User login
Prior head injury is associated with severe Parkinson’s disease phenotype
according to research presented online as part of the 2020 American Academy of Neurology Science Highlights.
Neurologists have identified various phenotypes among patients with Parkinson’s disease; however, the factors that determine these phenotypes, which may include genetic and environmental variables, are poorly understood. Ethan G. Brown, MD, assistant professor of neurology at the University of California, San Francisco, and colleagues hypothesized that head injury, which is a risk factor for Parkinson’s disease, would be associated with a more severe phenotype.
“Head injury is a risk factor for other conditions that involve cognitive impairment,” said Dr. Brown. “The mechanisms of how head injury contributes to neurodegenerative disease are not clear, but may be related to the initiation of an inflammatory cascade that can have a long-term, chronic effect. We hypothesized that these long-term sequelae may contribute to symptoms in Parkinson’s disease.”
An analysis of data from two cohorts
The researchers examined the relationship between head injury and clinical features by analyzing data for two cohorts of patients with Parkinson’s disease. Through an online survey, the investigators elicited information about head injury and other exposures from participants in the Parkinson’s Progression Markers Initiative (PPMI) and the Fox Insight (FI) study. Dr. Brown and colleagues determined disease phenotypes for participants in PPMI using baseline Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) score and 5-year change in Montreal Cognitive Assessment score. For participants in FI, the researchers determined phenotypes using baseline self-reported MDS-UPDRS-II score and self-reported cognitive impairment. They used parametric and nonparametric tests as appropriate and adjusted the results for age, sex, and smoking history.
In all, 267 participants with Parkinson’s disease in PPMI and 25,308 in FI submitted information about head injury. In the PPMI cohort, head injury before Parkinson’s disease diagnosis was associated with greater nonmotor symptom burden at enrollment. The mean MDS-UPDRS-I score was 7.73 among participants with any injury, compared with 6.19 among participants with no injury. Similarly, the mean MDS-UPDRS-I score was 8.29 among participants with severe head injury, compared with 6.19 among participants with no injury. Motor symptoms were worse among participants with severe injury (MDS-UPDRS-II score, 8.35). Among 110 participants who were followed for 5 years, patients who reported severe head injury before diagnosis had a decline in cognitive function. The mean change in Montreal Cognitive Assessment score was –0.60 for patients with severe head injury and 0.76 in those with no head injury.
“The improvement from baseline in the participants with Parkinson’s disease but without head injury was small and not statistically significant,” said Dr. Brown. The increase could have resulted from practice effect, although it is not certain, he added. “We are continuing to evaluate other, more sensitive tests of cognitive impairment to try to understand these results more completely in this population.”
In the FI cohort, participants who reported a prior head injury had more motor symptoms (MDS-UPDRS-II, 14.4), compared with those without head injury (MDS-UPDRS-II, 12.1). Also, the risk of self-reported cognitive impairment was elevated in participants who reported head injury (odds ratio, 1.58).
“The results most affected by the self-reported nature of [the] FI [data] are the cognitive impairment results,” said Dr. Brown. “Subjective cognitive impairment ... is very different from objective cognitive impairment, which could be measured through in-person testing in the PPMI cohort. Many factors may contribute to noticing cognitive decline, some of which can be measured and controlled for, but some cannot. There may be a correlation between subjective cognitive decline and true cognitive impairment, but this has not been fully studied in Parkinson’s disease.”
The search for the underlying mechanism
Clarifying whether the relationship between head injury and Parkinson’s disease phenotype is causal or whether falling is an early indication of worse symptoms will require more longitudinal data. “We would like to further characterize the differences between people with Parkinson’s disease with and without a history of head injury,” said Dr. Brown. “More detailed understanding of these phenotypic differences could point to an underlying mechanism, or whether or not other comorbid conditions are involved. We would also like to understand whether genetics plays a role.”
The PPMI and FI studies are funded by the Michael J. Fox Foundation. Dr. Brown has received compensation from HiOscar, NEJM Knowledge Plus, and Rune Labs and has received research support from Gateway Institute for Brain Research.
SOURCE: Brown EG et al. AAN 2020, Abstract S17.002.
according to research presented online as part of the 2020 American Academy of Neurology Science Highlights.
Neurologists have identified various phenotypes among patients with Parkinson’s disease; however, the factors that determine these phenotypes, which may include genetic and environmental variables, are poorly understood. Ethan G. Brown, MD, assistant professor of neurology at the University of California, San Francisco, and colleagues hypothesized that head injury, which is a risk factor for Parkinson’s disease, would be associated with a more severe phenotype.
“Head injury is a risk factor for other conditions that involve cognitive impairment,” said Dr. Brown. “The mechanisms of how head injury contributes to neurodegenerative disease are not clear, but may be related to the initiation of an inflammatory cascade that can have a long-term, chronic effect. We hypothesized that these long-term sequelae may contribute to symptoms in Parkinson’s disease.”
An analysis of data from two cohorts
The researchers examined the relationship between head injury and clinical features by analyzing data for two cohorts of patients with Parkinson’s disease. Through an online survey, the investigators elicited information about head injury and other exposures from participants in the Parkinson’s Progression Markers Initiative (PPMI) and the Fox Insight (FI) study. Dr. Brown and colleagues determined disease phenotypes for participants in PPMI using baseline Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) score and 5-year change in Montreal Cognitive Assessment score. For participants in FI, the researchers determined phenotypes using baseline self-reported MDS-UPDRS-II score and self-reported cognitive impairment. They used parametric and nonparametric tests as appropriate and adjusted the results for age, sex, and smoking history.
In all, 267 participants with Parkinson’s disease in PPMI and 25,308 in FI submitted information about head injury. In the PPMI cohort, head injury before Parkinson’s disease diagnosis was associated with greater nonmotor symptom burden at enrollment. The mean MDS-UPDRS-I score was 7.73 among participants with any injury, compared with 6.19 among participants with no injury. Similarly, the mean MDS-UPDRS-I score was 8.29 among participants with severe head injury, compared with 6.19 among participants with no injury. Motor symptoms were worse among participants with severe injury (MDS-UPDRS-II score, 8.35). Among 110 participants who were followed for 5 years, patients who reported severe head injury before diagnosis had a decline in cognitive function. The mean change in Montreal Cognitive Assessment score was –0.60 for patients with severe head injury and 0.76 in those with no head injury.
“The improvement from baseline in the participants with Parkinson’s disease but without head injury was small and not statistically significant,” said Dr. Brown. The increase could have resulted from practice effect, although it is not certain, he added. “We are continuing to evaluate other, more sensitive tests of cognitive impairment to try to understand these results more completely in this population.”
In the FI cohort, participants who reported a prior head injury had more motor symptoms (MDS-UPDRS-II, 14.4), compared with those without head injury (MDS-UPDRS-II, 12.1). Also, the risk of self-reported cognitive impairment was elevated in participants who reported head injury (odds ratio, 1.58).
“The results most affected by the self-reported nature of [the] FI [data] are the cognitive impairment results,” said Dr. Brown. “Subjective cognitive impairment ... is very different from objective cognitive impairment, which could be measured through in-person testing in the PPMI cohort. Many factors may contribute to noticing cognitive decline, some of which can be measured and controlled for, but some cannot. There may be a correlation between subjective cognitive decline and true cognitive impairment, but this has not been fully studied in Parkinson’s disease.”
The search for the underlying mechanism
Clarifying whether the relationship between head injury and Parkinson’s disease phenotype is causal or whether falling is an early indication of worse symptoms will require more longitudinal data. “We would like to further characterize the differences between people with Parkinson’s disease with and without a history of head injury,” said Dr. Brown. “More detailed understanding of these phenotypic differences could point to an underlying mechanism, or whether or not other comorbid conditions are involved. We would also like to understand whether genetics plays a role.”
The PPMI and FI studies are funded by the Michael J. Fox Foundation. Dr. Brown has received compensation from HiOscar, NEJM Knowledge Plus, and Rune Labs and has received research support from Gateway Institute for Brain Research.
SOURCE: Brown EG et al. AAN 2020, Abstract S17.002.
according to research presented online as part of the 2020 American Academy of Neurology Science Highlights.
Neurologists have identified various phenotypes among patients with Parkinson’s disease; however, the factors that determine these phenotypes, which may include genetic and environmental variables, are poorly understood. Ethan G. Brown, MD, assistant professor of neurology at the University of California, San Francisco, and colleagues hypothesized that head injury, which is a risk factor for Parkinson’s disease, would be associated with a more severe phenotype.
“Head injury is a risk factor for other conditions that involve cognitive impairment,” said Dr. Brown. “The mechanisms of how head injury contributes to neurodegenerative disease are not clear, but may be related to the initiation of an inflammatory cascade that can have a long-term, chronic effect. We hypothesized that these long-term sequelae may contribute to symptoms in Parkinson’s disease.”
An analysis of data from two cohorts
The researchers examined the relationship between head injury and clinical features by analyzing data for two cohorts of patients with Parkinson’s disease. Through an online survey, the investigators elicited information about head injury and other exposures from participants in the Parkinson’s Progression Markers Initiative (PPMI) and the Fox Insight (FI) study. Dr. Brown and colleagues determined disease phenotypes for participants in PPMI using baseline Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) score and 5-year change in Montreal Cognitive Assessment score. For participants in FI, the researchers determined phenotypes using baseline self-reported MDS-UPDRS-II score and self-reported cognitive impairment. They used parametric and nonparametric tests as appropriate and adjusted the results for age, sex, and smoking history.
In all, 267 participants with Parkinson’s disease in PPMI and 25,308 in FI submitted information about head injury. In the PPMI cohort, head injury before Parkinson’s disease diagnosis was associated with greater nonmotor symptom burden at enrollment. The mean MDS-UPDRS-I score was 7.73 among participants with any injury, compared with 6.19 among participants with no injury. Similarly, the mean MDS-UPDRS-I score was 8.29 among participants with severe head injury, compared with 6.19 among participants with no injury. Motor symptoms were worse among participants with severe injury (MDS-UPDRS-II score, 8.35). Among 110 participants who were followed for 5 years, patients who reported severe head injury before diagnosis had a decline in cognitive function. The mean change in Montreal Cognitive Assessment score was –0.60 for patients with severe head injury and 0.76 in those with no head injury.
“The improvement from baseline in the participants with Parkinson’s disease but without head injury was small and not statistically significant,” said Dr. Brown. The increase could have resulted from practice effect, although it is not certain, he added. “We are continuing to evaluate other, more sensitive tests of cognitive impairment to try to understand these results more completely in this population.”
In the FI cohort, participants who reported a prior head injury had more motor symptoms (MDS-UPDRS-II, 14.4), compared with those without head injury (MDS-UPDRS-II, 12.1). Also, the risk of self-reported cognitive impairment was elevated in participants who reported head injury (odds ratio, 1.58).
“The results most affected by the self-reported nature of [the] FI [data] are the cognitive impairment results,” said Dr. Brown. “Subjective cognitive impairment ... is very different from objective cognitive impairment, which could be measured through in-person testing in the PPMI cohort. Many factors may contribute to noticing cognitive decline, some of which can be measured and controlled for, but some cannot. There may be a correlation between subjective cognitive decline and true cognitive impairment, but this has not been fully studied in Parkinson’s disease.”
The search for the underlying mechanism
Clarifying whether the relationship between head injury and Parkinson’s disease phenotype is causal or whether falling is an early indication of worse symptoms will require more longitudinal data. “We would like to further characterize the differences between people with Parkinson’s disease with and without a history of head injury,” said Dr. Brown. “More detailed understanding of these phenotypic differences could point to an underlying mechanism, or whether or not other comorbid conditions are involved. We would also like to understand whether genetics plays a role.”
The PPMI and FI studies are funded by the Michael J. Fox Foundation. Dr. Brown has received compensation from HiOscar, NEJM Knowledge Plus, and Rune Labs and has received research support from Gateway Institute for Brain Research.
SOURCE: Brown EG et al. AAN 2020, Abstract S17.002.
FROM AAN 2020
Blue light improves concussion symptoms
a new study has found. Exposure to blue light in the morning through a special device may be a “critical factor” in resetting the circadian rhythm and helping people who have suffered a concussion, author William D. “Scott” Killgore, MD, professor of psychiatry, psychology, and medical imaging, the University of Arizona College of Medicine, Tucson, told Medscape Medical News.
“This is very new, so I wouldn’t say it’s the treatment of choice, but we should start looking at using this system as a nonpharmacologic way to perhaps help patients recover faster from a concussion,” he said.
The findings were released March 2 ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology. The AAN canceled the meeting and released abstracts and access to presenters for press coverage.
About half of patients with a concussion experience sleep problems, including problems falling asleep, staying asleep, and waking up in the middle of the night, said Dr. Killgore.
Poor sleep interrupts the brain’s repair mechanism. “Sleep is important for cleaning out the neurotoxins that build up in your brain during the day. Sleep also helps build oligodendrocyte precursor cells that provide insulation around nerve cells,” he said.
Master clock
Blue light stimulates receptors in the back of the retina that respond only to this wavelength of light, said Dr. Killgore. “It specifically projects to an area in the hypothalamus – essentially the brain’s master clock – that regulates your sleep-wake schedules. So exposure to that bright light essentially resets your circadian rhythm.”
That master clock involves regulating the brain’s production of melatonin. Morning exposure to blue light shifts that production to facilitate sleep at the appropriate time.
The ideal time to be exposed to blue light is from about 8:00 to 11:00 AM. “Timing is critical,” said Dr. Killgore. “If you get light at the wrong time, it will reset your circadian rhythm in the wrong direction.”
Previous research has shown that exposure to blue light leads to improved sleep, which is widely believed to lead to improved mood.
A separate study conducted by Dr. Killgore and colleagues that involved another group of mTBI patients was recently published in Neurobiology of Disease. That study showed that the participants who received blue light experienced a shift in circadian timing of about an hour. “They were going to sleep an hour earlier and waking up an hour earlier,” said Dr. Killgore.
The blue light also appeared to change brain structure and brain function, among other things, he said.
The current study included 35 patients who had suffered an mTBI within the previous 18 months. Most injuries were sports related and occurred while playing football or soccer or riding a bike.
Participants were randomly assigned to use a device fitted with a blue LED light (peak wavelength, 469 nm) or one fitted with an amber-colored LED light. They were instructed to use the device every morning for 30 minutes within 2 hours of waking.
The blue-light group comprised five men and 12 women (mean age, 25.5 years). The amber-light group comprised eight men and 10 women (mean age, 26.3 years).
Researchers told participants only that the study was exploring various aspects of light. “Subjects didn’t know if they were getting a control or active device,” said Dr. Killgore.
Researchers used the Beck Depression Inventory (BDI) to evaluate depression symptoms and the Rivermead Post-Concussion Symptom Questionnaire (RPCSQ). This 16-item questionnaire assesses symptoms in the acute stage as well as those that are more chronic.
After 6 weeks, the blue-light group had lower scores on the BDI compared to the amber-light group (P = .005).
“We found that in the amber-light group, there was essentially no change in terms of depression,” said Dr. Killgore. “But those who got the blue light showed a significant reduction in depressive symptoms, about a 22% decline overall relative to baseline, so a nice drop in overall depression.”
Changes in BDI scores were significantly positively associated with changes in the total chronic symptom score (P = .002) in the blue-light group but not the amber-light group. “Those who got blue light showed a significant reduction in the number of symptoms associated with concussion whereas those who got the amber light stayed the same,” said Dr. Killgore.
There were similar findings for somatic symptoms, such as headache and pain (P = .031), and for cognitive symptoms (P = .014) in the blue-light group.
“These subjects were having fewer problems remembering and paying attention, so their concentration seemed to be improving, at least subjectively,” commented Dr. Killgore.
There was no significant benefit from the blue light for emotional symptoms. “There was a decline, but it wasn’t statistically significant, even though there was a decline in depression,” said Dr. Killgore.
This, he explained, could be due to the small sample size and the greater sensitivity of the BDI for emotional symptoms relative to the RPCSQ. “The BDI has 21 items that are all focused on aspects of depression, whereas the RPCSQ only asks one item for depression and one item for irritability/anger.”
Less daytime sleepiness
The researchers also found a significant improvement in daytime sleepiness. “Subjects were much less sleepy by the end of the study if they got blue light than if they got amber light,” said Dr. Killgore.
Participants wore an actigraphy device that took sleep measurements. Early results indicate that blue-light recipients were getting more sleep by the end of the study.
Researchers are now analyzing additional data to see whether the improvements in depression and post-concussion symptoms are linked to improved sleep. They also gathered data from brain imaging that will be analyzed at a later date.
Dr. Killgore and his colleagues aim to determine what distinguishes people who respond to blue-light therapy from those who don’t. “We want to know what it is that would allow some people to be more responsive than others, so we’re going to be exploring skin color, eye color, genetic factors, and other factors,” he said.
They’re also conducting a study of blue-light therapy in patients with posttraumatic stress disorder, 90% of whom have sleep problems.
“This is quite fascinating,” said Dr. Killgore. “It looks like if you get blue light after your treatment, the treatment sticks better than if you didn’t get the blue light. We think that sleep is probably playing an important role in that.”
Several light devices are available, ranging in price from about $100 to $200.
Commenting on the research, concussion expert Frank Conidi, MD, director of the Florida Center for Headache and Sports Neurology, Port St. Lucie, said the study is interesting from a number of perspectives.
For one thing, it shows that blue-light therapy “provides an inexpensive and minimally invasive way to treat concussion,” he said.
Dr. Conidi said he would recommend blue-light therapy for concussion patients. “I could see neurology practices offering the device to patients as an in-office treatment or to take home for a small fee. I think athletes would be quite receptive to this, as they’re always looking for nonpharmacological ways to treat concussion.”
Dr. Conidi noted that the new results are consistent with other studies that show that decreased depression and improved sleep help with somatic symptoms.
From a research perspective, the study provides a “stepping stone” for larger trials, said Dr. Conidi. He would like to see more studies of acute concussion, such as studies as to whether the therapy shortens the duration of symptoms.
“I would also like to see controlled studies on headache and vestibular symptoms, which are the two most common,” he said.
The study was funded by the US Department of Defense. Killgore and Conidi have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
a new study has found. Exposure to blue light in the morning through a special device may be a “critical factor” in resetting the circadian rhythm and helping people who have suffered a concussion, author William D. “Scott” Killgore, MD, professor of psychiatry, psychology, and medical imaging, the University of Arizona College of Medicine, Tucson, told Medscape Medical News.
“This is very new, so I wouldn’t say it’s the treatment of choice, but we should start looking at using this system as a nonpharmacologic way to perhaps help patients recover faster from a concussion,” he said.
The findings were released March 2 ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology. The AAN canceled the meeting and released abstracts and access to presenters for press coverage.
About half of patients with a concussion experience sleep problems, including problems falling asleep, staying asleep, and waking up in the middle of the night, said Dr. Killgore.
Poor sleep interrupts the brain’s repair mechanism. “Sleep is important for cleaning out the neurotoxins that build up in your brain during the day. Sleep also helps build oligodendrocyte precursor cells that provide insulation around nerve cells,” he said.
Master clock
Blue light stimulates receptors in the back of the retina that respond only to this wavelength of light, said Dr. Killgore. “It specifically projects to an area in the hypothalamus – essentially the brain’s master clock – that regulates your sleep-wake schedules. So exposure to that bright light essentially resets your circadian rhythm.”
That master clock involves regulating the brain’s production of melatonin. Morning exposure to blue light shifts that production to facilitate sleep at the appropriate time.
The ideal time to be exposed to blue light is from about 8:00 to 11:00 AM. “Timing is critical,” said Dr. Killgore. “If you get light at the wrong time, it will reset your circadian rhythm in the wrong direction.”
Previous research has shown that exposure to blue light leads to improved sleep, which is widely believed to lead to improved mood.
A separate study conducted by Dr. Killgore and colleagues that involved another group of mTBI patients was recently published in Neurobiology of Disease. That study showed that the participants who received blue light experienced a shift in circadian timing of about an hour. “They were going to sleep an hour earlier and waking up an hour earlier,” said Dr. Killgore.
The blue light also appeared to change brain structure and brain function, among other things, he said.
The current study included 35 patients who had suffered an mTBI within the previous 18 months. Most injuries were sports related and occurred while playing football or soccer or riding a bike.
Participants were randomly assigned to use a device fitted with a blue LED light (peak wavelength, 469 nm) or one fitted with an amber-colored LED light. They were instructed to use the device every morning for 30 minutes within 2 hours of waking.
The blue-light group comprised five men and 12 women (mean age, 25.5 years). The amber-light group comprised eight men and 10 women (mean age, 26.3 years).
Researchers told participants only that the study was exploring various aspects of light. “Subjects didn’t know if they were getting a control or active device,” said Dr. Killgore.
Researchers used the Beck Depression Inventory (BDI) to evaluate depression symptoms and the Rivermead Post-Concussion Symptom Questionnaire (RPCSQ). This 16-item questionnaire assesses symptoms in the acute stage as well as those that are more chronic.
After 6 weeks, the blue-light group had lower scores on the BDI compared to the amber-light group (P = .005).
“We found that in the amber-light group, there was essentially no change in terms of depression,” said Dr. Killgore. “But those who got the blue light showed a significant reduction in depressive symptoms, about a 22% decline overall relative to baseline, so a nice drop in overall depression.”
Changes in BDI scores were significantly positively associated with changes in the total chronic symptom score (P = .002) in the blue-light group but not the amber-light group. “Those who got blue light showed a significant reduction in the number of symptoms associated with concussion whereas those who got the amber light stayed the same,” said Dr. Killgore.
There were similar findings for somatic symptoms, such as headache and pain (P = .031), and for cognitive symptoms (P = .014) in the blue-light group.
“These subjects were having fewer problems remembering and paying attention, so their concentration seemed to be improving, at least subjectively,” commented Dr. Killgore.
There was no significant benefit from the blue light for emotional symptoms. “There was a decline, but it wasn’t statistically significant, even though there was a decline in depression,” said Dr. Killgore.
This, he explained, could be due to the small sample size and the greater sensitivity of the BDI for emotional symptoms relative to the RPCSQ. “The BDI has 21 items that are all focused on aspects of depression, whereas the RPCSQ only asks one item for depression and one item for irritability/anger.”
Less daytime sleepiness
The researchers also found a significant improvement in daytime sleepiness. “Subjects were much less sleepy by the end of the study if they got blue light than if they got amber light,” said Dr. Killgore.
Participants wore an actigraphy device that took sleep measurements. Early results indicate that blue-light recipients were getting more sleep by the end of the study.
Researchers are now analyzing additional data to see whether the improvements in depression and post-concussion symptoms are linked to improved sleep. They also gathered data from brain imaging that will be analyzed at a later date.
Dr. Killgore and his colleagues aim to determine what distinguishes people who respond to blue-light therapy from those who don’t. “We want to know what it is that would allow some people to be more responsive than others, so we’re going to be exploring skin color, eye color, genetic factors, and other factors,” he said.
They’re also conducting a study of blue-light therapy in patients with posttraumatic stress disorder, 90% of whom have sleep problems.
“This is quite fascinating,” said Dr. Killgore. “It looks like if you get blue light after your treatment, the treatment sticks better than if you didn’t get the blue light. We think that sleep is probably playing an important role in that.”
Several light devices are available, ranging in price from about $100 to $200.
Commenting on the research, concussion expert Frank Conidi, MD, director of the Florida Center for Headache and Sports Neurology, Port St. Lucie, said the study is interesting from a number of perspectives.
For one thing, it shows that blue-light therapy “provides an inexpensive and minimally invasive way to treat concussion,” he said.
Dr. Conidi said he would recommend blue-light therapy for concussion patients. “I could see neurology practices offering the device to patients as an in-office treatment or to take home for a small fee. I think athletes would be quite receptive to this, as they’re always looking for nonpharmacological ways to treat concussion.”
Dr. Conidi noted that the new results are consistent with other studies that show that decreased depression and improved sleep help with somatic symptoms.
From a research perspective, the study provides a “stepping stone” for larger trials, said Dr. Conidi. He would like to see more studies of acute concussion, such as studies as to whether the therapy shortens the duration of symptoms.
“I would also like to see controlled studies on headache and vestibular symptoms, which are the two most common,” he said.
The study was funded by the US Department of Defense. Killgore and Conidi have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
a new study has found. Exposure to blue light in the morning through a special device may be a “critical factor” in resetting the circadian rhythm and helping people who have suffered a concussion, author William D. “Scott” Killgore, MD, professor of psychiatry, psychology, and medical imaging, the University of Arizona College of Medicine, Tucson, told Medscape Medical News.
“This is very new, so I wouldn’t say it’s the treatment of choice, but we should start looking at using this system as a nonpharmacologic way to perhaps help patients recover faster from a concussion,” he said.
The findings were released March 2 ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology. The AAN canceled the meeting and released abstracts and access to presenters for press coverage.
About half of patients with a concussion experience sleep problems, including problems falling asleep, staying asleep, and waking up in the middle of the night, said Dr. Killgore.
Poor sleep interrupts the brain’s repair mechanism. “Sleep is important for cleaning out the neurotoxins that build up in your brain during the day. Sleep also helps build oligodendrocyte precursor cells that provide insulation around nerve cells,” he said.
Master clock
Blue light stimulates receptors in the back of the retina that respond only to this wavelength of light, said Dr. Killgore. “It specifically projects to an area in the hypothalamus – essentially the brain’s master clock – that regulates your sleep-wake schedules. So exposure to that bright light essentially resets your circadian rhythm.”
That master clock involves regulating the brain’s production of melatonin. Morning exposure to blue light shifts that production to facilitate sleep at the appropriate time.
The ideal time to be exposed to blue light is from about 8:00 to 11:00 AM. “Timing is critical,” said Dr. Killgore. “If you get light at the wrong time, it will reset your circadian rhythm in the wrong direction.”
Previous research has shown that exposure to blue light leads to improved sleep, which is widely believed to lead to improved mood.
A separate study conducted by Dr. Killgore and colleagues that involved another group of mTBI patients was recently published in Neurobiology of Disease. That study showed that the participants who received blue light experienced a shift in circadian timing of about an hour. “They were going to sleep an hour earlier and waking up an hour earlier,” said Dr. Killgore.
The blue light also appeared to change brain structure and brain function, among other things, he said.
The current study included 35 patients who had suffered an mTBI within the previous 18 months. Most injuries were sports related and occurred while playing football or soccer or riding a bike.
Participants were randomly assigned to use a device fitted with a blue LED light (peak wavelength, 469 nm) or one fitted with an amber-colored LED light. They were instructed to use the device every morning for 30 minutes within 2 hours of waking.
The blue-light group comprised five men and 12 women (mean age, 25.5 years). The amber-light group comprised eight men and 10 women (mean age, 26.3 years).
Researchers told participants only that the study was exploring various aspects of light. “Subjects didn’t know if they were getting a control or active device,” said Dr. Killgore.
Researchers used the Beck Depression Inventory (BDI) to evaluate depression symptoms and the Rivermead Post-Concussion Symptom Questionnaire (RPCSQ). This 16-item questionnaire assesses symptoms in the acute stage as well as those that are more chronic.
After 6 weeks, the blue-light group had lower scores on the BDI compared to the amber-light group (P = .005).
“We found that in the amber-light group, there was essentially no change in terms of depression,” said Dr. Killgore. “But those who got the blue light showed a significant reduction in depressive symptoms, about a 22% decline overall relative to baseline, so a nice drop in overall depression.”
Changes in BDI scores were significantly positively associated with changes in the total chronic symptom score (P = .002) in the blue-light group but not the amber-light group. “Those who got blue light showed a significant reduction in the number of symptoms associated with concussion whereas those who got the amber light stayed the same,” said Dr. Killgore.
There were similar findings for somatic symptoms, such as headache and pain (P = .031), and for cognitive symptoms (P = .014) in the blue-light group.
“These subjects were having fewer problems remembering and paying attention, so their concentration seemed to be improving, at least subjectively,” commented Dr. Killgore.
There was no significant benefit from the blue light for emotional symptoms. “There was a decline, but it wasn’t statistically significant, even though there was a decline in depression,” said Dr. Killgore.
This, he explained, could be due to the small sample size and the greater sensitivity of the BDI for emotional symptoms relative to the RPCSQ. “The BDI has 21 items that are all focused on aspects of depression, whereas the RPCSQ only asks one item for depression and one item for irritability/anger.”
Less daytime sleepiness
The researchers also found a significant improvement in daytime sleepiness. “Subjects were much less sleepy by the end of the study if they got blue light than if they got amber light,” said Dr. Killgore.
Participants wore an actigraphy device that took sleep measurements. Early results indicate that blue-light recipients were getting more sleep by the end of the study.
Researchers are now analyzing additional data to see whether the improvements in depression and post-concussion symptoms are linked to improved sleep. They also gathered data from brain imaging that will be analyzed at a later date.
Dr. Killgore and his colleagues aim to determine what distinguishes people who respond to blue-light therapy from those who don’t. “We want to know what it is that would allow some people to be more responsive than others, so we’re going to be exploring skin color, eye color, genetic factors, and other factors,” he said.
They’re also conducting a study of blue-light therapy in patients with posttraumatic stress disorder, 90% of whom have sleep problems.
“This is quite fascinating,” said Dr. Killgore. “It looks like if you get blue light after your treatment, the treatment sticks better than if you didn’t get the blue light. We think that sleep is probably playing an important role in that.”
Several light devices are available, ranging in price from about $100 to $200.
Commenting on the research, concussion expert Frank Conidi, MD, director of the Florida Center for Headache and Sports Neurology, Port St. Lucie, said the study is interesting from a number of perspectives.
For one thing, it shows that blue-light therapy “provides an inexpensive and minimally invasive way to treat concussion,” he said.
Dr. Conidi said he would recommend blue-light therapy for concussion patients. “I could see neurology practices offering the device to patients as an in-office treatment or to take home for a small fee. I think athletes would be quite receptive to this, as they’re always looking for nonpharmacological ways to treat concussion.”
Dr. Conidi noted that the new results are consistent with other studies that show that decreased depression and improved sleep help with somatic symptoms.
From a research perspective, the study provides a “stepping stone” for larger trials, said Dr. Conidi. He would like to see more studies of acute concussion, such as studies as to whether the therapy shortens the duration of symptoms.
“I would also like to see controlled studies on headache and vestibular symptoms, which are the two most common,” he said.
The study was funded by the US Department of Defense. Killgore and Conidi have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
TBI deaths from falls on the rise
A 17% surge in mortality from fall-related traumatic brain injuries from 2008 to 2017 was driven largely by increases among those aged 75 years and older, according to investigators from the Centers for Disease Control and Prevention.
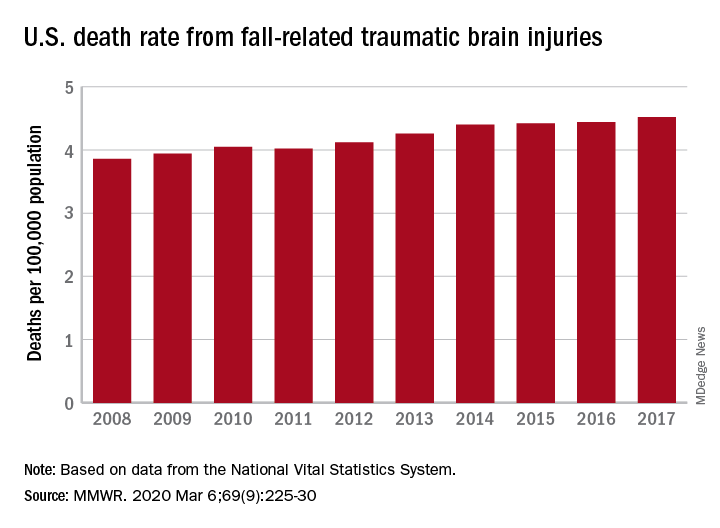
Nationally, the rate of deaths from traumatic brain injuries (TBIs) caused by unintentional falls rose from 3.86 per 100,000 population in 2008 to 4.52 per 100,000 in 2017, as the number of deaths went from 12,311 to 17,408, said Alexis B. Peterson, PhD, and Scott R. Kegler, PhD, of the CDC’s National Center for Injury Prevention and Control in Atlanta.
“This increase might be explained by longer survival following the onset of common diseases such as stroke, cancer, and heart disease or be attributable to the increasing population of older adults in the United States,” they suggested in the Mortality and Morbidity Weekly Report.
The rate of fall-related TBI among Americans aged 75 years and older increased by an average of 2.6% per year from 2008 to 2017, compared with 1.8% in those aged 55-74. Over that same time, death rates dropped for those aged 35-44 (–0.3%), 18-34 (–1.1%), and 0-17 (–4.3%), they said, based on data from the National Vital Statistics System’s multiple cause-of-death database.
The death rate increased fastest in residents of rural areas (2.9% per year), but deaths from fall-related TBI were up at all levels of urbanization. The largest central cities and fringe metro areas were up by 1.4% a year, with larger annual increases seen in medium-size cities (2.1%), small cities (2.2%), and small towns (2.1%), Dr. Peterson and Dr. Kegler said.
Rates of TBI-related mortality in general are higher in rural areas, they noted, and “heterogeneity in the availability and accessibility of resources (e.g., access to high-level trauma centers and rehabilitative services) can result in disparities in postinjury outcomes.”
State-specific rates increased in 45 states, although Alaska was excluded from the analysis because of its small number of cases (less than 20). Increases were significant in 29 states, but none of the changes were significant in the 4 states with lower rates at the end of the study period, the investigators reported.
“In older adults, evidence-based fall prevention strategies can prevent falls and avert costly medical expenditures,” Dr. Peterson and Dr. Kegler said, suggesting that health care providers “consider prescribing exercises that incorporate balance, strength and gait activities, such as tai chi, and reviewing and managing medications linked to falls.”
SOURCE: Peterson AB, Kegler SR. MMWR. 2019 Mar 6;69(9):225-30.
A 17% surge in mortality from fall-related traumatic brain injuries from 2008 to 2017 was driven largely by increases among those aged 75 years and older, according to investigators from the Centers for Disease Control and Prevention.

Nationally, the rate of deaths from traumatic brain injuries (TBIs) caused by unintentional falls rose from 3.86 per 100,000 population in 2008 to 4.52 per 100,000 in 2017, as the number of deaths went from 12,311 to 17,408, said Alexis B. Peterson, PhD, and Scott R. Kegler, PhD, of the CDC’s National Center for Injury Prevention and Control in Atlanta.
“This increase might be explained by longer survival following the onset of common diseases such as stroke, cancer, and heart disease or be attributable to the increasing population of older adults in the United States,” they suggested in the Mortality and Morbidity Weekly Report.
The rate of fall-related TBI among Americans aged 75 years and older increased by an average of 2.6% per year from 2008 to 2017, compared with 1.8% in those aged 55-74. Over that same time, death rates dropped for those aged 35-44 (–0.3%), 18-34 (–1.1%), and 0-17 (–4.3%), they said, based on data from the National Vital Statistics System’s multiple cause-of-death database.
The death rate increased fastest in residents of rural areas (2.9% per year), but deaths from fall-related TBI were up at all levels of urbanization. The largest central cities and fringe metro areas were up by 1.4% a year, with larger annual increases seen in medium-size cities (2.1%), small cities (2.2%), and small towns (2.1%), Dr. Peterson and Dr. Kegler said.
Rates of TBI-related mortality in general are higher in rural areas, they noted, and “heterogeneity in the availability and accessibility of resources (e.g., access to high-level trauma centers and rehabilitative services) can result in disparities in postinjury outcomes.”
State-specific rates increased in 45 states, although Alaska was excluded from the analysis because of its small number of cases (less than 20). Increases were significant in 29 states, but none of the changes were significant in the 4 states with lower rates at the end of the study period, the investigators reported.
“In older adults, evidence-based fall prevention strategies can prevent falls and avert costly medical expenditures,” Dr. Peterson and Dr. Kegler said, suggesting that health care providers “consider prescribing exercises that incorporate balance, strength and gait activities, such as tai chi, and reviewing and managing medications linked to falls.”
SOURCE: Peterson AB, Kegler SR. MMWR. 2019 Mar 6;69(9):225-30.
A 17% surge in mortality from fall-related traumatic brain injuries from 2008 to 2017 was driven largely by increases among those aged 75 years and older, according to investigators from the Centers for Disease Control and Prevention.

Nationally, the rate of deaths from traumatic brain injuries (TBIs) caused by unintentional falls rose from 3.86 per 100,000 population in 2008 to 4.52 per 100,000 in 2017, as the number of deaths went from 12,311 to 17,408, said Alexis B. Peterson, PhD, and Scott R. Kegler, PhD, of the CDC’s National Center for Injury Prevention and Control in Atlanta.
“This increase might be explained by longer survival following the onset of common diseases such as stroke, cancer, and heart disease or be attributable to the increasing population of older adults in the United States,” they suggested in the Mortality and Morbidity Weekly Report.
The rate of fall-related TBI among Americans aged 75 years and older increased by an average of 2.6% per year from 2008 to 2017, compared with 1.8% in those aged 55-74. Over that same time, death rates dropped for those aged 35-44 (–0.3%), 18-34 (–1.1%), and 0-17 (–4.3%), they said, based on data from the National Vital Statistics System’s multiple cause-of-death database.
The death rate increased fastest in residents of rural areas (2.9% per year), but deaths from fall-related TBI were up at all levels of urbanization. The largest central cities and fringe metro areas were up by 1.4% a year, with larger annual increases seen in medium-size cities (2.1%), small cities (2.2%), and small towns (2.1%), Dr. Peterson and Dr. Kegler said.
Rates of TBI-related mortality in general are higher in rural areas, they noted, and “heterogeneity in the availability and accessibility of resources (e.g., access to high-level trauma centers and rehabilitative services) can result in disparities in postinjury outcomes.”
State-specific rates increased in 45 states, although Alaska was excluded from the analysis because of its small number of cases (less than 20). Increases were significant in 29 states, but none of the changes were significant in the 4 states with lower rates at the end of the study period, the investigators reported.
“In older adults, evidence-based fall prevention strategies can prevent falls and avert costly medical expenditures,” Dr. Peterson and Dr. Kegler said, suggesting that health care providers “consider prescribing exercises that incorporate balance, strength and gait activities, such as tai chi, and reviewing and managing medications linked to falls.”
SOURCE: Peterson AB, Kegler SR. MMWR. 2019 Mar 6;69(9):225-30.
FROM MMWR
Demographic Profile and Service-Connection Trends of Posttraumatic Stress Disorder and Traumatic Brain Injury in US Veterans Pre- and Post-9/11
The nature of combat and associated injuries in Operation Iraqi Freedom (OIF), Operation Enduring Freedom (OEF), Operation New Dawn (OND), and Afghanistan War is different from previous conflicts. Multiple protracted deployments with infrequent breaks after September 11, 2001 (9/11) have further compounded the problem.
Posttraumatic stress disorder (PTSD) and traumatic brain injury (TBI) are the signature wounds of recent wars, with a higher incidence among the veterans of OEF and OIF compared with those from previous conflicts.1,2 More than 2.7 million who served in Iraq and Afghanistan suffer from PTSD.3,4 Symptoms of PTSD may appear within the first 3 months after exposure to a traumatic event or after many months and, in some cases, after a delay of many years and continue for life.5 Although delayed onset of PTSD in the absence of prior symptoms is rare,6,7 its incidence rises with increasing frequency of exposure to traumatic events8,9 and over time.10
According to the Brain Injury Association of America, TBI is “an alteration in brain function, or other evidence of brain pathology, caused by an external force.”8 TBI is often associated with increased risk of PTSD, depression, and posttraumatic headache,11-13 which may lead to broader cognitive, somatic, neurobiological, and psychosocial dysfunctions.14-17 According to Veterans Health Administration (VHA) data, 201,435 veterans from all eras enrolled with the US Department of Veterans Affairs (VA) have a diagnosis associated with TBI and 56,695 OEF/OIF veterans have been evaluated for a TBI-related condition.2 According to the Defense and Veterans Brain Injury Center (DVBIC), > 361,000 veterans have been diagnosed with TBI, with a peak of 32,000 cases in 2011.1,18 Moreover, the reported incidence and prevalence of PTSD and TBI among US veterans are not consistent. The incidence of PTSD has been estimated at 15% to 20% in recent wars3,19 compared with 10% to 30% in previous wars.3,19,20
When PTSD or TBI is deemed “related” to military service, the veteran may receive a service-connected disability rating ranging from 0% (no life-interfering symptoms due to injury) to 100% (totally disabling injury). The percentage of service connection associated with an injury is a quantifiable measure of the debilitating effect of injury on the individual. A significant majority (94%) of those who seek mental health services and treatment at VHA clinics apply for PTSD-related disability benefits.21 The estimated cost related to PTSD/TBI service-connected pensions is $20.28 billion per year and approximately $514 billion over 50 years.22 The cost of VA and Social Security disability payments combined with health care costs and treatment of PTSD is estimated to exceed $1 trillion over the next 30 years.22
The National Vietnam Veterans Readjustment Study (NVVRS) provided valuable information on prevalence rates of PTSD and other postwar psychological problems.23 Meanwhile, there have been no recent large-scale studies to compare the demographics of veterans diagnosed with PTSD and TBI who served prior to and after 9/11. A better understanding of demographic changes is considered essential for designing and tailoring therapeutic interventions to manage the rising cost.22
The present study focused on identifying changing trends in the demographics of veterans who served prior to and after 9/11 and who received a VA inpatient or outpatient diagnosis of PTSD and/or TBI. Specifically, this study addressed the changes in demographics of veterans with PTSD, TBI, or PTSD+TBI seen at the VHA clinics between December 1,1998 and May 31, 2014 (before and after September 11, 2001) for diagnosis, treatment and health care policy issues.
Methods
This study was approved by the Kansas City VA Medical Center Institutional Review Board. VHA data from the Corporate Data Warehouse (CDW) and the National Patient Care Database were extracted using the VA Informatics and Computing Infrastructure (VINCI) workspace. CDW uses a unique identifier to identify veterans across treatment episodes at more than 1,400 VHA centers organized under 21 Veterans Integrated Service Networks (VISNs). These sources of VA data are widely used for retrospective longitudinal studies.
Study Population
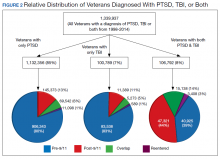
The study population consisted of 1,339,937 veterans with a VA inpatient/outpatient diagnosis of PTSD or TBI using International Statistical Classification of Diseases and Related Health Problems, Ninth Revision (ICD-9) codes between December 1, 1998 and May 31, 2014. Demographic (gender classification, race, ethnicity, marital status, age at date of data extraction, and date of death if indicated), service-connection disability rating, and geographic distribution within VISN data on each veteran were then extracted.
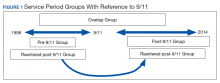
Veterans in the cohort were assigned to 1 of 4 US military services period groups. The pre-9/11 group included veterans who entered and left the military prior to September 11, 2001. This group mostly included veterans from World War II, Korean War, Vietnam War, and the first Gulf War (1990-1991). The post-9/11 group included veterans who first entered military services after September 11, 2001. The overlap group included veterans who entered military services prior to 9/11, remained in service and left after September 11, 2001. The reentered group included veterans who entered and left service prior to September 11, 2001, and then reentered military service after September 11, 2001 (Figure 1). Using ICD-9 codes, veterans also were placed into the following categories: PTSD alone (ICD-9 309.81 only), TBI alone (ICD-9 850.0-859.9, V15.52), and PTSD+TBI (any combination of ICD-9 codes from the other categories).
Statistical Analysis
Descriptive statistics were applied using proportions and means. Relationships between variables were examined using χ2 tests, t tests, analysis of variance, and nonparametric tests. All hypotheses were 2-sided at 95% CI. Results are presented as absolute numbers.
Results
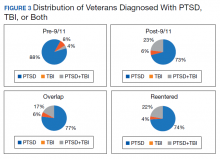
PTSD only (n = 1,132,356, 85%) was the predominant diagnosis category followed by PTSD+TBI (n = 106,792, 8%) and TBI only (n = 100,789, 7%) (Figure 2). Most of the veterans in the study served pre-9/11 (77%), followed by post-9/11 (15%); 7% were in the overlap group, and 1% in the reentered group (Table 1). It is notable that the proportion of veterans diagnosed with PTSD decreased from pre-9/11 (88%) to post-9/11 (71%), overlap (77%), and reentered (74%) service periods. Increases were noted in those with PTSD+TBI diagnosis category from pre-9/11 (4%) to post-9/11 (23%), overlap (17%), and reentered (22%) service periods (Figure 3). In general, the relative distribution of diagnostic categories in all the service periods showed a similar trend, with the majority of veterans diagnosed with PTSD only. Across all service periods, significantly smaller proportions of veterans were diagnosed with TBI only (P < .001).
Distribution by Gender and Age
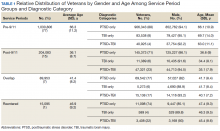
The cohort was 92% male (n = 1,239,295), but there was a marked increase in the percentage of nonmale veterans in post-9/11 groups. Study population ages ranged from 18 to 99 years based on date of birth to the date data were obtained; or date of birth to date of death, for those who were reported deceased at the time the data were obtained. The average (SD) ages for veterans in the pre-9/11 group were significantly older (66.3 [11.2] years) compared with the ages of veterans in the post-9/11 group (36.1 [8.7] years), the overlap group (41.4 [8.2] years), and the reentered group (46.9 [9.2] years), respectively.
Distribution by Race and Marital Status
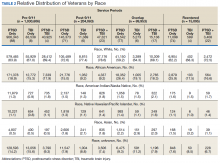
The cohort identified as 65.7% white and 18.2% African American with much smaller percentages of Asians, American Indian/Alaska Natives (AI/AN) and Native Hawaiian/Pacific Islanders (Table 2). The relative proportion of AI/AN and Native Hawaiian/Pacific Islanders remained constant across all groups, whereas the number of Asians diagnosed with PTSD, TBI, or PTSD+TBI increased in the post-9/11 group. The number of African Americans diagnosed with PTSD, TBI, or both markedly increased in the overlap and reentered groups when compared with the pre-9/11 group, yet it went down in the post-9/11/group.
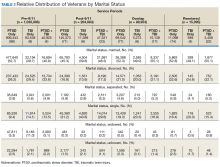
Half the cohort identified themselves as married (n = 675,145) (Table 3). A slightly larger proportion of those diagnosed with PTSD alone were married (51.7%), compared with those diagnosed with TBI only (40.3%), or PTSD+TBI (45.8%). Veterans in the post-9/11 group were less likely to identify as married (45.2%) compared with the pre-9/11 (51.2%), overlap (52.6%), or reentered (53.2%) groups. Divorce rates among pre-9/11 group, overlap group, and reentered group were higher compared with that of the post-9/11 group in all diagnosis categories.
Geographic Distribution
Veterans diagnosed with PTSD, TBI, or both were not evenly distributed across the VISNs VISNs 7, 8, 10, and 22 treated the most veterans, whereas VISN 9 and 15 treated the fewest. Taken together, the top 3 VISNs accounted for 27% to 28% of the total while lowest 3 accounted for 8% to 9% of the total cohort.
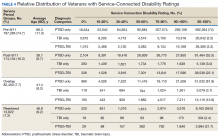
Service-Connected Disability
Of 1,339,937 veterans in the cohort, 1,067,691 had a service-connected disability rating for PTSD and/or TBI. Most were diagnosed with PTSD (n = 923,523, 86.5%) followed by both PTSD+TBI (n = 94,051, 8.8%). Three-quarters of the veterans with a service-connected disability were in the pre-9/11 group. Nearly 80% of veterans with a service-connected disability rating had a rating of > 50%. The average (SD) age of veterans with PTSD+TBI and a > 50% service-connected disability was 66.3 (11.2) years in the pre-9/11 group compared with 36.1 (8.7) years in the post-9/11 group.
Discussion
The demographic profile of veterans diagnosed with PTSD+TBI has changed across the service periods covered in this study. Compared with pre-9/11 veterans, the post-9/11 cohort: (1) higher percentage were diagnosed with PTSD+TBI; (2) higher proportion were nonmale veterans; (3) included more young veterans with > 50% service-connected disability; (4) were more racially diverse; and (5) were less likely to be married and divorced and more likely to be self-identified as single. Additionally, data revealed that veterans tended to locate more to some geographic regions than to others.
The nature of the warfare has changed remarkably over the past few decades. Gunshot wounds accounted for 65% of all injuries in World War I, 35% during Vietnam War, and 16% to 23% in the First Gulf War.24 In post-9/11 military conflicts, 81% of injuries were explosion related.24,25 Although improvements in personal protective gear and battlefield trauma care led to increased survival, several factors may have contributed to increased reporting of TBI, which peaked in 2011 at 32,000 cases.24-26
Increases in PTSD Diagnosis
Increasing media awareness, mandatory battlefield concussion screening programs instituted by the US Department of Defense (DoD), and stressful conditions that exacerbate mild TBI (mTBI) may have all contributed to the increase in numbers of veterans seeking evaluations and being diagnosed with PTSD and/or TBI in the post-9/11 groups. Additionally, the 2007 National Defense Authorization Act requested the Secretary of Defense to develop a comprehensive, systematic approach for the identification, treatment, disposition, and documentation of TBI in combat and peacetime. By a conservative estimate, significant numbers of veterans will continue to be seen for mTBI at about 20,000 new cases per year.25-27
More frequent diagnosis of mTBI may have contributed to the increase in veterans diagnosed with PTSD+TBI in the post-9/11 groups. A recent study found that almost 44% of US Army infantry soldiers in Iraq did not lose consciousness but reported symptoms consistent with TBI.14 Compared with veterans of previous wars, veterans of the post-9/11 conflicts (OIF, OED, and OND) have experienced multiple, protracted deployments with infrequent breaks that can have a cumulative effect on the development of PTSD.8-10
The findings from the NVVRS study led to creation of specialized PTSD programs in the late 1980s. Since then, there has been an explosion of knowledge and awareness about PTSD, TBI, and the associated service-connected disability ratings and benefits, leading to an increased number of veterans seeking care for PTSD. For example, media coverage of the 50th anniversary of the D-day celebrations resulted in a surge of World War II veterans seeking treatment for PTSD and a surge of Vietnam veterans sought treatment for PTSD during the wars in Iraq and Afghanistan.28 An increased number of veterans reporting PTSD symptoms prompted the DoD to increase screening for PTSD, and to encourage service members to seek treatment when appropriate.
The VA has instituted training programs for clinicians and psychologists to screen and provide care for PTSD. Beginning in 2007, the VA implemented mandatory TBI screening for all veterans who served in combat operations and separated from active-duty service after September 11, 2001. The 4-question screen identifies veterans who are at increased risk of TBI and who experience symptoms that may be related to specific event(s).29 A positive screen does not diagnose TBI but rather indicates a need for further evaluation, which may or may not be responsible for inflated reporting of TBI. Renewed research also has led providers to recognize and study PTSD resulting from noncombat trauma and moral injury. The possibility of delayed onset also drives up the number of veterans diagnosed with PTSD.5-7
Prevalence
A wide variability exists in the reported prevalence of PTSD among US war veterans with estimates ranging from 15% to 20% of veterans from recent conflicts3,20 and 10% to 30% of veterans from previous wars.3,19 These rates are higher than estimates from allied forces from other countries.19 Meta-analyses suggest that the prevalence of PTSD is 2% to 15% among Vietnam War veterans, 1% to 13% among first (pre-9/11) Gulf War veterans, 4% to 17% among OEF/OIF/OND veterans; these veterans have a lifetime prevalence of 6% to 31%.3,11,19,30-38 The prevalence of PTSD is 2 to 4 times higher among the US veterans19,39 when compared with that of civilians.40,41 According to one study, concomitant PTSD and TBI appears to be much higher in US war veterans (4%-17%) compared with United Kingdom Iraq War veterans (3%-6%).19
This study’s finding of an increase in nonmale soldiers with PTSD and/or TBI was not surprising. There is a paucity of data on the effect of war zone exposure on women veterans. Recently, women have been more actively involved in combat roles with 41,000 women deployed to a combat zone. Results of this study indicate a 2- to 3-fold increase in veterans identifying themselves as nonmale in post-9/11 groups with a higher proportion diagnosed with either PTSD alone or PTSD and TBI. Women are at a higher risk for PTSD than are men due in part to exposure to abuse/trauma prior to deployment, experience of higher rates of discrimination, and/or sexual assault.31-33 One study involving First Gulf War female veterans reported higher precombat psychiatric histories as well as higher rates of physical and sexual abuse when compared with that of men.31
In this study, the average age of veterans adjudicated and compensated for PTSD and/or TBI pre-9/11, was 66 years compared with 36 years for post-9/11 veterans. Sixty-six percent of veterans from the post-9/11 group had ≥ 50% service-connected disability at age 36 years; 75% of veterans from the overlap group had ≥ 50% service-connected disability at age 41 years; and 76% veterans from the reentered group had ≥ 50% service-connected disability at age 46 years. Younger age at diagnosis and higher rates of disability not only pose unique challenges for veterans and family members, but also suggest implications for career prospects, family earnings, loss of productivity, and disease-adjusted life years. Also noted in the results, this younger cohort has a higher percentage of single/unmarried veterans, suggesting familial support systems may be more parental than spousal. Treatment for this younger cohort will likely need to focus on early and sustained rehabilitation that can be integrated with career plans.
For treatment to be effective, there must be evidence for veterans enrolling, remaining, and reporting benefits from the treatment. Limited research has shown currently advocated evidence-based therapies to have low enrollment rates, high drop-out rates, and mixed outcomes.42
Results showing a gradual increase in the proportion of nonwhite, non-African American veterans diagnosed with PTSD alone, TBI alone, or both, likely reflect the changing demographic profile of the US as well as the Army. However, the reason that more African Americans were diagnosed with PTSD and/or TBI in the overlap and reentered groups when compared with the pre-9/11 group could not be ascertained. It is possible that more veterans identified themselves as African Americans as evident from a decrease in the number of veterans in the unknown category post-9/11 when compared with the pre-9/11 group. In 2016, the American Community Survey showed that Hispanic and African American veterans were more likely to use VA health care and other benefits than were any other racial group.40 Improved screening for PTSD and TBI diagnoses, increased awareness, and education about the availability of VA services and benefits may have contributed to the increased use of VA benefits in these groups.
Data from this study are concordant with data from the National Center for Veterans Analysis and Statistics reporting on the younger age of diagnosis and higher rates of initial service-connected disability in veterans with PTSD and PTSD+TBI.43 One study analyzing records from 1999 to 2004 showed that the number of PTSD cases grew by 79.5%, resulting in 148.7% increase in benefits payment from $1.7 billion to $4.3 billion per year.44 In contrast, the compensation cost for all other disability categories increased by only 41.7% over this period. This study also revealed that while veterans with PTSD represented only 8.7% of compensation recipients, they received 20.5% of all compensation payments, driven in large part by an increase in > 50% service-connected disability ratings.44
Thus, from financial as well as treatment points of view, the change in the demographic profile of the veteran must be considered when developing PTSD treatment strategies. While treatment in the past focused solely on addressing trauma-associated psychiatric issues, TBI and PTSD association will likely shift the focus to concurrent psychiatric and physical symptomology. Similarly, PTSD/TBI treatment modalities must consider that the profile of post-9/11 service members includes more women, younger age, and a greater racial diversity. For instance, younger age for a disabled veteran brings additional challenges, including reliance on parental or buddy support systems vs a spousal support system, integrating career with treatment, selecting geographic locations that can support both career and treatment, sustaining rehabilitation over time. The treatment needs of a 35-year-old soldier with PTSD and/or TBI, whether male or female, Asian or African American are likely to be very different from the treatment needs of a 65-year-old white male. Newer treatment approaches will have to address the needs of all soldiers.
Limitations
Our study may underestimate the actual PTSD and/or TBI disease burden because of the social stigma associated with diagnosis, military culture, limitations in data collection.45-50 In addition, in this retrospective database cohort study, we considered and tried to minimize the impact of any of the usual potential limitations, including (1) accuracy of data quality and linkage; (2) identifying cohort appropriately (study groups); (3) defining endpoints clearly to avoid misclassifications; and (4) incorporating all important confounders. We identified veterans utilizing medical services at VA hospitals during a defined period and diagnosed with PTSD and TBI using ICD-9 codes and divided in 4 well-defined groups. In addition, another limitation of our study is to not accurately capture the veterans who have alternative health coverage and may choose not to enroll and/or participate in VA health care. In addition, some service members leaving war zones may not disclose or downplay the mental health symptoms to avoid any delay in their return home.
Conclusions
This study highlights the changing profile of the soldier diagnosed with PTSD and/or TBI who served pre-9/11 compared with that of those who served post-9/11. Treatment modalities must address the changes in warfare and demographics of US service members. Future treatment will need to focus more on concurrent PTSD/TBI therapies, the needs of younger soldiers, the needs of women injured in combat, and the needs of a more racially and ethnically diverse population. Severe injuries at a younger age will require early detection and rehabilitation for return to optimum functioning over a lifetime. The current study underscores a need for identifying the gaps in ongoing programs and services, developing alternatives, and implementing improved systems of care. More studies are needed to identify the cost implications and the effectiveness of current therapies for PTSD and/or TBI.
Acknowledgments
This study was supported by VA Medical Center and Midwest BioMedical Research Foundation (MBRF), Kansas City, Missouri. The manuscript received support, in part, from NIH-RO1 DK107490. These agencies did not participate in the design/conduct of the study or, in the interpretation of the data.
1. Bagalman E. Traumatic brain injury among veterans. http://www.ncsl.org/documents/statefed/health/TBI_Vets2013.pdf. Published January 4, 2013. Accessed February 3, 2020.
2. Veterans Health Administration, Support Service Center. Workload files fiscal year 2008-fiscal year 2012. [Source not verified.]
3. Tanielian T, Jaycox LH, eds. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. Santa Monica, CA: RAND Corporation; 2008.
4. Bagalman E. Health care for veterans: traumatic brain injury. https://fas.org/sgp/crs/misc/R40941.pdf. Published March 9, 2015. Accessed February 4, 2020.
5. Ikin JF, Sim MR, McKenzie DP, et al. Anxiety, post-traumatic stress disorder and depression in Korean War veterans 50 years after the war. Br J Psychiatry. 2007;190(6):475-483.
6. Andrews B, Brewin CR, Philpott R, Stewart L. Delayed-onset posttraumatic stress disorder: a systematic review of the evidence. Am J Psychiatry. 2007;164(9):1319-1326.
7. Frueh BC, Grubaugh AL, Yeager DE, Magruder KM. Delayed-onset post-traumatic stress disorder among war veterans in primary care clinics. Br J Psychiatry. 2009;194(6):515-520.
8. McAllister TW. Neurobiological consequences of traumatic brain injury. Dialogues Clin Neurosci. 2011;13(3):287-300.
9. Schlenger WE, Kulka RA, Fairbank JA, et al. The prevalence of posttraumatic stress disorder in the Vietnam generation: a multimethod, multisource assessment of psychiatric disorder. J Trauma Stress. 1992;5(3):333-363.
10. Friedman MJ, Resick PA, Bryant RA, Strain J, Horowitz M, Spiegel D. Classification of trauma and stressor-related disorders in DSM-5. Depress Anxiety. 2011;28(9):737-749.
11. Lew HL, Otis JD, Tun C, Kerns RD, Clark ME, Cifu DX. Prevalence of chronic pain, posttraumatic stress disorder, and persistent postconcussive symptoms in OIF/OEF veterans: polytrauma clinical triad. J Rehabil Res Dev. 2009;46(6):697-702.
12. Carlson K, Kehle S, Meis L, et al. The Assessment and Treatment of Individuals with History of Traumatic Brain Injury and Post-Traumatic Stress Disorder: A Systematic Review of the Evidence. Washington, DC: US Department of Veterans Affairs; 2009.
13. Gironda RJ, Clark ME, Ruff RL, et al. Traumatic brain injury, polytrauma, and pain: challenges and treatment strategies for the polytrauma rehabilitation. Rehabil Psychol. 2009;54(3):247-258.
14. Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. soldiers returning from Iraq. N Engl J Med. 2008;358(5):453-463.
15. Bazarian JJ, Cernak I, Noble-Haeusslein L, Potolicchio S, Temkin N. Long-term neurologic outcomes after traumatic brain injury. J Head Trauma Rehabil. 2009;24(6):439-451.
16. Peskind ER, Brody D, Cernak I, McKee A, Ruff RL. Military- and sports-related mild traumatic brain injury: clinical presentation, management, and long-term consequences. J Clin Psychiatry. 2013;74(2):180-188.
17. Riggio S. Traumatic brain injury and its neurobehavioral sequelae. Neurol Clin. 2011;29(1):35-47, vii.
18. Helmick KM, Spells CA, Malik SZ, Davies CA, Marion DW, Hinds SR. Traumatic brain injury in the US military: epidemiology and key clinical and research programs. Brain Imaging Behav. 2015;9(3):358-366.
19. Richardson LK, Frueh BC, Acierno R. Prevalence estimates of combat-related post-traumatic stress disorder: critical review. Aust N Z J Psychiatry. 2010;44(1):4-19.
20. Thompson WW, Gottesman II, Zalewski C. Reconciling disparate prevalence rates of PTSD in large samples of US male Vietnam veterans and their controls. BMC Psychiatry. 2006;6:19.
21. Frueh BC, Elhai JD, Gold PB, et al Disability compensation seeking among veterans evaluated for posttraumatic stress disorder. Psychiatr Serv. 2003;54(1):84-91.
22. Thakur H, Oni O, Singh V, et al. Increases in the service connection disability and treatment costs associated with posttraumatic stress disorder and/or traumatic brain injury in United States veterans pre- and post-9/11: the strong need for a novel therapeutic approach. Epidemiology (Sunnyvale). 2018;8(4):353.
23. Schlenger WE, Kulka RA, Fairbank JA, et al. The prevalence of post-traumatic stress disorder in the Vietnam generation: a multimethod, multisource assessment of psychiatric disorder. J Trauma Stress. 1992;5(3):333-363.
24. Belmont PJ, Schoenfeld AJ, Goodman G. Epidemiology of combat wounds in Operation Iraqi Freedom and Operation Enduring Freedom: orthopaedic burden of disease. J Surg Orthop Adv. 2010;19(1):2-7.
25. Owens BD, Kragh JG Jr, Wenke JC, Macaitis J, Wade CE, Holcomb JB. Combat wounds in Operation Iraqi Freedom and Operation Enduring Freedom. J Trauma. 2008;64(2):295-299.
26. Defense Health Agency, Defense and Veterans Brain Injury Center. DOD worldwide numbers for TBI since 2000. https://dvbic.dcoe.mil/dod-worldwide-numbers-tbi. Updated February 14, 2020. Accessed February 14, 2020.
27. Armed Forces Health Surveillance Center. Deployment-related conditions of special surveillance interest, U.S. armed forces, by month and service, January 2003-December 2012 (data as of 22 January 2013). MSMR. 2013;20(1):16-19.
28. Harvey JH, Stein SK, Scott PK. Fifty years of grief: accounts and reported psychological reactions of Normandy invasion veterans. J Narrative Life History. 1995;5(4):321-332.
29. US Department of Veterans Affairs. Polytrauma/TBI system of care. https://www.polytrauma.va.gov/system-of-care/index.asp. Updated June 3, 2015. Accessed February 4, 2020.
30. Wolfe J, Erickson DJ, Sharkansky EJ, King DW, King LA. Course and predictors of posttraumatic stress disorder among Gulf War veterans: a prospective analysis. J Consult Clin Psychol. 1999;67(4):520-528.
31. Breslau N, Davis GC, Peterson EL, Schultz L. Psychiatric sequelae of posttraumatic stress disorder in women. Arch Gen Psychiatry. 1997;54(1):81-87.
32. Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52(12):1048-1060.
33. Wolfe J, Kimerling R. Gender issues in the assessment of posttraumatic stress disorder. In: Wilson J, Keane TM, eds. Assessing Psychological Trauma and PTSD. New York: Guilford; 2004:192-238.
34. Engel CC Jr, Engel AL, Campbell SJ, McFall ME, Russo J, Katon W. Posttraumatic stress disorder symptoms and precombat sexual and physical abuse in Desert Storm veterans. J Nerv Ment Dis. 1993;181(11):683-688.
35. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Profile of veterans: 2016 data from the American Community Survey. https://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2016.pdf. Published February 2018. Accessed February 4, 2020.
36. US Department of Commerce Economics and Statistics Administration, US Census Bureau, Geography Division. 2010 population distribution in the United States and Puerto Rico. https://www2.census.gov/geo/maps/dc10_thematic/2010_Nighttime_PopDist/2010_Nighttime_PopDist_Page_Map.pdf. Accessed February 4, 2020.
37. Cifu DX, Taylor BC, Carne WF, et al. Traumatic brain injury, posttraumatic stress disorder, and pain diagnoses in OIF/OEF/OND veterans. J Rehabil Res Dev. 2013;50(9):1169-1176.
38. Dohrenwend BP, Turner JB, Turse NA, Adams BG, Koenen KC, Marshall R. The psychological risks of Vietnam for U.S. veterans: a revisit with new data and methods. Science. 2006;313(5789):979-982.
39. Magruder KM, Frueh BC, Knapp RG, et al. Prevalence of posttraumatic stress disorder in Veterans Affairs primary care clinics. Gen Hosp Psychiatry. 2005;27(3):169-179.
40. Norris FH. Epidemiology of trauma: frequency and impact of different potentially traumatic events on different demographic groups. J Consult Clin Psychol. 1992;60(3):409-418.
41. Resnick HS, Kilpatrick DG, Dansky BS, Saunders BE, Best CL. Prevalence of civilian trauma and posttraumatic stress disorder in a representative national sample of women. J Consult Clin Psychol. 1993;61(6):984-991.
42. Najavits LM. The problem of dropout from “gold standard” PTSD therapies. F1000Prime Rep. 2015;7:43.
43. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Trends in veterans with a service-connected disability: 1985 to 2014. https://www.va.gov/vetdata/docs/QuickFacts/SCD_trends_FINAL_2014.PDF. Published June 2015. Accessed February 4, 2020.
44. US Department of Veterans Affairs, Office of Inspector General. Review of state variances in VA disability compensation payments. Report 05-00765-137. https://www.va.gov/oig/52/reports/2005/VAOIG-05-00765-137.pdf. Published May 19, 2015. Accessed February 4, 2020.
45. McNally RJ. Progress and controversy in the study of posttraumatic stress disorder. Annu Rev Psychol. 2003;54:229-252.
46. Freeman T, Powell M, Kimbrell T. Measuring symptom exaggeration in veterans with chronic posttraumatic stress disorder. Psychiatry Res. 2008;158(3):374-380.
47. Frueh BC, Elhai JD, Grubaugh AL, et al. Documented combat exposure of US veterans seeking treatment for combat-related post-traumatic stress disorder. Br J Psychiatry. 2005;186(6):467-475.
48. Frueh BC, Hamner MB, Cahill SP, Gold PB, Hamlin KL. Apparent symptom overreporting in combat veterans evaluated for PTSD. Clin Psychol Rev. 2000;20(7):853-885.
49. Sparr L, Pankratz LD. Factitious posttraumatic stress disorder. Am J Psychiatry. 1983;140(8):1016-1019.
50. Baggaley M. ‘Military Munchausen’s’: assessment of factitious claims of military service in psychiatric patients. Psychiatr Bull. 1998;22(3):153-154.
The nature of combat and associated injuries in Operation Iraqi Freedom (OIF), Operation Enduring Freedom (OEF), Operation New Dawn (OND), and Afghanistan War is different from previous conflicts. Multiple protracted deployments with infrequent breaks after September 11, 2001 (9/11) have further compounded the problem.
Posttraumatic stress disorder (PTSD) and traumatic brain injury (TBI) are the signature wounds of recent wars, with a higher incidence among the veterans of OEF and OIF compared with those from previous conflicts.1,2 More than 2.7 million who served in Iraq and Afghanistan suffer from PTSD.3,4 Symptoms of PTSD may appear within the first 3 months after exposure to a traumatic event or after many months and, in some cases, after a delay of many years and continue for life.5 Although delayed onset of PTSD in the absence of prior symptoms is rare,6,7 its incidence rises with increasing frequency of exposure to traumatic events8,9 and over time.10
According to the Brain Injury Association of America, TBI is “an alteration in brain function, or other evidence of brain pathology, caused by an external force.”8 TBI is often associated with increased risk of PTSD, depression, and posttraumatic headache,11-13 which may lead to broader cognitive, somatic, neurobiological, and psychosocial dysfunctions.14-17 According to Veterans Health Administration (VHA) data, 201,435 veterans from all eras enrolled with the US Department of Veterans Affairs (VA) have a diagnosis associated with TBI and 56,695 OEF/OIF veterans have been evaluated for a TBI-related condition.2 According to the Defense and Veterans Brain Injury Center (DVBIC), > 361,000 veterans have been diagnosed with TBI, with a peak of 32,000 cases in 2011.1,18 Moreover, the reported incidence and prevalence of PTSD and TBI among US veterans are not consistent. The incidence of PTSD has been estimated at 15% to 20% in recent wars3,19 compared with 10% to 30% in previous wars.3,19,20
When PTSD or TBI is deemed “related” to military service, the veteran may receive a service-connected disability rating ranging from 0% (no life-interfering symptoms due to injury) to 100% (totally disabling injury). The percentage of service connection associated with an injury is a quantifiable measure of the debilitating effect of injury on the individual. A significant majority (94%) of those who seek mental health services and treatment at VHA clinics apply for PTSD-related disability benefits.21 The estimated cost related to PTSD/TBI service-connected pensions is $20.28 billion per year and approximately $514 billion over 50 years.22 The cost of VA and Social Security disability payments combined with health care costs and treatment of PTSD is estimated to exceed $1 trillion over the next 30 years.22
The National Vietnam Veterans Readjustment Study (NVVRS) provided valuable information on prevalence rates of PTSD and other postwar psychological problems.23 Meanwhile, there have been no recent large-scale studies to compare the demographics of veterans diagnosed with PTSD and TBI who served prior to and after 9/11. A better understanding of demographic changes is considered essential for designing and tailoring therapeutic interventions to manage the rising cost.22
The present study focused on identifying changing trends in the demographics of veterans who served prior to and after 9/11 and who received a VA inpatient or outpatient diagnosis of PTSD and/or TBI. Specifically, this study addressed the changes in demographics of veterans with PTSD, TBI, or PTSD+TBI seen at the VHA clinics between December 1,1998 and May 31, 2014 (before and after September 11, 2001) for diagnosis, treatment and health care policy issues.
Methods
This study was approved by the Kansas City VA Medical Center Institutional Review Board. VHA data from the Corporate Data Warehouse (CDW) and the National Patient Care Database were extracted using the VA Informatics and Computing Infrastructure (VINCI) workspace. CDW uses a unique identifier to identify veterans across treatment episodes at more than 1,400 VHA centers organized under 21 Veterans Integrated Service Networks (VISNs). These sources of VA data are widely used for retrospective longitudinal studies.
Study Population
The study population consisted of 1,339,937 veterans with a VA inpatient/outpatient diagnosis of PTSD or TBI using International Statistical Classification of Diseases and Related Health Problems, Ninth Revision (ICD-9) codes between December 1, 1998 and May 31, 2014. Demographic (gender classification, race, ethnicity, marital status, age at date of data extraction, and date of death if indicated), service-connection disability rating, and geographic distribution within VISN data on each veteran were then extracted.
Veterans in the cohort were assigned to 1 of 4 US military services period groups. The pre-9/11 group included veterans who entered and left the military prior to September 11, 2001. This group mostly included veterans from World War II, Korean War, Vietnam War, and the first Gulf War (1990-1991). The post-9/11 group included veterans who first entered military services after September 11, 2001. The overlap group included veterans who entered military services prior to 9/11, remained in service and left after September 11, 2001. The reentered group included veterans who entered and left service prior to September 11, 2001, and then reentered military service after September 11, 2001 (Figure 1). Using ICD-9 codes, veterans also were placed into the following categories: PTSD alone (ICD-9 309.81 only), TBI alone (ICD-9 850.0-859.9, V15.52), and PTSD+TBI (any combination of ICD-9 codes from the other categories).
Statistical Analysis
Descriptive statistics were applied using proportions and means. Relationships between variables were examined using χ2 tests, t tests, analysis of variance, and nonparametric tests. All hypotheses were 2-sided at 95% CI. Results are presented as absolute numbers.
Results
PTSD only (n = 1,132,356, 85%) was the predominant diagnosis category followed by PTSD+TBI (n = 106,792, 8%) and TBI only (n = 100,789, 7%) (Figure 2). Most of the veterans in the study served pre-9/11 (77%), followed by post-9/11 (15%); 7% were in the overlap group, and 1% in the reentered group (Table 1). It is notable that the proportion of veterans diagnosed with PTSD decreased from pre-9/11 (88%) to post-9/11 (71%), overlap (77%), and reentered (74%) service periods. Increases were noted in those with PTSD+TBI diagnosis category from pre-9/11 (4%) to post-9/11 (23%), overlap (17%), and reentered (22%) service periods (Figure 3). In general, the relative distribution of diagnostic categories in all the service periods showed a similar trend, with the majority of veterans diagnosed with PTSD only. Across all service periods, significantly smaller proportions of veterans were diagnosed with TBI only (P < .001).
Distribution by Gender and Age
The cohort was 92% male (n = 1,239,295), but there was a marked increase in the percentage of nonmale veterans in post-9/11 groups. Study population ages ranged from 18 to 99 years based on date of birth to the date data were obtained; or date of birth to date of death, for those who were reported deceased at the time the data were obtained. The average (SD) ages for veterans in the pre-9/11 group were significantly older (66.3 [11.2] years) compared with the ages of veterans in the post-9/11 group (36.1 [8.7] years), the overlap group (41.4 [8.2] years), and the reentered group (46.9 [9.2] years), respectively.
Distribution by Race and Marital Status
The cohort identified as 65.7% white and 18.2% African American with much smaller percentages of Asians, American Indian/Alaska Natives (AI/AN) and Native Hawaiian/Pacific Islanders (Table 2). The relative proportion of AI/AN and Native Hawaiian/Pacific Islanders remained constant across all groups, whereas the number of Asians diagnosed with PTSD, TBI, or PTSD+TBI increased in the post-9/11 group. The number of African Americans diagnosed with PTSD, TBI, or both markedly increased in the overlap and reentered groups when compared with the pre-9/11 group, yet it went down in the post-9/11/group.
Half the cohort identified themselves as married (n = 675,145) (Table 3). A slightly larger proportion of those diagnosed with PTSD alone were married (51.7%), compared with those diagnosed with TBI only (40.3%), or PTSD+TBI (45.8%). Veterans in the post-9/11 group were less likely to identify as married (45.2%) compared with the pre-9/11 (51.2%), overlap (52.6%), or reentered (53.2%) groups. Divorce rates among pre-9/11 group, overlap group, and reentered group were higher compared with that of the post-9/11 group in all diagnosis categories.
Geographic Distribution
Veterans diagnosed with PTSD, TBI, or both were not evenly distributed across the VISNs VISNs 7, 8, 10, and 22 treated the most veterans, whereas VISN 9 and 15 treated the fewest. Taken together, the top 3 VISNs accounted for 27% to 28% of the total while lowest 3 accounted for 8% to 9% of the total cohort.
Service-Connected Disability
Of 1,339,937 veterans in the cohort, 1,067,691 had a service-connected disability rating for PTSD and/or TBI. Most were diagnosed with PTSD (n = 923,523, 86.5%) followed by both PTSD+TBI (n = 94,051, 8.8%). Three-quarters of the veterans with a service-connected disability were in the pre-9/11 group. Nearly 80% of veterans with a service-connected disability rating had a rating of > 50%. The average (SD) age of veterans with PTSD+TBI and a > 50% service-connected disability was 66.3 (11.2) years in the pre-9/11 group compared with 36.1 (8.7) years in the post-9/11 group.
Discussion
The demographic profile of veterans diagnosed with PTSD+TBI has changed across the service periods covered in this study. Compared with pre-9/11 veterans, the post-9/11 cohort: (1) higher percentage were diagnosed with PTSD+TBI; (2) higher proportion were nonmale veterans; (3) included more young veterans with > 50% service-connected disability; (4) were more racially diverse; and (5) were less likely to be married and divorced and more likely to be self-identified as single. Additionally, data revealed that veterans tended to locate more to some geographic regions than to others.
The nature of the warfare has changed remarkably over the past few decades. Gunshot wounds accounted for 65% of all injuries in World War I, 35% during Vietnam War, and 16% to 23% in the First Gulf War.24 In post-9/11 military conflicts, 81% of injuries were explosion related.24,25 Although improvements in personal protective gear and battlefield trauma care led to increased survival, several factors may have contributed to increased reporting of TBI, which peaked in 2011 at 32,000 cases.24-26
Increases in PTSD Diagnosis
Increasing media awareness, mandatory battlefield concussion screening programs instituted by the US Department of Defense (DoD), and stressful conditions that exacerbate mild TBI (mTBI) may have all contributed to the increase in numbers of veterans seeking evaluations and being diagnosed with PTSD and/or TBI in the post-9/11 groups. Additionally, the 2007 National Defense Authorization Act requested the Secretary of Defense to develop a comprehensive, systematic approach for the identification, treatment, disposition, and documentation of TBI in combat and peacetime. By a conservative estimate, significant numbers of veterans will continue to be seen for mTBI at about 20,000 new cases per year.25-27
More frequent diagnosis of mTBI may have contributed to the increase in veterans diagnosed with PTSD+TBI in the post-9/11 groups. A recent study found that almost 44% of US Army infantry soldiers in Iraq did not lose consciousness but reported symptoms consistent with TBI.14 Compared with veterans of previous wars, veterans of the post-9/11 conflicts (OIF, OED, and OND) have experienced multiple, protracted deployments with infrequent breaks that can have a cumulative effect on the development of PTSD.8-10
The findings from the NVVRS study led to creation of specialized PTSD programs in the late 1980s. Since then, there has been an explosion of knowledge and awareness about PTSD, TBI, and the associated service-connected disability ratings and benefits, leading to an increased number of veterans seeking care for PTSD. For example, media coverage of the 50th anniversary of the D-day celebrations resulted in a surge of World War II veterans seeking treatment for PTSD and a surge of Vietnam veterans sought treatment for PTSD during the wars in Iraq and Afghanistan.28 An increased number of veterans reporting PTSD symptoms prompted the DoD to increase screening for PTSD, and to encourage service members to seek treatment when appropriate.
The VA has instituted training programs for clinicians and psychologists to screen and provide care for PTSD. Beginning in 2007, the VA implemented mandatory TBI screening for all veterans who served in combat operations and separated from active-duty service after September 11, 2001. The 4-question screen identifies veterans who are at increased risk of TBI and who experience symptoms that may be related to specific event(s).29 A positive screen does not diagnose TBI but rather indicates a need for further evaluation, which may or may not be responsible for inflated reporting of TBI. Renewed research also has led providers to recognize and study PTSD resulting from noncombat trauma and moral injury. The possibility of delayed onset also drives up the number of veterans diagnosed with PTSD.5-7
Prevalence
A wide variability exists in the reported prevalence of PTSD among US war veterans with estimates ranging from 15% to 20% of veterans from recent conflicts3,20 and 10% to 30% of veterans from previous wars.3,19 These rates are higher than estimates from allied forces from other countries.19 Meta-analyses suggest that the prevalence of PTSD is 2% to 15% among Vietnam War veterans, 1% to 13% among first (pre-9/11) Gulf War veterans, 4% to 17% among OEF/OIF/OND veterans; these veterans have a lifetime prevalence of 6% to 31%.3,11,19,30-38 The prevalence of PTSD is 2 to 4 times higher among the US veterans19,39 when compared with that of civilians.40,41 According to one study, concomitant PTSD and TBI appears to be much higher in US war veterans (4%-17%) compared with United Kingdom Iraq War veterans (3%-6%).19
This study’s finding of an increase in nonmale soldiers with PTSD and/or TBI was not surprising. There is a paucity of data on the effect of war zone exposure on women veterans. Recently, women have been more actively involved in combat roles with 41,000 women deployed to a combat zone. Results of this study indicate a 2- to 3-fold increase in veterans identifying themselves as nonmale in post-9/11 groups with a higher proportion diagnosed with either PTSD alone or PTSD and TBI. Women are at a higher risk for PTSD than are men due in part to exposure to abuse/trauma prior to deployment, experience of higher rates of discrimination, and/or sexual assault.31-33 One study involving First Gulf War female veterans reported higher precombat psychiatric histories as well as higher rates of physical and sexual abuse when compared with that of men.31
In this study, the average age of veterans adjudicated and compensated for PTSD and/or TBI pre-9/11, was 66 years compared with 36 years for post-9/11 veterans. Sixty-six percent of veterans from the post-9/11 group had ≥ 50% service-connected disability at age 36 years; 75% of veterans from the overlap group had ≥ 50% service-connected disability at age 41 years; and 76% veterans from the reentered group had ≥ 50% service-connected disability at age 46 years. Younger age at diagnosis and higher rates of disability not only pose unique challenges for veterans and family members, but also suggest implications for career prospects, family earnings, loss of productivity, and disease-adjusted life years. Also noted in the results, this younger cohort has a higher percentage of single/unmarried veterans, suggesting familial support systems may be more parental than spousal. Treatment for this younger cohort will likely need to focus on early and sustained rehabilitation that can be integrated with career plans.
For treatment to be effective, there must be evidence for veterans enrolling, remaining, and reporting benefits from the treatment. Limited research has shown currently advocated evidence-based therapies to have low enrollment rates, high drop-out rates, and mixed outcomes.42
Results showing a gradual increase in the proportion of nonwhite, non-African American veterans diagnosed with PTSD alone, TBI alone, or both, likely reflect the changing demographic profile of the US as well as the Army. However, the reason that more African Americans were diagnosed with PTSD and/or TBI in the overlap and reentered groups when compared with the pre-9/11 group could not be ascertained. It is possible that more veterans identified themselves as African Americans as evident from a decrease in the number of veterans in the unknown category post-9/11 when compared with the pre-9/11 group. In 2016, the American Community Survey showed that Hispanic and African American veterans were more likely to use VA health care and other benefits than were any other racial group.40 Improved screening for PTSD and TBI diagnoses, increased awareness, and education about the availability of VA services and benefits may have contributed to the increased use of VA benefits in these groups.
Data from this study are concordant with data from the National Center for Veterans Analysis and Statistics reporting on the younger age of diagnosis and higher rates of initial service-connected disability in veterans with PTSD and PTSD+TBI.43 One study analyzing records from 1999 to 2004 showed that the number of PTSD cases grew by 79.5%, resulting in 148.7% increase in benefits payment from $1.7 billion to $4.3 billion per year.44 In contrast, the compensation cost for all other disability categories increased by only 41.7% over this period. This study also revealed that while veterans with PTSD represented only 8.7% of compensation recipients, they received 20.5% of all compensation payments, driven in large part by an increase in > 50% service-connected disability ratings.44
Thus, from financial as well as treatment points of view, the change in the demographic profile of the veteran must be considered when developing PTSD treatment strategies. While treatment in the past focused solely on addressing trauma-associated psychiatric issues, TBI and PTSD association will likely shift the focus to concurrent psychiatric and physical symptomology. Similarly, PTSD/TBI treatment modalities must consider that the profile of post-9/11 service members includes more women, younger age, and a greater racial diversity. For instance, younger age for a disabled veteran brings additional challenges, including reliance on parental or buddy support systems vs a spousal support system, integrating career with treatment, selecting geographic locations that can support both career and treatment, sustaining rehabilitation over time. The treatment needs of a 35-year-old soldier with PTSD and/or TBI, whether male or female, Asian or African American are likely to be very different from the treatment needs of a 65-year-old white male. Newer treatment approaches will have to address the needs of all soldiers.
Limitations
Our study may underestimate the actual PTSD and/or TBI disease burden because of the social stigma associated with diagnosis, military culture, limitations in data collection.45-50 In addition, in this retrospective database cohort study, we considered and tried to minimize the impact of any of the usual potential limitations, including (1) accuracy of data quality and linkage; (2) identifying cohort appropriately (study groups); (3) defining endpoints clearly to avoid misclassifications; and (4) incorporating all important confounders. We identified veterans utilizing medical services at VA hospitals during a defined period and diagnosed with PTSD and TBI using ICD-9 codes and divided in 4 well-defined groups. In addition, another limitation of our study is to not accurately capture the veterans who have alternative health coverage and may choose not to enroll and/or participate in VA health care. In addition, some service members leaving war zones may not disclose or downplay the mental health symptoms to avoid any delay in their return home.
Conclusions
This study highlights the changing profile of the soldier diagnosed with PTSD and/or TBI who served pre-9/11 compared with that of those who served post-9/11. Treatment modalities must address the changes in warfare and demographics of US service members. Future treatment will need to focus more on concurrent PTSD/TBI therapies, the needs of younger soldiers, the needs of women injured in combat, and the needs of a more racially and ethnically diverse population. Severe injuries at a younger age will require early detection and rehabilitation for return to optimum functioning over a lifetime. The current study underscores a need for identifying the gaps in ongoing programs and services, developing alternatives, and implementing improved systems of care. More studies are needed to identify the cost implications and the effectiveness of current therapies for PTSD and/or TBI.
Acknowledgments
This study was supported by VA Medical Center and Midwest BioMedical Research Foundation (MBRF), Kansas City, Missouri. The manuscript received support, in part, from NIH-RO1 DK107490. These agencies did not participate in the design/conduct of the study or, in the interpretation of the data.
The nature of combat and associated injuries in Operation Iraqi Freedom (OIF), Operation Enduring Freedom (OEF), Operation New Dawn (OND), and Afghanistan War is different from previous conflicts. Multiple protracted deployments with infrequent breaks after September 11, 2001 (9/11) have further compounded the problem.
Posttraumatic stress disorder (PTSD) and traumatic brain injury (TBI) are the signature wounds of recent wars, with a higher incidence among the veterans of OEF and OIF compared with those from previous conflicts.1,2 More than 2.7 million who served in Iraq and Afghanistan suffer from PTSD.3,4 Symptoms of PTSD may appear within the first 3 months after exposure to a traumatic event or after many months and, in some cases, after a delay of many years and continue for life.5 Although delayed onset of PTSD in the absence of prior symptoms is rare,6,7 its incidence rises with increasing frequency of exposure to traumatic events8,9 and over time.10
According to the Brain Injury Association of America, TBI is “an alteration in brain function, or other evidence of brain pathology, caused by an external force.”8 TBI is often associated with increased risk of PTSD, depression, and posttraumatic headache,11-13 which may lead to broader cognitive, somatic, neurobiological, and psychosocial dysfunctions.14-17 According to Veterans Health Administration (VHA) data, 201,435 veterans from all eras enrolled with the US Department of Veterans Affairs (VA) have a diagnosis associated with TBI and 56,695 OEF/OIF veterans have been evaluated for a TBI-related condition.2 According to the Defense and Veterans Brain Injury Center (DVBIC), > 361,000 veterans have been diagnosed with TBI, with a peak of 32,000 cases in 2011.1,18 Moreover, the reported incidence and prevalence of PTSD and TBI among US veterans are not consistent. The incidence of PTSD has been estimated at 15% to 20% in recent wars3,19 compared with 10% to 30% in previous wars.3,19,20
When PTSD or TBI is deemed “related” to military service, the veteran may receive a service-connected disability rating ranging from 0% (no life-interfering symptoms due to injury) to 100% (totally disabling injury). The percentage of service connection associated with an injury is a quantifiable measure of the debilitating effect of injury on the individual. A significant majority (94%) of those who seek mental health services and treatment at VHA clinics apply for PTSD-related disability benefits.21 The estimated cost related to PTSD/TBI service-connected pensions is $20.28 billion per year and approximately $514 billion over 50 years.22 The cost of VA and Social Security disability payments combined with health care costs and treatment of PTSD is estimated to exceed $1 trillion over the next 30 years.22
The National Vietnam Veterans Readjustment Study (NVVRS) provided valuable information on prevalence rates of PTSD and other postwar psychological problems.23 Meanwhile, there have been no recent large-scale studies to compare the demographics of veterans diagnosed with PTSD and TBI who served prior to and after 9/11. A better understanding of demographic changes is considered essential for designing and tailoring therapeutic interventions to manage the rising cost.22
The present study focused on identifying changing trends in the demographics of veterans who served prior to and after 9/11 and who received a VA inpatient or outpatient diagnosis of PTSD and/or TBI. Specifically, this study addressed the changes in demographics of veterans with PTSD, TBI, or PTSD+TBI seen at the VHA clinics between December 1,1998 and May 31, 2014 (before and after September 11, 2001) for diagnosis, treatment and health care policy issues.
Methods
This study was approved by the Kansas City VA Medical Center Institutional Review Board. VHA data from the Corporate Data Warehouse (CDW) and the National Patient Care Database were extracted using the VA Informatics and Computing Infrastructure (VINCI) workspace. CDW uses a unique identifier to identify veterans across treatment episodes at more than 1,400 VHA centers organized under 21 Veterans Integrated Service Networks (VISNs). These sources of VA data are widely used for retrospective longitudinal studies.
Study Population
The study population consisted of 1,339,937 veterans with a VA inpatient/outpatient diagnosis of PTSD or TBI using International Statistical Classification of Diseases and Related Health Problems, Ninth Revision (ICD-9) codes between December 1, 1998 and May 31, 2014. Demographic (gender classification, race, ethnicity, marital status, age at date of data extraction, and date of death if indicated), service-connection disability rating, and geographic distribution within VISN data on each veteran were then extracted.
Veterans in the cohort were assigned to 1 of 4 US military services period groups. The pre-9/11 group included veterans who entered and left the military prior to September 11, 2001. This group mostly included veterans from World War II, Korean War, Vietnam War, and the first Gulf War (1990-1991). The post-9/11 group included veterans who first entered military services after September 11, 2001. The overlap group included veterans who entered military services prior to 9/11, remained in service and left after September 11, 2001. The reentered group included veterans who entered and left service prior to September 11, 2001, and then reentered military service after September 11, 2001 (Figure 1). Using ICD-9 codes, veterans also were placed into the following categories: PTSD alone (ICD-9 309.81 only), TBI alone (ICD-9 850.0-859.9, V15.52), and PTSD+TBI (any combination of ICD-9 codes from the other categories).
Statistical Analysis
Descriptive statistics were applied using proportions and means. Relationships between variables were examined using χ2 tests, t tests, analysis of variance, and nonparametric tests. All hypotheses were 2-sided at 95% CI. Results are presented as absolute numbers.
Results
PTSD only (n = 1,132,356, 85%) was the predominant diagnosis category followed by PTSD+TBI (n = 106,792, 8%) and TBI only (n = 100,789, 7%) (Figure 2). Most of the veterans in the study served pre-9/11 (77%), followed by post-9/11 (15%); 7% were in the overlap group, and 1% in the reentered group (Table 1). It is notable that the proportion of veterans diagnosed with PTSD decreased from pre-9/11 (88%) to post-9/11 (71%), overlap (77%), and reentered (74%) service periods. Increases were noted in those with PTSD+TBI diagnosis category from pre-9/11 (4%) to post-9/11 (23%), overlap (17%), and reentered (22%) service periods (Figure 3). In general, the relative distribution of diagnostic categories in all the service periods showed a similar trend, with the majority of veterans diagnosed with PTSD only. Across all service periods, significantly smaller proportions of veterans were diagnosed with TBI only (P < .001).
Distribution by Gender and Age
The cohort was 92% male (n = 1,239,295), but there was a marked increase in the percentage of nonmale veterans in post-9/11 groups. Study population ages ranged from 18 to 99 years based on date of birth to the date data were obtained; or date of birth to date of death, for those who were reported deceased at the time the data were obtained. The average (SD) ages for veterans in the pre-9/11 group were significantly older (66.3 [11.2] years) compared with the ages of veterans in the post-9/11 group (36.1 [8.7] years), the overlap group (41.4 [8.2] years), and the reentered group (46.9 [9.2] years), respectively.
Distribution by Race and Marital Status
The cohort identified as 65.7% white and 18.2% African American with much smaller percentages of Asians, American Indian/Alaska Natives (AI/AN) and Native Hawaiian/Pacific Islanders (Table 2). The relative proportion of AI/AN and Native Hawaiian/Pacific Islanders remained constant across all groups, whereas the number of Asians diagnosed with PTSD, TBI, or PTSD+TBI increased in the post-9/11 group. The number of African Americans diagnosed with PTSD, TBI, or both markedly increased in the overlap and reentered groups when compared with the pre-9/11 group, yet it went down in the post-9/11/group.
Half the cohort identified themselves as married (n = 675,145) (Table 3). A slightly larger proportion of those diagnosed with PTSD alone were married (51.7%), compared with those diagnosed with TBI only (40.3%), or PTSD+TBI (45.8%). Veterans in the post-9/11 group were less likely to identify as married (45.2%) compared with the pre-9/11 (51.2%), overlap (52.6%), or reentered (53.2%) groups. Divorce rates among pre-9/11 group, overlap group, and reentered group were higher compared with that of the post-9/11 group in all diagnosis categories.
Geographic Distribution
Veterans diagnosed with PTSD, TBI, or both were not evenly distributed across the VISNs VISNs 7, 8, 10, and 22 treated the most veterans, whereas VISN 9 and 15 treated the fewest. Taken together, the top 3 VISNs accounted for 27% to 28% of the total while lowest 3 accounted for 8% to 9% of the total cohort.
Service-Connected Disability
Of 1,339,937 veterans in the cohort, 1,067,691 had a service-connected disability rating for PTSD and/or TBI. Most were diagnosed with PTSD (n = 923,523, 86.5%) followed by both PTSD+TBI (n = 94,051, 8.8%). Three-quarters of the veterans with a service-connected disability were in the pre-9/11 group. Nearly 80% of veterans with a service-connected disability rating had a rating of > 50%. The average (SD) age of veterans with PTSD+TBI and a > 50% service-connected disability was 66.3 (11.2) years in the pre-9/11 group compared with 36.1 (8.7) years in the post-9/11 group.
Discussion
The demographic profile of veterans diagnosed with PTSD+TBI has changed across the service periods covered in this study. Compared with pre-9/11 veterans, the post-9/11 cohort: (1) higher percentage were diagnosed with PTSD+TBI; (2) higher proportion were nonmale veterans; (3) included more young veterans with > 50% service-connected disability; (4) were more racially diverse; and (5) were less likely to be married and divorced and more likely to be self-identified as single. Additionally, data revealed that veterans tended to locate more to some geographic regions than to others.
The nature of the warfare has changed remarkably over the past few decades. Gunshot wounds accounted for 65% of all injuries in World War I, 35% during Vietnam War, and 16% to 23% in the First Gulf War.24 In post-9/11 military conflicts, 81% of injuries were explosion related.24,25 Although improvements in personal protective gear and battlefield trauma care led to increased survival, several factors may have contributed to increased reporting of TBI, which peaked in 2011 at 32,000 cases.24-26
Increases in PTSD Diagnosis
Increasing media awareness, mandatory battlefield concussion screening programs instituted by the US Department of Defense (DoD), and stressful conditions that exacerbate mild TBI (mTBI) may have all contributed to the increase in numbers of veterans seeking evaluations and being diagnosed with PTSD and/or TBI in the post-9/11 groups. Additionally, the 2007 National Defense Authorization Act requested the Secretary of Defense to develop a comprehensive, systematic approach for the identification, treatment, disposition, and documentation of TBI in combat and peacetime. By a conservative estimate, significant numbers of veterans will continue to be seen for mTBI at about 20,000 new cases per year.25-27
More frequent diagnosis of mTBI may have contributed to the increase in veterans diagnosed with PTSD+TBI in the post-9/11 groups. A recent study found that almost 44% of US Army infantry soldiers in Iraq did not lose consciousness but reported symptoms consistent with TBI.14 Compared with veterans of previous wars, veterans of the post-9/11 conflicts (OIF, OED, and OND) have experienced multiple, protracted deployments with infrequent breaks that can have a cumulative effect on the development of PTSD.8-10
The findings from the NVVRS study led to creation of specialized PTSD programs in the late 1980s. Since then, there has been an explosion of knowledge and awareness about PTSD, TBI, and the associated service-connected disability ratings and benefits, leading to an increased number of veterans seeking care for PTSD. For example, media coverage of the 50th anniversary of the D-day celebrations resulted in a surge of World War II veterans seeking treatment for PTSD and a surge of Vietnam veterans sought treatment for PTSD during the wars in Iraq and Afghanistan.28 An increased number of veterans reporting PTSD symptoms prompted the DoD to increase screening for PTSD, and to encourage service members to seek treatment when appropriate.
The VA has instituted training programs for clinicians and psychologists to screen and provide care for PTSD. Beginning in 2007, the VA implemented mandatory TBI screening for all veterans who served in combat operations and separated from active-duty service after September 11, 2001. The 4-question screen identifies veterans who are at increased risk of TBI and who experience symptoms that may be related to specific event(s).29 A positive screen does not diagnose TBI but rather indicates a need for further evaluation, which may or may not be responsible for inflated reporting of TBI. Renewed research also has led providers to recognize and study PTSD resulting from noncombat trauma and moral injury. The possibility of delayed onset also drives up the number of veterans diagnosed with PTSD.5-7
Prevalence
A wide variability exists in the reported prevalence of PTSD among US war veterans with estimates ranging from 15% to 20% of veterans from recent conflicts3,20 and 10% to 30% of veterans from previous wars.3,19 These rates are higher than estimates from allied forces from other countries.19 Meta-analyses suggest that the prevalence of PTSD is 2% to 15% among Vietnam War veterans, 1% to 13% among first (pre-9/11) Gulf War veterans, 4% to 17% among OEF/OIF/OND veterans; these veterans have a lifetime prevalence of 6% to 31%.3,11,19,30-38 The prevalence of PTSD is 2 to 4 times higher among the US veterans19,39 when compared with that of civilians.40,41 According to one study, concomitant PTSD and TBI appears to be much higher in US war veterans (4%-17%) compared with United Kingdom Iraq War veterans (3%-6%).19
This study’s finding of an increase in nonmale soldiers with PTSD and/or TBI was not surprising. There is a paucity of data on the effect of war zone exposure on women veterans. Recently, women have been more actively involved in combat roles with 41,000 women deployed to a combat zone. Results of this study indicate a 2- to 3-fold increase in veterans identifying themselves as nonmale in post-9/11 groups with a higher proportion diagnosed with either PTSD alone or PTSD and TBI. Women are at a higher risk for PTSD than are men due in part to exposure to abuse/trauma prior to deployment, experience of higher rates of discrimination, and/or sexual assault.31-33 One study involving First Gulf War female veterans reported higher precombat psychiatric histories as well as higher rates of physical and sexual abuse when compared with that of men.31
In this study, the average age of veterans adjudicated and compensated for PTSD and/or TBI pre-9/11, was 66 years compared with 36 years for post-9/11 veterans. Sixty-six percent of veterans from the post-9/11 group had ≥ 50% service-connected disability at age 36 years; 75% of veterans from the overlap group had ≥ 50% service-connected disability at age 41 years; and 76% veterans from the reentered group had ≥ 50% service-connected disability at age 46 years. Younger age at diagnosis and higher rates of disability not only pose unique challenges for veterans and family members, but also suggest implications for career prospects, family earnings, loss of productivity, and disease-adjusted life years. Also noted in the results, this younger cohort has a higher percentage of single/unmarried veterans, suggesting familial support systems may be more parental than spousal. Treatment for this younger cohort will likely need to focus on early and sustained rehabilitation that can be integrated with career plans.
For treatment to be effective, there must be evidence for veterans enrolling, remaining, and reporting benefits from the treatment. Limited research has shown currently advocated evidence-based therapies to have low enrollment rates, high drop-out rates, and mixed outcomes.42
Results showing a gradual increase in the proportion of nonwhite, non-African American veterans diagnosed with PTSD alone, TBI alone, or both, likely reflect the changing demographic profile of the US as well as the Army. However, the reason that more African Americans were diagnosed with PTSD and/or TBI in the overlap and reentered groups when compared with the pre-9/11 group could not be ascertained. It is possible that more veterans identified themselves as African Americans as evident from a decrease in the number of veterans in the unknown category post-9/11 when compared with the pre-9/11 group. In 2016, the American Community Survey showed that Hispanic and African American veterans were more likely to use VA health care and other benefits than were any other racial group.40 Improved screening for PTSD and TBI diagnoses, increased awareness, and education about the availability of VA services and benefits may have contributed to the increased use of VA benefits in these groups.
Data from this study are concordant with data from the National Center for Veterans Analysis and Statistics reporting on the younger age of diagnosis and higher rates of initial service-connected disability in veterans with PTSD and PTSD+TBI.43 One study analyzing records from 1999 to 2004 showed that the number of PTSD cases grew by 79.5%, resulting in 148.7% increase in benefits payment from $1.7 billion to $4.3 billion per year.44 In contrast, the compensation cost for all other disability categories increased by only 41.7% over this period. This study also revealed that while veterans with PTSD represented only 8.7% of compensation recipients, they received 20.5% of all compensation payments, driven in large part by an increase in > 50% service-connected disability ratings.44
Thus, from financial as well as treatment points of view, the change in the demographic profile of the veteran must be considered when developing PTSD treatment strategies. While treatment in the past focused solely on addressing trauma-associated psychiatric issues, TBI and PTSD association will likely shift the focus to concurrent psychiatric and physical symptomology. Similarly, PTSD/TBI treatment modalities must consider that the profile of post-9/11 service members includes more women, younger age, and a greater racial diversity. For instance, younger age for a disabled veteran brings additional challenges, including reliance on parental or buddy support systems vs a spousal support system, integrating career with treatment, selecting geographic locations that can support both career and treatment, sustaining rehabilitation over time. The treatment needs of a 35-year-old soldier with PTSD and/or TBI, whether male or female, Asian or African American are likely to be very different from the treatment needs of a 65-year-old white male. Newer treatment approaches will have to address the needs of all soldiers.
Limitations
Our study may underestimate the actual PTSD and/or TBI disease burden because of the social stigma associated with diagnosis, military culture, limitations in data collection.45-50 In addition, in this retrospective database cohort study, we considered and tried to minimize the impact of any of the usual potential limitations, including (1) accuracy of data quality and linkage; (2) identifying cohort appropriately (study groups); (3) defining endpoints clearly to avoid misclassifications; and (4) incorporating all important confounders. We identified veterans utilizing medical services at VA hospitals during a defined period and diagnosed with PTSD and TBI using ICD-9 codes and divided in 4 well-defined groups. In addition, another limitation of our study is to not accurately capture the veterans who have alternative health coverage and may choose not to enroll and/or participate in VA health care. In addition, some service members leaving war zones may not disclose or downplay the mental health symptoms to avoid any delay in their return home.
Conclusions
This study highlights the changing profile of the soldier diagnosed with PTSD and/or TBI who served pre-9/11 compared with that of those who served post-9/11. Treatment modalities must address the changes in warfare and demographics of US service members. Future treatment will need to focus more on concurrent PTSD/TBI therapies, the needs of younger soldiers, the needs of women injured in combat, and the needs of a more racially and ethnically diverse population. Severe injuries at a younger age will require early detection and rehabilitation for return to optimum functioning over a lifetime. The current study underscores a need for identifying the gaps in ongoing programs and services, developing alternatives, and implementing improved systems of care. More studies are needed to identify the cost implications and the effectiveness of current therapies for PTSD and/or TBI.
Acknowledgments
This study was supported by VA Medical Center and Midwest BioMedical Research Foundation (MBRF), Kansas City, Missouri. The manuscript received support, in part, from NIH-RO1 DK107490. These agencies did not participate in the design/conduct of the study or, in the interpretation of the data.
1. Bagalman E. Traumatic brain injury among veterans. http://www.ncsl.org/documents/statefed/health/TBI_Vets2013.pdf. Published January 4, 2013. Accessed February 3, 2020.
2. Veterans Health Administration, Support Service Center. Workload files fiscal year 2008-fiscal year 2012. [Source not verified.]
3. Tanielian T, Jaycox LH, eds. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. Santa Monica, CA: RAND Corporation; 2008.
4. Bagalman E. Health care for veterans: traumatic brain injury. https://fas.org/sgp/crs/misc/R40941.pdf. Published March 9, 2015. Accessed February 4, 2020.
5. Ikin JF, Sim MR, McKenzie DP, et al. Anxiety, post-traumatic stress disorder and depression in Korean War veterans 50 years after the war. Br J Psychiatry. 2007;190(6):475-483.
6. Andrews B, Brewin CR, Philpott R, Stewart L. Delayed-onset posttraumatic stress disorder: a systematic review of the evidence. Am J Psychiatry. 2007;164(9):1319-1326.
7. Frueh BC, Grubaugh AL, Yeager DE, Magruder KM. Delayed-onset post-traumatic stress disorder among war veterans in primary care clinics. Br J Psychiatry. 2009;194(6):515-520.
8. McAllister TW. Neurobiological consequences of traumatic brain injury. Dialogues Clin Neurosci. 2011;13(3):287-300.
9. Schlenger WE, Kulka RA, Fairbank JA, et al. The prevalence of posttraumatic stress disorder in the Vietnam generation: a multimethod, multisource assessment of psychiatric disorder. J Trauma Stress. 1992;5(3):333-363.
10. Friedman MJ, Resick PA, Bryant RA, Strain J, Horowitz M, Spiegel D. Classification of trauma and stressor-related disorders in DSM-5. Depress Anxiety. 2011;28(9):737-749.
11. Lew HL, Otis JD, Tun C, Kerns RD, Clark ME, Cifu DX. Prevalence of chronic pain, posttraumatic stress disorder, and persistent postconcussive symptoms in OIF/OEF veterans: polytrauma clinical triad. J Rehabil Res Dev. 2009;46(6):697-702.
12. Carlson K, Kehle S, Meis L, et al. The Assessment and Treatment of Individuals with History of Traumatic Brain Injury and Post-Traumatic Stress Disorder: A Systematic Review of the Evidence. Washington, DC: US Department of Veterans Affairs; 2009.
13. Gironda RJ, Clark ME, Ruff RL, et al. Traumatic brain injury, polytrauma, and pain: challenges and treatment strategies for the polytrauma rehabilitation. Rehabil Psychol. 2009;54(3):247-258.
14. Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. soldiers returning from Iraq. N Engl J Med. 2008;358(5):453-463.
15. Bazarian JJ, Cernak I, Noble-Haeusslein L, Potolicchio S, Temkin N. Long-term neurologic outcomes after traumatic brain injury. J Head Trauma Rehabil. 2009;24(6):439-451.
16. Peskind ER, Brody D, Cernak I, McKee A, Ruff RL. Military- and sports-related mild traumatic brain injury: clinical presentation, management, and long-term consequences. J Clin Psychiatry. 2013;74(2):180-188.
17. Riggio S. Traumatic brain injury and its neurobehavioral sequelae. Neurol Clin. 2011;29(1):35-47, vii.
18. Helmick KM, Spells CA, Malik SZ, Davies CA, Marion DW, Hinds SR. Traumatic brain injury in the US military: epidemiology and key clinical and research programs. Brain Imaging Behav. 2015;9(3):358-366.
19. Richardson LK, Frueh BC, Acierno R. Prevalence estimates of combat-related post-traumatic stress disorder: critical review. Aust N Z J Psychiatry. 2010;44(1):4-19.
20. Thompson WW, Gottesman II, Zalewski C. Reconciling disparate prevalence rates of PTSD in large samples of US male Vietnam veterans and their controls. BMC Psychiatry. 2006;6:19.
21. Frueh BC, Elhai JD, Gold PB, et al Disability compensation seeking among veterans evaluated for posttraumatic stress disorder. Psychiatr Serv. 2003;54(1):84-91.
22. Thakur H, Oni O, Singh V, et al. Increases in the service connection disability and treatment costs associated with posttraumatic stress disorder and/or traumatic brain injury in United States veterans pre- and post-9/11: the strong need for a novel therapeutic approach. Epidemiology (Sunnyvale). 2018;8(4):353.
23. Schlenger WE, Kulka RA, Fairbank JA, et al. The prevalence of post-traumatic stress disorder in the Vietnam generation: a multimethod, multisource assessment of psychiatric disorder. J Trauma Stress. 1992;5(3):333-363.
24. Belmont PJ, Schoenfeld AJ, Goodman G. Epidemiology of combat wounds in Operation Iraqi Freedom and Operation Enduring Freedom: orthopaedic burden of disease. J Surg Orthop Adv. 2010;19(1):2-7.
25. Owens BD, Kragh JG Jr, Wenke JC, Macaitis J, Wade CE, Holcomb JB. Combat wounds in Operation Iraqi Freedom and Operation Enduring Freedom. J Trauma. 2008;64(2):295-299.
26. Defense Health Agency, Defense and Veterans Brain Injury Center. DOD worldwide numbers for TBI since 2000. https://dvbic.dcoe.mil/dod-worldwide-numbers-tbi. Updated February 14, 2020. Accessed February 14, 2020.
27. Armed Forces Health Surveillance Center. Deployment-related conditions of special surveillance interest, U.S. armed forces, by month and service, January 2003-December 2012 (data as of 22 January 2013). MSMR. 2013;20(1):16-19.
28. Harvey JH, Stein SK, Scott PK. Fifty years of grief: accounts and reported psychological reactions of Normandy invasion veterans. J Narrative Life History. 1995;5(4):321-332.
29. US Department of Veterans Affairs. Polytrauma/TBI system of care. https://www.polytrauma.va.gov/system-of-care/index.asp. Updated June 3, 2015. Accessed February 4, 2020.
30. Wolfe J, Erickson DJ, Sharkansky EJ, King DW, King LA. Course and predictors of posttraumatic stress disorder among Gulf War veterans: a prospective analysis. J Consult Clin Psychol. 1999;67(4):520-528.
31. Breslau N, Davis GC, Peterson EL, Schultz L. Psychiatric sequelae of posttraumatic stress disorder in women. Arch Gen Psychiatry. 1997;54(1):81-87.
32. Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52(12):1048-1060.
33. Wolfe J, Kimerling R. Gender issues in the assessment of posttraumatic stress disorder. In: Wilson J, Keane TM, eds. Assessing Psychological Trauma and PTSD. New York: Guilford; 2004:192-238.
34. Engel CC Jr, Engel AL, Campbell SJ, McFall ME, Russo J, Katon W. Posttraumatic stress disorder symptoms and precombat sexual and physical abuse in Desert Storm veterans. J Nerv Ment Dis. 1993;181(11):683-688.
35. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Profile of veterans: 2016 data from the American Community Survey. https://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2016.pdf. Published February 2018. Accessed February 4, 2020.
36. US Department of Commerce Economics and Statistics Administration, US Census Bureau, Geography Division. 2010 population distribution in the United States and Puerto Rico. https://www2.census.gov/geo/maps/dc10_thematic/2010_Nighttime_PopDist/2010_Nighttime_PopDist_Page_Map.pdf. Accessed February 4, 2020.
37. Cifu DX, Taylor BC, Carne WF, et al. Traumatic brain injury, posttraumatic stress disorder, and pain diagnoses in OIF/OEF/OND veterans. J Rehabil Res Dev. 2013;50(9):1169-1176.
38. Dohrenwend BP, Turner JB, Turse NA, Adams BG, Koenen KC, Marshall R. The psychological risks of Vietnam for U.S. veterans: a revisit with new data and methods. Science. 2006;313(5789):979-982.
39. Magruder KM, Frueh BC, Knapp RG, et al. Prevalence of posttraumatic stress disorder in Veterans Affairs primary care clinics. Gen Hosp Psychiatry. 2005;27(3):169-179.
40. Norris FH. Epidemiology of trauma: frequency and impact of different potentially traumatic events on different demographic groups. J Consult Clin Psychol. 1992;60(3):409-418.
41. Resnick HS, Kilpatrick DG, Dansky BS, Saunders BE, Best CL. Prevalence of civilian trauma and posttraumatic stress disorder in a representative national sample of women. J Consult Clin Psychol. 1993;61(6):984-991.
42. Najavits LM. The problem of dropout from “gold standard” PTSD therapies. F1000Prime Rep. 2015;7:43.
43. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Trends in veterans with a service-connected disability: 1985 to 2014. https://www.va.gov/vetdata/docs/QuickFacts/SCD_trends_FINAL_2014.PDF. Published June 2015. Accessed February 4, 2020.
44. US Department of Veterans Affairs, Office of Inspector General. Review of state variances in VA disability compensation payments. Report 05-00765-137. https://www.va.gov/oig/52/reports/2005/VAOIG-05-00765-137.pdf. Published May 19, 2015. Accessed February 4, 2020.
45. McNally RJ. Progress and controversy in the study of posttraumatic stress disorder. Annu Rev Psychol. 2003;54:229-252.
46. Freeman T, Powell M, Kimbrell T. Measuring symptom exaggeration in veterans with chronic posttraumatic stress disorder. Psychiatry Res. 2008;158(3):374-380.
47. Frueh BC, Elhai JD, Grubaugh AL, et al. Documented combat exposure of US veterans seeking treatment for combat-related post-traumatic stress disorder. Br J Psychiatry. 2005;186(6):467-475.
48. Frueh BC, Hamner MB, Cahill SP, Gold PB, Hamlin KL. Apparent symptom overreporting in combat veterans evaluated for PTSD. Clin Psychol Rev. 2000;20(7):853-885.
49. Sparr L, Pankratz LD. Factitious posttraumatic stress disorder. Am J Psychiatry. 1983;140(8):1016-1019.
50. Baggaley M. ‘Military Munchausen’s’: assessment of factitious claims of military service in psychiatric patients. Psychiatr Bull. 1998;22(3):153-154.
1. Bagalman E. Traumatic brain injury among veterans. http://www.ncsl.org/documents/statefed/health/TBI_Vets2013.pdf. Published January 4, 2013. Accessed February 3, 2020.
2. Veterans Health Administration, Support Service Center. Workload files fiscal year 2008-fiscal year 2012. [Source not verified.]
3. Tanielian T, Jaycox LH, eds. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. Santa Monica, CA: RAND Corporation; 2008.
4. Bagalman E. Health care for veterans: traumatic brain injury. https://fas.org/sgp/crs/misc/R40941.pdf. Published March 9, 2015. Accessed February 4, 2020.
5. Ikin JF, Sim MR, McKenzie DP, et al. Anxiety, post-traumatic stress disorder and depression in Korean War veterans 50 years after the war. Br J Psychiatry. 2007;190(6):475-483.
6. Andrews B, Brewin CR, Philpott R, Stewart L. Delayed-onset posttraumatic stress disorder: a systematic review of the evidence. Am J Psychiatry. 2007;164(9):1319-1326.
7. Frueh BC, Grubaugh AL, Yeager DE, Magruder KM. Delayed-onset post-traumatic stress disorder among war veterans in primary care clinics. Br J Psychiatry. 2009;194(6):515-520.
8. McAllister TW. Neurobiological consequences of traumatic brain injury. Dialogues Clin Neurosci. 2011;13(3):287-300.
9. Schlenger WE, Kulka RA, Fairbank JA, et al. The prevalence of posttraumatic stress disorder in the Vietnam generation: a multimethod, multisource assessment of psychiatric disorder. J Trauma Stress. 1992;5(3):333-363.
10. Friedman MJ, Resick PA, Bryant RA, Strain J, Horowitz M, Spiegel D. Classification of trauma and stressor-related disorders in DSM-5. Depress Anxiety. 2011;28(9):737-749.
11. Lew HL, Otis JD, Tun C, Kerns RD, Clark ME, Cifu DX. Prevalence of chronic pain, posttraumatic stress disorder, and persistent postconcussive symptoms in OIF/OEF veterans: polytrauma clinical triad. J Rehabil Res Dev. 2009;46(6):697-702.
12. Carlson K, Kehle S, Meis L, et al. The Assessment and Treatment of Individuals with History of Traumatic Brain Injury and Post-Traumatic Stress Disorder: A Systematic Review of the Evidence. Washington, DC: US Department of Veterans Affairs; 2009.
13. Gironda RJ, Clark ME, Ruff RL, et al. Traumatic brain injury, polytrauma, and pain: challenges and treatment strategies for the polytrauma rehabilitation. Rehabil Psychol. 2009;54(3):247-258.
14. Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. soldiers returning from Iraq. N Engl J Med. 2008;358(5):453-463.
15. Bazarian JJ, Cernak I, Noble-Haeusslein L, Potolicchio S, Temkin N. Long-term neurologic outcomes after traumatic brain injury. J Head Trauma Rehabil. 2009;24(6):439-451.
16. Peskind ER, Brody D, Cernak I, McKee A, Ruff RL. Military- and sports-related mild traumatic brain injury: clinical presentation, management, and long-term consequences. J Clin Psychiatry. 2013;74(2):180-188.
17. Riggio S. Traumatic brain injury and its neurobehavioral sequelae. Neurol Clin. 2011;29(1):35-47, vii.
18. Helmick KM, Spells CA, Malik SZ, Davies CA, Marion DW, Hinds SR. Traumatic brain injury in the US military: epidemiology and key clinical and research programs. Brain Imaging Behav. 2015;9(3):358-366.
19. Richardson LK, Frueh BC, Acierno R. Prevalence estimates of combat-related post-traumatic stress disorder: critical review. Aust N Z J Psychiatry. 2010;44(1):4-19.
20. Thompson WW, Gottesman II, Zalewski C. Reconciling disparate prevalence rates of PTSD in large samples of US male Vietnam veterans and their controls. BMC Psychiatry. 2006;6:19.
21. Frueh BC, Elhai JD, Gold PB, et al Disability compensation seeking among veterans evaluated for posttraumatic stress disorder. Psychiatr Serv. 2003;54(1):84-91.
22. Thakur H, Oni O, Singh V, et al. Increases in the service connection disability and treatment costs associated with posttraumatic stress disorder and/or traumatic brain injury in United States veterans pre- and post-9/11: the strong need for a novel therapeutic approach. Epidemiology (Sunnyvale). 2018;8(4):353.
23. Schlenger WE, Kulka RA, Fairbank JA, et al. The prevalence of post-traumatic stress disorder in the Vietnam generation: a multimethod, multisource assessment of psychiatric disorder. J Trauma Stress. 1992;5(3):333-363.
24. Belmont PJ, Schoenfeld AJ, Goodman G. Epidemiology of combat wounds in Operation Iraqi Freedom and Operation Enduring Freedom: orthopaedic burden of disease. J Surg Orthop Adv. 2010;19(1):2-7.
25. Owens BD, Kragh JG Jr, Wenke JC, Macaitis J, Wade CE, Holcomb JB. Combat wounds in Operation Iraqi Freedom and Operation Enduring Freedom. J Trauma. 2008;64(2):295-299.
26. Defense Health Agency, Defense and Veterans Brain Injury Center. DOD worldwide numbers for TBI since 2000. https://dvbic.dcoe.mil/dod-worldwide-numbers-tbi. Updated February 14, 2020. Accessed February 14, 2020.
27. Armed Forces Health Surveillance Center. Deployment-related conditions of special surveillance interest, U.S. armed forces, by month and service, January 2003-December 2012 (data as of 22 January 2013). MSMR. 2013;20(1):16-19.
28. Harvey JH, Stein SK, Scott PK. Fifty years of grief: accounts and reported psychological reactions of Normandy invasion veterans. J Narrative Life History. 1995;5(4):321-332.
29. US Department of Veterans Affairs. Polytrauma/TBI system of care. https://www.polytrauma.va.gov/system-of-care/index.asp. Updated June 3, 2015. Accessed February 4, 2020.
30. Wolfe J, Erickson DJ, Sharkansky EJ, King DW, King LA. Course and predictors of posttraumatic stress disorder among Gulf War veterans: a prospective analysis. J Consult Clin Psychol. 1999;67(4):520-528.
31. Breslau N, Davis GC, Peterson EL, Schultz L. Psychiatric sequelae of posttraumatic stress disorder in women. Arch Gen Psychiatry. 1997;54(1):81-87.
32. Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52(12):1048-1060.
33. Wolfe J, Kimerling R. Gender issues in the assessment of posttraumatic stress disorder. In: Wilson J, Keane TM, eds. Assessing Psychological Trauma and PTSD. New York: Guilford; 2004:192-238.
34. Engel CC Jr, Engel AL, Campbell SJ, McFall ME, Russo J, Katon W. Posttraumatic stress disorder symptoms and precombat sexual and physical abuse in Desert Storm veterans. J Nerv Ment Dis. 1993;181(11):683-688.
35. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Profile of veterans: 2016 data from the American Community Survey. https://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2016.pdf. Published February 2018. Accessed February 4, 2020.
36. US Department of Commerce Economics and Statistics Administration, US Census Bureau, Geography Division. 2010 population distribution in the United States and Puerto Rico. https://www2.census.gov/geo/maps/dc10_thematic/2010_Nighttime_PopDist/2010_Nighttime_PopDist_Page_Map.pdf. Accessed February 4, 2020.
37. Cifu DX, Taylor BC, Carne WF, et al. Traumatic brain injury, posttraumatic stress disorder, and pain diagnoses in OIF/OEF/OND veterans. J Rehabil Res Dev. 2013;50(9):1169-1176.
38. Dohrenwend BP, Turner JB, Turse NA, Adams BG, Koenen KC, Marshall R. The psychological risks of Vietnam for U.S. veterans: a revisit with new data and methods. Science. 2006;313(5789):979-982.
39. Magruder KM, Frueh BC, Knapp RG, et al. Prevalence of posttraumatic stress disorder in Veterans Affairs primary care clinics. Gen Hosp Psychiatry. 2005;27(3):169-179.
40. Norris FH. Epidemiology of trauma: frequency and impact of different potentially traumatic events on different demographic groups. J Consult Clin Psychol. 1992;60(3):409-418.
41. Resnick HS, Kilpatrick DG, Dansky BS, Saunders BE, Best CL. Prevalence of civilian trauma and posttraumatic stress disorder in a representative national sample of women. J Consult Clin Psychol. 1993;61(6):984-991.
42. Najavits LM. The problem of dropout from “gold standard” PTSD therapies. F1000Prime Rep. 2015;7:43.
43. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Trends in veterans with a service-connected disability: 1985 to 2014. https://www.va.gov/vetdata/docs/QuickFacts/SCD_trends_FINAL_2014.PDF. Published June 2015. Accessed February 4, 2020.
44. US Department of Veterans Affairs, Office of Inspector General. Review of state variances in VA disability compensation payments. Report 05-00765-137. https://www.va.gov/oig/52/reports/2005/VAOIG-05-00765-137.pdf. Published May 19, 2015. Accessed February 4, 2020.
45. McNally RJ. Progress and controversy in the study of posttraumatic stress disorder. Annu Rev Psychol. 2003;54:229-252.
46. Freeman T, Powell M, Kimbrell T. Measuring symptom exaggeration in veterans with chronic posttraumatic stress disorder. Psychiatry Res. 2008;158(3):374-380.
47. Frueh BC, Elhai JD, Grubaugh AL, et al. Documented combat exposure of US veterans seeking treatment for combat-related post-traumatic stress disorder. Br J Psychiatry. 2005;186(6):467-475.
48. Frueh BC, Hamner MB, Cahill SP, Gold PB, Hamlin KL. Apparent symptom overreporting in combat veterans evaluated for PTSD. Clin Psychol Rev. 2000;20(7):853-885.
49. Sparr L, Pankratz LD. Factitious posttraumatic stress disorder. Am J Psychiatry. 1983;140(8):1016-1019.
50. Baggaley M. ‘Military Munchausen’s’: assessment of factitious claims of military service in psychiatric patients. Psychiatr Bull. 1998;22(3):153-154.
Genetic factor linked to impaired memory after heading many soccer balls
according to authors of a recent longitudinal study. Worse verbal memory was linked to high levels of ball heading among those players who were APOE e4–positive, compared with those who were APOE e4–negative, according to the authors, led by Liane E. Hunter, PhD, of the Gruss Magnetic Resonance Imaging Center at Albert Einstein College of Medicine, New York.
These findings, while preliminary, do raise the possibility that “safe levels for soccer heading” could be proposed to protect players from harm or that APOE e4-positive players might be advised to limit their exposure to head impacts, Dr. Hunter and coauthors wrote in a report in JAMA Neurology.
However, the findings should “in no way” be used to justify APOE testing to make clinical decisions regarding the safety of playing soccer, said Sarah J. Banks, PhD, of the University of California, San Diego, and Jesse Mez, MD, of Boston University in a related editorial (doi: 10.1001/jamaneurol.2019.4451). “Like most good science, the study provides an important, but incremental, step to understanding gene-environment interactions in sports,” Dr. Banks and Dr. Mez wrote in their editorial.
While there are some studies tying APOE e4 to poorer neuropsychiatric performance in boxers and U.S. football players, there are no such studies looking at the role of APOE e4 in soccer players exposed to repetitive “subconcussive” ball heading, according to Dr. Hunter and coresearchers. Accordingly, they sought to analyze APOE e4 and neuropsychological performance in relation to ball heading in 352 adult amateur soccer players enrolled in the Einstein Soccer Study between November 2013 and January 2018. About three-quarters of the players were male, and the median age at enrollment was 23 years.
The players completed a computer-based questionnaire designed to estimate their exposure to soccer heading at enrollment and at follow-up visits every 3-6 months. To test verbal memory at each visit, players were asked to memorize a 12-item grocery list, and then asked to recall the items 20 minutes later.
High levels of heading were linked to poorer performance on the verbal memory task, similar to one previously reported study, investigators said.
There was no association overall of APOE e4 and heading with performance on the shopping list task, according to investigators. By contrast, there was a 4.1-fold increased deficit in verbal memory for APOE e4–positive players with high heading exposure, compared with those with low exposure, investigators reported. Likewise, there was an 8.5-fold increased deficit in verbal memory for APOE e4–positive players with high versus moderate heading exposure.
That said, the absolute difference in performance was “subtle” and difficult to interpret in the context of a cross-sectional study, Dr. Banks and Dr. Mez said in their editorial.
In absolute terms, the mean decrease in scores on the 13-point shopping list task between the high and low heading exposure was 1.13 points greater for the APOE e4–positive group, compared with the APOE e4–negative group, and the decrease between the high and moderate heading exposure groups was 0.98 points greater, according to the report.
“The effect size of our interaction is relatively small,” Dr. Hunter and colleagues acknowledged in their report. “However, similar to the widely cited model of disease evolution in Alzheimer disease, our findings may be evidence of early subclinical effects, which could accumulate in APOE e4–positive players over a protracted time frame and ultimately be associated with overt clinical dysfunction.”
Several study authors said they had received grants from the National Institutes of Health and affiliated institutes, the Migraine Research Foundation, and the National Headache Foundation. They reported disclosures related to Amgen, Avanir, Biohaven Holdings, Biovision, Boston Scientific, Eli Lilly, eNeura Therapeutics, GlaxoSmithKline, Merck, and Pfizer, among others.
SOURCE: Hunter LE et al. JAMA Neurol. 2020 Jan 27. doi: 10.1001/jamaneurol.2019.4828.
according to authors of a recent longitudinal study. Worse verbal memory was linked to high levels of ball heading among those players who were APOE e4–positive, compared with those who were APOE e4–negative, according to the authors, led by Liane E. Hunter, PhD, of the Gruss Magnetic Resonance Imaging Center at Albert Einstein College of Medicine, New York.
These findings, while preliminary, do raise the possibility that “safe levels for soccer heading” could be proposed to protect players from harm or that APOE e4-positive players might be advised to limit their exposure to head impacts, Dr. Hunter and coauthors wrote in a report in JAMA Neurology.
However, the findings should “in no way” be used to justify APOE testing to make clinical decisions regarding the safety of playing soccer, said Sarah J. Banks, PhD, of the University of California, San Diego, and Jesse Mez, MD, of Boston University in a related editorial (doi: 10.1001/jamaneurol.2019.4451). “Like most good science, the study provides an important, but incremental, step to understanding gene-environment interactions in sports,” Dr. Banks and Dr. Mez wrote in their editorial.
While there are some studies tying APOE e4 to poorer neuropsychiatric performance in boxers and U.S. football players, there are no such studies looking at the role of APOE e4 in soccer players exposed to repetitive “subconcussive” ball heading, according to Dr. Hunter and coresearchers. Accordingly, they sought to analyze APOE e4 and neuropsychological performance in relation to ball heading in 352 adult amateur soccer players enrolled in the Einstein Soccer Study between November 2013 and January 2018. About three-quarters of the players were male, and the median age at enrollment was 23 years.
The players completed a computer-based questionnaire designed to estimate their exposure to soccer heading at enrollment and at follow-up visits every 3-6 months. To test verbal memory at each visit, players were asked to memorize a 12-item grocery list, and then asked to recall the items 20 minutes later.
High levels of heading were linked to poorer performance on the verbal memory task, similar to one previously reported study, investigators said.
There was no association overall of APOE e4 and heading with performance on the shopping list task, according to investigators. By contrast, there was a 4.1-fold increased deficit in verbal memory for APOE e4–positive players with high heading exposure, compared with those with low exposure, investigators reported. Likewise, there was an 8.5-fold increased deficit in verbal memory for APOE e4–positive players with high versus moderate heading exposure.
That said, the absolute difference in performance was “subtle” and difficult to interpret in the context of a cross-sectional study, Dr. Banks and Dr. Mez said in their editorial.
In absolute terms, the mean decrease in scores on the 13-point shopping list task between the high and low heading exposure was 1.13 points greater for the APOE e4–positive group, compared with the APOE e4–negative group, and the decrease between the high and moderate heading exposure groups was 0.98 points greater, according to the report.
“The effect size of our interaction is relatively small,” Dr. Hunter and colleagues acknowledged in their report. “However, similar to the widely cited model of disease evolution in Alzheimer disease, our findings may be evidence of early subclinical effects, which could accumulate in APOE e4–positive players over a protracted time frame and ultimately be associated with overt clinical dysfunction.”
Several study authors said they had received grants from the National Institutes of Health and affiliated institutes, the Migraine Research Foundation, and the National Headache Foundation. They reported disclosures related to Amgen, Avanir, Biohaven Holdings, Biovision, Boston Scientific, Eli Lilly, eNeura Therapeutics, GlaxoSmithKline, Merck, and Pfizer, among others.
SOURCE: Hunter LE et al. JAMA Neurol. 2020 Jan 27. doi: 10.1001/jamaneurol.2019.4828.
according to authors of a recent longitudinal study. Worse verbal memory was linked to high levels of ball heading among those players who were APOE e4–positive, compared with those who were APOE e4–negative, according to the authors, led by Liane E. Hunter, PhD, of the Gruss Magnetic Resonance Imaging Center at Albert Einstein College of Medicine, New York.
These findings, while preliminary, do raise the possibility that “safe levels for soccer heading” could be proposed to protect players from harm or that APOE e4-positive players might be advised to limit their exposure to head impacts, Dr. Hunter and coauthors wrote in a report in JAMA Neurology.
However, the findings should “in no way” be used to justify APOE testing to make clinical decisions regarding the safety of playing soccer, said Sarah J. Banks, PhD, of the University of California, San Diego, and Jesse Mez, MD, of Boston University in a related editorial (doi: 10.1001/jamaneurol.2019.4451). “Like most good science, the study provides an important, but incremental, step to understanding gene-environment interactions in sports,” Dr. Banks and Dr. Mez wrote in their editorial.
While there are some studies tying APOE e4 to poorer neuropsychiatric performance in boxers and U.S. football players, there are no such studies looking at the role of APOE e4 in soccer players exposed to repetitive “subconcussive” ball heading, according to Dr. Hunter and coresearchers. Accordingly, they sought to analyze APOE e4 and neuropsychological performance in relation to ball heading in 352 adult amateur soccer players enrolled in the Einstein Soccer Study between November 2013 and January 2018. About three-quarters of the players were male, and the median age at enrollment was 23 years.
The players completed a computer-based questionnaire designed to estimate their exposure to soccer heading at enrollment and at follow-up visits every 3-6 months. To test verbal memory at each visit, players were asked to memorize a 12-item grocery list, and then asked to recall the items 20 minutes later.
High levels of heading were linked to poorer performance on the verbal memory task, similar to one previously reported study, investigators said.
There was no association overall of APOE e4 and heading with performance on the shopping list task, according to investigators. By contrast, there was a 4.1-fold increased deficit in verbal memory for APOE e4–positive players with high heading exposure, compared with those with low exposure, investigators reported. Likewise, there was an 8.5-fold increased deficit in verbal memory for APOE e4–positive players with high versus moderate heading exposure.
That said, the absolute difference in performance was “subtle” and difficult to interpret in the context of a cross-sectional study, Dr. Banks and Dr. Mez said in their editorial.
In absolute terms, the mean decrease in scores on the 13-point shopping list task between the high and low heading exposure was 1.13 points greater for the APOE e4–positive group, compared with the APOE e4–negative group, and the decrease between the high and moderate heading exposure groups was 0.98 points greater, according to the report.
“The effect size of our interaction is relatively small,” Dr. Hunter and colleagues acknowledged in their report. “However, similar to the widely cited model of disease evolution in Alzheimer disease, our findings may be evidence of early subclinical effects, which could accumulate in APOE e4–positive players over a protracted time frame and ultimately be associated with overt clinical dysfunction.”
Several study authors said they had received grants from the National Institutes of Health and affiliated institutes, the Migraine Research Foundation, and the National Headache Foundation. They reported disclosures related to Amgen, Avanir, Biohaven Holdings, Biovision, Boston Scientific, Eli Lilly, eNeura Therapeutics, GlaxoSmithKline, Merck, and Pfizer, among others.
SOURCE: Hunter LE et al. JAMA Neurol. 2020 Jan 27. doi: 10.1001/jamaneurol.2019.4828.
FROM JAMA Neurology
Delayed clinical care after concussion may prolong recovery
, according to a study published Jan. 6 in JAMA Neurology.
In addition, more severe visual motion sensitivity symptoms are associated with longer recovery times, said lead author Anthony P. Kontos, PhD, and colleagues. Dr. Kontos is associate professor of orthopedic surgery at the University of Pittsburgh and the research director of the University of Pittsburgh Medical Center Sports Medicine Concussion Program.
“Without clinical guidance and behavioral management recommendations post injury, athletes may have been engaging in counterproductive recovery strategies, such as a strict rest or excessive physical activity,” the authors said. “This explanation is supported by the fact that athletes recovered in a similar amount of time after that first evaluation.”
Various factors may influence recovery after a concussion, including age, sex, and comorbidities. To examine the relationship between time since injury at the start of clinical care and recovery time, the investigators conducted a retrospective, cross-sectional study. They analyzed data from patients seen in a sports medicine clinic between August 2016 and March 2018. Eligible patients were aged 12-22 years and had a diagnosed, symptomatic, sport-related concussion. The researchers excluded patients with incomplete recovery data.
The investigators included 162 patients in their analyses; 98 of these patients were seen within 7 days of the injury, and 64 were seen 8-20 days after a concussion. Both groups had a mean age of 15 years and similar proportions of female patients (52% in the early-care group vs. 62.5% in the late-care group). At the first clinical visit, symptom severity and cognitive, ocular, and vestibular test results were similar in both groups.
The researchers defined recovery time as the number of days from injury until the date of clearance for a full return to play. Physicians cleared patients to return to play when they returned to preinjury levels of symptoms and preinjury performance on cognitive, ocular, and vestibular tests with no increase in symptoms after exertion.
Patients’ recovery times ranged from 9 to 299 days; the average recovery time was 57 days. Fifty-two percent of patients in the early-care group recovered in 30 days or fewer, compared with 19% of patients in late-care group. Patients in the early-care group had a mean recovery of 51.1 days, whereas patients in the late-care group had a mean recovery of 66 days.
In a logistic regression model, late care “was associated with a 5.8-times increased likelihood of a recovery longer than 30 days,” the researchers reported. Visual motion sensitivity symptoms also were associated with increased odds of protracted recovery (adjusted odds ratio, 4.5).
“Once care was established, time to recovery did not differ for athletes evaluated within the first week of injury compared with those evaluated 2-3 weeks post injury,” Dr. Kontos and colleagues concluded. “Education on injury and behavioral recommendations to optimize concussion recovery and earlier initiation of active rehabilitation strategies, including exertion and vestibular therapy, are plausible explanations for the association of a shorter recovery time with earlier care. However, future research should focus on the specific mechanisms by which earlier health care postconcussion promotes faster recovery and determine if these findings apply to other subpopulations, including military personnel.”
The researchers noted that they excluded from their analysis 254 patients who had incomplete recovery data but otherwise met inclusion criteria, which may have led to selection bias.
Dr. Kontos disclosed grants from the National Football League and personal fees from APA Books and University of Pittsburgh projects outside the scope of this study.
SOURCE: Kontos AP et al. JAMA Neurol. 2020 Jan 6. doi: 10.1001/jamaneurol.2019.4552.
, according to a study published Jan. 6 in JAMA Neurology.
In addition, more severe visual motion sensitivity symptoms are associated with longer recovery times, said lead author Anthony P. Kontos, PhD, and colleagues. Dr. Kontos is associate professor of orthopedic surgery at the University of Pittsburgh and the research director of the University of Pittsburgh Medical Center Sports Medicine Concussion Program.
“Without clinical guidance and behavioral management recommendations post injury, athletes may have been engaging in counterproductive recovery strategies, such as a strict rest or excessive physical activity,” the authors said. “This explanation is supported by the fact that athletes recovered in a similar amount of time after that first evaluation.”
Various factors may influence recovery after a concussion, including age, sex, and comorbidities. To examine the relationship between time since injury at the start of clinical care and recovery time, the investigators conducted a retrospective, cross-sectional study. They analyzed data from patients seen in a sports medicine clinic between August 2016 and March 2018. Eligible patients were aged 12-22 years and had a diagnosed, symptomatic, sport-related concussion. The researchers excluded patients with incomplete recovery data.
The investigators included 162 patients in their analyses; 98 of these patients were seen within 7 days of the injury, and 64 were seen 8-20 days after a concussion. Both groups had a mean age of 15 years and similar proportions of female patients (52% in the early-care group vs. 62.5% in the late-care group). At the first clinical visit, symptom severity and cognitive, ocular, and vestibular test results were similar in both groups.
The researchers defined recovery time as the number of days from injury until the date of clearance for a full return to play. Physicians cleared patients to return to play when they returned to preinjury levels of symptoms and preinjury performance on cognitive, ocular, and vestibular tests with no increase in symptoms after exertion.
Patients’ recovery times ranged from 9 to 299 days; the average recovery time was 57 days. Fifty-two percent of patients in the early-care group recovered in 30 days or fewer, compared with 19% of patients in late-care group. Patients in the early-care group had a mean recovery of 51.1 days, whereas patients in the late-care group had a mean recovery of 66 days.
In a logistic regression model, late care “was associated with a 5.8-times increased likelihood of a recovery longer than 30 days,” the researchers reported. Visual motion sensitivity symptoms also were associated with increased odds of protracted recovery (adjusted odds ratio, 4.5).
“Once care was established, time to recovery did not differ for athletes evaluated within the first week of injury compared with those evaluated 2-3 weeks post injury,” Dr. Kontos and colleagues concluded. “Education on injury and behavioral recommendations to optimize concussion recovery and earlier initiation of active rehabilitation strategies, including exertion and vestibular therapy, are plausible explanations for the association of a shorter recovery time with earlier care. However, future research should focus on the specific mechanisms by which earlier health care postconcussion promotes faster recovery and determine if these findings apply to other subpopulations, including military personnel.”
The researchers noted that they excluded from their analysis 254 patients who had incomplete recovery data but otherwise met inclusion criteria, which may have led to selection bias.
Dr. Kontos disclosed grants from the National Football League and personal fees from APA Books and University of Pittsburgh projects outside the scope of this study.
SOURCE: Kontos AP et al. JAMA Neurol. 2020 Jan 6. doi: 10.1001/jamaneurol.2019.4552.
, according to a study published Jan. 6 in JAMA Neurology.
In addition, more severe visual motion sensitivity symptoms are associated with longer recovery times, said lead author Anthony P. Kontos, PhD, and colleagues. Dr. Kontos is associate professor of orthopedic surgery at the University of Pittsburgh and the research director of the University of Pittsburgh Medical Center Sports Medicine Concussion Program.
“Without clinical guidance and behavioral management recommendations post injury, athletes may have been engaging in counterproductive recovery strategies, such as a strict rest or excessive physical activity,” the authors said. “This explanation is supported by the fact that athletes recovered in a similar amount of time after that first evaluation.”
Various factors may influence recovery after a concussion, including age, sex, and comorbidities. To examine the relationship between time since injury at the start of clinical care and recovery time, the investigators conducted a retrospective, cross-sectional study. They analyzed data from patients seen in a sports medicine clinic between August 2016 and March 2018. Eligible patients were aged 12-22 years and had a diagnosed, symptomatic, sport-related concussion. The researchers excluded patients with incomplete recovery data.
The investigators included 162 patients in their analyses; 98 of these patients were seen within 7 days of the injury, and 64 were seen 8-20 days after a concussion. Both groups had a mean age of 15 years and similar proportions of female patients (52% in the early-care group vs. 62.5% in the late-care group). At the first clinical visit, symptom severity and cognitive, ocular, and vestibular test results were similar in both groups.
The researchers defined recovery time as the number of days from injury until the date of clearance for a full return to play. Physicians cleared patients to return to play when they returned to preinjury levels of symptoms and preinjury performance on cognitive, ocular, and vestibular tests with no increase in symptoms after exertion.
Patients’ recovery times ranged from 9 to 299 days; the average recovery time was 57 days. Fifty-two percent of patients in the early-care group recovered in 30 days or fewer, compared with 19% of patients in late-care group. Patients in the early-care group had a mean recovery of 51.1 days, whereas patients in the late-care group had a mean recovery of 66 days.
In a logistic regression model, late care “was associated with a 5.8-times increased likelihood of a recovery longer than 30 days,” the researchers reported. Visual motion sensitivity symptoms also were associated with increased odds of protracted recovery (adjusted odds ratio, 4.5).
“Once care was established, time to recovery did not differ for athletes evaluated within the first week of injury compared with those evaluated 2-3 weeks post injury,” Dr. Kontos and colleagues concluded. “Education on injury and behavioral recommendations to optimize concussion recovery and earlier initiation of active rehabilitation strategies, including exertion and vestibular therapy, are plausible explanations for the association of a shorter recovery time with earlier care. However, future research should focus on the specific mechanisms by which earlier health care postconcussion promotes faster recovery and determine if these findings apply to other subpopulations, including military personnel.”
The researchers noted that they excluded from their analysis 254 patients who had incomplete recovery data but otherwise met inclusion criteria, which may have led to selection bias.
Dr. Kontos disclosed grants from the National Football League and personal fees from APA Books and University of Pittsburgh projects outside the scope of this study.
SOURCE: Kontos AP et al. JAMA Neurol. 2020 Jan 6. doi: 10.1001/jamaneurol.2019.4552.
FROM JAMA NEUROLOGY
Experts call to revise the Uniform Determination of Death Act
, according to an editorial published online Dec. 24, 2019, in Annals of Internal Medicine. Proposed revisions would identify the standards for determining death by neurologic criteria and address the question of whether consent is required to make this determination. If accepted, the revisions would enhance public trust in the determination of death by neurologic criteria, the authors said.
“There is a disconnect between the medical and legal standards for brain death,” said Ariane K. Lewis, MD, associate professor of neurology and neurosurgery at New York University and lead author of the editorial. The discrepancy must be remedied because it has led to lawsuits and has proved to be problematic from a societal standpoint, she added.
“We defend changing the law to match medical practice, rather than changing medical practice to match the law,” said Thaddeus Mason Pope, JD, PhD, director of the Health Law Institute at Mitchell Hamline School of Law in Saint Paul, Minnesota, and an author of the editorial.
Accepted medical standards are unclear
The UDDA was drafted in 1981 to establish a uniform legal standard for death by neurologic criteria. A person with “irreversible cessation of all functions of the entire brain, including the brainstem,” is dead, according to the statute. A determination of death, it adds, “must be made in accordance with accepted medical standards.”
But the medical standards used to determine death by neurologic cause have not been uniform. In 2015, the Supreme Court of Nevada ruled that it was not clear that the standard published by the American Academy of Neurology (AAN), which had been used in the case at issue, was the “accepted medical standard.” An AAN summit later affirmed that the accepted medical standards for determination of death by neurologic cause are the 2010 AAN standard for determination of brain death in adults and the 2011 Society of Critical Care Medicine (SCCM), American Academy of Pediatrics (AAP), and Child Neurology Society (CNS) standard for determination of brain death in children. The Nevada legislature amended the state UDDA to identify these standards as the accepted standards. A revised UDDA also should identify these standards and grant an administrative agency (i.e., the board of medicine) the power to review and update the accepted medical standards as needed, according to the editorial.
To the extent that hospitals are not following the AAN or SCCM/AAP/CNS standards for determining death by neurologic cause, “enshrining” these standards in a revised UDDA “should increase uniformity and consistency” in hospitals’ policies on brain death, Dr. Pope said.
The question of hormonal function
Lawsuits in California and Nevada raised the question of whether the pituitary gland and hypothalamus are parts of the brain. If so, then the accepted medical standards for death by neurologic cause are not consistent with the statutory requirements for the determination of death, since the former do not test for cessation of hormonal function.
The current edition of the adult standards for determining death by neurologic cause were published in 2010. “Whenever we measure brain death, we’re not measuring the cessation of all functions of the entire brain,” Dr. Pope said. “That’s not a new thing; that’s been the case for a long time.”
To address the discrepancy between medical practice and the legal statute, Dr. Lewis and colleagues proposed that the UDDA’s reference to “irreversible cessation of functions of the entire brain” be followed by the following clause: “including the brainstem, leading to unresponsive coma with loss of capacity for consciousness, brainstem areflexia, and the inability to breathe spontaneously.” An alternative revision would be to add the briefer phrase “... with the exception of hormonal function.”
Authors say consent is not required for testing
Other complications have arisen from the UDDA’s failure to specify whether consent is required for a determination of death by neurologic cause. Court rulings on this question have not been consistent. Dr. Lewis and colleagues propose adding the following text to the UDDA: “Reasonable efforts should be made to notify a patient’s legally authorized decision-maker before performing a determination of death by neurologic criteria, but consent is not required to initiate such an evaluation.”
The proposed revisions to the UDDA “might give [clinicians] more confidence to proceed with brain death testing, because it would clarify that they don’t need the parents’ [or the patient’s legally authorized decision-maker] consent to do the tests,” said Dr. Pope. “If anything, they might even have a duty to do the tests.”
The final problem with the UDDA that Dr. Lewis and colleagues cited is that it does not provide clear guidance about how to respond to religious objections to discontinuation of organ support after a determination of death by neurologic cause. “Because the issue is rather complicated, we have not advocated for a singular position related to this [question] in our revised UDDA,” Dr. Lewis said. “Rather, we recommended the need for a multidisciplinary group to come together to determine what is the best approach. In an ideal world, this [approach] would be universal throughout the country.”
Although a revised UDDA would provide greater clarity to physicians and promote uniformity of practice, it would not resolve ongoing theological and philosophical debates about whether brain death is biological death, Dr. Pope said. “The key thing is that it would give clinicians a green light or certainty and clarity that they may proceed to do the test in the first place. If the tests are positive and the patient really is dead, then they could proceed to organ procurement or to move to the morgue.”
Dr. Lewis is a member of various AAN committees and working groups but receives no compensation for her role. A coauthor received personal fees from the AAN that were unrelated to the editorial.
SOURCE: Lewis A et al. Ann Intern Med. 2019 Dec 24. doi: 10.7326/M19-2731.
, according to an editorial published online Dec. 24, 2019, in Annals of Internal Medicine. Proposed revisions would identify the standards for determining death by neurologic criteria and address the question of whether consent is required to make this determination. If accepted, the revisions would enhance public trust in the determination of death by neurologic criteria, the authors said.
“There is a disconnect between the medical and legal standards for brain death,” said Ariane K. Lewis, MD, associate professor of neurology and neurosurgery at New York University and lead author of the editorial. The discrepancy must be remedied because it has led to lawsuits and has proved to be problematic from a societal standpoint, she added.
“We defend changing the law to match medical practice, rather than changing medical practice to match the law,” said Thaddeus Mason Pope, JD, PhD, director of the Health Law Institute at Mitchell Hamline School of Law in Saint Paul, Minnesota, and an author of the editorial.
Accepted medical standards are unclear
The UDDA was drafted in 1981 to establish a uniform legal standard for death by neurologic criteria. A person with “irreversible cessation of all functions of the entire brain, including the brainstem,” is dead, according to the statute. A determination of death, it adds, “must be made in accordance with accepted medical standards.”
But the medical standards used to determine death by neurologic cause have not been uniform. In 2015, the Supreme Court of Nevada ruled that it was not clear that the standard published by the American Academy of Neurology (AAN), which had been used in the case at issue, was the “accepted medical standard.” An AAN summit later affirmed that the accepted medical standards for determination of death by neurologic cause are the 2010 AAN standard for determination of brain death in adults and the 2011 Society of Critical Care Medicine (SCCM), American Academy of Pediatrics (AAP), and Child Neurology Society (CNS) standard for determination of brain death in children. The Nevada legislature amended the state UDDA to identify these standards as the accepted standards. A revised UDDA also should identify these standards and grant an administrative agency (i.e., the board of medicine) the power to review and update the accepted medical standards as needed, according to the editorial.
To the extent that hospitals are not following the AAN or SCCM/AAP/CNS standards for determining death by neurologic cause, “enshrining” these standards in a revised UDDA “should increase uniformity and consistency” in hospitals’ policies on brain death, Dr. Pope said.
The question of hormonal function
Lawsuits in California and Nevada raised the question of whether the pituitary gland and hypothalamus are parts of the brain. If so, then the accepted medical standards for death by neurologic cause are not consistent with the statutory requirements for the determination of death, since the former do not test for cessation of hormonal function.
The current edition of the adult standards for determining death by neurologic cause were published in 2010. “Whenever we measure brain death, we’re not measuring the cessation of all functions of the entire brain,” Dr. Pope said. “That’s not a new thing; that’s been the case for a long time.”
To address the discrepancy between medical practice and the legal statute, Dr. Lewis and colleagues proposed that the UDDA’s reference to “irreversible cessation of functions of the entire brain” be followed by the following clause: “including the brainstem, leading to unresponsive coma with loss of capacity for consciousness, brainstem areflexia, and the inability to breathe spontaneously.” An alternative revision would be to add the briefer phrase “... with the exception of hormonal function.”
Authors say consent is not required for testing
Other complications have arisen from the UDDA’s failure to specify whether consent is required for a determination of death by neurologic cause. Court rulings on this question have not been consistent. Dr. Lewis and colleagues propose adding the following text to the UDDA: “Reasonable efforts should be made to notify a patient’s legally authorized decision-maker before performing a determination of death by neurologic criteria, but consent is not required to initiate such an evaluation.”
The proposed revisions to the UDDA “might give [clinicians] more confidence to proceed with brain death testing, because it would clarify that they don’t need the parents’ [or the patient’s legally authorized decision-maker] consent to do the tests,” said Dr. Pope. “If anything, they might even have a duty to do the tests.”
The final problem with the UDDA that Dr. Lewis and colleagues cited is that it does not provide clear guidance about how to respond to religious objections to discontinuation of organ support after a determination of death by neurologic cause. “Because the issue is rather complicated, we have not advocated for a singular position related to this [question] in our revised UDDA,” Dr. Lewis said. “Rather, we recommended the need for a multidisciplinary group to come together to determine what is the best approach. In an ideal world, this [approach] would be universal throughout the country.”
Although a revised UDDA would provide greater clarity to physicians and promote uniformity of practice, it would not resolve ongoing theological and philosophical debates about whether brain death is biological death, Dr. Pope said. “The key thing is that it would give clinicians a green light or certainty and clarity that they may proceed to do the test in the first place. If the tests are positive and the patient really is dead, then they could proceed to organ procurement or to move to the morgue.”
Dr. Lewis is a member of various AAN committees and working groups but receives no compensation for her role. A coauthor received personal fees from the AAN that were unrelated to the editorial.
SOURCE: Lewis A et al. Ann Intern Med. 2019 Dec 24. doi: 10.7326/M19-2731.
, according to an editorial published online Dec. 24, 2019, in Annals of Internal Medicine. Proposed revisions would identify the standards for determining death by neurologic criteria and address the question of whether consent is required to make this determination. If accepted, the revisions would enhance public trust in the determination of death by neurologic criteria, the authors said.
“There is a disconnect between the medical and legal standards for brain death,” said Ariane K. Lewis, MD, associate professor of neurology and neurosurgery at New York University and lead author of the editorial. The discrepancy must be remedied because it has led to lawsuits and has proved to be problematic from a societal standpoint, she added.
“We defend changing the law to match medical practice, rather than changing medical practice to match the law,” said Thaddeus Mason Pope, JD, PhD, director of the Health Law Institute at Mitchell Hamline School of Law in Saint Paul, Minnesota, and an author of the editorial.
Accepted medical standards are unclear
The UDDA was drafted in 1981 to establish a uniform legal standard for death by neurologic criteria. A person with “irreversible cessation of all functions of the entire brain, including the brainstem,” is dead, according to the statute. A determination of death, it adds, “must be made in accordance with accepted medical standards.”
But the medical standards used to determine death by neurologic cause have not been uniform. In 2015, the Supreme Court of Nevada ruled that it was not clear that the standard published by the American Academy of Neurology (AAN), which had been used in the case at issue, was the “accepted medical standard.” An AAN summit later affirmed that the accepted medical standards for determination of death by neurologic cause are the 2010 AAN standard for determination of brain death in adults and the 2011 Society of Critical Care Medicine (SCCM), American Academy of Pediatrics (AAP), and Child Neurology Society (CNS) standard for determination of brain death in children. The Nevada legislature amended the state UDDA to identify these standards as the accepted standards. A revised UDDA also should identify these standards and grant an administrative agency (i.e., the board of medicine) the power to review and update the accepted medical standards as needed, according to the editorial.
To the extent that hospitals are not following the AAN or SCCM/AAP/CNS standards for determining death by neurologic cause, “enshrining” these standards in a revised UDDA “should increase uniformity and consistency” in hospitals’ policies on brain death, Dr. Pope said.
The question of hormonal function
Lawsuits in California and Nevada raised the question of whether the pituitary gland and hypothalamus are parts of the brain. If so, then the accepted medical standards for death by neurologic cause are not consistent with the statutory requirements for the determination of death, since the former do not test for cessation of hormonal function.
The current edition of the adult standards for determining death by neurologic cause were published in 2010. “Whenever we measure brain death, we’re not measuring the cessation of all functions of the entire brain,” Dr. Pope said. “That’s not a new thing; that’s been the case for a long time.”
To address the discrepancy between medical practice and the legal statute, Dr. Lewis and colleagues proposed that the UDDA’s reference to “irreversible cessation of functions of the entire brain” be followed by the following clause: “including the brainstem, leading to unresponsive coma with loss of capacity for consciousness, brainstem areflexia, and the inability to breathe spontaneously.” An alternative revision would be to add the briefer phrase “... with the exception of hormonal function.”
Authors say consent is not required for testing
Other complications have arisen from the UDDA’s failure to specify whether consent is required for a determination of death by neurologic cause. Court rulings on this question have not been consistent. Dr. Lewis and colleagues propose adding the following text to the UDDA: “Reasonable efforts should be made to notify a patient’s legally authorized decision-maker before performing a determination of death by neurologic criteria, but consent is not required to initiate such an evaluation.”
The proposed revisions to the UDDA “might give [clinicians] more confidence to proceed with brain death testing, because it would clarify that they don’t need the parents’ [or the patient’s legally authorized decision-maker] consent to do the tests,” said Dr. Pope. “If anything, they might even have a duty to do the tests.”
The final problem with the UDDA that Dr. Lewis and colleagues cited is that it does not provide clear guidance about how to respond to religious objections to discontinuation of organ support after a determination of death by neurologic cause. “Because the issue is rather complicated, we have not advocated for a singular position related to this [question] in our revised UDDA,” Dr. Lewis said. “Rather, we recommended the need for a multidisciplinary group to come together to determine what is the best approach. In an ideal world, this [approach] would be universal throughout the country.”
Although a revised UDDA would provide greater clarity to physicians and promote uniformity of practice, it would not resolve ongoing theological and philosophical debates about whether brain death is biological death, Dr. Pope said. “The key thing is that it would give clinicians a green light or certainty and clarity that they may proceed to do the test in the first place. If the tests are positive and the patient really is dead, then they could proceed to organ procurement or to move to the morgue.”
Dr. Lewis is a member of various AAN committees and working groups but receives no compensation for her role. A coauthor received personal fees from the AAN that were unrelated to the editorial.
SOURCE: Lewis A et al. Ann Intern Med. 2019 Dec 24. doi: 10.7326/M19-2731.
FROM ANNALS OF INTERNAL MEDICINE
Oral contraceptive use associated with smaller hypothalamic and pituitary volumes
CHICAGO – Women taking oral contraceptives had, on average, a hypothalamus that was 6% smaller than those who didn’t, in a small study that used magnetic resonance imaging. Pituitary volume was also smaller.
Though the sample size was relatively small, 50 women in total, it’s the only study to date that looks at the relationship between hypothalamic volume and oral contraceptive (OC) use, and the largest examining pituitary volume, according to Ke Xun (Kevin) Chen, MD, who presented the findings at the annual meeting of the Radiological Society of North America.
Using MRI, Dr. Chen and his colleagues found that hypothalamic volume was significantly smaller in women taking oral contraceptives than those who were naturally cycling (b value = –64.1; P = .006). The pituitary gland also was significantly smaller in those taking OCs (b = –92.8; P = .007).
“I was quite surprised [at the finding], because the magnitude of the effect is not small,” especially in the context of changes in volume of other brain structures, senior author Michael L. Lipton, MD, PhD, said in an interview. In Alzheimer’s disease, for example, a volume loss of 4% annually can be expected.
However, “it’s not shocking to me in a negative way at all. I can’t tell you what it means in terms of how it’s going to affect people,” since this is a cross-sectional study that only detected a correlation and can’t say anything about a causative relationship, he added. “We don’t even know that [OCs] cause this effect. ... It’s plausible that this is just a plasticity-related change that’s simply showing us the effect of the drug.
“We’re going to be much more careful to consider oral contraceptive use as a covariate in future research studies; that’s for sure,” he said.
Although OCs have been available since their 1960 Food and Drug Administration approval, and their effects in some areas of physiology and health have been well studied, there’s still not much known about how oral contraceptives affect brain function, said Dr. Lipton, professor of neuroradiology and psychiatry and behavioral sciences at Albert Einstein College of Medicine, in the Montefiore medical system, New York.
The spark for this study came from one of Dr. Lipton’s main areas of research – sex differences in susceptibility to and recovery from traumatic brain injury. “Women are more likely to exhibit changes in their brain [after injury] – and changes in their brain function – than men,” he said.
In the present study, “we went at this trying to understand the effect to which the hormone effect might be doing something in regular, healthy people that we need to consider as part of the bigger picture,” he said.
Dr. Lipton, Dr. Chen (then a radiology resident at Albert Einstein College of Medicine), and their coauthors constructed the study to look for differences in brain structure between women who were experiencing natural menstrual cycles and those who were taking exogenous hormones, to begin to learn how oral contraceptive use might modify risk and susceptibility for neurologic disease and injury.
It had already been established that global brain volume didn’t differ between naturally cycling women and those using OCs. However, some studies had shown differences in volume of some specific brain regions, and one study had shown smaller pituitary volume in OC users, according to the presentation by Dr. Chen, who is now a radiology fellow at Brigham and Women’s Hospital, Boston. Accurately measuring hypothalamic volume represents a technical challenge, and the effect of OCs on the structure’s volume hadn’t previously been studied.
Sex hormones, said Dr. Lipton, have known trophic effects on brain tissue and ovarian sex hormones cross the blood brain barrier, so the idea that there would be some plasticity in the brains of those taking OCs wasn’t completely surprising, especially since there are hormone receptors that lie within the central nervous system. However, he said he was “very surprised” by the effect size seen in the study.
The study included 21 healthy women taking combined oral contraceptives, and 29 naturally cycling women. Participants’ mean age was 23 years for the OC users, and 21 for the naturally cycling women. Body mass index and smoking history didn’t differ between groups. Women on OCs were significantly more likely to use alcohol and to drink more frequently than those not taking OCs (P = .001). Participants were included only if they were taking a combined estrogen-progestin pill; those on noncyclical contraceptives such as implants and hormone-emitting intrauterine devices were excluded, as were naturally cycling women with very long or irregular menstrual cycles.
After multivariable statistical analysis, the only two significant predictors of hypothalamic volume were total intracranial volume and OC use. For pituitary volume, body mass index and OC use remained significant.
In addition to the MRI scans, participants also completed neurobehavioral testing to assess mood and cognition. An exploratory analysis showed no correlation between hypothalamic volume and the cognitive testing battery results, which included assessments for verbal learning and memory, executive function, and working memory.
However, a moderate positive association was seen between hypothalamic volume and anger scores (r = 0.34; P = .02). The investigators found a “strong positive correlation of hypothalamic volume with depression,” said Dr. Chen (r = 0.25; P = .09).
The investigators found no menstrual cycle-related changes in hypothalamic and pituitary volume among naturally cycling women.
Hypothalamic volume was obtained using manual segmentation of the MRIs; a combined automated-manual approach was used to obtain pituitary volume. Reliability was tested by having 5 raters each assess volumes for a randomly selected subset of the scans; inter-rater reliability fell between 0.78 and 0.86, values considered to indicate “good” reliability.
In addition to the small sample size, Dr. Chen acknowledged several limitations to the study. These included the lack of accounting for details of OC use including duration, exact type of OC, and whether women were taking the placebo phase of their pill packs at the time of scanning. Additionally, women who were naturally cycling were not asked about prior history of OC use.
Also, women’s menstrual phase was estimated from the self-reported date of the last menstrual period, rather than obtained by direct measurement via serum hormone levels.
Dr. Lipton’s perspective adds a strong note of caution to avoid overinterpretation from the study. Dr. Chen and Dr. Lipton agreed, however, that OC use should be accounted for when brain structure and function are studied in female participants.
Dr. Chen, Dr. Lipton, and their coauthors reported that they had no conflicts of interest. The authors reported no outside sources of funding.
SOURCE: Chen K et al. RSNA 2019. Presentation SSM-1904.
CHICAGO – Women taking oral contraceptives had, on average, a hypothalamus that was 6% smaller than those who didn’t, in a small study that used magnetic resonance imaging. Pituitary volume was also smaller.
Though the sample size was relatively small, 50 women in total, it’s the only study to date that looks at the relationship between hypothalamic volume and oral contraceptive (OC) use, and the largest examining pituitary volume, according to Ke Xun (Kevin) Chen, MD, who presented the findings at the annual meeting of the Radiological Society of North America.
Using MRI, Dr. Chen and his colleagues found that hypothalamic volume was significantly smaller in women taking oral contraceptives than those who were naturally cycling (b value = –64.1; P = .006). The pituitary gland also was significantly smaller in those taking OCs (b = –92.8; P = .007).
“I was quite surprised [at the finding], because the magnitude of the effect is not small,” especially in the context of changes in volume of other brain structures, senior author Michael L. Lipton, MD, PhD, said in an interview. In Alzheimer’s disease, for example, a volume loss of 4% annually can be expected.
However, “it’s not shocking to me in a negative way at all. I can’t tell you what it means in terms of how it’s going to affect people,” since this is a cross-sectional study that only detected a correlation and can’t say anything about a causative relationship, he added. “We don’t even know that [OCs] cause this effect. ... It’s plausible that this is just a plasticity-related change that’s simply showing us the effect of the drug.
“We’re going to be much more careful to consider oral contraceptive use as a covariate in future research studies; that’s for sure,” he said.
Although OCs have been available since their 1960 Food and Drug Administration approval, and their effects in some areas of physiology and health have been well studied, there’s still not much known about how oral contraceptives affect brain function, said Dr. Lipton, professor of neuroradiology and psychiatry and behavioral sciences at Albert Einstein College of Medicine, in the Montefiore medical system, New York.
The spark for this study came from one of Dr. Lipton’s main areas of research – sex differences in susceptibility to and recovery from traumatic brain injury. “Women are more likely to exhibit changes in their brain [after injury] – and changes in their brain function – than men,” he said.
In the present study, “we went at this trying to understand the effect to which the hormone effect might be doing something in regular, healthy people that we need to consider as part of the bigger picture,” he said.
Dr. Lipton, Dr. Chen (then a radiology resident at Albert Einstein College of Medicine), and their coauthors constructed the study to look for differences in brain structure between women who were experiencing natural menstrual cycles and those who were taking exogenous hormones, to begin to learn how oral contraceptive use might modify risk and susceptibility for neurologic disease and injury.
It had already been established that global brain volume didn’t differ between naturally cycling women and those using OCs. However, some studies had shown differences in volume of some specific brain regions, and one study had shown smaller pituitary volume in OC users, according to the presentation by Dr. Chen, who is now a radiology fellow at Brigham and Women’s Hospital, Boston. Accurately measuring hypothalamic volume represents a technical challenge, and the effect of OCs on the structure’s volume hadn’t previously been studied.
Sex hormones, said Dr. Lipton, have known trophic effects on brain tissue and ovarian sex hormones cross the blood brain barrier, so the idea that there would be some plasticity in the brains of those taking OCs wasn’t completely surprising, especially since there are hormone receptors that lie within the central nervous system. However, he said he was “very surprised” by the effect size seen in the study.
The study included 21 healthy women taking combined oral contraceptives, and 29 naturally cycling women. Participants’ mean age was 23 years for the OC users, and 21 for the naturally cycling women. Body mass index and smoking history didn’t differ between groups. Women on OCs were significantly more likely to use alcohol and to drink more frequently than those not taking OCs (P = .001). Participants were included only if they were taking a combined estrogen-progestin pill; those on noncyclical contraceptives such as implants and hormone-emitting intrauterine devices were excluded, as were naturally cycling women with very long or irregular menstrual cycles.
After multivariable statistical analysis, the only two significant predictors of hypothalamic volume were total intracranial volume and OC use. For pituitary volume, body mass index and OC use remained significant.
In addition to the MRI scans, participants also completed neurobehavioral testing to assess mood and cognition. An exploratory analysis showed no correlation between hypothalamic volume and the cognitive testing battery results, which included assessments for verbal learning and memory, executive function, and working memory.
However, a moderate positive association was seen between hypothalamic volume and anger scores (r = 0.34; P = .02). The investigators found a “strong positive correlation of hypothalamic volume with depression,” said Dr. Chen (r = 0.25; P = .09).
The investigators found no menstrual cycle-related changes in hypothalamic and pituitary volume among naturally cycling women.
Hypothalamic volume was obtained using manual segmentation of the MRIs; a combined automated-manual approach was used to obtain pituitary volume. Reliability was tested by having 5 raters each assess volumes for a randomly selected subset of the scans; inter-rater reliability fell between 0.78 and 0.86, values considered to indicate “good” reliability.
In addition to the small sample size, Dr. Chen acknowledged several limitations to the study. These included the lack of accounting for details of OC use including duration, exact type of OC, and whether women were taking the placebo phase of their pill packs at the time of scanning. Additionally, women who were naturally cycling were not asked about prior history of OC use.
Also, women’s menstrual phase was estimated from the self-reported date of the last menstrual period, rather than obtained by direct measurement via serum hormone levels.
Dr. Lipton’s perspective adds a strong note of caution to avoid overinterpretation from the study. Dr. Chen and Dr. Lipton agreed, however, that OC use should be accounted for when brain structure and function are studied in female participants.
Dr. Chen, Dr. Lipton, and their coauthors reported that they had no conflicts of interest. The authors reported no outside sources of funding.
SOURCE: Chen K et al. RSNA 2019. Presentation SSM-1904.
CHICAGO – Women taking oral contraceptives had, on average, a hypothalamus that was 6% smaller than those who didn’t, in a small study that used magnetic resonance imaging. Pituitary volume was also smaller.
Though the sample size was relatively small, 50 women in total, it’s the only study to date that looks at the relationship between hypothalamic volume and oral contraceptive (OC) use, and the largest examining pituitary volume, according to Ke Xun (Kevin) Chen, MD, who presented the findings at the annual meeting of the Radiological Society of North America.
Using MRI, Dr. Chen and his colleagues found that hypothalamic volume was significantly smaller in women taking oral contraceptives than those who were naturally cycling (b value = –64.1; P = .006). The pituitary gland also was significantly smaller in those taking OCs (b = –92.8; P = .007).
“I was quite surprised [at the finding], because the magnitude of the effect is not small,” especially in the context of changes in volume of other brain structures, senior author Michael L. Lipton, MD, PhD, said in an interview. In Alzheimer’s disease, for example, a volume loss of 4% annually can be expected.
However, “it’s not shocking to me in a negative way at all. I can’t tell you what it means in terms of how it’s going to affect people,” since this is a cross-sectional study that only detected a correlation and can’t say anything about a causative relationship, he added. “We don’t even know that [OCs] cause this effect. ... It’s plausible that this is just a plasticity-related change that’s simply showing us the effect of the drug.
“We’re going to be much more careful to consider oral contraceptive use as a covariate in future research studies; that’s for sure,” he said.
Although OCs have been available since their 1960 Food and Drug Administration approval, and their effects in some areas of physiology and health have been well studied, there’s still not much known about how oral contraceptives affect brain function, said Dr. Lipton, professor of neuroradiology and psychiatry and behavioral sciences at Albert Einstein College of Medicine, in the Montefiore medical system, New York.
The spark for this study came from one of Dr. Lipton’s main areas of research – sex differences in susceptibility to and recovery from traumatic brain injury. “Women are more likely to exhibit changes in their brain [after injury] – and changes in their brain function – than men,” he said.
In the present study, “we went at this trying to understand the effect to which the hormone effect might be doing something in regular, healthy people that we need to consider as part of the bigger picture,” he said.
Dr. Lipton, Dr. Chen (then a radiology resident at Albert Einstein College of Medicine), and their coauthors constructed the study to look for differences in brain structure between women who were experiencing natural menstrual cycles and those who were taking exogenous hormones, to begin to learn how oral contraceptive use might modify risk and susceptibility for neurologic disease and injury.
It had already been established that global brain volume didn’t differ between naturally cycling women and those using OCs. However, some studies had shown differences in volume of some specific brain regions, and one study had shown smaller pituitary volume in OC users, according to the presentation by Dr. Chen, who is now a radiology fellow at Brigham and Women’s Hospital, Boston. Accurately measuring hypothalamic volume represents a technical challenge, and the effect of OCs on the structure’s volume hadn’t previously been studied.
Sex hormones, said Dr. Lipton, have known trophic effects on brain tissue and ovarian sex hormones cross the blood brain barrier, so the idea that there would be some plasticity in the brains of those taking OCs wasn’t completely surprising, especially since there are hormone receptors that lie within the central nervous system. However, he said he was “very surprised” by the effect size seen in the study.
The study included 21 healthy women taking combined oral contraceptives, and 29 naturally cycling women. Participants’ mean age was 23 years for the OC users, and 21 for the naturally cycling women. Body mass index and smoking history didn’t differ between groups. Women on OCs were significantly more likely to use alcohol and to drink more frequently than those not taking OCs (P = .001). Participants were included only if they were taking a combined estrogen-progestin pill; those on noncyclical contraceptives such as implants and hormone-emitting intrauterine devices were excluded, as were naturally cycling women with very long or irregular menstrual cycles.
After multivariable statistical analysis, the only two significant predictors of hypothalamic volume were total intracranial volume and OC use. For pituitary volume, body mass index and OC use remained significant.
In addition to the MRI scans, participants also completed neurobehavioral testing to assess mood and cognition. An exploratory analysis showed no correlation between hypothalamic volume and the cognitive testing battery results, which included assessments for verbal learning and memory, executive function, and working memory.
However, a moderate positive association was seen between hypothalamic volume and anger scores (r = 0.34; P = .02). The investigators found a “strong positive correlation of hypothalamic volume with depression,” said Dr. Chen (r = 0.25; P = .09).
The investigators found no menstrual cycle-related changes in hypothalamic and pituitary volume among naturally cycling women.
Hypothalamic volume was obtained using manual segmentation of the MRIs; a combined automated-manual approach was used to obtain pituitary volume. Reliability was tested by having 5 raters each assess volumes for a randomly selected subset of the scans; inter-rater reliability fell between 0.78 and 0.86, values considered to indicate “good” reliability.
In addition to the small sample size, Dr. Chen acknowledged several limitations to the study. These included the lack of accounting for details of OC use including duration, exact type of OC, and whether women were taking the placebo phase of their pill packs at the time of scanning. Additionally, women who were naturally cycling were not asked about prior history of OC use.
Also, women’s menstrual phase was estimated from the self-reported date of the last menstrual period, rather than obtained by direct measurement via serum hormone levels.
Dr. Lipton’s perspective adds a strong note of caution to avoid overinterpretation from the study. Dr. Chen and Dr. Lipton agreed, however, that OC use should be accounted for when brain structure and function are studied in female participants.
Dr. Chen, Dr. Lipton, and their coauthors reported that they had no conflicts of interest. The authors reported no outside sources of funding.
SOURCE: Chen K et al. RSNA 2019. Presentation SSM-1904.
REPORTING FROM RSNA 2019
DoD Explores Virtual Health for Traumatic Brain Injury
NATIONAL HARBOR, MD – As it moves to expand the use of virtual health offerings, the US Department of Defense (DoD) Regional Health Command Europe piloted a virtual health (telehealth) program to treat service members with traumatic brain injury (TBI). Ronald Keen, FNP-C, and Steve Cain, PA, reported on the DoD use of virtual health at the 2019 AMSUS annual meeting in Maryland.
The study, conducted between October 2016 and May 2018, included 15 patients stationed in 4 countries, including Poland, Turkey, and Egypt and 67 total health care encounters. Patients were limited to service members in the direct care system or those who were in remote areas where gaps in care existed in the Tricare Network. The virtual health program was centered at Landstuhl Regional Medical Center in Germany and sought to determine whether virtual health was feasible to treat TBI and whether it would increase patient satisfaction. The multidisciplinary program brought together specialists in 7 different disciplines, including sleep medicine, optometry, behavioral health, and occupational therapy.
According to Keen, the results of the 15-patient pilot were promising. He conservatively estimated a savings of $3,700, and more important, the program saved 322 hours of on-duty time. Health care providers used the program an average 2.8 times, and patients used the system 1.6 times on average. Currently the DoD is requiring active permission from patients to receive a telehealth visit.
NATIONAL HARBOR, MD – As it moves to expand the use of virtual health offerings, the US Department of Defense (DoD) Regional Health Command Europe piloted a virtual health (telehealth) program to treat service members with traumatic brain injury (TBI). Ronald Keen, FNP-C, and Steve Cain, PA, reported on the DoD use of virtual health at the 2019 AMSUS annual meeting in Maryland.
The study, conducted between October 2016 and May 2018, included 15 patients stationed in 4 countries, including Poland, Turkey, and Egypt and 67 total health care encounters. Patients were limited to service members in the direct care system or those who were in remote areas where gaps in care existed in the Tricare Network. The virtual health program was centered at Landstuhl Regional Medical Center in Germany and sought to determine whether virtual health was feasible to treat TBI and whether it would increase patient satisfaction. The multidisciplinary program brought together specialists in 7 different disciplines, including sleep medicine, optometry, behavioral health, and occupational therapy.
According to Keen, the results of the 15-patient pilot were promising. He conservatively estimated a savings of $3,700, and more important, the program saved 322 hours of on-duty time. Health care providers used the program an average 2.8 times, and patients used the system 1.6 times on average. Currently the DoD is requiring active permission from patients to receive a telehealth visit.
NATIONAL HARBOR, MD – As it moves to expand the use of virtual health offerings, the US Department of Defense (DoD) Regional Health Command Europe piloted a virtual health (telehealth) program to treat service members with traumatic brain injury (TBI). Ronald Keen, FNP-C, and Steve Cain, PA, reported on the DoD use of virtual health at the 2019 AMSUS annual meeting in Maryland.
The study, conducted between October 2016 and May 2018, included 15 patients stationed in 4 countries, including Poland, Turkey, and Egypt and 67 total health care encounters. Patients were limited to service members in the direct care system or those who were in remote areas where gaps in care existed in the Tricare Network. The virtual health program was centered at Landstuhl Regional Medical Center in Germany and sought to determine whether virtual health was feasible to treat TBI and whether it would increase patient satisfaction. The multidisciplinary program brought together specialists in 7 different disciplines, including sleep medicine, optometry, behavioral health, and occupational therapy.
According to Keen, the results of the 15-patient pilot were promising. He conservatively estimated a savings of $3,700, and more important, the program saved 322 hours of on-duty time. Health care providers used the program an average 2.8 times, and patients used the system 1.6 times on average. Currently the DoD is requiring active permission from patients to receive a telehealth visit.
Children may develop prolonged headache after concussion
CHARLOTTE, N.C. – , according to research presented at the annual meeting of the Child Neurology Society. The headache may be migraine, chronic daily headache, tension-type headache, or a combination of these headaches.
“We strongly recommend that individuals who develop persistent headache after a concussion be evaluated and treated by a neurologist with experience in administering treatment for headache,” said Marcus Barissi, Weller Scholar at the Cleveland Clinic, and colleagues. “Using this approach, we hope that their prolonged headaches will be lessened.”
Few studies have examined prolonged pediatric postconcussion headache
The Centers for Disease Control and Prevention estimates that between 1.6 million and 3.8 million concussions occur annually during athletic and recreational activities in the United States. About 90% of concussions affect children or adolescents. The symptom most often reported after concussion is headache.
Few studies have focused on new persistent postconcussion headache (NPPCH) in children. Mr. Barissi and colleagues did not find any previous study that had examined prolonged headache following concussion in patients without prior chronic headache. They sought to ascertain the prognosis of patients with NPPCH and no history of prior headache, to describe this clinical entity, and to identify beneficial treatment methods.
The investigators retrospectively reviewed charts for approximately 2,000 patients who presented to the Cleveland Clinic pediatric neurology department between June 2017 and August 2018 for headaches. They identified 259 patients who received a diagnosis of concussion, 69 (27%) of whom had headaches for longer than 2 months after injury.
Mr. Barissi and colleagues emailed these patients, and 33 (48%) of them agreed to complete a questionnaire and participate in a 10-minute phone interview. Thirty-one patients (43%) could not be contacted, and eight (11%) declined to participate. All participants confirmed that they had not had consistent headache before the concussion and that chronic headache had arisen after concussion. To determine participants’ medical outcomes, the researchers compared participants’ initial assessment data with posttreatment data collected during the interview process.
Healthy behaviors increased after concussion
Of the 69 eligible participants, 38 (55%) were female. The population’s median age was 17. Twenty-eight (85%) of the 33 patients who completed the questionnaire considered the information and treatment that they had received to be beneficial. Twenty-five (78%) patients continued to have headache after several months, despite treatment.
Participants had withstood a mean of 1.72 concussions, and the mean age at first injury was 12.49 years. The most common cause of injury was a fall for males (36%) and an automobile accident for females (18%).
Forty-eight patients (70%) reported having two types of headache. Fifty-two patients (75%) had migraines, and 65 (94%) had chronic daily headache or tension-type headache. Forty-eight (70%) participants had a family history of headache.
In all, 64 patients (93%) had used a headache medication. The most common headache medications used were amitriptyline, topiramate, and cyproheptadine. Few patients were still taking these medications at several months after evaluation. The most common nonprescription medications used were Migravent (i.e., magnesium, riboflavin, coenzyme Q10, and butterbur), ondansetron, and melatonin. Furthermore, 61 patients (88%) participated in nonmedicinal therapy such as physical therapy, chiropractic therapy, and acupuncture.
After evaluation, patients engaged in several healthy behaviors (e.g., adequate exercise, proper use of over-the-counter medications, and drinking sufficient water) more frequently, but did not get adequate sleep. Sixty-five participants (94%) had undergone CT or MRI imaging, but the results did not improve understanding of headache etiology or treatment. Many patients missed several days of school, but average attendance improved after months of treatment.
Long-term outcomes
Thirty-one survey respondents (94%) reported that their emotional, cognitive, sleep, and somatic postconcussion symptoms had resolved. Nevertheless, a majority of participants still had headache. “The persistence of postconcussion symptoms is uncommon, but lasting headache is not,” said the researchers. “If patients are not properly educated, conditions may deteriorate, extending the duration of disability.” A longer study with a larger sample size could provide valuable information, said the researchers. Future work should examine objectively the efficacy of various medications used to treat NPPCH and determine the best methods of treatment for this syndrome, which “can cause prolonged pain, suffering, and lack of function,” they concluded.
The investigators did not report any study funding or disclosures.
SOURCE: Barissi M et al. CNS 2019, Abstract 95.
CHARLOTTE, N.C. – , according to research presented at the annual meeting of the Child Neurology Society. The headache may be migraine, chronic daily headache, tension-type headache, or a combination of these headaches.
“We strongly recommend that individuals who develop persistent headache after a concussion be evaluated and treated by a neurologist with experience in administering treatment for headache,” said Marcus Barissi, Weller Scholar at the Cleveland Clinic, and colleagues. “Using this approach, we hope that their prolonged headaches will be lessened.”
Few studies have examined prolonged pediatric postconcussion headache
The Centers for Disease Control and Prevention estimates that between 1.6 million and 3.8 million concussions occur annually during athletic and recreational activities in the United States. About 90% of concussions affect children or adolescents. The symptom most often reported after concussion is headache.
Few studies have focused on new persistent postconcussion headache (NPPCH) in children. Mr. Barissi and colleagues did not find any previous study that had examined prolonged headache following concussion in patients without prior chronic headache. They sought to ascertain the prognosis of patients with NPPCH and no history of prior headache, to describe this clinical entity, and to identify beneficial treatment methods.
The investigators retrospectively reviewed charts for approximately 2,000 patients who presented to the Cleveland Clinic pediatric neurology department between June 2017 and August 2018 for headaches. They identified 259 patients who received a diagnosis of concussion, 69 (27%) of whom had headaches for longer than 2 months after injury.
Mr. Barissi and colleagues emailed these patients, and 33 (48%) of them agreed to complete a questionnaire and participate in a 10-minute phone interview. Thirty-one patients (43%) could not be contacted, and eight (11%) declined to participate. All participants confirmed that they had not had consistent headache before the concussion and that chronic headache had arisen after concussion. To determine participants’ medical outcomes, the researchers compared participants’ initial assessment data with posttreatment data collected during the interview process.
Healthy behaviors increased after concussion
Of the 69 eligible participants, 38 (55%) were female. The population’s median age was 17. Twenty-eight (85%) of the 33 patients who completed the questionnaire considered the information and treatment that they had received to be beneficial. Twenty-five (78%) patients continued to have headache after several months, despite treatment.
Participants had withstood a mean of 1.72 concussions, and the mean age at first injury was 12.49 years. The most common cause of injury was a fall for males (36%) and an automobile accident for females (18%).
Forty-eight patients (70%) reported having two types of headache. Fifty-two patients (75%) had migraines, and 65 (94%) had chronic daily headache or tension-type headache. Forty-eight (70%) participants had a family history of headache.
In all, 64 patients (93%) had used a headache medication. The most common headache medications used were amitriptyline, topiramate, and cyproheptadine. Few patients were still taking these medications at several months after evaluation. The most common nonprescription medications used were Migravent (i.e., magnesium, riboflavin, coenzyme Q10, and butterbur), ondansetron, and melatonin. Furthermore, 61 patients (88%) participated in nonmedicinal therapy such as physical therapy, chiropractic therapy, and acupuncture.
After evaluation, patients engaged in several healthy behaviors (e.g., adequate exercise, proper use of over-the-counter medications, and drinking sufficient water) more frequently, but did not get adequate sleep. Sixty-five participants (94%) had undergone CT or MRI imaging, but the results did not improve understanding of headache etiology or treatment. Many patients missed several days of school, but average attendance improved after months of treatment.
Long-term outcomes
Thirty-one survey respondents (94%) reported that their emotional, cognitive, sleep, and somatic postconcussion symptoms had resolved. Nevertheless, a majority of participants still had headache. “The persistence of postconcussion symptoms is uncommon, but lasting headache is not,” said the researchers. “If patients are not properly educated, conditions may deteriorate, extending the duration of disability.” A longer study with a larger sample size could provide valuable information, said the researchers. Future work should examine objectively the efficacy of various medications used to treat NPPCH and determine the best methods of treatment for this syndrome, which “can cause prolonged pain, suffering, and lack of function,” they concluded.
The investigators did not report any study funding or disclosures.
SOURCE: Barissi M et al. CNS 2019, Abstract 95.
CHARLOTTE, N.C. – , according to research presented at the annual meeting of the Child Neurology Society. The headache may be migraine, chronic daily headache, tension-type headache, or a combination of these headaches.
“We strongly recommend that individuals who develop persistent headache after a concussion be evaluated and treated by a neurologist with experience in administering treatment for headache,” said Marcus Barissi, Weller Scholar at the Cleveland Clinic, and colleagues. “Using this approach, we hope that their prolonged headaches will be lessened.”
Few studies have examined prolonged pediatric postconcussion headache
The Centers for Disease Control and Prevention estimates that between 1.6 million and 3.8 million concussions occur annually during athletic and recreational activities in the United States. About 90% of concussions affect children or adolescents. The symptom most often reported after concussion is headache.
Few studies have focused on new persistent postconcussion headache (NPPCH) in children. Mr. Barissi and colleagues did not find any previous study that had examined prolonged headache following concussion in patients without prior chronic headache. They sought to ascertain the prognosis of patients with NPPCH and no history of prior headache, to describe this clinical entity, and to identify beneficial treatment methods.
The investigators retrospectively reviewed charts for approximately 2,000 patients who presented to the Cleveland Clinic pediatric neurology department between June 2017 and August 2018 for headaches. They identified 259 patients who received a diagnosis of concussion, 69 (27%) of whom had headaches for longer than 2 months after injury.
Mr. Barissi and colleagues emailed these patients, and 33 (48%) of them agreed to complete a questionnaire and participate in a 10-minute phone interview. Thirty-one patients (43%) could not be contacted, and eight (11%) declined to participate. All participants confirmed that they had not had consistent headache before the concussion and that chronic headache had arisen after concussion. To determine participants’ medical outcomes, the researchers compared participants’ initial assessment data with posttreatment data collected during the interview process.
Healthy behaviors increased after concussion
Of the 69 eligible participants, 38 (55%) were female. The population’s median age was 17. Twenty-eight (85%) of the 33 patients who completed the questionnaire considered the information and treatment that they had received to be beneficial. Twenty-five (78%) patients continued to have headache after several months, despite treatment.
Participants had withstood a mean of 1.72 concussions, and the mean age at first injury was 12.49 years. The most common cause of injury was a fall for males (36%) and an automobile accident for females (18%).
Forty-eight patients (70%) reported having two types of headache. Fifty-two patients (75%) had migraines, and 65 (94%) had chronic daily headache or tension-type headache. Forty-eight (70%) participants had a family history of headache.
In all, 64 patients (93%) had used a headache medication. The most common headache medications used were amitriptyline, topiramate, and cyproheptadine. Few patients were still taking these medications at several months after evaluation. The most common nonprescription medications used were Migravent (i.e., magnesium, riboflavin, coenzyme Q10, and butterbur), ondansetron, and melatonin. Furthermore, 61 patients (88%) participated in nonmedicinal therapy such as physical therapy, chiropractic therapy, and acupuncture.
After evaluation, patients engaged in several healthy behaviors (e.g., adequate exercise, proper use of over-the-counter medications, and drinking sufficient water) more frequently, but did not get adequate sleep. Sixty-five participants (94%) had undergone CT or MRI imaging, but the results did not improve understanding of headache etiology or treatment. Many patients missed several days of school, but average attendance improved after months of treatment.
Long-term outcomes
Thirty-one survey respondents (94%) reported that their emotional, cognitive, sleep, and somatic postconcussion symptoms had resolved. Nevertheless, a majority of participants still had headache. “The persistence of postconcussion symptoms is uncommon, but lasting headache is not,” said the researchers. “If patients are not properly educated, conditions may deteriorate, extending the duration of disability.” A longer study with a larger sample size could provide valuable information, said the researchers. Future work should examine objectively the efficacy of various medications used to treat NPPCH and determine the best methods of treatment for this syndrome, which “can cause prolonged pain, suffering, and lack of function,” they concluded.
The investigators did not report any study funding or disclosures.
SOURCE: Barissi M et al. CNS 2019, Abstract 95.
REPORTING FROM CNS 2019