User login
ASCO larynx-preservation guidelines reflect important practice changes
The latest edition of the clinical practice guideline on larynx preservation strategies for the treatment of laryngeal cancer from the American Society of Clinical Oncology (ASCO) emphasizes that larynx preservation in patients with early stage disease does not compromise survival compared with total laryngectomy.
“The nuances of treatment selection, assessments of pretreatment voice and swallowing, and public awareness of new organ-preservation treatment and decision making have increased to the point that careful and individualized discussion with patients and families with the multidisciplinary treatment team is a critical element of modern care,” wrote Arlene A. Forastiere, MD, of Johns Hopkins Medicine in Baltimore, and her colleagues. The report was published in the Journal of Clinical Oncology.
Changes since the last guideline on the subject, issued in 2006, include evidence-based support for the use of endoscopic resection in patients with limited stage (T1 and T2) disease, and as an initial total laryngectomy therapy both in patients with stage T4a disease, and in those with severe laryngeal dysfunction prior to treatment.
Also new since the last guideline are recommendations for the use of positron-emission tomography imaging for evaluating the status of regional nodes after treatment, as well as guidance on the best techniques for evaluating voice and swallowing function.
While the initial recommendation that all patients with T1 and T2 laryngeal cancer should be treated with the intent to preserve the larynx has not changed, there is a new recommendation (1.3) stating that surgery may be more effective than radiotherapy for initial larynx preservation therapy, although this recommendation is based on retrospective data and may be affected by patient selection factors, the authors acknowledged. The new recommendation also notes that in an experienced operator’s hands, endoscopic resections can have outcomes that are equal to or better than those with open partial laryngectomy.
The initial recommendation stating that “[e]very effort should be made to avoid combining surgery with radiation therapy because functional outcomes may be compromised by combined-modality therapy; single-modality treatment is effective for limited-stage, invasive cancer of the larynx” remains unchanged.
There is also an updated recommendation that tumor-free margins should be the goal when surgery with larynx preservation intent is performed (1.4).
“Surgery that anticipates the need for postoperative [radiation therapy] to treat close or involved tumor margins or widespread dysplasia is not an acceptable treatment approach,” the guideline authors noted.
There are two other new recommendations including the opinion, based on evidence of benefits vs. harms, that total laryngectomy rather than larynx preservation may be associated with better survival and quality of life in patients with extensive T3 lesions, large T4 lesions, or in those who have poor pretreatment laryngeal function.
The third new recommendation is that “[a]s part of a comprehensive pretreatment evaluation, all patients should undergo a baseline assessment of voice and swallowing function, voice (use and requirements), and counseling with regard to the potential effect of treatment options on voice, swallowing, and quality of life.”
Among the updated recommendations are the following:
• An emphasis on the importance of considering a multiplicity of factors when choosing therapy for patients with limited-stage disease (1.7).
• The option of specialized organ-preservation procedures for a small number of patients with T3 or T4 primary site disease (2.4).
• A strong recommendation for the use of concurrent chemoradiotherapy compared with radiotherapy alone or sequential therapy (2.5).
• Elective neck dissection is not required for patients with clinically involved regional cervical nodes treated with definitive radiotherapy of chemoradiotherapy who have complete clinical, radiologic, and metabolic imaging (3.3).
• “Selection of therapy for an individual patient requires assessment by the multidisciplinary team as well as consideration of voice and swallowing function; patient comorbidity, psychosocial situation, and preferences; and local therapeutic expertise” (4.2).
The guideline development process was supported by ASCO. Dr, Forastiere disclosed employment and stock ownership in NantHealth. Many of her coauthors disclosed institutional funding, consultation/advising, travel support and expenses, honoraria, and or patents/royalties with multiple entities.
The latest edition of the clinical practice guideline on larynx preservation strategies for the treatment of laryngeal cancer from the American Society of Clinical Oncology (ASCO) emphasizes that larynx preservation in patients with early stage disease does not compromise survival compared with total laryngectomy.
“The nuances of treatment selection, assessments of pretreatment voice and swallowing, and public awareness of new organ-preservation treatment and decision making have increased to the point that careful and individualized discussion with patients and families with the multidisciplinary treatment team is a critical element of modern care,” wrote Arlene A. Forastiere, MD, of Johns Hopkins Medicine in Baltimore, and her colleagues. The report was published in the Journal of Clinical Oncology.
Changes since the last guideline on the subject, issued in 2006, include evidence-based support for the use of endoscopic resection in patients with limited stage (T1 and T2) disease, and as an initial total laryngectomy therapy both in patients with stage T4a disease, and in those with severe laryngeal dysfunction prior to treatment.
Also new since the last guideline are recommendations for the use of positron-emission tomography imaging for evaluating the status of regional nodes after treatment, as well as guidance on the best techniques for evaluating voice and swallowing function.
While the initial recommendation that all patients with T1 and T2 laryngeal cancer should be treated with the intent to preserve the larynx has not changed, there is a new recommendation (1.3) stating that surgery may be more effective than radiotherapy for initial larynx preservation therapy, although this recommendation is based on retrospective data and may be affected by patient selection factors, the authors acknowledged. The new recommendation also notes that in an experienced operator’s hands, endoscopic resections can have outcomes that are equal to or better than those with open partial laryngectomy.
The initial recommendation stating that “[e]very effort should be made to avoid combining surgery with radiation therapy because functional outcomes may be compromised by combined-modality therapy; single-modality treatment is effective for limited-stage, invasive cancer of the larynx” remains unchanged.
There is also an updated recommendation that tumor-free margins should be the goal when surgery with larynx preservation intent is performed (1.4).
“Surgery that anticipates the need for postoperative [radiation therapy] to treat close or involved tumor margins or widespread dysplasia is not an acceptable treatment approach,” the guideline authors noted.
There are two other new recommendations including the opinion, based on evidence of benefits vs. harms, that total laryngectomy rather than larynx preservation may be associated with better survival and quality of life in patients with extensive T3 lesions, large T4 lesions, or in those who have poor pretreatment laryngeal function.
The third new recommendation is that “[a]s part of a comprehensive pretreatment evaluation, all patients should undergo a baseline assessment of voice and swallowing function, voice (use and requirements), and counseling with regard to the potential effect of treatment options on voice, swallowing, and quality of life.”
Among the updated recommendations are the following:
• An emphasis on the importance of considering a multiplicity of factors when choosing therapy for patients with limited-stage disease (1.7).
• The option of specialized organ-preservation procedures for a small number of patients with T3 or T4 primary site disease (2.4).
• A strong recommendation for the use of concurrent chemoradiotherapy compared with radiotherapy alone or sequential therapy (2.5).
• Elective neck dissection is not required for patients with clinically involved regional cervical nodes treated with definitive radiotherapy of chemoradiotherapy who have complete clinical, radiologic, and metabolic imaging (3.3).
• “Selection of therapy for an individual patient requires assessment by the multidisciplinary team as well as consideration of voice and swallowing function; patient comorbidity, psychosocial situation, and preferences; and local therapeutic expertise” (4.2).
The guideline development process was supported by ASCO. Dr, Forastiere disclosed employment and stock ownership in NantHealth. Many of her coauthors disclosed institutional funding, consultation/advising, travel support and expenses, honoraria, and or patents/royalties with multiple entities.
The latest edition of the clinical practice guideline on larynx preservation strategies for the treatment of laryngeal cancer from the American Society of Clinical Oncology (ASCO) emphasizes that larynx preservation in patients with early stage disease does not compromise survival compared with total laryngectomy.
“The nuances of treatment selection, assessments of pretreatment voice and swallowing, and public awareness of new organ-preservation treatment and decision making have increased to the point that careful and individualized discussion with patients and families with the multidisciplinary treatment team is a critical element of modern care,” wrote Arlene A. Forastiere, MD, of Johns Hopkins Medicine in Baltimore, and her colleagues. The report was published in the Journal of Clinical Oncology.
Changes since the last guideline on the subject, issued in 2006, include evidence-based support for the use of endoscopic resection in patients with limited stage (T1 and T2) disease, and as an initial total laryngectomy therapy both in patients with stage T4a disease, and in those with severe laryngeal dysfunction prior to treatment.
Also new since the last guideline are recommendations for the use of positron-emission tomography imaging for evaluating the status of regional nodes after treatment, as well as guidance on the best techniques for evaluating voice and swallowing function.
While the initial recommendation that all patients with T1 and T2 laryngeal cancer should be treated with the intent to preserve the larynx has not changed, there is a new recommendation (1.3) stating that surgery may be more effective than radiotherapy for initial larynx preservation therapy, although this recommendation is based on retrospective data and may be affected by patient selection factors, the authors acknowledged. The new recommendation also notes that in an experienced operator’s hands, endoscopic resections can have outcomes that are equal to or better than those with open partial laryngectomy.
The initial recommendation stating that “[e]very effort should be made to avoid combining surgery with radiation therapy because functional outcomes may be compromised by combined-modality therapy; single-modality treatment is effective for limited-stage, invasive cancer of the larynx” remains unchanged.
There is also an updated recommendation that tumor-free margins should be the goal when surgery with larynx preservation intent is performed (1.4).
“Surgery that anticipates the need for postoperative [radiation therapy] to treat close or involved tumor margins or widespread dysplasia is not an acceptable treatment approach,” the guideline authors noted.
There are two other new recommendations including the opinion, based on evidence of benefits vs. harms, that total laryngectomy rather than larynx preservation may be associated with better survival and quality of life in patients with extensive T3 lesions, large T4 lesions, or in those who have poor pretreatment laryngeal function.
The third new recommendation is that “[a]s part of a comprehensive pretreatment evaluation, all patients should undergo a baseline assessment of voice and swallowing function, voice (use and requirements), and counseling with regard to the potential effect of treatment options on voice, swallowing, and quality of life.”
Among the updated recommendations are the following:
• An emphasis on the importance of considering a multiplicity of factors when choosing therapy for patients with limited-stage disease (1.7).
• The option of specialized organ-preservation procedures for a small number of patients with T3 or T4 primary site disease (2.4).
• A strong recommendation for the use of concurrent chemoradiotherapy compared with radiotherapy alone or sequential therapy (2.5).
• Elective neck dissection is not required for patients with clinically involved regional cervical nodes treated with definitive radiotherapy of chemoradiotherapy who have complete clinical, radiologic, and metabolic imaging (3.3).
• “Selection of therapy for an individual patient requires assessment by the multidisciplinary team as well as consideration of voice and swallowing function; patient comorbidity, psychosocial situation, and preferences; and local therapeutic expertise” (4.2).
The guideline development process was supported by ASCO. Dr, Forastiere disclosed employment and stock ownership in NantHealth. Many of her coauthors disclosed institutional funding, consultation/advising, travel support and expenses, honoraria, and or patents/royalties with multiple entities.
FROM JCO
Defining quality in lung cancer surgery
Implementing quality initiatives and creating reporting mechanisms for lung cancer patients can lead to better outcomes, including overall survival. While barriers exist – namely the conflicting perspectives of providers, payers, hospitals, and patients – thoracic oncologic surgeons should seize the opportunity to establish robust quality and value metrics for lung cancer programs, said Whitney S. Brandt, MD, and her coauthors in an expert opinion in the Journal of Thoracic and Cardiovascular Surgery (2017;154:1397-403).
Dr. Brandt, a surgeon at Memorial Sloan Kettering Cancer Center in New York, and her coauthors examined the key elements of quality and value initiatives, categorizing them into preoperative, intraoperative, and postoperative components and primarily focusing on early stage lung cancer. The National Institutes of Health/National Cancer Center provided a grant for the authors’ work.
The preoperative evaluation should at least include CT imaging of the tumor and, for smokers, smoking cessation, said Dr. Brandt and her coauthors. All candidates for pulmonary lung resection should have spirometry and diffusion capacity tests; furthermore, both predicted postoperative forced expiratory volume in 1 second and diffusing capacity of the lungs for CO should be calculated. “Patients with a predicted postoperative value less than 40% for either measurement should be considered high risk for lobectomy and should be offered either sublobar resection or nonsurgical therapy,” they recommended.
Dr. Brandt and her colleagues also clarified preoperative management of patients with cardiac disease. Only patients with significant cardiac disease risk factors need to undergo cardiac testing before lung surgery, and patients with stable cardiac disease do not require revascularization beforehand.
For preoperative staging, the most comprehensive clinical guidelines come from the National Comprehensive Cancer Network, they stated. The guidelines recommend that all patients with a small cell lung cancer or stage II to IV non–small cell lung cancer (NSCLC) receive a brain MRI or – if that’s not available – a head CT with contrast to assess for brain metastasis.
Intraoperative quality measures take into account the surgical approach, including cost, resection and margins, and lymph node evaluation. With regard to surgical approach, trials have shown traditional video-assisted surgery (VATS) lobectomy results in shorter hospital stays and thereby lower costs, as well as fewer complications and deaths, than thoracotomy, said Dr. Brandt and her coauthors. But that cost advantage has not yet carried over to robotic-assisted VATS. That said, “robotic-assisted VATS remains a relatively new technology, and with time and increased robotic platform competition, costs will likely decrease.”
Dr. Brandt and her coauthors also noted that clinical trials support resection margins of 2 cm in patients having surgery for NSCLC and that adequate lymph node evaluation is a critical component of a lung cancer quality initiative. “Regardless of whether lymph nodes are sampled or dissected, we believe that systematic acquisition of mediastinal nodal tissue based on nodal station(s) is a useful quality metric, and, therefore, we recommend each program adopt a preferred approach and track adherence,” they said.
As for postoperative quality metrics, the most obvious are morbidity and mortality. “A quality program should track 30-day or in-hospital mortality, as well as 90-day mortality, following lung cancer resection.” Such metrics can serve as “starting points” for quality improvement initiatives. Length of stay has also emerged as an important metric because it is a surrogate of other metrics, such as patient comorbidities, age, and socioeconomic status. “Length-of-stay metrics likely need to be risk-stratified on the basis of these and other variables to be meaningful to a practicing surgeon,” Dr. Brandt and her coauthors said, adding that: “Studying the effectiveness of enhanced recovery after surgery programs in thoracic surgical oncology poses an opportunity for a well-designed trial.”
Two other key quality metrics for lung cancer programs that need further development were pointed out in the paper: hospital readmissions and tracking of adjuvant therapies. “Programmatic oncologic quality metrics to track appropriate and inappropriate referrals for adjuvant therapy and the number of patients who complete such therapy are important,” they said.
Another step programs should take: Participating in a national or regional database, as recommended by the Society of Thoracic Surgeons, and taking advantage of the “clear benefits to benchmarking your program to others.”
Dr. Brandt and her coauthors reported having no financial disclosures. The National Institutes of Health/National Cancer Center provided grant support.
Whitney S. Brandt, MD, and her coauthors pointed out the difficulty of finding a comprehensive quality metric because of the multitude of contributing indicators, said Alessandro Brunelli, MD, of St. James University Hospital in Leeds, England, in his invited commentary (J Thorac Cardiovasc Surg. 2017;154:1404-5). But he added that two nonclinical indicators needed further consideration: patient perspectives and costs.
“Satisfaction with care depends on multiple subjective factors and is affected by different socioeconomic and cultural backgrounds,” Dr. Brunelli said. “There have been very few attempts to use patient satisfaction scales as a measure of quality in our specialty.” Residual quality of life after surgery is another key measure of patient perspective. “Long-term survival in fact cannot be assessed in isolation and without taking into consideration the actual quality of life of the cancer survivors,” he said. That information would help inform surgical decision-making.
To be meaningful as a quality metric, cost requires clinical risk adjustment, Dr. Brunelli wrote, and surgeons should take the lead here “to prevent misleading evaluations by third parties.” He added, “There have been few studies reporting on financial risk models in our specialty, and more research is needed in this field.”
Dr. Brunelli reported having no financial disclosures.
Whitney S. Brandt, MD, and her coauthors pointed out the difficulty of finding a comprehensive quality metric because of the multitude of contributing indicators, said Alessandro Brunelli, MD, of St. James University Hospital in Leeds, England, in his invited commentary (J Thorac Cardiovasc Surg. 2017;154:1404-5). But he added that two nonclinical indicators needed further consideration: patient perspectives and costs.
“Satisfaction with care depends on multiple subjective factors and is affected by different socioeconomic and cultural backgrounds,” Dr. Brunelli said. “There have been very few attempts to use patient satisfaction scales as a measure of quality in our specialty.” Residual quality of life after surgery is another key measure of patient perspective. “Long-term survival in fact cannot be assessed in isolation and without taking into consideration the actual quality of life of the cancer survivors,” he said. That information would help inform surgical decision-making.
To be meaningful as a quality metric, cost requires clinical risk adjustment, Dr. Brunelli wrote, and surgeons should take the lead here “to prevent misleading evaluations by third parties.” He added, “There have been few studies reporting on financial risk models in our specialty, and more research is needed in this field.”
Dr. Brunelli reported having no financial disclosures.
Whitney S. Brandt, MD, and her coauthors pointed out the difficulty of finding a comprehensive quality metric because of the multitude of contributing indicators, said Alessandro Brunelli, MD, of St. James University Hospital in Leeds, England, in his invited commentary (J Thorac Cardiovasc Surg. 2017;154:1404-5). But he added that two nonclinical indicators needed further consideration: patient perspectives and costs.
“Satisfaction with care depends on multiple subjective factors and is affected by different socioeconomic and cultural backgrounds,” Dr. Brunelli said. “There have been very few attempts to use patient satisfaction scales as a measure of quality in our specialty.” Residual quality of life after surgery is another key measure of patient perspective. “Long-term survival in fact cannot be assessed in isolation and without taking into consideration the actual quality of life of the cancer survivors,” he said. That information would help inform surgical decision-making.
To be meaningful as a quality metric, cost requires clinical risk adjustment, Dr. Brunelli wrote, and surgeons should take the lead here “to prevent misleading evaluations by third parties.” He added, “There have been few studies reporting on financial risk models in our specialty, and more research is needed in this field.”
Dr. Brunelli reported having no financial disclosures.
Implementing quality initiatives and creating reporting mechanisms for lung cancer patients can lead to better outcomes, including overall survival. While barriers exist – namely the conflicting perspectives of providers, payers, hospitals, and patients – thoracic oncologic surgeons should seize the opportunity to establish robust quality and value metrics for lung cancer programs, said Whitney S. Brandt, MD, and her coauthors in an expert opinion in the Journal of Thoracic and Cardiovascular Surgery (2017;154:1397-403).
Dr. Brandt, a surgeon at Memorial Sloan Kettering Cancer Center in New York, and her coauthors examined the key elements of quality and value initiatives, categorizing them into preoperative, intraoperative, and postoperative components and primarily focusing on early stage lung cancer. The National Institutes of Health/National Cancer Center provided a grant for the authors’ work.
The preoperative evaluation should at least include CT imaging of the tumor and, for smokers, smoking cessation, said Dr. Brandt and her coauthors. All candidates for pulmonary lung resection should have spirometry and diffusion capacity tests; furthermore, both predicted postoperative forced expiratory volume in 1 second and diffusing capacity of the lungs for CO should be calculated. “Patients with a predicted postoperative value less than 40% for either measurement should be considered high risk for lobectomy and should be offered either sublobar resection or nonsurgical therapy,” they recommended.
Dr. Brandt and her colleagues also clarified preoperative management of patients with cardiac disease. Only patients with significant cardiac disease risk factors need to undergo cardiac testing before lung surgery, and patients with stable cardiac disease do not require revascularization beforehand.
For preoperative staging, the most comprehensive clinical guidelines come from the National Comprehensive Cancer Network, they stated. The guidelines recommend that all patients with a small cell lung cancer or stage II to IV non–small cell lung cancer (NSCLC) receive a brain MRI or – if that’s not available – a head CT with contrast to assess for brain metastasis.
Intraoperative quality measures take into account the surgical approach, including cost, resection and margins, and lymph node evaluation. With regard to surgical approach, trials have shown traditional video-assisted surgery (VATS) lobectomy results in shorter hospital stays and thereby lower costs, as well as fewer complications and deaths, than thoracotomy, said Dr. Brandt and her coauthors. But that cost advantage has not yet carried over to robotic-assisted VATS. That said, “robotic-assisted VATS remains a relatively new technology, and with time and increased robotic platform competition, costs will likely decrease.”
Dr. Brandt and her coauthors also noted that clinical trials support resection margins of 2 cm in patients having surgery for NSCLC and that adequate lymph node evaluation is a critical component of a lung cancer quality initiative. “Regardless of whether lymph nodes are sampled or dissected, we believe that systematic acquisition of mediastinal nodal tissue based on nodal station(s) is a useful quality metric, and, therefore, we recommend each program adopt a preferred approach and track adherence,” they said.
As for postoperative quality metrics, the most obvious are morbidity and mortality. “A quality program should track 30-day or in-hospital mortality, as well as 90-day mortality, following lung cancer resection.” Such metrics can serve as “starting points” for quality improvement initiatives. Length of stay has also emerged as an important metric because it is a surrogate of other metrics, such as patient comorbidities, age, and socioeconomic status. “Length-of-stay metrics likely need to be risk-stratified on the basis of these and other variables to be meaningful to a practicing surgeon,” Dr. Brandt and her coauthors said, adding that: “Studying the effectiveness of enhanced recovery after surgery programs in thoracic surgical oncology poses an opportunity for a well-designed trial.”
Two other key quality metrics for lung cancer programs that need further development were pointed out in the paper: hospital readmissions and tracking of adjuvant therapies. “Programmatic oncologic quality metrics to track appropriate and inappropriate referrals for adjuvant therapy and the number of patients who complete such therapy are important,” they said.
Another step programs should take: Participating in a national or regional database, as recommended by the Society of Thoracic Surgeons, and taking advantage of the “clear benefits to benchmarking your program to others.”
Dr. Brandt and her coauthors reported having no financial disclosures. The National Institutes of Health/National Cancer Center provided grant support.
Implementing quality initiatives and creating reporting mechanisms for lung cancer patients can lead to better outcomes, including overall survival. While barriers exist – namely the conflicting perspectives of providers, payers, hospitals, and patients – thoracic oncologic surgeons should seize the opportunity to establish robust quality and value metrics for lung cancer programs, said Whitney S. Brandt, MD, and her coauthors in an expert opinion in the Journal of Thoracic and Cardiovascular Surgery (2017;154:1397-403).
Dr. Brandt, a surgeon at Memorial Sloan Kettering Cancer Center in New York, and her coauthors examined the key elements of quality and value initiatives, categorizing them into preoperative, intraoperative, and postoperative components and primarily focusing on early stage lung cancer. The National Institutes of Health/National Cancer Center provided a grant for the authors’ work.
The preoperative evaluation should at least include CT imaging of the tumor and, for smokers, smoking cessation, said Dr. Brandt and her coauthors. All candidates for pulmonary lung resection should have spirometry and diffusion capacity tests; furthermore, both predicted postoperative forced expiratory volume in 1 second and diffusing capacity of the lungs for CO should be calculated. “Patients with a predicted postoperative value less than 40% for either measurement should be considered high risk for lobectomy and should be offered either sublobar resection or nonsurgical therapy,” they recommended.
Dr. Brandt and her colleagues also clarified preoperative management of patients with cardiac disease. Only patients with significant cardiac disease risk factors need to undergo cardiac testing before lung surgery, and patients with stable cardiac disease do not require revascularization beforehand.
For preoperative staging, the most comprehensive clinical guidelines come from the National Comprehensive Cancer Network, they stated. The guidelines recommend that all patients with a small cell lung cancer or stage II to IV non–small cell lung cancer (NSCLC) receive a brain MRI or – if that’s not available – a head CT with contrast to assess for brain metastasis.
Intraoperative quality measures take into account the surgical approach, including cost, resection and margins, and lymph node evaluation. With regard to surgical approach, trials have shown traditional video-assisted surgery (VATS) lobectomy results in shorter hospital stays and thereby lower costs, as well as fewer complications and deaths, than thoracotomy, said Dr. Brandt and her coauthors. But that cost advantage has not yet carried over to robotic-assisted VATS. That said, “robotic-assisted VATS remains a relatively new technology, and with time and increased robotic platform competition, costs will likely decrease.”
Dr. Brandt and her coauthors also noted that clinical trials support resection margins of 2 cm in patients having surgery for NSCLC and that adequate lymph node evaluation is a critical component of a lung cancer quality initiative. “Regardless of whether lymph nodes are sampled or dissected, we believe that systematic acquisition of mediastinal nodal tissue based on nodal station(s) is a useful quality metric, and, therefore, we recommend each program adopt a preferred approach and track adherence,” they said.
As for postoperative quality metrics, the most obvious are morbidity and mortality. “A quality program should track 30-day or in-hospital mortality, as well as 90-day mortality, following lung cancer resection.” Such metrics can serve as “starting points” for quality improvement initiatives. Length of stay has also emerged as an important metric because it is a surrogate of other metrics, such as patient comorbidities, age, and socioeconomic status. “Length-of-stay metrics likely need to be risk-stratified on the basis of these and other variables to be meaningful to a practicing surgeon,” Dr. Brandt and her coauthors said, adding that: “Studying the effectiveness of enhanced recovery after surgery programs in thoracic surgical oncology poses an opportunity for a well-designed trial.”
Two other key quality metrics for lung cancer programs that need further development were pointed out in the paper: hospital readmissions and tracking of adjuvant therapies. “Programmatic oncologic quality metrics to track appropriate and inappropriate referrals for adjuvant therapy and the number of patients who complete such therapy are important,” they said.
Another step programs should take: Participating in a national or regional database, as recommended by the Society of Thoracic Surgeons, and taking advantage of the “clear benefits to benchmarking your program to others.”
Dr. Brandt and her coauthors reported having no financial disclosures. The National Institutes of Health/National Cancer Center provided grant support.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Quality and value initiatives in lung cancer surgery are complex and multifaceted.
Major finding: Expert opinion identifies quality and value strategies for the preoperative, intraoperative, and postoperative stages.
Data source: Review of elements of quality and value for lung cancer surgery, including the Donabedian classification of structure, process and outcomes.
Disclosures: Dr. Brandt and co-authors reported having no financial disclosures. The National Institutes of Health/National Cancer Center provided grant support.
Breast cancer margin guidelines reduced re-excisions, cost
SCOTTSDALE, ARIZ. – In breast conservation surgery with whole-breast radiation, costs and the number of re-excisions performed at a single institution dropped after the implementation of 2014 consensus guidelines on excision margins.
The guidelines, created by a multidisciplinary margins panel convened by the Society of Surgical Oncology and the American Society for Radiation Oncology recommend “no ink on tumor” as an adequate margin in cases of invasive carcinoma.
The guidelines sought to reduce costs and re-excision rates and improve cosmetic outcomes. The results of the study carried out at the University of Louisville suggest that the guidelines may be successful in achieving these goals. The reduced need for re-excision is a key point. “That’s very traumatic for the patient. With this consensus, we were able to decrease that, improve patient satisfaction, and decrease the cost,” lead author Nicolás Ajkay, MD, assistant professor of surgery at the University of Louisville School of Medicine, said in an interview.
Dr. Ajkay presented the results of the study at the annual meeting of the Western Surgical Association.
“Surgeons need to be aware of the guidelines, and if the margin is close, they need to be in multidisciplinary discussions with other breast cancer experts to determine which patients would benefit from going back to the operating room,” he said.
The researchers examined the experiences of 237 patients with stage I or stage II invasive carcinoma who had a partial mastectomy. Of these patients, 126 underwent the procedure before the university incorporated the guidelines in March 2014 (PRE), while 111 were seen after that date (POST). The study excluded those who were diagnosed by excisional biopsy and those who were treated with neoadjuvant chemotherapy.
Per-patient operative costs went down on average after the guidelines were implemented ($4,247 vs. $5,465; difference, $1,218; P less than .001). The estimated savings for the entire POST cohort of 111 patients was approximately $135,000.
Patient satisfaction improved as measured by the breast satisfaction domain of the BREAST-Q survey tool (77/100 vs. 61/100; P = .03).
A multivariate analysis showed that the implementation of the consensus statement predicted lower re-excision rates (odds ratio, 0.17; 95% confidence interval, 0.08-0.38; P less than .001) as well as lower operative cost per patient (cost greater than $5,465 OR, 0.14; 95% CI, 0.07-0.30; P less than .001). Guideline implementation did not, however, predict decreased total resection volume, or probability of conversion to mastectomy.
Perhaps because diagnostic methods have improved over time, there were some significant differences between the two populations. The PRE group had a larger median tumor size (1.5 cm vs. 1.1 cm; P less than .001), and a lower proportion of the PRE group was diagnosed as stage I (62% vs. 77%; P = .005). The PRE group also had significantly larger initial resection volume (69.3 cm3 versus 47.1 cm3; P = .02), higher selective margin volume (50.0 cm3 vs. 11.3 cm3; P less than .001), and a larger final resection volume (81.0 cm3 vs. 51.5 cm3; P = .05). Additional selective margin resection was less frequent in the PRE group (76% vs. 41%; P less than .001).
Those differences may confound the findings, since outcomes might have been expected to improve anyway due to improvements in care.
One member of the audience asked whether the guidelines might boost rates of cancer recurrence. It’s too soon to tell, according to Dr. Ajkay, who said that researchers will need at least 4 or 5 years of clinical experience to make that determination. But he is optimistic. “Even though we’re excising less, I would predict we will not see an increase in recurrence, because adjuvant therapy is getting significantly better, and adjuvant therapy reduces the risk of recurrence just as margin re-excisions do,” he said.
The study received no external funding. Dr. Ajkay reported having no financial disclosures.
SCOTTSDALE, ARIZ. – In breast conservation surgery with whole-breast radiation, costs and the number of re-excisions performed at a single institution dropped after the implementation of 2014 consensus guidelines on excision margins.
The guidelines, created by a multidisciplinary margins panel convened by the Society of Surgical Oncology and the American Society for Radiation Oncology recommend “no ink on tumor” as an adequate margin in cases of invasive carcinoma.
The guidelines sought to reduce costs and re-excision rates and improve cosmetic outcomes. The results of the study carried out at the University of Louisville suggest that the guidelines may be successful in achieving these goals. The reduced need for re-excision is a key point. “That’s very traumatic for the patient. With this consensus, we were able to decrease that, improve patient satisfaction, and decrease the cost,” lead author Nicolás Ajkay, MD, assistant professor of surgery at the University of Louisville School of Medicine, said in an interview.
Dr. Ajkay presented the results of the study at the annual meeting of the Western Surgical Association.
“Surgeons need to be aware of the guidelines, and if the margin is close, they need to be in multidisciplinary discussions with other breast cancer experts to determine which patients would benefit from going back to the operating room,” he said.
The researchers examined the experiences of 237 patients with stage I or stage II invasive carcinoma who had a partial mastectomy. Of these patients, 126 underwent the procedure before the university incorporated the guidelines in March 2014 (PRE), while 111 were seen after that date (POST). The study excluded those who were diagnosed by excisional biopsy and those who were treated with neoadjuvant chemotherapy.
Per-patient operative costs went down on average after the guidelines were implemented ($4,247 vs. $5,465; difference, $1,218; P less than .001). The estimated savings for the entire POST cohort of 111 patients was approximately $135,000.
Patient satisfaction improved as measured by the breast satisfaction domain of the BREAST-Q survey tool (77/100 vs. 61/100; P = .03).
A multivariate analysis showed that the implementation of the consensus statement predicted lower re-excision rates (odds ratio, 0.17; 95% confidence interval, 0.08-0.38; P less than .001) as well as lower operative cost per patient (cost greater than $5,465 OR, 0.14; 95% CI, 0.07-0.30; P less than .001). Guideline implementation did not, however, predict decreased total resection volume, or probability of conversion to mastectomy.
Perhaps because diagnostic methods have improved over time, there were some significant differences between the two populations. The PRE group had a larger median tumor size (1.5 cm vs. 1.1 cm; P less than .001), and a lower proportion of the PRE group was diagnosed as stage I (62% vs. 77%; P = .005). The PRE group also had significantly larger initial resection volume (69.3 cm3 versus 47.1 cm3; P = .02), higher selective margin volume (50.0 cm3 vs. 11.3 cm3; P less than .001), and a larger final resection volume (81.0 cm3 vs. 51.5 cm3; P = .05). Additional selective margin resection was less frequent in the PRE group (76% vs. 41%; P less than .001).
Those differences may confound the findings, since outcomes might have been expected to improve anyway due to improvements in care.
One member of the audience asked whether the guidelines might boost rates of cancer recurrence. It’s too soon to tell, according to Dr. Ajkay, who said that researchers will need at least 4 or 5 years of clinical experience to make that determination. But he is optimistic. “Even though we’re excising less, I would predict we will not see an increase in recurrence, because adjuvant therapy is getting significantly better, and adjuvant therapy reduces the risk of recurrence just as margin re-excisions do,” he said.
The study received no external funding. Dr. Ajkay reported having no financial disclosures.
SCOTTSDALE, ARIZ. – In breast conservation surgery with whole-breast radiation, costs and the number of re-excisions performed at a single institution dropped after the implementation of 2014 consensus guidelines on excision margins.
The guidelines, created by a multidisciplinary margins panel convened by the Society of Surgical Oncology and the American Society for Radiation Oncology recommend “no ink on tumor” as an adequate margin in cases of invasive carcinoma.
The guidelines sought to reduce costs and re-excision rates and improve cosmetic outcomes. The results of the study carried out at the University of Louisville suggest that the guidelines may be successful in achieving these goals. The reduced need for re-excision is a key point. “That’s very traumatic for the patient. With this consensus, we were able to decrease that, improve patient satisfaction, and decrease the cost,” lead author Nicolás Ajkay, MD, assistant professor of surgery at the University of Louisville School of Medicine, said in an interview.
Dr. Ajkay presented the results of the study at the annual meeting of the Western Surgical Association.
“Surgeons need to be aware of the guidelines, and if the margin is close, they need to be in multidisciplinary discussions with other breast cancer experts to determine which patients would benefit from going back to the operating room,” he said.
The researchers examined the experiences of 237 patients with stage I or stage II invasive carcinoma who had a partial mastectomy. Of these patients, 126 underwent the procedure before the university incorporated the guidelines in March 2014 (PRE), while 111 were seen after that date (POST). The study excluded those who were diagnosed by excisional biopsy and those who were treated with neoadjuvant chemotherapy.
Per-patient operative costs went down on average after the guidelines were implemented ($4,247 vs. $5,465; difference, $1,218; P less than .001). The estimated savings for the entire POST cohort of 111 patients was approximately $135,000.
Patient satisfaction improved as measured by the breast satisfaction domain of the BREAST-Q survey tool (77/100 vs. 61/100; P = .03).
A multivariate analysis showed that the implementation of the consensus statement predicted lower re-excision rates (odds ratio, 0.17; 95% confidence interval, 0.08-0.38; P less than .001) as well as lower operative cost per patient (cost greater than $5,465 OR, 0.14; 95% CI, 0.07-0.30; P less than .001). Guideline implementation did not, however, predict decreased total resection volume, or probability of conversion to mastectomy.
Perhaps because diagnostic methods have improved over time, there were some significant differences between the two populations. The PRE group had a larger median tumor size (1.5 cm vs. 1.1 cm; P less than .001), and a lower proportion of the PRE group was diagnosed as stage I (62% vs. 77%; P = .005). The PRE group also had significantly larger initial resection volume (69.3 cm3 versus 47.1 cm3; P = .02), higher selective margin volume (50.0 cm3 vs. 11.3 cm3; P less than .001), and a larger final resection volume (81.0 cm3 vs. 51.5 cm3; P = .05). Additional selective margin resection was less frequent in the PRE group (76% vs. 41%; P less than .001).
Those differences may confound the findings, since outcomes might have been expected to improve anyway due to improvements in care.
One member of the audience asked whether the guidelines might boost rates of cancer recurrence. It’s too soon to tell, according to Dr. Ajkay, who said that researchers will need at least 4 or 5 years of clinical experience to make that determination. But he is optimistic. “Even though we’re excising less, I would predict we will not see an increase in recurrence, because adjuvant therapy is getting significantly better, and adjuvant therapy reduces the risk of recurrence just as margin re-excisions do,” he said.
The study received no external funding. Dr. Ajkay reported having no financial disclosures.
AT WSA 2017
Key clinical point: Breast cancer margin guidelines may help reduce re-excisions and lower costs.
Major finding: Operative costs per patient fell by $1,218 after the adoption of the “no ink on tumor” guidelines.
Data source: Retrospective analysis of 237 patients undergoing breast conservation surgery.
Disclosures: The study received no external funding. Dr. Ajkay reported having no financial disclosures.
New persistent opioid use common after cancer surgery
New and persistent opioid use is a common complication of surgery in patients with early-stage cancer, according to results of a retrospective cohort study.
The risk of new persistent opioid use was 10.4% (95% confidence interval, 10.1%-10.7%) among patients undergoing curative-intent cancer surgery, according to the report, which was based on examination of 68,463 deidentified insurance claims from employer health plans from 2010 to 2014.
“This problem requires changes to prescribing guidelines and patient counseling during the surveillance and survivorship phases of care,” wrote Jay Soong-Jin Lee, MD, and his colleagues at the University of Michigan, Ann Arbor (J Clin Oncol. 2017 Oct 19. doi: 10.1200/JCO.2017.74.1363).
One year after the surgery, patients who developed new persistent opioid use were still filling prescriptions at high daily opioid doses, equivalent to six hydrocodone 5-mg tablets per day, according Dr. Lee and his colleagues.
“This dose is similar to intermittent and chronic opioid users [in the insurance claim data], suggesting that patients with new persistent opioid use may transition to chronic opioid use,” they said in the study report.
Adjuvant chemotherapy was a “strong risk factor” for new persistent opioid use, they added, though use was still common among patients who had no adjuvant chemotherapy. Rates of new persistent opioid use ranged from 15% to 21% for adjuvant therapy patient groups, compared with 7%-11% for no advjuvant therapy, data show.
Previous studies suggested a 6%-8% risk of new persistent opioid use among surgical patients, but those studies either did not focus on cancer patients or excluded them entirely, Dr. Lee and his coauthors noted.
Strategies are needed to combat new persistent opioid use after curative-intent surgery, they added.
They recommended further study to develop evidence-based guidelines to reduce excessive opioid prescribing and screening tools to identify at-risk patients (e.g., those with psychosocial factors).
Surgeons should be more active in counseling patients on the potential risks of opioids and how to keep use to a minimum after surgery, they added.
“Given the high risk of new persistent opioid use in this population, physicians should consider universal precautions … including educating patients on safe use, storage, and disposal,” they wrote.
Dr. Lee disclosed no relationships relevant to the study, while several coauthors reported relationships with Neuros Medical, Merck, and Anesthesia Associates of Ann Arbor.
New and persistent opioid use is a common complication of surgery in patients with early-stage cancer, according to results of a retrospective cohort study.
The risk of new persistent opioid use was 10.4% (95% confidence interval, 10.1%-10.7%) among patients undergoing curative-intent cancer surgery, according to the report, which was based on examination of 68,463 deidentified insurance claims from employer health plans from 2010 to 2014.
“This problem requires changes to prescribing guidelines and patient counseling during the surveillance and survivorship phases of care,” wrote Jay Soong-Jin Lee, MD, and his colleagues at the University of Michigan, Ann Arbor (J Clin Oncol. 2017 Oct 19. doi: 10.1200/JCO.2017.74.1363).
One year after the surgery, patients who developed new persistent opioid use were still filling prescriptions at high daily opioid doses, equivalent to six hydrocodone 5-mg tablets per day, according Dr. Lee and his colleagues.
“This dose is similar to intermittent and chronic opioid users [in the insurance claim data], suggesting that patients with new persistent opioid use may transition to chronic opioid use,” they said in the study report.
Adjuvant chemotherapy was a “strong risk factor” for new persistent opioid use, they added, though use was still common among patients who had no adjuvant chemotherapy. Rates of new persistent opioid use ranged from 15% to 21% for adjuvant therapy patient groups, compared with 7%-11% for no advjuvant therapy, data show.
Previous studies suggested a 6%-8% risk of new persistent opioid use among surgical patients, but those studies either did not focus on cancer patients or excluded them entirely, Dr. Lee and his coauthors noted.
Strategies are needed to combat new persistent opioid use after curative-intent surgery, they added.
They recommended further study to develop evidence-based guidelines to reduce excessive opioid prescribing and screening tools to identify at-risk patients (e.g., those with psychosocial factors).
Surgeons should be more active in counseling patients on the potential risks of opioids and how to keep use to a minimum after surgery, they added.
“Given the high risk of new persistent opioid use in this population, physicians should consider universal precautions … including educating patients on safe use, storage, and disposal,” they wrote.
Dr. Lee disclosed no relationships relevant to the study, while several coauthors reported relationships with Neuros Medical, Merck, and Anesthesia Associates of Ann Arbor.
New and persistent opioid use is a common complication of surgery in patients with early-stage cancer, according to results of a retrospective cohort study.
The risk of new persistent opioid use was 10.4% (95% confidence interval, 10.1%-10.7%) among patients undergoing curative-intent cancer surgery, according to the report, which was based on examination of 68,463 deidentified insurance claims from employer health plans from 2010 to 2014.
“This problem requires changes to prescribing guidelines and patient counseling during the surveillance and survivorship phases of care,” wrote Jay Soong-Jin Lee, MD, and his colleagues at the University of Michigan, Ann Arbor (J Clin Oncol. 2017 Oct 19. doi: 10.1200/JCO.2017.74.1363).
One year after the surgery, patients who developed new persistent opioid use were still filling prescriptions at high daily opioid doses, equivalent to six hydrocodone 5-mg tablets per day, according Dr. Lee and his colleagues.
“This dose is similar to intermittent and chronic opioid users [in the insurance claim data], suggesting that patients with new persistent opioid use may transition to chronic opioid use,” they said in the study report.
Adjuvant chemotherapy was a “strong risk factor” for new persistent opioid use, they added, though use was still common among patients who had no adjuvant chemotherapy. Rates of new persistent opioid use ranged from 15% to 21% for adjuvant therapy patient groups, compared with 7%-11% for no advjuvant therapy, data show.
Previous studies suggested a 6%-8% risk of new persistent opioid use among surgical patients, but those studies either did not focus on cancer patients or excluded them entirely, Dr. Lee and his coauthors noted.
Strategies are needed to combat new persistent opioid use after curative-intent surgery, they added.
They recommended further study to develop evidence-based guidelines to reduce excessive opioid prescribing and screening tools to identify at-risk patients (e.g., those with psychosocial factors).
Surgeons should be more active in counseling patients on the potential risks of opioids and how to keep use to a minimum after surgery, they added.
“Given the high risk of new persistent opioid use in this population, physicians should consider universal precautions … including educating patients on safe use, storage, and disposal,” they wrote.
Dr. Lee disclosed no relationships relevant to the study, while several coauthors reported relationships with Neuros Medical, Merck, and Anesthesia Associates of Ann Arbor.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Prescribing guidelines and patient counseling need to change to combat new persistent opioid use, which authors confirmed is a common problem in patients undergoing surgery for early-stage cancer.
Major finding: The risk of new persistent opioid use was 10.4% (95% CI, 10.1%-10.7%) among patients undergoing curative-intent cancer surgery.
Data source: Retrospective cohort study based on examination of deidentified insurance claims from employer health plans from 2010 to 2014.
Disclosures: First author Jay Soong-Jin Lee, MD, had no relationships to disclose. Coauthors reported relationships with Neuros Medical and Merck and Anesthesia Associates of Ann Arbor.
Perioperative blood transfusion linked to worse outcomes in renal cell carcinoma
Perioperative blood transfusion (PBT) is associated with poorer outcomes among patients who underwent nephrectomy for renal cell carcinoma (RCC), according to a retrospective review of 1,159 patients.
Using multivariate analysis and controlling for potential confounders such as clinical and pathologic features, receipt of PBT was associated with significantly increased risk of tumor recurrence (HR = 2, P = .02, metastatic progression (HR = 2.5, P = .007), and death from RCC (HR = 2.5, P = .02).
Previous research suggests that PBT may be associated with worse oncological outcomes following cancer surgery, although the data have been inconsistent. In this study, Dr. Abu-Ghanem and colleagues conducted a retrospective study that examined effect of PBT on the prognosis of 1,159 patients who underwent radical nephrectomy or partial nephrectomy for RCC, between 1987 to 2013.
Within this cohort, 198 patients (17.1%) received a PBT, and the median follow-up was 63.2 months. Receipt of PBT was associated with a symptomatic presentation (P less than .001) and a higher rate of adverse pathological features that included larger tumors (P less than .001), high nuclear grade (P less than .001), presence of tumor necrosis (P less than .001), and capsular invasion (P less than .001). Patients who received PBT were also more likely to have undergone an open surgical procedure (P less than .05).
The authors found that receipt of a PBT was associated with significantly worse 5-year relapse free survival (81% vs. 92%, P less than .01) as well as metastatic free survival (79% vs. 93%, P less than .001). Receiving a PBT was also associated with a worse 5-year CSS (85% vs. 95%, P less than .001) and OS (73% vs. 81%, P less than .001) versus those who were not transfused.
A subgroup analysis showed that patients who underwent a partial nephrectomy also had worse outcomes if they received a PBT as compared to those who didn’t; 5-year relapse free survival was 81% vs. 90% (P = .014), CSS was 89% vs. 97% (P = .019) and OS was 82% vs. 92%, (P = .016).
There were no funding sources or author disclosures listed in the article.
Perioperative blood transfusion (PBT) is associated with poorer outcomes among patients who underwent nephrectomy for renal cell carcinoma (RCC), according to a retrospective review of 1,159 patients.
Using multivariate analysis and controlling for potential confounders such as clinical and pathologic features, receipt of PBT was associated with significantly increased risk of tumor recurrence (HR = 2, P = .02, metastatic progression (HR = 2.5, P = .007), and death from RCC (HR = 2.5, P = .02).
Previous research suggests that PBT may be associated with worse oncological outcomes following cancer surgery, although the data have been inconsistent. In this study, Dr. Abu-Ghanem and colleagues conducted a retrospective study that examined effect of PBT on the prognosis of 1,159 patients who underwent radical nephrectomy or partial nephrectomy for RCC, between 1987 to 2013.
Within this cohort, 198 patients (17.1%) received a PBT, and the median follow-up was 63.2 months. Receipt of PBT was associated with a symptomatic presentation (P less than .001) and a higher rate of adverse pathological features that included larger tumors (P less than .001), high nuclear grade (P less than .001), presence of tumor necrosis (P less than .001), and capsular invasion (P less than .001). Patients who received PBT were also more likely to have undergone an open surgical procedure (P less than .05).
The authors found that receipt of a PBT was associated with significantly worse 5-year relapse free survival (81% vs. 92%, P less than .01) as well as metastatic free survival (79% vs. 93%, P less than .001). Receiving a PBT was also associated with a worse 5-year CSS (85% vs. 95%, P less than .001) and OS (73% vs. 81%, P less than .001) versus those who were not transfused.
A subgroup analysis showed that patients who underwent a partial nephrectomy also had worse outcomes if they received a PBT as compared to those who didn’t; 5-year relapse free survival was 81% vs. 90% (P = .014), CSS was 89% vs. 97% (P = .019) and OS was 82% vs. 92%, (P = .016).
There were no funding sources or author disclosures listed in the article.
Perioperative blood transfusion (PBT) is associated with poorer outcomes among patients who underwent nephrectomy for renal cell carcinoma (RCC), according to a retrospective review of 1,159 patients.
Using multivariate analysis and controlling for potential confounders such as clinical and pathologic features, receipt of PBT was associated with significantly increased risk of tumor recurrence (HR = 2, P = .02, metastatic progression (HR = 2.5, P = .007), and death from RCC (HR = 2.5, P = .02).
Previous research suggests that PBT may be associated with worse oncological outcomes following cancer surgery, although the data have been inconsistent. In this study, Dr. Abu-Ghanem and colleagues conducted a retrospective study that examined effect of PBT on the prognosis of 1,159 patients who underwent radical nephrectomy or partial nephrectomy for RCC, between 1987 to 2013.
Within this cohort, 198 patients (17.1%) received a PBT, and the median follow-up was 63.2 months. Receipt of PBT was associated with a symptomatic presentation (P less than .001) and a higher rate of adverse pathological features that included larger tumors (P less than .001), high nuclear grade (P less than .001), presence of tumor necrosis (P less than .001), and capsular invasion (P less than .001). Patients who received PBT were also more likely to have undergone an open surgical procedure (P less than .05).
The authors found that receipt of a PBT was associated with significantly worse 5-year relapse free survival (81% vs. 92%, P less than .01) as well as metastatic free survival (79% vs. 93%, P less than .001). Receiving a PBT was also associated with a worse 5-year CSS (85% vs. 95%, P less than .001) and OS (73% vs. 81%, P less than .001) versus those who were not transfused.
A subgroup analysis showed that patients who underwent a partial nephrectomy also had worse outcomes if they received a PBT as compared to those who didn’t; 5-year relapse free survival was 81% vs. 90% (P = .014), CSS was 89% vs. 97% (P = .019) and OS was 82% vs. 92%, (P = .016).
There were no funding sources or author disclosures listed in the article.
FROM UROLOGIC ONCOLOGY
Key clinical point: Major finding: Receipt of PBT was associated with significantly increased risk of tumor recurrence (HR = 2, P = .02, metastatic progression (HR = 2.5, P = .007), and death from RCC (HR = 2.5, P = .02).
Data source: Retrospective study that included 1,159 patients with RCC who underwent nephrectomy and evaluated outcomes in those who received a PBT versus those who did not.
Disclosures: There are no funding sources or author disclosures listed.
Papillary thyroid microcarcinoma: Is ‘less is more’ the right approach?
Surgeons treated 95% of preoperatively diagnosed cases of papillary thyroid microcarcinoma with total thyroidectomy, compared with only 69% of postoperatively diagnosed cases, in to a single-center retrospective cohort study.
“During the study period, thyroid lobectomy was an acceptable alternative endorsed by the American Thyroid Association,” said Susan C. Pitt, MD, and her associates at the University of Wisconsin, Madison. “Nonetheless, documentation rarely stated that [thyroid lobectomy] was discussed as an option. Whether this finding indicates a true lack of discussion or a deficit in documentation is unclear, but emphasizes the need to improve the quality of the [electronic health record] and capture all elements of shared decision-making.”
Papillary thyroid microcarcinomas (PTMC) measure 1 cm or less, affect up to a third of adults, and explain about half of the recent rise in rates of papillary thyroid cancer, the investigators stated. Most cases are found incidentally and there is no evidence that they contribute to a rise in mortality, which stands at about 0.5 deaths per 100,000 diagnoses of thyroid carcinoma. Accordingly, in 2015, the American Thyroid Association (ATA) endorsed active surveillance and thyroid lobectomy as acceptable management strategies for most patients with PTMC (Thyroid. 2016 Jan 12;26[1]:1-133).
“The pendulum for the ATA guidelines has swung back and forth,” Dr. Pitt said in an interview. “I think the current 2015 ATA guidelines are still controversial – some surgeons and endocrinologists think we have swung too far [in the other direction]. Moving the field from total thyroidectomy to active surveillance is a big jump. Understanding the factors underlying current decisions will help us to implement less extensive management, like lobectomy and active surveillance.”
To do that, Dr. Pitt and her associates reviewed medical records from 125 patients with PTMC treated at the University of Wisconsin between 2008 and 2016. Most of the patients (90%) were white, 85% were female, average age was 50 years, and nearly all had classic or follicular-variant disease. Only 27% of patients underwent thyroid lobectomy; the rest underwent total thyroidectomy. Furthermore, among 19 patients diagnosed preoperatively, 95% underwent total thyroidectomy and 21% had a complication, including one (5%) case of permanent hypocalcemia that less extensive surgery might have avoided (J Surg Res. 2017;218:237-45).
“In all cases, documentation indicated that these preoperatively diagnosed patients followed the surgeon’s recommendation regarding the extent of surgery,” the researchers wrote. Surgeons cited various reasons for recommending total thyroidectomy, including – in about 20% of cases – a belief that it was the recommended treatment.
Only one of the 19 preoperatively diagnosed patients had a documented discussion of thyroid lobectomy, the researchers found.
While physicians might be concerned about recurrence or other “downstream” outcomes of a less-is-more approach to PTMC, Dr. Pitt noted that, in a recent large study, only 3.4% of these tumors metastasized over 10 years (World J Surg. 2010 Jan;34[1]:28-35).
“At the same time, I think that we have a better sense [that] patient-centered outcomes after thyroidectomy, such as health-related quality of life, swallowing, and voice outcomes, can be worse after a total thyroidectomy,” she added.
As surgical and medical therapies expand for PTMC and other nonmalignant diseases, it becomes increasingly vital that surgeons and patients undertake shared decision-making, she said. At the University of Wisconsin, physicians can enter free text in the EHR to document such discussions. She gave an example of how she does that: “‘Total thyroidectomy and lobectomy are both appropriate approaches for Ms. Smith. We discussed these options at length, including X, Y, and Z. Given Mrs. Smith’s (strong) preference to avoid X, we will proceed with a lobectomy.”
In her own practice, Dr. Pitt added, “when I look back at a note, I want to know what the decision was, and why it was made.”
Shared decision-making differs from informed consent by focusing on patient preferences, she noted. “I have used my notes in the operating room to help me decide what to do. I can look back and have a window into our conversation and what an individual patient values.” For PTMC, shared decisions should focus less on cancer risk and more on quality of life and outcomes a year later, she said.
“Patients don’t die from PTMC, and most live longer than the age-matched population. Given the risks of more extensive surgery and our current data on surgical and patient-centered outcomes, I think that thyroid lobectomy should be the initial treatment for most patients with PTMC, and surgeons should help their patients make informed decisions,” Dr. Pitt said.
The National Institutes of Health provided funding. The researchers reported having no conflicts of interest.
Surgeons treated 95% of preoperatively diagnosed cases of papillary thyroid microcarcinoma with total thyroidectomy, compared with only 69% of postoperatively diagnosed cases, in to a single-center retrospective cohort study.
“During the study period, thyroid lobectomy was an acceptable alternative endorsed by the American Thyroid Association,” said Susan C. Pitt, MD, and her associates at the University of Wisconsin, Madison. “Nonetheless, documentation rarely stated that [thyroid lobectomy] was discussed as an option. Whether this finding indicates a true lack of discussion or a deficit in documentation is unclear, but emphasizes the need to improve the quality of the [electronic health record] and capture all elements of shared decision-making.”
Papillary thyroid microcarcinomas (PTMC) measure 1 cm or less, affect up to a third of adults, and explain about half of the recent rise in rates of papillary thyroid cancer, the investigators stated. Most cases are found incidentally and there is no evidence that they contribute to a rise in mortality, which stands at about 0.5 deaths per 100,000 diagnoses of thyroid carcinoma. Accordingly, in 2015, the American Thyroid Association (ATA) endorsed active surveillance and thyroid lobectomy as acceptable management strategies for most patients with PTMC (Thyroid. 2016 Jan 12;26[1]:1-133).
“The pendulum for the ATA guidelines has swung back and forth,” Dr. Pitt said in an interview. “I think the current 2015 ATA guidelines are still controversial – some surgeons and endocrinologists think we have swung too far [in the other direction]. Moving the field from total thyroidectomy to active surveillance is a big jump. Understanding the factors underlying current decisions will help us to implement less extensive management, like lobectomy and active surveillance.”
To do that, Dr. Pitt and her associates reviewed medical records from 125 patients with PTMC treated at the University of Wisconsin between 2008 and 2016. Most of the patients (90%) were white, 85% were female, average age was 50 years, and nearly all had classic or follicular-variant disease. Only 27% of patients underwent thyroid lobectomy; the rest underwent total thyroidectomy. Furthermore, among 19 patients diagnosed preoperatively, 95% underwent total thyroidectomy and 21% had a complication, including one (5%) case of permanent hypocalcemia that less extensive surgery might have avoided (J Surg Res. 2017;218:237-45).
“In all cases, documentation indicated that these preoperatively diagnosed patients followed the surgeon’s recommendation regarding the extent of surgery,” the researchers wrote. Surgeons cited various reasons for recommending total thyroidectomy, including – in about 20% of cases – a belief that it was the recommended treatment.
Only one of the 19 preoperatively diagnosed patients had a documented discussion of thyroid lobectomy, the researchers found.
While physicians might be concerned about recurrence or other “downstream” outcomes of a less-is-more approach to PTMC, Dr. Pitt noted that, in a recent large study, only 3.4% of these tumors metastasized over 10 years (World J Surg. 2010 Jan;34[1]:28-35).
“At the same time, I think that we have a better sense [that] patient-centered outcomes after thyroidectomy, such as health-related quality of life, swallowing, and voice outcomes, can be worse after a total thyroidectomy,” she added.
As surgical and medical therapies expand for PTMC and other nonmalignant diseases, it becomes increasingly vital that surgeons and patients undertake shared decision-making, she said. At the University of Wisconsin, physicians can enter free text in the EHR to document such discussions. She gave an example of how she does that: “‘Total thyroidectomy and lobectomy are both appropriate approaches for Ms. Smith. We discussed these options at length, including X, Y, and Z. Given Mrs. Smith’s (strong) preference to avoid X, we will proceed with a lobectomy.”
In her own practice, Dr. Pitt added, “when I look back at a note, I want to know what the decision was, and why it was made.”
Shared decision-making differs from informed consent by focusing on patient preferences, she noted. “I have used my notes in the operating room to help me decide what to do. I can look back and have a window into our conversation and what an individual patient values.” For PTMC, shared decisions should focus less on cancer risk and more on quality of life and outcomes a year later, she said.
“Patients don’t die from PTMC, and most live longer than the age-matched population. Given the risks of more extensive surgery and our current data on surgical and patient-centered outcomes, I think that thyroid lobectomy should be the initial treatment for most patients with PTMC, and surgeons should help their patients make informed decisions,” Dr. Pitt said.
The National Institutes of Health provided funding. The researchers reported having no conflicts of interest.
Surgeons treated 95% of preoperatively diagnosed cases of papillary thyroid microcarcinoma with total thyroidectomy, compared with only 69% of postoperatively diagnosed cases, in to a single-center retrospective cohort study.
“During the study period, thyroid lobectomy was an acceptable alternative endorsed by the American Thyroid Association,” said Susan C. Pitt, MD, and her associates at the University of Wisconsin, Madison. “Nonetheless, documentation rarely stated that [thyroid lobectomy] was discussed as an option. Whether this finding indicates a true lack of discussion or a deficit in documentation is unclear, but emphasizes the need to improve the quality of the [electronic health record] and capture all elements of shared decision-making.”
Papillary thyroid microcarcinomas (PTMC) measure 1 cm or less, affect up to a third of adults, and explain about half of the recent rise in rates of papillary thyroid cancer, the investigators stated. Most cases are found incidentally and there is no evidence that they contribute to a rise in mortality, which stands at about 0.5 deaths per 100,000 diagnoses of thyroid carcinoma. Accordingly, in 2015, the American Thyroid Association (ATA) endorsed active surveillance and thyroid lobectomy as acceptable management strategies for most patients with PTMC (Thyroid. 2016 Jan 12;26[1]:1-133).
“The pendulum for the ATA guidelines has swung back and forth,” Dr. Pitt said in an interview. “I think the current 2015 ATA guidelines are still controversial – some surgeons and endocrinologists think we have swung too far [in the other direction]. Moving the field from total thyroidectomy to active surveillance is a big jump. Understanding the factors underlying current decisions will help us to implement less extensive management, like lobectomy and active surveillance.”
To do that, Dr. Pitt and her associates reviewed medical records from 125 patients with PTMC treated at the University of Wisconsin between 2008 and 2016. Most of the patients (90%) were white, 85% were female, average age was 50 years, and nearly all had classic or follicular-variant disease. Only 27% of patients underwent thyroid lobectomy; the rest underwent total thyroidectomy. Furthermore, among 19 patients diagnosed preoperatively, 95% underwent total thyroidectomy and 21% had a complication, including one (5%) case of permanent hypocalcemia that less extensive surgery might have avoided (J Surg Res. 2017;218:237-45).
“In all cases, documentation indicated that these preoperatively diagnosed patients followed the surgeon’s recommendation regarding the extent of surgery,” the researchers wrote. Surgeons cited various reasons for recommending total thyroidectomy, including – in about 20% of cases – a belief that it was the recommended treatment.
Only one of the 19 preoperatively diagnosed patients had a documented discussion of thyroid lobectomy, the researchers found.
While physicians might be concerned about recurrence or other “downstream” outcomes of a less-is-more approach to PTMC, Dr. Pitt noted that, in a recent large study, only 3.4% of these tumors metastasized over 10 years (World J Surg. 2010 Jan;34[1]:28-35).
“At the same time, I think that we have a better sense [that] patient-centered outcomes after thyroidectomy, such as health-related quality of life, swallowing, and voice outcomes, can be worse after a total thyroidectomy,” she added.
As surgical and medical therapies expand for PTMC and other nonmalignant diseases, it becomes increasingly vital that surgeons and patients undertake shared decision-making, she said. At the University of Wisconsin, physicians can enter free text in the EHR to document such discussions. She gave an example of how she does that: “‘Total thyroidectomy and lobectomy are both appropriate approaches for Ms. Smith. We discussed these options at length, including X, Y, and Z. Given Mrs. Smith’s (strong) preference to avoid X, we will proceed with a lobectomy.”
In her own practice, Dr. Pitt added, “when I look back at a note, I want to know what the decision was, and why it was made.”
Shared decision-making differs from informed consent by focusing on patient preferences, she noted. “I have used my notes in the operating room to help me decide what to do. I can look back and have a window into our conversation and what an individual patient values.” For PTMC, shared decisions should focus less on cancer risk and more on quality of life and outcomes a year later, she said.
“Patients don’t die from PTMC, and most live longer than the age-matched population. Given the risks of more extensive surgery and our current data on surgical and patient-centered outcomes, I think that thyroid lobectomy should be the initial treatment for most patients with PTMC, and surgeons should help their patients make informed decisions,” Dr. Pitt said.
The National Institutes of Health provided funding. The researchers reported having no conflicts of interest.
FROM THE JOURNAL OF SURGICAL RESEARCH
Key clinical point: Nearly all patients with a preoperative diagnosis of PTMC underwent total thyroidectomy, usually at their surgeon’s recommendation.
Major finding: 95% of preoperatively diagnosed patients underwent total thyroidectomy, versus 69% of those diagnosed postoperatively (P = .02). A discussion of thyroid lobectomy was documented in only one preoperatively diagnosed case.
Data source: A single-center retrospective study of 125 patients with papillary thyroid microcarcinoma.
Disclosures: The National Institutes of Health provided funding. The researchers reported having no conflicts of interest.
Reconstruction becoming more common after mastectomy
The rate of breast reconstruction surgery for mastectomy increased 62% from 2009 to 2014, while the mastectomy rate itself “remained relatively stable,” according to the Agency for Healthcare Research and Quality.
The rate of breast reconstructions in hospitals and ambulatory surgery settings rose steadily over the 6-year period, going from 21.7 per 100,000 women in 2009 to 35.1 per 100,000 in 2014. Meanwhile, the mastectomy rate dipped from 90.1 in 2009 to 83.2 in 2010 but varied less than 10% over the 2009-2014 time period, reaching 88.4 per 100,000 women in 2014. To put those numbers in a different context, the ratio of reconstructions to mastectomies went from 24-to-100 in 2009 to 40-to-100 in 2014, the AHRQ reported in a Statistical Brief.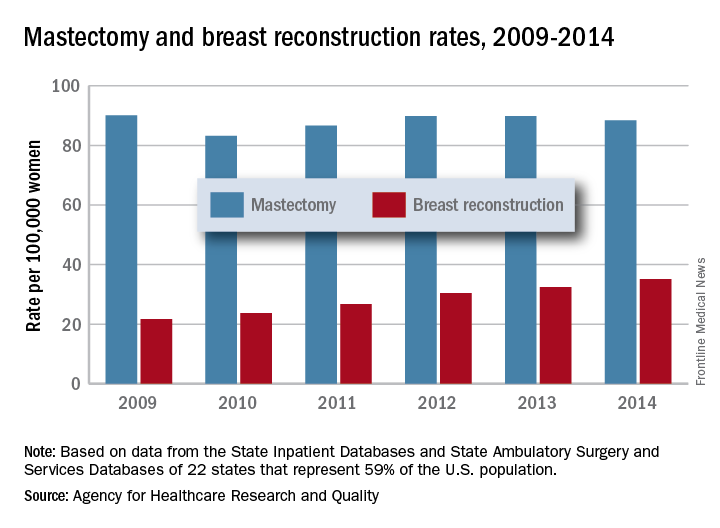
Those nonsimultaneous procedures were taking place much more often in ambulatory settings by 2014, as the rate of reconstructions at a separate visit increased 152% from 7.4 per 100,000 women in 2009 to 18.2. Simultaneous reconstructions in ambulatory settings were less common but increased at an even greater rate of 155%, going from 1.1 to 2.8 per 100,000 women. Inpatient reconstruction had little or no growth over the 6 years: Separate-visit procedures went from 6 to 6.8 and simultaneous reconstructions actually dropped from 7.4 per 100,000 women to 7.3, they reported.
The analysis was based on data from AHRQ State Inpatient Databases and State Ambulatory Surgery and Services Databases for 22 states that include 59% of the U.S. population: California, Colorado, Connecticut, Florida, Georgia, Iowa, Indiana, Maryland, Michigan, Minnesota, Missouri, Nebraska, New Jersey, New York, North Carolina, Ohio, South Carolina, South Dakota, Tennessee, Utah, Vermont, and Wisconsin.
The rate of breast reconstruction surgery for mastectomy increased 62% from 2009 to 2014, while the mastectomy rate itself “remained relatively stable,” according to the Agency for Healthcare Research and Quality.
The rate of breast reconstructions in hospitals and ambulatory surgery settings rose steadily over the 6-year period, going from 21.7 per 100,000 women in 2009 to 35.1 per 100,000 in 2014. Meanwhile, the mastectomy rate dipped from 90.1 in 2009 to 83.2 in 2010 but varied less than 10% over the 2009-2014 time period, reaching 88.4 per 100,000 women in 2014. To put those numbers in a different context, the ratio of reconstructions to mastectomies went from 24-to-100 in 2009 to 40-to-100 in 2014, the AHRQ reported in a Statistical Brief.
Those nonsimultaneous procedures were taking place much more often in ambulatory settings by 2014, as the rate of reconstructions at a separate visit increased 152% from 7.4 per 100,000 women in 2009 to 18.2. Simultaneous reconstructions in ambulatory settings were less common but increased at an even greater rate of 155%, going from 1.1 to 2.8 per 100,000 women. Inpatient reconstruction had little or no growth over the 6 years: Separate-visit procedures went from 6 to 6.8 and simultaneous reconstructions actually dropped from 7.4 per 100,000 women to 7.3, they reported.
The analysis was based on data from AHRQ State Inpatient Databases and State Ambulatory Surgery and Services Databases for 22 states that include 59% of the U.S. population: California, Colorado, Connecticut, Florida, Georgia, Iowa, Indiana, Maryland, Michigan, Minnesota, Missouri, Nebraska, New Jersey, New York, North Carolina, Ohio, South Carolina, South Dakota, Tennessee, Utah, Vermont, and Wisconsin.
The rate of breast reconstruction surgery for mastectomy increased 62% from 2009 to 2014, while the mastectomy rate itself “remained relatively stable,” according to the Agency for Healthcare Research and Quality.
The rate of breast reconstructions in hospitals and ambulatory surgery settings rose steadily over the 6-year period, going from 21.7 per 100,000 women in 2009 to 35.1 per 100,000 in 2014. Meanwhile, the mastectomy rate dipped from 90.1 in 2009 to 83.2 in 2010 but varied less than 10% over the 2009-2014 time period, reaching 88.4 per 100,000 women in 2014. To put those numbers in a different context, the ratio of reconstructions to mastectomies went from 24-to-100 in 2009 to 40-to-100 in 2014, the AHRQ reported in a Statistical Brief.
Those nonsimultaneous procedures were taking place much more often in ambulatory settings by 2014, as the rate of reconstructions at a separate visit increased 152% from 7.4 per 100,000 women in 2009 to 18.2. Simultaneous reconstructions in ambulatory settings were less common but increased at an even greater rate of 155%, going from 1.1 to 2.8 per 100,000 women. Inpatient reconstruction had little or no growth over the 6 years: Separate-visit procedures went from 6 to 6.8 and simultaneous reconstructions actually dropped from 7.4 per 100,000 women to 7.3, they reported.
The analysis was based on data from AHRQ State Inpatient Databases and State Ambulatory Surgery and Services Databases for 22 states that include 59% of the U.S. population: California, Colorado, Connecticut, Florida, Georgia, Iowa, Indiana, Maryland, Michigan, Minnesota, Missouri, Nebraska, New Jersey, New York, North Carolina, Ohio, South Carolina, South Dakota, Tennessee, Utah, Vermont, and Wisconsin.
Many new cancer drugs lack evidence of survival or QoL benefit
Even after several years on the market, only about half of cancer drug indications recently approved by the European Medicines Agency (EMA) had conclusive evidence that they can extend or improve quality of life, according to results of a retrospective cohort study.
With a median of 5.4 years of follow-up, significant improvements in overall survival or quality of life had been published for 35 of 68 (51%) cancer drug indications approved by the EMA, according to the report by Courtney Davis, MD, senior lecturer in the department of global health and social medicine, King’s College London, United Kingdom, and colleagues.
Furthermore, not all survival benefits were clinically meaningful, according to an analysis published in the report.
The dearth of evidence for survival or quality-of-life benefits has “negative implications” for both patients and public health, Dr. Davis and colleagues said in their article (BMJ 2017 Oct 5. doi:10.1136/bmj.j4530).
“When expensive drugs that lack clinically meaningful benefits are approved and paid for within publicly funded healthcare systems, individual patients can be harmed, important societal resources wasted, and the delivery of equitable and affordable care undermined,” they wrote.
Dr. Davis and associates systematically evaluated the evidence base for regulatory and scientific reports on 48 cancer drugs approved for 68 indications by the EMA between 2009-2013. Of those indications, 17 were for hematologic malignancies and 51 were for solid tumors.
Only 18 of 68 indications (26%) were supported by pivotal studies that had a primary outcome of overall survival, according to the investigators. That was an important finding for the investigators, who wrote that that EMA commonly accepts use of surrogate measures of drug benefit despite their own statements that overall survival is the “most persuasive outcome” in studies of new oncology drugs.
“To a large extent, regulatory evidence standards determine the clinical value of … new oncology drugs,” Dr. Davis and co-authors wrote. “Our study suggests these standards are failing to incentivize drug development that best meets the needs of patients, clinicians, and healthcare systems.”
The investigators also assessed the clinical value of reported improvements using the European Society for Medical Oncology-Magnitude of Clinical Benefit Scale (ESMO-MCBS). According to investigators, only 11 of the 23 drugs used to treat solid tumors (48%) reached the threshold for a meaningful survival benefit.
This report in BMJ echoes findings of an earlier study by Chul Kim, MD, and colleagues looking at cancer drugs approved by the U.S. Food and Drug Administration (FDA) between 2008 and 2012 (JAMA Intern Med. 2015;175(12):1992-4).
Dr. Kim, of the medical oncology service, National Cancer Institute, National Institutes of Health, Bethesda, Md., and colleagues found that 36 of 54 FDA approvals (67%) occurred with no evidence of survival or quality of life benefit. After a median of 4.4 years of follow-up, only 5 of those 36 (14%) had additional randomized study data that showed an improvement in overall survival, according to the published report.
The study was supported by Health Action International, which did not have a role in study design or data collection, analysis, or interpretation. The authors did not give financial disclosures.
The expense and toxicity of cancer drugs mean we have an obligation to expose patients to treatment only when they can reasonably expect an improvement in survival or quality of life. The study by Davis and colleagues suggests we may be falling far short of this important benchmark.
Few cancer drugs come to market with good evidence that they improve patient centered outcomes. If they do, they often offer marginal benefits that may be lost in the heterogeneous patients of the real world. Most approvals of cancer drugs are based on flimsy or untested surrogate endpoints, and postmarketing studies rarely validate the efficacy and safety of these drugs on patient centered endpoints.
In the United States, this broken system means huge expenditures on cancer drugs with certain toxicity but uncertain benefit. In Europe, payers yield the stick left unused by lax regulatoers. The National Institute for Health and Care Excellence (NICE) excludes from reimbursement drugs that provide only marginal or uncertain benefits at high cost. Their decisions are continually subjected to political scrutiny and public criticism.
What can be done? The default path to market for all cancer drugs should include rigorous testing against the best standard of care in randomized trials powered to rule in or rule out a clinically meaningful difference in patient centered outcomes in a representative population. The use of uncontrolled study designs or surrogate endpoints should be the exception, not the rule.
Vinay Prasad, MD, MPH, is assistant professor of medicine at Oregon Health and Science University, Portland. He declared a competing interest (royalties from his book Ending Medical Reversal). These comments are from his editorial (BMJ 2017 Oct 5. doi: 10.1136/bmj.j4528 )
The expense and toxicity of cancer drugs mean we have an obligation to expose patients to treatment only when they can reasonably expect an improvement in survival or quality of life. The study by Davis and colleagues suggests we may be falling far short of this important benchmark.
Few cancer drugs come to market with good evidence that they improve patient centered outcomes. If they do, they often offer marginal benefits that may be lost in the heterogeneous patients of the real world. Most approvals of cancer drugs are based on flimsy or untested surrogate endpoints, and postmarketing studies rarely validate the efficacy and safety of these drugs on patient centered endpoints.
In the United States, this broken system means huge expenditures on cancer drugs with certain toxicity but uncertain benefit. In Europe, payers yield the stick left unused by lax regulatoers. The National Institute for Health and Care Excellence (NICE) excludes from reimbursement drugs that provide only marginal or uncertain benefits at high cost. Their decisions are continually subjected to political scrutiny and public criticism.
What can be done? The default path to market for all cancer drugs should include rigorous testing against the best standard of care in randomized trials powered to rule in or rule out a clinically meaningful difference in patient centered outcomes in a representative population. The use of uncontrolled study designs or surrogate endpoints should be the exception, not the rule.
Vinay Prasad, MD, MPH, is assistant professor of medicine at Oregon Health and Science University, Portland. He declared a competing interest (royalties from his book Ending Medical Reversal). These comments are from his editorial (BMJ 2017 Oct 5. doi: 10.1136/bmj.j4528 )
The expense and toxicity of cancer drugs mean we have an obligation to expose patients to treatment only when they can reasonably expect an improvement in survival or quality of life. The study by Davis and colleagues suggests we may be falling far short of this important benchmark.
Few cancer drugs come to market with good evidence that they improve patient centered outcomes. If they do, they often offer marginal benefits that may be lost in the heterogeneous patients of the real world. Most approvals of cancer drugs are based on flimsy or untested surrogate endpoints, and postmarketing studies rarely validate the efficacy and safety of these drugs on patient centered endpoints.
In the United States, this broken system means huge expenditures on cancer drugs with certain toxicity but uncertain benefit. In Europe, payers yield the stick left unused by lax regulatoers. The National Institute for Health and Care Excellence (NICE) excludes from reimbursement drugs that provide only marginal or uncertain benefits at high cost. Their decisions are continually subjected to political scrutiny and public criticism.
What can be done? The default path to market for all cancer drugs should include rigorous testing against the best standard of care in randomized trials powered to rule in or rule out a clinically meaningful difference in patient centered outcomes in a representative population. The use of uncontrolled study designs or surrogate endpoints should be the exception, not the rule.
Vinay Prasad, MD, MPH, is assistant professor of medicine at Oregon Health and Science University, Portland. He declared a competing interest (royalties from his book Ending Medical Reversal). These comments are from his editorial (BMJ 2017 Oct 5. doi: 10.1136/bmj.j4528 )
Even after several years on the market, only about half of cancer drug indications recently approved by the European Medicines Agency (EMA) had conclusive evidence that they can extend or improve quality of life, according to results of a retrospective cohort study.
With a median of 5.4 years of follow-up, significant improvements in overall survival or quality of life had been published for 35 of 68 (51%) cancer drug indications approved by the EMA, according to the report by Courtney Davis, MD, senior lecturer in the department of global health and social medicine, King’s College London, United Kingdom, and colleagues.
Furthermore, not all survival benefits were clinically meaningful, according to an analysis published in the report.
The dearth of evidence for survival or quality-of-life benefits has “negative implications” for both patients and public health, Dr. Davis and colleagues said in their article (BMJ 2017 Oct 5. doi:10.1136/bmj.j4530).
“When expensive drugs that lack clinically meaningful benefits are approved and paid for within publicly funded healthcare systems, individual patients can be harmed, important societal resources wasted, and the delivery of equitable and affordable care undermined,” they wrote.
Dr. Davis and associates systematically evaluated the evidence base for regulatory and scientific reports on 48 cancer drugs approved for 68 indications by the EMA between 2009-2013. Of those indications, 17 were for hematologic malignancies and 51 were for solid tumors.
Only 18 of 68 indications (26%) were supported by pivotal studies that had a primary outcome of overall survival, according to the investigators. That was an important finding for the investigators, who wrote that that EMA commonly accepts use of surrogate measures of drug benefit despite their own statements that overall survival is the “most persuasive outcome” in studies of new oncology drugs.
“To a large extent, regulatory evidence standards determine the clinical value of … new oncology drugs,” Dr. Davis and co-authors wrote. “Our study suggests these standards are failing to incentivize drug development that best meets the needs of patients, clinicians, and healthcare systems.”
The investigators also assessed the clinical value of reported improvements using the European Society for Medical Oncology-Magnitude of Clinical Benefit Scale (ESMO-MCBS). According to investigators, only 11 of the 23 drugs used to treat solid tumors (48%) reached the threshold for a meaningful survival benefit.
This report in BMJ echoes findings of an earlier study by Chul Kim, MD, and colleagues looking at cancer drugs approved by the U.S. Food and Drug Administration (FDA) between 2008 and 2012 (JAMA Intern Med. 2015;175(12):1992-4).
Dr. Kim, of the medical oncology service, National Cancer Institute, National Institutes of Health, Bethesda, Md., and colleagues found that 36 of 54 FDA approvals (67%) occurred with no evidence of survival or quality of life benefit. After a median of 4.4 years of follow-up, only 5 of those 36 (14%) had additional randomized study data that showed an improvement in overall survival, according to the published report.
The study was supported by Health Action International, which did not have a role in study design or data collection, analysis, or interpretation. The authors did not give financial disclosures.
Even after several years on the market, only about half of cancer drug indications recently approved by the European Medicines Agency (EMA) had conclusive evidence that they can extend or improve quality of life, according to results of a retrospective cohort study.
With a median of 5.4 years of follow-up, significant improvements in overall survival or quality of life had been published for 35 of 68 (51%) cancer drug indications approved by the EMA, according to the report by Courtney Davis, MD, senior lecturer in the department of global health and social medicine, King’s College London, United Kingdom, and colleagues.
Furthermore, not all survival benefits were clinically meaningful, according to an analysis published in the report.
The dearth of evidence for survival or quality-of-life benefits has “negative implications” for both patients and public health, Dr. Davis and colleagues said in their article (BMJ 2017 Oct 5. doi:10.1136/bmj.j4530).
“When expensive drugs that lack clinically meaningful benefits are approved and paid for within publicly funded healthcare systems, individual patients can be harmed, important societal resources wasted, and the delivery of equitable and affordable care undermined,” they wrote.
Dr. Davis and associates systematically evaluated the evidence base for regulatory and scientific reports on 48 cancer drugs approved for 68 indications by the EMA between 2009-2013. Of those indications, 17 were for hematologic malignancies and 51 were for solid tumors.
Only 18 of 68 indications (26%) were supported by pivotal studies that had a primary outcome of overall survival, according to the investigators. That was an important finding for the investigators, who wrote that that EMA commonly accepts use of surrogate measures of drug benefit despite their own statements that overall survival is the “most persuasive outcome” in studies of new oncology drugs.
“To a large extent, regulatory evidence standards determine the clinical value of … new oncology drugs,” Dr. Davis and co-authors wrote. “Our study suggests these standards are failing to incentivize drug development that best meets the needs of patients, clinicians, and healthcare systems.”
The investigators also assessed the clinical value of reported improvements using the European Society for Medical Oncology-Magnitude of Clinical Benefit Scale (ESMO-MCBS). According to investigators, only 11 of the 23 drugs used to treat solid tumors (48%) reached the threshold for a meaningful survival benefit.
This report in BMJ echoes findings of an earlier study by Chul Kim, MD, and colleagues looking at cancer drugs approved by the U.S. Food and Drug Administration (FDA) between 2008 and 2012 (JAMA Intern Med. 2015;175(12):1992-4).
Dr. Kim, of the medical oncology service, National Cancer Institute, National Institutes of Health, Bethesda, Md., and colleagues found that 36 of 54 FDA approvals (67%) occurred with no evidence of survival or quality of life benefit. After a median of 4.4 years of follow-up, only 5 of those 36 (14%) had additional randomized study data that showed an improvement in overall survival, according to the published report.
The study was supported by Health Action International, which did not have a role in study design or data collection, analysis, or interpretation. The authors did not give financial disclosures.
From BMJ
Key clinical point: Even after several years on the market, only about half of cancer drug indications recently approved by the European Medicines Agency (EMA) lacked conclusive evidence that they can extend or improve quality of life.
Major finding: With a median of 5.4 years of follow-up, significant improvements in overall survival or quality of life had been published for 35 of 68 (51%) cancer drug indications approved by the EMA.
Data source: Retrospective cohort study of regulatory and scientific reports on 48 cancer drugs approved for 68 indications by the EMA between 2009-2013.
Disclosures: The study was supported by Health Action International, which did not have a role in study design or data collection, analysis, or interpretation.
Robot-assisted abdominoperineal resection outperforms open or laparoscopic surgery for rectal cancers
MADRID – The automaton uprising continues: Robot-assisted abdominoperineal resection (APR) can be safely performed in patients with rectal cancers within 5 cm of the anal verge, with surgical results equivalent to those seen with open or laparoscopic APR, investigators say.
Robot-assisted procedures were associated with a significantly lower rate of postoperative complications and with faster functional recovery than either laparoscopic or open surgery in a randomized trial, reported Jianmin Xu, MD, PhD., from Fudan University in Shanghai, China, and colleagues in a scientific poster presented at the European Society for Medical Oncology Congress.
“Retrospective studies have showed that robotic surgery was better than laparoscopic surgery in ensuring radical resection, reducing complications, and promoting recovery. However, there is no clinical trial reported for robotic surgery for rectal cancer. Thus, we conduct this randomized controlled trial to compare the safety and efficacy of robotic, laparoscopic, and open surgery for low rectal cancer,” they wrote.
Dr. Xu and colleagues enrolled 506 patients from 18 to 75 years of age with clinical stage T1 to T3 cancers within 5 cm of the anal verge and no distant metastases and randomly assigned them to resection with either a robot-assisted, laparoscopic, or open APR technique. Three of the 506 patients randomized did not undergo resection, leaving 503 for the per-protocol analysis presented here.
For the primary endpoint of complications rates within 30 days following surgery, the investigators found that patients assigned to robotic-assisted surgery (173 patients) had a total complication rate of 10.4%, compared with 18.8% for 176 patients assigned to laparoscopy (P = .027), and 26% for 154 assigned to open APR (P less than .001). The latter group included four patients assigned to laparoscopy whose procedures were converted to open surgery.
Among patients without complications, robot-assisted procedures were also associated with faster recovery, as measured by days to first flatus, at a median of 1 vs. 2 for laparoscopy and 3 for open procedures (P less than .001 for robots vs. each other surgery type). The robotic surgery was also significantly associated with fewer days to first automatic urination (median 2 vs. 4 for each of the other procedures; P less than .001), and with fewer days to discharge (median 5 vs. 7 for the other procedures; P less than .001).
"These are excellent postoperative complication rates reported, especially for the robotic treatment group, commented Thomas Gruenberger, MD, an oncologic surgeon at Rudolf Hospital in Vienna, in a poster discussion session.
“We all are now favoring laparoscopic surgery for these kinds of patients. The robot is a nice thing to have; however, we cannot use it in every hospital because it’s still quite expensive,” he said.
“We require – and this is a secondary endpoint of the study – long-term follow-up for local and distant outcomes,” he added,
The investigators did not report a funding source. All authors declared having no conflicts of interest. Dr. Gruenberger disclosed research funding, speakers bureau participation, and/or advisory roles with several companies.
MADRID – The automaton uprising continues: Robot-assisted abdominoperineal resection (APR) can be safely performed in patients with rectal cancers within 5 cm of the anal verge, with surgical results equivalent to those seen with open or laparoscopic APR, investigators say.
Robot-assisted procedures were associated with a significantly lower rate of postoperative complications and with faster functional recovery than either laparoscopic or open surgery in a randomized trial, reported Jianmin Xu, MD, PhD., from Fudan University in Shanghai, China, and colleagues in a scientific poster presented at the European Society for Medical Oncology Congress.
“Retrospective studies have showed that robotic surgery was better than laparoscopic surgery in ensuring radical resection, reducing complications, and promoting recovery. However, there is no clinical trial reported for robotic surgery for rectal cancer. Thus, we conduct this randomized controlled trial to compare the safety and efficacy of robotic, laparoscopic, and open surgery for low rectal cancer,” they wrote.
Dr. Xu and colleagues enrolled 506 patients from 18 to 75 years of age with clinical stage T1 to T3 cancers within 5 cm of the anal verge and no distant metastases and randomly assigned them to resection with either a robot-assisted, laparoscopic, or open APR technique. Three of the 506 patients randomized did not undergo resection, leaving 503 for the per-protocol analysis presented here.
For the primary endpoint of complications rates within 30 days following surgery, the investigators found that patients assigned to robotic-assisted surgery (173 patients) had a total complication rate of 10.4%, compared with 18.8% for 176 patients assigned to laparoscopy (P = .027), and 26% for 154 assigned to open APR (P less than .001). The latter group included four patients assigned to laparoscopy whose procedures were converted to open surgery.
Among patients without complications, robot-assisted procedures were also associated with faster recovery, as measured by days to first flatus, at a median of 1 vs. 2 for laparoscopy and 3 for open procedures (P less than .001 for robots vs. each other surgery type). The robotic surgery was also significantly associated with fewer days to first automatic urination (median 2 vs. 4 for each of the other procedures; P less than .001), and with fewer days to discharge (median 5 vs. 7 for the other procedures; P less than .001).
"These are excellent postoperative complication rates reported, especially for the robotic treatment group, commented Thomas Gruenberger, MD, an oncologic surgeon at Rudolf Hospital in Vienna, in a poster discussion session.
“We all are now favoring laparoscopic surgery for these kinds of patients. The robot is a nice thing to have; however, we cannot use it in every hospital because it’s still quite expensive,” he said.
“We require – and this is a secondary endpoint of the study – long-term follow-up for local and distant outcomes,” he added,
The investigators did not report a funding source. All authors declared having no conflicts of interest. Dr. Gruenberger disclosed research funding, speakers bureau participation, and/or advisory roles with several companies.
MADRID – The automaton uprising continues: Robot-assisted abdominoperineal resection (APR) can be safely performed in patients with rectal cancers within 5 cm of the anal verge, with surgical results equivalent to those seen with open or laparoscopic APR, investigators say.
Robot-assisted procedures were associated with a significantly lower rate of postoperative complications and with faster functional recovery than either laparoscopic or open surgery in a randomized trial, reported Jianmin Xu, MD, PhD., from Fudan University in Shanghai, China, and colleagues in a scientific poster presented at the European Society for Medical Oncology Congress.
“Retrospective studies have showed that robotic surgery was better than laparoscopic surgery in ensuring radical resection, reducing complications, and promoting recovery. However, there is no clinical trial reported for robotic surgery for rectal cancer. Thus, we conduct this randomized controlled trial to compare the safety and efficacy of robotic, laparoscopic, and open surgery for low rectal cancer,” they wrote.
Dr. Xu and colleagues enrolled 506 patients from 18 to 75 years of age with clinical stage T1 to T3 cancers within 5 cm of the anal verge and no distant metastases and randomly assigned them to resection with either a robot-assisted, laparoscopic, or open APR technique. Three of the 506 patients randomized did not undergo resection, leaving 503 for the per-protocol analysis presented here.
For the primary endpoint of complications rates within 30 days following surgery, the investigators found that patients assigned to robotic-assisted surgery (173 patients) had a total complication rate of 10.4%, compared with 18.8% for 176 patients assigned to laparoscopy (P = .027), and 26% for 154 assigned to open APR (P less than .001). The latter group included four patients assigned to laparoscopy whose procedures were converted to open surgery.
Among patients without complications, robot-assisted procedures were also associated with faster recovery, as measured by days to first flatus, at a median of 1 vs. 2 for laparoscopy and 3 for open procedures (P less than .001 for robots vs. each other surgery type). The robotic surgery was also significantly associated with fewer days to first automatic urination (median 2 vs. 4 for each of the other procedures; P less than .001), and with fewer days to discharge (median 5 vs. 7 for the other procedures; P less than .001).
"These are excellent postoperative complication rates reported, especially for the robotic treatment group, commented Thomas Gruenberger, MD, an oncologic surgeon at Rudolf Hospital in Vienna, in a poster discussion session.
“We all are now favoring laparoscopic surgery for these kinds of patients. The robot is a nice thing to have; however, we cannot use it in every hospital because it’s still quite expensive,” he said.
“We require – and this is a secondary endpoint of the study – long-term follow-up for local and distant outcomes,” he added,
The investigators did not report a funding source. All authors declared having no conflicts of interest. Dr. Gruenberger disclosed research funding, speakers bureau participation, and/or advisory roles with several companies.
AT ESMO 2017
Transoral robotic surgery assessed for oral lesions
A single-arm study is being conducted at the Ohio State University Comprehensive Cancer Center, Columbus, to assess transoral robotic surgery (TORS) for oral and laryngopharyngeal benign and malignant lesions using the da Vinci Robotic Surgical System.
The study started in 2007, and the estimated completion date is December 2020. Investigators hope to enroll 360 adults.
Patients are scheduled for regular postop visits to assess quality of life and other matters. Those unable to return to Ohio State University are contacted by phone or provided with the questionnaire by mail.
A single-arm study is being conducted at the Ohio State University Comprehensive Cancer Center, Columbus, to assess transoral robotic surgery (TORS) for oral and laryngopharyngeal benign and malignant lesions using the da Vinci Robotic Surgical System.
The study started in 2007, and the estimated completion date is December 2020. Investigators hope to enroll 360 adults.
Patients are scheduled for regular postop visits to assess quality of life and other matters. Those unable to return to Ohio State University are contacted by phone or provided with the questionnaire by mail.
A single-arm study is being conducted at the Ohio State University Comprehensive Cancer Center, Columbus, to assess transoral robotic surgery (TORS) for oral and laryngopharyngeal benign and malignant lesions using the da Vinci Robotic Surgical System.
The study started in 2007, and the estimated completion date is December 2020. Investigators hope to enroll 360 adults.
Patients are scheduled for regular postop visits to assess quality of life and other matters. Those unable to return to Ohio State University are contacted by phone or provided with the questionnaire by mail.





