levels may be decreased by heparin therapy.
Similarly, vitamin K antagonists (eg, warfarin) suppress protein C and S activity levels by inhibiting vitamin K epoxide reductase and may falsely indicate a protein C or S deficiency.
Direct oral anticoagulants can cause false-positive results on lupus anticoagulant assays (dilute Russell viper venom time, augmented partial thromboplastin time), raise protein C, protein S, and antithrombin activity levels, and normalize activated protein C resistance assays, leading to missed diagnoses.41
Since estrogen therapy and pregnancy lead to increases in C4b binding protein, resulting in decreased free protein S, these situations can result in clinicians falsely labeling patients as having congenital protein S deficiency when in fact the patient had a transient reduction in protein S levels.33
Therefore, to optimize accuracy and interpretation of results, thrombophilia testing should ideally be performed when the patient:
- Is past the acute event and out of the hospital
- Is not pregnant
- Has received the required 3 months of anticoagulation and is off this therapy.
For warfarin, most recommendations say that testing should be performed after the patient has been off therapy for 2 to 6 weeks.42 Low-molecular-weight heparins and direct oral anticoagulants should be discontinued for at least 48 to 72 hours, or longer if the patient has kidney impairment, as these medications are renally eliminated.
Genetic tests such as factor V Leiden and prothrombin gene mutation are not affected by these factors and do not require repeat or confirmatory testing.
What if the patient or family wants to understand why an event occurred?
Some experts advocate thrombophilia testing of asymptomatic family members to identify carriers who may need prophylaxis against venous thromboembolism in high-risk situations such as pregnancy, oral contraceptive use, hospitalization, and surgery.29 Asymptomatic family members of a first-degree relative with a history of venous thromboembolism have a 2 times higher risk of an index event.43 Thus, it may be argued that these asymptomatic individuals should receive prophylactic measures in any high-risk situation, based on the family history itself rather than results of thrombophilia testing.
Occasionally, patients and family members want to know the cause of the thrombotic event and want to be tested. In these instances, pretest counseling for the patient and family about the potential implications of testing and shared decision-making between the provider and patient are of utmost importance.29
What is the impact on family members if thrombophilia is diagnosed?
While positive test results can give patients some satisfaction, this knowledge may also cause unnecessary worry, as the patient knows he or she has a hematologic disorder and could possible die of venous thromboembolism.
Thrombophilia testing can have other adverse consequences. For example, while the Genetic Information Nondiscrimination Act of 2008 protects against denial of health insurance benefits based on genetic information, known carriers of thrombophilia may have trouble obtaining life or disability insurance.44
Unfortunately, it is not uncommon for thrombophilia testing to be inappropriately performed, interpreted, or followed up. These suboptimal approaches can lead to unnecessary exposure to high-risk therapeutic anticoagulation, excessive durations of therapy, and labeling with an unconfirmed or incorrect diagnosis. Additionally, there are significant costs associated with thrombophilia testing, including the cost of the tests and anticoagulant medications and management of adverse events such as bleeding.
WHAT ARE THE ALTERNATIVES TO THROMBOPHILIA TESTING?
Because discovered thrombophilias (eg, factor V Leiden mutation, prothrombin gene mutation) have not consistently shown a strong correlation with increased recurrence of venous thromboembolism, alternative approaches are emerging to determine the duration of therapy for unprovoked events.
Clinical prediction tools based on patient characteristics and laboratory markers that are more consistently associated with recurrent venous thromboembolism (eg, male sex, persistently elevated D-dimer) have been developed to aid clinicians dealing with this challenging question. Several prediction tools are available:
The “Men Continue and HERDOO2” rule (HERDOO2 = hyperpigmentation, edema, or redness in either leg; D-dimer level ≥ 250 μg/L; obesity with body mass index ≥ 30 kg/m2; or older age, ≥ 65)45
The DASH score (D-dimer, age, sex, and hormonal therapy)46
The Vienna score,47,48 at http://cemsiis.meduniwien.ac.at/en/kb/science-research/software/clinical-software/recurrent-vte/.
SUMMARY OF THROMBOPHILIA TESTING RECOMMENDATIONS
Test for thrombophilia only when…
- Discussing with a specialist (eg, hematologist) who has an understanding of thrombophilia
- Using the 4 Ps approach
- A patient requests testing to understand why a thrombotic event occurred, and the patient understands the implications of testing (ie, received counseling) for self and for family
- An expert deems identification of asymptomatic family members important for those who may be carriers of a detected thrombophilia
- The patient with a venous thromboembolic event has completed 3 months of anticoagulation and has been off anticoagulation for the appropriate length of time
- The results will change management.
Forgo thrombophilia testing when…
- A patient has a provoked venous thromboembolic event
- You do not intend to discontinue anticoagulation (ie, anticoagulation is indefinite)
- The patient is in the acute (eg, inpatient) setting
- The patient is on anticoagulants that may render test results uninterpretable
- The patient is pregnant or on oral contraceptives
- Use of alternative patient characteristics and laboratory markers to predict venous thromboembolism recurrence may be an option.
OPTIMIZING THE DIAGNOSIS
With the incidence of venous thromboembolism rapidly increasing, optimizing its diagnosis from both a financial and clinical perspective is becoming increasingly important. Clinicians should be familiar with the use of pretest probability scoring for venous thromboembolism, as well as which diagnostic tests are preferred if further workup is indicated. They should strive to minimize or avoid indiscriminate thrombophilia testing, which may lead to increased healthcare costs and patient exposure to potentially harmful anticoagulation.
Testing for thrombophilia should be based on whether a venous thromboembolic event was provoked or unprovoked. Patients with provoked venous thromboembolism or those receiving indefinite anticoagulation therapy should not be tested for thrombophilia. If testing is being considered in a patient with unprovoked venous thromboembolism, a specialist who is able to implement the 4 Ps approach should be consulted to ensure well-informed, shared decision-making with patients and family members.
When a patient presents with suspected venous thromboembolism, ie, deep vein thrombosis or pulmonary embolism, what diagnostic tests are needed to confirm the diagnosis? The clinical signs and symptoms of venous thromboembolism are nonspecific and often difficult to interpret. Therefore, it is essential for clinicians to use a standardized, structured approach to diagnosis that incorporates clinical findings and laboratory testing, as well as judicious use of diagnostic imaging. But while information is important, clinicians must also strive to avoid unnecessary testing, not only to decrease costs, but also to avoid potential harm.
If the diagnosis is confirmed, does the patient need testing for an underlying thrombophilic disorder? Such screening is often considered after a thromboembolic event occurs. However, a growing body of evidence indicates that the results of thrombophilia testing can be misinterpreted and potentially harmful.1 We need to understand the utility of this testing as well as when and how it should be used. Patients and thrombosis specialists should be involved in deciding whether to perform these tests.
In this article, we provide practical information about how to diagnose venous thromboembolism, including strategies to optimize testing in suspected cases. We also offer guidance on how to decide whether further thrombophilia testing is warranted.
COMMON AND SERIOUS
Venous thromboembolism is a major cause of morbidity and death. Approximately 900,000 cases of pulmonary embolism and deep vein thrombosis occur in the United States each year, causing 60,000 to 300,000 deaths,2 with the number of cases projected to double over the next 40 years.3
INITIAL APPROACH: PRETEST PROBABILITY
Given the morbidity and mortality associated with venous thromboembolism, prompt recognition and diagnosis are imperative. Clinical diagnosis alone is insufficient, with confirmed disease found in only 15% to 25% of patients suspected of having venous thromboembolism.4–8 Therefore, the pretest probability should be coupled with objective testing.
The Wells score shows good discrimination in the outpatient and emergency department settings, but it has been invalidated in the inpatient setting, and thus it should not be used in inpatients.10
LABORATORY TESTS FOR SUSPECTED VENOUS THROMBOEMBOLISM
Employing an understanding of diagnostic testing is fundamental to identifying patients with venous thromboembolism.
D-dimer is a byproduct of fibrinolysis.
D-dimer testing has very high sensitivity for venous thromboembolism (> 90%) but low specificity (about 50%), and levels can be elevated in a variety of situations such as advanced age, acute inflammation, and cancer.15 The standard threshold is 500 μg/L, but because the D-dimer level increases with age, some clinicians advocate using an age-adjusted threshold for patients age 50 or older (age in years × 10 μg/L) to increase the diagnostic yield.16
Of the laboratory tests for D-dimer, the enzyme-linked immunosorbent assay has the highest sensitivity and highest negative predictive value (100%) and may be preferred over the other test methodologies.17
With its high sensitivity, D-dimer testing is clinically useful for ruling out venous thromboembolism, particularly when the pretest probability is low, but it lacks the specificity required for diagnosing and treating the disease if positive. Thus, it is not useful for ruling in venous thromboembolism. If the patient has a high pretest probability, we can omit D-dimer testing in favor of imaging studies.
Other laboratory tests such as arterial blood gas and brain natriuretic peptide levels have been proposed as markers of pulmonary embolism, but studies suggest they have limited utility in predicting the presence of disease.18,19
DIAGNOSTIC TESTS FOR DEEP VEIN THROMBOSIS
Ultrasonography
If the pretest probability of deep vein thrombosis is high or a D-dimer test is found to be positive, the next step in evaluation is compression ultrasonography.
While some guidelines recommend scanning only the proximal leg, many facilities in the United States scan the whole leg, which may reveal distal deep vein thrombosis.20 The clinical significance of isolated distal deep vein thrombosis is unknown, and a selective anticoagulation approach may be used if this condition is discovered. The 2012 and 2016 American College of Chest Physicians (ACCP) guidelines on diagnosis and management of venous thromboembolism address this topic.20,21
Deep vein thrombosis in the arm should be evaluated in the same manner as in the lower extremities.
Venography
Invasive and therefore no longer often used, venography is considered the gold standard for diagnosing deep vein thrombosis. Computed tomographic (CT) or magnetic resonance (MR) venography is most useful if the patient has aberrant anatomy such as a deformity of the leg, or in situations where the use of ultrasonography is difficult or unreliable, such as in the setting of severe obesity. CT or MR venography may be considered when looking for thrombosis in noncompressible veins of the thorax and abdomen (eg, the subclavian vein, iliac vein, and inferior vena cava) if ultrasonography is negative but clinical suspicion is high. Venous-phase CT angiography is particularly useful in diagnosing deep vein thrombosis in the inferior vena cava and iliac vein when deep vein thrombosis is clinically suspected but cannot be visualized on duplex ultrasonography.
DIAGNOSTIC TESTS FOR PULMONARY EMBOLISM
Computed tomography
Imaging is warranted in patients who have a high pretest probability of pulmonary embolism, or in whom the D-dimer assay was positive but the pretest probability was low or moderate.
Once the gold standard, pulmonary angiography is no longer recommended for the initial diagnosis of pulmonary embolism because it is invasive, often unavailable, less sophisticated, and more expensive than noninvasive imaging techniques such as CT angiography. It is still used, however, in catheter-directed thrombolysis.
Thus, multiphasic CT angiography, as guided by pretest probability and the D-dimer level, is the imaging test of choice in the evaluation of pulmonary embolism. It can also offer insight into thrombotic burden and can reveal concurrent or alternative diagnoses (eg, pneumonia).
Ventilation-perfusion scanning
When CT angiography is unavailable or the patient should not be exposed to contrast medium (eg, due to concern for contrast-induced nephropathy or contrast allergy), ventilation-perfusion (V/Q) scanning remains an option for ruling out pulmonary embolism.22
Anderson et al23 compared CT angiography and V/Q scanning in a study in 1,417 patients considered likely to have acute pulmonary embolism. Rates of symptomatic pulmonary embolism during 3-month follow-up were similar in patients who initially had negative results on V/Q scanning compared with those who initially had negative results on CT angiography. However, this study used single-detector CT scanners for one-third of the patients. Therefore, the results may have been different if current technology had been used.
Limitations of V/Q scanning include length of time to perform (30–45 minutes), cost, inability to identify other causes of symptoms, and difficulty with interpretation when other pulmonary pathology is present (eg, lung infiltrate). V/Q scanning is helpful when negative but is often reported based on probability (low, intermediate, or high) and may not provide adequate guidance. Therefore, CT angiography should be used whenever possible for diagnosing pulmonary embolism.
Other tests for pulmonary embolism
Electrocardiography, transthoracic echocardiography, and chest radiography may aid in the search for alternative diagnoses and assess the degree of right heart strain as a sequela of pulmonary embolism, but they do not confirm the diagnosis.
ORDER IMAGING ONLY IF NEEDED
Diagnostic imaging can be optimized by avoiding unnecessary tests that carry both costs and clinical risks.
Most patients in whom acute pulmonary embolism is discovered will not need testing for deep vein thrombosis, as they will receive anticoagulation regardless. Similarly, many patients with acute symptomatic deep vein thrombosis do not need testing for pulmonary embolism with chest CT imaging, as they too will receive anticoagulation regardless.
Therefore, clinicians are encouraged to use diagnostic reasoning while practicing high-value care (including estimating pretest probability and measuring D-dimer when appropriate), ordering additional tests judiciously and only if indicated.
THROMBOEMBOLISM IS CONFIRMED—IS FURTHER TESTING WARRANTED?
Once acute venous thromboembolism is confirmed, key considerations include whether the event was provoked or unprovoked (ie, idiopathic) and whether the patient needs indefinite anticoagulation (eg, after 2 or more unprovoked events).
Was the event provoked or unprovoked?
Even in cases of unprovoked venous thromboembolism, no clear consensus exists as to which patients should be tested for thrombophilia. Experts do advocate, however, that it be done only in highly selected patients and that it be coordinated with the patient, family members, and an expert in this testing. Patients for whom further testing may be considered include those with venous thromboembolism in unusual sites (eg, the cavernous sinus), with warfarin-induced skin necrosis, or with recurrent pregnancy loss.
While screening for malignancy may seem prudent in the case of unexplained venous thromboembolism, the use of CT imaging for this purpose has been found to be of low yield. In one study,24 it was not found to detect additional neoplasms, and it can lead to additional cost and no added benefit for patients.
The American Board of Internal Medicine’s Choosing Wisely campaign strongly recommends consultation with an expert in thrombophilia (eg, a hematologist) before testing.25 Ordering multiple tests in bundles (hypercoagulability panels) is unlikely to alter management, could have a negative clinical impact on patients, and is generally not recommended.
The ‘4 Ps’ approach to testing
- Patient selection
- Pretest counseling
- Proper laboratory interpretation
- Provision of education and advice.
Importantly, testing should be reserved for patients in whom the pretest probability of the thrombophilic disease is moderate to high, such as testing for antiphospholipid antibody syndrome in patients with systemic lupus erythematosus or recurrent miscarriage.
Venous thromboembolism in a patient who is known to have a malignant disease does not typically warrant further thrombophilia testing, as the event was likely a sequela of the malignancy. The evaluation and management of venous thromboembolism with concurrent neoplasm is covered elsewhere.21
WHAT IF VENOUS THROMBOEMBOLISM IS DISCOVERED INCIDENTALLY?
Thrombophilia testing should be approached the same regardless of whether the venous thromboembolism was diagnosed intentionally or incidentally. First, determine whether the thrombosis was provoked or unprovoked, then order additional tests only if indicated, as recommended. Alternative approaches such as forgoing anticoagulation (but performing serial imaging, if indicated) may be reasonable if the thrombus is deemed clinically irrelevant (eg, nonocclusive, asymptomatic, subsegmental pulmonary embolism in the absence of proximal deep vein thrombosis; isolated distal deep vein thrombosis).25,27
It is still debatable whether the increasing incidence of asymptomatic pulmonary embolism due to enhanced sensitivity of noninvasive diagnostic imaging warrants a change in diagnostic approach.28
FACTORS TO CONSIDER BEFORE THROMBOPHILIA TESTING
Important factors to consider before testing for thrombophilia are29:
- How will the results affect the anticoagulation plan?
- How may the patient’s clinical status and medications influence the results?
- Has the patient expressed a desire to understand why venous thromboembolism occurred?
- Will the results have a potential impact on the patient’s family members?
How will the results of thrombophilia testing affect anticoagulation management?
Because the goal of any diagnostic test is to find out what type of care the patient needs, clinicians must determine whether knowledge of an underlying thrombophilia will alter the short-term or long-term anticoagulation therapy the patient is receiving for an acute venous thromboembolic event.
As most acute episodes of venous thromboembolism require an initial 3 months of anticoagulation (with the exception of some nonclinically relevant events such as isolated distal deep vein thrombosis without extension on reimaging), testing in the acute setting does not change the short-term management of anticoagulation. Many hospitals have advocated for outpatient-only thrombophilia testing (if testing does occur), as testing in the acute setting may render test results uninterpretable (see What factors can influence thrombophilia testing? below) and can inappropriately affect the long-term management of anticoagulation. We recommend against testing in the inpatient setting.
To determine the duration of anticoagulation, clinicians must balance the risk of recurrent venous thromboembolism and the risk of bleeding. If a patient is at significant risk of bleeding or does not tolerate anticoagulation, clinicians may consider stopping therapy instead of evaluating for thrombophilia. For patients with provoked venous thromboembolism, anticoagulation should generally be limited to 3 months, as the risk of recurrence does not outweigh the risk of bleeding with continued anticoagulation therapy.
Patients with unprovoked venous thromboembolism have a risk of recurrence twice as high as those with provoked venous thromboembolism and generally need a longer duration of anticoagulation.30,31 Once a patient with an unprovoked venous thromboembolic event has completed the initial 3 months of anticoagulation, a formal risk-benefit evaluation should be performed to determine whether to continue it.
Up to 42% of patients with unprovoked venous thromboembolism may have 1 or more thrombotic disorders, and some clinicians believe that detecting an underlying thrombophilia will aid in decisions regarding duration of therapy.32 However, the risk of recurrent venous thromboembolism in these patients does not differ significantly from that in patients without an underlying thrombophilia.33–35 As such, it has been suggested that the unprovoked character of the thrombotic event, rather than an underlying thrombophilia, determines the risk of future recurrence and should be used instead of testing to guide the duration of anticoagulation therapy.32
For more information, see the 2016 ACCP guideline update on antithrombotic therapy for venous thromboembolism.27
What factors can influence the results of thrombophilia testing?
For example, antithrombin is consumed during thrombus formation; therefore, antithrombin levels may be transiently suppressed in acute venous thromboembolism. Moreover, since antithrombin binds to unfractionated heparin, low-molecular-weight heparin, and fondaparinux and mediates their activity as anticoagulants, antithrombin levels may be decreased by heparin therapy.
Similarly, vitamin K antagonists (eg, warfarin) suppress protein C and S activity levels by inhibiting vitamin K epoxide reductase and may falsely indicate a protein C or S deficiency.
Direct oral anticoagulants can cause false-positive results on lupus anticoagulant assays (dilute Russell viper venom time, augmented partial thromboplastin time), raise protein C, protein S, and antithrombin activity levels, and normalize activated protein C resistance assays, leading to missed diagnoses.41
Since estrogen therapy and pregnancy lead to increases in C4b binding protein, resulting in decreased free protein S, these situations can result in clinicians falsely labeling patients as having congenital protein S deficiency when in fact the patient had a transient reduction in protein S levels.33
Therefore, to optimize accuracy and interpretation of results, thrombophilia testing should ideally be performed when the patient:
- Is past the acute event and out of the hospital
- Is not pregnant
- Has received the required 3 months of anticoagulation and is off this therapy.
For warfarin, most recommendations say that testing should be performed after the patient has been off therapy for 2 to 6 weeks.42 Low-molecular-weight heparins and direct oral anticoagulants should be discontinued for at least 48 to 72 hours, or longer if the patient has kidney impairment, as these medications are renally eliminated.
Genetic tests such as factor V Leiden and prothrombin gene mutation are not affected by these factors and do not require repeat or confirmatory testing.
What if the patient or family wants to understand why an event occurred?
Some experts advocate thrombophilia testing of asymptomatic family members to identify carriers who may need prophylaxis against venous thromboembolism in high-risk situations such as pregnancy, oral contraceptive use, hospitalization, and surgery.29 Asymptomatic family members of a first-degree relative with a history of venous thromboembolism have a 2 times higher risk of an index event.43 Thus, it may be argued that these asymptomatic individuals should receive prophylactic measures in any high-risk situation, based on the family history itself rather than results of thrombophilia testing.
Occasionally, patients and family members want to know the cause of the thrombotic event and want to be tested. In these instances, pretest counseling for the patient and family about the potential implications of testing and shared decision-making between the provider and patient are of utmost importance.29
What is the impact on family members if thrombophilia is diagnosed?
While positive test results can give patients some satisfaction, this knowledge may also cause unnecessary worry, as the patient knows he or she has a hematologic disorder and could possible die of venous thromboembolism.
Thrombophilia testing can have other adverse consequences. For example, while the Genetic Information Nondiscrimination Act of 2008 protects against denial of health insurance benefits based on genetic information, known carriers of thrombophilia may have trouble obtaining life or disability insurance.44
Unfortunately, it is not uncommon for thrombophilia testing to be inappropriately performed, interpreted, or followed up. These suboptimal approaches can lead to unnecessary exposure to high-risk therapeutic anticoagulation, excessive durations of therapy, and labeling with an unconfirmed or incorrect diagnosis. Additionally, there are significant costs associated with thrombophilia testing, including the cost of the tests and anticoagulant medications and management of adverse events such as bleeding.
WHAT ARE THE ALTERNATIVES TO THROMBOPHILIA TESTING?
Because discovered thrombophilias (eg, factor V Leiden mutation, prothrombin gene mutation) have not consistently shown a strong correlation with increased recurrence of venous thromboembolism, alternative approaches are emerging to determine the duration of therapy for unprovoked events.
Clinical prediction tools based on patient characteristics and laboratory markers that are more consistently associated with recurrent venous thromboembolism (eg, male sex, persistently elevated D-dimer) have been developed to aid clinicians dealing with this challenging question. Several prediction tools are available:
The “Men Continue and HERDOO2” rule (HERDOO2 = hyperpigmentation, edema, or redness in either leg; D-dimer level ≥ 250 μg/L; obesity with body mass index ≥ 30 kg/m2; or older age, ≥ 65)45
The DASH score (D-dimer, age, sex, and hormonal therapy)46
The Vienna score,47,48 at http://cemsiis.meduniwien.ac.at/en/kb/science-research/software/clinical-software/recurrent-vte/.
SUMMARY OF THROMBOPHILIA TESTING RECOMMENDATIONS
Test for thrombophilia only when…
- Discussing with a specialist (eg, hematologist) who has an understanding of thrombophilia
- Using the 4 Ps approach
- A patient requests testing to understand why a thrombotic event occurred, and the patient understands the implications of testing (ie, received counseling) for self and for family
- An expert deems identification of asymptomatic family members important for those who may be carriers of a detected thrombophilia
- The patient with a venous thromboembolic event has completed 3 months of anticoagulation and has been off anticoagulation for the appropriate length of time
- The results will change management.
Forgo thrombophilia testing when…
- A patient has a provoked venous thromboembolic event
- You do not intend to discontinue anticoagulation (ie, anticoagulation is indefinite)
- The patient is in the acute (eg, inpatient) setting
- The patient is on anticoagulants that may render test results uninterpretable
- The patient is pregnant or on oral contraceptives
- Use of alternative patient characteristics and laboratory markers to predict venous thromboembolism recurrence may be an option.
OPTIMIZING THE DIAGNOSIS
With the incidence of venous thromboembolism rapidly increasing, optimizing its diagnosis from both a financial and clinical perspective is becoming increasingly important. Clinicians should be familiar with the use of pretest probability scoring for venous thromboembolism, as well as which diagnostic tests are preferred if further workup is indicated. They should strive to minimize or avoid indiscriminate thrombophilia testing, which may lead to increased healthcare costs and patient exposure to potentially harmful anticoagulation.
Testing for thrombophilia should be based on whether a venous thromboembolic event was provoked or unprovoked. Patients with provoked venous thromboembolism or those receiving indefinite anticoagulation therapy should not be tested for thrombophilia. If testing is being considered in a patient with unprovoked venous thromboembolism, a specialist who is able to implement the 4 Ps approach should be consulted to ensure well-informed, shared decision-making with patients and family members.
- National Institute for Health and Care Excellence (NICE). Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. https://www.nice.org.uk/guidance/cg144. Accessed June 13, 2017.
- Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol 2008; 28:370–372.
- Deitelzweig SB, Johnson BH, Lin J, Schulman KL. Prevalence of clinical venous thromboembolism in the USA: current trends and future projections. Am J Hematol 2011; 86:217–220.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):e419S–e494S.
- Pengo V, Lensing AW, Prins MH, et al; Thromboembolic Pulmonary Hypertension Study Group. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004; 350:2257–2264.
- Kahn SR, Hirsch A, Shrier I. Effect of postthrombotic syndrome on health-related quality of life after deep venous thrombosis. Arch Intern Med 2002; 162:1144–1148.
- Wells PS, Owen C, Doucette S, Fergusson D, Tran H. Does this patient have deep vein thrombosis? JAMA 2006; 295:199–207.
- Ljungqvist M, Söderberg M, Moritz P, Ahlgren A, Lärfars G. Evaluation of Wells score and repeated D-dimer in diagnosing venous thromboembolism. Eur J Intern Med 2008; 19:285–288.
- Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med 2001; 135:98–107.
- Silveira PC, Ip IK, Goldhaber SZ, Piazza G, Benson CB, Khorasani R. Performance of Wells score for deep vein thrombosis in the inpatient setting. JAMA Intern Med 2015; 175:1112–1117.
- Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet 1997; 350:1795–1798.
- Wells PS, Anderson DR, Rodger M, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med 2003; 349:1227–1235.
- van Belle A, Büller HR, Huisman MV, et al. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. JAMA 2006; 295:172–179.
- Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemos 2000; 83:416–420.
- Schrecengost JE, LeGallo RD, Boyd JC, et al. Comparison of diagnostic accuracies in outpatients and hospitalized patients of D-dimer testing for the evaluation of suspected pulmonary embolism. Clin Chem 2003; 49:1483–1490.
- Righini M, Van Es J, Den Exter PL, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA 2014; 311:1117–1124.
- Pulivarthi S, Gurram MK. Effectiveness of D-dimer as a screening test for venous thromboembolism: an update. N Am J Med Sci 2014; 6:491–499.
- Söhne M, Ten Wolde M, Boomsma F, Reitsma JB, Douketis JD, Büller HR. Brain natriuretic peptide in hemodynamically stable acute pulmonary embolism. J Thromb Haemost 2006; 4:552–556.
- Stein PD, Goldhaber SZ, Henry JW, Miller AC. Arterial blood gas analysis in the assessment of suspected acute pulmonary embolism. Chest 1996; 109:78–81.
- Bates SM, Jaeschke R, Stevens SM, et al; American College of Chest Physicians. Diagnosis of DVT: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):e351S–e418S.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest 2016; 149:315–352.
- PIOPED Investigators. Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). JAMA 1990; 263:2753–2759.
- Anderson DR, Kahn SR, Rodger MA, et al. Computed tomographic pulmonary angiography vs ventilation-perfusion lung scanning in patients with suspected pulmonary embolism: a randomized controlled trial. JAMA 2007; 298:2743–2753.
- Carrier M. Cancer screening in unprovoked venous thromboembolism. N Engl J Med 2015; 373:2475.
- American Society of Hematology. Don’t test for thrombophilia in adult patients with venous thromboembolism (VTE) occurring in the setting of major transient risk factors (surgery, trauma or prolonged immobility). www.choosingwisely.org/clinician-lists/american-society-hematology-testing-for-thrombophilia-in-adults/. Accessed June 13, 2017.
- Cushman M. Thrombophilia testing in women with venous thrombosis: the 4 Ps approach. Clin Chem 2014; 60:134–137.
- Bates SM, Greer IA, Middeldorp S, Veenstra DL, Prabulos AM, Vandvik PO; American College of Chest Physicians. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl): e691S–e736S.
- Ritchie G, McGurk S, McCreath C, Graham C, Murchison JT. Prospective evaluation of unsuspected pulmonary embolism on contrast enhanced multidetector CT (MDCT) scanning. Thorax 2007; 62:536–540.
- Moll S. Thrombophilia: clinical-practical aspects. J Thromb Thrombolysis 2015; 39:367–378.
- Prandoni P, Noventa F, Ghirarduzzi A, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica 2007; 92:199–205.
- Boutitie F, Pinede L, Schulman S, et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants’ data from seven trials. BMJ 2011; 342:d3036.
- Kearon C, Julian JA, Kovacs MJ, et al; ELATE Investigators. Influence of thrombophilia on risk of recurrent venous thromboembolism while on warfarin: results from a randomized trial. Blood 2008; 112:4432–4436.
- Lijfering WM, Middeldorp S, Veeger NJ, et al. Risk of recurrent venous thrombosis in homozygous carriers and double heterozygous carriers of factor V Leiden and prothrombin G20210A. Circulation 2010; 121:1706–1712.
- Hron G, Eichinger S, Weltermann A, et al. Family history for venous thromboembolism and the risk for recurrence. Am J Med 2006; 119:50–53.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA 2005; 293:2352–2361.
- Lijfering WM. Selective testing for thrombophilia in patients with first venous thrombosis: results from a retrospective family cohort study on absolute thrombotic risk for currently known thrombophilic defects in 2479 relatives. Blood 2009; 113:5314–5322.
- Segal JB. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation. JAMA 2009; 301:2472–2485.
- Juul K. Factor V Leiden and the risk for venous thromboembolism in the adult Danish population. Ann Intern Med 2004; 140: 330–337.
- Emmerich J. Combined effect of factor V Leiden and prothrombin 20210A on the risk of venous thromboembolism: pooled analysis of 8 case-control studies including 2310 cases and 3204 controls. Thromb Haemost 2001; 86: 809–816.
- Garcia D. Antiphospholipid antibodies and the risk of recurrence after a first episode of venous thromboembolism: a systematic review. Blood 2013; 122:817–824.
- Gosselin R, Adcock DM. The laboratory’s 2015 perspective on direct oral anticoagulant testing. J Thromb Haemost 2016; 14:886–893.
- Marlar RA, Gausman JN. Protein S abnormalities: a diagnostic nightmare. Am J Hematol 2011; 86:418–421.
- Bezemer ID, van der Meer FJ, Eikenboom JC, Rosendaal FR, Doggen CJ. The value of family history as a risk indicator for venous thrombosis. Arch Intern Med 2009; 169:610–615.
- Middeldorp S. Evidence-based approach to thrombophilia testing. J Thromb Thrombolysis 2011; 31:275–281.
- Rodger MA, Le Gal G, Anderson DR, et al, for the REVERSE II Study Investigators. Validating the HERDOO2 rule to guide treatment duration for women with unprovoked venous thrombosis: multinational prospective cohort management study. BMJ 2017; 356:j1065.
- Tosetto A, Iorio A, Marcucci M, et al. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH). J Thromb Haemost 2012; 10:1019–1025.
- Eichinger S, Heinze G, Jandeck LM, Kyrle PA. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna prediction model. Circulation 2010; 121:1630–1636.
- Rodger MA, Kahn SR, Wells PS, et al. Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. CMAJ 2008; 179:417–426.
- National Institute for Health and Care Excellence (NICE). Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. https://www.nice.org.uk/guidance/cg144. Accessed June 13, 2017.
- Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol 2008; 28:370–372.
- Deitelzweig SB, Johnson BH, Lin J, Schulman KL. Prevalence of clinical venous thromboembolism in the USA: current trends and future projections. Am J Hematol 2011; 86:217–220.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):e419S–e494S.
- Pengo V, Lensing AW, Prins MH, et al; Thromboembolic Pulmonary Hypertension Study Group. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004; 350:2257–2264.
- Kahn SR, Hirsch A, Shrier I. Effect of postthrombotic syndrome on health-related quality of life after deep venous thrombosis. Arch Intern Med 2002; 162:1144–1148.
- Wells PS, Owen C, Doucette S, Fergusson D, Tran H. Does this patient have deep vein thrombosis? JAMA 2006; 295:199–207.
- Ljungqvist M, Söderberg M, Moritz P, Ahlgren A, Lärfars G. Evaluation of Wells score and repeated D-dimer in diagnosing venous thromboembolism. Eur J Intern Med 2008; 19:285–288.
- Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med 2001; 135:98–107.
- Silveira PC, Ip IK, Goldhaber SZ, Piazza G, Benson CB, Khorasani R. Performance of Wells score for deep vein thrombosis in the inpatient setting. JAMA Intern Med 2015; 175:1112–1117.
- Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet 1997; 350:1795–1798.
- Wells PS, Anderson DR, Rodger M, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med 2003; 349:1227–1235.
- van Belle A, Büller HR, Huisman MV, et al. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. JAMA 2006; 295:172–179.
- Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemos 2000; 83:416–420.
- Schrecengost JE, LeGallo RD, Boyd JC, et al. Comparison of diagnostic accuracies in outpatients and hospitalized patients of D-dimer testing for the evaluation of suspected pulmonary embolism. Clin Chem 2003; 49:1483–1490.
- Righini M, Van Es J, Den Exter PL, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA 2014; 311:1117–1124.
- Pulivarthi S, Gurram MK. Effectiveness of D-dimer as a screening test for venous thromboembolism: an update. N Am J Med Sci 2014; 6:491–499.
- Söhne M, Ten Wolde M, Boomsma F, Reitsma JB, Douketis JD, Büller HR. Brain natriuretic peptide in hemodynamically stable acute pulmonary embolism. J Thromb Haemost 2006; 4:552–556.
- Stein PD, Goldhaber SZ, Henry JW, Miller AC. Arterial blood gas analysis in the assessment of suspected acute pulmonary embolism. Chest 1996; 109:78–81.
- Bates SM, Jaeschke R, Stevens SM, et al; American College of Chest Physicians. Diagnosis of DVT: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):e351S–e418S.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest 2016; 149:315–352.
- PIOPED Investigators. Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). JAMA 1990; 263:2753–2759.
- Anderson DR, Kahn SR, Rodger MA, et al. Computed tomographic pulmonary angiography vs ventilation-perfusion lung scanning in patients with suspected pulmonary embolism: a randomized controlled trial. JAMA 2007; 298:2743–2753.
- Carrier M. Cancer screening in unprovoked venous thromboembolism. N Engl J Med 2015; 373:2475.
- American Society of Hematology. Don’t test for thrombophilia in adult patients with venous thromboembolism (VTE) occurring in the setting of major transient risk factors (surgery, trauma or prolonged immobility). www.choosingwisely.org/clinician-lists/american-society-hematology-testing-for-thrombophilia-in-adults/. Accessed June 13, 2017.
- Cushman M. Thrombophilia testing in women with venous thrombosis: the 4 Ps approach. Clin Chem 2014; 60:134–137.
- Bates SM, Greer IA, Middeldorp S, Veenstra DL, Prabulos AM, Vandvik PO; American College of Chest Physicians. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl): e691S–e736S.
- Ritchie G, McGurk S, McCreath C, Graham C, Murchison JT. Prospective evaluation of unsuspected pulmonary embolism on contrast enhanced multidetector CT (MDCT) scanning. Thorax 2007; 62:536–540.
- Moll S. Thrombophilia: clinical-practical aspects. J Thromb Thrombolysis 2015; 39:367–378.
- Prandoni P, Noventa F, Ghirarduzzi A, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica 2007; 92:199–205.
- Boutitie F, Pinede L, Schulman S, et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants’ data from seven trials. BMJ 2011; 342:d3036.
- Kearon C, Julian JA, Kovacs MJ, et al; ELATE Investigators. Influence of thrombophilia on risk of recurrent venous thromboembolism while on warfarin: results from a randomized trial. Blood 2008; 112:4432–4436.
- Lijfering WM, Middeldorp S, Veeger NJ, et al. Risk of recurrent venous thrombosis in homozygous carriers and double heterozygous carriers of factor V Leiden and prothrombin G20210A. Circulation 2010; 121:1706–1712.
- Hron G, Eichinger S, Weltermann A, et al. Family history for venous thromboembolism and the risk for recurrence. Am J Med 2006; 119:50–53.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA 2005; 293:2352–2361.
- Lijfering WM. Selective testing for thrombophilia in patients with first venous thrombosis: results from a retrospective family cohort study on absolute thrombotic risk for currently known thrombophilic defects in 2479 relatives. Blood 2009; 113:5314–5322.
- Segal JB. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation. JAMA 2009; 301:2472–2485.
- Juul K. Factor V Leiden and the risk for venous thromboembolism in the adult Danish population. Ann Intern Med 2004; 140: 330–337.
- Emmerich J. Combined effect of factor V Leiden and prothrombin 20210A on the risk of venous thromboembolism: pooled analysis of 8 case-control studies including 2310 cases and 3204 controls. Thromb Haemost 2001; 86: 809–816.
- Garcia D. Antiphospholipid antibodies and the risk of recurrence after a first episode of venous thromboembolism: a systematic review. Blood 2013; 122:817–824.
- Gosselin R, Adcock DM. The laboratory’s 2015 perspective on direct oral anticoagulant testing. J Thromb Haemost 2016; 14:886–893.
- Marlar RA, Gausman JN. Protein S abnormalities: a diagnostic nightmare. Am J Hematol 2011; 86:418–421.
- Bezemer ID, van der Meer FJ, Eikenboom JC, Rosendaal FR, Doggen CJ. The value of family history as a risk indicator for venous thrombosis. Arch Intern Med 2009; 169:610–615.
- Middeldorp S. Evidence-based approach to thrombophilia testing. J Thromb Thrombolysis 2011; 31:275–281.
- Rodger MA, Le Gal G, Anderson DR, et al, for the REVERSE II Study Investigators. Validating the HERDOO2 rule to guide treatment duration for women with unprovoked venous thrombosis: multinational prospective cohort management study. BMJ 2017; 356:j1065.
- Tosetto A, Iorio A, Marcucci M, et al. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH). J Thromb Haemost 2012; 10:1019–1025.
- Eichinger S, Heinze G, Jandeck LM, Kyrle PA. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna prediction model. Circulation 2010; 121:1630–1636.
- Rodger MA, Kahn SR, Wells PS, et al. Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. CMAJ 2008; 179:417–426.
KEY POINTS
- A pretest clinical prediction tool such as the Wells score can help in deciding whether a patient with suspected venous thromboembolism warrants further workup.
- A clinical prediction tool should be used in concert with additional laboratory testing (eg, D-dimer) and imaging in patients at risk.
- In many cases, screening for thrombophilia to determine the cause of a venous thromboembolic event may be unwarranted.
- Testing for thrombophilia should be based on whether a venous thromboembolic event was provoked or unprovoked.
A large mass in the right ventricle: Tumor or thrombus?
A 69-year-old woman with hypertension, diabetes mellitus, and chronic kidney disease presented with a 1-month history of worsening episodic dyspnea, lower-extremity edema, and dizziness. Two months earlier, she had been diagnosed with poorly differentiated pelvic adnexal sarcoma associated with a mature teratoma of the left ovary, and she had undergone bilateral salpingo-oophorectomy, pelvic and para-aortic lymph node dissection, and omentectomy.
Examination revealed tachypnea (23 breaths per minute) and bilateral pitting pedal edema. The neck veins were distended. There was no hepatomegaly. Results of laboratory testing were unremarkable.
EVALUATING A CARDIAC MASS
Thrombus, tumor, or vegetation?
If an intracardiac mass is discovered, we need to determine what it is.
Thrombosis is more likely if contrast echocardiography shows the mass has no stalk (thrombi almost never have a stalk), the atrial chamber is enlarged, cardiac output is low, there is stasis, the mass is avascular, and it responds to thrombolytic therapy. A giant organized thrombus can clinically mimic a tumor if it is immobile, is located close to the wall, and responds poorly to thrombolysis. A wall-motion abnormality adjacent to the mass, global hypokinesis, or a concomitant autoimmune condition such as lupus erythematosus or antiphospholipid antibody syndrome may also favor thrombosis.
Tumors in the heart are uncommon. The prevalence of primary cardiac tumors has been reported as 0.01% to 0.1% in autopsy studies. Metastases to the pericardium, myocardium, coronary arteries, or great vessels have been found at autopsy in 0.7% to 3.5% of the general population and in 9.1% of patients with known malignancy.1
Vegetations from infective endocarditis should also be considered early in the evaluation of an intracardiac mass. They can result from bacterial, fungal, or parasitic infection. Vegetations are generally irregular in appearance, mobile, and attached to a valve. Left-sided valves are generally involved, and a larger mass may indicate fungal origin. Abscess from tuberculosis may need to be considered in the appropriate setting. Whenever feasible, tissue diagnosis is desirable.
Occasionally, there may be an inflammatory component to masses detected in the setting of autoimmune disease.
CT and MRI
If echocardiography cannot clearly distinguish whether the mass is a tumor or a thrombus, MRI with gadolinium contrast is useful. MRI is superior to CT in depicting anatomic details and does not involve radiation.
Cardiac CT is increasingly used when other imaging findings are equivocal or to study a calcified mass. CT with contrast carries a small risk of contrast-induced nephropathy and has lower soft-tissue and temporal resolution. CT without contrast can detect the mass and reveal calcifications within the mass, but contrast is needed to assess the vascularity of the tumor. New-generation CT with electrocardiographic gating nearly matches MRI imaging, and CT is preferred for patients with contraindications to MRI.
CT provides additional information on the global assessment of the chest, lung and vascular structures.2 Cardiac CT and MRI help in precise anatomic delineation, characterization, and preoperative planning of treatment of a large cardiac mass.
TYPES OF CARDIAC TUMORS
Metastases account for most cardiac tumors and are often from primary cancers of the lung, breast, skin, thyroid, and kidney.
Primary cardiac tumors are most often myxomas, which are benign and generally found in the atrial chamber, solitary, with a stalk attached to the area of the fossa ovalis. Other primary cardiac tumors include sarcomas, angiosarcomas, rhabdomyosarcomas, papillary fibroelastomas, lipomas, hemangiomas, mesotheliomas, and rhabdomyomas.
TREATMENT OF CARDIAC TUMORS
For primary and secondary cardiac tumors, complete resection should be considered, provided there is no other organ involvement.3 For suspected lymphomas, image-guided biopsy should be performed before treatment.
For uncertain and diagnostically challenging cases, guided biopsy of the lesions using intracardiac echocardiography or transesophageal echocardiography has been reported to be helpful.4
Most often, the workup and management of cardiac masses calls for a team involving an internist, cardiologist, cardiothoracic surgeon, and vascular medicine specialist. Depending on the nature of the mass, the team may also include an oncologist, radiotherapist, and infectious disease specialist.
Because our patient had significant kidney disease, CT was done without contrast. However, it was not able to clearly delineate the mass in the right ventricle. Cardiac MRI was not performed. Biopsy with transesophageal or intracardiac echocardiographic guidance was not an option, as the patient’s condition was poor.
TAKE-HOME POINTS
The differential diagnosis of an intracardiac mass includes thrombus, benign or malignant tumors, and masses of infectious or inflammatory origin. While noninvasive imaging tests provide clues that can help narrow the differential diagnosis, tissue biopsy with histologic study is necessary to confirm the diagnosis. A team approach is paramount in managing cardiac masses.
- Goldberg AD, Blankstein R, Padera RF. Tumors metastatic to the heart. Circulation 2013; 128:1790–1794.
- Exarhos DN, Tavernaraki EA, Kyratzi F, et al. Imaging of cardiac tumours and masses. Hospital Chronicles 2010; 5:1–9.
- Hoffmeier A, Sindermann JR, Scheld HH, Martens S. Cardiac tumors—diagnosis and surgical treatment. Dtsch Arztebl Int 2014; 111:205–211.
- Park K-I, Kim MJ, Oh JK, et al. Intracardiac echocardiography to guide biopsy for two cases of intracardiac masses. Korean Circ J 2015; 45:165–168.
A 69-year-old woman with hypertension, diabetes mellitus, and chronic kidney disease presented with a 1-month history of worsening episodic dyspnea, lower-extremity edema, and dizziness. Two months earlier, she had been diagnosed with poorly differentiated pelvic adnexal sarcoma associated with a mature teratoma of the left ovary, and she had undergone bilateral salpingo-oophorectomy, pelvic and para-aortic lymph node dissection, and omentectomy.
Examination revealed tachypnea (23 breaths per minute) and bilateral pitting pedal edema. The neck veins were distended. There was no hepatomegaly. Results of laboratory testing were unremarkable.
EVALUATING A CARDIAC MASS
Thrombus, tumor, or vegetation?
If an intracardiac mass is discovered, we need to determine what it is.
Thrombosis is more likely if contrast echocardiography shows the mass has no stalk (thrombi almost never have a stalk), the atrial chamber is enlarged, cardiac output is low, there is stasis, the mass is avascular, and it responds to thrombolytic therapy. A giant organized thrombus can clinically mimic a tumor if it is immobile, is located close to the wall, and responds poorly to thrombolysis. A wall-motion abnormality adjacent to the mass, global hypokinesis, or a concomitant autoimmune condition such as lupus erythematosus or antiphospholipid antibody syndrome may also favor thrombosis.
Tumors in the heart are uncommon. The prevalence of primary cardiac tumors has been reported as 0.01% to 0.1% in autopsy studies. Metastases to the pericardium, myocardium, coronary arteries, or great vessels have been found at autopsy in 0.7% to 3.5% of the general population and in 9.1% of patients with known malignancy.1
Vegetations from infective endocarditis should also be considered early in the evaluation of an intracardiac mass. They can result from bacterial, fungal, or parasitic infection. Vegetations are generally irregular in appearance, mobile, and attached to a valve. Left-sided valves are generally involved, and a larger mass may indicate fungal origin. Abscess from tuberculosis may need to be considered in the appropriate setting. Whenever feasible, tissue diagnosis is desirable.
Occasionally, there may be an inflammatory component to masses detected in the setting of autoimmune disease.
CT and MRI
If echocardiography cannot clearly distinguish whether the mass is a tumor or a thrombus, MRI with gadolinium contrast is useful. MRI is superior to CT in depicting anatomic details and does not involve radiation.
Cardiac CT is increasingly used when other imaging findings are equivocal or to study a calcified mass. CT with contrast carries a small risk of contrast-induced nephropathy and has lower soft-tissue and temporal resolution. CT without contrast can detect the mass and reveal calcifications within the mass, but contrast is needed to assess the vascularity of the tumor. New-generation CT with electrocardiographic gating nearly matches MRI imaging, and CT is preferred for patients with contraindications to MRI.
CT provides additional information on the global assessment of the chest, lung and vascular structures.2 Cardiac CT and MRI help in precise anatomic delineation, characterization, and preoperative planning of treatment of a large cardiac mass.
TYPES OF CARDIAC TUMORS
Metastases account for most cardiac tumors and are often from primary cancers of the lung, breast, skin, thyroid, and kidney.
Primary cardiac tumors are most often myxomas, which are benign and generally found in the atrial chamber, solitary, with a stalk attached to the area of the fossa ovalis. Other primary cardiac tumors include sarcomas, angiosarcomas, rhabdomyosarcomas, papillary fibroelastomas, lipomas, hemangiomas, mesotheliomas, and rhabdomyomas.
TREATMENT OF CARDIAC TUMORS
For primary and secondary cardiac tumors, complete resection should be considered, provided there is no other organ involvement.3 For suspected lymphomas, image-guided biopsy should be performed before treatment.
For uncertain and diagnostically challenging cases, guided biopsy of the lesions using intracardiac echocardiography or transesophageal echocardiography has been reported to be helpful.4
Most often, the workup and management of cardiac masses calls for a team involving an internist, cardiologist, cardiothoracic surgeon, and vascular medicine specialist. Depending on the nature of the mass, the team may also include an oncologist, radiotherapist, and infectious disease specialist.
Because our patient had significant kidney disease, CT was done without contrast. However, it was not able to clearly delineate the mass in the right ventricle. Cardiac MRI was not performed. Biopsy with transesophageal or intracardiac echocardiographic guidance was not an option, as the patient’s condition was poor.
TAKE-HOME POINTS
The differential diagnosis of an intracardiac mass includes thrombus, benign or malignant tumors, and masses of infectious or inflammatory origin. While noninvasive imaging tests provide clues that can help narrow the differential diagnosis, tissue biopsy with histologic study is necessary to confirm the diagnosis. A team approach is paramount in managing cardiac masses.
A 69-year-old woman with hypertension, diabetes mellitus, and chronic kidney disease presented with a 1-month history of worsening episodic dyspnea, lower-extremity edema, and dizziness. Two months earlier, she had been diagnosed with poorly differentiated pelvic adnexal sarcoma associated with a mature teratoma of the left ovary, and she had undergone bilateral salpingo-oophorectomy, pelvic and para-aortic lymph node dissection, and omentectomy.
Examination revealed tachypnea (23 breaths per minute) and bilateral pitting pedal edema. The neck veins were distended. There was no hepatomegaly. Results of laboratory testing were unremarkable.
EVALUATING A CARDIAC MASS
Thrombus, tumor, or vegetation?
If an intracardiac mass is discovered, we need to determine what it is.
Thrombosis is more likely if contrast echocardiography shows the mass has no stalk (thrombi almost never have a stalk), the atrial chamber is enlarged, cardiac output is low, there is stasis, the mass is avascular, and it responds to thrombolytic therapy. A giant organized thrombus can clinically mimic a tumor if it is immobile, is located close to the wall, and responds poorly to thrombolysis. A wall-motion abnormality adjacent to the mass, global hypokinesis, or a concomitant autoimmune condition such as lupus erythematosus or antiphospholipid antibody syndrome may also favor thrombosis.
Tumors in the heart are uncommon. The prevalence of primary cardiac tumors has been reported as 0.01% to 0.1% in autopsy studies. Metastases to the pericardium, myocardium, coronary arteries, or great vessels have been found at autopsy in 0.7% to 3.5% of the general population and in 9.1% of patients with known malignancy.1
Vegetations from infective endocarditis should also be considered early in the evaluation of an intracardiac mass. They can result from bacterial, fungal, or parasitic infection. Vegetations are generally irregular in appearance, mobile, and attached to a valve. Left-sided valves are generally involved, and a larger mass may indicate fungal origin. Abscess from tuberculosis may need to be considered in the appropriate setting. Whenever feasible, tissue diagnosis is desirable.
Occasionally, there may be an inflammatory component to masses detected in the setting of autoimmune disease.
CT and MRI
If echocardiography cannot clearly distinguish whether the mass is a tumor or a thrombus, MRI with gadolinium contrast is useful. MRI is superior to CT in depicting anatomic details and does not involve radiation.
Cardiac CT is increasingly used when other imaging findings are equivocal or to study a calcified mass. CT with contrast carries a small risk of contrast-induced nephropathy and has lower soft-tissue and temporal resolution. CT without contrast can detect the mass and reveal calcifications within the mass, but contrast is needed to assess the vascularity of the tumor. New-generation CT with electrocardiographic gating nearly matches MRI imaging, and CT is preferred for patients with contraindications to MRI.
CT provides additional information on the global assessment of the chest, lung and vascular structures.2 Cardiac CT and MRI help in precise anatomic delineation, characterization, and preoperative planning of treatment of a large cardiac mass.
TYPES OF CARDIAC TUMORS
Metastases account for most cardiac tumors and are often from primary cancers of the lung, breast, skin, thyroid, and kidney.
Primary cardiac tumors are most often myxomas, which are benign and generally found in the atrial chamber, solitary, with a stalk attached to the area of the fossa ovalis. Other primary cardiac tumors include sarcomas, angiosarcomas, rhabdomyosarcomas, papillary fibroelastomas, lipomas, hemangiomas, mesotheliomas, and rhabdomyomas.
TREATMENT OF CARDIAC TUMORS
For primary and secondary cardiac tumors, complete resection should be considered, provided there is no other organ involvement.3 For suspected lymphomas, image-guided biopsy should be performed before treatment.
For uncertain and diagnostically challenging cases, guided biopsy of the lesions using intracardiac echocardiography or transesophageal echocardiography has been reported to be helpful.4
Most often, the workup and management of cardiac masses calls for a team involving an internist, cardiologist, cardiothoracic surgeon, and vascular medicine specialist. Depending on the nature of the mass, the team may also include an oncologist, radiotherapist, and infectious disease specialist.
Because our patient had significant kidney disease, CT was done without contrast. However, it was not able to clearly delineate the mass in the right ventricle. Cardiac MRI was not performed. Biopsy with transesophageal or intracardiac echocardiographic guidance was not an option, as the patient’s condition was poor.
TAKE-HOME POINTS
The differential diagnosis of an intracardiac mass includes thrombus, benign or malignant tumors, and masses of infectious or inflammatory origin. While noninvasive imaging tests provide clues that can help narrow the differential diagnosis, tissue biopsy with histologic study is necessary to confirm the diagnosis. A team approach is paramount in managing cardiac masses.
- Goldberg AD, Blankstein R, Padera RF. Tumors metastatic to the heart. Circulation 2013; 128:1790–1794.
- Exarhos DN, Tavernaraki EA, Kyratzi F, et al. Imaging of cardiac tumours and masses. Hospital Chronicles 2010; 5:1–9.
- Hoffmeier A, Sindermann JR, Scheld HH, Martens S. Cardiac tumors—diagnosis and surgical treatment. Dtsch Arztebl Int 2014; 111:205–211.
- Park K-I, Kim MJ, Oh JK, et al. Intracardiac echocardiography to guide biopsy for two cases of intracardiac masses. Korean Circ J 2015; 45:165–168.
- Goldberg AD, Blankstein R, Padera RF. Tumors metastatic to the heart. Circulation 2013; 128:1790–1794.
- Exarhos DN, Tavernaraki EA, Kyratzi F, et al. Imaging of cardiac tumours and masses. Hospital Chronicles 2010; 5:1–9.
- Hoffmeier A, Sindermann JR, Scheld HH, Martens S. Cardiac tumors—diagnosis and surgical treatment. Dtsch Arztebl Int 2014; 111:205–211.
- Park K-I, Kim MJ, Oh JK, et al. Intracardiac echocardiography to guide biopsy for two cases of intracardiac masses. Korean Circ J 2015; 45:165–168.
Very-low-volume vascular surgery practice linked to worse outcomes
The very-low-volume practice of surgeons performing no more than one open abdominal aortic aneurysm repair (OAR) or carotid endarterectomy (CEA) per year has persisted in New York State and was associated with worse postoperative outcomes and longer lengths of stay in a cohort study of statewide hospital data.
The study examined inpatient data on elective OARs and CEAs performed from 2000 to 2014 in every hospital in the state.
While the numbers and proportions of very-low-volume surgeons decreased (44.6%-23% for OAR and 35.2%-18.1% for CEA) and the number of procedures performed by these surgeons also decreased (QAR, 346-47; CEA, 395-90), the data are “concerning” and elucidate the “persistence” of very-low-volume practice in open vascular surgery, said Jialin Mao, MD, of Cornell University, New York, and associates (JAMA Surg. doi: 10:1001/jamasurg.2017.1100).
Very-low-volume surgeons were significantly less likely to be vascular surgeons, compared with higher-volume surgeons for both OAR (23.9% vs. 63.9%) and CEA (14.6% vs. 51.7%), they reported.
Compared with patients treated by higher-volume surgeons, those whose OAR was performed by very-low-volume surgeons had a twofold higher risk of postoperative death (6.7% vs. 3.5%) after adjusting for patient risk factors, surgeon specialty, and facility characteristics. Patients of very-low-volume surgeons also had significantly higher odds of sepsis or shock (odds ratio, 1.45), prolonged length of stay (OR, 1.37) and 30-day readmission (OR, 1.19), although the latter was not significant.
Similarly, patients whose CEA was performed by very-low-volume surgeons had a significant 1.8-fold higher odds of experiencing postoperative acute myocardial infarction (1.5% vs. 0.5%) and stroke (3.5% vs. 2.1%). They also were significantly more likely to have 30-day readmission (OR, 1.30).
With both procedures, patients treated by very-low-volume surgeons tended to be younger and healthier (less likely to have two or more comorbidities). They also were more likely to be nonwhite or insured by Medicaid.
“It is reasonable to speculate,” the researchers wrote, “that those treated by very-low-volume surgeons were more likely to be socioeconomically disadvantaged.”
Notably, 30% of the very-low-volume practice occurred in New York City, “where accessibility to high-volume practitioners should generally be higher,” they said.
The findings “indicate the need to eliminate this type of practice, to restrict the practice of these very-low-volume surgeons or to force referrals to higher-volume and specialized surgeons, and to improve disparity in access to high-quality care for all patients,” they said.
The study was funded in part by the U.S. Food and Drug Administration. The researchers reported having no relevant conflicts of interest.
The delineation of a threshold number of cases of OAR and CEA below which surgeons should not be credentialed remains unclear, despite much discussion of the volume-outcome relationship in vascular surgery.
In the current endovascular era, OAR in particular has become increasingly less frequent, with a dramatic effect on trainee experiences. It is often proposed that these cases be limited to high-volume surgeons. Some are concerned, however, that this action will leave rural surgeons unprepared to deal with ruptured abdominal aortic aneurysm and will force patients to travel long distances.
Sarah E. Deery, MD, and Marc L. Schermerhorn, MD, are in the division of vascular and endovascular surgery at Beth Israel Deaconess Medical Center in Boston. These remarks are adapted from an editorial accompanying the study. They reported having no disclosures.
The delineation of a threshold number of cases of OAR and CEA below which surgeons should not be credentialed remains unclear, despite much discussion of the volume-outcome relationship in vascular surgery.
In the current endovascular era, OAR in particular has become increasingly less frequent, with a dramatic effect on trainee experiences. It is often proposed that these cases be limited to high-volume surgeons. Some are concerned, however, that this action will leave rural surgeons unprepared to deal with ruptured abdominal aortic aneurysm and will force patients to travel long distances.
Sarah E. Deery, MD, and Marc L. Schermerhorn, MD, are in the division of vascular and endovascular surgery at Beth Israel Deaconess Medical Center in Boston. These remarks are adapted from an editorial accompanying the study. They reported having no disclosures.
The delineation of a threshold number of cases of OAR and CEA below which surgeons should not be credentialed remains unclear, despite much discussion of the volume-outcome relationship in vascular surgery.
In the current endovascular era, OAR in particular has become increasingly less frequent, with a dramatic effect on trainee experiences. It is often proposed that these cases be limited to high-volume surgeons. Some are concerned, however, that this action will leave rural surgeons unprepared to deal with ruptured abdominal aortic aneurysm and will force patients to travel long distances.
Sarah E. Deery, MD, and Marc L. Schermerhorn, MD, are in the division of vascular and endovascular surgery at Beth Israel Deaconess Medical Center in Boston. These remarks are adapted from an editorial accompanying the study. They reported having no disclosures.
The very-low-volume practice of surgeons performing no more than one open abdominal aortic aneurysm repair (OAR) or carotid endarterectomy (CEA) per year has persisted in New York State and was associated with worse postoperative outcomes and longer lengths of stay in a cohort study of statewide hospital data.
The study examined inpatient data on elective OARs and CEAs performed from 2000 to 2014 in every hospital in the state.
While the numbers and proportions of very-low-volume surgeons decreased (44.6%-23% for OAR and 35.2%-18.1% for CEA) and the number of procedures performed by these surgeons also decreased (QAR, 346-47; CEA, 395-90), the data are “concerning” and elucidate the “persistence” of very-low-volume practice in open vascular surgery, said Jialin Mao, MD, of Cornell University, New York, and associates (JAMA Surg. doi: 10:1001/jamasurg.2017.1100).
Very-low-volume surgeons were significantly less likely to be vascular surgeons, compared with higher-volume surgeons for both OAR (23.9% vs. 63.9%) and CEA (14.6% vs. 51.7%), they reported.
Compared with patients treated by higher-volume surgeons, those whose OAR was performed by very-low-volume surgeons had a twofold higher risk of postoperative death (6.7% vs. 3.5%) after adjusting for patient risk factors, surgeon specialty, and facility characteristics. Patients of very-low-volume surgeons also had significantly higher odds of sepsis or shock (odds ratio, 1.45), prolonged length of stay (OR, 1.37) and 30-day readmission (OR, 1.19), although the latter was not significant.
Similarly, patients whose CEA was performed by very-low-volume surgeons had a significant 1.8-fold higher odds of experiencing postoperative acute myocardial infarction (1.5% vs. 0.5%) and stroke (3.5% vs. 2.1%). They also were significantly more likely to have 30-day readmission (OR, 1.30).
With both procedures, patients treated by very-low-volume surgeons tended to be younger and healthier (less likely to have two or more comorbidities). They also were more likely to be nonwhite or insured by Medicaid.
“It is reasonable to speculate,” the researchers wrote, “that those treated by very-low-volume surgeons were more likely to be socioeconomically disadvantaged.”
Notably, 30% of the very-low-volume practice occurred in New York City, “where accessibility to high-volume practitioners should generally be higher,” they said.
The findings “indicate the need to eliminate this type of practice, to restrict the practice of these very-low-volume surgeons or to force referrals to higher-volume and specialized surgeons, and to improve disparity in access to high-quality care for all patients,” they said.
The study was funded in part by the U.S. Food and Drug Administration. The researchers reported having no relevant conflicts of interest.
The very-low-volume practice of surgeons performing no more than one open abdominal aortic aneurysm repair (OAR) or carotid endarterectomy (CEA) per year has persisted in New York State and was associated with worse postoperative outcomes and longer lengths of stay in a cohort study of statewide hospital data.
The study examined inpatient data on elective OARs and CEAs performed from 2000 to 2014 in every hospital in the state.
While the numbers and proportions of very-low-volume surgeons decreased (44.6%-23% for OAR and 35.2%-18.1% for CEA) and the number of procedures performed by these surgeons also decreased (QAR, 346-47; CEA, 395-90), the data are “concerning” and elucidate the “persistence” of very-low-volume practice in open vascular surgery, said Jialin Mao, MD, of Cornell University, New York, and associates (JAMA Surg. doi: 10:1001/jamasurg.2017.1100).
Very-low-volume surgeons were significantly less likely to be vascular surgeons, compared with higher-volume surgeons for both OAR (23.9% vs. 63.9%) and CEA (14.6% vs. 51.7%), they reported.
Compared with patients treated by higher-volume surgeons, those whose OAR was performed by very-low-volume surgeons had a twofold higher risk of postoperative death (6.7% vs. 3.5%) after adjusting for patient risk factors, surgeon specialty, and facility characteristics. Patients of very-low-volume surgeons also had significantly higher odds of sepsis or shock (odds ratio, 1.45), prolonged length of stay (OR, 1.37) and 30-day readmission (OR, 1.19), although the latter was not significant.
Similarly, patients whose CEA was performed by very-low-volume surgeons had a significant 1.8-fold higher odds of experiencing postoperative acute myocardial infarction (1.5% vs. 0.5%) and stroke (3.5% vs. 2.1%). They also were significantly more likely to have 30-day readmission (OR, 1.30).
With both procedures, patients treated by very-low-volume surgeons tended to be younger and healthier (less likely to have two or more comorbidities). They also were more likely to be nonwhite or insured by Medicaid.
“It is reasonable to speculate,” the researchers wrote, “that those treated by very-low-volume surgeons were more likely to be socioeconomically disadvantaged.”
Notably, 30% of the very-low-volume practice occurred in New York City, “where accessibility to high-volume practitioners should generally be higher,” they said.
The findings “indicate the need to eliminate this type of practice, to restrict the practice of these very-low-volume surgeons or to force referrals to higher-volume and specialized surgeons, and to improve disparity in access to high-quality care for all patients,” they said.
The study was funded in part by the U.S. Food and Drug Administration. The researchers reported having no relevant conflicts of interest.
FROM JAMA SURGERY
Key clinical point: The very-low-volume practice of open abdominal aortic aneurysm repair and carotid endarterectomy (one or fewer annual procedures) is associated with worse postoperative outcomes and greater length of stay.
Major finding: Patients whose procedure was performed by very-low-volume surgeons had a twofold higher risk of postoperative death after OAR or 1.8-fold higher odds of experiencing postoperative acute myocardial infarction or stroke after CEA.
Data source: The study was funded in part by the U.S. Food and Drug Administration.
Disclosures: The researchers reported having no relevant conflicts of interest.