User login
Thoracic Outlet Syndrome: Current Concepts, Imaging Features, and Therapeutic Strategies
Thoracic outlet syndrome (TOS) was first described by Coot in 1861,1,2 and the term was coined by Peet and colleagues3 in 1956 to cover a spectrum of conditions caused by dynamic compression of the brachial plexus (neurogenic), subclavian artery (arterial), or subclavian vein (venous). The estimated incidence of TOS is 10 in 100,000.4 However, cadaveric studies have suggested that up to 90% of the population may have what is considered abnormal anatomy of the thoracic outlet,5 which in turn suggests a multifactorial etiology for symptomatic disease. TOS is most commonly diagnosed in patients 20 to 40 years of age, with females affected in a 4:1 ratio.6 Although historically TOS is a clinical diagnosis, advanced imaging is often helpful in determining the nature and location of the structure undergoing compression and the structure producing compression, which help guide management. Computed tomography angiography (CTA) and magnetic resonance imaging (MRI) performed in association with postural maneuvers aid in the diagnosis in patients with dynamically acquired compression.7
Pathophysiology
The pathophysiology of TOS is attributable to the unique anatomy of the thoracic outlet. Compromise of the neurovascular structures can occur through congenital or acquired narrowing in 3 distinct compartments: the interscalene triangle, the costoclavicular space, and the retropectoralis minor space. The interscalene triangle is the most medial of the compartments. Containing the subclavian artery and the 3 trunks of the brachial plexus, it is bordered anteriorly by the anterior scalene muscle, posteriorly by the middle and posterior scalene muscles, and inferiorly by the first rib. The interscalene triangle is the most frequent site of neurologic compression.8 The middle compartment is the costoclavicular space, which is bordered superiorly by the clavicle, anteriorly by the subclavius muscle, and posteriorly by the first rib and the middle scalene muscle. The costoclavicular space is the most frequent site of arterial compression,8 where the artery lies directly anterior to the subclavian vein and is surrounded by the 3 cords of the brachial plexus. The most lateral compartment is the retropectoralis minor space, which is bordered anteriorly by the pectoralis minor muscle, superiorly by the subscapularis muscle, and inferiorly by the anterior chest wall. Sources of neurovascular compression within any of the spaces include cervical ribs9; elongated C7 transverse processes; hypertrophy of the anterior or middle scalene, subclavius, or pectoralis minor muscles10; anomalous scalenus minimus muscle; repetitive overhead arm movements (pitching, swimming)11; anomalous fascial bands; degenerative spine disease; bone destruction from primary or secondary neoplasms (Pancoast tumor); hyperextension/flexion injury of the neck12; and malunion of clavicle fractures, among others.13
Classification
Three distinct TOSs have been described, individually or combined, depending on the injured component: neurogenic from brachial plexus compression, arterial from subclavian artery compression, and venous from subclavian or axillary vein compression.14,15
Neurogenic TOS has 2 reported types: true (classic) and disputed. True neurogenic TOS is rare, with an estimated incidence of 1 in 1 million.16 First described in 1970 as a lower trunk plexopathy involving slowly progressive unilateral weakness of the intrinsic hand muscles and sensory abnormalities in the ulnar and medial antebrachial cutaneous nerve distributions, true neurogenic TOS was originally called Gilliatt-Sumner hand syndrome.17 A congenital band extending between the first rib and an elongated C7 transverse process was thought to be the location of brachial plexus injury in true neurogenic TOS. Conversely, disputed neurogenic TOS is the most common form of TOS, occurring in 3 to 80 per 100018 and accounting for 90% to 95% of all TOS cases.13,19 In contrast to true neurogenic TOS, in which anatomical and electrodiagnostic evidence supports the diagnosis, objective clinical findings are often lacking in the disputed form.18 Patients with disputed neurogenic TOS present with a diverse array of symptoms, including pain, numbness, and weakness affecting the neck, shoulder, and arm, exacerbated by activities requiring elevation or sustained use of the extremity.20
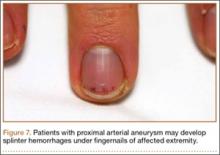
Arterial TOS accounts for 1% to 5% of all TOS cases.21 Arterial TOS typically affects patients who perform repetitive movements of the upper extremities with their arms above their shoulders, resulting in compression of the subclavian artery. Symptoms of arterial TOS include pain, weakness, coolness, pallor, and paresthesia.18,22 In severe cases of compression, subclavian artery damage can result in thrombosis with distal embolization, poststenotic aneurysm, or even retrograde extension causing stroke.22,23
Last, representing 2% to 3% of all TOS cases, venous TOS results from compression of the subclavian or axillary vein.18,24 Two mechanisms for vascular compromise have been described. The first involves compression of the vein between the clavicle and the first rib with overhead activities.18 Patients often experience intermittent “heaviness” of the extremity with repeated overhead use. The second mechanism involves repeated stress between the clavicle and vein, causing an intravascular thrombosis.18 Patients may experience pain, edema, cyanosis, venous distention, and even spontaneous venous thrombosis, referred to as Paget-Schroetter syndrome, which can lead to pulmonary embolism.6,25,26
Clinical Features
In cases of suspected TOS, clinicians should take a thorough history and perform a thorough physical examination. The differential diagnosis for unilateral, upper limb pain, numbness, tingling, and/or weakness exacerbated by movement includes shoulder and rotator cuff pathology, cervical spine injury, cervical radiculitis, distal compressive neuropathies (carpal or cubital tunnel syndrome), and neuralgic amyotrophy (Parsonage-Turner syndrome/acute brachial radiculitis).27,28 The clinician should pursue a history of trauma to the shoulder or neck as well as any occupational or recreational activities involving elevation of the upper extremity for extended periods.29 Physical examination must include an evaluation of the contralateral side and may begin with visual inspection to assess for muscle asymmetry, atrophy, color changes, edema, or deformities.18 Next, palpation should be used to assess for any tenderness, texture changes, masses, or vascular pulsations. Attention should be directed at examination of the cervical spine as well as neurologic and vascular assessments of the bilateral upper extremities, including range of motion and strength testing,18 to rule out alternative etiologies.
Four basic maneuvers—the Roos test,30 Adson test,31 Wright test,32 and costoclavicular test—traditionally have been used to diagnose TOS. A positive Roos test involves symptom reproduction with the patient slowly opening and closing the hand for 3 minutes with the arm externally rotated and abducted to 90°.33 However, the false-positive rate of the Roos test is as high as 77% in patients with carpal tunnel syndrome and up to 47% in normal subjects.34 The Adson test is performed by having the patient inhale deeply while the arm is kept in the anatomical position with the head extended and turned toward the involved extremity. The examiner monitors the radial pulse; an absent or diminished radial pulse suggests compression of the subclavian artery. The Adson test is not very reliable, however, because the pulse diminishes even in normal subjects,6,26 with a reported false-positive rate of 13.5%.35 A positive costoclavicular compression test occurs when depressing a patient’s shoulder reproduces symptoms. In one study, the false-positive rate of the costoclavicular compression test was 48% in patients with carpal tunnel syndrome and 16% in normal subjects.34 Last, the Wright test is performed by hyperabducting and externally rotating the affected shoulder. It is positive with a diminished pulse or reproduction of symptoms. One study found that the Wright test had 70% to 90% sensitivity and 29% to 53% specificity.36
Clinically distinguishing between the various forms of TOS may be difficult, and occasionally multiple types exist in a single patient, exacerbating one another and adding to the diagnostic difficulty. For example, arterial insufficiency may lead to disruption of the neural microcirculation, leading to concurrent arterial and neurogenic TOS. Because most cases present with nonspecific symptoms, advanced imaging modalities are often required to establish a definitive diagnosis and to target therapy to the appropriate site of compression.
Imaging Features
Plain Radiography
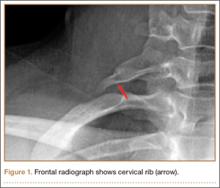
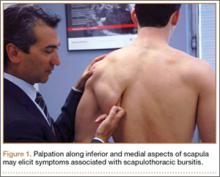
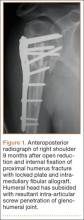
First, cervical spine and chest radiographs should be obtained to assess for bone abnormalities, including cervical ribs, long transverse processes, rib/clavicle fracture callus, rib anomalies, degenerative spine disease, and neoplasm (Pancoast/apical tumor) (Figure 1).18,25
Ultrasonography
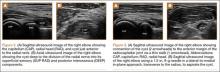
Ultrasonography is useful in evaluating arterial or venous TOS because of its low cost, noninvasive nature, and high specificity for vessel occlusion.37,38 In arterial TOS, ultrasound may demonstrate increased flow velocity through a stenosis or an aneurysmal degeneration distal to the stenosis.7 In venous TOS, duplex ultrasound can identify stasis and thrombus.7 Obtaining duplex ultrasound with the upper extremity in multiple positions allows clinicians to correlate dynamically induced symptoms with ultrasonographic findings of altered blood flow.39-41 Despite the purported benefits of ultrasound, its drawback is that it is operator-dependent,42 with some studies reporting a high false-positive rate24 for diagnosis of venous TOS.
Electrodiagnostic Testing
Ruling out etiologies such as cervical radiculitis (Parsonage-Turner syndrome), cervical radiculopathies, brachial plexus lesions, and other distal compressive neuropathies requires nerve conduction studies and electromyography.18,43-46 In true neurogenic TOS, a combination of decreased sensory nerve action potentials in the ulnar and medial antebrachial cutaneous nerves and decreased compound motor action potentials in the median nerve is often found.18 Specifically, an abnormal ulnar sensory nerve action potential suggests the lesion is situated away from the intraspinal canal, which argues against a diagnosis of radiculopathy or myelopathy.43,44 In the disputed form of neurogenic TOS, the role of electrodiagnostic testing is less clear.18
Conventional Arteriography and Venography
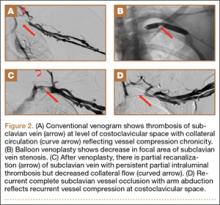
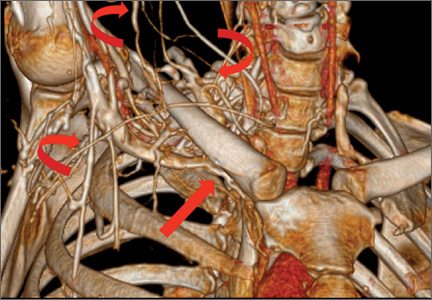
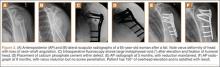
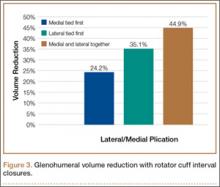
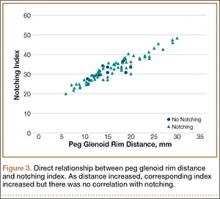
Although CTA has superseded conventional arteriography and venography in most treatment centers, it may still be used in patients with acute symptoms requiring immediate thrombolytic therapy. Catheter angiography and venography with postural maneuvers are often the first invasive treatment modality in cases of thoracic outlet vascular compression.22,24 Presence of intraluminal thrombus, vessel dilatation, and collateral vessels is readily demonstrated (Figure 2A). Recanalization of occluded vessels can be attempted using balloon angioplasty and venoplasty (Figure 2B), but it is usually only temporarily successful if the cause of extrinsic compression is not corrected (Figures 2C, 2D). CTA or conventional angiography, used if sophisticated CTA with 3-dimensional (3-D) reconstruction is unavailable, is the gold standard in diagnosis of TOS.
CTA and Venography
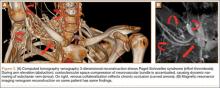
Computed tomography (CT) is a valuable modality because it can be performed rapidly and effectively to depict the relationship of vascular structures to surrounding bone and muscle.47 In addition, CTA and venography provide high-quality representations of the vasculature, and 3-D reconstruction reliably identifies areas of neurovascular compression in patients with TOS.47,48 Furthermore, CT may be performed in a dynamic fashion, with the upper extremity in various positions to reproduce dynamic compression of the neurovascular structures (Figure 3A). Comparison of the images with the upper extremities in the anatomical position and elevated allows the physician to evaluate narrowing of the compartments and dynamic compression of neurovascular structures.8 CT is particularly valuable in arterial and venous TOS. In arterial TOS, the cross-sectional area or diameter of the artery can be measured to calculate the degree of stenosis.8,47 In venous TOS, dynamic narrowing of the vein can be visualized and may be associated with venous thrombosis or collateral circulation (Figure 3B). Although a variety of maneuvers is possible during CTA, the size of the CT tunnel as well as mandatory supine positioning of the patient may limit the series. Drawbacks of CT for diagnosing TOS include difficulties in analyzing the brachial plexus because of limited contrast resolution. In addition, the risks of CT (ionizing radiation, administration of iodinated contrast medium) must be considered before image acquisition.
MRI
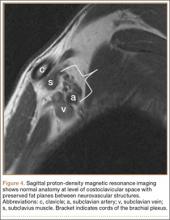
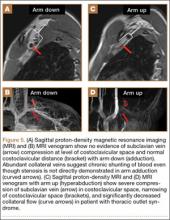
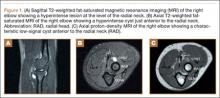
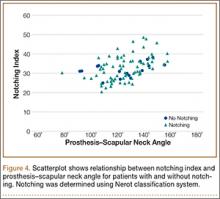
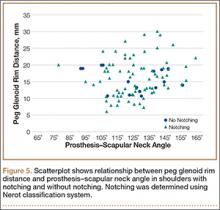
MRI is a noninvasive and nonionizing technique that offers good resolution of the anatomical components of the thoracic outlet8 and that, because of its superior soft-tissue contrast, is the modality of choice for imaging brachial plexus nerve compression in TOS (Figure 4). Neurologic compression is identified with MRI when the fat surrounding the brachial plexus disappears.8 MRI reliably identifies the source of compression, which may include bony structures, muscle hypertrophy (scalenus, scalenus minimus, subclavius, pectoralis minor), and fibrous bands.49 Because of their craniocaudal direction, the sagittal plane is often most useful in demonstrating neurovascular compression.42 Analyzing the caliber of the vessel along its course may evaluate vascular compression, and magnetic resonance (MR) angiography and venography (Figures 5A, 5B) can often complement the findings.50 Specifically, in arterial TOS, poststenotic aneurysmal dilatation may be seen, whereas thrombosis and collateral circulation can be visualized in cases of venous TOS.50 Limitations of MRI in the diagnosis of TOS historically were similar to those of CT, and included supine positioning as well as restricted upper extremity maneuvers because of the size of the tunnel and the presence of surface coils.42 However, newer higher channel surface coils and wider bores allow for imaging in a wider range of motion, including arm hyperabduction (Figures 5C, 5D), which is often necessary to elicit pathology.
Management
Generally, therapeutic options for TOS are aimed at relieving the source of neurovascular compression. It is important that treatment be directed only toward symptomatic patients, as many patients have anatomy consistent with TOS and remain asymptomatic.5 Treatment of TOS is predominately conservative and involves a combination of patient education, activity modification, medication, and rehabilitation to promote appropriate body mechanics and posture.18
Physical Therapy
Physical therapy should be aimed at decreasing pressure on the neurovascular structures of the thoracic outlet by relaxing the scalene muscles, strengthening the shoulder muscles, and working on postural exercises to help the patient sit and stand straighter.51 The scalene muscles are the primary targets for TOS rehabilitation, but focus should also be given to the upper trapezius, levator scapulae, sternocleidomastoid, pectoral, and suboccipital muscles.18 Physical therapy is often combined with hydrotherapy, massage, nonsteroidal anti-inflammatory drugs, and muscle relaxants for maximal symptomatic relief. Some patients have found relief with selective anesthetic or botulinum toxin A injections in the scalene muscles.18 A minimum of 4 to 6 weeks (often 4-6 months) of physical therapy and conservative treatment should be attempted before consideration of any invasive intervention.13,18
Anticoagulation
In venous TOS with evidence of thrombus but no obstructive clot, conservative management is typically sufficient. In rare cases, however, intimal damage secondary to vascular compression in arterial and venous TOS leads to thrombus formation, impairing upper extremity perfusion and producing symptoms. Treatment guidelines for venous TOS involve catheter-directed thrombolysis within 2 weeks of symptom onset.15 Thrombolysis replaced the prior recommendation of systemic anticoagulation combined with extremity rest and elevation because anticoagulation and rest alone result in up to 75% morbidity,52,53 whereas thrombolysis reestablishes vessel patency in nearly all patients.54 After thrombolysis, patients should receive intravenous heparin, and conversion to oral anticoagulation should occur as soon as manageable. In patients with arterial TOS, the goal of treatment is revascularization to prevent or decrease ischemia. In mild arterial ischemia, catheter-directed thrombolysis can be attempted. However, the threshold for surgical thromboembolectomy must remain low, as acute upper extremity ischemia may result in compartment syndrome and permanent loss of function.13 Fixed arterial lesions, whether occlusive or aneurysmal, are an absolute indication for thromboembolectomy with possible thoracic outlet decompression.13
Thoracic Outlet Decompression
Indications for surgical decompression are controversial. They include symptomatic patients who have vascular (arterial or venous) TOS and are not at high risk for surgery, patients with true neurologic TOS and acute progressive neurologic weakness or disabling pain,55 and patients who have disputed neurologic TOS and have failed conservative management—keeping in mind that high recurrence rates and iatrogenic brachial plexopathy have been reported in this population.56 In general, surgical procedures are aimed at reducing soft-tissue compression (scalene release or neurolysis) or bony compression (cervical or first thoracic rib excision). Three surgical approaches (transaxillary, supraclavicular, infraclavicular) are commonly used for decompression, and surgeons choose one over another depending on the anatomical abnormality causing the compression. The transaxillary approach requires limited dissection but still allows for adequate visualization of the rib during resection.57 In this approach, a transverse incision along the inferior border of the axilla extends from the pectoralis major to the latissimus dorsi. After dissection of the axillary vessels and the first thoracic nerve root, the first rib is identified and can be removed, when indicated. In contrast, the supraclavicular approach provides a wide exposure, and the site of compression is directly visualized, allowing for arterial reconstruction.58 Through this approach, the anterior and middle scalene muscles can be resected, and neurolysis of the brachial plexus can be performed. Last, the infraclavicular approach allows for exposure of the central veins through extension of the incision medially, which allows for venous reconstruction. Some patients with neurogenic or arterial TOS present with symptoms of sympathetic overactivity, in which case cervical sympathectomy can be used with decompression.
Outcomes of surgical decompression for TOS depend on the clinical type but are generally good. For instance, in cases of disputed neurogenic TOS, symptom resolution after decompression is reportedly between 80% and 90%.59 However, major depression, work-related injuries,60 and diffuse preoperative arm symptoms61 all influence long-term results. In true neurogenic TOS, postoperative pain relief is often substantial, though recovery of strength can be slow because of the axonal injury.55 In arterial TOS, outcomes are influenced by time to surgical intervention, with early surgery demonstrating better outcomes than later surgery.62 In one study, Cormier and colleagues14 evaluated 47 patients who underwent correction of subclavian-axillary artery compression; 91% were asymptomatic a mean of 5.7 months after decompression. Last, outcomes of successful thrombolysis and decompression for venous TOS demonstrated patency rates higher than 95% at 5-year follow-up.54,63
Conclusions
TOS is a spectrum of disorders caused by compression of the brachial plexus, subclavian artery, or subclavian vein. Early recognition of TOS is imperative, as diagnostic or treatment delays may be associated with significant morbidity. Clinical examination alone is often inadequate for determining the compression site and the structure causing compression. CTA and MRI performed in association with postural maneuvers may demonstrate dynamic compression of the neurovascular structures in the thoracic outlet. These imaging modalities reliably identify the structures causing compression and can be crucial for effective management.
1. Urschel HC Jr. The history of surgery for thoracic outlet syndrome. Chest Surg Clin North Am. 2000;10(1):183-188, x-xi.
2. Atasoy E. History of thoracic outlet syndrome. Hand Clin. 2004;20(1):15-16, v.
3. Peet RM, Henriksen JD, Anderson TP, Martin GM. Thoracic-outlet syndrome: evaluation of a therapeutic exercise program. Proc Staff Meet Mayo Clin. 1956;31(9):281-287.
4. Edwards DP, Mulkern E, Raja AN, Barker P. Trans-axillary first rib excision for thoracic outlet syndrome. J R Coll Surg Edinb. 1999;44(6):362-365.
5. Juvonen T, Satta J, Laitala P, Luukkonen K, Nissinen J. Anomalies at the thoracic outlet are frequent in the general population. Am J Surg. 1995;170(1):33-37.
6. Atasoy E. Thoracic outlet compression syndrome. Orthop Clin North Am. 1996;27(2):265-303.
7. Demondion X, Herbinet P, Van Sint Jan S, Boutry N, Chantelot C, Cotten A. Imaging assessment of thoracic outlet syndrome. Radiographics. 2006;26(6):1735-1750.
8. Demondion X, Bacqueville E, Paul C, Duquesnoy B, Hachulla E, Cotten A. Thoracic outlet: assessment with MR imaging in asymptomatic and symptomatic populations. Radiology. 2003;227(2):461-468.
9. Makhoul RG, Machleder HI. Developmental anomalies at the thoracic outlet: an analysis of 200 consecutive cases. J Vasc Surg. 1992;16(4):534-542.
10. Sanders RJ, Jackson CG, Banchero N, Pearce WH. Scalene muscle abnormalities in traumatic thoracic outlet syndrome. Am J Surg. 1990;159(2):231-236.
11. Katirji B, Hardy RW Jr. Classic neurogenic thoracic outlet syndrome in a competitive swimmer: a true scalenus anticus syndrome. Muscle Nerve. 1995;18(2):229-233.
12. Casbas L, Chauffour X, Cau J, et al. Post-traumatic thoracic outlet syndromes. Ann Vasc Surg. 2005;19(1):25-28.
13. Povlsen B, Belzberg A, Hansson T, Dorsi M. Treatment for thoracic outlet syndrome. Cochrane Database Syst Rev. 2010;(1):CD007218.
14. Cormier JM, Amrane M, Ward A, Laurian C, Gigou F. Arterial complications of the thoracic outlet syndrome: fifty-five operative cases. J Vasc Surg. 1989;9(6):778-787.
15. Hood DB, Kuehne J, Yellin AE, Weaver FA. Vascular complications of thoracic outlet syndrome. Am Surg. 1997;63(10):913-917.
16. Ferrante MA. Brachial plexopathies: classification, causes, and consequences. Muscle Nerve. 2004;30(5):547-568.
17. Gilliatt RW, Le Quesne PM, Logue V, Sumner AJ. Wasting of the hand associated with a cervical rib or band. J Neurol Neurosurg Psychiatry. 1970;33(5):615-624.
18. Ozoa G, Alves D, Fish DE. Thoracic outlet syndrome. Phys Med Rehabil Clin North Am. 2011;22(3):473-483, viii-ix.
19. Schwartzman RJ. Brachial plexus traction injuries. Hand Clin. 1991;7(3):547-556.
20. Christo PJ, McGreevy K. Updated perspectives on neurogenic thoracic outlet syndrome. Curr Pain Headache Rep. 2011;15(1):14-21.
21. Vanti C, Natalini L, Romeo A, Tosarelli D, Pillastrini P. Conservative treatment of thoracic outlet syndrome. A review of the literature. Eura Medicophys. 2007;43(1):55-70.22. Patton GM. Arterial thoracic outlet syndrome. Hand Clin. 2004;20(1):107-111, viii.
23. Lee TS, Hines GL. Cerebral embolic stroke and arm ischemia in a teenager with arterial thoracic outlet syndrome: a case report. Vasc Endovasc Surg. 2007;41(3):254-257.
24. Sanders RJ, Hammond SL. Venous thoracic outlet syndrome. Hand Clin. 2004;20(1):113-118, viii.
25. Sanders RJ, Hammond SL, Rao NM. Diagnosis of thoracic outlet syndrome. J Vasc Surg. 2007;46(3):601-604.
26. Luoma A, Nelems B. Thoracic outlet syndrome. Thoracic surgery perspective. Neurosurg Clin North Am. 1991;2(1):187-226.
27. Cup EH, Ijspeert J, Janssen RJ, et al. Residual complaints after neuralgic amyotrophy. Arch Phys Med Rehabil. 2013;94(1):67-73.
28. van Alfen N, van Engelen BG. The clinical spectrum of neuralgic amyotrophy in 246 cases. Brain. 2006;129(pt 2):438-450.
29. Nichols AW. The thoracic outlet syndrome in athletes. J Am Board Fam Pract. 1996;9(5):346-355.
30. Roos DB, Owens JC. Thoracic outlet syndrome. Arch Surg. 1966;93(1):71-74.
31. Adson AW, Coffey JR. Cervical rib: a method of anterior approach for relief of symptoms by division of the scalenus anticus. Ann Surg. 1927;85(6):839-857.
32. Wright IS. The neurovascular syndrome produced by hyperabduction of the arms. Am Heart J. 1945;29:1-19.
33. Rayan GM, Jensen C. Thoracic outlet syndrome: provocative examination maneuvers in a typical population. J Shoulder Elbow Surg. 1995;4(2):113-117.
34. Nord KM, Kapoor P, Fisher J, et al. False positive rate of thoracic outlet syndrome diagnostic maneuvers. Electromyogr Clin Neurophysiol. 2008;48(2):67-74.
35. Novak CB. Thoracic outlet syndrome. Clin Plast Surg. 2003;30(2):175-188.
36. Gillard J, Pérez-Cousin M, Hachulla E, et al. Diagnosing thoracic outlet syndrome: contribution of provocative tests, ultrasonography, electrophysiology, and helical computed tomography in 48 patients. Joint Bone Spine. 2001;68(5):416-424.
37. Baxter GM, Kincaid W, Jeffrey RF, Millar GM, Porteous C, Morley P. Comparison of colour Doppler ultrasound with venography in the diagnosis of axillary and subclavian vein thrombosis. Br J Radiol. 1991;64(765):777-781.
38. Passman MA, Criado E, Farber MA, et al. Efficacy of color flow duplex imaging for proximal upper extremity venous outflow obstruction in hemodialysis patients. J Vasc Surg. 1998;28(5):869-875.
39. Wadhwani R, Chaubal N, Sukthankar R, Shroff M, Agarwala S. Color Doppler and duplex sonography in 5 patients with thoracic outlet syndrome. J Ultrasound Med. 2001;20(7):795-801.
40. Napoli V, Vignali C, Braccini G, et al. Echography and echo-Doppler in the study of thoracic outlet syndrome. Correlation with angiographic data [in Italian]. Radiol Med. 1993;85(6):733-740.
41. Longley DG, Yedlicka JW, Molina EJ, Schwabacher S, Hunter DW, Letourneau JG. Thoracic outlet syndrome: evaluation of the subclavian vessels by color duplex sonography. AJR Am J Roentgenol. 1992;158(3):623-630.
42. Demondion X, Herbinet P, Boutry N, Fontaine C, Francke JP, Cotten A. Sonographic mapping of the normal brachial plexus. AJNR Am J Neuroradiol. 2003;24(7):1303-1309.
43. Cruz-Martinez A, Arpa J. Electrophysiological assessment in neurogenic thoracic outlet syndrome. Electromyogr Clin Neurophysiol. 2001;41(4):253-256.
44. Ferrante MA, Wilbourn AJ. The utility of various sensory nerve conduction responses in assessing brachial plexopathies. Muscle Nerve. 1995;18(8):879-889.
45. Aminoff MJ, Olney RK, Parry GJ, Raskin NH. Relative utility of different electrophysiologic techniques in the evaluation of brachial plexopathies. Neurology. 1988;38(4):546-550.
46. Komanetsky RM, Novak CB, Mackinnon SE, Russo MH, Padberg AM, Louis S. Somatosensory evoked potentials fail to diagnose thoracic outlet syndrome. J Hand Surg Am. 1996;21(4):662-666.
47. Remy-Jardin M, Remy J, Masson P, et al. Helical CT angiography of thoracic outlet syndrome: functional anatomy. AJR Am J Roentgenol. 2000;174(6):1667-1674.
48. Matsumura JS, Rilling WS, Pearce WH, Nemcek AA Jr, Vogelzang RL, Yao JS. Helical computed tomography of the normal thoracic outlet. J Vasc Surg. 1997;26(5):776-783.
49. Dymarkowski S, Bosmans H, Marchal G, Bogaert J. Three-dimensional MR angiography in the evaluation of thoracic outlet syndrome. AJR Am J Roentgenol. 1999;173(4):1005-1008.
50. Charon JP, Milne W, Sheppard DG, Houston JG. Evaluation of MR angiographic technique in the assessment of thoracic outlet syndrome. Clin Radiol. 2004;59(7):588-595.
51. Cuetter AC, Bartoszek DM. The thoracic outlet syndrome: controversies, overdiagnosis, overtreatment, and recommendations for management. Muscle Nerve. 1989;12(5):410-419.
52. Urschel HC Jr, Razzuk MA. Paget-Schroetter syndrome: what is the best management? Ann Thorac Surg. 2000;69(6):1663-1668.
53. Lee JT, Karwowski JK, Harris EJ, Haukoos JS, Olcott C 4th. Long-term thrombotic recurrence after nonoperative management of Paget-Schroetter syndrome. J Vasc Surg. 2006;43(6):1236-1243.
54. Molina JE, Hunter DW, Dietz CA. Paget-Schroetter syndrome treated with thrombolytics and immediate surgery. J Vasc Surg. 2007;45(2):328-334.
55. Le Forestier N, Mouton P, Maisonobe T, et al. True neurological thoracic outlet syndrome [in French]. Rev Neurol (Paris). 2000;156(1):34-40.
56. Wilbourn AJ. Thoracic outlet syndrome surgery causing severe brachial plexopathy. Muscle Nerve. 1988;11(1):66-74.
57. Likes K, Dapash T, Rochlin DH, Freischlag JA. Remaining or residual first ribs are the cause of recurrent thoracic outlet syndrome. Ann Vasc Surg. 2014;28(4):939-945.
58. Aljabri B, Al-Omran M. Surgical management of vascular thoracic outlet syndrome: a teaching hospital experience. Ann Vasc Dis. 2013;6(1):74-79.
59. Sanders RJ, Pearce WH. The treatment of thoracic outlet syndrome: a comparison of different operations. J Vasc Surg. 1989;10(6):626-634.
60. Franklin GM, Fulton-Kehoe D, Bradley C, Smith-Weller T. Outcome of surgery for thoracic outlet syndrome in Washington state workers’ compensation. Neurology. 2000;54(6):1252-1257.
61. Axelrod DA, Proctor MC, Geisser ME, Roth RS, Greenfield LJ. Outcomes after surgery for thoracic outlet syndrome. J Vasc Surg. 2001;33(6):1220-1225.
62. Taylor JM, Telford RJ, Kinsella DC, Watkinson AF, Thompson JF. Long-term clinical and functional outcome following treatment for Paget-Schroetter syndrome. Br J Surg. 2013;100(11):1459-1464.
63. Schneider DB, Dimuzio PJ, Martin ND, et al. Combination treatment of venous thoracic outlet syndrome: open surgical decompression and intraoperative angioplasty. J Vasc Surg. 2004;40(4):599-603.
Thoracic outlet syndrome (TOS) was first described by Coot in 1861,1,2 and the term was coined by Peet and colleagues3 in 1956 to cover a spectrum of conditions caused by dynamic compression of the brachial plexus (neurogenic), subclavian artery (arterial), or subclavian vein (venous). The estimated incidence of TOS is 10 in 100,000.4 However, cadaveric studies have suggested that up to 90% of the population may have what is considered abnormal anatomy of the thoracic outlet,5 which in turn suggests a multifactorial etiology for symptomatic disease. TOS is most commonly diagnosed in patients 20 to 40 years of age, with females affected in a 4:1 ratio.6 Although historically TOS is a clinical diagnosis, advanced imaging is often helpful in determining the nature and location of the structure undergoing compression and the structure producing compression, which help guide management. Computed tomography angiography (CTA) and magnetic resonance imaging (MRI) performed in association with postural maneuvers aid in the diagnosis in patients with dynamically acquired compression.7
Pathophysiology
The pathophysiology of TOS is attributable to the unique anatomy of the thoracic outlet. Compromise of the neurovascular structures can occur through congenital or acquired narrowing in 3 distinct compartments: the interscalene triangle, the costoclavicular space, and the retropectoralis minor space. The interscalene triangle is the most medial of the compartments. Containing the subclavian artery and the 3 trunks of the brachial plexus, it is bordered anteriorly by the anterior scalene muscle, posteriorly by the middle and posterior scalene muscles, and inferiorly by the first rib. The interscalene triangle is the most frequent site of neurologic compression.8 The middle compartment is the costoclavicular space, which is bordered superiorly by the clavicle, anteriorly by the subclavius muscle, and posteriorly by the first rib and the middle scalene muscle. The costoclavicular space is the most frequent site of arterial compression,8 where the artery lies directly anterior to the subclavian vein and is surrounded by the 3 cords of the brachial plexus. The most lateral compartment is the retropectoralis minor space, which is bordered anteriorly by the pectoralis minor muscle, superiorly by the subscapularis muscle, and inferiorly by the anterior chest wall. Sources of neurovascular compression within any of the spaces include cervical ribs9; elongated C7 transverse processes; hypertrophy of the anterior or middle scalene, subclavius, or pectoralis minor muscles10; anomalous scalenus minimus muscle; repetitive overhead arm movements (pitching, swimming)11; anomalous fascial bands; degenerative spine disease; bone destruction from primary or secondary neoplasms (Pancoast tumor); hyperextension/flexion injury of the neck12; and malunion of clavicle fractures, among others.13
Classification
Three distinct TOSs have been described, individually or combined, depending on the injured component: neurogenic from brachial plexus compression, arterial from subclavian artery compression, and venous from subclavian or axillary vein compression.14,15
Neurogenic TOS has 2 reported types: true (classic) and disputed. True neurogenic TOS is rare, with an estimated incidence of 1 in 1 million.16 First described in 1970 as a lower trunk plexopathy involving slowly progressive unilateral weakness of the intrinsic hand muscles and sensory abnormalities in the ulnar and medial antebrachial cutaneous nerve distributions, true neurogenic TOS was originally called Gilliatt-Sumner hand syndrome.17 A congenital band extending between the first rib and an elongated C7 transverse process was thought to be the location of brachial plexus injury in true neurogenic TOS. Conversely, disputed neurogenic TOS is the most common form of TOS, occurring in 3 to 80 per 100018 and accounting for 90% to 95% of all TOS cases.13,19 In contrast to true neurogenic TOS, in which anatomical and electrodiagnostic evidence supports the diagnosis, objective clinical findings are often lacking in the disputed form.18 Patients with disputed neurogenic TOS present with a diverse array of symptoms, including pain, numbness, and weakness affecting the neck, shoulder, and arm, exacerbated by activities requiring elevation or sustained use of the extremity.20
Arterial TOS accounts for 1% to 5% of all TOS cases.21 Arterial TOS typically affects patients who perform repetitive movements of the upper extremities with their arms above their shoulders, resulting in compression of the subclavian artery. Symptoms of arterial TOS include pain, weakness, coolness, pallor, and paresthesia.18,22 In severe cases of compression, subclavian artery damage can result in thrombosis with distal embolization, poststenotic aneurysm, or even retrograde extension causing stroke.22,23
Last, representing 2% to 3% of all TOS cases, venous TOS results from compression of the subclavian or axillary vein.18,24 Two mechanisms for vascular compromise have been described. The first involves compression of the vein between the clavicle and the first rib with overhead activities.18 Patients often experience intermittent “heaviness” of the extremity with repeated overhead use. The second mechanism involves repeated stress between the clavicle and vein, causing an intravascular thrombosis.18 Patients may experience pain, edema, cyanosis, venous distention, and even spontaneous venous thrombosis, referred to as Paget-Schroetter syndrome, which can lead to pulmonary embolism.6,25,26
Clinical Features
In cases of suspected TOS, clinicians should take a thorough history and perform a thorough physical examination. The differential diagnosis for unilateral, upper limb pain, numbness, tingling, and/or weakness exacerbated by movement includes shoulder and rotator cuff pathology, cervical spine injury, cervical radiculitis, distal compressive neuropathies (carpal or cubital tunnel syndrome), and neuralgic amyotrophy (Parsonage-Turner syndrome/acute brachial radiculitis).27,28 The clinician should pursue a history of trauma to the shoulder or neck as well as any occupational or recreational activities involving elevation of the upper extremity for extended periods.29 Physical examination must include an evaluation of the contralateral side and may begin with visual inspection to assess for muscle asymmetry, atrophy, color changes, edema, or deformities.18 Next, palpation should be used to assess for any tenderness, texture changes, masses, or vascular pulsations. Attention should be directed at examination of the cervical spine as well as neurologic and vascular assessments of the bilateral upper extremities, including range of motion and strength testing,18 to rule out alternative etiologies.
Four basic maneuvers—the Roos test,30 Adson test,31 Wright test,32 and costoclavicular test—traditionally have been used to diagnose TOS. A positive Roos test involves symptom reproduction with the patient slowly opening and closing the hand for 3 minutes with the arm externally rotated and abducted to 90°.33 However, the false-positive rate of the Roos test is as high as 77% in patients with carpal tunnel syndrome and up to 47% in normal subjects.34 The Adson test is performed by having the patient inhale deeply while the arm is kept in the anatomical position with the head extended and turned toward the involved extremity. The examiner monitors the radial pulse; an absent or diminished radial pulse suggests compression of the subclavian artery. The Adson test is not very reliable, however, because the pulse diminishes even in normal subjects,6,26 with a reported false-positive rate of 13.5%.35 A positive costoclavicular compression test occurs when depressing a patient’s shoulder reproduces symptoms. In one study, the false-positive rate of the costoclavicular compression test was 48% in patients with carpal tunnel syndrome and 16% in normal subjects.34 Last, the Wright test is performed by hyperabducting and externally rotating the affected shoulder. It is positive with a diminished pulse or reproduction of symptoms. One study found that the Wright test had 70% to 90% sensitivity and 29% to 53% specificity.36
Clinically distinguishing between the various forms of TOS may be difficult, and occasionally multiple types exist in a single patient, exacerbating one another and adding to the diagnostic difficulty. For example, arterial insufficiency may lead to disruption of the neural microcirculation, leading to concurrent arterial and neurogenic TOS. Because most cases present with nonspecific symptoms, advanced imaging modalities are often required to establish a definitive diagnosis and to target therapy to the appropriate site of compression.
Imaging Features
Plain Radiography
First, cervical spine and chest radiographs should be obtained to assess for bone abnormalities, including cervical ribs, long transverse processes, rib/clavicle fracture callus, rib anomalies, degenerative spine disease, and neoplasm (Pancoast/apical tumor) (Figure 1).18,25
Ultrasonography
Ultrasonography is useful in evaluating arterial or venous TOS because of its low cost, noninvasive nature, and high specificity for vessel occlusion.37,38 In arterial TOS, ultrasound may demonstrate increased flow velocity through a stenosis or an aneurysmal degeneration distal to the stenosis.7 In venous TOS, duplex ultrasound can identify stasis and thrombus.7 Obtaining duplex ultrasound with the upper extremity in multiple positions allows clinicians to correlate dynamically induced symptoms with ultrasonographic findings of altered blood flow.39-41 Despite the purported benefits of ultrasound, its drawback is that it is operator-dependent,42 with some studies reporting a high false-positive rate24 for diagnosis of venous TOS.
Electrodiagnostic Testing
Ruling out etiologies such as cervical radiculitis (Parsonage-Turner syndrome), cervical radiculopathies, brachial plexus lesions, and other distal compressive neuropathies requires nerve conduction studies and electromyography.18,43-46 In true neurogenic TOS, a combination of decreased sensory nerve action potentials in the ulnar and medial antebrachial cutaneous nerves and decreased compound motor action potentials in the median nerve is often found.18 Specifically, an abnormal ulnar sensory nerve action potential suggests the lesion is situated away from the intraspinal canal, which argues against a diagnosis of radiculopathy or myelopathy.43,44 In the disputed form of neurogenic TOS, the role of electrodiagnostic testing is less clear.18
Conventional Arteriography and Venography
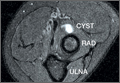
Although CTA has superseded conventional arteriography and venography in most treatment centers, it may still be used in patients with acute symptoms requiring immediate thrombolytic therapy. Catheter angiography and venography with postural maneuvers are often the first invasive treatment modality in cases of thoracic outlet vascular compression.22,24 Presence of intraluminal thrombus, vessel dilatation, and collateral vessels is readily demonstrated (Figure 2A). Recanalization of occluded vessels can be attempted using balloon angioplasty and venoplasty (Figure 2B), but it is usually only temporarily successful if the cause of extrinsic compression is not corrected (Figures 2C, 2D). CTA or conventional angiography, used if sophisticated CTA with 3-dimensional (3-D) reconstruction is unavailable, is the gold standard in diagnosis of TOS.
CTA and Venography
Computed tomography (CT) is a valuable modality because it can be performed rapidly and effectively to depict the relationship of vascular structures to surrounding bone and muscle.47 In addition, CTA and venography provide high-quality representations of the vasculature, and 3-D reconstruction reliably identifies areas of neurovascular compression in patients with TOS.47,48 Furthermore, CT may be performed in a dynamic fashion, with the upper extremity in various positions to reproduce dynamic compression of the neurovascular structures (Figure 3A). Comparison of the images with the upper extremities in the anatomical position and elevated allows the physician to evaluate narrowing of the compartments and dynamic compression of neurovascular structures.8 CT is particularly valuable in arterial and venous TOS. In arterial TOS, the cross-sectional area or diameter of the artery can be measured to calculate the degree of stenosis.8,47 In venous TOS, dynamic narrowing of the vein can be visualized and may be associated with venous thrombosis or collateral circulation (Figure 3B). Although a variety of maneuvers is possible during CTA, the size of the CT tunnel as well as mandatory supine positioning of the patient may limit the series. Drawbacks of CT for diagnosing TOS include difficulties in analyzing the brachial plexus because of limited contrast resolution. In addition, the risks of CT (ionizing radiation, administration of iodinated contrast medium) must be considered before image acquisition.
MRI
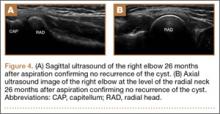
MRI is a noninvasive and nonionizing technique that offers good resolution of the anatomical components of the thoracic outlet8 and that, because of its superior soft-tissue contrast, is the modality of choice for imaging brachial plexus nerve compression in TOS (Figure 4). Neurologic compression is identified with MRI when the fat surrounding the brachial plexus disappears.8 MRI reliably identifies the source of compression, which may include bony structures, muscle hypertrophy (scalenus, scalenus minimus, subclavius, pectoralis minor), and fibrous bands.49 Because of their craniocaudal direction, the sagittal plane is often most useful in demonstrating neurovascular compression.42 Analyzing the caliber of the vessel along its course may evaluate vascular compression, and magnetic resonance (MR) angiography and venography (Figures 5A, 5B) can often complement the findings.50 Specifically, in arterial TOS, poststenotic aneurysmal dilatation may be seen, whereas thrombosis and collateral circulation can be visualized in cases of venous TOS.50 Limitations of MRI in the diagnosis of TOS historically were similar to those of CT, and included supine positioning as well as restricted upper extremity maneuvers because of the size of the tunnel and the presence of surface coils.42 However, newer higher channel surface coils and wider bores allow for imaging in a wider range of motion, including arm hyperabduction (Figures 5C, 5D), which is often necessary to elicit pathology.
Management
Generally, therapeutic options for TOS are aimed at relieving the source of neurovascular compression. It is important that treatment be directed only toward symptomatic patients, as many patients have anatomy consistent with TOS and remain asymptomatic.5 Treatment of TOS is predominately conservative and involves a combination of patient education, activity modification, medication, and rehabilitation to promote appropriate body mechanics and posture.18
Physical Therapy
Physical therapy should be aimed at decreasing pressure on the neurovascular structures of the thoracic outlet by relaxing the scalene muscles, strengthening the shoulder muscles, and working on postural exercises to help the patient sit and stand straighter.51 The scalene muscles are the primary targets for TOS rehabilitation, but focus should also be given to the upper trapezius, levator scapulae, sternocleidomastoid, pectoral, and suboccipital muscles.18 Physical therapy is often combined with hydrotherapy, massage, nonsteroidal anti-inflammatory drugs, and muscle relaxants for maximal symptomatic relief. Some patients have found relief with selective anesthetic or botulinum toxin A injections in the scalene muscles.18 A minimum of 4 to 6 weeks (often 4-6 months) of physical therapy and conservative treatment should be attempted before consideration of any invasive intervention.13,18
Anticoagulation
In venous TOS with evidence of thrombus but no obstructive clot, conservative management is typically sufficient. In rare cases, however, intimal damage secondary to vascular compression in arterial and venous TOS leads to thrombus formation, impairing upper extremity perfusion and producing symptoms. Treatment guidelines for venous TOS involve catheter-directed thrombolysis within 2 weeks of symptom onset.15 Thrombolysis replaced the prior recommendation of systemic anticoagulation combined with extremity rest and elevation because anticoagulation and rest alone result in up to 75% morbidity,52,53 whereas thrombolysis reestablishes vessel patency in nearly all patients.54 After thrombolysis, patients should receive intravenous heparin, and conversion to oral anticoagulation should occur as soon as manageable. In patients with arterial TOS, the goal of treatment is revascularization to prevent or decrease ischemia. In mild arterial ischemia, catheter-directed thrombolysis can be attempted. However, the threshold for surgical thromboembolectomy must remain low, as acute upper extremity ischemia may result in compartment syndrome and permanent loss of function.13 Fixed arterial lesions, whether occlusive or aneurysmal, are an absolute indication for thromboembolectomy with possible thoracic outlet decompression.13
Thoracic Outlet Decompression
Indications for surgical decompression are controversial. They include symptomatic patients who have vascular (arterial or venous) TOS and are not at high risk for surgery, patients with true neurologic TOS and acute progressive neurologic weakness or disabling pain,55 and patients who have disputed neurologic TOS and have failed conservative management—keeping in mind that high recurrence rates and iatrogenic brachial plexopathy have been reported in this population.56 In general, surgical procedures are aimed at reducing soft-tissue compression (scalene release or neurolysis) or bony compression (cervical or first thoracic rib excision). Three surgical approaches (transaxillary, supraclavicular, infraclavicular) are commonly used for decompression, and surgeons choose one over another depending on the anatomical abnormality causing the compression. The transaxillary approach requires limited dissection but still allows for adequate visualization of the rib during resection.57 In this approach, a transverse incision along the inferior border of the axilla extends from the pectoralis major to the latissimus dorsi. After dissection of the axillary vessels and the first thoracic nerve root, the first rib is identified and can be removed, when indicated. In contrast, the supraclavicular approach provides a wide exposure, and the site of compression is directly visualized, allowing for arterial reconstruction.58 Through this approach, the anterior and middle scalene muscles can be resected, and neurolysis of the brachial plexus can be performed. Last, the infraclavicular approach allows for exposure of the central veins through extension of the incision medially, which allows for venous reconstruction. Some patients with neurogenic or arterial TOS present with symptoms of sympathetic overactivity, in which case cervical sympathectomy can be used with decompression.
Outcomes of surgical decompression for TOS depend on the clinical type but are generally good. For instance, in cases of disputed neurogenic TOS, symptom resolution after decompression is reportedly between 80% and 90%.59 However, major depression, work-related injuries,60 and diffuse preoperative arm symptoms61 all influence long-term results. In true neurogenic TOS, postoperative pain relief is often substantial, though recovery of strength can be slow because of the axonal injury.55 In arterial TOS, outcomes are influenced by time to surgical intervention, with early surgery demonstrating better outcomes than later surgery.62 In one study, Cormier and colleagues14 evaluated 47 patients who underwent correction of subclavian-axillary artery compression; 91% were asymptomatic a mean of 5.7 months after decompression. Last, outcomes of successful thrombolysis and decompression for venous TOS demonstrated patency rates higher than 95% at 5-year follow-up.54,63
Conclusions
TOS is a spectrum of disorders caused by compression of the brachial plexus, subclavian artery, or subclavian vein. Early recognition of TOS is imperative, as diagnostic or treatment delays may be associated with significant morbidity. Clinical examination alone is often inadequate for determining the compression site and the structure causing compression. CTA and MRI performed in association with postural maneuvers may demonstrate dynamic compression of the neurovascular structures in the thoracic outlet. These imaging modalities reliably identify the structures causing compression and can be crucial for effective management.
Thoracic outlet syndrome (TOS) was first described by Coot in 1861,1,2 and the term was coined by Peet and colleagues3 in 1956 to cover a spectrum of conditions caused by dynamic compression of the brachial plexus (neurogenic), subclavian artery (arterial), or subclavian vein (venous). The estimated incidence of TOS is 10 in 100,000.4 However, cadaveric studies have suggested that up to 90% of the population may have what is considered abnormal anatomy of the thoracic outlet,5 which in turn suggests a multifactorial etiology for symptomatic disease. TOS is most commonly diagnosed in patients 20 to 40 years of age, with females affected in a 4:1 ratio.6 Although historically TOS is a clinical diagnosis, advanced imaging is often helpful in determining the nature and location of the structure undergoing compression and the structure producing compression, which help guide management. Computed tomography angiography (CTA) and magnetic resonance imaging (MRI) performed in association with postural maneuvers aid in the diagnosis in patients with dynamically acquired compression.7
Pathophysiology
The pathophysiology of TOS is attributable to the unique anatomy of the thoracic outlet. Compromise of the neurovascular structures can occur through congenital or acquired narrowing in 3 distinct compartments: the interscalene triangle, the costoclavicular space, and the retropectoralis minor space. The interscalene triangle is the most medial of the compartments. Containing the subclavian artery and the 3 trunks of the brachial plexus, it is bordered anteriorly by the anterior scalene muscle, posteriorly by the middle and posterior scalene muscles, and inferiorly by the first rib. The interscalene triangle is the most frequent site of neurologic compression.8 The middle compartment is the costoclavicular space, which is bordered superiorly by the clavicle, anteriorly by the subclavius muscle, and posteriorly by the first rib and the middle scalene muscle. The costoclavicular space is the most frequent site of arterial compression,8 where the artery lies directly anterior to the subclavian vein and is surrounded by the 3 cords of the brachial plexus. The most lateral compartment is the retropectoralis minor space, which is bordered anteriorly by the pectoralis minor muscle, superiorly by the subscapularis muscle, and inferiorly by the anterior chest wall. Sources of neurovascular compression within any of the spaces include cervical ribs9; elongated C7 transverse processes; hypertrophy of the anterior or middle scalene, subclavius, or pectoralis minor muscles10; anomalous scalenus minimus muscle; repetitive overhead arm movements (pitching, swimming)11; anomalous fascial bands; degenerative spine disease; bone destruction from primary or secondary neoplasms (Pancoast tumor); hyperextension/flexion injury of the neck12; and malunion of clavicle fractures, among others.13
Classification
Three distinct TOSs have been described, individually or combined, depending on the injured component: neurogenic from brachial plexus compression, arterial from subclavian artery compression, and venous from subclavian or axillary vein compression.14,15
Neurogenic TOS has 2 reported types: true (classic) and disputed. True neurogenic TOS is rare, with an estimated incidence of 1 in 1 million.16 First described in 1970 as a lower trunk plexopathy involving slowly progressive unilateral weakness of the intrinsic hand muscles and sensory abnormalities in the ulnar and medial antebrachial cutaneous nerve distributions, true neurogenic TOS was originally called Gilliatt-Sumner hand syndrome.17 A congenital band extending between the first rib and an elongated C7 transverse process was thought to be the location of brachial plexus injury in true neurogenic TOS. Conversely, disputed neurogenic TOS is the most common form of TOS, occurring in 3 to 80 per 100018 and accounting for 90% to 95% of all TOS cases.13,19 In contrast to true neurogenic TOS, in which anatomical and electrodiagnostic evidence supports the diagnosis, objective clinical findings are often lacking in the disputed form.18 Patients with disputed neurogenic TOS present with a diverse array of symptoms, including pain, numbness, and weakness affecting the neck, shoulder, and arm, exacerbated by activities requiring elevation or sustained use of the extremity.20
Arterial TOS accounts for 1% to 5% of all TOS cases.21 Arterial TOS typically affects patients who perform repetitive movements of the upper extremities with their arms above their shoulders, resulting in compression of the subclavian artery. Symptoms of arterial TOS include pain, weakness, coolness, pallor, and paresthesia.18,22 In severe cases of compression, subclavian artery damage can result in thrombosis with distal embolization, poststenotic aneurysm, or even retrograde extension causing stroke.22,23
Last, representing 2% to 3% of all TOS cases, venous TOS results from compression of the subclavian or axillary vein.18,24 Two mechanisms for vascular compromise have been described. The first involves compression of the vein between the clavicle and the first rib with overhead activities.18 Patients often experience intermittent “heaviness” of the extremity with repeated overhead use. The second mechanism involves repeated stress between the clavicle and vein, causing an intravascular thrombosis.18 Patients may experience pain, edema, cyanosis, venous distention, and even spontaneous venous thrombosis, referred to as Paget-Schroetter syndrome, which can lead to pulmonary embolism.6,25,26
Clinical Features
In cases of suspected TOS, clinicians should take a thorough history and perform a thorough physical examination. The differential diagnosis for unilateral, upper limb pain, numbness, tingling, and/or weakness exacerbated by movement includes shoulder and rotator cuff pathology, cervical spine injury, cervical radiculitis, distal compressive neuropathies (carpal or cubital tunnel syndrome), and neuralgic amyotrophy (Parsonage-Turner syndrome/acute brachial radiculitis).27,28 The clinician should pursue a history of trauma to the shoulder or neck as well as any occupational or recreational activities involving elevation of the upper extremity for extended periods.29 Physical examination must include an evaluation of the contralateral side and may begin with visual inspection to assess for muscle asymmetry, atrophy, color changes, edema, or deformities.18 Next, palpation should be used to assess for any tenderness, texture changes, masses, or vascular pulsations. Attention should be directed at examination of the cervical spine as well as neurologic and vascular assessments of the bilateral upper extremities, including range of motion and strength testing,18 to rule out alternative etiologies.
Four basic maneuvers—the Roos test,30 Adson test,31 Wright test,32 and costoclavicular test—traditionally have been used to diagnose TOS. A positive Roos test involves symptom reproduction with the patient slowly opening and closing the hand for 3 minutes with the arm externally rotated and abducted to 90°.33 However, the false-positive rate of the Roos test is as high as 77% in patients with carpal tunnel syndrome and up to 47% in normal subjects.34 The Adson test is performed by having the patient inhale deeply while the arm is kept in the anatomical position with the head extended and turned toward the involved extremity. The examiner monitors the radial pulse; an absent or diminished radial pulse suggests compression of the subclavian artery. The Adson test is not very reliable, however, because the pulse diminishes even in normal subjects,6,26 with a reported false-positive rate of 13.5%.35 A positive costoclavicular compression test occurs when depressing a patient’s shoulder reproduces symptoms. In one study, the false-positive rate of the costoclavicular compression test was 48% in patients with carpal tunnel syndrome and 16% in normal subjects.34 Last, the Wright test is performed by hyperabducting and externally rotating the affected shoulder. It is positive with a diminished pulse or reproduction of symptoms. One study found that the Wright test had 70% to 90% sensitivity and 29% to 53% specificity.36
Clinically distinguishing between the various forms of TOS may be difficult, and occasionally multiple types exist in a single patient, exacerbating one another and adding to the diagnostic difficulty. For example, arterial insufficiency may lead to disruption of the neural microcirculation, leading to concurrent arterial and neurogenic TOS. Because most cases present with nonspecific symptoms, advanced imaging modalities are often required to establish a definitive diagnosis and to target therapy to the appropriate site of compression.
Imaging Features
Plain Radiography
First, cervical spine and chest radiographs should be obtained to assess for bone abnormalities, including cervical ribs, long transverse processes, rib/clavicle fracture callus, rib anomalies, degenerative spine disease, and neoplasm (Pancoast/apical tumor) (Figure 1).18,25
Ultrasonography
Ultrasonography is useful in evaluating arterial or venous TOS because of its low cost, noninvasive nature, and high specificity for vessel occlusion.37,38 In arterial TOS, ultrasound may demonstrate increased flow velocity through a stenosis or an aneurysmal degeneration distal to the stenosis.7 In venous TOS, duplex ultrasound can identify stasis and thrombus.7 Obtaining duplex ultrasound with the upper extremity in multiple positions allows clinicians to correlate dynamically induced symptoms with ultrasonographic findings of altered blood flow.39-41 Despite the purported benefits of ultrasound, its drawback is that it is operator-dependent,42 with some studies reporting a high false-positive rate24 for diagnosis of venous TOS.
Electrodiagnostic Testing
Ruling out etiologies such as cervical radiculitis (Parsonage-Turner syndrome), cervical radiculopathies, brachial plexus lesions, and other distal compressive neuropathies requires nerve conduction studies and electromyography.18,43-46 In true neurogenic TOS, a combination of decreased sensory nerve action potentials in the ulnar and medial antebrachial cutaneous nerves and decreased compound motor action potentials in the median nerve is often found.18 Specifically, an abnormal ulnar sensory nerve action potential suggests the lesion is situated away from the intraspinal canal, which argues against a diagnosis of radiculopathy or myelopathy.43,44 In the disputed form of neurogenic TOS, the role of electrodiagnostic testing is less clear.18
Conventional Arteriography and Venography
Although CTA has superseded conventional arteriography and venography in most treatment centers, it may still be used in patients with acute symptoms requiring immediate thrombolytic therapy. Catheter angiography and venography with postural maneuvers are often the first invasive treatment modality in cases of thoracic outlet vascular compression.22,24 Presence of intraluminal thrombus, vessel dilatation, and collateral vessels is readily demonstrated (Figure 2A). Recanalization of occluded vessels can be attempted using balloon angioplasty and venoplasty (Figure 2B), but it is usually only temporarily successful if the cause of extrinsic compression is not corrected (Figures 2C, 2D). CTA or conventional angiography, used if sophisticated CTA with 3-dimensional (3-D) reconstruction is unavailable, is the gold standard in diagnosis of TOS.
CTA and Venography
Computed tomography (CT) is a valuable modality because it can be performed rapidly and effectively to depict the relationship of vascular structures to surrounding bone and muscle.47 In addition, CTA and venography provide high-quality representations of the vasculature, and 3-D reconstruction reliably identifies areas of neurovascular compression in patients with TOS.47,48 Furthermore, CT may be performed in a dynamic fashion, with the upper extremity in various positions to reproduce dynamic compression of the neurovascular structures (Figure 3A). Comparison of the images with the upper extremities in the anatomical position and elevated allows the physician to evaluate narrowing of the compartments and dynamic compression of neurovascular structures.8 CT is particularly valuable in arterial and venous TOS. In arterial TOS, the cross-sectional area or diameter of the artery can be measured to calculate the degree of stenosis.8,47 In venous TOS, dynamic narrowing of the vein can be visualized and may be associated with venous thrombosis or collateral circulation (Figure 3B). Although a variety of maneuvers is possible during CTA, the size of the CT tunnel as well as mandatory supine positioning of the patient may limit the series. Drawbacks of CT for diagnosing TOS include difficulties in analyzing the brachial plexus because of limited contrast resolution. In addition, the risks of CT (ionizing radiation, administration of iodinated contrast medium) must be considered before image acquisition.
MRI
MRI is a noninvasive and nonionizing technique that offers good resolution of the anatomical components of the thoracic outlet8 and that, because of its superior soft-tissue contrast, is the modality of choice for imaging brachial plexus nerve compression in TOS (Figure 4). Neurologic compression is identified with MRI when the fat surrounding the brachial plexus disappears.8 MRI reliably identifies the source of compression, which may include bony structures, muscle hypertrophy (scalenus, scalenus minimus, subclavius, pectoralis minor), and fibrous bands.49 Because of their craniocaudal direction, the sagittal plane is often most useful in demonstrating neurovascular compression.42 Analyzing the caliber of the vessel along its course may evaluate vascular compression, and magnetic resonance (MR) angiography and venography (Figures 5A, 5B) can often complement the findings.50 Specifically, in arterial TOS, poststenotic aneurysmal dilatation may be seen, whereas thrombosis and collateral circulation can be visualized in cases of venous TOS.50 Limitations of MRI in the diagnosis of TOS historically were similar to those of CT, and included supine positioning as well as restricted upper extremity maneuvers because of the size of the tunnel and the presence of surface coils.42 However, newer higher channel surface coils and wider bores allow for imaging in a wider range of motion, including arm hyperabduction (Figures 5C, 5D), which is often necessary to elicit pathology.
Management
Generally, therapeutic options for TOS are aimed at relieving the source of neurovascular compression. It is important that treatment be directed only toward symptomatic patients, as many patients have anatomy consistent with TOS and remain asymptomatic.5 Treatment of TOS is predominately conservative and involves a combination of patient education, activity modification, medication, and rehabilitation to promote appropriate body mechanics and posture.18
Physical Therapy
Physical therapy should be aimed at decreasing pressure on the neurovascular structures of the thoracic outlet by relaxing the scalene muscles, strengthening the shoulder muscles, and working on postural exercises to help the patient sit and stand straighter.51 The scalene muscles are the primary targets for TOS rehabilitation, but focus should also be given to the upper trapezius, levator scapulae, sternocleidomastoid, pectoral, and suboccipital muscles.18 Physical therapy is often combined with hydrotherapy, massage, nonsteroidal anti-inflammatory drugs, and muscle relaxants for maximal symptomatic relief. Some patients have found relief with selective anesthetic or botulinum toxin A injections in the scalene muscles.18 A minimum of 4 to 6 weeks (often 4-6 months) of physical therapy and conservative treatment should be attempted before consideration of any invasive intervention.13,18
Anticoagulation
In venous TOS with evidence of thrombus but no obstructive clot, conservative management is typically sufficient. In rare cases, however, intimal damage secondary to vascular compression in arterial and venous TOS leads to thrombus formation, impairing upper extremity perfusion and producing symptoms. Treatment guidelines for venous TOS involve catheter-directed thrombolysis within 2 weeks of symptom onset.15 Thrombolysis replaced the prior recommendation of systemic anticoagulation combined with extremity rest and elevation because anticoagulation and rest alone result in up to 75% morbidity,52,53 whereas thrombolysis reestablishes vessel patency in nearly all patients.54 After thrombolysis, patients should receive intravenous heparin, and conversion to oral anticoagulation should occur as soon as manageable. In patients with arterial TOS, the goal of treatment is revascularization to prevent or decrease ischemia. In mild arterial ischemia, catheter-directed thrombolysis can be attempted. However, the threshold for surgical thromboembolectomy must remain low, as acute upper extremity ischemia may result in compartment syndrome and permanent loss of function.13 Fixed arterial lesions, whether occlusive or aneurysmal, are an absolute indication for thromboembolectomy with possible thoracic outlet decompression.13
Thoracic Outlet Decompression
Indications for surgical decompression are controversial. They include symptomatic patients who have vascular (arterial or venous) TOS and are not at high risk for surgery, patients with true neurologic TOS and acute progressive neurologic weakness or disabling pain,55 and patients who have disputed neurologic TOS and have failed conservative management—keeping in mind that high recurrence rates and iatrogenic brachial plexopathy have been reported in this population.56 In general, surgical procedures are aimed at reducing soft-tissue compression (scalene release or neurolysis) or bony compression (cervical or first thoracic rib excision). Three surgical approaches (transaxillary, supraclavicular, infraclavicular) are commonly used for decompression, and surgeons choose one over another depending on the anatomical abnormality causing the compression. The transaxillary approach requires limited dissection but still allows for adequate visualization of the rib during resection.57 In this approach, a transverse incision along the inferior border of the axilla extends from the pectoralis major to the latissimus dorsi. After dissection of the axillary vessels and the first thoracic nerve root, the first rib is identified and can be removed, when indicated. In contrast, the supraclavicular approach provides a wide exposure, and the site of compression is directly visualized, allowing for arterial reconstruction.58 Through this approach, the anterior and middle scalene muscles can be resected, and neurolysis of the brachial plexus can be performed. Last, the infraclavicular approach allows for exposure of the central veins through extension of the incision medially, which allows for venous reconstruction. Some patients with neurogenic or arterial TOS present with symptoms of sympathetic overactivity, in which case cervical sympathectomy can be used with decompression.
Outcomes of surgical decompression for TOS depend on the clinical type but are generally good. For instance, in cases of disputed neurogenic TOS, symptom resolution after decompression is reportedly between 80% and 90%.59 However, major depression, work-related injuries,60 and diffuse preoperative arm symptoms61 all influence long-term results. In true neurogenic TOS, postoperative pain relief is often substantial, though recovery of strength can be slow because of the axonal injury.55 In arterial TOS, outcomes are influenced by time to surgical intervention, with early surgery demonstrating better outcomes than later surgery.62 In one study, Cormier and colleagues14 evaluated 47 patients who underwent correction of subclavian-axillary artery compression; 91% were asymptomatic a mean of 5.7 months after decompression. Last, outcomes of successful thrombolysis and decompression for venous TOS demonstrated patency rates higher than 95% at 5-year follow-up.54,63
Conclusions
TOS is a spectrum of disorders caused by compression of the brachial plexus, subclavian artery, or subclavian vein. Early recognition of TOS is imperative, as diagnostic or treatment delays may be associated with significant morbidity. Clinical examination alone is often inadequate for determining the compression site and the structure causing compression. CTA and MRI performed in association with postural maneuvers may demonstrate dynamic compression of the neurovascular structures in the thoracic outlet. These imaging modalities reliably identify the structures causing compression and can be crucial for effective management.
1. Urschel HC Jr. The history of surgery for thoracic outlet syndrome. Chest Surg Clin North Am. 2000;10(1):183-188, x-xi.
2. Atasoy E. History of thoracic outlet syndrome. Hand Clin. 2004;20(1):15-16, v.
3. Peet RM, Henriksen JD, Anderson TP, Martin GM. Thoracic-outlet syndrome: evaluation of a therapeutic exercise program. Proc Staff Meet Mayo Clin. 1956;31(9):281-287.
4. Edwards DP, Mulkern E, Raja AN, Barker P. Trans-axillary first rib excision for thoracic outlet syndrome. J R Coll Surg Edinb. 1999;44(6):362-365.
5. Juvonen T, Satta J, Laitala P, Luukkonen K, Nissinen J. Anomalies at the thoracic outlet are frequent in the general population. Am J Surg. 1995;170(1):33-37.
6. Atasoy E. Thoracic outlet compression syndrome. Orthop Clin North Am. 1996;27(2):265-303.
7. Demondion X, Herbinet P, Van Sint Jan S, Boutry N, Chantelot C, Cotten A. Imaging assessment of thoracic outlet syndrome. Radiographics. 2006;26(6):1735-1750.
8. Demondion X, Bacqueville E, Paul C, Duquesnoy B, Hachulla E, Cotten A. Thoracic outlet: assessment with MR imaging in asymptomatic and symptomatic populations. Radiology. 2003;227(2):461-468.
9. Makhoul RG, Machleder HI. Developmental anomalies at the thoracic outlet: an analysis of 200 consecutive cases. J Vasc Surg. 1992;16(4):534-542.
10. Sanders RJ, Jackson CG, Banchero N, Pearce WH. Scalene muscle abnormalities in traumatic thoracic outlet syndrome. Am J Surg. 1990;159(2):231-236.
11. Katirji B, Hardy RW Jr. Classic neurogenic thoracic outlet syndrome in a competitive swimmer: a true scalenus anticus syndrome. Muscle Nerve. 1995;18(2):229-233.
12. Casbas L, Chauffour X, Cau J, et al. Post-traumatic thoracic outlet syndromes. Ann Vasc Surg. 2005;19(1):25-28.
13. Povlsen B, Belzberg A, Hansson T, Dorsi M. Treatment for thoracic outlet syndrome. Cochrane Database Syst Rev. 2010;(1):CD007218.
14. Cormier JM, Amrane M, Ward A, Laurian C, Gigou F. Arterial complications of the thoracic outlet syndrome: fifty-five operative cases. J Vasc Surg. 1989;9(6):778-787.
15. Hood DB, Kuehne J, Yellin AE, Weaver FA. Vascular complications of thoracic outlet syndrome. Am Surg. 1997;63(10):913-917.
16. Ferrante MA. Brachial plexopathies: classification, causes, and consequences. Muscle Nerve. 2004;30(5):547-568.
17. Gilliatt RW, Le Quesne PM, Logue V, Sumner AJ. Wasting of the hand associated with a cervical rib or band. J Neurol Neurosurg Psychiatry. 1970;33(5):615-624.
18. Ozoa G, Alves D, Fish DE. Thoracic outlet syndrome. Phys Med Rehabil Clin North Am. 2011;22(3):473-483, viii-ix.
19. Schwartzman RJ. Brachial plexus traction injuries. Hand Clin. 1991;7(3):547-556.
20. Christo PJ, McGreevy K. Updated perspectives on neurogenic thoracic outlet syndrome. Curr Pain Headache Rep. 2011;15(1):14-21.
21. Vanti C, Natalini L, Romeo A, Tosarelli D, Pillastrini P. Conservative treatment of thoracic outlet syndrome. A review of the literature. Eura Medicophys. 2007;43(1):55-70.22. Patton GM. Arterial thoracic outlet syndrome. Hand Clin. 2004;20(1):107-111, viii.
23. Lee TS, Hines GL. Cerebral embolic stroke and arm ischemia in a teenager with arterial thoracic outlet syndrome: a case report. Vasc Endovasc Surg. 2007;41(3):254-257.
24. Sanders RJ, Hammond SL. Venous thoracic outlet syndrome. Hand Clin. 2004;20(1):113-118, viii.
25. Sanders RJ, Hammond SL, Rao NM. Diagnosis of thoracic outlet syndrome. J Vasc Surg. 2007;46(3):601-604.
26. Luoma A, Nelems B. Thoracic outlet syndrome. Thoracic surgery perspective. Neurosurg Clin North Am. 1991;2(1):187-226.
27. Cup EH, Ijspeert J, Janssen RJ, et al. Residual complaints after neuralgic amyotrophy. Arch Phys Med Rehabil. 2013;94(1):67-73.
28. van Alfen N, van Engelen BG. The clinical spectrum of neuralgic amyotrophy in 246 cases. Brain. 2006;129(pt 2):438-450.
29. Nichols AW. The thoracic outlet syndrome in athletes. J Am Board Fam Pract. 1996;9(5):346-355.
30. Roos DB, Owens JC. Thoracic outlet syndrome. Arch Surg. 1966;93(1):71-74.
31. Adson AW, Coffey JR. Cervical rib: a method of anterior approach for relief of symptoms by division of the scalenus anticus. Ann Surg. 1927;85(6):839-857.
32. Wright IS. The neurovascular syndrome produced by hyperabduction of the arms. Am Heart J. 1945;29:1-19.
33. Rayan GM, Jensen C. Thoracic outlet syndrome: provocative examination maneuvers in a typical population. J Shoulder Elbow Surg. 1995;4(2):113-117.
34. Nord KM, Kapoor P, Fisher J, et al. False positive rate of thoracic outlet syndrome diagnostic maneuvers. Electromyogr Clin Neurophysiol. 2008;48(2):67-74.
35. Novak CB. Thoracic outlet syndrome. Clin Plast Surg. 2003;30(2):175-188.
36. Gillard J, Pérez-Cousin M, Hachulla E, et al. Diagnosing thoracic outlet syndrome: contribution of provocative tests, ultrasonography, electrophysiology, and helical computed tomography in 48 patients. Joint Bone Spine. 2001;68(5):416-424.
37. Baxter GM, Kincaid W, Jeffrey RF, Millar GM, Porteous C, Morley P. Comparison of colour Doppler ultrasound with venography in the diagnosis of axillary and subclavian vein thrombosis. Br J Radiol. 1991;64(765):777-781.
38. Passman MA, Criado E, Farber MA, et al. Efficacy of color flow duplex imaging for proximal upper extremity venous outflow obstruction in hemodialysis patients. J Vasc Surg. 1998;28(5):869-875.
39. Wadhwani R, Chaubal N, Sukthankar R, Shroff M, Agarwala S. Color Doppler and duplex sonography in 5 patients with thoracic outlet syndrome. J Ultrasound Med. 2001;20(7):795-801.
40. Napoli V, Vignali C, Braccini G, et al. Echography and echo-Doppler in the study of thoracic outlet syndrome. Correlation with angiographic data [in Italian]. Radiol Med. 1993;85(6):733-740.
41. Longley DG, Yedlicka JW, Molina EJ, Schwabacher S, Hunter DW, Letourneau JG. Thoracic outlet syndrome: evaluation of the subclavian vessels by color duplex sonography. AJR Am J Roentgenol. 1992;158(3):623-630.
42. Demondion X, Herbinet P, Boutry N, Fontaine C, Francke JP, Cotten A. Sonographic mapping of the normal brachial plexus. AJNR Am J Neuroradiol. 2003;24(7):1303-1309.
43. Cruz-Martinez A, Arpa J. Electrophysiological assessment in neurogenic thoracic outlet syndrome. Electromyogr Clin Neurophysiol. 2001;41(4):253-256.
44. Ferrante MA, Wilbourn AJ. The utility of various sensory nerve conduction responses in assessing brachial plexopathies. Muscle Nerve. 1995;18(8):879-889.
45. Aminoff MJ, Olney RK, Parry GJ, Raskin NH. Relative utility of different electrophysiologic techniques in the evaluation of brachial plexopathies. Neurology. 1988;38(4):546-550.
46. Komanetsky RM, Novak CB, Mackinnon SE, Russo MH, Padberg AM, Louis S. Somatosensory evoked potentials fail to diagnose thoracic outlet syndrome. J Hand Surg Am. 1996;21(4):662-666.
47. Remy-Jardin M, Remy J, Masson P, et al. Helical CT angiography of thoracic outlet syndrome: functional anatomy. AJR Am J Roentgenol. 2000;174(6):1667-1674.
48. Matsumura JS, Rilling WS, Pearce WH, Nemcek AA Jr, Vogelzang RL, Yao JS. Helical computed tomography of the normal thoracic outlet. J Vasc Surg. 1997;26(5):776-783.
49. Dymarkowski S, Bosmans H, Marchal G, Bogaert J. Three-dimensional MR angiography in the evaluation of thoracic outlet syndrome. AJR Am J Roentgenol. 1999;173(4):1005-1008.
50. Charon JP, Milne W, Sheppard DG, Houston JG. Evaluation of MR angiographic technique in the assessment of thoracic outlet syndrome. Clin Radiol. 2004;59(7):588-595.
51. Cuetter AC, Bartoszek DM. The thoracic outlet syndrome: controversies, overdiagnosis, overtreatment, and recommendations for management. Muscle Nerve. 1989;12(5):410-419.
52. Urschel HC Jr, Razzuk MA. Paget-Schroetter syndrome: what is the best management? Ann Thorac Surg. 2000;69(6):1663-1668.
53. Lee JT, Karwowski JK, Harris EJ, Haukoos JS, Olcott C 4th. Long-term thrombotic recurrence after nonoperative management of Paget-Schroetter syndrome. J Vasc Surg. 2006;43(6):1236-1243.
54. Molina JE, Hunter DW, Dietz CA. Paget-Schroetter syndrome treated with thrombolytics and immediate surgery. J Vasc Surg. 2007;45(2):328-334.
55. Le Forestier N, Mouton P, Maisonobe T, et al. True neurological thoracic outlet syndrome [in French]. Rev Neurol (Paris). 2000;156(1):34-40.
56. Wilbourn AJ. Thoracic outlet syndrome surgery causing severe brachial plexopathy. Muscle Nerve. 1988;11(1):66-74.
57. Likes K, Dapash T, Rochlin DH, Freischlag JA. Remaining or residual first ribs are the cause of recurrent thoracic outlet syndrome. Ann Vasc Surg. 2014;28(4):939-945.
58. Aljabri B, Al-Omran M. Surgical management of vascular thoracic outlet syndrome: a teaching hospital experience. Ann Vasc Dis. 2013;6(1):74-79.
59. Sanders RJ, Pearce WH. The treatment of thoracic outlet syndrome: a comparison of different operations. J Vasc Surg. 1989;10(6):626-634.
60. Franklin GM, Fulton-Kehoe D, Bradley C, Smith-Weller T. Outcome of surgery for thoracic outlet syndrome in Washington state workers’ compensation. Neurology. 2000;54(6):1252-1257.
61. Axelrod DA, Proctor MC, Geisser ME, Roth RS, Greenfield LJ. Outcomes after surgery for thoracic outlet syndrome. J Vasc Surg. 2001;33(6):1220-1225.
62. Taylor JM, Telford RJ, Kinsella DC, Watkinson AF, Thompson JF. Long-term clinical and functional outcome following treatment for Paget-Schroetter syndrome. Br J Surg. 2013;100(11):1459-1464.
63. Schneider DB, Dimuzio PJ, Martin ND, et al. Combination treatment of venous thoracic outlet syndrome: open surgical decompression and intraoperative angioplasty. J Vasc Surg. 2004;40(4):599-603.
1. Urschel HC Jr. The history of surgery for thoracic outlet syndrome. Chest Surg Clin North Am. 2000;10(1):183-188, x-xi.
2. Atasoy E. History of thoracic outlet syndrome. Hand Clin. 2004;20(1):15-16, v.
3. Peet RM, Henriksen JD, Anderson TP, Martin GM. Thoracic-outlet syndrome: evaluation of a therapeutic exercise program. Proc Staff Meet Mayo Clin. 1956;31(9):281-287.
4. Edwards DP, Mulkern E, Raja AN, Barker P. Trans-axillary first rib excision for thoracic outlet syndrome. J R Coll Surg Edinb. 1999;44(6):362-365.
5. Juvonen T, Satta J, Laitala P, Luukkonen K, Nissinen J. Anomalies at the thoracic outlet are frequent in the general population. Am J Surg. 1995;170(1):33-37.
6. Atasoy E. Thoracic outlet compression syndrome. Orthop Clin North Am. 1996;27(2):265-303.
7. Demondion X, Herbinet P, Van Sint Jan S, Boutry N, Chantelot C, Cotten A. Imaging assessment of thoracic outlet syndrome. Radiographics. 2006;26(6):1735-1750.
8. Demondion X, Bacqueville E, Paul C, Duquesnoy B, Hachulla E, Cotten A. Thoracic outlet: assessment with MR imaging in asymptomatic and symptomatic populations. Radiology. 2003;227(2):461-468.
9. Makhoul RG, Machleder HI. Developmental anomalies at the thoracic outlet: an analysis of 200 consecutive cases. J Vasc Surg. 1992;16(4):534-542.
10. Sanders RJ, Jackson CG, Banchero N, Pearce WH. Scalene muscle abnormalities in traumatic thoracic outlet syndrome. Am J Surg. 1990;159(2):231-236.
11. Katirji B, Hardy RW Jr. Classic neurogenic thoracic outlet syndrome in a competitive swimmer: a true scalenus anticus syndrome. Muscle Nerve. 1995;18(2):229-233.
12. Casbas L, Chauffour X, Cau J, et al. Post-traumatic thoracic outlet syndromes. Ann Vasc Surg. 2005;19(1):25-28.
13. Povlsen B, Belzberg A, Hansson T, Dorsi M. Treatment for thoracic outlet syndrome. Cochrane Database Syst Rev. 2010;(1):CD007218.
14. Cormier JM, Amrane M, Ward A, Laurian C, Gigou F. Arterial complications of the thoracic outlet syndrome: fifty-five operative cases. J Vasc Surg. 1989;9(6):778-787.
15. Hood DB, Kuehne J, Yellin AE, Weaver FA. Vascular complications of thoracic outlet syndrome. Am Surg. 1997;63(10):913-917.
16. Ferrante MA. Brachial plexopathies: classification, causes, and consequences. Muscle Nerve. 2004;30(5):547-568.
17. Gilliatt RW, Le Quesne PM, Logue V, Sumner AJ. Wasting of the hand associated with a cervical rib or band. J Neurol Neurosurg Psychiatry. 1970;33(5):615-624.
18. Ozoa G, Alves D, Fish DE. Thoracic outlet syndrome. Phys Med Rehabil Clin North Am. 2011;22(3):473-483, viii-ix.
19. Schwartzman RJ. Brachial plexus traction injuries. Hand Clin. 1991;7(3):547-556.
20. Christo PJ, McGreevy K. Updated perspectives on neurogenic thoracic outlet syndrome. Curr Pain Headache Rep. 2011;15(1):14-21.
21. Vanti C, Natalini L, Romeo A, Tosarelli D, Pillastrini P. Conservative treatment of thoracic outlet syndrome. A review of the literature. Eura Medicophys. 2007;43(1):55-70.22. Patton GM. Arterial thoracic outlet syndrome. Hand Clin. 2004;20(1):107-111, viii.
23. Lee TS, Hines GL. Cerebral embolic stroke and arm ischemia in a teenager with arterial thoracic outlet syndrome: a case report. Vasc Endovasc Surg. 2007;41(3):254-257.
24. Sanders RJ, Hammond SL. Venous thoracic outlet syndrome. Hand Clin. 2004;20(1):113-118, viii.
25. Sanders RJ, Hammond SL, Rao NM. Diagnosis of thoracic outlet syndrome. J Vasc Surg. 2007;46(3):601-604.
26. Luoma A, Nelems B. Thoracic outlet syndrome. Thoracic surgery perspective. Neurosurg Clin North Am. 1991;2(1):187-226.
27. Cup EH, Ijspeert J, Janssen RJ, et al. Residual complaints after neuralgic amyotrophy. Arch Phys Med Rehabil. 2013;94(1):67-73.
28. van Alfen N, van Engelen BG. The clinical spectrum of neuralgic amyotrophy in 246 cases. Brain. 2006;129(pt 2):438-450.
29. Nichols AW. The thoracic outlet syndrome in athletes. J Am Board Fam Pract. 1996;9(5):346-355.
30. Roos DB, Owens JC. Thoracic outlet syndrome. Arch Surg. 1966;93(1):71-74.
31. Adson AW, Coffey JR. Cervical rib: a method of anterior approach for relief of symptoms by division of the scalenus anticus. Ann Surg. 1927;85(6):839-857.
32. Wright IS. The neurovascular syndrome produced by hyperabduction of the arms. Am Heart J. 1945;29:1-19.
33. Rayan GM, Jensen C. Thoracic outlet syndrome: provocative examination maneuvers in a typical population. J Shoulder Elbow Surg. 1995;4(2):113-117.
34. Nord KM, Kapoor P, Fisher J, et al. False positive rate of thoracic outlet syndrome diagnostic maneuvers. Electromyogr Clin Neurophysiol. 2008;48(2):67-74.
35. Novak CB. Thoracic outlet syndrome. Clin Plast Surg. 2003;30(2):175-188.
36. Gillard J, Pérez-Cousin M, Hachulla E, et al. Diagnosing thoracic outlet syndrome: contribution of provocative tests, ultrasonography, electrophysiology, and helical computed tomography in 48 patients. Joint Bone Spine. 2001;68(5):416-424.
37. Baxter GM, Kincaid W, Jeffrey RF, Millar GM, Porteous C, Morley P. Comparison of colour Doppler ultrasound with venography in the diagnosis of axillary and subclavian vein thrombosis. Br J Radiol. 1991;64(765):777-781.
38. Passman MA, Criado E, Farber MA, et al. Efficacy of color flow duplex imaging for proximal upper extremity venous outflow obstruction in hemodialysis patients. J Vasc Surg. 1998;28(5):869-875.
39. Wadhwani R, Chaubal N, Sukthankar R, Shroff M, Agarwala S. Color Doppler and duplex sonography in 5 patients with thoracic outlet syndrome. J Ultrasound Med. 2001;20(7):795-801.
40. Napoli V, Vignali C, Braccini G, et al. Echography and echo-Doppler in the study of thoracic outlet syndrome. Correlation with angiographic data [in Italian]. Radiol Med. 1993;85(6):733-740.
41. Longley DG, Yedlicka JW, Molina EJ, Schwabacher S, Hunter DW, Letourneau JG. Thoracic outlet syndrome: evaluation of the subclavian vessels by color duplex sonography. AJR Am J Roentgenol. 1992;158(3):623-630.
42. Demondion X, Herbinet P, Boutry N, Fontaine C, Francke JP, Cotten A. Sonographic mapping of the normal brachial plexus. AJNR Am J Neuroradiol. 2003;24(7):1303-1309.
43. Cruz-Martinez A, Arpa J. Electrophysiological assessment in neurogenic thoracic outlet syndrome. Electromyogr Clin Neurophysiol. 2001;41(4):253-256.
44. Ferrante MA, Wilbourn AJ. The utility of various sensory nerve conduction responses in assessing brachial plexopathies. Muscle Nerve. 1995;18(8):879-889.
45. Aminoff MJ, Olney RK, Parry GJ, Raskin NH. Relative utility of different electrophysiologic techniques in the evaluation of brachial plexopathies. Neurology. 1988;38(4):546-550.
46. Komanetsky RM, Novak CB, Mackinnon SE, Russo MH, Padberg AM, Louis S. Somatosensory evoked potentials fail to diagnose thoracic outlet syndrome. J Hand Surg Am. 1996;21(4):662-666.
47. Remy-Jardin M, Remy J, Masson P, et al. Helical CT angiography of thoracic outlet syndrome: functional anatomy. AJR Am J Roentgenol. 2000;174(6):1667-1674.
48. Matsumura JS, Rilling WS, Pearce WH, Nemcek AA Jr, Vogelzang RL, Yao JS. Helical computed tomography of the normal thoracic outlet. J Vasc Surg. 1997;26(5):776-783.
49. Dymarkowski S, Bosmans H, Marchal G, Bogaert J. Three-dimensional MR angiography in the evaluation of thoracic outlet syndrome. AJR Am J Roentgenol. 1999;173(4):1005-1008.
50. Charon JP, Milne W, Sheppard DG, Houston JG. Evaluation of MR angiographic technique in the assessment of thoracic outlet syndrome. Clin Radiol. 2004;59(7):588-595.
51. Cuetter AC, Bartoszek DM. The thoracic outlet syndrome: controversies, overdiagnosis, overtreatment, and recommendations for management. Muscle Nerve. 1989;12(5):410-419.
52. Urschel HC Jr, Razzuk MA. Paget-Schroetter syndrome: what is the best management? Ann Thorac Surg. 2000;69(6):1663-1668.
53. Lee JT, Karwowski JK, Harris EJ, Haukoos JS, Olcott C 4th. Long-term thrombotic recurrence after nonoperative management of Paget-Schroetter syndrome. J Vasc Surg. 2006;43(6):1236-1243.
54. Molina JE, Hunter DW, Dietz CA. Paget-Schroetter syndrome treated with thrombolytics and immediate surgery. J Vasc Surg. 2007;45(2):328-334.
55. Le Forestier N, Mouton P, Maisonobe T, et al. True neurological thoracic outlet syndrome [in French]. Rev Neurol (Paris). 2000;156(1):34-40.
56. Wilbourn AJ. Thoracic outlet syndrome surgery causing severe brachial plexopathy. Muscle Nerve. 1988;11(1):66-74.
57. Likes K, Dapash T, Rochlin DH, Freischlag JA. Remaining or residual first ribs are the cause of recurrent thoracic outlet syndrome. Ann Vasc Surg. 2014;28(4):939-945.
58. Aljabri B, Al-Omran M. Surgical management of vascular thoracic outlet syndrome: a teaching hospital experience. Ann Vasc Dis. 2013;6(1):74-79.
59. Sanders RJ, Pearce WH. The treatment of thoracic outlet syndrome: a comparison of different operations. J Vasc Surg. 1989;10(6):626-634.
60. Franklin GM, Fulton-Kehoe D, Bradley C, Smith-Weller T. Outcome of surgery for thoracic outlet syndrome in Washington state workers’ compensation. Neurology. 2000;54(6):1252-1257.
61. Axelrod DA, Proctor MC, Geisser ME, Roth RS, Greenfield LJ. Outcomes after surgery for thoracic outlet syndrome. J Vasc Surg. 2001;33(6):1220-1225.
62. Taylor JM, Telford RJ, Kinsella DC, Watkinson AF, Thompson JF. Long-term clinical and functional outcome following treatment for Paget-Schroetter syndrome. Br J Surg. 2013;100(11):1459-1464.
63. Schneider DB, Dimuzio PJ, Martin ND, et al. Combination treatment of venous thoracic outlet syndrome: open surgical decompression and intraoperative angioplasty. J Vasc Surg. 2004;40(4):599-603.
The Burden of Craft in Arthroscopic Rotator Cuff Repair: Where We Have Been and Where We Are Going
I am very honored that Dr. Rob Bell, past president of the American Shoulder and Elbow Surgeons, invited me to give last year’s Neer Lecture. Dr. Bell asked me to specifically address my role in the development of arthroscopic rotator cuff repair and to recount the significant resistance that the early arthroscopic shoulder surgeons faced from the shoulder establishment as we struggled to achieve mainstream acceptance for this new technology. Tasked with such a personal topic, I find myself in a position analogous to that of Winston Churchill at the end of World War II. When a journalist asked him to speculate on how historians would portray his role in the war, he replied without hesitation, “History will be kind to me because I intend to write it.”
So let’s start at the beginning. And for me it makes the most sense to travel back to the year I started my practice: 1981. The world then was very different from today’s world. On January 20, 1981, Ronald Reagan was inaugurated President of the United States. The same day, 52 US hostages in Iran were released after having been held captive for 442 days. In March 1981, Reagan survived an assassination attempt; 3 months earlier, John Lennon had not been so lucky. Lennon’s hit song “Starting Over” garnered the highest musical awards posthumously.
The world of shoulder surgery was also very different in 1981. The arthroscope was the “instrument of the devil,” according to Dr. Rockwood. And shoulder surgery was ruled by the Charlies—Dr. Charles Neer, Dr. Charlie Rockwood, and any other Charlie who felt compelled to marginalize shoulder arthroscopy.
My personal world in the early 1980s was daunting as well. I had just completed my residency at the Mayo Clinic and my sports medicine fellowship in Eugene, Oregon. I had a young son, a new daughter, and a new job with the San Antonio Orthopaedic Group. I had a new house with a 21% mortgage loan and a “new” used car with a 23% car loan.
I was simultaneously energized and intimidated by my new job, where I was doing general orthopedics with a “special interest” in shoulder surgery and sports medicine. I was initially very proud and humbled by the fact that my senior partners had entrusted me with the care of the most difficult shoulder cases within the practice. But that pride got cut down to its appropriate size the day after I had thanked one of my partners, Dr. Lamar Collie, for his confidence in my potential as a shoulder surgeon. Dr. Collie replied matter-of-factly, “Sure … but you need to understand that we always make the new guy the shoulder expert because shoulders never do worth a damn.”
For shoulder arthroscopy, the early 1980s were exciting. Most of us who were scoping shoulders had already been doing knee arthroscopy and were trying to adapt knee instruments to the shoulder. This worked for some simple excisional cases. For example, I recall excising the bucket-handle portion of a type III SLAP (superior labral tear from anterior to posterior) lesion in 1983. In general, however, shoulder problems were different from knee problems and usually involved repair rather than excision of damaged tissues. Therefore, the technology used in knee arthroscopy was often not directly transferable to the shoulder. Furthermore, treatment of the rotator cuff necessitated development of arthroscopic techniques in a virtual space, the subacromial space, and this was an entirely new arthroscopic concept.
Development of Arthroscopic Rotator Cuff Repair
A major mind-expanding turning point for me occurred in 1984 when I attended one of Dr. Jim Esch’s early San Diego shoulder courses. During that course, Dr. Harvard Ellman of Los Angeles demonstrated to me on a cadaver shoulder how he created a virtual subacromial working space that allowed enough visualization for an arthroscopic acromioplasty. At that moment, I knew that arthroscopic rotator cuff repair was just around the corner. Up until then, I had not been able to envision complex extra-articular reconstructive surgery, as all previous arthroscopic surgery had been intra-articular. But now, having realized a virtual working space could always be created, I knew it would be relatively straightforward to develop the portals to approach the cuff as well as the implants and the instruments to repair it. But I also knew that progression to all-arthroscopic repair techniques would have to be stepwise and that the final repair constructs would need to be at least as strong as those of open repair in order to be acceptable. With an undergraduate degree in mechanical engineering, I had a reasonably clear idea of the concepts I wanted to apply to the instrumentation and techniques, though I could never have envisioned how circuitous the route to the end result would be.
First Steps
I sketched out my ideas for arthroscopic suture passers and knot-tying instruments and presented them to a couple of the major arthroscopy companies in the United States, but the companies were not interested. They did not believe arthroscopy would have any meaningful applications in the shoulder. So, I enlisted the services of a local San Antonio aircraft machinist to fabricate instruments for me. By 1987, I was doing arthroscopic side-to-side margin convergence1 cuff repairs for U-shape tears on a regular basis. And I was doing these at the most hostile point in the universe for arthroscopic shoulder surgery: San Antonio, Texas.
Only a few surgeons were doing arthroscopic shoulder surgery in the 1980s and early 1990s, and without exception these surgeons became the leader-pioneers in the new discipline. In general, these were young surgeons who were in private practice and removed from academia and professional organizations, and thus relatively sheltered from the actions of the shoulder rule-makers of the day. They accepted their status as pariahs as they developed their techniques out of the view of mainstream orthopedics. These leaders included Jim Esch, Steve Snyder, Dick Caspari, Lanny Johnson, Gene Wolf, Gary Gartsman, Rob Bell, and Howard Sweeney. We shared our techniques and our ideas with one another, encouraged one another, and generally became good friends.
Thomas Kuhn, in his classic book The Structure of Scientific Revolutions,2 observed that paradigm shifts within a given field were usually achieved by practitioners who were either very young (naïve) or outside the established hierarchy in the field. The surgeons who contributed most to the shift of shoulder surgery from open to arthroscopic techniques were generally young men who were in private practice and had little to lose by inciting the disdain of the shoulder establishment. Predictably, resistance from the mainstream open shoulder surgeons increased as arthroscopic techniques became more successful and more threatening to the primacy of the open shoulder surgeons. The disdain yielded to disruption and finally to transformation as the paradigm shift occurred. The conflict between the open shoulder surgeons and the arthroscopic shoulder surgeons passed through all the phases that Mahatma Gandhi had described many years before. “First they ignore you; then they laugh at you; then they fight you; then you win.”
Building a Ship in a Bottle
At the start of the 1990s, I recognized that my progress in arthroscopic rotator cuff repair would be extremely slow unless I could find an industry partner who shared my vision for full-scale conversion to arthroscopic means of repair and would be willing to help make it a reality. In 1991, I happened to meet Reinhold Schmieding, the owner of Arthrex, a small arthroscopic device company in Naples, Florida. Reinhold invited me to visit him to discuss the feasibility of developing arthroscopic repair systems for the shoulder. At the time, the world headquarters of Arthrex was a 20×30-ft storage room in an office service center, and there were 2 employees. One employee, Don Grafton, was a talented engineer without medical experience. By the end of my first day there, Reinhold and Don and I had agreed that developing arthroscopic repair systems for shoulder instability and rotator cuff repair would become a top priority for Arthrex.
My initial bias toward arthroscopic cuff repair was that a transosseous bone tunnel technique not only would be possible but would be superior to suture anchor fixation. In fact, my first 2 patents with Arthrex were for instrumentation for an arthroscopic transosseous repair technique. I tested my hypothesis with 2 successive biomechanical studies. The first examined cyclic loading of bone tunnel repairs, and the second examined cyclic loading of anchor-based repairs.3,4 Evaluating the data from these 2 studies, I was surprised to find that anchor-based repairs were significantly stronger than bone tunnel repairs. In addition, anchors shifted the weak link from the bone–suture interface to the tendon–suture interface; in essence, anchors optimized bone fixation by shifting the weak link in the construct to the tendon. I was then completely convinced of the superiority of suture anchors over bone tunnels, and that conviction has become even stronger over the years. After these 2 cyclic loading studies, I shifted my focus, and that of Arthrex, toward arthroscopic suture anchor repair of the rotator cuff.
Reconciling Technique and Instrumentation With Anatomy and Biomechanics
Having recognized the importance of the rotator cable attachments both anatomically5 and biomechanically,6,7 I thought it important to reinforce them as a routine part of performing rotator cuff repairs. Our anatomical and biomechanical studies had had great translational implications in the development of our techniques and instrumentation.
As mentioned earlier, Don Grafton was the chief (and for a long time only) engineer at Arthrex. As he had no medical experience, I invited him to come to San Antonio to observe surgery. During Don’s many visits, I showed him pathology in the operating room and pointed out what I could do with the instruments I had and what I could not do. Then in the evening we went to my house and brainstormed how to perform the “missing” surgical manipulations, how to improve manipulations that were suboptimal, and how to optimize final surgical constructs.
Passing suture through tendon was an early challenge. One must remember that, in the early 1990s, it was not possible for machinists to fabricate complex shapes. Therefore, straight tubular retrograde suture passers were the logical first option. We initially developed spring-loaded retrograde hook retrievers (Figure 1) and then curved suture hooks with shuttling wires (Lasso). To me, the most unappealing feature of retrograde suture passage was the oblique angle of approach through the tendon, which caused a length–tension mismatch between the upper fibers and the lower fibers of the muscle–tendon unit. We recognized we could eliminate the mismatch if we passed the suture antegrade, such that it would pass perpendicular to the tendon fibers. These insights and efforts culminated in development of the Viper suture passer and then the FastPass Scorpion suture passer, which has a spring-loaded trapdoor on the upper jaw for ergonomic self-retrieving of the suture once it is passed through the tendon.
To develop a knot pusher that optimized knot tying (yielding the highest knot security and the tightest loop security), we used prototype instruments to tie and test literally thousands of knots in the laboratory. We were thus able to verify that the Surgeon’s Sixth Finger Knot Pusher (Arthrex) reproducibly tied optimized knots8,9 and also optimized knot fixation and bone fixation. However, our suture was not yet optimized and was prone to breakage, and our suture–tendon interface was not yet optimized. Clearly, improvement was needed in 2 more areas.
Don came up with the idea for a virtually unbreakable suture and developed that idea into FiberWire.10 Shortly thereafter, I contributed the idea and design for FiberTape, which dramatically enhanced suture pullout strength and footprint compression.
Anchor designs improved rapidly and dramatically. We made the second-generation BioCorkscrew fully threaded, which virtually eliminated anchor failure, even in soft bone.
Optimization of the suture–tendon interface took a giant step forward when Park and colleagues11,12 introduced linked double-row rotator cuff repair. Much as with a Chinese finger trap, the harder you pull, the stronger it becomes, with yield load approaching ultimate load.
At this point, it seemed we had optimized virtually every segment of the rotator cuff repair construct. Each component was just about as good as it could be. Or was it?
The Accidental Quest for Knotless Fixation
In November 1998, I made my first trip to China as a guest speaker at the Congress of the Hong Kong Orthopaedic Association. My first view of the magnificent Hong Kong skyline across Victoria Harbour was truly breathtaking. As I admired the gleaming glass towers and the concrete canyons of the city, I had no idea that the very next day these modern skyscrapers would reveal an ancient secret that would change my approach to arthroscopic rotator cuff repair.
The day after my arrival, Dr. James Lam took me to lunch. As we approached the restaurant, he pointed across the street to a tall building that was being renovated and had scaffolding supporting workers alongside the first 9 stories of the exterior wall. Dr. Lam said that, after lunch, he would take me to the construction site for a closer look at the scaffolding.
After lunch, we walked to the base of the scaffolding. Dr. Lam told me it was constructed entirely of bamboo poles held together with lashings but no knots (Figure 2). Lashings were secured by turning them back on themselves and wrapping them in an entirely knotless manner.13 I found it incredible that this knotless fixation was so secure that it could support the weight of workers many stories above the ground. I resolved to determine how this fixation method worked and see if the same mechanism might help us achieve reliable knotless fixation in surgery.
When I returned home, I broke out my college engineering books and reacquainted myself with the concept of cable friction. As has happened so often in the past, however, it took a practical lesson from the ranch to truly illustrate for me how cable friction works.
Every cowboy knows that a spirited horse cannot be restrained with only one lead rope. However, a cowboy can wrap a lead rope around a “snubbing post” and thereby gain complete control over the animal, despite the horse’s superior size and strength. The cable friction between the rope and the post creates such a large restraining force that the cowboy can easily hold the animal without the help of a knotted rope (Figure 3). In similar fashion in the Hong Kong scaffolding, fixation strength results from the significant amount of cable friction produced when the lashings wrap around one another and around the bamboo poles.
The cable friction concept was pivotal in the development of knotless fixation in arthroscopic rotator cuff repair. In lateral row fixation, the eyelet of the PushLock and SwiveLock suture anchors (Arthrex) produces significant cable friction at the eyelet–suture interface, in addition to frictional force wedging the suture between anchor and bone.
As with so many other devices in shoulder arthroscopy, the SwiveLock suture anchor developed in stages. In the first stage, a chainlike suture with consecutive intersecting links was used (FiberChain). The idea for an adjustable fixation construct came to me because I thought that a forked eyelet on a SwiveLock would provide a firm fixation point when inserted into the appropriate suture link, yet would be totally adjustable simply by choosing a tighter or looser link (Figure 4). Although the system worked very well, it was technically challenging. The process was greatly simplified after Don Grafton and I developed FiberTape and recognized that the power of cable friction was dramatically increased by the larger contact area between the eyelet and the braided FiberTape. The SpeedBridge construct (Arthrex), which enhanced cable friction fixation by means of passing FiberTape through the anchor eyelets, also provided a larger compressive interface at the repair site by using FiberTape rather than conventional suture. These incremental improvements led to what I would characterize as today’s gold standard for arthroscopic rotator cuff repair: a largely knotless linked double-row construct using FiberTape, with cinch-loop sutures at the anterior and posterior margins of the tear to reinforce the cable attachments and simultaneously reduce the dog-ears that typically occur in those locations, and a double-pulley medial mattress if tendon quality is poor (Figure 5).
The Burden of Craft
With all the recent enthusiasm for level I studies, I think we need to examine whether they will accelerate technological advancement in rotator cuff repair. The answer, in my opinion, is a resounding no. This answer is based on a major disconnect I have detected in how we evaluate these studies in rotator cuff disease and repair.
An irony related to technological advancement in surgery is that the more technically advanced the surgery becomes, the more skill is required. This fact is completely at odds with the public’s perception that technological advances make procedures easier. In arthroscopic rotator cuff repair, the surgeon must look, feel, and be aware to a greater degree than in open surgery.
Edward Tenner, in his book Why Things Bite Back, described the burden of the practitioner of any advanced technology as the burden of craft.14 The burden of craft is the inherent demand on all craftspeople, but particularly surgeons, to “up our game” if we are to be successful in our craft. For arthroscopic rotator cuff repair, the burden of craft requires patience, attention to detail, and the ability to work in a virtual space. Not everyone has these skills. But anyone who wants to practice in this discipline has an obligation to learn the skills required, and then to teach them to others and assess how well they are being applied.
The problem with relying on level I studies to assess the efficacy of a surgical procedure is that they are inherently biased by the surgeons involved. As results depend on surgeons’ skills, and surgeons’ skill levels are not equal, level I studies cannot prove what is possible, cannot demonstrate the limits of a technique, and cannot demonstrate the equivalence of techniques.
Amazingly enough, there are still rotator cuff repair “deniers” who confidently assert from the podium that a large percentage of massive cuff tears cannot be repaired and that, even if they can be repaired, they do not have the biological potential to heal. Given the disparity in surgeons’ skills and results, however, one must ask whether poor results are a consequence of a biological deficit in the patient, or of a skill deficit in the surgeon.
What I know is that we have techniques for predictable arthroscopic repair and healing of the vast majority of rotator cuff tears, even massive tears,15-17 and patients do very well clinically. Yet, among many orthopedic surgeons, there is a trend to go straight to reverse total shoulder arthroplasty (rTSA) for massive tears—despite the evidence against it. As reported in the literature, rTSA results are not as good as arthroscopic cuff repair results, and the complication rate for rTSA is much higher.
Why has this trend toward rTSA for massive tears gained so much momentum? The only reason I can surmise is that, for the average surgeon, rTSA is easier and quicker than arthroscopic repair for massive tears. But the reason for choosing a specific type of surgery for a given problem should not be that it is easiest for the surgeon; it should be that it is best for the patient.
The surgeon should start by asking what procedure he or she would want if the roles were reversed—if the surgeon were the patient with the massive rotator cuff tear. If a surgeon does not have the skill set for the best procedure for a particular patient, he or she is obligated to send that patient to a surgeon who does have the skills. In addition, given that infection is the most feared complication in most shoulder surgeries, the surgeon should ask which infection rate would be personally acceptable. Arthroscopic rotator cuff repair has a reported infection rate of 1.6 per 1000, or .0016,18 whereas rTSA has an infection rate about 25 times higher, or .04.19 Further, the surgeon must consider the relative severity of the consequences of infection. By any measure, an infected arthroscopy is a straightforward treatable complication, but an infected shoulder replacement is a human tragedy. Patients vastly prefer the minimally invasive arthroscopic approach, and through online searches can easily identify who can offer an arthroscopic solution.
To reproducibly achieve successful arthroscopic repair of massive rotator cuff tears, the surgeon must know advanced techniques, including subscapularis repair techniques,20,21 interval slides,22,23 and self-reinforcing constructs.24,25
“It’s a poor carpenter who blames his tools.” This 18th-century English proverb is as true today as it was 300 years ago. The tools for arthroscopic cuff repair exist, and they are excellent. The burden of craft is the surgeon’s burden and obligation. As surgeons, we must accept that obligation and the responsibility of that burden.
As mentioned earlier, Dr. Rob Bell’s charge to me when he invited me to give the Neer Lecture was to sum up my involvement in the development of arthroscopic shoulder surgery. The short version is that I have been doing shoulder arthroscopy for 31 years; have received 28 US patents related to shoulder instruments and implants and have 12 US patents pending; have published 167 peer-reviewed articles, a couple dozen book chapters, and 2 textbooks on shoulder arthroscopy; have trained 25 fellows; and have hosted approximately 3000 visiting surgeons in my operating room. My greatest professional dream was to see the standard of care for rotator cuff repair and shoulder instability transition from open to arthroscopic techniques, and I have been fortunate enough to have observed that paradigm shift during my career.
What do I envision over the next 31 years? As we all know, history runs in both directions, and some things simply have not happened yet. In terms of rotator cuff treatment, I think over the next few years the guiding principle of treatment will be joint preservation. All rotator cuff tears, even massive tears, will be repaired arthroscopically. Patients and insurers will demand arthroscopic repair, and surgeons without the skill set will migrate to other subspecialties. As for the role of arthroplasty in the treatment of rotator cuff tears, rTSA will be indicated only for pseudoparalysis after failed cuff repair in low-demand elderly patients.
In rotator cuff treatment, I envision a standard of care that is almost entirely arthroscopic. This standard will demand that surgeons who treat rotator cuff tears be proficient in arthroscopic repair of the full range of tears. Acquiring the skills for arthroscopic repair may not be easy, but then “there’s the easy way, and there’s the Cowboy Way.” As my dad used to tell me when I complained about working too hard, “No man ever drowned in his own sweat.” We shoulder surgeons must accept the burden of craft that accompanies the new standard of arthroscopic cuff repair, and we must offer our patients the same level of care we would choose for ourselves.
Happy trails!
1. Burkhart SS, Athanasiou KA, Wirth MA. Margin convergence: a method of reducing strain in massive rotator cuff tears. Arthroscopy. 1996:12(3):335-338.
2. Kuhn TS. The Structure of Scientific Revolutions. Chicago, IL: University of Chicago Press; 1962.
3. Burkhart SS, Johnson TC, Wirth MA, Athanasiou KA. Cyclic loading of transosseous rotator cuff repairs: tension overload as a possible cause of failure. Arthroscopy. 1997;13(2):172-176.
4. Burkhart SS, Diaz Pagàn JL, Wirth MA, Athansiou KA. Cyclic loading of anchor-based rotator cuff repairs: confirmation of the tension overload phenomenon and comparison of suture anchor fixation with transosseous fixation. Arthroscopy. 1997;13(6):720-724.
5. Burkhart SS, Esch JC, Jolson RS. The rotator crescent and rotator cable: an anatomic description of the shoulder’s “suspension bridge.” Arthroscopy. 1993;9(6):611-616.
6. Burkhart SS, Nottage WM, Ogilvie-Harris DJ, Kohn HS, Pachelli A. Partial repair of irreparable rotator cuff tears. Arthroscopy. 1994;10(4):363-370.
7. Halder AM, O’Driscoll SW, Heers G, et al. Biomechanical comparison of effects of supraspinatus tendon detachments, tendon defects, and muscle retractions. J Bone Joint Surg Am. 2002;84(5):780-785.
8. Lo IK, Burkhart SS, Chan KC, Athanasiou K. Arthroscopic knots: determining the optimal balance of loop security and knot security. Arthroscopy. 2004;20(5):489-502.
9. Lo IK, Ochoa E Jr, Burkhart SS. A comparison of knot security and loop security in arthroscopic knots tied with newer high-strength suture materials. Arthroscopy. 2010;26(9 suppl):S120-S126.
10. Lo IK, Burkhart SS, Athanasiou K. Abrasion resistance of two types of nonabsorbable braided suture. Arthroscopy. 2004;20(4):407-413.
11. Park MC, ElAttrache NS, Tibone JE, Ahmad CS, Jun BJ, Lee TQ. Part I: footprint contact characteristics for a “transosseous-equivalent” rotator cuff repair technique compared to a double-row technique. J Shoulder Elbow Surg. 2007;16(4):461-468.
12. Park MC, Tibone JE, ElAttrache NS, Ahmad CS, Jun BJ, Lee TQ. Part II: biomechanical assessment for a footprint-restoring transosseous-equivalent rotator cuff repair technique compared with a double-row repair technique. J Shoulder Elbow Surg. 2007;16(4):469-476.
13. Burkhart SS, Athanasiou KA. The twist-lock concept of tissue transport and suture fixation without knots: observations along the Hong Kong skyline. Arthroscopy. 2003;19(6):613-625.
14. Tenner E. Why Things Bite Back. New York, NY: Random House; 1996.
15. Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of arthroscopic massive rotator cuff repair: the importance of double-row fixation. Arthroscopy. 2012;28(7):909-915.
16. Denard PJ, Lädermann A, Jiwani AZ, Burkhart SS. Functional outcome after arthroscopic repair of massive rotator cuff tears in individuals with pseudoparalysis. Arthroscopy. 2012;28(9):1214-1219.
17. Lädermann A, Denard PJ, Burkhart SS. Revision arthroscopic rotator cuff repair: systematic review and authors’ preferred surgical technique. Arthroscopy. 2012;28(8):1160-1169.
18. Randelli P, Castagna A, Cabitza F, Cabitza P, Arrigoni P, Denti M. Infectious and thromboembolic complications of arthroscopic shoulder surgery. J Shoulder Elbow Surg. 2010;19(1):97-101.
19. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146-157.
20. Adams CR, Schoolfield JD, Burkhart SS. The results of arthroscopic subscapularis tendon repairs. Arthroscopy. 2008;24(12):1381-1389.
21. Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of a consecutive series of subscapularis tendon tears repaired arthroscopically. Arthroscopy. 2012;28(11):1587-1591.
22. Lo IK, Burkhart SS. Arthroscopic repair of massive, contracted, immobile rotator cuff tears using single and double interval slides: technique and preliminary results. Arthroscopy. 2004;20(1):22-33.
23. Lo IK, Burkhart SS. The interval slide in continuity: a method of mobilizing the anterosuperior rotator cuff without disrupting the tear margins. Arthroscopy. 2004;20(4):435-441.
24. Denard PJ, Burkhart SS. Techniques for managing poor quality tissue and bone during arthroscopic rotator cuff repair. Arthroscopy. 2011;27(10):1409-1421.
25. Burkhart SS, Denard PJ, Konicek J, Hanypsiak BT. Biomechanical validation of load-sharing rip-stop fixation for the repair of tissue-deficient rotator cuff tears. Am J Sports Med. 2014;42(2):457-462.
I am very honored that Dr. Rob Bell, past president of the American Shoulder and Elbow Surgeons, invited me to give last year’s Neer Lecture. Dr. Bell asked me to specifically address my role in the development of arthroscopic rotator cuff repair and to recount the significant resistance that the early arthroscopic shoulder surgeons faced from the shoulder establishment as we struggled to achieve mainstream acceptance for this new technology. Tasked with such a personal topic, I find myself in a position analogous to that of Winston Churchill at the end of World War II. When a journalist asked him to speculate on how historians would portray his role in the war, he replied without hesitation, “History will be kind to me because I intend to write it.”
So let’s start at the beginning. And for me it makes the most sense to travel back to the year I started my practice: 1981. The world then was very different from today’s world. On January 20, 1981, Ronald Reagan was inaugurated President of the United States. The same day, 52 US hostages in Iran were released after having been held captive for 442 days. In March 1981, Reagan survived an assassination attempt; 3 months earlier, John Lennon had not been so lucky. Lennon’s hit song “Starting Over” garnered the highest musical awards posthumously.
The world of shoulder surgery was also very different in 1981. The arthroscope was the “instrument of the devil,” according to Dr. Rockwood. And shoulder surgery was ruled by the Charlies—Dr. Charles Neer, Dr. Charlie Rockwood, and any other Charlie who felt compelled to marginalize shoulder arthroscopy.
My personal world in the early 1980s was daunting as well. I had just completed my residency at the Mayo Clinic and my sports medicine fellowship in Eugene, Oregon. I had a young son, a new daughter, and a new job with the San Antonio Orthopaedic Group. I had a new house with a 21% mortgage loan and a “new” used car with a 23% car loan.
I was simultaneously energized and intimidated by my new job, where I was doing general orthopedics with a “special interest” in shoulder surgery and sports medicine. I was initially very proud and humbled by the fact that my senior partners had entrusted me with the care of the most difficult shoulder cases within the practice. But that pride got cut down to its appropriate size the day after I had thanked one of my partners, Dr. Lamar Collie, for his confidence in my potential as a shoulder surgeon. Dr. Collie replied matter-of-factly, “Sure … but you need to understand that we always make the new guy the shoulder expert because shoulders never do worth a damn.”
For shoulder arthroscopy, the early 1980s were exciting. Most of us who were scoping shoulders had already been doing knee arthroscopy and were trying to adapt knee instruments to the shoulder. This worked for some simple excisional cases. For example, I recall excising the bucket-handle portion of a type III SLAP (superior labral tear from anterior to posterior) lesion in 1983. In general, however, shoulder problems were different from knee problems and usually involved repair rather than excision of damaged tissues. Therefore, the technology used in knee arthroscopy was often not directly transferable to the shoulder. Furthermore, treatment of the rotator cuff necessitated development of arthroscopic techniques in a virtual space, the subacromial space, and this was an entirely new arthroscopic concept.
Development of Arthroscopic Rotator Cuff Repair
A major mind-expanding turning point for me occurred in 1984 when I attended one of Dr. Jim Esch’s early San Diego shoulder courses. During that course, Dr. Harvard Ellman of Los Angeles demonstrated to me on a cadaver shoulder how he created a virtual subacromial working space that allowed enough visualization for an arthroscopic acromioplasty. At that moment, I knew that arthroscopic rotator cuff repair was just around the corner. Up until then, I had not been able to envision complex extra-articular reconstructive surgery, as all previous arthroscopic surgery had been intra-articular. But now, having realized a virtual working space could always be created, I knew it would be relatively straightforward to develop the portals to approach the cuff as well as the implants and the instruments to repair it. But I also knew that progression to all-arthroscopic repair techniques would have to be stepwise and that the final repair constructs would need to be at least as strong as those of open repair in order to be acceptable. With an undergraduate degree in mechanical engineering, I had a reasonably clear idea of the concepts I wanted to apply to the instrumentation and techniques, though I could never have envisioned how circuitous the route to the end result would be.
First Steps
I sketched out my ideas for arthroscopic suture passers and knot-tying instruments and presented them to a couple of the major arthroscopy companies in the United States, but the companies were not interested. They did not believe arthroscopy would have any meaningful applications in the shoulder. So, I enlisted the services of a local San Antonio aircraft machinist to fabricate instruments for me. By 1987, I was doing arthroscopic side-to-side margin convergence1 cuff repairs for U-shape tears on a regular basis. And I was doing these at the most hostile point in the universe for arthroscopic shoulder surgery: San Antonio, Texas.
Only a few surgeons were doing arthroscopic shoulder surgery in the 1980s and early 1990s, and without exception these surgeons became the leader-pioneers in the new discipline. In general, these were young surgeons who were in private practice and removed from academia and professional organizations, and thus relatively sheltered from the actions of the shoulder rule-makers of the day. They accepted their status as pariahs as they developed their techniques out of the view of mainstream orthopedics. These leaders included Jim Esch, Steve Snyder, Dick Caspari, Lanny Johnson, Gene Wolf, Gary Gartsman, Rob Bell, and Howard Sweeney. We shared our techniques and our ideas with one another, encouraged one another, and generally became good friends.
Thomas Kuhn, in his classic book The Structure of Scientific Revolutions,2 observed that paradigm shifts within a given field were usually achieved by practitioners who were either very young (naïve) or outside the established hierarchy in the field. The surgeons who contributed most to the shift of shoulder surgery from open to arthroscopic techniques were generally young men who were in private practice and had little to lose by inciting the disdain of the shoulder establishment. Predictably, resistance from the mainstream open shoulder surgeons increased as arthroscopic techniques became more successful and more threatening to the primacy of the open shoulder surgeons. The disdain yielded to disruption and finally to transformation as the paradigm shift occurred. The conflict between the open shoulder surgeons and the arthroscopic shoulder surgeons passed through all the phases that Mahatma Gandhi had described many years before. “First they ignore you; then they laugh at you; then they fight you; then you win.”
Building a Ship in a Bottle
At the start of the 1990s, I recognized that my progress in arthroscopic rotator cuff repair would be extremely slow unless I could find an industry partner who shared my vision for full-scale conversion to arthroscopic means of repair and would be willing to help make it a reality. In 1991, I happened to meet Reinhold Schmieding, the owner of Arthrex, a small arthroscopic device company in Naples, Florida. Reinhold invited me to visit him to discuss the feasibility of developing arthroscopic repair systems for the shoulder. At the time, the world headquarters of Arthrex was a 20×30-ft storage room in an office service center, and there were 2 employees. One employee, Don Grafton, was a talented engineer without medical experience. By the end of my first day there, Reinhold and Don and I had agreed that developing arthroscopic repair systems for shoulder instability and rotator cuff repair would become a top priority for Arthrex.
My initial bias toward arthroscopic cuff repair was that a transosseous bone tunnel technique not only would be possible but would be superior to suture anchor fixation. In fact, my first 2 patents with Arthrex were for instrumentation for an arthroscopic transosseous repair technique. I tested my hypothesis with 2 successive biomechanical studies. The first examined cyclic loading of bone tunnel repairs, and the second examined cyclic loading of anchor-based repairs.3,4 Evaluating the data from these 2 studies, I was surprised to find that anchor-based repairs were significantly stronger than bone tunnel repairs. In addition, anchors shifted the weak link from the bone–suture interface to the tendon–suture interface; in essence, anchors optimized bone fixation by shifting the weak link in the construct to the tendon. I was then completely convinced of the superiority of suture anchors over bone tunnels, and that conviction has become even stronger over the years. After these 2 cyclic loading studies, I shifted my focus, and that of Arthrex, toward arthroscopic suture anchor repair of the rotator cuff.
Reconciling Technique and Instrumentation With Anatomy and Biomechanics
Having recognized the importance of the rotator cable attachments both anatomically5 and biomechanically,6,7 I thought it important to reinforce them as a routine part of performing rotator cuff repairs. Our anatomical and biomechanical studies had had great translational implications in the development of our techniques and instrumentation.
As mentioned earlier, Don Grafton was the chief (and for a long time only) engineer at Arthrex. As he had no medical experience, I invited him to come to San Antonio to observe surgery. During Don’s many visits, I showed him pathology in the operating room and pointed out what I could do with the instruments I had and what I could not do. Then in the evening we went to my house and brainstormed how to perform the “missing” surgical manipulations, how to improve manipulations that were suboptimal, and how to optimize final surgical constructs.
Passing suture through tendon was an early challenge. One must remember that, in the early 1990s, it was not possible for machinists to fabricate complex shapes. Therefore, straight tubular retrograde suture passers were the logical first option. We initially developed spring-loaded retrograde hook retrievers (Figure 1) and then curved suture hooks with shuttling wires (Lasso). To me, the most unappealing feature of retrograde suture passage was the oblique angle of approach through the tendon, which caused a length–tension mismatch between the upper fibers and the lower fibers of the muscle–tendon unit. We recognized we could eliminate the mismatch if we passed the suture antegrade, such that it would pass perpendicular to the tendon fibers. These insights and efforts culminated in development of the Viper suture passer and then the FastPass Scorpion suture passer, which has a spring-loaded trapdoor on the upper jaw for ergonomic self-retrieving of the suture once it is passed through the tendon.
To develop a knot pusher that optimized knot tying (yielding the highest knot security and the tightest loop security), we used prototype instruments to tie and test literally thousands of knots in the laboratory. We were thus able to verify that the Surgeon’s Sixth Finger Knot Pusher (Arthrex) reproducibly tied optimized knots8,9 and also optimized knot fixation and bone fixation. However, our suture was not yet optimized and was prone to breakage, and our suture–tendon interface was not yet optimized. Clearly, improvement was needed in 2 more areas.
Don came up with the idea for a virtually unbreakable suture and developed that idea into FiberWire.10 Shortly thereafter, I contributed the idea and design for FiberTape, which dramatically enhanced suture pullout strength and footprint compression.
Anchor designs improved rapidly and dramatically. We made the second-generation BioCorkscrew fully threaded, which virtually eliminated anchor failure, even in soft bone.
Optimization of the suture–tendon interface took a giant step forward when Park and colleagues11,12 introduced linked double-row rotator cuff repair. Much as with a Chinese finger trap, the harder you pull, the stronger it becomes, with yield load approaching ultimate load.
At this point, it seemed we had optimized virtually every segment of the rotator cuff repair construct. Each component was just about as good as it could be. Or was it?
The Accidental Quest for Knotless Fixation
In November 1998, I made my first trip to China as a guest speaker at the Congress of the Hong Kong Orthopaedic Association. My first view of the magnificent Hong Kong skyline across Victoria Harbour was truly breathtaking. As I admired the gleaming glass towers and the concrete canyons of the city, I had no idea that the very next day these modern skyscrapers would reveal an ancient secret that would change my approach to arthroscopic rotator cuff repair.
The day after my arrival, Dr. James Lam took me to lunch. As we approached the restaurant, he pointed across the street to a tall building that was being renovated and had scaffolding supporting workers alongside the first 9 stories of the exterior wall. Dr. Lam said that, after lunch, he would take me to the construction site for a closer look at the scaffolding.
After lunch, we walked to the base of the scaffolding. Dr. Lam told me it was constructed entirely of bamboo poles held together with lashings but no knots (Figure 2). Lashings were secured by turning them back on themselves and wrapping them in an entirely knotless manner.13 I found it incredible that this knotless fixation was so secure that it could support the weight of workers many stories above the ground. I resolved to determine how this fixation method worked and see if the same mechanism might help us achieve reliable knotless fixation in surgery.
When I returned home, I broke out my college engineering books and reacquainted myself with the concept of cable friction. As has happened so often in the past, however, it took a practical lesson from the ranch to truly illustrate for me how cable friction works.
Every cowboy knows that a spirited horse cannot be restrained with only one lead rope. However, a cowboy can wrap a lead rope around a “snubbing post” and thereby gain complete control over the animal, despite the horse’s superior size and strength. The cable friction between the rope and the post creates such a large restraining force that the cowboy can easily hold the animal without the help of a knotted rope (Figure 3). In similar fashion in the Hong Kong scaffolding, fixation strength results from the significant amount of cable friction produced when the lashings wrap around one another and around the bamboo poles.
The cable friction concept was pivotal in the development of knotless fixation in arthroscopic rotator cuff repair. In lateral row fixation, the eyelet of the PushLock and SwiveLock suture anchors (Arthrex) produces significant cable friction at the eyelet–suture interface, in addition to frictional force wedging the suture between anchor and bone.
As with so many other devices in shoulder arthroscopy, the SwiveLock suture anchor developed in stages. In the first stage, a chainlike suture with consecutive intersecting links was used (FiberChain). The idea for an adjustable fixation construct came to me because I thought that a forked eyelet on a SwiveLock would provide a firm fixation point when inserted into the appropriate suture link, yet would be totally adjustable simply by choosing a tighter or looser link (Figure 4). Although the system worked very well, it was technically challenging. The process was greatly simplified after Don Grafton and I developed FiberTape and recognized that the power of cable friction was dramatically increased by the larger contact area between the eyelet and the braided FiberTape. The SpeedBridge construct (Arthrex), which enhanced cable friction fixation by means of passing FiberTape through the anchor eyelets, also provided a larger compressive interface at the repair site by using FiberTape rather than conventional suture. These incremental improvements led to what I would characterize as today’s gold standard for arthroscopic rotator cuff repair: a largely knotless linked double-row construct using FiberTape, with cinch-loop sutures at the anterior and posterior margins of the tear to reinforce the cable attachments and simultaneously reduce the dog-ears that typically occur in those locations, and a double-pulley medial mattress if tendon quality is poor (Figure 5).
The Burden of Craft
With all the recent enthusiasm for level I studies, I think we need to examine whether they will accelerate technological advancement in rotator cuff repair. The answer, in my opinion, is a resounding no. This answer is based on a major disconnect I have detected in how we evaluate these studies in rotator cuff disease and repair.
An irony related to technological advancement in surgery is that the more technically advanced the surgery becomes, the more skill is required. This fact is completely at odds with the public’s perception that technological advances make procedures easier. In arthroscopic rotator cuff repair, the surgeon must look, feel, and be aware to a greater degree than in open surgery.
Edward Tenner, in his book Why Things Bite Back, described the burden of the practitioner of any advanced technology as the burden of craft.14 The burden of craft is the inherent demand on all craftspeople, but particularly surgeons, to “up our game” if we are to be successful in our craft. For arthroscopic rotator cuff repair, the burden of craft requires patience, attention to detail, and the ability to work in a virtual space. Not everyone has these skills. But anyone who wants to practice in this discipline has an obligation to learn the skills required, and then to teach them to others and assess how well they are being applied.
The problem with relying on level I studies to assess the efficacy of a surgical procedure is that they are inherently biased by the surgeons involved. As results depend on surgeons’ skills, and surgeons’ skill levels are not equal, level I studies cannot prove what is possible, cannot demonstrate the limits of a technique, and cannot demonstrate the equivalence of techniques.
Amazingly enough, there are still rotator cuff repair “deniers” who confidently assert from the podium that a large percentage of massive cuff tears cannot be repaired and that, even if they can be repaired, they do not have the biological potential to heal. Given the disparity in surgeons’ skills and results, however, one must ask whether poor results are a consequence of a biological deficit in the patient, or of a skill deficit in the surgeon.
What I know is that we have techniques for predictable arthroscopic repair and healing of the vast majority of rotator cuff tears, even massive tears,15-17 and patients do very well clinically. Yet, among many orthopedic surgeons, there is a trend to go straight to reverse total shoulder arthroplasty (rTSA) for massive tears—despite the evidence against it. As reported in the literature, rTSA results are not as good as arthroscopic cuff repair results, and the complication rate for rTSA is much higher.
Why has this trend toward rTSA for massive tears gained so much momentum? The only reason I can surmise is that, for the average surgeon, rTSA is easier and quicker than arthroscopic repair for massive tears. But the reason for choosing a specific type of surgery for a given problem should not be that it is easiest for the surgeon; it should be that it is best for the patient.
The surgeon should start by asking what procedure he or she would want if the roles were reversed—if the surgeon were the patient with the massive rotator cuff tear. If a surgeon does not have the skill set for the best procedure for a particular patient, he or she is obligated to send that patient to a surgeon who does have the skills. In addition, given that infection is the most feared complication in most shoulder surgeries, the surgeon should ask which infection rate would be personally acceptable. Arthroscopic rotator cuff repair has a reported infection rate of 1.6 per 1000, or .0016,18 whereas rTSA has an infection rate about 25 times higher, or .04.19 Further, the surgeon must consider the relative severity of the consequences of infection. By any measure, an infected arthroscopy is a straightforward treatable complication, but an infected shoulder replacement is a human tragedy. Patients vastly prefer the minimally invasive arthroscopic approach, and through online searches can easily identify who can offer an arthroscopic solution.
To reproducibly achieve successful arthroscopic repair of massive rotator cuff tears, the surgeon must know advanced techniques, including subscapularis repair techniques,20,21 interval slides,22,23 and self-reinforcing constructs.24,25
“It’s a poor carpenter who blames his tools.” This 18th-century English proverb is as true today as it was 300 years ago. The tools for arthroscopic cuff repair exist, and they are excellent. The burden of craft is the surgeon’s burden and obligation. As surgeons, we must accept that obligation and the responsibility of that burden.
As mentioned earlier, Dr. Rob Bell’s charge to me when he invited me to give the Neer Lecture was to sum up my involvement in the development of arthroscopic shoulder surgery. The short version is that I have been doing shoulder arthroscopy for 31 years; have received 28 US patents related to shoulder instruments and implants and have 12 US patents pending; have published 167 peer-reviewed articles, a couple dozen book chapters, and 2 textbooks on shoulder arthroscopy; have trained 25 fellows; and have hosted approximately 3000 visiting surgeons in my operating room. My greatest professional dream was to see the standard of care for rotator cuff repair and shoulder instability transition from open to arthroscopic techniques, and I have been fortunate enough to have observed that paradigm shift during my career.
What do I envision over the next 31 years? As we all know, history runs in both directions, and some things simply have not happened yet. In terms of rotator cuff treatment, I think over the next few years the guiding principle of treatment will be joint preservation. All rotator cuff tears, even massive tears, will be repaired arthroscopically. Patients and insurers will demand arthroscopic repair, and surgeons without the skill set will migrate to other subspecialties. As for the role of arthroplasty in the treatment of rotator cuff tears, rTSA will be indicated only for pseudoparalysis after failed cuff repair in low-demand elderly patients.
In rotator cuff treatment, I envision a standard of care that is almost entirely arthroscopic. This standard will demand that surgeons who treat rotator cuff tears be proficient in arthroscopic repair of the full range of tears. Acquiring the skills for arthroscopic repair may not be easy, but then “there’s the easy way, and there’s the Cowboy Way.” As my dad used to tell me when I complained about working too hard, “No man ever drowned in his own sweat.” We shoulder surgeons must accept the burden of craft that accompanies the new standard of arthroscopic cuff repair, and we must offer our patients the same level of care we would choose for ourselves.
Happy trails!
I am very honored that Dr. Rob Bell, past president of the American Shoulder and Elbow Surgeons, invited me to give last year’s Neer Lecture. Dr. Bell asked me to specifically address my role in the development of arthroscopic rotator cuff repair and to recount the significant resistance that the early arthroscopic shoulder surgeons faced from the shoulder establishment as we struggled to achieve mainstream acceptance for this new technology. Tasked with such a personal topic, I find myself in a position analogous to that of Winston Churchill at the end of World War II. When a journalist asked him to speculate on how historians would portray his role in the war, he replied without hesitation, “History will be kind to me because I intend to write it.”
So let’s start at the beginning. And for me it makes the most sense to travel back to the year I started my practice: 1981. The world then was very different from today’s world. On January 20, 1981, Ronald Reagan was inaugurated President of the United States. The same day, 52 US hostages in Iran were released after having been held captive for 442 days. In March 1981, Reagan survived an assassination attempt; 3 months earlier, John Lennon had not been so lucky. Lennon’s hit song “Starting Over” garnered the highest musical awards posthumously.
The world of shoulder surgery was also very different in 1981. The arthroscope was the “instrument of the devil,” according to Dr. Rockwood. And shoulder surgery was ruled by the Charlies—Dr. Charles Neer, Dr. Charlie Rockwood, and any other Charlie who felt compelled to marginalize shoulder arthroscopy.
My personal world in the early 1980s was daunting as well. I had just completed my residency at the Mayo Clinic and my sports medicine fellowship in Eugene, Oregon. I had a young son, a new daughter, and a new job with the San Antonio Orthopaedic Group. I had a new house with a 21% mortgage loan and a “new” used car with a 23% car loan.
I was simultaneously energized and intimidated by my new job, where I was doing general orthopedics with a “special interest” in shoulder surgery and sports medicine. I was initially very proud and humbled by the fact that my senior partners had entrusted me with the care of the most difficult shoulder cases within the practice. But that pride got cut down to its appropriate size the day after I had thanked one of my partners, Dr. Lamar Collie, for his confidence in my potential as a shoulder surgeon. Dr. Collie replied matter-of-factly, “Sure … but you need to understand that we always make the new guy the shoulder expert because shoulders never do worth a damn.”
For shoulder arthroscopy, the early 1980s were exciting. Most of us who were scoping shoulders had already been doing knee arthroscopy and were trying to adapt knee instruments to the shoulder. This worked for some simple excisional cases. For example, I recall excising the bucket-handle portion of a type III SLAP (superior labral tear from anterior to posterior) lesion in 1983. In general, however, shoulder problems were different from knee problems and usually involved repair rather than excision of damaged tissues. Therefore, the technology used in knee arthroscopy was often not directly transferable to the shoulder. Furthermore, treatment of the rotator cuff necessitated development of arthroscopic techniques in a virtual space, the subacromial space, and this was an entirely new arthroscopic concept.
Development of Arthroscopic Rotator Cuff Repair
A major mind-expanding turning point for me occurred in 1984 when I attended one of Dr. Jim Esch’s early San Diego shoulder courses. During that course, Dr. Harvard Ellman of Los Angeles demonstrated to me on a cadaver shoulder how he created a virtual subacromial working space that allowed enough visualization for an arthroscopic acromioplasty. At that moment, I knew that arthroscopic rotator cuff repair was just around the corner. Up until then, I had not been able to envision complex extra-articular reconstructive surgery, as all previous arthroscopic surgery had been intra-articular. But now, having realized a virtual working space could always be created, I knew it would be relatively straightforward to develop the portals to approach the cuff as well as the implants and the instruments to repair it. But I also knew that progression to all-arthroscopic repair techniques would have to be stepwise and that the final repair constructs would need to be at least as strong as those of open repair in order to be acceptable. With an undergraduate degree in mechanical engineering, I had a reasonably clear idea of the concepts I wanted to apply to the instrumentation and techniques, though I could never have envisioned how circuitous the route to the end result would be.
First Steps
I sketched out my ideas for arthroscopic suture passers and knot-tying instruments and presented them to a couple of the major arthroscopy companies in the United States, but the companies were not interested. They did not believe arthroscopy would have any meaningful applications in the shoulder. So, I enlisted the services of a local San Antonio aircraft machinist to fabricate instruments for me. By 1987, I was doing arthroscopic side-to-side margin convergence1 cuff repairs for U-shape tears on a regular basis. And I was doing these at the most hostile point in the universe for arthroscopic shoulder surgery: San Antonio, Texas.
Only a few surgeons were doing arthroscopic shoulder surgery in the 1980s and early 1990s, and without exception these surgeons became the leader-pioneers in the new discipline. In general, these were young surgeons who were in private practice and removed from academia and professional organizations, and thus relatively sheltered from the actions of the shoulder rule-makers of the day. They accepted their status as pariahs as they developed their techniques out of the view of mainstream orthopedics. These leaders included Jim Esch, Steve Snyder, Dick Caspari, Lanny Johnson, Gene Wolf, Gary Gartsman, Rob Bell, and Howard Sweeney. We shared our techniques and our ideas with one another, encouraged one another, and generally became good friends.
Thomas Kuhn, in his classic book The Structure of Scientific Revolutions,2 observed that paradigm shifts within a given field were usually achieved by practitioners who were either very young (naïve) or outside the established hierarchy in the field. The surgeons who contributed most to the shift of shoulder surgery from open to arthroscopic techniques were generally young men who were in private practice and had little to lose by inciting the disdain of the shoulder establishment. Predictably, resistance from the mainstream open shoulder surgeons increased as arthroscopic techniques became more successful and more threatening to the primacy of the open shoulder surgeons. The disdain yielded to disruption and finally to transformation as the paradigm shift occurred. The conflict between the open shoulder surgeons and the arthroscopic shoulder surgeons passed through all the phases that Mahatma Gandhi had described many years before. “First they ignore you; then they laugh at you; then they fight you; then you win.”
Building a Ship in a Bottle
At the start of the 1990s, I recognized that my progress in arthroscopic rotator cuff repair would be extremely slow unless I could find an industry partner who shared my vision for full-scale conversion to arthroscopic means of repair and would be willing to help make it a reality. In 1991, I happened to meet Reinhold Schmieding, the owner of Arthrex, a small arthroscopic device company in Naples, Florida. Reinhold invited me to visit him to discuss the feasibility of developing arthroscopic repair systems for the shoulder. At the time, the world headquarters of Arthrex was a 20×30-ft storage room in an office service center, and there were 2 employees. One employee, Don Grafton, was a talented engineer without medical experience. By the end of my first day there, Reinhold and Don and I had agreed that developing arthroscopic repair systems for shoulder instability and rotator cuff repair would become a top priority for Arthrex.
My initial bias toward arthroscopic cuff repair was that a transosseous bone tunnel technique not only would be possible but would be superior to suture anchor fixation. In fact, my first 2 patents with Arthrex were for instrumentation for an arthroscopic transosseous repair technique. I tested my hypothesis with 2 successive biomechanical studies. The first examined cyclic loading of bone tunnel repairs, and the second examined cyclic loading of anchor-based repairs.3,4 Evaluating the data from these 2 studies, I was surprised to find that anchor-based repairs were significantly stronger than bone tunnel repairs. In addition, anchors shifted the weak link from the bone–suture interface to the tendon–suture interface; in essence, anchors optimized bone fixation by shifting the weak link in the construct to the tendon. I was then completely convinced of the superiority of suture anchors over bone tunnels, and that conviction has become even stronger over the years. After these 2 cyclic loading studies, I shifted my focus, and that of Arthrex, toward arthroscopic suture anchor repair of the rotator cuff.
Reconciling Technique and Instrumentation With Anatomy and Biomechanics
Having recognized the importance of the rotator cable attachments both anatomically5 and biomechanically,6,7 I thought it important to reinforce them as a routine part of performing rotator cuff repairs. Our anatomical and biomechanical studies had had great translational implications in the development of our techniques and instrumentation.
As mentioned earlier, Don Grafton was the chief (and for a long time only) engineer at Arthrex. As he had no medical experience, I invited him to come to San Antonio to observe surgery. During Don’s many visits, I showed him pathology in the operating room and pointed out what I could do with the instruments I had and what I could not do. Then in the evening we went to my house and brainstormed how to perform the “missing” surgical manipulations, how to improve manipulations that were suboptimal, and how to optimize final surgical constructs.
Passing suture through tendon was an early challenge. One must remember that, in the early 1990s, it was not possible for machinists to fabricate complex shapes. Therefore, straight tubular retrograde suture passers were the logical first option. We initially developed spring-loaded retrograde hook retrievers (Figure 1) and then curved suture hooks with shuttling wires (Lasso). To me, the most unappealing feature of retrograde suture passage was the oblique angle of approach through the tendon, which caused a length–tension mismatch between the upper fibers and the lower fibers of the muscle–tendon unit. We recognized we could eliminate the mismatch if we passed the suture antegrade, such that it would pass perpendicular to the tendon fibers. These insights and efforts culminated in development of the Viper suture passer and then the FastPass Scorpion suture passer, which has a spring-loaded trapdoor on the upper jaw for ergonomic self-retrieving of the suture once it is passed through the tendon.
To develop a knot pusher that optimized knot tying (yielding the highest knot security and the tightest loop security), we used prototype instruments to tie and test literally thousands of knots in the laboratory. We were thus able to verify that the Surgeon’s Sixth Finger Knot Pusher (Arthrex) reproducibly tied optimized knots8,9 and also optimized knot fixation and bone fixation. However, our suture was not yet optimized and was prone to breakage, and our suture–tendon interface was not yet optimized. Clearly, improvement was needed in 2 more areas.
Don came up with the idea for a virtually unbreakable suture and developed that idea into FiberWire.10 Shortly thereafter, I contributed the idea and design for FiberTape, which dramatically enhanced suture pullout strength and footprint compression.
Anchor designs improved rapidly and dramatically. We made the second-generation BioCorkscrew fully threaded, which virtually eliminated anchor failure, even in soft bone.
Optimization of the suture–tendon interface took a giant step forward when Park and colleagues11,12 introduced linked double-row rotator cuff repair. Much as with a Chinese finger trap, the harder you pull, the stronger it becomes, with yield load approaching ultimate load.
At this point, it seemed we had optimized virtually every segment of the rotator cuff repair construct. Each component was just about as good as it could be. Or was it?
The Accidental Quest for Knotless Fixation
In November 1998, I made my first trip to China as a guest speaker at the Congress of the Hong Kong Orthopaedic Association. My first view of the magnificent Hong Kong skyline across Victoria Harbour was truly breathtaking. As I admired the gleaming glass towers and the concrete canyons of the city, I had no idea that the very next day these modern skyscrapers would reveal an ancient secret that would change my approach to arthroscopic rotator cuff repair.
The day after my arrival, Dr. James Lam took me to lunch. As we approached the restaurant, he pointed across the street to a tall building that was being renovated and had scaffolding supporting workers alongside the first 9 stories of the exterior wall. Dr. Lam said that, after lunch, he would take me to the construction site for a closer look at the scaffolding.
After lunch, we walked to the base of the scaffolding. Dr. Lam told me it was constructed entirely of bamboo poles held together with lashings but no knots (Figure 2). Lashings were secured by turning them back on themselves and wrapping them in an entirely knotless manner.13 I found it incredible that this knotless fixation was so secure that it could support the weight of workers many stories above the ground. I resolved to determine how this fixation method worked and see if the same mechanism might help us achieve reliable knotless fixation in surgery.
When I returned home, I broke out my college engineering books and reacquainted myself with the concept of cable friction. As has happened so often in the past, however, it took a practical lesson from the ranch to truly illustrate for me how cable friction works.
Every cowboy knows that a spirited horse cannot be restrained with only one lead rope. However, a cowboy can wrap a lead rope around a “snubbing post” and thereby gain complete control over the animal, despite the horse’s superior size and strength. The cable friction between the rope and the post creates such a large restraining force that the cowboy can easily hold the animal without the help of a knotted rope (Figure 3). In similar fashion in the Hong Kong scaffolding, fixation strength results from the significant amount of cable friction produced when the lashings wrap around one another and around the bamboo poles.
The cable friction concept was pivotal in the development of knotless fixation in arthroscopic rotator cuff repair. In lateral row fixation, the eyelet of the PushLock and SwiveLock suture anchors (Arthrex) produces significant cable friction at the eyelet–suture interface, in addition to frictional force wedging the suture between anchor and bone.
As with so many other devices in shoulder arthroscopy, the SwiveLock suture anchor developed in stages. In the first stage, a chainlike suture with consecutive intersecting links was used (FiberChain). The idea for an adjustable fixation construct came to me because I thought that a forked eyelet on a SwiveLock would provide a firm fixation point when inserted into the appropriate suture link, yet would be totally adjustable simply by choosing a tighter or looser link (Figure 4). Although the system worked very well, it was technically challenging. The process was greatly simplified after Don Grafton and I developed FiberTape and recognized that the power of cable friction was dramatically increased by the larger contact area between the eyelet and the braided FiberTape. The SpeedBridge construct (Arthrex), which enhanced cable friction fixation by means of passing FiberTape through the anchor eyelets, also provided a larger compressive interface at the repair site by using FiberTape rather than conventional suture. These incremental improvements led to what I would characterize as today’s gold standard for arthroscopic rotator cuff repair: a largely knotless linked double-row construct using FiberTape, with cinch-loop sutures at the anterior and posterior margins of the tear to reinforce the cable attachments and simultaneously reduce the dog-ears that typically occur in those locations, and a double-pulley medial mattress if tendon quality is poor (Figure 5).
The Burden of Craft
With all the recent enthusiasm for level I studies, I think we need to examine whether they will accelerate technological advancement in rotator cuff repair. The answer, in my opinion, is a resounding no. This answer is based on a major disconnect I have detected in how we evaluate these studies in rotator cuff disease and repair.
An irony related to technological advancement in surgery is that the more technically advanced the surgery becomes, the more skill is required. This fact is completely at odds with the public’s perception that technological advances make procedures easier. In arthroscopic rotator cuff repair, the surgeon must look, feel, and be aware to a greater degree than in open surgery.
Edward Tenner, in his book Why Things Bite Back, described the burden of the practitioner of any advanced technology as the burden of craft.14 The burden of craft is the inherent demand on all craftspeople, but particularly surgeons, to “up our game” if we are to be successful in our craft. For arthroscopic rotator cuff repair, the burden of craft requires patience, attention to detail, and the ability to work in a virtual space. Not everyone has these skills. But anyone who wants to practice in this discipline has an obligation to learn the skills required, and then to teach them to others and assess how well they are being applied.
The problem with relying on level I studies to assess the efficacy of a surgical procedure is that they are inherently biased by the surgeons involved. As results depend on surgeons’ skills, and surgeons’ skill levels are not equal, level I studies cannot prove what is possible, cannot demonstrate the limits of a technique, and cannot demonstrate the equivalence of techniques.
Amazingly enough, there are still rotator cuff repair “deniers” who confidently assert from the podium that a large percentage of massive cuff tears cannot be repaired and that, even if they can be repaired, they do not have the biological potential to heal. Given the disparity in surgeons’ skills and results, however, one must ask whether poor results are a consequence of a biological deficit in the patient, or of a skill deficit in the surgeon.
What I know is that we have techniques for predictable arthroscopic repair and healing of the vast majority of rotator cuff tears, even massive tears,15-17 and patients do very well clinically. Yet, among many orthopedic surgeons, there is a trend to go straight to reverse total shoulder arthroplasty (rTSA) for massive tears—despite the evidence against it. As reported in the literature, rTSA results are not as good as arthroscopic cuff repair results, and the complication rate for rTSA is much higher.
Why has this trend toward rTSA for massive tears gained so much momentum? The only reason I can surmise is that, for the average surgeon, rTSA is easier and quicker than arthroscopic repair for massive tears. But the reason for choosing a specific type of surgery for a given problem should not be that it is easiest for the surgeon; it should be that it is best for the patient.
The surgeon should start by asking what procedure he or she would want if the roles were reversed—if the surgeon were the patient with the massive rotator cuff tear. If a surgeon does not have the skill set for the best procedure for a particular patient, he or she is obligated to send that patient to a surgeon who does have the skills. In addition, given that infection is the most feared complication in most shoulder surgeries, the surgeon should ask which infection rate would be personally acceptable. Arthroscopic rotator cuff repair has a reported infection rate of 1.6 per 1000, or .0016,18 whereas rTSA has an infection rate about 25 times higher, or .04.19 Further, the surgeon must consider the relative severity of the consequences of infection. By any measure, an infected arthroscopy is a straightforward treatable complication, but an infected shoulder replacement is a human tragedy. Patients vastly prefer the minimally invasive arthroscopic approach, and through online searches can easily identify who can offer an arthroscopic solution.
To reproducibly achieve successful arthroscopic repair of massive rotator cuff tears, the surgeon must know advanced techniques, including subscapularis repair techniques,20,21 interval slides,22,23 and self-reinforcing constructs.24,25
“It’s a poor carpenter who blames his tools.” This 18th-century English proverb is as true today as it was 300 years ago. The tools for arthroscopic cuff repair exist, and they are excellent. The burden of craft is the surgeon’s burden and obligation. As surgeons, we must accept that obligation and the responsibility of that burden.
As mentioned earlier, Dr. Rob Bell’s charge to me when he invited me to give the Neer Lecture was to sum up my involvement in the development of arthroscopic shoulder surgery. The short version is that I have been doing shoulder arthroscopy for 31 years; have received 28 US patents related to shoulder instruments and implants and have 12 US patents pending; have published 167 peer-reviewed articles, a couple dozen book chapters, and 2 textbooks on shoulder arthroscopy; have trained 25 fellows; and have hosted approximately 3000 visiting surgeons in my operating room. My greatest professional dream was to see the standard of care for rotator cuff repair and shoulder instability transition from open to arthroscopic techniques, and I have been fortunate enough to have observed that paradigm shift during my career.
What do I envision over the next 31 years? As we all know, history runs in both directions, and some things simply have not happened yet. In terms of rotator cuff treatment, I think over the next few years the guiding principle of treatment will be joint preservation. All rotator cuff tears, even massive tears, will be repaired arthroscopically. Patients and insurers will demand arthroscopic repair, and surgeons without the skill set will migrate to other subspecialties. As for the role of arthroplasty in the treatment of rotator cuff tears, rTSA will be indicated only for pseudoparalysis after failed cuff repair in low-demand elderly patients.
In rotator cuff treatment, I envision a standard of care that is almost entirely arthroscopic. This standard will demand that surgeons who treat rotator cuff tears be proficient in arthroscopic repair of the full range of tears. Acquiring the skills for arthroscopic repair may not be easy, but then “there’s the easy way, and there’s the Cowboy Way.” As my dad used to tell me when I complained about working too hard, “No man ever drowned in his own sweat.” We shoulder surgeons must accept the burden of craft that accompanies the new standard of arthroscopic cuff repair, and we must offer our patients the same level of care we would choose for ourselves.
Happy trails!
1. Burkhart SS, Athanasiou KA, Wirth MA. Margin convergence: a method of reducing strain in massive rotator cuff tears. Arthroscopy. 1996:12(3):335-338.
2. Kuhn TS. The Structure of Scientific Revolutions. Chicago, IL: University of Chicago Press; 1962.
3. Burkhart SS, Johnson TC, Wirth MA, Athanasiou KA. Cyclic loading of transosseous rotator cuff repairs: tension overload as a possible cause of failure. Arthroscopy. 1997;13(2):172-176.
4. Burkhart SS, Diaz Pagàn JL, Wirth MA, Athansiou KA. Cyclic loading of anchor-based rotator cuff repairs: confirmation of the tension overload phenomenon and comparison of suture anchor fixation with transosseous fixation. Arthroscopy. 1997;13(6):720-724.
5. Burkhart SS, Esch JC, Jolson RS. The rotator crescent and rotator cable: an anatomic description of the shoulder’s “suspension bridge.” Arthroscopy. 1993;9(6):611-616.
6. Burkhart SS, Nottage WM, Ogilvie-Harris DJ, Kohn HS, Pachelli A. Partial repair of irreparable rotator cuff tears. Arthroscopy. 1994;10(4):363-370.
7. Halder AM, O’Driscoll SW, Heers G, et al. Biomechanical comparison of effects of supraspinatus tendon detachments, tendon defects, and muscle retractions. J Bone Joint Surg Am. 2002;84(5):780-785.
8. Lo IK, Burkhart SS, Chan KC, Athanasiou K. Arthroscopic knots: determining the optimal balance of loop security and knot security. Arthroscopy. 2004;20(5):489-502.
9. Lo IK, Ochoa E Jr, Burkhart SS. A comparison of knot security and loop security in arthroscopic knots tied with newer high-strength suture materials. Arthroscopy. 2010;26(9 suppl):S120-S126.
10. Lo IK, Burkhart SS, Athanasiou K. Abrasion resistance of two types of nonabsorbable braided suture. Arthroscopy. 2004;20(4):407-413.
11. Park MC, ElAttrache NS, Tibone JE, Ahmad CS, Jun BJ, Lee TQ. Part I: footprint contact characteristics for a “transosseous-equivalent” rotator cuff repair technique compared to a double-row technique. J Shoulder Elbow Surg. 2007;16(4):461-468.
12. Park MC, Tibone JE, ElAttrache NS, Ahmad CS, Jun BJ, Lee TQ. Part II: biomechanical assessment for a footprint-restoring transosseous-equivalent rotator cuff repair technique compared with a double-row repair technique. J Shoulder Elbow Surg. 2007;16(4):469-476.
13. Burkhart SS, Athanasiou KA. The twist-lock concept of tissue transport and suture fixation without knots: observations along the Hong Kong skyline. Arthroscopy. 2003;19(6):613-625.
14. Tenner E. Why Things Bite Back. New York, NY: Random House; 1996.
15. Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of arthroscopic massive rotator cuff repair: the importance of double-row fixation. Arthroscopy. 2012;28(7):909-915.
16. Denard PJ, Lädermann A, Jiwani AZ, Burkhart SS. Functional outcome after arthroscopic repair of massive rotator cuff tears in individuals with pseudoparalysis. Arthroscopy. 2012;28(9):1214-1219.
17. Lädermann A, Denard PJ, Burkhart SS. Revision arthroscopic rotator cuff repair: systematic review and authors’ preferred surgical technique. Arthroscopy. 2012;28(8):1160-1169.
18. Randelli P, Castagna A, Cabitza F, Cabitza P, Arrigoni P, Denti M. Infectious and thromboembolic complications of arthroscopic shoulder surgery. J Shoulder Elbow Surg. 2010;19(1):97-101.
19. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146-157.
20. Adams CR, Schoolfield JD, Burkhart SS. The results of arthroscopic subscapularis tendon repairs. Arthroscopy. 2008;24(12):1381-1389.
21. Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of a consecutive series of subscapularis tendon tears repaired arthroscopically. Arthroscopy. 2012;28(11):1587-1591.
22. Lo IK, Burkhart SS. Arthroscopic repair of massive, contracted, immobile rotator cuff tears using single and double interval slides: technique and preliminary results. Arthroscopy. 2004;20(1):22-33.
23. Lo IK, Burkhart SS. The interval slide in continuity: a method of mobilizing the anterosuperior rotator cuff without disrupting the tear margins. Arthroscopy. 2004;20(4):435-441.
24. Denard PJ, Burkhart SS. Techniques for managing poor quality tissue and bone during arthroscopic rotator cuff repair. Arthroscopy. 2011;27(10):1409-1421.
25. Burkhart SS, Denard PJ, Konicek J, Hanypsiak BT. Biomechanical validation of load-sharing rip-stop fixation for the repair of tissue-deficient rotator cuff tears. Am J Sports Med. 2014;42(2):457-462.
1. Burkhart SS, Athanasiou KA, Wirth MA. Margin convergence: a method of reducing strain in massive rotator cuff tears. Arthroscopy. 1996:12(3):335-338.
2. Kuhn TS. The Structure of Scientific Revolutions. Chicago, IL: University of Chicago Press; 1962.
3. Burkhart SS, Johnson TC, Wirth MA, Athanasiou KA. Cyclic loading of transosseous rotator cuff repairs: tension overload as a possible cause of failure. Arthroscopy. 1997;13(2):172-176.
4. Burkhart SS, Diaz Pagàn JL, Wirth MA, Athansiou KA. Cyclic loading of anchor-based rotator cuff repairs: confirmation of the tension overload phenomenon and comparison of suture anchor fixation with transosseous fixation. Arthroscopy. 1997;13(6):720-724.
5. Burkhart SS, Esch JC, Jolson RS. The rotator crescent and rotator cable: an anatomic description of the shoulder’s “suspension bridge.” Arthroscopy. 1993;9(6):611-616.
6. Burkhart SS, Nottage WM, Ogilvie-Harris DJ, Kohn HS, Pachelli A. Partial repair of irreparable rotator cuff tears. Arthroscopy. 1994;10(4):363-370.
7. Halder AM, O’Driscoll SW, Heers G, et al. Biomechanical comparison of effects of supraspinatus tendon detachments, tendon defects, and muscle retractions. J Bone Joint Surg Am. 2002;84(5):780-785.
8. Lo IK, Burkhart SS, Chan KC, Athanasiou K. Arthroscopic knots: determining the optimal balance of loop security and knot security. Arthroscopy. 2004;20(5):489-502.
9. Lo IK, Ochoa E Jr, Burkhart SS. A comparison of knot security and loop security in arthroscopic knots tied with newer high-strength suture materials. Arthroscopy. 2010;26(9 suppl):S120-S126.
10. Lo IK, Burkhart SS, Athanasiou K. Abrasion resistance of two types of nonabsorbable braided suture. Arthroscopy. 2004;20(4):407-413.
11. Park MC, ElAttrache NS, Tibone JE, Ahmad CS, Jun BJ, Lee TQ. Part I: footprint contact characteristics for a “transosseous-equivalent” rotator cuff repair technique compared to a double-row technique. J Shoulder Elbow Surg. 2007;16(4):461-468.
12. Park MC, Tibone JE, ElAttrache NS, Ahmad CS, Jun BJ, Lee TQ. Part II: biomechanical assessment for a footprint-restoring transosseous-equivalent rotator cuff repair technique compared with a double-row repair technique. J Shoulder Elbow Surg. 2007;16(4):469-476.
13. Burkhart SS, Athanasiou KA. The twist-lock concept of tissue transport and suture fixation without knots: observations along the Hong Kong skyline. Arthroscopy. 2003;19(6):613-625.
14. Tenner E. Why Things Bite Back. New York, NY: Random House; 1996.
15. Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of arthroscopic massive rotator cuff repair: the importance of double-row fixation. Arthroscopy. 2012;28(7):909-915.
16. Denard PJ, Lädermann A, Jiwani AZ, Burkhart SS. Functional outcome after arthroscopic repair of massive rotator cuff tears in individuals with pseudoparalysis. Arthroscopy. 2012;28(9):1214-1219.
17. Lädermann A, Denard PJ, Burkhart SS. Revision arthroscopic rotator cuff repair: systematic review and authors’ preferred surgical technique. Arthroscopy. 2012;28(8):1160-1169.
18. Randelli P, Castagna A, Cabitza F, Cabitza P, Arrigoni P, Denti M. Infectious and thromboembolic complications of arthroscopic shoulder surgery. J Shoulder Elbow Surg. 2010;19(1):97-101.
19. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146-157.
20. Adams CR, Schoolfield JD, Burkhart SS. The results of arthroscopic subscapularis tendon repairs. Arthroscopy. 2008;24(12):1381-1389.
21. Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of a consecutive series of subscapularis tendon tears repaired arthroscopically. Arthroscopy. 2012;28(11):1587-1591.
22. Lo IK, Burkhart SS. Arthroscopic repair of massive, contracted, immobile rotator cuff tears using single and double interval slides: technique and preliminary results. Arthroscopy. 2004;20(1):22-33.
23. Lo IK, Burkhart SS. The interval slide in continuity: a method of mobilizing the anterosuperior rotator cuff without disrupting the tear margins. Arthroscopy. 2004;20(4):435-441.
24. Denard PJ, Burkhart SS. Techniques for managing poor quality tissue and bone during arthroscopic rotator cuff repair. Arthroscopy. 2011;27(10):1409-1421.
25. Burkhart SS, Denard PJ, Konicek J, Hanypsiak BT. Biomechanical validation of load-sharing rip-stop fixation for the repair of tissue-deficient rotator cuff tears. Am J Sports Med. 2014;42(2):457-462.
Shoulder Examination of the Overhead Athlete
The overhead athlete’s shoulder is exposed to extremes of stress and range of motion (ROM), predisposing this joint to unique injury patterns. Prompt diagnosis and management begin with a comprehensive history and a physical examination, supplemented by imaging studies as needed. Furthermore, the throwing shoulder undergoes adaptive changes, such as partial undersurface rotator cuff tears and capsular laxity. Imaging studies typically demonstrate abnormalities in asymptomatic throwers. Therefore, clinicians must be skilled in history taking and physical examination in throwing athletes to accurately determine the cause of symptoms and provide optimal treatment. This primer provides orthopedic surgeons with the key points in performing a thorough physical examination of the shoulder in overhead athletes.
When working with overhead athletes, surgeons must elicit the precise nature of symptoms. For example, it is important to distinguish pain from fatigue, as well as complaints related purely to decline in performance. Often, collaboration with the player’s parent or coach may help clarify the chief complaint. In addition, surgeons must have an intricate knowledge of the various stages of the overhead motion, as symptoms in specific stages (late cocking/early acceleration) may raise suspicion for distinctive pathology (labral/biceps complex). Last, it is imperative to understand that the shoulder represents only one part of the kinetic chain in overhead athletes. Successful throwing relies on integrity of the entire kinetic chain, starting with the lower extremity and trunk, extending through the spine, scapula, and shoulder, and terminating with the hand and fingers. Pathology anywhere in the chain must be evaluated and addressed.
When examining the shoulder in overhead athletes, surgeons must address several anatomical structures, both bony and soft tissue. Proper examination begins with comprehensive assessment of the ROM and strength of the various muscles around the shoulder, along with visual inspection to identify any asymmetry of these structures. In addition, the scapulothoracic structures must be examined in detail to rule out underlying dyskinesis. The capsular and ligamentous components of the shoulder joint must be further assessed to note any capsular contracture causing glenohumeral internal rotation deficit (GIRD) or any pathology with the rotator cuff or labral/biceps complex. Last, a comprehensive neurovascular examination should be performed to rule out any compression or neuropathy affecting the shoulder and overhead motion. Findings from the physical examination may then require further imaging to correlate the history and physical examination findings.
1. Inspection, palpation, strength testing
Every examination of the shoulder must begin with visual inspection, along with assessment of basic ROM and strength. The patient must be positioned and exposed adequately to promote visualization of the entire shoulder and scapular girdle, from both anterior and posterior. Visual inspection focuses on identifying any areas of asymmetry, such as position of the bony prominences or bulk of the muscular fossae. Asymmetry of the bony architecture may indicate prior trauma, and atrophy of the muscular fossae may indicate nerve compression. For example, atrophy of the infraspinatus fossa may be caused by compression of the suprascapular nerve at the spinoglenoid notch (likely by a cyst, often associated with labral pathology, but infraspinatus atrophy can result even without the presence of a compressive cyst1). Alternatively, atrophy of both the supraspinatus and infraspinatus fossae may indicate underlying compression of the suprascapular nerve at the suprascapular notch (either by a cyst or by the transverse scapular ligament). Static and dynamic observation of the posterior aspect of the shoulder may help identify gross pathology with scapular positioning or retraction, indicating underlying dyskinesis (discussed later). Deformity of the acromioclavicular joint may indicate prior trauma or separation. Last, all prior surgical scars should be noted.
Selective palpation may help identify pathology in the shoulder of the throwing athlete. Tenderness at the acromioclavicular joint may be especially common in patients who have had prior sprains of this joint or who have degenerative changes. Tenderness along the biceps tendon may be present in those with biceps tendinitis or partial tear. In addition, tenderness at the coracoid may be present in those with scapular dyskinesis. Posteriorly, palpation at the inferomedial aspect of the scapula (Figure 1), as with palpation along the medial border of the scapula, may elicit tenderness in those with scapulothoracic bursitis.
Strength testing in the shoulder is performed to elicit any deficiencies of the rotator cuff/musculature or surrounding structures. Weakness in forward elevation may indicate pathology in the supraspinatus, whereas weakness in external rotation may reflect deficiency in the infraspinatus or teres minor. Teres minor deficiency may be more isolated with weakness in a position of shoulder abduction to 90°. Last, weakness in internal rotation may indicate subscapularis deficiency. Lag signs and other provocative maneuvers are similarly elicited but typically are positive only in the event of large tears of the rotator cuff. These signs and maneuvers include the internal rotation lag sign or belly press test for subscapularis integrity, the drop-arm sign for supraspinatus function, the external rotation lag sign for infraspinatus function, and the hornblower sign for teres minor integrity. Supporting muscles of the shoulder may also be tested. Latissimus strength may be tested with resisted downward rotation of the arm with the shoulder in abduction and the elbow flexed to 90°.
2. ROM and GIRD assessment
After inspection and palpation, the shoulder should be ranged in all relevant planes of motion. Our standard examination includes forward elevation in the frontal and scapular planes, along with external rotation at the side and at 90° of abduction, as well as internal rotation behind the back with documentation of the highest spinal level that the patient can reach. This examination may be performed with the patient upright, but supine positioning can help stabilize the scapula and provide more accurate views of motion. Deficits of internal rotation may be a common finding in overhead athletes, and the degree of this deficit should be quantitatively noted.
Bony and soft-tissue remodeling of the shoulder (and associated structures) in the overhead athlete can lead to contracture of the posterior capsule. This contracture can cause excessive external rotation and subsequent decrease in internal rotation, leading to pain and anterior instability in the throwing shoulder.2 For precise measurements of the internal and external rotation arc, the scapula must be stabilized. This can be done with the patient supine on the examining table or seated upright with manual stabilization of the scapula by the examiner. Once the scapula is stabilized, the arc of internal and external rotation (with the arm in about 90° of abduction) can be measured with a goniometer, with maximum values obtained as the scapula begins to move along the posterior chest wall.2 The difference in internal rotation between the dominant and nondominant arms defines the extent of the athlete’s GIRD. Internal rotation can also be qualitatively assessed by having the athlete internally rotate each arm and reach up the spine while the examiner notes the difference in level achieved. However, this does not provide a quantitative assessment of the patient’s GIRD.
In general, the sum of the internal and external rotation arcs on the 2 sides should be symmetric. Consequently, in GIRD, excessive external rotation is balanced by decreased internal rotation. Symptomatic GIRD may be present when there is more than 25° of discrepancy in internal rotation between the athlete’s dominant and nondominant arms.2 The goal is to reduce this discrepancy to less than 20°.
3. Internal impingement: rotator cuff and labrum
In overhead athletes, an intricate relationship involving rotator cuff, labrum, and biceps tendon allows for efficient, pain-free force delivery at the shoulder. However, because of the significant external rotation and abduction required in the overhead motion, there may be internal impingement of the posterosuperior rotator cuff (infraspinatus and posterior aspect of supraspinatus) between the posterior labrum and the greater tuberosity. Detailed examination of these structures must be performed in any assessment of an overhead athlete. Symptomatic patients may complain of pain during the throwing cycle, particularly in late cocking and early acceleration.
The modified relocation examination is a common maneuver to detect internal impingement.3 In this examination, the patient’s arm is brought into a position of maximal external rotation and abduction mimicking that found in late cocking or early acceleration. In this position, a patient with internal impingement complains of pain in the posterior shoulder. A posteriorly directed force on the humerus relieves this pain.
There are also many examinations for detecting labral pathology, specifically a SLAP (superior labrum, anterior to posterior) lesion, which is commonly found in patients with internal impingement. One commonly tested maneuver is the O’Brien active compression test (Figures 2A, 2B), which has excellent sensitivity and specificity in detecting type II SLAP lesions.4 In this examination, the patient holds the arm in about 15° of adduction and 90° of forward elevation. A downward force is applied with the forearm pronated and subsequently supinated. If pain is noted on the force applied to the pronated arm, and if this pain decreases in the supinated examination, the test is positive for labral pathology.
Anterior instability is routinely found in these patients. Translation is measured with the anterior load and shift test. Anterior translation is tested with the patient supine, with the arm in abduction and external rotation, and with the examiner placing an anteriorly directed force on the humeral head. Translation is compared with the contralateral side and graded on a 3-point scale (1+ is translation to glenoid rim, 2+ is translation over glenoid rim but reduces, 3+ is translation over glenoid and locking). We also use the anterior release test, in which the patient is supine, the arm is brought into abduction and external rotation, and the examiner places a posteriorly directed force on the humeral head. When the examiner removes this force, the patient notices symptoms of instability caused by subluxation (Figures 3A, 3B).
Biceps tendon testing should also be performed to help elicit signs of labral pathology. The Speed test is performed by placing a downward force on the patient’s arm, which is held in 90° forward elevation, and with elbows in extension and forearm in supination. Pain in the long head of the biceps tendon is considered a positive sign and suggestive of SLAP lesion. Although not commonly found in these athletes, external impingement should also be elicited through both the Neer test and the Hawkins test. In the Neer test, the patient’s arm is brought to maximal forward elevation with the forearm supinated and elbow extended, while the scapula is stabilized by the examiner. Pain in the shoulder indicates a positive examination. In the Hawkins test, the patient’s arm is brought into a position of forward elevation, internal rotation, and elbow flexion. The arm is then further internally rotated, and shoulder pain defines a positive examination.
Any of these findings can be concomitant with scapular dyskinesis. Moreover, symptoms related to internal impingement may be exacerbated by concomitant scapular pathology, and therefore proper assessment of scapulothoracic motion must also be performed.
4. Scapulothoracic examination
Motion coupled between the scapula and the rest of the arm (scapular rhythm) allows for efficient use of the shoulder girdle. The scapula helps transfer the force generated by the core so that the hand can efficiently deliver it. Therefore, scapular pathology (or dyskinesis) results in inefficient functioning of the arm, which can be especially debilitating in an overhead athlete.
Scapular assessment begins with visual inspection of the patient, typically from the posterior view, which allows for assessment of the resting position of the scapula. Evidence of prominence of the medial or inferomedial border, coracoid malposition (or pain on palpation), or general scapular malposition should be noted. On active ROM, as the patient forward-elevates the arm, any asymmetric prominence of the inferomedial border of the scapula should be noted. Such asymmetry may indicate underlying scapular dyskinesis. In another important test, the lateral scapular slide test (described by Kibler5), the distance from the inferomedial angle of the scapula to the thoracic spine should be measured for both sides and in 3 difference positions, noting any asymmetry between the affected and nonaffected sides. These 3 positions (Figures 4A–4C) are with arms at side, with hands on hips (internal rotation of humerus in 45° abduction), and in 90° of shoulder abduction. Last, medial and lateral scapular winging—caused by long thoracic nerve and spinal accessory nerve pathology, respectively—can be detected by asking the patient to do a “push-up” against the wall while the examiner views from posterior.
After assessment of scapular position at rest and through motion, a series of provocative maneuvers6 may aid in the diagnosis of scapular dyskinesis. The first maneuver is the scapular assistance test, in which the examiner provides a gentle force at the inferomedial angle of the scapula, promoting upward rotation and posterior tilt as the patient elevates the arm (Figures 5A, 5B). If the patient experiences a decrease or absence of symptoms through this arc, the test is considered positive. The second maneuver is the scapular retraction test, in which strength testing of the supraspinatus is performed before and after retraction stabilization of the scapula. In the baseline state, the strength of the supraspinatus is tested in standard fashion, with resisted elevation of the internally rotated and abducted arm. The strength is then tested with the scapula stabilized in retraction (the examiner medially stabilizes the scapula). With scapular stabilization, an increase in strength or a decrease in symptoms is considered a positive test.
5. Neurovascular examination
It is essential to perform a comprehensive neurovascular examination in all overhead athletes. This includes basic cervical spine testing for any motor or sensory deficits, along with assessment of scapular winging to detect long thoracic or spinal accessory nerve palsy for medial and lateral winging, respectively. Although neurovascular injury may be a rare finding in the overhead athlete, a detailed examination must still be performed to rule it out.
Thoracic outlet syndrome
Thoracic syndrome is a compressive neuropathy of nerves and vasculature exiting the thorax and entering the upper extremity. Common symptoms include pain and tingling (sometimes vague) in the neck and upper extremity. These symptoms may be positional as well.
Diagnosis of thoracic outlet syndrome begins with visual inspection of the involved upper extremity, noting atrophy or asymmetry. Weakness may also be present. Additional provocative maneuvers can be used to detect decrease or loss of pulses, along with reproduction of symptoms, during a provocative maneuver with subsequent return of pulses and resolution of symptoms after the maneuver is completed.
One examination that can be used to detect thoracic outlet syndrome is the Adson test.7 During this maneuver, the radial pulse is palpated with the arm at rest on the patient’s side. The patient then turns to the symptomatic side, hyperextends the arm, and holds inspiration. A positive test coincides with both decreased pulse and reproduction of symptoms, indicating compression within the scalene triangle. In the Wright test,7 the pulse is again palpated at rest with the arm at the side. The patient then holds inspiration and places the arm in a position of abduction and external rotation. If the pulses decrease with this maneuver, the test is considered positive, indicating compression in the sub–pectoralis minor region deep to the coracoid. In a third test, the costoclavicular test, again pulses are measured before and during the provocative maneuver, which is with the shoulders thrust backward and depressed downward. A positive test indicates compression between the clavicle and the first rib. In our practice, we use a modified Wright test in which the arm is held in abduction and external rotation while radial pulses are palpated. The fist is then opened and clenched rapidly, and diminution of radial pulses is considered a positive examination (Figures 6A, 6B).
Effort thrombosis
Overhead athletes are at increased risk for developing effort thrombosis8 (Paget-Schroetter syndrome). This thrombosis, which results from repetitive motion involving the upper extremity, is not limited to overhead sports; it may be caused by underlying compression of or microtrauma to the venous infrastructure. On physical examination, there may be swelling of the affected limb, along with diffuse pain and fatigue, as well as dermatologic changes. Positive findings warrant further testing, such as coagulation profile testing and advanced imaging or venography.
Arterial aneurysm
Although rare, arterial aneurysms, especially of the axillary artery, must be ruled out in the overhead athlete with vague upper extremity pain (especially distally) and without clear diagnosis.9 Aneurysm of the axillary artery can result from repetitive microtrauma related to repetitive overhead motion of the upper extremity. This condition may cause showering of emboli distally to the vasculature of the hand and fingers (Figure 7). Patients may complain of pain in the fingers, difficulty with grip, cyanosis, or cold sensation. On examination, the sufficiency of the radial and ulnar arteries should be assessed, as with detailed sensorimotor examination of the fingers. The fingernails should be examined for splinter hemorrhages.
Conclusion
Overhead athletes place extreme stress on the shoulder during the throwing motion and are at high risk for injury because of repetitive stress on the shoulder girdle. When examining overhead athletes with shoulder pain, surgeons must consider the entire kinetic chain, as inefficiencies anywhere along the chain can lead to altered mechanics and pathology in the shoulder.
1. Cummins CA, Messer TM, Schafer MF. Infraspinatus muscle atrophy in professional baseball players. Am J Sports Med. 2004;32(1):116-120.
2. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
3. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part II: evaluation and treatment of SLAP lesions in throwers. Arthroscopy. 2003;19(5):531-539.
4. O’Brien SJ, Pagnani MJ, Fealy S, McGlynn SR, Wilson JB. The active compression test: a new and effective test for diagnosing labral tears and acromioclavicular joint abnormality. Am J Sports Med. 1998;26(5):610-613.
5. Kibler WB. The role of the scapula in athletic shoulder function. Am J Sports Med. 1998;26(2):325-337.
6. Kibler WB, Sciascia A, Wilkes T. Scapular dyskinesis and its relation to shoulder injury. J Am Acad Orthop Surg. 2012;20(6):364-372.
7. Leffert RD. Thoracic outlet syndrome. J Am Acad Orthop Surg. 1994;2(6):317-325.
8. Alla VM, Natarajan N, Kaushik M, Warrier R, Nair CK. Paget-Schroetter syndrome: review of pathogenesis and treatment of effort thrombosis. West J Emerg Med. 2010;11(4):358-362.
9. Baumgarten KM, Dines JS, Winchester PA, et al. Axillary artery aneurysm with distal embolization in a Major League Baseball pitcher. Am J Sports Med. 2007;35(4):650-653.
The overhead athlete’s shoulder is exposed to extremes of stress and range of motion (ROM), predisposing this joint to unique injury patterns. Prompt diagnosis and management begin with a comprehensive history and a physical examination, supplemented by imaging studies as needed. Furthermore, the throwing shoulder undergoes adaptive changes, such as partial undersurface rotator cuff tears and capsular laxity. Imaging studies typically demonstrate abnormalities in asymptomatic throwers. Therefore, clinicians must be skilled in history taking and physical examination in throwing athletes to accurately determine the cause of symptoms and provide optimal treatment. This primer provides orthopedic surgeons with the key points in performing a thorough physical examination of the shoulder in overhead athletes.
When working with overhead athletes, surgeons must elicit the precise nature of symptoms. For example, it is important to distinguish pain from fatigue, as well as complaints related purely to decline in performance. Often, collaboration with the player’s parent or coach may help clarify the chief complaint. In addition, surgeons must have an intricate knowledge of the various stages of the overhead motion, as symptoms in specific stages (late cocking/early acceleration) may raise suspicion for distinctive pathology (labral/biceps complex). Last, it is imperative to understand that the shoulder represents only one part of the kinetic chain in overhead athletes. Successful throwing relies on integrity of the entire kinetic chain, starting with the lower extremity and trunk, extending through the spine, scapula, and shoulder, and terminating with the hand and fingers. Pathology anywhere in the chain must be evaluated and addressed.
When examining the shoulder in overhead athletes, surgeons must address several anatomical structures, both bony and soft tissue. Proper examination begins with comprehensive assessment of the ROM and strength of the various muscles around the shoulder, along with visual inspection to identify any asymmetry of these structures. In addition, the scapulothoracic structures must be examined in detail to rule out underlying dyskinesis. The capsular and ligamentous components of the shoulder joint must be further assessed to note any capsular contracture causing glenohumeral internal rotation deficit (GIRD) or any pathology with the rotator cuff or labral/biceps complex. Last, a comprehensive neurovascular examination should be performed to rule out any compression or neuropathy affecting the shoulder and overhead motion. Findings from the physical examination may then require further imaging to correlate the history and physical examination findings.
1. Inspection, palpation, strength testing
Every examination of the shoulder must begin with visual inspection, along with assessment of basic ROM and strength. The patient must be positioned and exposed adequately to promote visualization of the entire shoulder and scapular girdle, from both anterior and posterior. Visual inspection focuses on identifying any areas of asymmetry, such as position of the bony prominences or bulk of the muscular fossae. Asymmetry of the bony architecture may indicate prior trauma, and atrophy of the muscular fossae may indicate nerve compression. For example, atrophy of the infraspinatus fossa may be caused by compression of the suprascapular nerve at the spinoglenoid notch (likely by a cyst, often associated with labral pathology, but infraspinatus atrophy can result even without the presence of a compressive cyst1). Alternatively, atrophy of both the supraspinatus and infraspinatus fossae may indicate underlying compression of the suprascapular nerve at the suprascapular notch (either by a cyst or by the transverse scapular ligament). Static and dynamic observation of the posterior aspect of the shoulder may help identify gross pathology with scapular positioning or retraction, indicating underlying dyskinesis (discussed later). Deformity of the acromioclavicular joint may indicate prior trauma or separation. Last, all prior surgical scars should be noted.
Selective palpation may help identify pathology in the shoulder of the throwing athlete. Tenderness at the acromioclavicular joint may be especially common in patients who have had prior sprains of this joint or who have degenerative changes. Tenderness along the biceps tendon may be present in those with biceps tendinitis or partial tear. In addition, tenderness at the coracoid may be present in those with scapular dyskinesis. Posteriorly, palpation at the inferomedial aspect of the scapula (Figure 1), as with palpation along the medial border of the scapula, may elicit tenderness in those with scapulothoracic bursitis.
Strength testing in the shoulder is performed to elicit any deficiencies of the rotator cuff/musculature or surrounding structures. Weakness in forward elevation may indicate pathology in the supraspinatus, whereas weakness in external rotation may reflect deficiency in the infraspinatus or teres minor. Teres minor deficiency may be more isolated with weakness in a position of shoulder abduction to 90°. Last, weakness in internal rotation may indicate subscapularis deficiency. Lag signs and other provocative maneuvers are similarly elicited but typically are positive only in the event of large tears of the rotator cuff. These signs and maneuvers include the internal rotation lag sign or belly press test for subscapularis integrity, the drop-arm sign for supraspinatus function, the external rotation lag sign for infraspinatus function, and the hornblower sign for teres minor integrity. Supporting muscles of the shoulder may also be tested. Latissimus strength may be tested with resisted downward rotation of the arm with the shoulder in abduction and the elbow flexed to 90°.
2. ROM and GIRD assessment
After inspection and palpation, the shoulder should be ranged in all relevant planes of motion. Our standard examination includes forward elevation in the frontal and scapular planes, along with external rotation at the side and at 90° of abduction, as well as internal rotation behind the back with documentation of the highest spinal level that the patient can reach. This examination may be performed with the patient upright, but supine positioning can help stabilize the scapula and provide more accurate views of motion. Deficits of internal rotation may be a common finding in overhead athletes, and the degree of this deficit should be quantitatively noted.
Bony and soft-tissue remodeling of the shoulder (and associated structures) in the overhead athlete can lead to contracture of the posterior capsule. This contracture can cause excessive external rotation and subsequent decrease in internal rotation, leading to pain and anterior instability in the throwing shoulder.2 For precise measurements of the internal and external rotation arc, the scapula must be stabilized. This can be done with the patient supine on the examining table or seated upright with manual stabilization of the scapula by the examiner. Once the scapula is stabilized, the arc of internal and external rotation (with the arm in about 90° of abduction) can be measured with a goniometer, with maximum values obtained as the scapula begins to move along the posterior chest wall.2 The difference in internal rotation between the dominant and nondominant arms defines the extent of the athlete’s GIRD. Internal rotation can also be qualitatively assessed by having the athlete internally rotate each arm and reach up the spine while the examiner notes the difference in level achieved. However, this does not provide a quantitative assessment of the patient’s GIRD.
In general, the sum of the internal and external rotation arcs on the 2 sides should be symmetric. Consequently, in GIRD, excessive external rotation is balanced by decreased internal rotation. Symptomatic GIRD may be present when there is more than 25° of discrepancy in internal rotation between the athlete’s dominant and nondominant arms.2 The goal is to reduce this discrepancy to less than 20°.
3. Internal impingement: rotator cuff and labrum
In overhead athletes, an intricate relationship involving rotator cuff, labrum, and biceps tendon allows for efficient, pain-free force delivery at the shoulder. However, because of the significant external rotation and abduction required in the overhead motion, there may be internal impingement of the posterosuperior rotator cuff (infraspinatus and posterior aspect of supraspinatus) between the posterior labrum and the greater tuberosity. Detailed examination of these structures must be performed in any assessment of an overhead athlete. Symptomatic patients may complain of pain during the throwing cycle, particularly in late cocking and early acceleration.
The modified relocation examination is a common maneuver to detect internal impingement.3 In this examination, the patient’s arm is brought into a position of maximal external rotation and abduction mimicking that found in late cocking or early acceleration. In this position, a patient with internal impingement complains of pain in the posterior shoulder. A posteriorly directed force on the humerus relieves this pain.
There are also many examinations for detecting labral pathology, specifically a SLAP (superior labrum, anterior to posterior) lesion, which is commonly found in patients with internal impingement. One commonly tested maneuver is the O’Brien active compression test (Figures 2A, 2B), which has excellent sensitivity and specificity in detecting type II SLAP lesions.4 In this examination, the patient holds the arm in about 15° of adduction and 90° of forward elevation. A downward force is applied with the forearm pronated and subsequently supinated. If pain is noted on the force applied to the pronated arm, and if this pain decreases in the supinated examination, the test is positive for labral pathology.
Anterior instability is routinely found in these patients. Translation is measured with the anterior load and shift test. Anterior translation is tested with the patient supine, with the arm in abduction and external rotation, and with the examiner placing an anteriorly directed force on the humeral head. Translation is compared with the contralateral side and graded on a 3-point scale (1+ is translation to glenoid rim, 2+ is translation over glenoid rim but reduces, 3+ is translation over glenoid and locking). We also use the anterior release test, in which the patient is supine, the arm is brought into abduction and external rotation, and the examiner places a posteriorly directed force on the humeral head. When the examiner removes this force, the patient notices symptoms of instability caused by subluxation (Figures 3A, 3B).
Biceps tendon testing should also be performed to help elicit signs of labral pathology. The Speed test is performed by placing a downward force on the patient’s arm, which is held in 90° forward elevation, and with elbows in extension and forearm in supination. Pain in the long head of the biceps tendon is considered a positive sign and suggestive of SLAP lesion. Although not commonly found in these athletes, external impingement should also be elicited through both the Neer test and the Hawkins test. In the Neer test, the patient’s arm is brought to maximal forward elevation with the forearm supinated and elbow extended, while the scapula is stabilized by the examiner. Pain in the shoulder indicates a positive examination. In the Hawkins test, the patient’s arm is brought into a position of forward elevation, internal rotation, and elbow flexion. The arm is then further internally rotated, and shoulder pain defines a positive examination.
Any of these findings can be concomitant with scapular dyskinesis. Moreover, symptoms related to internal impingement may be exacerbated by concomitant scapular pathology, and therefore proper assessment of scapulothoracic motion must also be performed.
4. Scapulothoracic examination
Motion coupled between the scapula and the rest of the arm (scapular rhythm) allows for efficient use of the shoulder girdle. The scapula helps transfer the force generated by the core so that the hand can efficiently deliver it. Therefore, scapular pathology (or dyskinesis) results in inefficient functioning of the arm, which can be especially debilitating in an overhead athlete.
Scapular assessment begins with visual inspection of the patient, typically from the posterior view, which allows for assessment of the resting position of the scapula. Evidence of prominence of the medial or inferomedial border, coracoid malposition (or pain on palpation), or general scapular malposition should be noted. On active ROM, as the patient forward-elevates the arm, any asymmetric prominence of the inferomedial border of the scapula should be noted. Such asymmetry may indicate underlying scapular dyskinesis. In another important test, the lateral scapular slide test (described by Kibler5), the distance from the inferomedial angle of the scapula to the thoracic spine should be measured for both sides and in 3 difference positions, noting any asymmetry between the affected and nonaffected sides. These 3 positions (Figures 4A–4C) are with arms at side, with hands on hips (internal rotation of humerus in 45° abduction), and in 90° of shoulder abduction. Last, medial and lateral scapular winging—caused by long thoracic nerve and spinal accessory nerve pathology, respectively—can be detected by asking the patient to do a “push-up” against the wall while the examiner views from posterior.
After assessment of scapular position at rest and through motion, a series of provocative maneuvers6 may aid in the diagnosis of scapular dyskinesis. The first maneuver is the scapular assistance test, in which the examiner provides a gentle force at the inferomedial angle of the scapula, promoting upward rotation and posterior tilt as the patient elevates the arm (Figures 5A, 5B). If the patient experiences a decrease or absence of symptoms through this arc, the test is considered positive. The second maneuver is the scapular retraction test, in which strength testing of the supraspinatus is performed before and after retraction stabilization of the scapula. In the baseline state, the strength of the supraspinatus is tested in standard fashion, with resisted elevation of the internally rotated and abducted arm. The strength is then tested with the scapula stabilized in retraction (the examiner medially stabilizes the scapula). With scapular stabilization, an increase in strength or a decrease in symptoms is considered a positive test.
5. Neurovascular examination
It is essential to perform a comprehensive neurovascular examination in all overhead athletes. This includes basic cervical spine testing for any motor or sensory deficits, along with assessment of scapular winging to detect long thoracic or spinal accessory nerve palsy for medial and lateral winging, respectively. Although neurovascular injury may be a rare finding in the overhead athlete, a detailed examination must still be performed to rule it out.
Thoracic outlet syndrome
Thoracic syndrome is a compressive neuropathy of nerves and vasculature exiting the thorax and entering the upper extremity. Common symptoms include pain and tingling (sometimes vague) in the neck and upper extremity. These symptoms may be positional as well.
Diagnosis of thoracic outlet syndrome begins with visual inspection of the involved upper extremity, noting atrophy or asymmetry. Weakness may also be present. Additional provocative maneuvers can be used to detect decrease or loss of pulses, along with reproduction of symptoms, during a provocative maneuver with subsequent return of pulses and resolution of symptoms after the maneuver is completed.
One examination that can be used to detect thoracic outlet syndrome is the Adson test.7 During this maneuver, the radial pulse is palpated with the arm at rest on the patient’s side. The patient then turns to the symptomatic side, hyperextends the arm, and holds inspiration. A positive test coincides with both decreased pulse and reproduction of symptoms, indicating compression within the scalene triangle. In the Wright test,7 the pulse is again palpated at rest with the arm at the side. The patient then holds inspiration and places the arm in a position of abduction and external rotation. If the pulses decrease with this maneuver, the test is considered positive, indicating compression in the sub–pectoralis minor region deep to the coracoid. In a third test, the costoclavicular test, again pulses are measured before and during the provocative maneuver, which is with the shoulders thrust backward and depressed downward. A positive test indicates compression between the clavicle and the first rib. In our practice, we use a modified Wright test in which the arm is held in abduction and external rotation while radial pulses are palpated. The fist is then opened and clenched rapidly, and diminution of radial pulses is considered a positive examination (Figures 6A, 6B).
Effort thrombosis
Overhead athletes are at increased risk for developing effort thrombosis8 (Paget-Schroetter syndrome). This thrombosis, which results from repetitive motion involving the upper extremity, is not limited to overhead sports; it may be caused by underlying compression of or microtrauma to the venous infrastructure. On physical examination, there may be swelling of the affected limb, along with diffuse pain and fatigue, as well as dermatologic changes. Positive findings warrant further testing, such as coagulation profile testing and advanced imaging or venography.
Arterial aneurysm
Although rare, arterial aneurysms, especially of the axillary artery, must be ruled out in the overhead athlete with vague upper extremity pain (especially distally) and without clear diagnosis.9 Aneurysm of the axillary artery can result from repetitive microtrauma related to repetitive overhead motion of the upper extremity. This condition may cause showering of emboli distally to the vasculature of the hand and fingers (Figure 7). Patients may complain of pain in the fingers, difficulty with grip, cyanosis, or cold sensation. On examination, the sufficiency of the radial and ulnar arteries should be assessed, as with detailed sensorimotor examination of the fingers. The fingernails should be examined for splinter hemorrhages.
Conclusion
Overhead athletes place extreme stress on the shoulder during the throwing motion and are at high risk for injury because of repetitive stress on the shoulder girdle. When examining overhead athletes with shoulder pain, surgeons must consider the entire kinetic chain, as inefficiencies anywhere along the chain can lead to altered mechanics and pathology in the shoulder.
The overhead athlete’s shoulder is exposed to extremes of stress and range of motion (ROM), predisposing this joint to unique injury patterns. Prompt diagnosis and management begin with a comprehensive history and a physical examination, supplemented by imaging studies as needed. Furthermore, the throwing shoulder undergoes adaptive changes, such as partial undersurface rotator cuff tears and capsular laxity. Imaging studies typically demonstrate abnormalities in asymptomatic throwers. Therefore, clinicians must be skilled in history taking and physical examination in throwing athletes to accurately determine the cause of symptoms and provide optimal treatment. This primer provides orthopedic surgeons with the key points in performing a thorough physical examination of the shoulder in overhead athletes.
When working with overhead athletes, surgeons must elicit the precise nature of symptoms. For example, it is important to distinguish pain from fatigue, as well as complaints related purely to decline in performance. Often, collaboration with the player’s parent or coach may help clarify the chief complaint. In addition, surgeons must have an intricate knowledge of the various stages of the overhead motion, as symptoms in specific stages (late cocking/early acceleration) may raise suspicion for distinctive pathology (labral/biceps complex). Last, it is imperative to understand that the shoulder represents only one part of the kinetic chain in overhead athletes. Successful throwing relies on integrity of the entire kinetic chain, starting with the lower extremity and trunk, extending through the spine, scapula, and shoulder, and terminating with the hand and fingers. Pathology anywhere in the chain must be evaluated and addressed.
When examining the shoulder in overhead athletes, surgeons must address several anatomical structures, both bony and soft tissue. Proper examination begins with comprehensive assessment of the ROM and strength of the various muscles around the shoulder, along with visual inspection to identify any asymmetry of these structures. In addition, the scapulothoracic structures must be examined in detail to rule out underlying dyskinesis. The capsular and ligamentous components of the shoulder joint must be further assessed to note any capsular contracture causing glenohumeral internal rotation deficit (GIRD) or any pathology with the rotator cuff or labral/biceps complex. Last, a comprehensive neurovascular examination should be performed to rule out any compression or neuropathy affecting the shoulder and overhead motion. Findings from the physical examination may then require further imaging to correlate the history and physical examination findings.
1. Inspection, palpation, strength testing
Every examination of the shoulder must begin with visual inspection, along with assessment of basic ROM and strength. The patient must be positioned and exposed adequately to promote visualization of the entire shoulder and scapular girdle, from both anterior and posterior. Visual inspection focuses on identifying any areas of asymmetry, such as position of the bony prominences or bulk of the muscular fossae. Asymmetry of the bony architecture may indicate prior trauma, and atrophy of the muscular fossae may indicate nerve compression. For example, atrophy of the infraspinatus fossa may be caused by compression of the suprascapular nerve at the spinoglenoid notch (likely by a cyst, often associated with labral pathology, but infraspinatus atrophy can result even without the presence of a compressive cyst1). Alternatively, atrophy of both the supraspinatus and infraspinatus fossae may indicate underlying compression of the suprascapular nerve at the suprascapular notch (either by a cyst or by the transverse scapular ligament). Static and dynamic observation of the posterior aspect of the shoulder may help identify gross pathology with scapular positioning or retraction, indicating underlying dyskinesis (discussed later). Deformity of the acromioclavicular joint may indicate prior trauma or separation. Last, all prior surgical scars should be noted.
Selective palpation may help identify pathology in the shoulder of the throwing athlete. Tenderness at the acromioclavicular joint may be especially common in patients who have had prior sprains of this joint or who have degenerative changes. Tenderness along the biceps tendon may be present in those with biceps tendinitis or partial tear. In addition, tenderness at the coracoid may be present in those with scapular dyskinesis. Posteriorly, palpation at the inferomedial aspect of the scapula (Figure 1), as with palpation along the medial border of the scapula, may elicit tenderness in those with scapulothoracic bursitis.
Strength testing in the shoulder is performed to elicit any deficiencies of the rotator cuff/musculature or surrounding structures. Weakness in forward elevation may indicate pathology in the supraspinatus, whereas weakness in external rotation may reflect deficiency in the infraspinatus or teres minor. Teres minor deficiency may be more isolated with weakness in a position of shoulder abduction to 90°. Last, weakness in internal rotation may indicate subscapularis deficiency. Lag signs and other provocative maneuvers are similarly elicited but typically are positive only in the event of large tears of the rotator cuff. These signs and maneuvers include the internal rotation lag sign or belly press test for subscapularis integrity, the drop-arm sign for supraspinatus function, the external rotation lag sign for infraspinatus function, and the hornblower sign for teres minor integrity. Supporting muscles of the shoulder may also be tested. Latissimus strength may be tested with resisted downward rotation of the arm with the shoulder in abduction and the elbow flexed to 90°.
2. ROM and GIRD assessment
After inspection and palpation, the shoulder should be ranged in all relevant planes of motion. Our standard examination includes forward elevation in the frontal and scapular planes, along with external rotation at the side and at 90° of abduction, as well as internal rotation behind the back with documentation of the highest spinal level that the patient can reach. This examination may be performed with the patient upright, but supine positioning can help stabilize the scapula and provide more accurate views of motion. Deficits of internal rotation may be a common finding in overhead athletes, and the degree of this deficit should be quantitatively noted.
Bony and soft-tissue remodeling of the shoulder (and associated structures) in the overhead athlete can lead to contracture of the posterior capsule. This contracture can cause excessive external rotation and subsequent decrease in internal rotation, leading to pain and anterior instability in the throwing shoulder.2 For precise measurements of the internal and external rotation arc, the scapula must be stabilized. This can be done with the patient supine on the examining table or seated upright with manual stabilization of the scapula by the examiner. Once the scapula is stabilized, the arc of internal and external rotation (with the arm in about 90° of abduction) can be measured with a goniometer, with maximum values obtained as the scapula begins to move along the posterior chest wall.2 The difference in internal rotation between the dominant and nondominant arms defines the extent of the athlete’s GIRD. Internal rotation can also be qualitatively assessed by having the athlete internally rotate each arm and reach up the spine while the examiner notes the difference in level achieved. However, this does not provide a quantitative assessment of the patient’s GIRD.
In general, the sum of the internal and external rotation arcs on the 2 sides should be symmetric. Consequently, in GIRD, excessive external rotation is balanced by decreased internal rotation. Symptomatic GIRD may be present when there is more than 25° of discrepancy in internal rotation between the athlete’s dominant and nondominant arms.2 The goal is to reduce this discrepancy to less than 20°.
3. Internal impingement: rotator cuff and labrum
In overhead athletes, an intricate relationship involving rotator cuff, labrum, and biceps tendon allows for efficient, pain-free force delivery at the shoulder. However, because of the significant external rotation and abduction required in the overhead motion, there may be internal impingement of the posterosuperior rotator cuff (infraspinatus and posterior aspect of supraspinatus) between the posterior labrum and the greater tuberosity. Detailed examination of these structures must be performed in any assessment of an overhead athlete. Symptomatic patients may complain of pain during the throwing cycle, particularly in late cocking and early acceleration.
The modified relocation examination is a common maneuver to detect internal impingement.3 In this examination, the patient’s arm is brought into a position of maximal external rotation and abduction mimicking that found in late cocking or early acceleration. In this position, a patient with internal impingement complains of pain in the posterior shoulder. A posteriorly directed force on the humerus relieves this pain.
There are also many examinations for detecting labral pathology, specifically a SLAP (superior labrum, anterior to posterior) lesion, which is commonly found in patients with internal impingement. One commonly tested maneuver is the O’Brien active compression test (Figures 2A, 2B), which has excellent sensitivity and specificity in detecting type II SLAP lesions.4 In this examination, the patient holds the arm in about 15° of adduction and 90° of forward elevation. A downward force is applied with the forearm pronated and subsequently supinated. If pain is noted on the force applied to the pronated arm, and if this pain decreases in the supinated examination, the test is positive for labral pathology.
Anterior instability is routinely found in these patients. Translation is measured with the anterior load and shift test. Anterior translation is tested with the patient supine, with the arm in abduction and external rotation, and with the examiner placing an anteriorly directed force on the humeral head. Translation is compared with the contralateral side and graded on a 3-point scale (1+ is translation to glenoid rim, 2+ is translation over glenoid rim but reduces, 3+ is translation over glenoid and locking). We also use the anterior release test, in which the patient is supine, the arm is brought into abduction and external rotation, and the examiner places a posteriorly directed force on the humeral head. When the examiner removes this force, the patient notices symptoms of instability caused by subluxation (Figures 3A, 3B).
Biceps tendon testing should also be performed to help elicit signs of labral pathology. The Speed test is performed by placing a downward force on the patient’s arm, which is held in 90° forward elevation, and with elbows in extension and forearm in supination. Pain in the long head of the biceps tendon is considered a positive sign and suggestive of SLAP lesion. Although not commonly found in these athletes, external impingement should also be elicited through both the Neer test and the Hawkins test. In the Neer test, the patient’s arm is brought to maximal forward elevation with the forearm supinated and elbow extended, while the scapula is stabilized by the examiner. Pain in the shoulder indicates a positive examination. In the Hawkins test, the patient’s arm is brought into a position of forward elevation, internal rotation, and elbow flexion. The arm is then further internally rotated, and shoulder pain defines a positive examination.
Any of these findings can be concomitant with scapular dyskinesis. Moreover, symptoms related to internal impingement may be exacerbated by concomitant scapular pathology, and therefore proper assessment of scapulothoracic motion must also be performed.
4. Scapulothoracic examination
Motion coupled between the scapula and the rest of the arm (scapular rhythm) allows for efficient use of the shoulder girdle. The scapula helps transfer the force generated by the core so that the hand can efficiently deliver it. Therefore, scapular pathology (or dyskinesis) results in inefficient functioning of the arm, which can be especially debilitating in an overhead athlete.
Scapular assessment begins with visual inspection of the patient, typically from the posterior view, which allows for assessment of the resting position of the scapula. Evidence of prominence of the medial or inferomedial border, coracoid malposition (or pain on palpation), or general scapular malposition should be noted. On active ROM, as the patient forward-elevates the arm, any asymmetric prominence of the inferomedial border of the scapula should be noted. Such asymmetry may indicate underlying scapular dyskinesis. In another important test, the lateral scapular slide test (described by Kibler5), the distance from the inferomedial angle of the scapula to the thoracic spine should be measured for both sides and in 3 difference positions, noting any asymmetry between the affected and nonaffected sides. These 3 positions (Figures 4A–4C) are with arms at side, with hands on hips (internal rotation of humerus in 45° abduction), and in 90° of shoulder abduction. Last, medial and lateral scapular winging—caused by long thoracic nerve and spinal accessory nerve pathology, respectively—can be detected by asking the patient to do a “push-up” against the wall while the examiner views from posterior.
After assessment of scapular position at rest and through motion, a series of provocative maneuvers6 may aid in the diagnosis of scapular dyskinesis. The first maneuver is the scapular assistance test, in which the examiner provides a gentle force at the inferomedial angle of the scapula, promoting upward rotation and posterior tilt as the patient elevates the arm (Figures 5A, 5B). If the patient experiences a decrease or absence of symptoms through this arc, the test is considered positive. The second maneuver is the scapular retraction test, in which strength testing of the supraspinatus is performed before and after retraction stabilization of the scapula. In the baseline state, the strength of the supraspinatus is tested in standard fashion, with resisted elevation of the internally rotated and abducted arm. The strength is then tested with the scapula stabilized in retraction (the examiner medially stabilizes the scapula). With scapular stabilization, an increase in strength or a decrease in symptoms is considered a positive test.
5. Neurovascular examination
It is essential to perform a comprehensive neurovascular examination in all overhead athletes. This includes basic cervical spine testing for any motor or sensory deficits, along with assessment of scapular winging to detect long thoracic or spinal accessory nerve palsy for medial and lateral winging, respectively. Although neurovascular injury may be a rare finding in the overhead athlete, a detailed examination must still be performed to rule it out.
Thoracic outlet syndrome
Thoracic syndrome is a compressive neuropathy of nerves and vasculature exiting the thorax and entering the upper extremity. Common symptoms include pain and tingling (sometimes vague) in the neck and upper extremity. These symptoms may be positional as well.
Diagnosis of thoracic outlet syndrome begins with visual inspection of the involved upper extremity, noting atrophy or asymmetry. Weakness may also be present. Additional provocative maneuvers can be used to detect decrease or loss of pulses, along with reproduction of symptoms, during a provocative maneuver with subsequent return of pulses and resolution of symptoms after the maneuver is completed.
One examination that can be used to detect thoracic outlet syndrome is the Adson test.7 During this maneuver, the radial pulse is palpated with the arm at rest on the patient’s side. The patient then turns to the symptomatic side, hyperextends the arm, and holds inspiration. A positive test coincides with both decreased pulse and reproduction of symptoms, indicating compression within the scalene triangle. In the Wright test,7 the pulse is again palpated at rest with the arm at the side. The patient then holds inspiration and places the arm in a position of abduction and external rotation. If the pulses decrease with this maneuver, the test is considered positive, indicating compression in the sub–pectoralis minor region deep to the coracoid. In a third test, the costoclavicular test, again pulses are measured before and during the provocative maneuver, which is with the shoulders thrust backward and depressed downward. A positive test indicates compression between the clavicle and the first rib. In our practice, we use a modified Wright test in which the arm is held in abduction and external rotation while radial pulses are palpated. The fist is then opened and clenched rapidly, and diminution of radial pulses is considered a positive examination (Figures 6A, 6B).
Effort thrombosis
Overhead athletes are at increased risk for developing effort thrombosis8 (Paget-Schroetter syndrome). This thrombosis, which results from repetitive motion involving the upper extremity, is not limited to overhead sports; it may be caused by underlying compression of or microtrauma to the venous infrastructure. On physical examination, there may be swelling of the affected limb, along with diffuse pain and fatigue, as well as dermatologic changes. Positive findings warrant further testing, such as coagulation profile testing and advanced imaging or venography.
Arterial aneurysm
Although rare, arterial aneurysms, especially of the axillary artery, must be ruled out in the overhead athlete with vague upper extremity pain (especially distally) and without clear diagnosis.9 Aneurysm of the axillary artery can result from repetitive microtrauma related to repetitive overhead motion of the upper extremity. This condition may cause showering of emboli distally to the vasculature of the hand and fingers (Figure 7). Patients may complain of pain in the fingers, difficulty with grip, cyanosis, or cold sensation. On examination, the sufficiency of the radial and ulnar arteries should be assessed, as with detailed sensorimotor examination of the fingers. The fingernails should be examined for splinter hemorrhages.
Conclusion
Overhead athletes place extreme stress on the shoulder during the throwing motion and are at high risk for injury because of repetitive stress on the shoulder girdle. When examining overhead athletes with shoulder pain, surgeons must consider the entire kinetic chain, as inefficiencies anywhere along the chain can lead to altered mechanics and pathology in the shoulder.
1. Cummins CA, Messer TM, Schafer MF. Infraspinatus muscle atrophy in professional baseball players. Am J Sports Med. 2004;32(1):116-120.
2. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
3. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part II: evaluation and treatment of SLAP lesions in throwers. Arthroscopy. 2003;19(5):531-539.
4. O’Brien SJ, Pagnani MJ, Fealy S, McGlynn SR, Wilson JB. The active compression test: a new and effective test for diagnosing labral tears and acromioclavicular joint abnormality. Am J Sports Med. 1998;26(5):610-613.
5. Kibler WB. The role of the scapula in athletic shoulder function. Am J Sports Med. 1998;26(2):325-337.
6. Kibler WB, Sciascia A, Wilkes T. Scapular dyskinesis and its relation to shoulder injury. J Am Acad Orthop Surg. 2012;20(6):364-372.
7. Leffert RD. Thoracic outlet syndrome. J Am Acad Orthop Surg. 1994;2(6):317-325.
8. Alla VM, Natarajan N, Kaushik M, Warrier R, Nair CK. Paget-Schroetter syndrome: review of pathogenesis and treatment of effort thrombosis. West J Emerg Med. 2010;11(4):358-362.
9. Baumgarten KM, Dines JS, Winchester PA, et al. Axillary artery aneurysm with distal embolization in a Major League Baseball pitcher. Am J Sports Med. 2007;35(4):650-653.
1. Cummins CA, Messer TM, Schafer MF. Infraspinatus muscle atrophy in professional baseball players. Am J Sports Med. 2004;32(1):116-120.
2. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
3. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part II: evaluation and treatment of SLAP lesions in throwers. Arthroscopy. 2003;19(5):531-539.
4. O’Brien SJ, Pagnani MJ, Fealy S, McGlynn SR, Wilson JB. The active compression test: a new and effective test for diagnosing labral tears and acromioclavicular joint abnormality. Am J Sports Med. 1998;26(5):610-613.
5. Kibler WB. The role of the scapula in athletic shoulder function. Am J Sports Med. 1998;26(2):325-337.
6. Kibler WB, Sciascia A, Wilkes T. Scapular dyskinesis and its relation to shoulder injury. J Am Acad Orthop Surg. 2012;20(6):364-372.
7. Leffert RD. Thoracic outlet syndrome. J Am Acad Orthop Surg. 1994;2(6):317-325.
8. Alla VM, Natarajan N, Kaushik M, Warrier R, Nair CK. Paget-Schroetter syndrome: review of pathogenesis and treatment of effort thrombosis. West J Emerg Med. 2010;11(4):358-362.
9. Baumgarten KM, Dines JS, Winchester PA, et al. Axillary artery aneurysm with distal embolization in a Major League Baseball pitcher. Am J Sports Med. 2007;35(4):650-653.
Supinator Cyst in a Young Female Softball Player Successfully Treated With Aspiration
Ganglion cysts around the elbow joint are unusual, with fewer than 25 citations (most of which are case reports) in the English-language literature. Among the many causes of elbow pain, cysts are chiefly diagnosed by advanced imaging. When an elbow ganglion or perineural cyst is symptomatic, treatment has ranged from nonoperative to surgical intervention. Our case report is the first documented ultrasound-guided aspiration and cortisone injection to successfully alleviate a patient’s symptoms. The procedures and outcomes of minimally invasive ultrasound-guided aspiration and steroid injections have not been described for cysts around the elbow. The patient and patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 14-year-old female freshman varsity softball pitcher on multiple teams presented with 6 months of vague right elbow pain. She was unable to pitch and had intermittent sharp pain localized to the lateral proximal forearm. She was, however, able to bat without pain and denied any radiating paresthesias. Despite a reduction in sports activities, the symptoms did not improve.
On physical examination, there was preserved strength that was symmetric with the contralateral side of all major muscles innervated by the radial nerve in the right arm, including full wrist, thumb, and finger extension. Sensation was intact to light touch in all major nervous distributions of the right and left upper extremities. She was tender to palpation at the radiocapitellar joint anteriorly, as well as just distally. The patient was also tender with motion through the proximal radial head. She had pain with resisted finger extension; however, resisted supination elicited no discomfort or pain.
The initial diagnostic workup included radiographs of the right elbow, a magnetic resonance imaging (MRI) scan, and an ultrasound. Elbow radiographs revealed no abnormalities. The MRI scan showed a well-circumscribed ovoid T2-hyperintense structure within the supinator muscle measuring 0.6×0.6×0.4 cm (longitudinal × anteroposterior × transverse), just deep to the split of the superficial and deep radial nerves (Figures 1A-1C). A musculoskeletal ultrasound was performed to further characterize and determine the relationship to neurovascular structures. Longitudinal (Figure 2A) and transverse (Figure 2B) images showed a hypoechoic cystic structure, separate from any local nerve, and without Doppler flow, consistent with what was seen on MRI. Additionally, there was an apparent stalk communicating with the anterior margin of the radiocapitellar articulation, seen on longitudinal images, suggesting an extension of the joint capsule (Figure 3A).
We diagnosed the patient with a radiocapitellar ganglion cyst. Her symptoms continued despite several sessions of physical therapy and cessation from all throwing. Given the ultrasound and MRI findings, and continuation of the symptoms despite conservative treatment, alternative treatment plans were discussed with the patient. These included continued activity modification and nonoperative treatment, open excision of the cyst, or aspiration of the cyst under ultrasound guidance. All appropriate risks and benefits were discussed, including possibility of nerve damage given the proximity of the cyst to the radial nerve branches. After a thorough discussion with both patient and family, a plan was made to undergo aspiration under ultrasound guidance. This was carried out using a lateral-to-medial in-plane approach, transverse to the radius. Using a 19-g, 1.5-inch needle (Figure 3B), 1 mL of serosanguinous fluid was aspirated from the cyst, followed by injection of 40 mg methylprednisolone sodium succinate.
The patient made a dramatic recovery within 8 days after aspiration. On examination, she had full strength to resisted flexion, extension, pronation, and supination; had no tenderness to palpation over the supinator; and no pain with resisted finger extension. She began dedicated physical therapy and a gradual return to throwing. She was able to return to her original level of softball activities 2 months after the aspiration. The patient continued to be symptom-free 26 months after the aspiration/injection. There was no evidence of recurrence of the ganglion on repeat ultrasound at her most recent follow-up (Figures 4A, 4B).
Discussion
Our review of the English-language literature identified 23 reports of cysts in and around the supinator muscle. Ganglion cysts are benign lesions that are uncommonly seen about the elbow. This highlights the rarity of this diagnosis, as well as the need for recognition of its existence. Cysts located in the substance of the nerve1-5 and extraneural ganglia causing symptomatic nerve compression have been described. These extraneural ganglia have been reported to cause compression of the ulnar nerve,1-4,6 posterior interosseous nerve (PIN),5,7-12 and radial nerve,13 and isolated compression of the radial sensory branch.14-17 Ganglion cyst compression in the elbow can result in pain, decreased motor function, and decreased sensation. The PIN syndrome is primarily a motor deficiency, whereas isolated compression of the sensory branches of the radial nerve presents as pain along the radial tunnel and extensor muscle mass.17
Most ganglion cysts are formed when joint fluid extrudes through a defect in the joint capsule; they have also been described originating from a nonunion site.18 When conservative treatment fails, surgical excision has been recommended.5,6,8-10,12-16 We present the first known case of successful ultrasound-guided aspiration and injection of a ganglion cyst from the proximal radiocapitellar joint.
In the earliest described case in 1955, Broomhead19 noted exploration was essential to establish the diagnosis of nerve palsy. In 1966, Bowen and Stone7 were the first to report PIN compression by a ganglion and that compression was likely where nerves pass through confined spaces. In keeping with the known potential for compression of the common peroneal nerve around the fibular head, Bowen and Stone7 posited that the same could be true of the PIN coursing through the supinator and around the radial neck.
Many authors have noted that nerve palsy either improves with rest or worsens with heavy manual work.3,20,21 These observations suggest that dynamic factors in addition to compression of the nerve by the ganglion may influence the occurrence of the nerve palsy.14 This is in line with our patient whose symptoms worsened after pitching.
Ogino and colleagues20 reported on the first use of ultrasonography as a screening examination for a ganglion, particularly when palpation was difficult. Ultrasound allows a detailed assessment of peripheral nerve continuity with a mass, differentiating an intraneural lesion from an adjacent extrinsic ganglion.13 Tonkin10 published the first description of MRI used for the diagnosis of an elbow cyst, and its use has been supported by others.5,8,20 The typical appearance of ganglion cysts on MRI include low signal on T1-weighted images and very high signal on T2-weighted images. Only the periphery of the mass is enhanced by gadolinium, if used.
As recently as 2009, Jou and associates13 suggested that surgical excision should be performed promptly to ensure optimal recovery from a nerve palsy. Many authors agree that early diagnosis and careful surgical excision is associated with a satisfactory outcome without recurrence of the cyst.5,6,8-10,12-15 There are only 4 published case reports14-17 of ganglions causing isolated compression of the superficial radial sensory nerve, as in our case. Their patients had pain with exertional trauma14 as did our patient, a positive Tinel sign,15 and resolution of symptoms after surgical excision without recurrence.14-16 Mileti and colleagues16 state that standard management for resistant radial tunnel syndrome is open decompression of the radial nerve.
In the last decade, a few reports of arthroscopic excision being a viable and safe alternative to open excision have been published.16,22,23 In 2000, Feldman22 described the benefits of an arthroscopic approach as decreased soft-tissue dissection, increased ability to identify intra-articular pathology, and similar recurrence rates to open procedures. He reported 1 transient neurapraxia of the superficial radial nerve from the arthroscopy, highlighting a risk of arthroscopic treatment.
An alternative to open or arthroscopic cyst decompression is aspiration. The only mention of aspiration in the literature comes from Broomhead19 in 1955 when he described 2 patients in whom treatment by aspiration was unsuccessful in relieving their symptoms. Yamazaki and colleagues12 noted that 1 of their 14 patients with PIN palsies caused by ganglions at the elbow underwent puncture of the ganglion with recovery of the paralysis. With the aid of ultrasound guidance, we were able to accurately locate the ganglion cyst, aspirate its contents, and inject methylprednisolone sodium succinate. Our patient continued to be symptom-free and was an active pitcher on a varsity softball team 26 months after aspiration.
Conclusion
This case report describes a rare location for a ganglion cyst in a high-level softball player. To our knowledge, successful treatment with ultrasound-guided aspiration and injection of a supinator cyst has not been reported in the literature. This case report highlights the importance of a careful diagnosis of this condition and an alternative treatment algorithm.
1. Boursinos LA, Dimitriou CG. Ulnar nerve compression in the cubital tunnel by an epineural ganglion: a case report. Hand (N Y). 2007;2(1):12-15.
2. Ferlic DC, Ries MD. Epineural ganglion of the ulnar nerve at the elbow. J Hand Surg Am. 1990;15(6):996-998.
3. Ming Chan K, Thompson S, Amirjani N, Satkunam L, Strohschlein FJ, Lobay GL. Compression of the ulnar nerve at the elbow by an intraneural ganglion. J Clin Neurosci. 2003;10(2):245-248.
4. Sharma RR, Pawar SJ, Delmendo A, Mahapatra AK. Symptomatic epineural ganglion cyst of the ulnar nerve in the cubital tunnel: a case report and brief review of the literature. J Clin Neurosci. 2000;7(6):542-543.
5. Hashizume H, Nishida K, Nanba Y, Inoue H, Konishiike T. Intraneural ganglion of the posterior interosseous nerve with lateral elbow pain. J Hand Surg Br. 1995;20(5):649-651.
6. Kato H, Hirayama T, Minami A, Iwasaki N, Hirachi K. Cubital tunnel syndrome associated with medial elbow Ganglia and osteoarthritis of the elbow. J Bone Joint Surg Am. 2002;84(8):1413-1419.
7. Bowen TL, Stone KH. Posterior interosseous nerve paralysis caused by a ganglion at the elbow. J Bone Joint Surg Br. 1966;48(4):774-776.
8. Ly JQ, Barrett TJ, Beall DP, Bertagnolli R. MRI diagnosis of occult ganglion compression of the posterior interosseous nerve and associated supinator muscle pathology. Clin Imaging. 2005;29(5):362-363.
9. McCollam SM, Corley FG, Green DP. Posterior interosseous nerve palsy caused by ganglions of the proximal radioulnar joint. J Hand Surg Am. 1988;13(5):725-728.
10. Tonkin MA. Posterior interosseous nerve axonotmesis from compression by a ganglion. J Hand Surg Br. 1990;15(4):491-493.
11. Tuygun H, Kose O, Gorgec M. Partial paralysis of the posterior interosseous nerve caused by a ganglion. J Hand Surg Eur. 2008;33(4):540-541.
12. Yamazaki H, Kato H, Hata Y, Murakami N, Saitoh S. The two locations of ganglions causing radial nerve palsy. J Hand Surg Eur. 2007;32(3):341-345.
13. Jou IM, Wang HN, Wang PH, Yong IS, Su WR. Compression of the radial nerve at the elbow by a ganglion: two case reports. J Med Case Rep. 2009;3:7258.
14. Hermansdorfer JD, Greider JL, Dell PC. A case report of a compressive neuropathy of the radial sensory nerve caused by a ganglion cyst at the elbow. Orthopedics. 1986;9(7):1005-1006.
15. McFarlane J, Trehan R, Olivera M, Jones C, Blease S, Davey P. A ganglion cyst at the elbow causing superficial radial nerve compression: a case report. J Med Case Rep. 2008;2:122.
16. Mileti J, Largacha M, O’Driscoll SW. Radial tunnel syndrome caused by ganglion cyst: treatment by arthroscopic cyst decompression. Arthroscopy. 2004;20(5):e39-e44.
17. Plancher KD, Peterson RK, Steichen JB. Compressive neuropathies and tendinopathies in the athletic elbow and wrist. Clin Sports Med. 1996;15(2):331-371.
18. Chim H, Yam AK, Teoh LC. Elbow ganglion arising from medial epicondyle pseudarthrosis. Hand Surg. 2007;12(3):155-158.
19. Broomhead IW. Ganglia associated with elbow and knee joints. Lancet. 1955;269(6885):317-319.
20. Ogino T, Minami A, Kato H. Diagnosis of radial nerve palsy caused by ganglion with use of different imaging techniques. J Hand Surg Am. 1991;16(2):230-235.
21. Spinner M, Spencer PS. Nerve compression lesions of the upper extremity. A clinical and experimental review. Clin Orthop Relat Res. 1974;(104):46-67.
22. Feldman MD. Arthroscopic excision of a ganglion cyst from the elbow. Arthroscopy. 2000;16(6):661-664.
23. Kirpalani PA, Lee HK, Lee YS, Han CW. Transarticular arthroscopic excision of an elbow cyst. Acta Orthop Belg. 2005;71(4):477-480.
Ganglion cysts around the elbow joint are unusual, with fewer than 25 citations (most of which are case reports) in the English-language literature. Among the many causes of elbow pain, cysts are chiefly diagnosed by advanced imaging. When an elbow ganglion or perineural cyst is symptomatic, treatment has ranged from nonoperative to surgical intervention. Our case report is the first documented ultrasound-guided aspiration and cortisone injection to successfully alleviate a patient’s symptoms. The procedures and outcomes of minimally invasive ultrasound-guided aspiration and steroid injections have not been described for cysts around the elbow. The patient and patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 14-year-old female freshman varsity softball pitcher on multiple teams presented with 6 months of vague right elbow pain. She was unable to pitch and had intermittent sharp pain localized to the lateral proximal forearm. She was, however, able to bat without pain and denied any radiating paresthesias. Despite a reduction in sports activities, the symptoms did not improve.
On physical examination, there was preserved strength that was symmetric with the contralateral side of all major muscles innervated by the radial nerve in the right arm, including full wrist, thumb, and finger extension. Sensation was intact to light touch in all major nervous distributions of the right and left upper extremities. She was tender to palpation at the radiocapitellar joint anteriorly, as well as just distally. The patient was also tender with motion through the proximal radial head. She had pain with resisted finger extension; however, resisted supination elicited no discomfort or pain.
The initial diagnostic workup included radiographs of the right elbow, a magnetic resonance imaging (MRI) scan, and an ultrasound. Elbow radiographs revealed no abnormalities. The MRI scan showed a well-circumscribed ovoid T2-hyperintense structure within the supinator muscle measuring 0.6×0.6×0.4 cm (longitudinal × anteroposterior × transverse), just deep to the split of the superficial and deep radial nerves (Figures 1A-1C). A musculoskeletal ultrasound was performed to further characterize and determine the relationship to neurovascular structures. Longitudinal (Figure 2A) and transverse (Figure 2B) images showed a hypoechoic cystic structure, separate from any local nerve, and without Doppler flow, consistent with what was seen on MRI. Additionally, there was an apparent stalk communicating with the anterior margin of the radiocapitellar articulation, seen on longitudinal images, suggesting an extension of the joint capsule (Figure 3A).
We diagnosed the patient with a radiocapitellar ganglion cyst. Her symptoms continued despite several sessions of physical therapy and cessation from all throwing. Given the ultrasound and MRI findings, and continuation of the symptoms despite conservative treatment, alternative treatment plans were discussed with the patient. These included continued activity modification and nonoperative treatment, open excision of the cyst, or aspiration of the cyst under ultrasound guidance. All appropriate risks and benefits were discussed, including possibility of nerve damage given the proximity of the cyst to the radial nerve branches. After a thorough discussion with both patient and family, a plan was made to undergo aspiration under ultrasound guidance. This was carried out using a lateral-to-medial in-plane approach, transverse to the radius. Using a 19-g, 1.5-inch needle (Figure 3B), 1 mL of serosanguinous fluid was aspirated from the cyst, followed by injection of 40 mg methylprednisolone sodium succinate.
The patient made a dramatic recovery within 8 days after aspiration. On examination, she had full strength to resisted flexion, extension, pronation, and supination; had no tenderness to palpation over the supinator; and no pain with resisted finger extension. She began dedicated physical therapy and a gradual return to throwing. She was able to return to her original level of softball activities 2 months after the aspiration. The patient continued to be symptom-free 26 months after the aspiration/injection. There was no evidence of recurrence of the ganglion on repeat ultrasound at her most recent follow-up (Figures 4A, 4B).
Discussion
Our review of the English-language literature identified 23 reports of cysts in and around the supinator muscle. Ganglion cysts are benign lesions that are uncommonly seen about the elbow. This highlights the rarity of this diagnosis, as well as the need for recognition of its existence. Cysts located in the substance of the nerve1-5 and extraneural ganglia causing symptomatic nerve compression have been described. These extraneural ganglia have been reported to cause compression of the ulnar nerve,1-4,6 posterior interosseous nerve (PIN),5,7-12 and radial nerve,13 and isolated compression of the radial sensory branch.14-17 Ganglion cyst compression in the elbow can result in pain, decreased motor function, and decreased sensation. The PIN syndrome is primarily a motor deficiency, whereas isolated compression of the sensory branches of the radial nerve presents as pain along the radial tunnel and extensor muscle mass.17
Most ganglion cysts are formed when joint fluid extrudes through a defect in the joint capsule; they have also been described originating from a nonunion site.18 When conservative treatment fails, surgical excision has been recommended.5,6,8-10,12-16 We present the first known case of successful ultrasound-guided aspiration and injection of a ganglion cyst from the proximal radiocapitellar joint.
In the earliest described case in 1955, Broomhead19 noted exploration was essential to establish the diagnosis of nerve palsy. In 1966, Bowen and Stone7 were the first to report PIN compression by a ganglion and that compression was likely where nerves pass through confined spaces. In keeping with the known potential for compression of the common peroneal nerve around the fibular head, Bowen and Stone7 posited that the same could be true of the PIN coursing through the supinator and around the radial neck.
Many authors have noted that nerve palsy either improves with rest or worsens with heavy manual work.3,20,21 These observations suggest that dynamic factors in addition to compression of the nerve by the ganglion may influence the occurrence of the nerve palsy.14 This is in line with our patient whose symptoms worsened after pitching.
Ogino and colleagues20 reported on the first use of ultrasonography as a screening examination for a ganglion, particularly when palpation was difficult. Ultrasound allows a detailed assessment of peripheral nerve continuity with a mass, differentiating an intraneural lesion from an adjacent extrinsic ganglion.13 Tonkin10 published the first description of MRI used for the diagnosis of an elbow cyst, and its use has been supported by others.5,8,20 The typical appearance of ganglion cysts on MRI include low signal on T1-weighted images and very high signal on T2-weighted images. Only the periphery of the mass is enhanced by gadolinium, if used.
As recently as 2009, Jou and associates13 suggested that surgical excision should be performed promptly to ensure optimal recovery from a nerve palsy. Many authors agree that early diagnosis and careful surgical excision is associated with a satisfactory outcome without recurrence of the cyst.5,6,8-10,12-15 There are only 4 published case reports14-17 of ganglions causing isolated compression of the superficial radial sensory nerve, as in our case. Their patients had pain with exertional trauma14 as did our patient, a positive Tinel sign,15 and resolution of symptoms after surgical excision without recurrence.14-16 Mileti and colleagues16 state that standard management for resistant radial tunnel syndrome is open decompression of the radial nerve.
In the last decade, a few reports of arthroscopic excision being a viable and safe alternative to open excision have been published.16,22,23 In 2000, Feldman22 described the benefits of an arthroscopic approach as decreased soft-tissue dissection, increased ability to identify intra-articular pathology, and similar recurrence rates to open procedures. He reported 1 transient neurapraxia of the superficial radial nerve from the arthroscopy, highlighting a risk of arthroscopic treatment.
An alternative to open or arthroscopic cyst decompression is aspiration. The only mention of aspiration in the literature comes from Broomhead19 in 1955 when he described 2 patients in whom treatment by aspiration was unsuccessful in relieving their symptoms. Yamazaki and colleagues12 noted that 1 of their 14 patients with PIN palsies caused by ganglions at the elbow underwent puncture of the ganglion with recovery of the paralysis. With the aid of ultrasound guidance, we were able to accurately locate the ganglion cyst, aspirate its contents, and inject methylprednisolone sodium succinate. Our patient continued to be symptom-free and was an active pitcher on a varsity softball team 26 months after aspiration.
Conclusion
This case report describes a rare location for a ganglion cyst in a high-level softball player. To our knowledge, successful treatment with ultrasound-guided aspiration and injection of a supinator cyst has not been reported in the literature. This case report highlights the importance of a careful diagnosis of this condition and an alternative treatment algorithm.
Ganglion cysts around the elbow joint are unusual, with fewer than 25 citations (most of which are case reports) in the English-language literature. Among the many causes of elbow pain, cysts are chiefly diagnosed by advanced imaging. When an elbow ganglion or perineural cyst is symptomatic, treatment has ranged from nonoperative to surgical intervention. Our case report is the first documented ultrasound-guided aspiration and cortisone injection to successfully alleviate a patient’s symptoms. The procedures and outcomes of minimally invasive ultrasound-guided aspiration and steroid injections have not been described for cysts around the elbow. The patient and patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 14-year-old female freshman varsity softball pitcher on multiple teams presented with 6 months of vague right elbow pain. She was unable to pitch and had intermittent sharp pain localized to the lateral proximal forearm. She was, however, able to bat without pain and denied any radiating paresthesias. Despite a reduction in sports activities, the symptoms did not improve.
On physical examination, there was preserved strength that was symmetric with the contralateral side of all major muscles innervated by the radial nerve in the right arm, including full wrist, thumb, and finger extension. Sensation was intact to light touch in all major nervous distributions of the right and left upper extremities. She was tender to palpation at the radiocapitellar joint anteriorly, as well as just distally. The patient was also tender with motion through the proximal radial head. She had pain with resisted finger extension; however, resisted supination elicited no discomfort or pain.
The initial diagnostic workup included radiographs of the right elbow, a magnetic resonance imaging (MRI) scan, and an ultrasound. Elbow radiographs revealed no abnormalities. The MRI scan showed a well-circumscribed ovoid T2-hyperintense structure within the supinator muscle measuring 0.6×0.6×0.4 cm (longitudinal × anteroposterior × transverse), just deep to the split of the superficial and deep radial nerves (Figures 1A-1C). A musculoskeletal ultrasound was performed to further characterize and determine the relationship to neurovascular structures. Longitudinal (Figure 2A) and transverse (Figure 2B) images showed a hypoechoic cystic structure, separate from any local nerve, and without Doppler flow, consistent with what was seen on MRI. Additionally, there was an apparent stalk communicating with the anterior margin of the radiocapitellar articulation, seen on longitudinal images, suggesting an extension of the joint capsule (Figure 3A).
We diagnosed the patient with a radiocapitellar ganglion cyst. Her symptoms continued despite several sessions of physical therapy and cessation from all throwing. Given the ultrasound and MRI findings, and continuation of the symptoms despite conservative treatment, alternative treatment plans were discussed with the patient. These included continued activity modification and nonoperative treatment, open excision of the cyst, or aspiration of the cyst under ultrasound guidance. All appropriate risks and benefits were discussed, including possibility of nerve damage given the proximity of the cyst to the radial nerve branches. After a thorough discussion with both patient and family, a plan was made to undergo aspiration under ultrasound guidance. This was carried out using a lateral-to-medial in-plane approach, transverse to the radius. Using a 19-g, 1.5-inch needle (Figure 3B), 1 mL of serosanguinous fluid was aspirated from the cyst, followed by injection of 40 mg methylprednisolone sodium succinate.
The patient made a dramatic recovery within 8 days after aspiration. On examination, she had full strength to resisted flexion, extension, pronation, and supination; had no tenderness to palpation over the supinator; and no pain with resisted finger extension. She began dedicated physical therapy and a gradual return to throwing. She was able to return to her original level of softball activities 2 months after the aspiration. The patient continued to be symptom-free 26 months after the aspiration/injection. There was no evidence of recurrence of the ganglion on repeat ultrasound at her most recent follow-up (Figures 4A, 4B).
Discussion
Our review of the English-language literature identified 23 reports of cysts in and around the supinator muscle. Ganglion cysts are benign lesions that are uncommonly seen about the elbow. This highlights the rarity of this diagnosis, as well as the need for recognition of its existence. Cysts located in the substance of the nerve1-5 and extraneural ganglia causing symptomatic nerve compression have been described. These extraneural ganglia have been reported to cause compression of the ulnar nerve,1-4,6 posterior interosseous nerve (PIN),5,7-12 and radial nerve,13 and isolated compression of the radial sensory branch.14-17 Ganglion cyst compression in the elbow can result in pain, decreased motor function, and decreased sensation. The PIN syndrome is primarily a motor deficiency, whereas isolated compression of the sensory branches of the radial nerve presents as pain along the radial tunnel and extensor muscle mass.17
Most ganglion cysts are formed when joint fluid extrudes through a defect in the joint capsule; they have also been described originating from a nonunion site.18 When conservative treatment fails, surgical excision has been recommended.5,6,8-10,12-16 We present the first known case of successful ultrasound-guided aspiration and injection of a ganglion cyst from the proximal radiocapitellar joint.
In the earliest described case in 1955, Broomhead19 noted exploration was essential to establish the diagnosis of nerve palsy. In 1966, Bowen and Stone7 were the first to report PIN compression by a ganglion and that compression was likely where nerves pass through confined spaces. In keeping with the known potential for compression of the common peroneal nerve around the fibular head, Bowen and Stone7 posited that the same could be true of the PIN coursing through the supinator and around the radial neck.
Many authors have noted that nerve palsy either improves with rest or worsens with heavy manual work.3,20,21 These observations suggest that dynamic factors in addition to compression of the nerve by the ganglion may influence the occurrence of the nerve palsy.14 This is in line with our patient whose symptoms worsened after pitching.
Ogino and colleagues20 reported on the first use of ultrasonography as a screening examination for a ganglion, particularly when palpation was difficult. Ultrasound allows a detailed assessment of peripheral nerve continuity with a mass, differentiating an intraneural lesion from an adjacent extrinsic ganglion.13 Tonkin10 published the first description of MRI used for the diagnosis of an elbow cyst, and its use has been supported by others.5,8,20 The typical appearance of ganglion cysts on MRI include low signal on T1-weighted images and very high signal on T2-weighted images. Only the periphery of the mass is enhanced by gadolinium, if used.
As recently as 2009, Jou and associates13 suggested that surgical excision should be performed promptly to ensure optimal recovery from a nerve palsy. Many authors agree that early diagnosis and careful surgical excision is associated with a satisfactory outcome without recurrence of the cyst.5,6,8-10,12-15 There are only 4 published case reports14-17 of ganglions causing isolated compression of the superficial radial sensory nerve, as in our case. Their patients had pain with exertional trauma14 as did our patient, a positive Tinel sign,15 and resolution of symptoms after surgical excision without recurrence.14-16 Mileti and colleagues16 state that standard management for resistant radial tunnel syndrome is open decompression of the radial nerve.
In the last decade, a few reports of arthroscopic excision being a viable and safe alternative to open excision have been published.16,22,23 In 2000, Feldman22 described the benefits of an arthroscopic approach as decreased soft-tissue dissection, increased ability to identify intra-articular pathology, and similar recurrence rates to open procedures. He reported 1 transient neurapraxia of the superficial radial nerve from the arthroscopy, highlighting a risk of arthroscopic treatment.
An alternative to open or arthroscopic cyst decompression is aspiration. The only mention of aspiration in the literature comes from Broomhead19 in 1955 when he described 2 patients in whom treatment by aspiration was unsuccessful in relieving their symptoms. Yamazaki and colleagues12 noted that 1 of their 14 patients with PIN palsies caused by ganglions at the elbow underwent puncture of the ganglion with recovery of the paralysis. With the aid of ultrasound guidance, we were able to accurately locate the ganglion cyst, aspirate its contents, and inject methylprednisolone sodium succinate. Our patient continued to be symptom-free and was an active pitcher on a varsity softball team 26 months after aspiration.
Conclusion
This case report describes a rare location for a ganglion cyst in a high-level softball player. To our knowledge, successful treatment with ultrasound-guided aspiration and injection of a supinator cyst has not been reported in the literature. This case report highlights the importance of a careful diagnosis of this condition and an alternative treatment algorithm.
1. Boursinos LA, Dimitriou CG. Ulnar nerve compression in the cubital tunnel by an epineural ganglion: a case report. Hand (N Y). 2007;2(1):12-15.
2. Ferlic DC, Ries MD. Epineural ganglion of the ulnar nerve at the elbow. J Hand Surg Am. 1990;15(6):996-998.
3. Ming Chan K, Thompson S, Amirjani N, Satkunam L, Strohschlein FJ, Lobay GL. Compression of the ulnar nerve at the elbow by an intraneural ganglion. J Clin Neurosci. 2003;10(2):245-248.
4. Sharma RR, Pawar SJ, Delmendo A, Mahapatra AK. Symptomatic epineural ganglion cyst of the ulnar nerve in the cubital tunnel: a case report and brief review of the literature. J Clin Neurosci. 2000;7(6):542-543.
5. Hashizume H, Nishida K, Nanba Y, Inoue H, Konishiike T. Intraneural ganglion of the posterior interosseous nerve with lateral elbow pain. J Hand Surg Br. 1995;20(5):649-651.
6. Kato H, Hirayama T, Minami A, Iwasaki N, Hirachi K. Cubital tunnel syndrome associated with medial elbow Ganglia and osteoarthritis of the elbow. J Bone Joint Surg Am. 2002;84(8):1413-1419.
7. Bowen TL, Stone KH. Posterior interosseous nerve paralysis caused by a ganglion at the elbow. J Bone Joint Surg Br. 1966;48(4):774-776.
8. Ly JQ, Barrett TJ, Beall DP, Bertagnolli R. MRI diagnosis of occult ganglion compression of the posterior interosseous nerve and associated supinator muscle pathology. Clin Imaging. 2005;29(5):362-363.
9. McCollam SM, Corley FG, Green DP. Posterior interosseous nerve palsy caused by ganglions of the proximal radioulnar joint. J Hand Surg Am. 1988;13(5):725-728.
10. Tonkin MA. Posterior interosseous nerve axonotmesis from compression by a ganglion. J Hand Surg Br. 1990;15(4):491-493.
11. Tuygun H, Kose O, Gorgec M. Partial paralysis of the posterior interosseous nerve caused by a ganglion. J Hand Surg Eur. 2008;33(4):540-541.
12. Yamazaki H, Kato H, Hata Y, Murakami N, Saitoh S. The two locations of ganglions causing radial nerve palsy. J Hand Surg Eur. 2007;32(3):341-345.
13. Jou IM, Wang HN, Wang PH, Yong IS, Su WR. Compression of the radial nerve at the elbow by a ganglion: two case reports. J Med Case Rep. 2009;3:7258.
14. Hermansdorfer JD, Greider JL, Dell PC. A case report of a compressive neuropathy of the radial sensory nerve caused by a ganglion cyst at the elbow. Orthopedics. 1986;9(7):1005-1006.
15. McFarlane J, Trehan R, Olivera M, Jones C, Blease S, Davey P. A ganglion cyst at the elbow causing superficial radial nerve compression: a case report. J Med Case Rep. 2008;2:122.
16. Mileti J, Largacha M, O’Driscoll SW. Radial tunnel syndrome caused by ganglion cyst: treatment by arthroscopic cyst decompression. Arthroscopy. 2004;20(5):e39-e44.
17. Plancher KD, Peterson RK, Steichen JB. Compressive neuropathies and tendinopathies in the athletic elbow and wrist. Clin Sports Med. 1996;15(2):331-371.
18. Chim H, Yam AK, Teoh LC. Elbow ganglion arising from medial epicondyle pseudarthrosis. Hand Surg. 2007;12(3):155-158.
19. Broomhead IW. Ganglia associated with elbow and knee joints. Lancet. 1955;269(6885):317-319.
20. Ogino T, Minami A, Kato H. Diagnosis of radial nerve palsy caused by ganglion with use of different imaging techniques. J Hand Surg Am. 1991;16(2):230-235.
21. Spinner M, Spencer PS. Nerve compression lesions of the upper extremity. A clinical and experimental review. Clin Orthop Relat Res. 1974;(104):46-67.
22. Feldman MD. Arthroscopic excision of a ganglion cyst from the elbow. Arthroscopy. 2000;16(6):661-664.
23. Kirpalani PA, Lee HK, Lee YS, Han CW. Transarticular arthroscopic excision of an elbow cyst. Acta Orthop Belg. 2005;71(4):477-480.
1. Boursinos LA, Dimitriou CG. Ulnar nerve compression in the cubital tunnel by an epineural ganglion: a case report. Hand (N Y). 2007;2(1):12-15.
2. Ferlic DC, Ries MD. Epineural ganglion of the ulnar nerve at the elbow. J Hand Surg Am. 1990;15(6):996-998.
3. Ming Chan K, Thompson S, Amirjani N, Satkunam L, Strohschlein FJ, Lobay GL. Compression of the ulnar nerve at the elbow by an intraneural ganglion. J Clin Neurosci. 2003;10(2):245-248.
4. Sharma RR, Pawar SJ, Delmendo A, Mahapatra AK. Symptomatic epineural ganglion cyst of the ulnar nerve in the cubital tunnel: a case report and brief review of the literature. J Clin Neurosci. 2000;7(6):542-543.
5. Hashizume H, Nishida K, Nanba Y, Inoue H, Konishiike T. Intraneural ganglion of the posterior interosseous nerve with lateral elbow pain. J Hand Surg Br. 1995;20(5):649-651.
6. Kato H, Hirayama T, Minami A, Iwasaki N, Hirachi K. Cubital tunnel syndrome associated with medial elbow Ganglia and osteoarthritis of the elbow. J Bone Joint Surg Am. 2002;84(8):1413-1419.
7. Bowen TL, Stone KH. Posterior interosseous nerve paralysis caused by a ganglion at the elbow. J Bone Joint Surg Br. 1966;48(4):774-776.
8. Ly JQ, Barrett TJ, Beall DP, Bertagnolli R. MRI diagnosis of occult ganglion compression of the posterior interosseous nerve and associated supinator muscle pathology. Clin Imaging. 2005;29(5):362-363.
9. McCollam SM, Corley FG, Green DP. Posterior interosseous nerve palsy caused by ganglions of the proximal radioulnar joint. J Hand Surg Am. 1988;13(5):725-728.
10. Tonkin MA. Posterior interosseous nerve axonotmesis from compression by a ganglion. J Hand Surg Br. 1990;15(4):491-493.
11. Tuygun H, Kose O, Gorgec M. Partial paralysis of the posterior interosseous nerve caused by a ganglion. J Hand Surg Eur. 2008;33(4):540-541.
12. Yamazaki H, Kato H, Hata Y, Murakami N, Saitoh S. The two locations of ganglions causing radial nerve palsy. J Hand Surg Eur. 2007;32(3):341-345.
13. Jou IM, Wang HN, Wang PH, Yong IS, Su WR. Compression of the radial nerve at the elbow by a ganglion: two case reports. J Med Case Rep. 2009;3:7258.
14. Hermansdorfer JD, Greider JL, Dell PC. A case report of a compressive neuropathy of the radial sensory nerve caused by a ganglion cyst at the elbow. Orthopedics. 1986;9(7):1005-1006.
15. McFarlane J, Trehan R, Olivera M, Jones C, Blease S, Davey P. A ganglion cyst at the elbow causing superficial radial nerve compression: a case report. J Med Case Rep. 2008;2:122.
16. Mileti J, Largacha M, O’Driscoll SW. Radial tunnel syndrome caused by ganglion cyst: treatment by arthroscopic cyst decompression. Arthroscopy. 2004;20(5):e39-e44.
17. Plancher KD, Peterson RK, Steichen JB. Compressive neuropathies and tendinopathies in the athletic elbow and wrist. Clin Sports Med. 1996;15(2):331-371.
18. Chim H, Yam AK, Teoh LC. Elbow ganglion arising from medial epicondyle pseudarthrosis. Hand Surg. 2007;12(3):155-158.
19. Broomhead IW. Ganglia associated with elbow and knee joints. Lancet. 1955;269(6885):317-319.
20. Ogino T, Minami A, Kato H. Diagnosis of radial nerve palsy caused by ganglion with use of different imaging techniques. J Hand Surg Am. 1991;16(2):230-235.
21. Spinner M, Spencer PS. Nerve compression lesions of the upper extremity. A clinical and experimental review. Clin Orthop Relat Res. 1974;(104):46-67.
22. Feldman MD. Arthroscopic excision of a ganglion cyst from the elbow. Arthroscopy. 2000;16(6):661-664.
23. Kirpalani PA, Lee HK, Lee YS, Han CW. Transarticular arthroscopic excision of an elbow cyst. Acta Orthop Belg. 2005;71(4):477-480.
Isolating Suture Slippage During Cadaveric Testing of Knotless Anchors
Knotless suture anchor fixation techniques continue to evolve as efficient, low-profile options for arthroscopic rotator cuff repair (RCR).1,2 Excellent outcomes have been reported for constructs that use knotless fixation laterally, typically in suture bridge-type configurations.2-4 Early comparative biomechanical and clinical studies have also demonstrated equivalent results for all-knotless versus conventional constructs for arthroscopic RCR.5-10 Given the increased use and availability of multiple implant designs, it is important to supplement our clinical knowledge of these devices with laboratory studies delineating the biomechanical properties of the anchors that are used to help guide appropriate clinical use of the implants in specific patient populations.
Several biomechanical studies have shown suture slippage to be the weak but crucial link in the design of knotless anchors and the most likely mode of in vivo failure.11,12 Other studies have demonstrated frequent anchor dislodgement from bone, but these analyses involved use of elderly cadaveric specimens and relatively high-force testing protocols.12,13 Because suture-retention force may have exceeded anchor resistance to pullout (imparted by weak cadaveric bone in such biomechanical settings), the focus on suture-retention properties was limited.11 It is thought that, in clinical practice, the majority of patients who undergo RCR tend not to generate the high forces (relative to resistance to bone pullout) used to cause the anchor pullouts observed in biomechanical studies, particularly in the early postoperative setting.11-15 Cadaveric testing, however, often involves use of specimens with diminished bone mineral density (BMD), relative to age, because of the illness and other factors leading to death and donation.
Using a novel testing apparatus, we isolated, analyzed, and compared suture slippage in 2 anchor designs, one with entirely press-fit suture clamping and the other reliant on an intrinsic suture-locking mechanism.
Materials and Methods
Six human cadaveric proximal humeri specimens were used for this biomechanical study. Mean (SD) age was 53.3 (5.7) years (range, 46-59 years). Middle-aged specimens were used in order to best represent the quality of bone typically encountered in RCR surgery. To approximate tissue in clinical use, we used fresh-frozen cadaver tissue. Specimens were maintained at –20°C until about 24 hours before use and then were thawed to room temperature for testing. Specimens were included only if they had a completely intact humeral head and no prior surgery or hardware placement. Before instrumentation, dual-energy x-ray absorptiometry with a QDR-1000 scanner (Hologic) was used to determine BMD of all proximal humeri.
Two knotless suture anchors were compared: PushLock (4.5×18.5 mm; Arthrex) and ReelX STT (5.5×19.4 mm; Stryker). These anchors have multiple surgical indications (including RCR), allow patient-specific tissue tensioning, and use polyetheretherketone eyelets. The clamping force for PushLock depends entirely on the interference fit achieved for the suture between the outside of the anchor and the surrounding trabecular/cortical bone after device insertion, whereas the suture in ReelX is secured within the anchor shaft entirely by an internal ratchet-locking mechanism.
For anchor insertion, shoulders were dissected down to the greater tuberosity of the proximal humerus, and all implants were inserted (by a fellowship-trained surgeon in accordance with manufacturer guidelines) at a 25° insertion angle with manufacturer-supplied instruments. One anchor of each type (Figure 1) was inserted into the center of the rotator cuff footprint on the greater tuberosity of each specimen. Anterior and posterior positions were randomized, and an anchor from the other group was inserted into the matching location on the contralateral matched-pair specimen. In all instances, distance between the anterior and posterior anchors was 2 cm, and anchors were placed midway between the articular margin and the lateral edge of the greater tuberosity (Figure 2). Two strands of size 2 ultrahigh-molecular-weight–polyethylene Force Fiber (Stryker) were loaded into all anchors.
A custom urethane fixture was secured over the center of each anchor to allow testing to focus on suture slippage by minimizing anchor migration (Figure 3). The small aperture of this device allowed suture tails to pass freely through the center of the fixture but prevented disengagement and proximal migration of the suture anchor from the underlying bone through contact of the urethane fixture with the anchor perimeter. Any system deformation observed during testing was restricted to the suture and/or the anchor’s suture-locking mechanism. Testing fixtures also oriented the suture anchor coaxial with the axis of tension, creating a worst-case loading scenario (Figure 3).
PushLock implants were inserted with 5 pounds of tension, as indicated, using a manufacturer-supplied suture tensioner, and ReelX devices were inserted and locked with 2 full rotations, as specified by the manufacturer. After one end of each suture was cut, as would be done in vivo, the 2 other suture ends, which would have been part of the RCR in vivo, were tied together to form an 8-cm circumference loop that was brought through the urethane fixture. Humeri were then mounted in a materials testing system (MTS 810; MTS Systems) servohydraulic load frame, and the suture loop was passed around a cross-bar on the actuator of the testing device. A mechanical testing protocol consisting of modest repetitive forces was carefully chosen to simulate expected activity during rehabilitation after RCR.15 In this protocol, a 60-second preload of 10 N was followed by tensile loading between 10 N and 90 N at a frequency of 0.5 Hz for 500 cycles.15 Cycle duration at 3 mm and 5 mm of suture slippage (threshold for clinical failure) was recorded.12,16,17 In addition, suture slippage was measured after 1, 10, 50, 100, 200, 300, 400, and 500 cycles. The first 5 test cycles were not counted in the analysis to control for initial knot slippage. Finally, after completion of dynamic testing, samples were loaded at a displacement rate of 0.5 mm/s for tension-to-failure testing in the custom fixtures. Maximum failure load, stiffness, and failure mode were recorded. Ultimate failure was defined as suture breakage or gross suture slippage.
Paired Student t test was used to determine significant differences in suture slippage distance between the 2 groups at various cycle durations. In addition, Kaplan-Meier survival test was used to determine statistical differences in sample survival during the dynamic loading test.
Results
Mean (SD) BMD of the cadaveric shoulder specimens was 0.55 (0.13) g/cm2 (range, 0.29-0.68 g/cm2). The testing fixtures isolated suture slippage from anchor–bone disengagement. All 6 PushLock implants demonstrated slippage of more than 3 mm, and 5 of the 6 demonstrated slippage of more than 5 mm. All 6 ReelX devices exhibited slippage of less than 3 mm. In addition, PushLock demonstrated more suture slippage at cycles 1, 10, and 100 (P < .05) and more maximum slippage after 500 cycles (mean, 11.2 mm; SD, 4.7 mm) compared with ReelX (mean, 1.9 mm; SD, 0.5 mm) (P = .004). Figure 4 shows mean suture slippage at each cycle.
Kaplan-Meier analysis revealed significantly (λ2 = 8.170; P = .0043) decreased survival after dynamic testing for PushLock versus ReelX (Figure 5). Survival was defined as suture slippage of less than 5 mm after completion of dynamic testing. Only 1 of the 6 PushLock anchors completed dynamic testing; the other 5 failed via complete suture slippage from the anchor before testing could be completed. All 6 ReelX devices survived dynamic testing.
Therefore, 1 PushLock implant and all 6 ReelX devices were available for subsequent load-to-failure testing. Failure in this setting was defined as suture slippage of more than 10 mm or suture breakage. The PushLock implant failed at a maximum force of 171.8 N with a stiffness of 74.4 N/mm and eventually exhibited gross suture slippage. All 6 ReelX devices failed at a mean (SD) maximum of 273.5 (20.2) N, with a mean (SD) stiffness of 74.1 (17) N/mm. Mechanism of failure for all ReelX devices was suture breakage during the tensile load-to-failure test.
Discussion
We evaluated a new technique designed to isolate suture slippage in knotless anchors used for RCR. The impetus for developing this new method was to provide a means for better analyzing the ability of a knotless anchor to resist suture slippage in the cadaveric biomechanical testing setting. Suture slippage is an important mode of failure during such analyses.11,12 Significant slippage occurred in a range of implants before half the anchor–bone pullout strength was reached in a study using young bovine femoral heads.11 In another study, using young, high-BMD cadaveric humeral heads, initial slippage and maximum failure loads were equivalent among numerous devices using various suture-retention mechanisms, and suture slippage was the most common failure mode.12 Nevertheless, other biomechanical studies have demonstrated frequent failure caused by anchor pullout in elderly human cadaveric specimens with diminished BMD, often with high-force testing protocols.12,13 In the more modest-force, in vivo rehabilitative environment, suture slippage rather than anchor dislodgement may be the main failure mode.11-15
We compared the PushLock implant and its entirely press-fit suture clamping design with the ReelX device, which relies on an intrinsic suture-locking mechanism. Middle-aged (mean, 53.3 years; SD, 5.7 years) cadaveric humeri were tested under physiologically relevant biomechanical conditions to begin to help identify how relatively osteopenic bone may affect suture-retention properties for a given implant. The results showed that the study methodology prevented implant failure via anchor–bone pullout. To our knowledge, this was the first study to exclusively analyze suture slippage in knotless anchors. The findings indicated that implants that rely heavily on a tight interference fit of the suture between the anchor and the surrounding bone may exhibit early slippage and failure after RCR in middle-aged patients with relative osteopenia.11,12 However, this study also demonstrated that devices with intrinsic clamping mechanisms that do not depend on the quality of surrounding bone may better resist suture slippage. It is not clear that all knotless anchors with intrinsic locking mechanisms function equivalently. For instance, Pietschmann and colleagues12 found that 2 of 10 implants with a different internal clamping device were unable to resist failure via suture slippage, even in healthy bone. Similarly, in a study comparing ReelX devices with implants having a different internal suture-retention mechanism, ReelX failed at higher ultimate loads, and typically via anchor dislodgement, versus suture slippage in the other implants.18
It is important to note that, in the present study, the loads at which sutures broke in the intrinsic clamping anchors approached the maximum contractile force of the supraspinatus muscle (302 N).19,20 In addition, these loads were above the resistance of the rotator cuff tendon to cut out with modern suture material.21
This study’s limitations include use of an in vitro human cadaveric model that precluded analysis of the effects of postoperative healing. Biomechanical testing was also performed in a single row-type suture configuration with the rotator cuff tendon removed. Fixtures used during testing oriented the load coaxially with the axis of tension, creating a worst-case loading scenario. Although this form of testing may limit its clinical applicability, its purpose was to critically isolate how well a knotless anchor could resist suture slippage. The methods we used were also limited because the stability of the bone–anchor interface was not assessed. For patients with osteopenia, anchor pullout rather than suture slippage could be the most limiting factor for knotless anchor construct failure, and therefore further testing of both failure modes is needed. Future biomechanical studies should compare various knotless anchors’ suture-slippage characteristics in other constructs in physiologic testing orientations, including double-row and suture-bridge configurations, as well as with intact rotator cuff tendons. In addition, use of labral tape as a substitute for polyblend suture has been suggested to limit suture slippage, and this technique theoretically could have changed the results of this study.22
Conclusion
An implant with an internal ratcheting mechanism for suture retention demonstrated significantly less suture slippage in an axial tension evaluation protocol than a device reliant on interference fit of the suture between the anchor and surrounding bone. In the clinical setting, this may allow for less gap formation during the healing phase following RCR with a knotless anchor. There was also increased maximum load to failure, demonstrating an increased load until catastrophic failure using a device with a ratcheting internal locking mechanism.
1. Thal R. A knotless suture anchor. Design, function, and biomechanical testing. Am J Sports Med. 2001;29(5):646-649.
2. Cole BJ, ElAttrache NS, Anbari A. Arthroscopic rotator cuff repairs: an anatomic and biomechanical rationale for different suture-anchor repair configurations. Arthroscopy. 2007;23(6):662-669.
3. Kim KC, Shin HD, Cha SM, Lee WY. Comparison of repair integrity and functional outcomes for 3 arthroscopic suture bridge rotator cuff repair techniques. Am J Sports Med. 2013;41(2):271-277.
4. Choi CH, Kim SK, Cho MR, et al. Functional outcomes and structural integrity after double-pulley suture bridge rotator cuff repair using serial ultrasonographic examination. J Shoulder Elbow Surg. 2012;21(12):1753-1763.
5. Brown BS, Cooper AD, McIff TE, Key VH, Toby EB. Initial fixation and cyclic loading stability of knotless suture anchors for rotator cuff repair. J Shoulder Elbow Surg. 2008;17(2):313-318.
6. Burkhart SS, Adams CR, Burkhart SS, Schoolfield JD. A biomechanical comparison of 2 techniques of footprint reconstruction for rotator cuff repair: the SwiveLock-FiberChain construct versus standard double-row repair. Arthroscopy. 2009;25(3):274-281.
7. Hepp P, Osterhoff G, Engel T, Marquass B, Klink T, Josten C. Biomechanical evaluation of knotless anatomical double-layer double-row rotator cuff repair: a comparative ex vivo study. Am J Sports Med. 2009;37(7):1363-1369.
8. Maguire M, Goldberg J, Bokor D, et al. Biomechanical evaluation of four different transosseous-equivalent/suture bridge rotator cuff repairs. Knee Surg Sports Traumatol Arthrosc. 2011;19(9):1582-1587.
9. Millar NL, Wu X, Tantau R, Silverstone E, Murrell GA. Open versus two forms of arthroscopic rotator cuff repair. Clin Orthop Relat Res. 2009;467(4):966-978.
10. Rhee YG, Cho NS, Parke CS. Arthroscopic rotator cuff repair using modified Mason-Allen medial row stitch: knotless versus knot-tying suture bridge technique. Am J Sports Med. 2012;40(11):2440-2447.
11. Wieser K, Farshad M, Vlachopoulos L, Ruffieux K, Gerber C, Meyer DC. Suture slippage in knotless suture anchors as a potential failure mechanism in rotator cuff repair. Arthroscopy. 2012;28(11):1622-1627.
12. Pietschmann MF, Gülecyüz MF, Fieseler S, et al. Biomechanical stability of knotless suture anchors used in rotator cuff repair in healthy and osteopenic bone. Arthroscopy. 2010;26(8):1035-1044.
13. Barber FA, Hapa O, Bynum JA. Comparative testing by cyclic loading of rotator cuff suture anchors containing multiple high-strength sutures. Arthroscopy. 2010;26(9 suppl):S134-S141.
14. Barber FA, Coons DA, Ruiz-Suarez M. Cyclic load testing of biodegradable suture anchors containing 2 high-strength sutures. Arthroscopy. 2007;23(4):355-360.
15. Bynum CK, Lee S, Mahar A, Tasto J, Pedowitz R. Failure mode of suture anchors as a function of insertion depth. Am J Sports Med. 2005;33(7):1030-1034.
16. Gerber C, Schneeberger AG, Beck M, Schlegel U. Mechanical strength of repairs of the rotator cuff. J Bone Joint Surg Br. 1994;76(3):371-380.
17. Schneeberger AG, von Roll A, Kalberer F, Jacob HA, Gerber C. Mechanical strength of arthroscopic rotator cuff repair techniques: an in vitro study. J Bone Joint Surg Am. 2002;84(12):2152-2160.
18. Efird C, Traub S, Baldini T, et al. Knotless single-row rotator cuff repair: a comparative biomechanical study of 2 knotless suture anchors. Orthopedics. 2013;36(8):e1033-e1037.
19. Wright PB, Budoff JE, Yeh ML, Kelm ZS, Luo ZP. Strength of damaged suture: an in vitro study. Arthroscopy. 2006;22(12):1270-1275.
20. Burkhart SS. A stepwise approach to arthroscopic rotator cuff repair based on biomechanical principles. Arthroscopy. 2000;16(1):82-90.
21. Bisson LJ, Manohar LM. A biomechanical comparison of the pullout strength of No. 2 FiberWire suture and 2-mm FiberWire tape in bovine rotator cuff tendons. Arthroscopy. 2010;26(11):1463-1468.
22. Burkhart SS, Denard PJ, Konicek J, Hanypsiak BT. Biomechanical validation of load-sharing rip-stop fixation for the repair of tissue-deficient rotator cuff tears. Am J Sports Med. 2014;42(2):457-462.
Knotless suture anchor fixation techniques continue to evolve as efficient, low-profile options for arthroscopic rotator cuff repair (RCR).1,2 Excellent outcomes have been reported for constructs that use knotless fixation laterally, typically in suture bridge-type configurations.2-4 Early comparative biomechanical and clinical studies have also demonstrated equivalent results for all-knotless versus conventional constructs for arthroscopic RCR.5-10 Given the increased use and availability of multiple implant designs, it is important to supplement our clinical knowledge of these devices with laboratory studies delineating the biomechanical properties of the anchors that are used to help guide appropriate clinical use of the implants in specific patient populations.
Several biomechanical studies have shown suture slippage to be the weak but crucial link in the design of knotless anchors and the most likely mode of in vivo failure.11,12 Other studies have demonstrated frequent anchor dislodgement from bone, but these analyses involved use of elderly cadaveric specimens and relatively high-force testing protocols.12,13 Because suture-retention force may have exceeded anchor resistance to pullout (imparted by weak cadaveric bone in such biomechanical settings), the focus on suture-retention properties was limited.11 It is thought that, in clinical practice, the majority of patients who undergo RCR tend not to generate the high forces (relative to resistance to bone pullout) used to cause the anchor pullouts observed in biomechanical studies, particularly in the early postoperative setting.11-15 Cadaveric testing, however, often involves use of specimens with diminished bone mineral density (BMD), relative to age, because of the illness and other factors leading to death and donation.
Using a novel testing apparatus, we isolated, analyzed, and compared suture slippage in 2 anchor designs, one with entirely press-fit suture clamping and the other reliant on an intrinsic suture-locking mechanism.
Materials and Methods
Six human cadaveric proximal humeri specimens were used for this biomechanical study. Mean (SD) age was 53.3 (5.7) years (range, 46-59 years). Middle-aged specimens were used in order to best represent the quality of bone typically encountered in RCR surgery. To approximate tissue in clinical use, we used fresh-frozen cadaver tissue. Specimens were maintained at –20°C until about 24 hours before use and then were thawed to room temperature for testing. Specimens were included only if they had a completely intact humeral head and no prior surgery or hardware placement. Before instrumentation, dual-energy x-ray absorptiometry with a QDR-1000 scanner (Hologic) was used to determine BMD of all proximal humeri.
Two knotless suture anchors were compared: PushLock (4.5×18.5 mm; Arthrex) and ReelX STT (5.5×19.4 mm; Stryker). These anchors have multiple surgical indications (including RCR), allow patient-specific tissue tensioning, and use polyetheretherketone eyelets. The clamping force for PushLock depends entirely on the interference fit achieved for the suture between the outside of the anchor and the surrounding trabecular/cortical bone after device insertion, whereas the suture in ReelX is secured within the anchor shaft entirely by an internal ratchet-locking mechanism.
For anchor insertion, shoulders were dissected down to the greater tuberosity of the proximal humerus, and all implants were inserted (by a fellowship-trained surgeon in accordance with manufacturer guidelines) at a 25° insertion angle with manufacturer-supplied instruments. One anchor of each type (Figure 1) was inserted into the center of the rotator cuff footprint on the greater tuberosity of each specimen. Anterior and posterior positions were randomized, and an anchor from the other group was inserted into the matching location on the contralateral matched-pair specimen. In all instances, distance between the anterior and posterior anchors was 2 cm, and anchors were placed midway between the articular margin and the lateral edge of the greater tuberosity (Figure 2). Two strands of size 2 ultrahigh-molecular-weight–polyethylene Force Fiber (Stryker) were loaded into all anchors.
A custom urethane fixture was secured over the center of each anchor to allow testing to focus on suture slippage by minimizing anchor migration (Figure 3). The small aperture of this device allowed suture tails to pass freely through the center of the fixture but prevented disengagement and proximal migration of the suture anchor from the underlying bone through contact of the urethane fixture with the anchor perimeter. Any system deformation observed during testing was restricted to the suture and/or the anchor’s suture-locking mechanism. Testing fixtures also oriented the suture anchor coaxial with the axis of tension, creating a worst-case loading scenario (Figure 3).
PushLock implants were inserted with 5 pounds of tension, as indicated, using a manufacturer-supplied suture tensioner, and ReelX devices were inserted and locked with 2 full rotations, as specified by the manufacturer. After one end of each suture was cut, as would be done in vivo, the 2 other suture ends, which would have been part of the RCR in vivo, were tied together to form an 8-cm circumference loop that was brought through the urethane fixture. Humeri were then mounted in a materials testing system (MTS 810; MTS Systems) servohydraulic load frame, and the suture loop was passed around a cross-bar on the actuator of the testing device. A mechanical testing protocol consisting of modest repetitive forces was carefully chosen to simulate expected activity during rehabilitation after RCR.15 In this protocol, a 60-second preload of 10 N was followed by tensile loading between 10 N and 90 N at a frequency of 0.5 Hz for 500 cycles.15 Cycle duration at 3 mm and 5 mm of suture slippage (threshold for clinical failure) was recorded.12,16,17 In addition, suture slippage was measured after 1, 10, 50, 100, 200, 300, 400, and 500 cycles. The first 5 test cycles were not counted in the analysis to control for initial knot slippage. Finally, after completion of dynamic testing, samples were loaded at a displacement rate of 0.5 mm/s for tension-to-failure testing in the custom fixtures. Maximum failure load, stiffness, and failure mode were recorded. Ultimate failure was defined as suture breakage or gross suture slippage.
Paired Student t test was used to determine significant differences in suture slippage distance between the 2 groups at various cycle durations. In addition, Kaplan-Meier survival test was used to determine statistical differences in sample survival during the dynamic loading test.
Results
Mean (SD) BMD of the cadaveric shoulder specimens was 0.55 (0.13) g/cm2 (range, 0.29-0.68 g/cm2). The testing fixtures isolated suture slippage from anchor–bone disengagement. All 6 PushLock implants demonstrated slippage of more than 3 mm, and 5 of the 6 demonstrated slippage of more than 5 mm. All 6 ReelX devices exhibited slippage of less than 3 mm. In addition, PushLock demonstrated more suture slippage at cycles 1, 10, and 100 (P < .05) and more maximum slippage after 500 cycles (mean, 11.2 mm; SD, 4.7 mm) compared with ReelX (mean, 1.9 mm; SD, 0.5 mm) (P = .004). Figure 4 shows mean suture slippage at each cycle.
Kaplan-Meier analysis revealed significantly (λ2 = 8.170; P = .0043) decreased survival after dynamic testing for PushLock versus ReelX (Figure 5). Survival was defined as suture slippage of less than 5 mm after completion of dynamic testing. Only 1 of the 6 PushLock anchors completed dynamic testing; the other 5 failed via complete suture slippage from the anchor before testing could be completed. All 6 ReelX devices survived dynamic testing.
Therefore, 1 PushLock implant and all 6 ReelX devices were available for subsequent load-to-failure testing. Failure in this setting was defined as suture slippage of more than 10 mm or suture breakage. The PushLock implant failed at a maximum force of 171.8 N with a stiffness of 74.4 N/mm and eventually exhibited gross suture slippage. All 6 ReelX devices failed at a mean (SD) maximum of 273.5 (20.2) N, with a mean (SD) stiffness of 74.1 (17) N/mm. Mechanism of failure for all ReelX devices was suture breakage during the tensile load-to-failure test.
Discussion
We evaluated a new technique designed to isolate suture slippage in knotless anchors used for RCR. The impetus for developing this new method was to provide a means for better analyzing the ability of a knotless anchor to resist suture slippage in the cadaveric biomechanical testing setting. Suture slippage is an important mode of failure during such analyses.11,12 Significant slippage occurred in a range of implants before half the anchor–bone pullout strength was reached in a study using young bovine femoral heads.11 In another study, using young, high-BMD cadaveric humeral heads, initial slippage and maximum failure loads were equivalent among numerous devices using various suture-retention mechanisms, and suture slippage was the most common failure mode.12 Nevertheless, other biomechanical studies have demonstrated frequent failure caused by anchor pullout in elderly human cadaveric specimens with diminished BMD, often with high-force testing protocols.12,13 In the more modest-force, in vivo rehabilitative environment, suture slippage rather than anchor dislodgement may be the main failure mode.11-15
We compared the PushLock implant and its entirely press-fit suture clamping design with the ReelX device, which relies on an intrinsic suture-locking mechanism. Middle-aged (mean, 53.3 years; SD, 5.7 years) cadaveric humeri were tested under physiologically relevant biomechanical conditions to begin to help identify how relatively osteopenic bone may affect suture-retention properties for a given implant. The results showed that the study methodology prevented implant failure via anchor–bone pullout. To our knowledge, this was the first study to exclusively analyze suture slippage in knotless anchors. The findings indicated that implants that rely heavily on a tight interference fit of the suture between the anchor and the surrounding bone may exhibit early slippage and failure after RCR in middle-aged patients with relative osteopenia.11,12 However, this study also demonstrated that devices with intrinsic clamping mechanisms that do not depend on the quality of surrounding bone may better resist suture slippage. It is not clear that all knotless anchors with intrinsic locking mechanisms function equivalently. For instance, Pietschmann and colleagues12 found that 2 of 10 implants with a different internal clamping device were unable to resist failure via suture slippage, even in healthy bone. Similarly, in a study comparing ReelX devices with implants having a different internal suture-retention mechanism, ReelX failed at higher ultimate loads, and typically via anchor dislodgement, versus suture slippage in the other implants.18
It is important to note that, in the present study, the loads at which sutures broke in the intrinsic clamping anchors approached the maximum contractile force of the supraspinatus muscle (302 N).19,20 In addition, these loads were above the resistance of the rotator cuff tendon to cut out with modern suture material.21
This study’s limitations include use of an in vitro human cadaveric model that precluded analysis of the effects of postoperative healing. Biomechanical testing was also performed in a single row-type suture configuration with the rotator cuff tendon removed. Fixtures used during testing oriented the load coaxially with the axis of tension, creating a worst-case loading scenario. Although this form of testing may limit its clinical applicability, its purpose was to critically isolate how well a knotless anchor could resist suture slippage. The methods we used were also limited because the stability of the bone–anchor interface was not assessed. For patients with osteopenia, anchor pullout rather than suture slippage could be the most limiting factor for knotless anchor construct failure, and therefore further testing of both failure modes is needed. Future biomechanical studies should compare various knotless anchors’ suture-slippage characteristics in other constructs in physiologic testing orientations, including double-row and suture-bridge configurations, as well as with intact rotator cuff tendons. In addition, use of labral tape as a substitute for polyblend suture has been suggested to limit suture slippage, and this technique theoretically could have changed the results of this study.22
Conclusion
An implant with an internal ratcheting mechanism for suture retention demonstrated significantly less suture slippage in an axial tension evaluation protocol than a device reliant on interference fit of the suture between the anchor and surrounding bone. In the clinical setting, this may allow for less gap formation during the healing phase following RCR with a knotless anchor. There was also increased maximum load to failure, demonstrating an increased load until catastrophic failure using a device with a ratcheting internal locking mechanism.
Knotless suture anchor fixation techniques continue to evolve as efficient, low-profile options for arthroscopic rotator cuff repair (RCR).1,2 Excellent outcomes have been reported for constructs that use knotless fixation laterally, typically in suture bridge-type configurations.2-4 Early comparative biomechanical and clinical studies have also demonstrated equivalent results for all-knotless versus conventional constructs for arthroscopic RCR.5-10 Given the increased use and availability of multiple implant designs, it is important to supplement our clinical knowledge of these devices with laboratory studies delineating the biomechanical properties of the anchors that are used to help guide appropriate clinical use of the implants in specific patient populations.
Several biomechanical studies have shown suture slippage to be the weak but crucial link in the design of knotless anchors and the most likely mode of in vivo failure.11,12 Other studies have demonstrated frequent anchor dislodgement from bone, but these analyses involved use of elderly cadaveric specimens and relatively high-force testing protocols.12,13 Because suture-retention force may have exceeded anchor resistance to pullout (imparted by weak cadaveric bone in such biomechanical settings), the focus on suture-retention properties was limited.11 It is thought that, in clinical practice, the majority of patients who undergo RCR tend not to generate the high forces (relative to resistance to bone pullout) used to cause the anchor pullouts observed in biomechanical studies, particularly in the early postoperative setting.11-15 Cadaveric testing, however, often involves use of specimens with diminished bone mineral density (BMD), relative to age, because of the illness and other factors leading to death and donation.
Using a novel testing apparatus, we isolated, analyzed, and compared suture slippage in 2 anchor designs, one with entirely press-fit suture clamping and the other reliant on an intrinsic suture-locking mechanism.
Materials and Methods
Six human cadaveric proximal humeri specimens were used for this biomechanical study. Mean (SD) age was 53.3 (5.7) years (range, 46-59 years). Middle-aged specimens were used in order to best represent the quality of bone typically encountered in RCR surgery. To approximate tissue in clinical use, we used fresh-frozen cadaver tissue. Specimens were maintained at –20°C until about 24 hours before use and then were thawed to room temperature for testing. Specimens were included only if they had a completely intact humeral head and no prior surgery or hardware placement. Before instrumentation, dual-energy x-ray absorptiometry with a QDR-1000 scanner (Hologic) was used to determine BMD of all proximal humeri.
Two knotless suture anchors were compared: PushLock (4.5×18.5 mm; Arthrex) and ReelX STT (5.5×19.4 mm; Stryker). These anchors have multiple surgical indications (including RCR), allow patient-specific tissue tensioning, and use polyetheretherketone eyelets. The clamping force for PushLock depends entirely on the interference fit achieved for the suture between the outside of the anchor and the surrounding trabecular/cortical bone after device insertion, whereas the suture in ReelX is secured within the anchor shaft entirely by an internal ratchet-locking mechanism.
For anchor insertion, shoulders were dissected down to the greater tuberosity of the proximal humerus, and all implants were inserted (by a fellowship-trained surgeon in accordance with manufacturer guidelines) at a 25° insertion angle with manufacturer-supplied instruments. One anchor of each type (Figure 1) was inserted into the center of the rotator cuff footprint on the greater tuberosity of each specimen. Anterior and posterior positions were randomized, and an anchor from the other group was inserted into the matching location on the contralateral matched-pair specimen. In all instances, distance between the anterior and posterior anchors was 2 cm, and anchors were placed midway between the articular margin and the lateral edge of the greater tuberosity (Figure 2). Two strands of size 2 ultrahigh-molecular-weight–polyethylene Force Fiber (Stryker) were loaded into all anchors.
A custom urethane fixture was secured over the center of each anchor to allow testing to focus on suture slippage by minimizing anchor migration (Figure 3). The small aperture of this device allowed suture tails to pass freely through the center of the fixture but prevented disengagement and proximal migration of the suture anchor from the underlying bone through contact of the urethane fixture with the anchor perimeter. Any system deformation observed during testing was restricted to the suture and/or the anchor’s suture-locking mechanism. Testing fixtures also oriented the suture anchor coaxial with the axis of tension, creating a worst-case loading scenario (Figure 3).
PushLock implants were inserted with 5 pounds of tension, as indicated, using a manufacturer-supplied suture tensioner, and ReelX devices were inserted and locked with 2 full rotations, as specified by the manufacturer. After one end of each suture was cut, as would be done in vivo, the 2 other suture ends, which would have been part of the RCR in vivo, were tied together to form an 8-cm circumference loop that was brought through the urethane fixture. Humeri were then mounted in a materials testing system (MTS 810; MTS Systems) servohydraulic load frame, and the suture loop was passed around a cross-bar on the actuator of the testing device. A mechanical testing protocol consisting of modest repetitive forces was carefully chosen to simulate expected activity during rehabilitation after RCR.15 In this protocol, a 60-second preload of 10 N was followed by tensile loading between 10 N and 90 N at a frequency of 0.5 Hz for 500 cycles.15 Cycle duration at 3 mm and 5 mm of suture slippage (threshold for clinical failure) was recorded.12,16,17 In addition, suture slippage was measured after 1, 10, 50, 100, 200, 300, 400, and 500 cycles. The first 5 test cycles were not counted in the analysis to control for initial knot slippage. Finally, after completion of dynamic testing, samples were loaded at a displacement rate of 0.5 mm/s for tension-to-failure testing in the custom fixtures. Maximum failure load, stiffness, and failure mode were recorded. Ultimate failure was defined as suture breakage or gross suture slippage.
Paired Student t test was used to determine significant differences in suture slippage distance between the 2 groups at various cycle durations. In addition, Kaplan-Meier survival test was used to determine statistical differences in sample survival during the dynamic loading test.
Results
Mean (SD) BMD of the cadaveric shoulder specimens was 0.55 (0.13) g/cm2 (range, 0.29-0.68 g/cm2). The testing fixtures isolated suture slippage from anchor–bone disengagement. All 6 PushLock implants demonstrated slippage of more than 3 mm, and 5 of the 6 demonstrated slippage of more than 5 mm. All 6 ReelX devices exhibited slippage of less than 3 mm. In addition, PushLock demonstrated more suture slippage at cycles 1, 10, and 100 (P < .05) and more maximum slippage after 500 cycles (mean, 11.2 mm; SD, 4.7 mm) compared with ReelX (mean, 1.9 mm; SD, 0.5 mm) (P = .004). Figure 4 shows mean suture slippage at each cycle.
Kaplan-Meier analysis revealed significantly (λ2 = 8.170; P = .0043) decreased survival after dynamic testing for PushLock versus ReelX (Figure 5). Survival was defined as suture slippage of less than 5 mm after completion of dynamic testing. Only 1 of the 6 PushLock anchors completed dynamic testing; the other 5 failed via complete suture slippage from the anchor before testing could be completed. All 6 ReelX devices survived dynamic testing.
Therefore, 1 PushLock implant and all 6 ReelX devices were available for subsequent load-to-failure testing. Failure in this setting was defined as suture slippage of more than 10 mm or suture breakage. The PushLock implant failed at a maximum force of 171.8 N with a stiffness of 74.4 N/mm and eventually exhibited gross suture slippage. All 6 ReelX devices failed at a mean (SD) maximum of 273.5 (20.2) N, with a mean (SD) stiffness of 74.1 (17) N/mm. Mechanism of failure for all ReelX devices was suture breakage during the tensile load-to-failure test.
Discussion
We evaluated a new technique designed to isolate suture slippage in knotless anchors used for RCR. The impetus for developing this new method was to provide a means for better analyzing the ability of a knotless anchor to resist suture slippage in the cadaveric biomechanical testing setting. Suture slippage is an important mode of failure during such analyses.11,12 Significant slippage occurred in a range of implants before half the anchor–bone pullout strength was reached in a study using young bovine femoral heads.11 In another study, using young, high-BMD cadaveric humeral heads, initial slippage and maximum failure loads were equivalent among numerous devices using various suture-retention mechanisms, and suture slippage was the most common failure mode.12 Nevertheless, other biomechanical studies have demonstrated frequent failure caused by anchor pullout in elderly human cadaveric specimens with diminished BMD, often with high-force testing protocols.12,13 In the more modest-force, in vivo rehabilitative environment, suture slippage rather than anchor dislodgement may be the main failure mode.11-15
We compared the PushLock implant and its entirely press-fit suture clamping design with the ReelX device, which relies on an intrinsic suture-locking mechanism. Middle-aged (mean, 53.3 years; SD, 5.7 years) cadaveric humeri were tested under physiologically relevant biomechanical conditions to begin to help identify how relatively osteopenic bone may affect suture-retention properties for a given implant. The results showed that the study methodology prevented implant failure via anchor–bone pullout. To our knowledge, this was the first study to exclusively analyze suture slippage in knotless anchors. The findings indicated that implants that rely heavily on a tight interference fit of the suture between the anchor and the surrounding bone may exhibit early slippage and failure after RCR in middle-aged patients with relative osteopenia.11,12 However, this study also demonstrated that devices with intrinsic clamping mechanisms that do not depend on the quality of surrounding bone may better resist suture slippage. It is not clear that all knotless anchors with intrinsic locking mechanisms function equivalently. For instance, Pietschmann and colleagues12 found that 2 of 10 implants with a different internal clamping device were unable to resist failure via suture slippage, even in healthy bone. Similarly, in a study comparing ReelX devices with implants having a different internal suture-retention mechanism, ReelX failed at higher ultimate loads, and typically via anchor dislodgement, versus suture slippage in the other implants.18
It is important to note that, in the present study, the loads at which sutures broke in the intrinsic clamping anchors approached the maximum contractile force of the supraspinatus muscle (302 N).19,20 In addition, these loads were above the resistance of the rotator cuff tendon to cut out with modern suture material.21
This study’s limitations include use of an in vitro human cadaveric model that precluded analysis of the effects of postoperative healing. Biomechanical testing was also performed in a single row-type suture configuration with the rotator cuff tendon removed. Fixtures used during testing oriented the load coaxially with the axis of tension, creating a worst-case loading scenario. Although this form of testing may limit its clinical applicability, its purpose was to critically isolate how well a knotless anchor could resist suture slippage. The methods we used were also limited because the stability of the bone–anchor interface was not assessed. For patients with osteopenia, anchor pullout rather than suture slippage could be the most limiting factor for knotless anchor construct failure, and therefore further testing of both failure modes is needed. Future biomechanical studies should compare various knotless anchors’ suture-slippage characteristics in other constructs in physiologic testing orientations, including double-row and suture-bridge configurations, as well as with intact rotator cuff tendons. In addition, use of labral tape as a substitute for polyblend suture has been suggested to limit suture slippage, and this technique theoretically could have changed the results of this study.22
Conclusion
An implant with an internal ratcheting mechanism for suture retention demonstrated significantly less suture slippage in an axial tension evaluation protocol than a device reliant on interference fit of the suture between the anchor and surrounding bone. In the clinical setting, this may allow for less gap formation during the healing phase following RCR with a knotless anchor. There was also increased maximum load to failure, demonstrating an increased load until catastrophic failure using a device with a ratcheting internal locking mechanism.
1. Thal R. A knotless suture anchor. Design, function, and biomechanical testing. Am J Sports Med. 2001;29(5):646-649.
2. Cole BJ, ElAttrache NS, Anbari A. Arthroscopic rotator cuff repairs: an anatomic and biomechanical rationale for different suture-anchor repair configurations. Arthroscopy. 2007;23(6):662-669.
3. Kim KC, Shin HD, Cha SM, Lee WY. Comparison of repair integrity and functional outcomes for 3 arthroscopic suture bridge rotator cuff repair techniques. Am J Sports Med. 2013;41(2):271-277.
4. Choi CH, Kim SK, Cho MR, et al. Functional outcomes and structural integrity after double-pulley suture bridge rotator cuff repair using serial ultrasonographic examination. J Shoulder Elbow Surg. 2012;21(12):1753-1763.
5. Brown BS, Cooper AD, McIff TE, Key VH, Toby EB. Initial fixation and cyclic loading stability of knotless suture anchors for rotator cuff repair. J Shoulder Elbow Surg. 2008;17(2):313-318.
6. Burkhart SS, Adams CR, Burkhart SS, Schoolfield JD. A biomechanical comparison of 2 techniques of footprint reconstruction for rotator cuff repair: the SwiveLock-FiberChain construct versus standard double-row repair. Arthroscopy. 2009;25(3):274-281.
7. Hepp P, Osterhoff G, Engel T, Marquass B, Klink T, Josten C. Biomechanical evaluation of knotless anatomical double-layer double-row rotator cuff repair: a comparative ex vivo study. Am J Sports Med. 2009;37(7):1363-1369.
8. Maguire M, Goldberg J, Bokor D, et al. Biomechanical evaluation of four different transosseous-equivalent/suture bridge rotator cuff repairs. Knee Surg Sports Traumatol Arthrosc. 2011;19(9):1582-1587.
9. Millar NL, Wu X, Tantau R, Silverstone E, Murrell GA. Open versus two forms of arthroscopic rotator cuff repair. Clin Orthop Relat Res. 2009;467(4):966-978.
10. Rhee YG, Cho NS, Parke CS. Arthroscopic rotator cuff repair using modified Mason-Allen medial row stitch: knotless versus knot-tying suture bridge technique. Am J Sports Med. 2012;40(11):2440-2447.
11. Wieser K, Farshad M, Vlachopoulos L, Ruffieux K, Gerber C, Meyer DC. Suture slippage in knotless suture anchors as a potential failure mechanism in rotator cuff repair. Arthroscopy. 2012;28(11):1622-1627.
12. Pietschmann MF, Gülecyüz MF, Fieseler S, et al. Biomechanical stability of knotless suture anchors used in rotator cuff repair in healthy and osteopenic bone. Arthroscopy. 2010;26(8):1035-1044.
13. Barber FA, Hapa O, Bynum JA. Comparative testing by cyclic loading of rotator cuff suture anchors containing multiple high-strength sutures. Arthroscopy. 2010;26(9 suppl):S134-S141.
14. Barber FA, Coons DA, Ruiz-Suarez M. Cyclic load testing of biodegradable suture anchors containing 2 high-strength sutures. Arthroscopy. 2007;23(4):355-360.
15. Bynum CK, Lee S, Mahar A, Tasto J, Pedowitz R. Failure mode of suture anchors as a function of insertion depth. Am J Sports Med. 2005;33(7):1030-1034.
16. Gerber C, Schneeberger AG, Beck M, Schlegel U. Mechanical strength of repairs of the rotator cuff. J Bone Joint Surg Br. 1994;76(3):371-380.
17. Schneeberger AG, von Roll A, Kalberer F, Jacob HA, Gerber C. Mechanical strength of arthroscopic rotator cuff repair techniques: an in vitro study. J Bone Joint Surg Am. 2002;84(12):2152-2160.
18. Efird C, Traub S, Baldini T, et al. Knotless single-row rotator cuff repair: a comparative biomechanical study of 2 knotless suture anchors. Orthopedics. 2013;36(8):e1033-e1037.
19. Wright PB, Budoff JE, Yeh ML, Kelm ZS, Luo ZP. Strength of damaged suture: an in vitro study. Arthroscopy. 2006;22(12):1270-1275.
20. Burkhart SS. A stepwise approach to arthroscopic rotator cuff repair based on biomechanical principles. Arthroscopy. 2000;16(1):82-90.
21. Bisson LJ, Manohar LM. A biomechanical comparison of the pullout strength of No. 2 FiberWire suture and 2-mm FiberWire tape in bovine rotator cuff tendons. Arthroscopy. 2010;26(11):1463-1468.
22. Burkhart SS, Denard PJ, Konicek J, Hanypsiak BT. Biomechanical validation of load-sharing rip-stop fixation for the repair of tissue-deficient rotator cuff tears. Am J Sports Med. 2014;42(2):457-462.
1. Thal R. A knotless suture anchor. Design, function, and biomechanical testing. Am J Sports Med. 2001;29(5):646-649.
2. Cole BJ, ElAttrache NS, Anbari A. Arthroscopic rotator cuff repairs: an anatomic and biomechanical rationale for different suture-anchor repair configurations. Arthroscopy. 2007;23(6):662-669.
3. Kim KC, Shin HD, Cha SM, Lee WY. Comparison of repair integrity and functional outcomes for 3 arthroscopic suture bridge rotator cuff repair techniques. Am J Sports Med. 2013;41(2):271-277.
4. Choi CH, Kim SK, Cho MR, et al. Functional outcomes and structural integrity after double-pulley suture bridge rotator cuff repair using serial ultrasonographic examination. J Shoulder Elbow Surg. 2012;21(12):1753-1763.
5. Brown BS, Cooper AD, McIff TE, Key VH, Toby EB. Initial fixation and cyclic loading stability of knotless suture anchors for rotator cuff repair. J Shoulder Elbow Surg. 2008;17(2):313-318.
6. Burkhart SS, Adams CR, Burkhart SS, Schoolfield JD. A biomechanical comparison of 2 techniques of footprint reconstruction for rotator cuff repair: the SwiveLock-FiberChain construct versus standard double-row repair. Arthroscopy. 2009;25(3):274-281.
7. Hepp P, Osterhoff G, Engel T, Marquass B, Klink T, Josten C. Biomechanical evaluation of knotless anatomical double-layer double-row rotator cuff repair: a comparative ex vivo study. Am J Sports Med. 2009;37(7):1363-1369.
8. Maguire M, Goldberg J, Bokor D, et al. Biomechanical evaluation of four different transosseous-equivalent/suture bridge rotator cuff repairs. Knee Surg Sports Traumatol Arthrosc. 2011;19(9):1582-1587.
9. Millar NL, Wu X, Tantau R, Silverstone E, Murrell GA. Open versus two forms of arthroscopic rotator cuff repair. Clin Orthop Relat Res. 2009;467(4):966-978.
10. Rhee YG, Cho NS, Parke CS. Arthroscopic rotator cuff repair using modified Mason-Allen medial row stitch: knotless versus knot-tying suture bridge technique. Am J Sports Med. 2012;40(11):2440-2447.
11. Wieser K, Farshad M, Vlachopoulos L, Ruffieux K, Gerber C, Meyer DC. Suture slippage in knotless suture anchors as a potential failure mechanism in rotator cuff repair. Arthroscopy. 2012;28(11):1622-1627.
12. Pietschmann MF, Gülecyüz MF, Fieseler S, et al. Biomechanical stability of knotless suture anchors used in rotator cuff repair in healthy and osteopenic bone. Arthroscopy. 2010;26(8):1035-1044.
13. Barber FA, Hapa O, Bynum JA. Comparative testing by cyclic loading of rotator cuff suture anchors containing multiple high-strength sutures. Arthroscopy. 2010;26(9 suppl):S134-S141.
14. Barber FA, Coons DA, Ruiz-Suarez M. Cyclic load testing of biodegradable suture anchors containing 2 high-strength sutures. Arthroscopy. 2007;23(4):355-360.
15. Bynum CK, Lee S, Mahar A, Tasto J, Pedowitz R. Failure mode of suture anchors as a function of insertion depth. Am J Sports Med. 2005;33(7):1030-1034.
16. Gerber C, Schneeberger AG, Beck M, Schlegel U. Mechanical strength of repairs of the rotator cuff. J Bone Joint Surg Br. 1994;76(3):371-380.
17. Schneeberger AG, von Roll A, Kalberer F, Jacob HA, Gerber C. Mechanical strength of arthroscopic rotator cuff repair techniques: an in vitro study. J Bone Joint Surg Am. 2002;84(12):2152-2160.
18. Efird C, Traub S, Baldini T, et al. Knotless single-row rotator cuff repair: a comparative biomechanical study of 2 knotless suture anchors. Orthopedics. 2013;36(8):e1033-e1037.
19. Wright PB, Budoff JE, Yeh ML, Kelm ZS, Luo ZP. Strength of damaged suture: an in vitro study. Arthroscopy. 2006;22(12):1270-1275.
20. Burkhart SS. A stepwise approach to arthroscopic rotator cuff repair based on biomechanical principles. Arthroscopy. 2000;16(1):82-90.
21. Bisson LJ, Manohar LM. A biomechanical comparison of the pullout strength of No. 2 FiberWire suture and 2-mm FiberWire tape in bovine rotator cuff tendons. Arthroscopy. 2010;26(11):1463-1468.
22. Burkhart SS, Denard PJ, Konicek J, Hanypsiak BT. Biomechanical validation of load-sharing rip-stop fixation for the repair of tissue-deficient rotator cuff tears. Am J Sports Med. 2014;42(2):457-462.
Revision Rotator Cuff Reconstruction for Large Tears With Retraction: A Novel Technique Using Autogenous Tendon and Autologous Marrow
Primary rotator cuff repair is a common procedure that consistently yields favorable clinical results.1 Revision rotator cuff repair and reconstruction yield less consistent clinical results and are associated with a significant incidence of recurrent cuff tearing.2 Possible factors contributing to the loss of tissue continuity have included poor quality or frank loss of rotator cuff tissue, diminished biological potential of the rotator cuff tendon, and excessive mechanical stress on or strain of the reconstructive surgical construct.3
I conducted a pilot study involving a technique that addresses these potential factors, amalgamating several contemporary surgical methods with the addition of a novel step: an autogenous tendon graft incubated in autologous bone marrow concentrate.
Materials and Methods
Ten consecutive patients (7 men, 3 women) enrolled in this retrospective case series. Mean age at time of surgery was 58 years (range, 47-65 years). Mean follow-up was 24 months (range, 12-44 months), and no patients were lost to follow-up. Mean time between original primary repair and current reconstruction was 36 months (range, 6-120 months). Criteria for enrollment included unremitting shoulder pain, radiographs showing no significant degenerative joint disease, magnetic resonance imaging confirming a large (3-5 cm) full-thickness rotator cuff tear with retraction, and history of prior rotator cuff repair on the affected shoulder without associated biceps tenodesis. The intraoperative inclusion criterion was direct visualization of a 3- to 5-cm full-thickness rotator cuff tear with retraction of at least 3 cm. Validated Constant, American Shoulder and Elbow Surgeons (ASES), and University of California Los Angeles (UCLA) shoulder scoring systems were used to collect range-of-motion, pain, strength, daily function, and patient satisfaction data before and after surgery. Standard error was calculated. Two-sample t test was used for preoperative–postoperative comparisons. Postoperative integrity of the rotator cuff reconstruction was evaluated by an independent full-time academic musculoskeletal radiologist using dynamic diagnostic ultrasound (iU22 xMatrix Ultrasound System [Philips Healthcare] at L 9-3 MHz). Informed consent was obtained from each patient. The study was approved by institutional review board.
After induction of general anesthesia, each patient was placed in the lateral decubitus position. Bone marrow (60 mL) was aspirated through a 14-gauge needle from a dorsal iliac table, just inferior to the iliac crest (Figure 1). The patient was then placed into the beach-chair position on a surgical shoulder table. The aspirated marrow was centrifuged at 2800 and 3800 rpm for 14 to 17 minutes (Magellan Autologous Platelet Separator; Arteriocyte Medical Systems) to yield 10 mL of a concentrated (4- to 5-fold) mixture of platelet-rich plasma (PRP) and mesenchymal stem cells. Surgery was performed through a 3-cm oblique anterior mini-open incision between the anterior corner of the acromion and the coracoid process, as I previously described.4 The deltoid muscle was split, not detached. Acromioplasty and release of the coracoacromial ligament were performed. The rotator cuff was inspected under ×4.5 optical magnification. The cuff tissue was mobilized and débrided back to a healthy-appearing margin. The size and shape of the rotator cuff defect were then estimated. The long head of the biceps was harvested from its origin just distal to the superior glenoid labrum unto the intertubercular sulcus on the proximal humerus. The remainder of the biceps tendon was tenodesed to the surgical neck of the humerus. The biceps tendon graft was then manipulated and fashioned (by longitudinal partial-thickness incision and expansion) to fit the cuff defect (Figures 2, 3). The expanded graft was incubated in the concentrated marrow (10 mL) for 60 minutes (Figure 4). Débridement at the base of the greater tuberosity down to bleeding cancellous bone was followed by insertion of multiple bone anchors bearing several strands of No. 2 synthetic suture. These strands were then passed through the biceps tendon graft for secure fixation (Figure 5). The débrided end of the rotator cuff was then sewn to the biceps tendon graft using locking stitches under zero tissue tension with the arm in full adduction. The free end of the graft was sewn to the subscapularis tendon (Figure 6). The remaining marrow concentrate was injected both deep and superficial to the rotator cuff construct. No additional wound irrigation fluid was injected or suction drain inserted. After surgery, the patient was placed into an abduction pillow for 1 month and then engaged in passive motion for 1 month. Active-assisted motion began 3 months after surgery.
Results
Clinically, all patients improved with respect to pain, motion, strength, function, and satisfaction by virtue of the reconstructive surgery. After surgery, mean Constant score was increased, from 13 to 71 (P < .001). Mean ASES score increased from 18 to 75 (P < .001). Mean UCLA score increased from 4 to 28 (P < .001) (Table). Ultrasound showed 0% incidence of full-thickness retearing. Dynamic scanning during abduction showed maintained reduction of the humeral head within the glenoid socket; superior subluxation of the humeral head was not detected. The biceps tendon graft was continuous with the rotator cuff tendon, indicative of graft integration: tissue healing at the graft–bone and graft–tendon interfaces (Figures 7, 8). There were no intraoperative or postoperative patient-related complications.
Discussion
Primary rotator cuff surgery is beneficial.5 Irrespective of technique, open versus arthroscopic,6 single- versus double-row repair,7 the clinical results have been satisfactory.8 Nevertheless, the “tissue failure” rate of rotator cuff surgery (full-thickness discontinuity of rotator cuff) has been as high as 31% in primary repairs.9 In revision rotator cuff repair and reconstruction, the radiographic tissue failure rate has been even higher, particularly in the setting of chronic large tears with retraction, with tissue failure rates up to 91%.10 Although small to medium full-thickness tears and retears are well tolerated by patients with reduced activity levels,11 and pain symptoms do not necessarily correlate with rotator cuff tear size,12 large retracted full-thickness tears in active patients seldom result in optimal clinical outcomes or patient satisfaction.13,14 In addition, although recurrent tearing does not preclude a satisfactory clinical result, maintenance of cuff tissue integrity tends to produce a better objective clinical score and a more desirable clinical outcome.2
Few evidence-based restorative solutions exist for large recurrent rotator cuff tears with retraction in active nongeriatric patients.15 The no-treatment option in this context may result in gradual enlargement of the tear, chronic pain, weakness, and progressive degeneration of the glenohumeral joint and acromiohumeral confluence—so-called rotator cuff arthropathy, for which reverse total shoulder arthroplasty is required.16,17 Partial repair of a large rotator cuff tear by margin convergence, interval slide, split deltoid flap, or nonanatomical reinsertion may improve clinical outcome scores but may not alter or prevent the progressive degenerative changes associated with rotator cuff arthropathy.18,19 Synthetic scaffolds with and without biological enhancement have been used with varying degrees of success, particularly pain improvement and tissue integration.20 Nevertheless, the failure rate has been reported to be 17% to 51%,21 and no evidence exists that allograft augmentation improves functional outcomes.22 Tendon transfer using the latissimus dorsi has also proved to be a surgical alternative in younger, active patients.23 However, dissection in this procedure is a major undertaking for both surgeon and patient—compared with the minimally invasive technique used in the present study.24
I selected a cohort of active, symptomatic patients for application of a synthesis of accepted surgical techniques through a mini-open incision in order to improve the reliability of the surgical construct while minimizing surgical morbidity. Débridement of marginal tissue, safe mobilization of remaining cuff, and tension-free suture line using locking sutures maximized the mechanical strength of the construct.25,26 Biological enhancement with autogenous tissue (the patient’s own biceps tendon) as graft material (scaffolding), as well as autologous concentrated marrow delivering viable responding cells and chemokine/cytokine biofactors, increased the probability of reparative activity at the graft site.27 The net effect was consistent tissue healing at a biologically challenging locus. Nonenhanced biceps tendon grafting in the setting of “irreparable” primary rotator cuff repair has had a 40-year history of orthopedic utility and an excellent record of clinical success.28 Nevertheless, the retear rate has been 7% to 30%.29 There are no previous reports of biologically enhanced autogenous biceps tendon grafting for reconstruction of a torn rotator cuff, either primary or in the setting of chronic revision surgery.
Previous well-designed PRP enhancement studies in the context of primary rotator cuff repair failed to demonstrate a consistent benefit with concentrated platelet-only augmentation.30,31 The shared experimental design of these published studies used intra-articular injection as the sole delivery method without guarantee that the injected platelets would migrate, adhere to, and persist at the intended destination, the healing edge of the rotator cuff. In the present study, extended exposure of the splayed tendon graft by incubation in concentrated marrow was specifically designed to increase the probability that biologically active components would settle at the desired location by cellular seeding and plasmatic imbibition.32 Furthermore, use of PRP for growth factor (platelet-derived, PDGF; basic fibroblast, bFGF; transforming, TGF-β; epidermal, EGF; vascular endothelial, VEGF; connective tissue, CTGF) therapy, in addition to pluripotential mesenchymal cells for marrow-derived stem cell therapy, is in theory biologically superior to use of PRP alone.33,34
The recent expansion of information about biologics has generated much interest in augmentation of soft-tissue healing. Unfortunately, the optimal technique of using cellular processing to upregulate stem-cell capacity at the graft interface is yet to be defined.35 Clinical studies using PRP and related products to promote tendon healing have been both inconsistent and contradictory with respect to benefit of outcome. As we have been unable to harness the biological potential of this medium, application of biologics in contemporary clinical orthopedics remains narrow, random, and infrequent. The technique presented in this clinical series appears to be a small advancement in a positive direction. The described construct provides a starting point for study, combining mechanical as well as biological steps to promote rotator cuff healing. The consistency of the outcome in a clinical model in which retearing is an expectation rather than an exception is noteworthy. The zero tissue failure rate at 1 to 4 years, compared with the literature values in similar patient cohorts, is very promising.36 The clinical outcome as measured by validated shoulder scores is also comparable to literature outcome values.19 Also noteworthy is the dynamic stability the construct gives to the glenohumeral joint. Ideally, the reconstructed rotator cuff provides active force coupling with the deltoid, simulating normal shoulder biomechanics. At a minimum, the reconstructed cuff provides a viable passive barrier to superior migration of the humeral head—thus supporting the mechanical efficiency of the deltoid and preventing rotator cuff arthropathy.
This study’s small sample (10 patients) puts its conclusions at risk for type I statistical error, in that too few patients were examined over a long enough period to demonstrate failure. Nevertheless, retears typically occur within 6 months of repair.37,38 Therefore, minimum follow-up of 1 year was deemed sufficient. None of the 10 patients had diabetes or another chronic comorbidity. Nine of the 10 had either no or only mild preoperative fatty atrophy of the rotator cuff muscles. Eight of the 10 were nonsmokers. These factors, which suggest optimal surgical candidates, may prove to be significant as the clinical series expands over time. Incubation of the autogenous biceps graft in concentrated marrow for 60 minutes was arbitrarily chosen. In future in vitro examination, marrow cell viability as a function of incubation time will be assessed.
Conclusion
In active, middle-aged patients with chronic recurrent large retracted rotator cuff tears, the technique presented here, using autogenous biceps tendon and autologous concentrated marrow containing PRP and mesenchymal cells, consistently yielded satisfactory clinical results and promoted rotator cuff tissue healing without full-thickness retearing.
1. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
2. Kim HM, Caldwell JM, Buza JA, et al. Factors affecting satisfaction and shoulder function in patients with a recurrent rotator cuff tear. J Bone Joint Surg Am. 2014;96(2):106-112.
3. George MS, Khazzam M. Current concepts review: revision rotator cuff repair. J Shoulder Elbow Surg. 2012;21(4):431-440.
4. Skoff HD. Conservative open acromioplasty. J Bone Joint Surg Br. 1995;77(6):933-936.
5. Mather RC 3rd, Koenig L, Acevedo D, et al. The societal and economic value of rotator cuff repair. J Bone Joint Surg Am. 2013;95(22):1993-2000.
6. Sauerbrey AM, Getz CL, Piancastelli M, Iannotti JP, Ramsey ML, Williams GR. Arthroscopic versus mini-open rotator cuff repair: a comparison of clinical outcome. Arthroscopy. 2005;21(12):1415-1420.
7. Koh KH, Kang KC, Lim TK, Shon MS, Yoo JC. Prospective randomized clinical trial of single- versus double-row suture anchor repair in 2- to 4-cm rotator cuff tears: clinical and magnetic resonance imaging results. Arthroscopy. 2011;27(4):453-462.
8. Galatz LM, Griggs S, Cameron BD, Iannotti JP. Prospective longitudinal analysis of post-operative shoulder function: a ten-year follow-up study of full thickness rotator cuff tears. J Bone Joint Surg Am. 2001;83(7):1052-1056.
9. Oh JH, Kim SH, Kang JY, Oh CH, Gong HS. Effect of age on functional and structural outcome after rotator cuff repair. Am J Sports Med. 2010;38(4):672-678.
10. Kim JH, Kim SH, Lee SK, Seo JW, Chun YMC. Arthroscopic repair of massive contracted rotator cuff tears: aggressive release with anterior and posterior interval slides do not improve cuff healing and integrity. J Bone Joint Surg Am. 2014;95(16):1482-1488.
11. Moosmayer S, Lund G, Seljom US, et al. Tendon repair compared with physiotherapy in the treatment of rotator cuff tears. J Bone Joint Surg Am. 2014;96(18):1504-1514.
12. Dunn WR, Kuhn JE, Sanders R, et al. Symptoms of pain do not correlate with rotator cuff tear severity. J Bone Joint Surg Am. 2014;96(10):793-800.
13. Lubiatowski P, Kaczmarek P, Dzianach M, et al. Clinical and biomechanical performance of patients with failed rotator cuff repair. Int Orthop. 2013;37(12):2395-2401.
14. Holtby R, Razmjou H. Relationship between clinical and surgical findings and reparability of large and massive rotator cuff tears: a longitudinal study. BMC Musculoskelet Disord. 2014;15:180.
15. Nho SJ, Delos D, Yadav H, et al. Biomechanical and biological augmentation for the treatment of massive rotator cuff tears. Am J Sports Med. 2010;38(3):619-629.
16. Moosmayer S, Tariq R, Stiris M, Smith HJ. The natural history of asymptomatic rotator cuff tears: a three-year follow-up of fifty cases. J Bone Joint Surg Am. 2013;95(14):1249-1255.
17. Neer CS 2nd, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65(9):1232-1244.
18. Bartl C, Louloumentas P, Konstantin H, et al. Long-term outcome and structural integrity following open repair of massive rotator cuff tears. Int J Shoulder Surg. 2012;6(1):1-8.
19. Paxton ES, Teefey SA, Dahiya N, Keener JD, Yamaguchi K, Galatz LM. Clinical and radiographic outcomes of failed repairs of large or massive rotator cuff tears: minimum ten-year follow-up. J Bone Joint Surg Am. 2013;95(7):627-632.
20. Longo UG, Lamberti A, Maffulli N, Denaro V. Tendon augmentation grafts: a systematic review. Br Med Bull. 2010;94:165-188.
21. Ciampi P, Scotti C, Nonis A, et al. The benefit of synthetic versus biological patch augmentation in the repair of posterosuperior massive rotator cuff tears: a 3-year follow-up study. Am J Sports Med. 2014;42(5):1169-1175.
22. Murhi AM. Rotator cuff tears and cuff tear arthropathy. In: Boyer MI, ed. AAOS Comprehensive Orthopedic Review. Vol 2. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2014:921-929.
23. Namdari S, Voleti P, Baldwin K, Glaser D, Huffman GR. Latissimus dorsi tendon transfer for irreparable rotator cuff tears: a systematic review. J Bone Joint Surg Am. 2012;94(10):891-898.
24. Gerber C, Rahm SA, Catanzaro S, Farshad M, Moor BK. Latissimus dorsi tendon transfer for treatment of irreparable posterosuperior rotator cuff tears: long-term results at a minimum follow-up of ten years. J Bone Joint Surg Am. 2013;95(21):1920-1926.
25. Wagner JP, Krushall RJ, Masqueloet A, Gerber C. Anatomy and relationships of the suprascapular nerve: anatomical constraints to mobilization of the supraspinatus and infraspinatus muscles in the management of massive rotator cuff tears. J Bone Joint Surg Am. 1992;74(1):36-45.
26. Ponce BA, Hosemann CD, Reghava P, Tate JP, Sheppard ED, Ebenhardt AW. A biomechanical analysis of controllable intraoperative variables affecting the strength of rotator cuff repairs at the suture–tendon interface. Am J Sports Med. 2013;41(10):2256-2261.
27. Thomopoulos S. Tendon and ligaments. In: Boyer MI, ed. AAOS Comprehensive Orthopedic Review. Vol 2. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2014:105-111.
28. Sano H, Mineta M, Kitz A, Itoi E. Tendon patch grafting using the long head of the biceps for irreparable massive rotator cuff tears. J Orthop Sci. 2010;15(3):310-316.
29. Rhee YG, Cho NS, Lim CT, Yi JW, Vishvanathan T. Bridging the gap in immobile massive rotator cuff tears: augmentation using the tenotomized biceps. Am J Sports Med. 2008;36(8):1511-1518.
30. Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39(2):258-265.
31. Rodeo SA, Delos, D, Williams, RJ, Adler RS, Pearle A, Warren RF. The effects of platelet-rich fibrin matrix on rotator cuff tendon healing: a prospective, randomized clinical study. Am J Sports Med. 2012;40(6):1234-1241.
32. Beitzel K, McCarthy MB, Cote MP, et al. Properties of biologic scaffolds and their response to mesenchymal stem cells. Arthroscopy. 2014;30(3):289-298.
33. Anz AW, Hackel JG, Nilssen ED, Andrews JR. Application of biologics in the treatment of rotator cuff, meniscus, cartilage and osteoarthritis. J Am Acad Orthop Surg. 2014;22(2):68-79.
34. Hernigou P, Flouzat Lachaniette CH, Delambre J, et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int Orthop. 2014;38(9):1811-1818.
35. Hsu WK, Mishra A, Rodeo SR, et al. Platelet-rich plasma in orthopaedic applications: evidence-based recommendations for treatment. J Am Acad Orthop Surg. 2013;21(12):739-748.
36. Kowalsky MS, Keener JD. Revision arthroscopic rotator cuff repair: repair integrity and clinical outcome: surgical technique. J Bone Joint Surg Am. 2011;93(suppl 1):62-74.
37. Iannotti JP, Deutsch A, Green A, et al. Time to failure after rotator cuff repair: a prospective imaging study. J Bone Joint Surg Am. 2013;95(11):965-971.
38. Le BT, Wu XL, Lam PH, Murrell GA. Factors predicting rotator cuff retears: an analysis of 1000 consecutive rotator cuff repairs. Am J Sports Med. 2014;42(5):1134-1142.
Primary rotator cuff repair is a common procedure that consistently yields favorable clinical results.1 Revision rotator cuff repair and reconstruction yield less consistent clinical results and are associated with a significant incidence of recurrent cuff tearing.2 Possible factors contributing to the loss of tissue continuity have included poor quality or frank loss of rotator cuff tissue, diminished biological potential of the rotator cuff tendon, and excessive mechanical stress on or strain of the reconstructive surgical construct.3
I conducted a pilot study involving a technique that addresses these potential factors, amalgamating several contemporary surgical methods with the addition of a novel step: an autogenous tendon graft incubated in autologous bone marrow concentrate.
Materials and Methods
Ten consecutive patients (7 men, 3 women) enrolled in this retrospective case series. Mean age at time of surgery was 58 years (range, 47-65 years). Mean follow-up was 24 months (range, 12-44 months), and no patients were lost to follow-up. Mean time between original primary repair and current reconstruction was 36 months (range, 6-120 months). Criteria for enrollment included unremitting shoulder pain, radiographs showing no significant degenerative joint disease, magnetic resonance imaging confirming a large (3-5 cm) full-thickness rotator cuff tear with retraction, and history of prior rotator cuff repair on the affected shoulder without associated biceps tenodesis. The intraoperative inclusion criterion was direct visualization of a 3- to 5-cm full-thickness rotator cuff tear with retraction of at least 3 cm. Validated Constant, American Shoulder and Elbow Surgeons (ASES), and University of California Los Angeles (UCLA) shoulder scoring systems were used to collect range-of-motion, pain, strength, daily function, and patient satisfaction data before and after surgery. Standard error was calculated. Two-sample t test was used for preoperative–postoperative comparisons. Postoperative integrity of the rotator cuff reconstruction was evaluated by an independent full-time academic musculoskeletal radiologist using dynamic diagnostic ultrasound (iU22 xMatrix Ultrasound System [Philips Healthcare] at L 9-3 MHz). Informed consent was obtained from each patient. The study was approved by institutional review board.
After induction of general anesthesia, each patient was placed in the lateral decubitus position. Bone marrow (60 mL) was aspirated through a 14-gauge needle from a dorsal iliac table, just inferior to the iliac crest (Figure 1). The patient was then placed into the beach-chair position on a surgical shoulder table. The aspirated marrow was centrifuged at 2800 and 3800 rpm for 14 to 17 minutes (Magellan Autologous Platelet Separator; Arteriocyte Medical Systems) to yield 10 mL of a concentrated (4- to 5-fold) mixture of platelet-rich plasma (PRP) and mesenchymal stem cells. Surgery was performed through a 3-cm oblique anterior mini-open incision between the anterior corner of the acromion and the coracoid process, as I previously described.4 The deltoid muscle was split, not detached. Acromioplasty and release of the coracoacromial ligament were performed. The rotator cuff was inspected under ×4.5 optical magnification. The cuff tissue was mobilized and débrided back to a healthy-appearing margin. The size and shape of the rotator cuff defect were then estimated. The long head of the biceps was harvested from its origin just distal to the superior glenoid labrum unto the intertubercular sulcus on the proximal humerus. The remainder of the biceps tendon was tenodesed to the surgical neck of the humerus. The biceps tendon graft was then manipulated and fashioned (by longitudinal partial-thickness incision and expansion) to fit the cuff defect (Figures 2, 3). The expanded graft was incubated in the concentrated marrow (10 mL) for 60 minutes (Figure 4). Débridement at the base of the greater tuberosity down to bleeding cancellous bone was followed by insertion of multiple bone anchors bearing several strands of No. 2 synthetic suture. These strands were then passed through the biceps tendon graft for secure fixation (Figure 5). The débrided end of the rotator cuff was then sewn to the biceps tendon graft using locking stitches under zero tissue tension with the arm in full adduction. The free end of the graft was sewn to the subscapularis tendon (Figure 6). The remaining marrow concentrate was injected both deep and superficial to the rotator cuff construct. No additional wound irrigation fluid was injected or suction drain inserted. After surgery, the patient was placed into an abduction pillow for 1 month and then engaged in passive motion for 1 month. Active-assisted motion began 3 months after surgery.
Results
Clinically, all patients improved with respect to pain, motion, strength, function, and satisfaction by virtue of the reconstructive surgery. After surgery, mean Constant score was increased, from 13 to 71 (P < .001). Mean ASES score increased from 18 to 75 (P < .001). Mean UCLA score increased from 4 to 28 (P < .001) (Table). Ultrasound showed 0% incidence of full-thickness retearing. Dynamic scanning during abduction showed maintained reduction of the humeral head within the glenoid socket; superior subluxation of the humeral head was not detected. The biceps tendon graft was continuous with the rotator cuff tendon, indicative of graft integration: tissue healing at the graft–bone and graft–tendon interfaces (Figures 7, 8). There were no intraoperative or postoperative patient-related complications.
Discussion
Primary rotator cuff surgery is beneficial.5 Irrespective of technique, open versus arthroscopic,6 single- versus double-row repair,7 the clinical results have been satisfactory.8 Nevertheless, the “tissue failure” rate of rotator cuff surgery (full-thickness discontinuity of rotator cuff) has been as high as 31% in primary repairs.9 In revision rotator cuff repair and reconstruction, the radiographic tissue failure rate has been even higher, particularly in the setting of chronic large tears with retraction, with tissue failure rates up to 91%.10 Although small to medium full-thickness tears and retears are well tolerated by patients with reduced activity levels,11 and pain symptoms do not necessarily correlate with rotator cuff tear size,12 large retracted full-thickness tears in active patients seldom result in optimal clinical outcomes or patient satisfaction.13,14 In addition, although recurrent tearing does not preclude a satisfactory clinical result, maintenance of cuff tissue integrity tends to produce a better objective clinical score and a more desirable clinical outcome.2
Few evidence-based restorative solutions exist for large recurrent rotator cuff tears with retraction in active nongeriatric patients.15 The no-treatment option in this context may result in gradual enlargement of the tear, chronic pain, weakness, and progressive degeneration of the glenohumeral joint and acromiohumeral confluence—so-called rotator cuff arthropathy, for which reverse total shoulder arthroplasty is required.16,17 Partial repair of a large rotator cuff tear by margin convergence, interval slide, split deltoid flap, or nonanatomical reinsertion may improve clinical outcome scores but may not alter or prevent the progressive degenerative changes associated with rotator cuff arthropathy.18,19 Synthetic scaffolds with and without biological enhancement have been used with varying degrees of success, particularly pain improvement and tissue integration.20 Nevertheless, the failure rate has been reported to be 17% to 51%,21 and no evidence exists that allograft augmentation improves functional outcomes.22 Tendon transfer using the latissimus dorsi has also proved to be a surgical alternative in younger, active patients.23 However, dissection in this procedure is a major undertaking for both surgeon and patient—compared with the minimally invasive technique used in the present study.24
I selected a cohort of active, symptomatic patients for application of a synthesis of accepted surgical techniques through a mini-open incision in order to improve the reliability of the surgical construct while minimizing surgical morbidity. Débridement of marginal tissue, safe mobilization of remaining cuff, and tension-free suture line using locking sutures maximized the mechanical strength of the construct.25,26 Biological enhancement with autogenous tissue (the patient’s own biceps tendon) as graft material (scaffolding), as well as autologous concentrated marrow delivering viable responding cells and chemokine/cytokine biofactors, increased the probability of reparative activity at the graft site.27 The net effect was consistent tissue healing at a biologically challenging locus. Nonenhanced biceps tendon grafting in the setting of “irreparable” primary rotator cuff repair has had a 40-year history of orthopedic utility and an excellent record of clinical success.28 Nevertheless, the retear rate has been 7% to 30%.29 There are no previous reports of biologically enhanced autogenous biceps tendon grafting for reconstruction of a torn rotator cuff, either primary or in the setting of chronic revision surgery.
Previous well-designed PRP enhancement studies in the context of primary rotator cuff repair failed to demonstrate a consistent benefit with concentrated platelet-only augmentation.30,31 The shared experimental design of these published studies used intra-articular injection as the sole delivery method without guarantee that the injected platelets would migrate, adhere to, and persist at the intended destination, the healing edge of the rotator cuff. In the present study, extended exposure of the splayed tendon graft by incubation in concentrated marrow was specifically designed to increase the probability that biologically active components would settle at the desired location by cellular seeding and plasmatic imbibition.32 Furthermore, use of PRP for growth factor (platelet-derived, PDGF; basic fibroblast, bFGF; transforming, TGF-β; epidermal, EGF; vascular endothelial, VEGF; connective tissue, CTGF) therapy, in addition to pluripotential mesenchymal cells for marrow-derived stem cell therapy, is in theory biologically superior to use of PRP alone.33,34
The recent expansion of information about biologics has generated much interest in augmentation of soft-tissue healing. Unfortunately, the optimal technique of using cellular processing to upregulate stem-cell capacity at the graft interface is yet to be defined.35 Clinical studies using PRP and related products to promote tendon healing have been both inconsistent and contradictory with respect to benefit of outcome. As we have been unable to harness the biological potential of this medium, application of biologics in contemporary clinical orthopedics remains narrow, random, and infrequent. The technique presented in this clinical series appears to be a small advancement in a positive direction. The described construct provides a starting point for study, combining mechanical as well as biological steps to promote rotator cuff healing. The consistency of the outcome in a clinical model in which retearing is an expectation rather than an exception is noteworthy. The zero tissue failure rate at 1 to 4 years, compared with the literature values in similar patient cohorts, is very promising.36 The clinical outcome as measured by validated shoulder scores is also comparable to literature outcome values.19 Also noteworthy is the dynamic stability the construct gives to the glenohumeral joint. Ideally, the reconstructed rotator cuff provides active force coupling with the deltoid, simulating normal shoulder biomechanics. At a minimum, the reconstructed cuff provides a viable passive barrier to superior migration of the humeral head—thus supporting the mechanical efficiency of the deltoid and preventing rotator cuff arthropathy.
This study’s small sample (10 patients) puts its conclusions at risk for type I statistical error, in that too few patients were examined over a long enough period to demonstrate failure. Nevertheless, retears typically occur within 6 months of repair.37,38 Therefore, minimum follow-up of 1 year was deemed sufficient. None of the 10 patients had diabetes or another chronic comorbidity. Nine of the 10 had either no or only mild preoperative fatty atrophy of the rotator cuff muscles. Eight of the 10 were nonsmokers. These factors, which suggest optimal surgical candidates, may prove to be significant as the clinical series expands over time. Incubation of the autogenous biceps graft in concentrated marrow for 60 minutes was arbitrarily chosen. In future in vitro examination, marrow cell viability as a function of incubation time will be assessed.
Conclusion
In active, middle-aged patients with chronic recurrent large retracted rotator cuff tears, the technique presented here, using autogenous biceps tendon and autologous concentrated marrow containing PRP and mesenchymal cells, consistently yielded satisfactory clinical results and promoted rotator cuff tissue healing without full-thickness retearing.
Primary rotator cuff repair is a common procedure that consistently yields favorable clinical results.1 Revision rotator cuff repair and reconstruction yield less consistent clinical results and are associated with a significant incidence of recurrent cuff tearing.2 Possible factors contributing to the loss of tissue continuity have included poor quality or frank loss of rotator cuff tissue, diminished biological potential of the rotator cuff tendon, and excessive mechanical stress on or strain of the reconstructive surgical construct.3
I conducted a pilot study involving a technique that addresses these potential factors, amalgamating several contemporary surgical methods with the addition of a novel step: an autogenous tendon graft incubated in autologous bone marrow concentrate.
Materials and Methods
Ten consecutive patients (7 men, 3 women) enrolled in this retrospective case series. Mean age at time of surgery was 58 years (range, 47-65 years). Mean follow-up was 24 months (range, 12-44 months), and no patients were lost to follow-up. Mean time between original primary repair and current reconstruction was 36 months (range, 6-120 months). Criteria for enrollment included unremitting shoulder pain, radiographs showing no significant degenerative joint disease, magnetic resonance imaging confirming a large (3-5 cm) full-thickness rotator cuff tear with retraction, and history of prior rotator cuff repair on the affected shoulder without associated biceps tenodesis. The intraoperative inclusion criterion was direct visualization of a 3- to 5-cm full-thickness rotator cuff tear with retraction of at least 3 cm. Validated Constant, American Shoulder and Elbow Surgeons (ASES), and University of California Los Angeles (UCLA) shoulder scoring systems were used to collect range-of-motion, pain, strength, daily function, and patient satisfaction data before and after surgery. Standard error was calculated. Two-sample t test was used for preoperative–postoperative comparisons. Postoperative integrity of the rotator cuff reconstruction was evaluated by an independent full-time academic musculoskeletal radiologist using dynamic diagnostic ultrasound (iU22 xMatrix Ultrasound System [Philips Healthcare] at L 9-3 MHz). Informed consent was obtained from each patient. The study was approved by institutional review board.
After induction of general anesthesia, each patient was placed in the lateral decubitus position. Bone marrow (60 mL) was aspirated through a 14-gauge needle from a dorsal iliac table, just inferior to the iliac crest (Figure 1). The patient was then placed into the beach-chair position on a surgical shoulder table. The aspirated marrow was centrifuged at 2800 and 3800 rpm for 14 to 17 minutes (Magellan Autologous Platelet Separator; Arteriocyte Medical Systems) to yield 10 mL of a concentrated (4- to 5-fold) mixture of platelet-rich plasma (PRP) and mesenchymal stem cells. Surgery was performed through a 3-cm oblique anterior mini-open incision between the anterior corner of the acromion and the coracoid process, as I previously described.4 The deltoid muscle was split, not detached. Acromioplasty and release of the coracoacromial ligament were performed. The rotator cuff was inspected under ×4.5 optical magnification. The cuff tissue was mobilized and débrided back to a healthy-appearing margin. The size and shape of the rotator cuff defect were then estimated. The long head of the biceps was harvested from its origin just distal to the superior glenoid labrum unto the intertubercular sulcus on the proximal humerus. The remainder of the biceps tendon was tenodesed to the surgical neck of the humerus. The biceps tendon graft was then manipulated and fashioned (by longitudinal partial-thickness incision and expansion) to fit the cuff defect (Figures 2, 3). The expanded graft was incubated in the concentrated marrow (10 mL) for 60 minutes (Figure 4). Débridement at the base of the greater tuberosity down to bleeding cancellous bone was followed by insertion of multiple bone anchors bearing several strands of No. 2 synthetic suture. These strands were then passed through the biceps tendon graft for secure fixation (Figure 5). The débrided end of the rotator cuff was then sewn to the biceps tendon graft using locking stitches under zero tissue tension with the arm in full adduction. The free end of the graft was sewn to the subscapularis tendon (Figure 6). The remaining marrow concentrate was injected both deep and superficial to the rotator cuff construct. No additional wound irrigation fluid was injected or suction drain inserted. After surgery, the patient was placed into an abduction pillow for 1 month and then engaged in passive motion for 1 month. Active-assisted motion began 3 months after surgery.
Results
Clinically, all patients improved with respect to pain, motion, strength, function, and satisfaction by virtue of the reconstructive surgery. After surgery, mean Constant score was increased, from 13 to 71 (P < .001). Mean ASES score increased from 18 to 75 (P < .001). Mean UCLA score increased from 4 to 28 (P < .001) (Table). Ultrasound showed 0% incidence of full-thickness retearing. Dynamic scanning during abduction showed maintained reduction of the humeral head within the glenoid socket; superior subluxation of the humeral head was not detected. The biceps tendon graft was continuous with the rotator cuff tendon, indicative of graft integration: tissue healing at the graft–bone and graft–tendon interfaces (Figures 7, 8). There were no intraoperative or postoperative patient-related complications.
Discussion
Primary rotator cuff surgery is beneficial.5 Irrespective of technique, open versus arthroscopic,6 single- versus double-row repair,7 the clinical results have been satisfactory.8 Nevertheless, the “tissue failure” rate of rotator cuff surgery (full-thickness discontinuity of rotator cuff) has been as high as 31% in primary repairs.9 In revision rotator cuff repair and reconstruction, the radiographic tissue failure rate has been even higher, particularly in the setting of chronic large tears with retraction, with tissue failure rates up to 91%.10 Although small to medium full-thickness tears and retears are well tolerated by patients with reduced activity levels,11 and pain symptoms do not necessarily correlate with rotator cuff tear size,12 large retracted full-thickness tears in active patients seldom result in optimal clinical outcomes or patient satisfaction.13,14 In addition, although recurrent tearing does not preclude a satisfactory clinical result, maintenance of cuff tissue integrity tends to produce a better objective clinical score and a more desirable clinical outcome.2
Few evidence-based restorative solutions exist for large recurrent rotator cuff tears with retraction in active nongeriatric patients.15 The no-treatment option in this context may result in gradual enlargement of the tear, chronic pain, weakness, and progressive degeneration of the glenohumeral joint and acromiohumeral confluence—so-called rotator cuff arthropathy, for which reverse total shoulder arthroplasty is required.16,17 Partial repair of a large rotator cuff tear by margin convergence, interval slide, split deltoid flap, or nonanatomical reinsertion may improve clinical outcome scores but may not alter or prevent the progressive degenerative changes associated with rotator cuff arthropathy.18,19 Synthetic scaffolds with and without biological enhancement have been used with varying degrees of success, particularly pain improvement and tissue integration.20 Nevertheless, the failure rate has been reported to be 17% to 51%,21 and no evidence exists that allograft augmentation improves functional outcomes.22 Tendon transfer using the latissimus dorsi has also proved to be a surgical alternative in younger, active patients.23 However, dissection in this procedure is a major undertaking for both surgeon and patient—compared with the minimally invasive technique used in the present study.24
I selected a cohort of active, symptomatic patients for application of a synthesis of accepted surgical techniques through a mini-open incision in order to improve the reliability of the surgical construct while minimizing surgical morbidity. Débridement of marginal tissue, safe mobilization of remaining cuff, and tension-free suture line using locking sutures maximized the mechanical strength of the construct.25,26 Biological enhancement with autogenous tissue (the patient’s own biceps tendon) as graft material (scaffolding), as well as autologous concentrated marrow delivering viable responding cells and chemokine/cytokine biofactors, increased the probability of reparative activity at the graft site.27 The net effect was consistent tissue healing at a biologically challenging locus. Nonenhanced biceps tendon grafting in the setting of “irreparable” primary rotator cuff repair has had a 40-year history of orthopedic utility and an excellent record of clinical success.28 Nevertheless, the retear rate has been 7% to 30%.29 There are no previous reports of biologically enhanced autogenous biceps tendon grafting for reconstruction of a torn rotator cuff, either primary or in the setting of chronic revision surgery.
Previous well-designed PRP enhancement studies in the context of primary rotator cuff repair failed to demonstrate a consistent benefit with concentrated platelet-only augmentation.30,31 The shared experimental design of these published studies used intra-articular injection as the sole delivery method without guarantee that the injected platelets would migrate, adhere to, and persist at the intended destination, the healing edge of the rotator cuff. In the present study, extended exposure of the splayed tendon graft by incubation in concentrated marrow was specifically designed to increase the probability that biologically active components would settle at the desired location by cellular seeding and plasmatic imbibition.32 Furthermore, use of PRP for growth factor (platelet-derived, PDGF; basic fibroblast, bFGF; transforming, TGF-β; epidermal, EGF; vascular endothelial, VEGF; connective tissue, CTGF) therapy, in addition to pluripotential mesenchymal cells for marrow-derived stem cell therapy, is in theory biologically superior to use of PRP alone.33,34
The recent expansion of information about biologics has generated much interest in augmentation of soft-tissue healing. Unfortunately, the optimal technique of using cellular processing to upregulate stem-cell capacity at the graft interface is yet to be defined.35 Clinical studies using PRP and related products to promote tendon healing have been both inconsistent and contradictory with respect to benefit of outcome. As we have been unable to harness the biological potential of this medium, application of biologics in contemporary clinical orthopedics remains narrow, random, and infrequent. The technique presented in this clinical series appears to be a small advancement in a positive direction. The described construct provides a starting point for study, combining mechanical as well as biological steps to promote rotator cuff healing. The consistency of the outcome in a clinical model in which retearing is an expectation rather than an exception is noteworthy. The zero tissue failure rate at 1 to 4 years, compared with the literature values in similar patient cohorts, is very promising.36 The clinical outcome as measured by validated shoulder scores is also comparable to literature outcome values.19 Also noteworthy is the dynamic stability the construct gives to the glenohumeral joint. Ideally, the reconstructed rotator cuff provides active force coupling with the deltoid, simulating normal shoulder biomechanics. At a minimum, the reconstructed cuff provides a viable passive barrier to superior migration of the humeral head—thus supporting the mechanical efficiency of the deltoid and preventing rotator cuff arthropathy.
This study’s small sample (10 patients) puts its conclusions at risk for type I statistical error, in that too few patients were examined over a long enough period to demonstrate failure. Nevertheless, retears typically occur within 6 months of repair.37,38 Therefore, minimum follow-up of 1 year was deemed sufficient. None of the 10 patients had diabetes or another chronic comorbidity. Nine of the 10 had either no or only mild preoperative fatty atrophy of the rotator cuff muscles. Eight of the 10 were nonsmokers. These factors, which suggest optimal surgical candidates, may prove to be significant as the clinical series expands over time. Incubation of the autogenous biceps graft in concentrated marrow for 60 minutes was arbitrarily chosen. In future in vitro examination, marrow cell viability as a function of incubation time will be assessed.
Conclusion
In active, middle-aged patients with chronic recurrent large retracted rotator cuff tears, the technique presented here, using autogenous biceps tendon and autologous concentrated marrow containing PRP and mesenchymal cells, consistently yielded satisfactory clinical results and promoted rotator cuff tissue healing without full-thickness retearing.
1. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
2. Kim HM, Caldwell JM, Buza JA, et al. Factors affecting satisfaction and shoulder function in patients with a recurrent rotator cuff tear. J Bone Joint Surg Am. 2014;96(2):106-112.
3. George MS, Khazzam M. Current concepts review: revision rotator cuff repair. J Shoulder Elbow Surg. 2012;21(4):431-440.
4. Skoff HD. Conservative open acromioplasty. J Bone Joint Surg Br. 1995;77(6):933-936.
5. Mather RC 3rd, Koenig L, Acevedo D, et al. The societal and economic value of rotator cuff repair. J Bone Joint Surg Am. 2013;95(22):1993-2000.
6. Sauerbrey AM, Getz CL, Piancastelli M, Iannotti JP, Ramsey ML, Williams GR. Arthroscopic versus mini-open rotator cuff repair: a comparison of clinical outcome. Arthroscopy. 2005;21(12):1415-1420.
7. Koh KH, Kang KC, Lim TK, Shon MS, Yoo JC. Prospective randomized clinical trial of single- versus double-row suture anchor repair in 2- to 4-cm rotator cuff tears: clinical and magnetic resonance imaging results. Arthroscopy. 2011;27(4):453-462.
8. Galatz LM, Griggs S, Cameron BD, Iannotti JP. Prospective longitudinal analysis of post-operative shoulder function: a ten-year follow-up study of full thickness rotator cuff tears. J Bone Joint Surg Am. 2001;83(7):1052-1056.
9. Oh JH, Kim SH, Kang JY, Oh CH, Gong HS. Effect of age on functional and structural outcome after rotator cuff repair. Am J Sports Med. 2010;38(4):672-678.
10. Kim JH, Kim SH, Lee SK, Seo JW, Chun YMC. Arthroscopic repair of massive contracted rotator cuff tears: aggressive release with anterior and posterior interval slides do not improve cuff healing and integrity. J Bone Joint Surg Am. 2014;95(16):1482-1488.
11. Moosmayer S, Lund G, Seljom US, et al. Tendon repair compared with physiotherapy in the treatment of rotator cuff tears. J Bone Joint Surg Am. 2014;96(18):1504-1514.
12. Dunn WR, Kuhn JE, Sanders R, et al. Symptoms of pain do not correlate with rotator cuff tear severity. J Bone Joint Surg Am. 2014;96(10):793-800.
13. Lubiatowski P, Kaczmarek P, Dzianach M, et al. Clinical and biomechanical performance of patients with failed rotator cuff repair. Int Orthop. 2013;37(12):2395-2401.
14. Holtby R, Razmjou H. Relationship between clinical and surgical findings and reparability of large and massive rotator cuff tears: a longitudinal study. BMC Musculoskelet Disord. 2014;15:180.
15. Nho SJ, Delos D, Yadav H, et al. Biomechanical and biological augmentation for the treatment of massive rotator cuff tears. Am J Sports Med. 2010;38(3):619-629.
16. Moosmayer S, Tariq R, Stiris M, Smith HJ. The natural history of asymptomatic rotator cuff tears: a three-year follow-up of fifty cases. J Bone Joint Surg Am. 2013;95(14):1249-1255.
17. Neer CS 2nd, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65(9):1232-1244.
18. Bartl C, Louloumentas P, Konstantin H, et al. Long-term outcome and structural integrity following open repair of massive rotator cuff tears. Int J Shoulder Surg. 2012;6(1):1-8.
19. Paxton ES, Teefey SA, Dahiya N, Keener JD, Yamaguchi K, Galatz LM. Clinical and radiographic outcomes of failed repairs of large or massive rotator cuff tears: minimum ten-year follow-up. J Bone Joint Surg Am. 2013;95(7):627-632.
20. Longo UG, Lamberti A, Maffulli N, Denaro V. Tendon augmentation grafts: a systematic review. Br Med Bull. 2010;94:165-188.
21. Ciampi P, Scotti C, Nonis A, et al. The benefit of synthetic versus biological patch augmentation in the repair of posterosuperior massive rotator cuff tears: a 3-year follow-up study. Am J Sports Med. 2014;42(5):1169-1175.
22. Murhi AM. Rotator cuff tears and cuff tear arthropathy. In: Boyer MI, ed. AAOS Comprehensive Orthopedic Review. Vol 2. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2014:921-929.
23. Namdari S, Voleti P, Baldwin K, Glaser D, Huffman GR. Latissimus dorsi tendon transfer for irreparable rotator cuff tears: a systematic review. J Bone Joint Surg Am. 2012;94(10):891-898.
24. Gerber C, Rahm SA, Catanzaro S, Farshad M, Moor BK. Latissimus dorsi tendon transfer for treatment of irreparable posterosuperior rotator cuff tears: long-term results at a minimum follow-up of ten years. J Bone Joint Surg Am. 2013;95(21):1920-1926.
25. Wagner JP, Krushall RJ, Masqueloet A, Gerber C. Anatomy and relationships of the suprascapular nerve: anatomical constraints to mobilization of the supraspinatus and infraspinatus muscles in the management of massive rotator cuff tears. J Bone Joint Surg Am. 1992;74(1):36-45.
26. Ponce BA, Hosemann CD, Reghava P, Tate JP, Sheppard ED, Ebenhardt AW. A biomechanical analysis of controllable intraoperative variables affecting the strength of rotator cuff repairs at the suture–tendon interface. Am J Sports Med. 2013;41(10):2256-2261.
27. Thomopoulos S. Tendon and ligaments. In: Boyer MI, ed. AAOS Comprehensive Orthopedic Review. Vol 2. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2014:105-111.
28. Sano H, Mineta M, Kitz A, Itoi E. Tendon patch grafting using the long head of the biceps for irreparable massive rotator cuff tears. J Orthop Sci. 2010;15(3):310-316.
29. Rhee YG, Cho NS, Lim CT, Yi JW, Vishvanathan T. Bridging the gap in immobile massive rotator cuff tears: augmentation using the tenotomized biceps. Am J Sports Med. 2008;36(8):1511-1518.
30. Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39(2):258-265.
31. Rodeo SA, Delos, D, Williams, RJ, Adler RS, Pearle A, Warren RF. The effects of platelet-rich fibrin matrix on rotator cuff tendon healing: a prospective, randomized clinical study. Am J Sports Med. 2012;40(6):1234-1241.
32. Beitzel K, McCarthy MB, Cote MP, et al. Properties of biologic scaffolds and their response to mesenchymal stem cells. Arthroscopy. 2014;30(3):289-298.
33. Anz AW, Hackel JG, Nilssen ED, Andrews JR. Application of biologics in the treatment of rotator cuff, meniscus, cartilage and osteoarthritis. J Am Acad Orthop Surg. 2014;22(2):68-79.
34. Hernigou P, Flouzat Lachaniette CH, Delambre J, et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int Orthop. 2014;38(9):1811-1818.
35. Hsu WK, Mishra A, Rodeo SR, et al. Platelet-rich plasma in orthopaedic applications: evidence-based recommendations for treatment. J Am Acad Orthop Surg. 2013;21(12):739-748.
36. Kowalsky MS, Keener JD. Revision arthroscopic rotator cuff repair: repair integrity and clinical outcome: surgical technique. J Bone Joint Surg Am. 2011;93(suppl 1):62-74.
37. Iannotti JP, Deutsch A, Green A, et al. Time to failure after rotator cuff repair: a prospective imaging study. J Bone Joint Surg Am. 2013;95(11):965-971.
38. Le BT, Wu XL, Lam PH, Murrell GA. Factors predicting rotator cuff retears: an analysis of 1000 consecutive rotator cuff repairs. Am J Sports Med. 2014;42(5):1134-1142.
1. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
2. Kim HM, Caldwell JM, Buza JA, et al. Factors affecting satisfaction and shoulder function in patients with a recurrent rotator cuff tear. J Bone Joint Surg Am. 2014;96(2):106-112.
3. George MS, Khazzam M. Current concepts review: revision rotator cuff repair. J Shoulder Elbow Surg. 2012;21(4):431-440.
4. Skoff HD. Conservative open acromioplasty. J Bone Joint Surg Br. 1995;77(6):933-936.
5. Mather RC 3rd, Koenig L, Acevedo D, et al. The societal and economic value of rotator cuff repair. J Bone Joint Surg Am. 2013;95(22):1993-2000.
6. Sauerbrey AM, Getz CL, Piancastelli M, Iannotti JP, Ramsey ML, Williams GR. Arthroscopic versus mini-open rotator cuff repair: a comparison of clinical outcome. Arthroscopy. 2005;21(12):1415-1420.
7. Koh KH, Kang KC, Lim TK, Shon MS, Yoo JC. Prospective randomized clinical trial of single- versus double-row suture anchor repair in 2- to 4-cm rotator cuff tears: clinical and magnetic resonance imaging results. Arthroscopy. 2011;27(4):453-462.
8. Galatz LM, Griggs S, Cameron BD, Iannotti JP. Prospective longitudinal analysis of post-operative shoulder function: a ten-year follow-up study of full thickness rotator cuff tears. J Bone Joint Surg Am. 2001;83(7):1052-1056.
9. Oh JH, Kim SH, Kang JY, Oh CH, Gong HS. Effect of age on functional and structural outcome after rotator cuff repair. Am J Sports Med. 2010;38(4):672-678.
10. Kim JH, Kim SH, Lee SK, Seo JW, Chun YMC. Arthroscopic repair of massive contracted rotator cuff tears: aggressive release with anterior and posterior interval slides do not improve cuff healing and integrity. J Bone Joint Surg Am. 2014;95(16):1482-1488.
11. Moosmayer S, Lund G, Seljom US, et al. Tendon repair compared with physiotherapy in the treatment of rotator cuff tears. J Bone Joint Surg Am. 2014;96(18):1504-1514.
12. Dunn WR, Kuhn JE, Sanders R, et al. Symptoms of pain do not correlate with rotator cuff tear severity. J Bone Joint Surg Am. 2014;96(10):793-800.
13. Lubiatowski P, Kaczmarek P, Dzianach M, et al. Clinical and biomechanical performance of patients with failed rotator cuff repair. Int Orthop. 2013;37(12):2395-2401.
14. Holtby R, Razmjou H. Relationship between clinical and surgical findings and reparability of large and massive rotator cuff tears: a longitudinal study. BMC Musculoskelet Disord. 2014;15:180.
15. Nho SJ, Delos D, Yadav H, et al. Biomechanical and biological augmentation for the treatment of massive rotator cuff tears. Am J Sports Med. 2010;38(3):619-629.
16. Moosmayer S, Tariq R, Stiris M, Smith HJ. The natural history of asymptomatic rotator cuff tears: a three-year follow-up of fifty cases. J Bone Joint Surg Am. 2013;95(14):1249-1255.
17. Neer CS 2nd, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65(9):1232-1244.
18. Bartl C, Louloumentas P, Konstantin H, et al. Long-term outcome and structural integrity following open repair of massive rotator cuff tears. Int J Shoulder Surg. 2012;6(1):1-8.
19. Paxton ES, Teefey SA, Dahiya N, Keener JD, Yamaguchi K, Galatz LM. Clinical and radiographic outcomes of failed repairs of large or massive rotator cuff tears: minimum ten-year follow-up. J Bone Joint Surg Am. 2013;95(7):627-632.
20. Longo UG, Lamberti A, Maffulli N, Denaro V. Tendon augmentation grafts: a systematic review. Br Med Bull. 2010;94:165-188.
21. Ciampi P, Scotti C, Nonis A, et al. The benefit of synthetic versus biological patch augmentation in the repair of posterosuperior massive rotator cuff tears: a 3-year follow-up study. Am J Sports Med. 2014;42(5):1169-1175.
22. Murhi AM. Rotator cuff tears and cuff tear arthropathy. In: Boyer MI, ed. AAOS Comprehensive Orthopedic Review. Vol 2. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2014:921-929.
23. Namdari S, Voleti P, Baldwin K, Glaser D, Huffman GR. Latissimus dorsi tendon transfer for irreparable rotator cuff tears: a systematic review. J Bone Joint Surg Am. 2012;94(10):891-898.
24. Gerber C, Rahm SA, Catanzaro S, Farshad M, Moor BK. Latissimus dorsi tendon transfer for treatment of irreparable posterosuperior rotator cuff tears: long-term results at a minimum follow-up of ten years. J Bone Joint Surg Am. 2013;95(21):1920-1926.
25. Wagner JP, Krushall RJ, Masqueloet A, Gerber C. Anatomy and relationships of the suprascapular nerve: anatomical constraints to mobilization of the supraspinatus and infraspinatus muscles in the management of massive rotator cuff tears. J Bone Joint Surg Am. 1992;74(1):36-45.
26. Ponce BA, Hosemann CD, Reghava P, Tate JP, Sheppard ED, Ebenhardt AW. A biomechanical analysis of controllable intraoperative variables affecting the strength of rotator cuff repairs at the suture–tendon interface. Am J Sports Med. 2013;41(10):2256-2261.
27. Thomopoulos S. Tendon and ligaments. In: Boyer MI, ed. AAOS Comprehensive Orthopedic Review. Vol 2. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2014:105-111.
28. Sano H, Mineta M, Kitz A, Itoi E. Tendon patch grafting using the long head of the biceps for irreparable massive rotator cuff tears. J Orthop Sci. 2010;15(3):310-316.
29. Rhee YG, Cho NS, Lim CT, Yi JW, Vishvanathan T. Bridging the gap in immobile massive rotator cuff tears: augmentation using the tenotomized biceps. Am J Sports Med. 2008;36(8):1511-1518.
30. Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39(2):258-265.
31. Rodeo SA, Delos, D, Williams, RJ, Adler RS, Pearle A, Warren RF. The effects of platelet-rich fibrin matrix on rotator cuff tendon healing: a prospective, randomized clinical study. Am J Sports Med. 2012;40(6):1234-1241.
32. Beitzel K, McCarthy MB, Cote MP, et al. Properties of biologic scaffolds and their response to mesenchymal stem cells. Arthroscopy. 2014;30(3):289-298.
33. Anz AW, Hackel JG, Nilssen ED, Andrews JR. Application of biologics in the treatment of rotator cuff, meniscus, cartilage and osteoarthritis. J Am Acad Orthop Surg. 2014;22(2):68-79.
34. Hernigou P, Flouzat Lachaniette CH, Delambre J, et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int Orthop. 2014;38(9):1811-1818.
35. Hsu WK, Mishra A, Rodeo SR, et al. Platelet-rich plasma in orthopaedic applications: evidence-based recommendations for treatment. J Am Acad Orthop Surg. 2013;21(12):739-748.
36. Kowalsky MS, Keener JD. Revision arthroscopic rotator cuff repair: repair integrity and clinical outcome: surgical technique. J Bone Joint Surg Am. 2011;93(suppl 1):62-74.
37. Iannotti JP, Deutsch A, Green A, et al. Time to failure after rotator cuff repair: a prospective imaging study. J Bone Joint Surg Am. 2013;95(11):965-971.
38. Le BT, Wu XL, Lam PH, Murrell GA. Factors predicting rotator cuff retears: an analysis of 1000 consecutive rotator cuff repairs. Am J Sports Med. 2014;42(5):1134-1142.
Reducing Postoperative Fracture Displacement After Locked Plating of Proximal Humerus Fractures: Current Concepts
Proximal humerus fractures account for 4% to 5% of all fractures.1 These fractures occur most frequently in the elderly—patients older than 60 years sustain 71% of these injuries2—and in females.1,3 Given an aging population, this incidence is predicted to increase 3-fold over the next 30 years.4 There is much debate regarding management of acute, displaced proximal humerus fractures. A recent Cochrane Review of published outcomes of operative and nonoperative treatment of displaced proximal humerus fractures found insufficient evidence supporting either modality, though surgery was associated with additional procedures.5 A review of 1000 proximal humerus fractures found that 49% had less than 1 cm of displacement of the major fragments or angulation of less than 45°.3 Other authors have reported similar findings.6,7 Although the incidence of proximal humerus fractures has remained stable over the past decade, from 1999 to 2005 there was a 25% relative increase in surgical management, including a relative increase of 29% in open reduction and internal fixation (ORIF) versus a 20% increase in arthroplasty.1
Locking plates have consistently demonstrated biomechanical superiority over other forms of fixation in osteoporotic bone.8-11 Egol and colleagues8 found that osteoporotic bone limited the torque of fixation to values less than what is required for adequate frictional force between the plate and the bone. This problem can be overcome with fixed-angle devices, such as locked plates.9 Compared with locked nail constructs, proximal humerus locking plates have demonstrated superiority in torsion, loading, and varus bending.10,11 Compared with blade plates, proximal humerus locking plates exhibited increased stiffness and torsional fatigue resistance.12 In a randomized clinical trial, Olerud and colleagues13 reported superior functional results with locking plate fixation compared with nonoperative treatment of displaced 3-part fractures in elderly patients with 2-year follow-up, though these clinical results were not supported by others.14 Two recent case–control studies comparing functional outcomes for 3- and 4-part fractures with follow-up of more than 2 years revealed higher Constant scores after locked plating compared with hemiarthroplasty, though complications were higher with locked plates.15,16 Adoption of locked proximal humerus plating has been correlated with good clinical outcomes and union rates, though this has been accompanied by a higher rate of reoperation.7 Reoperation rates from 1999 to 2005 increased both in the immediate postoperative period (odds ratio, 3.36) and at 1 year (odds ratio, 3.90).1
Complications of Locked Plating
Regardless of fixation type, reduced humeral head bone mass and quality may lead to implant loosening, fracture redisplacement, and, ultimately, poor outcomes. Baseline osteoporosis may predict likelihood of fixation failure.17 Multiple studies have reported on the implant-related complications associated with locking plate fixation—most commonly, intra-articular screw penetration, postoperative fracture displacement, and avascular necrosis (AVN)18-24 (Figure 1). A meta-analysis of 12 studies with a total of 514 proximal humerus fractures treated with locking plate fixation showed an overall complication rate of 49% and a 13.8% reoperation rate.25 The most common indication for reoperation involved intra-articular screw perforation. The most common complications were varus malunion (16%), osteonecrosis (10%), intra-articular screw penetration (8%), subacromial impingement (6%), and infection (4%).
Suboptimal intraoperative fracture reduction, specifically with residual varus, has been correlated with loss of fracture fixation. In a series of 153 fractures, loss of fixation occurred in 13.7% of cases, with the leading risk factor being varus malreduction.19 Failure rates were 30.4% and 11% when the head shaft angle was less than 120° and when it was 120° or more, respectively. Solberg and colleagues16 found that initial postoperative varus angulation of more than 20° resulted in universal loss of fixation. Conversion of these cases to hemiarthroplasty resulted in poor outcomes. Preoperative fracture alignment may also predict fixation failure.22 In one series, initial varus angulation healed with a mean 16° varus and a Constant score of 63, whereas initial valgus alignment healed with 6° varus and a Constant score of 71.22 Complications occurred in fractures that were initially in varus 79% of the time and initially in valgus 19% of the time. Screw perforation has been associated with loss of reduction 44% of the time.20
In an analysis of locking plate constructs revised after early (<4 weeks) failure in 8 patients with osteoporosis, Micic and colleagues21 found implant pullout leading to varus malalignment. All cases lacked medial support and subchondral screw purchase; 3 were initially malreduced. Owsley and Gorczyca23 retrospectively reviewed 53 cases of displaced proximal humerus fractures treated with locked plating. Despite the high rate of radiographic union, 36% developed complications, including screw cutout (23%), varus displacement of more than 10° (25%), and AVN (4%); 13% required revision. These complications disproportionately affected patients older than 60 years (57%) and negatively affected functional outcomes.
Augmentation Techniques
Despite its reported complications, proximal humerus locked plating remains the most widely used type of fixation.1 Advancements in locking plate design, improved understanding of fixation principles, and adoption of techniques augmenting proximal humerus locking plate fixation, particularly in osteoporotic bone, have reduced postoperative complications (Table 1).
Rotator Cuff Sutures
A widely adopted technique for neutralizing rotator cuff–deforming forces, which theoretically can cause fracture displacement, is incorporation of heavy nonabsorbable sutures. These sutures are placed through the rotator cuff–tuberosity junction and tied down after being passed through the plate. Obtaining and maintaining tuberosity reduction are essential in achieving good functional outcomes after fixation. In addition, tension band sutures may be particularly useful in the setting of initial varus deformity.26
Although clinical use of these sutures is common, biomechanical studies of their adjunctive contribution to fracture stability are lacking.27 The rotator cuff musculature has a maximal contractile force of 3.5 kg/cm2.28 Ricchetti and colleagues29 described a technique that involves using a locked plate and tagging the rotator cuff with heavy nonabsorbable sutures. Selective traction on the sutures can help obtain and maintain fracture reduction. Multiple studies have reported on suture use with locked plating for proximal humerus fractures.29-34 Badman and colleagues30 retrospectively reviewed 81 cases of metaphyseal defects or medial comminution treated with locked plating, rotator cuff sutures, and structural allograft. All cases healed within 6 months after surgery. The incidence of screw cutout was 3.7%, the incidence of AVN was 6.2%, and the incidence of varus collapse was 6%. A cadaveric study that used specimens (mean age, 77 years) with a simulated 3-part proximal humerus fracture treated with a locked plate both with and without cerclage sutures found no difference in interfragmentary motion between the groups.27 The authors concluded that additive sutures are not required for anatomically reduced fractures. Multiple sutures may counteract the deforming forces that act on bony segments that cannot be adequately maintained with screws, such as an osteoporotic greater tuberosity.
Medial Column Restoration
The importance of reducing and maintaining the medial calcar to provide biomechanical support for a laterally placed plate has been recognized.26,34-37 Gardner and colleagues26 suggested that medial support was achieved if the medial cortex was anatomically reduced, if the proximal fragment was impacted laterally onto the shaft, or if 1 or more inferomedial screws were placed. Cases that did not achieve medial support developed significantly more humeral head subsidence (5.8 mm vs 1.2 mm) and screw penetration. Krappinger and colleagues36 found that factors leading to fixation failure included age, local bone mineral density, anatomical reduction, and restoration of the medial cortical support. The authors concluded that anatomical reduction and restoration of the medial cortex were important in minimizing mechanical loads at the bone–implant interface. Biomechanically, Lescheid and colleagues37 found that the most stable construct was anatomical reduction with medial cortical contact. In the setting of comminution, however, it may be preferable to intentionally perform varus malreduction to achieve medial contact than to achieve anatomical reduction with a fracture gap. Badman and colleagues30 found that the incidence of screw penetration was 6% in patients with an intact medial calcar versus 29% in patients without medial support. In a retrospective analysis of patients treated with a locking plate and suture augmentation, Jung and colleagues35 concluded that restoring medial support was the most reliable factor in the prevention of loss of reduction with or without screw perforation. Last, Solberg and colleagues16 reported better clinical outcomes when the length of the metaphyseal segment attached to the articular fragment was more than 2 mm. A length of less than 2 mm was predictive of developing AVN.
Use of Bone Void Fillers
Allograft. Allograft is cancellous or corticocancellous chips or tricortical graft used as osteoconductive filler for metaphyseal defects.38 An increasingly popular technique involves using an endosteal fibular allograft strut to indirectly reduce the fracture and help support the medial calcar.39-42 Hettrich and colleagues40 reported on radiographic outcomes of displaced proximal humerus fractures with medial comminution treated with a locked plate and an endosteal fibular allograft or semitubular plate. The reduction was maintained in 96% of cases; there was 1 varus collapse. There were no cases of implant failure, screw perforation, or AVN. Other authors have also reported on successful use of fibular allograft in conjunction with a locked plate; the rate of reduction loss was low, and there were no cases of screw cutout or intra-articular screw penetration.30,41,42 These clinical outcomes are supported by results of biomechanical studies of the added benefit of intramedullary fibular allograft.43-46 Mathison and colleagues43 reported that a construct with fibular allograft and a locking plate increased the failure load by 1.72 times and the stiffness by 3.84 times compared with a control group of locking plate only. Bae and colleagues46 found significantly higher maximum failure load and construct stiffness with no varus collapse in specimens prepared with locked plate and fibular strut augmentation compared with a control group.
Others have successfully used cancellous allograft to fill humeral head bone defects.29,32,47-49 Duralde and Leddy47 reported 100% radiographic union and 81% good to excellent results in cases treated with a locking plate and morselized cancellous allograft to fill bone voids. Varus collapse and screw cutout did not occur, but there were 2 cases of AVN. Ricchetti and colleagues29 reviewed 54 cases treated with a locking plate and rotator cuff suture construct. Allograft cancellous chips and demineralized bone matrix were used in 3- and 4-part fractures (70% of cases) along with shorter screws in the humeral head. Major complications included AVN (1), fixation failure (3), and varus malunion (5). Others investigators have had less favorable results with use of cancellous bone graft. Schliemann and colleagues19 reported on 27 patients who were older than 65 years when they underwent ORIF with rotator cuff sutures to stabilize the tuberosities and either cancellous graft or a synthetic bone substitute in patients with massive metaphyseal defects. Patient-reported outcomes were superior to Constant scores. Complications included screw penetration (22.2%), reduction loss (44.4%), implant failure (3.7%), and AVN (29.6%).
Autograft. Autograft has both osteoconductive and osteoinductive properties and has been successfully used for metaphyseal defects.32,50 Kim and colleagues50 reported on patients with 4-part proximal humerus fractures treated with a locking plate and autologous iliac graft. All cases achieved union and had good or excellent outcomes. There were no cases of AVN, varus collapse, or hardware-related complications.
Bone Cement. Calcium phosphate cement has osteoconductive properties and enhances screw purchase in cancellous bone (Figures 2A–2F). It can be injected or molded into bone voids to provide improved compressive strength. It is resorbed through cell-mediated processes resembling bone remodeling and does not disappear until new bone forms. (Calcium sulfate cement, on the other hand, resorbs through a chemical process independent of new bone formation.51) Egol and colleagues52 reviewed the cases of 92 patients (mean age, 61 years) with 2-, 3-, and 4-part proximal humerus fractures treated with locked plate fixation. Metaphyseal defects were treated with no augmentation, augmentation with cancellous chips, or augmentation with calcium phosphate cement. Adding calcium phosphate cement was associated with lower incidence of intra-articular screw penetration and humeral head settling. In a recent cadaveric biomechanical study using 2-part proximal humerus fractures with metaphyseal comminution, the group augmented with calcium phosphate cement had enhanced axial stiffness and load to failure with reduced screw penetration.53 Other biomechanical studies have found increased screw pullout strength54 and decreased interfragmentary motion when specimens were augmented with calcium phosphate cement.55
Similar good clinical and radiographic outcomes have been observed with use of calcium sulfate cement.56,57 Somasundaram and colleagues56 reported good clinical outcomes in 82% of patients treated with locking plates and calcium sulfate cement used to fill metaphyseal voids. All fractures united without infection, fixation failure, subsequent malunion, tuberosity failure, or AVN. Lee and Shin57 compared outcomes of 14 patients who received calcium sulfate augmentation with outcomes of patients who did not receive this augmentation. Overall, 89% of patients had good or excellent results. Calcium sulfate cement did not affect the reduction failure rate or clinical outcomes in cases in which medial cortical reduction was achieved. However, postoperative displacement caused by lack of medial support was associated with poor outcomes.
Screw Placement
Screws optimally should be placed in the posterior-medial-inferior aspect of the humeral head to provide medial support for the fracture and mechanical stability.58 Cadaveric studies have shown the highest cancellous bone density in the proximal, posterior, and medial portions of the humeral head.59-63 Similarly, in a cadaveric study, Liew and colleagues61 found greater screw purchase and higher pullout strength when the screw was placed in the center of the humeral head, within subchondral bone; fixation was poorest when the screw was placed in the anterosuperior region of the humeral head. Tingart and colleagues62 reported that humeral head trabecular density significantly affected pullout strength of cancellous screws. In addition, the most pullout strength was at the center of the head, and the least within the anterosuperior head. Trabecular density was higher in the inferior and posterior regions than in the superior and anterior regions.
Most locking plate designs allow screws to be placed at the level of the medial calcar—the goal being to provide medial column support (Table 2). Zhang and colleagues58 treated 2-, 3-, and 4-part fractures with a locking plate and randomized them into receiving the plate with or without medial support screws. For 3- and 4-part fractures, the group with these screws had a significantly greater final neck-shaft angle and smaller angulation loss compared with the group without screws. No additional benefit was found for 2-part fractures. Erhardt and colleagues63 simulated unstable proximal humerus fractures using cadavers and testing different fixation methods using a polyaxial locking plate. They found that 5 screws in the head fragment and an inferomedial support screw significantly reduced the risk of screw perforation. Other authors have concluded that placing 1 or more inferomedial screws is important in cases of medial comminution or medial column malreduction.26 Interestingly, compared with use of a polyaxial implant, which allows for adjustment of screw direction, use of a monoaxial locking plate did not lead to a clinically different outcome or complication profile.64
Techniques have been used to achieve subchondral purchase of locking screws while reducing iatrogenic articular perforation.65 However, given the incidence of fracture settling and subsequent postoperative screw penetration, many authors currently recommend using shorter divergent screws combined with other augmentation techniques, described previously.17,29,32
Physical Therapy
There is no standardized physiotherapy regimen for postoperative management of proximal humerus fractures treated with locking plates.25 In older patients, immediate active range of motion (ROM) exercises should be delayed until early callus is noted, though there is a risk for stiffness. Lee and Shin57 found that a delay in rehabilitation after ORIF was an independent risk for poor clinical outcome. Namdari and colleagues17 recommended sling use only for comfort and initiated non-load-bearing activities and pendulum exercises immediately after surgery. Patients with adequate reduction at 4 to 6 weeks were advanced to full weight-bearing. Badman and colleagues30 initiated passive-assisted ROM exercises when the wound was healed at 2 weeks in 2-part fractures, whereas patients with 3- and 4-part fractures were immobilized until radiographic healing. Formal therapy was started after 6 weeks. Stiffness was reported in 5% of patients. For patients with stable fixation, Ricchetti and colleagues29 recommended passive shoulder ROM exercises on postoperative day 1; at 4 to 6 weeks, patients should start active shoulder ROM exercises, and then resistance exercises at 10 to 12 weeks. Other authors are more conservative—only sling immobilization and pendulum exercises the first month.66 Barlow and colleagues32 immobilized their patients (age, >75 years) for 6 weeks. No patient developed disabling stiffness. The authors suggested that patients older than 75 years may not be prone to stiffness.
Our Preferred Treatment Method
All proximal humerus fractures are approached anteriorly through the deltopectoral interval (Figure 3A). The long head biceps is identified and truncated for later tenodesis. Multiple No. 5 Ethibond sutures (Ethicon) are placed at the bone–tendon interface. The fracture is reduced with a Cobb elevator (Figure 3B), and provisional Kirschner wires are placed within the head (Figure 3C). The plate is affixed to the humeral head with its anterior border paralleling the posterior aspect of the bicipital groove. Multiple locking screws are placed within the superior and posterior humeral head. Nonlocking screws are then used to fix the plate to the shaft to reduce the specific deformity. Under fluoroscopy, any metaphyseal void is filled with calcium phosphate cement (Figure 3D). The remaining inferior screws are placed within the humeral head. Dr. Gruson uses screws 4 to 6 mm short of subchondral bone to reduce the risk for joint penetration. The rotator cuff sutures are tied down through the plate. Patients are started on progressive supine passive ROM exercises at 7 days, followed by supine active-assisted ROM exercises 6 weeks after fracture healing is confirmed radiographically.
Conclusion
Use of locked plating for proximal humerus fractures has increased, particularly in the elderly. Resulting complications include intra-articular screw penetration, postoperative fracture displacement, and AVN. Recognition of the importance of reducing and supporting the medial calcar, filling any metaphyseal defects, and selectively placing screws within the humeral head has lowered the incidence of these complications. Further comparative studies evaluating the efficacy of individual augmentation techniques are needed to determine their contribution to successful fracture healing and their cost-effectiveness. Results of such studies may help in the development of protocols for more standardized implementation of these techniques and in understanding which specific fracture patterns and patients would benefit from their use.
1. Bell JE, Leung BC, Spratt KF, et al. Trends and variation in incidence, surgical treatment, and repeat surgery of proximal humeral fractures in the elderly. J Bone Joint Surg Am. 2011;93(2):121-131.
2. Aaron D, Shatsky J, Paredes JC, Jiang C, Parsons BO, Flatow EL. Proximal humeral fractures: internal fixation. J Bone Joint Surg Am. 2012;94(24):2280-2288.
3. Court-Brown CM, Garg A, McQueen MM. The epidemiology of proximal humeral fractures. Acta Orthop Scand. 2001;72(4):365-371.
4. Kannus P, Palvanen M, Niemi S, Parkkari J, Jarvinen M, Vuori I. Increasing number and incidence of osteoporotic fractures of the proximal humerus in elderly people. BMJ. 1996;313(7064):1051-1052.
5. Handoll HH, Ollivere BJ, Rollins KE. Interventions for treating proximal humeral fractures in adults. Cochrane Database Syst Rev. 2012;12:CD000434.
6. Tamai K, Ishige N, Kuroda S, et al. Four-segment classification of proximal humeral fractures revisited: a multicenter study on 509 cases. J Shoulder Elbow Surg. 2009;18(6):845-850.
7. Rothberg D, Higgins T. Fractures of the proximal humerus. Orthop Clin North Am. 2013;44(1):9-19.
8. Egol KA, Kubiak EN, Fulkerson E, Kummer FJ, Koval KJ. Biomechanics of locked plates and screws. J Orthop Trauma. 2004;18(8):488-493.
9. Miranda MA. Locking plate technology and its role in osteoporotic fractures. Injury. 2007;38(suppl 3):35-39.
10. Foruria AM, Carrascal MT, Revilla C, Munuera L, Sanchez-Sotelo J. Proximal humerus fracture rotational stability after fixation using a locking plate or a fixed-angle locked nail: the role of implant stiffness. Clin Biomech. 2010;25(4):307-311.
11. Weinstein DM, Bratton DR, Ciccone WJ 2nd, Elias JJ. Locking plates improve torsional resistance in the stabilization of three-part proximal humeral fractures. J Shoulder Elbow Surg. 2006;15(2):239-243.
12. Siffri PC, Peindl RD, Coley ER, Norton J, Connor PM, Kellam JF. Biomechanical analysis of blade plate versus locking plate fixation for a proximal humerus fracture: comparison using cadaveric and synthetic humeri. J Orthop Trauma. 2006;20(8):547-554.
13. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Internal fixation versus nonoperative treatment of displaced 3-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(5):747-755.
14. Fjalestad T, Hole MO, Hovden IA, Blucher J, Stromsoe K. Surgical treatment with an angular stable plate for complex displaced proximal humeral fractures in elderly patients: a randomized controlled trial. J Orthop Trauma. 2012;26(2):98-106.
15. Wild JR, DeMers A, French R, et al. Functional outcomes for surgically treated 3- and 4-part proximal humerus fractures. Orthopedics. 2011;34(10):e629-e633.
16. Solberg BD, Moon CN, Franco DP, Paiement GD. Surgical treatment of three and four-part proximal humeral fractures. J Bone Joint Surg Am. 2009;91(7):1689-1697.
17. Namdari S, Voleti PB, Mehta S. Evaluation of the osteoporotic proximal humeral fracture and strategies for structural augmentation during surgical treatment. J Shoulder Elbow Surg. 2012;21(12):1787-1795.
18. Agudelo J, Schurmann M, Stahel P, et al. Analysis of efficacy and failure in proximal humerus fractures treated with locking plates. J Orthop Trauma. 2007;21(10):676-681.
19. Schliemann B, Siemoneit J, Theisen C, Kosters C, Weimann A, Raschke MJ. Complex fractures of the proximal humerus in the elderly—outcome and complications after locking plate fixation. Musculoskelet Surg. 2012;96(suppl 1):S3-S11.
20. Thanasas C, Kontakis G, Angoules A, Limb D, Giannoudis P. Treatment of proximal humerus fractures with locking plates: a systematic review. J Shoulder Elbow Surg. 2009;18(6):837-844.
21. Micic ID, Kim KC, Shin DJ, et al. Analysis of early failure of the locking compression plate in osteoporotic proximal humerus fractures. J Orthop Sci. 2009;14(5):596-601.
22. Solberg BD, Moon CN, Franco DP, Paiement GD. Locked plating of 3- and 4-part proximal humerus fractures in older patients: the effect of initial fracture pattern on outcome. J Orthop Trauma. 2009;23(2):113-119.
23. Owsley KC, Gorczyca JT. Fracture displacement and screw cutout after open reduction and locked plate fixation of proximal humeral fractures [corrected]. J Bone Joint Surg Am. 2008;90(2):233-240.
24. Fankhauser F, Boldin C, Schippinger G, Haunschmid C, Szyszkowitz R. A new locking plate for unstable fractures of the proximal humerus. Clin Orthop. 2005;(430):176-181.
25. Sproul RC, Iyengar JJ, Devcic Z, Feeley BT. A systematic review of locking plate fixation of proximal humerus fractures. Injury. 2011;42(4):408-413.
26. Gardner MJ, Weil Y, Barker JU, Kelly BT, Helfet DL, Lorich DG. The importance of medial support in locked plating of proximal humerus fractures. J Orthop Trauma. 2007;21(3):185-191.
27. Voigt C, Hurschler C, Rech L, Vossenrich R, Lill H. Additive fiber-cerclages in proximal humeral fractures stabilized by locking plates. No effect on fracture stabilization and rotator cuff function in human shoulder specimens. Acta Orthop. 2009;80(4):465-471.
28. Lo IK, Burkhart SS. Biomechanical principles of arthroscopic repair of the rotator cuff. Oper Tech Orthop. 2002;12(3):140-155.
29. Ricchetti ET, Warrender WJ, Abboud JA. Use of locking plates in the treatment of proximal humerus fractures. J Shoulder Elbow Surg. 2010;19(2 suppl):66-75.
30. Badman B, Frankle M, Keating C, Henderson L, Brooks J, Mighell M. Results of proximal humeral locked plating with supplemental suture fixation of rotator cuff. J Shoulder Elbow Surg. 2011;20(4):616-624.
31. Nho SJ, Brophy RH, Barker JU, Cornell CN, MacGillivray JD. Management of proximal humeral fractures based on current literature. J Bone Joint Surg Am. 2007;89(suppl 3):44-58.
32. Barlow JD, Sanchez-Sotelo J, Torchia M. Proximal humerus fractures in the elderly can be reliably fixed with a “hybrid” locked-plating technique. Clin Orthop. 2011;469(12):3281-3291.
33. Cho CH, Jung GH, Song KS. Tension suture fixation using 2 washers for proximal humeral fractures. Orthopedics. 2012;35(3):202-205.
34. Brunner F, Sommer C, Bahrs C, et al. Open reduction and internal fixation of proximal humerus fractures using a proximal humeral locked plate: a prospective multicenter analysis. J Orthop Trauma. 2009;23(3):163-172.
35. Jung WB, Moon ES, Kim SK, Kovacevic D, Kim MS. Does medial support decrease major complications of unstable proximal humerus fractures treated with locking plate? BMC Musculoskelet Disord. 2013;14:102.
36. Krappinger D, Bizzotto N, Riedmann S, Kammerlander C, Hengg C, Kralinger FS. Predicting failure after surgical fixation of proximal humerus fractures. Injury. 2011;42(11):1283-1288.
37. Lescheid J, Zdero R, Shah S, Kuzyk PR, Schemitsch EH. The biomechanics of locked plating for repairing proximal humerus fractures with or without medial cortical support. J Trauma. 2010;69(5):1235-1242.
38. De Long WG Jr, Einhorn TA, Koval K, et al. Bone grafts and bone graft substitutes in orthopaedic trauma surgery. A critical analysis. J Bone Joint Surg Am. 2007;89(3):649-658.
39. Gardner MJ, Boraiah S, Helfet DL, Lorich DG. Indirect medial reduction and strut support of proximal humerus fractures using an endosteal implant. J Orthop Trauma. 2008;22(3):195-200.
40. Hettrich CM, Neviaser A, Beamer BS, Paul O, Helfet DL, Lorich DG. Locked plating of the proximal humerus using an endosteal implant. J Orthop Trauma. 2012;26(4):212-215.
41. Matassi F, Angeloni R, Carulli C, et al. Locking plate and fibular allograft augmentation in unstable fractures of proximal humerus. Injury. 2012;43(11):1939-1942.
42. Neviaser AS, Hettrich CM, Beamer BS, Dines JS, Lorich DG. Endosteal strut augment reduces complications associated with proximal humeral locking plates. Clin Orthop. 2011;469(12):3300-3306.
43. Mathison C, Chaudhary R, Beaupre L, Reynolds M, Adeeb S, Bouliane M. Biomechanical analysis of proximal humeral fixation using locking plate fixation with an intramedullary fibular allograft. Clin Biomech. 2010;25(7):642-646.
44. Osterhoff G, Baumgartner D, Favre P, et al. Medial support by fibula bone graft in angular stable plate fixation of proximal humeral fractures: an in vitro study with synthetic bone. J Shoulder Elbow Surg. 2011;20(5):740-746.
45. Chow RM, Begum F, Beaupre LA, Carey JP, Adeeb S, Bouliane MJ. Proximal humeral fracture fixation: locking plate construct +/- intramedullary fibular allograft. J Shoulder Elbow Surg. 2012;21(7):894-901.
46. Bae JH, Oh JK, Chon CS, Oh CW, Hwang JH, Yoon YC. The biomechanical performance of locking plate fixation with intramedullary fibular strut graft augmentation in the treatment of unstable fractures of the proximal humerus. J Bone Joint Surg Br. 2011;93(7):937-941.
47. Duralde XA, Leddy LR. The results of ORIF of displaced unstable proximal humeral fractures using a locking plate. J Shoulder Elbow Surg. 2010;19(4):480-488.
48. Robinson CM, Wylie JR, Ray AG, et al. Proximal humeral fractures with a severe varus deformity treated by fixation with a locking plate. J Bone Joint Surg Br. 2010;92(5):672-678.
49. Ong C, Bechtel C, Walsh M, Zuckerman JD, Egol KA. Three- and four-part fractures have poorer function than one-part proximal humerus fractures. Clin Orthop. 2011;469(12):3292-3299.
50. Kim SH, Lee YH, Chung SW, et al. Outcomes for four-part proximal humerus fractures treated with a locking compression plate and an autologous iliac bone impaction graft. Injury. 2012;43(10):1724-1731.
51. Larsson S. Calcium phosphates: what is the evidence? J Orthop Trauma. 2010;24(suppl 1):S41-S45.
52. Egol KA, Sugi MT, Ong CC, Montero N, Davidovitch R, Zuckerman JD. Fracture site augmentation with calcium phosphate cement reduces screw penetration after open reduction–internal fixation of proximal humeral fractures. J Shoulder Elbow Surg. 2012;21(6):741-748.
53. Gradl G, Knobe M, Stoffel M, Prescher A, Dirrichs T, Pape HC. Biomechanical evaluation of locking plate fixation of proximal humeral fractures augmented with calcium phosphate cement. J Orthop Trauma. 2013;27(7):399-404.
54. Collinge C, Merk B, Lautenschlager EP. Mechanical evaluation of fracture fixation augmented with tricalcium phosphate bone cement in a porous osteoporotic cancellous bone model. J Orthop Trauma. 2007;21(2):124-128.
55. Kwon BK, Goertzen DJ, O’Brien PJ, Broekhuyse HM, Oxland TR. Biomechanical evaluation of proximal humeral fracture fixation supplemented with calcium phosphate cement. J Bone Joint Surg Am. 2002;84(6):951-961.
56. Somasundaram K, Huber CP, Babu V, Zadeh H. Proximal humeral fractures: the role of calcium sulphate augmentation and extended deltoid splitting approach in internal fixation using locking plates. Injury. 2013;44(4):481-487.
57. Lee CW, Shin SJ. Prognostic factors for unstable proximal humeral fractures treated with locking-plate fixation. J Shoulder Elbow Surg. 2009;18(1):83-88.
58. Zhang L, Zheng J, Wang W, et al. The clinical benefit of medial support screws in locking plating of proximal humerus fractures: a prospective randomized study. Int Orthop. 2011;35(11):1655-1661.
59. Brianza S, Roderer G, Schiuma D, et al. Where do locking screws purchase in the humeral head? Injury. 2012;43(6):850-855.
60. Hepp P, Lill H, Bail H, et al. Where should implants be anchored in the humeral head? Clin Orthop. 2003;(415):139-147.
61. Liew AS, Johnson JA, Patterson SD, King GJ, Chess DG. Effect of screw placement on fixation in the humeral head. J Shoulder Elbow Surg. 2000;9(5):423-426.
62. Tingart MJ, Lehtinen J, Zurakowski D, Warner JJ, Apreleva M. Proximal humeral fractures: regional differences in bone mineral density of the humeral head affect the fixation strength of cancellous screws. J Shoulder Elbow Surg. 2006;15(5):620-624.
63. Erhardt JB, Stoffel K, Kampshoff J, Badur N, Yates P, Kuster MS. The position and number of screws influence screw perforation of the humeral head in modern locking plates: a cadaver study. J Orthop Trauma. 2012;26(10):e188-e192.
64. Konigshausen M, Kubler L, Godry H, Citak M, Schildhauer TA, Seybold D. Clinical outcome and complications using a polyaxial locking plate in the treatment of displaced proximal humerus fractures. A reliable system? Injury. 2012;43(2):223-231.
65. Bengard MJ, Gardner MJ. Screw depth sounding in proximal humerus fractures to avoid iatrogenic intra-articular penetration. J Orthop Trauma. 2011;25(10):630-633.
66. Ring D. Current concepts in plate and screw fixation of osteoporotic proximal humerus fractures. Injury. 2007;38(3):S59-S68.
Proximal humerus fractures account for 4% to 5% of all fractures.1 These fractures occur most frequently in the elderly—patients older than 60 years sustain 71% of these injuries2—and in females.1,3 Given an aging population, this incidence is predicted to increase 3-fold over the next 30 years.4 There is much debate regarding management of acute, displaced proximal humerus fractures. A recent Cochrane Review of published outcomes of operative and nonoperative treatment of displaced proximal humerus fractures found insufficient evidence supporting either modality, though surgery was associated with additional procedures.5 A review of 1000 proximal humerus fractures found that 49% had less than 1 cm of displacement of the major fragments or angulation of less than 45°.3 Other authors have reported similar findings.6,7 Although the incidence of proximal humerus fractures has remained stable over the past decade, from 1999 to 2005 there was a 25% relative increase in surgical management, including a relative increase of 29% in open reduction and internal fixation (ORIF) versus a 20% increase in arthroplasty.1
Locking plates have consistently demonstrated biomechanical superiority over other forms of fixation in osteoporotic bone.8-11 Egol and colleagues8 found that osteoporotic bone limited the torque of fixation to values less than what is required for adequate frictional force between the plate and the bone. This problem can be overcome with fixed-angle devices, such as locked plates.9 Compared with locked nail constructs, proximal humerus locking plates have demonstrated superiority in torsion, loading, and varus bending.10,11 Compared with blade plates, proximal humerus locking plates exhibited increased stiffness and torsional fatigue resistance.12 In a randomized clinical trial, Olerud and colleagues13 reported superior functional results with locking plate fixation compared with nonoperative treatment of displaced 3-part fractures in elderly patients with 2-year follow-up, though these clinical results were not supported by others.14 Two recent case–control studies comparing functional outcomes for 3- and 4-part fractures with follow-up of more than 2 years revealed higher Constant scores after locked plating compared with hemiarthroplasty, though complications were higher with locked plates.15,16 Adoption of locked proximal humerus plating has been correlated with good clinical outcomes and union rates, though this has been accompanied by a higher rate of reoperation.7 Reoperation rates from 1999 to 2005 increased both in the immediate postoperative period (odds ratio, 3.36) and at 1 year (odds ratio, 3.90).1
Complications of Locked Plating
Regardless of fixation type, reduced humeral head bone mass and quality may lead to implant loosening, fracture redisplacement, and, ultimately, poor outcomes. Baseline osteoporosis may predict likelihood of fixation failure.17 Multiple studies have reported on the implant-related complications associated with locking plate fixation—most commonly, intra-articular screw penetration, postoperative fracture displacement, and avascular necrosis (AVN)18-24 (Figure 1). A meta-analysis of 12 studies with a total of 514 proximal humerus fractures treated with locking plate fixation showed an overall complication rate of 49% and a 13.8% reoperation rate.25 The most common indication for reoperation involved intra-articular screw perforation. The most common complications were varus malunion (16%), osteonecrosis (10%), intra-articular screw penetration (8%), subacromial impingement (6%), and infection (4%).
Suboptimal intraoperative fracture reduction, specifically with residual varus, has been correlated with loss of fracture fixation. In a series of 153 fractures, loss of fixation occurred in 13.7% of cases, with the leading risk factor being varus malreduction.19 Failure rates were 30.4% and 11% when the head shaft angle was less than 120° and when it was 120° or more, respectively. Solberg and colleagues16 found that initial postoperative varus angulation of more than 20° resulted in universal loss of fixation. Conversion of these cases to hemiarthroplasty resulted in poor outcomes. Preoperative fracture alignment may also predict fixation failure.22 In one series, initial varus angulation healed with a mean 16° varus and a Constant score of 63, whereas initial valgus alignment healed with 6° varus and a Constant score of 71.22 Complications occurred in fractures that were initially in varus 79% of the time and initially in valgus 19% of the time. Screw perforation has been associated with loss of reduction 44% of the time.20
In an analysis of locking plate constructs revised after early (<4 weeks) failure in 8 patients with osteoporosis, Micic and colleagues21 found implant pullout leading to varus malalignment. All cases lacked medial support and subchondral screw purchase; 3 were initially malreduced. Owsley and Gorczyca23 retrospectively reviewed 53 cases of displaced proximal humerus fractures treated with locked plating. Despite the high rate of radiographic union, 36% developed complications, including screw cutout (23%), varus displacement of more than 10° (25%), and AVN (4%); 13% required revision. These complications disproportionately affected patients older than 60 years (57%) and negatively affected functional outcomes.
Augmentation Techniques
Despite its reported complications, proximal humerus locked plating remains the most widely used type of fixation.1 Advancements in locking plate design, improved understanding of fixation principles, and adoption of techniques augmenting proximal humerus locking plate fixation, particularly in osteoporotic bone, have reduced postoperative complications (Table 1).
Rotator Cuff Sutures
A widely adopted technique for neutralizing rotator cuff–deforming forces, which theoretically can cause fracture displacement, is incorporation of heavy nonabsorbable sutures. These sutures are placed through the rotator cuff–tuberosity junction and tied down after being passed through the plate. Obtaining and maintaining tuberosity reduction are essential in achieving good functional outcomes after fixation. In addition, tension band sutures may be particularly useful in the setting of initial varus deformity.26
Although clinical use of these sutures is common, biomechanical studies of their adjunctive contribution to fracture stability are lacking.27 The rotator cuff musculature has a maximal contractile force of 3.5 kg/cm2.28 Ricchetti and colleagues29 described a technique that involves using a locked plate and tagging the rotator cuff with heavy nonabsorbable sutures. Selective traction on the sutures can help obtain and maintain fracture reduction. Multiple studies have reported on suture use with locked plating for proximal humerus fractures.29-34 Badman and colleagues30 retrospectively reviewed 81 cases of metaphyseal defects or medial comminution treated with locked plating, rotator cuff sutures, and structural allograft. All cases healed within 6 months after surgery. The incidence of screw cutout was 3.7%, the incidence of AVN was 6.2%, and the incidence of varus collapse was 6%. A cadaveric study that used specimens (mean age, 77 years) with a simulated 3-part proximal humerus fracture treated with a locked plate both with and without cerclage sutures found no difference in interfragmentary motion between the groups.27 The authors concluded that additive sutures are not required for anatomically reduced fractures. Multiple sutures may counteract the deforming forces that act on bony segments that cannot be adequately maintained with screws, such as an osteoporotic greater tuberosity.
Medial Column Restoration
The importance of reducing and maintaining the medial calcar to provide biomechanical support for a laterally placed plate has been recognized.26,34-37 Gardner and colleagues26 suggested that medial support was achieved if the medial cortex was anatomically reduced, if the proximal fragment was impacted laterally onto the shaft, or if 1 or more inferomedial screws were placed. Cases that did not achieve medial support developed significantly more humeral head subsidence (5.8 mm vs 1.2 mm) and screw penetration. Krappinger and colleagues36 found that factors leading to fixation failure included age, local bone mineral density, anatomical reduction, and restoration of the medial cortical support. The authors concluded that anatomical reduction and restoration of the medial cortex were important in minimizing mechanical loads at the bone–implant interface. Biomechanically, Lescheid and colleagues37 found that the most stable construct was anatomical reduction with medial cortical contact. In the setting of comminution, however, it may be preferable to intentionally perform varus malreduction to achieve medial contact than to achieve anatomical reduction with a fracture gap. Badman and colleagues30 found that the incidence of screw penetration was 6% in patients with an intact medial calcar versus 29% in patients without medial support. In a retrospective analysis of patients treated with a locking plate and suture augmentation, Jung and colleagues35 concluded that restoring medial support was the most reliable factor in the prevention of loss of reduction with or without screw perforation. Last, Solberg and colleagues16 reported better clinical outcomes when the length of the metaphyseal segment attached to the articular fragment was more than 2 mm. A length of less than 2 mm was predictive of developing AVN.
Use of Bone Void Fillers
Allograft. Allograft is cancellous or corticocancellous chips or tricortical graft used as osteoconductive filler for metaphyseal defects.38 An increasingly popular technique involves using an endosteal fibular allograft strut to indirectly reduce the fracture and help support the medial calcar.39-42 Hettrich and colleagues40 reported on radiographic outcomes of displaced proximal humerus fractures with medial comminution treated with a locked plate and an endosteal fibular allograft or semitubular plate. The reduction was maintained in 96% of cases; there was 1 varus collapse. There were no cases of implant failure, screw perforation, or AVN. Other authors have also reported on successful use of fibular allograft in conjunction with a locked plate; the rate of reduction loss was low, and there were no cases of screw cutout or intra-articular screw penetration.30,41,42 These clinical outcomes are supported by results of biomechanical studies of the added benefit of intramedullary fibular allograft.43-46 Mathison and colleagues43 reported that a construct with fibular allograft and a locking plate increased the failure load by 1.72 times and the stiffness by 3.84 times compared with a control group of locking plate only. Bae and colleagues46 found significantly higher maximum failure load and construct stiffness with no varus collapse in specimens prepared with locked plate and fibular strut augmentation compared with a control group.
Others have successfully used cancellous allograft to fill humeral head bone defects.29,32,47-49 Duralde and Leddy47 reported 100% radiographic union and 81% good to excellent results in cases treated with a locking plate and morselized cancellous allograft to fill bone voids. Varus collapse and screw cutout did not occur, but there were 2 cases of AVN. Ricchetti and colleagues29 reviewed 54 cases treated with a locking plate and rotator cuff suture construct. Allograft cancellous chips and demineralized bone matrix were used in 3- and 4-part fractures (70% of cases) along with shorter screws in the humeral head. Major complications included AVN (1), fixation failure (3), and varus malunion (5). Others investigators have had less favorable results with use of cancellous bone graft. Schliemann and colleagues19 reported on 27 patients who were older than 65 years when they underwent ORIF with rotator cuff sutures to stabilize the tuberosities and either cancellous graft or a synthetic bone substitute in patients with massive metaphyseal defects. Patient-reported outcomes were superior to Constant scores. Complications included screw penetration (22.2%), reduction loss (44.4%), implant failure (3.7%), and AVN (29.6%).
Autograft. Autograft has both osteoconductive and osteoinductive properties and has been successfully used for metaphyseal defects.32,50 Kim and colleagues50 reported on patients with 4-part proximal humerus fractures treated with a locking plate and autologous iliac graft. All cases achieved union and had good or excellent outcomes. There were no cases of AVN, varus collapse, or hardware-related complications.
Bone Cement. Calcium phosphate cement has osteoconductive properties and enhances screw purchase in cancellous bone (Figures 2A–2F). It can be injected or molded into bone voids to provide improved compressive strength. It is resorbed through cell-mediated processes resembling bone remodeling and does not disappear until new bone forms. (Calcium sulfate cement, on the other hand, resorbs through a chemical process independent of new bone formation.51) Egol and colleagues52 reviewed the cases of 92 patients (mean age, 61 years) with 2-, 3-, and 4-part proximal humerus fractures treated with locked plate fixation. Metaphyseal defects were treated with no augmentation, augmentation with cancellous chips, or augmentation with calcium phosphate cement. Adding calcium phosphate cement was associated with lower incidence of intra-articular screw penetration and humeral head settling. In a recent cadaveric biomechanical study using 2-part proximal humerus fractures with metaphyseal comminution, the group augmented with calcium phosphate cement had enhanced axial stiffness and load to failure with reduced screw penetration.53 Other biomechanical studies have found increased screw pullout strength54 and decreased interfragmentary motion when specimens were augmented with calcium phosphate cement.55
Similar good clinical and radiographic outcomes have been observed with use of calcium sulfate cement.56,57 Somasundaram and colleagues56 reported good clinical outcomes in 82% of patients treated with locking plates and calcium sulfate cement used to fill metaphyseal voids. All fractures united without infection, fixation failure, subsequent malunion, tuberosity failure, or AVN. Lee and Shin57 compared outcomes of 14 patients who received calcium sulfate augmentation with outcomes of patients who did not receive this augmentation. Overall, 89% of patients had good or excellent results. Calcium sulfate cement did not affect the reduction failure rate or clinical outcomes in cases in which medial cortical reduction was achieved. However, postoperative displacement caused by lack of medial support was associated with poor outcomes.
Screw Placement
Screws optimally should be placed in the posterior-medial-inferior aspect of the humeral head to provide medial support for the fracture and mechanical stability.58 Cadaveric studies have shown the highest cancellous bone density in the proximal, posterior, and medial portions of the humeral head.59-63 Similarly, in a cadaveric study, Liew and colleagues61 found greater screw purchase and higher pullout strength when the screw was placed in the center of the humeral head, within subchondral bone; fixation was poorest when the screw was placed in the anterosuperior region of the humeral head. Tingart and colleagues62 reported that humeral head trabecular density significantly affected pullout strength of cancellous screws. In addition, the most pullout strength was at the center of the head, and the least within the anterosuperior head. Trabecular density was higher in the inferior and posterior regions than in the superior and anterior regions.
Most locking plate designs allow screws to be placed at the level of the medial calcar—the goal being to provide medial column support (Table 2). Zhang and colleagues58 treated 2-, 3-, and 4-part fractures with a locking plate and randomized them into receiving the plate with or without medial support screws. For 3- and 4-part fractures, the group with these screws had a significantly greater final neck-shaft angle and smaller angulation loss compared with the group without screws. No additional benefit was found for 2-part fractures. Erhardt and colleagues63 simulated unstable proximal humerus fractures using cadavers and testing different fixation methods using a polyaxial locking plate. They found that 5 screws in the head fragment and an inferomedial support screw significantly reduced the risk of screw perforation. Other authors have concluded that placing 1 or more inferomedial screws is important in cases of medial comminution or medial column malreduction.26 Interestingly, compared with use of a polyaxial implant, which allows for adjustment of screw direction, use of a monoaxial locking plate did not lead to a clinically different outcome or complication profile.64
Techniques have been used to achieve subchondral purchase of locking screws while reducing iatrogenic articular perforation.65 However, given the incidence of fracture settling and subsequent postoperative screw penetration, many authors currently recommend using shorter divergent screws combined with other augmentation techniques, described previously.17,29,32
Physical Therapy
There is no standardized physiotherapy regimen for postoperative management of proximal humerus fractures treated with locking plates.25 In older patients, immediate active range of motion (ROM) exercises should be delayed until early callus is noted, though there is a risk for stiffness. Lee and Shin57 found that a delay in rehabilitation after ORIF was an independent risk for poor clinical outcome. Namdari and colleagues17 recommended sling use only for comfort and initiated non-load-bearing activities and pendulum exercises immediately after surgery. Patients with adequate reduction at 4 to 6 weeks were advanced to full weight-bearing. Badman and colleagues30 initiated passive-assisted ROM exercises when the wound was healed at 2 weeks in 2-part fractures, whereas patients with 3- and 4-part fractures were immobilized until radiographic healing. Formal therapy was started after 6 weeks. Stiffness was reported in 5% of patients. For patients with stable fixation, Ricchetti and colleagues29 recommended passive shoulder ROM exercises on postoperative day 1; at 4 to 6 weeks, patients should start active shoulder ROM exercises, and then resistance exercises at 10 to 12 weeks. Other authors are more conservative—only sling immobilization and pendulum exercises the first month.66 Barlow and colleagues32 immobilized their patients (age, >75 years) for 6 weeks. No patient developed disabling stiffness. The authors suggested that patients older than 75 years may not be prone to stiffness.
Our Preferred Treatment Method
All proximal humerus fractures are approached anteriorly through the deltopectoral interval (Figure 3A). The long head biceps is identified and truncated for later tenodesis. Multiple No. 5 Ethibond sutures (Ethicon) are placed at the bone–tendon interface. The fracture is reduced with a Cobb elevator (Figure 3B), and provisional Kirschner wires are placed within the head (Figure 3C). The plate is affixed to the humeral head with its anterior border paralleling the posterior aspect of the bicipital groove. Multiple locking screws are placed within the superior and posterior humeral head. Nonlocking screws are then used to fix the plate to the shaft to reduce the specific deformity. Under fluoroscopy, any metaphyseal void is filled with calcium phosphate cement (Figure 3D). The remaining inferior screws are placed within the humeral head. Dr. Gruson uses screws 4 to 6 mm short of subchondral bone to reduce the risk for joint penetration. The rotator cuff sutures are tied down through the plate. Patients are started on progressive supine passive ROM exercises at 7 days, followed by supine active-assisted ROM exercises 6 weeks after fracture healing is confirmed radiographically.
Conclusion
Use of locked plating for proximal humerus fractures has increased, particularly in the elderly. Resulting complications include intra-articular screw penetration, postoperative fracture displacement, and AVN. Recognition of the importance of reducing and supporting the medial calcar, filling any metaphyseal defects, and selectively placing screws within the humeral head has lowered the incidence of these complications. Further comparative studies evaluating the efficacy of individual augmentation techniques are needed to determine their contribution to successful fracture healing and their cost-effectiveness. Results of such studies may help in the development of protocols for more standardized implementation of these techniques and in understanding which specific fracture patterns and patients would benefit from their use.
Proximal humerus fractures account for 4% to 5% of all fractures.1 These fractures occur most frequently in the elderly—patients older than 60 years sustain 71% of these injuries2—and in females.1,3 Given an aging population, this incidence is predicted to increase 3-fold over the next 30 years.4 There is much debate regarding management of acute, displaced proximal humerus fractures. A recent Cochrane Review of published outcomes of operative and nonoperative treatment of displaced proximal humerus fractures found insufficient evidence supporting either modality, though surgery was associated with additional procedures.5 A review of 1000 proximal humerus fractures found that 49% had less than 1 cm of displacement of the major fragments or angulation of less than 45°.3 Other authors have reported similar findings.6,7 Although the incidence of proximal humerus fractures has remained stable over the past decade, from 1999 to 2005 there was a 25% relative increase in surgical management, including a relative increase of 29% in open reduction and internal fixation (ORIF) versus a 20% increase in arthroplasty.1
Locking plates have consistently demonstrated biomechanical superiority over other forms of fixation in osteoporotic bone.8-11 Egol and colleagues8 found that osteoporotic bone limited the torque of fixation to values less than what is required for adequate frictional force between the plate and the bone. This problem can be overcome with fixed-angle devices, such as locked plates.9 Compared with locked nail constructs, proximal humerus locking plates have demonstrated superiority in torsion, loading, and varus bending.10,11 Compared with blade plates, proximal humerus locking plates exhibited increased stiffness and torsional fatigue resistance.12 In a randomized clinical trial, Olerud and colleagues13 reported superior functional results with locking plate fixation compared with nonoperative treatment of displaced 3-part fractures in elderly patients with 2-year follow-up, though these clinical results were not supported by others.14 Two recent case–control studies comparing functional outcomes for 3- and 4-part fractures with follow-up of more than 2 years revealed higher Constant scores after locked plating compared with hemiarthroplasty, though complications were higher with locked plates.15,16 Adoption of locked proximal humerus plating has been correlated with good clinical outcomes and union rates, though this has been accompanied by a higher rate of reoperation.7 Reoperation rates from 1999 to 2005 increased both in the immediate postoperative period (odds ratio, 3.36) and at 1 year (odds ratio, 3.90).1
Complications of Locked Plating
Regardless of fixation type, reduced humeral head bone mass and quality may lead to implant loosening, fracture redisplacement, and, ultimately, poor outcomes. Baseline osteoporosis may predict likelihood of fixation failure.17 Multiple studies have reported on the implant-related complications associated with locking plate fixation—most commonly, intra-articular screw penetration, postoperative fracture displacement, and avascular necrosis (AVN)18-24 (Figure 1). A meta-analysis of 12 studies with a total of 514 proximal humerus fractures treated with locking plate fixation showed an overall complication rate of 49% and a 13.8% reoperation rate.25 The most common indication for reoperation involved intra-articular screw perforation. The most common complications were varus malunion (16%), osteonecrosis (10%), intra-articular screw penetration (8%), subacromial impingement (6%), and infection (4%).
Suboptimal intraoperative fracture reduction, specifically with residual varus, has been correlated with loss of fracture fixation. In a series of 153 fractures, loss of fixation occurred in 13.7% of cases, with the leading risk factor being varus malreduction.19 Failure rates were 30.4% and 11% when the head shaft angle was less than 120° and when it was 120° or more, respectively. Solberg and colleagues16 found that initial postoperative varus angulation of more than 20° resulted in universal loss of fixation. Conversion of these cases to hemiarthroplasty resulted in poor outcomes. Preoperative fracture alignment may also predict fixation failure.22 In one series, initial varus angulation healed with a mean 16° varus and a Constant score of 63, whereas initial valgus alignment healed with 6° varus and a Constant score of 71.22 Complications occurred in fractures that were initially in varus 79% of the time and initially in valgus 19% of the time. Screw perforation has been associated with loss of reduction 44% of the time.20
In an analysis of locking plate constructs revised after early (<4 weeks) failure in 8 patients with osteoporosis, Micic and colleagues21 found implant pullout leading to varus malalignment. All cases lacked medial support and subchondral screw purchase; 3 were initially malreduced. Owsley and Gorczyca23 retrospectively reviewed 53 cases of displaced proximal humerus fractures treated with locked plating. Despite the high rate of radiographic union, 36% developed complications, including screw cutout (23%), varus displacement of more than 10° (25%), and AVN (4%); 13% required revision. These complications disproportionately affected patients older than 60 years (57%) and negatively affected functional outcomes.
Augmentation Techniques
Despite its reported complications, proximal humerus locked plating remains the most widely used type of fixation.1 Advancements in locking plate design, improved understanding of fixation principles, and adoption of techniques augmenting proximal humerus locking plate fixation, particularly in osteoporotic bone, have reduced postoperative complications (Table 1).
Rotator Cuff Sutures
A widely adopted technique for neutralizing rotator cuff–deforming forces, which theoretically can cause fracture displacement, is incorporation of heavy nonabsorbable sutures. These sutures are placed through the rotator cuff–tuberosity junction and tied down after being passed through the plate. Obtaining and maintaining tuberosity reduction are essential in achieving good functional outcomes after fixation. In addition, tension band sutures may be particularly useful in the setting of initial varus deformity.26
Although clinical use of these sutures is common, biomechanical studies of their adjunctive contribution to fracture stability are lacking.27 The rotator cuff musculature has a maximal contractile force of 3.5 kg/cm2.28 Ricchetti and colleagues29 described a technique that involves using a locked plate and tagging the rotator cuff with heavy nonabsorbable sutures. Selective traction on the sutures can help obtain and maintain fracture reduction. Multiple studies have reported on suture use with locked plating for proximal humerus fractures.29-34 Badman and colleagues30 retrospectively reviewed 81 cases of metaphyseal defects or medial comminution treated with locked plating, rotator cuff sutures, and structural allograft. All cases healed within 6 months after surgery. The incidence of screw cutout was 3.7%, the incidence of AVN was 6.2%, and the incidence of varus collapse was 6%. A cadaveric study that used specimens (mean age, 77 years) with a simulated 3-part proximal humerus fracture treated with a locked plate both with and without cerclage sutures found no difference in interfragmentary motion between the groups.27 The authors concluded that additive sutures are not required for anatomically reduced fractures. Multiple sutures may counteract the deforming forces that act on bony segments that cannot be adequately maintained with screws, such as an osteoporotic greater tuberosity.
Medial Column Restoration
The importance of reducing and maintaining the medial calcar to provide biomechanical support for a laterally placed plate has been recognized.26,34-37 Gardner and colleagues26 suggested that medial support was achieved if the medial cortex was anatomically reduced, if the proximal fragment was impacted laterally onto the shaft, or if 1 or more inferomedial screws were placed. Cases that did not achieve medial support developed significantly more humeral head subsidence (5.8 mm vs 1.2 mm) and screw penetration. Krappinger and colleagues36 found that factors leading to fixation failure included age, local bone mineral density, anatomical reduction, and restoration of the medial cortical support. The authors concluded that anatomical reduction and restoration of the medial cortex were important in minimizing mechanical loads at the bone–implant interface. Biomechanically, Lescheid and colleagues37 found that the most stable construct was anatomical reduction with medial cortical contact. In the setting of comminution, however, it may be preferable to intentionally perform varus malreduction to achieve medial contact than to achieve anatomical reduction with a fracture gap. Badman and colleagues30 found that the incidence of screw penetration was 6% in patients with an intact medial calcar versus 29% in patients without medial support. In a retrospective analysis of patients treated with a locking plate and suture augmentation, Jung and colleagues35 concluded that restoring medial support was the most reliable factor in the prevention of loss of reduction with or without screw perforation. Last, Solberg and colleagues16 reported better clinical outcomes when the length of the metaphyseal segment attached to the articular fragment was more than 2 mm. A length of less than 2 mm was predictive of developing AVN.
Use of Bone Void Fillers
Allograft. Allograft is cancellous or corticocancellous chips or tricortical graft used as osteoconductive filler for metaphyseal defects.38 An increasingly popular technique involves using an endosteal fibular allograft strut to indirectly reduce the fracture and help support the medial calcar.39-42 Hettrich and colleagues40 reported on radiographic outcomes of displaced proximal humerus fractures with medial comminution treated with a locked plate and an endosteal fibular allograft or semitubular plate. The reduction was maintained in 96% of cases; there was 1 varus collapse. There were no cases of implant failure, screw perforation, or AVN. Other authors have also reported on successful use of fibular allograft in conjunction with a locked plate; the rate of reduction loss was low, and there were no cases of screw cutout or intra-articular screw penetration.30,41,42 These clinical outcomes are supported by results of biomechanical studies of the added benefit of intramedullary fibular allograft.43-46 Mathison and colleagues43 reported that a construct with fibular allograft and a locking plate increased the failure load by 1.72 times and the stiffness by 3.84 times compared with a control group of locking plate only. Bae and colleagues46 found significantly higher maximum failure load and construct stiffness with no varus collapse in specimens prepared with locked plate and fibular strut augmentation compared with a control group.
Others have successfully used cancellous allograft to fill humeral head bone defects.29,32,47-49 Duralde and Leddy47 reported 100% radiographic union and 81% good to excellent results in cases treated with a locking plate and morselized cancellous allograft to fill bone voids. Varus collapse and screw cutout did not occur, but there were 2 cases of AVN. Ricchetti and colleagues29 reviewed 54 cases treated with a locking plate and rotator cuff suture construct. Allograft cancellous chips and demineralized bone matrix were used in 3- and 4-part fractures (70% of cases) along with shorter screws in the humeral head. Major complications included AVN (1), fixation failure (3), and varus malunion (5). Others investigators have had less favorable results with use of cancellous bone graft. Schliemann and colleagues19 reported on 27 patients who were older than 65 years when they underwent ORIF with rotator cuff sutures to stabilize the tuberosities and either cancellous graft or a synthetic bone substitute in patients with massive metaphyseal defects. Patient-reported outcomes were superior to Constant scores. Complications included screw penetration (22.2%), reduction loss (44.4%), implant failure (3.7%), and AVN (29.6%).
Autograft. Autograft has both osteoconductive and osteoinductive properties and has been successfully used for metaphyseal defects.32,50 Kim and colleagues50 reported on patients with 4-part proximal humerus fractures treated with a locking plate and autologous iliac graft. All cases achieved union and had good or excellent outcomes. There were no cases of AVN, varus collapse, or hardware-related complications.
Bone Cement. Calcium phosphate cement has osteoconductive properties and enhances screw purchase in cancellous bone (Figures 2A–2F). It can be injected or molded into bone voids to provide improved compressive strength. It is resorbed through cell-mediated processes resembling bone remodeling and does not disappear until new bone forms. (Calcium sulfate cement, on the other hand, resorbs through a chemical process independent of new bone formation.51) Egol and colleagues52 reviewed the cases of 92 patients (mean age, 61 years) with 2-, 3-, and 4-part proximal humerus fractures treated with locked plate fixation. Metaphyseal defects were treated with no augmentation, augmentation with cancellous chips, or augmentation with calcium phosphate cement. Adding calcium phosphate cement was associated with lower incidence of intra-articular screw penetration and humeral head settling. In a recent cadaveric biomechanical study using 2-part proximal humerus fractures with metaphyseal comminution, the group augmented with calcium phosphate cement had enhanced axial stiffness and load to failure with reduced screw penetration.53 Other biomechanical studies have found increased screw pullout strength54 and decreased interfragmentary motion when specimens were augmented with calcium phosphate cement.55
Similar good clinical and radiographic outcomes have been observed with use of calcium sulfate cement.56,57 Somasundaram and colleagues56 reported good clinical outcomes in 82% of patients treated with locking plates and calcium sulfate cement used to fill metaphyseal voids. All fractures united without infection, fixation failure, subsequent malunion, tuberosity failure, or AVN. Lee and Shin57 compared outcomes of 14 patients who received calcium sulfate augmentation with outcomes of patients who did not receive this augmentation. Overall, 89% of patients had good or excellent results. Calcium sulfate cement did not affect the reduction failure rate or clinical outcomes in cases in which medial cortical reduction was achieved. However, postoperative displacement caused by lack of medial support was associated with poor outcomes.
Screw Placement
Screws optimally should be placed in the posterior-medial-inferior aspect of the humeral head to provide medial support for the fracture and mechanical stability.58 Cadaveric studies have shown the highest cancellous bone density in the proximal, posterior, and medial portions of the humeral head.59-63 Similarly, in a cadaveric study, Liew and colleagues61 found greater screw purchase and higher pullout strength when the screw was placed in the center of the humeral head, within subchondral bone; fixation was poorest when the screw was placed in the anterosuperior region of the humeral head. Tingart and colleagues62 reported that humeral head trabecular density significantly affected pullout strength of cancellous screws. In addition, the most pullout strength was at the center of the head, and the least within the anterosuperior head. Trabecular density was higher in the inferior and posterior regions than in the superior and anterior regions.
Most locking plate designs allow screws to be placed at the level of the medial calcar—the goal being to provide medial column support (Table 2). Zhang and colleagues58 treated 2-, 3-, and 4-part fractures with a locking plate and randomized them into receiving the plate with or without medial support screws. For 3- and 4-part fractures, the group with these screws had a significantly greater final neck-shaft angle and smaller angulation loss compared with the group without screws. No additional benefit was found for 2-part fractures. Erhardt and colleagues63 simulated unstable proximal humerus fractures using cadavers and testing different fixation methods using a polyaxial locking plate. They found that 5 screws in the head fragment and an inferomedial support screw significantly reduced the risk of screw perforation. Other authors have concluded that placing 1 or more inferomedial screws is important in cases of medial comminution or medial column malreduction.26 Interestingly, compared with use of a polyaxial implant, which allows for adjustment of screw direction, use of a monoaxial locking plate did not lead to a clinically different outcome or complication profile.64
Techniques have been used to achieve subchondral purchase of locking screws while reducing iatrogenic articular perforation.65 However, given the incidence of fracture settling and subsequent postoperative screw penetration, many authors currently recommend using shorter divergent screws combined with other augmentation techniques, described previously.17,29,32
Physical Therapy
There is no standardized physiotherapy regimen for postoperative management of proximal humerus fractures treated with locking plates.25 In older patients, immediate active range of motion (ROM) exercises should be delayed until early callus is noted, though there is a risk for stiffness. Lee and Shin57 found that a delay in rehabilitation after ORIF was an independent risk for poor clinical outcome. Namdari and colleagues17 recommended sling use only for comfort and initiated non-load-bearing activities and pendulum exercises immediately after surgery. Patients with adequate reduction at 4 to 6 weeks were advanced to full weight-bearing. Badman and colleagues30 initiated passive-assisted ROM exercises when the wound was healed at 2 weeks in 2-part fractures, whereas patients with 3- and 4-part fractures were immobilized until radiographic healing. Formal therapy was started after 6 weeks. Stiffness was reported in 5% of patients. For patients with stable fixation, Ricchetti and colleagues29 recommended passive shoulder ROM exercises on postoperative day 1; at 4 to 6 weeks, patients should start active shoulder ROM exercises, and then resistance exercises at 10 to 12 weeks. Other authors are more conservative—only sling immobilization and pendulum exercises the first month.66 Barlow and colleagues32 immobilized their patients (age, >75 years) for 6 weeks. No patient developed disabling stiffness. The authors suggested that patients older than 75 years may not be prone to stiffness.
Our Preferred Treatment Method
All proximal humerus fractures are approached anteriorly through the deltopectoral interval (Figure 3A). The long head biceps is identified and truncated for later tenodesis. Multiple No. 5 Ethibond sutures (Ethicon) are placed at the bone–tendon interface. The fracture is reduced with a Cobb elevator (Figure 3B), and provisional Kirschner wires are placed within the head (Figure 3C). The plate is affixed to the humeral head with its anterior border paralleling the posterior aspect of the bicipital groove. Multiple locking screws are placed within the superior and posterior humeral head. Nonlocking screws are then used to fix the plate to the shaft to reduce the specific deformity. Under fluoroscopy, any metaphyseal void is filled with calcium phosphate cement (Figure 3D). The remaining inferior screws are placed within the humeral head. Dr. Gruson uses screws 4 to 6 mm short of subchondral bone to reduce the risk for joint penetration. The rotator cuff sutures are tied down through the plate. Patients are started on progressive supine passive ROM exercises at 7 days, followed by supine active-assisted ROM exercises 6 weeks after fracture healing is confirmed radiographically.
Conclusion
Use of locked plating for proximal humerus fractures has increased, particularly in the elderly. Resulting complications include intra-articular screw penetration, postoperative fracture displacement, and AVN. Recognition of the importance of reducing and supporting the medial calcar, filling any metaphyseal defects, and selectively placing screws within the humeral head has lowered the incidence of these complications. Further comparative studies evaluating the efficacy of individual augmentation techniques are needed to determine their contribution to successful fracture healing and their cost-effectiveness. Results of such studies may help in the development of protocols for more standardized implementation of these techniques and in understanding which specific fracture patterns and patients would benefit from their use.
1. Bell JE, Leung BC, Spratt KF, et al. Trends and variation in incidence, surgical treatment, and repeat surgery of proximal humeral fractures in the elderly. J Bone Joint Surg Am. 2011;93(2):121-131.
2. Aaron D, Shatsky J, Paredes JC, Jiang C, Parsons BO, Flatow EL. Proximal humeral fractures: internal fixation. J Bone Joint Surg Am. 2012;94(24):2280-2288.
3. Court-Brown CM, Garg A, McQueen MM. The epidemiology of proximal humeral fractures. Acta Orthop Scand. 2001;72(4):365-371.
4. Kannus P, Palvanen M, Niemi S, Parkkari J, Jarvinen M, Vuori I. Increasing number and incidence of osteoporotic fractures of the proximal humerus in elderly people. BMJ. 1996;313(7064):1051-1052.
5. Handoll HH, Ollivere BJ, Rollins KE. Interventions for treating proximal humeral fractures in adults. Cochrane Database Syst Rev. 2012;12:CD000434.
6. Tamai K, Ishige N, Kuroda S, et al. Four-segment classification of proximal humeral fractures revisited: a multicenter study on 509 cases. J Shoulder Elbow Surg. 2009;18(6):845-850.
7. Rothberg D, Higgins T. Fractures of the proximal humerus. Orthop Clin North Am. 2013;44(1):9-19.
8. Egol KA, Kubiak EN, Fulkerson E, Kummer FJ, Koval KJ. Biomechanics of locked plates and screws. J Orthop Trauma. 2004;18(8):488-493.
9. Miranda MA. Locking plate technology and its role in osteoporotic fractures. Injury. 2007;38(suppl 3):35-39.
10. Foruria AM, Carrascal MT, Revilla C, Munuera L, Sanchez-Sotelo J. Proximal humerus fracture rotational stability after fixation using a locking plate or a fixed-angle locked nail: the role of implant stiffness. Clin Biomech. 2010;25(4):307-311.
11. Weinstein DM, Bratton DR, Ciccone WJ 2nd, Elias JJ. Locking plates improve torsional resistance in the stabilization of three-part proximal humeral fractures. J Shoulder Elbow Surg. 2006;15(2):239-243.
12. Siffri PC, Peindl RD, Coley ER, Norton J, Connor PM, Kellam JF. Biomechanical analysis of blade plate versus locking plate fixation for a proximal humerus fracture: comparison using cadaveric and synthetic humeri. J Orthop Trauma. 2006;20(8):547-554.
13. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Internal fixation versus nonoperative treatment of displaced 3-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(5):747-755.
14. Fjalestad T, Hole MO, Hovden IA, Blucher J, Stromsoe K. Surgical treatment with an angular stable plate for complex displaced proximal humeral fractures in elderly patients: a randomized controlled trial. J Orthop Trauma. 2012;26(2):98-106.
15. Wild JR, DeMers A, French R, et al. Functional outcomes for surgically treated 3- and 4-part proximal humerus fractures. Orthopedics. 2011;34(10):e629-e633.
16. Solberg BD, Moon CN, Franco DP, Paiement GD. Surgical treatment of three and four-part proximal humeral fractures. J Bone Joint Surg Am. 2009;91(7):1689-1697.
17. Namdari S, Voleti PB, Mehta S. Evaluation of the osteoporotic proximal humeral fracture and strategies for structural augmentation during surgical treatment. J Shoulder Elbow Surg. 2012;21(12):1787-1795.
18. Agudelo J, Schurmann M, Stahel P, et al. Analysis of efficacy and failure in proximal humerus fractures treated with locking plates. J Orthop Trauma. 2007;21(10):676-681.
19. Schliemann B, Siemoneit J, Theisen C, Kosters C, Weimann A, Raschke MJ. Complex fractures of the proximal humerus in the elderly—outcome and complications after locking plate fixation. Musculoskelet Surg. 2012;96(suppl 1):S3-S11.
20. Thanasas C, Kontakis G, Angoules A, Limb D, Giannoudis P. Treatment of proximal humerus fractures with locking plates: a systematic review. J Shoulder Elbow Surg. 2009;18(6):837-844.
21. Micic ID, Kim KC, Shin DJ, et al. Analysis of early failure of the locking compression plate in osteoporotic proximal humerus fractures. J Orthop Sci. 2009;14(5):596-601.
22. Solberg BD, Moon CN, Franco DP, Paiement GD. Locked plating of 3- and 4-part proximal humerus fractures in older patients: the effect of initial fracture pattern on outcome. J Orthop Trauma. 2009;23(2):113-119.
23. Owsley KC, Gorczyca JT. Fracture displacement and screw cutout after open reduction and locked plate fixation of proximal humeral fractures [corrected]. J Bone Joint Surg Am. 2008;90(2):233-240.
24. Fankhauser F, Boldin C, Schippinger G, Haunschmid C, Szyszkowitz R. A new locking plate for unstable fractures of the proximal humerus. Clin Orthop. 2005;(430):176-181.
25. Sproul RC, Iyengar JJ, Devcic Z, Feeley BT. A systematic review of locking plate fixation of proximal humerus fractures. Injury. 2011;42(4):408-413.
26. Gardner MJ, Weil Y, Barker JU, Kelly BT, Helfet DL, Lorich DG. The importance of medial support in locked plating of proximal humerus fractures. J Orthop Trauma. 2007;21(3):185-191.
27. Voigt C, Hurschler C, Rech L, Vossenrich R, Lill H. Additive fiber-cerclages in proximal humeral fractures stabilized by locking plates. No effect on fracture stabilization and rotator cuff function in human shoulder specimens. Acta Orthop. 2009;80(4):465-471.
28. Lo IK, Burkhart SS. Biomechanical principles of arthroscopic repair of the rotator cuff. Oper Tech Orthop. 2002;12(3):140-155.
29. Ricchetti ET, Warrender WJ, Abboud JA. Use of locking plates in the treatment of proximal humerus fractures. J Shoulder Elbow Surg. 2010;19(2 suppl):66-75.
30. Badman B, Frankle M, Keating C, Henderson L, Brooks J, Mighell M. Results of proximal humeral locked plating with supplemental suture fixation of rotator cuff. J Shoulder Elbow Surg. 2011;20(4):616-624.
31. Nho SJ, Brophy RH, Barker JU, Cornell CN, MacGillivray JD. Management of proximal humeral fractures based on current literature. J Bone Joint Surg Am. 2007;89(suppl 3):44-58.
32. Barlow JD, Sanchez-Sotelo J, Torchia M. Proximal humerus fractures in the elderly can be reliably fixed with a “hybrid” locked-plating technique. Clin Orthop. 2011;469(12):3281-3291.
33. Cho CH, Jung GH, Song KS. Tension suture fixation using 2 washers for proximal humeral fractures. Orthopedics. 2012;35(3):202-205.
34. Brunner F, Sommer C, Bahrs C, et al. Open reduction and internal fixation of proximal humerus fractures using a proximal humeral locked plate: a prospective multicenter analysis. J Orthop Trauma. 2009;23(3):163-172.
35. Jung WB, Moon ES, Kim SK, Kovacevic D, Kim MS. Does medial support decrease major complications of unstable proximal humerus fractures treated with locking plate? BMC Musculoskelet Disord. 2013;14:102.
36. Krappinger D, Bizzotto N, Riedmann S, Kammerlander C, Hengg C, Kralinger FS. Predicting failure after surgical fixation of proximal humerus fractures. Injury. 2011;42(11):1283-1288.
37. Lescheid J, Zdero R, Shah S, Kuzyk PR, Schemitsch EH. The biomechanics of locked plating for repairing proximal humerus fractures with or without medial cortical support. J Trauma. 2010;69(5):1235-1242.
38. De Long WG Jr, Einhorn TA, Koval K, et al. Bone grafts and bone graft substitutes in orthopaedic trauma surgery. A critical analysis. J Bone Joint Surg Am. 2007;89(3):649-658.
39. Gardner MJ, Boraiah S, Helfet DL, Lorich DG. Indirect medial reduction and strut support of proximal humerus fractures using an endosteal implant. J Orthop Trauma. 2008;22(3):195-200.
40. Hettrich CM, Neviaser A, Beamer BS, Paul O, Helfet DL, Lorich DG. Locked plating of the proximal humerus using an endosteal implant. J Orthop Trauma. 2012;26(4):212-215.
41. Matassi F, Angeloni R, Carulli C, et al. Locking plate and fibular allograft augmentation in unstable fractures of proximal humerus. Injury. 2012;43(11):1939-1942.
42. Neviaser AS, Hettrich CM, Beamer BS, Dines JS, Lorich DG. Endosteal strut augment reduces complications associated with proximal humeral locking plates. Clin Orthop. 2011;469(12):3300-3306.
43. Mathison C, Chaudhary R, Beaupre L, Reynolds M, Adeeb S, Bouliane M. Biomechanical analysis of proximal humeral fixation using locking plate fixation with an intramedullary fibular allograft. Clin Biomech. 2010;25(7):642-646.
44. Osterhoff G, Baumgartner D, Favre P, et al. Medial support by fibula bone graft in angular stable plate fixation of proximal humeral fractures: an in vitro study with synthetic bone. J Shoulder Elbow Surg. 2011;20(5):740-746.
45. Chow RM, Begum F, Beaupre LA, Carey JP, Adeeb S, Bouliane MJ. Proximal humeral fracture fixation: locking plate construct +/- intramedullary fibular allograft. J Shoulder Elbow Surg. 2012;21(7):894-901.
46. Bae JH, Oh JK, Chon CS, Oh CW, Hwang JH, Yoon YC. The biomechanical performance of locking plate fixation with intramedullary fibular strut graft augmentation in the treatment of unstable fractures of the proximal humerus. J Bone Joint Surg Br. 2011;93(7):937-941.
47. Duralde XA, Leddy LR. The results of ORIF of displaced unstable proximal humeral fractures using a locking plate. J Shoulder Elbow Surg. 2010;19(4):480-488.
48. Robinson CM, Wylie JR, Ray AG, et al. Proximal humeral fractures with a severe varus deformity treated by fixation with a locking plate. J Bone Joint Surg Br. 2010;92(5):672-678.
49. Ong C, Bechtel C, Walsh M, Zuckerman JD, Egol KA. Three- and four-part fractures have poorer function than one-part proximal humerus fractures. Clin Orthop. 2011;469(12):3292-3299.
50. Kim SH, Lee YH, Chung SW, et al. Outcomes for four-part proximal humerus fractures treated with a locking compression plate and an autologous iliac bone impaction graft. Injury. 2012;43(10):1724-1731.
51. Larsson S. Calcium phosphates: what is the evidence? J Orthop Trauma. 2010;24(suppl 1):S41-S45.
52. Egol KA, Sugi MT, Ong CC, Montero N, Davidovitch R, Zuckerman JD. Fracture site augmentation with calcium phosphate cement reduces screw penetration after open reduction–internal fixation of proximal humeral fractures. J Shoulder Elbow Surg. 2012;21(6):741-748.
53. Gradl G, Knobe M, Stoffel M, Prescher A, Dirrichs T, Pape HC. Biomechanical evaluation of locking plate fixation of proximal humeral fractures augmented with calcium phosphate cement. J Orthop Trauma. 2013;27(7):399-404.
54. Collinge C, Merk B, Lautenschlager EP. Mechanical evaluation of fracture fixation augmented with tricalcium phosphate bone cement in a porous osteoporotic cancellous bone model. J Orthop Trauma. 2007;21(2):124-128.
55. Kwon BK, Goertzen DJ, O’Brien PJ, Broekhuyse HM, Oxland TR. Biomechanical evaluation of proximal humeral fracture fixation supplemented with calcium phosphate cement. J Bone Joint Surg Am. 2002;84(6):951-961.
56. Somasundaram K, Huber CP, Babu V, Zadeh H. Proximal humeral fractures: the role of calcium sulphate augmentation and extended deltoid splitting approach in internal fixation using locking plates. Injury. 2013;44(4):481-487.
57. Lee CW, Shin SJ. Prognostic factors for unstable proximal humeral fractures treated with locking-plate fixation. J Shoulder Elbow Surg. 2009;18(1):83-88.
58. Zhang L, Zheng J, Wang W, et al. The clinical benefit of medial support screws in locking plating of proximal humerus fractures: a prospective randomized study. Int Orthop. 2011;35(11):1655-1661.
59. Brianza S, Roderer G, Schiuma D, et al. Where do locking screws purchase in the humeral head? Injury. 2012;43(6):850-855.
60. Hepp P, Lill H, Bail H, et al. Where should implants be anchored in the humeral head? Clin Orthop. 2003;(415):139-147.
61. Liew AS, Johnson JA, Patterson SD, King GJ, Chess DG. Effect of screw placement on fixation in the humeral head. J Shoulder Elbow Surg. 2000;9(5):423-426.
62. Tingart MJ, Lehtinen J, Zurakowski D, Warner JJ, Apreleva M. Proximal humeral fractures: regional differences in bone mineral density of the humeral head affect the fixation strength of cancellous screws. J Shoulder Elbow Surg. 2006;15(5):620-624.
63. Erhardt JB, Stoffel K, Kampshoff J, Badur N, Yates P, Kuster MS. The position and number of screws influence screw perforation of the humeral head in modern locking plates: a cadaver study. J Orthop Trauma. 2012;26(10):e188-e192.
64. Konigshausen M, Kubler L, Godry H, Citak M, Schildhauer TA, Seybold D. Clinical outcome and complications using a polyaxial locking plate in the treatment of displaced proximal humerus fractures. A reliable system? Injury. 2012;43(2):223-231.
65. Bengard MJ, Gardner MJ. Screw depth sounding in proximal humerus fractures to avoid iatrogenic intra-articular penetration. J Orthop Trauma. 2011;25(10):630-633.
66. Ring D. Current concepts in plate and screw fixation of osteoporotic proximal humerus fractures. Injury. 2007;38(3):S59-S68.
1. Bell JE, Leung BC, Spratt KF, et al. Trends and variation in incidence, surgical treatment, and repeat surgery of proximal humeral fractures in the elderly. J Bone Joint Surg Am. 2011;93(2):121-131.
2. Aaron D, Shatsky J, Paredes JC, Jiang C, Parsons BO, Flatow EL. Proximal humeral fractures: internal fixation. J Bone Joint Surg Am. 2012;94(24):2280-2288.
3. Court-Brown CM, Garg A, McQueen MM. The epidemiology of proximal humeral fractures. Acta Orthop Scand. 2001;72(4):365-371.
4. Kannus P, Palvanen M, Niemi S, Parkkari J, Jarvinen M, Vuori I. Increasing number and incidence of osteoporotic fractures of the proximal humerus in elderly people. BMJ. 1996;313(7064):1051-1052.
5. Handoll HH, Ollivere BJ, Rollins KE. Interventions for treating proximal humeral fractures in adults. Cochrane Database Syst Rev. 2012;12:CD000434.
6. Tamai K, Ishige N, Kuroda S, et al. Four-segment classification of proximal humeral fractures revisited: a multicenter study on 509 cases. J Shoulder Elbow Surg. 2009;18(6):845-850.
7. Rothberg D, Higgins T. Fractures of the proximal humerus. Orthop Clin North Am. 2013;44(1):9-19.
8. Egol KA, Kubiak EN, Fulkerson E, Kummer FJ, Koval KJ. Biomechanics of locked plates and screws. J Orthop Trauma. 2004;18(8):488-493.
9. Miranda MA. Locking plate technology and its role in osteoporotic fractures. Injury. 2007;38(suppl 3):35-39.
10. Foruria AM, Carrascal MT, Revilla C, Munuera L, Sanchez-Sotelo J. Proximal humerus fracture rotational stability after fixation using a locking plate or a fixed-angle locked nail: the role of implant stiffness. Clin Biomech. 2010;25(4):307-311.
11. Weinstein DM, Bratton DR, Ciccone WJ 2nd, Elias JJ. Locking plates improve torsional resistance in the stabilization of three-part proximal humeral fractures. J Shoulder Elbow Surg. 2006;15(2):239-243.
12. Siffri PC, Peindl RD, Coley ER, Norton J, Connor PM, Kellam JF. Biomechanical analysis of blade plate versus locking plate fixation for a proximal humerus fracture: comparison using cadaveric and synthetic humeri. J Orthop Trauma. 2006;20(8):547-554.
13. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Internal fixation versus nonoperative treatment of displaced 3-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(5):747-755.
14. Fjalestad T, Hole MO, Hovden IA, Blucher J, Stromsoe K. Surgical treatment with an angular stable plate for complex displaced proximal humeral fractures in elderly patients: a randomized controlled trial. J Orthop Trauma. 2012;26(2):98-106.
15. Wild JR, DeMers A, French R, et al. Functional outcomes for surgically treated 3- and 4-part proximal humerus fractures. Orthopedics. 2011;34(10):e629-e633.
16. Solberg BD, Moon CN, Franco DP, Paiement GD. Surgical treatment of three and four-part proximal humeral fractures. J Bone Joint Surg Am. 2009;91(7):1689-1697.
17. Namdari S, Voleti PB, Mehta S. Evaluation of the osteoporotic proximal humeral fracture and strategies for structural augmentation during surgical treatment. J Shoulder Elbow Surg. 2012;21(12):1787-1795.
18. Agudelo J, Schurmann M, Stahel P, et al. Analysis of efficacy and failure in proximal humerus fractures treated with locking plates. J Orthop Trauma. 2007;21(10):676-681.
19. Schliemann B, Siemoneit J, Theisen C, Kosters C, Weimann A, Raschke MJ. Complex fractures of the proximal humerus in the elderly—outcome and complications after locking plate fixation. Musculoskelet Surg. 2012;96(suppl 1):S3-S11.
20. Thanasas C, Kontakis G, Angoules A, Limb D, Giannoudis P. Treatment of proximal humerus fractures with locking plates: a systematic review. J Shoulder Elbow Surg. 2009;18(6):837-844.
21. Micic ID, Kim KC, Shin DJ, et al. Analysis of early failure of the locking compression plate in osteoporotic proximal humerus fractures. J Orthop Sci. 2009;14(5):596-601.
22. Solberg BD, Moon CN, Franco DP, Paiement GD. Locked plating of 3- and 4-part proximal humerus fractures in older patients: the effect of initial fracture pattern on outcome. J Orthop Trauma. 2009;23(2):113-119.
23. Owsley KC, Gorczyca JT. Fracture displacement and screw cutout after open reduction and locked plate fixation of proximal humeral fractures [corrected]. J Bone Joint Surg Am. 2008;90(2):233-240.
24. Fankhauser F, Boldin C, Schippinger G, Haunschmid C, Szyszkowitz R. A new locking plate for unstable fractures of the proximal humerus. Clin Orthop. 2005;(430):176-181.
25. Sproul RC, Iyengar JJ, Devcic Z, Feeley BT. A systematic review of locking plate fixation of proximal humerus fractures. Injury. 2011;42(4):408-413.
26. Gardner MJ, Weil Y, Barker JU, Kelly BT, Helfet DL, Lorich DG. The importance of medial support in locked plating of proximal humerus fractures. J Orthop Trauma. 2007;21(3):185-191.
27. Voigt C, Hurschler C, Rech L, Vossenrich R, Lill H. Additive fiber-cerclages in proximal humeral fractures stabilized by locking plates. No effect on fracture stabilization and rotator cuff function in human shoulder specimens. Acta Orthop. 2009;80(4):465-471.
28. Lo IK, Burkhart SS. Biomechanical principles of arthroscopic repair of the rotator cuff. Oper Tech Orthop. 2002;12(3):140-155.
29. Ricchetti ET, Warrender WJ, Abboud JA. Use of locking plates in the treatment of proximal humerus fractures. J Shoulder Elbow Surg. 2010;19(2 suppl):66-75.
30. Badman B, Frankle M, Keating C, Henderson L, Brooks J, Mighell M. Results of proximal humeral locked plating with supplemental suture fixation of rotator cuff. J Shoulder Elbow Surg. 2011;20(4):616-624.
31. Nho SJ, Brophy RH, Barker JU, Cornell CN, MacGillivray JD. Management of proximal humeral fractures based on current literature. J Bone Joint Surg Am. 2007;89(suppl 3):44-58.
32. Barlow JD, Sanchez-Sotelo J, Torchia M. Proximal humerus fractures in the elderly can be reliably fixed with a “hybrid” locked-plating technique. Clin Orthop. 2011;469(12):3281-3291.
33. Cho CH, Jung GH, Song KS. Tension suture fixation using 2 washers for proximal humeral fractures. Orthopedics. 2012;35(3):202-205.
34. Brunner F, Sommer C, Bahrs C, et al. Open reduction and internal fixation of proximal humerus fractures using a proximal humeral locked plate: a prospective multicenter analysis. J Orthop Trauma. 2009;23(3):163-172.
35. Jung WB, Moon ES, Kim SK, Kovacevic D, Kim MS. Does medial support decrease major complications of unstable proximal humerus fractures treated with locking plate? BMC Musculoskelet Disord. 2013;14:102.
36. Krappinger D, Bizzotto N, Riedmann S, Kammerlander C, Hengg C, Kralinger FS. Predicting failure after surgical fixation of proximal humerus fractures. Injury. 2011;42(11):1283-1288.
37. Lescheid J, Zdero R, Shah S, Kuzyk PR, Schemitsch EH. The biomechanics of locked plating for repairing proximal humerus fractures with or without medial cortical support. J Trauma. 2010;69(5):1235-1242.
38. De Long WG Jr, Einhorn TA, Koval K, et al. Bone grafts and bone graft substitutes in orthopaedic trauma surgery. A critical analysis. J Bone Joint Surg Am. 2007;89(3):649-658.
39. Gardner MJ, Boraiah S, Helfet DL, Lorich DG. Indirect medial reduction and strut support of proximal humerus fractures using an endosteal implant. J Orthop Trauma. 2008;22(3):195-200.
40. Hettrich CM, Neviaser A, Beamer BS, Paul O, Helfet DL, Lorich DG. Locked plating of the proximal humerus using an endosteal implant. J Orthop Trauma. 2012;26(4):212-215.
41. Matassi F, Angeloni R, Carulli C, et al. Locking plate and fibular allograft augmentation in unstable fractures of proximal humerus. Injury. 2012;43(11):1939-1942.
42. Neviaser AS, Hettrich CM, Beamer BS, Dines JS, Lorich DG. Endosteal strut augment reduces complications associated with proximal humeral locking plates. Clin Orthop. 2011;469(12):3300-3306.
43. Mathison C, Chaudhary R, Beaupre L, Reynolds M, Adeeb S, Bouliane M. Biomechanical analysis of proximal humeral fixation using locking plate fixation with an intramedullary fibular allograft. Clin Biomech. 2010;25(7):642-646.
44. Osterhoff G, Baumgartner D, Favre P, et al. Medial support by fibula bone graft in angular stable plate fixation of proximal humeral fractures: an in vitro study with synthetic bone. J Shoulder Elbow Surg. 2011;20(5):740-746.
45. Chow RM, Begum F, Beaupre LA, Carey JP, Adeeb S, Bouliane MJ. Proximal humeral fracture fixation: locking plate construct +/- intramedullary fibular allograft. J Shoulder Elbow Surg. 2012;21(7):894-901.
46. Bae JH, Oh JK, Chon CS, Oh CW, Hwang JH, Yoon YC. The biomechanical performance of locking plate fixation with intramedullary fibular strut graft augmentation in the treatment of unstable fractures of the proximal humerus. J Bone Joint Surg Br. 2011;93(7):937-941.
47. Duralde XA, Leddy LR. The results of ORIF of displaced unstable proximal humeral fractures using a locking plate. J Shoulder Elbow Surg. 2010;19(4):480-488.
48. Robinson CM, Wylie JR, Ray AG, et al. Proximal humeral fractures with a severe varus deformity treated by fixation with a locking plate. J Bone Joint Surg Br. 2010;92(5):672-678.
49. Ong C, Bechtel C, Walsh M, Zuckerman JD, Egol KA. Three- and four-part fractures have poorer function than one-part proximal humerus fractures. Clin Orthop. 2011;469(12):3292-3299.
50. Kim SH, Lee YH, Chung SW, et al. Outcomes for four-part proximal humerus fractures treated with a locking compression plate and an autologous iliac bone impaction graft. Injury. 2012;43(10):1724-1731.
51. Larsson S. Calcium phosphates: what is the evidence? J Orthop Trauma. 2010;24(suppl 1):S41-S45.
52. Egol KA, Sugi MT, Ong CC, Montero N, Davidovitch R, Zuckerman JD. Fracture site augmentation with calcium phosphate cement reduces screw penetration after open reduction–internal fixation of proximal humeral fractures. J Shoulder Elbow Surg. 2012;21(6):741-748.
53. Gradl G, Knobe M, Stoffel M, Prescher A, Dirrichs T, Pape HC. Biomechanical evaluation of locking plate fixation of proximal humeral fractures augmented with calcium phosphate cement. J Orthop Trauma. 2013;27(7):399-404.
54. Collinge C, Merk B, Lautenschlager EP. Mechanical evaluation of fracture fixation augmented with tricalcium phosphate bone cement in a porous osteoporotic cancellous bone model. J Orthop Trauma. 2007;21(2):124-128.
55. Kwon BK, Goertzen DJ, O’Brien PJ, Broekhuyse HM, Oxland TR. Biomechanical evaluation of proximal humeral fracture fixation supplemented with calcium phosphate cement. J Bone Joint Surg Am. 2002;84(6):951-961.
56. Somasundaram K, Huber CP, Babu V, Zadeh H. Proximal humeral fractures: the role of calcium sulphate augmentation and extended deltoid splitting approach in internal fixation using locking plates. Injury. 2013;44(4):481-487.
57. Lee CW, Shin SJ. Prognostic factors for unstable proximal humeral fractures treated with locking-plate fixation. J Shoulder Elbow Surg. 2009;18(1):83-88.
58. Zhang L, Zheng J, Wang W, et al. The clinical benefit of medial support screws in locking plating of proximal humerus fractures: a prospective randomized study. Int Orthop. 2011;35(11):1655-1661.
59. Brianza S, Roderer G, Schiuma D, et al. Where do locking screws purchase in the humeral head? Injury. 2012;43(6):850-855.
60. Hepp P, Lill H, Bail H, et al. Where should implants be anchored in the humeral head? Clin Orthop. 2003;(415):139-147.
61. Liew AS, Johnson JA, Patterson SD, King GJ, Chess DG. Effect of screw placement on fixation in the humeral head. J Shoulder Elbow Surg. 2000;9(5):423-426.
62. Tingart MJ, Lehtinen J, Zurakowski D, Warner JJ, Apreleva M. Proximal humeral fractures: regional differences in bone mineral density of the humeral head affect the fixation strength of cancellous screws. J Shoulder Elbow Surg. 2006;15(5):620-624.
63. Erhardt JB, Stoffel K, Kampshoff J, Badur N, Yates P, Kuster MS. The position and number of screws influence screw perforation of the humeral head in modern locking plates: a cadaver study. J Orthop Trauma. 2012;26(10):e188-e192.
64. Konigshausen M, Kubler L, Godry H, Citak M, Schildhauer TA, Seybold D. Clinical outcome and complications using a polyaxial locking plate in the treatment of displaced proximal humerus fractures. A reliable system? Injury. 2012;43(2):223-231.
65. Bengard MJ, Gardner MJ. Screw depth sounding in proximal humerus fractures to avoid iatrogenic intra-articular penetration. J Orthop Trauma. 2011;25(10):630-633.
66. Ring D. Current concepts in plate and screw fixation of osteoporotic proximal humerus fractures. Injury. 2007;38(3):S59-S68.
The Effect of Arthroscopic Rotator Interval Closure on Glenohumeral Volume
Since Neer described the rotator interval in 1970, its closure, often used in conjunction with capsulorrhaphy, has become an important surgical technique in managing shoulder instability.1-11 Numerous studies have sought to define the function of the rotator interval.1-3,6-20 The etiology of lesions of the rotator interval has been debated, and there is evidence that such lesions may be in part congenital.21 Increased rotator interval depth and width, along with increased size of the distended inferior and posteroinferior joint capsule on magnetic resonance arthrography, have been reported in cases of multidirectional shoulder instability.22 However, confusion remains about the role of the rotator interval in shoulder instability and about the effect its closure has on shoulder function. No one knows the degree of volume reduction that results from closure of the rotator interval and whether medial and lateral sutures differ in the volume reduction achieved.
Cadaveric studies have shown that the rotator interval has an important role in shoulder motion.6,13-16,19,20,23 Harryman and colleagues13 found that sectioning the coracohumeral ligament (CHL) increased shoulder range of motion (ROM), and medial-to-lateral closure of the rotator interval restricted motion in all planes. Most notably, interval closure limited inferior translation in the adducted shoulder, posterior translation in the flexed adducted shoulder, and external rotation in the neutral position. Subsequent studies,17,18 using rotator interval closure combined with thermal capsulorrhaphy, confirmed the results reported by Harryman and colleagues.13
More recent cadaveric studies using superior-to-inferior rotator interval closures have shown a decrease in anterior translation but not posterior translation.14-16,19-21 A superior-to-inferior interval closure technique limited external rotation less than a medial-to-lateral closure did.13-16,19-21 The majority of arthroscopically described rotator interval closures involve a superior-to-inferior technique and use 2 or 3 sutures.1,3,9-11
Plausinis and colleagues15 examined the effects of an isolated medial, an isolated lateral, and a medial combined with a lateral closure of the rotator interval. They noted that all 3 methods limited anterior translation and motion by means of 6° flexion and 10° external rotation; however, there was no statistical difference between methods. They also found that occasionally the medial interval closure resulted in massive loss of external rotation. Earlier, Jost and colleagues14 noted that a medial rotator interval could cause this massive loss by tethering the CHL, resulting in a medial-to-lateral imbrication of the CHL.
Arthroscopic rotator interval closure has clinically demonstrated an additive effect on shoulder stability. The recurrence rate was lower for arthroscopic Bankart repair combined with arthroscopic rotator interval closure (8%) than for arthroscopic Bankart repair alone (13%).24 In addition, time to recurrent dislocation was longer (42 vs 13 months) for the group that underwent the combination of Bankart repair and rotator interval closure. Regarding the concern about loss of motion after arthroscopic rotator interval closure, Chiang and colleagues25 recently noted no significant loss of motion 5 years after arthroscopic Bankart repair with rotator interval closure.
What effect rotator interval closure has on intra-articular glenohumeral volume (GHV) remains unknown. Using a cadaveric model, Yamamoto and colleagues20 showed that decreasing GHV can increase the responsiveness of the glenohumeral joint to the intra-articular pressure. Thus, reducing the volume can improve stability in vitro by increasing the magnitude of negative pressure stabilizing the glenohumeral joint.
We conducted a study to quantify the effects of arthroscopic rotator interval closure on capsular volume and to determine whether medial and lateral interval closures resulted in different degrees of volume reduction. Our hypothesis was that shoulder volume would be significantly reduced by closing the rotator interval.
Materials and Methods
Previous studies have not specifically evaluated GHV after rotator interval closure. Our power analysis was performed with data from a study by Karas and colleagues,26 who evaluated GHV after capsular plication. To detect a capsular volume reduction of 20% per stitch, with a 2-sided 5% significance level and a power of 80%, we needed a sample size of 5 specimens per group.
After receiving institutional review board approval for this study, we obtained 10 cadaveric shoulders (5 matched pairs). Exclusion criteria included arthroscopic evaluation revealing a full-thickness rotator cuff tear or significant osteoarthritis. Two shoulders had full-thickness cuff tears, leaving 8 shoulders to be tested; 6 of these were matched pairs. The shoulders were from 1 man (matched pair) and 4 women (2 matched pairs). Age ranged from 38 to 70 years (mean, 59.6 years). Differences in material properties between the specimens were accounted for by using primarily matched pairs.
The 2 study groups consisted of 4 shoulders each. After specimens were thawed, the skin, subcutaneous tissues, and periscapular muscles were removed from the shoulder. Only the capsule, biceps, and rotator cuff remained. For measurement purposes, the shoulders were mounted in a vice clamp in a beach-chair orientation. We placed a total of 2 portals with fully threaded 8.25-mm cannulas (Arthrex, Naples, Florida). A standard posterior portal was placed in the soft spot. A low anterior portal was then placed just superior to the subscapularis tendon. For arthroscopic examination and instrumentation in a saline environment, the shoulders were rotated into the lateral decubitus position, with suspension in 30° abduction and 20° forward flexion, by a rope attached to a pin in the distal shaft of the humerus.
In both groups, medial and lateral stitches with No. 2 FiberWire (Arthrex) were used to close the interval. The medial interval closure stitch was placed more than 10 mm away from the glenoid to prevent unpredictable CHL tethering; the lateral closure stitch was placed 10 mm lateral to the medial stitch (Figure 1).14 All sutures were placed intra-articularly under direct arthroscopic visualization, similar to the methods described in the literature.1,3,9-11 Sutures were passed through the superior glenohumeral ligament (SGHL) and through the upper subscapularis using a suture shuttle (SutureLasso; Arthrex) and Penetrator II Suture Retriever (Arthrex). The upper subscapularis was incorporated because of the unpredictable nature of the middle glenohumeral ligament (MGHL). Both rotator interval sutures were placed before tying either. In the medial group, the medial stitch was tied first, using alternating half-hitches, followed by the lateral stitch. In the lateral group, the lateral stitch was tied first, followed by the medial stitch. GHV was measured at baseline and after tying each stitch. Dr. Ponce instrumented all shoulders.
Modifying a beach-chair technique described by Miller and colleagues,27 we used a viscous fatty-acid sulfate solution, liquid soap, to measure GHV.27-29 A small slit in line with the fibers was made in the supraspinatus tendon just lateral to the musculotendinous junction. A 3-way stop-cock was placed into the joint though this defect. A 20-mL syringe with a 16-gauge needle was used to inject the soap. The needle was inserted into the rotator cuff interval, and the viscous solution was injected in 5-mL increments until there was active extravasation through the supraspinatus cannula (Figure 2). This technique, the “volcano method,” marked the maximum capacity of the joint. The joint was then copiously irrigated with normal saline and suctioned until all normal saline was evacuated. Dr. Rosenzweig took 2 measurements on each shoulder, and their mean was used for analysis.
The baseline measurement was taken with the 2 working cannulas in the shoulder joint. Measurements were obtained with cannulas to simulate normal clinical conditions. Subsequent measurements were done with the cannulas in place and inserted up to the same thread each time so as not to change the volume. The capsule and the rotator cuff were then dissected from the humerus so the size of the capsulolabral plication could be directly evaluated. Methylene blue was used to mark the capsular suture holes before removing the sutures. With use of a caliper, the size of the plication bite was measured (in millimeters).
Statistical Analysis
The primary outcome was percent reduction in GHV as a function of number of plications and size of plication. When only the first plication was tightened, the effect of position (medial or lateral) was also of interest. Percent volume reduction was calculated as (original – new) / original × 100. SAS 8.02 (SAS Institute, Cary, North Carolina) was used to fit a repeated random-intercept regression model for each outcome. This technique properly accounts for the paired nature of the specimens and the repeated measures (baseline plus 2 plications). Model fit was assessed by the method of difference in log likelihood.
Results
In the medial group, GHV was reduced by a mean of 24.2% with a single medial stitch; in the lateral group, GHV was reduced by a mean of 35.1% (Figure 3). The difference was significant (P < .02). In the medial group, when a second lateral stitch was used, GHV was reduced by another 18.7%; in the lateral group, when a medial stitch was added, GHV was reduced by another 11.4%. Final GHV for the medial and lateral groups was 42.9% and 46.5%, respectively. There was no statistical difference in final GHV, regardless of which stitch was placed first. When the 2 groups were combined, GHV was reduced by 44.9% with use of medial and lateral rotator interval closure stitches.
Mean amount of tissue purchased, or “bite size,” was 18 mm with a lateral suture and 15 mm with a medial suture (P < .05). In addition, an increase in bite size to GHV reduction was essentially linear, where an increase in bite size of 1 mm reduced GHV by about 1% (Figure 4).
Discussion
Although there have been numerous clinical series and biomechanical studies focused on isolated rotator interval closure (or its use as an adjunct) in shoulder stabilization, the precise function of the rotator interval remains poorly understood.1-3,6-11,19 Consequently, the in vivo effects of interval closure are unknown.
Initial studies proposed that rotator interval closure limited inferior and posterior translation.30 More recent studies have demonstrated that rotator interval closure confers little effect on posterior instability but increases anterior stability in cadaveric models.15,16 Clinical series have provided evidence that rotator interval closure can increase anterior stability.1,3,7,9,12 In a series of isolated rotator interval closures for multidirectional instability, Field and colleagues12 found that preoperative anterior and inferior symptoms predominated over posterior symptoms. Isolated closure of the rotator interval resulted in 100% excellent results with no cases of recurrent instability. Moon and colleagues31 reported that arthroscopic rotator interval closure with or without inferior capsular plication in multidirectional instability and predominant symptomatic inferior instability has shown benefit by improving function and stability. Other clinical reports of rotator interval closure in conjunction with arthroscopic Bankart repair have suggested it has an additive effect on anterior shoulder stability without limiting motion.24,25
In our study, arthroscopic closure of the rotator interval with 2 superior-to-inferior stitches reduced intracapsular volume by 45%. Even though open capsular shifts use different surgical techniques, similar technique volume reduction studies have reported reductions between 34% and 54% with open shifts.27,30 It is unknown if the stability resulting from decreased GHV is primarily from increasing intra-articular pressures or from restricting ROM, or from a combination of both. In shoulders with multidirectional instability, the joint volume may be increased, the joint capsule may be enlarged, or the glenohumeral ligaments may be lax and thin.4,6,32,33 Yamamoto and colleagues19 stated that intra-articular pressure is determined by 3 factors: load, joint volume, and material properties of the capsule. Load is a constant; joint volume and material properties can be changed.19 In our study, material properties were controlled by using a majority of matched specimens. Regardless of the stabilizing mechanism, our study results demonstrated that arthroscopic rotator interval closure may be a powerful tool in reducing shoulder volume, a consistent principle of surgical techniques used in reestablishing shoulder stability.19,20
When a single rotator interval closure stitch was used, volume reduction with a lateral stitch was superior to that with a medial stitch. This finding is logical, as anatomically the dimensions of the rotator interval are larger laterally as the CHL fans out to insert on the greater and lesser tuberosities.14 This finding has also been reported in open capsular shifts for multidirectional instability, with a lateral humeral shift having a larger volume reduction than a medial glenoid shift.27 Miller and colleagues27 used the image of a cone, with its larger opening facing the humerus and narrower side facing the glenoid, to illustrate this difference in open capsular shifts.
Our study also showed a larger volume reduction with 2 rotator interval closure stitches than with a single interval stitch. As ROM testing has not shown a difference between results with 1 and 2 sutures, we recommend a minimum of 2 sutures for arthroscopic rotator interval closure.15 If a single plication stitch is preferred, a lateral stitch (vs a medial stitch) can be used for a significantly larger reduction in shoulder volume. We think this is because of a larger amount of capsule being purchased with lateral closure (Figure 5). However, if a medial stitch is used, it is important to not place it too near the glenoid to avoid CHL tethering and subsequent excessive loss of external rotation.15
This study had several weaknesses. First, it was a cadaveric study, and use of specimens not known to have instability or specific rotator interval injury may make generalization to a clinical situation difficult. Second, although our power analysis called for 5 shoulders in each group, full-thickness rotator cuff tears rendered 2 shoulders unusable. This reduced our sample sizes and potentially decreased the power of the study, though the data demonstrated statistically significant differences. Third, we did not compare the effects of an open medial-to-lateral imbrication of the rotator interval on intracapsular volume with the effects of our arthroscopic method. We also did not assess our specimens’ ROM, effects of interval closure stitches on shoulder stability, or glenohumeral contact surface pressures, as these factors have already been studied.13-19 Instead, we focused on the effects of rotator interval closure on intracapsular volume, which had not been quantified until now. The clinical significance of such a volume reduction is unknown, especially with respect to influence on ROM, but the degree of volume reduction was larger than with previously reported arthroscopic instability repairs and smaller than with open capsular shifts, demonstrating that it may be a powerful tool in restoring stability in an unstable shoulder.26-30,34 Fourth, the role of isolated rotator interval closure is poorly defined, as only 1 clinical series of isolated rotator interval closure has been reported thus far.12 It has been far more common for rotator interval closure to be used with Bankart repair or capsulorrhaphy.1-3,7-9
In a cadaveric study by Provencher and colleagues,16 open rotator interval closure with medial-to-lateral imbrication of the interval altered shoulder kinematics differently from what occurred with arthroscopic closure of the MGHL to the SGHL, resulting in superior-to-inferior shift. Comparing the 2 methods may therefore be inappropriate. Currently we reserve rotator interval closure for infrequent cases of revision instability and cases in which glenoid bone loss is marginal (5%-15%) and there is a willingness to potentially sacrifice ROM to restore stability and avoid an open stabilization procedure. Continued investigation into the clinical role of rotator interval closure in shoulder stability is needed. We should identify the pathology in a patient with instability and use this technique as an adjuvant to other stabilization procedures.
Conclusion
Arthroscopic rotator interval closure with 2 plication stitches is a powerful tool in reducing the intracapsular volume of the shoulder. If a single plication stitch is preferred, a lateral rotator interval closure stitch (vs a medial stitch) can be used for a larger reduction in shoulder volume.
1. Creighton RA, Romeo AA, Brown FM, Hayden JK, Verma NN. Revision arthroscopic shoulder instability repair. Arthroscopy. 2007;23(7):703-709.
2. Gartsman GM, Roddey TS, Hammerman SM. Arthroscopic treatment of anterior-inferior glenohumeral instability. Two to five-year follow-up. J Bone Joint Surg Am. 2000;82(7):991-1003.
3. Gartsman GM, Taverna E, Hammerman SM. Arthroscopic rotator interval repair in glenohumeral instability: description of an operative technique. Arthroscopy. 1999;15(3):330-332.
4. Neer CS 2nd, Foster CR. Inferior capsular shift for involuntary inferior and multidirectional instability of the shoulder: a preliminary report. J Bone Joint Surg Am. 1980;62(6):897-908.
5. Neer CS 2nd. Displaced proximal humerus fractures: I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
6. Nobuhara K, Ikeda H. Rotator interval lesion. Clin Orthop. 1987;(223):44-50.
7. Rowe CR, Zarins B, Ciullo JV. Recurrent anterior dislocation of the shoulder after surgical repair. J Bone Joint Surg Am. 1984;66(2):159-168.
8. Rowe CR, Zarins B. Recurrent transient subluxation of the shoulder. J Bone Joint Surg Am. 1981;63(6):863-872.
9. Stokes DA, Savoie FH, Field LD. Arthroscopic repair of anterior glenohumeral instability and rotator interval lesions. Orthop Clin North Am. 2003;34(4):529-539.
10. Taverna E, Sansone V, Battistella F. Arthroscopic rotator interval repair: the three-step all-inside technique. Arthroscopy. 2004;20 Suppl 2:105-109.
11. Treacy SH, Field LD, Savoie FH. Rotator interval capsule closure: an arthroscopic technique. Arthroscopy. 1997;13(1):103-106.
12. Field LD, Warren RF, O’Brien SJ, Altcheck DW, Wickiewicz TL. Isolated closure of rotator interval defects for shoulder instability. Am J Sports Med. 1995;23(5):557-563.
13. Harryman DT 2nd, Sidles JA, Harris SL, Matsen FA 3rd. The role of the rotator interval capsule in passive motion and stability of the shoulder. J Bone Joint Surg Am. 1992;74(1):53-66.
14. Jost B, Koch PP, Gerber C. Anatomy and functional aspects of the rotator interval. J Shoulder Elbow Surg. 2000;9(4):336-341.
15. Plausinis D, Bravman JT, Heywood C, Kummer FJ, Kwon YM, Jazrawi LM. Arthroscopic rotator interval closure: effect of sutures on glenohumeral motion and anterior-posterior translation. Am J Sports Med. 2006;34(10):1656-1661.
16. Provencher MT, Mologne TS, Hongo M, Zhao K, Tasto JP, An KN. Arthroscopic versus open rotator interval closure: biomechanical evaluation of stability and motion. Arthroscopy. 2007;23(6):583-592.
17. Selecky MT, Tibone JE, Yang BY, et al. Glenohumeral joint translation after thermal capsuloplasty of the rotator interval. J Shoulder Elbow Surg. 2003;12(2):139-143.
18. Wolf R, Zheng N, Iero J, Weichel D. The effects of thermal capsulorrhaphy and rotator interval closure on multidirectional laxity in the glenohumeral joint: a cadaveric biomechanical study. Arthroscopy. 2004;20(10):1044-1049.
19. Yamamoto N, Itoi E, Tuoheti Y, et al. Effect of rotator interval closure on glenohumeral stability and motion: a cadaveric study. J Shoulder Elbow Surg. 2006;15(6):750-758.
20. Yamamoto N, Itoi E, Tuoheti Y, et al. The effect of the inferior capsular shift on shoulder intra-articular pressure: a cadaveric study. Am J Sports Med. 2006;34(6):939-944.
21. Cole BJ, Rodeo SA, O’Brien SJ, et al. The anatomy and histology of the rotator interval capsule of the shoulder. Clin Orthop. 2001;(390):129-137.
22. Lee HJ, Kim NR, Moon SG, Ko SM, Park JY. Multidirectional instability of the shoulder: rotator interval dimension and capsular laxity evaluation using MR arthrography. Skeletal Radiol. 2013;42(2):231-238.
23. Warner JP, Deng X, Warren RF, Torzilli PA, O’Brien SJ. Superoinferior translation in intact and vented glenohumeral joint. J Shoulder Elbow Surg. 1993;2(2):99-105.
24. Chechik O, Maman E, Dolkart O, Khashan M, Shabtai L, Mozes G. Arthroscopic rotator interval closure in shoulder instability repair: a retrospective study. J Shoulder Elbow Surg. 2010;19(7):1056-1062.
25. Chiang, E, Wang J, Wang S, et al. Arthroscopic posteroinferior capsular plication and rotator interval closure after Bankart repair in patients with traumatic anterior glenohumeral instability—a minimum follow-up of 5 years. Injury. 2010;41(10):1075-1078.
26. Karas SG, Creighton RA, DeMorat GJ. Glenohumeral volume reduction in arthroscopic shoulder reconstruction: a cadaveric analysis of suture plication and thermal capsulorrhaphy. Arthroscopy. 2004;20(2):179-184.
27. Miller MD, Larsen KM, Luke T, Leis HT, Plancher KD. Anterior capsular shift volume reduction: an in vitro comparison of 3 techniques. J Shoulder Elbow Surg. 2003;12(4):350-354.
28. Luke TA, Rovner AD, Karas SG, Hawkins RJ, Plancher KD. Volumetric change in the shoulder capsule after open inferior capsular shift versus arthroscopic thermal capsular shrinkage: a cadaveric model. J Shoulder Elbow Surg. 2004;13(2):146-149.
29. Ponce BA, Rosenzweig SD, Thompson KJ, Tokish J. Sequential volume reduction with capsular plications: relationship between cumulative size of plications and volumetric reduction for multidirectional instability of the shoulder. Am J Sports Med. 2011;39(3):526-531.
30. Lubowitz J, Bartolozzi A, Rubenstein D, et al. How much does inferior capsular shift reduce shoulder volume? Clin Orthop. 1996;(328):86-90.
31. Moon YL, Singh H, Yang H, Chul LK. Arthroscopic rotator interval closure by purse string suture for symptomatic inferior shoulder instability. Orthopedics. 2011;34(4).
32. Jerosch J, Castro WH. Shoulder instability in Ehlers-Danlos syndrome: an indication for surgical treatment? Acta Orthop Belg. 1990;56(2):451-453.
33. Schenk TJ, Brems JJ. Multidirectional instability of the shoulder: pathophysiology, diagnosis, and management. J Am Acad Orthop Surg. 1998;6(1):65-72.
34. Cohen SB, Wiley W, Goradia VK, Pearson S, Miller MD. Anterior capsulorrhaphy: an in vitro comparison of volume reduction. Arthroscopic plication versus open capsular shift. Arthroscopy. 2005;21(6):659-664.
Since Neer described the rotator interval in 1970, its closure, often used in conjunction with capsulorrhaphy, has become an important surgical technique in managing shoulder instability.1-11 Numerous studies have sought to define the function of the rotator interval.1-3,6-20 The etiology of lesions of the rotator interval has been debated, and there is evidence that such lesions may be in part congenital.21 Increased rotator interval depth and width, along with increased size of the distended inferior and posteroinferior joint capsule on magnetic resonance arthrography, have been reported in cases of multidirectional shoulder instability.22 However, confusion remains about the role of the rotator interval in shoulder instability and about the effect its closure has on shoulder function. No one knows the degree of volume reduction that results from closure of the rotator interval and whether medial and lateral sutures differ in the volume reduction achieved.
Cadaveric studies have shown that the rotator interval has an important role in shoulder motion.6,13-16,19,20,23 Harryman and colleagues13 found that sectioning the coracohumeral ligament (CHL) increased shoulder range of motion (ROM), and medial-to-lateral closure of the rotator interval restricted motion in all planes. Most notably, interval closure limited inferior translation in the adducted shoulder, posterior translation in the flexed adducted shoulder, and external rotation in the neutral position. Subsequent studies,17,18 using rotator interval closure combined with thermal capsulorrhaphy, confirmed the results reported by Harryman and colleagues.13
More recent cadaveric studies using superior-to-inferior rotator interval closures have shown a decrease in anterior translation but not posterior translation.14-16,19-21 A superior-to-inferior interval closure technique limited external rotation less than a medial-to-lateral closure did.13-16,19-21 The majority of arthroscopically described rotator interval closures involve a superior-to-inferior technique and use 2 or 3 sutures.1,3,9-11
Plausinis and colleagues15 examined the effects of an isolated medial, an isolated lateral, and a medial combined with a lateral closure of the rotator interval. They noted that all 3 methods limited anterior translation and motion by means of 6° flexion and 10° external rotation; however, there was no statistical difference between methods. They also found that occasionally the medial interval closure resulted in massive loss of external rotation. Earlier, Jost and colleagues14 noted that a medial rotator interval could cause this massive loss by tethering the CHL, resulting in a medial-to-lateral imbrication of the CHL.
Arthroscopic rotator interval closure has clinically demonstrated an additive effect on shoulder stability. The recurrence rate was lower for arthroscopic Bankart repair combined with arthroscopic rotator interval closure (8%) than for arthroscopic Bankart repair alone (13%).24 In addition, time to recurrent dislocation was longer (42 vs 13 months) for the group that underwent the combination of Bankart repair and rotator interval closure. Regarding the concern about loss of motion after arthroscopic rotator interval closure, Chiang and colleagues25 recently noted no significant loss of motion 5 years after arthroscopic Bankart repair with rotator interval closure.
What effect rotator interval closure has on intra-articular glenohumeral volume (GHV) remains unknown. Using a cadaveric model, Yamamoto and colleagues20 showed that decreasing GHV can increase the responsiveness of the glenohumeral joint to the intra-articular pressure. Thus, reducing the volume can improve stability in vitro by increasing the magnitude of negative pressure stabilizing the glenohumeral joint.
We conducted a study to quantify the effects of arthroscopic rotator interval closure on capsular volume and to determine whether medial and lateral interval closures resulted in different degrees of volume reduction. Our hypothesis was that shoulder volume would be significantly reduced by closing the rotator interval.
Materials and Methods
Previous studies have not specifically evaluated GHV after rotator interval closure. Our power analysis was performed with data from a study by Karas and colleagues,26 who evaluated GHV after capsular plication. To detect a capsular volume reduction of 20% per stitch, with a 2-sided 5% significance level and a power of 80%, we needed a sample size of 5 specimens per group.
After receiving institutional review board approval for this study, we obtained 10 cadaveric shoulders (5 matched pairs). Exclusion criteria included arthroscopic evaluation revealing a full-thickness rotator cuff tear or significant osteoarthritis. Two shoulders had full-thickness cuff tears, leaving 8 shoulders to be tested; 6 of these were matched pairs. The shoulders were from 1 man (matched pair) and 4 women (2 matched pairs). Age ranged from 38 to 70 years (mean, 59.6 years). Differences in material properties between the specimens were accounted for by using primarily matched pairs.
The 2 study groups consisted of 4 shoulders each. After specimens were thawed, the skin, subcutaneous tissues, and periscapular muscles were removed from the shoulder. Only the capsule, biceps, and rotator cuff remained. For measurement purposes, the shoulders were mounted in a vice clamp in a beach-chair orientation. We placed a total of 2 portals with fully threaded 8.25-mm cannulas (Arthrex, Naples, Florida). A standard posterior portal was placed in the soft spot. A low anterior portal was then placed just superior to the subscapularis tendon. For arthroscopic examination and instrumentation in a saline environment, the shoulders were rotated into the lateral decubitus position, with suspension in 30° abduction and 20° forward flexion, by a rope attached to a pin in the distal shaft of the humerus.
In both groups, medial and lateral stitches with No. 2 FiberWire (Arthrex) were used to close the interval. The medial interval closure stitch was placed more than 10 mm away from the glenoid to prevent unpredictable CHL tethering; the lateral closure stitch was placed 10 mm lateral to the medial stitch (Figure 1).14 All sutures were placed intra-articularly under direct arthroscopic visualization, similar to the methods described in the literature.1,3,9-11 Sutures were passed through the superior glenohumeral ligament (SGHL) and through the upper subscapularis using a suture shuttle (SutureLasso; Arthrex) and Penetrator II Suture Retriever (Arthrex). The upper subscapularis was incorporated because of the unpredictable nature of the middle glenohumeral ligament (MGHL). Both rotator interval sutures were placed before tying either. In the medial group, the medial stitch was tied first, using alternating half-hitches, followed by the lateral stitch. In the lateral group, the lateral stitch was tied first, followed by the medial stitch. GHV was measured at baseline and after tying each stitch. Dr. Ponce instrumented all shoulders.
Modifying a beach-chair technique described by Miller and colleagues,27 we used a viscous fatty-acid sulfate solution, liquid soap, to measure GHV.27-29 A small slit in line with the fibers was made in the supraspinatus tendon just lateral to the musculotendinous junction. A 3-way stop-cock was placed into the joint though this defect. A 20-mL syringe with a 16-gauge needle was used to inject the soap. The needle was inserted into the rotator cuff interval, and the viscous solution was injected in 5-mL increments until there was active extravasation through the supraspinatus cannula (Figure 2). This technique, the “volcano method,” marked the maximum capacity of the joint. The joint was then copiously irrigated with normal saline and suctioned until all normal saline was evacuated. Dr. Rosenzweig took 2 measurements on each shoulder, and their mean was used for analysis.
The baseline measurement was taken with the 2 working cannulas in the shoulder joint. Measurements were obtained with cannulas to simulate normal clinical conditions. Subsequent measurements were done with the cannulas in place and inserted up to the same thread each time so as not to change the volume. The capsule and the rotator cuff were then dissected from the humerus so the size of the capsulolabral plication could be directly evaluated. Methylene blue was used to mark the capsular suture holes before removing the sutures. With use of a caliper, the size of the plication bite was measured (in millimeters).
Statistical Analysis
The primary outcome was percent reduction in GHV as a function of number of plications and size of plication. When only the first plication was tightened, the effect of position (medial or lateral) was also of interest. Percent volume reduction was calculated as (original – new) / original × 100. SAS 8.02 (SAS Institute, Cary, North Carolina) was used to fit a repeated random-intercept regression model for each outcome. This technique properly accounts for the paired nature of the specimens and the repeated measures (baseline plus 2 plications). Model fit was assessed by the method of difference in log likelihood.
Results
In the medial group, GHV was reduced by a mean of 24.2% with a single medial stitch; in the lateral group, GHV was reduced by a mean of 35.1% (Figure 3). The difference was significant (P < .02). In the medial group, when a second lateral stitch was used, GHV was reduced by another 18.7%; in the lateral group, when a medial stitch was added, GHV was reduced by another 11.4%. Final GHV for the medial and lateral groups was 42.9% and 46.5%, respectively. There was no statistical difference in final GHV, regardless of which stitch was placed first. When the 2 groups were combined, GHV was reduced by 44.9% with use of medial and lateral rotator interval closure stitches.
Mean amount of tissue purchased, or “bite size,” was 18 mm with a lateral suture and 15 mm with a medial suture (P < .05). In addition, an increase in bite size to GHV reduction was essentially linear, where an increase in bite size of 1 mm reduced GHV by about 1% (Figure 4).
Discussion
Although there have been numerous clinical series and biomechanical studies focused on isolated rotator interval closure (or its use as an adjunct) in shoulder stabilization, the precise function of the rotator interval remains poorly understood.1-3,6-11,19 Consequently, the in vivo effects of interval closure are unknown.
Initial studies proposed that rotator interval closure limited inferior and posterior translation.30 More recent studies have demonstrated that rotator interval closure confers little effect on posterior instability but increases anterior stability in cadaveric models.15,16 Clinical series have provided evidence that rotator interval closure can increase anterior stability.1,3,7,9,12 In a series of isolated rotator interval closures for multidirectional instability, Field and colleagues12 found that preoperative anterior and inferior symptoms predominated over posterior symptoms. Isolated closure of the rotator interval resulted in 100% excellent results with no cases of recurrent instability. Moon and colleagues31 reported that arthroscopic rotator interval closure with or without inferior capsular plication in multidirectional instability and predominant symptomatic inferior instability has shown benefit by improving function and stability. Other clinical reports of rotator interval closure in conjunction with arthroscopic Bankart repair have suggested it has an additive effect on anterior shoulder stability without limiting motion.24,25
In our study, arthroscopic closure of the rotator interval with 2 superior-to-inferior stitches reduced intracapsular volume by 45%. Even though open capsular shifts use different surgical techniques, similar technique volume reduction studies have reported reductions between 34% and 54% with open shifts.27,30 It is unknown if the stability resulting from decreased GHV is primarily from increasing intra-articular pressures or from restricting ROM, or from a combination of both. In shoulders with multidirectional instability, the joint volume may be increased, the joint capsule may be enlarged, or the glenohumeral ligaments may be lax and thin.4,6,32,33 Yamamoto and colleagues19 stated that intra-articular pressure is determined by 3 factors: load, joint volume, and material properties of the capsule. Load is a constant; joint volume and material properties can be changed.19 In our study, material properties were controlled by using a majority of matched specimens. Regardless of the stabilizing mechanism, our study results demonstrated that arthroscopic rotator interval closure may be a powerful tool in reducing shoulder volume, a consistent principle of surgical techniques used in reestablishing shoulder stability.19,20
When a single rotator interval closure stitch was used, volume reduction with a lateral stitch was superior to that with a medial stitch. This finding is logical, as anatomically the dimensions of the rotator interval are larger laterally as the CHL fans out to insert on the greater and lesser tuberosities.14 This finding has also been reported in open capsular shifts for multidirectional instability, with a lateral humeral shift having a larger volume reduction than a medial glenoid shift.27 Miller and colleagues27 used the image of a cone, with its larger opening facing the humerus and narrower side facing the glenoid, to illustrate this difference in open capsular shifts.
Our study also showed a larger volume reduction with 2 rotator interval closure stitches than with a single interval stitch. As ROM testing has not shown a difference between results with 1 and 2 sutures, we recommend a minimum of 2 sutures for arthroscopic rotator interval closure.15 If a single plication stitch is preferred, a lateral stitch (vs a medial stitch) can be used for a significantly larger reduction in shoulder volume. We think this is because of a larger amount of capsule being purchased with lateral closure (Figure 5). However, if a medial stitch is used, it is important to not place it too near the glenoid to avoid CHL tethering and subsequent excessive loss of external rotation.15
This study had several weaknesses. First, it was a cadaveric study, and use of specimens not known to have instability or specific rotator interval injury may make generalization to a clinical situation difficult. Second, although our power analysis called for 5 shoulders in each group, full-thickness rotator cuff tears rendered 2 shoulders unusable. This reduced our sample sizes and potentially decreased the power of the study, though the data demonstrated statistically significant differences. Third, we did not compare the effects of an open medial-to-lateral imbrication of the rotator interval on intracapsular volume with the effects of our arthroscopic method. We also did not assess our specimens’ ROM, effects of interval closure stitches on shoulder stability, or glenohumeral contact surface pressures, as these factors have already been studied.13-19 Instead, we focused on the effects of rotator interval closure on intracapsular volume, which had not been quantified until now. The clinical significance of such a volume reduction is unknown, especially with respect to influence on ROM, but the degree of volume reduction was larger than with previously reported arthroscopic instability repairs and smaller than with open capsular shifts, demonstrating that it may be a powerful tool in restoring stability in an unstable shoulder.26-30,34 Fourth, the role of isolated rotator interval closure is poorly defined, as only 1 clinical series of isolated rotator interval closure has been reported thus far.12 It has been far more common for rotator interval closure to be used with Bankart repair or capsulorrhaphy.1-3,7-9
In a cadaveric study by Provencher and colleagues,16 open rotator interval closure with medial-to-lateral imbrication of the interval altered shoulder kinematics differently from what occurred with arthroscopic closure of the MGHL to the SGHL, resulting in superior-to-inferior shift. Comparing the 2 methods may therefore be inappropriate. Currently we reserve rotator interval closure for infrequent cases of revision instability and cases in which glenoid bone loss is marginal (5%-15%) and there is a willingness to potentially sacrifice ROM to restore stability and avoid an open stabilization procedure. Continued investigation into the clinical role of rotator interval closure in shoulder stability is needed. We should identify the pathology in a patient with instability and use this technique as an adjuvant to other stabilization procedures.
Conclusion
Arthroscopic rotator interval closure with 2 plication stitches is a powerful tool in reducing the intracapsular volume of the shoulder. If a single plication stitch is preferred, a lateral rotator interval closure stitch (vs a medial stitch) can be used for a larger reduction in shoulder volume.
Since Neer described the rotator interval in 1970, its closure, often used in conjunction with capsulorrhaphy, has become an important surgical technique in managing shoulder instability.1-11 Numerous studies have sought to define the function of the rotator interval.1-3,6-20 The etiology of lesions of the rotator interval has been debated, and there is evidence that such lesions may be in part congenital.21 Increased rotator interval depth and width, along with increased size of the distended inferior and posteroinferior joint capsule on magnetic resonance arthrography, have been reported in cases of multidirectional shoulder instability.22 However, confusion remains about the role of the rotator interval in shoulder instability and about the effect its closure has on shoulder function. No one knows the degree of volume reduction that results from closure of the rotator interval and whether medial and lateral sutures differ in the volume reduction achieved.
Cadaveric studies have shown that the rotator interval has an important role in shoulder motion.6,13-16,19,20,23 Harryman and colleagues13 found that sectioning the coracohumeral ligament (CHL) increased shoulder range of motion (ROM), and medial-to-lateral closure of the rotator interval restricted motion in all planes. Most notably, interval closure limited inferior translation in the adducted shoulder, posterior translation in the flexed adducted shoulder, and external rotation in the neutral position. Subsequent studies,17,18 using rotator interval closure combined with thermal capsulorrhaphy, confirmed the results reported by Harryman and colleagues.13
More recent cadaveric studies using superior-to-inferior rotator interval closures have shown a decrease in anterior translation but not posterior translation.14-16,19-21 A superior-to-inferior interval closure technique limited external rotation less than a medial-to-lateral closure did.13-16,19-21 The majority of arthroscopically described rotator interval closures involve a superior-to-inferior technique and use 2 or 3 sutures.1,3,9-11
Plausinis and colleagues15 examined the effects of an isolated medial, an isolated lateral, and a medial combined with a lateral closure of the rotator interval. They noted that all 3 methods limited anterior translation and motion by means of 6° flexion and 10° external rotation; however, there was no statistical difference between methods. They also found that occasionally the medial interval closure resulted in massive loss of external rotation. Earlier, Jost and colleagues14 noted that a medial rotator interval could cause this massive loss by tethering the CHL, resulting in a medial-to-lateral imbrication of the CHL.
Arthroscopic rotator interval closure has clinically demonstrated an additive effect on shoulder stability. The recurrence rate was lower for arthroscopic Bankart repair combined with arthroscopic rotator interval closure (8%) than for arthroscopic Bankart repair alone (13%).24 In addition, time to recurrent dislocation was longer (42 vs 13 months) for the group that underwent the combination of Bankart repair and rotator interval closure. Regarding the concern about loss of motion after arthroscopic rotator interval closure, Chiang and colleagues25 recently noted no significant loss of motion 5 years after arthroscopic Bankart repair with rotator interval closure.
What effect rotator interval closure has on intra-articular glenohumeral volume (GHV) remains unknown. Using a cadaveric model, Yamamoto and colleagues20 showed that decreasing GHV can increase the responsiveness of the glenohumeral joint to the intra-articular pressure. Thus, reducing the volume can improve stability in vitro by increasing the magnitude of negative pressure stabilizing the glenohumeral joint.
We conducted a study to quantify the effects of arthroscopic rotator interval closure on capsular volume and to determine whether medial and lateral interval closures resulted in different degrees of volume reduction. Our hypothesis was that shoulder volume would be significantly reduced by closing the rotator interval.
Materials and Methods
Previous studies have not specifically evaluated GHV after rotator interval closure. Our power analysis was performed with data from a study by Karas and colleagues,26 who evaluated GHV after capsular plication. To detect a capsular volume reduction of 20% per stitch, with a 2-sided 5% significance level and a power of 80%, we needed a sample size of 5 specimens per group.
After receiving institutional review board approval for this study, we obtained 10 cadaveric shoulders (5 matched pairs). Exclusion criteria included arthroscopic evaluation revealing a full-thickness rotator cuff tear or significant osteoarthritis. Two shoulders had full-thickness cuff tears, leaving 8 shoulders to be tested; 6 of these were matched pairs. The shoulders were from 1 man (matched pair) and 4 women (2 matched pairs). Age ranged from 38 to 70 years (mean, 59.6 years). Differences in material properties between the specimens were accounted for by using primarily matched pairs.
The 2 study groups consisted of 4 shoulders each. After specimens were thawed, the skin, subcutaneous tissues, and periscapular muscles were removed from the shoulder. Only the capsule, biceps, and rotator cuff remained. For measurement purposes, the shoulders were mounted in a vice clamp in a beach-chair orientation. We placed a total of 2 portals with fully threaded 8.25-mm cannulas (Arthrex, Naples, Florida). A standard posterior portal was placed in the soft spot. A low anterior portal was then placed just superior to the subscapularis tendon. For arthroscopic examination and instrumentation in a saline environment, the shoulders were rotated into the lateral decubitus position, with suspension in 30° abduction and 20° forward flexion, by a rope attached to a pin in the distal shaft of the humerus.
In both groups, medial and lateral stitches with No. 2 FiberWire (Arthrex) were used to close the interval. The medial interval closure stitch was placed more than 10 mm away from the glenoid to prevent unpredictable CHL tethering; the lateral closure stitch was placed 10 mm lateral to the medial stitch (Figure 1).14 All sutures were placed intra-articularly under direct arthroscopic visualization, similar to the methods described in the literature.1,3,9-11 Sutures were passed through the superior glenohumeral ligament (SGHL) and through the upper subscapularis using a suture shuttle (SutureLasso; Arthrex) and Penetrator II Suture Retriever (Arthrex). The upper subscapularis was incorporated because of the unpredictable nature of the middle glenohumeral ligament (MGHL). Both rotator interval sutures were placed before tying either. In the medial group, the medial stitch was tied first, using alternating half-hitches, followed by the lateral stitch. In the lateral group, the lateral stitch was tied first, followed by the medial stitch. GHV was measured at baseline and after tying each stitch. Dr. Ponce instrumented all shoulders.
Modifying a beach-chair technique described by Miller and colleagues,27 we used a viscous fatty-acid sulfate solution, liquid soap, to measure GHV.27-29 A small slit in line with the fibers was made in the supraspinatus tendon just lateral to the musculotendinous junction. A 3-way stop-cock was placed into the joint though this defect. A 20-mL syringe with a 16-gauge needle was used to inject the soap. The needle was inserted into the rotator cuff interval, and the viscous solution was injected in 5-mL increments until there was active extravasation through the supraspinatus cannula (Figure 2). This technique, the “volcano method,” marked the maximum capacity of the joint. The joint was then copiously irrigated with normal saline and suctioned until all normal saline was evacuated. Dr. Rosenzweig took 2 measurements on each shoulder, and their mean was used for analysis.
The baseline measurement was taken with the 2 working cannulas in the shoulder joint. Measurements were obtained with cannulas to simulate normal clinical conditions. Subsequent measurements were done with the cannulas in place and inserted up to the same thread each time so as not to change the volume. The capsule and the rotator cuff were then dissected from the humerus so the size of the capsulolabral plication could be directly evaluated. Methylene blue was used to mark the capsular suture holes before removing the sutures. With use of a caliper, the size of the plication bite was measured (in millimeters).
Statistical Analysis
The primary outcome was percent reduction in GHV as a function of number of plications and size of plication. When only the first plication was tightened, the effect of position (medial or lateral) was also of interest. Percent volume reduction was calculated as (original – new) / original × 100. SAS 8.02 (SAS Institute, Cary, North Carolina) was used to fit a repeated random-intercept regression model for each outcome. This technique properly accounts for the paired nature of the specimens and the repeated measures (baseline plus 2 plications). Model fit was assessed by the method of difference in log likelihood.
Results
In the medial group, GHV was reduced by a mean of 24.2% with a single medial stitch; in the lateral group, GHV was reduced by a mean of 35.1% (Figure 3). The difference was significant (P < .02). In the medial group, when a second lateral stitch was used, GHV was reduced by another 18.7%; in the lateral group, when a medial stitch was added, GHV was reduced by another 11.4%. Final GHV for the medial and lateral groups was 42.9% and 46.5%, respectively. There was no statistical difference in final GHV, regardless of which stitch was placed first. When the 2 groups were combined, GHV was reduced by 44.9% with use of medial and lateral rotator interval closure stitches.
Mean amount of tissue purchased, or “bite size,” was 18 mm with a lateral suture and 15 mm with a medial suture (P < .05). In addition, an increase in bite size to GHV reduction was essentially linear, where an increase in bite size of 1 mm reduced GHV by about 1% (Figure 4).
Discussion
Although there have been numerous clinical series and biomechanical studies focused on isolated rotator interval closure (or its use as an adjunct) in shoulder stabilization, the precise function of the rotator interval remains poorly understood.1-3,6-11,19 Consequently, the in vivo effects of interval closure are unknown.
Initial studies proposed that rotator interval closure limited inferior and posterior translation.30 More recent studies have demonstrated that rotator interval closure confers little effect on posterior instability but increases anterior stability in cadaveric models.15,16 Clinical series have provided evidence that rotator interval closure can increase anterior stability.1,3,7,9,12 In a series of isolated rotator interval closures for multidirectional instability, Field and colleagues12 found that preoperative anterior and inferior symptoms predominated over posterior symptoms. Isolated closure of the rotator interval resulted in 100% excellent results with no cases of recurrent instability. Moon and colleagues31 reported that arthroscopic rotator interval closure with or without inferior capsular plication in multidirectional instability and predominant symptomatic inferior instability has shown benefit by improving function and stability. Other clinical reports of rotator interval closure in conjunction with arthroscopic Bankart repair have suggested it has an additive effect on anterior shoulder stability without limiting motion.24,25
In our study, arthroscopic closure of the rotator interval with 2 superior-to-inferior stitches reduced intracapsular volume by 45%. Even though open capsular shifts use different surgical techniques, similar technique volume reduction studies have reported reductions between 34% and 54% with open shifts.27,30 It is unknown if the stability resulting from decreased GHV is primarily from increasing intra-articular pressures or from restricting ROM, or from a combination of both. In shoulders with multidirectional instability, the joint volume may be increased, the joint capsule may be enlarged, or the glenohumeral ligaments may be lax and thin.4,6,32,33 Yamamoto and colleagues19 stated that intra-articular pressure is determined by 3 factors: load, joint volume, and material properties of the capsule. Load is a constant; joint volume and material properties can be changed.19 In our study, material properties were controlled by using a majority of matched specimens. Regardless of the stabilizing mechanism, our study results demonstrated that arthroscopic rotator interval closure may be a powerful tool in reducing shoulder volume, a consistent principle of surgical techniques used in reestablishing shoulder stability.19,20
When a single rotator interval closure stitch was used, volume reduction with a lateral stitch was superior to that with a medial stitch. This finding is logical, as anatomically the dimensions of the rotator interval are larger laterally as the CHL fans out to insert on the greater and lesser tuberosities.14 This finding has also been reported in open capsular shifts for multidirectional instability, with a lateral humeral shift having a larger volume reduction than a medial glenoid shift.27 Miller and colleagues27 used the image of a cone, with its larger opening facing the humerus and narrower side facing the glenoid, to illustrate this difference in open capsular shifts.
Our study also showed a larger volume reduction with 2 rotator interval closure stitches than with a single interval stitch. As ROM testing has not shown a difference between results with 1 and 2 sutures, we recommend a minimum of 2 sutures for arthroscopic rotator interval closure.15 If a single plication stitch is preferred, a lateral stitch (vs a medial stitch) can be used for a significantly larger reduction in shoulder volume. We think this is because of a larger amount of capsule being purchased with lateral closure (Figure 5). However, if a medial stitch is used, it is important to not place it too near the glenoid to avoid CHL tethering and subsequent excessive loss of external rotation.15
This study had several weaknesses. First, it was a cadaveric study, and use of specimens not known to have instability or specific rotator interval injury may make generalization to a clinical situation difficult. Second, although our power analysis called for 5 shoulders in each group, full-thickness rotator cuff tears rendered 2 shoulders unusable. This reduced our sample sizes and potentially decreased the power of the study, though the data demonstrated statistically significant differences. Third, we did not compare the effects of an open medial-to-lateral imbrication of the rotator interval on intracapsular volume with the effects of our arthroscopic method. We also did not assess our specimens’ ROM, effects of interval closure stitches on shoulder stability, or glenohumeral contact surface pressures, as these factors have already been studied.13-19 Instead, we focused on the effects of rotator interval closure on intracapsular volume, which had not been quantified until now. The clinical significance of such a volume reduction is unknown, especially with respect to influence on ROM, but the degree of volume reduction was larger than with previously reported arthroscopic instability repairs and smaller than with open capsular shifts, demonstrating that it may be a powerful tool in restoring stability in an unstable shoulder.26-30,34 Fourth, the role of isolated rotator interval closure is poorly defined, as only 1 clinical series of isolated rotator interval closure has been reported thus far.12 It has been far more common for rotator interval closure to be used with Bankart repair or capsulorrhaphy.1-3,7-9
In a cadaveric study by Provencher and colleagues,16 open rotator interval closure with medial-to-lateral imbrication of the interval altered shoulder kinematics differently from what occurred with arthroscopic closure of the MGHL to the SGHL, resulting in superior-to-inferior shift. Comparing the 2 methods may therefore be inappropriate. Currently we reserve rotator interval closure for infrequent cases of revision instability and cases in which glenoid bone loss is marginal (5%-15%) and there is a willingness to potentially sacrifice ROM to restore stability and avoid an open stabilization procedure. Continued investigation into the clinical role of rotator interval closure in shoulder stability is needed. We should identify the pathology in a patient with instability and use this technique as an adjuvant to other stabilization procedures.
Conclusion
Arthroscopic rotator interval closure with 2 plication stitches is a powerful tool in reducing the intracapsular volume of the shoulder. If a single plication stitch is preferred, a lateral rotator interval closure stitch (vs a medial stitch) can be used for a larger reduction in shoulder volume.
1. Creighton RA, Romeo AA, Brown FM, Hayden JK, Verma NN. Revision arthroscopic shoulder instability repair. Arthroscopy. 2007;23(7):703-709.
2. Gartsman GM, Roddey TS, Hammerman SM. Arthroscopic treatment of anterior-inferior glenohumeral instability. Two to five-year follow-up. J Bone Joint Surg Am. 2000;82(7):991-1003.
3. Gartsman GM, Taverna E, Hammerman SM. Arthroscopic rotator interval repair in glenohumeral instability: description of an operative technique. Arthroscopy. 1999;15(3):330-332.
4. Neer CS 2nd, Foster CR. Inferior capsular shift for involuntary inferior and multidirectional instability of the shoulder: a preliminary report. J Bone Joint Surg Am. 1980;62(6):897-908.
5. Neer CS 2nd. Displaced proximal humerus fractures: I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
6. Nobuhara K, Ikeda H. Rotator interval lesion. Clin Orthop. 1987;(223):44-50.
7. Rowe CR, Zarins B, Ciullo JV. Recurrent anterior dislocation of the shoulder after surgical repair. J Bone Joint Surg Am. 1984;66(2):159-168.
8. Rowe CR, Zarins B. Recurrent transient subluxation of the shoulder. J Bone Joint Surg Am. 1981;63(6):863-872.
9. Stokes DA, Savoie FH, Field LD. Arthroscopic repair of anterior glenohumeral instability and rotator interval lesions. Orthop Clin North Am. 2003;34(4):529-539.
10. Taverna E, Sansone V, Battistella F. Arthroscopic rotator interval repair: the three-step all-inside technique. Arthroscopy. 2004;20 Suppl 2:105-109.
11. Treacy SH, Field LD, Savoie FH. Rotator interval capsule closure: an arthroscopic technique. Arthroscopy. 1997;13(1):103-106.
12. Field LD, Warren RF, O’Brien SJ, Altcheck DW, Wickiewicz TL. Isolated closure of rotator interval defects for shoulder instability. Am J Sports Med. 1995;23(5):557-563.
13. Harryman DT 2nd, Sidles JA, Harris SL, Matsen FA 3rd. The role of the rotator interval capsule in passive motion and stability of the shoulder. J Bone Joint Surg Am. 1992;74(1):53-66.
14. Jost B, Koch PP, Gerber C. Anatomy and functional aspects of the rotator interval. J Shoulder Elbow Surg. 2000;9(4):336-341.
15. Plausinis D, Bravman JT, Heywood C, Kummer FJ, Kwon YM, Jazrawi LM. Arthroscopic rotator interval closure: effect of sutures on glenohumeral motion and anterior-posterior translation. Am J Sports Med. 2006;34(10):1656-1661.
16. Provencher MT, Mologne TS, Hongo M, Zhao K, Tasto JP, An KN. Arthroscopic versus open rotator interval closure: biomechanical evaluation of stability and motion. Arthroscopy. 2007;23(6):583-592.
17. Selecky MT, Tibone JE, Yang BY, et al. Glenohumeral joint translation after thermal capsuloplasty of the rotator interval. J Shoulder Elbow Surg. 2003;12(2):139-143.
18. Wolf R, Zheng N, Iero J, Weichel D. The effects of thermal capsulorrhaphy and rotator interval closure on multidirectional laxity in the glenohumeral joint: a cadaveric biomechanical study. Arthroscopy. 2004;20(10):1044-1049.
19. Yamamoto N, Itoi E, Tuoheti Y, et al. Effect of rotator interval closure on glenohumeral stability and motion: a cadaveric study. J Shoulder Elbow Surg. 2006;15(6):750-758.
20. Yamamoto N, Itoi E, Tuoheti Y, et al. The effect of the inferior capsular shift on shoulder intra-articular pressure: a cadaveric study. Am J Sports Med. 2006;34(6):939-944.
21. Cole BJ, Rodeo SA, O’Brien SJ, et al. The anatomy and histology of the rotator interval capsule of the shoulder. Clin Orthop. 2001;(390):129-137.
22. Lee HJ, Kim NR, Moon SG, Ko SM, Park JY. Multidirectional instability of the shoulder: rotator interval dimension and capsular laxity evaluation using MR arthrography. Skeletal Radiol. 2013;42(2):231-238.
23. Warner JP, Deng X, Warren RF, Torzilli PA, O’Brien SJ. Superoinferior translation in intact and vented glenohumeral joint. J Shoulder Elbow Surg. 1993;2(2):99-105.
24. Chechik O, Maman E, Dolkart O, Khashan M, Shabtai L, Mozes G. Arthroscopic rotator interval closure in shoulder instability repair: a retrospective study. J Shoulder Elbow Surg. 2010;19(7):1056-1062.
25. Chiang, E, Wang J, Wang S, et al. Arthroscopic posteroinferior capsular plication and rotator interval closure after Bankart repair in patients with traumatic anterior glenohumeral instability—a minimum follow-up of 5 years. Injury. 2010;41(10):1075-1078.
26. Karas SG, Creighton RA, DeMorat GJ. Glenohumeral volume reduction in arthroscopic shoulder reconstruction: a cadaveric analysis of suture plication and thermal capsulorrhaphy. Arthroscopy. 2004;20(2):179-184.
27. Miller MD, Larsen KM, Luke T, Leis HT, Plancher KD. Anterior capsular shift volume reduction: an in vitro comparison of 3 techniques. J Shoulder Elbow Surg. 2003;12(4):350-354.
28. Luke TA, Rovner AD, Karas SG, Hawkins RJ, Plancher KD. Volumetric change in the shoulder capsule after open inferior capsular shift versus arthroscopic thermal capsular shrinkage: a cadaveric model. J Shoulder Elbow Surg. 2004;13(2):146-149.
29. Ponce BA, Rosenzweig SD, Thompson KJ, Tokish J. Sequential volume reduction with capsular plications: relationship between cumulative size of plications and volumetric reduction for multidirectional instability of the shoulder. Am J Sports Med. 2011;39(3):526-531.
30. Lubowitz J, Bartolozzi A, Rubenstein D, et al. How much does inferior capsular shift reduce shoulder volume? Clin Orthop. 1996;(328):86-90.
31. Moon YL, Singh H, Yang H, Chul LK. Arthroscopic rotator interval closure by purse string suture for symptomatic inferior shoulder instability. Orthopedics. 2011;34(4).
32. Jerosch J, Castro WH. Shoulder instability in Ehlers-Danlos syndrome: an indication for surgical treatment? Acta Orthop Belg. 1990;56(2):451-453.
33. Schenk TJ, Brems JJ. Multidirectional instability of the shoulder: pathophysiology, diagnosis, and management. J Am Acad Orthop Surg. 1998;6(1):65-72.
34. Cohen SB, Wiley W, Goradia VK, Pearson S, Miller MD. Anterior capsulorrhaphy: an in vitro comparison of volume reduction. Arthroscopic plication versus open capsular shift. Arthroscopy. 2005;21(6):659-664.
1. Creighton RA, Romeo AA, Brown FM, Hayden JK, Verma NN. Revision arthroscopic shoulder instability repair. Arthroscopy. 2007;23(7):703-709.
2. Gartsman GM, Roddey TS, Hammerman SM. Arthroscopic treatment of anterior-inferior glenohumeral instability. Two to five-year follow-up. J Bone Joint Surg Am. 2000;82(7):991-1003.
3. Gartsman GM, Taverna E, Hammerman SM. Arthroscopic rotator interval repair in glenohumeral instability: description of an operative technique. Arthroscopy. 1999;15(3):330-332.
4. Neer CS 2nd, Foster CR. Inferior capsular shift for involuntary inferior and multidirectional instability of the shoulder: a preliminary report. J Bone Joint Surg Am. 1980;62(6):897-908.
5. Neer CS 2nd. Displaced proximal humerus fractures: I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
6. Nobuhara K, Ikeda H. Rotator interval lesion. Clin Orthop. 1987;(223):44-50.
7. Rowe CR, Zarins B, Ciullo JV. Recurrent anterior dislocation of the shoulder after surgical repair. J Bone Joint Surg Am. 1984;66(2):159-168.
8. Rowe CR, Zarins B. Recurrent transient subluxation of the shoulder. J Bone Joint Surg Am. 1981;63(6):863-872.
9. Stokes DA, Savoie FH, Field LD. Arthroscopic repair of anterior glenohumeral instability and rotator interval lesions. Orthop Clin North Am. 2003;34(4):529-539.
10. Taverna E, Sansone V, Battistella F. Arthroscopic rotator interval repair: the three-step all-inside technique. Arthroscopy. 2004;20 Suppl 2:105-109.
11. Treacy SH, Field LD, Savoie FH. Rotator interval capsule closure: an arthroscopic technique. Arthroscopy. 1997;13(1):103-106.
12. Field LD, Warren RF, O’Brien SJ, Altcheck DW, Wickiewicz TL. Isolated closure of rotator interval defects for shoulder instability. Am J Sports Med. 1995;23(5):557-563.
13. Harryman DT 2nd, Sidles JA, Harris SL, Matsen FA 3rd. The role of the rotator interval capsule in passive motion and stability of the shoulder. J Bone Joint Surg Am. 1992;74(1):53-66.
14. Jost B, Koch PP, Gerber C. Anatomy and functional aspects of the rotator interval. J Shoulder Elbow Surg. 2000;9(4):336-341.
15. Plausinis D, Bravman JT, Heywood C, Kummer FJ, Kwon YM, Jazrawi LM. Arthroscopic rotator interval closure: effect of sutures on glenohumeral motion and anterior-posterior translation. Am J Sports Med. 2006;34(10):1656-1661.
16. Provencher MT, Mologne TS, Hongo M, Zhao K, Tasto JP, An KN. Arthroscopic versus open rotator interval closure: biomechanical evaluation of stability and motion. Arthroscopy. 2007;23(6):583-592.
17. Selecky MT, Tibone JE, Yang BY, et al. Glenohumeral joint translation after thermal capsuloplasty of the rotator interval. J Shoulder Elbow Surg. 2003;12(2):139-143.
18. Wolf R, Zheng N, Iero J, Weichel D. The effects of thermal capsulorrhaphy and rotator interval closure on multidirectional laxity in the glenohumeral joint: a cadaveric biomechanical study. Arthroscopy. 2004;20(10):1044-1049.
19. Yamamoto N, Itoi E, Tuoheti Y, et al. Effect of rotator interval closure on glenohumeral stability and motion: a cadaveric study. J Shoulder Elbow Surg. 2006;15(6):750-758.
20. Yamamoto N, Itoi E, Tuoheti Y, et al. The effect of the inferior capsular shift on shoulder intra-articular pressure: a cadaveric study. Am J Sports Med. 2006;34(6):939-944.
21. Cole BJ, Rodeo SA, O’Brien SJ, et al. The anatomy and histology of the rotator interval capsule of the shoulder. Clin Orthop. 2001;(390):129-137.
22. Lee HJ, Kim NR, Moon SG, Ko SM, Park JY. Multidirectional instability of the shoulder: rotator interval dimension and capsular laxity evaluation using MR arthrography. Skeletal Radiol. 2013;42(2):231-238.
23. Warner JP, Deng X, Warren RF, Torzilli PA, O’Brien SJ. Superoinferior translation in intact and vented glenohumeral joint. J Shoulder Elbow Surg. 1993;2(2):99-105.
24. Chechik O, Maman E, Dolkart O, Khashan M, Shabtai L, Mozes G. Arthroscopic rotator interval closure in shoulder instability repair: a retrospective study. J Shoulder Elbow Surg. 2010;19(7):1056-1062.
25. Chiang, E, Wang J, Wang S, et al. Arthroscopic posteroinferior capsular plication and rotator interval closure after Bankart repair in patients with traumatic anterior glenohumeral instability—a minimum follow-up of 5 years. Injury. 2010;41(10):1075-1078.
26. Karas SG, Creighton RA, DeMorat GJ. Glenohumeral volume reduction in arthroscopic shoulder reconstruction: a cadaveric analysis of suture plication and thermal capsulorrhaphy. Arthroscopy. 2004;20(2):179-184.
27. Miller MD, Larsen KM, Luke T, Leis HT, Plancher KD. Anterior capsular shift volume reduction: an in vitro comparison of 3 techniques. J Shoulder Elbow Surg. 2003;12(4):350-354.
28. Luke TA, Rovner AD, Karas SG, Hawkins RJ, Plancher KD. Volumetric change in the shoulder capsule after open inferior capsular shift versus arthroscopic thermal capsular shrinkage: a cadaveric model. J Shoulder Elbow Surg. 2004;13(2):146-149.
29. Ponce BA, Rosenzweig SD, Thompson KJ, Tokish J. Sequential volume reduction with capsular plications: relationship between cumulative size of plications and volumetric reduction for multidirectional instability of the shoulder. Am J Sports Med. 2011;39(3):526-531.
30. Lubowitz J, Bartolozzi A, Rubenstein D, et al. How much does inferior capsular shift reduce shoulder volume? Clin Orthop. 1996;(328):86-90.
31. Moon YL, Singh H, Yang H, Chul LK. Arthroscopic rotator interval closure by purse string suture for symptomatic inferior shoulder instability. Orthopedics. 2011;34(4).
32. Jerosch J, Castro WH. Shoulder instability in Ehlers-Danlos syndrome: an indication for surgical treatment? Acta Orthop Belg. 1990;56(2):451-453.
33. Schenk TJ, Brems JJ. Multidirectional instability of the shoulder: pathophysiology, diagnosis, and management. J Am Acad Orthop Surg. 1998;6(1):65-72.
34. Cohen SB, Wiley W, Goradia VK, Pearson S, Miller MD. Anterior capsulorrhaphy: an in vitro comparison of volume reduction. Arthroscopic plication versus open capsular shift. Arthroscopy. 2005;21(6):659-664.
Assessment of Scapular Morphology and Surgical Technique as Predictors of Notching in Reverse Shoulder Arthroplasty
Reverse shoulder arthroplasty (RSA) is a treatment option for a spectrum of diseases in shoulders with rotator cuff deficiency. There are distinct morphologic changes in the scapular and glenoid anatomy in patients with chronic rotator cuff tears.1 A muscular imbalance that occurs in the joint as a result of rotator cuff deficiency leads to morphologic changes that eliminate the compressive forces that hold the humeral head against the glenoid.2 RSA effectively stabilizes the glenohumeral joint in shoulders with deficient rotator cuffs.3,4 In early work, Grammont proposed that the glenosphere center of rotation should be medialized (concentric to the central axis of the metaglene or baseplate) and lowered.5 Although the medialized center of rotation in Grammont prostheses decreases shear forces and improves the deltoid lever arm, it also tends to result in mechanical impingement between the superomedial aspect of the humeral polyethylene insert and the scapular neck—so-called inferior scapular notching.6-9
Notching, which has been reported in 50% to 96% of patients who receive a Delta III prosthesis, typically appears within the first few months after surgery but may be seen as late as 14 months after surgery.5,10-12 Postmortem studies have shown that notching corresponds with erosion of the inferior pole of the glenoid and scapular neck, thought to be caused by the polyethylene cup of the implant.13 Although some studies have found that notching stabilizes after 1 year, others have shown notching progressing for up to 4 years after surgery.11,12,14 The clinical relevance of notching continues to be controversial, but notching has been associated with poorer clinical outcomes, polyethylene wear, and local osteolysis. Component loosening has also been reported with notching of grade 3 or more.8,10 Ultimately, there is concern that scapular notching could progress, ultimately leading to late glenoid loosening and potentially catastrophic failure.
Scapular anatomy has become an area of increased focus in rotator cuff disorders and in effects on RSA biomechanics.9 Recent reports have described important scapular morphology variations that suggest more individualized adjustments are needed during RSA.9,15 In addition, some investigators have reported that development of notching appears to depend on the height and inclination of the implanted glenoid component, where an inferior position of the glenosphere leads to less impingement and better range of motion.8,16 Simovitch and colleagues8 found the angle between the glenosphere and scapular neck and the craniocaudal position of the glenosphere to be highly correlated with inferior notching. They combined these 2 parameters into a predictive algorithm that provides a guideline (notching index, <35) for prevention of notching.
We conducted a study to evaluate the scapular notching index as a predictive tool and to consider other factors that may be associated with scapular notching occurring with use of Grammont reverse replacement systems. We hypothesized that patients with a notching index of less than 35 would not develop notching and that patients with an index of more than 35 would have increased incidence and severity of notching.
Materials and Methods
Patients treated with RSA for painful cuff tear arthropathy or irreparable rotator cuff tear with pseudoparesis (inability to actively elevate shoulder >90° in presence of free passive anterior elevation) were included in this retrospective review. All patients were treated between 2006 and 2010 by 1 of 2 established senior shoulder subspecialty surgeons. Patients treated with a Delta (DePuy Orthopaedics, Warsaw, Indiana) or an Aequalis (Tornier, Edina, Minnesota) reverse shoulder implant were included in the study. A standard polyethylene liner was used for all patients. These prostheses have the same neck shaft angle, 155º, as they have similar geometric designs, both based on the Grammont design—semiconstrained inverted with a fixed, lowered, medialized center of rotation. Standard instrumentation was used for all procedures. Patients were excluded if any nonstandard techniques or components were used (constrained or high-mobility liner, glenoid bone grafting). Patients who underwent revision for a previous reverse total arthroplasty, a total shoulder arthroplasty, or a hemiarthroplasty, or for treatment of acute fracture, posttraumatic deformity, or posttraumatic arthritis, were also excluded from our analyses. Minimum follow-up for study inclusion was 24 months.
All procedures were performed with the patient in the semi-beach-chair position and with use of a deltopectoral approach. The glenoid was prepared such that minimal reaming was needed to preserve the subchondral plate. The glenoid baseplate was positioned in the recommended inferior position to minimize notching and optimize functional outcomes.13 After surgery, all patients were managed with a simple soft immobilizer with or without a pillow with the arm at the patient’s side in internal rotation. Immediate passive mobilization was begun under the direction of physical therapists. Passive and active-assisted exercises were continued with gradual progression to independent activities of daily living at 6 weeks. Clinical evaluations were performed before and after surgery by the operating surgeon or independent research nurse. Active forward flexion, passive external rotation, strength, and visual analog scale (VAS) pain scores were reviewed and recorded. Case-specific complications were also reviewed.
Preoperative and postoperative anteroposterior radiographs were evaluated by 2 independent observers (attending surgeon, junior resident). Per standard technique, each radiograph was positioned horizontal to the scapular plane. Of the 91 patients, 66 had preoperative shoulder radiographs of acceptable quality, with complete visualization of scapular morphology. Radiographs were reviewed to measure the scapular neck angle (SNA), inferior scapular notching, prosthesis–scapular neck angle (PSNA), and peg glenoid rim distance (PGRD) (Figure 1). For the 66 patients with acceptable preoperative radiographs, SNA was determined by subtracting preoperative SNA from postoperative PSNA. Postoperative anteroposterior radiographs were used to classify degree of inferior scapular notching based on the Nerot grading scale (0-4). In addition, glenosphere overhang and glenosphere inclination were measured on postoperative radiographs.
The 91 shoulders were sorted into 2 groups based on degree of scapular notching: group 1, Nerot grade 0 (no inferior notching) and grade 1, and group 2, Nerot grades 2, 3, and 4. Group 1 had 37 patients with a size 36 glenosphere, 3 patients with size 38, and 8 patients with size 42; group 2 had 34 patients with a size 36 glenosphere, 1 patient with size 38, and 8 patients with size 42. All measurements were normalized to account for differences in glenosphere size. Groups 1 and 2 were compared on each radiographic parameter (inferior scapular notching, PSNA, PGRD, SNA).
Notching indexes were calculated ([PSNA × 0.13] + PGRD) and compared with the suggested index of 35.8 Simovitch and colleagues8 demonstrated that a notching index of more than 35 had 91% sensitivity and 88% specificity in predicting inferior notching, whereas a notching index of 35 or less avoided inferior notching 91% of the time. In this study, notching index was calculated for each patient, and then the mean values of groups 1 and 2 were compared (Table 1).
The effect of scapular notching and other individual radiographic parameters on outcomes was also evaluated with respect to forward flexion, external rotation, VAS pain score, complications, and external rotation lag sign. Mann-Whitney U test was used to test these variables; Spearman rank test was performed to determine correlation between each variable and scapular notching; logistic regression was used to explore the relationship of variables (PGRD, PSNA) as predictors of Nerot degree of inferior scapular notching, and postoperative complications; and independent-samples t test was used to determine group differences for each variable. For each investigation, the level of significance was set at P < .05. A biostatistician performed all statistical analyses using SPSS Version 19 (IBM, Armonk, New York).
Results
Our study cohort consisted of 91 shoulders. Mean follow-up was 41.8 months (range, 24.0-80.8 months). Seventy-five (82%) of the 91 shoulders developed scapular notching. Mean (SD) SNA on preoperative radiographs, used to assess preoperative scapular morphology, was 103.9° (14.5°). For all 91 shoulders, mean (SD) PSNA was 125.6° (16°), and mean (SD) PGRD was 16 (5.4) mm (Table 1). Inclination measurements were available for 86 patients. Mean (SD) inclination from 90° was 2.5° (10.3°) (range, 21°-30°). Mean (SD) SNA (postoperative PSNA minus preoperative SNA) for the 66 patients with acceptable preoperative radiographs was 24.3° (21.3°) (Table 1). Forty-eight of the 91 shoulders were placed in scapular notching group 1 (16 grade-0 shoulders, 32 grade-1 shoulders); the other 43 shoulders were placed in group 2 (33 grade-2 shoulders, 9 grade-3 shoulders, 1 grade-4 shoulder). Mean follow-up was 40 months for group 1 and 43 months for group 2.
There were no significant differences between groups 1 and 2 in SNA (102.8° vs 105.4°; P = .3), PGRD (15.4 vs 16.8 mm; P = . 47), or PSNA (125.8° vs 125.4°; P = .82) (Table 1). In addition, groups 1 and 2 had no significant differences (P > .05) in glenoid overhang and glenosphere inclination (other possible factors influencing notching).
Mean (SD) notching index was 31.8 (4.4) for group 1 and 33.1 (7.2) for group 2. These values were not significantly different (P = .29) (Table 1, Figure 2).8 Each was below the recommended threshold of 35 for prevention of notching (Table 1, Figure 2).
To try to understand why mean scapular notching index was low for both groups, we examined the contributing factors individually. Our cohort’s mean PGRD of 16.1 mm (15.4 and 16.8 mm for groups 1 and 2, respectively) was significantly lower than the cohort mean reported by Simovitch and colleagues8 (Table 2). Given that PGRD is more strongly weighted in the originally described notching index ([PSNA × 0.13] + PGRD),8 it was the primary driver for our notching index results, even though on average our results demonstrated a PSNA higher than that found by Simovitch and colleagues8 (Table 2; Figures 3, 4). Analyzing PGRD and PSNA together, we found no relationship between these variables and increased severity of inferior notching (Figure 5).
Regarding the effects of notching severity on outcomes in our study cohort, there were no significant differences between groups 1 and 2 in postoperative function, including forward flexion (123° vs 112.4°; P = .11), external rotation (18.8° vs 16.7°; P = .76), positive lag sign (P = .2), and VAS pain scores (1.2 vs 2.1; P = .15). There were also no significant differences between groups in the rate of complications (P = .92). Regression analysis determined that PSNA, PGRD, glenosphere inclination, glenosphere overhang, and implant manufacturer were not significant predictors of complications.
Discussion
RSA has provided good pain relief and restored function in patients with irreparable rotator cuff disease associated with arthritis.5,12,17,18 Scapular notching is a complex, multifactorial process. Nevertheless, surgeons remain cautious about the implications of inferior scapular notching, which is being reported by a significant number of patients. Our cohort’s high incidence of scapular notching (82%) in the early postoperative period clearly highlights the importance of predictive models, such as the notching index.8 Although concerns about consequences of notching have been expressed, notching severity did not affect outcomes or increase complications in this cohort.5,8,11,12,17-19
We conducted this study to examine use of a predictive tool for scapular notching, the notching index, in a large cohort of patients who underwent primary RSA. This index combines 2 well-established factors that contribute to notching—craniocaudal position and PSNA—into a predictive formula based on statistical analyses performed in a prospective cohort study.4,5,8,12,18 In their clinical study, Simovitch and colleagues8 found that both craniocaudal position and PSNA were tightly coupled with inferior scapular notching, and they developed a notching index that accounts for this relationship. We hypothesized that patients with a notching index of less than the recommended 35 would not develop notching and that patients with a notching index of more than 35 would have increased incidence and severity of notching. With our cohort, the recommended index of 35 was not an appropriate threshold predictive of notching. Furthermore, the 35 threshold applied to our cohort had 89% sensitivity and 21% specificity in predicting notching. Although the sensitivity is high, and correctly predicted true instances of notching, the low specificity compromises the precision of the notching formula ([PSNA × 0.13] + PGRD).
From the formula, it can be inferred that higher PSNA values can be compensated for by decreasing PGRD and inferiorizing the glenosphere. However, this recommendation appears limited based on increasing PSNA values, as in our cohort. The previously described notching formula cannot be universally applied to all patients treated with RSA because of the complexity of this relationship and patient-specific anatomy.
We assessed other possible anatomical and surgical factors, specific to scapular morphology, that could contribute to scapular notching. In other studies, reaming that produced an inferior tilt of the glenoid increased the likelihood of inferior notching.8,20,21 Furthermore, we expected less inferior glenoid overhang and smaller glenosphere would predispose patients to more notching.8,12,19 In our cohort, notching grade was not correlated with inferior tilt, glenoid overhang, or glenosphere size, which may be attributed to minimal variability in glenosphere size and a small range of glenosphere overhang.
There were limitations to this study. We examined only 2 types of RSA systems, and they had very similar Grammont designs. Other RSA designs might not have similar shortcomings with respect to inferior notching. In addition, we examined patient cases at a single time point and did not evaluate the effect of notching over time.
Overall, our results suggest that PGRD and PSNA have little effect on development of higher grade notching, particularly with use of Grammont prostheses. With newer surgical techniques, the recommendation is for inferior craniocaudal placement of the glenosphere, but this may not prevent notching with some types of patient-specific scapular morphology. Clearer surgical guidelines and techniques may help delineate the contribution of each parameter causing inferior scapular notching. Surgeons must weigh the evidence to determine how to correct patient-specific glenoid pathology and orient the glenosphere. Recent studies on bony increased-offset reverse shoulder arthroplasty (bio-RSA) techniques or newer prosthetic designs that considerably alter PSNA and the center of rotation may prevent inferior notching and provide a promising alternative to Grammont designs. Ultimately, longer follow-up is also needed to understand the clinical relevance of increased scapular notching.
1. Woodruff MJ, Cohen AP, Bradley JG. Arthroplasty of the shoulder in rheumatoid arthritis with rotator cuff dysfunction. Int Orthop. 2003;27(1):7-10.
2. Inman VT, Saunders JB, Abbott LC. Observations of the function of the shoulder joint. 1944. Clin Orthop. 1996;(330):3-12.
3. Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15(5):527-540.
4. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 suppl S):147S-161S.
5. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86(3):388-395.
6. Kowalsky MS, Galatz LM, Shia DS, Steger-May K, Keener JD. The relationship between scapular notching and reverse shoulder arthroplasty prosthesis design. J Shoulder Elbow Surg. 2012;21(10):1430-1441.
7. Lévigne C, Boileau P, Favard L, et al. Scapular notching in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2008;17(6):925-935.
8. Simovitch RW, Zumstein MA, Lohri E, Helmy N, Gerber C. Predictors of scapular notching in patients managed with the Delta III reverse total shoulder replacement. J Bone Joint Surg Am. 2007;89(3):588-600.
9. Torrens C, Corrales M, Gonzalez G, Solano A, Caceres E. Morphology of the scapula relative to the reverse shoulder prosthesis. J Orthop Surg (Hong Kong). 2009;17(2):146-150.
10. McFarland EG, Sanguanjit P, Tasaki A, Keyurapan E, Fishman EK, Fayad LM. The reverse shoulder prosthesis: a review of imaging features and complications. Skeletal Radiol. 2006;35(7):488-496.
11. Valenti PH, Boutens D, Nerot C. Delta 3 reversed prosthesis for osteoarthritis with massive rotator cuff tear: long-term results (>5 years). In: Walch G, Boileau P, Molé D, eds. Shoulder Prosthesis: Two to Ten Years Follow-Up. Montpellier, France: Sauramps Medical; 2001:253-259.
12. Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87(7):1476-1486.
13. Nyffeler RW, Werner CM, Simmen BR, Gerber C. Analysis of a retrieved Delta III total shoulder prosthesis. J Bone Joint Surg Br. 2004;86(8):1187-1191.
14. Grassi FA, Murena L, Valli F, Alberio R. Six-year experience with the Delta III reverse shoulder prosthesis. J Orthop Surg (Hong Kong). 2009;17(2):151-156.
15. Lévigne C, Garret J, Boileau P, Alami G, Favard L, Walch G. Scapular notching in reverse shoulder arthroplasty: is it important to avoid it and how? Clin Orthop. 2011;469(9):2512-2520.
16. Nyffeler RW, Werner CM, Gerber C. Biomechanical relevance of glenoid component positioning in the reverse Delta III total shoulder prosthesis. J Shoulder Elbow Surg. 2005;14(5):524-528.
17. Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22.
18. Vanhove B, Beugnies A. Grammont’s reverse shoulder prosthesis for rotator cuff arthropathy. A retrospective study of 32 cases. Acta Orthop Belg. 2004;70(3):219-225.
19. Kempton LB, Balasubramaniam M, Ankerson E, Wiater JM. A radiographic analysis of the effects of glenosphere position on scapular notching following reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(6):968-974.
20. Gutiérrez S, Greiwe RM, Frankle MA, Siegal S, Lee WE. Biomechanical comparison of component position and hardware failure in the reverse shoulder prosthesis. J Shoulder Elbow Surg. 2007;16(3 suppl):S9-S12.
21. Roche CP, Diep P, Hamilton M, et al. Impact of inferior glenoid tilt, humeral retroversion, bone grafting, and design parameters on muscle length and deltoid wrapping in reverse shoulder arthroplasty. Bull Hosp Jt Dis. 2013;71(4):284-293.
Reverse shoulder arthroplasty (RSA) is a treatment option for a spectrum of diseases in shoulders with rotator cuff deficiency. There are distinct morphologic changes in the scapular and glenoid anatomy in patients with chronic rotator cuff tears.1 A muscular imbalance that occurs in the joint as a result of rotator cuff deficiency leads to morphologic changes that eliminate the compressive forces that hold the humeral head against the glenoid.2 RSA effectively stabilizes the glenohumeral joint in shoulders with deficient rotator cuffs.3,4 In early work, Grammont proposed that the glenosphere center of rotation should be medialized (concentric to the central axis of the metaglene or baseplate) and lowered.5 Although the medialized center of rotation in Grammont prostheses decreases shear forces and improves the deltoid lever arm, it also tends to result in mechanical impingement between the superomedial aspect of the humeral polyethylene insert and the scapular neck—so-called inferior scapular notching.6-9
Notching, which has been reported in 50% to 96% of patients who receive a Delta III prosthesis, typically appears within the first few months after surgery but may be seen as late as 14 months after surgery.5,10-12 Postmortem studies have shown that notching corresponds with erosion of the inferior pole of the glenoid and scapular neck, thought to be caused by the polyethylene cup of the implant.13 Although some studies have found that notching stabilizes after 1 year, others have shown notching progressing for up to 4 years after surgery.11,12,14 The clinical relevance of notching continues to be controversial, but notching has been associated with poorer clinical outcomes, polyethylene wear, and local osteolysis. Component loosening has also been reported with notching of grade 3 or more.8,10 Ultimately, there is concern that scapular notching could progress, ultimately leading to late glenoid loosening and potentially catastrophic failure.
Scapular anatomy has become an area of increased focus in rotator cuff disorders and in effects on RSA biomechanics.9 Recent reports have described important scapular morphology variations that suggest more individualized adjustments are needed during RSA.9,15 In addition, some investigators have reported that development of notching appears to depend on the height and inclination of the implanted glenoid component, where an inferior position of the glenosphere leads to less impingement and better range of motion.8,16 Simovitch and colleagues8 found the angle between the glenosphere and scapular neck and the craniocaudal position of the glenosphere to be highly correlated with inferior notching. They combined these 2 parameters into a predictive algorithm that provides a guideline (notching index, <35) for prevention of notching.
We conducted a study to evaluate the scapular notching index as a predictive tool and to consider other factors that may be associated with scapular notching occurring with use of Grammont reverse replacement systems. We hypothesized that patients with a notching index of less than 35 would not develop notching and that patients with an index of more than 35 would have increased incidence and severity of notching.
Materials and Methods
Patients treated with RSA for painful cuff tear arthropathy or irreparable rotator cuff tear with pseudoparesis (inability to actively elevate shoulder >90° in presence of free passive anterior elevation) were included in this retrospective review. All patients were treated between 2006 and 2010 by 1 of 2 established senior shoulder subspecialty surgeons. Patients treated with a Delta (DePuy Orthopaedics, Warsaw, Indiana) or an Aequalis (Tornier, Edina, Minnesota) reverse shoulder implant were included in the study. A standard polyethylene liner was used for all patients. These prostheses have the same neck shaft angle, 155º, as they have similar geometric designs, both based on the Grammont design—semiconstrained inverted with a fixed, lowered, medialized center of rotation. Standard instrumentation was used for all procedures. Patients were excluded if any nonstandard techniques or components were used (constrained or high-mobility liner, glenoid bone grafting). Patients who underwent revision for a previous reverse total arthroplasty, a total shoulder arthroplasty, or a hemiarthroplasty, or for treatment of acute fracture, posttraumatic deformity, or posttraumatic arthritis, were also excluded from our analyses. Minimum follow-up for study inclusion was 24 months.
All procedures were performed with the patient in the semi-beach-chair position and with use of a deltopectoral approach. The glenoid was prepared such that minimal reaming was needed to preserve the subchondral plate. The glenoid baseplate was positioned in the recommended inferior position to minimize notching and optimize functional outcomes.13 After surgery, all patients were managed with a simple soft immobilizer with or without a pillow with the arm at the patient’s side in internal rotation. Immediate passive mobilization was begun under the direction of physical therapists. Passive and active-assisted exercises were continued with gradual progression to independent activities of daily living at 6 weeks. Clinical evaluations were performed before and after surgery by the operating surgeon or independent research nurse. Active forward flexion, passive external rotation, strength, and visual analog scale (VAS) pain scores were reviewed and recorded. Case-specific complications were also reviewed.
Preoperative and postoperative anteroposterior radiographs were evaluated by 2 independent observers (attending surgeon, junior resident). Per standard technique, each radiograph was positioned horizontal to the scapular plane. Of the 91 patients, 66 had preoperative shoulder radiographs of acceptable quality, with complete visualization of scapular morphology. Radiographs were reviewed to measure the scapular neck angle (SNA), inferior scapular notching, prosthesis–scapular neck angle (PSNA), and peg glenoid rim distance (PGRD) (Figure 1). For the 66 patients with acceptable preoperative radiographs, SNA was determined by subtracting preoperative SNA from postoperative PSNA. Postoperative anteroposterior radiographs were used to classify degree of inferior scapular notching based on the Nerot grading scale (0-4). In addition, glenosphere overhang and glenosphere inclination were measured on postoperative radiographs.
The 91 shoulders were sorted into 2 groups based on degree of scapular notching: group 1, Nerot grade 0 (no inferior notching) and grade 1, and group 2, Nerot grades 2, 3, and 4. Group 1 had 37 patients with a size 36 glenosphere, 3 patients with size 38, and 8 patients with size 42; group 2 had 34 patients with a size 36 glenosphere, 1 patient with size 38, and 8 patients with size 42. All measurements were normalized to account for differences in glenosphere size. Groups 1 and 2 were compared on each radiographic parameter (inferior scapular notching, PSNA, PGRD, SNA).
Notching indexes were calculated ([PSNA × 0.13] + PGRD) and compared with the suggested index of 35.8 Simovitch and colleagues8 demonstrated that a notching index of more than 35 had 91% sensitivity and 88% specificity in predicting inferior notching, whereas a notching index of 35 or less avoided inferior notching 91% of the time. In this study, notching index was calculated for each patient, and then the mean values of groups 1 and 2 were compared (Table 1).
The effect of scapular notching and other individual radiographic parameters on outcomes was also evaluated with respect to forward flexion, external rotation, VAS pain score, complications, and external rotation lag sign. Mann-Whitney U test was used to test these variables; Spearman rank test was performed to determine correlation between each variable and scapular notching; logistic regression was used to explore the relationship of variables (PGRD, PSNA) as predictors of Nerot degree of inferior scapular notching, and postoperative complications; and independent-samples t test was used to determine group differences for each variable. For each investigation, the level of significance was set at P < .05. A biostatistician performed all statistical analyses using SPSS Version 19 (IBM, Armonk, New York).
Results
Our study cohort consisted of 91 shoulders. Mean follow-up was 41.8 months (range, 24.0-80.8 months). Seventy-five (82%) of the 91 shoulders developed scapular notching. Mean (SD) SNA on preoperative radiographs, used to assess preoperative scapular morphology, was 103.9° (14.5°). For all 91 shoulders, mean (SD) PSNA was 125.6° (16°), and mean (SD) PGRD was 16 (5.4) mm (Table 1). Inclination measurements were available for 86 patients. Mean (SD) inclination from 90° was 2.5° (10.3°) (range, 21°-30°). Mean (SD) SNA (postoperative PSNA minus preoperative SNA) for the 66 patients with acceptable preoperative radiographs was 24.3° (21.3°) (Table 1). Forty-eight of the 91 shoulders were placed in scapular notching group 1 (16 grade-0 shoulders, 32 grade-1 shoulders); the other 43 shoulders were placed in group 2 (33 grade-2 shoulders, 9 grade-3 shoulders, 1 grade-4 shoulder). Mean follow-up was 40 months for group 1 and 43 months for group 2.
There were no significant differences between groups 1 and 2 in SNA (102.8° vs 105.4°; P = .3), PGRD (15.4 vs 16.8 mm; P = . 47), or PSNA (125.8° vs 125.4°; P = .82) (Table 1). In addition, groups 1 and 2 had no significant differences (P > .05) in glenoid overhang and glenosphere inclination (other possible factors influencing notching).
Mean (SD) notching index was 31.8 (4.4) for group 1 and 33.1 (7.2) for group 2. These values were not significantly different (P = .29) (Table 1, Figure 2).8 Each was below the recommended threshold of 35 for prevention of notching (Table 1, Figure 2).
To try to understand why mean scapular notching index was low for both groups, we examined the contributing factors individually. Our cohort’s mean PGRD of 16.1 mm (15.4 and 16.8 mm for groups 1 and 2, respectively) was significantly lower than the cohort mean reported by Simovitch and colleagues8 (Table 2). Given that PGRD is more strongly weighted in the originally described notching index ([PSNA × 0.13] + PGRD),8 it was the primary driver for our notching index results, even though on average our results demonstrated a PSNA higher than that found by Simovitch and colleagues8 (Table 2; Figures 3, 4). Analyzing PGRD and PSNA together, we found no relationship between these variables and increased severity of inferior notching (Figure 5).
Regarding the effects of notching severity on outcomes in our study cohort, there were no significant differences between groups 1 and 2 in postoperative function, including forward flexion (123° vs 112.4°; P = .11), external rotation (18.8° vs 16.7°; P = .76), positive lag sign (P = .2), and VAS pain scores (1.2 vs 2.1; P = .15). There were also no significant differences between groups in the rate of complications (P = .92). Regression analysis determined that PSNA, PGRD, glenosphere inclination, glenosphere overhang, and implant manufacturer were not significant predictors of complications.
Discussion
RSA has provided good pain relief and restored function in patients with irreparable rotator cuff disease associated with arthritis.5,12,17,18 Scapular notching is a complex, multifactorial process. Nevertheless, surgeons remain cautious about the implications of inferior scapular notching, which is being reported by a significant number of patients. Our cohort’s high incidence of scapular notching (82%) in the early postoperative period clearly highlights the importance of predictive models, such as the notching index.8 Although concerns about consequences of notching have been expressed, notching severity did not affect outcomes or increase complications in this cohort.5,8,11,12,17-19
We conducted this study to examine use of a predictive tool for scapular notching, the notching index, in a large cohort of patients who underwent primary RSA. This index combines 2 well-established factors that contribute to notching—craniocaudal position and PSNA—into a predictive formula based on statistical analyses performed in a prospective cohort study.4,5,8,12,18 In their clinical study, Simovitch and colleagues8 found that both craniocaudal position and PSNA were tightly coupled with inferior scapular notching, and they developed a notching index that accounts for this relationship. We hypothesized that patients with a notching index of less than the recommended 35 would not develop notching and that patients with a notching index of more than 35 would have increased incidence and severity of notching. With our cohort, the recommended index of 35 was not an appropriate threshold predictive of notching. Furthermore, the 35 threshold applied to our cohort had 89% sensitivity and 21% specificity in predicting notching. Although the sensitivity is high, and correctly predicted true instances of notching, the low specificity compromises the precision of the notching formula ([PSNA × 0.13] + PGRD).
From the formula, it can be inferred that higher PSNA values can be compensated for by decreasing PGRD and inferiorizing the glenosphere. However, this recommendation appears limited based on increasing PSNA values, as in our cohort. The previously described notching formula cannot be universally applied to all patients treated with RSA because of the complexity of this relationship and patient-specific anatomy.
We assessed other possible anatomical and surgical factors, specific to scapular morphology, that could contribute to scapular notching. In other studies, reaming that produced an inferior tilt of the glenoid increased the likelihood of inferior notching.8,20,21 Furthermore, we expected less inferior glenoid overhang and smaller glenosphere would predispose patients to more notching.8,12,19 In our cohort, notching grade was not correlated with inferior tilt, glenoid overhang, or glenosphere size, which may be attributed to minimal variability in glenosphere size and a small range of glenosphere overhang.
There were limitations to this study. We examined only 2 types of RSA systems, and they had very similar Grammont designs. Other RSA designs might not have similar shortcomings with respect to inferior notching. In addition, we examined patient cases at a single time point and did not evaluate the effect of notching over time.
Overall, our results suggest that PGRD and PSNA have little effect on development of higher grade notching, particularly with use of Grammont prostheses. With newer surgical techniques, the recommendation is for inferior craniocaudal placement of the glenosphere, but this may not prevent notching with some types of patient-specific scapular morphology. Clearer surgical guidelines and techniques may help delineate the contribution of each parameter causing inferior scapular notching. Surgeons must weigh the evidence to determine how to correct patient-specific glenoid pathology and orient the glenosphere. Recent studies on bony increased-offset reverse shoulder arthroplasty (bio-RSA) techniques or newer prosthetic designs that considerably alter PSNA and the center of rotation may prevent inferior notching and provide a promising alternative to Grammont designs. Ultimately, longer follow-up is also needed to understand the clinical relevance of increased scapular notching.
Reverse shoulder arthroplasty (RSA) is a treatment option for a spectrum of diseases in shoulders with rotator cuff deficiency. There are distinct morphologic changes in the scapular and glenoid anatomy in patients with chronic rotator cuff tears.1 A muscular imbalance that occurs in the joint as a result of rotator cuff deficiency leads to morphologic changes that eliminate the compressive forces that hold the humeral head against the glenoid.2 RSA effectively stabilizes the glenohumeral joint in shoulders with deficient rotator cuffs.3,4 In early work, Grammont proposed that the glenosphere center of rotation should be medialized (concentric to the central axis of the metaglene or baseplate) and lowered.5 Although the medialized center of rotation in Grammont prostheses decreases shear forces and improves the deltoid lever arm, it also tends to result in mechanical impingement between the superomedial aspect of the humeral polyethylene insert and the scapular neck—so-called inferior scapular notching.6-9
Notching, which has been reported in 50% to 96% of patients who receive a Delta III prosthesis, typically appears within the first few months after surgery but may be seen as late as 14 months after surgery.5,10-12 Postmortem studies have shown that notching corresponds with erosion of the inferior pole of the glenoid and scapular neck, thought to be caused by the polyethylene cup of the implant.13 Although some studies have found that notching stabilizes after 1 year, others have shown notching progressing for up to 4 years after surgery.11,12,14 The clinical relevance of notching continues to be controversial, but notching has been associated with poorer clinical outcomes, polyethylene wear, and local osteolysis. Component loosening has also been reported with notching of grade 3 or more.8,10 Ultimately, there is concern that scapular notching could progress, ultimately leading to late glenoid loosening and potentially catastrophic failure.
Scapular anatomy has become an area of increased focus in rotator cuff disorders and in effects on RSA biomechanics.9 Recent reports have described important scapular morphology variations that suggest more individualized adjustments are needed during RSA.9,15 In addition, some investigators have reported that development of notching appears to depend on the height and inclination of the implanted glenoid component, where an inferior position of the glenosphere leads to less impingement and better range of motion.8,16 Simovitch and colleagues8 found the angle between the glenosphere and scapular neck and the craniocaudal position of the glenosphere to be highly correlated with inferior notching. They combined these 2 parameters into a predictive algorithm that provides a guideline (notching index, <35) for prevention of notching.
We conducted a study to evaluate the scapular notching index as a predictive tool and to consider other factors that may be associated with scapular notching occurring with use of Grammont reverse replacement systems. We hypothesized that patients with a notching index of less than 35 would not develop notching and that patients with an index of more than 35 would have increased incidence and severity of notching.
Materials and Methods
Patients treated with RSA for painful cuff tear arthropathy or irreparable rotator cuff tear with pseudoparesis (inability to actively elevate shoulder >90° in presence of free passive anterior elevation) were included in this retrospective review. All patients were treated between 2006 and 2010 by 1 of 2 established senior shoulder subspecialty surgeons. Patients treated with a Delta (DePuy Orthopaedics, Warsaw, Indiana) or an Aequalis (Tornier, Edina, Minnesota) reverse shoulder implant were included in the study. A standard polyethylene liner was used for all patients. These prostheses have the same neck shaft angle, 155º, as they have similar geometric designs, both based on the Grammont design—semiconstrained inverted with a fixed, lowered, medialized center of rotation. Standard instrumentation was used for all procedures. Patients were excluded if any nonstandard techniques or components were used (constrained or high-mobility liner, glenoid bone grafting). Patients who underwent revision for a previous reverse total arthroplasty, a total shoulder arthroplasty, or a hemiarthroplasty, or for treatment of acute fracture, posttraumatic deformity, or posttraumatic arthritis, were also excluded from our analyses. Minimum follow-up for study inclusion was 24 months.
All procedures were performed with the patient in the semi-beach-chair position and with use of a deltopectoral approach. The glenoid was prepared such that minimal reaming was needed to preserve the subchondral plate. The glenoid baseplate was positioned in the recommended inferior position to minimize notching and optimize functional outcomes.13 After surgery, all patients were managed with a simple soft immobilizer with or without a pillow with the arm at the patient’s side in internal rotation. Immediate passive mobilization was begun under the direction of physical therapists. Passive and active-assisted exercises were continued with gradual progression to independent activities of daily living at 6 weeks. Clinical evaluations were performed before and after surgery by the operating surgeon or independent research nurse. Active forward flexion, passive external rotation, strength, and visual analog scale (VAS) pain scores were reviewed and recorded. Case-specific complications were also reviewed.
Preoperative and postoperative anteroposterior radiographs were evaluated by 2 independent observers (attending surgeon, junior resident). Per standard technique, each radiograph was positioned horizontal to the scapular plane. Of the 91 patients, 66 had preoperative shoulder radiographs of acceptable quality, with complete visualization of scapular morphology. Radiographs were reviewed to measure the scapular neck angle (SNA), inferior scapular notching, prosthesis–scapular neck angle (PSNA), and peg glenoid rim distance (PGRD) (Figure 1). For the 66 patients with acceptable preoperative radiographs, SNA was determined by subtracting preoperative SNA from postoperative PSNA. Postoperative anteroposterior radiographs were used to classify degree of inferior scapular notching based on the Nerot grading scale (0-4). In addition, glenosphere overhang and glenosphere inclination were measured on postoperative radiographs.
The 91 shoulders were sorted into 2 groups based on degree of scapular notching: group 1, Nerot grade 0 (no inferior notching) and grade 1, and group 2, Nerot grades 2, 3, and 4. Group 1 had 37 patients with a size 36 glenosphere, 3 patients with size 38, and 8 patients with size 42; group 2 had 34 patients with a size 36 glenosphere, 1 patient with size 38, and 8 patients with size 42. All measurements were normalized to account for differences in glenosphere size. Groups 1 and 2 were compared on each radiographic parameter (inferior scapular notching, PSNA, PGRD, SNA).
Notching indexes were calculated ([PSNA × 0.13] + PGRD) and compared with the suggested index of 35.8 Simovitch and colleagues8 demonstrated that a notching index of more than 35 had 91% sensitivity and 88% specificity in predicting inferior notching, whereas a notching index of 35 or less avoided inferior notching 91% of the time. In this study, notching index was calculated for each patient, and then the mean values of groups 1 and 2 were compared (Table 1).
The effect of scapular notching and other individual radiographic parameters on outcomes was also evaluated with respect to forward flexion, external rotation, VAS pain score, complications, and external rotation lag sign. Mann-Whitney U test was used to test these variables; Spearman rank test was performed to determine correlation between each variable and scapular notching; logistic regression was used to explore the relationship of variables (PGRD, PSNA) as predictors of Nerot degree of inferior scapular notching, and postoperative complications; and independent-samples t test was used to determine group differences for each variable. For each investigation, the level of significance was set at P < .05. A biostatistician performed all statistical analyses using SPSS Version 19 (IBM, Armonk, New York).
Results
Our study cohort consisted of 91 shoulders. Mean follow-up was 41.8 months (range, 24.0-80.8 months). Seventy-five (82%) of the 91 shoulders developed scapular notching. Mean (SD) SNA on preoperative radiographs, used to assess preoperative scapular morphology, was 103.9° (14.5°). For all 91 shoulders, mean (SD) PSNA was 125.6° (16°), and mean (SD) PGRD was 16 (5.4) mm (Table 1). Inclination measurements were available for 86 patients. Mean (SD) inclination from 90° was 2.5° (10.3°) (range, 21°-30°). Mean (SD) SNA (postoperative PSNA minus preoperative SNA) for the 66 patients with acceptable preoperative radiographs was 24.3° (21.3°) (Table 1). Forty-eight of the 91 shoulders were placed in scapular notching group 1 (16 grade-0 shoulders, 32 grade-1 shoulders); the other 43 shoulders were placed in group 2 (33 grade-2 shoulders, 9 grade-3 shoulders, 1 grade-4 shoulder). Mean follow-up was 40 months for group 1 and 43 months for group 2.
There were no significant differences between groups 1 and 2 in SNA (102.8° vs 105.4°; P = .3), PGRD (15.4 vs 16.8 mm; P = . 47), or PSNA (125.8° vs 125.4°; P = .82) (Table 1). In addition, groups 1 and 2 had no significant differences (P > .05) in glenoid overhang and glenosphere inclination (other possible factors influencing notching).
Mean (SD) notching index was 31.8 (4.4) for group 1 and 33.1 (7.2) for group 2. These values were not significantly different (P = .29) (Table 1, Figure 2).8 Each was below the recommended threshold of 35 for prevention of notching (Table 1, Figure 2).
To try to understand why mean scapular notching index was low for both groups, we examined the contributing factors individually. Our cohort’s mean PGRD of 16.1 mm (15.4 and 16.8 mm for groups 1 and 2, respectively) was significantly lower than the cohort mean reported by Simovitch and colleagues8 (Table 2). Given that PGRD is more strongly weighted in the originally described notching index ([PSNA × 0.13] + PGRD),8 it was the primary driver for our notching index results, even though on average our results demonstrated a PSNA higher than that found by Simovitch and colleagues8 (Table 2; Figures 3, 4). Analyzing PGRD and PSNA together, we found no relationship between these variables and increased severity of inferior notching (Figure 5).
Regarding the effects of notching severity on outcomes in our study cohort, there were no significant differences between groups 1 and 2 in postoperative function, including forward flexion (123° vs 112.4°; P = .11), external rotation (18.8° vs 16.7°; P = .76), positive lag sign (P = .2), and VAS pain scores (1.2 vs 2.1; P = .15). There were also no significant differences between groups in the rate of complications (P = .92). Regression analysis determined that PSNA, PGRD, glenosphere inclination, glenosphere overhang, and implant manufacturer were not significant predictors of complications.
Discussion
RSA has provided good pain relief and restored function in patients with irreparable rotator cuff disease associated with arthritis.5,12,17,18 Scapular notching is a complex, multifactorial process. Nevertheless, surgeons remain cautious about the implications of inferior scapular notching, which is being reported by a significant number of patients. Our cohort’s high incidence of scapular notching (82%) in the early postoperative period clearly highlights the importance of predictive models, such as the notching index.8 Although concerns about consequences of notching have been expressed, notching severity did not affect outcomes or increase complications in this cohort.5,8,11,12,17-19
We conducted this study to examine use of a predictive tool for scapular notching, the notching index, in a large cohort of patients who underwent primary RSA. This index combines 2 well-established factors that contribute to notching—craniocaudal position and PSNA—into a predictive formula based on statistical analyses performed in a prospective cohort study.4,5,8,12,18 In their clinical study, Simovitch and colleagues8 found that both craniocaudal position and PSNA were tightly coupled with inferior scapular notching, and they developed a notching index that accounts for this relationship. We hypothesized that patients with a notching index of less than the recommended 35 would not develop notching and that patients with a notching index of more than 35 would have increased incidence and severity of notching. With our cohort, the recommended index of 35 was not an appropriate threshold predictive of notching. Furthermore, the 35 threshold applied to our cohort had 89% sensitivity and 21% specificity in predicting notching. Although the sensitivity is high, and correctly predicted true instances of notching, the low specificity compromises the precision of the notching formula ([PSNA × 0.13] + PGRD).
From the formula, it can be inferred that higher PSNA values can be compensated for by decreasing PGRD and inferiorizing the glenosphere. However, this recommendation appears limited based on increasing PSNA values, as in our cohort. The previously described notching formula cannot be universally applied to all patients treated with RSA because of the complexity of this relationship and patient-specific anatomy.
We assessed other possible anatomical and surgical factors, specific to scapular morphology, that could contribute to scapular notching. In other studies, reaming that produced an inferior tilt of the glenoid increased the likelihood of inferior notching.8,20,21 Furthermore, we expected less inferior glenoid overhang and smaller glenosphere would predispose patients to more notching.8,12,19 In our cohort, notching grade was not correlated with inferior tilt, glenoid overhang, or glenosphere size, which may be attributed to minimal variability in glenosphere size and a small range of glenosphere overhang.
There were limitations to this study. We examined only 2 types of RSA systems, and they had very similar Grammont designs. Other RSA designs might not have similar shortcomings with respect to inferior notching. In addition, we examined patient cases at a single time point and did not evaluate the effect of notching over time.
Overall, our results suggest that PGRD and PSNA have little effect on development of higher grade notching, particularly with use of Grammont prostheses. With newer surgical techniques, the recommendation is for inferior craniocaudal placement of the glenosphere, but this may not prevent notching with some types of patient-specific scapular morphology. Clearer surgical guidelines and techniques may help delineate the contribution of each parameter causing inferior scapular notching. Surgeons must weigh the evidence to determine how to correct patient-specific glenoid pathology and orient the glenosphere. Recent studies on bony increased-offset reverse shoulder arthroplasty (bio-RSA) techniques or newer prosthetic designs that considerably alter PSNA and the center of rotation may prevent inferior notching and provide a promising alternative to Grammont designs. Ultimately, longer follow-up is also needed to understand the clinical relevance of increased scapular notching.
1. Woodruff MJ, Cohen AP, Bradley JG. Arthroplasty of the shoulder in rheumatoid arthritis with rotator cuff dysfunction. Int Orthop. 2003;27(1):7-10.
2. Inman VT, Saunders JB, Abbott LC. Observations of the function of the shoulder joint. 1944. Clin Orthop. 1996;(330):3-12.
3. Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15(5):527-540.
4. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 suppl S):147S-161S.
5. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86(3):388-395.
6. Kowalsky MS, Galatz LM, Shia DS, Steger-May K, Keener JD. The relationship between scapular notching and reverse shoulder arthroplasty prosthesis design. J Shoulder Elbow Surg. 2012;21(10):1430-1441.
7. Lévigne C, Boileau P, Favard L, et al. Scapular notching in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2008;17(6):925-935.
8. Simovitch RW, Zumstein MA, Lohri E, Helmy N, Gerber C. Predictors of scapular notching in patients managed with the Delta III reverse total shoulder replacement. J Bone Joint Surg Am. 2007;89(3):588-600.
9. Torrens C, Corrales M, Gonzalez G, Solano A, Caceres E. Morphology of the scapula relative to the reverse shoulder prosthesis. J Orthop Surg (Hong Kong). 2009;17(2):146-150.
10. McFarland EG, Sanguanjit P, Tasaki A, Keyurapan E, Fishman EK, Fayad LM. The reverse shoulder prosthesis: a review of imaging features and complications. Skeletal Radiol. 2006;35(7):488-496.
11. Valenti PH, Boutens D, Nerot C. Delta 3 reversed prosthesis for osteoarthritis with massive rotator cuff tear: long-term results (>5 years). In: Walch G, Boileau P, Molé D, eds. Shoulder Prosthesis: Two to Ten Years Follow-Up. Montpellier, France: Sauramps Medical; 2001:253-259.
12. Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87(7):1476-1486.
13. Nyffeler RW, Werner CM, Simmen BR, Gerber C. Analysis of a retrieved Delta III total shoulder prosthesis. J Bone Joint Surg Br. 2004;86(8):1187-1191.
14. Grassi FA, Murena L, Valli F, Alberio R. Six-year experience with the Delta III reverse shoulder prosthesis. J Orthop Surg (Hong Kong). 2009;17(2):151-156.
15. Lévigne C, Garret J, Boileau P, Alami G, Favard L, Walch G. Scapular notching in reverse shoulder arthroplasty: is it important to avoid it and how? Clin Orthop. 2011;469(9):2512-2520.
16. Nyffeler RW, Werner CM, Gerber C. Biomechanical relevance of glenoid component positioning in the reverse Delta III total shoulder prosthesis. J Shoulder Elbow Surg. 2005;14(5):524-528.
17. Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22.
18. Vanhove B, Beugnies A. Grammont’s reverse shoulder prosthesis for rotator cuff arthropathy. A retrospective study of 32 cases. Acta Orthop Belg. 2004;70(3):219-225.
19. Kempton LB, Balasubramaniam M, Ankerson E, Wiater JM. A radiographic analysis of the effects of glenosphere position on scapular notching following reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(6):968-974.
20. Gutiérrez S, Greiwe RM, Frankle MA, Siegal S, Lee WE. Biomechanical comparison of component position and hardware failure in the reverse shoulder prosthesis. J Shoulder Elbow Surg. 2007;16(3 suppl):S9-S12.
21. Roche CP, Diep P, Hamilton M, et al. Impact of inferior glenoid tilt, humeral retroversion, bone grafting, and design parameters on muscle length and deltoid wrapping in reverse shoulder arthroplasty. Bull Hosp Jt Dis. 2013;71(4):284-293.
1. Woodruff MJ, Cohen AP, Bradley JG. Arthroplasty of the shoulder in rheumatoid arthritis with rotator cuff dysfunction. Int Orthop. 2003;27(1):7-10.
2. Inman VT, Saunders JB, Abbott LC. Observations of the function of the shoulder joint. 1944. Clin Orthop. 1996;(330):3-12.
3. Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15(5):527-540.
4. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 suppl S):147S-161S.
5. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86(3):388-395.
6. Kowalsky MS, Galatz LM, Shia DS, Steger-May K, Keener JD. The relationship between scapular notching and reverse shoulder arthroplasty prosthesis design. J Shoulder Elbow Surg. 2012;21(10):1430-1441.
7. Lévigne C, Boileau P, Favard L, et al. Scapular notching in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2008;17(6):925-935.
8. Simovitch RW, Zumstein MA, Lohri E, Helmy N, Gerber C. Predictors of scapular notching in patients managed with the Delta III reverse total shoulder replacement. J Bone Joint Surg Am. 2007;89(3):588-600.
9. Torrens C, Corrales M, Gonzalez G, Solano A, Caceres E. Morphology of the scapula relative to the reverse shoulder prosthesis. J Orthop Surg (Hong Kong). 2009;17(2):146-150.
10. McFarland EG, Sanguanjit P, Tasaki A, Keyurapan E, Fishman EK, Fayad LM. The reverse shoulder prosthesis: a review of imaging features and complications. Skeletal Radiol. 2006;35(7):488-496.
11. Valenti PH, Boutens D, Nerot C. Delta 3 reversed prosthesis for osteoarthritis with massive rotator cuff tear: long-term results (>5 years). In: Walch G, Boileau P, Molé D, eds. Shoulder Prosthesis: Two to Ten Years Follow-Up. Montpellier, France: Sauramps Medical; 2001:253-259.
12. Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87(7):1476-1486.
13. Nyffeler RW, Werner CM, Simmen BR, Gerber C. Analysis of a retrieved Delta III total shoulder prosthesis. J Bone Joint Surg Br. 2004;86(8):1187-1191.
14. Grassi FA, Murena L, Valli F, Alberio R. Six-year experience with the Delta III reverse shoulder prosthesis. J Orthop Surg (Hong Kong). 2009;17(2):151-156.
15. Lévigne C, Garret J, Boileau P, Alami G, Favard L, Walch G. Scapular notching in reverse shoulder arthroplasty: is it important to avoid it and how? Clin Orthop. 2011;469(9):2512-2520.
16. Nyffeler RW, Werner CM, Gerber C. Biomechanical relevance of glenoid component positioning in the reverse Delta III total shoulder prosthesis. J Shoulder Elbow Surg. 2005;14(5):524-528.
17. Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22.
18. Vanhove B, Beugnies A. Grammont’s reverse shoulder prosthesis for rotator cuff arthropathy. A retrospective study of 32 cases. Acta Orthop Belg. 2004;70(3):219-225.
19. Kempton LB, Balasubramaniam M, Ankerson E, Wiater JM. A radiographic analysis of the effects of glenosphere position on scapular notching following reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(6):968-974.
20. Gutiérrez S, Greiwe RM, Frankle MA, Siegal S, Lee WE. Biomechanical comparison of component position and hardware failure in the reverse shoulder prosthesis. J Shoulder Elbow Surg. 2007;16(3 suppl):S9-S12.
21. Roche CP, Diep P, Hamilton M, et al. Impact of inferior glenoid tilt, humeral retroversion, bone grafting, and design parameters on muscle length and deltoid wrapping in reverse shoulder arthroplasty. Bull Hosp Jt Dis. 2013;71(4):284-293.
Risk Factors for In-Hospital Myocardial Infarction After Shoulder Arthroplasty
The incidence of shoulder arthroplasty in the United States is increasing annually,1-3 and the majority of these operations occur in older patients.4-6 Elderly patients with cardiovascular, pulmonary, cerebral, renal, and hepatic disease are increasingly susceptible to numerous surgical complications.4 Myocardial infarction (MI) is a complication that occurs in 0.7% of noncardiac surgeries. This figure increases to 1.1% in patients with coronary artery disease.7-11 Perioperative MI increases morbidity and mortality,8 and perioperative cardiac morbidity is the leading cause of death after anesthesia and surgery.12 The financial effects of perioperative cardiac morbidity and mortality must also be considered. A 2009 claims analysis study estimated charges associated with a perioperative MI at $15,000 and the cost of cardiac death at $21,909.13
Cardiovascular complications are associated with a significant degree of morbidity and mortality in patients who undergo arthroplasty.14-16 Although studies have elucidated 30- and 90-day morbidity and mortality rates after shoulder arthroplasty, in hip and knee arthroplasty17-19 little has been done to determine predictors of perioperative MI in a representative database of patients. Given the increasing incidence of shoulder arthroplasty in the United States, the elective nature of this procedure, and the percentage of the US population with cardiovascular risk factors,20 it is important to establish predictors of perioperative MI to ensure patients and physicians have the necessary resources to make informed decisions.
We conducted a study to examine the risk factors for perioperative MI in a large cohort of patients admitted for shoulder arthroplasty to US hospitals. We wanted to evaluate the association between perioperative MI and shoulder arthroplasty with respect to demographics, primary diagnosis, medical comorbidities, and perioperative complications. Specifically, we tested the null hypothesis that, among patients undergoing shoulder arthroplasty, and accounting for confounding variables, there would be no difference in risk factors for patients who have a perioperative MI.
Materials and Methods
This study was exempt from approval by our institutional review board. All data used in this project were deidentified before use.
Nationwide Inpatient Sample (NIS)
The Nationwide Inpatient Sample (NIS), an annual survey of hospitals, is conducted by the Healthcare Cost and Utilization Project (HCUP) and sponsored by the Agency for Healthcare Research and Quality (AHRQ). This database is the largest publicly available all-payer inpatient discharge database in the United States.21 Sampling 8 million hospital stays each year, NIS includes information from a representative batch of 20% of US hospitals. In 2011, 46 states and 1045 hospitals contributed information to the database, representing 97% of the US population.22 This large sample allows researchers to analyze a robust set of medical conditions and uncommon treatments. The survey, conducted each year since 1988, includes demographic, clinical, and resource use data.23 Discharge weight files are provided by NIS to arrive at valid national estimates.
This database is particularly useful because it provides information on up to 25 medical diagnoses and 15 procedures, which are recorded with International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. Researchers can use this database to analyze patient and hospital characteristics as well as inpatient outcomes.24,25 Numerous studies have used NIS to address pertinent queries across the medical landscape.22,26
Patient Selection and Analysis
We used NIS to isolate a population of 422,371 adults (≥18 years old) who underwent total shoulder arthroplasty (TSA) or hemi–shoulder arthroplasty (HSA) between January 1, 2002 and December 31, 2011. We then placed the patients in this population into 1 of 2 cohorts. The first cohort had an acute MI during the perioperative period after TSA, and the second, larger cohort did not have an acute MI after TSA. Acute MI was identified using ICD-9-CM code 410.xx. To identify a population of shoulder arthroplasty patients, we included discharges with an ICD-9-CM procedure code of 81.80 or 81.88 (both TSA) or 81.81 (HSA) in the sample. We then considered the degree to which each of 5 variables—primary diagnosis, age, sex, race, and select medical comorbidities—was predictive of in-hospital MI after TSA.
Statistical Analysis
Given the large sample used in this study, normal distribution of data was assumed. Using bivariate analysis, Pearson χ2 test for categorical data, and independent-samples t test for continuous data, we compared the nonacute MI and acute MI groups. Multivariable binary logistic regression analyses allowed us to isolate the extent that primary diagnosis, age, sex, race, and medical comorbidities were predictors of acute MI after shoulder arthroplasty. Statistical significance was set at P < .05. SPSS Version 22.0 (SPSS, Chicago, Illinois) was used for all statistical analyses and data modeling.
Results
Between January 1, 2002 and December 31, 2011, an estimated total of 422,371 patients underwent shoulder arthroplasty (59.3% TSA, 40.7% HSA). Of these patients, 1174 (0.28%) had a perioperative MI, and 421,197 (99.72%) did not (Table 1). Patients with a primary diagnosis of proximal humerus fracture (33.8% vs 16.6%; P < .001) or rotator cuff arthropathy (10.1% vs 9.9%; P < .001) were more likely than patients with other diagnoses to have an in-hospital MI.
Our review of the demographics found that patients who underwent shoulder arthroplasty and had a perioperative MI were likely older (75±8.9 years vs 69±11 years; P < .001), Caucasian (94.2% vs 91.9%; P = .002), male (43.2% vs 39.7%; P = .013), in the highest median household income bracket of $63,000 or more (30.8% vs 25.6%; P < .001), and using Medicare (80.9% vs 66.3%; P < .001). They were more likely to be treated in a medical center of medium size (25.6% vs 23.7%; P = .042) or larger (61.8% vs 61.2%; P = .042). MIs occurred more often in urban environments (91.4% vs 88.5%; P = .002) and in HSA patients (55% vs 40.6%; P < .001), resulting in longer hospital stays (9.4±7.9 days vs 2.7±2.5 days; P < .001) and higher probability of death (6.5% vs 0.1%; P < .001).
We then analyzed the 2 cohorts for medical comorbidities (Table 2). Patients in the MI cohort presented with a significantly higher incidence of congestive heart failure, previous MI, angina pectoris, chronic lung disease, hypertension, diabetes, renal failure, fluid and electrolyte disorders, pulmonary circulatory disease, coagulopathy, and deficiency anemia (P < .001) but not liver disease and obesity. Bivariate analysis of perioperative outcomes (Table 3) indicated that these patients also had a statistically higher rate of numerous other complications: pulmonary embolism (4.9% vs 0.2%; P < .001), pneumonia (15.1% vs 1.2%; P < .001), deep venous thrombosis (2.6% vs 0.2%; P < .001), cerebrovascular event (1.6% vs 0.1%; P < .001), acute renal failure (15.1% vs 1.2%; P < .001), gastrointestinal complication (1.2% vs 0.3%; P < .001), mechanical ventilation (1.2% vs 0.3%; P < .001), transfusion (33.4% vs 8.8%; P < .001), and nonroutine discharge (73.3% vs 36.0%; P < .001).
Multivariable logistic regression analysis was performed to determine independent predictors of perioperative MI after shoulder arthroplasty (Table 4). Patients with a primary diagnosis of proximal humerus fracture (odds ratio [OR], 1.38; 95% confidence interval [CI], 1.15-1.65; P < .001) were more likely than patients with a primary diagnosis of osteoarthritis to have an MI. The odds of postoperative MI increased with age (OR, 1.04 per year; 95% CI, 1.03-1.05; P < .001) and were higher in males (OR, 1.72; 95% CI, 1.52-1.96; P < .001). Compared with Caucasians, African Americans (OR, 0.19; 95% CI, 0.09-0.40; P < .001) were less likely to have an in-hospital MI after shoulder arthroplasty. After shoulder arthroplasty, the odds of MI in the perioperative period increased with each subsequent day of care (OR, 1.10; 95% CI, 1.10-1.11; P < .001).
Regarding independent comorbidities, multivariable logistic regression analysis also determined that history of congestive heart failure (OR, 4.86; 95% CI, 4.20-5.61; P < .001), angina pectoris (OR, 2.90; 95% CI, 2.02-4.17; P < .001), complicated diabetes (OR, 1.96; 95% CI, 1.49-2.57; P < .001), renal failure (OR, 1.42; 95% CI, 1.17-1.72; P < .001), fluid and electrolyte disorders (OR, 1.42; 95% CI, 1.21-1.67; P < .001), and deficiency anemia (OR, 1.62; 95% CI, 1.40-1.88; P < .001) were significant predictors of perioperative MI after shoulder arthroplasty.
Discussion
Results of other studies have elucidated 30- and 90-day mortality rates and postoperative complications after shoulder arthroplasty, but, relative to hip and knee arthroplasty,17-19 little has been done to determine predictors of perioperative MI in a large sample of shoulder arthroplasty patients. Given the increasing rates of shoulder arthroplasty1-3 and the demographics of this population,4-6 it is likely that postoperative cardiovascular events will increase in frequency. We found that, in order of decreasing significance, the top 4 risk predictors for acute MI after shoulder arthroplasty were congestive heart failure, angina pectoralis, complicated diabetes mellitus, and male sex. Other pertinent risk factors included older age, Caucasian ethnicity, and a primary diagnosis of proximal humerus fracture. The rate of acute MI in patients who were older than 75 years when they underwent HSA for proximal humerus fracture was 0.80%.
Demographics
We found that patients who had an acute MI after shoulder arthroplasty were likely older, male, and Caucasian. Age and male sex are well-established risk factors for increased cardiac complications after arthroplasty.27-29 Previous studies have indicated that the rate of cardiac events increases in arthroplasty patients older than 65 years.19,28,29 In our study, more than 50% of the patients who had an acute perioperative MI were older than 85 years. Less explainable is the increased occurrence of acute MI in Caucasian patients and wealthy patients, given that minorities in the United States have higher rates of cardiovascular disease.30 Shoulder arthroplasty is an elective procedure, more likely to be undertaken by Caucasians. Therefore, at-risk minority groups and financially challenged groups may be less likely to have this procedure.
Primary Diagnosis
In this series, patients with a primary diagnosis of proximal humerus fracture were more likely to have an in-hospital MI. This finding is consistent with previous studies indicating a higher rate of complications for proximal humerus fracture patients than for shoulder arthroplasty patients.31,32 Given that more than 75% of patients who present with a proximal humerus fracture are older than 70 years, it would be prudent to examine operative indications after this diagnosis,33 particularly as benefit from surgery for fractures has not been definitively demonstrated.34-37
Comorbidities
Many of the patients in our MI cohort presented with congestive heart failure, angina pectoris, complicated diabetes, renal failure, fluid and electrolyte disorders, or deficiency anemia. This is in keeping with other studies indicating that preexisting cardiovascular morbidity increases the rate of MI after various forms of arthroplasty.7-11 Patients in our MI cohort were also susceptible to a variety of post-MI perioperative complications, including pulmonary embolism, pneumonia, deep venous thrombosis, cerebrovascular event, acute renal failure, gastrointestinal complication, mechanical ventilation, transfusion, and nonroutine discharge, and their incidence of death was higher. These findings are consistent with reports that postoperative cardiovascular complications increase the degree of morbidity and mortality in arthroplasty patients.14-16 It is also worth noting that the odds of MI in the perioperative period increase with each subsequent day of care. This is understandable given that patients presenting with numerous comorbidities are at increased risk for perioperative complications38 resulting in hospital readmission.39
The literature indicates that MI occurs as a complication in 0.7% of patients who undergo noncardiac surgery,7 though some series have shown it is more prevalent after arthroplasty procedures.28,40 MI significantly increases the rate of perioperative morbidity and mortality,8 and perioperative cardiac morbidity is a leading cause of death after anesthesia and surgery.12 Furthermore, the most common cause of death after lower extremity arthroplasty is cardiovascular-related.41,42 In patients who presented for elective hip arthroplasty, cardiorespiratory disease was one of the main risk factors (with older age and male sex) shown to increase perioperative mortality.43
Perioperative cardiovascular complications increase postoperative morbidity and mortality.12 The rate of cardiovascular complications after shoulder arthroplasty ranges from 0.8% to 2.6%, and the incidence of MI hovers between 0.3% and 0.9%.17,19,28,40,44 A recent study in 793 patients found that, over a 30-day period, cardiovascular complications accounted for more than one-fourth of all complications.17 Singh and colleagues19 analyzed cardiopulmonary complications after primary shoulder arthroplasty in a total of 3480 patients (4019 arthroplasties) and found this group had a 90-day cardiac morbidity (MI, congestive heart failure, arrhythmia) rate of 2.6%. In that study, a Deyo-Charlson index of 1 or more was a significant independent risk factor for cardiac complications following surgery. Scores on this weighted index of 17 comorbidities are used to assess the complexities of a patient population. Given the severity of cardiovascular perioperative complications, it is important to preoperatively identify high-risk population groups and sufficiently study and optimize patients before shoulder arthroplasty.
There is much debate about the effectiveness of perioperative β-blockers in reducing perioperative cardiac morbidity and mortality.45-48 Such a discussion is outside of the scope of this article, but it may be prudent to seek a cardiology consultation for patients presenting with risk factors for perioperative MI. β-Blockers may prove useful in reducing cardiac morbidity in high-risk patients after noncardiac surgery.45,49
Many limitations are inherent in studies that use a nationally represented database such as NIS, which we used in this study. It is highly likely that NIS does not capture all potential postoperative complications, as this database is very large and subject to errors in data entry and clinical coding. In addition, detailed clinical information (eg, severity of certain comorbid diseases before shoulder arthroplasty, details about the intraoperative course) was not readily available for analysis. Another limitation, which may have led to an underestimate of complication rates, was our not being able to obtain information about postdischarge complications.
Despite these limitations, NIS and other databases have helped researchers answer questions about low-incidence conditions and generalize findings to a national population. In the present study, we analyzed 2 cohorts, patients with and without acute MI after shoulder arthroplasty, to determine predictors for and complications of postarthroplasty MI. We identified numerous predictors for acute MI: congestive heart failure, angina pectoris, complicated diabetes, renal failure, fluid and electrolyte disorders, and deficiency anemia prior to arthroplasty. As perioperative MI is associated with significant morbidity,14-16 it would be wise to screen patients for such comorbid conditions, assess the severity of these conditions, and offer shoulder arthroplasty with prudence.
Conclusion
The top 4 predictors for acute MI after shoulder arthroplasty were congestive heart failure, angina pectoralis, complicated diabetes mellitus, and male sex. Other pertinent risk factors included older age, Caucasian ethnicity, and primary diagnosis of proximal humerus fracture. Surgeons and patients must be aware of predictors for adverse surgical outcomes such as perioperative MI and understand the extent to which these events increase perioperative morbidity and mortality.
1. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120.
2. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
3. Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop. 2009;467(10):2606-2612.
4. Boettcher WG. Total hip arthroplasties in the elderly. Morbidity, mortality, and cost effectiveness. Clin Orthop. 1992;(274):30-34.
5. Greenfield S, Apolone G, McNeil BJ, Cleary PD. The importance of co-existent disease in the occurrence of postoperative complications and one-year recovery in patients undergoing total hip replacement. Comorbidity and outcomes after hip replacement. Med Care. 1993;31(2):141-154.
6. Kreder HJ, Williams JI, Jaglal S, Hu R, Axcell T, Stephen D. Are complication rates for elective primary total hip arthroplasty in Ontario related to surgeon and hospital volumes? A preliminary investigation. Can J Surg. 1998;41(6):431-437.
7. Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. 2014;120(3):564-578.
8. Mangano DT, Browner WS, Hollenberg M, London MJ, Tubau JF, Tateo IM. Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery. The Study of Perioperative Ischemia Research Group. N Engl J Med. 1990;323(26):1781-1788.
9. Tarhan S, Moffitt EA, Taylor WF, Giuliani ER. Myocardial infarction after general anesthesia. JAMA. 1972;220(11):1451-1454.
10. Landesberg G, Mosseri M, Zahger D, et al. Myocardial infarction after vascular surgery: the role of prolonged stress-induced, ST depression-type ischemia. J Am Coll Cardiol. 2001;37(7):1839-1845.
11. van Waes JA, Nathoe HM, de Graaff JC, et al. Myocardial injury after noncardiac surgery and its association with short-term mortality. Circulation. 2013;127(23):2264-2271.
12. Mangano DT. Perioperative cardiac morbidity. Anesthesiology. 1990;72(1):153-184.
13. Fleisher LA, Corbett W, Berry C, Poldermans D. Cost-effectiveness of differing perioperative beta-blockade strategies in vascular surgery patients. J Cardiothorac Vasc Anesth. 2004;18(1):7-13.
14. Aynardi M, Pulido L, Parvizi J, Sharkey PF, Rothman RH. Early mortality after modern total hip arthroplasty. Clin Orthop. 2009;467(1):213-218.
15. Gangireddy C, Rectenwald JR, Upchurch GR, et al. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. 2007;45(2):335-341.
16. Baser O, Supina D, Sengupta N, Wang L, Kwong L. Impact of postoperative venous thromboembolism on Medicare recipients undergoing total hip replacement or total knee replacement surgery. Am J Health Syst Pharm. 2010;67(17):1438-1445.
17. Fehringer EV, Mikuls TR, Michaud KD, Henderson WG, O’Dell JR. Shoulder arthroplasties have fewer complications than hip or knee arthroplasties in US veterans. Clin Orthop. 2010;468(3):717-722.
18. Farmer KW, Hammond JW, Queale WS, Keyurapan E, McFarland EG. Shoulder arthroplasty versus hip and knee arthroplasties: a comparison of outcomes. Clin Orthop. 2007;(455):183-189.
19. Singh JA, Sperling JW, Cofield RH. Cardiopulmonary complications after primary shoulder arthroplasty: a cohort study. Semin Arthritis Rheum. 2012;41(5):689-697.
20. Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28-e292.
21. Lin CA, Kuo AC, Takemoto S. Comorbidities and perioperative complications in HIV-positive patients undergoing primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2013;95(11):1028-1036.
22. Maynard C, Sales AE. Changes in the use of coronary artery revascularization procedures in the Department of Veterans Affairs, the National Hospital Discharge Survey, and the Nationwide Inpatient Sample, 1991–1999. BMC Health Serv Res. 2003;3(1):12.
23. Griffin JW, Novicoff WM, Browne JA, Brockmeier SF. Obstructive sleep apnea as a risk factor after shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(12):e6-e9.
24. Hambright D, Henderson RA, Cook C, Worrell T, Moorman CT, Bolognesi MP. A comparison of perioperative outcomes in patients with and without rheumatoid arthritis after receiving a total shoulder replacement arthroplasty. J Shoulder Elbow Surg. 2011;20(1):77-85.
25. Odum SM, Troyer JL, Kelly MP, Dedini RD, Bozic KJ. A cost-utility analysis comparing the cost-effectiveness of simultaneous and staged bilateral total knee arthroplasty. J Bone Joint Surg Am. 2013;95(16):1441-1449.
26. Ponce BA, Menendez ME, Oladeji LO, Soldado F. Diabetes as a risk factor for poorer early postoperative outcomes after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(5):671-678.
27. Alfonso DT, Toussaint RJ, Alfonso BD, Strauss EJ, Steiger DT, Di Cesare PE. Nonsurgical complications after total hip and knee arthroplasty. Am J Orthop. 2006;35(11):503-510.
28. Mantilla CB, Horlocker TT, Schroeder DR, Berry DJ, Brown DL. Frequency of myocardial infarction, pulmonary embolism, deep venous thrombosis, and death following primary hip or knee arthroplasty. Anesthesiology. 2002;96(5):1140-1146.
29. Singh JA, Jensen MR, Harmsen WS, Gabriel SE, Lewallen DG. Cardiac and thromboembolic complications and mortality in patients undergoing total hip and total knee arthroplasty. Ann Rheum Dis. 2011;70(12):2082-2088.
30. Kurian AK, Cardarelli KM. Racial and ethnic differences in cardiovascular disease risk factors: a systematic review. Ethn Dis. 2007;17(1):143-152.
31. Zhang AL, Schairer WW, Feeley BT. Hospital readmissions after surgical treatment of proximal humerus fractures: is arthroplasty safer than open reduction internal fixation? Clin Orthop. 2014;472(8):2317-2324.
32. Schairer WW, Zhang AL, Feeley BT. Hospital readmissions after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(9):1349-1355.
33. de Kruijf M, Vroemen JP, de Leur K, van der Voort EA, Vos DI, Van der Laan L. Proximal fractures of the humerus in patients older than 75 years of age: should we consider operative treatment? J Orthop Traumatol. 2014;15(2):111-115.
34. Hauschild O, Konrad G, Audige L, et al. Operative versus non-operative treatment for two-part surgical neck fractures of the proximal humerus. Arch Orthop Trauma Surg. 2013;133(10):1385-1393.
35. Hanson B, Neidenbach P, de Boer P, Stengel D. Functional outcomes after nonoperative management of fractures of the proximal humerus. J Shoulder Elbow Surg. 2009;18(4):612-621.
36. Handoll HH, Ollivere BJ, Rollins KE. Interventions for treating proximal humeral fractures in adults. Cochrane Database Syst Rev. 2012;12:CD000434.
37. Court-Brown CM, Cattermole H, McQueen MM. Impacted valgus fractures (B1.1) of the proximal humerus. The results of non-operative treatment. J Bone Joint Surg Br. 2002;84(4):504-508.
38. Chalmers PN, Gupta AK, Rahman Z, Bruce B, Romeo AA, Nicholson GP. Predictors of early complications of total shoulder arthroplasty. J Arthroplasty. 2014;29(4):856-860.
39. Mahoney A, Bosco JA 3rd, Zuckerman JD. Readmission after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):377-381.
40. Khan SK, Malviya A, Muller SD, et al. Reduced short-term complications and mortality following Enhanced Recovery primary hip and knee arthroplasty: results from 6,000 consecutive procedures. Acta Orthop. 2014;85(1):26-31.
41. Paavolainen P, Pukkala E, Pulkkinen P, Visuri T. Causes of death after total hip arthroplasty: a nationwide cohort study with 24,638 patients. J Arthroplasty. 2002;17(3):274-281.
42. Sharrock NE, Cazan MG, Hargett MJ, Williams-Russo P, Wilson PD Jr. Changes in mortality after total hip and knee arthroplasty over a ten-year period. Anesth Analg. 1995;80(2):242-248.
43. Parvizi J, Johnson BG, Rowland C, Ereth MH, Lewallen DG. Thirty-day mortality after elective total hip arthroplasty. J Bone Joint Surg Am. 2001;83(10):1524-1528.
44. Morris MJ, Molli RG, Berend KR, Lombardi AV Jr. Mortality and perioperative complications after unicompartmental knee arthroplasty. Knee. 2013;20(3):218-220.
45. Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353(4):349-361.
46. Wijeysundera DN, Beattie WS, Wijeysundera HC, Yun L, Austin PC, Ko DT. Duration of preoperative beta-blockade and outcomes after major elective noncardiac surgery. Can J Cardiol. 2014;30(2):217-223.
47. Andersson C, Merie C, Jorgensen M, et al. Association of beta-blocker therapy with risks of adverse cardiovascular events and deaths in patients with ischemic heart disease undergoing noncardiac surgery: a Danish nationwide cohort study. JAMA Int Med. 2014;174(3):336-344.
48. Bakker EJ, Ravensbergen NJ, Poldermans D. Perioperative cardiac evaluation, monitoring, and risk reduction strategies in noncardiac surgery patients. Curr Opin Crit Care. 2011;17(5):409-415.
49. Auerbach AD, Goldman L. Beta-blockers and reduction of cardiac events in noncardiac surgery: scientific review. JAMA. 2002;287(11):1435-1444.
The incidence of shoulder arthroplasty in the United States is increasing annually,1-3 and the majority of these operations occur in older patients.4-6 Elderly patients with cardiovascular, pulmonary, cerebral, renal, and hepatic disease are increasingly susceptible to numerous surgical complications.4 Myocardial infarction (MI) is a complication that occurs in 0.7% of noncardiac surgeries. This figure increases to 1.1% in patients with coronary artery disease.7-11 Perioperative MI increases morbidity and mortality,8 and perioperative cardiac morbidity is the leading cause of death after anesthesia and surgery.12 The financial effects of perioperative cardiac morbidity and mortality must also be considered. A 2009 claims analysis study estimated charges associated with a perioperative MI at $15,000 and the cost of cardiac death at $21,909.13
Cardiovascular complications are associated with a significant degree of morbidity and mortality in patients who undergo arthroplasty.14-16 Although studies have elucidated 30- and 90-day morbidity and mortality rates after shoulder arthroplasty, in hip and knee arthroplasty17-19 little has been done to determine predictors of perioperative MI in a representative database of patients. Given the increasing incidence of shoulder arthroplasty in the United States, the elective nature of this procedure, and the percentage of the US population with cardiovascular risk factors,20 it is important to establish predictors of perioperative MI to ensure patients and physicians have the necessary resources to make informed decisions.
We conducted a study to examine the risk factors for perioperative MI in a large cohort of patients admitted for shoulder arthroplasty to US hospitals. We wanted to evaluate the association between perioperative MI and shoulder arthroplasty with respect to demographics, primary diagnosis, medical comorbidities, and perioperative complications. Specifically, we tested the null hypothesis that, among patients undergoing shoulder arthroplasty, and accounting for confounding variables, there would be no difference in risk factors for patients who have a perioperative MI.
Materials and Methods
This study was exempt from approval by our institutional review board. All data used in this project were deidentified before use.
Nationwide Inpatient Sample (NIS)
The Nationwide Inpatient Sample (NIS), an annual survey of hospitals, is conducted by the Healthcare Cost and Utilization Project (HCUP) and sponsored by the Agency for Healthcare Research and Quality (AHRQ). This database is the largest publicly available all-payer inpatient discharge database in the United States.21 Sampling 8 million hospital stays each year, NIS includes information from a representative batch of 20% of US hospitals. In 2011, 46 states and 1045 hospitals contributed information to the database, representing 97% of the US population.22 This large sample allows researchers to analyze a robust set of medical conditions and uncommon treatments. The survey, conducted each year since 1988, includes demographic, clinical, and resource use data.23 Discharge weight files are provided by NIS to arrive at valid national estimates.
This database is particularly useful because it provides information on up to 25 medical diagnoses and 15 procedures, which are recorded with International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. Researchers can use this database to analyze patient and hospital characteristics as well as inpatient outcomes.24,25 Numerous studies have used NIS to address pertinent queries across the medical landscape.22,26
Patient Selection and Analysis
We used NIS to isolate a population of 422,371 adults (≥18 years old) who underwent total shoulder arthroplasty (TSA) or hemi–shoulder arthroplasty (HSA) between January 1, 2002 and December 31, 2011. We then placed the patients in this population into 1 of 2 cohorts. The first cohort had an acute MI during the perioperative period after TSA, and the second, larger cohort did not have an acute MI after TSA. Acute MI was identified using ICD-9-CM code 410.xx. To identify a population of shoulder arthroplasty patients, we included discharges with an ICD-9-CM procedure code of 81.80 or 81.88 (both TSA) or 81.81 (HSA) in the sample. We then considered the degree to which each of 5 variables—primary diagnosis, age, sex, race, and select medical comorbidities—was predictive of in-hospital MI after TSA.
Statistical Analysis
Given the large sample used in this study, normal distribution of data was assumed. Using bivariate analysis, Pearson χ2 test for categorical data, and independent-samples t test for continuous data, we compared the nonacute MI and acute MI groups. Multivariable binary logistic regression analyses allowed us to isolate the extent that primary diagnosis, age, sex, race, and medical comorbidities were predictors of acute MI after shoulder arthroplasty. Statistical significance was set at P < .05. SPSS Version 22.0 (SPSS, Chicago, Illinois) was used for all statistical analyses and data modeling.
Results
Between January 1, 2002 and December 31, 2011, an estimated total of 422,371 patients underwent shoulder arthroplasty (59.3% TSA, 40.7% HSA). Of these patients, 1174 (0.28%) had a perioperative MI, and 421,197 (99.72%) did not (Table 1). Patients with a primary diagnosis of proximal humerus fracture (33.8% vs 16.6%; P < .001) or rotator cuff arthropathy (10.1% vs 9.9%; P < .001) were more likely than patients with other diagnoses to have an in-hospital MI.
Our review of the demographics found that patients who underwent shoulder arthroplasty and had a perioperative MI were likely older (75±8.9 years vs 69±11 years; P < .001), Caucasian (94.2% vs 91.9%; P = .002), male (43.2% vs 39.7%; P = .013), in the highest median household income bracket of $63,000 or more (30.8% vs 25.6%; P < .001), and using Medicare (80.9% vs 66.3%; P < .001). They were more likely to be treated in a medical center of medium size (25.6% vs 23.7%; P = .042) or larger (61.8% vs 61.2%; P = .042). MIs occurred more often in urban environments (91.4% vs 88.5%; P = .002) and in HSA patients (55% vs 40.6%; P < .001), resulting in longer hospital stays (9.4±7.9 days vs 2.7±2.5 days; P < .001) and higher probability of death (6.5% vs 0.1%; P < .001).
We then analyzed the 2 cohorts for medical comorbidities (Table 2). Patients in the MI cohort presented with a significantly higher incidence of congestive heart failure, previous MI, angina pectoris, chronic lung disease, hypertension, diabetes, renal failure, fluid and electrolyte disorders, pulmonary circulatory disease, coagulopathy, and deficiency anemia (P < .001) but not liver disease and obesity. Bivariate analysis of perioperative outcomes (Table 3) indicated that these patients also had a statistically higher rate of numerous other complications: pulmonary embolism (4.9% vs 0.2%; P < .001), pneumonia (15.1% vs 1.2%; P < .001), deep venous thrombosis (2.6% vs 0.2%; P < .001), cerebrovascular event (1.6% vs 0.1%; P < .001), acute renal failure (15.1% vs 1.2%; P < .001), gastrointestinal complication (1.2% vs 0.3%; P < .001), mechanical ventilation (1.2% vs 0.3%; P < .001), transfusion (33.4% vs 8.8%; P < .001), and nonroutine discharge (73.3% vs 36.0%; P < .001).
Multivariable logistic regression analysis was performed to determine independent predictors of perioperative MI after shoulder arthroplasty (Table 4). Patients with a primary diagnosis of proximal humerus fracture (odds ratio [OR], 1.38; 95% confidence interval [CI], 1.15-1.65; P < .001) were more likely than patients with a primary diagnosis of osteoarthritis to have an MI. The odds of postoperative MI increased with age (OR, 1.04 per year; 95% CI, 1.03-1.05; P < .001) and were higher in males (OR, 1.72; 95% CI, 1.52-1.96; P < .001). Compared with Caucasians, African Americans (OR, 0.19; 95% CI, 0.09-0.40; P < .001) were less likely to have an in-hospital MI after shoulder arthroplasty. After shoulder arthroplasty, the odds of MI in the perioperative period increased with each subsequent day of care (OR, 1.10; 95% CI, 1.10-1.11; P < .001).
Regarding independent comorbidities, multivariable logistic regression analysis also determined that history of congestive heart failure (OR, 4.86; 95% CI, 4.20-5.61; P < .001), angina pectoris (OR, 2.90; 95% CI, 2.02-4.17; P < .001), complicated diabetes (OR, 1.96; 95% CI, 1.49-2.57; P < .001), renal failure (OR, 1.42; 95% CI, 1.17-1.72; P < .001), fluid and electrolyte disorders (OR, 1.42; 95% CI, 1.21-1.67; P < .001), and deficiency anemia (OR, 1.62; 95% CI, 1.40-1.88; P < .001) were significant predictors of perioperative MI after shoulder arthroplasty.
Discussion
Results of other studies have elucidated 30- and 90-day mortality rates and postoperative complications after shoulder arthroplasty, but, relative to hip and knee arthroplasty,17-19 little has been done to determine predictors of perioperative MI in a large sample of shoulder arthroplasty patients. Given the increasing rates of shoulder arthroplasty1-3 and the demographics of this population,4-6 it is likely that postoperative cardiovascular events will increase in frequency. We found that, in order of decreasing significance, the top 4 risk predictors for acute MI after shoulder arthroplasty were congestive heart failure, angina pectoralis, complicated diabetes mellitus, and male sex. Other pertinent risk factors included older age, Caucasian ethnicity, and a primary diagnosis of proximal humerus fracture. The rate of acute MI in patients who were older than 75 years when they underwent HSA for proximal humerus fracture was 0.80%.
Demographics
We found that patients who had an acute MI after shoulder arthroplasty were likely older, male, and Caucasian. Age and male sex are well-established risk factors for increased cardiac complications after arthroplasty.27-29 Previous studies have indicated that the rate of cardiac events increases in arthroplasty patients older than 65 years.19,28,29 In our study, more than 50% of the patients who had an acute perioperative MI were older than 85 years. Less explainable is the increased occurrence of acute MI in Caucasian patients and wealthy patients, given that minorities in the United States have higher rates of cardiovascular disease.30 Shoulder arthroplasty is an elective procedure, more likely to be undertaken by Caucasians. Therefore, at-risk minority groups and financially challenged groups may be less likely to have this procedure.
Primary Diagnosis
In this series, patients with a primary diagnosis of proximal humerus fracture were more likely to have an in-hospital MI. This finding is consistent with previous studies indicating a higher rate of complications for proximal humerus fracture patients than for shoulder arthroplasty patients.31,32 Given that more than 75% of patients who present with a proximal humerus fracture are older than 70 years, it would be prudent to examine operative indications after this diagnosis,33 particularly as benefit from surgery for fractures has not been definitively demonstrated.34-37
Comorbidities
Many of the patients in our MI cohort presented with congestive heart failure, angina pectoris, complicated diabetes, renal failure, fluid and electrolyte disorders, or deficiency anemia. This is in keeping with other studies indicating that preexisting cardiovascular morbidity increases the rate of MI after various forms of arthroplasty.7-11 Patients in our MI cohort were also susceptible to a variety of post-MI perioperative complications, including pulmonary embolism, pneumonia, deep venous thrombosis, cerebrovascular event, acute renal failure, gastrointestinal complication, mechanical ventilation, transfusion, and nonroutine discharge, and their incidence of death was higher. These findings are consistent with reports that postoperative cardiovascular complications increase the degree of morbidity and mortality in arthroplasty patients.14-16 It is also worth noting that the odds of MI in the perioperative period increase with each subsequent day of care. This is understandable given that patients presenting with numerous comorbidities are at increased risk for perioperative complications38 resulting in hospital readmission.39
The literature indicates that MI occurs as a complication in 0.7% of patients who undergo noncardiac surgery,7 though some series have shown it is more prevalent after arthroplasty procedures.28,40 MI significantly increases the rate of perioperative morbidity and mortality,8 and perioperative cardiac morbidity is a leading cause of death after anesthesia and surgery.12 Furthermore, the most common cause of death after lower extremity arthroplasty is cardiovascular-related.41,42 In patients who presented for elective hip arthroplasty, cardiorespiratory disease was one of the main risk factors (with older age and male sex) shown to increase perioperative mortality.43
Perioperative cardiovascular complications increase postoperative morbidity and mortality.12 The rate of cardiovascular complications after shoulder arthroplasty ranges from 0.8% to 2.6%, and the incidence of MI hovers between 0.3% and 0.9%.17,19,28,40,44 A recent study in 793 patients found that, over a 30-day period, cardiovascular complications accounted for more than one-fourth of all complications.17 Singh and colleagues19 analyzed cardiopulmonary complications after primary shoulder arthroplasty in a total of 3480 patients (4019 arthroplasties) and found this group had a 90-day cardiac morbidity (MI, congestive heart failure, arrhythmia) rate of 2.6%. In that study, a Deyo-Charlson index of 1 or more was a significant independent risk factor for cardiac complications following surgery. Scores on this weighted index of 17 comorbidities are used to assess the complexities of a patient population. Given the severity of cardiovascular perioperative complications, it is important to preoperatively identify high-risk population groups and sufficiently study and optimize patients before shoulder arthroplasty.
There is much debate about the effectiveness of perioperative β-blockers in reducing perioperative cardiac morbidity and mortality.45-48 Such a discussion is outside of the scope of this article, but it may be prudent to seek a cardiology consultation for patients presenting with risk factors for perioperative MI. β-Blockers may prove useful in reducing cardiac morbidity in high-risk patients after noncardiac surgery.45,49
Many limitations are inherent in studies that use a nationally represented database such as NIS, which we used in this study. It is highly likely that NIS does not capture all potential postoperative complications, as this database is very large and subject to errors in data entry and clinical coding. In addition, detailed clinical information (eg, severity of certain comorbid diseases before shoulder arthroplasty, details about the intraoperative course) was not readily available for analysis. Another limitation, which may have led to an underestimate of complication rates, was our not being able to obtain information about postdischarge complications.
Despite these limitations, NIS and other databases have helped researchers answer questions about low-incidence conditions and generalize findings to a national population. In the present study, we analyzed 2 cohorts, patients with and without acute MI after shoulder arthroplasty, to determine predictors for and complications of postarthroplasty MI. We identified numerous predictors for acute MI: congestive heart failure, angina pectoris, complicated diabetes, renal failure, fluid and electrolyte disorders, and deficiency anemia prior to arthroplasty. As perioperative MI is associated with significant morbidity,14-16 it would be wise to screen patients for such comorbid conditions, assess the severity of these conditions, and offer shoulder arthroplasty with prudence.
Conclusion
The top 4 predictors for acute MI after shoulder arthroplasty were congestive heart failure, angina pectoralis, complicated diabetes mellitus, and male sex. Other pertinent risk factors included older age, Caucasian ethnicity, and primary diagnosis of proximal humerus fracture. Surgeons and patients must be aware of predictors for adverse surgical outcomes such as perioperative MI and understand the extent to which these events increase perioperative morbidity and mortality.
The incidence of shoulder arthroplasty in the United States is increasing annually,1-3 and the majority of these operations occur in older patients.4-6 Elderly patients with cardiovascular, pulmonary, cerebral, renal, and hepatic disease are increasingly susceptible to numerous surgical complications.4 Myocardial infarction (MI) is a complication that occurs in 0.7% of noncardiac surgeries. This figure increases to 1.1% in patients with coronary artery disease.7-11 Perioperative MI increases morbidity and mortality,8 and perioperative cardiac morbidity is the leading cause of death after anesthesia and surgery.12 The financial effects of perioperative cardiac morbidity and mortality must also be considered. A 2009 claims analysis study estimated charges associated with a perioperative MI at $15,000 and the cost of cardiac death at $21,909.13
Cardiovascular complications are associated with a significant degree of morbidity and mortality in patients who undergo arthroplasty.14-16 Although studies have elucidated 30- and 90-day morbidity and mortality rates after shoulder arthroplasty, in hip and knee arthroplasty17-19 little has been done to determine predictors of perioperative MI in a representative database of patients. Given the increasing incidence of shoulder arthroplasty in the United States, the elective nature of this procedure, and the percentage of the US population with cardiovascular risk factors,20 it is important to establish predictors of perioperative MI to ensure patients and physicians have the necessary resources to make informed decisions.
We conducted a study to examine the risk factors for perioperative MI in a large cohort of patients admitted for shoulder arthroplasty to US hospitals. We wanted to evaluate the association between perioperative MI and shoulder arthroplasty with respect to demographics, primary diagnosis, medical comorbidities, and perioperative complications. Specifically, we tested the null hypothesis that, among patients undergoing shoulder arthroplasty, and accounting for confounding variables, there would be no difference in risk factors for patients who have a perioperative MI.
Materials and Methods
This study was exempt from approval by our institutional review board. All data used in this project were deidentified before use.
Nationwide Inpatient Sample (NIS)
The Nationwide Inpatient Sample (NIS), an annual survey of hospitals, is conducted by the Healthcare Cost and Utilization Project (HCUP) and sponsored by the Agency for Healthcare Research and Quality (AHRQ). This database is the largest publicly available all-payer inpatient discharge database in the United States.21 Sampling 8 million hospital stays each year, NIS includes information from a representative batch of 20% of US hospitals. In 2011, 46 states and 1045 hospitals contributed information to the database, representing 97% of the US population.22 This large sample allows researchers to analyze a robust set of medical conditions and uncommon treatments. The survey, conducted each year since 1988, includes demographic, clinical, and resource use data.23 Discharge weight files are provided by NIS to arrive at valid national estimates.
This database is particularly useful because it provides information on up to 25 medical diagnoses and 15 procedures, which are recorded with International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. Researchers can use this database to analyze patient and hospital characteristics as well as inpatient outcomes.24,25 Numerous studies have used NIS to address pertinent queries across the medical landscape.22,26
Patient Selection and Analysis
We used NIS to isolate a population of 422,371 adults (≥18 years old) who underwent total shoulder arthroplasty (TSA) or hemi–shoulder arthroplasty (HSA) between January 1, 2002 and December 31, 2011. We then placed the patients in this population into 1 of 2 cohorts. The first cohort had an acute MI during the perioperative period after TSA, and the second, larger cohort did not have an acute MI after TSA. Acute MI was identified using ICD-9-CM code 410.xx. To identify a population of shoulder arthroplasty patients, we included discharges with an ICD-9-CM procedure code of 81.80 or 81.88 (both TSA) or 81.81 (HSA) in the sample. We then considered the degree to which each of 5 variables—primary diagnosis, age, sex, race, and select medical comorbidities—was predictive of in-hospital MI after TSA.
Statistical Analysis
Given the large sample used in this study, normal distribution of data was assumed. Using bivariate analysis, Pearson χ2 test for categorical data, and independent-samples t test for continuous data, we compared the nonacute MI and acute MI groups. Multivariable binary logistic regression analyses allowed us to isolate the extent that primary diagnosis, age, sex, race, and medical comorbidities were predictors of acute MI after shoulder arthroplasty. Statistical significance was set at P < .05. SPSS Version 22.0 (SPSS, Chicago, Illinois) was used for all statistical analyses and data modeling.
Results
Between January 1, 2002 and December 31, 2011, an estimated total of 422,371 patients underwent shoulder arthroplasty (59.3% TSA, 40.7% HSA). Of these patients, 1174 (0.28%) had a perioperative MI, and 421,197 (99.72%) did not (Table 1). Patients with a primary diagnosis of proximal humerus fracture (33.8% vs 16.6%; P < .001) or rotator cuff arthropathy (10.1% vs 9.9%; P < .001) were more likely than patients with other diagnoses to have an in-hospital MI.
Our review of the demographics found that patients who underwent shoulder arthroplasty and had a perioperative MI were likely older (75±8.9 years vs 69±11 years; P < .001), Caucasian (94.2% vs 91.9%; P = .002), male (43.2% vs 39.7%; P = .013), in the highest median household income bracket of $63,000 or more (30.8% vs 25.6%; P < .001), and using Medicare (80.9% vs 66.3%; P < .001). They were more likely to be treated in a medical center of medium size (25.6% vs 23.7%; P = .042) or larger (61.8% vs 61.2%; P = .042). MIs occurred more often in urban environments (91.4% vs 88.5%; P = .002) and in HSA patients (55% vs 40.6%; P < .001), resulting in longer hospital stays (9.4±7.9 days vs 2.7±2.5 days; P < .001) and higher probability of death (6.5% vs 0.1%; P < .001).
We then analyzed the 2 cohorts for medical comorbidities (Table 2). Patients in the MI cohort presented with a significantly higher incidence of congestive heart failure, previous MI, angina pectoris, chronic lung disease, hypertension, diabetes, renal failure, fluid and electrolyte disorders, pulmonary circulatory disease, coagulopathy, and deficiency anemia (P < .001) but not liver disease and obesity. Bivariate analysis of perioperative outcomes (Table 3) indicated that these patients also had a statistically higher rate of numerous other complications: pulmonary embolism (4.9% vs 0.2%; P < .001), pneumonia (15.1% vs 1.2%; P < .001), deep venous thrombosis (2.6% vs 0.2%; P < .001), cerebrovascular event (1.6% vs 0.1%; P < .001), acute renal failure (15.1% vs 1.2%; P < .001), gastrointestinal complication (1.2% vs 0.3%; P < .001), mechanical ventilation (1.2% vs 0.3%; P < .001), transfusion (33.4% vs 8.8%; P < .001), and nonroutine discharge (73.3% vs 36.0%; P < .001).
Multivariable logistic regression analysis was performed to determine independent predictors of perioperative MI after shoulder arthroplasty (Table 4). Patients with a primary diagnosis of proximal humerus fracture (odds ratio [OR], 1.38; 95% confidence interval [CI], 1.15-1.65; P < .001) were more likely than patients with a primary diagnosis of osteoarthritis to have an MI. The odds of postoperative MI increased with age (OR, 1.04 per year; 95% CI, 1.03-1.05; P < .001) and were higher in males (OR, 1.72; 95% CI, 1.52-1.96; P < .001). Compared with Caucasians, African Americans (OR, 0.19; 95% CI, 0.09-0.40; P < .001) were less likely to have an in-hospital MI after shoulder arthroplasty. After shoulder arthroplasty, the odds of MI in the perioperative period increased with each subsequent day of care (OR, 1.10; 95% CI, 1.10-1.11; P < .001).
Regarding independent comorbidities, multivariable logistic regression analysis also determined that history of congestive heart failure (OR, 4.86; 95% CI, 4.20-5.61; P < .001), angina pectoris (OR, 2.90; 95% CI, 2.02-4.17; P < .001), complicated diabetes (OR, 1.96; 95% CI, 1.49-2.57; P < .001), renal failure (OR, 1.42; 95% CI, 1.17-1.72; P < .001), fluid and electrolyte disorders (OR, 1.42; 95% CI, 1.21-1.67; P < .001), and deficiency anemia (OR, 1.62; 95% CI, 1.40-1.88; P < .001) were significant predictors of perioperative MI after shoulder arthroplasty.
Discussion
Results of other studies have elucidated 30- and 90-day mortality rates and postoperative complications after shoulder arthroplasty, but, relative to hip and knee arthroplasty,17-19 little has been done to determine predictors of perioperative MI in a large sample of shoulder arthroplasty patients. Given the increasing rates of shoulder arthroplasty1-3 and the demographics of this population,4-6 it is likely that postoperative cardiovascular events will increase in frequency. We found that, in order of decreasing significance, the top 4 risk predictors for acute MI after shoulder arthroplasty were congestive heart failure, angina pectoralis, complicated diabetes mellitus, and male sex. Other pertinent risk factors included older age, Caucasian ethnicity, and a primary diagnosis of proximal humerus fracture. The rate of acute MI in patients who were older than 75 years when they underwent HSA for proximal humerus fracture was 0.80%.
Demographics
We found that patients who had an acute MI after shoulder arthroplasty were likely older, male, and Caucasian. Age and male sex are well-established risk factors for increased cardiac complications after arthroplasty.27-29 Previous studies have indicated that the rate of cardiac events increases in arthroplasty patients older than 65 years.19,28,29 In our study, more than 50% of the patients who had an acute perioperative MI were older than 85 years. Less explainable is the increased occurrence of acute MI in Caucasian patients and wealthy patients, given that minorities in the United States have higher rates of cardiovascular disease.30 Shoulder arthroplasty is an elective procedure, more likely to be undertaken by Caucasians. Therefore, at-risk minority groups and financially challenged groups may be less likely to have this procedure.
Primary Diagnosis
In this series, patients with a primary diagnosis of proximal humerus fracture were more likely to have an in-hospital MI. This finding is consistent with previous studies indicating a higher rate of complications for proximal humerus fracture patients than for shoulder arthroplasty patients.31,32 Given that more than 75% of patients who present with a proximal humerus fracture are older than 70 years, it would be prudent to examine operative indications after this diagnosis,33 particularly as benefit from surgery for fractures has not been definitively demonstrated.34-37
Comorbidities
Many of the patients in our MI cohort presented with congestive heart failure, angina pectoris, complicated diabetes, renal failure, fluid and electrolyte disorders, or deficiency anemia. This is in keeping with other studies indicating that preexisting cardiovascular morbidity increases the rate of MI after various forms of arthroplasty.7-11 Patients in our MI cohort were also susceptible to a variety of post-MI perioperative complications, including pulmonary embolism, pneumonia, deep venous thrombosis, cerebrovascular event, acute renal failure, gastrointestinal complication, mechanical ventilation, transfusion, and nonroutine discharge, and their incidence of death was higher. These findings are consistent with reports that postoperative cardiovascular complications increase the degree of morbidity and mortality in arthroplasty patients.14-16 It is also worth noting that the odds of MI in the perioperative period increase with each subsequent day of care. This is understandable given that patients presenting with numerous comorbidities are at increased risk for perioperative complications38 resulting in hospital readmission.39
The literature indicates that MI occurs as a complication in 0.7% of patients who undergo noncardiac surgery,7 though some series have shown it is more prevalent after arthroplasty procedures.28,40 MI significantly increases the rate of perioperative morbidity and mortality,8 and perioperative cardiac morbidity is a leading cause of death after anesthesia and surgery.12 Furthermore, the most common cause of death after lower extremity arthroplasty is cardiovascular-related.41,42 In patients who presented for elective hip arthroplasty, cardiorespiratory disease was one of the main risk factors (with older age and male sex) shown to increase perioperative mortality.43
Perioperative cardiovascular complications increase postoperative morbidity and mortality.12 The rate of cardiovascular complications after shoulder arthroplasty ranges from 0.8% to 2.6%, and the incidence of MI hovers between 0.3% and 0.9%.17,19,28,40,44 A recent study in 793 patients found that, over a 30-day period, cardiovascular complications accounted for more than one-fourth of all complications.17 Singh and colleagues19 analyzed cardiopulmonary complications after primary shoulder arthroplasty in a total of 3480 patients (4019 arthroplasties) and found this group had a 90-day cardiac morbidity (MI, congestive heart failure, arrhythmia) rate of 2.6%. In that study, a Deyo-Charlson index of 1 or more was a significant independent risk factor for cardiac complications following surgery. Scores on this weighted index of 17 comorbidities are used to assess the complexities of a patient population. Given the severity of cardiovascular perioperative complications, it is important to preoperatively identify high-risk population groups and sufficiently study and optimize patients before shoulder arthroplasty.
There is much debate about the effectiveness of perioperative β-blockers in reducing perioperative cardiac morbidity and mortality.45-48 Such a discussion is outside of the scope of this article, but it may be prudent to seek a cardiology consultation for patients presenting with risk factors for perioperative MI. β-Blockers may prove useful in reducing cardiac morbidity in high-risk patients after noncardiac surgery.45,49
Many limitations are inherent in studies that use a nationally represented database such as NIS, which we used in this study. It is highly likely that NIS does not capture all potential postoperative complications, as this database is very large and subject to errors in data entry and clinical coding. In addition, detailed clinical information (eg, severity of certain comorbid diseases before shoulder arthroplasty, details about the intraoperative course) was not readily available for analysis. Another limitation, which may have led to an underestimate of complication rates, was our not being able to obtain information about postdischarge complications.
Despite these limitations, NIS and other databases have helped researchers answer questions about low-incidence conditions and generalize findings to a national population. In the present study, we analyzed 2 cohorts, patients with and without acute MI after shoulder arthroplasty, to determine predictors for and complications of postarthroplasty MI. We identified numerous predictors for acute MI: congestive heart failure, angina pectoris, complicated diabetes, renal failure, fluid and electrolyte disorders, and deficiency anemia prior to arthroplasty. As perioperative MI is associated with significant morbidity,14-16 it would be wise to screen patients for such comorbid conditions, assess the severity of these conditions, and offer shoulder arthroplasty with prudence.
Conclusion
The top 4 predictors for acute MI after shoulder arthroplasty were congestive heart failure, angina pectoralis, complicated diabetes mellitus, and male sex. Other pertinent risk factors included older age, Caucasian ethnicity, and primary diagnosis of proximal humerus fracture. Surgeons and patients must be aware of predictors for adverse surgical outcomes such as perioperative MI and understand the extent to which these events increase perioperative morbidity and mortality.
1. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120.
2. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
3. Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop. 2009;467(10):2606-2612.
4. Boettcher WG. Total hip arthroplasties in the elderly. Morbidity, mortality, and cost effectiveness. Clin Orthop. 1992;(274):30-34.
5. Greenfield S, Apolone G, McNeil BJ, Cleary PD. The importance of co-existent disease in the occurrence of postoperative complications and one-year recovery in patients undergoing total hip replacement. Comorbidity and outcomes after hip replacement. Med Care. 1993;31(2):141-154.
6. Kreder HJ, Williams JI, Jaglal S, Hu R, Axcell T, Stephen D. Are complication rates for elective primary total hip arthroplasty in Ontario related to surgeon and hospital volumes? A preliminary investigation. Can J Surg. 1998;41(6):431-437.
7. Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. 2014;120(3):564-578.
8. Mangano DT, Browner WS, Hollenberg M, London MJ, Tubau JF, Tateo IM. Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery. The Study of Perioperative Ischemia Research Group. N Engl J Med. 1990;323(26):1781-1788.
9. Tarhan S, Moffitt EA, Taylor WF, Giuliani ER. Myocardial infarction after general anesthesia. JAMA. 1972;220(11):1451-1454.
10. Landesberg G, Mosseri M, Zahger D, et al. Myocardial infarction after vascular surgery: the role of prolonged stress-induced, ST depression-type ischemia. J Am Coll Cardiol. 2001;37(7):1839-1845.
11. van Waes JA, Nathoe HM, de Graaff JC, et al. Myocardial injury after noncardiac surgery and its association with short-term mortality. Circulation. 2013;127(23):2264-2271.
12. Mangano DT. Perioperative cardiac morbidity. Anesthesiology. 1990;72(1):153-184.
13. Fleisher LA, Corbett W, Berry C, Poldermans D. Cost-effectiveness of differing perioperative beta-blockade strategies in vascular surgery patients. J Cardiothorac Vasc Anesth. 2004;18(1):7-13.
14. Aynardi M, Pulido L, Parvizi J, Sharkey PF, Rothman RH. Early mortality after modern total hip arthroplasty. Clin Orthop. 2009;467(1):213-218.
15. Gangireddy C, Rectenwald JR, Upchurch GR, et al. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. 2007;45(2):335-341.
16. Baser O, Supina D, Sengupta N, Wang L, Kwong L. Impact of postoperative venous thromboembolism on Medicare recipients undergoing total hip replacement or total knee replacement surgery. Am J Health Syst Pharm. 2010;67(17):1438-1445.
17. Fehringer EV, Mikuls TR, Michaud KD, Henderson WG, O’Dell JR. Shoulder arthroplasties have fewer complications than hip or knee arthroplasties in US veterans. Clin Orthop. 2010;468(3):717-722.
18. Farmer KW, Hammond JW, Queale WS, Keyurapan E, McFarland EG. Shoulder arthroplasty versus hip and knee arthroplasties: a comparison of outcomes. Clin Orthop. 2007;(455):183-189.
19. Singh JA, Sperling JW, Cofield RH. Cardiopulmonary complications after primary shoulder arthroplasty: a cohort study. Semin Arthritis Rheum. 2012;41(5):689-697.
20. Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28-e292.
21. Lin CA, Kuo AC, Takemoto S. Comorbidities and perioperative complications in HIV-positive patients undergoing primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2013;95(11):1028-1036.
22. Maynard C, Sales AE. Changes in the use of coronary artery revascularization procedures in the Department of Veterans Affairs, the National Hospital Discharge Survey, and the Nationwide Inpatient Sample, 1991–1999. BMC Health Serv Res. 2003;3(1):12.
23. Griffin JW, Novicoff WM, Browne JA, Brockmeier SF. Obstructive sleep apnea as a risk factor after shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(12):e6-e9.
24. Hambright D, Henderson RA, Cook C, Worrell T, Moorman CT, Bolognesi MP. A comparison of perioperative outcomes in patients with and without rheumatoid arthritis after receiving a total shoulder replacement arthroplasty. J Shoulder Elbow Surg. 2011;20(1):77-85.
25. Odum SM, Troyer JL, Kelly MP, Dedini RD, Bozic KJ. A cost-utility analysis comparing the cost-effectiveness of simultaneous and staged bilateral total knee arthroplasty. J Bone Joint Surg Am. 2013;95(16):1441-1449.
26. Ponce BA, Menendez ME, Oladeji LO, Soldado F. Diabetes as a risk factor for poorer early postoperative outcomes after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(5):671-678.
27. Alfonso DT, Toussaint RJ, Alfonso BD, Strauss EJ, Steiger DT, Di Cesare PE. Nonsurgical complications after total hip and knee arthroplasty. Am J Orthop. 2006;35(11):503-510.
28. Mantilla CB, Horlocker TT, Schroeder DR, Berry DJ, Brown DL. Frequency of myocardial infarction, pulmonary embolism, deep venous thrombosis, and death following primary hip or knee arthroplasty. Anesthesiology. 2002;96(5):1140-1146.
29. Singh JA, Jensen MR, Harmsen WS, Gabriel SE, Lewallen DG. Cardiac and thromboembolic complications and mortality in patients undergoing total hip and total knee arthroplasty. Ann Rheum Dis. 2011;70(12):2082-2088.
30. Kurian AK, Cardarelli KM. Racial and ethnic differences in cardiovascular disease risk factors: a systematic review. Ethn Dis. 2007;17(1):143-152.
31. Zhang AL, Schairer WW, Feeley BT. Hospital readmissions after surgical treatment of proximal humerus fractures: is arthroplasty safer than open reduction internal fixation? Clin Orthop. 2014;472(8):2317-2324.
32. Schairer WW, Zhang AL, Feeley BT. Hospital readmissions after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(9):1349-1355.
33. de Kruijf M, Vroemen JP, de Leur K, van der Voort EA, Vos DI, Van der Laan L. Proximal fractures of the humerus in patients older than 75 years of age: should we consider operative treatment? J Orthop Traumatol. 2014;15(2):111-115.
34. Hauschild O, Konrad G, Audige L, et al. Operative versus non-operative treatment for two-part surgical neck fractures of the proximal humerus. Arch Orthop Trauma Surg. 2013;133(10):1385-1393.
35. Hanson B, Neidenbach P, de Boer P, Stengel D. Functional outcomes after nonoperative management of fractures of the proximal humerus. J Shoulder Elbow Surg. 2009;18(4):612-621.
36. Handoll HH, Ollivere BJ, Rollins KE. Interventions for treating proximal humeral fractures in adults. Cochrane Database Syst Rev. 2012;12:CD000434.
37. Court-Brown CM, Cattermole H, McQueen MM. Impacted valgus fractures (B1.1) of the proximal humerus. The results of non-operative treatment. J Bone Joint Surg Br. 2002;84(4):504-508.
38. Chalmers PN, Gupta AK, Rahman Z, Bruce B, Romeo AA, Nicholson GP. Predictors of early complications of total shoulder arthroplasty. J Arthroplasty. 2014;29(4):856-860.
39. Mahoney A, Bosco JA 3rd, Zuckerman JD. Readmission after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):377-381.
40. Khan SK, Malviya A, Muller SD, et al. Reduced short-term complications and mortality following Enhanced Recovery primary hip and knee arthroplasty: results from 6,000 consecutive procedures. Acta Orthop. 2014;85(1):26-31.
41. Paavolainen P, Pukkala E, Pulkkinen P, Visuri T. Causes of death after total hip arthroplasty: a nationwide cohort study with 24,638 patients. J Arthroplasty. 2002;17(3):274-281.
42. Sharrock NE, Cazan MG, Hargett MJ, Williams-Russo P, Wilson PD Jr. Changes in mortality after total hip and knee arthroplasty over a ten-year period. Anesth Analg. 1995;80(2):242-248.
43. Parvizi J, Johnson BG, Rowland C, Ereth MH, Lewallen DG. Thirty-day mortality after elective total hip arthroplasty. J Bone Joint Surg Am. 2001;83(10):1524-1528.
44. Morris MJ, Molli RG, Berend KR, Lombardi AV Jr. Mortality and perioperative complications after unicompartmental knee arthroplasty. Knee. 2013;20(3):218-220.
45. Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353(4):349-361.
46. Wijeysundera DN, Beattie WS, Wijeysundera HC, Yun L, Austin PC, Ko DT. Duration of preoperative beta-blockade and outcomes after major elective noncardiac surgery. Can J Cardiol. 2014;30(2):217-223.
47. Andersson C, Merie C, Jorgensen M, et al. Association of beta-blocker therapy with risks of adverse cardiovascular events and deaths in patients with ischemic heart disease undergoing noncardiac surgery: a Danish nationwide cohort study. JAMA Int Med. 2014;174(3):336-344.
48. Bakker EJ, Ravensbergen NJ, Poldermans D. Perioperative cardiac evaluation, monitoring, and risk reduction strategies in noncardiac surgery patients. Curr Opin Crit Care. 2011;17(5):409-415.
49. Auerbach AD, Goldman L. Beta-blockers and reduction of cardiac events in noncardiac surgery: scientific review. JAMA. 2002;287(11):1435-1444.
1. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120.
2. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
3. Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop. 2009;467(10):2606-2612.
4. Boettcher WG. Total hip arthroplasties in the elderly. Morbidity, mortality, and cost effectiveness. Clin Orthop. 1992;(274):30-34.
5. Greenfield S, Apolone G, McNeil BJ, Cleary PD. The importance of co-existent disease in the occurrence of postoperative complications and one-year recovery in patients undergoing total hip replacement. Comorbidity and outcomes after hip replacement. Med Care. 1993;31(2):141-154.
6. Kreder HJ, Williams JI, Jaglal S, Hu R, Axcell T, Stephen D. Are complication rates for elective primary total hip arthroplasty in Ontario related to surgeon and hospital volumes? A preliminary investigation. Can J Surg. 1998;41(6):431-437.
7. Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. 2014;120(3):564-578.
8. Mangano DT, Browner WS, Hollenberg M, London MJ, Tubau JF, Tateo IM. Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery. The Study of Perioperative Ischemia Research Group. N Engl J Med. 1990;323(26):1781-1788.
9. Tarhan S, Moffitt EA, Taylor WF, Giuliani ER. Myocardial infarction after general anesthesia. JAMA. 1972;220(11):1451-1454.
10. Landesberg G, Mosseri M, Zahger D, et al. Myocardial infarction after vascular surgery: the role of prolonged stress-induced, ST depression-type ischemia. J Am Coll Cardiol. 2001;37(7):1839-1845.
11. van Waes JA, Nathoe HM, de Graaff JC, et al. Myocardial injury after noncardiac surgery and its association with short-term mortality. Circulation. 2013;127(23):2264-2271.
12. Mangano DT. Perioperative cardiac morbidity. Anesthesiology. 1990;72(1):153-184.
13. Fleisher LA, Corbett W, Berry C, Poldermans D. Cost-effectiveness of differing perioperative beta-blockade strategies in vascular surgery patients. J Cardiothorac Vasc Anesth. 2004;18(1):7-13.
14. Aynardi M, Pulido L, Parvizi J, Sharkey PF, Rothman RH. Early mortality after modern total hip arthroplasty. Clin Orthop. 2009;467(1):213-218.
15. Gangireddy C, Rectenwald JR, Upchurch GR, et al. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. 2007;45(2):335-341.
16. Baser O, Supina D, Sengupta N, Wang L, Kwong L. Impact of postoperative venous thromboembolism on Medicare recipients undergoing total hip replacement or total knee replacement surgery. Am J Health Syst Pharm. 2010;67(17):1438-1445.
17. Fehringer EV, Mikuls TR, Michaud KD, Henderson WG, O’Dell JR. Shoulder arthroplasties have fewer complications than hip or knee arthroplasties in US veterans. Clin Orthop. 2010;468(3):717-722.
18. Farmer KW, Hammond JW, Queale WS, Keyurapan E, McFarland EG. Shoulder arthroplasty versus hip and knee arthroplasties: a comparison of outcomes. Clin Orthop. 2007;(455):183-189.
19. Singh JA, Sperling JW, Cofield RH. Cardiopulmonary complications after primary shoulder arthroplasty: a cohort study. Semin Arthritis Rheum. 2012;41(5):689-697.
20. Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28-e292.
21. Lin CA, Kuo AC, Takemoto S. Comorbidities and perioperative complications in HIV-positive patients undergoing primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2013;95(11):1028-1036.
22. Maynard C, Sales AE. Changes in the use of coronary artery revascularization procedures in the Department of Veterans Affairs, the National Hospital Discharge Survey, and the Nationwide Inpatient Sample, 1991–1999. BMC Health Serv Res. 2003;3(1):12.
23. Griffin JW, Novicoff WM, Browne JA, Brockmeier SF. Obstructive sleep apnea as a risk factor after shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(12):e6-e9.
24. Hambright D, Henderson RA, Cook C, Worrell T, Moorman CT, Bolognesi MP. A comparison of perioperative outcomes in patients with and without rheumatoid arthritis after receiving a total shoulder replacement arthroplasty. J Shoulder Elbow Surg. 2011;20(1):77-85.
25. Odum SM, Troyer JL, Kelly MP, Dedini RD, Bozic KJ. A cost-utility analysis comparing the cost-effectiveness of simultaneous and staged bilateral total knee arthroplasty. J Bone Joint Surg Am. 2013;95(16):1441-1449.
26. Ponce BA, Menendez ME, Oladeji LO, Soldado F. Diabetes as a risk factor for poorer early postoperative outcomes after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(5):671-678.
27. Alfonso DT, Toussaint RJ, Alfonso BD, Strauss EJ, Steiger DT, Di Cesare PE. Nonsurgical complications after total hip and knee arthroplasty. Am J Orthop. 2006;35(11):503-510.
28. Mantilla CB, Horlocker TT, Schroeder DR, Berry DJ, Brown DL. Frequency of myocardial infarction, pulmonary embolism, deep venous thrombosis, and death following primary hip or knee arthroplasty. Anesthesiology. 2002;96(5):1140-1146.
29. Singh JA, Jensen MR, Harmsen WS, Gabriel SE, Lewallen DG. Cardiac and thromboembolic complications and mortality in patients undergoing total hip and total knee arthroplasty. Ann Rheum Dis. 2011;70(12):2082-2088.
30. Kurian AK, Cardarelli KM. Racial and ethnic differences in cardiovascular disease risk factors: a systematic review. Ethn Dis. 2007;17(1):143-152.
31. Zhang AL, Schairer WW, Feeley BT. Hospital readmissions after surgical treatment of proximal humerus fractures: is arthroplasty safer than open reduction internal fixation? Clin Orthop. 2014;472(8):2317-2324.
32. Schairer WW, Zhang AL, Feeley BT. Hospital readmissions after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(9):1349-1355.
33. de Kruijf M, Vroemen JP, de Leur K, van der Voort EA, Vos DI, Van der Laan L. Proximal fractures of the humerus in patients older than 75 years of age: should we consider operative treatment? J Orthop Traumatol. 2014;15(2):111-115.
34. Hauschild O, Konrad G, Audige L, et al. Operative versus non-operative treatment for two-part surgical neck fractures of the proximal humerus. Arch Orthop Trauma Surg. 2013;133(10):1385-1393.
35. Hanson B, Neidenbach P, de Boer P, Stengel D. Functional outcomes after nonoperative management of fractures of the proximal humerus. J Shoulder Elbow Surg. 2009;18(4):612-621.
36. Handoll HH, Ollivere BJ, Rollins KE. Interventions for treating proximal humeral fractures in adults. Cochrane Database Syst Rev. 2012;12:CD000434.
37. Court-Brown CM, Cattermole H, McQueen MM. Impacted valgus fractures (B1.1) of the proximal humerus. The results of non-operative treatment. J Bone Joint Surg Br. 2002;84(4):504-508.
38. Chalmers PN, Gupta AK, Rahman Z, Bruce B, Romeo AA, Nicholson GP. Predictors of early complications of total shoulder arthroplasty. J Arthroplasty. 2014;29(4):856-860.
39. Mahoney A, Bosco JA 3rd, Zuckerman JD. Readmission after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):377-381.
40. Khan SK, Malviya A, Muller SD, et al. Reduced short-term complications and mortality following Enhanced Recovery primary hip and knee arthroplasty: results from 6,000 consecutive procedures. Acta Orthop. 2014;85(1):26-31.
41. Paavolainen P, Pukkala E, Pulkkinen P, Visuri T. Causes of death after total hip arthroplasty: a nationwide cohort study with 24,638 patients. J Arthroplasty. 2002;17(3):274-281.
42. Sharrock NE, Cazan MG, Hargett MJ, Williams-Russo P, Wilson PD Jr. Changes in mortality after total hip and knee arthroplasty over a ten-year period. Anesth Analg. 1995;80(2):242-248.
43. Parvizi J, Johnson BG, Rowland C, Ereth MH, Lewallen DG. Thirty-day mortality after elective total hip arthroplasty. J Bone Joint Surg Am. 2001;83(10):1524-1528.
44. Morris MJ, Molli RG, Berend KR, Lombardi AV Jr. Mortality and perioperative complications after unicompartmental knee arthroplasty. Knee. 2013;20(3):218-220.
45. Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353(4):349-361.
46. Wijeysundera DN, Beattie WS, Wijeysundera HC, Yun L, Austin PC, Ko DT. Duration of preoperative beta-blockade and outcomes after major elective noncardiac surgery. Can J Cardiol. 2014;30(2):217-223.
47. Andersson C, Merie C, Jorgensen M, et al. Association of beta-blocker therapy with risks of adverse cardiovascular events and deaths in patients with ischemic heart disease undergoing noncardiac surgery: a Danish nationwide cohort study. JAMA Int Med. 2014;174(3):336-344.
48. Bakker EJ, Ravensbergen NJ, Poldermans D. Perioperative cardiac evaluation, monitoring, and risk reduction strategies in noncardiac surgery patients. Curr Opin Crit Care. 2011;17(5):409-415.
49. Auerbach AD, Goldman L. Beta-blockers and reduction of cardiac events in noncardiac surgery: scientific review. JAMA. 2002;287(11):1435-1444.