User login
Cutaneous vasculitis curtails quality of life
, and its measurement with an organ-specific instrument may catch important disease outcomes better than a generic health-related quality of life index, according to survey responses from participants in the Vasculitis Patient-Powered Research Network (VPPRN).
Although cutaneous vasculitis often causes itching, pain, and ulceration, the impact of the disease on specific health-related quality of life (HRQOL) outcomes has not been systematically assessed, wrote Sarah Mann, MD, of the University of Pittsburgh, and colleagues.
In a study published in JAMA Dermatology, the researchers used the VPPRN to conduct an online survey of adults aged 18 years and older with cutaneous manifestations of vasculitis. The survey was conducted between January 2020 and August 2021.
The primary outcomes of HRQOL were determined using two validated measures. One measured skin-related HRQOL (the Effects of Skin Disease on Quality-of-Life Survey [Skindex-29]), and the other measured general health and well-being (36-Item Short Form Health Survey [SF-36]).
The final analysis included 190 survey responses. The mean age of the respondents was 50.5 years, 84.1% were female, and approximately two-thirds reported a duration of vasculitis of at least 5 years. Respondents’ vasculitides included cutaneous small-vessel vasculitis (14%), IgA vasculitis (6.5%), urticarial vasculitis (8.4%), granulomatosis with polyangiitis (17.6%), microscopic polyangiitis (10.3%), eosinophilic vasculitis (15%), polyarteritis nodosa (3.7%), and other vasculitis types (24.2%).
On the Skindex-29 domains, severely or very severely diminished HRQOL was reported by 77.6% of respondents for emotions, 78.5% for symptoms, 60.7% for functioning, and 75.7% for overall HRQOL.
On the SF-36, the HRQOL was below average on six of eight domains, and approximately half of the patients had summative physical component scores (56%) and mental component scores (52%) below 50.
The HRQOL outcomes of cutaneous vasculitis were worse on the Skindex-29 than the SF-36, the researchers noted. “This discordance may reflect the value of disease or organ-specific measures, which may be able to capture important outcomes of disease even when generic measures do not,” they said.
The study findings were limited by several factors, including the potential lack of generalizability to broader populations of vasculitis patients, the researchers noted. Other limitations included the underrepresentation of male patients and the lack of a disease-specific patient-reported outcome measure, they said.
In addition, “Because half of patients reported having disease which was in remission or mildly active, the study findings may underestimate the true role of active cutaneous vasculitis on HRQOL,” the researchers said.
More studies are needed to assess how HRQOL measures respond to disease treatment and control, the researchers wrote in their discussion. However, the results suggest that cutaneous vasculitis has a significant effect on patients’ perception of their health, as well as on their well-being and symptoms, they said.
The study was supported by the Patient-Centered Outcomes Research Institute and GlaxoSmithKline. Dr. Mann had no financial conflicts to disclose. Several coauthors disclosed relationships with multiple companies, including GlaxoSmithKline.
, and its measurement with an organ-specific instrument may catch important disease outcomes better than a generic health-related quality of life index, according to survey responses from participants in the Vasculitis Patient-Powered Research Network (VPPRN).
Although cutaneous vasculitis often causes itching, pain, and ulceration, the impact of the disease on specific health-related quality of life (HRQOL) outcomes has not been systematically assessed, wrote Sarah Mann, MD, of the University of Pittsburgh, and colleagues.
In a study published in JAMA Dermatology, the researchers used the VPPRN to conduct an online survey of adults aged 18 years and older with cutaneous manifestations of vasculitis. The survey was conducted between January 2020 and August 2021.
The primary outcomes of HRQOL were determined using two validated measures. One measured skin-related HRQOL (the Effects of Skin Disease on Quality-of-Life Survey [Skindex-29]), and the other measured general health and well-being (36-Item Short Form Health Survey [SF-36]).
The final analysis included 190 survey responses. The mean age of the respondents was 50.5 years, 84.1% were female, and approximately two-thirds reported a duration of vasculitis of at least 5 years. Respondents’ vasculitides included cutaneous small-vessel vasculitis (14%), IgA vasculitis (6.5%), urticarial vasculitis (8.4%), granulomatosis with polyangiitis (17.6%), microscopic polyangiitis (10.3%), eosinophilic vasculitis (15%), polyarteritis nodosa (3.7%), and other vasculitis types (24.2%).
On the Skindex-29 domains, severely or very severely diminished HRQOL was reported by 77.6% of respondents for emotions, 78.5% for symptoms, 60.7% for functioning, and 75.7% for overall HRQOL.
On the SF-36, the HRQOL was below average on six of eight domains, and approximately half of the patients had summative physical component scores (56%) and mental component scores (52%) below 50.
The HRQOL outcomes of cutaneous vasculitis were worse on the Skindex-29 than the SF-36, the researchers noted. “This discordance may reflect the value of disease or organ-specific measures, which may be able to capture important outcomes of disease even when generic measures do not,” they said.
The study findings were limited by several factors, including the potential lack of generalizability to broader populations of vasculitis patients, the researchers noted. Other limitations included the underrepresentation of male patients and the lack of a disease-specific patient-reported outcome measure, they said.
In addition, “Because half of patients reported having disease which was in remission or mildly active, the study findings may underestimate the true role of active cutaneous vasculitis on HRQOL,” the researchers said.
More studies are needed to assess how HRQOL measures respond to disease treatment and control, the researchers wrote in their discussion. However, the results suggest that cutaneous vasculitis has a significant effect on patients’ perception of their health, as well as on their well-being and symptoms, they said.
The study was supported by the Patient-Centered Outcomes Research Institute and GlaxoSmithKline. Dr. Mann had no financial conflicts to disclose. Several coauthors disclosed relationships with multiple companies, including GlaxoSmithKline.
, and its measurement with an organ-specific instrument may catch important disease outcomes better than a generic health-related quality of life index, according to survey responses from participants in the Vasculitis Patient-Powered Research Network (VPPRN).
Although cutaneous vasculitis often causes itching, pain, and ulceration, the impact of the disease on specific health-related quality of life (HRQOL) outcomes has not been systematically assessed, wrote Sarah Mann, MD, of the University of Pittsburgh, and colleagues.
In a study published in JAMA Dermatology, the researchers used the VPPRN to conduct an online survey of adults aged 18 years and older with cutaneous manifestations of vasculitis. The survey was conducted between January 2020 and August 2021.
The primary outcomes of HRQOL were determined using two validated measures. One measured skin-related HRQOL (the Effects of Skin Disease on Quality-of-Life Survey [Skindex-29]), and the other measured general health and well-being (36-Item Short Form Health Survey [SF-36]).
The final analysis included 190 survey responses. The mean age of the respondents was 50.5 years, 84.1% were female, and approximately two-thirds reported a duration of vasculitis of at least 5 years. Respondents’ vasculitides included cutaneous small-vessel vasculitis (14%), IgA vasculitis (6.5%), urticarial vasculitis (8.4%), granulomatosis with polyangiitis (17.6%), microscopic polyangiitis (10.3%), eosinophilic vasculitis (15%), polyarteritis nodosa (3.7%), and other vasculitis types (24.2%).
On the Skindex-29 domains, severely or very severely diminished HRQOL was reported by 77.6% of respondents for emotions, 78.5% for symptoms, 60.7% for functioning, and 75.7% for overall HRQOL.
On the SF-36, the HRQOL was below average on six of eight domains, and approximately half of the patients had summative physical component scores (56%) and mental component scores (52%) below 50.
The HRQOL outcomes of cutaneous vasculitis were worse on the Skindex-29 than the SF-36, the researchers noted. “This discordance may reflect the value of disease or organ-specific measures, which may be able to capture important outcomes of disease even when generic measures do not,” they said.
The study findings were limited by several factors, including the potential lack of generalizability to broader populations of vasculitis patients, the researchers noted. Other limitations included the underrepresentation of male patients and the lack of a disease-specific patient-reported outcome measure, they said.
In addition, “Because half of patients reported having disease which was in remission or mildly active, the study findings may underestimate the true role of active cutaneous vasculitis on HRQOL,” the researchers said.
More studies are needed to assess how HRQOL measures respond to disease treatment and control, the researchers wrote in their discussion. However, the results suggest that cutaneous vasculitis has a significant effect on patients’ perception of their health, as well as on their well-being and symptoms, they said.
The study was supported by the Patient-Centered Outcomes Research Institute and GlaxoSmithKline. Dr. Mann had no financial conflicts to disclose. Several coauthors disclosed relationships with multiple companies, including GlaxoSmithKline.
FROM JAMA DERMATOLOGY
IVIG shows no impact on VTE risk in dermatomyositis patients
Use of intravenous immunoglobulin (IVIG) had no apparent effect on the risk of venous thromboembolism (VTE) in adults with dermatomyositis (DM), based on data from more than 400 individuals.
DM has been associated with an increased risk of VTE in previous studies, wrote Elizabeth T. Rotrosen, of Boston University and Brigham and Women’s Hospital, Boston, and colleagues. Although IVIG is often effective for DM patients with recalcitrant disease, it carries a boxed warning for increased thrombosis risk; however, the association between IVIG use and VTE risk in DM has not been well examined, the researchers said.
In a study published in JAMA Dermatology, the researchers identified 458 adults with DM based on the European Alliance of Associations for Reumatology/American College of Rheumatology criteria. The mean age of the participants was 51.8 years, 76% were female, and 82% were White. Of these, 178 were treated with IVIG and 280 were not. The mean duration of IVIG treatment was 32.9 months. The researchers used the chi square test to test for independence between binary variables, the Pearson chi square test to test for independence between categorical variables, and the unpaired t test to compare continuous variables in their statistical analysis.
A total of 23 patients experienced DM-associated VTEs; 6 in the IVIG group and 17 in the non-IVIG group (3.4% vs. 5.7%, P = .20), a nonsignificant difference. The patients in the IVIG group who experienced a DM-associated VTE all underwent IVIG treatment within 4 weeks before the event.
The most common risk factors for VTE in both the IVIG and non-IVIG groups were malignant neoplasm (66.7% and 58.8%, respectively), followed by immobilization (16.7% and 35.3%, respectively) and tobacco use (16.7% and 23.5%, respectively).
“Notably, 5 of the IVIG-treated patients with DM who experienced a VTE also had at least 1 additional underlying risk factor for VTE, including 4 with malignant neoplasm,” the researchers wrote.
A total of 76 patients had cancer-associated DM, including 12 treated with IVIG and 64 not treated with IVIG. Of these, 14 experienced a VTE (4 IVIG patients and 10 non-IVIG patients).
The study findings were limited by several factors, including the retrospective design and small number of VTEs. Prospective studies are needed for better assessment of the VTE risk in patients with DM treated with IVIG, the researchers noted. However, the study is the largest known to explore the association between IVIG use and VTE risk in patients with DM, they said, and the results suggest that clinicians may continue IVIG use in these patients with considerations of risks and benefits on an individual basis.
The study received no outside funding. Ms. Rotrosen had no financial conflicts to disclose. Two coauthors reported financial relationships with Pfizer unrelated to this study.
Use of intravenous immunoglobulin (IVIG) had no apparent effect on the risk of venous thromboembolism (VTE) in adults with dermatomyositis (DM), based on data from more than 400 individuals.
DM has been associated with an increased risk of VTE in previous studies, wrote Elizabeth T. Rotrosen, of Boston University and Brigham and Women’s Hospital, Boston, and colleagues. Although IVIG is often effective for DM patients with recalcitrant disease, it carries a boxed warning for increased thrombosis risk; however, the association between IVIG use and VTE risk in DM has not been well examined, the researchers said.
In a study published in JAMA Dermatology, the researchers identified 458 adults with DM based on the European Alliance of Associations for Reumatology/American College of Rheumatology criteria. The mean age of the participants was 51.8 years, 76% were female, and 82% were White. Of these, 178 were treated with IVIG and 280 were not. The mean duration of IVIG treatment was 32.9 months. The researchers used the chi square test to test for independence between binary variables, the Pearson chi square test to test for independence between categorical variables, and the unpaired t test to compare continuous variables in their statistical analysis.
A total of 23 patients experienced DM-associated VTEs; 6 in the IVIG group and 17 in the non-IVIG group (3.4% vs. 5.7%, P = .20), a nonsignificant difference. The patients in the IVIG group who experienced a DM-associated VTE all underwent IVIG treatment within 4 weeks before the event.
The most common risk factors for VTE in both the IVIG and non-IVIG groups were malignant neoplasm (66.7% and 58.8%, respectively), followed by immobilization (16.7% and 35.3%, respectively) and tobacco use (16.7% and 23.5%, respectively).
“Notably, 5 of the IVIG-treated patients with DM who experienced a VTE also had at least 1 additional underlying risk factor for VTE, including 4 with malignant neoplasm,” the researchers wrote.
A total of 76 patients had cancer-associated DM, including 12 treated with IVIG and 64 not treated with IVIG. Of these, 14 experienced a VTE (4 IVIG patients and 10 non-IVIG patients).
The study findings were limited by several factors, including the retrospective design and small number of VTEs. Prospective studies are needed for better assessment of the VTE risk in patients with DM treated with IVIG, the researchers noted. However, the study is the largest known to explore the association between IVIG use and VTE risk in patients with DM, they said, and the results suggest that clinicians may continue IVIG use in these patients with considerations of risks and benefits on an individual basis.
The study received no outside funding. Ms. Rotrosen had no financial conflicts to disclose. Two coauthors reported financial relationships with Pfizer unrelated to this study.
Use of intravenous immunoglobulin (IVIG) had no apparent effect on the risk of venous thromboembolism (VTE) in adults with dermatomyositis (DM), based on data from more than 400 individuals.
DM has been associated with an increased risk of VTE in previous studies, wrote Elizabeth T. Rotrosen, of Boston University and Brigham and Women’s Hospital, Boston, and colleagues. Although IVIG is often effective for DM patients with recalcitrant disease, it carries a boxed warning for increased thrombosis risk; however, the association between IVIG use and VTE risk in DM has not been well examined, the researchers said.
In a study published in JAMA Dermatology, the researchers identified 458 adults with DM based on the European Alliance of Associations for Reumatology/American College of Rheumatology criteria. The mean age of the participants was 51.8 years, 76% were female, and 82% were White. Of these, 178 were treated with IVIG and 280 were not. The mean duration of IVIG treatment was 32.9 months. The researchers used the chi square test to test for independence between binary variables, the Pearson chi square test to test for independence between categorical variables, and the unpaired t test to compare continuous variables in their statistical analysis.
A total of 23 patients experienced DM-associated VTEs; 6 in the IVIG group and 17 in the non-IVIG group (3.4% vs. 5.7%, P = .20), a nonsignificant difference. The patients in the IVIG group who experienced a DM-associated VTE all underwent IVIG treatment within 4 weeks before the event.
The most common risk factors for VTE in both the IVIG and non-IVIG groups were malignant neoplasm (66.7% and 58.8%, respectively), followed by immobilization (16.7% and 35.3%, respectively) and tobacco use (16.7% and 23.5%, respectively).
“Notably, 5 of the IVIG-treated patients with DM who experienced a VTE also had at least 1 additional underlying risk factor for VTE, including 4 with malignant neoplasm,” the researchers wrote.
A total of 76 patients had cancer-associated DM, including 12 treated with IVIG and 64 not treated with IVIG. Of these, 14 experienced a VTE (4 IVIG patients and 10 non-IVIG patients).
The study findings were limited by several factors, including the retrospective design and small number of VTEs. Prospective studies are needed for better assessment of the VTE risk in patients with DM treated with IVIG, the researchers noted. However, the study is the largest known to explore the association between IVIG use and VTE risk in patients with DM, they said, and the results suggest that clinicians may continue IVIG use in these patients with considerations of risks and benefits on an individual basis.
The study received no outside funding. Ms. Rotrosen had no financial conflicts to disclose. Two coauthors reported financial relationships with Pfizer unrelated to this study.
FROM JAMA DERMATOLOGY
Low disease state for childhood lupus approaches validation
MANCHESTER, ENGLAND – An age-appropriate version of the Lupus Low Disease Activity State (LLDAS) has been developed by an international task force that will hopefully enable childhood-onset systemic lupus erythematosus (cSLE) to be treated to target in the near future.
The new childhood LLDAS (cLLDAS) has been purposefully developed to align with that already used for adults, Eve Smith, MBChB, PhD, explained at the annual meeting of the British Society for Rheumatology.
“There’s a lot of compelling data that’s accumulating from adult lupus and increasingly from childhood lupus that [treat to target] might be a good idea,” said Dr. Smith, who is a senior clinical fellow and honorary consultant at the University of Liverpool (England) and Alder Hey Children’s NHS Foundation Trust Hospital, also in Liverpool.
Urgent need to improve childhood lupus outcomes
“We urgently need to do something to try and improve outcomes for children,” Dr. Smith said.
“We know that childhood lupus patients have got higher disease activity as compared to adults; they have a greater medication burden, particularly steroids; and they tend to have more severe organ manifestations,” she added.
Moreover, data show that one-fifth of pediatric patients with lupus have already accrued early damage, and there is much higher mortality associated with childhood lupus than there is with adult lupus.
“So, really we want to use treat to target as a way to try and improve on these aspects,” Dr. Smith said.
The treat-to-target (T2T) approach is not a new idea in lupus, with a lot of work already done in adult patients. One large study of more than 3,300 patients conducted in 13 countries has shown that patients who never achieve LLDAS are more likely to have high levels of damage, greater glucocorticoid use, worse quality of life, and higher mortality than are those who do.
Conversely, data have also shown that achieving a LLDAS is associated with a reduction in the risk for new damage, flares, and hospitalization, as well as reducing health care costs and improving patients’ overall health-related quality of life.
T2T is a recognized approach in European adult SLE guidelines, Dr. Smith said, although the approach has not really been fully realized as of yet, even in adult practice.
The cSLE T2T international task force and cLLDAS definition
With evidence accumulating on the benefits of getting children with SLE to a low disease activity state, Dr. Smith and colleague Michael Beresford, MBChB, PhD, Brough Chair, Professor of Child Health at the University of Liverpool, put out a call to develop a task force to look into the feasibility of a T2T approach.
“We had a really enthusiastic response internationally, which we were really encouraged by,” Dr. Smith said, “and we now lead a task force of 20 experts from across all five continents, and we have really strong patient involvement.”
Through a consensus process, an international cSLE T2T Task Force agreed on overarching principles and points to consider that will “lay the foundation for future T2T approaches in cSLE,” according to the recommendations statement, which was endorsed by the Paediatric Rheumatology European Society.
Next, they looked to develop an age-appropriate definition for low disease activity.
“We’re deliberately wanting to maintain sufficient unity with the adult definition, so that we could facilitate life-course studies,” said Dr. Smith, who presented the results of a literature review and series of Delphi surveys at the meeting.
The conceptual definition of cLLDAS is similar to adults in describing it as a sustained state that is associated with a low likelihood of adverse outcome, Dr. Smith said, but with the added wording of “considering disease activity, damage, and medication toxicity.”
The definition is achieved when the SLE Disease Activity Index-2K is ≤ 4 and there is no activity in major organ systems; there are no new features of lupus disease activity since the last assessment; there is a score of ≤ 1 on Physician Global Assessment; steroid doses are ≤ 0.15 mg/kg/day or a maximum of 7.5 mg/day (whichever is lower); and immunosuppressive treatment is stable, with any changes to medication only because of side effects, adherence, changes in weight, or when in the process of reaching a target dose.
“It’s all very well having a definition, but you need to think about how that will work in practice,” Dr. Smith said. This is something that the task force is thinking about very carefully.
The task force next aims to validate the cLLDAS definition, form an extensive research agenda to inform the T2T methods, and develop innovative methods to apply the approach in practice.
The work is supported by the Wellcome Trust, National Institutes for Health Research, Versus Arthritis, and the University of Liverpool, Alder Hey Children’s NHS Foundation Trust and the Alder Hey Charity. Dr. Smith reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MANCHESTER, ENGLAND – An age-appropriate version of the Lupus Low Disease Activity State (LLDAS) has been developed by an international task force that will hopefully enable childhood-onset systemic lupus erythematosus (cSLE) to be treated to target in the near future.
The new childhood LLDAS (cLLDAS) has been purposefully developed to align with that already used for adults, Eve Smith, MBChB, PhD, explained at the annual meeting of the British Society for Rheumatology.
“There’s a lot of compelling data that’s accumulating from adult lupus and increasingly from childhood lupus that [treat to target] might be a good idea,” said Dr. Smith, who is a senior clinical fellow and honorary consultant at the University of Liverpool (England) and Alder Hey Children’s NHS Foundation Trust Hospital, also in Liverpool.
Urgent need to improve childhood lupus outcomes
“We urgently need to do something to try and improve outcomes for children,” Dr. Smith said.
“We know that childhood lupus patients have got higher disease activity as compared to adults; they have a greater medication burden, particularly steroids; and they tend to have more severe organ manifestations,” she added.
Moreover, data show that one-fifth of pediatric patients with lupus have already accrued early damage, and there is much higher mortality associated with childhood lupus than there is with adult lupus.
“So, really we want to use treat to target as a way to try and improve on these aspects,” Dr. Smith said.
The treat-to-target (T2T) approach is not a new idea in lupus, with a lot of work already done in adult patients. One large study of more than 3,300 patients conducted in 13 countries has shown that patients who never achieve LLDAS are more likely to have high levels of damage, greater glucocorticoid use, worse quality of life, and higher mortality than are those who do.
Conversely, data have also shown that achieving a LLDAS is associated with a reduction in the risk for new damage, flares, and hospitalization, as well as reducing health care costs and improving patients’ overall health-related quality of life.
T2T is a recognized approach in European adult SLE guidelines, Dr. Smith said, although the approach has not really been fully realized as of yet, even in adult practice.
The cSLE T2T international task force and cLLDAS definition
With evidence accumulating on the benefits of getting children with SLE to a low disease activity state, Dr. Smith and colleague Michael Beresford, MBChB, PhD, Brough Chair, Professor of Child Health at the University of Liverpool, put out a call to develop a task force to look into the feasibility of a T2T approach.
“We had a really enthusiastic response internationally, which we were really encouraged by,” Dr. Smith said, “and we now lead a task force of 20 experts from across all five continents, and we have really strong patient involvement.”
Through a consensus process, an international cSLE T2T Task Force agreed on overarching principles and points to consider that will “lay the foundation for future T2T approaches in cSLE,” according to the recommendations statement, which was endorsed by the Paediatric Rheumatology European Society.
Next, they looked to develop an age-appropriate definition for low disease activity.
“We’re deliberately wanting to maintain sufficient unity with the adult definition, so that we could facilitate life-course studies,” said Dr. Smith, who presented the results of a literature review and series of Delphi surveys at the meeting.
The conceptual definition of cLLDAS is similar to adults in describing it as a sustained state that is associated with a low likelihood of adverse outcome, Dr. Smith said, but with the added wording of “considering disease activity, damage, and medication toxicity.”
The definition is achieved when the SLE Disease Activity Index-2K is ≤ 4 and there is no activity in major organ systems; there are no new features of lupus disease activity since the last assessment; there is a score of ≤ 1 on Physician Global Assessment; steroid doses are ≤ 0.15 mg/kg/day or a maximum of 7.5 mg/day (whichever is lower); and immunosuppressive treatment is stable, with any changes to medication only because of side effects, adherence, changes in weight, or when in the process of reaching a target dose.
“It’s all very well having a definition, but you need to think about how that will work in practice,” Dr. Smith said. This is something that the task force is thinking about very carefully.
The task force next aims to validate the cLLDAS definition, form an extensive research agenda to inform the T2T methods, and develop innovative methods to apply the approach in practice.
The work is supported by the Wellcome Trust, National Institutes for Health Research, Versus Arthritis, and the University of Liverpool, Alder Hey Children’s NHS Foundation Trust and the Alder Hey Charity. Dr. Smith reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MANCHESTER, ENGLAND – An age-appropriate version of the Lupus Low Disease Activity State (LLDAS) has been developed by an international task force that will hopefully enable childhood-onset systemic lupus erythematosus (cSLE) to be treated to target in the near future.
The new childhood LLDAS (cLLDAS) has been purposefully developed to align with that already used for adults, Eve Smith, MBChB, PhD, explained at the annual meeting of the British Society for Rheumatology.
“There’s a lot of compelling data that’s accumulating from adult lupus and increasingly from childhood lupus that [treat to target] might be a good idea,” said Dr. Smith, who is a senior clinical fellow and honorary consultant at the University of Liverpool (England) and Alder Hey Children’s NHS Foundation Trust Hospital, also in Liverpool.
Urgent need to improve childhood lupus outcomes
“We urgently need to do something to try and improve outcomes for children,” Dr. Smith said.
“We know that childhood lupus patients have got higher disease activity as compared to adults; they have a greater medication burden, particularly steroids; and they tend to have more severe organ manifestations,” she added.
Moreover, data show that one-fifth of pediatric patients with lupus have already accrued early damage, and there is much higher mortality associated with childhood lupus than there is with adult lupus.
“So, really we want to use treat to target as a way to try and improve on these aspects,” Dr. Smith said.
The treat-to-target (T2T) approach is not a new idea in lupus, with a lot of work already done in adult patients. One large study of more than 3,300 patients conducted in 13 countries has shown that patients who never achieve LLDAS are more likely to have high levels of damage, greater glucocorticoid use, worse quality of life, and higher mortality than are those who do.
Conversely, data have also shown that achieving a LLDAS is associated with a reduction in the risk for new damage, flares, and hospitalization, as well as reducing health care costs and improving patients’ overall health-related quality of life.
T2T is a recognized approach in European adult SLE guidelines, Dr. Smith said, although the approach has not really been fully realized as of yet, even in adult practice.
The cSLE T2T international task force and cLLDAS definition
With evidence accumulating on the benefits of getting children with SLE to a low disease activity state, Dr. Smith and colleague Michael Beresford, MBChB, PhD, Brough Chair, Professor of Child Health at the University of Liverpool, put out a call to develop a task force to look into the feasibility of a T2T approach.
“We had a really enthusiastic response internationally, which we were really encouraged by,” Dr. Smith said, “and we now lead a task force of 20 experts from across all five continents, and we have really strong patient involvement.”
Through a consensus process, an international cSLE T2T Task Force agreed on overarching principles and points to consider that will “lay the foundation for future T2T approaches in cSLE,” according to the recommendations statement, which was endorsed by the Paediatric Rheumatology European Society.
Next, they looked to develop an age-appropriate definition for low disease activity.
“We’re deliberately wanting to maintain sufficient unity with the adult definition, so that we could facilitate life-course studies,” said Dr. Smith, who presented the results of a literature review and series of Delphi surveys at the meeting.
The conceptual definition of cLLDAS is similar to adults in describing it as a sustained state that is associated with a low likelihood of adverse outcome, Dr. Smith said, but with the added wording of “considering disease activity, damage, and medication toxicity.”
The definition is achieved when the SLE Disease Activity Index-2K is ≤ 4 and there is no activity in major organ systems; there are no new features of lupus disease activity since the last assessment; there is a score of ≤ 1 on Physician Global Assessment; steroid doses are ≤ 0.15 mg/kg/day or a maximum of 7.5 mg/day (whichever is lower); and immunosuppressive treatment is stable, with any changes to medication only because of side effects, adherence, changes in weight, or when in the process of reaching a target dose.
“It’s all very well having a definition, but you need to think about how that will work in practice,” Dr. Smith said. This is something that the task force is thinking about very carefully.
The task force next aims to validate the cLLDAS definition, form an extensive research agenda to inform the T2T methods, and develop innovative methods to apply the approach in practice.
The work is supported by the Wellcome Trust, National Institutes for Health Research, Versus Arthritis, and the University of Liverpool, Alder Hey Children’s NHS Foundation Trust and the Alder Hey Charity. Dr. Smith reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT BSR 2023
Study shifts burden of IgG4-related disease to women
The incidence and prevalence of IgG4-related disease each rose considerably from 2015 to 2019 in the United States, and the risk of death in those with the immune-mediated condition is about 2.5 times higher than those who are not affected, based on an analysis of claims data from commercially insured adults.
The first population-based study of IgG4-RD incidence, prevalence, and mortality establishes “key benchmarks for informing the diagnosis and management of patients” with a condition “that causes fibrosing inflammatory lesions at nearly any anatomic site,” and wasn’t initially described until 2001, Zachary S. Wallace, MD, and associates said in Annals of the Rheumatic Diseases.
The increases in incidence and prevalence likely reflected increased disease awareness, they suggested. Overall U.S. incidence was 1.2 per 100,000 person-years for the 5-year period of 2015-2019, rising 86% from 0.78 per 100,000 person-years to 1.45 in 2018 before dropping to 1.39 in 2019. The change in prevalence was even greater, increasing 122% from 2.41 per 100,000 persons in 2015 to 5.34 per 100,000 in 2019, the investigators said.
Previous studies had indicated that the majority of patients with IgG4-RD were male, but the current study, using Optum’s Clinformatics Data Mart, which includes commercial health plan and Medicare Advantage members in all 50 states, showed that both incidence and prevalence (see graph) were higher among women, noted Dr. Wallace of Massachusetts General Hospital, Boston, and associates. They identified 524 patients (57.6% female) in the database who met the criteria for IgG4-RD from Jan. 1, 2010, to Dec. 31, 2019.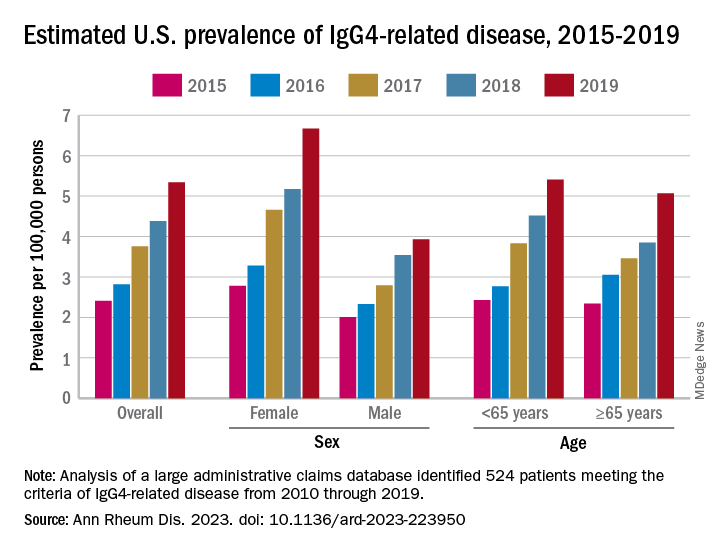
Incidence over the course of the study “was similar in patients identified as Asian or White but lower in those identified as Black or Hispanic,” they noted, adding that “the prevalence of IgG4-RD during this period reflected similar trends.” A jump in prevalence from 2018 to 2019, however, left White patients with a much higher rate (6.13 per 100,000 persons) than Asian patients (4.54 per 100,000), Black patients (3.42), and Hispanic patients (3.02).
For the mortality analysis, 516 patients with IgG4-RD were age-, sex-, and race-matched with 5,160 patients without IgG4-RD. Mortality was 3.42 and 1.46 per 100 person-years, respectively, over the 5.5 years of follow-up, so IgG4-RD was associated with a 2.5-fold higher risk of death. “The association of IgG4-RD with a higher risk of death was observed across the age spectrum and among both male and female patients,” the researchers said.
“Clinicians across specialties should be aware of IgG4-RD given the incidence, prevalence, and excess risk of death associated with this condition. ... Additional studies are urgently needed to define optimal management strategies to improve survival,” they wrote.
The study was supported by a grant to Massachusetts General Hospital from Sanofi, and Dr. Wallace received funding from the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Rheumatology Research Foundation. He has received research support and consulting fees from several companies, and four coinvestigators are employees of Sanofi.
The incidence and prevalence of IgG4-related disease each rose considerably from 2015 to 2019 in the United States, and the risk of death in those with the immune-mediated condition is about 2.5 times higher than those who are not affected, based on an analysis of claims data from commercially insured adults.
The first population-based study of IgG4-RD incidence, prevalence, and mortality establishes “key benchmarks for informing the diagnosis and management of patients” with a condition “that causes fibrosing inflammatory lesions at nearly any anatomic site,” and wasn’t initially described until 2001, Zachary S. Wallace, MD, and associates said in Annals of the Rheumatic Diseases.
The increases in incidence and prevalence likely reflected increased disease awareness, they suggested. Overall U.S. incidence was 1.2 per 100,000 person-years for the 5-year period of 2015-2019, rising 86% from 0.78 per 100,000 person-years to 1.45 in 2018 before dropping to 1.39 in 2019. The change in prevalence was even greater, increasing 122% from 2.41 per 100,000 persons in 2015 to 5.34 per 100,000 in 2019, the investigators said.
Previous studies had indicated that the majority of patients with IgG4-RD were male, but the current study, using Optum’s Clinformatics Data Mart, which includes commercial health plan and Medicare Advantage members in all 50 states, showed that both incidence and prevalence (see graph) were higher among women, noted Dr. Wallace of Massachusetts General Hospital, Boston, and associates. They identified 524 patients (57.6% female) in the database who met the criteria for IgG4-RD from Jan. 1, 2010, to Dec. 31, 2019.
Incidence over the course of the study “was similar in patients identified as Asian or White but lower in those identified as Black or Hispanic,” they noted, adding that “the prevalence of IgG4-RD during this period reflected similar trends.” A jump in prevalence from 2018 to 2019, however, left White patients with a much higher rate (6.13 per 100,000 persons) than Asian patients (4.54 per 100,000), Black patients (3.42), and Hispanic patients (3.02).
For the mortality analysis, 516 patients with IgG4-RD were age-, sex-, and race-matched with 5,160 patients without IgG4-RD. Mortality was 3.42 and 1.46 per 100 person-years, respectively, over the 5.5 years of follow-up, so IgG4-RD was associated with a 2.5-fold higher risk of death. “The association of IgG4-RD with a higher risk of death was observed across the age spectrum and among both male and female patients,” the researchers said.
“Clinicians across specialties should be aware of IgG4-RD given the incidence, prevalence, and excess risk of death associated with this condition. ... Additional studies are urgently needed to define optimal management strategies to improve survival,” they wrote.
The study was supported by a grant to Massachusetts General Hospital from Sanofi, and Dr. Wallace received funding from the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Rheumatology Research Foundation. He has received research support and consulting fees from several companies, and four coinvestigators are employees of Sanofi.
The incidence and prevalence of IgG4-related disease each rose considerably from 2015 to 2019 in the United States, and the risk of death in those with the immune-mediated condition is about 2.5 times higher than those who are not affected, based on an analysis of claims data from commercially insured adults.
The first population-based study of IgG4-RD incidence, prevalence, and mortality establishes “key benchmarks for informing the diagnosis and management of patients” with a condition “that causes fibrosing inflammatory lesions at nearly any anatomic site,” and wasn’t initially described until 2001, Zachary S. Wallace, MD, and associates said in Annals of the Rheumatic Diseases.
The increases in incidence and prevalence likely reflected increased disease awareness, they suggested. Overall U.S. incidence was 1.2 per 100,000 person-years for the 5-year period of 2015-2019, rising 86% from 0.78 per 100,000 person-years to 1.45 in 2018 before dropping to 1.39 in 2019. The change in prevalence was even greater, increasing 122% from 2.41 per 100,000 persons in 2015 to 5.34 per 100,000 in 2019, the investigators said.
Previous studies had indicated that the majority of patients with IgG4-RD were male, but the current study, using Optum’s Clinformatics Data Mart, which includes commercial health plan and Medicare Advantage members in all 50 states, showed that both incidence and prevalence (see graph) were higher among women, noted Dr. Wallace of Massachusetts General Hospital, Boston, and associates. They identified 524 patients (57.6% female) in the database who met the criteria for IgG4-RD from Jan. 1, 2010, to Dec. 31, 2019.
Incidence over the course of the study “was similar in patients identified as Asian or White but lower in those identified as Black or Hispanic,” they noted, adding that “the prevalence of IgG4-RD during this period reflected similar trends.” A jump in prevalence from 2018 to 2019, however, left White patients with a much higher rate (6.13 per 100,000 persons) than Asian patients (4.54 per 100,000), Black patients (3.42), and Hispanic patients (3.02).
For the mortality analysis, 516 patients with IgG4-RD were age-, sex-, and race-matched with 5,160 patients without IgG4-RD. Mortality was 3.42 and 1.46 per 100 person-years, respectively, over the 5.5 years of follow-up, so IgG4-RD was associated with a 2.5-fold higher risk of death. “The association of IgG4-RD with a higher risk of death was observed across the age spectrum and among both male and female patients,” the researchers said.
“Clinicians across specialties should be aware of IgG4-RD given the incidence, prevalence, and excess risk of death associated with this condition. ... Additional studies are urgently needed to define optimal management strategies to improve survival,” they wrote.
The study was supported by a grant to Massachusetts General Hospital from Sanofi, and Dr. Wallace received funding from the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Rheumatology Research Foundation. He has received research support and consulting fees from several companies, and four coinvestigators are employees of Sanofi.
FROM ANNALS OF THE RHEUMATIC DISEASES
New outbreaks of Marburg virus disease: What clinicians need to know
What do green monkeys, fruit bats, and python caves all have in common? All have been implicated in outbreaks as transmission sources of the rare but deadly Marburg virus. Marburg virus is in the same Filoviridae family of highly pathogenic RNA viruses as Ebola virus, and similarly can cause a rapidly progressive and fatal viral hemorrhagic fever.
In the first reported Marburg outbreak in 1967, laboratory workers in Marburg and Frankfurt, Germany, and in Belgrade, Yugoslavia, developed severe febrile illnesses with massive hemorrhage and multiorgan system dysfunction after contact with infected African green monkeys imported from Uganda.
The majority of MVD outbreaks have occurred in sub-Saharan Africa, and primarily in three African countries: Angola, the Democratic Republic of Congo, and Uganda. In sub-Saharan Africa, these sporadic outbreaks have had high case fatality rates (up to 80%-90%) and been linked to human exposure to the oral secretions or urinary/fecal droppings of Egyptian fruit bats (Rousettus aegyptiacus), the animal reservoir for Marburg virus. These exposures have primarily occurred among miners or tourists frequenting bat-infested mines or caves, including Uganda’s python cave, where Centers for Disease Control and Prevention investigators have conducted ecological studies on Marburg-infected bats. Person-to-person transmission occurs from direct contact with the blood or bodily fluids of an infected person or contact with a contaminated object (for example, unsterilized needles and syringes in a large nosocomial outbreak in Angola).
On April 6, 2023, the CDC issued a Health Advisory for U.S. clinicians and public health departments regarding two separate MVD outbreaks in Equatorial Guinea and Tanzania. These first-ever MVD outbreaks in both West and East African countries appear to be epidemiologically unrelated. As of March 24, 2023, in Equatorial Guinea, a total of 15 confirmed cases, including 11 deaths, and 23 probable cases, all deceased, have been identified in multiple districts since the outbreak declaration in February 2023. In Tanzania, a total of eight cases, including five deaths, have been reported among villagers in a northwest region since the outbreak declaration in March 2023. While so far cases in the Tanzania MVD outbreak have been epidemiologically linked, in Equatorial Guinea some cases have no identified epidemiological links, raising concern for ongoing community spread.
To date, no cases in these outbreaks have been reported in the United States or outside the affected countries. Overall, the risk of MVD in nonendemic countries, like the United States, is low but there is still a risk of importation. As of May 2, 2023, CDC has issued a Level 2 travel alert (practice enhanced precautions) for Marburg in Equatorial Guinea and a Level 1 travel watch (practice usual precautions) for Marburg in Tanzania. Travelers to these countries are advised to avoid nonessential travel to areas with active outbreaks and practice preventative measures, including avoiding contact with sick people, blood and bodily fluids, dead bodies, fruit bats, and nonhuman primates. International travelers returning to the United States from these countries are advised to self-monitor for Marburg symptoms during travel and for 21 days after country departure. Travelers who develop signs or symptoms of MVD should immediately self-isolate and contact their local health department or clinician.
So, how should clinicians manage such return travelers? In the setting of these new MVD outbreaks in sub-Saharan Africa, what do U.S. clinicians need to know? Clinicians should consider MVD in the differential diagnosis of ill patients with a compatible exposure history and clinical presentation. A detailed exposure history should be obtained to determine if patients have been to an area with an active MVD outbreak during their incubation period (in the past 21 days), had concerning epidemiologic risk factors (for example, presence at funerals, health care facilities, in mines/caves) while in the affected area, and/or had contact with a suspected or confirmed MVD case.
Clinical diagnosis of MVD is challenging as the initial dry symptoms of infection are nonspecific (fever, influenza-like illness, malaise, anorexia, etc.) and can resemble other febrile infectious illnesses. Similarly, presenting alternative or concurrent infections, particularly in febrile return travelers, include malaria, Lassa fever, typhoid, and measles. From these nonspecific symptoms, patients with MVD can then progress to the more severe wet symptoms (for example, vomiting, diarrhea, and bleeding). Common clinical features of MVD have been described based on the clinical presentation and course of cases in MVD outbreaks. Notably, in the original Marburg outbreak, maculopapular rash and conjunctival injection were early patient symptoms and most patient deaths occurred during the second week of illness progression.
Supportive care, including aggressive fluid replacement, is the mainstay of therapy for MVD. Currently, there are no Food and Drug Administration–approved antiviral treatments or vaccines for Marburg virus. Despite their viral similarities, vaccines against Ebola virus have not been shown to be protective against Marburg virus. Marburg virus vaccine development is ongoing, with a few promising candidate vaccines in early phase 1 and 2 clinical trials. In 2022, in response to MVD outbreaks in Ghana and Guinea, the World Health Organization convened an international Marburg virus vaccine consortium which is working to promote global research collaboration for more rapid vaccine development.
In the absence of definitive therapies, early identification of patients with suspected MVD is critical for preventing the spread of infection to close contacts. Like Ebola virus–infected patients, only symptomatic MVD patients are infectious and all patients with suspected MVD should be isolated in a private room and cared for in accordance with infection control procedures. As MVD is a nationally notifiable disease, suspected cases should be reported to local or state health departments as per jurisdictional requirements. Clinicians should also consult with their local or state health department and CDC for guidance on testing patients with suspected MVD and consider prompt evaluation for other infectious etiologies in the patient’s differential diagnosis. Comprehensive guidance for clinicians on screening and diagnosing patients with MVD is available on the CDC website at https://www.cdc.gov/vhf/marburg/index.html.
Dr. Appiah (she/her) is a medical epidemiologist in the division of global migration and quarantine at the CDC. Dr. Appiah holds adjunct faculty appointment in the division of infectious diseases at Emory University, Atlanta. She also holds a commission in the U.S. Public Health Service and is a resident advisor, Uganda, U.S. President’s Malaria Initiative, at the CDC.
What do green monkeys, fruit bats, and python caves all have in common? All have been implicated in outbreaks as transmission sources of the rare but deadly Marburg virus. Marburg virus is in the same Filoviridae family of highly pathogenic RNA viruses as Ebola virus, and similarly can cause a rapidly progressive and fatal viral hemorrhagic fever.
In the first reported Marburg outbreak in 1967, laboratory workers in Marburg and Frankfurt, Germany, and in Belgrade, Yugoslavia, developed severe febrile illnesses with massive hemorrhage and multiorgan system dysfunction after contact with infected African green monkeys imported from Uganda.
The majority of MVD outbreaks have occurred in sub-Saharan Africa, and primarily in three African countries: Angola, the Democratic Republic of Congo, and Uganda. In sub-Saharan Africa, these sporadic outbreaks have had high case fatality rates (up to 80%-90%) and been linked to human exposure to the oral secretions or urinary/fecal droppings of Egyptian fruit bats (Rousettus aegyptiacus), the animal reservoir for Marburg virus. These exposures have primarily occurred among miners or tourists frequenting bat-infested mines or caves, including Uganda’s python cave, where Centers for Disease Control and Prevention investigators have conducted ecological studies on Marburg-infected bats. Person-to-person transmission occurs from direct contact with the blood or bodily fluids of an infected person or contact with a contaminated object (for example, unsterilized needles and syringes in a large nosocomial outbreak in Angola).
On April 6, 2023, the CDC issued a Health Advisory for U.S. clinicians and public health departments regarding two separate MVD outbreaks in Equatorial Guinea and Tanzania. These first-ever MVD outbreaks in both West and East African countries appear to be epidemiologically unrelated. As of March 24, 2023, in Equatorial Guinea, a total of 15 confirmed cases, including 11 deaths, and 23 probable cases, all deceased, have been identified in multiple districts since the outbreak declaration in February 2023. In Tanzania, a total of eight cases, including five deaths, have been reported among villagers in a northwest region since the outbreak declaration in March 2023. While so far cases in the Tanzania MVD outbreak have been epidemiologically linked, in Equatorial Guinea some cases have no identified epidemiological links, raising concern for ongoing community spread.
To date, no cases in these outbreaks have been reported in the United States or outside the affected countries. Overall, the risk of MVD in nonendemic countries, like the United States, is low but there is still a risk of importation. As of May 2, 2023, CDC has issued a Level 2 travel alert (practice enhanced precautions) for Marburg in Equatorial Guinea and a Level 1 travel watch (practice usual precautions) for Marburg in Tanzania. Travelers to these countries are advised to avoid nonessential travel to areas with active outbreaks and practice preventative measures, including avoiding contact with sick people, blood and bodily fluids, dead bodies, fruit bats, and nonhuman primates. International travelers returning to the United States from these countries are advised to self-monitor for Marburg symptoms during travel and for 21 days after country departure. Travelers who develop signs or symptoms of MVD should immediately self-isolate and contact their local health department or clinician.
So, how should clinicians manage such return travelers? In the setting of these new MVD outbreaks in sub-Saharan Africa, what do U.S. clinicians need to know? Clinicians should consider MVD in the differential diagnosis of ill patients with a compatible exposure history and clinical presentation. A detailed exposure history should be obtained to determine if patients have been to an area with an active MVD outbreak during their incubation period (in the past 21 days), had concerning epidemiologic risk factors (for example, presence at funerals, health care facilities, in mines/caves) while in the affected area, and/or had contact with a suspected or confirmed MVD case.
Clinical diagnosis of MVD is challenging as the initial dry symptoms of infection are nonspecific (fever, influenza-like illness, malaise, anorexia, etc.) and can resemble other febrile infectious illnesses. Similarly, presenting alternative or concurrent infections, particularly in febrile return travelers, include malaria, Lassa fever, typhoid, and measles. From these nonspecific symptoms, patients with MVD can then progress to the more severe wet symptoms (for example, vomiting, diarrhea, and bleeding). Common clinical features of MVD have been described based on the clinical presentation and course of cases in MVD outbreaks. Notably, in the original Marburg outbreak, maculopapular rash and conjunctival injection were early patient symptoms and most patient deaths occurred during the second week of illness progression.
Supportive care, including aggressive fluid replacement, is the mainstay of therapy for MVD. Currently, there are no Food and Drug Administration–approved antiviral treatments or vaccines for Marburg virus. Despite their viral similarities, vaccines against Ebola virus have not been shown to be protective against Marburg virus. Marburg virus vaccine development is ongoing, with a few promising candidate vaccines in early phase 1 and 2 clinical trials. In 2022, in response to MVD outbreaks in Ghana and Guinea, the World Health Organization convened an international Marburg virus vaccine consortium which is working to promote global research collaboration for more rapid vaccine development.
In the absence of definitive therapies, early identification of patients with suspected MVD is critical for preventing the spread of infection to close contacts. Like Ebola virus–infected patients, only symptomatic MVD patients are infectious and all patients with suspected MVD should be isolated in a private room and cared for in accordance with infection control procedures. As MVD is a nationally notifiable disease, suspected cases should be reported to local or state health departments as per jurisdictional requirements. Clinicians should also consult with their local or state health department and CDC for guidance on testing patients with suspected MVD and consider prompt evaluation for other infectious etiologies in the patient’s differential diagnosis. Comprehensive guidance for clinicians on screening and diagnosing patients with MVD is available on the CDC website at https://www.cdc.gov/vhf/marburg/index.html.
Dr. Appiah (she/her) is a medical epidemiologist in the division of global migration and quarantine at the CDC. Dr. Appiah holds adjunct faculty appointment in the division of infectious diseases at Emory University, Atlanta. She also holds a commission in the U.S. Public Health Service and is a resident advisor, Uganda, U.S. President’s Malaria Initiative, at the CDC.
What do green monkeys, fruit bats, and python caves all have in common? All have been implicated in outbreaks as transmission sources of the rare but deadly Marburg virus. Marburg virus is in the same Filoviridae family of highly pathogenic RNA viruses as Ebola virus, and similarly can cause a rapidly progressive and fatal viral hemorrhagic fever.
In the first reported Marburg outbreak in 1967, laboratory workers in Marburg and Frankfurt, Germany, and in Belgrade, Yugoslavia, developed severe febrile illnesses with massive hemorrhage and multiorgan system dysfunction after contact with infected African green monkeys imported from Uganda.
The majority of MVD outbreaks have occurred in sub-Saharan Africa, and primarily in three African countries: Angola, the Democratic Republic of Congo, and Uganda. In sub-Saharan Africa, these sporadic outbreaks have had high case fatality rates (up to 80%-90%) and been linked to human exposure to the oral secretions or urinary/fecal droppings of Egyptian fruit bats (Rousettus aegyptiacus), the animal reservoir for Marburg virus. These exposures have primarily occurred among miners or tourists frequenting bat-infested mines or caves, including Uganda’s python cave, where Centers for Disease Control and Prevention investigators have conducted ecological studies on Marburg-infected bats. Person-to-person transmission occurs from direct contact with the blood or bodily fluids of an infected person or contact with a contaminated object (for example, unsterilized needles and syringes in a large nosocomial outbreak in Angola).
On April 6, 2023, the CDC issued a Health Advisory for U.S. clinicians and public health departments regarding two separate MVD outbreaks in Equatorial Guinea and Tanzania. These first-ever MVD outbreaks in both West and East African countries appear to be epidemiologically unrelated. As of March 24, 2023, in Equatorial Guinea, a total of 15 confirmed cases, including 11 deaths, and 23 probable cases, all deceased, have been identified in multiple districts since the outbreak declaration in February 2023. In Tanzania, a total of eight cases, including five deaths, have been reported among villagers in a northwest region since the outbreak declaration in March 2023. While so far cases in the Tanzania MVD outbreak have been epidemiologically linked, in Equatorial Guinea some cases have no identified epidemiological links, raising concern for ongoing community spread.
To date, no cases in these outbreaks have been reported in the United States or outside the affected countries. Overall, the risk of MVD in nonendemic countries, like the United States, is low but there is still a risk of importation. As of May 2, 2023, CDC has issued a Level 2 travel alert (practice enhanced precautions) for Marburg in Equatorial Guinea and a Level 1 travel watch (practice usual precautions) for Marburg in Tanzania. Travelers to these countries are advised to avoid nonessential travel to areas with active outbreaks and practice preventative measures, including avoiding contact with sick people, blood and bodily fluids, dead bodies, fruit bats, and nonhuman primates. International travelers returning to the United States from these countries are advised to self-monitor for Marburg symptoms during travel and for 21 days after country departure. Travelers who develop signs or symptoms of MVD should immediately self-isolate and contact their local health department or clinician.
So, how should clinicians manage such return travelers? In the setting of these new MVD outbreaks in sub-Saharan Africa, what do U.S. clinicians need to know? Clinicians should consider MVD in the differential diagnosis of ill patients with a compatible exposure history and clinical presentation. A detailed exposure history should be obtained to determine if patients have been to an area with an active MVD outbreak during their incubation period (in the past 21 days), had concerning epidemiologic risk factors (for example, presence at funerals, health care facilities, in mines/caves) while in the affected area, and/or had contact with a suspected or confirmed MVD case.
Clinical diagnosis of MVD is challenging as the initial dry symptoms of infection are nonspecific (fever, influenza-like illness, malaise, anorexia, etc.) and can resemble other febrile infectious illnesses. Similarly, presenting alternative or concurrent infections, particularly in febrile return travelers, include malaria, Lassa fever, typhoid, and measles. From these nonspecific symptoms, patients with MVD can then progress to the more severe wet symptoms (for example, vomiting, diarrhea, and bleeding). Common clinical features of MVD have been described based on the clinical presentation and course of cases in MVD outbreaks. Notably, in the original Marburg outbreak, maculopapular rash and conjunctival injection were early patient symptoms and most patient deaths occurred during the second week of illness progression.
Supportive care, including aggressive fluid replacement, is the mainstay of therapy for MVD. Currently, there are no Food and Drug Administration–approved antiviral treatments or vaccines for Marburg virus. Despite their viral similarities, vaccines against Ebola virus have not been shown to be protective against Marburg virus. Marburg virus vaccine development is ongoing, with a few promising candidate vaccines in early phase 1 and 2 clinical trials. In 2022, in response to MVD outbreaks in Ghana and Guinea, the World Health Organization convened an international Marburg virus vaccine consortium which is working to promote global research collaboration for more rapid vaccine development.
In the absence of definitive therapies, early identification of patients with suspected MVD is critical for preventing the spread of infection to close contacts. Like Ebola virus–infected patients, only symptomatic MVD patients are infectious and all patients with suspected MVD should be isolated in a private room and cared for in accordance with infection control procedures. As MVD is a nationally notifiable disease, suspected cases should be reported to local or state health departments as per jurisdictional requirements. Clinicians should also consult with their local or state health department and CDC for guidance on testing patients with suspected MVD and consider prompt evaluation for other infectious etiologies in the patient’s differential diagnosis. Comprehensive guidance for clinicians on screening and diagnosing patients with MVD is available on the CDC website at https://www.cdc.gov/vhf/marburg/index.html.
Dr. Appiah (she/her) is a medical epidemiologist in the division of global migration and quarantine at the CDC. Dr. Appiah holds adjunct faculty appointment in the division of infectious diseases at Emory University, Atlanta. She also holds a commission in the U.S. Public Health Service and is a resident advisor, Uganda, U.S. President’s Malaria Initiative, at the CDC.
U.S. incidence, prevalence of myasthenia gravis is rising
, an analysis of new claims data shows. Investigators speculate the rise of this rare disorder may be due to “increased diagnosis and more awareness of the disease over time, which has been shown in several studies,” study investigator Ema Rodrigues, DSc, MPH, with Alexion Pharmaceuticals, Boston.
Dr. Rodrigues presented her research at the 2023 annual meeting of the American Academy of Neurology.
Myasthenia gravis is a rare neuromuscular disease characterized by muscle weakness and fatigue caused by the binding of autoantibodies at the neuromuscular junction. It affects the voluntary muscles of the body, especially those that control the eyes, mouth, throat, and limbs.
In Europe, the incidence and prevalence of myasthenia gravis has increased for the past several decades. In the United States, increasing prevalence has also been observed, but recent estimates are lacking, making it tough to gauge the true burden of disease, Dr. Rodrigues explained.
Claims-based analysis
To investigate, Dr. Rodrigues and colleagues analyzed claims data (commercial, Medicare, and Medicaid) and electronic health records representing over 300 million patients in the United States from 2011 to present.
They calculated sex- and age-specific incidence and prevalence of myasthenia gravis for the year 2021 using U.S. Census data.
Prevalent patients were identified as having one or more myasthenia gravis records in 2021 and two or more myasthenia gravis records, at least 30 days apart, from 2016 to 2021. This cohort had 78,225 patients.
Incident patients were identified as those with a myasthenia gravis record in 2021 and no previous myasthenia gravis record from 2019 to 2020. This cohort had 4,214 patients.
For both the prevalent and incident cohort, the distribution of male and female patients was roughly 50/50, with a slightly higher proportion of females in the incident cohort, Dr. Rodrigues reported.
When looking at age groups, there were “very few pediatric patients,” she noted, with less than 1% of the patients under the age of 12. The highest proportion of patients were 65 years or older. The mean age was 67 in the prevalent cohort and 68 in the incident cohort.
In 2021, the overall incidence of myasthenia gravis was 3.2 per 100,000 with similar estimates for males and females (3.2 vs. 3.1 per 100,000, respectively).
Total prevalence was estimated to be 37.0 per 100,000 with sex-specific estimates being comparable at 37.3 and 36.7 per 100,000 for males and females, respectively.
The incidence and prevalence of myasthenia gravis increased with age, ranging from 0.3 and 0.4 per 100,000, respectively, in children younger than age 2 years, to 10.2 and 116.8 per 100,000, respectively, in people 65 and older.
These estimates are “significantly higher” than those from a prior U.S. analysis from 2003, Dr. Rodrigues told attendees, but they are quite similar to the estimates that were reported in Sweden in 2020.
A limitation of the analysis is that patients who do not seek care regularly may have not been identified due to inclusion criteria, potentially leading to underestimates. Also, no information was available on the myasthenia gravis subtype (ocular vs. generalized).
Underestimated burden
Reached for comment, Richard J. Nowak, MD, MS, director of the Yale Myasthenia Gravis Clinic, Yale School of Medicine, New Haven, Conn., noted that the new report, “albeit limited as a claims-based analysis, presents modern data on incidence and prevalence of myasthenia gravis in the United States.”
“It suggests that the current estimates of myasthenia gravis in the United States are too low and that the true impact/burden of myasthenia gravis is greater. While we are unable to verify the accuracy of the diagnosis, the total myasthenia gravis population is likely to be about 100,000, which is higher than prior estimates.”
“This, in fact, might be driven by greater disease awareness and increased diagnosis along with decreased mortality and longer life expectancy,” Dr. Nowak said.
“Anecdotally, we are most certainly seeing patients with new-onset myasthenia gravis in their 70s, 80s, and even 90s in recent years. The EXPLORE-MG registry published data from a tertiary center on age of onset breakdown showing myasthenia gravis can present at any age,” Dr. Nowak added.
Funding for the study was provided by Alexion, AstraZeneca Rare Disease. Dr. Rodrigues receives compensation and owns stock as an employee of Alexion, AstraZeneca Rare Diseases. Dr. Nowak has no relevant disclosures.
A version of this article originally appeared on Medscape.com.
, an analysis of new claims data shows. Investigators speculate the rise of this rare disorder may be due to “increased diagnosis and more awareness of the disease over time, which has been shown in several studies,” study investigator Ema Rodrigues, DSc, MPH, with Alexion Pharmaceuticals, Boston.
Dr. Rodrigues presented her research at the 2023 annual meeting of the American Academy of Neurology.
Myasthenia gravis is a rare neuromuscular disease characterized by muscle weakness and fatigue caused by the binding of autoantibodies at the neuromuscular junction. It affects the voluntary muscles of the body, especially those that control the eyes, mouth, throat, and limbs.
In Europe, the incidence and prevalence of myasthenia gravis has increased for the past several decades. In the United States, increasing prevalence has also been observed, but recent estimates are lacking, making it tough to gauge the true burden of disease, Dr. Rodrigues explained.
Claims-based analysis
To investigate, Dr. Rodrigues and colleagues analyzed claims data (commercial, Medicare, and Medicaid) and electronic health records representing over 300 million patients in the United States from 2011 to present.
They calculated sex- and age-specific incidence and prevalence of myasthenia gravis for the year 2021 using U.S. Census data.
Prevalent patients were identified as having one or more myasthenia gravis records in 2021 and two or more myasthenia gravis records, at least 30 days apart, from 2016 to 2021. This cohort had 78,225 patients.
Incident patients were identified as those with a myasthenia gravis record in 2021 and no previous myasthenia gravis record from 2019 to 2020. This cohort had 4,214 patients.
For both the prevalent and incident cohort, the distribution of male and female patients was roughly 50/50, with a slightly higher proportion of females in the incident cohort, Dr. Rodrigues reported.
When looking at age groups, there were “very few pediatric patients,” she noted, with less than 1% of the patients under the age of 12. The highest proportion of patients were 65 years or older. The mean age was 67 in the prevalent cohort and 68 in the incident cohort.
In 2021, the overall incidence of myasthenia gravis was 3.2 per 100,000 with similar estimates for males and females (3.2 vs. 3.1 per 100,000, respectively).
Total prevalence was estimated to be 37.0 per 100,000 with sex-specific estimates being comparable at 37.3 and 36.7 per 100,000 for males and females, respectively.
The incidence and prevalence of myasthenia gravis increased with age, ranging from 0.3 and 0.4 per 100,000, respectively, in children younger than age 2 years, to 10.2 and 116.8 per 100,000, respectively, in people 65 and older.
These estimates are “significantly higher” than those from a prior U.S. analysis from 2003, Dr. Rodrigues told attendees, but they are quite similar to the estimates that were reported in Sweden in 2020.
A limitation of the analysis is that patients who do not seek care regularly may have not been identified due to inclusion criteria, potentially leading to underestimates. Also, no information was available on the myasthenia gravis subtype (ocular vs. generalized).
Underestimated burden
Reached for comment, Richard J. Nowak, MD, MS, director of the Yale Myasthenia Gravis Clinic, Yale School of Medicine, New Haven, Conn., noted that the new report, “albeit limited as a claims-based analysis, presents modern data on incidence and prevalence of myasthenia gravis in the United States.”
“It suggests that the current estimates of myasthenia gravis in the United States are too low and that the true impact/burden of myasthenia gravis is greater. While we are unable to verify the accuracy of the diagnosis, the total myasthenia gravis population is likely to be about 100,000, which is higher than prior estimates.”
“This, in fact, might be driven by greater disease awareness and increased diagnosis along with decreased mortality and longer life expectancy,” Dr. Nowak said.
“Anecdotally, we are most certainly seeing patients with new-onset myasthenia gravis in their 70s, 80s, and even 90s in recent years. The EXPLORE-MG registry published data from a tertiary center on age of onset breakdown showing myasthenia gravis can present at any age,” Dr. Nowak added.
Funding for the study was provided by Alexion, AstraZeneca Rare Disease. Dr. Rodrigues receives compensation and owns stock as an employee of Alexion, AstraZeneca Rare Diseases. Dr. Nowak has no relevant disclosures.
A version of this article originally appeared on Medscape.com.
, an analysis of new claims data shows. Investigators speculate the rise of this rare disorder may be due to “increased diagnosis and more awareness of the disease over time, which has been shown in several studies,” study investigator Ema Rodrigues, DSc, MPH, with Alexion Pharmaceuticals, Boston.
Dr. Rodrigues presented her research at the 2023 annual meeting of the American Academy of Neurology.
Myasthenia gravis is a rare neuromuscular disease characterized by muscle weakness and fatigue caused by the binding of autoantibodies at the neuromuscular junction. It affects the voluntary muscles of the body, especially those that control the eyes, mouth, throat, and limbs.
In Europe, the incidence and prevalence of myasthenia gravis has increased for the past several decades. In the United States, increasing prevalence has also been observed, but recent estimates are lacking, making it tough to gauge the true burden of disease, Dr. Rodrigues explained.
Claims-based analysis
To investigate, Dr. Rodrigues and colleagues analyzed claims data (commercial, Medicare, and Medicaid) and electronic health records representing over 300 million patients in the United States from 2011 to present.
They calculated sex- and age-specific incidence and prevalence of myasthenia gravis for the year 2021 using U.S. Census data.
Prevalent patients were identified as having one or more myasthenia gravis records in 2021 and two or more myasthenia gravis records, at least 30 days apart, from 2016 to 2021. This cohort had 78,225 patients.
Incident patients were identified as those with a myasthenia gravis record in 2021 and no previous myasthenia gravis record from 2019 to 2020. This cohort had 4,214 patients.
For both the prevalent and incident cohort, the distribution of male and female patients was roughly 50/50, with a slightly higher proportion of females in the incident cohort, Dr. Rodrigues reported.
When looking at age groups, there were “very few pediatric patients,” she noted, with less than 1% of the patients under the age of 12. The highest proportion of patients were 65 years or older. The mean age was 67 in the prevalent cohort and 68 in the incident cohort.
In 2021, the overall incidence of myasthenia gravis was 3.2 per 100,000 with similar estimates for males and females (3.2 vs. 3.1 per 100,000, respectively).
Total prevalence was estimated to be 37.0 per 100,000 with sex-specific estimates being comparable at 37.3 and 36.7 per 100,000 for males and females, respectively.
The incidence and prevalence of myasthenia gravis increased with age, ranging from 0.3 and 0.4 per 100,000, respectively, in children younger than age 2 years, to 10.2 and 116.8 per 100,000, respectively, in people 65 and older.
These estimates are “significantly higher” than those from a prior U.S. analysis from 2003, Dr. Rodrigues told attendees, but they are quite similar to the estimates that were reported in Sweden in 2020.
A limitation of the analysis is that patients who do not seek care regularly may have not been identified due to inclusion criteria, potentially leading to underestimates. Also, no information was available on the myasthenia gravis subtype (ocular vs. generalized).
Underestimated burden
Reached for comment, Richard J. Nowak, MD, MS, director of the Yale Myasthenia Gravis Clinic, Yale School of Medicine, New Haven, Conn., noted that the new report, “albeit limited as a claims-based analysis, presents modern data on incidence and prevalence of myasthenia gravis in the United States.”
“It suggests that the current estimates of myasthenia gravis in the United States are too low and that the true impact/burden of myasthenia gravis is greater. While we are unable to verify the accuracy of the diagnosis, the total myasthenia gravis population is likely to be about 100,000, which is higher than prior estimates.”
“This, in fact, might be driven by greater disease awareness and increased diagnosis along with decreased mortality and longer life expectancy,” Dr. Nowak said.
“Anecdotally, we are most certainly seeing patients with new-onset myasthenia gravis in their 70s, 80s, and even 90s in recent years. The EXPLORE-MG registry published data from a tertiary center on age of onset breakdown showing myasthenia gravis can present at any age,” Dr. Nowak added.
Funding for the study was provided by Alexion, AstraZeneca Rare Disease. Dr. Rodrigues receives compensation and owns stock as an employee of Alexion, AstraZeneca Rare Diseases. Dr. Nowak has no relevant disclosures.
A version of this article originally appeared on Medscape.com.
From AAN 2023
FDA gives fast-track approval to new ALS drug
the debilitating and deadly disease for which there is no cure.
Most people with ALS die within 3-5 years of when symptoms appear, usually of respiratory failure.
The newly approved drug, called Qalsody, is made by the Swiss company Biogen. The FDA fast-tracked the approval based on early trial results. The agency said in a news release that its decision was based on the demonstrated ability of the drug to reduce a protein in the blood that is a sign of degeneration of brain and nerve cells.
While the drug was shown to impact the chemical process in the body linked to degeneration, there was no significant change in people’s symptoms during the first 28 weeks that they took the drug, Biogen said in a news release. But the company noted that some patients did see improved functioning after starting treatment.
“I have observed the positive impact Qalsody has on slowing the progression of ALS in people with SOD1 mutations,” Timothy M. Miller, MD, PhD, researcher and codirector of the ALS Center at Washington University in St. Louis, said in a statement released by Biogen. “The FDA’s approval of Qalsody gives me hope that people living with this rare form of ALS could experience a reduction in decline in strength, clinical function, and respiratory function.”
Qalsody is given to people via a spinal injection, with an initial course of three injections every 2 weeks. People then get the injection once every 28 days.
The new treatment is approved only for people with a rare kind of ALS called SOD1-ALS, which is known for a genetic mutation. While ALS affects up to 32,000 people in the United States, just 2% of people with ALS have the SOD1 gene mutation. The FDA says the number of people in the United States who could use Qalsody is about 500.
In trials, 147 people received either Qalsody or a placebo, and the treatment significantly reduced the level of a protein in people’s blood that is associated with the loss of control of voluntary muscles.
Because Qalsody received a fast-track approval from the FDA, it must still provide more research data in the future, including from a trial examining how the drug affects people who carry the SOD1 gene but do not yet show symptoms of ALS.
A version of this article first appeared on Medscape.com.
the debilitating and deadly disease for which there is no cure.
Most people with ALS die within 3-5 years of when symptoms appear, usually of respiratory failure.
The newly approved drug, called Qalsody, is made by the Swiss company Biogen. The FDA fast-tracked the approval based on early trial results. The agency said in a news release that its decision was based on the demonstrated ability of the drug to reduce a protein in the blood that is a sign of degeneration of brain and nerve cells.
While the drug was shown to impact the chemical process in the body linked to degeneration, there was no significant change in people’s symptoms during the first 28 weeks that they took the drug, Biogen said in a news release. But the company noted that some patients did see improved functioning after starting treatment.
“I have observed the positive impact Qalsody has on slowing the progression of ALS in people with SOD1 mutations,” Timothy M. Miller, MD, PhD, researcher and codirector of the ALS Center at Washington University in St. Louis, said in a statement released by Biogen. “The FDA’s approval of Qalsody gives me hope that people living with this rare form of ALS could experience a reduction in decline in strength, clinical function, and respiratory function.”
Qalsody is given to people via a spinal injection, with an initial course of three injections every 2 weeks. People then get the injection once every 28 days.
The new treatment is approved only for people with a rare kind of ALS called SOD1-ALS, which is known for a genetic mutation. While ALS affects up to 32,000 people in the United States, just 2% of people with ALS have the SOD1 gene mutation. The FDA says the number of people in the United States who could use Qalsody is about 500.
In trials, 147 people received either Qalsody or a placebo, and the treatment significantly reduced the level of a protein in people’s blood that is associated with the loss of control of voluntary muscles.
Because Qalsody received a fast-track approval from the FDA, it must still provide more research data in the future, including from a trial examining how the drug affects people who carry the SOD1 gene but do not yet show symptoms of ALS.
A version of this article first appeared on Medscape.com.
the debilitating and deadly disease for which there is no cure.
Most people with ALS die within 3-5 years of when symptoms appear, usually of respiratory failure.
The newly approved drug, called Qalsody, is made by the Swiss company Biogen. The FDA fast-tracked the approval based on early trial results. The agency said in a news release that its decision was based on the demonstrated ability of the drug to reduce a protein in the blood that is a sign of degeneration of brain and nerve cells.
While the drug was shown to impact the chemical process in the body linked to degeneration, there was no significant change in people’s symptoms during the first 28 weeks that they took the drug, Biogen said in a news release. But the company noted that some patients did see improved functioning after starting treatment.
“I have observed the positive impact Qalsody has on slowing the progression of ALS in people with SOD1 mutations,” Timothy M. Miller, MD, PhD, researcher and codirector of the ALS Center at Washington University in St. Louis, said in a statement released by Biogen. “The FDA’s approval of Qalsody gives me hope that people living with this rare form of ALS could experience a reduction in decline in strength, clinical function, and respiratory function.”
Qalsody is given to people via a spinal injection, with an initial course of three injections every 2 weeks. People then get the injection once every 28 days.
The new treatment is approved only for people with a rare kind of ALS called SOD1-ALS, which is known for a genetic mutation. While ALS affects up to 32,000 people in the United States, just 2% of people with ALS have the SOD1 gene mutation. The FDA says the number of people in the United States who could use Qalsody is about 500.
In trials, 147 people received either Qalsody or a placebo, and the treatment significantly reduced the level of a protein in people’s blood that is associated with the loss of control of voluntary muscles.
Because Qalsody received a fast-track approval from the FDA, it must still provide more research data in the future, including from a trial examining how the drug affects people who carry the SOD1 gene but do not yet show symptoms of ALS.
A version of this article first appeared on Medscape.com.
Deoxycholic Acid for Dercum Disease: Repurposing a Cosmetic Agent to Treat a Rare Disease
Dercum disease (or adiposis dolorosa) is a rare condition of unknown etiology characterized by multiple painful lipomas localized throughout the body.1,2 It typically presents in adults aged 35 to 50 years and is at least 5 times more common in women.3 It often is associated with comorbidities such as obesity, fatigue and weakness.1 There currently are no approved treatments for Dercum disease, only therapies tried with little to no efficacy for symptom management, including analgesics, excision, liposuction,1 lymphatic drainage,4 hypobaric pressure,5 and frequency rhythmic electrical modulation systems.6 For patients who continually develop widespread lesions, surgical excision is not feasible, which poses a therapeutic challenge. Deoxycholic acid (DCA), a bile acid that is approved to treat submental fat, disrupts the integrity of cell membranes, induces adipocyte lysis, and solubilizes fat when injected subcutaneously.7 We used DCA to mitigate pain and reduce lipoma size in patients with Dercum disease, which demonstrated lipoma reduction via ultrasonography in 3 patients.
Case Reports
Three patients presented to clinic with multiple painful subcutaneous nodules throughout several areas of the body and were screened using radiography. Ultrasonography demonstrated numerous lipomas consistent with Dercum disease. The lipomas were measured by ultrasonography to obtain 3-dimensional measurements of each lesion. The most painful lipomas identified by the patients were either treated with 2 mL of DCA (10 mg/mL) or served as a control with no treatment. Patients returned for symptom monitoring and repeat measurements of both treated and untreated lipomas. Two physicians with expertise in ultrasonography measured lesions in a blinded fashion. Photographs were obtained with patient consent.
Patient 1—A 45-year-old woman with a family history of lipomas was diagnosed with Dercum disease that was confirmed via ultrasonography. A painful 1.63×1.64×0.55-cm lipoma was measured on the volar aspect of the left forearm, and a 1.17×1.26×0.39-cm lipoma was measured on the volar aspect of the right wrist. At a follow-up visit 11 months later, 2 mL of DCA was administered to the lipoma on the volar aspect of the left forearm, while the lipoma on the volar aspect of the right wrist was monitored as an untreated control. Following the procedure, the patient reported 1 week of swelling and tenderness of the treated area. Repeat imaging 4 months after administration of DCA revealed reduction of the treated lesion to 0.80×1.48×0.60 cm and growth of the untreated lesion to 1.32×2.17×0.52 cm. The treated lipoma reduced in volume by 34.55%, while the lipoma in the untreated control increased in volume from its original measurement by 111.11% (Table). The patient also reported decreased pain in the treated area at all follow-up visits in the 1 year following the procedure.
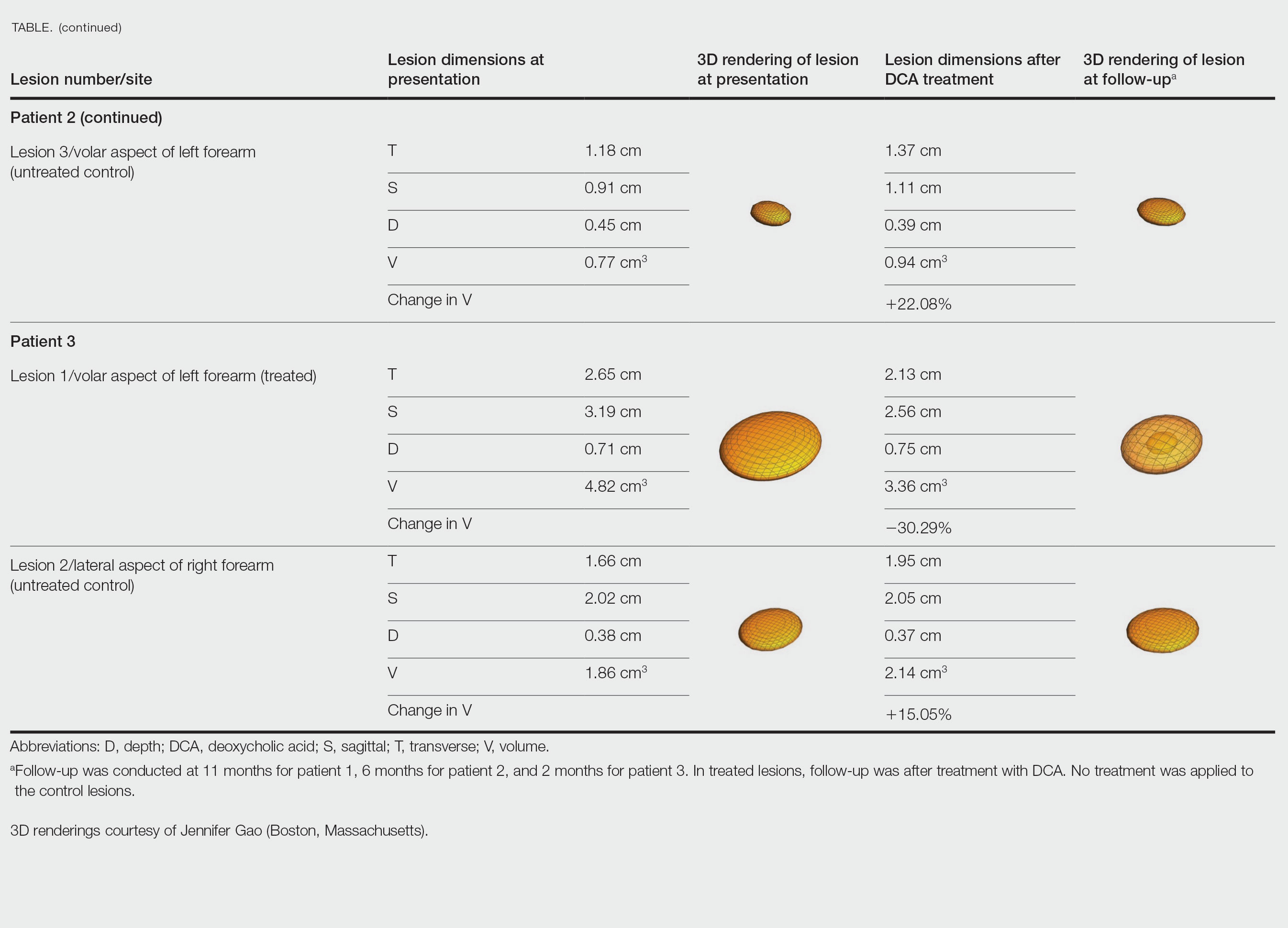
Patient 2—A 42-year-old woman with Dercum disease received administration of 2 mL of DCA to a 1.90×1.70×0.90-cm lipoma of the lateral aspect of the left mid thigh and 2 mL of DCA to a 2.40×3.07×0.60-cm lipoma on the volar aspect of the right forearm 2 weeks later. A 1.18×0.91×0.45-cm lipoma of the volar aspect of the left forearm was monitored as an untreated control. The patient reported bruising and discoloration a few weeks following the procedure. At subsequent 1-month and 3-month follow-ups, the patient reported induration in the volar aspect of the right forearm and noticeable reduction in size of the lesion in the lateral aspect of the left mid thigh. At the 6-month follow-up, the patient reported reduction in size of both lesions and improvement of the previously noted side effects. Repeat ultrasonography approximately 6 months after administration of DCA demonstrated reduction of the treated lesion on the lateral aspect of the left mid thigh to 0.92×0.96×0.57 cm and the volar aspect of the right forearm to 1.56×2.18×0.79 cm, with growth of the untreated lesion on the volar aspect of the left forearm to 1.37×1.11×0.39 cm. The treated lipomas reduced in volume by 68.42% and 41.25%, respectively, and the untreated control increased in volume by 22.08% (Table).
Patient 3—A 75-year-old woman with a family history of lipomas was diagnosed with Dercum disease verified by ultrasonography. The patient was administered 2 mL of DCA to a 2.65×3.19×0.71-cm lipoma of the volar aspect of the left forearm. A 1.66×2.02×0.38-cm lipoma of the lateral aspect of the right forearm was monitored as an untreated control. Following the procedure, the patient reported initial swelling that persisted for a few weeks followed by notable pain relief and a decrease in lipoma size. At 2-month follow-up, the patient reported no pain or other adverse effects, while repeat imaging demonstrated reduction of the treated lesion on the volar aspect of the left forearm to 2.13×2.56×0.75 cm and growth of the untreated lesion on the lateral aspect of the right forearm to 1.95×2.05×0.37 cm. The treated lipoma reduced in volume by 30.29%, and the untreated control increased in volume by 15.05% (Table).
Comment
Deoxycholic acid is a bile acid naturally found in the body that helps to emulsify and solubilize fats in the intestines. When injected subcutaneously, DCA becomes an adipolytic agent that induces inflammation and targets adipose degradation by macrophages, and it has been manufactured to reduce submental fat.7 Off-label use of DCA has been explored for nonsurgical body contouring and lipomas with promising results in some cases; however, these prior studies have been limited by the lack of quantitative objective measurements to effectively demonstrate the impact of treatment.8,9
We present 3 patients who requested treatment for numerous painful lipomas. Given the extent of their disease, surgical options were not feasible, and the patients opted to try a nonsurgical alternative. In each case, the painful lipomas that were chosen for treatment were injected with 2 mL of DCA. Injection-associated symptoms included swelling, tenderness, discoloration, and induration, which resolved over a period of months. Patient 1 had a treated lipoma that reduced in volume by approximately 35%, while the control continued to grow and doubled in volume. In patient 2, the treated lesion on the lateral aspect of the mid thigh reduced in volume by almost 70%, and the treated lesion on the volar aspect of the right forearm reduced in volume by more than 40%, while the control grew by more than 20%. In patient 3, the volume of the treated lipoma decreased by 30%, and the control increased by 15%. The follow-up interval was shortest in patient 3—2 months as opposed to 11 months and 6 months for patients 1 and 2, respectively; therefore, more progress may be seen in patient 3 with more time. Interestingly, a change in shape of the lipoma was noted in patient 3 (Figure)—an increase in its depth while the center became anechoic, which is a sign of hollowing in the center due to the saponification of fat and a possible cause for the change from an elliptical to a more spherical or doughnutlike shape. Intralesional administration of DCA may offer patients with extensive lipomas, such as those seen in patients with Dercum disease, an alternative, less-invasive option to assist with pain and tumor burden when excision is not feasible. Although treatments with DCA can be associated with side effects, including pain, swelling, bruising, erythema, induration, and numbness, all 3 of our patients had ultimate mitigation of pain and reduction in lipoma size within months of the injection. Additional studies should be explored to determine the optimal dose and frequency of administration of DCA that could benefit patients with Dercum disease.
- National Organization for Rare Disorders. Dercum’s disease. Updated March 26, 2020. Accessed March 27, 2023. https://rarediseases.org/rare-diseases/dercums-disease/.
- Kucharz EJ, Kopec´-Me˛drek M, Kramza J, et al. Dercum’s disease (adiposis dolorosa): a review of clinical presentation and management. Reumatologia. 2019;57:281-287. doi:10.5114/reum.2019.89521
- Hansson E, Svensson H, Brorson H. Review of Dercum’s disease and proposal of diagnostic criteria, diagnostic methods, classification and management. Orphanet J Rare Dis. 2012;7:23. doi:10.1186/1750-1172-7-23
- Lange U, Oelzner P, Uhlemann C. Dercum’s disease (Lipomatosis dolorosa): successful therapy with pregabalin and manual lymphatic drainage and a current overview. Rheumatol Int. 2008;29:17-22. doi:10.1007/s00296-008-0635-3
- Herbst KL, Rutledge T. Pilot study: rapidly cycling hypobaric pressure improves pain after 5 days in adiposis dolorosa. J Pain Res. 2010;3:147-153. doi:10.2147/JPR.S12351
- Martinenghi S, Caretto A, Losio C, et al. Successful treatment of Dercum’s disease by transcutaneous electrical stimulation: a case report. Medicine (Baltimore). 2015;94:e950. doi:10.1097/MD.0000000000000950
- National Center for Biotechnology Information. PubChem compound summary for CID 222528, deoxycholic acid. https://pubchem.ncbi.nlm.nih.gov/compound/Deoxycholic-acid. Accessed November 11, 2021.
- Liu C, Li MK, Alster TS. Alternative cosmetic and medical applications of injectable deoxycholic acid: a systematic review. Dermatol Surg. 2021;47:1466-1472. doi:10.1097/DSS.0000000000003159
- Santiago-Vázquez M, Michelen-Gómez EA, Carrasquillo-Bonilla D, et al. Intralesional deoxycholic acid: a potential therapeutic alternative for the treatment of lipomas arising in the face. JAAD Case Rep. 2021;13:112-114. doi:10.1016/j.jdcr.2021.04.037
Dercum disease (or adiposis dolorosa) is a rare condition of unknown etiology characterized by multiple painful lipomas localized throughout the body.1,2 It typically presents in adults aged 35 to 50 years and is at least 5 times more common in women.3 It often is associated with comorbidities such as obesity, fatigue and weakness.1 There currently are no approved treatments for Dercum disease, only therapies tried with little to no efficacy for symptom management, including analgesics, excision, liposuction,1 lymphatic drainage,4 hypobaric pressure,5 and frequency rhythmic electrical modulation systems.6 For patients who continually develop widespread lesions, surgical excision is not feasible, which poses a therapeutic challenge. Deoxycholic acid (DCA), a bile acid that is approved to treat submental fat, disrupts the integrity of cell membranes, induces adipocyte lysis, and solubilizes fat when injected subcutaneously.7 We used DCA to mitigate pain and reduce lipoma size in patients with Dercum disease, which demonstrated lipoma reduction via ultrasonography in 3 patients.
Case Reports
Three patients presented to clinic with multiple painful subcutaneous nodules throughout several areas of the body and were screened using radiography. Ultrasonography demonstrated numerous lipomas consistent with Dercum disease. The lipomas were measured by ultrasonography to obtain 3-dimensional measurements of each lesion. The most painful lipomas identified by the patients were either treated with 2 mL of DCA (10 mg/mL) or served as a control with no treatment. Patients returned for symptom monitoring and repeat measurements of both treated and untreated lipomas. Two physicians with expertise in ultrasonography measured lesions in a blinded fashion. Photographs were obtained with patient consent.
Patient 1—A 45-year-old woman with a family history of lipomas was diagnosed with Dercum disease that was confirmed via ultrasonography. A painful 1.63×1.64×0.55-cm lipoma was measured on the volar aspect of the left forearm, and a 1.17×1.26×0.39-cm lipoma was measured on the volar aspect of the right wrist. At a follow-up visit 11 months later, 2 mL of DCA was administered to the lipoma on the volar aspect of the left forearm, while the lipoma on the volar aspect of the right wrist was monitored as an untreated control. Following the procedure, the patient reported 1 week of swelling and tenderness of the treated area. Repeat imaging 4 months after administration of DCA revealed reduction of the treated lesion to 0.80×1.48×0.60 cm and growth of the untreated lesion to 1.32×2.17×0.52 cm. The treated lipoma reduced in volume by 34.55%, while the lipoma in the untreated control increased in volume from its original measurement by 111.11% (Table). The patient also reported decreased pain in the treated area at all follow-up visits in the 1 year following the procedure.


Patient 2—A 42-year-old woman with Dercum disease received administration of 2 mL of DCA to a 1.90×1.70×0.90-cm lipoma of the lateral aspect of the left mid thigh and 2 mL of DCA to a 2.40×3.07×0.60-cm lipoma on the volar aspect of the right forearm 2 weeks later. A 1.18×0.91×0.45-cm lipoma of the volar aspect of the left forearm was monitored as an untreated control. The patient reported bruising and discoloration a few weeks following the procedure. At subsequent 1-month and 3-month follow-ups, the patient reported induration in the volar aspect of the right forearm and noticeable reduction in size of the lesion in the lateral aspect of the left mid thigh. At the 6-month follow-up, the patient reported reduction in size of both lesions and improvement of the previously noted side effects. Repeat ultrasonography approximately 6 months after administration of DCA demonstrated reduction of the treated lesion on the lateral aspect of the left mid thigh to 0.92×0.96×0.57 cm and the volar aspect of the right forearm to 1.56×2.18×0.79 cm, with growth of the untreated lesion on the volar aspect of the left forearm to 1.37×1.11×0.39 cm. The treated lipomas reduced in volume by 68.42% and 41.25%, respectively, and the untreated control increased in volume by 22.08% (Table).
Patient 3—A 75-year-old woman with a family history of lipomas was diagnosed with Dercum disease verified by ultrasonography. The patient was administered 2 mL of DCA to a 2.65×3.19×0.71-cm lipoma of the volar aspect of the left forearm. A 1.66×2.02×0.38-cm lipoma of the lateral aspect of the right forearm was monitored as an untreated control. Following the procedure, the patient reported initial swelling that persisted for a few weeks followed by notable pain relief and a decrease in lipoma size. At 2-month follow-up, the patient reported no pain or other adverse effects, while repeat imaging demonstrated reduction of the treated lesion on the volar aspect of the left forearm to 2.13×2.56×0.75 cm and growth of the untreated lesion on the lateral aspect of the right forearm to 1.95×2.05×0.37 cm. The treated lipoma reduced in volume by 30.29%, and the untreated control increased in volume by 15.05% (Table).
Comment
Deoxycholic acid is a bile acid naturally found in the body that helps to emulsify and solubilize fats in the intestines. When injected subcutaneously, DCA becomes an adipolytic agent that induces inflammation and targets adipose degradation by macrophages, and it has been manufactured to reduce submental fat.7 Off-label use of DCA has been explored for nonsurgical body contouring and lipomas with promising results in some cases; however, these prior studies have been limited by the lack of quantitative objective measurements to effectively demonstrate the impact of treatment.8,9
We present 3 patients who requested treatment for numerous painful lipomas. Given the extent of their disease, surgical options were not feasible, and the patients opted to try a nonsurgical alternative. In each case, the painful lipomas that were chosen for treatment were injected with 2 mL of DCA. Injection-associated symptoms included swelling, tenderness, discoloration, and induration, which resolved over a period of months. Patient 1 had a treated lipoma that reduced in volume by approximately 35%, while the control continued to grow and doubled in volume. In patient 2, the treated lesion on the lateral aspect of the mid thigh reduced in volume by almost 70%, and the treated lesion on the volar aspect of the right forearm reduced in volume by more than 40%, while the control grew by more than 20%. In patient 3, the volume of the treated lipoma decreased by 30%, and the control increased by 15%. The follow-up interval was shortest in patient 3—2 months as opposed to 11 months and 6 months for patients 1 and 2, respectively; therefore, more progress may be seen in patient 3 with more time. Interestingly, a change in shape of the lipoma was noted in patient 3 (Figure)—an increase in its depth while the center became anechoic, which is a sign of hollowing in the center due to the saponification of fat and a possible cause for the change from an elliptical to a more spherical or doughnutlike shape. Intralesional administration of DCA may offer patients with extensive lipomas, such as those seen in patients with Dercum disease, an alternative, less-invasive option to assist with pain and tumor burden when excision is not feasible. Although treatments with DCA can be associated with side effects, including pain, swelling, bruising, erythema, induration, and numbness, all 3 of our patients had ultimate mitigation of pain and reduction in lipoma size within months of the injection. Additional studies should be explored to determine the optimal dose and frequency of administration of DCA that could benefit patients with Dercum disease.

Dercum disease (or adiposis dolorosa) is a rare condition of unknown etiology characterized by multiple painful lipomas localized throughout the body.1,2 It typically presents in adults aged 35 to 50 years and is at least 5 times more common in women.3 It often is associated with comorbidities such as obesity, fatigue and weakness.1 There currently are no approved treatments for Dercum disease, only therapies tried with little to no efficacy for symptom management, including analgesics, excision, liposuction,1 lymphatic drainage,4 hypobaric pressure,5 and frequency rhythmic electrical modulation systems.6 For patients who continually develop widespread lesions, surgical excision is not feasible, which poses a therapeutic challenge. Deoxycholic acid (DCA), a bile acid that is approved to treat submental fat, disrupts the integrity of cell membranes, induces adipocyte lysis, and solubilizes fat when injected subcutaneously.7 We used DCA to mitigate pain and reduce lipoma size in patients with Dercum disease, which demonstrated lipoma reduction via ultrasonography in 3 patients.
Case Reports
Three patients presented to clinic with multiple painful subcutaneous nodules throughout several areas of the body and were screened using radiography. Ultrasonography demonstrated numerous lipomas consistent with Dercum disease. The lipomas were measured by ultrasonography to obtain 3-dimensional measurements of each lesion. The most painful lipomas identified by the patients were either treated with 2 mL of DCA (10 mg/mL) or served as a control with no treatment. Patients returned for symptom monitoring and repeat measurements of both treated and untreated lipomas. Two physicians with expertise in ultrasonography measured lesions in a blinded fashion. Photographs were obtained with patient consent.
Patient 1—A 45-year-old woman with a family history of lipomas was diagnosed with Dercum disease that was confirmed via ultrasonography. A painful 1.63×1.64×0.55-cm lipoma was measured on the volar aspect of the left forearm, and a 1.17×1.26×0.39-cm lipoma was measured on the volar aspect of the right wrist. At a follow-up visit 11 months later, 2 mL of DCA was administered to the lipoma on the volar aspect of the left forearm, while the lipoma on the volar aspect of the right wrist was monitored as an untreated control. Following the procedure, the patient reported 1 week of swelling and tenderness of the treated area. Repeat imaging 4 months after administration of DCA revealed reduction of the treated lesion to 0.80×1.48×0.60 cm and growth of the untreated lesion to 1.32×2.17×0.52 cm. The treated lipoma reduced in volume by 34.55%, while the lipoma in the untreated control increased in volume from its original measurement by 111.11% (Table). The patient also reported decreased pain in the treated area at all follow-up visits in the 1 year following the procedure.


Patient 2—A 42-year-old woman with Dercum disease received administration of 2 mL of DCA to a 1.90×1.70×0.90-cm lipoma of the lateral aspect of the left mid thigh and 2 mL of DCA to a 2.40×3.07×0.60-cm lipoma on the volar aspect of the right forearm 2 weeks later. A 1.18×0.91×0.45-cm lipoma of the volar aspect of the left forearm was monitored as an untreated control. The patient reported bruising and discoloration a few weeks following the procedure. At subsequent 1-month and 3-month follow-ups, the patient reported induration in the volar aspect of the right forearm and noticeable reduction in size of the lesion in the lateral aspect of the left mid thigh. At the 6-month follow-up, the patient reported reduction in size of both lesions and improvement of the previously noted side effects. Repeat ultrasonography approximately 6 months after administration of DCA demonstrated reduction of the treated lesion on the lateral aspect of the left mid thigh to 0.92×0.96×0.57 cm and the volar aspect of the right forearm to 1.56×2.18×0.79 cm, with growth of the untreated lesion on the volar aspect of the left forearm to 1.37×1.11×0.39 cm. The treated lipomas reduced in volume by 68.42% and 41.25%, respectively, and the untreated control increased in volume by 22.08% (Table).
Patient 3—A 75-year-old woman with a family history of lipomas was diagnosed with Dercum disease verified by ultrasonography. The patient was administered 2 mL of DCA to a 2.65×3.19×0.71-cm lipoma of the volar aspect of the left forearm. A 1.66×2.02×0.38-cm lipoma of the lateral aspect of the right forearm was monitored as an untreated control. Following the procedure, the patient reported initial swelling that persisted for a few weeks followed by notable pain relief and a decrease in lipoma size. At 2-month follow-up, the patient reported no pain or other adverse effects, while repeat imaging demonstrated reduction of the treated lesion on the volar aspect of the left forearm to 2.13×2.56×0.75 cm and growth of the untreated lesion on the lateral aspect of the right forearm to 1.95×2.05×0.37 cm. The treated lipoma reduced in volume by 30.29%, and the untreated control increased in volume by 15.05% (Table).
Comment
Deoxycholic acid is a bile acid naturally found in the body that helps to emulsify and solubilize fats in the intestines. When injected subcutaneously, DCA becomes an adipolytic agent that induces inflammation and targets adipose degradation by macrophages, and it has been manufactured to reduce submental fat.7 Off-label use of DCA has been explored for nonsurgical body contouring and lipomas with promising results in some cases; however, these prior studies have been limited by the lack of quantitative objective measurements to effectively demonstrate the impact of treatment.8,9
We present 3 patients who requested treatment for numerous painful lipomas. Given the extent of their disease, surgical options were not feasible, and the patients opted to try a nonsurgical alternative. In each case, the painful lipomas that were chosen for treatment were injected with 2 mL of DCA. Injection-associated symptoms included swelling, tenderness, discoloration, and induration, which resolved over a period of months. Patient 1 had a treated lipoma that reduced in volume by approximately 35%, while the control continued to grow and doubled in volume. In patient 2, the treated lesion on the lateral aspect of the mid thigh reduced in volume by almost 70%, and the treated lesion on the volar aspect of the right forearm reduced in volume by more than 40%, while the control grew by more than 20%. In patient 3, the volume of the treated lipoma decreased by 30%, and the control increased by 15%. The follow-up interval was shortest in patient 3—2 months as opposed to 11 months and 6 months for patients 1 and 2, respectively; therefore, more progress may be seen in patient 3 with more time. Interestingly, a change in shape of the lipoma was noted in patient 3 (Figure)—an increase in its depth while the center became anechoic, which is a sign of hollowing in the center due to the saponification of fat and a possible cause for the change from an elliptical to a more spherical or doughnutlike shape. Intralesional administration of DCA may offer patients with extensive lipomas, such as those seen in patients with Dercum disease, an alternative, less-invasive option to assist with pain and tumor burden when excision is not feasible. Although treatments with DCA can be associated with side effects, including pain, swelling, bruising, erythema, induration, and numbness, all 3 of our patients had ultimate mitigation of pain and reduction in lipoma size within months of the injection. Additional studies should be explored to determine the optimal dose and frequency of administration of DCA that could benefit patients with Dercum disease.

- National Organization for Rare Disorders. Dercum’s disease. Updated March 26, 2020. Accessed March 27, 2023. https://rarediseases.org/rare-diseases/dercums-disease/.
- Kucharz EJ, Kopec´-Me˛drek M, Kramza J, et al. Dercum’s disease (adiposis dolorosa): a review of clinical presentation and management. Reumatologia. 2019;57:281-287. doi:10.5114/reum.2019.89521
- Hansson E, Svensson H, Brorson H. Review of Dercum’s disease and proposal of diagnostic criteria, diagnostic methods, classification and management. Orphanet J Rare Dis. 2012;7:23. doi:10.1186/1750-1172-7-23
- Lange U, Oelzner P, Uhlemann C. Dercum’s disease (Lipomatosis dolorosa): successful therapy with pregabalin and manual lymphatic drainage and a current overview. Rheumatol Int. 2008;29:17-22. doi:10.1007/s00296-008-0635-3
- Herbst KL, Rutledge T. Pilot study: rapidly cycling hypobaric pressure improves pain after 5 days in adiposis dolorosa. J Pain Res. 2010;3:147-153. doi:10.2147/JPR.S12351
- Martinenghi S, Caretto A, Losio C, et al. Successful treatment of Dercum’s disease by transcutaneous electrical stimulation: a case report. Medicine (Baltimore). 2015;94:e950. doi:10.1097/MD.0000000000000950
- National Center for Biotechnology Information. PubChem compound summary for CID 222528, deoxycholic acid. https://pubchem.ncbi.nlm.nih.gov/compound/Deoxycholic-acid. Accessed November 11, 2021.
- Liu C, Li MK, Alster TS. Alternative cosmetic and medical applications of injectable deoxycholic acid: a systematic review. Dermatol Surg. 2021;47:1466-1472. doi:10.1097/DSS.0000000000003159
- Santiago-Vázquez M, Michelen-Gómez EA, Carrasquillo-Bonilla D, et al. Intralesional deoxycholic acid: a potential therapeutic alternative for the treatment of lipomas arising in the face. JAAD Case Rep. 2021;13:112-114. doi:10.1016/j.jdcr.2021.04.037
- National Organization for Rare Disorders. Dercum’s disease. Updated March 26, 2020. Accessed March 27, 2023. https://rarediseases.org/rare-diseases/dercums-disease/.
- Kucharz EJ, Kopec´-Me˛drek M, Kramza J, et al. Dercum’s disease (adiposis dolorosa): a review of clinical presentation and management. Reumatologia. 2019;57:281-287. doi:10.5114/reum.2019.89521
- Hansson E, Svensson H, Brorson H. Review of Dercum’s disease and proposal of diagnostic criteria, diagnostic methods, classification and management. Orphanet J Rare Dis. 2012;7:23. doi:10.1186/1750-1172-7-23
- Lange U, Oelzner P, Uhlemann C. Dercum’s disease (Lipomatosis dolorosa): successful therapy with pregabalin and manual lymphatic drainage and a current overview. Rheumatol Int. 2008;29:17-22. doi:10.1007/s00296-008-0635-3
- Herbst KL, Rutledge T. Pilot study: rapidly cycling hypobaric pressure improves pain after 5 days in adiposis dolorosa. J Pain Res. 2010;3:147-153. doi:10.2147/JPR.S12351
- Martinenghi S, Caretto A, Losio C, et al. Successful treatment of Dercum’s disease by transcutaneous electrical stimulation: a case report. Medicine (Baltimore). 2015;94:e950. doi:10.1097/MD.0000000000000950
- National Center for Biotechnology Information. PubChem compound summary for CID 222528, deoxycholic acid. https://pubchem.ncbi.nlm.nih.gov/compound/Deoxycholic-acid. Accessed November 11, 2021.
- Liu C, Li MK, Alster TS. Alternative cosmetic and medical applications of injectable deoxycholic acid: a systematic review. Dermatol Surg. 2021;47:1466-1472. doi:10.1097/DSS.0000000000003159
- Santiago-Vázquez M, Michelen-Gómez EA, Carrasquillo-Bonilla D, et al. Intralesional deoxycholic acid: a potential therapeutic alternative for the treatment of lipomas arising in the face. JAAD Case Rep. 2021;13:112-114. doi:10.1016/j.jdcr.2021.04.037
Practice Points
- Dermatologists should consider Dercum disease when encountering a patient with numerous painful lipomas.
- Subcutaneous administration of deoxycholic acid resulted in a notable reduction in pain and size of lipomas by 30% to 68% per radiographic review.
- Deoxycholic acid may provide an alternative therapeutic option for patients who have Dercum disease with substantial tumor burden.
Pembrolizumab monotherapy effective for rare melanoma
The findings could represent a new standard of treatment for this extremely rare tumor.
The study was inspired by a previous retrospective analysis which found an overall response rate of 77% and a complete response of 32% to anti–PD-1 monotherapy.
The ORR is about double what is seen in melanoma more generally, according to Kari Kendra, MD, PhD, who presented the study at the annual meeting of the American Association for Cancer Research.
“Our study was a positive study. Of note, in the retrospective study, they saw a complete response rate of 32%, which was amazingly similar to what we found. [The findings support] the use of single agent anti–PD-1 immunotherapy as first line treatment for most patients with unresectable desmoplastic melanoma. [There was 89% overall response and we saw] dramatic responses across the board,” said Dr. Kendra, who is a medical oncologist at Ohio State University Wexner Medical Center, Columbus.
The findings drew a strong reaction. “In a rare tumor session, to see response curves like that, it’s just outstanding,” said the session’s cochair Brian Van Tine, MD, PhD, who is a professor of medical oncology at Washington University in St. Louis.
“This really is one of the highest tumor response rates to immunotherapy that we are seeing in any cancer. And I think may also highlight the fact that we shouldn’t think of all cutaneous melanomas as one disease, given the heterogeneity in tumor responses based on some of the pathologic and molecular characteristics,” said Zeynep Aroglu, MD, who served as a discussant but was also one of the investigators who enrolled patients for the trial.
Desmoplastic melanoma represents about 4% of all cutaneous melanoma diagnoses, and its unique pathology can make it difficult to diagnose. That often leads to a late diagnosis, according to Dr. Aroglu. They typically occur in elderly patients, in the head and neck area, and are associated with sun exposure. DM also tends to have a high mutation burden, Dr. Aroglu said during the session.
It remains to be seen why there is such a high response rate in this tumor type, even among tumor types with mutation burdens that are nearly as high. DM tumors are often driven by neurofibromatosis type 1, but other tumors driven by NF-1 don’t have as high of a response rate to immunotherapy. The tumor environment could also play a role, she said.
“Is it a combination of all these factors? I think some of the ongoing analysis of tumor samples that Dr. Kendra mentioned may help to answer some of these questions,” Dr. Aroglu continued.
She also noted that the melanoma field is increasingly turning to combination of anti–PD-1 therapy with agents like that target LAG3 or CTLA4. Such combinations can achieve higher response rates, but at a cost of higher rates of grade 3-4 adverse events than anti–PD-1 inhibitors alone. “I wonder if for desmoplastic melanomas in light of this data, do we consider de-escalating therapy, given these very high response rates to PD-1 alone, given also the elderly age of many of these patients, because even the PD-1–LAG3 combo still has a higher rate of toxicity than PD-1 monotherapy. Perhaps the immunotherapy combinations can be reserved for those rare desmoplastic patients who are resistant to PD-1 alone,” said Dr. Aroglu.
Study details and adverse events
Twenty-seven patients were enrolled in the study; 93% were male, all were White, and 22% had elevated baseline lactate dehydrogenase. About 63% had disease located in the head and neck area, 33% experienced a complete response (P < .001), and 56% had a partial response for an ORR of 89%. The result surpassed the primary endpoint target of at least a 20% complete response rate.
The 2-year progression-free survival was 74%, and 2-year overall survival was 89%. The most common toxicities were fatigue (56%), diarrhea (33%), maculopapular rash (30%), pruritus (22%), anemia (19%), arthralgia (19%), and decreased lymphocyte count (19%). There were two grade 4 adverse events: a lipase increase and a lung infection accompanied by sepsis.
The researchers also carried out whole exome sequencing of biopsies and found that 67% had NF-1 loss of function mutations.
Dr. Aroglu has served on advisory boards for Pfizer, Array, Eisai, Genentech, Natera, Novartis, OncoSec, and Regeneron. She has received research support from Boehringer Ingelheim, Pfizer, and Novartis. Dr. Kendra has received institutional support from Bristol Myers-Squibb and trial support from CheckMate Pharmaceuticals, GlaxoSmithKline, Immunocore, Medspace, Merck, Novartis, and Varian Medical Systems. Dr. Van Tine has financial relationships with a wide range of pharmaceutical companies.
The findings could represent a new standard of treatment for this extremely rare tumor.
The study was inspired by a previous retrospective analysis which found an overall response rate of 77% and a complete response of 32% to anti–PD-1 monotherapy.
The ORR is about double what is seen in melanoma more generally, according to Kari Kendra, MD, PhD, who presented the study at the annual meeting of the American Association for Cancer Research.
“Our study was a positive study. Of note, in the retrospective study, they saw a complete response rate of 32%, which was amazingly similar to what we found. [The findings support] the use of single agent anti–PD-1 immunotherapy as first line treatment for most patients with unresectable desmoplastic melanoma. [There was 89% overall response and we saw] dramatic responses across the board,” said Dr. Kendra, who is a medical oncologist at Ohio State University Wexner Medical Center, Columbus.
The findings drew a strong reaction. “In a rare tumor session, to see response curves like that, it’s just outstanding,” said the session’s cochair Brian Van Tine, MD, PhD, who is a professor of medical oncology at Washington University in St. Louis.
“This really is one of the highest tumor response rates to immunotherapy that we are seeing in any cancer. And I think may also highlight the fact that we shouldn’t think of all cutaneous melanomas as one disease, given the heterogeneity in tumor responses based on some of the pathologic and molecular characteristics,” said Zeynep Aroglu, MD, who served as a discussant but was also one of the investigators who enrolled patients for the trial.
Desmoplastic melanoma represents about 4% of all cutaneous melanoma diagnoses, and its unique pathology can make it difficult to diagnose. That often leads to a late diagnosis, according to Dr. Aroglu. They typically occur in elderly patients, in the head and neck area, and are associated with sun exposure. DM also tends to have a high mutation burden, Dr. Aroglu said during the session.
It remains to be seen why there is such a high response rate in this tumor type, even among tumor types with mutation burdens that are nearly as high. DM tumors are often driven by neurofibromatosis type 1, but other tumors driven by NF-1 don’t have as high of a response rate to immunotherapy. The tumor environment could also play a role, she said.
“Is it a combination of all these factors? I think some of the ongoing analysis of tumor samples that Dr. Kendra mentioned may help to answer some of these questions,” Dr. Aroglu continued.
She also noted that the melanoma field is increasingly turning to combination of anti–PD-1 therapy with agents like that target LAG3 or CTLA4. Such combinations can achieve higher response rates, but at a cost of higher rates of grade 3-4 adverse events than anti–PD-1 inhibitors alone. “I wonder if for desmoplastic melanomas in light of this data, do we consider de-escalating therapy, given these very high response rates to PD-1 alone, given also the elderly age of many of these patients, because even the PD-1–LAG3 combo still has a higher rate of toxicity than PD-1 monotherapy. Perhaps the immunotherapy combinations can be reserved for those rare desmoplastic patients who are resistant to PD-1 alone,” said Dr. Aroglu.
Study details and adverse events
Twenty-seven patients were enrolled in the study; 93% were male, all were White, and 22% had elevated baseline lactate dehydrogenase. About 63% had disease located in the head and neck area, 33% experienced a complete response (P < .001), and 56% had a partial response for an ORR of 89%. The result surpassed the primary endpoint target of at least a 20% complete response rate.
The 2-year progression-free survival was 74%, and 2-year overall survival was 89%. The most common toxicities were fatigue (56%), diarrhea (33%), maculopapular rash (30%), pruritus (22%), anemia (19%), arthralgia (19%), and decreased lymphocyte count (19%). There were two grade 4 adverse events: a lipase increase and a lung infection accompanied by sepsis.
The researchers also carried out whole exome sequencing of biopsies and found that 67% had NF-1 loss of function mutations.
Dr. Aroglu has served on advisory boards for Pfizer, Array, Eisai, Genentech, Natera, Novartis, OncoSec, and Regeneron. She has received research support from Boehringer Ingelheim, Pfizer, and Novartis. Dr. Kendra has received institutional support from Bristol Myers-Squibb and trial support from CheckMate Pharmaceuticals, GlaxoSmithKline, Immunocore, Medspace, Merck, Novartis, and Varian Medical Systems. Dr. Van Tine has financial relationships with a wide range of pharmaceutical companies.
The findings could represent a new standard of treatment for this extremely rare tumor.
The study was inspired by a previous retrospective analysis which found an overall response rate of 77% and a complete response of 32% to anti–PD-1 monotherapy.
The ORR is about double what is seen in melanoma more generally, according to Kari Kendra, MD, PhD, who presented the study at the annual meeting of the American Association for Cancer Research.
“Our study was a positive study. Of note, in the retrospective study, they saw a complete response rate of 32%, which was amazingly similar to what we found. [The findings support] the use of single agent anti–PD-1 immunotherapy as first line treatment for most patients with unresectable desmoplastic melanoma. [There was 89% overall response and we saw] dramatic responses across the board,” said Dr. Kendra, who is a medical oncologist at Ohio State University Wexner Medical Center, Columbus.
The findings drew a strong reaction. “In a rare tumor session, to see response curves like that, it’s just outstanding,” said the session’s cochair Brian Van Tine, MD, PhD, who is a professor of medical oncology at Washington University in St. Louis.
“This really is one of the highest tumor response rates to immunotherapy that we are seeing in any cancer. And I think may also highlight the fact that we shouldn’t think of all cutaneous melanomas as one disease, given the heterogeneity in tumor responses based on some of the pathologic and molecular characteristics,” said Zeynep Aroglu, MD, who served as a discussant but was also one of the investigators who enrolled patients for the trial.
Desmoplastic melanoma represents about 4% of all cutaneous melanoma diagnoses, and its unique pathology can make it difficult to diagnose. That often leads to a late diagnosis, according to Dr. Aroglu. They typically occur in elderly patients, in the head and neck area, and are associated with sun exposure. DM also tends to have a high mutation burden, Dr. Aroglu said during the session.
It remains to be seen why there is such a high response rate in this tumor type, even among tumor types with mutation burdens that are nearly as high. DM tumors are often driven by neurofibromatosis type 1, but other tumors driven by NF-1 don’t have as high of a response rate to immunotherapy. The tumor environment could also play a role, she said.
“Is it a combination of all these factors? I think some of the ongoing analysis of tumor samples that Dr. Kendra mentioned may help to answer some of these questions,” Dr. Aroglu continued.
She also noted that the melanoma field is increasingly turning to combination of anti–PD-1 therapy with agents like that target LAG3 or CTLA4. Such combinations can achieve higher response rates, but at a cost of higher rates of grade 3-4 adverse events than anti–PD-1 inhibitors alone. “I wonder if for desmoplastic melanomas in light of this data, do we consider de-escalating therapy, given these very high response rates to PD-1 alone, given also the elderly age of many of these patients, because even the PD-1–LAG3 combo still has a higher rate of toxicity than PD-1 monotherapy. Perhaps the immunotherapy combinations can be reserved for those rare desmoplastic patients who are resistant to PD-1 alone,” said Dr. Aroglu.
Study details and adverse events
Twenty-seven patients were enrolled in the study; 93% were male, all were White, and 22% had elevated baseline lactate dehydrogenase. About 63% had disease located in the head and neck area, 33% experienced a complete response (P < .001), and 56% had a partial response for an ORR of 89%. The result surpassed the primary endpoint target of at least a 20% complete response rate.
The 2-year progression-free survival was 74%, and 2-year overall survival was 89%. The most common toxicities were fatigue (56%), diarrhea (33%), maculopapular rash (30%), pruritus (22%), anemia (19%), arthralgia (19%), and decreased lymphocyte count (19%). There were two grade 4 adverse events: a lipase increase and a lung infection accompanied by sepsis.
The researchers also carried out whole exome sequencing of biopsies and found that 67% had NF-1 loss of function mutations.
Dr. Aroglu has served on advisory boards for Pfizer, Array, Eisai, Genentech, Natera, Novartis, OncoSec, and Regeneron. She has received research support from Boehringer Ingelheim, Pfizer, and Novartis. Dr. Kendra has received institutional support from Bristol Myers-Squibb and trial support from CheckMate Pharmaceuticals, GlaxoSmithKline, Immunocore, Medspace, Merck, Novartis, and Varian Medical Systems. Dr. Van Tine has financial relationships with a wide range of pharmaceutical companies.
FROM AACR 2023
Cocaine damage can be misdiagnosed as nasal vasculitis
Nasal damage from cocaine use can be misdiagnosed as a rare, nonthreatening nasal disease, according to researchers from the United Kingdom.
Granulomatosis with polyangiitis (GPA), a disorder which causes inflammation in the nose, sinuses, throat, lungs, and kidneys, can have similar symptoms to cocaine-induced vasculitis, the researchers wrote. Drug testing can help identify patients who have cocaine-induced disease, they argued.
“Patients with destructive nasal lesions, especially young patients, should have urine toxicology performed for cocaine before diagnosing GPA and considering immunosuppressive therapy,” the authors wrote.
The paper was published in Rheumatology Advances in Practice.
Cocaine is the second-most popular drug in the United Kingdom, with 2.0% of people aged 16-59 years reporting using the drug in the past year. In the United States, about 1.7% of people aged 12 years and older (about 4.8 million people) used cocaine in the last 12 months, according to the 2021 National Survey on Drug Use and Health. The drug can cause midline destructive lesions, skin rash, and other vascular problems, and it can also trigger the production of antineutrophil cytoplasmic antibodies (ANCA) that lead to a clinical presentation that mimics GPA, which can make diagnosis more difficult. Treating cocaine-induced disease with immunosuppressant medication can be ineffective if the patient does not stop using the drug, and can have dangerous side effects, previous case studies suggest.
To better understand cocaine-induced disease, researchers conducted a review of patients who visited vasculitis clinics at Queen Elizabeth Hospital in Birmingham, England, and at the Royal Free Hospital in London between 2016 and 2021. They identified 42 patients with GPA-like symptoms who disclosed cocaine use or tested positive for the drug in urine toxicology test. The study included 23 men, 18 women, and 1 individual who did not identify with either gender. The median age was 41 years, and most patients were white.
Of those who underwent drug testing, more than 85% were positive. Nine patients who denied ever using cocaine were positive for the drug and 11 patients who said they were ex-users also tested positive via urine analysis. During clinical examinations, 30 patients had evidence of septal perforation, of which 6 had oronasal fistulas. Most patients’ symptoms were limited to the upper respiratory tract, though 12 did have other systemic symptoms, including skin lesions, joint pain, breathlessness, fatigue, and diplopia. Of the patients who received blood tests for ANCA, 87.5% tested positive for the antibodies.
The researchers noted that patients who continued cocaine use did not see improvement of symptoms, even if they were treated with immunosuppressant drugs.
“The experience in our two different centers suggests that discontinuation of cocaine is required to manage patients and that symptoms will persist despite immunosuppression if there is ongoing cocaine use,” the authors wrote.
“It can feel like chasing your tail at times if you’re trying to treat the inflammation but the real culprit – what’s driving the inflammation – is persistent,” Lindsay S. Lally, MD, a rheumatologist at the Hospital for Special Surgery in New York, said in an interview. She was not involved with the work.
Dr. Lally said the paper had a decent-sized cohort, and “helps us recognize that cocaine use is probably an under-recognized mimic of GPA, even though it’s something we all learn about and talk about.” She added that routine toxicology screening for patients deserves some consideration, though asking patients to complete a drug test could also undermine trust in the doctor-patient relationship. Patients who deny cocaine use may leave the office without providing a urine sample.
If Dr. Lally does suspect cocaine may be the cause of a patient’s systems, having a candid conversation with the patient may have a better chance at getting a patient to open up about their potential drug use. In practice, this means explaining “why it’s so important for me as their partner in this treatment to understand what factors are at play, and how dangerous it could potentially be if I was giving strong immunosuppressive medications [for a condition] that is being induced by a drug,” she said. “I do think that partnership and talking to the patients, at least in many patients, is more helpful than sort of the ‘gotcha’ moment” that can happen with drug testing.
The study authors disclosed no relevant financial relationships. Dr. Lally reported receiving consulting fees from Amgen.
A version of this article first appeared on Medscape.com.
Nasal damage from cocaine use can be misdiagnosed as a rare, nonthreatening nasal disease, according to researchers from the United Kingdom.
Granulomatosis with polyangiitis (GPA), a disorder which causes inflammation in the nose, sinuses, throat, lungs, and kidneys, can have similar symptoms to cocaine-induced vasculitis, the researchers wrote. Drug testing can help identify patients who have cocaine-induced disease, they argued.
“Patients with destructive nasal lesions, especially young patients, should have urine toxicology performed for cocaine before diagnosing GPA and considering immunosuppressive therapy,” the authors wrote.
The paper was published in Rheumatology Advances in Practice.
Cocaine is the second-most popular drug in the United Kingdom, with 2.0% of people aged 16-59 years reporting using the drug in the past year. In the United States, about 1.7% of people aged 12 years and older (about 4.8 million people) used cocaine in the last 12 months, according to the 2021 National Survey on Drug Use and Health. The drug can cause midline destructive lesions, skin rash, and other vascular problems, and it can also trigger the production of antineutrophil cytoplasmic antibodies (ANCA) that lead to a clinical presentation that mimics GPA, which can make diagnosis more difficult. Treating cocaine-induced disease with immunosuppressant medication can be ineffective if the patient does not stop using the drug, and can have dangerous side effects, previous case studies suggest.
To better understand cocaine-induced disease, researchers conducted a review of patients who visited vasculitis clinics at Queen Elizabeth Hospital in Birmingham, England, and at the Royal Free Hospital in London between 2016 and 2021. They identified 42 patients with GPA-like symptoms who disclosed cocaine use or tested positive for the drug in urine toxicology test. The study included 23 men, 18 women, and 1 individual who did not identify with either gender. The median age was 41 years, and most patients were white.
Of those who underwent drug testing, more than 85% were positive. Nine patients who denied ever using cocaine were positive for the drug and 11 patients who said they were ex-users also tested positive via urine analysis. During clinical examinations, 30 patients had evidence of septal perforation, of which 6 had oronasal fistulas. Most patients’ symptoms were limited to the upper respiratory tract, though 12 did have other systemic symptoms, including skin lesions, joint pain, breathlessness, fatigue, and diplopia. Of the patients who received blood tests for ANCA, 87.5% tested positive for the antibodies.
The researchers noted that patients who continued cocaine use did not see improvement of symptoms, even if they were treated with immunosuppressant drugs.
“The experience in our two different centers suggests that discontinuation of cocaine is required to manage patients and that symptoms will persist despite immunosuppression if there is ongoing cocaine use,” the authors wrote.
“It can feel like chasing your tail at times if you’re trying to treat the inflammation but the real culprit – what’s driving the inflammation – is persistent,” Lindsay S. Lally, MD, a rheumatologist at the Hospital for Special Surgery in New York, said in an interview. She was not involved with the work.
Dr. Lally said the paper had a decent-sized cohort, and “helps us recognize that cocaine use is probably an under-recognized mimic of GPA, even though it’s something we all learn about and talk about.” She added that routine toxicology screening for patients deserves some consideration, though asking patients to complete a drug test could also undermine trust in the doctor-patient relationship. Patients who deny cocaine use may leave the office without providing a urine sample.
If Dr. Lally does suspect cocaine may be the cause of a patient’s systems, having a candid conversation with the patient may have a better chance at getting a patient to open up about their potential drug use. In practice, this means explaining “why it’s so important for me as their partner in this treatment to understand what factors are at play, and how dangerous it could potentially be if I was giving strong immunosuppressive medications [for a condition] that is being induced by a drug,” she said. “I do think that partnership and talking to the patients, at least in many patients, is more helpful than sort of the ‘gotcha’ moment” that can happen with drug testing.
The study authors disclosed no relevant financial relationships. Dr. Lally reported receiving consulting fees from Amgen.
A version of this article first appeared on Medscape.com.
Nasal damage from cocaine use can be misdiagnosed as a rare, nonthreatening nasal disease, according to researchers from the United Kingdom.
Granulomatosis with polyangiitis (GPA), a disorder which causes inflammation in the nose, sinuses, throat, lungs, and kidneys, can have similar symptoms to cocaine-induced vasculitis, the researchers wrote. Drug testing can help identify patients who have cocaine-induced disease, they argued.
“Patients with destructive nasal lesions, especially young patients, should have urine toxicology performed for cocaine before diagnosing GPA and considering immunosuppressive therapy,” the authors wrote.
The paper was published in Rheumatology Advances in Practice.
Cocaine is the second-most popular drug in the United Kingdom, with 2.0% of people aged 16-59 years reporting using the drug in the past year. In the United States, about 1.7% of people aged 12 years and older (about 4.8 million people) used cocaine in the last 12 months, according to the 2021 National Survey on Drug Use and Health. The drug can cause midline destructive lesions, skin rash, and other vascular problems, and it can also trigger the production of antineutrophil cytoplasmic antibodies (ANCA) that lead to a clinical presentation that mimics GPA, which can make diagnosis more difficult. Treating cocaine-induced disease with immunosuppressant medication can be ineffective if the patient does not stop using the drug, and can have dangerous side effects, previous case studies suggest.
To better understand cocaine-induced disease, researchers conducted a review of patients who visited vasculitis clinics at Queen Elizabeth Hospital in Birmingham, England, and at the Royal Free Hospital in London between 2016 and 2021. They identified 42 patients with GPA-like symptoms who disclosed cocaine use or tested positive for the drug in urine toxicology test. The study included 23 men, 18 women, and 1 individual who did not identify with either gender. The median age was 41 years, and most patients were white.
Of those who underwent drug testing, more than 85% were positive. Nine patients who denied ever using cocaine were positive for the drug and 11 patients who said they were ex-users also tested positive via urine analysis. During clinical examinations, 30 patients had evidence of septal perforation, of which 6 had oronasal fistulas. Most patients’ symptoms were limited to the upper respiratory tract, though 12 did have other systemic symptoms, including skin lesions, joint pain, breathlessness, fatigue, and diplopia. Of the patients who received blood tests for ANCA, 87.5% tested positive for the antibodies.
The researchers noted that patients who continued cocaine use did not see improvement of symptoms, even if they were treated with immunosuppressant drugs.
“The experience in our two different centers suggests that discontinuation of cocaine is required to manage patients and that symptoms will persist despite immunosuppression if there is ongoing cocaine use,” the authors wrote.
“It can feel like chasing your tail at times if you’re trying to treat the inflammation but the real culprit – what’s driving the inflammation – is persistent,” Lindsay S. Lally, MD, a rheumatologist at the Hospital for Special Surgery in New York, said in an interview. She was not involved with the work.
Dr. Lally said the paper had a decent-sized cohort, and “helps us recognize that cocaine use is probably an under-recognized mimic of GPA, even though it’s something we all learn about and talk about.” She added that routine toxicology screening for patients deserves some consideration, though asking patients to complete a drug test could also undermine trust in the doctor-patient relationship. Patients who deny cocaine use may leave the office without providing a urine sample.
If Dr. Lally does suspect cocaine may be the cause of a patient’s systems, having a candid conversation with the patient may have a better chance at getting a patient to open up about their potential drug use. In practice, this means explaining “why it’s so important for me as their partner in this treatment to understand what factors are at play, and how dangerous it could potentially be if I was giving strong immunosuppressive medications [for a condition] that is being induced by a drug,” she said. “I do think that partnership and talking to the patients, at least in many patients, is more helpful than sort of the ‘gotcha’ moment” that can happen with drug testing.
The study authors disclosed no relevant financial relationships. Dr. Lally reported receiving consulting fees from Amgen.
A version of this article first appeared on Medscape.com.
FROM RHEUMATOLOGY ADVANCES IN PRACTICE