User login
Cerebral blood flow may predict children’s recovery from persistent postconcussion symptoms
, according to a study presented at the 2020 CNS-ICNA Conjoint Meeting, held virtually this year. Furthermore, cerebral blood flow at 4-6 weeks predicts recovery during the next 4 weeks in 77% of children.
“This is the first study to examine cerebral blood flow changes in children with persistent postconcussion symptoms,” said Karen Barlow, MBChB, associate professor of biomedical sciences at the University of Queensland in St. Lucia, Australia. “Our findings support the link between neurovascular unit dysfunction and persistent postconcussion symptoms in children, potentially because of injury or dysfunction in the GABAergic interneurons.”
Quantifying cerebral tissue perfusion
At least 25% of children with concussion have persistent postconcussion symptoms at 1 month post injury. Understanding the factors that influence the speed of recovery may help clarify the biology of postconcussion symptoms and suggest new treatments. In previous research, Dr. Barlow and colleagues found that children with early recovery (i.e., recovery by 4 weeks post injury) have decreases in cerebral blood flow, when compared with normal children. Children with persistent symptoms, however, have increases in cerebral blood flow. Dr. Barlow and colleagues conducted a new study to examine how cerebral blood flow changes in children with persistent postconcussion symptoms.
The investigators recruited participants through the randomized controlled Play Game trial, which examined melatonin as a treatment for persistent postconcussion symptoms. Among the exclusion criteria were history of assault, drug or alcohol use, significant past medical or psychiatric history, concussion within the previous 3 months, and use of psychoactive medications.
Children entered the study at 4-8 weeks after injury and received treatment for 4 weeks. Participants underwent 3-D pseudo-continuous arterial spin–labeled MRI before and after the treatment period (i.e., at 5 and 10 weeks post injury). This imaging technique provides a quantitative assessment of cerebral tissue perfusion. “You can do it without manipulating the cerebral circulation, making it particularly useful for research and in children,” said Dr. Barlow.
She and her colleagues evaluated recovery using the Post-Concussion Symptom Inventory. They defined good recovery as a total score at or below baseline at 10 weeks post injury. They considered any children who did not meet this criterion to have poor recovery.
Speed of blood-flow change varied
In all, 124 children were eligible for the study, and 76 had MRIs at both time points. Fourteen participants were excluded because of motion artifacts, slice truncation, and normalization failure. The population’s average age was approximately 14 years. About half of participants were males. The first MRI was performed at 37 days post injury, and the second MRI at around 70 days post injury. Twenty-three children had good recovery.
Children with poor recovery at 10 weeks had higher relative cerebral blood flow, compared with children with good recovery. Treatment group, age, and sex did not affect the changes in relative cerebral blood flow over time. Dr. Barlow and colleagues also measured mean total gray matter cerebral blood flow. Children with poor recovery had higher cerebral blood flow at 5 and 10 weeks post injury, compared with children with good recovery. In addition, cerebral blood flow changed more slowly in participants with poor recovery, compared with those with good recovery. Logistic regression analysis indicated that the mean absolute gray matter cerebral blood flow at 4-6 weeks post injury significantly predicted which children would recover by 10 weeks post injury, with an area under the receiver operating characteristic curve of 77%.
Funders for the study included Alberta Children’s Hospital, the Canadian Institutes of Health Research, and the University of Calgary. Dr. Barlow had no disclosures or conflicts of interest.
SOURCE: Barlow K et al. CNS-ICNA 2020. Abstract PL100.
, according to a study presented at the 2020 CNS-ICNA Conjoint Meeting, held virtually this year. Furthermore, cerebral blood flow at 4-6 weeks predicts recovery during the next 4 weeks in 77% of children.
“This is the first study to examine cerebral blood flow changes in children with persistent postconcussion symptoms,” said Karen Barlow, MBChB, associate professor of biomedical sciences at the University of Queensland in St. Lucia, Australia. “Our findings support the link between neurovascular unit dysfunction and persistent postconcussion symptoms in children, potentially because of injury or dysfunction in the GABAergic interneurons.”
Quantifying cerebral tissue perfusion
At least 25% of children with concussion have persistent postconcussion symptoms at 1 month post injury. Understanding the factors that influence the speed of recovery may help clarify the biology of postconcussion symptoms and suggest new treatments. In previous research, Dr. Barlow and colleagues found that children with early recovery (i.e., recovery by 4 weeks post injury) have decreases in cerebral blood flow, when compared with normal children. Children with persistent symptoms, however, have increases in cerebral blood flow. Dr. Barlow and colleagues conducted a new study to examine how cerebral blood flow changes in children with persistent postconcussion symptoms.
The investigators recruited participants through the randomized controlled Play Game trial, which examined melatonin as a treatment for persistent postconcussion symptoms. Among the exclusion criteria were history of assault, drug or alcohol use, significant past medical or psychiatric history, concussion within the previous 3 months, and use of psychoactive medications.
Children entered the study at 4-8 weeks after injury and received treatment for 4 weeks. Participants underwent 3-D pseudo-continuous arterial spin–labeled MRI before and after the treatment period (i.e., at 5 and 10 weeks post injury). This imaging technique provides a quantitative assessment of cerebral tissue perfusion. “You can do it without manipulating the cerebral circulation, making it particularly useful for research and in children,” said Dr. Barlow.
She and her colleagues evaluated recovery using the Post-Concussion Symptom Inventory. They defined good recovery as a total score at or below baseline at 10 weeks post injury. They considered any children who did not meet this criterion to have poor recovery.
Speed of blood-flow change varied
In all, 124 children were eligible for the study, and 76 had MRIs at both time points. Fourteen participants were excluded because of motion artifacts, slice truncation, and normalization failure. The population’s average age was approximately 14 years. About half of participants were males. The first MRI was performed at 37 days post injury, and the second MRI at around 70 days post injury. Twenty-three children had good recovery.
Children with poor recovery at 10 weeks had higher relative cerebral blood flow, compared with children with good recovery. Treatment group, age, and sex did not affect the changes in relative cerebral blood flow over time. Dr. Barlow and colleagues also measured mean total gray matter cerebral blood flow. Children with poor recovery had higher cerebral blood flow at 5 and 10 weeks post injury, compared with children with good recovery. In addition, cerebral blood flow changed more slowly in participants with poor recovery, compared with those with good recovery. Logistic regression analysis indicated that the mean absolute gray matter cerebral blood flow at 4-6 weeks post injury significantly predicted which children would recover by 10 weeks post injury, with an area under the receiver operating characteristic curve of 77%.
Funders for the study included Alberta Children’s Hospital, the Canadian Institutes of Health Research, and the University of Calgary. Dr. Barlow had no disclosures or conflicts of interest.
SOURCE: Barlow K et al. CNS-ICNA 2020. Abstract PL100.
, according to a study presented at the 2020 CNS-ICNA Conjoint Meeting, held virtually this year. Furthermore, cerebral blood flow at 4-6 weeks predicts recovery during the next 4 weeks in 77% of children.
“This is the first study to examine cerebral blood flow changes in children with persistent postconcussion symptoms,” said Karen Barlow, MBChB, associate professor of biomedical sciences at the University of Queensland in St. Lucia, Australia. “Our findings support the link between neurovascular unit dysfunction and persistent postconcussion symptoms in children, potentially because of injury or dysfunction in the GABAergic interneurons.”
Quantifying cerebral tissue perfusion
At least 25% of children with concussion have persistent postconcussion symptoms at 1 month post injury. Understanding the factors that influence the speed of recovery may help clarify the biology of postconcussion symptoms and suggest new treatments. In previous research, Dr. Barlow and colleagues found that children with early recovery (i.e., recovery by 4 weeks post injury) have decreases in cerebral blood flow, when compared with normal children. Children with persistent symptoms, however, have increases in cerebral blood flow. Dr. Barlow and colleagues conducted a new study to examine how cerebral blood flow changes in children with persistent postconcussion symptoms.
The investigators recruited participants through the randomized controlled Play Game trial, which examined melatonin as a treatment for persistent postconcussion symptoms. Among the exclusion criteria were history of assault, drug or alcohol use, significant past medical or psychiatric history, concussion within the previous 3 months, and use of psychoactive medications.
Children entered the study at 4-8 weeks after injury and received treatment for 4 weeks. Participants underwent 3-D pseudo-continuous arterial spin–labeled MRI before and after the treatment period (i.e., at 5 and 10 weeks post injury). This imaging technique provides a quantitative assessment of cerebral tissue perfusion. “You can do it without manipulating the cerebral circulation, making it particularly useful for research and in children,” said Dr. Barlow.
She and her colleagues evaluated recovery using the Post-Concussion Symptom Inventory. They defined good recovery as a total score at or below baseline at 10 weeks post injury. They considered any children who did not meet this criterion to have poor recovery.
Speed of blood-flow change varied
In all, 124 children were eligible for the study, and 76 had MRIs at both time points. Fourteen participants were excluded because of motion artifacts, slice truncation, and normalization failure. The population’s average age was approximately 14 years. About half of participants were males. The first MRI was performed at 37 days post injury, and the second MRI at around 70 days post injury. Twenty-three children had good recovery.
Children with poor recovery at 10 weeks had higher relative cerebral blood flow, compared with children with good recovery. Treatment group, age, and sex did not affect the changes in relative cerebral blood flow over time. Dr. Barlow and colleagues also measured mean total gray matter cerebral blood flow. Children with poor recovery had higher cerebral blood flow at 5 and 10 weeks post injury, compared with children with good recovery. In addition, cerebral blood flow changed more slowly in participants with poor recovery, compared with those with good recovery. Logistic regression analysis indicated that the mean absolute gray matter cerebral blood flow at 4-6 weeks post injury significantly predicted which children would recover by 10 weeks post injury, with an area under the receiver operating characteristic curve of 77%.
Funders for the study included Alberta Children’s Hospital, the Canadian Institutes of Health Research, and the University of Calgary. Dr. Barlow had no disclosures or conflicts of interest.
SOURCE: Barlow K et al. CNS-ICNA 2020. Abstract PL100.
FROM CNS-ICNA 2020
Blood biomarkers could help predict when athletes recover from concussions
, according to a new study of collegiate athletes and recovery time. “Although preliminary, the current results highlight the potential role of biomarkers in tracking neuronal recovery, which may be associated with duration of [return to sport],” wrote Cassandra L. Pattinson, PhD, of the University of Queensland, Brisbane, Australia, and the National Institutes of Health, Bethesda, Md., along with coauthors. The study was published in JAMA Network Open.
To determine if three specific blood biomarkers – total tau protein, glial fibrillary acidic protein (GFAP), and neurofilament light chain protein (NfL) – can help predict when athletes should return from sports-related concussions, a multicenter, prospective diagnostic study was launched and led by the Advanced Research Core (ARC) of the Concussion Assessment, Research, and Education (CARE) Consortium. The consortium is a joint effort of the National Collegiate Athletics Association (NCAA) and the U.S. Department of Defense.
From among the CARE ARC database, researchers evaluated 127 eligible student athletes who had experienced a sports-related concussion, underwent clinical testing and blood collection before and after their injuries, and returned to their sports. Their average age was 18.9 years old, 76% were men, and 65% were White. Biomarker levels were measured from nonfasting blood samples via ultrasensitive single molecule array technology. As current NCAA guidelines indicate that most athletes will be asymptomatic roughly 2 weeks after a concussion, the study used 14 days as a cutoff period.
Among the 127 athletes, the median return-to-sport time was 14 days; 65 returned to their sports in less than 14 days while 62 returned to their sports in 14 days or more. According to the study’s linear mixed models, athletes with a return-to-sport time of 14 days or longer had significantly higher total tau levels at 24-48 hours post injury (mean difference –0.51 pg/mL, 95% confidence interval, –0.88 to –0.14; P = .008) and when symptoms had resolved (mean difference –0.71 pg/mL, 95% CI, –1.09 to –0.34; P < .001) compared with athletes with a return-to-sport time of less than 14 days. Athletes who returned in 14 days or more also had comparatively lower levels of GFAP postinjury than did those who returned in under 14 days (4.39 pg/mL versus 4.72 pg/mL; P = .04).
Preliminary steps toward an appropriate point-of-care test
“This particular study is one of several emerging studies on what these biomarkers look like,” Brian W. Hainline, MD, chief medical officer of the NCAA, said in an interview. “It’s all still very preliminary – you couldn’t make policy changes based on what we have – but the data is accumulating. Ultimately, we should be able to perform a multivariate analysis of all the different objective biomarkers, looking at repetitive head impact exposure, looking at imaging, looking at these blood-based biomarkers. Then you can say, ‘OK, what can we do? Can we actually predict recovery, who is likely or less likely to do well?’ ”
“It’s not realistic to be taking blood samples all the time,” said Dr. Hainline, who was not involved in the study. “Another goal, once we know which biomarkers are valuable, is to convert to a point-of-care test. You get a finger prick or even a salivary test and we get the result immediately; that’s the direction that all of this is heading. But first, we have to lay out the groundwork. We envision a day, in the not too distant future, where we can get this information much more quickly.”
The authors acknowledged their study’s limitations, including an inability to standardize the time of biomarker collection and the fact that they analyzed a “relatively small number of athletes” who met their specific criteria. That said, they emphasized that their work is based on “the largest prospective sample of sports-related concussions in athletes to date” and that they “anticipate that we will be able to continue to gather a more representative sample” in the future to better generalize to the larger collegiate community.
The study was supported by the Grand Alliance Concussion Assessment, Research, and Education Consortium, which was funded in part by the NCAA and the Department of Defense. The authors disclosed receiving grants and travel reimbursements from – or working as advisers or consultants for – various organizations, college programs, and sports leagues.
SOURCE: Pattinson CL, et al. JAMA Netw Open. 2020 Aug 27. doi: 10.1001/jamanetworkopen.2020.13191.
, according to a new study of collegiate athletes and recovery time. “Although preliminary, the current results highlight the potential role of biomarkers in tracking neuronal recovery, which may be associated with duration of [return to sport],” wrote Cassandra L. Pattinson, PhD, of the University of Queensland, Brisbane, Australia, and the National Institutes of Health, Bethesda, Md., along with coauthors. The study was published in JAMA Network Open.
To determine if three specific blood biomarkers – total tau protein, glial fibrillary acidic protein (GFAP), and neurofilament light chain protein (NfL) – can help predict when athletes should return from sports-related concussions, a multicenter, prospective diagnostic study was launched and led by the Advanced Research Core (ARC) of the Concussion Assessment, Research, and Education (CARE) Consortium. The consortium is a joint effort of the National Collegiate Athletics Association (NCAA) and the U.S. Department of Defense.
From among the CARE ARC database, researchers evaluated 127 eligible student athletes who had experienced a sports-related concussion, underwent clinical testing and blood collection before and after their injuries, and returned to their sports. Their average age was 18.9 years old, 76% were men, and 65% were White. Biomarker levels were measured from nonfasting blood samples via ultrasensitive single molecule array technology. As current NCAA guidelines indicate that most athletes will be asymptomatic roughly 2 weeks after a concussion, the study used 14 days as a cutoff period.
Among the 127 athletes, the median return-to-sport time was 14 days; 65 returned to their sports in less than 14 days while 62 returned to their sports in 14 days or more. According to the study’s linear mixed models, athletes with a return-to-sport time of 14 days or longer had significantly higher total tau levels at 24-48 hours post injury (mean difference –0.51 pg/mL, 95% confidence interval, –0.88 to –0.14; P = .008) and when symptoms had resolved (mean difference –0.71 pg/mL, 95% CI, –1.09 to –0.34; P < .001) compared with athletes with a return-to-sport time of less than 14 days. Athletes who returned in 14 days or more also had comparatively lower levels of GFAP postinjury than did those who returned in under 14 days (4.39 pg/mL versus 4.72 pg/mL; P = .04).
Preliminary steps toward an appropriate point-of-care test
“This particular study is one of several emerging studies on what these biomarkers look like,” Brian W. Hainline, MD, chief medical officer of the NCAA, said in an interview. “It’s all still very preliminary – you couldn’t make policy changes based on what we have – but the data is accumulating. Ultimately, we should be able to perform a multivariate analysis of all the different objective biomarkers, looking at repetitive head impact exposure, looking at imaging, looking at these blood-based biomarkers. Then you can say, ‘OK, what can we do? Can we actually predict recovery, who is likely or less likely to do well?’ ”
“It’s not realistic to be taking blood samples all the time,” said Dr. Hainline, who was not involved in the study. “Another goal, once we know which biomarkers are valuable, is to convert to a point-of-care test. You get a finger prick or even a salivary test and we get the result immediately; that’s the direction that all of this is heading. But first, we have to lay out the groundwork. We envision a day, in the not too distant future, where we can get this information much more quickly.”
The authors acknowledged their study’s limitations, including an inability to standardize the time of biomarker collection and the fact that they analyzed a “relatively small number of athletes” who met their specific criteria. That said, they emphasized that their work is based on “the largest prospective sample of sports-related concussions in athletes to date” and that they “anticipate that we will be able to continue to gather a more representative sample” in the future to better generalize to the larger collegiate community.
The study was supported by the Grand Alliance Concussion Assessment, Research, and Education Consortium, which was funded in part by the NCAA and the Department of Defense. The authors disclosed receiving grants and travel reimbursements from – or working as advisers or consultants for – various organizations, college programs, and sports leagues.
SOURCE: Pattinson CL, et al. JAMA Netw Open. 2020 Aug 27. doi: 10.1001/jamanetworkopen.2020.13191.
, according to a new study of collegiate athletes and recovery time. “Although preliminary, the current results highlight the potential role of biomarkers in tracking neuronal recovery, which may be associated with duration of [return to sport],” wrote Cassandra L. Pattinson, PhD, of the University of Queensland, Brisbane, Australia, and the National Institutes of Health, Bethesda, Md., along with coauthors. The study was published in JAMA Network Open.
To determine if three specific blood biomarkers – total tau protein, glial fibrillary acidic protein (GFAP), and neurofilament light chain protein (NfL) – can help predict when athletes should return from sports-related concussions, a multicenter, prospective diagnostic study was launched and led by the Advanced Research Core (ARC) of the Concussion Assessment, Research, and Education (CARE) Consortium. The consortium is a joint effort of the National Collegiate Athletics Association (NCAA) and the U.S. Department of Defense.
From among the CARE ARC database, researchers evaluated 127 eligible student athletes who had experienced a sports-related concussion, underwent clinical testing and blood collection before and after their injuries, and returned to their sports. Their average age was 18.9 years old, 76% were men, and 65% were White. Biomarker levels were measured from nonfasting blood samples via ultrasensitive single molecule array technology. As current NCAA guidelines indicate that most athletes will be asymptomatic roughly 2 weeks after a concussion, the study used 14 days as a cutoff period.
Among the 127 athletes, the median return-to-sport time was 14 days; 65 returned to their sports in less than 14 days while 62 returned to their sports in 14 days or more. According to the study’s linear mixed models, athletes with a return-to-sport time of 14 days or longer had significantly higher total tau levels at 24-48 hours post injury (mean difference –0.51 pg/mL, 95% confidence interval, –0.88 to –0.14; P = .008) and when symptoms had resolved (mean difference –0.71 pg/mL, 95% CI, –1.09 to –0.34; P < .001) compared with athletes with a return-to-sport time of less than 14 days. Athletes who returned in 14 days or more also had comparatively lower levels of GFAP postinjury than did those who returned in under 14 days (4.39 pg/mL versus 4.72 pg/mL; P = .04).
Preliminary steps toward an appropriate point-of-care test
“This particular study is one of several emerging studies on what these biomarkers look like,” Brian W. Hainline, MD, chief medical officer of the NCAA, said in an interview. “It’s all still very preliminary – you couldn’t make policy changes based on what we have – but the data is accumulating. Ultimately, we should be able to perform a multivariate analysis of all the different objective biomarkers, looking at repetitive head impact exposure, looking at imaging, looking at these blood-based biomarkers. Then you can say, ‘OK, what can we do? Can we actually predict recovery, who is likely or less likely to do well?’ ”
“It’s not realistic to be taking blood samples all the time,” said Dr. Hainline, who was not involved in the study. “Another goal, once we know which biomarkers are valuable, is to convert to a point-of-care test. You get a finger prick or even a salivary test and we get the result immediately; that’s the direction that all of this is heading. But first, we have to lay out the groundwork. We envision a day, in the not too distant future, where we can get this information much more quickly.”
The authors acknowledged their study’s limitations, including an inability to standardize the time of biomarker collection and the fact that they analyzed a “relatively small number of athletes” who met their specific criteria. That said, they emphasized that their work is based on “the largest prospective sample of sports-related concussions in athletes to date” and that they “anticipate that we will be able to continue to gather a more representative sample” in the future to better generalize to the larger collegiate community.
The study was supported by the Grand Alliance Concussion Assessment, Research, and Education Consortium, which was funded in part by the NCAA and the Department of Defense. The authors disclosed receiving grants and travel reimbursements from – or working as advisers or consultants for – various organizations, college programs, and sports leagues.
SOURCE: Pattinson CL, et al. JAMA Netw Open. 2020 Aug 27. doi: 10.1001/jamanetworkopen.2020.13191.
FROM JAMA NETWORK OPEN
Mild TBI/Concussion Clinical Tools for Providers Used Within the Department of Defense and Defense Health Agency
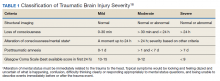
Traumatic brain injury (TBI) is a major health concern that can cause significant disability as well as economic and social burden. The Centers for Disease Control and Prevention (CDC) reported a 58% increase in the number of TBI-related emergency department visits, hospitalizations, and deaths from 2006 to 2014.1 In the CDC report, falls and motor vehicle accidents accounted for 52.3% and 20.4%, respectively, of all civilian TBI-related hospitalizations. In 2014, 56,800 TBIs in the US resulted in death. A large proportion of severe TBI survivors continue to experience long-term physical, cognitive, and psychologic disorders and require extensive rehabilitation, which may disrupt relationships and prevent return to work.2 About 37% of people with mild TBI (mTBI) cases and 51% of severe cases were unable to return to previous jobs. A study examining psychosocial burden found that people with a history of TBI reported greater feelings of loneliness compared with individuals without TBI.3
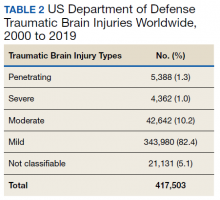
Within the US military, the Defense and Veterans Brain Injury Center (DVBIC) indicates that > 417,503 service members (SMs) have been diagnosed with TBI since November 2000.4 Of these, 82.4% were classified as having a mTBI, or concussion (Tables 1 and 2). The nature of combat and military training to which SMs are routinely exposed may increase the risk for sustaining a TBI. Specifically, the increased use of improvised explosives devices by enemy combatants in the recent military conflicts (ie, Operation Enduring Freedom, Operation Iraqi Freedom and Operation New Dawn) resulted in TBI being recognized as the signature injury of these conflicts and brought attention to the prevalence of concussion within the US military.5,6 In the military, the effects of concussion can decrease individual and unit effectiveness, emphasizing the importance of prompt diagnosis and proper management.7
Typically, patients recover from concussion within a few weeks of injury; however, some individuals experience symptoms that persist for months or years. Studies found that early intervention after concussion may aid in expediting recovery, stressing the importance of identifying concussion as promptly as possible.8,9 Active treatment is centered on patient education and symptom management, in addition to a progressive return to activities, as tolerated. Patient education may help validate the symptoms of some patients, as well as help to reattribute the symptoms to benign causes, leading to better outcomes.10 Since TBI is such a relevant health concern within the DoD, it is paramount for practitioners to understand what resources are available in order to identify and initiate treatment expeditiously.
This article focuses on the clinical tools used in evaluating and treating concussion, and best practices treatment guidelines for health care providers (HCPs) who are required to evaluate and treat military populations. While these resources are used for military SMs, they can also be used in veteran and civilian populations. This article showcases 3 DoD clinical tools that assist HCPs in evaluating and treating patients with TBI: (1) the Military Acute Concussion Evaluation 2 (MACE 2); (2) the Progressive Return to Activity (PRA) Clinical Recommendation (CR); and (3) the Concussion Management Tool (CMT). Additional DoD clinical tools and resources are discussed, and resources and links for the practitioner are provided for easy access and reference.
Military Acute Concussion Evaluation 2
Early concussion identification and evaluation are important steps in the treatment process to ensure timely recovery and return to duty for SMs. As such, DVBIC assembled a working group of military and civilian brain injury experts to create an evidence-based clinical practice guideline for the assessment and management of concussion in a military operational setting that could be learned and effectively used by corpsmen and combat medics in the battlefield to screen for a possible concussion.7 This team created the first version of the MACE, a clinical tool that prompted a systematic assessment of concussion related symptoms, neurologic signs, and cognitive deficits. The cognitive assessment portion was based on the standardized assessment of concussion (SAC) that had been reported by McCrea and colleagues in 1998.11 Soon after its creation, field utilization of the MACE for screening of concussion was mandated by the Army through an All Army Action (ALARACT 178/2008) and for all of the Services through the DoD Instruction (DoDI) 6490.11 published in 2014.12
The MACE has been updated several times since the original version. Most recently, the MACE was revised in 2018 to include a vestibular oculomotor assessment section, and red flags that immediately alert the HCP to the need for immediate triage referral and treatment of the patient possibly at a higher echelon of care or with more emergent evaluation.13-15 Additionally, the neurologic examination was expanded to increase clarity and comprehensiveness, including speech and balance testing. Updates made to the tool were intended to provide a more thorough and informative evaluation of the SM with suspected concussion.
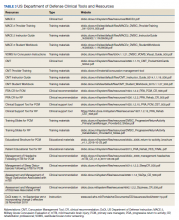
This latest version, MACE 2, is designed to be used by any HCP who is treating SMs with a suspected or potential TBI, not just corpsmen and combat medics in theater. The MACE 2 is a comprehensive evaluation within a set of portable pocket cards designed to assist end-users in the proper triage of potentially concussed individuals. The DoD has specified 4 events that require a MACE 2 evaluation: (1) SM was in a vehicle associated with a blast event, collision, or roll over; (2) SM was within 50 meters of a blast; (3) anyone who sustained a direct blow to the head; or (4) when command provides direction (eg, repeated exposures to the events above or in accordance with protocols).12 Sleep deprivation, medications, and pain may affect MACE 2 results, in addition to deployment related stress, chronic stress, high adrenaline sustained over time, and additional comorbidities. This tool is most effective when used as close to the time of injury as possible but also may be used later (after 24 hours of rest) to reevaluate symptoms. The MACE 2 Instructor Guide, a student workbook, HCP training, and Vestibular/Ocular-Motor Screening (VOMS) for Concussion instructions can be found on the DVBIC website (Table 3).
Description
The MACE 2 is a brief multimodal screening tool that assists medics, corpsman, and primary care managers (PCMs) in the assessment and identification of a potential concussion (Figure 1). Embedded in the MACE 2 is the Standardized Assessment of Concussion (SAC), a well-validated sports concussion tool, and the VOMS tool as portions of the 2-part cognitive examination. The entirety of the tool has 5 sections: (1) red flags; (2) acute concussion screening; (3) cognitive examination, part 1; (4) neurologic examination; and (5) cognitive examination, part 2. The end of the MACE 2 includes sections on the scoring, instructions for International Classification of Diseases, Tenth Revision, TBI coding, and next steps following completion of the MACE 2. The latest version of this screening tool impacts TBI care in several noteworthy ways. First, it broadens the scope of users by expanding use to all medically trained personnel, allowing any provider to treat SMs in the field. Second, it combines state-of-the-science advances from the research field and reflects feedback from end-users collected during the development. Last, the MACE 2 is updated as changes in the field occur, and is currently undergoing research to better identify end-user utility and usability.
Screening Tools
• Red Flags. The red flags section aids in identifying potentially serious underlying conditions in patients presenting with Glasgow Coma Scale (GCS) between 13 and 15. A positive red flag prompts the practitioner to stop administering the MACE 2 and immediately consult a higher level of care and consider urgent evacuation. While the red flags are completed first, and advancement to later sections of the MACE 2 is dependent upon the absence of red flags, the red flags should be monitored throughout the completion of the MACE 2. Upon completion of patient demographics and red flags, the remaining sections of the MACE 2 are dedicated to acute concussion screening.
• Acute Concussion Screening. The acute concussion screening portion consists of 4 sections: description of the incident; alteration of consciousness or memory; a “check all that apply” symptom inventory; and a patient history that includes concussions within the past 12 months, headache disorders, and/or behavioral health concerns. The final portion of the acute concussion screening section provides an algorithm to identify a positive or negative concussion screen. When a negative screen is identified, the user is prompted to prescribe a 24-hour rest period and follow up with the SM based on the guidance in the CMT. A positive screen warrants the user to continue administration of the MACE 2.
Neurologic and CognitiveExaminations
• Cognitive Exam Part 1. The initial cognitive examination is designed to assess orientation to time (eg, What is the day of the week, day of the month, the month, the year, and the timeof day?) as well as immediate recall of a short list of concrete words (5 words total, repeated for 3 trials). These tests are based on other neuropsychological measures designed to assess cognitive/mental status and short-term memory.
• The Neurological Exam. The neurological exam section of the MACE 2 includes brief neuropsychologic tests such as speech fluency and word finding. Other sections within the neurological exam assess the
following: grip strength, vestibular function/balance (eg, tandem gait and single leg stance), as well as motor function (eg, pronator drift), autonomic nervous system function (eg, pupil response), and vestibular function (eye-tracking).
• Cognitive Exam Part 2. After completion of the first cognitive examination and the neurologic examination, the second part of the cognitive examination is initiated. Part 2 includes measures of short-term and working memory (eg, digits-reverse tasks, listing the months in reverse order, and a delayed recall task of the short list of concrete words presented in the first part). The final assessment is the administration of the VOMS, a tool developed from the sports concussion field and designed to measure vestibular-ocular function.13 It is critical to note that the VOMS is contraindicated if there is concern of an unstable cervical spine or absence of a trained HCP. An examination summary provides guidance on test scoring and yields a positive or negative indication for concussive injury. A positive test refers users to guidelines listed in the Concussion Management Tool for recommendations. The final page provides coding instructions for entering the results into the patient’s electronic medical record for documentation and future reference.
Progressive Return To Activities Clinical Recommendation
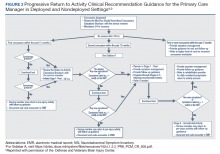
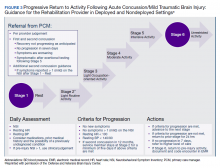
The Progressive Return to Activities Clinical Recommendation (PRA CR) also was developed by DVBIC for the DoD to assist military HCPs in managing SMs with concussion by providing systematic and evidence-based guidance to both prevent extended rest and promote return to full duty as quickly and safely as clinically indicated. The general guidance is to monitor the SM at each of the 6 stages in the process and safely and gradually increase activity to the next stage as tolerated. Daily symptoms are measured using the Neurobehavioral Symptom Inventory (NSI), which SMs self-administer every morning at each stage within the process.
Prior to initiation of the progressive return to activity, SM education using the educational brochure is strongly encouraged, as previous evidence suggests that it is an effective intervention during the acute stages of injury.10,11 Return to activity follows a 6 stage process, from stage 1 (rest) through stage 6 (unrestricted activity) (Table 4). Referral to rehabilitation providers (RPs) or higher care is left to the discretion of the PCM when (1) recovery is not progressing as anticipated; (2) progression is not being made within a 7-day period; or (3) symptoms worsen with time. The guidance outlined in the PRA CR is consistent with current policies and medical literature, and undergoes reviews as updates in the field emerge. The PRA for PCM, PRA for RP, Clinical Support Tool for PCM, Clinical Support Tool for RP, Training Slides for PCM, Training Slides for RP, Educational Brochure for PCM, and Patient Educational Tool for RP can be found on the DVBIC website (dvbic.dcoe.mil).
Description
To improve the clinical utility, 2 separate PRA CRs were developed specifically for PCMs (Figure 2) and RPs (Figure 3). The PRA CR for PCMs provides the initial framework to monitor SMs during recovery and gradually increase physical, cognitive, and vestibular/balance activities as symptoms improve in order to return to preinjury activities. The PRA CR for RPs outlines the approach for treating SMs who meet 1 of the following criteria: recovery is not progressing as anticipated, there is no progression in 7 days, symptoms are worsening, the SM is symptomatic after exertional testing following stage 5, or referral made per PCM judgment. Following the mandatory 24-hour rest period after a diagnosis of a concussion, progression through the PRA algorithm is based on history of concussion within the past 12 months (ie, 1, 2, or ≥ 3 concussions) and symptomatology, with varying treatment pathways depending on the SM’s responses to history and symptomology.
Guidelines
• One Concussion within Past 12 Months. Following the mandatory 24-hour rest period, if the SM is asymptomatic, then exertional testing (eg, activities such as push-ups, sit-ups, running in place, step aerobics, stationary bike, treadmill and/or hand crank) is performed at 65 to 85% of target heart rate for 2 minutes and symptoms are reassessed. If still asymptomatic, the SM may return to preinjury activity; however, if exertional testing provokes symptoms > 1 (mild) on the NSI, the SM should return to stage 1 with an additional 24 hours of rest. A second exertional test can be performed after stage 1, and if symptoms are provoked, progression through the remaining stages 2 to 5 is encouraged. Symptoms are continually monitored throughout each stage to determine whether the SM is recovered sufficiently to proceed to the next stage.
• Two Concussion Within Past 12 Months. Following the mandatory 24-hour rest period, no exertional testing is performed, and SMs move directly into stage 1 and remain at stage 1 or stage 2 for 7 consecutive days with no symptoms > 1 on the NSI before advancing through the remaining stages. Some defining features are longer rest periods (eg, 5 additional days of rest at stage 2) and additional patient education, symptom management, and follow-up.
• Three or more Concussions Within Past 12 Months. Following the 24 hour mandatory rest period, in cases where ≥ 3 concussions have occurred within a 12 month period, the recommendation is to provide guidance for symptom management rest and refer the SM to a higher level of care.
Concussion Management Tool
Beyond the initial assessment and concussion evaluation and the promotion of SMs’ timely return to duty, the DoD developed a tool to help endpoint users manage concussion, to include those with more protracted symptoms (Figure 4). The CMT assists HCPs and the SMs they treat in the management of symptoms before and after they return to duty. Specifically, the CMT is designed to be given in combination with guidelines issued by the DoD in the PRA CR but extends management of concussion to include those symptoms experienced more long-term, or symptoms that are not solely addressed during the timeline of the PRA CR. Together, the MACE 2, PRA CR, and the CMT provide endpoint users with a set of tools to comprehensively evaluate, treat, and manage concussions in SMs.
Description
The CMT provides step-by-step guidance for the initial and comprehensive management of concussion, once a diagnosis is made using assessments in the MACE 2. All types of HCPs, particularly those with limited training, such as Navy Hospital Corpsman and Army Combat Medics, are the intended clinical audience for the CMT. This tool was revised in 2019 to better align with the MACE 2, PRA CR, and other DVBIC CRs, and replaces the 2012 Concussion Management Algorithm and the 2014 Army Concussion Management in Garrison Setting Algorithm. The first 2 sections of the CMT are action cards, which provide management guidelines for acute injuries up to 7 days following injury and for comprehensive management beyond 1 week. Guidelines within the CMT partially overlap with those in the PRA CR; however, the PRA is designed for a more acute timeline, whereas the CMT focuses on symptom management following a more protracted recovery. The CMT clinical tool, provider training, instructor guide, and student workbook all can be found on the DVBIC website (Table 3).
Discussion
It is important for HCPs to have the skills and clinically relevant tools to optimize accurate TBI assessment. Early and accurate assessment and effective symptom management allows SMs to receive timely treatment based on clinical recommendations, and prevent and/or minimize secondary injury and prolonged recovery. Several longitudinal studies emphasize the benefits of early diagnosis and systematic follow-up.16-18 Prompt diagnosis, patient education, and early initiation to treatment may help optimize triage to care, mitigate prolonged symptoms by educating the patient on what to expect, and target specific symptoms early.8,10 Beyond the health outcomes of an individual SM, TBI recovery impacts unit readiness and consequently force readiness. As such, health outcomes and medical readiness are a priority of the Defense Health Agency (DHA).
The DHA priorities are, in part, based on DoD policy guidance for the management of concussion in the deployed setting. According to DoD instruction, “Medically documented mTBI/concussion in service members shall be clinically evaluated, treated, and managed according to the most current DoD clinical practice guidance for the deployed environment found in the Defense and Veterans Brain Injury Center (DVBIC) guidance, ‘Medical Providers: Clinical Tools.’”12 In 2018, the Deputy Secretary of Defense issued a memorandum regarding the comprehensive strategy and action plan for warfighter brain health.12 Therein, the memorandum acknowledges the enduring responsibility of the DoD to promote and protect the health and well-being of members of the nation’s armed forces. Particular emphasis was placed on issuing a response to the effects caused by concussive impacts and exposure to blast waves. This response resulted in a commitment by the DoD to understanding, preventing, diagnosing, and treating TBI in all forms. Taken together, the message from the secretary of defense and instruction from the DoD is clear and makes imperative the use of DoD clinical tools to accomplish this commitment.
Conclusion
This article showcases 3 of the DoD’s TBI clinical tools (MACE 2, PRA CR, and CMT) that assist HCPs in identifying and treating concussion. Over time, these tools undergo revisions according to the state of the science, and are adapted to meet the needs of clinicians and the SMs they treat. Studies are currently ongoing to better understand the effectiveness of these tools as well as to assist clinicians in making return-to-duty and/or medical separation decisions. These tools assist clinicians throughout the recovery process; from initial assessment and treatment (acute phase), as well as with symptom management (acute and protracted symptoms).
Concussion is not a homogenous condition and the experiences of the SM, including events that may cause emotional distress, other injuries and/or other factors, may further complicate the injury. Accordingly, there is no single clinical tool that can conclusively determine return-to-duty status; rather, these tools can help characterize injury, validate, and treat symptoms, which have been suggested to improve outcomes. More research and data are needed confirm the effectiveness of these tools to improve outcomes.
It is beyond the scope of this article to provide a more in-depth discussion on TBI prevention or longer term effects/care. However, there are additional, personalized tools for specific symptoms, deficits, or dysfunctions following concussion. These tools include the Management of Headache Following mTBI for PCM CR, Management of Sleep Disturbances Following mTBI for PCM CR, Assessment and Management of Visual Dysfunction Associated with mTBI CR, and Assessment and Management of Dizziness Associated mTBI CR. These tools enable endpoint users to evaluate and treat SMs as well as know when to elevate to higher levels of care.
The DoD commitment toward treating TBI influenced the development of the clinical tools highlighted in this article. They are the result of collective efforts among military and civilian TBI subject matter experts, data from medical literature and state-of-the-science research, and feedback from endpoint users to create the most effective, evidence-based tools. These tools undergo continuous review and revision to ensure alignment with the most up-to-date science within the field, to meet the needs of SMs and to continue the commitment to DoD concussion care.
Acknowledgments
This work was prepared under Contract (HT0014-19-C-0004) General Dynamics Information Technology and (W81XWH-16-F-0330) Credence Management Solutions, and is defined as U.S. Government work under Title 17 U.S.C.§101. Per Title 17 U.S.C.§105, copyright protection is not available for any work of the U.S. Government. For more information, please contact [email protected].
1. Centers for Disease Control and Prevention. Surveillance report of traumatic brain injury-related emergency department visits, hospitalizations, and deaths. https://www.cdc.gov/traumaticbraininjury/pdf/TBI-Surveillance-Report-FINAL_508.pdf. Published 2014. Accessed August 18, 2020.
2. Stocchetti N, Zanier ER. Chronic impact of traumatic brain injury on outcome and quality of life: a narrative review. Crit Care. 2016;20(1):148. Published 2016 Jun 21. doi:10.1186/s13054-016-1318-1
3. Kumar RG, Ornstein KA, Bollens-Lund E, et al. Lifetime history of traumatic brain injury is associated with increased loneliness in adults: A US nationally representative study. Int J Geriatr Psychiatry. 2020;35(5):553-563. doi:10.1002/gps.5271
4. Defense and Veterans Brain Injury Center. Worldwide DoD numbers for traumatic brain injury. 2020; https://dvbic.dcoe.mil/sites/default/files/tbi-numbers/DVBIC_WorldwideTotal_2000-2019.pdf. Updated March 10, 2020. Accessed August 18, 2020.
5. Kennedy JE, Lu LH, Reid MW, Leal FO, Cooper DB. Correlates of depression in U.S. military service members with a history of mild traumatic brain injury. Mil Med. 2019;184(suppl 1):148-154. doi:10.1093/milmed/usy321
6. Marshall KR, Holland SL, Meyer KS, Martin EM, Wilmore M, Grimes JB. Mild traumatic brain injury screening, diagnosis, and treatment. Mil Med. 2012;177(suppl 8):67-75. doi:10.7205/milmed-d-12-00110
7. French L, McCrea M., Baggett M. The Military Acute Concussion Evaluation. J Spec Oper Med. 2008;8(1):68-77. https://www.jsomonline.org/Publications/2008168French.pdf. Accessed August 18, 2020.
8. Kontos AP, Jorgensen-Wagers K, Trbovich AM, et al. Association of time since injury to the first clinic visit with recovery following concussion. JAMA Neurol. 2020;77(4):435-440. doi:10.1001/jamaneurol.2019.4552
9. Ponsford J, Willmott C, Rothwell A, et al. Impact of early intervention on outcome following mild head injury in adults. J Neurol Neurosurg Psychiatry. 2002;73(3):330-332. doi:10.1136/jnnp.73.3.33010.
10. Mittenberg W, Canyock EM, Condit D, Patton C. Treatment of post-concussion syndrome following mild head injury. J Clin Exp Neuropsychol. 2001;23(6):829-836. doi:10.1076/jcen.23.6.829.1022
11. McCrea M, Kelly JP, Randolph C, et al. Standardized assessment of concussion (SAC): on-site mental status evaluation of the athlete. J Head Trauma Rehabil. 1998;13(2):27-35. doi:10.1097/00001199-199804000-00005
12. US Department of Defense. Department of Defense Instruction, Number 6490.11. Policy guidance for management of mild traumatic brain injury/concussion in the deployed setting. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/649011p.pdf. Updated November 26, 2019. Accessed August 18, 2020.
13. Mucha A, Collins MW, Elbin RJ, et al. A brief Vestibular/Ocular Motor Screening (VOMS) assessment to evaluate concussions: preliminary findings. Am J Sports Med. 2014;42(10):2479-2486. doi:10.1177/0363546514543775
14. Defense and Veterans Brain Injury Center. Military Acute Concussion Evaluation 2 (MACE 2). https://dvbic.dcoe.mil/material/military-acute-concussion-evaluation-2-mace-2. Updated August 18, 2020. Accessed August 18, 2020.
15. US Department of Defense, Defense Health Agency. Defense and Veterans Brain Injury Center releases new concussion screening tool. https://www.health.mil/News/Articles/2019/03/15/Defense-and-Veterans-Brain-Injury-Center-releases-new-concussion-screening-tool. Published March 15, 2019. Accessed August 18, 2020.
16. Schwab K, Terrio HP, Brenner LA, et al. Epidemiology and prognosis of mild traumatic brain injury in returning soldiers: a cohort study. Neurology. 2017;88(16):1571-1579. doi:10.1212/WNL.0000000000003839
17. Mac Donald CL, Johnson AM, Wierzechowski L, et al. Outcome trends after US military concussive traumatic brain injury. J Neurotrauma. 2017;34(14):2206-2219. doi:10.1089/neu.2016.4434
18. Andelic N, Howe EI, Hellstrøm T, et al. Disability and quality of life 20 years after traumatic brain injury. Brain Behav. 2018;8(7):e01018. doi:10.1002/brb3.1018
Traumatic brain injury (TBI) is a major health concern that can cause significant disability as well as economic and social burden. The Centers for Disease Control and Prevention (CDC) reported a 58% increase in the number of TBI-related emergency department visits, hospitalizations, and deaths from 2006 to 2014.1 In the CDC report, falls and motor vehicle accidents accounted for 52.3% and 20.4%, respectively, of all civilian TBI-related hospitalizations. In 2014, 56,800 TBIs in the US resulted in death. A large proportion of severe TBI survivors continue to experience long-term physical, cognitive, and psychologic disorders and require extensive rehabilitation, which may disrupt relationships and prevent return to work.2 About 37% of people with mild TBI (mTBI) cases and 51% of severe cases were unable to return to previous jobs. A study examining psychosocial burden found that people with a history of TBI reported greater feelings of loneliness compared with individuals without TBI.3
Within the US military, the Defense and Veterans Brain Injury Center (DVBIC) indicates that > 417,503 service members (SMs) have been diagnosed with TBI since November 2000.4 Of these, 82.4% were classified as having a mTBI, or concussion (Tables 1 and 2). The nature of combat and military training to which SMs are routinely exposed may increase the risk for sustaining a TBI. Specifically, the increased use of improvised explosives devices by enemy combatants in the recent military conflicts (ie, Operation Enduring Freedom, Operation Iraqi Freedom and Operation New Dawn) resulted in TBI being recognized as the signature injury of these conflicts and brought attention to the prevalence of concussion within the US military.5,6 In the military, the effects of concussion can decrease individual and unit effectiveness, emphasizing the importance of prompt diagnosis and proper management.7
Typically, patients recover from concussion within a few weeks of injury; however, some individuals experience symptoms that persist for months or years. Studies found that early intervention after concussion may aid in expediting recovery, stressing the importance of identifying concussion as promptly as possible.8,9 Active treatment is centered on patient education and symptom management, in addition to a progressive return to activities, as tolerated. Patient education may help validate the symptoms of some patients, as well as help to reattribute the symptoms to benign causes, leading to better outcomes.10 Since TBI is such a relevant health concern within the DoD, it is paramount for practitioners to understand what resources are available in order to identify and initiate treatment expeditiously.
This article focuses on the clinical tools used in evaluating and treating concussion, and best practices treatment guidelines for health care providers (HCPs) who are required to evaluate and treat military populations. While these resources are used for military SMs, they can also be used in veteran and civilian populations. This article showcases 3 DoD clinical tools that assist HCPs in evaluating and treating patients with TBI: (1) the Military Acute Concussion Evaluation 2 (MACE 2); (2) the Progressive Return to Activity (PRA) Clinical Recommendation (CR); and (3) the Concussion Management Tool (CMT). Additional DoD clinical tools and resources are discussed, and resources and links for the practitioner are provided for easy access and reference.
Military Acute Concussion Evaluation 2
Early concussion identification and evaluation are important steps in the treatment process to ensure timely recovery and return to duty for SMs. As such, DVBIC assembled a working group of military and civilian brain injury experts to create an evidence-based clinical practice guideline for the assessment and management of concussion in a military operational setting that could be learned and effectively used by corpsmen and combat medics in the battlefield to screen for a possible concussion.7 This team created the first version of the MACE, a clinical tool that prompted a systematic assessment of concussion related symptoms, neurologic signs, and cognitive deficits. The cognitive assessment portion was based on the standardized assessment of concussion (SAC) that had been reported by McCrea and colleagues in 1998.11 Soon after its creation, field utilization of the MACE for screening of concussion was mandated by the Army through an All Army Action (ALARACT 178/2008) and for all of the Services through the DoD Instruction (DoDI) 6490.11 published in 2014.12
The MACE has been updated several times since the original version. Most recently, the MACE was revised in 2018 to include a vestibular oculomotor assessment section, and red flags that immediately alert the HCP to the need for immediate triage referral and treatment of the patient possibly at a higher echelon of care or with more emergent evaluation.13-15 Additionally, the neurologic examination was expanded to increase clarity and comprehensiveness, including speech and balance testing. Updates made to the tool were intended to provide a more thorough and informative evaluation of the SM with suspected concussion.
This latest version, MACE 2, is designed to be used by any HCP who is treating SMs with a suspected or potential TBI, not just corpsmen and combat medics in theater. The MACE 2 is a comprehensive evaluation within a set of portable pocket cards designed to assist end-users in the proper triage of potentially concussed individuals. The DoD has specified 4 events that require a MACE 2 evaluation: (1) SM was in a vehicle associated with a blast event, collision, or roll over; (2) SM was within 50 meters of a blast; (3) anyone who sustained a direct blow to the head; or (4) when command provides direction (eg, repeated exposures to the events above or in accordance with protocols).12 Sleep deprivation, medications, and pain may affect MACE 2 results, in addition to deployment related stress, chronic stress, high adrenaline sustained over time, and additional comorbidities. This tool is most effective when used as close to the time of injury as possible but also may be used later (after 24 hours of rest) to reevaluate symptoms. The MACE 2 Instructor Guide, a student workbook, HCP training, and Vestibular/Ocular-Motor Screening (VOMS) for Concussion instructions can be found on the DVBIC website (Table 3).
Description
The MACE 2 is a brief multimodal screening tool that assists medics, corpsman, and primary care managers (PCMs) in the assessment and identification of a potential concussion (Figure 1). Embedded in the MACE 2 is the Standardized Assessment of Concussion (SAC), a well-validated sports concussion tool, and the VOMS tool as portions of the 2-part cognitive examination. The entirety of the tool has 5 sections: (1) red flags; (2) acute concussion screening; (3) cognitive examination, part 1; (4) neurologic examination; and (5) cognitive examination, part 2. The end of the MACE 2 includes sections on the scoring, instructions for International Classification of Diseases, Tenth Revision, TBI coding, and next steps following completion of the MACE 2. The latest version of this screening tool impacts TBI care in several noteworthy ways. First, it broadens the scope of users by expanding use to all medically trained personnel, allowing any provider to treat SMs in the field. Second, it combines state-of-the-science advances from the research field and reflects feedback from end-users collected during the development. Last, the MACE 2 is updated as changes in the field occur, and is currently undergoing research to better identify end-user utility and usability.
Screening Tools
• Red Flags. The red flags section aids in identifying potentially serious underlying conditions in patients presenting with Glasgow Coma Scale (GCS) between 13 and 15. A positive red flag prompts the practitioner to stop administering the MACE 2 and immediately consult a higher level of care and consider urgent evacuation. While the red flags are completed first, and advancement to later sections of the MACE 2 is dependent upon the absence of red flags, the red flags should be monitored throughout the completion of the MACE 2. Upon completion of patient demographics and red flags, the remaining sections of the MACE 2 are dedicated to acute concussion screening.
• Acute Concussion Screening. The acute concussion screening portion consists of 4 sections: description of the incident; alteration of consciousness or memory; a “check all that apply” symptom inventory; and a patient history that includes concussions within the past 12 months, headache disorders, and/or behavioral health concerns. The final portion of the acute concussion screening section provides an algorithm to identify a positive or negative concussion screen. When a negative screen is identified, the user is prompted to prescribe a 24-hour rest period and follow up with the SM based on the guidance in the CMT. A positive screen warrants the user to continue administration of the MACE 2.
Neurologic and CognitiveExaminations
• Cognitive Exam Part 1. The initial cognitive examination is designed to assess orientation to time (eg, What is the day of the week, day of the month, the month, the year, and the timeof day?) as well as immediate recall of a short list of concrete words (5 words total, repeated for 3 trials). These tests are based on other neuropsychological measures designed to assess cognitive/mental status and short-term memory.
• The Neurological Exam. The neurological exam section of the MACE 2 includes brief neuropsychologic tests such as speech fluency and word finding. Other sections within the neurological exam assess the
following: grip strength, vestibular function/balance (eg, tandem gait and single leg stance), as well as motor function (eg, pronator drift), autonomic nervous system function (eg, pupil response), and vestibular function (eye-tracking).
• Cognitive Exam Part 2. After completion of the first cognitive examination and the neurologic examination, the second part of the cognitive examination is initiated. Part 2 includes measures of short-term and working memory (eg, digits-reverse tasks, listing the months in reverse order, and a delayed recall task of the short list of concrete words presented in the first part). The final assessment is the administration of the VOMS, a tool developed from the sports concussion field and designed to measure vestibular-ocular function.13 It is critical to note that the VOMS is contraindicated if there is concern of an unstable cervical spine or absence of a trained HCP. An examination summary provides guidance on test scoring and yields a positive or negative indication for concussive injury. A positive test refers users to guidelines listed in the Concussion Management Tool for recommendations. The final page provides coding instructions for entering the results into the patient’s electronic medical record for documentation and future reference.
Progressive Return To Activities Clinical Recommendation
The Progressive Return to Activities Clinical Recommendation (PRA CR) also was developed by DVBIC for the DoD to assist military HCPs in managing SMs with concussion by providing systematic and evidence-based guidance to both prevent extended rest and promote return to full duty as quickly and safely as clinically indicated. The general guidance is to monitor the SM at each of the 6 stages in the process and safely and gradually increase activity to the next stage as tolerated. Daily symptoms are measured using the Neurobehavioral Symptom Inventory (NSI), which SMs self-administer every morning at each stage within the process.
Prior to initiation of the progressive return to activity, SM education using the educational brochure is strongly encouraged, as previous evidence suggests that it is an effective intervention during the acute stages of injury.10,11 Return to activity follows a 6 stage process, from stage 1 (rest) through stage 6 (unrestricted activity) (Table 4). Referral to rehabilitation providers (RPs) or higher care is left to the discretion of the PCM when (1) recovery is not progressing as anticipated; (2) progression is not being made within a 7-day period; or (3) symptoms worsen with time. The guidance outlined in the PRA CR is consistent with current policies and medical literature, and undergoes reviews as updates in the field emerge. The PRA for PCM, PRA for RP, Clinical Support Tool for PCM, Clinical Support Tool for RP, Training Slides for PCM, Training Slides for RP, Educational Brochure for PCM, and Patient Educational Tool for RP can be found on the DVBIC website (dvbic.dcoe.mil).
Description
To improve the clinical utility, 2 separate PRA CRs were developed specifically for PCMs (Figure 2) and RPs (Figure 3). The PRA CR for PCMs provides the initial framework to monitor SMs during recovery and gradually increase physical, cognitive, and vestibular/balance activities as symptoms improve in order to return to preinjury activities. The PRA CR for RPs outlines the approach for treating SMs who meet 1 of the following criteria: recovery is not progressing as anticipated, there is no progression in 7 days, symptoms are worsening, the SM is symptomatic after exertional testing following stage 5, or referral made per PCM judgment. Following the mandatory 24-hour rest period after a diagnosis of a concussion, progression through the PRA algorithm is based on history of concussion within the past 12 months (ie, 1, 2, or ≥ 3 concussions) and symptomatology, with varying treatment pathways depending on the SM’s responses to history and symptomology.
Guidelines
• One Concussion within Past 12 Months. Following the mandatory 24-hour rest period, if the SM is asymptomatic, then exertional testing (eg, activities such as push-ups, sit-ups, running in place, step aerobics, stationary bike, treadmill and/or hand crank) is performed at 65 to 85% of target heart rate for 2 minutes and symptoms are reassessed. If still asymptomatic, the SM may return to preinjury activity; however, if exertional testing provokes symptoms > 1 (mild) on the NSI, the SM should return to stage 1 with an additional 24 hours of rest. A second exertional test can be performed after stage 1, and if symptoms are provoked, progression through the remaining stages 2 to 5 is encouraged. Symptoms are continually monitored throughout each stage to determine whether the SM is recovered sufficiently to proceed to the next stage.
• Two Concussion Within Past 12 Months. Following the mandatory 24-hour rest period, no exertional testing is performed, and SMs move directly into stage 1 and remain at stage 1 or stage 2 for 7 consecutive days with no symptoms > 1 on the NSI before advancing through the remaining stages. Some defining features are longer rest periods (eg, 5 additional days of rest at stage 2) and additional patient education, symptom management, and follow-up.
• Three or more Concussions Within Past 12 Months. Following the 24 hour mandatory rest period, in cases where ≥ 3 concussions have occurred within a 12 month period, the recommendation is to provide guidance for symptom management rest and refer the SM to a higher level of care.
Concussion Management Tool
Beyond the initial assessment and concussion evaluation and the promotion of SMs’ timely return to duty, the DoD developed a tool to help endpoint users manage concussion, to include those with more protracted symptoms (Figure 4). The CMT assists HCPs and the SMs they treat in the management of symptoms before and after they return to duty. Specifically, the CMT is designed to be given in combination with guidelines issued by the DoD in the PRA CR but extends management of concussion to include those symptoms experienced more long-term, or symptoms that are not solely addressed during the timeline of the PRA CR. Together, the MACE 2, PRA CR, and the CMT provide endpoint users with a set of tools to comprehensively evaluate, treat, and manage concussions in SMs.
Description
The CMT provides step-by-step guidance for the initial and comprehensive management of concussion, once a diagnosis is made using assessments in the MACE 2. All types of HCPs, particularly those with limited training, such as Navy Hospital Corpsman and Army Combat Medics, are the intended clinical audience for the CMT. This tool was revised in 2019 to better align with the MACE 2, PRA CR, and other DVBIC CRs, and replaces the 2012 Concussion Management Algorithm and the 2014 Army Concussion Management in Garrison Setting Algorithm. The first 2 sections of the CMT are action cards, which provide management guidelines for acute injuries up to 7 days following injury and for comprehensive management beyond 1 week. Guidelines within the CMT partially overlap with those in the PRA CR; however, the PRA is designed for a more acute timeline, whereas the CMT focuses on symptom management following a more protracted recovery. The CMT clinical tool, provider training, instructor guide, and student workbook all can be found on the DVBIC website (Table 3).
Discussion
It is important for HCPs to have the skills and clinically relevant tools to optimize accurate TBI assessment. Early and accurate assessment and effective symptom management allows SMs to receive timely treatment based on clinical recommendations, and prevent and/or minimize secondary injury and prolonged recovery. Several longitudinal studies emphasize the benefits of early diagnosis and systematic follow-up.16-18 Prompt diagnosis, patient education, and early initiation to treatment may help optimize triage to care, mitigate prolonged symptoms by educating the patient on what to expect, and target specific symptoms early.8,10 Beyond the health outcomes of an individual SM, TBI recovery impacts unit readiness and consequently force readiness. As such, health outcomes and medical readiness are a priority of the Defense Health Agency (DHA).
The DHA priorities are, in part, based on DoD policy guidance for the management of concussion in the deployed setting. According to DoD instruction, “Medically documented mTBI/concussion in service members shall be clinically evaluated, treated, and managed according to the most current DoD clinical practice guidance for the deployed environment found in the Defense and Veterans Brain Injury Center (DVBIC) guidance, ‘Medical Providers: Clinical Tools.’”12 In 2018, the Deputy Secretary of Defense issued a memorandum regarding the comprehensive strategy and action plan for warfighter brain health.12 Therein, the memorandum acknowledges the enduring responsibility of the DoD to promote and protect the health and well-being of members of the nation’s armed forces. Particular emphasis was placed on issuing a response to the effects caused by concussive impacts and exposure to blast waves. This response resulted in a commitment by the DoD to understanding, preventing, diagnosing, and treating TBI in all forms. Taken together, the message from the secretary of defense and instruction from the DoD is clear and makes imperative the use of DoD clinical tools to accomplish this commitment.
Conclusion
This article showcases 3 of the DoD’s TBI clinical tools (MACE 2, PRA CR, and CMT) that assist HCPs in identifying and treating concussion. Over time, these tools undergo revisions according to the state of the science, and are adapted to meet the needs of clinicians and the SMs they treat. Studies are currently ongoing to better understand the effectiveness of these tools as well as to assist clinicians in making return-to-duty and/or medical separation decisions. These tools assist clinicians throughout the recovery process; from initial assessment and treatment (acute phase), as well as with symptom management (acute and protracted symptoms).
Concussion is not a homogenous condition and the experiences of the SM, including events that may cause emotional distress, other injuries and/or other factors, may further complicate the injury. Accordingly, there is no single clinical tool that can conclusively determine return-to-duty status; rather, these tools can help characterize injury, validate, and treat symptoms, which have been suggested to improve outcomes. More research and data are needed confirm the effectiveness of these tools to improve outcomes.
It is beyond the scope of this article to provide a more in-depth discussion on TBI prevention or longer term effects/care. However, there are additional, personalized tools for specific symptoms, deficits, or dysfunctions following concussion. These tools include the Management of Headache Following mTBI for PCM CR, Management of Sleep Disturbances Following mTBI for PCM CR, Assessment and Management of Visual Dysfunction Associated with mTBI CR, and Assessment and Management of Dizziness Associated mTBI CR. These tools enable endpoint users to evaluate and treat SMs as well as know when to elevate to higher levels of care.
The DoD commitment toward treating TBI influenced the development of the clinical tools highlighted in this article. They are the result of collective efforts among military and civilian TBI subject matter experts, data from medical literature and state-of-the-science research, and feedback from endpoint users to create the most effective, evidence-based tools. These tools undergo continuous review and revision to ensure alignment with the most up-to-date science within the field, to meet the needs of SMs and to continue the commitment to DoD concussion care.
Acknowledgments
This work was prepared under Contract (HT0014-19-C-0004) General Dynamics Information Technology and (W81XWH-16-F-0330) Credence Management Solutions, and is defined as U.S. Government work under Title 17 U.S.C.§101. Per Title 17 U.S.C.§105, copyright protection is not available for any work of the U.S. Government. For more information, please contact [email protected].
Traumatic brain injury (TBI) is a major health concern that can cause significant disability as well as economic and social burden. The Centers for Disease Control and Prevention (CDC) reported a 58% increase in the number of TBI-related emergency department visits, hospitalizations, and deaths from 2006 to 2014.1 In the CDC report, falls and motor vehicle accidents accounted for 52.3% and 20.4%, respectively, of all civilian TBI-related hospitalizations. In 2014, 56,800 TBIs in the US resulted in death. A large proportion of severe TBI survivors continue to experience long-term physical, cognitive, and psychologic disorders and require extensive rehabilitation, which may disrupt relationships and prevent return to work.2 About 37% of people with mild TBI (mTBI) cases and 51% of severe cases were unable to return to previous jobs. A study examining psychosocial burden found that people with a history of TBI reported greater feelings of loneliness compared with individuals without TBI.3
Within the US military, the Defense and Veterans Brain Injury Center (DVBIC) indicates that > 417,503 service members (SMs) have been diagnosed with TBI since November 2000.4 Of these, 82.4% were classified as having a mTBI, or concussion (Tables 1 and 2). The nature of combat and military training to which SMs are routinely exposed may increase the risk for sustaining a TBI. Specifically, the increased use of improvised explosives devices by enemy combatants in the recent military conflicts (ie, Operation Enduring Freedom, Operation Iraqi Freedom and Operation New Dawn) resulted in TBI being recognized as the signature injury of these conflicts and brought attention to the prevalence of concussion within the US military.5,6 In the military, the effects of concussion can decrease individual and unit effectiveness, emphasizing the importance of prompt diagnosis and proper management.7
Typically, patients recover from concussion within a few weeks of injury; however, some individuals experience symptoms that persist for months or years. Studies found that early intervention after concussion may aid in expediting recovery, stressing the importance of identifying concussion as promptly as possible.8,9 Active treatment is centered on patient education and symptom management, in addition to a progressive return to activities, as tolerated. Patient education may help validate the symptoms of some patients, as well as help to reattribute the symptoms to benign causes, leading to better outcomes.10 Since TBI is such a relevant health concern within the DoD, it is paramount for practitioners to understand what resources are available in order to identify and initiate treatment expeditiously.
This article focuses on the clinical tools used in evaluating and treating concussion, and best practices treatment guidelines for health care providers (HCPs) who are required to evaluate and treat military populations. While these resources are used for military SMs, they can also be used in veteran and civilian populations. This article showcases 3 DoD clinical tools that assist HCPs in evaluating and treating patients with TBI: (1) the Military Acute Concussion Evaluation 2 (MACE 2); (2) the Progressive Return to Activity (PRA) Clinical Recommendation (CR); and (3) the Concussion Management Tool (CMT). Additional DoD clinical tools and resources are discussed, and resources and links for the practitioner are provided for easy access and reference.
Military Acute Concussion Evaluation 2
Early concussion identification and evaluation are important steps in the treatment process to ensure timely recovery and return to duty for SMs. As such, DVBIC assembled a working group of military and civilian brain injury experts to create an evidence-based clinical practice guideline for the assessment and management of concussion in a military operational setting that could be learned and effectively used by corpsmen and combat medics in the battlefield to screen for a possible concussion.7 This team created the first version of the MACE, a clinical tool that prompted a systematic assessment of concussion related symptoms, neurologic signs, and cognitive deficits. The cognitive assessment portion was based on the standardized assessment of concussion (SAC) that had been reported by McCrea and colleagues in 1998.11 Soon after its creation, field utilization of the MACE for screening of concussion was mandated by the Army through an All Army Action (ALARACT 178/2008) and for all of the Services through the DoD Instruction (DoDI) 6490.11 published in 2014.12
The MACE has been updated several times since the original version. Most recently, the MACE was revised in 2018 to include a vestibular oculomotor assessment section, and red flags that immediately alert the HCP to the need for immediate triage referral and treatment of the patient possibly at a higher echelon of care or with more emergent evaluation.13-15 Additionally, the neurologic examination was expanded to increase clarity and comprehensiveness, including speech and balance testing. Updates made to the tool were intended to provide a more thorough and informative evaluation of the SM with suspected concussion.
This latest version, MACE 2, is designed to be used by any HCP who is treating SMs with a suspected or potential TBI, not just corpsmen and combat medics in theater. The MACE 2 is a comprehensive evaluation within a set of portable pocket cards designed to assist end-users in the proper triage of potentially concussed individuals. The DoD has specified 4 events that require a MACE 2 evaluation: (1) SM was in a vehicle associated with a blast event, collision, or roll over; (2) SM was within 50 meters of a blast; (3) anyone who sustained a direct blow to the head; or (4) when command provides direction (eg, repeated exposures to the events above or in accordance with protocols).12 Sleep deprivation, medications, and pain may affect MACE 2 results, in addition to deployment related stress, chronic stress, high adrenaline sustained over time, and additional comorbidities. This tool is most effective when used as close to the time of injury as possible but also may be used later (after 24 hours of rest) to reevaluate symptoms. The MACE 2 Instructor Guide, a student workbook, HCP training, and Vestibular/Ocular-Motor Screening (VOMS) for Concussion instructions can be found on the DVBIC website (Table 3).
Description
The MACE 2 is a brief multimodal screening tool that assists medics, corpsman, and primary care managers (PCMs) in the assessment and identification of a potential concussion (Figure 1). Embedded in the MACE 2 is the Standardized Assessment of Concussion (SAC), a well-validated sports concussion tool, and the VOMS tool as portions of the 2-part cognitive examination. The entirety of the tool has 5 sections: (1) red flags; (2) acute concussion screening; (3) cognitive examination, part 1; (4) neurologic examination; and (5) cognitive examination, part 2. The end of the MACE 2 includes sections on the scoring, instructions for International Classification of Diseases, Tenth Revision, TBI coding, and next steps following completion of the MACE 2. The latest version of this screening tool impacts TBI care in several noteworthy ways. First, it broadens the scope of users by expanding use to all medically trained personnel, allowing any provider to treat SMs in the field. Second, it combines state-of-the-science advances from the research field and reflects feedback from end-users collected during the development. Last, the MACE 2 is updated as changes in the field occur, and is currently undergoing research to better identify end-user utility and usability.
Screening Tools
• Red Flags. The red flags section aids in identifying potentially serious underlying conditions in patients presenting with Glasgow Coma Scale (GCS) between 13 and 15. A positive red flag prompts the practitioner to stop administering the MACE 2 and immediately consult a higher level of care and consider urgent evacuation. While the red flags are completed first, and advancement to later sections of the MACE 2 is dependent upon the absence of red flags, the red flags should be monitored throughout the completion of the MACE 2. Upon completion of patient demographics and red flags, the remaining sections of the MACE 2 are dedicated to acute concussion screening.
• Acute Concussion Screening. The acute concussion screening portion consists of 4 sections: description of the incident; alteration of consciousness or memory; a “check all that apply” symptom inventory; and a patient history that includes concussions within the past 12 months, headache disorders, and/or behavioral health concerns. The final portion of the acute concussion screening section provides an algorithm to identify a positive or negative concussion screen. When a negative screen is identified, the user is prompted to prescribe a 24-hour rest period and follow up with the SM based on the guidance in the CMT. A positive screen warrants the user to continue administration of the MACE 2.
Neurologic and CognitiveExaminations
• Cognitive Exam Part 1. The initial cognitive examination is designed to assess orientation to time (eg, What is the day of the week, day of the month, the month, the year, and the timeof day?) as well as immediate recall of a short list of concrete words (5 words total, repeated for 3 trials). These tests are based on other neuropsychological measures designed to assess cognitive/mental status and short-term memory.
• The Neurological Exam. The neurological exam section of the MACE 2 includes brief neuropsychologic tests such as speech fluency and word finding. Other sections within the neurological exam assess the
following: grip strength, vestibular function/balance (eg, tandem gait and single leg stance), as well as motor function (eg, pronator drift), autonomic nervous system function (eg, pupil response), and vestibular function (eye-tracking).
• Cognitive Exam Part 2. After completion of the first cognitive examination and the neurologic examination, the second part of the cognitive examination is initiated. Part 2 includes measures of short-term and working memory (eg, digits-reverse tasks, listing the months in reverse order, and a delayed recall task of the short list of concrete words presented in the first part). The final assessment is the administration of the VOMS, a tool developed from the sports concussion field and designed to measure vestibular-ocular function.13 It is critical to note that the VOMS is contraindicated if there is concern of an unstable cervical spine or absence of a trained HCP. An examination summary provides guidance on test scoring and yields a positive or negative indication for concussive injury. A positive test refers users to guidelines listed in the Concussion Management Tool for recommendations. The final page provides coding instructions for entering the results into the patient’s electronic medical record for documentation and future reference.
Progressive Return To Activities Clinical Recommendation
The Progressive Return to Activities Clinical Recommendation (PRA CR) also was developed by DVBIC for the DoD to assist military HCPs in managing SMs with concussion by providing systematic and evidence-based guidance to both prevent extended rest and promote return to full duty as quickly and safely as clinically indicated. The general guidance is to monitor the SM at each of the 6 stages in the process and safely and gradually increase activity to the next stage as tolerated. Daily symptoms are measured using the Neurobehavioral Symptom Inventory (NSI), which SMs self-administer every morning at each stage within the process.
Prior to initiation of the progressive return to activity, SM education using the educational brochure is strongly encouraged, as previous evidence suggests that it is an effective intervention during the acute stages of injury.10,11 Return to activity follows a 6 stage process, from stage 1 (rest) through stage 6 (unrestricted activity) (Table 4). Referral to rehabilitation providers (RPs) or higher care is left to the discretion of the PCM when (1) recovery is not progressing as anticipated; (2) progression is not being made within a 7-day period; or (3) symptoms worsen with time. The guidance outlined in the PRA CR is consistent with current policies and medical literature, and undergoes reviews as updates in the field emerge. The PRA for PCM, PRA for RP, Clinical Support Tool for PCM, Clinical Support Tool for RP, Training Slides for PCM, Training Slides for RP, Educational Brochure for PCM, and Patient Educational Tool for RP can be found on the DVBIC website (dvbic.dcoe.mil).
Description
To improve the clinical utility, 2 separate PRA CRs were developed specifically for PCMs (Figure 2) and RPs (Figure 3). The PRA CR for PCMs provides the initial framework to monitor SMs during recovery and gradually increase physical, cognitive, and vestibular/balance activities as symptoms improve in order to return to preinjury activities. The PRA CR for RPs outlines the approach for treating SMs who meet 1 of the following criteria: recovery is not progressing as anticipated, there is no progression in 7 days, symptoms are worsening, the SM is symptomatic after exertional testing following stage 5, or referral made per PCM judgment. Following the mandatory 24-hour rest period after a diagnosis of a concussion, progression through the PRA algorithm is based on history of concussion within the past 12 months (ie, 1, 2, or ≥ 3 concussions) and symptomatology, with varying treatment pathways depending on the SM’s responses to history and symptomology.
Guidelines
• One Concussion within Past 12 Months. Following the mandatory 24-hour rest period, if the SM is asymptomatic, then exertional testing (eg, activities such as push-ups, sit-ups, running in place, step aerobics, stationary bike, treadmill and/or hand crank) is performed at 65 to 85% of target heart rate for 2 minutes and symptoms are reassessed. If still asymptomatic, the SM may return to preinjury activity; however, if exertional testing provokes symptoms > 1 (mild) on the NSI, the SM should return to stage 1 with an additional 24 hours of rest. A second exertional test can be performed after stage 1, and if symptoms are provoked, progression through the remaining stages 2 to 5 is encouraged. Symptoms are continually monitored throughout each stage to determine whether the SM is recovered sufficiently to proceed to the next stage.
• Two Concussion Within Past 12 Months. Following the mandatory 24-hour rest period, no exertional testing is performed, and SMs move directly into stage 1 and remain at stage 1 or stage 2 for 7 consecutive days with no symptoms > 1 on the NSI before advancing through the remaining stages. Some defining features are longer rest periods (eg, 5 additional days of rest at stage 2) and additional patient education, symptom management, and follow-up.
• Three or more Concussions Within Past 12 Months. Following the 24 hour mandatory rest period, in cases where ≥ 3 concussions have occurred within a 12 month period, the recommendation is to provide guidance for symptom management rest and refer the SM to a higher level of care.
Concussion Management Tool
Beyond the initial assessment and concussion evaluation and the promotion of SMs’ timely return to duty, the DoD developed a tool to help endpoint users manage concussion, to include those with more protracted symptoms (Figure 4). The CMT assists HCPs and the SMs they treat in the management of symptoms before and after they return to duty. Specifically, the CMT is designed to be given in combination with guidelines issued by the DoD in the PRA CR but extends management of concussion to include those symptoms experienced more long-term, or symptoms that are not solely addressed during the timeline of the PRA CR. Together, the MACE 2, PRA CR, and the CMT provide endpoint users with a set of tools to comprehensively evaluate, treat, and manage concussions in SMs.
Description
The CMT provides step-by-step guidance for the initial and comprehensive management of concussion, once a diagnosis is made using assessments in the MACE 2. All types of HCPs, particularly those with limited training, such as Navy Hospital Corpsman and Army Combat Medics, are the intended clinical audience for the CMT. This tool was revised in 2019 to better align with the MACE 2, PRA CR, and other DVBIC CRs, and replaces the 2012 Concussion Management Algorithm and the 2014 Army Concussion Management in Garrison Setting Algorithm. The first 2 sections of the CMT are action cards, which provide management guidelines for acute injuries up to 7 days following injury and for comprehensive management beyond 1 week. Guidelines within the CMT partially overlap with those in the PRA CR; however, the PRA is designed for a more acute timeline, whereas the CMT focuses on symptom management following a more protracted recovery. The CMT clinical tool, provider training, instructor guide, and student workbook all can be found on the DVBIC website (Table 3).
Discussion
It is important for HCPs to have the skills and clinically relevant tools to optimize accurate TBI assessment. Early and accurate assessment and effective symptom management allows SMs to receive timely treatment based on clinical recommendations, and prevent and/or minimize secondary injury and prolonged recovery. Several longitudinal studies emphasize the benefits of early diagnosis and systematic follow-up.16-18 Prompt diagnosis, patient education, and early initiation to treatment may help optimize triage to care, mitigate prolonged symptoms by educating the patient on what to expect, and target specific symptoms early.8,10 Beyond the health outcomes of an individual SM, TBI recovery impacts unit readiness and consequently force readiness. As such, health outcomes and medical readiness are a priority of the Defense Health Agency (DHA).
The DHA priorities are, in part, based on DoD policy guidance for the management of concussion in the deployed setting. According to DoD instruction, “Medically documented mTBI/concussion in service members shall be clinically evaluated, treated, and managed according to the most current DoD clinical practice guidance for the deployed environment found in the Defense and Veterans Brain Injury Center (DVBIC) guidance, ‘Medical Providers: Clinical Tools.’”12 In 2018, the Deputy Secretary of Defense issued a memorandum regarding the comprehensive strategy and action plan for warfighter brain health.12 Therein, the memorandum acknowledges the enduring responsibility of the DoD to promote and protect the health and well-being of members of the nation’s armed forces. Particular emphasis was placed on issuing a response to the effects caused by concussive impacts and exposure to blast waves. This response resulted in a commitment by the DoD to understanding, preventing, diagnosing, and treating TBI in all forms. Taken together, the message from the secretary of defense and instruction from the DoD is clear and makes imperative the use of DoD clinical tools to accomplish this commitment.
Conclusion
This article showcases 3 of the DoD’s TBI clinical tools (MACE 2, PRA CR, and CMT) that assist HCPs in identifying and treating concussion. Over time, these tools undergo revisions according to the state of the science, and are adapted to meet the needs of clinicians and the SMs they treat. Studies are currently ongoing to better understand the effectiveness of these tools as well as to assist clinicians in making return-to-duty and/or medical separation decisions. These tools assist clinicians throughout the recovery process; from initial assessment and treatment (acute phase), as well as with symptom management (acute and protracted symptoms).
Concussion is not a homogenous condition and the experiences of the SM, including events that may cause emotional distress, other injuries and/or other factors, may further complicate the injury. Accordingly, there is no single clinical tool that can conclusively determine return-to-duty status; rather, these tools can help characterize injury, validate, and treat symptoms, which have been suggested to improve outcomes. More research and data are needed confirm the effectiveness of these tools to improve outcomes.
It is beyond the scope of this article to provide a more in-depth discussion on TBI prevention or longer term effects/care. However, there are additional, personalized tools for specific symptoms, deficits, or dysfunctions following concussion. These tools include the Management of Headache Following mTBI for PCM CR, Management of Sleep Disturbances Following mTBI for PCM CR, Assessment and Management of Visual Dysfunction Associated with mTBI CR, and Assessment and Management of Dizziness Associated mTBI CR. These tools enable endpoint users to evaluate and treat SMs as well as know when to elevate to higher levels of care.
The DoD commitment toward treating TBI influenced the development of the clinical tools highlighted in this article. They are the result of collective efforts among military and civilian TBI subject matter experts, data from medical literature and state-of-the-science research, and feedback from endpoint users to create the most effective, evidence-based tools. These tools undergo continuous review and revision to ensure alignment with the most up-to-date science within the field, to meet the needs of SMs and to continue the commitment to DoD concussion care.
Acknowledgments
This work was prepared under Contract (HT0014-19-C-0004) General Dynamics Information Technology and (W81XWH-16-F-0330) Credence Management Solutions, and is defined as U.S. Government work under Title 17 U.S.C.§101. Per Title 17 U.S.C.§105, copyright protection is not available for any work of the U.S. Government. For more information, please contact [email protected].
1. Centers for Disease Control and Prevention. Surveillance report of traumatic brain injury-related emergency department visits, hospitalizations, and deaths. https://www.cdc.gov/traumaticbraininjury/pdf/TBI-Surveillance-Report-FINAL_508.pdf. Published 2014. Accessed August 18, 2020.
2. Stocchetti N, Zanier ER. Chronic impact of traumatic brain injury on outcome and quality of life: a narrative review. Crit Care. 2016;20(1):148. Published 2016 Jun 21. doi:10.1186/s13054-016-1318-1
3. Kumar RG, Ornstein KA, Bollens-Lund E, et al. Lifetime history of traumatic brain injury is associated with increased loneliness in adults: A US nationally representative study. Int J Geriatr Psychiatry. 2020;35(5):553-563. doi:10.1002/gps.5271
4. Defense and Veterans Brain Injury Center. Worldwide DoD numbers for traumatic brain injury. 2020; https://dvbic.dcoe.mil/sites/default/files/tbi-numbers/DVBIC_WorldwideTotal_2000-2019.pdf. Updated March 10, 2020. Accessed August 18, 2020.
5. Kennedy JE, Lu LH, Reid MW, Leal FO, Cooper DB. Correlates of depression in U.S. military service members with a history of mild traumatic brain injury. Mil Med. 2019;184(suppl 1):148-154. doi:10.1093/milmed/usy321
6. Marshall KR, Holland SL, Meyer KS, Martin EM, Wilmore M, Grimes JB. Mild traumatic brain injury screening, diagnosis, and treatment. Mil Med. 2012;177(suppl 8):67-75. doi:10.7205/milmed-d-12-00110
7. French L, McCrea M., Baggett M. The Military Acute Concussion Evaluation. J Spec Oper Med. 2008;8(1):68-77. https://www.jsomonline.org/Publications/2008168French.pdf. Accessed August 18, 2020.
8. Kontos AP, Jorgensen-Wagers K, Trbovich AM, et al. Association of time since injury to the first clinic visit with recovery following concussion. JAMA Neurol. 2020;77(4):435-440. doi:10.1001/jamaneurol.2019.4552
9. Ponsford J, Willmott C, Rothwell A, et al. Impact of early intervention on outcome following mild head injury in adults. J Neurol Neurosurg Psychiatry. 2002;73(3):330-332. doi:10.1136/jnnp.73.3.33010.
10. Mittenberg W, Canyock EM, Condit D, Patton C. Treatment of post-concussion syndrome following mild head injury. J Clin Exp Neuropsychol. 2001;23(6):829-836. doi:10.1076/jcen.23.6.829.1022
11. McCrea M, Kelly JP, Randolph C, et al. Standardized assessment of concussion (SAC): on-site mental status evaluation of the athlete. J Head Trauma Rehabil. 1998;13(2):27-35. doi:10.1097/00001199-199804000-00005
12. US Department of Defense. Department of Defense Instruction, Number 6490.11. Policy guidance for management of mild traumatic brain injury/concussion in the deployed setting. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/649011p.pdf. Updated November 26, 2019. Accessed August 18, 2020.
13. Mucha A, Collins MW, Elbin RJ, et al. A brief Vestibular/Ocular Motor Screening (VOMS) assessment to evaluate concussions: preliminary findings. Am J Sports Med. 2014;42(10):2479-2486. doi:10.1177/0363546514543775
14. Defense and Veterans Brain Injury Center. Military Acute Concussion Evaluation 2 (MACE 2). https://dvbic.dcoe.mil/material/military-acute-concussion-evaluation-2-mace-2. Updated August 18, 2020. Accessed August 18, 2020.
15. US Department of Defense, Defense Health Agency. Defense and Veterans Brain Injury Center releases new concussion screening tool. https://www.health.mil/News/Articles/2019/03/15/Defense-and-Veterans-Brain-Injury-Center-releases-new-concussion-screening-tool. Published March 15, 2019. Accessed August 18, 2020.
16. Schwab K, Terrio HP, Brenner LA, et al. Epidemiology and prognosis of mild traumatic brain injury in returning soldiers: a cohort study. Neurology. 2017;88(16):1571-1579. doi:10.1212/WNL.0000000000003839
17. Mac Donald CL, Johnson AM, Wierzechowski L, et al. Outcome trends after US military concussive traumatic brain injury. J Neurotrauma. 2017;34(14):2206-2219. doi:10.1089/neu.2016.4434
18. Andelic N, Howe EI, Hellstrøm T, et al. Disability and quality of life 20 years after traumatic brain injury. Brain Behav. 2018;8(7):e01018. doi:10.1002/brb3.1018
1. Centers for Disease Control and Prevention. Surveillance report of traumatic brain injury-related emergency department visits, hospitalizations, and deaths. https://www.cdc.gov/traumaticbraininjury/pdf/TBI-Surveillance-Report-FINAL_508.pdf. Published 2014. Accessed August 18, 2020.
2. Stocchetti N, Zanier ER. Chronic impact of traumatic brain injury on outcome and quality of life: a narrative review. Crit Care. 2016;20(1):148. Published 2016 Jun 21. doi:10.1186/s13054-016-1318-1
3. Kumar RG, Ornstein KA, Bollens-Lund E, et al. Lifetime history of traumatic brain injury is associated with increased loneliness in adults: A US nationally representative study. Int J Geriatr Psychiatry. 2020;35(5):553-563. doi:10.1002/gps.5271
4. Defense and Veterans Brain Injury Center. Worldwide DoD numbers for traumatic brain injury. 2020; https://dvbic.dcoe.mil/sites/default/files/tbi-numbers/DVBIC_WorldwideTotal_2000-2019.pdf. Updated March 10, 2020. Accessed August 18, 2020.
5. Kennedy JE, Lu LH, Reid MW, Leal FO, Cooper DB. Correlates of depression in U.S. military service members with a history of mild traumatic brain injury. Mil Med. 2019;184(suppl 1):148-154. doi:10.1093/milmed/usy321
6. Marshall KR, Holland SL, Meyer KS, Martin EM, Wilmore M, Grimes JB. Mild traumatic brain injury screening, diagnosis, and treatment. Mil Med. 2012;177(suppl 8):67-75. doi:10.7205/milmed-d-12-00110
7. French L, McCrea M., Baggett M. The Military Acute Concussion Evaluation. J Spec Oper Med. 2008;8(1):68-77. https://www.jsomonline.org/Publications/2008168French.pdf. Accessed August 18, 2020.
8. Kontos AP, Jorgensen-Wagers K, Trbovich AM, et al. Association of time since injury to the first clinic visit with recovery following concussion. JAMA Neurol. 2020;77(4):435-440. doi:10.1001/jamaneurol.2019.4552
9. Ponsford J, Willmott C, Rothwell A, et al. Impact of early intervention on outcome following mild head injury in adults. J Neurol Neurosurg Psychiatry. 2002;73(3):330-332. doi:10.1136/jnnp.73.3.33010.
10. Mittenberg W, Canyock EM, Condit D, Patton C. Treatment of post-concussion syndrome following mild head injury. J Clin Exp Neuropsychol. 2001;23(6):829-836. doi:10.1076/jcen.23.6.829.1022
11. McCrea M, Kelly JP, Randolph C, et al. Standardized assessment of concussion (SAC): on-site mental status evaluation of the athlete. J Head Trauma Rehabil. 1998;13(2):27-35. doi:10.1097/00001199-199804000-00005
12. US Department of Defense. Department of Defense Instruction, Number 6490.11. Policy guidance for management of mild traumatic brain injury/concussion in the deployed setting. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/649011p.pdf. Updated November 26, 2019. Accessed August 18, 2020.
13. Mucha A, Collins MW, Elbin RJ, et al. A brief Vestibular/Ocular Motor Screening (VOMS) assessment to evaluate concussions: preliminary findings. Am J Sports Med. 2014;42(10):2479-2486. doi:10.1177/0363546514543775
14. Defense and Veterans Brain Injury Center. Military Acute Concussion Evaluation 2 (MACE 2). https://dvbic.dcoe.mil/material/military-acute-concussion-evaluation-2-mace-2. Updated August 18, 2020. Accessed August 18, 2020.
15. US Department of Defense, Defense Health Agency. Defense and Veterans Brain Injury Center releases new concussion screening tool. https://www.health.mil/News/Articles/2019/03/15/Defense-and-Veterans-Brain-Injury-Center-releases-new-concussion-screening-tool. Published March 15, 2019. Accessed August 18, 2020.
16. Schwab K, Terrio HP, Brenner LA, et al. Epidemiology and prognosis of mild traumatic brain injury in returning soldiers: a cohort study. Neurology. 2017;88(16):1571-1579. doi:10.1212/WNL.0000000000003839
17. Mac Donald CL, Johnson AM, Wierzechowski L, et al. Outcome trends after US military concussive traumatic brain injury. J Neurotrauma. 2017;34(14):2206-2219. doi:10.1089/neu.2016.4434
18. Andelic N, Howe EI, Hellstrøm T, et al. Disability and quality of life 20 years after traumatic brain injury. Brain Behav. 2018;8(7):e01018. doi:10.1002/brb3.1018
ADHD and dyslexia may affect evaluation of concussion
, a new study shows.
“Our results suggest kids with certain learning disorders may respond differently to concussion tests, and this needs to be taken into account when advising on recovery times and when they can return to sport,” said lead author Mathew Stokes, MD. Dr. Stokes is assistant professor of pediatrics and neurology/neurotherapeutics at the University of Texas–Southwestern Medical Center, Dallas.
The study was presented at the American Academy of Neurology Sports Concussion Virtual Conference, held online July 31 to Aug. 1.
Learning disorders affected scores
The researchers analyzed data from participants aged 10-18 years who were enrolled in the North Texas Concussion Registry (ConTex). Participants had been diagnosed with a concussion that was sustained within 30 days of enrollment. The researchers investigated whether there were differences between patients who had no history of learning disorders and those with a history of dyslexia and/or ADD/ADHD with regard to results of clinical testing following concussion.
Of the 1,298 individuals in the study, 58 had been diagnosed with dyslexia, 158 had been diagnosed with ADD/ADHD, and 35 had been diagnosed with both conditions. There was no difference in age, time since injury, or history of concussion between those with learning disorders and those without, but there were more male patients in the ADD/ADHD group.
Results showed that in the dyslexia group, mean time was slower (P = .011), and there was an increase in error scores on the King-Devick (KD) test (P = .028). That test assesses eye movements and involves the rapid naming of numbers that are spaced differently. In addition, those with ADD/ADHD had significantly higher impulse control scores (P = .007) on the ImPACT series of tests, which are commonly used in the evaluation of concussion. Participants with both dyslexia and ADHD demonstrated slower KD times (P = .009) and had higher depression scores and anxiety scores.
Dr. Stokes noted that a limiting factor of the study was that baseline scores were not available. “It is possible that kids with ADD have less impulse control even at baseline, and this would need to be taken into account,” he said. “You may perhaps also expect someone with dyslexia to have a worse score on the KD tests, so we need more data on how these scores are affected from baseline in these individuals. But our results show that when evaluating kids pre- or post concussion, it is important to know about learning disorders, as this will affect how we interpret the data.”
At 3-month follow-up, there were no longer significant differences in anxiety and depression scores for those with and those without learning disorders. “This suggests anxiety and depression may well be worse temporarily after concussion for those with ADD/ADHD but gets better with time,” Dr. Stokes said.
Follow-up data were not available for the other cognitive tests.
Are recovery times longer?
Asked whether young people with these learning disorders needed a longer time to recover after concussion, Dr. Stokes said: “That is a million-dollar question. Studies so far on this have shown conflicting results. Our results add to a growing body of literature on this.” He stressed that it is important to include anxiety and depression scores on both baseline and postconcussion tests. “People don’t tend to think of these symptoms as being associated with concussion, but they are actually very prominent in this situation,” he noted. “Our results suggest that individuals with ADHD may be more prone to anxiety and depression, and a blow to the head may tip them more into these symptoms.”
Discussing the study at a virtual press conference as part of the AAN Sports Concussion meeting, the codirector of the meeting, David Dodick, MD, Mayo Clinic, Scottsdale, Ariz., said: “This is a very interesting and important study which suggests there are differences between adolescents with a history of dyslexia/ADHD and those without these conditions in performance in concussion tests. Understanding the differences in these groups will help health care providers in evaluating these athletes and assisting in counseling them and their families with regard to their risk of injury.
“It is important to recognize that athletes with ADHD, whether or not they are on medication, may take longer to recover from a concussion,” Dr. Dodick added. They also exhibit greater reductions in cognitive skills and visual motor speed regarding hand-eye coordination, he said. There is an increase in the severity of symptoms. “Symptoms that exist in both groups tend to more severe in those individuals with ADHD,” he noted.
“Ascertaining the presence or absence of ADHD or dyslexia in those who are participating in sport is important, especially when trying to interpret the results of baseline testing, the results of postinjury testing, decisions on when to return to play, and assessing for individuals and their families the risk of long-term repeat concussions and adverse outcomes,” he concluded.
The other codirector of the AAN meeting, Brian Hainline, MD, chief medical officer of the National Collegiate Athletic Association, added: “It appears that athletes with ADHD may suffer more with concussion and have a longer recovery time. This can inform our decision making and help these individuals to understand that they are at higher risk.”
Dr. Hainline said this raises another important point: “Concussion is not a homogeneous entity. It is a brain injury that can manifest in multiple parts of the brain, and the way the brain is from a premorbid or comorbid point of view can influence the manifestation of concussion as well,” he said. “All these things need to be taken into account.”
Attentional deficit may itself make an individual more susceptible to sustaining an injury in the first place, he said. “All of this is an evolving body of research which is helping clinicians to make better-informed decisions for athletes who may manifest differently.”
A version of this article originally appeared on Medscape.com.
, a new study shows.
“Our results suggest kids with certain learning disorders may respond differently to concussion tests, and this needs to be taken into account when advising on recovery times and when they can return to sport,” said lead author Mathew Stokes, MD. Dr. Stokes is assistant professor of pediatrics and neurology/neurotherapeutics at the University of Texas–Southwestern Medical Center, Dallas.
The study was presented at the American Academy of Neurology Sports Concussion Virtual Conference, held online July 31 to Aug. 1.
Learning disorders affected scores
The researchers analyzed data from participants aged 10-18 years who were enrolled in the North Texas Concussion Registry (ConTex). Participants had been diagnosed with a concussion that was sustained within 30 days of enrollment. The researchers investigated whether there were differences between patients who had no history of learning disorders and those with a history of dyslexia and/or ADD/ADHD with regard to results of clinical testing following concussion.
Of the 1,298 individuals in the study, 58 had been diagnosed with dyslexia, 158 had been diagnosed with ADD/ADHD, and 35 had been diagnosed with both conditions. There was no difference in age, time since injury, or history of concussion between those with learning disorders and those without, but there were more male patients in the ADD/ADHD group.
Results showed that in the dyslexia group, mean time was slower (P = .011), and there was an increase in error scores on the King-Devick (KD) test (P = .028). That test assesses eye movements and involves the rapid naming of numbers that are spaced differently. In addition, those with ADD/ADHD had significantly higher impulse control scores (P = .007) on the ImPACT series of tests, which are commonly used in the evaluation of concussion. Participants with both dyslexia and ADHD demonstrated slower KD times (P = .009) and had higher depression scores and anxiety scores.
Dr. Stokes noted that a limiting factor of the study was that baseline scores were not available. “It is possible that kids with ADD have less impulse control even at baseline, and this would need to be taken into account,” he said. “You may perhaps also expect someone with dyslexia to have a worse score on the KD tests, so we need more data on how these scores are affected from baseline in these individuals. But our results show that when evaluating kids pre- or post concussion, it is important to know about learning disorders, as this will affect how we interpret the data.”
At 3-month follow-up, there were no longer significant differences in anxiety and depression scores for those with and those without learning disorders. “This suggests anxiety and depression may well be worse temporarily after concussion for those with ADD/ADHD but gets better with time,” Dr. Stokes said.
Follow-up data were not available for the other cognitive tests.
Are recovery times longer?
Asked whether young people with these learning disorders needed a longer time to recover after concussion, Dr. Stokes said: “That is a million-dollar question. Studies so far on this have shown conflicting results. Our results add to a growing body of literature on this.” He stressed that it is important to include anxiety and depression scores on both baseline and postconcussion tests. “People don’t tend to think of these symptoms as being associated with concussion, but they are actually very prominent in this situation,” he noted. “Our results suggest that individuals with ADHD may be more prone to anxiety and depression, and a blow to the head may tip them more into these symptoms.”
Discussing the study at a virtual press conference as part of the AAN Sports Concussion meeting, the codirector of the meeting, David Dodick, MD, Mayo Clinic, Scottsdale, Ariz., said: “This is a very interesting and important study which suggests there are differences between adolescents with a history of dyslexia/ADHD and those without these conditions in performance in concussion tests. Understanding the differences in these groups will help health care providers in evaluating these athletes and assisting in counseling them and their families with regard to their risk of injury.
“It is important to recognize that athletes with ADHD, whether or not they are on medication, may take longer to recover from a concussion,” Dr. Dodick added. They also exhibit greater reductions in cognitive skills and visual motor speed regarding hand-eye coordination, he said. There is an increase in the severity of symptoms. “Symptoms that exist in both groups tend to more severe in those individuals with ADHD,” he noted.
“Ascertaining the presence or absence of ADHD or dyslexia in those who are participating in sport is important, especially when trying to interpret the results of baseline testing, the results of postinjury testing, decisions on when to return to play, and assessing for individuals and their families the risk of long-term repeat concussions and adverse outcomes,” he concluded.
The other codirector of the AAN meeting, Brian Hainline, MD, chief medical officer of the National Collegiate Athletic Association, added: “It appears that athletes with ADHD may suffer more with concussion and have a longer recovery time. This can inform our decision making and help these individuals to understand that they are at higher risk.”
Dr. Hainline said this raises another important point: “Concussion is not a homogeneous entity. It is a brain injury that can manifest in multiple parts of the brain, and the way the brain is from a premorbid or comorbid point of view can influence the manifestation of concussion as well,” he said. “All these things need to be taken into account.”
Attentional deficit may itself make an individual more susceptible to sustaining an injury in the first place, he said. “All of this is an evolving body of research which is helping clinicians to make better-informed decisions for athletes who may manifest differently.”
A version of this article originally appeared on Medscape.com.
, a new study shows.
“Our results suggest kids with certain learning disorders may respond differently to concussion tests, and this needs to be taken into account when advising on recovery times and when they can return to sport,” said lead author Mathew Stokes, MD. Dr. Stokes is assistant professor of pediatrics and neurology/neurotherapeutics at the University of Texas–Southwestern Medical Center, Dallas.
The study was presented at the American Academy of Neurology Sports Concussion Virtual Conference, held online July 31 to Aug. 1.
Learning disorders affected scores
The researchers analyzed data from participants aged 10-18 years who were enrolled in the North Texas Concussion Registry (ConTex). Participants had been diagnosed with a concussion that was sustained within 30 days of enrollment. The researchers investigated whether there were differences between patients who had no history of learning disorders and those with a history of dyslexia and/or ADD/ADHD with regard to results of clinical testing following concussion.
Of the 1,298 individuals in the study, 58 had been diagnosed with dyslexia, 158 had been diagnosed with ADD/ADHD, and 35 had been diagnosed with both conditions. There was no difference in age, time since injury, or history of concussion between those with learning disorders and those without, but there were more male patients in the ADD/ADHD group.
Results showed that in the dyslexia group, mean time was slower (P = .011), and there was an increase in error scores on the King-Devick (KD) test (P = .028). That test assesses eye movements and involves the rapid naming of numbers that are spaced differently. In addition, those with ADD/ADHD had significantly higher impulse control scores (P = .007) on the ImPACT series of tests, which are commonly used in the evaluation of concussion. Participants with both dyslexia and ADHD demonstrated slower KD times (P = .009) and had higher depression scores and anxiety scores.
Dr. Stokes noted that a limiting factor of the study was that baseline scores were not available. “It is possible that kids with ADD have less impulse control even at baseline, and this would need to be taken into account,” he said. “You may perhaps also expect someone with dyslexia to have a worse score on the KD tests, so we need more data on how these scores are affected from baseline in these individuals. But our results show that when evaluating kids pre- or post concussion, it is important to know about learning disorders, as this will affect how we interpret the data.”
At 3-month follow-up, there were no longer significant differences in anxiety and depression scores for those with and those without learning disorders. “This suggests anxiety and depression may well be worse temporarily after concussion for those with ADD/ADHD but gets better with time,” Dr. Stokes said.
Follow-up data were not available for the other cognitive tests.
Are recovery times longer?
Asked whether young people with these learning disorders needed a longer time to recover after concussion, Dr. Stokes said: “That is a million-dollar question. Studies so far on this have shown conflicting results. Our results add to a growing body of literature on this.” He stressed that it is important to include anxiety and depression scores on both baseline and postconcussion tests. “People don’t tend to think of these symptoms as being associated with concussion, but they are actually very prominent in this situation,” he noted. “Our results suggest that individuals with ADHD may be more prone to anxiety and depression, and a blow to the head may tip them more into these symptoms.”
Discussing the study at a virtual press conference as part of the AAN Sports Concussion meeting, the codirector of the meeting, David Dodick, MD, Mayo Clinic, Scottsdale, Ariz., said: “This is a very interesting and important study which suggests there are differences between adolescents with a history of dyslexia/ADHD and those without these conditions in performance in concussion tests. Understanding the differences in these groups will help health care providers in evaluating these athletes and assisting in counseling them and their families with regard to their risk of injury.
“It is important to recognize that athletes with ADHD, whether or not they are on medication, may take longer to recover from a concussion,” Dr. Dodick added. They also exhibit greater reductions in cognitive skills and visual motor speed regarding hand-eye coordination, he said. There is an increase in the severity of symptoms. “Symptoms that exist in both groups tend to more severe in those individuals with ADHD,” he noted.
“Ascertaining the presence or absence of ADHD or dyslexia in those who are participating in sport is important, especially when trying to interpret the results of baseline testing, the results of postinjury testing, decisions on when to return to play, and assessing for individuals and their families the risk of long-term repeat concussions and adverse outcomes,” he concluded.
The other codirector of the AAN meeting, Brian Hainline, MD, chief medical officer of the National Collegiate Athletic Association, added: “It appears that athletes with ADHD may suffer more with concussion and have a longer recovery time. This can inform our decision making and help these individuals to understand that they are at higher risk.”
Dr. Hainline said this raises another important point: “Concussion is not a homogeneous entity. It is a brain injury that can manifest in multiple parts of the brain, and the way the brain is from a premorbid or comorbid point of view can influence the manifestation of concussion as well,” he said. “All these things need to be taken into account.”
Attentional deficit may itself make an individual more susceptible to sustaining an injury in the first place, he said. “All of this is an evolving body of research which is helping clinicians to make better-informed decisions for athletes who may manifest differently.”
A version of this article originally appeared on Medscape.com.
From AAN Sports Concussion Conference
Concussion linked to risk for dementia, Parkinson’s disease, and ADHD
new research suggests. Results from a retrospective, population-based cohort study showed that controlling for socioeconomic status and overall health did not significantly affect this association.
The link between concussion and risk for ADHD and for mood and anxiety disorder was stronger in the women than in the men. In addition, having a history of multiple concussions strengthened the association between concussion and subsequent mood and anxiety disorder, dementia, and Parkinson’s disease compared with experiencing just one concussion.
The findings are similar to those of previous studies, noted lead author Marc P. Morissette, PhD, research assistant at the Pan Am Clinic Foundation in Winnipeg, Manitoba, Canada. “The main methodological differences separating our study from previous studies in this area is a focus on concussion-specific injuries identified from medical records and the potential for study participants to have up to 25 years of follow-up data,” said Dr. Morissette.
The findings were published online July 27 in Family Medicine and Community Health, a BMJ journal.
Almost 190,000 participants
Several studies have shown associations between head injury and increased risk for ADHD, depression, anxiety, Alzheimer’s disease, and Parkinson’s disease. However, many of these studies relied on self-reported medical history, included all forms of traumatic brain injury, and failed to adjust for preexisting health conditions.
An improved understanding of concussion and the risks associated with it could help physicians manage their patients’ long-term needs, the investigators noted.
In the current study, the researchers examined anonymized administrative health data collected between the periods of 1990–1991 and 2014–2015 in the Manitoba Population Research Data Repository at the Manitoba Center for Health Policy.
Eligible patients had been diagnosed with concussion in accordance with standard criteria. Participants were excluded if they had been diagnosed with dementia or Parkinson’s disease before the incident concussion during the study period. The investigators matched three control participants to each included patient on the basis of age, sex, and location.
Study outcome was time from index date (date of first concussion) to diagnosis of ADHD, mood and anxiety disorder, dementia, or Parkinson’s disease. The researchers controlled for socioeconomic status using the Socioeconomic Factor Index, version 2 (SEFI2), and for preexisting medical conditions using the Charlson Comorbidity Index (CCI).
The study included 28,021 men (mean age, 25 years) and 19,462 women (mean age, 30 years) in the concussion group and 81,871 men (mean age, 25 years) and 57,159 women (mean age, 30 years) in the control group. Mean SEFI2 score was approximately −0.05, and mean CCI score was approximately 0.2.
Dose effect?
Results showed that concussion was associated with an increased risk for ADHD (hazard ratio [HR], 1.39), mood and anxiety disorder (HR, 1.72), dementia (HR, 1.72), and Parkinson’s disease (HR, 1.57).
After a concussion, the risk of developing ADHD was 28% higher and the risk of developing mood and anxiety disorder was 7% higher among women than among men. Gender was not associated with risk for dementia or Parkinson’s disease after concussion.
Sustaining a second concussion increased the strength of the association with risk for dementia compared with sustaining a single concussion (HR, 1.62). Similarly, sustaining more than three concussions increased the strength of the association with the risk for mood and anxiety disorders (HR for more than three vs one concussion, 1.22) and Parkinson›s disease (HR, 3.27).
A sensitivity analysis found similar associations between concussion and risk for mood and anxiety disorder among all age groups. Younger participants were at greater risk for ADHD, however, and older participants were at greater risk for dementia and Parkinson’s disease.
Increased awareness of concussion and the outcomes of interest, along with improved diagnostic tools, may have influenced the study’s findings, Dr. Morissette noted. “The sex-based differences may be due to either pathophysiological differences in response to concussive injuries or potentially a difference in willingness to seek medical care or share symptoms, concussion-related or otherwise, with a medical professional,” he said.
“We are hopeful that our findings will encourage practitioners to be cognizant of various conditions that may present in individuals who have previously experienced a concussion,” Dr. Morissette added. “If physicians are aware of the various associations identified following a concussion, it may lead to more thorough clinical examination at initial presentation, along with more dedicated care throughout the patient’s life.”
Association versus causation
Commenting on the research, Steven Erickson, MD, sports medicine specialist at Banner–University Medicine Neuroscience Institute, Phoenix, Ariz., noted that although the study showed an association between concussion and subsequent diagnosis of ADHD, anxiety, and Parkinson’s disease, “this association should not be misconstrued as causation.” He added that the study’s conclusions “are just as likely to be due to labeling theory” or a self-fulfilling prophecy.
“Patients diagnosed with ADHD, anxiety, or Parkinson’s disease may recall concussion and associate the two diagnoses; but patients who have not previously been diagnosed with a concussion cannot draw that conclusion,” said Dr. Erickson, who was not involved with the research.
Citing the apparent gender difference in the strength of the association between concussion and the outcomes of interest, Dr. Erickson noted that women are more likely to report symptoms in general “and therefore are more likely to be diagnosed with ADHD and anxiety disorders” because of differences in reporting rather than incidence of disease.
“Further research needs to be done to definitively determine a causal relationship between concussion and any psychiatric or neurologic diagnosis,” Dr. Erickson concluded.
The study was funded by the Pan Am Clinic Foundation. Dr. Morissette and Dr. Erickson have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests. Results from a retrospective, population-based cohort study showed that controlling for socioeconomic status and overall health did not significantly affect this association.
The link between concussion and risk for ADHD and for mood and anxiety disorder was stronger in the women than in the men. In addition, having a history of multiple concussions strengthened the association between concussion and subsequent mood and anxiety disorder, dementia, and Parkinson’s disease compared with experiencing just one concussion.
The findings are similar to those of previous studies, noted lead author Marc P. Morissette, PhD, research assistant at the Pan Am Clinic Foundation in Winnipeg, Manitoba, Canada. “The main methodological differences separating our study from previous studies in this area is a focus on concussion-specific injuries identified from medical records and the potential for study participants to have up to 25 years of follow-up data,” said Dr. Morissette.
The findings were published online July 27 in Family Medicine and Community Health, a BMJ journal.
Almost 190,000 participants
Several studies have shown associations between head injury and increased risk for ADHD, depression, anxiety, Alzheimer’s disease, and Parkinson’s disease. However, many of these studies relied on self-reported medical history, included all forms of traumatic brain injury, and failed to adjust for preexisting health conditions.
An improved understanding of concussion and the risks associated with it could help physicians manage their patients’ long-term needs, the investigators noted.
In the current study, the researchers examined anonymized administrative health data collected between the periods of 1990–1991 and 2014–2015 in the Manitoba Population Research Data Repository at the Manitoba Center for Health Policy.
Eligible patients had been diagnosed with concussion in accordance with standard criteria. Participants were excluded if they had been diagnosed with dementia or Parkinson’s disease before the incident concussion during the study period. The investigators matched three control participants to each included patient on the basis of age, sex, and location.
Study outcome was time from index date (date of first concussion) to diagnosis of ADHD, mood and anxiety disorder, dementia, or Parkinson’s disease. The researchers controlled for socioeconomic status using the Socioeconomic Factor Index, version 2 (SEFI2), and for preexisting medical conditions using the Charlson Comorbidity Index (CCI).
The study included 28,021 men (mean age, 25 years) and 19,462 women (mean age, 30 years) in the concussion group and 81,871 men (mean age, 25 years) and 57,159 women (mean age, 30 years) in the control group. Mean SEFI2 score was approximately −0.05, and mean CCI score was approximately 0.2.
Dose effect?
Results showed that concussion was associated with an increased risk for ADHD (hazard ratio [HR], 1.39), mood and anxiety disorder (HR, 1.72), dementia (HR, 1.72), and Parkinson’s disease (HR, 1.57).
After a concussion, the risk of developing ADHD was 28% higher and the risk of developing mood and anxiety disorder was 7% higher among women than among men. Gender was not associated with risk for dementia or Parkinson’s disease after concussion.
Sustaining a second concussion increased the strength of the association with risk for dementia compared with sustaining a single concussion (HR, 1.62). Similarly, sustaining more than three concussions increased the strength of the association with the risk for mood and anxiety disorders (HR for more than three vs one concussion, 1.22) and Parkinson›s disease (HR, 3.27).
A sensitivity analysis found similar associations between concussion and risk for mood and anxiety disorder among all age groups. Younger participants were at greater risk for ADHD, however, and older participants were at greater risk for dementia and Parkinson’s disease.
Increased awareness of concussion and the outcomes of interest, along with improved diagnostic tools, may have influenced the study’s findings, Dr. Morissette noted. “The sex-based differences may be due to either pathophysiological differences in response to concussive injuries or potentially a difference in willingness to seek medical care or share symptoms, concussion-related or otherwise, with a medical professional,” he said.
“We are hopeful that our findings will encourage practitioners to be cognizant of various conditions that may present in individuals who have previously experienced a concussion,” Dr. Morissette added. “If physicians are aware of the various associations identified following a concussion, it may lead to more thorough clinical examination at initial presentation, along with more dedicated care throughout the patient’s life.”
Association versus causation
Commenting on the research, Steven Erickson, MD, sports medicine specialist at Banner–University Medicine Neuroscience Institute, Phoenix, Ariz., noted that although the study showed an association between concussion and subsequent diagnosis of ADHD, anxiety, and Parkinson’s disease, “this association should not be misconstrued as causation.” He added that the study’s conclusions “are just as likely to be due to labeling theory” or a self-fulfilling prophecy.
“Patients diagnosed with ADHD, anxiety, or Parkinson’s disease may recall concussion and associate the two diagnoses; but patients who have not previously been diagnosed with a concussion cannot draw that conclusion,” said Dr. Erickson, who was not involved with the research.
Citing the apparent gender difference in the strength of the association between concussion and the outcomes of interest, Dr. Erickson noted that women are more likely to report symptoms in general “and therefore are more likely to be diagnosed with ADHD and anxiety disorders” because of differences in reporting rather than incidence of disease.
“Further research needs to be done to definitively determine a causal relationship between concussion and any psychiatric or neurologic diagnosis,” Dr. Erickson concluded.
The study was funded by the Pan Am Clinic Foundation. Dr. Morissette and Dr. Erickson have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests. Results from a retrospective, population-based cohort study showed that controlling for socioeconomic status and overall health did not significantly affect this association.
The link between concussion and risk for ADHD and for mood and anxiety disorder was stronger in the women than in the men. In addition, having a history of multiple concussions strengthened the association between concussion and subsequent mood and anxiety disorder, dementia, and Parkinson’s disease compared with experiencing just one concussion.
The findings are similar to those of previous studies, noted lead author Marc P. Morissette, PhD, research assistant at the Pan Am Clinic Foundation in Winnipeg, Manitoba, Canada. “The main methodological differences separating our study from previous studies in this area is a focus on concussion-specific injuries identified from medical records and the potential for study participants to have up to 25 years of follow-up data,” said Dr. Morissette.
The findings were published online July 27 in Family Medicine and Community Health, a BMJ journal.
Almost 190,000 participants
Several studies have shown associations between head injury and increased risk for ADHD, depression, anxiety, Alzheimer’s disease, and Parkinson’s disease. However, many of these studies relied on self-reported medical history, included all forms of traumatic brain injury, and failed to adjust for preexisting health conditions.
An improved understanding of concussion and the risks associated with it could help physicians manage their patients’ long-term needs, the investigators noted.
In the current study, the researchers examined anonymized administrative health data collected between the periods of 1990–1991 and 2014–2015 in the Manitoba Population Research Data Repository at the Manitoba Center for Health Policy.
Eligible patients had been diagnosed with concussion in accordance with standard criteria. Participants were excluded if they had been diagnosed with dementia or Parkinson’s disease before the incident concussion during the study period. The investigators matched three control participants to each included patient on the basis of age, sex, and location.
Study outcome was time from index date (date of first concussion) to diagnosis of ADHD, mood and anxiety disorder, dementia, or Parkinson’s disease. The researchers controlled for socioeconomic status using the Socioeconomic Factor Index, version 2 (SEFI2), and for preexisting medical conditions using the Charlson Comorbidity Index (CCI).
The study included 28,021 men (mean age, 25 years) and 19,462 women (mean age, 30 years) in the concussion group and 81,871 men (mean age, 25 years) and 57,159 women (mean age, 30 years) in the control group. Mean SEFI2 score was approximately −0.05, and mean CCI score was approximately 0.2.
Dose effect?
Results showed that concussion was associated with an increased risk for ADHD (hazard ratio [HR], 1.39), mood and anxiety disorder (HR, 1.72), dementia (HR, 1.72), and Parkinson’s disease (HR, 1.57).
After a concussion, the risk of developing ADHD was 28% higher and the risk of developing mood and anxiety disorder was 7% higher among women than among men. Gender was not associated with risk for dementia or Parkinson’s disease after concussion.
Sustaining a second concussion increased the strength of the association with risk for dementia compared with sustaining a single concussion (HR, 1.62). Similarly, sustaining more than three concussions increased the strength of the association with the risk for mood and anxiety disorders (HR for more than three vs one concussion, 1.22) and Parkinson›s disease (HR, 3.27).
A sensitivity analysis found similar associations between concussion and risk for mood and anxiety disorder among all age groups. Younger participants were at greater risk for ADHD, however, and older participants were at greater risk for dementia and Parkinson’s disease.
Increased awareness of concussion and the outcomes of interest, along with improved diagnostic tools, may have influenced the study’s findings, Dr. Morissette noted. “The sex-based differences may be due to either pathophysiological differences in response to concussive injuries or potentially a difference in willingness to seek medical care or share symptoms, concussion-related or otherwise, with a medical professional,” he said.
“We are hopeful that our findings will encourage practitioners to be cognizant of various conditions that may present in individuals who have previously experienced a concussion,” Dr. Morissette added. “If physicians are aware of the various associations identified following a concussion, it may lead to more thorough clinical examination at initial presentation, along with more dedicated care throughout the patient’s life.”
Association versus causation
Commenting on the research, Steven Erickson, MD, sports medicine specialist at Banner–University Medicine Neuroscience Institute, Phoenix, Ariz., noted that although the study showed an association between concussion and subsequent diagnosis of ADHD, anxiety, and Parkinson’s disease, “this association should not be misconstrued as causation.” He added that the study’s conclusions “are just as likely to be due to labeling theory” or a self-fulfilling prophecy.
“Patients diagnosed with ADHD, anxiety, or Parkinson’s disease may recall concussion and associate the two diagnoses; but patients who have not previously been diagnosed with a concussion cannot draw that conclusion,” said Dr. Erickson, who was not involved with the research.
Citing the apparent gender difference in the strength of the association between concussion and the outcomes of interest, Dr. Erickson noted that women are more likely to report symptoms in general “and therefore are more likely to be diagnosed with ADHD and anxiety disorders” because of differences in reporting rather than incidence of disease.
“Further research needs to be done to definitively determine a causal relationship between concussion and any psychiatric or neurologic diagnosis,” Dr. Erickson concluded.
The study was funded by the Pan Am Clinic Foundation. Dr. Morissette and Dr. Erickson have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
From Family Medicine and Community Health
Consensus document reviews determination of brain death
The document, a result of the World Brain Death Project, surveys the clinical aspects of this determination, such as clinical testing, apnea testing, and the number of examinations required, as well as its social and legal aspects, including documentation, qualifications for making the determination, and religious attitudes toward BD/DNC.
The recommendations are the minimum criteria for BD/DNC, and countries and professional societies may choose to adopt stricter criteria, the authors noted. Seventeen supplements to the consensus statement contain detailed reports on topics the statement examines, including focuses on both adults and children.
“Perhaps the most important points of this project are, first, to show the worldwide acceptance of the concept of BD/DNC and what the minimum requirements are for BD/DNC,” said corresponding author Gene Sung, MD, MPH, director of the neurocritical care and stroke division at the University of Southern California, Los Angeles. Second, “this standard is centered around a clinical determination without the need for other testing.”
The consensus document and supplements were published online Aug. 3 in JAMA.
Comprehensive review
A lack of rigor has led to many differences in the determination of BD/DNC, said Dr. Sung. “Some of the variance that is common are the numbers of exams and examiners that are required and whether ancillary tests are required for determination of BD/DNC. In addition, a lot of guidelines and protocols that are in use are not thorough in detailing how to do the examinations and what to do in different circumstances.”
Professional societies such as the World Federation of Intensive and Critical Care recruited experts in BD/DNC to develop recommendations, which were based on relevant articles that they identified during a literature search. “We wanted to develop a fairly comprehensive document that, along with the 17 supplements, builds a foundation to show how to determine BD/DNC – what the minimum clinical criteria needed are and what to do in special circumstances,” Dr. Sung said.
Major sections of the statement include recommendations for the minimum clinical standards for the determination of BD/DNC in adults and children.
Determination must begin by establishing that the patient has sustained an irreversible brain injury that resulted in the loss of all brain function, according to the authors. Confounders such as pharmacologic paralysis and the effect of CNS depressant medications should be ruled out.
In addition, clinical evaluation must include an assessment for coma and an evaluation for brain stem areflexia. Among other criteria, the pupils should be fixed and nonresponsive to light, the face should not move in response to noxious cranial stimulation, and the gag and cough reflexes should be absent. Apnea testing is recommended to evaluate the responsiveness of respiratory centers in the medulla.
Although the definition of BD/DNC is the same in children as in adults, less evidence is available for the determination of BD/DNC in the very young. The authors thus advised a cautious approach to the evaluation of infants and younger children.
Recommendations vary by age and often require serial examinations, including apnea testing, they noted.
Ancillary testing
The consensus statement also reviews ancillary testing, which the authors recommend be required when the minimum clinical examination, including the apnea test, cannot be completed and when it is in the presence of confounding conditions that cannot be resolved.
The authors recommended digital subtraction angiography, radionuclide studies, and transcranial Doppler ultrasonography as ancillary tests based on blood flow in the brain. However, CT angiography and magnetic resonance angiography not be used.
A lack of guidance makes performing an apnea test in patients receiving extracorporeal membrane oxygenation (ECMO) challenging, according to the authors. Nevertheless, they recommended that the same principles of BD/DNC be applied to adults and children receiving ECMO.
They further recommended a period of preoxygenation before the apnea test, and the document describes in detail the method for administering this test to people receiving ECMO.
Another potentially challenging situation pointed out in the consensus document is the determination of BD/DNC in patients who have been treated with targeted temperature management. Therapeutic hypothermia, particularly if it is preceded or accompanied by sedation, can temporarily impair brain stem reflexes, thus mimicking BD/DNC.
The new document includes a flowchart and step-by-step recommendations as well as suggestions for determining BD/DNC under these circumstances.
Among document limitations acknowledged by the authors is the lack of high-quality data from randomized, controlled trials on which to base their recommendations.
In addition, economic, technological, or personnel limitations may reduce the available options for ancillary testing, they added. Also, the recommendations do not incorporate contributions from patients or social or religious groups, although the authors were mindful of their concerns.
To promote the national and international harmonization of BD/DNC criteria, “medical societies and countries can evaluate their own policies in relation to this document and fix any deficiencies,” Dr. Sung said.
“Many countries do not have any BD/DNC policies and can use the documents from this project to create their own. There may need to be discussions with legal, governmental, religious, and societal leaders to help understand and accept BD/DNC and to help enact policies in different communities,” he added.
Divergent definitions
The determination of death is not simply a scientific question, but also a philosophical, religious, and cultural question, wrote Robert D. Truog, MD, director of the Harvard Center for Bioethics, Boston, and colleagues in an accompanying editorial. Future research should consider cultural differences over these questions.
“Most important is that there be a clear and logical consistency between the definition of death and the tests that are used to diagnose it,” Dr. Truog said.
The concept of whole brain death was advanced as an equivalent to biological death, “such that, when the brain dies, the body literally disintegrates, just as it does after cardiac arrest,” but evidence indicates that this claim is untrue, Dr. Truog said. Current tests also do not diagnose the death of the whole brain.
Another hypothesis is that brain stem death represents the irreversible loss of consciousness and the capacity for spontaneous respiration.
“Instead of focusing on biology, [this definition] focuses on values and is based on the claim that when a person is in a state of irreversible apneic unconsciousness, we may consider them to be dead,” said Dr. Truog. He and his coeditorialists argued that the concept of whole brain death should be replaced with that of brain stem death.
“This report should be a call for our profession, as well as for federal and state lawmakers, to reform our laws so that they are consistent with our diagnostic criteria,” Dr. Truog said.
“The most straightforward way of doing this would be to change U.S. law and adopt the British standard of brain stem death, and then refine our testing to make the diagnosis of irreversible apneic unconsciousness as reliable and safe as possible,” he concluded.
The drafting of the consensus statement was not supported by outside funding. Dr. Sung reported no relevant financial relationships. Dr. Truog reported receiving compensation from Sanofi and Covance for participating in data and safety monitoring boards unrelated to the consensus document.
A version of this article originally appeared on Medscape.com.
The document, a result of the World Brain Death Project, surveys the clinical aspects of this determination, such as clinical testing, apnea testing, and the number of examinations required, as well as its social and legal aspects, including documentation, qualifications for making the determination, and religious attitudes toward BD/DNC.
The recommendations are the minimum criteria for BD/DNC, and countries and professional societies may choose to adopt stricter criteria, the authors noted. Seventeen supplements to the consensus statement contain detailed reports on topics the statement examines, including focuses on both adults and children.
“Perhaps the most important points of this project are, first, to show the worldwide acceptance of the concept of BD/DNC and what the minimum requirements are for BD/DNC,” said corresponding author Gene Sung, MD, MPH, director of the neurocritical care and stroke division at the University of Southern California, Los Angeles. Second, “this standard is centered around a clinical determination without the need for other testing.”
The consensus document and supplements were published online Aug. 3 in JAMA.
Comprehensive review
A lack of rigor has led to many differences in the determination of BD/DNC, said Dr. Sung. “Some of the variance that is common are the numbers of exams and examiners that are required and whether ancillary tests are required for determination of BD/DNC. In addition, a lot of guidelines and protocols that are in use are not thorough in detailing how to do the examinations and what to do in different circumstances.”
Professional societies such as the World Federation of Intensive and Critical Care recruited experts in BD/DNC to develop recommendations, which were based on relevant articles that they identified during a literature search. “We wanted to develop a fairly comprehensive document that, along with the 17 supplements, builds a foundation to show how to determine BD/DNC – what the minimum clinical criteria needed are and what to do in special circumstances,” Dr. Sung said.
Major sections of the statement include recommendations for the minimum clinical standards for the determination of BD/DNC in adults and children.
Determination must begin by establishing that the patient has sustained an irreversible brain injury that resulted in the loss of all brain function, according to the authors. Confounders such as pharmacologic paralysis and the effect of CNS depressant medications should be ruled out.
In addition, clinical evaluation must include an assessment for coma and an evaluation for brain stem areflexia. Among other criteria, the pupils should be fixed and nonresponsive to light, the face should not move in response to noxious cranial stimulation, and the gag and cough reflexes should be absent. Apnea testing is recommended to evaluate the responsiveness of respiratory centers in the medulla.
Although the definition of BD/DNC is the same in children as in adults, less evidence is available for the determination of BD/DNC in the very young. The authors thus advised a cautious approach to the evaluation of infants and younger children.
Recommendations vary by age and often require serial examinations, including apnea testing, they noted.
Ancillary testing
The consensus statement also reviews ancillary testing, which the authors recommend be required when the minimum clinical examination, including the apnea test, cannot be completed and when it is in the presence of confounding conditions that cannot be resolved.
The authors recommended digital subtraction angiography, radionuclide studies, and transcranial Doppler ultrasonography as ancillary tests based on blood flow in the brain. However, CT angiography and magnetic resonance angiography not be used.
A lack of guidance makes performing an apnea test in patients receiving extracorporeal membrane oxygenation (ECMO) challenging, according to the authors. Nevertheless, they recommended that the same principles of BD/DNC be applied to adults and children receiving ECMO.
They further recommended a period of preoxygenation before the apnea test, and the document describes in detail the method for administering this test to people receiving ECMO.
Another potentially challenging situation pointed out in the consensus document is the determination of BD/DNC in patients who have been treated with targeted temperature management. Therapeutic hypothermia, particularly if it is preceded or accompanied by sedation, can temporarily impair brain stem reflexes, thus mimicking BD/DNC.
The new document includes a flowchart and step-by-step recommendations as well as suggestions for determining BD/DNC under these circumstances.
Among document limitations acknowledged by the authors is the lack of high-quality data from randomized, controlled trials on which to base their recommendations.
In addition, economic, technological, or personnel limitations may reduce the available options for ancillary testing, they added. Also, the recommendations do not incorporate contributions from patients or social or religious groups, although the authors were mindful of their concerns.
To promote the national and international harmonization of BD/DNC criteria, “medical societies and countries can evaluate their own policies in relation to this document and fix any deficiencies,” Dr. Sung said.
“Many countries do not have any BD/DNC policies and can use the documents from this project to create their own. There may need to be discussions with legal, governmental, religious, and societal leaders to help understand and accept BD/DNC and to help enact policies in different communities,” he added.
Divergent definitions
The determination of death is not simply a scientific question, but also a philosophical, religious, and cultural question, wrote Robert D. Truog, MD, director of the Harvard Center for Bioethics, Boston, and colleagues in an accompanying editorial. Future research should consider cultural differences over these questions.
“Most important is that there be a clear and logical consistency between the definition of death and the tests that are used to diagnose it,” Dr. Truog said.
The concept of whole brain death was advanced as an equivalent to biological death, “such that, when the brain dies, the body literally disintegrates, just as it does after cardiac arrest,” but evidence indicates that this claim is untrue, Dr. Truog said. Current tests also do not diagnose the death of the whole brain.
Another hypothesis is that brain stem death represents the irreversible loss of consciousness and the capacity for spontaneous respiration.
“Instead of focusing on biology, [this definition] focuses on values and is based on the claim that when a person is in a state of irreversible apneic unconsciousness, we may consider them to be dead,” said Dr. Truog. He and his coeditorialists argued that the concept of whole brain death should be replaced with that of brain stem death.
“This report should be a call for our profession, as well as for federal and state lawmakers, to reform our laws so that they are consistent with our diagnostic criteria,” Dr. Truog said.
“The most straightforward way of doing this would be to change U.S. law and adopt the British standard of brain stem death, and then refine our testing to make the diagnosis of irreversible apneic unconsciousness as reliable and safe as possible,” he concluded.
The drafting of the consensus statement was not supported by outside funding. Dr. Sung reported no relevant financial relationships. Dr. Truog reported receiving compensation from Sanofi and Covance for participating in data and safety monitoring boards unrelated to the consensus document.
A version of this article originally appeared on Medscape.com.
The document, a result of the World Brain Death Project, surveys the clinical aspects of this determination, such as clinical testing, apnea testing, and the number of examinations required, as well as its social and legal aspects, including documentation, qualifications for making the determination, and religious attitudes toward BD/DNC.
The recommendations are the minimum criteria for BD/DNC, and countries and professional societies may choose to adopt stricter criteria, the authors noted. Seventeen supplements to the consensus statement contain detailed reports on topics the statement examines, including focuses on both adults and children.
“Perhaps the most important points of this project are, first, to show the worldwide acceptance of the concept of BD/DNC and what the minimum requirements are for BD/DNC,” said corresponding author Gene Sung, MD, MPH, director of the neurocritical care and stroke division at the University of Southern California, Los Angeles. Second, “this standard is centered around a clinical determination without the need for other testing.”
The consensus document and supplements were published online Aug. 3 in JAMA.
Comprehensive review
A lack of rigor has led to many differences in the determination of BD/DNC, said Dr. Sung. “Some of the variance that is common are the numbers of exams and examiners that are required and whether ancillary tests are required for determination of BD/DNC. In addition, a lot of guidelines and protocols that are in use are not thorough in detailing how to do the examinations and what to do in different circumstances.”
Professional societies such as the World Federation of Intensive and Critical Care recruited experts in BD/DNC to develop recommendations, which were based on relevant articles that they identified during a literature search. “We wanted to develop a fairly comprehensive document that, along with the 17 supplements, builds a foundation to show how to determine BD/DNC – what the minimum clinical criteria needed are and what to do in special circumstances,” Dr. Sung said.
Major sections of the statement include recommendations for the minimum clinical standards for the determination of BD/DNC in adults and children.
Determination must begin by establishing that the patient has sustained an irreversible brain injury that resulted in the loss of all brain function, according to the authors. Confounders such as pharmacologic paralysis and the effect of CNS depressant medications should be ruled out.
In addition, clinical evaluation must include an assessment for coma and an evaluation for brain stem areflexia. Among other criteria, the pupils should be fixed and nonresponsive to light, the face should not move in response to noxious cranial stimulation, and the gag and cough reflexes should be absent. Apnea testing is recommended to evaluate the responsiveness of respiratory centers in the medulla.
Although the definition of BD/DNC is the same in children as in adults, less evidence is available for the determination of BD/DNC in the very young. The authors thus advised a cautious approach to the evaluation of infants and younger children.
Recommendations vary by age and often require serial examinations, including apnea testing, they noted.
Ancillary testing
The consensus statement also reviews ancillary testing, which the authors recommend be required when the minimum clinical examination, including the apnea test, cannot be completed and when it is in the presence of confounding conditions that cannot be resolved.
The authors recommended digital subtraction angiography, radionuclide studies, and transcranial Doppler ultrasonography as ancillary tests based on blood flow in the brain. However, CT angiography and magnetic resonance angiography not be used.
A lack of guidance makes performing an apnea test in patients receiving extracorporeal membrane oxygenation (ECMO) challenging, according to the authors. Nevertheless, they recommended that the same principles of BD/DNC be applied to adults and children receiving ECMO.
They further recommended a period of preoxygenation before the apnea test, and the document describes in detail the method for administering this test to people receiving ECMO.
Another potentially challenging situation pointed out in the consensus document is the determination of BD/DNC in patients who have been treated with targeted temperature management. Therapeutic hypothermia, particularly if it is preceded or accompanied by sedation, can temporarily impair brain stem reflexes, thus mimicking BD/DNC.
The new document includes a flowchart and step-by-step recommendations as well as suggestions for determining BD/DNC under these circumstances.
Among document limitations acknowledged by the authors is the lack of high-quality data from randomized, controlled trials on which to base their recommendations.
In addition, economic, technological, or personnel limitations may reduce the available options for ancillary testing, they added. Also, the recommendations do not incorporate contributions from patients or social or religious groups, although the authors were mindful of their concerns.
To promote the national and international harmonization of BD/DNC criteria, “medical societies and countries can evaluate their own policies in relation to this document and fix any deficiencies,” Dr. Sung said.
“Many countries do not have any BD/DNC policies and can use the documents from this project to create their own. There may need to be discussions with legal, governmental, religious, and societal leaders to help understand and accept BD/DNC and to help enact policies in different communities,” he added.
Divergent definitions
The determination of death is not simply a scientific question, but also a philosophical, religious, and cultural question, wrote Robert D. Truog, MD, director of the Harvard Center for Bioethics, Boston, and colleagues in an accompanying editorial. Future research should consider cultural differences over these questions.
“Most important is that there be a clear and logical consistency between the definition of death and the tests that are used to diagnose it,” Dr. Truog said.
The concept of whole brain death was advanced as an equivalent to biological death, “such that, when the brain dies, the body literally disintegrates, just as it does after cardiac arrest,” but evidence indicates that this claim is untrue, Dr. Truog said. Current tests also do not diagnose the death of the whole brain.
Another hypothesis is that brain stem death represents the irreversible loss of consciousness and the capacity for spontaneous respiration.
“Instead of focusing on biology, [this definition] focuses on values and is based on the claim that when a person is in a state of irreversible apneic unconsciousness, we may consider them to be dead,” said Dr. Truog. He and his coeditorialists argued that the concept of whole brain death should be replaced with that of brain stem death.
“This report should be a call for our profession, as well as for federal and state lawmakers, to reform our laws so that they are consistent with our diagnostic criteria,” Dr. Truog said.
“The most straightforward way of doing this would be to change U.S. law and adopt the British standard of brain stem death, and then refine our testing to make the diagnosis of irreversible apneic unconsciousness as reliable and safe as possible,” he concluded.
The drafting of the consensus statement was not supported by outside funding. Dr. Sung reported no relevant financial relationships. Dr. Truog reported receiving compensation from Sanofi and Covance for participating in data and safety monitoring boards unrelated to the consensus document.
A version of this article originally appeared on Medscape.com.
Repetitive hits to the head tied to depression, poor cognition in later life
A history of repetitive hits to the head (RHI), even without noticeable symptoms, is linked to a significantly increased risk of depression and poorer cognition later in life, new research shows.
“We found that a history of exposure to [repetitive hits to the head] from contact sports, military service, or physical abuse, as well as a history of TBI (traumatic brain injury), corresponded to more symptoms of later life depression and worse cognitive function,” lead author Michael Alosco, PhD, associate professor of neurology and codirector of the Boston University Alzheimer’s Disease Center Clinical Core, told Medscape Medical News.
He added that the findings underscore the importance of assessing repetitive head impacts (RHI).
The study was published online June 26 in Neurology.
Largest study to date
It is well known that sustaining a TBI is associated with worse later life cognition or mood problems, said Alosco. However, in the current research the investigators hypothesized that RHI may be a key driver of some of these outcomes, Alosco said.
Previous studies have been small or have only examined male former football players.
“What’s unique about our study is that we focused on a history of RHIs, and it is the largest study of its kind, incorporating over 30,000 males and females with different types of exposure to these RHIs.”
The researchers used data from the Brain Health Registry, an internet-based registry that longitudinally monitors cognition and functioning of participants (age 40 years and older).
Participants completed the Ohio State University TBI Identification Method (OSU TBI-ID) and answered a yes/no question: “Have you ever had a period of time in which you experienced multiple, repeated impacts to your head (eg, history of abuse, contact sports, military duty)?”
Participants also completed the Geriatric Depression Scale (GDS-15), the CogState Battery (CBB), and the Lumos Labs NeuroCognitive Performance Tests (NCPT). Demographic information included age, sex, race/ethnicity, and level of education.
Negative synergistic effect
Of the total sample (N = 13,323, mean age 62 years, 72.5% female, 88.6% White) 725 participants (5%) reported exposure to RHI, with contact sports as the most common cause, followed by physical abuse and then military duty; about 55% (7277 participants) reported TBI.
The researchers noted that 44.4% of those exposed to RHI and 70.3% of those who reported TBI were female. However, those with a history of contact sports were predominantly male and those reporting a history of abuse were predominantly women.
Among study participants who completed the GDS-15, 16.4% reported symptoms of depression, similar to rates reported among community-dwelling older adults.
Compared to the unexposed group, participants who reported TBI with loss of consciousness (LOC) and participants who reported TBI without LOC both had higher scores on the GDS-15 (beta = 0.75 [95% CI, 0.59-0.91] and beta = 0.43 [95% CI, 0.31-0.54], respectively).
A history of RHI was associated with an even higher depression score (beta = 1.24 [95% CI, 0.36-2.12).
Depression increased in tandem with increased exposure, with the lowest GDS-15 scores found in the unexposed group and subsequent increases in scores as exposure to RHI was introduced and TBI severity increased. The GDS scores were highest in those who had RHI plus TBI with LOC.
Participants with a history of RHI and/or TBI also had worse scores on tests of memory, learning, processing speed, and reaction time, compared with unexposed participants.
In particular, TBI with LOC had the most neuropsychological associations.
TBI without LOC had a negative effect on CogState tests measuring Identification and processing speed (beta = 0.004 [95% CI, 0-0.01] and beta = 0.004 [95% CI, 0.0002-0.01], respectively), whereas RHI predicted a worse processing speed score (beta = .02 [95% CI, 0.01-0.05]).
The presence of both RHI and TBI (with or without LOC) had a “synergistic negative effect” on neuropsychological performance, with a “consistent statistically significant finding” for worse neuropsychological test performance for those who had RHI and TBI with LOC, compared with those who had not sustained RHI.
Alosco said the findings highlight the need for clinicians to educate and inform parents/guardians of kids playing (or considering playing) contact sports about the research and potential risks associated with these activities.
If we want to prevent long-term problems, one way is not to expose [people] to these hits. Everyone takes risks in life with everything, but the more we can understand and mitigate the risks, the better,” Alosco said.
“A significant contribution”
Commenting on the findings for Medscape Medical News, Temitayo Oyegbile-Chidi, MD, PhD, a pediatric neurologist with Health Peak Inc, McLean, Virginia, and a member of the American Academy of Neurology, said the study “makes a significant contribution to the literature, as neurologists who specialized in TBI have long yearned to understand the long-term effects of repeated head impact on the brain and cognition.”
Clinicians should “inquire about a history of prior head impacts on all our patients, regardless of age, especially if they are experiencing or showing signs of unexpected cognitive dysfunction or mental health concerns,” said Oyegbile-Chidi, who was not involved with the study.
For those who have sustained single or repeated head impacts with or without associated LOC in the past, “it is important … to keep in mind that depression and cognitive dysfunction may persist or present even many years after the impact was sustained,” she added.
The study was supported by a grant from the National Institutes of Health. Alosco has disclosed no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Oyegbile-Chidi has disclosed no relevant financial relationships.
A history of repetitive hits to the head (RHI), even without noticeable symptoms, is linked to a significantly increased risk of depression and poorer cognition later in life, new research shows.
“We found that a history of exposure to [repetitive hits to the head] from contact sports, military service, or physical abuse, as well as a history of TBI (traumatic brain injury), corresponded to more symptoms of later life depression and worse cognitive function,” lead author Michael Alosco, PhD, associate professor of neurology and codirector of the Boston University Alzheimer’s Disease Center Clinical Core, told Medscape Medical News.
He added that the findings underscore the importance of assessing repetitive head impacts (RHI).
The study was published online June 26 in Neurology.
Largest study to date
It is well known that sustaining a TBI is associated with worse later life cognition or mood problems, said Alosco. However, in the current research the investigators hypothesized that RHI may be a key driver of some of these outcomes, Alosco said.
Previous studies have been small or have only examined male former football players.
“What’s unique about our study is that we focused on a history of RHIs, and it is the largest study of its kind, incorporating over 30,000 males and females with different types of exposure to these RHIs.”
The researchers used data from the Brain Health Registry, an internet-based registry that longitudinally monitors cognition and functioning of participants (age 40 years and older).
Participants completed the Ohio State University TBI Identification Method (OSU TBI-ID) and answered a yes/no question: “Have you ever had a period of time in which you experienced multiple, repeated impacts to your head (eg, history of abuse, contact sports, military duty)?”
Participants also completed the Geriatric Depression Scale (GDS-15), the CogState Battery (CBB), and the Lumos Labs NeuroCognitive Performance Tests (NCPT). Demographic information included age, sex, race/ethnicity, and level of education.
Negative synergistic effect
Of the total sample (N = 13,323, mean age 62 years, 72.5% female, 88.6% White) 725 participants (5%) reported exposure to RHI, with contact sports as the most common cause, followed by physical abuse and then military duty; about 55% (7277 participants) reported TBI.
The researchers noted that 44.4% of those exposed to RHI and 70.3% of those who reported TBI were female. However, those with a history of contact sports were predominantly male and those reporting a history of abuse were predominantly women.
Among study participants who completed the GDS-15, 16.4% reported symptoms of depression, similar to rates reported among community-dwelling older adults.
Compared to the unexposed group, participants who reported TBI with loss of consciousness (LOC) and participants who reported TBI without LOC both had higher scores on the GDS-15 (beta = 0.75 [95% CI, 0.59-0.91] and beta = 0.43 [95% CI, 0.31-0.54], respectively).
A history of RHI was associated with an even higher depression score (beta = 1.24 [95% CI, 0.36-2.12).
Depression increased in tandem with increased exposure, with the lowest GDS-15 scores found in the unexposed group and subsequent increases in scores as exposure to RHI was introduced and TBI severity increased. The GDS scores were highest in those who had RHI plus TBI with LOC.
Participants with a history of RHI and/or TBI also had worse scores on tests of memory, learning, processing speed, and reaction time, compared with unexposed participants.
In particular, TBI with LOC had the most neuropsychological associations.
TBI without LOC had a negative effect on CogState tests measuring Identification and processing speed (beta = 0.004 [95% CI, 0-0.01] and beta = 0.004 [95% CI, 0.0002-0.01], respectively), whereas RHI predicted a worse processing speed score (beta = .02 [95% CI, 0.01-0.05]).
The presence of both RHI and TBI (with or without LOC) had a “synergistic negative effect” on neuropsychological performance, with a “consistent statistically significant finding” for worse neuropsychological test performance for those who had RHI and TBI with LOC, compared with those who had not sustained RHI.
Alosco said the findings highlight the need for clinicians to educate and inform parents/guardians of kids playing (or considering playing) contact sports about the research and potential risks associated with these activities.
If we want to prevent long-term problems, one way is not to expose [people] to these hits. Everyone takes risks in life with everything, but the more we can understand and mitigate the risks, the better,” Alosco said.
“A significant contribution”
Commenting on the findings for Medscape Medical News, Temitayo Oyegbile-Chidi, MD, PhD, a pediatric neurologist with Health Peak Inc, McLean, Virginia, and a member of the American Academy of Neurology, said the study “makes a significant contribution to the literature, as neurologists who specialized in TBI have long yearned to understand the long-term effects of repeated head impact on the brain and cognition.”
Clinicians should “inquire about a history of prior head impacts on all our patients, regardless of age, especially if they are experiencing or showing signs of unexpected cognitive dysfunction or mental health concerns,” said Oyegbile-Chidi, who was not involved with the study.
For those who have sustained single or repeated head impacts with or without associated LOC in the past, “it is important … to keep in mind that depression and cognitive dysfunction may persist or present even many years after the impact was sustained,” she added.
The study was supported by a grant from the National Institutes of Health. Alosco has disclosed no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Oyegbile-Chidi has disclosed no relevant financial relationships.
A history of repetitive hits to the head (RHI), even without noticeable symptoms, is linked to a significantly increased risk of depression and poorer cognition later in life, new research shows.
“We found that a history of exposure to [repetitive hits to the head] from contact sports, military service, or physical abuse, as well as a history of TBI (traumatic brain injury), corresponded to more symptoms of later life depression and worse cognitive function,” lead author Michael Alosco, PhD, associate professor of neurology and codirector of the Boston University Alzheimer’s Disease Center Clinical Core, told Medscape Medical News.
He added that the findings underscore the importance of assessing repetitive head impacts (RHI).
The study was published online June 26 in Neurology.
Largest study to date
It is well known that sustaining a TBI is associated with worse later life cognition or mood problems, said Alosco. However, in the current research the investigators hypothesized that RHI may be a key driver of some of these outcomes, Alosco said.
Previous studies have been small or have only examined male former football players.
“What’s unique about our study is that we focused on a history of RHIs, and it is the largest study of its kind, incorporating over 30,000 males and females with different types of exposure to these RHIs.”
The researchers used data from the Brain Health Registry, an internet-based registry that longitudinally monitors cognition and functioning of participants (age 40 years and older).
Participants completed the Ohio State University TBI Identification Method (OSU TBI-ID) and answered a yes/no question: “Have you ever had a period of time in which you experienced multiple, repeated impacts to your head (eg, history of abuse, contact sports, military duty)?”
Participants also completed the Geriatric Depression Scale (GDS-15), the CogState Battery (CBB), and the Lumos Labs NeuroCognitive Performance Tests (NCPT). Demographic information included age, sex, race/ethnicity, and level of education.
Negative synergistic effect
Of the total sample (N = 13,323, mean age 62 years, 72.5% female, 88.6% White) 725 participants (5%) reported exposure to RHI, with contact sports as the most common cause, followed by physical abuse and then military duty; about 55% (7277 participants) reported TBI.
The researchers noted that 44.4% of those exposed to RHI and 70.3% of those who reported TBI were female. However, those with a history of contact sports were predominantly male and those reporting a history of abuse were predominantly women.
Among study participants who completed the GDS-15, 16.4% reported symptoms of depression, similar to rates reported among community-dwelling older adults.
Compared to the unexposed group, participants who reported TBI with loss of consciousness (LOC) and participants who reported TBI without LOC both had higher scores on the GDS-15 (beta = 0.75 [95% CI, 0.59-0.91] and beta = 0.43 [95% CI, 0.31-0.54], respectively).
A history of RHI was associated with an even higher depression score (beta = 1.24 [95% CI, 0.36-2.12).
Depression increased in tandem with increased exposure, with the lowest GDS-15 scores found in the unexposed group and subsequent increases in scores as exposure to RHI was introduced and TBI severity increased. The GDS scores were highest in those who had RHI plus TBI with LOC.
Participants with a history of RHI and/or TBI also had worse scores on tests of memory, learning, processing speed, and reaction time, compared with unexposed participants.
In particular, TBI with LOC had the most neuropsychological associations.
TBI without LOC had a negative effect on CogState tests measuring Identification and processing speed (beta = 0.004 [95% CI, 0-0.01] and beta = 0.004 [95% CI, 0.0002-0.01], respectively), whereas RHI predicted a worse processing speed score (beta = .02 [95% CI, 0.01-0.05]).
The presence of both RHI and TBI (with or without LOC) had a “synergistic negative effect” on neuropsychological performance, with a “consistent statistically significant finding” for worse neuropsychological test performance for those who had RHI and TBI with LOC, compared with those who had not sustained RHI.
Alosco said the findings highlight the need for clinicians to educate and inform parents/guardians of kids playing (or considering playing) contact sports about the research and potential risks associated with these activities.
If we want to prevent long-term problems, one way is not to expose [people] to these hits. Everyone takes risks in life with everything, but the more we can understand and mitigate the risks, the better,” Alosco said.
“A significant contribution”
Commenting on the findings for Medscape Medical News, Temitayo Oyegbile-Chidi, MD, PhD, a pediatric neurologist with Health Peak Inc, McLean, Virginia, and a member of the American Academy of Neurology, said the study “makes a significant contribution to the literature, as neurologists who specialized in TBI have long yearned to understand the long-term effects of repeated head impact on the brain and cognition.”
Clinicians should “inquire about a history of prior head impacts on all our patients, regardless of age, especially if they are experiencing or showing signs of unexpected cognitive dysfunction or mental health concerns,” said Oyegbile-Chidi, who was not involved with the study.
For those who have sustained single or repeated head impacts with or without associated LOC in the past, “it is important … to keep in mind that depression and cognitive dysfunction may persist or present even many years after the impact was sustained,” she added.
The study was supported by a grant from the National Institutes of Health. Alosco has disclosed no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Oyegbile-Chidi has disclosed no relevant financial relationships.
Blood biomarker detects concussion, shows severity, predicts recovery
(TBI), new research indicates.
“Blood NfL may be used to aid in the diagnosis of patients with concussion or mild TBI [and] to identify individuals at increased risk of developing persistent postconcussive symptoms following TBI,” said lead author Pashtun Shahim, MD, PhD, National Institutes of Health Clinical Center, Bethesda, Md.
“This study is the first to do a detailed assessment of serum NfL chain and advanced brain imaging in multiple cohorts, brain injury severities, and time points after injury. The cohorts included professional athletes and nonathletes, and over time up to 5 years after TBI,” Dr. Shahim added.
The study was published online July 8 in Neurology.
Rapid indicator of neuronal damage
The researchers studied two cohorts of patients with head injuries. In the first, they determined serum and CSF NfL chain levels in professional Swedish ice hockey players (median age, 27 years), including 45 with acute concussion, 31 with repetitive concussions and persistent post-concussive symptoms (PCS), 28 who contributed samples during preseason with no recent concussion, and 14 healthy nonathletes.
CSF and serum NfL concentrations were closely correlated (r = 0.71; P < .0001). Serum NfL distinguished players with persistent PCS due to repetitive concussions from preseason concussion-free players, with an area under the receiver operating characteristic curve of 0.97. Higher CSF and serum NfL levels were associated with a higher number of concussions and severity of PCS after 1 year.
The second cohort involved 230 clinic-based adults (mean age, 43 years), including 162 with TBI and 68 healthy controls. In this cohort, patients with TBI had increased serum NfL concentrations compared with controls for up to 5 years, and these concentrations were able to distinguish between mild, moderate, and severe TBI. Serum NfL also correlated with measures of functional outcome, MRI brain atrophy, and diffusion tensor imaging estimates of traumatic axonal injury.
“Our findings suggest that NfL concentrations in serum offer rapid and accessible means of assessing and predicting neuronal damage in patients with TBI,” the investigators wrote.
What’s needed going forward, said Dr. Shahim, is “validation in larger cohorts for determining what levels of NfL in blood may be associated with a specific type of TBI, and what the levels are in healthy individuals of different ages.”
Not ready for prime time
In an accompanying editorial, Christopher Filley, MD, University of Colorado at Denver, Aurora, noted that NfL “may prove useful in identifying TBI patients at risk for prolonged symptoms and in enabling more focused treatment for these individuals.”
“These reports are richly laden with acute and longitudinal data that not only support the use of NfL as a convenient diagnostic test for TBI, but plausibly correlate with the neuropathology of TBI that is thought to play a major role in immediate and lasting cognitive disability,” he wrote.
Although the origin of TBI-induced cognitive decline is not entirely explained by traumatic axonal injury, “NfL appears to have much promise as a blood test that relates directly to the ubiquitous white matter damage of TBI, revealing a great deal about not only whether a TBI occurred, but also the extent of injury sustained, and how this injury may affect patient outcome for years thereafter,” Dr. Filley wrote.
However, he cautioned more research is needed before the blood test can be routinely applied to TBI diagnosis in clinical practice. “Among the hurdles still ahead are the standardization of measurement techniques across analytical platforms, and the determination of precise cutoffs between normal and abnormal values in different ages groups and at varying levels of TBI severity,” Dr. Filley noted.
The research was supported by the National Institutes of Health, the Department of Defense, the Center for Neuroscience and Regenerative Medicine at the Uniformed Services University, and the Swedish Research Council. Dr. Shahim and Dr. Filley have reported no relevant financial relationships.
This article first appeared on Medscape.com.
(TBI), new research indicates.
“Blood NfL may be used to aid in the diagnosis of patients with concussion or mild TBI [and] to identify individuals at increased risk of developing persistent postconcussive symptoms following TBI,” said lead author Pashtun Shahim, MD, PhD, National Institutes of Health Clinical Center, Bethesda, Md.
“This study is the first to do a detailed assessment of serum NfL chain and advanced brain imaging in multiple cohorts, brain injury severities, and time points after injury. The cohorts included professional athletes and nonathletes, and over time up to 5 years after TBI,” Dr. Shahim added.
The study was published online July 8 in Neurology.
Rapid indicator of neuronal damage
The researchers studied two cohorts of patients with head injuries. In the first, they determined serum and CSF NfL chain levels in professional Swedish ice hockey players (median age, 27 years), including 45 with acute concussion, 31 with repetitive concussions and persistent post-concussive symptoms (PCS), 28 who contributed samples during preseason with no recent concussion, and 14 healthy nonathletes.
CSF and serum NfL concentrations were closely correlated (r = 0.71; P < .0001). Serum NfL distinguished players with persistent PCS due to repetitive concussions from preseason concussion-free players, with an area under the receiver operating characteristic curve of 0.97. Higher CSF and serum NfL levels were associated with a higher number of concussions and severity of PCS after 1 year.
The second cohort involved 230 clinic-based adults (mean age, 43 years), including 162 with TBI and 68 healthy controls. In this cohort, patients with TBI had increased serum NfL concentrations compared with controls for up to 5 years, and these concentrations were able to distinguish between mild, moderate, and severe TBI. Serum NfL also correlated with measures of functional outcome, MRI brain atrophy, and diffusion tensor imaging estimates of traumatic axonal injury.
“Our findings suggest that NfL concentrations in serum offer rapid and accessible means of assessing and predicting neuronal damage in patients with TBI,” the investigators wrote.
What’s needed going forward, said Dr. Shahim, is “validation in larger cohorts for determining what levels of NfL in blood may be associated with a specific type of TBI, and what the levels are in healthy individuals of different ages.”
Not ready for prime time
In an accompanying editorial, Christopher Filley, MD, University of Colorado at Denver, Aurora, noted that NfL “may prove useful in identifying TBI patients at risk for prolonged symptoms and in enabling more focused treatment for these individuals.”
“These reports are richly laden with acute and longitudinal data that not only support the use of NfL as a convenient diagnostic test for TBI, but plausibly correlate with the neuropathology of TBI that is thought to play a major role in immediate and lasting cognitive disability,” he wrote.
Although the origin of TBI-induced cognitive decline is not entirely explained by traumatic axonal injury, “NfL appears to have much promise as a blood test that relates directly to the ubiquitous white matter damage of TBI, revealing a great deal about not only whether a TBI occurred, but also the extent of injury sustained, and how this injury may affect patient outcome for years thereafter,” Dr. Filley wrote.
However, he cautioned more research is needed before the blood test can be routinely applied to TBI diagnosis in clinical practice. “Among the hurdles still ahead are the standardization of measurement techniques across analytical platforms, and the determination of precise cutoffs between normal and abnormal values in different ages groups and at varying levels of TBI severity,” Dr. Filley noted.
The research was supported by the National Institutes of Health, the Department of Defense, the Center for Neuroscience and Regenerative Medicine at the Uniformed Services University, and the Swedish Research Council. Dr. Shahim and Dr. Filley have reported no relevant financial relationships.
This article first appeared on Medscape.com.
(TBI), new research indicates.
“Blood NfL may be used to aid in the diagnosis of patients with concussion or mild TBI [and] to identify individuals at increased risk of developing persistent postconcussive symptoms following TBI,” said lead author Pashtun Shahim, MD, PhD, National Institutes of Health Clinical Center, Bethesda, Md.
“This study is the first to do a detailed assessment of serum NfL chain and advanced brain imaging in multiple cohorts, brain injury severities, and time points after injury. The cohorts included professional athletes and nonathletes, and over time up to 5 years after TBI,” Dr. Shahim added.
The study was published online July 8 in Neurology.
Rapid indicator of neuronal damage
The researchers studied two cohorts of patients with head injuries. In the first, they determined serum and CSF NfL chain levels in professional Swedish ice hockey players (median age, 27 years), including 45 with acute concussion, 31 with repetitive concussions and persistent post-concussive symptoms (PCS), 28 who contributed samples during preseason with no recent concussion, and 14 healthy nonathletes.
CSF and serum NfL concentrations were closely correlated (r = 0.71; P < .0001). Serum NfL distinguished players with persistent PCS due to repetitive concussions from preseason concussion-free players, with an area under the receiver operating characteristic curve of 0.97. Higher CSF and serum NfL levels were associated with a higher number of concussions and severity of PCS after 1 year.
The second cohort involved 230 clinic-based adults (mean age, 43 years), including 162 with TBI and 68 healthy controls. In this cohort, patients with TBI had increased serum NfL concentrations compared with controls for up to 5 years, and these concentrations were able to distinguish between mild, moderate, and severe TBI. Serum NfL also correlated with measures of functional outcome, MRI brain atrophy, and diffusion tensor imaging estimates of traumatic axonal injury.
“Our findings suggest that NfL concentrations in serum offer rapid and accessible means of assessing and predicting neuronal damage in patients with TBI,” the investigators wrote.
What’s needed going forward, said Dr. Shahim, is “validation in larger cohorts for determining what levels of NfL in blood may be associated with a specific type of TBI, and what the levels are in healthy individuals of different ages.”
Not ready for prime time
In an accompanying editorial, Christopher Filley, MD, University of Colorado at Denver, Aurora, noted that NfL “may prove useful in identifying TBI patients at risk for prolonged symptoms and in enabling more focused treatment for these individuals.”
“These reports are richly laden with acute and longitudinal data that not only support the use of NfL as a convenient diagnostic test for TBI, but plausibly correlate with the neuropathology of TBI that is thought to play a major role in immediate and lasting cognitive disability,” he wrote.
Although the origin of TBI-induced cognitive decline is not entirely explained by traumatic axonal injury, “NfL appears to have much promise as a blood test that relates directly to the ubiquitous white matter damage of TBI, revealing a great deal about not only whether a TBI occurred, but also the extent of injury sustained, and how this injury may affect patient outcome for years thereafter,” Dr. Filley wrote.
However, he cautioned more research is needed before the blood test can be routinely applied to TBI diagnosis in clinical practice. “Among the hurdles still ahead are the standardization of measurement techniques across analytical platforms, and the determination of precise cutoffs between normal and abnormal values in different ages groups and at varying levels of TBI severity,” Dr. Filley noted.
The research was supported by the National Institutes of Health, the Department of Defense, the Center for Neuroscience and Regenerative Medicine at the Uniformed Services University, and the Swedish Research Council. Dr. Shahim and Dr. Filley have reported no relevant financial relationships.
This article first appeared on Medscape.com.
Epilepsy after TBI linked to worse 12-month outcomes
findings from an analysis of a large, prospective database suggest. “We found that patients essentially have a 10-times greater risk of developing posttraumatic epilepsy and seizures at 12 months [post injury] if the presenting Glasgow Coma Scale GCS) is less than 8,” said lead author John F. Burke, MD, PhD, University of California, San Francisco, in presenting the findings as part of the virtual annual meeting of the American Association of Neurological Surgeons.
Assessing risk factors
While posttraumatic epilepsy represents an estimated 20% of all cases of symptomatic epilepsy, many questions remain on those most at risk and on the long-term effects of posttraumatic epilepsy on TBI outcomes. To probe those issues, Dr. Burke and colleagues turned to the multicenter TRACK-TBI database, which has prospective, longitudinal data on more than 2,700 patients with traumatic brain injuries and is considered the largest source of prospective data on posttraumatic epilepsy.
Using the criteria of no previous epilepsy and having 12 months of follow-up, the team identified 1,493 patients with TBI. In addition, investigators identified 182 orthopedic controls (included and prospectively followed because they have injuries but not specifically head trauma) and 210 controls who are friends of the patients and who do not have injuries but allow researchers to control for socioeconomic and environmental factors.
Of the 1,493 patients with TBI, 41 (2.7%) were determined to have posttraumatic epilepsy, assessed according to a National Institute of Neurological Disorders and Stroke epilepsy screening questionnaire, which is designed to identify patients with posttraumatic epilepsy symptoms. There were no reports of epilepsy symptoms using the screening tool among the controls. Dr. Burke noted that the 2.7% was in agreement with historical reports.
In comparing patients with TBI who did and did not have posttraumatic epilepsy, no differences were observed in the groups in terms of gender, although there was a trend toward younger age among those with PTE (mean age, 35.4 years with posttraumatic injury vs. 41.5 without; P = .05).
A major risk factor for the development of posttraumatic epilepsy was presenting GCS scores. Among those with scores of less than 8, indicative of severe injury, the rate of posttraumatic epilepsy was 6% at 6 months and 12.5% at 12 months. In contrast, those with TBI presenting with GCS scores between 13 and 15, indicative of minor injury, had an incidence of posttraumatic epilepsy of 0.9% at 6 months and 1.4% at 12 months.
Imaging findings in the two groups showed that hemorrhage detected on CT imaging was associated with a significantly higher risk for posttraumatic epilepsy (P < .001).
“The main takeaway is that any hemorrhage in the brain is a major risk factor for developing seizures,” Dr. Burke said. “Whether it is subdural, epidural blood, subarachnoid or contusion, any blood confers a very [high] risk for developing seizures.”
Posttraumatic epilepsy was linked to poorer longer-term outcomes even for patients with lesser injury: Among those with TBI and GCS of 13-15, the mean Glasgow Outcome Scale Extended (GOSE) score at 12 months among those without posttraumatic epilepsy was 7, indicative of a good recovery with minor defects, whereas the mean GOSE score for those with PTE was 4.6, indicative of moderate to severe disability (P < .001).
“It was surprising to us that PTE-positive patients had a very significant decrease in GOSE, compared to PTE-negative patients,” Dr. Burke said. “There was a nearly 2-point drop in the GOSE and that was extremely significant.”
A multivariate analysis showed there was still a significant independent risk for a poor GOSE score with posttraumatic epilepsy after controlling for GCS score, head CT findings, and age (P < .001).
The authors also looked at mood outcomes using the Brief Symptom Inventory–18, which showed significant worse effect in those with posttraumatic epilepsy after multivariate adjustment (P = .01). Additionally, a highly significant worse effect in cognitive outcomes on the Rivermead cognitive metric was observed with posttraumatic epilepsy (P = .001).
“On all metrics tested, posttraumatic epilepsy worsened outcomes,” Dr. Burke said.
He noted that the study has some key limitations, including the 12-month follow-up. A previous study showed a linear increase in posttraumatic follow-up up to 30 years. “The fact that we found 41 patients at 12 months indicates there are probably more that are out there who are going to develop seizures, but because we don’t have the follow-up we can’t look at that.”
Although the screening questionnaires are effective, “the issue is these people are not being seen by an epileptologist or having scalp EEG done, and we need a more accurate way to do this,” he said. A new study, TRACK-TBI EPI, will address those limitations and a host of other issues with a 5-year follow-up.
Capturing the nuances of brain injury
Commenting on the study as a discussant, neurosurgeon Uzma Samadani, MD, PhD, of the Minneapolis Veterans Affairs Medical Center and CentraCare in Minneapolis, suggested that the future work should focus on issues including the wide-ranging mechanisms that could explain the seizure activity.
“For example, it’s known that posttraumatic epilepsy or seizures can be triggered by abnormal conductivity due to multiple different mechanisms associated with brain injury, such as endocrine dysfunction, cortical-spreading depression, and many others,” said Dr. Samadani, who has been a researcher on the TRACK-TBI study.
Factors ranging from genetic differences to comorbid conditions such as alcoholism can play a role in brain injury susceptibility, Dr. Samadani added. Furthermore, outcome measures currently available simply may not capture the unknown nuances of brain injury.
“We have to ask, are these an all-or-none phenomena, or is aberrant electrical activity after brain injury a continuum of dysfunction?” Dr. Samadani speculated.
“I would caution that we are likely underestimating the non–easily measurable consequences of brain injury,” she said. “And the better we can quantitate susceptibility, classify the nature of injury and target acute management, the less posttraumatic epilepsy/aberrant electrical activity our patients will have.”
Dr. Burke and Dr. Samadani disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
findings from an analysis of a large, prospective database suggest. “We found that patients essentially have a 10-times greater risk of developing posttraumatic epilepsy and seizures at 12 months [post injury] if the presenting Glasgow Coma Scale GCS) is less than 8,” said lead author John F. Burke, MD, PhD, University of California, San Francisco, in presenting the findings as part of the virtual annual meeting of the American Association of Neurological Surgeons.
Assessing risk factors
While posttraumatic epilepsy represents an estimated 20% of all cases of symptomatic epilepsy, many questions remain on those most at risk and on the long-term effects of posttraumatic epilepsy on TBI outcomes. To probe those issues, Dr. Burke and colleagues turned to the multicenter TRACK-TBI database, which has prospective, longitudinal data on more than 2,700 patients with traumatic brain injuries and is considered the largest source of prospective data on posttraumatic epilepsy.
Using the criteria of no previous epilepsy and having 12 months of follow-up, the team identified 1,493 patients with TBI. In addition, investigators identified 182 orthopedic controls (included and prospectively followed because they have injuries but not specifically head trauma) and 210 controls who are friends of the patients and who do not have injuries but allow researchers to control for socioeconomic and environmental factors.
Of the 1,493 patients with TBI, 41 (2.7%) were determined to have posttraumatic epilepsy, assessed according to a National Institute of Neurological Disorders and Stroke epilepsy screening questionnaire, which is designed to identify patients with posttraumatic epilepsy symptoms. There were no reports of epilepsy symptoms using the screening tool among the controls. Dr. Burke noted that the 2.7% was in agreement with historical reports.
In comparing patients with TBI who did and did not have posttraumatic epilepsy, no differences were observed in the groups in terms of gender, although there was a trend toward younger age among those with PTE (mean age, 35.4 years with posttraumatic injury vs. 41.5 without; P = .05).
A major risk factor for the development of posttraumatic epilepsy was presenting GCS scores. Among those with scores of less than 8, indicative of severe injury, the rate of posttraumatic epilepsy was 6% at 6 months and 12.5% at 12 months. In contrast, those with TBI presenting with GCS scores between 13 and 15, indicative of minor injury, had an incidence of posttraumatic epilepsy of 0.9% at 6 months and 1.4% at 12 months.
Imaging findings in the two groups showed that hemorrhage detected on CT imaging was associated with a significantly higher risk for posttraumatic epilepsy (P < .001).
“The main takeaway is that any hemorrhage in the brain is a major risk factor for developing seizures,” Dr. Burke said. “Whether it is subdural, epidural blood, subarachnoid or contusion, any blood confers a very [high] risk for developing seizures.”
Posttraumatic epilepsy was linked to poorer longer-term outcomes even for patients with lesser injury: Among those with TBI and GCS of 13-15, the mean Glasgow Outcome Scale Extended (GOSE) score at 12 months among those without posttraumatic epilepsy was 7, indicative of a good recovery with minor defects, whereas the mean GOSE score for those with PTE was 4.6, indicative of moderate to severe disability (P < .001).
“It was surprising to us that PTE-positive patients had a very significant decrease in GOSE, compared to PTE-negative patients,” Dr. Burke said. “There was a nearly 2-point drop in the GOSE and that was extremely significant.”
A multivariate analysis showed there was still a significant independent risk for a poor GOSE score with posttraumatic epilepsy after controlling for GCS score, head CT findings, and age (P < .001).
The authors also looked at mood outcomes using the Brief Symptom Inventory–18, which showed significant worse effect in those with posttraumatic epilepsy after multivariate adjustment (P = .01). Additionally, a highly significant worse effect in cognitive outcomes on the Rivermead cognitive metric was observed with posttraumatic epilepsy (P = .001).
“On all metrics tested, posttraumatic epilepsy worsened outcomes,” Dr. Burke said.
He noted that the study has some key limitations, including the 12-month follow-up. A previous study showed a linear increase in posttraumatic follow-up up to 30 years. “The fact that we found 41 patients at 12 months indicates there are probably more that are out there who are going to develop seizures, but because we don’t have the follow-up we can’t look at that.”
Although the screening questionnaires are effective, “the issue is these people are not being seen by an epileptologist or having scalp EEG done, and we need a more accurate way to do this,” he said. A new study, TRACK-TBI EPI, will address those limitations and a host of other issues with a 5-year follow-up.
Capturing the nuances of brain injury
Commenting on the study as a discussant, neurosurgeon Uzma Samadani, MD, PhD, of the Minneapolis Veterans Affairs Medical Center and CentraCare in Minneapolis, suggested that the future work should focus on issues including the wide-ranging mechanisms that could explain the seizure activity.
“For example, it’s known that posttraumatic epilepsy or seizures can be triggered by abnormal conductivity due to multiple different mechanisms associated with brain injury, such as endocrine dysfunction, cortical-spreading depression, and many others,” said Dr. Samadani, who has been a researcher on the TRACK-TBI study.
Factors ranging from genetic differences to comorbid conditions such as alcoholism can play a role in brain injury susceptibility, Dr. Samadani added. Furthermore, outcome measures currently available simply may not capture the unknown nuances of brain injury.
“We have to ask, are these an all-or-none phenomena, or is aberrant electrical activity after brain injury a continuum of dysfunction?” Dr. Samadani speculated.
“I would caution that we are likely underestimating the non–easily measurable consequences of brain injury,” she said. “And the better we can quantitate susceptibility, classify the nature of injury and target acute management, the less posttraumatic epilepsy/aberrant electrical activity our patients will have.”
Dr. Burke and Dr. Samadani disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
findings from an analysis of a large, prospective database suggest. “We found that patients essentially have a 10-times greater risk of developing posttraumatic epilepsy and seizures at 12 months [post injury] if the presenting Glasgow Coma Scale GCS) is less than 8,” said lead author John F. Burke, MD, PhD, University of California, San Francisco, in presenting the findings as part of the virtual annual meeting of the American Association of Neurological Surgeons.
Assessing risk factors
While posttraumatic epilepsy represents an estimated 20% of all cases of symptomatic epilepsy, many questions remain on those most at risk and on the long-term effects of posttraumatic epilepsy on TBI outcomes. To probe those issues, Dr. Burke and colleagues turned to the multicenter TRACK-TBI database, which has prospective, longitudinal data on more than 2,700 patients with traumatic brain injuries and is considered the largest source of prospective data on posttraumatic epilepsy.
Using the criteria of no previous epilepsy and having 12 months of follow-up, the team identified 1,493 patients with TBI. In addition, investigators identified 182 orthopedic controls (included and prospectively followed because they have injuries but not specifically head trauma) and 210 controls who are friends of the patients and who do not have injuries but allow researchers to control for socioeconomic and environmental factors.
Of the 1,493 patients with TBI, 41 (2.7%) were determined to have posttraumatic epilepsy, assessed according to a National Institute of Neurological Disorders and Stroke epilepsy screening questionnaire, which is designed to identify patients with posttraumatic epilepsy symptoms. There were no reports of epilepsy symptoms using the screening tool among the controls. Dr. Burke noted that the 2.7% was in agreement with historical reports.
In comparing patients with TBI who did and did not have posttraumatic epilepsy, no differences were observed in the groups in terms of gender, although there was a trend toward younger age among those with PTE (mean age, 35.4 years with posttraumatic injury vs. 41.5 without; P = .05).
A major risk factor for the development of posttraumatic epilepsy was presenting GCS scores. Among those with scores of less than 8, indicative of severe injury, the rate of posttraumatic epilepsy was 6% at 6 months and 12.5% at 12 months. In contrast, those with TBI presenting with GCS scores between 13 and 15, indicative of minor injury, had an incidence of posttraumatic epilepsy of 0.9% at 6 months and 1.4% at 12 months.
Imaging findings in the two groups showed that hemorrhage detected on CT imaging was associated with a significantly higher risk for posttraumatic epilepsy (P < .001).
“The main takeaway is that any hemorrhage in the brain is a major risk factor for developing seizures,” Dr. Burke said. “Whether it is subdural, epidural blood, subarachnoid or contusion, any blood confers a very [high] risk for developing seizures.”
Posttraumatic epilepsy was linked to poorer longer-term outcomes even for patients with lesser injury: Among those with TBI and GCS of 13-15, the mean Glasgow Outcome Scale Extended (GOSE) score at 12 months among those without posttraumatic epilepsy was 7, indicative of a good recovery with minor defects, whereas the mean GOSE score for those with PTE was 4.6, indicative of moderate to severe disability (P < .001).
“It was surprising to us that PTE-positive patients had a very significant decrease in GOSE, compared to PTE-negative patients,” Dr. Burke said. “There was a nearly 2-point drop in the GOSE and that was extremely significant.”
A multivariate analysis showed there was still a significant independent risk for a poor GOSE score with posttraumatic epilepsy after controlling for GCS score, head CT findings, and age (P < .001).
The authors also looked at mood outcomes using the Brief Symptom Inventory–18, which showed significant worse effect in those with posttraumatic epilepsy after multivariate adjustment (P = .01). Additionally, a highly significant worse effect in cognitive outcomes on the Rivermead cognitive metric was observed with posttraumatic epilepsy (P = .001).
“On all metrics tested, posttraumatic epilepsy worsened outcomes,” Dr. Burke said.
He noted that the study has some key limitations, including the 12-month follow-up. A previous study showed a linear increase in posttraumatic follow-up up to 30 years. “The fact that we found 41 patients at 12 months indicates there are probably more that are out there who are going to develop seizures, but because we don’t have the follow-up we can’t look at that.”
Although the screening questionnaires are effective, “the issue is these people are not being seen by an epileptologist or having scalp EEG done, and we need a more accurate way to do this,” he said. A new study, TRACK-TBI EPI, will address those limitations and a host of other issues with a 5-year follow-up.
Capturing the nuances of brain injury
Commenting on the study as a discussant, neurosurgeon Uzma Samadani, MD, PhD, of the Minneapolis Veterans Affairs Medical Center and CentraCare in Minneapolis, suggested that the future work should focus on issues including the wide-ranging mechanisms that could explain the seizure activity.
“For example, it’s known that posttraumatic epilepsy or seizures can be triggered by abnormal conductivity due to multiple different mechanisms associated with brain injury, such as endocrine dysfunction, cortical-spreading depression, and many others,” said Dr. Samadani, who has been a researcher on the TRACK-TBI study.
Factors ranging from genetic differences to comorbid conditions such as alcoholism can play a role in brain injury susceptibility, Dr. Samadani added. Furthermore, outcome measures currently available simply may not capture the unknown nuances of brain injury.
“We have to ask, are these an all-or-none phenomena, or is aberrant electrical activity after brain injury a continuum of dysfunction?” Dr. Samadani speculated.
“I would caution that we are likely underestimating the non–easily measurable consequences of brain injury,” she said. “And the better we can quantitate susceptibility, classify the nature of injury and target acute management, the less posttraumatic epilepsy/aberrant electrical activity our patients will have.”
Dr. Burke and Dr. Samadani disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM AANS 2020
Persistent posttraumatic headache risk factors confirmed
It also revealed a surprisingly high frequency of misdiagnosis. The original sample included 500 patients drawn from the Stanford Research Repository Cohort Discovery Tool, but a review found 200 records that were misdiagnosed and had to be excluded.
“It’s very easy to label someone who suffered a head injury and say this is the reason why they have this (headache),” said lead author Tommy Chan, MBBS, a headache fellow in the department of neurology at Stanford (Calif.) University, in an interview. Such patients are often seen by ED or primary care physicians who do not have a lot of experience with posttraumatic headache, and that can lead to negative consequences if a low-pressure headache is mistaken as stemming from a skull fracture. “It’s a very different treatment plan for one versus the other,” said Dr. Chan in an interview.
He noted that it can help to take a patient history that includes the preaccident headache frequency and determine if there was a change in frequency post injury.
Dr. Chan presented the results at the virtual annual meeting of the American Headache Society.
“The results are what one might expect, although we haven’t studied it enough to really know. We haven’t systematically characterized these risk factors for chronic posttraumatic headache very well, [so] it’s useful to have this information,” said Andrew Charles, MD, professor neurology at the University of California, Los Angeles, and director of the UCLA Goldberg Migraine Program, who was not involved in the study. However, Dr. Charles emphasized the need to confirm the results prospectively.
Defining risk factors
The analysis found that a history of migraines, medication overuse, psychological disorders, and new posttraumatic headache–associated comorbidities were all associated with a greater risk for persistent posttraumatic headache. None of those came as a surprise, “but we live in a world where medicine is practiced based on evidence, and providers want to see data to support that. I think that this will help with resource allocation. It’s important to address [a patient’s] overuse of medications, or if they’re having psychological symptoms,” said Dr. Chan.
A total of 150 patients in the analysis had acute posttraumatic headache (mean duration, 0.7 months) while 150 had persistent posttraumatic headache (mean duration, 24 months; P < .00001). Clinical factors associated with risk of persistent headache included a history migraine (relative risk, 2.4; P < .0001), a previous head injury (odds ratio, 5.8; P < .0001), medication overuse (RR, 2.6; P < .0001), preexisting psychological history (OR, 5; P < .0001), and new posttraumatic headache–associated comorbidities, such as vertigo or posttraumatic stress disorder (RR, 9.8; P < .0001).
Identifying patient subgroups
The researchers also identified four subcategories of patients with persistent posttraumatic headache, each with differing risk factors and clinical characteristics. It’s too soon to use these identifiers to make clinical recommendations, but Dr. Chan hopes that further study of these groups will be informative. “It might point us toward (the idea) that each patient population is actually different, even within the chronic persistent posttraumatic headache population, we can’t group them all under the same umbrella term. If we can tease out that a patient has truly had a head injury, but no history of migraine, no overuse of medication, no psychological history, and no other associated symptoms, this would be a very interesting population to study because they would help us understand the pathophysiology [of persistent posttraumatic headache].”
Although the study was conducted by defining persistent posttraumatic headache as lasting at least 3 months, Dr. Chan took issue with that commonly held definition. That choice is arbitrary, with no pathophysiological basis or data to support it, and is based more on clinical trials testing preventative treatments. But when it is used in clinical practice, it can muddy communication with patients. “When this timeline is told to a patient, and when it’s not achieved, they might become disappointed. We should not put too much emphasis on time. Everybody is different,” he said.
The study did not receive any funding. Dr. Chan had no relevant financial disclosures. Dr. Charles consults for consults for Amgen, BioHaven, Eli Lilly, Novartis, and Lundbeck.
It also revealed a surprisingly high frequency of misdiagnosis. The original sample included 500 patients drawn from the Stanford Research Repository Cohort Discovery Tool, but a review found 200 records that were misdiagnosed and had to be excluded.
“It’s very easy to label someone who suffered a head injury and say this is the reason why they have this (headache),” said lead author Tommy Chan, MBBS, a headache fellow in the department of neurology at Stanford (Calif.) University, in an interview. Such patients are often seen by ED or primary care physicians who do not have a lot of experience with posttraumatic headache, and that can lead to negative consequences if a low-pressure headache is mistaken as stemming from a skull fracture. “It’s a very different treatment plan for one versus the other,” said Dr. Chan in an interview.
He noted that it can help to take a patient history that includes the preaccident headache frequency and determine if there was a change in frequency post injury.
Dr. Chan presented the results at the virtual annual meeting of the American Headache Society.
“The results are what one might expect, although we haven’t studied it enough to really know. We haven’t systematically characterized these risk factors for chronic posttraumatic headache very well, [so] it’s useful to have this information,” said Andrew Charles, MD, professor neurology at the University of California, Los Angeles, and director of the UCLA Goldberg Migraine Program, who was not involved in the study. However, Dr. Charles emphasized the need to confirm the results prospectively.
Defining risk factors
The analysis found that a history of migraines, medication overuse, psychological disorders, and new posttraumatic headache–associated comorbidities were all associated with a greater risk for persistent posttraumatic headache. None of those came as a surprise, “but we live in a world where medicine is practiced based on evidence, and providers want to see data to support that. I think that this will help with resource allocation. It’s important to address [a patient’s] overuse of medications, or if they’re having psychological symptoms,” said Dr. Chan.
A total of 150 patients in the analysis had acute posttraumatic headache (mean duration, 0.7 months) while 150 had persistent posttraumatic headache (mean duration, 24 months; P < .00001). Clinical factors associated with risk of persistent headache included a history migraine (relative risk, 2.4; P < .0001), a previous head injury (odds ratio, 5.8; P < .0001), medication overuse (RR, 2.6; P < .0001), preexisting psychological history (OR, 5; P < .0001), and new posttraumatic headache–associated comorbidities, such as vertigo or posttraumatic stress disorder (RR, 9.8; P < .0001).
Identifying patient subgroups
The researchers also identified four subcategories of patients with persistent posttraumatic headache, each with differing risk factors and clinical characteristics. It’s too soon to use these identifiers to make clinical recommendations, but Dr. Chan hopes that further study of these groups will be informative. “It might point us toward (the idea) that each patient population is actually different, even within the chronic persistent posttraumatic headache population, we can’t group them all under the same umbrella term. If we can tease out that a patient has truly had a head injury, but no history of migraine, no overuse of medication, no psychological history, and no other associated symptoms, this would be a very interesting population to study because they would help us understand the pathophysiology [of persistent posttraumatic headache].”
Although the study was conducted by defining persistent posttraumatic headache as lasting at least 3 months, Dr. Chan took issue with that commonly held definition. That choice is arbitrary, with no pathophysiological basis or data to support it, and is based more on clinical trials testing preventative treatments. But when it is used in clinical practice, it can muddy communication with patients. “When this timeline is told to a patient, and when it’s not achieved, they might become disappointed. We should not put too much emphasis on time. Everybody is different,” he said.
The study did not receive any funding. Dr. Chan had no relevant financial disclosures. Dr. Charles consults for consults for Amgen, BioHaven, Eli Lilly, Novartis, and Lundbeck.
It also revealed a surprisingly high frequency of misdiagnosis. The original sample included 500 patients drawn from the Stanford Research Repository Cohort Discovery Tool, but a review found 200 records that were misdiagnosed and had to be excluded.
“It’s very easy to label someone who suffered a head injury and say this is the reason why they have this (headache),” said lead author Tommy Chan, MBBS, a headache fellow in the department of neurology at Stanford (Calif.) University, in an interview. Such patients are often seen by ED or primary care physicians who do not have a lot of experience with posttraumatic headache, and that can lead to negative consequences if a low-pressure headache is mistaken as stemming from a skull fracture. “It’s a very different treatment plan for one versus the other,” said Dr. Chan in an interview.
He noted that it can help to take a patient history that includes the preaccident headache frequency and determine if there was a change in frequency post injury.
Dr. Chan presented the results at the virtual annual meeting of the American Headache Society.
“The results are what one might expect, although we haven’t studied it enough to really know. We haven’t systematically characterized these risk factors for chronic posttraumatic headache very well, [so] it’s useful to have this information,” said Andrew Charles, MD, professor neurology at the University of California, Los Angeles, and director of the UCLA Goldberg Migraine Program, who was not involved in the study. However, Dr. Charles emphasized the need to confirm the results prospectively.
Defining risk factors
The analysis found that a history of migraines, medication overuse, psychological disorders, and new posttraumatic headache–associated comorbidities were all associated with a greater risk for persistent posttraumatic headache. None of those came as a surprise, “but we live in a world where medicine is practiced based on evidence, and providers want to see data to support that. I think that this will help with resource allocation. It’s important to address [a patient’s] overuse of medications, or if they’re having psychological symptoms,” said Dr. Chan.
A total of 150 patients in the analysis had acute posttraumatic headache (mean duration, 0.7 months) while 150 had persistent posttraumatic headache (mean duration, 24 months; P < .00001). Clinical factors associated with risk of persistent headache included a history migraine (relative risk, 2.4; P < .0001), a previous head injury (odds ratio, 5.8; P < .0001), medication overuse (RR, 2.6; P < .0001), preexisting psychological history (OR, 5; P < .0001), and new posttraumatic headache–associated comorbidities, such as vertigo or posttraumatic stress disorder (RR, 9.8; P < .0001).
Identifying patient subgroups
The researchers also identified four subcategories of patients with persistent posttraumatic headache, each with differing risk factors and clinical characteristics. It’s too soon to use these identifiers to make clinical recommendations, but Dr. Chan hopes that further study of these groups will be informative. “It might point us toward (the idea) that each patient population is actually different, even within the chronic persistent posttraumatic headache population, we can’t group them all under the same umbrella term. If we can tease out that a patient has truly had a head injury, but no history of migraine, no overuse of medication, no psychological history, and no other associated symptoms, this would be a very interesting population to study because they would help us understand the pathophysiology [of persistent posttraumatic headache].”
Although the study was conducted by defining persistent posttraumatic headache as lasting at least 3 months, Dr. Chan took issue with that commonly held definition. That choice is arbitrary, with no pathophysiological basis or data to support it, and is based more on clinical trials testing preventative treatments. But when it is used in clinical practice, it can muddy communication with patients. “When this timeline is told to a patient, and when it’s not achieved, they might become disappointed. We should not put too much emphasis on time. Everybody is different,” he said.
The study did not receive any funding. Dr. Chan had no relevant financial disclosures. Dr. Charles consults for consults for Amgen, BioHaven, Eli Lilly, Novartis, and Lundbeck.
FROM AHS 2020