User login
Arthroscopic Posterior-Inferior Capsular Release in the Treatment of Overhead Athletes
Glenohumeral internal rotation deficit (GIRD) can be observed in overhead athletes and is thought to play a role in generating pain and rotator cuff weakness in the dominant shoulder with sport. It is unclear what is an acceptable value of GIRD in a population of overhead athletes and whether it should be based solely on internal rotation deficit or should include total range of motion (ROM) deficit.1,2 Acquired GIRD in the athlete’s throwing shoulder has been thoroughly documented in the literature as a loss of internal rotation relative to the nonthrowing shoulder, with etiologies including bony adaptations (increased humeral retroversion), muscular tightness, and posterior capsular tightness.1,3-11 In particular, the repetitive torsional stresses acting on the throwing shoulder of baseball players is thought to produce, over the long term, structural adaptations such as increased humeral retroversion.5,12-14 Further, for shoulders with posterior-inferior capsular tightness, cadaveric studies have shown increased contact pressure at the coracoacromial arch during simulated follow-through.15 Athletes of other overhead and throwing sports, such as football, softball, tennis, and volleyball, may show similar adaptations in overhead motion.9,16,17
GIRD has been associated with a variety of pathologic conditions, including scapular dyskinesis, internal and secondary impingement, partial articular-sided rotator cuff tears, damage to the biceps–labral complex, and ulnar collateral ligament insufficiency.10,12,18-22
Restriction from engaging in exacerbating activities (eg, throwing) and compliance with a specific stretching program reduces or eliminates GIRD in the majority of cases.1,23-28 In the few cases in which conservative management fails, operative intervention may be indicated.1,23,29,30 Few investigators have detailed an operative technique for selective arthroscopic capsular release of the posterior-inferior capsule or evaluated the ability of athletes to return to sport after such surgery.
In this article, we present our technique for arthroscopic posterior-inferior capsular release and report the results of applying this technique in a population of athletes with symptomatic GIRD that was unresponsive to nonoperative treatment and was preventing them from returning to sport.
We hypothesized that selective arthroscopic surgical release of the posterior-inferior capsule would improve symptomatic GIRD and result in a return to sport in the majority of cases unresponsive to nonoperative treatment.
Materials and Methods
Patients
After obtaining institutional review board approval, we retrospectively reviewed patient charts and collected data. Study inclusion criteria were arthroscopic selective posterior-inferior capsular release between 2004 and 2008; failure to resume sport after minimum 3 months of physical therapy, including use of sleeper stretch, active joint mobilization by licensed physical therapist, and sport-specific restriction from exacerbating activities (eg, throwing for baseball players); and active participation in overhead sport.1,27 Exclusion criteria were generalized adhesive capsulitis, labral pathology producing glenohumeral joint instability (Bankart or reverse Bankart lesion), high-grade or full-thickness tearing of rotator cuff, and clinically significant partial-thickness tearing or instability of long head of biceps tendon.
Assessment
One of 3 authors (Dr. Buss, Dr. Codding, or Dr. Dahm) used a bubble goniometer to measure passive internal rotation. Patients were positioned supine with 90° of thoracohumeral abduction and 90° of elbow flexion. The examiner’s hand stabilized the scapula against the examination table, in accordance with published techniques.1,26 Active internal rotation was measured at 0° of thoracohumeral abduction by noting the most superior spinal segment reached. Before and after surgery, passive internal rotation measurements were taken on both arms. GIRD was determined by the difference between dominant and nondominant arm measurements; segmental differences were obtained by subtracting segments achieved between the dominant and nondominant arms.
Before surgery and at minimum 2-year follow-up after surgery, patients completed a subjective questionnaire, which included the American Shoulder and Elbow Surgeons (ASES) Standardized Shoulder Assessment Form, for assessment of both arms. ASES scores are reliable, valid, and responsive in evaluating shoulder pain and function.15,31 Patients also answered questions about their ability to return to play, their level of play after surgery, and whether they would undergo the procedure again.
Surgical Technique
After induction of general anesthesia and standard preparation and draping, the patient is placed in a standard beach-chair position and examined. Diagnostic arthroscopy is then performed. In all patients, intra-articular evaluation revealed a thickened, contracted posterior band of the inferior glenohumeral ligament. This finding is consistent with other studies of patients with significant GIRD.1,14,22,30
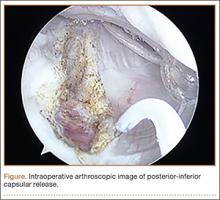
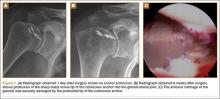
On completion of the diagnostic portion of the arthroscopy, attention is turned to the selective posterior-inferior capsular release. Key to proper execution of the release is establishing a posterior-inferior accessory portal. This is accomplished while viewing from a standard posterior (“soft spot”) portal and determining the appropriate location and angle of entry by spinal needle localization. Typically, an entry point is selected about 4 cm distal and 1 cm lateral to the standard posterior portal. An 18-gauge spinal needle introduced at this location is angled about 15° superiorly and about 20° medially. Once the appropriate vector is determined, a skin incision is made, and a Wissinger rod is introduced, over which a small-diameter cannula is passed. A hooked-tip electrocautery device is used to divide the posterior capsule from the glenoid labrum between the 8- and 6-o’clock positions in the right shoulder (Figure). Care is taken to perform the release immediately adjacent to the glenoid labrum and using short bursts of cautery in order to minimize risk of injury to the teres minor branch of the axillary nerve. Adequate release is confirmed by reassessing passive internal rotation under anesthesia. Additional procedures are performed, if necessary, after completion of the capsular release.
Postoperative rehabilitation consists initially of pendulum exercises and scapular retraction starting on postoperative day 1. Once the swelling from the surgical procedure subsides, typically within 1 week, passive and active-assisted ROM and gentle posterior capsular mobilization are initiated under the direction of a licensed physical therapist. Active ROM is allowed once the patient regains normal scapulothoracic rhythm. Strengthening consists initially of isometrics followed by light resistance strengthening for the rotator cuff and scapular stabilizers once active ROM and scapulothoracic rhythm return to normal. Passive internal rotation stretching, including use of the sleeper stretch, is implemented as soon as tolerated and continues throughout the rehabilitation process.32
Statistical Analysis
Statistical analysis was performed with Stata Release 11 (StataCorp, College Station, Texas). Paired t tests were used to assess preoperative and postoperative mean differences in ASES scores, in passive glenohumeral internal rotation, and in active glenohumeral internal rotation; independent-samples t tests were used to assess side-to-side differences. Significance was set at P < .05.
Results
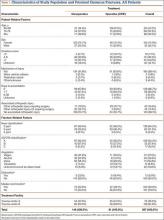
Fifteen overhead athletes met the study inclusion criteria. Two were lost to follow-up. Of the remaining 13 patients, 6 underwent isolated arthroscopic posterior-inferior capsular release, and 7 had concomitant procedures (6 subacromial decompressions, 1 superior labrum anterior-posterior [SLAP] repair). There were 11 male athletes and 2 female athletes. Twelve of the 13 patients were right-hand–dominant. Mean age at time of surgery was 21 years (range, 16-33 years). There were 10 baseball players (6 pitchers, 4 position players); the other 3 patients played softball (1), volleyball (1), or tennis (1). Six patients played at high school level, 5 at college level, 1 at professional level, and 1 at amateur level. All 13 patients underwent a minimum of 3 months of comprehensive rehabilitation, which included use of the sleeper stretch, active joint mobilization by a licensed physical therapist, and sport-specific restriction from exacerbating activities. Mean duration of symptoms before surgery was 18 months (range, 4-48 months). Mean postoperative follow-up was 31 months (range, 24-59 months). Mean ASES score was 71.5 (range, 33-95) before surgery and 86.9 (range, 60-100) after surgery (P < .001). Mean GIRD improved from 43.1° (range, 30°-60°) before surgery to 9.7° (range, –7° to 40°) after surgery (P < .001). Mean active internal rotation difference improved from 3.8 vertebral segments before surgery to 2.6 vertebral segments after surgery; this difference was not statistically significant (P = .459). Ten (77%) of the 13 patients returned to their preoperative level of play or a higher level; the other 3 (23%) did not return to their preoperative level of play but continued to compete in a different position (Table). Eleven patients (85%) stated they would repeat the procedure. One of the 2 patients who would not repeat the procedure was in the isolated posterior-inferior capsular release group; the other was in the concomitant-procedure group (subacromial decompression). Total glenohumeral ROM of dominant arm was 122° before surgery and 136° after surgery (P = .04). There was no significant difference in total ROM between dominant and nondominant arms after surgery (136° and 141°; P = .12), but the preoperative difference was significant (122° vs 141°; P = .022).
Discussion
GIRD has been associated with various pathologic conditions of the upper extremity. In 1991, Verna28 found that a majority of 39 professional baseball pitchers with significant GIRD had shoulder problems that affected playing time. More recently, GIRD has been associated with a progression of injuries, including scapular dyskinesia, internal and secondary impingement, articular-sided partial rotator cuff tears, rotator cuff weakness, damage to the biceps–labral complex, and ulnar collateral ligament insufficiency.12,18-22 In a cadaveric study of humeral head translation, Harryman and colleagues33 noted an anterosuperior migration of the humeral head during flexion and concluded it resulted from a loose anterior and tight posterior glenohumeral capsule, leading to loss of glenohumeral internal rotation. More recently, posterosuperior migration of the humeral head has been postulated, with GIRD secondary to an essential posterior capsular contracture.1 Tyler and colleagues34 clinically linked posterior capsular tightness with GIRD, and both cadaveric and magnetic resonance imaging studies have supported the finding that posterior capsular contracture leads to posterosuperior humeral head migration in association with GIRD.14,20 Such a disruption in normal glenohumeral joint mechanics could produce phenomena of internal or secondary acromiohumeral impingement and pain.
More recently, in a large cohort of professional baseball pitchers, a significant correlation was found between the incidence of rotator cuff strength deficits and GIRD.35 More than 40% of the pitchers with GIRD of at least 35° had a measureable rotator cuff strength deficit in the throwing shoulder.
Burkhart and colleagues23 concluded that the shoulder most at risk for developing “dead arm” has GIRD and an advanced form of scapular dyskinesia known as SICK scapula (the phenomenon involves Scapula malposition, Inferior medial border prominence, Coracoid pain and malposition, and dysKinesis of scapular movement).
Most athletes with symptoms attributed to GIRD respond to conservative management. A posterior-inferior capsular stretching program focused on regaining internal rotation in the throwing arm has been shown to return about 90% of athletes to play.1 Numerous studies have indicated that enrollment in a compliant stretching program reduces GIRD.1,23-27 However, nonoperative treatment fails in a reported 10% of patients with GIRD; these patients may respond to operative treatment.1
More specifically, for patients who do not respond to conservative treatment, a posterior-inferior capsular release may be indicated.1,29 Ticker and colleagues22 identified 9 patients who had lost internal rotation and had a posterior capsular contracture at arthroscopy. That study, however, was not performed on overhead or throwing athletes. Yoneda and colleagues30 followed 16 overhead throwing athletes after arthroscopic posterior-inferior capsular release and found favorable preliminary clinical results. Eleven of the 16 patients returned to their preinjury level of performance; the other 5 returned to a lower level. In addition, all 4 patients who underwent isolated arthroscopic capsular release had throwing power restored to between 90% and 100%.
In the present study, 10 of 13 patients who underwent arthroscopic posterior-inferior capsular release returned to their preoperative level of play or a higher level. Mean passive GIRD improved significantly from before surgery to after surgery. ASES scores likewise were significantly improved from before surgery to after surgery. The active internal rotation difference as measured by vertebral segment level was not significantly changed after surgery. This lack of improvement may stem from the more complex musculoligamentous interactions governing active internal rotation versus isolated, passive internal rotation. Another possible explanation for lack of improvement is that the interobserver and intraobserver reliability of this method is lower.36
At 2-year follow-up, the patient who had undergone concomitant SLAP repair demonstrated a 23% improvement in ASES score and more internal rotation on the dominant arm relative to the nondominant arm. This patient returned to a level of play at least as good as his preoperative level. Although we could not determine its statistical significance, this patient’s improvement suggests that the SLAP repair did not reduce the efficacy of the posterior-inferior capsular release.
Limitations of this study include its relatively small cohort (precluded statistical comparisons between groups), the proportion of patients (7/13) who had concomitant surgeries, and the limited options for patient outcome scores. Although the ASES score is a validated outcome score, the Kerlan-Jobe Orthopaedic Clinic Shoulder and Elbow (KJOC) score or the Disabilities of the Arm, Shoulder, and Hand (DASH) score may be more appropriate in an athletic population. In addition, although all study patients had GIRD that was unresponsive to a concerted trial of nonoperative management, we did not have a control group (nonoperatively treated patients) for comparison. Finally, we did not obtain computed tomography scans or account for the potential contribution of humeral retroversion to GIRD in this group of patients.
Conclusion
Selective arthroscopic posterior-inferior capsular release can be recommended as a reasonable operative solution for overhead athletes with symptomatic GIRD that has not responded to conservative management. In the present study, ASES scores improved significantly, and 77% of our athlete-patients returned to sport at their preoperative level of play or a higher level.
1. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
2. Wilk KE, Macrina LC, Fleisig GS, et al. Correlation of glenohumeral internal rotation deficit and total rotational motion to shoulder injuries in professional baseball pitchers. Am J Sports Med. 2011;39(2):329-335.
3. Bigliani LU, Codd TP, Connor PM, Levine WN, Littlefield MA, Hershon SJ. Shoulder motion and laxity in the professional baseball player. Am J Sports Med. 1997;25(5):609-613.
4. Brown LP, Niehues SL, Harrah A, Yavorsky P, Hirshman HP. Upper extremity range of motion and isokinetic strength of the internal and external shoulder rotators in Major League baseball players. Am J Sports Med. 1988;16(6):577-585.
5. Crockett HC, Gross LB, Wilk KE, et al. Osseous adaptation and range of motion at the glenohumeral joint in professional baseball pitchers. Am J Sports Med. 2002;30(1):20-26.
6. Kibler WB, Chandler TJ, Livingston BP, Roetert EP. Shoulder range of motion in elite tennis players. Effect of age and years of tournament play. Am J Sports Med. 1996;24(3):279-285.
7. Meister K. Injuries to the shoulder in the throwing athlete. Part one: biomechanics/pathophysiology/classification of injury. Am J Sports Med. 2000;28(2):265-275.
8. Osbahr DC, Cannon DL, Speer KP. Retroversion of the humerus in the throwing shoulder of college baseball pitchers. Am J Sports Med. 2002;30(3):347-353.
9. Torres RR, Gomes JL. Measurement of glenohumeral internal rotation in asymptomatic tennis players and swimmers. Am J Sports Med. 2009;37(5):1017-1023.
10. Tyler TF, Nicholas SJ, Lee SJ, Mullaney M, McHugh MP. Correction of posterior shoulder tightness is associated with symptom resolution in patients with internal impingement. Am J Sports Med. 2010;28(1):114-119.
11. Wilk KE, Meister K, Andrews JR. Current concepts in the rehabilitation of the overhead throwing athlete. Am J Sports Med. 2002;30(1):136-151.
12. Braun S, Kokmeyer D, Millett PJ. Shoulder injuries in the throwing athlete. J Bone Joint Surg Am. 2009;91(4):966-978.
13. Reagan KM, Meister K, Horodyski MB, Werner DW, Carruthers C, Wilk K. Humeral retroversion and its relationship to glenohumeral rotation in the shoulder of college baseball players. Am J Sports Med. 2002;30(3):354-360.
14. Tehranzadeh AD, Fronek J, Resnick D. Posterior capsular fibrosis in professional baseball pitchers: case series of MR arthrographic findings in six patients with glenohumeral internal rotational deficit. Clin Imaging. 2007;31(5):343-348.
15. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
16. Curtis AS, Deshmukh R. Throwing injuries: diagnosis and treatment. Arthroscopy. 2003;19(suppl 1):80-85.
17. Lajtai G, Pfirrmann CW, Aitzetmuller G, Pirkl C, Gerber C, Jost B. The shoulders of fully competitive professional beach volleyball players: high prevalence of infraspinatus atrophy. Am J Sports Med. 2009;37(7):1375-1383.
18. Burkhart SS, Morgan CD. The peel-back mechanism: its role in producing and extending posterior type II SLAP lesions and its effect on SLAP repair rehabilitation. Arthroscopy. 1998;14(6):637-640.
19. Dines JS, Frank JB, Akerman M, Yocum LA. Glenohumeral internal rotation deficits in baseball players with ulnar collateral ligament insufficiency. Am J Sports Med. 2009;37(3):566-570.
20. Grossman MG, Tibone JE, McGarry MH, Schneider DJ, Veneziani S, Lee TQ. A cadaveric model of the throwing shoulder: a possible etiology of superior labrum anterior-to-posterior lesions. J Bone Joint Surg Am. 2005;87(4):824-831.
21. Myers JB, Laudner KG, Pasquale MR, Bradley JP, Lephart SM. Glenohumeral range of motion deficits and posterior shoulder tightness in throwers with pathologic internal impingement. Am J Sports Med. 2006;34(3):385-391.
22. Ticker JB, Beim GM, Warner JJ. Recognition and treatment of refractory posterior capsular contracture of the shoulder. Arthroscopy. 2000;16(1):27-34.
23. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part III: the SICK scapula, scapular dyskinesis, the kinetic chain, and rehabilitation. Arthroscopy. 2003;19(6):641-661.
24. Kibler WB, McMullen J. Scapular dyskinesis and its relation to shoulder pain. J Am Acad Orthop Surg. 2003;11(2):142-151.
25. Kibler WB. The relationship of glenohumeral internal rotation deficit to shoulder and elbow injuries in tennis players: a prospective evaluation of posterior capsular stretching. Presented at: American Shoulder and Elbow Surgeons 15th Annual Closed Meeting; November 6, 1998; New York, NY.
26. Lintner D, Mayol M, Uzodinma O, Jones R, Labossiere D. Glenohumeral internal rotation deficits in professional pitchers enrolled in an internal rotation stretching program. Am J Sports Med. 2007;35(4):617-621.
27. McClure P, Balaicuis J, Heiland D, Broersma ME, Thorndike CK, Wood A. A randomized controlled comparison of stretching procedures for posterior shoulder tightness. J Orthop Sports Phys Ther. 2007;37(3):108-114.
28. Verna C. Shoulder flexibility to reduce impingement. Presented at: 3rd Annual Professional Baseball Athletic Trainer Society Meeting; March 1991; Mesa, AZ.
29. Bach HG, Goldberg BA. Posterior capsular contracture of the shoulder. J Am Acad Orthop Surg. 2006;14(5):265-277.
30. Yoneda M, Nakagawa S, Mizuno N, et al. Arthroscopic capsular release for painful throwing shoulder with posterior capsular tightness. Arthroscopy. 2006;22(7):801e1-801e5.
31. Kocher MS, Horan MP, Briggs KK, Richardson TR, O’Holleran J, Hawkins RJ. Reliability, validity, and responsiveness of the American Shoulder and Elbow Surgeons subjective shoulder scale in patients with shoulder instability, rotator cuff disease, and glenohumeral arthritis. J Bone Joint Surg Am. 2005;87(9):2006-2011.
32. Johansen RL, Callis M, Potts J, Shall LM. A modified internal rotation stretching technique for overhand and throwing athletes. J Orthop Sports Phys Ther. 1995;21(4):216-219.
33. Harryman DT 2nd, Sidles JA, Clark JM, McQuade KJ, Gibb TD, Matsen FA 3rd. Translation of the humeral head on the glenoid with passive glenohumeral motion. J Bone Joint Surg Am. 1990;72(9):1334-1343.
34. Tyler TF, Nicholas SJ, Roy T, Gleim GW. Quantification of posterior capsule tightness and motion loss in patients with shoulder impingement. Am J Sports Med. 2000;28(5):668-673.
35. McCarty LP, Buss DD, Giveans MR. Correlation between throwing arm strength deficit and glenohumeral internal rotation deficit in professional baseball pitchers, and differences between Latino and non-Latino pitchers. Presented at: American Academy of Orthopaedic Surgeons Annual Meeting; February 2012; San Francisco, CA.
36. Edwards TB, Bostick RD, Greene CC, Baratta RV, Drez D. Interobserver and intraobserver reliability of the measurement of shoulder internal rotation by vertebral level. J Shoulder Elbow Surg. 2002;11(1):40-42.
Glenohumeral internal rotation deficit (GIRD) can be observed in overhead athletes and is thought to play a role in generating pain and rotator cuff weakness in the dominant shoulder with sport. It is unclear what is an acceptable value of GIRD in a population of overhead athletes and whether it should be based solely on internal rotation deficit or should include total range of motion (ROM) deficit.1,2 Acquired GIRD in the athlete’s throwing shoulder has been thoroughly documented in the literature as a loss of internal rotation relative to the nonthrowing shoulder, with etiologies including bony adaptations (increased humeral retroversion), muscular tightness, and posterior capsular tightness.1,3-11 In particular, the repetitive torsional stresses acting on the throwing shoulder of baseball players is thought to produce, over the long term, structural adaptations such as increased humeral retroversion.5,12-14 Further, for shoulders with posterior-inferior capsular tightness, cadaveric studies have shown increased contact pressure at the coracoacromial arch during simulated follow-through.15 Athletes of other overhead and throwing sports, such as football, softball, tennis, and volleyball, may show similar adaptations in overhead motion.9,16,17
GIRD has been associated with a variety of pathologic conditions, including scapular dyskinesis, internal and secondary impingement, partial articular-sided rotator cuff tears, damage to the biceps–labral complex, and ulnar collateral ligament insufficiency.10,12,18-22
Restriction from engaging in exacerbating activities (eg, throwing) and compliance with a specific stretching program reduces or eliminates GIRD in the majority of cases.1,23-28 In the few cases in which conservative management fails, operative intervention may be indicated.1,23,29,30 Few investigators have detailed an operative technique for selective arthroscopic capsular release of the posterior-inferior capsule or evaluated the ability of athletes to return to sport after such surgery.
In this article, we present our technique for arthroscopic posterior-inferior capsular release and report the results of applying this technique in a population of athletes with symptomatic GIRD that was unresponsive to nonoperative treatment and was preventing them from returning to sport.
We hypothesized that selective arthroscopic surgical release of the posterior-inferior capsule would improve symptomatic GIRD and result in a return to sport in the majority of cases unresponsive to nonoperative treatment.
Materials and Methods
Patients
After obtaining institutional review board approval, we retrospectively reviewed patient charts and collected data. Study inclusion criteria were arthroscopic selective posterior-inferior capsular release between 2004 and 2008; failure to resume sport after minimum 3 months of physical therapy, including use of sleeper stretch, active joint mobilization by licensed physical therapist, and sport-specific restriction from exacerbating activities (eg, throwing for baseball players); and active participation in overhead sport.1,27 Exclusion criteria were generalized adhesive capsulitis, labral pathology producing glenohumeral joint instability (Bankart or reverse Bankart lesion), high-grade or full-thickness tearing of rotator cuff, and clinically significant partial-thickness tearing or instability of long head of biceps tendon.
Assessment
One of 3 authors (Dr. Buss, Dr. Codding, or Dr. Dahm) used a bubble goniometer to measure passive internal rotation. Patients were positioned supine with 90° of thoracohumeral abduction and 90° of elbow flexion. The examiner’s hand stabilized the scapula against the examination table, in accordance with published techniques.1,26 Active internal rotation was measured at 0° of thoracohumeral abduction by noting the most superior spinal segment reached. Before and after surgery, passive internal rotation measurements were taken on both arms. GIRD was determined by the difference between dominant and nondominant arm measurements; segmental differences were obtained by subtracting segments achieved between the dominant and nondominant arms.
Before surgery and at minimum 2-year follow-up after surgery, patients completed a subjective questionnaire, which included the American Shoulder and Elbow Surgeons (ASES) Standardized Shoulder Assessment Form, for assessment of both arms. ASES scores are reliable, valid, and responsive in evaluating shoulder pain and function.15,31 Patients also answered questions about their ability to return to play, their level of play after surgery, and whether they would undergo the procedure again.
Surgical Technique
After induction of general anesthesia and standard preparation and draping, the patient is placed in a standard beach-chair position and examined. Diagnostic arthroscopy is then performed. In all patients, intra-articular evaluation revealed a thickened, contracted posterior band of the inferior glenohumeral ligament. This finding is consistent with other studies of patients with significant GIRD.1,14,22,30
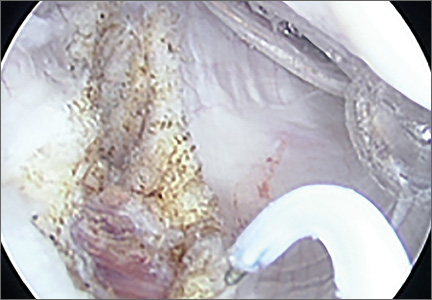
On completion of the diagnostic portion of the arthroscopy, attention is turned to the selective posterior-inferior capsular release. Key to proper execution of the release is establishing a posterior-inferior accessory portal. This is accomplished while viewing from a standard posterior (“soft spot”) portal and determining the appropriate location and angle of entry by spinal needle localization. Typically, an entry point is selected about 4 cm distal and 1 cm lateral to the standard posterior portal. An 18-gauge spinal needle introduced at this location is angled about 15° superiorly and about 20° medially. Once the appropriate vector is determined, a skin incision is made, and a Wissinger rod is introduced, over which a small-diameter cannula is passed. A hooked-tip electrocautery device is used to divide the posterior capsule from the glenoid labrum between the 8- and 6-o’clock positions in the right shoulder (Figure). Care is taken to perform the release immediately adjacent to the glenoid labrum and using short bursts of cautery in order to minimize risk of injury to the teres minor branch of the axillary nerve. Adequate release is confirmed by reassessing passive internal rotation under anesthesia. Additional procedures are performed, if necessary, after completion of the capsular release.
Postoperative rehabilitation consists initially of pendulum exercises and scapular retraction starting on postoperative day 1. Once the swelling from the surgical procedure subsides, typically within 1 week, passive and active-assisted ROM and gentle posterior capsular mobilization are initiated under the direction of a licensed physical therapist. Active ROM is allowed once the patient regains normal scapulothoracic rhythm. Strengthening consists initially of isometrics followed by light resistance strengthening for the rotator cuff and scapular stabilizers once active ROM and scapulothoracic rhythm return to normal. Passive internal rotation stretching, including use of the sleeper stretch, is implemented as soon as tolerated and continues throughout the rehabilitation process.32
Statistical Analysis
Statistical analysis was performed with Stata Release 11 (StataCorp, College Station, Texas). Paired t tests were used to assess preoperative and postoperative mean differences in ASES scores, in passive glenohumeral internal rotation, and in active glenohumeral internal rotation; independent-samples t tests were used to assess side-to-side differences. Significance was set at P < .05.
Results
Fifteen overhead athletes met the study inclusion criteria. Two were lost to follow-up. Of the remaining 13 patients, 6 underwent isolated arthroscopic posterior-inferior capsular release, and 7 had concomitant procedures (6 subacromial decompressions, 1 superior labrum anterior-posterior [SLAP] repair). There were 11 male athletes and 2 female athletes. Twelve of the 13 patients were right-hand–dominant. Mean age at time of surgery was 21 years (range, 16-33 years). There were 10 baseball players (6 pitchers, 4 position players); the other 3 patients played softball (1), volleyball (1), or tennis (1). Six patients played at high school level, 5 at college level, 1 at professional level, and 1 at amateur level. All 13 patients underwent a minimum of 3 months of comprehensive rehabilitation, which included use of the sleeper stretch, active joint mobilization by a licensed physical therapist, and sport-specific restriction from exacerbating activities. Mean duration of symptoms before surgery was 18 months (range, 4-48 months). Mean postoperative follow-up was 31 months (range, 24-59 months). Mean ASES score was 71.5 (range, 33-95) before surgery and 86.9 (range, 60-100) after surgery (P < .001). Mean GIRD improved from 43.1° (range, 30°-60°) before surgery to 9.7° (range, –7° to 40°) after surgery (P < .001). Mean active internal rotation difference improved from 3.8 vertebral segments before surgery to 2.6 vertebral segments after surgery; this difference was not statistically significant (P = .459). Ten (77%) of the 13 patients returned to their preoperative level of play or a higher level; the other 3 (23%) did not return to their preoperative level of play but continued to compete in a different position (Table). Eleven patients (85%) stated they would repeat the procedure. One of the 2 patients who would not repeat the procedure was in the isolated posterior-inferior capsular release group; the other was in the concomitant-procedure group (subacromial decompression). Total glenohumeral ROM of dominant arm was 122° before surgery and 136° after surgery (P = .04). There was no significant difference in total ROM between dominant and nondominant arms after surgery (136° and 141°; P = .12), but the preoperative difference was significant (122° vs 141°; P = .022).
Discussion
GIRD has been associated with various pathologic conditions of the upper extremity. In 1991, Verna28 found that a majority of 39 professional baseball pitchers with significant GIRD had shoulder problems that affected playing time. More recently, GIRD has been associated with a progression of injuries, including scapular dyskinesia, internal and secondary impingement, articular-sided partial rotator cuff tears, rotator cuff weakness, damage to the biceps–labral complex, and ulnar collateral ligament insufficiency.12,18-22 In a cadaveric study of humeral head translation, Harryman and colleagues33 noted an anterosuperior migration of the humeral head during flexion and concluded it resulted from a loose anterior and tight posterior glenohumeral capsule, leading to loss of glenohumeral internal rotation. More recently, posterosuperior migration of the humeral head has been postulated, with GIRD secondary to an essential posterior capsular contracture.1 Tyler and colleagues34 clinically linked posterior capsular tightness with GIRD, and both cadaveric and magnetic resonance imaging studies have supported the finding that posterior capsular contracture leads to posterosuperior humeral head migration in association with GIRD.14,20 Such a disruption in normal glenohumeral joint mechanics could produce phenomena of internal or secondary acromiohumeral impingement and pain.
More recently, in a large cohort of professional baseball pitchers, a significant correlation was found between the incidence of rotator cuff strength deficits and GIRD.35 More than 40% of the pitchers with GIRD of at least 35° had a measureable rotator cuff strength deficit in the throwing shoulder.
Burkhart and colleagues23 concluded that the shoulder most at risk for developing “dead arm” has GIRD and an advanced form of scapular dyskinesia known as SICK scapula (the phenomenon involves Scapula malposition, Inferior medial border prominence, Coracoid pain and malposition, and dysKinesis of scapular movement).
Most athletes with symptoms attributed to GIRD respond to conservative management. A posterior-inferior capsular stretching program focused on regaining internal rotation in the throwing arm has been shown to return about 90% of athletes to play.1 Numerous studies have indicated that enrollment in a compliant stretching program reduces GIRD.1,23-27 However, nonoperative treatment fails in a reported 10% of patients with GIRD; these patients may respond to operative treatment.1
More specifically, for patients who do not respond to conservative treatment, a posterior-inferior capsular release may be indicated.1,29 Ticker and colleagues22 identified 9 patients who had lost internal rotation and had a posterior capsular contracture at arthroscopy. That study, however, was not performed on overhead or throwing athletes. Yoneda and colleagues30 followed 16 overhead throwing athletes after arthroscopic posterior-inferior capsular release and found favorable preliminary clinical results. Eleven of the 16 patients returned to their preinjury level of performance; the other 5 returned to a lower level. In addition, all 4 patients who underwent isolated arthroscopic capsular release had throwing power restored to between 90% and 100%.
In the present study, 10 of 13 patients who underwent arthroscopic posterior-inferior capsular release returned to their preoperative level of play or a higher level. Mean passive GIRD improved significantly from before surgery to after surgery. ASES scores likewise were significantly improved from before surgery to after surgery. The active internal rotation difference as measured by vertebral segment level was not significantly changed after surgery. This lack of improvement may stem from the more complex musculoligamentous interactions governing active internal rotation versus isolated, passive internal rotation. Another possible explanation for lack of improvement is that the interobserver and intraobserver reliability of this method is lower.36
At 2-year follow-up, the patient who had undergone concomitant SLAP repair demonstrated a 23% improvement in ASES score and more internal rotation on the dominant arm relative to the nondominant arm. This patient returned to a level of play at least as good as his preoperative level. Although we could not determine its statistical significance, this patient’s improvement suggests that the SLAP repair did not reduce the efficacy of the posterior-inferior capsular release.
Limitations of this study include its relatively small cohort (precluded statistical comparisons between groups), the proportion of patients (7/13) who had concomitant surgeries, and the limited options for patient outcome scores. Although the ASES score is a validated outcome score, the Kerlan-Jobe Orthopaedic Clinic Shoulder and Elbow (KJOC) score or the Disabilities of the Arm, Shoulder, and Hand (DASH) score may be more appropriate in an athletic population. In addition, although all study patients had GIRD that was unresponsive to a concerted trial of nonoperative management, we did not have a control group (nonoperatively treated patients) for comparison. Finally, we did not obtain computed tomography scans or account for the potential contribution of humeral retroversion to GIRD in this group of patients.
Conclusion
Selective arthroscopic posterior-inferior capsular release can be recommended as a reasonable operative solution for overhead athletes with symptomatic GIRD that has not responded to conservative management. In the present study, ASES scores improved significantly, and 77% of our athlete-patients returned to sport at their preoperative level of play or a higher level.
Glenohumeral internal rotation deficit (GIRD) can be observed in overhead athletes and is thought to play a role in generating pain and rotator cuff weakness in the dominant shoulder with sport. It is unclear what is an acceptable value of GIRD in a population of overhead athletes and whether it should be based solely on internal rotation deficit or should include total range of motion (ROM) deficit.1,2 Acquired GIRD in the athlete’s throwing shoulder has been thoroughly documented in the literature as a loss of internal rotation relative to the nonthrowing shoulder, with etiologies including bony adaptations (increased humeral retroversion), muscular tightness, and posterior capsular tightness.1,3-11 In particular, the repetitive torsional stresses acting on the throwing shoulder of baseball players is thought to produce, over the long term, structural adaptations such as increased humeral retroversion.5,12-14 Further, for shoulders with posterior-inferior capsular tightness, cadaveric studies have shown increased contact pressure at the coracoacromial arch during simulated follow-through.15 Athletes of other overhead and throwing sports, such as football, softball, tennis, and volleyball, may show similar adaptations in overhead motion.9,16,17
GIRD has been associated with a variety of pathologic conditions, including scapular dyskinesis, internal and secondary impingement, partial articular-sided rotator cuff tears, damage to the biceps–labral complex, and ulnar collateral ligament insufficiency.10,12,18-22
Restriction from engaging in exacerbating activities (eg, throwing) and compliance with a specific stretching program reduces or eliminates GIRD in the majority of cases.1,23-28 In the few cases in which conservative management fails, operative intervention may be indicated.1,23,29,30 Few investigators have detailed an operative technique for selective arthroscopic capsular release of the posterior-inferior capsule or evaluated the ability of athletes to return to sport after such surgery.
In this article, we present our technique for arthroscopic posterior-inferior capsular release and report the results of applying this technique in a population of athletes with symptomatic GIRD that was unresponsive to nonoperative treatment and was preventing them from returning to sport.
We hypothesized that selective arthroscopic surgical release of the posterior-inferior capsule would improve symptomatic GIRD and result in a return to sport in the majority of cases unresponsive to nonoperative treatment.
Materials and Methods
Patients
After obtaining institutional review board approval, we retrospectively reviewed patient charts and collected data. Study inclusion criteria were arthroscopic selective posterior-inferior capsular release between 2004 and 2008; failure to resume sport after minimum 3 months of physical therapy, including use of sleeper stretch, active joint mobilization by licensed physical therapist, and sport-specific restriction from exacerbating activities (eg, throwing for baseball players); and active participation in overhead sport.1,27 Exclusion criteria were generalized adhesive capsulitis, labral pathology producing glenohumeral joint instability (Bankart or reverse Bankart lesion), high-grade or full-thickness tearing of rotator cuff, and clinically significant partial-thickness tearing or instability of long head of biceps tendon.
Assessment
One of 3 authors (Dr. Buss, Dr. Codding, or Dr. Dahm) used a bubble goniometer to measure passive internal rotation. Patients were positioned supine with 90° of thoracohumeral abduction and 90° of elbow flexion. The examiner’s hand stabilized the scapula against the examination table, in accordance with published techniques.1,26 Active internal rotation was measured at 0° of thoracohumeral abduction by noting the most superior spinal segment reached. Before and after surgery, passive internal rotation measurements were taken on both arms. GIRD was determined by the difference between dominant and nondominant arm measurements; segmental differences were obtained by subtracting segments achieved between the dominant and nondominant arms.
Before surgery and at minimum 2-year follow-up after surgery, patients completed a subjective questionnaire, which included the American Shoulder and Elbow Surgeons (ASES) Standardized Shoulder Assessment Form, for assessment of both arms. ASES scores are reliable, valid, and responsive in evaluating shoulder pain and function.15,31 Patients also answered questions about their ability to return to play, their level of play after surgery, and whether they would undergo the procedure again.
Surgical Technique
After induction of general anesthesia and standard preparation and draping, the patient is placed in a standard beach-chair position and examined. Diagnostic arthroscopy is then performed. In all patients, intra-articular evaluation revealed a thickened, contracted posterior band of the inferior glenohumeral ligament. This finding is consistent with other studies of patients with significant GIRD.1,14,22,30
On completion of the diagnostic portion of the arthroscopy, attention is turned to the selective posterior-inferior capsular release. Key to proper execution of the release is establishing a posterior-inferior accessory portal. This is accomplished while viewing from a standard posterior (“soft spot”) portal and determining the appropriate location and angle of entry by spinal needle localization. Typically, an entry point is selected about 4 cm distal and 1 cm lateral to the standard posterior portal. An 18-gauge spinal needle introduced at this location is angled about 15° superiorly and about 20° medially. Once the appropriate vector is determined, a skin incision is made, and a Wissinger rod is introduced, over which a small-diameter cannula is passed. A hooked-tip electrocautery device is used to divide the posterior capsule from the glenoid labrum between the 8- and 6-o’clock positions in the right shoulder (Figure). Care is taken to perform the release immediately adjacent to the glenoid labrum and using short bursts of cautery in order to minimize risk of injury to the teres minor branch of the axillary nerve. Adequate release is confirmed by reassessing passive internal rotation under anesthesia. Additional procedures are performed, if necessary, after completion of the capsular release.
Postoperative rehabilitation consists initially of pendulum exercises and scapular retraction starting on postoperative day 1. Once the swelling from the surgical procedure subsides, typically within 1 week, passive and active-assisted ROM and gentle posterior capsular mobilization are initiated under the direction of a licensed physical therapist. Active ROM is allowed once the patient regains normal scapulothoracic rhythm. Strengthening consists initially of isometrics followed by light resistance strengthening for the rotator cuff and scapular stabilizers once active ROM and scapulothoracic rhythm return to normal. Passive internal rotation stretching, including use of the sleeper stretch, is implemented as soon as tolerated and continues throughout the rehabilitation process.32
Statistical Analysis
Statistical analysis was performed with Stata Release 11 (StataCorp, College Station, Texas). Paired t tests were used to assess preoperative and postoperative mean differences in ASES scores, in passive glenohumeral internal rotation, and in active glenohumeral internal rotation; independent-samples t tests were used to assess side-to-side differences. Significance was set at P < .05.
Results
Fifteen overhead athletes met the study inclusion criteria. Two were lost to follow-up. Of the remaining 13 patients, 6 underwent isolated arthroscopic posterior-inferior capsular release, and 7 had concomitant procedures (6 subacromial decompressions, 1 superior labrum anterior-posterior [SLAP] repair). There were 11 male athletes and 2 female athletes. Twelve of the 13 patients were right-hand–dominant. Mean age at time of surgery was 21 years (range, 16-33 years). There were 10 baseball players (6 pitchers, 4 position players); the other 3 patients played softball (1), volleyball (1), or tennis (1). Six patients played at high school level, 5 at college level, 1 at professional level, and 1 at amateur level. All 13 patients underwent a minimum of 3 months of comprehensive rehabilitation, which included use of the sleeper stretch, active joint mobilization by a licensed physical therapist, and sport-specific restriction from exacerbating activities. Mean duration of symptoms before surgery was 18 months (range, 4-48 months). Mean postoperative follow-up was 31 months (range, 24-59 months). Mean ASES score was 71.5 (range, 33-95) before surgery and 86.9 (range, 60-100) after surgery (P < .001). Mean GIRD improved from 43.1° (range, 30°-60°) before surgery to 9.7° (range, –7° to 40°) after surgery (P < .001). Mean active internal rotation difference improved from 3.8 vertebral segments before surgery to 2.6 vertebral segments after surgery; this difference was not statistically significant (P = .459). Ten (77%) of the 13 patients returned to their preoperative level of play or a higher level; the other 3 (23%) did not return to their preoperative level of play but continued to compete in a different position (Table). Eleven patients (85%) stated they would repeat the procedure. One of the 2 patients who would not repeat the procedure was in the isolated posterior-inferior capsular release group; the other was in the concomitant-procedure group (subacromial decompression). Total glenohumeral ROM of dominant arm was 122° before surgery and 136° after surgery (P = .04). There was no significant difference in total ROM between dominant and nondominant arms after surgery (136° and 141°; P = .12), but the preoperative difference was significant (122° vs 141°; P = .022).
Discussion
GIRD has been associated with various pathologic conditions of the upper extremity. In 1991, Verna28 found that a majority of 39 professional baseball pitchers with significant GIRD had shoulder problems that affected playing time. More recently, GIRD has been associated with a progression of injuries, including scapular dyskinesia, internal and secondary impingement, articular-sided partial rotator cuff tears, rotator cuff weakness, damage to the biceps–labral complex, and ulnar collateral ligament insufficiency.12,18-22 In a cadaveric study of humeral head translation, Harryman and colleagues33 noted an anterosuperior migration of the humeral head during flexion and concluded it resulted from a loose anterior and tight posterior glenohumeral capsule, leading to loss of glenohumeral internal rotation. More recently, posterosuperior migration of the humeral head has been postulated, with GIRD secondary to an essential posterior capsular contracture.1 Tyler and colleagues34 clinically linked posterior capsular tightness with GIRD, and both cadaveric and magnetic resonance imaging studies have supported the finding that posterior capsular contracture leads to posterosuperior humeral head migration in association with GIRD.14,20 Such a disruption in normal glenohumeral joint mechanics could produce phenomena of internal or secondary acromiohumeral impingement and pain.
More recently, in a large cohort of professional baseball pitchers, a significant correlation was found between the incidence of rotator cuff strength deficits and GIRD.35 More than 40% of the pitchers with GIRD of at least 35° had a measureable rotator cuff strength deficit in the throwing shoulder.
Burkhart and colleagues23 concluded that the shoulder most at risk for developing “dead arm” has GIRD and an advanced form of scapular dyskinesia known as SICK scapula (the phenomenon involves Scapula malposition, Inferior medial border prominence, Coracoid pain and malposition, and dysKinesis of scapular movement).
Most athletes with symptoms attributed to GIRD respond to conservative management. A posterior-inferior capsular stretching program focused on regaining internal rotation in the throwing arm has been shown to return about 90% of athletes to play.1 Numerous studies have indicated that enrollment in a compliant stretching program reduces GIRD.1,23-27 However, nonoperative treatment fails in a reported 10% of patients with GIRD; these patients may respond to operative treatment.1
More specifically, for patients who do not respond to conservative treatment, a posterior-inferior capsular release may be indicated.1,29 Ticker and colleagues22 identified 9 patients who had lost internal rotation and had a posterior capsular contracture at arthroscopy. That study, however, was not performed on overhead or throwing athletes. Yoneda and colleagues30 followed 16 overhead throwing athletes after arthroscopic posterior-inferior capsular release and found favorable preliminary clinical results. Eleven of the 16 patients returned to their preinjury level of performance; the other 5 returned to a lower level. In addition, all 4 patients who underwent isolated arthroscopic capsular release had throwing power restored to between 90% and 100%.
In the present study, 10 of 13 patients who underwent arthroscopic posterior-inferior capsular release returned to their preoperative level of play or a higher level. Mean passive GIRD improved significantly from before surgery to after surgery. ASES scores likewise were significantly improved from before surgery to after surgery. The active internal rotation difference as measured by vertebral segment level was not significantly changed after surgery. This lack of improvement may stem from the more complex musculoligamentous interactions governing active internal rotation versus isolated, passive internal rotation. Another possible explanation for lack of improvement is that the interobserver and intraobserver reliability of this method is lower.36
At 2-year follow-up, the patient who had undergone concomitant SLAP repair demonstrated a 23% improvement in ASES score and more internal rotation on the dominant arm relative to the nondominant arm. This patient returned to a level of play at least as good as his preoperative level. Although we could not determine its statistical significance, this patient’s improvement suggests that the SLAP repair did not reduce the efficacy of the posterior-inferior capsular release.
Limitations of this study include its relatively small cohort (precluded statistical comparisons between groups), the proportion of patients (7/13) who had concomitant surgeries, and the limited options for patient outcome scores. Although the ASES score is a validated outcome score, the Kerlan-Jobe Orthopaedic Clinic Shoulder and Elbow (KJOC) score or the Disabilities of the Arm, Shoulder, and Hand (DASH) score may be more appropriate in an athletic population. In addition, although all study patients had GIRD that was unresponsive to a concerted trial of nonoperative management, we did not have a control group (nonoperatively treated patients) for comparison. Finally, we did not obtain computed tomography scans or account for the potential contribution of humeral retroversion to GIRD in this group of patients.
Conclusion
Selective arthroscopic posterior-inferior capsular release can be recommended as a reasonable operative solution for overhead athletes with symptomatic GIRD that has not responded to conservative management. In the present study, ASES scores improved significantly, and 77% of our athlete-patients returned to sport at their preoperative level of play or a higher level.
1. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
2. Wilk KE, Macrina LC, Fleisig GS, et al. Correlation of glenohumeral internal rotation deficit and total rotational motion to shoulder injuries in professional baseball pitchers. Am J Sports Med. 2011;39(2):329-335.
3. Bigliani LU, Codd TP, Connor PM, Levine WN, Littlefield MA, Hershon SJ. Shoulder motion and laxity in the professional baseball player. Am J Sports Med. 1997;25(5):609-613.
4. Brown LP, Niehues SL, Harrah A, Yavorsky P, Hirshman HP. Upper extremity range of motion and isokinetic strength of the internal and external shoulder rotators in Major League baseball players. Am J Sports Med. 1988;16(6):577-585.
5. Crockett HC, Gross LB, Wilk KE, et al. Osseous adaptation and range of motion at the glenohumeral joint in professional baseball pitchers. Am J Sports Med. 2002;30(1):20-26.
6. Kibler WB, Chandler TJ, Livingston BP, Roetert EP. Shoulder range of motion in elite tennis players. Effect of age and years of tournament play. Am J Sports Med. 1996;24(3):279-285.
7. Meister K. Injuries to the shoulder in the throwing athlete. Part one: biomechanics/pathophysiology/classification of injury. Am J Sports Med. 2000;28(2):265-275.
8. Osbahr DC, Cannon DL, Speer KP. Retroversion of the humerus in the throwing shoulder of college baseball pitchers. Am J Sports Med. 2002;30(3):347-353.
9. Torres RR, Gomes JL. Measurement of glenohumeral internal rotation in asymptomatic tennis players and swimmers. Am J Sports Med. 2009;37(5):1017-1023.
10. Tyler TF, Nicholas SJ, Lee SJ, Mullaney M, McHugh MP. Correction of posterior shoulder tightness is associated with symptom resolution in patients with internal impingement. Am J Sports Med. 2010;28(1):114-119.
11. Wilk KE, Meister K, Andrews JR. Current concepts in the rehabilitation of the overhead throwing athlete. Am J Sports Med. 2002;30(1):136-151.
12. Braun S, Kokmeyer D, Millett PJ. Shoulder injuries in the throwing athlete. J Bone Joint Surg Am. 2009;91(4):966-978.
13. Reagan KM, Meister K, Horodyski MB, Werner DW, Carruthers C, Wilk K. Humeral retroversion and its relationship to glenohumeral rotation in the shoulder of college baseball players. Am J Sports Med. 2002;30(3):354-360.
14. Tehranzadeh AD, Fronek J, Resnick D. Posterior capsular fibrosis in professional baseball pitchers: case series of MR arthrographic findings in six patients with glenohumeral internal rotational deficit. Clin Imaging. 2007;31(5):343-348.
15. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
16. Curtis AS, Deshmukh R. Throwing injuries: diagnosis and treatment. Arthroscopy. 2003;19(suppl 1):80-85.
17. Lajtai G, Pfirrmann CW, Aitzetmuller G, Pirkl C, Gerber C, Jost B. The shoulders of fully competitive professional beach volleyball players: high prevalence of infraspinatus atrophy. Am J Sports Med. 2009;37(7):1375-1383.
18. Burkhart SS, Morgan CD. The peel-back mechanism: its role in producing and extending posterior type II SLAP lesions and its effect on SLAP repair rehabilitation. Arthroscopy. 1998;14(6):637-640.
19. Dines JS, Frank JB, Akerman M, Yocum LA. Glenohumeral internal rotation deficits in baseball players with ulnar collateral ligament insufficiency. Am J Sports Med. 2009;37(3):566-570.
20. Grossman MG, Tibone JE, McGarry MH, Schneider DJ, Veneziani S, Lee TQ. A cadaveric model of the throwing shoulder: a possible etiology of superior labrum anterior-to-posterior lesions. J Bone Joint Surg Am. 2005;87(4):824-831.
21. Myers JB, Laudner KG, Pasquale MR, Bradley JP, Lephart SM. Glenohumeral range of motion deficits and posterior shoulder tightness in throwers with pathologic internal impingement. Am J Sports Med. 2006;34(3):385-391.
22. Ticker JB, Beim GM, Warner JJ. Recognition and treatment of refractory posterior capsular contracture of the shoulder. Arthroscopy. 2000;16(1):27-34.
23. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part III: the SICK scapula, scapular dyskinesis, the kinetic chain, and rehabilitation. Arthroscopy. 2003;19(6):641-661.
24. Kibler WB, McMullen J. Scapular dyskinesis and its relation to shoulder pain. J Am Acad Orthop Surg. 2003;11(2):142-151.
25. Kibler WB. The relationship of glenohumeral internal rotation deficit to shoulder and elbow injuries in tennis players: a prospective evaluation of posterior capsular stretching. Presented at: American Shoulder and Elbow Surgeons 15th Annual Closed Meeting; November 6, 1998; New York, NY.
26. Lintner D, Mayol M, Uzodinma O, Jones R, Labossiere D. Glenohumeral internal rotation deficits in professional pitchers enrolled in an internal rotation stretching program. Am J Sports Med. 2007;35(4):617-621.
27. McClure P, Balaicuis J, Heiland D, Broersma ME, Thorndike CK, Wood A. A randomized controlled comparison of stretching procedures for posterior shoulder tightness. J Orthop Sports Phys Ther. 2007;37(3):108-114.
28. Verna C. Shoulder flexibility to reduce impingement. Presented at: 3rd Annual Professional Baseball Athletic Trainer Society Meeting; March 1991; Mesa, AZ.
29. Bach HG, Goldberg BA. Posterior capsular contracture of the shoulder. J Am Acad Orthop Surg. 2006;14(5):265-277.
30. Yoneda M, Nakagawa S, Mizuno N, et al. Arthroscopic capsular release for painful throwing shoulder with posterior capsular tightness. Arthroscopy. 2006;22(7):801e1-801e5.
31. Kocher MS, Horan MP, Briggs KK, Richardson TR, O’Holleran J, Hawkins RJ. Reliability, validity, and responsiveness of the American Shoulder and Elbow Surgeons subjective shoulder scale in patients with shoulder instability, rotator cuff disease, and glenohumeral arthritis. J Bone Joint Surg Am. 2005;87(9):2006-2011.
32. Johansen RL, Callis M, Potts J, Shall LM. A modified internal rotation stretching technique for overhand and throwing athletes. J Orthop Sports Phys Ther. 1995;21(4):216-219.
33. Harryman DT 2nd, Sidles JA, Clark JM, McQuade KJ, Gibb TD, Matsen FA 3rd. Translation of the humeral head on the glenoid with passive glenohumeral motion. J Bone Joint Surg Am. 1990;72(9):1334-1343.
34. Tyler TF, Nicholas SJ, Roy T, Gleim GW. Quantification of posterior capsule tightness and motion loss in patients with shoulder impingement. Am J Sports Med. 2000;28(5):668-673.
35. McCarty LP, Buss DD, Giveans MR. Correlation between throwing arm strength deficit and glenohumeral internal rotation deficit in professional baseball pitchers, and differences between Latino and non-Latino pitchers. Presented at: American Academy of Orthopaedic Surgeons Annual Meeting; February 2012; San Francisco, CA.
36. Edwards TB, Bostick RD, Greene CC, Baratta RV, Drez D. Interobserver and intraobserver reliability of the measurement of shoulder internal rotation by vertebral level. J Shoulder Elbow Surg. 2002;11(1):40-42.
1. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
2. Wilk KE, Macrina LC, Fleisig GS, et al. Correlation of glenohumeral internal rotation deficit and total rotational motion to shoulder injuries in professional baseball pitchers. Am J Sports Med. 2011;39(2):329-335.
3. Bigliani LU, Codd TP, Connor PM, Levine WN, Littlefield MA, Hershon SJ. Shoulder motion and laxity in the professional baseball player. Am J Sports Med. 1997;25(5):609-613.
4. Brown LP, Niehues SL, Harrah A, Yavorsky P, Hirshman HP. Upper extremity range of motion and isokinetic strength of the internal and external shoulder rotators in Major League baseball players. Am J Sports Med. 1988;16(6):577-585.
5. Crockett HC, Gross LB, Wilk KE, et al. Osseous adaptation and range of motion at the glenohumeral joint in professional baseball pitchers. Am J Sports Med. 2002;30(1):20-26.
6. Kibler WB, Chandler TJ, Livingston BP, Roetert EP. Shoulder range of motion in elite tennis players. Effect of age and years of tournament play. Am J Sports Med. 1996;24(3):279-285.
7. Meister K. Injuries to the shoulder in the throwing athlete. Part one: biomechanics/pathophysiology/classification of injury. Am J Sports Med. 2000;28(2):265-275.
8. Osbahr DC, Cannon DL, Speer KP. Retroversion of the humerus in the throwing shoulder of college baseball pitchers. Am J Sports Med. 2002;30(3):347-353.
9. Torres RR, Gomes JL. Measurement of glenohumeral internal rotation in asymptomatic tennis players and swimmers. Am J Sports Med. 2009;37(5):1017-1023.
10. Tyler TF, Nicholas SJ, Lee SJ, Mullaney M, McHugh MP. Correction of posterior shoulder tightness is associated with symptom resolution in patients with internal impingement. Am J Sports Med. 2010;28(1):114-119.
11. Wilk KE, Meister K, Andrews JR. Current concepts in the rehabilitation of the overhead throwing athlete. Am J Sports Med. 2002;30(1):136-151.
12. Braun S, Kokmeyer D, Millett PJ. Shoulder injuries in the throwing athlete. J Bone Joint Surg Am. 2009;91(4):966-978.
13. Reagan KM, Meister K, Horodyski MB, Werner DW, Carruthers C, Wilk K. Humeral retroversion and its relationship to glenohumeral rotation in the shoulder of college baseball players. Am J Sports Med. 2002;30(3):354-360.
14. Tehranzadeh AD, Fronek J, Resnick D. Posterior capsular fibrosis in professional baseball pitchers: case series of MR arthrographic findings in six patients with glenohumeral internal rotational deficit. Clin Imaging. 2007;31(5):343-348.
15. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
16. Curtis AS, Deshmukh R. Throwing injuries: diagnosis and treatment. Arthroscopy. 2003;19(suppl 1):80-85.
17. Lajtai G, Pfirrmann CW, Aitzetmuller G, Pirkl C, Gerber C, Jost B. The shoulders of fully competitive professional beach volleyball players: high prevalence of infraspinatus atrophy. Am J Sports Med. 2009;37(7):1375-1383.
18. Burkhart SS, Morgan CD. The peel-back mechanism: its role in producing and extending posterior type II SLAP lesions and its effect on SLAP repair rehabilitation. Arthroscopy. 1998;14(6):637-640.
19. Dines JS, Frank JB, Akerman M, Yocum LA. Glenohumeral internal rotation deficits in baseball players with ulnar collateral ligament insufficiency. Am J Sports Med. 2009;37(3):566-570.
20. Grossman MG, Tibone JE, McGarry MH, Schneider DJ, Veneziani S, Lee TQ. A cadaveric model of the throwing shoulder: a possible etiology of superior labrum anterior-to-posterior lesions. J Bone Joint Surg Am. 2005;87(4):824-831.
21. Myers JB, Laudner KG, Pasquale MR, Bradley JP, Lephart SM. Glenohumeral range of motion deficits and posterior shoulder tightness in throwers with pathologic internal impingement. Am J Sports Med. 2006;34(3):385-391.
22. Ticker JB, Beim GM, Warner JJ. Recognition and treatment of refractory posterior capsular contracture of the shoulder. Arthroscopy. 2000;16(1):27-34.
23. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part III: the SICK scapula, scapular dyskinesis, the kinetic chain, and rehabilitation. Arthroscopy. 2003;19(6):641-661.
24. Kibler WB, McMullen J. Scapular dyskinesis and its relation to shoulder pain. J Am Acad Orthop Surg. 2003;11(2):142-151.
25. Kibler WB. The relationship of glenohumeral internal rotation deficit to shoulder and elbow injuries in tennis players: a prospective evaluation of posterior capsular stretching. Presented at: American Shoulder and Elbow Surgeons 15th Annual Closed Meeting; November 6, 1998; New York, NY.
26. Lintner D, Mayol M, Uzodinma O, Jones R, Labossiere D. Glenohumeral internal rotation deficits in professional pitchers enrolled in an internal rotation stretching program. Am J Sports Med. 2007;35(4):617-621.
27. McClure P, Balaicuis J, Heiland D, Broersma ME, Thorndike CK, Wood A. A randomized controlled comparison of stretching procedures for posterior shoulder tightness. J Orthop Sports Phys Ther. 2007;37(3):108-114.
28. Verna C. Shoulder flexibility to reduce impingement. Presented at: 3rd Annual Professional Baseball Athletic Trainer Society Meeting; March 1991; Mesa, AZ.
29. Bach HG, Goldberg BA. Posterior capsular contracture of the shoulder. J Am Acad Orthop Surg. 2006;14(5):265-277.
30. Yoneda M, Nakagawa S, Mizuno N, et al. Arthroscopic capsular release for painful throwing shoulder with posterior capsular tightness. Arthroscopy. 2006;22(7):801e1-801e5.
31. Kocher MS, Horan MP, Briggs KK, Richardson TR, O’Holleran J, Hawkins RJ. Reliability, validity, and responsiveness of the American Shoulder and Elbow Surgeons subjective shoulder scale in patients with shoulder instability, rotator cuff disease, and glenohumeral arthritis. J Bone Joint Surg Am. 2005;87(9):2006-2011.
32. Johansen RL, Callis M, Potts J, Shall LM. A modified internal rotation stretching technique for overhand and throwing athletes. J Orthop Sports Phys Ther. 1995;21(4):216-219.
33. Harryman DT 2nd, Sidles JA, Clark JM, McQuade KJ, Gibb TD, Matsen FA 3rd. Translation of the humeral head on the glenoid with passive glenohumeral motion. J Bone Joint Surg Am. 1990;72(9):1334-1343.
34. Tyler TF, Nicholas SJ, Roy T, Gleim GW. Quantification of posterior capsule tightness and motion loss in patients with shoulder impingement. Am J Sports Med. 2000;28(5):668-673.
35. McCarty LP, Buss DD, Giveans MR. Correlation between throwing arm strength deficit and glenohumeral internal rotation deficit in professional baseball pitchers, and differences between Latino and non-Latino pitchers. Presented at: American Academy of Orthopaedic Surgeons Annual Meeting; February 2012; San Francisco, CA.
36. Edwards TB, Bostick RD, Greene CC, Baratta RV, Drez D. Interobserver and intraobserver reliability of the measurement of shoulder internal rotation by vertebral level. J Shoulder Elbow Surg. 2002;11(1):40-42.
Enhancement of Acute Tendon Repair Using Chitosan Matrix
Rotator cuff tears (RCTs) are common tendon injuries that can cause chronic pain and severe functional disability. Massive RCTs do not heal spontaneously and, in many cases, result in poor clinical outcomes. Specifically, muscle atrophy and fatty infiltration correlate with poor outcomes after surgical repair.1 Fatty infiltration of the rotator cuff is a common phenomenon that can lead to permanent structural alterations within the tendon. It has been suggested that changes in muscle fiber orientation (the pennation angle) can cause mesenchymal stem cells to migrate to the interface between muscle fibers and the region of fatty infiltration of the muscle.2 Understanding the factors involved in muscle degeneration and atrophy, and in fatty infiltration, may lead to treatments that improve outcomes for patients with massive RCTs. One proposed treatment involves placing continuous mechanical traction on the ends of the torn tendon.2 Findings from this research have indicated that acute tears that become chronic tears are typified by inelasticity and poor function of the muscle–tendon unit. It is therefore important to develop a method that speeds tendon healing without causing the muscle fiber atrophy and pennation angle changes that lead to fatty atrophy, which appears to be an irreversible structural change.
On the basis of the theory that adding mesenchymal cells may improve tendon healing, investigators have studied use of transcription factors (eg, scleraxis) specific to tendogenesis in the embryonal stage.3,4 Nevertheless, certain transcription factors are associated with formation of fibrocartilage in higher concentrations.4 Moreover, decalcified bone matrix increases cartilage formation when added to the tendon repair site.5 Cartilage formation, however, is associated with poorer functional results.6 Thus, there is a need for a method that facilitates faster tendon healing with higher quality tissue formation and less muscle atrophy.
Chitosan, a linear polysaccharide, is associated with scarless healing of soft tissues and prevention of adhesion formation both intraperitoneally and during tendon healing after surgery.7,8 Chitosan tends to precipitate in physiologic pH, thereby mitigating its potency. Fortunately, a chitosan solution that does not precipitate in physiologic conditions was recently developed.9 The solution’s lack of precipitation, coupled with its in situ gelling, allows it to adhere to the repair site long enough to take effect. These characteristics could allow for intimate contact between gel and tendon, facilitating guided-tissue regeneration and preventing adhesion of the rotator cuff to surrounding tissue. By contrast, other biological agents (eg, platelet-rich plasma) are administered as fluid rather than gel and are therefore more susceptible to diffusing from the repair site, mitigating their effects. Thus, chitosan gel is fairly unique among agents.
In the study reported here, we histologically investigated whether a chitosan gel would help improve healing of rotator cuff tendon (acute supraspinatus) tears in a rat model.
Materials and Methods
Supraspinatus Surgical Model
Forty Wistar rats, each weighing between 300 and 400 g, were used in this study. All procedures were approved by the Institutional Animal Care and Use Committee at Rabin Medical Center in Petah Tikva, Israel. The rats were anesthetized with ketamine 90 mg/kg and xylazine 10 mg/kg, both administered intramuscularly, and anesthesia was prolonged as needed with 2% isoflurane, administered by nose cone. The skin was incised 5 cm along the upper back following the midline of the spine. The resulting skin flaps were retracted and the scapula exposed. Careful blunt dissection allowed visualization of the rotator cuff and the trans-scapular arch. A full-thickness incision of the supraspinatus tendon was then made 2 mm distal to the arch. This procedure was performed on both shoulders. For the right supraspinatus tendon, a bioabsorbable chitosan–hydrochloric acid solution (>70% de-acetylated chitosan, molecular weight of 600 kDa; Heppe Medical Chitosan GmbH, Halle, Germany) was sterilely applied to the ends of the tendon (total volume, 0.5 mL) and automatically gelled in situ by heating to about 37°C (rat’s internal body temperature). The tendon ends were subsequently approximated with a single 4-0 Prolene suture (Ethicon, Somerville, New Jersey). The left shoulder (tendon repaired with suture only) served as a control.
The rats were housed for a maximum of 12 weeks after surgery. They were sacrificed (in groups of 5 each) 2 hours, 3 days, 1 week, 2 weeks, 4 weeks, 6 weeks, 8 weeks, and 12 weeks after surgery. After each rat was sacrificed, both shoulder girdles were harvested, and the sutures were removed from the supraspinatus tendons.
Histologic Analysis
After routine fixation with 4% formalin for 48 hours and decalcification with 10% ethylenediaminetetraacetic acid (EDTA) for 3 weeks, the specimens were sectioned with a microtome blade. Care was taken to ensure the plane of the microtome blade was parallel with the longitudinal plane of the supraspinatus muscle and tendon to allow for evaluation of pennation angle. Hematoxylin-eosin staining and Masson trichrome staining were subsequently performed.
A variety of histologic measurements were obtained with use of ImageJ software (US National Institutes of Health). Percentage of fibrous tissue was determined by examining the slides at low magnification fields (×25) at the tendon healing site. Three such fields were evaluated per specimen. The fibrous tissue was circled manually, and percentage of tissue area was assessed and compared with total region of interest. Cellularity was carefully outlined and measured as percentage of total tendon area occupied by cells. Fatty atrophy was defined as either present or absent. Muscle fiber diameter was defined as average diameter of 10 muscle fibers measured within 2 mm of the tendon laceration site. Inflammatory cell collections were defined as either large (>100 µm in diameter) or small (<100 µm in diameter) and were dichotomized to either present or absent. Pennation angle was defined as average angle between muscle fibers and longitudinal axis of supraspinatus muscle and tendon unit. Ten fibers proximal to and within 2 mm of the laceration site were randomly selected, measured, and averaged.
Statistical Analysis
Statistical analysis was performed with Analyse-it 2.20 for Microsoft Excel 2010 (Analyse-it Software, Leeds, United Kingdom). Data were initially analyzed with the Kolmogorov-Smirnov test to assess for normality of distribution. The t test was used to compare continuous variables when the data were normally distributed and the Mann-Whitney test when the data were not normally distributed.
Results
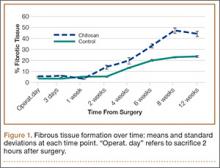
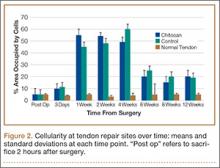
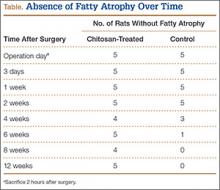
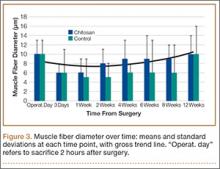
All tendons (both groups) healed within 12 weeks. Generally, the tissue formed at the repair site exhibited a mixture of tenocyte-like cells (fibrotic tissue) and granulation tissue without clear orientation. As noted in Figure 1, the tendons treated with chitosan had more fibrotic tissue (overall mean, 21.5%) relative to the control group (mean, 12.3%), and the difference was significant (P = .003). The most notable differences were found at time points later than 1 week after surgery. In addition, amount of cellularity (Figure 2) was higher in chitosan-treated tendon and control tendon than in the normal, uninjured adjacent tendon at all time points (P < .001). Chitosan-treated tendons had significantly higher cellularity than untreated control tendons from 1 to 2 weeks (P < .001), and control tendons were significantly hypercellular compared with chitosan-treated tendons from 4 to 8 weeks (P < .001), but both groups exhibited similar cellularity by 12 weeks (P > .05). Fatty atrophy was found at significantly higher rates in control rats than in chitosan-treated rats (P = .001; Table). Furthermore, as noted in Figure 3, muscle fiber diameter decreased in both groups after injury (P < .001).
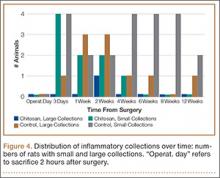
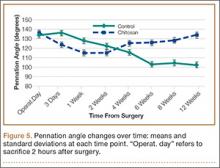
Figure 4 shows that the amount of inflammatory collections was significantly smaller in the chitosan-treated group than in the control group over the course of the study (P = .01). In addition, pennation angle steadily decreased in the control group throughout the study period, whereas it transiently decreased in the chitosan-treated group (until 2 weeks) before returning to its immediate postoperative level by 12 weeks (Figure 5). Overall, the chitosan-treated group maintained a higher pennation angle than the control group did (P < .001).
Discussion
RCTs affect more than 40% of patients over age 60 years and are a common cause of debilitating pain, reduced shoulder function, and weakness.10 Thirty thousand to 75,000 rotator cuff repairs are performed annually in the United States.11 Although the best treatment for this disorder remains a topic of debate, arthroscopic and (when necessary) open surgical repair is the accepted gold standard for the treatment of tears that do not improve with conservative management. Despite advances in the surgical treatment of these tears, the surgical failure rates are high (range, 20%-90%), with failures attributed to factors beyond patient age, tear size and chronicity, muscle atrophy and degeneration, tendon quality, repair technique, and postoperative rehabilitation.12,13 Repair strategies that biologically enhance the patient’s intrinsic healing potential are needed.
In tendon repair, choice of repair material (eg, graft) is crucial in determining the success of tissue engineering approaches. The ideal scaffold is biocompatible and does not elicit a host inflammatory response. The selected scaffold in its composition and fabricated form must be capable of holding and supporting cells. In addition, the scaffold should be biodegradable, serving as a temporary support for such cells and mechanically augmenting the repaired tendon while allowing for eventual replacement by matrix components. Moreover, the scaffold should have high porosity and a large surface area. Furthermore, the material should mimic the native tendon extracellular matrix (ECM) architecture to allow cells to be distributed throughout the scaffold and to facilitate diffusion of nutrients and factors that promote cellular proliferation and ECM production.
Given the importance of glycosaminoglycans (GAGs) in supporting the reticular structure of the matrix, use of GAGs or GAG-analogues as components of a tendon tissue scaffold for enhancing repair is well documented.14 One such candidate is chitosan, a partially de-acetylated derivative of chitin found in arthropod exoskeletons. Structurally, chitosan shares some characteristics with various GAGs and hyaluronic acid.15 More specifically, chitosan is a linear polysaccharide composed of glucosamine and N-acetyl glucosamine units linked by β-glycosidic bonds. Investigators have studied the properties of chitosan, including its biocompatibility, biodegradability, antibacterial activity, mucoadhesivity, and wound healing.16,17
One of the most promising features of chitosan is that it can be processed into porous structures for use in cell transplantation and tissue regeneration.18,19 Porous chitosan structures can be formed by freezing and lyophilizing chitosan-acetic acid solutions; chondrogenic cell adhesion and proliferation onto these structures have been reported.20,21 This chitosan scaffolding method has also been used to test different composites with collagens, gelatins, GAGs, and hyaluronic acid, all of which have also been proposed as useful 3-dimensional materials for tissue repair.22
In the present study, we used chitosan matrix in RCT repair. We hypothesized that chitosan matrix could enhance rotator cuff repair the same way it enhances repair in epidermal tissues.16 Histologic findings demonstrated that the percentage of fibrous tissue was significantly higher in the chitosan-treated group than in the control group. This improved fibroblastic response may be attributed to the ability of chitosan to enhance cell migration and serve as a scaffold for repair. Other studies have indicated that chitin, of which chitosan is the primary derivative, accelerated the healing of skin and subcutaneous tissues by increased cell migration.23 Moreover, Okamoto and colleagues24 reported that chitin implants stimulated abundant angiogenesis through the same mechanism.
Inadequate initial strength of a repair may lead to a recurrent cuff tear or a disability of rotator cuff function in the early healing stages. In our study, the chitosan matrix tended to be absorbed by 6 weeks after surgery. Its adherence to and ultimate absorption at the repair site may be challenged by the flow of irrigation fluid through the subacromial space in the setting of arthroscopic surgery. However, because the chitosan remains in a more robust gel form, it is better able to resist being washed from the repair site. For augmentation, it may be possible to apply a biocompatible patch over the gel to further protect it from being dislodged. In addition, histologic findings showed that the fibrous repair tissue gradually increased until reaching a peak 8 weeks after surgery—an indication that the absorption rate of the chitosan scaffold lags behind full recovery of the repair tissue. Given this relationship, further studies are needed to determine the mechanical strength of the repair between 6 and 8 weeks, which is important for avoiding recurrent tears.
This study had a few limitations. First, as with any animal model, the anatomy and function of the rat shoulder differ from those of the human shoulder. The acromial arch differs in quadruped animals, with less coverage of the supraspinatus and more of the subscapularis.25 These anatomical differences could yield altered stress mechanics that could affect tendon repair. Furthermore, rats and humans differ in their RCT healing rates. Thus, the pathophysiology of muscle atrophy and fat infiltration in rats may slightly differ from that in humans. In addition, no mechanical testing was performed to compare chitosan-treated and untreated rotator cuff repairs, and such testing is needed to clarify the biomechanical importance of augmentation. Furthermore, no immunohistochemical analysis was performed for collagen. In the repair of rotator cuff tendons, surgeons must consider not only the number of cells but also the production of ECM. Although not directly confirmed in this study, chitosan induced fibrous tissue proliferation that mirrored production of a large amount of collagen fibers. Last, we used an open RTC model. As an arthroscopic model was not used, no definitive conclusions can be drawn regarding use of chitosan in arthroscopy.
Conclusion
Use of chitosan as an acellular matrix improved formation of healing fibrous tissue, increased the number of cells, and prevented fatty atrophy and inflammatory aggregates inside repair sites while facilitating recovery of the natural pennation angle of the tissue. These results demonstrate that chitosan can enhance tendon healing in the setting of acute RCT. Further research, including biomechanical testing of repaired tendons, is needed to further delineate the utility of chitosan in regenerating irreparable RCTs.
1. Shen PH, Lien SB, Shen HC, Lee CH, Wu SS, Lin LC. Long-term functional outcomes after repair of rotator cuff tears correlated with atrophy of the supraspinatus muscles on magnetic resonance images. J Shoulder Elbow Surg. 2008;17(1 suppl):1S-7S.
2. Meyer DC, Hoppeler H, von Rechenberg B, Gerber C. A pathomechanical concept explains muscle loss and fatty muscular changes following surgical tendon release. J Orthop Res. 2004;22(5):1004-1007.
3. Gulotta LV, Kovacevic D, Packer JD, Deng XH, Rodeo SA. Bone marrow–derived mesenchymal stem cells transduced with scleraxis improve rotator cuff healing in a rat model. Am J Sports Med. 2011;39(6):1282-1289.
4. Gulotta LV, Rodeo SA. Emerging ideas: evaluation of stem cells genetically modified with scleraxis to improve rotator cuff healing. Clin Orthop. 2011;469(10):2977-2980.
5. Sundar S, Pendegrass CJ, Blunn GW. Tendon bone healing can be enhanced by demineralized bone matrix: a functional and histological study. J Biomed Mater Res B Appl Biomater. 2009;88(1):115-122.
6. Kumagai J, Sarkar K, Uhthoff HK. The collagen types in the attachment zone of rotator cuff tendons in the elderly: an immunohistochemical study. J Rheumatol. 1994;21(11):2096-2100.
7. Wang D, Mo J, Pan S, Chen H, Zhen H. Prevention of postoperative peritoneal adhesions by O-carboxymethyl chitosan in a rat cecal abrasion model. Clin Invest Med. 2010;33(4):E254-E260.
8. Zhang H, Sheng ZJ, Hou CL. Effect of chitosan membrane on tendon adhesion and healing [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 1999;13(6):382-385.
9. Cho MH, Kim KS, Ahn HH, et al. Chitosan gel as an in situ–forming scaffold for rat bone marrow mesenchymal stem cells in vivo. Tissue Eng Part A. 2008;14(6):1099-1108.
10. Yamaguchi K, Tetro AM, Blam O, Evanoff BA, Teefey SA, Middleton WD. Natural history of asymptomatic rotator cuff tears: a longitudinal analysis of asymptomatic tears detected sonographically. J Shoulder Elbow Surg. 2001;10(3):199-203.
11. Vitale MA, Vitale MG, Zivin JG, Braman JP, Bigliani LU, Flatow EL. Rotator cuff repair: an analysis of utility scores and cost-effectiveness. J Shoulder Elbow Surg. 2007;16(2):181-187.
12. Accousti KJ, Flatow EL. Technical pearls on how to maximize healing of the rotator cuff. Instr Course Lect. 2007;56:3-12.
13. Bishop J, Klepps S, Lo IK, Bird J, Gladstone JN, Flatow EL. Cuff integrity after arthroscopic versus open rotator cuff repair: a prospective study. J Shoulder Elbow Surg. 2006;15(3):290-299.
14. Hunziker E, Spector M, Libera J, et al. Translation from research to applications. Tissue Eng. 2006;12(12):3341-3364.
15. Suh JK, Matthew HW. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: a review. Biomaterials. 2000;21(24):2589-2598.
16. Kumar MN, Muzzarelli RA, Muzzarelli C, Sashiwa H, Domb AJ. Chitosan chemistry and pharmaceutical perspectives. Chem Rev. 2004;104(12):6017-6084.
17. Shi C, Zhu Y, Ran X, Wang M, Su Y, Cheng T. Therapeutic potential of chitosan and its derivatives in regenerative medicine. J Surg Res. 2006;133(2):185-192.
18. Hsieh WC, Chang CP, Lin SM. Morphology and characterization of 3D micro-porous structured chitosan scaffolds for tissue engineering. Colloids Surf B Biointerfaces. 2007;57(2):250-255.
19. Madihally SV, Matthew HW. Porous chitosan scaffolds for tissue engineering. Biomaterials. 1999;20(12):1133-1142.
20. Nettles DL, Elder SH, Gilbert JA. Potential use of chitosan as a cell scaffold material for cartilage tissue engineering. Tissue Eng. 2002;8(6):1009-1016.
21. Griffon DJ, Sedighi MR, Schaeffer DV, Eurell JA, Johnson AL. Chitosan scaffolds: interconnective pore size and cartilage engineering. Acta Biomater. 2006;2(3):313-320.
22. Manjubala I, Scheler S, Bossert J, Jandt KD. Mineralisation of chitosan scaffolds with nano-apatite formation by double diffusion technique. Acta Biomater. 2006;2(1):75-84.
23. Su CH, Sun CS, Juan SW, Ho HO, Hu CH, Sheu MT. Development of fungal mycelia as skin substitutes: effects on wound healing and fibroblast. Biomaterials. 1999;20(1):61-68.
24. Okamoto Y, Southwood L, Stashak TS. Effect of chitin on nonwoven fabric implant in tendon healing. Carbohydr Polym. 1997;33:33-38.
25. Gupta R, Lee TQ. Contributions of the different rabbit models to our understanding of rotator cuff pathology. J Shoulder Elbow Surg. 2007;16(5 suppl):S149-S157.
Rotator cuff tears (RCTs) are common tendon injuries that can cause chronic pain and severe functional disability. Massive RCTs do not heal spontaneously and, in many cases, result in poor clinical outcomes. Specifically, muscle atrophy and fatty infiltration correlate with poor outcomes after surgical repair.1 Fatty infiltration of the rotator cuff is a common phenomenon that can lead to permanent structural alterations within the tendon. It has been suggested that changes in muscle fiber orientation (the pennation angle) can cause mesenchymal stem cells to migrate to the interface between muscle fibers and the region of fatty infiltration of the muscle.2 Understanding the factors involved in muscle degeneration and atrophy, and in fatty infiltration, may lead to treatments that improve outcomes for patients with massive RCTs. One proposed treatment involves placing continuous mechanical traction on the ends of the torn tendon.2 Findings from this research have indicated that acute tears that become chronic tears are typified by inelasticity and poor function of the muscle–tendon unit. It is therefore important to develop a method that speeds tendon healing without causing the muscle fiber atrophy and pennation angle changes that lead to fatty atrophy, which appears to be an irreversible structural change.
On the basis of the theory that adding mesenchymal cells may improve tendon healing, investigators have studied use of transcription factors (eg, scleraxis) specific to tendogenesis in the embryonal stage.3,4 Nevertheless, certain transcription factors are associated with formation of fibrocartilage in higher concentrations.4 Moreover, decalcified bone matrix increases cartilage formation when added to the tendon repair site.5 Cartilage formation, however, is associated with poorer functional results.6 Thus, there is a need for a method that facilitates faster tendon healing with higher quality tissue formation and less muscle atrophy.
Chitosan, a linear polysaccharide, is associated with scarless healing of soft tissues and prevention of adhesion formation both intraperitoneally and during tendon healing after surgery.7,8 Chitosan tends to precipitate in physiologic pH, thereby mitigating its potency. Fortunately, a chitosan solution that does not precipitate in physiologic conditions was recently developed.9 The solution’s lack of precipitation, coupled with its in situ gelling, allows it to adhere to the repair site long enough to take effect. These characteristics could allow for intimate contact between gel and tendon, facilitating guided-tissue regeneration and preventing adhesion of the rotator cuff to surrounding tissue. By contrast, other biological agents (eg, platelet-rich plasma) are administered as fluid rather than gel and are therefore more susceptible to diffusing from the repair site, mitigating their effects. Thus, chitosan gel is fairly unique among agents.
In the study reported here, we histologically investigated whether a chitosan gel would help improve healing of rotator cuff tendon (acute supraspinatus) tears in a rat model.
Materials and Methods
Supraspinatus Surgical Model
Forty Wistar rats, each weighing between 300 and 400 g, were used in this study. All procedures were approved by the Institutional Animal Care and Use Committee at Rabin Medical Center in Petah Tikva, Israel. The rats were anesthetized with ketamine 90 mg/kg and xylazine 10 mg/kg, both administered intramuscularly, and anesthesia was prolonged as needed with 2% isoflurane, administered by nose cone. The skin was incised 5 cm along the upper back following the midline of the spine. The resulting skin flaps were retracted and the scapula exposed. Careful blunt dissection allowed visualization of the rotator cuff and the trans-scapular arch. A full-thickness incision of the supraspinatus tendon was then made 2 mm distal to the arch. This procedure was performed on both shoulders. For the right supraspinatus tendon, a bioabsorbable chitosan–hydrochloric acid solution (>70% de-acetylated chitosan, molecular weight of 600 kDa; Heppe Medical Chitosan GmbH, Halle, Germany) was sterilely applied to the ends of the tendon (total volume, 0.5 mL) and automatically gelled in situ by heating to about 37°C (rat’s internal body temperature). The tendon ends were subsequently approximated with a single 4-0 Prolene suture (Ethicon, Somerville, New Jersey). The left shoulder (tendon repaired with suture only) served as a control.
The rats were housed for a maximum of 12 weeks after surgery. They were sacrificed (in groups of 5 each) 2 hours, 3 days, 1 week, 2 weeks, 4 weeks, 6 weeks, 8 weeks, and 12 weeks after surgery. After each rat was sacrificed, both shoulder girdles were harvested, and the sutures were removed from the supraspinatus tendons.
Histologic Analysis
After routine fixation with 4% formalin for 48 hours and decalcification with 10% ethylenediaminetetraacetic acid (EDTA) for 3 weeks, the specimens were sectioned with a microtome blade. Care was taken to ensure the plane of the microtome blade was parallel with the longitudinal plane of the supraspinatus muscle and tendon to allow for evaluation of pennation angle. Hematoxylin-eosin staining and Masson trichrome staining were subsequently performed.
A variety of histologic measurements were obtained with use of ImageJ software (US National Institutes of Health). Percentage of fibrous tissue was determined by examining the slides at low magnification fields (×25) at the tendon healing site. Three such fields were evaluated per specimen. The fibrous tissue was circled manually, and percentage of tissue area was assessed and compared with total region of interest. Cellularity was carefully outlined and measured as percentage of total tendon area occupied by cells. Fatty atrophy was defined as either present or absent. Muscle fiber diameter was defined as average diameter of 10 muscle fibers measured within 2 mm of the tendon laceration site. Inflammatory cell collections were defined as either large (>100 µm in diameter) or small (<100 µm in diameter) and were dichotomized to either present or absent. Pennation angle was defined as average angle between muscle fibers and longitudinal axis of supraspinatus muscle and tendon unit. Ten fibers proximal to and within 2 mm of the laceration site were randomly selected, measured, and averaged.
Statistical Analysis
Statistical analysis was performed with Analyse-it 2.20 for Microsoft Excel 2010 (Analyse-it Software, Leeds, United Kingdom). Data were initially analyzed with the Kolmogorov-Smirnov test to assess for normality of distribution. The t test was used to compare continuous variables when the data were normally distributed and the Mann-Whitney test when the data were not normally distributed.
Results
All tendons (both groups) healed within 12 weeks. Generally, the tissue formed at the repair site exhibited a mixture of tenocyte-like cells (fibrotic tissue) and granulation tissue without clear orientation. As noted in Figure 1, the tendons treated with chitosan had more fibrotic tissue (overall mean, 21.5%) relative to the control group (mean, 12.3%), and the difference was significant (P = .003). The most notable differences were found at time points later than 1 week after surgery. In addition, amount of cellularity (Figure 2) was higher in chitosan-treated tendon and control tendon than in the normal, uninjured adjacent tendon at all time points (P < .001). Chitosan-treated tendons had significantly higher cellularity than untreated control tendons from 1 to 2 weeks (P < .001), and control tendons were significantly hypercellular compared with chitosan-treated tendons from 4 to 8 weeks (P < .001), but both groups exhibited similar cellularity by 12 weeks (P > .05). Fatty atrophy was found at significantly higher rates in control rats than in chitosan-treated rats (P = .001; Table). Furthermore, as noted in Figure 3, muscle fiber diameter decreased in both groups after injury (P < .001).
Figure 4 shows that the amount of inflammatory collections was significantly smaller in the chitosan-treated group than in the control group over the course of the study (P = .01). In addition, pennation angle steadily decreased in the control group throughout the study period, whereas it transiently decreased in the chitosan-treated group (until 2 weeks) before returning to its immediate postoperative level by 12 weeks (Figure 5). Overall, the chitosan-treated group maintained a higher pennation angle than the control group did (P < .001).
Discussion
RCTs affect more than 40% of patients over age 60 years and are a common cause of debilitating pain, reduced shoulder function, and weakness.10 Thirty thousand to 75,000 rotator cuff repairs are performed annually in the United States.11 Although the best treatment for this disorder remains a topic of debate, arthroscopic and (when necessary) open surgical repair is the accepted gold standard for the treatment of tears that do not improve with conservative management. Despite advances in the surgical treatment of these tears, the surgical failure rates are high (range, 20%-90%), with failures attributed to factors beyond patient age, tear size and chronicity, muscle atrophy and degeneration, tendon quality, repair technique, and postoperative rehabilitation.12,13 Repair strategies that biologically enhance the patient’s intrinsic healing potential are needed.
In tendon repair, choice of repair material (eg, graft) is crucial in determining the success of tissue engineering approaches. The ideal scaffold is biocompatible and does not elicit a host inflammatory response. The selected scaffold in its composition and fabricated form must be capable of holding and supporting cells. In addition, the scaffold should be biodegradable, serving as a temporary support for such cells and mechanically augmenting the repaired tendon while allowing for eventual replacement by matrix components. Moreover, the scaffold should have high porosity and a large surface area. Furthermore, the material should mimic the native tendon extracellular matrix (ECM) architecture to allow cells to be distributed throughout the scaffold and to facilitate diffusion of nutrients and factors that promote cellular proliferation and ECM production.
Given the importance of glycosaminoglycans (GAGs) in supporting the reticular structure of the matrix, use of GAGs or GAG-analogues as components of a tendon tissue scaffold for enhancing repair is well documented.14 One such candidate is chitosan, a partially de-acetylated derivative of chitin found in arthropod exoskeletons. Structurally, chitosan shares some characteristics with various GAGs and hyaluronic acid.15 More specifically, chitosan is a linear polysaccharide composed of glucosamine and N-acetyl glucosamine units linked by β-glycosidic bonds. Investigators have studied the properties of chitosan, including its biocompatibility, biodegradability, antibacterial activity, mucoadhesivity, and wound healing.16,17
One of the most promising features of chitosan is that it can be processed into porous structures for use in cell transplantation and tissue regeneration.18,19 Porous chitosan structures can be formed by freezing and lyophilizing chitosan-acetic acid solutions; chondrogenic cell adhesion and proliferation onto these structures have been reported.20,21 This chitosan scaffolding method has also been used to test different composites with collagens, gelatins, GAGs, and hyaluronic acid, all of which have also been proposed as useful 3-dimensional materials for tissue repair.22
In the present study, we used chitosan matrix in RCT repair. We hypothesized that chitosan matrix could enhance rotator cuff repair the same way it enhances repair in epidermal tissues.16 Histologic findings demonstrated that the percentage of fibrous tissue was significantly higher in the chitosan-treated group than in the control group. This improved fibroblastic response may be attributed to the ability of chitosan to enhance cell migration and serve as a scaffold for repair. Other studies have indicated that chitin, of which chitosan is the primary derivative, accelerated the healing of skin and subcutaneous tissues by increased cell migration.23 Moreover, Okamoto and colleagues24 reported that chitin implants stimulated abundant angiogenesis through the same mechanism.
Inadequate initial strength of a repair may lead to a recurrent cuff tear or a disability of rotator cuff function in the early healing stages. In our study, the chitosan matrix tended to be absorbed by 6 weeks after surgery. Its adherence to and ultimate absorption at the repair site may be challenged by the flow of irrigation fluid through the subacromial space in the setting of arthroscopic surgery. However, because the chitosan remains in a more robust gel form, it is better able to resist being washed from the repair site. For augmentation, it may be possible to apply a biocompatible patch over the gel to further protect it from being dislodged. In addition, histologic findings showed that the fibrous repair tissue gradually increased until reaching a peak 8 weeks after surgery—an indication that the absorption rate of the chitosan scaffold lags behind full recovery of the repair tissue. Given this relationship, further studies are needed to determine the mechanical strength of the repair between 6 and 8 weeks, which is important for avoiding recurrent tears.
This study had a few limitations. First, as with any animal model, the anatomy and function of the rat shoulder differ from those of the human shoulder. The acromial arch differs in quadruped animals, with less coverage of the supraspinatus and more of the subscapularis.25 These anatomical differences could yield altered stress mechanics that could affect tendon repair. Furthermore, rats and humans differ in their RCT healing rates. Thus, the pathophysiology of muscle atrophy and fat infiltration in rats may slightly differ from that in humans. In addition, no mechanical testing was performed to compare chitosan-treated and untreated rotator cuff repairs, and such testing is needed to clarify the biomechanical importance of augmentation. Furthermore, no immunohistochemical analysis was performed for collagen. In the repair of rotator cuff tendons, surgeons must consider not only the number of cells but also the production of ECM. Although not directly confirmed in this study, chitosan induced fibrous tissue proliferation that mirrored production of a large amount of collagen fibers. Last, we used an open RTC model. As an arthroscopic model was not used, no definitive conclusions can be drawn regarding use of chitosan in arthroscopy.
Conclusion
Use of chitosan as an acellular matrix improved formation of healing fibrous tissue, increased the number of cells, and prevented fatty atrophy and inflammatory aggregates inside repair sites while facilitating recovery of the natural pennation angle of the tissue. These results demonstrate that chitosan can enhance tendon healing in the setting of acute RCT. Further research, including biomechanical testing of repaired tendons, is needed to further delineate the utility of chitosan in regenerating irreparable RCTs.
Rotator cuff tears (RCTs) are common tendon injuries that can cause chronic pain and severe functional disability. Massive RCTs do not heal spontaneously and, in many cases, result in poor clinical outcomes. Specifically, muscle atrophy and fatty infiltration correlate with poor outcomes after surgical repair.1 Fatty infiltration of the rotator cuff is a common phenomenon that can lead to permanent structural alterations within the tendon. It has been suggested that changes in muscle fiber orientation (the pennation angle) can cause mesenchymal stem cells to migrate to the interface between muscle fibers and the region of fatty infiltration of the muscle.2 Understanding the factors involved in muscle degeneration and atrophy, and in fatty infiltration, may lead to treatments that improve outcomes for patients with massive RCTs. One proposed treatment involves placing continuous mechanical traction on the ends of the torn tendon.2 Findings from this research have indicated that acute tears that become chronic tears are typified by inelasticity and poor function of the muscle–tendon unit. It is therefore important to develop a method that speeds tendon healing without causing the muscle fiber atrophy and pennation angle changes that lead to fatty atrophy, which appears to be an irreversible structural change.
On the basis of the theory that adding mesenchymal cells may improve tendon healing, investigators have studied use of transcription factors (eg, scleraxis) specific to tendogenesis in the embryonal stage.3,4 Nevertheless, certain transcription factors are associated with formation of fibrocartilage in higher concentrations.4 Moreover, decalcified bone matrix increases cartilage formation when added to the tendon repair site.5 Cartilage formation, however, is associated with poorer functional results.6 Thus, there is a need for a method that facilitates faster tendon healing with higher quality tissue formation and less muscle atrophy.
Chitosan, a linear polysaccharide, is associated with scarless healing of soft tissues and prevention of adhesion formation both intraperitoneally and during tendon healing after surgery.7,8 Chitosan tends to precipitate in physiologic pH, thereby mitigating its potency. Fortunately, a chitosan solution that does not precipitate in physiologic conditions was recently developed.9 The solution’s lack of precipitation, coupled with its in situ gelling, allows it to adhere to the repair site long enough to take effect. These characteristics could allow for intimate contact between gel and tendon, facilitating guided-tissue regeneration and preventing adhesion of the rotator cuff to surrounding tissue. By contrast, other biological agents (eg, platelet-rich plasma) are administered as fluid rather than gel and are therefore more susceptible to diffusing from the repair site, mitigating their effects. Thus, chitosan gel is fairly unique among agents.
In the study reported here, we histologically investigated whether a chitosan gel would help improve healing of rotator cuff tendon (acute supraspinatus) tears in a rat model.
Materials and Methods
Supraspinatus Surgical Model
Forty Wistar rats, each weighing between 300 and 400 g, were used in this study. All procedures were approved by the Institutional Animal Care and Use Committee at Rabin Medical Center in Petah Tikva, Israel. The rats were anesthetized with ketamine 90 mg/kg and xylazine 10 mg/kg, both administered intramuscularly, and anesthesia was prolonged as needed with 2% isoflurane, administered by nose cone. The skin was incised 5 cm along the upper back following the midline of the spine. The resulting skin flaps were retracted and the scapula exposed. Careful blunt dissection allowed visualization of the rotator cuff and the trans-scapular arch. A full-thickness incision of the supraspinatus tendon was then made 2 mm distal to the arch. This procedure was performed on both shoulders. For the right supraspinatus tendon, a bioabsorbable chitosan–hydrochloric acid solution (>70% de-acetylated chitosan, molecular weight of 600 kDa; Heppe Medical Chitosan GmbH, Halle, Germany) was sterilely applied to the ends of the tendon (total volume, 0.5 mL) and automatically gelled in situ by heating to about 37°C (rat’s internal body temperature). The tendon ends were subsequently approximated with a single 4-0 Prolene suture (Ethicon, Somerville, New Jersey). The left shoulder (tendon repaired with suture only) served as a control.
The rats were housed for a maximum of 12 weeks after surgery. They were sacrificed (in groups of 5 each) 2 hours, 3 days, 1 week, 2 weeks, 4 weeks, 6 weeks, 8 weeks, and 12 weeks after surgery. After each rat was sacrificed, both shoulder girdles were harvested, and the sutures were removed from the supraspinatus tendons.
Histologic Analysis
After routine fixation with 4% formalin for 48 hours and decalcification with 10% ethylenediaminetetraacetic acid (EDTA) for 3 weeks, the specimens were sectioned with a microtome blade. Care was taken to ensure the plane of the microtome blade was parallel with the longitudinal plane of the supraspinatus muscle and tendon to allow for evaluation of pennation angle. Hematoxylin-eosin staining and Masson trichrome staining were subsequently performed.
A variety of histologic measurements were obtained with use of ImageJ software (US National Institutes of Health). Percentage of fibrous tissue was determined by examining the slides at low magnification fields (×25) at the tendon healing site. Three such fields were evaluated per specimen. The fibrous tissue was circled manually, and percentage of tissue area was assessed and compared with total region of interest. Cellularity was carefully outlined and measured as percentage of total tendon area occupied by cells. Fatty atrophy was defined as either present or absent. Muscle fiber diameter was defined as average diameter of 10 muscle fibers measured within 2 mm of the tendon laceration site. Inflammatory cell collections were defined as either large (>100 µm in diameter) or small (<100 µm in diameter) and were dichotomized to either present or absent. Pennation angle was defined as average angle between muscle fibers and longitudinal axis of supraspinatus muscle and tendon unit. Ten fibers proximal to and within 2 mm of the laceration site were randomly selected, measured, and averaged.
Statistical Analysis
Statistical analysis was performed with Analyse-it 2.20 for Microsoft Excel 2010 (Analyse-it Software, Leeds, United Kingdom). Data were initially analyzed with the Kolmogorov-Smirnov test to assess for normality of distribution. The t test was used to compare continuous variables when the data were normally distributed and the Mann-Whitney test when the data were not normally distributed.
Results
All tendons (both groups) healed within 12 weeks. Generally, the tissue formed at the repair site exhibited a mixture of tenocyte-like cells (fibrotic tissue) and granulation tissue without clear orientation. As noted in Figure 1, the tendons treated with chitosan had more fibrotic tissue (overall mean, 21.5%) relative to the control group (mean, 12.3%), and the difference was significant (P = .003). The most notable differences were found at time points later than 1 week after surgery. In addition, amount of cellularity (Figure 2) was higher in chitosan-treated tendon and control tendon than in the normal, uninjured adjacent tendon at all time points (P < .001). Chitosan-treated tendons had significantly higher cellularity than untreated control tendons from 1 to 2 weeks (P < .001), and control tendons were significantly hypercellular compared with chitosan-treated tendons from 4 to 8 weeks (P < .001), but both groups exhibited similar cellularity by 12 weeks (P > .05). Fatty atrophy was found at significantly higher rates in control rats than in chitosan-treated rats (P = .001; Table). Furthermore, as noted in Figure 3, muscle fiber diameter decreased in both groups after injury (P < .001).
Figure 4 shows that the amount of inflammatory collections was significantly smaller in the chitosan-treated group than in the control group over the course of the study (P = .01). In addition, pennation angle steadily decreased in the control group throughout the study period, whereas it transiently decreased in the chitosan-treated group (until 2 weeks) before returning to its immediate postoperative level by 12 weeks (Figure 5). Overall, the chitosan-treated group maintained a higher pennation angle than the control group did (P < .001).
Discussion
RCTs affect more than 40% of patients over age 60 years and are a common cause of debilitating pain, reduced shoulder function, and weakness.10 Thirty thousand to 75,000 rotator cuff repairs are performed annually in the United States.11 Although the best treatment for this disorder remains a topic of debate, arthroscopic and (when necessary) open surgical repair is the accepted gold standard for the treatment of tears that do not improve with conservative management. Despite advances in the surgical treatment of these tears, the surgical failure rates are high (range, 20%-90%), with failures attributed to factors beyond patient age, tear size and chronicity, muscle atrophy and degeneration, tendon quality, repair technique, and postoperative rehabilitation.12,13 Repair strategies that biologically enhance the patient’s intrinsic healing potential are needed.
In tendon repair, choice of repair material (eg, graft) is crucial in determining the success of tissue engineering approaches. The ideal scaffold is biocompatible and does not elicit a host inflammatory response. The selected scaffold in its composition and fabricated form must be capable of holding and supporting cells. In addition, the scaffold should be biodegradable, serving as a temporary support for such cells and mechanically augmenting the repaired tendon while allowing for eventual replacement by matrix components. Moreover, the scaffold should have high porosity and a large surface area. Furthermore, the material should mimic the native tendon extracellular matrix (ECM) architecture to allow cells to be distributed throughout the scaffold and to facilitate diffusion of nutrients and factors that promote cellular proliferation and ECM production.
Given the importance of glycosaminoglycans (GAGs) in supporting the reticular structure of the matrix, use of GAGs or GAG-analogues as components of a tendon tissue scaffold for enhancing repair is well documented.14 One such candidate is chitosan, a partially de-acetylated derivative of chitin found in arthropod exoskeletons. Structurally, chitosan shares some characteristics with various GAGs and hyaluronic acid.15 More specifically, chitosan is a linear polysaccharide composed of glucosamine and N-acetyl glucosamine units linked by β-glycosidic bonds. Investigators have studied the properties of chitosan, including its biocompatibility, biodegradability, antibacterial activity, mucoadhesivity, and wound healing.16,17
One of the most promising features of chitosan is that it can be processed into porous structures for use in cell transplantation and tissue regeneration.18,19 Porous chitosan structures can be formed by freezing and lyophilizing chitosan-acetic acid solutions; chondrogenic cell adhesion and proliferation onto these structures have been reported.20,21 This chitosan scaffolding method has also been used to test different composites with collagens, gelatins, GAGs, and hyaluronic acid, all of which have also been proposed as useful 3-dimensional materials for tissue repair.22
In the present study, we used chitosan matrix in RCT repair. We hypothesized that chitosan matrix could enhance rotator cuff repair the same way it enhances repair in epidermal tissues.16 Histologic findings demonstrated that the percentage of fibrous tissue was significantly higher in the chitosan-treated group than in the control group. This improved fibroblastic response may be attributed to the ability of chitosan to enhance cell migration and serve as a scaffold for repair. Other studies have indicated that chitin, of which chitosan is the primary derivative, accelerated the healing of skin and subcutaneous tissues by increased cell migration.23 Moreover, Okamoto and colleagues24 reported that chitin implants stimulated abundant angiogenesis through the same mechanism.
Inadequate initial strength of a repair may lead to a recurrent cuff tear or a disability of rotator cuff function in the early healing stages. In our study, the chitosan matrix tended to be absorbed by 6 weeks after surgery. Its adherence to and ultimate absorption at the repair site may be challenged by the flow of irrigation fluid through the subacromial space in the setting of arthroscopic surgery. However, because the chitosan remains in a more robust gel form, it is better able to resist being washed from the repair site. For augmentation, it may be possible to apply a biocompatible patch over the gel to further protect it from being dislodged. In addition, histologic findings showed that the fibrous repair tissue gradually increased until reaching a peak 8 weeks after surgery—an indication that the absorption rate of the chitosan scaffold lags behind full recovery of the repair tissue. Given this relationship, further studies are needed to determine the mechanical strength of the repair between 6 and 8 weeks, which is important for avoiding recurrent tears.
This study had a few limitations. First, as with any animal model, the anatomy and function of the rat shoulder differ from those of the human shoulder. The acromial arch differs in quadruped animals, with less coverage of the supraspinatus and more of the subscapularis.25 These anatomical differences could yield altered stress mechanics that could affect tendon repair. Furthermore, rats and humans differ in their RCT healing rates. Thus, the pathophysiology of muscle atrophy and fat infiltration in rats may slightly differ from that in humans. In addition, no mechanical testing was performed to compare chitosan-treated and untreated rotator cuff repairs, and such testing is needed to clarify the biomechanical importance of augmentation. Furthermore, no immunohistochemical analysis was performed for collagen. In the repair of rotator cuff tendons, surgeons must consider not only the number of cells but also the production of ECM. Although not directly confirmed in this study, chitosan induced fibrous tissue proliferation that mirrored production of a large amount of collagen fibers. Last, we used an open RTC model. As an arthroscopic model was not used, no definitive conclusions can be drawn regarding use of chitosan in arthroscopy.
Conclusion
Use of chitosan as an acellular matrix improved formation of healing fibrous tissue, increased the number of cells, and prevented fatty atrophy and inflammatory aggregates inside repair sites while facilitating recovery of the natural pennation angle of the tissue. These results demonstrate that chitosan can enhance tendon healing in the setting of acute RCT. Further research, including biomechanical testing of repaired tendons, is needed to further delineate the utility of chitosan in regenerating irreparable RCTs.
1. Shen PH, Lien SB, Shen HC, Lee CH, Wu SS, Lin LC. Long-term functional outcomes after repair of rotator cuff tears correlated with atrophy of the supraspinatus muscles on magnetic resonance images. J Shoulder Elbow Surg. 2008;17(1 suppl):1S-7S.
2. Meyer DC, Hoppeler H, von Rechenberg B, Gerber C. A pathomechanical concept explains muscle loss and fatty muscular changes following surgical tendon release. J Orthop Res. 2004;22(5):1004-1007.
3. Gulotta LV, Kovacevic D, Packer JD, Deng XH, Rodeo SA. Bone marrow–derived mesenchymal stem cells transduced with scleraxis improve rotator cuff healing in a rat model. Am J Sports Med. 2011;39(6):1282-1289.
4. Gulotta LV, Rodeo SA. Emerging ideas: evaluation of stem cells genetically modified with scleraxis to improve rotator cuff healing. Clin Orthop. 2011;469(10):2977-2980.
5. Sundar S, Pendegrass CJ, Blunn GW. Tendon bone healing can be enhanced by demineralized bone matrix: a functional and histological study. J Biomed Mater Res B Appl Biomater. 2009;88(1):115-122.
6. Kumagai J, Sarkar K, Uhthoff HK. The collagen types in the attachment zone of rotator cuff tendons in the elderly: an immunohistochemical study. J Rheumatol. 1994;21(11):2096-2100.
7. Wang D, Mo J, Pan S, Chen H, Zhen H. Prevention of postoperative peritoneal adhesions by O-carboxymethyl chitosan in a rat cecal abrasion model. Clin Invest Med. 2010;33(4):E254-E260.
8. Zhang H, Sheng ZJ, Hou CL. Effect of chitosan membrane on tendon adhesion and healing [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 1999;13(6):382-385.
9. Cho MH, Kim KS, Ahn HH, et al. Chitosan gel as an in situ–forming scaffold for rat bone marrow mesenchymal stem cells in vivo. Tissue Eng Part A. 2008;14(6):1099-1108.
10. Yamaguchi K, Tetro AM, Blam O, Evanoff BA, Teefey SA, Middleton WD. Natural history of asymptomatic rotator cuff tears: a longitudinal analysis of asymptomatic tears detected sonographically. J Shoulder Elbow Surg. 2001;10(3):199-203.
11. Vitale MA, Vitale MG, Zivin JG, Braman JP, Bigliani LU, Flatow EL. Rotator cuff repair: an analysis of utility scores and cost-effectiveness. J Shoulder Elbow Surg. 2007;16(2):181-187.
12. Accousti KJ, Flatow EL. Technical pearls on how to maximize healing of the rotator cuff. Instr Course Lect. 2007;56:3-12.
13. Bishop J, Klepps S, Lo IK, Bird J, Gladstone JN, Flatow EL. Cuff integrity after arthroscopic versus open rotator cuff repair: a prospective study. J Shoulder Elbow Surg. 2006;15(3):290-299.
14. Hunziker E, Spector M, Libera J, et al. Translation from research to applications. Tissue Eng. 2006;12(12):3341-3364.
15. Suh JK, Matthew HW. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: a review. Biomaterials. 2000;21(24):2589-2598.
16. Kumar MN, Muzzarelli RA, Muzzarelli C, Sashiwa H, Domb AJ. Chitosan chemistry and pharmaceutical perspectives. Chem Rev. 2004;104(12):6017-6084.
17. Shi C, Zhu Y, Ran X, Wang M, Su Y, Cheng T. Therapeutic potential of chitosan and its derivatives in regenerative medicine. J Surg Res. 2006;133(2):185-192.
18. Hsieh WC, Chang CP, Lin SM. Morphology and characterization of 3D micro-porous structured chitosan scaffolds for tissue engineering. Colloids Surf B Biointerfaces. 2007;57(2):250-255.
19. Madihally SV, Matthew HW. Porous chitosan scaffolds for tissue engineering. Biomaterials. 1999;20(12):1133-1142.
20. Nettles DL, Elder SH, Gilbert JA. Potential use of chitosan as a cell scaffold material for cartilage tissue engineering. Tissue Eng. 2002;8(6):1009-1016.
21. Griffon DJ, Sedighi MR, Schaeffer DV, Eurell JA, Johnson AL. Chitosan scaffolds: interconnective pore size and cartilage engineering. Acta Biomater. 2006;2(3):313-320.
22. Manjubala I, Scheler S, Bossert J, Jandt KD. Mineralisation of chitosan scaffolds with nano-apatite formation by double diffusion technique. Acta Biomater. 2006;2(1):75-84.
23. Su CH, Sun CS, Juan SW, Ho HO, Hu CH, Sheu MT. Development of fungal mycelia as skin substitutes: effects on wound healing and fibroblast. Biomaterials. 1999;20(1):61-68.
24. Okamoto Y, Southwood L, Stashak TS. Effect of chitin on nonwoven fabric implant in tendon healing. Carbohydr Polym. 1997;33:33-38.
25. Gupta R, Lee TQ. Contributions of the different rabbit models to our understanding of rotator cuff pathology. J Shoulder Elbow Surg. 2007;16(5 suppl):S149-S157.
1. Shen PH, Lien SB, Shen HC, Lee CH, Wu SS, Lin LC. Long-term functional outcomes after repair of rotator cuff tears correlated with atrophy of the supraspinatus muscles on magnetic resonance images. J Shoulder Elbow Surg. 2008;17(1 suppl):1S-7S.
2. Meyer DC, Hoppeler H, von Rechenberg B, Gerber C. A pathomechanical concept explains muscle loss and fatty muscular changes following surgical tendon release. J Orthop Res. 2004;22(5):1004-1007.
3. Gulotta LV, Kovacevic D, Packer JD, Deng XH, Rodeo SA. Bone marrow–derived mesenchymal stem cells transduced with scleraxis improve rotator cuff healing in a rat model. Am J Sports Med. 2011;39(6):1282-1289.
4. Gulotta LV, Rodeo SA. Emerging ideas: evaluation of stem cells genetically modified with scleraxis to improve rotator cuff healing. Clin Orthop. 2011;469(10):2977-2980.
5. Sundar S, Pendegrass CJ, Blunn GW. Tendon bone healing can be enhanced by demineralized bone matrix: a functional and histological study. J Biomed Mater Res B Appl Biomater. 2009;88(1):115-122.
6. Kumagai J, Sarkar K, Uhthoff HK. The collagen types in the attachment zone of rotator cuff tendons in the elderly: an immunohistochemical study. J Rheumatol. 1994;21(11):2096-2100.
7. Wang D, Mo J, Pan S, Chen H, Zhen H. Prevention of postoperative peritoneal adhesions by O-carboxymethyl chitosan in a rat cecal abrasion model. Clin Invest Med. 2010;33(4):E254-E260.
8. Zhang H, Sheng ZJ, Hou CL. Effect of chitosan membrane on tendon adhesion and healing [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 1999;13(6):382-385.
9. Cho MH, Kim KS, Ahn HH, et al. Chitosan gel as an in situ–forming scaffold for rat bone marrow mesenchymal stem cells in vivo. Tissue Eng Part A. 2008;14(6):1099-1108.
10. Yamaguchi K, Tetro AM, Blam O, Evanoff BA, Teefey SA, Middleton WD. Natural history of asymptomatic rotator cuff tears: a longitudinal analysis of asymptomatic tears detected sonographically. J Shoulder Elbow Surg. 2001;10(3):199-203.
11. Vitale MA, Vitale MG, Zivin JG, Braman JP, Bigliani LU, Flatow EL. Rotator cuff repair: an analysis of utility scores and cost-effectiveness. J Shoulder Elbow Surg. 2007;16(2):181-187.
12. Accousti KJ, Flatow EL. Technical pearls on how to maximize healing of the rotator cuff. Instr Course Lect. 2007;56:3-12.
13. Bishop J, Klepps S, Lo IK, Bird J, Gladstone JN, Flatow EL. Cuff integrity after arthroscopic versus open rotator cuff repair: a prospective study. J Shoulder Elbow Surg. 2006;15(3):290-299.
14. Hunziker E, Spector M, Libera J, et al. Translation from research to applications. Tissue Eng. 2006;12(12):3341-3364.
15. Suh JK, Matthew HW. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: a review. Biomaterials. 2000;21(24):2589-2598.
16. Kumar MN, Muzzarelli RA, Muzzarelli C, Sashiwa H, Domb AJ. Chitosan chemistry and pharmaceutical perspectives. Chem Rev. 2004;104(12):6017-6084.
17. Shi C, Zhu Y, Ran X, Wang M, Su Y, Cheng T. Therapeutic potential of chitosan and its derivatives in regenerative medicine. J Surg Res. 2006;133(2):185-192.
18. Hsieh WC, Chang CP, Lin SM. Morphology and characterization of 3D micro-porous structured chitosan scaffolds for tissue engineering. Colloids Surf B Biointerfaces. 2007;57(2):250-255.
19. Madihally SV, Matthew HW. Porous chitosan scaffolds for tissue engineering. Biomaterials. 1999;20(12):1133-1142.
20. Nettles DL, Elder SH, Gilbert JA. Potential use of chitosan as a cell scaffold material for cartilage tissue engineering. Tissue Eng. 2002;8(6):1009-1016.
21. Griffon DJ, Sedighi MR, Schaeffer DV, Eurell JA, Johnson AL. Chitosan scaffolds: interconnective pore size and cartilage engineering. Acta Biomater. 2006;2(3):313-320.
22. Manjubala I, Scheler S, Bossert J, Jandt KD. Mineralisation of chitosan scaffolds with nano-apatite formation by double diffusion technique. Acta Biomater. 2006;2(1):75-84.
23. Su CH, Sun CS, Juan SW, Ho HO, Hu CH, Sheu MT. Development of fungal mycelia as skin substitutes: effects on wound healing and fibroblast. Biomaterials. 1999;20(1):61-68.
24. Okamoto Y, Southwood L, Stashak TS. Effect of chitin on nonwoven fabric implant in tendon healing. Carbohydr Polym. 1997;33:33-38.
25. Gupta R, Lee TQ. Contributions of the different rabbit models to our understanding of rotator cuff pathology. J Shoulder Elbow Surg. 2007;16(5 suppl):S149-S157.
Comparison of Locked Plate Fixation and Nonoperative Management for Displaced Proximal Humerus Fractures in Elderly Patients
Proximal humerus fractures are increasingly common in the elderly population,1 accounting for 10% of all these patients’ fractures.2 The injuries result in substantial morbidity and are associated with significantly higher mortality rates for up to 4 years.3 With the recent advent of anatomical locking plates,4,5 operative fixation of proximal humerus fractures in elderly patients has become more common.6 Although early clinical studies reported favorable outcomes, high complication rates have also been documented.7-22
Investigators have recently compared outcomes of locked plate fixation and nonoperative treatment of proximal humerus fractures in elderly patients.23-26 Fjalestad and colleagues23 conducted a randomized clinical trial of locked plating versus nonoperative treatment of 3- and 4-part fractures in 50 patients age 60 years or older and found no significant differences in Constant score or patient self-assessment at 1 year. Similarly, Olerud and colleagues25 conducted a randomized clinical trial of locked plating versus nonoperative treatment of 3-part fractures in 60 patients age 55 years or older. Although outcomes were better in the operative group, differences did not reach statistical significance, and the operative group’s reoperation rate was 30%.
Given this lack of conclusive outcomes data, optimal treatment of displaced proximal humerus fractures in elderly patients remains unknown. We conducted a study to compare outcomes of operative (locked plate fixation) and nonoperative management of displaced proximal humerus fractures in patients older than 60 years. Our hypothesis was that the clinical outcomes of these 2 treatment methods would be similar.
Materials and Methods
Selection Criteria
Our research protocol was approved by the Partners Human Research Committee. To determine the operative cohort, we queried our trauma database to identify all patients over age 60 years who sustained a displaced proximal humerus fracture between 2006 and 2009 and underwent surgical fixation. Cases were excluded if they presented more than 4 weeks after injury; if they represented a refracture, nonunion, or pathologic fracture; if the fracture was an isolated greater or lesser tuberosity fracture; if there was an associated neurovascular injury; if the injury radiographs were absent or inadequate; or if a fixation method other than locked plating was used. Applying these inclusion and exclusion criteria yielded 61 patients over age 60 years who underwent locked plating of a displaced proximal humerus fracture between 2006 and 2009.
The comparison group consisted of all patients who presented to our institutions with a displaced proximal humerus fracture during the same time period but instead had nonoperative treatment. To identify this group, we performed another database search for all patients over age 60 years who sustained a proximal humerus fracture between 2006 and 2009 (n = 452). Twenty-two patients were excluded for inadequate radiographs. To determine which of the other 430 patients had displaced fractures, Dr. Okike and Dr. Lee (orthopedic surgeons) reviewed injury radiographs and any computed tomography scans in duplicate and resolved discrepancies by consensus. Neer’s criteria were used to define displacement: Fractures displaced 1 cm or more and/or with angulation of 45° or more were displaced, and fractures not meeting these criteria were nondisplaced. In the assessment of displacement, interobserver reliability was substantial (overall agreement, 87.0% [374/430]; κ = 0.68). With use of these methods, 311 fractures were classified displaced and 119 nondisplaced. As with the operative group, cases were excluded if they presented more than 4 weeks after injury; if they represented a refracture, nonunion, or pathologic fracture; if the fracture was an isolated greater or lesser tuberosity fracture; if there was an associated neurovascular injury; if injury radiographs were absent or inadequate; or if the treatment method was operative or unknown. Applying these inclusion and exclusion criteria yielded 146 patients over age 60 years who had nonoperative treatment of a displaced proximal humerus fracture between 2006 and 2009.
Patient Characteristics
Dr. Makanji retrospectively reviewed the charts of all 207 patients (61 operative, 146 nonoperative). Information was recorded on patient age and sex, mechanism of injury, number of days between injury and presentation, any associated orthopedic injuries, side of injury, and treatment facility (trauma center A, trauma center B). In addition, a Charlson score was assigned to each patient on the basis of medical comorbidities.27
Radiographs and any computed tomography scans were also assessed by Dr. Okike and Dr. Lee. Each fracture was assigned a Neer classification (2-part, 3-part, 4-part) and an AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) classification (A, B, C).28 Displacement was categorized as varus angulation (neck–shaft angle, <130°), valgus angulation (neck–shaft angle, >140°), neutral angulation (neck–shaft angle, 135° ± 5°), or translation alone. In addition, all fractures were assessed for dislocation and medial comminution.29
Outcome Measures
All follow-up radiographs were reviewed to assess for nonunion (defined as lack of healing by 12 months), malunion, and humeral head avascular necrosis. Operative patients’ follow-up radiographs were reviewed to determine frequency of screw perforation and/or loss of fixation, and their medical records were reviewed to assess for other complications, including infection, neurovascular injury, and return to operating room for any other reason. Nonoperative patients’ medical records were reviewed to determine if surgical treatment was subsequently required.
To determine clinical outcomes, we asked patients to return for clinical evaluation, which included use of several questionnaires: Constant; DASH (Disabilities of the Arm, Shoulder, and Hand); SMFA (Short Musculoskeletal Functional Assessment); and Patient Reported Outcomes Measurement Information System (PROMIS) Physical Function Computer Adaptive Test.
Statistical Analysis
Chi-square test was used to compare the characteristics of patients who returned for clinical evaluation, Fisher exact test was used for tables with multiple cells less than 5, Student t test was used to compare clinical outcomes between operative and nonoperative groups. P < .05 was considered statistically significant, and all tests were 2-sided. Statistical analysis was performed using SAS Version 9 (SAS, Cary, North Carolina).
Results
Of the 207 patients who met the inclusion and exclusion criteria, 61 were treated operatively (locked plate open reduction and internal fixation) and 146 nonoperatively. Mean age was 76.9 years. One hundred fifty-five (74.9%) of the patients were female. Medical comorbidities were common (average Charlson score, 6.6). Most patients (185/207; 89.4%) were injured in a fall. There were 129 two-part fractures, 63 three-part fractures, and 9 four-part fractures (Table 1).
Operative patients’ complications included screw perforation (35.6%; 21 of the 59 cases with radiographs) and loss of fixation (17.5%; 10/57). Four (6.6%) of the 61 operative patients developed an infection. In sum, 8 (13.1%) of operative patients required another surgery (Table 2).
Among nonoperative patients, malunion at time of healing was common (86.9%; 113 of the 130 cases with radiographs). Eighty-six malunions (66.2% of the 130 cases) healed in varus, 25 (19.2%) in valgus, and 2 (1.5%) with translation alone. Uncommon among nonoperative patients were nonunion (1.4%; 2/143) and avascular necrosis (2.2%; 3/136). Two (1.4%) of the 146 nonoperative patients subsequently underwent surgery for malunion (Table 2).
Forty-seven patients accepted our invitation to return for clinical evaluation. Mean follow-up was 3.3 years (range, 1.4-6.4 years). Of these patients, 25 had been treated operatively (Figures 1A, 1B) and 22 nonoperatively (Figures 2A, 2B). Complication rates for patients who returned for clinical evaluation were similar to those for the entire cohort, with the exception of secondary surgical procedures (Table 3). There were no significant differences between operative and nonoperative patients in the group that returned for clinical evaluation (Table 4).
Regarding clinical outcome scores, there were no significant differences between operative and nonoperative patients (Table 5). In particular, there were no differences in SMFA Functional index (18.4 vs 19.7; P = .78), SMFA Bothersome index (20.8 vs 23.6; P = .61), DASH scores (26.5 vs 25.1; P = .79), Constant scores (58.0 vs 59.7; P = .74), or PROMIS Physical Function Computer Adaptive Test scores (43.9 vs 45.0; P = .70).
Discussion
In this observational study of displaced proximal humerus fractures in an elderly population, operative treatment (vs nonoperative treatment) had a lower malunion rate but was associated with more complications, including screw perforation, loss of fixation, and unplanned return to the operating room. Among patients who returned for clinical evaluation at a mean follow-up of 3.3 years, there were no significant operative–nonoperative differences.
Our results are similar to those recently reported by other investigators. In Norway, Fjalestad and colleagues23 conducted a randomized controlled trial of locked plating versus nonoperative treatment in 50 patients over age 60 years with a 3- or 4-part proximal humerus fracture. At 12 months, there was no significant difference between the operative and nonoperative groups’ Constant scores.
Similarly, Olerud and colleagues25 in Sweden conducted a trial in which 60 patients over age 55 years with a 3-part fracture of the proximal humerus were randomized to locked plating or nonoperative treatment. At 2 years, there were no significant operative–nonoperative differences on several outcome measures: Constant scores, DASH scores, EQ-5D (EuroQol) scores. Thirty percent of operative patients required a secondary procedure to treat infection, nonunion, avascular necrosis, screw perforation, stiffness, or impingement.
Our study benefited from having a large sample size (207) of consecutive patients with displaced proximal humerus fractures, but it also had its limitations. In this retrospective study, treatment assignment was not randomized. We were also limited by the large number of patients who did not return for clinical evaluation (160/207; 77.3%), including 52 (25.1%) found to be deceased, 27 (13.0%) who could not be reached, and 81 (39.1%) who declined our request (in many cases because of difficulties traveling to the trauma center). These challenges are inherent to research in the elderly population. As a result, the number of patients who returned for clinical evaluation (47/207; 22.7%) was lower than expected, which may have underpowered the study. In addition, treatment protocols were not standardized; patients were managed by a number of different surgeons. On the other hand, this wide variety of surgeons, including orthopedic trauma and upper extremity specialists, may increase the generalizability of our results.
Conclusion
Although use of locked plate fixation in treating proximal humerus fractures in elderly patients has increased markedly over recent years, definitive evidence supporting such management is lacking. In the present study, the outcomes of locked plate fixation were similar to those of nonoperative treatment. In addition, rates of complications and secondary surgical procedures were higher for operative patients than for nonoperative patients. Research is needed to identify the circumstances under which locked plating improves treatment outcomes for displaced proximal humerus fractures in elderly patients.
1. Palvanen M, Kannus P, Niemi S, Parkkari J. Update in the epidemiology of proximal humeral fractures. Clin Orthop. 2006;(442):87-92.
2. Baron JA, Karagas M, Barrett J, et al. Basic epidemiology of fractures of the upper and lower limb among Americans over 65 years of age. Epidemiology. 1996;7(6):612-618.
3. Johnell O, Kanis JA, Oden A, et al. Mortality after osteoporotic fractures. Osteoporos Int. 2004;15(1):38-42.
4. Badman BL, Mighell M. Fixed-angle locked plating of two-, three-, and four-part proximal humerus fractures. J Am Acad Orthop Surg. 2008;16(5):294-302.
5. Nho SJ, Brophy RH, Barker JU, Cornell CN, MacGillivray JD. Innovations in the management of displaced proximal humerus fractures. J Am Acad Orthop Surg. 2007;15(1):12-26.
6. Bell JE, Leung BC, Spratt KF, et al. Trends and variation in incidence, surgical treatment, and repeat surgery of proximal humeral fractures in the elderly. J Bone Joint Surg Am. 2011;93(2):121-131.
7. Agudelo J, Schurmann M, Stahel P, et al. Analysis of efficacy and failure in proximal humerus fractures treated with locking plates. J Orthop Trauma. 2007;21(10):676-681.
8. Bigorre N, Talha A, Cronier P, Hubert L, Toulemonde JL, Massin P. A prospective study of a new locking plate for proximal humeral fracture. Injury. 2009;40(2):192-196.
9. Bjorkenheim JM, Pajarinen J, Savolainen V. Internal fixation of proximal humeral fractures with a locking compression plate: a retrospective evaluation of 72 patients followed for a minimum of 1 year. Acta Orthop Scand. 2004;75(6):741-745.
10. Brunner F, Sommer C, Bahrs C, et al. Open reduction and internal fixation of proximal humerus fractures using a proximal humeral locked plate: a prospective multicenter analysis. J Orthop Trauma. 2009;23(3):163-172.
11. Charalambous CP, Siddique I, Valluripalli K, et al. Proximal humeral internal locking system (PHILOS) for the treatment of proximal humeral fractures. Arch Orthop Trauma Surg. 2007;127(3):205-210.
12. Egol KA, Ong CC, Walsh M, Jazrawi LM, Tejwani NC, Zuckerman JD. Early complications in proximal humerus fractures (OTA types 11) treated with locked plates. J Orthop Trauma. 2008;22(3):159-164.
13. Fankhauser F, Boldin C, Schippinger G, Haunschmid C, Szyszkowitz R. A new locking plate for unstable fractures of the proximal humerus. Clin Orthop. 2005;(430):176-181.
14. Hepp P, Theopold J, Osterhoff G, Marquass B, Voigt C, Josten C. Bone quality measured by the radiogrammetric parameter “cortical index” and reoperations after locking plate osteosynthesis in patients sustaining proximal humerus fractures. Arch Orthop Trauma Surg. 2009;129(9):1251-1259.
15. Koukakis A, Apostolou CD, Taneja T, Korres DS, Amini A. Fixation of proximal humerus fractures using the PHILOS plate: early experience. Clin Orthop. 2006;(442):115-120.
16. Moonot P, Ashwood N, Hamlet M. Early results for treatment of three- and four-part fractures of the proximal humerus using the PHILOS plate system. J Bone Joint Surg Br. 2007;89(9):1206-1209.
17. Owsley KC, Gorczyca JT. Fracture displacement and screw cutout after open reduction and locked plate fixation of proximal humeral fractures. J Bone Joint Surg Am. 2008;90(2):233-240.
18. Rose PS, Adams CR, Torchia ME, Jacofsky DJ, Haidukewych GG, Steinmann SP. Locking plate fixation for proximal humeral fractures: initial results with a new implant. J Shoulder Elbow Surg. 2007;16(2):202-207.
19. Shahid R, Mushtaq A, Northover J, Maqsood M. Outcome of proximal humerus fractures treated by PHILOS plate internal fixation. Experience of a district general hospital. Acta Orthop Belg. 2008;74(5):602-608.
20. Smith AM, Mardones RM, Sperling JW, Cofield RH. Early complications of operatively treated proximal humeral fractures. J Shoulder Elbow Surg. 2007;16(1):14-24.
21. Sudkamp N, Bayer J, Hepp P, et al. Open reduction and internal fixation of proximal humeral fractures with use of the locking proximal humerus plate. Results of a prospective, multicenter, observational study. J Bone Joint Surg Am. 2009;91(6):1320-1328.
22. Thalhammer G, Platzer P, Oberleitner G, Fialka C, Greitbauer M, Vecsei V. Angular stable fixation of proximal humeral fractures. J Trauma. 2009;66(1):204-210.
23. Fjalestad T, Hole MO, Hovden IA, Blucher J, Stromsoe K. Surgical treatment with an angular stable plate for complex displaced proximal humeral fractures in elderly patients: a randomized controlled trial. J Orthop Trauma. 2012;26(2):98-106.
24. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Hemiarthroplasty versus nonoperative treatment of displaced 4-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(7):1025-1033.
25. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Internal fixation versus nonoperative treatment of displaced 3-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(5):747-755.
26. Sanders RJ, Thissen LG, Teepen JC, van Kampen A, Jaarsma RL. Locking plate versus nonsurgical treatment for proximal humeral fractures: better midterm outcome with nonsurgical treatment. J Shoulder Elbow Surg. 2011;20(7):1118-1124
27. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.
28. Muller ME, Nazarus C, Koch P, Schatzker J. The Comprehensive Classification of Fractures of Long Bones. Berlin, Germany: Springer-Verlag; 1990.
29. Gardner MJ, Weil Y, Barker JU, Kelly BT, Helfet DL, Lorich DG. The importance of medial support in locked plating of proximal humerus fractures. J Orthop Trauma. 2007;21(3):185-191.
Proximal humerus fractures are increasingly common in the elderly population,1 accounting for 10% of all these patients’ fractures.2 The injuries result in substantial morbidity and are associated with significantly higher mortality rates for up to 4 years.3 With the recent advent of anatomical locking plates,4,5 operative fixation of proximal humerus fractures in elderly patients has become more common.6 Although early clinical studies reported favorable outcomes, high complication rates have also been documented.7-22
Investigators have recently compared outcomes of locked plate fixation and nonoperative treatment of proximal humerus fractures in elderly patients.23-26 Fjalestad and colleagues23 conducted a randomized clinical trial of locked plating versus nonoperative treatment of 3- and 4-part fractures in 50 patients age 60 years or older and found no significant differences in Constant score or patient self-assessment at 1 year. Similarly, Olerud and colleagues25 conducted a randomized clinical trial of locked plating versus nonoperative treatment of 3-part fractures in 60 patients age 55 years or older. Although outcomes were better in the operative group, differences did not reach statistical significance, and the operative group’s reoperation rate was 30%.
Given this lack of conclusive outcomes data, optimal treatment of displaced proximal humerus fractures in elderly patients remains unknown. We conducted a study to compare outcomes of operative (locked plate fixation) and nonoperative management of displaced proximal humerus fractures in patients older than 60 years. Our hypothesis was that the clinical outcomes of these 2 treatment methods would be similar.
Materials and Methods
Selection Criteria
Our research protocol was approved by the Partners Human Research Committee. To determine the operative cohort, we queried our trauma database to identify all patients over age 60 years who sustained a displaced proximal humerus fracture between 2006 and 2009 and underwent surgical fixation. Cases were excluded if they presented more than 4 weeks after injury; if they represented a refracture, nonunion, or pathologic fracture; if the fracture was an isolated greater or lesser tuberosity fracture; if there was an associated neurovascular injury; if the injury radiographs were absent or inadequate; or if a fixation method other than locked plating was used. Applying these inclusion and exclusion criteria yielded 61 patients over age 60 years who underwent locked plating of a displaced proximal humerus fracture between 2006 and 2009.
The comparison group consisted of all patients who presented to our institutions with a displaced proximal humerus fracture during the same time period but instead had nonoperative treatment. To identify this group, we performed another database search for all patients over age 60 years who sustained a proximal humerus fracture between 2006 and 2009 (n = 452). Twenty-two patients were excluded for inadequate radiographs. To determine which of the other 430 patients had displaced fractures, Dr. Okike and Dr. Lee (orthopedic surgeons) reviewed injury radiographs and any computed tomography scans in duplicate and resolved discrepancies by consensus. Neer’s criteria were used to define displacement: Fractures displaced 1 cm or more and/or with angulation of 45° or more were displaced, and fractures not meeting these criteria were nondisplaced. In the assessment of displacement, interobserver reliability was substantial (overall agreement, 87.0% [374/430]; κ = 0.68). With use of these methods, 311 fractures were classified displaced and 119 nondisplaced. As with the operative group, cases were excluded if they presented more than 4 weeks after injury; if they represented a refracture, nonunion, or pathologic fracture; if the fracture was an isolated greater or lesser tuberosity fracture; if there was an associated neurovascular injury; if injury radiographs were absent or inadequate; or if the treatment method was operative or unknown. Applying these inclusion and exclusion criteria yielded 146 patients over age 60 years who had nonoperative treatment of a displaced proximal humerus fracture between 2006 and 2009.
Patient Characteristics
Dr. Makanji retrospectively reviewed the charts of all 207 patients (61 operative, 146 nonoperative). Information was recorded on patient age and sex, mechanism of injury, number of days between injury and presentation, any associated orthopedic injuries, side of injury, and treatment facility (trauma center A, trauma center B). In addition, a Charlson score was assigned to each patient on the basis of medical comorbidities.27
Radiographs and any computed tomography scans were also assessed by Dr. Okike and Dr. Lee. Each fracture was assigned a Neer classification (2-part, 3-part, 4-part) and an AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) classification (A, B, C).28 Displacement was categorized as varus angulation (neck–shaft angle, <130°), valgus angulation (neck–shaft angle, >140°), neutral angulation (neck–shaft angle, 135° ± 5°), or translation alone. In addition, all fractures were assessed for dislocation and medial comminution.29
Outcome Measures
All follow-up radiographs were reviewed to assess for nonunion (defined as lack of healing by 12 months), malunion, and humeral head avascular necrosis. Operative patients’ follow-up radiographs were reviewed to determine frequency of screw perforation and/or loss of fixation, and their medical records were reviewed to assess for other complications, including infection, neurovascular injury, and return to operating room for any other reason. Nonoperative patients’ medical records were reviewed to determine if surgical treatment was subsequently required.
To determine clinical outcomes, we asked patients to return for clinical evaluation, which included use of several questionnaires: Constant; DASH (Disabilities of the Arm, Shoulder, and Hand); SMFA (Short Musculoskeletal Functional Assessment); and Patient Reported Outcomes Measurement Information System (PROMIS) Physical Function Computer Adaptive Test.
Statistical Analysis
Chi-square test was used to compare the characteristics of patients who returned for clinical evaluation, Fisher exact test was used for tables with multiple cells less than 5, Student t test was used to compare clinical outcomes between operative and nonoperative groups. P < .05 was considered statistically significant, and all tests were 2-sided. Statistical analysis was performed using SAS Version 9 (SAS, Cary, North Carolina).
Results
Of the 207 patients who met the inclusion and exclusion criteria, 61 were treated operatively (locked plate open reduction and internal fixation) and 146 nonoperatively. Mean age was 76.9 years. One hundred fifty-five (74.9%) of the patients were female. Medical comorbidities were common (average Charlson score, 6.6). Most patients (185/207; 89.4%) were injured in a fall. There were 129 two-part fractures, 63 three-part fractures, and 9 four-part fractures (Table 1).
Operative patients’ complications included screw perforation (35.6%; 21 of the 59 cases with radiographs) and loss of fixation (17.5%; 10/57). Four (6.6%) of the 61 operative patients developed an infection. In sum, 8 (13.1%) of operative patients required another surgery (Table 2).
Among nonoperative patients, malunion at time of healing was common (86.9%; 113 of the 130 cases with radiographs). Eighty-six malunions (66.2% of the 130 cases) healed in varus, 25 (19.2%) in valgus, and 2 (1.5%) with translation alone. Uncommon among nonoperative patients were nonunion (1.4%; 2/143) and avascular necrosis (2.2%; 3/136). Two (1.4%) of the 146 nonoperative patients subsequently underwent surgery for malunion (Table 2).
Forty-seven patients accepted our invitation to return for clinical evaluation. Mean follow-up was 3.3 years (range, 1.4-6.4 years). Of these patients, 25 had been treated operatively (Figures 1A, 1B) and 22 nonoperatively (Figures 2A, 2B). Complication rates for patients who returned for clinical evaluation were similar to those for the entire cohort, with the exception of secondary surgical procedures (Table 3). There were no significant differences between operative and nonoperative patients in the group that returned for clinical evaluation (Table 4).
Regarding clinical outcome scores, there were no significant differences between operative and nonoperative patients (Table 5). In particular, there were no differences in SMFA Functional index (18.4 vs 19.7; P = .78), SMFA Bothersome index (20.8 vs 23.6; P = .61), DASH scores (26.5 vs 25.1; P = .79), Constant scores (58.0 vs 59.7; P = .74), or PROMIS Physical Function Computer Adaptive Test scores (43.9 vs 45.0; P = .70).
Discussion
In this observational study of displaced proximal humerus fractures in an elderly population, operative treatment (vs nonoperative treatment) had a lower malunion rate but was associated with more complications, including screw perforation, loss of fixation, and unplanned return to the operating room. Among patients who returned for clinical evaluation at a mean follow-up of 3.3 years, there were no significant operative–nonoperative differences.
Our results are similar to those recently reported by other investigators. In Norway, Fjalestad and colleagues23 conducted a randomized controlled trial of locked plating versus nonoperative treatment in 50 patients over age 60 years with a 3- or 4-part proximal humerus fracture. At 12 months, there was no significant difference between the operative and nonoperative groups’ Constant scores.
Similarly, Olerud and colleagues25 in Sweden conducted a trial in which 60 patients over age 55 years with a 3-part fracture of the proximal humerus were randomized to locked plating or nonoperative treatment. At 2 years, there were no significant operative–nonoperative differences on several outcome measures: Constant scores, DASH scores, EQ-5D (EuroQol) scores. Thirty percent of operative patients required a secondary procedure to treat infection, nonunion, avascular necrosis, screw perforation, stiffness, or impingement.
Our study benefited from having a large sample size (207) of consecutive patients with displaced proximal humerus fractures, but it also had its limitations. In this retrospective study, treatment assignment was not randomized. We were also limited by the large number of patients who did not return for clinical evaluation (160/207; 77.3%), including 52 (25.1%) found to be deceased, 27 (13.0%) who could not be reached, and 81 (39.1%) who declined our request (in many cases because of difficulties traveling to the trauma center). These challenges are inherent to research in the elderly population. As a result, the number of patients who returned for clinical evaluation (47/207; 22.7%) was lower than expected, which may have underpowered the study. In addition, treatment protocols were not standardized; patients were managed by a number of different surgeons. On the other hand, this wide variety of surgeons, including orthopedic trauma and upper extremity specialists, may increase the generalizability of our results.
Conclusion
Although use of locked plate fixation in treating proximal humerus fractures in elderly patients has increased markedly over recent years, definitive evidence supporting such management is lacking. In the present study, the outcomes of locked plate fixation were similar to those of nonoperative treatment. In addition, rates of complications and secondary surgical procedures were higher for operative patients than for nonoperative patients. Research is needed to identify the circumstances under which locked plating improves treatment outcomes for displaced proximal humerus fractures in elderly patients.
Proximal humerus fractures are increasingly common in the elderly population,1 accounting for 10% of all these patients’ fractures.2 The injuries result in substantial morbidity and are associated with significantly higher mortality rates for up to 4 years.3 With the recent advent of anatomical locking plates,4,5 operative fixation of proximal humerus fractures in elderly patients has become more common.6 Although early clinical studies reported favorable outcomes, high complication rates have also been documented.7-22
Investigators have recently compared outcomes of locked plate fixation and nonoperative treatment of proximal humerus fractures in elderly patients.23-26 Fjalestad and colleagues23 conducted a randomized clinical trial of locked plating versus nonoperative treatment of 3- and 4-part fractures in 50 patients age 60 years or older and found no significant differences in Constant score or patient self-assessment at 1 year. Similarly, Olerud and colleagues25 conducted a randomized clinical trial of locked plating versus nonoperative treatment of 3-part fractures in 60 patients age 55 years or older. Although outcomes were better in the operative group, differences did not reach statistical significance, and the operative group’s reoperation rate was 30%.
Given this lack of conclusive outcomes data, optimal treatment of displaced proximal humerus fractures in elderly patients remains unknown. We conducted a study to compare outcomes of operative (locked plate fixation) and nonoperative management of displaced proximal humerus fractures in patients older than 60 years. Our hypothesis was that the clinical outcomes of these 2 treatment methods would be similar.
Materials and Methods
Selection Criteria
Our research protocol was approved by the Partners Human Research Committee. To determine the operative cohort, we queried our trauma database to identify all patients over age 60 years who sustained a displaced proximal humerus fracture between 2006 and 2009 and underwent surgical fixation. Cases were excluded if they presented more than 4 weeks after injury; if they represented a refracture, nonunion, or pathologic fracture; if the fracture was an isolated greater or lesser tuberosity fracture; if there was an associated neurovascular injury; if the injury radiographs were absent or inadequate; or if a fixation method other than locked plating was used. Applying these inclusion and exclusion criteria yielded 61 patients over age 60 years who underwent locked plating of a displaced proximal humerus fracture between 2006 and 2009.
The comparison group consisted of all patients who presented to our institutions with a displaced proximal humerus fracture during the same time period but instead had nonoperative treatment. To identify this group, we performed another database search for all patients over age 60 years who sustained a proximal humerus fracture between 2006 and 2009 (n = 452). Twenty-two patients were excluded for inadequate radiographs. To determine which of the other 430 patients had displaced fractures, Dr. Okike and Dr. Lee (orthopedic surgeons) reviewed injury radiographs and any computed tomography scans in duplicate and resolved discrepancies by consensus. Neer’s criteria were used to define displacement: Fractures displaced 1 cm or more and/or with angulation of 45° or more were displaced, and fractures not meeting these criteria were nondisplaced. In the assessment of displacement, interobserver reliability was substantial (overall agreement, 87.0% [374/430]; κ = 0.68). With use of these methods, 311 fractures were classified displaced and 119 nondisplaced. As with the operative group, cases were excluded if they presented more than 4 weeks after injury; if they represented a refracture, nonunion, or pathologic fracture; if the fracture was an isolated greater or lesser tuberosity fracture; if there was an associated neurovascular injury; if injury radiographs were absent or inadequate; or if the treatment method was operative or unknown. Applying these inclusion and exclusion criteria yielded 146 patients over age 60 years who had nonoperative treatment of a displaced proximal humerus fracture between 2006 and 2009.
Patient Characteristics
Dr. Makanji retrospectively reviewed the charts of all 207 patients (61 operative, 146 nonoperative). Information was recorded on patient age and sex, mechanism of injury, number of days between injury and presentation, any associated orthopedic injuries, side of injury, and treatment facility (trauma center A, trauma center B). In addition, a Charlson score was assigned to each patient on the basis of medical comorbidities.27
Radiographs and any computed tomography scans were also assessed by Dr. Okike and Dr. Lee. Each fracture was assigned a Neer classification (2-part, 3-part, 4-part) and an AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) classification (A, B, C).28 Displacement was categorized as varus angulation (neck–shaft angle, <130°), valgus angulation (neck–shaft angle, >140°), neutral angulation (neck–shaft angle, 135° ± 5°), or translation alone. In addition, all fractures were assessed for dislocation and medial comminution.29
Outcome Measures
All follow-up radiographs were reviewed to assess for nonunion (defined as lack of healing by 12 months), malunion, and humeral head avascular necrosis. Operative patients’ follow-up radiographs were reviewed to determine frequency of screw perforation and/or loss of fixation, and their medical records were reviewed to assess for other complications, including infection, neurovascular injury, and return to operating room for any other reason. Nonoperative patients’ medical records were reviewed to determine if surgical treatment was subsequently required.
To determine clinical outcomes, we asked patients to return for clinical evaluation, which included use of several questionnaires: Constant; DASH (Disabilities of the Arm, Shoulder, and Hand); SMFA (Short Musculoskeletal Functional Assessment); and Patient Reported Outcomes Measurement Information System (PROMIS) Physical Function Computer Adaptive Test.
Statistical Analysis
Chi-square test was used to compare the characteristics of patients who returned for clinical evaluation, Fisher exact test was used for tables with multiple cells less than 5, Student t test was used to compare clinical outcomes between operative and nonoperative groups. P < .05 was considered statistically significant, and all tests were 2-sided. Statistical analysis was performed using SAS Version 9 (SAS, Cary, North Carolina).
Results
Of the 207 patients who met the inclusion and exclusion criteria, 61 were treated operatively (locked plate open reduction and internal fixation) and 146 nonoperatively. Mean age was 76.9 years. One hundred fifty-five (74.9%) of the patients were female. Medical comorbidities were common (average Charlson score, 6.6). Most patients (185/207; 89.4%) were injured in a fall. There were 129 two-part fractures, 63 three-part fractures, and 9 four-part fractures (Table 1).
Operative patients’ complications included screw perforation (35.6%; 21 of the 59 cases with radiographs) and loss of fixation (17.5%; 10/57). Four (6.6%) of the 61 operative patients developed an infection. In sum, 8 (13.1%) of operative patients required another surgery (Table 2).
Among nonoperative patients, malunion at time of healing was common (86.9%; 113 of the 130 cases with radiographs). Eighty-six malunions (66.2% of the 130 cases) healed in varus, 25 (19.2%) in valgus, and 2 (1.5%) with translation alone. Uncommon among nonoperative patients were nonunion (1.4%; 2/143) and avascular necrosis (2.2%; 3/136). Two (1.4%) of the 146 nonoperative patients subsequently underwent surgery for malunion (Table 2).
Forty-seven patients accepted our invitation to return for clinical evaluation. Mean follow-up was 3.3 years (range, 1.4-6.4 years). Of these patients, 25 had been treated operatively (Figures 1A, 1B) and 22 nonoperatively (Figures 2A, 2B). Complication rates for patients who returned for clinical evaluation were similar to those for the entire cohort, with the exception of secondary surgical procedures (Table 3). There were no significant differences between operative and nonoperative patients in the group that returned for clinical evaluation (Table 4).
Regarding clinical outcome scores, there were no significant differences between operative and nonoperative patients (Table 5). In particular, there were no differences in SMFA Functional index (18.4 vs 19.7; P = .78), SMFA Bothersome index (20.8 vs 23.6; P = .61), DASH scores (26.5 vs 25.1; P = .79), Constant scores (58.0 vs 59.7; P = .74), or PROMIS Physical Function Computer Adaptive Test scores (43.9 vs 45.0; P = .70).
Discussion
In this observational study of displaced proximal humerus fractures in an elderly population, operative treatment (vs nonoperative treatment) had a lower malunion rate but was associated with more complications, including screw perforation, loss of fixation, and unplanned return to the operating room. Among patients who returned for clinical evaluation at a mean follow-up of 3.3 years, there were no significant operative–nonoperative differences.
Our results are similar to those recently reported by other investigators. In Norway, Fjalestad and colleagues23 conducted a randomized controlled trial of locked plating versus nonoperative treatment in 50 patients over age 60 years with a 3- or 4-part proximal humerus fracture. At 12 months, there was no significant difference between the operative and nonoperative groups’ Constant scores.
Similarly, Olerud and colleagues25 in Sweden conducted a trial in which 60 patients over age 55 years with a 3-part fracture of the proximal humerus were randomized to locked plating or nonoperative treatment. At 2 years, there were no significant operative–nonoperative differences on several outcome measures: Constant scores, DASH scores, EQ-5D (EuroQol) scores. Thirty percent of operative patients required a secondary procedure to treat infection, nonunion, avascular necrosis, screw perforation, stiffness, or impingement.
Our study benefited from having a large sample size (207) of consecutive patients with displaced proximal humerus fractures, but it also had its limitations. In this retrospective study, treatment assignment was not randomized. We were also limited by the large number of patients who did not return for clinical evaluation (160/207; 77.3%), including 52 (25.1%) found to be deceased, 27 (13.0%) who could not be reached, and 81 (39.1%) who declined our request (in many cases because of difficulties traveling to the trauma center). These challenges are inherent to research in the elderly population. As a result, the number of patients who returned for clinical evaluation (47/207; 22.7%) was lower than expected, which may have underpowered the study. In addition, treatment protocols were not standardized; patients were managed by a number of different surgeons. On the other hand, this wide variety of surgeons, including orthopedic trauma and upper extremity specialists, may increase the generalizability of our results.
Conclusion
Although use of locked plate fixation in treating proximal humerus fractures in elderly patients has increased markedly over recent years, definitive evidence supporting such management is lacking. In the present study, the outcomes of locked plate fixation were similar to those of nonoperative treatment. In addition, rates of complications and secondary surgical procedures were higher for operative patients than for nonoperative patients. Research is needed to identify the circumstances under which locked plating improves treatment outcomes for displaced proximal humerus fractures in elderly patients.
1. Palvanen M, Kannus P, Niemi S, Parkkari J. Update in the epidemiology of proximal humeral fractures. Clin Orthop. 2006;(442):87-92.
2. Baron JA, Karagas M, Barrett J, et al. Basic epidemiology of fractures of the upper and lower limb among Americans over 65 years of age. Epidemiology. 1996;7(6):612-618.
3. Johnell O, Kanis JA, Oden A, et al. Mortality after osteoporotic fractures. Osteoporos Int. 2004;15(1):38-42.
4. Badman BL, Mighell M. Fixed-angle locked plating of two-, three-, and four-part proximal humerus fractures. J Am Acad Orthop Surg. 2008;16(5):294-302.
5. Nho SJ, Brophy RH, Barker JU, Cornell CN, MacGillivray JD. Innovations in the management of displaced proximal humerus fractures. J Am Acad Orthop Surg. 2007;15(1):12-26.
6. Bell JE, Leung BC, Spratt KF, et al. Trends and variation in incidence, surgical treatment, and repeat surgery of proximal humeral fractures in the elderly. J Bone Joint Surg Am. 2011;93(2):121-131.
7. Agudelo J, Schurmann M, Stahel P, et al. Analysis of efficacy and failure in proximal humerus fractures treated with locking plates. J Orthop Trauma. 2007;21(10):676-681.
8. Bigorre N, Talha A, Cronier P, Hubert L, Toulemonde JL, Massin P. A prospective study of a new locking plate for proximal humeral fracture. Injury. 2009;40(2):192-196.
9. Bjorkenheim JM, Pajarinen J, Savolainen V. Internal fixation of proximal humeral fractures with a locking compression plate: a retrospective evaluation of 72 patients followed for a minimum of 1 year. Acta Orthop Scand. 2004;75(6):741-745.
10. Brunner F, Sommer C, Bahrs C, et al. Open reduction and internal fixation of proximal humerus fractures using a proximal humeral locked plate: a prospective multicenter analysis. J Orthop Trauma. 2009;23(3):163-172.
11. Charalambous CP, Siddique I, Valluripalli K, et al. Proximal humeral internal locking system (PHILOS) for the treatment of proximal humeral fractures. Arch Orthop Trauma Surg. 2007;127(3):205-210.
12. Egol KA, Ong CC, Walsh M, Jazrawi LM, Tejwani NC, Zuckerman JD. Early complications in proximal humerus fractures (OTA types 11) treated with locked plates. J Orthop Trauma. 2008;22(3):159-164.
13. Fankhauser F, Boldin C, Schippinger G, Haunschmid C, Szyszkowitz R. A new locking plate for unstable fractures of the proximal humerus. Clin Orthop. 2005;(430):176-181.
14. Hepp P, Theopold J, Osterhoff G, Marquass B, Voigt C, Josten C. Bone quality measured by the radiogrammetric parameter “cortical index” and reoperations after locking plate osteosynthesis in patients sustaining proximal humerus fractures. Arch Orthop Trauma Surg. 2009;129(9):1251-1259.
15. Koukakis A, Apostolou CD, Taneja T, Korres DS, Amini A. Fixation of proximal humerus fractures using the PHILOS plate: early experience. Clin Orthop. 2006;(442):115-120.
16. Moonot P, Ashwood N, Hamlet M. Early results for treatment of three- and four-part fractures of the proximal humerus using the PHILOS plate system. J Bone Joint Surg Br. 2007;89(9):1206-1209.
17. Owsley KC, Gorczyca JT. Fracture displacement and screw cutout after open reduction and locked plate fixation of proximal humeral fractures. J Bone Joint Surg Am. 2008;90(2):233-240.
18. Rose PS, Adams CR, Torchia ME, Jacofsky DJ, Haidukewych GG, Steinmann SP. Locking plate fixation for proximal humeral fractures: initial results with a new implant. J Shoulder Elbow Surg. 2007;16(2):202-207.
19. Shahid R, Mushtaq A, Northover J, Maqsood M. Outcome of proximal humerus fractures treated by PHILOS plate internal fixation. Experience of a district general hospital. Acta Orthop Belg. 2008;74(5):602-608.
20. Smith AM, Mardones RM, Sperling JW, Cofield RH. Early complications of operatively treated proximal humeral fractures. J Shoulder Elbow Surg. 2007;16(1):14-24.
21. Sudkamp N, Bayer J, Hepp P, et al. Open reduction and internal fixation of proximal humeral fractures with use of the locking proximal humerus plate. Results of a prospective, multicenter, observational study. J Bone Joint Surg Am. 2009;91(6):1320-1328.
22. Thalhammer G, Platzer P, Oberleitner G, Fialka C, Greitbauer M, Vecsei V. Angular stable fixation of proximal humeral fractures. J Trauma. 2009;66(1):204-210.
23. Fjalestad T, Hole MO, Hovden IA, Blucher J, Stromsoe K. Surgical treatment with an angular stable plate for complex displaced proximal humeral fractures in elderly patients: a randomized controlled trial. J Orthop Trauma. 2012;26(2):98-106.
24. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Hemiarthroplasty versus nonoperative treatment of displaced 4-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(7):1025-1033.
25. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Internal fixation versus nonoperative treatment of displaced 3-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(5):747-755.
26. Sanders RJ, Thissen LG, Teepen JC, van Kampen A, Jaarsma RL. Locking plate versus nonsurgical treatment for proximal humeral fractures: better midterm outcome with nonsurgical treatment. J Shoulder Elbow Surg. 2011;20(7):1118-1124
27. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.
28. Muller ME, Nazarus C, Koch P, Schatzker J. The Comprehensive Classification of Fractures of Long Bones. Berlin, Germany: Springer-Verlag; 1990.
29. Gardner MJ, Weil Y, Barker JU, Kelly BT, Helfet DL, Lorich DG. The importance of medial support in locked plating of proximal humerus fractures. J Orthop Trauma. 2007;21(3):185-191.
1. Palvanen M, Kannus P, Niemi S, Parkkari J. Update in the epidemiology of proximal humeral fractures. Clin Orthop. 2006;(442):87-92.
2. Baron JA, Karagas M, Barrett J, et al. Basic epidemiology of fractures of the upper and lower limb among Americans over 65 years of age. Epidemiology. 1996;7(6):612-618.
3. Johnell O, Kanis JA, Oden A, et al. Mortality after osteoporotic fractures. Osteoporos Int. 2004;15(1):38-42.
4. Badman BL, Mighell M. Fixed-angle locked plating of two-, three-, and four-part proximal humerus fractures. J Am Acad Orthop Surg. 2008;16(5):294-302.
5. Nho SJ, Brophy RH, Barker JU, Cornell CN, MacGillivray JD. Innovations in the management of displaced proximal humerus fractures. J Am Acad Orthop Surg. 2007;15(1):12-26.
6. Bell JE, Leung BC, Spratt KF, et al. Trends and variation in incidence, surgical treatment, and repeat surgery of proximal humeral fractures in the elderly. J Bone Joint Surg Am. 2011;93(2):121-131.
7. Agudelo J, Schurmann M, Stahel P, et al. Analysis of efficacy and failure in proximal humerus fractures treated with locking plates. J Orthop Trauma. 2007;21(10):676-681.
8. Bigorre N, Talha A, Cronier P, Hubert L, Toulemonde JL, Massin P. A prospective study of a new locking plate for proximal humeral fracture. Injury. 2009;40(2):192-196.
9. Bjorkenheim JM, Pajarinen J, Savolainen V. Internal fixation of proximal humeral fractures with a locking compression plate: a retrospective evaluation of 72 patients followed for a minimum of 1 year. Acta Orthop Scand. 2004;75(6):741-745.
10. Brunner F, Sommer C, Bahrs C, et al. Open reduction and internal fixation of proximal humerus fractures using a proximal humeral locked plate: a prospective multicenter analysis. J Orthop Trauma. 2009;23(3):163-172.
11. Charalambous CP, Siddique I, Valluripalli K, et al. Proximal humeral internal locking system (PHILOS) for the treatment of proximal humeral fractures. Arch Orthop Trauma Surg. 2007;127(3):205-210.
12. Egol KA, Ong CC, Walsh M, Jazrawi LM, Tejwani NC, Zuckerman JD. Early complications in proximal humerus fractures (OTA types 11) treated with locked plates. J Orthop Trauma. 2008;22(3):159-164.
13. Fankhauser F, Boldin C, Schippinger G, Haunschmid C, Szyszkowitz R. A new locking plate for unstable fractures of the proximal humerus. Clin Orthop. 2005;(430):176-181.
14. Hepp P, Theopold J, Osterhoff G, Marquass B, Voigt C, Josten C. Bone quality measured by the radiogrammetric parameter “cortical index” and reoperations after locking plate osteosynthesis in patients sustaining proximal humerus fractures. Arch Orthop Trauma Surg. 2009;129(9):1251-1259.
15. Koukakis A, Apostolou CD, Taneja T, Korres DS, Amini A. Fixation of proximal humerus fractures using the PHILOS plate: early experience. Clin Orthop. 2006;(442):115-120.
16. Moonot P, Ashwood N, Hamlet M. Early results for treatment of three- and four-part fractures of the proximal humerus using the PHILOS plate system. J Bone Joint Surg Br. 2007;89(9):1206-1209.
17. Owsley KC, Gorczyca JT. Fracture displacement and screw cutout after open reduction and locked plate fixation of proximal humeral fractures. J Bone Joint Surg Am. 2008;90(2):233-240.
18. Rose PS, Adams CR, Torchia ME, Jacofsky DJ, Haidukewych GG, Steinmann SP. Locking plate fixation for proximal humeral fractures: initial results with a new implant. J Shoulder Elbow Surg. 2007;16(2):202-207.
19. Shahid R, Mushtaq A, Northover J, Maqsood M. Outcome of proximal humerus fractures treated by PHILOS plate internal fixation. Experience of a district general hospital. Acta Orthop Belg. 2008;74(5):602-608.
20. Smith AM, Mardones RM, Sperling JW, Cofield RH. Early complications of operatively treated proximal humeral fractures. J Shoulder Elbow Surg. 2007;16(1):14-24.
21. Sudkamp N, Bayer J, Hepp P, et al. Open reduction and internal fixation of proximal humeral fractures with use of the locking proximal humerus plate. Results of a prospective, multicenter, observational study. J Bone Joint Surg Am. 2009;91(6):1320-1328.
22. Thalhammer G, Platzer P, Oberleitner G, Fialka C, Greitbauer M, Vecsei V. Angular stable fixation of proximal humeral fractures. J Trauma. 2009;66(1):204-210.
23. Fjalestad T, Hole MO, Hovden IA, Blucher J, Stromsoe K. Surgical treatment with an angular stable plate for complex displaced proximal humeral fractures in elderly patients: a randomized controlled trial. J Orthop Trauma. 2012;26(2):98-106.
24. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Hemiarthroplasty versus nonoperative treatment of displaced 4-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(7):1025-1033.
25. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Internal fixation versus nonoperative treatment of displaced 3-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(5):747-755.
26. Sanders RJ, Thissen LG, Teepen JC, van Kampen A, Jaarsma RL. Locking plate versus nonsurgical treatment for proximal humeral fractures: better midterm outcome with nonsurgical treatment. J Shoulder Elbow Surg. 2011;20(7):1118-1124
27. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.
28. Muller ME, Nazarus C, Koch P, Schatzker J. The Comprehensive Classification of Fractures of Long Bones. Berlin, Germany: Springer-Verlag; 1990.
29. Gardner MJ, Weil Y, Barker JU, Kelly BT, Helfet DL, Lorich DG. The importance of medial support in locked plating of proximal humerus fractures. J Orthop Trauma. 2007;21(3):185-191.
Stem Cells May Significantly Improve Tendon Healing, Reduce Retear Risk in Rotator Cuff Surgery
LAS VEGAS—An injection of a patient’s bone marrow stem cells during rotator cuff surgery significantly improved healing and tendon durability, according to a study presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS).
The French study, of which a portion appeared in the September 2014 issue of International Orthopaedics, included 90 patients who underwent rotator cuff surgery. Forty-five of the patients received injections of bone marrow concentrate (BMC) mesenchymal stem cells (MSCs) at the surgical site, and 45 had their rotator cuff repaired or reattached without MSCs. Researchers tried to make the 2 groups as equivalent as possible based on rotator cuff tear size, tendon rupture location, dominate shoulder, gender, and age.
Patient ultrasound images were obtained each month following surgery for 24 months. In addition, magnetic resonance imaging was obtained of patient shoulders at 3 and 6 months following surgery, and at 1 year, 2 years, and 10 years following surgery.
At 6 months, all 45 of the patients who received MSCs had healed rotator cuff tendons, compared to 30 (67%) of the patients who did not receive MSCs. The use of BMC also prevented further ruptures or retears. At 10 years after surgery, intact rotator cuffs were found in 39 (87%) of the MSC patients, but just 20 (44%) of the non-MSC patients.
In addition, “some retears or new tears occurred after 1 year,” said lead study author Philippe Hernigou, MD, an orthopedic surgeon at the University of Paris. “These retears were more frequently associated with the control group patients who were not treated with MSCs.
“While the risk of a retear after arthroscopic repair of the rotator cuff has been well documented, publications with long-term follow-up (more than 3 years) are relatively limited,” said Dr. Hernigou. “Many patients undergoing rotator cuff repair surgery show advanced degeneration of the tendons, which are thinner and atrophic, probably explaining why negative results are so often reported in the literature, with frequent post-operative complications, especially retear. Observations in the MSC treatment group support the potential that MSC treatment has both a short-term and long-term benefit in reducing the rate of tendon retear.”
LAS VEGAS—An injection of a patient’s bone marrow stem cells during rotator cuff surgery significantly improved healing and tendon durability, according to a study presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS).
The French study, of which a portion appeared in the September 2014 issue of International Orthopaedics, included 90 patients who underwent rotator cuff surgery. Forty-five of the patients received injections of bone marrow concentrate (BMC) mesenchymal stem cells (MSCs) at the surgical site, and 45 had their rotator cuff repaired or reattached without MSCs. Researchers tried to make the 2 groups as equivalent as possible based on rotator cuff tear size, tendon rupture location, dominate shoulder, gender, and age.
Patient ultrasound images were obtained each month following surgery for 24 months. In addition, magnetic resonance imaging was obtained of patient shoulders at 3 and 6 months following surgery, and at 1 year, 2 years, and 10 years following surgery.
At 6 months, all 45 of the patients who received MSCs had healed rotator cuff tendons, compared to 30 (67%) of the patients who did not receive MSCs. The use of BMC also prevented further ruptures or retears. At 10 years after surgery, intact rotator cuffs were found in 39 (87%) of the MSC patients, but just 20 (44%) of the non-MSC patients.
In addition, “some retears or new tears occurred after 1 year,” said lead study author Philippe Hernigou, MD, an orthopedic surgeon at the University of Paris. “These retears were more frequently associated with the control group patients who were not treated with MSCs.
“While the risk of a retear after arthroscopic repair of the rotator cuff has been well documented, publications with long-term follow-up (more than 3 years) are relatively limited,” said Dr. Hernigou. “Many patients undergoing rotator cuff repair surgery show advanced degeneration of the tendons, which are thinner and atrophic, probably explaining why negative results are so often reported in the literature, with frequent post-operative complications, especially retear. Observations in the MSC treatment group support the potential that MSC treatment has both a short-term and long-term benefit in reducing the rate of tendon retear.”
LAS VEGAS—An injection of a patient’s bone marrow stem cells during rotator cuff surgery significantly improved healing and tendon durability, according to a study presented at the 2015 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS).
The French study, of which a portion appeared in the September 2014 issue of International Orthopaedics, included 90 patients who underwent rotator cuff surgery. Forty-five of the patients received injections of bone marrow concentrate (BMC) mesenchymal stem cells (MSCs) at the surgical site, and 45 had their rotator cuff repaired or reattached without MSCs. Researchers tried to make the 2 groups as equivalent as possible based on rotator cuff tear size, tendon rupture location, dominate shoulder, gender, and age.
Patient ultrasound images were obtained each month following surgery for 24 months. In addition, magnetic resonance imaging was obtained of patient shoulders at 3 and 6 months following surgery, and at 1 year, 2 years, and 10 years following surgery.
At 6 months, all 45 of the patients who received MSCs had healed rotator cuff tendons, compared to 30 (67%) of the patients who did not receive MSCs. The use of BMC also prevented further ruptures or retears. At 10 years after surgery, intact rotator cuffs were found in 39 (87%) of the MSC patients, but just 20 (44%) of the non-MSC patients.
In addition, “some retears or new tears occurred after 1 year,” said lead study author Philippe Hernigou, MD, an orthopedic surgeon at the University of Paris. “These retears were more frequently associated with the control group patients who were not treated with MSCs.
“While the risk of a retear after arthroscopic repair of the rotator cuff has been well documented, publications with long-term follow-up (more than 3 years) are relatively limited,” said Dr. Hernigou. “Many patients undergoing rotator cuff repair surgery show advanced degeneration of the tendons, which are thinner and atrophic, probably explaining why negative results are so often reported in the literature, with frequent post-operative complications, especially retear. Observations in the MSC treatment group support the potential that MSC treatment has both a short-term and long-term benefit in reducing the rate of tendon retear.”
Greater Auricular Nerve Palsy After Arthroscopic Anterior-Inferior and Posterior-Inferior Labral Tear Repair Using Beach-Chair Positioning and a Standard Universal Headrest
Anterior-inferior and posterior-inferior labral tears are common injuries treated with arthroscopic surgery1 typically performed with beach-chair2,3 or lateral decubitus1,2 positioning and variable headrest positioning. Iatrogenic nerve damage that occurs after arthroscopic shoulder surgery—including damage to the suprascapular, axillary, musculocutaneous, subscapular, and spinal accessory nerves—has recently been reported to be more common than previously recognized.2,4
Although iatrogenic nerve injuries are in general being recognized,1,2,4 reports of greater auricular nerve injuries are limited. The greater auricular nerve is a superficial cutaneous nerve that arises from the cervical plexus at the C2 and C3 spinal nerves, obliquely crosses the sternocleidomastoid muscle, and splits into anterior and posterior portions that innervate the skin over the mastoid process and parotid gland.5,6 In particular, as illustrated by Ginsberg and Eicher6 (Figure 1), its superficial anatomy lies very near where a headrest is positioned during arthroscopic surgery, and increased pressure on the nerve throughout arthroscopic shoulder surgery may lead to neurapraxia.6,7 In 2 case series, authors reported on a total of 5 patients who had greater auricular nerve palsy after uncomplicated shoulder surgery using beach-chair positioning and a horseshoe headrest.7,8 The authors attributed these palsies to the horseshoe headrest, which they believed was compressing the greater auricular nerve during the entire surgery.7,8 However, standard universal headrests, which are thought to distribute pressures that would theoretically place the greater auricular nerve at risk for palsy, previously had not been described as contributing to palsy of the greater auricular nerve.
In this article, we report on a case of greater auricular nerve palsy that occurred after the patient’s anterior-inferior and posterior-inferior labral tear was arthroscopically repaired using beach-chair positioning and a standard universal headrest. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old right-hand–dominant high school American football player was referred for orthopedic evaluation of left chronic glenohumeral instability after 6 months of physical therapy. Physical examination revealed a positive apprehension test with the shoulder abducted and externally rotated at 90° and a positive relocation test. The patient complained of pain and instability when his arm was placed in a cross-chest adducted position and a posteroinferiorly directed axial load was applied. Magnetic resonance arthrogram showed an anterior-inferior labral Bankart tear with a small Hill-Sachs lesion to the humeral head but did not clearly reveal the posterior-inferior labral tear. Because of persistent left shoulder instability with most overhead activities and continued pain, the patient decided to undergo left shoulder arthroscopic Bankart repair with inferior capsular shift and posterior-inferior labral repair with capsulorraphy. He had no significant past medical history or known drug allergies.
The patient was placed in the standard beach-chair position: upright at 45° to the floor, hips flexed at 60°, knees flexed at 30°.1 Pneumatic compression devices were placed on his lower extremities. His head was secured in neutral position to a standard universal headrest (model A-90026; Allen Medical Systems, Acton, Massachusetts) (Figures 2, 3). Care was taken to protect the deep neurovascular structures and bony prominences throughout. The patient was in this position for 122 minutes of the operation, from positioning and draping to wound closure and dressing application. Before draping, the anesthesiologist, head nurse, and circulating nurse ensured that head and neck were in neutral position. The anesthesiologist monitored positioning throughout the perioperative period to ensure head and neck were in neutral, and the head did not need to be repositioned during surgery. Standard preoperative intravenous antibiotics were given.
General anesthesia and postoperative interscalene block were used. Standard preparation and draping were performed. Three standard arthroscopic portal incisions were used: posterior, anterior, and anterosuperolateral. Findings included extensive labral pathology, small bony Hill-Sachs lesion to humeral head, small bony anterior glenoid deficiency, and deficient anterior-inferior and posterior-inferior labral remnant. These were repaired arthroscopically in a standard fashion using bioabsorbable suture anchors. There were no arthroscopic complications. After surgery, a standard well-fitted shoulder immobilizer was placed. The anesthesiologist provided interscalene regional analgesia (15 mL of bupivacaine 0.5%) in the recovery area after surgery.
Postoperative neurovascular examination in the recovery room revealed no discomfort. The patient was discharged the same day. At a scheduled 1-week follow-up, he complained of numbness and dysesthesia on the left side of the greater auricular nerve distribution. A diagnosis of greater auricular nerve palsy was made by physical examination; the symptoms were along the classic greater auricular nerve distribution affecting the lower face and ear (Figure 4). The patient had no pain, skin lesions, or soft-tissue swelling. Otolaryngology confirmed the diagnosis and recommended observation-only treatment of symptoms. Symptoms lessened over the next 3 months, and the altered sensation resolved without deficit by 6 months. In addition, by 6 months the patient had returned to full activities (including collision sports) pain-free and with normal left shoulder function. Because symptoms continued to improve, the patient was followed with clinical observation, and a formal work-up was not necessary.
Discussion
The most important finding in this case is the greater auricular nerve palsy that occurred after arthroscopic anterior-inferior and posterior-inferior labral repairs in beach-chair positioning. This greater auricular nerve palsy was the first encountered by Dr. Foad, who over 17 years in a primarily shoulder practice setting has used beach-chair positioning exclusively. Previous reports have described a palsy occurring after arthroscopic shoulder surgery using beach-chair positioning and a horseshoe headrest.7,8 Ng and Page7 discontinued and recommended against use of this headrest because of the complications of the palsy, and Park and Kim8 recommended a headrest redesign. We think the present case report is the first to describe a greater auricular nerve palsy that occurred after arthroscopic surgery using a standard universal headrest, which theoretically should prevent compression of the greater auricular nerve. Increased awareness of the possibility of greater auricular nerve palsy, even when proper precautions are taken,1 is therefore warranted.
Based on the location of our patient’s palsy, we think his paralysis was most likely the result of nerve compression by the headrest during the shoulder surgery. Other factors, though unlikely, may have played a role. These include operative time (increases duration of nerve compression) and head positioning. However, 122 minutes is not unusually long for a patient’s head to be in this position during a procedure, and over the past 10 years the same anesthesiologist, head nurse, and circulating nurse have routinely used the same beach-chair positioning and headrest for Dr. Foad’s patients. Second, the postoperative interscalene block theoretically could have caused the palsy, but we think this is unlikely, as the block is placed lower on the neck, at the C6 level, and the greater auricular nerve branches off the C2–C3 spinal nerves. As described by Rains and colleagues,9 other authors have reported transient neuropathies to the brachial plexus, which originates in the C5–C8 region, but not to the greater auricular nerve. Last, it cannot be ruled out that a variant of the greater auricular nerve could have played a role, given the variation in the greater auricular nerve.10,11 However, Brennan and colleagues10 reported that 2 of 25 neck dissections involved a variant in which the anterior division of the greater auricular nerve passed into the submandibular triangle and joined the mandibular region of the facial nerve. Stimulation of this nerve resulted in activity of the depressor of the lower lip, which was not the location of our patient’s palsy. In addition, our patient’s symptoms followed a classic nerve distribution of the greater auricular nerve (Figures 1, 4), which would seem to decrease the likelihood that a variant was the source of the palsy.
The superficial nature of the greater auricular nerve, which runs roughly parallel with the sternocleidomastoid muscle and innervates much of the superficial region of the skin over the mastoid, parotid gland, and mandible,5-7 theoretically places the nerve at risk for compressive forces from the headrest during arthroscopic shoulder surgery. Skyhar and colleagues3 first described beach-chair positioning as an alternative to lateral decubitus positioning, which had been reported to result in neurologic injury in about 10% of surgical cases.9 The theoretical advantages of beach-chair positioning are lack of traction needed and ease of setup, which would therefore decrease the possibility of neuropathy.1,3 However, as seen in this and other case reports,7,8 greater auricular nerve neuropathy should still be considered a possible complication, even when using beach-chair positioning.
Besides neuropathy after arthroscopic shoulder surgery, as described in previous case reports7,8 and in the present report, greater auricular nerve injury has been described as arising from other stimuli. Greater auricular nerve injury has arisen after perineural tumor metastasis,6 neuroma of greater auricular nerve after endolympathic shunt surgery,12 internal fixation of mandibular condyle,13 and carotid endarterectomy.14,15 Given the superficial nature of the greater auricular nerve, it may not be all that surprising that a palsy could also develop after headrest compression during arthroscopic shoulder surgery.
This case report brings to light a possible complication of greater auricular nerve palsy during arthroscopic shoulder surgery using beach-chair positioning and a standard universal headrest. Studies should now investigate whether greater auricular nerve palsy is more common than realized, especially with regard to specific headrests in beach-chair positioning. For now, though, Dr. Foad’s intention is not to change to a different headrest or discontinue beach-chair positioning but to draw attention to this rare complication. Additional attention should be given to the location of the headrest in relation to the greater auricular nerve, especially in cases in which operative time may be longer.
Conclusion
We have reported a greater auricular nerve palsy that occurred after arthroscopic shoulder surgery for an anterior-inferior and posterior-inferior labral tear. This is the first report of a greater auricular nerve palsy occurring with beach-chair positioning and a standard universal headrest. Symptoms resolved within 6 months. New studies should investigate the incidence of greater auricular nerve palsy after arthroscopic shoulder surgery.
1. Paxton ES, Backus J, Keener J, Brophy RH. Shoulder arthroscopy: basic principles of positioning, anesthesia, and portal anatomy. J Am Acad Orthop Surg. 2013;21(6):332-342.
2. Scully WF, Wilson DJ, Parada SA, Arrington ED. Iatrogenic nerve injuries in shoulder surgery. J Am Acad Orthop Surg. 2013;21(12):717-726.
3. Skyhar MJ, Altchek DW, Warren RF, Wickiewicz TL, O’Brien SJ. Shoulder arthroscopy with the patient in the beach-chair position. Arthroscopy. 1988;4(4):256-259.
4. Zhang J, Moore AE, Stringer MD. Iatrogenic upper limb nerve injuries: a systematic review. ANZ J Surg. 2011;81(4):227-236.
5. Alberti PW. The greater auricular nerve. Donor for facial nerve grafts: a note on its topographical anatomy. Arch Otolaryngol. 1962;76:422-424.
6. Ginsberg LE, Eicher SA. Great auricular nerve: anatomy and imaging in a case of perineural tumor spread. AJNR Am J Neuroradiol. 2000;21(3):568-571.
7. Ng AK, Page RS. Greater auricular nerve neuropraxia with beach chair positioning during shoulder surgery. Int J Shoulder Surg. 2010;4(2):48-50.
8. Park TS, Kim YS. Neuropraxia of the cutaneous nerve of the cervical plexus after shoulder arthroscopy. Arthroscopy. 2005;21(5):631.e1-e3.
9. Rains DD, Rooke GA, Wahl CJ. Pathomechanisms and complications related to patient positioning and anesthesia during shoulder arthroscopy. Arthroscopy. 2011;27(4):532-541.
10. Brennan PA, Al Gholmy M, Ounnas H, Zaki GA, Puxeddu R, Standring S. Communication of the anterior branch of the great auricular nerve with the marginal mandibular nerve: a prospective study of 25 neck dissections. Br J Oral Maxillofac Surg. 2010;48(6):431-433.
11. Sand T, Becser N. Neurophysiological and anatomical variability of the greater auricular nerve. Acta Neurol Scand. 1998;98(5):333-339.
12. Vorobeichik L, Fallucco MA, Hagan RR. Chronic daily headaches secondary to greater auricular and lesser occipital neuromas following endolymphatic shunt surgery. BMJ Case Rep. 2012;2012. pii: bcr-2012-007189. doi:10.1136/bcr-2012-007189.
13. Sverzut CE, Trivellato AE, Serra EC, Ferraz EP, Sverzut AT. Frey’s syndrome after condylar fracture: case report. Braz Dent J. 2004;15(2):159-162.
14. AbuRahma AF, Choueiri MA. Cranial and cervical nerve injuries after repeat carotid endarterectomy. J Vasc Surg. 2000;32(4):649-654.
15. Ballotta E, Da Giau G, Renon L, et al. Cranial and cervical nerve injuries after carotid endarterectomy: a prospective study. Surgery. 1999;125(1):85-91.
Anterior-inferior and posterior-inferior labral tears are common injuries treated with arthroscopic surgery1 typically performed with beach-chair2,3 or lateral decubitus1,2 positioning and variable headrest positioning. Iatrogenic nerve damage that occurs after arthroscopic shoulder surgery—including damage to the suprascapular, axillary, musculocutaneous, subscapular, and spinal accessory nerves—has recently been reported to be more common than previously recognized.2,4
Although iatrogenic nerve injuries are in general being recognized,1,2,4 reports of greater auricular nerve injuries are limited. The greater auricular nerve is a superficial cutaneous nerve that arises from the cervical plexus at the C2 and C3 spinal nerves, obliquely crosses the sternocleidomastoid muscle, and splits into anterior and posterior portions that innervate the skin over the mastoid process and parotid gland.5,6 In particular, as illustrated by Ginsberg and Eicher6 (Figure 1), its superficial anatomy lies very near where a headrest is positioned during arthroscopic surgery, and increased pressure on the nerve throughout arthroscopic shoulder surgery may lead to neurapraxia.6,7 In 2 case series, authors reported on a total of 5 patients who had greater auricular nerve palsy after uncomplicated shoulder surgery using beach-chair positioning and a horseshoe headrest.7,8 The authors attributed these palsies to the horseshoe headrest, which they believed was compressing the greater auricular nerve during the entire surgery.7,8 However, standard universal headrests, which are thought to distribute pressures that would theoretically place the greater auricular nerve at risk for palsy, previously had not been described as contributing to palsy of the greater auricular nerve.
In this article, we report on a case of greater auricular nerve palsy that occurred after the patient’s anterior-inferior and posterior-inferior labral tear was arthroscopically repaired using beach-chair positioning and a standard universal headrest. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old right-hand–dominant high school American football player was referred for orthopedic evaluation of left chronic glenohumeral instability after 6 months of physical therapy. Physical examination revealed a positive apprehension test with the shoulder abducted and externally rotated at 90° and a positive relocation test. The patient complained of pain and instability when his arm was placed in a cross-chest adducted position and a posteroinferiorly directed axial load was applied. Magnetic resonance arthrogram showed an anterior-inferior labral Bankart tear with a small Hill-Sachs lesion to the humeral head but did not clearly reveal the posterior-inferior labral tear. Because of persistent left shoulder instability with most overhead activities and continued pain, the patient decided to undergo left shoulder arthroscopic Bankart repair with inferior capsular shift and posterior-inferior labral repair with capsulorraphy. He had no significant past medical history or known drug allergies.
The patient was placed in the standard beach-chair position: upright at 45° to the floor, hips flexed at 60°, knees flexed at 30°.1 Pneumatic compression devices were placed on his lower extremities. His head was secured in neutral position to a standard universal headrest (model A-90026; Allen Medical Systems, Acton, Massachusetts) (Figures 2, 3). Care was taken to protect the deep neurovascular structures and bony prominences throughout. The patient was in this position for 122 minutes of the operation, from positioning and draping to wound closure and dressing application. Before draping, the anesthesiologist, head nurse, and circulating nurse ensured that head and neck were in neutral position. The anesthesiologist monitored positioning throughout the perioperative period to ensure head and neck were in neutral, and the head did not need to be repositioned during surgery. Standard preoperative intravenous antibiotics were given.
General anesthesia and postoperative interscalene block were used. Standard preparation and draping were performed. Three standard arthroscopic portal incisions were used: posterior, anterior, and anterosuperolateral. Findings included extensive labral pathology, small bony Hill-Sachs lesion to humeral head, small bony anterior glenoid deficiency, and deficient anterior-inferior and posterior-inferior labral remnant. These were repaired arthroscopically in a standard fashion using bioabsorbable suture anchors. There were no arthroscopic complications. After surgery, a standard well-fitted shoulder immobilizer was placed. The anesthesiologist provided interscalene regional analgesia (15 mL of bupivacaine 0.5%) in the recovery area after surgery.
Postoperative neurovascular examination in the recovery room revealed no discomfort. The patient was discharged the same day. At a scheduled 1-week follow-up, he complained of numbness and dysesthesia on the left side of the greater auricular nerve distribution. A diagnosis of greater auricular nerve palsy was made by physical examination; the symptoms were along the classic greater auricular nerve distribution affecting the lower face and ear (Figure 4). The patient had no pain, skin lesions, or soft-tissue swelling. Otolaryngology confirmed the diagnosis and recommended observation-only treatment of symptoms. Symptoms lessened over the next 3 months, and the altered sensation resolved without deficit by 6 months. In addition, by 6 months the patient had returned to full activities (including collision sports) pain-free and with normal left shoulder function. Because symptoms continued to improve, the patient was followed with clinical observation, and a formal work-up was not necessary.
Discussion
The most important finding in this case is the greater auricular nerve palsy that occurred after arthroscopic anterior-inferior and posterior-inferior labral repairs in beach-chair positioning. This greater auricular nerve palsy was the first encountered by Dr. Foad, who over 17 years in a primarily shoulder practice setting has used beach-chair positioning exclusively. Previous reports have described a palsy occurring after arthroscopic shoulder surgery using beach-chair positioning and a horseshoe headrest.7,8 Ng and Page7 discontinued and recommended against use of this headrest because of the complications of the palsy, and Park and Kim8 recommended a headrest redesign. We think the present case report is the first to describe a greater auricular nerve palsy that occurred after arthroscopic surgery using a standard universal headrest, which theoretically should prevent compression of the greater auricular nerve. Increased awareness of the possibility of greater auricular nerve palsy, even when proper precautions are taken,1 is therefore warranted.
Based on the location of our patient’s palsy, we think his paralysis was most likely the result of nerve compression by the headrest during the shoulder surgery. Other factors, though unlikely, may have played a role. These include operative time (increases duration of nerve compression) and head positioning. However, 122 minutes is not unusually long for a patient’s head to be in this position during a procedure, and over the past 10 years the same anesthesiologist, head nurse, and circulating nurse have routinely used the same beach-chair positioning and headrest for Dr. Foad’s patients. Second, the postoperative interscalene block theoretically could have caused the palsy, but we think this is unlikely, as the block is placed lower on the neck, at the C6 level, and the greater auricular nerve branches off the C2–C3 spinal nerves. As described by Rains and colleagues,9 other authors have reported transient neuropathies to the brachial plexus, which originates in the C5–C8 region, but not to the greater auricular nerve. Last, it cannot be ruled out that a variant of the greater auricular nerve could have played a role, given the variation in the greater auricular nerve.10,11 However, Brennan and colleagues10 reported that 2 of 25 neck dissections involved a variant in which the anterior division of the greater auricular nerve passed into the submandibular triangle and joined the mandibular region of the facial nerve. Stimulation of this nerve resulted in activity of the depressor of the lower lip, which was not the location of our patient’s palsy. In addition, our patient’s symptoms followed a classic nerve distribution of the greater auricular nerve (Figures 1, 4), which would seem to decrease the likelihood that a variant was the source of the palsy.
The superficial nature of the greater auricular nerve, which runs roughly parallel with the sternocleidomastoid muscle and innervates much of the superficial region of the skin over the mastoid, parotid gland, and mandible,5-7 theoretically places the nerve at risk for compressive forces from the headrest during arthroscopic shoulder surgery. Skyhar and colleagues3 first described beach-chair positioning as an alternative to lateral decubitus positioning, which had been reported to result in neurologic injury in about 10% of surgical cases.9 The theoretical advantages of beach-chair positioning are lack of traction needed and ease of setup, which would therefore decrease the possibility of neuropathy.1,3 However, as seen in this and other case reports,7,8 greater auricular nerve neuropathy should still be considered a possible complication, even when using beach-chair positioning.
Besides neuropathy after arthroscopic shoulder surgery, as described in previous case reports7,8 and in the present report, greater auricular nerve injury has been described as arising from other stimuli. Greater auricular nerve injury has arisen after perineural tumor metastasis,6 neuroma of greater auricular nerve after endolympathic shunt surgery,12 internal fixation of mandibular condyle,13 and carotid endarterectomy.14,15 Given the superficial nature of the greater auricular nerve, it may not be all that surprising that a palsy could also develop after headrest compression during arthroscopic shoulder surgery.
This case report brings to light a possible complication of greater auricular nerve palsy during arthroscopic shoulder surgery using beach-chair positioning and a standard universal headrest. Studies should now investigate whether greater auricular nerve palsy is more common than realized, especially with regard to specific headrests in beach-chair positioning. For now, though, Dr. Foad’s intention is not to change to a different headrest or discontinue beach-chair positioning but to draw attention to this rare complication. Additional attention should be given to the location of the headrest in relation to the greater auricular nerve, especially in cases in which operative time may be longer.
Conclusion
We have reported a greater auricular nerve palsy that occurred after arthroscopic shoulder surgery for an anterior-inferior and posterior-inferior labral tear. This is the first report of a greater auricular nerve palsy occurring with beach-chair positioning and a standard universal headrest. Symptoms resolved within 6 months. New studies should investigate the incidence of greater auricular nerve palsy after arthroscopic shoulder surgery.
Anterior-inferior and posterior-inferior labral tears are common injuries treated with arthroscopic surgery1 typically performed with beach-chair2,3 or lateral decubitus1,2 positioning and variable headrest positioning. Iatrogenic nerve damage that occurs after arthroscopic shoulder surgery—including damage to the suprascapular, axillary, musculocutaneous, subscapular, and spinal accessory nerves—has recently been reported to be more common than previously recognized.2,4
Although iatrogenic nerve injuries are in general being recognized,1,2,4 reports of greater auricular nerve injuries are limited. The greater auricular nerve is a superficial cutaneous nerve that arises from the cervical plexus at the C2 and C3 spinal nerves, obliquely crosses the sternocleidomastoid muscle, and splits into anterior and posterior portions that innervate the skin over the mastoid process and parotid gland.5,6 In particular, as illustrated by Ginsberg and Eicher6 (Figure 1), its superficial anatomy lies very near where a headrest is positioned during arthroscopic surgery, and increased pressure on the nerve throughout arthroscopic shoulder surgery may lead to neurapraxia.6,7 In 2 case series, authors reported on a total of 5 patients who had greater auricular nerve palsy after uncomplicated shoulder surgery using beach-chair positioning and a horseshoe headrest.7,8 The authors attributed these palsies to the horseshoe headrest, which they believed was compressing the greater auricular nerve during the entire surgery.7,8 However, standard universal headrests, which are thought to distribute pressures that would theoretically place the greater auricular nerve at risk for palsy, previously had not been described as contributing to palsy of the greater auricular nerve.
In this article, we report on a case of greater auricular nerve palsy that occurred after the patient’s anterior-inferior and posterior-inferior labral tear was arthroscopically repaired using beach-chair positioning and a standard universal headrest. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old right-hand–dominant high school American football player was referred for orthopedic evaluation of left chronic glenohumeral instability after 6 months of physical therapy. Physical examination revealed a positive apprehension test with the shoulder abducted and externally rotated at 90° and a positive relocation test. The patient complained of pain and instability when his arm was placed in a cross-chest adducted position and a posteroinferiorly directed axial load was applied. Magnetic resonance arthrogram showed an anterior-inferior labral Bankart tear with a small Hill-Sachs lesion to the humeral head but did not clearly reveal the posterior-inferior labral tear. Because of persistent left shoulder instability with most overhead activities and continued pain, the patient decided to undergo left shoulder arthroscopic Bankart repair with inferior capsular shift and posterior-inferior labral repair with capsulorraphy. He had no significant past medical history or known drug allergies.
The patient was placed in the standard beach-chair position: upright at 45° to the floor, hips flexed at 60°, knees flexed at 30°.1 Pneumatic compression devices were placed on his lower extremities. His head was secured in neutral position to a standard universal headrest (model A-90026; Allen Medical Systems, Acton, Massachusetts) (Figures 2, 3). Care was taken to protect the deep neurovascular structures and bony prominences throughout. The patient was in this position for 122 minutes of the operation, from positioning and draping to wound closure and dressing application. Before draping, the anesthesiologist, head nurse, and circulating nurse ensured that head and neck were in neutral position. The anesthesiologist monitored positioning throughout the perioperative period to ensure head and neck were in neutral, and the head did not need to be repositioned during surgery. Standard preoperative intravenous antibiotics were given.
General anesthesia and postoperative interscalene block were used. Standard preparation and draping were performed. Three standard arthroscopic portal incisions were used: posterior, anterior, and anterosuperolateral. Findings included extensive labral pathology, small bony Hill-Sachs lesion to humeral head, small bony anterior glenoid deficiency, and deficient anterior-inferior and posterior-inferior labral remnant. These were repaired arthroscopically in a standard fashion using bioabsorbable suture anchors. There were no arthroscopic complications. After surgery, a standard well-fitted shoulder immobilizer was placed. The anesthesiologist provided interscalene regional analgesia (15 mL of bupivacaine 0.5%) in the recovery area after surgery.
Postoperative neurovascular examination in the recovery room revealed no discomfort. The patient was discharged the same day. At a scheduled 1-week follow-up, he complained of numbness and dysesthesia on the left side of the greater auricular nerve distribution. A diagnosis of greater auricular nerve palsy was made by physical examination; the symptoms were along the classic greater auricular nerve distribution affecting the lower face and ear (Figure 4). The patient had no pain, skin lesions, or soft-tissue swelling. Otolaryngology confirmed the diagnosis and recommended observation-only treatment of symptoms. Symptoms lessened over the next 3 months, and the altered sensation resolved without deficit by 6 months. In addition, by 6 months the patient had returned to full activities (including collision sports) pain-free and with normal left shoulder function. Because symptoms continued to improve, the patient was followed with clinical observation, and a formal work-up was not necessary.
Discussion
The most important finding in this case is the greater auricular nerve palsy that occurred after arthroscopic anterior-inferior and posterior-inferior labral repairs in beach-chair positioning. This greater auricular nerve palsy was the first encountered by Dr. Foad, who over 17 years in a primarily shoulder practice setting has used beach-chair positioning exclusively. Previous reports have described a palsy occurring after arthroscopic shoulder surgery using beach-chair positioning and a horseshoe headrest.7,8 Ng and Page7 discontinued and recommended against use of this headrest because of the complications of the palsy, and Park and Kim8 recommended a headrest redesign. We think the present case report is the first to describe a greater auricular nerve palsy that occurred after arthroscopic surgery using a standard universal headrest, which theoretically should prevent compression of the greater auricular nerve. Increased awareness of the possibility of greater auricular nerve palsy, even when proper precautions are taken,1 is therefore warranted.
Based on the location of our patient’s palsy, we think his paralysis was most likely the result of nerve compression by the headrest during the shoulder surgery. Other factors, though unlikely, may have played a role. These include operative time (increases duration of nerve compression) and head positioning. However, 122 minutes is not unusually long for a patient’s head to be in this position during a procedure, and over the past 10 years the same anesthesiologist, head nurse, and circulating nurse have routinely used the same beach-chair positioning and headrest for Dr. Foad’s patients. Second, the postoperative interscalene block theoretically could have caused the palsy, but we think this is unlikely, as the block is placed lower on the neck, at the C6 level, and the greater auricular nerve branches off the C2–C3 spinal nerves. As described by Rains and colleagues,9 other authors have reported transient neuropathies to the brachial plexus, which originates in the C5–C8 region, but not to the greater auricular nerve. Last, it cannot be ruled out that a variant of the greater auricular nerve could have played a role, given the variation in the greater auricular nerve.10,11 However, Brennan and colleagues10 reported that 2 of 25 neck dissections involved a variant in which the anterior division of the greater auricular nerve passed into the submandibular triangle and joined the mandibular region of the facial nerve. Stimulation of this nerve resulted in activity of the depressor of the lower lip, which was not the location of our patient’s palsy. In addition, our patient’s symptoms followed a classic nerve distribution of the greater auricular nerve (Figures 1, 4), which would seem to decrease the likelihood that a variant was the source of the palsy.
The superficial nature of the greater auricular nerve, which runs roughly parallel with the sternocleidomastoid muscle and innervates much of the superficial region of the skin over the mastoid, parotid gland, and mandible,5-7 theoretically places the nerve at risk for compressive forces from the headrest during arthroscopic shoulder surgery. Skyhar and colleagues3 first described beach-chair positioning as an alternative to lateral decubitus positioning, which had been reported to result in neurologic injury in about 10% of surgical cases.9 The theoretical advantages of beach-chair positioning are lack of traction needed and ease of setup, which would therefore decrease the possibility of neuropathy.1,3 However, as seen in this and other case reports,7,8 greater auricular nerve neuropathy should still be considered a possible complication, even when using beach-chair positioning.
Besides neuropathy after arthroscopic shoulder surgery, as described in previous case reports7,8 and in the present report, greater auricular nerve injury has been described as arising from other stimuli. Greater auricular nerve injury has arisen after perineural tumor metastasis,6 neuroma of greater auricular nerve after endolympathic shunt surgery,12 internal fixation of mandibular condyle,13 and carotid endarterectomy.14,15 Given the superficial nature of the greater auricular nerve, it may not be all that surprising that a palsy could also develop after headrest compression during arthroscopic shoulder surgery.
This case report brings to light a possible complication of greater auricular nerve palsy during arthroscopic shoulder surgery using beach-chair positioning and a standard universal headrest. Studies should now investigate whether greater auricular nerve palsy is more common than realized, especially with regard to specific headrests in beach-chair positioning. For now, though, Dr. Foad’s intention is not to change to a different headrest or discontinue beach-chair positioning but to draw attention to this rare complication. Additional attention should be given to the location of the headrest in relation to the greater auricular nerve, especially in cases in which operative time may be longer.
Conclusion
We have reported a greater auricular nerve palsy that occurred after arthroscopic shoulder surgery for an anterior-inferior and posterior-inferior labral tear. This is the first report of a greater auricular nerve palsy occurring with beach-chair positioning and a standard universal headrest. Symptoms resolved within 6 months. New studies should investigate the incidence of greater auricular nerve palsy after arthroscopic shoulder surgery.
1. Paxton ES, Backus J, Keener J, Brophy RH. Shoulder arthroscopy: basic principles of positioning, anesthesia, and portal anatomy. J Am Acad Orthop Surg. 2013;21(6):332-342.
2. Scully WF, Wilson DJ, Parada SA, Arrington ED. Iatrogenic nerve injuries in shoulder surgery. J Am Acad Orthop Surg. 2013;21(12):717-726.
3. Skyhar MJ, Altchek DW, Warren RF, Wickiewicz TL, O’Brien SJ. Shoulder arthroscopy with the patient in the beach-chair position. Arthroscopy. 1988;4(4):256-259.
4. Zhang J, Moore AE, Stringer MD. Iatrogenic upper limb nerve injuries: a systematic review. ANZ J Surg. 2011;81(4):227-236.
5. Alberti PW. The greater auricular nerve. Donor for facial nerve grafts: a note on its topographical anatomy. Arch Otolaryngol. 1962;76:422-424.
6. Ginsberg LE, Eicher SA. Great auricular nerve: anatomy and imaging in a case of perineural tumor spread. AJNR Am J Neuroradiol. 2000;21(3):568-571.
7. Ng AK, Page RS. Greater auricular nerve neuropraxia with beach chair positioning during shoulder surgery. Int J Shoulder Surg. 2010;4(2):48-50.
8. Park TS, Kim YS. Neuropraxia of the cutaneous nerve of the cervical plexus after shoulder arthroscopy. Arthroscopy. 2005;21(5):631.e1-e3.
9. Rains DD, Rooke GA, Wahl CJ. Pathomechanisms and complications related to patient positioning and anesthesia during shoulder arthroscopy. Arthroscopy. 2011;27(4):532-541.
10. Brennan PA, Al Gholmy M, Ounnas H, Zaki GA, Puxeddu R, Standring S. Communication of the anterior branch of the great auricular nerve with the marginal mandibular nerve: a prospective study of 25 neck dissections. Br J Oral Maxillofac Surg. 2010;48(6):431-433.
11. Sand T, Becser N. Neurophysiological and anatomical variability of the greater auricular nerve. Acta Neurol Scand. 1998;98(5):333-339.
12. Vorobeichik L, Fallucco MA, Hagan RR. Chronic daily headaches secondary to greater auricular and lesser occipital neuromas following endolymphatic shunt surgery. BMJ Case Rep. 2012;2012. pii: bcr-2012-007189. doi:10.1136/bcr-2012-007189.
13. Sverzut CE, Trivellato AE, Serra EC, Ferraz EP, Sverzut AT. Frey’s syndrome after condylar fracture: case report. Braz Dent J. 2004;15(2):159-162.
14. AbuRahma AF, Choueiri MA. Cranial and cervical nerve injuries after repeat carotid endarterectomy. J Vasc Surg. 2000;32(4):649-654.
15. Ballotta E, Da Giau G, Renon L, et al. Cranial and cervical nerve injuries after carotid endarterectomy: a prospective study. Surgery. 1999;125(1):85-91.
1. Paxton ES, Backus J, Keener J, Brophy RH. Shoulder arthroscopy: basic principles of positioning, anesthesia, and portal anatomy. J Am Acad Orthop Surg. 2013;21(6):332-342.
2. Scully WF, Wilson DJ, Parada SA, Arrington ED. Iatrogenic nerve injuries in shoulder surgery. J Am Acad Orthop Surg. 2013;21(12):717-726.
3. Skyhar MJ, Altchek DW, Warren RF, Wickiewicz TL, O’Brien SJ. Shoulder arthroscopy with the patient in the beach-chair position. Arthroscopy. 1988;4(4):256-259.
4. Zhang J, Moore AE, Stringer MD. Iatrogenic upper limb nerve injuries: a systematic review. ANZ J Surg. 2011;81(4):227-236.
5. Alberti PW. The greater auricular nerve. Donor for facial nerve grafts: a note on its topographical anatomy. Arch Otolaryngol. 1962;76:422-424.
6. Ginsberg LE, Eicher SA. Great auricular nerve: anatomy and imaging in a case of perineural tumor spread. AJNR Am J Neuroradiol. 2000;21(3):568-571.
7. Ng AK, Page RS. Greater auricular nerve neuropraxia with beach chair positioning during shoulder surgery. Int J Shoulder Surg. 2010;4(2):48-50.
8. Park TS, Kim YS. Neuropraxia of the cutaneous nerve of the cervical plexus after shoulder arthroscopy. Arthroscopy. 2005;21(5):631.e1-e3.
9. Rains DD, Rooke GA, Wahl CJ. Pathomechanisms and complications related to patient positioning and anesthesia during shoulder arthroscopy. Arthroscopy. 2011;27(4):532-541.
10. Brennan PA, Al Gholmy M, Ounnas H, Zaki GA, Puxeddu R, Standring S. Communication of the anterior branch of the great auricular nerve with the marginal mandibular nerve: a prospective study of 25 neck dissections. Br J Oral Maxillofac Surg. 2010;48(6):431-433.
11. Sand T, Becser N. Neurophysiological and anatomical variability of the greater auricular nerve. Acta Neurol Scand. 1998;98(5):333-339.
12. Vorobeichik L, Fallucco MA, Hagan RR. Chronic daily headaches secondary to greater auricular and lesser occipital neuromas following endolymphatic shunt surgery. BMJ Case Rep. 2012;2012. pii: bcr-2012-007189. doi:10.1136/bcr-2012-007189.
13. Sverzut CE, Trivellato AE, Serra EC, Ferraz EP, Sverzut AT. Frey’s syndrome after condylar fracture: case report. Braz Dent J. 2004;15(2):159-162.
14. AbuRahma AF, Choueiri MA. Cranial and cervical nerve injuries after repeat carotid endarterectomy. J Vasc Surg. 2000;32(4):649-654.
15. Ballotta E, Da Giau G, Renon L, et al. Cranial and cervical nerve injuries after carotid endarterectomy: a prospective study. Surgery. 1999;125(1):85-91.
Glenoid Damage From Articular Protrusion of Metal Suture Anchor After Arthroscopic Rotator Cuff Repair
Complications with the use of anchor screws in shoulder surgery have been well-documented1,2 and can be divided into 3 categories: insertion (eg, incomplete seating, inadequate insertion, and migration), biologic (eg, large tacks producing synovitis and bone reaction), and, less commonly, mechanical (eg, intra- and extra-articular bone pull-out with migration) complications.
Prominent hardware, including suture anchors, as a cause of arthritis and joint damage has been well-documented in shoulder surgery.3,4 For example, anchors placed on the glenoid rim have been implicated in severe cartilage loss if they protrude above the level of the glenoid rim.3 However, to the authors’ knowledge, prominent anchor placement after rotator cuff repair has not been reported as a cause of arthritis unless the anchor dislodges into the glenohumeral joint. The authors present a case in which a suture anchor used for rotator cuff repair protruded through the humeral head, resulting in glenohumeral arthritis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 61-year-old woman presented with complaints of persistent right shoulder pain for 5 months after a fall from a bicycle. She had taken nonsteroidal anti-inflammatory medication without pain relief. On presentation, she had no atrophy or deformity, was neurologically intact for sensation and reflexes, and had full range of motion (ROM) but a painful arc. She had tenderness over the greater tuberosity and positive Neer and Hawkins-Kennedy impingement signs. She had pain but no weakness to resisted abduction or to resisted external rotation with the arms at the sides.
Preoperative conventional radiographs of the shoulder were normal. A gadolinium-enhanced magnetic resonance arthrogram showed a high-grade articular partial tear of the supraspinatus, which was judged to be at least two-thirds of the tendon width. Because nonoperative methods had failed, the patient elected operative intervention for this tear.
Diagnostic arthroscopy (with the patient in a lateral decubitus position) showed a normal joint except for a high-grade, 8×8-mm, greater than 6 mm deep, partial tear of the articular side of the supraspinatus tendon. The subacromial space had moderate to severe bursal tissue inflammation but no full-thickness component to the rotator cuff tear. A bursectomy, coracoacromial ligament release, and partial anterolateral acromioplasty were performed.
A transtendinous technique was used to repair this high-grade tear. For an anatomically rigid repair, we used 3 suture anchors with a straight configuration because each metal anchor has only 1 suture. According to the standard arthroscopic transtendinous repair technique, the suture anchors were placed through the rotator cuff tendon (at the lateral articular margin at the medial extent of the footprint) after localization of the angle with a spinal needle. A shuttle relay was used to pass the sutures, and the knot was pulled into the subacromial space, cinching the rotator cuff on top of the suture anchors and reestablishing the contact of the tendon to the footprint. We used two 2.4-mm FASTak suture anchors (Arthrex, Naples, Florida) and one 3.5-mm Corkscrew suture anchor (Arthrex). This process was repeated for the remaining suture limbs. The placement of the suture anchors adequately reduced the articular part of the cuff to the footprint.
After surgery, the patient had no complications, and radiographs taken the next day suggested no abnormalities (Figure 1A). The shoulder was immobilized for 4 weeks after surgery, and passive, gentle ROM exercise was supervised by a physical therapist twice a week during this period. After the first 4 weeks, an active ROM program was begun. However, shortly after initiating motion in the shoulder, the patient complained of a recurrence of pain that she described as a sharp and grinding sensation.
The patient was reevaluated 8 weeks after surgery. Her pain was worsening, and she was having difficulty regaining ROM. Conventional radiographs showed the tip of the metal anchor protruding through the articular cartilage of the humeral head (Figure 1B). The patient was informed of the findings, and immediate surgery was performed to remove the anchor.
Arthroscopic examination showed extensive damage to the glenoid cartilage (Figure 1C) and an intra-articularly intact rotator cuff repair. The cartilage damage was located in the posterior and inferior half of the glenoid, which is related to the forward flexion of the arm; the depth of the cartilage defect was approximately 2 mm. Under the image intensifier, an empty suture anchor driver was inserted into the previous screw insertion hole, and the anchor was screwed back out and removed.
After surgery, the patient’s arm was placed in a sling, and an ROM program began 4 weeks later. The sensation of grinding was eliminated, and her pain gradually improved. Three years after surgery, she had no pain, no weakness, and full ROM without limitations (Figure 2).
Discussion
Protrusion and migration of suture anchors in shoulder surgery has been documented extensively.3,4 Zuckerman and Matsen4 divided these complications into 4 groups: (1) incorrect placement, (2) migration after placement, (3) loosening, and (4) device breakage. These complications may be frequently related to surgical technique, and all these studies describe backward migration of the anchor out of the drill hole. In the current case, the anchor tip penetrated the articular surface of the humeral head, not because of anchor migration but because the anchor was inserted too far. To the authors’ knowledge, there is only 1 reported case of anchor protrusion through the humeral head; it involved a different type of anchor insertion system.5 In that case, there was only mild cartilage damage to the glenoid, and the patient recovered after removal of the anchors.
Several factors contributed to the improper insertion of the anchor in the current patient. First, repairing a high-grade articular side defect or partial articular supraspinatus tendon avulsion lesion can be technically challenging because rotator cuff tissue obscures the view when inserting the anchor. Second, the anchor was inserted too medially on the greater tuberosity, which made the distance from the tuberosity to the joint shorter. Wong and colleagues5 performed an analysis of the angle of insertion that would be safe using a PEEK PushLock SP system (Arthrex), but they emphasized that the angle depends on the configuration of the particular insertion system. The current case also shows that the surgeon should be cognizant of the fact that penetration of the humeral head by the anchor can occur if the surgeon is unaware of the distance from the anchor to the laser line on the insertion device or of the distance from the tuberosity to the articular surface of the humeral head.
The current case also shows that the type of anchor and delivery system may contribute to this complication. Double-loaded suture anchors can decrease the number of anchors needed for secure fixation. Bioabsorbable anchors can be used for this purpose, but they may be technically more difficult to use for repairing partial tears of the rotator cuff. Better visualization of the laser line on the anchor may be facilitated by using a probe from an anterior portal to hold the cuff up while the anchor is inserted.
This case has shown the importance of obtaining postoperative radiographic studies in patients who have metal anchors placed during shoulder surgery, especially if they complain of continued pain, new pain, crepitus, or grinding. When conventional radiography is insufficient for locating the anchor or its proximity to the joint line, computed tomography can be helpful.1
Conclusion
Removing failed suture anchors can be challenging, especially when they protrude into the joint on the humeral side.1,6 The best way to prevent this complication is through careful technique. The anchors should not be inserted beyond the depth of the laser line on the anchors, and every attempt should be made to make sure the laser line is visible at the time of anchor insertion. Postoperative radiographs should be considered for patients with metal anchors in the shoulder, especially if the patient continues to have symptoms or develops new symptoms in the shoulder after surgery.
1. Park HB, Keyurapan E, Gill HS, Selhi HS, McFarland EG. Suture anchors and tacks for shoulder surgery. Part II: The prevention and treatment of complications. Am J Sports Med. 2006;34(1):136-144.
2. McFarland EG, Park HB, Keyurapan E, Gill HS, Selhi HS. Suture anchors and tacks for shoulder surgery. Part I: Biology and biomechanics. Am J Sports Med. 2005;33(12):1918-1923.
3. Rhee YG, Lee DH, Chun IH, Bae SC. Glenohumeral arthropathy after arthroscopic anterior shoulder stabilization. Arthroscopy. 2004;20(4):402-406.
4. Zuckerman JD, Matsen FA III. Complications about the glenohumeral joint related to the use of screws and staples. J Bone Joint Surg Am. 1984;66(2):175-180.
5. Wong AS, Kokkalis ZT, Schmidt CC. Proper insertion angle is essential to prevent intra-articular protrusion of a knotless suture anchor in shoulder rotator cuff repair. Arthroscopy. 2010;26(2):286-290.
6. Grutter PW, McFarland EG, Zikria BA, Dai Z, Petersen SA. Techniques for suture anchor removal in shoulder surgery. Am J Sports Med. 2010;38(8):1706-1710.
Complications with the use of anchor screws in shoulder surgery have been well-documented1,2 and can be divided into 3 categories: insertion (eg, incomplete seating, inadequate insertion, and migration), biologic (eg, large tacks producing synovitis and bone reaction), and, less commonly, mechanical (eg, intra- and extra-articular bone pull-out with migration) complications.
Prominent hardware, including suture anchors, as a cause of arthritis and joint damage has been well-documented in shoulder surgery.3,4 For example, anchors placed on the glenoid rim have been implicated in severe cartilage loss if they protrude above the level of the glenoid rim.3 However, to the authors’ knowledge, prominent anchor placement after rotator cuff repair has not been reported as a cause of arthritis unless the anchor dislodges into the glenohumeral joint. The authors present a case in which a suture anchor used for rotator cuff repair protruded through the humeral head, resulting in glenohumeral arthritis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 61-year-old woman presented with complaints of persistent right shoulder pain for 5 months after a fall from a bicycle. She had taken nonsteroidal anti-inflammatory medication without pain relief. On presentation, she had no atrophy or deformity, was neurologically intact for sensation and reflexes, and had full range of motion (ROM) but a painful arc. She had tenderness over the greater tuberosity and positive Neer and Hawkins-Kennedy impingement signs. She had pain but no weakness to resisted abduction or to resisted external rotation with the arms at the sides.
Preoperative conventional radiographs of the shoulder were normal. A gadolinium-enhanced magnetic resonance arthrogram showed a high-grade articular partial tear of the supraspinatus, which was judged to be at least two-thirds of the tendon width. Because nonoperative methods had failed, the patient elected operative intervention for this tear.
Diagnostic arthroscopy (with the patient in a lateral decubitus position) showed a normal joint except for a high-grade, 8×8-mm, greater than 6 mm deep, partial tear of the articular side of the supraspinatus tendon. The subacromial space had moderate to severe bursal tissue inflammation but no full-thickness component to the rotator cuff tear. A bursectomy, coracoacromial ligament release, and partial anterolateral acromioplasty were performed.
A transtendinous technique was used to repair this high-grade tear. For an anatomically rigid repair, we used 3 suture anchors with a straight configuration because each metal anchor has only 1 suture. According to the standard arthroscopic transtendinous repair technique, the suture anchors were placed through the rotator cuff tendon (at the lateral articular margin at the medial extent of the footprint) after localization of the angle with a spinal needle. A shuttle relay was used to pass the sutures, and the knot was pulled into the subacromial space, cinching the rotator cuff on top of the suture anchors and reestablishing the contact of the tendon to the footprint. We used two 2.4-mm FASTak suture anchors (Arthrex, Naples, Florida) and one 3.5-mm Corkscrew suture anchor (Arthrex). This process was repeated for the remaining suture limbs. The placement of the suture anchors adequately reduced the articular part of the cuff to the footprint.
After surgery, the patient had no complications, and radiographs taken the next day suggested no abnormalities (Figure 1A). The shoulder was immobilized for 4 weeks after surgery, and passive, gentle ROM exercise was supervised by a physical therapist twice a week during this period. After the first 4 weeks, an active ROM program was begun. However, shortly after initiating motion in the shoulder, the patient complained of a recurrence of pain that she described as a sharp and grinding sensation.
The patient was reevaluated 8 weeks after surgery. Her pain was worsening, and she was having difficulty regaining ROM. Conventional radiographs showed the tip of the metal anchor protruding through the articular cartilage of the humeral head (Figure 1B). The patient was informed of the findings, and immediate surgery was performed to remove the anchor.
Arthroscopic examination showed extensive damage to the glenoid cartilage (Figure 1C) and an intra-articularly intact rotator cuff repair. The cartilage damage was located in the posterior and inferior half of the glenoid, which is related to the forward flexion of the arm; the depth of the cartilage defect was approximately 2 mm. Under the image intensifier, an empty suture anchor driver was inserted into the previous screw insertion hole, and the anchor was screwed back out and removed.
After surgery, the patient’s arm was placed in a sling, and an ROM program began 4 weeks later. The sensation of grinding was eliminated, and her pain gradually improved. Three years after surgery, she had no pain, no weakness, and full ROM without limitations (Figure 2).
Discussion
Protrusion and migration of suture anchors in shoulder surgery has been documented extensively.3,4 Zuckerman and Matsen4 divided these complications into 4 groups: (1) incorrect placement, (2) migration after placement, (3) loosening, and (4) device breakage. These complications may be frequently related to surgical technique, and all these studies describe backward migration of the anchor out of the drill hole. In the current case, the anchor tip penetrated the articular surface of the humeral head, not because of anchor migration but because the anchor was inserted too far. To the authors’ knowledge, there is only 1 reported case of anchor protrusion through the humeral head; it involved a different type of anchor insertion system.5 In that case, there was only mild cartilage damage to the glenoid, and the patient recovered after removal of the anchors.
Several factors contributed to the improper insertion of the anchor in the current patient. First, repairing a high-grade articular side defect or partial articular supraspinatus tendon avulsion lesion can be technically challenging because rotator cuff tissue obscures the view when inserting the anchor. Second, the anchor was inserted too medially on the greater tuberosity, which made the distance from the tuberosity to the joint shorter. Wong and colleagues5 performed an analysis of the angle of insertion that would be safe using a PEEK PushLock SP system (Arthrex), but they emphasized that the angle depends on the configuration of the particular insertion system. The current case also shows that the surgeon should be cognizant of the fact that penetration of the humeral head by the anchor can occur if the surgeon is unaware of the distance from the anchor to the laser line on the insertion device or of the distance from the tuberosity to the articular surface of the humeral head.
The current case also shows that the type of anchor and delivery system may contribute to this complication. Double-loaded suture anchors can decrease the number of anchors needed for secure fixation. Bioabsorbable anchors can be used for this purpose, but they may be technically more difficult to use for repairing partial tears of the rotator cuff. Better visualization of the laser line on the anchor may be facilitated by using a probe from an anterior portal to hold the cuff up while the anchor is inserted.
This case has shown the importance of obtaining postoperative radiographic studies in patients who have metal anchors placed during shoulder surgery, especially if they complain of continued pain, new pain, crepitus, or grinding. When conventional radiography is insufficient for locating the anchor or its proximity to the joint line, computed tomography can be helpful.1
Conclusion
Removing failed suture anchors can be challenging, especially when they protrude into the joint on the humeral side.1,6 The best way to prevent this complication is through careful technique. The anchors should not be inserted beyond the depth of the laser line on the anchors, and every attempt should be made to make sure the laser line is visible at the time of anchor insertion. Postoperative radiographs should be considered for patients with metal anchors in the shoulder, especially if the patient continues to have symptoms or develops new symptoms in the shoulder after surgery.
Complications with the use of anchor screws in shoulder surgery have been well-documented1,2 and can be divided into 3 categories: insertion (eg, incomplete seating, inadequate insertion, and migration), biologic (eg, large tacks producing synovitis and bone reaction), and, less commonly, mechanical (eg, intra- and extra-articular bone pull-out with migration) complications.
Prominent hardware, including suture anchors, as a cause of arthritis and joint damage has been well-documented in shoulder surgery.3,4 For example, anchors placed on the glenoid rim have been implicated in severe cartilage loss if they protrude above the level of the glenoid rim.3 However, to the authors’ knowledge, prominent anchor placement after rotator cuff repair has not been reported as a cause of arthritis unless the anchor dislodges into the glenohumeral joint. The authors present a case in which a suture anchor used for rotator cuff repair protruded through the humeral head, resulting in glenohumeral arthritis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 61-year-old woman presented with complaints of persistent right shoulder pain for 5 months after a fall from a bicycle. She had taken nonsteroidal anti-inflammatory medication without pain relief. On presentation, she had no atrophy or deformity, was neurologically intact for sensation and reflexes, and had full range of motion (ROM) but a painful arc. She had tenderness over the greater tuberosity and positive Neer and Hawkins-Kennedy impingement signs. She had pain but no weakness to resisted abduction or to resisted external rotation with the arms at the sides.
Preoperative conventional radiographs of the shoulder were normal. A gadolinium-enhanced magnetic resonance arthrogram showed a high-grade articular partial tear of the supraspinatus, which was judged to be at least two-thirds of the tendon width. Because nonoperative methods had failed, the patient elected operative intervention for this tear.
Diagnostic arthroscopy (with the patient in a lateral decubitus position) showed a normal joint except for a high-grade, 8×8-mm, greater than 6 mm deep, partial tear of the articular side of the supraspinatus tendon. The subacromial space had moderate to severe bursal tissue inflammation but no full-thickness component to the rotator cuff tear. A bursectomy, coracoacromial ligament release, and partial anterolateral acromioplasty were performed.
A transtendinous technique was used to repair this high-grade tear. For an anatomically rigid repair, we used 3 suture anchors with a straight configuration because each metal anchor has only 1 suture. According to the standard arthroscopic transtendinous repair technique, the suture anchors were placed through the rotator cuff tendon (at the lateral articular margin at the medial extent of the footprint) after localization of the angle with a spinal needle. A shuttle relay was used to pass the sutures, and the knot was pulled into the subacromial space, cinching the rotator cuff on top of the suture anchors and reestablishing the contact of the tendon to the footprint. We used two 2.4-mm FASTak suture anchors (Arthrex, Naples, Florida) and one 3.5-mm Corkscrew suture anchor (Arthrex). This process was repeated for the remaining suture limbs. The placement of the suture anchors adequately reduced the articular part of the cuff to the footprint.
After surgery, the patient had no complications, and radiographs taken the next day suggested no abnormalities (Figure 1A). The shoulder was immobilized for 4 weeks after surgery, and passive, gentle ROM exercise was supervised by a physical therapist twice a week during this period. After the first 4 weeks, an active ROM program was begun. However, shortly after initiating motion in the shoulder, the patient complained of a recurrence of pain that she described as a sharp and grinding sensation.
The patient was reevaluated 8 weeks after surgery. Her pain was worsening, and she was having difficulty regaining ROM. Conventional radiographs showed the tip of the metal anchor protruding through the articular cartilage of the humeral head (Figure 1B). The patient was informed of the findings, and immediate surgery was performed to remove the anchor.
Arthroscopic examination showed extensive damage to the glenoid cartilage (Figure 1C) and an intra-articularly intact rotator cuff repair. The cartilage damage was located in the posterior and inferior half of the glenoid, which is related to the forward flexion of the arm; the depth of the cartilage defect was approximately 2 mm. Under the image intensifier, an empty suture anchor driver was inserted into the previous screw insertion hole, and the anchor was screwed back out and removed.
After surgery, the patient’s arm was placed in a sling, and an ROM program began 4 weeks later. The sensation of grinding was eliminated, and her pain gradually improved. Three years after surgery, she had no pain, no weakness, and full ROM without limitations (Figure 2).
Discussion
Protrusion and migration of suture anchors in shoulder surgery has been documented extensively.3,4 Zuckerman and Matsen4 divided these complications into 4 groups: (1) incorrect placement, (2) migration after placement, (3) loosening, and (4) device breakage. These complications may be frequently related to surgical technique, and all these studies describe backward migration of the anchor out of the drill hole. In the current case, the anchor tip penetrated the articular surface of the humeral head, not because of anchor migration but because the anchor was inserted too far. To the authors’ knowledge, there is only 1 reported case of anchor protrusion through the humeral head; it involved a different type of anchor insertion system.5 In that case, there was only mild cartilage damage to the glenoid, and the patient recovered after removal of the anchors.
Several factors contributed to the improper insertion of the anchor in the current patient. First, repairing a high-grade articular side defect or partial articular supraspinatus tendon avulsion lesion can be technically challenging because rotator cuff tissue obscures the view when inserting the anchor. Second, the anchor was inserted too medially on the greater tuberosity, which made the distance from the tuberosity to the joint shorter. Wong and colleagues5 performed an analysis of the angle of insertion that would be safe using a PEEK PushLock SP system (Arthrex), but they emphasized that the angle depends on the configuration of the particular insertion system. The current case also shows that the surgeon should be cognizant of the fact that penetration of the humeral head by the anchor can occur if the surgeon is unaware of the distance from the anchor to the laser line on the insertion device or of the distance from the tuberosity to the articular surface of the humeral head.
The current case also shows that the type of anchor and delivery system may contribute to this complication. Double-loaded suture anchors can decrease the number of anchors needed for secure fixation. Bioabsorbable anchors can be used for this purpose, but they may be technically more difficult to use for repairing partial tears of the rotator cuff. Better visualization of the laser line on the anchor may be facilitated by using a probe from an anterior portal to hold the cuff up while the anchor is inserted.
This case has shown the importance of obtaining postoperative radiographic studies in patients who have metal anchors placed during shoulder surgery, especially if they complain of continued pain, new pain, crepitus, or grinding. When conventional radiography is insufficient for locating the anchor or its proximity to the joint line, computed tomography can be helpful.1
Conclusion
Removing failed suture anchors can be challenging, especially when they protrude into the joint on the humeral side.1,6 The best way to prevent this complication is through careful technique. The anchors should not be inserted beyond the depth of the laser line on the anchors, and every attempt should be made to make sure the laser line is visible at the time of anchor insertion. Postoperative radiographs should be considered for patients with metal anchors in the shoulder, especially if the patient continues to have symptoms or develops new symptoms in the shoulder after surgery.
1. Park HB, Keyurapan E, Gill HS, Selhi HS, McFarland EG. Suture anchors and tacks for shoulder surgery. Part II: The prevention and treatment of complications. Am J Sports Med. 2006;34(1):136-144.
2. McFarland EG, Park HB, Keyurapan E, Gill HS, Selhi HS. Suture anchors and tacks for shoulder surgery. Part I: Biology and biomechanics. Am J Sports Med. 2005;33(12):1918-1923.
3. Rhee YG, Lee DH, Chun IH, Bae SC. Glenohumeral arthropathy after arthroscopic anterior shoulder stabilization. Arthroscopy. 2004;20(4):402-406.
4. Zuckerman JD, Matsen FA III. Complications about the glenohumeral joint related to the use of screws and staples. J Bone Joint Surg Am. 1984;66(2):175-180.
5. Wong AS, Kokkalis ZT, Schmidt CC. Proper insertion angle is essential to prevent intra-articular protrusion of a knotless suture anchor in shoulder rotator cuff repair. Arthroscopy. 2010;26(2):286-290.
6. Grutter PW, McFarland EG, Zikria BA, Dai Z, Petersen SA. Techniques for suture anchor removal in shoulder surgery. Am J Sports Med. 2010;38(8):1706-1710.
1. Park HB, Keyurapan E, Gill HS, Selhi HS, McFarland EG. Suture anchors and tacks for shoulder surgery. Part II: The prevention and treatment of complications. Am J Sports Med. 2006;34(1):136-144.
2. McFarland EG, Park HB, Keyurapan E, Gill HS, Selhi HS. Suture anchors and tacks for shoulder surgery. Part I: Biology and biomechanics. Am J Sports Med. 2005;33(12):1918-1923.
3. Rhee YG, Lee DH, Chun IH, Bae SC. Glenohumeral arthropathy after arthroscopic anterior shoulder stabilization. Arthroscopy. 2004;20(4):402-406.
4. Zuckerman JD, Matsen FA III. Complications about the glenohumeral joint related to the use of screws and staples. J Bone Joint Surg Am. 1984;66(2):175-180.
5. Wong AS, Kokkalis ZT, Schmidt CC. Proper insertion angle is essential to prevent intra-articular protrusion of a knotless suture anchor in shoulder rotator cuff repair. Arthroscopy. 2010;26(2):286-290.
6. Grutter PW, McFarland EG, Zikria BA, Dai Z, Petersen SA. Techniques for suture anchor removal in shoulder surgery. Am J Sports Med. 2010;38(8):1706-1710.
Cutaneous Burn Caused by Radiofrequency Ablation Probe During Shoulder Arthroscopy
Cautery and radiofrequency ablation (RFA) devices are commonly used in shoulder arthroscopic surgery for hemostasis and ablation of soft tissue. Although these devices are easily used and applied, complications (eg, extensive release of deltoid muscle,1 nerve damage,2 tendon damage,3 cartilage damage from heat transfer4) can occur during arthroscopic surgery. Radiofrequency devices can elevate fluid temperatures to unsafe levels and directly or indirectly injure surrounding tissue.5,6 Skin complications from using these devices include direct burns to the subcutaneous tissues from the joint to the skin surface7 and skin burns related to overheated arthroscopic fluid.8
In our English-language literature review, however, we found no report of a skin burn secondary to contact between a RFA device and a spinal needle used in identifying structures during an arthroscopic acromioplasty. We report such a case here. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old woman injured her left, nondominant shoulder when a descending garage door hit her directly on the superior aspect of the shoulder. She had immediate onset of pain on the top and lateral side of the shoulder and was evaluated by a primary care physician. Radiographs and magnetic resonance imaging (MRI) were normal. The patient was referred to an orthopedic surgeon for further evaluation.
The orthopedic surgeon found her to be in good health, with no history of diabetes, vascular conditions, or skin disorders. The initial diagnosis after history taking and physical examination was impingement syndrome with subacromial bursitis. The surgeon recommended nonoperative treatment: ice, nonsteroidal anti-inflammatory drugs, and physical therapy. After 3 months, the patient’s examination was unchanged, and there was no improvement in pain. Cortisone injected into the subacromial space helped for a few weeks, but the pain returned. After 2 more cortisone injections over 9 months failed, repeat MRI showed no tears of the rotator cuff or any other salient abnormalities. The treatment options were discussed with the patient, and, because the physical examination findings were consistent with impingement syndrome and nonoperative measures had failed, she consented to arthroscopic evaluation of the shoulder and arthroscopic partial anterior-lateral acromioplasty.
The procedure was performed 8 months after initial injury. With the patient under general anesthesia and in a lateral decubitus position, her arm was placed in an arm holder. Before the partial acromioplasty, two 18-gauge spinal needles were inserted from the skin surface into the subacromial space to help localize the anterolateral acromion and the acromioclavicular joint. The procedure was performed with a pump using saline bags kept at room temperature. A bipolar radiofrequency device (Stryker Energy Radiofrequency Ablation System; Stryker, Mahwah, New Jersey) was used to débride the subacromial bursa and the periosteum of the undersurface of the acromion. While the bursa was being débrided, the radiofrequency device inadvertently touched the anterior lateral needle probe, and a small skin burn formed around the needle on the surface of the shoulder (Figure). The radiofrequency device did not directly contact the skin, and the deltoid fascia was intact. The spinal needle was removed, and the skin around the burn was excised; the muscle beneath the skin was intact and showed no signs of thermal damage. The skin was mobilized and closed with interrupted simple sutures using a 4-0 nylon suture. The procedure was then completed with no other complications.
After surgery, the patient recovered without complications, and the skin lesion healed with no signs of infection and no skin or muscle defects. Some stiffness was treated with medication and physical therapy. Nine months after surgery, the patient reported mild shoulder stiffness and remained dissatisfied with the appearance of the skin in the area of the burn.
Discussion
Our patient’s case is a reminder that contact between a radiofrequency device and metal needles can transfer heat to tissues and cause skin burns. When using a radiofrequency device around metal needles or cannulas, surgeons should be sure to avoid prolonged contact with the metal. Our patient’s case is the first reported case of a thermal skin injury occurring when a spinal needle was heated by an arthroscopic ablater.
Other authors have reported indirect thermal skin injuries caused by radiofrequency devices during arthroscopic surgery, but the causes were postulated to be direct contact between device and skin7 and overheating of the arthroscopy fluid.5,6,8 Huang and colleagues8 reported that full-thickness skin burns occurred when normal saline used during routine knee arthroscopy overheated from use of a radiofrequency device. Burn lesions, noted on their patient’s leg within 1 day after surgery, required subsequent débridement, a muscle flap, and split-skin grafting. Skin burns caused by overheated fluid have occurred irrespective of type of fluid used (eg, 1.5% glycine or lactated Ringer solution).6 There was no evidence that our patient’s burn resulted from extravasated overheated fluid, as the lesion was localized to the area immediately around the needle and was not geographic, as was described by Huang and colleagues.8
Other possible causes of skin burns during arthroscopic surgery have been described, but none applies in our patient’s case. Segami and colleagues7 described a burn resulting from direct transfer of heat from the radiofrequency device to the skin because of their proximity. This mechanism was not the cause in our patient’s case; there was no evidence of a defect or burned deltoid muscle at time of surgery. Lau and Dao9 reported 2 small full-thickness skin burns caused by a fiberoptic-light cable tip placed on a patient’s leg; in addition, the hot (>170°C) cables caused the paper drapes to combust.9 Skin burns secondary to use of skin antiseptics have been reported,10 but such lesions typically are located beneath tourniquets or in areas of friction from surgical drapes. In some cases, lesions described as skin burns may actually have been pressure lesions secondary to moist skin and friction.11
Whether type of radiofrequency device contributes to the occurrence of heat-related lesions during arthroscopic surgery is unknown. Some investigators have suggested there is more potential for harm with bipolar RFA devices than with monopolar devices.12,13 Monopolar devices pass energy between a probe and a grounding plate, whereas bipolar devices pass energy through 2 points on the probe.14 Because the heat for the monopolar probe derives from the frictional resistance of tissues to each other rather than from the probe itself, the bipolar probe theoretically allows for better temperature control. In addition, bipolar probes require less current to achieve the same heating effect. However, recent studies have suggested that, compared with monopolar radiofrequency devices, bipolar radiofrequency devices are associated with larger increases in temperature at equal depths after an equal number of applications.12,13
To our knowledge, no one has specifically investigated the type of bipolar device used in the present case. This case report, the first to describe a thermal skin injury caused by direct contact between a radiofrequency device and a metal needle inserted in the skin, is a reminder that contact between radiofrequency devices and spinal needles or other metal cannulas used in arthroscopic surgery should be avoided.
1. Bonsell S. Detached deltoid during arthroscopic subacromial decompression. Arthroscopy. 2000;16(7):745-748.
2. Mohammed KD, Hayes MG, Saies AD. Unusual complications of shoulder arthroscopy. J Shoulder Elbow Surg. 2000;9(4):350-353.
3. Pell RF 4th, Uhl RL. Complications of thermal ablation in wrist arthroscopy. Arthroscopy. 2004;20(suppl 2):84-86.
4. Lu Y, Hayashi K, Hecht P, et al. The effect of monopolar radiofrequency energy on partial-thickness defects of articular cartilage. Arthroscopy. 2000;16(5):527-536.
5. Kouk SN, Zoric B, Stetson WB. Complication of the use of a radiofrequency device in arthroscopic shoulder surgery: second-degree burn of the shoulder girdle. Arthroscopy. 2011;27(1):136-141.
6. Lord MJ, Maltry JA, Shall LM. Thermal injury resulting from arthroscopic lateral retinacular release by electrocautery: report of three cases and a review of the literature. Arthroscopy. 1991;7(1):33-37.
7. Segami N, Yamada T, Nishimura M. Thermal injury during temporomandibular joint arthroscopy: a case report. J Oral Maxillofac Surg. 2004;62(4):508-510.
8. Huang S, Gateley D, Moss ALH. Accidental burn injury during knee arthroscopy. Arthroscopy. 2007;23(12):1363.e1-e3.
9. Lau YJ, Dao Q. Cutaneous burns from a fiberoptic cable tip during arthroscopy of the knee. Knee. 2008;15(4):333-335.
10. Sanders TH, Hawken SM. Chlorhexidine burns after shoulder arthroscopy. Am J Orthop. 2012;41(4):172-174.
11. Keyurapan E, Hu SJ, Redett R, McCarthy EF, McFarland EG. Pressure ulcers of the thorax after shoulder surgery. Knee Surg Sports Traumatol Arthrosc. 2007;15(12):1489-1493.
12. Edwards RB 3rd, Lu Y, Rodriguez E, Markel MD. Thermometric determination of cartilage matrix temperatures during thermal chondroplasty: comparison of bipolar and monopolar radiofrequency devices. Arthroscopy. 2002;18(4):339-346.
13. Figueroa D, Calvo R, Vaisman A, et al. Bipolar radiofrequency in the human meniscus. Comparative study between patients younger and older than 40 years of age. Knee. 2007;14(5):357-360.
14. Sahasrabudhe A, McMahon PJ. Thermal probes: what’s available in 2004. Oper Tech Sports Med. 2004;12:206-209.
Cautery and radiofrequency ablation (RFA) devices are commonly used in shoulder arthroscopic surgery for hemostasis and ablation of soft tissue. Although these devices are easily used and applied, complications (eg, extensive release of deltoid muscle,1 nerve damage,2 tendon damage,3 cartilage damage from heat transfer4) can occur during arthroscopic surgery. Radiofrequency devices can elevate fluid temperatures to unsafe levels and directly or indirectly injure surrounding tissue.5,6 Skin complications from using these devices include direct burns to the subcutaneous tissues from the joint to the skin surface7 and skin burns related to overheated arthroscopic fluid.8
In our English-language literature review, however, we found no report of a skin burn secondary to contact between a RFA device and a spinal needle used in identifying structures during an arthroscopic acromioplasty. We report such a case here. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old woman injured her left, nondominant shoulder when a descending garage door hit her directly on the superior aspect of the shoulder. She had immediate onset of pain on the top and lateral side of the shoulder and was evaluated by a primary care physician. Radiographs and magnetic resonance imaging (MRI) were normal. The patient was referred to an orthopedic surgeon for further evaluation.
The orthopedic surgeon found her to be in good health, with no history of diabetes, vascular conditions, or skin disorders. The initial diagnosis after history taking and physical examination was impingement syndrome with subacromial bursitis. The surgeon recommended nonoperative treatment: ice, nonsteroidal anti-inflammatory drugs, and physical therapy. After 3 months, the patient’s examination was unchanged, and there was no improvement in pain. Cortisone injected into the subacromial space helped for a few weeks, but the pain returned. After 2 more cortisone injections over 9 months failed, repeat MRI showed no tears of the rotator cuff or any other salient abnormalities. The treatment options were discussed with the patient, and, because the physical examination findings were consistent with impingement syndrome and nonoperative measures had failed, she consented to arthroscopic evaluation of the shoulder and arthroscopic partial anterior-lateral acromioplasty.
The procedure was performed 8 months after initial injury. With the patient under general anesthesia and in a lateral decubitus position, her arm was placed in an arm holder. Before the partial acromioplasty, two 18-gauge spinal needles were inserted from the skin surface into the subacromial space to help localize the anterolateral acromion and the acromioclavicular joint. The procedure was performed with a pump using saline bags kept at room temperature. A bipolar radiofrequency device (Stryker Energy Radiofrequency Ablation System; Stryker, Mahwah, New Jersey) was used to débride the subacromial bursa and the periosteum of the undersurface of the acromion. While the bursa was being débrided, the radiofrequency device inadvertently touched the anterior lateral needle probe, and a small skin burn formed around the needle on the surface of the shoulder (Figure). The radiofrequency device did not directly contact the skin, and the deltoid fascia was intact. The spinal needle was removed, and the skin around the burn was excised; the muscle beneath the skin was intact and showed no signs of thermal damage. The skin was mobilized and closed with interrupted simple sutures using a 4-0 nylon suture. The procedure was then completed with no other complications.
After surgery, the patient recovered without complications, and the skin lesion healed with no signs of infection and no skin or muscle defects. Some stiffness was treated with medication and physical therapy. Nine months after surgery, the patient reported mild shoulder stiffness and remained dissatisfied with the appearance of the skin in the area of the burn.
Discussion
Our patient’s case is a reminder that contact between a radiofrequency device and metal needles can transfer heat to tissues and cause skin burns. When using a radiofrequency device around metal needles or cannulas, surgeons should be sure to avoid prolonged contact with the metal. Our patient’s case is the first reported case of a thermal skin injury occurring when a spinal needle was heated by an arthroscopic ablater.
Other authors have reported indirect thermal skin injuries caused by radiofrequency devices during arthroscopic surgery, but the causes were postulated to be direct contact between device and skin7 and overheating of the arthroscopy fluid.5,6,8 Huang and colleagues8 reported that full-thickness skin burns occurred when normal saline used during routine knee arthroscopy overheated from use of a radiofrequency device. Burn lesions, noted on their patient’s leg within 1 day after surgery, required subsequent débridement, a muscle flap, and split-skin grafting. Skin burns caused by overheated fluid have occurred irrespective of type of fluid used (eg, 1.5% glycine or lactated Ringer solution).6 There was no evidence that our patient’s burn resulted from extravasated overheated fluid, as the lesion was localized to the area immediately around the needle and was not geographic, as was described by Huang and colleagues.8
Other possible causes of skin burns during arthroscopic surgery have been described, but none applies in our patient’s case. Segami and colleagues7 described a burn resulting from direct transfer of heat from the radiofrequency device to the skin because of their proximity. This mechanism was not the cause in our patient’s case; there was no evidence of a defect or burned deltoid muscle at time of surgery. Lau and Dao9 reported 2 small full-thickness skin burns caused by a fiberoptic-light cable tip placed on a patient’s leg; in addition, the hot (>170°C) cables caused the paper drapes to combust.9 Skin burns secondary to use of skin antiseptics have been reported,10 but such lesions typically are located beneath tourniquets or in areas of friction from surgical drapes. In some cases, lesions described as skin burns may actually have been pressure lesions secondary to moist skin and friction.11
Whether type of radiofrequency device contributes to the occurrence of heat-related lesions during arthroscopic surgery is unknown. Some investigators have suggested there is more potential for harm with bipolar RFA devices than with monopolar devices.12,13 Monopolar devices pass energy between a probe and a grounding plate, whereas bipolar devices pass energy through 2 points on the probe.14 Because the heat for the monopolar probe derives from the frictional resistance of tissues to each other rather than from the probe itself, the bipolar probe theoretically allows for better temperature control. In addition, bipolar probes require less current to achieve the same heating effect. However, recent studies have suggested that, compared with monopolar radiofrequency devices, bipolar radiofrequency devices are associated with larger increases in temperature at equal depths after an equal number of applications.12,13
To our knowledge, no one has specifically investigated the type of bipolar device used in the present case. This case report, the first to describe a thermal skin injury caused by direct contact between a radiofrequency device and a metal needle inserted in the skin, is a reminder that contact between radiofrequency devices and spinal needles or other metal cannulas used in arthroscopic surgery should be avoided.
Cautery and radiofrequency ablation (RFA) devices are commonly used in shoulder arthroscopic surgery for hemostasis and ablation of soft tissue. Although these devices are easily used and applied, complications (eg, extensive release of deltoid muscle,1 nerve damage,2 tendon damage,3 cartilage damage from heat transfer4) can occur during arthroscopic surgery. Radiofrequency devices can elevate fluid temperatures to unsafe levels and directly or indirectly injure surrounding tissue.5,6 Skin complications from using these devices include direct burns to the subcutaneous tissues from the joint to the skin surface7 and skin burns related to overheated arthroscopic fluid.8
In our English-language literature review, however, we found no report of a skin burn secondary to contact between a RFA device and a spinal needle used in identifying structures during an arthroscopic acromioplasty. We report such a case here. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old woman injured her left, nondominant shoulder when a descending garage door hit her directly on the superior aspect of the shoulder. She had immediate onset of pain on the top and lateral side of the shoulder and was evaluated by a primary care physician. Radiographs and magnetic resonance imaging (MRI) were normal. The patient was referred to an orthopedic surgeon for further evaluation.
The orthopedic surgeon found her to be in good health, with no history of diabetes, vascular conditions, or skin disorders. The initial diagnosis after history taking and physical examination was impingement syndrome with subacromial bursitis. The surgeon recommended nonoperative treatment: ice, nonsteroidal anti-inflammatory drugs, and physical therapy. After 3 months, the patient’s examination was unchanged, and there was no improvement in pain. Cortisone injected into the subacromial space helped for a few weeks, but the pain returned. After 2 more cortisone injections over 9 months failed, repeat MRI showed no tears of the rotator cuff or any other salient abnormalities. The treatment options were discussed with the patient, and, because the physical examination findings were consistent with impingement syndrome and nonoperative measures had failed, she consented to arthroscopic evaluation of the shoulder and arthroscopic partial anterior-lateral acromioplasty.
The procedure was performed 8 months after initial injury. With the patient under general anesthesia and in a lateral decubitus position, her arm was placed in an arm holder. Before the partial acromioplasty, two 18-gauge spinal needles were inserted from the skin surface into the subacromial space to help localize the anterolateral acromion and the acromioclavicular joint. The procedure was performed with a pump using saline bags kept at room temperature. A bipolar radiofrequency device (Stryker Energy Radiofrequency Ablation System; Stryker, Mahwah, New Jersey) was used to débride the subacromial bursa and the periosteum of the undersurface of the acromion. While the bursa was being débrided, the radiofrequency device inadvertently touched the anterior lateral needle probe, and a small skin burn formed around the needle on the surface of the shoulder (Figure). The radiofrequency device did not directly contact the skin, and the deltoid fascia was intact. The spinal needle was removed, and the skin around the burn was excised; the muscle beneath the skin was intact and showed no signs of thermal damage. The skin was mobilized and closed with interrupted simple sutures using a 4-0 nylon suture. The procedure was then completed with no other complications.
After surgery, the patient recovered without complications, and the skin lesion healed with no signs of infection and no skin or muscle defects. Some stiffness was treated with medication and physical therapy. Nine months after surgery, the patient reported mild shoulder stiffness and remained dissatisfied with the appearance of the skin in the area of the burn.
Discussion
Our patient’s case is a reminder that contact between a radiofrequency device and metal needles can transfer heat to tissues and cause skin burns. When using a radiofrequency device around metal needles or cannulas, surgeons should be sure to avoid prolonged contact with the metal. Our patient’s case is the first reported case of a thermal skin injury occurring when a spinal needle was heated by an arthroscopic ablater.
Other authors have reported indirect thermal skin injuries caused by radiofrequency devices during arthroscopic surgery, but the causes were postulated to be direct contact between device and skin7 and overheating of the arthroscopy fluid.5,6,8 Huang and colleagues8 reported that full-thickness skin burns occurred when normal saline used during routine knee arthroscopy overheated from use of a radiofrequency device. Burn lesions, noted on their patient’s leg within 1 day after surgery, required subsequent débridement, a muscle flap, and split-skin grafting. Skin burns caused by overheated fluid have occurred irrespective of type of fluid used (eg, 1.5% glycine or lactated Ringer solution).6 There was no evidence that our patient’s burn resulted from extravasated overheated fluid, as the lesion was localized to the area immediately around the needle and was not geographic, as was described by Huang and colleagues.8
Other possible causes of skin burns during arthroscopic surgery have been described, but none applies in our patient’s case. Segami and colleagues7 described a burn resulting from direct transfer of heat from the radiofrequency device to the skin because of their proximity. This mechanism was not the cause in our patient’s case; there was no evidence of a defect or burned deltoid muscle at time of surgery. Lau and Dao9 reported 2 small full-thickness skin burns caused by a fiberoptic-light cable tip placed on a patient’s leg; in addition, the hot (>170°C) cables caused the paper drapes to combust.9 Skin burns secondary to use of skin antiseptics have been reported,10 but such lesions typically are located beneath tourniquets or in areas of friction from surgical drapes. In some cases, lesions described as skin burns may actually have been pressure lesions secondary to moist skin and friction.11
Whether type of radiofrequency device contributes to the occurrence of heat-related lesions during arthroscopic surgery is unknown. Some investigators have suggested there is more potential for harm with bipolar RFA devices than with monopolar devices.12,13 Monopolar devices pass energy between a probe and a grounding plate, whereas bipolar devices pass energy through 2 points on the probe.14 Because the heat for the monopolar probe derives from the frictional resistance of tissues to each other rather than from the probe itself, the bipolar probe theoretically allows for better temperature control. In addition, bipolar probes require less current to achieve the same heating effect. However, recent studies have suggested that, compared with monopolar radiofrequency devices, bipolar radiofrequency devices are associated with larger increases in temperature at equal depths after an equal number of applications.12,13
To our knowledge, no one has specifically investigated the type of bipolar device used in the present case. This case report, the first to describe a thermal skin injury caused by direct contact between a radiofrequency device and a metal needle inserted in the skin, is a reminder that contact between radiofrequency devices and spinal needles or other metal cannulas used in arthroscopic surgery should be avoided.
1. Bonsell S. Detached deltoid during arthroscopic subacromial decompression. Arthroscopy. 2000;16(7):745-748.
2. Mohammed KD, Hayes MG, Saies AD. Unusual complications of shoulder arthroscopy. J Shoulder Elbow Surg. 2000;9(4):350-353.
3. Pell RF 4th, Uhl RL. Complications of thermal ablation in wrist arthroscopy. Arthroscopy. 2004;20(suppl 2):84-86.
4. Lu Y, Hayashi K, Hecht P, et al. The effect of monopolar radiofrequency energy on partial-thickness defects of articular cartilage. Arthroscopy. 2000;16(5):527-536.
5. Kouk SN, Zoric B, Stetson WB. Complication of the use of a radiofrequency device in arthroscopic shoulder surgery: second-degree burn of the shoulder girdle. Arthroscopy. 2011;27(1):136-141.
6. Lord MJ, Maltry JA, Shall LM. Thermal injury resulting from arthroscopic lateral retinacular release by electrocautery: report of three cases and a review of the literature. Arthroscopy. 1991;7(1):33-37.
7. Segami N, Yamada T, Nishimura M. Thermal injury during temporomandibular joint arthroscopy: a case report. J Oral Maxillofac Surg. 2004;62(4):508-510.
8. Huang S, Gateley D, Moss ALH. Accidental burn injury during knee arthroscopy. Arthroscopy. 2007;23(12):1363.e1-e3.
9. Lau YJ, Dao Q. Cutaneous burns from a fiberoptic cable tip during arthroscopy of the knee. Knee. 2008;15(4):333-335.
10. Sanders TH, Hawken SM. Chlorhexidine burns after shoulder arthroscopy. Am J Orthop. 2012;41(4):172-174.
11. Keyurapan E, Hu SJ, Redett R, McCarthy EF, McFarland EG. Pressure ulcers of the thorax after shoulder surgery. Knee Surg Sports Traumatol Arthrosc. 2007;15(12):1489-1493.
12. Edwards RB 3rd, Lu Y, Rodriguez E, Markel MD. Thermometric determination of cartilage matrix temperatures during thermal chondroplasty: comparison of bipolar and monopolar radiofrequency devices. Arthroscopy. 2002;18(4):339-346.
13. Figueroa D, Calvo R, Vaisman A, et al. Bipolar radiofrequency in the human meniscus. Comparative study between patients younger and older than 40 years of age. Knee. 2007;14(5):357-360.
14. Sahasrabudhe A, McMahon PJ. Thermal probes: what’s available in 2004. Oper Tech Sports Med. 2004;12:206-209.
1. Bonsell S. Detached deltoid during arthroscopic subacromial decompression. Arthroscopy. 2000;16(7):745-748.
2. Mohammed KD, Hayes MG, Saies AD. Unusual complications of shoulder arthroscopy. J Shoulder Elbow Surg. 2000;9(4):350-353.
3. Pell RF 4th, Uhl RL. Complications of thermal ablation in wrist arthroscopy. Arthroscopy. 2004;20(suppl 2):84-86.
4. Lu Y, Hayashi K, Hecht P, et al. The effect of monopolar radiofrequency energy on partial-thickness defects of articular cartilage. Arthroscopy. 2000;16(5):527-536.
5. Kouk SN, Zoric B, Stetson WB. Complication of the use of a radiofrequency device in arthroscopic shoulder surgery: second-degree burn of the shoulder girdle. Arthroscopy. 2011;27(1):136-141.
6. Lord MJ, Maltry JA, Shall LM. Thermal injury resulting from arthroscopic lateral retinacular release by electrocautery: report of three cases and a review of the literature. Arthroscopy. 1991;7(1):33-37.
7. Segami N, Yamada T, Nishimura M. Thermal injury during temporomandibular joint arthroscopy: a case report. J Oral Maxillofac Surg. 2004;62(4):508-510.
8. Huang S, Gateley D, Moss ALH. Accidental burn injury during knee arthroscopy. Arthroscopy. 2007;23(12):1363.e1-e3.
9. Lau YJ, Dao Q. Cutaneous burns from a fiberoptic cable tip during arthroscopy of the knee. Knee. 2008;15(4):333-335.
10. Sanders TH, Hawken SM. Chlorhexidine burns after shoulder arthroscopy. Am J Orthop. 2012;41(4):172-174.
11. Keyurapan E, Hu SJ, Redett R, McCarthy EF, McFarland EG. Pressure ulcers of the thorax after shoulder surgery. Knee Surg Sports Traumatol Arthrosc. 2007;15(12):1489-1493.
12. Edwards RB 3rd, Lu Y, Rodriguez E, Markel MD. Thermometric determination of cartilage matrix temperatures during thermal chondroplasty: comparison of bipolar and monopolar radiofrequency devices. Arthroscopy. 2002;18(4):339-346.
13. Figueroa D, Calvo R, Vaisman A, et al. Bipolar radiofrequency in the human meniscus. Comparative study between patients younger and older than 40 years of age. Knee. 2007;14(5):357-360.
14. Sahasrabudhe A, McMahon PJ. Thermal probes: what’s available in 2004. Oper Tech Sports Med. 2004;12:206-209.
Treatment of Proximal Humerus Fractures: Comparison of Shoulder and Trauma Surgeons
Proximal humerus fractures (PHFs), AO/OTA (Ar beitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) type 11,1 are common, representing 4% to 5% of all fractures in adults.2 However, there is no consensus as to optimal management of these injuries, with some reports supporting and others rejecting the various fixation methods,3 and there are no evidence-based practice guidelines informing treatment decisions.4 Not surprisingly, orthopedic surgeons do not agree on ideal treatment for PHFs5,6 and differ by region in their rates of surgical management.2 In addition, analyses of national databases have found variation in choice of surgical treatment for PHFs between surgeons and between hospitals of different patient volumes.4 Few studies have assessed surgeon agreement on treatment decisions. Findings from these limited investigations indicate there is little agreement on treatment choices, but training may have some impact.5-7 In 3 studies,5-7 shoulder and trauma fellowship–trained surgeons differed in their management of PHFs both in terms of rates of operative treatment5,7 and specific operative management choices.5,6 No study has assessed surgeon agreement on radiographic outcomes.
We conducted a study to compare expert shoulder and trauma surgeons’ treatment decision-making and agreement on final radiographic outcomes of surgically treated PHFs. We hypothesized there would be poor agreement on treatment decisions and better agreement on radiographic outcomes, with a difference between shoulder and trauma fellowship–trained surgeons.
Materials and Methods
After receiving institutional review board approval for this study, we collected data on 100 consecutive PHFs (AO/OTA type 111) surgically treated at 2 affiliated level I trauma centers between January 2004 and July 2008. None of the cases in the series was managed by any of the surgeons participating in this study.
We created a PowerPoint (Microsoft, Redmond, Washington) survey that included radiographs (preoperative, immediate postoperative, final postoperative) and, if available, a computed tomography image. This survey was sent to 4 orthopedic surgeons: Drs. Gardner, Gerber, Lorich, and Walch. Two of these authors are fellowship-trained in shoulder surgery, the other 2 in orthopedic traumatology with specialization in treating PHFs. All are internationally renowned in PHF management. Using the survey images and a 4-point Likert scale ranging from disagree strongly to agree strongly, the examiners rated their agreement with treatment decisions (arthroplasty vs fixation). They also rated (very poor to very good) immediate postoperative reduction or arthroplasty placement, immediate postoperative fixation methods for fractures treated with open reduction and internal fixation (ORIF), and final radiographic outcomes.
Interobserver agreement was calculated using the intraclass correlation coefficient (ICC),8,9 with scores of <0.2 (poor), 0.21 to 0.4 (fair), 0.41 to 0.6 (moderate), 0.61 to 0.8 (good), and >0.8 (excellent) used to indicate agreement among observers. ICC scores were determined by treating the 4 examiners as independent entities. Subgroup analyses were also performed to determine ICC scores comparing the 2 shoulder surgeons, comparing the 2 trauma surgeons, and comparing the shoulder surgeons and trauma surgeons as 2 separate groups. ICC scores were used instead of κ coefficients to assess agreement because ICC scores treat ratings as continuous variables, allow for comparison of 2 or more raters, and allow for assessment of correlation among raters, whereas κ coefficients treat data as categorical variables and assume the ratings have no natural ordering. ICC scores were generated by SAS 9.1.3 software (SAS Institute, Cary, North Carolina).
Results
The 4 surgeons’ overall ICC scores for agreement with the rating of immediate reduction or arthroplasty placement and the rating of final radiographic outcome indicated moderate levels of agreement (Table 1). Regarding treatment decision-making and ratings of fixation, the surgeons demonstrated poor and fair levels of agreement, respectively.
The ICC scores comparing the shoulder and trauma surgeons revealed similar levels of agreement (Table 2): moderate levels of agreement for ratings of both immediate postoperative reduction or arthroplasty placement and final radiographic outcomes, but poor and fair levels of agreement regarding treatment decision-making and the rating of immediate postoperative fixation methods for fractures treated with ORIF, respectively.
Subgroup analysis revealed that the 2 shoulder surgeons had poor and fair levels of agreement for treatment decisions and rating of immediate postoperative fixation, respectively, though they moderately agreed on rating of immediate postoperative reduction or arthroplasty placement and rating of final radiographic outcome (Table 3). When the 2 trauma surgeons were compared with each other, ICC scores revealed higher levels of agreement overall (Table 4). In other words, the 2 trauma surgeons agreed with each other more than the 2 shoulder surgeons agreed with each other.
Discussion
This study had 3 major findings: (1) Surgeons do not agree on treatment decisions, including fixation methods, regarding PHFs; (2) regardless of their opinions on ideal treatment, they moderately agree on reductions and final radiographic outcomes; (3) expert trauma surgeons may agree more on treatment decisions than expert shoulder surgeons do. In other words, surgeons do not agree on the best treatment, but they radiographically recognize when a procedure has been performed technically well or poorly. These results support our hypothesis and the limited current literature.
An analysis of Medicare databases showed marked regional variation in rates of operative treatment of PHFs.2 Similarly, a Nationwide Inpatient Sample analysis revealed nationwide variation in operative management of PHFs.4 Both findings are consistent with our results of poor agreement about treatment decisions and ratings of postoperative fixation of PHFs. In 2010, Petit and colleagues6 reported that surgeons do not agree on PHF management. In 2011, Foroohar and colleagues10 similarly reported low interobserver agreement for treatment recommendations made by 4 upper extremity orthopedic specialists, 4 general orthopedic surgeons, 4 senior residents, and 4 junior residents, for a series of 16 PHFs—also consistent with our findings.
The lack of agreement about PHF treatment may reflect a difference in training, particularly in light of the recent expansion of shoulder and elbow fellowships.2 Three separate studies performed at 2 affiliated level I trauma centers demonstrated significant differences in treatment decision-making between shoulder and trauma fellowship–trained surgeons.5-7 Our results are consistent with the hypothesis that training affects treatment decision-making, as we found poor agreement between shoulder and trauma fellowship–trained surgeons regarding treatment decision for PHFs. Subanalyses revealed that expert trauma surgeons agreed with each other on treatment decisions more than expert shoulder surgeons agreed with each other, further suggesting that training may affect how surgeons manage PHFs. Differences in fellowship training even within the same specialty may account for the observed lesser levels of agreement between the shoulder surgeons, even among experts in the field.
The evidence for optimal treatment historically has been poor,4,6 with few high-quality prospective, randomized controlled studies on the topic up until the past few years. The most recent Cochrane Review on optimal PHF treatment concluded that there is insufficient evidence to make an evidence-based recommendation and that the long-term benefit of surgery is unclear.11 However, at least 5 controlled trials on the topic have been published within the past 5 years.12-16 The evidence is striking and generally supports nonoperative treatment for most PHFs, including some displaced fractures—contrary to general orthopedic practice in many parts of the United States,2 which hitherto had been based mainly on individual surgeon experience and the limited literature. Without strong evidence to support one treatment option over another, surgeons are left with no objective, scientific way of coming to agreement.
Related to the poor status quo of evidence for PHF treatments is new technology (eg, locking plates, reverse total shoulder arthroplasty) that has expanded surgical indications.2,17 Although such developments have the potential to improve surgical treatments, they may also exacerbate the disagreement between surgeons regarding optimal operative treatment of PHFs. This potential consequence of new technology may be reflected in our finding of disagreement among surgeons on immediate postoperative fixation methods. Precisely because they are new, such technological innovations have limited evidence supporting their use. This leaves surgeons with little to nothing to inform their decisions to use these devices, other than familiarity with and impressions of the new technology.
Our study had several limitations. First is the small sample size, of surgeons who are leaders in the field. Our sample therefore may not be generalizable to the general population of shoulder and trauma surgeons. Second, we did not calculate intraobserver variability. Third, inherent to studies of interobserver agreement is the uncertainty of their clinical relevance. In the clinical setting, a surgeon has much more information at hand (eg, patient history, physical examination findings, colleague consultations), thus raising the possibility of underestimations of interobserver agreements.18 Fourth, our comparison of surgeons’ ratings of outcomes was purely radiographic, which may or may not represent or be indicative of clinical outcomes (eg, pain relief, function, range of motion, patient satisfaction). The conclusions we may draw are accordingly limited, as we did not directly evaluate clinical outcome parameters.
Our study had several strengths as well. First, to our knowledge this is the first study to assess interobserver variability in surgeons’ ratings of radiographic outcomes. Its findings may provide further insight into the reasons for poor agreement among orthopedic surgeons on both classification and treatment of PHFs. Second, our surveying of internationally renowned expert surgeons from 4 different institutions may have helped reduce single-institution bias, and it presents the highest level of expertise in the treatment of PHFs.
Although the surgeons in our study moderately agreed on final radiographic outcomes of PHFs, such levels of agreement may still be clinically unacceptable.19 The overall disagreement on treatment decisions highlights the need for better evidence for optimal treatment of PHFs in order to improve consensus, particularly with anticipated increases in age and comorbidities in the population in coming years.4 Subgroup analysis suggested trauma fellowships may contribute to better treatment agreement, though this idea requires further study, perhaps by surveying shoulder and trauma fellowship directors and their curricula for variability in teaching treatment decision-making. The surgeons in our study agreed more on what they consider acceptable final radiographic outcomes, which is encouraging. However, treatment consensus is the primary goal. The recent publication of prospective, randomized studies is helping with this issue, but more studies are needed. It is encouraging that several are planned or under way.20-22
Conclusion
The surgeons surveyed in this study did not agree on ideal treatment for PHFs but moderately agreed on quality of radiographic outcomes. These differences may reflect a difference in training. We conducted this study to compare experienced shoulder and trauma fellowship–trained surgeons’ treatment decision-making and ratings of radiographic outcomes of PHFs when presented with the same group of patients managed at 2 level I trauma centers. We hypothesized there would be little agreement on treatment decisions, better agreement on final radiographic outcome, and a difference between decision-making and ratings of radiographic outcomes between expert shoulder and trauma surgeons. Our results showed that surgeons do not agree on the best treatment for PHFs but radiographically recognize when an operative treatment has been performed well or poorly. Regarding treatment decisions, our results also showed that expert trauma surgeons may agree more with each other than shoulder surgeons agree with each other. These results support our hypothesis and the limited current literature. The overall disagreement among the surgeons in our study and an aging population that grows sicker each year highlight the need for better evidence for the optimal treatment of PHFs in order to improve consensus.
1. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium – 2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21(10 suppl):S1-S133.
2. Bell JE, Leung BC, Spratt KF, et al. Trends and variation in incidence, surgical treatment, and repeat surgery of proximal humeral fractures in the elderly. J Bone Joint Surg Am. 2011;93(2):121-131.
3. McLaurin TM. Proximal humerus fractures in the elderly are we operating on too many? Bull Hosp Jt Dis. 2004;62(1-2):24-32.
4. Jain NB, Kuye I, Higgins LD, Warner JJP. Surgeon volume is associated with cost and variation in surgical treatment of proximal humeral fractures. Clin Orthop. 2012;471(2):655-664.
5. Boykin RE, Jawa A, O’Brien T, Higgins LD, Warner JJP. Variability in operative management of proximal humerus fractures. Shoulder Elbow. 2011;3(4):197-201.
6. Petit CJ, Millett PJ, Endres NK, Diller D, Harris MB, Warner JJP. Management of proximal humeral fractures: surgeons don’t agree. J Shoulder Elbow Surg. 2010;19(3):446-451.
7. Okike K, Lee OC, Makanji H, Harris MB, Vrahas MS. Factors associated with the decision for operative versus non-operative treatment of displaced proximal humerus fractures in the elderly. Injury. 2013;44(4):448-455.
8. Kodali P, Jones MH, Polster J, Miniaci A, Fening SD. Accuracy of measurement of Hill-Sachs lesions with computed tomography. J Shoulder Elbow Surg. 2011;20(8):1328-1334.
9. Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420-428.
10. Foroohar A, Tosti R, Richmond JM, Gaughan JP, Ilyas AM. Classification and treatment of proximal humerus fractures: inter-observer reliability and agreement across imaging modalities and experience. J Orthop Surg Res. 2011;6:38.
11. Handoll HH, Ollivere BJ. Interventions for treating proximal humeral fractures in adults. Cochrane Database Syst Rev. 2010;(12):CD000434.
12. Boons HW, Goosen JH, van Grinsven S, van Susante JL, van Loon CJ. Hemiarthroplasty for humeral four-part fractures for patients 65 years and older: a randomized controlled trial. Clin Orthop. 2012;470(12):3483-3491.
13. Fjalestad T, Hole MØ, Hovden IAH, Blücher J, Strømsøe K. Surgical treatment with an angular stable plate for complex displaced proximal humeral fractures in elderly patients: a randomized controlled trial. J Orthop Trauma. 2012;26(2):98-106.
14. Fjalestad T, Hole MØ, Jørgensen JJ, Strømsøe K, Kristiansen IS. Health and cost consequences of surgical versus conservative treatment for a comminuted proximal humeral fracture in elderly patients. Injury. 2010;41(6):599-605.
15. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Internal fixation versus nonoperative treatment of displaced 3-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(5):747-755.
16. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Hemiarthroplasty versus nonoperative treatment of displaced 4-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(7):1025-1033.
17. Agudelo J, Schürmann M, Stahel P, et al. Analysis of efficacy and failure in proximal humerus fractures treated with locking plates. J Orthop Trauma. 2007;21(10):676-681.
18. Brorson S, Hróbjartsson A. Training improves agreement among doctors using the Neer system for proximal humeral fractures in a systematic review. J Clin Epidemiol. 2008;61(1):7-16.
19. Brorson S, Olsen BS, Frich LH, et al. Surgeons agree more on treatment recommendations than on classification of proximal humeral fractures. BMC Musculoskelet Disord. 2012;13:114.
20. Handoll H, Brealey S, Rangan A, et al. Protocol for the ProFHER (PROximal Fracture of the Humerus: Evaluation by Randomisation) trial: a pragmatic multi-centre randomised controlled trial of surgical versus non-surgical treatment for proximal fracture of the humerus in adults. BMC Musculoskelet Disord. 2009;10:140.
21. Den Hartog D, Van Lieshout EMM, Tuinebreijer WE, et al. Primary hemiarthroplasty versus conservative treatment for comminuted fractures of the proximal humerus in the elderly (ProCon): a multicenter randomized controlled trial. BMC Musculoskelet Disord. 2010;11:97.
22. Verbeek PA, van den Akker-Scheek I, Wendt KW, Diercks RL. Hemiarthroplasty versus angle-stable locking compression plate osteosynthesis in the treatment of three- and four-part fractures of the proximal humerus in the elderly: design of a randomized controlled trial. BMC Musculoskelet Disord. 2012;13:16.
Proximal humerus fractures (PHFs), AO/OTA (Ar beitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) type 11,1 are common, representing 4% to 5% of all fractures in adults.2 However, there is no consensus as to optimal management of these injuries, with some reports supporting and others rejecting the various fixation methods,3 and there are no evidence-based practice guidelines informing treatment decisions.4 Not surprisingly, orthopedic surgeons do not agree on ideal treatment for PHFs5,6 and differ by region in their rates of surgical management.2 In addition, analyses of national databases have found variation in choice of surgical treatment for PHFs between surgeons and between hospitals of different patient volumes.4 Few studies have assessed surgeon agreement on treatment decisions. Findings from these limited investigations indicate there is little agreement on treatment choices, but training may have some impact.5-7 In 3 studies,5-7 shoulder and trauma fellowship–trained surgeons differed in their management of PHFs both in terms of rates of operative treatment5,7 and specific operative management choices.5,6 No study has assessed surgeon agreement on radiographic outcomes.
We conducted a study to compare expert shoulder and trauma surgeons’ treatment decision-making and agreement on final radiographic outcomes of surgically treated PHFs. We hypothesized there would be poor agreement on treatment decisions and better agreement on radiographic outcomes, with a difference between shoulder and trauma fellowship–trained surgeons.
Materials and Methods
After receiving institutional review board approval for this study, we collected data on 100 consecutive PHFs (AO/OTA type 111) surgically treated at 2 affiliated level I trauma centers between January 2004 and July 2008. None of the cases in the series was managed by any of the surgeons participating in this study.
We created a PowerPoint (Microsoft, Redmond, Washington) survey that included radiographs (preoperative, immediate postoperative, final postoperative) and, if available, a computed tomography image. This survey was sent to 4 orthopedic surgeons: Drs. Gardner, Gerber, Lorich, and Walch. Two of these authors are fellowship-trained in shoulder surgery, the other 2 in orthopedic traumatology with specialization in treating PHFs. All are internationally renowned in PHF management. Using the survey images and a 4-point Likert scale ranging from disagree strongly to agree strongly, the examiners rated their agreement with treatment decisions (arthroplasty vs fixation). They also rated (very poor to very good) immediate postoperative reduction or arthroplasty placement, immediate postoperative fixation methods for fractures treated with open reduction and internal fixation (ORIF), and final radiographic outcomes.
Interobserver agreement was calculated using the intraclass correlation coefficient (ICC),8,9 with scores of <0.2 (poor), 0.21 to 0.4 (fair), 0.41 to 0.6 (moderate), 0.61 to 0.8 (good), and >0.8 (excellent) used to indicate agreement among observers. ICC scores were determined by treating the 4 examiners as independent entities. Subgroup analyses were also performed to determine ICC scores comparing the 2 shoulder surgeons, comparing the 2 trauma surgeons, and comparing the shoulder surgeons and trauma surgeons as 2 separate groups. ICC scores were used instead of κ coefficients to assess agreement because ICC scores treat ratings as continuous variables, allow for comparison of 2 or more raters, and allow for assessment of correlation among raters, whereas κ coefficients treat data as categorical variables and assume the ratings have no natural ordering. ICC scores were generated by SAS 9.1.3 software (SAS Institute, Cary, North Carolina).
Results
The 4 surgeons’ overall ICC scores for agreement with the rating of immediate reduction or arthroplasty placement and the rating of final radiographic outcome indicated moderate levels of agreement (Table 1). Regarding treatment decision-making and ratings of fixation, the surgeons demonstrated poor and fair levels of agreement, respectively.
The ICC scores comparing the shoulder and trauma surgeons revealed similar levels of agreement (Table 2): moderate levels of agreement for ratings of both immediate postoperative reduction or arthroplasty placement and final radiographic outcomes, but poor and fair levels of agreement regarding treatment decision-making and the rating of immediate postoperative fixation methods for fractures treated with ORIF, respectively.
Subgroup analysis revealed that the 2 shoulder surgeons had poor and fair levels of agreement for treatment decisions and rating of immediate postoperative fixation, respectively, though they moderately agreed on rating of immediate postoperative reduction or arthroplasty placement and rating of final radiographic outcome (Table 3). When the 2 trauma surgeons were compared with each other, ICC scores revealed higher levels of agreement overall (Table 4). In other words, the 2 trauma surgeons agreed with each other more than the 2 shoulder surgeons agreed with each other.
Discussion
This study had 3 major findings: (1) Surgeons do not agree on treatment decisions, including fixation methods, regarding PHFs; (2) regardless of their opinions on ideal treatment, they moderately agree on reductions and final radiographic outcomes; (3) expert trauma surgeons may agree more on treatment decisions than expert shoulder surgeons do. In other words, surgeons do not agree on the best treatment, but they radiographically recognize when a procedure has been performed technically well or poorly. These results support our hypothesis and the limited current literature.
An analysis of Medicare databases showed marked regional variation in rates of operative treatment of PHFs.2 Similarly, a Nationwide Inpatient Sample analysis revealed nationwide variation in operative management of PHFs.4 Both findings are consistent with our results of poor agreement about treatment decisions and ratings of postoperative fixation of PHFs. In 2010, Petit and colleagues6 reported that surgeons do not agree on PHF management. In 2011, Foroohar and colleagues10 similarly reported low interobserver agreement for treatment recommendations made by 4 upper extremity orthopedic specialists, 4 general orthopedic surgeons, 4 senior residents, and 4 junior residents, for a series of 16 PHFs—also consistent with our findings.
The lack of agreement about PHF treatment may reflect a difference in training, particularly in light of the recent expansion of shoulder and elbow fellowships.2 Three separate studies performed at 2 affiliated level I trauma centers demonstrated significant differences in treatment decision-making between shoulder and trauma fellowship–trained surgeons.5-7 Our results are consistent with the hypothesis that training affects treatment decision-making, as we found poor agreement between shoulder and trauma fellowship–trained surgeons regarding treatment decision for PHFs. Subanalyses revealed that expert trauma surgeons agreed with each other on treatment decisions more than expert shoulder surgeons agreed with each other, further suggesting that training may affect how surgeons manage PHFs. Differences in fellowship training even within the same specialty may account for the observed lesser levels of agreement between the shoulder surgeons, even among experts in the field.
The evidence for optimal treatment historically has been poor,4,6 with few high-quality prospective, randomized controlled studies on the topic up until the past few years. The most recent Cochrane Review on optimal PHF treatment concluded that there is insufficient evidence to make an evidence-based recommendation and that the long-term benefit of surgery is unclear.11 However, at least 5 controlled trials on the topic have been published within the past 5 years.12-16 The evidence is striking and generally supports nonoperative treatment for most PHFs, including some displaced fractures—contrary to general orthopedic practice in many parts of the United States,2 which hitherto had been based mainly on individual surgeon experience and the limited literature. Without strong evidence to support one treatment option over another, surgeons are left with no objective, scientific way of coming to agreement.
Related to the poor status quo of evidence for PHF treatments is new technology (eg, locking plates, reverse total shoulder arthroplasty) that has expanded surgical indications.2,17 Although such developments have the potential to improve surgical treatments, they may also exacerbate the disagreement between surgeons regarding optimal operative treatment of PHFs. This potential consequence of new technology may be reflected in our finding of disagreement among surgeons on immediate postoperative fixation methods. Precisely because they are new, such technological innovations have limited evidence supporting their use. This leaves surgeons with little to nothing to inform their decisions to use these devices, other than familiarity with and impressions of the new technology.
Our study had several limitations. First is the small sample size, of surgeons who are leaders in the field. Our sample therefore may not be generalizable to the general population of shoulder and trauma surgeons. Second, we did not calculate intraobserver variability. Third, inherent to studies of interobserver agreement is the uncertainty of their clinical relevance. In the clinical setting, a surgeon has much more information at hand (eg, patient history, physical examination findings, colleague consultations), thus raising the possibility of underestimations of interobserver agreements.18 Fourth, our comparison of surgeons’ ratings of outcomes was purely radiographic, which may or may not represent or be indicative of clinical outcomes (eg, pain relief, function, range of motion, patient satisfaction). The conclusions we may draw are accordingly limited, as we did not directly evaluate clinical outcome parameters.
Our study had several strengths as well. First, to our knowledge this is the first study to assess interobserver variability in surgeons’ ratings of radiographic outcomes. Its findings may provide further insight into the reasons for poor agreement among orthopedic surgeons on both classification and treatment of PHFs. Second, our surveying of internationally renowned expert surgeons from 4 different institutions may have helped reduce single-institution bias, and it presents the highest level of expertise in the treatment of PHFs.
Although the surgeons in our study moderately agreed on final radiographic outcomes of PHFs, such levels of agreement may still be clinically unacceptable.19 The overall disagreement on treatment decisions highlights the need for better evidence for optimal treatment of PHFs in order to improve consensus, particularly with anticipated increases in age and comorbidities in the population in coming years.4 Subgroup analysis suggested trauma fellowships may contribute to better treatment agreement, though this idea requires further study, perhaps by surveying shoulder and trauma fellowship directors and their curricula for variability in teaching treatment decision-making. The surgeons in our study agreed more on what they consider acceptable final radiographic outcomes, which is encouraging. However, treatment consensus is the primary goal. The recent publication of prospective, randomized studies is helping with this issue, but more studies are needed. It is encouraging that several are planned or under way.20-22
Conclusion
The surgeons surveyed in this study did not agree on ideal treatment for PHFs but moderately agreed on quality of radiographic outcomes. These differences may reflect a difference in training. We conducted this study to compare experienced shoulder and trauma fellowship–trained surgeons’ treatment decision-making and ratings of radiographic outcomes of PHFs when presented with the same group of patients managed at 2 level I trauma centers. We hypothesized there would be little agreement on treatment decisions, better agreement on final radiographic outcome, and a difference between decision-making and ratings of radiographic outcomes between expert shoulder and trauma surgeons. Our results showed that surgeons do not agree on the best treatment for PHFs but radiographically recognize when an operative treatment has been performed well or poorly. Regarding treatment decisions, our results also showed that expert trauma surgeons may agree more with each other than shoulder surgeons agree with each other. These results support our hypothesis and the limited current literature. The overall disagreement among the surgeons in our study and an aging population that grows sicker each year highlight the need for better evidence for the optimal treatment of PHFs in order to improve consensus.
Proximal humerus fractures (PHFs), AO/OTA (Ar beitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) type 11,1 are common, representing 4% to 5% of all fractures in adults.2 However, there is no consensus as to optimal management of these injuries, with some reports supporting and others rejecting the various fixation methods,3 and there are no evidence-based practice guidelines informing treatment decisions.4 Not surprisingly, orthopedic surgeons do not agree on ideal treatment for PHFs5,6 and differ by region in their rates of surgical management.2 In addition, analyses of national databases have found variation in choice of surgical treatment for PHFs between surgeons and between hospitals of different patient volumes.4 Few studies have assessed surgeon agreement on treatment decisions. Findings from these limited investigations indicate there is little agreement on treatment choices, but training may have some impact.5-7 In 3 studies,5-7 shoulder and trauma fellowship–trained surgeons differed in their management of PHFs both in terms of rates of operative treatment5,7 and specific operative management choices.5,6 No study has assessed surgeon agreement on radiographic outcomes.
We conducted a study to compare expert shoulder and trauma surgeons’ treatment decision-making and agreement on final radiographic outcomes of surgically treated PHFs. We hypothesized there would be poor agreement on treatment decisions and better agreement on radiographic outcomes, with a difference between shoulder and trauma fellowship–trained surgeons.
Materials and Methods
After receiving institutional review board approval for this study, we collected data on 100 consecutive PHFs (AO/OTA type 111) surgically treated at 2 affiliated level I trauma centers between January 2004 and July 2008. None of the cases in the series was managed by any of the surgeons participating in this study.
We created a PowerPoint (Microsoft, Redmond, Washington) survey that included radiographs (preoperative, immediate postoperative, final postoperative) and, if available, a computed tomography image. This survey was sent to 4 orthopedic surgeons: Drs. Gardner, Gerber, Lorich, and Walch. Two of these authors are fellowship-trained in shoulder surgery, the other 2 in orthopedic traumatology with specialization in treating PHFs. All are internationally renowned in PHF management. Using the survey images and a 4-point Likert scale ranging from disagree strongly to agree strongly, the examiners rated their agreement with treatment decisions (arthroplasty vs fixation). They also rated (very poor to very good) immediate postoperative reduction or arthroplasty placement, immediate postoperative fixation methods for fractures treated with open reduction and internal fixation (ORIF), and final radiographic outcomes.
Interobserver agreement was calculated using the intraclass correlation coefficient (ICC),8,9 with scores of <0.2 (poor), 0.21 to 0.4 (fair), 0.41 to 0.6 (moderate), 0.61 to 0.8 (good), and >0.8 (excellent) used to indicate agreement among observers. ICC scores were determined by treating the 4 examiners as independent entities. Subgroup analyses were also performed to determine ICC scores comparing the 2 shoulder surgeons, comparing the 2 trauma surgeons, and comparing the shoulder surgeons and trauma surgeons as 2 separate groups. ICC scores were used instead of κ coefficients to assess agreement because ICC scores treat ratings as continuous variables, allow for comparison of 2 or more raters, and allow for assessment of correlation among raters, whereas κ coefficients treat data as categorical variables and assume the ratings have no natural ordering. ICC scores were generated by SAS 9.1.3 software (SAS Institute, Cary, North Carolina).
Results
The 4 surgeons’ overall ICC scores for agreement with the rating of immediate reduction or arthroplasty placement and the rating of final radiographic outcome indicated moderate levels of agreement (Table 1). Regarding treatment decision-making and ratings of fixation, the surgeons demonstrated poor and fair levels of agreement, respectively.
The ICC scores comparing the shoulder and trauma surgeons revealed similar levels of agreement (Table 2): moderate levels of agreement for ratings of both immediate postoperative reduction or arthroplasty placement and final radiographic outcomes, but poor and fair levels of agreement regarding treatment decision-making and the rating of immediate postoperative fixation methods for fractures treated with ORIF, respectively.
Subgroup analysis revealed that the 2 shoulder surgeons had poor and fair levels of agreement for treatment decisions and rating of immediate postoperative fixation, respectively, though they moderately agreed on rating of immediate postoperative reduction or arthroplasty placement and rating of final radiographic outcome (Table 3). When the 2 trauma surgeons were compared with each other, ICC scores revealed higher levels of agreement overall (Table 4). In other words, the 2 trauma surgeons agreed with each other more than the 2 shoulder surgeons agreed with each other.
Discussion
This study had 3 major findings: (1) Surgeons do not agree on treatment decisions, including fixation methods, regarding PHFs; (2) regardless of their opinions on ideal treatment, they moderately agree on reductions and final radiographic outcomes; (3) expert trauma surgeons may agree more on treatment decisions than expert shoulder surgeons do. In other words, surgeons do not agree on the best treatment, but they radiographically recognize when a procedure has been performed technically well or poorly. These results support our hypothesis and the limited current literature.
An analysis of Medicare databases showed marked regional variation in rates of operative treatment of PHFs.2 Similarly, a Nationwide Inpatient Sample analysis revealed nationwide variation in operative management of PHFs.4 Both findings are consistent with our results of poor agreement about treatment decisions and ratings of postoperative fixation of PHFs. In 2010, Petit and colleagues6 reported that surgeons do not agree on PHF management. In 2011, Foroohar and colleagues10 similarly reported low interobserver agreement for treatment recommendations made by 4 upper extremity orthopedic specialists, 4 general orthopedic surgeons, 4 senior residents, and 4 junior residents, for a series of 16 PHFs—also consistent with our findings.
The lack of agreement about PHF treatment may reflect a difference in training, particularly in light of the recent expansion of shoulder and elbow fellowships.2 Three separate studies performed at 2 affiliated level I trauma centers demonstrated significant differences in treatment decision-making between shoulder and trauma fellowship–trained surgeons.5-7 Our results are consistent with the hypothesis that training affects treatment decision-making, as we found poor agreement between shoulder and trauma fellowship–trained surgeons regarding treatment decision for PHFs. Subanalyses revealed that expert trauma surgeons agreed with each other on treatment decisions more than expert shoulder surgeons agreed with each other, further suggesting that training may affect how surgeons manage PHFs. Differences in fellowship training even within the same specialty may account for the observed lesser levels of agreement between the shoulder surgeons, even among experts in the field.
The evidence for optimal treatment historically has been poor,4,6 with few high-quality prospective, randomized controlled studies on the topic up until the past few years. The most recent Cochrane Review on optimal PHF treatment concluded that there is insufficient evidence to make an evidence-based recommendation and that the long-term benefit of surgery is unclear.11 However, at least 5 controlled trials on the topic have been published within the past 5 years.12-16 The evidence is striking and generally supports nonoperative treatment for most PHFs, including some displaced fractures—contrary to general orthopedic practice in many parts of the United States,2 which hitherto had been based mainly on individual surgeon experience and the limited literature. Without strong evidence to support one treatment option over another, surgeons are left with no objective, scientific way of coming to agreement.
Related to the poor status quo of evidence for PHF treatments is new technology (eg, locking plates, reverse total shoulder arthroplasty) that has expanded surgical indications.2,17 Although such developments have the potential to improve surgical treatments, they may also exacerbate the disagreement between surgeons regarding optimal operative treatment of PHFs. This potential consequence of new technology may be reflected in our finding of disagreement among surgeons on immediate postoperative fixation methods. Precisely because they are new, such technological innovations have limited evidence supporting their use. This leaves surgeons with little to nothing to inform their decisions to use these devices, other than familiarity with and impressions of the new technology.
Our study had several limitations. First is the small sample size, of surgeons who are leaders in the field. Our sample therefore may not be generalizable to the general population of shoulder and trauma surgeons. Second, we did not calculate intraobserver variability. Third, inherent to studies of interobserver agreement is the uncertainty of their clinical relevance. In the clinical setting, a surgeon has much more information at hand (eg, patient history, physical examination findings, colleague consultations), thus raising the possibility of underestimations of interobserver agreements.18 Fourth, our comparison of surgeons’ ratings of outcomes was purely radiographic, which may or may not represent or be indicative of clinical outcomes (eg, pain relief, function, range of motion, patient satisfaction). The conclusions we may draw are accordingly limited, as we did not directly evaluate clinical outcome parameters.
Our study had several strengths as well. First, to our knowledge this is the first study to assess interobserver variability in surgeons’ ratings of radiographic outcomes. Its findings may provide further insight into the reasons for poor agreement among orthopedic surgeons on both classification and treatment of PHFs. Second, our surveying of internationally renowned expert surgeons from 4 different institutions may have helped reduce single-institution bias, and it presents the highest level of expertise in the treatment of PHFs.
Although the surgeons in our study moderately agreed on final radiographic outcomes of PHFs, such levels of agreement may still be clinically unacceptable.19 The overall disagreement on treatment decisions highlights the need for better evidence for optimal treatment of PHFs in order to improve consensus, particularly with anticipated increases in age and comorbidities in the population in coming years.4 Subgroup analysis suggested trauma fellowships may contribute to better treatment agreement, though this idea requires further study, perhaps by surveying shoulder and trauma fellowship directors and their curricula for variability in teaching treatment decision-making. The surgeons in our study agreed more on what they consider acceptable final radiographic outcomes, which is encouraging. However, treatment consensus is the primary goal. The recent publication of prospective, randomized studies is helping with this issue, but more studies are needed. It is encouraging that several are planned or under way.20-22
Conclusion
The surgeons surveyed in this study did not agree on ideal treatment for PHFs but moderately agreed on quality of radiographic outcomes. These differences may reflect a difference in training. We conducted this study to compare experienced shoulder and trauma fellowship–trained surgeons’ treatment decision-making and ratings of radiographic outcomes of PHFs when presented with the same group of patients managed at 2 level I trauma centers. We hypothesized there would be little agreement on treatment decisions, better agreement on final radiographic outcome, and a difference between decision-making and ratings of radiographic outcomes between expert shoulder and trauma surgeons. Our results showed that surgeons do not agree on the best treatment for PHFs but radiographically recognize when an operative treatment has been performed well or poorly. Regarding treatment decisions, our results also showed that expert trauma surgeons may agree more with each other than shoulder surgeons agree with each other. These results support our hypothesis and the limited current literature. The overall disagreement among the surgeons in our study and an aging population that grows sicker each year highlight the need for better evidence for the optimal treatment of PHFs in order to improve consensus.
1. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium – 2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21(10 suppl):S1-S133.
2. Bell JE, Leung BC, Spratt KF, et al. Trends and variation in incidence, surgical treatment, and repeat surgery of proximal humeral fractures in the elderly. J Bone Joint Surg Am. 2011;93(2):121-131.
3. McLaurin TM. Proximal humerus fractures in the elderly are we operating on too many? Bull Hosp Jt Dis. 2004;62(1-2):24-32.
4. Jain NB, Kuye I, Higgins LD, Warner JJP. Surgeon volume is associated with cost and variation in surgical treatment of proximal humeral fractures. Clin Orthop. 2012;471(2):655-664.
5. Boykin RE, Jawa A, O’Brien T, Higgins LD, Warner JJP. Variability in operative management of proximal humerus fractures. Shoulder Elbow. 2011;3(4):197-201.
6. Petit CJ, Millett PJ, Endres NK, Diller D, Harris MB, Warner JJP. Management of proximal humeral fractures: surgeons don’t agree. J Shoulder Elbow Surg. 2010;19(3):446-451.
7. Okike K, Lee OC, Makanji H, Harris MB, Vrahas MS. Factors associated with the decision for operative versus non-operative treatment of displaced proximal humerus fractures in the elderly. Injury. 2013;44(4):448-455.
8. Kodali P, Jones MH, Polster J, Miniaci A, Fening SD. Accuracy of measurement of Hill-Sachs lesions with computed tomography. J Shoulder Elbow Surg. 2011;20(8):1328-1334.
9. Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420-428.
10. Foroohar A, Tosti R, Richmond JM, Gaughan JP, Ilyas AM. Classification and treatment of proximal humerus fractures: inter-observer reliability and agreement across imaging modalities and experience. J Orthop Surg Res. 2011;6:38.
11. Handoll HH, Ollivere BJ. Interventions for treating proximal humeral fractures in adults. Cochrane Database Syst Rev. 2010;(12):CD000434.
12. Boons HW, Goosen JH, van Grinsven S, van Susante JL, van Loon CJ. Hemiarthroplasty for humeral four-part fractures for patients 65 years and older: a randomized controlled trial. Clin Orthop. 2012;470(12):3483-3491.
13. Fjalestad T, Hole MØ, Hovden IAH, Blücher J, Strømsøe K. Surgical treatment with an angular stable plate for complex displaced proximal humeral fractures in elderly patients: a randomized controlled trial. J Orthop Trauma. 2012;26(2):98-106.
14. Fjalestad T, Hole MØ, Jørgensen JJ, Strømsøe K, Kristiansen IS. Health and cost consequences of surgical versus conservative treatment for a comminuted proximal humeral fracture in elderly patients. Injury. 2010;41(6):599-605.
15. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Internal fixation versus nonoperative treatment of displaced 3-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(5):747-755.
16. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Hemiarthroplasty versus nonoperative treatment of displaced 4-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(7):1025-1033.
17. Agudelo J, Schürmann M, Stahel P, et al. Analysis of efficacy and failure in proximal humerus fractures treated with locking plates. J Orthop Trauma. 2007;21(10):676-681.
18. Brorson S, Hróbjartsson A. Training improves agreement among doctors using the Neer system for proximal humeral fractures in a systematic review. J Clin Epidemiol. 2008;61(1):7-16.
19. Brorson S, Olsen BS, Frich LH, et al. Surgeons agree more on treatment recommendations than on classification of proximal humeral fractures. BMC Musculoskelet Disord. 2012;13:114.
20. Handoll H, Brealey S, Rangan A, et al. Protocol for the ProFHER (PROximal Fracture of the Humerus: Evaluation by Randomisation) trial: a pragmatic multi-centre randomised controlled trial of surgical versus non-surgical treatment for proximal fracture of the humerus in adults. BMC Musculoskelet Disord. 2009;10:140.
21. Den Hartog D, Van Lieshout EMM, Tuinebreijer WE, et al. Primary hemiarthroplasty versus conservative treatment for comminuted fractures of the proximal humerus in the elderly (ProCon): a multicenter randomized controlled trial. BMC Musculoskelet Disord. 2010;11:97.
22. Verbeek PA, van den Akker-Scheek I, Wendt KW, Diercks RL. Hemiarthroplasty versus angle-stable locking compression plate osteosynthesis in the treatment of three- and four-part fractures of the proximal humerus in the elderly: design of a randomized controlled trial. BMC Musculoskelet Disord. 2012;13:16.
1. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium – 2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21(10 suppl):S1-S133.
2. Bell JE, Leung BC, Spratt KF, et al. Trends and variation in incidence, surgical treatment, and repeat surgery of proximal humeral fractures in the elderly. J Bone Joint Surg Am. 2011;93(2):121-131.
3. McLaurin TM. Proximal humerus fractures in the elderly are we operating on too many? Bull Hosp Jt Dis. 2004;62(1-2):24-32.
4. Jain NB, Kuye I, Higgins LD, Warner JJP. Surgeon volume is associated with cost and variation in surgical treatment of proximal humeral fractures. Clin Orthop. 2012;471(2):655-664.
5. Boykin RE, Jawa A, O’Brien T, Higgins LD, Warner JJP. Variability in operative management of proximal humerus fractures. Shoulder Elbow. 2011;3(4):197-201.
6. Petit CJ, Millett PJ, Endres NK, Diller D, Harris MB, Warner JJP. Management of proximal humeral fractures: surgeons don’t agree. J Shoulder Elbow Surg. 2010;19(3):446-451.
7. Okike K, Lee OC, Makanji H, Harris MB, Vrahas MS. Factors associated with the decision for operative versus non-operative treatment of displaced proximal humerus fractures in the elderly. Injury. 2013;44(4):448-455.
8. Kodali P, Jones MH, Polster J, Miniaci A, Fening SD. Accuracy of measurement of Hill-Sachs lesions with computed tomography. J Shoulder Elbow Surg. 2011;20(8):1328-1334.
9. Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420-428.
10. Foroohar A, Tosti R, Richmond JM, Gaughan JP, Ilyas AM. Classification and treatment of proximal humerus fractures: inter-observer reliability and agreement across imaging modalities and experience. J Orthop Surg Res. 2011;6:38.
11. Handoll HH, Ollivere BJ. Interventions for treating proximal humeral fractures in adults. Cochrane Database Syst Rev. 2010;(12):CD000434.
12. Boons HW, Goosen JH, van Grinsven S, van Susante JL, van Loon CJ. Hemiarthroplasty for humeral four-part fractures for patients 65 years and older: a randomized controlled trial. Clin Orthop. 2012;470(12):3483-3491.
13. Fjalestad T, Hole MØ, Hovden IAH, Blücher J, Strømsøe K. Surgical treatment with an angular stable plate for complex displaced proximal humeral fractures in elderly patients: a randomized controlled trial. J Orthop Trauma. 2012;26(2):98-106.
14. Fjalestad T, Hole MØ, Jørgensen JJ, Strømsøe K, Kristiansen IS. Health and cost consequences of surgical versus conservative treatment for a comminuted proximal humeral fracture in elderly patients. Injury. 2010;41(6):599-605.
15. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Internal fixation versus nonoperative treatment of displaced 3-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(5):747-755.
16. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Hemiarthroplasty versus nonoperative treatment of displaced 4-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(7):1025-1033.
17. Agudelo J, Schürmann M, Stahel P, et al. Analysis of efficacy and failure in proximal humerus fractures treated with locking plates. J Orthop Trauma. 2007;21(10):676-681.
18. Brorson S, Hróbjartsson A. Training improves agreement among doctors using the Neer system for proximal humeral fractures in a systematic review. J Clin Epidemiol. 2008;61(1):7-16.
19. Brorson S, Olsen BS, Frich LH, et al. Surgeons agree more on treatment recommendations than on classification of proximal humeral fractures. BMC Musculoskelet Disord. 2012;13:114.
20. Handoll H, Brealey S, Rangan A, et al. Protocol for the ProFHER (PROximal Fracture of the Humerus: Evaluation by Randomisation) trial: a pragmatic multi-centre randomised controlled trial of surgical versus non-surgical treatment for proximal fracture of the humerus in adults. BMC Musculoskelet Disord. 2009;10:140.
21. Den Hartog D, Van Lieshout EMM, Tuinebreijer WE, et al. Primary hemiarthroplasty versus conservative treatment for comminuted fractures of the proximal humerus in the elderly (ProCon): a multicenter randomized controlled trial. BMC Musculoskelet Disord. 2010;11:97.
22. Verbeek PA, van den Akker-Scheek I, Wendt KW, Diercks RL. Hemiarthroplasty versus angle-stable locking compression plate osteosynthesis in the treatment of three- and four-part fractures of the proximal humerus in the elderly: design of a randomized controlled trial. BMC Musculoskelet Disord. 2012;13:16.
The Effect of Humeral Rotation on Elbow Range-of-Motion Measurements
Elbow motion is crucial for activities of daily living and full function of the upper extremity.1 Measuring the elbow flexion arc accurately and consistently is an important part of the physical examination of patients with elbow pathology. Orthopedic surgeons rely on these measurements to follow patients over time, and they often base their treatment decisions on the range and progression/regression of motion arc.
In the clinical setting, elbow range of motion (ROM) is commonly measured with a handheld goniometer.2,3 The literature also suggests that goniometric measurements are highly reliable in the clinical setting and that intrarater reliability of elbow ROM measurements is high.2-4 Despite the routine use and clinical importance of flexion arc assessment, there is no universal recommendation regarding optimal measurement position. Textbooks and journal articles commonly do not specify arm position at time of elbow ROM measurements,5-8 and a literature review found no studies directly addressing this issue.
From a biomechanical standpoint, humeral rotation is often affected by forearm pronosupination position. Although forearm pronosupination is a product of the motion at the radioulnar joints, forearm position during elbow flexion arc measurement can influence the relationship of the distal humeral intercondylar axis to the plane of measurement. Full forearm supination rotates the distal humeral intercondylar axis externally to a position parallel to the floor and in line with the plane of measurement. Humeral rotation with the forearm in neutral pronosupination places the humeral condyles internally rotated relative to the floor. Therefore, for the purposes of this study, we defined full humeral external rotation and true plane of ulnohumeral motion as full forearm supination, and relative humeral and ulnohumeral joint internal rotation as neutral pronosupination.
Because of the potential for elbow ROM measurement changes caused by differences in the motion plane in which measurements are taken, some have advocated taking flexion arc measurements with the arm in full supination to allow measurements to be taken in the true plane of motion. We hypothesized that elbow flexion arc measurements taken with the forearm in neutral rotation would underestimate the extent of elbow flexion contractures compared with measurements taken in full supination.
Materials and Methods
This study received institutional review board approval. Eighty-four patients who presented with elbow dysfunction to a single shoulder and elbow orthopedic surgeon enrolled in the study. Skeletally immature patients and patients with a fracture or other disorder that prohibited elbow ROM were excluded. A standard goniometer was used to measure elbow flexion and extension with the humerus in 2 positions: full external rotation and neutral rotation.
All goniometer measurements were made by the same surgeon (to eliminate interobserver reliability error) using a standardized technique with the patient sitting upright. The goniometer was positioned laterally with its center of rotation over the lateral epicondyle, aligned proximally with the humeral head and distally with the center of the wrist. Measurements were obtained sequentially with the hand in both positions. For external rotation measurements, the patient’s arm was fully supinated to bring the humeral condyles parallel to the floor. For neutral positioning, the patient’s arm was placed in the “thumb-up” position with the hand perpendicular to the horizontal axis of the floor (Figures 1A–1C).
Data collected included demographics, diagnosis, hand dominance, affected side, and elbow ROM measurements with the hand in the 2 positions. These data were compiled and analyzed for all patients and then stratified into 3 groups by extent of elbow flexion contracture in the supinated position (group 1, hyperextension; group 2, 0°-29° elbow extension; group 3, ≥30° flexion contracture).
Statistically, paired t tests were used to identify differences between the 2 elbow ROM measurement methods. P < .05 was considered significant.
Results
Eighty-four (44 male, 40 female) consecutive patients (85 elbows) met the inclusion and exclusion criteria. Mean age was 51 years (range, 19-84 years). Seventy-six patients were right-handed, 7 were left-handed, and dominance was unknown in 1 patient. The right elbow was affected in 45 patients, the left in 38, and both in 1 patient. There were 25 different diagnoses, the most common of which was lateral epicondylitis; 7 patients had multiple disorders (Table).
The first set of data, elbow ROM measurements, was taken with all 84 patients analyzed as a single group. In neutral humeral rotation, mean elbow extension was 14° (range, 10°-72°), and mean elbow flexion was 134° (range, 72°-145°). In external rotation, mean elbow extension was 20° (range, 12°-87°), and mean elbow flexion was 134° (range, 72°-145°). For the group, mean absolute difference in elbow extension was 8° (range, 0°-30°; P < .0001); there was no difference between external rotation and neutral rotation in flexion (Figure 2).
The data were reanalyzed after being stratified into 3 groups based on extent of elbow flexion contracture measured in supination.
The 9 elbows in group 1 (hyperextension) had mean extension of –2° (range, 10°-2°) and mean flexion of 141° (range, 130°-145°) in the neutral position. In external rotation, mean extension was –9° (range, –12° to –1°), and mean flexion was 141° (range, 130°-145°). When the 2 measurement positions were compared, group 1 had mean elbow ROM differences of –6° (range, –14° to 0°; P = .0033) for elbow extension and 0° for elbow flexion (Figure 3A).
The 50 elbows in group 2 (0°-29° flexion contracture) had mean extension of 7° (range, 0°-20°) and mean flexion of 138° (range, 100°-145°) in the neutral position. In external rotation, mean extension was 13° (range, 0°-26°), and mean flexion was 138° (range, 100°-145°). Mean difference between neutral and external rotation measurements was 6° (range, 0°-20°; P < .0001) in extension and 0° in flexion (Figure 3B).
The 26 elbows in group 3 (≥30° flexion contracture) had mean extension of 33° (range, 0°-72°) and mean flexion of 124° (range, 72°-145°) in the neutral position. In external rotation, mean extension was 45° (range, 30°-87°), and mean flexion was 124° (range, 72°-145°). Mean difference between neutral and external rotation measurements was 12° (range, 0°-30°; P < .0001) in extension and 0° in flexion (Figure 3C).
Discussion
Elbow flexion arc measurements are crucial for patient outcomes and activities of daily living. Commonly cited as functional ROM, the 30°-to-130° flexion arc often is used to guide clinical decisions in patients with elbow disorders.1 However, our data indicate that humeral position can alter elbow ROM measurements. Specifically, because of neutral forearm pronosupination, measurements made with the humerus in neutral rotation underestimate both the extent of elbow hyperextension and the degree of flexion contracture (Figures 4A, 4B). The more severe the flexion contracture, the more values are altered by measurements taken in this position. The same does not apply for elbow flexion measurements, as varying humeral rotation did not significantly affect those values.
Our results indicate that patients evaluated with the arm in neutral humeral rotation had flexion contractures underestimated by a mean of 8°, while there was a negligible difference in flexion measurements. Stratifying our data into 3 groups, we found that neutral humeral rotation kept elbow extension measurements closer to 0° for patients with both hyperextension and contractures. With increasing severity of flexion contractures in groups 2 and 3, the measurement errors were magnified. The differences in extension measurement values between these 2 groups based on humeral rotation increased more than 4°—an indication that, as flexion contracture severity increases, so does the degree of measurement error when elbow extension is measured with the humerus in neutral rotation rather than external rotation.
Our literature review found no studies on ROM value differences based on position of humeral rotation. Most texts, in their descriptions of elbow ROM and biomechanics, make no reference to position of pronosupination at time of flexion arc measurement.5-8 Although many elbow authorities recommend taking elbow ROM measurements in full external rotation, we found no corroborating evidence.
Other investigators have evaluated the reliability of goniometer measurements.2,3 Rothstein and colleagues3 concluded that elbow and knee goniometric measurements are highly reliable in the clinical setting when taken by the same person. In particular, intratester reliability for elbow extension measurements was high. Armstrong and colleagues2 specifically examined intratester, intertester, and interdevice reliability and found that intratester reliability was much higher than intertester reliability for universal goniometry. In our study, all patients were measured with the same technique by the same orthopedic surgeon to eliminate any intertester reliability error. Armstrong and colleagues2 also found that intratester changes vis-a-vis extension measurements are meaningful when goniometric differences are more than 7°. In our study, the difference in extension measurements between the 2 humeral positions averaged 8° overall and 12° in group 3. This suggests that the data reported here reflect a true difference dependent on humeral rotation and are not a result of goniometer intratester variability.
Other studies have examined measurement devices other than the standard universal goniometer. Cleffken and colleagues4 found that the electronic digital inclinometer was reliable for elbow ROM measurements. Blonna and colleagues9 used digital photography–based goniometry to measure patient outcomes without doctor–patient contact at tertiary-care centers and found it to be more accurate and reliable than clinical goniometry in measuring elbow flexion and extension. Chapleau and colleagues10 compared the validity of goniometric elbow measurements in radiographic methods and concluded that the maximal error of goniometric measurements in extension was 10.3°. However, they also found high intraclass correlation coefficients for goniometric measurements. With the accepted clinical reliability of universal goniometry,2-4,10 we believe it to be the best clinical tool for this study because of its availability, minimal cost, and ease of use.
In the clinical setting, elbow flexion arc measurements are a major factor in treatment decisions and often dictate whether to proceed with operative interventions such as capsular release. In addition, ROM measurements are crucial in determining the success of treatments and the progression of disease. Erroneous elbow extension measurements can have significant consequences if they falsely indicate functional ROM when taken in neutral position. This is particularly true for patients with elbow flexion contractures of more than 30°, in whom differences in humeral rotation resulted in about 12° of variance between measured values. For instance, a patient with a true 40° flexion contracture in the externally rotated position could be determined to have functional ROM based on measurements made in the neutral position.
Limitations of this study include those involving goniometer reliability and intraobserver variability (already described) and the validity of goniometer measurements compared with radiographic measurements.
Conclusion
Because elbow goniometer extension measurements taken in neutral humeral rotation underestimate both the degree of elbow hyperextension and the degree of elbow flexion contracture, we recommend taking elbow flexion arc measurements in the true plane of motion, with the humerus externally rotated by fully supinating the forearm, such that the distal humeral condyles are parallel to the floor.
1. Morrey BF, Askew LJ, Chao EY. A biomechanical study of normal functional elbow motion. J Bone Joint Surg Am. 1981;63(6):872-877.
2. Armstrong AD, MacDermid JC, Chinchalkar S, Stevens RS, King GJ. Reliability of range-of-motion measurement in the elbow and forearm. J Shoulder Elbow Surg. 1998;7(6):573-580.
3. Rothstein JM, Miller PJ, Roettger RF. Goniometric reliability in a clinical setting. Elbow and knee measurements. Phys Ther. 1983;63(10):1611-1615.
4. Cleffken B, van Breukelen G, van Mameren H, Brink P, Olde Damink S. Test–retest reproducibility of elbow goniometric measurements in a rigid double-blinded protocol: intervals for distinguishing between measurement error and clinical change. J Shoulder Elbow Surg. 2007;16(6):788-794.
5. Hoppenfeld S. Physical Examination of the Spine and Extremities. Englewood Cliffs, NJ: Prentice-Hall; 1976.
6. Miller RM 3rd, Azar FM, Throckmorton TW. Shoulder and elbow injuries. In: Canale S, Beaty J, eds. Campbell’s Operative Orthopaedics. 12th ed. Philadelphia, PA: Mosby Elsevier; 2013:2241-2253.
7. Ring D. Elbow fractures and dislocations. In: Bucholz R, Heckman J, Court-Brown C, eds. Rockwood and Green’s Fractures in Adults. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006:901-991.
8. Katolik LI, Cohen MS. Lateral columnar release for extracapsular elbow contracture. In: Wiesel S, ed. Operative Techniques in Orthopaedic Surgery. Philadelphia, PA: Lippincott Williams & Wilkins; 2011:3406-3407.
9. Blonna D, Zarkadas PC, Fitzsimmons JS, O’Driscoll SW. Validation of a photography-based goniometry method for measuring joint range of motion. J Shoulder Elbow Surg. 2012;21(1):29-35.
10. Chapleau J, Canet F, Petit Y, Laflamme G, Rouleau D. Validity of elbow goniometer measurements. Comparative study with a radiographic method. Clin Orthop. 2001;(469):3134-3140.
Elbow motion is crucial for activities of daily living and full function of the upper extremity.1 Measuring the elbow flexion arc accurately and consistently is an important part of the physical examination of patients with elbow pathology. Orthopedic surgeons rely on these measurements to follow patients over time, and they often base their treatment decisions on the range and progression/regression of motion arc.
In the clinical setting, elbow range of motion (ROM) is commonly measured with a handheld goniometer.2,3 The literature also suggests that goniometric measurements are highly reliable in the clinical setting and that intrarater reliability of elbow ROM measurements is high.2-4 Despite the routine use and clinical importance of flexion arc assessment, there is no universal recommendation regarding optimal measurement position. Textbooks and journal articles commonly do not specify arm position at time of elbow ROM measurements,5-8 and a literature review found no studies directly addressing this issue.
From a biomechanical standpoint, humeral rotation is often affected by forearm pronosupination position. Although forearm pronosupination is a product of the motion at the radioulnar joints, forearm position during elbow flexion arc measurement can influence the relationship of the distal humeral intercondylar axis to the plane of measurement. Full forearm supination rotates the distal humeral intercondylar axis externally to a position parallel to the floor and in line with the plane of measurement. Humeral rotation with the forearm in neutral pronosupination places the humeral condyles internally rotated relative to the floor. Therefore, for the purposes of this study, we defined full humeral external rotation and true plane of ulnohumeral motion as full forearm supination, and relative humeral and ulnohumeral joint internal rotation as neutral pronosupination.
Because of the potential for elbow ROM measurement changes caused by differences in the motion plane in which measurements are taken, some have advocated taking flexion arc measurements with the arm in full supination to allow measurements to be taken in the true plane of motion. We hypothesized that elbow flexion arc measurements taken with the forearm in neutral rotation would underestimate the extent of elbow flexion contractures compared with measurements taken in full supination.
Materials and Methods
This study received institutional review board approval. Eighty-four patients who presented with elbow dysfunction to a single shoulder and elbow orthopedic surgeon enrolled in the study. Skeletally immature patients and patients with a fracture or other disorder that prohibited elbow ROM were excluded. A standard goniometer was used to measure elbow flexion and extension with the humerus in 2 positions: full external rotation and neutral rotation.
All goniometer measurements were made by the same surgeon (to eliminate interobserver reliability error) using a standardized technique with the patient sitting upright. The goniometer was positioned laterally with its center of rotation over the lateral epicondyle, aligned proximally with the humeral head and distally with the center of the wrist. Measurements were obtained sequentially with the hand in both positions. For external rotation measurements, the patient’s arm was fully supinated to bring the humeral condyles parallel to the floor. For neutral positioning, the patient’s arm was placed in the “thumb-up” position with the hand perpendicular to the horizontal axis of the floor (Figures 1A–1C).
Data collected included demographics, diagnosis, hand dominance, affected side, and elbow ROM measurements with the hand in the 2 positions. These data were compiled and analyzed for all patients and then stratified into 3 groups by extent of elbow flexion contracture in the supinated position (group 1, hyperextension; group 2, 0°-29° elbow extension; group 3, ≥30° flexion contracture).
Statistically, paired t tests were used to identify differences between the 2 elbow ROM measurement methods. P < .05 was considered significant.
Results
Eighty-four (44 male, 40 female) consecutive patients (85 elbows) met the inclusion and exclusion criteria. Mean age was 51 years (range, 19-84 years). Seventy-six patients were right-handed, 7 were left-handed, and dominance was unknown in 1 patient. The right elbow was affected in 45 patients, the left in 38, and both in 1 patient. There were 25 different diagnoses, the most common of which was lateral epicondylitis; 7 patients had multiple disorders (Table).
The first set of data, elbow ROM measurements, was taken with all 84 patients analyzed as a single group. In neutral humeral rotation, mean elbow extension was 14° (range, 10°-72°), and mean elbow flexion was 134° (range, 72°-145°). In external rotation, mean elbow extension was 20° (range, 12°-87°), and mean elbow flexion was 134° (range, 72°-145°). For the group, mean absolute difference in elbow extension was 8° (range, 0°-30°; P < .0001); there was no difference between external rotation and neutral rotation in flexion (Figure 2).
The data were reanalyzed after being stratified into 3 groups based on extent of elbow flexion contracture measured in supination.
The 9 elbows in group 1 (hyperextension) had mean extension of –2° (range, 10°-2°) and mean flexion of 141° (range, 130°-145°) in the neutral position. In external rotation, mean extension was –9° (range, –12° to –1°), and mean flexion was 141° (range, 130°-145°). When the 2 measurement positions were compared, group 1 had mean elbow ROM differences of –6° (range, –14° to 0°; P = .0033) for elbow extension and 0° for elbow flexion (Figure 3A).
The 50 elbows in group 2 (0°-29° flexion contracture) had mean extension of 7° (range, 0°-20°) and mean flexion of 138° (range, 100°-145°) in the neutral position. In external rotation, mean extension was 13° (range, 0°-26°), and mean flexion was 138° (range, 100°-145°). Mean difference between neutral and external rotation measurements was 6° (range, 0°-20°; P < .0001) in extension and 0° in flexion (Figure 3B).
The 26 elbows in group 3 (≥30° flexion contracture) had mean extension of 33° (range, 0°-72°) and mean flexion of 124° (range, 72°-145°) in the neutral position. In external rotation, mean extension was 45° (range, 30°-87°), and mean flexion was 124° (range, 72°-145°). Mean difference between neutral and external rotation measurements was 12° (range, 0°-30°; P < .0001) in extension and 0° in flexion (Figure 3C).
Discussion
Elbow flexion arc measurements are crucial for patient outcomes and activities of daily living. Commonly cited as functional ROM, the 30°-to-130° flexion arc often is used to guide clinical decisions in patients with elbow disorders.1 However, our data indicate that humeral position can alter elbow ROM measurements. Specifically, because of neutral forearm pronosupination, measurements made with the humerus in neutral rotation underestimate both the extent of elbow hyperextension and the degree of flexion contracture (Figures 4A, 4B). The more severe the flexion contracture, the more values are altered by measurements taken in this position. The same does not apply for elbow flexion measurements, as varying humeral rotation did not significantly affect those values.
Our results indicate that patients evaluated with the arm in neutral humeral rotation had flexion contractures underestimated by a mean of 8°, while there was a negligible difference in flexion measurements. Stratifying our data into 3 groups, we found that neutral humeral rotation kept elbow extension measurements closer to 0° for patients with both hyperextension and contractures. With increasing severity of flexion contractures in groups 2 and 3, the measurement errors were magnified. The differences in extension measurement values between these 2 groups based on humeral rotation increased more than 4°—an indication that, as flexion contracture severity increases, so does the degree of measurement error when elbow extension is measured with the humerus in neutral rotation rather than external rotation.
Our literature review found no studies on ROM value differences based on position of humeral rotation. Most texts, in their descriptions of elbow ROM and biomechanics, make no reference to position of pronosupination at time of flexion arc measurement.5-8 Although many elbow authorities recommend taking elbow ROM measurements in full external rotation, we found no corroborating evidence.
Other investigators have evaluated the reliability of goniometer measurements.2,3 Rothstein and colleagues3 concluded that elbow and knee goniometric measurements are highly reliable in the clinical setting when taken by the same person. In particular, intratester reliability for elbow extension measurements was high. Armstrong and colleagues2 specifically examined intratester, intertester, and interdevice reliability and found that intratester reliability was much higher than intertester reliability for universal goniometry. In our study, all patients were measured with the same technique by the same orthopedic surgeon to eliminate any intertester reliability error. Armstrong and colleagues2 also found that intratester changes vis-a-vis extension measurements are meaningful when goniometric differences are more than 7°. In our study, the difference in extension measurements between the 2 humeral positions averaged 8° overall and 12° in group 3. This suggests that the data reported here reflect a true difference dependent on humeral rotation and are not a result of goniometer intratester variability.
Other studies have examined measurement devices other than the standard universal goniometer. Cleffken and colleagues4 found that the electronic digital inclinometer was reliable for elbow ROM measurements. Blonna and colleagues9 used digital photography–based goniometry to measure patient outcomes without doctor–patient contact at tertiary-care centers and found it to be more accurate and reliable than clinical goniometry in measuring elbow flexion and extension. Chapleau and colleagues10 compared the validity of goniometric elbow measurements in radiographic methods and concluded that the maximal error of goniometric measurements in extension was 10.3°. However, they also found high intraclass correlation coefficients for goniometric measurements. With the accepted clinical reliability of universal goniometry,2-4,10 we believe it to be the best clinical tool for this study because of its availability, minimal cost, and ease of use.
In the clinical setting, elbow flexion arc measurements are a major factor in treatment decisions and often dictate whether to proceed with operative interventions such as capsular release. In addition, ROM measurements are crucial in determining the success of treatments and the progression of disease. Erroneous elbow extension measurements can have significant consequences if they falsely indicate functional ROM when taken in neutral position. This is particularly true for patients with elbow flexion contractures of more than 30°, in whom differences in humeral rotation resulted in about 12° of variance between measured values. For instance, a patient with a true 40° flexion contracture in the externally rotated position could be determined to have functional ROM based on measurements made in the neutral position.
Limitations of this study include those involving goniometer reliability and intraobserver variability (already described) and the validity of goniometer measurements compared with radiographic measurements.
Conclusion
Because elbow goniometer extension measurements taken in neutral humeral rotation underestimate both the degree of elbow hyperextension and the degree of elbow flexion contracture, we recommend taking elbow flexion arc measurements in the true plane of motion, with the humerus externally rotated by fully supinating the forearm, such that the distal humeral condyles are parallel to the floor.
Elbow motion is crucial for activities of daily living and full function of the upper extremity.1 Measuring the elbow flexion arc accurately and consistently is an important part of the physical examination of patients with elbow pathology. Orthopedic surgeons rely on these measurements to follow patients over time, and they often base their treatment decisions on the range and progression/regression of motion arc.
In the clinical setting, elbow range of motion (ROM) is commonly measured with a handheld goniometer.2,3 The literature also suggests that goniometric measurements are highly reliable in the clinical setting and that intrarater reliability of elbow ROM measurements is high.2-4 Despite the routine use and clinical importance of flexion arc assessment, there is no universal recommendation regarding optimal measurement position. Textbooks and journal articles commonly do not specify arm position at time of elbow ROM measurements,5-8 and a literature review found no studies directly addressing this issue.
From a biomechanical standpoint, humeral rotation is often affected by forearm pronosupination position. Although forearm pronosupination is a product of the motion at the radioulnar joints, forearm position during elbow flexion arc measurement can influence the relationship of the distal humeral intercondylar axis to the plane of measurement. Full forearm supination rotates the distal humeral intercondylar axis externally to a position parallel to the floor and in line with the plane of measurement. Humeral rotation with the forearm in neutral pronosupination places the humeral condyles internally rotated relative to the floor. Therefore, for the purposes of this study, we defined full humeral external rotation and true plane of ulnohumeral motion as full forearm supination, and relative humeral and ulnohumeral joint internal rotation as neutral pronosupination.
Because of the potential for elbow ROM measurement changes caused by differences in the motion plane in which measurements are taken, some have advocated taking flexion arc measurements with the arm in full supination to allow measurements to be taken in the true plane of motion. We hypothesized that elbow flexion arc measurements taken with the forearm in neutral rotation would underestimate the extent of elbow flexion contractures compared with measurements taken in full supination.
Materials and Methods
This study received institutional review board approval. Eighty-four patients who presented with elbow dysfunction to a single shoulder and elbow orthopedic surgeon enrolled in the study. Skeletally immature patients and patients with a fracture or other disorder that prohibited elbow ROM were excluded. A standard goniometer was used to measure elbow flexion and extension with the humerus in 2 positions: full external rotation and neutral rotation.
All goniometer measurements were made by the same surgeon (to eliminate interobserver reliability error) using a standardized technique with the patient sitting upright. The goniometer was positioned laterally with its center of rotation over the lateral epicondyle, aligned proximally with the humeral head and distally with the center of the wrist. Measurements were obtained sequentially with the hand in both positions. For external rotation measurements, the patient’s arm was fully supinated to bring the humeral condyles parallel to the floor. For neutral positioning, the patient’s arm was placed in the “thumb-up” position with the hand perpendicular to the horizontal axis of the floor (Figures 1A–1C).
Data collected included demographics, diagnosis, hand dominance, affected side, and elbow ROM measurements with the hand in the 2 positions. These data were compiled and analyzed for all patients and then stratified into 3 groups by extent of elbow flexion contracture in the supinated position (group 1, hyperextension; group 2, 0°-29° elbow extension; group 3, ≥30° flexion contracture).
Statistically, paired t tests were used to identify differences between the 2 elbow ROM measurement methods. P < .05 was considered significant.
Results
Eighty-four (44 male, 40 female) consecutive patients (85 elbows) met the inclusion and exclusion criteria. Mean age was 51 years (range, 19-84 years). Seventy-six patients were right-handed, 7 were left-handed, and dominance was unknown in 1 patient. The right elbow was affected in 45 patients, the left in 38, and both in 1 patient. There were 25 different diagnoses, the most common of which was lateral epicondylitis; 7 patients had multiple disorders (Table).
The first set of data, elbow ROM measurements, was taken with all 84 patients analyzed as a single group. In neutral humeral rotation, mean elbow extension was 14° (range, 10°-72°), and mean elbow flexion was 134° (range, 72°-145°). In external rotation, mean elbow extension was 20° (range, 12°-87°), and mean elbow flexion was 134° (range, 72°-145°). For the group, mean absolute difference in elbow extension was 8° (range, 0°-30°; P < .0001); there was no difference between external rotation and neutral rotation in flexion (Figure 2).
The data were reanalyzed after being stratified into 3 groups based on extent of elbow flexion contracture measured in supination.
The 9 elbows in group 1 (hyperextension) had mean extension of –2° (range, 10°-2°) and mean flexion of 141° (range, 130°-145°) in the neutral position. In external rotation, mean extension was –9° (range, –12° to –1°), and mean flexion was 141° (range, 130°-145°). When the 2 measurement positions were compared, group 1 had mean elbow ROM differences of –6° (range, –14° to 0°; P = .0033) for elbow extension and 0° for elbow flexion (Figure 3A).
The 50 elbows in group 2 (0°-29° flexion contracture) had mean extension of 7° (range, 0°-20°) and mean flexion of 138° (range, 100°-145°) in the neutral position. In external rotation, mean extension was 13° (range, 0°-26°), and mean flexion was 138° (range, 100°-145°). Mean difference between neutral and external rotation measurements was 6° (range, 0°-20°; P < .0001) in extension and 0° in flexion (Figure 3B).
The 26 elbows in group 3 (≥30° flexion contracture) had mean extension of 33° (range, 0°-72°) and mean flexion of 124° (range, 72°-145°) in the neutral position. In external rotation, mean extension was 45° (range, 30°-87°), and mean flexion was 124° (range, 72°-145°). Mean difference between neutral and external rotation measurements was 12° (range, 0°-30°; P < .0001) in extension and 0° in flexion (Figure 3C).
Discussion
Elbow flexion arc measurements are crucial for patient outcomes and activities of daily living. Commonly cited as functional ROM, the 30°-to-130° flexion arc often is used to guide clinical decisions in patients with elbow disorders.1 However, our data indicate that humeral position can alter elbow ROM measurements. Specifically, because of neutral forearm pronosupination, measurements made with the humerus in neutral rotation underestimate both the extent of elbow hyperextension and the degree of flexion contracture (Figures 4A, 4B). The more severe the flexion contracture, the more values are altered by measurements taken in this position. The same does not apply for elbow flexion measurements, as varying humeral rotation did not significantly affect those values.
Our results indicate that patients evaluated with the arm in neutral humeral rotation had flexion contractures underestimated by a mean of 8°, while there was a negligible difference in flexion measurements. Stratifying our data into 3 groups, we found that neutral humeral rotation kept elbow extension measurements closer to 0° for patients with both hyperextension and contractures. With increasing severity of flexion contractures in groups 2 and 3, the measurement errors were magnified. The differences in extension measurement values between these 2 groups based on humeral rotation increased more than 4°—an indication that, as flexion contracture severity increases, so does the degree of measurement error when elbow extension is measured with the humerus in neutral rotation rather than external rotation.
Our literature review found no studies on ROM value differences based on position of humeral rotation. Most texts, in their descriptions of elbow ROM and biomechanics, make no reference to position of pronosupination at time of flexion arc measurement.5-8 Although many elbow authorities recommend taking elbow ROM measurements in full external rotation, we found no corroborating evidence.
Other investigators have evaluated the reliability of goniometer measurements.2,3 Rothstein and colleagues3 concluded that elbow and knee goniometric measurements are highly reliable in the clinical setting when taken by the same person. In particular, intratester reliability for elbow extension measurements was high. Armstrong and colleagues2 specifically examined intratester, intertester, and interdevice reliability and found that intratester reliability was much higher than intertester reliability for universal goniometry. In our study, all patients were measured with the same technique by the same orthopedic surgeon to eliminate any intertester reliability error. Armstrong and colleagues2 also found that intratester changes vis-a-vis extension measurements are meaningful when goniometric differences are more than 7°. In our study, the difference in extension measurements between the 2 humeral positions averaged 8° overall and 12° in group 3. This suggests that the data reported here reflect a true difference dependent on humeral rotation and are not a result of goniometer intratester variability.
Other studies have examined measurement devices other than the standard universal goniometer. Cleffken and colleagues4 found that the electronic digital inclinometer was reliable for elbow ROM measurements. Blonna and colleagues9 used digital photography–based goniometry to measure patient outcomes without doctor–patient contact at tertiary-care centers and found it to be more accurate and reliable than clinical goniometry in measuring elbow flexion and extension. Chapleau and colleagues10 compared the validity of goniometric elbow measurements in radiographic methods and concluded that the maximal error of goniometric measurements in extension was 10.3°. However, they also found high intraclass correlation coefficients for goniometric measurements. With the accepted clinical reliability of universal goniometry,2-4,10 we believe it to be the best clinical tool for this study because of its availability, minimal cost, and ease of use.
In the clinical setting, elbow flexion arc measurements are a major factor in treatment decisions and often dictate whether to proceed with operative interventions such as capsular release. In addition, ROM measurements are crucial in determining the success of treatments and the progression of disease. Erroneous elbow extension measurements can have significant consequences if they falsely indicate functional ROM when taken in neutral position. This is particularly true for patients with elbow flexion contractures of more than 30°, in whom differences in humeral rotation resulted in about 12° of variance between measured values. For instance, a patient with a true 40° flexion contracture in the externally rotated position could be determined to have functional ROM based on measurements made in the neutral position.
Limitations of this study include those involving goniometer reliability and intraobserver variability (already described) and the validity of goniometer measurements compared with radiographic measurements.
Conclusion
Because elbow goniometer extension measurements taken in neutral humeral rotation underestimate both the degree of elbow hyperextension and the degree of elbow flexion contracture, we recommend taking elbow flexion arc measurements in the true plane of motion, with the humerus externally rotated by fully supinating the forearm, such that the distal humeral condyles are parallel to the floor.
1. Morrey BF, Askew LJ, Chao EY. A biomechanical study of normal functional elbow motion. J Bone Joint Surg Am. 1981;63(6):872-877.
2. Armstrong AD, MacDermid JC, Chinchalkar S, Stevens RS, King GJ. Reliability of range-of-motion measurement in the elbow and forearm. J Shoulder Elbow Surg. 1998;7(6):573-580.
3. Rothstein JM, Miller PJ, Roettger RF. Goniometric reliability in a clinical setting. Elbow and knee measurements. Phys Ther. 1983;63(10):1611-1615.
4. Cleffken B, van Breukelen G, van Mameren H, Brink P, Olde Damink S. Test–retest reproducibility of elbow goniometric measurements in a rigid double-blinded protocol: intervals for distinguishing between measurement error and clinical change. J Shoulder Elbow Surg. 2007;16(6):788-794.
5. Hoppenfeld S. Physical Examination of the Spine and Extremities. Englewood Cliffs, NJ: Prentice-Hall; 1976.
6. Miller RM 3rd, Azar FM, Throckmorton TW. Shoulder and elbow injuries. In: Canale S, Beaty J, eds. Campbell’s Operative Orthopaedics. 12th ed. Philadelphia, PA: Mosby Elsevier; 2013:2241-2253.
7. Ring D. Elbow fractures and dislocations. In: Bucholz R, Heckman J, Court-Brown C, eds. Rockwood and Green’s Fractures in Adults. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006:901-991.
8. Katolik LI, Cohen MS. Lateral columnar release for extracapsular elbow contracture. In: Wiesel S, ed. Operative Techniques in Orthopaedic Surgery. Philadelphia, PA: Lippincott Williams & Wilkins; 2011:3406-3407.
9. Blonna D, Zarkadas PC, Fitzsimmons JS, O’Driscoll SW. Validation of a photography-based goniometry method for measuring joint range of motion. J Shoulder Elbow Surg. 2012;21(1):29-35.
10. Chapleau J, Canet F, Petit Y, Laflamme G, Rouleau D. Validity of elbow goniometer measurements. Comparative study with a radiographic method. Clin Orthop. 2001;(469):3134-3140.
1. Morrey BF, Askew LJ, Chao EY. A biomechanical study of normal functional elbow motion. J Bone Joint Surg Am. 1981;63(6):872-877.
2. Armstrong AD, MacDermid JC, Chinchalkar S, Stevens RS, King GJ. Reliability of range-of-motion measurement in the elbow and forearm. J Shoulder Elbow Surg. 1998;7(6):573-580.
3. Rothstein JM, Miller PJ, Roettger RF. Goniometric reliability in a clinical setting. Elbow and knee measurements. Phys Ther. 1983;63(10):1611-1615.
4. Cleffken B, van Breukelen G, van Mameren H, Brink P, Olde Damink S. Test–retest reproducibility of elbow goniometric measurements in a rigid double-blinded protocol: intervals for distinguishing between measurement error and clinical change. J Shoulder Elbow Surg. 2007;16(6):788-794.
5. Hoppenfeld S. Physical Examination of the Spine and Extremities. Englewood Cliffs, NJ: Prentice-Hall; 1976.
6. Miller RM 3rd, Azar FM, Throckmorton TW. Shoulder and elbow injuries. In: Canale S, Beaty J, eds. Campbell’s Operative Orthopaedics. 12th ed. Philadelphia, PA: Mosby Elsevier; 2013:2241-2253.
7. Ring D. Elbow fractures and dislocations. In: Bucholz R, Heckman J, Court-Brown C, eds. Rockwood and Green’s Fractures in Adults. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006:901-991.
8. Katolik LI, Cohen MS. Lateral columnar release for extracapsular elbow contracture. In: Wiesel S, ed. Operative Techniques in Orthopaedic Surgery. Philadelphia, PA: Lippincott Williams & Wilkins; 2011:3406-3407.
9. Blonna D, Zarkadas PC, Fitzsimmons JS, O’Driscoll SW. Validation of a photography-based goniometry method for measuring joint range of motion. J Shoulder Elbow Surg. 2012;21(1):29-35.
10. Chapleau J, Canet F, Petit Y, Laflamme G, Rouleau D. Validity of elbow goniometer measurements. Comparative study with a radiographic method. Clin Orthop. 2001;(469):3134-3140.
Sports Activity After Reverse Total Shoulder Arthroplasty With Minimum 2-Year Follow-Up
The treatment of patients with severe shoulder pain and disability combined with a nonfunctional rotator cuff was a clinical challenge until the development of the reverse total shoulder arthroplasty (RTSA).1-3 Massive rotator cuff tears can leave patients with a pseudoparalytic upper extremity and may result in advanced arthritis of the joint because of altered mechanical and nutritional factors.4 In this setting, simply replacing the arthritic joint with standard total shoulder arthroplasty (TSA) is not recommended because it does not address the functional deficits, and it has poor long-term outcomes.3,5 RTSA works by changing the center of rotation of the shoulder joint so that the deltoid muscle can be used to elevate the arm.6,7 The 4 rotator cuff muscles are not required for forward elevation or stability of this constrained implant.6,8
Current indications for RTSA are cuff tear arthropathy, complex proximal humerus fractures, and revision from hemiarthroplasty or TSA with rotator cuff dysfunction. Patients with advanced cuff tear arthropathy have minimal forward elevation and pseudoparalysis. Previous studies have shown mean preoperative forward flexion of 55º and mean ASES (American Shoulder and Elbow Surgeons) Standardized Shoulder Assessment Form score of 34.3.9 Thus, minimal overhead activity is possible without RTSA. Advances in the RTSA technique have led to promising results (excellent functional improvement), but there is limited information regarding the activity levels patients can achieve after surgery.7,9-11
We conducted a study of the types of sporting activities in which patients with RTSA could participate. We hypothesized that, relative to historic controls, patients with RTSA could return to low-intensity sporting activities with improvement in motion and ASES scores.
Materials and Methods
After this study received institutional review board approval, patients who had undergone RTSA at our institution between January 1, 2004 and December 31, 2010 were identified by the billing codes used for the procedure. Each patient who had RTSA performed during the study period was included in the study. Charts were then reviewed to extract demographic data, preoperative diagnosis, surgery date, operative side, dominant side, type of implant used, operative complications, and subsequent revisions. A questionnaire (Appendix) was designed and used to assess activity, functional status, pain, and satisfaction levels after RTSA. Patients had to be willing and able to complete this questionnaire in order to be included in the study.
The questionnaire included demographic questions; a list of 42 activities patients could choose from to describe their current activity level, activities they were able to perform before the surgery, and activities they wish they could perform; a list of reasons for any limitations; and questions about overall pain, strength, and satisfaction with the procedure. In addition, there was an open-ended question for activities that may not have been listed. The questionnaire also included a validated method for assessing shoulder range of motion (ROM) at home, where patients rated their overhead motion according to standardized physical landmarks, including the level of the shoulder, chin, eyebrows, top of head, and above head.12-14 Also provided was the ASES Standardized Shoulder Assessment Form, which features a 100-point visual analog scale for pain plus functional ability questions, with higher scores indicating less pain and better function.15,16 The minimal clinical significance in the ASES score is 6.4 points.17,18 Scores were recorded and analyzed. Student t test was used to calculate statistical differences between patients who had primary RTSA performed and patients who underwent revision RTSA.
Study personnel contacted patients by telephone and direct mailing. Patients who could not be reached initially were called at least 4 more times: twice during the weekday, once during the evening, and once on the weekend. Patients who could not be contacted by telephone were then cross-referenced with the Social Security database to see if any were deceased. Response data were tabulated, and patients were stratified into high-, moderate-, and low-intensity activity.
One of the 3 senior authors (Dr. Ahmad, Dr. Bigliani, Dr. Levine) performed the 95 RTSAs: 84 Zimmer (Warsaw, Indiana), 7 DePuy (Warsaw, Indiana), 4 Tornier (Minneapolis, Minnesota). The DePuy and Tornier implants were used when a 30-mm glenoid peg was required (before Zimmer offered this length in its system). The procedure was done with a deltopectoral approach with 20° of retroversion. In revision cases, the same approach was used, the hardware or implants were removed, and the position of the humeral component was determined based on the pectoralis major insertion and the deltoid tension. In 80% of cases, the subscapularis was not repaired; in the other 20%, it was. Whether it was repaired depended on tendon viability and surgeon preference, as subscapularis repair status has been shown not to affect functional outcome.19-21 No combined latissimus transfers were performed. Patients wore a sling the first 4 weeks after surgery (only wrist and elbow motion allowed) and then advanced to active shoulder ROM. Eight weeks after surgery, they began gentle shoulder strengthening.
Results
One hundred nine consecutive patients underwent RTSA at a single institution. Fifteen patients subsequently died, 14 could not be contacted, and 2 declined, leaving 78 patients available for clinical follow-up. Mean follow-up was 4.8 years (range 2-9 years). Mean (SD) age at surgery was 75.3 (7.5) years. Seventy-five percent of the patients were women. Sixty-one percent underwent surgery for cuff tear arthropathy, 31% for revision of previous arthroplasty or internal fixation, 7% for complex fractures, and 1% for tumor. Of the 24 revisions, 15 were for failed hemiarthroplasty, 3 were for failed TSA with rotator cuff dysfunction, 4 were for fracture with failed internal fixation, and 2 were for failed RTSA referred from other institutions. The dominant shoulder was involved 62% of the time. Preoperative active forward shoulder elevation was less than 90° in all patients. There were 10 complications: 2 dislocations that were closed-reduced and remained stable, 1 dislocation that required revision of the liner, 1 aseptic loosening in a patient who has declined revision, 2 dissociated glenosphere baseplates, 2 deep infections that required 2-stage exchanges, 1 deep infection that required a 2-stage exchange that was then complicated by dissociation of the glenosphere baseplate requiring revision, and 1 superficial infection that resolved with oral antibiotics.
After surgery, mean active forward elevation was 140°, mean active external rotation was 48°, and mean active internal rotation was to S1. Mean (SD) postoperative ASES score was 77.5 (23.4). Satisfaction level was high (mean, 8.3/10), and mean pain levels were low: 2.3 out of 10 on the visual analog scale and 44.0 (SD, 11.7) on the ASES pain component. Strength was rated a mean of good. Table 1 lists the clinical data for the primary and revision surgery patients.
Eighteen patients (23.1%) returned to 24 different high-intensity activities, such as hunting, golf, and skiing; 38 patients (48.7%) returned to moderate-intensity activities, such as swimming, bowling, and raking leaves; and 22 patients (28.2%) returned to low-intensity activities, such as riding a stationary bike, playing a musical instrument, and walking (Table 2). Four patients played golf before and after RTSA, but neither of the 2 patients who played tennis before RTSA were able to do so after. Patients reported they engaged in their favorite leisure activity a mean of 4.8 times per week and a mean of 1.5 hours each time.
A medical problem was cited by 58% of patients as the reason for limited activity. These patients reported physical decline resulting from cardiac disease, diabetes, asthma/chronic obstructive pulmonary disease, or arthritis in other joints. Reasons for activity limitation are listed in Table 3. Post-RTSA activities that patients could not do for any reason are listed in Table 4. Activity limitations that patients attributed to the RTSA are listed in Table 5.
The majority of patients (57.7%) reported no change, from before RTSA to after RTSA, in being unable to do certain desired activities (eg, softball, target shooting, horseback riding, running, traveling). Sixteen patients (20.8%) reported being unable to return to an activity (eg, tennis, swimming, baseball, kayaking) they had been able to do before surgery. Most (69%) of those patients reported being unable to return to a moderate- or high-intensity activity after RTSA, but 81.8% were able to return to different moderate- or high-intensity activities.
Revision patients, who reported lower overhead activity levels, constituted 73% of the patients who felt their shoulder mechanically limited their activity, despite the fact that revisions constituted only 25% of the cases overall. Mean active ROM was statistically lower for revision patients than for primary patients (P < .05). Mean ASES score was statistically lower for the revision group (P < .001) and represented a clinically significant difference. Mean pain level was low (3.3) and satisfaction still generally high (7.4), but pain, satisfaction, and strength were about 1 point worse on average in the revision group than in the primary group.
Discussion
In the United States and other countries, RTSA implant survivorship is good.9,22 In this article, we have reported on post-RTSA activity levels, on the significant impact of comorbidities on this group, and on the negative effect of revisions on postoperative activity. Patients in this population reported that concomitant medical problems were the most important factor limiting their post-RTSA activity levels. Understanding and interpreting quality-of-life or functional scores in this elderly group must take into account the impact of comorbidities.23
Patients should have realistic postoperative expectations.24 In this study, some patients engaged in high-intensity overhead activities, such as golf, chopping wood, and shooting. However, the most difficulty was encountered trying to return to activities (eg, tennis, kayaking, archery, combing hair) that required external rotation in abduction.
Patients who had a previous implant (eg, hemiarthroplasty, TSA, failed internal fixation) revised to RTSA had lower activity levels and were 9 times more likely than primary patients to report having a mechanical shoulder limitation affecting their activity. Revision patients also had worse forward elevation, external rotation, pain, and satisfaction.
This study is limited in that it is retrospective. Subsequent prospective studies focused on younger patients who undergo primary RTSA may be useful if indications expand. In addition, subscapularis status and especially infraspinatus status may affect activity levels and could be analyzed in a study. Another limitation is that we did not specifically record detailed preoperative data, though all patients were known to have preoperative forward elevation of less than 90°.
In general, the primary measure of success for RTSA has been pain relief. Some studies have also reported on strength and ROM.2,20,25,26 A recent study using similar methodology demonstrated comparable ROM and low pain after RTSA, though revisions were not included in that study.26 In contrast to the present study, no patient in that study was able to play tennis or golf, but the reasons for the limited activity were not explored. In both studies, post-RTSA sports were generally of lower intensity than those played by patients after anatomical TSA.27
Overall, the majority of patients were very satisfied with their low pain level after RTSA. In addition, many patients not limited by other medical conditions were able to return to their pre-RTSA moderate-intensity recreational activities.
1. Baulot E, Chabernaud D, Grammont PM. Results of Grammont’s inverted prosthesis in omarthritis associated with major cuff destruction. Apropos of 16 cases [in French]. Acta Orthop Belg. 1995;61(suppl 1):112-119.
2. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86(3):388-395.
3. Franklin JL, Barrett WP, Jackins SE, Matsen FA 3rd. Glenoid loosening in total shoulder arthroplasty. Association with rotator cuff deficiency. J Arthroplasty. 1988;3(1):39-46.
4. Neer CS 2nd, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65(9):1232-1244.
5. Edwards TB, Boulahia A, Kempf JF, Boileau P, Nemoz C, Walch G. The influence of rotator cuff disease on the results of shoulder arthroplasty for primary osteoarthritis: results of a multicenter study. J Bone Joint Surg Am. 2002;84(12):2240-2248.
6. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 suppl S):147S-161S.
7. Nam D, Kepler CK, Neviaser AS, et al. Reverse total shoulder arthroplasty: current concepts, results, and component wear analysis. J Bone Joint Surg Am. 2010;92(suppl 2):23-35.
8. Ackland DC, Roshan-Zamir S, Richardson M, Pandy MG. Moment arms of the shoulder musculature after reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(5):1221-1230.
9. Frankle M, Siegal S, Pupello D, Saleem A, Mighell M, Vasey M. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients. J Bone Joint Surg Am. 2005;87(8):1697-1705.
10. Cazeneuve JF, Cristofari DJ. Long term functional outcome following reverse shoulder arthroplasty in the elderly. Orthop Traumatol Surg Res. 2011;97(6):583-589.
11. Gerber C, Pennington, SD, Nyffeler RW. Reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2009;17(5):284-295.
12. Brophy RH, Beauvais RL, Jones EC, Cordasco FA, Marx RG. Measurement of shoulder activity level. Clin Orthop. 2005;(439):101-108.
13. Smith AM, Barnes SA, Sperling JW, Farrell CM, Cummings JD, Cofield RH. Patient and physician-assessed shoulder function after arthroplasty. J Bone Joint Surg Am. 2006;88(3):508-513.
14. Zarkadas PC, Throckmorton TQ, Dahm DL, Sperling J, Schleck CD, Cofield R. Patient reported activities after shoulder replacement: total and hemiarthroplasty. J Shoulder Elbow Surg. 2011;20(2):273-280.
15. Kocher, MS, Horan MP, Briggs KK, Richardson TR, O’Holleran J, Hawkins RJ. Reliability, validity, and responsiveness of the American Shoulder and Elbow Surgeons subjective shoulder scale in patients with shoulder instability, rotator cuff disease, and glenohumeral arthritis. J Bone Joint Surg Am. 2005;87(9):2006-2011.
16. Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3(6):347-352.
17. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
18. Hunsaker FG, Cioffi DA, Amadio PC, Wright JG, Caughlin B. The American Academy of Orthopaedic Surgeons outcomes instruments: normative values from the general population. J Bone Joint Surg Am. 2002;84(2):208-215.
19. Molé D, Favard L. Excentered scapulohumeral osteoarthritis [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2007;93(6 suppl):37-94.
20. Clark JC, Ritchie J, Song FS, et al. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg. 2012;21(1):36-41.
21. Boulahia A, Edwards TB, Walch G, Baratta RV. Early results of a reverse design prosthesis in the treatment of arthritis of the shoulder in elderly patients with a large rotator cuff tear. Orthopedics. 2002;25(2):129-133.
22. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747.
23. Antuña SA, Sperling JW, Sánchez-Sotelo J, Cofield RH. Shoulder arthroplasty for proximal humeral nonunions. J Shoulder Elbow Surg. 2002;11(2):114-121.
24. Cheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011;19(7):439-449.
25. Nolan BM, Ankerson E, Wiater JM. Reverse total shoulder arthroplasty improves function in cuff tear arthropathy. Clin Orthop. 2011;469(9):2476-2482.
26. Lawrence TM, Ahmadi S, Sanchez-Sotelo J, Sperling JW, Cofield RH. Patient reported activities after reverse shoulder arthroplasty: part II. J Shoulder Elbow Surg. 2012;21(11):1464-1469.
27. Schumann K, Flury MP, Schwyzer HK, Simmen BR, Drerup S, Goldhahn J. Sports activity after anatomical total shoulder arthroplasty. Am J Sports Med. 2010;38(10):2097-2105.
The treatment of patients with severe shoulder pain and disability combined with a nonfunctional rotator cuff was a clinical challenge until the development of the reverse total shoulder arthroplasty (RTSA).1-3 Massive rotator cuff tears can leave patients with a pseudoparalytic upper extremity and may result in advanced arthritis of the joint because of altered mechanical and nutritional factors.4 In this setting, simply replacing the arthritic joint with standard total shoulder arthroplasty (TSA) is not recommended because it does not address the functional deficits, and it has poor long-term outcomes.3,5 RTSA works by changing the center of rotation of the shoulder joint so that the deltoid muscle can be used to elevate the arm.6,7 The 4 rotator cuff muscles are not required for forward elevation or stability of this constrained implant.6,8
Current indications for RTSA are cuff tear arthropathy, complex proximal humerus fractures, and revision from hemiarthroplasty or TSA with rotator cuff dysfunction. Patients with advanced cuff tear arthropathy have minimal forward elevation and pseudoparalysis. Previous studies have shown mean preoperative forward flexion of 55º and mean ASES (American Shoulder and Elbow Surgeons) Standardized Shoulder Assessment Form score of 34.3.9 Thus, minimal overhead activity is possible without RTSA. Advances in the RTSA technique have led to promising results (excellent functional improvement), but there is limited information regarding the activity levels patients can achieve after surgery.7,9-11
We conducted a study of the types of sporting activities in which patients with RTSA could participate. We hypothesized that, relative to historic controls, patients with RTSA could return to low-intensity sporting activities with improvement in motion and ASES scores.
Materials and Methods
After this study received institutional review board approval, patients who had undergone RTSA at our institution between January 1, 2004 and December 31, 2010 were identified by the billing codes used for the procedure. Each patient who had RTSA performed during the study period was included in the study. Charts were then reviewed to extract demographic data, preoperative diagnosis, surgery date, operative side, dominant side, type of implant used, operative complications, and subsequent revisions. A questionnaire (Appendix) was designed and used to assess activity, functional status, pain, and satisfaction levels after RTSA. Patients had to be willing and able to complete this questionnaire in order to be included in the study.
The questionnaire included demographic questions; a list of 42 activities patients could choose from to describe their current activity level, activities they were able to perform before the surgery, and activities they wish they could perform; a list of reasons for any limitations; and questions about overall pain, strength, and satisfaction with the procedure. In addition, there was an open-ended question for activities that may not have been listed. The questionnaire also included a validated method for assessing shoulder range of motion (ROM) at home, where patients rated their overhead motion according to standardized physical landmarks, including the level of the shoulder, chin, eyebrows, top of head, and above head.12-14 Also provided was the ASES Standardized Shoulder Assessment Form, which features a 100-point visual analog scale for pain plus functional ability questions, with higher scores indicating less pain and better function.15,16 The minimal clinical significance in the ASES score is 6.4 points.17,18 Scores were recorded and analyzed. Student t test was used to calculate statistical differences between patients who had primary RTSA performed and patients who underwent revision RTSA.
Study personnel contacted patients by telephone and direct mailing. Patients who could not be reached initially were called at least 4 more times: twice during the weekday, once during the evening, and once on the weekend. Patients who could not be contacted by telephone were then cross-referenced with the Social Security database to see if any were deceased. Response data were tabulated, and patients were stratified into high-, moderate-, and low-intensity activity.
One of the 3 senior authors (Dr. Ahmad, Dr. Bigliani, Dr. Levine) performed the 95 RTSAs: 84 Zimmer (Warsaw, Indiana), 7 DePuy (Warsaw, Indiana), 4 Tornier (Minneapolis, Minnesota). The DePuy and Tornier implants were used when a 30-mm glenoid peg was required (before Zimmer offered this length in its system). The procedure was done with a deltopectoral approach with 20° of retroversion. In revision cases, the same approach was used, the hardware or implants were removed, and the position of the humeral component was determined based on the pectoralis major insertion and the deltoid tension. In 80% of cases, the subscapularis was not repaired; in the other 20%, it was. Whether it was repaired depended on tendon viability and surgeon preference, as subscapularis repair status has been shown not to affect functional outcome.19-21 No combined latissimus transfers were performed. Patients wore a sling the first 4 weeks after surgery (only wrist and elbow motion allowed) and then advanced to active shoulder ROM. Eight weeks after surgery, they began gentle shoulder strengthening.
Results
One hundred nine consecutive patients underwent RTSA at a single institution. Fifteen patients subsequently died, 14 could not be contacted, and 2 declined, leaving 78 patients available for clinical follow-up. Mean follow-up was 4.8 years (range 2-9 years). Mean (SD) age at surgery was 75.3 (7.5) years. Seventy-five percent of the patients were women. Sixty-one percent underwent surgery for cuff tear arthropathy, 31% for revision of previous arthroplasty or internal fixation, 7% for complex fractures, and 1% for tumor. Of the 24 revisions, 15 were for failed hemiarthroplasty, 3 were for failed TSA with rotator cuff dysfunction, 4 were for fracture with failed internal fixation, and 2 were for failed RTSA referred from other institutions. The dominant shoulder was involved 62% of the time. Preoperative active forward shoulder elevation was less than 90° in all patients. There were 10 complications: 2 dislocations that were closed-reduced and remained stable, 1 dislocation that required revision of the liner, 1 aseptic loosening in a patient who has declined revision, 2 dissociated glenosphere baseplates, 2 deep infections that required 2-stage exchanges, 1 deep infection that required a 2-stage exchange that was then complicated by dissociation of the glenosphere baseplate requiring revision, and 1 superficial infection that resolved with oral antibiotics.
After surgery, mean active forward elevation was 140°, mean active external rotation was 48°, and mean active internal rotation was to S1. Mean (SD) postoperative ASES score was 77.5 (23.4). Satisfaction level was high (mean, 8.3/10), and mean pain levels were low: 2.3 out of 10 on the visual analog scale and 44.0 (SD, 11.7) on the ASES pain component. Strength was rated a mean of good. Table 1 lists the clinical data for the primary and revision surgery patients.
Eighteen patients (23.1%) returned to 24 different high-intensity activities, such as hunting, golf, and skiing; 38 patients (48.7%) returned to moderate-intensity activities, such as swimming, bowling, and raking leaves; and 22 patients (28.2%) returned to low-intensity activities, such as riding a stationary bike, playing a musical instrument, and walking (Table 2). Four patients played golf before and after RTSA, but neither of the 2 patients who played tennis before RTSA were able to do so after. Patients reported they engaged in their favorite leisure activity a mean of 4.8 times per week and a mean of 1.5 hours each time.
A medical problem was cited by 58% of patients as the reason for limited activity. These patients reported physical decline resulting from cardiac disease, diabetes, asthma/chronic obstructive pulmonary disease, or arthritis in other joints. Reasons for activity limitation are listed in Table 3. Post-RTSA activities that patients could not do for any reason are listed in Table 4. Activity limitations that patients attributed to the RTSA are listed in Table 5.
The majority of patients (57.7%) reported no change, from before RTSA to after RTSA, in being unable to do certain desired activities (eg, softball, target shooting, horseback riding, running, traveling). Sixteen patients (20.8%) reported being unable to return to an activity (eg, tennis, swimming, baseball, kayaking) they had been able to do before surgery. Most (69%) of those patients reported being unable to return to a moderate- or high-intensity activity after RTSA, but 81.8% were able to return to different moderate- or high-intensity activities.
Revision patients, who reported lower overhead activity levels, constituted 73% of the patients who felt their shoulder mechanically limited their activity, despite the fact that revisions constituted only 25% of the cases overall. Mean active ROM was statistically lower for revision patients than for primary patients (P < .05). Mean ASES score was statistically lower for the revision group (P < .001) and represented a clinically significant difference. Mean pain level was low (3.3) and satisfaction still generally high (7.4), but pain, satisfaction, and strength were about 1 point worse on average in the revision group than in the primary group.
Discussion
In the United States and other countries, RTSA implant survivorship is good.9,22 In this article, we have reported on post-RTSA activity levels, on the significant impact of comorbidities on this group, and on the negative effect of revisions on postoperative activity. Patients in this population reported that concomitant medical problems were the most important factor limiting their post-RTSA activity levels. Understanding and interpreting quality-of-life or functional scores in this elderly group must take into account the impact of comorbidities.23
Patients should have realistic postoperative expectations.24 In this study, some patients engaged in high-intensity overhead activities, such as golf, chopping wood, and shooting. However, the most difficulty was encountered trying to return to activities (eg, tennis, kayaking, archery, combing hair) that required external rotation in abduction.
Patients who had a previous implant (eg, hemiarthroplasty, TSA, failed internal fixation) revised to RTSA had lower activity levels and were 9 times more likely than primary patients to report having a mechanical shoulder limitation affecting their activity. Revision patients also had worse forward elevation, external rotation, pain, and satisfaction.
This study is limited in that it is retrospective. Subsequent prospective studies focused on younger patients who undergo primary RTSA may be useful if indications expand. In addition, subscapularis status and especially infraspinatus status may affect activity levels and could be analyzed in a study. Another limitation is that we did not specifically record detailed preoperative data, though all patients were known to have preoperative forward elevation of less than 90°.
In general, the primary measure of success for RTSA has been pain relief. Some studies have also reported on strength and ROM.2,20,25,26 A recent study using similar methodology demonstrated comparable ROM and low pain after RTSA, though revisions were not included in that study.26 In contrast to the present study, no patient in that study was able to play tennis or golf, but the reasons for the limited activity were not explored. In both studies, post-RTSA sports were generally of lower intensity than those played by patients after anatomical TSA.27
Overall, the majority of patients were very satisfied with their low pain level after RTSA. In addition, many patients not limited by other medical conditions were able to return to their pre-RTSA moderate-intensity recreational activities.
The treatment of patients with severe shoulder pain and disability combined with a nonfunctional rotator cuff was a clinical challenge until the development of the reverse total shoulder arthroplasty (RTSA).1-3 Massive rotator cuff tears can leave patients with a pseudoparalytic upper extremity and may result in advanced arthritis of the joint because of altered mechanical and nutritional factors.4 In this setting, simply replacing the arthritic joint with standard total shoulder arthroplasty (TSA) is not recommended because it does not address the functional deficits, and it has poor long-term outcomes.3,5 RTSA works by changing the center of rotation of the shoulder joint so that the deltoid muscle can be used to elevate the arm.6,7 The 4 rotator cuff muscles are not required for forward elevation or stability of this constrained implant.6,8
Current indications for RTSA are cuff tear arthropathy, complex proximal humerus fractures, and revision from hemiarthroplasty or TSA with rotator cuff dysfunction. Patients with advanced cuff tear arthropathy have minimal forward elevation and pseudoparalysis. Previous studies have shown mean preoperative forward flexion of 55º and mean ASES (American Shoulder and Elbow Surgeons) Standardized Shoulder Assessment Form score of 34.3.9 Thus, minimal overhead activity is possible without RTSA. Advances in the RTSA technique have led to promising results (excellent functional improvement), but there is limited information regarding the activity levels patients can achieve after surgery.7,9-11
We conducted a study of the types of sporting activities in which patients with RTSA could participate. We hypothesized that, relative to historic controls, patients with RTSA could return to low-intensity sporting activities with improvement in motion and ASES scores.
Materials and Methods
After this study received institutional review board approval, patients who had undergone RTSA at our institution between January 1, 2004 and December 31, 2010 were identified by the billing codes used for the procedure. Each patient who had RTSA performed during the study period was included in the study. Charts were then reviewed to extract demographic data, preoperative diagnosis, surgery date, operative side, dominant side, type of implant used, operative complications, and subsequent revisions. A questionnaire (Appendix) was designed and used to assess activity, functional status, pain, and satisfaction levels after RTSA. Patients had to be willing and able to complete this questionnaire in order to be included in the study.
The questionnaire included demographic questions; a list of 42 activities patients could choose from to describe their current activity level, activities they were able to perform before the surgery, and activities they wish they could perform; a list of reasons for any limitations; and questions about overall pain, strength, and satisfaction with the procedure. In addition, there was an open-ended question for activities that may not have been listed. The questionnaire also included a validated method for assessing shoulder range of motion (ROM) at home, where patients rated their overhead motion according to standardized physical landmarks, including the level of the shoulder, chin, eyebrows, top of head, and above head.12-14 Also provided was the ASES Standardized Shoulder Assessment Form, which features a 100-point visual analog scale for pain plus functional ability questions, with higher scores indicating less pain and better function.15,16 The minimal clinical significance in the ASES score is 6.4 points.17,18 Scores were recorded and analyzed. Student t test was used to calculate statistical differences between patients who had primary RTSA performed and patients who underwent revision RTSA.
Study personnel contacted patients by telephone and direct mailing. Patients who could not be reached initially were called at least 4 more times: twice during the weekday, once during the evening, and once on the weekend. Patients who could not be contacted by telephone were then cross-referenced with the Social Security database to see if any were deceased. Response data were tabulated, and patients were stratified into high-, moderate-, and low-intensity activity.
One of the 3 senior authors (Dr. Ahmad, Dr. Bigliani, Dr. Levine) performed the 95 RTSAs: 84 Zimmer (Warsaw, Indiana), 7 DePuy (Warsaw, Indiana), 4 Tornier (Minneapolis, Minnesota). The DePuy and Tornier implants were used when a 30-mm glenoid peg was required (before Zimmer offered this length in its system). The procedure was done with a deltopectoral approach with 20° of retroversion. In revision cases, the same approach was used, the hardware or implants were removed, and the position of the humeral component was determined based on the pectoralis major insertion and the deltoid tension. In 80% of cases, the subscapularis was not repaired; in the other 20%, it was. Whether it was repaired depended on tendon viability and surgeon preference, as subscapularis repair status has been shown not to affect functional outcome.19-21 No combined latissimus transfers were performed. Patients wore a sling the first 4 weeks after surgery (only wrist and elbow motion allowed) and then advanced to active shoulder ROM. Eight weeks after surgery, they began gentle shoulder strengthening.
Results
One hundred nine consecutive patients underwent RTSA at a single institution. Fifteen patients subsequently died, 14 could not be contacted, and 2 declined, leaving 78 patients available for clinical follow-up. Mean follow-up was 4.8 years (range 2-9 years). Mean (SD) age at surgery was 75.3 (7.5) years. Seventy-five percent of the patients were women. Sixty-one percent underwent surgery for cuff tear arthropathy, 31% for revision of previous arthroplasty or internal fixation, 7% for complex fractures, and 1% for tumor. Of the 24 revisions, 15 were for failed hemiarthroplasty, 3 were for failed TSA with rotator cuff dysfunction, 4 were for fracture with failed internal fixation, and 2 were for failed RTSA referred from other institutions. The dominant shoulder was involved 62% of the time. Preoperative active forward shoulder elevation was less than 90° in all patients. There were 10 complications: 2 dislocations that were closed-reduced and remained stable, 1 dislocation that required revision of the liner, 1 aseptic loosening in a patient who has declined revision, 2 dissociated glenosphere baseplates, 2 deep infections that required 2-stage exchanges, 1 deep infection that required a 2-stage exchange that was then complicated by dissociation of the glenosphere baseplate requiring revision, and 1 superficial infection that resolved with oral antibiotics.
After surgery, mean active forward elevation was 140°, mean active external rotation was 48°, and mean active internal rotation was to S1. Mean (SD) postoperative ASES score was 77.5 (23.4). Satisfaction level was high (mean, 8.3/10), and mean pain levels were low: 2.3 out of 10 on the visual analog scale and 44.0 (SD, 11.7) on the ASES pain component. Strength was rated a mean of good. Table 1 lists the clinical data for the primary and revision surgery patients.
Eighteen patients (23.1%) returned to 24 different high-intensity activities, such as hunting, golf, and skiing; 38 patients (48.7%) returned to moderate-intensity activities, such as swimming, bowling, and raking leaves; and 22 patients (28.2%) returned to low-intensity activities, such as riding a stationary bike, playing a musical instrument, and walking (Table 2). Four patients played golf before and after RTSA, but neither of the 2 patients who played tennis before RTSA were able to do so after. Patients reported they engaged in their favorite leisure activity a mean of 4.8 times per week and a mean of 1.5 hours each time.
A medical problem was cited by 58% of patients as the reason for limited activity. These patients reported physical decline resulting from cardiac disease, diabetes, asthma/chronic obstructive pulmonary disease, or arthritis in other joints. Reasons for activity limitation are listed in Table 3. Post-RTSA activities that patients could not do for any reason are listed in Table 4. Activity limitations that patients attributed to the RTSA are listed in Table 5.
The majority of patients (57.7%) reported no change, from before RTSA to after RTSA, in being unable to do certain desired activities (eg, softball, target shooting, horseback riding, running, traveling). Sixteen patients (20.8%) reported being unable to return to an activity (eg, tennis, swimming, baseball, kayaking) they had been able to do before surgery. Most (69%) of those patients reported being unable to return to a moderate- or high-intensity activity after RTSA, but 81.8% were able to return to different moderate- or high-intensity activities.
Revision patients, who reported lower overhead activity levels, constituted 73% of the patients who felt their shoulder mechanically limited their activity, despite the fact that revisions constituted only 25% of the cases overall. Mean active ROM was statistically lower for revision patients than for primary patients (P < .05). Mean ASES score was statistically lower for the revision group (P < .001) and represented a clinically significant difference. Mean pain level was low (3.3) and satisfaction still generally high (7.4), but pain, satisfaction, and strength were about 1 point worse on average in the revision group than in the primary group.
Discussion
In the United States and other countries, RTSA implant survivorship is good.9,22 In this article, we have reported on post-RTSA activity levels, on the significant impact of comorbidities on this group, and on the negative effect of revisions on postoperative activity. Patients in this population reported that concomitant medical problems were the most important factor limiting their post-RTSA activity levels. Understanding and interpreting quality-of-life or functional scores in this elderly group must take into account the impact of comorbidities.23
Patients should have realistic postoperative expectations.24 In this study, some patients engaged in high-intensity overhead activities, such as golf, chopping wood, and shooting. However, the most difficulty was encountered trying to return to activities (eg, tennis, kayaking, archery, combing hair) that required external rotation in abduction.
Patients who had a previous implant (eg, hemiarthroplasty, TSA, failed internal fixation) revised to RTSA had lower activity levels and were 9 times more likely than primary patients to report having a mechanical shoulder limitation affecting their activity. Revision patients also had worse forward elevation, external rotation, pain, and satisfaction.
This study is limited in that it is retrospective. Subsequent prospective studies focused on younger patients who undergo primary RTSA may be useful if indications expand. In addition, subscapularis status and especially infraspinatus status may affect activity levels and could be analyzed in a study. Another limitation is that we did not specifically record detailed preoperative data, though all patients were known to have preoperative forward elevation of less than 90°.
In general, the primary measure of success for RTSA has been pain relief. Some studies have also reported on strength and ROM.2,20,25,26 A recent study using similar methodology demonstrated comparable ROM and low pain after RTSA, though revisions were not included in that study.26 In contrast to the present study, no patient in that study was able to play tennis or golf, but the reasons for the limited activity were not explored. In both studies, post-RTSA sports were generally of lower intensity than those played by patients after anatomical TSA.27
Overall, the majority of patients were very satisfied with their low pain level after RTSA. In addition, many patients not limited by other medical conditions were able to return to their pre-RTSA moderate-intensity recreational activities.
1. Baulot E, Chabernaud D, Grammont PM. Results of Grammont’s inverted prosthesis in omarthritis associated with major cuff destruction. Apropos of 16 cases [in French]. Acta Orthop Belg. 1995;61(suppl 1):112-119.
2. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86(3):388-395.
3. Franklin JL, Barrett WP, Jackins SE, Matsen FA 3rd. Glenoid loosening in total shoulder arthroplasty. Association with rotator cuff deficiency. J Arthroplasty. 1988;3(1):39-46.
4. Neer CS 2nd, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65(9):1232-1244.
5. Edwards TB, Boulahia A, Kempf JF, Boileau P, Nemoz C, Walch G. The influence of rotator cuff disease on the results of shoulder arthroplasty for primary osteoarthritis: results of a multicenter study. J Bone Joint Surg Am. 2002;84(12):2240-2248.
6. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 suppl S):147S-161S.
7. Nam D, Kepler CK, Neviaser AS, et al. Reverse total shoulder arthroplasty: current concepts, results, and component wear analysis. J Bone Joint Surg Am. 2010;92(suppl 2):23-35.
8. Ackland DC, Roshan-Zamir S, Richardson M, Pandy MG. Moment arms of the shoulder musculature after reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(5):1221-1230.
9. Frankle M, Siegal S, Pupello D, Saleem A, Mighell M, Vasey M. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients. J Bone Joint Surg Am. 2005;87(8):1697-1705.
10. Cazeneuve JF, Cristofari DJ. Long term functional outcome following reverse shoulder arthroplasty in the elderly. Orthop Traumatol Surg Res. 2011;97(6):583-589.
11. Gerber C, Pennington, SD, Nyffeler RW. Reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2009;17(5):284-295.
12. Brophy RH, Beauvais RL, Jones EC, Cordasco FA, Marx RG. Measurement of shoulder activity level. Clin Orthop. 2005;(439):101-108.
13. Smith AM, Barnes SA, Sperling JW, Farrell CM, Cummings JD, Cofield RH. Patient and physician-assessed shoulder function after arthroplasty. J Bone Joint Surg Am. 2006;88(3):508-513.
14. Zarkadas PC, Throckmorton TQ, Dahm DL, Sperling J, Schleck CD, Cofield R. Patient reported activities after shoulder replacement: total and hemiarthroplasty. J Shoulder Elbow Surg. 2011;20(2):273-280.
15. Kocher, MS, Horan MP, Briggs KK, Richardson TR, O’Holleran J, Hawkins RJ. Reliability, validity, and responsiveness of the American Shoulder and Elbow Surgeons subjective shoulder scale in patients with shoulder instability, rotator cuff disease, and glenohumeral arthritis. J Bone Joint Surg Am. 2005;87(9):2006-2011.
16. Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3(6):347-352.
17. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
18. Hunsaker FG, Cioffi DA, Amadio PC, Wright JG, Caughlin B. The American Academy of Orthopaedic Surgeons outcomes instruments: normative values from the general population. J Bone Joint Surg Am. 2002;84(2):208-215.
19. Molé D, Favard L. Excentered scapulohumeral osteoarthritis [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2007;93(6 suppl):37-94.
20. Clark JC, Ritchie J, Song FS, et al. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg. 2012;21(1):36-41.
21. Boulahia A, Edwards TB, Walch G, Baratta RV. Early results of a reverse design prosthesis in the treatment of arthritis of the shoulder in elderly patients with a large rotator cuff tear. Orthopedics. 2002;25(2):129-133.
22. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747.
23. Antuña SA, Sperling JW, Sánchez-Sotelo J, Cofield RH. Shoulder arthroplasty for proximal humeral nonunions. J Shoulder Elbow Surg. 2002;11(2):114-121.
24. Cheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011;19(7):439-449.
25. Nolan BM, Ankerson E, Wiater JM. Reverse total shoulder arthroplasty improves function in cuff tear arthropathy. Clin Orthop. 2011;469(9):2476-2482.
26. Lawrence TM, Ahmadi S, Sanchez-Sotelo J, Sperling JW, Cofield RH. Patient reported activities after reverse shoulder arthroplasty: part II. J Shoulder Elbow Surg. 2012;21(11):1464-1469.
27. Schumann K, Flury MP, Schwyzer HK, Simmen BR, Drerup S, Goldhahn J. Sports activity after anatomical total shoulder arthroplasty. Am J Sports Med. 2010;38(10):2097-2105.
1. Baulot E, Chabernaud D, Grammont PM. Results of Grammont’s inverted prosthesis in omarthritis associated with major cuff destruction. Apropos of 16 cases [in French]. Acta Orthop Belg. 1995;61(suppl 1):112-119.
2. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86(3):388-395.
3. Franklin JL, Barrett WP, Jackins SE, Matsen FA 3rd. Glenoid loosening in total shoulder arthroplasty. Association with rotator cuff deficiency. J Arthroplasty. 1988;3(1):39-46.
4. Neer CS 2nd, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65(9):1232-1244.
5. Edwards TB, Boulahia A, Kempf JF, Boileau P, Nemoz C, Walch G. The influence of rotator cuff disease on the results of shoulder arthroplasty for primary osteoarthritis: results of a multicenter study. J Bone Joint Surg Am. 2002;84(12):2240-2248.
6. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 suppl S):147S-161S.
7. Nam D, Kepler CK, Neviaser AS, et al. Reverse total shoulder arthroplasty: current concepts, results, and component wear analysis. J Bone Joint Surg Am. 2010;92(suppl 2):23-35.
8. Ackland DC, Roshan-Zamir S, Richardson M, Pandy MG. Moment arms of the shoulder musculature after reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(5):1221-1230.
9. Frankle M, Siegal S, Pupello D, Saleem A, Mighell M, Vasey M. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients. J Bone Joint Surg Am. 2005;87(8):1697-1705.
10. Cazeneuve JF, Cristofari DJ. Long term functional outcome following reverse shoulder arthroplasty in the elderly. Orthop Traumatol Surg Res. 2011;97(6):583-589.
11. Gerber C, Pennington, SD, Nyffeler RW. Reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2009;17(5):284-295.
12. Brophy RH, Beauvais RL, Jones EC, Cordasco FA, Marx RG. Measurement of shoulder activity level. Clin Orthop. 2005;(439):101-108.
13. Smith AM, Barnes SA, Sperling JW, Farrell CM, Cummings JD, Cofield RH. Patient and physician-assessed shoulder function after arthroplasty. J Bone Joint Surg Am. 2006;88(3):508-513.
14. Zarkadas PC, Throckmorton TQ, Dahm DL, Sperling J, Schleck CD, Cofield R. Patient reported activities after shoulder replacement: total and hemiarthroplasty. J Shoulder Elbow Surg. 2011;20(2):273-280.
15. Kocher, MS, Horan MP, Briggs KK, Richardson TR, O’Holleran J, Hawkins RJ. Reliability, validity, and responsiveness of the American Shoulder and Elbow Surgeons subjective shoulder scale in patients with shoulder instability, rotator cuff disease, and glenohumeral arthritis. J Bone Joint Surg Am. 2005;87(9):2006-2011.
16. Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3(6):347-352.
17. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
18. Hunsaker FG, Cioffi DA, Amadio PC, Wright JG, Caughlin B. The American Academy of Orthopaedic Surgeons outcomes instruments: normative values from the general population. J Bone Joint Surg Am. 2002;84(2):208-215.
19. Molé D, Favard L. Excentered scapulohumeral osteoarthritis [in French]. Rev Chir Orthop Reparatrice Appar Mot. 2007;93(6 suppl):37-94.
20. Clark JC, Ritchie J, Song FS, et al. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg. 2012;21(1):36-41.
21. Boulahia A, Edwards TB, Walch G, Baratta RV. Early results of a reverse design prosthesis in the treatment of arthritis of the shoulder in elderly patients with a large rotator cuff tear. Orthopedics. 2002;25(2):129-133.
22. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747.
23. Antuña SA, Sperling JW, Sánchez-Sotelo J, Cofield RH. Shoulder arthroplasty for proximal humeral nonunions. J Shoulder Elbow Surg. 2002;11(2):114-121.
24. Cheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011;19(7):439-449.
25. Nolan BM, Ankerson E, Wiater JM. Reverse total shoulder arthroplasty improves function in cuff tear arthropathy. Clin Orthop. 2011;469(9):2476-2482.
26. Lawrence TM, Ahmadi S, Sanchez-Sotelo J, Sperling JW, Cofield RH. Patient reported activities after reverse shoulder arthroplasty: part II. J Shoulder Elbow Surg. 2012;21(11):1464-1469.
27. Schumann K, Flury MP, Schwyzer HK, Simmen BR, Drerup S, Goldhahn J. Sports activity after anatomical total shoulder arthroplasty. Am J Sports Med. 2010;38(10):2097-2105.