User login
Direct oral anticoagulants: Competition brought no cost relief
Medicare Part D spending for oral anticoagulants has risen by almost 1,600% since 2011, while the number of users has increased by just 95%, according to a new study.
In 2011, the year after the first direct oral anticoagulant (DOACs) was approved, Medicare Part D spent $0.44 billion on all oral anticoagulants. By 2019, when there a total of four DOACs on the market, spending was $7.38 billion, an increase of 1,577%, Aaron Troy, MD, MPH, and Timothy S. Anderson, MD, MAS, said in JAMA Health Forum.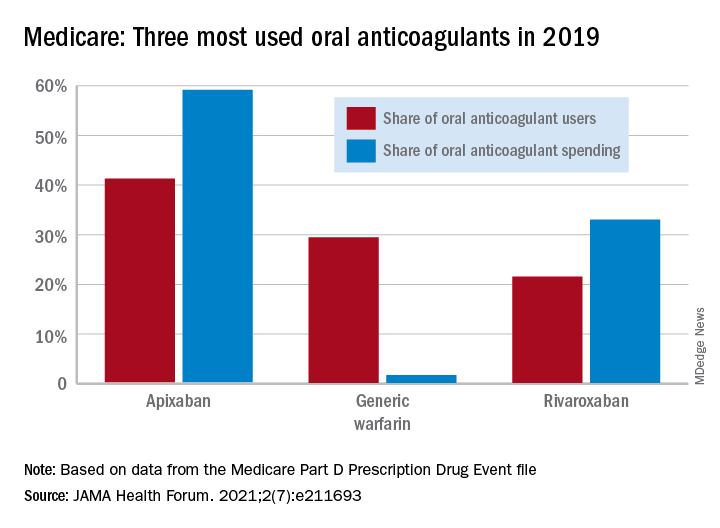
Over that same time, the number of beneficiaries using oral anticoagulants went from 2.68 million to 5.24 million, they said, based on data from the Medicare Part D Prescription Drug Event file.
“While higher prices for novel therapeutics like DOACs, which offer clear benefits, such as decreased drug-drug interactions and improved persistence, may partly reflect value and help drive innovation, the patterns and effects of spending on novel medications still merit attention,” they noted.
One pattern of use looked like this: 0.2 million Medicare beneficiaries took DOACs in 2011,compared with 3.5 million in 2019, while the number of warfarin users dropped from 2.48 million to 1.74 million, the investigators reported.
As for spending over the study period, the cost to treat one beneficiary with atrial fibrillation increased by 9.3% each year for apixaban (a DOAC that was the most popular oral anticoagulant in 2019), decreased 27.6% per year for generic warfarin, and increased 9.5% per year for rivaroxaban, said Dr. Troy and Dr. Anderson of Beth Israel Deaconess Medical Center, Boston.
Rising Part D enrollment had an effect on spending growth, as did increased use of oral anticoagulants in general. The introduction of competing DOACs, however, “did not substantially curb annual spending increases, suggesting a lack of price competition, which is consistent with trends observed in other therapeutic categories,” they wrote.
Dr. Anderson has received research grants from the National Institute on Aging and the American College of Cardiology outside of this study and honoraria from Alosa Health. No other disclosures were reported.
Medicare Part D spending for oral anticoagulants has risen by almost 1,600% since 2011, while the number of users has increased by just 95%, according to a new study.
In 2011, the year after the first direct oral anticoagulant (DOACs) was approved, Medicare Part D spent $0.44 billion on all oral anticoagulants. By 2019, when there a total of four DOACs on the market, spending was $7.38 billion, an increase of 1,577%, Aaron Troy, MD, MPH, and Timothy S. Anderson, MD, MAS, said in JAMA Health Forum.
Over that same time, the number of beneficiaries using oral anticoagulants went from 2.68 million to 5.24 million, they said, based on data from the Medicare Part D Prescription Drug Event file.
“While higher prices for novel therapeutics like DOACs, which offer clear benefits, such as decreased drug-drug interactions and improved persistence, may partly reflect value and help drive innovation, the patterns and effects of spending on novel medications still merit attention,” they noted.
One pattern of use looked like this: 0.2 million Medicare beneficiaries took DOACs in 2011,compared with 3.5 million in 2019, while the number of warfarin users dropped from 2.48 million to 1.74 million, the investigators reported.
As for spending over the study period, the cost to treat one beneficiary with atrial fibrillation increased by 9.3% each year for apixaban (a DOAC that was the most popular oral anticoagulant in 2019), decreased 27.6% per year for generic warfarin, and increased 9.5% per year for rivaroxaban, said Dr. Troy and Dr. Anderson of Beth Israel Deaconess Medical Center, Boston.
Rising Part D enrollment had an effect on spending growth, as did increased use of oral anticoagulants in general. The introduction of competing DOACs, however, “did not substantially curb annual spending increases, suggesting a lack of price competition, which is consistent with trends observed in other therapeutic categories,” they wrote.
Dr. Anderson has received research grants from the National Institute on Aging and the American College of Cardiology outside of this study and honoraria from Alosa Health. No other disclosures were reported.
Medicare Part D spending for oral anticoagulants has risen by almost 1,600% since 2011, while the number of users has increased by just 95%, according to a new study.
In 2011, the year after the first direct oral anticoagulant (DOACs) was approved, Medicare Part D spent $0.44 billion on all oral anticoagulants. By 2019, when there a total of four DOACs on the market, spending was $7.38 billion, an increase of 1,577%, Aaron Troy, MD, MPH, and Timothy S. Anderson, MD, MAS, said in JAMA Health Forum.
Over that same time, the number of beneficiaries using oral anticoagulants went from 2.68 million to 5.24 million, they said, based on data from the Medicare Part D Prescription Drug Event file.
“While higher prices for novel therapeutics like DOACs, which offer clear benefits, such as decreased drug-drug interactions and improved persistence, may partly reflect value and help drive innovation, the patterns and effects of spending on novel medications still merit attention,” they noted.
One pattern of use looked like this: 0.2 million Medicare beneficiaries took DOACs in 2011,compared with 3.5 million in 2019, while the number of warfarin users dropped from 2.48 million to 1.74 million, the investigators reported.
As for spending over the study period, the cost to treat one beneficiary with atrial fibrillation increased by 9.3% each year for apixaban (a DOAC that was the most popular oral anticoagulant in 2019), decreased 27.6% per year for generic warfarin, and increased 9.5% per year for rivaroxaban, said Dr. Troy and Dr. Anderson of Beth Israel Deaconess Medical Center, Boston.
Rising Part D enrollment had an effect on spending growth, as did increased use of oral anticoagulants in general. The introduction of competing DOACs, however, “did not substantially curb annual spending increases, suggesting a lack of price competition, which is consistent with trends observed in other therapeutic categories,” they wrote.
Dr. Anderson has received research grants from the National Institute on Aging and the American College of Cardiology outside of this study and honoraria from Alosa Health. No other disclosures were reported.
FROM JAMA HEALTH FORUM
Younger adults with HIV have higher CVD risk but low ASCVD scores
People age 40 and younger living with HIV have a higher risk for heart disease than even their over-40 peers living with HIV – and that risk was 54% higher than the general public.
And this was among people without traditional heart disease risks, such as smoking and obesity.
“What’s surprising is that not only do we see that, yes, they do have increased risk, but this is after controlling for all of that – which means the mechanism underlying this risk,” said Tiffany Gooden, MPH and a PhD candidate at the University of Birmingham, England, who presented the data at the 11th International AIDS Society Conference on HIV Science (IAS 2021).
“If we’re using a non–HIV-validated assessment tool, you should always know that there could be a risk that you are under-recognizing,” she added.
Right now, there’s not a lot to aid clinicians in ferreting out this increased risk. Traditional cardiovascular risk assessment tools, like Framingham risk scores and atherosclerotic cardiovascular disease risk score from the American College of Cardiology, have been found to overlook the real risk of cardiovascular disease in people living with HIV. Plus, most guidelines, including those from the British HIV Medical Association and the American College of Cardiology, primarily focus screening on people 40 or older.
Ms. Gooden’s study drew data from The Health Improvement Network (THIN) database, which combines data from 800 primary care practices in the United Kingdom. Looking at data between January 2000 and January 2020, the investigators compared each person living with HIV with four peers not living with HIV, matched for age, gender, and practice. In total, 9,233 people living with HIV and 35,721 people without HIV were included in the analysis. Median age of participants was 41 years in people living with HIV and 40.4 years in people without HIV. About 35% of participants in both arms were women, and a greater proportion of participants living with HIV were Black, accounting for 22.5% of people living with HIV, versus 3.8% of the general population. Fewer people living with HIV were overweight or obese compared to people without HIV.
Researchers then tracked participants over time to identify the incidence of heart attack, stroke, peripheral vascular disease, and heart failure, as well as common risk factors for heart problems, such as high blood pressure, type 2 diabetes, chronic kidney disease, atrial fibrillation, and use of a lipid-lowering drug such as a statin.
The investigators then sectioned the data on heart disease risk by decade – 2000-2009 and 2010-2019 – in order to separate the potential impact of antiretroviral treatment (ART) drugs, from early combinations that have been associated with cardiovascular disease, to current drugs that are less likely to have that effect.
Overall risk for any kind of cardiovascular disease was 54% higher among people living with HIV of any age, compared to their age- and risk-matched peers. And when they broke the data down by age, they found that people younger than 40 had nearly twice the risk for any heart disease as their HIV-negative peers, which was a numerically higher risk than for people older than 40 – though not significantly so.
People living with HIV also had a 49% increased risk for stroke and a 59% increased risk for ischemic heart disease but no increased risk for peripheral vascular disease, heart failure, or heart attack. But the confidence intervals here were wide, “which may indicate lack of power and therefore not be conclusive,” Ms. Gooden said.
People living with HIV also had a 37% increased risk for hypertension, were 96% more likely to be prescribed lipid-lowering drugs, 2.4-times more likely to have chronic kidney disease, and 2.68-times more likely to experience all-cause mortality. The study couldn’t account for the type of HIV medications people living with the virus used, their viral load, or their CD4 counts – all of which have been found in previous studies to contribute to heart disease in people with HIV.
“That was the biggest limitation of our study,” Ms. Gooden said in an interview. “The fact that the risk of cardiovascular disease remains the same in the [first decade] and the later decade goes to show that even if antiretroviral therapies contributed to that … now or 20 years ago, it’s still not the entire reason for the risk.”
Steven Grinspoon, MD, of Harvard Medical School, Boston, is the lead author on the REPRIEVE trial, now testing statins as a treatment for people like those in this study. He told this news organization that this large analysis had one of the youngest cohorts of people living with HIV he’d seen to explore these issues. Additionally, it backs up what the team recently reported in the Journal of the American Medical Association – that plaque was present in 49% of 755 people living with HIV, despite having risk scores for cardiovascular disease in the normal range. This was as true for people younger than 40 as those older than 40.
For primary care clinicians, the message is that even relatively young people with HIV should be counseled early and often about amending traditional risk factors, while we wait for the results of REPRIEVE to say whether statins improve outcomes for people living with HIV, Dr. Grinspoon said in an interview.
“Sometimes physicians and primary care providers say, ‘Well I’ll focus my hypertension efforts on older people, who are closer to having heart attacks,’” Dr. Grinspoon said. “But this data suggests we should pay attention even in young people … and pay particular attention to women who wouldn’t have traditional risk scores that were very high at all, largely because they are women.”
The study was funded by Merck. Ms. Gooden has disclosed no relevant financial relationships. Dr. Grinspoon reports receiving personal and consulting fees from Theratechnologies and ViiV Healthcare.
A version of this article first appeared on Medscape.com.
People age 40 and younger living with HIV have a higher risk for heart disease than even their over-40 peers living with HIV – and that risk was 54% higher than the general public.
And this was among people without traditional heart disease risks, such as smoking and obesity.
“What’s surprising is that not only do we see that, yes, they do have increased risk, but this is after controlling for all of that – which means the mechanism underlying this risk,” said Tiffany Gooden, MPH and a PhD candidate at the University of Birmingham, England, who presented the data at the 11th International AIDS Society Conference on HIV Science (IAS 2021).
“If we’re using a non–HIV-validated assessment tool, you should always know that there could be a risk that you are under-recognizing,” she added.
Right now, there’s not a lot to aid clinicians in ferreting out this increased risk. Traditional cardiovascular risk assessment tools, like Framingham risk scores and atherosclerotic cardiovascular disease risk score from the American College of Cardiology, have been found to overlook the real risk of cardiovascular disease in people living with HIV. Plus, most guidelines, including those from the British HIV Medical Association and the American College of Cardiology, primarily focus screening on people 40 or older.
Ms. Gooden’s study drew data from The Health Improvement Network (THIN) database, which combines data from 800 primary care practices in the United Kingdom. Looking at data between January 2000 and January 2020, the investigators compared each person living with HIV with four peers not living with HIV, matched for age, gender, and practice. In total, 9,233 people living with HIV and 35,721 people without HIV were included in the analysis. Median age of participants was 41 years in people living with HIV and 40.4 years in people without HIV. About 35% of participants in both arms were women, and a greater proportion of participants living with HIV were Black, accounting for 22.5% of people living with HIV, versus 3.8% of the general population. Fewer people living with HIV were overweight or obese compared to people without HIV.
Researchers then tracked participants over time to identify the incidence of heart attack, stroke, peripheral vascular disease, and heart failure, as well as common risk factors for heart problems, such as high blood pressure, type 2 diabetes, chronic kidney disease, atrial fibrillation, and use of a lipid-lowering drug such as a statin.
The investigators then sectioned the data on heart disease risk by decade – 2000-2009 and 2010-2019 – in order to separate the potential impact of antiretroviral treatment (ART) drugs, from early combinations that have been associated with cardiovascular disease, to current drugs that are less likely to have that effect.
Overall risk for any kind of cardiovascular disease was 54% higher among people living with HIV of any age, compared to their age- and risk-matched peers. And when they broke the data down by age, they found that people younger than 40 had nearly twice the risk for any heart disease as their HIV-negative peers, which was a numerically higher risk than for people older than 40 – though not significantly so.
People living with HIV also had a 49% increased risk for stroke and a 59% increased risk for ischemic heart disease but no increased risk for peripheral vascular disease, heart failure, or heart attack. But the confidence intervals here were wide, “which may indicate lack of power and therefore not be conclusive,” Ms. Gooden said.
People living with HIV also had a 37% increased risk for hypertension, were 96% more likely to be prescribed lipid-lowering drugs, 2.4-times more likely to have chronic kidney disease, and 2.68-times more likely to experience all-cause mortality. The study couldn’t account for the type of HIV medications people living with the virus used, their viral load, or their CD4 counts – all of which have been found in previous studies to contribute to heart disease in people with HIV.
“That was the biggest limitation of our study,” Ms. Gooden said in an interview. “The fact that the risk of cardiovascular disease remains the same in the [first decade] and the later decade goes to show that even if antiretroviral therapies contributed to that … now or 20 years ago, it’s still not the entire reason for the risk.”
Steven Grinspoon, MD, of Harvard Medical School, Boston, is the lead author on the REPRIEVE trial, now testing statins as a treatment for people like those in this study. He told this news organization that this large analysis had one of the youngest cohorts of people living with HIV he’d seen to explore these issues. Additionally, it backs up what the team recently reported in the Journal of the American Medical Association – that plaque was present in 49% of 755 people living with HIV, despite having risk scores for cardiovascular disease in the normal range. This was as true for people younger than 40 as those older than 40.
For primary care clinicians, the message is that even relatively young people with HIV should be counseled early and often about amending traditional risk factors, while we wait for the results of REPRIEVE to say whether statins improve outcomes for people living with HIV, Dr. Grinspoon said in an interview.
“Sometimes physicians and primary care providers say, ‘Well I’ll focus my hypertension efforts on older people, who are closer to having heart attacks,’” Dr. Grinspoon said. “But this data suggests we should pay attention even in young people … and pay particular attention to women who wouldn’t have traditional risk scores that were very high at all, largely because they are women.”
The study was funded by Merck. Ms. Gooden has disclosed no relevant financial relationships. Dr. Grinspoon reports receiving personal and consulting fees from Theratechnologies and ViiV Healthcare.
A version of this article first appeared on Medscape.com.
People age 40 and younger living with HIV have a higher risk for heart disease than even their over-40 peers living with HIV – and that risk was 54% higher than the general public.
And this was among people without traditional heart disease risks, such as smoking and obesity.
“What’s surprising is that not only do we see that, yes, they do have increased risk, but this is after controlling for all of that – which means the mechanism underlying this risk,” said Tiffany Gooden, MPH and a PhD candidate at the University of Birmingham, England, who presented the data at the 11th International AIDS Society Conference on HIV Science (IAS 2021).
“If we’re using a non–HIV-validated assessment tool, you should always know that there could be a risk that you are under-recognizing,” she added.
Right now, there’s not a lot to aid clinicians in ferreting out this increased risk. Traditional cardiovascular risk assessment tools, like Framingham risk scores and atherosclerotic cardiovascular disease risk score from the American College of Cardiology, have been found to overlook the real risk of cardiovascular disease in people living with HIV. Plus, most guidelines, including those from the British HIV Medical Association and the American College of Cardiology, primarily focus screening on people 40 or older.
Ms. Gooden’s study drew data from The Health Improvement Network (THIN) database, which combines data from 800 primary care practices in the United Kingdom. Looking at data between January 2000 and January 2020, the investigators compared each person living with HIV with four peers not living with HIV, matched for age, gender, and practice. In total, 9,233 people living with HIV and 35,721 people without HIV were included in the analysis. Median age of participants was 41 years in people living with HIV and 40.4 years in people without HIV. About 35% of participants in both arms were women, and a greater proportion of participants living with HIV were Black, accounting for 22.5% of people living with HIV, versus 3.8% of the general population. Fewer people living with HIV were overweight or obese compared to people without HIV.
Researchers then tracked participants over time to identify the incidence of heart attack, stroke, peripheral vascular disease, and heart failure, as well as common risk factors for heart problems, such as high blood pressure, type 2 diabetes, chronic kidney disease, atrial fibrillation, and use of a lipid-lowering drug such as a statin.
The investigators then sectioned the data on heart disease risk by decade – 2000-2009 and 2010-2019 – in order to separate the potential impact of antiretroviral treatment (ART) drugs, from early combinations that have been associated with cardiovascular disease, to current drugs that are less likely to have that effect.
Overall risk for any kind of cardiovascular disease was 54% higher among people living with HIV of any age, compared to their age- and risk-matched peers. And when they broke the data down by age, they found that people younger than 40 had nearly twice the risk for any heart disease as their HIV-negative peers, which was a numerically higher risk than for people older than 40 – though not significantly so.
People living with HIV also had a 49% increased risk for stroke and a 59% increased risk for ischemic heart disease but no increased risk for peripheral vascular disease, heart failure, or heart attack. But the confidence intervals here were wide, “which may indicate lack of power and therefore not be conclusive,” Ms. Gooden said.
People living with HIV also had a 37% increased risk for hypertension, were 96% more likely to be prescribed lipid-lowering drugs, 2.4-times more likely to have chronic kidney disease, and 2.68-times more likely to experience all-cause mortality. The study couldn’t account for the type of HIV medications people living with the virus used, their viral load, or their CD4 counts – all of which have been found in previous studies to contribute to heart disease in people with HIV.
“That was the biggest limitation of our study,” Ms. Gooden said in an interview. “The fact that the risk of cardiovascular disease remains the same in the [first decade] and the later decade goes to show that even if antiretroviral therapies contributed to that … now or 20 years ago, it’s still not the entire reason for the risk.”
Steven Grinspoon, MD, of Harvard Medical School, Boston, is the lead author on the REPRIEVE trial, now testing statins as a treatment for people like those in this study. He told this news organization that this large analysis had one of the youngest cohorts of people living with HIV he’d seen to explore these issues. Additionally, it backs up what the team recently reported in the Journal of the American Medical Association – that plaque was present in 49% of 755 people living with HIV, despite having risk scores for cardiovascular disease in the normal range. This was as true for people younger than 40 as those older than 40.
For primary care clinicians, the message is that even relatively young people with HIV should be counseled early and often about amending traditional risk factors, while we wait for the results of REPRIEVE to say whether statins improve outcomes for people living with HIV, Dr. Grinspoon said in an interview.
“Sometimes physicians and primary care providers say, ‘Well I’ll focus my hypertension efforts on older people, who are closer to having heart attacks,’” Dr. Grinspoon said. “But this data suggests we should pay attention even in young people … and pay particular attention to women who wouldn’t have traditional risk scores that were very high at all, largely because they are women.”
The study was funded by Merck. Ms. Gooden has disclosed no relevant financial relationships. Dr. Grinspoon reports receiving personal and consulting fees from Theratechnologies and ViiV Healthcare.
A version of this article first appeared on Medscape.com.
Statins again linked to lower COVID-19 mortality
Among patients hospitalized for COVID-19, those who had been taking statins had a substantially lower risk of death in a new large observational study.
Results showed that use of statins prior to admission was linked to a greater than 40% reduction in mortality and a greater than 25% reduction in risk of developing a severe outcome.
The findings come an analysis of data from the American Heart Association’s COVID-19 Cardiovascular Disease Registry on more than 10,000 patients hospitalized with COVID-19 at 104 hospitals across the United States published in PLoS One.
While several other studies have suggested benefits of statins in COVID-19, this is by far the largest study so far on this topic.
“I would say this is the most reliable study on statins in COVID-19 to date, with the results adjusted for many confounders, including socioeconomic factors and insurance type,” lead author Lori B. Daniels, MD, told this news organization. “However, it still an observational study and therefore falls short of a randomized study. But I would think a randomized study of statins in COVID-19 is probably not feasible, so this study provides excellent data at an observational level.”
After propensity matching for cardiovascular disease, results showed that most of the benefit of statins occurred in patients with known cardiovascular disease.
“While most patients taking statins will have cardiovascular disease, there are also many patients who take these drugs who don’t have heart disease but do have cardiovascular risk factors, such as those with raised cholesterol, or a family history of cardiovascular disease. For [such patients], the effect of statins was also in the same direction but it was not significant. This doesn’t exclude an effect,” noted Dr. Daniels, who is professor of medicine and director of cardiovascular intensive care at the University of California, San Diego.
“We are not saying that everyone should rush out and take a statin if they do not have risk factors for cardiovascular in order to lower their risk of dying from COVID. But if individuals do have an indication for a statin and are not taking one of these dugs this is another good reason to start taking them now,” she added.
The investigators embarked on the study because, although previous observational studies have found that statins may reduce the severity of COVID-19 infection, these studies have been limited in size with mostly single-center or regional studies, and some results have been conflicting. They therefore conducted the current, much larger analysis, in the AHA COVID-19 CVD Registry which systematically collected hospitalized patient–level data in a broad and diverse hospital and patient population across the United States.
For the analysis, the researchers analyzed data from 10,541 patients hospitalized with COVID-19 through September 2020 at 104 U.S. hospitals enrolled in the AHA registry to evaluate the associations between statin use and outcomes.
Most patients (71%) had either cardiovascular disease, hypertension, or both. Prior to admission, 42% of subjects used statins, with 7% being on statins alone and 35% on statins plus antihypertensives. Death (or discharge to hospice) occurred in 2,212 subjects (21%).
Results showed that outpatient use of statins, either alone or with antihypertensives, was associated with a 41% reduced risk of death (odds ratio, 0.59; 95% confidence interval, 0.50-0.69), after adjusting for demographic characteristics, underlying conditions, insurance status, hospital site, and concurrent medications. Statin use was also associated with a roughly 25% lower adjusted odds of developing severe disease.
Noting that patients on statins are also likely to be on antihypertensive medication, the researchers found that the statin benefit on mortality was seen in both patients taking a statin alone (OR, 0.54) and in those taking statins with an antihypertensive medication (OR, 0.60).
Use of antihypertensive drugs was associated with a smaller, albeit still substantial, 27% lower odds of death (OR, 0.73; 95% CI, 0.62-0.87).
In propensity-matched analyses, use of statins and/or antihypertensives was tied to a 32% reduced risk of death among those with a history of CVD and/or hypertension (OR, 0.68; 95% CI, 0.58-0.81). An observed 16% reduction in odds of death with statins and/or antihypertensive drugs among those without cardiovascular disease and/or hypertension was not statistically significant (OR, 0.84; 95% CI, 0.58-1.22).
Stabilizing the underlying disease
The researchers pointed out that the results of the propensity matching analysis are consistent with the hypothesis that the major benefit of these medications accrues from treating and/or stabilizing underlying disease.
“Although it is well known that statins improve long-term outcomes among patients with or at elevated risk for cardiovascular disease, the association with a large short-term benefit which accrues in the setting of hospitalization for COVID-19 is a new and intriguing finding,” they said.
They cited several “plausible mechanisms whereby statins could directly mitigate outcomes in COVID-19 beyond treating underlying disease conditions,” including anti-inflammatory effects and a direct inhibitory effect on the SARS-CoV-2 virus.
Dr. Daniels elaborated more on the potential mechanism at play in an interview: “I think what is happening is that the statin is stabilizing the coronary disease so patients are less likely to die from MI or stroke, and this gives them more time and strength to recover from COVID-19.”
She added: “Statins may also have some direct anti-COVID effects such as an anti-inflammatory actions, but I would guess that this is probably not the primary effect behind what we’re seeing here.”
‘Important clinical implications’
The authors say their findings have “important clinical implications.”
They noted that early in the pandemic there was speculation that certain medications, including statins, and the ACE inhibitor/angiotensin receptor blocker (ARB) classes of antihypertensives may confer an increased susceptibility to COVID-19 positivity and/or severity.
“Our study reinforces the AHA and others’ recommendations that not only is it safe to remain on these medications, but they may substantially reduce risk of severe COVID-19 and especially death from COVID-19, particularly statins, and particularly among those with associated underlying conditions,” the authors stressed.
Dr. Daniels added that, although statins are very safe drugs, there are always some patients who prefer not to take medication even if indicated, and others who may have borderline indications and decide not to take a statin at present.
“This study may persuade these patients that taking a statin is the right thing to do. It may give those patients on the cusp of thinking about taking one of these drugs a reason to go ahead,” she said.
‘Provocative but not definitive’
Commenting on the study, Robert Harrington, MD, professor of medicine and chair of the department of medicine at Stanford (Calif.) University, said: “These are interesting observational data but as such have all the limitations of nonrandomized comparisons despite the best attempts to adjust for a variety of potential confounders. For example, is this an effect of statins (perhaps through some anti-inflammatory mechanism) or is it more an effect that can be attributed to the patients who are prescribed and taking a statin, compared with those who are not?”
He added: “The primary clinical benefit of statins, based on many large randomized clinical trials, seems to be derived from their LDL lowering effect. Observational studies have suggested potential benefits from anti-inflammatory effects of statins, but the randomized trials have not confirmed these observations. So, the current data are interesting, even provocative, but ultimately hypothesis generating rather than definitive.”
Also commenting on the study, Steven Nissen, MD, professor of medicine at the Cleveland Clinic, said: “While statins have many established benefits, their role in preventing COVID-19 complications is very speculative. Like all observational studies, the current study must be viewed as hypothesis generating, not definitive evidence of benefit. There are many potential confounders. I’m skeptical.”
The authors of this study received no specific funding for this work and report no competing interests. Dr. Harrington was AHA president when the COVID registry was created and he is still a member of the AHA board, which has oversight over the project.
Among patients hospitalized for COVID-19, those who had been taking statins had a substantially lower risk of death in a new large observational study.
Results showed that use of statins prior to admission was linked to a greater than 40% reduction in mortality and a greater than 25% reduction in risk of developing a severe outcome.
The findings come an analysis of data from the American Heart Association’s COVID-19 Cardiovascular Disease Registry on more than 10,000 patients hospitalized with COVID-19 at 104 hospitals across the United States published in PLoS One.
While several other studies have suggested benefits of statins in COVID-19, this is by far the largest study so far on this topic.
“I would say this is the most reliable study on statins in COVID-19 to date, with the results adjusted for many confounders, including socioeconomic factors and insurance type,” lead author Lori B. Daniels, MD, told this news organization. “However, it still an observational study and therefore falls short of a randomized study. But I would think a randomized study of statins in COVID-19 is probably not feasible, so this study provides excellent data at an observational level.”
After propensity matching for cardiovascular disease, results showed that most of the benefit of statins occurred in patients with known cardiovascular disease.
“While most patients taking statins will have cardiovascular disease, there are also many patients who take these drugs who don’t have heart disease but do have cardiovascular risk factors, such as those with raised cholesterol, or a family history of cardiovascular disease. For [such patients], the effect of statins was also in the same direction but it was not significant. This doesn’t exclude an effect,” noted Dr. Daniels, who is professor of medicine and director of cardiovascular intensive care at the University of California, San Diego.
“We are not saying that everyone should rush out and take a statin if they do not have risk factors for cardiovascular in order to lower their risk of dying from COVID. But if individuals do have an indication for a statin and are not taking one of these dugs this is another good reason to start taking them now,” she added.
The investigators embarked on the study because, although previous observational studies have found that statins may reduce the severity of COVID-19 infection, these studies have been limited in size with mostly single-center or regional studies, and some results have been conflicting. They therefore conducted the current, much larger analysis, in the AHA COVID-19 CVD Registry which systematically collected hospitalized patient–level data in a broad and diverse hospital and patient population across the United States.
For the analysis, the researchers analyzed data from 10,541 patients hospitalized with COVID-19 through September 2020 at 104 U.S. hospitals enrolled in the AHA registry to evaluate the associations between statin use and outcomes.
Most patients (71%) had either cardiovascular disease, hypertension, or both. Prior to admission, 42% of subjects used statins, with 7% being on statins alone and 35% on statins plus antihypertensives. Death (or discharge to hospice) occurred in 2,212 subjects (21%).
Results showed that outpatient use of statins, either alone or with antihypertensives, was associated with a 41% reduced risk of death (odds ratio, 0.59; 95% confidence interval, 0.50-0.69), after adjusting for demographic characteristics, underlying conditions, insurance status, hospital site, and concurrent medications. Statin use was also associated with a roughly 25% lower adjusted odds of developing severe disease.
Noting that patients on statins are also likely to be on antihypertensive medication, the researchers found that the statin benefit on mortality was seen in both patients taking a statin alone (OR, 0.54) and in those taking statins with an antihypertensive medication (OR, 0.60).
Use of antihypertensive drugs was associated with a smaller, albeit still substantial, 27% lower odds of death (OR, 0.73; 95% CI, 0.62-0.87).
In propensity-matched analyses, use of statins and/or antihypertensives was tied to a 32% reduced risk of death among those with a history of CVD and/or hypertension (OR, 0.68; 95% CI, 0.58-0.81). An observed 16% reduction in odds of death with statins and/or antihypertensive drugs among those without cardiovascular disease and/or hypertension was not statistically significant (OR, 0.84; 95% CI, 0.58-1.22).
Stabilizing the underlying disease
The researchers pointed out that the results of the propensity matching analysis are consistent with the hypothesis that the major benefit of these medications accrues from treating and/or stabilizing underlying disease.
“Although it is well known that statins improve long-term outcomes among patients with or at elevated risk for cardiovascular disease, the association with a large short-term benefit which accrues in the setting of hospitalization for COVID-19 is a new and intriguing finding,” they said.
They cited several “plausible mechanisms whereby statins could directly mitigate outcomes in COVID-19 beyond treating underlying disease conditions,” including anti-inflammatory effects and a direct inhibitory effect on the SARS-CoV-2 virus.
Dr. Daniels elaborated more on the potential mechanism at play in an interview: “I think what is happening is that the statin is stabilizing the coronary disease so patients are less likely to die from MI or stroke, and this gives them more time and strength to recover from COVID-19.”
She added: “Statins may also have some direct anti-COVID effects such as an anti-inflammatory actions, but I would guess that this is probably not the primary effect behind what we’re seeing here.”
‘Important clinical implications’
The authors say their findings have “important clinical implications.”
They noted that early in the pandemic there was speculation that certain medications, including statins, and the ACE inhibitor/angiotensin receptor blocker (ARB) classes of antihypertensives may confer an increased susceptibility to COVID-19 positivity and/or severity.
“Our study reinforces the AHA and others’ recommendations that not only is it safe to remain on these medications, but they may substantially reduce risk of severe COVID-19 and especially death from COVID-19, particularly statins, and particularly among those with associated underlying conditions,” the authors stressed.
Dr. Daniels added that, although statins are very safe drugs, there are always some patients who prefer not to take medication even if indicated, and others who may have borderline indications and decide not to take a statin at present.
“This study may persuade these patients that taking a statin is the right thing to do. It may give those patients on the cusp of thinking about taking one of these drugs a reason to go ahead,” she said.
‘Provocative but not definitive’
Commenting on the study, Robert Harrington, MD, professor of medicine and chair of the department of medicine at Stanford (Calif.) University, said: “These are interesting observational data but as such have all the limitations of nonrandomized comparisons despite the best attempts to adjust for a variety of potential confounders. For example, is this an effect of statins (perhaps through some anti-inflammatory mechanism) or is it more an effect that can be attributed to the patients who are prescribed and taking a statin, compared with those who are not?”
He added: “The primary clinical benefit of statins, based on many large randomized clinical trials, seems to be derived from their LDL lowering effect. Observational studies have suggested potential benefits from anti-inflammatory effects of statins, but the randomized trials have not confirmed these observations. So, the current data are interesting, even provocative, but ultimately hypothesis generating rather than definitive.”
Also commenting on the study, Steven Nissen, MD, professor of medicine at the Cleveland Clinic, said: “While statins have many established benefits, their role in preventing COVID-19 complications is very speculative. Like all observational studies, the current study must be viewed as hypothesis generating, not definitive evidence of benefit. There are many potential confounders. I’m skeptical.”
The authors of this study received no specific funding for this work and report no competing interests. Dr. Harrington was AHA president when the COVID registry was created and he is still a member of the AHA board, which has oversight over the project.
Among patients hospitalized for COVID-19, those who had been taking statins had a substantially lower risk of death in a new large observational study.
Results showed that use of statins prior to admission was linked to a greater than 40% reduction in mortality and a greater than 25% reduction in risk of developing a severe outcome.
The findings come an analysis of data from the American Heart Association’s COVID-19 Cardiovascular Disease Registry on more than 10,000 patients hospitalized with COVID-19 at 104 hospitals across the United States published in PLoS One.
While several other studies have suggested benefits of statins in COVID-19, this is by far the largest study so far on this topic.
“I would say this is the most reliable study on statins in COVID-19 to date, with the results adjusted for many confounders, including socioeconomic factors and insurance type,” lead author Lori B. Daniels, MD, told this news organization. “However, it still an observational study and therefore falls short of a randomized study. But I would think a randomized study of statins in COVID-19 is probably not feasible, so this study provides excellent data at an observational level.”
After propensity matching for cardiovascular disease, results showed that most of the benefit of statins occurred in patients with known cardiovascular disease.
“While most patients taking statins will have cardiovascular disease, there are also many patients who take these drugs who don’t have heart disease but do have cardiovascular risk factors, such as those with raised cholesterol, or a family history of cardiovascular disease. For [such patients], the effect of statins was also in the same direction but it was not significant. This doesn’t exclude an effect,” noted Dr. Daniels, who is professor of medicine and director of cardiovascular intensive care at the University of California, San Diego.
“We are not saying that everyone should rush out and take a statin if they do not have risk factors for cardiovascular in order to lower their risk of dying from COVID. But if individuals do have an indication for a statin and are not taking one of these dugs this is another good reason to start taking them now,” she added.
The investigators embarked on the study because, although previous observational studies have found that statins may reduce the severity of COVID-19 infection, these studies have been limited in size with mostly single-center or regional studies, and some results have been conflicting. They therefore conducted the current, much larger analysis, in the AHA COVID-19 CVD Registry which systematically collected hospitalized patient–level data in a broad and diverse hospital and patient population across the United States.
For the analysis, the researchers analyzed data from 10,541 patients hospitalized with COVID-19 through September 2020 at 104 U.S. hospitals enrolled in the AHA registry to evaluate the associations between statin use and outcomes.
Most patients (71%) had either cardiovascular disease, hypertension, or both. Prior to admission, 42% of subjects used statins, with 7% being on statins alone and 35% on statins plus antihypertensives. Death (or discharge to hospice) occurred in 2,212 subjects (21%).
Results showed that outpatient use of statins, either alone or with antihypertensives, was associated with a 41% reduced risk of death (odds ratio, 0.59; 95% confidence interval, 0.50-0.69), after adjusting for demographic characteristics, underlying conditions, insurance status, hospital site, and concurrent medications. Statin use was also associated with a roughly 25% lower adjusted odds of developing severe disease.
Noting that patients on statins are also likely to be on antihypertensive medication, the researchers found that the statin benefit on mortality was seen in both patients taking a statin alone (OR, 0.54) and in those taking statins with an antihypertensive medication (OR, 0.60).
Use of antihypertensive drugs was associated with a smaller, albeit still substantial, 27% lower odds of death (OR, 0.73; 95% CI, 0.62-0.87).
In propensity-matched analyses, use of statins and/or antihypertensives was tied to a 32% reduced risk of death among those with a history of CVD and/or hypertension (OR, 0.68; 95% CI, 0.58-0.81). An observed 16% reduction in odds of death with statins and/or antihypertensive drugs among those without cardiovascular disease and/or hypertension was not statistically significant (OR, 0.84; 95% CI, 0.58-1.22).
Stabilizing the underlying disease
The researchers pointed out that the results of the propensity matching analysis are consistent with the hypothesis that the major benefit of these medications accrues from treating and/or stabilizing underlying disease.
“Although it is well known that statins improve long-term outcomes among patients with or at elevated risk for cardiovascular disease, the association with a large short-term benefit which accrues in the setting of hospitalization for COVID-19 is a new and intriguing finding,” they said.
They cited several “plausible mechanisms whereby statins could directly mitigate outcomes in COVID-19 beyond treating underlying disease conditions,” including anti-inflammatory effects and a direct inhibitory effect on the SARS-CoV-2 virus.
Dr. Daniels elaborated more on the potential mechanism at play in an interview: “I think what is happening is that the statin is stabilizing the coronary disease so patients are less likely to die from MI or stroke, and this gives them more time and strength to recover from COVID-19.”
She added: “Statins may also have some direct anti-COVID effects such as an anti-inflammatory actions, but I would guess that this is probably not the primary effect behind what we’re seeing here.”
‘Important clinical implications’
The authors say their findings have “important clinical implications.”
They noted that early in the pandemic there was speculation that certain medications, including statins, and the ACE inhibitor/angiotensin receptor blocker (ARB) classes of antihypertensives may confer an increased susceptibility to COVID-19 positivity and/or severity.
“Our study reinforces the AHA and others’ recommendations that not only is it safe to remain on these medications, but they may substantially reduce risk of severe COVID-19 and especially death from COVID-19, particularly statins, and particularly among those with associated underlying conditions,” the authors stressed.
Dr. Daniels added that, although statins are very safe drugs, there are always some patients who prefer not to take medication even if indicated, and others who may have borderline indications and decide not to take a statin at present.
“This study may persuade these patients that taking a statin is the right thing to do. It may give those patients on the cusp of thinking about taking one of these drugs a reason to go ahead,” she said.
‘Provocative but not definitive’
Commenting on the study, Robert Harrington, MD, professor of medicine and chair of the department of medicine at Stanford (Calif.) University, said: “These are interesting observational data but as such have all the limitations of nonrandomized comparisons despite the best attempts to adjust for a variety of potential confounders. For example, is this an effect of statins (perhaps through some anti-inflammatory mechanism) or is it more an effect that can be attributed to the patients who are prescribed and taking a statin, compared with those who are not?”
He added: “The primary clinical benefit of statins, based on many large randomized clinical trials, seems to be derived from their LDL lowering effect. Observational studies have suggested potential benefits from anti-inflammatory effects of statins, but the randomized trials have not confirmed these observations. So, the current data are interesting, even provocative, but ultimately hypothesis generating rather than definitive.”
Also commenting on the study, Steven Nissen, MD, professor of medicine at the Cleveland Clinic, said: “While statins have many established benefits, their role in preventing COVID-19 complications is very speculative. Like all observational studies, the current study must be viewed as hypothesis generating, not definitive evidence of benefit. There are many potential confounders. I’m skeptical.”
The authors of this study received no specific funding for this work and report no competing interests. Dr. Harrington was AHA president when the COVID registry was created and he is still a member of the AHA board, which has oversight over the project.
FROM PLOS ONE
Five risk factors may predict thrombus on LAA occlusion implants
, itself an important risk factor for cerebrovascular events, in patients with implants for left atrial appendage occlusion (LAAO), new research suggests.
The identified independent predictors of DRT in the largest dedicated multicenter LAAO-DRT registry to date were presence of a hypercoagulability disorder, pericardial effusion, renal insufficiency, an implantation depth greater than 10 mm from the pulmonary ridge, and presence of nonparoxysmal atrial fibrillation (AFib).
“Unfortunately, most of them are not modifiable, like hypercoaguable disorders or nonparoxysmal atrial fibrillation. But we can avoid deep implants because that’s been associated with creating a little bit of a crater or valley where the clot can form,” senior author Mohamad Alkhouli, MD, said in an interview.
But most important, and “really why we wanted to do this,” he said, is that “we want to give the patient a realistic prediction of adverse events for this procedure.”
LAAO has taken off in recent years for preventing thrombus formation and stroke in patients with AFib. Predicting DRT is a priority for the LAAO field, the authors note, especially given its expansion to younger, lower-risk patients and the increasing procedural volumes.
“This is a problem, DRT, that’s been discussed a lot because this is a preventative procedure,” observed Dr. Alkhouli, professor of medicine at Mayo Medical School, Rochester, Minn.
“The actual stroke risk every year – even if you don’t take any blood thinner and you have a CHADsVASc score of 9, the highest – is 11%. So if the chance of having thrombus is close, then that’s not a good tradeoff.”
Previous studies have also identified implantation depth and nonparoxysmal AFib as risk factors for DRT. But most of them have been small, he noted, with one of the largest reporting 65 DRTs in four prospective trials.
To cast a wider net, the investigators, led by Trevor Simard, MD, also from the Mayo Clinic, invited more than 50 international sites to contribute data to the registry. Of these, 37 centers reported on 237 DRTs and 474 device-matched control subjects from the same site.
Three-fourths of patients received a first-generation Watchman or a FLEX device (Boston Scientific).
Medical regimens were similar between the DRT and control cohorts at discharge after LAA closure. Most patients were managed with single (36.3%) or dual antiplatelet therapy (26.2%) at the time of DRT diagnosis.
As reported July 19 in the Journal of the American College of Cardiology, the timing of DRT development varied widely, with 24.9% appearing in the first 45 days, 38.8% between days 45 and 180, 16.0% between days 180 to 365, and 20.3% beyond 1 year. At last known follow-up, one-quarter of patients had DRT.
The odds ratios for DRT associated with the five identified risk factors were:
- 17.50 (95% confidence interval, 3.39-90.45) for hypercoagulability disorder
- 13.45 (95% CI, 1.46-123.52) for pericardial effusion
- 4.02 (95% CI, 1.22-13.25) for renal insufficiency
- 2.41 (95% CI, 1.57-3.69) for implantation depth >10 mm
- 1.90 (95% CI, 1.22-2.97) for nonparoxysmal AFib
The risk for a composite of death, ischemic stroke, and systemic embolization was twofold higher in the DRT cohort than in the control cohort (29.5% vs. 14.4%; hazard ratio, 2.37; 95% CI, 1.58-3.56) and driven by a higher rate of ischemic stroke (16.9% vs. 3.6%; HR, 3.49; 95% CI, 1.35-9.00).
The incidence of bleeding and intracerebral hemorrhage, however, was similar in the DRT and control cohorts.
One of the surprises of the study was that medications prescribed in the short term after LAA closure were not associated with DRT, Dr. Alkhouli said. A previous meta-analysis of 66 studies by the investigators also found that antithrombotic regimen did not explain the heterogeneity of DRT formation.
“I think we’ll have to take that with a grain of salt, because there’s so many variations in the practice, and this is observational data. But that, in my mind, brings up a mechanistic issue,” he said.
It’s often recommended “that we should put patients on blood thinners for 3 months or 6 weeks, or whatever it is, to decrease the chance of thrombus, assuming the patients will have a normal endothelialization of the device,” Dr. Alkhouli said.
“Well, we know that’s not the reality,” he continued. “We know many patients don’t endothelialize, and, even if some patients do, there may be some endothelial damage. So I think the whole mechanism of prescribing a little bit of a blood thinner to avoid that risk may be missing the point. It’s a bit more complex than that, evidenced also by the fact that three-fourths of all the DRTs happened after 45 days, when patients are typically not taking a blood thinner.”
Based on the five independent risk factors, the investigators created a clinical DRT risk score that assigned 1 point for renal insufficiency, implantation depth greater than 10 mm from the pulmonary ridge, and nonparoxysmal AFib; and 4 points for iatrogenic pericardial effusion and for hypercoagulability disorder. Low risk was categorized as 1 point and high risk as 2 or more points.
The presence of one major risk factor or two minor risk factors, for example, led to a 2.1-fold increased risk for DRT, compared with those with no DRT risk factors.
The risk score will require validation in a prospective cohort but is “a step forward in addressing DRT” and triaging patients, Dr. Alkhouli said. The findings highlight the need to avoid deep device implantation and the importance of shared decision-making with patients, especially with those at high risk.
“And third, which is most important, I think, in my mind, is that it tells us not to put a blind eye to this topic and just say with improved devices it will go away,” he said. “That’s a bit unrealistic.”
In an accompanying editorial, Oussama Wazni, MD, Walid Saliba, MD, and Ayman A. Hussein, MD, all from the Cleveland Clinic, write that “the study sheds light on this yet unresolved issue, and the observations may help with risk stratification and optimization of procedural techniques.”
Whereas many of the nonmodifiable risk factors are helpful in shared decision-making decisions, they continue, “knowledge of these risk factors may not preclude implantation in patients who are otherwise at risk of both stroke off anticoagulation and bleeding on anticoagulation.”
Dr. Wazni and colleagues acknowledge that the small number of events in the study limits statistical power for definitive conclusions and say that further studies are needed to clarify the natural history of DRTs and their management, resolution, and impact on cardiovascular events.
Practitioners should also continue to cautiously assess for LAAO clinical indications for implant, according to the editorialists, who point out that the regulatory approval language in the United States was “flexible and nonspecific.”
“As the field grows wider, enhancing LAAO safety with optimal design, implantation, and periprocedural management is critically important, yet the main focus should remain on optimal patient selection for the purpose of achieving safe and successful outcomes,” the editorialists conclude.
Dr. Alkhouli has served as a consultant for Boston Scientific. Coauthor disclosures are listed in the paper. Dr. Wazni and Dr. Hussein have received research grant support from Boston Scientific. Dr. Wazni and Dr. Saliba have been consultants for Boston Scientific.
A version of this article first appeared on Medscape.com.
, itself an important risk factor for cerebrovascular events, in patients with implants for left atrial appendage occlusion (LAAO), new research suggests.
The identified independent predictors of DRT in the largest dedicated multicenter LAAO-DRT registry to date were presence of a hypercoagulability disorder, pericardial effusion, renal insufficiency, an implantation depth greater than 10 mm from the pulmonary ridge, and presence of nonparoxysmal atrial fibrillation (AFib).
“Unfortunately, most of them are not modifiable, like hypercoaguable disorders or nonparoxysmal atrial fibrillation. But we can avoid deep implants because that’s been associated with creating a little bit of a crater or valley where the clot can form,” senior author Mohamad Alkhouli, MD, said in an interview.
But most important, and “really why we wanted to do this,” he said, is that “we want to give the patient a realistic prediction of adverse events for this procedure.”
LAAO has taken off in recent years for preventing thrombus formation and stroke in patients with AFib. Predicting DRT is a priority for the LAAO field, the authors note, especially given its expansion to younger, lower-risk patients and the increasing procedural volumes.
“This is a problem, DRT, that’s been discussed a lot because this is a preventative procedure,” observed Dr. Alkhouli, professor of medicine at Mayo Medical School, Rochester, Minn.
“The actual stroke risk every year – even if you don’t take any blood thinner and you have a CHADsVASc score of 9, the highest – is 11%. So if the chance of having thrombus is close, then that’s not a good tradeoff.”
Previous studies have also identified implantation depth and nonparoxysmal AFib as risk factors for DRT. But most of them have been small, he noted, with one of the largest reporting 65 DRTs in four prospective trials.
To cast a wider net, the investigators, led by Trevor Simard, MD, also from the Mayo Clinic, invited more than 50 international sites to contribute data to the registry. Of these, 37 centers reported on 237 DRTs and 474 device-matched control subjects from the same site.
Three-fourths of patients received a first-generation Watchman or a FLEX device (Boston Scientific).
Medical regimens were similar between the DRT and control cohorts at discharge after LAA closure. Most patients were managed with single (36.3%) or dual antiplatelet therapy (26.2%) at the time of DRT diagnosis.
As reported July 19 in the Journal of the American College of Cardiology, the timing of DRT development varied widely, with 24.9% appearing in the first 45 days, 38.8% between days 45 and 180, 16.0% between days 180 to 365, and 20.3% beyond 1 year. At last known follow-up, one-quarter of patients had DRT.
The odds ratios for DRT associated with the five identified risk factors were:
- 17.50 (95% confidence interval, 3.39-90.45) for hypercoagulability disorder
- 13.45 (95% CI, 1.46-123.52) for pericardial effusion
- 4.02 (95% CI, 1.22-13.25) for renal insufficiency
- 2.41 (95% CI, 1.57-3.69) for implantation depth >10 mm
- 1.90 (95% CI, 1.22-2.97) for nonparoxysmal AFib
The risk for a composite of death, ischemic stroke, and systemic embolization was twofold higher in the DRT cohort than in the control cohort (29.5% vs. 14.4%; hazard ratio, 2.37; 95% CI, 1.58-3.56) and driven by a higher rate of ischemic stroke (16.9% vs. 3.6%; HR, 3.49; 95% CI, 1.35-9.00).
The incidence of bleeding and intracerebral hemorrhage, however, was similar in the DRT and control cohorts.
One of the surprises of the study was that medications prescribed in the short term after LAA closure were not associated with DRT, Dr. Alkhouli said. A previous meta-analysis of 66 studies by the investigators also found that antithrombotic regimen did not explain the heterogeneity of DRT formation.
“I think we’ll have to take that with a grain of salt, because there’s so many variations in the practice, and this is observational data. But that, in my mind, brings up a mechanistic issue,” he said.
It’s often recommended “that we should put patients on blood thinners for 3 months or 6 weeks, or whatever it is, to decrease the chance of thrombus, assuming the patients will have a normal endothelialization of the device,” Dr. Alkhouli said.
“Well, we know that’s not the reality,” he continued. “We know many patients don’t endothelialize, and, even if some patients do, there may be some endothelial damage. So I think the whole mechanism of prescribing a little bit of a blood thinner to avoid that risk may be missing the point. It’s a bit more complex than that, evidenced also by the fact that three-fourths of all the DRTs happened after 45 days, when patients are typically not taking a blood thinner.”
Based on the five independent risk factors, the investigators created a clinical DRT risk score that assigned 1 point for renal insufficiency, implantation depth greater than 10 mm from the pulmonary ridge, and nonparoxysmal AFib; and 4 points for iatrogenic pericardial effusion and for hypercoagulability disorder. Low risk was categorized as 1 point and high risk as 2 or more points.
The presence of one major risk factor or two minor risk factors, for example, led to a 2.1-fold increased risk for DRT, compared with those with no DRT risk factors.
The risk score will require validation in a prospective cohort but is “a step forward in addressing DRT” and triaging patients, Dr. Alkhouli said. The findings highlight the need to avoid deep device implantation and the importance of shared decision-making with patients, especially with those at high risk.
“And third, which is most important, I think, in my mind, is that it tells us not to put a blind eye to this topic and just say with improved devices it will go away,” he said. “That’s a bit unrealistic.”
In an accompanying editorial, Oussama Wazni, MD, Walid Saliba, MD, and Ayman A. Hussein, MD, all from the Cleveland Clinic, write that “the study sheds light on this yet unresolved issue, and the observations may help with risk stratification and optimization of procedural techniques.”
Whereas many of the nonmodifiable risk factors are helpful in shared decision-making decisions, they continue, “knowledge of these risk factors may not preclude implantation in patients who are otherwise at risk of both stroke off anticoagulation and bleeding on anticoagulation.”
Dr. Wazni and colleagues acknowledge that the small number of events in the study limits statistical power for definitive conclusions and say that further studies are needed to clarify the natural history of DRTs and their management, resolution, and impact on cardiovascular events.
Practitioners should also continue to cautiously assess for LAAO clinical indications for implant, according to the editorialists, who point out that the regulatory approval language in the United States was “flexible and nonspecific.”
“As the field grows wider, enhancing LAAO safety with optimal design, implantation, and periprocedural management is critically important, yet the main focus should remain on optimal patient selection for the purpose of achieving safe and successful outcomes,” the editorialists conclude.
Dr. Alkhouli has served as a consultant for Boston Scientific. Coauthor disclosures are listed in the paper. Dr. Wazni and Dr. Hussein have received research grant support from Boston Scientific. Dr. Wazni and Dr. Saliba have been consultants for Boston Scientific.
A version of this article first appeared on Medscape.com.
, itself an important risk factor for cerebrovascular events, in patients with implants for left atrial appendage occlusion (LAAO), new research suggests.
The identified independent predictors of DRT in the largest dedicated multicenter LAAO-DRT registry to date were presence of a hypercoagulability disorder, pericardial effusion, renal insufficiency, an implantation depth greater than 10 mm from the pulmonary ridge, and presence of nonparoxysmal atrial fibrillation (AFib).
“Unfortunately, most of them are not modifiable, like hypercoaguable disorders or nonparoxysmal atrial fibrillation. But we can avoid deep implants because that’s been associated with creating a little bit of a crater or valley where the clot can form,” senior author Mohamad Alkhouli, MD, said in an interview.
But most important, and “really why we wanted to do this,” he said, is that “we want to give the patient a realistic prediction of adverse events for this procedure.”
LAAO has taken off in recent years for preventing thrombus formation and stroke in patients with AFib. Predicting DRT is a priority for the LAAO field, the authors note, especially given its expansion to younger, lower-risk patients and the increasing procedural volumes.
“This is a problem, DRT, that’s been discussed a lot because this is a preventative procedure,” observed Dr. Alkhouli, professor of medicine at Mayo Medical School, Rochester, Minn.
“The actual stroke risk every year – even if you don’t take any blood thinner and you have a CHADsVASc score of 9, the highest – is 11%. So if the chance of having thrombus is close, then that’s not a good tradeoff.”
Previous studies have also identified implantation depth and nonparoxysmal AFib as risk factors for DRT. But most of them have been small, he noted, with one of the largest reporting 65 DRTs in four prospective trials.
To cast a wider net, the investigators, led by Trevor Simard, MD, also from the Mayo Clinic, invited more than 50 international sites to contribute data to the registry. Of these, 37 centers reported on 237 DRTs and 474 device-matched control subjects from the same site.
Three-fourths of patients received a first-generation Watchman or a FLEX device (Boston Scientific).
Medical regimens were similar between the DRT and control cohorts at discharge after LAA closure. Most patients were managed with single (36.3%) or dual antiplatelet therapy (26.2%) at the time of DRT diagnosis.
As reported July 19 in the Journal of the American College of Cardiology, the timing of DRT development varied widely, with 24.9% appearing in the first 45 days, 38.8% between days 45 and 180, 16.0% between days 180 to 365, and 20.3% beyond 1 year. At last known follow-up, one-quarter of patients had DRT.
The odds ratios for DRT associated with the five identified risk factors were:
- 17.50 (95% confidence interval, 3.39-90.45) for hypercoagulability disorder
- 13.45 (95% CI, 1.46-123.52) for pericardial effusion
- 4.02 (95% CI, 1.22-13.25) for renal insufficiency
- 2.41 (95% CI, 1.57-3.69) for implantation depth >10 mm
- 1.90 (95% CI, 1.22-2.97) for nonparoxysmal AFib
The risk for a composite of death, ischemic stroke, and systemic embolization was twofold higher in the DRT cohort than in the control cohort (29.5% vs. 14.4%; hazard ratio, 2.37; 95% CI, 1.58-3.56) and driven by a higher rate of ischemic stroke (16.9% vs. 3.6%; HR, 3.49; 95% CI, 1.35-9.00).
The incidence of bleeding and intracerebral hemorrhage, however, was similar in the DRT and control cohorts.
One of the surprises of the study was that medications prescribed in the short term after LAA closure were not associated with DRT, Dr. Alkhouli said. A previous meta-analysis of 66 studies by the investigators also found that antithrombotic regimen did not explain the heterogeneity of DRT formation.
“I think we’ll have to take that with a grain of salt, because there’s so many variations in the practice, and this is observational data. But that, in my mind, brings up a mechanistic issue,” he said.
It’s often recommended “that we should put patients on blood thinners for 3 months or 6 weeks, or whatever it is, to decrease the chance of thrombus, assuming the patients will have a normal endothelialization of the device,” Dr. Alkhouli said.
“Well, we know that’s not the reality,” he continued. “We know many patients don’t endothelialize, and, even if some patients do, there may be some endothelial damage. So I think the whole mechanism of prescribing a little bit of a blood thinner to avoid that risk may be missing the point. It’s a bit more complex than that, evidenced also by the fact that three-fourths of all the DRTs happened after 45 days, when patients are typically not taking a blood thinner.”
Based on the five independent risk factors, the investigators created a clinical DRT risk score that assigned 1 point for renal insufficiency, implantation depth greater than 10 mm from the pulmonary ridge, and nonparoxysmal AFib; and 4 points for iatrogenic pericardial effusion and for hypercoagulability disorder. Low risk was categorized as 1 point and high risk as 2 or more points.
The presence of one major risk factor or two minor risk factors, for example, led to a 2.1-fold increased risk for DRT, compared with those with no DRT risk factors.
The risk score will require validation in a prospective cohort but is “a step forward in addressing DRT” and triaging patients, Dr. Alkhouli said. The findings highlight the need to avoid deep device implantation and the importance of shared decision-making with patients, especially with those at high risk.
“And third, which is most important, I think, in my mind, is that it tells us not to put a blind eye to this topic and just say with improved devices it will go away,” he said. “That’s a bit unrealistic.”
In an accompanying editorial, Oussama Wazni, MD, Walid Saliba, MD, and Ayman A. Hussein, MD, all from the Cleveland Clinic, write that “the study sheds light on this yet unresolved issue, and the observations may help with risk stratification and optimization of procedural techniques.”
Whereas many of the nonmodifiable risk factors are helpful in shared decision-making decisions, they continue, “knowledge of these risk factors may not preclude implantation in patients who are otherwise at risk of both stroke off anticoagulation and bleeding on anticoagulation.”
Dr. Wazni and colleagues acknowledge that the small number of events in the study limits statistical power for definitive conclusions and say that further studies are needed to clarify the natural history of DRTs and their management, resolution, and impact on cardiovascular events.
Practitioners should also continue to cautiously assess for LAAO clinical indications for implant, according to the editorialists, who point out that the regulatory approval language in the United States was “flexible and nonspecific.”
“As the field grows wider, enhancing LAAO safety with optimal design, implantation, and periprocedural management is critically important, yet the main focus should remain on optimal patient selection for the purpose of achieving safe and successful outcomes,” the editorialists conclude.
Dr. Alkhouli has served as a consultant for Boston Scientific. Coauthor disclosures are listed in the paper. Dr. Wazni and Dr. Hussein have received research grant support from Boston Scientific. Dr. Wazni and Dr. Saliba have been consultants for Boston Scientific.
A version of this article first appeared on Medscape.com.
Testosterone replacement shows CV benefit in hypogonadal men
Data from a long-term study suggest that testosterone replacement therapy (TRT) for men with hypogonadism may reduce the risk for major adverse cardiovascular events. Previous studies have yielded conflicting results on whether there is a benefit.
The latest results come from a study of 805 men with hypogonadism from Germany and Qatar who were followed for nearly a decade. For those who received parenteral testosterone 1,000 mg every 12 weeks, there were improvements in classical cardiovascular risk factors, such as obesity, lipid level, and inflammatory markers, whereas among those who chose not to take testosterone (control patients), all of these factors worsened.
In addition, there were only 16 deaths among patients in the TRT group, and none of the deaths were from myocardial infarction or stroke. In contrast, there were 74 deaths among the control patients, as well as 70 cases of MI and 59 strokes.
The men in the study were all at relatively high risk for cardiovascular adverse events. In the TRT group, the mean Framingham Risk score was 15.5; in the control group, it was 15.8. This translates into mean 10-year risks of 22.7% and 23.5%, respectively.
“Given that all these men would normally have been expected to suffer a heart attack or stroke in the next 5-10 years with no other intervention, it was a real surprise to see no cardiovascular events at all in the group on testosterone therapy. It’s clear that this treatment can significantly reduce the risks in this particular group,” commented lead investigator Omar Aboumarzouk, MD, from Hamad Medical in Doha, Qatar.
He presented the new data at the 2021 annual congress of the European Association of Urology.
Dr. Aboumarzouk emphasized, however, that, “while men need testosterone for certain psychological and biological functions, only those with low levels who display other symptoms are likely to benefit from testosterone therapy.”
Maarten Albersen, MD, a urologist at the University of Leuven (Belgium), who was not involved in the study, noted that, although the study showed a reduction in major adverse cardiovascular events and mortality among the men who received TRT, the risk scores were in the intermediate range, and the men in the TRT group were slightly younger and were at slightly lower risk at baseline.
“The study was long enough to see differences in the rate of cardiovascular events. However, the numbers involved and the fact that the trial was not randomized mean it’s still difficult to draw any hard conclusions,” he said.
Registry study
The data came from a cumulative registry study begun in 2004 to assess the long-term efficacy and safety of TRT every 3 months in men with hypogonadism. The study, conducted in Bremen, Dresden, and Muenster in Germany, as well as in Doha, Qatar, is ongoing.
At total of 805 men were enrolled; 412 received TRT, and 393 declined testosterone replacement and served as control patients.
The investigators reported 10-year data. Statistical models controlled for age, body mass index, smoking, alcohol, total and HDL cholesterol level, systolic blood pressure, and type 2 diabetes.
The median age at baseline was lower among those in the TRT arm, at 57.7 years versus 63.7 years for control patients (P < .001).
All classical cardiovascular risk factors, including obesity, glycemic control, lipid pattern, and C-reactive protein, improved in the TRT group and worsened in the control group.
Dr. Albersen noted that “a new trial is now underway, aiming to recruit 6,000 participants, and this should provide definitive answers on the cardiovascular risks or even benefits of hormone therapy in men with low testosterone.”
No funding source for the study was reported. Dr. Aboumarzouk and Dr. Albersen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Data from a long-term study suggest that testosterone replacement therapy (TRT) for men with hypogonadism may reduce the risk for major adverse cardiovascular events. Previous studies have yielded conflicting results on whether there is a benefit.
The latest results come from a study of 805 men with hypogonadism from Germany and Qatar who were followed for nearly a decade. For those who received parenteral testosterone 1,000 mg every 12 weeks, there were improvements in classical cardiovascular risk factors, such as obesity, lipid level, and inflammatory markers, whereas among those who chose not to take testosterone (control patients), all of these factors worsened.
In addition, there were only 16 deaths among patients in the TRT group, and none of the deaths were from myocardial infarction or stroke. In contrast, there were 74 deaths among the control patients, as well as 70 cases of MI and 59 strokes.
The men in the study were all at relatively high risk for cardiovascular adverse events. In the TRT group, the mean Framingham Risk score was 15.5; in the control group, it was 15.8. This translates into mean 10-year risks of 22.7% and 23.5%, respectively.
“Given that all these men would normally have been expected to suffer a heart attack or stroke in the next 5-10 years with no other intervention, it was a real surprise to see no cardiovascular events at all in the group on testosterone therapy. It’s clear that this treatment can significantly reduce the risks in this particular group,” commented lead investigator Omar Aboumarzouk, MD, from Hamad Medical in Doha, Qatar.
He presented the new data at the 2021 annual congress of the European Association of Urology.
Dr. Aboumarzouk emphasized, however, that, “while men need testosterone for certain psychological and biological functions, only those with low levels who display other symptoms are likely to benefit from testosterone therapy.”
Maarten Albersen, MD, a urologist at the University of Leuven (Belgium), who was not involved in the study, noted that, although the study showed a reduction in major adverse cardiovascular events and mortality among the men who received TRT, the risk scores were in the intermediate range, and the men in the TRT group were slightly younger and were at slightly lower risk at baseline.
“The study was long enough to see differences in the rate of cardiovascular events. However, the numbers involved and the fact that the trial was not randomized mean it’s still difficult to draw any hard conclusions,” he said.
Registry study
The data came from a cumulative registry study begun in 2004 to assess the long-term efficacy and safety of TRT every 3 months in men with hypogonadism. The study, conducted in Bremen, Dresden, and Muenster in Germany, as well as in Doha, Qatar, is ongoing.
At total of 805 men were enrolled; 412 received TRT, and 393 declined testosterone replacement and served as control patients.
The investigators reported 10-year data. Statistical models controlled for age, body mass index, smoking, alcohol, total and HDL cholesterol level, systolic blood pressure, and type 2 diabetes.
The median age at baseline was lower among those in the TRT arm, at 57.7 years versus 63.7 years for control patients (P < .001).
All classical cardiovascular risk factors, including obesity, glycemic control, lipid pattern, and C-reactive protein, improved in the TRT group and worsened in the control group.
Dr. Albersen noted that “a new trial is now underway, aiming to recruit 6,000 participants, and this should provide definitive answers on the cardiovascular risks or even benefits of hormone therapy in men with low testosterone.”
No funding source for the study was reported. Dr. Aboumarzouk and Dr. Albersen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Data from a long-term study suggest that testosterone replacement therapy (TRT) for men with hypogonadism may reduce the risk for major adverse cardiovascular events. Previous studies have yielded conflicting results on whether there is a benefit.
The latest results come from a study of 805 men with hypogonadism from Germany and Qatar who were followed for nearly a decade. For those who received parenteral testosterone 1,000 mg every 12 weeks, there were improvements in classical cardiovascular risk factors, such as obesity, lipid level, and inflammatory markers, whereas among those who chose not to take testosterone (control patients), all of these factors worsened.
In addition, there were only 16 deaths among patients in the TRT group, and none of the deaths were from myocardial infarction or stroke. In contrast, there were 74 deaths among the control patients, as well as 70 cases of MI and 59 strokes.
The men in the study were all at relatively high risk for cardiovascular adverse events. In the TRT group, the mean Framingham Risk score was 15.5; in the control group, it was 15.8. This translates into mean 10-year risks of 22.7% and 23.5%, respectively.
“Given that all these men would normally have been expected to suffer a heart attack or stroke in the next 5-10 years with no other intervention, it was a real surprise to see no cardiovascular events at all in the group on testosterone therapy. It’s clear that this treatment can significantly reduce the risks in this particular group,” commented lead investigator Omar Aboumarzouk, MD, from Hamad Medical in Doha, Qatar.
He presented the new data at the 2021 annual congress of the European Association of Urology.
Dr. Aboumarzouk emphasized, however, that, “while men need testosterone for certain psychological and biological functions, only those with low levels who display other symptoms are likely to benefit from testosterone therapy.”
Maarten Albersen, MD, a urologist at the University of Leuven (Belgium), who was not involved in the study, noted that, although the study showed a reduction in major adverse cardiovascular events and mortality among the men who received TRT, the risk scores were in the intermediate range, and the men in the TRT group were slightly younger and were at slightly lower risk at baseline.
“The study was long enough to see differences in the rate of cardiovascular events. However, the numbers involved and the fact that the trial was not randomized mean it’s still difficult to draw any hard conclusions,” he said.
Registry study
The data came from a cumulative registry study begun in 2004 to assess the long-term efficacy and safety of TRT every 3 months in men with hypogonadism. The study, conducted in Bremen, Dresden, and Muenster in Germany, as well as in Doha, Qatar, is ongoing.
At total of 805 men were enrolled; 412 received TRT, and 393 declined testosterone replacement and served as control patients.
The investigators reported 10-year data. Statistical models controlled for age, body mass index, smoking, alcohol, total and HDL cholesterol level, systolic blood pressure, and type 2 diabetes.
The median age at baseline was lower among those in the TRT arm, at 57.7 years versus 63.7 years for control patients (P < .001).
All classical cardiovascular risk factors, including obesity, glycemic control, lipid pattern, and C-reactive protein, improved in the TRT group and worsened in the control group.
Dr. Albersen noted that “a new trial is now underway, aiming to recruit 6,000 participants, and this should provide definitive answers on the cardiovascular risks or even benefits of hormone therapy in men with low testosterone.”
No funding source for the study was reported. Dr. Aboumarzouk and Dr. Albersen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CABANA: Ablation bests drugs for AFib in racial/ethnic minorities
CABANA, which was undertaken to compare catheter ablation and rate-control or rhythm-control drug therapy for AFib, concluded there was no significant difference between the two strategies in improving the trial’s composite primary outcome of death, disabling stroke, serious bleeding, or cardiac arrest.
But a closer look at a subgroup of participants reveals an important difference in outcome among racial and ethnic minorities.
In that group, which made up about 10% of the CABANA study population, catheter ablation was significantly better at treating AFib than was drug therapy, producing roughly a 70% relative reduction in the primary endpoint and all-cause mortality.
The benefit for catheter ablation, which was not seen in the nonminority participants, appeared to be due to worse outcomes with drug therapy, the investigators report in an article published July 5 in the Journal of the American College of Cardiology.
“The study really highlights the importance of trying to secure an inclusive and diverse population in clinical trials,” lead author Kevin L. Thomas, MD, Duke University, Durham, N.C., said in an interview.
“When we focused on the racial and ethnic minorities who were included in CABANA, the findings were different. This was a surprise,” Dr. Thomas said.
“The findings from the secondary analysis of CABANA suggest that racial and ethnic minorities that are treated with drugs compared with ablation do worse,” he said. “If we can validate this in a larger sample of patients and this does in fact turn out to be true, then we would change how we practice medicine. We would have discussions with these populations about the benefits of ablation over drugs, and this would be important information to help guide our practice.”
The investigators analyzed data from 1,280 participants enrolled in the North American arm of CABANA. Of these, 127 (9.9%) were of racial or ethnic minorities, as defined by the National Institutes of Health, and were randomly assigned to receive ablation (n = 62) or drug therapy (n = 65).
Compared with nonminorities, participants of racial and ethnic minorities were younger (median age, 65.5 years, vs. 68.5 years) and were more likely to have NYHA functional class greater than or equal to II symptoms (37.0% vs. 22.0%), hypertension (92.1% vs. 76.8%), and an ejection fraction less than 40% (20.8% vs. 7.1%).
The overall median follow-up was 54.9 months. Among ethnic and minority participants, the median follow-up was 48 months, compared with 55.5 months for the nonminority participants.
Although there was no significant difference in the primary composite endpoint in the main CABANA trial, among racial and ethnic minorities treated with ablation, there was a 68% relative reduction in the trial’s primary endpoint (adjusted hazard ratio, 0.32; 95% confidence interval, 0.13-0.78) and a 72% relative reduction in all-cause mortality (aHR, 0.28; 95% CI, 0.10-0.79).
The 4-year Kaplan-Meier primary event rates were similar in both racial/ethnic minority and nonminority groups that received catheter ablation (12.3% vs. 9.9%).
However, the 4-year event rate was much higher among nonminority participants than among racial and ethnic minorities who received drug therapy (27.4% vs. 9.4%).
The corresponding all-cause 4-year mortality rates were 8.1% and 6.7%, respectively, in the ablation arm and 20.2% and 4.5%, respectively, in the drug arm.
Dr. Thomas and colleagues point out that heart failure in racial and ethnic minorities, particularly Black patients, is typically due to hypertensive heart disease, whereas in non-Hispanic White patients, it is overwhelmingly associated with coronary artery disease. “Our results in CABANA, therefore, raise the possibility that the variations in the prevalence of the heart diseases associated with AFib might account for differences in the benefits observed with ablation therapy.”
Prior data suggest that AFib in the setting of heart failure with either reduced or preserved ejection fraction has substantially better clinical outcomes with ablation versus drug therapy, but most studies either do not report racial/ethnic demographics or enroll very low numbers of minorities, they note.
Andrea M. Russo, MD, a professor of medicine at Rowan University, Camden, New Jersey, asks why drug therapy might result in worse outcomes in racial and ethnic minorities in an accompanying editorial.
“Those who received ablation did better than those who received drugs, and the main reason for that is not that ablation works better in minorities than nonminorities, it’s because drugs are worse in minority patients than they are in nonminority patients. This means that either the way we are using the drugs or the ones that we are using in minority patients are resulting in worse overall outcomes,” Dr. Russo told this news organization.
“The minority patients were younger and yet had more hypertension at baseline. There could be all kinds of factors contributing to their health,” she said.
Dr. Russo agrees with Dr. Thomas on the need to enroll diverse populations in clinical trials.
“Dr. Thomas should be commended. He did a fabulous job of looking at this issue. It’s only 10% of the group, but it is better than what we have had so far, and this is a start,” Dr. Russo said. “It’s bringing recognition to how important it is to make sure that we include underrepresented populations in these trials and also that we offer all appropriate therapies to everyone.”
Dr. Thomas reports financial relationships with Janssen, Pfizer, Biosense Webster. Dr. Russo reports no relevant financial relationships. The study was funded by the National Institutes of Health, St. Jude Medical Foundation and Corporation, Biosense Webster, Medtronic, and Boston Scientific.
A version of this article first appeared on Medscape.com.
CABANA, which was undertaken to compare catheter ablation and rate-control or rhythm-control drug therapy for AFib, concluded there was no significant difference between the two strategies in improving the trial’s composite primary outcome of death, disabling stroke, serious bleeding, or cardiac arrest.
But a closer look at a subgroup of participants reveals an important difference in outcome among racial and ethnic minorities.
In that group, which made up about 10% of the CABANA study population, catheter ablation was significantly better at treating AFib than was drug therapy, producing roughly a 70% relative reduction in the primary endpoint and all-cause mortality.
The benefit for catheter ablation, which was not seen in the nonminority participants, appeared to be due to worse outcomes with drug therapy, the investigators report in an article published July 5 in the Journal of the American College of Cardiology.
“The study really highlights the importance of trying to secure an inclusive and diverse population in clinical trials,” lead author Kevin L. Thomas, MD, Duke University, Durham, N.C., said in an interview.
“When we focused on the racial and ethnic minorities who were included in CABANA, the findings were different. This was a surprise,” Dr. Thomas said.
“The findings from the secondary analysis of CABANA suggest that racial and ethnic minorities that are treated with drugs compared with ablation do worse,” he said. “If we can validate this in a larger sample of patients and this does in fact turn out to be true, then we would change how we practice medicine. We would have discussions with these populations about the benefits of ablation over drugs, and this would be important information to help guide our practice.”
The investigators analyzed data from 1,280 participants enrolled in the North American arm of CABANA. Of these, 127 (9.9%) were of racial or ethnic minorities, as defined by the National Institutes of Health, and were randomly assigned to receive ablation (n = 62) or drug therapy (n = 65).
Compared with nonminorities, participants of racial and ethnic minorities were younger (median age, 65.5 years, vs. 68.5 years) and were more likely to have NYHA functional class greater than or equal to II symptoms (37.0% vs. 22.0%), hypertension (92.1% vs. 76.8%), and an ejection fraction less than 40% (20.8% vs. 7.1%).
The overall median follow-up was 54.9 months. Among ethnic and minority participants, the median follow-up was 48 months, compared with 55.5 months for the nonminority participants.
Although there was no significant difference in the primary composite endpoint in the main CABANA trial, among racial and ethnic minorities treated with ablation, there was a 68% relative reduction in the trial’s primary endpoint (adjusted hazard ratio, 0.32; 95% confidence interval, 0.13-0.78) and a 72% relative reduction in all-cause mortality (aHR, 0.28; 95% CI, 0.10-0.79).
The 4-year Kaplan-Meier primary event rates were similar in both racial/ethnic minority and nonminority groups that received catheter ablation (12.3% vs. 9.9%).
However, the 4-year event rate was much higher among nonminority participants than among racial and ethnic minorities who received drug therapy (27.4% vs. 9.4%).
The corresponding all-cause 4-year mortality rates were 8.1% and 6.7%, respectively, in the ablation arm and 20.2% and 4.5%, respectively, in the drug arm.
Dr. Thomas and colleagues point out that heart failure in racial and ethnic minorities, particularly Black patients, is typically due to hypertensive heart disease, whereas in non-Hispanic White patients, it is overwhelmingly associated with coronary artery disease. “Our results in CABANA, therefore, raise the possibility that the variations in the prevalence of the heart diseases associated with AFib might account for differences in the benefits observed with ablation therapy.”
Prior data suggest that AFib in the setting of heart failure with either reduced or preserved ejection fraction has substantially better clinical outcomes with ablation versus drug therapy, but most studies either do not report racial/ethnic demographics or enroll very low numbers of minorities, they note.
Andrea M. Russo, MD, a professor of medicine at Rowan University, Camden, New Jersey, asks why drug therapy might result in worse outcomes in racial and ethnic minorities in an accompanying editorial.
“Those who received ablation did better than those who received drugs, and the main reason for that is not that ablation works better in minorities than nonminorities, it’s because drugs are worse in minority patients than they are in nonminority patients. This means that either the way we are using the drugs or the ones that we are using in minority patients are resulting in worse overall outcomes,” Dr. Russo told this news organization.
“The minority patients were younger and yet had more hypertension at baseline. There could be all kinds of factors contributing to their health,” she said.
Dr. Russo agrees with Dr. Thomas on the need to enroll diverse populations in clinical trials.
“Dr. Thomas should be commended. He did a fabulous job of looking at this issue. It’s only 10% of the group, but it is better than what we have had so far, and this is a start,” Dr. Russo said. “It’s bringing recognition to how important it is to make sure that we include underrepresented populations in these trials and also that we offer all appropriate therapies to everyone.”
Dr. Thomas reports financial relationships with Janssen, Pfizer, Biosense Webster. Dr. Russo reports no relevant financial relationships. The study was funded by the National Institutes of Health, St. Jude Medical Foundation and Corporation, Biosense Webster, Medtronic, and Boston Scientific.
A version of this article first appeared on Medscape.com.
CABANA, which was undertaken to compare catheter ablation and rate-control or rhythm-control drug therapy for AFib, concluded there was no significant difference between the two strategies in improving the trial’s composite primary outcome of death, disabling stroke, serious bleeding, or cardiac arrest.
But a closer look at a subgroup of participants reveals an important difference in outcome among racial and ethnic minorities.
In that group, which made up about 10% of the CABANA study population, catheter ablation was significantly better at treating AFib than was drug therapy, producing roughly a 70% relative reduction in the primary endpoint and all-cause mortality.
The benefit for catheter ablation, which was not seen in the nonminority participants, appeared to be due to worse outcomes with drug therapy, the investigators report in an article published July 5 in the Journal of the American College of Cardiology.
“The study really highlights the importance of trying to secure an inclusive and diverse population in clinical trials,” lead author Kevin L. Thomas, MD, Duke University, Durham, N.C., said in an interview.
“When we focused on the racial and ethnic minorities who were included in CABANA, the findings were different. This was a surprise,” Dr. Thomas said.
“The findings from the secondary analysis of CABANA suggest that racial and ethnic minorities that are treated with drugs compared with ablation do worse,” he said. “If we can validate this in a larger sample of patients and this does in fact turn out to be true, then we would change how we practice medicine. We would have discussions with these populations about the benefits of ablation over drugs, and this would be important information to help guide our practice.”
The investigators analyzed data from 1,280 participants enrolled in the North American arm of CABANA. Of these, 127 (9.9%) were of racial or ethnic minorities, as defined by the National Institutes of Health, and were randomly assigned to receive ablation (n = 62) or drug therapy (n = 65).
Compared with nonminorities, participants of racial and ethnic minorities were younger (median age, 65.5 years, vs. 68.5 years) and were more likely to have NYHA functional class greater than or equal to II symptoms (37.0% vs. 22.0%), hypertension (92.1% vs. 76.8%), and an ejection fraction less than 40% (20.8% vs. 7.1%).
The overall median follow-up was 54.9 months. Among ethnic and minority participants, the median follow-up was 48 months, compared with 55.5 months for the nonminority participants.
Although there was no significant difference in the primary composite endpoint in the main CABANA trial, among racial and ethnic minorities treated with ablation, there was a 68% relative reduction in the trial’s primary endpoint (adjusted hazard ratio, 0.32; 95% confidence interval, 0.13-0.78) and a 72% relative reduction in all-cause mortality (aHR, 0.28; 95% CI, 0.10-0.79).
The 4-year Kaplan-Meier primary event rates were similar in both racial/ethnic minority and nonminority groups that received catheter ablation (12.3% vs. 9.9%).
However, the 4-year event rate was much higher among nonminority participants than among racial and ethnic minorities who received drug therapy (27.4% vs. 9.4%).
The corresponding all-cause 4-year mortality rates were 8.1% and 6.7%, respectively, in the ablation arm and 20.2% and 4.5%, respectively, in the drug arm.
Dr. Thomas and colleagues point out that heart failure in racial and ethnic minorities, particularly Black patients, is typically due to hypertensive heart disease, whereas in non-Hispanic White patients, it is overwhelmingly associated with coronary artery disease. “Our results in CABANA, therefore, raise the possibility that the variations in the prevalence of the heart diseases associated with AFib might account for differences in the benefits observed with ablation therapy.”
Prior data suggest that AFib in the setting of heart failure with either reduced or preserved ejection fraction has substantially better clinical outcomes with ablation versus drug therapy, but most studies either do not report racial/ethnic demographics or enroll very low numbers of minorities, they note.
Andrea M. Russo, MD, a professor of medicine at Rowan University, Camden, New Jersey, asks why drug therapy might result in worse outcomes in racial and ethnic minorities in an accompanying editorial.
“Those who received ablation did better than those who received drugs, and the main reason for that is not that ablation works better in minorities than nonminorities, it’s because drugs are worse in minority patients than they are in nonminority patients. This means that either the way we are using the drugs or the ones that we are using in minority patients are resulting in worse overall outcomes,” Dr. Russo told this news organization.
“The minority patients were younger and yet had more hypertension at baseline. There could be all kinds of factors contributing to their health,” she said.
Dr. Russo agrees with Dr. Thomas on the need to enroll diverse populations in clinical trials.
“Dr. Thomas should be commended. He did a fabulous job of looking at this issue. It’s only 10% of the group, but it is better than what we have had so far, and this is a start,” Dr. Russo said. “It’s bringing recognition to how important it is to make sure that we include underrepresented populations in these trials and also that we offer all appropriate therapies to everyone.”
Dr. Thomas reports financial relationships with Janssen, Pfizer, Biosense Webster. Dr. Russo reports no relevant financial relationships. The study was funded by the National Institutes of Health, St. Jude Medical Foundation and Corporation, Biosense Webster, Medtronic, and Boston Scientific.
A version of this article first appeared on Medscape.com.
Midlife change in wealth may be costly for heart health
It found that upward wealth mobility relative to peers was independently associated with protection against cardiovascular disease after age 65. In contrast, downward wealth mobility during middle age was linked to an increased risk of adverse cardiovascular events.
“A lot of studies have shown an inverse relationship between wealth and health in cross section at a single timepoint. What we really wanted to understand is whether this risk is modifiable and if this relationship changes over time,” senior author Muthiah Vaduganathan, MD, MPH, Brigham and Women’s Hospital Heart & Vascular Center, Boston, said in an interview.
The results were published online June 15 in JAMA Cardiology.
For the primary analysis, the researchers collected data from 5,579 U.S. adults aged 50 years and older with no known cardiovascular disease at baseline who participated in the RAND Health and Retirement Study, a longitudinal survey that measures changes in health and wealth every 2 years. The participants had been interviewed in at least two of three 5-year age intervals (50-54, 55-59, 60-64 years) and had follow-up data available after age 65. Survey data from Jan. 1, 1992 to Dec. 31, 2016 was used.
Participants were grouped into quintiles based on wealth, defined as total nonhousing assets in 2012 U.S. dollars, and were further stratified by birth cohort (1931-1935, 1936-1940, 1941-1945, 1946-1950). Upward relative wealth mobility involved an increase of one or more wealth quintiles during the observation period, while downward relative wealth mobility was defined as a decrease of one or more wealth quintiles. Participants who remained in the same quintile were described as having stable wealth.
Across the birth cohorts, the bottom wealth quintile ranged from -$581,447 to $7,460 and the top wealth quintile ranged from $327,064 to $22,661,450.
Over a mean 16.9 years of follow-up, the primary outcome of cardiovascular death or a nonfatal cardiovascular event such as a heart attack or stroke occurred in 1,336 participants, including 22.5% whose wealth increased by one quintile versus 28.1% whose wealth decreased by one quintile.
In adjusted analyses, higher initial wealth was associated with lower cardiovascular risk after turning 65 (adjusted hazard ratio per quintile, 0.89; 95% confidence interval, 0.84-0.95; P = .001). Additionally, experiencing relative upward wealth mobility by at least one quintile was independently associated with a lower risk of a nonfatal cardiovascular event or cardiovascular death, compared with stable wealth (aHR, 0.84; 95% CI, 0.73-0.97; P = .02).
Downward wealth mobility was associated with worse cardiovascular outcomes (aHR, 1.15; 95% CI, 1.00-1.32; P = .046). This effect was also observed on the risk of cardiovascular death in a secondary analysis of 3,360 participants who had a previous history of cardiovascular disease (aHR, 1.48; 95% CI, 1.13-1.93; P = .004).
“We estimate that each $100,000 increase in wealth was associated with a roughly 1% lower hazard of cardiovascular outcome in follow-up,” the authors write.
The protective effect of wealth on cardiovascular health may be the result of factors such as “better access to care, having more time to adhere to a healthier diet or exercise regularly, and reduced stress,” Kiarri Kershaw, PhD, a social epidemiologist at Northwestern University, Chicago, said in an interview. Dr. Kershaw, who was not involved in the study, added that “stress can affect health through both biological and behavioral pathways.”
The study did not find a statistical relationship between race, wealth, and health. However, it was observed that the overall risk of cardiovascular events among non-Hispanic Black and Black participants was lower. The authors noted that “these findings are likely a byproduct of collider bias, in which Black and Hispanic participants who experience downward wealth mobility are more likely to experience barriers to care and subsequently less likely to receive a diagnosis of cardiovascular disease.”
Moving forward, the researchers plan to investigate health policy interventions that “best promote and sustain economic opportunity and wealth formed among low-income individuals,” Dr. Vaduganathan said.
The study was funded independently. Dr. Vaduganathan and Dr. Kershaw have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It found that upward wealth mobility relative to peers was independently associated with protection against cardiovascular disease after age 65. In contrast, downward wealth mobility during middle age was linked to an increased risk of adverse cardiovascular events.
“A lot of studies have shown an inverse relationship between wealth and health in cross section at a single timepoint. What we really wanted to understand is whether this risk is modifiable and if this relationship changes over time,” senior author Muthiah Vaduganathan, MD, MPH, Brigham and Women’s Hospital Heart & Vascular Center, Boston, said in an interview.
The results were published online June 15 in JAMA Cardiology.
For the primary analysis, the researchers collected data from 5,579 U.S. adults aged 50 years and older with no known cardiovascular disease at baseline who participated in the RAND Health and Retirement Study, a longitudinal survey that measures changes in health and wealth every 2 years. The participants had been interviewed in at least two of three 5-year age intervals (50-54, 55-59, 60-64 years) and had follow-up data available after age 65. Survey data from Jan. 1, 1992 to Dec. 31, 2016 was used.
Participants were grouped into quintiles based on wealth, defined as total nonhousing assets in 2012 U.S. dollars, and were further stratified by birth cohort (1931-1935, 1936-1940, 1941-1945, 1946-1950). Upward relative wealth mobility involved an increase of one or more wealth quintiles during the observation period, while downward relative wealth mobility was defined as a decrease of one or more wealth quintiles. Participants who remained in the same quintile were described as having stable wealth.
Across the birth cohorts, the bottom wealth quintile ranged from -$581,447 to $7,460 and the top wealth quintile ranged from $327,064 to $22,661,450.
Over a mean 16.9 years of follow-up, the primary outcome of cardiovascular death or a nonfatal cardiovascular event such as a heart attack or stroke occurred in 1,336 participants, including 22.5% whose wealth increased by one quintile versus 28.1% whose wealth decreased by one quintile.
In adjusted analyses, higher initial wealth was associated with lower cardiovascular risk after turning 65 (adjusted hazard ratio per quintile, 0.89; 95% confidence interval, 0.84-0.95; P = .001). Additionally, experiencing relative upward wealth mobility by at least one quintile was independently associated with a lower risk of a nonfatal cardiovascular event or cardiovascular death, compared with stable wealth (aHR, 0.84; 95% CI, 0.73-0.97; P = .02).
Downward wealth mobility was associated with worse cardiovascular outcomes (aHR, 1.15; 95% CI, 1.00-1.32; P = .046). This effect was also observed on the risk of cardiovascular death in a secondary analysis of 3,360 participants who had a previous history of cardiovascular disease (aHR, 1.48; 95% CI, 1.13-1.93; P = .004).
“We estimate that each $100,000 increase in wealth was associated with a roughly 1% lower hazard of cardiovascular outcome in follow-up,” the authors write.
The protective effect of wealth on cardiovascular health may be the result of factors such as “better access to care, having more time to adhere to a healthier diet or exercise regularly, and reduced stress,” Kiarri Kershaw, PhD, a social epidemiologist at Northwestern University, Chicago, said in an interview. Dr. Kershaw, who was not involved in the study, added that “stress can affect health through both biological and behavioral pathways.”
The study did not find a statistical relationship between race, wealth, and health. However, it was observed that the overall risk of cardiovascular events among non-Hispanic Black and Black participants was lower. The authors noted that “these findings are likely a byproduct of collider bias, in which Black and Hispanic participants who experience downward wealth mobility are more likely to experience barriers to care and subsequently less likely to receive a diagnosis of cardiovascular disease.”
Moving forward, the researchers plan to investigate health policy interventions that “best promote and sustain economic opportunity and wealth formed among low-income individuals,” Dr. Vaduganathan said.
The study was funded independently. Dr. Vaduganathan and Dr. Kershaw have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It found that upward wealth mobility relative to peers was independently associated with protection against cardiovascular disease after age 65. In contrast, downward wealth mobility during middle age was linked to an increased risk of adverse cardiovascular events.
“A lot of studies have shown an inverse relationship between wealth and health in cross section at a single timepoint. What we really wanted to understand is whether this risk is modifiable and if this relationship changes over time,” senior author Muthiah Vaduganathan, MD, MPH, Brigham and Women’s Hospital Heart & Vascular Center, Boston, said in an interview.
The results were published online June 15 in JAMA Cardiology.
For the primary analysis, the researchers collected data from 5,579 U.S. adults aged 50 years and older with no known cardiovascular disease at baseline who participated in the RAND Health and Retirement Study, a longitudinal survey that measures changes in health and wealth every 2 years. The participants had been interviewed in at least two of three 5-year age intervals (50-54, 55-59, 60-64 years) and had follow-up data available after age 65. Survey data from Jan. 1, 1992 to Dec. 31, 2016 was used.
Participants were grouped into quintiles based on wealth, defined as total nonhousing assets in 2012 U.S. dollars, and were further stratified by birth cohort (1931-1935, 1936-1940, 1941-1945, 1946-1950). Upward relative wealth mobility involved an increase of one or more wealth quintiles during the observation period, while downward relative wealth mobility was defined as a decrease of one or more wealth quintiles. Participants who remained in the same quintile were described as having stable wealth.
Across the birth cohorts, the bottom wealth quintile ranged from -$581,447 to $7,460 and the top wealth quintile ranged from $327,064 to $22,661,450.
Over a mean 16.9 years of follow-up, the primary outcome of cardiovascular death or a nonfatal cardiovascular event such as a heart attack or stroke occurred in 1,336 participants, including 22.5% whose wealth increased by one quintile versus 28.1% whose wealth decreased by one quintile.
In adjusted analyses, higher initial wealth was associated with lower cardiovascular risk after turning 65 (adjusted hazard ratio per quintile, 0.89; 95% confidence interval, 0.84-0.95; P = .001). Additionally, experiencing relative upward wealth mobility by at least one quintile was independently associated with a lower risk of a nonfatal cardiovascular event or cardiovascular death, compared with stable wealth (aHR, 0.84; 95% CI, 0.73-0.97; P = .02).
Downward wealth mobility was associated with worse cardiovascular outcomes (aHR, 1.15; 95% CI, 1.00-1.32; P = .046). This effect was also observed on the risk of cardiovascular death in a secondary analysis of 3,360 participants who had a previous history of cardiovascular disease (aHR, 1.48; 95% CI, 1.13-1.93; P = .004).
“We estimate that each $100,000 increase in wealth was associated with a roughly 1% lower hazard of cardiovascular outcome in follow-up,” the authors write.
The protective effect of wealth on cardiovascular health may be the result of factors such as “better access to care, having more time to adhere to a healthier diet or exercise regularly, and reduced stress,” Kiarri Kershaw, PhD, a social epidemiologist at Northwestern University, Chicago, said in an interview. Dr. Kershaw, who was not involved in the study, added that “stress can affect health through both biological and behavioral pathways.”
The study did not find a statistical relationship between race, wealth, and health. However, it was observed that the overall risk of cardiovascular events among non-Hispanic Black and Black participants was lower. The authors noted that “these findings are likely a byproduct of collider bias, in which Black and Hispanic participants who experience downward wealth mobility are more likely to experience barriers to care and subsequently less likely to receive a diagnosis of cardiovascular disease.”
Moving forward, the researchers plan to investigate health policy interventions that “best promote and sustain economic opportunity and wealth formed among low-income individuals,” Dr. Vaduganathan said.
The study was funded independently. Dr. Vaduganathan and Dr. Kershaw have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Is walking speed following stroke a good predictor of recovery?
, suggests new research backed by imaging data.
In secondary analysis of a previous study, training enabled both “good” and “limited” walkers to increase travel distance during a 2-minute walk. However, for “dual-task” walking, good walkers improved their distance by approximately 10 m after training, whereas limited walkers improved by only 1 m. Brain imaging showed increased brain activity in the limited walkers, which could reduce cognitive resources available for performing a second task while walking.
These findings, which were published online May 30 in Clinical Rehabilitation, may explain the apparent lack of superiority, shown previously, of dual-task training compared with single-task training for patients with stroke and impaired walking ability, researchers noted.
“Imaging data were consistent with our hypothesis that walking automaticity might explain these results,” said lead author Johnny Collett, PhD, senior clinical research fellow at Oxford Brookes University, United Kingdom.
At baseline, participants who walked slowly had increased resting state connectivity between contralesional M1 and cortical areas associated with conscious gait control.
“In response to the intervention, we found increased connectivity with the precuneus in those who walked slowly at baseline, an adaptation that might support walking in more complex situations,” Dr. Collett said.
Benefits questioned
After stroke, many patients have difficulty walking while performing a second task, such as holding a conversation. Training in dual-task walking has provided uncertain benefits, according to clinical research.
In healthy individuals, walking is believed to be a largely automatic process that requires minimal executive resources. Previous studies have suggested that a certain minimum walking speed is required to enable automatic control of walking in the brain.
“We know that those with better walking ability after stroke are better able to cope with additional cognitive loads while walking,” said Dr. Collett. “Here, we proposed that increased automatic gait control may provide a mechanism whereby executive resources are freed up to attend to additional tasks,” he added.
The investigators further hypothesized that greater walking speed is required for automatic gait control. To test these hypotheses, they analyzed data from a previously conducted randomized trial of single- and dual-task walking interventions.
Trial participants were aged 18 years or older, had survived a stroke that had occurred at least 6 months before enrollment, had reduced 2-minute walk distance relative to their peers, and had no comorbid neurologic or psychologic disorders.
Over 10 weeks, participants underwent 20 sessions that included 30 minutes of walking on a treadmill. They were randomly assigned to undergo single-task walking or dual-task walking. The latter incorporated cognitive tasks as distractions.
Good versus limited walkers
In the current study, investigators analyzed various assessments that had been conducted at baseline and after completion of the training sessions, including distance on 2-minute walks with and without a distracting task. In addition, participants underwent imaging with functional near-infrared spectroscopy (fNIRS) and fMRI.
Using previous research as a basis, the researchers defined good walking speed as 0.8 m/sec. They categorized all participants, regardless of their intervention assignments, as having good walking capacity (0.8 m/sec or more) or limited walking capacity (less than 0.79 m/sec).
A total of 50 participants enrolled in the study (mean age, 62 years), and 45 completed the interventions. Of those who completed the interventions, 22 were randomly assigned to undergo single-task training, and 23 were assigned to dual-task training.
The researchers categorized 21 participants as having good walking capacity and 24 as having limited walking capacity. Participants in each category were divided approximately evenly between treatment assignments.
Barthel index score, which assesses functional independence, was higher in the group of good walkers.
Increased travel distance
Results showed that after the interventions, distance traveled during the single-task 2-minute walk increased by 8.9 m for good walkers and by 5.3 m for limited walkers. For the dual-task 2-minute walk, the distance traveled increased by 10.4 m among good walkers and by 1.3 m for limited walkers. Change from baseline on the dual-task walk was not significant for limited walkers.
There was no significant difference between good walkers and limited walkers in their perceptions of participation in community walking. Neither group increased its walking activity significantly following the interventions.
At baseline, limited walkers, in comparison with good walkers, had significantly greater activation in the contralesional hemisphere during dual-task walking, which consisted of incorporating a planning task.
In contrast, for many good walkers, there was a decrease in activation during dual-task walking. Activation in the contralesional hemisphere correlated negatively with dual-task 2-minute walk distance.
The researchers also found a negative correlation between activation and dual-task 2-minute walk distance when the second task was the Stroop task.
Initial step
“The original trial was never designed or powered to compare groups formed by walking speed or test our automaticity hypothesis, and the results need to be viewed within this context,” said Dr. Collett. The small sample size did not allow the researchers to detect small effects of the intervention, especially in the imaging data, he added.
It also prevented the investigators from comparing limited walking and good walking groups according to whether they underwent the single-task or dual-task intervention, “which would be a superior way to investigate our hypotheses,” Dr. Collett said.
“The result of this study should be seen as exploratory, with further investigation needed,” he noted.
Helping stroke survivors to walk in the community is challenging, and new interventions that enable them to navigate complex surroundings need to be designed, said Dr. Collett. “Research is required to better understand the conscious and automatic contribution to gait control, especially with neurological impairment,” he added.
Overall, “our results suggest that improving automatic walking may be an initial step to improve capacity to respond to more complex walking interventions. However, [future] trials are required to test this,” he concluded.
The next frontier?
Commenting on the findings, Louis R. Caplan, MD, professor of neurology at Harvard University and senior neurologist at Beth Israel Deaconess Medical Center, Boston, said that “recovery and rehab are going to be the next frontier in stroke neurology, because there has to be a limitation in the present emphasis on acute care.”
Some patients do not receive acute care on time, and current treatment is not curative, added Dr. Caplan, who was not involved with the research.
Little scientific attention has been paid to how doctors can enhance recovery after stroke, what interventions delay recovery, and what the natural history of recovery is, he said. “This is a very nice study about that.”
Although the study’s methodology was sound, there were some limitations, including that strokes and underlying brain lesions were heterogeneous and that the study population was relatively small, Dr. Caplan said.
He added that “it’s a difficult study to do” and that it is difficult to organize participants into homogeneous groups.
Another limitation cited was lack of long-term follow-up that could indicate whether training provided sustained improvements in walking.
“It would be nice to revisit the same people later and see if their walking has improved, if they’re doing it differently, and if their subjective responses are different,” said Dr. Caplan.
In addition, the study did not examine whether the interventions made it easier for participants to walk with other people or to socialize more. “It may be that it really requires some time for them to gain confidence and for them to integrate that into their social network,” Dr. Caplan said.
“I would call it a proof-of-principle study, not a final study,” he noted. “It’s a study that shows that you can scientifically study rehab” and indicates the possible methodology that could be used.
The study was funded by the Stroke Association. Dr. Collett and Dr. Caplan have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, suggests new research backed by imaging data.
In secondary analysis of a previous study, training enabled both “good” and “limited” walkers to increase travel distance during a 2-minute walk. However, for “dual-task” walking, good walkers improved their distance by approximately 10 m after training, whereas limited walkers improved by only 1 m. Brain imaging showed increased brain activity in the limited walkers, which could reduce cognitive resources available for performing a second task while walking.
These findings, which were published online May 30 in Clinical Rehabilitation, may explain the apparent lack of superiority, shown previously, of dual-task training compared with single-task training for patients with stroke and impaired walking ability, researchers noted.
“Imaging data were consistent with our hypothesis that walking automaticity might explain these results,” said lead author Johnny Collett, PhD, senior clinical research fellow at Oxford Brookes University, United Kingdom.
At baseline, participants who walked slowly had increased resting state connectivity between contralesional M1 and cortical areas associated with conscious gait control.
“In response to the intervention, we found increased connectivity with the precuneus in those who walked slowly at baseline, an adaptation that might support walking in more complex situations,” Dr. Collett said.
Benefits questioned
After stroke, many patients have difficulty walking while performing a second task, such as holding a conversation. Training in dual-task walking has provided uncertain benefits, according to clinical research.
In healthy individuals, walking is believed to be a largely automatic process that requires minimal executive resources. Previous studies have suggested that a certain minimum walking speed is required to enable automatic control of walking in the brain.
“We know that those with better walking ability after stroke are better able to cope with additional cognitive loads while walking,” said Dr. Collett. “Here, we proposed that increased automatic gait control may provide a mechanism whereby executive resources are freed up to attend to additional tasks,” he added.
The investigators further hypothesized that greater walking speed is required for automatic gait control. To test these hypotheses, they analyzed data from a previously conducted randomized trial of single- and dual-task walking interventions.
Trial participants were aged 18 years or older, had survived a stroke that had occurred at least 6 months before enrollment, had reduced 2-minute walk distance relative to their peers, and had no comorbid neurologic or psychologic disorders.
Over 10 weeks, participants underwent 20 sessions that included 30 minutes of walking on a treadmill. They were randomly assigned to undergo single-task walking or dual-task walking. The latter incorporated cognitive tasks as distractions.
Good versus limited walkers
In the current study, investigators analyzed various assessments that had been conducted at baseline and after completion of the training sessions, including distance on 2-minute walks with and without a distracting task. In addition, participants underwent imaging with functional near-infrared spectroscopy (fNIRS) and fMRI.
Using previous research as a basis, the researchers defined good walking speed as 0.8 m/sec. They categorized all participants, regardless of their intervention assignments, as having good walking capacity (0.8 m/sec or more) or limited walking capacity (less than 0.79 m/sec).
A total of 50 participants enrolled in the study (mean age, 62 years), and 45 completed the interventions. Of those who completed the interventions, 22 were randomly assigned to undergo single-task training, and 23 were assigned to dual-task training.
The researchers categorized 21 participants as having good walking capacity and 24 as having limited walking capacity. Participants in each category were divided approximately evenly between treatment assignments.
Barthel index score, which assesses functional independence, was higher in the group of good walkers.
Increased travel distance
Results showed that after the interventions, distance traveled during the single-task 2-minute walk increased by 8.9 m for good walkers and by 5.3 m for limited walkers. For the dual-task 2-minute walk, the distance traveled increased by 10.4 m among good walkers and by 1.3 m for limited walkers. Change from baseline on the dual-task walk was not significant for limited walkers.
There was no significant difference between good walkers and limited walkers in their perceptions of participation in community walking. Neither group increased its walking activity significantly following the interventions.
At baseline, limited walkers, in comparison with good walkers, had significantly greater activation in the contralesional hemisphere during dual-task walking, which consisted of incorporating a planning task.
In contrast, for many good walkers, there was a decrease in activation during dual-task walking. Activation in the contralesional hemisphere correlated negatively with dual-task 2-minute walk distance.
The researchers also found a negative correlation between activation and dual-task 2-minute walk distance when the second task was the Stroop task.
Initial step
“The original trial was never designed or powered to compare groups formed by walking speed or test our automaticity hypothesis, and the results need to be viewed within this context,” said Dr. Collett. The small sample size did not allow the researchers to detect small effects of the intervention, especially in the imaging data, he added.
It also prevented the investigators from comparing limited walking and good walking groups according to whether they underwent the single-task or dual-task intervention, “which would be a superior way to investigate our hypotheses,” Dr. Collett said.
“The result of this study should be seen as exploratory, with further investigation needed,” he noted.
Helping stroke survivors to walk in the community is challenging, and new interventions that enable them to navigate complex surroundings need to be designed, said Dr. Collett. “Research is required to better understand the conscious and automatic contribution to gait control, especially with neurological impairment,” he added.
Overall, “our results suggest that improving automatic walking may be an initial step to improve capacity to respond to more complex walking interventions. However, [future] trials are required to test this,” he concluded.
The next frontier?
Commenting on the findings, Louis R. Caplan, MD, professor of neurology at Harvard University and senior neurologist at Beth Israel Deaconess Medical Center, Boston, said that “recovery and rehab are going to be the next frontier in stroke neurology, because there has to be a limitation in the present emphasis on acute care.”
Some patients do not receive acute care on time, and current treatment is not curative, added Dr. Caplan, who was not involved with the research.
Little scientific attention has been paid to how doctors can enhance recovery after stroke, what interventions delay recovery, and what the natural history of recovery is, he said. “This is a very nice study about that.”
Although the study’s methodology was sound, there were some limitations, including that strokes and underlying brain lesions were heterogeneous and that the study population was relatively small, Dr. Caplan said.
He added that “it’s a difficult study to do” and that it is difficult to organize participants into homogeneous groups.
Another limitation cited was lack of long-term follow-up that could indicate whether training provided sustained improvements in walking.
“It would be nice to revisit the same people later and see if their walking has improved, if they’re doing it differently, and if their subjective responses are different,” said Dr. Caplan.
In addition, the study did not examine whether the interventions made it easier for participants to walk with other people or to socialize more. “It may be that it really requires some time for them to gain confidence and for them to integrate that into their social network,” Dr. Caplan said.
“I would call it a proof-of-principle study, not a final study,” he noted. “It’s a study that shows that you can scientifically study rehab” and indicates the possible methodology that could be used.
The study was funded by the Stroke Association. Dr. Collett and Dr. Caplan have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, suggests new research backed by imaging data.
In secondary analysis of a previous study, training enabled both “good” and “limited” walkers to increase travel distance during a 2-minute walk. However, for “dual-task” walking, good walkers improved their distance by approximately 10 m after training, whereas limited walkers improved by only 1 m. Brain imaging showed increased brain activity in the limited walkers, which could reduce cognitive resources available for performing a second task while walking.
These findings, which were published online May 30 in Clinical Rehabilitation, may explain the apparent lack of superiority, shown previously, of dual-task training compared with single-task training for patients with stroke and impaired walking ability, researchers noted.
“Imaging data were consistent with our hypothesis that walking automaticity might explain these results,” said lead author Johnny Collett, PhD, senior clinical research fellow at Oxford Brookes University, United Kingdom.
At baseline, participants who walked slowly had increased resting state connectivity between contralesional M1 and cortical areas associated with conscious gait control.
“In response to the intervention, we found increased connectivity with the precuneus in those who walked slowly at baseline, an adaptation that might support walking in more complex situations,” Dr. Collett said.
Benefits questioned
After stroke, many patients have difficulty walking while performing a second task, such as holding a conversation. Training in dual-task walking has provided uncertain benefits, according to clinical research.
In healthy individuals, walking is believed to be a largely automatic process that requires minimal executive resources. Previous studies have suggested that a certain minimum walking speed is required to enable automatic control of walking in the brain.
“We know that those with better walking ability after stroke are better able to cope with additional cognitive loads while walking,” said Dr. Collett. “Here, we proposed that increased automatic gait control may provide a mechanism whereby executive resources are freed up to attend to additional tasks,” he added.
The investigators further hypothesized that greater walking speed is required for automatic gait control. To test these hypotheses, they analyzed data from a previously conducted randomized trial of single- and dual-task walking interventions.
Trial participants were aged 18 years or older, had survived a stroke that had occurred at least 6 months before enrollment, had reduced 2-minute walk distance relative to their peers, and had no comorbid neurologic or psychologic disorders.
Over 10 weeks, participants underwent 20 sessions that included 30 minutes of walking on a treadmill. They were randomly assigned to undergo single-task walking or dual-task walking. The latter incorporated cognitive tasks as distractions.
Good versus limited walkers
In the current study, investigators analyzed various assessments that had been conducted at baseline and after completion of the training sessions, including distance on 2-minute walks with and without a distracting task. In addition, participants underwent imaging with functional near-infrared spectroscopy (fNIRS) and fMRI.
Using previous research as a basis, the researchers defined good walking speed as 0.8 m/sec. They categorized all participants, regardless of their intervention assignments, as having good walking capacity (0.8 m/sec or more) or limited walking capacity (less than 0.79 m/sec).
A total of 50 participants enrolled in the study (mean age, 62 years), and 45 completed the interventions. Of those who completed the interventions, 22 were randomly assigned to undergo single-task training, and 23 were assigned to dual-task training.
The researchers categorized 21 participants as having good walking capacity and 24 as having limited walking capacity. Participants in each category were divided approximately evenly between treatment assignments.
Barthel index score, which assesses functional independence, was higher in the group of good walkers.
Increased travel distance
Results showed that after the interventions, distance traveled during the single-task 2-minute walk increased by 8.9 m for good walkers and by 5.3 m for limited walkers. For the dual-task 2-minute walk, the distance traveled increased by 10.4 m among good walkers and by 1.3 m for limited walkers. Change from baseline on the dual-task walk was not significant for limited walkers.
There was no significant difference between good walkers and limited walkers in their perceptions of participation in community walking. Neither group increased its walking activity significantly following the interventions.
At baseline, limited walkers, in comparison with good walkers, had significantly greater activation in the contralesional hemisphere during dual-task walking, which consisted of incorporating a planning task.
In contrast, for many good walkers, there was a decrease in activation during dual-task walking. Activation in the contralesional hemisphere correlated negatively with dual-task 2-minute walk distance.
The researchers also found a negative correlation between activation and dual-task 2-minute walk distance when the second task was the Stroop task.
Initial step
“The original trial was never designed or powered to compare groups formed by walking speed or test our automaticity hypothesis, and the results need to be viewed within this context,” said Dr. Collett. The small sample size did not allow the researchers to detect small effects of the intervention, especially in the imaging data, he added.
It also prevented the investigators from comparing limited walking and good walking groups according to whether they underwent the single-task or dual-task intervention, “which would be a superior way to investigate our hypotheses,” Dr. Collett said.
“The result of this study should be seen as exploratory, with further investigation needed,” he noted.
Helping stroke survivors to walk in the community is challenging, and new interventions that enable them to navigate complex surroundings need to be designed, said Dr. Collett. “Research is required to better understand the conscious and automatic contribution to gait control, especially with neurological impairment,” he added.
Overall, “our results suggest that improving automatic walking may be an initial step to improve capacity to respond to more complex walking interventions. However, [future] trials are required to test this,” he concluded.
The next frontier?
Commenting on the findings, Louis R. Caplan, MD, professor of neurology at Harvard University and senior neurologist at Beth Israel Deaconess Medical Center, Boston, said that “recovery and rehab are going to be the next frontier in stroke neurology, because there has to be a limitation in the present emphasis on acute care.”
Some patients do not receive acute care on time, and current treatment is not curative, added Dr. Caplan, who was not involved with the research.
Little scientific attention has been paid to how doctors can enhance recovery after stroke, what interventions delay recovery, and what the natural history of recovery is, he said. “This is a very nice study about that.”
Although the study’s methodology was sound, there were some limitations, including that strokes and underlying brain lesions were heterogeneous and that the study population was relatively small, Dr. Caplan said.
He added that “it’s a difficult study to do” and that it is difficult to organize participants into homogeneous groups.
Another limitation cited was lack of long-term follow-up that could indicate whether training provided sustained improvements in walking.
“It would be nice to revisit the same people later and see if their walking has improved, if they’re doing it differently, and if their subjective responses are different,” said Dr. Caplan.
In addition, the study did not examine whether the interventions made it easier for participants to walk with other people or to socialize more. “It may be that it really requires some time for them to gain confidence and for them to integrate that into their social network,” Dr. Caplan said.
“I would call it a proof-of-principle study, not a final study,” he noted. “It’s a study that shows that you can scientifically study rehab” and indicates the possible methodology that could be used.
The study was funded by the Stroke Association. Dr. Collett and Dr. Caplan have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
From Clinical Rehabilitation
What’s best for diabetes after metformin? GRADE outdated at outset
Liraglutide and insulin glargine outperformed glimepiride and sitagliptin as single add-on agents to metformin for treating patients with type 2 diabetes in a multicenter U.S. trial that randomized just over 5,000 patients.
. Results were reported at the virtual American Diabetes Association (ADA) 81st Scientific Sessions.
The comparison included two oral medications – the sulfonylurea glimepiride and dipeptidyl peptidase-4 (DPP-4) inhibitor sitagliptin – and two injectable medications – insulin glargine and glucagon-like peptide 1 (GLP-1) receptor agonist liraglutide.
The primary endpoint was change in A1c level and overall glycemic control. Secondary endpoints include changes in weight, as well as cardiovascular, renal, gastrointestinal, and other complications.
For the primary endpoint – keeping A1c levels below 7% – liraglutide and the basal insulin glargine both did this best and were almost equivalent.
During the average 5-year follow-up, the rates of patients progressing to a confirmed A1c of 7% or higher were 67% among patients randomized to insulin glargine, 68% maintained on liraglutide, 72% taking the sulfonylurea glimepiride, and 77% taking sitagliptin, reported John M. Lachin, ScD, a biostatistician at George Washington University, Washington.
Too soon for take-aways, or are the data already obsolete?
“The ultimate goal of GRADE is to help clinicians select the therapies that will work best for individual patients, as diabetes care is not a one-size-fits all approach,” noted David M. Nathan, MD, chair of the study and director of the Diabetes Center at Massachusetts General Hospital, in an ADA press release.
Dr. Nathan, as well as several other members of the GRADE trial steering committee who presented results, repeatedly cautioned that the findings were preliminary because they represent 90% of outcomes, with the remaining 10% still to be adjudicated.
“We undertook this study to fill a gap in the guidelines,” said investigator Deborah J. Wexler, MD, clinical director of the Diabetes Center at Massachusetts General Hospital in Boston. “I would like to have all the results in ... before I comment on how the guidelines should change.”
“The metabolic data are solid, but the cardiovascular disease data are preliminary,” warned Dr. Nathan.
But that didn’t stop some from drawing their own conclusions, with Julio Rosenstock, MD, who comoderated the session but was not involved with the study, giving his own opinion.
“A pleasant surprise was the performance of basal insulin,” he said, calling the findings “a vindication” for basal insulin as a treatment for the types of patients with type 2 diabetes that enrolled in the study.
Steven E. Kahn, MB, ChB, another GRADE co-investigator agreed. “Based on the results, guidelines should say that you add insulin early on,” he observed.
A generic basal insulin and a generic sulfonylurea are both reasonable options, after metformin, for patients with limited resources, added Dr. Kahn, an endocrinologist and professor at the University of Washington, Seattle.
Dr. Rosenstock, director of the Dallas Diabetes Research Center, also saw the results as an indictment of agents in the DDP-4 inhibitor class, such as sitagliptin.
The DPP-4 inhibitors generate $9 billion a year, he said, wondering whether it “is justifiable to put them on the same level as other agents?”
Meanwhile the assigned discussant, David R. Matthews, DPhil, a professor of diabetes medicine at the University of Oxford, England – while congratulating the investigators on certain aspects of the study – said it ultimately fell short because it didn’t include an arm with an SGLT2 inhibitor.
“We should kick the authors for missing out on SGLT2 inhibitors,” Dr. Matthews said. “The omission means that the GRADE data are already obsolescent.”
In reply, Dr. Nathan admitted “we feel bad we did not include” an SGLT2 inhibitor, but he vigorously defended the dilemma faced by the trial’s organizers.
Oral SGLT2 inhibitors were not “well-established drugs” for type 2 diabetes when enrollment launched in 2013, and the researchers were wary of including what could turn out to be a problematic agent soon after controversy over the safety of agents in the thiazolidinedione drug class (such as rosiglitazone), he explained.
They also realized that adding a fifth drug to the study would necessitate doubling enrollment size, which would have undercut the funding plans already in place.
Dr. Matthews also derided GRADE as being underpowered to adequately address the impact of the tested agents on major adverse cardiovascular events (MACE) and hospitalizations for heart failure and too U.S.-centric to be generalizable elsewhere.
A study with lots of data
The roughly 5,000 patients enrolled in GRADE were an average age of 57 years old, 64% were men, 66% were White, and 20% were Black. They had had type 2 diabetes, on average, for 4.2 years. Mean body mass index at entry was about 34 kg/m2, average A1c was 7.5%, and average estimated glomerular filtration rate was 95 mL/min/1.73m2. The trial included a 6-12 week run-in period during which background metformin treatment was optimized and led to average A1c levels less than 7%.
Patients were then randomized to one of the four agents as add-on treatment.
Both liraglutide and insulin glargine performed well on many of the numerous metrics in the data-rich trial, largely funded by two branches of the National Institutes of Health, with commercial involvement limited to free supplies of the study drugs.
The secondary metabolic outcome, of disease progressing to a confirmed A1c of 7.5%, was reached by 39% of patients taking insulin glargine, significantly lower than the rate of 46% among patients taking liraglutide, and that rate, in turn, was significantly below the 50% rate among patients taking glimepiride and the 55% rate of those taking sitagliptin.
Mean doses of the second-line agents after 4 years of treatment were 38.3 units/day for glargine, 3.5 mg/day for glimepiride, 1.3 mg/day for subcutaneous liraglutide, and 82.9 mg/day for sitagliptin.
A trio of cardiovascular outcomes showed one significant benefit of liraglutide over the other three drugs for the endpoint of any cardiovascular event, which included not only major adverse cardiovascular events (MACE; cardiovascular death, myocardial infarction, or stroke), but also several other event types, including heart failure requiring hospitalization, unstable angina requiring hospitalization, revascularization or any arterial repairs, stent thrombosis, or transient ischemic attack.
For the endpoint of any cardiovascular event, the rate was 5.8% for patients taking liraglutide, significantly less than the rate of 7.6% of those taking insulin glargine, 8.0% for glimepiride, and 8.6% for sitagliptin, reported John B. Buse, MD, PhD, professor, chief of endocrinology, and director of the Diabetes Center at the University of North Carolina at Chapel Hill.
For each of the other two main cardiovascular endpoints – MACE and hospitalization for heart failure – liraglutide had a numeric advantage over the other three drugs but failed to reach significance.
Patients taking liraglutide also had a smaller but not significantly different point estimate for all-cause death, at 2.1%, compared with 3.1%-3.4% in the other three groups.
And, Dr. Nathan emphasized, the cardiovascular disease data are still considered preliminary.
Liraglutide scored a pair of additional outcome victories. Its use resulted in a significantly lower rate of patients who progressed during follow-up to either needing antihypertensive medications or having their blood pressure rise above 140/90 mm Hg compared with the other three drugs. (At baseline, average blood pressure for all patients was 128/77 mm Hg.)
And after 4 years, patients taking liraglutide lost an average of about 4 kg (8.8 lb) from their baseline weight (which averaged about 100 kg [220 lb]), roughly the same as patients taking sitagliptin but significantly better than with glimepiride or insulin glargine. Patients taking glargine gained a small amount of weight on average during their first couple of years of treatment, roughly 1 kg, but returned to around their baseline weight by the end of 4 years.
Four drugs performed equally well for some outcomes
Finally, the four drugs had similar results for some outcomes. This included their effects on renal function, distal sensory polyneuropathy, and low-density lipoprotein (LDL) cholesterol.
The four agents also had roughly similar safety profiles, with rates of serious adverse events all falling within the tight range of 33%-37%.
But the rate of severe hypoglycemic episodes that required assistance to treat showed significant separation, ranging from 2.3% for glimepiride, 1.4% for glargine, 0.9% for liraglutide, and 0.7% for sitagliptin. Gastrointestinal symptoms occurred in about 50% of patients in three of the treatment groups but were significantly higher in those taking liraglutide, affecting 60%.
GRADE received no commercial funding. Dr. Wexler has reported serving on data monitoring committees for Novo Nordisk. Dr. Buse has reported being a consultant for and holding stock in numerous companies. Dr. Rosenstock has reported being an advisor or consultant to Applied Therapeutics, Boehringer Ingelheim, Hanmi Pharmaceutical, Intarcia Therapeutics, Lilly, Novo Nordisk, Oramed, and Sanofi and has received research support from numerous companies. Dr. Kahn has reported being an advisor to or speaker on behalf of Bayer, Boehringer Ingelheim, Casma Therapeutics, Intarcia Therapeutics, Lilly, Merck, Novo Nordisk, Pfizer, and Third Rock Ventures. Dr. Matthews has reported receiving lecture and advisor fees from Merck, Novartis, Novo Nordisk, Sanofi Aventis, and Servier. Dr. Lachin and Dr. Nathan have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Liraglutide and insulin glargine outperformed glimepiride and sitagliptin as single add-on agents to metformin for treating patients with type 2 diabetes in a multicenter U.S. trial that randomized just over 5,000 patients.
. Results were reported at the virtual American Diabetes Association (ADA) 81st Scientific Sessions.
The comparison included two oral medications – the sulfonylurea glimepiride and dipeptidyl peptidase-4 (DPP-4) inhibitor sitagliptin – and two injectable medications – insulin glargine and glucagon-like peptide 1 (GLP-1) receptor agonist liraglutide.
The primary endpoint was change in A1c level and overall glycemic control. Secondary endpoints include changes in weight, as well as cardiovascular, renal, gastrointestinal, and other complications.
For the primary endpoint – keeping A1c levels below 7% – liraglutide and the basal insulin glargine both did this best and were almost equivalent.
During the average 5-year follow-up, the rates of patients progressing to a confirmed A1c of 7% or higher were 67% among patients randomized to insulin glargine, 68% maintained on liraglutide, 72% taking the sulfonylurea glimepiride, and 77% taking sitagliptin, reported John M. Lachin, ScD, a biostatistician at George Washington University, Washington.
Too soon for take-aways, or are the data already obsolete?
“The ultimate goal of GRADE is to help clinicians select the therapies that will work best for individual patients, as diabetes care is not a one-size-fits all approach,” noted David M. Nathan, MD, chair of the study and director of the Diabetes Center at Massachusetts General Hospital, in an ADA press release.
Dr. Nathan, as well as several other members of the GRADE trial steering committee who presented results, repeatedly cautioned that the findings were preliminary because they represent 90% of outcomes, with the remaining 10% still to be adjudicated.
“We undertook this study to fill a gap in the guidelines,” said investigator Deborah J. Wexler, MD, clinical director of the Diabetes Center at Massachusetts General Hospital in Boston. “I would like to have all the results in ... before I comment on how the guidelines should change.”
“The metabolic data are solid, but the cardiovascular disease data are preliminary,” warned Dr. Nathan.
But that didn’t stop some from drawing their own conclusions, with Julio Rosenstock, MD, who comoderated the session but was not involved with the study, giving his own opinion.
“A pleasant surprise was the performance of basal insulin,” he said, calling the findings “a vindication” for basal insulin as a treatment for the types of patients with type 2 diabetes that enrolled in the study.
Steven E. Kahn, MB, ChB, another GRADE co-investigator agreed. “Based on the results, guidelines should say that you add insulin early on,” he observed.
A generic basal insulin and a generic sulfonylurea are both reasonable options, after metformin, for patients with limited resources, added Dr. Kahn, an endocrinologist and professor at the University of Washington, Seattle.
Dr. Rosenstock, director of the Dallas Diabetes Research Center, also saw the results as an indictment of agents in the DDP-4 inhibitor class, such as sitagliptin.
The DPP-4 inhibitors generate $9 billion a year, he said, wondering whether it “is justifiable to put them on the same level as other agents?”
Meanwhile the assigned discussant, David R. Matthews, DPhil, a professor of diabetes medicine at the University of Oxford, England – while congratulating the investigators on certain aspects of the study – said it ultimately fell short because it didn’t include an arm with an SGLT2 inhibitor.
“We should kick the authors for missing out on SGLT2 inhibitors,” Dr. Matthews said. “The omission means that the GRADE data are already obsolescent.”
In reply, Dr. Nathan admitted “we feel bad we did not include” an SGLT2 inhibitor, but he vigorously defended the dilemma faced by the trial’s organizers.
Oral SGLT2 inhibitors were not “well-established drugs” for type 2 diabetes when enrollment launched in 2013, and the researchers were wary of including what could turn out to be a problematic agent soon after controversy over the safety of agents in the thiazolidinedione drug class (such as rosiglitazone), he explained.
They also realized that adding a fifth drug to the study would necessitate doubling enrollment size, which would have undercut the funding plans already in place.
Dr. Matthews also derided GRADE as being underpowered to adequately address the impact of the tested agents on major adverse cardiovascular events (MACE) and hospitalizations for heart failure and too U.S.-centric to be generalizable elsewhere.
A study with lots of data
The roughly 5,000 patients enrolled in GRADE were an average age of 57 years old, 64% were men, 66% were White, and 20% were Black. They had had type 2 diabetes, on average, for 4.2 years. Mean body mass index at entry was about 34 kg/m2, average A1c was 7.5%, and average estimated glomerular filtration rate was 95 mL/min/1.73m2. The trial included a 6-12 week run-in period during which background metformin treatment was optimized and led to average A1c levels less than 7%.
Patients were then randomized to one of the four agents as add-on treatment.
Both liraglutide and insulin glargine performed well on many of the numerous metrics in the data-rich trial, largely funded by two branches of the National Institutes of Health, with commercial involvement limited to free supplies of the study drugs.
The secondary metabolic outcome, of disease progressing to a confirmed A1c of 7.5%, was reached by 39% of patients taking insulin glargine, significantly lower than the rate of 46% among patients taking liraglutide, and that rate, in turn, was significantly below the 50% rate among patients taking glimepiride and the 55% rate of those taking sitagliptin.
Mean doses of the second-line agents after 4 years of treatment were 38.3 units/day for glargine, 3.5 mg/day for glimepiride, 1.3 mg/day for subcutaneous liraglutide, and 82.9 mg/day for sitagliptin.
A trio of cardiovascular outcomes showed one significant benefit of liraglutide over the other three drugs for the endpoint of any cardiovascular event, which included not only major adverse cardiovascular events (MACE; cardiovascular death, myocardial infarction, or stroke), but also several other event types, including heart failure requiring hospitalization, unstable angina requiring hospitalization, revascularization or any arterial repairs, stent thrombosis, or transient ischemic attack.
For the endpoint of any cardiovascular event, the rate was 5.8% for patients taking liraglutide, significantly less than the rate of 7.6% of those taking insulin glargine, 8.0% for glimepiride, and 8.6% for sitagliptin, reported John B. Buse, MD, PhD, professor, chief of endocrinology, and director of the Diabetes Center at the University of North Carolina at Chapel Hill.
For each of the other two main cardiovascular endpoints – MACE and hospitalization for heart failure – liraglutide had a numeric advantage over the other three drugs but failed to reach significance.
Patients taking liraglutide also had a smaller but not significantly different point estimate for all-cause death, at 2.1%, compared with 3.1%-3.4% in the other three groups.
And, Dr. Nathan emphasized, the cardiovascular disease data are still considered preliminary.
Liraglutide scored a pair of additional outcome victories. Its use resulted in a significantly lower rate of patients who progressed during follow-up to either needing antihypertensive medications or having their blood pressure rise above 140/90 mm Hg compared with the other three drugs. (At baseline, average blood pressure for all patients was 128/77 mm Hg.)
And after 4 years, patients taking liraglutide lost an average of about 4 kg (8.8 lb) from their baseline weight (which averaged about 100 kg [220 lb]), roughly the same as patients taking sitagliptin but significantly better than with glimepiride or insulin glargine. Patients taking glargine gained a small amount of weight on average during their first couple of years of treatment, roughly 1 kg, but returned to around their baseline weight by the end of 4 years.
Four drugs performed equally well for some outcomes
Finally, the four drugs had similar results for some outcomes. This included their effects on renal function, distal sensory polyneuropathy, and low-density lipoprotein (LDL) cholesterol.
The four agents also had roughly similar safety profiles, with rates of serious adverse events all falling within the tight range of 33%-37%.
But the rate of severe hypoglycemic episodes that required assistance to treat showed significant separation, ranging from 2.3% for glimepiride, 1.4% for glargine, 0.9% for liraglutide, and 0.7% for sitagliptin. Gastrointestinal symptoms occurred in about 50% of patients in three of the treatment groups but were significantly higher in those taking liraglutide, affecting 60%.
GRADE received no commercial funding. Dr. Wexler has reported serving on data monitoring committees for Novo Nordisk. Dr. Buse has reported being a consultant for and holding stock in numerous companies. Dr. Rosenstock has reported being an advisor or consultant to Applied Therapeutics, Boehringer Ingelheim, Hanmi Pharmaceutical, Intarcia Therapeutics, Lilly, Novo Nordisk, Oramed, and Sanofi and has received research support from numerous companies. Dr. Kahn has reported being an advisor to or speaker on behalf of Bayer, Boehringer Ingelheim, Casma Therapeutics, Intarcia Therapeutics, Lilly, Merck, Novo Nordisk, Pfizer, and Third Rock Ventures. Dr. Matthews has reported receiving lecture and advisor fees from Merck, Novartis, Novo Nordisk, Sanofi Aventis, and Servier. Dr. Lachin and Dr. Nathan have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Liraglutide and insulin glargine outperformed glimepiride and sitagliptin as single add-on agents to metformin for treating patients with type 2 diabetes in a multicenter U.S. trial that randomized just over 5,000 patients.
. Results were reported at the virtual American Diabetes Association (ADA) 81st Scientific Sessions.
The comparison included two oral medications – the sulfonylurea glimepiride and dipeptidyl peptidase-4 (DPP-4) inhibitor sitagliptin – and two injectable medications – insulin glargine and glucagon-like peptide 1 (GLP-1) receptor agonist liraglutide.
The primary endpoint was change in A1c level and overall glycemic control. Secondary endpoints include changes in weight, as well as cardiovascular, renal, gastrointestinal, and other complications.
For the primary endpoint – keeping A1c levels below 7% – liraglutide and the basal insulin glargine both did this best and were almost equivalent.
During the average 5-year follow-up, the rates of patients progressing to a confirmed A1c of 7% or higher were 67% among patients randomized to insulin glargine, 68% maintained on liraglutide, 72% taking the sulfonylurea glimepiride, and 77% taking sitagliptin, reported John M. Lachin, ScD, a biostatistician at George Washington University, Washington.
Too soon for take-aways, or are the data already obsolete?
“The ultimate goal of GRADE is to help clinicians select the therapies that will work best for individual patients, as diabetes care is not a one-size-fits all approach,” noted David M. Nathan, MD, chair of the study and director of the Diabetes Center at Massachusetts General Hospital, in an ADA press release.
Dr. Nathan, as well as several other members of the GRADE trial steering committee who presented results, repeatedly cautioned that the findings were preliminary because they represent 90% of outcomes, with the remaining 10% still to be adjudicated.
“We undertook this study to fill a gap in the guidelines,” said investigator Deborah J. Wexler, MD, clinical director of the Diabetes Center at Massachusetts General Hospital in Boston. “I would like to have all the results in ... before I comment on how the guidelines should change.”
“The metabolic data are solid, but the cardiovascular disease data are preliminary,” warned Dr. Nathan.
But that didn’t stop some from drawing their own conclusions, with Julio Rosenstock, MD, who comoderated the session but was not involved with the study, giving his own opinion.
“A pleasant surprise was the performance of basal insulin,” he said, calling the findings “a vindication” for basal insulin as a treatment for the types of patients with type 2 diabetes that enrolled in the study.
Steven E. Kahn, MB, ChB, another GRADE co-investigator agreed. “Based on the results, guidelines should say that you add insulin early on,” he observed.
A generic basal insulin and a generic sulfonylurea are both reasonable options, after metformin, for patients with limited resources, added Dr. Kahn, an endocrinologist and professor at the University of Washington, Seattle.
Dr. Rosenstock, director of the Dallas Diabetes Research Center, also saw the results as an indictment of agents in the DDP-4 inhibitor class, such as sitagliptin.
The DPP-4 inhibitors generate $9 billion a year, he said, wondering whether it “is justifiable to put them on the same level as other agents?”
Meanwhile the assigned discussant, David R. Matthews, DPhil, a professor of diabetes medicine at the University of Oxford, England – while congratulating the investigators on certain aspects of the study – said it ultimately fell short because it didn’t include an arm with an SGLT2 inhibitor.
“We should kick the authors for missing out on SGLT2 inhibitors,” Dr. Matthews said. “The omission means that the GRADE data are already obsolescent.”
In reply, Dr. Nathan admitted “we feel bad we did not include” an SGLT2 inhibitor, but he vigorously defended the dilemma faced by the trial’s organizers.
Oral SGLT2 inhibitors were not “well-established drugs” for type 2 diabetes when enrollment launched in 2013, and the researchers were wary of including what could turn out to be a problematic agent soon after controversy over the safety of agents in the thiazolidinedione drug class (such as rosiglitazone), he explained.
They also realized that adding a fifth drug to the study would necessitate doubling enrollment size, which would have undercut the funding plans already in place.
Dr. Matthews also derided GRADE as being underpowered to adequately address the impact of the tested agents on major adverse cardiovascular events (MACE) and hospitalizations for heart failure and too U.S.-centric to be generalizable elsewhere.
A study with lots of data
The roughly 5,000 patients enrolled in GRADE were an average age of 57 years old, 64% were men, 66% were White, and 20% were Black. They had had type 2 diabetes, on average, for 4.2 years. Mean body mass index at entry was about 34 kg/m2, average A1c was 7.5%, and average estimated glomerular filtration rate was 95 mL/min/1.73m2. The trial included a 6-12 week run-in period during which background metformin treatment was optimized and led to average A1c levels less than 7%.
Patients were then randomized to one of the four agents as add-on treatment.
Both liraglutide and insulin glargine performed well on many of the numerous metrics in the data-rich trial, largely funded by two branches of the National Institutes of Health, with commercial involvement limited to free supplies of the study drugs.
The secondary metabolic outcome, of disease progressing to a confirmed A1c of 7.5%, was reached by 39% of patients taking insulin glargine, significantly lower than the rate of 46% among patients taking liraglutide, and that rate, in turn, was significantly below the 50% rate among patients taking glimepiride and the 55% rate of those taking sitagliptin.
Mean doses of the second-line agents after 4 years of treatment were 38.3 units/day for glargine, 3.5 mg/day for glimepiride, 1.3 mg/day for subcutaneous liraglutide, and 82.9 mg/day for sitagliptin.
A trio of cardiovascular outcomes showed one significant benefit of liraglutide over the other three drugs for the endpoint of any cardiovascular event, which included not only major adverse cardiovascular events (MACE; cardiovascular death, myocardial infarction, or stroke), but also several other event types, including heart failure requiring hospitalization, unstable angina requiring hospitalization, revascularization or any arterial repairs, stent thrombosis, or transient ischemic attack.
For the endpoint of any cardiovascular event, the rate was 5.8% for patients taking liraglutide, significantly less than the rate of 7.6% of those taking insulin glargine, 8.0% for glimepiride, and 8.6% for sitagliptin, reported John B. Buse, MD, PhD, professor, chief of endocrinology, and director of the Diabetes Center at the University of North Carolina at Chapel Hill.
For each of the other two main cardiovascular endpoints – MACE and hospitalization for heart failure – liraglutide had a numeric advantage over the other three drugs but failed to reach significance.
Patients taking liraglutide also had a smaller but not significantly different point estimate for all-cause death, at 2.1%, compared with 3.1%-3.4% in the other three groups.
And, Dr. Nathan emphasized, the cardiovascular disease data are still considered preliminary.
Liraglutide scored a pair of additional outcome victories. Its use resulted in a significantly lower rate of patients who progressed during follow-up to either needing antihypertensive medications or having their blood pressure rise above 140/90 mm Hg compared with the other three drugs. (At baseline, average blood pressure for all patients was 128/77 mm Hg.)
And after 4 years, patients taking liraglutide lost an average of about 4 kg (8.8 lb) from their baseline weight (which averaged about 100 kg [220 lb]), roughly the same as patients taking sitagliptin but significantly better than with glimepiride or insulin glargine. Patients taking glargine gained a small amount of weight on average during their first couple of years of treatment, roughly 1 kg, but returned to around their baseline weight by the end of 4 years.
Four drugs performed equally well for some outcomes
Finally, the four drugs had similar results for some outcomes. This included their effects on renal function, distal sensory polyneuropathy, and low-density lipoprotein (LDL) cholesterol.
The four agents also had roughly similar safety profiles, with rates of serious adverse events all falling within the tight range of 33%-37%.
But the rate of severe hypoglycemic episodes that required assistance to treat showed significant separation, ranging from 2.3% for glimepiride, 1.4% for glargine, 0.9% for liraglutide, and 0.7% for sitagliptin. Gastrointestinal symptoms occurred in about 50% of patients in three of the treatment groups but were significantly higher in those taking liraglutide, affecting 60%.
GRADE received no commercial funding. Dr. Wexler has reported serving on data monitoring committees for Novo Nordisk. Dr. Buse has reported being a consultant for and holding stock in numerous companies. Dr. Rosenstock has reported being an advisor or consultant to Applied Therapeutics, Boehringer Ingelheim, Hanmi Pharmaceutical, Intarcia Therapeutics, Lilly, Novo Nordisk, Oramed, and Sanofi and has received research support from numerous companies. Dr. Kahn has reported being an advisor to or speaker on behalf of Bayer, Boehringer Ingelheim, Casma Therapeutics, Intarcia Therapeutics, Lilly, Merck, Novo Nordisk, Pfizer, and Third Rock Ventures. Dr. Matthews has reported receiving lecture and advisor fees from Merck, Novartis, Novo Nordisk, Sanofi Aventis, and Servier. Dr. Lachin and Dr. Nathan have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Magnesium is strongly tied to lower risk for intracranial aneurysm
The effects may be partially mediated by magnesium’s influence on systolic blood pressure, new research suggests.
“The modifiable risk factors for intracranial aneurysm are largely unknown. Our findings provided evidence of a causal association between increased serum magnesium levels and reduced risk of intracranial aneurysm,” said Susanna Larsson, PhD, Karolinska Institutet, Stockholm.
These results suggest that raising serum magnesium levels – through a magnesium-rich diet or magnesium supplementation – “may play a role in the primary prevention of intracranial aneurysm and associated hemorrhage,” Dr. Larsson added.
The study was published online June 22 in Neurology.
Lower risk for rupture
The researchers leveraged randomly allocated genetic variants related to serum magnesium concentrations in a two-sample Mendelian randomization (MR) study to assess whether higher genetically predicted serum magnesium correlates with reduced risk for intracranial aneurysm. They also performed a multivariable MR analysis to assess the role blood pressure might play in this association.
Source data came from a genome-wide association study (GWAS) involving 23,829 individuals that previously identified five single-nucleotide polymorphisms associated with serum magnesium. Genetic association estimates for intracranial aneurysm were derived from a GWAS in 79,429 people (7,495 case patients and 71,934 control patients), and genetic association estimates for systolic blood pressure were derived from a GWAS of 757,601 individuals.
The researchers found that higher genetically predicted serum magnesium concentrations were associated with lower risk for intracranial aneurysm.
The odds ratios per 0.1 mmol/L increment in genetically predicted serum magnesium concentrations were 0.66 (95% confidence interval, 0.49-0.91) for intracranial aneurysm (unruptured and ruptured combined), 0.57 (95% CI, 0.30-1.06) for unruptured intracranial aneurysm, and 0.67 (95% CI, 0.48-0.92) for aneurysmal subarachnoid hemorrhage.
Adjustment for genetically predicted systolic blood pressure partially attenuated the associations of genetically predicted serum magnesium with all three outcomes, suggesting that magnesium’s influence was at least partially mediated by systolic blood pressure.
“In addition to a blood pressure lowering effect, increased magnesium concentrations may reduce the risk of intracranial aneurysm rupture by improving endothelial function and reducing oxidative stress,” the investigators noted.
They caution that the data were derived from people of European ancestry, which limits the generalizability to other populations. “Caution should be taken when extrapolating findings from MR to infer the effect of a clinical intervention, and clinical trials are warranted to guide optimal practice,” they added.
Critical role in vascular health
In an accompanying editorial, Joanna Pera, MD, PhD, of Jagiellonian University Medical College, Krakow, Poland, and Christopher Anderson, MD, of Brigham and Women’s Hospital, Boston, noted that the study “adds to our understanding of the importance of magnesium in vascular health particularly related to cerebral aneurysms.”
There is a need for “both mechanistic and potentially therapeutic investigation into the role that magnesium plays in subarachnoid hemorrhage,” they added.
Further, they wrote, the results “raise interesting new questions about the links between circulating magnesium, intracranial aneurysms, and blood pressure. Arterial hypertension is a well-recognized risk factor for intracranial aneurysm development and rupture. Magnesium supplementation may lower blood pressure values.
“Could this mineral prove useful in developing interventions that could prevent intracranial aneurysm development and/or rupture over and above a simple lowering of blood pressure, perhaps through pleiotropic effects on endothelial function or other mechanisms? With these results in hand, work is clearly needed to learn more about the biology of magnesium in the vascular system and in intracranial aneurysm biology in particular,” Dr. Pera and Dr. Anderson concluded.
This study was supported by the Swedish Research Council for Health, Working Life and Welfare, the British Heart Foundation Research Center of Excellence at Imperial College London, and the National Institute for Health Research Clinical Lectureship at St. George’s, University of London. Dr. Larsson has disclosed no relevant financial relationships. Study coauthor Dipender Gill, PhD, is employed part time by Novo Nordisk. Dr. Pera has disclosed no relevant financial relationships. Dr. Anderson has received research support from the Bayer AG and has consulted for ApoPharma and Invitae.
A version of this article first appeared on Medscape.com.
The effects may be partially mediated by magnesium’s influence on systolic blood pressure, new research suggests.
“The modifiable risk factors for intracranial aneurysm are largely unknown. Our findings provided evidence of a causal association between increased serum magnesium levels and reduced risk of intracranial aneurysm,” said Susanna Larsson, PhD, Karolinska Institutet, Stockholm.
These results suggest that raising serum magnesium levels – through a magnesium-rich diet or magnesium supplementation – “may play a role in the primary prevention of intracranial aneurysm and associated hemorrhage,” Dr. Larsson added.
The study was published online June 22 in Neurology.
Lower risk for rupture
The researchers leveraged randomly allocated genetic variants related to serum magnesium concentrations in a two-sample Mendelian randomization (MR) study to assess whether higher genetically predicted serum magnesium correlates with reduced risk for intracranial aneurysm. They also performed a multivariable MR analysis to assess the role blood pressure might play in this association.
Source data came from a genome-wide association study (GWAS) involving 23,829 individuals that previously identified five single-nucleotide polymorphisms associated with serum magnesium. Genetic association estimates for intracranial aneurysm were derived from a GWAS in 79,429 people (7,495 case patients and 71,934 control patients), and genetic association estimates for systolic blood pressure were derived from a GWAS of 757,601 individuals.
The researchers found that higher genetically predicted serum magnesium concentrations were associated with lower risk for intracranial aneurysm.
The odds ratios per 0.1 mmol/L increment in genetically predicted serum magnesium concentrations were 0.66 (95% confidence interval, 0.49-0.91) for intracranial aneurysm (unruptured and ruptured combined), 0.57 (95% CI, 0.30-1.06) for unruptured intracranial aneurysm, and 0.67 (95% CI, 0.48-0.92) for aneurysmal subarachnoid hemorrhage.
Adjustment for genetically predicted systolic blood pressure partially attenuated the associations of genetically predicted serum magnesium with all three outcomes, suggesting that magnesium’s influence was at least partially mediated by systolic blood pressure.
“In addition to a blood pressure lowering effect, increased magnesium concentrations may reduce the risk of intracranial aneurysm rupture by improving endothelial function and reducing oxidative stress,” the investigators noted.
They caution that the data were derived from people of European ancestry, which limits the generalizability to other populations. “Caution should be taken when extrapolating findings from MR to infer the effect of a clinical intervention, and clinical trials are warranted to guide optimal practice,” they added.
Critical role in vascular health
In an accompanying editorial, Joanna Pera, MD, PhD, of Jagiellonian University Medical College, Krakow, Poland, and Christopher Anderson, MD, of Brigham and Women’s Hospital, Boston, noted that the study “adds to our understanding of the importance of magnesium in vascular health particularly related to cerebral aneurysms.”
There is a need for “both mechanistic and potentially therapeutic investigation into the role that magnesium plays in subarachnoid hemorrhage,” they added.
Further, they wrote, the results “raise interesting new questions about the links between circulating magnesium, intracranial aneurysms, and blood pressure. Arterial hypertension is a well-recognized risk factor for intracranial aneurysm development and rupture. Magnesium supplementation may lower blood pressure values.
“Could this mineral prove useful in developing interventions that could prevent intracranial aneurysm development and/or rupture over and above a simple lowering of blood pressure, perhaps through pleiotropic effects on endothelial function or other mechanisms? With these results in hand, work is clearly needed to learn more about the biology of magnesium in the vascular system and in intracranial aneurysm biology in particular,” Dr. Pera and Dr. Anderson concluded.
This study was supported by the Swedish Research Council for Health, Working Life and Welfare, the British Heart Foundation Research Center of Excellence at Imperial College London, and the National Institute for Health Research Clinical Lectureship at St. George’s, University of London. Dr. Larsson has disclosed no relevant financial relationships. Study coauthor Dipender Gill, PhD, is employed part time by Novo Nordisk. Dr. Pera has disclosed no relevant financial relationships. Dr. Anderson has received research support from the Bayer AG and has consulted for ApoPharma and Invitae.
A version of this article first appeared on Medscape.com.
The effects may be partially mediated by magnesium’s influence on systolic blood pressure, new research suggests.
“The modifiable risk factors for intracranial aneurysm are largely unknown. Our findings provided evidence of a causal association between increased serum magnesium levels and reduced risk of intracranial aneurysm,” said Susanna Larsson, PhD, Karolinska Institutet, Stockholm.
These results suggest that raising serum magnesium levels – through a magnesium-rich diet or magnesium supplementation – “may play a role in the primary prevention of intracranial aneurysm and associated hemorrhage,” Dr. Larsson added.
The study was published online June 22 in Neurology.
Lower risk for rupture
The researchers leveraged randomly allocated genetic variants related to serum magnesium concentrations in a two-sample Mendelian randomization (MR) study to assess whether higher genetically predicted serum magnesium correlates with reduced risk for intracranial aneurysm. They also performed a multivariable MR analysis to assess the role blood pressure might play in this association.
Source data came from a genome-wide association study (GWAS) involving 23,829 individuals that previously identified five single-nucleotide polymorphisms associated with serum magnesium. Genetic association estimates for intracranial aneurysm were derived from a GWAS in 79,429 people (7,495 case patients and 71,934 control patients), and genetic association estimates for systolic blood pressure were derived from a GWAS of 757,601 individuals.
The researchers found that higher genetically predicted serum magnesium concentrations were associated with lower risk for intracranial aneurysm.
The odds ratios per 0.1 mmol/L increment in genetically predicted serum magnesium concentrations were 0.66 (95% confidence interval, 0.49-0.91) for intracranial aneurysm (unruptured and ruptured combined), 0.57 (95% CI, 0.30-1.06) for unruptured intracranial aneurysm, and 0.67 (95% CI, 0.48-0.92) for aneurysmal subarachnoid hemorrhage.
Adjustment for genetically predicted systolic blood pressure partially attenuated the associations of genetically predicted serum magnesium with all three outcomes, suggesting that magnesium’s influence was at least partially mediated by systolic blood pressure.
“In addition to a blood pressure lowering effect, increased magnesium concentrations may reduce the risk of intracranial aneurysm rupture by improving endothelial function and reducing oxidative stress,” the investigators noted.
They caution that the data were derived from people of European ancestry, which limits the generalizability to other populations. “Caution should be taken when extrapolating findings from MR to infer the effect of a clinical intervention, and clinical trials are warranted to guide optimal practice,” they added.
Critical role in vascular health
In an accompanying editorial, Joanna Pera, MD, PhD, of Jagiellonian University Medical College, Krakow, Poland, and Christopher Anderson, MD, of Brigham and Women’s Hospital, Boston, noted that the study “adds to our understanding of the importance of magnesium in vascular health particularly related to cerebral aneurysms.”
There is a need for “both mechanistic and potentially therapeutic investigation into the role that magnesium plays in subarachnoid hemorrhage,” they added.
Further, they wrote, the results “raise interesting new questions about the links between circulating magnesium, intracranial aneurysms, and blood pressure. Arterial hypertension is a well-recognized risk factor for intracranial aneurysm development and rupture. Magnesium supplementation may lower blood pressure values.
“Could this mineral prove useful in developing interventions that could prevent intracranial aneurysm development and/or rupture over and above a simple lowering of blood pressure, perhaps through pleiotropic effects on endothelial function or other mechanisms? With these results in hand, work is clearly needed to learn more about the biology of magnesium in the vascular system and in intracranial aneurysm biology in particular,” Dr. Pera and Dr. Anderson concluded.
This study was supported by the Swedish Research Council for Health, Working Life and Welfare, the British Heart Foundation Research Center of Excellence at Imperial College London, and the National Institute for Health Research Clinical Lectureship at St. George’s, University of London. Dr. Larsson has disclosed no relevant financial relationships. Study coauthor Dipender Gill, PhD, is employed part time by Novo Nordisk. Dr. Pera has disclosed no relevant financial relationships. Dr. Anderson has received research support from the Bayer AG and has consulted for ApoPharma and Invitae.
A version of this article first appeared on Medscape.com.
From Neurology



