User login
Gray hair goes away and squids go to space
Goodbye stress, goodbye gray hair
Last year was a doozy, so it wouldn’t be too surprising if we all had a few new gray strands in our hair. But what if we told you that you don’t need to start dying them or plucking them out? What if they could magically go back to the way they were? Well, it may be possible, sans magic and sans stress.
Investigators recently discovered that the age-old belief that stress will permanently turn your hair gray may not be true after all. There’s a strong possibility that it could turn back to its original color once the stressful agent is eliminated.
“Understanding the mechanisms that allow ‘old’ gray hairs to return to their ‘young’ pigmented states could yield new clues about the malleability of human aging in general and how it is influenced by stress,” said senior author Martin Picard, PhD, of Columbia University, New York.
For the study, 14 volunteers were asked to keep a stress diary and review their levels of stress throughout the week. The researchers used a new method of viewing and capturing the images of tiny parts of the hairs to see how much graying took place in each part of the strand. And what they found – some strands naturally turning back to the original color – had never been documented before.
How did it happen? Our good friend the mitochondria. We haven’t really heard that word since eighth-grade biology, but it’s actually the key link between stress hormones and hair pigmentation. Think of them as little radars picking up all different kinds of signals in your body, like mental/emotional stress. They get a big enough alert and they’re going to react, thus gray hair.
So that’s all it takes? Cut the stress and a full head of gray can go back to brown? Not exactly. The researchers said there may be a “threshold because of biological age and other factors.” They believe middle age is near that threshold and it could easily be pushed over due to stress and could potentially go back. But if you’ve been rocking the salt and pepper or silver fox for a number of years and are looking for change, you might want to just eliminate the stress and pick up a bottle of dye.
One small step for squid
Space does a number on the human body. Forget the obvious like going for a walk outside without a spacesuit, or even the well-known risks like the degradation of bone in microgravity; there are numerous smaller but still important changes to the body during spaceflight, like the disruption of the symbiotic relationship between gut bacteria and the human body. This causes the immune system to lose the ability to recognize threats, and illnesses spread more easily.
Naturally, if astronauts are going to undertake years-long journeys to Mars and beyond, a thorough understanding of this disturbance is necessary, and that’s why NASA has sent a bunch of squid to the International Space Station.
When it comes to animal studies, squid aren’t the usual culprits, but there’s a reason NASA chose calamari over the alternatives: The Hawaiian bobtail squid has a symbiotic relationship with bacteria that regulate their bioluminescence in much the same way that we have a symbiotic relationship with our gut bacteria, but the squid is a much simpler animal. If the bioluminescence-regulating bacteria are disturbed during their time in space, it will be much easier to figure out what’s going wrong.
The experiment is ongoing, but we should salute the brave squid who have taken a giant leap for squidkind. Though if NASA didn’t send them up in a giant bubble, we’re going to be very disappointed.
Less plastic, more vanilla
Have you been racked by guilt over the number of plastic water bottles you use? What about the amount of ice cream you eat? Well, this one’s for you.
Plastic isn’t the first thing you think about when you open up a pint of vanilla ice cream and catch the sweet, spicy vanilla scent, or when you smell those fresh vanilla scones coming out of the oven at the coffee shop, but a new study shows that the flavor of vanilla can come from water bottles.
Here’s the deal. A compound called vanillin is responsible for the scent of vanilla, and it can come naturally from the bean or it can be made synthetically. Believe it or not, 85% of vanillin is made synthetically from fossil fuels!
We’ve definitely grown accustomed to our favorite vanilla scents, foods, and cosmetics. In 2018, the global demand for vanillin was about 40,800 tons and is expected to grow to 65,000 tons by 2025, which far exceeds the supply of natural vanilla.
So what can we do? Well, we can use genetically engineered bacteria to turn plastic water bottles into vanillin, according to a study published in the journal Green Chemistry.
The plastic can be broken down into terephthalic acid, which is very similar, chemically speaking, to vanillin. Similar enough that a bit of bioengineering produced Escherichia coli that could convert the acid into the tasty treat, according to researchers at the University of Edinburgh.
A perfect solution? Decreasing plastic waste while producing a valued food product? The thought of consuming plastic isn’t appetizing, so just eat your ice cream and try to forget about it.
No withdrawals from this bank
Into each life, some milestones must fall: High school graduation, birth of a child, first house, 50th wedding anniversary, COVID-19. One LOTME staffer got really excited – way too excited, actually – when his Nissan Sentra reached 300,000 miles.
Well, there are milestones, and then there are milestones. “1,000 Reasons for Hope” is a report celebrating the first 1,000 brains donated to the VA-BU-CLF Brain Bank. For those of you keeping score at home, that would be the Department of Veterans Affairs, Boston University, and the Concussion Legacy Foundation.
The Brain Bank, created in 2008 to study concussions and chronic traumatic encephalopathy, is the brainchild – yes, we went there – of Chris Nowinski, PhD, a former professional wrestler, and Ann McKee, MD, an expert on neurogenerative disease. “Our discoveries have already inspired changes to sports that will prevent many future cases of CTE in the next generation of athletes,” Dr. Nowinski, the CEO of CLF, said in a written statement.
Data from the first thousand brains show that 706 men, including 305 former NFL players, had football as their primary exposure to head impacts. Women were underrepresented, making up only 2.8% of brain donations, so recruiting females is a priority. Anyone interested in pledging can go to PledgeMyBrain.org or call 617-992-0615 for the 24-hour emergency donation pager.
LOTME wanted to help, so we called the Brain Bank to find out about donating. They asked a few questions and we told them what we do for a living. “Oh, you’re with LOTME? Yeah, we’ve … um, seen that before. It’s, um … funny. Can we put you on hold?” We’re starting to get a little sick of the on-hold music by now.
Goodbye stress, goodbye gray hair
Last year was a doozy, so it wouldn’t be too surprising if we all had a few new gray strands in our hair. But what if we told you that you don’t need to start dying them or plucking them out? What if they could magically go back to the way they were? Well, it may be possible, sans magic and sans stress.
Investigators recently discovered that the age-old belief that stress will permanently turn your hair gray may not be true after all. There’s a strong possibility that it could turn back to its original color once the stressful agent is eliminated.
“Understanding the mechanisms that allow ‘old’ gray hairs to return to their ‘young’ pigmented states could yield new clues about the malleability of human aging in general and how it is influenced by stress,” said senior author Martin Picard, PhD, of Columbia University, New York.
For the study, 14 volunteers were asked to keep a stress diary and review their levels of stress throughout the week. The researchers used a new method of viewing and capturing the images of tiny parts of the hairs to see how much graying took place in each part of the strand. And what they found – some strands naturally turning back to the original color – had never been documented before.
How did it happen? Our good friend the mitochondria. We haven’t really heard that word since eighth-grade biology, but it’s actually the key link between stress hormones and hair pigmentation. Think of them as little radars picking up all different kinds of signals in your body, like mental/emotional stress. They get a big enough alert and they’re going to react, thus gray hair.
So that’s all it takes? Cut the stress and a full head of gray can go back to brown? Not exactly. The researchers said there may be a “threshold because of biological age and other factors.” They believe middle age is near that threshold and it could easily be pushed over due to stress and could potentially go back. But if you’ve been rocking the salt and pepper or silver fox for a number of years and are looking for change, you might want to just eliminate the stress and pick up a bottle of dye.
One small step for squid
Space does a number on the human body. Forget the obvious like going for a walk outside without a spacesuit, or even the well-known risks like the degradation of bone in microgravity; there are numerous smaller but still important changes to the body during spaceflight, like the disruption of the symbiotic relationship between gut bacteria and the human body. This causes the immune system to lose the ability to recognize threats, and illnesses spread more easily.
Naturally, if astronauts are going to undertake years-long journeys to Mars and beyond, a thorough understanding of this disturbance is necessary, and that’s why NASA has sent a bunch of squid to the International Space Station.
When it comes to animal studies, squid aren’t the usual culprits, but there’s a reason NASA chose calamari over the alternatives: The Hawaiian bobtail squid has a symbiotic relationship with bacteria that regulate their bioluminescence in much the same way that we have a symbiotic relationship with our gut bacteria, but the squid is a much simpler animal. If the bioluminescence-regulating bacteria are disturbed during their time in space, it will be much easier to figure out what’s going wrong.
The experiment is ongoing, but we should salute the brave squid who have taken a giant leap for squidkind. Though if NASA didn’t send them up in a giant bubble, we’re going to be very disappointed.
Less plastic, more vanilla
Have you been racked by guilt over the number of plastic water bottles you use? What about the amount of ice cream you eat? Well, this one’s for you.
Plastic isn’t the first thing you think about when you open up a pint of vanilla ice cream and catch the sweet, spicy vanilla scent, or when you smell those fresh vanilla scones coming out of the oven at the coffee shop, but a new study shows that the flavor of vanilla can come from water bottles.
Here’s the deal. A compound called vanillin is responsible for the scent of vanilla, and it can come naturally from the bean or it can be made synthetically. Believe it or not, 85% of vanillin is made synthetically from fossil fuels!
We’ve definitely grown accustomed to our favorite vanilla scents, foods, and cosmetics. In 2018, the global demand for vanillin was about 40,800 tons and is expected to grow to 65,000 tons by 2025, which far exceeds the supply of natural vanilla.
So what can we do? Well, we can use genetically engineered bacteria to turn plastic water bottles into vanillin, according to a study published in the journal Green Chemistry.
The plastic can be broken down into terephthalic acid, which is very similar, chemically speaking, to vanillin. Similar enough that a bit of bioengineering produced Escherichia coli that could convert the acid into the tasty treat, according to researchers at the University of Edinburgh.
A perfect solution? Decreasing plastic waste while producing a valued food product? The thought of consuming plastic isn’t appetizing, so just eat your ice cream and try to forget about it.
No withdrawals from this bank
Into each life, some milestones must fall: High school graduation, birth of a child, first house, 50th wedding anniversary, COVID-19. One LOTME staffer got really excited – way too excited, actually – when his Nissan Sentra reached 300,000 miles.
Well, there are milestones, and then there are milestones. “1,000 Reasons for Hope” is a report celebrating the first 1,000 brains donated to the VA-BU-CLF Brain Bank. For those of you keeping score at home, that would be the Department of Veterans Affairs, Boston University, and the Concussion Legacy Foundation.
The Brain Bank, created in 2008 to study concussions and chronic traumatic encephalopathy, is the brainchild – yes, we went there – of Chris Nowinski, PhD, a former professional wrestler, and Ann McKee, MD, an expert on neurogenerative disease. “Our discoveries have already inspired changes to sports that will prevent many future cases of CTE in the next generation of athletes,” Dr. Nowinski, the CEO of CLF, said in a written statement.
Data from the first thousand brains show that 706 men, including 305 former NFL players, had football as their primary exposure to head impacts. Women were underrepresented, making up only 2.8% of brain donations, so recruiting females is a priority. Anyone interested in pledging can go to PledgeMyBrain.org or call 617-992-0615 for the 24-hour emergency donation pager.
LOTME wanted to help, so we called the Brain Bank to find out about donating. They asked a few questions and we told them what we do for a living. “Oh, you’re with LOTME? Yeah, we’ve … um, seen that before. It’s, um … funny. Can we put you on hold?” We’re starting to get a little sick of the on-hold music by now.
Goodbye stress, goodbye gray hair
Last year was a doozy, so it wouldn’t be too surprising if we all had a few new gray strands in our hair. But what if we told you that you don’t need to start dying them or plucking them out? What if they could magically go back to the way they were? Well, it may be possible, sans magic and sans stress.
Investigators recently discovered that the age-old belief that stress will permanently turn your hair gray may not be true after all. There’s a strong possibility that it could turn back to its original color once the stressful agent is eliminated.
“Understanding the mechanisms that allow ‘old’ gray hairs to return to their ‘young’ pigmented states could yield new clues about the malleability of human aging in general and how it is influenced by stress,” said senior author Martin Picard, PhD, of Columbia University, New York.
For the study, 14 volunteers were asked to keep a stress diary and review their levels of stress throughout the week. The researchers used a new method of viewing and capturing the images of tiny parts of the hairs to see how much graying took place in each part of the strand. And what they found – some strands naturally turning back to the original color – had never been documented before.
How did it happen? Our good friend the mitochondria. We haven’t really heard that word since eighth-grade biology, but it’s actually the key link between stress hormones and hair pigmentation. Think of them as little radars picking up all different kinds of signals in your body, like mental/emotional stress. They get a big enough alert and they’re going to react, thus gray hair.
So that’s all it takes? Cut the stress and a full head of gray can go back to brown? Not exactly. The researchers said there may be a “threshold because of biological age and other factors.” They believe middle age is near that threshold and it could easily be pushed over due to stress and could potentially go back. But if you’ve been rocking the salt and pepper or silver fox for a number of years and are looking for change, you might want to just eliminate the stress and pick up a bottle of dye.
One small step for squid
Space does a number on the human body. Forget the obvious like going for a walk outside without a spacesuit, or even the well-known risks like the degradation of bone in microgravity; there are numerous smaller but still important changes to the body during spaceflight, like the disruption of the symbiotic relationship between gut bacteria and the human body. This causes the immune system to lose the ability to recognize threats, and illnesses spread more easily.
Naturally, if astronauts are going to undertake years-long journeys to Mars and beyond, a thorough understanding of this disturbance is necessary, and that’s why NASA has sent a bunch of squid to the International Space Station.
When it comes to animal studies, squid aren’t the usual culprits, but there’s a reason NASA chose calamari over the alternatives: The Hawaiian bobtail squid has a symbiotic relationship with bacteria that regulate their bioluminescence in much the same way that we have a symbiotic relationship with our gut bacteria, but the squid is a much simpler animal. If the bioluminescence-regulating bacteria are disturbed during their time in space, it will be much easier to figure out what’s going wrong.
The experiment is ongoing, but we should salute the brave squid who have taken a giant leap for squidkind. Though if NASA didn’t send them up in a giant bubble, we’re going to be very disappointed.
Less plastic, more vanilla
Have you been racked by guilt over the number of plastic water bottles you use? What about the amount of ice cream you eat? Well, this one’s for you.
Plastic isn’t the first thing you think about when you open up a pint of vanilla ice cream and catch the sweet, spicy vanilla scent, or when you smell those fresh vanilla scones coming out of the oven at the coffee shop, but a new study shows that the flavor of vanilla can come from water bottles.
Here’s the deal. A compound called vanillin is responsible for the scent of vanilla, and it can come naturally from the bean or it can be made synthetically. Believe it or not, 85% of vanillin is made synthetically from fossil fuels!
We’ve definitely grown accustomed to our favorite vanilla scents, foods, and cosmetics. In 2018, the global demand for vanillin was about 40,800 tons and is expected to grow to 65,000 tons by 2025, which far exceeds the supply of natural vanilla.
So what can we do? Well, we can use genetically engineered bacteria to turn plastic water bottles into vanillin, according to a study published in the journal Green Chemistry.
The plastic can be broken down into terephthalic acid, which is very similar, chemically speaking, to vanillin. Similar enough that a bit of bioengineering produced Escherichia coli that could convert the acid into the tasty treat, according to researchers at the University of Edinburgh.
A perfect solution? Decreasing plastic waste while producing a valued food product? The thought of consuming plastic isn’t appetizing, so just eat your ice cream and try to forget about it.
No withdrawals from this bank
Into each life, some milestones must fall: High school graduation, birth of a child, first house, 50th wedding anniversary, COVID-19. One LOTME staffer got really excited – way too excited, actually – when his Nissan Sentra reached 300,000 miles.
Well, there are milestones, and then there are milestones. “1,000 Reasons for Hope” is a report celebrating the first 1,000 brains donated to the VA-BU-CLF Brain Bank. For those of you keeping score at home, that would be the Department of Veterans Affairs, Boston University, and the Concussion Legacy Foundation.
The Brain Bank, created in 2008 to study concussions and chronic traumatic encephalopathy, is the brainchild – yes, we went there – of Chris Nowinski, PhD, a former professional wrestler, and Ann McKee, MD, an expert on neurogenerative disease. “Our discoveries have already inspired changes to sports that will prevent many future cases of CTE in the next generation of athletes,” Dr. Nowinski, the CEO of CLF, said in a written statement.
Data from the first thousand brains show that 706 men, including 305 former NFL players, had football as their primary exposure to head impacts. Women were underrepresented, making up only 2.8% of brain donations, so recruiting females is a priority. Anyone interested in pledging can go to PledgeMyBrain.org or call 617-992-0615 for the 24-hour emergency donation pager.
LOTME wanted to help, so we called the Brain Bank to find out about donating. They asked a few questions and we told them what we do for a living. “Oh, you’re with LOTME? Yeah, we’ve … um, seen that before. It’s, um … funny. Can we put you on hold?” We’re starting to get a little sick of the on-hold music by now.
Many comatose TBI patients recover consciousness during rehab
according to a study of 3 decades of TBI survivors.
“Caution is warranted in consideration of withdrawing or withholding life-sustaining therapies in patients with severe TBI and DoC,” wrote Robert G. Kowalski, MBBCh, MS, of the department of neurology at the University of Colorado at Denver, Aurora, and colleagues. The study was published in JAMA Neurology.
To determine the likelihood of returning to consciousness in the weeks that follow a serious brain injury, along with any notable contributing factors, the researchers launched a retrospective analysis of 17,470 patients with moderate to severe TBI. All participants had been enrolled in the Traumatic Brain Injury Model Systems database from January 1989 to June 2019 after being admitted to any 1 of 23 inpatient rehabilitation centers. The cohort had a median age of 39 (interquartile range, 25-56), with 74% being male and 66% being white. Their median duration of acute hospital care was 16 days (IQR, 9-26).
Unconsciousness was defined by the researchers as not being able to follow commands or having a Glasgow Coma Scale motor score in the ED of lower than 6 or a Disability Rating Scale motor score greater than 0. Of the overall cohort, 7,547 (57%) patients initially lost consciousness and 2,058 (12%) remained unconscious as they were admitted to rehab. Of that subgroup, 1,674 (82%) recovered consciousness during rehab. The 414 patients who still had a DoC at completion of rehab had a longer median stay (37 days; IQR, 22-65), compared with the patients who recovered consciousness (19 days; IQR, 12-30; P < .001). After multivariable analysis, the factors most associated with recovery of consciousness were the absence of intraventricular hemorrhage (adjusted odds ratio, 0.678; 95% confidence interval, 0.532-0.863; P = .002) and the absence of intracranial mass effect (aOR, 0.759; 95% CI, 0.595-0.968; P = .03).
Though all patients experienced an improvement in functional status during rehabilitation, patients with DoC had an increase in median Functional Independence Measure total score from 19 to 71 while patients without DoC increased from 54 to 96 (change in total score, +43 versus +37; P = .002). After multivariate analysis, younger age and male sex were both associated with better functional outcomes during rehab and at discharge.
When it comes to TBI patients, don’t give up hope
The choice to withdraw care in TBI patients is a complicated and daunting one, and this study is further evidence that physicians should delay that decision in many scenarios, wrote Jennifer A. Kim, MD, PhD, and Kevin N. Sheth, MD, of Yale University, New Haven, Conn., in an accompanying editorial.
“By showing that a large proportion of patients with persistent DoC recover during acute rehabilitation, this article further challenges our potential toward overly nihilistic notions of who may or may not ultimately recover consciousness long term,” they added.
That said, they also recognized the questions that still persist: What are the reasons for late-stage withdrawal of lifesaving therapy? What is the recovery rate of all hospitalized patients with TBI, not just those in rehabilitation facilities? And is it possible to detect covert consciousness using MRI and electroencephalography, which this study did not include?
“Defining both good and poor prognostic risk factors is critical to portending recovery,” they wrote, emphasizing the need for physicians to rely on scientifically based predictions when making such important assessments.
Patience is a virtue for TBI specialists
“A lot of people write notes on hospital charts, ‘poor prognosis.’ You don’t know, that early in the game, in the acute care setting, how TBI patients are going to do,” said Jamie S. Ullman, MD, of the department of neurosurgery at Hofstra University, Hempstead, N.Y., in an interview. “It’s over the long term that we really have to judge that.”
“Of course, there may be some characteristics that patients might have that may portend for a worse outcome, like brain stem damage,” she added. “But in general, there is plenty of literature to suggest that not only can even the worst-looking patients have some kind of functional outcome but that it takes 18 months or more to actually realize an outcome from a traumatic brain injury.”
She emphasized that each patient with TBI is unique; beyond their current status, you have to consider the significance of their injury, the thoughts of their families or partner, and their own previously stated wishes and willingness to tolerate disability. Nonetheless, this study is another step toward distilling the “nihilistic thinking” that can lead physicians to expect the worst regarding patients who may still have a path toward a functional life.
“As traumatic brain injury specialists,” she said, “we need to see what we can do to give patients as good a chance as possible at a recovery.”
The authors acknowledged their study’s limitations, including an inability to account for 3 decades of variations in treatment regimens and its limited generalizability because of the cohort being composed of only TBI survivors admitted to inpatient rehab. In addition, they noted a possible referential bias for the study’s mostly young TBI patients in rehab facilities, another reason why these findings “may not be directly applicable to the overall population of patients with moderate or severe TBI.”
The study was funded by grants from the National Institute on Disability, Independent Living, and Rehabilitation Research; the Department of Health & Human Services; and the Veterans Health Administration Central Office VA TBI Model Systems Program of Research. The authors reported several potential conflicts of interest, including receiving grants and support from various government agencies and pharmaceutical companies.
according to a study of 3 decades of TBI survivors.
“Caution is warranted in consideration of withdrawing or withholding life-sustaining therapies in patients with severe TBI and DoC,” wrote Robert G. Kowalski, MBBCh, MS, of the department of neurology at the University of Colorado at Denver, Aurora, and colleagues. The study was published in JAMA Neurology.
To determine the likelihood of returning to consciousness in the weeks that follow a serious brain injury, along with any notable contributing factors, the researchers launched a retrospective analysis of 17,470 patients with moderate to severe TBI. All participants had been enrolled in the Traumatic Brain Injury Model Systems database from January 1989 to June 2019 after being admitted to any 1 of 23 inpatient rehabilitation centers. The cohort had a median age of 39 (interquartile range, 25-56), with 74% being male and 66% being white. Their median duration of acute hospital care was 16 days (IQR, 9-26).
Unconsciousness was defined by the researchers as not being able to follow commands or having a Glasgow Coma Scale motor score in the ED of lower than 6 or a Disability Rating Scale motor score greater than 0. Of the overall cohort, 7,547 (57%) patients initially lost consciousness and 2,058 (12%) remained unconscious as they were admitted to rehab. Of that subgroup, 1,674 (82%) recovered consciousness during rehab. The 414 patients who still had a DoC at completion of rehab had a longer median stay (37 days; IQR, 22-65), compared with the patients who recovered consciousness (19 days; IQR, 12-30; P < .001). After multivariable analysis, the factors most associated with recovery of consciousness were the absence of intraventricular hemorrhage (adjusted odds ratio, 0.678; 95% confidence interval, 0.532-0.863; P = .002) and the absence of intracranial mass effect (aOR, 0.759; 95% CI, 0.595-0.968; P = .03).
Though all patients experienced an improvement in functional status during rehabilitation, patients with DoC had an increase in median Functional Independence Measure total score from 19 to 71 while patients without DoC increased from 54 to 96 (change in total score, +43 versus +37; P = .002). After multivariate analysis, younger age and male sex were both associated with better functional outcomes during rehab and at discharge.
When it comes to TBI patients, don’t give up hope
The choice to withdraw care in TBI patients is a complicated and daunting one, and this study is further evidence that physicians should delay that decision in many scenarios, wrote Jennifer A. Kim, MD, PhD, and Kevin N. Sheth, MD, of Yale University, New Haven, Conn., in an accompanying editorial.
“By showing that a large proportion of patients with persistent DoC recover during acute rehabilitation, this article further challenges our potential toward overly nihilistic notions of who may or may not ultimately recover consciousness long term,” they added.
That said, they also recognized the questions that still persist: What are the reasons for late-stage withdrawal of lifesaving therapy? What is the recovery rate of all hospitalized patients with TBI, not just those in rehabilitation facilities? And is it possible to detect covert consciousness using MRI and electroencephalography, which this study did not include?
“Defining both good and poor prognostic risk factors is critical to portending recovery,” they wrote, emphasizing the need for physicians to rely on scientifically based predictions when making such important assessments.
Patience is a virtue for TBI specialists
“A lot of people write notes on hospital charts, ‘poor prognosis.’ You don’t know, that early in the game, in the acute care setting, how TBI patients are going to do,” said Jamie S. Ullman, MD, of the department of neurosurgery at Hofstra University, Hempstead, N.Y., in an interview. “It’s over the long term that we really have to judge that.”
“Of course, there may be some characteristics that patients might have that may portend for a worse outcome, like brain stem damage,” she added. “But in general, there is plenty of literature to suggest that not only can even the worst-looking patients have some kind of functional outcome but that it takes 18 months or more to actually realize an outcome from a traumatic brain injury.”
She emphasized that each patient with TBI is unique; beyond their current status, you have to consider the significance of their injury, the thoughts of their families or partner, and their own previously stated wishes and willingness to tolerate disability. Nonetheless, this study is another step toward distilling the “nihilistic thinking” that can lead physicians to expect the worst regarding patients who may still have a path toward a functional life.
“As traumatic brain injury specialists,” she said, “we need to see what we can do to give patients as good a chance as possible at a recovery.”
The authors acknowledged their study’s limitations, including an inability to account for 3 decades of variations in treatment regimens and its limited generalizability because of the cohort being composed of only TBI survivors admitted to inpatient rehab. In addition, they noted a possible referential bias for the study’s mostly young TBI patients in rehab facilities, another reason why these findings “may not be directly applicable to the overall population of patients with moderate or severe TBI.”
The study was funded by grants from the National Institute on Disability, Independent Living, and Rehabilitation Research; the Department of Health & Human Services; and the Veterans Health Administration Central Office VA TBI Model Systems Program of Research. The authors reported several potential conflicts of interest, including receiving grants and support from various government agencies and pharmaceutical companies.
according to a study of 3 decades of TBI survivors.
“Caution is warranted in consideration of withdrawing or withholding life-sustaining therapies in patients with severe TBI and DoC,” wrote Robert G. Kowalski, MBBCh, MS, of the department of neurology at the University of Colorado at Denver, Aurora, and colleagues. The study was published in JAMA Neurology.
To determine the likelihood of returning to consciousness in the weeks that follow a serious brain injury, along with any notable contributing factors, the researchers launched a retrospective analysis of 17,470 patients with moderate to severe TBI. All participants had been enrolled in the Traumatic Brain Injury Model Systems database from January 1989 to June 2019 after being admitted to any 1 of 23 inpatient rehabilitation centers. The cohort had a median age of 39 (interquartile range, 25-56), with 74% being male and 66% being white. Their median duration of acute hospital care was 16 days (IQR, 9-26).
Unconsciousness was defined by the researchers as not being able to follow commands or having a Glasgow Coma Scale motor score in the ED of lower than 6 or a Disability Rating Scale motor score greater than 0. Of the overall cohort, 7,547 (57%) patients initially lost consciousness and 2,058 (12%) remained unconscious as they were admitted to rehab. Of that subgroup, 1,674 (82%) recovered consciousness during rehab. The 414 patients who still had a DoC at completion of rehab had a longer median stay (37 days; IQR, 22-65), compared with the patients who recovered consciousness (19 days; IQR, 12-30; P < .001). After multivariable analysis, the factors most associated with recovery of consciousness were the absence of intraventricular hemorrhage (adjusted odds ratio, 0.678; 95% confidence interval, 0.532-0.863; P = .002) and the absence of intracranial mass effect (aOR, 0.759; 95% CI, 0.595-0.968; P = .03).
Though all patients experienced an improvement in functional status during rehabilitation, patients with DoC had an increase in median Functional Independence Measure total score from 19 to 71 while patients without DoC increased from 54 to 96 (change in total score, +43 versus +37; P = .002). After multivariate analysis, younger age and male sex were both associated with better functional outcomes during rehab and at discharge.
When it comes to TBI patients, don’t give up hope
The choice to withdraw care in TBI patients is a complicated and daunting one, and this study is further evidence that physicians should delay that decision in many scenarios, wrote Jennifer A. Kim, MD, PhD, and Kevin N. Sheth, MD, of Yale University, New Haven, Conn., in an accompanying editorial.
“By showing that a large proportion of patients with persistent DoC recover during acute rehabilitation, this article further challenges our potential toward overly nihilistic notions of who may or may not ultimately recover consciousness long term,” they added.
That said, they also recognized the questions that still persist: What are the reasons for late-stage withdrawal of lifesaving therapy? What is the recovery rate of all hospitalized patients with TBI, not just those in rehabilitation facilities? And is it possible to detect covert consciousness using MRI and electroencephalography, which this study did not include?
“Defining both good and poor prognostic risk factors is critical to portending recovery,” they wrote, emphasizing the need for physicians to rely on scientifically based predictions when making such important assessments.
Patience is a virtue for TBI specialists
“A lot of people write notes on hospital charts, ‘poor prognosis.’ You don’t know, that early in the game, in the acute care setting, how TBI patients are going to do,” said Jamie S. Ullman, MD, of the department of neurosurgery at Hofstra University, Hempstead, N.Y., in an interview. “It’s over the long term that we really have to judge that.”
“Of course, there may be some characteristics that patients might have that may portend for a worse outcome, like brain stem damage,” she added. “But in general, there is plenty of literature to suggest that not only can even the worst-looking patients have some kind of functional outcome but that it takes 18 months or more to actually realize an outcome from a traumatic brain injury.”
She emphasized that each patient with TBI is unique; beyond their current status, you have to consider the significance of their injury, the thoughts of their families or partner, and their own previously stated wishes and willingness to tolerate disability. Nonetheless, this study is another step toward distilling the “nihilistic thinking” that can lead physicians to expect the worst regarding patients who may still have a path toward a functional life.
“As traumatic brain injury specialists,” she said, “we need to see what we can do to give patients as good a chance as possible at a recovery.”
The authors acknowledged their study’s limitations, including an inability to account for 3 decades of variations in treatment regimens and its limited generalizability because of the cohort being composed of only TBI survivors admitted to inpatient rehab. In addition, they noted a possible referential bias for the study’s mostly young TBI patients in rehab facilities, another reason why these findings “may not be directly applicable to the overall population of patients with moderate or severe TBI.”
The study was funded by grants from the National Institute on Disability, Independent Living, and Rehabilitation Research; the Department of Health & Human Services; and the Veterans Health Administration Central Office VA TBI Model Systems Program of Research. The authors reported several potential conflicts of interest, including receiving grants and support from various government agencies and pharmaceutical companies.
FROM JAMA NEUROLOGY
A new biomarker of traumatic brain injury?
, new research suggests. “Reliable detection of this biomarker at very early time points may allow for prompt TBI detection and therefore intervention,” said study investigator Rachel Elizabeth Thomas, MD, PhD, a neurology resident at the University of Pennsylvania, Philadelphia, while presenting study findings at the American Academy of Neurology’s 2021 annual meeting.
“The level reflects the degree of severity and provides some degree of prognostic information,” she added.
A specific marker of acute injury?
Von Willebrand factor is a glycoprotein released in the endothelium in response to local trauma. It plays a part in hemostasis and inflammation and is an indicator of traumatic microvascular injury. Research has shown that it is a biomarker of cerebrovascular pathology. In addition, increased expression of the factor is associated with vascular and neurodegenerative dementia.
The researchers examined whether von Willebrand factor is a biomarker of mild, repetitive TBI. They measured plasma levels of von Willebrand factor in 17 professional boxers before and after boxing bouts.
Eligible participants were between the ages of 18 and 35 years. They had a score of greater than or equal to 1 on the Rivermead Post-Concussion Symptoms Questionnaire (RPQ-3), had competed in at least three 3-minute bouts, and had withstood 25 or more blows to the head.
The investigators compared the plasma levels of von Willebrand factor of the boxers with those of 42 patients who presented to the University of Pennsylvania Trauma Center with TBI and with those of 23 uninjured control persons.
There was no significant difference in plasma levels of von Willebrand factor between boxers before the bout (13.15 µg/mL) and the control persons (6.16 µg/mL). Among the boxers, levels of von Willebrand factor increased by a factor of 1.8 within 30 minutes after bouts, compared with the levels among the control persons. The mean post-bout von Willebrand factor level was 25.09 µg/mL.
“Von Willebrand factor may be more specific for acute injuries, given that it does not seem to stay chronically elevated,” said Dr. Thomas.
In addition, the researchers found a significant positive correlation (r = 0.51; P = .03) between the fold change in plasma von Willebrand factor levels and the number of blows to the head that the athletes sustained.
They also found a significant positive correlation between fold change in von Willebrand factor and RPQ-3 score (r = 0.69; P = .002). These objective and subjective data suggest that levels of von Willebrand factor reflect injury severity, said Dr. Thomas.
Among patients hospitalized with TBI, levels of von Willebrand factor were significantly higher than among control persons (73.2 µg/mL vs. 40.8 µg/mL; P < .0009). The investigators found a linear correlation between plasma von Willebrand factor level and RPQ-3 score (r = 0.24) that was not statistically significant.
Levels of von Willebrand factor among patients hospitalized with TBI were higher on average and demonstrated a greater degree of variability than the levels among boxers immediately after a bout.
“This is not unexpected, given that this group represents a more heterogeneous population with varied forms of acute blunt injury, as compared to the boxers, who have undergone relatively repetitive, milder trauma,” Dr. Thomas said.
The traditional biomarkers of neurotrauma reflect neuronal and glial injury, whereas von Willebrand factor is an indicator of vascular trauma.
“Although on its own, von Willebrand factor is not specific to intracranial vascular injury, paired together with markers such as neurofilament light, GFAP [glial fibrillary acidic protein], and tau, it could be utilized to identify TBI-associated microvascular injury and thus delineate between specific TBI endophenotypes,” said Dr. Thomas. It could distinguish, for example, predominantly neuronal injury from predominantly vascular injury.
Because von Willebrand factor plays a role in the neurovascular unit and is a marker of microvascular injury, the investigators intend to pair measurements of plasma von Willebrand factor with advanced imaging techniques to evaluate cerebral blood flow or cerebrovascular reactivity. Such a study could help determine whether von Willebrand factor levels correlate with the degree of vascular injury and cerebrovascular dysregulation.
Point-of-care test?
Commenting on the findings, Kristine O’Phelan, MD, professor of clinical neurology and director of neurocritical care in the department of neurology at the University of Miami, said von Willebrand factor’s likely utility would be as a marker of injury in patients with mild TBI or sports-related concussion.
Imaging and clinical exams do not always reveal these injuries, Dr. O’Phelan added. “Having a biomarker that you can easily test in the blood would be extremely helpful,” she said.
The most exciting part of this study is that it indicates the potential to develop a point-of-care test for use on the athletic field or the battlefield for early detection of mild TBI, she added.
The fact that the test for von Willebrand factor has already been developed is an advantage, said Dr. O’Phelan. The normal and abnormal values of the test are clearly understood. “I do think that they will still need to calibrate it for head injury, because that’s not usually what the test is used for,” said Dr. O’Phelan.
One of the study’s strengths is that the investigators compared patients with TBI with control persons who had exercised, she added, because such a comparison helps clarify the biomarker’s relationship to the injury. Another strength is the application of the test to injuries of various types and of different degrees of severity.
But the biomarker will need to be tested in a larger population, said Dr. O’Phelan. In addition, there is a need to identify the right patient population for this test, as well as the best time frame for its application and potential factors that could confound the test results.
“I do worry a little bit about using early biomarkers for prognosis, particularly in severe TBI, because there’s so many variables that go into outcome,” said Dr. O’Phelan. This test likely would be administered in the first hours after injury, but many factors might affect patients’ outcomes, she added.
One influential factor is age. “If you have a von Willebrand factor of whatever number, that might have different importance in a 30-year-old than in an 80-year-old,” said Dr. O’Phelan. “We need to understand how to interpret those findings better.”
The study was supported by the National Institute for Neurological Disorders and Stroke, the U.S. Department of Defense, and the Pennsylvania Department of Health. Dr. Thomas and Dr. O’Phelan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. “Reliable detection of this biomarker at very early time points may allow for prompt TBI detection and therefore intervention,” said study investigator Rachel Elizabeth Thomas, MD, PhD, a neurology resident at the University of Pennsylvania, Philadelphia, while presenting study findings at the American Academy of Neurology’s 2021 annual meeting.
“The level reflects the degree of severity and provides some degree of prognostic information,” she added.
A specific marker of acute injury?
Von Willebrand factor is a glycoprotein released in the endothelium in response to local trauma. It plays a part in hemostasis and inflammation and is an indicator of traumatic microvascular injury. Research has shown that it is a biomarker of cerebrovascular pathology. In addition, increased expression of the factor is associated with vascular and neurodegenerative dementia.
The researchers examined whether von Willebrand factor is a biomarker of mild, repetitive TBI. They measured plasma levels of von Willebrand factor in 17 professional boxers before and after boxing bouts.
Eligible participants were between the ages of 18 and 35 years. They had a score of greater than or equal to 1 on the Rivermead Post-Concussion Symptoms Questionnaire (RPQ-3), had competed in at least three 3-minute bouts, and had withstood 25 or more blows to the head.
The investigators compared the plasma levels of von Willebrand factor of the boxers with those of 42 patients who presented to the University of Pennsylvania Trauma Center with TBI and with those of 23 uninjured control persons.
There was no significant difference in plasma levels of von Willebrand factor between boxers before the bout (13.15 µg/mL) and the control persons (6.16 µg/mL). Among the boxers, levels of von Willebrand factor increased by a factor of 1.8 within 30 minutes after bouts, compared with the levels among the control persons. The mean post-bout von Willebrand factor level was 25.09 µg/mL.
“Von Willebrand factor may be more specific for acute injuries, given that it does not seem to stay chronically elevated,” said Dr. Thomas.
In addition, the researchers found a significant positive correlation (r = 0.51; P = .03) between the fold change in plasma von Willebrand factor levels and the number of blows to the head that the athletes sustained.
They also found a significant positive correlation between fold change in von Willebrand factor and RPQ-3 score (r = 0.69; P = .002). These objective and subjective data suggest that levels of von Willebrand factor reflect injury severity, said Dr. Thomas.
Among patients hospitalized with TBI, levels of von Willebrand factor were significantly higher than among control persons (73.2 µg/mL vs. 40.8 µg/mL; P < .0009). The investigators found a linear correlation between plasma von Willebrand factor level and RPQ-3 score (r = 0.24) that was not statistically significant.
Levels of von Willebrand factor among patients hospitalized with TBI were higher on average and demonstrated a greater degree of variability than the levels among boxers immediately after a bout.
“This is not unexpected, given that this group represents a more heterogeneous population with varied forms of acute blunt injury, as compared to the boxers, who have undergone relatively repetitive, milder trauma,” Dr. Thomas said.
The traditional biomarkers of neurotrauma reflect neuronal and glial injury, whereas von Willebrand factor is an indicator of vascular trauma.
“Although on its own, von Willebrand factor is not specific to intracranial vascular injury, paired together with markers such as neurofilament light, GFAP [glial fibrillary acidic protein], and tau, it could be utilized to identify TBI-associated microvascular injury and thus delineate between specific TBI endophenotypes,” said Dr. Thomas. It could distinguish, for example, predominantly neuronal injury from predominantly vascular injury.
Because von Willebrand factor plays a role in the neurovascular unit and is a marker of microvascular injury, the investigators intend to pair measurements of plasma von Willebrand factor with advanced imaging techniques to evaluate cerebral blood flow or cerebrovascular reactivity. Such a study could help determine whether von Willebrand factor levels correlate with the degree of vascular injury and cerebrovascular dysregulation.
Point-of-care test?
Commenting on the findings, Kristine O’Phelan, MD, professor of clinical neurology and director of neurocritical care in the department of neurology at the University of Miami, said von Willebrand factor’s likely utility would be as a marker of injury in patients with mild TBI or sports-related concussion.
Imaging and clinical exams do not always reveal these injuries, Dr. O’Phelan added. “Having a biomarker that you can easily test in the blood would be extremely helpful,” she said.
The most exciting part of this study is that it indicates the potential to develop a point-of-care test for use on the athletic field or the battlefield for early detection of mild TBI, she added.
The fact that the test for von Willebrand factor has already been developed is an advantage, said Dr. O’Phelan. The normal and abnormal values of the test are clearly understood. “I do think that they will still need to calibrate it for head injury, because that’s not usually what the test is used for,” said Dr. O’Phelan.
One of the study’s strengths is that the investigators compared patients with TBI with control persons who had exercised, she added, because such a comparison helps clarify the biomarker’s relationship to the injury. Another strength is the application of the test to injuries of various types and of different degrees of severity.
But the biomarker will need to be tested in a larger population, said Dr. O’Phelan. In addition, there is a need to identify the right patient population for this test, as well as the best time frame for its application and potential factors that could confound the test results.
“I do worry a little bit about using early biomarkers for prognosis, particularly in severe TBI, because there’s so many variables that go into outcome,” said Dr. O’Phelan. This test likely would be administered in the first hours after injury, but many factors might affect patients’ outcomes, she added.
One influential factor is age. “If you have a von Willebrand factor of whatever number, that might have different importance in a 30-year-old than in an 80-year-old,” said Dr. O’Phelan. “We need to understand how to interpret those findings better.”
The study was supported by the National Institute for Neurological Disorders and Stroke, the U.S. Department of Defense, and the Pennsylvania Department of Health. Dr. Thomas and Dr. O’Phelan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. “Reliable detection of this biomarker at very early time points may allow for prompt TBI detection and therefore intervention,” said study investigator Rachel Elizabeth Thomas, MD, PhD, a neurology resident at the University of Pennsylvania, Philadelphia, while presenting study findings at the American Academy of Neurology’s 2021 annual meeting.
“The level reflects the degree of severity and provides some degree of prognostic information,” she added.
A specific marker of acute injury?
Von Willebrand factor is a glycoprotein released in the endothelium in response to local trauma. It plays a part in hemostasis and inflammation and is an indicator of traumatic microvascular injury. Research has shown that it is a biomarker of cerebrovascular pathology. In addition, increased expression of the factor is associated with vascular and neurodegenerative dementia.
The researchers examined whether von Willebrand factor is a biomarker of mild, repetitive TBI. They measured plasma levels of von Willebrand factor in 17 professional boxers before and after boxing bouts.
Eligible participants were between the ages of 18 and 35 years. They had a score of greater than or equal to 1 on the Rivermead Post-Concussion Symptoms Questionnaire (RPQ-3), had competed in at least three 3-minute bouts, and had withstood 25 or more blows to the head.
The investigators compared the plasma levels of von Willebrand factor of the boxers with those of 42 patients who presented to the University of Pennsylvania Trauma Center with TBI and with those of 23 uninjured control persons.
There was no significant difference in plasma levels of von Willebrand factor between boxers before the bout (13.15 µg/mL) and the control persons (6.16 µg/mL). Among the boxers, levels of von Willebrand factor increased by a factor of 1.8 within 30 minutes after bouts, compared with the levels among the control persons. The mean post-bout von Willebrand factor level was 25.09 µg/mL.
“Von Willebrand factor may be more specific for acute injuries, given that it does not seem to stay chronically elevated,” said Dr. Thomas.
In addition, the researchers found a significant positive correlation (r = 0.51; P = .03) between the fold change in plasma von Willebrand factor levels and the number of blows to the head that the athletes sustained.
They also found a significant positive correlation between fold change in von Willebrand factor and RPQ-3 score (r = 0.69; P = .002). These objective and subjective data suggest that levels of von Willebrand factor reflect injury severity, said Dr. Thomas.
Among patients hospitalized with TBI, levels of von Willebrand factor were significantly higher than among control persons (73.2 µg/mL vs. 40.8 µg/mL; P < .0009). The investigators found a linear correlation between plasma von Willebrand factor level and RPQ-3 score (r = 0.24) that was not statistically significant.
Levels of von Willebrand factor among patients hospitalized with TBI were higher on average and demonstrated a greater degree of variability than the levels among boxers immediately after a bout.
“This is not unexpected, given that this group represents a more heterogeneous population with varied forms of acute blunt injury, as compared to the boxers, who have undergone relatively repetitive, milder trauma,” Dr. Thomas said.
The traditional biomarkers of neurotrauma reflect neuronal and glial injury, whereas von Willebrand factor is an indicator of vascular trauma.
“Although on its own, von Willebrand factor is not specific to intracranial vascular injury, paired together with markers such as neurofilament light, GFAP [glial fibrillary acidic protein], and tau, it could be utilized to identify TBI-associated microvascular injury and thus delineate between specific TBI endophenotypes,” said Dr. Thomas. It could distinguish, for example, predominantly neuronal injury from predominantly vascular injury.
Because von Willebrand factor plays a role in the neurovascular unit and is a marker of microvascular injury, the investigators intend to pair measurements of plasma von Willebrand factor with advanced imaging techniques to evaluate cerebral blood flow or cerebrovascular reactivity. Such a study could help determine whether von Willebrand factor levels correlate with the degree of vascular injury and cerebrovascular dysregulation.
Point-of-care test?
Commenting on the findings, Kristine O’Phelan, MD, professor of clinical neurology and director of neurocritical care in the department of neurology at the University of Miami, said von Willebrand factor’s likely utility would be as a marker of injury in patients with mild TBI or sports-related concussion.
Imaging and clinical exams do not always reveal these injuries, Dr. O’Phelan added. “Having a biomarker that you can easily test in the blood would be extremely helpful,” she said.
The most exciting part of this study is that it indicates the potential to develop a point-of-care test for use on the athletic field or the battlefield for early detection of mild TBI, she added.
The fact that the test for von Willebrand factor has already been developed is an advantage, said Dr. O’Phelan. The normal and abnormal values of the test are clearly understood. “I do think that they will still need to calibrate it for head injury, because that’s not usually what the test is used for,” said Dr. O’Phelan.
One of the study’s strengths is that the investigators compared patients with TBI with control persons who had exercised, she added, because such a comparison helps clarify the biomarker’s relationship to the injury. Another strength is the application of the test to injuries of various types and of different degrees of severity.
But the biomarker will need to be tested in a larger population, said Dr. O’Phelan. In addition, there is a need to identify the right patient population for this test, as well as the best time frame for its application and potential factors that could confound the test results.
“I do worry a little bit about using early biomarkers for prognosis, particularly in severe TBI, because there’s so many variables that go into outcome,” said Dr. O’Phelan. This test likely would be administered in the first hours after injury, but many factors might affect patients’ outcomes, she added.
One influential factor is age. “If you have a von Willebrand factor of whatever number, that might have different importance in a 30-year-old than in an 80-year-old,” said Dr. O’Phelan. “We need to understand how to interpret those findings better.”
The study was supported by the National Institute for Neurological Disorders and Stroke, the U.S. Department of Defense, and the Pennsylvania Department of Health. Dr. Thomas and Dr. O’Phelan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
From AAN 2021
Gastrointestinal Symptoms and Lactic Acidosis in a Chronic Marijuana User
A 57-year-old woman with a history of traumatic brain injury, posttraumatic stress disorder, depression, migraines, hypothyroidism, and a hiatal hernia repair presented to the emergency department with a 1-day history of nausea, vomiting, and diffuse abdominal pain. She reported that her symptoms were relieved by hot showers. She also reported having similar symptoms and a previous gastric-emptying study that showed a slow-emptying stomach. Her history also consisted of frequent cannabis use for mood and appetite stimulation along with eliminating meat and fish from her diet, an increase in consumption of simple carbohydrates in the past year, and no alcohol use. Her medications included topiramate 100 mg and clonidine 0.3 mg nightly for migraines; levothyroxine 200 mcg daily for hypothyroidism; tizanidine 4 mg twice a day for muscle spasm; famotidine 40 mg twice a day as needed for gastric reflux; and bupropion 50 mg daily, citalopram 20 mg daily, and lamotrigine 25 mg nightly for mood.
The patient’s physical examination was notable for bradycardia (43 beats/min) and epigastric tenderness. Admission laboratory results were notable for an elevated lactic acid level of 4.8 (normal range, 0.50-2.20) mmol/L and a leukocytosis count of 10.8×109 cells/L. Serum alcohol level and blood cultures were negative. Liver function test, hemoglobin A1c, and lipase test were unremarkable. Her electrocardiogram showed an unchanged right bundle branch block. Chest X-ray, computed tomography (CT) of her abdomen/pelvis and echocardiogram were unremarkable.
What is your diagnosis?
How would you treat this patient?
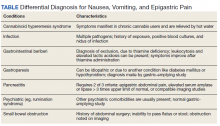
This patient was diagnosed with gastrointestinal beriberi. Because of her dietary changes, lactic acidosis, and bradycardia, thiamine deficiency was suspected after ruling out other possibilities on the differential diagnosis (Table). The patient’s symptoms resolved after administration of high-dose IV thiamine 500 mg 3 times daily for 4 days. Her white blood cell count and lactic acid level normalized. Unfortunately, thiamine levels were not obtained for the patient before treatment was initiated. After administration of IV thiamine, her plasma thiamine level was > 1,200 (normal range, 8-30) nmol/L.
Her differential diagnosis included infectious etiology. Given her leukocytosis and lactic acidosis, vancomycin and piperacillin/tazobactam were started on admission. One day later, her leukocytosis count doubled to 20.7×109 cells/L. However, after 48 hours of negative blood cultures, antibiotics were discontinued.
Small bowel obstruction was suspected due to the patient’s history of abdominal surgery but was ruled out with CT imaging. Similarly, pancreatitis was ruled out based on negative CT imaging and the patient’s normal lipase level. Gastroparesis also was considered because of the patient’s history of hypothyroidism, tobacco use, and her prior gastric-emptying study. The patient was treated for gastroparesis with a course of metoclopramide and erythromycin without improvement in symptoms. Additionally, gastroparesis would not explain the patient’s leukocytosis.
Cannabinoid hyperemesis syndrome (CHS) was suspected because the patient’s symptoms improved with cannabis discontinuation and hot showers.1 In chronic users, however, tetrahydrocannabinol levels have a half-life of 5 to 13 days.2 Although lactic acidosis and leukocytosis have been previously reported with cannabis use, it is unlikely that the patient would have such significant improvement within the first 4 days after discontinuation.1,3,4 Although the patient had many psychiatric comorbidities with previous hospitalizations describing concern for somatization disorder, her leukocytosis and elevated lactic acid levels were suggestive of an organic rather than a psychiatric etiology of her symptoms.
Discussion
Gastrointestinal beriberi has been reported in chronic cannabis users who present with nausea, vomiting, epigastric pain, leukocytosis, and lactic acidosis; all these symptoms rapidly improve after thiamine administration.5,6 The patient’s dietary change also eliminated her intake of vitamin B12, which compounded her condition. Thiamine deficiency produces lactic acidosis by disrupting pyruvate metabolism.7 Bradycardia also can be a sign of thiamine deficiency, although the patient’s use of clonidine for migraines is a confounder.8
Chronically ill patients are prone to nutritional deficiencies, including deficiencies of thiamine.7,9 Many patients with chronic illnesses also use cannabis to ameliorate physical and neuropsychiatric symptoms.2 Recent reports suggest cannabis users are prone to gastrointestinal beriberi and Wernicke encephalopathy.5,10 Treating gastrointestinal symptoms in these patients can be challenging to diagnose because gastrointestinal beriberi and CHS share many clinical manifestations.
The patient’s presentation is likely multifactorial resulting from the combination of gastrointestinal beriberi and CHS. However, thiamine deficiency seems to play the dominant role.
There is no standard treatment regimen for thiamine deficiency with neurologic deficits, and patients only retain about 10 to 15% of intramuscular (IM) injections of cyanocobalamin.11,12 The British Committee for Standards in Haematology recommends IM injections of 1,000 mcg of cyanocobalamin 3 times a week for 2 weeks and then reassess the need for continued treatment.13 The British Columbia guidelines also recommend IM injections of 1,000 mcg daily for 1 to 5 days before transitioning to oral repletion.14 European Neurology guidelines for the treatment of Wernicke encephalopathy recommend IV cyanocobalamin 200 mg 3 times daily.15 Low-level evidence with observational studies informs these decisions and is why there is variation.
The patient’s serum lactate and leukocytosis normalized 1 day after the administration of thiamine. Thiamine deficiency classically causes Wernicke encephalopathy and wet beriberi.16 The patient did not present with Wernicke encephalopathy’s triad: ophthalmoplegia, ataxia, or confusion. She also was euvolemic without signs or symptoms of wet beriberi.
Conclusions
Thiamine deficiency is principally a clinical diagnosis. Thiamine laboratory testing may not be readily available in all medical centers, and confirming a diagnosis of thiamine deficiency should not delay treatment when thiamine deficiency is suspected. This patient’s thiamine levels resulted a week after collection. The administration of thiamine before sampling also can alter the result as it did in this case. Additionally, laboratories may offer whole blood and serum testing. Whole blood testing is more accurate because most bioactive thiamine is found in red blood cells.17
1. Price SL, Fisher C, Kumar R, Hilgerson A. Cannabinoid hyperemesis syndrome as the underlying cause of intractable nausea and vomiting. J Am Osteopath Assoc. 2011;111(3):166-169. doi:10.7556/jaoa.2011.111.3.166
2. Sharma P, Murthy P, Bharath MM. Chemistry, metabolism, and toxicology of cannabis: clinical implications. Iran J Psychiatry. 2012;7(4):149-156.
3. Antill T, Jakkoju A, Dieguez J, Laskhmiprasad L. Lactic acidosis: a rare manifestation of synthetic marijuana intoxication. J La State Med Soc. 2015;167(3):155.
4. Sullivan S. Cannabinoid hyperemesis. Can J Gastroenterol. 2010;24(5):284-285. doi:10.1155/2010/481940
5. Duca J, Lum CJ, Lo AM. Elevated lactate secondary to gastrointestinal beriberi. J Gen Intern Med. 2016;31(1):133-136. doi:10.1007/s11606-015-3326-2
6. Prakash S. Gastrointestinal beriberi: a forme fruste of Wernicke’s encephalopathy? BMJ Case Rep. 2018;bcr2018224841. doi:10.1136/bcr-2018-224841
7. Friedenberg AS, Brandoff DE, Schiffman FJ. Type B lactic acidosis as a severe metabolic complication in lymphoma and leukemia: a case series from a single institution and literature review. Medicine (Baltimore). 2007;86(4):225-232. doi:10.1097/MD.0b013e318125759a
8. Liang CC. Bradycardia in thiamin deficiency and the role of glyoxylate. J Nutrition Sci Vitaminology. 1977;23(1):1-6. doi:10.3177/jnsv.23.1
9. Attaluri P, Castillo A, Edriss H, Nugent K. Thiamine deficiency: an important consideration in critically ill patients. Am J Med Sci. 2018;356(4):382-390. doi:10.1016/j.amjms.2018.06.015
10. Chaudhari A, Li ZY, Long A, Afshinnik A. Heavy cannabis use associated with Wernicke’s encephalopathy. Cureus. 2019;11(7):e5109. doi:10.7759/cureus.5109
11. Stabler SP. Vitamin B12 deficiency. N Engl J Med. 2013;368(2):149-160. doi:10.1056/NEJMcp1113996
12. Green R, Allen LH, Bjørke-Monsen A-L, et al. Vitamin B12 deficiency. Nat Rev Dis Primers. 2017;3(1):17040. doi:10.1038/nrdp.2017.40
13. Devalia V, Hamilton MS, Molloy AM. Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br J Haematol. 2014;166(4):496-513. doi:10.1111/bjh.12959
14. British Columbia Ministry of Health; Guidelines and Protocols and Advisory Committee. Guidelines and protocols cobalamin (vitamin B12) deficiency–investigation & management. Effective January 1, 2012. Revised May 1, 2013. Accessed March 10, 2021. https://www2.gov.bc.ca/gov/content/health/practitioner-professional-resources/bc-guidelines/vitamin-b12
15. Galvin R, Brathen G, Ivashynka A, Hillbom M, Tanasescu R, Leone MA. EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. Eur J Neurol. 2010;17(12):1408-1418. doi:10.1111/j.1468-1331.2010.03153.x
16. Wiley KD, Gupta M. Vitamin B1 thiamine deficiency (beriberi). In: StatPearls. StatPearls Publishing LLC; 2019.
17. Jenco J, Krcmova LK, Solichova D, Solich P. Recent trends in determination of thiamine and its derivatives in clinical practice. J Chromatogra A. 2017;1510:1-12. doi:10.1016/j.chroma.2017.06.048
A 57-year-old woman with a history of traumatic brain injury, posttraumatic stress disorder, depression, migraines, hypothyroidism, and a hiatal hernia repair presented to the emergency department with a 1-day history of nausea, vomiting, and diffuse abdominal pain. She reported that her symptoms were relieved by hot showers. She also reported having similar symptoms and a previous gastric-emptying study that showed a slow-emptying stomach. Her history also consisted of frequent cannabis use for mood and appetite stimulation along with eliminating meat and fish from her diet, an increase in consumption of simple carbohydrates in the past year, and no alcohol use. Her medications included topiramate 100 mg and clonidine 0.3 mg nightly for migraines; levothyroxine 200 mcg daily for hypothyroidism; tizanidine 4 mg twice a day for muscle spasm; famotidine 40 mg twice a day as needed for gastric reflux; and bupropion 50 mg daily, citalopram 20 mg daily, and lamotrigine 25 mg nightly for mood.
The patient’s physical examination was notable for bradycardia (43 beats/min) and epigastric tenderness. Admission laboratory results were notable for an elevated lactic acid level of 4.8 (normal range, 0.50-2.20) mmol/L and a leukocytosis count of 10.8×109 cells/L. Serum alcohol level and blood cultures were negative. Liver function test, hemoglobin A1c, and lipase test were unremarkable. Her electrocardiogram showed an unchanged right bundle branch block. Chest X-ray, computed tomography (CT) of her abdomen/pelvis and echocardiogram were unremarkable.
What is your diagnosis?
How would you treat this patient?
This patient was diagnosed with gastrointestinal beriberi. Because of her dietary changes, lactic acidosis, and bradycardia, thiamine deficiency was suspected after ruling out other possibilities on the differential diagnosis (Table). The patient’s symptoms resolved after administration of high-dose IV thiamine 500 mg 3 times daily for 4 days. Her white blood cell count and lactic acid level normalized. Unfortunately, thiamine levels were not obtained for the patient before treatment was initiated. After administration of IV thiamine, her plasma thiamine level was > 1,200 (normal range, 8-30) nmol/L.
Her differential diagnosis included infectious etiology. Given her leukocytosis and lactic acidosis, vancomycin and piperacillin/tazobactam were started on admission. One day later, her leukocytosis count doubled to 20.7×109 cells/L. However, after 48 hours of negative blood cultures, antibiotics were discontinued.
Small bowel obstruction was suspected due to the patient’s history of abdominal surgery but was ruled out with CT imaging. Similarly, pancreatitis was ruled out based on negative CT imaging and the patient’s normal lipase level. Gastroparesis also was considered because of the patient’s history of hypothyroidism, tobacco use, and her prior gastric-emptying study. The patient was treated for gastroparesis with a course of metoclopramide and erythromycin without improvement in symptoms. Additionally, gastroparesis would not explain the patient’s leukocytosis.
Cannabinoid hyperemesis syndrome (CHS) was suspected because the patient’s symptoms improved with cannabis discontinuation and hot showers.1 In chronic users, however, tetrahydrocannabinol levels have a half-life of 5 to 13 days.2 Although lactic acidosis and leukocytosis have been previously reported with cannabis use, it is unlikely that the patient would have such significant improvement within the first 4 days after discontinuation.1,3,4 Although the patient had many psychiatric comorbidities with previous hospitalizations describing concern for somatization disorder, her leukocytosis and elevated lactic acid levels were suggestive of an organic rather than a psychiatric etiology of her symptoms.
Discussion
Gastrointestinal beriberi has been reported in chronic cannabis users who present with nausea, vomiting, epigastric pain, leukocytosis, and lactic acidosis; all these symptoms rapidly improve after thiamine administration.5,6 The patient’s dietary change also eliminated her intake of vitamin B12, which compounded her condition. Thiamine deficiency produces lactic acidosis by disrupting pyruvate metabolism.7 Bradycardia also can be a sign of thiamine deficiency, although the patient’s use of clonidine for migraines is a confounder.8
Chronically ill patients are prone to nutritional deficiencies, including deficiencies of thiamine.7,9 Many patients with chronic illnesses also use cannabis to ameliorate physical and neuropsychiatric symptoms.2 Recent reports suggest cannabis users are prone to gastrointestinal beriberi and Wernicke encephalopathy.5,10 Treating gastrointestinal symptoms in these patients can be challenging to diagnose because gastrointestinal beriberi and CHS share many clinical manifestations.
The patient’s presentation is likely multifactorial resulting from the combination of gastrointestinal beriberi and CHS. However, thiamine deficiency seems to play the dominant role.
There is no standard treatment regimen for thiamine deficiency with neurologic deficits, and patients only retain about 10 to 15% of intramuscular (IM) injections of cyanocobalamin.11,12 The British Committee for Standards in Haematology recommends IM injections of 1,000 mcg of cyanocobalamin 3 times a week for 2 weeks and then reassess the need for continued treatment.13 The British Columbia guidelines also recommend IM injections of 1,000 mcg daily for 1 to 5 days before transitioning to oral repletion.14 European Neurology guidelines for the treatment of Wernicke encephalopathy recommend IV cyanocobalamin 200 mg 3 times daily.15 Low-level evidence with observational studies informs these decisions and is why there is variation.
The patient’s serum lactate and leukocytosis normalized 1 day after the administration of thiamine. Thiamine deficiency classically causes Wernicke encephalopathy and wet beriberi.16 The patient did not present with Wernicke encephalopathy’s triad: ophthalmoplegia, ataxia, or confusion. She also was euvolemic without signs or symptoms of wet beriberi.
Conclusions
Thiamine deficiency is principally a clinical diagnosis. Thiamine laboratory testing may not be readily available in all medical centers, and confirming a diagnosis of thiamine deficiency should not delay treatment when thiamine deficiency is suspected. This patient’s thiamine levels resulted a week after collection. The administration of thiamine before sampling also can alter the result as it did in this case. Additionally, laboratories may offer whole blood and serum testing. Whole blood testing is more accurate because most bioactive thiamine is found in red blood cells.17
A 57-year-old woman with a history of traumatic brain injury, posttraumatic stress disorder, depression, migraines, hypothyroidism, and a hiatal hernia repair presented to the emergency department with a 1-day history of nausea, vomiting, and diffuse abdominal pain. She reported that her symptoms were relieved by hot showers. She also reported having similar symptoms and a previous gastric-emptying study that showed a slow-emptying stomach. Her history also consisted of frequent cannabis use for mood and appetite stimulation along with eliminating meat and fish from her diet, an increase in consumption of simple carbohydrates in the past year, and no alcohol use. Her medications included topiramate 100 mg and clonidine 0.3 mg nightly for migraines; levothyroxine 200 mcg daily for hypothyroidism; tizanidine 4 mg twice a day for muscle spasm; famotidine 40 mg twice a day as needed for gastric reflux; and bupropion 50 mg daily, citalopram 20 mg daily, and lamotrigine 25 mg nightly for mood.
The patient’s physical examination was notable for bradycardia (43 beats/min) and epigastric tenderness. Admission laboratory results were notable for an elevated lactic acid level of 4.8 (normal range, 0.50-2.20) mmol/L and a leukocytosis count of 10.8×109 cells/L. Serum alcohol level and blood cultures were negative. Liver function test, hemoglobin A1c, and lipase test were unremarkable. Her electrocardiogram showed an unchanged right bundle branch block. Chest X-ray, computed tomography (CT) of her abdomen/pelvis and echocardiogram were unremarkable.
What is your diagnosis?
How would you treat this patient?
This patient was diagnosed with gastrointestinal beriberi. Because of her dietary changes, lactic acidosis, and bradycardia, thiamine deficiency was suspected after ruling out other possibilities on the differential diagnosis (Table). The patient’s symptoms resolved after administration of high-dose IV thiamine 500 mg 3 times daily for 4 days. Her white blood cell count and lactic acid level normalized. Unfortunately, thiamine levels were not obtained for the patient before treatment was initiated. After administration of IV thiamine, her plasma thiamine level was > 1,200 (normal range, 8-30) nmol/L.
Her differential diagnosis included infectious etiology. Given her leukocytosis and lactic acidosis, vancomycin and piperacillin/tazobactam were started on admission. One day later, her leukocytosis count doubled to 20.7×109 cells/L. However, after 48 hours of negative blood cultures, antibiotics were discontinued.
Small bowel obstruction was suspected due to the patient’s history of abdominal surgery but was ruled out with CT imaging. Similarly, pancreatitis was ruled out based on negative CT imaging and the patient’s normal lipase level. Gastroparesis also was considered because of the patient’s history of hypothyroidism, tobacco use, and her prior gastric-emptying study. The patient was treated for gastroparesis with a course of metoclopramide and erythromycin without improvement in symptoms. Additionally, gastroparesis would not explain the patient’s leukocytosis.
Cannabinoid hyperemesis syndrome (CHS) was suspected because the patient’s symptoms improved with cannabis discontinuation and hot showers.1 In chronic users, however, tetrahydrocannabinol levels have a half-life of 5 to 13 days.2 Although lactic acidosis and leukocytosis have been previously reported with cannabis use, it is unlikely that the patient would have such significant improvement within the first 4 days after discontinuation.1,3,4 Although the patient had many psychiatric comorbidities with previous hospitalizations describing concern for somatization disorder, her leukocytosis and elevated lactic acid levels were suggestive of an organic rather than a psychiatric etiology of her symptoms.
Discussion
Gastrointestinal beriberi has been reported in chronic cannabis users who present with nausea, vomiting, epigastric pain, leukocytosis, and lactic acidosis; all these symptoms rapidly improve after thiamine administration.5,6 The patient’s dietary change also eliminated her intake of vitamin B12, which compounded her condition. Thiamine deficiency produces lactic acidosis by disrupting pyruvate metabolism.7 Bradycardia also can be a sign of thiamine deficiency, although the patient’s use of clonidine for migraines is a confounder.8
Chronically ill patients are prone to nutritional deficiencies, including deficiencies of thiamine.7,9 Many patients with chronic illnesses also use cannabis to ameliorate physical and neuropsychiatric symptoms.2 Recent reports suggest cannabis users are prone to gastrointestinal beriberi and Wernicke encephalopathy.5,10 Treating gastrointestinal symptoms in these patients can be challenging to diagnose because gastrointestinal beriberi and CHS share many clinical manifestations.
The patient’s presentation is likely multifactorial resulting from the combination of gastrointestinal beriberi and CHS. However, thiamine deficiency seems to play the dominant role.
There is no standard treatment regimen for thiamine deficiency with neurologic deficits, and patients only retain about 10 to 15% of intramuscular (IM) injections of cyanocobalamin.11,12 The British Committee for Standards in Haematology recommends IM injections of 1,000 mcg of cyanocobalamin 3 times a week for 2 weeks and then reassess the need for continued treatment.13 The British Columbia guidelines also recommend IM injections of 1,000 mcg daily for 1 to 5 days before transitioning to oral repletion.14 European Neurology guidelines for the treatment of Wernicke encephalopathy recommend IV cyanocobalamin 200 mg 3 times daily.15 Low-level evidence with observational studies informs these decisions and is why there is variation.
The patient’s serum lactate and leukocytosis normalized 1 day after the administration of thiamine. Thiamine deficiency classically causes Wernicke encephalopathy and wet beriberi.16 The patient did not present with Wernicke encephalopathy’s triad: ophthalmoplegia, ataxia, or confusion. She also was euvolemic without signs or symptoms of wet beriberi.
Conclusions
Thiamine deficiency is principally a clinical diagnosis. Thiamine laboratory testing may not be readily available in all medical centers, and confirming a diagnosis of thiamine deficiency should not delay treatment when thiamine deficiency is suspected. This patient’s thiamine levels resulted a week after collection. The administration of thiamine before sampling also can alter the result as it did in this case. Additionally, laboratories may offer whole blood and serum testing. Whole blood testing is more accurate because most bioactive thiamine is found in red blood cells.17
1. Price SL, Fisher C, Kumar R, Hilgerson A. Cannabinoid hyperemesis syndrome as the underlying cause of intractable nausea and vomiting. J Am Osteopath Assoc. 2011;111(3):166-169. doi:10.7556/jaoa.2011.111.3.166
2. Sharma P, Murthy P, Bharath MM. Chemistry, metabolism, and toxicology of cannabis: clinical implications. Iran J Psychiatry. 2012;7(4):149-156.
3. Antill T, Jakkoju A, Dieguez J, Laskhmiprasad L. Lactic acidosis: a rare manifestation of synthetic marijuana intoxication. J La State Med Soc. 2015;167(3):155.
4. Sullivan S. Cannabinoid hyperemesis. Can J Gastroenterol. 2010;24(5):284-285. doi:10.1155/2010/481940
5. Duca J, Lum CJ, Lo AM. Elevated lactate secondary to gastrointestinal beriberi. J Gen Intern Med. 2016;31(1):133-136. doi:10.1007/s11606-015-3326-2
6. Prakash S. Gastrointestinal beriberi: a forme fruste of Wernicke’s encephalopathy? BMJ Case Rep. 2018;bcr2018224841. doi:10.1136/bcr-2018-224841
7. Friedenberg AS, Brandoff DE, Schiffman FJ. Type B lactic acidosis as a severe metabolic complication in lymphoma and leukemia: a case series from a single institution and literature review. Medicine (Baltimore). 2007;86(4):225-232. doi:10.1097/MD.0b013e318125759a
8. Liang CC. Bradycardia in thiamin deficiency and the role of glyoxylate. J Nutrition Sci Vitaminology. 1977;23(1):1-6. doi:10.3177/jnsv.23.1
9. Attaluri P, Castillo A, Edriss H, Nugent K. Thiamine deficiency: an important consideration in critically ill patients. Am J Med Sci. 2018;356(4):382-390. doi:10.1016/j.amjms.2018.06.015
10. Chaudhari A, Li ZY, Long A, Afshinnik A. Heavy cannabis use associated with Wernicke’s encephalopathy. Cureus. 2019;11(7):e5109. doi:10.7759/cureus.5109
11. Stabler SP. Vitamin B12 deficiency. N Engl J Med. 2013;368(2):149-160. doi:10.1056/NEJMcp1113996
12. Green R, Allen LH, Bjørke-Monsen A-L, et al. Vitamin B12 deficiency. Nat Rev Dis Primers. 2017;3(1):17040. doi:10.1038/nrdp.2017.40
13. Devalia V, Hamilton MS, Molloy AM. Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br J Haematol. 2014;166(4):496-513. doi:10.1111/bjh.12959
14. British Columbia Ministry of Health; Guidelines and Protocols and Advisory Committee. Guidelines and protocols cobalamin (vitamin B12) deficiency–investigation & management. Effective January 1, 2012. Revised May 1, 2013. Accessed March 10, 2021. https://www2.gov.bc.ca/gov/content/health/practitioner-professional-resources/bc-guidelines/vitamin-b12
15. Galvin R, Brathen G, Ivashynka A, Hillbom M, Tanasescu R, Leone MA. EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. Eur J Neurol. 2010;17(12):1408-1418. doi:10.1111/j.1468-1331.2010.03153.x
16. Wiley KD, Gupta M. Vitamin B1 thiamine deficiency (beriberi). In: StatPearls. StatPearls Publishing LLC; 2019.
17. Jenco J, Krcmova LK, Solichova D, Solich P. Recent trends in determination of thiamine and its derivatives in clinical practice. J Chromatogra A. 2017;1510:1-12. doi:10.1016/j.chroma.2017.06.048
1. Price SL, Fisher C, Kumar R, Hilgerson A. Cannabinoid hyperemesis syndrome as the underlying cause of intractable nausea and vomiting. J Am Osteopath Assoc. 2011;111(3):166-169. doi:10.7556/jaoa.2011.111.3.166
2. Sharma P, Murthy P, Bharath MM. Chemistry, metabolism, and toxicology of cannabis: clinical implications. Iran J Psychiatry. 2012;7(4):149-156.
3. Antill T, Jakkoju A, Dieguez J, Laskhmiprasad L. Lactic acidosis: a rare manifestation of synthetic marijuana intoxication. J La State Med Soc. 2015;167(3):155.
4. Sullivan S. Cannabinoid hyperemesis. Can J Gastroenterol. 2010;24(5):284-285. doi:10.1155/2010/481940
5. Duca J, Lum CJ, Lo AM. Elevated lactate secondary to gastrointestinal beriberi. J Gen Intern Med. 2016;31(1):133-136. doi:10.1007/s11606-015-3326-2
6. Prakash S. Gastrointestinal beriberi: a forme fruste of Wernicke’s encephalopathy? BMJ Case Rep. 2018;bcr2018224841. doi:10.1136/bcr-2018-224841
7. Friedenberg AS, Brandoff DE, Schiffman FJ. Type B lactic acidosis as a severe metabolic complication in lymphoma and leukemia: a case series from a single institution and literature review. Medicine (Baltimore). 2007;86(4):225-232. doi:10.1097/MD.0b013e318125759a
8. Liang CC. Bradycardia in thiamin deficiency and the role of glyoxylate. J Nutrition Sci Vitaminology. 1977;23(1):1-6. doi:10.3177/jnsv.23.1
9. Attaluri P, Castillo A, Edriss H, Nugent K. Thiamine deficiency: an important consideration in critically ill patients. Am J Med Sci. 2018;356(4):382-390. doi:10.1016/j.amjms.2018.06.015
10. Chaudhari A, Li ZY, Long A, Afshinnik A. Heavy cannabis use associated with Wernicke’s encephalopathy. Cureus. 2019;11(7):e5109. doi:10.7759/cureus.5109
11. Stabler SP. Vitamin B12 deficiency. N Engl J Med. 2013;368(2):149-160. doi:10.1056/NEJMcp1113996
12. Green R, Allen LH, Bjørke-Monsen A-L, et al. Vitamin B12 deficiency. Nat Rev Dis Primers. 2017;3(1):17040. doi:10.1038/nrdp.2017.40
13. Devalia V, Hamilton MS, Molloy AM. Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br J Haematol. 2014;166(4):496-513. doi:10.1111/bjh.12959
14. British Columbia Ministry of Health; Guidelines and Protocols and Advisory Committee. Guidelines and protocols cobalamin (vitamin B12) deficiency–investigation & management. Effective January 1, 2012. Revised May 1, 2013. Accessed March 10, 2021. https://www2.gov.bc.ca/gov/content/health/practitioner-professional-resources/bc-guidelines/vitamin-b12
15. Galvin R, Brathen G, Ivashynka A, Hillbom M, Tanasescu R, Leone MA. EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. Eur J Neurol. 2010;17(12):1408-1418. doi:10.1111/j.1468-1331.2010.03153.x
16. Wiley KD, Gupta M. Vitamin B1 thiamine deficiency (beriberi). In: StatPearls. StatPearls Publishing LLC; 2019.
17. Jenco J, Krcmova LK, Solichova D, Solich P. Recent trends in determination of thiamine and its derivatives in clinical practice. J Chromatogra A. 2017;1510:1-12. doi:10.1016/j.chroma.2017.06.048
Role of Speech Pathology in a Multidisciplinary Approach to a Patient With Mild Traumatic Brain Injury
Speech-language pathologists can fill a unique need in the treatment of patients with several conditions that are seen regularly in primary care.
Speech-language pathologists (SLPs) are integral to the comprehensive treatment of mild traumatic brain injury (mTBI), yet the evaluation and treatment options they offer may not be known to all primary care providers (PCPs). As the research on the management and treatment of mTBI continues to evolve, the PCPs role in referring patients with mTBI to the appropriate resources becomes imperative.
mTBI is a common injury in both military and civilian settings, but it can be difficult to diagnose and is not always well understood. Long-term debilitating effects have been associated with mTBI, with literature linking it to an increased risk of developing Alzheimer disease, motor neuron disease, and Parkinson disease.1 In addition, mTBI is a strong predictor for the development of posttraumatic stress disorder (PTSD). Among returning Iraq and Afghanistan service members, the incidence of mTBI associated mental health conditions have been reported to be as high as 22.8%, affecting > 320,000 veterans.2-5
The US Department of Veteran Affairs (VA) health care system offers these returning veterans a comprehensive, multidisciplinary treatment strategy. The care is often coordinated by the veteran’s patient aligned care team (PACT) that consists of a PCP, nurses, and a medical support associate. The US Department of Defense (DoD) and VA also facilitated the development of a clinical practice guideline (CPG) that can be used by the PACT and other health care providers to support evidence based patient-centered care. This CPG is extensive and has recommendations for evaluation and treatment of mTBI and the symptoms associated such as impaired memory and alterations in executive function.6
The following hypothetical case is based on an actual patient. This case illustrates the role of speech pathology in caring for patients with mTBI.
Case Presentation
A 25-year-old male combat veteran presented to his VA PACT team for a new patient visit. As part of the screening of his medical history, mTBI was fully defined for the patient to include “alteration” in consciousness. This reminded the patient of an injury that occurred 1 year prior to presentation during a routine convoy mission. He was riding in the back of a Humvee when it hit a large pothole slamming his head into the side of the vehicle. He reported that he felt “dazed and dizzy” with “ringing” in his ears immediately following the event, without an overt loss of consciousness. He was unable to seek medical attention secondary to the urgency of the convoy mission, so he “shook it off” and kept going. Later that week he noted headache and insomnia. He was seen and evaluated by his health care provider for insomnia, but when questioned he reported no head trauma as he had forgotten the incident. Upon follow-up with his PCP, he reported his headaches were manageable, and his insomnia was somewhat improved with recommended life-style modifications and good sleep hygiene.
He still had frequent headaches, dizziness, and some insomnia. However, his chief concern was that he was struggling with new schoolwork. He noted that he was a straight-A student prior to his military service. A review of his medical history in his medical chart showed that a previous PCP had treated his associated symptoms of insomnia and headache without improvement. In addition, he had recently been diagnosed with PTSD. As his symptoms had lasted > 90 days, not resolved with initial treatment in primary care, and were causing a significant impact on his activities of daily living, his PCP placed a consult to Speech Pathology for cognitive-linguistic assessment and treatment, if indicated, noting that he may have had a mTBI.6 Although not intended to be comprehensive, Table 1 describes several clinical areas where a speech pathology referral may be appropriate.
The Role of the Speech-Language Pathologist
The speech-language pathologist takes an additional history of the patient. This better quantifies specific details of the veteran’s functional concerns pertaining to possible difficulty with attention, memory, executive function, visuospatial awareness, etc. Examples might include difficulty with attention/memory, including not remembering what to get at the store, forgetting to take medications, forgetting appointments, and difficulty in school, among many others. Reports of feeling “stupid” also are common. Assessment varies by clinician, but it is not uncommon for the SLP to administer a battery of evaluations to help identify a range of possible impairments. Choosing testing that is sensitive to even mild impairment is important and should be used in combination with subjective complaints. Mild deficits can sometimes be missed in those with average performance, but whose premorbid intelligence was above average. One combination of test batteries sometimes utilized is the Wechsler Test of Adult Reading (WTAR), the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), the Ruff Figural Fluency Test (RFFT), the Controlled Oral Word Association Test (COWAT), and Trails A and B (Table 2).
The initial testing results are discussed with the veteran. If patient concerns and/or testing reveal impairment that is amenable to treatment and the veteran wishes to proceed, subsequent treatment sessions are scheduled. The first treatment session is spent establishing and prioritizing functional goals specific to that individual and their needs (eg, for daily life, work, school). In a case of subacute or older mTBI, as is often seen in veterans coming to the VA, intervention often targets strategies and techniques that can help the individual compensate for current deficits.
Many patients already own a smartphone, so this device often is used functionally as a cognitive prosthetic as early as the first treatment session. In an effort to immediately start addressing important issues like medication management and attending appointments, the veteran is educated to the benefit of entering important information into the calendar and/or reminder apps on their phone and setting associated alarms that would serve as a reminder for what was entered. Patients are often encouraged by the positive impact of these initial strategies and look forward to future treatment sessions to address compensation for their functional deficits.
If a veteran with TBI has numerous needs, it can be beneficial for the care team to discuss the care plan at an interdisciplinary team meeting. It is not uncommon for veterans like the one discussed above to be referred to neurology (persistent headaches and further neurological evaluation); mental health (PTSD treatment and family support/counseling options); occupational therapy (visuospatial needs); and audiology (vestibular concerns). Social work involvement is often extremely beneficial for coordination of care in more complex cases. If patient is having difficulty making healthy eating choices or with meal preparation, a consult to a dietitian may prove invaluable. Concerns related to trouble with medication adherence (beyond memory-related adherence issues that speech pathology would address) or polypharmacy can be directed to a clinical pharmacy specialist, who could prepare a medication chart, review optimal medication timing, and provide education on adverse effects. A veteran's communication with the team can be facilitated through secure messaging (a method of secure emailing) and encouraging use of the My HealtheVet portal. With this modality, patients could review chart notes and results and share them with non-VA health care providers and/or family members as indicated.
A whole health approach also may appeal to some mTBI patients. This approach focuses on the totality of patient needs for healthy living and on patient-centered goal setting. Services provided may differ at various VA medical centers, but the PACT team can connect the veteran to the services of interest.
Conclusions
A team approach to veterans with mTBI provides a comprehensive way to treat the various problems associated with the condition. Further research into the role of multidisciplinary teams in the management of mTBI was recommended in the 2016 CPG.6 The unique role that the speech-language pathologist plays as part of this team has been highlighted, as it is important that PCP’s be aware of the extent of evaluation and treatment services they offer. Beyond mTBI, speech pathologists evaluate and treat patients with several conditions that are seen regularly in primary care.
1. McKee AC, Robinson ME. Military-related traumatic brain injury and neurodegeneration. Alzheimers Dement. 2014;10(3 suppl):S242-S253. doi:10.1016/j.jalz.2014.04.003
2. Yurgil KA, Barkauskas DA, Vasterling JJ, et al. Association between traumatic brain injury and risk of posttraumatic stress disorder in active-duty Marines. JAMA Psychiatry. 2014;71(2):149-157. doi:10.1001/jamapsychiatry.2013.3080
3. Chin DL, Zeber JE. Mental Health Outcomes Among Military Service Members After Severe Injury in Combat and TBI. Mil Med. 2020;185(5-6):e711-e718. doi:10.1093/milmed/usz440
4. Hoge CW, Auchterlonie JL, Milliken CS. Mental health problems, use of mental health services, and attrition from military service after returning from deployment to Iraq or Afghanistan. JAMA. 2006;295(9):1023-1032. doi:10.1001/jama.295.9.1023
5. Miles SR, Harik JM, Hundt NE, et al. Delivery of mental health treatment to combat veterans with psychiatric diagnoses and TBI histories. PLoS One. 2017;12(9):e0184265. Published 2017 Sep 8. doi:10.1371/journal.pone.0184265
6. US Department of Defense, US Department of Veterans Affairs; Management of Concussion/mTBI Working Group. VA/DoD clinical practice guideline for management of concussion/mild traumatic brain injury. Version 2.0. Published February 2016. Accessed February 8, 2021. https://www.healthquality.va.gov/guidelines/Rehab/mtbi/mTBICPGFullCPG50821816.pdf
Speech-language pathologists can fill a unique need in the treatment of patients with several conditions that are seen regularly in primary care.
Speech-language pathologists can fill a unique need in the treatment of patients with several conditions that are seen regularly in primary care.
Speech-language pathologists (SLPs) are integral to the comprehensive treatment of mild traumatic brain injury (mTBI), yet the evaluation and treatment options they offer may not be known to all primary care providers (PCPs). As the research on the management and treatment of mTBI continues to evolve, the PCPs role in referring patients with mTBI to the appropriate resources becomes imperative.
mTBI is a common injury in both military and civilian settings, but it can be difficult to diagnose and is not always well understood. Long-term debilitating effects have been associated with mTBI, with literature linking it to an increased risk of developing Alzheimer disease, motor neuron disease, and Parkinson disease.1 In addition, mTBI is a strong predictor for the development of posttraumatic stress disorder (PTSD). Among returning Iraq and Afghanistan service members, the incidence of mTBI associated mental health conditions have been reported to be as high as 22.8%, affecting > 320,000 veterans.2-5
The US Department of Veteran Affairs (VA) health care system offers these returning veterans a comprehensive, multidisciplinary treatment strategy. The care is often coordinated by the veteran’s patient aligned care team (PACT) that consists of a PCP, nurses, and a medical support associate. The US Department of Defense (DoD) and VA also facilitated the development of a clinical practice guideline (CPG) that can be used by the PACT and other health care providers to support evidence based patient-centered care. This CPG is extensive and has recommendations for evaluation and treatment of mTBI and the symptoms associated such as impaired memory and alterations in executive function.6
The following hypothetical case is based on an actual patient. This case illustrates the role of speech pathology in caring for patients with mTBI.
Case Presentation
A 25-year-old male combat veteran presented to his VA PACT team for a new patient visit. As part of the screening of his medical history, mTBI was fully defined for the patient to include “alteration” in consciousness. This reminded the patient of an injury that occurred 1 year prior to presentation during a routine convoy mission. He was riding in the back of a Humvee when it hit a large pothole slamming his head into the side of the vehicle. He reported that he felt “dazed and dizzy” with “ringing” in his ears immediately following the event, without an overt loss of consciousness. He was unable to seek medical attention secondary to the urgency of the convoy mission, so he “shook it off” and kept going. Later that week he noted headache and insomnia. He was seen and evaluated by his health care provider for insomnia, but when questioned he reported no head trauma as he had forgotten the incident. Upon follow-up with his PCP, he reported his headaches were manageable, and his insomnia was somewhat improved with recommended life-style modifications and good sleep hygiene.
He still had frequent headaches, dizziness, and some insomnia. However, his chief concern was that he was struggling with new schoolwork. He noted that he was a straight-A student prior to his military service. A review of his medical history in his medical chart showed that a previous PCP had treated his associated symptoms of insomnia and headache without improvement. In addition, he had recently been diagnosed with PTSD. As his symptoms had lasted > 90 days, not resolved with initial treatment in primary care, and were causing a significant impact on his activities of daily living, his PCP placed a consult to Speech Pathology for cognitive-linguistic assessment and treatment, if indicated, noting that he may have had a mTBI.6 Although not intended to be comprehensive, Table 1 describes several clinical areas where a speech pathology referral may be appropriate.
The Role of the Speech-Language Pathologist
The speech-language pathologist takes an additional history of the patient. This better quantifies specific details of the veteran’s functional concerns pertaining to possible difficulty with attention, memory, executive function, visuospatial awareness, etc. Examples might include difficulty with attention/memory, including not remembering what to get at the store, forgetting to take medications, forgetting appointments, and difficulty in school, among many others. Reports of feeling “stupid” also are common. Assessment varies by clinician, but it is not uncommon for the SLP to administer a battery of evaluations to help identify a range of possible impairments. Choosing testing that is sensitive to even mild impairment is important and should be used in combination with subjective complaints. Mild deficits can sometimes be missed in those with average performance, but whose premorbid intelligence was above average. One combination of test batteries sometimes utilized is the Wechsler Test of Adult Reading (WTAR), the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), the Ruff Figural Fluency Test (RFFT), the Controlled Oral Word Association Test (COWAT), and Trails A and B (Table 2).
The initial testing results are discussed with the veteran. If patient concerns and/or testing reveal impairment that is amenable to treatment and the veteran wishes to proceed, subsequent treatment sessions are scheduled. The first treatment session is spent establishing and prioritizing functional goals specific to that individual and their needs (eg, for daily life, work, school). In a case of subacute or older mTBI, as is often seen in veterans coming to the VA, intervention often targets strategies and techniques that can help the individual compensate for current deficits.
Many patients already own a smartphone, so this device often is used functionally as a cognitive prosthetic as early as the first treatment session. In an effort to immediately start addressing important issues like medication management and attending appointments, the veteran is educated to the benefit of entering important information into the calendar and/or reminder apps on their phone and setting associated alarms that would serve as a reminder for what was entered. Patients are often encouraged by the positive impact of these initial strategies and look forward to future treatment sessions to address compensation for their functional deficits.
If a veteran with TBI has numerous needs, it can be beneficial for the care team to discuss the care plan at an interdisciplinary team meeting. It is not uncommon for veterans like the one discussed above to be referred to neurology (persistent headaches and further neurological evaluation); mental health (PTSD treatment and family support/counseling options); occupational therapy (visuospatial needs); and audiology (vestibular concerns). Social work involvement is often extremely beneficial for coordination of care in more complex cases. If patient is having difficulty making healthy eating choices or with meal preparation, a consult to a dietitian may prove invaluable. Concerns related to trouble with medication adherence (beyond memory-related adherence issues that speech pathology would address) or polypharmacy can be directed to a clinical pharmacy specialist, who could prepare a medication chart, review optimal medication timing, and provide education on adverse effects. A veteran's communication with the team can be facilitated through secure messaging (a method of secure emailing) and encouraging use of the My HealtheVet portal. With this modality, patients could review chart notes and results and share them with non-VA health care providers and/or family members as indicated.
A whole health approach also may appeal to some mTBI patients. This approach focuses on the totality of patient needs for healthy living and on patient-centered goal setting. Services provided may differ at various VA medical centers, but the PACT team can connect the veteran to the services of interest.
Conclusions
A team approach to veterans with mTBI provides a comprehensive way to treat the various problems associated with the condition. Further research into the role of multidisciplinary teams in the management of mTBI was recommended in the 2016 CPG.6 The unique role that the speech-language pathologist plays as part of this team has been highlighted, as it is important that PCP’s be aware of the extent of evaluation and treatment services they offer. Beyond mTBI, speech pathologists evaluate and treat patients with several conditions that are seen regularly in primary care.
Speech-language pathologists (SLPs) are integral to the comprehensive treatment of mild traumatic brain injury (mTBI), yet the evaluation and treatment options they offer may not be known to all primary care providers (PCPs). As the research on the management and treatment of mTBI continues to evolve, the PCPs role in referring patients with mTBI to the appropriate resources becomes imperative.
mTBI is a common injury in both military and civilian settings, but it can be difficult to diagnose and is not always well understood. Long-term debilitating effects have been associated with mTBI, with literature linking it to an increased risk of developing Alzheimer disease, motor neuron disease, and Parkinson disease.1 In addition, mTBI is a strong predictor for the development of posttraumatic stress disorder (PTSD). Among returning Iraq and Afghanistan service members, the incidence of mTBI associated mental health conditions have been reported to be as high as 22.8%, affecting > 320,000 veterans.2-5
The US Department of Veteran Affairs (VA) health care system offers these returning veterans a comprehensive, multidisciplinary treatment strategy. The care is often coordinated by the veteran’s patient aligned care team (PACT) that consists of a PCP, nurses, and a medical support associate. The US Department of Defense (DoD) and VA also facilitated the development of a clinical practice guideline (CPG) that can be used by the PACT and other health care providers to support evidence based patient-centered care. This CPG is extensive and has recommendations for evaluation and treatment of mTBI and the symptoms associated such as impaired memory and alterations in executive function.6
The following hypothetical case is based on an actual patient. This case illustrates the role of speech pathology in caring for patients with mTBI.
Case Presentation
A 25-year-old male combat veteran presented to his VA PACT team for a new patient visit. As part of the screening of his medical history, mTBI was fully defined for the patient to include “alteration” in consciousness. This reminded the patient of an injury that occurred 1 year prior to presentation during a routine convoy mission. He was riding in the back of a Humvee when it hit a large pothole slamming his head into the side of the vehicle. He reported that he felt “dazed and dizzy” with “ringing” in his ears immediately following the event, without an overt loss of consciousness. He was unable to seek medical attention secondary to the urgency of the convoy mission, so he “shook it off” and kept going. Later that week he noted headache and insomnia. He was seen and evaluated by his health care provider for insomnia, but when questioned he reported no head trauma as he had forgotten the incident. Upon follow-up with his PCP, he reported his headaches were manageable, and his insomnia was somewhat improved with recommended life-style modifications and good sleep hygiene.
He still had frequent headaches, dizziness, and some insomnia. However, his chief concern was that he was struggling with new schoolwork. He noted that he was a straight-A student prior to his military service. A review of his medical history in his medical chart showed that a previous PCP had treated his associated symptoms of insomnia and headache without improvement. In addition, he had recently been diagnosed with PTSD. As his symptoms had lasted > 90 days, not resolved with initial treatment in primary care, and were causing a significant impact on his activities of daily living, his PCP placed a consult to Speech Pathology for cognitive-linguistic assessment and treatment, if indicated, noting that he may have had a mTBI.6 Although not intended to be comprehensive, Table 1 describes several clinical areas where a speech pathology referral may be appropriate.
The Role of the Speech-Language Pathologist
The speech-language pathologist takes an additional history of the patient. This better quantifies specific details of the veteran’s functional concerns pertaining to possible difficulty with attention, memory, executive function, visuospatial awareness, etc. Examples might include difficulty with attention/memory, including not remembering what to get at the store, forgetting to take medications, forgetting appointments, and difficulty in school, among many others. Reports of feeling “stupid” also are common. Assessment varies by clinician, but it is not uncommon for the SLP to administer a battery of evaluations to help identify a range of possible impairments. Choosing testing that is sensitive to even mild impairment is important and should be used in combination with subjective complaints. Mild deficits can sometimes be missed in those with average performance, but whose premorbid intelligence was above average. One combination of test batteries sometimes utilized is the Wechsler Test of Adult Reading (WTAR), the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), the Ruff Figural Fluency Test (RFFT), the Controlled Oral Word Association Test (COWAT), and Trails A and B (Table 2).
The initial testing results are discussed with the veteran. If patient concerns and/or testing reveal impairment that is amenable to treatment and the veteran wishes to proceed, subsequent treatment sessions are scheduled. The first treatment session is spent establishing and prioritizing functional goals specific to that individual and their needs (eg, for daily life, work, school). In a case of subacute or older mTBI, as is often seen in veterans coming to the VA, intervention often targets strategies and techniques that can help the individual compensate for current deficits.
Many patients already own a smartphone, so this device often is used functionally as a cognitive prosthetic as early as the first treatment session. In an effort to immediately start addressing important issues like medication management and attending appointments, the veteran is educated to the benefit of entering important information into the calendar and/or reminder apps on their phone and setting associated alarms that would serve as a reminder for what was entered. Patients are often encouraged by the positive impact of these initial strategies and look forward to future treatment sessions to address compensation for their functional deficits.
If a veteran with TBI has numerous needs, it can be beneficial for the care team to discuss the care plan at an interdisciplinary team meeting. It is not uncommon for veterans like the one discussed above to be referred to neurology (persistent headaches and further neurological evaluation); mental health (PTSD treatment and family support/counseling options); occupational therapy (visuospatial needs); and audiology (vestibular concerns). Social work involvement is often extremely beneficial for coordination of care in more complex cases. If patient is having difficulty making healthy eating choices or with meal preparation, a consult to a dietitian may prove invaluable. Concerns related to trouble with medication adherence (beyond memory-related adherence issues that speech pathology would address) or polypharmacy can be directed to a clinical pharmacy specialist, who could prepare a medication chart, review optimal medication timing, and provide education on adverse effects. A veteran's communication with the team can be facilitated through secure messaging (a method of secure emailing) and encouraging use of the My HealtheVet portal. With this modality, patients could review chart notes and results and share them with non-VA health care providers and/or family members as indicated.
A whole health approach also may appeal to some mTBI patients. This approach focuses on the totality of patient needs for healthy living and on patient-centered goal setting. Services provided may differ at various VA medical centers, but the PACT team can connect the veteran to the services of interest.
Conclusions
A team approach to veterans with mTBI provides a comprehensive way to treat the various problems associated with the condition. Further research into the role of multidisciplinary teams in the management of mTBI was recommended in the 2016 CPG.6 The unique role that the speech-language pathologist plays as part of this team has been highlighted, as it is important that PCP’s be aware of the extent of evaluation and treatment services they offer. Beyond mTBI, speech pathologists evaluate and treat patients with several conditions that are seen regularly in primary care.
1. McKee AC, Robinson ME. Military-related traumatic brain injury and neurodegeneration. Alzheimers Dement. 2014;10(3 suppl):S242-S253. doi:10.1016/j.jalz.2014.04.003
2. Yurgil KA, Barkauskas DA, Vasterling JJ, et al. Association between traumatic brain injury and risk of posttraumatic stress disorder in active-duty Marines. JAMA Psychiatry. 2014;71(2):149-157. doi:10.1001/jamapsychiatry.2013.3080
3. Chin DL, Zeber JE. Mental Health Outcomes Among Military Service Members After Severe Injury in Combat and TBI. Mil Med. 2020;185(5-6):e711-e718. doi:10.1093/milmed/usz440
4. Hoge CW, Auchterlonie JL, Milliken CS. Mental health problems, use of mental health services, and attrition from military service after returning from deployment to Iraq or Afghanistan. JAMA. 2006;295(9):1023-1032. doi:10.1001/jama.295.9.1023
5. Miles SR, Harik JM, Hundt NE, et al. Delivery of mental health treatment to combat veterans with psychiatric diagnoses and TBI histories. PLoS One. 2017;12(9):e0184265. Published 2017 Sep 8. doi:10.1371/journal.pone.0184265
6. US Department of Defense, US Department of Veterans Affairs; Management of Concussion/mTBI Working Group. VA/DoD clinical practice guideline for management of concussion/mild traumatic brain injury. Version 2.0. Published February 2016. Accessed February 8, 2021. https://www.healthquality.va.gov/guidelines/Rehab/mtbi/mTBICPGFullCPG50821816.pdf
1. McKee AC, Robinson ME. Military-related traumatic brain injury and neurodegeneration. Alzheimers Dement. 2014;10(3 suppl):S242-S253. doi:10.1016/j.jalz.2014.04.003
2. Yurgil KA, Barkauskas DA, Vasterling JJ, et al. Association between traumatic brain injury and risk of posttraumatic stress disorder in active-duty Marines. JAMA Psychiatry. 2014;71(2):149-157. doi:10.1001/jamapsychiatry.2013.3080
3. Chin DL, Zeber JE. Mental Health Outcomes Among Military Service Members After Severe Injury in Combat and TBI. Mil Med. 2020;185(5-6):e711-e718. doi:10.1093/milmed/usz440
4. Hoge CW, Auchterlonie JL, Milliken CS. Mental health problems, use of mental health services, and attrition from military service after returning from deployment to Iraq or Afghanistan. JAMA. 2006;295(9):1023-1032. doi:10.1001/jama.295.9.1023
5. Miles SR, Harik JM, Hundt NE, et al. Delivery of mental health treatment to combat veterans with psychiatric diagnoses and TBI histories. PLoS One. 2017;12(9):e0184265. Published 2017 Sep 8. doi:10.1371/journal.pone.0184265
6. US Department of Defense, US Department of Veterans Affairs; Management of Concussion/mTBI Working Group. VA/DoD clinical practice guideline for management of concussion/mild traumatic brain injury. Version 2.0. Published February 2016. Accessed February 8, 2021. https://www.healthquality.va.gov/guidelines/Rehab/mtbi/mTBICPGFullCPG50821816.pdf
Neurologic disorders ubiquitous and rising in the U.S.
, according to new findings derived from the 2017 Global Burden of Disease study.
The authors of the analysis, led by Valery Feigin, MD, PhD, of New Zealand’s National Institute for Stroke and Applied Neurosciences, and published in the February 2021 issue of JAMA Neurology, looked at prevalence, incidence, mortality, and disability-adjusted life years for 14 neurological disorders across 50 states between 1990 and 2017. The diseases included in the analysis were stroke, Alzheimer’s disease and other dementias, Parkinson’s disease, epilepsy, multiple sclerosis, motor neuron disease, headaches, traumatic brain injury, spinal cord injuries, brain and other nervous system cancers, meningitis, encephalitis, and tetanus.
Tracking the burden of neurologic diseases
Dr. Feigin and colleagues estimated that a full 60% of the U.S. population lives with one or more of these disorders, a figure much greater than previous estimates for neurological disease burden nationwide. Tension-type headache and migraine were the most prevalent in the analysis by Dr. Feigin and colleagues. During the study period, they found, prevalence, incidence, and disability burden of nearly all the included disorders increased, with the exception of brain and spinal cord injuries, meningitis, and encephalitis.
The researchers attributed most of the rise in noncommunicable neurological diseases to population aging. An age-standardized analysis found trends for stroke and Alzheimer’s disease and other dementias to be declining or flat. Age-standardized stroke incidence dropped by 16% from 1990 to 2017, while stroke mortality declined by nearly a third, and stroke disability by a quarter. Age-standardized incidence of Alzheimer’s disease and other dementias dropped by 12%, and their prevalence by 13%, during the study period, though dementia mortality and disability were seen increasing.
The authors surmised that the age-standardized declines in stroke and dementias could reflect that “primary prevention of these disorders are beginning to show an influence.” With dementia, which is linked to cognitive reserve and education, “improving educational levels of cohort reaching the age groups at greatest risk of disease may also be contributing to a modest decline over time,” Dr. Feigin and his colleagues wrote.
Parkinson’s disease and multiple sclerosis, meanwhile, were both seen rising in incidence, prevalence, and disability adjusted life years (DALYs) even with age-standardized figures. The United States saw comparatively more disability in 2017 from dementias, Parkinson’s disease, epilepsy, multiple sclerosis, motor neuron disease, and headache disorders, which together comprised 6.7% of DALYs, compared with 4.4% globally; these also accounted for a higher share of mortality in the U.S. than worldwide. The authors attributed at least some of the difference to better case ascertainment in the U.S.
Regional variations
The researchers also reported variations in disease burden by state and region. While previous studies have identified a “stroke belt” concentrated in North Carolina, South Carolina, and Georgia, the new findings point to stroke disability highest in Alabama, Arkansas, and Mississippi, and mortality highest in Alabama, Mississippi, and South Carolina. The researchers noted increases in dementia mortality in these states, “likely attributable to the reciprocal association between stroke and dementia.”
Northern states saw higher burdens of multiple sclerosis compared with the rest of the country, while eastern states had higher rates of Parkinson’s disease.
Such regional and state-by state variations, Dr. Feigin and colleagues wrote in their analysis, “may be associated with differences in the case ascertainment, as well as access to health care; racial/ethnic, genetic, and socioeconomic diversity; quality and comprehensiveness of preventive strategies; and risk factor distribution.”
The researchers noted as a limitation of their study that the 14 diseases captured were not an exhaustive list of neurological conditions; chronic lower back pain, a condition included in a previous major study of the burden of neurological disease in the United States, was omitted, as were restless legs syndrome and peripheral neuropathy. The researchers cited changes to coding practice in the U.S. and accuracy of medical claims data as potential limitations of their analysis. The Global Burden of Disease study is funded by the Bill and Melinda Gates Foundation, and several of Dr. Feigin’s coauthors reported financial relationships with industry.
Time to adjust the stroke belt?
Amelia Boehme, PhD, a stroke epidemiologist at Columbia University Mailman School of Public Health in New York, said in an interview that the current study added to recent findings showing surprising local variability in stroke prevalence, incidence, and mortality. “What we had always conceptually thought of as the ‘stroke belt’ isn’t necessarily the case,” Dr. Boehme said, but is rather subject to local, county-by-county variations. “Looking at the data here in conjunction with what previous authors have found, it raises some questions as to whether or not state-level data is giving a completely accurate picture, and whether we need to start looking at the county level and adjust for populations and age.” Importantly, Dr. Boehme said, data collected in the Global Burden of Disease study tends to be exceptionally rigorous and systematic, adding weight to Dr. Feigin and colleagues’ suggestions that prevention efforts may be making a dent in stroke and dementia.
“More data is always needed before we start to say we’re seeing things change,” Dr. Boehme noted. “But any glimmer of optimism is welcome, especially with regard to interventions that have been put in place, to allow us to build on those interventions.”
Dr. Boehme disclosed no financial conflicts of interest.
, according to new findings derived from the 2017 Global Burden of Disease study.
The authors of the analysis, led by Valery Feigin, MD, PhD, of New Zealand’s National Institute for Stroke and Applied Neurosciences, and published in the February 2021 issue of JAMA Neurology, looked at prevalence, incidence, mortality, and disability-adjusted life years for 14 neurological disorders across 50 states between 1990 and 2017. The diseases included in the analysis were stroke, Alzheimer’s disease and other dementias, Parkinson’s disease, epilepsy, multiple sclerosis, motor neuron disease, headaches, traumatic brain injury, spinal cord injuries, brain and other nervous system cancers, meningitis, encephalitis, and tetanus.
Tracking the burden of neurologic diseases
Dr. Feigin and colleagues estimated that a full 60% of the U.S. population lives with one or more of these disorders, a figure much greater than previous estimates for neurological disease burden nationwide. Tension-type headache and migraine were the most prevalent in the analysis by Dr. Feigin and colleagues. During the study period, they found, prevalence, incidence, and disability burden of nearly all the included disorders increased, with the exception of brain and spinal cord injuries, meningitis, and encephalitis.
The researchers attributed most of the rise in noncommunicable neurological diseases to population aging. An age-standardized analysis found trends for stroke and Alzheimer’s disease and other dementias to be declining or flat. Age-standardized stroke incidence dropped by 16% from 1990 to 2017, while stroke mortality declined by nearly a third, and stroke disability by a quarter. Age-standardized incidence of Alzheimer’s disease and other dementias dropped by 12%, and their prevalence by 13%, during the study period, though dementia mortality and disability were seen increasing.
The authors surmised that the age-standardized declines in stroke and dementias could reflect that “primary prevention of these disorders are beginning to show an influence.” With dementia, which is linked to cognitive reserve and education, “improving educational levels of cohort reaching the age groups at greatest risk of disease may also be contributing to a modest decline over time,” Dr. Feigin and his colleagues wrote.
Parkinson’s disease and multiple sclerosis, meanwhile, were both seen rising in incidence, prevalence, and disability adjusted life years (DALYs) even with age-standardized figures. The United States saw comparatively more disability in 2017 from dementias, Parkinson’s disease, epilepsy, multiple sclerosis, motor neuron disease, and headache disorders, which together comprised 6.7% of DALYs, compared with 4.4% globally; these also accounted for a higher share of mortality in the U.S. than worldwide. The authors attributed at least some of the difference to better case ascertainment in the U.S.
Regional variations
The researchers also reported variations in disease burden by state and region. While previous studies have identified a “stroke belt” concentrated in North Carolina, South Carolina, and Georgia, the new findings point to stroke disability highest in Alabama, Arkansas, and Mississippi, and mortality highest in Alabama, Mississippi, and South Carolina. The researchers noted increases in dementia mortality in these states, “likely attributable to the reciprocal association between stroke and dementia.”
Northern states saw higher burdens of multiple sclerosis compared with the rest of the country, while eastern states had higher rates of Parkinson’s disease.
Such regional and state-by state variations, Dr. Feigin and colleagues wrote in their analysis, “may be associated with differences in the case ascertainment, as well as access to health care; racial/ethnic, genetic, and socioeconomic diversity; quality and comprehensiveness of preventive strategies; and risk factor distribution.”
The researchers noted as a limitation of their study that the 14 diseases captured were not an exhaustive list of neurological conditions; chronic lower back pain, a condition included in a previous major study of the burden of neurological disease in the United States, was omitted, as were restless legs syndrome and peripheral neuropathy. The researchers cited changes to coding practice in the U.S. and accuracy of medical claims data as potential limitations of their analysis. The Global Burden of Disease study is funded by the Bill and Melinda Gates Foundation, and several of Dr. Feigin’s coauthors reported financial relationships with industry.
Time to adjust the stroke belt?
Amelia Boehme, PhD, a stroke epidemiologist at Columbia University Mailman School of Public Health in New York, said in an interview that the current study added to recent findings showing surprising local variability in stroke prevalence, incidence, and mortality. “What we had always conceptually thought of as the ‘stroke belt’ isn’t necessarily the case,” Dr. Boehme said, but is rather subject to local, county-by-county variations. “Looking at the data here in conjunction with what previous authors have found, it raises some questions as to whether or not state-level data is giving a completely accurate picture, and whether we need to start looking at the county level and adjust for populations and age.” Importantly, Dr. Boehme said, data collected in the Global Burden of Disease study tends to be exceptionally rigorous and systematic, adding weight to Dr. Feigin and colleagues’ suggestions that prevention efforts may be making a dent in stroke and dementia.
“More data is always needed before we start to say we’re seeing things change,” Dr. Boehme noted. “But any glimmer of optimism is welcome, especially with regard to interventions that have been put in place, to allow us to build on those interventions.”
Dr. Boehme disclosed no financial conflicts of interest.
, according to new findings derived from the 2017 Global Burden of Disease study.
The authors of the analysis, led by Valery Feigin, MD, PhD, of New Zealand’s National Institute for Stroke and Applied Neurosciences, and published in the February 2021 issue of JAMA Neurology, looked at prevalence, incidence, mortality, and disability-adjusted life years for 14 neurological disorders across 50 states between 1990 and 2017. The diseases included in the analysis were stroke, Alzheimer’s disease and other dementias, Parkinson’s disease, epilepsy, multiple sclerosis, motor neuron disease, headaches, traumatic brain injury, spinal cord injuries, brain and other nervous system cancers, meningitis, encephalitis, and tetanus.
Tracking the burden of neurologic diseases
Dr. Feigin and colleagues estimated that a full 60% of the U.S. population lives with one or more of these disorders, a figure much greater than previous estimates for neurological disease burden nationwide. Tension-type headache and migraine were the most prevalent in the analysis by Dr. Feigin and colleagues. During the study period, they found, prevalence, incidence, and disability burden of nearly all the included disorders increased, with the exception of brain and spinal cord injuries, meningitis, and encephalitis.
The researchers attributed most of the rise in noncommunicable neurological diseases to population aging. An age-standardized analysis found trends for stroke and Alzheimer’s disease and other dementias to be declining or flat. Age-standardized stroke incidence dropped by 16% from 1990 to 2017, while stroke mortality declined by nearly a third, and stroke disability by a quarter. Age-standardized incidence of Alzheimer’s disease and other dementias dropped by 12%, and their prevalence by 13%, during the study period, though dementia mortality and disability were seen increasing.
The authors surmised that the age-standardized declines in stroke and dementias could reflect that “primary prevention of these disorders are beginning to show an influence.” With dementia, which is linked to cognitive reserve and education, “improving educational levels of cohort reaching the age groups at greatest risk of disease may also be contributing to a modest decline over time,” Dr. Feigin and his colleagues wrote.
Parkinson’s disease and multiple sclerosis, meanwhile, were both seen rising in incidence, prevalence, and disability adjusted life years (DALYs) even with age-standardized figures. The United States saw comparatively more disability in 2017 from dementias, Parkinson’s disease, epilepsy, multiple sclerosis, motor neuron disease, and headache disorders, which together comprised 6.7% of DALYs, compared with 4.4% globally; these also accounted for a higher share of mortality in the U.S. than worldwide. The authors attributed at least some of the difference to better case ascertainment in the U.S.
Regional variations
The researchers also reported variations in disease burden by state and region. While previous studies have identified a “stroke belt” concentrated in North Carolina, South Carolina, and Georgia, the new findings point to stroke disability highest in Alabama, Arkansas, and Mississippi, and mortality highest in Alabama, Mississippi, and South Carolina. The researchers noted increases in dementia mortality in these states, “likely attributable to the reciprocal association between stroke and dementia.”
Northern states saw higher burdens of multiple sclerosis compared with the rest of the country, while eastern states had higher rates of Parkinson’s disease.
Such regional and state-by state variations, Dr. Feigin and colleagues wrote in their analysis, “may be associated with differences in the case ascertainment, as well as access to health care; racial/ethnic, genetic, and socioeconomic diversity; quality and comprehensiveness of preventive strategies; and risk factor distribution.”
The researchers noted as a limitation of their study that the 14 diseases captured were not an exhaustive list of neurological conditions; chronic lower back pain, a condition included in a previous major study of the burden of neurological disease in the United States, was omitted, as were restless legs syndrome and peripheral neuropathy. The researchers cited changes to coding practice in the U.S. and accuracy of medical claims data as potential limitations of their analysis. The Global Burden of Disease study is funded by the Bill and Melinda Gates Foundation, and several of Dr. Feigin’s coauthors reported financial relationships with industry.
Time to adjust the stroke belt?
Amelia Boehme, PhD, a stroke epidemiologist at Columbia University Mailman School of Public Health in New York, said in an interview that the current study added to recent findings showing surprising local variability in stroke prevalence, incidence, and mortality. “What we had always conceptually thought of as the ‘stroke belt’ isn’t necessarily the case,” Dr. Boehme said, but is rather subject to local, county-by-county variations. “Looking at the data here in conjunction with what previous authors have found, it raises some questions as to whether or not state-level data is giving a completely accurate picture, and whether we need to start looking at the county level and adjust for populations and age.” Importantly, Dr. Boehme said, data collected in the Global Burden of Disease study tends to be exceptionally rigorous and systematic, adding weight to Dr. Feigin and colleagues’ suggestions that prevention efforts may be making a dent in stroke and dementia.
“More data is always needed before we start to say we’re seeing things change,” Dr. Boehme noted. “But any glimmer of optimism is welcome, especially with regard to interventions that have been put in place, to allow us to build on those interventions.”
Dr. Boehme disclosed no financial conflicts of interest.
FROM JAMA NEUROLOGY
New data may help intercept head injuries in college football
Novel research from the Concussion Assessment, Research and Education (CARE) Consortium sheds new light on how to effectively reduce the incidence of concussion and head injury exposure in college football.
The study, led by neurotrauma experts Michael McCrea, PhD, and Brian Stemper, PhD, professors of neurosurgery at the Medical College of Wisconsin in Milwaukee, reports data from hundreds of college football players across five seasons and shows
The research also reveals that such injuries occur more often during practices than games.
“We think that with the findings from this paper, there’s a role for everybody to play in reducing injury,” Dr. McCrea said. “We hope these data help inform broad-based policy about practice and preseason training policies in collegiate football. We also think there’s a role for athletic administrators, coaches, and even athletes themselves.”
The study was published online Feb. 1 in JAMA Neurology.
More injuries in preseason
Concussion is one of the most common injuries in football. Beyond these harms are growing concerns that repetitive HIE may increase the risk of long-term neurologic health problems including chronic traumatic encephalopathy (CTE).
The CARE Consortium, which has been conducting research with college athletes across 26 sports and military cadets since 2014, has been interested in multiple facets of concussion and brain trauma.
“We’ve enrolled more than 50,000 athletes and service academy cadets into the consortium over the last 6 years to research all involved aspects including the clinical core, the imaging core, the blood biomarker core, and the genetic core, and we have a head impact measurement core.”
To investigate the pattern of concussion incidence across the football season in college players, the investigators used impact measurement technology across six Division I NCAA football programs participating in the CARE Consortium from 2015 to 2019.
A total of 658 players – all male, mean age 19 years – were fitted with the Head Impact Telemetry System (HITS) sensor arrays in their helmets to measure head impact frequency, location, and magnitude during play.
“This particular study had built-in algorithms that weeded out impacts that were below 10G of linear magnitude, because those have been determined not likely to be real impacts,” Dr. McCrea said.
Across the five seasons studied, 528,684 head impacts recorded met the quality standards for analysis. Players sustained a median of 415 (interquartile range [IQR], 190-727) impacts per season.
Of those, 68 players sustained a diagnosed concussion. In total, 48.5% of concussions occurred during preseason training, despite preseason representing only 20.8% of the football season. Total head injury exposure in the preseason occurred at twice the proportion of the regular season (324.9 vs. 162.4 impacts per team per day; mean difference, 162.6 impacts; 95% confidence interval, 110.9-214.3; P < .001).
“Preseason training often has a much higher intensity to it, in terms of the total hours, the actual training, and the heavy emphasis on full-contact drills like tackling and blocking,” said Dr. McCrea. “Even the volume of players that are participating is greater.”
Results also showed that in each of the five seasons, head injury exposure per athlete was highest in August (preseason) (median, 146.0 impacts; IQR, 63.0-247.8) and lowest in November (median, 80.0 impacts; IQR, 35.0-148.0). In the studied period, 72% of concussions and 66.9% of head injury exposure occurred in practice. Even within the regular season, total head injury exposure in practices was 84.2% higher than in games.
“This incredible dataset we have on head impact measurement also gives us the opportunity to compare it with our other research looking at the correlation between a single head impact and changes in brain structure and function on MRI, on blood biomarkers, giving us the ability to look at the connection between mechanism of effect of injury and recovery from injury,” said Dr. McCrea.
These findings also provide an opportunity to modify approaches to preseason training and football practices to keep players safer, said Dr. McCrea, noting that about half of the variance in head injury exposure is at the level of the individual athlete.
“With this large body of athletes we’ve instrumented, we can look at, for instance, all of the running backs and understand the athlete and what his head injury exposure looks like compared to all other running backs. If we find out that an athlete has a rate of head injury exposure that’s 300% higher than most other players that play the same position, we can take that data directly to the athlete to work on their technique and approach to the game.
“Every researcher wishes that their basic science or their clinical research findings will have some impact on the health and well-being of the population they’re studying. By modifying practices and preseason training, football teams could greatly reduce the risk of injury and exposure for their players, while still maintaining the competitive nature of game play,” he added.
Through a combination of policy and education, similar strategies could be implemented to help prevent concussion and HIE in high school and youth football too, said Dr. McCrea.
‘Shocking’ findings
In an accompanying editorial, Christopher J. Nowinski, PhD, of the Concussion Legacy Foundation, Boston, and Robert C. Cantu, MD, department of neurosurgery, Emerson Hospital, Concord, Massachusetts, said the findings could have significant policy implications and offer a valuable expansion of prior research.
“From 2005 to 2010, studies on college football revealed that about two-thirds of head impacts occurred in practice,” they noted. “We cited this data in 2010 when we proposed to the NFL Players Association that the most effective way to reduce the risks of negative neurological outcomes was to reduce hitting in practice. They agreed, and in 2011 collectively bargained for severe contact limits in practice, with 14 full-contact practices allowed during the 17-week season. Since that rule was implemented, only 18% of NFL concussions have occurred in practice.”
“Against this backdrop, the results of the study by McCrea et al. are shocking,” they added. “It reveals that college football players still experience 72% of their concussions and 67% of their total head injury exposure in practice.”
Even more shocking, noted Dr. Nowinski and Dr. Cantu, is that these numbers are almost certainly an underestimate of the dangers of practice.
“As a former college football player and a former team physician, respectively, we find this situation inexcusable. Concussions in games are inevitable, but concussions in practice are preventable,” they wrote.
“Laudably,” they added “the investigators call on the NCAA and football conferences to explore policy and rule changes to reduce concussion incidence and HIE and to create robust educational offerings to encourage change from coaches and college administrators.”
A version of this article first appeared on Medscape.com.
Novel research from the Concussion Assessment, Research and Education (CARE) Consortium sheds new light on how to effectively reduce the incidence of concussion and head injury exposure in college football.
The study, led by neurotrauma experts Michael McCrea, PhD, and Brian Stemper, PhD, professors of neurosurgery at the Medical College of Wisconsin in Milwaukee, reports data from hundreds of college football players across five seasons and shows
The research also reveals that such injuries occur more often during practices than games.
“We think that with the findings from this paper, there’s a role for everybody to play in reducing injury,” Dr. McCrea said. “We hope these data help inform broad-based policy about practice and preseason training policies in collegiate football. We also think there’s a role for athletic administrators, coaches, and even athletes themselves.”
The study was published online Feb. 1 in JAMA Neurology.
More injuries in preseason
Concussion is one of the most common injuries in football. Beyond these harms are growing concerns that repetitive HIE may increase the risk of long-term neurologic health problems including chronic traumatic encephalopathy (CTE).
The CARE Consortium, which has been conducting research with college athletes across 26 sports and military cadets since 2014, has been interested in multiple facets of concussion and brain trauma.
“We’ve enrolled more than 50,000 athletes and service academy cadets into the consortium over the last 6 years to research all involved aspects including the clinical core, the imaging core, the blood biomarker core, and the genetic core, and we have a head impact measurement core.”
To investigate the pattern of concussion incidence across the football season in college players, the investigators used impact measurement technology across six Division I NCAA football programs participating in the CARE Consortium from 2015 to 2019.
A total of 658 players – all male, mean age 19 years – were fitted with the Head Impact Telemetry System (HITS) sensor arrays in their helmets to measure head impact frequency, location, and magnitude during play.
“This particular study had built-in algorithms that weeded out impacts that were below 10G of linear magnitude, because those have been determined not likely to be real impacts,” Dr. McCrea said.
Across the five seasons studied, 528,684 head impacts recorded met the quality standards for analysis. Players sustained a median of 415 (interquartile range [IQR], 190-727) impacts per season.
Of those, 68 players sustained a diagnosed concussion. In total, 48.5% of concussions occurred during preseason training, despite preseason representing only 20.8% of the football season. Total head injury exposure in the preseason occurred at twice the proportion of the regular season (324.9 vs. 162.4 impacts per team per day; mean difference, 162.6 impacts; 95% confidence interval, 110.9-214.3; P < .001).
“Preseason training often has a much higher intensity to it, in terms of the total hours, the actual training, and the heavy emphasis on full-contact drills like tackling and blocking,” said Dr. McCrea. “Even the volume of players that are participating is greater.”
Results also showed that in each of the five seasons, head injury exposure per athlete was highest in August (preseason) (median, 146.0 impacts; IQR, 63.0-247.8) and lowest in November (median, 80.0 impacts; IQR, 35.0-148.0). In the studied period, 72% of concussions and 66.9% of head injury exposure occurred in practice. Even within the regular season, total head injury exposure in practices was 84.2% higher than in games.
“This incredible dataset we have on head impact measurement also gives us the opportunity to compare it with our other research looking at the correlation between a single head impact and changes in brain structure and function on MRI, on blood biomarkers, giving us the ability to look at the connection between mechanism of effect of injury and recovery from injury,” said Dr. McCrea.
These findings also provide an opportunity to modify approaches to preseason training and football practices to keep players safer, said Dr. McCrea, noting that about half of the variance in head injury exposure is at the level of the individual athlete.
“With this large body of athletes we’ve instrumented, we can look at, for instance, all of the running backs and understand the athlete and what his head injury exposure looks like compared to all other running backs. If we find out that an athlete has a rate of head injury exposure that’s 300% higher than most other players that play the same position, we can take that data directly to the athlete to work on their technique and approach to the game.
“Every researcher wishes that their basic science or their clinical research findings will have some impact on the health and well-being of the population they’re studying. By modifying practices and preseason training, football teams could greatly reduce the risk of injury and exposure for their players, while still maintaining the competitive nature of game play,” he added.
Through a combination of policy and education, similar strategies could be implemented to help prevent concussion and HIE in high school and youth football too, said Dr. McCrea.
‘Shocking’ findings
In an accompanying editorial, Christopher J. Nowinski, PhD, of the Concussion Legacy Foundation, Boston, and Robert C. Cantu, MD, department of neurosurgery, Emerson Hospital, Concord, Massachusetts, said the findings could have significant policy implications and offer a valuable expansion of prior research.
“From 2005 to 2010, studies on college football revealed that about two-thirds of head impacts occurred in practice,” they noted. “We cited this data in 2010 when we proposed to the NFL Players Association that the most effective way to reduce the risks of negative neurological outcomes was to reduce hitting in practice. They agreed, and in 2011 collectively bargained for severe contact limits in practice, with 14 full-contact practices allowed during the 17-week season. Since that rule was implemented, only 18% of NFL concussions have occurred in practice.”
“Against this backdrop, the results of the study by McCrea et al. are shocking,” they added. “It reveals that college football players still experience 72% of their concussions and 67% of their total head injury exposure in practice.”
Even more shocking, noted Dr. Nowinski and Dr. Cantu, is that these numbers are almost certainly an underestimate of the dangers of practice.
“As a former college football player and a former team physician, respectively, we find this situation inexcusable. Concussions in games are inevitable, but concussions in practice are preventable,” they wrote.
“Laudably,” they added “the investigators call on the NCAA and football conferences to explore policy and rule changes to reduce concussion incidence and HIE and to create robust educational offerings to encourage change from coaches and college administrators.”
A version of this article first appeared on Medscape.com.
Novel research from the Concussion Assessment, Research and Education (CARE) Consortium sheds new light on how to effectively reduce the incidence of concussion and head injury exposure in college football.
The study, led by neurotrauma experts Michael McCrea, PhD, and Brian Stemper, PhD, professors of neurosurgery at the Medical College of Wisconsin in Milwaukee, reports data from hundreds of college football players across five seasons and shows
The research also reveals that such injuries occur more often during practices than games.
“We think that with the findings from this paper, there’s a role for everybody to play in reducing injury,” Dr. McCrea said. “We hope these data help inform broad-based policy about practice and preseason training policies in collegiate football. We also think there’s a role for athletic administrators, coaches, and even athletes themselves.”
The study was published online Feb. 1 in JAMA Neurology.
More injuries in preseason
Concussion is one of the most common injuries in football. Beyond these harms are growing concerns that repetitive HIE may increase the risk of long-term neurologic health problems including chronic traumatic encephalopathy (CTE).
The CARE Consortium, which has been conducting research with college athletes across 26 sports and military cadets since 2014, has been interested in multiple facets of concussion and brain trauma.
“We’ve enrolled more than 50,000 athletes and service academy cadets into the consortium over the last 6 years to research all involved aspects including the clinical core, the imaging core, the blood biomarker core, and the genetic core, and we have a head impact measurement core.”
To investigate the pattern of concussion incidence across the football season in college players, the investigators used impact measurement technology across six Division I NCAA football programs participating in the CARE Consortium from 2015 to 2019.
A total of 658 players – all male, mean age 19 years – were fitted with the Head Impact Telemetry System (HITS) sensor arrays in their helmets to measure head impact frequency, location, and magnitude during play.
“This particular study had built-in algorithms that weeded out impacts that were below 10G of linear magnitude, because those have been determined not likely to be real impacts,” Dr. McCrea said.
Across the five seasons studied, 528,684 head impacts recorded met the quality standards for analysis. Players sustained a median of 415 (interquartile range [IQR], 190-727) impacts per season.
Of those, 68 players sustained a diagnosed concussion. In total, 48.5% of concussions occurred during preseason training, despite preseason representing only 20.8% of the football season. Total head injury exposure in the preseason occurred at twice the proportion of the regular season (324.9 vs. 162.4 impacts per team per day; mean difference, 162.6 impacts; 95% confidence interval, 110.9-214.3; P < .001).
“Preseason training often has a much higher intensity to it, in terms of the total hours, the actual training, and the heavy emphasis on full-contact drills like tackling and blocking,” said Dr. McCrea. “Even the volume of players that are participating is greater.”
Results also showed that in each of the five seasons, head injury exposure per athlete was highest in August (preseason) (median, 146.0 impacts; IQR, 63.0-247.8) and lowest in November (median, 80.0 impacts; IQR, 35.0-148.0). In the studied period, 72% of concussions and 66.9% of head injury exposure occurred in practice. Even within the regular season, total head injury exposure in practices was 84.2% higher than in games.
“This incredible dataset we have on head impact measurement also gives us the opportunity to compare it with our other research looking at the correlation between a single head impact and changes in brain structure and function on MRI, on blood biomarkers, giving us the ability to look at the connection between mechanism of effect of injury and recovery from injury,” said Dr. McCrea.
These findings also provide an opportunity to modify approaches to preseason training and football practices to keep players safer, said Dr. McCrea, noting that about half of the variance in head injury exposure is at the level of the individual athlete.
“With this large body of athletes we’ve instrumented, we can look at, for instance, all of the running backs and understand the athlete and what his head injury exposure looks like compared to all other running backs. If we find out that an athlete has a rate of head injury exposure that’s 300% higher than most other players that play the same position, we can take that data directly to the athlete to work on their technique and approach to the game.
“Every researcher wishes that their basic science or their clinical research findings will have some impact on the health and well-being of the population they’re studying. By modifying practices and preseason training, football teams could greatly reduce the risk of injury and exposure for their players, while still maintaining the competitive nature of game play,” he added.
Through a combination of policy and education, similar strategies could be implemented to help prevent concussion and HIE in high school and youth football too, said Dr. McCrea.
‘Shocking’ findings
In an accompanying editorial, Christopher J. Nowinski, PhD, of the Concussion Legacy Foundation, Boston, and Robert C. Cantu, MD, department of neurosurgery, Emerson Hospital, Concord, Massachusetts, said the findings could have significant policy implications and offer a valuable expansion of prior research.
“From 2005 to 2010, studies on college football revealed that about two-thirds of head impacts occurred in practice,” they noted. “We cited this data in 2010 when we proposed to the NFL Players Association that the most effective way to reduce the risks of negative neurological outcomes was to reduce hitting in practice. They agreed, and in 2011 collectively bargained for severe contact limits in practice, with 14 full-contact practices allowed during the 17-week season. Since that rule was implemented, only 18% of NFL concussions have occurred in practice.”
“Against this backdrop, the results of the study by McCrea et al. are shocking,” they added. “It reveals that college football players still experience 72% of their concussions and 67% of their total head injury exposure in practice.”
Even more shocking, noted Dr. Nowinski and Dr. Cantu, is that these numbers are almost certainly an underestimate of the dangers of practice.
“As a former college football player and a former team physician, respectively, we find this situation inexcusable. Concussions in games are inevitable, but concussions in practice are preventable,” they wrote.
“Laudably,” they added “the investigators call on the NCAA and football conferences to explore policy and rule changes to reduce concussion incidence and HIE and to create robust educational offerings to encourage change from coaches and college administrators.”
A version of this article first appeared on Medscape.com.
FROM JAMA NEUROLOGY
Bedside EEG test aids prognosis in patients with brain injury
results of a new study suggest. The study showed that the use of a paradigm that measures the strength of responses to speech improved the accuracy of prognosis for these patients, compared with prognoses made solely on the basis of standard clinical characteristics.
“What we found is really compelling evidence” of the usefulness of the test, lead study author Rodika Sokoliuk, PhD, a postdoctoral researcher at the Center for Human Brain Health, University of Birmingham (England), said in an interview.
The passive measure of comprehension, which doesn’t require any other response from the patient, can reduce uncertainty at a critical phase of decision-making in the ICU, said Dr. Sokoliuk.
The study was published online Dec. 23, 2020, in Annals of Neurology.
Useful information at a time of ‘considerable prognostic uncertainty’
Accurate, early prognostication is vital for efficient stratification of patients after a TBI, the authors wrote. This can often be achieved from patient behavior and CT at admission, but some patients continue to fail to obey commands after washout of sedation.
These patients pose a significant challenge for neurologic prognostication, they noted. In these cases, clinicians and families must decide whether to “wait and see” or consider treatment withdrawal.
The authors noted that a lack of command following early in the postsedation period is associated with poor outcome, including vegetative state/unresponsive wakefulness syndrome (VS/UWS). This, they said, represents a “window of opportunity” for cessation of life-sustaining therapy at a time of considerable prognostic uncertainty.
Recent research shows that a significant proportion of unresponsive patients retain a level of cognition, and even consciousness, that isn’t evident from their external behavior – the so-called cognitive-motor dissociation.
The new study included 28 adult patients who had experienced a TBI and were admitted to the ICU of the Queen Elizabeth Hospital in Birmingham, England. The patients had a Glasgow Coma Scale motor score less than 6 (i.e., they were incapable of obeying commands). They had been sedation free for 2-7 days.
For the paradigm, researchers constructed 288 English words using the male voice of the Apple synthesizer. The words required the same amount of time to be generated (320 ms) and were monosyllabic, so the rhythms of the sounds were the same.
The words were presented in a specific order: an adjective, then a noun, then a verb, then a noun. Two words – for example, an adjective and noun – “would build a meaningful phrase,” and four words would build a sentence, said Dr. Sokoliuk.
The researchers built 72 of these four-word sentences. A trial comprised 12 of these sentences, resulting in a total of 864 four-word sentences.
Dr. Sokoliuk likened the paradigm to a rap song with a specific beat that is continually repeated. “Basically, we play 12 of these four-word sentences in a row, without any gaps,” she said.
Each sentence was played to patients, in random order, a minimum of eight and a maximum of nine times per patient throughout the experiment. The patients’ brain activity was recorded on EEG.
Dr. Sokoliuk noted that brain activity in healthy people synchronizes only with the rhythm of phrases and sentences when listeners consciously comprehend the speech. The researchers assessed the level of comprehension in the unresponsive patients by measuring the strength of this synchronicity or brain pattern.
After exclusions, 17 patients were available for outcome assessment 3 months post EEG, and 16 patients were available 6 months post EEG.
The analysis showed that outcome significantly correlated with the strength of patients’ acute cortical tracking of phrases and sentences (r > 0.6; P < .007), quantified by intertrial phase coherence.
Linear regressions revealed that the strength of this comprehension response (beta, 0.603; P = .006) significantly improved the accuracy of prognoses relative to clinical characteristics alone, such as the Glasgow Coma Scale or CT grade.
Previous studies showed that, if there is no understanding of the language used or if the subject is asleep, the brain doesn’t have the “signature” of tracking phrases and sentences, so it doesn’t have the synchronicity or the pattern of individuals with normal cognition, said Dr. Sokoliuk.
“You need a certain level of consciousness, and you need to understand the language, so your brain can actually track sentences or phrases,” she said.
Dr. Sokoliuk explained that the paradigm shows that patients are understanding the sentences and are not just hearing them.
“It’s not showing us that they only hear it, because there are no obvious gaps between the sentences; if there were gaps between sentences, it would probably only show that they hear it. It could be both, that they hear and understand it, but we wouldn’t know.”
A receiver operating characteristics analysis indicated 100% sensitivity and 80% specificity for a distinction between bad outcome (death, VS/UWS) and good outcome at 6 months.
“We could actually define a threshold of the tracking,” said Dr. Sokoliuk. “Patients who had phrases and sentences tracking below this threshold had worse outcome than those whose tracking value was above this threshold.”
The study illustrates that some posttraumatic patients who remain in an unresponsive state despite being sedation free may nevertheless comprehend speech.
The EEG paradigm approach, the authors said, may significantly reduce prognostic uncertainty in a critical phase of medical decision-making. It could also help clinicians make more appropriate decisions about whether or not to continue life-sustaining therapy and ensure more appropriate distribution of limited rehabilitation resources to patients most likely to benefit.
Dr. Sokoliuk stressed that the paradigm could be used at the bedside soon after a brain injury. “The critical thing is, we can actually use it during the acute phase, which is very important for clinical decisions about life-sustaining methods, therapy, and long-term care.”
A prognostic tool
The simple approach promises to be more accessible than fMRI, said Dr. Sokoliuk. “Putting an unresponsive coma patient in a scanner is very difficult and also much more expensive,” she said.
The next step, said Dr. Sokoliuk, is to repeat the study with a larger sample. “The number in the current study was quite small, and we can’t say if the sensitivity of the paradigm is strong enough to use it as a standard prognostic tool.”
To use it in clinical setting, “we really have to have robust measures,” she added.
She aims to conduct a collaborative study involving several institutions and more patients.
The research team plans to eventually build “an open-access toolbox” that would include the auditory streams to be played during EEG recordings and a program to analyze the data, said Dr. Sokoliuk. “Then, in the end, you would get a threshold or a value of tracking for phrases and sentences, and this could then classify a patient to be in a good-outcome or in bad-outcome group.”
She stressed this is a prognostic tool, not a diagnostic tool, and it should not be used in isolation. “It’s important to know that no clinician should only use this paradigm to prognosticate a patient; our paradigm should be part of a bigger battery of tests.”
But it could go a long way toward helping families as well as physicians. “If they know that the patient would be better in 3 months’ time, it’s easier for them to decide what should come next,” she said.
And it’s heartening to know that when families talk to their unresponsive loved one, the patient understands them, she added.
Promising basic research
Commenting on the study in an interview, Christine Blume, PhD, of the Center for Chronobiology, University of Basel (Switzerland), whose research interests include cognitive processing of patients with disorders of consciousness, described it as “very elegant and appealing” and the paradigm it used as “really promising.”
“However, we do, of course, not yet know about the prognostic value on a single-subject level, as the authors performed only group analyses,” said Dr. Blume. “This will require more extensive and perhaps even multicenter studies.”
It would also require developing a “solution” that “allows clinicians with limited time resources and perhaps lacking expert knowledge on the paradigm and the necessary analyses to apply the paradigm at bedside,” said Dr. Blume.
She agreed that a passive paradigm that helps determine whether a patient consciously understands speech, without the need for further processing, “has the potential to really improve the diagnostic process and uncover covert consciousness.”
One should bear in mind, though, that the paradigm “makes one essential assumption: that patients can understand speech,” said Dr. Blume. “For example, an aphasic patient might not understand but still be conscious.”
In this context, she added, “it’s essential to note that while the presence of a response suggests consciousness, the absence of a response does not suggest the absence of consciousness.”
Dr. Blume cautioned that the approach used in the study “is still at the stage of basic research.” Although the paradigm is promising, “I do not think it is ‘around the corner,’ ” she said.
The study was funded by the Medical Research Council. It was further supported by the National Institute for Health Research Surgical Reconstruction and Microbiology Research Center. Dr. Sokoliuk and Dr. Blume have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
results of a new study suggest. The study showed that the use of a paradigm that measures the strength of responses to speech improved the accuracy of prognosis for these patients, compared with prognoses made solely on the basis of standard clinical characteristics.
“What we found is really compelling evidence” of the usefulness of the test, lead study author Rodika Sokoliuk, PhD, a postdoctoral researcher at the Center for Human Brain Health, University of Birmingham (England), said in an interview.
The passive measure of comprehension, which doesn’t require any other response from the patient, can reduce uncertainty at a critical phase of decision-making in the ICU, said Dr. Sokoliuk.
The study was published online Dec. 23, 2020, in Annals of Neurology.
Useful information at a time of ‘considerable prognostic uncertainty’
Accurate, early prognostication is vital for efficient stratification of patients after a TBI, the authors wrote. This can often be achieved from patient behavior and CT at admission, but some patients continue to fail to obey commands after washout of sedation.
These patients pose a significant challenge for neurologic prognostication, they noted. In these cases, clinicians and families must decide whether to “wait and see” or consider treatment withdrawal.
The authors noted that a lack of command following early in the postsedation period is associated with poor outcome, including vegetative state/unresponsive wakefulness syndrome (VS/UWS). This, they said, represents a “window of opportunity” for cessation of life-sustaining therapy at a time of considerable prognostic uncertainty.
Recent research shows that a significant proportion of unresponsive patients retain a level of cognition, and even consciousness, that isn’t evident from their external behavior – the so-called cognitive-motor dissociation.
The new study included 28 adult patients who had experienced a TBI and were admitted to the ICU of the Queen Elizabeth Hospital in Birmingham, England. The patients had a Glasgow Coma Scale motor score less than 6 (i.e., they were incapable of obeying commands). They had been sedation free for 2-7 days.
For the paradigm, researchers constructed 288 English words using the male voice of the Apple synthesizer. The words required the same amount of time to be generated (320 ms) and were monosyllabic, so the rhythms of the sounds were the same.
The words were presented in a specific order: an adjective, then a noun, then a verb, then a noun. Two words – for example, an adjective and noun – “would build a meaningful phrase,” and four words would build a sentence, said Dr. Sokoliuk.
The researchers built 72 of these four-word sentences. A trial comprised 12 of these sentences, resulting in a total of 864 four-word sentences.
Dr. Sokoliuk likened the paradigm to a rap song with a specific beat that is continually repeated. “Basically, we play 12 of these four-word sentences in a row, without any gaps,” she said.
Each sentence was played to patients, in random order, a minimum of eight and a maximum of nine times per patient throughout the experiment. The patients’ brain activity was recorded on EEG.
Dr. Sokoliuk noted that brain activity in healthy people synchronizes only with the rhythm of phrases and sentences when listeners consciously comprehend the speech. The researchers assessed the level of comprehension in the unresponsive patients by measuring the strength of this synchronicity or brain pattern.
After exclusions, 17 patients were available for outcome assessment 3 months post EEG, and 16 patients were available 6 months post EEG.
The analysis showed that outcome significantly correlated with the strength of patients’ acute cortical tracking of phrases and sentences (r > 0.6; P < .007), quantified by intertrial phase coherence.
Linear regressions revealed that the strength of this comprehension response (beta, 0.603; P = .006) significantly improved the accuracy of prognoses relative to clinical characteristics alone, such as the Glasgow Coma Scale or CT grade.
Previous studies showed that, if there is no understanding of the language used or if the subject is asleep, the brain doesn’t have the “signature” of tracking phrases and sentences, so it doesn’t have the synchronicity or the pattern of individuals with normal cognition, said Dr. Sokoliuk.
“You need a certain level of consciousness, and you need to understand the language, so your brain can actually track sentences or phrases,” she said.
Dr. Sokoliuk explained that the paradigm shows that patients are understanding the sentences and are not just hearing them.
“It’s not showing us that they only hear it, because there are no obvious gaps between the sentences; if there were gaps between sentences, it would probably only show that they hear it. It could be both, that they hear and understand it, but we wouldn’t know.”
A receiver operating characteristics analysis indicated 100% sensitivity and 80% specificity for a distinction between bad outcome (death, VS/UWS) and good outcome at 6 months.
“We could actually define a threshold of the tracking,” said Dr. Sokoliuk. “Patients who had phrases and sentences tracking below this threshold had worse outcome than those whose tracking value was above this threshold.”
The study illustrates that some posttraumatic patients who remain in an unresponsive state despite being sedation free may nevertheless comprehend speech.
The EEG paradigm approach, the authors said, may significantly reduce prognostic uncertainty in a critical phase of medical decision-making. It could also help clinicians make more appropriate decisions about whether or not to continue life-sustaining therapy and ensure more appropriate distribution of limited rehabilitation resources to patients most likely to benefit.
Dr. Sokoliuk stressed that the paradigm could be used at the bedside soon after a brain injury. “The critical thing is, we can actually use it during the acute phase, which is very important for clinical decisions about life-sustaining methods, therapy, and long-term care.”
A prognostic tool
The simple approach promises to be more accessible than fMRI, said Dr. Sokoliuk. “Putting an unresponsive coma patient in a scanner is very difficult and also much more expensive,” she said.
The next step, said Dr. Sokoliuk, is to repeat the study with a larger sample. “The number in the current study was quite small, and we can’t say if the sensitivity of the paradigm is strong enough to use it as a standard prognostic tool.”
To use it in clinical setting, “we really have to have robust measures,” she added.
She aims to conduct a collaborative study involving several institutions and more patients.
The research team plans to eventually build “an open-access toolbox” that would include the auditory streams to be played during EEG recordings and a program to analyze the data, said Dr. Sokoliuk. “Then, in the end, you would get a threshold or a value of tracking for phrases and sentences, and this could then classify a patient to be in a good-outcome or in bad-outcome group.”
She stressed this is a prognostic tool, not a diagnostic tool, and it should not be used in isolation. “It’s important to know that no clinician should only use this paradigm to prognosticate a patient; our paradigm should be part of a bigger battery of tests.”
But it could go a long way toward helping families as well as physicians. “If they know that the patient would be better in 3 months’ time, it’s easier for them to decide what should come next,” she said.
And it’s heartening to know that when families talk to their unresponsive loved one, the patient understands them, she added.
Promising basic research
Commenting on the study in an interview, Christine Blume, PhD, of the Center for Chronobiology, University of Basel (Switzerland), whose research interests include cognitive processing of patients with disorders of consciousness, described it as “very elegant and appealing” and the paradigm it used as “really promising.”
“However, we do, of course, not yet know about the prognostic value on a single-subject level, as the authors performed only group analyses,” said Dr. Blume. “This will require more extensive and perhaps even multicenter studies.”
It would also require developing a “solution” that “allows clinicians with limited time resources and perhaps lacking expert knowledge on the paradigm and the necessary analyses to apply the paradigm at bedside,” said Dr. Blume.
She agreed that a passive paradigm that helps determine whether a patient consciously understands speech, without the need for further processing, “has the potential to really improve the diagnostic process and uncover covert consciousness.”
One should bear in mind, though, that the paradigm “makes one essential assumption: that patients can understand speech,” said Dr. Blume. “For example, an aphasic patient might not understand but still be conscious.”
In this context, she added, “it’s essential to note that while the presence of a response suggests consciousness, the absence of a response does not suggest the absence of consciousness.”
Dr. Blume cautioned that the approach used in the study “is still at the stage of basic research.” Although the paradigm is promising, “I do not think it is ‘around the corner,’ ” she said.
The study was funded by the Medical Research Council. It was further supported by the National Institute for Health Research Surgical Reconstruction and Microbiology Research Center. Dr. Sokoliuk and Dr. Blume have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
results of a new study suggest. The study showed that the use of a paradigm that measures the strength of responses to speech improved the accuracy of prognosis for these patients, compared with prognoses made solely on the basis of standard clinical characteristics.
“What we found is really compelling evidence” of the usefulness of the test, lead study author Rodika Sokoliuk, PhD, a postdoctoral researcher at the Center for Human Brain Health, University of Birmingham (England), said in an interview.
The passive measure of comprehension, which doesn’t require any other response from the patient, can reduce uncertainty at a critical phase of decision-making in the ICU, said Dr. Sokoliuk.
The study was published online Dec. 23, 2020, in Annals of Neurology.
Useful information at a time of ‘considerable prognostic uncertainty’
Accurate, early prognostication is vital for efficient stratification of patients after a TBI, the authors wrote. This can often be achieved from patient behavior and CT at admission, but some patients continue to fail to obey commands after washout of sedation.
These patients pose a significant challenge for neurologic prognostication, they noted. In these cases, clinicians and families must decide whether to “wait and see” or consider treatment withdrawal.
The authors noted that a lack of command following early in the postsedation period is associated with poor outcome, including vegetative state/unresponsive wakefulness syndrome (VS/UWS). This, they said, represents a “window of opportunity” for cessation of life-sustaining therapy at a time of considerable prognostic uncertainty.
Recent research shows that a significant proportion of unresponsive patients retain a level of cognition, and even consciousness, that isn’t evident from their external behavior – the so-called cognitive-motor dissociation.
The new study included 28 adult patients who had experienced a TBI and were admitted to the ICU of the Queen Elizabeth Hospital in Birmingham, England. The patients had a Glasgow Coma Scale motor score less than 6 (i.e., they were incapable of obeying commands). They had been sedation free for 2-7 days.
For the paradigm, researchers constructed 288 English words using the male voice of the Apple synthesizer. The words required the same amount of time to be generated (320 ms) and were monosyllabic, so the rhythms of the sounds were the same.
The words were presented in a specific order: an adjective, then a noun, then a verb, then a noun. Two words – for example, an adjective and noun – “would build a meaningful phrase,” and four words would build a sentence, said Dr. Sokoliuk.
The researchers built 72 of these four-word sentences. A trial comprised 12 of these sentences, resulting in a total of 864 four-word sentences.
Dr. Sokoliuk likened the paradigm to a rap song with a specific beat that is continually repeated. “Basically, we play 12 of these four-word sentences in a row, without any gaps,” she said.
Each sentence was played to patients, in random order, a minimum of eight and a maximum of nine times per patient throughout the experiment. The patients’ brain activity was recorded on EEG.
Dr. Sokoliuk noted that brain activity in healthy people synchronizes only with the rhythm of phrases and sentences when listeners consciously comprehend the speech. The researchers assessed the level of comprehension in the unresponsive patients by measuring the strength of this synchronicity or brain pattern.
After exclusions, 17 patients were available for outcome assessment 3 months post EEG, and 16 patients were available 6 months post EEG.
The analysis showed that outcome significantly correlated with the strength of patients’ acute cortical tracking of phrases and sentences (r > 0.6; P < .007), quantified by intertrial phase coherence.
Linear regressions revealed that the strength of this comprehension response (beta, 0.603; P = .006) significantly improved the accuracy of prognoses relative to clinical characteristics alone, such as the Glasgow Coma Scale or CT grade.
Previous studies showed that, if there is no understanding of the language used or if the subject is asleep, the brain doesn’t have the “signature” of tracking phrases and sentences, so it doesn’t have the synchronicity or the pattern of individuals with normal cognition, said Dr. Sokoliuk.
“You need a certain level of consciousness, and you need to understand the language, so your brain can actually track sentences or phrases,” she said.
Dr. Sokoliuk explained that the paradigm shows that patients are understanding the sentences and are not just hearing them.
“It’s not showing us that they only hear it, because there are no obvious gaps between the sentences; if there were gaps between sentences, it would probably only show that they hear it. It could be both, that they hear and understand it, but we wouldn’t know.”
A receiver operating characteristics analysis indicated 100% sensitivity and 80% specificity for a distinction between bad outcome (death, VS/UWS) and good outcome at 6 months.
“We could actually define a threshold of the tracking,” said Dr. Sokoliuk. “Patients who had phrases and sentences tracking below this threshold had worse outcome than those whose tracking value was above this threshold.”
The study illustrates that some posttraumatic patients who remain in an unresponsive state despite being sedation free may nevertheless comprehend speech.
The EEG paradigm approach, the authors said, may significantly reduce prognostic uncertainty in a critical phase of medical decision-making. It could also help clinicians make more appropriate decisions about whether or not to continue life-sustaining therapy and ensure more appropriate distribution of limited rehabilitation resources to patients most likely to benefit.
Dr. Sokoliuk stressed that the paradigm could be used at the bedside soon after a brain injury. “The critical thing is, we can actually use it during the acute phase, which is very important for clinical decisions about life-sustaining methods, therapy, and long-term care.”
A prognostic tool
The simple approach promises to be more accessible than fMRI, said Dr. Sokoliuk. “Putting an unresponsive coma patient in a scanner is very difficult and also much more expensive,” she said.
The next step, said Dr. Sokoliuk, is to repeat the study with a larger sample. “The number in the current study was quite small, and we can’t say if the sensitivity of the paradigm is strong enough to use it as a standard prognostic tool.”
To use it in clinical setting, “we really have to have robust measures,” she added.
She aims to conduct a collaborative study involving several institutions and more patients.
The research team plans to eventually build “an open-access toolbox” that would include the auditory streams to be played during EEG recordings and a program to analyze the data, said Dr. Sokoliuk. “Then, in the end, you would get a threshold or a value of tracking for phrases and sentences, and this could then classify a patient to be in a good-outcome or in bad-outcome group.”
She stressed this is a prognostic tool, not a diagnostic tool, and it should not be used in isolation. “It’s important to know that no clinician should only use this paradigm to prognosticate a patient; our paradigm should be part of a bigger battery of tests.”
But it could go a long way toward helping families as well as physicians. “If they know that the patient would be better in 3 months’ time, it’s easier for them to decide what should come next,” she said.
And it’s heartening to know that when families talk to their unresponsive loved one, the patient understands them, she added.
Promising basic research
Commenting on the study in an interview, Christine Blume, PhD, of the Center for Chronobiology, University of Basel (Switzerland), whose research interests include cognitive processing of patients with disorders of consciousness, described it as “very elegant and appealing” and the paradigm it used as “really promising.”
“However, we do, of course, not yet know about the prognostic value on a single-subject level, as the authors performed only group analyses,” said Dr. Blume. “This will require more extensive and perhaps even multicenter studies.”
It would also require developing a “solution” that “allows clinicians with limited time resources and perhaps lacking expert knowledge on the paradigm and the necessary analyses to apply the paradigm at bedside,” said Dr. Blume.
She agreed that a passive paradigm that helps determine whether a patient consciously understands speech, without the need for further processing, “has the potential to really improve the diagnostic process and uncover covert consciousness.”
One should bear in mind, though, that the paradigm “makes one essential assumption: that patients can understand speech,” said Dr. Blume. “For example, an aphasic patient might not understand but still be conscious.”
In this context, she added, “it’s essential to note that while the presence of a response suggests consciousness, the absence of a response does not suggest the absence of consciousness.”
Dr. Blume cautioned that the approach used in the study “is still at the stage of basic research.” Although the paradigm is promising, “I do not think it is ‘around the corner,’ ” she said.
The study was funded by the Medical Research Council. It was further supported by the National Institute for Health Research Surgical Reconstruction and Microbiology Research Center. Dr. Sokoliuk and Dr. Blume have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF NEUROLOGY
Concussion linked to risk for dementia, Parkinson’s disease, and ADHD
new research suggests. Results from a retrospective, population-based cohort study showed that controlling for socioeconomic status and overall health did not significantly affect this association.
The link between concussion and risk for ADHD and for mood and anxiety disorder was stronger in the women than in the men. In addition, having a history of multiple concussions strengthened the association between concussion and subsequent mood and anxiety disorder, dementia, and Parkinson’s disease compared with experiencing just one concussion.
The findings are similar to those of previous studies, noted lead author Marc P. Morissette, PhD, research assistant at the Pan Am Clinic Foundation in Winnipeg, Manitoba, Canada. “The main methodological differences separating our study from previous studies in this area is a focus on concussion-specific injuries identified from medical records and the potential for study participants to have up to 25 years of follow-up data,” said Dr. Morissette.
The findings were published online July 27 in Family Medicine and Community Health, a BMJ journal.
Almost 190,000 participants
Several studies have shown associations between head injury and increased risk for ADHD, depression, anxiety, Alzheimer’s disease, and Parkinson’s disease. However, many of these studies relied on self-reported medical history, included all forms of traumatic brain injury, and failed to adjust for preexisting health conditions.
An improved understanding of concussion and the risks associated with it could help physicians manage their patients’ long-term needs, the investigators noted.
In the current study, the researchers examined anonymized administrative health data collected between the periods of 1990–1991 and 2014–2015 in the Manitoba Population Research Data Repository at the Manitoba Center for Health Policy.
Eligible patients had been diagnosed with concussion in accordance with standard criteria. Participants were excluded if they had been diagnosed with dementia or Parkinson’s disease before the incident concussion during the study period. The investigators matched three control participants to each included patient on the basis of age, sex, and location.
Study outcome was time from index date (date of first concussion) to diagnosis of ADHD, mood and anxiety disorder, dementia, or Parkinson’s disease. The researchers controlled for socioeconomic status using the Socioeconomic Factor Index, version 2 (SEFI2), and for preexisting medical conditions using the Charlson Comorbidity Index (CCI).
The study included 28,021 men (mean age, 25 years) and 19,462 women (mean age, 30 years) in the concussion group and 81,871 men (mean age, 25 years) and 57,159 women (mean age, 30 years) in the control group. Mean SEFI2 score was approximately −0.05, and mean CCI score was approximately 0.2.
Dose effect?
Results showed that concussion was associated with an increased risk for ADHD (hazard ratio [HR], 1.39), mood and anxiety disorder (HR, 1.72), dementia (HR, 1.72), and Parkinson’s disease (HR, 1.57).
After a concussion, the risk of developing ADHD was 28% higher and the risk of developing mood and anxiety disorder was 7% higher among women than among men. Gender was not associated with risk for dementia or Parkinson’s disease after concussion.
Sustaining a second concussion increased the strength of the association with risk for dementia compared with sustaining a single concussion (HR, 1.62). Similarly, sustaining more than three concussions increased the strength of the association with the risk for mood and anxiety disorders (HR for more than three vs one concussion, 1.22) and Parkinson›s disease (HR, 3.27).
A sensitivity analysis found similar associations between concussion and risk for mood and anxiety disorder among all age groups. Younger participants were at greater risk for ADHD, however, and older participants were at greater risk for dementia and Parkinson’s disease.
Increased awareness of concussion and the outcomes of interest, along with improved diagnostic tools, may have influenced the study’s findings, Dr. Morissette noted. “The sex-based differences may be due to either pathophysiological differences in response to concussive injuries or potentially a difference in willingness to seek medical care or share symptoms, concussion-related or otherwise, with a medical professional,” he said.
“We are hopeful that our findings will encourage practitioners to be cognizant of various conditions that may present in individuals who have previously experienced a concussion,” Dr. Morissette added. “If physicians are aware of the various associations identified following a concussion, it may lead to more thorough clinical examination at initial presentation, along with more dedicated care throughout the patient’s life.”
Association versus causation
Commenting on the research, Steven Erickson, MD, sports medicine specialist at Banner–University Medicine Neuroscience Institute, Phoenix, Ariz., noted that although the study showed an association between concussion and subsequent diagnosis of ADHD, anxiety, and Parkinson’s disease, “this association should not be misconstrued as causation.” He added that the study’s conclusions “are just as likely to be due to labeling theory” or a self-fulfilling prophecy.
“Patients diagnosed with ADHD, anxiety, or Parkinson’s disease may recall concussion and associate the two diagnoses; but patients who have not previously been diagnosed with a concussion cannot draw that conclusion,” said Dr. Erickson, who was not involved with the research.
Citing the apparent gender difference in the strength of the association between concussion and the outcomes of interest, Dr. Erickson noted that women are more likely to report symptoms in general “and therefore are more likely to be diagnosed with ADHD and anxiety disorders” because of differences in reporting rather than incidence of disease.
“Further research needs to be done to definitively determine a causal relationship between concussion and any psychiatric or neurologic diagnosis,” Dr. Erickson concluded.
The study was funded by the Pan Am Clinic Foundation. Dr. Morissette and Dr. Erickson have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests. Results from a retrospective, population-based cohort study showed that controlling for socioeconomic status and overall health did not significantly affect this association.
The link between concussion and risk for ADHD and for mood and anxiety disorder was stronger in the women than in the men. In addition, having a history of multiple concussions strengthened the association between concussion and subsequent mood and anxiety disorder, dementia, and Parkinson’s disease compared with experiencing just one concussion.
The findings are similar to those of previous studies, noted lead author Marc P. Morissette, PhD, research assistant at the Pan Am Clinic Foundation in Winnipeg, Manitoba, Canada. “The main methodological differences separating our study from previous studies in this area is a focus on concussion-specific injuries identified from medical records and the potential for study participants to have up to 25 years of follow-up data,” said Dr. Morissette.
The findings were published online July 27 in Family Medicine and Community Health, a BMJ journal.
Almost 190,000 participants
Several studies have shown associations between head injury and increased risk for ADHD, depression, anxiety, Alzheimer’s disease, and Parkinson’s disease. However, many of these studies relied on self-reported medical history, included all forms of traumatic brain injury, and failed to adjust for preexisting health conditions.
An improved understanding of concussion and the risks associated with it could help physicians manage their patients’ long-term needs, the investigators noted.
In the current study, the researchers examined anonymized administrative health data collected between the periods of 1990–1991 and 2014–2015 in the Manitoba Population Research Data Repository at the Manitoba Center for Health Policy.
Eligible patients had been diagnosed with concussion in accordance with standard criteria. Participants were excluded if they had been diagnosed with dementia or Parkinson’s disease before the incident concussion during the study period. The investigators matched three control participants to each included patient on the basis of age, sex, and location.
Study outcome was time from index date (date of first concussion) to diagnosis of ADHD, mood and anxiety disorder, dementia, or Parkinson’s disease. The researchers controlled for socioeconomic status using the Socioeconomic Factor Index, version 2 (SEFI2), and for preexisting medical conditions using the Charlson Comorbidity Index (CCI).
The study included 28,021 men (mean age, 25 years) and 19,462 women (mean age, 30 years) in the concussion group and 81,871 men (mean age, 25 years) and 57,159 women (mean age, 30 years) in the control group. Mean SEFI2 score was approximately −0.05, and mean CCI score was approximately 0.2.
Dose effect?
Results showed that concussion was associated with an increased risk for ADHD (hazard ratio [HR], 1.39), mood and anxiety disorder (HR, 1.72), dementia (HR, 1.72), and Parkinson’s disease (HR, 1.57).
After a concussion, the risk of developing ADHD was 28% higher and the risk of developing mood and anxiety disorder was 7% higher among women than among men. Gender was not associated with risk for dementia or Parkinson’s disease after concussion.
Sustaining a second concussion increased the strength of the association with risk for dementia compared with sustaining a single concussion (HR, 1.62). Similarly, sustaining more than three concussions increased the strength of the association with the risk for mood and anxiety disorders (HR for more than three vs one concussion, 1.22) and Parkinson›s disease (HR, 3.27).
A sensitivity analysis found similar associations between concussion and risk for mood and anxiety disorder among all age groups. Younger participants were at greater risk for ADHD, however, and older participants were at greater risk for dementia and Parkinson’s disease.
Increased awareness of concussion and the outcomes of interest, along with improved diagnostic tools, may have influenced the study’s findings, Dr. Morissette noted. “The sex-based differences may be due to either pathophysiological differences in response to concussive injuries or potentially a difference in willingness to seek medical care or share symptoms, concussion-related or otherwise, with a medical professional,” he said.
“We are hopeful that our findings will encourage practitioners to be cognizant of various conditions that may present in individuals who have previously experienced a concussion,” Dr. Morissette added. “If physicians are aware of the various associations identified following a concussion, it may lead to more thorough clinical examination at initial presentation, along with more dedicated care throughout the patient’s life.”
Association versus causation
Commenting on the research, Steven Erickson, MD, sports medicine specialist at Banner–University Medicine Neuroscience Institute, Phoenix, Ariz., noted that although the study showed an association between concussion and subsequent diagnosis of ADHD, anxiety, and Parkinson’s disease, “this association should not be misconstrued as causation.” He added that the study’s conclusions “are just as likely to be due to labeling theory” or a self-fulfilling prophecy.
“Patients diagnosed with ADHD, anxiety, or Parkinson’s disease may recall concussion and associate the two diagnoses; but patients who have not previously been diagnosed with a concussion cannot draw that conclusion,” said Dr. Erickson, who was not involved with the research.
Citing the apparent gender difference in the strength of the association between concussion and the outcomes of interest, Dr. Erickson noted that women are more likely to report symptoms in general “and therefore are more likely to be diagnosed with ADHD and anxiety disorders” because of differences in reporting rather than incidence of disease.
“Further research needs to be done to definitively determine a causal relationship between concussion and any psychiatric or neurologic diagnosis,” Dr. Erickson concluded.
The study was funded by the Pan Am Clinic Foundation. Dr. Morissette and Dr. Erickson have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests. Results from a retrospective, population-based cohort study showed that controlling for socioeconomic status and overall health did not significantly affect this association.
The link between concussion and risk for ADHD and for mood and anxiety disorder was stronger in the women than in the men. In addition, having a history of multiple concussions strengthened the association between concussion and subsequent mood and anxiety disorder, dementia, and Parkinson’s disease compared with experiencing just one concussion.
The findings are similar to those of previous studies, noted lead author Marc P. Morissette, PhD, research assistant at the Pan Am Clinic Foundation in Winnipeg, Manitoba, Canada. “The main methodological differences separating our study from previous studies in this area is a focus on concussion-specific injuries identified from medical records and the potential for study participants to have up to 25 years of follow-up data,” said Dr. Morissette.
The findings were published online July 27 in Family Medicine and Community Health, a BMJ journal.
Almost 190,000 participants
Several studies have shown associations between head injury and increased risk for ADHD, depression, anxiety, Alzheimer’s disease, and Parkinson’s disease. However, many of these studies relied on self-reported medical history, included all forms of traumatic brain injury, and failed to adjust for preexisting health conditions.
An improved understanding of concussion and the risks associated with it could help physicians manage their patients’ long-term needs, the investigators noted.
In the current study, the researchers examined anonymized administrative health data collected between the periods of 1990–1991 and 2014–2015 in the Manitoba Population Research Data Repository at the Manitoba Center for Health Policy.
Eligible patients had been diagnosed with concussion in accordance with standard criteria. Participants were excluded if they had been diagnosed with dementia or Parkinson’s disease before the incident concussion during the study period. The investigators matched three control participants to each included patient on the basis of age, sex, and location.
Study outcome was time from index date (date of first concussion) to diagnosis of ADHD, mood and anxiety disorder, dementia, or Parkinson’s disease. The researchers controlled for socioeconomic status using the Socioeconomic Factor Index, version 2 (SEFI2), and for preexisting medical conditions using the Charlson Comorbidity Index (CCI).
The study included 28,021 men (mean age, 25 years) and 19,462 women (mean age, 30 years) in the concussion group and 81,871 men (mean age, 25 years) and 57,159 women (mean age, 30 years) in the control group. Mean SEFI2 score was approximately −0.05, and mean CCI score was approximately 0.2.
Dose effect?
Results showed that concussion was associated with an increased risk for ADHD (hazard ratio [HR], 1.39), mood and anxiety disorder (HR, 1.72), dementia (HR, 1.72), and Parkinson’s disease (HR, 1.57).
After a concussion, the risk of developing ADHD was 28% higher and the risk of developing mood and anxiety disorder was 7% higher among women than among men. Gender was not associated with risk for dementia or Parkinson’s disease after concussion.
Sustaining a second concussion increased the strength of the association with risk for dementia compared with sustaining a single concussion (HR, 1.62). Similarly, sustaining more than three concussions increased the strength of the association with the risk for mood and anxiety disorders (HR for more than three vs one concussion, 1.22) and Parkinson›s disease (HR, 3.27).
A sensitivity analysis found similar associations between concussion and risk for mood and anxiety disorder among all age groups. Younger participants were at greater risk for ADHD, however, and older participants were at greater risk for dementia and Parkinson’s disease.
Increased awareness of concussion and the outcomes of interest, along with improved diagnostic tools, may have influenced the study’s findings, Dr. Morissette noted. “The sex-based differences may be due to either pathophysiological differences in response to concussive injuries or potentially a difference in willingness to seek medical care or share symptoms, concussion-related or otherwise, with a medical professional,” he said.
“We are hopeful that our findings will encourage practitioners to be cognizant of various conditions that may present in individuals who have previously experienced a concussion,” Dr. Morissette added. “If physicians are aware of the various associations identified following a concussion, it may lead to more thorough clinical examination at initial presentation, along with more dedicated care throughout the patient’s life.”
Association versus causation
Commenting on the research, Steven Erickson, MD, sports medicine specialist at Banner–University Medicine Neuroscience Institute, Phoenix, Ariz., noted that although the study showed an association between concussion and subsequent diagnosis of ADHD, anxiety, and Parkinson’s disease, “this association should not be misconstrued as causation.” He added that the study’s conclusions “are just as likely to be due to labeling theory” or a self-fulfilling prophecy.
“Patients diagnosed with ADHD, anxiety, or Parkinson’s disease may recall concussion and associate the two diagnoses; but patients who have not previously been diagnosed with a concussion cannot draw that conclusion,” said Dr. Erickson, who was not involved with the research.
Citing the apparent gender difference in the strength of the association between concussion and the outcomes of interest, Dr. Erickson noted that women are more likely to report symptoms in general “and therefore are more likely to be diagnosed with ADHD and anxiety disorders” because of differences in reporting rather than incidence of disease.
“Further research needs to be done to definitively determine a causal relationship between concussion and any psychiatric or neurologic diagnosis,” Dr. Erickson concluded.
The study was funded by the Pan Am Clinic Foundation. Dr. Morissette and Dr. Erickson have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
From Family Medicine and Community Health
‘Landmark’ study pushed detection of covert consciousness in TBI
Compelling advances in the ability to detect signs of consciousness in unconscious patients who have experienced traumatic brain injury (TBI) are leading to unprecedented changes in the field. There is now hope of improving outcomes and even sparing lives of patients who may otherwise have been mistakenly assessed as having no chance of recovery.
That research, published in the New England Journal of Medicine in June 2019, linked the promising signals of consciousness in comatose patients, detected only on imaging, with remarkable outcomes a year later.
“This was a landmark study,” said Brian L. Edlow, MD, in a presentation on the issue of covert consciousness at the virtual annual meeting of the American Neurological Association.
“Importantly, it is the first compelling evidence that early detection of covert consciousness also predicts 1-year outcomes in the Glasgow Outcome Scale Extended (GOSE), showing that covert consciousness in the ICU appears to be relevant for predicting long-term outcomes,” said Dr. Edlow, who is associate director of the Center for Neurotechnology and Neurorecovery, Massachusetts General Hospital, in Boston.
The researchers showed that 15% of unconscious patients with acute brain injury in the study exhibited significant brain activity on EEG in response to stimuli that included verbal commands such as envisioning that they are playing tennis.
Although other studies have shown similar effects with task-based stimuli, the New England Journal of Medicine study further showed that a year later, the patients who had shown signs of covert consciousness, also called “cognitive motor dissociation” (CMD), were significantly more likely to have a good functional outcome, said the study’s senior author, Jan Claassen, MD, director of critical care neurology at Columbia University, New York, who also presented at the ANA session.
“Importantly, a year later after injury, we found that 44% of patients with CMD and only 14% of non-CMD patients had a good functional outcome, defined as a GOSE score indicating a state where they can at least take care of themselves for 8 hours in a day,” he said.
“[Whether] these patients in a CMD state represent a parallel state or a transitory state on the road to recovery remains to be shown,” he said.
Jennifer Frontera, MD, a professor in the department of neurology at NYU Langone Health in New York and comoderator of the session, agreed that the research is “remarkable.”
“Also,” she said, “it is practical, since many could potentially apply and validate his algorithms, since EEG technology is portable and widely available.”
Research has ushered in a ‘sea change’ in neurocritical care
The research has helped push forward recommendations on the treatment of unconscious patients, Dr. Edlow said. “This has led to a sea change in our field just over the last 2 years, with multiple guidelines published suggesting that it may be time for us to consider incorporating task-based fMRI and EEG techniques into our clinical assessment of patients with disorders of consciousness,” Dr. Edlow said.
Among those updating their recommendations was the American Academy of Neurology, which revised guidelines on practice parameters for patients in a persistent vegetative state. Those guidelines had not been updated since 1995.
Although concluding that “no diagnostic assessment procedure had moderate or strong evidence for use,” the guidelines acknowledge that “it is possible that a positive electromyographic (EMG) response to command, EEG reactivity to sensory stimuli, laser-evoked potentials, and the Perturbational Complexity Index can distinguish a minimally conscious state from vegetative state/unresponsive wakefulness syndrome (VS/UWS).”
Earlier this year, the European Academy of Neurology followed suit with updated guidelines of its own. In the EAN guideline, the academy’s Panel on Coma, Disorders of Consciousness recommends that task-based fMRI, EEG, and other advanced assessments be performed as part of a composite assessment of consciousness and that a patient’s best performance or highest level of consciousness on any of those tests should be a reflection of their diagnosis, Dr. Edlow explained.
“What this means is that our field is moving toward a multimodal assessment of consciousness in the ICU as well as beyond, in the subacute to chronic setting, whereby the behavioral exam, advanced DG, and advanced MRI methods all also contribute to the diagnosis of consciousness,” he said.
The standard for assessment of disorders of consciousness is the Coma Recovery Scale–Revised, with a 25-item scale for diagnosis, prediction of outcome, and assessment of potential treatment efficacy.
But much uncertainty can remain despite the assessment, Dr. Claassen said. “Behavioral assessments of patients with acute brain injury are challenging because examinations fluctuate, and there’s variability between assessors,” he said. “Nevertheless, patients and their families demand guidance from us.”
Dr. Edlow pointed out that the largest study to date of the causes of death among patients with TBI in the ICU underscores the need for better assessments.
The study of more than 600 patients at six level l trauma centers in Canada showed that 70% of patients who died in the ICU from TBI did so as the result of the withdrawal of life-sustaining therapy. However, only about a half (57%) had an unreactive pupil, and only about a quarter (23.7%) had evidence of herniation on CT, findings that are commonly associated with a poor prognosis.
“What emerges from this is that the manner in which the clinicians communicated the prognosis to families was a primary determinant of decisions to withdraw life-sustaining therapy,” Dr. Edlow said.
Negative response not necessarily conclusive
Dr. Edlow added a word of caution that the science is still far from perfect. He noted that, for 25% of healthy patients who are given a motor imagery task, neuroimaging might not show a response, implying that the lack of a signal may not be conclusive.
He described the case of a patient who was comatose at the time she was scanned on day 3 after injury and who showed no responses to language, music, or motor imagery during the MRI, yet a year later, she was functionally independent, back in the workforce, and had very few residual symptoms from her trauma.
“So if a patient does not show a response, that does not prove the patient is not conscious, and it does not prove that the patient is likely to have a poor outcome,” Dr. Edlow said. Such cases underscore the need for more advances in understanding the inner workings of brain injury.
Dr. Edlow and his colleagues are embarking on a trial of the effects of intravenous methylphenidate in targeting the stimulation of dopaminergic circuits within the subcortical ascending arousal network in patients with severe brain injuries.
“The scientific premise of the trial is that personalized brain network mapping in the ICU can identify patients whose connectomes are amenable to neuromodulation,” Dr. Edlow and his colleague report in an article in Neurocritical Care.
The trial, called STIMPACT (Stimulant Therapy Targeted to Individualized Connectivity Maps to Promote ReACTivation of Consciousness), is part of the newly launched Connectome-based Clinical Trial Platform, which the authors describe as “a new paradigm for developing and testing targeted therapies that promote early recovery of consciousness in the ICU.”
Such efforts are essential, given the high stakes of TBI outcomes, Dr. Edlow said.
“Let’s be clear about the stakes of an incorrect prognosis,” he said. “If we’re overly pessimistic, then a patient who could have potential for meaningful recovery will likely die in our ICU. On the other hand, if we are overly optimistic, then a patient could end up in a vegetative or minimally conscious state that he or she may never have found to be acceptable,” he said.
Access to technologies a ‘civil right?’
Some ethicists in the field are recommending that patients be given access to the advanced techniques as a civil right, similar to the rights described in the Convention on the Rights of Persons With Disabilities, which was adopted by the United Nations in 2008, Dr. Edlow noted.
“So the question that we as clinicians are going to face moving forward from an ethical standpoint is, if we have access to these techniques, is it an ethical obligation to offer them now?” he said.
Dr. Edlow underscored the need to consider the reality that “there are profound issues relating to resource allocation and access to these advanced techniques, but we’re going to have to consider this together as we move forward.”
Dr. Edlow has received funding from the National Institutes of Health. Dr. Claassen is a minority shareholder with ICE Neurosystems. Dr. Frontera has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Compelling advances in the ability to detect signs of consciousness in unconscious patients who have experienced traumatic brain injury (TBI) are leading to unprecedented changes in the field. There is now hope of improving outcomes and even sparing lives of patients who may otherwise have been mistakenly assessed as having no chance of recovery.
That research, published in the New England Journal of Medicine in June 2019, linked the promising signals of consciousness in comatose patients, detected only on imaging, with remarkable outcomes a year later.
“This was a landmark study,” said Brian L. Edlow, MD, in a presentation on the issue of covert consciousness at the virtual annual meeting of the American Neurological Association.
“Importantly, it is the first compelling evidence that early detection of covert consciousness also predicts 1-year outcomes in the Glasgow Outcome Scale Extended (GOSE), showing that covert consciousness in the ICU appears to be relevant for predicting long-term outcomes,” said Dr. Edlow, who is associate director of the Center for Neurotechnology and Neurorecovery, Massachusetts General Hospital, in Boston.
The researchers showed that 15% of unconscious patients with acute brain injury in the study exhibited significant brain activity on EEG in response to stimuli that included verbal commands such as envisioning that they are playing tennis.
Although other studies have shown similar effects with task-based stimuli, the New England Journal of Medicine study further showed that a year later, the patients who had shown signs of covert consciousness, also called “cognitive motor dissociation” (CMD), were significantly more likely to have a good functional outcome, said the study’s senior author, Jan Claassen, MD, director of critical care neurology at Columbia University, New York, who also presented at the ANA session.
“Importantly, a year later after injury, we found that 44% of patients with CMD and only 14% of non-CMD patients had a good functional outcome, defined as a GOSE score indicating a state where they can at least take care of themselves for 8 hours in a day,” he said.
“[Whether] these patients in a CMD state represent a parallel state or a transitory state on the road to recovery remains to be shown,” he said.
Jennifer Frontera, MD, a professor in the department of neurology at NYU Langone Health in New York and comoderator of the session, agreed that the research is “remarkable.”
“Also,” she said, “it is practical, since many could potentially apply and validate his algorithms, since EEG technology is portable and widely available.”
Research has ushered in a ‘sea change’ in neurocritical care
The research has helped push forward recommendations on the treatment of unconscious patients, Dr. Edlow said. “This has led to a sea change in our field just over the last 2 years, with multiple guidelines published suggesting that it may be time for us to consider incorporating task-based fMRI and EEG techniques into our clinical assessment of patients with disorders of consciousness,” Dr. Edlow said.
Among those updating their recommendations was the American Academy of Neurology, which revised guidelines on practice parameters for patients in a persistent vegetative state. Those guidelines had not been updated since 1995.
Although concluding that “no diagnostic assessment procedure had moderate or strong evidence for use,” the guidelines acknowledge that “it is possible that a positive electromyographic (EMG) response to command, EEG reactivity to sensory stimuli, laser-evoked potentials, and the Perturbational Complexity Index can distinguish a minimally conscious state from vegetative state/unresponsive wakefulness syndrome (VS/UWS).”
Earlier this year, the European Academy of Neurology followed suit with updated guidelines of its own. In the EAN guideline, the academy’s Panel on Coma, Disorders of Consciousness recommends that task-based fMRI, EEG, and other advanced assessments be performed as part of a composite assessment of consciousness and that a patient’s best performance or highest level of consciousness on any of those tests should be a reflection of their diagnosis, Dr. Edlow explained.
“What this means is that our field is moving toward a multimodal assessment of consciousness in the ICU as well as beyond, in the subacute to chronic setting, whereby the behavioral exam, advanced DG, and advanced MRI methods all also contribute to the diagnosis of consciousness,” he said.
The standard for assessment of disorders of consciousness is the Coma Recovery Scale–Revised, with a 25-item scale for diagnosis, prediction of outcome, and assessment of potential treatment efficacy.
But much uncertainty can remain despite the assessment, Dr. Claassen said. “Behavioral assessments of patients with acute brain injury are challenging because examinations fluctuate, and there’s variability between assessors,” he said. “Nevertheless, patients and their families demand guidance from us.”
Dr. Edlow pointed out that the largest study to date of the causes of death among patients with TBI in the ICU underscores the need for better assessments.
The study of more than 600 patients at six level l trauma centers in Canada showed that 70% of patients who died in the ICU from TBI did so as the result of the withdrawal of life-sustaining therapy. However, only about a half (57%) had an unreactive pupil, and only about a quarter (23.7%) had evidence of herniation on CT, findings that are commonly associated with a poor prognosis.
“What emerges from this is that the manner in which the clinicians communicated the prognosis to families was a primary determinant of decisions to withdraw life-sustaining therapy,” Dr. Edlow said.
Negative response not necessarily conclusive
Dr. Edlow added a word of caution that the science is still far from perfect. He noted that, for 25% of healthy patients who are given a motor imagery task, neuroimaging might not show a response, implying that the lack of a signal may not be conclusive.
He described the case of a patient who was comatose at the time she was scanned on day 3 after injury and who showed no responses to language, music, or motor imagery during the MRI, yet a year later, she was functionally independent, back in the workforce, and had very few residual symptoms from her trauma.
“So if a patient does not show a response, that does not prove the patient is not conscious, and it does not prove that the patient is likely to have a poor outcome,” Dr. Edlow said. Such cases underscore the need for more advances in understanding the inner workings of brain injury.
Dr. Edlow and his colleagues are embarking on a trial of the effects of intravenous methylphenidate in targeting the stimulation of dopaminergic circuits within the subcortical ascending arousal network in patients with severe brain injuries.
“The scientific premise of the trial is that personalized brain network mapping in the ICU can identify patients whose connectomes are amenable to neuromodulation,” Dr. Edlow and his colleague report in an article in Neurocritical Care.
The trial, called STIMPACT (Stimulant Therapy Targeted to Individualized Connectivity Maps to Promote ReACTivation of Consciousness), is part of the newly launched Connectome-based Clinical Trial Platform, which the authors describe as “a new paradigm for developing and testing targeted therapies that promote early recovery of consciousness in the ICU.”
Such efforts are essential, given the high stakes of TBI outcomes, Dr. Edlow said.
“Let’s be clear about the stakes of an incorrect prognosis,” he said. “If we’re overly pessimistic, then a patient who could have potential for meaningful recovery will likely die in our ICU. On the other hand, if we are overly optimistic, then a patient could end up in a vegetative or minimally conscious state that he or she may never have found to be acceptable,” he said.
Access to technologies a ‘civil right?’
Some ethicists in the field are recommending that patients be given access to the advanced techniques as a civil right, similar to the rights described in the Convention on the Rights of Persons With Disabilities, which was adopted by the United Nations in 2008, Dr. Edlow noted.
“So the question that we as clinicians are going to face moving forward from an ethical standpoint is, if we have access to these techniques, is it an ethical obligation to offer them now?” he said.
Dr. Edlow underscored the need to consider the reality that “there are profound issues relating to resource allocation and access to these advanced techniques, but we’re going to have to consider this together as we move forward.”
Dr. Edlow has received funding from the National Institutes of Health. Dr. Claassen is a minority shareholder with ICE Neurosystems. Dr. Frontera has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Compelling advances in the ability to detect signs of consciousness in unconscious patients who have experienced traumatic brain injury (TBI) are leading to unprecedented changes in the field. There is now hope of improving outcomes and even sparing lives of patients who may otherwise have been mistakenly assessed as having no chance of recovery.
That research, published in the New England Journal of Medicine in June 2019, linked the promising signals of consciousness in comatose patients, detected only on imaging, with remarkable outcomes a year later.
“This was a landmark study,” said Brian L. Edlow, MD, in a presentation on the issue of covert consciousness at the virtual annual meeting of the American Neurological Association.
“Importantly, it is the first compelling evidence that early detection of covert consciousness also predicts 1-year outcomes in the Glasgow Outcome Scale Extended (GOSE), showing that covert consciousness in the ICU appears to be relevant for predicting long-term outcomes,” said Dr. Edlow, who is associate director of the Center for Neurotechnology and Neurorecovery, Massachusetts General Hospital, in Boston.
The researchers showed that 15% of unconscious patients with acute brain injury in the study exhibited significant brain activity on EEG in response to stimuli that included verbal commands such as envisioning that they are playing tennis.
Although other studies have shown similar effects with task-based stimuli, the New England Journal of Medicine study further showed that a year later, the patients who had shown signs of covert consciousness, also called “cognitive motor dissociation” (CMD), were significantly more likely to have a good functional outcome, said the study’s senior author, Jan Claassen, MD, director of critical care neurology at Columbia University, New York, who also presented at the ANA session.
“Importantly, a year later after injury, we found that 44% of patients with CMD and only 14% of non-CMD patients had a good functional outcome, defined as a GOSE score indicating a state where they can at least take care of themselves for 8 hours in a day,” he said.
“[Whether] these patients in a CMD state represent a parallel state or a transitory state on the road to recovery remains to be shown,” he said.
Jennifer Frontera, MD, a professor in the department of neurology at NYU Langone Health in New York and comoderator of the session, agreed that the research is “remarkable.”
“Also,” she said, “it is practical, since many could potentially apply and validate his algorithms, since EEG technology is portable and widely available.”
Research has ushered in a ‘sea change’ in neurocritical care
The research has helped push forward recommendations on the treatment of unconscious patients, Dr. Edlow said. “This has led to a sea change in our field just over the last 2 years, with multiple guidelines published suggesting that it may be time for us to consider incorporating task-based fMRI and EEG techniques into our clinical assessment of patients with disorders of consciousness,” Dr. Edlow said.
Among those updating their recommendations was the American Academy of Neurology, which revised guidelines on practice parameters for patients in a persistent vegetative state. Those guidelines had not been updated since 1995.
Although concluding that “no diagnostic assessment procedure had moderate or strong evidence for use,” the guidelines acknowledge that “it is possible that a positive electromyographic (EMG) response to command, EEG reactivity to sensory stimuli, laser-evoked potentials, and the Perturbational Complexity Index can distinguish a minimally conscious state from vegetative state/unresponsive wakefulness syndrome (VS/UWS).”
Earlier this year, the European Academy of Neurology followed suit with updated guidelines of its own. In the EAN guideline, the academy’s Panel on Coma, Disorders of Consciousness recommends that task-based fMRI, EEG, and other advanced assessments be performed as part of a composite assessment of consciousness and that a patient’s best performance or highest level of consciousness on any of those tests should be a reflection of their diagnosis, Dr. Edlow explained.
“What this means is that our field is moving toward a multimodal assessment of consciousness in the ICU as well as beyond, in the subacute to chronic setting, whereby the behavioral exam, advanced DG, and advanced MRI methods all also contribute to the diagnosis of consciousness,” he said.
The standard for assessment of disorders of consciousness is the Coma Recovery Scale–Revised, with a 25-item scale for diagnosis, prediction of outcome, and assessment of potential treatment efficacy.
But much uncertainty can remain despite the assessment, Dr. Claassen said. “Behavioral assessments of patients with acute brain injury are challenging because examinations fluctuate, and there’s variability between assessors,” he said. “Nevertheless, patients and their families demand guidance from us.”
Dr. Edlow pointed out that the largest study to date of the causes of death among patients with TBI in the ICU underscores the need for better assessments.
The study of more than 600 patients at six level l trauma centers in Canada showed that 70% of patients who died in the ICU from TBI did so as the result of the withdrawal of life-sustaining therapy. However, only about a half (57%) had an unreactive pupil, and only about a quarter (23.7%) had evidence of herniation on CT, findings that are commonly associated with a poor prognosis.
“What emerges from this is that the manner in which the clinicians communicated the prognosis to families was a primary determinant of decisions to withdraw life-sustaining therapy,” Dr. Edlow said.
Negative response not necessarily conclusive
Dr. Edlow added a word of caution that the science is still far from perfect. He noted that, for 25% of healthy patients who are given a motor imagery task, neuroimaging might not show a response, implying that the lack of a signal may not be conclusive.
He described the case of a patient who was comatose at the time she was scanned on day 3 after injury and who showed no responses to language, music, or motor imagery during the MRI, yet a year later, she was functionally independent, back in the workforce, and had very few residual symptoms from her trauma.
“So if a patient does not show a response, that does not prove the patient is not conscious, and it does not prove that the patient is likely to have a poor outcome,” Dr. Edlow said. Such cases underscore the need for more advances in understanding the inner workings of brain injury.
Dr. Edlow and his colleagues are embarking on a trial of the effects of intravenous methylphenidate in targeting the stimulation of dopaminergic circuits within the subcortical ascending arousal network in patients with severe brain injuries.
“The scientific premise of the trial is that personalized brain network mapping in the ICU can identify patients whose connectomes are amenable to neuromodulation,” Dr. Edlow and his colleague report in an article in Neurocritical Care.
The trial, called STIMPACT (Stimulant Therapy Targeted to Individualized Connectivity Maps to Promote ReACTivation of Consciousness), is part of the newly launched Connectome-based Clinical Trial Platform, which the authors describe as “a new paradigm for developing and testing targeted therapies that promote early recovery of consciousness in the ICU.”
Such efforts are essential, given the high stakes of TBI outcomes, Dr. Edlow said.
“Let’s be clear about the stakes of an incorrect prognosis,” he said. “If we’re overly pessimistic, then a patient who could have potential for meaningful recovery will likely die in our ICU. On the other hand, if we are overly optimistic, then a patient could end up in a vegetative or minimally conscious state that he or she may never have found to be acceptable,” he said.
Access to technologies a ‘civil right?’
Some ethicists in the field are recommending that patients be given access to the advanced techniques as a civil right, similar to the rights described in the Convention on the Rights of Persons With Disabilities, which was adopted by the United Nations in 2008, Dr. Edlow noted.
“So the question that we as clinicians are going to face moving forward from an ethical standpoint is, if we have access to these techniques, is it an ethical obligation to offer them now?” he said.
Dr. Edlow underscored the need to consider the reality that “there are profound issues relating to resource allocation and access to these advanced techniques, but we’re going to have to consider this together as we move forward.”
Dr. Edlow has received funding from the National Institutes of Health. Dr. Claassen is a minority shareholder with ICE Neurosystems. Dr. Frontera has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM ANA 2020