User login
Robot-assisted prostatectomy providing better outcomes
Robot-assisted radical prostatectomy shows better early postoperative outcomes than does laparoscopic radical prostatectomy, but the differences between the two surgical approaches disappeared by the 6-month follow-up.
Dr. Hiroyuki Koike and his colleagues at Wakayama (Japan) Medical University Hospital conducted a study of two groups of patients treated for localized prostate cancer. One group of 229 patients underwent laparoscopic radical prostatectomy (LRP) between July 2007 and July 2013. The other group of 115 patients had robot-assisted radical prostatectomy (RARP) between December 2012 and August 2014 (J Robot Surg. 2017;11[3]:325-31).
The patients were given health-related quality of life self-assessment surveys prior to surgery and at 3, 6, and 12 months post surgery. In addition, a generic questionnaire, the eight-item Short-Form Health Survey, was used to assess a physical component summary (PCS) and a mental component summary (MCS). The Expanded Prostate Cancer Index of Prostate, which covers four domains – urinary, sexual, bowel, and hormonal – was used as a disease-specific measure, and the response rates for both LRP and RARP at each follow-up interval were over 80%.
“The RARP group showed significantly better scores in urinary summary and all urinary subscales at postoperative 3-month follow-up. However, these differences disappeared at postoperative 6 and 12-month follow-up,” the investigators wrote. For the urinary summary score, LRP significantly underperformed, compared with RARP, with scores of 63.3 vs. 75.8, respectively, after 3 months. In addition, the bowel function score was superior for RARP, compared with LRP, at 96.9 vs. 92.9, respectively. Sexual function results were similar, with RARP and LRP scores of 2.8 vs. 0.
The general measures of the PCS and MCS also favored RARP. At the 3-month follow-up, PCS (51.3 vs. 48.1) and MCS (50 vs. 47.8) scores were higher for RARP, compared with LRP.
“It is unclear why our superiority of urinary function in RARP was observed only in early period. However, we can speculate several reasons for better urinary function in RARP group. First, we were able to treat the apex area more delicately with RARP. Second, some of the new techniques which we employed after the introduction of RARP could influence the urinary continence recovery,” the investigators wrote.
The authors had no relevant financial disclosures.
Robot-assisted radical prostatectomy shows better early postoperative outcomes than does laparoscopic radical prostatectomy, but the differences between the two surgical approaches disappeared by the 6-month follow-up.
Dr. Hiroyuki Koike and his colleagues at Wakayama (Japan) Medical University Hospital conducted a study of two groups of patients treated for localized prostate cancer. One group of 229 patients underwent laparoscopic radical prostatectomy (LRP) between July 2007 and July 2013. The other group of 115 patients had robot-assisted radical prostatectomy (RARP) between December 2012 and August 2014 (J Robot Surg. 2017;11[3]:325-31).
The patients were given health-related quality of life self-assessment surveys prior to surgery and at 3, 6, and 12 months post surgery. In addition, a generic questionnaire, the eight-item Short-Form Health Survey, was used to assess a physical component summary (PCS) and a mental component summary (MCS). The Expanded Prostate Cancer Index of Prostate, which covers four domains – urinary, sexual, bowel, and hormonal – was used as a disease-specific measure, and the response rates for both LRP and RARP at each follow-up interval were over 80%.
“The RARP group showed significantly better scores in urinary summary and all urinary subscales at postoperative 3-month follow-up. However, these differences disappeared at postoperative 6 and 12-month follow-up,” the investigators wrote. For the urinary summary score, LRP significantly underperformed, compared with RARP, with scores of 63.3 vs. 75.8, respectively, after 3 months. In addition, the bowel function score was superior for RARP, compared with LRP, at 96.9 vs. 92.9, respectively. Sexual function results were similar, with RARP and LRP scores of 2.8 vs. 0.
The general measures of the PCS and MCS also favored RARP. At the 3-month follow-up, PCS (51.3 vs. 48.1) and MCS (50 vs. 47.8) scores were higher for RARP, compared with LRP.
“It is unclear why our superiority of urinary function in RARP was observed only in early period. However, we can speculate several reasons for better urinary function in RARP group. First, we were able to treat the apex area more delicately with RARP. Second, some of the new techniques which we employed after the introduction of RARP could influence the urinary continence recovery,” the investigators wrote.
The authors had no relevant financial disclosures.
Robot-assisted radical prostatectomy shows better early postoperative outcomes than does laparoscopic radical prostatectomy, but the differences between the two surgical approaches disappeared by the 6-month follow-up.
Dr. Hiroyuki Koike and his colleagues at Wakayama (Japan) Medical University Hospital conducted a study of two groups of patients treated for localized prostate cancer. One group of 229 patients underwent laparoscopic radical prostatectomy (LRP) between July 2007 and July 2013. The other group of 115 patients had robot-assisted radical prostatectomy (RARP) between December 2012 and August 2014 (J Robot Surg. 2017;11[3]:325-31).
The patients were given health-related quality of life self-assessment surveys prior to surgery and at 3, 6, and 12 months post surgery. In addition, a generic questionnaire, the eight-item Short-Form Health Survey, was used to assess a physical component summary (PCS) and a mental component summary (MCS). The Expanded Prostate Cancer Index of Prostate, which covers four domains – urinary, sexual, bowel, and hormonal – was used as a disease-specific measure, and the response rates for both LRP and RARP at each follow-up interval were over 80%.
“The RARP group showed significantly better scores in urinary summary and all urinary subscales at postoperative 3-month follow-up. However, these differences disappeared at postoperative 6 and 12-month follow-up,” the investigators wrote. For the urinary summary score, LRP significantly underperformed, compared with RARP, with scores of 63.3 vs. 75.8, respectively, after 3 months. In addition, the bowel function score was superior for RARP, compared with LRP, at 96.9 vs. 92.9, respectively. Sexual function results were similar, with RARP and LRP scores of 2.8 vs. 0.
The general measures of the PCS and MCS also favored RARP. At the 3-month follow-up, PCS (51.3 vs. 48.1) and MCS (50 vs. 47.8) scores were higher for RARP, compared with LRP.
“It is unclear why our superiority of urinary function in RARP was observed only in early period. However, we can speculate several reasons for better urinary function in RARP group. First, we were able to treat the apex area more delicately with RARP. Second, some of the new techniques which we employed after the introduction of RARP could influence the urinary continence recovery,” the investigators wrote.
The authors had no relevant financial disclosures.
FROM JOURNAL OF ROBOTIC SURGERY
Key clinical point:
Major finding: Quality-of-life score for robotic-assisted radical prostatectomy was higher in all urinary categories after 3 months.
Data source: Postop survey results from patients with localized prostate cancer who underwent laparoscopic radical prostatectomy (n = 229) or robot-assisted radical prostatectomy (n = 115).
Disclosures: The investigators had no financial disclosures to report.
Consider adding chemotherapy after GI surgery
Adjuvant chemotherapy was associated with improved overall survival rates at 3 years in patients who had surgery for gastroesophageal cancer, based on retrospective data from more than 10,000 adults.
Preoperative chemoradiotherapy and resection has shown benefits in patients with gastroesophageal adenocarcinoma, but the potential benefits of adjuvant chemotherapy (AC) after surgery in these patients has not been well studied, wrote Ali A. El Mokdad, MD, of the University of Texas Southwestern Medical Center, Dallas, and his colleagues (JAMA Oncol. 2017 Sep 21. doi: 10.1001/jamaoncol.2017.2805).
The researchers reviewed data from 10,086 patients in the National Cancer Database during 2006-2013. Of these, 814 (8%) received adjuvant chemotherapy after surgery and 9,272 (94%) received no additional intervention beyond postoperative observation. The average age of the patients was 61 years, and 88% were men.
The average survival rates at 3 years after surgery were 40 months for the adjuvant group and 34 months for the observation group (hazard ratio, 0.79). The overall survival rates in the adjuvant group were 94%, 54%, and 38% at 1,3, and 5 years, respectively, compared with rates of 88%, 47%, and 34%, in the observation group.
The findings were limited in part by the retrospective nature of the study, the researchers said. In addition, “the estimated effect of AC on overall survival is subject to selection bias and immortal time bias given that the study was observational,” they noted.
However, the results support the addition of chemotherapy for gastroesophageal surgery patients, and “provide compelling motivation to explore the potential benefit of adjuvant chemotherapy in a randomized clinical trial,” they said. “A two-arm phase 2 trial design using recurrence-free survival as a primary endpoint is an appealing first step,” they added.
The researchers had no financial conflicts to disclose.
The study findings “seem to indicate that additional systemic chemotherapy could be advantageous for patients treated with neoadjuvant chemoradiotherapy for resectable gastroesophageal cancer,” wrote David Cunningham, MD, FMedSci, and Elizabeth C. Smyth, MB, BCh., MSc., in an accompanying editorial.
“The small percentage of patients treated with adjuvant chemotherapy is reassuring; neoadjuvant chemoradiotherapy and surgery followed by adjuvant chemotherapy is not a treatment approach endorsed by current national or international guidelines,” they noted. The findings suggest that the 4% increase in overall survival at 3 years is promising because most gastroesophageal cancer recurrences arise within 3 years of surgery, they said. “However, these results require validation in the form of a randomized clinical trial,” they emphasized (JAMA Oncol. 2017 Sep 21. doi: 10.1001/jamaoncol.2017.2792).
Dr. Cunningham and Ms. Smyth are affiliated with the department of gastrointestinal oncology and lymphoma at the Royal Marsden Hospital, London. Dr. Cunningham disclosed institutional research funding from Amgen, AstraZeneca, Bayer, Celgene, MedImmune, Merck Serono, Merrimack, and Sanofi. Ms. Smyth disclosed honoraria for advisory roles with Five Prime Therapeutics, Bristol-Myers Squibb, and Gritstone Oncology.
The study findings “seem to indicate that additional systemic chemotherapy could be advantageous for patients treated with neoadjuvant chemoradiotherapy for resectable gastroesophageal cancer,” wrote David Cunningham, MD, FMedSci, and Elizabeth C. Smyth, MB, BCh., MSc., in an accompanying editorial.
“The small percentage of patients treated with adjuvant chemotherapy is reassuring; neoadjuvant chemoradiotherapy and surgery followed by adjuvant chemotherapy is not a treatment approach endorsed by current national or international guidelines,” they noted. The findings suggest that the 4% increase in overall survival at 3 years is promising because most gastroesophageal cancer recurrences arise within 3 years of surgery, they said. “However, these results require validation in the form of a randomized clinical trial,” they emphasized (JAMA Oncol. 2017 Sep 21. doi: 10.1001/jamaoncol.2017.2792).
Dr. Cunningham and Ms. Smyth are affiliated with the department of gastrointestinal oncology and lymphoma at the Royal Marsden Hospital, London. Dr. Cunningham disclosed institutional research funding from Amgen, AstraZeneca, Bayer, Celgene, MedImmune, Merck Serono, Merrimack, and Sanofi. Ms. Smyth disclosed honoraria for advisory roles with Five Prime Therapeutics, Bristol-Myers Squibb, and Gritstone Oncology.
The study findings “seem to indicate that additional systemic chemotherapy could be advantageous for patients treated with neoadjuvant chemoradiotherapy for resectable gastroesophageal cancer,” wrote David Cunningham, MD, FMedSci, and Elizabeth C. Smyth, MB, BCh., MSc., in an accompanying editorial.
“The small percentage of patients treated with adjuvant chemotherapy is reassuring; neoadjuvant chemoradiotherapy and surgery followed by adjuvant chemotherapy is not a treatment approach endorsed by current national or international guidelines,” they noted. The findings suggest that the 4% increase in overall survival at 3 years is promising because most gastroesophageal cancer recurrences arise within 3 years of surgery, they said. “However, these results require validation in the form of a randomized clinical trial,” they emphasized (JAMA Oncol. 2017 Sep 21. doi: 10.1001/jamaoncol.2017.2792).
Dr. Cunningham and Ms. Smyth are affiliated with the department of gastrointestinal oncology and lymphoma at the Royal Marsden Hospital, London. Dr. Cunningham disclosed institutional research funding from Amgen, AstraZeneca, Bayer, Celgene, MedImmune, Merck Serono, Merrimack, and Sanofi. Ms. Smyth disclosed honoraria for advisory roles with Five Prime Therapeutics, Bristol-Myers Squibb, and Gritstone Oncology.
Adjuvant chemotherapy was associated with improved overall survival rates at 3 years in patients who had surgery for gastroesophageal cancer, based on retrospective data from more than 10,000 adults.
Preoperative chemoradiotherapy and resection has shown benefits in patients with gastroesophageal adenocarcinoma, but the potential benefits of adjuvant chemotherapy (AC) after surgery in these patients has not been well studied, wrote Ali A. El Mokdad, MD, of the University of Texas Southwestern Medical Center, Dallas, and his colleagues (JAMA Oncol. 2017 Sep 21. doi: 10.1001/jamaoncol.2017.2805).
The researchers reviewed data from 10,086 patients in the National Cancer Database during 2006-2013. Of these, 814 (8%) received adjuvant chemotherapy after surgery and 9,272 (94%) received no additional intervention beyond postoperative observation. The average age of the patients was 61 years, and 88% were men.
The average survival rates at 3 years after surgery were 40 months for the adjuvant group and 34 months for the observation group (hazard ratio, 0.79). The overall survival rates in the adjuvant group were 94%, 54%, and 38% at 1,3, and 5 years, respectively, compared with rates of 88%, 47%, and 34%, in the observation group.
The findings were limited in part by the retrospective nature of the study, the researchers said. In addition, “the estimated effect of AC on overall survival is subject to selection bias and immortal time bias given that the study was observational,” they noted.
However, the results support the addition of chemotherapy for gastroesophageal surgery patients, and “provide compelling motivation to explore the potential benefit of adjuvant chemotherapy in a randomized clinical trial,” they said. “A two-arm phase 2 trial design using recurrence-free survival as a primary endpoint is an appealing first step,” they added.
The researchers had no financial conflicts to disclose.
Adjuvant chemotherapy was associated with improved overall survival rates at 3 years in patients who had surgery for gastroesophageal cancer, based on retrospective data from more than 10,000 adults.
Preoperative chemoradiotherapy and resection has shown benefits in patients with gastroesophageal adenocarcinoma, but the potential benefits of adjuvant chemotherapy (AC) after surgery in these patients has not been well studied, wrote Ali A. El Mokdad, MD, of the University of Texas Southwestern Medical Center, Dallas, and his colleagues (JAMA Oncol. 2017 Sep 21. doi: 10.1001/jamaoncol.2017.2805).
The researchers reviewed data from 10,086 patients in the National Cancer Database during 2006-2013. Of these, 814 (8%) received adjuvant chemotherapy after surgery and 9,272 (94%) received no additional intervention beyond postoperative observation. The average age of the patients was 61 years, and 88% were men.
The average survival rates at 3 years after surgery were 40 months for the adjuvant group and 34 months for the observation group (hazard ratio, 0.79). The overall survival rates in the adjuvant group were 94%, 54%, and 38% at 1,3, and 5 years, respectively, compared with rates of 88%, 47%, and 34%, in the observation group.
The findings were limited in part by the retrospective nature of the study, the researchers said. In addition, “the estimated effect of AC on overall survival is subject to selection bias and immortal time bias given that the study was observational,” they noted.
However, the results support the addition of chemotherapy for gastroesophageal surgery patients, and “provide compelling motivation to explore the potential benefit of adjuvant chemotherapy in a randomized clinical trial,” they said. “A two-arm phase 2 trial design using recurrence-free survival as a primary endpoint is an appealing first step,” they added.
The researchers had no financial conflicts to disclose.
FROM JAMA ONCOLOGY
Key clinical point: Patients undergoing surgery for gastroesophageal cancer may benefit from additional chemotherapy.
Major finding: Overall survival rates improved in patients who received adjuvant chemotherapy, compared with those who did not (40 months vs. 34 months, respectively).
Data source: A review of 10,086 adults in the National Cancer Database who underwent gastroesophageal cancer surgery during 2006-2013.
Disclosures: The researchers had no financial conflicts to disclose.
‘Very daring study’ of neoadjuvant AI/CDKi combo in early BC is hypothesis generating
MADRID – For women with luminal breast cancer who are not initially candidates for breast-conserving surgery, neoadjuvant therapy with an aromatase inhibitor and a cyclin-dependent kinases 4/6 (CDK4/6) inhibitor offered a slightly higher residual cancer burden prior to surgery, but a significantly better safety profile than conventional chemotherapy with similar near-term safety outcomes, results of a phase 2 parallel group, noncomparative trial suggested.
Among 60 patients evaluable for response in an interim analysis of the UNICANCER NeoPAL trial, one patient (3.3%) treated with a combination of letrozole (Femara) and palbociclib (Ibrance) had a residual cancer burden (RCB) score of 0 (equivalent to a pathologic complete response; pCR), whereas three patients (10%) treated with FEC 100 chemotherapy (5-fluorouracil, epirubicin, and cyclophosphamide) had RCB 0 or I, reported Paul-Henri Cottu, MD, of the Institut Curie in Paris.
Following the interim analysis, the independent data monitoring committee for the NeoPAL trial recommended halting accrual; accrual was stopped in November 2016, after 106 patients had been randomized.
The IDMC also recommended that patients in the letrozole/palbociclib arm who did not have an RCB of 0 or I be offered adjuvant chemotherapy.
“Please note that 70% of those patients refused adjuvant chemotherapy,” Dr. Cottu said.
The investigators set out to test whether letrozole and palbociclib, which have been shown to have synergistic antiproliferative activity against advanced luminal breast cancer, could have similar benefits in the neoadjuvant setting.
They screened for women with luminal breast cancer who had newly diagnosed stage II or III breast cancer with biopsy-proven endocrine receptor–positive, human epidermal growth factor receptor 2–negative tumors, using the Prosigna test, based on the PAM50 gene signature assay. Women with node-positive luminal A or luminal B disease were enrolled and randomized to receive either letrozole 2.5 mg and palbociclib 125 mg daily for 3 out of every 4 weeks over 19 weeks, or three cycles of FEC 100, followed by three cycles of docetaxel 100 mg/m2 every 3 weeks, followed by surgery.
An interim analysis was planned after 30 patients were evaluable for RCB in the experimental arm, and, as prespecified, the trial was stopped for futility when fewer than five patients had an RCB of 0 or I.
The safety analysis, conducted with all 106 patients randomized, showed that letrozole/palbocilib was associated with more frequent grade 3 neutropenia (23% vs. 10% of patients with FEC), but less grade 4 neutropenia (1% vs. 11%, respectively), and no febrile neutropenia vs. 6% in the chemotherapy arm.
There were 2 serious adverse events with the AI/CDK-inhibitor combination vs. 17 with chemotherapy. Dose reductions or interruptions were less frequent with letrozole/palbociclib (10 and 16), and only two patients in the experimental arm required premature cessation of therapy vs. seven in the chemotherapy arm.
The final response analysis in 103 patients showed that the rate of RCB 0 or I was 7.7% with letrozole/palbociclib and 15.7% with chemotherapy. Respective rates of RCB II-III were 92.3% and 84.3%.
Clinical response rates were similar in each study arm, with approximately 30% complete responses and 44% partial responses.
In each arm, slightly less than one-third of patients underwent mastectomy, and a little more than two-thirds were able to have breast-conserving surgery after neoadjuvant therapy.
The patients will be followed out to at least 3 years to see whether those patients in the letrozole/palbociclib arm who turned down subsequent chemotherapy will have worse survival than patients who decided to undergo it, Dr. Cottu said.
Invited discussant Nadia Harbeck, MD, PhD, of the University of Munich called the NeoPAL trial “a very daring study.”
“This is not a practice-changing trial, but it’s a very, very interesting hypothesis-generating trial,” she said.
She said that the choice of RCB was probably not the best endpoint in a trial of endocrine-based therapy vs. chemotherapy.
“I think the challenge remains to identify those patients with luminal early breast cancer for whom an endocrine-based approach – not endocrine, but endocrine-based – will improve outcome, either replacing chemotherapy in the intermediate-risk setting or as an add-on in high-risk disease,” she said.
The study was funded by Pfizer and Nanostring. Dr. Cottu disclosed advisory board participation and travel support from Pfizer and others, and research support from Roche, Novartis, and AstraZeneca. Dr. Harbeck disclosed advising and consulting fees from Pfizer, Nanostring, and other companies.
MADRID – For women with luminal breast cancer who are not initially candidates for breast-conserving surgery, neoadjuvant therapy with an aromatase inhibitor and a cyclin-dependent kinases 4/6 (CDK4/6) inhibitor offered a slightly higher residual cancer burden prior to surgery, but a significantly better safety profile than conventional chemotherapy with similar near-term safety outcomes, results of a phase 2 parallel group, noncomparative trial suggested.
Among 60 patients evaluable for response in an interim analysis of the UNICANCER NeoPAL trial, one patient (3.3%) treated with a combination of letrozole (Femara) and palbociclib (Ibrance) had a residual cancer burden (RCB) score of 0 (equivalent to a pathologic complete response; pCR), whereas three patients (10%) treated with FEC 100 chemotherapy (5-fluorouracil, epirubicin, and cyclophosphamide) had RCB 0 or I, reported Paul-Henri Cottu, MD, of the Institut Curie in Paris.
Following the interim analysis, the independent data monitoring committee for the NeoPAL trial recommended halting accrual; accrual was stopped in November 2016, after 106 patients had been randomized.
The IDMC also recommended that patients in the letrozole/palbociclib arm who did not have an RCB of 0 or I be offered adjuvant chemotherapy.
“Please note that 70% of those patients refused adjuvant chemotherapy,” Dr. Cottu said.
The investigators set out to test whether letrozole and palbociclib, which have been shown to have synergistic antiproliferative activity against advanced luminal breast cancer, could have similar benefits in the neoadjuvant setting.
They screened for women with luminal breast cancer who had newly diagnosed stage II or III breast cancer with biopsy-proven endocrine receptor–positive, human epidermal growth factor receptor 2–negative tumors, using the Prosigna test, based on the PAM50 gene signature assay. Women with node-positive luminal A or luminal B disease were enrolled and randomized to receive either letrozole 2.5 mg and palbociclib 125 mg daily for 3 out of every 4 weeks over 19 weeks, or three cycles of FEC 100, followed by three cycles of docetaxel 100 mg/m2 every 3 weeks, followed by surgery.
An interim analysis was planned after 30 patients were evaluable for RCB in the experimental arm, and, as prespecified, the trial was stopped for futility when fewer than five patients had an RCB of 0 or I.
The safety analysis, conducted with all 106 patients randomized, showed that letrozole/palbocilib was associated with more frequent grade 3 neutropenia (23% vs. 10% of patients with FEC), but less grade 4 neutropenia (1% vs. 11%, respectively), and no febrile neutropenia vs. 6% in the chemotherapy arm.
There were 2 serious adverse events with the AI/CDK-inhibitor combination vs. 17 with chemotherapy. Dose reductions or interruptions were less frequent with letrozole/palbociclib (10 and 16), and only two patients in the experimental arm required premature cessation of therapy vs. seven in the chemotherapy arm.
The final response analysis in 103 patients showed that the rate of RCB 0 or I was 7.7% with letrozole/palbociclib and 15.7% with chemotherapy. Respective rates of RCB II-III were 92.3% and 84.3%.
Clinical response rates were similar in each study arm, with approximately 30% complete responses and 44% partial responses.
In each arm, slightly less than one-third of patients underwent mastectomy, and a little more than two-thirds were able to have breast-conserving surgery after neoadjuvant therapy.
The patients will be followed out to at least 3 years to see whether those patients in the letrozole/palbociclib arm who turned down subsequent chemotherapy will have worse survival than patients who decided to undergo it, Dr. Cottu said.
Invited discussant Nadia Harbeck, MD, PhD, of the University of Munich called the NeoPAL trial “a very daring study.”
“This is not a practice-changing trial, but it’s a very, very interesting hypothesis-generating trial,” she said.
She said that the choice of RCB was probably not the best endpoint in a trial of endocrine-based therapy vs. chemotherapy.
“I think the challenge remains to identify those patients with luminal early breast cancer for whom an endocrine-based approach – not endocrine, but endocrine-based – will improve outcome, either replacing chemotherapy in the intermediate-risk setting or as an add-on in high-risk disease,” she said.
The study was funded by Pfizer and Nanostring. Dr. Cottu disclosed advisory board participation and travel support from Pfizer and others, and research support from Roche, Novartis, and AstraZeneca. Dr. Harbeck disclosed advising and consulting fees from Pfizer, Nanostring, and other companies.
MADRID – For women with luminal breast cancer who are not initially candidates for breast-conserving surgery, neoadjuvant therapy with an aromatase inhibitor and a cyclin-dependent kinases 4/6 (CDK4/6) inhibitor offered a slightly higher residual cancer burden prior to surgery, but a significantly better safety profile than conventional chemotherapy with similar near-term safety outcomes, results of a phase 2 parallel group, noncomparative trial suggested.
Among 60 patients evaluable for response in an interim analysis of the UNICANCER NeoPAL trial, one patient (3.3%) treated with a combination of letrozole (Femara) and palbociclib (Ibrance) had a residual cancer burden (RCB) score of 0 (equivalent to a pathologic complete response; pCR), whereas three patients (10%) treated with FEC 100 chemotherapy (5-fluorouracil, epirubicin, and cyclophosphamide) had RCB 0 or I, reported Paul-Henri Cottu, MD, of the Institut Curie in Paris.
Following the interim analysis, the independent data monitoring committee for the NeoPAL trial recommended halting accrual; accrual was stopped in November 2016, after 106 patients had been randomized.
The IDMC also recommended that patients in the letrozole/palbociclib arm who did not have an RCB of 0 or I be offered adjuvant chemotherapy.
“Please note that 70% of those patients refused adjuvant chemotherapy,” Dr. Cottu said.
The investigators set out to test whether letrozole and palbociclib, which have been shown to have synergistic antiproliferative activity against advanced luminal breast cancer, could have similar benefits in the neoadjuvant setting.
They screened for women with luminal breast cancer who had newly diagnosed stage II or III breast cancer with biopsy-proven endocrine receptor–positive, human epidermal growth factor receptor 2–negative tumors, using the Prosigna test, based on the PAM50 gene signature assay. Women with node-positive luminal A or luminal B disease were enrolled and randomized to receive either letrozole 2.5 mg and palbociclib 125 mg daily for 3 out of every 4 weeks over 19 weeks, or three cycles of FEC 100, followed by three cycles of docetaxel 100 mg/m2 every 3 weeks, followed by surgery.
An interim analysis was planned after 30 patients were evaluable for RCB in the experimental arm, and, as prespecified, the trial was stopped for futility when fewer than five patients had an RCB of 0 or I.
The safety analysis, conducted with all 106 patients randomized, showed that letrozole/palbocilib was associated with more frequent grade 3 neutropenia (23% vs. 10% of patients with FEC), but less grade 4 neutropenia (1% vs. 11%, respectively), and no febrile neutropenia vs. 6% in the chemotherapy arm.
There were 2 serious adverse events with the AI/CDK-inhibitor combination vs. 17 with chemotherapy. Dose reductions or interruptions were less frequent with letrozole/palbociclib (10 and 16), and only two patients in the experimental arm required premature cessation of therapy vs. seven in the chemotherapy arm.
The final response analysis in 103 patients showed that the rate of RCB 0 or I was 7.7% with letrozole/palbociclib and 15.7% with chemotherapy. Respective rates of RCB II-III were 92.3% and 84.3%.
Clinical response rates were similar in each study arm, with approximately 30% complete responses and 44% partial responses.
In each arm, slightly less than one-third of patients underwent mastectomy, and a little more than two-thirds were able to have breast-conserving surgery after neoadjuvant therapy.
The patients will be followed out to at least 3 years to see whether those patients in the letrozole/palbociclib arm who turned down subsequent chemotherapy will have worse survival than patients who decided to undergo it, Dr. Cottu said.
Invited discussant Nadia Harbeck, MD, PhD, of the University of Munich called the NeoPAL trial “a very daring study.”
“This is not a practice-changing trial, but it’s a very, very interesting hypothesis-generating trial,” she said.
She said that the choice of RCB was probably not the best endpoint in a trial of endocrine-based therapy vs. chemotherapy.
“I think the challenge remains to identify those patients with luminal early breast cancer for whom an endocrine-based approach – not endocrine, but endocrine-based – will improve outcome, either replacing chemotherapy in the intermediate-risk setting or as an add-on in high-risk disease,” she said.
The study was funded by Pfizer and Nanostring. Dr. Cottu disclosed advisory board participation and travel support from Pfizer and others, and research support from Roche, Novartis, and AstraZeneca. Dr. Harbeck disclosed advising and consulting fees from Pfizer, Nanostring, and other companies.
AT ESMO 2017
Key clinical point:
Major finding: One patient (3.3%) assigned to letrozole/palbociclib had a residual cancer burden score of 0 or I, compared with three patients (10%) assigned to chemotherapy.
Data source: Interim analysis of a phase 2 parallel group trial with 60 patients evaluable for response and 106 evaluable for safety.
Disclosures: The study was funded by Pfizer and Nanostring. Dr. Cottu disclosed advisory board participation and travel support from Pfizer and others and research support from Roche, Novartis, and AstraZeneca. Dr. Harbeck disclosed advising and consulting fees from Pfizer, Nanostring, and other companies.
Calcitonin-to-CEA ratio predicts medullary thyroid cancer survival
BOSTON – The ratio of serum calcitonin to the serum level of carcinoembryonic antigen in patients with medullary thyroid cancer can predict which patients have a better chance for survival following thyroidectomy, based on retrospective findings from 164 presurgical patients at one U.S. center.
A lower serum calcitonin–to–serum carcinoembryonic antigen (CEA) ratio following thyroidectomy is a second marker of good postsurgical survival, Tania Jaber, MD, said at the World Congress on Thyroid Cancer.
“Patients want to know whether surgery will cure them, and we have had no prognostic markers to predict this. Depending on the ratio, we can now tell patients whether or not they have a good chance of cure,” said Dr. Jaber, an endocrinological oncologist at MD Anderson in Houston. “Surgery remains the standard of care, so the ratio does not affect the decision of whether to undergo surgery, but it helps patients know what to expect” after surgery, she said in an interview.
“If their ratio is favorable it can be reassuring, and if their ratio is unfavorable it helps set expectations. We are also studying whether the ratio can be a marker for the need for systemic therapy following surgery. Right now, our prognostic tools for medullary thyroid cancer are very limited, so any additional information we can give patients based on their calcitonin-to-CEA ratio is very valuable.”
Her study included 164 patients treated at MD Anderson who had their serum drawn before thyroidectomy, and 187 patients with specimens taken 3-9 months after surgery. Median patient follow-up after surgery was 5 years. Calcitonin levels were measured as pg/mL and CEA levels as ng/mL; despite this difference in unit size the researchers calculated the ratios by a direct numerical comparison that ignored the units.
Among the preoperative patients and specifically among those with a low serum CEA level of less than 25 ng/ML a calcitonin-to-CEA ratio of less than 43 had the best survival rate, Dr. Jaber reported. Among preoperative patients with a CEA level of 25 ng/mL or greater a ratio of less than 18 flagged patients with the best survival rate following thyroidectomy.
Among postoperative patients the ratios that linked with better survival also depended on the CEA level. In patients with a low postoperative CEA a ratio of less than 149 linked with better survival. In patients with a high CEA level a ratio of less than 12 linked with better postoperative survival.
[email protected]
On Twitter @mitchelzoler
BOSTON – The ratio of serum calcitonin to the serum level of carcinoembryonic antigen in patients with medullary thyroid cancer can predict which patients have a better chance for survival following thyroidectomy, based on retrospective findings from 164 presurgical patients at one U.S. center.
A lower serum calcitonin–to–serum carcinoembryonic antigen (CEA) ratio following thyroidectomy is a second marker of good postsurgical survival, Tania Jaber, MD, said at the World Congress on Thyroid Cancer.
“Patients want to know whether surgery will cure them, and we have had no prognostic markers to predict this. Depending on the ratio, we can now tell patients whether or not they have a good chance of cure,” said Dr. Jaber, an endocrinological oncologist at MD Anderson in Houston. “Surgery remains the standard of care, so the ratio does not affect the decision of whether to undergo surgery, but it helps patients know what to expect” after surgery, she said in an interview.
“If their ratio is favorable it can be reassuring, and if their ratio is unfavorable it helps set expectations. We are also studying whether the ratio can be a marker for the need for systemic therapy following surgery. Right now, our prognostic tools for medullary thyroid cancer are very limited, so any additional information we can give patients based on their calcitonin-to-CEA ratio is very valuable.”
Her study included 164 patients treated at MD Anderson who had their serum drawn before thyroidectomy, and 187 patients with specimens taken 3-9 months after surgery. Median patient follow-up after surgery was 5 years. Calcitonin levels were measured as pg/mL and CEA levels as ng/mL; despite this difference in unit size the researchers calculated the ratios by a direct numerical comparison that ignored the units.
Among the preoperative patients and specifically among those with a low serum CEA level of less than 25 ng/ML a calcitonin-to-CEA ratio of less than 43 had the best survival rate, Dr. Jaber reported. Among preoperative patients with a CEA level of 25 ng/mL or greater a ratio of less than 18 flagged patients with the best survival rate following thyroidectomy.
Among postoperative patients the ratios that linked with better survival also depended on the CEA level. In patients with a low postoperative CEA a ratio of less than 149 linked with better survival. In patients with a high CEA level a ratio of less than 12 linked with better postoperative survival.
[email protected]
On Twitter @mitchelzoler
BOSTON – The ratio of serum calcitonin to the serum level of carcinoembryonic antigen in patients with medullary thyroid cancer can predict which patients have a better chance for survival following thyroidectomy, based on retrospective findings from 164 presurgical patients at one U.S. center.
A lower serum calcitonin–to–serum carcinoembryonic antigen (CEA) ratio following thyroidectomy is a second marker of good postsurgical survival, Tania Jaber, MD, said at the World Congress on Thyroid Cancer.
“Patients want to know whether surgery will cure them, and we have had no prognostic markers to predict this. Depending on the ratio, we can now tell patients whether or not they have a good chance of cure,” said Dr. Jaber, an endocrinological oncologist at MD Anderson in Houston. “Surgery remains the standard of care, so the ratio does not affect the decision of whether to undergo surgery, but it helps patients know what to expect” after surgery, she said in an interview.
“If their ratio is favorable it can be reassuring, and if their ratio is unfavorable it helps set expectations. We are also studying whether the ratio can be a marker for the need for systemic therapy following surgery. Right now, our prognostic tools for medullary thyroid cancer are very limited, so any additional information we can give patients based on their calcitonin-to-CEA ratio is very valuable.”
Her study included 164 patients treated at MD Anderson who had their serum drawn before thyroidectomy, and 187 patients with specimens taken 3-9 months after surgery. Median patient follow-up after surgery was 5 years. Calcitonin levels were measured as pg/mL and CEA levels as ng/mL; despite this difference in unit size the researchers calculated the ratios by a direct numerical comparison that ignored the units.
Among the preoperative patients and specifically among those with a low serum CEA level of less than 25 ng/ML a calcitonin-to-CEA ratio of less than 43 had the best survival rate, Dr. Jaber reported. Among preoperative patients with a CEA level of 25 ng/mL or greater a ratio of less than 18 flagged patients with the best survival rate following thyroidectomy.
Among postoperative patients the ratios that linked with better survival also depended on the CEA level. In patients with a low postoperative CEA a ratio of less than 149 linked with better survival. In patients with a high CEA level a ratio of less than 12 linked with better postoperative survival.
[email protected]
On Twitter @mitchelzoler
AT WCTC 2017
Key clinical point:
Major finding: Presurgery, a calcitonin-to-CEA ratio below 18 was linked with superior survival in patients whose CEA was at least 25 ng/Ml.
Data source: A single-center, retrospective study with 164 patients assessed before thyroidectomy and 187 assessed after surgery.
Disclosures: Dr. Jaber had no disclosures.
Mitotic rate not tied to SLN biopsy results in thin melanomas
SAN FRANCISCO –
The finding supports the 2017 revision in the American Joint Committee on Cancer guideline, which dropped mitotic rate from its criteria for upstaging thin melanomas.
An earlier version of the guideline, published in 2010, had called for upgrading thin (less than 1 mm), nonulcerated melanomas with a mitotic rate (MR) of at least 1/mm2 to T1B, which could then trigger an SLN biopsy.
SLN biopsy is controversial in thin melanomas, because there is no evidence that it has a survival benefit in these populations, though it is useful as a prognostic measure. However, the procedure carries a risk of complications.
“This makes judicious selection of patients for the procedures even more important,” Heidi Wat, MD, of the division of dermatology at the University of Alberta, Edmonton, said during her presentation of the research at the annual meeting of the Pacific Dermatologic Association.
The researchers set out to determine the predictive value of mitotic rate (the number of cells undergoing cell division) on SLN status, particularly when stratified by tumor thickness. They analyzed 990 SLN biopsy procedures performed in Alberta from January 2007 through December 2013, which were pulled from the Cancer Surgery Alberta tumor database and provincial pathology records. The mean age of the patients was 57 years (range, 15-93 years), and 55% were male; 171 records involved thin melanomas.
Overall, 25.4% of SLN biopsies came back positive, including 8.8% of thin melanomas. Among all cases, there was a statistically significant association between a mitotic rate of 1 or higher and a positive SLN biopsy.
However, when the researchers stratified the results by thickness, they found a statistically significant association only between mitotic rate and SLN biopsy positivity in thicker tumors (1-2 mm, P = .01).
Further analysis of factors including age, ulceration, and tumor location showed that MR and thickness measures were not independent, and the potential for MR to predict SLN biopsy positivity declined at lower thickness values.
“Performing sentinel lymph node biopsy in thin melanomas upstaged purely because of the finding of a single mitotic (event) has questionable clinical value,” said Dr. Wat.
The 2010 AJCC guidelines called for upgrading thin tumors with an MR of 1 or higher, or ulceration, to T1b. The new AJCC guidelines restrict the definition of T1b to tumors 0.8-1.0 mm in size with or without ulceration, or tumors 0.8 mm or smaller with ulceration.
“The results really confirm the latest recommendations,” said Nina Botto, MD, of the department of dermatology at the University of California, San Francisco, who chaired the session in which the research was presented.
SLN status remains a useful prognostic indicator, Dr. Wat said, and MR may still be useful for intermediate and thick melanomas.
Dr. Wat and Dr. Botto reported no relevant financial disclosures.
SAN FRANCISCO –
The finding supports the 2017 revision in the American Joint Committee on Cancer guideline, which dropped mitotic rate from its criteria for upstaging thin melanomas.
An earlier version of the guideline, published in 2010, had called for upgrading thin (less than 1 mm), nonulcerated melanomas with a mitotic rate (MR) of at least 1/mm2 to T1B, which could then trigger an SLN biopsy.
SLN biopsy is controversial in thin melanomas, because there is no evidence that it has a survival benefit in these populations, though it is useful as a prognostic measure. However, the procedure carries a risk of complications.
“This makes judicious selection of patients for the procedures even more important,” Heidi Wat, MD, of the division of dermatology at the University of Alberta, Edmonton, said during her presentation of the research at the annual meeting of the Pacific Dermatologic Association.
The researchers set out to determine the predictive value of mitotic rate (the number of cells undergoing cell division) on SLN status, particularly when stratified by tumor thickness. They analyzed 990 SLN biopsy procedures performed in Alberta from January 2007 through December 2013, which were pulled from the Cancer Surgery Alberta tumor database and provincial pathology records. The mean age of the patients was 57 years (range, 15-93 years), and 55% were male; 171 records involved thin melanomas.
Overall, 25.4% of SLN biopsies came back positive, including 8.8% of thin melanomas. Among all cases, there was a statistically significant association between a mitotic rate of 1 or higher and a positive SLN biopsy.
However, when the researchers stratified the results by thickness, they found a statistically significant association only between mitotic rate and SLN biopsy positivity in thicker tumors (1-2 mm, P = .01).
Further analysis of factors including age, ulceration, and tumor location showed that MR and thickness measures were not independent, and the potential for MR to predict SLN biopsy positivity declined at lower thickness values.
“Performing sentinel lymph node biopsy in thin melanomas upstaged purely because of the finding of a single mitotic (event) has questionable clinical value,” said Dr. Wat.
The 2010 AJCC guidelines called for upgrading thin tumors with an MR of 1 or higher, or ulceration, to T1b. The new AJCC guidelines restrict the definition of T1b to tumors 0.8-1.0 mm in size with or without ulceration, or tumors 0.8 mm or smaller with ulceration.
“The results really confirm the latest recommendations,” said Nina Botto, MD, of the department of dermatology at the University of California, San Francisco, who chaired the session in which the research was presented.
SLN status remains a useful prognostic indicator, Dr. Wat said, and MR may still be useful for intermediate and thick melanomas.
Dr. Wat and Dr. Botto reported no relevant financial disclosures.
SAN FRANCISCO –
The finding supports the 2017 revision in the American Joint Committee on Cancer guideline, which dropped mitotic rate from its criteria for upstaging thin melanomas.
An earlier version of the guideline, published in 2010, had called for upgrading thin (less than 1 mm), nonulcerated melanomas with a mitotic rate (MR) of at least 1/mm2 to T1B, which could then trigger an SLN biopsy.
SLN biopsy is controversial in thin melanomas, because there is no evidence that it has a survival benefit in these populations, though it is useful as a prognostic measure. However, the procedure carries a risk of complications.
“This makes judicious selection of patients for the procedures even more important,” Heidi Wat, MD, of the division of dermatology at the University of Alberta, Edmonton, said during her presentation of the research at the annual meeting of the Pacific Dermatologic Association.
The researchers set out to determine the predictive value of mitotic rate (the number of cells undergoing cell division) on SLN status, particularly when stratified by tumor thickness. They analyzed 990 SLN biopsy procedures performed in Alberta from January 2007 through December 2013, which were pulled from the Cancer Surgery Alberta tumor database and provincial pathology records. The mean age of the patients was 57 years (range, 15-93 years), and 55% were male; 171 records involved thin melanomas.
Overall, 25.4% of SLN biopsies came back positive, including 8.8% of thin melanomas. Among all cases, there was a statistically significant association between a mitotic rate of 1 or higher and a positive SLN biopsy.
However, when the researchers stratified the results by thickness, they found a statistically significant association only between mitotic rate and SLN biopsy positivity in thicker tumors (1-2 mm, P = .01).
Further analysis of factors including age, ulceration, and tumor location showed that MR and thickness measures were not independent, and the potential for MR to predict SLN biopsy positivity declined at lower thickness values.
“Performing sentinel lymph node biopsy in thin melanomas upstaged purely because of the finding of a single mitotic (event) has questionable clinical value,” said Dr. Wat.
The 2010 AJCC guidelines called for upgrading thin tumors with an MR of 1 or higher, or ulceration, to T1b. The new AJCC guidelines restrict the definition of T1b to tumors 0.8-1.0 mm in size with or without ulceration, or tumors 0.8 mm or smaller with ulceration.
“The results really confirm the latest recommendations,” said Nina Botto, MD, of the department of dermatology at the University of California, San Francisco, who chaired the session in which the research was presented.
SLN status remains a useful prognostic indicator, Dr. Wat said, and MR may still be useful for intermediate and thick melanomas.
Dr. Wat and Dr. Botto reported no relevant financial disclosures.
AT PDA 2017
Key clinical point: The results support the latest guidelines, which exclude mitotic rate in the criteria for upstaging thin melanomas.
Major finding: There was no association between mitotic rate and positive sentinel lymph node biopsy results.
Data source: A retrospective analysis of 990 patient records in Alberta, Canada.
Disclosures: Dr. Wat and Dr. Botto reported no relevant financial disclosures.
Surgeons strongly influenced chances of contralateral prophylactic mastectomy
Surgeons, not clinical factors, accounted for 20% of variation in rates of contralateral prophylactic mastectomy (CPM), according to the results of a large survey study.
Only 4% of patients elected CPM when their surgeons were among those who least favored it overall and most preferred breast-conserving treatment, according to Steven J. Katz, MD, MPH, of the University of Michigan, Ann Arbor, and his associates. But 34% of patients chose CPM when their surgeons least favored BCT and were most willing to perform CPM, the researchers found. “Attending surgeons exert strong influence on the likelihood of receipt of CPM after diagnosis of breast cancer,” highlighting “the need to help surgeons address this growing clinical conundrum in the examination room,” they wrote (JAMA Surg. 2017 Sep 13. doi: 10.1001/jamasurg.2017.3415).
Rates of CPM have risen markedly in the United States although it has not been shown to confer a survival advantage for average-risk women. To examine how surgeons themselves affected rates of CPM, the investigators sent surveys to 7,810 women treated for stage 0 to II breast cancer from 2013 to 2015 and included in the Surveillance, Epidemiology, and End Results (SEER) registries of Georgia and Los Angeles County. (Among the 7,810 women, 507 were ineligible.) The researchers also surveyed 488 attending surgeons of these patients.
Response rates were high – 70% among patients (5,080 of 7,303) and 77% (377 of 488) among surgeons, the investigators reported. The average age of the patients was 62 years; 28% had an elevated risk of second primary cancer, and 16% underwent CPM. Patients whose surgeons’ rates of CPM exceeded the mean by at least one standard deviation had nearly threefold greater odds of undergoing CPM themselves (odds ratio, 2.8; 95% confidence interval, 2.1-3.4) regardless of age, date of diagnosis, BRCA mutation status, or risk of second primary cancer.
“One quarter of the surgeon influence was explained by attending attitudes about initial recommendations for surgery and responses to patient requests for CPM,” the researchers wrote. Additional predictors of CPM included elevated risk of second primary breast cancer, BRCA mutation, and younger age.
“We observed a range of reasons why a surgeon would be willing to perform CPM if asked: give peace of mind, yield better cosmetic outcomes, avoid conflict with patient, reduce need for surveillance, improve long-term quality of life, reduce recurrence of invasive disease, avoid losing patient to another surgeon, or improve survival (in order of endorsement),” the researchers wrote. “Our findings reinforce the need to address better ways to communicate with patients with regard to their beliefs about the benefits of more extensive surgery and their reactions to the management plan including surgeon training and deployment of decision aids.”
The National Cancer Institute provided funding. The researchers reported having no conflicts of interest.
Patients who are provided education tools regarding the decision between [breast conserving therapy] and mastectomy are more likely to opt for BCT. However, this discussion is arduous and time consuming. We offer decision-making autonomy to patients, but, in creating that autonomy, we have resigned to overtreatment, motivated by the desire to avoid creating conflict in our relationship with the patient.
How do we overcome this hurdle? Consensus statements reinforce that contralateral prophylactic mastectomy should be discouraged in average-risk patients, but it is time to move beyond consensus statements and create communication tools that guide the surgeon and patient through a stepwise informed discussion. We are participating in a multi-institutional randomized trial to develop such an aid, and we believe this will effect real change in the way surgeons counsel patients. The goal is to standardize the methods and information patients receive to ensure that their decisions are based on facts, not fear.
Julie A. Margenthaler, MD, and Amy E. Cyr, MD, are in the department of surgery, Washington University, St. Louis. They reported no conflicts of interest. These comments are from their editorial (JAMA Surg. 2017 Sep 13. doi: 10.1001/jamasurg.2017.3435).
Patients who are provided education tools regarding the decision between [breast conserving therapy] and mastectomy are more likely to opt for BCT. However, this discussion is arduous and time consuming. We offer decision-making autonomy to patients, but, in creating that autonomy, we have resigned to overtreatment, motivated by the desire to avoid creating conflict in our relationship with the patient.
How do we overcome this hurdle? Consensus statements reinforce that contralateral prophylactic mastectomy should be discouraged in average-risk patients, but it is time to move beyond consensus statements and create communication tools that guide the surgeon and patient through a stepwise informed discussion. We are participating in a multi-institutional randomized trial to develop such an aid, and we believe this will effect real change in the way surgeons counsel patients. The goal is to standardize the methods and information patients receive to ensure that their decisions are based on facts, not fear.
Julie A. Margenthaler, MD, and Amy E. Cyr, MD, are in the department of surgery, Washington University, St. Louis. They reported no conflicts of interest. These comments are from their editorial (JAMA Surg. 2017 Sep 13. doi: 10.1001/jamasurg.2017.3435).
Patients who are provided education tools regarding the decision between [breast conserving therapy] and mastectomy are more likely to opt for BCT. However, this discussion is arduous and time consuming. We offer decision-making autonomy to patients, but, in creating that autonomy, we have resigned to overtreatment, motivated by the desire to avoid creating conflict in our relationship with the patient.
How do we overcome this hurdle? Consensus statements reinforce that contralateral prophylactic mastectomy should be discouraged in average-risk patients, but it is time to move beyond consensus statements and create communication tools that guide the surgeon and patient through a stepwise informed discussion. We are participating in a multi-institutional randomized trial to develop such an aid, and we believe this will effect real change in the way surgeons counsel patients. The goal is to standardize the methods and information patients receive to ensure that their decisions are based on facts, not fear.
Julie A. Margenthaler, MD, and Amy E. Cyr, MD, are in the department of surgery, Washington University, St. Louis. They reported no conflicts of interest. These comments are from their editorial (JAMA Surg. 2017 Sep 13. doi: 10.1001/jamasurg.2017.3435).
Surgeons, not clinical factors, accounted for 20% of variation in rates of contralateral prophylactic mastectomy (CPM), according to the results of a large survey study.
Only 4% of patients elected CPM when their surgeons were among those who least favored it overall and most preferred breast-conserving treatment, according to Steven J. Katz, MD, MPH, of the University of Michigan, Ann Arbor, and his associates. But 34% of patients chose CPM when their surgeons least favored BCT and were most willing to perform CPM, the researchers found. “Attending surgeons exert strong influence on the likelihood of receipt of CPM after diagnosis of breast cancer,” highlighting “the need to help surgeons address this growing clinical conundrum in the examination room,” they wrote (JAMA Surg. 2017 Sep 13. doi: 10.1001/jamasurg.2017.3415).
Rates of CPM have risen markedly in the United States although it has not been shown to confer a survival advantage for average-risk women. To examine how surgeons themselves affected rates of CPM, the investigators sent surveys to 7,810 women treated for stage 0 to II breast cancer from 2013 to 2015 and included in the Surveillance, Epidemiology, and End Results (SEER) registries of Georgia and Los Angeles County. (Among the 7,810 women, 507 were ineligible.) The researchers also surveyed 488 attending surgeons of these patients.
Response rates were high – 70% among patients (5,080 of 7,303) and 77% (377 of 488) among surgeons, the investigators reported. The average age of the patients was 62 years; 28% had an elevated risk of second primary cancer, and 16% underwent CPM. Patients whose surgeons’ rates of CPM exceeded the mean by at least one standard deviation had nearly threefold greater odds of undergoing CPM themselves (odds ratio, 2.8; 95% confidence interval, 2.1-3.4) regardless of age, date of diagnosis, BRCA mutation status, or risk of second primary cancer.
“One quarter of the surgeon influence was explained by attending attitudes about initial recommendations for surgery and responses to patient requests for CPM,” the researchers wrote. Additional predictors of CPM included elevated risk of second primary breast cancer, BRCA mutation, and younger age.
“We observed a range of reasons why a surgeon would be willing to perform CPM if asked: give peace of mind, yield better cosmetic outcomes, avoid conflict with patient, reduce need for surveillance, improve long-term quality of life, reduce recurrence of invasive disease, avoid losing patient to another surgeon, or improve survival (in order of endorsement),” the researchers wrote. “Our findings reinforce the need to address better ways to communicate with patients with regard to their beliefs about the benefits of more extensive surgery and their reactions to the management plan including surgeon training and deployment of decision aids.”
The National Cancer Institute provided funding. The researchers reported having no conflicts of interest.
Surgeons, not clinical factors, accounted for 20% of variation in rates of contralateral prophylactic mastectomy (CPM), according to the results of a large survey study.
Only 4% of patients elected CPM when their surgeons were among those who least favored it overall and most preferred breast-conserving treatment, according to Steven J. Katz, MD, MPH, of the University of Michigan, Ann Arbor, and his associates. But 34% of patients chose CPM when their surgeons least favored BCT and were most willing to perform CPM, the researchers found. “Attending surgeons exert strong influence on the likelihood of receipt of CPM after diagnosis of breast cancer,” highlighting “the need to help surgeons address this growing clinical conundrum in the examination room,” they wrote (JAMA Surg. 2017 Sep 13. doi: 10.1001/jamasurg.2017.3415).
Rates of CPM have risen markedly in the United States although it has not been shown to confer a survival advantage for average-risk women. To examine how surgeons themselves affected rates of CPM, the investigators sent surveys to 7,810 women treated for stage 0 to II breast cancer from 2013 to 2015 and included in the Surveillance, Epidemiology, and End Results (SEER) registries of Georgia and Los Angeles County. (Among the 7,810 women, 507 were ineligible.) The researchers also surveyed 488 attending surgeons of these patients.
Response rates were high – 70% among patients (5,080 of 7,303) and 77% (377 of 488) among surgeons, the investigators reported. The average age of the patients was 62 years; 28% had an elevated risk of second primary cancer, and 16% underwent CPM. Patients whose surgeons’ rates of CPM exceeded the mean by at least one standard deviation had nearly threefold greater odds of undergoing CPM themselves (odds ratio, 2.8; 95% confidence interval, 2.1-3.4) regardless of age, date of diagnosis, BRCA mutation status, or risk of second primary cancer.
“One quarter of the surgeon influence was explained by attending attitudes about initial recommendations for surgery and responses to patient requests for CPM,” the researchers wrote. Additional predictors of CPM included elevated risk of second primary breast cancer, BRCA mutation, and younger age.
“We observed a range of reasons why a surgeon would be willing to perform CPM if asked: give peace of mind, yield better cosmetic outcomes, avoid conflict with patient, reduce need for surveillance, improve long-term quality of life, reduce recurrence of invasive disease, avoid losing patient to another surgeon, or improve survival (in order of endorsement),” the researchers wrote. “Our findings reinforce the need to address better ways to communicate with patients with regard to their beliefs about the benefits of more extensive surgery and their reactions to the management plan including surgeon training and deployment of decision aids.”
The National Cancer Institute provided funding. The researchers reported having no conflicts of interest.
FROM JAMA SURGERY
Key clinical point: Attending surgeons explained 20% of variation in rates of contralateral prophylactic mastectomy.
Major finding: Only 4% of patients elected CPM when their surgeons were among those who least favored it and most preferred breast-conserving treatment (BCT). However, 34% of patients chose CPM when their surgeons least favored initial BCT and were most willing to perform CPM.
Data source: Surveys of 5,080 patients with stage 0-II breast cancer and 339 attending surgeons.
Disclosures: The National Cancer Institute provided funding. The researchers reported having no conflicts of interest.
Ten-year outcomes support skipping axillary lymph node dissection with positive sentinel nodes
A follow-up to a study showing the noninferiority of sentinel lymph node dissection to axillary lymph node dissection for breast cancer in overall and disease-free survival at a median of 6.3 years found similar noninferiority in overall survival at 10 years.
Axillary lymph node dissection has a risk of complications including lymphedema, numbness, axillary web syndrome, and decreased upper-extremity range of motion. The American College of Surgeons Oncology Group Z0011 trial sought to determine if the procedure could be avoided without inferior survival outcomes.
Criticism of the study focused on the potential for later recurrence, particularly in patients with hormone receptor–positive breast cancer. All enrolled patients had one or two sentinel nodes with metastases. At randomization, 436 received sentinel lymph node dissection alone, and 420 received the additional axillary lymph node dissection. The patients were assessed every 6 months for the first 3 years, then annually.
After a median of 9.3 years, 110 of the patients had died of any cause – 51 in the sentinel lymph node dissection group and 59 in the axillary lymph node dissection group – a 10-year overall survival rate of 86.3% and 83.6%, respectively. This met the study’s primary endpoint of showing noninferior overall survival without the riskier procedure. In the study’s secondary endpoint, disease-free survival, there was not a significant difference either (80.2% vs. 78.2%).
“Axillary dissections are associated with considerable morbidity, and the results of this trial demonstrated that this morbidity can be avoided without decreasing cancer control. … These findings do not support routine use of axillary lymph node dissection in this patient population based on 10-year outcomes,” wrote Armando E. Guiliano, MD, of Cedars-Sinai Medical, Los Angeles and his coauthors (JAMA. 2017;318[10]:918-26. doi: 10.1001/jama.2017.11470).
A follow-up to a study showing the noninferiority of sentinel lymph node dissection to axillary lymph node dissection for breast cancer in overall and disease-free survival at a median of 6.3 years found similar noninferiority in overall survival at 10 years.
Axillary lymph node dissection has a risk of complications including lymphedema, numbness, axillary web syndrome, and decreased upper-extremity range of motion. The American College of Surgeons Oncology Group Z0011 trial sought to determine if the procedure could be avoided without inferior survival outcomes.
Criticism of the study focused on the potential for later recurrence, particularly in patients with hormone receptor–positive breast cancer. All enrolled patients had one or two sentinel nodes with metastases. At randomization, 436 received sentinel lymph node dissection alone, and 420 received the additional axillary lymph node dissection. The patients were assessed every 6 months for the first 3 years, then annually.
After a median of 9.3 years, 110 of the patients had died of any cause – 51 in the sentinel lymph node dissection group and 59 in the axillary lymph node dissection group – a 10-year overall survival rate of 86.3% and 83.6%, respectively. This met the study’s primary endpoint of showing noninferior overall survival without the riskier procedure. In the study’s secondary endpoint, disease-free survival, there was not a significant difference either (80.2% vs. 78.2%).
“Axillary dissections are associated with considerable morbidity, and the results of this trial demonstrated that this morbidity can be avoided without decreasing cancer control. … These findings do not support routine use of axillary lymph node dissection in this patient population based on 10-year outcomes,” wrote Armando E. Guiliano, MD, of Cedars-Sinai Medical, Los Angeles and his coauthors (JAMA. 2017;318[10]:918-26. doi: 10.1001/jama.2017.11470).
A follow-up to a study showing the noninferiority of sentinel lymph node dissection to axillary lymph node dissection for breast cancer in overall and disease-free survival at a median of 6.3 years found similar noninferiority in overall survival at 10 years.
Axillary lymph node dissection has a risk of complications including lymphedema, numbness, axillary web syndrome, and decreased upper-extremity range of motion. The American College of Surgeons Oncology Group Z0011 trial sought to determine if the procedure could be avoided without inferior survival outcomes.
Criticism of the study focused on the potential for later recurrence, particularly in patients with hormone receptor–positive breast cancer. All enrolled patients had one or two sentinel nodes with metastases. At randomization, 436 received sentinel lymph node dissection alone, and 420 received the additional axillary lymph node dissection. The patients were assessed every 6 months for the first 3 years, then annually.
After a median of 9.3 years, 110 of the patients had died of any cause – 51 in the sentinel lymph node dissection group and 59 in the axillary lymph node dissection group – a 10-year overall survival rate of 86.3% and 83.6%, respectively. This met the study’s primary endpoint of showing noninferior overall survival without the riskier procedure. In the study’s secondary endpoint, disease-free survival, there was not a significant difference either (80.2% vs. 78.2%).
“Axillary dissections are associated with considerable morbidity, and the results of this trial demonstrated that this morbidity can be avoided without decreasing cancer control. … These findings do not support routine use of axillary lymph node dissection in this patient population based on 10-year outcomes,” wrote Armando E. Guiliano, MD, of Cedars-Sinai Medical, Los Angeles and his coauthors (JAMA. 2017;318[10]:918-26. doi: 10.1001/jama.2017.11470).
FROM JAMA
Minimally invasive esophagectomy may mean less major morbidity
COLORADO SPRINGS – Minimally invasive esophagectomy was associated with a significantly lower rate of postoperative major morbidity as well as a mean 1-day briefer length of stay than open esophagectomy in a propensity-matched analysis of the real-world American College of Surgeons-National Quality Improvement Program database, Mark F. Berry, MD, reported at the annual meeting of the Western Thoracic Surgical Association.
However, both of the study’s discussants questioned whether the reported modest absolute reduction in major morbidity was really attributable to the minimally invasive approach or could instead have resulted from one of several potential confounders that couldn’t be fully adjusted for, given inherent limitations of the ACS-NSQIP database.
“There was a statistically significant difference in morbidity,” replied Dr. Berry of Stanford (Calif.) University. “It was a 4% absolute difference, which I think is probably clinically meaningful, but certainly it’s not really, really dramatic.”
“What I think we found is that it’s safe to do a minimally invasive esophagectomy and safe for people to introduce it into their practice. But it’s not necessarily something that’s a game changer, unlike what’s been seen with minimally invasive approaches for some other things,” said Dr. Berry, who added that he didn’t wish to overstate the importance of the observed difference in morbidity.
Studies from high-volume centers show that minimally-invasive esophagectomy (MIE) reduces length of stay, postoperative major morbidity, and features equivalent or even slightly lower mortality than traditional open esophagectomy, the generalizability of these findings beyond such centers is questionable. That’s why Dr. Berry and his coinvestigators turned to the ACS-NSQIP database, which includes all esophagectomies performed for esophageal cancer at roughly 700 U.S. hospitals, not just those done by board-certified thoracic surgeons.
He presented a retrospective cohort study of 3,901 esophagectomy patients during 2005-2013 who met study criteria, 16.4% of whom had MIE. The use of this approach increased steadily from 6.5% of all esophagectomies in 2005 to 22.3% in 2013. A propensity-matched analysis designed to neutralize potentially confounding differences included 638 MIE and 1,914 open esophagectomy patients.
The primary outcome was the 30-day rate of composite major morbidity in the realms of various wound, respiratory, renal, and cardiovascular complications. The rate was 36.1% in the MIE group and 40.5% with open esophagectomy in the propensity-matched analysis, an absolute risk reduction of 4.4% and a relative risk reduction of 17%. Although rates were consistently slightly lower in each of the categories of major morbidity, those individual differences didn’t achieve statistical significance. The difference in major morbidity became significant only when major morbidity was considered as a whole.
Mean length of stay was 9 days with MIE and 10 days with open surgery.
There was no significant difference between the two study groups in 30-day rates of readmission, reoperation, or mortality.
Discussant Donald E. Low said “esophagectomy is being analysed regarding its place in all sorts of presentations, stages, and situations, so the aspect of making sure that we’re delivering the services as efficiently as possible is going to become more important, not less important.”
That being said, he noted that there is no specific CPT code for MIE. That raises the possibility of an uncertain amount of procedural misclassification in the ACS-NSQIP database.
Also, the only significant difference in major morbidity between the two study groups was in the subcategory of intra- or postoperative bleeding requiring transfusion, which occurred in 10.8% of the MIE and 16.7% of the open esophagectomy groups, observed Dr. Low, director of the Esophageal Center of Excellence at Virginia Mason Medical Center, Seattle.
“Some of us believe that blood utilization and transfusion requirement is really a quality measure and not a complication,” the surgeon said. And if that outcome is excluded from consideration, then there is no significant difference in major morbidity.
Discussant Douglas E. Wood, MD, professor and chair of the department of surgery at the University of Washington, Seattle, took the opportunity to share a self-described “pet peeve” about analyses of national surgical databases: these databases typically don’t contain key details necessary to correct for provider and hospital characteristics.
“The small differences that you demonstrate could easily have been completely driven by providers who choose to do minimally invasive esophagectomy and are in higher-volume, more specialized centers,” he said. “I’m not convinced of your conclusion that MIE produces less morbidity based on a 4% difference and no analysis of provider characteristics.”
COLORADO SPRINGS – Minimally invasive esophagectomy was associated with a significantly lower rate of postoperative major morbidity as well as a mean 1-day briefer length of stay than open esophagectomy in a propensity-matched analysis of the real-world American College of Surgeons-National Quality Improvement Program database, Mark F. Berry, MD, reported at the annual meeting of the Western Thoracic Surgical Association.
However, both of the study’s discussants questioned whether the reported modest absolute reduction in major morbidity was really attributable to the minimally invasive approach or could instead have resulted from one of several potential confounders that couldn’t be fully adjusted for, given inherent limitations of the ACS-NSQIP database.
“There was a statistically significant difference in morbidity,” replied Dr. Berry of Stanford (Calif.) University. “It was a 4% absolute difference, which I think is probably clinically meaningful, but certainly it’s not really, really dramatic.”
“What I think we found is that it’s safe to do a minimally invasive esophagectomy and safe for people to introduce it into their practice. But it’s not necessarily something that’s a game changer, unlike what’s been seen with minimally invasive approaches for some other things,” said Dr. Berry, who added that he didn’t wish to overstate the importance of the observed difference in morbidity.
Studies from high-volume centers show that minimally-invasive esophagectomy (MIE) reduces length of stay, postoperative major morbidity, and features equivalent or even slightly lower mortality than traditional open esophagectomy, the generalizability of these findings beyond such centers is questionable. That’s why Dr. Berry and his coinvestigators turned to the ACS-NSQIP database, which includes all esophagectomies performed for esophageal cancer at roughly 700 U.S. hospitals, not just those done by board-certified thoracic surgeons.
He presented a retrospective cohort study of 3,901 esophagectomy patients during 2005-2013 who met study criteria, 16.4% of whom had MIE. The use of this approach increased steadily from 6.5% of all esophagectomies in 2005 to 22.3% in 2013. A propensity-matched analysis designed to neutralize potentially confounding differences included 638 MIE and 1,914 open esophagectomy patients.
The primary outcome was the 30-day rate of composite major morbidity in the realms of various wound, respiratory, renal, and cardiovascular complications. The rate was 36.1% in the MIE group and 40.5% with open esophagectomy in the propensity-matched analysis, an absolute risk reduction of 4.4% and a relative risk reduction of 17%. Although rates were consistently slightly lower in each of the categories of major morbidity, those individual differences didn’t achieve statistical significance. The difference in major morbidity became significant only when major morbidity was considered as a whole.
Mean length of stay was 9 days with MIE and 10 days with open surgery.
There was no significant difference between the two study groups in 30-day rates of readmission, reoperation, or mortality.
Discussant Donald E. Low said “esophagectomy is being analysed regarding its place in all sorts of presentations, stages, and situations, so the aspect of making sure that we’re delivering the services as efficiently as possible is going to become more important, not less important.”
That being said, he noted that there is no specific CPT code for MIE. That raises the possibility of an uncertain amount of procedural misclassification in the ACS-NSQIP database.
Also, the only significant difference in major morbidity between the two study groups was in the subcategory of intra- or postoperative bleeding requiring transfusion, which occurred in 10.8% of the MIE and 16.7% of the open esophagectomy groups, observed Dr. Low, director of the Esophageal Center of Excellence at Virginia Mason Medical Center, Seattle.
“Some of us believe that blood utilization and transfusion requirement is really a quality measure and not a complication,” the surgeon said. And if that outcome is excluded from consideration, then there is no significant difference in major morbidity.
Discussant Douglas E. Wood, MD, professor and chair of the department of surgery at the University of Washington, Seattle, took the opportunity to share a self-described “pet peeve” about analyses of national surgical databases: these databases typically don’t contain key details necessary to correct for provider and hospital characteristics.
“The small differences that you demonstrate could easily have been completely driven by providers who choose to do minimally invasive esophagectomy and are in higher-volume, more specialized centers,” he said. “I’m not convinced of your conclusion that MIE produces less morbidity based on a 4% difference and no analysis of provider characteristics.”
COLORADO SPRINGS – Minimally invasive esophagectomy was associated with a significantly lower rate of postoperative major morbidity as well as a mean 1-day briefer length of stay than open esophagectomy in a propensity-matched analysis of the real-world American College of Surgeons-National Quality Improvement Program database, Mark F. Berry, MD, reported at the annual meeting of the Western Thoracic Surgical Association.
However, both of the study’s discussants questioned whether the reported modest absolute reduction in major morbidity was really attributable to the minimally invasive approach or could instead have resulted from one of several potential confounders that couldn’t be fully adjusted for, given inherent limitations of the ACS-NSQIP database.
“There was a statistically significant difference in morbidity,” replied Dr. Berry of Stanford (Calif.) University. “It was a 4% absolute difference, which I think is probably clinically meaningful, but certainly it’s not really, really dramatic.”
“What I think we found is that it’s safe to do a minimally invasive esophagectomy and safe for people to introduce it into their practice. But it’s not necessarily something that’s a game changer, unlike what’s been seen with minimally invasive approaches for some other things,” said Dr. Berry, who added that he didn’t wish to overstate the importance of the observed difference in morbidity.
Studies from high-volume centers show that minimally-invasive esophagectomy (MIE) reduces length of stay, postoperative major morbidity, and features equivalent or even slightly lower mortality than traditional open esophagectomy, the generalizability of these findings beyond such centers is questionable. That’s why Dr. Berry and his coinvestigators turned to the ACS-NSQIP database, which includes all esophagectomies performed for esophageal cancer at roughly 700 U.S. hospitals, not just those done by board-certified thoracic surgeons.
He presented a retrospective cohort study of 3,901 esophagectomy patients during 2005-2013 who met study criteria, 16.4% of whom had MIE. The use of this approach increased steadily from 6.5% of all esophagectomies in 2005 to 22.3% in 2013. A propensity-matched analysis designed to neutralize potentially confounding differences included 638 MIE and 1,914 open esophagectomy patients.
The primary outcome was the 30-day rate of composite major morbidity in the realms of various wound, respiratory, renal, and cardiovascular complications. The rate was 36.1% in the MIE group and 40.5% with open esophagectomy in the propensity-matched analysis, an absolute risk reduction of 4.4% and a relative risk reduction of 17%. Although rates were consistently slightly lower in each of the categories of major morbidity, those individual differences didn’t achieve statistical significance. The difference in major morbidity became significant only when major morbidity was considered as a whole.
Mean length of stay was 9 days with MIE and 10 days with open surgery.
There was no significant difference between the two study groups in 30-day rates of readmission, reoperation, or mortality.
Discussant Donald E. Low said “esophagectomy is being analysed regarding its place in all sorts of presentations, stages, and situations, so the aspect of making sure that we’re delivering the services as efficiently as possible is going to become more important, not less important.”
That being said, he noted that there is no specific CPT code for MIE. That raises the possibility of an uncertain amount of procedural misclassification in the ACS-NSQIP database.
Also, the only significant difference in major morbidity between the two study groups was in the subcategory of intra- or postoperative bleeding requiring transfusion, which occurred in 10.8% of the MIE and 16.7% of the open esophagectomy groups, observed Dr. Low, director of the Esophageal Center of Excellence at Virginia Mason Medical Center, Seattle.
“Some of us believe that blood utilization and transfusion requirement is really a quality measure and not a complication,” the surgeon said. And if that outcome is excluded from consideration, then there is no significant difference in major morbidity.
Discussant Douglas E. Wood, MD, professor and chair of the department of surgery at the University of Washington, Seattle, took the opportunity to share a self-described “pet peeve” about analyses of national surgical databases: these databases typically don’t contain key details necessary to correct for provider and hospital characteristics.
“The small differences that you demonstrate could easily have been completely driven by providers who choose to do minimally invasive esophagectomy and are in higher-volume, more specialized centers,” he said. “I’m not convinced of your conclusion that MIE produces less morbidity based on a 4% difference and no analysis of provider characteristics.”
AT WTSA 2017
Key clinical point:
Major finding: The 30-day rate of major morbidity was 36.1% in patients who underwent minimally invasive esophagectomy, significantly lower than the 40.5% rate with open esophagectomy in a propensity-matched analysis.
Data source: This retrospective cohort study included 3,901 patients who underwent esophagectomy for esophageal cancer as recorded in the American College of Surgeons-National Quality Improvement Program database for 2005-2013.
Disclosures: The study presenter reported having no financial conflicts of interest.
Big changes coming for thyroid cancer staging
BOSTON – When the American Joint Committee on Cancer’s Eighth Edition Cancer Staging Manual becomes effective for U.S. practice on Jan. 1, 2018, substantially more patients with thyroid cancer will meet the definition for stage I disease, but their survival prognosis will remain as good as it was for the smaller slice of patients defined with stage I thyroid cancer by the seventh edition, Bryan R. Haugen, MD, predicted during a talk at the World Congress on Thyroid Cancer.
Under current stage definitions in the seventh edition, roughly 60% of thyroid cancer patients have stage I disease, but this will kick up to about 80% under the eighth edition, said Dr. Haugen, professor of medicine and head of the division of endocrinology, metabolism, and diabetes at the University of Colorado in Aurora. Despite this influx of more patients, “survival rates in stage I patients haven’t changed,” with a disease-specific survival (DSS) of 98%-100% for stage I patients in the eighth edition compared with 97%-100% in the seventh edition, he noted.
Dr. Haugen credited this apparent paradox to the revised staging system’s superior discrimination among various grades of disease progression. “The eighth edition better separates patients based on their projected survival.” As more patients fit stage I classification with its highest level of projected survival, fewer patients will classify with more advanced disease and its worse projected survival.
For example, in the seventh edition patients with stage IV disease had a projected DSS rate of 50%-75%; in the eighth edition that rate is now less than 50%. The projected DSS rate for patients with stage II disease has down shifted from 97%-100% in the seventh edition to 85%-95% in the eighth. For patients with stage III thyroid cancer the DSS rate of 88%-95% in the seventh edition became 60%-70% in the eighth edition.
‘The new system will take some getting used to,” Dr. Haugen admitted, and it involves even more “big” changes, he warned. These include:
• Changing the cutpoint separating younger from older patients to 55 years of age in the eighth edition, a rise from the 45-year-old cutpoint in the seventh edition.
• Allowing tumors classified as stage I to be as large as 4 cm, up from the 2 cm or less defining stage I in the seventh edition.
• Reserving stage II designation for patients with tumors larger than 4 cm. In the seventh edition tumors had to be 2-4 cm in size.
• Expanding stage II disease to include not only patients with disease confined to their thyroid, but also patients with N1 lymph node spread or gross extrathyroidal extension. In the seventh edition tumor spread like this put patients into stage III.
• Specifying in the eighth edition that stage III disease must feature gross extrathyroidal extension into the larynx, trachea, esophagus, or recurrent laryngial nerve. To qualify for stage IV in the eighth edition, spread must extend into prevertebral fascia or encase major vessels, for stage IVA, or involve distant metastases for stage IVB.
• Paring down three stage IV subgroups, A, B, and C, in the seventh edition to just an A or B subgroup in the eighth edition.
Dr. Haugen coauthored a recent editorial that laid out an assessment of the eighth edition in greater detail (Thyroid. 2017 Jun;27[6]:751-6).
[email protected]
On Twitter @mitchelzoler
BOSTON – When the American Joint Committee on Cancer’s Eighth Edition Cancer Staging Manual becomes effective for U.S. practice on Jan. 1, 2018, substantially more patients with thyroid cancer will meet the definition for stage I disease, but their survival prognosis will remain as good as it was for the smaller slice of patients defined with stage I thyroid cancer by the seventh edition, Bryan R. Haugen, MD, predicted during a talk at the World Congress on Thyroid Cancer.
Under current stage definitions in the seventh edition, roughly 60% of thyroid cancer patients have stage I disease, but this will kick up to about 80% under the eighth edition, said Dr. Haugen, professor of medicine and head of the division of endocrinology, metabolism, and diabetes at the University of Colorado in Aurora. Despite this influx of more patients, “survival rates in stage I patients haven’t changed,” with a disease-specific survival (DSS) of 98%-100% for stage I patients in the eighth edition compared with 97%-100% in the seventh edition, he noted.
Dr. Haugen credited this apparent paradox to the revised staging system’s superior discrimination among various grades of disease progression. “The eighth edition better separates patients based on their projected survival.” As more patients fit stage I classification with its highest level of projected survival, fewer patients will classify with more advanced disease and its worse projected survival.
For example, in the seventh edition patients with stage IV disease had a projected DSS rate of 50%-75%; in the eighth edition that rate is now less than 50%. The projected DSS rate for patients with stage II disease has down shifted from 97%-100% in the seventh edition to 85%-95% in the eighth. For patients with stage III thyroid cancer the DSS rate of 88%-95% in the seventh edition became 60%-70% in the eighth edition.
‘The new system will take some getting used to,” Dr. Haugen admitted, and it involves even more “big” changes, he warned. These include:
• Changing the cutpoint separating younger from older patients to 55 years of age in the eighth edition, a rise from the 45-year-old cutpoint in the seventh edition.
• Allowing tumors classified as stage I to be as large as 4 cm, up from the 2 cm or less defining stage I in the seventh edition.
• Reserving stage II designation for patients with tumors larger than 4 cm. In the seventh edition tumors had to be 2-4 cm in size.
• Expanding stage II disease to include not only patients with disease confined to their thyroid, but also patients with N1 lymph node spread or gross extrathyroidal extension. In the seventh edition tumor spread like this put patients into stage III.
• Specifying in the eighth edition that stage III disease must feature gross extrathyroidal extension into the larynx, trachea, esophagus, or recurrent laryngial nerve. To qualify for stage IV in the eighth edition, spread must extend into prevertebral fascia or encase major vessels, for stage IVA, or involve distant metastases for stage IVB.
• Paring down three stage IV subgroups, A, B, and C, in the seventh edition to just an A or B subgroup in the eighth edition.
Dr. Haugen coauthored a recent editorial that laid out an assessment of the eighth edition in greater detail (Thyroid. 2017 Jun;27[6]:751-6).
[email protected]
On Twitter @mitchelzoler
BOSTON – When the American Joint Committee on Cancer’s Eighth Edition Cancer Staging Manual becomes effective for U.S. practice on Jan. 1, 2018, substantially more patients with thyroid cancer will meet the definition for stage I disease, but their survival prognosis will remain as good as it was for the smaller slice of patients defined with stage I thyroid cancer by the seventh edition, Bryan R. Haugen, MD, predicted during a talk at the World Congress on Thyroid Cancer.
Under current stage definitions in the seventh edition, roughly 60% of thyroid cancer patients have stage I disease, but this will kick up to about 80% under the eighth edition, said Dr. Haugen, professor of medicine and head of the division of endocrinology, metabolism, and diabetes at the University of Colorado in Aurora. Despite this influx of more patients, “survival rates in stage I patients haven’t changed,” with a disease-specific survival (DSS) of 98%-100% for stage I patients in the eighth edition compared with 97%-100% in the seventh edition, he noted.
Dr. Haugen credited this apparent paradox to the revised staging system’s superior discrimination among various grades of disease progression. “The eighth edition better separates patients based on their projected survival.” As more patients fit stage I classification with its highest level of projected survival, fewer patients will classify with more advanced disease and its worse projected survival.
For example, in the seventh edition patients with stage IV disease had a projected DSS rate of 50%-75%; in the eighth edition that rate is now less than 50%. The projected DSS rate for patients with stage II disease has down shifted from 97%-100% in the seventh edition to 85%-95% in the eighth. For patients with stage III thyroid cancer the DSS rate of 88%-95% in the seventh edition became 60%-70% in the eighth edition.
‘The new system will take some getting used to,” Dr. Haugen admitted, and it involves even more “big” changes, he warned. These include:
• Changing the cutpoint separating younger from older patients to 55 years of age in the eighth edition, a rise from the 45-year-old cutpoint in the seventh edition.
• Allowing tumors classified as stage I to be as large as 4 cm, up from the 2 cm or less defining stage I in the seventh edition.
• Reserving stage II designation for patients with tumors larger than 4 cm. In the seventh edition tumors had to be 2-4 cm in size.
• Expanding stage II disease to include not only patients with disease confined to their thyroid, but also patients with N1 lymph node spread or gross extrathyroidal extension. In the seventh edition tumor spread like this put patients into stage III.
• Specifying in the eighth edition that stage III disease must feature gross extrathyroidal extension into the larynx, trachea, esophagus, or recurrent laryngial nerve. To qualify for stage IV in the eighth edition, spread must extend into prevertebral fascia or encase major vessels, for stage IVA, or involve distant metastases for stage IVB.
• Paring down three stage IV subgroups, A, B, and C, in the seventh edition to just an A or B subgroup in the eighth edition.
Dr. Haugen coauthored a recent editorial that laid out an assessment of the eighth edition in greater detail (Thyroid. 2017 Jun;27[6]:751-6).
[email protected]
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM WCTC 2017
Cancer the most common diagnosis in palliative care patients
More than a quarter of the patients in palliative care have a primary diagnosis of cancer, according to the Center to Advance Palliative Care.
A survey of 351 palliative care programs showed that 27% of their patients had been diagnosed with cancer in 2016, more than twice as many patients who had a cardiac (13%) or pulmonary (12%) diagnosis. The next most common primary diagnosis category in 2016 was neurologic at 8%, with a tie at 6% between diagnoses classified as infectious or complex chronic, followed by patients with dementia at 5%, Maggie Rogers and Tamara Dumanovsky, PhD, of the CAPC reported.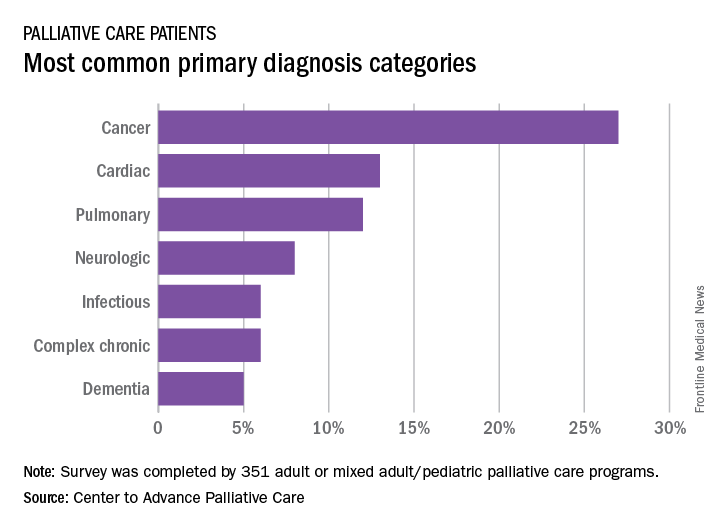
A medical/surgical unit was the referring site for 43% of palliative care referrals in 2016, with 26% of patients coming from an intensive care unit, 13% from a step-down unit, and 8% from an oncology unit, they noted.
More than a quarter of the patients in palliative care have a primary diagnosis of cancer, according to the Center to Advance Palliative Care.
A survey of 351 palliative care programs showed that 27% of their patients had been diagnosed with cancer in 2016, more than twice as many patients who had a cardiac (13%) or pulmonary (12%) diagnosis. The next most common primary diagnosis category in 2016 was neurologic at 8%, with a tie at 6% between diagnoses classified as infectious or complex chronic, followed by patients with dementia at 5%, Maggie Rogers and Tamara Dumanovsky, PhD, of the CAPC reported.
A medical/surgical unit was the referring site for 43% of palliative care referrals in 2016, with 26% of patients coming from an intensive care unit, 13% from a step-down unit, and 8% from an oncology unit, they noted.
More than a quarter of the patients in palliative care have a primary diagnosis of cancer, according to the Center to Advance Palliative Care.
A survey of 351 palliative care programs showed that 27% of their patients had been diagnosed with cancer in 2016, more than twice as many patients who had a cardiac (13%) or pulmonary (12%) diagnosis. The next most common primary diagnosis category in 2016 was neurologic at 8%, with a tie at 6% between diagnoses classified as infectious or complex chronic, followed by patients with dementia at 5%, Maggie Rogers and Tamara Dumanovsky, PhD, of the CAPC reported.
A medical/surgical unit was the referring site for 43% of palliative care referrals in 2016, with 26% of patients coming from an intensive care unit, 13% from a step-down unit, and 8% from an oncology unit, they noted.