User login
Brachioradial Pruritus: An Etiologic Review and Treatment Summary
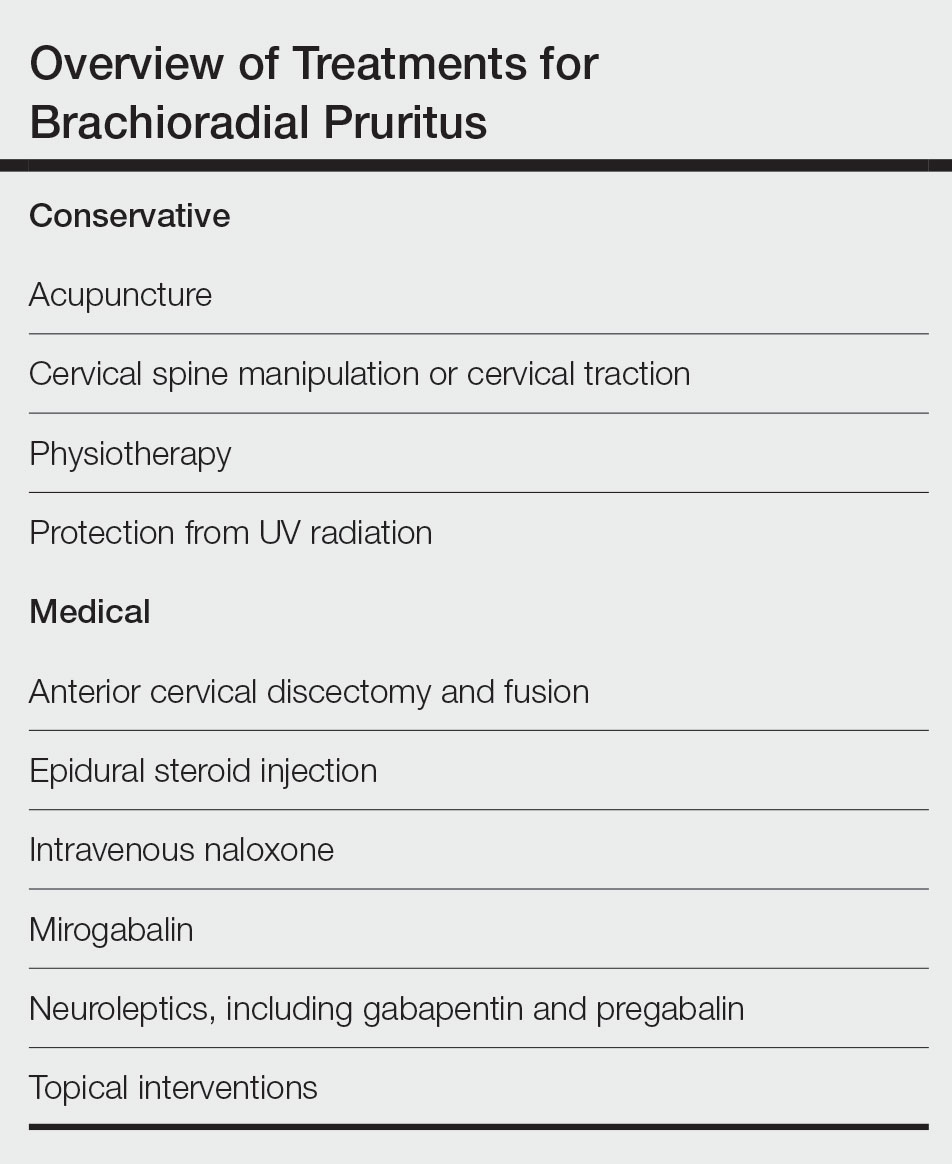
Brachioradial pruritus (BRP) is a neuropathic condition typically characterized by localized dysesthesia of the dorsolateral arms.1 This dysesthesia has been described as a persistent painful itching, burning, tingling, or stinging sensation2-4 and has a median duration of expression of 24 months.5,6 The condition may be unilateral or bilateral in nature but tends to have a predilection for a bilateral distribution along the C5 to C6 dermatomes.1,7,8 There are no primary skin lesions associated with BRP; however, excoriations, prurigo nodules, and lichenification may arise secondary to scratching of the irritated skin.1,4,5,9 Brachioradial pruritus tends to have a predilection for adult females (3:1 ratio) with lighter skin. The mean age at diagnosis is 59 years, but cases have been reported in patients aged 12 to 84 years.1,5 The diagnosis of BRP is based on clinical signs and symptoms, though the ice-pack sign tends to be pathognomonic for the diagnosis.10,11 Although there is no clear evidence on the exact cause of BRP, there are 2 prevalent theories: cervical radiculopathy secondary to cervical spine pathology and/or excessive exposure to UV radiation (UVR) in the summer months.3-5,12 Brachioradial pruritus remains poorly described in the literature, and even its origin is under debate. As such, the clinician may have difficulty deciding on the best course of management. The goal of this article is to identify and discuss known treatment options for BRP (Table).
Etiology
Cervical Spine Pathology—A correlation appears to exist between BRP and cervical spine changes seen on plain film radiographs at the levels of C3 to C7, with increased incidence at the C5 to C6 levels. These plain film radiographs typically show degenerative joint disease and neural foraminal stenosis at levels that correlate to the dermatomal distribution of BRP.1,7,10,12-14 In addition to plain film radiography, some studies have utilized magnetic resonance imaging to view the cervical spine and have documented evidence of intervertebral disc protrusion/bulging, central canal stenosis, neuroforaminal stenosis, and spondylosis at the affected regions.5,15-17 Moreover, supporting the theory that the cervical spine is responsible for the emergence of BRP, Marziniak et al17 investigated 41 patients with BRP utilizing magnetic resonance tomography to find that 33 patients (80.5%) had changes in nerve compression, and 8 patients (19.5%) had degenerative changes. In addition to these findings, they found that there was a significant correlation (P<.01) between the dermatomal expression of BRP and the location of cervical anatomical changes.17 Further validating the relationship between cervical spine pathology and BRP is a case study of a patient who saw rapid and complete resolution of the pruritus following spinal decompression surgery.10 Another case study described an intramedullary tumor found in a patient with BRP that was diagnosed as an ependymoma after magnetic resonance imaging revealed an intramedullary lesion within the spinal cord between C4 and C7. The location of the tumor and dermatomal pattern of the neuropathic itch pointed to a possible association between nerve compression and BRP.14 Electromyography studies performed on individuals with BRP have shown an increase in polyphasic units, decreased motor units, and/or denervation changes along the C5/C6 or C6 nerve roots, which provides additional support for the theory of cervical spine pathology as a causative factor for BRP.16
UVR Exposure—Another etiologic theory for BRP is that UVR exposure may be responsible for the genesis of pruritus. Previously known as solar pruritus, BRP was deemed a clinical condition, as there was increased prevalence in patients living in warmer climates, such as Florida.9 Wallengren and Dahlbäck18 reported that sun exposure is a notable factor in the onset of BRP, as they saw an increase in symptoms during the late summer and a decrease in symptoms over the winter months. To further support the theory that UVR is linked to BRP, several studies have shown that the utilization of sun protection is linked to a reduction of symptoms, specifically in patients who showed seasonal variations of their symptoms.9,12,19 Additionally, a study by Mirzoyev and Davis5 retrospectively reviewed 111 patients diagnosed with BRP. Of these patients, 84 (75.7%) presented with bilateral symptoms, and 54 (48.6%) reported prolonged sun exposure. Both of these findings demonstrate correlation between UVR and BRP.5 Interestingly, UV light exposure is known to release β-endorphin in the skin and may theoretically provide an area of exploration between UVR and cervical spine theories.
Conservative Treatment
Chiropractic Manipulation—Because one etiologic theory includes disease of the cervical spine, there is evidence that targeting this region with treatment is beneficial.7 Two case reports found in the literature noted that cervical spine manipulation and cervical traction yielded positive results.20,21 It has been established that pain generated by disc lesions can be the result of local nociceptive fiber activation, direct mechanical compression of the nerve roots, or inflammatory mediators.22 There are several postulated models describing the hypoalgesic effects of spinal manipulation, which contains both biomechanical and neurophysiological mechanisms. Biomechanical changes theorized to elicit analgesia include restoration of faulty biomechanical movement patterns, breaking up of periarticular adhesions, and reflexogenic muscle inhibition of hypertonic musculature. Hypothesized neurophysiological effects of joint manipulation include an increase in afferent information overwhelming the nociceptive input, reduction of temporal summation, and autonomic activation leading to non–opioid-induced hypoalgesia.23 Cervical traction is another plausible treatment for BRP, wherein the physiological effects of traction allow for a separation of vertebral bodies and expansion of the intervertebral foramen circumference, thus decreasing compression of the nerve roots.24
Acupuncture—Neurogenic pruritus, including BRP, is a group of conditions that have been treated using acupuncture. Acupuncture treatment consists of intramuscular needle stimulation and has been found to alleviate itching in patients with neurogenic pruritus. In 1 retrospective case series, acupuncture was used to treat 16 patients who were identified as having segmental pruritus. Acupuncture targeted the spasmed paravertebral muscles of the affected dermatomal levels as well as other regions of the body, and it was found that 12 patients (75%) experienced full resolution of symptoms. However, relapse did occur in 6 patients (37%) within 1 to 12 months following treatment.25 Multiple theories exist as to why acupuncture may help. One is that it relieves muscle spasms, which in turn relieves neural irritation of the spinal nerves as they traverse the respective paraspinal musculature. Another is that acupuncture decreases nociception by stimulating release of opioid peptides in the dorsal horn.26 A third proposed theory is that acupuncture acts on the afferent nerve fibers responsible for transmitting pain—Aδ and C fibers—activating these afferent nerves to produce an analgesic effect.27
Physiotherapy—The literature suggests that possible first-line therapies for neurogenic pruritus, including BRP and notalgia paresthetica, consist of noninvasive nondermatologic treatments that target cervical spine disease. Notalgia paresthetica and BRP have similar proposed mechanisms of nerve impingement; therefore, they often are grouped together when discussing proposed manual treatment options. Physiotherapy treatment includes cervical muscle strengthening, increased range of motion, application of cervical soft collars, massage, transcutaneous electronic nerve stimulation, and cervical traction.7 A study of 12 patients by Raison-Peyron et al28 in 1999 discussed the use of spinal and paraspinal ultrasound or radiation physiotherapy. Six patients underwent this treatment, and the symptoms subsided in 4 cases.28 Another study by Fleischer et al29 in 2011 discussed improvement in 2 patients with notalgia paresthetica by exercise involving active range of motion and strengthening.
Photoprotection—Avoidance of UVR exposure has been beneficial to some patients to reduce symptoms. Use of sunscreen and long-sleeved UV-protective clothing during outdoor activities or the warmer summer months may be beneficial.1
Medical Treatment
Medication—Because of the nonspecific clinical presentation of BRP, initial treatment often involves prescription of first-line antipruritic agents, including steroid creams and systemic antihistamines, both of which generally fail to provide symptom relief.1,30 Medications with neurologic mechanisms of action appear to provide potentially superior outcomes.
Topical interventions for BRP and related neurogenic pruritus have shown limited success. A case series evaluating capsaicin for pruritus offered only transient relief, likely because of its temporary hyperstimulatory and desensitizing effect on neuropeptides.7,33 In small populations, the use of topical antidepressants has yielded cutaneous and pathological relief for BRP. A case study of a 70-year-old woman evaluated the efficacy of a combination cream of ketamine and amitriptyline (a tricyclic antidepressant) yielding moderate pruritus improvement and notable improvement of secondary brachial skin lesions.34 Oral steroids also have shown success in the treatment of chronic pruritus; however, limited research is available on the efficacy of such medications for BRP, and the long-term use of oral steroids is limited by many side effects.30
Interventional Pain Procedure—A 2018 case series investigated 3 patients with a clinical diagnosis of BRP who were treated between 2010 and 2016 with
Surgery—There are multiple case studies in the literature that discuss
Conclusion
The pathogenesis of BRP continues to be an area of debate—it may be secondary to cervical spine disease or UVR. This review found there is more research pointing to cervical spine disease. There is an abundance of literature discussing both conservative and invasive treatment strategies, both of which carry benefits. Further research is needed to better establish the etiology of BRP so that formal treatment guidelines may be established.
Neuropathic itch can be a frustrating condition for providers and patients, and many treatment modalities often are tried before arriving at a helpful treatment for a particular patient. Clinicians who may encounter BRP in practice benefit from up-to-date literature reviews that provide a summary of management strategies.
- Robbins BA, Schmieder GJ. Brachioradial pruritus. StatPearls Publishing; 2020. Updated September 12, 2022. Accessed July 25, 2023. https://www.ncbi.nlm.nih.gov/books/NBK459321/
- Crevits L. Brachioradial pruritus—a peculiar neuropathic disorder. Clin Neurol Neurosurg. 2006;108:803-805.
- Lane J, McKenzie J, Spiegel J. Brachioradial pruritus: a case report and review of the literature. Cutis. 2008;81:37-40.
- Wallengren J. Brachioradial pruritus: a recurrent solar dermopathy. J Am Acad Dermatol. 1998;39:803-806.
- Mirzoyev S, Davis M. Brachioradial pruritus: Mayo Clinic experience over the past decade. Br J Dermatol. 2013;169:1007-1015.
- Pinto AC, Wachholz PA, Masuda PY, et al. Clinical, epidemiological and therapeutic profile of patients with brachioradial pruritus in a reference service in dermatology. An Bras Dermatol. 2016;91:549-551. doi:10.1590/abd1806-4841.201644767
- Alai NN, Skinner HB. Concurrent notalgia paresthetica and brachioradial pruritus associated with cervical degenerative disc disease. Cutis. 2018;102:185, 186, 189, 190.
- Atis¸ G, Bilir Kaya B. Pregabalin treatment of three cases with brachioradial pruritus. Dermatol Ther. 2017;30:e12459.
- Waisman M. Solar pruritus of the elbows (brachioradial summer pruritus). Arch Dermatol. 1968;98:481-485.
- Binder A, Fölster-Holst R, Sahan G, et al. A case of neuropathic brachioradial pruritus caused by cervical disc herniation. Nat Clin Pract Neurol. 2008;4:338-342.
- Bernhard JD, Bordeaux JS. Medical pearl: the ice-pack sign in brachioradial pruritus. J Am Acad Dermatol. 2005;52:1073.
- Veien N, Laurberg G. Brachioradial pruritus: a follow-up of 76 patients. Acta Derm Venereol. 2011;91:183-185.
- Mataix J, Silvestre JF, Climent JM, et al. Brachioradial pruritus as a symptom of cervical radiculopathy. Article in Spanish. Actas Dermosifiliogr. 2008;99:719-722.
- Kavak A, Dosoglu M. Can a spinal cord tumor cause brachioradial pruritus? J Am Acad Dermatol. 2002;46:437-440.
- Zeidler C, Pereira MP, Ständer S. Brachioradial pruritus successfully treated with intravenous naloxone. J Eur Acad Dermatol Venereol. 2023;37:e87-e89. doi:10.1111/jdv.18553
- Shields LB, Iyer VG, Zhang Y, et al. Brachioradial pruritus: clinical, electromyographic, and cervical MRI features in nine patients. Cureus. 2022;14:e21811. doi:10.7759/cureus.21811
- Marziniak M, Phan NQ, Raap U, et al. Brachioradial pruritus as a result of cervical spine pathology: the results of a magneticresonance tomography study. J Am Acad Dermatol. 2011;65:756-762. doi:10.1016/j.jaad.2010.07.036
- Wallengren J, Dahlbäck K. Familial brachioradial pruritus. Br J Dermatol. 2005;153:1016-1018.
- Salzmann SN, Okano I, Shue J, et al. Disabling pruritus in a patient with cervical stenosis. J Am Acad Orthop Surg Glob Res Rev. 2020;4:e19.00178. doi:10.5435/JAAOSGlobal-D-19-00178
- Golden KJ, Diana RM. A case of brachioradial pruritus treated with chiropractic and acupuncture. Case Rep Dermatol. 2022;14:93-97. doi:10.1159/000524054
- Tait CP, Grigg E, Quirk CJ. Brachioradial pruritus and cervical spine manipulation. Australas J Dermatol. 1998;39:168-170. doi:10.1111/j.1440-0960.1998.tb01274.x
- Freynhagen R, Baron R. The evaluation of neuropathic components in low back pain. Curr Pain Headache Rep. 2009;13:185-190. doi:10.1007/s11916-009-0032-y
- Gyer G, Michael J, Inklebarger J, et al. Spinal manipulation therapy: is it all about the brain? A current review of the neurophysiological effects of manipulation. J Integr Med. 2019;17:328-337. doi:10.1016/j.joim.2019.05.004
- Graham N, Gross A, Goldsmith CH, et al. Mechanical traction for neck pain with or without radiculopathy. Cochrane Database Syst Rev. 2008:CD006408. doi:10.1002/14651858.CD006408.pub2
- Stellon A. Neurogenic pruritus: an unrecognised problem? A retrospective case series of treatment by acupuncture. Acupunct Med. 2002;20:186-190. doi:10.1136/aim.20.4.186
- Bowsher D. Mechanisms of acupuncture. In: Filshie J, White A, eds. Medical Acupuncture: A Western Scientific Approach. Churchill Livingstone; 1998:69-82.
- Lim TK, Ma Y, Berger F, et al. Acupuncture and neural mechanism in the management of low back pain-an update. Medicines (Basel). 2018;5:63.
- Raison-Peyron N, Meunier L, Acevedo M, et al. Notalgia paresthetica: clinical, physiopathological and therapeutic aspects. a study of 12 cases. J Eur Acad Dermatol Venereol. 1999;12:215-221.
- Fleischer AB, Meade TJ, Fleischer AB. Notalgia paresthetica: successful treatment with exercises. Acta Derm Venereol. 2011;91:356-357. doi:10.2340/00015555-1039
- Kouwenhoven TA, van de Kerkhof PCM, Kamsteeg M. Use of oral antidepressants in patients with chronic pruritus: a systematic review. J Am Acad Dermatol. 2017;77:1068-1073.e7. doi:10.1016/j.jaad.2017.08.025
- Matsuda KM, Sharma D, Schonfeld AR, et al. Gabapentin and pregabalin for the treatment of chronic pruritus. J Am Acad Dermatol. 2016;75:619-625.e6. doi:10.1016/j.jaad.2016.02.1237
- Okuno S, Hashimoto T, Satoh T. Case of neuropathic itch-associated prurigo nodules on the bilateral upper arms after unilateral herpes zoster in a patient with cervical herniated discs: successful treatment with mirogabalin. J Dermatol. 2021;48:e585-e586.
- Papoiu AD, Yosipovitch G. Topical capsaicin. The fire of a ‘hot’ medicine is reignited. Expert Opin Pharmacother. 2010;11:1359-1371. doi:10.1517/14656566.2010.481670
- Magazin M, Daze RP, Okeson N. Treatment refractory brachioradial pruritus treated with topical amitriptyline and ketamine. Cureus. 2019;11:e5117. doi:10.7759/cureus.5117
- Weinberg BD, Amans M, Deviren S, et al. Brachioradial pruritus treated with computed tomography-guided cervical nerve root block: a case series. JAAD Case Rep. 2018;4:640-644. doi:10.1016/j.jdcr.2018.03.025
- De Ridder D, Hans G, Pals P, et al. A C-fiber-mediated neuropathic brachioradial pruritus. J Neurosurg. 2010;113:118-121. doi:10.3171/2009.9.JNS09620
- Morosanu CO, Etim G, Alalade AF. Brachioradial pruritus secondary to cervical disc protrusion—a case report. J Surg Case Rep. 2022:rjac277. doi:10.1093/jscr/rjac277
Brachioradial pruritus (BRP) is a neuropathic condition typically characterized by localized dysesthesia of the dorsolateral arms.1 This dysesthesia has been described as a persistent painful itching, burning, tingling, or stinging sensation2-4 and has a median duration of expression of 24 months.5,6 The condition may be unilateral or bilateral in nature but tends to have a predilection for a bilateral distribution along the C5 to C6 dermatomes.1,7,8 There are no primary skin lesions associated with BRP; however, excoriations, prurigo nodules, and lichenification may arise secondary to scratching of the irritated skin.1,4,5,9 Brachioradial pruritus tends to have a predilection for adult females (3:1 ratio) with lighter skin. The mean age at diagnosis is 59 years, but cases have been reported in patients aged 12 to 84 years.1,5 The diagnosis of BRP is based on clinical signs and symptoms, though the ice-pack sign tends to be pathognomonic for the diagnosis.10,11 Although there is no clear evidence on the exact cause of BRP, there are 2 prevalent theories: cervical radiculopathy secondary to cervical spine pathology and/or excessive exposure to UV radiation (UVR) in the summer months.3-5,12 Brachioradial pruritus remains poorly described in the literature, and even its origin is under debate. As such, the clinician may have difficulty deciding on the best course of management. The goal of this article is to identify and discuss known treatment options for BRP (Table).

Etiology
Cervical Spine Pathology—A correlation appears to exist between BRP and cervical spine changes seen on plain film radiographs at the levels of C3 to C7, with increased incidence at the C5 to C6 levels. These plain film radiographs typically show degenerative joint disease and neural foraminal stenosis at levels that correlate to the dermatomal distribution of BRP.1,7,10,12-14 In addition to plain film radiography, some studies have utilized magnetic resonance imaging to view the cervical spine and have documented evidence of intervertebral disc protrusion/bulging, central canal stenosis, neuroforaminal stenosis, and spondylosis at the affected regions.5,15-17 Moreover, supporting the theory that the cervical spine is responsible for the emergence of BRP, Marziniak et al17 investigated 41 patients with BRP utilizing magnetic resonance tomography to find that 33 patients (80.5%) had changes in nerve compression, and 8 patients (19.5%) had degenerative changes. In addition to these findings, they found that there was a significant correlation (P<.01) between the dermatomal expression of BRP and the location of cervical anatomical changes.17 Further validating the relationship between cervical spine pathology and BRP is a case study of a patient who saw rapid and complete resolution of the pruritus following spinal decompression surgery.10 Another case study described an intramedullary tumor found in a patient with BRP that was diagnosed as an ependymoma after magnetic resonance imaging revealed an intramedullary lesion within the spinal cord between C4 and C7. The location of the tumor and dermatomal pattern of the neuropathic itch pointed to a possible association between nerve compression and BRP.14 Electromyography studies performed on individuals with BRP have shown an increase in polyphasic units, decreased motor units, and/or denervation changes along the C5/C6 or C6 nerve roots, which provides additional support for the theory of cervical spine pathology as a causative factor for BRP.16
UVR Exposure—Another etiologic theory for BRP is that UVR exposure may be responsible for the genesis of pruritus. Previously known as solar pruritus, BRP was deemed a clinical condition, as there was increased prevalence in patients living in warmer climates, such as Florida.9 Wallengren and Dahlbäck18 reported that sun exposure is a notable factor in the onset of BRP, as they saw an increase in symptoms during the late summer and a decrease in symptoms over the winter months. To further support the theory that UVR is linked to BRP, several studies have shown that the utilization of sun protection is linked to a reduction of symptoms, specifically in patients who showed seasonal variations of their symptoms.9,12,19 Additionally, a study by Mirzoyev and Davis5 retrospectively reviewed 111 patients diagnosed with BRP. Of these patients, 84 (75.7%) presented with bilateral symptoms, and 54 (48.6%) reported prolonged sun exposure. Both of these findings demonstrate correlation between UVR and BRP.5 Interestingly, UV light exposure is known to release β-endorphin in the skin and may theoretically provide an area of exploration between UVR and cervical spine theories.
Conservative Treatment
Chiropractic Manipulation—Because one etiologic theory includes disease of the cervical spine, there is evidence that targeting this region with treatment is beneficial.7 Two case reports found in the literature noted that cervical spine manipulation and cervical traction yielded positive results.20,21 It has been established that pain generated by disc lesions can be the result of local nociceptive fiber activation, direct mechanical compression of the nerve roots, or inflammatory mediators.22 There are several postulated models describing the hypoalgesic effects of spinal manipulation, which contains both biomechanical and neurophysiological mechanisms. Biomechanical changes theorized to elicit analgesia include restoration of faulty biomechanical movement patterns, breaking up of periarticular adhesions, and reflexogenic muscle inhibition of hypertonic musculature. Hypothesized neurophysiological effects of joint manipulation include an increase in afferent information overwhelming the nociceptive input, reduction of temporal summation, and autonomic activation leading to non–opioid-induced hypoalgesia.23 Cervical traction is another plausible treatment for BRP, wherein the physiological effects of traction allow for a separation of vertebral bodies and expansion of the intervertebral foramen circumference, thus decreasing compression of the nerve roots.24
Acupuncture—Neurogenic pruritus, including BRP, is a group of conditions that have been treated using acupuncture. Acupuncture treatment consists of intramuscular needle stimulation and has been found to alleviate itching in patients with neurogenic pruritus. In 1 retrospective case series, acupuncture was used to treat 16 patients who were identified as having segmental pruritus. Acupuncture targeted the spasmed paravertebral muscles of the affected dermatomal levels as well as other regions of the body, and it was found that 12 patients (75%) experienced full resolution of symptoms. However, relapse did occur in 6 patients (37%) within 1 to 12 months following treatment.25 Multiple theories exist as to why acupuncture may help. One is that it relieves muscle spasms, which in turn relieves neural irritation of the spinal nerves as they traverse the respective paraspinal musculature. Another is that acupuncture decreases nociception by stimulating release of opioid peptides in the dorsal horn.26 A third proposed theory is that acupuncture acts on the afferent nerve fibers responsible for transmitting pain—Aδ and C fibers—activating these afferent nerves to produce an analgesic effect.27
Physiotherapy—The literature suggests that possible first-line therapies for neurogenic pruritus, including BRP and notalgia paresthetica, consist of noninvasive nondermatologic treatments that target cervical spine disease. Notalgia paresthetica and BRP have similar proposed mechanisms of nerve impingement; therefore, they often are grouped together when discussing proposed manual treatment options. Physiotherapy treatment includes cervical muscle strengthening, increased range of motion, application of cervical soft collars, massage, transcutaneous electronic nerve stimulation, and cervical traction.7 A study of 12 patients by Raison-Peyron et al28 in 1999 discussed the use of spinal and paraspinal ultrasound or radiation physiotherapy. Six patients underwent this treatment, and the symptoms subsided in 4 cases.28 Another study by Fleischer et al29 in 2011 discussed improvement in 2 patients with notalgia paresthetica by exercise involving active range of motion and strengthening.
Photoprotection—Avoidance of UVR exposure has been beneficial to some patients to reduce symptoms. Use of sunscreen and long-sleeved UV-protective clothing during outdoor activities or the warmer summer months may be beneficial.1
Medical Treatment
Medication—Because of the nonspecific clinical presentation of BRP, initial treatment often involves prescription of first-line antipruritic agents, including steroid creams and systemic antihistamines, both of which generally fail to provide symptom relief.1,30 Medications with neurologic mechanisms of action appear to provide potentially superior outcomes.
Topical interventions for BRP and related neurogenic pruritus have shown limited success. A case series evaluating capsaicin for pruritus offered only transient relief, likely because of its temporary hyperstimulatory and desensitizing effect on neuropeptides.7,33 In small populations, the use of topical antidepressants has yielded cutaneous and pathological relief for BRP. A case study of a 70-year-old woman evaluated the efficacy of a combination cream of ketamine and amitriptyline (a tricyclic antidepressant) yielding moderate pruritus improvement and notable improvement of secondary brachial skin lesions.34 Oral steroids also have shown success in the treatment of chronic pruritus; however, limited research is available on the efficacy of such medications for BRP, and the long-term use of oral steroids is limited by many side effects.30
Interventional Pain Procedure—A 2018 case series investigated 3 patients with a clinical diagnosis of BRP who were treated between 2010 and 2016 with
Surgery—There are multiple case studies in the literature that discuss
Conclusion
The pathogenesis of BRP continues to be an area of debate—it may be secondary to cervical spine disease or UVR. This review found there is more research pointing to cervical spine disease. There is an abundance of literature discussing both conservative and invasive treatment strategies, both of which carry benefits. Further research is needed to better establish the etiology of BRP so that formal treatment guidelines may be established.
Neuropathic itch can be a frustrating condition for providers and patients, and many treatment modalities often are tried before arriving at a helpful treatment for a particular patient. Clinicians who may encounter BRP in practice benefit from up-to-date literature reviews that provide a summary of management strategies.
Brachioradial pruritus (BRP) is a neuropathic condition typically characterized by localized dysesthesia of the dorsolateral arms.1 This dysesthesia has been described as a persistent painful itching, burning, tingling, or stinging sensation2-4 and has a median duration of expression of 24 months.5,6 The condition may be unilateral or bilateral in nature but tends to have a predilection for a bilateral distribution along the C5 to C6 dermatomes.1,7,8 There are no primary skin lesions associated with BRP; however, excoriations, prurigo nodules, and lichenification may arise secondary to scratching of the irritated skin.1,4,5,9 Brachioradial pruritus tends to have a predilection for adult females (3:1 ratio) with lighter skin. The mean age at diagnosis is 59 years, but cases have been reported in patients aged 12 to 84 years.1,5 The diagnosis of BRP is based on clinical signs and symptoms, though the ice-pack sign tends to be pathognomonic for the diagnosis.10,11 Although there is no clear evidence on the exact cause of BRP, there are 2 prevalent theories: cervical radiculopathy secondary to cervical spine pathology and/or excessive exposure to UV radiation (UVR) in the summer months.3-5,12 Brachioradial pruritus remains poorly described in the literature, and even its origin is under debate. As such, the clinician may have difficulty deciding on the best course of management. The goal of this article is to identify and discuss known treatment options for BRP (Table).

Etiology
Cervical Spine Pathology—A correlation appears to exist between BRP and cervical spine changes seen on plain film radiographs at the levels of C3 to C7, with increased incidence at the C5 to C6 levels. These plain film radiographs typically show degenerative joint disease and neural foraminal stenosis at levels that correlate to the dermatomal distribution of BRP.1,7,10,12-14 In addition to plain film radiography, some studies have utilized magnetic resonance imaging to view the cervical spine and have documented evidence of intervertebral disc protrusion/bulging, central canal stenosis, neuroforaminal stenosis, and spondylosis at the affected regions.5,15-17 Moreover, supporting the theory that the cervical spine is responsible for the emergence of BRP, Marziniak et al17 investigated 41 patients with BRP utilizing magnetic resonance tomography to find that 33 patients (80.5%) had changes in nerve compression, and 8 patients (19.5%) had degenerative changes. In addition to these findings, they found that there was a significant correlation (P<.01) between the dermatomal expression of BRP and the location of cervical anatomical changes.17 Further validating the relationship between cervical spine pathology and BRP is a case study of a patient who saw rapid and complete resolution of the pruritus following spinal decompression surgery.10 Another case study described an intramedullary tumor found in a patient with BRP that was diagnosed as an ependymoma after magnetic resonance imaging revealed an intramedullary lesion within the spinal cord between C4 and C7. The location of the tumor and dermatomal pattern of the neuropathic itch pointed to a possible association between nerve compression and BRP.14 Electromyography studies performed on individuals with BRP have shown an increase in polyphasic units, decreased motor units, and/or denervation changes along the C5/C6 or C6 nerve roots, which provides additional support for the theory of cervical spine pathology as a causative factor for BRP.16
UVR Exposure—Another etiologic theory for BRP is that UVR exposure may be responsible for the genesis of pruritus. Previously known as solar pruritus, BRP was deemed a clinical condition, as there was increased prevalence in patients living in warmer climates, such as Florida.9 Wallengren and Dahlbäck18 reported that sun exposure is a notable factor in the onset of BRP, as they saw an increase in symptoms during the late summer and a decrease in symptoms over the winter months. To further support the theory that UVR is linked to BRP, several studies have shown that the utilization of sun protection is linked to a reduction of symptoms, specifically in patients who showed seasonal variations of their symptoms.9,12,19 Additionally, a study by Mirzoyev and Davis5 retrospectively reviewed 111 patients diagnosed with BRP. Of these patients, 84 (75.7%) presented with bilateral symptoms, and 54 (48.6%) reported prolonged sun exposure. Both of these findings demonstrate correlation between UVR and BRP.5 Interestingly, UV light exposure is known to release β-endorphin in the skin and may theoretically provide an area of exploration between UVR and cervical spine theories.
Conservative Treatment
Chiropractic Manipulation—Because one etiologic theory includes disease of the cervical spine, there is evidence that targeting this region with treatment is beneficial.7 Two case reports found in the literature noted that cervical spine manipulation and cervical traction yielded positive results.20,21 It has been established that pain generated by disc lesions can be the result of local nociceptive fiber activation, direct mechanical compression of the nerve roots, or inflammatory mediators.22 There are several postulated models describing the hypoalgesic effects of spinal manipulation, which contains both biomechanical and neurophysiological mechanisms. Biomechanical changes theorized to elicit analgesia include restoration of faulty biomechanical movement patterns, breaking up of periarticular adhesions, and reflexogenic muscle inhibition of hypertonic musculature. Hypothesized neurophysiological effects of joint manipulation include an increase in afferent information overwhelming the nociceptive input, reduction of temporal summation, and autonomic activation leading to non–opioid-induced hypoalgesia.23 Cervical traction is another plausible treatment for BRP, wherein the physiological effects of traction allow for a separation of vertebral bodies and expansion of the intervertebral foramen circumference, thus decreasing compression of the nerve roots.24
Acupuncture—Neurogenic pruritus, including BRP, is a group of conditions that have been treated using acupuncture. Acupuncture treatment consists of intramuscular needle stimulation and has been found to alleviate itching in patients with neurogenic pruritus. In 1 retrospective case series, acupuncture was used to treat 16 patients who were identified as having segmental pruritus. Acupuncture targeted the spasmed paravertebral muscles of the affected dermatomal levels as well as other regions of the body, and it was found that 12 patients (75%) experienced full resolution of symptoms. However, relapse did occur in 6 patients (37%) within 1 to 12 months following treatment.25 Multiple theories exist as to why acupuncture may help. One is that it relieves muscle spasms, which in turn relieves neural irritation of the spinal nerves as they traverse the respective paraspinal musculature. Another is that acupuncture decreases nociception by stimulating release of opioid peptides in the dorsal horn.26 A third proposed theory is that acupuncture acts on the afferent nerve fibers responsible for transmitting pain—Aδ and C fibers—activating these afferent nerves to produce an analgesic effect.27
Physiotherapy—The literature suggests that possible first-line therapies for neurogenic pruritus, including BRP and notalgia paresthetica, consist of noninvasive nondermatologic treatments that target cervical spine disease. Notalgia paresthetica and BRP have similar proposed mechanisms of nerve impingement; therefore, they often are grouped together when discussing proposed manual treatment options. Physiotherapy treatment includes cervical muscle strengthening, increased range of motion, application of cervical soft collars, massage, transcutaneous electronic nerve stimulation, and cervical traction.7 A study of 12 patients by Raison-Peyron et al28 in 1999 discussed the use of spinal and paraspinal ultrasound or radiation physiotherapy. Six patients underwent this treatment, and the symptoms subsided in 4 cases.28 Another study by Fleischer et al29 in 2011 discussed improvement in 2 patients with notalgia paresthetica by exercise involving active range of motion and strengthening.
Photoprotection—Avoidance of UVR exposure has been beneficial to some patients to reduce symptoms. Use of sunscreen and long-sleeved UV-protective clothing during outdoor activities or the warmer summer months may be beneficial.1
Medical Treatment
Medication—Because of the nonspecific clinical presentation of BRP, initial treatment often involves prescription of first-line antipruritic agents, including steroid creams and systemic antihistamines, both of which generally fail to provide symptom relief.1,30 Medications with neurologic mechanisms of action appear to provide potentially superior outcomes.
Topical interventions for BRP and related neurogenic pruritus have shown limited success. A case series evaluating capsaicin for pruritus offered only transient relief, likely because of its temporary hyperstimulatory and desensitizing effect on neuropeptides.7,33 In small populations, the use of topical antidepressants has yielded cutaneous and pathological relief for BRP. A case study of a 70-year-old woman evaluated the efficacy of a combination cream of ketamine and amitriptyline (a tricyclic antidepressant) yielding moderate pruritus improvement and notable improvement of secondary brachial skin lesions.34 Oral steroids also have shown success in the treatment of chronic pruritus; however, limited research is available on the efficacy of such medications for BRP, and the long-term use of oral steroids is limited by many side effects.30
Interventional Pain Procedure—A 2018 case series investigated 3 patients with a clinical diagnosis of BRP who were treated between 2010 and 2016 with
Surgery—There are multiple case studies in the literature that discuss
Conclusion
The pathogenesis of BRP continues to be an area of debate—it may be secondary to cervical spine disease or UVR. This review found there is more research pointing to cervical spine disease. There is an abundance of literature discussing both conservative and invasive treatment strategies, both of which carry benefits. Further research is needed to better establish the etiology of BRP so that formal treatment guidelines may be established.
Neuropathic itch can be a frustrating condition for providers and patients, and many treatment modalities often are tried before arriving at a helpful treatment for a particular patient. Clinicians who may encounter BRP in practice benefit from up-to-date literature reviews that provide a summary of management strategies.
- Robbins BA, Schmieder GJ. Brachioradial pruritus. StatPearls Publishing; 2020. Updated September 12, 2022. Accessed July 25, 2023. https://www.ncbi.nlm.nih.gov/books/NBK459321/
- Crevits L. Brachioradial pruritus—a peculiar neuropathic disorder. Clin Neurol Neurosurg. 2006;108:803-805.
- Lane J, McKenzie J, Spiegel J. Brachioradial pruritus: a case report and review of the literature. Cutis. 2008;81:37-40.
- Wallengren J. Brachioradial pruritus: a recurrent solar dermopathy. J Am Acad Dermatol. 1998;39:803-806.
- Mirzoyev S, Davis M. Brachioradial pruritus: Mayo Clinic experience over the past decade. Br J Dermatol. 2013;169:1007-1015.
- Pinto AC, Wachholz PA, Masuda PY, et al. Clinical, epidemiological and therapeutic profile of patients with brachioradial pruritus in a reference service in dermatology. An Bras Dermatol. 2016;91:549-551. doi:10.1590/abd1806-4841.201644767
- Alai NN, Skinner HB. Concurrent notalgia paresthetica and brachioradial pruritus associated with cervical degenerative disc disease. Cutis. 2018;102:185, 186, 189, 190.
- Atis¸ G, Bilir Kaya B. Pregabalin treatment of three cases with brachioradial pruritus. Dermatol Ther. 2017;30:e12459.
- Waisman M. Solar pruritus of the elbows (brachioradial summer pruritus). Arch Dermatol. 1968;98:481-485.
- Binder A, Fölster-Holst R, Sahan G, et al. A case of neuropathic brachioradial pruritus caused by cervical disc herniation. Nat Clin Pract Neurol. 2008;4:338-342.
- Bernhard JD, Bordeaux JS. Medical pearl: the ice-pack sign in brachioradial pruritus. J Am Acad Dermatol. 2005;52:1073.
- Veien N, Laurberg G. Brachioradial pruritus: a follow-up of 76 patients. Acta Derm Venereol. 2011;91:183-185.
- Mataix J, Silvestre JF, Climent JM, et al. Brachioradial pruritus as a symptom of cervical radiculopathy. Article in Spanish. Actas Dermosifiliogr. 2008;99:719-722.
- Kavak A, Dosoglu M. Can a spinal cord tumor cause brachioradial pruritus? J Am Acad Dermatol. 2002;46:437-440.
- Zeidler C, Pereira MP, Ständer S. Brachioradial pruritus successfully treated with intravenous naloxone. J Eur Acad Dermatol Venereol. 2023;37:e87-e89. doi:10.1111/jdv.18553
- Shields LB, Iyer VG, Zhang Y, et al. Brachioradial pruritus: clinical, electromyographic, and cervical MRI features in nine patients. Cureus. 2022;14:e21811. doi:10.7759/cureus.21811
- Marziniak M, Phan NQ, Raap U, et al. Brachioradial pruritus as a result of cervical spine pathology: the results of a magneticresonance tomography study. J Am Acad Dermatol. 2011;65:756-762. doi:10.1016/j.jaad.2010.07.036
- Wallengren J, Dahlbäck K. Familial brachioradial pruritus. Br J Dermatol. 2005;153:1016-1018.
- Salzmann SN, Okano I, Shue J, et al. Disabling pruritus in a patient with cervical stenosis. J Am Acad Orthop Surg Glob Res Rev. 2020;4:e19.00178. doi:10.5435/JAAOSGlobal-D-19-00178
- Golden KJ, Diana RM. A case of brachioradial pruritus treated with chiropractic and acupuncture. Case Rep Dermatol. 2022;14:93-97. doi:10.1159/000524054
- Tait CP, Grigg E, Quirk CJ. Brachioradial pruritus and cervical spine manipulation. Australas J Dermatol. 1998;39:168-170. doi:10.1111/j.1440-0960.1998.tb01274.x
- Freynhagen R, Baron R. The evaluation of neuropathic components in low back pain. Curr Pain Headache Rep. 2009;13:185-190. doi:10.1007/s11916-009-0032-y
- Gyer G, Michael J, Inklebarger J, et al. Spinal manipulation therapy: is it all about the brain? A current review of the neurophysiological effects of manipulation. J Integr Med. 2019;17:328-337. doi:10.1016/j.joim.2019.05.004
- Graham N, Gross A, Goldsmith CH, et al. Mechanical traction for neck pain with or without radiculopathy. Cochrane Database Syst Rev. 2008:CD006408. doi:10.1002/14651858.CD006408.pub2
- Stellon A. Neurogenic pruritus: an unrecognised problem? A retrospective case series of treatment by acupuncture. Acupunct Med. 2002;20:186-190. doi:10.1136/aim.20.4.186
- Bowsher D. Mechanisms of acupuncture. In: Filshie J, White A, eds. Medical Acupuncture: A Western Scientific Approach. Churchill Livingstone; 1998:69-82.
- Lim TK, Ma Y, Berger F, et al. Acupuncture and neural mechanism in the management of low back pain-an update. Medicines (Basel). 2018;5:63.
- Raison-Peyron N, Meunier L, Acevedo M, et al. Notalgia paresthetica: clinical, physiopathological and therapeutic aspects. a study of 12 cases. J Eur Acad Dermatol Venereol. 1999;12:215-221.
- Fleischer AB, Meade TJ, Fleischer AB. Notalgia paresthetica: successful treatment with exercises. Acta Derm Venereol. 2011;91:356-357. doi:10.2340/00015555-1039
- Kouwenhoven TA, van de Kerkhof PCM, Kamsteeg M. Use of oral antidepressants in patients with chronic pruritus: a systematic review. J Am Acad Dermatol. 2017;77:1068-1073.e7. doi:10.1016/j.jaad.2017.08.025
- Matsuda KM, Sharma D, Schonfeld AR, et al. Gabapentin and pregabalin for the treatment of chronic pruritus. J Am Acad Dermatol. 2016;75:619-625.e6. doi:10.1016/j.jaad.2016.02.1237
- Okuno S, Hashimoto T, Satoh T. Case of neuropathic itch-associated prurigo nodules on the bilateral upper arms after unilateral herpes zoster in a patient with cervical herniated discs: successful treatment with mirogabalin. J Dermatol. 2021;48:e585-e586.
- Papoiu AD, Yosipovitch G. Topical capsaicin. The fire of a ‘hot’ medicine is reignited. Expert Opin Pharmacother. 2010;11:1359-1371. doi:10.1517/14656566.2010.481670
- Magazin M, Daze RP, Okeson N. Treatment refractory brachioradial pruritus treated with topical amitriptyline and ketamine. Cureus. 2019;11:e5117. doi:10.7759/cureus.5117
- Weinberg BD, Amans M, Deviren S, et al. Brachioradial pruritus treated with computed tomography-guided cervical nerve root block: a case series. JAAD Case Rep. 2018;4:640-644. doi:10.1016/j.jdcr.2018.03.025
- De Ridder D, Hans G, Pals P, et al. A C-fiber-mediated neuropathic brachioradial pruritus. J Neurosurg. 2010;113:118-121. doi:10.3171/2009.9.JNS09620
- Morosanu CO, Etim G, Alalade AF. Brachioradial pruritus secondary to cervical disc protrusion—a case report. J Surg Case Rep. 2022:rjac277. doi:10.1093/jscr/rjac277
- Robbins BA, Schmieder GJ. Brachioradial pruritus. StatPearls Publishing; 2020. Updated September 12, 2022. Accessed July 25, 2023. https://www.ncbi.nlm.nih.gov/books/NBK459321/
- Crevits L. Brachioradial pruritus—a peculiar neuropathic disorder. Clin Neurol Neurosurg. 2006;108:803-805.
- Lane J, McKenzie J, Spiegel J. Brachioradial pruritus: a case report and review of the literature. Cutis. 2008;81:37-40.
- Wallengren J. Brachioradial pruritus: a recurrent solar dermopathy. J Am Acad Dermatol. 1998;39:803-806.
- Mirzoyev S, Davis M. Brachioradial pruritus: Mayo Clinic experience over the past decade. Br J Dermatol. 2013;169:1007-1015.
- Pinto AC, Wachholz PA, Masuda PY, et al. Clinical, epidemiological and therapeutic profile of patients with brachioradial pruritus in a reference service in dermatology. An Bras Dermatol. 2016;91:549-551. doi:10.1590/abd1806-4841.201644767
- Alai NN, Skinner HB. Concurrent notalgia paresthetica and brachioradial pruritus associated with cervical degenerative disc disease. Cutis. 2018;102:185, 186, 189, 190.
- Atis¸ G, Bilir Kaya B. Pregabalin treatment of three cases with brachioradial pruritus. Dermatol Ther. 2017;30:e12459.
- Waisman M. Solar pruritus of the elbows (brachioradial summer pruritus). Arch Dermatol. 1968;98:481-485.
- Binder A, Fölster-Holst R, Sahan G, et al. A case of neuropathic brachioradial pruritus caused by cervical disc herniation. Nat Clin Pract Neurol. 2008;4:338-342.
- Bernhard JD, Bordeaux JS. Medical pearl: the ice-pack sign in brachioradial pruritus. J Am Acad Dermatol. 2005;52:1073.
- Veien N, Laurberg G. Brachioradial pruritus: a follow-up of 76 patients. Acta Derm Venereol. 2011;91:183-185.
- Mataix J, Silvestre JF, Climent JM, et al. Brachioradial pruritus as a symptom of cervical radiculopathy. Article in Spanish. Actas Dermosifiliogr. 2008;99:719-722.
- Kavak A, Dosoglu M. Can a spinal cord tumor cause brachioradial pruritus? J Am Acad Dermatol. 2002;46:437-440.
- Zeidler C, Pereira MP, Ständer S. Brachioradial pruritus successfully treated with intravenous naloxone. J Eur Acad Dermatol Venereol. 2023;37:e87-e89. doi:10.1111/jdv.18553
- Shields LB, Iyer VG, Zhang Y, et al. Brachioradial pruritus: clinical, electromyographic, and cervical MRI features in nine patients. Cureus. 2022;14:e21811. doi:10.7759/cureus.21811
- Marziniak M, Phan NQ, Raap U, et al. Brachioradial pruritus as a result of cervical spine pathology: the results of a magneticresonance tomography study. J Am Acad Dermatol. 2011;65:756-762. doi:10.1016/j.jaad.2010.07.036
- Wallengren J, Dahlbäck K. Familial brachioradial pruritus. Br J Dermatol. 2005;153:1016-1018.
- Salzmann SN, Okano I, Shue J, et al. Disabling pruritus in a patient with cervical stenosis. J Am Acad Orthop Surg Glob Res Rev. 2020;4:e19.00178. doi:10.5435/JAAOSGlobal-D-19-00178
- Golden KJ, Diana RM. A case of brachioradial pruritus treated with chiropractic and acupuncture. Case Rep Dermatol. 2022;14:93-97. doi:10.1159/000524054
- Tait CP, Grigg E, Quirk CJ. Brachioradial pruritus and cervical spine manipulation. Australas J Dermatol. 1998;39:168-170. doi:10.1111/j.1440-0960.1998.tb01274.x
- Freynhagen R, Baron R. The evaluation of neuropathic components in low back pain. Curr Pain Headache Rep. 2009;13:185-190. doi:10.1007/s11916-009-0032-y
- Gyer G, Michael J, Inklebarger J, et al. Spinal manipulation therapy: is it all about the brain? A current review of the neurophysiological effects of manipulation. J Integr Med. 2019;17:328-337. doi:10.1016/j.joim.2019.05.004
- Graham N, Gross A, Goldsmith CH, et al. Mechanical traction for neck pain with or without radiculopathy. Cochrane Database Syst Rev. 2008:CD006408. doi:10.1002/14651858.CD006408.pub2
- Stellon A. Neurogenic pruritus: an unrecognised problem? A retrospective case series of treatment by acupuncture. Acupunct Med. 2002;20:186-190. doi:10.1136/aim.20.4.186
- Bowsher D. Mechanisms of acupuncture. In: Filshie J, White A, eds. Medical Acupuncture: A Western Scientific Approach. Churchill Livingstone; 1998:69-82.
- Lim TK, Ma Y, Berger F, et al. Acupuncture and neural mechanism in the management of low back pain-an update. Medicines (Basel). 2018;5:63.
- Raison-Peyron N, Meunier L, Acevedo M, et al. Notalgia paresthetica: clinical, physiopathological and therapeutic aspects. a study of 12 cases. J Eur Acad Dermatol Venereol. 1999;12:215-221.
- Fleischer AB, Meade TJ, Fleischer AB. Notalgia paresthetica: successful treatment with exercises. Acta Derm Venereol. 2011;91:356-357. doi:10.2340/00015555-1039
- Kouwenhoven TA, van de Kerkhof PCM, Kamsteeg M. Use of oral antidepressants in patients with chronic pruritus: a systematic review. J Am Acad Dermatol. 2017;77:1068-1073.e7. doi:10.1016/j.jaad.2017.08.025
- Matsuda KM, Sharma D, Schonfeld AR, et al. Gabapentin and pregabalin for the treatment of chronic pruritus. J Am Acad Dermatol. 2016;75:619-625.e6. doi:10.1016/j.jaad.2016.02.1237
- Okuno S, Hashimoto T, Satoh T. Case of neuropathic itch-associated prurigo nodules on the bilateral upper arms after unilateral herpes zoster in a patient with cervical herniated discs: successful treatment with mirogabalin. J Dermatol. 2021;48:e585-e586.
- Papoiu AD, Yosipovitch G. Topical capsaicin. The fire of a ‘hot’ medicine is reignited. Expert Opin Pharmacother. 2010;11:1359-1371. doi:10.1517/14656566.2010.481670
- Magazin M, Daze RP, Okeson N. Treatment refractory brachioradial pruritus treated with topical amitriptyline and ketamine. Cureus. 2019;11:e5117. doi:10.7759/cureus.5117
- Weinberg BD, Amans M, Deviren S, et al. Brachioradial pruritus treated with computed tomography-guided cervical nerve root block: a case series. JAAD Case Rep. 2018;4:640-644. doi:10.1016/j.jdcr.2018.03.025
- De Ridder D, Hans G, Pals P, et al. A C-fiber-mediated neuropathic brachioradial pruritus. J Neurosurg. 2010;113:118-121. doi:10.3171/2009.9.JNS09620
- Morosanu CO, Etim G, Alalade AF. Brachioradial pruritus secondary to cervical disc protrusion—a case report. J Surg Case Rep. 2022:rjac277. doi:10.1093/jscr/rjac277
Practice Points
- The etiology of brachioradial pruritus (BRP) has been associated with cervical spine pathology and/or UV radiation exposure.
- Treatment options for BRP range from conservative to invasive, and clinicians should consider the evidence for all options to decide what is best for each patient.
Study evaluating in utero treatment for hypohidrotic ectodermal dysplasia seeks enrollees
A multicenter, international phase 2 trial known as EDELIFE is underway to investigate the safety and efficacy of an in utero treatment for developing males with X-linked hypohidrotic ectodermal dysplasia (XLHED).
This condition is caused by mutations in the gene coding for ectodysplasin A (EDA), a protein that signals the epithelial-mesenchymal transition during embryogenesis. EDA loss or dysfunction precludes binding to its endogenous EDA1 receptor (EDAR), and downstream development of teeth, hair, nails, and skin adnexae, most notably eccrine glands.
The treatment, ER004, is a first-in-class signaling protein EDA replacement molecule now under investigation by the EspeRare Foundation, with support from the Pierre Fabre Foundation. The pioneering clinical trial is evaluating the delivery of ER004 protein replacement in utero to affected fetuses, allowing antenatal binding to the EDAR. According to the EDELIFE web site, when ER004 is administered to XLHED-affected males in utero, it “should act as a replacement for the missing EDA and trigger the process that leads to the normal development of a baby’s skin, teeth, hair, and sweat glands, leading to better formation of these structures.”
The protein is delivered into the amniotic fluid via a needle and syringe under ultrasound guidance. In a report on this treatment used in a pair of affected twins and a third XLHED-affected male published in 2018, the authors reported that the three babies were able to sweat normally after birth, “and XLHED-related illness had not developed by 14-22 months of age.”
The goal of the prospective, open-label, genotype match–controlled EDELIFE trial is to confirm the efficacy and safety results for ER004 in a larger group of boys, and to determine if it can lead to robust, and long-lasting improvement in XLHED-associated defects.
In the United States, the first pregnant woman to join the study received the treatment in February 2023 at Washington University in St. Louis. Other clinical sites are located in France, Germany, Italy, Spain, and the United Kingdom. Led by principal investigator Holm Schneider, MD, of the University Erlanger-Nurnberg (Germany), researchers are seeking to enroll mothers aged 18 years and older who are genetically confirmed carriers of the XLHED mutation and pregnant with a boy or considering pregnancy. The control group will include XLHED-affected males, 6 months to 60 years old, who are blood relatives of the pregnant woman participating in the study.
“This is an unprecedented approach to preventing a significant morbidity affecting boys with XLHED, and a potential model for in utero correction of genetic defects involving embryogenesis,” Elaine Siegfried, MD, professor of pediatrics and dermatology at Saint Louis University, said in an interview. Dr. Siegfried, who has served on the scientific advisory board of the National Foundation for Ectodermal Dysplasias since 1997, added that many years of effort “has finally yielded sufficient funding and identified an international network of experts to support this ambitious trial. We are now seeking participation of the most important collaborators: mothers willing to help establish safety and efficacy of this approach.”
Mary Fete, MSN, RN, executive director of the NFED, said that the EDELIFE clinical trial “provides enormous hope for our families affected by XLHED. It’s extraordinary to think that the baby boys affected by XLHED who have received ER004 are sweating normally and have other improved symptoms. The NFED is proud to have begun and fostered the research for 30-plus years that developed ER004.”
Dr. Siegfried is a member of the independent data monitoring committee for the EDELIFE trial.
Clinicians treating affected families or potentially eligible subjects are encouraged to contact the trial investigators at this link.
A multicenter, international phase 2 trial known as EDELIFE is underway to investigate the safety and efficacy of an in utero treatment for developing males with X-linked hypohidrotic ectodermal dysplasia (XLHED).
This condition is caused by mutations in the gene coding for ectodysplasin A (EDA), a protein that signals the epithelial-mesenchymal transition during embryogenesis. EDA loss or dysfunction precludes binding to its endogenous EDA1 receptor (EDAR), and downstream development of teeth, hair, nails, and skin adnexae, most notably eccrine glands.
The treatment, ER004, is a first-in-class signaling protein EDA replacement molecule now under investigation by the EspeRare Foundation, with support from the Pierre Fabre Foundation. The pioneering clinical trial is evaluating the delivery of ER004 protein replacement in utero to affected fetuses, allowing antenatal binding to the EDAR. According to the EDELIFE web site, when ER004 is administered to XLHED-affected males in utero, it “should act as a replacement for the missing EDA and trigger the process that leads to the normal development of a baby’s skin, teeth, hair, and sweat glands, leading to better formation of these structures.”
The protein is delivered into the amniotic fluid via a needle and syringe under ultrasound guidance. In a report on this treatment used in a pair of affected twins and a third XLHED-affected male published in 2018, the authors reported that the three babies were able to sweat normally after birth, “and XLHED-related illness had not developed by 14-22 months of age.”
The goal of the prospective, open-label, genotype match–controlled EDELIFE trial is to confirm the efficacy and safety results for ER004 in a larger group of boys, and to determine if it can lead to robust, and long-lasting improvement in XLHED-associated defects.
In the United States, the first pregnant woman to join the study received the treatment in February 2023 at Washington University in St. Louis. Other clinical sites are located in France, Germany, Italy, Spain, and the United Kingdom. Led by principal investigator Holm Schneider, MD, of the University Erlanger-Nurnberg (Germany), researchers are seeking to enroll mothers aged 18 years and older who are genetically confirmed carriers of the XLHED mutation and pregnant with a boy or considering pregnancy. The control group will include XLHED-affected males, 6 months to 60 years old, who are blood relatives of the pregnant woman participating in the study.
“This is an unprecedented approach to preventing a significant morbidity affecting boys with XLHED, and a potential model for in utero correction of genetic defects involving embryogenesis,” Elaine Siegfried, MD, professor of pediatrics and dermatology at Saint Louis University, said in an interview. Dr. Siegfried, who has served on the scientific advisory board of the National Foundation for Ectodermal Dysplasias since 1997, added that many years of effort “has finally yielded sufficient funding and identified an international network of experts to support this ambitious trial. We are now seeking participation of the most important collaborators: mothers willing to help establish safety and efficacy of this approach.”
Mary Fete, MSN, RN, executive director of the NFED, said that the EDELIFE clinical trial “provides enormous hope for our families affected by XLHED. It’s extraordinary to think that the baby boys affected by XLHED who have received ER004 are sweating normally and have other improved symptoms. The NFED is proud to have begun and fostered the research for 30-plus years that developed ER004.”
Dr. Siegfried is a member of the independent data monitoring committee for the EDELIFE trial.
Clinicians treating affected families or potentially eligible subjects are encouraged to contact the trial investigators at this link.
A multicenter, international phase 2 trial known as EDELIFE is underway to investigate the safety and efficacy of an in utero treatment for developing males with X-linked hypohidrotic ectodermal dysplasia (XLHED).
This condition is caused by mutations in the gene coding for ectodysplasin A (EDA), a protein that signals the epithelial-mesenchymal transition during embryogenesis. EDA loss or dysfunction precludes binding to its endogenous EDA1 receptor (EDAR), and downstream development of teeth, hair, nails, and skin adnexae, most notably eccrine glands.
The treatment, ER004, is a first-in-class signaling protein EDA replacement molecule now under investigation by the EspeRare Foundation, with support from the Pierre Fabre Foundation. The pioneering clinical trial is evaluating the delivery of ER004 protein replacement in utero to affected fetuses, allowing antenatal binding to the EDAR. According to the EDELIFE web site, when ER004 is administered to XLHED-affected males in utero, it “should act as a replacement for the missing EDA and trigger the process that leads to the normal development of a baby’s skin, teeth, hair, and sweat glands, leading to better formation of these structures.”
The protein is delivered into the amniotic fluid via a needle and syringe under ultrasound guidance. In a report on this treatment used in a pair of affected twins and a third XLHED-affected male published in 2018, the authors reported that the three babies were able to sweat normally after birth, “and XLHED-related illness had not developed by 14-22 months of age.”
The goal of the prospective, open-label, genotype match–controlled EDELIFE trial is to confirm the efficacy and safety results for ER004 in a larger group of boys, and to determine if it can lead to robust, and long-lasting improvement in XLHED-associated defects.
In the United States, the first pregnant woman to join the study received the treatment in February 2023 at Washington University in St. Louis. Other clinical sites are located in France, Germany, Italy, Spain, and the United Kingdom. Led by principal investigator Holm Schneider, MD, of the University Erlanger-Nurnberg (Germany), researchers are seeking to enroll mothers aged 18 years and older who are genetically confirmed carriers of the XLHED mutation and pregnant with a boy or considering pregnancy. The control group will include XLHED-affected males, 6 months to 60 years old, who are blood relatives of the pregnant woman participating in the study.
“This is an unprecedented approach to preventing a significant morbidity affecting boys with XLHED, and a potential model for in utero correction of genetic defects involving embryogenesis,” Elaine Siegfried, MD, professor of pediatrics and dermatology at Saint Louis University, said in an interview. Dr. Siegfried, who has served on the scientific advisory board of the National Foundation for Ectodermal Dysplasias since 1997, added that many years of effort “has finally yielded sufficient funding and identified an international network of experts to support this ambitious trial. We are now seeking participation of the most important collaborators: mothers willing to help establish safety and efficacy of this approach.”
Mary Fete, MSN, RN, executive director of the NFED, said that the EDELIFE clinical trial “provides enormous hope for our families affected by XLHED. It’s extraordinary to think that the baby boys affected by XLHED who have received ER004 are sweating normally and have other improved symptoms. The NFED is proud to have begun and fostered the research for 30-plus years that developed ER004.”
Dr. Siegfried is a member of the independent data monitoring committee for the EDELIFE trial.
Clinicians treating affected families or potentially eligible subjects are encouraged to contact the trial investigators at this link.
Woman with transplanted uterus gives birth to boy
It’s the first time that a baby has been born to a woman with a transplanted uterus outside of a clinical trial. Officials from University of Alabama–Birmingham Hospital, where the 2-year process took place, said in a statement on July 24 that the birth sets its uterus transplant program on track to perhaps become covered under insurance plans.
The process of uterus transplant, in vitro fertilization, and pregnancy involves 50 medical providers and is open to women who have uterine factor infertility (UFI). The condition may affect up to 5% of reproductive-age women worldwide. Women with UFI cannot carry a pregnancy to term because they were either born without a uterus, had it removed via hysterectomy, or have a uterus that does not function properly.
The woman, whom the hospital identified as Mallory, moved with her family to the Birmingham area to enter the transplant program, which is one of four programs operating in the United States. Mallory learned when she was 17 years old that she was born without a uterus because of Mayer-Rokitansky-Küster-Hauser syndrome. Her first child, a daughter, was born after her sister carried the pregnancy as a surrogate.
Mallory received her uterus from a deceased donor. Her son was born in May.
“As with other types of organ transplants, the woman must take immunosuppressive medications to prevent the body from rejecting the transplanted uterus,” the transplant program’s website states. “After the baby is born and if the woman does not want more children, the transplanted uterus is removed with a hysterectomy procedure, and the woman no longer needs to take antirejection medications.”
“There are all different ways to grow your family if you have uterine factor infertility, but this [uterus transplantation] is what I feel like I knew that I was supposed to do,” Mallory said in a statement. “I mean, just hearing the cry at first was just, you know, mind blowing.”
A version of this article first appeared on WebMD.com.
It’s the first time that a baby has been born to a woman with a transplanted uterus outside of a clinical trial. Officials from University of Alabama–Birmingham Hospital, where the 2-year process took place, said in a statement on July 24 that the birth sets its uterus transplant program on track to perhaps become covered under insurance plans.
The process of uterus transplant, in vitro fertilization, and pregnancy involves 50 medical providers and is open to women who have uterine factor infertility (UFI). The condition may affect up to 5% of reproductive-age women worldwide. Women with UFI cannot carry a pregnancy to term because they were either born without a uterus, had it removed via hysterectomy, or have a uterus that does not function properly.
The woman, whom the hospital identified as Mallory, moved with her family to the Birmingham area to enter the transplant program, which is one of four programs operating in the United States. Mallory learned when she was 17 years old that she was born without a uterus because of Mayer-Rokitansky-Küster-Hauser syndrome. Her first child, a daughter, was born after her sister carried the pregnancy as a surrogate.
Mallory received her uterus from a deceased donor. Her son was born in May.
“As with other types of organ transplants, the woman must take immunosuppressive medications to prevent the body from rejecting the transplanted uterus,” the transplant program’s website states. “After the baby is born and if the woman does not want more children, the transplanted uterus is removed with a hysterectomy procedure, and the woman no longer needs to take antirejection medications.”
“There are all different ways to grow your family if you have uterine factor infertility, but this [uterus transplantation] is what I feel like I knew that I was supposed to do,” Mallory said in a statement. “I mean, just hearing the cry at first was just, you know, mind blowing.”
A version of this article first appeared on WebMD.com.
It’s the first time that a baby has been born to a woman with a transplanted uterus outside of a clinical trial. Officials from University of Alabama–Birmingham Hospital, where the 2-year process took place, said in a statement on July 24 that the birth sets its uterus transplant program on track to perhaps become covered under insurance plans.
The process of uterus transplant, in vitro fertilization, and pregnancy involves 50 medical providers and is open to women who have uterine factor infertility (UFI). The condition may affect up to 5% of reproductive-age women worldwide. Women with UFI cannot carry a pregnancy to term because they were either born without a uterus, had it removed via hysterectomy, or have a uterus that does not function properly.
The woman, whom the hospital identified as Mallory, moved with her family to the Birmingham area to enter the transplant program, which is one of four programs operating in the United States. Mallory learned when she was 17 years old that she was born without a uterus because of Mayer-Rokitansky-Küster-Hauser syndrome. Her first child, a daughter, was born after her sister carried the pregnancy as a surrogate.
Mallory received her uterus from a deceased donor. Her son was born in May.
“As with other types of organ transplants, the woman must take immunosuppressive medications to prevent the body from rejecting the transplanted uterus,” the transplant program’s website states. “After the baby is born and if the woman does not want more children, the transplanted uterus is removed with a hysterectomy procedure, and the woman no longer needs to take antirejection medications.”
“There are all different ways to grow your family if you have uterine factor infertility, but this [uterus transplantation] is what I feel like I knew that I was supposed to do,” Mallory said in a statement. “I mean, just hearing the cry at first was just, you know, mind blowing.”
A version of this article first appeared on WebMD.com.
Progress seen on five fronts for substantially improving treatment of epidermolysis bullosa
ASHEVILLE, N.C. – , according to a prominent EB researcher.
Not only are recent developments in EB “exciting,” the progress on multiple fronts for control of disease or its symptoms suggests “we are on the cusp of a new era,” Jemima Mellerio, BSc, MD, a consultant dermatologist, St. John’s Institute of Dermatology, London, said at the annual meeting of the Society for Pediatric Dermatology.
Published clinical studies of cell therapies and gene therapies date back at least 15 years, according to a review by Dr. Mellerio on why developments are starting to move so quickly. The difference now is that many obstacles to routine use of these options are being resolved so that viable strategies have reached or are reaching phase 3 trials.
In addition to cell therapies and gene therapies, Dr. Mellerio discussed progress in three additional areas: gene editing, protein therapy, and drug repurposing.
Summarizing progress in each, she described improvement in levels of collagen VII, an important deficit in most types of EB, that were achieved with fibroblast injections that improved levels of collagen VII and anchoring fibrils in a study published in the Journal of Investigative Dermatology. Injection of mesenchymal stromal cells (MSC) have been associated with reduced pain and itch in a series of studies, one of the earliest of which was published in the New England Journal of Medicine.
Since that time, there have been several approaches using MSC.
Of these approaches, intravenous injection of ABCB5+ MSCs might be the first to gain regulatory approval. According to Dr. Mellerio, there is an ongoing phase 3 crossover trial evaluating this approach, which followed several earlier phase studies that demonstrated adequate safety and tolerability while reducing severity scores, relieving pain and itch, and improving wound closure in patients with EB.
In 2006, correction of junctional EB (JEB) was achieved by transplantation of genetically modified epidermal cells to replace the LAMB3 gene, thereby restoring production of laminin 332, which is an essential component of the dermal-epidermal junction, according to Dr. Mellerio, citing a study in Nature Medicine.
The next attempt with this approach did not take place until 2015, resurrected to save the life of a 7-year-old Syrian boy – to generate epidermal sheets that eventually covered 80% of his body. The success is supporting further work on this approach but has also been an inspiration to other gene therapies, including a topical gene therapy recently approved in the United States.
Topically applied beremagene geperpavec (Vyjuvek, formerly known as B-VEC) was approved by the FDA in May for treating wounds in patients 6 months of age and older, with recessive or dominant dystrophic EB, on the basis of a phase 3 trial published in the New England Journal of Medicine, but others are coming. Dr. Mellerio also described a recently completed phase 3 trial with introduction of ex vivo gene-corrected keratinocytes, which has been associated with long-term improvements among patients with recessive dystrophic EB (RDEB). The responses in early phase studies included wound healing and reduction in pain and itch.
Perhaps less advanced but still promising, protein therapy, gene editing, and repurposing of existing therapies are all approaches that are moving forward. Many are supported by at least some clinical data, according to Dr. Mellerio.
As an example of protein therapy, a completed phase I/II trial associated recombinant human collagen with wound healing and pain reduction in RDEB. This study provided proof of principle for a therapy that could be applied topically or intravenously. Further development is anticipated.
Multiple platforms for gene editing have been described with the goal of simply excising pathogenic mutations or antisense oligonucleotides for sustained or permanent control of EB expression. Clinical evidence is limited, but Dr. Mellerio suggested that the theoretical potential for eliminating the source of abnormal transcription is the restoration of functional proteins essential for reversing skin fragility.
In some cases, existing drugs have the same potential. Dr. Mellerio described efforts to use an aminoglycoside to circumvent nonsense mutations that produce messenger RNA decay and impaired production of the proteins that prevent EB. In a pilot study evaluating topical gentamicin in RDEB, there were substantial improvements at 1 month and 3 months in several measures of skin fragility and encouraged studies that are now ongoing in both RDEB and JEB.
More than promising, a multinational randomized phase 3 study with birch bark extract recently published in the British Journal of Dermatology, associated treatment with this topical gel, known as Oleogel-S10, with higher rates of complete wound closure at 45 days (41.3% vs. 28.9% in the control vehicle arm) and a low risk of adverse events.
“This therapy is now approved in Europe and the United Kingdom, although, unfortunately, it is not yet available in the United States,” Dr. Mellerio noted.
Importantly, none of these therapies are necessarily effective across subtypes of EB, which often have different underlying pathogenic mechanisms, she said. However, the growing sophistication with which the pathophysiology of these subtypes is understood makes the numerous treatments in the pipeline “exciting.”
“We are at a point where we can really start to think of personalized medicine in EB,” Dr. Mellerio said. With the clinical advances already available and those expected, she suggested the recently approved treatment options are just the beginning. She expects the treatment landscape to evolve quickly over the next few years.
This does not appear to be a personal opinion. Another prominent researcher in EB, M. Peter Marinkovich, MD, director of the Stanford Bullous Disease and Psoriasis Clinics at Stanford (Calif.) University, is seeing the same real-world promise of therapies that have been in gestation for a decade or more.
“Dr. Mellerio is right. This is an exciting time for EB patients,” Dr. Marinkovich said in an interview. While the approval of B-VEC, the first gene therapy for EB, is the proof, Dr. Marinkovich, the lead author of the NEJM paper on B-VEC, noted that “many other potential EB therapies are being studied right now.” Based on promise in earlier clinical studies with many of these agents, he, like Dr. Mellerio, expects progress in real-world treatments for EB to accelerate.
Dr. Mellerio reported financial relationships with Amryt Pharma and Krystal Biotech. Dr. Marinkovich receives research support from Abeona Therapeutics, Castle Creek Pharmaceuticals, Krystal Biotech, Phoenix Tissue Repair, and WINGS Therapeutics.
A version of this article first appeared on Medscape.com.
ASHEVILLE, N.C. – , according to a prominent EB researcher.
Not only are recent developments in EB “exciting,” the progress on multiple fronts for control of disease or its symptoms suggests “we are on the cusp of a new era,” Jemima Mellerio, BSc, MD, a consultant dermatologist, St. John’s Institute of Dermatology, London, said at the annual meeting of the Society for Pediatric Dermatology.
Published clinical studies of cell therapies and gene therapies date back at least 15 years, according to a review by Dr. Mellerio on why developments are starting to move so quickly. The difference now is that many obstacles to routine use of these options are being resolved so that viable strategies have reached or are reaching phase 3 trials.
In addition to cell therapies and gene therapies, Dr. Mellerio discussed progress in three additional areas: gene editing, protein therapy, and drug repurposing.
Summarizing progress in each, she described improvement in levels of collagen VII, an important deficit in most types of EB, that were achieved with fibroblast injections that improved levels of collagen VII and anchoring fibrils in a study published in the Journal of Investigative Dermatology. Injection of mesenchymal stromal cells (MSC) have been associated with reduced pain and itch in a series of studies, one of the earliest of which was published in the New England Journal of Medicine.
Since that time, there have been several approaches using MSC.
Of these approaches, intravenous injection of ABCB5+ MSCs might be the first to gain regulatory approval. According to Dr. Mellerio, there is an ongoing phase 3 crossover trial evaluating this approach, which followed several earlier phase studies that demonstrated adequate safety and tolerability while reducing severity scores, relieving pain and itch, and improving wound closure in patients with EB.
In 2006, correction of junctional EB (JEB) was achieved by transplantation of genetically modified epidermal cells to replace the LAMB3 gene, thereby restoring production of laminin 332, which is an essential component of the dermal-epidermal junction, according to Dr. Mellerio, citing a study in Nature Medicine.
The next attempt with this approach did not take place until 2015, resurrected to save the life of a 7-year-old Syrian boy – to generate epidermal sheets that eventually covered 80% of his body. The success is supporting further work on this approach but has also been an inspiration to other gene therapies, including a topical gene therapy recently approved in the United States.
Topically applied beremagene geperpavec (Vyjuvek, formerly known as B-VEC) was approved by the FDA in May for treating wounds in patients 6 months of age and older, with recessive or dominant dystrophic EB, on the basis of a phase 3 trial published in the New England Journal of Medicine, but others are coming. Dr. Mellerio also described a recently completed phase 3 trial with introduction of ex vivo gene-corrected keratinocytes, which has been associated with long-term improvements among patients with recessive dystrophic EB (RDEB). The responses in early phase studies included wound healing and reduction in pain and itch.
Perhaps less advanced but still promising, protein therapy, gene editing, and repurposing of existing therapies are all approaches that are moving forward. Many are supported by at least some clinical data, according to Dr. Mellerio.
As an example of protein therapy, a completed phase I/II trial associated recombinant human collagen with wound healing and pain reduction in RDEB. This study provided proof of principle for a therapy that could be applied topically or intravenously. Further development is anticipated.
Multiple platforms for gene editing have been described with the goal of simply excising pathogenic mutations or antisense oligonucleotides for sustained or permanent control of EB expression. Clinical evidence is limited, but Dr. Mellerio suggested that the theoretical potential for eliminating the source of abnormal transcription is the restoration of functional proteins essential for reversing skin fragility.
In some cases, existing drugs have the same potential. Dr. Mellerio described efforts to use an aminoglycoside to circumvent nonsense mutations that produce messenger RNA decay and impaired production of the proteins that prevent EB. In a pilot study evaluating topical gentamicin in RDEB, there were substantial improvements at 1 month and 3 months in several measures of skin fragility and encouraged studies that are now ongoing in both RDEB and JEB.
More than promising, a multinational randomized phase 3 study with birch bark extract recently published in the British Journal of Dermatology, associated treatment with this topical gel, known as Oleogel-S10, with higher rates of complete wound closure at 45 days (41.3% vs. 28.9% in the control vehicle arm) and a low risk of adverse events.
“This therapy is now approved in Europe and the United Kingdom, although, unfortunately, it is not yet available in the United States,” Dr. Mellerio noted.
Importantly, none of these therapies are necessarily effective across subtypes of EB, which often have different underlying pathogenic mechanisms, she said. However, the growing sophistication with which the pathophysiology of these subtypes is understood makes the numerous treatments in the pipeline “exciting.”
“We are at a point where we can really start to think of personalized medicine in EB,” Dr. Mellerio said. With the clinical advances already available and those expected, she suggested the recently approved treatment options are just the beginning. She expects the treatment landscape to evolve quickly over the next few years.
This does not appear to be a personal opinion. Another prominent researcher in EB, M. Peter Marinkovich, MD, director of the Stanford Bullous Disease and Psoriasis Clinics at Stanford (Calif.) University, is seeing the same real-world promise of therapies that have been in gestation for a decade or more.
“Dr. Mellerio is right. This is an exciting time for EB patients,” Dr. Marinkovich said in an interview. While the approval of B-VEC, the first gene therapy for EB, is the proof, Dr. Marinkovich, the lead author of the NEJM paper on B-VEC, noted that “many other potential EB therapies are being studied right now.” Based on promise in earlier clinical studies with many of these agents, he, like Dr. Mellerio, expects progress in real-world treatments for EB to accelerate.
Dr. Mellerio reported financial relationships with Amryt Pharma and Krystal Biotech. Dr. Marinkovich receives research support from Abeona Therapeutics, Castle Creek Pharmaceuticals, Krystal Biotech, Phoenix Tissue Repair, and WINGS Therapeutics.
A version of this article first appeared on Medscape.com.
ASHEVILLE, N.C. – , according to a prominent EB researcher.
Not only are recent developments in EB “exciting,” the progress on multiple fronts for control of disease or its symptoms suggests “we are on the cusp of a new era,” Jemima Mellerio, BSc, MD, a consultant dermatologist, St. John’s Institute of Dermatology, London, said at the annual meeting of the Society for Pediatric Dermatology.
Published clinical studies of cell therapies and gene therapies date back at least 15 years, according to a review by Dr. Mellerio on why developments are starting to move so quickly. The difference now is that many obstacles to routine use of these options are being resolved so that viable strategies have reached or are reaching phase 3 trials.
In addition to cell therapies and gene therapies, Dr. Mellerio discussed progress in three additional areas: gene editing, protein therapy, and drug repurposing.
Summarizing progress in each, she described improvement in levels of collagen VII, an important deficit in most types of EB, that were achieved with fibroblast injections that improved levels of collagen VII and anchoring fibrils in a study published in the Journal of Investigative Dermatology. Injection of mesenchymal stromal cells (MSC) have been associated with reduced pain and itch in a series of studies, one of the earliest of which was published in the New England Journal of Medicine.
Since that time, there have been several approaches using MSC.
Of these approaches, intravenous injection of ABCB5+ MSCs might be the first to gain regulatory approval. According to Dr. Mellerio, there is an ongoing phase 3 crossover trial evaluating this approach, which followed several earlier phase studies that demonstrated adequate safety and tolerability while reducing severity scores, relieving pain and itch, and improving wound closure in patients with EB.
In 2006, correction of junctional EB (JEB) was achieved by transplantation of genetically modified epidermal cells to replace the LAMB3 gene, thereby restoring production of laminin 332, which is an essential component of the dermal-epidermal junction, according to Dr. Mellerio, citing a study in Nature Medicine.
The next attempt with this approach did not take place until 2015, resurrected to save the life of a 7-year-old Syrian boy – to generate epidermal sheets that eventually covered 80% of his body. The success is supporting further work on this approach but has also been an inspiration to other gene therapies, including a topical gene therapy recently approved in the United States.
Topically applied beremagene geperpavec (Vyjuvek, formerly known as B-VEC) was approved by the FDA in May for treating wounds in patients 6 months of age and older, with recessive or dominant dystrophic EB, on the basis of a phase 3 trial published in the New England Journal of Medicine, but others are coming. Dr. Mellerio also described a recently completed phase 3 trial with introduction of ex vivo gene-corrected keratinocytes, which has been associated with long-term improvements among patients with recessive dystrophic EB (RDEB). The responses in early phase studies included wound healing and reduction in pain and itch.
Perhaps less advanced but still promising, protein therapy, gene editing, and repurposing of existing therapies are all approaches that are moving forward. Many are supported by at least some clinical data, according to Dr. Mellerio.
As an example of protein therapy, a completed phase I/II trial associated recombinant human collagen with wound healing and pain reduction in RDEB. This study provided proof of principle for a therapy that could be applied topically or intravenously. Further development is anticipated.
Multiple platforms for gene editing have been described with the goal of simply excising pathogenic mutations or antisense oligonucleotides for sustained or permanent control of EB expression. Clinical evidence is limited, but Dr. Mellerio suggested that the theoretical potential for eliminating the source of abnormal transcription is the restoration of functional proteins essential for reversing skin fragility.
In some cases, existing drugs have the same potential. Dr. Mellerio described efforts to use an aminoglycoside to circumvent nonsense mutations that produce messenger RNA decay and impaired production of the proteins that prevent EB. In a pilot study evaluating topical gentamicin in RDEB, there were substantial improvements at 1 month and 3 months in several measures of skin fragility and encouraged studies that are now ongoing in both RDEB and JEB.
More than promising, a multinational randomized phase 3 study with birch bark extract recently published in the British Journal of Dermatology, associated treatment with this topical gel, known as Oleogel-S10, with higher rates of complete wound closure at 45 days (41.3% vs. 28.9% in the control vehicle arm) and a low risk of adverse events.
“This therapy is now approved in Europe and the United Kingdom, although, unfortunately, it is not yet available in the United States,” Dr. Mellerio noted.
Importantly, none of these therapies are necessarily effective across subtypes of EB, which often have different underlying pathogenic mechanisms, she said. However, the growing sophistication with which the pathophysiology of these subtypes is understood makes the numerous treatments in the pipeline “exciting.”
“We are at a point where we can really start to think of personalized medicine in EB,” Dr. Mellerio said. With the clinical advances already available and those expected, she suggested the recently approved treatment options are just the beginning. She expects the treatment landscape to evolve quickly over the next few years.
This does not appear to be a personal opinion. Another prominent researcher in EB, M. Peter Marinkovich, MD, director of the Stanford Bullous Disease and Psoriasis Clinics at Stanford (Calif.) University, is seeing the same real-world promise of therapies that have been in gestation for a decade or more.
“Dr. Mellerio is right. This is an exciting time for EB patients,” Dr. Marinkovich said in an interview. While the approval of B-VEC, the first gene therapy for EB, is the proof, Dr. Marinkovich, the lead author of the NEJM paper on B-VEC, noted that “many other potential EB therapies are being studied right now.” Based on promise in earlier clinical studies with many of these agents, he, like Dr. Mellerio, expects progress in real-world treatments for EB to accelerate.
Dr. Mellerio reported financial relationships with Amryt Pharma and Krystal Biotech. Dr. Marinkovich receives research support from Abeona Therapeutics, Castle Creek Pharmaceuticals, Krystal Biotech, Phoenix Tissue Repair, and WINGS Therapeutics.
A version of this article first appeared on Medscape.com.
AT SPD 2023
Case report describes pediatric RIME triggered by norovirus
, according to a newly published case report.
Lead author Anna Yasmine Kirkorian, MD, chief of dermatology at Children’s National Hospital in Washington, said she wanted to get the word out in part because it seems like RIME is occurring more frequently. “I do feel like we’re seeing more cases and from a more diverse number of pathogens,” Dr. Kirkorian told this news organization.
There was a decrease in RIME during the early stages of the COVID-19 pandemic when people were isolating more, Dr. Kirkorian said. SARS-CoV-2 has been a trigger for some cases, but she did not find that remarkable, given that respiratory viruses are known RIME precursors. The question is why RIME is being triggered more frequently now that people have essentially gone back to their normal lives, she said.
Dr. Kirkorian and colleagues at Children’s National Hospital and George Washington University, Washington, wrote about a 5-year-old boy with norovirus-triggered RIME in a case report published in Pediatric Dermatology.
RIME – previously known as Mycoplasma pneumoniae–induced rash and mucositis (MIRM) – tends to arise after a viral infection, with upper respiratory viruses such as mycoplasma and Chlamydophila pneumoniae, influenza, and enterovirus among the common triggers. “We think this is actually your own immune system overreacting to a pathogen,” Dr. Kirkorian said in an interview, adding that the mechanism of RIME is still not understood.
While the norovirus discovery was a surprise, it shows that much is still unknown about this rare condition. “I don’t think we know what is usual and what is unusual,” Dr. Kirkorian said.
In this case, the boy swiftly declined, with progressive conjunctivitis, high fever, and rapidly developing mucositis. By the time the 5-year-old got to Children’s National Hospital, he had a spreading, painful rash, including tense vesicles and bullae involving more than 30% of his total body surface area, and areas of denuded skin on both cheeks and the back of his neck.
He had hemorrhagic mucositis of the lips, a large erosion at the urethral meatus, and hemorrhagic conjunctivitis of both eyes with thick yellow crusting on the eyelids.
The clinicians intubated the boy and admitted him to the intensive care unit. He was given a one-time injection of etanercept (25 mg) followed by 8 days of intravenous cyclosporine at a dose of 5 mg per kilogram, divided twice daily, which helped calm the mucositis and stopped the rash from progressing. There is not an accepted protocol or list of evidence-based therapeutics for RIME, Dr. Kirkorian noted.
The severe eye damage required amniotic membrane grafts. The patient was extubated after 9 days but remained in the hospital for a total of 26 days because he needed to receive nutritional support (the mucositis kept him from eating), and for pain control and weaning of sedation.
As the clinicians searched for a potential triggering virus, they came up empty. Results were negative for adenovirus, Epstein Barr virus, cytomegalovirus, herpes simplex, and varicella zoster. But they noted that the child’s household contacts had all been sick a week before with presumed viral gastroenteritis. They decided to run a stool screen and the polymerase chain reaction for norovirus was positive. The boy never had GI symptoms.
Dr. Kirkorian said in the interview that she has seen other RIME cases where a child did not have symptoms associated with the original virus but did have a sudden onset of mucositis.
Although the definition of RIME is evolving, it is defined in part by mucositis in at least two of three areas: the mouth, eyes, and genitals. “Once you have the inflammation of the mucous membranes you should be on alert to think about more serious conditions,” like RIME, said Dr. Kirkorian. “Why does it manifest with the mucositis? I don’t think we know that,” she added.
RIME recurrence has also been vexing for patients, families and clinicians. In May, at the annual Atlantic Dermatology Conference, held in Baltimore, Dr. Kirkorian also discussed an 11-year-old patient who had RIME after SARS-CoV-2 infection early in the pandemic, resulting in a 22-day hospitalization and placement of a peripherally inserted central catheter and a feeding tube. He improved with cyclosporine and was discharged on systemic tacrolimus.
He was fine for several years, until another COVID infection. He again responded to medication. But not long after, an undetermined viral infection triggered another episode of RIME.
Dr. Kirkorian said there is no way to predict recurrence – making a devastating condition all the more worrisome. “Knowing that it might come back and it’s totally haphazard as to what might make it come back – that is very stressful for families,” she said in the interview.
“Some of the most perplexing patients with RIME are those with recurrent disease,” wrote Warren R. Heymann, MD, professor of dermatology and pediatrics at Rowan University, Camden, N.J., wrote in an online column on RIME in the American Academy of Dermatology’s “Dermatology World Insights and Inquiries”.
“Recurrent RIME is of particular interest, given that we could potentially intervene and prevent additional disease,” wrote Camille Introcaso, MD, associate professor of medicine at Rowan University, in response to Dr. Heymann’s remarks. “Although multiple possible mechanisms for the clinical findings of RIME have been proposed, including molecular mimicry between infectious agent proteins and keratinocyte antigens, immune complex deposition, and combinations of medication and infection, the pathophysiology is unknown,” she added.
In the interview, Dr. Kirkorian said that she and colleagues in the Pediatric Dermatology Research Alliance (PeDRA) are trying to assemble more multicenter trials to assess the underlying pathology of RIME, effectiveness of various treatments, and to “find some predictive factors.” Given that RIME is an acute-onset emergency, it is not easy to conduct randomized controlled trials, she added.
Dr. Kirkorian, Dr. Heymann, and Dr. Introcaso report no relevant financial relationships.
, according to a newly published case report.
Lead author Anna Yasmine Kirkorian, MD, chief of dermatology at Children’s National Hospital in Washington, said she wanted to get the word out in part because it seems like RIME is occurring more frequently. “I do feel like we’re seeing more cases and from a more diverse number of pathogens,” Dr. Kirkorian told this news organization.
There was a decrease in RIME during the early stages of the COVID-19 pandemic when people were isolating more, Dr. Kirkorian said. SARS-CoV-2 has been a trigger for some cases, but she did not find that remarkable, given that respiratory viruses are known RIME precursors. The question is why RIME is being triggered more frequently now that people have essentially gone back to their normal lives, she said.
Dr. Kirkorian and colleagues at Children’s National Hospital and George Washington University, Washington, wrote about a 5-year-old boy with norovirus-triggered RIME in a case report published in Pediatric Dermatology.
RIME – previously known as Mycoplasma pneumoniae–induced rash and mucositis (MIRM) – tends to arise after a viral infection, with upper respiratory viruses such as mycoplasma and Chlamydophila pneumoniae, influenza, and enterovirus among the common triggers. “We think this is actually your own immune system overreacting to a pathogen,” Dr. Kirkorian said in an interview, adding that the mechanism of RIME is still not understood.
While the norovirus discovery was a surprise, it shows that much is still unknown about this rare condition. “I don’t think we know what is usual and what is unusual,” Dr. Kirkorian said.
In this case, the boy swiftly declined, with progressive conjunctivitis, high fever, and rapidly developing mucositis. By the time the 5-year-old got to Children’s National Hospital, he had a spreading, painful rash, including tense vesicles and bullae involving more than 30% of his total body surface area, and areas of denuded skin on both cheeks and the back of his neck.
He had hemorrhagic mucositis of the lips, a large erosion at the urethral meatus, and hemorrhagic conjunctivitis of both eyes with thick yellow crusting on the eyelids.
The clinicians intubated the boy and admitted him to the intensive care unit. He was given a one-time injection of etanercept (25 mg) followed by 8 days of intravenous cyclosporine at a dose of 5 mg per kilogram, divided twice daily, which helped calm the mucositis and stopped the rash from progressing. There is not an accepted protocol or list of evidence-based therapeutics for RIME, Dr. Kirkorian noted.
The severe eye damage required amniotic membrane grafts. The patient was extubated after 9 days but remained in the hospital for a total of 26 days because he needed to receive nutritional support (the mucositis kept him from eating), and for pain control and weaning of sedation.
As the clinicians searched for a potential triggering virus, they came up empty. Results were negative for adenovirus, Epstein Barr virus, cytomegalovirus, herpes simplex, and varicella zoster. But they noted that the child’s household contacts had all been sick a week before with presumed viral gastroenteritis. They decided to run a stool screen and the polymerase chain reaction for norovirus was positive. The boy never had GI symptoms.
Dr. Kirkorian said in the interview that she has seen other RIME cases where a child did not have symptoms associated with the original virus but did have a sudden onset of mucositis.
Although the definition of RIME is evolving, it is defined in part by mucositis in at least two of three areas: the mouth, eyes, and genitals. “Once you have the inflammation of the mucous membranes you should be on alert to think about more serious conditions,” like RIME, said Dr. Kirkorian. “Why does it manifest with the mucositis? I don’t think we know that,” she added.
RIME recurrence has also been vexing for patients, families and clinicians. In May, at the annual Atlantic Dermatology Conference, held in Baltimore, Dr. Kirkorian also discussed an 11-year-old patient who had RIME after SARS-CoV-2 infection early in the pandemic, resulting in a 22-day hospitalization and placement of a peripherally inserted central catheter and a feeding tube. He improved with cyclosporine and was discharged on systemic tacrolimus.
He was fine for several years, until another COVID infection. He again responded to medication. But not long after, an undetermined viral infection triggered another episode of RIME.
Dr. Kirkorian said there is no way to predict recurrence – making a devastating condition all the more worrisome. “Knowing that it might come back and it’s totally haphazard as to what might make it come back – that is very stressful for families,” she said in the interview.
“Some of the most perplexing patients with RIME are those with recurrent disease,” wrote Warren R. Heymann, MD, professor of dermatology and pediatrics at Rowan University, Camden, N.J., wrote in an online column on RIME in the American Academy of Dermatology’s “Dermatology World Insights and Inquiries”.
“Recurrent RIME is of particular interest, given that we could potentially intervene and prevent additional disease,” wrote Camille Introcaso, MD, associate professor of medicine at Rowan University, in response to Dr. Heymann’s remarks. “Although multiple possible mechanisms for the clinical findings of RIME have been proposed, including molecular mimicry between infectious agent proteins and keratinocyte antigens, immune complex deposition, and combinations of medication and infection, the pathophysiology is unknown,” she added.
In the interview, Dr. Kirkorian said that she and colleagues in the Pediatric Dermatology Research Alliance (PeDRA) are trying to assemble more multicenter trials to assess the underlying pathology of RIME, effectiveness of various treatments, and to “find some predictive factors.” Given that RIME is an acute-onset emergency, it is not easy to conduct randomized controlled trials, she added.
Dr. Kirkorian, Dr. Heymann, and Dr. Introcaso report no relevant financial relationships.
, according to a newly published case report.
Lead author Anna Yasmine Kirkorian, MD, chief of dermatology at Children’s National Hospital in Washington, said she wanted to get the word out in part because it seems like RIME is occurring more frequently. “I do feel like we’re seeing more cases and from a more diverse number of pathogens,” Dr. Kirkorian told this news organization.
There was a decrease in RIME during the early stages of the COVID-19 pandemic when people were isolating more, Dr. Kirkorian said. SARS-CoV-2 has been a trigger for some cases, but she did not find that remarkable, given that respiratory viruses are known RIME precursors. The question is why RIME is being triggered more frequently now that people have essentially gone back to their normal lives, she said.
Dr. Kirkorian and colleagues at Children’s National Hospital and George Washington University, Washington, wrote about a 5-year-old boy with norovirus-triggered RIME in a case report published in Pediatric Dermatology.
RIME – previously known as Mycoplasma pneumoniae–induced rash and mucositis (MIRM) – tends to arise after a viral infection, with upper respiratory viruses such as mycoplasma and Chlamydophila pneumoniae, influenza, and enterovirus among the common triggers. “We think this is actually your own immune system overreacting to a pathogen,” Dr. Kirkorian said in an interview, adding that the mechanism of RIME is still not understood.
While the norovirus discovery was a surprise, it shows that much is still unknown about this rare condition. “I don’t think we know what is usual and what is unusual,” Dr. Kirkorian said.
In this case, the boy swiftly declined, with progressive conjunctivitis, high fever, and rapidly developing mucositis. By the time the 5-year-old got to Children’s National Hospital, he had a spreading, painful rash, including tense vesicles and bullae involving more than 30% of his total body surface area, and areas of denuded skin on both cheeks and the back of his neck.
He had hemorrhagic mucositis of the lips, a large erosion at the urethral meatus, and hemorrhagic conjunctivitis of both eyes with thick yellow crusting on the eyelids.
The clinicians intubated the boy and admitted him to the intensive care unit. He was given a one-time injection of etanercept (25 mg) followed by 8 days of intravenous cyclosporine at a dose of 5 mg per kilogram, divided twice daily, which helped calm the mucositis and stopped the rash from progressing. There is not an accepted protocol or list of evidence-based therapeutics for RIME, Dr. Kirkorian noted.
The severe eye damage required amniotic membrane grafts. The patient was extubated after 9 days but remained in the hospital for a total of 26 days because he needed to receive nutritional support (the mucositis kept him from eating), and for pain control and weaning of sedation.
As the clinicians searched for a potential triggering virus, they came up empty. Results were negative for adenovirus, Epstein Barr virus, cytomegalovirus, herpes simplex, and varicella zoster. But they noted that the child’s household contacts had all been sick a week before with presumed viral gastroenteritis. They decided to run a stool screen and the polymerase chain reaction for norovirus was positive. The boy never had GI symptoms.
Dr. Kirkorian said in the interview that she has seen other RIME cases where a child did not have symptoms associated with the original virus but did have a sudden onset of mucositis.
Although the definition of RIME is evolving, it is defined in part by mucositis in at least two of three areas: the mouth, eyes, and genitals. “Once you have the inflammation of the mucous membranes you should be on alert to think about more serious conditions,” like RIME, said Dr. Kirkorian. “Why does it manifest with the mucositis? I don’t think we know that,” she added.
RIME recurrence has also been vexing for patients, families and clinicians. In May, at the annual Atlantic Dermatology Conference, held in Baltimore, Dr. Kirkorian also discussed an 11-year-old patient who had RIME after SARS-CoV-2 infection early in the pandemic, resulting in a 22-day hospitalization and placement of a peripherally inserted central catheter and a feeding tube. He improved with cyclosporine and was discharged on systemic tacrolimus.
He was fine for several years, until another COVID infection. He again responded to medication. But not long after, an undetermined viral infection triggered another episode of RIME.
Dr. Kirkorian said there is no way to predict recurrence – making a devastating condition all the more worrisome. “Knowing that it might come back and it’s totally haphazard as to what might make it come back – that is very stressful for families,” she said in the interview.
“Some of the most perplexing patients with RIME are those with recurrent disease,” wrote Warren R. Heymann, MD, professor of dermatology and pediatrics at Rowan University, Camden, N.J., wrote in an online column on RIME in the American Academy of Dermatology’s “Dermatology World Insights and Inquiries”.
“Recurrent RIME is of particular interest, given that we could potentially intervene and prevent additional disease,” wrote Camille Introcaso, MD, associate professor of medicine at Rowan University, in response to Dr. Heymann’s remarks. “Although multiple possible mechanisms for the clinical findings of RIME have been proposed, including molecular mimicry between infectious agent proteins and keratinocyte antigens, immune complex deposition, and combinations of medication and infection, the pathophysiology is unknown,” she added.
In the interview, Dr. Kirkorian said that she and colleagues in the Pediatric Dermatology Research Alliance (PeDRA) are trying to assemble more multicenter trials to assess the underlying pathology of RIME, effectiveness of various treatments, and to “find some predictive factors.” Given that RIME is an acute-onset emergency, it is not easy to conduct randomized controlled trials, she added.
Dr. Kirkorian, Dr. Heymann, and Dr. Introcaso report no relevant financial relationships.
In new era of gene therapy, PCPs are ‘boots on the ground’
In Colorado and Wyoming, nearly every baby born since 2020 is tested for signs of a mutation in the SMN1 gene, an indicator of spinal muscular atrophy (SMA). And in 4 years, genetic counselor Melissa Gibbons has seen 24 positive results. She has prepped 24 different pediatricians and family doctors to deliver the news: A seemingly perfect newborn likely has a lethal genetic disease.
Most of these clinicians had never cared for a child with SMA before, nor did they know that lifesaving gene therapy for the condition now exists. Still, the physicians were foundational to getting babies emergency treatment and monitoring the child’s safety after the fact.
“They are boots on the ground for this kind of [work],” Ms. Gibbons, who is the newborn screen coordinator for SMA in both states, told this news organization. “I’m not even sure they realize it.” As of today, the U.S. Food and Drug Administration has approved 16 gene therapies for the treatment of rare and debilitating diseases once considered lethal, such as SMA and cerebral adrenoleukodystrophy.
The newest addition to the list of approvals is Elevidys, Sarepta’s gene therapy for Duchenne muscular dystrophy (DMD). These conditions can now be mitigated, abated for years at a time, and even cured using treatments that tweak a patient’s DNA or RNA.
Hundreds of treatments are under development using the same mechanism. Viruses, liposomes, and other vectors of all kinds are being used to usher new genes into cells, correcting faulty copies or equipping a cell to fight disease. Cells gain the ability to make lifesaving proteins – proteins that heal wounds, restore muscle function, and fight cancer.
Within the decade, a significant fraction of the pediatric population will have gone through gene therapy, experts told this news organization. And primary care stands to be a linchpin in the scale-up of this kind of precision genetic medicine. Pediatricians and general practitioners will be central to finding and monitoring the patients that need these treatments. But the time and support doctors will need to fill that role remain scarce.
“This is a world we are creating right now, quite literally,” said Stanley Nelson, MD, director of the center for Duchenne muscular dystrophy at the University of California, Los Angeles. These cases – some before gene therapy and some after – will show up in primary care offices before the textbook is written.
Unknown side effects, new diseases
Even now, gene therapy is sequestered away in large academic medical research centers. The diagnosis, decision-making, and aftercare are handled by subspecialists working on clinical trials. While the research is ongoing, trial sponsors are keeping a close eye on enrolled patients. But that’s only until these drugs get market approval, Phil Beales, MD, chief medical officer at Congenica, a digital health company specializing in genome analysis support, said. Afterward, “the trialists will no longer have a role in looking after those patients.”
At that point, the role of primary care clinicians will be critically important. Although they probably will not manage gene-therapy patients on their own – comanaging them instead with subspecialists – they will be involved in the ordering and monitoring of safety labs and other tests.
General practitioners “need to know side effects because they are going to deal with side effects when someone calls them in the middle of the night,” said Dr. Beales, who also is chief executive officer of Axovia Therapeutics, a biotech company developing gene therapies.
Some of the side effects that come with gene therapy are established. Adeno-associated virus (AAV) or AAV-mediated gene therapies carry an increased risk for damage to the heart and liver, Dr. Nelson said. Other side effects are less well known and could be specific to the treatment and the tissue it targets. Primary care will be critical in detecting these unexpected side effects and expediting visits with subspecialists, he said.
In rural Wyoming, pediatricians and family doctors are especially important, Ms. Gibbons said. In the 30-90 days after gene therapy, patients need a lot of follow-up for safety reasons.
But aftercare for gene therapy will be more than just monitoring and managing side effects. The diseases themselves will change. Patients will be living with conditions that once were lethal.
In some cases, gene therapy may largely eliminate the disease. The data suggest that thalassemia, for example, can be largely cured for decades with one infusion of a patient’s genetically modified hematopoietic stem cells made using bluebird bio’s Zynteglo, according to Christy Duncan, MD, medical director of clinical research at the gene therapy program at Boston Children’s Hospital.
But other gene therapies, like the one for DMD, will offer a “spectrum of benefits,” Dr. Nelson said. They will be lifesaving, but the signs of the disease will linger. Clinicians will be learning alongside specialists what the new disease state for DMD and other rare diseases looks like after gene therapy.
“As we get hundreds of such therapies, [post–gene therapy] will amount to a substantial part of the pediatric population,” Dr. Nelson said.
Finding patients
Many of these rare diseases that plague young patients are unmistakable. Children with moderate or severe dystrophic epidermolysis bullosa, for instance, carry a mutation that prevents them from making type VII collagen. The babies suffer wounds and excessive bleeding and tend to receive a quick diagnosis within the first 6 months of life, according to Andy Orth, chief commercial officer at Krystal Bio, manufacturer of a new wound-healing gene therapy, Vyjuvek, for the disorder.
Other rare neurologic or muscular diseases can go undiagnosed for years. Until recently, drug companies and researchers have had little motivation to speed up the timeline because early diagnosis of a disease like DMD would not change the outcome, Dr. Nelson said.
But with gene therapy, prognoses are changing. And finding diseases early could soon mean preserving muscular function or preventing neurologic damage, Dr. Duncan said.
Newborn sequencing “is not standard of care yet, but it’s certainly coming,” Josh Peterson, MD, MPH, director of the center for precision medicine at Vanderbilt University Medical Center, in Nashville, Tenn., told this news organization.
A recent survey of 238 specialists in rare diseases found that roughly 90% believe whole-genome sequencing should be available to all newborns. And 80% of those experts endorse 42 genes as disease predictors. Screening for rare diseases at birth could reveal a host of conditions in the first week of life and expedite treatment. But this strategy will often rely on primary care and pediatricians interpreting the results.
Most pediatricians think sequencing is a great idea, but they do not feel comfortable doing it themselves, Dr. Peterson said. The good news, he said, is that manufacturers have made screening tests straightforward. Some drug companies even offer free screenings for gene therapy candidates.
Dr. Peterson predicts pediatricians will need to be equipped to deliver negative results on their own, which will be the case for around 97%-99% of patients. They also will need to be clear on whether a negative result is definitive or if more testing is warranted.
Positive results are more nuanced. Genetic counseling is the ideal resource when delivering this kind of news to patients, but counselors are a scarce resource nationally – and particularly in rural areas, Dr. Nelson said. Physicians likely will have to rely on their own counseling training to some degree.
“I feel very strongly that genetic counselors are in short supply,” Ms. Gibbons in Colorado said. Patients need a friendly resource who can talk them through the disease and how it works. And that discussion is not a one-off, she said.
The number of board-certified genetic counselors in the United States has doubled to more than 6,000 in the past 10 years – a pace that is expected to continue, according to the National Society of Genetic Counselors. “However, the geographical distribution of genetic counselors is most concentrated in urban centers.”
Equally important to the counseling experience, according to Dr. Duncan at Boston Children’s, is a primary care physician’s network of connections. The best newborn screening rollouts across the country have succeeded because clinicians knew where to send people next and how to get families the help they needed, she said.
But she also cautioned that this learning curve will soon be overwhelming. As gene therapy expands, it may be difficult for primary care doctors to keep up with the science, treatment studies, and commercially available therapies. “It’s asking too much,” Dr. Duncan said.
The structure of primary care already stretches practitioners thin and will “affect how well precision medicine can be adopted and disseminated,” Dr. Peterson said. “I think that is a key issue.”
Artificial intelligence may offer a partial solution. Some genetic counseling models already exist, but their utility for clinicians so far is limited, Dr. Beales said. But he said he expects these tools to improve rapidly to help clinicians and patients. On the patient’s end, they may be able to answer questions and supplement basic genetic counseling. On the physician’s end, algorithms could help triage patients and help move them along to the next steps in the care pathway for these rare diseases.
The whole patient
Primary care physicians will not be expected to be experts in gene therapy or solely in charge of patient safety. They will have support from industry and subspecialists leading the development of these treatments, experts agreed.
But generalists should expect to be drawn into multidisciplinary care teams, be the sounding boards for patients making decisions about gene therapy, help arrange insurance coverage, and be the recipients of late-night phone calls about side effects.
All that, while never losing sight of the child’s holistic health. In children so sick, specialists, subspecialists, and even parents tend to focus only on the rare disease. The team can “get distracted from good normal routine care,” Dr. Nelson said. But these children aren’t exempt from check-ups, vaccine regimens, or the other diseases of childhood.
“In a world where we mitigate that core disease,” he said, “we need a partner in the general pediatrics community” investing in their long-term health.
A version of this article first appeared on Medscape.com.
In Colorado and Wyoming, nearly every baby born since 2020 is tested for signs of a mutation in the SMN1 gene, an indicator of spinal muscular atrophy (SMA). And in 4 years, genetic counselor Melissa Gibbons has seen 24 positive results. She has prepped 24 different pediatricians and family doctors to deliver the news: A seemingly perfect newborn likely has a lethal genetic disease.
Most of these clinicians had never cared for a child with SMA before, nor did they know that lifesaving gene therapy for the condition now exists. Still, the physicians were foundational to getting babies emergency treatment and monitoring the child’s safety after the fact.
“They are boots on the ground for this kind of [work],” Ms. Gibbons, who is the newborn screen coordinator for SMA in both states, told this news organization. “I’m not even sure they realize it.” As of today, the U.S. Food and Drug Administration has approved 16 gene therapies for the treatment of rare and debilitating diseases once considered lethal, such as SMA and cerebral adrenoleukodystrophy.
The newest addition to the list of approvals is Elevidys, Sarepta’s gene therapy for Duchenne muscular dystrophy (DMD). These conditions can now be mitigated, abated for years at a time, and even cured using treatments that tweak a patient’s DNA or RNA.
Hundreds of treatments are under development using the same mechanism. Viruses, liposomes, and other vectors of all kinds are being used to usher new genes into cells, correcting faulty copies or equipping a cell to fight disease. Cells gain the ability to make lifesaving proteins – proteins that heal wounds, restore muscle function, and fight cancer.
Within the decade, a significant fraction of the pediatric population will have gone through gene therapy, experts told this news organization. And primary care stands to be a linchpin in the scale-up of this kind of precision genetic medicine. Pediatricians and general practitioners will be central to finding and monitoring the patients that need these treatments. But the time and support doctors will need to fill that role remain scarce.
“This is a world we are creating right now, quite literally,” said Stanley Nelson, MD, director of the center for Duchenne muscular dystrophy at the University of California, Los Angeles. These cases – some before gene therapy and some after – will show up in primary care offices before the textbook is written.
Unknown side effects, new diseases
Even now, gene therapy is sequestered away in large academic medical research centers. The diagnosis, decision-making, and aftercare are handled by subspecialists working on clinical trials. While the research is ongoing, trial sponsors are keeping a close eye on enrolled patients. But that’s only until these drugs get market approval, Phil Beales, MD, chief medical officer at Congenica, a digital health company specializing in genome analysis support, said. Afterward, “the trialists will no longer have a role in looking after those patients.”
At that point, the role of primary care clinicians will be critically important. Although they probably will not manage gene-therapy patients on their own – comanaging them instead with subspecialists – they will be involved in the ordering and monitoring of safety labs and other tests.
General practitioners “need to know side effects because they are going to deal with side effects when someone calls them in the middle of the night,” said Dr. Beales, who also is chief executive officer of Axovia Therapeutics, a biotech company developing gene therapies.
Some of the side effects that come with gene therapy are established. Adeno-associated virus (AAV) or AAV-mediated gene therapies carry an increased risk for damage to the heart and liver, Dr. Nelson said. Other side effects are less well known and could be specific to the treatment and the tissue it targets. Primary care will be critical in detecting these unexpected side effects and expediting visits with subspecialists, he said.
In rural Wyoming, pediatricians and family doctors are especially important, Ms. Gibbons said. In the 30-90 days after gene therapy, patients need a lot of follow-up for safety reasons.
But aftercare for gene therapy will be more than just monitoring and managing side effects. The diseases themselves will change. Patients will be living with conditions that once were lethal.
In some cases, gene therapy may largely eliminate the disease. The data suggest that thalassemia, for example, can be largely cured for decades with one infusion of a patient’s genetically modified hematopoietic stem cells made using bluebird bio’s Zynteglo, according to Christy Duncan, MD, medical director of clinical research at the gene therapy program at Boston Children’s Hospital.
But other gene therapies, like the one for DMD, will offer a “spectrum of benefits,” Dr. Nelson said. They will be lifesaving, but the signs of the disease will linger. Clinicians will be learning alongside specialists what the new disease state for DMD and other rare diseases looks like after gene therapy.
“As we get hundreds of such therapies, [post–gene therapy] will amount to a substantial part of the pediatric population,” Dr. Nelson said.
Finding patients
Many of these rare diseases that plague young patients are unmistakable. Children with moderate or severe dystrophic epidermolysis bullosa, for instance, carry a mutation that prevents them from making type VII collagen. The babies suffer wounds and excessive bleeding and tend to receive a quick diagnosis within the first 6 months of life, according to Andy Orth, chief commercial officer at Krystal Bio, manufacturer of a new wound-healing gene therapy, Vyjuvek, for the disorder.
Other rare neurologic or muscular diseases can go undiagnosed for years. Until recently, drug companies and researchers have had little motivation to speed up the timeline because early diagnosis of a disease like DMD would not change the outcome, Dr. Nelson said.
But with gene therapy, prognoses are changing. And finding diseases early could soon mean preserving muscular function or preventing neurologic damage, Dr. Duncan said.
Newborn sequencing “is not standard of care yet, but it’s certainly coming,” Josh Peterson, MD, MPH, director of the center for precision medicine at Vanderbilt University Medical Center, in Nashville, Tenn., told this news organization.
A recent survey of 238 specialists in rare diseases found that roughly 90% believe whole-genome sequencing should be available to all newborns. And 80% of those experts endorse 42 genes as disease predictors. Screening for rare diseases at birth could reveal a host of conditions in the first week of life and expedite treatment. But this strategy will often rely on primary care and pediatricians interpreting the results.
Most pediatricians think sequencing is a great idea, but they do not feel comfortable doing it themselves, Dr. Peterson said. The good news, he said, is that manufacturers have made screening tests straightforward. Some drug companies even offer free screenings for gene therapy candidates.
Dr. Peterson predicts pediatricians will need to be equipped to deliver negative results on their own, which will be the case for around 97%-99% of patients. They also will need to be clear on whether a negative result is definitive or if more testing is warranted.
Positive results are more nuanced. Genetic counseling is the ideal resource when delivering this kind of news to patients, but counselors are a scarce resource nationally – and particularly in rural areas, Dr. Nelson said. Physicians likely will have to rely on their own counseling training to some degree.
“I feel very strongly that genetic counselors are in short supply,” Ms. Gibbons in Colorado said. Patients need a friendly resource who can talk them through the disease and how it works. And that discussion is not a one-off, she said.
The number of board-certified genetic counselors in the United States has doubled to more than 6,000 in the past 10 years – a pace that is expected to continue, according to the National Society of Genetic Counselors. “However, the geographical distribution of genetic counselors is most concentrated in urban centers.”
Equally important to the counseling experience, according to Dr. Duncan at Boston Children’s, is a primary care physician’s network of connections. The best newborn screening rollouts across the country have succeeded because clinicians knew where to send people next and how to get families the help they needed, she said.
But she also cautioned that this learning curve will soon be overwhelming. As gene therapy expands, it may be difficult for primary care doctors to keep up with the science, treatment studies, and commercially available therapies. “It’s asking too much,” Dr. Duncan said.
The structure of primary care already stretches practitioners thin and will “affect how well precision medicine can be adopted and disseminated,” Dr. Peterson said. “I think that is a key issue.”
Artificial intelligence may offer a partial solution. Some genetic counseling models already exist, but their utility for clinicians so far is limited, Dr. Beales said. But he said he expects these tools to improve rapidly to help clinicians and patients. On the patient’s end, they may be able to answer questions and supplement basic genetic counseling. On the physician’s end, algorithms could help triage patients and help move them along to the next steps in the care pathway for these rare diseases.
The whole patient
Primary care physicians will not be expected to be experts in gene therapy or solely in charge of patient safety. They will have support from industry and subspecialists leading the development of these treatments, experts agreed.
But generalists should expect to be drawn into multidisciplinary care teams, be the sounding boards for patients making decisions about gene therapy, help arrange insurance coverage, and be the recipients of late-night phone calls about side effects.
All that, while never losing sight of the child’s holistic health. In children so sick, specialists, subspecialists, and even parents tend to focus only on the rare disease. The team can “get distracted from good normal routine care,” Dr. Nelson said. But these children aren’t exempt from check-ups, vaccine regimens, or the other diseases of childhood.
“In a world where we mitigate that core disease,” he said, “we need a partner in the general pediatrics community” investing in their long-term health.
A version of this article first appeared on Medscape.com.
In Colorado and Wyoming, nearly every baby born since 2020 is tested for signs of a mutation in the SMN1 gene, an indicator of spinal muscular atrophy (SMA). And in 4 years, genetic counselor Melissa Gibbons has seen 24 positive results. She has prepped 24 different pediatricians and family doctors to deliver the news: A seemingly perfect newborn likely has a lethal genetic disease.
Most of these clinicians had never cared for a child with SMA before, nor did they know that lifesaving gene therapy for the condition now exists. Still, the physicians were foundational to getting babies emergency treatment and monitoring the child’s safety after the fact.
“They are boots on the ground for this kind of [work],” Ms. Gibbons, who is the newborn screen coordinator for SMA in both states, told this news organization. “I’m not even sure they realize it.” As of today, the U.S. Food and Drug Administration has approved 16 gene therapies for the treatment of rare and debilitating diseases once considered lethal, such as SMA and cerebral adrenoleukodystrophy.
The newest addition to the list of approvals is Elevidys, Sarepta’s gene therapy for Duchenne muscular dystrophy (DMD). These conditions can now be mitigated, abated for years at a time, and even cured using treatments that tweak a patient’s DNA or RNA.
Hundreds of treatments are under development using the same mechanism. Viruses, liposomes, and other vectors of all kinds are being used to usher new genes into cells, correcting faulty copies or equipping a cell to fight disease. Cells gain the ability to make lifesaving proteins – proteins that heal wounds, restore muscle function, and fight cancer.
Within the decade, a significant fraction of the pediatric population will have gone through gene therapy, experts told this news organization. And primary care stands to be a linchpin in the scale-up of this kind of precision genetic medicine. Pediatricians and general practitioners will be central to finding and monitoring the patients that need these treatments. But the time and support doctors will need to fill that role remain scarce.
“This is a world we are creating right now, quite literally,” said Stanley Nelson, MD, director of the center for Duchenne muscular dystrophy at the University of California, Los Angeles. These cases – some before gene therapy and some after – will show up in primary care offices before the textbook is written.
Unknown side effects, new diseases
Even now, gene therapy is sequestered away in large academic medical research centers. The diagnosis, decision-making, and aftercare are handled by subspecialists working on clinical trials. While the research is ongoing, trial sponsors are keeping a close eye on enrolled patients. But that’s only until these drugs get market approval, Phil Beales, MD, chief medical officer at Congenica, a digital health company specializing in genome analysis support, said. Afterward, “the trialists will no longer have a role in looking after those patients.”
At that point, the role of primary care clinicians will be critically important. Although they probably will not manage gene-therapy patients on their own – comanaging them instead with subspecialists – they will be involved in the ordering and monitoring of safety labs and other tests.
General practitioners “need to know side effects because they are going to deal with side effects when someone calls them in the middle of the night,” said Dr. Beales, who also is chief executive officer of Axovia Therapeutics, a biotech company developing gene therapies.
Some of the side effects that come with gene therapy are established. Adeno-associated virus (AAV) or AAV-mediated gene therapies carry an increased risk for damage to the heart and liver, Dr. Nelson said. Other side effects are less well known and could be specific to the treatment and the tissue it targets. Primary care will be critical in detecting these unexpected side effects and expediting visits with subspecialists, he said.
In rural Wyoming, pediatricians and family doctors are especially important, Ms. Gibbons said. In the 30-90 days after gene therapy, patients need a lot of follow-up for safety reasons.
But aftercare for gene therapy will be more than just monitoring and managing side effects. The diseases themselves will change. Patients will be living with conditions that once were lethal.
In some cases, gene therapy may largely eliminate the disease. The data suggest that thalassemia, for example, can be largely cured for decades with one infusion of a patient’s genetically modified hematopoietic stem cells made using bluebird bio’s Zynteglo, according to Christy Duncan, MD, medical director of clinical research at the gene therapy program at Boston Children’s Hospital.
But other gene therapies, like the one for DMD, will offer a “spectrum of benefits,” Dr. Nelson said. They will be lifesaving, but the signs of the disease will linger. Clinicians will be learning alongside specialists what the new disease state for DMD and other rare diseases looks like after gene therapy.
“As we get hundreds of such therapies, [post–gene therapy] will amount to a substantial part of the pediatric population,” Dr. Nelson said.
Finding patients
Many of these rare diseases that plague young patients are unmistakable. Children with moderate or severe dystrophic epidermolysis bullosa, for instance, carry a mutation that prevents them from making type VII collagen. The babies suffer wounds and excessive bleeding and tend to receive a quick diagnosis within the first 6 months of life, according to Andy Orth, chief commercial officer at Krystal Bio, manufacturer of a new wound-healing gene therapy, Vyjuvek, for the disorder.
Other rare neurologic or muscular diseases can go undiagnosed for years. Until recently, drug companies and researchers have had little motivation to speed up the timeline because early diagnosis of a disease like DMD would not change the outcome, Dr. Nelson said.
But with gene therapy, prognoses are changing. And finding diseases early could soon mean preserving muscular function or preventing neurologic damage, Dr. Duncan said.
Newborn sequencing “is not standard of care yet, but it’s certainly coming,” Josh Peterson, MD, MPH, director of the center for precision medicine at Vanderbilt University Medical Center, in Nashville, Tenn., told this news organization.
A recent survey of 238 specialists in rare diseases found that roughly 90% believe whole-genome sequencing should be available to all newborns. And 80% of those experts endorse 42 genes as disease predictors. Screening for rare diseases at birth could reveal a host of conditions in the first week of life and expedite treatment. But this strategy will often rely on primary care and pediatricians interpreting the results.
Most pediatricians think sequencing is a great idea, but they do not feel comfortable doing it themselves, Dr. Peterson said. The good news, he said, is that manufacturers have made screening tests straightforward. Some drug companies even offer free screenings for gene therapy candidates.
Dr. Peterson predicts pediatricians will need to be equipped to deliver negative results on their own, which will be the case for around 97%-99% of patients. They also will need to be clear on whether a negative result is definitive or if more testing is warranted.
Positive results are more nuanced. Genetic counseling is the ideal resource when delivering this kind of news to patients, but counselors are a scarce resource nationally – and particularly in rural areas, Dr. Nelson said. Physicians likely will have to rely on their own counseling training to some degree.
“I feel very strongly that genetic counselors are in short supply,” Ms. Gibbons in Colorado said. Patients need a friendly resource who can talk them through the disease and how it works. And that discussion is not a one-off, she said.
The number of board-certified genetic counselors in the United States has doubled to more than 6,000 in the past 10 years – a pace that is expected to continue, according to the National Society of Genetic Counselors. “However, the geographical distribution of genetic counselors is most concentrated in urban centers.”
Equally important to the counseling experience, according to Dr. Duncan at Boston Children’s, is a primary care physician’s network of connections. The best newborn screening rollouts across the country have succeeded because clinicians knew where to send people next and how to get families the help they needed, she said.
But she also cautioned that this learning curve will soon be overwhelming. As gene therapy expands, it may be difficult for primary care doctors to keep up with the science, treatment studies, and commercially available therapies. “It’s asking too much,” Dr. Duncan said.
The structure of primary care already stretches practitioners thin and will “affect how well precision medicine can be adopted and disseminated,” Dr. Peterson said. “I think that is a key issue.”
Artificial intelligence may offer a partial solution. Some genetic counseling models already exist, but their utility for clinicians so far is limited, Dr. Beales said. But he said he expects these tools to improve rapidly to help clinicians and patients. On the patient’s end, they may be able to answer questions and supplement basic genetic counseling. On the physician’s end, algorithms could help triage patients and help move them along to the next steps in the care pathway for these rare diseases.
The whole patient
Primary care physicians will not be expected to be experts in gene therapy or solely in charge of patient safety. They will have support from industry and subspecialists leading the development of these treatments, experts agreed.
But generalists should expect to be drawn into multidisciplinary care teams, be the sounding boards for patients making decisions about gene therapy, help arrange insurance coverage, and be the recipients of late-night phone calls about side effects.
All that, while never losing sight of the child’s holistic health. In children so sick, specialists, subspecialists, and even parents tend to focus only on the rare disease. The team can “get distracted from good normal routine care,” Dr. Nelson said. But these children aren’t exempt from check-ups, vaccine regimens, or the other diseases of childhood.
“In a world where we mitigate that core disease,” he said, “we need a partner in the general pediatrics community” investing in their long-term health.
A version of this article first appeared on Medscape.com.
Camp Discovery: A place for children to be comfortable in their own skin
The talent show, the grand finale of the 1-week camp, was nearly 7 years ago, but Emily Haygood of Houston, now 17 and about to start her senior year, remembers it in detail. She sang “Death of a Bachelor,” an R&B pop song and Billboard No. 1 hit at the time about a former bachelor who had happily married. These days, she said, if she watched the video of her 10-year-old singing self, “I would probably throw up.” But she still treasures the audience response, “having all those people I’d gotten close to cheer for me.”
Emily was at , but share one feature: they are the kind of dermatologic issues that can make doing everyday kid or teen activities like swimming difficult and can elicit mean comments from classmates and other would-be friends.

Emily was first diagnosed with atopic dermatitis at age 4, her mother, Amber Haygood, says. By age 9, it had become severe. Emily remembers being teased some in elementary school. “I did feel bad a lot of the time, when asked insensitive questions.” Her mother still bristles that adults often could be cruel, too.
But at Camp Discovery, those issues were nonexistent. “Camp was so cool,” Emily said. Besides the usual camp activities, it had things that “normal” camp didn’t, like other kids who didn’t stare at your skin condition or make fun of it.
30th anniversary season begins
This year is the 30th anniversary of Camp Discovery. Sessions began July 23 and continue through Aug. 18, with locations in Crosslake, Minn.; Hebron, Conn.; and Millville, Pa., in addition to Burton, Tex. About 300 campers will attend this year, according to the AAD, and 6,151 campers have attended from 1993 to 2022.
The 1-week camp accepts youth with conditions ranging from eczema and psoriasis to vitiligo, alopecia, epidermolysis bullosa, and ichthyosis, according to the academy. A dermatologist first refers a child, downloading and completing the referral form and sending it to the academy.
The 1-week session, including travel, is free for the campers, thanks to donors. As a nonprofit and membership-based organization, the AAD does not release the detailed financial information about the operating budget for the camp. Dermatologists, nurses, and counselors volunteer their time.
In his presidential address at the AAD’s annual meeting in March, outgoing president Mark D. Kaufmann, MD, of the department of dermatology at the Icahn School of Medicine at Mount Sinai in New York, referred to camp volunteering as an antidote to professional burnout. Remembering why as a dermatologist one entered the profession can be one solution, he said, and described his own recent 3-day volunteer stint at the camp.
“Those 3 magical days, being with kids as they discovered they weren’t alone in the world, sharing their experiences and ideas, reminded me why I became a physician in the first place,” he told the audience of meeting attendees. He vowed to expand the program, with a goal of having every dermatology resident attend Camp Discovery.
Mental health effects of skin conditions
Much research has focused on the mental health fallout from living with chronic skin conditions, and even young children can be adversely affected. In one review of the literature, researchers concluded that pediatric skin disease, including acne, atopic dermatitis, and psoriasis, can affect quality of life, carry stigma, and lead to bullying and eventually even suicidal behavior. Another study, published earlier this year, found that atopic dermatitis affected children’s quality of life, impacting sleep and leading to feelings of being ashamed.
“It’s not necessarily about what their skin condition is and more about the psychosocial impact,’’ said Samantha Hill, MD, a pediatric and general dermatologist in Lynchburg, Va., who is the medical director of Camp Discovery in Minnesota this year.
Camp activities, reactions
The overriding theme of camp is allowing all the youth to be “just one of the kids at camp,” Dr. Hill said in an interview. “They come to do all kinds of things they don’t do in normal life because people don’t give them the credit to [be able to] do it.”
Every year, she said, “I tell my staff we are in the business of making things happen, so if there is a kid bandaged head to toe [because of a skin condition] and they want to go tubing and get in the lake, we figure out how to make it happen. We have done that multiple times.”
Newcomers are initially nervous, Dr. Hill acknowledged, but in time let their guard down. Returnees are a different story. “When kids who have been at camp before arrive, you can see them start breathing again, looking for their friends. You can see them relax right before your eyes.”
“The single most empowering thing is the realization you are not alone,” said Meena Julapalli, MD, a Houston dermatologist who is a medical team member and long-time volunteer at Camp Discovery. That, she said, and “You get to be a kid, and you don’t have to have people staring at you.”
Dr. Julapalli remembers one of her patients with keratitis-ichthyosis-deafness (KID) syndrome. “She needed more than what I could offer,” she said. “She needed camp.” At camp, the organizers found a counselor who knew sign language to accompany her. At first, she was quiet and didn’t smile much. By the end of the week, as she was about to observe her birthday, things changed. After breakfast, she was led to the stage, where fellow campers began singing – and signing the song they had just learned.
Camp staff gets it
Allyson Garin, who was diagnosed with vitiligo at age 6 months, is a camp program director at Camp Discovery in Crosslake, Minn. She first went to camp in 1990 at age 11, returning until she “aged out” at 16, then worked as a counselor. She gets it when campers tell her they hear rude comments about their skin conditions.
“I remember being in swimming pools, in lines at fairgrounds or amusement parks,” she said in an interview, “and hearing people say, ‘Don’t touch her,’ ’’ fearing contagion, perhaps. “People would make jokes about cows, since they are spotted,” she said, or people would simply step back.
All those years ago, her mother found out about the camp and decided to figure out how to get her there. She got there, and she met a fellow camper with vitiligo, and they became pen pals. “We still talk,” she said.
Meeting someone with the same skin condition, she said, isn’t just about commiserating. “There is a lot of information sharing,” on topics such as best treatments, strategies, and other conversations.
Other lessons
While campers can feel comfortable around others who also have skin conditions, and understand, the lesson extends beyond that, Ms. Garin said. “It gave me a perspective,” she said of her camp experience. “I always felt, ‘Woe is me.’ ” But when she met others with, as she said, conditions “way worse than vitiligo, it really grounds you.”
Dr. Hill agreed. Campers get the benefit of others accepting and including them, but also practicing that same attitude toward fellow campers, she said. “It insures that we are providing this environment of inclusion, but that they are practicing it as well. They need to practice it like everyone else.”
Getting parents on board
The idea of camp, especially for those at the younger end of the 8- to 16-years age range accepted for Camp Discovery, can take some getting used to for some parents. Ms. Haygood, Emily’s mother, relates to that. Her daughter’s dermatologist at the time, who is now retired, had first suggested the camp. Her first reaction? “I am not sending my chronically ill child to camp with strangers.” She also acknowledged that she, like other parents of children with a chronic illness, can be a helicopter parent.
Then, she noticed that Emily seemed interested, so she got more information, finding out that it was staffed by doctors. It all sounded good, she said, and the social interaction, she knew, would be beneficial. “Then my husband was a no,” she said, concerned about their daughter being with strangers. “Eventually he came around,” Ms. Haygood said. All along, Emily said, “it seemed fun. I was probably trying to talk them into it.” She admits she was very nervous at first, but calmed down when she realized her own dermatologist was going to be there.
Vanessa Hadley of Spring, Tex., was on board the moment she heard about Camp Discovery. “I just thought it was amazing,” she said. Her daughter Isabelle, 13, has been to the camp. “She has alopecia areata and severe eczema,” Ms. Hadley said. Now, Isabelle is returning to camp and coaching her sister Penelope, 8, who has eczema and mild alopecia and is a first-timer this summer.
One tip the 8-year-old has learned so far: Turn to your counselor for support if you’re nervous. That worked, Isabelle said, the first year when she was wary of the zipline – then surprised herself and conquered it.
Dr. Hill and Dr. Julapalli have no disclosures.
The talent show, the grand finale of the 1-week camp, was nearly 7 years ago, but Emily Haygood of Houston, now 17 and about to start her senior year, remembers it in detail. She sang “Death of a Bachelor,” an R&B pop song and Billboard No. 1 hit at the time about a former bachelor who had happily married. These days, she said, if she watched the video of her 10-year-old singing self, “I would probably throw up.” But she still treasures the audience response, “having all those people I’d gotten close to cheer for me.”
Emily was at , but share one feature: they are the kind of dermatologic issues that can make doing everyday kid or teen activities like swimming difficult and can elicit mean comments from classmates and other would-be friends.

Emily was first diagnosed with atopic dermatitis at age 4, her mother, Amber Haygood, says. By age 9, it had become severe. Emily remembers being teased some in elementary school. “I did feel bad a lot of the time, when asked insensitive questions.” Her mother still bristles that adults often could be cruel, too.
But at Camp Discovery, those issues were nonexistent. “Camp was so cool,” Emily said. Besides the usual camp activities, it had things that “normal” camp didn’t, like other kids who didn’t stare at your skin condition or make fun of it.
30th anniversary season begins
This year is the 30th anniversary of Camp Discovery. Sessions began July 23 and continue through Aug. 18, with locations in Crosslake, Minn.; Hebron, Conn.; and Millville, Pa., in addition to Burton, Tex. About 300 campers will attend this year, according to the AAD, and 6,151 campers have attended from 1993 to 2022.
The 1-week camp accepts youth with conditions ranging from eczema and psoriasis to vitiligo, alopecia, epidermolysis bullosa, and ichthyosis, according to the academy. A dermatologist first refers a child, downloading and completing the referral form and sending it to the academy.
The 1-week session, including travel, is free for the campers, thanks to donors. As a nonprofit and membership-based organization, the AAD does not release the detailed financial information about the operating budget for the camp. Dermatologists, nurses, and counselors volunteer their time.
In his presidential address at the AAD’s annual meeting in March, outgoing president Mark D. Kaufmann, MD, of the department of dermatology at the Icahn School of Medicine at Mount Sinai in New York, referred to camp volunteering as an antidote to professional burnout. Remembering why as a dermatologist one entered the profession can be one solution, he said, and described his own recent 3-day volunteer stint at the camp.
“Those 3 magical days, being with kids as they discovered they weren’t alone in the world, sharing their experiences and ideas, reminded me why I became a physician in the first place,” he told the audience of meeting attendees. He vowed to expand the program, with a goal of having every dermatology resident attend Camp Discovery.
Mental health effects of skin conditions
Much research has focused on the mental health fallout from living with chronic skin conditions, and even young children can be adversely affected. In one review of the literature, researchers concluded that pediatric skin disease, including acne, atopic dermatitis, and psoriasis, can affect quality of life, carry stigma, and lead to bullying and eventually even suicidal behavior. Another study, published earlier this year, found that atopic dermatitis affected children’s quality of life, impacting sleep and leading to feelings of being ashamed.
“It’s not necessarily about what their skin condition is and more about the psychosocial impact,’’ said Samantha Hill, MD, a pediatric and general dermatologist in Lynchburg, Va., who is the medical director of Camp Discovery in Minnesota this year.
Camp activities, reactions
The overriding theme of camp is allowing all the youth to be “just one of the kids at camp,” Dr. Hill said in an interview. “They come to do all kinds of things they don’t do in normal life because people don’t give them the credit to [be able to] do it.”
Every year, she said, “I tell my staff we are in the business of making things happen, so if there is a kid bandaged head to toe [because of a skin condition] and they want to go tubing and get in the lake, we figure out how to make it happen. We have done that multiple times.”
Newcomers are initially nervous, Dr. Hill acknowledged, but in time let their guard down. Returnees are a different story. “When kids who have been at camp before arrive, you can see them start breathing again, looking for their friends. You can see them relax right before your eyes.”
“The single most empowering thing is the realization you are not alone,” said Meena Julapalli, MD, a Houston dermatologist who is a medical team member and long-time volunteer at Camp Discovery. That, she said, and “You get to be a kid, and you don’t have to have people staring at you.”
Dr. Julapalli remembers one of her patients with keratitis-ichthyosis-deafness (KID) syndrome. “She needed more than what I could offer,” she said. “She needed camp.” At camp, the organizers found a counselor who knew sign language to accompany her. At first, she was quiet and didn’t smile much. By the end of the week, as she was about to observe her birthday, things changed. After breakfast, she was led to the stage, where fellow campers began singing – and signing the song they had just learned.
Camp staff gets it
Allyson Garin, who was diagnosed with vitiligo at age 6 months, is a camp program director at Camp Discovery in Crosslake, Minn. She first went to camp in 1990 at age 11, returning until she “aged out” at 16, then worked as a counselor. She gets it when campers tell her they hear rude comments about their skin conditions.
“I remember being in swimming pools, in lines at fairgrounds or amusement parks,” she said in an interview, “and hearing people say, ‘Don’t touch her,’ ’’ fearing contagion, perhaps. “People would make jokes about cows, since they are spotted,” she said, or people would simply step back.
All those years ago, her mother found out about the camp and decided to figure out how to get her there. She got there, and she met a fellow camper with vitiligo, and they became pen pals. “We still talk,” she said.
Meeting someone with the same skin condition, she said, isn’t just about commiserating. “There is a lot of information sharing,” on topics such as best treatments, strategies, and other conversations.
Other lessons
While campers can feel comfortable around others who also have skin conditions, and understand, the lesson extends beyond that, Ms. Garin said. “It gave me a perspective,” she said of her camp experience. “I always felt, ‘Woe is me.’ ” But when she met others with, as she said, conditions “way worse than vitiligo, it really grounds you.”
Dr. Hill agreed. Campers get the benefit of others accepting and including them, but also practicing that same attitude toward fellow campers, she said. “It insures that we are providing this environment of inclusion, but that they are practicing it as well. They need to practice it like everyone else.”
Getting parents on board
The idea of camp, especially for those at the younger end of the 8- to 16-years age range accepted for Camp Discovery, can take some getting used to for some parents. Ms. Haygood, Emily’s mother, relates to that. Her daughter’s dermatologist at the time, who is now retired, had first suggested the camp. Her first reaction? “I am not sending my chronically ill child to camp with strangers.” She also acknowledged that she, like other parents of children with a chronic illness, can be a helicopter parent.
Then, she noticed that Emily seemed interested, so she got more information, finding out that it was staffed by doctors. It all sounded good, she said, and the social interaction, she knew, would be beneficial. “Then my husband was a no,” she said, concerned about their daughter being with strangers. “Eventually he came around,” Ms. Haygood said. All along, Emily said, “it seemed fun. I was probably trying to talk them into it.” She admits she was very nervous at first, but calmed down when she realized her own dermatologist was going to be there.
Vanessa Hadley of Spring, Tex., was on board the moment she heard about Camp Discovery. “I just thought it was amazing,” she said. Her daughter Isabelle, 13, has been to the camp. “She has alopecia areata and severe eczema,” Ms. Hadley said. Now, Isabelle is returning to camp and coaching her sister Penelope, 8, who has eczema and mild alopecia and is a first-timer this summer.
One tip the 8-year-old has learned so far: Turn to your counselor for support if you’re nervous. That worked, Isabelle said, the first year when she was wary of the zipline – then surprised herself and conquered it.
Dr. Hill and Dr. Julapalli have no disclosures.
The talent show, the grand finale of the 1-week camp, was nearly 7 years ago, but Emily Haygood of Houston, now 17 and about to start her senior year, remembers it in detail. She sang “Death of a Bachelor,” an R&B pop song and Billboard No. 1 hit at the time about a former bachelor who had happily married. These days, she said, if she watched the video of her 10-year-old singing self, “I would probably throw up.” But she still treasures the audience response, “having all those people I’d gotten close to cheer for me.”
Emily was at , but share one feature: they are the kind of dermatologic issues that can make doing everyday kid or teen activities like swimming difficult and can elicit mean comments from classmates and other would-be friends.

Emily was first diagnosed with atopic dermatitis at age 4, her mother, Amber Haygood, says. By age 9, it had become severe. Emily remembers being teased some in elementary school. “I did feel bad a lot of the time, when asked insensitive questions.” Her mother still bristles that adults often could be cruel, too.
But at Camp Discovery, those issues were nonexistent. “Camp was so cool,” Emily said. Besides the usual camp activities, it had things that “normal” camp didn’t, like other kids who didn’t stare at your skin condition or make fun of it.
30th anniversary season begins
This year is the 30th anniversary of Camp Discovery. Sessions began July 23 and continue through Aug. 18, with locations in Crosslake, Minn.; Hebron, Conn.; and Millville, Pa., in addition to Burton, Tex. About 300 campers will attend this year, according to the AAD, and 6,151 campers have attended from 1993 to 2022.
The 1-week camp accepts youth with conditions ranging from eczema and psoriasis to vitiligo, alopecia, epidermolysis bullosa, and ichthyosis, according to the academy. A dermatologist first refers a child, downloading and completing the referral form and sending it to the academy.
The 1-week session, including travel, is free for the campers, thanks to donors. As a nonprofit and membership-based organization, the AAD does not release the detailed financial information about the operating budget for the camp. Dermatologists, nurses, and counselors volunteer their time.
In his presidential address at the AAD’s annual meeting in March, outgoing president Mark D. Kaufmann, MD, of the department of dermatology at the Icahn School of Medicine at Mount Sinai in New York, referred to camp volunteering as an antidote to professional burnout. Remembering why as a dermatologist one entered the profession can be one solution, he said, and described his own recent 3-day volunteer stint at the camp.
“Those 3 magical days, being with kids as they discovered they weren’t alone in the world, sharing their experiences and ideas, reminded me why I became a physician in the first place,” he told the audience of meeting attendees. He vowed to expand the program, with a goal of having every dermatology resident attend Camp Discovery.
Mental health effects of skin conditions
Much research has focused on the mental health fallout from living with chronic skin conditions, and even young children can be adversely affected. In one review of the literature, researchers concluded that pediatric skin disease, including acne, atopic dermatitis, and psoriasis, can affect quality of life, carry stigma, and lead to bullying and eventually even suicidal behavior. Another study, published earlier this year, found that atopic dermatitis affected children’s quality of life, impacting sleep and leading to feelings of being ashamed.
“It’s not necessarily about what their skin condition is and more about the psychosocial impact,’’ said Samantha Hill, MD, a pediatric and general dermatologist in Lynchburg, Va., who is the medical director of Camp Discovery in Minnesota this year.
Camp activities, reactions
The overriding theme of camp is allowing all the youth to be “just one of the kids at camp,” Dr. Hill said in an interview. “They come to do all kinds of things they don’t do in normal life because people don’t give them the credit to [be able to] do it.”
Every year, she said, “I tell my staff we are in the business of making things happen, so if there is a kid bandaged head to toe [because of a skin condition] and they want to go tubing and get in the lake, we figure out how to make it happen. We have done that multiple times.”
Newcomers are initially nervous, Dr. Hill acknowledged, but in time let their guard down. Returnees are a different story. “When kids who have been at camp before arrive, you can see them start breathing again, looking for their friends. You can see them relax right before your eyes.”
“The single most empowering thing is the realization you are not alone,” said Meena Julapalli, MD, a Houston dermatologist who is a medical team member and long-time volunteer at Camp Discovery. That, she said, and “You get to be a kid, and you don’t have to have people staring at you.”
Dr. Julapalli remembers one of her patients with keratitis-ichthyosis-deafness (KID) syndrome. “She needed more than what I could offer,” she said. “She needed camp.” At camp, the organizers found a counselor who knew sign language to accompany her. At first, she was quiet and didn’t smile much. By the end of the week, as she was about to observe her birthday, things changed. After breakfast, she was led to the stage, where fellow campers began singing – and signing the song they had just learned.
Camp staff gets it
Allyson Garin, who was diagnosed with vitiligo at age 6 months, is a camp program director at Camp Discovery in Crosslake, Minn. She first went to camp in 1990 at age 11, returning until she “aged out” at 16, then worked as a counselor. She gets it when campers tell her they hear rude comments about their skin conditions.
“I remember being in swimming pools, in lines at fairgrounds or amusement parks,” she said in an interview, “and hearing people say, ‘Don’t touch her,’ ’’ fearing contagion, perhaps. “People would make jokes about cows, since they are spotted,” she said, or people would simply step back.
All those years ago, her mother found out about the camp and decided to figure out how to get her there. She got there, and she met a fellow camper with vitiligo, and they became pen pals. “We still talk,” she said.
Meeting someone with the same skin condition, she said, isn’t just about commiserating. “There is a lot of information sharing,” on topics such as best treatments, strategies, and other conversations.
Other lessons
While campers can feel comfortable around others who also have skin conditions, and understand, the lesson extends beyond that, Ms. Garin said. “It gave me a perspective,” she said of her camp experience. “I always felt, ‘Woe is me.’ ” But when she met others with, as she said, conditions “way worse than vitiligo, it really grounds you.”
Dr. Hill agreed. Campers get the benefit of others accepting and including them, but also practicing that same attitude toward fellow campers, she said. “It insures that we are providing this environment of inclusion, but that they are practicing it as well. They need to practice it like everyone else.”
Getting parents on board
The idea of camp, especially for those at the younger end of the 8- to 16-years age range accepted for Camp Discovery, can take some getting used to for some parents. Ms. Haygood, Emily’s mother, relates to that. Her daughter’s dermatologist at the time, who is now retired, had first suggested the camp. Her first reaction? “I am not sending my chronically ill child to camp with strangers.” She also acknowledged that she, like other parents of children with a chronic illness, can be a helicopter parent.
Then, she noticed that Emily seemed interested, so she got more information, finding out that it was staffed by doctors. It all sounded good, she said, and the social interaction, she knew, would be beneficial. “Then my husband was a no,” she said, concerned about their daughter being with strangers. “Eventually he came around,” Ms. Haygood said. All along, Emily said, “it seemed fun. I was probably trying to talk them into it.” She admits she was very nervous at first, but calmed down when she realized her own dermatologist was going to be there.
Vanessa Hadley of Spring, Tex., was on board the moment she heard about Camp Discovery. “I just thought it was amazing,” she said. Her daughter Isabelle, 13, has been to the camp. “She has alopecia areata and severe eczema,” Ms. Hadley said. Now, Isabelle is returning to camp and coaching her sister Penelope, 8, who has eczema and mild alopecia and is a first-timer this summer.
One tip the 8-year-old has learned so far: Turn to your counselor for support if you’re nervous. That worked, Isabelle said, the first year when she was wary of the zipline – then surprised herself and conquered it.
Dr. Hill and Dr. Julapalli have no disclosures.
Gene therapy promising for reversal of hereditary vision loss
who received the therapy as part of an early access program.
Results of a study of more than 60 patients who received lenadogene nolparvovec (Lumevoq, GenSight Biologics) as a unilateral or bilateral intravitreal injection showed that at 2-year follow-up, 60% had experienced a clinically relevant improvement in the number of letters they could read on a visual acuity chart.
The results, said study presenter Chiara La Morgia, MD, PhD, IRCCS Istituto delle Scienze Neurologiche di Bologna (Italy), confirm in a “real-life setting” the efficacy and safety of the treatment as previously shown in clinical trials.
The findings were presented at the Congress of the European Academy of Neurology (EAN) 2023.
Severe disease
LHON is a maternally inherited genetic condition that leads to rapid loss of vision. It is caused by alterations in mitochondrial DNA that increase oxidative stress in retinal ganglion cells, leading to cell damage and death. The m.11778G>A MT-ND4 mutation (MT-ND4-LHON) is the most common and is seen in approximately 75% of LHON patients in Europe and North America.
It also leads to the most severe form of the disease, in which patients experience rapid, progressive, and painless bilateral loss of vision, either simultaneously or sequentially in both eyes. Within 1 year of onset, 97% of patients have bilateral involvement.
Lenadogene nolparvovec uses an adeno-associated virus vector to deliver the wild-type ND4 gene directly to the mitochondrial membrane of the retinal ganglion cells. This compensates for the mutation and leads to protein synthesis and restoration of energy production.
Dr. La Morgia said that, so far, five clinical studies are or have been conducted with the gene therapy with patients in France, Italy, the United Kingdom, and the United States.
In the current analysis, patients who received the therapy as part of an early access program underwent unilateral or bilateral intravitreal injection at a dose of 9x1010 viral genomes per eye. Efficacy and safety data, as well as patient baseline characteristics, were collected.
Between August 2018 and March 2022, 63 patients with MT-ND4-LHON received lenadogene nolparvovec. Individual-level data were pooled and analyzed. The mean age at first injection was 33.7 years; 90.5% of participants were aged 60 years or younger. The majority (77.8%) were male; just over half (55.6%) were from France, while 28.6% were from the United States.
The average disease duration at first injection was 11.3 years. Sixty-seven percent of patients were injected in both eyes, and 81.0% had previously received idebenone, a short-chain benzoquinone, the only treatment for LHON that has marketing authorization.
At 2-year follow-up, there was a marked improvement in best-corrected visual acuity (BCVA) scores. Moreover, among all 90 treated eyes for which data were available, the mean change in BCVA from nadir at 1 year was –0.45 log of the minimum angle of resolution (LogMAR), or +22.5 Early Treatment Diabetic Retinopathy Study (ETDRS) letters on a chart.
Among 58 bilaterally treated eyes, the mean improvement was –0.49 LogMAR, or +24.5 ETDRS letters, over the same period. In 32 unilaterally treated eyes, mean improvement was –0.39 LogMAR, or +19.5 ETDRS letters.
Overall, 64.4% of treated eyes showed an improvement from nadir of ≥ 0.3 LogMAR. A clinically relevant response, defined as an improvement of ≥ 10 ETDRS letters, was achieved by 60.0% of patients.
Regarding safety, Dr. La Morgia showed that 42.9% of eyes had at least one episode of intraocular inflammation, a rate she described as “quite high.” The episodes lasted for an average of 155.8 days.
“But all of this inflammation was very easily treatable in a majority of cases without using oral steroids,” she added, “just topical steroids.”
She also noted that in most cases, the inflammation was not severe.
Approval status
Session cochair Gianfranco De Stefano, MD, department of human neuroscience, Sapienza University of Rome, asked Dr. La Morgia about the current approval status of lenadogene nolparvovec.
She said that it was presented to the European Medicines Agency for approval, but the application was withdrawn earlier this year. The “main criticism” was that bilateral improvement was seen even in patients who received only a unilateral injection.
This is “not easily explainable,” said Dr. La Morgia, although it was found that the viral vector was present in the uninjected eye.
There was also a question regarding the heterogeneity of the patient data, which it is hoped will be addressed in future clinical trials.
Commenting after the session, De Stefano said in an interview that the results are “very interesting” and “very promising.”
He pointed out that idebenone may be the only currently available therapy for LHON, but it is “not very effective, and it’s something you give the patient just for the sake of doing something” in light of the possibility that he or she might have “even a small improvement” in eyesight.
However, he believes that lenadogene nolparvovec is a long way from becoming available in the clinic, primarily because longer follow-up is required to determine whether just one injection is enough.
“It may be likely that this gene therapy does not have a long-lasting effect,” he explained.
Currently, the longest follow-up is just 2 years; “I don’t know if there will be a need for repeat injection,” De Stefano said.
No funding was declared. Dr. La Morgia has relationships with Chiesi Farmaceutici, GenSight Biologics, Regulatory Pharma Net, Thenewway, Santhera Pharmaceuticals, First Class srl, Biologix, Stoke Therapeutics, and Reneo.
A version of this article first appeared on Medscape.com.
who received the therapy as part of an early access program.
Results of a study of more than 60 patients who received lenadogene nolparvovec (Lumevoq, GenSight Biologics) as a unilateral or bilateral intravitreal injection showed that at 2-year follow-up, 60% had experienced a clinically relevant improvement in the number of letters they could read on a visual acuity chart.
The results, said study presenter Chiara La Morgia, MD, PhD, IRCCS Istituto delle Scienze Neurologiche di Bologna (Italy), confirm in a “real-life setting” the efficacy and safety of the treatment as previously shown in clinical trials.
The findings were presented at the Congress of the European Academy of Neurology (EAN) 2023.
Severe disease
LHON is a maternally inherited genetic condition that leads to rapid loss of vision. It is caused by alterations in mitochondrial DNA that increase oxidative stress in retinal ganglion cells, leading to cell damage and death. The m.11778G>A MT-ND4 mutation (MT-ND4-LHON) is the most common and is seen in approximately 75% of LHON patients in Europe and North America.
It also leads to the most severe form of the disease, in which patients experience rapid, progressive, and painless bilateral loss of vision, either simultaneously or sequentially in both eyes. Within 1 year of onset, 97% of patients have bilateral involvement.
Lenadogene nolparvovec uses an adeno-associated virus vector to deliver the wild-type ND4 gene directly to the mitochondrial membrane of the retinal ganglion cells. This compensates for the mutation and leads to protein synthesis and restoration of energy production.
Dr. La Morgia said that, so far, five clinical studies are or have been conducted with the gene therapy with patients in France, Italy, the United Kingdom, and the United States.
In the current analysis, patients who received the therapy as part of an early access program underwent unilateral or bilateral intravitreal injection at a dose of 9x1010 viral genomes per eye. Efficacy and safety data, as well as patient baseline characteristics, were collected.
Between August 2018 and March 2022, 63 patients with MT-ND4-LHON received lenadogene nolparvovec. Individual-level data were pooled and analyzed. The mean age at first injection was 33.7 years; 90.5% of participants were aged 60 years or younger. The majority (77.8%) were male; just over half (55.6%) were from France, while 28.6% were from the United States.
The average disease duration at first injection was 11.3 years. Sixty-seven percent of patients were injected in both eyes, and 81.0% had previously received idebenone, a short-chain benzoquinone, the only treatment for LHON that has marketing authorization.
At 2-year follow-up, there was a marked improvement in best-corrected visual acuity (BCVA) scores. Moreover, among all 90 treated eyes for which data were available, the mean change in BCVA from nadir at 1 year was –0.45 log of the minimum angle of resolution (LogMAR), or +22.5 Early Treatment Diabetic Retinopathy Study (ETDRS) letters on a chart.
Among 58 bilaterally treated eyes, the mean improvement was –0.49 LogMAR, or +24.5 ETDRS letters, over the same period. In 32 unilaterally treated eyes, mean improvement was –0.39 LogMAR, or +19.5 ETDRS letters.
Overall, 64.4% of treated eyes showed an improvement from nadir of ≥ 0.3 LogMAR. A clinically relevant response, defined as an improvement of ≥ 10 ETDRS letters, was achieved by 60.0% of patients.
Regarding safety, Dr. La Morgia showed that 42.9% of eyes had at least one episode of intraocular inflammation, a rate she described as “quite high.” The episodes lasted for an average of 155.8 days.
“But all of this inflammation was very easily treatable in a majority of cases without using oral steroids,” she added, “just topical steroids.”
She also noted that in most cases, the inflammation was not severe.
Approval status
Session cochair Gianfranco De Stefano, MD, department of human neuroscience, Sapienza University of Rome, asked Dr. La Morgia about the current approval status of lenadogene nolparvovec.
She said that it was presented to the European Medicines Agency for approval, but the application was withdrawn earlier this year. The “main criticism” was that bilateral improvement was seen even in patients who received only a unilateral injection.
This is “not easily explainable,” said Dr. La Morgia, although it was found that the viral vector was present in the uninjected eye.
There was also a question regarding the heterogeneity of the patient data, which it is hoped will be addressed in future clinical trials.
Commenting after the session, De Stefano said in an interview that the results are “very interesting” and “very promising.”
He pointed out that idebenone may be the only currently available therapy for LHON, but it is “not very effective, and it’s something you give the patient just for the sake of doing something” in light of the possibility that he or she might have “even a small improvement” in eyesight.
However, he believes that lenadogene nolparvovec is a long way from becoming available in the clinic, primarily because longer follow-up is required to determine whether just one injection is enough.
“It may be likely that this gene therapy does not have a long-lasting effect,” he explained.
Currently, the longest follow-up is just 2 years; “I don’t know if there will be a need for repeat injection,” De Stefano said.
No funding was declared. Dr. La Morgia has relationships with Chiesi Farmaceutici, GenSight Biologics, Regulatory Pharma Net, Thenewway, Santhera Pharmaceuticals, First Class srl, Biologix, Stoke Therapeutics, and Reneo.
A version of this article first appeared on Medscape.com.
who received the therapy as part of an early access program.
Results of a study of more than 60 patients who received lenadogene nolparvovec (Lumevoq, GenSight Biologics) as a unilateral or bilateral intravitreal injection showed that at 2-year follow-up, 60% had experienced a clinically relevant improvement in the number of letters they could read on a visual acuity chart.
The results, said study presenter Chiara La Morgia, MD, PhD, IRCCS Istituto delle Scienze Neurologiche di Bologna (Italy), confirm in a “real-life setting” the efficacy and safety of the treatment as previously shown in clinical trials.
The findings were presented at the Congress of the European Academy of Neurology (EAN) 2023.
Severe disease
LHON is a maternally inherited genetic condition that leads to rapid loss of vision. It is caused by alterations in mitochondrial DNA that increase oxidative stress in retinal ganglion cells, leading to cell damage and death. The m.11778G>A MT-ND4 mutation (MT-ND4-LHON) is the most common and is seen in approximately 75% of LHON patients in Europe and North America.
It also leads to the most severe form of the disease, in which patients experience rapid, progressive, and painless bilateral loss of vision, either simultaneously or sequentially in both eyes. Within 1 year of onset, 97% of patients have bilateral involvement.
Lenadogene nolparvovec uses an adeno-associated virus vector to deliver the wild-type ND4 gene directly to the mitochondrial membrane of the retinal ganglion cells. This compensates for the mutation and leads to protein synthesis and restoration of energy production.
Dr. La Morgia said that, so far, five clinical studies are or have been conducted with the gene therapy with patients in France, Italy, the United Kingdom, and the United States.
In the current analysis, patients who received the therapy as part of an early access program underwent unilateral or bilateral intravitreal injection at a dose of 9x1010 viral genomes per eye. Efficacy and safety data, as well as patient baseline characteristics, were collected.
Between August 2018 and March 2022, 63 patients with MT-ND4-LHON received lenadogene nolparvovec. Individual-level data were pooled and analyzed. The mean age at first injection was 33.7 years; 90.5% of participants were aged 60 years or younger. The majority (77.8%) were male; just over half (55.6%) were from France, while 28.6% were from the United States.
The average disease duration at first injection was 11.3 years. Sixty-seven percent of patients were injected in both eyes, and 81.0% had previously received idebenone, a short-chain benzoquinone, the only treatment for LHON that has marketing authorization.
At 2-year follow-up, there was a marked improvement in best-corrected visual acuity (BCVA) scores. Moreover, among all 90 treated eyes for which data were available, the mean change in BCVA from nadir at 1 year was –0.45 log of the minimum angle of resolution (LogMAR), or +22.5 Early Treatment Diabetic Retinopathy Study (ETDRS) letters on a chart.
Among 58 bilaterally treated eyes, the mean improvement was –0.49 LogMAR, or +24.5 ETDRS letters, over the same period. In 32 unilaterally treated eyes, mean improvement was –0.39 LogMAR, or +19.5 ETDRS letters.
Overall, 64.4% of treated eyes showed an improvement from nadir of ≥ 0.3 LogMAR. A clinically relevant response, defined as an improvement of ≥ 10 ETDRS letters, was achieved by 60.0% of patients.
Regarding safety, Dr. La Morgia showed that 42.9% of eyes had at least one episode of intraocular inflammation, a rate she described as “quite high.” The episodes lasted for an average of 155.8 days.
“But all of this inflammation was very easily treatable in a majority of cases without using oral steroids,” she added, “just topical steroids.”
She also noted that in most cases, the inflammation was not severe.
Approval status
Session cochair Gianfranco De Stefano, MD, department of human neuroscience, Sapienza University of Rome, asked Dr. La Morgia about the current approval status of lenadogene nolparvovec.
She said that it was presented to the European Medicines Agency for approval, but the application was withdrawn earlier this year. The “main criticism” was that bilateral improvement was seen even in patients who received only a unilateral injection.
This is “not easily explainable,” said Dr. La Morgia, although it was found that the viral vector was present in the uninjected eye.
There was also a question regarding the heterogeneity of the patient data, which it is hoped will be addressed in future clinical trials.
Commenting after the session, De Stefano said in an interview that the results are “very interesting” and “very promising.”
He pointed out that idebenone may be the only currently available therapy for LHON, but it is “not very effective, and it’s something you give the patient just for the sake of doing something” in light of the possibility that he or she might have “even a small improvement” in eyesight.
However, he believes that lenadogene nolparvovec is a long way from becoming available in the clinic, primarily because longer follow-up is required to determine whether just one injection is enough.
“It may be likely that this gene therapy does not have a long-lasting effect,” he explained.
Currently, the longest follow-up is just 2 years; “I don’t know if there will be a need for repeat injection,” De Stefano said.
No funding was declared. Dr. La Morgia has relationships with Chiesi Farmaceutici, GenSight Biologics, Regulatory Pharma Net, Thenewway, Santhera Pharmaceuticals, First Class srl, Biologix, Stoke Therapeutics, and Reneo.
A version of this article first appeared on Medscape.com.
FROM EAN 2023
New guidelines for MTX use in pediatric inflammatory skin disease unveiled
While the typical dose of methotrexate (MTX) for inflammatory disease in pediatric patients varies in published studies, the maximum dose is considered to be 1 mg/kg and not to exceed 25 mg/week. In addition, test doses are not necessary for pediatric patients starting low dose (1 mg/kg or less) MTX for inflammatory skin disease, and the onset of efficacy with MTX may take 8-16 weeks.
and published online in Pediatric Dermatology.
“Methotrexate is a cost-effective, readily accessible, well-tolerated, useful, and time-honored option for children with a spectrum of inflammatory skin diseases,” project cochair Elaine C. Siegfried, MD, professor of pediatrics and dermatology at Saint Louis University, told this news organization. “Although considered an ‘immune suppressant’ by some, it is more accurately classified as an immune modulator and has been widely used for more than 50 years, and remains the standard of care when administered at very high doses and intrathecally in children with acute lymphoblastic leukemia – a practice that supports safety. But many details that support optimized treatment are not widely appreciated.”
In their guidelines document, Dr. Siegfried and her 22 coauthors noted that Food and Drug Administration labeling does not include approved indications for the use of MTX for many inflammatory skin diseases in pediatric patients, including morphea, psoriasis, atopic dermatitis, and alopecia areata. “Furthermore, some clinicians may be unfamiliar or uncomfortable prescribing medications off label for pediatric patients, causing delayed initiation, premature drug discontinuation, or use of less advantageous alternatives,” they wrote.
To address this unmet need, Dr. Siegfried and the other committee members used a modified Delphi process to reach agreement on recommendations related to five key topic areas: indications and contraindications, dosing, interactions with immunizations and medications, potential for and management of adverse effects, and monitoring needs. Consensus was predefined as at least 70% of participants rating a statement as 7-9 on the Likert scale. The effort to develop 46 recommendations has been a work in progress for almost 5 years, “somewhat delayed by the pandemic,” Dr. Siegfried, past president and director of the American Board of Dermatology, said in an interview. “But it remains relevant, despite the emergence of biologics and JAK inhibitors for treating inflammatory skin conditions in children. Although the mechanism-of-action of low-dose MTX is not clear, it may overlap with the newer small molecules.”
The guidelines contain several pearls to guide optimal dosing, including the following key points:
- MTX can be discontinued abruptly without adverse effects, other than the risk of disease worsening.
- Folic acid supplementation (starting at 1 mg/day, regardless of weight) is an effective approach to minimizing associated gastrointestinal adverse effects.
- Concomitant use of MTX and antibiotics (including trimethoprim-sulfamethoxazole) and NSAIDS are not contraindicated for most pediatric patients treated for inflammatory skin disease.
- Live virus vaccine boosters such as varicella-zoster virus (VZV) and measles, mumps, and rubella (MMR) are not contraindicated in patients taking MTX; there are insufficient data to make recommendations for or against primary immunization with MMR vaccine in patients taking MTX; inactivated vaccines should be given to patients taking MTX.
- Routine surveillance laboratory monitoring (i.e., CBC with differential, alanine transaminase, aspartate aminotransferase, creatinine) is recommended at baseline, after 1 month of treatment, and every 3-4 months thereafter.
- Transient transaminase elevation (≤ 3 upper limit normal for < 3 months) is not uncommon with low-dose MTX and does not usually require interruption of MTX. The most likely causes are concomitant viral infection, MTX dosing within 24 hours prior to phlebotomy, recent administration of other medications (such as acetaminophen), and/or recent alcohol consumption.
- Liver biopsy is not indicated for routine monitoring of pediatric patients taking low-dose MTX.
According to Dr. Siegfried, consensus of the committee members was lowest on the need for a test dose of MTX.
Overall, she said in the interview, helping to craft the guidelines caused her to reflect on how her approach to using MTX has evolved over the past 35 years, after treating “many hundreds” of patients. “I was gratified to confirm similar practice patterns among my colleagues,” she added.
The project’s other cochair was Heather Brandling-Bennett, MD, a dermatologist at Seattle Children’s Hospital. This work was supported by a grant from the Pediatric Dermatology Research Alliance (PeDRA), with additional funding from the National Eczema Association and the National Psoriasis Foundation. Dr. Siegfried disclosed ties with AbbVie, Boehringer Ingelheim, Incyte, LEO Pharma, Novan, Novartis, Pierre Fabre, Pfizer, Regeneron, Sanofi Genzyme, UCB, and Verrica. She has participated in contracted research for AI Therapeutics, and has served as principal investigator for Janssen. Many of the guideline coauthors disclosed having received grant support and other funding from pharmaceutical companies.
While the typical dose of methotrexate (MTX) for inflammatory disease in pediatric patients varies in published studies, the maximum dose is considered to be 1 mg/kg and not to exceed 25 mg/week. In addition, test doses are not necessary for pediatric patients starting low dose (1 mg/kg or less) MTX for inflammatory skin disease, and the onset of efficacy with MTX may take 8-16 weeks.
and published online in Pediatric Dermatology.
“Methotrexate is a cost-effective, readily accessible, well-tolerated, useful, and time-honored option for children with a spectrum of inflammatory skin diseases,” project cochair Elaine C. Siegfried, MD, professor of pediatrics and dermatology at Saint Louis University, told this news organization. “Although considered an ‘immune suppressant’ by some, it is more accurately classified as an immune modulator and has been widely used for more than 50 years, and remains the standard of care when administered at very high doses and intrathecally in children with acute lymphoblastic leukemia – a practice that supports safety. But many details that support optimized treatment are not widely appreciated.”
In their guidelines document, Dr. Siegfried and her 22 coauthors noted that Food and Drug Administration labeling does not include approved indications for the use of MTX for many inflammatory skin diseases in pediatric patients, including morphea, psoriasis, atopic dermatitis, and alopecia areata. “Furthermore, some clinicians may be unfamiliar or uncomfortable prescribing medications off label for pediatric patients, causing delayed initiation, premature drug discontinuation, or use of less advantageous alternatives,” they wrote.
To address this unmet need, Dr. Siegfried and the other committee members used a modified Delphi process to reach agreement on recommendations related to five key topic areas: indications and contraindications, dosing, interactions with immunizations and medications, potential for and management of adverse effects, and monitoring needs. Consensus was predefined as at least 70% of participants rating a statement as 7-9 on the Likert scale. The effort to develop 46 recommendations has been a work in progress for almost 5 years, “somewhat delayed by the pandemic,” Dr. Siegfried, past president and director of the American Board of Dermatology, said in an interview. “But it remains relevant, despite the emergence of biologics and JAK inhibitors for treating inflammatory skin conditions in children. Although the mechanism-of-action of low-dose MTX is not clear, it may overlap with the newer small molecules.”
The guidelines contain several pearls to guide optimal dosing, including the following key points:
- MTX can be discontinued abruptly without adverse effects, other than the risk of disease worsening.
- Folic acid supplementation (starting at 1 mg/day, regardless of weight) is an effective approach to minimizing associated gastrointestinal adverse effects.
- Concomitant use of MTX and antibiotics (including trimethoprim-sulfamethoxazole) and NSAIDS are not contraindicated for most pediatric patients treated for inflammatory skin disease.
- Live virus vaccine boosters such as varicella-zoster virus (VZV) and measles, mumps, and rubella (MMR) are not contraindicated in patients taking MTX; there are insufficient data to make recommendations for or against primary immunization with MMR vaccine in patients taking MTX; inactivated vaccines should be given to patients taking MTX.
- Routine surveillance laboratory monitoring (i.e., CBC with differential, alanine transaminase, aspartate aminotransferase, creatinine) is recommended at baseline, after 1 month of treatment, and every 3-4 months thereafter.
- Transient transaminase elevation (≤ 3 upper limit normal for < 3 months) is not uncommon with low-dose MTX and does not usually require interruption of MTX. The most likely causes are concomitant viral infection, MTX dosing within 24 hours prior to phlebotomy, recent administration of other medications (such as acetaminophen), and/or recent alcohol consumption.
- Liver biopsy is not indicated for routine monitoring of pediatric patients taking low-dose MTX.
According to Dr. Siegfried, consensus of the committee members was lowest on the need for a test dose of MTX.
Overall, she said in the interview, helping to craft the guidelines caused her to reflect on how her approach to using MTX has evolved over the past 35 years, after treating “many hundreds” of patients. “I was gratified to confirm similar practice patterns among my colleagues,” she added.
The project’s other cochair was Heather Brandling-Bennett, MD, a dermatologist at Seattle Children’s Hospital. This work was supported by a grant from the Pediatric Dermatology Research Alliance (PeDRA), with additional funding from the National Eczema Association and the National Psoriasis Foundation. Dr. Siegfried disclosed ties with AbbVie, Boehringer Ingelheim, Incyte, LEO Pharma, Novan, Novartis, Pierre Fabre, Pfizer, Regeneron, Sanofi Genzyme, UCB, and Verrica. She has participated in contracted research for AI Therapeutics, and has served as principal investigator for Janssen. Many of the guideline coauthors disclosed having received grant support and other funding from pharmaceutical companies.
While the typical dose of methotrexate (MTX) for inflammatory disease in pediatric patients varies in published studies, the maximum dose is considered to be 1 mg/kg and not to exceed 25 mg/week. In addition, test doses are not necessary for pediatric patients starting low dose (1 mg/kg or less) MTX for inflammatory skin disease, and the onset of efficacy with MTX may take 8-16 weeks.
and published online in Pediatric Dermatology.
“Methotrexate is a cost-effective, readily accessible, well-tolerated, useful, and time-honored option for children with a spectrum of inflammatory skin diseases,” project cochair Elaine C. Siegfried, MD, professor of pediatrics and dermatology at Saint Louis University, told this news organization. “Although considered an ‘immune suppressant’ by some, it is more accurately classified as an immune modulator and has been widely used for more than 50 years, and remains the standard of care when administered at very high doses and intrathecally in children with acute lymphoblastic leukemia – a practice that supports safety. But many details that support optimized treatment are not widely appreciated.”
In their guidelines document, Dr. Siegfried and her 22 coauthors noted that Food and Drug Administration labeling does not include approved indications for the use of MTX for many inflammatory skin diseases in pediatric patients, including morphea, psoriasis, atopic dermatitis, and alopecia areata. “Furthermore, some clinicians may be unfamiliar or uncomfortable prescribing medications off label for pediatric patients, causing delayed initiation, premature drug discontinuation, or use of less advantageous alternatives,” they wrote.
To address this unmet need, Dr. Siegfried and the other committee members used a modified Delphi process to reach agreement on recommendations related to five key topic areas: indications and contraindications, dosing, interactions with immunizations and medications, potential for and management of adverse effects, and monitoring needs. Consensus was predefined as at least 70% of participants rating a statement as 7-9 on the Likert scale. The effort to develop 46 recommendations has been a work in progress for almost 5 years, “somewhat delayed by the pandemic,” Dr. Siegfried, past president and director of the American Board of Dermatology, said in an interview. “But it remains relevant, despite the emergence of biologics and JAK inhibitors for treating inflammatory skin conditions in children. Although the mechanism-of-action of low-dose MTX is not clear, it may overlap with the newer small molecules.”
The guidelines contain several pearls to guide optimal dosing, including the following key points:
- MTX can be discontinued abruptly without adverse effects, other than the risk of disease worsening.
- Folic acid supplementation (starting at 1 mg/day, regardless of weight) is an effective approach to minimizing associated gastrointestinal adverse effects.
- Concomitant use of MTX and antibiotics (including trimethoprim-sulfamethoxazole) and NSAIDS are not contraindicated for most pediatric patients treated for inflammatory skin disease.
- Live virus vaccine boosters such as varicella-zoster virus (VZV) and measles, mumps, and rubella (MMR) are not contraindicated in patients taking MTX; there are insufficient data to make recommendations for or against primary immunization with MMR vaccine in patients taking MTX; inactivated vaccines should be given to patients taking MTX.
- Routine surveillance laboratory monitoring (i.e., CBC with differential, alanine transaminase, aspartate aminotransferase, creatinine) is recommended at baseline, after 1 month of treatment, and every 3-4 months thereafter.
- Transient transaminase elevation (≤ 3 upper limit normal for < 3 months) is not uncommon with low-dose MTX and does not usually require interruption of MTX. The most likely causes are concomitant viral infection, MTX dosing within 24 hours prior to phlebotomy, recent administration of other medications (such as acetaminophen), and/or recent alcohol consumption.
- Liver biopsy is not indicated for routine monitoring of pediatric patients taking low-dose MTX.
According to Dr. Siegfried, consensus of the committee members was lowest on the need for a test dose of MTX.
Overall, she said in the interview, helping to craft the guidelines caused her to reflect on how her approach to using MTX has evolved over the past 35 years, after treating “many hundreds” of patients. “I was gratified to confirm similar practice patterns among my colleagues,” she added.
The project’s other cochair was Heather Brandling-Bennett, MD, a dermatologist at Seattle Children’s Hospital. This work was supported by a grant from the Pediatric Dermatology Research Alliance (PeDRA), with additional funding from the National Eczema Association and the National Psoriasis Foundation. Dr. Siegfried disclosed ties with AbbVie, Boehringer Ingelheim, Incyte, LEO Pharma, Novan, Novartis, Pierre Fabre, Pfizer, Regeneron, Sanofi Genzyme, UCB, and Verrica. She has participated in contracted research for AI Therapeutics, and has served as principal investigator for Janssen. Many of the guideline coauthors disclosed having received grant support and other funding from pharmaceutical companies.
FROM PEDIATRIC DERMATOLOGY
Myasthenia gravis drug gets FDA nod
, the drug’s manufacturer, UCB, has announced.
Rozanolixizumab is a subcutaneous-infused humanized IgG4 monoclonal antibody that binds to the neonatal Fc receptor (FcRn), reducing the concentration of pathogenic IgG autoantibodies.
U.S. approval is based on results of the phase 3 MycarinG study involving 200 patients with AChR or MuSK autoantibody-positive gMG. Patients were randomly assigned to one of two rozanolixizumab groups (7 mg/kg or 10 mg/kg) or placebo for 6 weeks.
As reported last month in Lancet Neurology, rozanolixizumab led to statistically significant improvements in gMG-specific outcomes, including everyday activities such as breathing, talking, swallowing, and being able to rise from a chair.
“There is a significant need for new, innovative treatment options to reduce the day-to-day burden of gMG,” lead investigator Vera Bril, MD, professor of medicine (neurology), University of Toronto, said in a news release.
Rozanolixizumab is “a new treatment option, targeting one of the mechanisms of disease to provide symptom improvement in patient- and physician-reported outcomes at day 43,” Dr. Bril added.
The most common adverse reactions (reported in at least 10% of patients treated with rozanolixizumab) were headache, infections, diarrhea, pyrexia, hypersensitivity reactions, and nausea.
The company expects rozanolixizumab to be available in the United States during the third quarter of 2023.
The FDA granted the application for rozanolixizumab in gMG priority review.
A version of this article first appeared on Medscape.com.
, the drug’s manufacturer, UCB, has announced.
Rozanolixizumab is a subcutaneous-infused humanized IgG4 monoclonal antibody that binds to the neonatal Fc receptor (FcRn), reducing the concentration of pathogenic IgG autoantibodies.
U.S. approval is based on results of the phase 3 MycarinG study involving 200 patients with AChR or MuSK autoantibody-positive gMG. Patients were randomly assigned to one of two rozanolixizumab groups (7 mg/kg or 10 mg/kg) or placebo for 6 weeks.
As reported last month in Lancet Neurology, rozanolixizumab led to statistically significant improvements in gMG-specific outcomes, including everyday activities such as breathing, talking, swallowing, and being able to rise from a chair.
“There is a significant need for new, innovative treatment options to reduce the day-to-day burden of gMG,” lead investigator Vera Bril, MD, professor of medicine (neurology), University of Toronto, said in a news release.
Rozanolixizumab is “a new treatment option, targeting one of the mechanisms of disease to provide symptom improvement in patient- and physician-reported outcomes at day 43,” Dr. Bril added.
The most common adverse reactions (reported in at least 10% of patients treated with rozanolixizumab) were headache, infections, diarrhea, pyrexia, hypersensitivity reactions, and nausea.
The company expects rozanolixizumab to be available in the United States during the third quarter of 2023.
The FDA granted the application for rozanolixizumab in gMG priority review.
A version of this article first appeared on Medscape.com.
, the drug’s manufacturer, UCB, has announced.
Rozanolixizumab is a subcutaneous-infused humanized IgG4 monoclonal antibody that binds to the neonatal Fc receptor (FcRn), reducing the concentration of pathogenic IgG autoantibodies.
U.S. approval is based on results of the phase 3 MycarinG study involving 200 patients with AChR or MuSK autoantibody-positive gMG. Patients were randomly assigned to one of two rozanolixizumab groups (7 mg/kg or 10 mg/kg) or placebo for 6 weeks.
As reported last month in Lancet Neurology, rozanolixizumab led to statistically significant improvements in gMG-specific outcomes, including everyday activities such as breathing, talking, swallowing, and being able to rise from a chair.
“There is a significant need for new, innovative treatment options to reduce the day-to-day burden of gMG,” lead investigator Vera Bril, MD, professor of medicine (neurology), University of Toronto, said in a news release.
Rozanolixizumab is “a new treatment option, targeting one of the mechanisms of disease to provide symptom improvement in patient- and physician-reported outcomes at day 43,” Dr. Bril added.
The most common adverse reactions (reported in at least 10% of patients treated with rozanolixizumab) were headache, infections, diarrhea, pyrexia, hypersensitivity reactions, and nausea.
The company expects rozanolixizumab to be available in the United States during the third quarter of 2023.
The FDA granted the application for rozanolixizumab in gMG priority review.
A version of this article first appeared on Medscape.com.