User login
Lung disease raises mortality risk in older RA patients
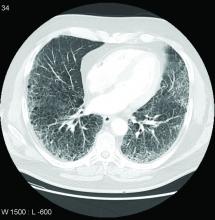
Patients with rheumatoid arthritis–associated interstitial lung disease showed increases in overall mortality, respiratory mortality, and cancer mortality, compared with RA patients without interstitial lung disease, based on data from more than 500,000 patients in a nationwide cohort study.
RA-associated interstitial lung disease (RA-ILD) has been associated with worse survival rates as well as reduced quality of life, functional impairment, and increased health care use and costs, wrote Jeffrey A. Sparks, MD, of Brigham and Women’s Hospital, Boston, and colleagues. However, data on the incidence and prevalence of RA-ILD have been inconsistent and large studies are lacking.
In a study published online in Rheumatology, the researchers identified 509,787 RA patients aged 65 years and older from Medicare claims data. The average age of the patients was 72.6 years, and 76.2% were women.
At baseline, 10,306 (2%) of the study population had RA-ILD, and 13,372 (2.7%) developed RA-ILD over an average of 3.8 years’ follow-up per person (total of 1,873,127 person-years of follow-up). The overall incidence of RA-ILD was 7.14 per 1,000 person-years.
Overall mortality was significantly higher among RA-ILD patients than in those with RA alone in a multivariate analysis (38.7% vs. 20.7%; hazard ratio, 1.66).
In addition, RA-ILD was associated with an increased risk of respiratory mortality (HR, 4.39) and cancer mortality (HR, 1.56), compared with RA without ILD. For these hazard regression analyses, the researchers used Fine and Gray subdistribution HRs “to handle competing risks of alternative causes of mortality. For example, the risk of respiratory mortality for patients with RA-ILD, compared with RA without ILD also accounted for the competing risk of cardiovascular, cancer, infection and other types of mortality.”
In another multivariate analysis, male gender, smoking, asthma, chronic obstructive pulmonary disorder, and medication use (specifically biologic disease-modifying antirheumatic drugs, targeted synthetic DMARDs, and glucocorticoids) were independently associated with increased incident RA-ILD at baseline. However, “the associations of RA-related medications with incident RA-ILD risk should be interpreted with caution since they may be explained by unmeasured factors, including RA disease activity, severity, comorbidities, and prior or concomitant medication use,” the researchers noted.
The study findings were limited by several factors, including the lack of data on disease activity, disease duration, disease severity, and RA-related autoantibodies, the researchers noted. However, the results support data from previous studies and were strengthened by the large sample size and data on demographics and health care use.
“Ours is the first to study the epidemiology and mortality outcomes of RA-ILD using a validated claims algorithm to identify RA and RA-ILD,” and “to quantify the mortality burden of RA-ILD and to identify a potentially novel association of RA-ILD with cancer mortality,” they noted.
The study was supported by an investigator-initiated grant from Bristol-Myers Squibb. Lead author Dr. Sparks disclosed support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Rheumatology Research Foundation, the Brigham Research Institute, and the R. Bruce and Joan M. Mickey Research Scholar Fund. Dr. Sparks also disclosed serving as a consultant to Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer for work unrelated to the current study. Other authors reported research funding from Bristol-Myers Squibb, involvement in a clinical trial funded by Genentech and Bristol-Myers Squibb, and receiving research support to Brigham and Women’s Hospital for other studies from AbbVie, Bayer, Bristol-Myers Squibb, Novartis, Pfizer, Roche, and Vertex.
Patients with rheumatoid arthritis–associated interstitial lung disease showed increases in overall mortality, respiratory mortality, and cancer mortality, compared with RA patients without interstitial lung disease, based on data from more than 500,000 patients in a nationwide cohort study.
RA-associated interstitial lung disease (RA-ILD) has been associated with worse survival rates as well as reduced quality of life, functional impairment, and increased health care use and costs, wrote Jeffrey A. Sparks, MD, of Brigham and Women’s Hospital, Boston, and colleagues. However, data on the incidence and prevalence of RA-ILD have been inconsistent and large studies are lacking.
In a study published online in Rheumatology, the researchers identified 509,787 RA patients aged 65 years and older from Medicare claims data. The average age of the patients was 72.6 years, and 76.2% were women.
At baseline, 10,306 (2%) of the study population had RA-ILD, and 13,372 (2.7%) developed RA-ILD over an average of 3.8 years’ follow-up per person (total of 1,873,127 person-years of follow-up). The overall incidence of RA-ILD was 7.14 per 1,000 person-years.
Overall mortality was significantly higher among RA-ILD patients than in those with RA alone in a multivariate analysis (38.7% vs. 20.7%; hazard ratio, 1.66).
In addition, RA-ILD was associated with an increased risk of respiratory mortality (HR, 4.39) and cancer mortality (HR, 1.56), compared with RA without ILD. For these hazard regression analyses, the researchers used Fine and Gray subdistribution HRs “to handle competing risks of alternative causes of mortality. For example, the risk of respiratory mortality for patients with RA-ILD, compared with RA without ILD also accounted for the competing risk of cardiovascular, cancer, infection and other types of mortality.”
In another multivariate analysis, male gender, smoking, asthma, chronic obstructive pulmonary disorder, and medication use (specifically biologic disease-modifying antirheumatic drugs, targeted synthetic DMARDs, and glucocorticoids) were independently associated with increased incident RA-ILD at baseline. However, “the associations of RA-related medications with incident RA-ILD risk should be interpreted with caution since they may be explained by unmeasured factors, including RA disease activity, severity, comorbidities, and prior or concomitant medication use,” the researchers noted.
The study findings were limited by several factors, including the lack of data on disease activity, disease duration, disease severity, and RA-related autoantibodies, the researchers noted. However, the results support data from previous studies and were strengthened by the large sample size and data on demographics and health care use.
“Ours is the first to study the epidemiology and mortality outcomes of RA-ILD using a validated claims algorithm to identify RA and RA-ILD,” and “to quantify the mortality burden of RA-ILD and to identify a potentially novel association of RA-ILD with cancer mortality,” they noted.
The study was supported by an investigator-initiated grant from Bristol-Myers Squibb. Lead author Dr. Sparks disclosed support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Rheumatology Research Foundation, the Brigham Research Institute, and the R. Bruce and Joan M. Mickey Research Scholar Fund. Dr. Sparks also disclosed serving as a consultant to Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer for work unrelated to the current study. Other authors reported research funding from Bristol-Myers Squibb, involvement in a clinical trial funded by Genentech and Bristol-Myers Squibb, and receiving research support to Brigham and Women’s Hospital for other studies from AbbVie, Bayer, Bristol-Myers Squibb, Novartis, Pfizer, Roche, and Vertex.
Patients with rheumatoid arthritis–associated interstitial lung disease showed increases in overall mortality, respiratory mortality, and cancer mortality, compared with RA patients without interstitial lung disease, based on data from more than 500,000 patients in a nationwide cohort study.
RA-associated interstitial lung disease (RA-ILD) has been associated with worse survival rates as well as reduced quality of life, functional impairment, and increased health care use and costs, wrote Jeffrey A. Sparks, MD, of Brigham and Women’s Hospital, Boston, and colleagues. However, data on the incidence and prevalence of RA-ILD have been inconsistent and large studies are lacking.
In a study published online in Rheumatology, the researchers identified 509,787 RA patients aged 65 years and older from Medicare claims data. The average age of the patients was 72.6 years, and 76.2% were women.
At baseline, 10,306 (2%) of the study population had RA-ILD, and 13,372 (2.7%) developed RA-ILD over an average of 3.8 years’ follow-up per person (total of 1,873,127 person-years of follow-up). The overall incidence of RA-ILD was 7.14 per 1,000 person-years.
Overall mortality was significantly higher among RA-ILD patients than in those with RA alone in a multivariate analysis (38.7% vs. 20.7%; hazard ratio, 1.66).
In addition, RA-ILD was associated with an increased risk of respiratory mortality (HR, 4.39) and cancer mortality (HR, 1.56), compared with RA without ILD. For these hazard regression analyses, the researchers used Fine and Gray subdistribution HRs “to handle competing risks of alternative causes of mortality. For example, the risk of respiratory mortality for patients with RA-ILD, compared with RA without ILD also accounted for the competing risk of cardiovascular, cancer, infection and other types of mortality.”
In another multivariate analysis, male gender, smoking, asthma, chronic obstructive pulmonary disorder, and medication use (specifically biologic disease-modifying antirheumatic drugs, targeted synthetic DMARDs, and glucocorticoids) were independently associated with increased incident RA-ILD at baseline. However, “the associations of RA-related medications with incident RA-ILD risk should be interpreted with caution since they may be explained by unmeasured factors, including RA disease activity, severity, comorbidities, and prior or concomitant medication use,” the researchers noted.
The study findings were limited by several factors, including the lack of data on disease activity, disease duration, disease severity, and RA-related autoantibodies, the researchers noted. However, the results support data from previous studies and were strengthened by the large sample size and data on demographics and health care use.
“Ours is the first to study the epidemiology and mortality outcomes of RA-ILD using a validated claims algorithm to identify RA and RA-ILD,” and “to quantify the mortality burden of RA-ILD and to identify a potentially novel association of RA-ILD with cancer mortality,” they noted.
The study was supported by an investigator-initiated grant from Bristol-Myers Squibb. Lead author Dr. Sparks disclosed support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Rheumatology Research Foundation, the Brigham Research Institute, and the R. Bruce and Joan M. Mickey Research Scholar Fund. Dr. Sparks also disclosed serving as a consultant to Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer for work unrelated to the current study. Other authors reported research funding from Bristol-Myers Squibb, involvement in a clinical trial funded by Genentech and Bristol-Myers Squibb, and receiving research support to Brigham and Women’s Hospital for other studies from AbbVie, Bayer, Bristol-Myers Squibb, Novartis, Pfizer, Roche, and Vertex.
FROM RHEUMATOLOGY
Severe renal arteriosclerosis may indicate cardiovascular risk in lupus nephritis
Severe renal arteriosclerosis was associated with a ninefold increased risk of atherosclerotic cardiovascular disease in patients with lupus nephritis, based on data from an observational study of 189 individuals.
Atherosclerotic cardiovascular disease (ASCVD) has traditionally been thought to be a late complication of systemic lupus erythematosus (SLE), but this has been challenged in recent population-based studies of patients with SLE and lupus nephritis (LN) that indicated an early and increased risk of ASCVD at the time of diagnosis. However, it is unclear which early risk factors may predispose patients to ASCVD, Shivani Garg, MD, of the University of Wisconsin, Madison, and colleagues wrote in a study published in Arthritis Care & Research.
In patients with IgA nephropathy and renal transplantation, previous studies have shown that severe renal arteriosclerosis (r-ASCL) based on kidney biopsies at the time of diagnosis predicts ASCVD, but “a few studies including LN biopsies failed to report a similar association between the presence of severe r-ASCL and ASCVD occurrence,” possibly because of underreporting of r-ASCL. Dr. Garg and colleagues also noted the problem of underreporting of r-ASCL in their own previous study of its prevalence in LN patients at the time of diagnosis.
To get a more detailed view of how r-ASCL may be linked to early occurrence of ASCVD in LN patients, Dr. Garg and coauthors identified 189 consecutive patients with incident LN who underwent diagnostic biopsies between 1994 and 2017. The median age of the patients was 25 years, 78% were women, and 73% were white. The researchers developed a composite score for r-ASCL severity based on reported and overread biopsies.
Overall, 31% of the patients had any reported r-ASCL, and 7% had moderate-severe r-ASCL. After incorporating systematically reexamined r-ASCL grades, the prevalence of any and moderate-severe r-ASCL increased to 39% and 12%, respectively.
Based on their composite of reported and overread r-ASCL grade, severe r-ASCL in diagnostic LN biopsies was associated with a ninefold increased risk of ASCVD.
The researchers identified 22 incident ASCVD events over an 11-year follow-up for an overall 12% incidence of ASCVD in LN. ASCVD was defined as ischemic heart disease (including myocardial infarction, coronary artery revascularization, abnormal stress test, abnormal angiogram, and events documented by a cardiologist); stroke and transient ischemic attack (TIA); and peripheral vascular disease. Incident ASCVD was defined as the first ASCVD event between 1 and 10 years after LN diagnosis.
The most common ASCVD events were stroke or TIA (12 patients), events related to ischemic heart disease (7 patients), and events related to peripheral vascular disease (3 patients).
Lack of statin use
The researchers also hypothesized that the presence of gaps in statin use among eligible LN patients would be present in their study population. “Among the 20 patients with incident ASCVD events after LN diagnosis in our cohort, none was on statin therapy at the time of LN diagnosis,” the researchers said, noting that current guidelines from the American College of Rheumatology and the European League Against Rheumatism (now known as the European Alliance of Associations for Rheumatology) recommend initiating statin therapy at the time of LN diagnosis in all patients who have hyperlipidemia and chronic kidney disease (CKD) stage ≥3. “Further, 11 patients (55%) met high-risk criteria (hyperlipidemia and CKD stage ≥3) to implement statin therapy at the time of LN diagnosis, yet only one patient (9%) was initiated on statin therapy.” In addition, patients with stage 3 or higher CKD were more likely to develop ASCVD than patients without stage 3 or higher CKD, they said.
The study findings were limited by several factors including the majority white study population, the ability to overread only 25% of the biopsies, and the lack of data on the potential role of chronic lesions in ASCVD, the researchers noted. However, the results were strengthened by the use of a validated LN cohort, and the data provide “the basis to establish severe composite r-ASCL as a predictor of ASCVD events using a larger sample size in different cohorts,” they said.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Severe renal arteriosclerosis was associated with a ninefold increased risk of atherosclerotic cardiovascular disease in patients with lupus nephritis, based on data from an observational study of 189 individuals.
Atherosclerotic cardiovascular disease (ASCVD) has traditionally been thought to be a late complication of systemic lupus erythematosus (SLE), but this has been challenged in recent population-based studies of patients with SLE and lupus nephritis (LN) that indicated an early and increased risk of ASCVD at the time of diagnosis. However, it is unclear which early risk factors may predispose patients to ASCVD, Shivani Garg, MD, of the University of Wisconsin, Madison, and colleagues wrote in a study published in Arthritis Care & Research.
In patients with IgA nephropathy and renal transplantation, previous studies have shown that severe renal arteriosclerosis (r-ASCL) based on kidney biopsies at the time of diagnosis predicts ASCVD, but “a few studies including LN biopsies failed to report a similar association between the presence of severe r-ASCL and ASCVD occurrence,” possibly because of underreporting of r-ASCL. Dr. Garg and colleagues also noted the problem of underreporting of r-ASCL in their own previous study of its prevalence in LN patients at the time of diagnosis.
To get a more detailed view of how r-ASCL may be linked to early occurrence of ASCVD in LN patients, Dr. Garg and coauthors identified 189 consecutive patients with incident LN who underwent diagnostic biopsies between 1994 and 2017. The median age of the patients was 25 years, 78% were women, and 73% were white. The researchers developed a composite score for r-ASCL severity based on reported and overread biopsies.
Overall, 31% of the patients had any reported r-ASCL, and 7% had moderate-severe r-ASCL. After incorporating systematically reexamined r-ASCL grades, the prevalence of any and moderate-severe r-ASCL increased to 39% and 12%, respectively.
Based on their composite of reported and overread r-ASCL grade, severe r-ASCL in diagnostic LN biopsies was associated with a ninefold increased risk of ASCVD.
The researchers identified 22 incident ASCVD events over an 11-year follow-up for an overall 12% incidence of ASCVD in LN. ASCVD was defined as ischemic heart disease (including myocardial infarction, coronary artery revascularization, abnormal stress test, abnormal angiogram, and events documented by a cardiologist); stroke and transient ischemic attack (TIA); and peripheral vascular disease. Incident ASCVD was defined as the first ASCVD event between 1 and 10 years after LN diagnosis.
The most common ASCVD events were stroke or TIA (12 patients), events related to ischemic heart disease (7 patients), and events related to peripheral vascular disease (3 patients).
Lack of statin use
The researchers also hypothesized that the presence of gaps in statin use among eligible LN patients would be present in their study population. “Among the 20 patients with incident ASCVD events after LN diagnosis in our cohort, none was on statin therapy at the time of LN diagnosis,” the researchers said, noting that current guidelines from the American College of Rheumatology and the European League Against Rheumatism (now known as the European Alliance of Associations for Rheumatology) recommend initiating statin therapy at the time of LN diagnosis in all patients who have hyperlipidemia and chronic kidney disease (CKD) stage ≥3. “Further, 11 patients (55%) met high-risk criteria (hyperlipidemia and CKD stage ≥3) to implement statin therapy at the time of LN diagnosis, yet only one patient (9%) was initiated on statin therapy.” In addition, patients with stage 3 or higher CKD were more likely to develop ASCVD than patients without stage 3 or higher CKD, they said.
The study findings were limited by several factors including the majority white study population, the ability to overread only 25% of the biopsies, and the lack of data on the potential role of chronic lesions in ASCVD, the researchers noted. However, the results were strengthened by the use of a validated LN cohort, and the data provide “the basis to establish severe composite r-ASCL as a predictor of ASCVD events using a larger sample size in different cohorts,” they said.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Severe renal arteriosclerosis was associated with a ninefold increased risk of atherosclerotic cardiovascular disease in patients with lupus nephritis, based on data from an observational study of 189 individuals.
Atherosclerotic cardiovascular disease (ASCVD) has traditionally been thought to be a late complication of systemic lupus erythematosus (SLE), but this has been challenged in recent population-based studies of patients with SLE and lupus nephritis (LN) that indicated an early and increased risk of ASCVD at the time of diagnosis. However, it is unclear which early risk factors may predispose patients to ASCVD, Shivani Garg, MD, of the University of Wisconsin, Madison, and colleagues wrote in a study published in Arthritis Care & Research.
In patients with IgA nephropathy and renal transplantation, previous studies have shown that severe renal arteriosclerosis (r-ASCL) based on kidney biopsies at the time of diagnosis predicts ASCVD, but “a few studies including LN biopsies failed to report a similar association between the presence of severe r-ASCL and ASCVD occurrence,” possibly because of underreporting of r-ASCL. Dr. Garg and colleagues also noted the problem of underreporting of r-ASCL in their own previous study of its prevalence in LN patients at the time of diagnosis.
To get a more detailed view of how r-ASCL may be linked to early occurrence of ASCVD in LN patients, Dr. Garg and coauthors identified 189 consecutive patients with incident LN who underwent diagnostic biopsies between 1994 and 2017. The median age of the patients was 25 years, 78% were women, and 73% were white. The researchers developed a composite score for r-ASCL severity based on reported and overread biopsies.
Overall, 31% of the patients had any reported r-ASCL, and 7% had moderate-severe r-ASCL. After incorporating systematically reexamined r-ASCL grades, the prevalence of any and moderate-severe r-ASCL increased to 39% and 12%, respectively.
Based on their composite of reported and overread r-ASCL grade, severe r-ASCL in diagnostic LN biopsies was associated with a ninefold increased risk of ASCVD.
The researchers identified 22 incident ASCVD events over an 11-year follow-up for an overall 12% incidence of ASCVD in LN. ASCVD was defined as ischemic heart disease (including myocardial infarction, coronary artery revascularization, abnormal stress test, abnormal angiogram, and events documented by a cardiologist); stroke and transient ischemic attack (TIA); and peripheral vascular disease. Incident ASCVD was defined as the first ASCVD event between 1 and 10 years after LN diagnosis.
The most common ASCVD events were stroke or TIA (12 patients), events related to ischemic heart disease (7 patients), and events related to peripheral vascular disease (3 patients).
Lack of statin use
The researchers also hypothesized that the presence of gaps in statin use among eligible LN patients would be present in their study population. “Among the 20 patients with incident ASCVD events after LN diagnosis in our cohort, none was on statin therapy at the time of LN diagnosis,” the researchers said, noting that current guidelines from the American College of Rheumatology and the European League Against Rheumatism (now known as the European Alliance of Associations for Rheumatology) recommend initiating statin therapy at the time of LN diagnosis in all patients who have hyperlipidemia and chronic kidney disease (CKD) stage ≥3. “Further, 11 patients (55%) met high-risk criteria (hyperlipidemia and CKD stage ≥3) to implement statin therapy at the time of LN diagnosis, yet only one patient (9%) was initiated on statin therapy.” In addition, patients with stage 3 or higher CKD were more likely to develop ASCVD than patients without stage 3 or higher CKD, they said.
The study findings were limited by several factors including the majority white study population, the ability to overread only 25% of the biopsies, and the lack of data on the potential role of chronic lesions in ASCVD, the researchers noted. However, the results were strengthened by the use of a validated LN cohort, and the data provide “the basis to establish severe composite r-ASCL as a predictor of ASCVD events using a larger sample size in different cohorts,” they said.
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM ARTHRITIS CARE & RESEARCH
Meta-analysis: No evidence that SNRIs relieve back pain
While some guidelines support serotonin norepinephrine reuptake inhibitors (SNRIs) as treatments for back pain, a new systematic review and meta-analysis of existing research found no firm evidence of a benefit. Adverse effects, however, are common.
“Our review shows that, although these medicines are effective, the effect is small and unlikely to be considered clinically important by most patients,” wrote the authors of the review, which appeared Jan. 20 in the BMJ. “Our review also showed that about two-thirds of patients using SNRIs experience adverse events.”
However, the report hinted that certain classes of antidepressants may provide significant relief in knee OA and sciatica.
According to a 2018 review, 10 of 15 clinical guidelines from around the world – including those of the American College of Physicians – recommended antidepressants as treatments for low back pain, and 2 advised against them. “Evidence supporting the use of antidepressants is, however, uncertain,” wrote the authors of the new review, led by Giovanni E. Ferreira, PhD, of the University of Sydney. “Systematic reviews of antidepressants for back pain and osteoarthritis have either not included several published trials, considered only one type of antidepressant (e.g., duloxetine), or failed to assess the certainty of evidence.”
For the new review, the authors analyzed 33 randomized, controlled trials with a total of 5,318 subjects. Both published data and unpublished data from clinical trial registries were included.
Back pain trials
A total of 19 trials examined back pain, mostly lower back pain (16 trials), and none lasted more than 1 year. Fifteen examined SNRIs while others looked at other kinds of antidepressants.
The researchers found that “the effect of SNRIs was small [on back pain] and below this review’s predetermined threshold of clinical importance. ... Evidence ranging from low to very low certainty showed no benefit of a range of antidepressant classes, including SSRIs [selective serotonin reuptake inhibitors], tetracyclic antidepressants, SARIs [serotonin antagonist and reuptake inhibitors], and NDRIs [norepinephrine and dopamine reuptake inhibitors] for pain and disability across follow-ups of 2 weeks or less, 3-13 weeks, and 3-12 months.”
Sciatica trials
Six trials examined antidepressants as treatments for sciatica. Very-low-certainty evidence suggested that SNRIs reduced pain at up to 2 weeks (1 trial, n = 50) but not at 3-13 weeks (3 trials, n = 96). The results of trials of tricyclic antidepressants (TCAs) were the opposite: low- to very-low-certainty evidence suggested the drugs didn’t reduce pain at up to 2 weeks (2 trials, n = 94) but did at 3-13 weeks (2 trials, n = 114) and 3-12 months (1 trial, n = 60).
“All sciatica trials were small, had imprecise estimates, and were at high risk of bias, which reduced the certainty of evidence to low and very low,” the authors cautioned. “This level of uncertainty indicates that the true estimate of effect of TCAs and SNRIs for sciatica is likely to be substantially different from what we estimated in our review.”
Knee OA trials
Eight trials examined SNRIs in knee OA. Moderate-certainty evidence linked the drugs to less pain at up to 2 weeks (four trials, n = 1,328) and low-certainty evidence linked them to less pain at 3-13 weeks (eight trials, n = 1,941). Low-certainty evidence also linked the drugs to less disability at 2 weeks or less (one trial, n = 353) and 3-13 weeks (seven trials, n = 1,810).
In knee OA, “the effect of SNRIs was small and below this review’s predetermined threshold of clinical importance,” the researchers wrote. “However, the lower limit of the confidence interval did contain clinically important effects for pain, but not for disability.”
Antidepressant side effects in trials
A total of 21 trials (n = 4,107) looked at side effects when antidepressants were studied as treatments for back pain and OA. Low-certainty evidence in 13 SNRI trials (n = 3,447) suggested a higher risk of any adverse events in antidepressant versus placebo (62.5% vs. 49.7%; relative risk, 1.23, 95% confidence interval, 1.16-1.30), but there was no significantly higher risk of serious adverse events in 10 SNRI trials with 3,309 subjects (1.6% vs. 1.3%; RR, 1.12, 95% CI, 0.61-2.07).
As for adverse effects of non-SNRIs, “the number of studies evaluating the safety of other antidepressant classes was small, trials were underpowered to detect harm, and the certainty of evidence ranged from low to very low,” the researchers wrote.
Going forward, the authors said that “large, definitive randomized trials that are free of industry ties are urgently needed to resolve uncertainties about the efficacy of antidepressants for sciatica and osteoarthritis highlighted by this review.”
‘Largely ineffective’ drug treatments
In an accompanying commentary, Martin Underwood, of the University of Warwick in Coventry, England, and Colin Tysall, of the University Hospitals of Coventry and Warwickshire, also in Coventry, noted that “drug treatments are largely ineffective for back pain and osteoarthritis and have the potential for serious harm. We need to work harder to help people with these disorders to live better with their pain without recourse to the prescription pad.”
However, they noted that SNRIs may still be helpful for patients with back pain or OA. “Absolute effect sizes for physical treatments for low-back pain are of similar magnitudes to those reported here and translate into numbers needed to treat of between five and nine. If the same were true for SNRIs, some people might choose to a try that option for a 1 in 10 chance of a worthwhile reduction in pain after 3 months. They can easily stop if treatment is ineffective or does not suit them.”
The research received no specific funding. The review authors disclosed relationships with GlaxoSmithKline (postgraduate scholarship), Pfizer (investigational product for two trials), and Flexeze (provision of heat wraps for a trial). Mr. Underwood reported being a director and shareholder of Clinvivo. Mr. Tysall reported no disclosures.
While some guidelines support serotonin norepinephrine reuptake inhibitors (SNRIs) as treatments for back pain, a new systematic review and meta-analysis of existing research found no firm evidence of a benefit. Adverse effects, however, are common.
“Our review shows that, although these medicines are effective, the effect is small and unlikely to be considered clinically important by most patients,” wrote the authors of the review, which appeared Jan. 20 in the BMJ. “Our review also showed that about two-thirds of patients using SNRIs experience adverse events.”
However, the report hinted that certain classes of antidepressants may provide significant relief in knee OA and sciatica.
According to a 2018 review, 10 of 15 clinical guidelines from around the world – including those of the American College of Physicians – recommended antidepressants as treatments for low back pain, and 2 advised against them. “Evidence supporting the use of antidepressants is, however, uncertain,” wrote the authors of the new review, led by Giovanni E. Ferreira, PhD, of the University of Sydney. “Systematic reviews of antidepressants for back pain and osteoarthritis have either not included several published trials, considered only one type of antidepressant (e.g., duloxetine), or failed to assess the certainty of evidence.”
For the new review, the authors analyzed 33 randomized, controlled trials with a total of 5,318 subjects. Both published data and unpublished data from clinical trial registries were included.
Back pain trials
A total of 19 trials examined back pain, mostly lower back pain (16 trials), and none lasted more than 1 year. Fifteen examined SNRIs while others looked at other kinds of antidepressants.
The researchers found that “the effect of SNRIs was small [on back pain] and below this review’s predetermined threshold of clinical importance. ... Evidence ranging from low to very low certainty showed no benefit of a range of antidepressant classes, including SSRIs [selective serotonin reuptake inhibitors], tetracyclic antidepressants, SARIs [serotonin antagonist and reuptake inhibitors], and NDRIs [norepinephrine and dopamine reuptake inhibitors] for pain and disability across follow-ups of 2 weeks or less, 3-13 weeks, and 3-12 months.”
Sciatica trials
Six trials examined antidepressants as treatments for sciatica. Very-low-certainty evidence suggested that SNRIs reduced pain at up to 2 weeks (1 trial, n = 50) but not at 3-13 weeks (3 trials, n = 96). The results of trials of tricyclic antidepressants (TCAs) were the opposite: low- to very-low-certainty evidence suggested the drugs didn’t reduce pain at up to 2 weeks (2 trials, n = 94) but did at 3-13 weeks (2 trials, n = 114) and 3-12 months (1 trial, n = 60).
“All sciatica trials were small, had imprecise estimates, and were at high risk of bias, which reduced the certainty of evidence to low and very low,” the authors cautioned. “This level of uncertainty indicates that the true estimate of effect of TCAs and SNRIs for sciatica is likely to be substantially different from what we estimated in our review.”
Knee OA trials
Eight trials examined SNRIs in knee OA. Moderate-certainty evidence linked the drugs to less pain at up to 2 weeks (four trials, n = 1,328) and low-certainty evidence linked them to less pain at 3-13 weeks (eight trials, n = 1,941). Low-certainty evidence also linked the drugs to less disability at 2 weeks or less (one trial, n = 353) and 3-13 weeks (seven trials, n = 1,810).
In knee OA, “the effect of SNRIs was small and below this review’s predetermined threshold of clinical importance,” the researchers wrote. “However, the lower limit of the confidence interval did contain clinically important effects for pain, but not for disability.”
Antidepressant side effects in trials
A total of 21 trials (n = 4,107) looked at side effects when antidepressants were studied as treatments for back pain and OA. Low-certainty evidence in 13 SNRI trials (n = 3,447) suggested a higher risk of any adverse events in antidepressant versus placebo (62.5% vs. 49.7%; relative risk, 1.23, 95% confidence interval, 1.16-1.30), but there was no significantly higher risk of serious adverse events in 10 SNRI trials with 3,309 subjects (1.6% vs. 1.3%; RR, 1.12, 95% CI, 0.61-2.07).
As for adverse effects of non-SNRIs, “the number of studies evaluating the safety of other antidepressant classes was small, trials were underpowered to detect harm, and the certainty of evidence ranged from low to very low,” the researchers wrote.
Going forward, the authors said that “large, definitive randomized trials that are free of industry ties are urgently needed to resolve uncertainties about the efficacy of antidepressants for sciatica and osteoarthritis highlighted by this review.”
‘Largely ineffective’ drug treatments
In an accompanying commentary, Martin Underwood, of the University of Warwick in Coventry, England, and Colin Tysall, of the University Hospitals of Coventry and Warwickshire, also in Coventry, noted that “drug treatments are largely ineffective for back pain and osteoarthritis and have the potential for serious harm. We need to work harder to help people with these disorders to live better with their pain without recourse to the prescription pad.”
However, they noted that SNRIs may still be helpful for patients with back pain or OA. “Absolute effect sizes for physical treatments for low-back pain are of similar magnitudes to those reported here and translate into numbers needed to treat of between five and nine. If the same were true for SNRIs, some people might choose to a try that option for a 1 in 10 chance of a worthwhile reduction in pain after 3 months. They can easily stop if treatment is ineffective or does not suit them.”
The research received no specific funding. The review authors disclosed relationships with GlaxoSmithKline (postgraduate scholarship), Pfizer (investigational product for two trials), and Flexeze (provision of heat wraps for a trial). Mr. Underwood reported being a director and shareholder of Clinvivo. Mr. Tysall reported no disclosures.
While some guidelines support serotonin norepinephrine reuptake inhibitors (SNRIs) as treatments for back pain, a new systematic review and meta-analysis of existing research found no firm evidence of a benefit. Adverse effects, however, are common.
“Our review shows that, although these medicines are effective, the effect is small and unlikely to be considered clinically important by most patients,” wrote the authors of the review, which appeared Jan. 20 in the BMJ. “Our review also showed that about two-thirds of patients using SNRIs experience adverse events.”
However, the report hinted that certain classes of antidepressants may provide significant relief in knee OA and sciatica.
According to a 2018 review, 10 of 15 clinical guidelines from around the world – including those of the American College of Physicians – recommended antidepressants as treatments for low back pain, and 2 advised against them. “Evidence supporting the use of antidepressants is, however, uncertain,” wrote the authors of the new review, led by Giovanni E. Ferreira, PhD, of the University of Sydney. “Systematic reviews of antidepressants for back pain and osteoarthritis have either not included several published trials, considered only one type of antidepressant (e.g., duloxetine), or failed to assess the certainty of evidence.”
For the new review, the authors analyzed 33 randomized, controlled trials with a total of 5,318 subjects. Both published data and unpublished data from clinical trial registries were included.
Back pain trials
A total of 19 trials examined back pain, mostly lower back pain (16 trials), and none lasted more than 1 year. Fifteen examined SNRIs while others looked at other kinds of antidepressants.
The researchers found that “the effect of SNRIs was small [on back pain] and below this review’s predetermined threshold of clinical importance. ... Evidence ranging from low to very low certainty showed no benefit of a range of antidepressant classes, including SSRIs [selective serotonin reuptake inhibitors], tetracyclic antidepressants, SARIs [serotonin antagonist and reuptake inhibitors], and NDRIs [norepinephrine and dopamine reuptake inhibitors] for pain and disability across follow-ups of 2 weeks or less, 3-13 weeks, and 3-12 months.”
Sciatica trials
Six trials examined antidepressants as treatments for sciatica. Very-low-certainty evidence suggested that SNRIs reduced pain at up to 2 weeks (1 trial, n = 50) but not at 3-13 weeks (3 trials, n = 96). The results of trials of tricyclic antidepressants (TCAs) were the opposite: low- to very-low-certainty evidence suggested the drugs didn’t reduce pain at up to 2 weeks (2 trials, n = 94) but did at 3-13 weeks (2 trials, n = 114) and 3-12 months (1 trial, n = 60).
“All sciatica trials were small, had imprecise estimates, and were at high risk of bias, which reduced the certainty of evidence to low and very low,” the authors cautioned. “This level of uncertainty indicates that the true estimate of effect of TCAs and SNRIs for sciatica is likely to be substantially different from what we estimated in our review.”
Knee OA trials
Eight trials examined SNRIs in knee OA. Moderate-certainty evidence linked the drugs to less pain at up to 2 weeks (four trials, n = 1,328) and low-certainty evidence linked them to less pain at 3-13 weeks (eight trials, n = 1,941). Low-certainty evidence also linked the drugs to less disability at 2 weeks or less (one trial, n = 353) and 3-13 weeks (seven trials, n = 1,810).
In knee OA, “the effect of SNRIs was small and below this review’s predetermined threshold of clinical importance,” the researchers wrote. “However, the lower limit of the confidence interval did contain clinically important effects for pain, but not for disability.”
Antidepressant side effects in trials
A total of 21 trials (n = 4,107) looked at side effects when antidepressants were studied as treatments for back pain and OA. Low-certainty evidence in 13 SNRI trials (n = 3,447) suggested a higher risk of any adverse events in antidepressant versus placebo (62.5% vs. 49.7%; relative risk, 1.23, 95% confidence interval, 1.16-1.30), but there was no significantly higher risk of serious adverse events in 10 SNRI trials with 3,309 subjects (1.6% vs. 1.3%; RR, 1.12, 95% CI, 0.61-2.07).
As for adverse effects of non-SNRIs, “the number of studies evaluating the safety of other antidepressant classes was small, trials were underpowered to detect harm, and the certainty of evidence ranged from low to very low,” the researchers wrote.
Going forward, the authors said that “large, definitive randomized trials that are free of industry ties are urgently needed to resolve uncertainties about the efficacy of antidepressants for sciatica and osteoarthritis highlighted by this review.”
‘Largely ineffective’ drug treatments
In an accompanying commentary, Martin Underwood, of the University of Warwick in Coventry, England, and Colin Tysall, of the University Hospitals of Coventry and Warwickshire, also in Coventry, noted that “drug treatments are largely ineffective for back pain and osteoarthritis and have the potential for serious harm. We need to work harder to help people with these disorders to live better with their pain without recourse to the prescription pad.”
However, they noted that SNRIs may still be helpful for patients with back pain or OA. “Absolute effect sizes for physical treatments for low-back pain are of similar magnitudes to those reported here and translate into numbers needed to treat of between five and nine. If the same were true for SNRIs, some people might choose to a try that option for a 1 in 10 chance of a worthwhile reduction in pain after 3 months. They can easily stop if treatment is ineffective or does not suit them.”
The research received no specific funding. The review authors disclosed relationships with GlaxoSmithKline (postgraduate scholarship), Pfizer (investigational product for two trials), and Flexeze (provision of heat wraps for a trial). Mr. Underwood reported being a director and shareholder of Clinvivo. Mr. Tysall reported no disclosures.
FROM THE BMJ
Arthritis drugs ‘impressive’ for severe COVID but not ‘magic cure’
New findings suggest that monoclonal antibodies used to treat RA could improve severe COVID-19 outcomes, including risk for death.
Given within 24 hours of critical illness, tocilizumab (Actemra) was associated with a median of 10 days free of respiratory and cardiovascular support up to day 21, the primary outcome. Similarly, sarilumab (Kevzara) was linked to a median of 11 days. In contrast, the usual care control group experienced zero such days in the hospital.
However, the Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) trial comes with a caveat. The preprint findings have not yet been peer reviewed and “should not be used to guide clinical practice,” the authors stated.
The results were published online Jan. 7 in MedRxiv.
Nevertheless, the trial also revealed a mortality benefit associated with the two interleukin-6 antagonists. The hospital mortality rate was 22% with sarilumab, 28% with tocilizumab, and almost 36% with usual care.
“That’s a big change in survival. They are both lifesaving drugs,” lead coinvestigator Anthony Gordon, an Imperial College London professor of anesthesia and critical care, commented in a recent story by Reuters.
Consider the big picture
“What I think is important is ... this is one of many trials,” Paul Auwaerter, MD, MBA, said in an interview. Many other studies looking at monoclonal antibody therapy for people with COVID-19 were halted because they did not show improvement.
One exception is the EMPACTA trial, which suggested that tocilizumab was effective if given before a person becomes ill enough to be placed on a ventilator, said Dr. Auwaerter, clinical director of the division of infectious diseases at Johns Hopkins Medicine and a contributor to this news organization. “It appeared to reduce the need for mechanical ventilation or death.”
“These two trials are the first randomized, prospective trials that show a benefit on a background of others which have not,” Dr. Auwaerter added.
Interim findings
The REMAP-CAP investigators randomly assigned adults within 24 hours of critical care for COVID-19 to 8 mg/kg tocilizumab, 400 mg sarilumab, or usual care at 113 sites in six countries. There were 353 participants in the tocilizumab arm, 48 in the sarilumab group, and 402 in the control group.
Compared with the control group, the 10 days free of organ support in the tocilizumab cohort was associated with an adjusted odds ratio of 1.64 (95% confidence interval, 1.25-2.14). The 11 days free of organ support in the sarilumab cohort was likewise superior to control (adjusted odds ratio, 1.76; 95% CI, 1.17-2.91).
“All secondary outcomes and analyses supported efficacy of these IL-6 receptor antagonists,” the authors note. These endpoints included 90-day survival, time to intensive care unit discharge, and hospital discharge.
Cautious optimism?
“The results were quite impressive – having 10 or 11 fewer days in the ICU, compared to standard of care,” Deepa Gotur, MD, said in an interview. “Choosing the right patient population and providing the anti-IL-6 treatment at the right time would be the key here.”
In addition to not yet receiving peer review, an open-label design, a relatively short follow-up of 21 days, and steroids becoming standard of care about halfway through the trial are potential limitations, said Dr. Gotur, an intensivist at Houston Methodist Hospital and associate professor of clinical medicine at Weill Cornell Medicine, New York.
“This is an interesting study,” Carl J. Fichtenbaum, MD, professor of clinical medicine at the University of Cincinnati, said in a comment.
Additional detail on how many participants in each group received steroids is warranted, Dr. Fichtenbaum said. “The analysis did not carefully adjust for the use of steroids that might have influenced outcomes.”
Dr. Fichtenbaum said it’s important to look at what is distinctive about REMAP-CAP because “there are several other studies showing opposite results.”
Dr. Gotur was an investigator on a previous study evaluating tocilizumab for patients already on mechanical ventilation. “One of the key differences between this and other studies is that they included more of the ICU population,” she said. “They also included patients within 24 hours of requiring organ support, cardiac, as well as respiratory support.” Some other research included less-acute patients, including all comers into the ED who required oxygen and received tocilizumab.
The prior studies also evaluated cytokine or inflammatory markers. In contrast, REMAP-CAP researchers “looked at organ failure itself ... which I think makes sense,” Dr. Gotur said.
Cytokine release syndrome can cause organ damage or organ failure, she added, “but these markers are all over the place. I’ve seen patients who are very, very sick despite having a low [C-reactive protein] or IL-6 level.”
Backing from the British
Citing the combined 24% decrease in the risk for death associated with these agents in the REMAP-CAP trial, the U.K. government announced Jan. 7 it will work to make tocilizumab and sarilumab available to citizens with severe COVID-19.
Experts in the United Kingdom shared their perspectives on the REMAP-CAP interim findings through the U.K. Science Media Centre.
“There are few treatments for severe COVID-19,” said Robin Ferner, MD, honorary professor of clinical pharmacology at the University of Birmingham (England) and honorary consultant physician at City Hospital Birmingham. “If the published data from REMAP-CAP are supported by further studies, this suggests that two IL-6 receptor antagonists can reduce the death rate in the most severely ill patients.”
Dr. Ferner added that the findings are not a “magic cure,” however. He pointed out that of 401 patients given the drugs, 109 died, and with standard treatment, 144 out of 402 died.
Peter Horby, MD, PhD, was more optimistic. “It is great to see a positive result at a time that we really need good news and more tools to fight COVID. This is great achievement for REMAP-CAP,” he said.
“We hope to soon have results from RECOVERY on the effect of tocilizumab in less severely ill patients in the hospital,” said Dr. Horby, cochief investigator of the RECOVERY trial and professor of emerging infectious diseases at the Centre for Tropical Medicine and Global Health at the University of Oxford (England).
Stephen Evans, BA, MSc, FRCP, professor of pharmacoepidemiology at the London School of Hygiene & Tropical Medicine, said, “This is a high-quality trial, and although published as a preprint, is of much higher quality than many non–peer-reviewed papers.”
Dr. Evans also noted the addition of steroid therapy for many participants. “Partway through the trial, the RECOVERY trial findings showed that the corticosteroid drug dexamethasone had notable mortality benefits. Consequently, quite a number of the patients in this trial had also received a corticosteroid.”
“It does look as though these drugs give some additional benefit beyond that given by dexamethasone,” he added.
Awaiting peer review
“We need to wait for the final results and ensure it was adequately powered with enough observations to make us confident in the results,” Dr. Fichtenbaum said.
“We in the United States have to step back and look at the entire set of studies and also, for this particular one, REMAP-CAP, to be in a peer-reviewed publication,” Dr. Auwaerter said. Preprints are often released “in the setting of the pandemic, where there may be important findings, especially if they impact mortality or severity of illness.”
“We need to make sure these findings, as outlined, hold up,” he said.
In the meantime, Dr. Auwaerter added, “Exactly how this will fit in is unclear. But it’s important to me as another potential drug that can help our critically ill patients.”
The REMAP-CAP study is ongoing and updated results will be provided online.
Dr. Auwaerter disclosed that he is a consultant for EMD Serono and a member of the data monitoring safety board for Humanigen. Dr. Gotur, Dr. Fichtenbaum, Dr. Ferner, and Dr. Evans disclosed no relevant financial relationships. Dr. Horby reported that Oxford University receives funding for the RECOVERY trial from U.K. Research and Innovation and the National Institute for Health Research. Roche Products and Sanofi supported REMAP-CAP through provision of tocilizumab and sarilumab in the United Kingdom.
A version of this article first appeared on Medscape.com.
New findings suggest that monoclonal antibodies used to treat RA could improve severe COVID-19 outcomes, including risk for death.
Given within 24 hours of critical illness, tocilizumab (Actemra) was associated with a median of 10 days free of respiratory and cardiovascular support up to day 21, the primary outcome. Similarly, sarilumab (Kevzara) was linked to a median of 11 days. In contrast, the usual care control group experienced zero such days in the hospital.
However, the Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) trial comes with a caveat. The preprint findings have not yet been peer reviewed and “should not be used to guide clinical practice,” the authors stated.
The results were published online Jan. 7 in MedRxiv.
Nevertheless, the trial also revealed a mortality benefit associated with the two interleukin-6 antagonists. The hospital mortality rate was 22% with sarilumab, 28% with tocilizumab, and almost 36% with usual care.
“That’s a big change in survival. They are both lifesaving drugs,” lead coinvestigator Anthony Gordon, an Imperial College London professor of anesthesia and critical care, commented in a recent story by Reuters.
Consider the big picture
“What I think is important is ... this is one of many trials,” Paul Auwaerter, MD, MBA, said in an interview. Many other studies looking at monoclonal antibody therapy for people with COVID-19 were halted because they did not show improvement.
One exception is the EMPACTA trial, which suggested that tocilizumab was effective if given before a person becomes ill enough to be placed on a ventilator, said Dr. Auwaerter, clinical director of the division of infectious diseases at Johns Hopkins Medicine and a contributor to this news organization. “It appeared to reduce the need for mechanical ventilation or death.”
“These two trials are the first randomized, prospective trials that show a benefit on a background of others which have not,” Dr. Auwaerter added.
Interim findings
The REMAP-CAP investigators randomly assigned adults within 24 hours of critical care for COVID-19 to 8 mg/kg tocilizumab, 400 mg sarilumab, or usual care at 113 sites in six countries. There were 353 participants in the tocilizumab arm, 48 in the sarilumab group, and 402 in the control group.
Compared with the control group, the 10 days free of organ support in the tocilizumab cohort was associated with an adjusted odds ratio of 1.64 (95% confidence interval, 1.25-2.14). The 11 days free of organ support in the sarilumab cohort was likewise superior to control (adjusted odds ratio, 1.76; 95% CI, 1.17-2.91).
“All secondary outcomes and analyses supported efficacy of these IL-6 receptor antagonists,” the authors note. These endpoints included 90-day survival, time to intensive care unit discharge, and hospital discharge.
Cautious optimism?
“The results were quite impressive – having 10 or 11 fewer days in the ICU, compared to standard of care,” Deepa Gotur, MD, said in an interview. “Choosing the right patient population and providing the anti-IL-6 treatment at the right time would be the key here.”
In addition to not yet receiving peer review, an open-label design, a relatively short follow-up of 21 days, and steroids becoming standard of care about halfway through the trial are potential limitations, said Dr. Gotur, an intensivist at Houston Methodist Hospital and associate professor of clinical medicine at Weill Cornell Medicine, New York.
“This is an interesting study,” Carl J. Fichtenbaum, MD, professor of clinical medicine at the University of Cincinnati, said in a comment.
Additional detail on how many participants in each group received steroids is warranted, Dr. Fichtenbaum said. “The analysis did not carefully adjust for the use of steroids that might have influenced outcomes.”
Dr. Fichtenbaum said it’s important to look at what is distinctive about REMAP-CAP because “there are several other studies showing opposite results.”
Dr. Gotur was an investigator on a previous study evaluating tocilizumab for patients already on mechanical ventilation. “One of the key differences between this and other studies is that they included more of the ICU population,” she said. “They also included patients within 24 hours of requiring organ support, cardiac, as well as respiratory support.” Some other research included less-acute patients, including all comers into the ED who required oxygen and received tocilizumab.
The prior studies also evaluated cytokine or inflammatory markers. In contrast, REMAP-CAP researchers “looked at organ failure itself ... which I think makes sense,” Dr. Gotur said.
Cytokine release syndrome can cause organ damage or organ failure, she added, “but these markers are all over the place. I’ve seen patients who are very, very sick despite having a low [C-reactive protein] or IL-6 level.”
Backing from the British
Citing the combined 24% decrease in the risk for death associated with these agents in the REMAP-CAP trial, the U.K. government announced Jan. 7 it will work to make tocilizumab and sarilumab available to citizens with severe COVID-19.
Experts in the United Kingdom shared their perspectives on the REMAP-CAP interim findings through the U.K. Science Media Centre.
“There are few treatments for severe COVID-19,” said Robin Ferner, MD, honorary professor of clinical pharmacology at the University of Birmingham (England) and honorary consultant physician at City Hospital Birmingham. “If the published data from REMAP-CAP are supported by further studies, this suggests that two IL-6 receptor antagonists can reduce the death rate in the most severely ill patients.”
Dr. Ferner added that the findings are not a “magic cure,” however. He pointed out that of 401 patients given the drugs, 109 died, and with standard treatment, 144 out of 402 died.
Peter Horby, MD, PhD, was more optimistic. “It is great to see a positive result at a time that we really need good news and more tools to fight COVID. This is great achievement for REMAP-CAP,” he said.
“We hope to soon have results from RECOVERY on the effect of tocilizumab in less severely ill patients in the hospital,” said Dr. Horby, cochief investigator of the RECOVERY trial and professor of emerging infectious diseases at the Centre for Tropical Medicine and Global Health at the University of Oxford (England).
Stephen Evans, BA, MSc, FRCP, professor of pharmacoepidemiology at the London School of Hygiene & Tropical Medicine, said, “This is a high-quality trial, and although published as a preprint, is of much higher quality than many non–peer-reviewed papers.”
Dr. Evans also noted the addition of steroid therapy for many participants. “Partway through the trial, the RECOVERY trial findings showed that the corticosteroid drug dexamethasone had notable mortality benefits. Consequently, quite a number of the patients in this trial had also received a corticosteroid.”
“It does look as though these drugs give some additional benefit beyond that given by dexamethasone,” he added.
Awaiting peer review
“We need to wait for the final results and ensure it was adequately powered with enough observations to make us confident in the results,” Dr. Fichtenbaum said.
“We in the United States have to step back and look at the entire set of studies and also, for this particular one, REMAP-CAP, to be in a peer-reviewed publication,” Dr. Auwaerter said. Preprints are often released “in the setting of the pandemic, where there may be important findings, especially if they impact mortality or severity of illness.”
“We need to make sure these findings, as outlined, hold up,” he said.
In the meantime, Dr. Auwaerter added, “Exactly how this will fit in is unclear. But it’s important to me as another potential drug that can help our critically ill patients.”
The REMAP-CAP study is ongoing and updated results will be provided online.
Dr. Auwaerter disclosed that he is a consultant for EMD Serono and a member of the data monitoring safety board for Humanigen. Dr. Gotur, Dr. Fichtenbaum, Dr. Ferner, and Dr. Evans disclosed no relevant financial relationships. Dr. Horby reported that Oxford University receives funding for the RECOVERY trial from U.K. Research and Innovation and the National Institute for Health Research. Roche Products and Sanofi supported REMAP-CAP through provision of tocilizumab and sarilumab in the United Kingdom.
A version of this article first appeared on Medscape.com.
New findings suggest that monoclonal antibodies used to treat RA could improve severe COVID-19 outcomes, including risk for death.
Given within 24 hours of critical illness, tocilizumab (Actemra) was associated with a median of 10 days free of respiratory and cardiovascular support up to day 21, the primary outcome. Similarly, sarilumab (Kevzara) was linked to a median of 11 days. In contrast, the usual care control group experienced zero such days in the hospital.
However, the Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) trial comes with a caveat. The preprint findings have not yet been peer reviewed and “should not be used to guide clinical practice,” the authors stated.
The results were published online Jan. 7 in MedRxiv.
Nevertheless, the trial also revealed a mortality benefit associated with the two interleukin-6 antagonists. The hospital mortality rate was 22% with sarilumab, 28% with tocilizumab, and almost 36% with usual care.
“That’s a big change in survival. They are both lifesaving drugs,” lead coinvestigator Anthony Gordon, an Imperial College London professor of anesthesia and critical care, commented in a recent story by Reuters.
Consider the big picture
“What I think is important is ... this is one of many trials,” Paul Auwaerter, MD, MBA, said in an interview. Many other studies looking at monoclonal antibody therapy for people with COVID-19 were halted because they did not show improvement.
One exception is the EMPACTA trial, which suggested that tocilizumab was effective if given before a person becomes ill enough to be placed on a ventilator, said Dr. Auwaerter, clinical director of the division of infectious diseases at Johns Hopkins Medicine and a contributor to this news organization. “It appeared to reduce the need for mechanical ventilation or death.”
“These two trials are the first randomized, prospective trials that show a benefit on a background of others which have not,” Dr. Auwaerter added.
Interim findings
The REMAP-CAP investigators randomly assigned adults within 24 hours of critical care for COVID-19 to 8 mg/kg tocilizumab, 400 mg sarilumab, or usual care at 113 sites in six countries. There were 353 participants in the tocilizumab arm, 48 in the sarilumab group, and 402 in the control group.
Compared with the control group, the 10 days free of organ support in the tocilizumab cohort was associated with an adjusted odds ratio of 1.64 (95% confidence interval, 1.25-2.14). The 11 days free of organ support in the sarilumab cohort was likewise superior to control (adjusted odds ratio, 1.76; 95% CI, 1.17-2.91).
“All secondary outcomes and analyses supported efficacy of these IL-6 receptor antagonists,” the authors note. These endpoints included 90-day survival, time to intensive care unit discharge, and hospital discharge.
Cautious optimism?
“The results were quite impressive – having 10 or 11 fewer days in the ICU, compared to standard of care,” Deepa Gotur, MD, said in an interview. “Choosing the right patient population and providing the anti-IL-6 treatment at the right time would be the key here.”
In addition to not yet receiving peer review, an open-label design, a relatively short follow-up of 21 days, and steroids becoming standard of care about halfway through the trial are potential limitations, said Dr. Gotur, an intensivist at Houston Methodist Hospital and associate professor of clinical medicine at Weill Cornell Medicine, New York.
“This is an interesting study,” Carl J. Fichtenbaum, MD, professor of clinical medicine at the University of Cincinnati, said in a comment.
Additional detail on how many participants in each group received steroids is warranted, Dr. Fichtenbaum said. “The analysis did not carefully adjust for the use of steroids that might have influenced outcomes.”
Dr. Fichtenbaum said it’s important to look at what is distinctive about REMAP-CAP because “there are several other studies showing opposite results.”
Dr. Gotur was an investigator on a previous study evaluating tocilizumab for patients already on mechanical ventilation. “One of the key differences between this and other studies is that they included more of the ICU population,” she said. “They also included patients within 24 hours of requiring organ support, cardiac, as well as respiratory support.” Some other research included less-acute patients, including all comers into the ED who required oxygen and received tocilizumab.
The prior studies also evaluated cytokine or inflammatory markers. In contrast, REMAP-CAP researchers “looked at organ failure itself ... which I think makes sense,” Dr. Gotur said.
Cytokine release syndrome can cause organ damage or organ failure, she added, “but these markers are all over the place. I’ve seen patients who are very, very sick despite having a low [C-reactive protein] or IL-6 level.”
Backing from the British
Citing the combined 24% decrease in the risk for death associated with these agents in the REMAP-CAP trial, the U.K. government announced Jan. 7 it will work to make tocilizumab and sarilumab available to citizens with severe COVID-19.
Experts in the United Kingdom shared their perspectives on the REMAP-CAP interim findings through the U.K. Science Media Centre.
“There are few treatments for severe COVID-19,” said Robin Ferner, MD, honorary professor of clinical pharmacology at the University of Birmingham (England) and honorary consultant physician at City Hospital Birmingham. “If the published data from REMAP-CAP are supported by further studies, this suggests that two IL-6 receptor antagonists can reduce the death rate in the most severely ill patients.”
Dr. Ferner added that the findings are not a “magic cure,” however. He pointed out that of 401 patients given the drugs, 109 died, and with standard treatment, 144 out of 402 died.
Peter Horby, MD, PhD, was more optimistic. “It is great to see a positive result at a time that we really need good news and more tools to fight COVID. This is great achievement for REMAP-CAP,” he said.
“We hope to soon have results from RECOVERY on the effect of tocilizumab in less severely ill patients in the hospital,” said Dr. Horby, cochief investigator of the RECOVERY trial and professor of emerging infectious diseases at the Centre for Tropical Medicine and Global Health at the University of Oxford (England).
Stephen Evans, BA, MSc, FRCP, professor of pharmacoepidemiology at the London School of Hygiene & Tropical Medicine, said, “This is a high-quality trial, and although published as a preprint, is of much higher quality than many non–peer-reviewed papers.”
Dr. Evans also noted the addition of steroid therapy for many participants. “Partway through the trial, the RECOVERY trial findings showed that the corticosteroid drug dexamethasone had notable mortality benefits. Consequently, quite a number of the patients in this trial had also received a corticosteroid.”
“It does look as though these drugs give some additional benefit beyond that given by dexamethasone,” he added.
Awaiting peer review
“We need to wait for the final results and ensure it was adequately powered with enough observations to make us confident in the results,” Dr. Fichtenbaum said.
“We in the United States have to step back and look at the entire set of studies and also, for this particular one, REMAP-CAP, to be in a peer-reviewed publication,” Dr. Auwaerter said. Preprints are often released “in the setting of the pandemic, where there may be important findings, especially if they impact mortality or severity of illness.”
“We need to make sure these findings, as outlined, hold up,” he said.
In the meantime, Dr. Auwaerter added, “Exactly how this will fit in is unclear. But it’s important to me as another potential drug that can help our critically ill patients.”
The REMAP-CAP study is ongoing and updated results will be provided online.
Dr. Auwaerter disclosed that he is a consultant for EMD Serono and a member of the data monitoring safety board for Humanigen. Dr. Gotur, Dr. Fichtenbaum, Dr. Ferner, and Dr. Evans disclosed no relevant financial relationships. Dr. Horby reported that Oxford University receives funding for the RECOVERY trial from U.K. Research and Innovation and the National Institute for Health Research. Roche Products and Sanofi supported REMAP-CAP through provision of tocilizumab and sarilumab in the United Kingdom.
A version of this article first appeared on Medscape.com.
Could an osteoporosis drug reduce need for hip revision surgery?
A single injection of denosumab (Prolia, Amgen), frequently used to treat osteoporosis, may reduce the need for revision surgery in patients with symptomatic osteolysis following total hip arthroplasty, a new proof-of-concept study suggests.
Aseptic loosening is the result of wear-induced osteolysis caused by the prosthetic hip and is a major contributor to the need for revision surgery in many parts of the world.
“The only established treatment for prosthesis-related osteolysis after joint replacement is revision surgery, which carries substantially greater morbidity and mortality than primary joint replacement,” Mohit M. Mahatma, MRes, of the University of Sheffield, England, and colleagues wrote in their article, published online Jan. 11 in The Lancet Rheumatology.
As well as an increased risk of infection and other complications, revision surgery is much more costly than a first-time operation, they added.
“The results of this proof-of-concept clinical trial indicate that denosumab is effective at reducing bone resorption activity within osteolytic lesion tissue and is well tolerated within the limitations of the single dose used here,” they concluded.
Commenting on the findings, Antonia Chen, MD, associate professor of orthopedic surgery, Harvard Medical School, Boston, emphasized that further studies are needed to assess the effectiveness of this strategy to reduce the need for hip revision surgery.
Nevertheless, “osteolysis is still unfortunately a problem we do have to deal with and we do not have any other way to prevent it,” she said in an interview. “So it’s a good start ... although further studies are definitely needed,” Dr. Chen added.
In an accompanying editorial, Hannu Aro, MD, Turku University Hospital in Finland, agreed: “Without a doubt, the trial is a breakthrough, but it represents only the first step in the development of pharmacological therapy aiming to slow, prevent, or even reverse the process of wear-induced periprosthetic osteolysis.”
Small single-center study
The phase 2, single-center, randomized, controlled trial involved 22 patients who had previously undergone hip replacement surgery at Sheffield Teaching Hospitals and were scheduled for revision surgery due to symptomatic osteolysis. They were randomized to a single subcutaneous injection of denosumab at a dose of 60 mg, or placebo, on their second hospital visit.
“The primary outcome was the between-group difference in the number of osteoclasts per mm of osteolytic membrane at the osteolytic membrane-bone interface at week 8,” the authors noted.
At this time point, there were 83% fewer osteoclasts at the interface in the denosumab group compared with placebo, at a median of 0.05 per mm in the treatment group compared with 0.30 per mm in the placebo group (P = .011).
Secondary histological outcomes were also significantly improved in favor of the denosumab group compared with placebo.
Potential to prevent half of all hip revision surgeries?
Patients who received denosumab also demonstrated an acute fall in serum and urinary markers of bone resorption following administration of the drug, reaching a nadir at week 4, which was maintained until revision surgery at week 8.
In contrast, “no change in these markers was observed in the placebo group [P < .0003 for all biomarkers],” the investigators noted. Rates of adverse events were comparable in both treatment groups.
As the authors explained, osteolysis occurs following joint replacement surgery when particles of plastic wear off from the prosthesis, triggering an immune reaction that attacks the bone around the implant, causing the joint to loosen.
“It is very clear from our bone biopsies and bone imaging that the [denosumab] injection stops the bone absorbing the microplastic particles from the replacement joint and therefore could prevent the bone from being eaten away and the need for revision surgery,” senior author Mark Wilkinson, MBChB, PhD, honorary consultant orthopedic surgeon, Sheffield Teaching Hospitals, said in a press release from his institution.
“This study is a significant breakthrough as we’ve demonstrated that there is a drug, already available and successful in the treatment of osteoporosis, that has the potential to prevent up to half of all revised replacement surgeries which are caused by osteolysis,” he added.
Dr. Wilkinson and coauthors said their results justify the need for future trials targeting earlier-stage disease to further test the use of denosumab to prevent or reduce the need for revision surgery.
In 2018, aseptic loosening accounted for over half of all revision procedures, as reported to the National Joint Registry in England and Wales.
Older polyethylene prostheses are the main culprit
Commenting further on the study, Dr. Chen noted that osteolysis still plagues orthopedic surgeons because the original polyethylene prostheses were not very good. A better prosthesis developed at Massachusetts General Hospital is made up of highly crossed-link polyethylene and still wears over time but to a much lesser extent than the older polyethylene prostheses.
Metal and ceramic prostheses also can induce osteolysis, but again to a much lesser extent than the older polyethylene implants.
“Any particle can technically cause osteolysis but plastic produces the most particles,” Dr. Chen explained. Although hip revision rates in the United States are low to begin with, aseptic loosening is still one of the main reasons that patients need to undergo revision surgery, she observed.
“A lot of patients are still living with the old plastic [implants] so there is still a need for something like this,” she stressed.
However, many questions about this potential new strategy remain to be answered, including when best to initiate treatment and how to manage patients at risk for osteolysis 20-30 years after they have received their original implant.
In his editorial, Dr. Aro said that serious adverse consequences often become evident 10-20 years after patients have undergone the original hip replacement procedures, when they are potentially less physically fit than they were at the time of the operation and thus less able to withstand the rigors of a difficult revision surgery.
“In this context, the concept of nonsurgical pharmacological treatment of periprosthetic osteolysis ... brings a new hope for the ever-increasing population of patients with total hip arthroplasty to avoid revision surgery,” Dr. Aro suggested.
However, Dr. Aro cautioned that reduction of bone turnover by antiresorptive agents such as denosumab has been associated with the development of atypical femoral fractures.
The study was funded by Amgen. Dr. Wilkinson has reported receiving a grant from Amgen. Dr. Chen has reported serving as a consultant for Striker and b-One Ortho. Dr. Aro has reported receiving a grant to his institution from Amgen Finland and the Academy of Finland. He has also served as a member of an advisory scientific board for Amgen Finland.
A version of this article first appeared on Medscape.com.
A single injection of denosumab (Prolia, Amgen), frequently used to treat osteoporosis, may reduce the need for revision surgery in patients with symptomatic osteolysis following total hip arthroplasty, a new proof-of-concept study suggests.
Aseptic loosening is the result of wear-induced osteolysis caused by the prosthetic hip and is a major contributor to the need for revision surgery in many parts of the world.
“The only established treatment for prosthesis-related osteolysis after joint replacement is revision surgery, which carries substantially greater morbidity and mortality than primary joint replacement,” Mohit M. Mahatma, MRes, of the University of Sheffield, England, and colleagues wrote in their article, published online Jan. 11 in The Lancet Rheumatology.
As well as an increased risk of infection and other complications, revision surgery is much more costly than a first-time operation, they added.
“The results of this proof-of-concept clinical trial indicate that denosumab is effective at reducing bone resorption activity within osteolytic lesion tissue and is well tolerated within the limitations of the single dose used here,” they concluded.
Commenting on the findings, Antonia Chen, MD, associate professor of orthopedic surgery, Harvard Medical School, Boston, emphasized that further studies are needed to assess the effectiveness of this strategy to reduce the need for hip revision surgery.
Nevertheless, “osteolysis is still unfortunately a problem we do have to deal with and we do not have any other way to prevent it,” she said in an interview. “So it’s a good start ... although further studies are definitely needed,” Dr. Chen added.
In an accompanying editorial, Hannu Aro, MD, Turku University Hospital in Finland, agreed: “Without a doubt, the trial is a breakthrough, but it represents only the first step in the development of pharmacological therapy aiming to slow, prevent, or even reverse the process of wear-induced periprosthetic osteolysis.”
Small single-center study
The phase 2, single-center, randomized, controlled trial involved 22 patients who had previously undergone hip replacement surgery at Sheffield Teaching Hospitals and were scheduled for revision surgery due to symptomatic osteolysis. They were randomized to a single subcutaneous injection of denosumab at a dose of 60 mg, or placebo, on their second hospital visit.
“The primary outcome was the between-group difference in the number of osteoclasts per mm of osteolytic membrane at the osteolytic membrane-bone interface at week 8,” the authors noted.
At this time point, there were 83% fewer osteoclasts at the interface in the denosumab group compared with placebo, at a median of 0.05 per mm in the treatment group compared with 0.30 per mm in the placebo group (P = .011).
Secondary histological outcomes were also significantly improved in favor of the denosumab group compared with placebo.
Potential to prevent half of all hip revision surgeries?
Patients who received denosumab also demonstrated an acute fall in serum and urinary markers of bone resorption following administration of the drug, reaching a nadir at week 4, which was maintained until revision surgery at week 8.
In contrast, “no change in these markers was observed in the placebo group [P < .0003 for all biomarkers],” the investigators noted. Rates of adverse events were comparable in both treatment groups.
As the authors explained, osteolysis occurs following joint replacement surgery when particles of plastic wear off from the prosthesis, triggering an immune reaction that attacks the bone around the implant, causing the joint to loosen.
“It is very clear from our bone biopsies and bone imaging that the [denosumab] injection stops the bone absorbing the microplastic particles from the replacement joint and therefore could prevent the bone from being eaten away and the need for revision surgery,” senior author Mark Wilkinson, MBChB, PhD, honorary consultant orthopedic surgeon, Sheffield Teaching Hospitals, said in a press release from his institution.
“This study is a significant breakthrough as we’ve demonstrated that there is a drug, already available and successful in the treatment of osteoporosis, that has the potential to prevent up to half of all revised replacement surgeries which are caused by osteolysis,” he added.
Dr. Wilkinson and coauthors said their results justify the need for future trials targeting earlier-stage disease to further test the use of denosumab to prevent or reduce the need for revision surgery.
In 2018, aseptic loosening accounted for over half of all revision procedures, as reported to the National Joint Registry in England and Wales.
Older polyethylene prostheses are the main culprit
Commenting further on the study, Dr. Chen noted that osteolysis still plagues orthopedic surgeons because the original polyethylene prostheses were not very good. A better prosthesis developed at Massachusetts General Hospital is made up of highly crossed-link polyethylene and still wears over time but to a much lesser extent than the older polyethylene prostheses.
Metal and ceramic prostheses also can induce osteolysis, but again to a much lesser extent than the older polyethylene implants.
“Any particle can technically cause osteolysis but plastic produces the most particles,” Dr. Chen explained. Although hip revision rates in the United States are low to begin with, aseptic loosening is still one of the main reasons that patients need to undergo revision surgery, she observed.
“A lot of patients are still living with the old plastic [implants] so there is still a need for something like this,” she stressed.
However, many questions about this potential new strategy remain to be answered, including when best to initiate treatment and how to manage patients at risk for osteolysis 20-30 years after they have received their original implant.
In his editorial, Dr. Aro said that serious adverse consequences often become evident 10-20 years after patients have undergone the original hip replacement procedures, when they are potentially less physically fit than they were at the time of the operation and thus less able to withstand the rigors of a difficult revision surgery.
“In this context, the concept of nonsurgical pharmacological treatment of periprosthetic osteolysis ... brings a new hope for the ever-increasing population of patients with total hip arthroplasty to avoid revision surgery,” Dr. Aro suggested.
However, Dr. Aro cautioned that reduction of bone turnover by antiresorptive agents such as denosumab has been associated with the development of atypical femoral fractures.
The study was funded by Amgen. Dr. Wilkinson has reported receiving a grant from Amgen. Dr. Chen has reported serving as a consultant for Striker and b-One Ortho. Dr. Aro has reported receiving a grant to his institution from Amgen Finland and the Academy of Finland. He has also served as a member of an advisory scientific board for Amgen Finland.
A version of this article first appeared on Medscape.com.
A single injection of denosumab (Prolia, Amgen), frequently used to treat osteoporosis, may reduce the need for revision surgery in patients with symptomatic osteolysis following total hip arthroplasty, a new proof-of-concept study suggests.
Aseptic loosening is the result of wear-induced osteolysis caused by the prosthetic hip and is a major contributor to the need for revision surgery in many parts of the world.
“The only established treatment for prosthesis-related osteolysis after joint replacement is revision surgery, which carries substantially greater morbidity and mortality than primary joint replacement,” Mohit M. Mahatma, MRes, of the University of Sheffield, England, and colleagues wrote in their article, published online Jan. 11 in The Lancet Rheumatology.
As well as an increased risk of infection and other complications, revision surgery is much more costly than a first-time operation, they added.
“The results of this proof-of-concept clinical trial indicate that denosumab is effective at reducing bone resorption activity within osteolytic lesion tissue and is well tolerated within the limitations of the single dose used here,” they concluded.
Commenting on the findings, Antonia Chen, MD, associate professor of orthopedic surgery, Harvard Medical School, Boston, emphasized that further studies are needed to assess the effectiveness of this strategy to reduce the need for hip revision surgery.
Nevertheless, “osteolysis is still unfortunately a problem we do have to deal with and we do not have any other way to prevent it,” she said in an interview. “So it’s a good start ... although further studies are definitely needed,” Dr. Chen added.
In an accompanying editorial, Hannu Aro, MD, Turku University Hospital in Finland, agreed: “Without a doubt, the trial is a breakthrough, but it represents only the first step in the development of pharmacological therapy aiming to slow, prevent, or even reverse the process of wear-induced periprosthetic osteolysis.”
Small single-center study
The phase 2, single-center, randomized, controlled trial involved 22 patients who had previously undergone hip replacement surgery at Sheffield Teaching Hospitals and were scheduled for revision surgery due to symptomatic osteolysis. They were randomized to a single subcutaneous injection of denosumab at a dose of 60 mg, or placebo, on their second hospital visit.
“The primary outcome was the between-group difference in the number of osteoclasts per mm of osteolytic membrane at the osteolytic membrane-bone interface at week 8,” the authors noted.
At this time point, there were 83% fewer osteoclasts at the interface in the denosumab group compared with placebo, at a median of 0.05 per mm in the treatment group compared with 0.30 per mm in the placebo group (P = .011).
Secondary histological outcomes were also significantly improved in favor of the denosumab group compared with placebo.
Potential to prevent half of all hip revision surgeries?
Patients who received denosumab also demonstrated an acute fall in serum and urinary markers of bone resorption following administration of the drug, reaching a nadir at week 4, which was maintained until revision surgery at week 8.
In contrast, “no change in these markers was observed in the placebo group [P < .0003 for all biomarkers],” the investigators noted. Rates of adverse events were comparable in both treatment groups.
As the authors explained, osteolysis occurs following joint replacement surgery when particles of plastic wear off from the prosthesis, triggering an immune reaction that attacks the bone around the implant, causing the joint to loosen.
“It is very clear from our bone biopsies and bone imaging that the [denosumab] injection stops the bone absorbing the microplastic particles from the replacement joint and therefore could prevent the bone from being eaten away and the need for revision surgery,” senior author Mark Wilkinson, MBChB, PhD, honorary consultant orthopedic surgeon, Sheffield Teaching Hospitals, said in a press release from his institution.
“This study is a significant breakthrough as we’ve demonstrated that there is a drug, already available and successful in the treatment of osteoporosis, that has the potential to prevent up to half of all revised replacement surgeries which are caused by osteolysis,” he added.
Dr. Wilkinson and coauthors said their results justify the need for future trials targeting earlier-stage disease to further test the use of denosumab to prevent or reduce the need for revision surgery.
In 2018, aseptic loosening accounted for over half of all revision procedures, as reported to the National Joint Registry in England and Wales.
Older polyethylene prostheses are the main culprit
Commenting further on the study, Dr. Chen noted that osteolysis still plagues orthopedic surgeons because the original polyethylene prostheses were not very good. A better prosthesis developed at Massachusetts General Hospital is made up of highly crossed-link polyethylene and still wears over time but to a much lesser extent than the older polyethylene prostheses.
Metal and ceramic prostheses also can induce osteolysis, but again to a much lesser extent than the older polyethylene implants.
“Any particle can technically cause osteolysis but plastic produces the most particles,” Dr. Chen explained. Although hip revision rates in the United States are low to begin with, aseptic loosening is still one of the main reasons that patients need to undergo revision surgery, she observed.
“A lot of patients are still living with the old plastic [implants] so there is still a need for something like this,” she stressed.
However, many questions about this potential new strategy remain to be answered, including when best to initiate treatment and how to manage patients at risk for osteolysis 20-30 years after they have received their original implant.
In his editorial, Dr. Aro said that serious adverse consequences often become evident 10-20 years after patients have undergone the original hip replacement procedures, when they are potentially less physically fit than they were at the time of the operation and thus less able to withstand the rigors of a difficult revision surgery.
“In this context, the concept of nonsurgical pharmacological treatment of periprosthetic osteolysis ... brings a new hope for the ever-increasing population of patients with total hip arthroplasty to avoid revision surgery,” Dr. Aro suggested.
However, Dr. Aro cautioned that reduction of bone turnover by antiresorptive agents such as denosumab has been associated with the development of atypical femoral fractures.
The study was funded by Amgen. Dr. Wilkinson has reported receiving a grant from Amgen. Dr. Chen has reported serving as a consultant for Striker and b-One Ortho. Dr. Aro has reported receiving a grant to his institution from Amgen Finland and the Academy of Finland. He has also served as a member of an advisory scientific board for Amgen Finland.
A version of this article first appeared on Medscape.com.
New findings add to questions about existence of gouty nephropathy
Is gouty nephropathy real? It’s a question that has been posed often in rheumatology over the last several decades.
A new study found 36% of patients with untreated gout at a medical center in Vietnam have diffuse hyperechoic renal medulla as seen on ultrasound, which could indicate the presence of microcrystalline nephropathy. However, the results, published in Kidney International, may raise more questions than answers about the existence of gouty nephropathy and its relation to chronic kidney disease (CKD).
In their study, Thomas Bardin, MD, of the department of rheumatology at Lariboisière Hospital in Paris and colleagues evaluated 502 consecutive patients from Vien Gut Medical Center in Ho Chi Minh City, Vietnam, using B-mode renal ultrasound. The patients were mostly men with a median age of 46 years, body mass index of 25 kg/m2, estimated disease duration of 4 years, and uricemia of 423.2 micromol/L (7.11 mg/dL). Patients had a median estimated glomerular filtration rate (eGFR) of 78 mL/min per 1.73 m2. There was a history of hypertension in 112 patients (22.3%), type 2 diabetes in 58 patients (11.5%), renal lithiasis in 28 patients (5.6%), and coronary heart disease in 5 patients (1%).
While 39% of patients had previously used allopurinol for “a generally short period,” patients were not on urate-lowering therapy at the time of the study. Clinical tophi were present in 279 patients (55.6%), urate arthropathies in 154 patients (30.7%), and 43 patients (10.4%) used steroids daily.
B-mode renal ultrasound showed 181 patients (36%; 95% confidence interval, 32%-40%) had “hyperechoic pattern of Malpighi pyramids compared with the adjacent cortex,” which was “associated with twinkling artifacts” visible on color Doppler ultrasound. There was a significant association between renal medulla hyperechogenicity and patient age, disease duration, use of steroids, clinical tophi, and urate arthropathy (P less than .0001 for all). A significant association was also seen between renal medulla hyperechogenicity and decreased eGFR (P < .0001), proteinuria (P = .0006), leukocyturia (P = .0008), hypertension (P = .0008), hyperuricemia (P = .002), and coronary heart disease (P = .006).
In a multivariate analysis, there was a significant association between renal medulla hyperechogenicity and clinical tophi (odds ratio, 7.27; 95% CI, 3.68–15.19; P < .0001), urate arthropathy (OR, 3.46; 95% CI, 1.99–6.09; P < .0001), estimated gout duration (OR, 2.13; 95% CI, 1.55–2.96; P < .0001), double contour thickness (OR, 1.45; 95% CI, 1.06–1.97; P < .02), and eGFR (OR, 0.30; 95% CI, 0.09–0.89; P < .034).
“The finding was observed mainly in tophaceous gout, which involved a large proportion of our patients who had received very little treatment with urate-lowering drugs and was associated with moderately impaired renal function and urinary features compatible with tubulointerstitial nephritis,” Dr. Bardin and colleagues wrote in the study. The researchers also found “similar features” in 4 of 10 French patients at the Paris Necker Hospital in Paris, and noted that similar findings have been reported in Japan and Korea, which they said may mean hyperechoic medulla “is not unique to Vietnamese patients.”
Relation to CKD still unclear
In a related editorial, Federica Piani, MD, and Richard J. Johnson, MD, of the division of renal diseases and hypertension at the University of Colorado at Denver, Aurora, explained that gout was considered by some clinicians to be a cause of CKD in a time before urate-lowering therapies, because as many as 25% of patients with gout went on to experience kidney failure and about half experienced lower kidney function.
The association between gout and CKD was thought to be attributable to “frequent deposition of urate crystals in the tubular lumens and interstitium in the outer medulla of these patients,” but the concept was later challenged because “the crystals were generally found focally and did not readily explain the kidney damage.”
But even as interest in rheumatology moved away from the concept of gouty nephropathy to how serum uric acid impacts CKD, “the possibility that urate crystal deposition in the kidney could also be contributing to the kidney injury was never ruled out,” according to Dr. Piani and Dr. Johnson.
Kidney biopsies can sometimes miss urate crystals because the crystals dissolve if alcohol fixation is not used and because the biopsy site is often in the renal cortex, the authors noted. Recent research has identified that dual-energy CT scans can distinguish between calcium deposits and urate crystals better than ultrasound, and previous research from Thomas Bardin, MD, and colleagues in two patients noted a correlation between dual-energy CT scan findings of urate crystals and hyperechoic medulla findings on renal ultrasound.
The results by Dr. Bardin and associates, they said, “have reawakened the entity of urate microcrystalline nephropathy as a possible cause of CKD.”
Robert Terkeltaub, MD, professor of medicine at the University of California, San Diego, and section chief of Rheumatology at the San Diego VA Medical Center, said in an interview that he also believes the findings by Dr. Bardin and associates are real. He cited a study by Isabelle Ayoub, MD, and colleagues in Clinical Nephrology from 2016 that evaluated kidney biopsies in Germany and found medullary tophi were more likely to be present in patients with CKD than without.
“Chronic gouty nephropathy did not disappear. It still exists,” said Dr. Terkeltaub, who was not involved in the study by Dr. Bardin and colleagues.
The prospect that, if “you look hard enough, you’re going to see urate crystals and a pattern that’s attributed in the renal medulla” in patients with untreated gout is “very provocative, and interesting, and clinically relevant, and merits more investigation,” noted Dr. Terkeltaub, who is also president of the Gout, Hyperuricemia and Crystal-Associated Disease Network.
If verified, the results have important implications for patients with gout and its relationship to CKD, Dr. Terkeltaub said, but they raise “more questions than answers.
“I think it’s a really good wake-up call to start looking, doing good detective work here, and looking especially in people who have gout as opposed to just people with chronic kidney disease,” he said.
The authors reported no relevant conflicts of interest. Dr. Johnson, who coauthored an accompanying editorial, reported having equity in XORTX Therapeutics, serving as a consultant for Horizon Pharma, and having equity in Colorado Research Partners LLC. Dr. Terkeltaub reported receiving a research grant from AstraZeneca in the field of hyperuricemia and consultancies with AstraZeneca, Horizon, Sobi, Selecta Biosciences.
Is gouty nephropathy real? It’s a question that has been posed often in rheumatology over the last several decades.
A new study found 36% of patients with untreated gout at a medical center in Vietnam have diffuse hyperechoic renal medulla as seen on ultrasound, which could indicate the presence of microcrystalline nephropathy. However, the results, published in Kidney International, may raise more questions than answers about the existence of gouty nephropathy and its relation to chronic kidney disease (CKD).
In their study, Thomas Bardin, MD, of the department of rheumatology at Lariboisière Hospital in Paris and colleagues evaluated 502 consecutive patients from Vien Gut Medical Center in Ho Chi Minh City, Vietnam, using B-mode renal ultrasound. The patients were mostly men with a median age of 46 years, body mass index of 25 kg/m2, estimated disease duration of 4 years, and uricemia of 423.2 micromol/L (7.11 mg/dL). Patients had a median estimated glomerular filtration rate (eGFR) of 78 mL/min per 1.73 m2. There was a history of hypertension in 112 patients (22.3%), type 2 diabetes in 58 patients (11.5%), renal lithiasis in 28 patients (5.6%), and coronary heart disease in 5 patients (1%).
While 39% of patients had previously used allopurinol for “a generally short period,” patients were not on urate-lowering therapy at the time of the study. Clinical tophi were present in 279 patients (55.6%), urate arthropathies in 154 patients (30.7%), and 43 patients (10.4%) used steroids daily.
B-mode renal ultrasound showed 181 patients (36%; 95% confidence interval, 32%-40%) had “hyperechoic pattern of Malpighi pyramids compared with the adjacent cortex,” which was “associated with twinkling artifacts” visible on color Doppler ultrasound. There was a significant association between renal medulla hyperechogenicity and patient age, disease duration, use of steroids, clinical tophi, and urate arthropathy (P less than .0001 for all). A significant association was also seen between renal medulla hyperechogenicity and decreased eGFR (P < .0001), proteinuria (P = .0006), leukocyturia (P = .0008), hypertension (P = .0008), hyperuricemia (P = .002), and coronary heart disease (P = .006).
In a multivariate analysis, there was a significant association between renal medulla hyperechogenicity and clinical tophi (odds ratio, 7.27; 95% CI, 3.68–15.19; P < .0001), urate arthropathy (OR, 3.46; 95% CI, 1.99–6.09; P < .0001), estimated gout duration (OR, 2.13; 95% CI, 1.55–2.96; P < .0001), double contour thickness (OR, 1.45; 95% CI, 1.06–1.97; P < .02), and eGFR (OR, 0.30; 95% CI, 0.09–0.89; P < .034).
“The finding was observed mainly in tophaceous gout, which involved a large proportion of our patients who had received very little treatment with urate-lowering drugs and was associated with moderately impaired renal function and urinary features compatible with tubulointerstitial nephritis,” Dr. Bardin and colleagues wrote in the study. The researchers also found “similar features” in 4 of 10 French patients at the Paris Necker Hospital in Paris, and noted that similar findings have been reported in Japan and Korea, which they said may mean hyperechoic medulla “is not unique to Vietnamese patients.”
Relation to CKD still unclear
In a related editorial, Federica Piani, MD, and Richard J. Johnson, MD, of the division of renal diseases and hypertension at the University of Colorado at Denver, Aurora, explained that gout was considered by some clinicians to be a cause of CKD in a time before urate-lowering therapies, because as many as 25% of patients with gout went on to experience kidney failure and about half experienced lower kidney function.
The association between gout and CKD was thought to be attributable to “frequent deposition of urate crystals in the tubular lumens and interstitium in the outer medulla of these patients,” but the concept was later challenged because “the crystals were generally found focally and did not readily explain the kidney damage.”
But even as interest in rheumatology moved away from the concept of gouty nephropathy to how serum uric acid impacts CKD, “the possibility that urate crystal deposition in the kidney could also be contributing to the kidney injury was never ruled out,” according to Dr. Piani and Dr. Johnson.
Kidney biopsies can sometimes miss urate crystals because the crystals dissolve if alcohol fixation is not used and because the biopsy site is often in the renal cortex, the authors noted. Recent research has identified that dual-energy CT scans can distinguish between calcium deposits and urate crystals better than ultrasound, and previous research from Thomas Bardin, MD, and colleagues in two patients noted a correlation between dual-energy CT scan findings of urate crystals and hyperechoic medulla findings on renal ultrasound.
The results by Dr. Bardin and associates, they said, “have reawakened the entity of urate microcrystalline nephropathy as a possible cause of CKD.”
Robert Terkeltaub, MD, professor of medicine at the University of California, San Diego, and section chief of Rheumatology at the San Diego VA Medical Center, said in an interview that he also believes the findings by Dr. Bardin and associates are real. He cited a study by Isabelle Ayoub, MD, and colleagues in Clinical Nephrology from 2016 that evaluated kidney biopsies in Germany and found medullary tophi were more likely to be present in patients with CKD than without.
“Chronic gouty nephropathy did not disappear. It still exists,” said Dr. Terkeltaub, who was not involved in the study by Dr. Bardin and colleagues.
The prospect that, if “you look hard enough, you’re going to see urate crystals and a pattern that’s attributed in the renal medulla” in patients with untreated gout is “very provocative, and interesting, and clinically relevant, and merits more investigation,” noted Dr. Terkeltaub, who is also president of the Gout, Hyperuricemia and Crystal-Associated Disease Network.
If verified, the results have important implications for patients with gout and its relationship to CKD, Dr. Terkeltaub said, but they raise “more questions than answers.
“I think it’s a really good wake-up call to start looking, doing good detective work here, and looking especially in people who have gout as opposed to just people with chronic kidney disease,” he said.
The authors reported no relevant conflicts of interest. Dr. Johnson, who coauthored an accompanying editorial, reported having equity in XORTX Therapeutics, serving as a consultant for Horizon Pharma, and having equity in Colorado Research Partners LLC. Dr. Terkeltaub reported receiving a research grant from AstraZeneca in the field of hyperuricemia and consultancies with AstraZeneca, Horizon, Sobi, Selecta Biosciences.
Is gouty nephropathy real? It’s a question that has been posed often in rheumatology over the last several decades.
A new study found 36% of patients with untreated gout at a medical center in Vietnam have diffuse hyperechoic renal medulla as seen on ultrasound, which could indicate the presence of microcrystalline nephropathy. However, the results, published in Kidney International, may raise more questions than answers about the existence of gouty nephropathy and its relation to chronic kidney disease (CKD).
In their study, Thomas Bardin, MD, of the department of rheumatology at Lariboisière Hospital in Paris and colleagues evaluated 502 consecutive patients from Vien Gut Medical Center in Ho Chi Minh City, Vietnam, using B-mode renal ultrasound. The patients were mostly men with a median age of 46 years, body mass index of 25 kg/m2, estimated disease duration of 4 years, and uricemia of 423.2 micromol/L (7.11 mg/dL). Patients had a median estimated glomerular filtration rate (eGFR) of 78 mL/min per 1.73 m2. There was a history of hypertension in 112 patients (22.3%), type 2 diabetes in 58 patients (11.5%), renal lithiasis in 28 patients (5.6%), and coronary heart disease in 5 patients (1%).
While 39% of patients had previously used allopurinol for “a generally short period,” patients were not on urate-lowering therapy at the time of the study. Clinical tophi were present in 279 patients (55.6%), urate arthropathies in 154 patients (30.7%), and 43 patients (10.4%) used steroids daily.
B-mode renal ultrasound showed 181 patients (36%; 95% confidence interval, 32%-40%) had “hyperechoic pattern of Malpighi pyramids compared with the adjacent cortex,” which was “associated with twinkling artifacts” visible on color Doppler ultrasound. There was a significant association between renal medulla hyperechogenicity and patient age, disease duration, use of steroids, clinical tophi, and urate arthropathy (P less than .0001 for all). A significant association was also seen between renal medulla hyperechogenicity and decreased eGFR (P < .0001), proteinuria (P = .0006), leukocyturia (P = .0008), hypertension (P = .0008), hyperuricemia (P = .002), and coronary heart disease (P = .006).
In a multivariate analysis, there was a significant association between renal medulla hyperechogenicity and clinical tophi (odds ratio, 7.27; 95% CI, 3.68–15.19; P < .0001), urate arthropathy (OR, 3.46; 95% CI, 1.99–6.09; P < .0001), estimated gout duration (OR, 2.13; 95% CI, 1.55–2.96; P < .0001), double contour thickness (OR, 1.45; 95% CI, 1.06–1.97; P < .02), and eGFR (OR, 0.30; 95% CI, 0.09–0.89; P < .034).
“The finding was observed mainly in tophaceous gout, which involved a large proportion of our patients who had received very little treatment with urate-lowering drugs and was associated with moderately impaired renal function and urinary features compatible with tubulointerstitial nephritis,” Dr. Bardin and colleagues wrote in the study. The researchers also found “similar features” in 4 of 10 French patients at the Paris Necker Hospital in Paris, and noted that similar findings have been reported in Japan and Korea, which they said may mean hyperechoic medulla “is not unique to Vietnamese patients.”
Relation to CKD still unclear
In a related editorial, Federica Piani, MD, and Richard J. Johnson, MD, of the division of renal diseases and hypertension at the University of Colorado at Denver, Aurora, explained that gout was considered by some clinicians to be a cause of CKD in a time before urate-lowering therapies, because as many as 25% of patients with gout went on to experience kidney failure and about half experienced lower kidney function.
The association between gout and CKD was thought to be attributable to “frequent deposition of urate crystals in the tubular lumens and interstitium in the outer medulla of these patients,” but the concept was later challenged because “the crystals were generally found focally and did not readily explain the kidney damage.”
But even as interest in rheumatology moved away from the concept of gouty nephropathy to how serum uric acid impacts CKD, “the possibility that urate crystal deposition in the kidney could also be contributing to the kidney injury was never ruled out,” according to Dr. Piani and Dr. Johnson.
Kidney biopsies can sometimes miss urate crystals because the crystals dissolve if alcohol fixation is not used and because the biopsy site is often in the renal cortex, the authors noted. Recent research has identified that dual-energy CT scans can distinguish between calcium deposits and urate crystals better than ultrasound, and previous research from Thomas Bardin, MD, and colleagues in two patients noted a correlation between dual-energy CT scan findings of urate crystals and hyperechoic medulla findings on renal ultrasound.
The results by Dr. Bardin and associates, they said, “have reawakened the entity of urate microcrystalline nephropathy as a possible cause of CKD.”
Robert Terkeltaub, MD, professor of medicine at the University of California, San Diego, and section chief of Rheumatology at the San Diego VA Medical Center, said in an interview that he also believes the findings by Dr. Bardin and associates are real. He cited a study by Isabelle Ayoub, MD, and colleagues in Clinical Nephrology from 2016 that evaluated kidney biopsies in Germany and found medullary tophi were more likely to be present in patients with CKD than without.
“Chronic gouty nephropathy did not disappear. It still exists,” said Dr. Terkeltaub, who was not involved in the study by Dr. Bardin and colleagues.
The prospect that, if “you look hard enough, you’re going to see urate crystals and a pattern that’s attributed in the renal medulla” in patients with untreated gout is “very provocative, and interesting, and clinically relevant, and merits more investigation,” noted Dr. Terkeltaub, who is also president of the Gout, Hyperuricemia and Crystal-Associated Disease Network.
If verified, the results have important implications for patients with gout and its relationship to CKD, Dr. Terkeltaub said, but they raise “more questions than answers.
“I think it’s a really good wake-up call to start looking, doing good detective work here, and looking especially in people who have gout as opposed to just people with chronic kidney disease,” he said.
The authors reported no relevant conflicts of interest. Dr. Johnson, who coauthored an accompanying editorial, reported having equity in XORTX Therapeutics, serving as a consultant for Horizon Pharma, and having equity in Colorado Research Partners LLC. Dr. Terkeltaub reported receiving a research grant from AstraZeneca in the field of hyperuricemia and consultancies with AstraZeneca, Horizon, Sobi, Selecta Biosciences.
FROM KIDNEY INTERNATIONAL
Ehlers-Danlos syndrome associated with various complications in hospitalized patients
Hospitalized patients with Ehlers-Danlos syndrome (EDS) are more likely to have gastrointestinal, cardiovascular, autonomic, and allergic disorders than are hospitalized patients who do not have EDS, according to a new study of hospital outcomes in these four areas.
“Further research is necessary to explore the prevalence of these manifestations in the different subtypes of EDS and in outpatient population,” wrote Rachel S. Brooks of the University of Connecticut, Farmington, and her coauthors. The study was published in Rheumatology.
To investigate previously observed connections between EDS and these four types of complications, the researchers launched a case-control study using hospital records from the 2016 National Inpatient Sample. A total of 2,007 patients with EDS were identified via ICD-10 code and matched with 4,014 non-EDS patients according to 5-year age intervals, sex, and month of admission. EDS patients had an average age of nearly 37, and 84% were female. The average hospitalization was lengthier for EDS patients (4.77 days) than for controls (4.07 days).
GI conditions were found in 44% of EDS patients, compared with 18% of controls (odds ratio, 3.57; 95% confidence interval, 3.17-4.02; P < .0001). Among the more likely conditions were functional disorders of the stomach (OR, 5.18; 95% CI, 2.16-12.42; P < .0001), unspecified abdominal pain (OR, 3.97; 95% CI, 2.34-6.73; P < .0001), irritable bowel syndrome (OR, 7.44; 95% CI, 5.07-10.94; P < .0001), and nausea (OR, 3.20; 95% CI, 1.95-5.24; P < .0001).
Autonomic dysfunction was found in 20% of EDS patients, compared with 6% of controls (OR, 4.45; 95% CI, 3.71-5.32; P < .0001). They were significantly more likely to have postural orthostatic tachycardia syndrome (OR, 223.77; 95% CI, 31.21-1604.46; P < .0001), orthostatic hypotension (OR, 8.98; 95% CI, 5.36-15.03; P < .0001), syncope (OR, 3.62; 95% CI, 2.23-5.82; P < .0001), and other autonomic nervous system disorders (OR, 54.72; 95% CI, 7.43-403.00; P < .0001).
Food allergies were also considerably more likely to occur in EDS patients (OR, 3.88; 95% CI, 2.65-5.66; P < .0001), as were cardiovascular complications like mitral valve disorders, aortic aneurysm, and cardiac dysrhythmias (OR, 6.16; 95% CI, 4.60-8.23; P < .0001). Although EDS patients were more likely to have hospital stays that lasted longer than 4 days, there was no notable difference in mortality (OR, 0.79; 95% CI, 0.41-1.50; P = .47).
After multivariate regression analysis that adjusted for age, sex, race, and smoking status, EDS patients were more likely to have GI (OR, 3.53; 95% CI, 3.08-4.03; P < .0001), autonomic (OR, 4.13; 95% CI, 3.40-5.01; P < .0001), allergic (OR, 3.92; 95% CI, 2.57-5.98; P < .0001), and cardiovascular complications (OR, 5.82; 95% CI, 4.21-8.03; P < .0001).
Shining a much-needed light on the conditions associated with EDS
“Anyone who takes care of patients with EDS has likely seen some of these complications before and knows they can occur,” Jordan T. Jones, DO, a pediatric rheumatologist at Children’s Mercy Hospital in Kansas City, Mo., said in an interview. “I think this study legitimizes what many who take care of patients with EDS know to be true, and for those who don’t, it brings a lot of attention to many of the symptoms and associated conditions.”
He did, however, draw a conclusion that differed from one of the researchers’ chief observations.
“They note that these patients have a longer-than-average hospital stay, suggesting that EDS may be linked to adverse complications during hospitalization,” he said. “I think the reason for longer-than-average hospital stays is due to the number of symptoms and complexity of these patients, which can lead to delays in diagnosis. The complexity can lead to more involved evaluation that keeps them in the hospital longer than usual. Another reason for longer-than-average hospital stays that I’ve seen is the presentation of severe and chronic pain, which can be difficult to treat in the hospital and then transition to outpatient therapy. An inpatient hospitalization is not always the best place to treat chronic pain symptoms, which can drag out a hospital stay.”
He also highlighted the lack of discussion regarding musculoskeletal complications, which he sees as one of the most common symptoms related to EDS.
“As a rheumatologist, I see many patients with EDS present with chronic pain, chronic muscle weakness, and chronic fatigue. If you think about the joint laxity with EDS, these patients are a perfect setup to develop tight, weak muscles, which leads to a lot of musculoskeletal pain and fatigue.”
That said, he ultimately emphasized the clear benefits of such a large study on such an under-researched subject.
“We think EDS is more common than is reported,” he said. “But despite that, there are still a lot of people who don’t know about EDS, understand it, or appreciate how to evaluate for it. One of the best things this study does is bring more visibility to this disease and the associated conditions related to it.”
The authors declared no potential conflicts of interest.
SOURCE: Brooks RS et al. Rheumatology. 2021 Jan 7. doi: 10.1093/rheumatology/keaa926.
Hospitalized patients with Ehlers-Danlos syndrome (EDS) are more likely to have gastrointestinal, cardiovascular, autonomic, and allergic disorders than are hospitalized patients who do not have EDS, according to a new study of hospital outcomes in these four areas.
“Further research is necessary to explore the prevalence of these manifestations in the different subtypes of EDS and in outpatient population,” wrote Rachel S. Brooks of the University of Connecticut, Farmington, and her coauthors. The study was published in Rheumatology.
To investigate previously observed connections between EDS and these four types of complications, the researchers launched a case-control study using hospital records from the 2016 National Inpatient Sample. A total of 2,007 patients with EDS were identified via ICD-10 code and matched with 4,014 non-EDS patients according to 5-year age intervals, sex, and month of admission. EDS patients had an average age of nearly 37, and 84% were female. The average hospitalization was lengthier for EDS patients (4.77 days) than for controls (4.07 days).
GI conditions were found in 44% of EDS patients, compared with 18% of controls (odds ratio, 3.57; 95% confidence interval, 3.17-4.02; P < .0001). Among the more likely conditions were functional disorders of the stomach (OR, 5.18; 95% CI, 2.16-12.42; P < .0001), unspecified abdominal pain (OR, 3.97; 95% CI, 2.34-6.73; P < .0001), irritable bowel syndrome (OR, 7.44; 95% CI, 5.07-10.94; P < .0001), and nausea (OR, 3.20; 95% CI, 1.95-5.24; P < .0001).
Autonomic dysfunction was found in 20% of EDS patients, compared with 6% of controls (OR, 4.45; 95% CI, 3.71-5.32; P < .0001). They were significantly more likely to have postural orthostatic tachycardia syndrome (OR, 223.77; 95% CI, 31.21-1604.46; P < .0001), orthostatic hypotension (OR, 8.98; 95% CI, 5.36-15.03; P < .0001), syncope (OR, 3.62; 95% CI, 2.23-5.82; P < .0001), and other autonomic nervous system disorders (OR, 54.72; 95% CI, 7.43-403.00; P < .0001).
Food allergies were also considerably more likely to occur in EDS patients (OR, 3.88; 95% CI, 2.65-5.66; P < .0001), as were cardiovascular complications like mitral valve disorders, aortic aneurysm, and cardiac dysrhythmias (OR, 6.16; 95% CI, 4.60-8.23; P < .0001). Although EDS patients were more likely to have hospital stays that lasted longer than 4 days, there was no notable difference in mortality (OR, 0.79; 95% CI, 0.41-1.50; P = .47).
After multivariate regression analysis that adjusted for age, sex, race, and smoking status, EDS patients were more likely to have GI (OR, 3.53; 95% CI, 3.08-4.03; P < .0001), autonomic (OR, 4.13; 95% CI, 3.40-5.01; P < .0001), allergic (OR, 3.92; 95% CI, 2.57-5.98; P < .0001), and cardiovascular complications (OR, 5.82; 95% CI, 4.21-8.03; P < .0001).
Shining a much-needed light on the conditions associated with EDS
“Anyone who takes care of patients with EDS has likely seen some of these complications before and knows they can occur,” Jordan T. Jones, DO, a pediatric rheumatologist at Children’s Mercy Hospital in Kansas City, Mo., said in an interview. “I think this study legitimizes what many who take care of patients with EDS know to be true, and for those who don’t, it brings a lot of attention to many of the symptoms and associated conditions.”
He did, however, draw a conclusion that differed from one of the researchers’ chief observations.
“They note that these patients have a longer-than-average hospital stay, suggesting that EDS may be linked to adverse complications during hospitalization,” he said. “I think the reason for longer-than-average hospital stays is due to the number of symptoms and complexity of these patients, which can lead to delays in diagnosis. The complexity can lead to more involved evaluation that keeps them in the hospital longer than usual. Another reason for longer-than-average hospital stays that I’ve seen is the presentation of severe and chronic pain, which can be difficult to treat in the hospital and then transition to outpatient therapy. An inpatient hospitalization is not always the best place to treat chronic pain symptoms, which can drag out a hospital stay.”
He also highlighted the lack of discussion regarding musculoskeletal complications, which he sees as one of the most common symptoms related to EDS.
“As a rheumatologist, I see many patients with EDS present with chronic pain, chronic muscle weakness, and chronic fatigue. If you think about the joint laxity with EDS, these patients are a perfect setup to develop tight, weak muscles, which leads to a lot of musculoskeletal pain and fatigue.”
That said, he ultimately emphasized the clear benefits of such a large study on such an under-researched subject.
“We think EDS is more common than is reported,” he said. “But despite that, there are still a lot of people who don’t know about EDS, understand it, or appreciate how to evaluate for it. One of the best things this study does is bring more visibility to this disease and the associated conditions related to it.”
The authors declared no potential conflicts of interest.
SOURCE: Brooks RS et al. Rheumatology. 2021 Jan 7. doi: 10.1093/rheumatology/keaa926.
Hospitalized patients with Ehlers-Danlos syndrome (EDS) are more likely to have gastrointestinal, cardiovascular, autonomic, and allergic disorders than are hospitalized patients who do not have EDS, according to a new study of hospital outcomes in these four areas.
“Further research is necessary to explore the prevalence of these manifestations in the different subtypes of EDS and in outpatient population,” wrote Rachel S. Brooks of the University of Connecticut, Farmington, and her coauthors. The study was published in Rheumatology.
To investigate previously observed connections between EDS and these four types of complications, the researchers launched a case-control study using hospital records from the 2016 National Inpatient Sample. A total of 2,007 patients with EDS were identified via ICD-10 code and matched with 4,014 non-EDS patients according to 5-year age intervals, sex, and month of admission. EDS patients had an average age of nearly 37, and 84% were female. The average hospitalization was lengthier for EDS patients (4.77 days) than for controls (4.07 days).
GI conditions were found in 44% of EDS patients, compared with 18% of controls (odds ratio, 3.57; 95% confidence interval, 3.17-4.02; P < .0001). Among the more likely conditions were functional disorders of the stomach (OR, 5.18; 95% CI, 2.16-12.42; P < .0001), unspecified abdominal pain (OR, 3.97; 95% CI, 2.34-6.73; P < .0001), irritable bowel syndrome (OR, 7.44; 95% CI, 5.07-10.94; P < .0001), and nausea (OR, 3.20; 95% CI, 1.95-5.24; P < .0001).
Autonomic dysfunction was found in 20% of EDS patients, compared with 6% of controls (OR, 4.45; 95% CI, 3.71-5.32; P < .0001). They were significantly more likely to have postural orthostatic tachycardia syndrome (OR, 223.77; 95% CI, 31.21-1604.46; P < .0001), orthostatic hypotension (OR, 8.98; 95% CI, 5.36-15.03; P < .0001), syncope (OR, 3.62; 95% CI, 2.23-5.82; P < .0001), and other autonomic nervous system disorders (OR, 54.72; 95% CI, 7.43-403.00; P < .0001).
Food allergies were also considerably more likely to occur in EDS patients (OR, 3.88; 95% CI, 2.65-5.66; P < .0001), as were cardiovascular complications like mitral valve disorders, aortic aneurysm, and cardiac dysrhythmias (OR, 6.16; 95% CI, 4.60-8.23; P < .0001). Although EDS patients were more likely to have hospital stays that lasted longer than 4 days, there was no notable difference in mortality (OR, 0.79; 95% CI, 0.41-1.50; P = .47).
After multivariate regression analysis that adjusted for age, sex, race, and smoking status, EDS patients were more likely to have GI (OR, 3.53; 95% CI, 3.08-4.03; P < .0001), autonomic (OR, 4.13; 95% CI, 3.40-5.01; P < .0001), allergic (OR, 3.92; 95% CI, 2.57-5.98; P < .0001), and cardiovascular complications (OR, 5.82; 95% CI, 4.21-8.03; P < .0001).
Shining a much-needed light on the conditions associated with EDS
“Anyone who takes care of patients with EDS has likely seen some of these complications before and knows they can occur,” Jordan T. Jones, DO, a pediatric rheumatologist at Children’s Mercy Hospital in Kansas City, Mo., said in an interview. “I think this study legitimizes what many who take care of patients with EDS know to be true, and for those who don’t, it brings a lot of attention to many of the symptoms and associated conditions.”
He did, however, draw a conclusion that differed from one of the researchers’ chief observations.
“They note that these patients have a longer-than-average hospital stay, suggesting that EDS may be linked to adverse complications during hospitalization,” he said. “I think the reason for longer-than-average hospital stays is due to the number of symptoms and complexity of these patients, which can lead to delays in diagnosis. The complexity can lead to more involved evaluation that keeps them in the hospital longer than usual. Another reason for longer-than-average hospital stays that I’ve seen is the presentation of severe and chronic pain, which can be difficult to treat in the hospital and then transition to outpatient therapy. An inpatient hospitalization is not always the best place to treat chronic pain symptoms, which can drag out a hospital stay.”
He also highlighted the lack of discussion regarding musculoskeletal complications, which he sees as one of the most common symptoms related to EDS.
“As a rheumatologist, I see many patients with EDS present with chronic pain, chronic muscle weakness, and chronic fatigue. If you think about the joint laxity with EDS, these patients are a perfect setup to develop tight, weak muscles, which leads to a lot of musculoskeletal pain and fatigue.”
That said, he ultimately emphasized the clear benefits of such a large study on such an under-researched subject.
“We think EDS is more common than is reported,” he said. “But despite that, there are still a lot of people who don’t know about EDS, understand it, or appreciate how to evaluate for it. One of the best things this study does is bring more visibility to this disease and the associated conditions related to it.”
The authors declared no potential conflicts of interest.
SOURCE: Brooks RS et al. Rheumatology. 2021 Jan 7. doi: 10.1093/rheumatology/keaa926.
FROM RHEUMATOLOGY
Data call for biologics trials in undertreated juvenile arthritis subtype
Children with enthesitis-related arthritis often have a high burden of disease and could benefit from medications currently approved for adults with spondyloarthritis, according to a review published in Arthritis Care & Research.
“Enthesitis-related arthritis (ERA) was the JIA [juvenile idiopathic arthritis] category applied to children with spondyloarthritis (SpA), recognizing enthesitis as a defining characteristic,” wrote Pamela F. Weiss, MD, of Children’s Hospital of Philadelphia, and colleagues.
The ERA criteria include “arthritis plus enthesitis; or arthritis or enthesitis plus at least two of the following: sacroiliac tenderness or inflammatory back pain, HLA-B27 positivity, first-degree relative with HLA-B27–associated disease, acute anterior uveitis, and arthritis in a male older than 6 years,” the review authors noted.
“None of the [Food and Drug Administration]–approved therapies for peripheral SpA or nonradiographic axial SpA” have been studied or approved for use in children with ERA, but data support biologic similarity to SpA in adults; notably, studies of the HLA-B27 allele have identified it as a risk factor for both SpA and ERA, they said.
Common factors in adult and childhood conditions
“The principal commonalities of children with ERA and axial arthritis, and adults with nonradiographic axial SpA, include enthesitis, arthritis, inflammatory back pain, anterior uveitis, HLA-B27 positivity, and family history of HLA-B27–associated disease,” the review authors wrote.
The first-line treatment for both ERA with axial arthritis and nonradiographic axial SpA is NSAIDs, followed by tumor necrosis factor (TNF) inhibitors if needed, they said. However, conventional disease-modifying antirheumatic drugs (cDMARDs) may be used in cases of peripheral disease affecting five or more joints. Studies of treatment response show similarities between ERA in children and SpA in adults, the authors added, with nearly half of adults with axial disease unable to achieve remission and approximately one-third of children with ERA failing to respond to therapy.
Clinical trials could improve options and outcomes for those with ERA who need advanced therapy and such trials should evaluate response of axial and peripheral disease separately, the review authors emphasized. For example, “Eligibility criteria for children with ERA and axial features could include the presence of some of the following disease features: active inflammatory sacroiliitis based on typical MRI changes according to ASAS/OMERACT [Assessment of SpondyloArthritis international Society/Outcome Measures in Rheumatology Clinical Trials] criteria; elevated CRP [C-reactive protein]; and inadequate response or intolerance to NSAIDs,” they noted. “Considering the similarities between adult spondyloarthritis and ERA in terms of etiology, genetics, pathogenesis, and clinical manifestations, it is evident that medications approved for axial or peripheral SpA should be studied in children with ERA involving axial or peripheral joints, respectively, with the intent to achieve labeling for use in children,” they concluded.
New data highlight ERA disease burden
The need for additional therapies for ERA patients gained more support from a recent study in which a majority of children with ERA or juvenile psoriatic arthritis (jPsA) used biologics, but those with sacroiliitis in particular showed a significant disease burden despite high biologic use.
The International Leagues Against Rheumatism criteria include seven categories of juvenile idiopathic arthritis, of which ERA and jPsA are the most common; however, characteristics of these children have not been well described, wrote Dax G. Rumsey, MD, of the University of Alberta, Edmonton, and colleagues.
“Children with ERA are more likely to have a clinical picture with predominantly peripheral arthritis, typically described as an oligoarthritis involving the lower limbs with high risk of axial disease, relative to the other categories of JIA,” and report more intense pain and worse health status, compared with children in other categories, the researchers wrote.
To more completely characterize children with ERA and jPsA, the researchers assessed 522 children with ERA and 380 with jPsA. The children were enrolled in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry. The findings were published in a brief report in Arthritis Care & Research.
Overall, 69% of the children took at least one biologic, including 72% with ERA and 64% with jPsA. Biologic use was even higher (81%) among the 28% of patients with sacroiliitis (40% of ERA patients and 12% of jPsA patients). Approximately 36% of the patients with sacroiliitis were positive for HLA-B27. In addition, Physician Global Assessment scores and clinical Juvenile Arthritis Disease Activity Score-10 (cJADAS10) scores were significantly higher at the first clinical visit with sacroiliitis, compared with the first visit without, which confirms “the clinical impression that active sacroiliitis significantly impacts children and their families,” the researchers said.
The average age at diagnosis was 10.8 years for ERA and 8.2 years for jPsA, and significantly more ERA patients were male (56% vs. 38%). However, more of the patients with sacroiliitis (54%) were female. More than half of the patients reported polyarticular involvement.
The study findings were limited by several factors, including the classification of ERA or jPsA and the reliance on physician diagnoses, as well as the variation in identifying sacroiliitis, the researchers said. However, the results increase understanding of the pathophysiology of ERA and jPsA to help determine optimal treatment, they concluded.
Data highlight research and treatment gaps
“Recent research demonstrates a large, unmet medical need in the treatment of JIA with 52%-65% of all JIA patients, including those with ERA and jPsA, having been treated with at least one biologic DMARD and 15%-19% having been treated with an FDA-unapproved biologic. In those with ERA or jPsA, 72%-79% of the children had been treated with a biologic DMARD, although no biologic DMARD has ever been FDA approved for these JIA categories,” Daniel J. Lovell, MD, and Hermine I. Brunner, MD, both with Cincinnati Children’s Hospital Medical Center, wrote in an editorial that accompanied the new study. Dr. Lovell and Dr. Brunner also were coauthors of the review article.
The new study supports findings from other recent publications, the editorialists noted. The new results showed “a significant proportion of the JIA population with active sacroiliitis with high disease burden despite very frequent (over 80% of the population) [treatment] with unstudied and unapproved biologic DMARDs,” they said. “These children with sacroiliitis had significantly greater disease burden with higher physician assessment of disease activity, higher parent assessment of disease impact, and higher disease activity as measured by the Juvenile Idiopathic Arthritis Disease Activity Score, compared to the children with ERA or jPsA without sacroiliitis,” they noted.
Previously, “the FDA granted pharmaceutical companies studying new treatments in adult SpA automatic full waivers from doing studies in children for new medications for ‘axial spondyloarthropathies including ankylosing spondylitis’ up until July 2020,” the editorialists said. However, “It is now time now for the pharmaceutical industry to perform FDA-monitored clinical trials of children and adolescents with SpA,” they emphasized. “This will allow for the scientific assessment of proper dosing, efficacy, and safety of the increasing number of new medications that are being licensed by the FDA for the treatment of SpA, such as the anti-TNF, anti–IL[interleukin]-17, and anti–IL-23 biologics, and perhaps JAK [Janus kinase] agents, to address this unmet medical need in these patients with juvenile SpA,” they concluded.
Dr. Weiss disclosed grant support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), and financial relationships with Eli Lilly and Pfizer. Dr. Lovell disclosed relationships with companies including Abbott, AbbVie Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Hoffmann-La Roche, Janssen, Novartis, Pfizer, Takeda, UCB, and Wyeth, as well as serving on the data and safety monitoring board for Forest Research and NIAMS. Dr. Brunner disclosed relationships with companies including Ablynx, AbbVie, AstraZeneca-MedImmune, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eli Lilly, EMD Serono, F. Hoffmann-La Roche, Genzyme, GlaxoSmithKline, Merck, Novartis, R-Pharm, and Sanofi. The study by Dr. Rumsey and colleagues was supported by Amgen. Dr. Rumsey and colleagues had no relevant financial conflicts to disclose.
SOURCES: Weiss PF et al. Arthritis Care Res. 2020 Dec 5. doi: 10.1002/acr.24529; Rumsey DG et al. Arthritis Care Res. 2020 Dec. 16. doi: 10.1002/acr.24537; Lovell DJ and Brunner HI. Arthritis Care Res. 2020 Dec 16. doi: 10.1002/acr.24536.
Children with enthesitis-related arthritis often have a high burden of disease and could benefit from medications currently approved for adults with spondyloarthritis, according to a review published in Arthritis Care & Research.
“Enthesitis-related arthritis (ERA) was the JIA [juvenile idiopathic arthritis] category applied to children with spondyloarthritis (SpA), recognizing enthesitis as a defining characteristic,” wrote Pamela F. Weiss, MD, of Children’s Hospital of Philadelphia, and colleagues.
The ERA criteria include “arthritis plus enthesitis; or arthritis or enthesitis plus at least two of the following: sacroiliac tenderness or inflammatory back pain, HLA-B27 positivity, first-degree relative with HLA-B27–associated disease, acute anterior uveitis, and arthritis in a male older than 6 years,” the review authors noted.
“None of the [Food and Drug Administration]–approved therapies for peripheral SpA or nonradiographic axial SpA” have been studied or approved for use in children with ERA, but data support biologic similarity to SpA in adults; notably, studies of the HLA-B27 allele have identified it as a risk factor for both SpA and ERA, they said.
Common factors in adult and childhood conditions
“The principal commonalities of children with ERA and axial arthritis, and adults with nonradiographic axial SpA, include enthesitis, arthritis, inflammatory back pain, anterior uveitis, HLA-B27 positivity, and family history of HLA-B27–associated disease,” the review authors wrote.
The first-line treatment for both ERA with axial arthritis and nonradiographic axial SpA is NSAIDs, followed by tumor necrosis factor (TNF) inhibitors if needed, they said. However, conventional disease-modifying antirheumatic drugs (cDMARDs) may be used in cases of peripheral disease affecting five or more joints. Studies of treatment response show similarities between ERA in children and SpA in adults, the authors added, with nearly half of adults with axial disease unable to achieve remission and approximately one-third of children with ERA failing to respond to therapy.
Clinical trials could improve options and outcomes for those with ERA who need advanced therapy and such trials should evaluate response of axial and peripheral disease separately, the review authors emphasized. For example, “Eligibility criteria for children with ERA and axial features could include the presence of some of the following disease features: active inflammatory sacroiliitis based on typical MRI changes according to ASAS/OMERACT [Assessment of SpondyloArthritis international Society/Outcome Measures in Rheumatology Clinical Trials] criteria; elevated CRP [C-reactive protein]; and inadequate response or intolerance to NSAIDs,” they noted. “Considering the similarities between adult spondyloarthritis and ERA in terms of etiology, genetics, pathogenesis, and clinical manifestations, it is evident that medications approved for axial or peripheral SpA should be studied in children with ERA involving axial or peripheral joints, respectively, with the intent to achieve labeling for use in children,” they concluded.
New data highlight ERA disease burden
The need for additional therapies for ERA patients gained more support from a recent study in which a majority of children with ERA or juvenile psoriatic arthritis (jPsA) used biologics, but those with sacroiliitis in particular showed a significant disease burden despite high biologic use.
The International Leagues Against Rheumatism criteria include seven categories of juvenile idiopathic arthritis, of which ERA and jPsA are the most common; however, characteristics of these children have not been well described, wrote Dax G. Rumsey, MD, of the University of Alberta, Edmonton, and colleagues.
“Children with ERA are more likely to have a clinical picture with predominantly peripheral arthritis, typically described as an oligoarthritis involving the lower limbs with high risk of axial disease, relative to the other categories of JIA,” and report more intense pain and worse health status, compared with children in other categories, the researchers wrote.
To more completely characterize children with ERA and jPsA, the researchers assessed 522 children with ERA and 380 with jPsA. The children were enrolled in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry. The findings were published in a brief report in Arthritis Care & Research.
Overall, 69% of the children took at least one biologic, including 72% with ERA and 64% with jPsA. Biologic use was even higher (81%) among the 28% of patients with sacroiliitis (40% of ERA patients and 12% of jPsA patients). Approximately 36% of the patients with sacroiliitis were positive for HLA-B27. In addition, Physician Global Assessment scores and clinical Juvenile Arthritis Disease Activity Score-10 (cJADAS10) scores were significantly higher at the first clinical visit with sacroiliitis, compared with the first visit without, which confirms “the clinical impression that active sacroiliitis significantly impacts children and their families,” the researchers said.
The average age at diagnosis was 10.8 years for ERA and 8.2 years for jPsA, and significantly more ERA patients were male (56% vs. 38%). However, more of the patients with sacroiliitis (54%) were female. More than half of the patients reported polyarticular involvement.
The study findings were limited by several factors, including the classification of ERA or jPsA and the reliance on physician diagnoses, as well as the variation in identifying sacroiliitis, the researchers said. However, the results increase understanding of the pathophysiology of ERA and jPsA to help determine optimal treatment, they concluded.
Data highlight research and treatment gaps
“Recent research demonstrates a large, unmet medical need in the treatment of JIA with 52%-65% of all JIA patients, including those with ERA and jPsA, having been treated with at least one biologic DMARD and 15%-19% having been treated with an FDA-unapproved biologic. In those with ERA or jPsA, 72%-79% of the children had been treated with a biologic DMARD, although no biologic DMARD has ever been FDA approved for these JIA categories,” Daniel J. Lovell, MD, and Hermine I. Brunner, MD, both with Cincinnati Children’s Hospital Medical Center, wrote in an editorial that accompanied the new study. Dr. Lovell and Dr. Brunner also were coauthors of the review article.
The new study supports findings from other recent publications, the editorialists noted. The new results showed “a significant proportion of the JIA population with active sacroiliitis with high disease burden despite very frequent (over 80% of the population) [treatment] with unstudied and unapproved biologic DMARDs,” they said. “These children with sacroiliitis had significantly greater disease burden with higher physician assessment of disease activity, higher parent assessment of disease impact, and higher disease activity as measured by the Juvenile Idiopathic Arthritis Disease Activity Score, compared to the children with ERA or jPsA without sacroiliitis,” they noted.
Previously, “the FDA granted pharmaceutical companies studying new treatments in adult SpA automatic full waivers from doing studies in children for new medications for ‘axial spondyloarthropathies including ankylosing spondylitis’ up until July 2020,” the editorialists said. However, “It is now time now for the pharmaceutical industry to perform FDA-monitored clinical trials of children and adolescents with SpA,” they emphasized. “This will allow for the scientific assessment of proper dosing, efficacy, and safety of the increasing number of new medications that are being licensed by the FDA for the treatment of SpA, such as the anti-TNF, anti–IL[interleukin]-17, and anti–IL-23 biologics, and perhaps JAK [Janus kinase] agents, to address this unmet medical need in these patients with juvenile SpA,” they concluded.
Dr. Weiss disclosed grant support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), and financial relationships with Eli Lilly and Pfizer. Dr. Lovell disclosed relationships with companies including Abbott, AbbVie Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Hoffmann-La Roche, Janssen, Novartis, Pfizer, Takeda, UCB, and Wyeth, as well as serving on the data and safety monitoring board for Forest Research and NIAMS. Dr. Brunner disclosed relationships with companies including Ablynx, AbbVie, AstraZeneca-MedImmune, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eli Lilly, EMD Serono, F. Hoffmann-La Roche, Genzyme, GlaxoSmithKline, Merck, Novartis, R-Pharm, and Sanofi. The study by Dr. Rumsey and colleagues was supported by Amgen. Dr. Rumsey and colleagues had no relevant financial conflicts to disclose.
SOURCES: Weiss PF et al. Arthritis Care Res. 2020 Dec 5. doi: 10.1002/acr.24529; Rumsey DG et al. Arthritis Care Res. 2020 Dec. 16. doi: 10.1002/acr.24537; Lovell DJ and Brunner HI. Arthritis Care Res. 2020 Dec 16. doi: 10.1002/acr.24536.
Children with enthesitis-related arthritis often have a high burden of disease and could benefit from medications currently approved for adults with spondyloarthritis, according to a review published in Arthritis Care & Research.
“Enthesitis-related arthritis (ERA) was the JIA [juvenile idiopathic arthritis] category applied to children with spondyloarthritis (SpA), recognizing enthesitis as a defining characteristic,” wrote Pamela F. Weiss, MD, of Children’s Hospital of Philadelphia, and colleagues.
The ERA criteria include “arthritis plus enthesitis; or arthritis or enthesitis plus at least two of the following: sacroiliac tenderness or inflammatory back pain, HLA-B27 positivity, first-degree relative with HLA-B27–associated disease, acute anterior uveitis, and arthritis in a male older than 6 years,” the review authors noted.
“None of the [Food and Drug Administration]–approved therapies for peripheral SpA or nonradiographic axial SpA” have been studied or approved for use in children with ERA, but data support biologic similarity to SpA in adults; notably, studies of the HLA-B27 allele have identified it as a risk factor for both SpA and ERA, they said.
Common factors in adult and childhood conditions
“The principal commonalities of children with ERA and axial arthritis, and adults with nonradiographic axial SpA, include enthesitis, arthritis, inflammatory back pain, anterior uveitis, HLA-B27 positivity, and family history of HLA-B27–associated disease,” the review authors wrote.
The first-line treatment for both ERA with axial arthritis and nonradiographic axial SpA is NSAIDs, followed by tumor necrosis factor (TNF) inhibitors if needed, they said. However, conventional disease-modifying antirheumatic drugs (cDMARDs) may be used in cases of peripheral disease affecting five or more joints. Studies of treatment response show similarities between ERA in children and SpA in adults, the authors added, with nearly half of adults with axial disease unable to achieve remission and approximately one-third of children with ERA failing to respond to therapy.
Clinical trials could improve options and outcomes for those with ERA who need advanced therapy and such trials should evaluate response of axial and peripheral disease separately, the review authors emphasized. For example, “Eligibility criteria for children with ERA and axial features could include the presence of some of the following disease features: active inflammatory sacroiliitis based on typical MRI changes according to ASAS/OMERACT [Assessment of SpondyloArthritis international Society/Outcome Measures in Rheumatology Clinical Trials] criteria; elevated CRP [C-reactive protein]; and inadequate response or intolerance to NSAIDs,” they noted. “Considering the similarities between adult spondyloarthritis and ERA in terms of etiology, genetics, pathogenesis, and clinical manifestations, it is evident that medications approved for axial or peripheral SpA should be studied in children with ERA involving axial or peripheral joints, respectively, with the intent to achieve labeling for use in children,” they concluded.
New data highlight ERA disease burden
The need for additional therapies for ERA patients gained more support from a recent study in which a majority of children with ERA or juvenile psoriatic arthritis (jPsA) used biologics, but those with sacroiliitis in particular showed a significant disease burden despite high biologic use.
The International Leagues Against Rheumatism criteria include seven categories of juvenile idiopathic arthritis, of which ERA and jPsA are the most common; however, characteristics of these children have not been well described, wrote Dax G. Rumsey, MD, of the University of Alberta, Edmonton, and colleagues.
“Children with ERA are more likely to have a clinical picture with predominantly peripheral arthritis, typically described as an oligoarthritis involving the lower limbs with high risk of axial disease, relative to the other categories of JIA,” and report more intense pain and worse health status, compared with children in other categories, the researchers wrote.
To more completely characterize children with ERA and jPsA, the researchers assessed 522 children with ERA and 380 with jPsA. The children were enrolled in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry. The findings were published in a brief report in Arthritis Care & Research.
Overall, 69% of the children took at least one biologic, including 72% with ERA and 64% with jPsA. Biologic use was even higher (81%) among the 28% of patients with sacroiliitis (40% of ERA patients and 12% of jPsA patients). Approximately 36% of the patients with sacroiliitis were positive for HLA-B27. In addition, Physician Global Assessment scores and clinical Juvenile Arthritis Disease Activity Score-10 (cJADAS10) scores were significantly higher at the first clinical visit with sacroiliitis, compared with the first visit without, which confirms “the clinical impression that active sacroiliitis significantly impacts children and their families,” the researchers said.
The average age at diagnosis was 10.8 years for ERA and 8.2 years for jPsA, and significantly more ERA patients were male (56% vs. 38%). However, more of the patients with sacroiliitis (54%) were female. More than half of the patients reported polyarticular involvement.
The study findings were limited by several factors, including the classification of ERA or jPsA and the reliance on physician diagnoses, as well as the variation in identifying sacroiliitis, the researchers said. However, the results increase understanding of the pathophysiology of ERA and jPsA to help determine optimal treatment, they concluded.
Data highlight research and treatment gaps
“Recent research demonstrates a large, unmet medical need in the treatment of JIA with 52%-65% of all JIA patients, including those with ERA and jPsA, having been treated with at least one biologic DMARD and 15%-19% having been treated with an FDA-unapproved biologic. In those with ERA or jPsA, 72%-79% of the children had been treated with a biologic DMARD, although no biologic DMARD has ever been FDA approved for these JIA categories,” Daniel J. Lovell, MD, and Hermine I. Brunner, MD, both with Cincinnati Children’s Hospital Medical Center, wrote in an editorial that accompanied the new study. Dr. Lovell and Dr. Brunner also were coauthors of the review article.
The new study supports findings from other recent publications, the editorialists noted. The new results showed “a significant proportion of the JIA population with active sacroiliitis with high disease burden despite very frequent (over 80% of the population) [treatment] with unstudied and unapproved biologic DMARDs,” they said. “These children with sacroiliitis had significantly greater disease burden with higher physician assessment of disease activity, higher parent assessment of disease impact, and higher disease activity as measured by the Juvenile Idiopathic Arthritis Disease Activity Score, compared to the children with ERA or jPsA without sacroiliitis,” they noted.
Previously, “the FDA granted pharmaceutical companies studying new treatments in adult SpA automatic full waivers from doing studies in children for new medications for ‘axial spondyloarthropathies including ankylosing spondylitis’ up until July 2020,” the editorialists said. However, “It is now time now for the pharmaceutical industry to perform FDA-monitored clinical trials of children and adolescents with SpA,” they emphasized. “This will allow for the scientific assessment of proper dosing, efficacy, and safety of the increasing number of new medications that are being licensed by the FDA for the treatment of SpA, such as the anti-TNF, anti–IL[interleukin]-17, and anti–IL-23 biologics, and perhaps JAK [Janus kinase] agents, to address this unmet medical need in these patients with juvenile SpA,” they concluded.
Dr. Weiss disclosed grant support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), and financial relationships with Eli Lilly and Pfizer. Dr. Lovell disclosed relationships with companies including Abbott, AbbVie Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Hoffmann-La Roche, Janssen, Novartis, Pfizer, Takeda, UCB, and Wyeth, as well as serving on the data and safety monitoring board for Forest Research and NIAMS. Dr. Brunner disclosed relationships with companies including Ablynx, AbbVie, AstraZeneca-MedImmune, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eli Lilly, EMD Serono, F. Hoffmann-La Roche, Genzyme, GlaxoSmithKline, Merck, Novartis, R-Pharm, and Sanofi. The study by Dr. Rumsey and colleagues was supported by Amgen. Dr. Rumsey and colleagues had no relevant financial conflicts to disclose.
SOURCES: Weiss PF et al. Arthritis Care Res. 2020 Dec 5. doi: 10.1002/acr.24529; Rumsey DG et al. Arthritis Care Res. 2020 Dec. 16. doi: 10.1002/acr.24537; Lovell DJ and Brunner HI. Arthritis Care Res. 2020 Dec 16. doi: 10.1002/acr.24536.
FROM ARTHRITIS CARE & RESEARCH
Osteoporosis prevalence in PsA similar to general population
The rates of osteopenia and osteoporosis among individuals with psoriatic arthritis are comparable to those seen in the general population, research suggests.
The cohort study, published in Arthritis Care & Research, also found that clinicians are likely to refer patients for bone mineral density (BMD) testing based on osteoporosis risk factors or psoriatic arthritis disease severity markers.
Timothy S.H. Kwok, MD, of the University of Toronto, and coauthors wrote that previous research suggested a possible link between psoriatic arthritis and osteoporosis or osteopenia. However, no cohort studies appear to have examined this association.
The study involved 201 individuals with psoriatic arthritis attending a single specialist clinic, who were enrolled in a longitudinal study of psoriatic arthritis (PsA) and who were also referred for BMD testing with dual-energy x-ray absorptiometry.
Of these participants, 13% had a BMD in the osteoporotic range, 45% were in the osteopenic range, and 42% were in the normal range for BMD. The prevalence of osteoporosis observed in the general population aged 50 or above, observed in an earlier large prospective study, ranged from 7% to 16%, and osteopenia ranged from 27% to 46%.
“Our study suggests that patients with PsA have similar BMDs compared to the general population,” the authors wrote.
Researchers did note the suggestion that patients with polyarthritis had lower BMDs over time. Because of the small number of events, this did not achieve statistical significance, but “this relationship warrants further research, given that multiple cohort studies have independently demonstrated polyarticular onset of disease predicting clinical deformities and erosive disease in PsA,” they wrote.
They also saw that patients with increased body mass index had a significant 21% lower odds of having a BMD in the osteoporotic range, while those using biologics had a significant 83% lower odds.
Among participants with BMD scores in the osteopenic or osteoporotic range, these scores were seen in the lumbar spine in 63% of measurements, the femoral neck in 88%, and the total hip in 39%. Mean T-scores for the lumbar spine were –0.30±0.32, and for the femoral neck were –1.10±1.04 and the total hip, –0.45±0.42.
The study also examined what factors were associated with referral for BMD testing. They found that increasing age, menopause, elevated acute phase reactants, or use of biologics, methotrexate, and systemic glucocorticoids were associated with a higher likelihood of undergoing BMD testing.
Noting that the latest Canadian clinical practice guidelines on BMD testing advise that age, menopause, and use of systemic glucocorticoids use are risk factors that should prompt testing, the authors suggested clinicians were using a combination of traditional osteoporosis risk factors and markers of psoriatic disease severity to underpin their decision to refer.
However, they commented that none of the factors associated with a higher likelihood of having a BMD test were actually associated with lower BMD scores.
“This suggests that clinicians may be over-screening patients with PsA for osteopenia/osteoporosis, as they do not appear to be at baseline higher risk for lower BMD scores than the general population,” they wrote. “This is of importance, as there are currently no formal recommendations with regards to the optimal interval or time to commence BMD testing within the recent major PsA guidelines.”
The study was supported by a grant from the Krembil Foundation. No conflicts of interest were declared.
SOURCE: Kwok TSH et al. Arthritis Care Res. 2020 Dec 16. doi: 10.1002/acr.24538.
The rates of osteopenia and osteoporosis among individuals with psoriatic arthritis are comparable to those seen in the general population, research suggests.
The cohort study, published in Arthritis Care & Research, also found that clinicians are likely to refer patients for bone mineral density (BMD) testing based on osteoporosis risk factors or psoriatic arthritis disease severity markers.
Timothy S.H. Kwok, MD, of the University of Toronto, and coauthors wrote that previous research suggested a possible link between psoriatic arthritis and osteoporosis or osteopenia. However, no cohort studies appear to have examined this association.
The study involved 201 individuals with psoriatic arthritis attending a single specialist clinic, who were enrolled in a longitudinal study of psoriatic arthritis (PsA) and who were also referred for BMD testing with dual-energy x-ray absorptiometry.
Of these participants, 13% had a BMD in the osteoporotic range, 45% were in the osteopenic range, and 42% were in the normal range for BMD. The prevalence of osteoporosis observed in the general population aged 50 or above, observed in an earlier large prospective study, ranged from 7% to 16%, and osteopenia ranged from 27% to 46%.
“Our study suggests that patients with PsA have similar BMDs compared to the general population,” the authors wrote.
Researchers did note the suggestion that patients with polyarthritis had lower BMDs over time. Because of the small number of events, this did not achieve statistical significance, but “this relationship warrants further research, given that multiple cohort studies have independently demonstrated polyarticular onset of disease predicting clinical deformities and erosive disease in PsA,” they wrote.
They also saw that patients with increased body mass index had a significant 21% lower odds of having a BMD in the osteoporotic range, while those using biologics had a significant 83% lower odds.
Among participants with BMD scores in the osteopenic or osteoporotic range, these scores were seen in the lumbar spine in 63% of measurements, the femoral neck in 88%, and the total hip in 39%. Mean T-scores for the lumbar spine were –0.30±0.32, and for the femoral neck were –1.10±1.04 and the total hip, –0.45±0.42.
The study also examined what factors were associated with referral for BMD testing. They found that increasing age, menopause, elevated acute phase reactants, or use of biologics, methotrexate, and systemic glucocorticoids were associated with a higher likelihood of undergoing BMD testing.
Noting that the latest Canadian clinical practice guidelines on BMD testing advise that age, menopause, and use of systemic glucocorticoids use are risk factors that should prompt testing, the authors suggested clinicians were using a combination of traditional osteoporosis risk factors and markers of psoriatic disease severity to underpin their decision to refer.
However, they commented that none of the factors associated with a higher likelihood of having a BMD test were actually associated with lower BMD scores.
“This suggests that clinicians may be over-screening patients with PsA for osteopenia/osteoporosis, as they do not appear to be at baseline higher risk for lower BMD scores than the general population,” they wrote. “This is of importance, as there are currently no formal recommendations with regards to the optimal interval or time to commence BMD testing within the recent major PsA guidelines.”
The study was supported by a grant from the Krembil Foundation. No conflicts of interest were declared.
SOURCE: Kwok TSH et al. Arthritis Care Res. 2020 Dec 16. doi: 10.1002/acr.24538.
The rates of osteopenia and osteoporosis among individuals with psoriatic arthritis are comparable to those seen in the general population, research suggests.
The cohort study, published in Arthritis Care & Research, also found that clinicians are likely to refer patients for bone mineral density (BMD) testing based on osteoporosis risk factors or psoriatic arthritis disease severity markers.
Timothy S.H. Kwok, MD, of the University of Toronto, and coauthors wrote that previous research suggested a possible link between psoriatic arthritis and osteoporosis or osteopenia. However, no cohort studies appear to have examined this association.
The study involved 201 individuals with psoriatic arthritis attending a single specialist clinic, who were enrolled in a longitudinal study of psoriatic arthritis (PsA) and who were also referred for BMD testing with dual-energy x-ray absorptiometry.
Of these participants, 13% had a BMD in the osteoporotic range, 45% were in the osteopenic range, and 42% were in the normal range for BMD. The prevalence of osteoporosis observed in the general population aged 50 or above, observed in an earlier large prospective study, ranged from 7% to 16%, and osteopenia ranged from 27% to 46%.
“Our study suggests that patients with PsA have similar BMDs compared to the general population,” the authors wrote.
Researchers did note the suggestion that patients with polyarthritis had lower BMDs over time. Because of the small number of events, this did not achieve statistical significance, but “this relationship warrants further research, given that multiple cohort studies have independently demonstrated polyarticular onset of disease predicting clinical deformities and erosive disease in PsA,” they wrote.
They also saw that patients with increased body mass index had a significant 21% lower odds of having a BMD in the osteoporotic range, while those using biologics had a significant 83% lower odds.
Among participants with BMD scores in the osteopenic or osteoporotic range, these scores were seen in the lumbar spine in 63% of measurements, the femoral neck in 88%, and the total hip in 39%. Mean T-scores for the lumbar spine were –0.30±0.32, and for the femoral neck were –1.10±1.04 and the total hip, –0.45±0.42.
The study also examined what factors were associated with referral for BMD testing. They found that increasing age, menopause, elevated acute phase reactants, or use of biologics, methotrexate, and systemic glucocorticoids were associated with a higher likelihood of undergoing BMD testing.
Noting that the latest Canadian clinical practice guidelines on BMD testing advise that age, menopause, and use of systemic glucocorticoids use are risk factors that should prompt testing, the authors suggested clinicians were using a combination of traditional osteoporosis risk factors and markers of psoriatic disease severity to underpin their decision to refer.
However, they commented that none of the factors associated with a higher likelihood of having a BMD test were actually associated with lower BMD scores.
“This suggests that clinicians may be over-screening patients with PsA for osteopenia/osteoporosis, as they do not appear to be at baseline higher risk for lower BMD scores than the general population,” they wrote. “This is of importance, as there are currently no formal recommendations with regards to the optimal interval or time to commence BMD testing within the recent major PsA guidelines.”
The study was supported by a grant from the Krembil Foundation. No conflicts of interest were declared.
SOURCE: Kwok TSH et al. Arthritis Care Res. 2020 Dec 16. doi: 10.1002/acr.24538.
FROM ARTHRITIS CARE & RESEARCH
High hydroxychloroquine blood level may lower thrombosis risk in lupus
Maintaining an average hydroxychloroquine whole blood level above 1,068 ng/mL significantly reduced the risk of thrombosis in adults with systemic lupus erythematosus, based on data from 739 patients.
Hydroxychloroquine (HCQ) is a common treatment for systemic lupus erythematosus (SLE); studies suggest that it may protect against thrombosis, but the optimal dosing for this purpose remains unknown, wrote Michelle Petri, MD, of Johns Hopkins University, Baltimore, and colleagues. In a study published in Arthritis & Rheumatology, the researchers examined data on HCQ levels from 739 adults with SLE who were part of the Hopkins Lupus Cohort, a longitudinal study of outcomes in SLE patients. Of these, 38 (5.1%) developed thrombosis during 2,330 person-years of follow-up.
Overall, the average HCQ blood level was significantly lower in patients who experienced thrombosis, compared to those who did not (720 ng/mL vs. 935 ng/mL; P = .025). “Prescribed hydroxychloroquine doses did not predict hydroxychloroquine blood levels,” the researchers noted.
In addition, Dr. Petri and associates found a dose-response relationship in which the thrombosis rate declined approximately 13% for every 200-ng/mL increase in the mean HCQ blood level measurement and for the most recent HCQ blood level measurement after controlling for factors that included age, ethnicity, lupus anticoagulant, low C3, and hypertension.
In a multivariate analysis, thrombotic events decreased by 69% in patients with mean HCQ blood levels greater than 1,068 ng/mL, compared to those with average HCQ blood levels less than 648 ng/mL.
The average age of the patients at the time HCQ measurements began was 43 years, 93% were female, and 46% were White. Patients visited a clinic every 3 months, and HCQ levels were determined by liquid chromatography-tandem mass spectrometry.
“Between-person and within-person correlation coefficients were used to measure the strength of the linear association between HCQ blood levels and commonly prescribed HCQ doses from 4.5 to 6.5 mg/kg,” the researchers said.
Higher doses of HCQ have been associated with increased risk for retinopathy, and current guidelines recommend using less than 5 mg/kg of ideal body weight, the researchers said. “Importantly, there was no correlation between the prescribed dose and the hydroxychloroquine blood level over the range (4.5 to 6.5 mg/kg) used in clinical practice, highlighting the need for personalized hydroxychloroquine drug level-guided therapy and dose adjustment,” they emphasized.
The study findings were limited by several factors, including the observational design and potential confounding from variables not included in the model, as well as the small sample size, single site, and single rheumatologist involved in the study, the researchers noted.
The results suggest that aiming for a blood HCQ level of 1,068 ng/mL can be done safely to help prevent thrombosis in patients with SLE, the researchers said. “Routine clinical integration of hydroxychloroquine blood level measurement offers an opportunity for personalized drug dosing and risk management beyond rigid empirical dosing recommendations in patients with SLE,” they concluded.
The study was supported in part by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The researchers had no relevant financial conflicts to disclose.
SOURCE: Petri M et al. Arthritis Rheumatol. 2021 Jan 6. doi: 10.1002/ART.41621.
Maintaining an average hydroxychloroquine whole blood level above 1,068 ng/mL significantly reduced the risk of thrombosis in adults with systemic lupus erythematosus, based on data from 739 patients.
Hydroxychloroquine (HCQ) is a common treatment for systemic lupus erythematosus (SLE); studies suggest that it may protect against thrombosis, but the optimal dosing for this purpose remains unknown, wrote Michelle Petri, MD, of Johns Hopkins University, Baltimore, and colleagues. In a study published in Arthritis & Rheumatology, the researchers examined data on HCQ levels from 739 adults with SLE who were part of the Hopkins Lupus Cohort, a longitudinal study of outcomes in SLE patients. Of these, 38 (5.1%) developed thrombosis during 2,330 person-years of follow-up.
Overall, the average HCQ blood level was significantly lower in patients who experienced thrombosis, compared to those who did not (720 ng/mL vs. 935 ng/mL; P = .025). “Prescribed hydroxychloroquine doses did not predict hydroxychloroquine blood levels,” the researchers noted.
In addition, Dr. Petri and associates found a dose-response relationship in which the thrombosis rate declined approximately 13% for every 200-ng/mL increase in the mean HCQ blood level measurement and for the most recent HCQ blood level measurement after controlling for factors that included age, ethnicity, lupus anticoagulant, low C3, and hypertension.
In a multivariate analysis, thrombotic events decreased by 69% in patients with mean HCQ blood levels greater than 1,068 ng/mL, compared to those with average HCQ blood levels less than 648 ng/mL.
The average age of the patients at the time HCQ measurements began was 43 years, 93% were female, and 46% were White. Patients visited a clinic every 3 months, and HCQ levels were determined by liquid chromatography-tandem mass spectrometry.
“Between-person and within-person correlation coefficients were used to measure the strength of the linear association between HCQ blood levels and commonly prescribed HCQ doses from 4.5 to 6.5 mg/kg,” the researchers said.
Higher doses of HCQ have been associated with increased risk for retinopathy, and current guidelines recommend using less than 5 mg/kg of ideal body weight, the researchers said. “Importantly, there was no correlation between the prescribed dose and the hydroxychloroquine blood level over the range (4.5 to 6.5 mg/kg) used in clinical practice, highlighting the need for personalized hydroxychloroquine drug level-guided therapy and dose adjustment,” they emphasized.
The study findings were limited by several factors, including the observational design and potential confounding from variables not included in the model, as well as the small sample size, single site, and single rheumatologist involved in the study, the researchers noted.
The results suggest that aiming for a blood HCQ level of 1,068 ng/mL can be done safely to help prevent thrombosis in patients with SLE, the researchers said. “Routine clinical integration of hydroxychloroquine blood level measurement offers an opportunity for personalized drug dosing and risk management beyond rigid empirical dosing recommendations in patients with SLE,” they concluded.
The study was supported in part by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The researchers had no relevant financial conflicts to disclose.
SOURCE: Petri M et al. Arthritis Rheumatol. 2021 Jan 6. doi: 10.1002/ART.41621.
Maintaining an average hydroxychloroquine whole blood level above 1,068 ng/mL significantly reduced the risk of thrombosis in adults with systemic lupus erythematosus, based on data from 739 patients.
Hydroxychloroquine (HCQ) is a common treatment for systemic lupus erythematosus (SLE); studies suggest that it may protect against thrombosis, but the optimal dosing for this purpose remains unknown, wrote Michelle Petri, MD, of Johns Hopkins University, Baltimore, and colleagues. In a study published in Arthritis & Rheumatology, the researchers examined data on HCQ levels from 739 adults with SLE who were part of the Hopkins Lupus Cohort, a longitudinal study of outcomes in SLE patients. Of these, 38 (5.1%) developed thrombosis during 2,330 person-years of follow-up.
Overall, the average HCQ blood level was significantly lower in patients who experienced thrombosis, compared to those who did not (720 ng/mL vs. 935 ng/mL; P = .025). “Prescribed hydroxychloroquine doses did not predict hydroxychloroquine blood levels,” the researchers noted.
In addition, Dr. Petri and associates found a dose-response relationship in which the thrombosis rate declined approximately 13% for every 200-ng/mL increase in the mean HCQ blood level measurement and for the most recent HCQ blood level measurement after controlling for factors that included age, ethnicity, lupus anticoagulant, low C3, and hypertension.
In a multivariate analysis, thrombotic events decreased by 69% in patients with mean HCQ blood levels greater than 1,068 ng/mL, compared to those with average HCQ blood levels less than 648 ng/mL.
The average age of the patients at the time HCQ measurements began was 43 years, 93% were female, and 46% were White. Patients visited a clinic every 3 months, and HCQ levels were determined by liquid chromatography-tandem mass spectrometry.
“Between-person and within-person correlation coefficients were used to measure the strength of the linear association between HCQ blood levels and commonly prescribed HCQ doses from 4.5 to 6.5 mg/kg,” the researchers said.
Higher doses of HCQ have been associated with increased risk for retinopathy, and current guidelines recommend using less than 5 mg/kg of ideal body weight, the researchers said. “Importantly, there was no correlation between the prescribed dose and the hydroxychloroquine blood level over the range (4.5 to 6.5 mg/kg) used in clinical practice, highlighting the need for personalized hydroxychloroquine drug level-guided therapy and dose adjustment,” they emphasized.
The study findings were limited by several factors, including the observational design and potential confounding from variables not included in the model, as well as the small sample size, single site, and single rheumatologist involved in the study, the researchers noted.
The results suggest that aiming for a blood HCQ level of 1,068 ng/mL can be done safely to help prevent thrombosis in patients with SLE, the researchers said. “Routine clinical integration of hydroxychloroquine blood level measurement offers an opportunity for personalized drug dosing and risk management beyond rigid empirical dosing recommendations in patients with SLE,” they concluded.
The study was supported in part by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The researchers had no relevant financial conflicts to disclose.
SOURCE: Petri M et al. Arthritis Rheumatol. 2021 Jan 6. doi: 10.1002/ART.41621.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point: Higher blood levels of hydroxychloroquine (HCQ) were protective against thrombosis in adults with systemic lupus erythematosus (SLE).
Major finding: The average HCQ in SLE patients who developed thrombosis was 720 ng/mL, compared to 935 ng/mL in those without thrombosis (P = .025).
Study details: The data come from an observational study of 739 adults with SLE; 5.1% developed thrombosis during the study period.
Disclosures: The study was supported in part by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The researchers had no relevant financial conflicts to disclose.
Source: Petri M et al. Arthritis Rheumatol. 2021 Jan 6. doi: 10.1002/ART.41621.