User login
The Epidemic of Tommy John Surgery: The Role of the Orthopedic Surgeon
Ulnar collateral ligament (UCL) reconstruction, commonly referred to as Tommy John surgery, is a well-described surgical treatment for elite athletes with a symptomatic, deficient UCL.1, 2 The procedure was first performed by the late Dr. Frank Jobe in 1974, described in 1986, and has undergone several modifications over the past 30 years.3 Different graft choices, tunnel positions, graft configurations, and tunnel fixation methods are just some of the alterations that have been made to the original Jobe technique.4-6 With time, the index procedure has become more refined, with predictable outcomes in Major League Baseball (MLB) pitchers as well as other elite overhead throwing athletes.2,7,8 However, though this surgery was originally described for elite athletes suffering from UCL deficiency, recent times have seen an increase of over 50% in the number of UCL reconstructions performed on high school–aged and younger athletes.9 Furthermore, in 2000, a total of 13 MLB pitchers underwent UCL reconstruction, while in 2012 this number increased nearly threefold to 32.2 This paradigm shift of performing UCL reconstructions more frequently and on younger athletes raises a very important question: what is the role of the orthopedic surgeon in this epidemic?
UCL reconstruction has become a reliable procedure for MLB pitchers and other overhead throwing athletes.7,10,11 Recent studies have reported that MLB pitchers who undergo UCL reconstruction return to pitch in the MLB 83% of the time, whereas only 3% fail to return to pitch in either MLB or the minor league.2 Furthermore, pitchers who undergo UCL reconstruction perform similarly after surgery as prior to their UCL reconstruction, with fewer innings pitched after surgery, but, more importantly, a lower earned run average (ERA) and walks plus hits per inning pitched (WHIP) after surgery. These last 2 statistics, known as sabermetrics, evaluate the pitcher’s effectiveness; the fact that these are improved after surgery is reassuring for pitchers who undergo this procedure. However, it must be recognized that these pitchers pitched fewer innings after surgery.
There has been a sharp increase in the number of MLB pitchers who have undergone UCL reconstruction in recent years, especially the past 3 seasons, in which over 60 pitchers have had Tommy John surgery.2 This increase, however, is not confined to MLB pitchers. High school–aged pitchers have also been part of this drastic rise in the number of UCL reconstructions performed throughout the country. Dr. James Andrews and colleagues noted a 50% increase from 1988-1994 to 1995-2003 in the proportion of high school–aged pitchers who underwent UCL reconstruction (while the absolute number increased from 7 to 77 in high school–aged players compared with 85 to 609 in adult athletes).9 Given the increase in MLB pitchers over the past few years, it is likely this number has also increased among adolescent pitchers.
This data again raises the question: what is the role of the orthopedic surgeon in this epidemic? There are many plausible responses, but in my opinion, there is one answer that surpasses the others. As a trained professional, surgeons are tasked with the responsibility of looking out for the best interest of their patients, even when this conflicts with the patient’s, or the patient’s parent’s or coach’s desires. This includes injury prevention, such as instituting pitch counts and developing products that allow coaches to determine when a pitcher may be at risk for injury from fatigue, as well as injury treatment.12 It is difficult for a patient to understand the gravity of surgery and the rehabilitation process, specifically a procedure as involved as UCL reconstruction, and especially if the patient is an adolescent who has their outlook clouded by the fact that they believe they will be the next MLB star pitcher. The reality is that the National Collegiate Athletic Association (NCAA)13 has released data that has demonstrated that only 6.8% of high school baseball players will play baseball in college. Furthermore, only 9.4% of college baseball players will reach the professional level. That equates to 0.5%, or 1 in 200 high school players who will eventually play professional baseball.13 However, the reverse of this is also true, that out of every 200 players, 1 will make it to the major leagues, and that 1 player could be the patient in question. Hence, the purpose of this data is to show parents and athletes that, while they do have a chance of playing professional, and certainly collegiate, baseball, that percentage must be weighed against the risks of surgery.
MLB pitchers who have an endless supply of rehabilitation facilities, trainers, etc, do not return to pitching competitively and consistently in the majors for more than 15 months after UCL reconstruction.2 The time commitment and rehabilitation required for these patients is staggering.14,15 Furthermore, parents of these children who are consenting for them also have a difficult time comprehending the workload they are signing their child up for. Some parents believe this surgery will help their child throw faster, longer, and more accurately—beliefs that numerous studies have shown to be flat-out inaccurate. In fact, pitchers tend to lose a slight amount of velocity and accuracy after UCL reconstruction.11,16 Ahmad and colleagues17 administered a questionnaire to 189 players, 15 coaches, and 31 parents about the indications, risks, benefits, etc, regarding UCL reconstruction to determine the public’s perception regarding this surgery. The results demonstrated that the public, including coaches, have a significantly skewed perception of exactly how serious this surgery is. The study showed that 28% of players and 20% of coaches believed the pitcher’s performance would be improved after surgery, and, more strikingly, 26% of collegiate athletes, 30% percent of coaches, 37% of parents, and 51% of high school athletes believed UCL reconstruction should be performed as a prophylactic procedure to enhance performance in an uninjured athlete.17
Henceforth, it becomes the surgeon’s responsibility to ensure that both the patient and the parents understand what the surgery and rehabilitation process entails, to keep the expectations of the patient and his or her family realistic, and to counsel these patients on alternative options with lower risks. As Ahmad and colleagues17 demonstrated, this is not an easy task given the public’s preconceived notions. Many patients, especially patients of the younger generation, seem to be willing to jump to surgery as the first option for treatment without having truly tried any nonoperative measures, because they believe surgery to be a quick, easy, and definitive answer. This is not always the case, and a trial of nonoperative treatment, including rest, ice, physical therapy, and possibly platelet-rich plasma (PRP), should be instituted for high school–aged players who present with UCL insufficiency prior to discussing surgery.18,19
Medial UCL reconstruction is a successful procedure for elite MLB athletes. However, UCL reconstruction is becoming a victim of its own success as younger and younger athletes who will likely never play at the major league level are undergoing this procedure at an alarming rate. This is an epidemic which must be addressed by surgeons, coaches, and parents alike to curb the beliefs that UCL reconstruction will make high school–aged pitchers more successful. This procedure should not be performed prophylactically on an athlete of any age, especially those in high school. Further studies on the effectiveness of both nonoperative rest and rehabilitation and of PRP on partial-thickness UCL tears are warranted. New technology in the form of a compression sleeve with imbedded sensors to track the biomechanics of a pitcher’s elbow has been released and will hopefully provide information to coaches about when pitchers’ elbows begin to fatigue based on several biomechanical parameters.12 The future of UCL reconstruction is still fluid, and with proper prevention strategies, nonoperative treatment, indications, and preoperative discussions, the Tommy John epidemic can be cured. ◾
1. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes. Treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
2. Erickson BJ, Gupta AK, Harris JD, et al. Rate of return to pitching and performance after Tommy John surgery in Major League Baseball pitchers. Am J Sports Med. 2014;42(3):536-543.
3. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
4. Jackson TJ, Adamson GJ, Peterson A, Patton J, McGarry MH, Lee TQ. Ulnar collateral ligament reconstruction using bisuspensory fixation: a biomechanical comparison with the docking technique. Am J Sports Med. 2013;41(5):1158-1164.
5. Dines JS, ElAttrache NS, Conway JE, Smith W, Ahmad CS. Clinical outcomes of the DANE TJ technique to treat ulnar collateral ligament insufficiency of the elbow. Am J Sports Med. 2007;35(12):2039-2044.
6. Andrews JR, Jost PW, Cain EL. The ulnar collateral ligament procedure revisited: the procedure we use. Sports Health. 2012;4(5):438-441.
7. Dines JS, Jones KJ, Kahlenberg C, Rosenbaum A, Osbahr DC, Altchek DW. Elbow ulnar collateral ligament reconstruction in javelin throwers at a minimum 2-year follow-up. Am J Sports Med. 2012;40(1):148-151.
8. Gibson BW, Webner D, Huffman GR, Sennett BJ. Ulnar collateral ligament reconstruction in major league baseball pitchers. Am J Sports Med. 2007;35(4):575-581.
9. Petty DH, Andrews JR, Fleisig GS, Cain EL. Ulnar collateral ligament reconstruction in high school baseball players: clinical results and injury risk factors. Am J Sports Med. 2004;32(5):1158-1164.
10. Osbahr DC, Cain EL Jr, Raines BT, Fortenbaugh D, Dugas JR, Andrews JR. Long-term outcomes after ulnar collateral ligament reconstruction in competitive baseball players: minimum 10-year follow-up. Am J Sports Med. 2014;42(6):1333-1342.
11. Jiang JJ, Leland JM. Analysis of pitching velocity in major league baseball players before and after ulnar collateral ligament reconstruction. Am J Sports Med. 2014;42(4):880-885.
12. Carroll W. The sleeve that could save baseball: exclusive look at new MLB technology. Bleacher Report. http://bleacherreport.com/articles/2097866-the-sleeve-that-could-save-baseball-exclusive-look-at-new-mlb-technology?utm_campaign=tsipad&utm_medium=referral&utm_source=teamstream. Published July 2, 2014. Accessed November 12, 2014.
13. National Collegiate Athletic Association. Estimated probability of competing in athletics beyond the high school interscholastic level. https://www.ncaa.org/sites/default/files/Probability-of-going-pro-methodology_Update2013.pdf. Updated September 24, 2013. Accessed November 12, 2014.
14. Wilk KE, Macrina LC, Cain EL, Dugas JR, Andrews JR. Rehabilitation of the overhead athlete’s elbow. Sports Health. 2012;4(5):404-414.
15. Wilk KE, Reinold MM, Andrews JR. Rehabilitation of the thrower’s elbow. Tech Hand Up Extrem Surg. 2003;7(4):197-216.
16. Makhni EC, Lee RW, Morrow ZS, Gualtieri AP, Gorroochurn P, Ahmad CS. Performance, return to competition, and reinjury after Tommy John surgery in Major League Baseball pitchers: a review of 147 cases. Am J Sports Med. 2014;42(6):1323-1332.
17. Ahmad CS, Grantham WJ, Greiwe RM. Public perceptions of Tommy John surgery. Phys Sportsmed. 2012;40(2):64-72.
18. Rettig AC, Sherrill C, Snead DS, Mendler JC, Mieling P. Nonoperative treatment of ulnar collateral ligament injuries in throwing athletes. Am J Sports Med. 2001;29(1):15-17.
19. Podesta L, Crow SA, Volkmer D, Bert T, Yocum LA. Treatment of partial ulnar collateral ligament tears in the elbow with platelet-rich plasma. Am J Sports Med. 2013;41(7):1689-1694.
Ulnar collateral ligament (UCL) reconstruction, commonly referred to as Tommy John surgery, is a well-described surgical treatment for elite athletes with a symptomatic, deficient UCL.1, 2 The procedure was first performed by the late Dr. Frank Jobe in 1974, described in 1986, and has undergone several modifications over the past 30 years.3 Different graft choices, tunnel positions, graft configurations, and tunnel fixation methods are just some of the alterations that have been made to the original Jobe technique.4-6 With time, the index procedure has become more refined, with predictable outcomes in Major League Baseball (MLB) pitchers as well as other elite overhead throwing athletes.2,7,8 However, though this surgery was originally described for elite athletes suffering from UCL deficiency, recent times have seen an increase of over 50% in the number of UCL reconstructions performed on high school–aged and younger athletes.9 Furthermore, in 2000, a total of 13 MLB pitchers underwent UCL reconstruction, while in 2012 this number increased nearly threefold to 32.2 This paradigm shift of performing UCL reconstructions more frequently and on younger athletes raises a very important question: what is the role of the orthopedic surgeon in this epidemic?
UCL reconstruction has become a reliable procedure for MLB pitchers and other overhead throwing athletes.7,10,11 Recent studies have reported that MLB pitchers who undergo UCL reconstruction return to pitch in the MLB 83% of the time, whereas only 3% fail to return to pitch in either MLB or the minor league.2 Furthermore, pitchers who undergo UCL reconstruction perform similarly after surgery as prior to their UCL reconstruction, with fewer innings pitched after surgery, but, more importantly, a lower earned run average (ERA) and walks plus hits per inning pitched (WHIP) after surgery. These last 2 statistics, known as sabermetrics, evaluate the pitcher’s effectiveness; the fact that these are improved after surgery is reassuring for pitchers who undergo this procedure. However, it must be recognized that these pitchers pitched fewer innings after surgery.
There has been a sharp increase in the number of MLB pitchers who have undergone UCL reconstruction in recent years, especially the past 3 seasons, in which over 60 pitchers have had Tommy John surgery.2 This increase, however, is not confined to MLB pitchers. High school–aged pitchers have also been part of this drastic rise in the number of UCL reconstructions performed throughout the country. Dr. James Andrews and colleagues noted a 50% increase from 1988-1994 to 1995-2003 in the proportion of high school–aged pitchers who underwent UCL reconstruction (while the absolute number increased from 7 to 77 in high school–aged players compared with 85 to 609 in adult athletes).9 Given the increase in MLB pitchers over the past few years, it is likely this number has also increased among adolescent pitchers.
This data again raises the question: what is the role of the orthopedic surgeon in this epidemic? There are many plausible responses, but in my opinion, there is one answer that surpasses the others. As a trained professional, surgeons are tasked with the responsibility of looking out for the best interest of their patients, even when this conflicts with the patient’s, or the patient’s parent’s or coach’s desires. This includes injury prevention, such as instituting pitch counts and developing products that allow coaches to determine when a pitcher may be at risk for injury from fatigue, as well as injury treatment.12 It is difficult for a patient to understand the gravity of surgery and the rehabilitation process, specifically a procedure as involved as UCL reconstruction, and especially if the patient is an adolescent who has their outlook clouded by the fact that they believe they will be the next MLB star pitcher. The reality is that the National Collegiate Athletic Association (NCAA)13 has released data that has demonstrated that only 6.8% of high school baseball players will play baseball in college. Furthermore, only 9.4% of college baseball players will reach the professional level. That equates to 0.5%, or 1 in 200 high school players who will eventually play professional baseball.13 However, the reverse of this is also true, that out of every 200 players, 1 will make it to the major leagues, and that 1 player could be the patient in question. Hence, the purpose of this data is to show parents and athletes that, while they do have a chance of playing professional, and certainly collegiate, baseball, that percentage must be weighed against the risks of surgery.
MLB pitchers who have an endless supply of rehabilitation facilities, trainers, etc, do not return to pitching competitively and consistently in the majors for more than 15 months after UCL reconstruction.2 The time commitment and rehabilitation required for these patients is staggering.14,15 Furthermore, parents of these children who are consenting for them also have a difficult time comprehending the workload they are signing their child up for. Some parents believe this surgery will help their child throw faster, longer, and more accurately—beliefs that numerous studies have shown to be flat-out inaccurate. In fact, pitchers tend to lose a slight amount of velocity and accuracy after UCL reconstruction.11,16 Ahmad and colleagues17 administered a questionnaire to 189 players, 15 coaches, and 31 parents about the indications, risks, benefits, etc, regarding UCL reconstruction to determine the public’s perception regarding this surgery. The results demonstrated that the public, including coaches, have a significantly skewed perception of exactly how serious this surgery is. The study showed that 28% of players and 20% of coaches believed the pitcher’s performance would be improved after surgery, and, more strikingly, 26% of collegiate athletes, 30% percent of coaches, 37% of parents, and 51% of high school athletes believed UCL reconstruction should be performed as a prophylactic procedure to enhance performance in an uninjured athlete.17
Henceforth, it becomes the surgeon’s responsibility to ensure that both the patient and the parents understand what the surgery and rehabilitation process entails, to keep the expectations of the patient and his or her family realistic, and to counsel these patients on alternative options with lower risks. As Ahmad and colleagues17 demonstrated, this is not an easy task given the public’s preconceived notions. Many patients, especially patients of the younger generation, seem to be willing to jump to surgery as the first option for treatment without having truly tried any nonoperative measures, because they believe surgery to be a quick, easy, and definitive answer. This is not always the case, and a trial of nonoperative treatment, including rest, ice, physical therapy, and possibly platelet-rich plasma (PRP), should be instituted for high school–aged players who present with UCL insufficiency prior to discussing surgery.18,19
Medial UCL reconstruction is a successful procedure for elite MLB athletes. However, UCL reconstruction is becoming a victim of its own success as younger and younger athletes who will likely never play at the major league level are undergoing this procedure at an alarming rate. This is an epidemic which must be addressed by surgeons, coaches, and parents alike to curb the beliefs that UCL reconstruction will make high school–aged pitchers more successful. This procedure should not be performed prophylactically on an athlete of any age, especially those in high school. Further studies on the effectiveness of both nonoperative rest and rehabilitation and of PRP on partial-thickness UCL tears are warranted. New technology in the form of a compression sleeve with imbedded sensors to track the biomechanics of a pitcher’s elbow has been released and will hopefully provide information to coaches about when pitchers’ elbows begin to fatigue based on several biomechanical parameters.12 The future of UCL reconstruction is still fluid, and with proper prevention strategies, nonoperative treatment, indications, and preoperative discussions, the Tommy John epidemic can be cured. ◾
Ulnar collateral ligament (UCL) reconstruction, commonly referred to as Tommy John surgery, is a well-described surgical treatment for elite athletes with a symptomatic, deficient UCL.1, 2 The procedure was first performed by the late Dr. Frank Jobe in 1974, described in 1986, and has undergone several modifications over the past 30 years.3 Different graft choices, tunnel positions, graft configurations, and tunnel fixation methods are just some of the alterations that have been made to the original Jobe technique.4-6 With time, the index procedure has become more refined, with predictable outcomes in Major League Baseball (MLB) pitchers as well as other elite overhead throwing athletes.2,7,8 However, though this surgery was originally described for elite athletes suffering from UCL deficiency, recent times have seen an increase of over 50% in the number of UCL reconstructions performed on high school–aged and younger athletes.9 Furthermore, in 2000, a total of 13 MLB pitchers underwent UCL reconstruction, while in 2012 this number increased nearly threefold to 32.2 This paradigm shift of performing UCL reconstructions more frequently and on younger athletes raises a very important question: what is the role of the orthopedic surgeon in this epidemic?
UCL reconstruction has become a reliable procedure for MLB pitchers and other overhead throwing athletes.7,10,11 Recent studies have reported that MLB pitchers who undergo UCL reconstruction return to pitch in the MLB 83% of the time, whereas only 3% fail to return to pitch in either MLB or the minor league.2 Furthermore, pitchers who undergo UCL reconstruction perform similarly after surgery as prior to their UCL reconstruction, with fewer innings pitched after surgery, but, more importantly, a lower earned run average (ERA) and walks plus hits per inning pitched (WHIP) after surgery. These last 2 statistics, known as sabermetrics, evaluate the pitcher’s effectiveness; the fact that these are improved after surgery is reassuring for pitchers who undergo this procedure. However, it must be recognized that these pitchers pitched fewer innings after surgery.
There has been a sharp increase in the number of MLB pitchers who have undergone UCL reconstruction in recent years, especially the past 3 seasons, in which over 60 pitchers have had Tommy John surgery.2 This increase, however, is not confined to MLB pitchers. High school–aged pitchers have also been part of this drastic rise in the number of UCL reconstructions performed throughout the country. Dr. James Andrews and colleagues noted a 50% increase from 1988-1994 to 1995-2003 in the proportion of high school–aged pitchers who underwent UCL reconstruction (while the absolute number increased from 7 to 77 in high school–aged players compared with 85 to 609 in adult athletes).9 Given the increase in MLB pitchers over the past few years, it is likely this number has also increased among adolescent pitchers.
This data again raises the question: what is the role of the orthopedic surgeon in this epidemic? There are many plausible responses, but in my opinion, there is one answer that surpasses the others. As a trained professional, surgeons are tasked with the responsibility of looking out for the best interest of their patients, even when this conflicts with the patient’s, or the patient’s parent’s or coach’s desires. This includes injury prevention, such as instituting pitch counts and developing products that allow coaches to determine when a pitcher may be at risk for injury from fatigue, as well as injury treatment.12 It is difficult for a patient to understand the gravity of surgery and the rehabilitation process, specifically a procedure as involved as UCL reconstruction, and especially if the patient is an adolescent who has their outlook clouded by the fact that they believe they will be the next MLB star pitcher. The reality is that the National Collegiate Athletic Association (NCAA)13 has released data that has demonstrated that only 6.8% of high school baseball players will play baseball in college. Furthermore, only 9.4% of college baseball players will reach the professional level. That equates to 0.5%, or 1 in 200 high school players who will eventually play professional baseball.13 However, the reverse of this is also true, that out of every 200 players, 1 will make it to the major leagues, and that 1 player could be the patient in question. Hence, the purpose of this data is to show parents and athletes that, while they do have a chance of playing professional, and certainly collegiate, baseball, that percentage must be weighed against the risks of surgery.
MLB pitchers who have an endless supply of rehabilitation facilities, trainers, etc, do not return to pitching competitively and consistently in the majors for more than 15 months after UCL reconstruction.2 The time commitment and rehabilitation required for these patients is staggering.14,15 Furthermore, parents of these children who are consenting for them also have a difficult time comprehending the workload they are signing their child up for. Some parents believe this surgery will help their child throw faster, longer, and more accurately—beliefs that numerous studies have shown to be flat-out inaccurate. In fact, pitchers tend to lose a slight amount of velocity and accuracy after UCL reconstruction.11,16 Ahmad and colleagues17 administered a questionnaire to 189 players, 15 coaches, and 31 parents about the indications, risks, benefits, etc, regarding UCL reconstruction to determine the public’s perception regarding this surgery. The results demonstrated that the public, including coaches, have a significantly skewed perception of exactly how serious this surgery is. The study showed that 28% of players and 20% of coaches believed the pitcher’s performance would be improved after surgery, and, more strikingly, 26% of collegiate athletes, 30% percent of coaches, 37% of parents, and 51% of high school athletes believed UCL reconstruction should be performed as a prophylactic procedure to enhance performance in an uninjured athlete.17
Henceforth, it becomes the surgeon’s responsibility to ensure that both the patient and the parents understand what the surgery and rehabilitation process entails, to keep the expectations of the patient and his or her family realistic, and to counsel these patients on alternative options with lower risks. As Ahmad and colleagues17 demonstrated, this is not an easy task given the public’s preconceived notions. Many patients, especially patients of the younger generation, seem to be willing to jump to surgery as the first option for treatment without having truly tried any nonoperative measures, because they believe surgery to be a quick, easy, and definitive answer. This is not always the case, and a trial of nonoperative treatment, including rest, ice, physical therapy, and possibly platelet-rich plasma (PRP), should be instituted for high school–aged players who present with UCL insufficiency prior to discussing surgery.18,19
Medial UCL reconstruction is a successful procedure for elite MLB athletes. However, UCL reconstruction is becoming a victim of its own success as younger and younger athletes who will likely never play at the major league level are undergoing this procedure at an alarming rate. This is an epidemic which must be addressed by surgeons, coaches, and parents alike to curb the beliefs that UCL reconstruction will make high school–aged pitchers more successful. This procedure should not be performed prophylactically on an athlete of any age, especially those in high school. Further studies on the effectiveness of both nonoperative rest and rehabilitation and of PRP on partial-thickness UCL tears are warranted. New technology in the form of a compression sleeve with imbedded sensors to track the biomechanics of a pitcher’s elbow has been released and will hopefully provide information to coaches about when pitchers’ elbows begin to fatigue based on several biomechanical parameters.12 The future of UCL reconstruction is still fluid, and with proper prevention strategies, nonoperative treatment, indications, and preoperative discussions, the Tommy John epidemic can be cured. ◾
1. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes. Treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
2. Erickson BJ, Gupta AK, Harris JD, et al. Rate of return to pitching and performance after Tommy John surgery in Major League Baseball pitchers. Am J Sports Med. 2014;42(3):536-543.
3. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
4. Jackson TJ, Adamson GJ, Peterson A, Patton J, McGarry MH, Lee TQ. Ulnar collateral ligament reconstruction using bisuspensory fixation: a biomechanical comparison with the docking technique. Am J Sports Med. 2013;41(5):1158-1164.
5. Dines JS, ElAttrache NS, Conway JE, Smith W, Ahmad CS. Clinical outcomes of the DANE TJ technique to treat ulnar collateral ligament insufficiency of the elbow. Am J Sports Med. 2007;35(12):2039-2044.
6. Andrews JR, Jost PW, Cain EL. The ulnar collateral ligament procedure revisited: the procedure we use. Sports Health. 2012;4(5):438-441.
7. Dines JS, Jones KJ, Kahlenberg C, Rosenbaum A, Osbahr DC, Altchek DW. Elbow ulnar collateral ligament reconstruction in javelin throwers at a minimum 2-year follow-up. Am J Sports Med. 2012;40(1):148-151.
8. Gibson BW, Webner D, Huffman GR, Sennett BJ. Ulnar collateral ligament reconstruction in major league baseball pitchers. Am J Sports Med. 2007;35(4):575-581.
9. Petty DH, Andrews JR, Fleisig GS, Cain EL. Ulnar collateral ligament reconstruction in high school baseball players: clinical results and injury risk factors. Am J Sports Med. 2004;32(5):1158-1164.
10. Osbahr DC, Cain EL Jr, Raines BT, Fortenbaugh D, Dugas JR, Andrews JR. Long-term outcomes after ulnar collateral ligament reconstruction in competitive baseball players: minimum 10-year follow-up. Am J Sports Med. 2014;42(6):1333-1342.
11. Jiang JJ, Leland JM. Analysis of pitching velocity in major league baseball players before and after ulnar collateral ligament reconstruction. Am J Sports Med. 2014;42(4):880-885.
12. Carroll W. The sleeve that could save baseball: exclusive look at new MLB technology. Bleacher Report. http://bleacherreport.com/articles/2097866-the-sleeve-that-could-save-baseball-exclusive-look-at-new-mlb-technology?utm_campaign=tsipad&utm_medium=referral&utm_source=teamstream. Published July 2, 2014. Accessed November 12, 2014.
13. National Collegiate Athletic Association. Estimated probability of competing in athletics beyond the high school interscholastic level. https://www.ncaa.org/sites/default/files/Probability-of-going-pro-methodology_Update2013.pdf. Updated September 24, 2013. Accessed November 12, 2014.
14. Wilk KE, Macrina LC, Cain EL, Dugas JR, Andrews JR. Rehabilitation of the overhead athlete’s elbow. Sports Health. 2012;4(5):404-414.
15. Wilk KE, Reinold MM, Andrews JR. Rehabilitation of the thrower’s elbow. Tech Hand Up Extrem Surg. 2003;7(4):197-216.
16. Makhni EC, Lee RW, Morrow ZS, Gualtieri AP, Gorroochurn P, Ahmad CS. Performance, return to competition, and reinjury after Tommy John surgery in Major League Baseball pitchers: a review of 147 cases. Am J Sports Med. 2014;42(6):1323-1332.
17. Ahmad CS, Grantham WJ, Greiwe RM. Public perceptions of Tommy John surgery. Phys Sportsmed. 2012;40(2):64-72.
18. Rettig AC, Sherrill C, Snead DS, Mendler JC, Mieling P. Nonoperative treatment of ulnar collateral ligament injuries in throwing athletes. Am J Sports Med. 2001;29(1):15-17.
19. Podesta L, Crow SA, Volkmer D, Bert T, Yocum LA. Treatment of partial ulnar collateral ligament tears in the elbow with platelet-rich plasma. Am J Sports Med. 2013;41(7):1689-1694.
1. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes. Treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
2. Erickson BJ, Gupta AK, Harris JD, et al. Rate of return to pitching and performance after Tommy John surgery in Major League Baseball pitchers. Am J Sports Med. 2014;42(3):536-543.
3. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
4. Jackson TJ, Adamson GJ, Peterson A, Patton J, McGarry MH, Lee TQ. Ulnar collateral ligament reconstruction using bisuspensory fixation: a biomechanical comparison with the docking technique. Am J Sports Med. 2013;41(5):1158-1164.
5. Dines JS, ElAttrache NS, Conway JE, Smith W, Ahmad CS. Clinical outcomes of the DANE TJ technique to treat ulnar collateral ligament insufficiency of the elbow. Am J Sports Med. 2007;35(12):2039-2044.
6. Andrews JR, Jost PW, Cain EL. The ulnar collateral ligament procedure revisited: the procedure we use. Sports Health. 2012;4(5):438-441.
7. Dines JS, Jones KJ, Kahlenberg C, Rosenbaum A, Osbahr DC, Altchek DW. Elbow ulnar collateral ligament reconstruction in javelin throwers at a minimum 2-year follow-up. Am J Sports Med. 2012;40(1):148-151.
8. Gibson BW, Webner D, Huffman GR, Sennett BJ. Ulnar collateral ligament reconstruction in major league baseball pitchers. Am J Sports Med. 2007;35(4):575-581.
9. Petty DH, Andrews JR, Fleisig GS, Cain EL. Ulnar collateral ligament reconstruction in high school baseball players: clinical results and injury risk factors. Am J Sports Med. 2004;32(5):1158-1164.
10. Osbahr DC, Cain EL Jr, Raines BT, Fortenbaugh D, Dugas JR, Andrews JR. Long-term outcomes after ulnar collateral ligament reconstruction in competitive baseball players: minimum 10-year follow-up. Am J Sports Med. 2014;42(6):1333-1342.
11. Jiang JJ, Leland JM. Analysis of pitching velocity in major league baseball players before and after ulnar collateral ligament reconstruction. Am J Sports Med. 2014;42(4):880-885.
12. Carroll W. The sleeve that could save baseball: exclusive look at new MLB technology. Bleacher Report. http://bleacherreport.com/articles/2097866-the-sleeve-that-could-save-baseball-exclusive-look-at-new-mlb-technology?utm_campaign=tsipad&utm_medium=referral&utm_source=teamstream. Published July 2, 2014. Accessed November 12, 2014.
13. National Collegiate Athletic Association. Estimated probability of competing in athletics beyond the high school interscholastic level. https://www.ncaa.org/sites/default/files/Probability-of-going-pro-methodology_Update2013.pdf. Updated September 24, 2013. Accessed November 12, 2014.
14. Wilk KE, Macrina LC, Cain EL, Dugas JR, Andrews JR. Rehabilitation of the overhead athlete’s elbow. Sports Health. 2012;4(5):404-414.
15. Wilk KE, Reinold MM, Andrews JR. Rehabilitation of the thrower’s elbow. Tech Hand Up Extrem Surg. 2003;7(4):197-216.
16. Makhni EC, Lee RW, Morrow ZS, Gualtieri AP, Gorroochurn P, Ahmad CS. Performance, return to competition, and reinjury after Tommy John surgery in Major League Baseball pitchers: a review of 147 cases. Am J Sports Med. 2014;42(6):1323-1332.
17. Ahmad CS, Grantham WJ, Greiwe RM. Public perceptions of Tommy John surgery. Phys Sportsmed. 2012;40(2):64-72.
18. Rettig AC, Sherrill C, Snead DS, Mendler JC, Mieling P. Nonoperative treatment of ulnar collateral ligament injuries in throwing athletes. Am J Sports Med. 2001;29(1):15-17.
19. Podesta L, Crow SA, Volkmer D, Bert T, Yocum LA. Treatment of partial ulnar collateral ligament tears in the elbow with platelet-rich plasma. Am J Sports Med. 2013;41(7):1689-1694.
Severe Neurologic Manifestations of Fat Embolism Syndrome in a Polytrauma Patient
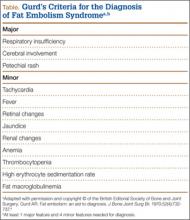
Fat embolism syndrome (FES) was first described by Von Bergmann in 1873 in a patient with a fractured femur.1 While fat within the circulation (fat embolism) is relatively common following long-bone fracture, the clinical pattern of symptoms that make up FES is less so, occurring in 1% to 3% of isolated long-bone fractures and 5% to 10% of patients with multiple skeletal trauma.1 A variety of clinical, laboratory, and imaging criteria has been described, classically by Gurd in 1970 (Table).1-6 Most commonly, however, it is a diagnosis of exclusion when the classic triad of respiratory difficulty, neurologic abnormalities, and a characteristic petechial rash are present in the appropriate clinical setting.6
The neurologic sequelae of this syndrome can range from headache, confusion, and agitation to stupor, focal neurologic signs, and, less commonly, coma.7 Onset of these symptoms usually occurs between 24 hours and 48 hours (mean, 40 hours) after trauma.1 While these neurologic manifestations occur in up to 86% of patients with FES, it is rare for them to be present without the pulmonary symptoms of dyspnea, hypoxemia, and tachypnea, which are the most common presenting symptoms of the disease.1-6 In this case report, we describe severe, rapid-onset neurologic manifestations, without the typical pulmonary involvement, as the primary clinical presentation of FES in a polytrauma patient. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
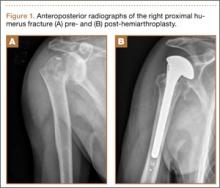
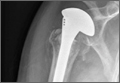
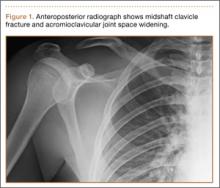
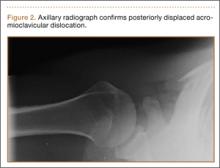
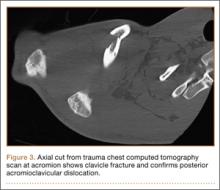
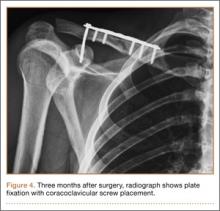
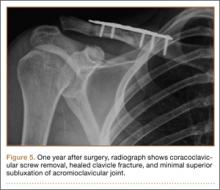
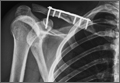
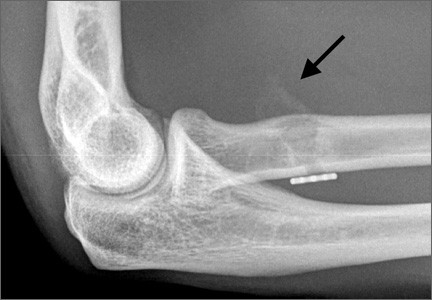
A previously healthy 50-year-old man presented to the emergency room in transfer from an outside hospital after a rollover motor vehicle collision in which he was ejected approximately 50 feet. Injuries included a right proximal humerus fracture/dislocation (Figure 1), right ulnar styloid fracture, L1 compression fracture, and multiple rib fractures. On admission, the patient had an ethanol level of 969 mg/L (.097%) and a urine drug screen positive only for opioids, presumably because of pain medication given that day. He denied a history of alcohol abuse and reported consuming 2 to 3 beers per week. The patient was awake, alert, and oriented with a Glasgow Coma Scale (GCS) of 15. He was tachycardic (heart rate, 126), tachypneic (respiratory rate, 24), and febrile (temperature, 38.6°C [101.5°F]), and his white blood cell count was elevated at 29.5×109/L. On examination, his right arm was found to be neurovascularly intact; it was placed in a sling with a forearm splint, and the patient was admitted to the intermediate special care unit on spine precautions with a plan for right shoulder hemiarthroplasty the following day.
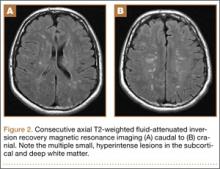
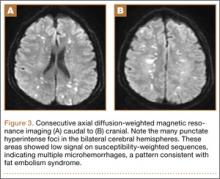
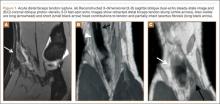
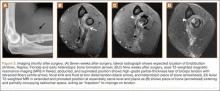
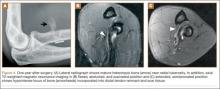
Overnight the patient’s mental status began to deteriorate, and approximately 10 hours after initial assessment, he was not answering questions but was able to respond to some commands. On hospital day 2, approximately 20 hours after initial assessment, the patient had a GCS of 8, was not responding to commands, and moved only in response to painful stimuli. The patient had been prescribed morphine by patient-controlled analgesia and had received intravenous hydromorphone on the day of admission, although the amount of medication delivered was not thought adequate to explain this deterioration. On the morning of hospital day 2, noncontrast brain computed tomography (CT) was normal with no evidence of intracranial hemorrhage or infarct. This was followed by brain magnetic resonance imaging (MRI), with the T2-weighted images showing numerous, small hyperintense lesions in subcortical and periventricular white matter, corpus callosum, basal ganglia, brain stem, and cerebellar hemispheres (Figure 2). The lesions also showed hyperintensity on diffusion-weighted MRI and were interpreted to be consistent with multiple, tiny infarcts (Figure 3). In addition, susceptibility-weighted sequences showed low signal in the same areas, suggesting multiple microhemorrhages, a pattern consistent with FES. Oxygen saturations remained 95% to 99%, and chest radiograph revealed clear lung fields without infiltrate. On hospital day 2, the patient was transferred to the intensive care unit and intubated for airway protection owing to an inability to clear secretions, although arterial blood gas levels remained normal. An echocardiogram revealed no right-to-left shunt, such as a patent foramen ovale (PFO); an electroencephalogram showed no seizure-like activity. No petechial rash was noted on skin examination. The patient was treated with supportive care. Right shoulder hemiarthroplasty was performed on hospital day 7 without complications (Figure 1). On hospital day 13, the patient was following commands and on day 14 he was extubated. His mental status continued to improve, and he was discharged to a rehabilitation facility after 36 days. On last follow-up, 6 months after initial injury, the patient was recovering well with no residual neurologic deficits and only minor limitation in range of motion of the right shoulder.
Discussion
This case presented an interesting diagnostic challenge regarding the patient’s rapid decline in mental status, with a differential diagnosis including diffuse axonal injury (DAI), anoxic brain injury, posttraumatic seizure, other intracranial pathology, such as stroke or hemorrhage, and FES. FES was diagnosed, when other possibilities were ruled out, given the characteristic findings on brain MRI described above in the context of multiple fractures.
Pathophysiology
Despite its recognition in 1873, there is no consensus on the pathophysiological mechanism that causes the clinical symptoms of FES. In the setting of trauma, there are 2 predominant theories. The mechanical theory postulates that fat globules enter the circulation through disrupted venules after the fracture of marrow-containing bones, passing to the arterial circulation through pulmonary vasculature, or paradoxically, by way of a right-to-left shunt, such as a PFO.1,3 The presence of fat in the heart, visualized as echogenic material in the right and left atria on transesophageal echocardiography, has been confirmed in multiple studies during orthopedic procedures, including total knee arthroplasty and femoral reaming.8,9 These fat particles can lodge as microembolisms in target organs such as the skin and brain. However, autopsy studies have shown a lack of correlation of the severity of symptoms and the quantity of intravascular fat.1 In addition, the typical 24- to 72-hour delay in the onset of symptoms after initial trauma would argue against a solely mechanical explanation.10
Alternatively or concomitantly, the biochemical theory proposes that embolized fat may be degraded to toxic intermediaries, such as free fatty acids and C-reactive protein, which cause end-organ damage.3 This has been shown in an animal model, in which intravascular injection of free fatty acids was associated with endothelial damage and increased capillary permeability in the lung, leading to acute respiratory distress syndrome (ARDS).11 The same mechanism could explain injury to other end organs and is consistent with the delay in onset of symptoms after acute injury. In our patient’s case, the absence of pulmonary involvement, lack of a right-to-left vascular shunt such as a PFO, and presence of a systemic inflammatory response on admission may implicate the production of toxic intermediaries from the metabolism of embolized fat as the source of this patient’s FES.
Clinical Presentation
The initial presentation of FES usually manifests as respiratory distress and hypoxia.10 Chest radiographs are often normal, as in our patient, but can show bilateral diffuse interstitial or alveolar infiltrates.2,6 CT more often has findings, including bilateral ground-glass opacities with interlobar septal thickening.12 A petechial rash can be found on the head, neck, anterior thorax, axillae, subconjunctiva, and oral mucous membranes, although it occurs in only 20% to 50% of cases.1,2,13 Neurologic sequelae are present in up to 80% of patients,7 with onset typically following pulmonary symptoms.1,10 These sequelae can range from headache, confusion, and agitation to stupor, focal neurologic signs, and, less commonly, coma.7 Onset of symptoms generally occurs between 24 and 48 hours after trauma,1 although they have been reported as early as 12 hours.10 This case is an example of an atypical course, with the initial presentation of neurologic symptoms at approximately 14 hours after trauma with rapid progression to coma without classic pulmonary symptoms.
Diagnosis
Owing to the nonspecific clinical features of FES, a variety of clinical, laboratory, and imaging criteria has been described. Of these criteria, the most frequently referenced is by Gurd in 1970,4,5 who divided the features into major and minor, with 1 major and 4 minor features required to make the diagnosis (Table). In applying these criteria to our patient, we found that he exhibited the major criteria of cerebral involvement and minor criteria of tachycardia, fever, and thrombocytopenia. Respiratory insufficiency and petechial rash, as well as jaundice, renal changes, and anemia were negative features. Retinal changes, elevated erythrocyte sedimentation rate, and fat macroglobulinemia were not tested or examined. Although in our case the clinical and laboratory criteria for the diagnosis of FES as defined by Gurd were not met, the sensitivity of Gurd’s and other criteria is debated.10
Laboratory tests specific for the disease have not been developed. Although elevated serum levels of lipase, increased blood lipid levels, and fat globules in the urine, sputum, and blood have all been proposed, they are found in trauma patients with and without FES.2,5,6
The nonspecific nature of the signs and symptoms of FES and the lack of reliable laboratory tests for diagnosis of the syndrome highlight the importance of radiographic evaluation in patients with neurologic symptoms. Brain CT scans are usually negative,14 although, in some cases, they may show diffuse edema with scattered low attenuating areas and hemorrhage.15 MRI is more sensitive, and T2-weighted images typically reveal multiple small, nonconfluent hyperintense lesions, usually in the periventricular, subcortical, and deep white matter, sometimes referred to as the “starfield” pattern.14,16 The differential diagnosis for these findings is broad and, in addition to FES, includes DAI, vasogenic edema with microinfarcts, and demyelinating disease.14 Sensitivity and specificity may be increased with the addition of diffusion-weighted MRI, which shows scattered bright spots on a dark background in a similar “starfield” pattern as on T2-weighted images.15 Susceptibility-weighted MRI has recently been introduced as having utility in the diagnosis of FES, with areas of low-signal intensity indicating diffuse microhemorrhages.17 DAI can show a similar pattern; however, the autopsy-confirmed locations of the abnormalities are distinct, with those of FES being found in cerebral and cerebellar white matter and splenium of the corpus callosum and radiographic abnormalities of DAI being found in the gray-white matter junction, dorsolateral brainstem, and splenium of corpus callosum.17
Prevention and Treatment
Of primary importance in the prevention of FES is early stabilization of fractures. Several studies have shown a decreased incidence of FES when long-bone fractures are treated with immediate operative fixation.18,19 However, in the setting of polytrauma, the desire for early definitive treatment must be balanced against the risks for the exaggerated immune response from prolonged surgery.20 The timing of fracture fixation to prevent sequelae of the inflammatory response, such as ARDS and multiple organ dysfunction syndrome, is still debated. In a review article, Pape and colleagues20 suggest classifying the multiply injured patient as stable, borderline, unstable, and in extremis based on clinical and laboratory criteria. They recommend early definitive fixation for stable patients and those patients who are borderline or unstable and responsive to resuscitation, whereas damage-control orthopedics and staged fracture fixation should be considered in the other groups.
Several pharmacologic interventions have been described, although their effects are highly variable and none have clear indications.1-3,6 The most heavily researched is corticosteroids, with the proposed mechanisms of action including blunting of the inflammatory response, stabilizing the pulmonary capillary membrane to reduce interstitial edema, preventing activation of the complement system, and retarding platelet aggregation.21 A recent meta-analysis to assess this intervention examined 6 studies with a total of 386 patients with long-bone fractures who were randomized to treatment with corticosteroids or supportive care only.22 They found a reduced risk for FES in those patients who received corticosteroids, but there was no difference in mortality between groups. Given these results, the utility of corticosteroids is still debated.
Once FES has occurred, treatment options usually focus on supportive care, with most patients having a full recovery.1,3 No specific treatments are available, and symptomatic treatment is the suggested approach, including ensuring adequate oxygenation and ventilation and providing hemodynamic support and volume and blood-product resuscitation as needed.1-3,6
Conclusion
We have presented a case of FES unique in its rapid onset, an initial presentation with neurologic manifestations without typical pulmonary involvement, and the mechanism of end-organ damage without a right-to-left shunt. This case emphasizes the importance of considering FES in the patient with deteriorating mental status in the setting of multiple fractures, particularly in the absence of other characteristic clinical findings, such as pulmonary distress and the pathognomonic petechial rash. Brain MRI can play an important role in diagnosing those patients presenting with predominantly neurological symptoms. Early recognition of this condition allows for the anticipation of complications of the disease process, such as respiratory distress, and the potential need for mechanical ventilation and hemodynamic support.
1. Johnson MJ, Lucas GL. Fat embolism syndrome. Orthopedics. 1996;19(1):41-49.
2. Levy D. The fat embolism syndrome. A review. Clin Orthop. 1990;261:281-286.
3. Mellor A, Soni N. Fat embolism. Anaesthesia. 2001;56(2):145-154.
4. Gurd AR. Fat embolism: an aid to diagnosis. J Bone Joint Surg Br. 1970:52(4):732-737.
5. Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56(3):408-416.
6. Bulger EM, Smith DG, Maier RV, Jurkovich GJ. Fat embolism syndrome. A 10-year review. Arch Surg. 1997;132(4):435-439.
7. Jacobson DM, Terrence CF, Reinmuth OM. The neurologic manifestations of fat embolism. Neurology. 1986;36(6):847-851.
8. Sulek CA, Davies LK, Enneking FK, Gearen PA, Lobato EB. Cerebral microembolism diagnosed by transcranial Doppler during total knee arthroplasty: correlation with transesophageal echocardiography. Anesthesiology. 1999;91(3):672-676.
9. Volgas DA, Burch T, Stannard JP, Ellis T, Bilotta J, Alonso JE. Fat embolus in femur fractures: a comparison of two reaming systems. Injury. 2010;41(Suppl 2):S90-S93.
10. Gupta B, D’souza N, Sawhney C, et al. Analyzing fat embolism syndrome in trauma patients at AIIMS Apex Trauma Center, New Delhi, India. J Emerg Trauma Shock. 2011;4(3):337–341.
11. King EG, Wagner WW Jr, Ashbaugh DG, Latham LP, Halsey DR. Alterations in pulmonary microanatomy after fat embolism. In vivo observations via thoracic window of the oleic acid-embolized canine lung. Chest. 1971:59(5):524-530.
12. Malagari K, Economopoulos N, Stoupis C, et al. High-resolution CT findings in mild pulmonary fat embolism. Chest. 2003:123(4):1196-1201.
13. King MB, Harmon KR. Unusual forms of pulmonary embolism. Clin Chest Med. 1994;15(3):561-580.
14. Parizel PM, Demey HE, Veeckmans G, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern). Stroke. 2001;32(12):2942-2944.
15. Simon AD, Ulmer JL, Strottmann JM. Contrast-enhanced MR imaging of cerebral fat embolism: case report and review of the literature. AJNR Am J Neuroradiol. 2003;24(1):97-101.
16. Butteriss DJ, Mahad D, Soh C, Walls T, Weir D, Birchall D. Reversible cytotoxic cerebral edema in cerebral fat embolism. AJNR Am J Neuroradiol. 2006;27(3):620-623.
17. Zaitsu Y, Terae S, Kudo K, et al. Susceptibility-weighted imaging of cerebral fat embolism. J Comput Assist Tomogr. 2010;34(1):107-112.
18. Riska EB, Myllynen P. Fat embolism in patients with multiple injuries. J Trauma. 1982;22(11):891-894.
19. Svenningsen S, Nesse O, Finsen V, Hole A, Benum P. Prevention of fat embolism syndrome in patients with femoral fractures–immediate or delayed operative fixation? Ann Chir Gynaecol. 1987;76(3):163-166.
20. Pape HC, Tornetta P, Tarkin I, Tzioupis C, Sabeson V, Olson SA. Timing of fracture fixation in multitrauma patients: the role of early total care and damage control surgery. J Am Acad Orthop Surg. 2009;17(9):541-549.
21. Gosseling HR, Pellegrini VD Jr. Fat embolism syndrome: a review of the pathophysiology and physiological basis of treatment. Clin Orthop. 1982;165:68-82.
22. Bederman SS, Bhandari M, McKee MD, Schemitsch EH. Do corticosteroids reduce the risk of fat embolism syndrome in patients with long-bone fractures? A meta-analysis. Can J Surg. 2009:52(5):386-393.
Fat embolism syndrome (FES) was first described by Von Bergmann in 1873 in a patient with a fractured femur.1 While fat within the circulation (fat embolism) is relatively common following long-bone fracture, the clinical pattern of symptoms that make up FES is less so, occurring in 1% to 3% of isolated long-bone fractures and 5% to 10% of patients with multiple skeletal trauma.1 A variety of clinical, laboratory, and imaging criteria has been described, classically by Gurd in 1970 (Table).1-6 Most commonly, however, it is a diagnosis of exclusion when the classic triad of respiratory difficulty, neurologic abnormalities, and a characteristic petechial rash are present in the appropriate clinical setting.6
The neurologic sequelae of this syndrome can range from headache, confusion, and agitation to stupor, focal neurologic signs, and, less commonly, coma.7 Onset of these symptoms usually occurs between 24 hours and 48 hours (mean, 40 hours) after trauma.1 While these neurologic manifestations occur in up to 86% of patients with FES, it is rare for them to be present without the pulmonary symptoms of dyspnea, hypoxemia, and tachypnea, which are the most common presenting symptoms of the disease.1-6 In this case report, we describe severe, rapid-onset neurologic manifestations, without the typical pulmonary involvement, as the primary clinical presentation of FES in a polytrauma patient. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A previously healthy 50-year-old man presented to the emergency room in transfer from an outside hospital after a rollover motor vehicle collision in which he was ejected approximately 50 feet. Injuries included a right proximal humerus fracture/dislocation (Figure 1), right ulnar styloid fracture, L1 compression fracture, and multiple rib fractures. On admission, the patient had an ethanol level of 969 mg/L (.097%) and a urine drug screen positive only for opioids, presumably because of pain medication given that day. He denied a history of alcohol abuse and reported consuming 2 to 3 beers per week. The patient was awake, alert, and oriented with a Glasgow Coma Scale (GCS) of 15. He was tachycardic (heart rate, 126), tachypneic (respiratory rate, 24), and febrile (temperature, 38.6°C [101.5°F]), and his white blood cell count was elevated at 29.5×109/L. On examination, his right arm was found to be neurovascularly intact; it was placed in a sling with a forearm splint, and the patient was admitted to the intermediate special care unit on spine precautions with a plan for right shoulder hemiarthroplasty the following day.
Overnight the patient’s mental status began to deteriorate, and approximately 10 hours after initial assessment, he was not answering questions but was able to respond to some commands. On hospital day 2, approximately 20 hours after initial assessment, the patient had a GCS of 8, was not responding to commands, and moved only in response to painful stimuli. The patient had been prescribed morphine by patient-controlled analgesia and had received intravenous hydromorphone on the day of admission, although the amount of medication delivered was not thought adequate to explain this deterioration. On the morning of hospital day 2, noncontrast brain computed tomography (CT) was normal with no evidence of intracranial hemorrhage or infarct. This was followed by brain magnetic resonance imaging (MRI), with the T2-weighted images showing numerous, small hyperintense lesions in subcortical and periventricular white matter, corpus callosum, basal ganglia, brain stem, and cerebellar hemispheres (Figure 2). The lesions also showed hyperintensity on diffusion-weighted MRI and were interpreted to be consistent with multiple, tiny infarcts (Figure 3). In addition, susceptibility-weighted sequences showed low signal in the same areas, suggesting multiple microhemorrhages, a pattern consistent with FES. Oxygen saturations remained 95% to 99%, and chest radiograph revealed clear lung fields without infiltrate. On hospital day 2, the patient was transferred to the intensive care unit and intubated for airway protection owing to an inability to clear secretions, although arterial blood gas levels remained normal. An echocardiogram revealed no right-to-left shunt, such as a patent foramen ovale (PFO); an electroencephalogram showed no seizure-like activity. No petechial rash was noted on skin examination. The patient was treated with supportive care. Right shoulder hemiarthroplasty was performed on hospital day 7 without complications (Figure 1). On hospital day 13, the patient was following commands and on day 14 he was extubated. His mental status continued to improve, and he was discharged to a rehabilitation facility after 36 days. On last follow-up, 6 months after initial injury, the patient was recovering well with no residual neurologic deficits and only minor limitation in range of motion of the right shoulder.
Discussion
This case presented an interesting diagnostic challenge regarding the patient’s rapid decline in mental status, with a differential diagnosis including diffuse axonal injury (DAI), anoxic brain injury, posttraumatic seizure, other intracranial pathology, such as stroke or hemorrhage, and FES. FES was diagnosed, when other possibilities were ruled out, given the characteristic findings on brain MRI described above in the context of multiple fractures.
Pathophysiology
Despite its recognition in 1873, there is no consensus on the pathophysiological mechanism that causes the clinical symptoms of FES. In the setting of trauma, there are 2 predominant theories. The mechanical theory postulates that fat globules enter the circulation through disrupted venules after the fracture of marrow-containing bones, passing to the arterial circulation through pulmonary vasculature, or paradoxically, by way of a right-to-left shunt, such as a PFO.1,3 The presence of fat in the heart, visualized as echogenic material in the right and left atria on transesophageal echocardiography, has been confirmed in multiple studies during orthopedic procedures, including total knee arthroplasty and femoral reaming.8,9 These fat particles can lodge as microembolisms in target organs such as the skin and brain. However, autopsy studies have shown a lack of correlation of the severity of symptoms and the quantity of intravascular fat.1 In addition, the typical 24- to 72-hour delay in the onset of symptoms after initial trauma would argue against a solely mechanical explanation.10
Alternatively or concomitantly, the biochemical theory proposes that embolized fat may be degraded to toxic intermediaries, such as free fatty acids and C-reactive protein, which cause end-organ damage.3 This has been shown in an animal model, in which intravascular injection of free fatty acids was associated with endothelial damage and increased capillary permeability in the lung, leading to acute respiratory distress syndrome (ARDS).11 The same mechanism could explain injury to other end organs and is consistent with the delay in onset of symptoms after acute injury. In our patient’s case, the absence of pulmonary involvement, lack of a right-to-left vascular shunt such as a PFO, and presence of a systemic inflammatory response on admission may implicate the production of toxic intermediaries from the metabolism of embolized fat as the source of this patient’s FES.
Clinical Presentation
The initial presentation of FES usually manifests as respiratory distress and hypoxia.10 Chest radiographs are often normal, as in our patient, but can show bilateral diffuse interstitial or alveolar infiltrates.2,6 CT more often has findings, including bilateral ground-glass opacities with interlobar septal thickening.12 A petechial rash can be found on the head, neck, anterior thorax, axillae, subconjunctiva, and oral mucous membranes, although it occurs in only 20% to 50% of cases.1,2,13 Neurologic sequelae are present in up to 80% of patients,7 with onset typically following pulmonary symptoms.1,10 These sequelae can range from headache, confusion, and agitation to stupor, focal neurologic signs, and, less commonly, coma.7 Onset of symptoms generally occurs between 24 and 48 hours after trauma,1 although they have been reported as early as 12 hours.10 This case is an example of an atypical course, with the initial presentation of neurologic symptoms at approximately 14 hours after trauma with rapid progression to coma without classic pulmonary symptoms.
Diagnosis
Owing to the nonspecific clinical features of FES, a variety of clinical, laboratory, and imaging criteria has been described. Of these criteria, the most frequently referenced is by Gurd in 1970,4,5 who divided the features into major and minor, with 1 major and 4 minor features required to make the diagnosis (Table). In applying these criteria to our patient, we found that he exhibited the major criteria of cerebral involvement and minor criteria of tachycardia, fever, and thrombocytopenia. Respiratory insufficiency and petechial rash, as well as jaundice, renal changes, and anemia were negative features. Retinal changes, elevated erythrocyte sedimentation rate, and fat macroglobulinemia were not tested or examined. Although in our case the clinical and laboratory criteria for the diagnosis of FES as defined by Gurd were not met, the sensitivity of Gurd’s and other criteria is debated.10
Laboratory tests specific for the disease have not been developed. Although elevated serum levels of lipase, increased blood lipid levels, and fat globules in the urine, sputum, and blood have all been proposed, they are found in trauma patients with and without FES.2,5,6
The nonspecific nature of the signs and symptoms of FES and the lack of reliable laboratory tests for diagnosis of the syndrome highlight the importance of radiographic evaluation in patients with neurologic symptoms. Brain CT scans are usually negative,14 although, in some cases, they may show diffuse edema with scattered low attenuating areas and hemorrhage.15 MRI is more sensitive, and T2-weighted images typically reveal multiple small, nonconfluent hyperintense lesions, usually in the periventricular, subcortical, and deep white matter, sometimes referred to as the “starfield” pattern.14,16 The differential diagnosis for these findings is broad and, in addition to FES, includes DAI, vasogenic edema with microinfarcts, and demyelinating disease.14 Sensitivity and specificity may be increased with the addition of diffusion-weighted MRI, which shows scattered bright spots on a dark background in a similar “starfield” pattern as on T2-weighted images.15 Susceptibility-weighted MRI has recently been introduced as having utility in the diagnosis of FES, with areas of low-signal intensity indicating diffuse microhemorrhages.17 DAI can show a similar pattern; however, the autopsy-confirmed locations of the abnormalities are distinct, with those of FES being found in cerebral and cerebellar white matter and splenium of the corpus callosum and radiographic abnormalities of DAI being found in the gray-white matter junction, dorsolateral brainstem, and splenium of corpus callosum.17
Prevention and Treatment
Of primary importance in the prevention of FES is early stabilization of fractures. Several studies have shown a decreased incidence of FES when long-bone fractures are treated with immediate operative fixation.18,19 However, in the setting of polytrauma, the desire for early definitive treatment must be balanced against the risks for the exaggerated immune response from prolonged surgery.20 The timing of fracture fixation to prevent sequelae of the inflammatory response, such as ARDS and multiple organ dysfunction syndrome, is still debated. In a review article, Pape and colleagues20 suggest classifying the multiply injured patient as stable, borderline, unstable, and in extremis based on clinical and laboratory criteria. They recommend early definitive fixation for stable patients and those patients who are borderline or unstable and responsive to resuscitation, whereas damage-control orthopedics and staged fracture fixation should be considered in the other groups.
Several pharmacologic interventions have been described, although their effects are highly variable and none have clear indications.1-3,6 The most heavily researched is corticosteroids, with the proposed mechanisms of action including blunting of the inflammatory response, stabilizing the pulmonary capillary membrane to reduce interstitial edema, preventing activation of the complement system, and retarding platelet aggregation.21 A recent meta-analysis to assess this intervention examined 6 studies with a total of 386 patients with long-bone fractures who were randomized to treatment with corticosteroids or supportive care only.22 They found a reduced risk for FES in those patients who received corticosteroids, but there was no difference in mortality between groups. Given these results, the utility of corticosteroids is still debated.
Once FES has occurred, treatment options usually focus on supportive care, with most patients having a full recovery.1,3 No specific treatments are available, and symptomatic treatment is the suggested approach, including ensuring adequate oxygenation and ventilation and providing hemodynamic support and volume and blood-product resuscitation as needed.1-3,6
Conclusion
We have presented a case of FES unique in its rapid onset, an initial presentation with neurologic manifestations without typical pulmonary involvement, and the mechanism of end-organ damage without a right-to-left shunt. This case emphasizes the importance of considering FES in the patient with deteriorating mental status in the setting of multiple fractures, particularly in the absence of other characteristic clinical findings, such as pulmonary distress and the pathognomonic petechial rash. Brain MRI can play an important role in diagnosing those patients presenting with predominantly neurological symptoms. Early recognition of this condition allows for the anticipation of complications of the disease process, such as respiratory distress, and the potential need for mechanical ventilation and hemodynamic support.
Fat embolism syndrome (FES) was first described by Von Bergmann in 1873 in a patient with a fractured femur.1 While fat within the circulation (fat embolism) is relatively common following long-bone fracture, the clinical pattern of symptoms that make up FES is less so, occurring in 1% to 3% of isolated long-bone fractures and 5% to 10% of patients with multiple skeletal trauma.1 A variety of clinical, laboratory, and imaging criteria has been described, classically by Gurd in 1970 (Table).1-6 Most commonly, however, it is a diagnosis of exclusion when the classic triad of respiratory difficulty, neurologic abnormalities, and a characteristic petechial rash are present in the appropriate clinical setting.6
The neurologic sequelae of this syndrome can range from headache, confusion, and agitation to stupor, focal neurologic signs, and, less commonly, coma.7 Onset of these symptoms usually occurs between 24 hours and 48 hours (mean, 40 hours) after trauma.1 While these neurologic manifestations occur in up to 86% of patients with FES, it is rare for them to be present without the pulmonary symptoms of dyspnea, hypoxemia, and tachypnea, which are the most common presenting symptoms of the disease.1-6 In this case report, we describe severe, rapid-onset neurologic manifestations, without the typical pulmonary involvement, as the primary clinical presentation of FES in a polytrauma patient. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A previously healthy 50-year-old man presented to the emergency room in transfer from an outside hospital after a rollover motor vehicle collision in which he was ejected approximately 50 feet. Injuries included a right proximal humerus fracture/dislocation (Figure 1), right ulnar styloid fracture, L1 compression fracture, and multiple rib fractures. On admission, the patient had an ethanol level of 969 mg/L (.097%) and a urine drug screen positive only for opioids, presumably because of pain medication given that day. He denied a history of alcohol abuse and reported consuming 2 to 3 beers per week. The patient was awake, alert, and oriented with a Glasgow Coma Scale (GCS) of 15. He was tachycardic (heart rate, 126), tachypneic (respiratory rate, 24), and febrile (temperature, 38.6°C [101.5°F]), and his white blood cell count was elevated at 29.5×109/L. On examination, his right arm was found to be neurovascularly intact; it was placed in a sling with a forearm splint, and the patient was admitted to the intermediate special care unit on spine precautions with a plan for right shoulder hemiarthroplasty the following day.
Overnight the patient’s mental status began to deteriorate, and approximately 10 hours after initial assessment, he was not answering questions but was able to respond to some commands. On hospital day 2, approximately 20 hours after initial assessment, the patient had a GCS of 8, was not responding to commands, and moved only in response to painful stimuli. The patient had been prescribed morphine by patient-controlled analgesia and had received intravenous hydromorphone on the day of admission, although the amount of medication delivered was not thought adequate to explain this deterioration. On the morning of hospital day 2, noncontrast brain computed tomography (CT) was normal with no evidence of intracranial hemorrhage or infarct. This was followed by brain magnetic resonance imaging (MRI), with the T2-weighted images showing numerous, small hyperintense lesions in subcortical and periventricular white matter, corpus callosum, basal ganglia, brain stem, and cerebellar hemispheres (Figure 2). The lesions also showed hyperintensity on diffusion-weighted MRI and were interpreted to be consistent with multiple, tiny infarcts (Figure 3). In addition, susceptibility-weighted sequences showed low signal in the same areas, suggesting multiple microhemorrhages, a pattern consistent with FES. Oxygen saturations remained 95% to 99%, and chest radiograph revealed clear lung fields without infiltrate. On hospital day 2, the patient was transferred to the intensive care unit and intubated for airway protection owing to an inability to clear secretions, although arterial blood gas levels remained normal. An echocardiogram revealed no right-to-left shunt, such as a patent foramen ovale (PFO); an electroencephalogram showed no seizure-like activity. No petechial rash was noted on skin examination. The patient was treated with supportive care. Right shoulder hemiarthroplasty was performed on hospital day 7 without complications (Figure 1). On hospital day 13, the patient was following commands and on day 14 he was extubated. His mental status continued to improve, and he was discharged to a rehabilitation facility after 36 days. On last follow-up, 6 months after initial injury, the patient was recovering well with no residual neurologic deficits and only minor limitation in range of motion of the right shoulder.
Discussion
This case presented an interesting diagnostic challenge regarding the patient’s rapid decline in mental status, with a differential diagnosis including diffuse axonal injury (DAI), anoxic brain injury, posttraumatic seizure, other intracranial pathology, such as stroke or hemorrhage, and FES. FES was diagnosed, when other possibilities were ruled out, given the characteristic findings on brain MRI described above in the context of multiple fractures.
Pathophysiology
Despite its recognition in 1873, there is no consensus on the pathophysiological mechanism that causes the clinical symptoms of FES. In the setting of trauma, there are 2 predominant theories. The mechanical theory postulates that fat globules enter the circulation through disrupted venules after the fracture of marrow-containing bones, passing to the arterial circulation through pulmonary vasculature, or paradoxically, by way of a right-to-left shunt, such as a PFO.1,3 The presence of fat in the heart, visualized as echogenic material in the right and left atria on transesophageal echocardiography, has been confirmed in multiple studies during orthopedic procedures, including total knee arthroplasty and femoral reaming.8,9 These fat particles can lodge as microembolisms in target organs such as the skin and brain. However, autopsy studies have shown a lack of correlation of the severity of symptoms and the quantity of intravascular fat.1 In addition, the typical 24- to 72-hour delay in the onset of symptoms after initial trauma would argue against a solely mechanical explanation.10
Alternatively or concomitantly, the biochemical theory proposes that embolized fat may be degraded to toxic intermediaries, such as free fatty acids and C-reactive protein, which cause end-organ damage.3 This has been shown in an animal model, in which intravascular injection of free fatty acids was associated with endothelial damage and increased capillary permeability in the lung, leading to acute respiratory distress syndrome (ARDS).11 The same mechanism could explain injury to other end organs and is consistent with the delay in onset of symptoms after acute injury. In our patient’s case, the absence of pulmonary involvement, lack of a right-to-left vascular shunt such as a PFO, and presence of a systemic inflammatory response on admission may implicate the production of toxic intermediaries from the metabolism of embolized fat as the source of this patient’s FES.
Clinical Presentation
The initial presentation of FES usually manifests as respiratory distress and hypoxia.10 Chest radiographs are often normal, as in our patient, but can show bilateral diffuse interstitial or alveolar infiltrates.2,6 CT more often has findings, including bilateral ground-glass opacities with interlobar septal thickening.12 A petechial rash can be found on the head, neck, anterior thorax, axillae, subconjunctiva, and oral mucous membranes, although it occurs in only 20% to 50% of cases.1,2,13 Neurologic sequelae are present in up to 80% of patients,7 with onset typically following pulmonary symptoms.1,10 These sequelae can range from headache, confusion, and agitation to stupor, focal neurologic signs, and, less commonly, coma.7 Onset of symptoms generally occurs between 24 and 48 hours after trauma,1 although they have been reported as early as 12 hours.10 This case is an example of an atypical course, with the initial presentation of neurologic symptoms at approximately 14 hours after trauma with rapid progression to coma without classic pulmonary symptoms.
Diagnosis
Owing to the nonspecific clinical features of FES, a variety of clinical, laboratory, and imaging criteria has been described. Of these criteria, the most frequently referenced is by Gurd in 1970,4,5 who divided the features into major and minor, with 1 major and 4 minor features required to make the diagnosis (Table). In applying these criteria to our patient, we found that he exhibited the major criteria of cerebral involvement and minor criteria of tachycardia, fever, and thrombocytopenia. Respiratory insufficiency and petechial rash, as well as jaundice, renal changes, and anemia were negative features. Retinal changes, elevated erythrocyte sedimentation rate, and fat macroglobulinemia were not tested or examined. Although in our case the clinical and laboratory criteria for the diagnosis of FES as defined by Gurd were not met, the sensitivity of Gurd’s and other criteria is debated.10
Laboratory tests specific for the disease have not been developed. Although elevated serum levels of lipase, increased blood lipid levels, and fat globules in the urine, sputum, and blood have all been proposed, they are found in trauma patients with and without FES.2,5,6
The nonspecific nature of the signs and symptoms of FES and the lack of reliable laboratory tests for diagnosis of the syndrome highlight the importance of radiographic evaluation in patients with neurologic symptoms. Brain CT scans are usually negative,14 although, in some cases, they may show diffuse edema with scattered low attenuating areas and hemorrhage.15 MRI is more sensitive, and T2-weighted images typically reveal multiple small, nonconfluent hyperintense lesions, usually in the periventricular, subcortical, and deep white matter, sometimes referred to as the “starfield” pattern.14,16 The differential diagnosis for these findings is broad and, in addition to FES, includes DAI, vasogenic edema with microinfarcts, and demyelinating disease.14 Sensitivity and specificity may be increased with the addition of diffusion-weighted MRI, which shows scattered bright spots on a dark background in a similar “starfield” pattern as on T2-weighted images.15 Susceptibility-weighted MRI has recently been introduced as having utility in the diagnosis of FES, with areas of low-signal intensity indicating diffuse microhemorrhages.17 DAI can show a similar pattern; however, the autopsy-confirmed locations of the abnormalities are distinct, with those of FES being found in cerebral and cerebellar white matter and splenium of the corpus callosum and radiographic abnormalities of DAI being found in the gray-white matter junction, dorsolateral brainstem, and splenium of corpus callosum.17
Prevention and Treatment
Of primary importance in the prevention of FES is early stabilization of fractures. Several studies have shown a decreased incidence of FES when long-bone fractures are treated with immediate operative fixation.18,19 However, in the setting of polytrauma, the desire for early definitive treatment must be balanced against the risks for the exaggerated immune response from prolonged surgery.20 The timing of fracture fixation to prevent sequelae of the inflammatory response, such as ARDS and multiple organ dysfunction syndrome, is still debated. In a review article, Pape and colleagues20 suggest classifying the multiply injured patient as stable, borderline, unstable, and in extremis based on clinical and laboratory criteria. They recommend early definitive fixation for stable patients and those patients who are borderline or unstable and responsive to resuscitation, whereas damage-control orthopedics and staged fracture fixation should be considered in the other groups.
Several pharmacologic interventions have been described, although their effects are highly variable and none have clear indications.1-3,6 The most heavily researched is corticosteroids, with the proposed mechanisms of action including blunting of the inflammatory response, stabilizing the pulmonary capillary membrane to reduce interstitial edema, preventing activation of the complement system, and retarding platelet aggregation.21 A recent meta-analysis to assess this intervention examined 6 studies with a total of 386 patients with long-bone fractures who were randomized to treatment with corticosteroids or supportive care only.22 They found a reduced risk for FES in those patients who received corticosteroids, but there was no difference in mortality between groups. Given these results, the utility of corticosteroids is still debated.
Once FES has occurred, treatment options usually focus on supportive care, with most patients having a full recovery.1,3 No specific treatments are available, and symptomatic treatment is the suggested approach, including ensuring adequate oxygenation and ventilation and providing hemodynamic support and volume and blood-product resuscitation as needed.1-3,6
Conclusion
We have presented a case of FES unique in its rapid onset, an initial presentation with neurologic manifestations without typical pulmonary involvement, and the mechanism of end-organ damage without a right-to-left shunt. This case emphasizes the importance of considering FES in the patient with deteriorating mental status in the setting of multiple fractures, particularly in the absence of other characteristic clinical findings, such as pulmonary distress and the pathognomonic petechial rash. Brain MRI can play an important role in diagnosing those patients presenting with predominantly neurological symptoms. Early recognition of this condition allows for the anticipation of complications of the disease process, such as respiratory distress, and the potential need for mechanical ventilation and hemodynamic support.
1. Johnson MJ, Lucas GL. Fat embolism syndrome. Orthopedics. 1996;19(1):41-49.
2. Levy D. The fat embolism syndrome. A review. Clin Orthop. 1990;261:281-286.
3. Mellor A, Soni N. Fat embolism. Anaesthesia. 2001;56(2):145-154.
4. Gurd AR. Fat embolism: an aid to diagnosis. J Bone Joint Surg Br. 1970:52(4):732-737.
5. Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56(3):408-416.
6. Bulger EM, Smith DG, Maier RV, Jurkovich GJ. Fat embolism syndrome. A 10-year review. Arch Surg. 1997;132(4):435-439.
7. Jacobson DM, Terrence CF, Reinmuth OM. The neurologic manifestations of fat embolism. Neurology. 1986;36(6):847-851.
8. Sulek CA, Davies LK, Enneking FK, Gearen PA, Lobato EB. Cerebral microembolism diagnosed by transcranial Doppler during total knee arthroplasty: correlation with transesophageal echocardiography. Anesthesiology. 1999;91(3):672-676.
9. Volgas DA, Burch T, Stannard JP, Ellis T, Bilotta J, Alonso JE. Fat embolus in femur fractures: a comparison of two reaming systems. Injury. 2010;41(Suppl 2):S90-S93.
10. Gupta B, D’souza N, Sawhney C, et al. Analyzing fat embolism syndrome in trauma patients at AIIMS Apex Trauma Center, New Delhi, India. J Emerg Trauma Shock. 2011;4(3):337–341.
11. King EG, Wagner WW Jr, Ashbaugh DG, Latham LP, Halsey DR. Alterations in pulmonary microanatomy after fat embolism. In vivo observations via thoracic window of the oleic acid-embolized canine lung. Chest. 1971:59(5):524-530.
12. Malagari K, Economopoulos N, Stoupis C, et al. High-resolution CT findings in mild pulmonary fat embolism. Chest. 2003:123(4):1196-1201.
13. King MB, Harmon KR. Unusual forms of pulmonary embolism. Clin Chest Med. 1994;15(3):561-580.
14. Parizel PM, Demey HE, Veeckmans G, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern). Stroke. 2001;32(12):2942-2944.
15. Simon AD, Ulmer JL, Strottmann JM. Contrast-enhanced MR imaging of cerebral fat embolism: case report and review of the literature. AJNR Am J Neuroradiol. 2003;24(1):97-101.
16. Butteriss DJ, Mahad D, Soh C, Walls T, Weir D, Birchall D. Reversible cytotoxic cerebral edema in cerebral fat embolism. AJNR Am J Neuroradiol. 2006;27(3):620-623.
17. Zaitsu Y, Terae S, Kudo K, et al. Susceptibility-weighted imaging of cerebral fat embolism. J Comput Assist Tomogr. 2010;34(1):107-112.
18. Riska EB, Myllynen P. Fat embolism in patients with multiple injuries. J Trauma. 1982;22(11):891-894.
19. Svenningsen S, Nesse O, Finsen V, Hole A, Benum P. Prevention of fat embolism syndrome in patients with femoral fractures–immediate or delayed operative fixation? Ann Chir Gynaecol. 1987;76(3):163-166.
20. Pape HC, Tornetta P, Tarkin I, Tzioupis C, Sabeson V, Olson SA. Timing of fracture fixation in multitrauma patients: the role of early total care and damage control surgery. J Am Acad Orthop Surg. 2009;17(9):541-549.
21. Gosseling HR, Pellegrini VD Jr. Fat embolism syndrome: a review of the pathophysiology and physiological basis of treatment. Clin Orthop. 1982;165:68-82.
22. Bederman SS, Bhandari M, McKee MD, Schemitsch EH. Do corticosteroids reduce the risk of fat embolism syndrome in patients with long-bone fractures? A meta-analysis. Can J Surg. 2009:52(5):386-393.
1. Johnson MJ, Lucas GL. Fat embolism syndrome. Orthopedics. 1996;19(1):41-49.
2. Levy D. The fat embolism syndrome. A review. Clin Orthop. 1990;261:281-286.
3. Mellor A, Soni N. Fat embolism. Anaesthesia. 2001;56(2):145-154.
4. Gurd AR. Fat embolism: an aid to diagnosis. J Bone Joint Surg Br. 1970:52(4):732-737.
5. Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56(3):408-416.
6. Bulger EM, Smith DG, Maier RV, Jurkovich GJ. Fat embolism syndrome. A 10-year review. Arch Surg. 1997;132(4):435-439.
7. Jacobson DM, Terrence CF, Reinmuth OM. The neurologic manifestations of fat embolism. Neurology. 1986;36(6):847-851.
8. Sulek CA, Davies LK, Enneking FK, Gearen PA, Lobato EB. Cerebral microembolism diagnosed by transcranial Doppler during total knee arthroplasty: correlation with transesophageal echocardiography. Anesthesiology. 1999;91(3):672-676.
9. Volgas DA, Burch T, Stannard JP, Ellis T, Bilotta J, Alonso JE. Fat embolus in femur fractures: a comparison of two reaming systems. Injury. 2010;41(Suppl 2):S90-S93.
10. Gupta B, D’souza N, Sawhney C, et al. Analyzing fat embolism syndrome in trauma patients at AIIMS Apex Trauma Center, New Delhi, India. J Emerg Trauma Shock. 2011;4(3):337–341.
11. King EG, Wagner WW Jr, Ashbaugh DG, Latham LP, Halsey DR. Alterations in pulmonary microanatomy after fat embolism. In vivo observations via thoracic window of the oleic acid-embolized canine lung. Chest. 1971:59(5):524-530.
12. Malagari K, Economopoulos N, Stoupis C, et al. High-resolution CT findings in mild pulmonary fat embolism. Chest. 2003:123(4):1196-1201.
13. King MB, Harmon KR. Unusual forms of pulmonary embolism. Clin Chest Med. 1994;15(3):561-580.
14. Parizel PM, Demey HE, Veeckmans G, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern). Stroke. 2001;32(12):2942-2944.
15. Simon AD, Ulmer JL, Strottmann JM. Contrast-enhanced MR imaging of cerebral fat embolism: case report and review of the literature. AJNR Am J Neuroradiol. 2003;24(1):97-101.
16. Butteriss DJ, Mahad D, Soh C, Walls T, Weir D, Birchall D. Reversible cytotoxic cerebral edema in cerebral fat embolism. AJNR Am J Neuroradiol. 2006;27(3):620-623.
17. Zaitsu Y, Terae S, Kudo K, et al. Susceptibility-weighted imaging of cerebral fat embolism. J Comput Assist Tomogr. 2010;34(1):107-112.
18. Riska EB, Myllynen P. Fat embolism in patients with multiple injuries. J Trauma. 1982;22(11):891-894.
19. Svenningsen S, Nesse O, Finsen V, Hole A, Benum P. Prevention of fat embolism syndrome in patients with femoral fractures–immediate or delayed operative fixation? Ann Chir Gynaecol. 1987;76(3):163-166.
20. Pape HC, Tornetta P, Tarkin I, Tzioupis C, Sabeson V, Olson SA. Timing of fracture fixation in multitrauma patients: the role of early total care and damage control surgery. J Am Acad Orthop Surg. 2009;17(9):541-549.
21. Gosseling HR, Pellegrini VD Jr. Fat embolism syndrome: a review of the pathophysiology and physiological basis of treatment. Clin Orthop. 1982;165:68-82.
22. Bederman SS, Bhandari M, McKee MD, Schemitsch EH. Do corticosteroids reduce the risk of fat embolism syndrome in patients with long-bone fractures? A meta-analysis. Can J Surg. 2009:52(5):386-393.
Physical Examination of the Throwing Athlete’s Elbow
Understanding the pathomechanics of throwing and the accompanying elbow injuries is the groundwork for conducting a directed history taking and a physical examination that produce an accurate diagnosis of elbow injuries in throwing athletes. Advances in physical examination techniques have improved our ability to accurately diagnose and treat throwers’ athletic elbow disorders.
Throwing imposes an extremely high valgus stress (approaching 60-65 Nm) across the elbow. This high stress occurs during the cocking and acceleration phases of the overhead throwing motion.1-3 The valgus stress generates tension on the medial elbow, compression on the lateral elbow, and shear on the posterior aspect of the elbow. These forces cause predictable injury patterns in different parts of throwers’ elbows. Physical examination performed in a systematic anatomical fashion can enhance predictable and accurate elbow injury diagnosis. In this article, we outline 5 points in a systematic approach to physical examination of a throwing athlete’s elbow.
1. Perform a general upper extremity examination
Cervical spine and shoulder girdle
In the initial examination, the cervical spine and the entire affected upper extremity should be quickly assessed. Assessment of the cervical spine should include palpation, range of motion (ROM), and basic provocative testing, such as the Spurling test, to evaluate for radiculopathy caused by foraminal compression. Posture, asymmetry, atrophy, edema, ecchymosis, and any other deformity should be noted. For example, atrophy of the neck and shoulders suggests underlying neuropathy. In addition, fullness of the supraclavicular region and local tenderness or bruit suggest vasculopathy. Symptomatic compression of the subclavian artery and vein between the anterior and middle scalene muscles may present as weakness, fullness, heaviness, and early fatigue. Physical signs include coolness, pallor, claudication, engorgement, and edema in the arm.4 Thoracic outlet syndrome can manifest as effort-induced vague pain at the arm and elbow.5 If this syndrome is suspected, an Adson test should be performed. With the patient’s neck extended and rotated away from the affected side, the examiner, standing next to the patient, palpates the radial pulse with the patient’s elbow extended (Figure 1A). Next, the examiner abducts, extends, and externally rotates the patient’s shoulder (Figure 1B) while the patient alternates between opening and closing the fist (Figure 1C). A decrease or absence in pulse strength from the starting position is a positive test result.
Last, the shoulder and scapulae should be assessed, as an affected shoulder or dyskinetic scapula can lead to improper mechanics of the kinetic chain at the elbow. The shoulder should be palpated, and ROM, strength, and stability should be assessed. Glenohumeral internal rotation deficit is associated with medial collateral ligament (MCL) tears; if present, this deficit should be addressed.6
Elbow
Inspection should reveal a normal carrying angle of about 11° to 14° of valgus in men and 13° to 16° in women. In immature athletes, increased valgus stresses from repetitive overhead throwing can cause medial epicondylar hypertrophy, and carrying angles of more than 15° are common.7-9
Active and passive ROM should be assessed. Normal ROM is about 0° extension and 140° flexion with 80° of supination and pronation. For determination of pathologic differences, ROM should always be compared between the affected and the contralateral sides. Painful loss of motion may be caused by soft-tissue swelling or contracture, effusion, bony impingement, or loose bodies. Crepitus, locking, catching, or another mechanical symptom may indicate loose bodies or chondral injury. Firm, mechanical blocks to ROM during flexion may indicate osteophyte formation in the coronoid fossa, and mechanical blocks to ROM during extension may indicate osteophyte formation in the olecranon fossa. Pain elicited at the end points of motion is caused by osteophytes and impingement, whereas pain elicited during the mid-arc of motion is often caused by osteochondral lesions. Terminal extension, often the first motion lost after injury, may signal intra-articular pathology, if symptomatic. However, throwing athletes may present with developmental flexion contractures of up to 20°.10
2. Examine the medial aspect of the elbow
The medial epicondyle, easy to recognize as a bony prominence on the medial side of the distal humerus, serves as an attachment site for the MCL, pronator teres, and the common flexor tendon. In throwers, assessing the MCL is crucial. The MCL should be palpated from its origin on the inferior aspect of the medial epicondyle moving distally to the sublime tubercle of the proximal ulna. Tenderness at any point along the ligament can indicate a range of ligament pathology, from attenuation to complete rupture.
The MCL is further assessed with stress tests, most commonly the valgus stress test, the milking maneuver, and the moving valgus stress test. Of these 3 procedures, the moving valgus stress test is perhaps the most sensitive and specific for MCL injury, and is the test preferred by the authors.11 This test takes into account shoulder position, simulates the position of throwing, and can account for bony structures that provide stability at more than 120° of flexion. We prefer to position the patient supine on the examining table to help stabilize the shoulder and humerus and to relax the patient. The shoulder is placed in abduction and external rotation while the examiner holds the thumb with one hand and supports the elbow with the other. The elbow is extended (Figure 2A) and flexed (Figure 2B) while valgus stress is applied. A positive test elicits pain localized to the MCL at the arc of motion between 80° to 120°.12 Pain at positions near full extension with the moving valgus stress test may also indicate chondral damage at the posteromedial trochlea.13
During pitching, the tensile demand on the MCL is reduced by the action of the flexor-pronator mass. It is common to see a flexor-pronator mass injury concurrent with MCL injury.14 Medial epicondyle tenderness that increases with resisted wrist flexion may signal flexor-pronator injury, though, classically, flexor-pronator muscle strains and tears produce pain anterior and distal to the medial epicondyle.15
Traction, compression, and friction at the medial elbow can irritate the ulnar nerve. This nerve should be inspected and palpated along its course at the cubital tunnel to determine its location and stability. Ulnar nerve hypermobility, which has been identified in 37% of elbows, can be determined by having the patient actively flex the elbow with the forearm in supination, placing a finger at the posteromedial aspect of the medial humeral epicondyle, and having the patient actively extend the elbow.16 The nerve dislocates if trapped anterior to the examiner’s finger, perches if under the examiner’s finger, or is stable if still palpable in the groove posterior to the medial epicondyle.16
The distal band of the medial triceps tendon may also sublux over the medial epicondyle with elbow flexion. This subluxation, also known as snapping triceps syndrome, may cause pain or ulnar nerve symptoms.17 Bringing the elbow from extension to flexion may produce subluxation, first of the ulnar nerve and then of the medial triceps, in 2 separate “snaps.” Tenderness can be elicited along the medial triceps muscle.
Ulnar neuritis is caused by traction injury, such as with dynamic pitching, nerve subluxation, or compression at the cubital tunnel. With MCL injury and valgus instability, the ulnar nerve can become irritated as it becomes stretched because of medial elbow laxity.18 The nerve can also be damaged during flexion as the cubital tunnel retinaculum tightens, decreasing the space available for the nerve.19 This concept is applied during the elbow flexion compression test. A positive test may elicit tingling radiating toward the small finger or pain at the elbow or medial forearm when manual pressure is directly applied over the ulnar nerve between the posteromedial olecranon and the medial humeral epicondyle as the elbow is maximally flexed.20
3. Examine the lateral aspect of the elbow
Palpation of the lateral epicondyle, the radial head, and the olecranon tip assists in defining injury to the underlying anatomy. The anconeus “soft spot” (infracondylar recess) within the triangle formed by these 3 bony landmarks should be palpated for fullness, indicating a joint effusion, hemarthrosis, or even a subluxed or dislocated radial head.
While the medial elbow endures a large tensile load, throwing imposes a tremendous compressive force at the lateral elbow, particularly at the radiocapitellar joint. This joint may be tender and produce clicking with pronation and supination in patients with radiocapitellar arthrosis, symptomatic posterolateral synovial plica, or an inflamed radial bursa. Tenderness with crepitus that can be exacerbated with forceful flexion and extension may indicate radiocapitellar overload or loose bodies.
Long-term load transmission and subsequent degeneration of the articular surface may advance to osteochondritis dissecans (OCD). Examination for capitellar OCD reveals tenderness over the radiocapitellar joint and commonly a loss of 15° to 20° of extension. The active radiocapitellar compression test is positive for OCD lesions and elicits pain in the lateral compartment of the elbow when the patient pronates (Figure 3A) and supinates (Figure 3B) the forearm with the elbow axially loaded in extension.21
Microtrauma and inflammation may occur with repetitive eccentric overload. Although rare in throwing athletes, “tennis elbow” causes pain with gripping, and decreased grip strength. Tenderness caused by lateral epicondylitis is just anterior and distal to the epicondyle, at the origin of the extensor carpi radialis brevis. Pain is reproducible with passive wrist flexion and resisted wrist extension with the elbow extended (Cozen test).
Less commonly, athletes may complain of mechanical symptoms, such as snapping or catching with posterolateral elbow pain.22 These symptoms may be due to thickened or inflamed synovial plica causing impingement. A posterior radiocapitellar plica can be examined by bringing the elbow to full extension while applying valgus stress with the forearm in supination. Conversely, an anterior radiocapitellar plica can be examined with a valgus load on the elbow and passive flexion with the forearm in pronation.23 A palpable painful snap over the radiocapitellar joint is a positive test.
4. Examine the posterior aspect of the elbow
Posteriorly, palpation is focused on the triceps tendon and the olecranon tip. The elbow should be flexed to 30° to relax the triceps, isolate the olecranon, and allow for palpation of the olecranon fossa on either side of the triceps tendon. Tenderness at the posterolateral or posteromedial aspect of the olecranon should be noted. Warmth, fluctuance, or distension at the elbow may be caused by olecranon bursitis. The 3 heads of the triceps muscle should be palpated where they converge to form an aponeurosis, and tenderness or a palpable gap on any of the heads should be noted.
A combination of valgus force and a rapidly decelerating arm at the follow-through phase of pitching causes a shear force between the medial aspect of the olecranon tip and the olecranon fossa. This shear force can result in chondrolysis, osteophyte formation, and loose bodies, particularly in the posteromedial elbow. This valgus extension overload (VEO) syndrome often results in loss of full extension and symptoms, which may be attributed to osteophytes or fractured and nonunited fragments in the olecranon fossa or the olecranon tip. Frank crepitus may also be present with extension testing caused by loose bodies or synovial reaction over osteophytes. Assessing for VEO using the extension impingement test, the examiner places continuous valgus stress on the elbow while quickly extending from 20° to 30° of flexion (Figure 4A) to terminal extension (Figure 4B) repeatedly. The examiner repeats this without valgus load while palpating the posteromedial olecranon for tenderness to differentiate impingement caused by instability from pain over the medial olecranon without instability (Figure 4C). Particular attention should be focused posteriorly in athletes with medial instability, as MCL injuries and VEO syndrome often occur in conjunction in the throwing athlete.
Repetitive acceleration and deceleration of the arm can also cause stress fractures. With stress fractures, pain is often noted more distal and lateral on the olecranon, but tenderness may be palpable medially from posteromedial impaction that occurs from the valgus load during the overhead throwing motion. In immature athletes, the repetitive sudden snap of full extension in the deceleration phase of throwing can cause olecranon apophysitis. Frank avulsions can occur as well but are usually preceded by chronic posterior elbow pain with possible loss of full extension.
The late cocking phase of the throwing motion (just before throwing) hyperextends the elbow and places significant strain on the elbow. Repetitive strain can cause painful posterior impingement. The arm bar test is extremely sensitive (Figure 5).13 With the patient’s elbow extended, shoulder internally rotated, and hand on the examiner’s shoulder, the examiner pulls down on the olecranon to simulate forced extension and reproduces the pain associated with posteromedial impingement.
Last, though triceps tendon injuries are rare, ruptures most often occur at the origin of the lateral head of the triceps. As the initial swelling and ecchymosis subside, a palpable gap is pathognomonic for rupture. Extensor weakness can often be observed, but extension may still be possible from anconeus triceps expansion with the aid of gravity. With the elbow overhead, the athlete must extend the elbow against gravity and will exhibit weakness against resistance.
5. Examine the anterior aspect of the elbow
Anteriorly, the bulk of the flexor-pronator group restricts the extent of joint palpation, and the soft tissues are usually injured. The antecubital fossa is a triangular area on the anterior aspect of the elbow that is bounded superiorly by a horizontal line connecting the medial epicondyle to the lateral epicondyle of the humerus, medially by the lateral border of the pronator teres muscle and laterally by the medial border of the brachioradialis muscle. From lateral to medial, the antecubital fossa contains the radial nerve, the biceps brachii tendon, the brachial artery, and the median nerve. Evaluating this area is important because a visible defect, change in muscle contour, or proximal retraction of a muscle belly can indicate a muscular rupture. In particular, a distal biceps rupture (rare) may be accompanied by weakness and pain in supination and, to a lesser degree, in flexion. It is important to note that, in the case of a partial biceps rupture, ecchymosis may not appear, as the hematoma is confined by the intact lacertus fibrosis.24 The hook test can be used to evaluate for the presence of an intact distal biceps tendon (Figure 6).25 The patient abducts the shoulder, flexes the elbow to 90°, and actively supinates the forearm while the examiner attempts to hook an index finger laterally under the tendon. The test is negative if the finger can be inserted 1 cm under the tendon and positive if no cordlike structure can be hooked. Partial biceps tendon ruptures or tendinitis may exhibit tenderness of the distal biceps tendon and pain on resisted supination with a negative hook test. Often, resisted elbow flexion with the elbow at maximal extension elicits pain at the biceps insertion. Clicking with forearm rotation near the insertion of the tendon, which may be caused by an inflamed radial bursa between the distal biceps tendon and the radial tuberosity, may be associated with impending rupture.
Conclusion
Physical examination combined with thorough history taking usually provides a solid basis for a diagnosis, which in turn makes the value of surgical treatment more assured.
1. Elliott B, Fleisig G, Nicholls R, Escamilia R. Technique effects on upper limb loading in the tennis serve. J Sci Med Sport. 2003;6(1):76-87.
2. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
3. Werner SL, Fleisig GS, Dillman CJ, Andrews JR. Biomechanics of the elbow during baseball pitching. J Orthop Sports Phys Ther. 1993;17(6):274-278.
4. Aval SM, Durand P Jr, Shankwiler JA. Neurovascular injuries to the athlete’s shoulder: part II. J Am Acad Orthop Surg. 2007;15(5):281-289.
5. Strukel RJ, Garrick JG. Thoracic outlet compression in athletes: a report of four cases. Am J Sports Med. 1978;6(2):35-39.
6. Dines JS, Frank JB, Akerman M, Yocum LA. Glenohumeral internal rotation deficits in baseball players with ulnar collateral ligament insufficiency. Am J Sports Med. 2009;37(3):566-570.
7. Adams JE. Injury to the throwing arm. A study of traumatic changes in the elbow joints of boy baseball players. Calif Med. 1965;102:127-132.
8. Hang DW, Chao CM, Hang YS. A clinical and roentgenographic study of Little League elbow. Am J Sports Med. 2004;32(1):79-84.
9. King JW, Brelsford HJ, Tullos HS. Analysis of the pitching arm of the professional baseball pitcher. Clin Orthop. 1969;(67):116-123.
10. Cain EL Jr, Dugas JR, Wolf RS, Andrews JR. Elbow injuries in throwing athletes: a current concepts review. Am J Sports Med. 2003;31(4):621-635.
11. Safran M, Ahmad CS, Elattrache NS. Ulnar collateral ligament of the elbow. Arthroscopy. 2005;21(11):1381-1395.
12. O’Driscoll SW, Lawton RL, Smith AM. The “moving valgus stress test” for medial collateral ligament tears of the elbow. Am J Sports Med. 2005;33(2):231-239.
13. O’Driscoll SW. Valgus extension overload and plica. In: Levine WN, ed. The Athlete’s Elbow. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008:71-83.
14. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes. Treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
15. Andrews JR, Whiteside JA, Buettner CM. Clinical evaluation of the elbow in throwers. Oper Tech Sports Med. 1996;4(2):77-83.
16. Calfee RP, Manske PR, Gelberman RH, Van Steyn MO, Steffen J, Goldfarb CA. Clinical assessment of the ulnar nerve at the elbow: reliability of instability testing and the association of hypermobility with clinical symptoms. J Bone Joint Surg Am. 2010;92(17):2801-2808.
17. Spinner RJ, Goldner RD. Snapping of the medial head of the triceps and recurrent dislocation of the ulnar nerve. Anatomical and dynamic factors. J Bone Joint Surg Am. 1998;80(2):239-247.
18. Guerra JJ, Timmerman LA. Clinical anatomy, histology, & pathomechanics of the elbow in sports. Oper Tech Sports Med. 1996;4(2):69-76.
19. O’Driscoll SW, Horii E, Carmichael SW, Morrey BF. The cubital tunnel and ulnar neuropathy. J Bone Joint Surg Br. 1991;73(4):613-617.
20. Novak CB, Lee GW, Mackinnon SE, Lay L. Provocative testing for cubital tunnel syndrome. J Hand Surg Am. 1994;19(5):817-820.
21. Andrews JR. Bony injuries about the elbow in the throwing athlete. Instr Course Lect. 1985;34:323-331.
22. Kim DH, Gambardella RA, Elattrache NS, Yocum LA, Jobe FW. Arthroscopic treatment of posterolateral elbow impingement from lateral synovial plicae in throwing athletes and golfers. Am J Sports Med. 2006;34(3):438-444.
23. Antuna SA, O’Driscoll SW. Snapping plicae associated with radiocapitellar chondromalacia. Arthroscopy. 2001;17(5):491-495.
24. Bernstein AD, Breslow MJ, Jazrawi LM. Distal biceps tendon ruptures: a historical perspective and current concepts. Am J Orthop. 2001;30(3):
193-200.
25. O’Driscoll SW, Goncalves LB, Dietz P. The hook test for distal biceps tendon avulsion. Am J Sports Med. 2007;35(11):1865-1869.
Understanding the pathomechanics of throwing and the accompanying elbow injuries is the groundwork for conducting a directed history taking and a physical examination that produce an accurate diagnosis of elbow injuries in throwing athletes. Advances in physical examination techniques have improved our ability to accurately diagnose and treat throwers’ athletic elbow disorders.
Throwing imposes an extremely high valgus stress (approaching 60-65 Nm) across the elbow. This high stress occurs during the cocking and acceleration phases of the overhead throwing motion.1-3 The valgus stress generates tension on the medial elbow, compression on the lateral elbow, and shear on the posterior aspect of the elbow. These forces cause predictable injury patterns in different parts of throwers’ elbows. Physical examination performed in a systematic anatomical fashion can enhance predictable and accurate elbow injury diagnosis. In this article, we outline 5 points in a systematic approach to physical examination of a throwing athlete’s elbow.
1. Perform a general upper extremity examination
Cervical spine and shoulder girdle
In the initial examination, the cervical spine and the entire affected upper extremity should be quickly assessed. Assessment of the cervical spine should include palpation, range of motion (ROM), and basic provocative testing, such as the Spurling test, to evaluate for radiculopathy caused by foraminal compression. Posture, asymmetry, atrophy, edema, ecchymosis, and any other deformity should be noted. For example, atrophy of the neck and shoulders suggests underlying neuropathy. In addition, fullness of the supraclavicular region and local tenderness or bruit suggest vasculopathy. Symptomatic compression of the subclavian artery and vein between the anterior and middle scalene muscles may present as weakness, fullness, heaviness, and early fatigue. Physical signs include coolness, pallor, claudication, engorgement, and edema in the arm.4 Thoracic outlet syndrome can manifest as effort-induced vague pain at the arm and elbow.5 If this syndrome is suspected, an Adson test should be performed. With the patient’s neck extended and rotated away from the affected side, the examiner, standing next to the patient, palpates the radial pulse with the patient’s elbow extended (Figure 1A). Next, the examiner abducts, extends, and externally rotates the patient’s shoulder (Figure 1B) while the patient alternates between opening and closing the fist (Figure 1C). A decrease or absence in pulse strength from the starting position is a positive test result.
Last, the shoulder and scapulae should be assessed, as an affected shoulder or dyskinetic scapula can lead to improper mechanics of the kinetic chain at the elbow. The shoulder should be palpated, and ROM, strength, and stability should be assessed. Glenohumeral internal rotation deficit is associated with medial collateral ligament (MCL) tears; if present, this deficit should be addressed.6
Elbow
Inspection should reveal a normal carrying angle of about 11° to 14° of valgus in men and 13° to 16° in women. In immature athletes, increased valgus stresses from repetitive overhead throwing can cause medial epicondylar hypertrophy, and carrying angles of more than 15° are common.7-9
Active and passive ROM should be assessed. Normal ROM is about 0° extension and 140° flexion with 80° of supination and pronation. For determination of pathologic differences, ROM should always be compared between the affected and the contralateral sides. Painful loss of motion may be caused by soft-tissue swelling or contracture, effusion, bony impingement, or loose bodies. Crepitus, locking, catching, or another mechanical symptom may indicate loose bodies or chondral injury. Firm, mechanical blocks to ROM during flexion may indicate osteophyte formation in the coronoid fossa, and mechanical blocks to ROM during extension may indicate osteophyte formation in the olecranon fossa. Pain elicited at the end points of motion is caused by osteophytes and impingement, whereas pain elicited during the mid-arc of motion is often caused by osteochondral lesions. Terminal extension, often the first motion lost after injury, may signal intra-articular pathology, if symptomatic. However, throwing athletes may present with developmental flexion contractures of up to 20°.10
2. Examine the medial aspect of the elbow
The medial epicondyle, easy to recognize as a bony prominence on the medial side of the distal humerus, serves as an attachment site for the MCL, pronator teres, and the common flexor tendon. In throwers, assessing the MCL is crucial. The MCL should be palpated from its origin on the inferior aspect of the medial epicondyle moving distally to the sublime tubercle of the proximal ulna. Tenderness at any point along the ligament can indicate a range of ligament pathology, from attenuation to complete rupture.
The MCL is further assessed with stress tests, most commonly the valgus stress test, the milking maneuver, and the moving valgus stress test. Of these 3 procedures, the moving valgus stress test is perhaps the most sensitive and specific for MCL injury, and is the test preferred by the authors.11 This test takes into account shoulder position, simulates the position of throwing, and can account for bony structures that provide stability at more than 120° of flexion. We prefer to position the patient supine on the examining table to help stabilize the shoulder and humerus and to relax the patient. The shoulder is placed in abduction and external rotation while the examiner holds the thumb with one hand and supports the elbow with the other. The elbow is extended (Figure 2A) and flexed (Figure 2B) while valgus stress is applied. A positive test elicits pain localized to the MCL at the arc of motion between 80° to 120°.12 Pain at positions near full extension with the moving valgus stress test may also indicate chondral damage at the posteromedial trochlea.13
During pitching, the tensile demand on the MCL is reduced by the action of the flexor-pronator mass. It is common to see a flexor-pronator mass injury concurrent with MCL injury.14 Medial epicondyle tenderness that increases with resisted wrist flexion may signal flexor-pronator injury, though, classically, flexor-pronator muscle strains and tears produce pain anterior and distal to the medial epicondyle.15
Traction, compression, and friction at the medial elbow can irritate the ulnar nerve. This nerve should be inspected and palpated along its course at the cubital tunnel to determine its location and stability. Ulnar nerve hypermobility, which has been identified in 37% of elbows, can be determined by having the patient actively flex the elbow with the forearm in supination, placing a finger at the posteromedial aspect of the medial humeral epicondyle, and having the patient actively extend the elbow.16 The nerve dislocates if trapped anterior to the examiner’s finger, perches if under the examiner’s finger, or is stable if still palpable in the groove posterior to the medial epicondyle.16
The distal band of the medial triceps tendon may also sublux over the medial epicondyle with elbow flexion. This subluxation, also known as snapping triceps syndrome, may cause pain or ulnar nerve symptoms.17 Bringing the elbow from extension to flexion may produce subluxation, first of the ulnar nerve and then of the medial triceps, in 2 separate “snaps.” Tenderness can be elicited along the medial triceps muscle.
Ulnar neuritis is caused by traction injury, such as with dynamic pitching, nerve subluxation, or compression at the cubital tunnel. With MCL injury and valgus instability, the ulnar nerve can become irritated as it becomes stretched because of medial elbow laxity.18 The nerve can also be damaged during flexion as the cubital tunnel retinaculum tightens, decreasing the space available for the nerve.19 This concept is applied during the elbow flexion compression test. A positive test may elicit tingling radiating toward the small finger or pain at the elbow or medial forearm when manual pressure is directly applied over the ulnar nerve between the posteromedial olecranon and the medial humeral epicondyle as the elbow is maximally flexed.20
3. Examine the lateral aspect of the elbow
Palpation of the lateral epicondyle, the radial head, and the olecranon tip assists in defining injury to the underlying anatomy. The anconeus “soft spot” (infracondylar recess) within the triangle formed by these 3 bony landmarks should be palpated for fullness, indicating a joint effusion, hemarthrosis, or even a subluxed or dislocated radial head.
While the medial elbow endures a large tensile load, throwing imposes a tremendous compressive force at the lateral elbow, particularly at the radiocapitellar joint. This joint may be tender and produce clicking with pronation and supination in patients with radiocapitellar arthrosis, symptomatic posterolateral synovial plica, or an inflamed radial bursa. Tenderness with crepitus that can be exacerbated with forceful flexion and extension may indicate radiocapitellar overload or loose bodies.
Long-term load transmission and subsequent degeneration of the articular surface may advance to osteochondritis dissecans (OCD). Examination for capitellar OCD reveals tenderness over the radiocapitellar joint and commonly a loss of 15° to 20° of extension. The active radiocapitellar compression test is positive for OCD lesions and elicits pain in the lateral compartment of the elbow when the patient pronates (Figure 3A) and supinates (Figure 3B) the forearm with the elbow axially loaded in extension.21
Microtrauma and inflammation may occur with repetitive eccentric overload. Although rare in throwing athletes, “tennis elbow” causes pain with gripping, and decreased grip strength. Tenderness caused by lateral epicondylitis is just anterior and distal to the epicondyle, at the origin of the extensor carpi radialis brevis. Pain is reproducible with passive wrist flexion and resisted wrist extension with the elbow extended (Cozen test).
Less commonly, athletes may complain of mechanical symptoms, such as snapping or catching with posterolateral elbow pain.22 These symptoms may be due to thickened or inflamed synovial plica causing impingement. A posterior radiocapitellar plica can be examined by bringing the elbow to full extension while applying valgus stress with the forearm in supination. Conversely, an anterior radiocapitellar plica can be examined with a valgus load on the elbow and passive flexion with the forearm in pronation.23 A palpable painful snap over the radiocapitellar joint is a positive test.
4. Examine the posterior aspect of the elbow
Posteriorly, palpation is focused on the triceps tendon and the olecranon tip. The elbow should be flexed to 30° to relax the triceps, isolate the olecranon, and allow for palpation of the olecranon fossa on either side of the triceps tendon. Tenderness at the posterolateral or posteromedial aspect of the olecranon should be noted. Warmth, fluctuance, or distension at the elbow may be caused by olecranon bursitis. The 3 heads of the triceps muscle should be palpated where they converge to form an aponeurosis, and tenderness or a palpable gap on any of the heads should be noted.
A combination of valgus force and a rapidly decelerating arm at the follow-through phase of pitching causes a shear force between the medial aspect of the olecranon tip and the olecranon fossa. This shear force can result in chondrolysis, osteophyte formation, and loose bodies, particularly in the posteromedial elbow. This valgus extension overload (VEO) syndrome often results in loss of full extension and symptoms, which may be attributed to osteophytes or fractured and nonunited fragments in the olecranon fossa or the olecranon tip. Frank crepitus may also be present with extension testing caused by loose bodies or synovial reaction over osteophytes. Assessing for VEO using the extension impingement test, the examiner places continuous valgus stress on the elbow while quickly extending from 20° to 30° of flexion (Figure 4A) to terminal extension (Figure 4B) repeatedly. The examiner repeats this without valgus load while palpating the posteromedial olecranon for tenderness to differentiate impingement caused by instability from pain over the medial olecranon without instability (Figure 4C). Particular attention should be focused posteriorly in athletes with medial instability, as MCL injuries and VEO syndrome often occur in conjunction in the throwing athlete.
Repetitive acceleration and deceleration of the arm can also cause stress fractures. With stress fractures, pain is often noted more distal and lateral on the olecranon, but tenderness may be palpable medially from posteromedial impaction that occurs from the valgus load during the overhead throwing motion. In immature athletes, the repetitive sudden snap of full extension in the deceleration phase of throwing can cause olecranon apophysitis. Frank avulsions can occur as well but are usually preceded by chronic posterior elbow pain with possible loss of full extension.
The late cocking phase of the throwing motion (just before throwing) hyperextends the elbow and places significant strain on the elbow. Repetitive strain can cause painful posterior impingement. The arm bar test is extremely sensitive (Figure 5).13 With the patient’s elbow extended, shoulder internally rotated, and hand on the examiner’s shoulder, the examiner pulls down on the olecranon to simulate forced extension and reproduces the pain associated with posteromedial impingement.
Last, though triceps tendon injuries are rare, ruptures most often occur at the origin of the lateral head of the triceps. As the initial swelling and ecchymosis subside, a palpable gap is pathognomonic for rupture. Extensor weakness can often be observed, but extension may still be possible from anconeus triceps expansion with the aid of gravity. With the elbow overhead, the athlete must extend the elbow against gravity and will exhibit weakness against resistance.
5. Examine the anterior aspect of the elbow
Anteriorly, the bulk of the flexor-pronator group restricts the extent of joint palpation, and the soft tissues are usually injured. The antecubital fossa is a triangular area on the anterior aspect of the elbow that is bounded superiorly by a horizontal line connecting the medial epicondyle to the lateral epicondyle of the humerus, medially by the lateral border of the pronator teres muscle and laterally by the medial border of the brachioradialis muscle. From lateral to medial, the antecubital fossa contains the radial nerve, the biceps brachii tendon, the brachial artery, and the median nerve. Evaluating this area is important because a visible defect, change in muscle contour, or proximal retraction of a muscle belly can indicate a muscular rupture. In particular, a distal biceps rupture (rare) may be accompanied by weakness and pain in supination and, to a lesser degree, in flexion. It is important to note that, in the case of a partial biceps rupture, ecchymosis may not appear, as the hematoma is confined by the intact lacertus fibrosis.24 The hook test can be used to evaluate for the presence of an intact distal biceps tendon (Figure 6).25 The patient abducts the shoulder, flexes the elbow to 90°, and actively supinates the forearm while the examiner attempts to hook an index finger laterally under the tendon. The test is negative if the finger can be inserted 1 cm under the tendon and positive if no cordlike structure can be hooked. Partial biceps tendon ruptures or tendinitis may exhibit tenderness of the distal biceps tendon and pain on resisted supination with a negative hook test. Often, resisted elbow flexion with the elbow at maximal extension elicits pain at the biceps insertion. Clicking with forearm rotation near the insertion of the tendon, which may be caused by an inflamed radial bursa between the distal biceps tendon and the radial tuberosity, may be associated with impending rupture.
Conclusion
Physical examination combined with thorough history taking usually provides a solid basis for a diagnosis, which in turn makes the value of surgical treatment more assured.
Understanding the pathomechanics of throwing and the accompanying elbow injuries is the groundwork for conducting a directed history taking and a physical examination that produce an accurate diagnosis of elbow injuries in throwing athletes. Advances in physical examination techniques have improved our ability to accurately diagnose and treat throwers’ athletic elbow disorders.
Throwing imposes an extremely high valgus stress (approaching 60-65 Nm) across the elbow. This high stress occurs during the cocking and acceleration phases of the overhead throwing motion.1-3 The valgus stress generates tension on the medial elbow, compression on the lateral elbow, and shear on the posterior aspect of the elbow. These forces cause predictable injury patterns in different parts of throwers’ elbows. Physical examination performed in a systematic anatomical fashion can enhance predictable and accurate elbow injury diagnosis. In this article, we outline 5 points in a systematic approach to physical examination of a throwing athlete’s elbow.
1. Perform a general upper extremity examination
Cervical spine and shoulder girdle
In the initial examination, the cervical spine and the entire affected upper extremity should be quickly assessed. Assessment of the cervical spine should include palpation, range of motion (ROM), and basic provocative testing, such as the Spurling test, to evaluate for radiculopathy caused by foraminal compression. Posture, asymmetry, atrophy, edema, ecchymosis, and any other deformity should be noted. For example, atrophy of the neck and shoulders suggests underlying neuropathy. In addition, fullness of the supraclavicular region and local tenderness or bruit suggest vasculopathy. Symptomatic compression of the subclavian artery and vein between the anterior and middle scalene muscles may present as weakness, fullness, heaviness, and early fatigue. Physical signs include coolness, pallor, claudication, engorgement, and edema in the arm.4 Thoracic outlet syndrome can manifest as effort-induced vague pain at the arm and elbow.5 If this syndrome is suspected, an Adson test should be performed. With the patient’s neck extended and rotated away from the affected side, the examiner, standing next to the patient, palpates the radial pulse with the patient’s elbow extended (Figure 1A). Next, the examiner abducts, extends, and externally rotates the patient’s shoulder (Figure 1B) while the patient alternates between opening and closing the fist (Figure 1C). A decrease or absence in pulse strength from the starting position is a positive test result.
Last, the shoulder and scapulae should be assessed, as an affected shoulder or dyskinetic scapula can lead to improper mechanics of the kinetic chain at the elbow. The shoulder should be palpated, and ROM, strength, and stability should be assessed. Glenohumeral internal rotation deficit is associated with medial collateral ligament (MCL) tears; if present, this deficit should be addressed.6
Elbow
Inspection should reveal a normal carrying angle of about 11° to 14° of valgus in men and 13° to 16° in women. In immature athletes, increased valgus stresses from repetitive overhead throwing can cause medial epicondylar hypertrophy, and carrying angles of more than 15° are common.7-9
Active and passive ROM should be assessed. Normal ROM is about 0° extension and 140° flexion with 80° of supination and pronation. For determination of pathologic differences, ROM should always be compared between the affected and the contralateral sides. Painful loss of motion may be caused by soft-tissue swelling or contracture, effusion, bony impingement, or loose bodies. Crepitus, locking, catching, or another mechanical symptom may indicate loose bodies or chondral injury. Firm, mechanical blocks to ROM during flexion may indicate osteophyte formation in the coronoid fossa, and mechanical blocks to ROM during extension may indicate osteophyte formation in the olecranon fossa. Pain elicited at the end points of motion is caused by osteophytes and impingement, whereas pain elicited during the mid-arc of motion is often caused by osteochondral lesions. Terminal extension, often the first motion lost after injury, may signal intra-articular pathology, if symptomatic. However, throwing athletes may present with developmental flexion contractures of up to 20°.10
2. Examine the medial aspect of the elbow
The medial epicondyle, easy to recognize as a bony prominence on the medial side of the distal humerus, serves as an attachment site for the MCL, pronator teres, and the common flexor tendon. In throwers, assessing the MCL is crucial. The MCL should be palpated from its origin on the inferior aspect of the medial epicondyle moving distally to the sublime tubercle of the proximal ulna. Tenderness at any point along the ligament can indicate a range of ligament pathology, from attenuation to complete rupture.
The MCL is further assessed with stress tests, most commonly the valgus stress test, the milking maneuver, and the moving valgus stress test. Of these 3 procedures, the moving valgus stress test is perhaps the most sensitive and specific for MCL injury, and is the test preferred by the authors.11 This test takes into account shoulder position, simulates the position of throwing, and can account for bony structures that provide stability at more than 120° of flexion. We prefer to position the patient supine on the examining table to help stabilize the shoulder and humerus and to relax the patient. The shoulder is placed in abduction and external rotation while the examiner holds the thumb with one hand and supports the elbow with the other. The elbow is extended (Figure 2A) and flexed (Figure 2B) while valgus stress is applied. A positive test elicits pain localized to the MCL at the arc of motion between 80° to 120°.12 Pain at positions near full extension with the moving valgus stress test may also indicate chondral damage at the posteromedial trochlea.13
During pitching, the tensile demand on the MCL is reduced by the action of the flexor-pronator mass. It is common to see a flexor-pronator mass injury concurrent with MCL injury.14 Medial epicondyle tenderness that increases with resisted wrist flexion may signal flexor-pronator injury, though, classically, flexor-pronator muscle strains and tears produce pain anterior and distal to the medial epicondyle.15
Traction, compression, and friction at the medial elbow can irritate the ulnar nerve. This nerve should be inspected and palpated along its course at the cubital tunnel to determine its location and stability. Ulnar nerve hypermobility, which has been identified in 37% of elbows, can be determined by having the patient actively flex the elbow with the forearm in supination, placing a finger at the posteromedial aspect of the medial humeral epicondyle, and having the patient actively extend the elbow.16 The nerve dislocates if trapped anterior to the examiner’s finger, perches if under the examiner’s finger, or is stable if still palpable in the groove posterior to the medial epicondyle.16
The distal band of the medial triceps tendon may also sublux over the medial epicondyle with elbow flexion. This subluxation, also known as snapping triceps syndrome, may cause pain or ulnar nerve symptoms.17 Bringing the elbow from extension to flexion may produce subluxation, first of the ulnar nerve and then of the medial triceps, in 2 separate “snaps.” Tenderness can be elicited along the medial triceps muscle.
Ulnar neuritis is caused by traction injury, such as with dynamic pitching, nerve subluxation, or compression at the cubital tunnel. With MCL injury and valgus instability, the ulnar nerve can become irritated as it becomes stretched because of medial elbow laxity.18 The nerve can also be damaged during flexion as the cubital tunnel retinaculum tightens, decreasing the space available for the nerve.19 This concept is applied during the elbow flexion compression test. A positive test may elicit tingling radiating toward the small finger or pain at the elbow or medial forearm when manual pressure is directly applied over the ulnar nerve between the posteromedial olecranon and the medial humeral epicondyle as the elbow is maximally flexed.20
3. Examine the lateral aspect of the elbow
Palpation of the lateral epicondyle, the radial head, and the olecranon tip assists in defining injury to the underlying anatomy. The anconeus “soft spot” (infracondylar recess) within the triangle formed by these 3 bony landmarks should be palpated for fullness, indicating a joint effusion, hemarthrosis, or even a subluxed or dislocated radial head.
While the medial elbow endures a large tensile load, throwing imposes a tremendous compressive force at the lateral elbow, particularly at the radiocapitellar joint. This joint may be tender and produce clicking with pronation and supination in patients with radiocapitellar arthrosis, symptomatic posterolateral synovial plica, or an inflamed radial bursa. Tenderness with crepitus that can be exacerbated with forceful flexion and extension may indicate radiocapitellar overload or loose bodies.
Long-term load transmission and subsequent degeneration of the articular surface may advance to osteochondritis dissecans (OCD). Examination for capitellar OCD reveals tenderness over the radiocapitellar joint and commonly a loss of 15° to 20° of extension. The active radiocapitellar compression test is positive for OCD lesions and elicits pain in the lateral compartment of the elbow when the patient pronates (Figure 3A) and supinates (Figure 3B) the forearm with the elbow axially loaded in extension.21
Microtrauma and inflammation may occur with repetitive eccentric overload. Although rare in throwing athletes, “tennis elbow” causes pain with gripping, and decreased grip strength. Tenderness caused by lateral epicondylitis is just anterior and distal to the epicondyle, at the origin of the extensor carpi radialis brevis. Pain is reproducible with passive wrist flexion and resisted wrist extension with the elbow extended (Cozen test).
Less commonly, athletes may complain of mechanical symptoms, such as snapping or catching with posterolateral elbow pain.22 These symptoms may be due to thickened or inflamed synovial plica causing impingement. A posterior radiocapitellar plica can be examined by bringing the elbow to full extension while applying valgus stress with the forearm in supination. Conversely, an anterior radiocapitellar plica can be examined with a valgus load on the elbow and passive flexion with the forearm in pronation.23 A palpable painful snap over the radiocapitellar joint is a positive test.
4. Examine the posterior aspect of the elbow
Posteriorly, palpation is focused on the triceps tendon and the olecranon tip. The elbow should be flexed to 30° to relax the triceps, isolate the olecranon, and allow for palpation of the olecranon fossa on either side of the triceps tendon. Tenderness at the posterolateral or posteromedial aspect of the olecranon should be noted. Warmth, fluctuance, or distension at the elbow may be caused by olecranon bursitis. The 3 heads of the triceps muscle should be palpated where they converge to form an aponeurosis, and tenderness or a palpable gap on any of the heads should be noted.
A combination of valgus force and a rapidly decelerating arm at the follow-through phase of pitching causes a shear force between the medial aspect of the olecranon tip and the olecranon fossa. This shear force can result in chondrolysis, osteophyte formation, and loose bodies, particularly in the posteromedial elbow. This valgus extension overload (VEO) syndrome often results in loss of full extension and symptoms, which may be attributed to osteophytes or fractured and nonunited fragments in the olecranon fossa or the olecranon tip. Frank crepitus may also be present with extension testing caused by loose bodies or synovial reaction over osteophytes. Assessing for VEO using the extension impingement test, the examiner places continuous valgus stress on the elbow while quickly extending from 20° to 30° of flexion (Figure 4A) to terminal extension (Figure 4B) repeatedly. The examiner repeats this without valgus load while palpating the posteromedial olecranon for tenderness to differentiate impingement caused by instability from pain over the medial olecranon without instability (Figure 4C). Particular attention should be focused posteriorly in athletes with medial instability, as MCL injuries and VEO syndrome often occur in conjunction in the throwing athlete.
Repetitive acceleration and deceleration of the arm can also cause stress fractures. With stress fractures, pain is often noted more distal and lateral on the olecranon, but tenderness may be palpable medially from posteromedial impaction that occurs from the valgus load during the overhead throwing motion. In immature athletes, the repetitive sudden snap of full extension in the deceleration phase of throwing can cause olecranon apophysitis. Frank avulsions can occur as well but are usually preceded by chronic posterior elbow pain with possible loss of full extension.
The late cocking phase of the throwing motion (just before throwing) hyperextends the elbow and places significant strain on the elbow. Repetitive strain can cause painful posterior impingement. The arm bar test is extremely sensitive (Figure 5).13 With the patient’s elbow extended, shoulder internally rotated, and hand on the examiner’s shoulder, the examiner pulls down on the olecranon to simulate forced extension and reproduces the pain associated with posteromedial impingement.
Last, though triceps tendon injuries are rare, ruptures most often occur at the origin of the lateral head of the triceps. As the initial swelling and ecchymosis subside, a palpable gap is pathognomonic for rupture. Extensor weakness can often be observed, but extension may still be possible from anconeus triceps expansion with the aid of gravity. With the elbow overhead, the athlete must extend the elbow against gravity and will exhibit weakness against resistance.
5. Examine the anterior aspect of the elbow
Anteriorly, the bulk of the flexor-pronator group restricts the extent of joint palpation, and the soft tissues are usually injured. The antecubital fossa is a triangular area on the anterior aspect of the elbow that is bounded superiorly by a horizontal line connecting the medial epicondyle to the lateral epicondyle of the humerus, medially by the lateral border of the pronator teres muscle and laterally by the medial border of the brachioradialis muscle. From lateral to medial, the antecubital fossa contains the radial nerve, the biceps brachii tendon, the brachial artery, and the median nerve. Evaluating this area is important because a visible defect, change in muscle contour, or proximal retraction of a muscle belly can indicate a muscular rupture. In particular, a distal biceps rupture (rare) may be accompanied by weakness and pain in supination and, to a lesser degree, in flexion. It is important to note that, in the case of a partial biceps rupture, ecchymosis may not appear, as the hematoma is confined by the intact lacertus fibrosis.24 The hook test can be used to evaluate for the presence of an intact distal biceps tendon (Figure 6).25 The patient abducts the shoulder, flexes the elbow to 90°, and actively supinates the forearm while the examiner attempts to hook an index finger laterally under the tendon. The test is negative if the finger can be inserted 1 cm under the tendon and positive if no cordlike structure can be hooked. Partial biceps tendon ruptures or tendinitis may exhibit tenderness of the distal biceps tendon and pain on resisted supination with a negative hook test. Often, resisted elbow flexion with the elbow at maximal extension elicits pain at the biceps insertion. Clicking with forearm rotation near the insertion of the tendon, which may be caused by an inflamed radial bursa between the distal biceps tendon and the radial tuberosity, may be associated with impending rupture.
Conclusion
Physical examination combined with thorough history taking usually provides a solid basis for a diagnosis, which in turn makes the value of surgical treatment more assured.
1. Elliott B, Fleisig G, Nicholls R, Escamilia R. Technique effects on upper limb loading in the tennis serve. J Sci Med Sport. 2003;6(1):76-87.
2. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
3. Werner SL, Fleisig GS, Dillman CJ, Andrews JR. Biomechanics of the elbow during baseball pitching. J Orthop Sports Phys Ther. 1993;17(6):274-278.
4. Aval SM, Durand P Jr, Shankwiler JA. Neurovascular injuries to the athlete’s shoulder: part II. J Am Acad Orthop Surg. 2007;15(5):281-289.
5. Strukel RJ, Garrick JG. Thoracic outlet compression in athletes: a report of four cases. Am J Sports Med. 1978;6(2):35-39.
6. Dines JS, Frank JB, Akerman M, Yocum LA. Glenohumeral internal rotation deficits in baseball players with ulnar collateral ligament insufficiency. Am J Sports Med. 2009;37(3):566-570.
7. Adams JE. Injury to the throwing arm. A study of traumatic changes in the elbow joints of boy baseball players. Calif Med. 1965;102:127-132.
8. Hang DW, Chao CM, Hang YS. A clinical and roentgenographic study of Little League elbow. Am J Sports Med. 2004;32(1):79-84.
9. King JW, Brelsford HJ, Tullos HS. Analysis of the pitching arm of the professional baseball pitcher. Clin Orthop. 1969;(67):116-123.
10. Cain EL Jr, Dugas JR, Wolf RS, Andrews JR. Elbow injuries in throwing athletes: a current concepts review. Am J Sports Med. 2003;31(4):621-635.
11. Safran M, Ahmad CS, Elattrache NS. Ulnar collateral ligament of the elbow. Arthroscopy. 2005;21(11):1381-1395.
12. O’Driscoll SW, Lawton RL, Smith AM. The “moving valgus stress test” for medial collateral ligament tears of the elbow. Am J Sports Med. 2005;33(2):231-239.
13. O’Driscoll SW. Valgus extension overload and plica. In: Levine WN, ed. The Athlete’s Elbow. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008:71-83.
14. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes. Treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
15. Andrews JR, Whiteside JA, Buettner CM. Clinical evaluation of the elbow in throwers. Oper Tech Sports Med. 1996;4(2):77-83.
16. Calfee RP, Manske PR, Gelberman RH, Van Steyn MO, Steffen J, Goldfarb CA. Clinical assessment of the ulnar nerve at the elbow: reliability of instability testing and the association of hypermobility with clinical symptoms. J Bone Joint Surg Am. 2010;92(17):2801-2808.
17. Spinner RJ, Goldner RD. Snapping of the medial head of the triceps and recurrent dislocation of the ulnar nerve. Anatomical and dynamic factors. J Bone Joint Surg Am. 1998;80(2):239-247.
18. Guerra JJ, Timmerman LA. Clinical anatomy, histology, & pathomechanics of the elbow in sports. Oper Tech Sports Med. 1996;4(2):69-76.
19. O’Driscoll SW, Horii E, Carmichael SW, Morrey BF. The cubital tunnel and ulnar neuropathy. J Bone Joint Surg Br. 1991;73(4):613-617.
20. Novak CB, Lee GW, Mackinnon SE, Lay L. Provocative testing for cubital tunnel syndrome. J Hand Surg Am. 1994;19(5):817-820.
21. Andrews JR. Bony injuries about the elbow in the throwing athlete. Instr Course Lect. 1985;34:323-331.
22. Kim DH, Gambardella RA, Elattrache NS, Yocum LA, Jobe FW. Arthroscopic treatment of posterolateral elbow impingement from lateral synovial plicae in throwing athletes and golfers. Am J Sports Med. 2006;34(3):438-444.
23. Antuna SA, O’Driscoll SW. Snapping plicae associated with radiocapitellar chondromalacia. Arthroscopy. 2001;17(5):491-495.
24. Bernstein AD, Breslow MJ, Jazrawi LM. Distal biceps tendon ruptures: a historical perspective and current concepts. Am J Orthop. 2001;30(3):
193-200.
25. O’Driscoll SW, Goncalves LB, Dietz P. The hook test for distal biceps tendon avulsion. Am J Sports Med. 2007;35(11):1865-1869.
1. Elliott B, Fleisig G, Nicholls R, Escamilia R. Technique effects on upper limb loading in the tennis serve. J Sci Med Sport. 2003;6(1):76-87.
2. Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23(2):233-239.
3. Werner SL, Fleisig GS, Dillman CJ, Andrews JR. Biomechanics of the elbow during baseball pitching. J Orthop Sports Phys Ther. 1993;17(6):274-278.
4. Aval SM, Durand P Jr, Shankwiler JA. Neurovascular injuries to the athlete’s shoulder: part II. J Am Acad Orthop Surg. 2007;15(5):281-289.
5. Strukel RJ, Garrick JG. Thoracic outlet compression in athletes: a report of four cases. Am J Sports Med. 1978;6(2):35-39.
6. Dines JS, Frank JB, Akerman M, Yocum LA. Glenohumeral internal rotation deficits in baseball players with ulnar collateral ligament insufficiency. Am J Sports Med. 2009;37(3):566-570.
7. Adams JE. Injury to the throwing arm. A study of traumatic changes in the elbow joints of boy baseball players. Calif Med. 1965;102:127-132.
8. Hang DW, Chao CM, Hang YS. A clinical and roentgenographic study of Little League elbow. Am J Sports Med. 2004;32(1):79-84.
9. King JW, Brelsford HJ, Tullos HS. Analysis of the pitching arm of the professional baseball pitcher. Clin Orthop. 1969;(67):116-123.
10. Cain EL Jr, Dugas JR, Wolf RS, Andrews JR. Elbow injuries in throwing athletes: a current concepts review. Am J Sports Med. 2003;31(4):621-635.
11. Safran M, Ahmad CS, Elattrache NS. Ulnar collateral ligament of the elbow. Arthroscopy. 2005;21(11):1381-1395.
12. O’Driscoll SW, Lawton RL, Smith AM. The “moving valgus stress test” for medial collateral ligament tears of the elbow. Am J Sports Med. 2005;33(2):231-239.
13. O’Driscoll SW. Valgus extension overload and plica. In: Levine WN, ed. The Athlete’s Elbow. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008:71-83.
14. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes. Treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
15. Andrews JR, Whiteside JA, Buettner CM. Clinical evaluation of the elbow in throwers. Oper Tech Sports Med. 1996;4(2):77-83.
16. Calfee RP, Manske PR, Gelberman RH, Van Steyn MO, Steffen J, Goldfarb CA. Clinical assessment of the ulnar nerve at the elbow: reliability of instability testing and the association of hypermobility with clinical symptoms. J Bone Joint Surg Am. 2010;92(17):2801-2808.
17. Spinner RJ, Goldner RD. Snapping of the medial head of the triceps and recurrent dislocation of the ulnar nerve. Anatomical and dynamic factors. J Bone Joint Surg Am. 1998;80(2):239-247.
18. Guerra JJ, Timmerman LA. Clinical anatomy, histology, & pathomechanics of the elbow in sports. Oper Tech Sports Med. 1996;4(2):69-76.
19. O’Driscoll SW, Horii E, Carmichael SW, Morrey BF. The cubital tunnel and ulnar neuropathy. J Bone Joint Surg Br. 1991;73(4):613-617.
20. Novak CB, Lee GW, Mackinnon SE, Lay L. Provocative testing for cubital tunnel syndrome. J Hand Surg Am. 1994;19(5):817-820.
21. Andrews JR. Bony injuries about the elbow in the throwing athlete. Instr Course Lect. 1985;34:323-331.
22. Kim DH, Gambardella RA, Elattrache NS, Yocum LA, Jobe FW. Arthroscopic treatment of posterolateral elbow impingement from lateral synovial plicae in throwing athletes and golfers. Am J Sports Med. 2006;34(3):438-444.
23. Antuna SA, O’Driscoll SW. Snapping plicae associated with radiocapitellar chondromalacia. Arthroscopy. 2001;17(5):491-495.
24. Bernstein AD, Breslow MJ, Jazrawi LM. Distal biceps tendon ruptures: a historical perspective and current concepts. Am J Orthop. 2001;30(3):
193-200.
25. O’Driscoll SW, Goncalves LB, Dietz P. The hook test for distal biceps tendon avulsion. Am J Sports Med. 2007;35(11):1865-1869.
Teenage Baseball Pitchers at Increased Risk of Permanent Shoulder Injury
Young baseball pitchers who throw more than 100 pitches per week are at risk for a newly identified overuse injury that can impede normal shoulder development and lead to additional problems, including rotator cuff tears, according to a study published online ahead of print October 14 in Radiology.
The injury, termed acromial apophysiolysis by the researchers, is characterized by incomplete fusion and tenderness at the acromion. The acromion, which forms the bone at the top of the shoulder, typically develops from four individual bones into one bone during the teenage years.
“We kept seeing this injury over and over again in young athletes who come to the hospital at the end of the baseball season with shoulder pain and edema at the acromion on MRI, but no other imaging findings,” said Johannes B. Roedl, MD, a radiologist in the Musculoskeletal Division at Thomas Jefferson University Hospital in Philadelphia.
Dr. Roedl and a team of researchers conducted a retrospective study of 2,372 consecutive patients between the ages of 15 and 25, who underwent magnetic resonance imaging (MRI) for shoulder pain between 1998 and 2012. The majority of the patients, which included both males and females, were baseball pitchers.
Patients with edema at the acromial apophyses and no other abnormalities on MRI were included in the study group. Association of acromial edema with incomplete fusion , pitching, and clinical findings was determined in the study group and in an age- and sex-matched control group. Association with the development of an os acromial and rotator cuff tears later in life was assessed with follow-up imaging after age 25.
Edema at the acromial apophyses was found in 2.6% (61 of 2,372) and was associated with incomplete fusion of the acromial apophyses and superior shoulder tenderness.
A pitch count of more than 100 pitches per week was a substantial risk factor for developing acromial apophysiolysis (odds ratio 6.5). Among the patients with this overuse injury, 40% threw more than 100 pitches per week, compared to 8% in the control group.
All 61 injured patients took a three-month rest from pitching. One patient underwent surgery while the remaining 60 patients were treated conservatively with non-steroidal pain medication. Follow-up imaging conducted a minimum of two years later after the patients turned 25 were available for 29 of the 61 injured patients and for 23 of the 61 controls. Follow-up imaging revealed that 25 of the 29 patients (86%) with the overuse injury showed incomplete fusion of the acromion, compared to only one of the 23 (4%) controls.
Twenty-one of the 29 patients with the overuse injury continued pitching after the rest period, and all 21 showed incomplete bone fusion at the acromion. Rotator cuff tears were significantly more common among this group than in the control group (68% versus 29%, respectively). The severity of the rotator cuff tears was also higher in the overuse injury group compared with the control group.
“More and more kids are entering sports earlier in life and are overtraining,” said Dr. Roedl. “Baseball players who pitch too much are at risk of developing a stress response and overuse injury to the acromion. It is important to limit stress to the growing bones to allow them to develop normally.”
Suggested Reading
Roedl JB, Morrison WB, Ciccotti MG, Zoga AC. Acromial apophysiolysis: superior shoulder pain and acromial nonfusion in the young throwing athlete. Radiology. 2014 Oct 14:140587 [Epub ahead of print].
Young baseball pitchers who throw more than 100 pitches per week are at risk for a newly identified overuse injury that can impede normal shoulder development and lead to additional problems, including rotator cuff tears, according to a study published online ahead of print October 14 in Radiology.
The injury, termed acromial apophysiolysis by the researchers, is characterized by incomplete fusion and tenderness at the acromion. The acromion, which forms the bone at the top of the shoulder, typically develops from four individual bones into one bone during the teenage years.
“We kept seeing this injury over and over again in young athletes who come to the hospital at the end of the baseball season with shoulder pain and edema at the acromion on MRI, but no other imaging findings,” said Johannes B. Roedl, MD, a radiologist in the Musculoskeletal Division at Thomas Jefferson University Hospital in Philadelphia.
Dr. Roedl and a team of researchers conducted a retrospective study of 2,372 consecutive patients between the ages of 15 and 25, who underwent magnetic resonance imaging (MRI) for shoulder pain between 1998 and 2012. The majority of the patients, which included both males and females, were baseball pitchers.
Patients with edema at the acromial apophyses and no other abnormalities on MRI were included in the study group. Association of acromial edema with incomplete fusion , pitching, and clinical findings was determined in the study group and in an age- and sex-matched control group. Association with the development of an os acromial and rotator cuff tears later in life was assessed with follow-up imaging after age 25.
Edema at the acromial apophyses was found in 2.6% (61 of 2,372) and was associated with incomplete fusion of the acromial apophyses and superior shoulder tenderness.
A pitch count of more than 100 pitches per week was a substantial risk factor for developing acromial apophysiolysis (odds ratio 6.5). Among the patients with this overuse injury, 40% threw more than 100 pitches per week, compared to 8% in the control group.
All 61 injured patients took a three-month rest from pitching. One patient underwent surgery while the remaining 60 patients were treated conservatively with non-steroidal pain medication. Follow-up imaging conducted a minimum of two years later after the patients turned 25 were available for 29 of the 61 injured patients and for 23 of the 61 controls. Follow-up imaging revealed that 25 of the 29 patients (86%) with the overuse injury showed incomplete fusion of the acromion, compared to only one of the 23 (4%) controls.
Twenty-one of the 29 patients with the overuse injury continued pitching after the rest period, and all 21 showed incomplete bone fusion at the acromion. Rotator cuff tears were significantly more common among this group than in the control group (68% versus 29%, respectively). The severity of the rotator cuff tears was also higher in the overuse injury group compared with the control group.
“More and more kids are entering sports earlier in life and are overtraining,” said Dr. Roedl. “Baseball players who pitch too much are at risk of developing a stress response and overuse injury to the acromion. It is important to limit stress to the growing bones to allow them to develop normally.”
Young baseball pitchers who throw more than 100 pitches per week are at risk for a newly identified overuse injury that can impede normal shoulder development and lead to additional problems, including rotator cuff tears, according to a study published online ahead of print October 14 in Radiology.
The injury, termed acromial apophysiolysis by the researchers, is characterized by incomplete fusion and tenderness at the acromion. The acromion, which forms the bone at the top of the shoulder, typically develops from four individual bones into one bone during the teenage years.
“We kept seeing this injury over and over again in young athletes who come to the hospital at the end of the baseball season with shoulder pain and edema at the acromion on MRI, but no other imaging findings,” said Johannes B. Roedl, MD, a radiologist in the Musculoskeletal Division at Thomas Jefferson University Hospital in Philadelphia.
Dr. Roedl and a team of researchers conducted a retrospective study of 2,372 consecutive patients between the ages of 15 and 25, who underwent magnetic resonance imaging (MRI) for shoulder pain between 1998 and 2012. The majority of the patients, which included both males and females, were baseball pitchers.
Patients with edema at the acromial apophyses and no other abnormalities on MRI were included in the study group. Association of acromial edema with incomplete fusion , pitching, and clinical findings was determined in the study group and in an age- and sex-matched control group. Association with the development of an os acromial and rotator cuff tears later in life was assessed with follow-up imaging after age 25.
Edema at the acromial apophyses was found in 2.6% (61 of 2,372) and was associated with incomplete fusion of the acromial apophyses and superior shoulder tenderness.
A pitch count of more than 100 pitches per week was a substantial risk factor for developing acromial apophysiolysis (odds ratio 6.5). Among the patients with this overuse injury, 40% threw more than 100 pitches per week, compared to 8% in the control group.
All 61 injured patients took a three-month rest from pitching. One patient underwent surgery while the remaining 60 patients were treated conservatively with non-steroidal pain medication. Follow-up imaging conducted a minimum of two years later after the patients turned 25 were available for 29 of the 61 injured patients and for 23 of the 61 controls. Follow-up imaging revealed that 25 of the 29 patients (86%) with the overuse injury showed incomplete fusion of the acromion, compared to only one of the 23 (4%) controls.
Twenty-one of the 29 patients with the overuse injury continued pitching after the rest period, and all 21 showed incomplete bone fusion at the acromion. Rotator cuff tears were significantly more common among this group than in the control group (68% versus 29%, respectively). The severity of the rotator cuff tears was also higher in the overuse injury group compared with the control group.
“More and more kids are entering sports earlier in life and are overtraining,” said Dr. Roedl. “Baseball players who pitch too much are at risk of developing a stress response and overuse injury to the acromion. It is important to limit stress to the growing bones to allow them to develop normally.”
Suggested Reading
Roedl JB, Morrison WB, Ciccotti MG, Zoga AC. Acromial apophysiolysis: superior shoulder pain and acromial nonfusion in the young throwing athlete. Radiology. 2014 Oct 14:140587 [Epub ahead of print].
Suggested Reading
Roedl JB, Morrison WB, Ciccotti MG, Zoga AC. Acromial apophysiolysis: superior shoulder pain and acromial nonfusion in the young throwing athlete. Radiology. 2014 Oct 14:140587 [Epub ahead of print].
Concurrent Treatment of a Middle-Third Clavicle Fracture and Type IV Acromioclavicular Dislocation
Acromioclavicular (AC) dislocations and displaced fractures of the middle third of the clavicle rarely occur together. Isolated AC joint separation is often treated nonoperatively with internal coracoclavicular (CC) fixation or reconstruction considered for type IV-VI AC dislocations and some type III injuries.1 Isolated clavicle fractures traditionally have been treated nonoperatively. The current trend is toward internal fixation for displaced and shortened fractures.2 There have been only a handful of reports of concomitant AC dislocation and midshaft clavicle fracture.3-6 Previous treatments have included nonoperative treatment, AC fixation, or internal fixation of the clavicle with ligamentous reconstruction.
We present a previously undescribed technique for internal fixation of this rare shoulder injury. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
While driving an all-terrain vehicle, a healthy 19-year-old right-hand–dominant man hit a bridge and sustained direct impact to his right shoulder. He presented to the emergency department complaining of right shoulder pain and deformity without skin disruption, vascular insufficiency, or neurologic symptoms. Anteroposterior (AP) radiograph showed an oblique, displaced, middle-third clavicle shaft fracture (Figure 1). An associated type IV AC dislocation was confirmed on axillary radiograph (Figure 2) and on an axial cut from a trauma chest computed tomography (CT) scan (Figure 3). The patient was discharged home from the trauma service the next day with a sling for comfort and plans for delayed, elective operative fixation 1 week later.
The patient was placed in a beach-chair position. Through a longitudinal incision extending laterally over the AC joint, the clavicle was exposed for fracture reduction, with care taken to retain soft-tissue attachments. The distal clavicle was buttonholed posteriorly through the trapezius muscle and fascia. The distal fracture fragment was devoid of any remaining CC ligamentous attachment. After satisfactory reduction, a low-profile precontoured clavicle plate (Superior Midshaft Clavicle Plate; Acumed, Hillsboro, Oregon) was placed superiorly; the fracture was compressed through the plate and internally fixed with three 3.5-mm bicortical screws on both sides of the fracture. Approximately 5 mm of the distal clavicle was resected at the AC joint to facilitate adequate AC and CC reduction without disruption of the clavicle fracture. With an adequate CC reduction, a 3.5-mm fully threaded cortical screw was placed through the most distal hole in the clavicle plate, clavicle, and coracoid.
After surgery, the patient was placed into an ARC shoulder immobilizer (Bledsoe, Grand Prairie, Texas) for 6 weeks, removing the immobilizer only for elbow and wrist range of motion (ROM) exercises. Radiographs at 3-month follow-up (Figure 4) showed a healed fracture with no loss of AC or CC reduction. Three months after surgery, another procedure was performed to remove the CC screw. One year after the initial surgery, the patient complained of intermittent soreness over the lateral shoulder but was not limited in his activities and was back to performing manual labor without difficulty. He had full ROM in forward flexion, abduction, internal rotation, and external rotation without weakness, tenderness, or any neurovascular deficit. After CC screw removal, no deformity returned at the shoulder. Radiographs showed a healed fracture with minimal superior subluxation at the AC joint without significant change from the 3-month follow-up (Figure 5).
Discussion
The combined injury pattern of a type IV AC dislocation and a displaced middle-third clavicle shaft fracture is rare. The usual mechanism of injury, as seen in the present case, is a direct blow to the shoulder at the tip of the acromion, though indirect forces from a fall on an outstretched hand are also described.7 Disruption of the CC ligaments with AC separation likely dissipates the stress necessary to create a clavicle fracture in most cases,1 explaining the rarity of this injury. It is imperative to evaluate patients for injury to both the osseous and ligamentous structures.
Previous case reports of concomitant AC separation and midshaft clavicle fracture have described a variety of treatment options, but to date our case represents the only episode in which both the clavicle fracture and the AC joint were treated with open reduction and internal fixation (ORIF). Wurtz and colleagues5 reported on a series of 4 patients with AC disruption and middle-third clavicle fracture. Three of the 4 patients had type IV AC separation; all 3 were treated, 2 acutely and 1 chronically, with open reduction of the AC and CC joints; 2 of these patients had CC screw fixation only after reduction, and the third had 2 Steinmann pins placed across the AC joint without CC screw fixation. All hardware was removed after 12 weeks. The fourth patient had a type II AC dislocation and was treated with closed reduction of the clavicle with no intervention for the AC joint. None of the clavicle fractures in this series were treated with internal fixation. All patients had full and pain-free ROM at 1- to 3-year follow-up.
Juhn and Simonian3 reported on a case of type VI separation with greenstick midshaft clavicle fracture in a hockey player seen 7 days after injury. The patient described some tingling in the upper extremity and had shoulder pain on initial presentation but was noted to have minimal displacement of both the AC joint and the midshaft clavicle fracture. Both injuries were treated nonsurgically with good outcome, and the patient returned to full activity (including hockey) within 14 weeks after injury.
Lancourt4 described the case of a patient with a type V AC dislocation and a displaced midshaft clavicle fracture. The AC joint was treated with Steinmann pin fixation, and the clavicle fracture was treated nonoperatively. The author cited high complication rates of plate fixation for clavicle fractures as the reason for not performing the additional procedure. The pins were removed 8 weeks after surgery. At 3-year follow-up, the patient had good radiographic and clinical outcome.
Yeh and colleagues6 described a patient who sustained a displaced midshaft clavicle fracture and a type IV AC dislocation in a fall from a horse. The patient underwent ORIF of the clavicle fracture with plate fixation. After the procedure, the AC joint was still unstable intraoperatively, and the AC and CC ligaments were reconstructed with semitendinosus allograft. The patient had full and painless ROM at 1-year follow-up.
The present case report serves as a reminder to obtain adequate shoulder radiographs when evaluating “just another clavicle fracture.” The radiographs should include a good axillary view to ensure there is not an associated AC dislocation. Increasingly, some authors have been advocating internal fixation for clavicle fractures, with reports of improved functional outcomes, improved cosmesis, and increased union rates.2 Indications for operative fixation include shortening and 100% displacement,8 and relative indications include open fractures.1 Operative fixation is perhaps more important for younger, athletic, and manual-labor populations. The trend in treatment of clavicle fractures toward operative fixation lends itself well to ORIF of the AC and CC joints; hence, a modern treatment for this rarely described combination injury should include internal plate fixation of the clavicle in addition to CC fixation. This additional procedure requires little extra time and energy in an operative scenario already requiring anesthesia, with easy insertion of the CC screw through the clavicle plate. Use of a CC screw obviates any potential risks associated with use of allograft tissue, and there is no anticipated difficulty with screw removal at 12 weeks.
Alternative options for AC stability include CC reconstruction with ligamentous allograft, ligamentous autograft, or suture/tightrope techniques. A noted advantage of these alternative techniques is less need to return to the operating room for the hardware removal that is recommended with CC screw fixation. However, these procedures potentially increase surgical exposure and operating time. In addition, screw fixation minimizes the possibility of donor-site morbidity from autograft transfers and potential complications from allograft tissue.
Hook plate fixation of the AC joint has also been described. In a recent case report of a similar injury pattern, plate fixation of the clavicle with simultaneous hook plate fixation of the AC joint was described.9 The patient did well but required removal of hardware of the hook plate and the clavicle plate 1 and 3 years after surgery, respectively. Although screw fixation is biomechanically stronger, debate persists about the clinical importance of this increase in strength.1 In the setting of plate fixation for the clavicle, these alternative AC fixations would require technique adjustments, including length of grafts and/or sutures, and raise concerns regarding interaction of the metal with the fixation material.
Critical evaluation of our technique revealed a lucency larger than the screw (Figure 5). However, the screw was not clinically loose at removal. This potential complication, in combination with the bent screw (Figure 4) before removal, highlights the concern for screw breakage with this technique, given the increased construct stiffness caused by the added plate.
Conclusion
As in the other reports mentioned, our patient had an excellent clinical and radiographic outcome. It could be inferred that, if fixation for isolated clavicle fractures demonstrates improved function, better outcomes would be seen for higher-energy fractures associated with AC dislocation. Given the current trend toward surgical fixation for certain clavicle fractures, we recommend that clavicle fractures associated with type IV AC dislocation be treated with ORIF of both injuries.
1. Ring D, Jupiter J. Injuries to the shoulder girdle. In: Browner, BD. Skeletal Trauma. Philadelphia, PA: Elsevier Health Sciences; 2008:1755-1778.
2. Altamimi SA, McKee MD; Canadian Orthopaedic Trauma Society. Nonoperative treatment compared with plate fixation of displaced midshaft clavicular fractures. Surgical technique. J Bone Joint Surg Am. 2008;90(suppl 2 pt 1):1-8.
3. Juhn MS, Simonian PT. Type VI acromioclavicular separation with middle-third clavicle fracture in an ice hockey player. Clin J Sports Med. 2002;12(5):315-317.
4. Lancourt JE. Acromioclavicular dislocation with adjacent clavicular fracture in a horseback rider. A case report. Am J Sports Med. 1990;18(3):321-322.
5. Wurtz LD, Lyons FA, Rockwood CA Jr. Fracture of the middle third of the clavicle and dislocation of the acromioclavicular joint. A report of four cases. J Bone Joint Surg Am. 1992;74(1):133-137.
6. Yeh PC, Miller SR, Cunningham JG, Sethi PM. Midshaft clavicle fracture and acromioclavicular dislocation: a case report of a rare injury. J Shoulder Elbow Surg. 2009;18(5):e1-e4.
7. Stanley D, Trowbridge EA, Norris SH. The mechanism of clavicular fracture. A clinical and biomechanical analysis. J Bone Joint Surg Br. 1988;70(3):461-464.
8. Kim W, McKee MD. Management of acute clavicle fractures. Orthop Clin North Am. 2008;39(4):491-505.
9. Woolf SK, Valentine BJ, Barfield WR, Hartsock LA. Middle-third clavicle fracture with associated type IV acromioclavicular separation: case report and literature review. J Surg Orthop Adv. 2013;22(2):183-186.
Acromioclavicular (AC) dislocations and displaced fractures of the middle third of the clavicle rarely occur together. Isolated AC joint separation is often treated nonoperatively with internal coracoclavicular (CC) fixation or reconstruction considered for type IV-VI AC dislocations and some type III injuries.1 Isolated clavicle fractures traditionally have been treated nonoperatively. The current trend is toward internal fixation for displaced and shortened fractures.2 There have been only a handful of reports of concomitant AC dislocation and midshaft clavicle fracture.3-6 Previous treatments have included nonoperative treatment, AC fixation, or internal fixation of the clavicle with ligamentous reconstruction.
We present a previously undescribed technique for internal fixation of this rare shoulder injury. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
While driving an all-terrain vehicle, a healthy 19-year-old right-hand–dominant man hit a bridge and sustained direct impact to his right shoulder. He presented to the emergency department complaining of right shoulder pain and deformity without skin disruption, vascular insufficiency, or neurologic symptoms. Anteroposterior (AP) radiograph showed an oblique, displaced, middle-third clavicle shaft fracture (Figure 1). An associated type IV AC dislocation was confirmed on axillary radiograph (Figure 2) and on an axial cut from a trauma chest computed tomography (CT) scan (Figure 3). The patient was discharged home from the trauma service the next day with a sling for comfort and plans for delayed, elective operative fixation 1 week later.
The patient was placed in a beach-chair position. Through a longitudinal incision extending laterally over the AC joint, the clavicle was exposed for fracture reduction, with care taken to retain soft-tissue attachments. The distal clavicle was buttonholed posteriorly through the trapezius muscle and fascia. The distal fracture fragment was devoid of any remaining CC ligamentous attachment. After satisfactory reduction, a low-profile precontoured clavicle plate (Superior Midshaft Clavicle Plate; Acumed, Hillsboro, Oregon) was placed superiorly; the fracture was compressed through the plate and internally fixed with three 3.5-mm bicortical screws on both sides of the fracture. Approximately 5 mm of the distal clavicle was resected at the AC joint to facilitate adequate AC and CC reduction without disruption of the clavicle fracture. With an adequate CC reduction, a 3.5-mm fully threaded cortical screw was placed through the most distal hole in the clavicle plate, clavicle, and coracoid.
After surgery, the patient was placed into an ARC shoulder immobilizer (Bledsoe, Grand Prairie, Texas) for 6 weeks, removing the immobilizer only for elbow and wrist range of motion (ROM) exercises. Radiographs at 3-month follow-up (Figure 4) showed a healed fracture with no loss of AC or CC reduction. Three months after surgery, another procedure was performed to remove the CC screw. One year after the initial surgery, the patient complained of intermittent soreness over the lateral shoulder but was not limited in his activities and was back to performing manual labor without difficulty. He had full ROM in forward flexion, abduction, internal rotation, and external rotation without weakness, tenderness, or any neurovascular deficit. After CC screw removal, no deformity returned at the shoulder. Radiographs showed a healed fracture with minimal superior subluxation at the AC joint without significant change from the 3-month follow-up (Figure 5).
Discussion
The combined injury pattern of a type IV AC dislocation and a displaced middle-third clavicle shaft fracture is rare. The usual mechanism of injury, as seen in the present case, is a direct blow to the shoulder at the tip of the acromion, though indirect forces from a fall on an outstretched hand are also described.7 Disruption of the CC ligaments with AC separation likely dissipates the stress necessary to create a clavicle fracture in most cases,1 explaining the rarity of this injury. It is imperative to evaluate patients for injury to both the osseous and ligamentous structures.
Previous case reports of concomitant AC separation and midshaft clavicle fracture have described a variety of treatment options, but to date our case represents the only episode in which both the clavicle fracture and the AC joint were treated with open reduction and internal fixation (ORIF). Wurtz and colleagues5 reported on a series of 4 patients with AC disruption and middle-third clavicle fracture. Three of the 4 patients had type IV AC separation; all 3 were treated, 2 acutely and 1 chronically, with open reduction of the AC and CC joints; 2 of these patients had CC screw fixation only after reduction, and the third had 2 Steinmann pins placed across the AC joint without CC screw fixation. All hardware was removed after 12 weeks. The fourth patient had a type II AC dislocation and was treated with closed reduction of the clavicle with no intervention for the AC joint. None of the clavicle fractures in this series were treated with internal fixation. All patients had full and pain-free ROM at 1- to 3-year follow-up.
Juhn and Simonian3 reported on a case of type VI separation with greenstick midshaft clavicle fracture in a hockey player seen 7 days after injury. The patient described some tingling in the upper extremity and had shoulder pain on initial presentation but was noted to have minimal displacement of both the AC joint and the midshaft clavicle fracture. Both injuries were treated nonsurgically with good outcome, and the patient returned to full activity (including hockey) within 14 weeks after injury.
Lancourt4 described the case of a patient with a type V AC dislocation and a displaced midshaft clavicle fracture. The AC joint was treated with Steinmann pin fixation, and the clavicle fracture was treated nonoperatively. The author cited high complication rates of plate fixation for clavicle fractures as the reason for not performing the additional procedure. The pins were removed 8 weeks after surgery. At 3-year follow-up, the patient had good radiographic and clinical outcome.
Yeh and colleagues6 described a patient who sustained a displaced midshaft clavicle fracture and a type IV AC dislocation in a fall from a horse. The patient underwent ORIF of the clavicle fracture with plate fixation. After the procedure, the AC joint was still unstable intraoperatively, and the AC and CC ligaments were reconstructed with semitendinosus allograft. The patient had full and painless ROM at 1-year follow-up.
The present case report serves as a reminder to obtain adequate shoulder radiographs when evaluating “just another clavicle fracture.” The radiographs should include a good axillary view to ensure there is not an associated AC dislocation. Increasingly, some authors have been advocating internal fixation for clavicle fractures, with reports of improved functional outcomes, improved cosmesis, and increased union rates.2 Indications for operative fixation include shortening and 100% displacement,8 and relative indications include open fractures.1 Operative fixation is perhaps more important for younger, athletic, and manual-labor populations. The trend in treatment of clavicle fractures toward operative fixation lends itself well to ORIF of the AC and CC joints; hence, a modern treatment for this rarely described combination injury should include internal plate fixation of the clavicle in addition to CC fixation. This additional procedure requires little extra time and energy in an operative scenario already requiring anesthesia, with easy insertion of the CC screw through the clavicle plate. Use of a CC screw obviates any potential risks associated with use of allograft tissue, and there is no anticipated difficulty with screw removal at 12 weeks.
Alternative options for AC stability include CC reconstruction with ligamentous allograft, ligamentous autograft, or suture/tightrope techniques. A noted advantage of these alternative techniques is less need to return to the operating room for the hardware removal that is recommended with CC screw fixation. However, these procedures potentially increase surgical exposure and operating time. In addition, screw fixation minimizes the possibility of donor-site morbidity from autograft transfers and potential complications from allograft tissue.
Hook plate fixation of the AC joint has also been described. In a recent case report of a similar injury pattern, plate fixation of the clavicle with simultaneous hook plate fixation of the AC joint was described.9 The patient did well but required removal of hardware of the hook plate and the clavicle plate 1 and 3 years after surgery, respectively. Although screw fixation is biomechanically stronger, debate persists about the clinical importance of this increase in strength.1 In the setting of plate fixation for the clavicle, these alternative AC fixations would require technique adjustments, including length of grafts and/or sutures, and raise concerns regarding interaction of the metal with the fixation material.
Critical evaluation of our technique revealed a lucency larger than the screw (Figure 5). However, the screw was not clinically loose at removal. This potential complication, in combination with the bent screw (Figure 4) before removal, highlights the concern for screw breakage with this technique, given the increased construct stiffness caused by the added plate.
Conclusion
As in the other reports mentioned, our patient had an excellent clinical and radiographic outcome. It could be inferred that, if fixation for isolated clavicle fractures demonstrates improved function, better outcomes would be seen for higher-energy fractures associated with AC dislocation. Given the current trend toward surgical fixation for certain clavicle fractures, we recommend that clavicle fractures associated with type IV AC dislocation be treated with ORIF of both injuries.
Acromioclavicular (AC) dislocations and displaced fractures of the middle third of the clavicle rarely occur together. Isolated AC joint separation is often treated nonoperatively with internal coracoclavicular (CC) fixation or reconstruction considered for type IV-VI AC dislocations and some type III injuries.1 Isolated clavicle fractures traditionally have been treated nonoperatively. The current trend is toward internal fixation for displaced and shortened fractures.2 There have been only a handful of reports of concomitant AC dislocation and midshaft clavicle fracture.3-6 Previous treatments have included nonoperative treatment, AC fixation, or internal fixation of the clavicle with ligamentous reconstruction.
We present a previously undescribed technique for internal fixation of this rare shoulder injury. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
While driving an all-terrain vehicle, a healthy 19-year-old right-hand–dominant man hit a bridge and sustained direct impact to his right shoulder. He presented to the emergency department complaining of right shoulder pain and deformity without skin disruption, vascular insufficiency, or neurologic symptoms. Anteroposterior (AP) radiograph showed an oblique, displaced, middle-third clavicle shaft fracture (Figure 1). An associated type IV AC dislocation was confirmed on axillary radiograph (Figure 2) and on an axial cut from a trauma chest computed tomography (CT) scan (Figure 3). The patient was discharged home from the trauma service the next day with a sling for comfort and plans for delayed, elective operative fixation 1 week later.
The patient was placed in a beach-chair position. Through a longitudinal incision extending laterally over the AC joint, the clavicle was exposed for fracture reduction, with care taken to retain soft-tissue attachments. The distal clavicle was buttonholed posteriorly through the trapezius muscle and fascia. The distal fracture fragment was devoid of any remaining CC ligamentous attachment. After satisfactory reduction, a low-profile precontoured clavicle plate (Superior Midshaft Clavicle Plate; Acumed, Hillsboro, Oregon) was placed superiorly; the fracture was compressed through the plate and internally fixed with three 3.5-mm bicortical screws on both sides of the fracture. Approximately 5 mm of the distal clavicle was resected at the AC joint to facilitate adequate AC and CC reduction without disruption of the clavicle fracture. With an adequate CC reduction, a 3.5-mm fully threaded cortical screw was placed through the most distal hole in the clavicle plate, clavicle, and coracoid.
After surgery, the patient was placed into an ARC shoulder immobilizer (Bledsoe, Grand Prairie, Texas) for 6 weeks, removing the immobilizer only for elbow and wrist range of motion (ROM) exercises. Radiographs at 3-month follow-up (Figure 4) showed a healed fracture with no loss of AC or CC reduction. Three months after surgery, another procedure was performed to remove the CC screw. One year after the initial surgery, the patient complained of intermittent soreness over the lateral shoulder but was not limited in his activities and was back to performing manual labor without difficulty. He had full ROM in forward flexion, abduction, internal rotation, and external rotation without weakness, tenderness, or any neurovascular deficit. After CC screw removal, no deformity returned at the shoulder. Radiographs showed a healed fracture with minimal superior subluxation at the AC joint without significant change from the 3-month follow-up (Figure 5).
Discussion
The combined injury pattern of a type IV AC dislocation and a displaced middle-third clavicle shaft fracture is rare. The usual mechanism of injury, as seen in the present case, is a direct blow to the shoulder at the tip of the acromion, though indirect forces from a fall on an outstretched hand are also described.7 Disruption of the CC ligaments with AC separation likely dissipates the stress necessary to create a clavicle fracture in most cases,1 explaining the rarity of this injury. It is imperative to evaluate patients for injury to both the osseous and ligamentous structures.
Previous case reports of concomitant AC separation and midshaft clavicle fracture have described a variety of treatment options, but to date our case represents the only episode in which both the clavicle fracture and the AC joint were treated with open reduction and internal fixation (ORIF). Wurtz and colleagues5 reported on a series of 4 patients with AC disruption and middle-third clavicle fracture. Three of the 4 patients had type IV AC separation; all 3 were treated, 2 acutely and 1 chronically, with open reduction of the AC and CC joints; 2 of these patients had CC screw fixation only after reduction, and the third had 2 Steinmann pins placed across the AC joint without CC screw fixation. All hardware was removed after 12 weeks. The fourth patient had a type II AC dislocation and was treated with closed reduction of the clavicle with no intervention for the AC joint. None of the clavicle fractures in this series were treated with internal fixation. All patients had full and pain-free ROM at 1- to 3-year follow-up.
Juhn and Simonian3 reported on a case of type VI separation with greenstick midshaft clavicle fracture in a hockey player seen 7 days after injury. The patient described some tingling in the upper extremity and had shoulder pain on initial presentation but was noted to have minimal displacement of both the AC joint and the midshaft clavicle fracture. Both injuries were treated nonsurgically with good outcome, and the patient returned to full activity (including hockey) within 14 weeks after injury.
Lancourt4 described the case of a patient with a type V AC dislocation and a displaced midshaft clavicle fracture. The AC joint was treated with Steinmann pin fixation, and the clavicle fracture was treated nonoperatively. The author cited high complication rates of plate fixation for clavicle fractures as the reason for not performing the additional procedure. The pins were removed 8 weeks after surgery. At 3-year follow-up, the patient had good radiographic and clinical outcome.
Yeh and colleagues6 described a patient who sustained a displaced midshaft clavicle fracture and a type IV AC dislocation in a fall from a horse. The patient underwent ORIF of the clavicle fracture with plate fixation. After the procedure, the AC joint was still unstable intraoperatively, and the AC and CC ligaments were reconstructed with semitendinosus allograft. The patient had full and painless ROM at 1-year follow-up.
The present case report serves as a reminder to obtain adequate shoulder radiographs when evaluating “just another clavicle fracture.” The radiographs should include a good axillary view to ensure there is not an associated AC dislocation. Increasingly, some authors have been advocating internal fixation for clavicle fractures, with reports of improved functional outcomes, improved cosmesis, and increased union rates.2 Indications for operative fixation include shortening and 100% displacement,8 and relative indications include open fractures.1 Operative fixation is perhaps more important for younger, athletic, and manual-labor populations. The trend in treatment of clavicle fractures toward operative fixation lends itself well to ORIF of the AC and CC joints; hence, a modern treatment for this rarely described combination injury should include internal plate fixation of the clavicle in addition to CC fixation. This additional procedure requires little extra time and energy in an operative scenario already requiring anesthesia, with easy insertion of the CC screw through the clavicle plate. Use of a CC screw obviates any potential risks associated with use of allograft tissue, and there is no anticipated difficulty with screw removal at 12 weeks.
Alternative options for AC stability include CC reconstruction with ligamentous allograft, ligamentous autograft, or suture/tightrope techniques. A noted advantage of these alternative techniques is less need to return to the operating room for the hardware removal that is recommended with CC screw fixation. However, these procedures potentially increase surgical exposure and operating time. In addition, screw fixation minimizes the possibility of donor-site morbidity from autograft transfers and potential complications from allograft tissue.
Hook plate fixation of the AC joint has also been described. In a recent case report of a similar injury pattern, plate fixation of the clavicle with simultaneous hook plate fixation of the AC joint was described.9 The patient did well but required removal of hardware of the hook plate and the clavicle plate 1 and 3 years after surgery, respectively. Although screw fixation is biomechanically stronger, debate persists about the clinical importance of this increase in strength.1 In the setting of plate fixation for the clavicle, these alternative AC fixations would require technique adjustments, including length of grafts and/or sutures, and raise concerns regarding interaction of the metal with the fixation material.
Critical evaluation of our technique revealed a lucency larger than the screw (Figure 5). However, the screw was not clinically loose at removal. This potential complication, in combination with the bent screw (Figure 4) before removal, highlights the concern for screw breakage with this technique, given the increased construct stiffness caused by the added plate.
Conclusion
As in the other reports mentioned, our patient had an excellent clinical and radiographic outcome. It could be inferred that, if fixation for isolated clavicle fractures demonstrates improved function, better outcomes would be seen for higher-energy fractures associated with AC dislocation. Given the current trend toward surgical fixation for certain clavicle fractures, we recommend that clavicle fractures associated with type IV AC dislocation be treated with ORIF of both injuries.
1. Ring D, Jupiter J. Injuries to the shoulder girdle. In: Browner, BD. Skeletal Trauma. Philadelphia, PA: Elsevier Health Sciences; 2008:1755-1778.
2. Altamimi SA, McKee MD; Canadian Orthopaedic Trauma Society. Nonoperative treatment compared with plate fixation of displaced midshaft clavicular fractures. Surgical technique. J Bone Joint Surg Am. 2008;90(suppl 2 pt 1):1-8.
3. Juhn MS, Simonian PT. Type VI acromioclavicular separation with middle-third clavicle fracture in an ice hockey player. Clin J Sports Med. 2002;12(5):315-317.
4. Lancourt JE. Acromioclavicular dislocation with adjacent clavicular fracture in a horseback rider. A case report. Am J Sports Med. 1990;18(3):321-322.
5. Wurtz LD, Lyons FA, Rockwood CA Jr. Fracture of the middle third of the clavicle and dislocation of the acromioclavicular joint. A report of four cases. J Bone Joint Surg Am. 1992;74(1):133-137.
6. Yeh PC, Miller SR, Cunningham JG, Sethi PM. Midshaft clavicle fracture and acromioclavicular dislocation: a case report of a rare injury. J Shoulder Elbow Surg. 2009;18(5):e1-e4.
7. Stanley D, Trowbridge EA, Norris SH. The mechanism of clavicular fracture. A clinical and biomechanical analysis. J Bone Joint Surg Br. 1988;70(3):461-464.
8. Kim W, McKee MD. Management of acute clavicle fractures. Orthop Clin North Am. 2008;39(4):491-505.
9. Woolf SK, Valentine BJ, Barfield WR, Hartsock LA. Middle-third clavicle fracture with associated type IV acromioclavicular separation: case report and literature review. J Surg Orthop Adv. 2013;22(2):183-186.
1. Ring D, Jupiter J. Injuries to the shoulder girdle. In: Browner, BD. Skeletal Trauma. Philadelphia, PA: Elsevier Health Sciences; 2008:1755-1778.
2. Altamimi SA, McKee MD; Canadian Orthopaedic Trauma Society. Nonoperative treatment compared with plate fixation of displaced midshaft clavicular fractures. Surgical technique. J Bone Joint Surg Am. 2008;90(suppl 2 pt 1):1-8.
3. Juhn MS, Simonian PT. Type VI acromioclavicular separation with middle-third clavicle fracture in an ice hockey player. Clin J Sports Med. 2002;12(5):315-317.
4. Lancourt JE. Acromioclavicular dislocation with adjacent clavicular fracture in a horseback rider. A case report. Am J Sports Med. 1990;18(3):321-322.
5. Wurtz LD, Lyons FA, Rockwood CA Jr. Fracture of the middle third of the clavicle and dislocation of the acromioclavicular joint. A report of four cases. J Bone Joint Surg Am. 1992;74(1):133-137.
6. Yeh PC, Miller SR, Cunningham JG, Sethi PM. Midshaft clavicle fracture and acromioclavicular dislocation: a case report of a rare injury. J Shoulder Elbow Surg. 2009;18(5):e1-e4.
7. Stanley D, Trowbridge EA, Norris SH. The mechanism of clavicular fracture. A clinical and biomechanical analysis. J Bone Joint Surg Br. 1988;70(3):461-464.
8. Kim W, McKee MD. Management of acute clavicle fractures. Orthop Clin North Am. 2008;39(4):491-505.
9. Woolf SK, Valentine BJ, Barfield WR, Hartsock LA. Middle-third clavicle fracture with associated type IV acromioclavicular separation: case report and literature review. J Surg Orthop Adv. 2013;22(2):183-186.
Dynamic Magnetic Resonance Imaging of Partial-Thickness Retearing of Distal Biceps Tendon After Endobutton Repair
Retearing after repair of the distal biceps tendon is rare.1 Heterotopic ossification (HO) is also considered uncommon, though reported rates in the literature vary widely, depending on repair and follow-up methods.1-3
In this article, we report a case of ruptured distal biceps tendon repaired with a 1-incision Endobutton technique with longitudinal clinical and imaging follow-up, and we discuss the potential biomechanical and rehabilitative implications of clinically occult retearing after repair.
This case is unique in that the patient was a physician who procured multiple magnetic resonance imaging (MRI) examinations during the postoperative period and again at 1-year follow-up. We witnessed formation of a small focus of HO, which entered and significantly narrowed the radioulnar space on forearm pronation on dynamic MRI. There was no obvious clinical evidence for retearing; high-grade partial-thickness tendon retearing was diagnosed on MRI. This prompted a gentler rehabilitation protocol. Subsequent scar formation and tendon remodeling allowed the patient to return to full activity by 1-year follow-up, confirming recent reports that intrasubstance signal abnormalities4 and even rerupture on MRI are not correlated with symptoms.5 The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A healthy right-hand–dominant 32-year-old man was rock climbing when he heard a pop and felt sudden weakness in his right elbow. The injury occurred during eccentric contraction, while he was climbing a 45° overhanging wall with his right elbow fully extended and forearm maximally pronated. Immediately after injury, he noticed obvious deformity in the right arm. Before this incident, there was no history of elbow symptoms or any medication use.
Physical examination revealed distortion of the biceps with a palpable defect in the right elbow consistent with a complete biceps tendon rupture. This was confirmed on MRI, which showed avulsion of the distal biceps tendon from its insertion on the radius. There was 4 cm of proximal retraction of the tendon, which was kept at the level of the joint line by a partially intact lacertus fibrosis (Figure 1).
As the patient was physically active, operative treatment was chosen with the expectation of restoration to full function and strength. Six days after injury, surgery was performed using a 1-incision anterior approach with an Endobutton technique, as first described by Bain and colleagues6 and subsequently detailed by other authors.7 The diameter of the distal biceps tendon after attachment to the Endobutton (Arthrex, Naples, Florida) was measured, and a corresponding 7-mm unicortical tunnel was drilled into the radial tuberosity. During surgery, there was full range of motion (ROM) at the elbow and forearm. Before closure, the wound was copiously irrigated to minimize the potential of HO. In our practice, we do not routinely administer prophylactic anti-inflammatory drugs to low-risk patients because of the theoretical risks for delayed tendon–bone healing8 and inferior healing strength.9 The theoretical, expected postoperative appearance is illustrated in Figure 2.
For 7 days after surgery, the patient wore a posterior elbow splint in a flexed, supinated position. Afterward, rehabilitation initially consisted of passive ROM progressing to active ROM at postoperative week 4. Pronation was slow to return, but essentially full ROM was regained by 7 weeks after surgery. Seven weeks after surgery, a radiograph showed a small amount of HO near the radial tuberosity (Figure 3A). However, the patient was clinically progressing well, and by 9 weeks was comfortably performing slow, controlled arm curls with a 10-lb weight. Despite the clinical improvements, MRI 9 weeks after surgery showed high-grade partial-thickness retearing of the distal biceps tendon without significant retraction. With dynamic MRI, it was evident that the focus of HO near but external to the distal tendon entered the radioulnar space on pronation (Figures 3B–3D). On axial images of the center of the cortical tunnel, the short-axis diameter of the heterotopic bone measured 2.5 mm, and the bone clearly was occupying part of the radioulnar space during pronation. As the patient was not having pain and was increasing in strength, the clinical team resumed rehabilitation, albeit at a gentler pace.
By 1-year follow-up, the patient had returned to preinjury activity levels, which included rock climbing and weightlifting without pain or loss of strength. One year after surgery, radiographs and MRI showed maturation of heterotopic bone, which was incorporated with scar tissue along the remodeled distal biceps tendon remnant (Figures 4A-4C).
Discussion
Distal biceps tendon ruptures historically have been considered relatively rare injuries. Postrepair complications are uncommon but well known. HO has been described with all distal biceps tendon repair techniques, but rates vary depending on follow-up method. Given the data reported, HO is thought to have a higher incidence with the 2-incision technique than with the 1-incision technique.10 The literature includes fewer reports of HO with the Endobutton technique11,12 than with the suture anchor technique.3 Incidence of HO after distal biceps tendon repair has been reported to be as high as 50%, with Marnitz and colleagues5 suggesting that its presence does not necessarily affect clinical outcome. This was confirmed in our patient’s case.
A much rarer complication of repair is rerupture, which can be asymptomatic or symptomatic.5 The most common failure site, discovered during surgery, is the fixation site.2,13 The true incidence of rerupture is unknown, as MRI typically is not obtained for asymptomatic patients. However, Marnitz and colleagues5 recently found increased intratendinous signal and thickness of repaired tendons in the majority of intact postoperative cases and no significant correlation between any MRI features, including tendon rerupture, and clinical measures. This was confirmed in our patient’s case, in which the MRI-based diagnosis of partial retear was not correlated with adverse clinical outcome at 1-year follow-up. Marnitz and colleagues5 hypothesized that the increased thickness of the repaired tendon would predispose the patient to impingement.
Our patient had no demonstrable loss of motion during surgery. However, postoperative dynamic MRI clearly showed insufficient room in the pronated radioulnar space for both heterotopic bone and repaired biceps tendon. It is possible that a space-occupying peritendinous hematoma or HO soon after surgery caused early loss of pronation. In a study of 10 volunteers, mean radioulnar distance was 4.0 mm (range, 2.1-6.0 mm) at its minimum in pronation.14 We used the same technique to measure our patient’s radioulnar space in active semipronation: 7 mm. This diameter was the same as that of the distal biceps tendon during surgery (Figure 3D). Had our patient been in maximum pronation during imaging, we would have expected a further decrease in radioulnar distance. Given the insufficient room in this case, it is possible that, during the attempt to regain full pronation, attritional wear of the repaired biceps tendon occurred with a corresponding maturation of the focus of heterotopic bone. Supporting this theory is the patient’s lack of history of traumatic loading, which would have suggested tensile failure of the repair. By 1-year follow-up, scar-tissue maturation and remodeling had occurred, and there was sufficient overall biomechanical strength to withstand return to normal activity.
The literature includes multiple reports of in vitro biomechanical studies of various types of distal biceps tendon fixation,15-17 and multiple authors have demonstrated the superior pullout strength of cortical fixation buttons,18,19 such as the Endobutton. It is important to note that all biomechanical tests are performed in cadaveric specimens and are therefore likely applicable only at time zero, after in vivo repair. In part stemming from the results of these cadaveric biomechanical tests, earlier and more aggressive rehabilitation protocols have been developed with the assumption that time zero is the weakest point.20 If in fact the native repaired biceps tendon is retorn and remodeled, there will exist a nadir in strength because of the high concentration of biomechanically inferior type III collagen in scar tissue (as opposed to the very strong type I collagen in native tendons).21 In the absence of complete rerupture, biomechanical strength would continue to improve during scar maturation and continued healing, leading to the typical excellent clinical result, as seen in our case.
This case report illustrates the dynamic MRI appearance of a small focus of HO after distal biceps tendon repair and adds to the time-zero cadaveric data of distal biceps tendon repair. The small focus of HO near the repaired distal tendon may have caused tendon impingement in pronation because of its space-occupying nature and possible attritional tendon wear. A gentler rehabilitation protocol for this pattern of HO, during a period in which biomechanically inferior scar tissue is maturing, may be warranted. Despite the high rates of clinical success with distal biceps tendon repair, there is lack of agreement between ex vivo cadaveric studies and the in vivo scenario. A prospective study involving a larger series of patients with postoperative dynamic MRI examinations would be useful to better understand the true in vivo course of distal biceps tendon repair.
1. Cohen MS. Complications of distal biceps tendon repairs. Sports Med Arthrosc. 2008;16(3):148-153.
2. Bisson L, Moyer M, Lanighan K, Marzo J. Complications associated with repair of a distal biceps rupture using the modified two-incision technique. J Shoulder Elbow Surg. 2008;17(1 suppl):67S-71S.
3. Gallinet D, Dietsch E, Barbier-Brion B, Lerais JM, Obert L. Suture anchor reinsertion of distal biceps rupture: clinical results and radiological assessment of tendon healing. Orthop Traumatol Surg Res. 2011;97(3):252-259.
4. Schmidt CC, Diaz VA, Weir DM, Latona CR, Miller MC. Repaired distal biceps magnetic resonance imaging anatomy compared with outcome. J Shoulder Elbow Surg. 2012;21(12):1623-1631.
5. Marnitz T, Spiegel D, Hug K, et al. MR imaging findings in flexed abducted supinated (FABS) position and clinical presentation following refixation of distal biceps tendon rupture using bioabsorbable suture anchors. Rofo. 2012;184(5):432-436.
6. Bain GI, Prem H, Heptinstall RJ, Verhellen R, Paix D. Repair of distal biceps tendon rupture: a new technique using the Endobutton. J Shoulder Elbow Surg. 2000;9(2):120-126.
7. King J, Bollier M. Repair of distal biceps tendon ruptures using the Endobutton. J Am Acad Orthop Surg. 2008;16(8):490-494.
8. Cohen DB, Kawamura S, Ehteshami JR, Rodeo SA. Indomethacin and celecoxib impair rotator cuff tendon-to-bone healing. Am J Sports Med. 2006;34(3):362-369.
9. Ferry ST, Dahners LE, Afshari HM, Weinhold PS. The effects of common anti-inflammatory drugs on the healing rat patellar tendon. Am J Sports Med. 2007;35(8):1326-1333.
10. Miyamoto RG, Elser F, Millett PJ. Distal biceps tendon injuries. J Bone Joint Surg Am. 2010;92(11):2128-2138.
11. Dillon MT, Lepore DJ. Heterotopic ossification after single-incision distal biceps tendon repair with an Endobutton. J Surg Orthop Adv. 2011;20(3):198-201.
12. Peeters T, Ching-Soon NG, Jansen N, Sneyers C, Declercq G, Verstreken F. Functional outcome after repair of distal biceps tendon ruptures using the Endobutton technique. J Shoulder Elbow Surg. 2009;18(2):283-287.
13. Katolik LI, Fernandez J, Cohen MS. Acute failure of distal biceps reconstruction: a case report. J Shoulder Elbow Surg. 2007;16(5):e10-e12.
14. Seiler JG 3rd, Parker LM, Chamberland PD, Sherbourne GM, Carpenter WA. The distal biceps tendon. Two potential mechanisms involved in its rupture: arterial supply and mechanical impingement. J Shoulder Elbow Surg. 1995;4(3):149-156.
15. Siebenlist S, Lenich A, Buchholz A, et al. Biomechanical in vitro validation of intramedullary cortical button fixation for distal biceps tendon repair: a new technique. Am J Sports Med. 2011;39(8):1762-1768.
16. Pereira DS, Kvitne RS, Liang M, Giacobetti FB, Ebramzadeh E. Surgical repair of distal biceps tendon ruptures: a biomechanical comparison of two techniques. Am J Sports Med. 2002;30(3):432-436.
17. Lemos SE, Ebramzedeh E, Kvitne RS. A new technique: in vitro suture anchor fixation has superior yield strength to bone tunnel fixation for distal biceps tendon repair. Am J Sports Med. 2004;32(2):406-410.
18. Kettler M, Lunger J, Kuhn V, Mutschler W, Tingart MJ. Failure strengths in distal biceps tendon repair. Am J Sports Med. 2007;35(9):1544-1548.
19. Mazzocca AD, Burton KJ, Romeo AA, Santangelo S, Adams DA, Arciero RA. Biomechanical evaluation of 4 techniques of distal biceps brachii tendon repair. Am J Sports Med. 2007;35(2):252-258.
20. Spencer EE Jr, Tisdale A, Kostka K, Ivy RE. Is therapy necessary after distal biceps tendon repair? Hand (N Y). 2008;3(4):316-319.
21. Maffulli N, Ewen SWB, Waterston SW, Reaper J, Barrass V. Tenocytes from ruptured and tendinopathic Achilles tendons produce greater quantities of type III collagen than tenocytes from normal Achilles tendons. Am J Sports Med. 2000;28(4):499-505.
Retearing after repair of the distal biceps tendon is rare.1 Heterotopic ossification (HO) is also considered uncommon, though reported rates in the literature vary widely, depending on repair and follow-up methods.1-3
In this article, we report a case of ruptured distal biceps tendon repaired with a 1-incision Endobutton technique with longitudinal clinical and imaging follow-up, and we discuss the potential biomechanical and rehabilitative implications of clinically occult retearing after repair.
This case is unique in that the patient was a physician who procured multiple magnetic resonance imaging (MRI) examinations during the postoperative period and again at 1-year follow-up. We witnessed formation of a small focus of HO, which entered and significantly narrowed the radioulnar space on forearm pronation on dynamic MRI. There was no obvious clinical evidence for retearing; high-grade partial-thickness tendon retearing was diagnosed on MRI. This prompted a gentler rehabilitation protocol. Subsequent scar formation and tendon remodeling allowed the patient to return to full activity by 1-year follow-up, confirming recent reports that intrasubstance signal abnormalities4 and even rerupture on MRI are not correlated with symptoms.5 The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A healthy right-hand–dominant 32-year-old man was rock climbing when he heard a pop and felt sudden weakness in his right elbow. The injury occurred during eccentric contraction, while he was climbing a 45° overhanging wall with his right elbow fully extended and forearm maximally pronated. Immediately after injury, he noticed obvious deformity in the right arm. Before this incident, there was no history of elbow symptoms or any medication use.
Physical examination revealed distortion of the biceps with a palpable defect in the right elbow consistent with a complete biceps tendon rupture. This was confirmed on MRI, which showed avulsion of the distal biceps tendon from its insertion on the radius. There was 4 cm of proximal retraction of the tendon, which was kept at the level of the joint line by a partially intact lacertus fibrosis (Figure 1).
As the patient was physically active, operative treatment was chosen with the expectation of restoration to full function and strength. Six days after injury, surgery was performed using a 1-incision anterior approach with an Endobutton technique, as first described by Bain and colleagues6 and subsequently detailed by other authors.7 The diameter of the distal biceps tendon after attachment to the Endobutton (Arthrex, Naples, Florida) was measured, and a corresponding 7-mm unicortical tunnel was drilled into the radial tuberosity. During surgery, there was full range of motion (ROM) at the elbow and forearm. Before closure, the wound was copiously irrigated to minimize the potential of HO. In our practice, we do not routinely administer prophylactic anti-inflammatory drugs to low-risk patients because of the theoretical risks for delayed tendon–bone healing8 and inferior healing strength.9 The theoretical, expected postoperative appearance is illustrated in Figure 2.
For 7 days after surgery, the patient wore a posterior elbow splint in a flexed, supinated position. Afterward, rehabilitation initially consisted of passive ROM progressing to active ROM at postoperative week 4. Pronation was slow to return, but essentially full ROM was regained by 7 weeks after surgery. Seven weeks after surgery, a radiograph showed a small amount of HO near the radial tuberosity (Figure 3A). However, the patient was clinically progressing well, and by 9 weeks was comfortably performing slow, controlled arm curls with a 10-lb weight. Despite the clinical improvements, MRI 9 weeks after surgery showed high-grade partial-thickness retearing of the distal biceps tendon without significant retraction. With dynamic MRI, it was evident that the focus of HO near but external to the distal tendon entered the radioulnar space on pronation (Figures 3B–3D). On axial images of the center of the cortical tunnel, the short-axis diameter of the heterotopic bone measured 2.5 mm, and the bone clearly was occupying part of the radioulnar space during pronation. As the patient was not having pain and was increasing in strength, the clinical team resumed rehabilitation, albeit at a gentler pace.
By 1-year follow-up, the patient had returned to preinjury activity levels, which included rock climbing and weightlifting without pain or loss of strength. One year after surgery, radiographs and MRI showed maturation of heterotopic bone, which was incorporated with scar tissue along the remodeled distal biceps tendon remnant (Figures 4A-4C).
Discussion
Distal biceps tendon ruptures historically have been considered relatively rare injuries. Postrepair complications are uncommon but well known. HO has been described with all distal biceps tendon repair techniques, but rates vary depending on follow-up method. Given the data reported, HO is thought to have a higher incidence with the 2-incision technique than with the 1-incision technique.10 The literature includes fewer reports of HO with the Endobutton technique11,12 than with the suture anchor technique.3 Incidence of HO after distal biceps tendon repair has been reported to be as high as 50%, with Marnitz and colleagues5 suggesting that its presence does not necessarily affect clinical outcome. This was confirmed in our patient’s case.
A much rarer complication of repair is rerupture, which can be asymptomatic or symptomatic.5 The most common failure site, discovered during surgery, is the fixation site.2,13 The true incidence of rerupture is unknown, as MRI typically is not obtained for asymptomatic patients. However, Marnitz and colleagues5 recently found increased intratendinous signal and thickness of repaired tendons in the majority of intact postoperative cases and no significant correlation between any MRI features, including tendon rerupture, and clinical measures. This was confirmed in our patient’s case, in which the MRI-based diagnosis of partial retear was not correlated with adverse clinical outcome at 1-year follow-up. Marnitz and colleagues5 hypothesized that the increased thickness of the repaired tendon would predispose the patient to impingement.
Our patient had no demonstrable loss of motion during surgery. However, postoperative dynamic MRI clearly showed insufficient room in the pronated radioulnar space for both heterotopic bone and repaired biceps tendon. It is possible that a space-occupying peritendinous hematoma or HO soon after surgery caused early loss of pronation. In a study of 10 volunteers, mean radioulnar distance was 4.0 mm (range, 2.1-6.0 mm) at its minimum in pronation.14 We used the same technique to measure our patient’s radioulnar space in active semipronation: 7 mm. This diameter was the same as that of the distal biceps tendon during surgery (Figure 3D). Had our patient been in maximum pronation during imaging, we would have expected a further decrease in radioulnar distance. Given the insufficient room in this case, it is possible that, during the attempt to regain full pronation, attritional wear of the repaired biceps tendon occurred with a corresponding maturation of the focus of heterotopic bone. Supporting this theory is the patient’s lack of history of traumatic loading, which would have suggested tensile failure of the repair. By 1-year follow-up, scar-tissue maturation and remodeling had occurred, and there was sufficient overall biomechanical strength to withstand return to normal activity.
The literature includes multiple reports of in vitro biomechanical studies of various types of distal biceps tendon fixation,15-17 and multiple authors have demonstrated the superior pullout strength of cortical fixation buttons,18,19 such as the Endobutton. It is important to note that all biomechanical tests are performed in cadaveric specimens and are therefore likely applicable only at time zero, after in vivo repair. In part stemming from the results of these cadaveric biomechanical tests, earlier and more aggressive rehabilitation protocols have been developed with the assumption that time zero is the weakest point.20 If in fact the native repaired biceps tendon is retorn and remodeled, there will exist a nadir in strength because of the high concentration of biomechanically inferior type III collagen in scar tissue (as opposed to the very strong type I collagen in native tendons).21 In the absence of complete rerupture, biomechanical strength would continue to improve during scar maturation and continued healing, leading to the typical excellent clinical result, as seen in our case.
This case report illustrates the dynamic MRI appearance of a small focus of HO after distal biceps tendon repair and adds to the time-zero cadaveric data of distal biceps tendon repair. The small focus of HO near the repaired distal tendon may have caused tendon impingement in pronation because of its space-occupying nature and possible attritional tendon wear. A gentler rehabilitation protocol for this pattern of HO, during a period in which biomechanically inferior scar tissue is maturing, may be warranted. Despite the high rates of clinical success with distal biceps tendon repair, there is lack of agreement between ex vivo cadaveric studies and the in vivo scenario. A prospective study involving a larger series of patients with postoperative dynamic MRI examinations would be useful to better understand the true in vivo course of distal biceps tendon repair.
Retearing after repair of the distal biceps tendon is rare.1 Heterotopic ossification (HO) is also considered uncommon, though reported rates in the literature vary widely, depending on repair and follow-up methods.1-3
In this article, we report a case of ruptured distal biceps tendon repaired with a 1-incision Endobutton technique with longitudinal clinical and imaging follow-up, and we discuss the potential biomechanical and rehabilitative implications of clinically occult retearing after repair.
This case is unique in that the patient was a physician who procured multiple magnetic resonance imaging (MRI) examinations during the postoperative period and again at 1-year follow-up. We witnessed formation of a small focus of HO, which entered and significantly narrowed the radioulnar space on forearm pronation on dynamic MRI. There was no obvious clinical evidence for retearing; high-grade partial-thickness tendon retearing was diagnosed on MRI. This prompted a gentler rehabilitation protocol. Subsequent scar formation and tendon remodeling allowed the patient to return to full activity by 1-year follow-up, confirming recent reports that intrasubstance signal abnormalities4 and even rerupture on MRI are not correlated with symptoms.5 The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A healthy right-hand–dominant 32-year-old man was rock climbing when he heard a pop and felt sudden weakness in his right elbow. The injury occurred during eccentric contraction, while he was climbing a 45° overhanging wall with his right elbow fully extended and forearm maximally pronated. Immediately after injury, he noticed obvious deformity in the right arm. Before this incident, there was no history of elbow symptoms or any medication use.
Physical examination revealed distortion of the biceps with a palpable defect in the right elbow consistent with a complete biceps tendon rupture. This was confirmed on MRI, which showed avulsion of the distal biceps tendon from its insertion on the radius. There was 4 cm of proximal retraction of the tendon, which was kept at the level of the joint line by a partially intact lacertus fibrosis (Figure 1).
As the patient was physically active, operative treatment was chosen with the expectation of restoration to full function and strength. Six days after injury, surgery was performed using a 1-incision anterior approach with an Endobutton technique, as first described by Bain and colleagues6 and subsequently detailed by other authors.7 The diameter of the distal biceps tendon after attachment to the Endobutton (Arthrex, Naples, Florida) was measured, and a corresponding 7-mm unicortical tunnel was drilled into the radial tuberosity. During surgery, there was full range of motion (ROM) at the elbow and forearm. Before closure, the wound was copiously irrigated to minimize the potential of HO. In our practice, we do not routinely administer prophylactic anti-inflammatory drugs to low-risk patients because of the theoretical risks for delayed tendon–bone healing8 and inferior healing strength.9 The theoretical, expected postoperative appearance is illustrated in Figure 2.
For 7 days after surgery, the patient wore a posterior elbow splint in a flexed, supinated position. Afterward, rehabilitation initially consisted of passive ROM progressing to active ROM at postoperative week 4. Pronation was slow to return, but essentially full ROM was regained by 7 weeks after surgery. Seven weeks after surgery, a radiograph showed a small amount of HO near the radial tuberosity (Figure 3A). However, the patient was clinically progressing well, and by 9 weeks was comfortably performing slow, controlled arm curls with a 10-lb weight. Despite the clinical improvements, MRI 9 weeks after surgery showed high-grade partial-thickness retearing of the distal biceps tendon without significant retraction. With dynamic MRI, it was evident that the focus of HO near but external to the distal tendon entered the radioulnar space on pronation (Figures 3B–3D). On axial images of the center of the cortical tunnel, the short-axis diameter of the heterotopic bone measured 2.5 mm, and the bone clearly was occupying part of the radioulnar space during pronation. As the patient was not having pain and was increasing in strength, the clinical team resumed rehabilitation, albeit at a gentler pace.
By 1-year follow-up, the patient had returned to preinjury activity levels, which included rock climbing and weightlifting without pain or loss of strength. One year after surgery, radiographs and MRI showed maturation of heterotopic bone, which was incorporated with scar tissue along the remodeled distal biceps tendon remnant (Figures 4A-4C).
Discussion
Distal biceps tendon ruptures historically have been considered relatively rare injuries. Postrepair complications are uncommon but well known. HO has been described with all distal biceps tendon repair techniques, but rates vary depending on follow-up method. Given the data reported, HO is thought to have a higher incidence with the 2-incision technique than with the 1-incision technique.10 The literature includes fewer reports of HO with the Endobutton technique11,12 than with the suture anchor technique.3 Incidence of HO after distal biceps tendon repair has been reported to be as high as 50%, with Marnitz and colleagues5 suggesting that its presence does not necessarily affect clinical outcome. This was confirmed in our patient’s case.
A much rarer complication of repair is rerupture, which can be asymptomatic or symptomatic.5 The most common failure site, discovered during surgery, is the fixation site.2,13 The true incidence of rerupture is unknown, as MRI typically is not obtained for asymptomatic patients. However, Marnitz and colleagues5 recently found increased intratendinous signal and thickness of repaired tendons in the majority of intact postoperative cases and no significant correlation between any MRI features, including tendon rerupture, and clinical measures. This was confirmed in our patient’s case, in which the MRI-based diagnosis of partial retear was not correlated with adverse clinical outcome at 1-year follow-up. Marnitz and colleagues5 hypothesized that the increased thickness of the repaired tendon would predispose the patient to impingement.
Our patient had no demonstrable loss of motion during surgery. However, postoperative dynamic MRI clearly showed insufficient room in the pronated radioulnar space for both heterotopic bone and repaired biceps tendon. It is possible that a space-occupying peritendinous hematoma or HO soon after surgery caused early loss of pronation. In a study of 10 volunteers, mean radioulnar distance was 4.0 mm (range, 2.1-6.0 mm) at its minimum in pronation.14 We used the same technique to measure our patient’s radioulnar space in active semipronation: 7 mm. This diameter was the same as that of the distal biceps tendon during surgery (Figure 3D). Had our patient been in maximum pronation during imaging, we would have expected a further decrease in radioulnar distance. Given the insufficient room in this case, it is possible that, during the attempt to regain full pronation, attritional wear of the repaired biceps tendon occurred with a corresponding maturation of the focus of heterotopic bone. Supporting this theory is the patient’s lack of history of traumatic loading, which would have suggested tensile failure of the repair. By 1-year follow-up, scar-tissue maturation and remodeling had occurred, and there was sufficient overall biomechanical strength to withstand return to normal activity.
The literature includes multiple reports of in vitro biomechanical studies of various types of distal biceps tendon fixation,15-17 and multiple authors have demonstrated the superior pullout strength of cortical fixation buttons,18,19 such as the Endobutton. It is important to note that all biomechanical tests are performed in cadaveric specimens and are therefore likely applicable only at time zero, after in vivo repair. In part stemming from the results of these cadaveric biomechanical tests, earlier and more aggressive rehabilitation protocols have been developed with the assumption that time zero is the weakest point.20 If in fact the native repaired biceps tendon is retorn and remodeled, there will exist a nadir in strength because of the high concentration of biomechanically inferior type III collagen in scar tissue (as opposed to the very strong type I collagen in native tendons).21 In the absence of complete rerupture, biomechanical strength would continue to improve during scar maturation and continued healing, leading to the typical excellent clinical result, as seen in our case.
This case report illustrates the dynamic MRI appearance of a small focus of HO after distal biceps tendon repair and adds to the time-zero cadaveric data of distal biceps tendon repair. The small focus of HO near the repaired distal tendon may have caused tendon impingement in pronation because of its space-occupying nature and possible attritional tendon wear. A gentler rehabilitation protocol for this pattern of HO, during a period in which biomechanically inferior scar tissue is maturing, may be warranted. Despite the high rates of clinical success with distal biceps tendon repair, there is lack of agreement between ex vivo cadaveric studies and the in vivo scenario. A prospective study involving a larger series of patients with postoperative dynamic MRI examinations would be useful to better understand the true in vivo course of distal biceps tendon repair.
1. Cohen MS. Complications of distal biceps tendon repairs. Sports Med Arthrosc. 2008;16(3):148-153.
2. Bisson L, Moyer M, Lanighan K, Marzo J. Complications associated with repair of a distal biceps rupture using the modified two-incision technique. J Shoulder Elbow Surg. 2008;17(1 suppl):67S-71S.
3. Gallinet D, Dietsch E, Barbier-Brion B, Lerais JM, Obert L. Suture anchor reinsertion of distal biceps rupture: clinical results and radiological assessment of tendon healing. Orthop Traumatol Surg Res. 2011;97(3):252-259.
4. Schmidt CC, Diaz VA, Weir DM, Latona CR, Miller MC. Repaired distal biceps magnetic resonance imaging anatomy compared with outcome. J Shoulder Elbow Surg. 2012;21(12):1623-1631.
5. Marnitz T, Spiegel D, Hug K, et al. MR imaging findings in flexed abducted supinated (FABS) position and clinical presentation following refixation of distal biceps tendon rupture using bioabsorbable suture anchors. Rofo. 2012;184(5):432-436.
6. Bain GI, Prem H, Heptinstall RJ, Verhellen R, Paix D. Repair of distal biceps tendon rupture: a new technique using the Endobutton. J Shoulder Elbow Surg. 2000;9(2):120-126.
7. King J, Bollier M. Repair of distal biceps tendon ruptures using the Endobutton. J Am Acad Orthop Surg. 2008;16(8):490-494.
8. Cohen DB, Kawamura S, Ehteshami JR, Rodeo SA. Indomethacin and celecoxib impair rotator cuff tendon-to-bone healing. Am J Sports Med. 2006;34(3):362-369.
9. Ferry ST, Dahners LE, Afshari HM, Weinhold PS. The effects of common anti-inflammatory drugs on the healing rat patellar tendon. Am J Sports Med. 2007;35(8):1326-1333.
10. Miyamoto RG, Elser F, Millett PJ. Distal biceps tendon injuries. J Bone Joint Surg Am. 2010;92(11):2128-2138.
11. Dillon MT, Lepore DJ. Heterotopic ossification after single-incision distal biceps tendon repair with an Endobutton. J Surg Orthop Adv. 2011;20(3):198-201.
12. Peeters T, Ching-Soon NG, Jansen N, Sneyers C, Declercq G, Verstreken F. Functional outcome after repair of distal biceps tendon ruptures using the Endobutton technique. J Shoulder Elbow Surg. 2009;18(2):283-287.
13. Katolik LI, Fernandez J, Cohen MS. Acute failure of distal biceps reconstruction: a case report. J Shoulder Elbow Surg. 2007;16(5):e10-e12.
14. Seiler JG 3rd, Parker LM, Chamberland PD, Sherbourne GM, Carpenter WA. The distal biceps tendon. Two potential mechanisms involved in its rupture: arterial supply and mechanical impingement. J Shoulder Elbow Surg. 1995;4(3):149-156.
15. Siebenlist S, Lenich A, Buchholz A, et al. Biomechanical in vitro validation of intramedullary cortical button fixation for distal biceps tendon repair: a new technique. Am J Sports Med. 2011;39(8):1762-1768.
16. Pereira DS, Kvitne RS, Liang M, Giacobetti FB, Ebramzadeh E. Surgical repair of distal biceps tendon ruptures: a biomechanical comparison of two techniques. Am J Sports Med. 2002;30(3):432-436.
17. Lemos SE, Ebramzedeh E, Kvitne RS. A new technique: in vitro suture anchor fixation has superior yield strength to bone tunnel fixation for distal biceps tendon repair. Am J Sports Med. 2004;32(2):406-410.
18. Kettler M, Lunger J, Kuhn V, Mutschler W, Tingart MJ. Failure strengths in distal biceps tendon repair. Am J Sports Med. 2007;35(9):1544-1548.
19. Mazzocca AD, Burton KJ, Romeo AA, Santangelo S, Adams DA, Arciero RA. Biomechanical evaluation of 4 techniques of distal biceps brachii tendon repair. Am J Sports Med. 2007;35(2):252-258.
20. Spencer EE Jr, Tisdale A, Kostka K, Ivy RE. Is therapy necessary after distal biceps tendon repair? Hand (N Y). 2008;3(4):316-319.
21. Maffulli N, Ewen SWB, Waterston SW, Reaper J, Barrass V. Tenocytes from ruptured and tendinopathic Achilles tendons produce greater quantities of type III collagen than tenocytes from normal Achilles tendons. Am J Sports Med. 2000;28(4):499-505.
1. Cohen MS. Complications of distal biceps tendon repairs. Sports Med Arthrosc. 2008;16(3):148-153.
2. Bisson L, Moyer M, Lanighan K, Marzo J. Complications associated with repair of a distal biceps rupture using the modified two-incision technique. J Shoulder Elbow Surg. 2008;17(1 suppl):67S-71S.
3. Gallinet D, Dietsch E, Barbier-Brion B, Lerais JM, Obert L. Suture anchor reinsertion of distal biceps rupture: clinical results and radiological assessment of tendon healing. Orthop Traumatol Surg Res. 2011;97(3):252-259.
4. Schmidt CC, Diaz VA, Weir DM, Latona CR, Miller MC. Repaired distal biceps magnetic resonance imaging anatomy compared with outcome. J Shoulder Elbow Surg. 2012;21(12):1623-1631.
5. Marnitz T, Spiegel D, Hug K, et al. MR imaging findings in flexed abducted supinated (FABS) position and clinical presentation following refixation of distal biceps tendon rupture using bioabsorbable suture anchors. Rofo. 2012;184(5):432-436.
6. Bain GI, Prem H, Heptinstall RJ, Verhellen R, Paix D. Repair of distal biceps tendon rupture: a new technique using the Endobutton. J Shoulder Elbow Surg. 2000;9(2):120-126.
7. King J, Bollier M. Repair of distal biceps tendon ruptures using the Endobutton. J Am Acad Orthop Surg. 2008;16(8):490-494.
8. Cohen DB, Kawamura S, Ehteshami JR, Rodeo SA. Indomethacin and celecoxib impair rotator cuff tendon-to-bone healing. Am J Sports Med. 2006;34(3):362-369.
9. Ferry ST, Dahners LE, Afshari HM, Weinhold PS. The effects of common anti-inflammatory drugs on the healing rat patellar tendon. Am J Sports Med. 2007;35(8):1326-1333.
10. Miyamoto RG, Elser F, Millett PJ. Distal biceps tendon injuries. J Bone Joint Surg Am. 2010;92(11):2128-2138.
11. Dillon MT, Lepore DJ. Heterotopic ossification after single-incision distal biceps tendon repair with an Endobutton. J Surg Orthop Adv. 2011;20(3):198-201.
12. Peeters T, Ching-Soon NG, Jansen N, Sneyers C, Declercq G, Verstreken F. Functional outcome after repair of distal biceps tendon ruptures using the Endobutton technique. J Shoulder Elbow Surg. 2009;18(2):283-287.
13. Katolik LI, Fernandez J, Cohen MS. Acute failure of distal biceps reconstruction: a case report. J Shoulder Elbow Surg. 2007;16(5):e10-e12.
14. Seiler JG 3rd, Parker LM, Chamberland PD, Sherbourne GM, Carpenter WA. The distal biceps tendon. Two potential mechanisms involved in its rupture: arterial supply and mechanical impingement. J Shoulder Elbow Surg. 1995;4(3):149-156.
15. Siebenlist S, Lenich A, Buchholz A, et al. Biomechanical in vitro validation of intramedullary cortical button fixation for distal biceps tendon repair: a new technique. Am J Sports Med. 2011;39(8):1762-1768.
16. Pereira DS, Kvitne RS, Liang M, Giacobetti FB, Ebramzadeh E. Surgical repair of distal biceps tendon ruptures: a biomechanical comparison of two techniques. Am J Sports Med. 2002;30(3):432-436.
17. Lemos SE, Ebramzedeh E, Kvitne RS. A new technique: in vitro suture anchor fixation has superior yield strength to bone tunnel fixation for distal biceps tendon repair. Am J Sports Med. 2004;32(2):406-410.
18. Kettler M, Lunger J, Kuhn V, Mutschler W, Tingart MJ. Failure strengths in distal biceps tendon repair. Am J Sports Med. 2007;35(9):1544-1548.
19. Mazzocca AD, Burton KJ, Romeo AA, Santangelo S, Adams DA, Arciero RA. Biomechanical evaluation of 4 techniques of distal biceps brachii tendon repair. Am J Sports Med. 2007;35(2):252-258.
20. Spencer EE Jr, Tisdale A, Kostka K, Ivy RE. Is therapy necessary after distal biceps tendon repair? Hand (N Y). 2008;3(4):316-319.
21. Maffulli N, Ewen SWB, Waterston SW, Reaper J, Barrass V. Tenocytes from ruptured and tendinopathic Achilles tendons produce greater quantities of type III collagen than tenocytes from normal Achilles tendons. Am J Sports Med. 2000;28(4):499-505.
Lateral Femoral Cutaneous Nerve Palsy Following Shoulder Surgery in the Beach Chair Position: A Report of 4 Cases
Reverse Shoulder Arthroplasty Using an Implant With a Lateral Center of Rotation: Outcomes, Complications, and the Influence of Experience
Timing of Forearm Deformity Correction in a Child With Multiple Hereditary Exostosis
Recurrent Instability Seen in Intercollegiate Athletes With Shoulder Injury After Return to Play
SEATTLE—Among mid-season contact athletes with shoulder instability, 73% of athletes return to play after 1 week, according to a study presented at the 2014 Annual Meeting of the American Orthopaedic Society for Sports Medicine. Regardless of whether the initial injury was a subluxation or dislocation, 63% of the cases developed recurrent instability.
There is no consensus on the optimal treatment of young in-season athletes with anterior shoulder instability and limited data are available to guide return to play and treatment. MAJ Jonathan F. Dickens, MD, from the John A. Feagin Jr. Sports Medicine Fellowship and Keller Army Hospital in West Point, New York, and colleagues conducted a study to examine the likelihood of return to sport following an in-season shoulder instability event based on the type of instability (subluxation vs. dislocation). Additionally, injury factors and patient reported outcome scores administered at the time of injury were evaluated to assess the predictability of eventual successful return to sport and time to return to sport during the competitive season following injury.
Dr. Dickens and colleagues examined 45 male and female intercollegiate athletes over 2 academic years, to assess return to play following in-season anterior glenohumeral instability. Athletes included in the sample were both male and female and participated in sports including basketball, soccer, lacrosse, and football. All observed athletes underwent a standardized accelerated rehabilitation program without shoulder immobilization, following the initial shoulder instability event. Subjects were followed during the course of their competitive season to determine return to play success and recurrent instability.
Of the 45 athletes who suffered an anterior shoulder instability event, 33 (73%) returned to play for at least part of the season after a median 5 days lost from competition. “While a large portion of the athletes in this observational study return to mid-season sport, only 36% completed the season without subsequent instability,” said Dr. Dickens. Athletes with a subluxation injury (partial dislocation) of the shoulder were 5.3 times more likely to return in the same season compared to those with a complete dislocation. The most common reason for athletes not returning was the inability to reach sufficient shoulder function for athletic participation.
“These early results should be valuable to physicians caring for the in-season athlete with shoulder instability, as we have not yet reached a consensus treatment approach on these injuries,” said Dr. Dickens. “More research is needed to determine the effect of multiple recurrent instability events on long-term outcomes and this study will hopefully be a first good step in understanding this relationship.”
SEATTLE—Among mid-season contact athletes with shoulder instability, 73% of athletes return to play after 1 week, according to a study presented at the 2014 Annual Meeting of the American Orthopaedic Society for Sports Medicine. Regardless of whether the initial injury was a subluxation or dislocation, 63% of the cases developed recurrent instability.
There is no consensus on the optimal treatment of young in-season athletes with anterior shoulder instability and limited data are available to guide return to play and treatment. MAJ Jonathan F. Dickens, MD, from the John A. Feagin Jr. Sports Medicine Fellowship and Keller Army Hospital in West Point, New York, and colleagues conducted a study to examine the likelihood of return to sport following an in-season shoulder instability event based on the type of instability (subluxation vs. dislocation). Additionally, injury factors and patient reported outcome scores administered at the time of injury were evaluated to assess the predictability of eventual successful return to sport and time to return to sport during the competitive season following injury.
Dr. Dickens and colleagues examined 45 male and female intercollegiate athletes over 2 academic years, to assess return to play following in-season anterior glenohumeral instability. Athletes included in the sample were both male and female and participated in sports including basketball, soccer, lacrosse, and football. All observed athletes underwent a standardized accelerated rehabilitation program without shoulder immobilization, following the initial shoulder instability event. Subjects were followed during the course of their competitive season to determine return to play success and recurrent instability.
Of the 45 athletes who suffered an anterior shoulder instability event, 33 (73%) returned to play for at least part of the season after a median 5 days lost from competition. “While a large portion of the athletes in this observational study return to mid-season sport, only 36% completed the season without subsequent instability,” said Dr. Dickens. Athletes with a subluxation injury (partial dislocation) of the shoulder were 5.3 times more likely to return in the same season compared to those with a complete dislocation. The most common reason for athletes not returning was the inability to reach sufficient shoulder function for athletic participation.
“These early results should be valuable to physicians caring for the in-season athlete with shoulder instability, as we have not yet reached a consensus treatment approach on these injuries,” said Dr. Dickens. “More research is needed to determine the effect of multiple recurrent instability events on long-term outcomes and this study will hopefully be a first good step in understanding this relationship.”
SEATTLE—Among mid-season contact athletes with shoulder instability, 73% of athletes return to play after 1 week, according to a study presented at the 2014 Annual Meeting of the American Orthopaedic Society for Sports Medicine. Regardless of whether the initial injury was a subluxation or dislocation, 63% of the cases developed recurrent instability.
There is no consensus on the optimal treatment of young in-season athletes with anterior shoulder instability and limited data are available to guide return to play and treatment. MAJ Jonathan F. Dickens, MD, from the John A. Feagin Jr. Sports Medicine Fellowship and Keller Army Hospital in West Point, New York, and colleagues conducted a study to examine the likelihood of return to sport following an in-season shoulder instability event based on the type of instability (subluxation vs. dislocation). Additionally, injury factors and patient reported outcome scores administered at the time of injury were evaluated to assess the predictability of eventual successful return to sport and time to return to sport during the competitive season following injury.
Dr. Dickens and colleagues examined 45 male and female intercollegiate athletes over 2 academic years, to assess return to play following in-season anterior glenohumeral instability. Athletes included in the sample were both male and female and participated in sports including basketball, soccer, lacrosse, and football. All observed athletes underwent a standardized accelerated rehabilitation program without shoulder immobilization, following the initial shoulder instability event. Subjects were followed during the course of their competitive season to determine return to play success and recurrent instability.
Of the 45 athletes who suffered an anterior shoulder instability event, 33 (73%) returned to play for at least part of the season after a median 5 days lost from competition. “While a large portion of the athletes in this observational study return to mid-season sport, only 36% completed the season without subsequent instability,” said Dr. Dickens. Athletes with a subluxation injury (partial dislocation) of the shoulder were 5.3 times more likely to return in the same season compared to those with a complete dislocation. The most common reason for athletes not returning was the inability to reach sufficient shoulder function for athletic participation.
“These early results should be valuable to physicians caring for the in-season athlete with shoulder instability, as we have not yet reached a consensus treatment approach on these injuries,” said Dr. Dickens. “More research is needed to determine the effect of multiple recurrent instability events on long-term outcomes and this study will hopefully be a first good step in understanding this relationship.”