User login
Malpractice Counsel
Carbon Monoxide Poisoning
A 72-year-old man was brought to the ED by paramedics with inability to move his left leg and difficulty speaking. The patient had been heating his home with a generator placed inside the house during an ice storm, and paramedics reported a strong smell of gas inside the house.
The patient was unable to describe the time of onset of his symptoms. He complained of headache, slurred speech, and inability to move his left leg. He also said he felt the urge to urinate, but was unable to do so. He denied chest pain or shortness of breath. His medical history was significant only for hypertension, which was controlled with hydrochlorothiazide and lisinopril. He admitted to smoking a few cigarettes daily, but denied any alcohol use.
On physical examination, the patient’s vital signs were: blood pressure (BP) 162/98 mm Hg; heart rate (HR), 110 beats/minute; respiratory rate (RR), 20 breaths/minute; and temperature (T), 98.6˚F. The patient had 100% oxygen (O2) saturation on 4L O2 via nasal cannula. The head, eyes, ears, nose, and throat examination was normal. There was no facial droop; his speech was slurred, but he was easily understandable. The cardiopulmonary examination revealed tachycardia without murmurs, rubs, or gallop; the lungs were clear to auscultation bilaterally. The neurological examination revealed 5/5 motor strength in the upper extremities and symmetrical; there was no pronator drift. The left leg had 2/5 motor strength compared to 5/5 in the right lower extremity. There was also fullness and tenderness over his suprapubic region.
The emergency physician (EP) ordered a complete blood count, basic metabolic profile, carboxyhemoglobin (COHb) test, electrocardiogram (ECG), portable chest X-ray (CXR), and a noncontrast computed tomography (CT) scan of the head. Since the history and physical examination suggested urinary retention, a Foley catheter was placed; a total of 1,200 cc of clear urine was obtained, after which the patient expressed a feeling of relief.
The patient’s COHb level was 8.5%. The portable CXR and CT scan of the head were both reported as normal by the radiologist. Likewise, the results of the rest of the laboratory evaluation were normal. The ECG revealed sinus tachycardia without evidence of strain or injury.
The EP diagnosed an acute cerebrovascular accident (CVA) and admitted the patient to the hospital. He did not feel that carbon monoxide (CO) contributed to the event given the low level in a cigarette smoker. After an uneventful hospital stay, the patient was transferred to a physical rehabilitation unit. He was ultimately discharged with a neurogenic bladder and weak left leg.
The patient sued the EP for negligence in the failure to diagnose CO poisoning and prompt initiation of 100% O2 therapy. The EP argued that CO poisoning had been properly ruled out and that the diagnosis of CVA was correct. The defense also claimed that even if the patient had suffered CO poisoning, the length of the exposure would have led to the same outcome. A defense verdict was returned.
Discussion
Carbon monoxide poisoning is one of the leading causes of poisoning morbidity and mortality in the United States. This is in part due to the fact that CO is a colorless, odorless, and tasteless gas. The peak incidence for CO poisoning is in the fall and winter, when people are more likely to use space heaters, wood burning stoves, or portable generators inside without adequate ventilation.
The clinical presentation of CO poisoning can range from mild (eg, headache, flu-like symptoms) to devastating (eg, coma, death). The central nervous system is the organ system that is most sensitive to CO poisoning. Symptoms can range from a dull frontal headache, dizziness, and ataxia, to syncope, seizures, focal neurological deficit, and coma. In fact, the most serious complication of CO poisoning may be persistent or delayed neurological or neurocognitive sequelae, which can occur in up to 50% of patients with symptomatic acute poisoning.1 Unfortunately, COHb levels and symptoms do not always correlate well. In fact, particular COHb levels are not predictive of symptoms or outcome.1
The treatment for CO poisoning consists of administering 100% O2 as soon as the diagnosis is considered. If 100% O2 is administered, the half-life of COHb can be reduced from 5 hours (room air) to approximately 1 hour.1 While some argue that treatment with hyperbaric O2 (HBO) therapy should be considered standard of care, it has not yet been determined which patient population benefits from HBO therapy; moreover, there is currently no established optimum timing of therapy. Regardless, the jury came to the correct decision in this case as it is impossible to determine, with any degree of medical certainty, if the patient’s neurological deficits were due to the natural course of an ischemic stroke, or if CO contributed to or was the sole cause of the CVA.
Death in the Emergency Department
A 43-year-old man presented to the ED with the chief complaint of a lower lip laceration. The patient stated he had gotten into an altercation with his girlfriend just prior to arrival. She had punched the patient in the face with her fist, resulting in the lip laceration. The patient denied any loss of consciousness or other pain. He did, however, smell of alcohol and was emotionally labile, crying one moment and yelling the next.
The patient was instructed to remove all of his clothes, change into a hospital gown and give all of his belongings to hospital security. He removed his clothes, but refused to turn them over to security. This prompted a physical altercation between the patient and hospital security. Three hospital security guards wrestled the patient to the ground and placed him face down; one guard placed the patient in a choke hold while the other two guards sat on top of him. Within a few moments, the patient became unresponsive. He was placed immediately on a stretcher and intubated by the EP. After successful intubation and bagging with 100% O2, the patient regained a palpable pulse, but remained unresponsive.
The patient was admitted to the intensive care unit, but never regained consciousness and died 5 days later. The cause of death was thought to be anoxic brain injury due to asphyxiation. The family of the patient sued the hospital and the EP for causing asphyxiation and death in this patient seeking medical care. The hospital denied responsibility for the death because the patient both instigated the altercation and had a preexisting heart condition. According to published reports, a $2.5 million settlement was reached.
Discussion
This unfortunate case did not involve the EP; all of the important events transpired prior to the EP’s initial interaction with the patient. There are not enough details to explain how this situation escalated so rapidly, or why hospital security felt this was the best way to subdue the patient.
Unfortunately, EPs are no strangers to agitated patients. Behavioral emergencies account for approximately 5% of all ED visits, and these usually involve some form of violence or agitation.1 Every physician and nurse working in the ED must be prepared to deal with patients who have the potential to become violent. Clearly, training of all patient-care personnel to handle such patients in the ED is important to ensuring both staff and patient safety. Having the patient undress and change into a hospital gown is the correct first step. This allows for removal of real or potential weapons, and makes it much less likely for the patient to leave before his or her evaluation and management is complete. Doing this properly, however, is key. Providing the patient with a warm blanket or food, or just talking to him or her in a calm and reassuring voice, can often prevent escalation. Simply arguing with the patient rarely works, and often has the opposite desired effect.
If the situation continues to escalate, and it appears either physical or chemical restraint will be necessary, a “show of force” should be made. A restraint team consisting of at least five trained members should be assembled, with the EP acting as the team leader. The team should all enter the room at the same time, explain what will happen, and then move quickly.1 The leader should move to the head of the bed and direct the team, while the remaining four members each take a limb. To preserve the physician-patient relationship, it is best if the EP is not actively involved in placing the physical restraints.
The choke hold should only be considered as a method of last resort. Many police departments in the country prohibit use of the choke hold because of complications such as those observed in this case. The use of choke holds became a topic of intense debate this summer with the death of Eric Garner in Staten Island, New York; it was thought that his pre-existing conditions of obesity, asthma, and heart disease were all aggravated by the choke hold. Although obese patients are often at a higher risk for complications due to pre-existing issues with adequate oxygenation, it is unclear whether the patient in this case was obese.
An alternative strategy in handling an agitated patient would be the use of a taser by trained security personnel. In one study, 99.75% of tasered patients had no significant injury as a result of the device.2 In 2009, the American Medical Association found that tasers, “when used appropriately, can save lives during interventions that would have otherwise involved the use of deadly force.” While the safety of patients and the ED staff (nurses, physicians, and technicians) is paramount, the clinician should always adhere to the principle of “primum non nocere”—“first, do no harm.”
Reference - Carbon Monoxide Poisoning
- Tomaszewski C: Carbon monoxide. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:1658.
Reference - Death in the Emergency Department
- Rossi J, Swan MC, Issacs ED. The violent or agitated patient. Emerg Med Clin North Am. 2010;28(1):235-256.
- Bozeman WP, Hauda WE 2nd, Heck JJ, Graham DD Jr, Martin BP, Winslow JE. Safety and injury profile of conducted electrical weapons used by law enforcement officers against criminal suspects. Ann Emerg Med. 2009;53(4):480-489.
Carbon Monoxide Poisoning
A 72-year-old man was brought to the ED by paramedics with inability to move his left leg and difficulty speaking. The patient had been heating his home with a generator placed inside the house during an ice storm, and paramedics reported a strong smell of gas inside the house.
The patient was unable to describe the time of onset of his symptoms. He complained of headache, slurred speech, and inability to move his left leg. He also said he felt the urge to urinate, but was unable to do so. He denied chest pain or shortness of breath. His medical history was significant only for hypertension, which was controlled with hydrochlorothiazide and lisinopril. He admitted to smoking a few cigarettes daily, but denied any alcohol use.
On physical examination, the patient’s vital signs were: blood pressure (BP) 162/98 mm Hg; heart rate (HR), 110 beats/minute; respiratory rate (RR), 20 breaths/minute; and temperature (T), 98.6˚F. The patient had 100% oxygen (O2) saturation on 4L O2 via nasal cannula. The head, eyes, ears, nose, and throat examination was normal. There was no facial droop; his speech was slurred, but he was easily understandable. The cardiopulmonary examination revealed tachycardia without murmurs, rubs, or gallop; the lungs were clear to auscultation bilaterally. The neurological examination revealed 5/5 motor strength in the upper extremities and symmetrical; there was no pronator drift. The left leg had 2/5 motor strength compared to 5/5 in the right lower extremity. There was also fullness and tenderness over his suprapubic region.
The emergency physician (EP) ordered a complete blood count, basic metabolic profile, carboxyhemoglobin (COHb) test, electrocardiogram (ECG), portable chest X-ray (CXR), and a noncontrast computed tomography (CT) scan of the head. Since the history and physical examination suggested urinary retention, a Foley catheter was placed; a total of 1,200 cc of clear urine was obtained, after which the patient expressed a feeling of relief.
The patient’s COHb level was 8.5%. The portable CXR and CT scan of the head were both reported as normal by the radiologist. Likewise, the results of the rest of the laboratory evaluation were normal. The ECG revealed sinus tachycardia without evidence of strain or injury.
The EP diagnosed an acute cerebrovascular accident (CVA) and admitted the patient to the hospital. He did not feel that carbon monoxide (CO) contributed to the event given the low level in a cigarette smoker. After an uneventful hospital stay, the patient was transferred to a physical rehabilitation unit. He was ultimately discharged with a neurogenic bladder and weak left leg.
The patient sued the EP for negligence in the failure to diagnose CO poisoning and prompt initiation of 100% O2 therapy. The EP argued that CO poisoning had been properly ruled out and that the diagnosis of CVA was correct. The defense also claimed that even if the patient had suffered CO poisoning, the length of the exposure would have led to the same outcome. A defense verdict was returned.
Discussion
Carbon monoxide poisoning is one of the leading causes of poisoning morbidity and mortality in the United States. This is in part due to the fact that CO is a colorless, odorless, and tasteless gas. The peak incidence for CO poisoning is in the fall and winter, when people are more likely to use space heaters, wood burning stoves, or portable generators inside without adequate ventilation.
The clinical presentation of CO poisoning can range from mild (eg, headache, flu-like symptoms) to devastating (eg, coma, death). The central nervous system is the organ system that is most sensitive to CO poisoning. Symptoms can range from a dull frontal headache, dizziness, and ataxia, to syncope, seizures, focal neurological deficit, and coma. In fact, the most serious complication of CO poisoning may be persistent or delayed neurological or neurocognitive sequelae, which can occur in up to 50% of patients with symptomatic acute poisoning.1 Unfortunately, COHb levels and symptoms do not always correlate well. In fact, particular COHb levels are not predictive of symptoms or outcome.1
The treatment for CO poisoning consists of administering 100% O2 as soon as the diagnosis is considered. If 100% O2 is administered, the half-life of COHb can be reduced from 5 hours (room air) to approximately 1 hour.1 While some argue that treatment with hyperbaric O2 (HBO) therapy should be considered standard of care, it has not yet been determined which patient population benefits from HBO therapy; moreover, there is currently no established optimum timing of therapy. Regardless, the jury came to the correct decision in this case as it is impossible to determine, with any degree of medical certainty, if the patient’s neurological deficits were due to the natural course of an ischemic stroke, or if CO contributed to or was the sole cause of the CVA.
Death in the Emergency Department
A 43-year-old man presented to the ED with the chief complaint of a lower lip laceration. The patient stated he had gotten into an altercation with his girlfriend just prior to arrival. She had punched the patient in the face with her fist, resulting in the lip laceration. The patient denied any loss of consciousness or other pain. He did, however, smell of alcohol and was emotionally labile, crying one moment and yelling the next.
The patient was instructed to remove all of his clothes, change into a hospital gown and give all of his belongings to hospital security. He removed his clothes, but refused to turn them over to security. This prompted a physical altercation between the patient and hospital security. Three hospital security guards wrestled the patient to the ground and placed him face down; one guard placed the patient in a choke hold while the other two guards sat on top of him. Within a few moments, the patient became unresponsive. He was placed immediately on a stretcher and intubated by the EP. After successful intubation and bagging with 100% O2, the patient regained a palpable pulse, but remained unresponsive.
The patient was admitted to the intensive care unit, but never regained consciousness and died 5 days later. The cause of death was thought to be anoxic brain injury due to asphyxiation. The family of the patient sued the hospital and the EP for causing asphyxiation and death in this patient seeking medical care. The hospital denied responsibility for the death because the patient both instigated the altercation and had a preexisting heart condition. According to published reports, a $2.5 million settlement was reached.
Discussion
This unfortunate case did not involve the EP; all of the important events transpired prior to the EP’s initial interaction with the patient. There are not enough details to explain how this situation escalated so rapidly, or why hospital security felt this was the best way to subdue the patient.
Unfortunately, EPs are no strangers to agitated patients. Behavioral emergencies account for approximately 5% of all ED visits, and these usually involve some form of violence or agitation.1 Every physician and nurse working in the ED must be prepared to deal with patients who have the potential to become violent. Clearly, training of all patient-care personnel to handle such patients in the ED is important to ensuring both staff and patient safety. Having the patient undress and change into a hospital gown is the correct first step. This allows for removal of real or potential weapons, and makes it much less likely for the patient to leave before his or her evaluation and management is complete. Doing this properly, however, is key. Providing the patient with a warm blanket or food, or just talking to him or her in a calm and reassuring voice, can often prevent escalation. Simply arguing with the patient rarely works, and often has the opposite desired effect.
If the situation continues to escalate, and it appears either physical or chemical restraint will be necessary, a “show of force” should be made. A restraint team consisting of at least five trained members should be assembled, with the EP acting as the team leader. The team should all enter the room at the same time, explain what will happen, and then move quickly.1 The leader should move to the head of the bed and direct the team, while the remaining four members each take a limb. To preserve the physician-patient relationship, it is best if the EP is not actively involved in placing the physical restraints.
The choke hold should only be considered as a method of last resort. Many police departments in the country prohibit use of the choke hold because of complications such as those observed in this case. The use of choke holds became a topic of intense debate this summer with the death of Eric Garner in Staten Island, New York; it was thought that his pre-existing conditions of obesity, asthma, and heart disease were all aggravated by the choke hold. Although obese patients are often at a higher risk for complications due to pre-existing issues with adequate oxygenation, it is unclear whether the patient in this case was obese.
An alternative strategy in handling an agitated patient would be the use of a taser by trained security personnel. In one study, 99.75% of tasered patients had no significant injury as a result of the device.2 In 2009, the American Medical Association found that tasers, “when used appropriately, can save lives during interventions that would have otherwise involved the use of deadly force.” While the safety of patients and the ED staff (nurses, physicians, and technicians) is paramount, the clinician should always adhere to the principle of “primum non nocere”—“first, do no harm.”
Carbon Monoxide Poisoning
A 72-year-old man was brought to the ED by paramedics with inability to move his left leg and difficulty speaking. The patient had been heating his home with a generator placed inside the house during an ice storm, and paramedics reported a strong smell of gas inside the house.
The patient was unable to describe the time of onset of his symptoms. He complained of headache, slurred speech, and inability to move his left leg. He also said he felt the urge to urinate, but was unable to do so. He denied chest pain or shortness of breath. His medical history was significant only for hypertension, which was controlled with hydrochlorothiazide and lisinopril. He admitted to smoking a few cigarettes daily, but denied any alcohol use.
On physical examination, the patient’s vital signs were: blood pressure (BP) 162/98 mm Hg; heart rate (HR), 110 beats/minute; respiratory rate (RR), 20 breaths/minute; and temperature (T), 98.6˚F. The patient had 100% oxygen (O2) saturation on 4L O2 via nasal cannula. The head, eyes, ears, nose, and throat examination was normal. There was no facial droop; his speech was slurred, but he was easily understandable. The cardiopulmonary examination revealed tachycardia without murmurs, rubs, or gallop; the lungs were clear to auscultation bilaterally. The neurological examination revealed 5/5 motor strength in the upper extremities and symmetrical; there was no pronator drift. The left leg had 2/5 motor strength compared to 5/5 in the right lower extremity. There was also fullness and tenderness over his suprapubic region.
The emergency physician (EP) ordered a complete blood count, basic metabolic profile, carboxyhemoglobin (COHb) test, electrocardiogram (ECG), portable chest X-ray (CXR), and a noncontrast computed tomography (CT) scan of the head. Since the history and physical examination suggested urinary retention, a Foley catheter was placed; a total of 1,200 cc of clear urine was obtained, after which the patient expressed a feeling of relief.
The patient’s COHb level was 8.5%. The portable CXR and CT scan of the head were both reported as normal by the radiologist. Likewise, the results of the rest of the laboratory evaluation were normal. The ECG revealed sinus tachycardia without evidence of strain or injury.
The EP diagnosed an acute cerebrovascular accident (CVA) and admitted the patient to the hospital. He did not feel that carbon monoxide (CO) contributed to the event given the low level in a cigarette smoker. After an uneventful hospital stay, the patient was transferred to a physical rehabilitation unit. He was ultimately discharged with a neurogenic bladder and weak left leg.
The patient sued the EP for negligence in the failure to diagnose CO poisoning and prompt initiation of 100% O2 therapy. The EP argued that CO poisoning had been properly ruled out and that the diagnosis of CVA was correct. The defense also claimed that even if the patient had suffered CO poisoning, the length of the exposure would have led to the same outcome. A defense verdict was returned.
Discussion
Carbon monoxide poisoning is one of the leading causes of poisoning morbidity and mortality in the United States. This is in part due to the fact that CO is a colorless, odorless, and tasteless gas. The peak incidence for CO poisoning is in the fall and winter, when people are more likely to use space heaters, wood burning stoves, or portable generators inside without adequate ventilation.
The clinical presentation of CO poisoning can range from mild (eg, headache, flu-like symptoms) to devastating (eg, coma, death). The central nervous system is the organ system that is most sensitive to CO poisoning. Symptoms can range from a dull frontal headache, dizziness, and ataxia, to syncope, seizures, focal neurological deficit, and coma. In fact, the most serious complication of CO poisoning may be persistent or delayed neurological or neurocognitive sequelae, which can occur in up to 50% of patients with symptomatic acute poisoning.1 Unfortunately, COHb levels and symptoms do not always correlate well. In fact, particular COHb levels are not predictive of symptoms or outcome.1
The treatment for CO poisoning consists of administering 100% O2 as soon as the diagnosis is considered. If 100% O2 is administered, the half-life of COHb can be reduced from 5 hours (room air) to approximately 1 hour.1 While some argue that treatment with hyperbaric O2 (HBO) therapy should be considered standard of care, it has not yet been determined which patient population benefits from HBO therapy; moreover, there is currently no established optimum timing of therapy. Regardless, the jury came to the correct decision in this case as it is impossible to determine, with any degree of medical certainty, if the patient’s neurological deficits were due to the natural course of an ischemic stroke, or if CO contributed to or was the sole cause of the CVA.
Death in the Emergency Department
A 43-year-old man presented to the ED with the chief complaint of a lower lip laceration. The patient stated he had gotten into an altercation with his girlfriend just prior to arrival. She had punched the patient in the face with her fist, resulting in the lip laceration. The patient denied any loss of consciousness or other pain. He did, however, smell of alcohol and was emotionally labile, crying one moment and yelling the next.
The patient was instructed to remove all of his clothes, change into a hospital gown and give all of his belongings to hospital security. He removed his clothes, but refused to turn them over to security. This prompted a physical altercation between the patient and hospital security. Three hospital security guards wrestled the patient to the ground and placed him face down; one guard placed the patient in a choke hold while the other two guards sat on top of him. Within a few moments, the patient became unresponsive. He was placed immediately on a stretcher and intubated by the EP. After successful intubation and bagging with 100% O2, the patient regained a palpable pulse, but remained unresponsive.
The patient was admitted to the intensive care unit, but never regained consciousness and died 5 days later. The cause of death was thought to be anoxic brain injury due to asphyxiation. The family of the patient sued the hospital and the EP for causing asphyxiation and death in this patient seeking medical care. The hospital denied responsibility for the death because the patient both instigated the altercation and had a preexisting heart condition. According to published reports, a $2.5 million settlement was reached.
Discussion
This unfortunate case did not involve the EP; all of the important events transpired prior to the EP’s initial interaction with the patient. There are not enough details to explain how this situation escalated so rapidly, or why hospital security felt this was the best way to subdue the patient.
Unfortunately, EPs are no strangers to agitated patients. Behavioral emergencies account for approximately 5% of all ED visits, and these usually involve some form of violence or agitation.1 Every physician and nurse working in the ED must be prepared to deal with patients who have the potential to become violent. Clearly, training of all patient-care personnel to handle such patients in the ED is important to ensuring both staff and patient safety. Having the patient undress and change into a hospital gown is the correct first step. This allows for removal of real or potential weapons, and makes it much less likely for the patient to leave before his or her evaluation and management is complete. Doing this properly, however, is key. Providing the patient with a warm blanket or food, or just talking to him or her in a calm and reassuring voice, can often prevent escalation. Simply arguing with the patient rarely works, and often has the opposite desired effect.
If the situation continues to escalate, and it appears either physical or chemical restraint will be necessary, a “show of force” should be made. A restraint team consisting of at least five trained members should be assembled, with the EP acting as the team leader. The team should all enter the room at the same time, explain what will happen, and then move quickly.1 The leader should move to the head of the bed and direct the team, while the remaining four members each take a limb. To preserve the physician-patient relationship, it is best if the EP is not actively involved in placing the physical restraints.
The choke hold should only be considered as a method of last resort. Many police departments in the country prohibit use of the choke hold because of complications such as those observed in this case. The use of choke holds became a topic of intense debate this summer with the death of Eric Garner in Staten Island, New York; it was thought that his pre-existing conditions of obesity, asthma, and heart disease were all aggravated by the choke hold. Although obese patients are often at a higher risk for complications due to pre-existing issues with adequate oxygenation, it is unclear whether the patient in this case was obese.
An alternative strategy in handling an agitated patient would be the use of a taser by trained security personnel. In one study, 99.75% of tasered patients had no significant injury as a result of the device.2 In 2009, the American Medical Association found that tasers, “when used appropriately, can save lives during interventions that would have otherwise involved the use of deadly force.” While the safety of patients and the ED staff (nurses, physicians, and technicians) is paramount, the clinician should always adhere to the principle of “primum non nocere”—“first, do no harm.”
Reference - Carbon Monoxide Poisoning
- Tomaszewski C: Carbon monoxide. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:1658.
Reference - Death in the Emergency Department
- Rossi J, Swan MC, Issacs ED. The violent or agitated patient. Emerg Med Clin North Am. 2010;28(1):235-256.
- Bozeman WP, Hauda WE 2nd, Heck JJ, Graham DD Jr, Martin BP, Winslow JE. Safety and injury profile of conducted electrical weapons used by law enforcement officers against criminal suspects. Ann Emerg Med. 2009;53(4):480-489.
Reference - Carbon Monoxide Poisoning
- Tomaszewski C: Carbon monoxide. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:1658.
Reference - Death in the Emergency Department
- Rossi J, Swan MC, Issacs ED. The violent or agitated patient. Emerg Med Clin North Am. 2010;28(1):235-256.
- Bozeman WP, Hauda WE 2nd, Heck JJ, Graham DD Jr, Martin BP, Winslow JE. Safety and injury profile of conducted electrical weapons used by law enforcement officers against criminal suspects. Ann Emerg Med. 2009;53(4):480-489.
FDA approves Anthrasil to treat inhalational anthrax
The Food and Drug Administration has approved Anthrasil, Anthrax Immune Globulin Intravenous (Human), for treatment of inhalational anthrax when used with appropriate antibacterial drugs.
Inhalational anthrax is caused by breathing in Bacillus anthracis spores, which can occur after exposure to infected animals or contaminated animal products, or as a result of an intentional release of spores. In a statement, Dr. Karen Midthun – director of the FDA’s Center for Biologics Evaluation and Research – explained that Anthrasil “will be stored in U.S. Strategic National Stockpile to facilitate its availability in response to an anthrax emergency.”
Anthrasil was purchased by the U.S. Department of Health & Human Services’ Biomedical Advanced Research and Development Authority (BARDA) in 2011, but because it was not approved, its use prior to FDA approval would have required an emergency use authorization from the FDA.
The efficacy of Anthrasil was studied in animals because it was not feasible or ethical to conduct adequately controlled efficacy studies in humans, the FDA said. Monkeys and rabbits were exposed to Bacillus anthracis spores, and subsequently given either Anthrasil or a placebo. The survival rate for monkeys given Anthrasil was between 36% and 70%, with a trend toward increased survival at higher doses of Anthrasil. None of the monkeys given placebo survived. Rabbits had a 26% survival rate when given the drug, compared to 2% of those given placebo. A separate study exposed rabbits to Bacillus anthracis and treated them with either antibiotics or a combination of antibiotics and Anthrasil; survival rates were 71% for those treated with the combination and 25% for those treated with antibiotics only.
Safety was tested in 74 healthy human volunteers and the most commonly reported side effects were headache, back pain, nausea, and pain and swelling at the infusion site.
Anthrasil is manufactured by Cangene Corporation, based in Winnipeg, Canada, which developed the drug in collaboration with BARDA.
The Food and Drug Administration has approved Anthrasil, Anthrax Immune Globulin Intravenous (Human), for treatment of inhalational anthrax when used with appropriate antibacterial drugs.
Inhalational anthrax is caused by breathing in Bacillus anthracis spores, which can occur after exposure to infected animals or contaminated animal products, or as a result of an intentional release of spores. In a statement, Dr. Karen Midthun – director of the FDA’s Center for Biologics Evaluation and Research – explained that Anthrasil “will be stored in U.S. Strategic National Stockpile to facilitate its availability in response to an anthrax emergency.”
Anthrasil was purchased by the U.S. Department of Health & Human Services’ Biomedical Advanced Research and Development Authority (BARDA) in 2011, but because it was not approved, its use prior to FDA approval would have required an emergency use authorization from the FDA.
The efficacy of Anthrasil was studied in animals because it was not feasible or ethical to conduct adequately controlled efficacy studies in humans, the FDA said. Monkeys and rabbits were exposed to Bacillus anthracis spores, and subsequently given either Anthrasil or a placebo. The survival rate for monkeys given Anthrasil was between 36% and 70%, with a trend toward increased survival at higher doses of Anthrasil. None of the monkeys given placebo survived. Rabbits had a 26% survival rate when given the drug, compared to 2% of those given placebo. A separate study exposed rabbits to Bacillus anthracis and treated them with either antibiotics or a combination of antibiotics and Anthrasil; survival rates were 71% for those treated with the combination and 25% for those treated with antibiotics only.
Safety was tested in 74 healthy human volunteers and the most commonly reported side effects were headache, back pain, nausea, and pain and swelling at the infusion site.
Anthrasil is manufactured by Cangene Corporation, based in Winnipeg, Canada, which developed the drug in collaboration with BARDA.
The Food and Drug Administration has approved Anthrasil, Anthrax Immune Globulin Intravenous (Human), for treatment of inhalational anthrax when used with appropriate antibacterial drugs.
Inhalational anthrax is caused by breathing in Bacillus anthracis spores, which can occur after exposure to infected animals or contaminated animal products, or as a result of an intentional release of spores. In a statement, Dr. Karen Midthun – director of the FDA’s Center for Biologics Evaluation and Research – explained that Anthrasil “will be stored in U.S. Strategic National Stockpile to facilitate its availability in response to an anthrax emergency.”
Anthrasil was purchased by the U.S. Department of Health & Human Services’ Biomedical Advanced Research and Development Authority (BARDA) in 2011, but because it was not approved, its use prior to FDA approval would have required an emergency use authorization from the FDA.
The efficacy of Anthrasil was studied in animals because it was not feasible or ethical to conduct adequately controlled efficacy studies in humans, the FDA said. Monkeys and rabbits were exposed to Bacillus anthracis spores, and subsequently given either Anthrasil or a placebo. The survival rate for monkeys given Anthrasil was between 36% and 70%, with a trend toward increased survival at higher doses of Anthrasil. None of the monkeys given placebo survived. Rabbits had a 26% survival rate when given the drug, compared to 2% of those given placebo. A separate study exposed rabbits to Bacillus anthracis and treated them with either antibiotics or a combination of antibiotics and Anthrasil; survival rates were 71% for those treated with the combination and 25% for those treated with antibiotics only.
Safety was tested in 74 healthy human volunteers and the most commonly reported side effects were headache, back pain, nausea, and pain and swelling at the infusion site.
Anthrasil is manufactured by Cangene Corporation, based in Winnipeg, Canada, which developed the drug in collaboration with BARDA.
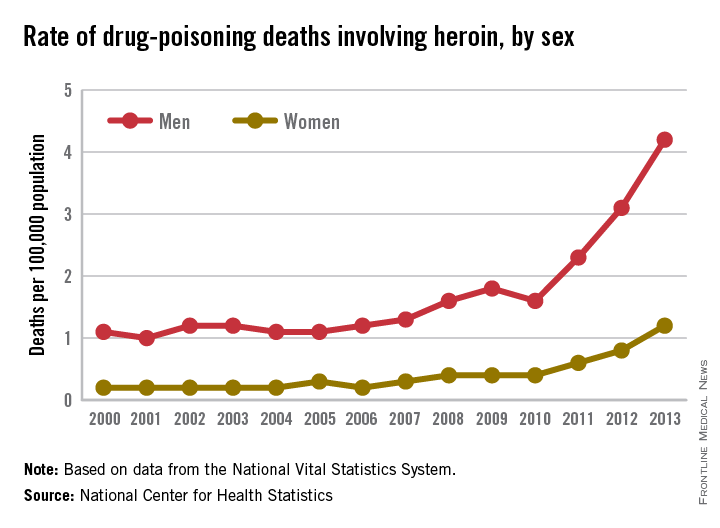
Heroin overdoses up dramatically since 2010
Drug-poisoning deaths involving heroin have soared since 2000, and most of the increase occurred since 2010, according to a report from the National Center for Health Statistics.
From 2010 to 2013, the rate of heroin overdose deaths increased 163% for men, from a rate of 1.6/100,000 population in 2010 to 4.2 in 2013. For women, the death rate increased by 200%, from 0.4/100,000 in 2010 to 1.2/100,000 in 2013. From 2000 to 2010, however, the rate of increase was much slower, with the death rate increasing from 1.1 to 1.6 for men and from 0.2 to 0.4 for women.

The overall rate for heroin overdose from 2000 to 2013 increased from 0.7 to 2.7/100,000. Most of this increase occurred from 2010 to 2013: From 2000 to 2010, the death rate increased to only 1/100,000, a growth rate of 6%, but after 2010, the rate grew by 37% per year, the NCHS reported.
In 2013, non-Hispanic whites aged 18-44 years had the highest heroin poisoning death rate among measured racial/ethnic groups at 7/100,000. In 2000, older, non-Hispanic blacks aged 45-64 years had the highest death rate among the reported racial/ethnic groups at 2/100,000. The death rate for whites aged 18-44 in 2000 was 1.2/100,000, meaning that the death rate increased by 483% from 2000 to 2013. For non-Hispanic blacks aged 45-64, the death rate in 2013 was 4.9, an increase of 145%.
The number of heroin-related overdose deaths climbed in every region of the country from 2000 through 2013. The largest change in heroin overdose by region occurred in the Midwest, where the death rate rose from 0.4/100,000 in 2000 to 4.3 in 2013, an increase of 975%, said the NCHS report, which used data collected by the National Vital Statistics System.
Drug-poisoning deaths involving heroin have soared since 2000, and most of the increase occurred since 2010, according to a report from the National Center for Health Statistics.
From 2010 to 2013, the rate of heroin overdose deaths increased 163% for men, from a rate of 1.6/100,000 population in 2010 to 4.2 in 2013. For women, the death rate increased by 200%, from 0.4/100,000 in 2010 to 1.2/100,000 in 2013. From 2000 to 2010, however, the rate of increase was much slower, with the death rate increasing from 1.1 to 1.6 for men and from 0.2 to 0.4 for women.

The overall rate for heroin overdose from 2000 to 2013 increased from 0.7 to 2.7/100,000. Most of this increase occurred from 2010 to 2013: From 2000 to 2010, the death rate increased to only 1/100,000, a growth rate of 6%, but after 2010, the rate grew by 37% per year, the NCHS reported.
In 2013, non-Hispanic whites aged 18-44 years had the highest heroin poisoning death rate among measured racial/ethnic groups at 7/100,000. In 2000, older, non-Hispanic blacks aged 45-64 years had the highest death rate among the reported racial/ethnic groups at 2/100,000. The death rate for whites aged 18-44 in 2000 was 1.2/100,000, meaning that the death rate increased by 483% from 2000 to 2013. For non-Hispanic blacks aged 45-64, the death rate in 2013 was 4.9, an increase of 145%.
The number of heroin-related overdose deaths climbed in every region of the country from 2000 through 2013. The largest change in heroin overdose by region occurred in the Midwest, where the death rate rose from 0.4/100,000 in 2000 to 4.3 in 2013, an increase of 975%, said the NCHS report, which used data collected by the National Vital Statistics System.
Drug-poisoning deaths involving heroin have soared since 2000, and most of the increase occurred since 2010, according to a report from the National Center for Health Statistics.
From 2010 to 2013, the rate of heroin overdose deaths increased 163% for men, from a rate of 1.6/100,000 population in 2010 to 4.2 in 2013. For women, the death rate increased by 200%, from 0.4/100,000 in 2010 to 1.2/100,000 in 2013. From 2000 to 2010, however, the rate of increase was much slower, with the death rate increasing from 1.1 to 1.6 for men and from 0.2 to 0.4 for women.

The overall rate for heroin overdose from 2000 to 2013 increased from 0.7 to 2.7/100,000. Most of this increase occurred from 2010 to 2013: From 2000 to 2010, the death rate increased to only 1/100,000, a growth rate of 6%, but after 2010, the rate grew by 37% per year, the NCHS reported.
In 2013, non-Hispanic whites aged 18-44 years had the highest heroin poisoning death rate among measured racial/ethnic groups at 7/100,000. In 2000, older, non-Hispanic blacks aged 45-64 years had the highest death rate among the reported racial/ethnic groups at 2/100,000. The death rate for whites aged 18-44 in 2000 was 1.2/100,000, meaning that the death rate increased by 483% from 2000 to 2013. For non-Hispanic blacks aged 45-64, the death rate in 2013 was 4.9, an increase of 145%.
The number of heroin-related overdose deaths climbed in every region of the country from 2000 through 2013. The largest change in heroin overdose by region occurred in the Midwest, where the death rate rose from 0.4/100,000 in 2000 to 4.3 in 2013, an increase of 975%, said the NCHS report, which used data collected by the National Vital Statistics System.
Environmental factors could increase U.S. anthrax cases
WASHINGTON– Recent isolated cases of anthrax in Minnesota and elsewhere, along with the disease’s relative ease of transmission from animals or plants to humans, should heighten U.S. physicians’ awareness of anthrax’s symptoms and treatments, warned Dr. Jason K. Blackburn.
“[Anthrax] is something that our international partners deal with on an annual basis [as] we can see the disease reemerging, or at least increasing, in annual reports on humans in a number of countries,” explained Dr. Blackburn of the University of Florida in Gainesville, at a meeting on biodefense and emerging diseases sponsored by the American Society for Microbiology. “Here in the United States, we’re seeing it shift from a livestock disease [to] a wildlife disease, where we have these populations that we can’t reach with vaccines, and where surveillance is very logistically challenging.”
Environmental factors can drive higher incidences of anthrax cases. Temperature, precipitation, and vegetation indices are key variables that facilitate anthrax transmission and spread of the disease. Geographically, lowland areas also have higher prevalences of the disease.
For example, Dr. Blackburn and his colleagues used predictive models to quantify the theory that anthrax case rates increase during years that have wet springs followed by hot, dry summers in the region of western Texas.
Using these data would allow scientists and health care providers to predict which years would have an increased risk for anthrax cases in humans, Dr. Blackburn said, and could help hospitals and clinics effectively prepare to treat a higher influx of these cases and prevent possible outbreaks.
Although relatively large numbers of human anthrax cases persist in parts of world – particularly in Africa and central Asia – cases in the United States have been primarily relegated to livestock.
However, during the last decade, there has been a noticeable shift in cases from livestock to wildlife, particularly in western Texas and Montana, where local populations of elk, bison, and white-tailed deer have been affected. The newfound prevalence in wildlife species, along with continued presence in domestic animals such as cattle and sheep, mean that transmission to humans could become even easier.
“Human cases are usually driven by direct human interaction with mammalian hosts,” said Dr. Blackburn, citing farms and meat factories as prime examples of where the Bacillus anthracis organism would easily spread. In addition, Dr. Blackburn specifically noted a scenario in which flies can transmit the disease from sheep to humans, and have also been found to carry anthrax from carcasses to wildlife and vegetation.
From 2010 to 2012, anthrax cases in Europe, particularly Georgia and Turkey, increased, compared with numbers over a similar time frame between 2000 and 2009. While case reporting can be partly attributed to this increase, Dr. Blackburn indicated that it was most likely evidence of an associative trend between livestock and human anthrax cases.
Dr. Blackburn did not report any disclosures.
WASHINGTON– Recent isolated cases of anthrax in Minnesota and elsewhere, along with the disease’s relative ease of transmission from animals or plants to humans, should heighten U.S. physicians’ awareness of anthrax’s symptoms and treatments, warned Dr. Jason K. Blackburn.
“[Anthrax] is something that our international partners deal with on an annual basis [as] we can see the disease reemerging, or at least increasing, in annual reports on humans in a number of countries,” explained Dr. Blackburn of the University of Florida in Gainesville, at a meeting on biodefense and emerging diseases sponsored by the American Society for Microbiology. “Here in the United States, we’re seeing it shift from a livestock disease [to] a wildlife disease, where we have these populations that we can’t reach with vaccines, and where surveillance is very logistically challenging.”
Environmental factors can drive higher incidences of anthrax cases. Temperature, precipitation, and vegetation indices are key variables that facilitate anthrax transmission and spread of the disease. Geographically, lowland areas also have higher prevalences of the disease.
For example, Dr. Blackburn and his colleagues used predictive models to quantify the theory that anthrax case rates increase during years that have wet springs followed by hot, dry summers in the region of western Texas.
Using these data would allow scientists and health care providers to predict which years would have an increased risk for anthrax cases in humans, Dr. Blackburn said, and could help hospitals and clinics effectively prepare to treat a higher influx of these cases and prevent possible outbreaks.
Although relatively large numbers of human anthrax cases persist in parts of world – particularly in Africa and central Asia – cases in the United States have been primarily relegated to livestock.
However, during the last decade, there has been a noticeable shift in cases from livestock to wildlife, particularly in western Texas and Montana, where local populations of elk, bison, and white-tailed deer have been affected. The newfound prevalence in wildlife species, along with continued presence in domestic animals such as cattle and sheep, mean that transmission to humans could become even easier.
“Human cases are usually driven by direct human interaction with mammalian hosts,” said Dr. Blackburn, citing farms and meat factories as prime examples of where the Bacillus anthracis organism would easily spread. In addition, Dr. Blackburn specifically noted a scenario in which flies can transmit the disease from sheep to humans, and have also been found to carry anthrax from carcasses to wildlife and vegetation.
From 2010 to 2012, anthrax cases in Europe, particularly Georgia and Turkey, increased, compared with numbers over a similar time frame between 2000 and 2009. While case reporting can be partly attributed to this increase, Dr. Blackburn indicated that it was most likely evidence of an associative trend between livestock and human anthrax cases.
Dr. Blackburn did not report any disclosures.
WASHINGTON– Recent isolated cases of anthrax in Minnesota and elsewhere, along with the disease’s relative ease of transmission from animals or plants to humans, should heighten U.S. physicians’ awareness of anthrax’s symptoms and treatments, warned Dr. Jason K. Blackburn.
“[Anthrax] is something that our international partners deal with on an annual basis [as] we can see the disease reemerging, or at least increasing, in annual reports on humans in a number of countries,” explained Dr. Blackburn of the University of Florida in Gainesville, at a meeting on biodefense and emerging diseases sponsored by the American Society for Microbiology. “Here in the United States, we’re seeing it shift from a livestock disease [to] a wildlife disease, where we have these populations that we can’t reach with vaccines, and where surveillance is very logistically challenging.”
Environmental factors can drive higher incidences of anthrax cases. Temperature, precipitation, and vegetation indices are key variables that facilitate anthrax transmission and spread of the disease. Geographically, lowland areas also have higher prevalences of the disease.
For example, Dr. Blackburn and his colleagues used predictive models to quantify the theory that anthrax case rates increase during years that have wet springs followed by hot, dry summers in the region of western Texas.
Using these data would allow scientists and health care providers to predict which years would have an increased risk for anthrax cases in humans, Dr. Blackburn said, and could help hospitals and clinics effectively prepare to treat a higher influx of these cases and prevent possible outbreaks.
Although relatively large numbers of human anthrax cases persist in parts of world – particularly in Africa and central Asia – cases in the United States have been primarily relegated to livestock.
However, during the last decade, there has been a noticeable shift in cases from livestock to wildlife, particularly in western Texas and Montana, where local populations of elk, bison, and white-tailed deer have been affected. The newfound prevalence in wildlife species, along with continued presence in domestic animals such as cattle and sheep, mean that transmission to humans could become even easier.
“Human cases are usually driven by direct human interaction with mammalian hosts,” said Dr. Blackburn, citing farms and meat factories as prime examples of where the Bacillus anthracis organism would easily spread. In addition, Dr. Blackburn specifically noted a scenario in which flies can transmit the disease from sheep to humans, and have also been found to carry anthrax from carcasses to wildlife and vegetation.
From 2010 to 2012, anthrax cases in Europe, particularly Georgia and Turkey, increased, compared with numbers over a similar time frame between 2000 and 2009. While case reporting can be partly attributed to this increase, Dr. Blackburn indicated that it was most likely evidence of an associative trend between livestock and human anthrax cases.
Dr. Blackburn did not report any disclosures.
AT THE ASM BIODEFENSE MEETING
Case Studies in Toxicology: Double Take—Is Re-exposure Necessary to Explain Delayed Recurrent Opioid Toxicity?
Case
A previously healthy 10-month-old girl was brought to the ED by her mother, who noted that the child had been excessively drowsy throughout the day. She reported that her husband had dropped an unknown amount of his morphine sulfate extended-release 60-mg tablets and oxycodone 10-mg/acetaminophen 325-mg tablets on the floor 5 days earlier. Although unsure of how many tablets he had dropped, the father believed he had located all of them. The mother, however, found some of the tablets around the crib in their daughter’s room.
When the child arrived to the ED, her vital signs were: blood pressure, 95/60 mm Hg; heart rate, 102 beats/minute; respiratory rate (RR), 18 breaths/minute; and temperature, 98.4°F. Oxygen saturation was 98% on room air. On physical examination, the child was lethargic, her pupils were less than 1 mm in diameter, and her bowel sounds were absent. After the administration of intravenous (IV) naloxone 0.4 mg, the patient became less drowsy and her RR normalized. Approximately 1 hour later, though, the child again became lethargic; she was given a repeat dose of IV naloxone 0.4 mg, and a naloxone infusion was initiated at 0.3 mg/h. Over approximately 20 hours, the infusion was tapered and discontinued. Three hours after the infusion was stopped, the child’s vital signs and behavior were both normal. After a social worker and representative from the Administration for Children’s Services reviewed the patient’s case, she was discharged home with her parents.
Less than 1 hour later, however, the mother returned to the ED with the child, who was again unresponsive. Although the girl’s RR was normal, she had pinpoint pupils. After she was given IV naloxone 0.4 mg, the child awoke and remained responsive for 20 minutes before returning to a somnolent state. Another IV dose of naloxone 0.4 mg was administered, which showed partial improvement in responsiveness. A naloxone infusion was then initiated and titrated up to 1 mg/h to maintain wakefulness and ventilation. In the pediatric intensive care unit, the child required titration of the naloxone infusion to 2 mg/h to which she responded well. Over the next 12 hours, the infusion was tapered off and the child was discharged home with her parents.
Blood samples from both the initial visit and the return visit were sent for toxicologic analysis by gas chromatography-mass spectrometry (GC-MS). Serum from the first visit contained morphine at a concentration of 3,000 ng/mL; serum from the second visit contained morphine at 420 ng/mL. Both samples were negative for oxycodone or any of the other substances checked on the extended GC-MS screen.
What is the toxicologic differential?
Although this patient’s extreme somnolence was suspected to be opioid-induced, and was confirmed by an appropriate response to naloxone, children may present to the ED somnolent for a variety of unknown reasons. Even with a fairly clear history, the clinician should also consider metabolic, neurological, infectious, traumatic, and psychiatric causes of altered mental status.1 The toxicologic causes of altered mental status are expansive and include the effects of many medications used therapeutically or in overdose. Opioids, benzodiazepines, barbiturates, α-2 agonists (eg, clonidine), sleep aids (eg, zolpidem, diphenhydramine), and ethanol are common causes of induced an altered mental status. When taking a toxicologic history, it is important to inquire not only about the patient’s medications but also the medications of other members of the household to which the patient may have access. This includes not only prescription medications but also over-the-counter, complementary, and herbal preparations.
Why did this child have delayed recurrent opioid toxicity?
When used as directed, opioids cause analgesia and euphoria. Analgesia is mediated by agonism at the μ- , κ-, and δ-opioid receptors throughout the brain and spinal cord. The majority of morphine’s analgesic activity comes from activation of the μ-opioid receptors.2 In overdose, opioids classically cause a toxidrome characterized by miosis, coma, decreased bowel sounds, and respiratory depression. These signs can give clues to a patient’s exposure.
Supportive care is the cornerstone of treatment for patients with opioid toxicity, and maintaining the airway and monitoring the respiratory status are extremely important. When ventilation decreases due to the actions of opioids (typically denoted by a RR of <12 breaths/minute in adults, but may be marked by a reduction in depth of breathing as well), the use of an opioid antagonist is appropriate.4 The most commonly used antagonist is naloxone, an antidote with antagonism at all opioid receptor subtypes.5
In patients who are not dependent on opioids, IV naloxone 0.4 mg is an appropriate initial dose—regardless of patient size or specifics of the exposure. Patients with opioid dependency (eg, patients taking opioids for chronic pain or palliative care, or in those with suspected or confirmed opioid abuse), should receive smaller initial doses of naloxone (eg, 0.04 mg); the dose should be titrated up to effect to avoid precipitating acute opioid withdrawal. The goal of opioid antagonism is to allow the patient to breathe spontaneously and at an appropriate rate and depth without precipitating withdrawal. The duration of action of naloxone is 20 to 90 minutes in adults.
Patients presenting with heroin overdose should be monitored for at least 2 hours after naloxone administration (some suggest 3 hours) to determine whether or not additional dosing will be necessary. After oral opioid exposures, particularly with extended-release or long-acting formulations, longer periods of observation are required (this is unrelated to the naloxone pharmacokinetics, but rather to the slow rise in blood levels from some of these formulations). If repeated opioid toxicity occurs in adults, a naloxone infusion may be helpful to reduce the need for repetitive re-dosing. Initially, an hourly infusion equal to two-thirds of the dose of naloxone that reversed the patient’s respiratory depression is suggested6
Naloxone is eliminated by conjugation with glucuronic acid before is it excreted from the body. Due to decreased hepatic conjugation and prolonged metabolization of drugs in pediatric patients, naloxone may have a longer half-life in children—especially neonates and infants7; in children, the half-life of naloxone may extend up to three times that of adults.8 This extended half-life can lead to a false sense of assurance that a child is free of opioid effects 120 minutes after receiving naloxone—the time by which an adult patient would likely be without significant systemic effects of naloxone—when in fact the effect of naloxone has not yet sufficiently waned. This in turn may prompt discharge before sufficient time has passed to exclude recrudescence of opioid toxicity: The presence of persistent opioid agonist concentrations in the blood, even at consequential amounts, remains masked by the persistent presence of naloxone.
The goal of opioid antagonism is to allow the patient to breathe spontaneously and at an appropriate rate and depth without precipitating withdrawal. In this patient, it is not surprising that the the ingestion of an extended-relief form of morphine should produce a prolonged opioid effect. At therapeutic concentrations in children (~10 ng/mL), the half-life of morphine is slightly longer than in adults (~3 hours vs 2 hours) and is likely even longer with very high serum concentrations. It is metabolized to morphine 6-glucuronide, which is active and longer lasting than the parent compound. This may account for additional clinical effects beyond the time that the serum morphine concentration falls, and is particularly relevant following immediate-release morphine overdose.
In this case it is also important to consider whether or not the patient was re-exposed to an opioid between the first and second ED visit. The dramatically elevated initial serum morphine concentrations and the relatively appropriate fall in magnitude of the second sample suggest that the recurrence of respiratory depression was not the result of re-exposure. The patient’s recurrent effects, even a day out from exposure, can be explained by the immediate-release morphine exposure and the discharge prior to waning of the naloxone. In children with opioid toxicity, another potential option, though not directly studied, is to administer the long-acting opioid antagonist naltrexone to the patient prior to discharge.
Case Conclusion
When used appropriately and under the correct circumstances, naloxone is safe and effective for the reversal of opioid toxicity. As with any antidote, patients must be appropriately monitored for any adverse effects or recurrence of toxicity. Moreover, the clinician should be mindful of the pharmacokinetic differences between adults and young children and the possibility of a later-than-expected recurrence of opioid toxicity in pediatric patients.
This case is a reminder of the importance of safe medication storage. Infants and young children who are crawling and exploring their environment are especially vulnerable to toxicity from medications found on the floor. Regardless of age, quick recognition of opioid-induced respiratory depression and appropriate use of naloxone can help to decrease the morbidity associated with excessive opioid exposures in all patients.
Dr Berman is a senior medical toxicology fellow at North Shore-Long Island Jewish Medical Center, New York. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board. Dr Majlesi is the director of medical toxicology at Staten Island University Hospital, New York.
- Lehman RK, Mink J. Altered mental status. Clin Pediatr Emerg Med. 2008;9:68-75.
- Chang SH, Maney KM, Phillips JP, Langford RM, Mehta V. A comparison of the respiratory effects of oxycodone versus morphine: a randomised, double-blind, placebo-controlled investigation. Anaesthesia. 2010;65(10):1007-1012.
- Holstege CP, Borek HA. Toxidromes. Crit Care Clin. 2012;28(4):479-498.
- Hoffman JR, Schriger DL, Luo JS. The empiric use of naloxone in patients with altered mental status: a reappraisal. Ann Emerg Men. 1991;20(3):246-252.
- Howland MA, Nelson LS. Chapter A6. Opioid antagonists. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:579-585.
- Goldfrank L, Weisman RS, Errick JK, Lo MW. A dosing nomogram for continuous infusion intravenous naloxone. Ann Emerg Med. 1986;15(5):566-570.
- Moreland TA, Brice JE, Walker CH, Parija AC. Naloxone pharmacokinetics in the newborn. Br J Clin Pharmacol. 1980;9(6):609-612.
- Ngai SH, Berkowitz BA, Yang JC, et al. Pharmacokinetics of naloxone in rats and in man: basis for its potency and short duration of action. Anesthesiology. 1976;44(5):398-401.
Case
A previously healthy 10-month-old girl was brought to the ED by her mother, who noted that the child had been excessively drowsy throughout the day. She reported that her husband had dropped an unknown amount of his morphine sulfate extended-release 60-mg tablets and oxycodone 10-mg/acetaminophen 325-mg tablets on the floor 5 days earlier. Although unsure of how many tablets he had dropped, the father believed he had located all of them. The mother, however, found some of the tablets around the crib in their daughter’s room.
When the child arrived to the ED, her vital signs were: blood pressure, 95/60 mm Hg; heart rate, 102 beats/minute; respiratory rate (RR), 18 breaths/minute; and temperature, 98.4°F. Oxygen saturation was 98% on room air. On physical examination, the child was lethargic, her pupils were less than 1 mm in diameter, and her bowel sounds were absent. After the administration of intravenous (IV) naloxone 0.4 mg, the patient became less drowsy and her RR normalized. Approximately 1 hour later, though, the child again became lethargic; she was given a repeat dose of IV naloxone 0.4 mg, and a naloxone infusion was initiated at 0.3 mg/h. Over approximately 20 hours, the infusion was tapered and discontinued. Three hours after the infusion was stopped, the child’s vital signs and behavior were both normal. After a social worker and representative from the Administration for Children’s Services reviewed the patient’s case, she was discharged home with her parents.
Less than 1 hour later, however, the mother returned to the ED with the child, who was again unresponsive. Although the girl’s RR was normal, she had pinpoint pupils. After she was given IV naloxone 0.4 mg, the child awoke and remained responsive for 20 minutes before returning to a somnolent state. Another IV dose of naloxone 0.4 mg was administered, which showed partial improvement in responsiveness. A naloxone infusion was then initiated and titrated up to 1 mg/h to maintain wakefulness and ventilation. In the pediatric intensive care unit, the child required titration of the naloxone infusion to 2 mg/h to which she responded well. Over the next 12 hours, the infusion was tapered off and the child was discharged home with her parents.
Blood samples from both the initial visit and the return visit were sent for toxicologic analysis by gas chromatography-mass spectrometry (GC-MS). Serum from the first visit contained morphine at a concentration of 3,000 ng/mL; serum from the second visit contained morphine at 420 ng/mL. Both samples were negative for oxycodone or any of the other substances checked on the extended GC-MS screen.
What is the toxicologic differential?
Although this patient’s extreme somnolence was suspected to be opioid-induced, and was confirmed by an appropriate response to naloxone, children may present to the ED somnolent for a variety of unknown reasons. Even with a fairly clear history, the clinician should also consider metabolic, neurological, infectious, traumatic, and psychiatric causes of altered mental status.1 The toxicologic causes of altered mental status are expansive and include the effects of many medications used therapeutically or in overdose. Opioids, benzodiazepines, barbiturates, α-2 agonists (eg, clonidine), sleep aids (eg, zolpidem, diphenhydramine), and ethanol are common causes of induced an altered mental status. When taking a toxicologic history, it is important to inquire not only about the patient’s medications but also the medications of other members of the household to which the patient may have access. This includes not only prescription medications but also over-the-counter, complementary, and herbal preparations.
Why did this child have delayed recurrent opioid toxicity?
When used as directed, opioids cause analgesia and euphoria. Analgesia is mediated by agonism at the μ- , κ-, and δ-opioid receptors throughout the brain and spinal cord. The majority of morphine’s analgesic activity comes from activation of the μ-opioid receptors.2 In overdose, opioids classically cause a toxidrome characterized by miosis, coma, decreased bowel sounds, and respiratory depression. These signs can give clues to a patient’s exposure.
Supportive care is the cornerstone of treatment for patients with opioid toxicity, and maintaining the airway and monitoring the respiratory status are extremely important. When ventilation decreases due to the actions of opioids (typically denoted by a RR of <12 breaths/minute in adults, but may be marked by a reduction in depth of breathing as well), the use of an opioid antagonist is appropriate.4 The most commonly used antagonist is naloxone, an antidote with antagonism at all opioid receptor subtypes.5
In patients who are not dependent on opioids, IV naloxone 0.4 mg is an appropriate initial dose—regardless of patient size or specifics of the exposure. Patients with opioid dependency (eg, patients taking opioids for chronic pain or palliative care, or in those with suspected or confirmed opioid abuse), should receive smaller initial doses of naloxone (eg, 0.04 mg); the dose should be titrated up to effect to avoid precipitating acute opioid withdrawal. The goal of opioid antagonism is to allow the patient to breathe spontaneously and at an appropriate rate and depth without precipitating withdrawal. The duration of action of naloxone is 20 to 90 minutes in adults.
Patients presenting with heroin overdose should be monitored for at least 2 hours after naloxone administration (some suggest 3 hours) to determine whether or not additional dosing will be necessary. After oral opioid exposures, particularly with extended-release or long-acting formulations, longer periods of observation are required (this is unrelated to the naloxone pharmacokinetics, but rather to the slow rise in blood levels from some of these formulations). If repeated opioid toxicity occurs in adults, a naloxone infusion may be helpful to reduce the need for repetitive re-dosing. Initially, an hourly infusion equal to two-thirds of the dose of naloxone that reversed the patient’s respiratory depression is suggested6
Naloxone is eliminated by conjugation with glucuronic acid before is it excreted from the body. Due to decreased hepatic conjugation and prolonged metabolization of drugs in pediatric patients, naloxone may have a longer half-life in children—especially neonates and infants7; in children, the half-life of naloxone may extend up to three times that of adults.8 This extended half-life can lead to a false sense of assurance that a child is free of opioid effects 120 minutes after receiving naloxone—the time by which an adult patient would likely be without significant systemic effects of naloxone—when in fact the effect of naloxone has not yet sufficiently waned. This in turn may prompt discharge before sufficient time has passed to exclude recrudescence of opioid toxicity: The presence of persistent opioid agonist concentrations in the blood, even at consequential amounts, remains masked by the persistent presence of naloxone.
The goal of opioid antagonism is to allow the patient to breathe spontaneously and at an appropriate rate and depth without precipitating withdrawal. In this patient, it is not surprising that the the ingestion of an extended-relief form of morphine should produce a prolonged opioid effect. At therapeutic concentrations in children (~10 ng/mL), the half-life of morphine is slightly longer than in adults (~3 hours vs 2 hours) and is likely even longer with very high serum concentrations. It is metabolized to morphine 6-glucuronide, which is active and longer lasting than the parent compound. This may account for additional clinical effects beyond the time that the serum morphine concentration falls, and is particularly relevant following immediate-release morphine overdose.
In this case it is also important to consider whether or not the patient was re-exposed to an opioid between the first and second ED visit. The dramatically elevated initial serum morphine concentrations and the relatively appropriate fall in magnitude of the second sample suggest that the recurrence of respiratory depression was not the result of re-exposure. The patient’s recurrent effects, even a day out from exposure, can be explained by the immediate-release morphine exposure and the discharge prior to waning of the naloxone. In children with opioid toxicity, another potential option, though not directly studied, is to administer the long-acting opioid antagonist naltrexone to the patient prior to discharge.
Case Conclusion
When used appropriately and under the correct circumstances, naloxone is safe and effective for the reversal of opioid toxicity. As with any antidote, patients must be appropriately monitored for any adverse effects or recurrence of toxicity. Moreover, the clinician should be mindful of the pharmacokinetic differences between adults and young children and the possibility of a later-than-expected recurrence of opioid toxicity in pediatric patients.
This case is a reminder of the importance of safe medication storage. Infants and young children who are crawling and exploring their environment are especially vulnerable to toxicity from medications found on the floor. Regardless of age, quick recognition of opioid-induced respiratory depression and appropriate use of naloxone can help to decrease the morbidity associated with excessive opioid exposures in all patients.
Dr Berman is a senior medical toxicology fellow at North Shore-Long Island Jewish Medical Center, New York. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board. Dr Majlesi is the director of medical toxicology at Staten Island University Hospital, New York.
Case
A previously healthy 10-month-old girl was brought to the ED by her mother, who noted that the child had been excessively drowsy throughout the day. She reported that her husband had dropped an unknown amount of his morphine sulfate extended-release 60-mg tablets and oxycodone 10-mg/acetaminophen 325-mg tablets on the floor 5 days earlier. Although unsure of how many tablets he had dropped, the father believed he had located all of them. The mother, however, found some of the tablets around the crib in their daughter’s room.
When the child arrived to the ED, her vital signs were: blood pressure, 95/60 mm Hg; heart rate, 102 beats/minute; respiratory rate (RR), 18 breaths/minute; and temperature, 98.4°F. Oxygen saturation was 98% on room air. On physical examination, the child was lethargic, her pupils were less than 1 mm in diameter, and her bowel sounds were absent. After the administration of intravenous (IV) naloxone 0.4 mg, the patient became less drowsy and her RR normalized. Approximately 1 hour later, though, the child again became lethargic; she was given a repeat dose of IV naloxone 0.4 mg, and a naloxone infusion was initiated at 0.3 mg/h. Over approximately 20 hours, the infusion was tapered and discontinued. Three hours after the infusion was stopped, the child’s vital signs and behavior were both normal. After a social worker and representative from the Administration for Children’s Services reviewed the patient’s case, she was discharged home with her parents.
Less than 1 hour later, however, the mother returned to the ED with the child, who was again unresponsive. Although the girl’s RR was normal, she had pinpoint pupils. After she was given IV naloxone 0.4 mg, the child awoke and remained responsive for 20 minutes before returning to a somnolent state. Another IV dose of naloxone 0.4 mg was administered, which showed partial improvement in responsiveness. A naloxone infusion was then initiated and titrated up to 1 mg/h to maintain wakefulness and ventilation. In the pediatric intensive care unit, the child required titration of the naloxone infusion to 2 mg/h to which she responded well. Over the next 12 hours, the infusion was tapered off and the child was discharged home with her parents.
Blood samples from both the initial visit and the return visit were sent for toxicologic analysis by gas chromatography-mass spectrometry (GC-MS). Serum from the first visit contained morphine at a concentration of 3,000 ng/mL; serum from the second visit contained morphine at 420 ng/mL. Both samples were negative for oxycodone or any of the other substances checked on the extended GC-MS screen.
What is the toxicologic differential?
Although this patient’s extreme somnolence was suspected to be opioid-induced, and was confirmed by an appropriate response to naloxone, children may present to the ED somnolent for a variety of unknown reasons. Even with a fairly clear history, the clinician should also consider metabolic, neurological, infectious, traumatic, and psychiatric causes of altered mental status.1 The toxicologic causes of altered mental status are expansive and include the effects of many medications used therapeutically or in overdose. Opioids, benzodiazepines, barbiturates, α-2 agonists (eg, clonidine), sleep aids (eg, zolpidem, diphenhydramine), and ethanol are common causes of induced an altered mental status. When taking a toxicologic history, it is important to inquire not only about the patient’s medications but also the medications of other members of the household to which the patient may have access. This includes not only prescription medications but also over-the-counter, complementary, and herbal preparations.
Why did this child have delayed recurrent opioid toxicity?
When used as directed, opioids cause analgesia and euphoria. Analgesia is mediated by agonism at the μ- , κ-, and δ-opioid receptors throughout the brain and spinal cord. The majority of morphine’s analgesic activity comes from activation of the μ-opioid receptors.2 In overdose, opioids classically cause a toxidrome characterized by miosis, coma, decreased bowel sounds, and respiratory depression. These signs can give clues to a patient’s exposure.
Supportive care is the cornerstone of treatment for patients with opioid toxicity, and maintaining the airway and monitoring the respiratory status are extremely important. When ventilation decreases due to the actions of opioids (typically denoted by a RR of <12 breaths/minute in adults, but may be marked by a reduction in depth of breathing as well), the use of an opioid antagonist is appropriate.4 The most commonly used antagonist is naloxone, an antidote with antagonism at all opioid receptor subtypes.5
In patients who are not dependent on opioids, IV naloxone 0.4 mg is an appropriate initial dose—regardless of patient size or specifics of the exposure. Patients with opioid dependency (eg, patients taking opioids for chronic pain or palliative care, or in those with suspected or confirmed opioid abuse), should receive smaller initial doses of naloxone (eg, 0.04 mg); the dose should be titrated up to effect to avoid precipitating acute opioid withdrawal. The goal of opioid antagonism is to allow the patient to breathe spontaneously and at an appropriate rate and depth without precipitating withdrawal. The duration of action of naloxone is 20 to 90 minutes in adults.
Patients presenting with heroin overdose should be monitored for at least 2 hours after naloxone administration (some suggest 3 hours) to determine whether or not additional dosing will be necessary. After oral opioid exposures, particularly with extended-release or long-acting formulations, longer periods of observation are required (this is unrelated to the naloxone pharmacokinetics, but rather to the slow rise in blood levels from some of these formulations). If repeated opioid toxicity occurs in adults, a naloxone infusion may be helpful to reduce the need for repetitive re-dosing. Initially, an hourly infusion equal to two-thirds of the dose of naloxone that reversed the patient’s respiratory depression is suggested6
Naloxone is eliminated by conjugation with glucuronic acid before is it excreted from the body. Due to decreased hepatic conjugation and prolonged metabolization of drugs in pediatric patients, naloxone may have a longer half-life in children—especially neonates and infants7; in children, the half-life of naloxone may extend up to three times that of adults.8 This extended half-life can lead to a false sense of assurance that a child is free of opioid effects 120 minutes after receiving naloxone—the time by which an adult patient would likely be without significant systemic effects of naloxone—when in fact the effect of naloxone has not yet sufficiently waned. This in turn may prompt discharge before sufficient time has passed to exclude recrudescence of opioid toxicity: The presence of persistent opioid agonist concentrations in the blood, even at consequential amounts, remains masked by the persistent presence of naloxone.
The goal of opioid antagonism is to allow the patient to breathe spontaneously and at an appropriate rate and depth without precipitating withdrawal. In this patient, it is not surprising that the the ingestion of an extended-relief form of morphine should produce a prolonged opioid effect. At therapeutic concentrations in children (~10 ng/mL), the half-life of morphine is slightly longer than in adults (~3 hours vs 2 hours) and is likely even longer with very high serum concentrations. It is metabolized to morphine 6-glucuronide, which is active and longer lasting than the parent compound. This may account for additional clinical effects beyond the time that the serum morphine concentration falls, and is particularly relevant following immediate-release morphine overdose.
In this case it is also important to consider whether or not the patient was re-exposed to an opioid between the first and second ED visit. The dramatically elevated initial serum morphine concentrations and the relatively appropriate fall in magnitude of the second sample suggest that the recurrence of respiratory depression was not the result of re-exposure. The patient’s recurrent effects, even a day out from exposure, can be explained by the immediate-release morphine exposure and the discharge prior to waning of the naloxone. In children with opioid toxicity, another potential option, though not directly studied, is to administer the long-acting opioid antagonist naltrexone to the patient prior to discharge.
Case Conclusion
When used appropriately and under the correct circumstances, naloxone is safe and effective for the reversal of opioid toxicity. As with any antidote, patients must be appropriately monitored for any adverse effects or recurrence of toxicity. Moreover, the clinician should be mindful of the pharmacokinetic differences between adults and young children and the possibility of a later-than-expected recurrence of opioid toxicity in pediatric patients.
This case is a reminder of the importance of safe medication storage. Infants and young children who are crawling and exploring their environment are especially vulnerable to toxicity from medications found on the floor. Regardless of age, quick recognition of opioid-induced respiratory depression and appropriate use of naloxone can help to decrease the morbidity associated with excessive opioid exposures in all patients.
Dr Berman is a senior medical toxicology fellow at North Shore-Long Island Jewish Medical Center, New York. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board. Dr Majlesi is the director of medical toxicology at Staten Island University Hospital, New York.
- Lehman RK, Mink J. Altered mental status. Clin Pediatr Emerg Med. 2008;9:68-75.
- Chang SH, Maney KM, Phillips JP, Langford RM, Mehta V. A comparison of the respiratory effects of oxycodone versus morphine: a randomised, double-blind, placebo-controlled investigation. Anaesthesia. 2010;65(10):1007-1012.
- Holstege CP, Borek HA. Toxidromes. Crit Care Clin. 2012;28(4):479-498.
- Hoffman JR, Schriger DL, Luo JS. The empiric use of naloxone in patients with altered mental status: a reappraisal. Ann Emerg Men. 1991;20(3):246-252.
- Howland MA, Nelson LS. Chapter A6. Opioid antagonists. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:579-585.
- Goldfrank L, Weisman RS, Errick JK, Lo MW. A dosing nomogram for continuous infusion intravenous naloxone. Ann Emerg Med. 1986;15(5):566-570.
- Moreland TA, Brice JE, Walker CH, Parija AC. Naloxone pharmacokinetics in the newborn. Br J Clin Pharmacol. 1980;9(6):609-612.
- Ngai SH, Berkowitz BA, Yang JC, et al. Pharmacokinetics of naloxone in rats and in man: basis for its potency and short duration of action. Anesthesiology. 1976;44(5):398-401.
- Lehman RK, Mink J. Altered mental status. Clin Pediatr Emerg Med. 2008;9:68-75.
- Chang SH, Maney KM, Phillips JP, Langford RM, Mehta V. A comparison of the respiratory effects of oxycodone versus morphine: a randomised, double-blind, placebo-controlled investigation. Anaesthesia. 2010;65(10):1007-1012.
- Holstege CP, Borek HA. Toxidromes. Crit Care Clin. 2012;28(4):479-498.
- Hoffman JR, Schriger DL, Luo JS. The empiric use of naloxone in patients with altered mental status: a reappraisal. Ann Emerg Men. 1991;20(3):246-252.
- Howland MA, Nelson LS. Chapter A6. Opioid antagonists. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:579-585.
- Goldfrank L, Weisman RS, Errick JK, Lo MW. A dosing nomogram for continuous infusion intravenous naloxone. Ann Emerg Med. 1986;15(5):566-570.
- Moreland TA, Brice JE, Walker CH, Parija AC. Naloxone pharmacokinetics in the newborn. Br J Clin Pharmacol. 1980;9(6):609-612.
- Ngai SH, Berkowitz BA, Yang JC, et al. Pharmacokinetics of naloxone in rats and in man: basis for its potency and short duration of action. Anesthesiology. 1976;44(5):398-401.
Icatibant rapidly resolved ACE inhibitor–induced angioedema
Angioedema caused by ACE inhibitors resolved 70% more rapidly with icatibant than did standard therapy in a multicenter phase II study in Germany, which was reported online Jan. 29 in the New England Journal of Medicine.
Because of the increasing use of ACE inhibitors, approximately one-third of all cases of angioedema treated in emergency departments now are attributed to these agents. The current standard ED treatment of ACE inhibitor–induced angioedema is glucocorticoids plus antihistamines. However, patients generally don’t respond to this therapy, likely because this form of angioedema isn’t a histamine-mediated reaction. Instead, it is thought by some to be a bradykinin-mediated reaction, said Dr. Murat Bas of the department of otorhinolaryngology, Technische Universität München (Germany), and his associates.
Icatibant injections (Firazyr) are approved by the Food and Drug Administration for the treatment of acute attacks of hereditary angioedema in adults 18 years of age and older. The drug also is being studied in the United States for the treatment of ACE-inhibitor–induced angioedema.
Since ACE inhibitors interfere with the breakdown of bradykinin, and bradykinin-mediated hereditary angioedema is usually treated with bradykinin-receptor antagonists such as icatibant, the investigators performed a double-blind randomized trial comparing subcutaneous icatibant against standard treatment in 27 adults who presented to four German EDs during a 1.5-year period.
The primary endpoint – the time to complete resolution of ACE inhibitor–induced angioedema – was 8 hours with icatibant and 27 hours with standard therapy. Angioedema resolved within 4 hours in five patients (38%) given icatibant; none of the patients given standard therapy responded that quickly. The onset of symptom relief was 2 hours with icatibant and 12 hours with standard glucocorticoids plus antihistamines, a significant difference as judged by the study participants and the researchers. Also, the physician-assessed severity of angioedema began to abate within 1 hour of icatibant administration and within 8 hours for standard treatment (N. Engl. J. Med. 2015 Jan. 29 [doi:10.1056/NEJMoa1312524]).
“Although the sample size in this trial was too small to allow for a robust evaluation of safety, no patient discontinued participation in the study owing to adverse events,” Dr. Bas and his associates added.
Dr. Bas reported receiving grants and personal fees from Shire, the maker of icatibant, as did some of his associates.
Angioedema caused by ACE inhibitors resolved 70% more rapidly with icatibant than did standard therapy in a multicenter phase II study in Germany, which was reported online Jan. 29 in the New England Journal of Medicine.
Because of the increasing use of ACE inhibitors, approximately one-third of all cases of angioedema treated in emergency departments now are attributed to these agents. The current standard ED treatment of ACE inhibitor–induced angioedema is glucocorticoids plus antihistamines. However, patients generally don’t respond to this therapy, likely because this form of angioedema isn’t a histamine-mediated reaction. Instead, it is thought by some to be a bradykinin-mediated reaction, said Dr. Murat Bas of the department of otorhinolaryngology, Technische Universität München (Germany), and his associates.
Icatibant injections (Firazyr) are approved by the Food and Drug Administration for the treatment of acute attacks of hereditary angioedema in adults 18 years of age and older. The drug also is being studied in the United States for the treatment of ACE-inhibitor–induced angioedema.
Since ACE inhibitors interfere with the breakdown of bradykinin, and bradykinin-mediated hereditary angioedema is usually treated with bradykinin-receptor antagonists such as icatibant, the investigators performed a double-blind randomized trial comparing subcutaneous icatibant against standard treatment in 27 adults who presented to four German EDs during a 1.5-year period.
The primary endpoint – the time to complete resolution of ACE inhibitor–induced angioedema – was 8 hours with icatibant and 27 hours with standard therapy. Angioedema resolved within 4 hours in five patients (38%) given icatibant; none of the patients given standard therapy responded that quickly. The onset of symptom relief was 2 hours with icatibant and 12 hours with standard glucocorticoids plus antihistamines, a significant difference as judged by the study participants and the researchers. Also, the physician-assessed severity of angioedema began to abate within 1 hour of icatibant administration and within 8 hours for standard treatment (N. Engl. J. Med. 2015 Jan. 29 [doi:10.1056/NEJMoa1312524]).
“Although the sample size in this trial was too small to allow for a robust evaluation of safety, no patient discontinued participation in the study owing to adverse events,” Dr. Bas and his associates added.
Dr. Bas reported receiving grants and personal fees from Shire, the maker of icatibant, as did some of his associates.
Angioedema caused by ACE inhibitors resolved 70% more rapidly with icatibant than did standard therapy in a multicenter phase II study in Germany, which was reported online Jan. 29 in the New England Journal of Medicine.
Because of the increasing use of ACE inhibitors, approximately one-third of all cases of angioedema treated in emergency departments now are attributed to these agents. The current standard ED treatment of ACE inhibitor–induced angioedema is glucocorticoids plus antihistamines. However, patients generally don’t respond to this therapy, likely because this form of angioedema isn’t a histamine-mediated reaction. Instead, it is thought by some to be a bradykinin-mediated reaction, said Dr. Murat Bas of the department of otorhinolaryngology, Technische Universität München (Germany), and his associates.
Icatibant injections (Firazyr) are approved by the Food and Drug Administration for the treatment of acute attacks of hereditary angioedema in adults 18 years of age and older. The drug also is being studied in the United States for the treatment of ACE-inhibitor–induced angioedema.
Since ACE inhibitors interfere with the breakdown of bradykinin, and bradykinin-mediated hereditary angioedema is usually treated with bradykinin-receptor antagonists such as icatibant, the investigators performed a double-blind randomized trial comparing subcutaneous icatibant against standard treatment in 27 adults who presented to four German EDs during a 1.5-year period.
The primary endpoint – the time to complete resolution of ACE inhibitor–induced angioedema – was 8 hours with icatibant and 27 hours with standard therapy. Angioedema resolved within 4 hours in five patients (38%) given icatibant; none of the patients given standard therapy responded that quickly. The onset of symptom relief was 2 hours with icatibant and 12 hours with standard glucocorticoids plus antihistamines, a significant difference as judged by the study participants and the researchers. Also, the physician-assessed severity of angioedema began to abate within 1 hour of icatibant administration and within 8 hours for standard treatment (N. Engl. J. Med. 2015 Jan. 29 [doi:10.1056/NEJMoa1312524]).
“Although the sample size in this trial was too small to allow for a robust evaluation of safety, no patient discontinued participation in the study owing to adverse events,” Dr. Bas and his associates added.
Dr. Bas reported receiving grants and personal fees from Shire, the maker of icatibant, as did some of his associates.
Key clinical point: Icatibant may prove to be a more effective treatment than glucocorticoids and antihistamines for ACE inhibitor–induced angioedema.
Major finding: The time to complete resolution of ACE inhibitor–induced angioedema was 8 hours with icatibant and 27 hours with standard therapy.
Data source: A multicenter double-blind randomized phase II clinical trial involving 27 adults hospitalized in Germany for ACE inhibitor–induced angioedema during a 1.5-year period.
Disclosures: This study was supported by an educational grant from Shire and by the Federal Ministry of Education and Research of Germany. Dr. Bas reported receiving grants and personal fees from Shire, the maker of icatibant, as did some of his associates.
Increased heroin use may not be linked to rise in prescription opioid use
The increase in nonmedical prescription opioid use in the United States does not appear to be strongly related to the concurrent increase in heroin use, according to a review by Dr. Wilson Compton of the National Institute on Drug Abuse, Bethesda, Md., and his associates.
While heroin users are 3.9 times more likely to have used nonmedical prescription opioids than are those who haven’t used heroin, heroin use only occurs in a small number of nonmedical prescription opioid users. The researchers cited studies showing that 3.6% of opioid users began using heroin within 5 years of beginning opioid use, and 4.2% of opioid users reported also using heroin in the past year.
A more likely driver for the increased use of heroin and heroin death rate is decreased cost and increased availability, the investigators wrote. For every $100 decrease in price per gram of heroin, hospitalizations for heroin overdose increase by 2.9%. In addition, heroin use has grown significantly in areas of the United States that were not typically centers for heroin distribution, the researchers reported.
“Fundamentally, prescription opioids and heroin are each elements of a larger epidemic of opioid-related disorders and death. Viewing them from a unified perspective is essential to improving public health. The perniciousness of this epidemic requires a multipronged interventional approach that engages all sectors of society,” the investigators wrote.
Dr. Compton has ties with General Electric, 3M, and Pfizer. No other conflicts were reported. Find the study in the New England Journal of Medicine (doi: 10.1056/NEJMra1508490).
The increase in nonmedical prescription opioid use in the United States does not appear to be strongly related to the concurrent increase in heroin use, according to a review by Dr. Wilson Compton of the National Institute on Drug Abuse, Bethesda, Md., and his associates.
While heroin users are 3.9 times more likely to have used nonmedical prescription opioids than are those who haven’t used heroin, heroin use only occurs in a small number of nonmedical prescription opioid users. The researchers cited studies showing that 3.6% of opioid users began using heroin within 5 years of beginning opioid use, and 4.2% of opioid users reported also using heroin in the past year.
A more likely driver for the increased use of heroin and heroin death rate is decreased cost and increased availability, the investigators wrote. For every $100 decrease in price per gram of heroin, hospitalizations for heroin overdose increase by 2.9%. In addition, heroin use has grown significantly in areas of the United States that were not typically centers for heroin distribution, the researchers reported.
“Fundamentally, prescription opioids and heroin are each elements of a larger epidemic of opioid-related disorders and death. Viewing them from a unified perspective is essential to improving public health. The perniciousness of this epidemic requires a multipronged interventional approach that engages all sectors of society,” the investigators wrote.
Dr. Compton has ties with General Electric, 3M, and Pfizer. No other conflicts were reported. Find the study in the New England Journal of Medicine (doi: 10.1056/NEJMra1508490).
The increase in nonmedical prescription opioid use in the United States does not appear to be strongly related to the concurrent increase in heroin use, according to a review by Dr. Wilson Compton of the National Institute on Drug Abuse, Bethesda, Md., and his associates.
While heroin users are 3.9 times more likely to have used nonmedical prescription opioids than are those who haven’t used heroin, heroin use only occurs in a small number of nonmedical prescription opioid users. The researchers cited studies showing that 3.6% of opioid users began using heroin within 5 years of beginning opioid use, and 4.2% of opioid users reported also using heroin in the past year.
A more likely driver for the increased use of heroin and heroin death rate is decreased cost and increased availability, the investigators wrote. For every $100 decrease in price per gram of heroin, hospitalizations for heroin overdose increase by 2.9%. In addition, heroin use has grown significantly in areas of the United States that were not typically centers for heroin distribution, the researchers reported.
“Fundamentally, prescription opioids and heroin are each elements of a larger epidemic of opioid-related disorders and death. Viewing them from a unified perspective is essential to improving public health. The perniciousness of this epidemic requires a multipronged interventional approach that engages all sectors of society,” the investigators wrote.
Dr. Compton has ties with General Electric, 3M, and Pfizer. No other conflicts were reported. Find the study in the New England Journal of Medicine (doi: 10.1056/NEJMra1508490).
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Increased heroin use may not be linked to rise in prescription opioid use
The increase in nonmedical prescription opioid use in the United States does not appear to be strongly related to the concurrent increase in heroin use, according to a review by Dr. Wilson Compton of the National Institute on Drug Abuse, Bethesda, Md., and his associates.
While heroin users are 3.9 times more likely to have used nonmedical prescription opioids than are those who haven’t used heroin, heroin use does occur in a small number of nonmedical prescription opioid users. The researchers cited studies showing that 3.6% of opioid users began using heroin within 5 years of beginning opioid use, and 4.2% of opioid users reported also using heroin in the past year.
A more likely driver for the increased use of heroin and heroin death rate is decreased cost and increased availability, the investigators wrote. For every $100 decrease in price per gram of heroin, hospitalizations for heroin overdose increase by 2.9%. In addition, heroin use has grown significantly in areas of the United States that were not typically centers for heroin distribution, the researchers reported.
“Fundamentally, prescription opioids and heroin are each elements of a larger epidemic of opioid-related disorders and death. Viewing them from a unified perspective is essential to improving public health. The perniciousness of this epidemic requires a multipronged interventional approach that engages all sectors of society,” the investigators wrote.
Dr. Compton has ties with General Electric, 3M, and Pfizer. No other conflicts were reported. Find the study in the New England Journal of Medicine (doi: 10.1056/NEJMra1508490).
The increase in nonmedical prescription opioid use in the United States does not appear to be strongly related to the concurrent increase in heroin use, according to a review by Dr. Wilson Compton of the National Institute on Drug Abuse, Bethesda, Md., and his associates.
While heroin users are 3.9 times more likely to have used nonmedical prescription opioids than are those who haven’t used heroin, heroin use does occur in a small number of nonmedical prescription opioid users. The researchers cited studies showing that 3.6% of opioid users began using heroin within 5 years of beginning opioid use, and 4.2% of opioid users reported also using heroin in the past year.
A more likely driver for the increased use of heroin and heroin death rate is decreased cost and increased availability, the investigators wrote. For every $100 decrease in price per gram of heroin, hospitalizations for heroin overdose increase by 2.9%. In addition, heroin use has grown significantly in areas of the United States that were not typically centers for heroin distribution, the researchers reported.
“Fundamentally, prescription opioids and heroin are each elements of a larger epidemic of opioid-related disorders and death. Viewing them from a unified perspective is essential to improving public health. The perniciousness of this epidemic requires a multipronged interventional approach that engages all sectors of society,” the investigators wrote.
Dr. Compton has ties with General Electric, 3M, and Pfizer. No other conflicts were reported. Find the study in the New England Journal of Medicine (doi: 10.1056/NEJMra1508490).
The increase in nonmedical prescription opioid use in the United States does not appear to be strongly related to the concurrent increase in heroin use, according to a review by Dr. Wilson Compton of the National Institute on Drug Abuse, Bethesda, Md., and his associates.
While heroin users are 3.9 times more likely to have used nonmedical prescription opioids than are those who haven’t used heroin, heroin use does occur in a small number of nonmedical prescription opioid users. The researchers cited studies showing that 3.6% of opioid users began using heroin within 5 years of beginning opioid use, and 4.2% of opioid users reported also using heroin in the past year.
A more likely driver for the increased use of heroin and heroin death rate is decreased cost and increased availability, the investigators wrote. For every $100 decrease in price per gram of heroin, hospitalizations for heroin overdose increase by 2.9%. In addition, heroin use has grown significantly in areas of the United States that were not typically centers for heroin distribution, the researchers reported.
“Fundamentally, prescription opioids and heroin are each elements of a larger epidemic of opioid-related disorders and death. Viewing them from a unified perspective is essential to improving public health. The perniciousness of this epidemic requires a multipronged interventional approach that engages all sectors of society,” the investigators wrote.
Dr. Compton has ties with General Electric, 3M, and Pfizer. No other conflicts were reported. Find the study in the New England Journal of Medicine (doi: 10.1056/NEJMra1508490).
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Alcohol poisoning kills an average of six people each day
Every day, an average of six people in the United States die from alcohol poisoning—the majority of them middle-aged men, according to a new Vital Signs report from the Centers for Disease Control and Prevention.
“This is likely to be an underestimate,” the CDC’s Deputy Principal Director, Ileana Arias, Ph.D., said during a Jan. 6, 2015 press briefing.
Dr. Arias highlighted findings from a study of alcohol poisoning among people aged 15 and older that coauthor Dr. Robert D. Brewer and associates conducted using multiple cause-of-death data from the National Vital Statistics for 2010-2012. They found that more than 2,200 Americans died each year of alcohol poisoning, for an average of six deaths every day each year. Three in four alcohol poisoning deaths involved adults 35-54 years old, mostly men.
The researchers determined that binge drinking, defined as consuming four or more drinks for women and five or more drinks for men during a period of 2-3 hours, accounted for most of the deaths. “Despite the risks, more than 38 million U.S. adults report binge drinking about four times per month and consume an average of eight drinks per binge,” Dr. Arias said. “Alcohol poisoning is caused by consuming a very large amount of alcohol in a very short period of time.”
A person’s response to alcohol can vary depending on many factors, including the grade of alcohol consumed, the health of the drinker, and whether the drinker has consumed other drugs. “But the key point is this: The more you drink, the greater you are at risk of poisoning and of death,” she said.
Dr. Arias noted that a 12-ounce can of 5% beer contains the same amount of alcohol as a 5-ounce glass of 12% wine or 1.5 ounces of 80-proof distilled spirits. “It’s also best to avoid drinks with unknown alcohol content and be very cautious when mixing alcohol with energy drinks,” she said. “Caffeine can mask alcohol’s effects, causing you to drink more than you intended [to].”
When assessed by race and ethnicity, the majority of alcohol-poisoning deaths occurred among non-Hispanic whites. However, American Indians and Alaska Natives had the most alcohol-poisoning deaths per million people. Alcohol-poisoning deaths also varied widely across states, ranging from 5.3 deaths per million residents in Alabama to 46.5 deaths per million residents in Alaska. “Alcohol dependence was identified as a factor in 30% of these deaths and other drugs contributed to 3% of the deaths,” she said.
Life-threatening signs of alcohol poisoning include the inability to wake up from sleep, vomiting, seizures, slow or irregular breathing or heart rate, and low body temperature, bluish skin color, or pallor. Dr. Arias said that health professionals can play a role in the prevention of deaths related to alcohol poisoning by screening all adult patients for excessive drinking, counseling those who do so to help them drink less, and referring excessive drinkers who are alcohol dependent for specialized treatment.
“The bottom line is that binge drinking can be lethal,” she said. “Alcohol poisoning is killing people across the lifespan, but in particular men in the prime of their lives.”
None of the researchers reported having relevant financial disclosures.
On Twitter @dougbrunk
Every day, an average of six people in the United States die from alcohol poisoning—the majority of them middle-aged men, according to a new Vital Signs report from the Centers for Disease Control and Prevention.
“This is likely to be an underestimate,” the CDC’s Deputy Principal Director, Ileana Arias, Ph.D., said during a Jan. 6, 2015 press briefing.
Dr. Arias highlighted findings from a study of alcohol poisoning among people aged 15 and older that coauthor Dr. Robert D. Brewer and associates conducted using multiple cause-of-death data from the National Vital Statistics for 2010-2012. They found that more than 2,200 Americans died each year of alcohol poisoning, for an average of six deaths every day each year. Three in four alcohol poisoning deaths involved adults 35-54 years old, mostly men.
The researchers determined that binge drinking, defined as consuming four or more drinks for women and five or more drinks for men during a period of 2-3 hours, accounted for most of the deaths. “Despite the risks, more than 38 million U.S. adults report binge drinking about four times per month and consume an average of eight drinks per binge,” Dr. Arias said. “Alcohol poisoning is caused by consuming a very large amount of alcohol in a very short period of time.”
A person’s response to alcohol can vary depending on many factors, including the grade of alcohol consumed, the health of the drinker, and whether the drinker has consumed other drugs. “But the key point is this: The more you drink, the greater you are at risk of poisoning and of death,” she said.
Dr. Arias noted that a 12-ounce can of 5% beer contains the same amount of alcohol as a 5-ounce glass of 12% wine or 1.5 ounces of 80-proof distilled spirits. “It’s also best to avoid drinks with unknown alcohol content and be very cautious when mixing alcohol with energy drinks,” she said. “Caffeine can mask alcohol’s effects, causing you to drink more than you intended [to].”
When assessed by race and ethnicity, the majority of alcohol-poisoning deaths occurred among non-Hispanic whites. However, American Indians and Alaska Natives had the most alcohol-poisoning deaths per million people. Alcohol-poisoning deaths also varied widely across states, ranging from 5.3 deaths per million residents in Alabama to 46.5 deaths per million residents in Alaska. “Alcohol dependence was identified as a factor in 30% of these deaths and other drugs contributed to 3% of the deaths,” she said.
Life-threatening signs of alcohol poisoning include the inability to wake up from sleep, vomiting, seizures, slow or irregular breathing or heart rate, and low body temperature, bluish skin color, or pallor. Dr. Arias said that health professionals can play a role in the prevention of deaths related to alcohol poisoning by screening all adult patients for excessive drinking, counseling those who do so to help them drink less, and referring excessive drinkers who are alcohol dependent for specialized treatment.
“The bottom line is that binge drinking can be lethal,” she said. “Alcohol poisoning is killing people across the lifespan, but in particular men in the prime of their lives.”
None of the researchers reported having relevant financial disclosures.
On Twitter @dougbrunk
Every day, an average of six people in the United States die from alcohol poisoning—the majority of them middle-aged men, according to a new Vital Signs report from the Centers for Disease Control and Prevention.
“This is likely to be an underestimate,” the CDC’s Deputy Principal Director, Ileana Arias, Ph.D., said during a Jan. 6, 2015 press briefing.
Dr. Arias highlighted findings from a study of alcohol poisoning among people aged 15 and older that coauthor Dr. Robert D. Brewer and associates conducted using multiple cause-of-death data from the National Vital Statistics for 2010-2012. They found that more than 2,200 Americans died each year of alcohol poisoning, for an average of six deaths every day each year. Three in four alcohol poisoning deaths involved adults 35-54 years old, mostly men.
The researchers determined that binge drinking, defined as consuming four or more drinks for women and five or more drinks for men during a period of 2-3 hours, accounted for most of the deaths. “Despite the risks, more than 38 million U.S. adults report binge drinking about four times per month and consume an average of eight drinks per binge,” Dr. Arias said. “Alcohol poisoning is caused by consuming a very large amount of alcohol in a very short period of time.”
A person’s response to alcohol can vary depending on many factors, including the grade of alcohol consumed, the health of the drinker, and whether the drinker has consumed other drugs. “But the key point is this: The more you drink, the greater you are at risk of poisoning and of death,” she said.
Dr. Arias noted that a 12-ounce can of 5% beer contains the same amount of alcohol as a 5-ounce glass of 12% wine or 1.5 ounces of 80-proof distilled spirits. “It’s also best to avoid drinks with unknown alcohol content and be very cautious when mixing alcohol with energy drinks,” she said. “Caffeine can mask alcohol’s effects, causing you to drink more than you intended [to].”
When assessed by race and ethnicity, the majority of alcohol-poisoning deaths occurred among non-Hispanic whites. However, American Indians and Alaska Natives had the most alcohol-poisoning deaths per million people. Alcohol-poisoning deaths also varied widely across states, ranging from 5.3 deaths per million residents in Alabama to 46.5 deaths per million residents in Alaska. “Alcohol dependence was identified as a factor in 30% of these deaths and other drugs contributed to 3% of the deaths,” she said.
Life-threatening signs of alcohol poisoning include the inability to wake up from sleep, vomiting, seizures, slow or irregular breathing or heart rate, and low body temperature, bluish skin color, or pallor. Dr. Arias said that health professionals can play a role in the prevention of deaths related to alcohol poisoning by screening all adult patients for excessive drinking, counseling those who do so to help them drink less, and referring excessive drinkers who are alcohol dependent for specialized treatment.
“The bottom line is that binge drinking can be lethal,” she said. “Alcohol poisoning is killing people across the lifespan, but in particular men in the prime of their lives.”
None of the researchers reported having relevant financial disclosures.
On Twitter @dougbrunk
Key clinical point: An estimated six people in the United States die each day from alcohol poisoning.
Major finding: More than 2,200 Americans die each year of alcohol poisoning, or an average of six deaths every day each year.
Data source: An analysis of multiple cause-of-death data from the National Vital Statistics for 2010-2012.
Disclosures: None of the researchers reported having relevant financial disclosures.
Case Studies in Toxicology: You Can’t See Dragonfly or Hear NBOMe, but They Can Still Hurt You
Case
A 24-year-old man was brought to the ED by emergency medical services (EMS) for altered mental status. The EMS crew reported they had picked up the patient at a nearby arts festival and concert series. A bystander at the event reported that the patient had taken something called “dragonfly.”
Initial assessment revealed the patient to be disoriented, with nonlinear thought patterns and an inability to follow commands. His vital signs were: blood pressure, 160/100 mm Hg; heart rate, 120 beats/minute; respiratory rate, 24 breaths/minute; and temperature, 102.2˚F. Oxygen saturation was 99% on room air. He was diaphoretic and agitated, and the nursing staff was concerned he would become aggressive and potentially violent. A quick Web search revealed that the agent the bystander mentioned was most likely Bromo-DragonFLY (BDF).
What is Bromo-DragonFLY?
In the 1960s, an American chemist named Alexander Shulgin ushered in a new era of psychedelic drug use by establishing a simple synthesis of 3,4-methylenedioxy-methamphetamine (MDMA). Following this discovery, he suggested a therapist friend use the drug therapeutically.1 Shulgin then began a process of homologation (ie, creating novel compounds by slightly altering existing ones in an organized fashion) and developed systems for rating the drug experiences and naming the drugs in shorthand, both of which are still in use. The chemical structure common to nearly all of the drugs he studied is phenylethylamine. The Figure shows the structures of several phenylethylamine derivatives that were created by adding functional groups to the phenylethylamine backbone. Although the popularity of psychedelic drugs surged during this time period, 2,5-dimethoxy-N-(2-methoxybenzyl)phenylethylamine) (NBOMe), one of a number of newly popular psychedelics, only became available in 2003.
What is known about the pharmacology of Bromo-DragonFLY and NBOMe?
The major target of psychedelic drugs is the serotonin (5-HT2) receptor, specifically the central 5-HT2A subtype. Bromo-DragonFLY is a classic example of designer pharmacology in that the it was intended to potently exert its effect at this specific receptor site.
As its name suggests, BDF adds the “wings of the fly” to the phenylethylamine backbone furanyl rings at positions 2 and 5, and a halogen (bromine) at position 4. The furanyl ring impairs enzymatic clearance of the drug,2 resulting in a duration of action of up to 3 days.3 The addition of halogens increases drug potency, but the mechanism is not clear. The psychedelic agent NBOMe results from chemical additions of methoxy groups at position 2 and 5, and the halogen moiety (iodine in this case) at position 4 of the phenyl ring of the phenylethylamine structure.4
Through the work of Shulgin, some of his colleagues, and many disparate street chemists, a vast family of substituted phenylethylamines have been synthesized and used. Shulgin’s semiautobiographical book PiHKAL: A Chemical Love Story includes his laboratory notes for the synthesis and initial test-dose experience of 179 compounds1; this does not include research done by others or any work since its publication in 1995.
Notable popular drugs chemically similar to NBOMe and BDF are mescaline (found in peyote), cathinones (“bath salts”), and MDMA (found in ecstasy) (Figure). Naturally occurring (and more complex) compounds with similar effects include ayahuasca, a plant-derived beverage consisting of Banisteriopsis caapi and either Psychotria viridis or Diplopterys cabrerana from the Brazilian rainforest (see Emerg Med. 2014;46[12]:553-556); psilocybin (“magic mushrooms”); and lysergic acid diethylamide.
How are these drugs used and what are their clinical effects?
Most phenylethylamine compounds are well absorbed across the buccal mucosa, which is why BDF and NBOMe are commonly used in liquid form or on blotter paper. Dosing guides also exist for insufflation and claim equipotent dosing for this route.5 Regardless of delivery route, given the high potency, inadvertent exposures to these drugs should be expected.
Users simply seeking to hallucinate may not be aware of the significant risks associated with these potent serotonergic agents, which include both life- and limb-threatening effects.6 The high 5-HT2A potency results both in vasoconstriction and promotion of clot formation due to the presence of 5HT2A receptors on small blood vessels and platelets, respectively. Ergotism, historically called Saint Anthony’s fire, is an example of serotonergic vasoconstriction and hallucination.7 Chronic users of substituted amphetamines can develop necrotic ulcers in distal vascular beds such as the hands and feet; these ulcers may progress to amputation despite treatment attempts with vasodilators.
In addition to the vasoconstrictive properties, there are multiple reports of serotonin toxicity (serotonin syndrome) associated with use of these designer serotonergic amphetamines. This syndrome includes severe psychomotor agitation that can lead to personal injury, along with muscle rigidity, tremor, hyperthermia, rhabdomyolysis, and seizures.8
How are patients with phenylethylamine exposures managed?
Management of a patient with a substituted phenylethylamine exposure is similar to management of those with cocaine overdose. Attention to the life-threatening clinical effects of psychomotor agitation, hyperthermia, and seizures is paramount. Appropriate supportive care includes intravenous (IV) benzodiazepines to control agitation and muscle rigidity, replacement of lost volume with crystalloids, and active cooling measures. Failure of benzodiazepines (preferably in conjunction with continuous electroencephalogram monitoring) to control rigidity may lead to the need for propofol and/or result in paralysis. Similar to patients with cocaine intoxication, some may experience ischemic chest pain, and the usual protocol of sedation, nitroglycerin, morphine, and an antiplatelet drug is appropriate.
Identification of phenylethylamines typically requires specialized laboratory testing since most will not trigger a positive result on a standard urine immunoassay. Many specialized laboratories have test catalogs on their Web sites listing under the “stimulants panel” which drugs can be identified. However, none of these assays is likely truly comprehensive, and minor alterations or substitutions to the compounds result in new analogs that may not be in the reference laboratory’s identification library.
The patient was initially restrained and given 5 mg IV diazepam, which was followed by escalating doses every 5 minutes to a total of 35 mg for effect. He had a rectal temperature of 102.5˚F and was externally cooled after sedation. After 20 minutes, he had a generalized convulsion; an additional 10 mg of IV diazepam terminated the seizure, but he remained hyperthermic at 104˚F. The patient was intubated, placed on a propofol infusion, and admitted to the intensive care unit where his temperature was carefully monitored. The following day his temperature had normalized and he was weaned from the ventilator and discharged to the floor for monitoring. On hospital day 3, he was discharged in stable condition.
Mr Waldrop is a fourth-year medical student at the State University of New York, Upstate Medical University, Syracuse. Dr Nacca is a fellow in medical toxicology, department of emergency medicine, State University of New York, Upstate Medical University, Syracuse. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine, and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Shulgin A, Shulgin A. PiHKAL: A Chemical Love Story. Berkeley, CA: Transform Press; 1995.
- Andreasen MF, Telving R, Birkler RI, Schumacher B, Johannsen M. A fatal poisoning involving bromo-dragonfly. Forensic Sci Int. 2009;183(1-3):91-96.
- Hill SL, Thomas SH. Clinical toxicology of newer recreational drugs. Clin Toxicol (Phila). 2011;49(8):705-719.
- Gentry CL, Egleton RD, Gillespie T, et al. The effect of halogenation on blood-brain barrier permeability of a novel peptide drug. Peptides. 1999;20(10):1229-1238.
- Erowid. Bromo-Dragonfly Dosage. http://www.erowid.org/chemicals/bromo_dragonfly/bromo_dragonfly_dose.shtml. Accessed January 14, 2015.
- Baumann MH, Ayestas MA Jr, Partilla JS, et al. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology. 2012;37(5):1192-1203.
- Walterscheid JP, Phillips GT, Lopez AE, Gonsoulin ML, Chen HH, Sanchez LA. Pathological findings in 2 cases of fatal 25I-NBOMe toxicity. Am J Forensic Med Pathol. 2014;35(1):20-25.
- Wood DM, Looker JJ, Shaikh L, et al. Delayed onset of seizures and toxicity associated with recreational use of Bromo-dragonFLY. J Med Toxicol. 2009;5(4):226-229.
Case
A 24-year-old man was brought to the ED by emergency medical services (EMS) for altered mental status. The EMS crew reported they had picked up the patient at a nearby arts festival and concert series. A bystander at the event reported that the patient had taken something called “dragonfly.”
Initial assessment revealed the patient to be disoriented, with nonlinear thought patterns and an inability to follow commands. His vital signs were: blood pressure, 160/100 mm Hg; heart rate, 120 beats/minute; respiratory rate, 24 breaths/minute; and temperature, 102.2˚F. Oxygen saturation was 99% on room air. He was diaphoretic and agitated, and the nursing staff was concerned he would become aggressive and potentially violent. A quick Web search revealed that the agent the bystander mentioned was most likely Bromo-DragonFLY (BDF).
What is Bromo-DragonFLY?
In the 1960s, an American chemist named Alexander Shulgin ushered in a new era of psychedelic drug use by establishing a simple synthesis of 3,4-methylenedioxy-methamphetamine (MDMA). Following this discovery, he suggested a therapist friend use the drug therapeutically.1 Shulgin then began a process of homologation (ie, creating novel compounds by slightly altering existing ones in an organized fashion) and developed systems for rating the drug experiences and naming the drugs in shorthand, both of which are still in use. The chemical structure common to nearly all of the drugs he studied is phenylethylamine. The Figure shows the structures of several phenylethylamine derivatives that were created by adding functional groups to the phenylethylamine backbone. Although the popularity of psychedelic drugs surged during this time period, 2,5-dimethoxy-N-(2-methoxybenzyl)phenylethylamine) (NBOMe), one of a number of newly popular psychedelics, only became available in 2003.
What is known about the pharmacology of Bromo-DragonFLY and NBOMe?
The major target of psychedelic drugs is the serotonin (5-HT2) receptor, specifically the central 5-HT2A subtype. Bromo-DragonFLY is a classic example of designer pharmacology in that the it was intended to potently exert its effect at this specific receptor site.
As its name suggests, BDF adds the “wings of the fly” to the phenylethylamine backbone furanyl rings at positions 2 and 5, and a halogen (bromine) at position 4. The furanyl ring impairs enzymatic clearance of the drug,2 resulting in a duration of action of up to 3 days.3 The addition of halogens increases drug potency, but the mechanism is not clear. The psychedelic agent NBOMe results from chemical additions of methoxy groups at position 2 and 5, and the halogen moiety (iodine in this case) at position 4 of the phenyl ring of the phenylethylamine structure.4
Through the work of Shulgin, some of his colleagues, and many disparate street chemists, a vast family of substituted phenylethylamines have been synthesized and used. Shulgin’s semiautobiographical book PiHKAL: A Chemical Love Story includes his laboratory notes for the synthesis and initial test-dose experience of 179 compounds1; this does not include research done by others or any work since its publication in 1995.
Notable popular drugs chemically similar to NBOMe and BDF are mescaline (found in peyote), cathinones (“bath salts”), and MDMA (found in ecstasy) (Figure). Naturally occurring (and more complex) compounds with similar effects include ayahuasca, a plant-derived beverage consisting of Banisteriopsis caapi and either Psychotria viridis or Diplopterys cabrerana from the Brazilian rainforest (see Emerg Med. 2014;46[12]:553-556); psilocybin (“magic mushrooms”); and lysergic acid diethylamide.
How are these drugs used and what are their clinical effects?
Most phenylethylamine compounds are well absorbed across the buccal mucosa, which is why BDF and NBOMe are commonly used in liquid form or on blotter paper. Dosing guides also exist for insufflation and claim equipotent dosing for this route.5 Regardless of delivery route, given the high potency, inadvertent exposures to these drugs should be expected.
Users simply seeking to hallucinate may not be aware of the significant risks associated with these potent serotonergic agents, which include both life- and limb-threatening effects.6 The high 5-HT2A potency results both in vasoconstriction and promotion of clot formation due to the presence of 5HT2A receptors on small blood vessels and platelets, respectively. Ergotism, historically called Saint Anthony’s fire, is an example of serotonergic vasoconstriction and hallucination.7 Chronic users of substituted amphetamines can develop necrotic ulcers in distal vascular beds such as the hands and feet; these ulcers may progress to amputation despite treatment attempts with vasodilators.
In addition to the vasoconstrictive properties, there are multiple reports of serotonin toxicity (serotonin syndrome) associated with use of these designer serotonergic amphetamines. This syndrome includes severe psychomotor agitation that can lead to personal injury, along with muscle rigidity, tremor, hyperthermia, rhabdomyolysis, and seizures.8
How are patients with phenylethylamine exposures managed?
Management of a patient with a substituted phenylethylamine exposure is similar to management of those with cocaine overdose. Attention to the life-threatening clinical effects of psychomotor agitation, hyperthermia, and seizures is paramount. Appropriate supportive care includes intravenous (IV) benzodiazepines to control agitation and muscle rigidity, replacement of lost volume with crystalloids, and active cooling measures. Failure of benzodiazepines (preferably in conjunction with continuous electroencephalogram monitoring) to control rigidity may lead to the need for propofol and/or result in paralysis. Similar to patients with cocaine intoxication, some may experience ischemic chest pain, and the usual protocol of sedation, nitroglycerin, morphine, and an antiplatelet drug is appropriate.
Identification of phenylethylamines typically requires specialized laboratory testing since most will not trigger a positive result on a standard urine immunoassay. Many specialized laboratories have test catalogs on their Web sites listing under the “stimulants panel” which drugs can be identified. However, none of these assays is likely truly comprehensive, and minor alterations or substitutions to the compounds result in new analogs that may not be in the reference laboratory’s identification library.
The patient was initially restrained and given 5 mg IV diazepam, which was followed by escalating doses every 5 minutes to a total of 35 mg for effect. He had a rectal temperature of 102.5˚F and was externally cooled after sedation. After 20 minutes, he had a generalized convulsion; an additional 10 mg of IV diazepam terminated the seizure, but he remained hyperthermic at 104˚F. The patient was intubated, placed on a propofol infusion, and admitted to the intensive care unit where his temperature was carefully monitored. The following day his temperature had normalized and he was weaned from the ventilator and discharged to the floor for monitoring. On hospital day 3, he was discharged in stable condition.
Mr Waldrop is a fourth-year medical student at the State University of New York, Upstate Medical University, Syracuse. Dr Nacca is a fellow in medical toxicology, department of emergency medicine, State University of New York, Upstate Medical University, Syracuse. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine, and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
Case
A 24-year-old man was brought to the ED by emergency medical services (EMS) for altered mental status. The EMS crew reported they had picked up the patient at a nearby arts festival and concert series. A bystander at the event reported that the patient had taken something called “dragonfly.”
Initial assessment revealed the patient to be disoriented, with nonlinear thought patterns and an inability to follow commands. His vital signs were: blood pressure, 160/100 mm Hg; heart rate, 120 beats/minute; respiratory rate, 24 breaths/minute; and temperature, 102.2˚F. Oxygen saturation was 99% on room air. He was diaphoretic and agitated, and the nursing staff was concerned he would become aggressive and potentially violent. A quick Web search revealed that the agent the bystander mentioned was most likely Bromo-DragonFLY (BDF).
What is Bromo-DragonFLY?
In the 1960s, an American chemist named Alexander Shulgin ushered in a new era of psychedelic drug use by establishing a simple synthesis of 3,4-methylenedioxy-methamphetamine (MDMA). Following this discovery, he suggested a therapist friend use the drug therapeutically.1 Shulgin then began a process of homologation (ie, creating novel compounds by slightly altering existing ones in an organized fashion) and developed systems for rating the drug experiences and naming the drugs in shorthand, both of which are still in use. The chemical structure common to nearly all of the drugs he studied is phenylethylamine. The Figure shows the structures of several phenylethylamine derivatives that were created by adding functional groups to the phenylethylamine backbone. Although the popularity of psychedelic drugs surged during this time period, 2,5-dimethoxy-N-(2-methoxybenzyl)phenylethylamine) (NBOMe), one of a number of newly popular psychedelics, only became available in 2003.
What is known about the pharmacology of Bromo-DragonFLY and NBOMe?
The major target of psychedelic drugs is the serotonin (5-HT2) receptor, specifically the central 5-HT2A subtype. Bromo-DragonFLY is a classic example of designer pharmacology in that the it was intended to potently exert its effect at this specific receptor site.
As its name suggests, BDF adds the “wings of the fly” to the phenylethylamine backbone furanyl rings at positions 2 and 5, and a halogen (bromine) at position 4. The furanyl ring impairs enzymatic clearance of the drug,2 resulting in a duration of action of up to 3 days.3 The addition of halogens increases drug potency, but the mechanism is not clear. The psychedelic agent NBOMe results from chemical additions of methoxy groups at position 2 and 5, and the halogen moiety (iodine in this case) at position 4 of the phenyl ring of the phenylethylamine structure.4
Through the work of Shulgin, some of his colleagues, and many disparate street chemists, a vast family of substituted phenylethylamines have been synthesized and used. Shulgin’s semiautobiographical book PiHKAL: A Chemical Love Story includes his laboratory notes for the synthesis and initial test-dose experience of 179 compounds1; this does not include research done by others or any work since its publication in 1995.
Notable popular drugs chemically similar to NBOMe and BDF are mescaline (found in peyote), cathinones (“bath salts”), and MDMA (found in ecstasy) (Figure). Naturally occurring (and more complex) compounds with similar effects include ayahuasca, a plant-derived beverage consisting of Banisteriopsis caapi and either Psychotria viridis or Diplopterys cabrerana from the Brazilian rainforest (see Emerg Med. 2014;46[12]:553-556); psilocybin (“magic mushrooms”); and lysergic acid diethylamide.
How are these drugs used and what are their clinical effects?
Most phenylethylamine compounds are well absorbed across the buccal mucosa, which is why BDF and NBOMe are commonly used in liquid form or on blotter paper. Dosing guides also exist for insufflation and claim equipotent dosing for this route.5 Regardless of delivery route, given the high potency, inadvertent exposures to these drugs should be expected.
Users simply seeking to hallucinate may not be aware of the significant risks associated with these potent serotonergic agents, which include both life- and limb-threatening effects.6 The high 5-HT2A potency results both in vasoconstriction and promotion of clot formation due to the presence of 5HT2A receptors on small blood vessels and platelets, respectively. Ergotism, historically called Saint Anthony’s fire, is an example of serotonergic vasoconstriction and hallucination.7 Chronic users of substituted amphetamines can develop necrotic ulcers in distal vascular beds such as the hands and feet; these ulcers may progress to amputation despite treatment attempts with vasodilators.
In addition to the vasoconstrictive properties, there are multiple reports of serotonin toxicity (serotonin syndrome) associated with use of these designer serotonergic amphetamines. This syndrome includes severe psychomotor agitation that can lead to personal injury, along with muscle rigidity, tremor, hyperthermia, rhabdomyolysis, and seizures.8
How are patients with phenylethylamine exposures managed?
Management of a patient with a substituted phenylethylamine exposure is similar to management of those with cocaine overdose. Attention to the life-threatening clinical effects of psychomotor agitation, hyperthermia, and seizures is paramount. Appropriate supportive care includes intravenous (IV) benzodiazepines to control agitation and muscle rigidity, replacement of lost volume with crystalloids, and active cooling measures. Failure of benzodiazepines (preferably in conjunction with continuous electroencephalogram monitoring) to control rigidity may lead to the need for propofol and/or result in paralysis. Similar to patients with cocaine intoxication, some may experience ischemic chest pain, and the usual protocol of sedation, nitroglycerin, morphine, and an antiplatelet drug is appropriate.
Identification of phenylethylamines typically requires specialized laboratory testing since most will not trigger a positive result on a standard urine immunoassay. Many specialized laboratories have test catalogs on their Web sites listing under the “stimulants panel” which drugs can be identified. However, none of these assays is likely truly comprehensive, and minor alterations or substitutions to the compounds result in new analogs that may not be in the reference laboratory’s identification library.
The patient was initially restrained and given 5 mg IV diazepam, which was followed by escalating doses every 5 minutes to a total of 35 mg for effect. He had a rectal temperature of 102.5˚F and was externally cooled after sedation. After 20 minutes, he had a generalized convulsion; an additional 10 mg of IV diazepam terminated the seizure, but he remained hyperthermic at 104˚F. The patient was intubated, placed on a propofol infusion, and admitted to the intensive care unit where his temperature was carefully monitored. The following day his temperature had normalized and he was weaned from the ventilator and discharged to the floor for monitoring. On hospital day 3, he was discharged in stable condition.
Mr Waldrop is a fourth-year medical student at the State University of New York, Upstate Medical University, Syracuse. Dr Nacca is a fellow in medical toxicology, department of emergency medicine, State University of New York, Upstate Medical University, Syracuse. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine, and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Shulgin A, Shulgin A. PiHKAL: A Chemical Love Story. Berkeley, CA: Transform Press; 1995.
- Andreasen MF, Telving R, Birkler RI, Schumacher B, Johannsen M. A fatal poisoning involving bromo-dragonfly. Forensic Sci Int. 2009;183(1-3):91-96.
- Hill SL, Thomas SH. Clinical toxicology of newer recreational drugs. Clin Toxicol (Phila). 2011;49(8):705-719.
- Gentry CL, Egleton RD, Gillespie T, et al. The effect of halogenation on blood-brain barrier permeability of a novel peptide drug. Peptides. 1999;20(10):1229-1238.
- Erowid. Bromo-Dragonfly Dosage. http://www.erowid.org/chemicals/bromo_dragonfly/bromo_dragonfly_dose.shtml. Accessed January 14, 2015.
- Baumann MH, Ayestas MA Jr, Partilla JS, et al. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology. 2012;37(5):1192-1203.
- Walterscheid JP, Phillips GT, Lopez AE, Gonsoulin ML, Chen HH, Sanchez LA. Pathological findings in 2 cases of fatal 25I-NBOMe toxicity. Am J Forensic Med Pathol. 2014;35(1):20-25.
- Wood DM, Looker JJ, Shaikh L, et al. Delayed onset of seizures and toxicity associated with recreational use of Bromo-dragonFLY. J Med Toxicol. 2009;5(4):226-229.
- Shulgin A, Shulgin A. PiHKAL: A Chemical Love Story. Berkeley, CA: Transform Press; 1995.
- Andreasen MF, Telving R, Birkler RI, Schumacher B, Johannsen M. A fatal poisoning involving bromo-dragonfly. Forensic Sci Int. 2009;183(1-3):91-96.
- Hill SL, Thomas SH. Clinical toxicology of newer recreational drugs. Clin Toxicol (Phila). 2011;49(8):705-719.
- Gentry CL, Egleton RD, Gillespie T, et al. The effect of halogenation on blood-brain barrier permeability of a novel peptide drug. Peptides. 1999;20(10):1229-1238.
- Erowid. Bromo-Dragonfly Dosage. http://www.erowid.org/chemicals/bromo_dragonfly/bromo_dragonfly_dose.shtml. Accessed January 14, 2015.
- Baumann MH, Ayestas MA Jr, Partilla JS, et al. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology. 2012;37(5):1192-1203.
- Walterscheid JP, Phillips GT, Lopez AE, Gonsoulin ML, Chen HH, Sanchez LA. Pathological findings in 2 cases of fatal 25I-NBOMe toxicity. Am J Forensic Med Pathol. 2014;35(1):20-25.
- Wood DM, Looker JJ, Shaikh L, et al. Delayed onset of seizures and toxicity associated with recreational use of Bromo-dragonFLY. J Med Toxicol. 2009;5(4):226-229.