User login
PSYCHIATRY UPDATE 2016
View summaries from the event on the following pages.
Thursday, March 10, 2016
Make Way for Possibilities of an Adjunctive Treatment for Major Depressive Disorder
Roueen Rafeyan, MD, Feinberg School of Medicine at Northwestern University
Successful Aging
George T. Grossberg, MD, Saint Louis University
Psychopharmacology and Pregnancy: The New Labeling Changes and Implications for Clinical Practice
Marlene P. Freeman, MD, Massachusetts General Hospital
Anxiety Disorders in Women Across the Lifecycle
Marlene P. Freeman, MD, Massachusetts General Hospital
Mild Cognitive Impairment: “Senior Moments” and DSM-5
George T. Grossberg, MD, Saint Louis University
Assessing major depressive disorder and an option for treatment
Jay D. Fawver, MD, Indiana University School of Medicine
Innovative Treatments of Anxiety, Part 1 (Use of Benzodiazepines)
Mark H. Pollack, MD, Rush University Medical Center
Innovative Treatments of Anxiety, Part 2 (Other Standard and Novel Therapeutic Approaches)
Mark H. Pollack, MD, Rush University Medical Center
Treatment of Chronic Depression
Andrew A. Nierenberg, MD, Massachusetts General Hospital
Friday, March 11, 2016
Subtypes of Depression
Andrew A. Nierenberg, MD, Massachusetts General Hospital, Alexian Brothers Behavioral Health Hospital for Violence Prevention Clinic/Program and ADHD Clinic
Managing ADHD: What Matters Most When Selecting a Treatment Option
Michael Feld, MD, Alexian Brothers Behavioral Health Hospital for Violence Prevention Clinic/Program and ADHD Clinic
Dr. Feld discussed the utility of the brand-name extended-release (ER) methylphenidate HCl (Aptensio) for its value in children—specifically, its ability to “extend the day” without additional dosing of a short-action medication. The design of Aptensio—a multilayered beaded delivery system in which every bead is both an immediate- and an extended-release vehicle—allows an early peak serum drug level and later peak level (at 8 hours). Aptensio is administered by sprinkling the contents of a capsule on applesauce; it is is safe practice, Dr. Feld explained, to augment the ER drug delivery with an immediate-release agent when deemed necessary, by observing how difficult it is for the patient to make it through the day at home, school, or work.
Overview of Autism Spectrum Disorder
Robert L. Hendren, DO, University of California, San Francisco
Comorbidity of Schizophrenia and Substance Abuse
Henry A. Nasrallah, MD, Saint Louis University
Overview of PTSD
Carol S. North, MD, MPE, DFAPA, University of Texas Southwestern Medical Center
Bipolar Depression: Presentation, Diagnosis, and Treatment in the Outpatient Psychiatry Practice Setting
Peter J. Weiden, MD, University of Illinois at Chicago
Neuroinflammation and Oxidative Stress in Schizophrenia and Mood Disorders: Biomarkers and Therapeutic Targets
Henry A. Nasrallah, MD, Saint Louis University
Clinical Management of Autism Spectrum Disorders: What Happens Over Time/Borderline Intellectual Functioning
Robert L. Hendren, DO, University of California, San Francisco
Management of PTSD
Carol S. North, MD, MPE, DFAPA, University of Texas Southwestern Medical Center
Saturday, March 12, 2015
Managing the Difficult Child
Anthony L. Rostain, MD, MA, University of Pennsylvania
Major Depression With Subsyndromal Mania/Hypomania: Implications for Diagnosis and Management
Trisha Suppes, MD, PhD, Stanford University School of Medicine, Roger S. McIntyre, MD, FRCPC, University of Toronto, and J. Craig Nelson, MD, University of California, San Francisco
General Overview of Sleep Disorders
Thomas Roth, PhD, Henry Ford Hospital
Comorbid ADHD with Substance Abuse
Anthony L. Rostain, MD, MA, University of Pennsylvania
How to Treat Patients with Insomnia
Thomas Roth, PhD, Henry Ford Hospital
personality disorder, DSM-5, adults with ADHD, residual depressive symptoms, treatment-resistant depression,antisocial personality disorder, bipolar disorder, schizophrenia, psychotic disorder, clozapine, bipolar disorder and substance abuse, mood disorders during pregnancy, premenstrual dysphoric disorder, depressive symptoms in perimenopause, smoking and the mentally ill, help patients with mental illness lose weight, substance abuse in older adults
View summaries from the event on the following pages.
Thursday, March 10, 2016
Make Way for Possibilities of an Adjunctive Treatment for Major Depressive Disorder
Roueen Rafeyan, MD, Feinberg School of Medicine at Northwestern University
Successful Aging
George T. Grossberg, MD, Saint Louis University
Psychopharmacology and Pregnancy: The New Labeling Changes and Implications for Clinical Practice
Marlene P. Freeman, MD, Massachusetts General Hospital
Anxiety Disorders in Women Across the Lifecycle
Marlene P. Freeman, MD, Massachusetts General Hospital
Mild Cognitive Impairment: “Senior Moments” and DSM-5
George T. Grossberg, MD, Saint Louis University
Assessing major depressive disorder and an option for treatment
Jay D. Fawver, MD, Indiana University School of Medicine
Innovative Treatments of Anxiety, Part 1 (Use of Benzodiazepines)
Mark H. Pollack, MD, Rush University Medical Center
Innovative Treatments of Anxiety, Part 2 (Other Standard and Novel Therapeutic Approaches)
Mark H. Pollack, MD, Rush University Medical Center
Treatment of Chronic Depression
Andrew A. Nierenberg, MD, Massachusetts General Hospital
Friday, March 11, 2016
Subtypes of Depression
Andrew A. Nierenberg, MD, Massachusetts General Hospital, Alexian Brothers Behavioral Health Hospital for Violence Prevention Clinic/Program and ADHD Clinic
Managing ADHD: What Matters Most When Selecting a Treatment Option
Michael Feld, MD, Alexian Brothers Behavioral Health Hospital for Violence Prevention Clinic/Program and ADHD Clinic
Dr. Feld discussed the utility of the brand-name extended-release (ER) methylphenidate HCl (Aptensio) for its value in children—specifically, its ability to “extend the day” without additional dosing of a short-action medication. The design of Aptensio—a multilayered beaded delivery system in which every bead is both an immediate- and an extended-release vehicle—allows an early peak serum drug level and later peak level (at 8 hours). Aptensio is administered by sprinkling the contents of a capsule on applesauce; it is is safe practice, Dr. Feld explained, to augment the ER drug delivery with an immediate-release agent when deemed necessary, by observing how difficult it is for the patient to make it through the day at home, school, or work.
Overview of Autism Spectrum Disorder
Robert L. Hendren, DO, University of California, San Francisco
Comorbidity of Schizophrenia and Substance Abuse
Henry A. Nasrallah, MD, Saint Louis University
Overview of PTSD
Carol S. North, MD, MPE, DFAPA, University of Texas Southwestern Medical Center
Bipolar Depression: Presentation, Diagnosis, and Treatment in the Outpatient Psychiatry Practice Setting
Peter J. Weiden, MD, University of Illinois at Chicago
Neuroinflammation and Oxidative Stress in Schizophrenia and Mood Disorders: Biomarkers and Therapeutic Targets
Henry A. Nasrallah, MD, Saint Louis University
Clinical Management of Autism Spectrum Disorders: What Happens Over Time/Borderline Intellectual Functioning
Robert L. Hendren, DO, University of California, San Francisco
Management of PTSD
Carol S. North, MD, MPE, DFAPA, University of Texas Southwestern Medical Center
Saturday, March 12, 2015
Managing the Difficult Child
Anthony L. Rostain, MD, MA, University of Pennsylvania
Major Depression With Subsyndromal Mania/Hypomania: Implications for Diagnosis and Management
Trisha Suppes, MD, PhD, Stanford University School of Medicine, Roger S. McIntyre, MD, FRCPC, University of Toronto, and J. Craig Nelson, MD, University of California, San Francisco
General Overview of Sleep Disorders
Thomas Roth, PhD, Henry Ford Hospital
Comorbid ADHD with Substance Abuse
Anthony L. Rostain, MD, MA, University of Pennsylvania
How to Treat Patients with Insomnia
Thomas Roth, PhD, Henry Ford Hospital
View summaries from the event on the following pages.
Thursday, March 10, 2016
Make Way for Possibilities of an Adjunctive Treatment for Major Depressive Disorder
Roueen Rafeyan, MD, Feinberg School of Medicine at Northwestern University
Successful Aging
George T. Grossberg, MD, Saint Louis University
Psychopharmacology and Pregnancy: The New Labeling Changes and Implications for Clinical Practice
Marlene P. Freeman, MD, Massachusetts General Hospital
Anxiety Disorders in Women Across the Lifecycle
Marlene P. Freeman, MD, Massachusetts General Hospital
Mild Cognitive Impairment: “Senior Moments” and DSM-5
George T. Grossberg, MD, Saint Louis University
Assessing major depressive disorder and an option for treatment
Jay D. Fawver, MD, Indiana University School of Medicine
Innovative Treatments of Anxiety, Part 1 (Use of Benzodiazepines)
Mark H. Pollack, MD, Rush University Medical Center
Innovative Treatments of Anxiety, Part 2 (Other Standard and Novel Therapeutic Approaches)
Mark H. Pollack, MD, Rush University Medical Center
Treatment of Chronic Depression
Andrew A. Nierenberg, MD, Massachusetts General Hospital
Friday, March 11, 2016
Subtypes of Depression
Andrew A. Nierenberg, MD, Massachusetts General Hospital, Alexian Brothers Behavioral Health Hospital for Violence Prevention Clinic/Program and ADHD Clinic
Managing ADHD: What Matters Most When Selecting a Treatment Option
Michael Feld, MD, Alexian Brothers Behavioral Health Hospital for Violence Prevention Clinic/Program and ADHD Clinic
Dr. Feld discussed the utility of the brand-name extended-release (ER) methylphenidate HCl (Aptensio) for its value in children—specifically, its ability to “extend the day” without additional dosing of a short-action medication. The design of Aptensio—a multilayered beaded delivery system in which every bead is both an immediate- and an extended-release vehicle—allows an early peak serum drug level and later peak level (at 8 hours). Aptensio is administered by sprinkling the contents of a capsule on applesauce; it is is safe practice, Dr. Feld explained, to augment the ER drug delivery with an immediate-release agent when deemed necessary, by observing how difficult it is for the patient to make it through the day at home, school, or work.
Overview of Autism Spectrum Disorder
Robert L. Hendren, DO, University of California, San Francisco
Comorbidity of Schizophrenia and Substance Abuse
Henry A. Nasrallah, MD, Saint Louis University
Overview of PTSD
Carol S. North, MD, MPE, DFAPA, University of Texas Southwestern Medical Center
Bipolar Depression: Presentation, Diagnosis, and Treatment in the Outpatient Psychiatry Practice Setting
Peter J. Weiden, MD, University of Illinois at Chicago
Neuroinflammation and Oxidative Stress in Schizophrenia and Mood Disorders: Biomarkers and Therapeutic Targets
Henry A. Nasrallah, MD, Saint Louis University
Clinical Management of Autism Spectrum Disorders: What Happens Over Time/Borderline Intellectual Functioning
Robert L. Hendren, DO, University of California, San Francisco
Management of PTSD
Carol S. North, MD, MPE, DFAPA, University of Texas Southwestern Medical Center
Saturday, March 12, 2015
Managing the Difficult Child
Anthony L. Rostain, MD, MA, University of Pennsylvania
Major Depression With Subsyndromal Mania/Hypomania: Implications for Diagnosis and Management
Trisha Suppes, MD, PhD, Stanford University School of Medicine, Roger S. McIntyre, MD, FRCPC, University of Toronto, and J. Craig Nelson, MD, University of California, San Francisco
General Overview of Sleep Disorders
Thomas Roth, PhD, Henry Ford Hospital
Comorbid ADHD with Substance Abuse
Anthony L. Rostain, MD, MA, University of Pennsylvania
How to Treat Patients with Insomnia
Thomas Roth, PhD, Henry Ford Hospital
personality disorder, DSM-5, adults with ADHD, residual depressive symptoms, treatment-resistant depression,antisocial personality disorder, bipolar disorder, schizophrenia, psychotic disorder, clozapine, bipolar disorder and substance abuse, mood disorders during pregnancy, premenstrual dysphoric disorder, depressive symptoms in perimenopause, smoking and the mentally ill, help patients with mental illness lose weight, substance abuse in older adults
personality disorder, DSM-5, adults with ADHD, residual depressive symptoms, treatment-resistant depression,antisocial personality disorder, bipolar disorder, schizophrenia, psychotic disorder, clozapine, bipolar disorder and substance abuse, mood disorders during pregnancy, premenstrual dysphoric disorder, depressive symptoms in perimenopause, smoking and the mentally ill, help patients with mental illness lose weight, substance abuse in older adults
Comorbid depression worsens asthma outcomes in older adults
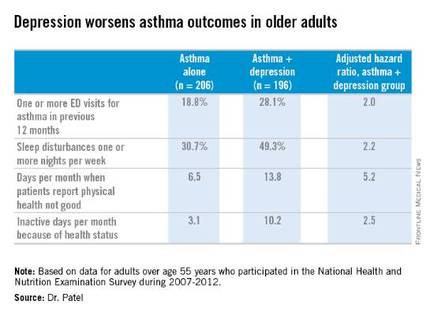
LOS ANGELES – Adults over age 55 with asthma and depression have nearly twice as many emergency department visits for asthma as do asthma patients without depression, based on findings in 402 asthma patients, Dr. Pooja O. Patel reported at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Comorbid depression also is associated with more asthma-related sleep disturbances and worse health-related quality of life, even though spirometry findings are similar in asthma patients with and without depression, added Dr. Patel of the University of Michigan, Ann Arbor.
She analyzed data on 7,256 adults over age 55 who participated in the National Health and Nutrition Examination Survey during 2007-2012. The prevalence of physician-diagnosed asthma in this nationally representative group of older adults was 5.5%. And 196 of those 402 asthma patients, or fully 49%, had comorbid depression as defined by their scores on the Patient Health Questionnaire-9 (PHQ-9), a validated brief and reliable self-administered measure of depression severity.
One or more emergency department visits for asthma within the last 12 months occurred in 18.8% of the group with asthma alone, compared with 28.1% in those with comorbid depression. In a multivariate regression analysis adjusted for the demographic differences, this translated to a twofold increased likelihood of ED visits specifically for asthma in the group with asthma and depression.
These data make a compelling case for routine screening for depression in older adults with asthma. The PHQ-9 is a good, simple tool for this purpose. Future studies will need to be done in order to learn whether early identification and treatment of comorbid depression in older asthmatic adults will result in improved asthma outcomes, but that is a reasonable hope, Dr. Patel added.
Why is asthma control worse in older adults with depression? In an interview, Dr. Patel said she thinks demographic differences may play a role. The older asthma patients with depression had less education and lower socioeconomic status than those without depression. Also, depression could adversely affect adherence to asthma controller medications, as depression is linked to worse medication adherence.
Dr. Patel reported having no financial conflicts regarding her study.
LOS ANGELES – Adults over age 55 with asthma and depression have nearly twice as many emergency department visits for asthma as do asthma patients without depression, based on findings in 402 asthma patients, Dr. Pooja O. Patel reported at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Comorbid depression also is associated with more asthma-related sleep disturbances and worse health-related quality of life, even though spirometry findings are similar in asthma patients with and without depression, added Dr. Patel of the University of Michigan, Ann Arbor.
She analyzed data on 7,256 adults over age 55 who participated in the National Health and Nutrition Examination Survey during 2007-2012. The prevalence of physician-diagnosed asthma in this nationally representative group of older adults was 5.5%. And 196 of those 402 asthma patients, or fully 49%, had comorbid depression as defined by their scores on the Patient Health Questionnaire-9 (PHQ-9), a validated brief and reliable self-administered measure of depression severity.
One or more emergency department visits for asthma within the last 12 months occurred in 18.8% of the group with asthma alone, compared with 28.1% in those with comorbid depression. In a multivariate regression analysis adjusted for the demographic differences, this translated to a twofold increased likelihood of ED visits specifically for asthma in the group with asthma and depression.
These data make a compelling case for routine screening for depression in older adults with asthma. The PHQ-9 is a good, simple tool for this purpose. Future studies will need to be done in order to learn whether early identification and treatment of comorbid depression in older asthmatic adults will result in improved asthma outcomes, but that is a reasonable hope, Dr. Patel added.

Why is asthma control worse in older adults with depression? In an interview, Dr. Patel said she thinks demographic differences may play a role. The older asthma patients with depression had less education and lower socioeconomic status than those without depression. Also, depression could adversely affect adherence to asthma controller medications, as depression is linked to worse medication adherence.
Dr. Patel reported having no financial conflicts regarding her study.
LOS ANGELES – Adults over age 55 with asthma and depression have nearly twice as many emergency department visits for asthma as do asthma patients without depression, based on findings in 402 asthma patients, Dr. Pooja O. Patel reported at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Comorbid depression also is associated with more asthma-related sleep disturbances and worse health-related quality of life, even though spirometry findings are similar in asthma patients with and without depression, added Dr. Patel of the University of Michigan, Ann Arbor.
She analyzed data on 7,256 adults over age 55 who participated in the National Health and Nutrition Examination Survey during 2007-2012. The prevalence of physician-diagnosed asthma in this nationally representative group of older adults was 5.5%. And 196 of those 402 asthma patients, or fully 49%, had comorbid depression as defined by their scores on the Patient Health Questionnaire-9 (PHQ-9), a validated brief and reliable self-administered measure of depression severity.
One or more emergency department visits for asthma within the last 12 months occurred in 18.8% of the group with asthma alone, compared with 28.1% in those with comorbid depression. In a multivariate regression analysis adjusted for the demographic differences, this translated to a twofold increased likelihood of ED visits specifically for asthma in the group with asthma and depression.
These data make a compelling case for routine screening for depression in older adults with asthma. The PHQ-9 is a good, simple tool for this purpose. Future studies will need to be done in order to learn whether early identification and treatment of comorbid depression in older asthmatic adults will result in improved asthma outcomes, but that is a reasonable hope, Dr. Patel added.

Why is asthma control worse in older adults with depression? In an interview, Dr. Patel said she thinks demographic differences may play a role. The older asthma patients with depression had less education and lower socioeconomic status than those without depression. Also, depression could adversely affect adherence to asthma controller medications, as depression is linked to worse medication adherence.
Dr. Patel reported having no financial conflicts regarding her study.
AT 2016 AAAAI ANNUAL MEETING
Key clinical point: Screening for depression in older adults with asthma has a high yield, with a 49% prevalence found in a nationally representative population.
Major finding: Older adults with asthma and depression were twice as likely as adults with asthma alone to have one or more emergency department visits for asthma in the past year.
Data source: An analysis of cross-sectional data on a nationally representative sample of 402 adults with physician-diagnosed asthma who participated in the National Health and Nutrition Examination Survey during 2007-2012.
Disclosures: The presenter reported having no financial conflicts regarding her study.
Financial toxicity is prevalent among patients with head and neck cancer
SCOTTSDALE, ARIZ. – Costs of care are a major burden in patients undergoing treatment for head and neck cancer, especially those who are socially isolated, finds a study reported at the Multidisciplinary Head and Neck Cancer Symposium.
More than two-thirds of the 73 patients with locally advanced disease had adopted life-altering strategies, such as tapping their savings or using credit to help pay for treatment, according to data presented in a poster session and related press briefing.
Patients who had a high perceived level of social isolation were more than 10 times as likely to have taken such actions than counterparts who had a medium or low level of isolation.
“Based on our study, a majority of patients rely on lifestyle-altering, cost-coping strategies to manage the financial side effects of head and neck cancer care. Financial side effects should be considered a morbidity of head and neck cancer,” commented lead author Sunny Kung, a second-year medical student at the University of Chicago and lead author on the study. “A lack of social support, coupled with increased loneliness is a risk factor for suboptimal medication adherence, missed appointments, and longer length of hospital stay.”
“Our study demonstrates that it is important for physicians to assess risk factors such as financial burden, loneliness, and [lack of] social support in order to provide optimal care for our patients,” she added. “Additional studies should be done to identify patient-specific interventions in order to help these patients optimize their care.”
Press briefing moderator Dr. Randall J. Kimple of the University of Wisconsin–Madison, noted, “One of the questions that our patients often ask and one of the things that we often spend time talking to our patients about is how long can they continue working after treatment. Was your dataset able to provide any insight into that?”
“Our study confirms that many of our patients are actually unable to continue working,” Ms. Kung replied. “I think head and neck cancer has some of the highest rates of disability of all the cancers – it’s something above 50% or 60%. I’m not sure about the exact number, but it’s quite high.”
In the study, the investigators followed patients starting treatment at the University of Chicago, surveying them monthly for 6 months about out-of-pocket costs, coping strategies, medication compliance, health care use, and perceived social isolation (loneliness and lack of social support).
During that 6-month period, 69% of patients overall used one or more lifestyle-altering strategies to cope with the costs of treatment. Specifically, 62% used part or all of their savings, 42% borrowed money or used credit, 25% sold possessions or property, and 23% had family members work more hours.
“We only assessed whether or not they used their savings, not how much of their savings they used,” Ms. Kung noted. “So this is a limitation of our study.”
The patients’ mean total monthly out-of-pocket costs totaled to $1,589. This was mainly driven by direct medical costs such as deductibles and hospital bills ($1,286), but insurance premiums also contributed ($303). Median values were lower but still substantial.
In multivariate analyses that controlled for potential confounders including factors such as marital status, patients were significantly and markedly more likely to resort to cost-coping strategies if they had Medicaid as compared with private insurance (odds ratio, 42.3). The likelihood rose with each $1,000 increment in total out-of-pocket costs (OR, 1.07) and fell with each $10,000 increment in wealth status (OR, 0.95). Patients who had a high perceived level of social isolation before starting treatment were also dramatically more likely to use these strategies (OR, 11.5).
Furthermore, on average, patients with a high level of social isolation skipped medication on more days (21.4 vs. 5.5; P = .03) and missed more appointments (7 vs. 3; P = .008).
“I believe it’s important for physicians to start screening patients – just as we do for depression – and identifying patients who have high perceived social isolation so that we can intervene earlier on, before they experience these negative financial side effects of their care,” concluded Ms. Kung.
SCOTTSDALE, ARIZ. – Costs of care are a major burden in patients undergoing treatment for head and neck cancer, especially those who are socially isolated, finds a study reported at the Multidisciplinary Head and Neck Cancer Symposium.
More than two-thirds of the 73 patients with locally advanced disease had adopted life-altering strategies, such as tapping their savings or using credit to help pay for treatment, according to data presented in a poster session and related press briefing.
Patients who had a high perceived level of social isolation were more than 10 times as likely to have taken such actions than counterparts who had a medium or low level of isolation.
“Based on our study, a majority of patients rely on lifestyle-altering, cost-coping strategies to manage the financial side effects of head and neck cancer care. Financial side effects should be considered a morbidity of head and neck cancer,” commented lead author Sunny Kung, a second-year medical student at the University of Chicago and lead author on the study. “A lack of social support, coupled with increased loneliness is a risk factor for suboptimal medication adherence, missed appointments, and longer length of hospital stay.”
“Our study demonstrates that it is important for physicians to assess risk factors such as financial burden, loneliness, and [lack of] social support in order to provide optimal care for our patients,” she added. “Additional studies should be done to identify patient-specific interventions in order to help these patients optimize their care.”
Press briefing moderator Dr. Randall J. Kimple of the University of Wisconsin–Madison, noted, “One of the questions that our patients often ask and one of the things that we often spend time talking to our patients about is how long can they continue working after treatment. Was your dataset able to provide any insight into that?”
“Our study confirms that many of our patients are actually unable to continue working,” Ms. Kung replied. “I think head and neck cancer has some of the highest rates of disability of all the cancers – it’s something above 50% or 60%. I’m not sure about the exact number, but it’s quite high.”
In the study, the investigators followed patients starting treatment at the University of Chicago, surveying them monthly for 6 months about out-of-pocket costs, coping strategies, medication compliance, health care use, and perceived social isolation (loneliness and lack of social support).
During that 6-month period, 69% of patients overall used one or more lifestyle-altering strategies to cope with the costs of treatment. Specifically, 62% used part or all of their savings, 42% borrowed money or used credit, 25% sold possessions or property, and 23% had family members work more hours.
“We only assessed whether or not they used their savings, not how much of their savings they used,” Ms. Kung noted. “So this is a limitation of our study.”
The patients’ mean total monthly out-of-pocket costs totaled to $1,589. This was mainly driven by direct medical costs such as deductibles and hospital bills ($1,286), but insurance premiums also contributed ($303). Median values were lower but still substantial.
In multivariate analyses that controlled for potential confounders including factors such as marital status, patients were significantly and markedly more likely to resort to cost-coping strategies if they had Medicaid as compared with private insurance (odds ratio, 42.3). The likelihood rose with each $1,000 increment in total out-of-pocket costs (OR, 1.07) and fell with each $10,000 increment in wealth status (OR, 0.95). Patients who had a high perceived level of social isolation before starting treatment were also dramatically more likely to use these strategies (OR, 11.5).
Furthermore, on average, patients with a high level of social isolation skipped medication on more days (21.4 vs. 5.5; P = .03) and missed more appointments (7 vs. 3; P = .008).
“I believe it’s important for physicians to start screening patients – just as we do for depression – and identifying patients who have high perceived social isolation so that we can intervene earlier on, before they experience these negative financial side effects of their care,” concluded Ms. Kung.
SCOTTSDALE, ARIZ. – Costs of care are a major burden in patients undergoing treatment for head and neck cancer, especially those who are socially isolated, finds a study reported at the Multidisciplinary Head and Neck Cancer Symposium.
More than two-thirds of the 73 patients with locally advanced disease had adopted life-altering strategies, such as tapping their savings or using credit to help pay for treatment, according to data presented in a poster session and related press briefing.
Patients who had a high perceived level of social isolation were more than 10 times as likely to have taken such actions than counterparts who had a medium or low level of isolation.
“Based on our study, a majority of patients rely on lifestyle-altering, cost-coping strategies to manage the financial side effects of head and neck cancer care. Financial side effects should be considered a morbidity of head and neck cancer,” commented lead author Sunny Kung, a second-year medical student at the University of Chicago and lead author on the study. “A lack of social support, coupled with increased loneliness is a risk factor for suboptimal medication adherence, missed appointments, and longer length of hospital stay.”
“Our study demonstrates that it is important for physicians to assess risk factors such as financial burden, loneliness, and [lack of] social support in order to provide optimal care for our patients,” she added. “Additional studies should be done to identify patient-specific interventions in order to help these patients optimize their care.”
Press briefing moderator Dr. Randall J. Kimple of the University of Wisconsin–Madison, noted, “One of the questions that our patients often ask and one of the things that we often spend time talking to our patients about is how long can they continue working after treatment. Was your dataset able to provide any insight into that?”
“Our study confirms that many of our patients are actually unable to continue working,” Ms. Kung replied. “I think head and neck cancer has some of the highest rates of disability of all the cancers – it’s something above 50% or 60%. I’m not sure about the exact number, but it’s quite high.”
In the study, the investigators followed patients starting treatment at the University of Chicago, surveying them monthly for 6 months about out-of-pocket costs, coping strategies, medication compliance, health care use, and perceived social isolation (loneliness and lack of social support).
During that 6-month period, 69% of patients overall used one or more lifestyle-altering strategies to cope with the costs of treatment. Specifically, 62% used part or all of their savings, 42% borrowed money or used credit, 25% sold possessions or property, and 23% had family members work more hours.
“We only assessed whether or not they used their savings, not how much of their savings they used,” Ms. Kung noted. “So this is a limitation of our study.”
The patients’ mean total monthly out-of-pocket costs totaled to $1,589. This was mainly driven by direct medical costs such as deductibles and hospital bills ($1,286), but insurance premiums also contributed ($303). Median values were lower but still substantial.
In multivariate analyses that controlled for potential confounders including factors such as marital status, patients were significantly and markedly more likely to resort to cost-coping strategies if they had Medicaid as compared with private insurance (odds ratio, 42.3). The likelihood rose with each $1,000 increment in total out-of-pocket costs (OR, 1.07) and fell with each $10,000 increment in wealth status (OR, 0.95). Patients who had a high perceived level of social isolation before starting treatment were also dramatically more likely to use these strategies (OR, 11.5).
Furthermore, on average, patients with a high level of social isolation skipped medication on more days (21.4 vs. 5.5; P = .03) and missed more appointments (7 vs. 3; P = .008).
“I believe it’s important for physicians to start screening patients – just as we do for depression – and identifying patients who have high perceived social isolation so that we can intervene earlier on, before they experience these negative financial side effects of their care,” concluded Ms. Kung.
AT THE MULTIDISCIPLINARY HEAD AND NECK CANCER SYMPOSIUM
Key clinical point: The majority of patients with head and neck cancer resort to steps such as tapping their savings to help pay for their care.
Major finding: Overall, 69% of patients used life-altering strategies to cope with costs, and those with a high level of social isolation were more likely to do so.
Data source: A prospective longitudinal cohort study of 73 patients with locally advanced head and neck cancer.
Disclosures: Ms. Kung disclosed that she had no relevant conflicts of interest.
Fibromyalgia doesn’t fit the disease model



‘We need to protect the brain’ Addressing the growing problem of chronic traumatic encephalopathy
The National Football League (NFL) had its highest concussion tally last year: 182 such injuries reported1 in the 2014-2015 regular season. The true rate of concussion in the NFL is likely higher, as a result of multiple factors (fear of “letting the team [or the coach] down,” fear of retaliation from team owners,2 etc.).
To simply call a head injury a “concussion” is a disservice to players and their family: Any blow to the head, severe or otherwise, has the potential to cause microvascular disruption in the brain; repeated blows to the head undoubtedly cause further damage.
In reality, a “concussion” is a mild traumatic brain injury (mTBI). With repeated blows, an mTBI can lead to chronic traumatic encephalopathy (CTE). In 2015, eighty-seven of 91 brains from autopsied former NFL players displayed some stage of CTE.3
Pathophysiology and presentation
CTE comprises 4 histological stages; Stage 4 is the most advanced. Alzheimer’s disease (AD) and CTE display similarities, which suggests a separate classification of CTE-AD; the presence of amyloid β plaques correlates with (1) more severe hyperphosphorylated tau (pTau) pathology and (2) advanced stages of the disease and clinical presentations. Death tends to occur 10 years earlier in CTE-AD than in AD, suggesting that repetitive mTBI might change the deposition and accumulation of amyloid β plaques, and even accelerate the aging process in the brain.4
Symptoms. The case series by Omalu et al4 (which inspired the 2015 motion picture Concussion) and the case series presented by McKee et al5 described severe psychiatric symptoms associated with CTE:
- decreased speed of information processing
- increase in religiosity
- lack of insight
- poor judgment
- involvement in illegal activities
- substance abuse
- indiscretion
- verbal and physical abuse
- problems with interpersonal relationships
- isolation
- restlessness and hyperactivity
- somatic complaints.
The 2 groups of researchers also noted hopelessness, social phobia, anxiety, agitation, mania, labile mood, insomnia, explosivity, and suicidal ideation, attempt, and completion.4,5
By Stage 4, all affected patients are symptomatic. Cognitive impairment is severe; many are described as having “severe memory loss with dementia,”5 “profound” inattention and loss of concentration,5 and dysarthria. Paranoia may develop. Mood symptoms can be severe: Approximately 31% of subjects studied have contemplated suicide; of those, 26% had “suicidal tendencies” and 14% completed suicide.5
Two distinct types of CTE progression are apparent:
- patients who display cognitive deficits first; they progress to dementia but live longer
- patients who display mood and behavioral symptoms first; they tend to be younger, more violent, depressed, and explosive.6
CTE cannot be diagnosed with imaging. There are, however, a few positron emission tomography (PET) ligands for pTau that show promise:
- [F-18]FDDNP, which consistently identifies pTau deposits in brains in which CTE is clinically suspected, in the same distribution of pTau neurofibrillary tangles on autopsy.
- [11C]DPA-713, which detected TBI-related inflammation of neurons in 9 former NFL players in whom CTE was suspected based on the clinical presentation.
- PiB amyloid ligand, under investigation for use in PET neuroimaging.7
Casualties
In January 2016 alone, at least 3 former NFL players were found to have CTE posthumously.
Earl Morrall. Former quarterback who had a 21-year NFL career. Official cause of death in 2014 at age 79 was recorded as “complications of Parkinson’s disease.” In 2016, Stage-4 CTE was discovered on autopsy.8
Ken Stabler. Former quarterback for several NFL teams over 15 seasons. Died of colon cancer at age 69 in 2015. On autopsy, was found to have Stage-3 CTE.9
Tyler Sash. Former University of Iowa and New York Giants football player. Died in September 2015 at age 27 of an apparent drug overdose; posthumously, determined to have Stage-2 CTE. His family reported memory loss, minor fits of rage, confusion, inattention, lack of focus, and chronic pain.
Sash’s mother said, “My son knew something was wrong, but he couldn’t express it. He was such a good person, and it’s sad that he struggled so with this—not knowing where to go with it. Now it makes sense.”10 Sash played 16 years of football in all, sustaining at least 5 concussions. (“If you’ve played football, you know there are often other incidents [of head trauma],” Sash’s father said.10)
Cultural and medical mindsets about contact sports
In the United States, children as young as age 5, with a low weight limit of 35 pounds, routinely are introduced to football.11 Reports of 5 high school players dying from football-related injury in the 2014 season, and 3 deaths in the 2015 season, led a St. Louis, Missouri, area school district to defund their football program entirely. The district’s 2015 homecoming game was a soccer match; students and parents seemed to embrace the change.12
On its face, soccer seems a good alternative to football. When children are instructed to “head” the ball, however, concern arises about CTE: Mild CTE changes have been reported in 2 young soccer players, and late-stage CTE changes were seen in a retired soccer player with dementia.13
Perhaps most disturbing is that players who develop symptoms of CTE, or are at risk, are unlikely to seek psychiatric help. We, as psychiatric clinicians, must be diligent about questioning young patients about their extracurricular activities. It is not enough to simply ask about a history of head trauma: Ask patients about any blow to the head, and don’t limit your questioning to whether they sustained a “concussion” during practice or play.
When speaking with adult and geriatric patients, ask about a history of playing interscholastic or collegiate contact sports, such as football, hockey, and soccer.
Is the solution to better shield the head?
That is not a solution: Helmets and other protective headgear appear to be insufficient to protect the brain from traumatic injury. Perhaps keeping children from engaging in violent sports that put them at high risk of CTE later is the preventive approach that merits the most attention.
1. Blackstone J. NFL tackles alarming increase in concussions. CBS News. http://www.cbsnews.com/news/nfl-studying-how-to-tackle-alarming-increase-in-concussions. Published February 2, 2016. Accessed February 3, 2016.
2. McNamee M, Partridge B, Anderson L. Concussion ethics and sports medicine. Clin Sports Med. 2015;35(2):257-267.
3. Abreu MA, Cromartie FJ, Spradley BD; United States Sports Academy. Chronic traumatic encephalopathy (CTE) and former National Football League player suicides. The Sport Journal. http://thesportjournal.org/article/chronic-traumatic-encephalopathy-cte-and-former-national-football-league-player-suicides. Published January 29, 2016. Accessed January 29, 2016.
4. Omalu B, Bailes J, Hamilton RL, et al. Emerging histomorphologic phenotypes of chronic traumatic encephalopathy in american athletes. Neurosurgery. 2011;69(1):173-183; discussion 183.
5. McKee AC, Stern RA, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136(pt 1):43-64.
6. Stern RA, Daneshvar DH, Baugh CM, et al. Clinical presentation of chronic traumatic encephalopathy. Neurology. 2013;81(13):1122-1129.
7. Eisenmenger LB, Huo EJ, Hoffman JM, et al. Advances in PET imaging of degenerative, cerebrovascular, and traumatic causes of dementia. Semin Nucl Med. 2016;46(1):57-87.
8. Jackson B. Report: former Miami Dolphins QB Earl Morrall had brain disease CTE. Miami Herald. http://www.miamiherald.com/sports/nfl/miami-dolphins/article58794523.html. Published February 5, 2016. Accessed February 6, 2016.
9. Fantz A. Ex-NFL player Ken Stabler had concussion disease CTE, doctor says. CNN. http://www.cnn.com/2016/02/03/health/ken-stabler-cte. Updated February 4, 2016. Accessed February 9, 2016.
10. Pennington B. C.T.E. is found in an Ex-Giant Tyler Sash, who died at 27. The New York Times. http://www.nytimes.com/2016/01/27/sports/football/former-giants-safety-tyler-sash-found-to-have-cte.html?_r=0. Published January 26, 2016. Accessed January 27, 2016.
11. Pop Warner Little Scholars, Inc. Ages and weights for tackle football programs. http://www.popwarner.com/football/footballstructure.htm. Accessed February 5, 2016.
12. Fowler L. No football for homecoming? No problem at Maplewood-Richmond Heights High. St. Louis Post Dispatch. http://www.stltoday.com/news/local/education/no-football-for-homecoming-no-problem-at-maplewood-richmond-heights/article_cc8dc31b-5097-5114-ba9b-9b3584f478b9.html. Published October 9, 2015. Accessed February 3, 2016.
13. Hales C, Neill S, Gearing M, et al. Late-stage CTE pathology in a retired soccer player with dementia. Neurology. 2014;83(24):2307-2309. doi: 10.1212/WNL.0000000000001081.
The National Football League (NFL) had its highest concussion tally last year: 182 such injuries reported1 in the 2014-2015 regular season. The true rate of concussion in the NFL is likely higher, as a result of multiple factors (fear of “letting the team [or the coach] down,” fear of retaliation from team owners,2 etc.).
To simply call a head injury a “concussion” is a disservice to players and their family: Any blow to the head, severe or otherwise, has the potential to cause microvascular disruption in the brain; repeated blows to the head undoubtedly cause further damage.
In reality, a “concussion” is a mild traumatic brain injury (mTBI). With repeated blows, an mTBI can lead to chronic traumatic encephalopathy (CTE). In 2015, eighty-seven of 91 brains from autopsied former NFL players displayed some stage of CTE.3
Pathophysiology and presentation
CTE comprises 4 histological stages; Stage 4 is the most advanced. Alzheimer’s disease (AD) and CTE display similarities, which suggests a separate classification of CTE-AD; the presence of amyloid β plaques correlates with (1) more severe hyperphosphorylated tau (pTau) pathology and (2) advanced stages of the disease and clinical presentations. Death tends to occur 10 years earlier in CTE-AD than in AD, suggesting that repetitive mTBI might change the deposition and accumulation of amyloid β plaques, and even accelerate the aging process in the brain.4
Symptoms. The case series by Omalu et al4 (which inspired the 2015 motion picture Concussion) and the case series presented by McKee et al5 described severe psychiatric symptoms associated with CTE:
- decreased speed of information processing
- increase in religiosity
- lack of insight
- poor judgment
- involvement in illegal activities
- substance abuse
- indiscretion
- verbal and physical abuse
- problems with interpersonal relationships
- isolation
- restlessness and hyperactivity
- somatic complaints.
The 2 groups of researchers also noted hopelessness, social phobia, anxiety, agitation, mania, labile mood, insomnia, explosivity, and suicidal ideation, attempt, and completion.4,5
By Stage 4, all affected patients are symptomatic. Cognitive impairment is severe; many are described as having “severe memory loss with dementia,”5 “profound” inattention and loss of concentration,5 and dysarthria. Paranoia may develop. Mood symptoms can be severe: Approximately 31% of subjects studied have contemplated suicide; of those, 26% had “suicidal tendencies” and 14% completed suicide.5
Two distinct types of CTE progression are apparent:
- patients who display cognitive deficits first; they progress to dementia but live longer
- patients who display mood and behavioral symptoms first; they tend to be younger, more violent, depressed, and explosive.6
CTE cannot be diagnosed with imaging. There are, however, a few positron emission tomography (PET) ligands for pTau that show promise:
- [F-18]FDDNP, which consistently identifies pTau deposits in brains in which CTE is clinically suspected, in the same distribution of pTau neurofibrillary tangles on autopsy.
- [11C]DPA-713, which detected TBI-related inflammation of neurons in 9 former NFL players in whom CTE was suspected based on the clinical presentation.
- PiB amyloid ligand, under investigation for use in PET neuroimaging.7
Casualties
In January 2016 alone, at least 3 former NFL players were found to have CTE posthumously.
Earl Morrall. Former quarterback who had a 21-year NFL career. Official cause of death in 2014 at age 79 was recorded as “complications of Parkinson’s disease.” In 2016, Stage-4 CTE was discovered on autopsy.8
Ken Stabler. Former quarterback for several NFL teams over 15 seasons. Died of colon cancer at age 69 in 2015. On autopsy, was found to have Stage-3 CTE.9
Tyler Sash. Former University of Iowa and New York Giants football player. Died in September 2015 at age 27 of an apparent drug overdose; posthumously, determined to have Stage-2 CTE. His family reported memory loss, minor fits of rage, confusion, inattention, lack of focus, and chronic pain.
Sash’s mother said, “My son knew something was wrong, but he couldn’t express it. He was such a good person, and it’s sad that he struggled so with this—not knowing where to go with it. Now it makes sense.”10 Sash played 16 years of football in all, sustaining at least 5 concussions. (“If you’ve played football, you know there are often other incidents [of head trauma],” Sash’s father said.10)
Cultural and medical mindsets about contact sports
In the United States, children as young as age 5, with a low weight limit of 35 pounds, routinely are introduced to football.11 Reports of 5 high school players dying from football-related injury in the 2014 season, and 3 deaths in the 2015 season, led a St. Louis, Missouri, area school district to defund their football program entirely. The district’s 2015 homecoming game was a soccer match; students and parents seemed to embrace the change.12
On its face, soccer seems a good alternative to football. When children are instructed to “head” the ball, however, concern arises about CTE: Mild CTE changes have been reported in 2 young soccer players, and late-stage CTE changes were seen in a retired soccer player with dementia.13
Perhaps most disturbing is that players who develop symptoms of CTE, or are at risk, are unlikely to seek psychiatric help. We, as psychiatric clinicians, must be diligent about questioning young patients about their extracurricular activities. It is not enough to simply ask about a history of head trauma: Ask patients about any blow to the head, and don’t limit your questioning to whether they sustained a “concussion” during practice or play.
When speaking with adult and geriatric patients, ask about a history of playing interscholastic or collegiate contact sports, such as football, hockey, and soccer.
Is the solution to better shield the head?
That is not a solution: Helmets and other protective headgear appear to be insufficient to protect the brain from traumatic injury. Perhaps keeping children from engaging in violent sports that put them at high risk of CTE later is the preventive approach that merits the most attention.
The National Football League (NFL) had its highest concussion tally last year: 182 such injuries reported1 in the 2014-2015 regular season. The true rate of concussion in the NFL is likely higher, as a result of multiple factors (fear of “letting the team [or the coach] down,” fear of retaliation from team owners,2 etc.).
To simply call a head injury a “concussion” is a disservice to players and their family: Any blow to the head, severe or otherwise, has the potential to cause microvascular disruption in the brain; repeated blows to the head undoubtedly cause further damage.
In reality, a “concussion” is a mild traumatic brain injury (mTBI). With repeated blows, an mTBI can lead to chronic traumatic encephalopathy (CTE). In 2015, eighty-seven of 91 brains from autopsied former NFL players displayed some stage of CTE.3
Pathophysiology and presentation
CTE comprises 4 histological stages; Stage 4 is the most advanced. Alzheimer’s disease (AD) and CTE display similarities, which suggests a separate classification of CTE-AD; the presence of amyloid β plaques correlates with (1) more severe hyperphosphorylated tau (pTau) pathology and (2) advanced stages of the disease and clinical presentations. Death tends to occur 10 years earlier in CTE-AD than in AD, suggesting that repetitive mTBI might change the deposition and accumulation of amyloid β plaques, and even accelerate the aging process in the brain.4
Symptoms. The case series by Omalu et al4 (which inspired the 2015 motion picture Concussion) and the case series presented by McKee et al5 described severe psychiatric symptoms associated with CTE:
- decreased speed of information processing
- increase in religiosity
- lack of insight
- poor judgment
- involvement in illegal activities
- substance abuse
- indiscretion
- verbal and physical abuse
- problems with interpersonal relationships
- isolation
- restlessness and hyperactivity
- somatic complaints.
The 2 groups of researchers also noted hopelessness, social phobia, anxiety, agitation, mania, labile mood, insomnia, explosivity, and suicidal ideation, attempt, and completion.4,5
By Stage 4, all affected patients are symptomatic. Cognitive impairment is severe; many are described as having “severe memory loss with dementia,”5 “profound” inattention and loss of concentration,5 and dysarthria. Paranoia may develop. Mood symptoms can be severe: Approximately 31% of subjects studied have contemplated suicide; of those, 26% had “suicidal tendencies” and 14% completed suicide.5
Two distinct types of CTE progression are apparent:
- patients who display cognitive deficits first; they progress to dementia but live longer
- patients who display mood and behavioral symptoms first; they tend to be younger, more violent, depressed, and explosive.6
CTE cannot be diagnosed with imaging. There are, however, a few positron emission tomography (PET) ligands for pTau that show promise:
- [F-18]FDDNP, which consistently identifies pTau deposits in brains in which CTE is clinically suspected, in the same distribution of pTau neurofibrillary tangles on autopsy.
- [11C]DPA-713, which detected TBI-related inflammation of neurons in 9 former NFL players in whom CTE was suspected based on the clinical presentation.
- PiB amyloid ligand, under investigation for use in PET neuroimaging.7
Casualties
In January 2016 alone, at least 3 former NFL players were found to have CTE posthumously.
Earl Morrall. Former quarterback who had a 21-year NFL career. Official cause of death in 2014 at age 79 was recorded as “complications of Parkinson’s disease.” In 2016, Stage-4 CTE was discovered on autopsy.8
Ken Stabler. Former quarterback for several NFL teams over 15 seasons. Died of colon cancer at age 69 in 2015. On autopsy, was found to have Stage-3 CTE.9
Tyler Sash. Former University of Iowa and New York Giants football player. Died in September 2015 at age 27 of an apparent drug overdose; posthumously, determined to have Stage-2 CTE. His family reported memory loss, minor fits of rage, confusion, inattention, lack of focus, and chronic pain.
Sash’s mother said, “My son knew something was wrong, but he couldn’t express it. He was such a good person, and it’s sad that he struggled so with this—not knowing where to go with it. Now it makes sense.”10 Sash played 16 years of football in all, sustaining at least 5 concussions. (“If you’ve played football, you know there are often other incidents [of head trauma],” Sash’s father said.10)
Cultural and medical mindsets about contact sports
In the United States, children as young as age 5, with a low weight limit of 35 pounds, routinely are introduced to football.11 Reports of 5 high school players dying from football-related injury in the 2014 season, and 3 deaths in the 2015 season, led a St. Louis, Missouri, area school district to defund their football program entirely. The district’s 2015 homecoming game was a soccer match; students and parents seemed to embrace the change.12
On its face, soccer seems a good alternative to football. When children are instructed to “head” the ball, however, concern arises about CTE: Mild CTE changes have been reported in 2 young soccer players, and late-stage CTE changes were seen in a retired soccer player with dementia.13
Perhaps most disturbing is that players who develop symptoms of CTE, or are at risk, are unlikely to seek psychiatric help. We, as psychiatric clinicians, must be diligent about questioning young patients about their extracurricular activities. It is not enough to simply ask about a history of head trauma: Ask patients about any blow to the head, and don’t limit your questioning to whether they sustained a “concussion” during practice or play.
When speaking with adult and geriatric patients, ask about a history of playing interscholastic or collegiate contact sports, such as football, hockey, and soccer.
Is the solution to better shield the head?
That is not a solution: Helmets and other protective headgear appear to be insufficient to protect the brain from traumatic injury. Perhaps keeping children from engaging in violent sports that put them at high risk of CTE later is the preventive approach that merits the most attention.
1. Blackstone J. NFL tackles alarming increase in concussions. CBS News. http://www.cbsnews.com/news/nfl-studying-how-to-tackle-alarming-increase-in-concussions. Published February 2, 2016. Accessed February 3, 2016.
2. McNamee M, Partridge B, Anderson L. Concussion ethics and sports medicine. Clin Sports Med. 2015;35(2):257-267.
3. Abreu MA, Cromartie FJ, Spradley BD; United States Sports Academy. Chronic traumatic encephalopathy (CTE) and former National Football League player suicides. The Sport Journal. http://thesportjournal.org/article/chronic-traumatic-encephalopathy-cte-and-former-national-football-league-player-suicides. Published January 29, 2016. Accessed January 29, 2016.
4. Omalu B, Bailes J, Hamilton RL, et al. Emerging histomorphologic phenotypes of chronic traumatic encephalopathy in american athletes. Neurosurgery. 2011;69(1):173-183; discussion 183.
5. McKee AC, Stern RA, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136(pt 1):43-64.
6. Stern RA, Daneshvar DH, Baugh CM, et al. Clinical presentation of chronic traumatic encephalopathy. Neurology. 2013;81(13):1122-1129.
7. Eisenmenger LB, Huo EJ, Hoffman JM, et al. Advances in PET imaging of degenerative, cerebrovascular, and traumatic causes of dementia. Semin Nucl Med. 2016;46(1):57-87.
8. Jackson B. Report: former Miami Dolphins QB Earl Morrall had brain disease CTE. Miami Herald. http://www.miamiherald.com/sports/nfl/miami-dolphins/article58794523.html. Published February 5, 2016. Accessed February 6, 2016.
9. Fantz A. Ex-NFL player Ken Stabler had concussion disease CTE, doctor says. CNN. http://www.cnn.com/2016/02/03/health/ken-stabler-cte. Updated February 4, 2016. Accessed February 9, 2016.
10. Pennington B. C.T.E. is found in an Ex-Giant Tyler Sash, who died at 27. The New York Times. http://www.nytimes.com/2016/01/27/sports/football/former-giants-safety-tyler-sash-found-to-have-cte.html?_r=0. Published January 26, 2016. Accessed January 27, 2016.
11. Pop Warner Little Scholars, Inc. Ages and weights for tackle football programs. http://www.popwarner.com/football/footballstructure.htm. Accessed February 5, 2016.
12. Fowler L. No football for homecoming? No problem at Maplewood-Richmond Heights High. St. Louis Post Dispatch. http://www.stltoday.com/news/local/education/no-football-for-homecoming-no-problem-at-maplewood-richmond-heights/article_cc8dc31b-5097-5114-ba9b-9b3584f478b9.html. Published October 9, 2015. Accessed February 3, 2016.
13. Hales C, Neill S, Gearing M, et al. Late-stage CTE pathology in a retired soccer player with dementia. Neurology. 2014;83(24):2307-2309. doi: 10.1212/WNL.0000000000001081.
1. Blackstone J. NFL tackles alarming increase in concussions. CBS News. http://www.cbsnews.com/news/nfl-studying-how-to-tackle-alarming-increase-in-concussions. Published February 2, 2016. Accessed February 3, 2016.
2. McNamee M, Partridge B, Anderson L. Concussion ethics and sports medicine. Clin Sports Med. 2015;35(2):257-267.
3. Abreu MA, Cromartie FJ, Spradley BD; United States Sports Academy. Chronic traumatic encephalopathy (CTE) and former National Football League player suicides. The Sport Journal. http://thesportjournal.org/article/chronic-traumatic-encephalopathy-cte-and-former-national-football-league-player-suicides. Published January 29, 2016. Accessed January 29, 2016.
4. Omalu B, Bailes J, Hamilton RL, et al. Emerging histomorphologic phenotypes of chronic traumatic encephalopathy in american athletes. Neurosurgery. 2011;69(1):173-183; discussion 183.
5. McKee AC, Stern RA, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136(pt 1):43-64.
6. Stern RA, Daneshvar DH, Baugh CM, et al. Clinical presentation of chronic traumatic encephalopathy. Neurology. 2013;81(13):1122-1129.
7. Eisenmenger LB, Huo EJ, Hoffman JM, et al. Advances in PET imaging of degenerative, cerebrovascular, and traumatic causes of dementia. Semin Nucl Med. 2016;46(1):57-87.
8. Jackson B. Report: former Miami Dolphins QB Earl Morrall had brain disease CTE. Miami Herald. http://www.miamiherald.com/sports/nfl/miami-dolphins/article58794523.html. Published February 5, 2016. Accessed February 6, 2016.
9. Fantz A. Ex-NFL player Ken Stabler had concussion disease CTE, doctor says. CNN. http://www.cnn.com/2016/02/03/health/ken-stabler-cte. Updated February 4, 2016. Accessed February 9, 2016.
10. Pennington B. C.T.E. is found in an Ex-Giant Tyler Sash, who died at 27. The New York Times. http://www.nytimes.com/2016/01/27/sports/football/former-giants-safety-tyler-sash-found-to-have-cte.html?_r=0. Published January 26, 2016. Accessed January 27, 2016.
11. Pop Warner Little Scholars, Inc. Ages and weights for tackle football programs. http://www.popwarner.com/football/footballstructure.htm. Accessed February 5, 2016.
12. Fowler L. No football for homecoming? No problem at Maplewood-Richmond Heights High. St. Louis Post Dispatch. http://www.stltoday.com/news/local/education/no-football-for-homecoming-no-problem-at-maplewood-richmond-heights/article_cc8dc31b-5097-5114-ba9b-9b3584f478b9.html. Published October 9, 2015. Accessed February 3, 2016.
13. Hales C, Neill S, Gearing M, et al. Late-stage CTE pathology in a retired soccer player with dementia. Neurology. 2014;83(24):2307-2309. doi: 10.1212/WNL.0000000000001081.
Tailor chronic pain interventions to the patient’s clinical profile



Chronic pain and psychiatric illness: Managing comorbid conditions
Pain is one of the most common symptoms for which patients seek medical care, with an associated estimated annual cost of $600 billion.1 Using a multimodal approach to care—thorough evaluation, cognitive-behavioral and psychophysiological therapy, physical therapy, medications, and other interventions—can help patients effectively manage their condition and achieve healthier outcomes.
Evaluating a patient with pain
When developing a safe, comprehensive, and effective treatment plan for patients with chronic pain, first perform a thorough history and physical exam using the following elements:
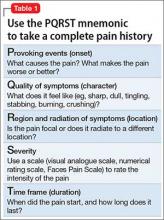
Pain history. The PQRST mnemonic (Table 1) can help you obtain critical information and assist in determining the appropriate diagnosis and cause of the patient’s pain complaints.
Psychiatric history. Document the mental health history of the patient and first-degree relatives.
Medical history. Knowing the medical history could reveal comorbidities contributing to a patient’s pain complaint.
Treatment history. Listing past and current treatments for pain, including effectiveness, helps the clinician understand if an existing treatment plan should be modified.
Functional status. Document current level of daily activity, how life activities are affected by pain; strategies used to help cope with pain; level of physical and emotional support provided in home, work, and school environments; and active stressors (eg, financial, interpersonal).
Psychosocial history. Document historical information related to coping skills, trauma history, family of origin, abuse, interpersonal relationships, social support, and academic and vocational functioning.
Substance use or abuse. Assess for use of controlled substances (ie, early refills; lost medications; obtaining medications from multiple prescribers, friends, families, or strangers; use of prescribed and non-prescribed medications for non-medical and medical purposes), nicotine, alcohol, illicit substances, and caffeine. A thorough inventory can help to identify substances a patient is using that could affect daily functioning and pain level.
Behavioral observations. Assessing mental status (eg, insight, pain behavior, cooperation) can be useful. Paying attention to pain behaviors, such as complaints of pain, decreased activity, increased medication intake, or altered facial expressions or body posture, can help the clinician gain insight to the extent that pain affects the patient’s quality of life.
The information gathered in the patient evaluation can be used to design a multimodal treatment plan to achieve maximum effectiveness.
Assessing psychiatric illness
Current approaches to pain evaluation and treatment recommend use of a biopsychosocial orientation because psychological, behavioral, and social factors can influence the experience and impact of pain, regardless of the primary cause.2 A comprehensive psychiatric evaluation, diagnosis, and treatment plan should consider the broader context in which a patient’s pain occurs.
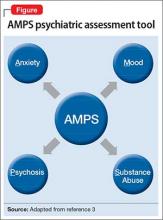
Regarding psychiatric illness, past and current symptoms, treatment history, and risk assessment should all be included. Using the “AMPS approach” (Figure)3—assessing Anxiety, Mood (depression and mania), Psychotic symptoms (paranoid ideation and hallucinations), and Substance use—helps screen for comorbid psychiatric conditions in patients with chronic pain.
Sleep assessment
Chronic pain patients often experience significant sleep disturbance that could be caused by physiological aspects of the pain condition, environmental factors (eg, uncomfortable bedding), a comorbid sleep disorder (eg, sleep apnea), a psychiatric disorder, or a combination of the above.
Obstructive and central sleep apnea are characterized by nighttime hypoxia, which leads to frequent disruption of the sleep-wake cycle and often manifests as daytime fatigue, irritability, depression, drowsiness, headaches, and increased pain sensitivity. Changes in sleep arousal can lead to neuropsychological changes during the day, such as decreased attention, memory problems, impaired executive functioning, and reduced impulse control.
Screen patients for central and obstructive sleep apnea before prescribing opioids or benzodiazepines for pain because these medications can cause or exacerbate underlying sleep apnea. Although many screening tools, such as the Epworth Sleepiness Scale, assess daytime somnolence,4 the STOP-BANG questionnaire is a quick, validated, and efficient screening tool that often is used to assess sleep apnea risk5,6 (Table 2). The presence of ≥3 risk factors identifies patients at increased risk and warrants consideration for further workup by a sleep specialist.7,8
Pharmacotherapy for chronic pain
Non-opioid medications. Pain can be broadly categorized as neuropathic or nociceptive. Neuropathic pain can be described by patients as numbness, burning, electric-like, and tingling, and is associated with nerve damage. Nociceptive pain commonly is described as similar to a toothache with descriptors such as stabbing, sharp, or a dull aching sensation; it is often, but not always, associated with acute injury or ongoing trauma to tissue. Drug treatment is most successful when the appropriate class of medication is matched to the specific type of pain.
Nociceptive pain often is successfully treated with non-steroidal anti-inflammatory drugs and acetaminophen. Non-selective COX inhibitors (eg, ibuprofen, indomethacin, ketorolac) and COX-2 selective inhibitors (eg, celecoxib) have been associated with cardiovascular, gastrointestinal, and renal disease; acetaminophen is associated with liver dysfunction.9-11 However, the absolute risk for complications in healthy patients is low.12 To minimize risk, use these agents for the shortest duration and at the lowest effective dosage possible.
Neuropathic pain can be addressed with certain antidepressants13—specifically, those that increase serotonin and norepinephrine (eg, tricyclic antidepressants [TCAs] and serotonin-norepinephrine reuptake inhibitors [SNRIs]), or medications that block ion channels (eg, anticonvulsants). TCAs (eg, desipramine, nortriptyline, amitriptyline) are among the best studied and most cost effective medications for treating neuropathic pain,14,15 but they can have sedating and anticholinergic effects, as well as cardiac adverse effects (ie, prolongation of the QTc interval). SNRIs (eg, venlafaxine, desvenlafaxine, duloxetine, and milnacipran) can be effective and often are better tolerated than TCAs.14
Some newer anticonvulsants (eg, gabapentin and pregabalin) have been found to be more effective than placebo for a variety of neuropathic pain conditions.16,17 Although they have few drug-drug interactions, anticonvulsants can cause dizziness, forgetfulness, and sedation. These adverse effects can be minimized by starting at a low dosage and titrating carefully. Because hepatic or renal impairment can affect metabolism or excretion of these drugs, review the prescribing information to determine safe dosing.
Targeted injection of medications to major pain generators (eg, an epidural steroid for radicular neck and back pain; facet injections for facet-related neck and back pain; trigger point injections for myofascial pain; occipital nerve blocks for occipital neuralgia; and botulinum toxin A injections for chronic migraine headache) can be effective in reducing discomfort and increasing function in patients with chronic pain. A detailed discussion of such therapies is beyond the scope of this article, but have been reviewed extensively elsewhere.18,19
Opioids. Although there is little evidence of long-term efficacy with chronic opioid therapy for most patients, a trial of opioids might be warranted for select patients who do not respond to other medications. Because the risk–benefit ratio for chronic opioid therapy is high,20-22 a decision to initiate a trial of a low-dosage opioid should be made only after careful consideration of those risks. It is generally agreed that treatment of chronic pain with low-dosage opioid therapy is more likely to be successful when it is used as an adjuvant to non-opioid modalities (eg, physical reconditioning, injection therapies, spinal cord stimulation, neurobehavioral interventions, non-opioid medications).
The Federation of State Medical Boards has stated that excessive reliance on opioid medications for treating chronic pain is a deviation from best practices.23 To maximize benefit and minimize risk, clinicians should carefully select appropriate patients, establish functional goals, and regularly monitor for efficacy and compliance. Thoroughly document these steps in the patient’s record for later reference.23
After establishing a clinical diagnosis for the cause of the pain, you should determine the risk of opioid abuse or misuse by using any one of the available risk assessment tools (Box). Understand, however, that no single tool has been shown to be more effective than others.
Although patients and some clinicians tend to overvalue the benefits of chronic opioid therapy, many do not fully appreciate the risks (eg, respiratory depression and death), which can be exacerbated if the patient is using other substance that suppress respiration (eg, benzodiazepines, alcohol, and illicit substances). Written informed consent and treatment agreement is highly recommended; components of such a document are listed in Table 3.23
Develop a treatment plan that emphasizes functional goals based on the patient’s physical limitations and that incorporates some type of daily, atraumatic physical activity. This plan should be documented and reviewed regularly to help monitor treatment effectiveness.
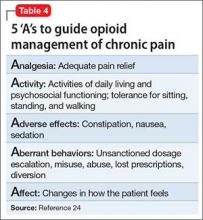
After an initial trial of a few weeks, the patient and clinician should meet to review the 5 “A”s (Table 4)24 to determine the success of the opioid regimen. Consulting your state’s prescription drug monitoring program (if one is available), obtaining a random urine drug test, and doing a pill count can provide useful, objective data. If the patient has not made progress but has experienced no adverse effects, then a small dosage increase might be warranted. If any of the 5 “A”s indicates lack of improvement or increased risk, consider stopping opioid therapy and exploring non-opioid options to manage chronic pain.
Referrals to a pain specialist or an addiction specialist, or both, might be needed, depending on the patient’s condition at any given follow-up visit. Such referral decisions, as well as all treatment plans, should be documented clearly in the medical record to prevent any misunderstanding, false accusations, or medicolegal repercussions regarding the rationale for continuing or terminating opioid-based treatment.
Non-pharmaceutical therapy for treating pain
The pain management field has successfully integrated the biopsychosocial model into regular practice. This model advocates the use of multimodal non-drug interventions in conjunction with opioid and non-opioid medications. Such interventions address behavioral, cognitive, sociocultural (psychosocial), lifestyle, and physiological dimensions of pain. A partial list of non-drug interventions is provided in Table 5.
Integration of these interventions within a biopsychosocial framework can assist you in making a comprehensive treatment plan. For example, patients with focal myofascial shoulder and back pain might derive only transient benefit from trigger point injection. However, concurrent referral to a pain psychologist and physical therapist could substantially improve functional outcomes by addressing factors that directly and indirectly influence myofascial pain. Inclusion of cognitive-behavioral therapy (addressing psychosocial and lifestyle dimensions), surface electromyography, psychophysiological interventions/biofeedback (addressing psychosocial, lifestyle, and physiological dimensions), and physical therapy (addressing lifestyle and physiological dimensions) allows the patient to learn coping skills, decrease physiological arousal that can lead to unnecessary tensing of muscles, and strengthen core muscle groups.
1. Institute of Medicine. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. http://www.iom.edu/~/media/Files/Report%20 Files/2011/Relieving-Pain-in-America-A-Blueprint-for- Transforming-Prevention-Care-Education-Research/ Pain%20Research%202011%20Report%20Brief.pdf. Published June 2011. Accessed April 15, 2015.
2. Jensen MP, Moore MR, Bockow TB, et al. Psychosocial factors and adjustment to chronic pain in persons with physical disabilities: a systematic review. Arch Phys Med Rehabil. 2011;92(1):146-160.
3. McCarron R, Xiong G, Bourgeois J. Lippincott’s primary care psychiatry. Philadelphia, PA: Lippincott Williams & Wilkins; 2009.
4. Abrishami A, Khajehdehi A, Chung F. A systematic review of screening questionnaires for obstructive sleep apnea. Can J Anaesth. 2010;57(5):423-438.
5. Boynton G, Vahabzadeh A, Hammoud S, et al. Validation of the STOP-BANG questionnaire among patients referred for suspected obstructive sleep apnea. J Sleep Disord Treat Care. 2013;2(4). doi: 10.4172/2325-9639.1000121.
6. Vana KD, Silva GE, Goldberg R. Predictive abilities of the STOP-Bang and Epworth Sleepiness Scale in identifying sleep clinic patients at high risk for obstructive sleep apnea. Res Nurs Health. 2013;36(1):84-94.
7. Chung F, Elsaid H. Screening for obstructive sleep apnea before surgery: why is it important? Curr Opin Anaesthesiol. 2009;22(3):405-411.
8. Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108(5):812-821.
9. Forman JP, Rimm EB, Curhan GC. Frequency of analgesic use and risk of hypertension among men. Arch Intern Med. 2007;167(4):394-399.
10. Sudano I, Flammer AJ, Périat D, et al. Acetaminophen increases blood pressure in patients with coronary artery disease. Circulation. 2010;122(18):1789-1796.
11. U.S. Food and Drug Administration. Questions and answers about oral prescription acetaminophen products to be limited to 325 mg per dosage unit. http://www.fda.gov/ drugs/drugsafety/informationbydrugclass/ucm239871. htm. Updated December 11, 2014. Accessed February 23, 2015.
12. Bhala N, Emberson J, Merhi A, et al; Coxib and traditional NSAID Trialists’ (CNT) Collaboration. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet. 2013;382(9894):769-779.
13. Sullivan MD, Robinson JP. Antidepressant and anticonvulsant medication for chronic pain. Phys Med Rehabil Clin N Am. 2006;17(2):381-400, vi-vii.
14. Sindrup SH, Otto M, Finnerup NB, et al. Antidepressants in the treatment of neuropathic pain. Basic Clin Pharmacol Toxicol. 2005;96(6):399-409.
15. Pilowsky I, Hallett EC, Bassett DL, et al. A controlled study of amitriptyline in the treatment of chronic pain. Pain. 1982;14(2):169-179.
16. Finnerup NB, Sindrup SH, Jensen TS. The evidence for pharmacological treatment of neuropathic pain. Pain. 2010;150(3):573-581.
17. Dworkin RH, O’Connor AB, Backonja M, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132(3):237-251.
18. Manchikanti L, Abdi S, Atluri S, et al. An update of comprehensive evidence-based guidelines for interventional techniques in chronic spinal pain. Part II: guidance and recommendations. Pain Physician. 2013;16(suppl 2):S49-S283.
19. Singh V, Trescot A, Nishio I. Injections for chronic pain. Phys Med Rehabil Clin N Am. 2015;26(2):249-261.
20. Centers for Disease Control and Prevention (CDC). Vital signs: overdoses of prescription opioid pain relievers— United States, 1999–2008. MMWR Morb Mortal Wkly Rep. 2011;60(43):1487-1492.
21. Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013;309(7):657-659.
22. Chen L, Vo T, Seefeld L, et al. Lack of correlation between opioid dose adjustment and pain score change in a group of chronic pain patients. J Pain. 2013;14(4):384-392.
23. Federation of State Medical Boards. Model policy for the use of opioid analgesics in the treatment of chronic pain. http:// www.fsmb.org/Media/Default/PDF/FSMB/Advocacy/ pain_policy_july2013.pdf. Published July 2013. Accessed December 18, 2015.
24. Passik SD, Weinreb HJ. Managing chronic nonmalignant pain: overcoming obstacles to the use of opioids. Adv Ther. 2000;17(2):70-83.
Pain is one of the most common symptoms for which patients seek medical care, with an associated estimated annual cost of $600 billion.1 Using a multimodal approach to care—thorough evaluation, cognitive-behavioral and psychophysiological therapy, physical therapy, medications, and other interventions—can help patients effectively manage their condition and achieve healthier outcomes.
Evaluating a patient with pain
When developing a safe, comprehensive, and effective treatment plan for patients with chronic pain, first perform a thorough history and physical exam using the following elements:
Pain history. The PQRST mnemonic (Table 1) can help you obtain critical information and assist in determining the appropriate diagnosis and cause of the patient’s pain complaints.
Psychiatric history. Document the mental health history of the patient and first-degree relatives.
Medical history. Knowing the medical history could reveal comorbidities contributing to a patient’s pain complaint.
Treatment history. Listing past and current treatments for pain, including effectiveness, helps the clinician understand if an existing treatment plan should be modified.
Functional status. Document current level of daily activity, how life activities are affected by pain; strategies used to help cope with pain; level of physical and emotional support provided in home, work, and school environments; and active stressors (eg, financial, interpersonal).
Psychosocial history. Document historical information related to coping skills, trauma history, family of origin, abuse, interpersonal relationships, social support, and academic and vocational functioning.
Substance use or abuse. Assess for use of controlled substances (ie, early refills; lost medications; obtaining medications from multiple prescribers, friends, families, or strangers; use of prescribed and non-prescribed medications for non-medical and medical purposes), nicotine, alcohol, illicit substances, and caffeine. A thorough inventory can help to identify substances a patient is using that could affect daily functioning and pain level.
Behavioral observations. Assessing mental status (eg, insight, pain behavior, cooperation) can be useful. Paying attention to pain behaviors, such as complaints of pain, decreased activity, increased medication intake, or altered facial expressions or body posture, can help the clinician gain insight to the extent that pain affects the patient’s quality of life.
The information gathered in the patient evaluation can be used to design a multimodal treatment plan to achieve maximum effectiveness.
Assessing psychiatric illness
Current approaches to pain evaluation and treatment recommend use of a biopsychosocial orientation because psychological, behavioral, and social factors can influence the experience and impact of pain, regardless of the primary cause.2 A comprehensive psychiatric evaluation, diagnosis, and treatment plan should consider the broader context in which a patient’s pain occurs.
Regarding psychiatric illness, past and current symptoms, treatment history, and risk assessment should all be included. Using the “AMPS approach” (Figure)3—assessing Anxiety, Mood (depression and mania), Psychotic symptoms (paranoid ideation and hallucinations), and Substance use—helps screen for comorbid psychiatric conditions in patients with chronic pain.
Sleep assessment
Chronic pain patients often experience significant sleep disturbance that could be caused by physiological aspects of the pain condition, environmental factors (eg, uncomfortable bedding), a comorbid sleep disorder (eg, sleep apnea), a psychiatric disorder, or a combination of the above.
Obstructive and central sleep apnea are characterized by nighttime hypoxia, which leads to frequent disruption of the sleep-wake cycle and often manifests as daytime fatigue, irritability, depression, drowsiness, headaches, and increased pain sensitivity. Changes in sleep arousal can lead to neuropsychological changes during the day, such as decreased attention, memory problems, impaired executive functioning, and reduced impulse control.
Screen patients for central and obstructive sleep apnea before prescribing opioids or benzodiazepines for pain because these medications can cause or exacerbate underlying sleep apnea. Although many screening tools, such as the Epworth Sleepiness Scale, assess daytime somnolence,4 the STOP-BANG questionnaire is a quick, validated, and efficient screening tool that often is used to assess sleep apnea risk5,6 (Table 2). The presence of ≥3 risk factors identifies patients at increased risk and warrants consideration for further workup by a sleep specialist.7,8
Pharmacotherapy for chronic pain
Non-opioid medications. Pain can be broadly categorized as neuropathic or nociceptive. Neuropathic pain can be described by patients as numbness, burning, electric-like, and tingling, and is associated with nerve damage. Nociceptive pain commonly is described as similar to a toothache with descriptors such as stabbing, sharp, or a dull aching sensation; it is often, but not always, associated with acute injury or ongoing trauma to tissue. Drug treatment is most successful when the appropriate class of medication is matched to the specific type of pain.
Nociceptive pain often is successfully treated with non-steroidal anti-inflammatory drugs and acetaminophen. Non-selective COX inhibitors (eg, ibuprofen, indomethacin, ketorolac) and COX-2 selective inhibitors (eg, celecoxib) have been associated with cardiovascular, gastrointestinal, and renal disease; acetaminophen is associated with liver dysfunction.9-11 However, the absolute risk for complications in healthy patients is low.12 To minimize risk, use these agents for the shortest duration and at the lowest effective dosage possible.
Neuropathic pain can be addressed with certain antidepressants13—specifically, those that increase serotonin and norepinephrine (eg, tricyclic antidepressants [TCAs] and serotonin-norepinephrine reuptake inhibitors [SNRIs]), or medications that block ion channels (eg, anticonvulsants). TCAs (eg, desipramine, nortriptyline, amitriptyline) are among the best studied and most cost effective medications for treating neuropathic pain,14,15 but they can have sedating and anticholinergic effects, as well as cardiac adverse effects (ie, prolongation of the QTc interval). SNRIs (eg, venlafaxine, desvenlafaxine, duloxetine, and milnacipran) can be effective and often are better tolerated than TCAs.14
Some newer anticonvulsants (eg, gabapentin and pregabalin) have been found to be more effective than placebo for a variety of neuropathic pain conditions.16,17 Although they have few drug-drug interactions, anticonvulsants can cause dizziness, forgetfulness, and sedation. These adverse effects can be minimized by starting at a low dosage and titrating carefully. Because hepatic or renal impairment can affect metabolism or excretion of these drugs, review the prescribing information to determine safe dosing.
Targeted injection of medications to major pain generators (eg, an epidural steroid for radicular neck and back pain; facet injections for facet-related neck and back pain; trigger point injections for myofascial pain; occipital nerve blocks for occipital neuralgia; and botulinum toxin A injections for chronic migraine headache) can be effective in reducing discomfort and increasing function in patients with chronic pain. A detailed discussion of such therapies is beyond the scope of this article, but have been reviewed extensively elsewhere.18,19
Opioids. Although there is little evidence of long-term efficacy with chronic opioid therapy for most patients, a trial of opioids might be warranted for select patients who do not respond to other medications. Because the risk–benefit ratio for chronic opioid therapy is high,20-22 a decision to initiate a trial of a low-dosage opioid should be made only after careful consideration of those risks. It is generally agreed that treatment of chronic pain with low-dosage opioid therapy is more likely to be successful when it is used as an adjuvant to non-opioid modalities (eg, physical reconditioning, injection therapies, spinal cord stimulation, neurobehavioral interventions, non-opioid medications).
The Federation of State Medical Boards has stated that excessive reliance on opioid medications for treating chronic pain is a deviation from best practices.23 To maximize benefit and minimize risk, clinicians should carefully select appropriate patients, establish functional goals, and regularly monitor for efficacy and compliance. Thoroughly document these steps in the patient’s record for later reference.23
After establishing a clinical diagnosis for the cause of the pain, you should determine the risk of opioid abuse or misuse by using any one of the available risk assessment tools (Box). Understand, however, that no single tool has been shown to be more effective than others.
Although patients and some clinicians tend to overvalue the benefits of chronic opioid therapy, many do not fully appreciate the risks (eg, respiratory depression and death), which can be exacerbated if the patient is using other substance that suppress respiration (eg, benzodiazepines, alcohol, and illicit substances). Written informed consent and treatment agreement is highly recommended; components of such a document are listed in Table 3.23
Develop a treatment plan that emphasizes functional goals based on the patient’s physical limitations and that incorporates some type of daily, atraumatic physical activity. This plan should be documented and reviewed regularly to help monitor treatment effectiveness.
After an initial trial of a few weeks, the patient and clinician should meet to review the 5 “A”s (Table 4)24 to determine the success of the opioid regimen. Consulting your state’s prescription drug monitoring program (if one is available), obtaining a random urine drug test, and doing a pill count can provide useful, objective data. If the patient has not made progress but has experienced no adverse effects, then a small dosage increase might be warranted. If any of the 5 “A”s indicates lack of improvement or increased risk, consider stopping opioid therapy and exploring non-opioid options to manage chronic pain.
Referrals to a pain specialist or an addiction specialist, or both, might be needed, depending on the patient’s condition at any given follow-up visit. Such referral decisions, as well as all treatment plans, should be documented clearly in the medical record to prevent any misunderstanding, false accusations, or medicolegal repercussions regarding the rationale for continuing or terminating opioid-based treatment.
Non-pharmaceutical therapy for treating pain
The pain management field has successfully integrated the biopsychosocial model into regular practice. This model advocates the use of multimodal non-drug interventions in conjunction with opioid and non-opioid medications. Such interventions address behavioral, cognitive, sociocultural (psychosocial), lifestyle, and physiological dimensions of pain. A partial list of non-drug interventions is provided in Table 5.
Integration of these interventions within a biopsychosocial framework can assist you in making a comprehensive treatment plan. For example, patients with focal myofascial shoulder and back pain might derive only transient benefit from trigger point injection. However, concurrent referral to a pain psychologist and physical therapist could substantially improve functional outcomes by addressing factors that directly and indirectly influence myofascial pain. Inclusion of cognitive-behavioral therapy (addressing psychosocial and lifestyle dimensions), surface electromyography, psychophysiological interventions/biofeedback (addressing psychosocial, lifestyle, and physiological dimensions), and physical therapy (addressing lifestyle and physiological dimensions) allows the patient to learn coping skills, decrease physiological arousal that can lead to unnecessary tensing of muscles, and strengthen core muscle groups.
Pain is one of the most common symptoms for which patients seek medical care, with an associated estimated annual cost of $600 billion.1 Using a multimodal approach to care—thorough evaluation, cognitive-behavioral and psychophysiological therapy, physical therapy, medications, and other interventions—can help patients effectively manage their condition and achieve healthier outcomes.
Evaluating a patient with pain
When developing a safe, comprehensive, and effective treatment plan for patients with chronic pain, first perform a thorough history and physical exam using the following elements:
Pain history. The PQRST mnemonic (Table 1) can help you obtain critical information and assist in determining the appropriate diagnosis and cause of the patient’s pain complaints.
Psychiatric history. Document the mental health history of the patient and first-degree relatives.
Medical history. Knowing the medical history could reveal comorbidities contributing to a patient’s pain complaint.
Treatment history. Listing past and current treatments for pain, including effectiveness, helps the clinician understand if an existing treatment plan should be modified.
Functional status. Document current level of daily activity, how life activities are affected by pain; strategies used to help cope with pain; level of physical and emotional support provided in home, work, and school environments; and active stressors (eg, financial, interpersonal).
Psychosocial history. Document historical information related to coping skills, trauma history, family of origin, abuse, interpersonal relationships, social support, and academic and vocational functioning.
Substance use or abuse. Assess for use of controlled substances (ie, early refills; lost medications; obtaining medications from multiple prescribers, friends, families, or strangers; use of prescribed and non-prescribed medications for non-medical and medical purposes), nicotine, alcohol, illicit substances, and caffeine. A thorough inventory can help to identify substances a patient is using that could affect daily functioning and pain level.
Behavioral observations. Assessing mental status (eg, insight, pain behavior, cooperation) can be useful. Paying attention to pain behaviors, such as complaints of pain, decreased activity, increased medication intake, or altered facial expressions or body posture, can help the clinician gain insight to the extent that pain affects the patient’s quality of life.
The information gathered in the patient evaluation can be used to design a multimodal treatment plan to achieve maximum effectiveness.
Assessing psychiatric illness
Current approaches to pain evaluation and treatment recommend use of a biopsychosocial orientation because psychological, behavioral, and social factors can influence the experience and impact of pain, regardless of the primary cause.2 A comprehensive psychiatric evaluation, diagnosis, and treatment plan should consider the broader context in which a patient’s pain occurs.
Regarding psychiatric illness, past and current symptoms, treatment history, and risk assessment should all be included. Using the “AMPS approach” (Figure)3—assessing Anxiety, Mood (depression and mania), Psychotic symptoms (paranoid ideation and hallucinations), and Substance use—helps screen for comorbid psychiatric conditions in patients with chronic pain.
Sleep assessment
Chronic pain patients often experience significant sleep disturbance that could be caused by physiological aspects of the pain condition, environmental factors (eg, uncomfortable bedding), a comorbid sleep disorder (eg, sleep apnea), a psychiatric disorder, or a combination of the above.
Obstructive and central sleep apnea are characterized by nighttime hypoxia, which leads to frequent disruption of the sleep-wake cycle and often manifests as daytime fatigue, irritability, depression, drowsiness, headaches, and increased pain sensitivity. Changes in sleep arousal can lead to neuropsychological changes during the day, such as decreased attention, memory problems, impaired executive functioning, and reduced impulse control.
Screen patients for central and obstructive sleep apnea before prescribing opioids or benzodiazepines for pain because these medications can cause or exacerbate underlying sleep apnea. Although many screening tools, such as the Epworth Sleepiness Scale, assess daytime somnolence,4 the STOP-BANG questionnaire is a quick, validated, and efficient screening tool that often is used to assess sleep apnea risk5,6 (Table 2). The presence of ≥3 risk factors identifies patients at increased risk and warrants consideration for further workup by a sleep specialist.7,8
Pharmacotherapy for chronic pain
Non-opioid medications. Pain can be broadly categorized as neuropathic or nociceptive. Neuropathic pain can be described by patients as numbness, burning, electric-like, and tingling, and is associated with nerve damage. Nociceptive pain commonly is described as similar to a toothache with descriptors such as stabbing, sharp, or a dull aching sensation; it is often, but not always, associated with acute injury or ongoing trauma to tissue. Drug treatment is most successful when the appropriate class of medication is matched to the specific type of pain.
Nociceptive pain often is successfully treated with non-steroidal anti-inflammatory drugs and acetaminophen. Non-selective COX inhibitors (eg, ibuprofen, indomethacin, ketorolac) and COX-2 selective inhibitors (eg, celecoxib) have been associated with cardiovascular, gastrointestinal, and renal disease; acetaminophen is associated with liver dysfunction.9-11 However, the absolute risk for complications in healthy patients is low.12 To minimize risk, use these agents for the shortest duration and at the lowest effective dosage possible.
Neuropathic pain can be addressed with certain antidepressants13—specifically, those that increase serotonin and norepinephrine (eg, tricyclic antidepressants [TCAs] and serotonin-norepinephrine reuptake inhibitors [SNRIs]), or medications that block ion channels (eg, anticonvulsants). TCAs (eg, desipramine, nortriptyline, amitriptyline) are among the best studied and most cost effective medications for treating neuropathic pain,14,15 but they can have sedating and anticholinergic effects, as well as cardiac adverse effects (ie, prolongation of the QTc interval). SNRIs (eg, venlafaxine, desvenlafaxine, duloxetine, and milnacipran) can be effective and often are better tolerated than TCAs.14
Some newer anticonvulsants (eg, gabapentin and pregabalin) have been found to be more effective than placebo for a variety of neuropathic pain conditions.16,17 Although they have few drug-drug interactions, anticonvulsants can cause dizziness, forgetfulness, and sedation. These adverse effects can be minimized by starting at a low dosage and titrating carefully. Because hepatic or renal impairment can affect metabolism or excretion of these drugs, review the prescribing information to determine safe dosing.
Targeted injection of medications to major pain generators (eg, an epidural steroid for radicular neck and back pain; facet injections for facet-related neck and back pain; trigger point injections for myofascial pain; occipital nerve blocks for occipital neuralgia; and botulinum toxin A injections for chronic migraine headache) can be effective in reducing discomfort and increasing function in patients with chronic pain. A detailed discussion of such therapies is beyond the scope of this article, but have been reviewed extensively elsewhere.18,19
Opioids. Although there is little evidence of long-term efficacy with chronic opioid therapy for most patients, a trial of opioids might be warranted for select patients who do not respond to other medications. Because the risk–benefit ratio for chronic opioid therapy is high,20-22 a decision to initiate a trial of a low-dosage opioid should be made only after careful consideration of those risks. It is generally agreed that treatment of chronic pain with low-dosage opioid therapy is more likely to be successful when it is used as an adjuvant to non-opioid modalities (eg, physical reconditioning, injection therapies, spinal cord stimulation, neurobehavioral interventions, non-opioid medications).
The Federation of State Medical Boards has stated that excessive reliance on opioid medications for treating chronic pain is a deviation from best practices.23 To maximize benefit and minimize risk, clinicians should carefully select appropriate patients, establish functional goals, and regularly monitor for efficacy and compliance. Thoroughly document these steps in the patient’s record for later reference.23
After establishing a clinical diagnosis for the cause of the pain, you should determine the risk of opioid abuse or misuse by using any one of the available risk assessment tools (Box). Understand, however, that no single tool has been shown to be more effective than others.
Although patients and some clinicians tend to overvalue the benefits of chronic opioid therapy, many do not fully appreciate the risks (eg, respiratory depression and death), which can be exacerbated if the patient is using other substance that suppress respiration (eg, benzodiazepines, alcohol, and illicit substances). Written informed consent and treatment agreement is highly recommended; components of such a document are listed in Table 3.23
Develop a treatment plan that emphasizes functional goals based on the patient’s physical limitations and that incorporates some type of daily, atraumatic physical activity. This plan should be documented and reviewed regularly to help monitor treatment effectiveness.
After an initial trial of a few weeks, the patient and clinician should meet to review the 5 “A”s (Table 4)24 to determine the success of the opioid regimen. Consulting your state’s prescription drug monitoring program (if one is available), obtaining a random urine drug test, and doing a pill count can provide useful, objective data. If the patient has not made progress but has experienced no adverse effects, then a small dosage increase might be warranted. If any of the 5 “A”s indicates lack of improvement or increased risk, consider stopping opioid therapy and exploring non-opioid options to manage chronic pain.
Referrals to a pain specialist or an addiction specialist, or both, might be needed, depending on the patient’s condition at any given follow-up visit. Such referral decisions, as well as all treatment plans, should be documented clearly in the medical record to prevent any misunderstanding, false accusations, or medicolegal repercussions regarding the rationale for continuing or terminating opioid-based treatment.
Non-pharmaceutical therapy for treating pain
The pain management field has successfully integrated the biopsychosocial model into regular practice. This model advocates the use of multimodal non-drug interventions in conjunction with opioid and non-opioid medications. Such interventions address behavioral, cognitive, sociocultural (psychosocial), lifestyle, and physiological dimensions of pain. A partial list of non-drug interventions is provided in Table 5.
Integration of these interventions within a biopsychosocial framework can assist you in making a comprehensive treatment plan. For example, patients with focal myofascial shoulder and back pain might derive only transient benefit from trigger point injection. However, concurrent referral to a pain psychologist and physical therapist could substantially improve functional outcomes by addressing factors that directly and indirectly influence myofascial pain. Inclusion of cognitive-behavioral therapy (addressing psychosocial and lifestyle dimensions), surface electromyography, psychophysiological interventions/biofeedback (addressing psychosocial, lifestyle, and physiological dimensions), and physical therapy (addressing lifestyle and physiological dimensions) allows the patient to learn coping skills, decrease physiological arousal that can lead to unnecessary tensing of muscles, and strengthen core muscle groups.
1. Institute of Medicine. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. http://www.iom.edu/~/media/Files/Report%20 Files/2011/Relieving-Pain-in-America-A-Blueprint-for- Transforming-Prevention-Care-Education-Research/ Pain%20Research%202011%20Report%20Brief.pdf. Published June 2011. Accessed April 15, 2015.
2. Jensen MP, Moore MR, Bockow TB, et al. Psychosocial factors and adjustment to chronic pain in persons with physical disabilities: a systematic review. Arch Phys Med Rehabil. 2011;92(1):146-160.
3. McCarron R, Xiong G, Bourgeois J. Lippincott’s primary care psychiatry. Philadelphia, PA: Lippincott Williams & Wilkins; 2009.
4. Abrishami A, Khajehdehi A, Chung F. A systematic review of screening questionnaires for obstructive sleep apnea. Can J Anaesth. 2010;57(5):423-438.
5. Boynton G, Vahabzadeh A, Hammoud S, et al. Validation of the STOP-BANG questionnaire among patients referred for suspected obstructive sleep apnea. J Sleep Disord Treat Care. 2013;2(4). doi: 10.4172/2325-9639.1000121.
6. Vana KD, Silva GE, Goldberg R. Predictive abilities of the STOP-Bang and Epworth Sleepiness Scale in identifying sleep clinic patients at high risk for obstructive sleep apnea. Res Nurs Health. 2013;36(1):84-94.
7. Chung F, Elsaid H. Screening for obstructive sleep apnea before surgery: why is it important? Curr Opin Anaesthesiol. 2009;22(3):405-411.
8. Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108(5):812-821.
9. Forman JP, Rimm EB, Curhan GC. Frequency of analgesic use and risk of hypertension among men. Arch Intern Med. 2007;167(4):394-399.
10. Sudano I, Flammer AJ, Périat D, et al. Acetaminophen increases blood pressure in patients with coronary artery disease. Circulation. 2010;122(18):1789-1796.
11. U.S. Food and Drug Administration. Questions and answers about oral prescription acetaminophen products to be limited to 325 mg per dosage unit. http://www.fda.gov/ drugs/drugsafety/informationbydrugclass/ucm239871. htm. Updated December 11, 2014. Accessed February 23, 2015.
12. Bhala N, Emberson J, Merhi A, et al; Coxib and traditional NSAID Trialists’ (CNT) Collaboration. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet. 2013;382(9894):769-779.
13. Sullivan MD, Robinson JP. Antidepressant and anticonvulsant medication for chronic pain. Phys Med Rehabil Clin N Am. 2006;17(2):381-400, vi-vii.
14. Sindrup SH, Otto M, Finnerup NB, et al. Antidepressants in the treatment of neuropathic pain. Basic Clin Pharmacol Toxicol. 2005;96(6):399-409.
15. Pilowsky I, Hallett EC, Bassett DL, et al. A controlled study of amitriptyline in the treatment of chronic pain. Pain. 1982;14(2):169-179.
16. Finnerup NB, Sindrup SH, Jensen TS. The evidence for pharmacological treatment of neuropathic pain. Pain. 2010;150(3):573-581.
17. Dworkin RH, O’Connor AB, Backonja M, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132(3):237-251.
18. Manchikanti L, Abdi S, Atluri S, et al. An update of comprehensive evidence-based guidelines for interventional techniques in chronic spinal pain. Part II: guidance and recommendations. Pain Physician. 2013;16(suppl 2):S49-S283.
19. Singh V, Trescot A, Nishio I. Injections for chronic pain. Phys Med Rehabil Clin N Am. 2015;26(2):249-261.
20. Centers for Disease Control and Prevention (CDC). Vital signs: overdoses of prescription opioid pain relievers— United States, 1999–2008. MMWR Morb Mortal Wkly Rep. 2011;60(43):1487-1492.
21. Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013;309(7):657-659.
22. Chen L, Vo T, Seefeld L, et al. Lack of correlation between opioid dose adjustment and pain score change in a group of chronic pain patients. J Pain. 2013;14(4):384-392.
23. Federation of State Medical Boards. Model policy for the use of opioid analgesics in the treatment of chronic pain. http:// www.fsmb.org/Media/Default/PDF/FSMB/Advocacy/ pain_policy_july2013.pdf. Published July 2013. Accessed December 18, 2015.
24. Passik SD, Weinreb HJ. Managing chronic nonmalignant pain: overcoming obstacles to the use of opioids. Adv Ther. 2000;17(2):70-83.
1. Institute of Medicine. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. http://www.iom.edu/~/media/Files/Report%20 Files/2011/Relieving-Pain-in-America-A-Blueprint-for- Transforming-Prevention-Care-Education-Research/ Pain%20Research%202011%20Report%20Brief.pdf. Published June 2011. Accessed April 15, 2015.
2. Jensen MP, Moore MR, Bockow TB, et al. Psychosocial factors and adjustment to chronic pain in persons with physical disabilities: a systematic review. Arch Phys Med Rehabil. 2011;92(1):146-160.
3. McCarron R, Xiong G, Bourgeois J. Lippincott’s primary care psychiatry. Philadelphia, PA: Lippincott Williams & Wilkins; 2009.
4. Abrishami A, Khajehdehi A, Chung F. A systematic review of screening questionnaires for obstructive sleep apnea. Can J Anaesth. 2010;57(5):423-438.
5. Boynton G, Vahabzadeh A, Hammoud S, et al. Validation of the STOP-BANG questionnaire among patients referred for suspected obstructive sleep apnea. J Sleep Disord Treat Care. 2013;2(4). doi: 10.4172/2325-9639.1000121.
6. Vana KD, Silva GE, Goldberg R. Predictive abilities of the STOP-Bang and Epworth Sleepiness Scale in identifying sleep clinic patients at high risk for obstructive sleep apnea. Res Nurs Health. 2013;36(1):84-94.
7. Chung F, Elsaid H. Screening for obstructive sleep apnea before surgery: why is it important? Curr Opin Anaesthesiol. 2009;22(3):405-411.
8. Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108(5):812-821.
9. Forman JP, Rimm EB, Curhan GC. Frequency of analgesic use and risk of hypertension among men. Arch Intern Med. 2007;167(4):394-399.
10. Sudano I, Flammer AJ, Périat D, et al. Acetaminophen increases blood pressure in patients with coronary artery disease. Circulation. 2010;122(18):1789-1796.
11. U.S. Food and Drug Administration. Questions and answers about oral prescription acetaminophen products to be limited to 325 mg per dosage unit. http://www.fda.gov/ drugs/drugsafety/informationbydrugclass/ucm239871. htm. Updated December 11, 2014. Accessed February 23, 2015.
12. Bhala N, Emberson J, Merhi A, et al; Coxib and traditional NSAID Trialists’ (CNT) Collaboration. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet. 2013;382(9894):769-779.
13. Sullivan MD, Robinson JP. Antidepressant and anticonvulsant medication for chronic pain. Phys Med Rehabil Clin N Am. 2006;17(2):381-400, vi-vii.
14. Sindrup SH, Otto M, Finnerup NB, et al. Antidepressants in the treatment of neuropathic pain. Basic Clin Pharmacol Toxicol. 2005;96(6):399-409.
15. Pilowsky I, Hallett EC, Bassett DL, et al. A controlled study of amitriptyline in the treatment of chronic pain. Pain. 1982;14(2):169-179.
16. Finnerup NB, Sindrup SH, Jensen TS. The evidence for pharmacological treatment of neuropathic pain. Pain. 2010;150(3):573-581.
17. Dworkin RH, O’Connor AB, Backonja M, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132(3):237-251.
18. Manchikanti L, Abdi S, Atluri S, et al. An update of comprehensive evidence-based guidelines for interventional techniques in chronic spinal pain. Part II: guidance and recommendations. Pain Physician. 2013;16(suppl 2):S49-S283.
19. Singh V, Trescot A, Nishio I. Injections for chronic pain. Phys Med Rehabil Clin N Am. 2015;26(2):249-261.
20. Centers for Disease Control and Prevention (CDC). Vital signs: overdoses of prescription opioid pain relievers— United States, 1999–2008. MMWR Morb Mortal Wkly Rep. 2011;60(43):1487-1492.
21. Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013;309(7):657-659.
22. Chen L, Vo T, Seefeld L, et al. Lack of correlation between opioid dose adjustment and pain score change in a group of chronic pain patients. J Pain. 2013;14(4):384-392.
23. Federation of State Medical Boards. Model policy for the use of opioid analgesics in the treatment of chronic pain. http:// www.fsmb.org/Media/Default/PDF/FSMB/Advocacy/ pain_policy_july2013.pdf. Published July 2013. Accessed December 18, 2015.
24. Passik SD, Weinreb HJ. Managing chronic nonmalignant pain: overcoming obstacles to the use of opioids. Adv Ther. 2000;17(2):70-83.
Migraines more severe in PNES patients than in epilepsy patients
Psychogenic nonepileptic seizure (PNES) patients reported having more frequent and longer-lasting migraines than patients diagnosed with epilepsy, in an observational study conducted in the United States.
Researchers questioned 29 patients with epilepsy and 43 PNES patients about their migraines and seizures through the use of standardized questionnaires and a standardized interview. All study participants were found in a clinician database of patients who had been evaluated in the Mayo Clinic epilepsy monitoring unit between 2008 and 2014. Their ages ranged from 20 years to 82 years. Patients who were diagnosed with both PNES and epilepsy were excluded from the research project.
PNES patients reported having significantly more migraine attacks and longer-duration migraines (when untreated) than patients with epilepsy. Specifically, on average, PNES patients said they experienced 6.5 migraine attacks per month and migraines with a length of 39.5 hours, whereas patients with epilepsy said they had, on average, 3.8 migraine attacks per month and migraines lasting 27.3 hours. Another significant difference between the two groups of patients occurred in the numbers of nonvisual migraine aura symptoms reported. While 22 of the PNES patients (78.6%) reported experiencing such symptoms, 7 of the epilepsy patients (46.7%) reported having nonvisual aura symptoms (P = .033).
“Our study adds to the existing literature [on the relationship between PNES and migraine] by detailing specific migraine characteristics in patients with PNES,” wrote Morgan A. Shepard and colleagues. The researchers noted that PNES patients could have overreported the severity of their migraine symptoms and that a high level of somatization has been found in patients with PNES.
The results of this research project “justify the need for clinicians to assess PNES patients for the presence of migraine and when present, to treat them appropriately for migraine,” according to the researchers.
The study’s authors did not report any conflicts of interest.
Read the study in Seizure (doi: 10.1016/j.seizure.2015.12.006).
Psychogenic nonepileptic seizure (PNES) patients reported having more frequent and longer-lasting migraines than patients diagnosed with epilepsy, in an observational study conducted in the United States.
Researchers questioned 29 patients with epilepsy and 43 PNES patients about their migraines and seizures through the use of standardized questionnaires and a standardized interview. All study participants were found in a clinician database of patients who had been evaluated in the Mayo Clinic epilepsy monitoring unit between 2008 and 2014. Their ages ranged from 20 years to 82 years. Patients who were diagnosed with both PNES and epilepsy were excluded from the research project.
PNES patients reported having significantly more migraine attacks and longer-duration migraines (when untreated) than patients with epilepsy. Specifically, on average, PNES patients said they experienced 6.5 migraine attacks per month and migraines with a length of 39.5 hours, whereas patients with epilepsy said they had, on average, 3.8 migraine attacks per month and migraines lasting 27.3 hours. Another significant difference between the two groups of patients occurred in the numbers of nonvisual migraine aura symptoms reported. While 22 of the PNES patients (78.6%) reported experiencing such symptoms, 7 of the epilepsy patients (46.7%) reported having nonvisual aura symptoms (P = .033).
“Our study adds to the existing literature [on the relationship between PNES and migraine] by detailing specific migraine characteristics in patients with PNES,” wrote Morgan A. Shepard and colleagues. The researchers noted that PNES patients could have overreported the severity of their migraine symptoms and that a high level of somatization has been found in patients with PNES.
The results of this research project “justify the need for clinicians to assess PNES patients for the presence of migraine and when present, to treat them appropriately for migraine,” according to the researchers.
The study’s authors did not report any conflicts of interest.
Read the study in Seizure (doi: 10.1016/j.seizure.2015.12.006).
Psychogenic nonepileptic seizure (PNES) patients reported having more frequent and longer-lasting migraines than patients diagnosed with epilepsy, in an observational study conducted in the United States.
Researchers questioned 29 patients with epilepsy and 43 PNES patients about their migraines and seizures through the use of standardized questionnaires and a standardized interview. All study participants were found in a clinician database of patients who had been evaluated in the Mayo Clinic epilepsy monitoring unit between 2008 and 2014. Their ages ranged from 20 years to 82 years. Patients who were diagnosed with both PNES and epilepsy were excluded from the research project.
PNES patients reported having significantly more migraine attacks and longer-duration migraines (when untreated) than patients with epilepsy. Specifically, on average, PNES patients said they experienced 6.5 migraine attacks per month and migraines with a length of 39.5 hours, whereas patients with epilepsy said they had, on average, 3.8 migraine attacks per month and migraines lasting 27.3 hours. Another significant difference between the two groups of patients occurred in the numbers of nonvisual migraine aura symptoms reported. While 22 of the PNES patients (78.6%) reported experiencing such symptoms, 7 of the epilepsy patients (46.7%) reported having nonvisual aura symptoms (P = .033).
“Our study adds to the existing literature [on the relationship between PNES and migraine] by detailing specific migraine characteristics in patients with PNES,” wrote Morgan A. Shepard and colleagues. The researchers noted that PNES patients could have overreported the severity of their migraine symptoms and that a high level of somatization has been found in patients with PNES.
The results of this research project “justify the need for clinicians to assess PNES patients for the presence of migraine and when present, to treat them appropriately for migraine,” according to the researchers.
The study’s authors did not report any conflicts of interest.
Read the study in Seizure (doi: 10.1016/j.seizure.2015.12.006).
FROM SEIZURE
Breast cancer
Manic and nonadherent, with a diagnosis of breast cancer
CASE Diagnosis, mood changes
Ms. A, age 58, is a white female with a history of chronic bipolar I disorder who is being evaluated as a new patient in an academic psychiatric clinic. Recently, she was diagnosed with ER+, PR+, and HER2+ ductal carcinoma. She does not take her prescribed mood stabilizers.
After her cancer diagnosis, Ms. A experiences new-onset agitation, including irritable mood, suicidal thoughts, tearfulness, decreased need for sleep, fast speech, excessive spending, and anorexia. She reports that she hears the voice of God telling her that she could cure her breast cancer through prayer and herbal remedies. Her treatment team, comprising her primary care provider and surgical oncologist, consider several medication adjustments, but are unsure of their effects on Ms. A’s mental health, progression of cancer, and cancer treatment.
What is the most likely cause of Ms. A’s psychiatric symptoms?
a) anxiety from having a diagnosis of cancer
b) stress reaction
c) panic attack
d) manic or mixed phase of bipolar I disorder
The authors’ observations
Treating breast cancer with concurrent severe mental illness is complex and challenging for the patient, family, and health care providers. Mental health and oncology clinicians must collaborate when treating these patients because of overlapping pathophysiology and medication interactions. A comprehensive evaluation is required to tease apart whether a patient is simply demoralized by her new diagnosis, or if a more serious mood disorder is present.
Worldwide, breast cancer is the most frequently diagnosed cancer and the leading cause of cancer death among women.1 The mean age of women diagnosed with breast cancer is 61 years; 61% of these women are alive 15 years after diagnosis, representing the largest group of female cancer survivors.
The incidence of breast cancer is reported to be higher in women with bipolar disorder compared with the general population.2-4 This positive correlation might be associated with a high rate of smoking, poor health-related behaviors, and, possibly, medication side effects. A genome-wide association study found significant associations between bipolar disorder and the breast cancer-related genes BRCA2 and PALB2.5
Antipsychotics and prolactin
Antipsychotics play an important role in managing bipolar disorder; several, however, are known to raise the serum prolactin level 10- to 20-fold. A high prolactin level could be associated with progression of breast cancer. All antipsychotics have label warnings regarding their use in women with breast cancer.
The prolactin receptor is overexpressed in >95% of breast cancer cells, regardless of estrogen-receptor status. The role of prolactin in development of new breast cancer is open to debate. The effect of a high prolactin level in women with diagnosed breast cancer is unknown, although available preclinical data suggest that high levels should be avoided. Psychiatric clinicians should consider checking the serum prolactin level or switching to a treatment strategy that avoids iatrogenic prolactin elevation. This risk must be carefully weighed against the mood-stabilizing properties of antipsychotics.6
TREATMENT Consider comorbidities
Ms. A receives supportive psychotherapy in addition to quetiapine, 400 mg/d, and valproic acid, 1,500 mg/d. This regimen helps her successfully complete the initial phase of breast cancer treatment, which consists of a single mastectomy, adjuvant chemotherapy (doxorubicin and cyclophosphamide followed by paclitaxel and trastuzumab). She is now on endocrine therapy with tamoxifen.
Ms. A, calm, much improved mood symptoms, and euthymic, has questions regarding her mental health, cancer prognosis, and potential medication side effects with continued cancer treatment.
Which drug used to treat breast cancer might relieve Ms. A’s manic symptoms?
a) cyclophosphamide
b) tamoxifen
c) trastuzumab
d) pamidronate
The authors’ observations
Recent evidence suggests that tamoxifen reduces symptoms of bipolar mania more rapidly than many standard medications for bipolar disorder. Tamoxifen is the only available centrally active protein kinase C (PKC) inhibitor,7 although lithium and valproic acid also might inhibit PKC activity. PKC regulates presynaptic and postsynaptic neurotransmission, neuronal excitability, and neurotransmitter release. PKC is thought to be overactive during mania, possibly because of an increase in membrane-bound PKC and PKC translocation from the cytosol to membrane.7,8
Preliminary clinical trials suggest that tamoxifen significantly reduces manic symptoms in patients with bipolar disorder within 5 days of initiation.7 These findings have been confirmed in animal studies and in 1 single-blind and 4 double-blind placebo-controlled clinical studies over the past 15 years.9
Tamoxifen is a selective estrogen-receptor modulator used to prevent recurrence in receptor-positive breast cancer. Cytochrome P450 (CYP) 2D6 is the principal enzyme that converts tamoxifen to its active metabolite, endoxifen. Inhibition of tamoxifen conversion to endoxifen by CYP2D6 inhibitors could decrease the efficacy of tamoxifen therapy and might increase the risk of breast cancer recurrence. Although antidepressants generally are not recommended as a first-line agent for bipolar disorder, several selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors are potent, moderate, or mild inhibitors of CYP2D610 (Table 1). Approximately 7% of women have nonfunctional CYP2D6 alleles and have a lower endoxifen level.11
Treating breast cancer
The mainstays of breast cancer treatment are surgery, radiation therapy, chemotherapy, hormone therapy, and targeted monoclonal antibody therapy. The protocol of choice depends on the stage of cancer, estrogen receptor status, expression of human epidermal growth factor receptor 2 (HER-2), treatment history, and the patient’s menopausal status. Overexpression of HER-2 oncoprotein, found in 25% to 30% of breast cancers, has been shown to promote cell transformation. HER-2 overexpression is associated with aggressive tumor phenotypes, lymph node involvement, and resistance to chemotherapy and endocrine therapy. Therefore, the HER-2 oncoprotein is a key target for treatment. Often, several therapies are combined to prevent recurrence of disease.
Breast cancer treatment often can cause demoralization, menopausal symptoms, sleep disturbance, impaired sexual function, infertility, and disturbed body image. It also can trigger psychiatric symptoms in patients with, or without, a history of mental illness.
Trastuzumab is a recombinant humanized monoclonal antibody against HER-2, and is approved for treating HER-2 positive breast cancer. However, approximately 50% of patients with HER-2 overexpression do not respond to trastuzumab alone or combined with chemotherapy, and nearly all patients develop resistance to trastuzumab, leading to recurrence.12 This medication is still used in practice, and research regarding antiepileptic drugs working in synergy with this monoclonal antibody is underway.
OUTCOME Stability achieved
Quetiapine and valproic acid are first-line choices for Ms. A because (1) she would be on long-term tamoxifen to maintain cancer remission maintenance and (2) she is in a manic phase of bipolar disorder. Tamoxifen also could improve her manic symptoms. This medication regimen might enhance the action of cancer treatments and also could reduce adverse effects of cancer treatment, such as insomnia associated with tamoxifen.
After the team educates Ms. A about how her psychiatric medications could benefit her cancer treatment, she becomes more motivated to stay on her regimen. Ms. A does well on these medications and after 18 months has not experienced exacerbation of psychiatric symptoms or recurrence of cancer.
The authors’ observations
There are 3 major classes of mood stabilizers for treating bipolar disorder: lithium, antiepileptic drugs, and atypical antipsychotics.13 In a setting of cancer, mood stabilizers are prescribed for managing mania or drug-induced agitation or anxiety associated with steroid use, brain metastases, and other medical conditions. They also can be used to treat neuropathic pain and hot flashes and seizure prophylaxis.13
Valproic acid
Valproic acid can help treat mood lability, impulsivity, and disinhibition, whether these symptoms are due to primary psychiatric illness or secondary to cancer metastasis. It is a first-line agent for manic and mixed bipolar states, and can be titrated quickly to achieve optimal benefit. Valproic acid also has been described as a histone deacetylase (HDAC) inhibitor, known to attenuate apoptotic activity, making it of interest as a treatment for cancer.14 HDAC inhibitors have been shown to:
- induce differentiation and cell cycle arrest
- activate the extrinsic or intrinsic pathways of apoptosis
- inhibit invasion, migration, and angiogenesis in different cancer cell lines.15
In regard to breast cancer, valproic acid inhibits growth of cell lines independent of estrogen receptors, increases the action of such breast cancer treatments as tamoxifen, raloxifene, fulvestrant, and letrozole, and induces solid tumor regression.14 Valproic acid also reduces cancer cell viability and could act as a powerful antiproliferative agent in estrogen-sensitive breast cancer cells.16
Valproic acid reduces cell growth-inducing apoptosis and cell cycle arrest in ERα-positive breast cancer cells, although it has no significant apoptotic effect in ERα-negative cells.16 However, evidence does support the ability of valproic acid to restore an estrogen-sensitive phenotype in ERα-negative breast cancer cells, allowing successful treatment with the anti-estrogen tamoxifen in vitro.10
Antipsychotics
Antipsychotics act as dopamine D2 receptor antagonists within the hypothalamic-pituitary-adrenal axis, thus increasing the serum prolactin level. Among atypicals, risperidone and its active metabolite, paliperidone, produce the greatest increase in the prolactin level, whereas quetiapine, clozapine, and aripiprazole minimally elevate the prolactin level.
Hyperprolactinemia correlates with rapid breast cancer progression and inferior prognosis, regardless of breast cancer receptor typing. Therefore, prolactin-sparing antipsychotics are preferred when treating a patient with comorbid bipolar disorder and breast cancer. Checking the serum prolactin level might help guide treatment. The literature is mixed regarding antipsychotic use and new mammary tumorigenesis; current research does not support antipsychotic choice based on future risk of breast cancer.6
Other adverse effects from antipsychotic use for bipolar disorder could have an impact on patients with breast cancer. Several of these medications could ameliorate side effects of advanced cancer and chemotherapy. Quetiapine, for example, might improve tamoxifen-induced insomnia in women with breast cancer because of its high affinity for serotonergic receptors, thus enhancing central serotonergic neurotransmitters and decreasing excitatory glutamatergic transmission.17
In any type of advanced cancer, nausea and vomiting are common, independent of chemotherapy and medication regimens. Metabolic derangement, vestibular dysfunction, CNS disorders, and visceral metastasis all contribute to hyperemesis. Olanzapine has been shown to significantly reduce refractory nausea and can cause weight gain and improved appetite, which benefits cachectic patients.18
Last, clozapine is one of the more effective antipsychotic medications, but also carries a risk of neutropenia. In patients with neutropenia secondary to chemotherapy, clozapine could increase the risk of infection in an immunocompromised patient.19 Granulocyte colony stimulating factor might be useful as a rescue medication for treatment-emergent neutropenia.19
Treatment considerations
Cancer patients might be unable or unwilling to seek services for mental health during their cancer treatment, and many who have a diagnosis of psychiatric illness might stop following up with psychiatric care when cancer treatment takes priority. It is critical for clinicians to be aware of the current literature regarding the impact of mood-stabilizing medication on cancer treatment. Monitoring for drug interactions is essential, and electronic drug interaction tools, such as Lexicomp, may be useful for this purpose.13 Because of special vulnerabilities in this population, cautious and judicious prescribing practices are advised.
The risk-benefit profile for medications for bipolar disorder must be considered before they are initiated or changes are made to the regimen (Table 2). Changing an effective mood stabilizer to gain benefits in breast cancer prognosis is not recommended in most cases, because benefits have been shown to be only significant in preclinical research; currently, there are no clinical guidelines. However, medication adjustments should be made with these theoretical benefits in mind, as long as the treatment of bipolar disorder remains effective.
Regardless of what treatment regimen the health team decides on, several underlying issues that affect patient care must be considered in this population. Successfully treating breast cancer in a woman with severe mental illness only can be accomplished when her mental illness is under control. Once she is psychiatrically stable, it is important for her to have a basic understanding of how cancer can affect the body and know the reasons behind treatment.
It is imperative that physicians provide their patients with a general understanding of their comorbid disorders, and find ways to help patients remain adherent with treatment of both diseases. Many patients feel demoralized by a cancer diagnosis and adherence to a medication regimen might be a difficult task among those with bipolar disorder who also are socially isolated, lack education, or have poor recall of treatment recommendations.20
Bottom Line
Managing comorbid bipolar disorder and breast cancer might seem daunting,
but treatments for the 2 diseases can work in synergy. You have an opportunity to
educate patients and colleagues in treating bipolar disorder and comorbid breast
cancer. Optimizing care using known psychopharmacologic data can not only lead
to better outcomes, but might additionally offer some hope and reason to remain
treatment-adherent for patients suffering from this complex comorbidity.
Related Resources
• Agarwala P, Riba MB. Tailoring depression treatment for women with breast cancer. Current Psychiatry. 2010;9(11): 39-40,45-46,48-49.
• Cunningham R, Sarfati D, Stanley J, et al. Cancer survival in the context of mental illness: a national cohort study. Gen Hosp Psychiatry. 2015;37(6):501-506.
Drug Brand Names
Amiodarone • Cordarone
Aripiprazole • Abilify
Asenapine • Saphris
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Citalopram • Celexa
Clozapine • Clozaril
Cyclophosphamide • Cytoxan, Neosar
Doxorubicin • Doxil, Adriamycin
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
Fulvestrant • Faslodex
Iloperidone • Fanapt
Lamotrigine • Lamictal
Letrozole • Femara
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Olanzapine • Zyprexa
Paclitaxel • Onxol
Paliperidone • Invega
Pamidronate • Aredia
Paroxetine • Paxil
Quetiapine • Seroquel
Raloxifene • Evista
Risperidone • Risperdal
Sertraline • Zoloft
Tamoxifen • Nolvadex
Thioridazine • Mellaril
Trastuzumab • Herceptin
Valproic acid • Depakene
Venlafaxine • Effexor
Ziprasidone • Geodon
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69-90.
2. American Cancer Society. Cancer facts and figures 2014. Atlanta, GA: American Cancer Society; 2014.
3. BarChana M, Levav I, Lipshitz I, et al. Enhanced cancer risk among patients with bipolar disorder. J Affect Disord. 2008;108(1-2):43-48.
4. Hung YP, Liu CJ, Tsai CF, et al. Incidence and risk of mood disorders in patients with breast cancers in Taiwan: a nationwide population-based study. Psychooncology. 2013;22(10):2227-2234.
5. Tesli M, Athanasiu L, Mattingsdal M, et al. Association analysis of PALB2 and BRCA2 in bipolar disorder and schizophrenia in a scandinavian case–control sample. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(7):1276-1282.
6. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
7. Armani F, Andersen ML, Galduróz JC. Tamoxifen use for the management of mania: a review of current preclinical evidence. Psychopharmacology (Berl). 2014;231(4):639-649.
8. Zarate CA Jr, Singh JB, Carlson PJ, et al. Efficacy of a protein kinase C inhibitor (tamoxifen) in the treatment of acute mania: a pilot study. Bipolar Disord. 2007;9(6):561-570.
9. Zarate CA, Manji HK. Protein kinase C inhibitors: rationale for use and potential in the treatment of bipolar disorder. CNS Drugs. 2009;23(7):569-582.
10. Fortunati N, Bertino S, Costantino L, et al. Valproic acid restores ER alpha and antiestrogen sensitivity to ER alpha-negative breast cancer cells. Mol Cell Endocrinol. 2010;314(1):17-22.
11. Thekdi SM, Trinidad A, Roth A. Psychopharmacology in cancer. Curr Psychiatry Rep. 2014;17(1):529.
12. Meng Q, Chen X, Sun L, et al. Carbamazepine promotes Her-2 protein degradation in breast cancer cells by modulating HDAC6 activity and acetylation of Hsp90. Mol Cell Biochem. 2011;348(1-2):165-171.
13. Altamura AC, Lietti L, Dobrea C, et al. Mood stabilizers for patients with bipolar disorder: the state of the art. Expert Rev Neurother. 2011;11(1):85-99.
14. Chateauvieux S, Morceau F, Dicato M, et al. Molecular and therapeutic potential and toxicity of valproic acid [published online July 29, 2010]. J Biomed Biotechnol. doi: 10.1155/2010/479364.
15. Jafary H, Ahmadian S, Soleimani M. The enhanced apoptosis and antiproliferative response to combined treatment with valproate and nicotinamide in MCF-7 breast cancer cells. Tumour Biol. 2013;35(3):2701-2710.
16. Fortunati N, Bertino S, Costantino L, et al. Valproic acid is a selective antiproliferative agent in estrogen-sensitive breast cancer cells. Cancer Lett. 2008;259(2):156-164.
17. Pasquini M, Speca A, Biondi M. Quetiapine for tamoxifen-induced insomnia in women with breast cancer. Psychosomatics. 2009;50(2):159-161.
18. Srivastava M, Brito-Dellan N, Davis MP, et al. Olanzapine as an antiemetic in refractory nausea and vomiting in advanced cancer. J Pain Symptom Manage. 2003;25(6):578-582.
19. Sankaranarayanan A, Mulchandani M, Tirupati S. Clozapine, cancer chemotherapy and neutropenia - dilemmas in management. Psychiatr Danub. 2013;25(4):419-422.
20. Cole M, Padmanabhan A. Breast cancer treatment of women with schizophrenia and bipolar disorder from Philadelphia, PA: lessons learned and suggestions for improvement. J Cancer Educ. 2012;27(4):774-779.
CASE Diagnosis, mood changes
Ms. A, age 58, is a white female with a history of chronic bipolar I disorder who is being evaluated as a new patient in an academic psychiatric clinic. Recently, she was diagnosed with ER+, PR+, and HER2+ ductal carcinoma. She does not take her prescribed mood stabilizers.
After her cancer diagnosis, Ms. A experiences new-onset agitation, including irritable mood, suicidal thoughts, tearfulness, decreased need for sleep, fast speech, excessive spending, and anorexia. She reports that she hears the voice of God telling her that she could cure her breast cancer through prayer and herbal remedies. Her treatment team, comprising her primary care provider and surgical oncologist, consider several medication adjustments, but are unsure of their effects on Ms. A’s mental health, progression of cancer, and cancer treatment.
What is the most likely cause of Ms. A’s psychiatric symptoms?
a) anxiety from having a diagnosis of cancer
b) stress reaction
c) panic attack
d) manic or mixed phase of bipolar I disorder
The authors’ observations
Treating breast cancer with concurrent severe mental illness is complex and challenging for the patient, family, and health care providers. Mental health and oncology clinicians must collaborate when treating these patients because of overlapping pathophysiology and medication interactions. A comprehensive evaluation is required to tease apart whether a patient is simply demoralized by her new diagnosis, or if a more serious mood disorder is present.
Worldwide, breast cancer is the most frequently diagnosed cancer and the leading cause of cancer death among women.1 The mean age of women diagnosed with breast cancer is 61 years; 61% of these women are alive 15 years after diagnosis, representing the largest group of female cancer survivors.
The incidence of breast cancer is reported to be higher in women with bipolar disorder compared with the general population.2-4 This positive correlation might be associated with a high rate of smoking, poor health-related behaviors, and, possibly, medication side effects. A genome-wide association study found significant associations between bipolar disorder and the breast cancer-related genes BRCA2 and PALB2.5
Antipsychotics and prolactin
Antipsychotics play an important role in managing bipolar disorder; several, however, are known to raise the serum prolactin level 10- to 20-fold. A high prolactin level could be associated with progression of breast cancer. All antipsychotics have label warnings regarding their use in women with breast cancer.
The prolactin receptor is overexpressed in >95% of breast cancer cells, regardless of estrogen-receptor status. The role of prolactin in development of new breast cancer is open to debate. The effect of a high prolactin level in women with diagnosed breast cancer is unknown, although available preclinical data suggest that high levels should be avoided. Psychiatric clinicians should consider checking the serum prolactin level or switching to a treatment strategy that avoids iatrogenic prolactin elevation. This risk must be carefully weighed against the mood-stabilizing properties of antipsychotics.6
TREATMENT Consider comorbidities
Ms. A receives supportive psychotherapy in addition to quetiapine, 400 mg/d, and valproic acid, 1,500 mg/d. This regimen helps her successfully complete the initial phase of breast cancer treatment, which consists of a single mastectomy, adjuvant chemotherapy (doxorubicin and cyclophosphamide followed by paclitaxel and trastuzumab). She is now on endocrine therapy with tamoxifen.
Ms. A, calm, much improved mood symptoms, and euthymic, has questions regarding her mental health, cancer prognosis, and potential medication side effects with continued cancer treatment.
Which drug used to treat breast cancer might relieve Ms. A’s manic symptoms?
a) cyclophosphamide
b) tamoxifen
c) trastuzumab
d) pamidronate
The authors’ observations
Recent evidence suggests that tamoxifen reduces symptoms of bipolar mania more rapidly than many standard medications for bipolar disorder. Tamoxifen is the only available centrally active protein kinase C (PKC) inhibitor,7 although lithium and valproic acid also might inhibit PKC activity. PKC regulates presynaptic and postsynaptic neurotransmission, neuronal excitability, and neurotransmitter release. PKC is thought to be overactive during mania, possibly because of an increase in membrane-bound PKC and PKC translocation from the cytosol to membrane.7,8
Preliminary clinical trials suggest that tamoxifen significantly reduces manic symptoms in patients with bipolar disorder within 5 days of initiation.7 These findings have been confirmed in animal studies and in 1 single-blind and 4 double-blind placebo-controlled clinical studies over the past 15 years.9
Tamoxifen is a selective estrogen-receptor modulator used to prevent recurrence in receptor-positive breast cancer. Cytochrome P450 (CYP) 2D6 is the principal enzyme that converts tamoxifen to its active metabolite, endoxifen. Inhibition of tamoxifen conversion to endoxifen by CYP2D6 inhibitors could decrease the efficacy of tamoxifen therapy and might increase the risk of breast cancer recurrence. Although antidepressants generally are not recommended as a first-line agent for bipolar disorder, several selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors are potent, moderate, or mild inhibitors of CYP2D610 (Table 1). Approximately 7% of women have nonfunctional CYP2D6 alleles and have a lower endoxifen level.11
Treating breast cancer
The mainstays of breast cancer treatment are surgery, radiation therapy, chemotherapy, hormone therapy, and targeted monoclonal antibody therapy. The protocol of choice depends on the stage of cancer, estrogen receptor status, expression of human epidermal growth factor receptor 2 (HER-2), treatment history, and the patient’s menopausal status. Overexpression of HER-2 oncoprotein, found in 25% to 30% of breast cancers, has been shown to promote cell transformation. HER-2 overexpression is associated with aggressive tumor phenotypes, lymph node involvement, and resistance to chemotherapy and endocrine therapy. Therefore, the HER-2 oncoprotein is a key target for treatment. Often, several therapies are combined to prevent recurrence of disease.
Breast cancer treatment often can cause demoralization, menopausal symptoms, sleep disturbance, impaired sexual function, infertility, and disturbed body image. It also can trigger psychiatric symptoms in patients with, or without, a history of mental illness.
Trastuzumab is a recombinant humanized monoclonal antibody against HER-2, and is approved for treating HER-2 positive breast cancer. However, approximately 50% of patients with HER-2 overexpression do not respond to trastuzumab alone or combined with chemotherapy, and nearly all patients develop resistance to trastuzumab, leading to recurrence.12 This medication is still used in practice, and research regarding antiepileptic drugs working in synergy with this monoclonal antibody is underway.
OUTCOME Stability achieved
Quetiapine and valproic acid are first-line choices for Ms. A because (1) she would be on long-term tamoxifen to maintain cancer remission maintenance and (2) she is in a manic phase of bipolar disorder. Tamoxifen also could improve her manic symptoms. This medication regimen might enhance the action of cancer treatments and also could reduce adverse effects of cancer treatment, such as insomnia associated with tamoxifen.
After the team educates Ms. A about how her psychiatric medications could benefit her cancer treatment, she becomes more motivated to stay on her regimen. Ms. A does well on these medications and after 18 months has not experienced exacerbation of psychiatric symptoms or recurrence of cancer.
The authors’ observations
There are 3 major classes of mood stabilizers for treating bipolar disorder: lithium, antiepileptic drugs, and atypical antipsychotics.13 In a setting of cancer, mood stabilizers are prescribed for managing mania or drug-induced agitation or anxiety associated with steroid use, brain metastases, and other medical conditions. They also can be used to treat neuropathic pain and hot flashes and seizure prophylaxis.13
Valproic acid
Valproic acid can help treat mood lability, impulsivity, and disinhibition, whether these symptoms are due to primary psychiatric illness or secondary to cancer metastasis. It is a first-line agent for manic and mixed bipolar states, and can be titrated quickly to achieve optimal benefit. Valproic acid also has been described as a histone deacetylase (HDAC) inhibitor, known to attenuate apoptotic activity, making it of interest as a treatment for cancer.14 HDAC inhibitors have been shown to:
- induce differentiation and cell cycle arrest
- activate the extrinsic or intrinsic pathways of apoptosis
- inhibit invasion, migration, and angiogenesis in different cancer cell lines.15
In regard to breast cancer, valproic acid inhibits growth of cell lines independent of estrogen receptors, increases the action of such breast cancer treatments as tamoxifen, raloxifene, fulvestrant, and letrozole, and induces solid tumor regression.14 Valproic acid also reduces cancer cell viability and could act as a powerful antiproliferative agent in estrogen-sensitive breast cancer cells.16
Valproic acid reduces cell growth-inducing apoptosis and cell cycle arrest in ERα-positive breast cancer cells, although it has no significant apoptotic effect in ERα-negative cells.16 However, evidence does support the ability of valproic acid to restore an estrogen-sensitive phenotype in ERα-negative breast cancer cells, allowing successful treatment with the anti-estrogen tamoxifen in vitro.10
Antipsychotics
Antipsychotics act as dopamine D2 receptor antagonists within the hypothalamic-pituitary-adrenal axis, thus increasing the serum prolactin level. Among atypicals, risperidone and its active metabolite, paliperidone, produce the greatest increase in the prolactin level, whereas quetiapine, clozapine, and aripiprazole minimally elevate the prolactin level.
Hyperprolactinemia correlates with rapid breast cancer progression and inferior prognosis, regardless of breast cancer receptor typing. Therefore, prolactin-sparing antipsychotics are preferred when treating a patient with comorbid bipolar disorder and breast cancer. Checking the serum prolactin level might help guide treatment. The literature is mixed regarding antipsychotic use and new mammary tumorigenesis; current research does not support antipsychotic choice based on future risk of breast cancer.6
Other adverse effects from antipsychotic use for bipolar disorder could have an impact on patients with breast cancer. Several of these medications could ameliorate side effects of advanced cancer and chemotherapy. Quetiapine, for example, might improve tamoxifen-induced insomnia in women with breast cancer because of its high affinity for serotonergic receptors, thus enhancing central serotonergic neurotransmitters and decreasing excitatory glutamatergic transmission.17
In any type of advanced cancer, nausea and vomiting are common, independent of chemotherapy and medication regimens. Metabolic derangement, vestibular dysfunction, CNS disorders, and visceral metastasis all contribute to hyperemesis. Olanzapine has been shown to significantly reduce refractory nausea and can cause weight gain and improved appetite, which benefits cachectic patients.18
Last, clozapine is one of the more effective antipsychotic medications, but also carries a risk of neutropenia. In patients with neutropenia secondary to chemotherapy, clozapine could increase the risk of infection in an immunocompromised patient.19 Granulocyte colony stimulating factor might be useful as a rescue medication for treatment-emergent neutropenia.19
Treatment considerations
Cancer patients might be unable or unwilling to seek services for mental health during their cancer treatment, and many who have a diagnosis of psychiatric illness might stop following up with psychiatric care when cancer treatment takes priority. It is critical for clinicians to be aware of the current literature regarding the impact of mood-stabilizing medication on cancer treatment. Monitoring for drug interactions is essential, and electronic drug interaction tools, such as Lexicomp, may be useful for this purpose.13 Because of special vulnerabilities in this population, cautious and judicious prescribing practices are advised.
The risk-benefit profile for medications for bipolar disorder must be considered before they are initiated or changes are made to the regimen (Table 2). Changing an effective mood stabilizer to gain benefits in breast cancer prognosis is not recommended in most cases, because benefits have been shown to be only significant in preclinical research; currently, there are no clinical guidelines. However, medication adjustments should be made with these theoretical benefits in mind, as long as the treatment of bipolar disorder remains effective.
Regardless of what treatment regimen the health team decides on, several underlying issues that affect patient care must be considered in this population. Successfully treating breast cancer in a woman with severe mental illness only can be accomplished when her mental illness is under control. Once she is psychiatrically stable, it is important for her to have a basic understanding of how cancer can affect the body and know the reasons behind treatment.
It is imperative that physicians provide their patients with a general understanding of their comorbid disorders, and find ways to help patients remain adherent with treatment of both diseases. Many patients feel demoralized by a cancer diagnosis and adherence to a medication regimen might be a difficult task among those with bipolar disorder who also are socially isolated, lack education, or have poor recall of treatment recommendations.20
Bottom Line
Managing comorbid bipolar disorder and breast cancer might seem daunting,
but treatments for the 2 diseases can work in synergy. You have an opportunity to
educate patients and colleagues in treating bipolar disorder and comorbid breast
cancer. Optimizing care using known psychopharmacologic data can not only lead
to better outcomes, but might additionally offer some hope and reason to remain
treatment-adherent for patients suffering from this complex comorbidity.
Related Resources
• Agarwala P, Riba MB. Tailoring depression treatment for women with breast cancer. Current Psychiatry. 2010;9(11): 39-40,45-46,48-49.
• Cunningham R, Sarfati D, Stanley J, et al. Cancer survival in the context of mental illness: a national cohort study. Gen Hosp Psychiatry. 2015;37(6):501-506.
Drug Brand Names
Amiodarone • Cordarone
Aripiprazole • Abilify
Asenapine • Saphris
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Citalopram • Celexa
Clozapine • Clozaril
Cyclophosphamide • Cytoxan, Neosar
Doxorubicin • Doxil, Adriamycin
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
Fulvestrant • Faslodex
Iloperidone • Fanapt
Lamotrigine • Lamictal
Letrozole • Femara
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Olanzapine • Zyprexa
Paclitaxel • Onxol
Paliperidone • Invega
Pamidronate • Aredia
Paroxetine • Paxil
Quetiapine • Seroquel
Raloxifene • Evista
Risperidone • Risperdal
Sertraline • Zoloft
Tamoxifen • Nolvadex
Thioridazine • Mellaril
Trastuzumab • Herceptin
Valproic acid • Depakene
Venlafaxine • Effexor
Ziprasidone • Geodon
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Diagnosis, mood changes
Ms. A, age 58, is a white female with a history of chronic bipolar I disorder who is being evaluated as a new patient in an academic psychiatric clinic. Recently, she was diagnosed with ER+, PR+, and HER2+ ductal carcinoma. She does not take her prescribed mood stabilizers.
After her cancer diagnosis, Ms. A experiences new-onset agitation, including irritable mood, suicidal thoughts, tearfulness, decreased need for sleep, fast speech, excessive spending, and anorexia. She reports that she hears the voice of God telling her that she could cure her breast cancer through prayer and herbal remedies. Her treatment team, comprising her primary care provider and surgical oncologist, consider several medication adjustments, but are unsure of their effects on Ms. A’s mental health, progression of cancer, and cancer treatment.
What is the most likely cause of Ms. A’s psychiatric symptoms?
a) anxiety from having a diagnosis of cancer
b) stress reaction
c) panic attack
d) manic or mixed phase of bipolar I disorder
The authors’ observations
Treating breast cancer with concurrent severe mental illness is complex and challenging for the patient, family, and health care providers. Mental health and oncology clinicians must collaborate when treating these patients because of overlapping pathophysiology and medication interactions. A comprehensive evaluation is required to tease apart whether a patient is simply demoralized by her new diagnosis, or if a more serious mood disorder is present.
Worldwide, breast cancer is the most frequently diagnosed cancer and the leading cause of cancer death among women.1 The mean age of women diagnosed with breast cancer is 61 years; 61% of these women are alive 15 years after diagnosis, representing the largest group of female cancer survivors.
The incidence of breast cancer is reported to be higher in women with bipolar disorder compared with the general population.2-4 This positive correlation might be associated with a high rate of smoking, poor health-related behaviors, and, possibly, medication side effects. A genome-wide association study found significant associations between bipolar disorder and the breast cancer-related genes BRCA2 and PALB2.5
Antipsychotics and prolactin
Antipsychotics play an important role in managing bipolar disorder; several, however, are known to raise the serum prolactin level 10- to 20-fold. A high prolactin level could be associated with progression of breast cancer. All antipsychotics have label warnings regarding their use in women with breast cancer.
The prolactin receptor is overexpressed in >95% of breast cancer cells, regardless of estrogen-receptor status. The role of prolactin in development of new breast cancer is open to debate. The effect of a high prolactin level in women with diagnosed breast cancer is unknown, although available preclinical data suggest that high levels should be avoided. Psychiatric clinicians should consider checking the serum prolactin level or switching to a treatment strategy that avoids iatrogenic prolactin elevation. This risk must be carefully weighed against the mood-stabilizing properties of antipsychotics.6
TREATMENT Consider comorbidities
Ms. A receives supportive psychotherapy in addition to quetiapine, 400 mg/d, and valproic acid, 1,500 mg/d. This regimen helps her successfully complete the initial phase of breast cancer treatment, which consists of a single mastectomy, adjuvant chemotherapy (doxorubicin and cyclophosphamide followed by paclitaxel and trastuzumab). She is now on endocrine therapy with tamoxifen.
Ms. A, calm, much improved mood symptoms, and euthymic, has questions regarding her mental health, cancer prognosis, and potential medication side effects with continued cancer treatment.
Which drug used to treat breast cancer might relieve Ms. A’s manic symptoms?
a) cyclophosphamide
b) tamoxifen
c) trastuzumab
d) pamidronate
The authors’ observations
Recent evidence suggests that tamoxifen reduces symptoms of bipolar mania more rapidly than many standard medications for bipolar disorder. Tamoxifen is the only available centrally active protein kinase C (PKC) inhibitor,7 although lithium and valproic acid also might inhibit PKC activity. PKC regulates presynaptic and postsynaptic neurotransmission, neuronal excitability, and neurotransmitter release. PKC is thought to be overactive during mania, possibly because of an increase in membrane-bound PKC and PKC translocation from the cytosol to membrane.7,8
Preliminary clinical trials suggest that tamoxifen significantly reduces manic symptoms in patients with bipolar disorder within 5 days of initiation.7 These findings have been confirmed in animal studies and in 1 single-blind and 4 double-blind placebo-controlled clinical studies over the past 15 years.9
Tamoxifen is a selective estrogen-receptor modulator used to prevent recurrence in receptor-positive breast cancer. Cytochrome P450 (CYP) 2D6 is the principal enzyme that converts tamoxifen to its active metabolite, endoxifen. Inhibition of tamoxifen conversion to endoxifen by CYP2D6 inhibitors could decrease the efficacy of tamoxifen therapy and might increase the risk of breast cancer recurrence. Although antidepressants generally are not recommended as a first-line agent for bipolar disorder, several selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors are potent, moderate, or mild inhibitors of CYP2D610 (Table 1). Approximately 7% of women have nonfunctional CYP2D6 alleles and have a lower endoxifen level.11
Treating breast cancer
The mainstays of breast cancer treatment are surgery, radiation therapy, chemotherapy, hormone therapy, and targeted monoclonal antibody therapy. The protocol of choice depends on the stage of cancer, estrogen receptor status, expression of human epidermal growth factor receptor 2 (HER-2), treatment history, and the patient’s menopausal status. Overexpression of HER-2 oncoprotein, found in 25% to 30% of breast cancers, has been shown to promote cell transformation. HER-2 overexpression is associated with aggressive tumor phenotypes, lymph node involvement, and resistance to chemotherapy and endocrine therapy. Therefore, the HER-2 oncoprotein is a key target for treatment. Often, several therapies are combined to prevent recurrence of disease.
Breast cancer treatment often can cause demoralization, menopausal symptoms, sleep disturbance, impaired sexual function, infertility, and disturbed body image. It also can trigger psychiatric symptoms in patients with, or without, a history of mental illness.
Trastuzumab is a recombinant humanized monoclonal antibody against HER-2, and is approved for treating HER-2 positive breast cancer. However, approximately 50% of patients with HER-2 overexpression do not respond to trastuzumab alone or combined with chemotherapy, and nearly all patients develop resistance to trastuzumab, leading to recurrence.12 This medication is still used in practice, and research regarding antiepileptic drugs working in synergy with this monoclonal antibody is underway.
OUTCOME Stability achieved
Quetiapine and valproic acid are first-line choices for Ms. A because (1) she would be on long-term tamoxifen to maintain cancer remission maintenance and (2) she is in a manic phase of bipolar disorder. Tamoxifen also could improve her manic symptoms. This medication regimen might enhance the action of cancer treatments and also could reduce adverse effects of cancer treatment, such as insomnia associated with tamoxifen.
After the team educates Ms. A about how her psychiatric medications could benefit her cancer treatment, she becomes more motivated to stay on her regimen. Ms. A does well on these medications and after 18 months has not experienced exacerbation of psychiatric symptoms or recurrence of cancer.
The authors’ observations
There are 3 major classes of mood stabilizers for treating bipolar disorder: lithium, antiepileptic drugs, and atypical antipsychotics.13 In a setting of cancer, mood stabilizers are prescribed for managing mania or drug-induced agitation or anxiety associated with steroid use, brain metastases, and other medical conditions. They also can be used to treat neuropathic pain and hot flashes and seizure prophylaxis.13
Valproic acid
Valproic acid can help treat mood lability, impulsivity, and disinhibition, whether these symptoms are due to primary psychiatric illness or secondary to cancer metastasis. It is a first-line agent for manic and mixed bipolar states, and can be titrated quickly to achieve optimal benefit. Valproic acid also has been described as a histone deacetylase (HDAC) inhibitor, known to attenuate apoptotic activity, making it of interest as a treatment for cancer.14 HDAC inhibitors have been shown to:
- induce differentiation and cell cycle arrest
- activate the extrinsic or intrinsic pathways of apoptosis
- inhibit invasion, migration, and angiogenesis in different cancer cell lines.15
In regard to breast cancer, valproic acid inhibits growth of cell lines independent of estrogen receptors, increases the action of such breast cancer treatments as tamoxifen, raloxifene, fulvestrant, and letrozole, and induces solid tumor regression.14 Valproic acid also reduces cancer cell viability and could act as a powerful antiproliferative agent in estrogen-sensitive breast cancer cells.16
Valproic acid reduces cell growth-inducing apoptosis and cell cycle arrest in ERα-positive breast cancer cells, although it has no significant apoptotic effect in ERα-negative cells.16 However, evidence does support the ability of valproic acid to restore an estrogen-sensitive phenotype in ERα-negative breast cancer cells, allowing successful treatment with the anti-estrogen tamoxifen in vitro.10
Antipsychotics
Antipsychotics act as dopamine D2 receptor antagonists within the hypothalamic-pituitary-adrenal axis, thus increasing the serum prolactin level. Among atypicals, risperidone and its active metabolite, paliperidone, produce the greatest increase in the prolactin level, whereas quetiapine, clozapine, and aripiprazole minimally elevate the prolactin level.
Hyperprolactinemia correlates with rapid breast cancer progression and inferior prognosis, regardless of breast cancer receptor typing. Therefore, prolactin-sparing antipsychotics are preferred when treating a patient with comorbid bipolar disorder and breast cancer. Checking the serum prolactin level might help guide treatment. The literature is mixed regarding antipsychotic use and new mammary tumorigenesis; current research does not support antipsychotic choice based on future risk of breast cancer.6
Other adverse effects from antipsychotic use for bipolar disorder could have an impact on patients with breast cancer. Several of these medications could ameliorate side effects of advanced cancer and chemotherapy. Quetiapine, for example, might improve tamoxifen-induced insomnia in women with breast cancer because of its high affinity for serotonergic receptors, thus enhancing central serotonergic neurotransmitters and decreasing excitatory glutamatergic transmission.17
In any type of advanced cancer, nausea and vomiting are common, independent of chemotherapy and medication regimens. Metabolic derangement, vestibular dysfunction, CNS disorders, and visceral metastasis all contribute to hyperemesis. Olanzapine has been shown to significantly reduce refractory nausea and can cause weight gain and improved appetite, which benefits cachectic patients.18
Last, clozapine is one of the more effective antipsychotic medications, but also carries a risk of neutropenia. In patients with neutropenia secondary to chemotherapy, clozapine could increase the risk of infection in an immunocompromised patient.19 Granulocyte colony stimulating factor might be useful as a rescue medication for treatment-emergent neutropenia.19
Treatment considerations
Cancer patients might be unable or unwilling to seek services for mental health during their cancer treatment, and many who have a diagnosis of psychiatric illness might stop following up with psychiatric care when cancer treatment takes priority. It is critical for clinicians to be aware of the current literature regarding the impact of mood-stabilizing medication on cancer treatment. Monitoring for drug interactions is essential, and electronic drug interaction tools, such as Lexicomp, may be useful for this purpose.13 Because of special vulnerabilities in this population, cautious and judicious prescribing practices are advised.
The risk-benefit profile for medications for bipolar disorder must be considered before they are initiated or changes are made to the regimen (Table 2). Changing an effective mood stabilizer to gain benefits in breast cancer prognosis is not recommended in most cases, because benefits have been shown to be only significant in preclinical research; currently, there are no clinical guidelines. However, medication adjustments should be made with these theoretical benefits in mind, as long as the treatment of bipolar disorder remains effective.
Regardless of what treatment regimen the health team decides on, several underlying issues that affect patient care must be considered in this population. Successfully treating breast cancer in a woman with severe mental illness only can be accomplished when her mental illness is under control. Once she is psychiatrically stable, it is important for her to have a basic understanding of how cancer can affect the body and know the reasons behind treatment.
It is imperative that physicians provide their patients with a general understanding of their comorbid disorders, and find ways to help patients remain adherent with treatment of both diseases. Many patients feel demoralized by a cancer diagnosis and adherence to a medication regimen might be a difficult task among those with bipolar disorder who also are socially isolated, lack education, or have poor recall of treatment recommendations.20
Bottom Line
Managing comorbid bipolar disorder and breast cancer might seem daunting,
but treatments for the 2 diseases can work in synergy. You have an opportunity to
educate patients and colleagues in treating bipolar disorder and comorbid breast
cancer. Optimizing care using known psychopharmacologic data can not only lead
to better outcomes, but might additionally offer some hope and reason to remain
treatment-adherent for patients suffering from this complex comorbidity.
Related Resources
• Agarwala P, Riba MB. Tailoring depression treatment for women with breast cancer. Current Psychiatry. 2010;9(11): 39-40,45-46,48-49.
• Cunningham R, Sarfati D, Stanley J, et al. Cancer survival in the context of mental illness: a national cohort study. Gen Hosp Psychiatry. 2015;37(6):501-506.
Drug Brand Names
Amiodarone • Cordarone
Aripiprazole • Abilify
Asenapine • Saphris
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Citalopram • Celexa
Clozapine • Clozaril
Cyclophosphamide • Cytoxan, Neosar
Doxorubicin • Doxil, Adriamycin
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
Fulvestrant • Faslodex
Iloperidone • Fanapt
Lamotrigine • Lamictal
Letrozole • Femara
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Olanzapine • Zyprexa
Paclitaxel • Onxol
Paliperidone • Invega
Pamidronate • Aredia
Paroxetine • Paxil
Quetiapine • Seroquel
Raloxifene • Evista
Risperidone • Risperdal
Sertraline • Zoloft
Tamoxifen • Nolvadex
Thioridazine • Mellaril
Trastuzumab • Herceptin
Valproic acid • Depakene
Venlafaxine • Effexor
Ziprasidone • Geodon
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69-90.
2. American Cancer Society. Cancer facts and figures 2014. Atlanta, GA: American Cancer Society; 2014.
3. BarChana M, Levav I, Lipshitz I, et al. Enhanced cancer risk among patients with bipolar disorder. J Affect Disord. 2008;108(1-2):43-48.
4. Hung YP, Liu CJ, Tsai CF, et al. Incidence and risk of mood disorders in patients with breast cancers in Taiwan: a nationwide population-based study. Psychooncology. 2013;22(10):2227-2234.
5. Tesli M, Athanasiu L, Mattingsdal M, et al. Association analysis of PALB2 and BRCA2 in bipolar disorder and schizophrenia in a scandinavian case–control sample. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(7):1276-1282.
6. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
7. Armani F, Andersen ML, Galduróz JC. Tamoxifen use for the management of mania: a review of current preclinical evidence. Psychopharmacology (Berl). 2014;231(4):639-649.
8. Zarate CA Jr, Singh JB, Carlson PJ, et al. Efficacy of a protein kinase C inhibitor (tamoxifen) in the treatment of acute mania: a pilot study. Bipolar Disord. 2007;9(6):561-570.
9. Zarate CA, Manji HK. Protein kinase C inhibitors: rationale for use and potential in the treatment of bipolar disorder. CNS Drugs. 2009;23(7):569-582.
10. Fortunati N, Bertino S, Costantino L, et al. Valproic acid restores ER alpha and antiestrogen sensitivity to ER alpha-negative breast cancer cells. Mol Cell Endocrinol. 2010;314(1):17-22.
11. Thekdi SM, Trinidad A, Roth A. Psychopharmacology in cancer. Curr Psychiatry Rep. 2014;17(1):529.
12. Meng Q, Chen X, Sun L, et al. Carbamazepine promotes Her-2 protein degradation in breast cancer cells by modulating HDAC6 activity and acetylation of Hsp90. Mol Cell Biochem. 2011;348(1-2):165-171.
13. Altamura AC, Lietti L, Dobrea C, et al. Mood stabilizers for patients with bipolar disorder: the state of the art. Expert Rev Neurother. 2011;11(1):85-99.
14. Chateauvieux S, Morceau F, Dicato M, et al. Molecular and therapeutic potential and toxicity of valproic acid [published online July 29, 2010]. J Biomed Biotechnol. doi: 10.1155/2010/479364.
15. Jafary H, Ahmadian S, Soleimani M. The enhanced apoptosis and antiproliferative response to combined treatment with valproate and nicotinamide in MCF-7 breast cancer cells. Tumour Biol. 2013;35(3):2701-2710.
16. Fortunati N, Bertino S, Costantino L, et al. Valproic acid is a selective antiproliferative agent in estrogen-sensitive breast cancer cells. Cancer Lett. 2008;259(2):156-164.
17. Pasquini M, Speca A, Biondi M. Quetiapine for tamoxifen-induced insomnia in women with breast cancer. Psychosomatics. 2009;50(2):159-161.
18. Srivastava M, Brito-Dellan N, Davis MP, et al. Olanzapine as an antiemetic in refractory nausea and vomiting in advanced cancer. J Pain Symptom Manage. 2003;25(6):578-582.
19. Sankaranarayanan A, Mulchandani M, Tirupati S. Clozapine, cancer chemotherapy and neutropenia - dilemmas in management. Psychiatr Danub. 2013;25(4):419-422.
20. Cole M, Padmanabhan A. Breast cancer treatment of women with schizophrenia and bipolar disorder from Philadelphia, PA: lessons learned and suggestions for improvement. J Cancer Educ. 2012;27(4):774-779.
1. Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69-90.
2. American Cancer Society. Cancer facts and figures 2014. Atlanta, GA: American Cancer Society; 2014.
3. BarChana M, Levav I, Lipshitz I, et al. Enhanced cancer risk among patients with bipolar disorder. J Affect Disord. 2008;108(1-2):43-48.
4. Hung YP, Liu CJ, Tsai CF, et al. Incidence and risk of mood disorders in patients with breast cancers in Taiwan: a nationwide population-based study. Psychooncology. 2013;22(10):2227-2234.
5. Tesli M, Athanasiu L, Mattingsdal M, et al. Association analysis of PALB2 and BRCA2 in bipolar disorder and schizophrenia in a scandinavian case–control sample. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(7):1276-1282.
6. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
7. Armani F, Andersen ML, Galduróz JC. Tamoxifen use for the management of mania: a review of current preclinical evidence. Psychopharmacology (Berl). 2014;231(4):639-649.
8. Zarate CA Jr, Singh JB, Carlson PJ, et al. Efficacy of a protein kinase C inhibitor (tamoxifen) in the treatment of acute mania: a pilot study. Bipolar Disord. 2007;9(6):561-570.
9. Zarate CA, Manji HK. Protein kinase C inhibitors: rationale for use and potential in the treatment of bipolar disorder. CNS Drugs. 2009;23(7):569-582.
10. Fortunati N, Bertino S, Costantino L, et al. Valproic acid restores ER alpha and antiestrogen sensitivity to ER alpha-negative breast cancer cells. Mol Cell Endocrinol. 2010;314(1):17-22.
11. Thekdi SM, Trinidad A, Roth A. Psychopharmacology in cancer. Curr Psychiatry Rep. 2014;17(1):529.
12. Meng Q, Chen X, Sun L, et al. Carbamazepine promotes Her-2 protein degradation in breast cancer cells by modulating HDAC6 activity and acetylation of Hsp90. Mol Cell Biochem. 2011;348(1-2):165-171.
13. Altamura AC, Lietti L, Dobrea C, et al. Mood stabilizers for patients with bipolar disorder: the state of the art. Expert Rev Neurother. 2011;11(1):85-99.
14. Chateauvieux S, Morceau F, Dicato M, et al. Molecular and therapeutic potential and toxicity of valproic acid [published online July 29, 2010]. J Biomed Biotechnol. doi: 10.1155/2010/479364.
15. Jafary H, Ahmadian S, Soleimani M. The enhanced apoptosis and antiproliferative response to combined treatment with valproate and nicotinamide in MCF-7 breast cancer cells. Tumour Biol. 2013;35(3):2701-2710.
16. Fortunati N, Bertino S, Costantino L, et al. Valproic acid is a selective antiproliferative agent in estrogen-sensitive breast cancer cells. Cancer Lett. 2008;259(2):156-164.
17. Pasquini M, Speca A, Biondi M. Quetiapine for tamoxifen-induced insomnia in women with breast cancer. Psychosomatics. 2009;50(2):159-161.
18. Srivastava M, Brito-Dellan N, Davis MP, et al. Olanzapine as an antiemetic in refractory nausea and vomiting in advanced cancer. J Pain Symptom Manage. 2003;25(6):578-582.
19. Sankaranarayanan A, Mulchandani M, Tirupati S. Clozapine, cancer chemotherapy and neutropenia - dilemmas in management. Psychiatr Danub. 2013;25(4):419-422.
20. Cole M, Padmanabhan A. Breast cancer treatment of women with schizophrenia and bipolar disorder from Philadelphia, PA: lessons learned and suggestions for improvement. J Cancer Educ. 2012;27(4):774-779.