User login
Understanding messenger RNA and other SARS-CoV-2 vaccines
In mid-November, Pfizer/BioNTech were the first with surprising positive protection interim data for their coronavirus vaccine, BNT162b2. A week later, Moderna released interim efficacy results showing its coronavirus vaccine, mRNA-1273, also protected patients from developing SARS-CoV-2 infections. Both studies included mostly healthy adults. A diverse ethnic and racial vaccinated population was included. A reasonable number of persons aged over 65 years, and persons with stable compromising medical conditions were included. Adolescents aged 16 years and over were included. Younger adolescents have been vaccinated or such studies are in the planning or early implementation stage as 2020 came to a close.
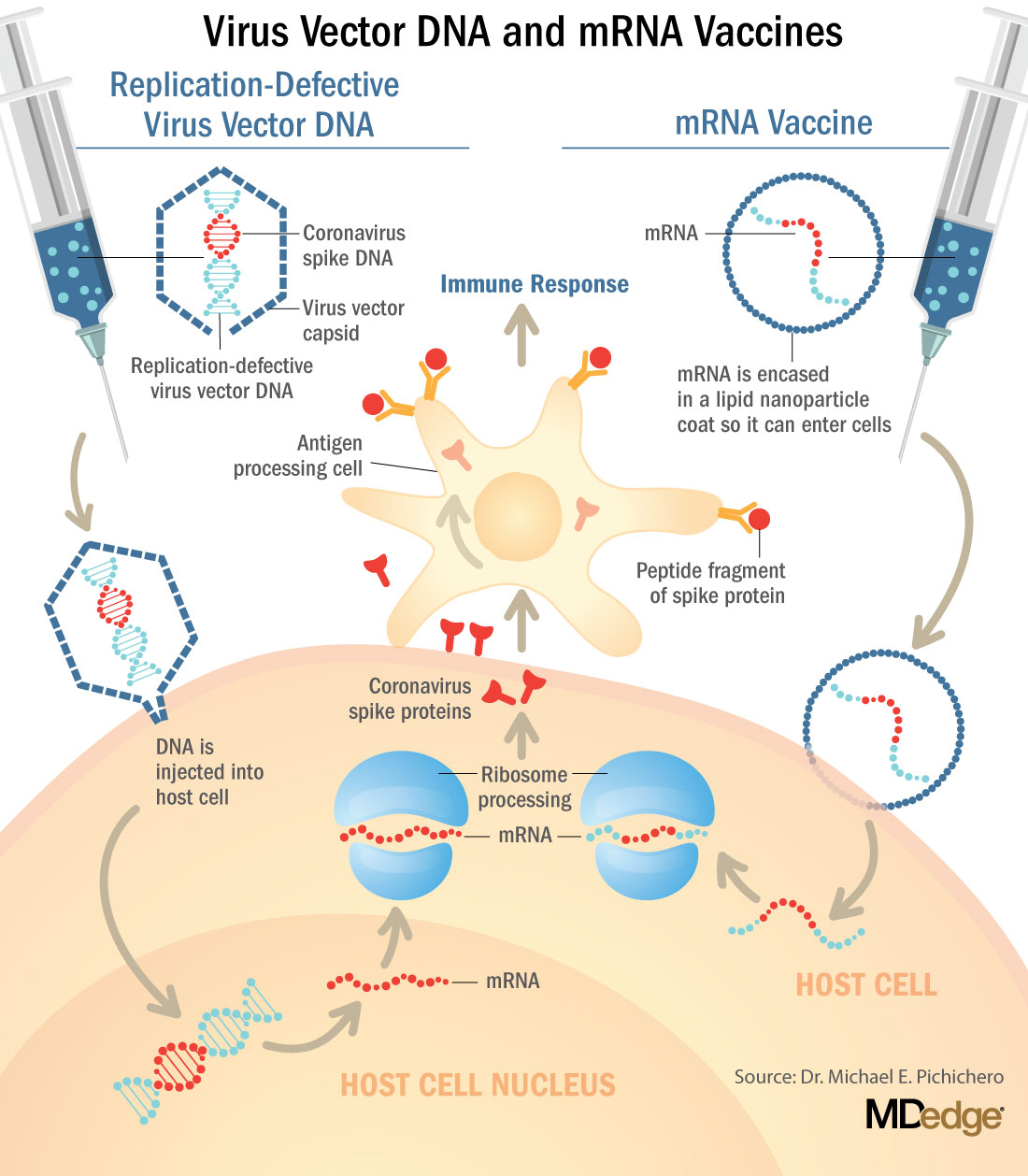
These are new and revolutionary vaccines, although the ability to inject mRNA into animals dates back to 1990, technological advances today make it a reality.1 Traditional vaccines typically involve injection with antigens such as purified proteins or polysaccharides or inactivated/attenuated viruses. In the case of Pfizer’s and Moderna’s vaccines, the mRNA provides the genetic information to synthesize the spike protein that the SARS-CoV-2 virus uses to attach to and infect human cells. Each type of vaccine is packaged in proprietary lipid nanoparticles to protect the mRNA from rapid degradation, and the nanoparticles serve as an adjuvant to attract immune cells to the site of injection. (The properties of the respective lipid nanoparticle packaging may be the factor that impacts storage requirements discussed below.) When injected into muscle (myocyte), the lipid nanoparticles containing the mRNA inside are taken into muscle cells, where the cytoplasmic ribosomes detect and decode the mRNA resulting in the production of the spike protein antigen. It should be noted that the mRNA does not enter the nucleus, where the genetic information (DNA) of a cell is located, and can’t be reproduced or integrated into the DNA. The antigen is exported to the myocyte cell surface where the immune system’s antigen presenting cells detect the protein, ingest it, and take it to regional lymph nodes where interactions with T cells and B cells results in antibodies, T cell–mediated immunity, and generation of immune memory T cells and B cells. A particular subset of T cells – cytotoxic or killer T cells – destroy cells that have been infected by a pathogen. The SARS-CoV-2 mRNA vaccine from Pfizer was reported to induce powerful cytotoxic T-cell responses. Results for Moderna’s vaccine had not been reported at the time this column was prepared, but I anticipate the same positive results.
The revolutionary aspect of mRNA vaccines is the speed at which they can be designed and produced. This is why they lead the pack among the SARS-CoV-2 vaccine candidates and why the National Institute of Allergy and Infectious Diseases provided financial, technical, and/or clinical support. Indeed, once the amino acid sequence of a protein can be determined (a relatively easy task these days) it’s straightforward to synthesize mRNA in the lab – and it can be done incredibly fast. It is reported that the mRNA code for the vaccine by Moderna was made in 2 days and production development was completed in about 2 months.2
A 2007 World Health Organization report noted that infectious diseases are emerging at “the historically unprecedented rate of one per year.”3 Severe acute respiratory syndrome (SARS), Zika, Ebola, and avian and swine flu are recent examples. For most vaccines against emerging diseases, the challenge is about speed: developing and manufacturing a vaccine and getting it to persons who need it as quickly as possible. The current seasonal flu vaccine takes about 6 months to develop; it takes years for most of the traditional vaccines. That’s why once the infrastructure is in place, mRNA vaccines may prove to offer a big advantage as vaccines against emerging pathogens.
Early efficacy results have been surprising
Both vaccines were reported to produce about 95% efficacy in the final analysis. That was unexpectedly high because most vaccines for respiratory illness achieve efficacy of 60%-80%, e.g., flu vaccines. However, the efficacy rate may drop as time goes by because stimulation of short-term immunity would be in the earliest reported results.
Preventing SARS-CoV-2 cases is an important aspect of a coronavirus vaccine, but preventing severe illness is especially important considering that severe cases can result in prolonged intubation/artificial ventilation, prolonged disability and death. Pfizer/BioNTech had not released any data on the breakdown of severe cases as this column was finalized. In Moderna’s clinical trial, a secondary endpoint analyzed severe cases of COVID-19 and included 30 severe cases (as defined in the study protocol) in this analysis. All 30 cases occurred in the placebo group and none in the mRNA-1273–vaccinated group. In the Pfizer/BioNTech trial there were too few cases of severe illness to calculate efficacy.
Duration of immunity and need to revaccinate after initial primary vaccination are unknowns. Study of induction of B- and T-cell memory and levels of long-term protection have not been reported thus far.
Could mRNA COVID-19 vaccines be dangerous in the long term?
These will be the first-ever mRNA vaccines brought to market for humans. In order to receive Food and Drug Administration approval, the companies had to prove there were no immediate or short-term negative adverse effects from the vaccines. The companies reported that their independent data-monitoring committees hadn’t “reported any serious safety concerns.” However, fairly significant local reactions at the site of injection, fever, malaise, and fatigue occur with modest frequency following vaccinations with these products, reportedly in 10%-15% of vaccinees. Overall, the immediate reaction profile appears to be more severe than what occurs following seasonal influenza vaccination. When mass inoculations with these completely new and revolutionary vaccines begins, we will know virtually nothing about their long-term side effects. The possibility of systemic inflammatory responses that could lead to autoimmune conditions, persistence of the induced immunogen expression, development of autoreactive antibodies, and toxic effects of delivery components have been raised as theoretical concerns.4-6 None of these theoretical risks have been observed to date and postmarketing phase 4 safety monitoring studies are in place from the Centers for Disease Control and Prevention and the companies that produce the vaccines. This is a risk public health authorities are willing to take because the risk to benefit calculation strongly favors taking theoretical risks, compared with clear benefits in preventing severe illnesses and death.
What about availability?
Pfizer/BioNTech expects to be able to produce up to 50 million vaccine doses in 2020 and up to 1.3 billion doses in 2021. Moderna expects to produce 20 million doses by the end of 2020, and 500 million to 1 billion doses in 2021. Storage requirements are inherent to the composition of the vaccines with their differing lipid nanoparticle delivery systems. Pfizer/BioNTech’s BNT162b2 has to be stored and transported at –80° C, which requires specialized freezers, which most doctors’ offices and pharmacies are unlikely to have on site, or dry ice containers. Once the vaccine is thawed, it can only remain in the refrigerator for 24 hours. Moderna’s mRNA-1273 will be much easier to distribute. The vaccine is stable in a standard freezer at –20° C for up to 6 months, in a refrigerator for up to 30 days within that 6-month shelf life, and at room temperature for up to 12 hours.
Timelines and testing other vaccines
Strong efficacy data from the two leading SARS-CoV-2 vaccines and emergency-use authorization Food and Drug Administration approval suggest the window for testing additional vaccine candidates in the United States could soon start to close. Of the more than 200 vaccines in development for SARS-CoV-2, at least 7 have a chance of gathering pivotal data before the front-runners become broadly available.
Testing diverse vaccine candidates, based on different technologies, is important for ensuring sufficient supply and could lead to products with tolerability and safety profiles that make them better suited, or more attractive, to subsets of the population. Different vaccine antigens and technologies also may yield different durations of protection, a question that will not be answered until long after the first products are on the market.
AstraZeneca enrolled about 23,000 subjects into its two phase 3 trials of AZD1222 (ChAdOx1 nCoV-19): a 40,000-subject U.S. trial and a 10,000-subject study in Brazil. AstraZeneca’s AZD1222, developed with the University of Oxford (England), uses a replication defective simian adenovirus vector called ChAdOx1.AZD1222 which encodes the SARS-CoV-2 spike protein. After injection, the viral vector delivers recombinant DNA that is decoded to mRNA, followed by mRNA decoding to become a protein. A serendipitous manufacturing error for the first 3,000 doses resulted in a half dose for those subjects before the error was discovered. Full doses were given to those subjects on second injections and those subjects showed 90% efficacy. Subjects who received 2 full doses showed 62% efficacy. A vaccine cannot be licensed based on 3,000 subjects so AstraZeneca has started a new phase 3 trial involving many more subjects to receive the combination lower dose followed by the full dose.
Johnson and Johnson (J&J) started its phase 3 trial evaluating a single dose of JNJ-78436735 in September. Phase 3 data may be reported by the end of2020. In November, J&J announced it was starting a second phase 3 trial to test two doses of the candidate. J&J’s JNJ-78436735 encodes the SARS-CoV-2 spike protein in an adenovirus serotype 26 (Ad26) vector, which is one of the two adenovirus vectors used in Sputnik V, the Russian vaccine reported to have 90% efficacy at an early interim analysis.
Sanofi and Novavax are both developing protein-based vaccines, a proven modality. Sanofi, in partnership with GlaxoSmithKline started a phase 1/2 clinical trial in the Fall 2020 with plans to commence a phase 3 trial in late December. Sanofi developed the protein ingredients and GlaxoSmithKline added one of their novel adjuvants. Novavax expects data from a U.K. phase 3 trial of NVX-CoV2373 in early 2021 and began a U.S. phase 3 study in late November. NVX-CoV2373 was created using Novavax’ recombinant nanoparticle technology to generate antigen derived from the coronavirus spike protein and contains Novavax’s patented saponin-based Matrix-M adjuvant.
Inovio Pharmaceuticals was gearing up to start a U.S. phase 2/3 trial of DNA vaccine INO-4800 by the end of 2020.
After Moderna and Pfizer-BioNTech, CureVac has the next most advanced mRNA vaccine. It was planned that a phase 2b/3 trial of CVnCoV would be conducted in Europe, Latin America, Africa, and Asia. Sanofi is also developing a mRNA vaccine as a second product in addition to its protein vaccine.
Vaxxinity planned to begin phase 3 testing of UB-612, a multitope peptide–based vaccine, in Brazil by the end of 2020.
However, emergency-use authorizations for the Pfizer and Moderna vaccines could hinder trial recruitment in at least two ways. Given the gravity of the pandemic, some stakeholders believe it would be ethical to unblind ongoing trials to give subjects the opportunity to switch to a vaccine proven to be effective. Even if unblinding doesn’t occur, as the two authorized vaccines start to become widely available, volunteering for clinical trials may become less attractive.
Dr. Pichichero is a specialist in pediatric infectious diseases, and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he has no relevant financial disclosures. Email Dr. Pichichero at [email protected].
References
1. Wolff JA et al. Science. 1990 Mar 23. doi: 10.1126/science.1690918.
2. Jackson LA et al. N Engl J Med. 2020 Nov 12. doi: 10.1056/NEJMoa2022483.
3. Prentice T and Reinders LT. The world health report 2007. (Geneva Switzerland: World Health Organization, 2007).
4. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
5. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
6. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
In mid-November, Pfizer/BioNTech were the first with surprising positive protection interim data for their coronavirus vaccine, BNT162b2. A week later, Moderna released interim efficacy results showing its coronavirus vaccine, mRNA-1273, also protected patients from developing SARS-CoV-2 infections. Both studies included mostly healthy adults. A diverse ethnic and racial vaccinated population was included. A reasonable number of persons aged over 65 years, and persons with stable compromising medical conditions were included. Adolescents aged 16 years and over were included. Younger adolescents have been vaccinated or such studies are in the planning or early implementation stage as 2020 came to a close.

These are new and revolutionary vaccines, although the ability to inject mRNA into animals dates back to 1990, technological advances today make it a reality.1 Traditional vaccines typically involve injection with antigens such as purified proteins or polysaccharides or inactivated/attenuated viruses. In the case of Pfizer’s and Moderna’s vaccines, the mRNA provides the genetic information to synthesize the spike protein that the SARS-CoV-2 virus uses to attach to and infect human cells. Each type of vaccine is packaged in proprietary lipid nanoparticles to protect the mRNA from rapid degradation, and the nanoparticles serve as an adjuvant to attract immune cells to the site of injection. (The properties of the respective lipid nanoparticle packaging may be the factor that impacts storage requirements discussed below.) When injected into muscle (myocyte), the lipid nanoparticles containing the mRNA inside are taken into muscle cells, where the cytoplasmic ribosomes detect and decode the mRNA resulting in the production of the spike protein antigen. It should be noted that the mRNA does not enter the nucleus, where the genetic information (DNA) of a cell is located, and can’t be reproduced or integrated into the DNA. The antigen is exported to the myocyte cell surface where the immune system’s antigen presenting cells detect the protein, ingest it, and take it to regional lymph nodes where interactions with T cells and B cells results in antibodies, T cell–mediated immunity, and generation of immune memory T cells and B cells. A particular subset of T cells – cytotoxic or killer T cells – destroy cells that have been infected by a pathogen. The SARS-CoV-2 mRNA vaccine from Pfizer was reported to induce powerful cytotoxic T-cell responses. Results for Moderna’s vaccine had not been reported at the time this column was prepared, but I anticipate the same positive results.
The revolutionary aspect of mRNA vaccines is the speed at which they can be designed and produced. This is why they lead the pack among the SARS-CoV-2 vaccine candidates and why the National Institute of Allergy and Infectious Diseases provided financial, technical, and/or clinical support. Indeed, once the amino acid sequence of a protein can be determined (a relatively easy task these days) it’s straightforward to synthesize mRNA in the lab – and it can be done incredibly fast. It is reported that the mRNA code for the vaccine by Moderna was made in 2 days and production development was completed in about 2 months.2
A 2007 World Health Organization report noted that infectious diseases are emerging at “the historically unprecedented rate of one per year.”3 Severe acute respiratory syndrome (SARS), Zika, Ebola, and avian and swine flu are recent examples. For most vaccines against emerging diseases, the challenge is about speed: developing and manufacturing a vaccine and getting it to persons who need it as quickly as possible. The current seasonal flu vaccine takes about 6 months to develop; it takes years for most of the traditional vaccines. That’s why once the infrastructure is in place, mRNA vaccines may prove to offer a big advantage as vaccines against emerging pathogens.
Early efficacy results have been surprising
Both vaccines were reported to produce about 95% efficacy in the final analysis. That was unexpectedly high because most vaccines for respiratory illness achieve efficacy of 60%-80%, e.g., flu vaccines. However, the efficacy rate may drop as time goes by because stimulation of short-term immunity would be in the earliest reported results.
Preventing SARS-CoV-2 cases is an important aspect of a coronavirus vaccine, but preventing severe illness is especially important considering that severe cases can result in prolonged intubation/artificial ventilation, prolonged disability and death. Pfizer/BioNTech had not released any data on the breakdown of severe cases as this column was finalized. In Moderna’s clinical trial, a secondary endpoint analyzed severe cases of COVID-19 and included 30 severe cases (as defined in the study protocol) in this analysis. All 30 cases occurred in the placebo group and none in the mRNA-1273–vaccinated group. In the Pfizer/BioNTech trial there were too few cases of severe illness to calculate efficacy.
Duration of immunity and need to revaccinate after initial primary vaccination are unknowns. Study of induction of B- and T-cell memory and levels of long-term protection have not been reported thus far.
Could mRNA COVID-19 vaccines be dangerous in the long term?
These will be the first-ever mRNA vaccines brought to market for humans. In order to receive Food and Drug Administration approval, the companies had to prove there were no immediate or short-term negative adverse effects from the vaccines. The companies reported that their independent data-monitoring committees hadn’t “reported any serious safety concerns.” However, fairly significant local reactions at the site of injection, fever, malaise, and fatigue occur with modest frequency following vaccinations with these products, reportedly in 10%-15% of vaccinees. Overall, the immediate reaction profile appears to be more severe than what occurs following seasonal influenza vaccination. When mass inoculations with these completely new and revolutionary vaccines begins, we will know virtually nothing about their long-term side effects. The possibility of systemic inflammatory responses that could lead to autoimmune conditions, persistence of the induced immunogen expression, development of autoreactive antibodies, and toxic effects of delivery components have been raised as theoretical concerns.4-6 None of these theoretical risks have been observed to date and postmarketing phase 4 safety monitoring studies are in place from the Centers for Disease Control and Prevention and the companies that produce the vaccines. This is a risk public health authorities are willing to take because the risk to benefit calculation strongly favors taking theoretical risks, compared with clear benefits in preventing severe illnesses and death.
What about availability?
Pfizer/BioNTech expects to be able to produce up to 50 million vaccine doses in 2020 and up to 1.3 billion doses in 2021. Moderna expects to produce 20 million doses by the end of 2020, and 500 million to 1 billion doses in 2021. Storage requirements are inherent to the composition of the vaccines with their differing lipid nanoparticle delivery systems. Pfizer/BioNTech’s BNT162b2 has to be stored and transported at –80° C, which requires specialized freezers, which most doctors’ offices and pharmacies are unlikely to have on site, or dry ice containers. Once the vaccine is thawed, it can only remain in the refrigerator for 24 hours. Moderna’s mRNA-1273 will be much easier to distribute. The vaccine is stable in a standard freezer at –20° C for up to 6 months, in a refrigerator for up to 30 days within that 6-month shelf life, and at room temperature for up to 12 hours.
Timelines and testing other vaccines
Strong efficacy data from the two leading SARS-CoV-2 vaccines and emergency-use authorization Food and Drug Administration approval suggest the window for testing additional vaccine candidates in the United States could soon start to close. Of the more than 200 vaccines in development for SARS-CoV-2, at least 7 have a chance of gathering pivotal data before the front-runners become broadly available.
Testing diverse vaccine candidates, based on different technologies, is important for ensuring sufficient supply and could lead to products with tolerability and safety profiles that make them better suited, or more attractive, to subsets of the population. Different vaccine antigens and technologies also may yield different durations of protection, a question that will not be answered until long after the first products are on the market.
AstraZeneca enrolled about 23,000 subjects into its two phase 3 trials of AZD1222 (ChAdOx1 nCoV-19): a 40,000-subject U.S. trial and a 10,000-subject study in Brazil. AstraZeneca’s AZD1222, developed with the University of Oxford (England), uses a replication defective simian adenovirus vector called ChAdOx1.AZD1222 which encodes the SARS-CoV-2 spike protein. After injection, the viral vector delivers recombinant DNA that is decoded to mRNA, followed by mRNA decoding to become a protein. A serendipitous manufacturing error for the first 3,000 doses resulted in a half dose for those subjects before the error was discovered. Full doses were given to those subjects on second injections and those subjects showed 90% efficacy. Subjects who received 2 full doses showed 62% efficacy. A vaccine cannot be licensed based on 3,000 subjects so AstraZeneca has started a new phase 3 trial involving many more subjects to receive the combination lower dose followed by the full dose.
Johnson and Johnson (J&J) started its phase 3 trial evaluating a single dose of JNJ-78436735 in September. Phase 3 data may be reported by the end of2020. In November, J&J announced it was starting a second phase 3 trial to test two doses of the candidate. J&J’s JNJ-78436735 encodes the SARS-CoV-2 spike protein in an adenovirus serotype 26 (Ad26) vector, which is one of the two adenovirus vectors used in Sputnik V, the Russian vaccine reported to have 90% efficacy at an early interim analysis.
Sanofi and Novavax are both developing protein-based vaccines, a proven modality. Sanofi, in partnership with GlaxoSmithKline started a phase 1/2 clinical trial in the Fall 2020 with plans to commence a phase 3 trial in late December. Sanofi developed the protein ingredients and GlaxoSmithKline added one of their novel adjuvants. Novavax expects data from a U.K. phase 3 trial of NVX-CoV2373 in early 2021 and began a U.S. phase 3 study in late November. NVX-CoV2373 was created using Novavax’ recombinant nanoparticle technology to generate antigen derived from the coronavirus spike protein and contains Novavax’s patented saponin-based Matrix-M adjuvant.
Inovio Pharmaceuticals was gearing up to start a U.S. phase 2/3 trial of DNA vaccine INO-4800 by the end of 2020.
After Moderna and Pfizer-BioNTech, CureVac has the next most advanced mRNA vaccine. It was planned that a phase 2b/3 trial of CVnCoV would be conducted in Europe, Latin America, Africa, and Asia. Sanofi is also developing a mRNA vaccine as a second product in addition to its protein vaccine.
Vaxxinity planned to begin phase 3 testing of UB-612, a multitope peptide–based vaccine, in Brazil by the end of 2020.
However, emergency-use authorizations for the Pfizer and Moderna vaccines could hinder trial recruitment in at least two ways. Given the gravity of the pandemic, some stakeholders believe it would be ethical to unblind ongoing trials to give subjects the opportunity to switch to a vaccine proven to be effective. Even if unblinding doesn’t occur, as the two authorized vaccines start to become widely available, volunteering for clinical trials may become less attractive.
Dr. Pichichero is a specialist in pediatric infectious diseases, and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he has no relevant financial disclosures. Email Dr. Pichichero at [email protected].
References
1. Wolff JA et al. Science. 1990 Mar 23. doi: 10.1126/science.1690918.
2. Jackson LA et al. N Engl J Med. 2020 Nov 12. doi: 10.1056/NEJMoa2022483.
3. Prentice T and Reinders LT. The world health report 2007. (Geneva Switzerland: World Health Organization, 2007).
4. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
5. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
6. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
In mid-November, Pfizer/BioNTech were the first with surprising positive protection interim data for their coronavirus vaccine, BNT162b2. A week later, Moderna released interim efficacy results showing its coronavirus vaccine, mRNA-1273, also protected patients from developing SARS-CoV-2 infections. Both studies included mostly healthy adults. A diverse ethnic and racial vaccinated population was included. A reasonable number of persons aged over 65 years, and persons with stable compromising medical conditions were included. Adolescents aged 16 years and over were included. Younger adolescents have been vaccinated or such studies are in the planning or early implementation stage as 2020 came to a close.

These are new and revolutionary vaccines, although the ability to inject mRNA into animals dates back to 1990, technological advances today make it a reality.1 Traditional vaccines typically involve injection with antigens such as purified proteins or polysaccharides or inactivated/attenuated viruses. In the case of Pfizer’s and Moderna’s vaccines, the mRNA provides the genetic information to synthesize the spike protein that the SARS-CoV-2 virus uses to attach to and infect human cells. Each type of vaccine is packaged in proprietary lipid nanoparticles to protect the mRNA from rapid degradation, and the nanoparticles serve as an adjuvant to attract immune cells to the site of injection. (The properties of the respective lipid nanoparticle packaging may be the factor that impacts storage requirements discussed below.) When injected into muscle (myocyte), the lipid nanoparticles containing the mRNA inside are taken into muscle cells, where the cytoplasmic ribosomes detect and decode the mRNA resulting in the production of the spike protein antigen. It should be noted that the mRNA does not enter the nucleus, where the genetic information (DNA) of a cell is located, and can’t be reproduced or integrated into the DNA. The antigen is exported to the myocyte cell surface where the immune system’s antigen presenting cells detect the protein, ingest it, and take it to regional lymph nodes where interactions with T cells and B cells results in antibodies, T cell–mediated immunity, and generation of immune memory T cells and B cells. A particular subset of T cells – cytotoxic or killer T cells – destroy cells that have been infected by a pathogen. The SARS-CoV-2 mRNA vaccine from Pfizer was reported to induce powerful cytotoxic T-cell responses. Results for Moderna’s vaccine had not been reported at the time this column was prepared, but I anticipate the same positive results.
The revolutionary aspect of mRNA vaccines is the speed at which they can be designed and produced. This is why they lead the pack among the SARS-CoV-2 vaccine candidates and why the National Institute of Allergy and Infectious Diseases provided financial, technical, and/or clinical support. Indeed, once the amino acid sequence of a protein can be determined (a relatively easy task these days) it’s straightforward to synthesize mRNA in the lab – and it can be done incredibly fast. It is reported that the mRNA code for the vaccine by Moderna was made in 2 days and production development was completed in about 2 months.2
A 2007 World Health Organization report noted that infectious diseases are emerging at “the historically unprecedented rate of one per year.”3 Severe acute respiratory syndrome (SARS), Zika, Ebola, and avian and swine flu are recent examples. For most vaccines against emerging diseases, the challenge is about speed: developing and manufacturing a vaccine and getting it to persons who need it as quickly as possible. The current seasonal flu vaccine takes about 6 months to develop; it takes years for most of the traditional vaccines. That’s why once the infrastructure is in place, mRNA vaccines may prove to offer a big advantage as vaccines against emerging pathogens.
Early efficacy results have been surprising
Both vaccines were reported to produce about 95% efficacy in the final analysis. That was unexpectedly high because most vaccines for respiratory illness achieve efficacy of 60%-80%, e.g., flu vaccines. However, the efficacy rate may drop as time goes by because stimulation of short-term immunity would be in the earliest reported results.
Preventing SARS-CoV-2 cases is an important aspect of a coronavirus vaccine, but preventing severe illness is especially important considering that severe cases can result in prolonged intubation/artificial ventilation, prolonged disability and death. Pfizer/BioNTech had not released any data on the breakdown of severe cases as this column was finalized. In Moderna’s clinical trial, a secondary endpoint analyzed severe cases of COVID-19 and included 30 severe cases (as defined in the study protocol) in this analysis. All 30 cases occurred in the placebo group and none in the mRNA-1273–vaccinated group. In the Pfizer/BioNTech trial there were too few cases of severe illness to calculate efficacy.
Duration of immunity and need to revaccinate after initial primary vaccination are unknowns. Study of induction of B- and T-cell memory and levels of long-term protection have not been reported thus far.
Could mRNA COVID-19 vaccines be dangerous in the long term?
These will be the first-ever mRNA vaccines brought to market for humans. In order to receive Food and Drug Administration approval, the companies had to prove there were no immediate or short-term negative adverse effects from the vaccines. The companies reported that their independent data-monitoring committees hadn’t “reported any serious safety concerns.” However, fairly significant local reactions at the site of injection, fever, malaise, and fatigue occur with modest frequency following vaccinations with these products, reportedly in 10%-15% of vaccinees. Overall, the immediate reaction profile appears to be more severe than what occurs following seasonal influenza vaccination. When mass inoculations with these completely new and revolutionary vaccines begins, we will know virtually nothing about their long-term side effects. The possibility of systemic inflammatory responses that could lead to autoimmune conditions, persistence of the induced immunogen expression, development of autoreactive antibodies, and toxic effects of delivery components have been raised as theoretical concerns.4-6 None of these theoretical risks have been observed to date and postmarketing phase 4 safety monitoring studies are in place from the Centers for Disease Control and Prevention and the companies that produce the vaccines. This is a risk public health authorities are willing to take because the risk to benefit calculation strongly favors taking theoretical risks, compared with clear benefits in preventing severe illnesses and death.
What about availability?
Pfizer/BioNTech expects to be able to produce up to 50 million vaccine doses in 2020 and up to 1.3 billion doses in 2021. Moderna expects to produce 20 million doses by the end of 2020, and 500 million to 1 billion doses in 2021. Storage requirements are inherent to the composition of the vaccines with their differing lipid nanoparticle delivery systems. Pfizer/BioNTech’s BNT162b2 has to be stored and transported at –80° C, which requires specialized freezers, which most doctors’ offices and pharmacies are unlikely to have on site, or dry ice containers. Once the vaccine is thawed, it can only remain in the refrigerator for 24 hours. Moderna’s mRNA-1273 will be much easier to distribute. The vaccine is stable in a standard freezer at –20° C for up to 6 months, in a refrigerator for up to 30 days within that 6-month shelf life, and at room temperature for up to 12 hours.
Timelines and testing other vaccines
Strong efficacy data from the two leading SARS-CoV-2 vaccines and emergency-use authorization Food and Drug Administration approval suggest the window for testing additional vaccine candidates in the United States could soon start to close. Of the more than 200 vaccines in development for SARS-CoV-2, at least 7 have a chance of gathering pivotal data before the front-runners become broadly available.
Testing diverse vaccine candidates, based on different technologies, is important for ensuring sufficient supply and could lead to products with tolerability and safety profiles that make them better suited, or more attractive, to subsets of the population. Different vaccine antigens and technologies also may yield different durations of protection, a question that will not be answered until long after the first products are on the market.
AstraZeneca enrolled about 23,000 subjects into its two phase 3 trials of AZD1222 (ChAdOx1 nCoV-19): a 40,000-subject U.S. trial and a 10,000-subject study in Brazil. AstraZeneca’s AZD1222, developed with the University of Oxford (England), uses a replication defective simian adenovirus vector called ChAdOx1.AZD1222 which encodes the SARS-CoV-2 spike protein. After injection, the viral vector delivers recombinant DNA that is decoded to mRNA, followed by mRNA decoding to become a protein. A serendipitous manufacturing error for the first 3,000 doses resulted in a half dose for those subjects before the error was discovered. Full doses were given to those subjects on second injections and those subjects showed 90% efficacy. Subjects who received 2 full doses showed 62% efficacy. A vaccine cannot be licensed based on 3,000 subjects so AstraZeneca has started a new phase 3 trial involving many more subjects to receive the combination lower dose followed by the full dose.
Johnson and Johnson (J&J) started its phase 3 trial evaluating a single dose of JNJ-78436735 in September. Phase 3 data may be reported by the end of2020. In November, J&J announced it was starting a second phase 3 trial to test two doses of the candidate. J&J’s JNJ-78436735 encodes the SARS-CoV-2 spike protein in an adenovirus serotype 26 (Ad26) vector, which is one of the two adenovirus vectors used in Sputnik V, the Russian vaccine reported to have 90% efficacy at an early interim analysis.
Sanofi and Novavax are both developing protein-based vaccines, a proven modality. Sanofi, in partnership with GlaxoSmithKline started a phase 1/2 clinical trial in the Fall 2020 with plans to commence a phase 3 trial in late December. Sanofi developed the protein ingredients and GlaxoSmithKline added one of their novel adjuvants. Novavax expects data from a U.K. phase 3 trial of NVX-CoV2373 in early 2021 and began a U.S. phase 3 study in late November. NVX-CoV2373 was created using Novavax’ recombinant nanoparticle technology to generate antigen derived from the coronavirus spike protein and contains Novavax’s patented saponin-based Matrix-M adjuvant.
Inovio Pharmaceuticals was gearing up to start a U.S. phase 2/3 trial of DNA vaccine INO-4800 by the end of 2020.
After Moderna and Pfizer-BioNTech, CureVac has the next most advanced mRNA vaccine. It was planned that a phase 2b/3 trial of CVnCoV would be conducted in Europe, Latin America, Africa, and Asia. Sanofi is also developing a mRNA vaccine as a second product in addition to its protein vaccine.
Vaxxinity planned to begin phase 3 testing of UB-612, a multitope peptide–based vaccine, in Brazil by the end of 2020.
However, emergency-use authorizations for the Pfizer and Moderna vaccines could hinder trial recruitment in at least two ways. Given the gravity of the pandemic, some stakeholders believe it would be ethical to unblind ongoing trials to give subjects the opportunity to switch to a vaccine proven to be effective. Even if unblinding doesn’t occur, as the two authorized vaccines start to become widely available, volunteering for clinical trials may become less attractive.
Dr. Pichichero is a specialist in pediatric infectious diseases, and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he has no relevant financial disclosures. Email Dr. Pichichero at [email protected].
References
1. Wolff JA et al. Science. 1990 Mar 23. doi: 10.1126/science.1690918.
2. Jackson LA et al. N Engl J Med. 2020 Nov 12. doi: 10.1056/NEJMoa2022483.
3. Prentice T and Reinders LT. The world health report 2007. (Geneva Switzerland: World Health Organization, 2007).
4. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
5. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
6. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
How to identify and treat common bites and stings
Insect, arachnid, and other arthropod bites and stings are common patient complaints in a primary care office. A thorough history and physical exam can often isolate the specific offender and guide management. In this article, we outline how to identify, diagnose, and treat common bites and stings from bees and wasps; centipedes and spiders; fleas; flies and biting midges; mosquitoes; and ticks, and discuss how high-risk patients should be triaged and referred for additional testing and treatment, such as venom immunotherapy (VIT).
Insects and arachnids:Background and epidemiology
Insects are arthropods with 3-part exoskeletons: head, thorax, and abdomen. They have 6 jointed legs, compound eyes, and antennae. There are approximately 91,000 insect species in the United States, the most abundant orders being Coleoptera (beetles), Diptera (flies), and Hymenoptera (includes ants, bees, wasps, and sawflies).1
The reported incidence of insect bites and stings varies widely because most people experience mild symptoms and therefore do not seek medical care. Best statistics are for Hymenoptera stings, which are more likely to cause a severe reaction. In Europe, 56% to 94% of the general population has reported being bitten or stung by one of the Hymenoptera species.2 In many areas of Australia, the incidence of jack jumper ant stings is only 2% to 3%3; in the United States, 55% of people report being stung by nonnative fire ants within 3 weeks of moving into an endemic area.4
Arachnids are some of the earliest terrestrial organisms, of the class Arachnida, which includes scorpions, ticks, spiders, mites, and daddy longlegs (harvestmen).5 Arachnids are wingless and characterized by segmented bodies, jointed appendages, and exoskeletons.6,7 In most, the body is separated into 2 segments (the cephalothorax and abdomen), except for mites, ticks, and daddy longlegs, in which the entire body comprises a single segment.5
Arthropod bites are common in the United States; almost one-half are caused by spiders.7 Brown recluse (Loxosceles spp) and black widow (Latrodectus spp) spider bites are the most concerning: Although usually mild, these bites can be life-threatening but are rarely fatal. In 2013, almost 3500 bites by black widow and brown recluse spiders were reported.8
Risk factors
Risk factors for insect, arachnid, and other arthropod bites and stings are primarily environmental. People who live or work in proximity of biting or stinging insects (eg, gardeners and beekeepers) are more likely to be affected; so are those who work with animals or live next to standing water or grassy or wooded locales.
Continue to: There are also risk factors...
There are also risk factors for a systemic sting reaction:
- A sting reaction < 2 months earlier increases the risk of a subsequent systemic sting reaction by ≥ 50%.9
- Among beekeepers, paradoxically, the risk of a systemic reaction is higher in those stung < 15 times a year than in those stung > 200 times.10
- Patients with an elevated baseline serum level of tryptase (reference range, < 11.4 ng/mL), which is part of the allergenic response, or with biopsy-proven systemic mastocytosis are at increased risk of a systemic sting reaction.11
Presentation: Signs and symptomsvary with severity
Insect bites and stings usually cause transient local inflammation and, occasionally, a toxic reaction. Allergic hypersensitivity can result in a large local reaction or a generalized systemic reaction12:
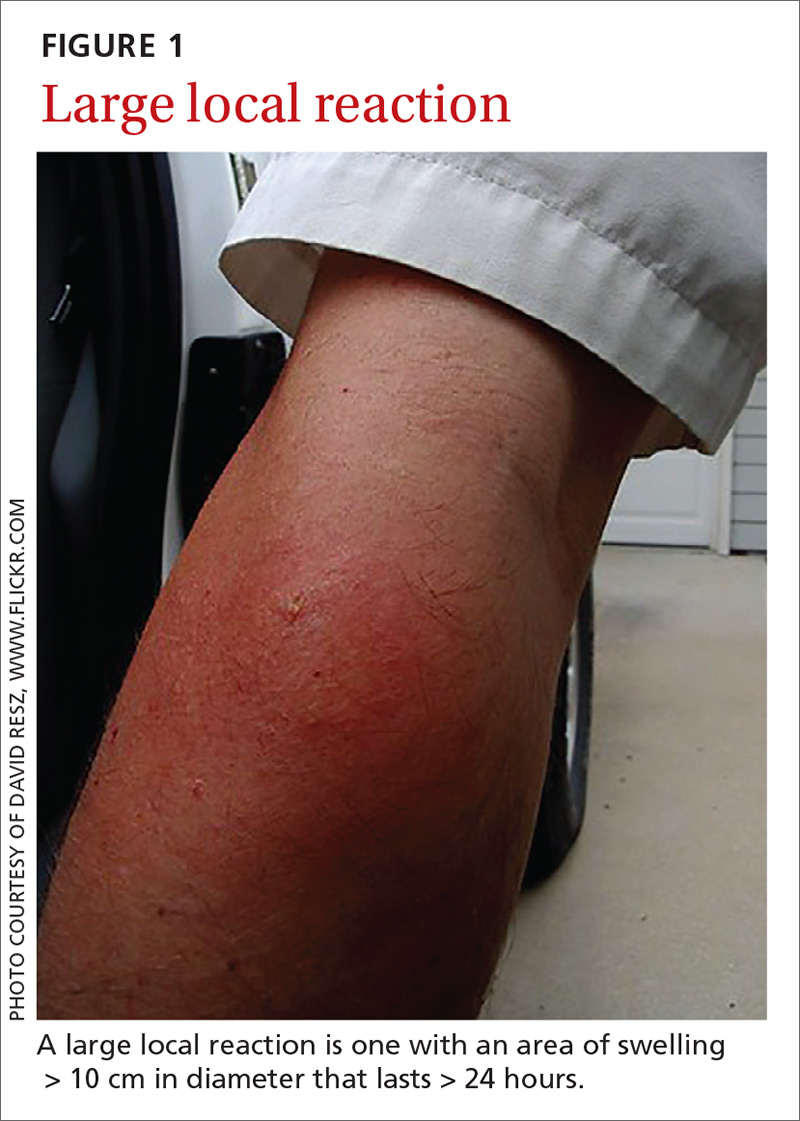
- A small local reaction is transient and mild, develops directly at the site of the sting, and can last several days.13
- A large (or significant) local reaction, defined as swelling > 10 cm in diameter (FIGURE 1) and lasting > 24 hours, occurs in 2% to 26% of people who have been bitten or stung.14 This is an immunoglobulin (Ig) E–mediated late-phase reaction that can be accompanied by fatigue and nausea.12,13,15 For a patient with a large local reaction, the risk of a concomitant systemic reaction is 4% to 10%, typically beginning within 30 minutes after envenomation or, possibly, delayed for several hours or marked by a biphasic interval.16
- Characteristics of a systemic reaction are urticaria, angioedema, bronchospasm, large-airway edema, hypotension, and other clinical manifestations of anaphylaxis.17 In the United States, a systemic sting reaction is reported to occur in approximately 3% of bite and sting victims. Mortality among the general population from a systemic bite or sting reaction is 0.16 for every 100,000 people,2 and at least 40 to 100 die every year in the United States from anaphylaxis resulting from an insect bite or sting.18
- The most severe anaphylactic reactions involve the cardiovascular and respiratory systems, commonly including hypotension and symptoms of upper- or lower-airway obstruction. Laryngeal edema and circulatory failure are the most common mechanisms of anaphylactic death.19
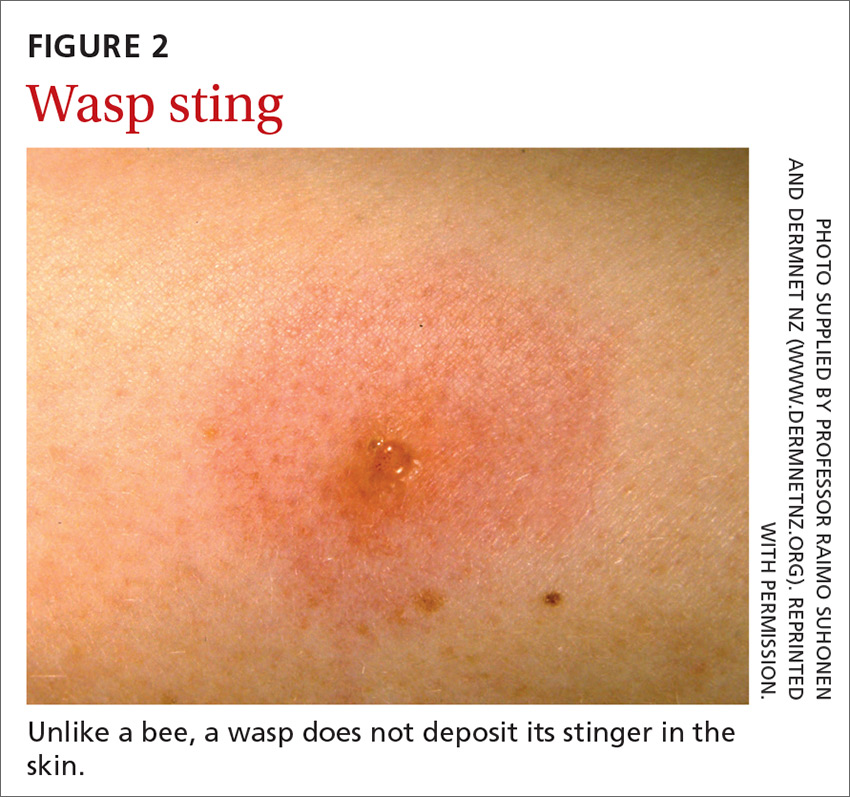
Bees and wasps
Hymenoptera stinging insects include the family Apidae (honey bee, bumblebee, and sweat bee) and Vespidae (yellow jacket, yellow- and white-faced hornets, and paper wasp). A worker honey bee can sting only once, leaving its barbed stinger in the skin; a wasp, hornet, and yellow jacket can sting multiple times (FIGURE 2).2
Continue to: Bee and wasp sting...
Bee and wasp sting allergies are the most common insect venom allergic reactions. A bee sting is more likely to lead to a severe allergic reaction than a wasp sting. Allergic reactions to hornet and bumblebee stings are less common but can occur in patients already sensitized to wasp and honey bee stings.20,21
Management. Remove honey bee stingers by scraping the skin with a fingernail or credit card. Ideally, the stinger should be removed in the first 30 seconds, before the venom sac empties. Otherwise, intense local inflammation, with possible lymphangitic streaking, can result.22
For guidance on localized symptomatic care of bee and wasp stings and bites and stings from other sources discussed in this article, see “Providing relief and advanced care” on page E6.
Centipedes and spiders
Centipedes are arthropods of the class Chilopoda, subphylum Myriapoda, that are characterized by repeating linear (metameric) segments, each containing 1 pair of legs.23 Centipedes have a pair of poison claws behind the head that are used to paralyze prey—usually, small insects.23,24 The bite of a larger centipede can cause a painful reaction that generally subsides after a few hours but can last several days. Centipede bites are usually nonfatal to humans.23
Spiders belong to the class Arachnida, order Araneae. They have 8 legs with chelicerae (mouthpiece, or “jaws”) that inject venom into prey.25 Most spiders found in the United States cannot bite through human skin.26,27 Common exceptions are black widow and brown recluse spiders, which each produce a distinct toxic venom that can cause significant morbidity in humans through a bite, although bites are rarely fatal.26,27
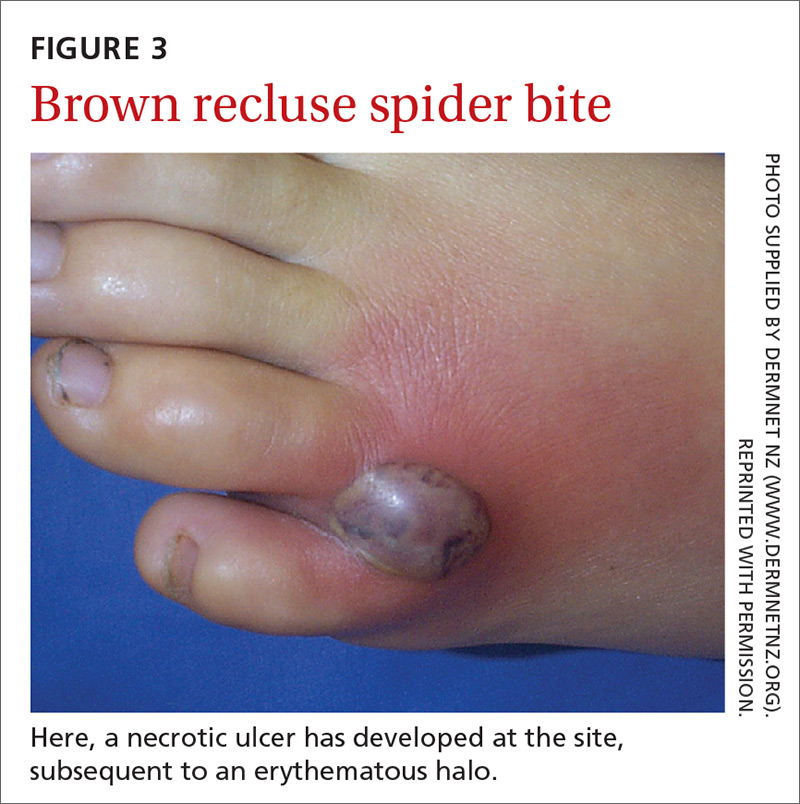
The brown recluse spider is described as having a violin-shaped marking on the abdomen; the body is yellowish, tan, or dark brown. A bite can produce tiny fang marks and cause dull pain at the site of the bite that spreads quickly; myalgia; and pain in the stomach, back, chest, and legs.28,29 The bite takes approximately 7 days to resolve. In a minority of cases, a tender erythematous halo develops, followed by a severe necrotic ulcer, or loxoscelism (FIGURE 3; 40% of cases) or scarring (13%), or both.29,30
Continue to: In contrast...
In contrast, the body of a black widow spider is black; females exhibit a distinctive red or yellow hourglass marking on their ventral aspect.28,31 The pinprick sensation of a bite leads to symptoms that can include erythema, swelling, pain, stiffness, chills, fever, nausea, and stomach pain.30,32
Management. Again, see “Providing relief and advanced care” on page E6. Consider providing antivenin treatment for moderate or severe bites of brown recluse and black widow spiders.
Fleas
Fleas are members of the order Siphonaptera. They are small (1.5-3.2 mm long), reddish brown, wingless, blood-sucking insects with long legs that allow them to jump far (12 or 13 inches) and high (6 or 7 inches).33 Domesticated cats and dogs are the source of most flea infestations, resulting in an increased risk of exposure for humans.34,35 Flea bites, which generally occur on lower extremities, develop into a small, erythematous papule with a halo (FIGURE 4) and associated mild edema, and cause intense pruritus 30 minutes after the bite.35-37
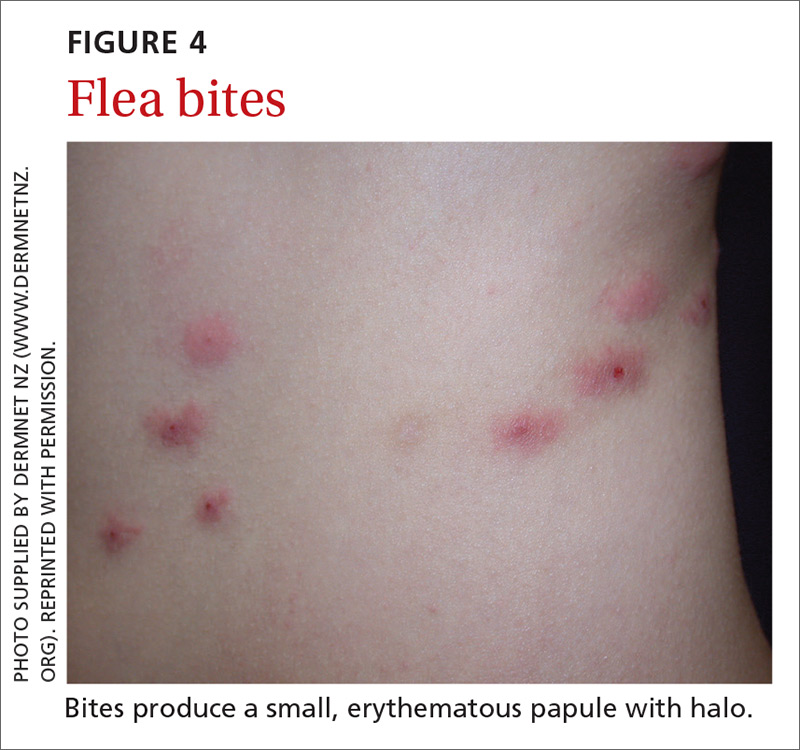
Fleas are a vector for severe microbial infections, including bartonellosis, bubonic plague, cat-flea typhus, murine typhus, cat-scratch disease, rickettsial disease, and tularemia. Tungiasis is an inflammatory burrowing flea infestation—not a secondary infection for which the flea is a vector.34,35
Preventive management. Repellents, including products that contain DEET (N,N-diethyl-meta-toluamide), picaridin (2-[2-hydroxyethyl]-1-piperidinecarboxylic acid 1-methylpropyl ester), and PMD (p-menthane-3,8-diol, a chemical constituent of Eucalyptus citriodora oil) can be used to prevent flea bites in humans.33,38 Studies show that the scent of other botanic oils, including lavender, cedarwood, and peppermint, can also help prevent infestation by fleas; however, these compounds are not as effective as traditional insect repellents.33,38
Flea control is difficult, requiring a multimodal approach to treating the infested animal and its environment.39 Treatment of the infested domestic animal is the primary method of preventing human bites. Nonpesticidal control involves frequent cleaning of carpeting, furniture, animal bedding, and kennels. Insecticides can be applied throughout the house to combat severe infestation.33,38
Continue to: The Centers for Disease Control and Prevention...
The Centers for Disease Control and Prevention provide a general introduction to getting rid of fleas for pet owners.40 For specific guidance on flea-eradication strategies and specific flea-control products, advise patients to seek the advice of their veterinarian.
Flies and biting midges
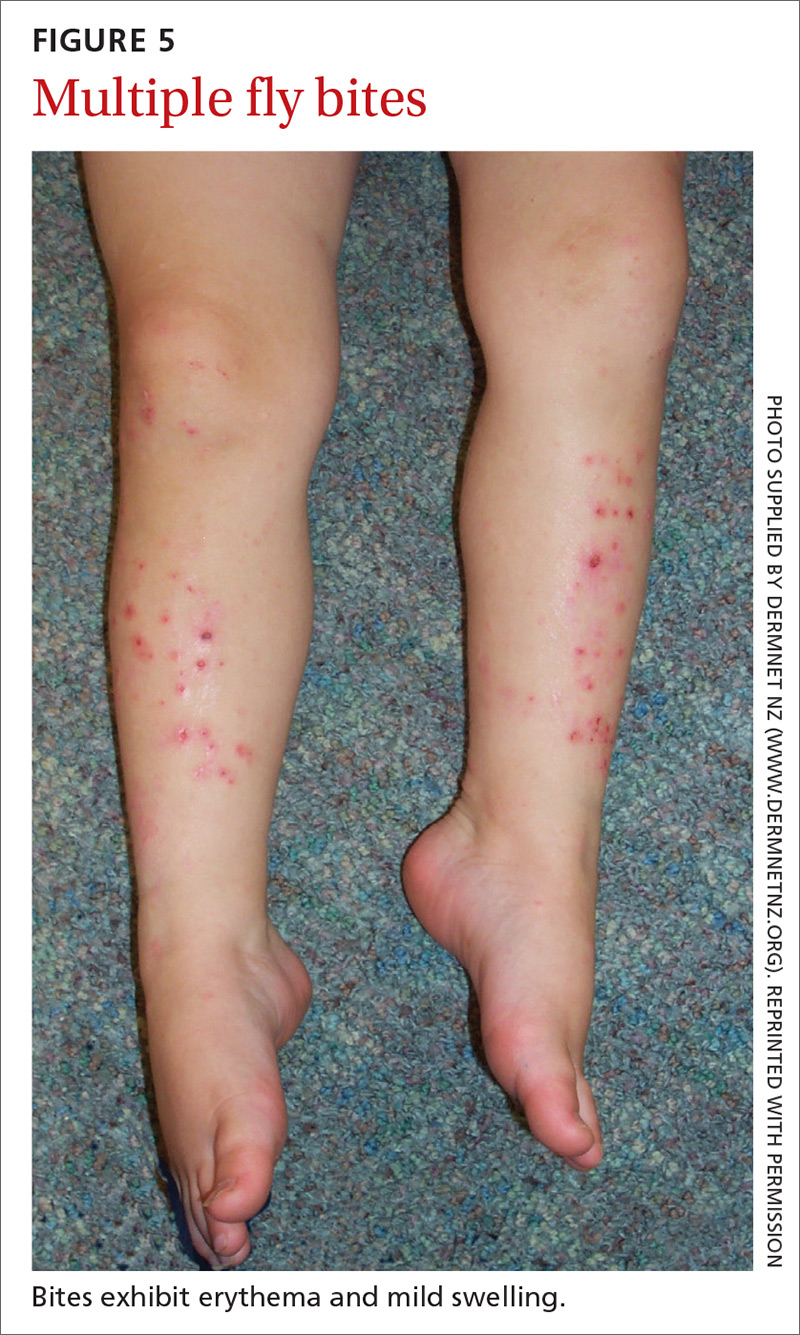
Flies are 2-winged insects belonging to the order Diptera. Several fly species can bite, causing a local inflammatory reaction; these include black flies, deer flies, horse flies, and sand flies. Signs and symptoms of a fly bite include pain, pruritus, erythema, and mild swelling (FIGURE 5).41,42 Flies can transmit several infections, including bartonellosis, enteric bacterial disease (eg, caused by Campylobacter spp), leishmaniasis, loiasis, onchocerciasis, and trypanosomiasis.43
Biting midges, also called “no-see-ums,” biting gnats, moose flies, and “punkies,”44 are tiny (1-3 mm long) blood-sucking flies.45 Bitten patients often report not having seen the midge because it is so small. The bite typically starts as a small, erythematous papule that develops into a dome-shaped blister and can be extraordinarily pruritic and painful.44 The majority of people who have been bitten develop a hypersensitivity reaction, which usually resolves in a few weeks.
Management. Suppressing adult biting midges with an environmental insecticide is typically insufficient because the insecticide must be sprayed daily to eradicate active midges and generally does not affect larval habitat. Insect repellents and biopesticides, such as oil of lemon eucalyptus, can be effective in reducing the risk of bites.44,45
Mosquitoes
Mosquitoes are flying, blood-sucking insects of the order Diptera and family Culicidae. Anopheles, Culex, and Aedes genera are responsible for most bites of humans.
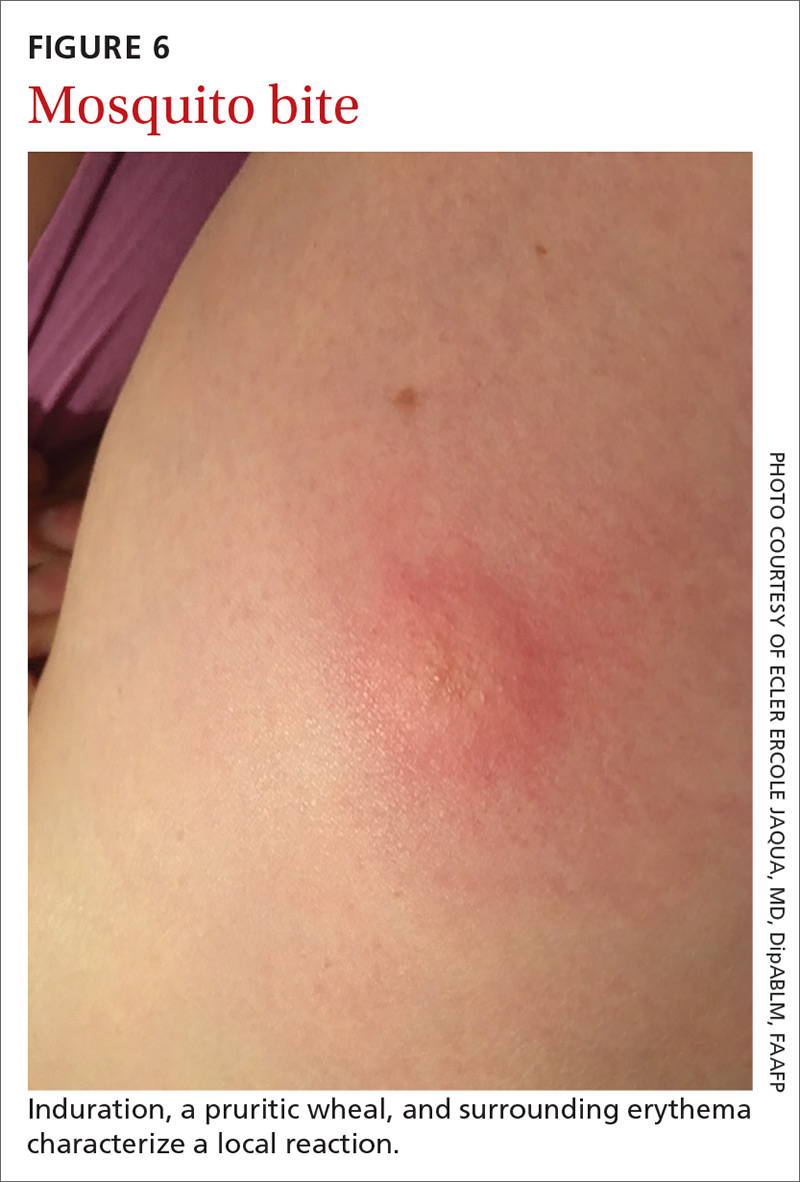
The bite of a mosquito produces an indurated, limited local reaction characterized by a pruritic wheal (3-29 mm in diameter) with surrounding erythema (FIGURE 6) that peaks in approximately 30 minutes, although patients might have a delayed reaction hours later.46 Immunocompromised patients might experience a more significant local inflammatory reaction that is accompanied by low-grade fever, hives, or swollen lymph nodes.46,47
Mosquitoes are a vector for serious infections, including dengue, Japanese encephalitis, malaria, and yellow fever, and disease caused by Chikungunya, West Nile, and Zika viruses.
Continue to: Management
Management. Advise patients to reduce their risk by using insect repellent, sleeping under mosquito netting, and wearing a long-sleeve shirt and long pants when traveling to endemic areas or when a local outbreak occurs.48
Ticks
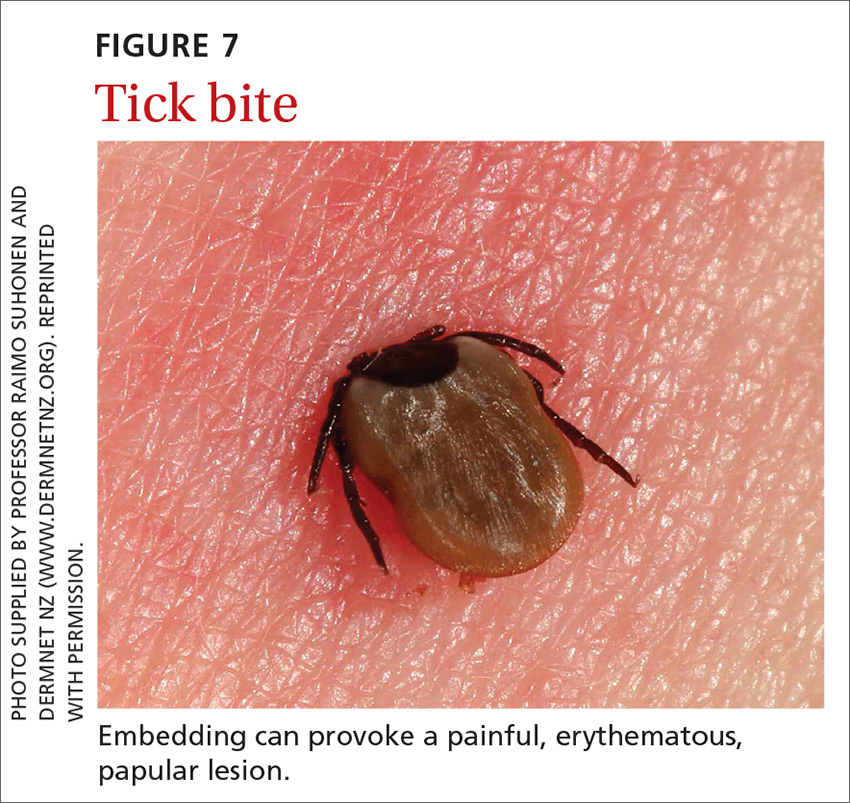
Ticks belong to the order Parasitiformes and families Ixodidae and Argasidae. Hard ticks are found in brushy fields and tall grasses and can bite and feed on humans for days. Soft ticks are generally found around animal nests.29 Tick bites can cause a local reaction that includes painful, erythematous, inflammatory papular lesions (FIGURE 7).49
Ticks can transmit several infectious diseases. Depending on the microbial pathogen and the genus and species of tick, it takes 2 to 96 hours for the tick to attach to skin and transmit the pathogen to the human host. The TABLE29,49,50 provides an overview of tick species in the United States, diseases that they can transmit, and the geographic distribution of those diseases.
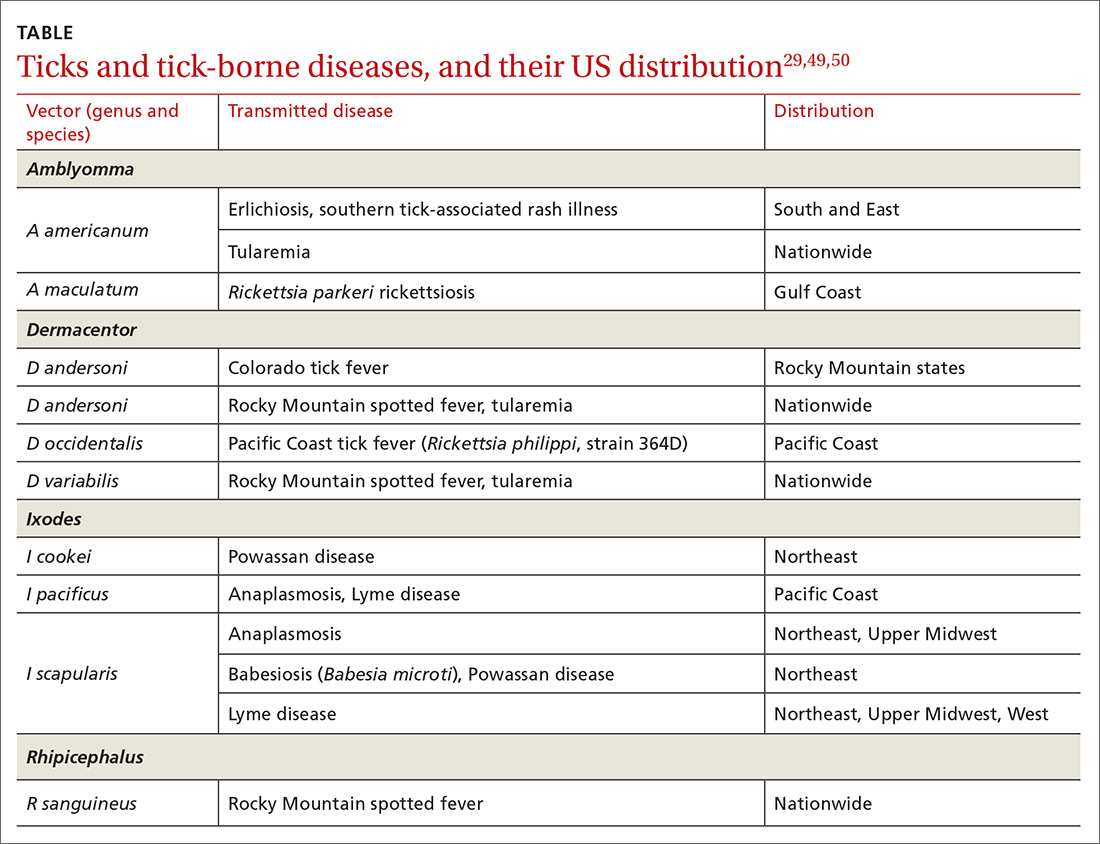
Management. Ticks should be removed with fine-tipped tweezers. Grasp the body of the tick close to the skin and pull upward while applying steady, even pressure. After removing the tick, clean the bite and the surrounding area with alcohol or with soap and water. Dispose of a live tick by flushing it down the toilet; or, kill it in alcohol and either seal it in a bag with tape or place it in a container.50
Diagnosis and the utilityof special testing
The diagnosis of insect, arachnid, and other arthropod bites and stings depends on the history, including obtaining a record of possible exposure and a travel history; the timing of the bite or sting; and associated signs and symptoms.18,51
Venom skin testing. For Hymenoptera stings, intradermal tests using a venom concentration of 0.001 to 1 μg/mL are positive in 65% to 80% of patients with a history of a systemic insect-sting allergic reaction. A negative venom skin test can occur during the 3-to-6-week refractory period after a sting reaction or many years later, which represents a loss of sensitivity. Positive venom skin tests are used to confirm allergy and identify specific insects to which the patient is allergic.11,12
Continue to: Allergen-specific IgE antibody testing.
Allergen-specific IgE antibody testing. These serum assays—typically, radioallergosorbent testing (RAST)—are less sensitive than venom skin tests. RAST is useful when venom skin testing cannot be performed or when skin testing is negative in a patient who has had a severe allergic reaction to an insect bite or sting. Serum IgE-specific antibody testing is preferred over venom skin testing in patients who are at high risk of anaphylaxis.52,53
Providing reliefand advanced care
Symptomatic treatment of mild bites and stings includes washing the affected area with soap and water and applying a cold compress to reduce swelling.54 For painful lesions, an oral analgesic can be prescribed.
For mild or moderate pruritus, a low- to midpotency topical corticosteroid (eg, hydrocortisone valerate cream 0.2% bid), topical calamine, or pramoxine can be applied,or a nonsedating oral antihistamine, such as loratadine (10 mg/d) or cetirizine (10 mg/d), can be used.14,55 For severe itching, a sedating antihistamine, such as hydroxyzine (10-25 mg every 4 to 6 hours prn), might help relieve symptoms; H1- and H2-receptor antagonists can be used concomitantly.54,55
Significant local symptoms. Large local reactions are treated with a midpotency topical corticosteroid (eg, triamcinolone acetonide cream 0.1% bid) plus an oral antihistamine to relieve pruritus and reduce allergic inflammation. For a more severe reaction, an oral corticosteroid (prednisone 1 mg/kg; maximum dosage, 50 mg/d) can be given for 5 to 7 days.54-56
Management of a necrotic ulcer secondary to a brown recluse spider bite is symptomatic and supportive. The size of these wounds can increase for as long as 10 days after the bite; resolution can require months of wound care, possibly with debridement. Rarely, skin grafting is required.27,28,31
VIT. Some studies show that VIT can improve quality of life in patients with prolonged, frequent, and worsening reactions to insect bites or stings and repeated, unavoidable exposures.55,56 VIT is recommended for patients with systemic hypersensitivity and a positive venom skin test result. It is approximately 95% effective in preventing or reducing severe systemic reactions and reduces the risk of anaphylaxis (see next section) and death.57 The maintenance dosage of VIT is usually 100 μg every 4 to 6 weeks; optimal duration of treatment is 3 to 5 years.58
Continue to: After VIT is complete...
After VIT is complete, counsel patients that a mild systemic reaction is still possible after an insect bite or sting. More prolonged, even lifetime, treatment should be considered for patients who have58,59
- a history of severe, life-threatening allergic reactions to bites and stings
- honey bee sting allergy
- mast-cell disease
- a history of anaphylaxis while receiving VIT.
Absolute contraindications to VIT include a history of serious immune disease, chronic infection, or cancer.58,59
Managing anaphylaxis
This severe allergic reaction can lead to death if untreated. First-line therapy is intramuscular epinephrine, 0.01 mg/kg (maximum single dose, 0.5 mg) given every 5 to 15 minutes.14,60 Epinephrine auto-injectors deliver a fixed dose and are labeled according to weight. Administration of O2 and intravenous fluids is recommended for hemodynamically unstable patients.60,61 Antihistamines and corticosteroids can be used as secondary treatment but should not replace epinephrine.56
After preliminary improvement, patients might decompensate when the epinephrine dose wears off. Furthermore, a biphasic reaction, variously reported in < 5% to as many as 20% of patients,61,62 occurs hours after the initial anaphylactic reaction. Patients should be monitored, therefore, for at least 6 to 8 hours after an anaphylactic reaction, preferably in a facility equipped to treat anaphylaxis.17,56
Before discharge, patients who have had an anaphylactic reaction should be given a prescription for epinephrine and training in the use of an epinephrine auto-injector. Allergen avoidance, along with an emergency plan in the event of a bite or sting, is recommended. Follow-up evaluation with an allergist or immunologist is essential for proper diagnosis and to determine whether the patient is a candidate for VIT.14,17
CORRESPONDENCE
Ecler Ercole Jaqua, MD, DipABLM, FAAFP, 1200 California Street, Suite 240, Redlands, CA 92374; [email protected].
1. Numbers of insects (species and individuals). Smithsonian BugInfo Web site. www.si.edu/spotlight/buginfo/bugnos. Accessed November 25, 2020.
2. Antonicelli L, Bilò MB, Bonifazi F. Epidemiology of Hymenoptera allergy. Curr Opin Allergy Clin Immunol. 2002;2:341-346.
3. Jack jumper ant allergy. Australasian Society of Clinical Immunology and Allergy (ASCIA) Web site. Updated October 19, 2019. www.allergy.org.au/patients/insect-allergy-bites-and-stings/jack-jumper-ant-allergy. Accessed November 25, 2020.
4. Kemp SF, deShazo RD, Moffit JE, et al. Expanding habitat of the imported fire ant (Solenopsis invicta): a public health concern. J Allergy Clin Immunol. 2000;105:683-691.
5. Goodnight ML. Arachnid. In: Encyclopædia Britannica. 2012. www.britannica.com/animal/arachnid. Accessed November 25, 2020.
6. Despommier DD, Gwadz RW, Hotez PJ. Arachnids. In: Despommier DD, Gwadz RW, Hotez PJ. Parasitic Diseases. 3rd ed. Springer-Verlag; 1995:268-283.
7. Diaz JH, Leblanc KE. Common spider bites. Am Fam Physician. 2007;75:869-873.
8. Mowry JB, Spyker DA, Cantilena LR Jr, McMillan N, Ford M. 2013 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 31st Annual Report. Clin Toxicol (Phila). 2014;52:1032-1283.
9. Pucci S, Antonicelli L, Bilò MB, et al. Shortness of interval between two stings as risk factor for developing Hymenoptera venom allergy. Allergy.1994;49:894-896.
10. Müller UR. Bee venom allergy in beekeepers and their family members. Curr Opin Allergy Clin Immunol. 2005;5:343-347.
11. Müller UR. Cardiovascular disease and anaphylaxis. Curr Opin Allergy Clin Immunol. 2007;7:337-341.
12. Golden DBK. Stinging insect allergy. Am Fam Physician. 2003;67:2541-2546.
13. Golden DBK, Demain T, Freeman T, et al. Stinging insect hypersensitivity: a practice parameter update 2016. Ann Allergy Asthma Immunol. 2017;118:28-54.
14. Bilò BM, Rueff F, Mosbech H, et al; EAACI Interest Group on Insect Venom Hypersensitivity. Diagnosis of Hymenoptera venom allergy. Allergy. 2005;60:1339-1349.
15. Reisman RE. Insect stings. N Engl J Med. 1994;331:523-527.
16. Pucci S, D’Alò S, De Pasquale T, et al. Risk of anaphylaxis in patients with large local reactions to hymenoptera stings: a retrospective and prospective study. Clin Mol Allergy. 2015;13:21.
17. Golden DBK. Large local reactions to insect stings. J Allergy Clin Immunol Pract. 2015;3:331-334.
18. Clark S, Camargo CA Jr. Emergency treatment and prevention of insect-sting anaphylaxis. Curr Opin Allergy Clin Immunol. 2006;6:279-283.
19. Stinging insect allergy. In: Volcheck GW. Clinical Allergy: Diagnosis and Management. Humana Press; 2009:465-479.
20. Järvinen KM, Celestin J. Anaphylaxis avoidance and management: educating patients and their caregivers. J Asthma Allergy. 2014;7:95-104.
21. Institute for Quality and Efficiency in Health Care (IQWiG). Insect venom allergies: overview. InformedHealth.org. Updated May 7, 2020. www.ncbi.nlm.nih.gov/pubmedhealth/PMH0096282/. Accessed November 25, 2020.
22. Casale TB, Burks AW. Clinical practice. Hymenoptera-sting hypersensitivity. N Engl J Med. 2014;370:1432-1439.
23. Shelley RM. Centipedes and millipedes with emphasis on North American fauna. Kansas School Naturalist. 1999;45:1-16. https://sites.google.com/g.emporia.edu/ksn/ksn-home/vol-45-no-3-centipedes-and-millipedes-with-emphasis-on-n-america-fauna#h.p_JEf3uDlTg0jw. Accessed November 25, 2020.
24. Ogg B. Centipedes and millipedes. Nebraska Extension in Lancaster County Web site. https://lancaster.unl.edu/pest/resources/CentipedeMillipede012.shtml. Accessed November 25, 2020.
25. Cushing PE. Spiders (Arachnida: Araneae). In: Capinera JL, ed. Encyclopedia of Entomology. Springer, Dordrecht; 2008:226.
26. Diaz JH, Leblanc KE. Common spider bites. Am Fam Physician. 2007;75:869-873.
27. The National Institute for Occupational Safety and Health (NIOSH), Centers for Disease Control and Prevention. Venomous spiders. www.cdc.gov/niosh/topics/spiders/. Accessed November 25, 2020.
28. Starr S. What you need to know to prevent a poisonous spider bite. AAP News. 2013;34:42. www.aappublications.org/content/aapnews/34/9/42.5.full.pdf. Accessed November 25, 2020.
29. Spider bites. Mayo Clinic Web site. www.mayoclinic.org/diseases-conditions/spider-bites/symptoms-causes/syc-20352371. Accessed November 25, 2020.
30. Barish RA, Arnold T. Spider bites. In: Merck Manual (Professional Version). Merck Sharp & Dohme Corp.; 2016. www.merckmanuals.com/professional/injuries-poisoning/bites-and-stings/spider-bites. Accessed November 25, 2020.
31. Juckett G. Arthropod bites. Am Fam Physician. 2013;88:841-847.
32. Clark RF, Wethern-Kestner S, Vance MV, et al. Clinical presentation and treatment of black widow spider envenomation: a review of 163 cases. Ann Emerg Med. 1992;21:782-787.
33. Koehler PG, Pereira RM, Diclaro JW II. Fleas. Publication ENY-025. University of Florida IFAS Extension. Revised January 2012. https://edis.ifas.ufl.edu/ig087. Accessed November 25, 2020.
34. Bitam I, Dittmar K, Parola P, et al. Fleas and flea-borne diseases. Int J Infect Dis. 2010;14:e667-e676.
35. Leulmi H, Socolovschi C, Laudisoit A, et al. Detection of Rickettsia felis, Rickettsia typhi, Bartonella species and Yersinia pestis in fleas (Siphonaptera) from Africa. PLoS Negl Trop Dis. 2014;8:e3152.
36. Naimer SA, Cohen AD, Mumcuoglu KY, et al. Household papular urticaria. Isr Med Assoc J. 2002;4(11 suppl):911-913.
37. Golomb MR, Golomb HS. What’s eating you? Cat flea (Ctenocephalides felis). Cutis. 2010;85:10-11.
38. Dryden MW. Flea and tick control in the 21st century: challenges and opportunities. Vet Dermatol. 2009;20:435-440.
39. Dryden MW. Fleas in dogs and cats. Merck Sharp & Dohme Corporation: Merck Manual Veterinary Manual. Updated December 2014. www.merckvetmanual.com/integumentary-system/fleas-and-flea-allergy-dermatitis/fleas-in-dogs-and-cats. Accessed November 25, 2020.
40. Centers for Disease Control and Prevention. Getting rid of fleas. www.cdc.gov/fleas/getting_rid.html. Accessed November 25, 2020.
41. Chattopadhyay P, Goyary D, Dhiman S, et al. Immunomodulating effects and hypersensitivity reactions caused by Northeast Indian black fly salivary gland extract. J Immunotoxicol. 2014;11:126-132.
42. Hrabak TM, Dice JP. Use of immunotherapy in the management of presumed anaphylaxis to the deer fly. Ann Allergy Asthma Immunol. 2003;90:351-354.
43. Royden A, Wedley A, Merga JY, et al. A role for flies (Diptera) in the transmission of Campylobacter to broilers? Epidemiol Infect. 2016;144:3326-3334.
44. Fradin MS, Day JF. Comparative efficacy of insect repellents against mosquito bites. N Engl J Med. 2002;347:13-18.

45. Carpenter S, Groschup MH, Garros C, et al. Culicoides biting midges, arboviruses and public health in Europe. Antiviral Res. 2013;100:102-113.
46. Peng Z, Yang M, Simons FE. Immunologic mechanisms in mosquito allergy: correlation of skin reactions with specific IgE and IgG anti-bodies and lymphocyte proliferation response to mosquito antigens. Ann Allergy Asthma Immunol. 1996;77:238-244.
47. Simons FE, Peng Z. Skeeter syndrome. J Allergy Clin Immunol. 1999;104:705-707.
48. Centers for Disease Control and Prevention. Travelers’ health. Clinician resources. wwwnc.cdc.gov/travel/page/clinician-information-center. Accessed November 25, 2020.
49. Gauci M, Loh RK, Stone BF, et al. Allergic reactions to the Australian paralysis tick, Ixodes holocyclus: diagnostic evaluation by skin test and radioimmunoassay. Clin Exp Allergy. 1989;19:279-283.
50. Centers for Disease Control and Prevention. Ticks. Removing a tick. www.cdc.gov/ticks/removing_a_tick.html. Accessed November 25, 2020.
51. Golden DB, Kagey-Sobotka A, Norman PS, et al. Insect sting allergy with negative venom skin test responses. J Allergy Clin Immunol. 2001;107:897-901.
52. Arzt L, Bokanovic D, Schrautzer C, et al. Immunological differences between insect venom-allergic patients with and without immunotherapy and asymptomatically sensitized subjects. Allergy. 2018;73:1223-1231.
53. Heddle R, Golden DBK. Allergy to insect stings and bites. World Allergy Organization Web site. Updated August 2015. www.worldallergy.org/education-and-programs/education/allergic-disease-resource-center/professionals/allergy-to-insect-stings-and-bites. Accessed November 25, 2020.
54. RuëffF, Przybilla B, Müller U, et al. The sting challenge test in Hymenoptera venom allergy. Position paper of the Subcommittee on Insect Venom Allergy of the European Academy of Allergology and Clinical Immunology. Allergy. 1996;51:216-225.
55. Management of simple insect bites: where’s the evidence? Drug Ther Bull. 2012;50:45-48.
56. Tracy JM. Insect allergy. Mt Sinai J Med. 2011;78:773-783.
57. Golden DBK. Insect sting allergy and venom immunotherapy: a model and a mystery. J Allergy Clin Immunol. 2005;115:439-447.
58. Winther L, Arnved J, Malling H-J, et al. Side-effects of allergen-specific immunotherapy: a prospective multi-centre study. Clin Exp Allergy. 2006;36:254-260.
59. Mellerup MT, Hahn GW, Poulsen LK, et al. Safety of allergen-specific immunotherapy. Relation between dosage regimen, allergen extract, disease and systemic side-effects during induction treatment. Clin Exp Allergy. 2000;30:1423-1429.
60. Anaphylaxis and insect stings and bites. Med Lett Drugs Ther. 2017;59:e79-e82.
61. Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report—second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. Ann Emerg Med. 2006;47:373-380.
62. Pflipsen MC, Vega Colon KM. Anaphylaxis: recognition and management. Am Fam Physician. 2020;102:355-362. Accessed November 25, 2020.
Insect, arachnid, and other arthropod bites and stings are common patient complaints in a primary care office. A thorough history and physical exam can often isolate the specific offender and guide management. In this article, we outline how to identify, diagnose, and treat common bites and stings from bees and wasps; centipedes and spiders; fleas; flies and biting midges; mosquitoes; and ticks, and discuss how high-risk patients should be triaged and referred for additional testing and treatment, such as venom immunotherapy (VIT).
Insects and arachnids:Background and epidemiology
Insects are arthropods with 3-part exoskeletons: head, thorax, and abdomen. They have 6 jointed legs, compound eyes, and antennae. There are approximately 91,000 insect species in the United States, the most abundant orders being Coleoptera (beetles), Diptera (flies), and Hymenoptera (includes ants, bees, wasps, and sawflies).1
The reported incidence of insect bites and stings varies widely because most people experience mild symptoms and therefore do not seek medical care. Best statistics are for Hymenoptera stings, which are more likely to cause a severe reaction. In Europe, 56% to 94% of the general population has reported being bitten or stung by one of the Hymenoptera species.2 In many areas of Australia, the incidence of jack jumper ant stings is only 2% to 3%3; in the United States, 55% of people report being stung by nonnative fire ants within 3 weeks of moving into an endemic area.4
Arachnids are some of the earliest terrestrial organisms, of the class Arachnida, which includes scorpions, ticks, spiders, mites, and daddy longlegs (harvestmen).5 Arachnids are wingless and characterized by segmented bodies, jointed appendages, and exoskeletons.6,7 In most, the body is separated into 2 segments (the cephalothorax and abdomen), except for mites, ticks, and daddy longlegs, in which the entire body comprises a single segment.5
Arthropod bites are common in the United States; almost one-half are caused by spiders.7 Brown recluse (Loxosceles spp) and black widow (Latrodectus spp) spider bites are the most concerning: Although usually mild, these bites can be life-threatening but are rarely fatal. In 2013, almost 3500 bites by black widow and brown recluse spiders were reported.8
Risk factors
Risk factors for insect, arachnid, and other arthropod bites and stings are primarily environmental. People who live or work in proximity of biting or stinging insects (eg, gardeners and beekeepers) are more likely to be affected; so are those who work with animals or live next to standing water or grassy or wooded locales.
Continue to: There are also risk factors...
There are also risk factors for a systemic sting reaction:
- A sting reaction < 2 months earlier increases the risk of a subsequent systemic sting reaction by ≥ 50%.9
- Among beekeepers, paradoxically, the risk of a systemic reaction is higher in those stung < 15 times a year than in those stung > 200 times.10
- Patients with an elevated baseline serum level of tryptase (reference range, < 11.4 ng/mL), which is part of the allergenic response, or with biopsy-proven systemic mastocytosis are at increased risk of a systemic sting reaction.11
Presentation: Signs and symptomsvary with severity
Insect bites and stings usually cause transient local inflammation and, occasionally, a toxic reaction. Allergic hypersensitivity can result in a large local reaction or a generalized systemic reaction12:
- A small local reaction is transient and mild, develops directly at the site of the sting, and can last several days.13
- A large (or significant) local reaction, defined as swelling > 10 cm in diameter (FIGURE 1) and lasting > 24 hours, occurs in 2% to 26% of people who have been bitten or stung.14 This is an immunoglobulin (Ig) E–mediated late-phase reaction that can be accompanied by fatigue and nausea.12,13,15 For a patient with a large local reaction, the risk of a concomitant systemic reaction is 4% to 10%, typically beginning within 30 minutes after envenomation or, possibly, delayed for several hours or marked by a biphasic interval.16
- Characteristics of a systemic reaction are urticaria, angioedema, bronchospasm, large-airway edema, hypotension, and other clinical manifestations of anaphylaxis.17 In the United States, a systemic sting reaction is reported to occur in approximately 3% of bite and sting victims. Mortality among the general population from a systemic bite or sting reaction is 0.16 for every 100,000 people,2 and at least 40 to 100 die every year in the United States from anaphylaxis resulting from an insect bite or sting.18
- The most severe anaphylactic reactions involve the cardiovascular and respiratory systems, commonly including hypotension and symptoms of upper- or lower-airway obstruction. Laryngeal edema and circulatory failure are the most common mechanisms of anaphylactic death.19

Bees and wasps
Hymenoptera stinging insects include the family Apidae (honey bee, bumblebee, and sweat bee) and Vespidae (yellow jacket, yellow- and white-faced hornets, and paper wasp). A worker honey bee can sting only once, leaving its barbed stinger in the skin; a wasp, hornet, and yellow jacket can sting multiple times (FIGURE 2).2

Continue to: Bee and wasp sting...
Bee and wasp sting allergies are the most common insect venom allergic reactions. A bee sting is more likely to lead to a severe allergic reaction than a wasp sting. Allergic reactions to hornet and bumblebee stings are less common but can occur in patients already sensitized to wasp and honey bee stings.20,21
Management. Remove honey bee stingers by scraping the skin with a fingernail or credit card. Ideally, the stinger should be removed in the first 30 seconds, before the venom sac empties. Otherwise, intense local inflammation, with possible lymphangitic streaking, can result.22
For guidance on localized symptomatic care of bee and wasp stings and bites and stings from other sources discussed in this article, see “Providing relief and advanced care” on page E6.
Centipedes and spiders
Centipedes are arthropods of the class Chilopoda, subphylum Myriapoda, that are characterized by repeating linear (metameric) segments, each containing 1 pair of legs.23 Centipedes have a pair of poison claws behind the head that are used to paralyze prey—usually, small insects.23,24 The bite of a larger centipede can cause a painful reaction that generally subsides after a few hours but can last several days. Centipede bites are usually nonfatal to humans.23
Spiders belong to the class Arachnida, order Araneae. They have 8 legs with chelicerae (mouthpiece, or “jaws”) that inject venom into prey.25 Most spiders found in the United States cannot bite through human skin.26,27 Common exceptions are black widow and brown recluse spiders, which each produce a distinct toxic venom that can cause significant morbidity in humans through a bite, although bites are rarely fatal.26,27
The brown recluse spider is described as having a violin-shaped marking on the abdomen; the body is yellowish, tan, or dark brown. A bite can produce tiny fang marks and cause dull pain at the site of the bite that spreads quickly; myalgia; and pain in the stomach, back, chest, and legs.28,29 The bite takes approximately 7 days to resolve. In a minority of cases, a tender erythematous halo develops, followed by a severe necrotic ulcer, or loxoscelism (FIGURE 3; 40% of cases) or scarring (13%), or both.29,30

Continue to: In contrast...
In contrast, the body of a black widow spider is black; females exhibit a distinctive red or yellow hourglass marking on their ventral aspect.28,31 The pinprick sensation of a bite leads to symptoms that can include erythema, swelling, pain, stiffness, chills, fever, nausea, and stomach pain.30,32
Management. Again, see “Providing relief and advanced care” on page E6. Consider providing antivenin treatment for moderate or severe bites of brown recluse and black widow spiders.
Fleas
Fleas are members of the order Siphonaptera. They are small (1.5-3.2 mm long), reddish brown, wingless, blood-sucking insects with long legs that allow them to jump far (12 or 13 inches) and high (6 or 7 inches).33 Domesticated cats and dogs are the source of most flea infestations, resulting in an increased risk of exposure for humans.34,35 Flea bites, which generally occur on lower extremities, develop into a small, erythematous papule with a halo (FIGURE 4) and associated mild edema, and cause intense pruritus 30 minutes after the bite.35-37

Fleas are a vector for severe microbial infections, including bartonellosis, bubonic plague, cat-flea typhus, murine typhus, cat-scratch disease, rickettsial disease, and tularemia. Tungiasis is an inflammatory burrowing flea infestation—not a secondary infection for which the flea is a vector.34,35
Preventive management. Repellents, including products that contain DEET (N,N-diethyl-meta-toluamide), picaridin (2-[2-hydroxyethyl]-1-piperidinecarboxylic acid 1-methylpropyl ester), and PMD (p-menthane-3,8-diol, a chemical constituent of Eucalyptus citriodora oil) can be used to prevent flea bites in humans.33,38 Studies show that the scent of other botanic oils, including lavender, cedarwood, and peppermint, can also help prevent infestation by fleas; however, these compounds are not as effective as traditional insect repellents.33,38
Flea control is difficult, requiring a multimodal approach to treating the infested animal and its environment.39 Treatment of the infested domestic animal is the primary method of preventing human bites. Nonpesticidal control involves frequent cleaning of carpeting, furniture, animal bedding, and kennels. Insecticides can be applied throughout the house to combat severe infestation.33,38
Continue to: The Centers for Disease Control and Prevention...
The Centers for Disease Control and Prevention provide a general introduction to getting rid of fleas for pet owners.40 For specific guidance on flea-eradication strategies and specific flea-control products, advise patients to seek the advice of their veterinarian.
Flies and biting midges
Flies are 2-winged insects belonging to the order Diptera. Several fly species can bite, causing a local inflammatory reaction; these include black flies, deer flies, horse flies, and sand flies. Signs and symptoms of a fly bite include pain, pruritus, erythema, and mild swelling (FIGURE 5).41,42 Flies can transmit several infections, including bartonellosis, enteric bacterial disease (eg, caused by Campylobacter spp), leishmaniasis, loiasis, onchocerciasis, and trypanosomiasis.43

Biting midges, also called “no-see-ums,” biting gnats, moose flies, and “punkies,”44 are tiny (1-3 mm long) blood-sucking flies.45 Bitten patients often report not having seen the midge because it is so small. The bite typically starts as a small, erythematous papule that develops into a dome-shaped blister and can be extraordinarily pruritic and painful.44 The majority of people who have been bitten develop a hypersensitivity reaction, which usually resolves in a few weeks.
Management. Suppressing adult biting midges with an environmental insecticide is typically insufficient because the insecticide must be sprayed daily to eradicate active midges and generally does not affect larval habitat. Insect repellents and biopesticides, such as oil of lemon eucalyptus, can be effective in reducing the risk of bites.44,45
Mosquitoes
Mosquitoes are flying, blood-sucking insects of the order Diptera and family Culicidae. Anopheles, Culex, and Aedes genera are responsible for most bites of humans.
The bite of a mosquito produces an indurated, limited local reaction characterized by a pruritic wheal (3-29 mm in diameter) with surrounding erythema (FIGURE 6) that peaks in approximately 30 minutes, although patients might have a delayed reaction hours later.46 Immunocompromised patients might experience a more significant local inflammatory reaction that is accompanied by low-grade fever, hives, or swollen lymph nodes.46,47

Mosquitoes are a vector for serious infections, including dengue, Japanese encephalitis, malaria, and yellow fever, and disease caused by Chikungunya, West Nile, and Zika viruses.
Continue to: Management
Management. Advise patients to reduce their risk by using insect repellent, sleeping under mosquito netting, and wearing a long-sleeve shirt and long pants when traveling to endemic areas or when a local outbreak occurs.48
Ticks
Ticks belong to the order Parasitiformes and families Ixodidae and Argasidae. Hard ticks are found in brushy fields and tall grasses and can bite and feed on humans for days. Soft ticks are generally found around animal nests.29 Tick bites can cause a local reaction that includes painful, erythematous, inflammatory papular lesions (FIGURE 7).49

Ticks can transmit several infectious diseases. Depending on the microbial pathogen and the genus and species of tick, it takes 2 to 96 hours for the tick to attach to skin and transmit the pathogen to the human host. The TABLE29,49,50 provides an overview of tick species in the United States, diseases that they can transmit, and the geographic distribution of those diseases.

Management. Ticks should be removed with fine-tipped tweezers. Grasp the body of the tick close to the skin and pull upward while applying steady, even pressure. After removing the tick, clean the bite and the surrounding area with alcohol or with soap and water. Dispose of a live tick by flushing it down the toilet; or, kill it in alcohol and either seal it in a bag with tape or place it in a container.50
Diagnosis and the utilityof special testing
The diagnosis of insect, arachnid, and other arthropod bites and stings depends on the history, including obtaining a record of possible exposure and a travel history; the timing of the bite or sting; and associated signs and symptoms.18,51
Venom skin testing. For Hymenoptera stings, intradermal tests using a venom concentration of 0.001 to 1 μg/mL are positive in 65% to 80% of patients with a history of a systemic insect-sting allergic reaction. A negative venom skin test can occur during the 3-to-6-week refractory period after a sting reaction or many years later, which represents a loss of sensitivity. Positive venom skin tests are used to confirm allergy and identify specific insects to which the patient is allergic.11,12
Continue to: Allergen-specific IgE antibody testing.
Allergen-specific IgE antibody testing. These serum assays—typically, radioallergosorbent testing (RAST)—are less sensitive than venom skin tests. RAST is useful when venom skin testing cannot be performed or when skin testing is negative in a patient who has had a severe allergic reaction to an insect bite or sting. Serum IgE-specific antibody testing is preferred over venom skin testing in patients who are at high risk of anaphylaxis.52,53
Providing reliefand advanced care
Symptomatic treatment of mild bites and stings includes washing the affected area with soap and water and applying a cold compress to reduce swelling.54 For painful lesions, an oral analgesic can be prescribed.
For mild or moderate pruritus, a low- to midpotency topical corticosteroid (eg, hydrocortisone valerate cream 0.2% bid), topical calamine, or pramoxine can be applied,or a nonsedating oral antihistamine, such as loratadine (10 mg/d) or cetirizine (10 mg/d), can be used.14,55 For severe itching, a sedating antihistamine, such as hydroxyzine (10-25 mg every 4 to 6 hours prn), might help relieve symptoms; H1- and H2-receptor antagonists can be used concomitantly.54,55
Significant local symptoms. Large local reactions are treated with a midpotency topical corticosteroid (eg, triamcinolone acetonide cream 0.1% bid) plus an oral antihistamine to relieve pruritus and reduce allergic inflammation. For a more severe reaction, an oral corticosteroid (prednisone 1 mg/kg; maximum dosage, 50 mg/d) can be given for 5 to 7 days.54-56
Management of a necrotic ulcer secondary to a brown recluse spider bite is symptomatic and supportive. The size of these wounds can increase for as long as 10 days after the bite; resolution can require months of wound care, possibly with debridement. Rarely, skin grafting is required.27,28,31
VIT. Some studies show that VIT can improve quality of life in patients with prolonged, frequent, and worsening reactions to insect bites or stings and repeated, unavoidable exposures.55,56 VIT is recommended for patients with systemic hypersensitivity and a positive venom skin test result. It is approximately 95% effective in preventing or reducing severe systemic reactions and reduces the risk of anaphylaxis (see next section) and death.57 The maintenance dosage of VIT is usually 100 μg every 4 to 6 weeks; optimal duration of treatment is 3 to 5 years.58
Continue to: After VIT is complete...
After VIT is complete, counsel patients that a mild systemic reaction is still possible after an insect bite or sting. More prolonged, even lifetime, treatment should be considered for patients who have58,59
- a history of severe, life-threatening allergic reactions to bites and stings
- honey bee sting allergy
- mast-cell disease
- a history of anaphylaxis while receiving VIT.
Absolute contraindications to VIT include a history of serious immune disease, chronic infection, or cancer.58,59
Managing anaphylaxis
This severe allergic reaction can lead to death if untreated. First-line therapy is intramuscular epinephrine, 0.01 mg/kg (maximum single dose, 0.5 mg) given every 5 to 15 minutes.14,60 Epinephrine auto-injectors deliver a fixed dose and are labeled according to weight. Administration of O2 and intravenous fluids is recommended for hemodynamically unstable patients.60,61 Antihistamines and corticosteroids can be used as secondary treatment but should not replace epinephrine.56
After preliminary improvement, patients might decompensate when the epinephrine dose wears off. Furthermore, a biphasic reaction, variously reported in < 5% to as many as 20% of patients,61,62 occurs hours after the initial anaphylactic reaction. Patients should be monitored, therefore, for at least 6 to 8 hours after an anaphylactic reaction, preferably in a facility equipped to treat anaphylaxis.17,56
Before discharge, patients who have had an anaphylactic reaction should be given a prescription for epinephrine and training in the use of an epinephrine auto-injector. Allergen avoidance, along with an emergency plan in the event of a bite or sting, is recommended. Follow-up evaluation with an allergist or immunologist is essential for proper diagnosis and to determine whether the patient is a candidate for VIT.14,17
CORRESPONDENCE
Ecler Ercole Jaqua, MD, DipABLM, FAAFP, 1200 California Street, Suite 240, Redlands, CA 92374; [email protected].
Insect, arachnid, and other arthropod bites and stings are common patient complaints in a primary care office. A thorough history and physical exam can often isolate the specific offender and guide management. In this article, we outline how to identify, diagnose, and treat common bites and stings from bees and wasps; centipedes and spiders; fleas; flies and biting midges; mosquitoes; and ticks, and discuss how high-risk patients should be triaged and referred for additional testing and treatment, such as venom immunotherapy (VIT).
Insects and arachnids:Background and epidemiology
Insects are arthropods with 3-part exoskeletons: head, thorax, and abdomen. They have 6 jointed legs, compound eyes, and antennae. There are approximately 91,000 insect species in the United States, the most abundant orders being Coleoptera (beetles), Diptera (flies), and Hymenoptera (includes ants, bees, wasps, and sawflies).1
The reported incidence of insect bites and stings varies widely because most people experience mild symptoms and therefore do not seek medical care. Best statistics are for Hymenoptera stings, which are more likely to cause a severe reaction. In Europe, 56% to 94% of the general population has reported being bitten or stung by one of the Hymenoptera species.2 In many areas of Australia, the incidence of jack jumper ant stings is only 2% to 3%3; in the United States, 55% of people report being stung by nonnative fire ants within 3 weeks of moving into an endemic area.4
Arachnids are some of the earliest terrestrial organisms, of the class Arachnida, which includes scorpions, ticks, spiders, mites, and daddy longlegs (harvestmen).5 Arachnids are wingless and characterized by segmented bodies, jointed appendages, and exoskeletons.6,7 In most, the body is separated into 2 segments (the cephalothorax and abdomen), except for mites, ticks, and daddy longlegs, in which the entire body comprises a single segment.5
Arthropod bites are common in the United States; almost one-half are caused by spiders.7 Brown recluse (Loxosceles spp) and black widow (Latrodectus spp) spider bites are the most concerning: Although usually mild, these bites can be life-threatening but are rarely fatal. In 2013, almost 3500 bites by black widow and brown recluse spiders were reported.8
Risk factors
Risk factors for insect, arachnid, and other arthropod bites and stings are primarily environmental. People who live or work in proximity of biting or stinging insects (eg, gardeners and beekeepers) are more likely to be affected; so are those who work with animals or live next to standing water or grassy or wooded locales.
Continue to: There are also risk factors...
There are also risk factors for a systemic sting reaction:
- A sting reaction < 2 months earlier increases the risk of a subsequent systemic sting reaction by ≥ 50%.9
- Among beekeepers, paradoxically, the risk of a systemic reaction is higher in those stung < 15 times a year than in those stung > 200 times.10
- Patients with an elevated baseline serum level of tryptase (reference range, < 11.4 ng/mL), which is part of the allergenic response, or with biopsy-proven systemic mastocytosis are at increased risk of a systemic sting reaction.11
Presentation: Signs and symptomsvary with severity
Insect bites and stings usually cause transient local inflammation and, occasionally, a toxic reaction. Allergic hypersensitivity can result in a large local reaction or a generalized systemic reaction12:
- A small local reaction is transient and mild, develops directly at the site of the sting, and can last several days.13
- A large (or significant) local reaction, defined as swelling > 10 cm in diameter (FIGURE 1) and lasting > 24 hours, occurs in 2% to 26% of people who have been bitten or stung.14 This is an immunoglobulin (Ig) E–mediated late-phase reaction that can be accompanied by fatigue and nausea.12,13,15 For a patient with a large local reaction, the risk of a concomitant systemic reaction is 4% to 10%, typically beginning within 30 minutes after envenomation or, possibly, delayed for several hours or marked by a biphasic interval.16
- Characteristics of a systemic reaction are urticaria, angioedema, bronchospasm, large-airway edema, hypotension, and other clinical manifestations of anaphylaxis.17 In the United States, a systemic sting reaction is reported to occur in approximately 3% of bite and sting victims. Mortality among the general population from a systemic bite or sting reaction is 0.16 for every 100,000 people,2 and at least 40 to 100 die every year in the United States from anaphylaxis resulting from an insect bite or sting.18
- The most severe anaphylactic reactions involve the cardiovascular and respiratory systems, commonly including hypotension and symptoms of upper- or lower-airway obstruction. Laryngeal edema and circulatory failure are the most common mechanisms of anaphylactic death.19

Bees and wasps
Hymenoptera stinging insects include the family Apidae (honey bee, bumblebee, and sweat bee) and Vespidae (yellow jacket, yellow- and white-faced hornets, and paper wasp). A worker honey bee can sting only once, leaving its barbed stinger in the skin; a wasp, hornet, and yellow jacket can sting multiple times (FIGURE 2).2

Continue to: Bee and wasp sting...
Bee and wasp sting allergies are the most common insect venom allergic reactions. A bee sting is more likely to lead to a severe allergic reaction than a wasp sting. Allergic reactions to hornet and bumblebee stings are less common but can occur in patients already sensitized to wasp and honey bee stings.20,21
Management. Remove honey bee stingers by scraping the skin with a fingernail or credit card. Ideally, the stinger should be removed in the first 30 seconds, before the venom sac empties. Otherwise, intense local inflammation, with possible lymphangitic streaking, can result.22
For guidance on localized symptomatic care of bee and wasp stings and bites and stings from other sources discussed in this article, see “Providing relief and advanced care” on page E6.
Centipedes and spiders
Centipedes are arthropods of the class Chilopoda, subphylum Myriapoda, that are characterized by repeating linear (metameric) segments, each containing 1 pair of legs.23 Centipedes have a pair of poison claws behind the head that are used to paralyze prey—usually, small insects.23,24 The bite of a larger centipede can cause a painful reaction that generally subsides after a few hours but can last several days. Centipede bites are usually nonfatal to humans.23
Spiders belong to the class Arachnida, order Araneae. They have 8 legs with chelicerae (mouthpiece, or “jaws”) that inject venom into prey.25 Most spiders found in the United States cannot bite through human skin.26,27 Common exceptions are black widow and brown recluse spiders, which each produce a distinct toxic venom that can cause significant morbidity in humans through a bite, although bites are rarely fatal.26,27
The brown recluse spider is described as having a violin-shaped marking on the abdomen; the body is yellowish, tan, or dark brown. A bite can produce tiny fang marks and cause dull pain at the site of the bite that spreads quickly; myalgia; and pain in the stomach, back, chest, and legs.28,29 The bite takes approximately 7 days to resolve. In a minority of cases, a tender erythematous halo develops, followed by a severe necrotic ulcer, or loxoscelism (FIGURE 3; 40% of cases) or scarring (13%), or both.29,30

Continue to: In contrast...
In contrast, the body of a black widow spider is black; females exhibit a distinctive red or yellow hourglass marking on their ventral aspect.28,31 The pinprick sensation of a bite leads to symptoms that can include erythema, swelling, pain, stiffness, chills, fever, nausea, and stomach pain.30,32
Management. Again, see “Providing relief and advanced care” on page E6. Consider providing antivenin treatment for moderate or severe bites of brown recluse and black widow spiders.
Fleas
Fleas are members of the order Siphonaptera. They are small (1.5-3.2 mm long), reddish brown, wingless, blood-sucking insects with long legs that allow them to jump far (12 or 13 inches) and high (6 or 7 inches).33 Domesticated cats and dogs are the source of most flea infestations, resulting in an increased risk of exposure for humans.34,35 Flea bites, which generally occur on lower extremities, develop into a small, erythematous papule with a halo (FIGURE 4) and associated mild edema, and cause intense pruritus 30 minutes after the bite.35-37

Fleas are a vector for severe microbial infections, including bartonellosis, bubonic plague, cat-flea typhus, murine typhus, cat-scratch disease, rickettsial disease, and tularemia. Tungiasis is an inflammatory burrowing flea infestation—not a secondary infection for which the flea is a vector.34,35
Preventive management. Repellents, including products that contain DEET (N,N-diethyl-meta-toluamide), picaridin (2-[2-hydroxyethyl]-1-piperidinecarboxylic acid 1-methylpropyl ester), and PMD (p-menthane-3,8-diol, a chemical constituent of Eucalyptus citriodora oil) can be used to prevent flea bites in humans.33,38 Studies show that the scent of other botanic oils, including lavender, cedarwood, and peppermint, can also help prevent infestation by fleas; however, these compounds are not as effective as traditional insect repellents.33,38
Flea control is difficult, requiring a multimodal approach to treating the infested animal and its environment.39 Treatment of the infested domestic animal is the primary method of preventing human bites. Nonpesticidal control involves frequent cleaning of carpeting, furniture, animal bedding, and kennels. Insecticides can be applied throughout the house to combat severe infestation.33,38
Continue to: The Centers for Disease Control and Prevention...
The Centers for Disease Control and Prevention provide a general introduction to getting rid of fleas for pet owners.40 For specific guidance on flea-eradication strategies and specific flea-control products, advise patients to seek the advice of their veterinarian.
Flies and biting midges
Flies are 2-winged insects belonging to the order Diptera. Several fly species can bite, causing a local inflammatory reaction; these include black flies, deer flies, horse flies, and sand flies. Signs and symptoms of a fly bite include pain, pruritus, erythema, and mild swelling (FIGURE 5).41,42 Flies can transmit several infections, including bartonellosis, enteric bacterial disease (eg, caused by Campylobacter spp), leishmaniasis, loiasis, onchocerciasis, and trypanosomiasis.43

Biting midges, also called “no-see-ums,” biting gnats, moose flies, and “punkies,”44 are tiny (1-3 mm long) blood-sucking flies.45 Bitten patients often report not having seen the midge because it is so small. The bite typically starts as a small, erythematous papule that develops into a dome-shaped blister and can be extraordinarily pruritic and painful.44 The majority of people who have been bitten develop a hypersensitivity reaction, which usually resolves in a few weeks.
Management. Suppressing adult biting midges with an environmental insecticide is typically insufficient because the insecticide must be sprayed daily to eradicate active midges and generally does not affect larval habitat. Insect repellents and biopesticides, such as oil of lemon eucalyptus, can be effective in reducing the risk of bites.44,45
Mosquitoes
Mosquitoes are flying, blood-sucking insects of the order Diptera and family Culicidae. Anopheles, Culex, and Aedes genera are responsible for most bites of humans.
The bite of a mosquito produces an indurated, limited local reaction characterized by a pruritic wheal (3-29 mm in diameter) with surrounding erythema (FIGURE 6) that peaks in approximately 30 minutes, although patients might have a delayed reaction hours later.46 Immunocompromised patients might experience a more significant local inflammatory reaction that is accompanied by low-grade fever, hives, or swollen lymph nodes.46,47

Mosquitoes are a vector for serious infections, including dengue, Japanese encephalitis, malaria, and yellow fever, and disease caused by Chikungunya, West Nile, and Zika viruses.
Continue to: Management
Management. Advise patients to reduce their risk by using insect repellent, sleeping under mosquito netting, and wearing a long-sleeve shirt and long pants when traveling to endemic areas or when a local outbreak occurs.48
Ticks
Ticks belong to the order Parasitiformes and families Ixodidae and Argasidae. Hard ticks are found in brushy fields and tall grasses and can bite and feed on humans for days. Soft ticks are generally found around animal nests.29 Tick bites can cause a local reaction that includes painful, erythematous, inflammatory papular lesions (FIGURE 7).49

Ticks can transmit several infectious diseases. Depending on the microbial pathogen and the genus and species of tick, it takes 2 to 96 hours for the tick to attach to skin and transmit the pathogen to the human host. The TABLE29,49,50 provides an overview of tick species in the United States, diseases that they can transmit, and the geographic distribution of those diseases.

Management. Ticks should be removed with fine-tipped tweezers. Grasp the body of the tick close to the skin and pull upward while applying steady, even pressure. After removing the tick, clean the bite and the surrounding area with alcohol or with soap and water. Dispose of a live tick by flushing it down the toilet; or, kill it in alcohol and either seal it in a bag with tape or place it in a container.50
Diagnosis and the utilityof special testing
The diagnosis of insect, arachnid, and other arthropod bites and stings depends on the history, including obtaining a record of possible exposure and a travel history; the timing of the bite or sting; and associated signs and symptoms.18,51
Venom skin testing. For Hymenoptera stings, intradermal tests using a venom concentration of 0.001 to 1 μg/mL are positive in 65% to 80% of patients with a history of a systemic insect-sting allergic reaction. A negative venom skin test can occur during the 3-to-6-week refractory period after a sting reaction or many years later, which represents a loss of sensitivity. Positive venom skin tests are used to confirm allergy and identify specific insects to which the patient is allergic.11,12
Continue to: Allergen-specific IgE antibody testing.
Allergen-specific IgE antibody testing. These serum assays—typically, radioallergosorbent testing (RAST)—are less sensitive than venom skin tests. RAST is useful when venom skin testing cannot be performed or when skin testing is negative in a patient who has had a severe allergic reaction to an insect bite or sting. Serum IgE-specific antibody testing is preferred over venom skin testing in patients who are at high risk of anaphylaxis.52,53
Providing reliefand advanced care
Symptomatic treatment of mild bites and stings includes washing the affected area with soap and water and applying a cold compress to reduce swelling.54 For painful lesions, an oral analgesic can be prescribed.
For mild or moderate pruritus, a low- to midpotency topical corticosteroid (eg, hydrocortisone valerate cream 0.2% bid), topical calamine, or pramoxine can be applied,or a nonsedating oral antihistamine, such as loratadine (10 mg/d) or cetirizine (10 mg/d), can be used.14,55 For severe itching, a sedating antihistamine, such as hydroxyzine (10-25 mg every 4 to 6 hours prn), might help relieve symptoms; H1- and H2-receptor antagonists can be used concomitantly.54,55
Significant local symptoms. Large local reactions are treated with a midpotency topical corticosteroid (eg, triamcinolone acetonide cream 0.1% bid) plus an oral antihistamine to relieve pruritus and reduce allergic inflammation. For a more severe reaction, an oral corticosteroid (prednisone 1 mg/kg; maximum dosage, 50 mg/d) can be given for 5 to 7 days.54-56
Management of a necrotic ulcer secondary to a brown recluse spider bite is symptomatic and supportive. The size of these wounds can increase for as long as 10 days after the bite; resolution can require months of wound care, possibly with debridement. Rarely, skin grafting is required.27,28,31
VIT. Some studies show that VIT can improve quality of life in patients with prolonged, frequent, and worsening reactions to insect bites or stings and repeated, unavoidable exposures.55,56 VIT is recommended for patients with systemic hypersensitivity and a positive venom skin test result. It is approximately 95% effective in preventing or reducing severe systemic reactions and reduces the risk of anaphylaxis (see next section) and death.57 The maintenance dosage of VIT is usually 100 μg every 4 to 6 weeks; optimal duration of treatment is 3 to 5 years.58
Continue to: After VIT is complete...
After VIT is complete, counsel patients that a mild systemic reaction is still possible after an insect bite or sting. More prolonged, even lifetime, treatment should be considered for patients who have58,59
- a history of severe, life-threatening allergic reactions to bites and stings
- honey bee sting allergy
- mast-cell disease
- a history of anaphylaxis while receiving VIT.
Absolute contraindications to VIT include a history of serious immune disease, chronic infection, or cancer.58,59
Managing anaphylaxis
This severe allergic reaction can lead to death if untreated. First-line therapy is intramuscular epinephrine, 0.01 mg/kg (maximum single dose, 0.5 mg) given every 5 to 15 minutes.14,60 Epinephrine auto-injectors deliver a fixed dose and are labeled according to weight. Administration of O2 and intravenous fluids is recommended for hemodynamically unstable patients.60,61 Antihistamines and corticosteroids can be used as secondary treatment but should not replace epinephrine.56
After preliminary improvement, patients might decompensate when the epinephrine dose wears off. Furthermore, a biphasic reaction, variously reported in < 5% to as many as 20% of patients,61,62 occurs hours after the initial anaphylactic reaction. Patients should be monitored, therefore, for at least 6 to 8 hours after an anaphylactic reaction, preferably in a facility equipped to treat anaphylaxis.17,56
Before discharge, patients who have had an anaphylactic reaction should be given a prescription for epinephrine and training in the use of an epinephrine auto-injector. Allergen avoidance, along with an emergency plan in the event of a bite or sting, is recommended. Follow-up evaluation with an allergist or immunologist is essential for proper diagnosis and to determine whether the patient is a candidate for VIT.14,17
CORRESPONDENCE
Ecler Ercole Jaqua, MD, DipABLM, FAAFP, 1200 California Street, Suite 240, Redlands, CA 92374; [email protected].
1. Numbers of insects (species and individuals). Smithsonian BugInfo Web site. www.si.edu/spotlight/buginfo/bugnos. Accessed November 25, 2020.
2. Antonicelli L, Bilò MB, Bonifazi F. Epidemiology of Hymenoptera allergy. Curr Opin Allergy Clin Immunol. 2002;2:341-346.
3. Jack jumper ant allergy. Australasian Society of Clinical Immunology and Allergy (ASCIA) Web site. Updated October 19, 2019. www.allergy.org.au/patients/insect-allergy-bites-and-stings/jack-jumper-ant-allergy. Accessed November 25, 2020.
4. Kemp SF, deShazo RD, Moffit JE, et al. Expanding habitat of the imported fire ant (Solenopsis invicta): a public health concern. J Allergy Clin Immunol. 2000;105:683-691.
5. Goodnight ML. Arachnid. In: Encyclopædia Britannica. 2012. www.britannica.com/animal/arachnid. Accessed November 25, 2020.
6. Despommier DD, Gwadz RW, Hotez PJ. Arachnids. In: Despommier DD, Gwadz RW, Hotez PJ. Parasitic Diseases. 3rd ed. Springer-Verlag; 1995:268-283.
7. Diaz JH, Leblanc KE. Common spider bites. Am Fam Physician. 2007;75:869-873.
8. Mowry JB, Spyker DA, Cantilena LR Jr, McMillan N, Ford M. 2013 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 31st Annual Report. Clin Toxicol (Phila). 2014;52:1032-1283.
9. Pucci S, Antonicelli L, Bilò MB, et al. Shortness of interval between two stings as risk factor for developing Hymenoptera venom allergy. Allergy.1994;49:894-896.
10. Müller UR. Bee venom allergy in beekeepers and their family members. Curr Opin Allergy Clin Immunol. 2005;5:343-347.
11. Müller UR. Cardiovascular disease and anaphylaxis. Curr Opin Allergy Clin Immunol. 2007;7:337-341.
12. Golden DBK. Stinging insect allergy. Am Fam Physician. 2003;67:2541-2546.
13. Golden DBK, Demain T, Freeman T, et al. Stinging insect hypersensitivity: a practice parameter update 2016. Ann Allergy Asthma Immunol. 2017;118:28-54.
14. Bilò BM, Rueff F, Mosbech H, et al; EAACI Interest Group on Insect Venom Hypersensitivity. Diagnosis of Hymenoptera venom allergy. Allergy. 2005;60:1339-1349.
15. Reisman RE. Insect stings. N Engl J Med. 1994;331:523-527.
16. Pucci S, D’Alò S, De Pasquale T, et al. Risk of anaphylaxis in patients with large local reactions to hymenoptera stings: a retrospective and prospective study. Clin Mol Allergy. 2015;13:21.
17. Golden DBK. Large local reactions to insect stings. J Allergy Clin Immunol Pract. 2015;3:331-334.
18. Clark S, Camargo CA Jr. Emergency treatment and prevention of insect-sting anaphylaxis. Curr Opin Allergy Clin Immunol. 2006;6:279-283.
19. Stinging insect allergy. In: Volcheck GW. Clinical Allergy: Diagnosis and Management. Humana Press; 2009:465-479.
20. Järvinen KM, Celestin J. Anaphylaxis avoidance and management: educating patients and their caregivers. J Asthma Allergy. 2014;7:95-104.
21. Institute for Quality and Efficiency in Health Care (IQWiG). Insect venom allergies: overview. InformedHealth.org. Updated May 7, 2020. www.ncbi.nlm.nih.gov/pubmedhealth/PMH0096282/. Accessed November 25, 2020.
22. Casale TB, Burks AW. Clinical practice. Hymenoptera-sting hypersensitivity. N Engl J Med. 2014;370:1432-1439.
23. Shelley RM. Centipedes and millipedes with emphasis on North American fauna. Kansas School Naturalist. 1999;45:1-16. https://sites.google.com/g.emporia.edu/ksn/ksn-home/vol-45-no-3-centipedes-and-millipedes-with-emphasis-on-n-america-fauna#h.p_JEf3uDlTg0jw. Accessed November 25, 2020.
24. Ogg B. Centipedes and millipedes. Nebraska Extension in Lancaster County Web site. https://lancaster.unl.edu/pest/resources/CentipedeMillipede012.shtml. Accessed November 25, 2020.
25. Cushing PE. Spiders (Arachnida: Araneae). In: Capinera JL, ed. Encyclopedia of Entomology. Springer, Dordrecht; 2008:226.
26. Diaz JH, Leblanc KE. Common spider bites. Am Fam Physician. 2007;75:869-873.
27. The National Institute for Occupational Safety and Health (NIOSH), Centers for Disease Control and Prevention. Venomous spiders. www.cdc.gov/niosh/topics/spiders/. Accessed November 25, 2020.
28. Starr S. What you need to know to prevent a poisonous spider bite. AAP News. 2013;34:42. www.aappublications.org/content/aapnews/34/9/42.5.full.pdf. Accessed November 25, 2020.
29. Spider bites. Mayo Clinic Web site. www.mayoclinic.org/diseases-conditions/spider-bites/symptoms-causes/syc-20352371. Accessed November 25, 2020.
30. Barish RA, Arnold T. Spider bites. In: Merck Manual (Professional Version). Merck Sharp & Dohme Corp.; 2016. www.merckmanuals.com/professional/injuries-poisoning/bites-and-stings/spider-bites. Accessed November 25, 2020.
31. Juckett G. Arthropod bites. Am Fam Physician. 2013;88:841-847.
32. Clark RF, Wethern-Kestner S, Vance MV, et al. Clinical presentation and treatment of black widow spider envenomation: a review of 163 cases. Ann Emerg Med. 1992;21:782-787.
33. Koehler PG, Pereira RM, Diclaro JW II. Fleas. Publication ENY-025. University of Florida IFAS Extension. Revised January 2012. https://edis.ifas.ufl.edu/ig087. Accessed November 25, 2020.
34. Bitam I, Dittmar K, Parola P, et al. Fleas and flea-borne diseases. Int J Infect Dis. 2010;14:e667-e676.
35. Leulmi H, Socolovschi C, Laudisoit A, et al. Detection of Rickettsia felis, Rickettsia typhi, Bartonella species and Yersinia pestis in fleas (Siphonaptera) from Africa. PLoS Negl Trop Dis. 2014;8:e3152.
36. Naimer SA, Cohen AD, Mumcuoglu KY, et al. Household papular urticaria. Isr Med Assoc J. 2002;4(11 suppl):911-913.
37. Golomb MR, Golomb HS. What’s eating you? Cat flea (Ctenocephalides felis). Cutis. 2010;85:10-11.
38. Dryden MW. Flea and tick control in the 21st century: challenges and opportunities. Vet Dermatol. 2009;20:435-440.
39. Dryden MW. Fleas in dogs and cats. Merck Sharp & Dohme Corporation: Merck Manual Veterinary Manual. Updated December 2014. www.merckvetmanual.com/integumentary-system/fleas-and-flea-allergy-dermatitis/fleas-in-dogs-and-cats. Accessed November 25, 2020.
40. Centers for Disease Control and Prevention. Getting rid of fleas. www.cdc.gov/fleas/getting_rid.html. Accessed November 25, 2020.
41. Chattopadhyay P, Goyary D, Dhiman S, et al. Immunomodulating effects and hypersensitivity reactions caused by Northeast Indian black fly salivary gland extract. J Immunotoxicol. 2014;11:126-132.
42. Hrabak TM, Dice JP. Use of immunotherapy in the management of presumed anaphylaxis to the deer fly. Ann Allergy Asthma Immunol. 2003;90:351-354.
43. Royden A, Wedley A, Merga JY, et al. A role for flies (Diptera) in the transmission of Campylobacter to broilers? Epidemiol Infect. 2016;144:3326-3334.
44. Fradin MS, Day JF. Comparative efficacy of insect repellents against mosquito bites. N Engl J Med. 2002;347:13-18.

45. Carpenter S, Groschup MH, Garros C, et al. Culicoides biting midges, arboviruses and public health in Europe. Antiviral Res. 2013;100:102-113.
46. Peng Z, Yang M, Simons FE. Immunologic mechanisms in mosquito allergy: correlation of skin reactions with specific IgE and IgG anti-bodies and lymphocyte proliferation response to mosquito antigens. Ann Allergy Asthma Immunol. 1996;77:238-244.
47. Simons FE, Peng Z. Skeeter syndrome. J Allergy Clin Immunol. 1999;104:705-707.
48. Centers for Disease Control and Prevention. Travelers’ health. Clinician resources. wwwnc.cdc.gov/travel/page/clinician-information-center. Accessed November 25, 2020.
49. Gauci M, Loh RK, Stone BF, et al. Allergic reactions to the Australian paralysis tick, Ixodes holocyclus: diagnostic evaluation by skin test and radioimmunoassay. Clin Exp Allergy. 1989;19:279-283.
50. Centers for Disease Control and Prevention. Ticks. Removing a tick. www.cdc.gov/ticks/removing_a_tick.html. Accessed November 25, 2020.
51. Golden DB, Kagey-Sobotka A, Norman PS, et al. Insect sting allergy with negative venom skin test responses. J Allergy Clin Immunol. 2001;107:897-901.
52. Arzt L, Bokanovic D, Schrautzer C, et al. Immunological differences between insect venom-allergic patients with and without immunotherapy and asymptomatically sensitized subjects. Allergy. 2018;73:1223-1231.
53. Heddle R, Golden DBK. Allergy to insect stings and bites. World Allergy Organization Web site. Updated August 2015. www.worldallergy.org/education-and-programs/education/allergic-disease-resource-center/professionals/allergy-to-insect-stings-and-bites. Accessed November 25, 2020.
54. RuëffF, Przybilla B, Müller U, et al. The sting challenge test in Hymenoptera venom allergy. Position paper of the Subcommittee on Insect Venom Allergy of the European Academy of Allergology and Clinical Immunology. Allergy. 1996;51:216-225.
55. Management of simple insect bites: where’s the evidence? Drug Ther Bull. 2012;50:45-48.
56. Tracy JM. Insect allergy. Mt Sinai J Med. 2011;78:773-783.
57. Golden DBK. Insect sting allergy and venom immunotherapy: a model and a mystery. J Allergy Clin Immunol. 2005;115:439-447.
58. Winther L, Arnved J, Malling H-J, et al. Side-effects of allergen-specific immunotherapy: a prospective multi-centre study. Clin Exp Allergy. 2006;36:254-260.
59. Mellerup MT, Hahn GW, Poulsen LK, et al. Safety of allergen-specific immunotherapy. Relation between dosage regimen, allergen extract, disease and systemic side-effects during induction treatment. Clin Exp Allergy. 2000;30:1423-1429.
60. Anaphylaxis and insect stings and bites. Med Lett Drugs Ther. 2017;59:e79-e82.
61. Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report—second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. Ann Emerg Med. 2006;47:373-380.
62. Pflipsen MC, Vega Colon KM. Anaphylaxis: recognition and management. Am Fam Physician. 2020;102:355-362. Accessed November 25, 2020.
1. Numbers of insects (species and individuals). Smithsonian BugInfo Web site. www.si.edu/spotlight/buginfo/bugnos. Accessed November 25, 2020.
2. Antonicelli L, Bilò MB, Bonifazi F. Epidemiology of Hymenoptera allergy. Curr Opin Allergy Clin Immunol. 2002;2:341-346.
3. Jack jumper ant allergy. Australasian Society of Clinical Immunology and Allergy (ASCIA) Web site. Updated October 19, 2019. www.allergy.org.au/patients/insect-allergy-bites-and-stings/jack-jumper-ant-allergy. Accessed November 25, 2020.
4. Kemp SF, deShazo RD, Moffit JE, et al. Expanding habitat of the imported fire ant (Solenopsis invicta): a public health concern. J Allergy Clin Immunol. 2000;105:683-691.
5. Goodnight ML. Arachnid. In: Encyclopædia Britannica. 2012. www.britannica.com/animal/arachnid. Accessed November 25, 2020.
6. Despommier DD, Gwadz RW, Hotez PJ. Arachnids. In: Despommier DD, Gwadz RW, Hotez PJ. Parasitic Diseases. 3rd ed. Springer-Verlag; 1995:268-283.
7. Diaz JH, Leblanc KE. Common spider bites. Am Fam Physician. 2007;75:869-873.
8. Mowry JB, Spyker DA, Cantilena LR Jr, McMillan N, Ford M. 2013 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 31st Annual Report. Clin Toxicol (Phila). 2014;52:1032-1283.
9. Pucci S, Antonicelli L, Bilò MB, et al. Shortness of interval between two stings as risk factor for developing Hymenoptera venom allergy. Allergy.1994;49:894-896.
10. Müller UR. Bee venom allergy in beekeepers and their family members. Curr Opin Allergy Clin Immunol. 2005;5:343-347.
11. Müller UR. Cardiovascular disease and anaphylaxis. Curr Opin Allergy Clin Immunol. 2007;7:337-341.
12. Golden DBK. Stinging insect allergy. Am Fam Physician. 2003;67:2541-2546.
13. Golden DBK, Demain T, Freeman T, et al. Stinging insect hypersensitivity: a practice parameter update 2016. Ann Allergy Asthma Immunol. 2017;118:28-54.
14. Bilò BM, Rueff F, Mosbech H, et al; EAACI Interest Group on Insect Venom Hypersensitivity. Diagnosis of Hymenoptera venom allergy. Allergy. 2005;60:1339-1349.
15. Reisman RE. Insect stings. N Engl J Med. 1994;331:523-527.
16. Pucci S, D’Alò S, De Pasquale T, et al. Risk of anaphylaxis in patients with large local reactions to hymenoptera stings: a retrospective and prospective study. Clin Mol Allergy. 2015;13:21.
17. Golden DBK. Large local reactions to insect stings. J Allergy Clin Immunol Pract. 2015;3:331-334.
18. Clark S, Camargo CA Jr. Emergency treatment and prevention of insect-sting anaphylaxis. Curr Opin Allergy Clin Immunol. 2006;6:279-283.
19. Stinging insect allergy. In: Volcheck GW. Clinical Allergy: Diagnosis and Management. Humana Press; 2009:465-479.
20. Järvinen KM, Celestin J. Anaphylaxis avoidance and management: educating patients and their caregivers. J Asthma Allergy. 2014;7:95-104.
21. Institute for Quality and Efficiency in Health Care (IQWiG). Insect venom allergies: overview. InformedHealth.org. Updated May 7, 2020. www.ncbi.nlm.nih.gov/pubmedhealth/PMH0096282/. Accessed November 25, 2020.
22. Casale TB, Burks AW. Clinical practice. Hymenoptera-sting hypersensitivity. N Engl J Med. 2014;370:1432-1439.
23. Shelley RM. Centipedes and millipedes with emphasis on North American fauna. Kansas School Naturalist. 1999;45:1-16. https://sites.google.com/g.emporia.edu/ksn/ksn-home/vol-45-no-3-centipedes-and-millipedes-with-emphasis-on-n-america-fauna#h.p_JEf3uDlTg0jw. Accessed November 25, 2020.
24. Ogg B. Centipedes and millipedes. Nebraska Extension in Lancaster County Web site. https://lancaster.unl.edu/pest/resources/CentipedeMillipede012.shtml. Accessed November 25, 2020.
25. Cushing PE. Spiders (Arachnida: Araneae). In: Capinera JL, ed. Encyclopedia of Entomology. Springer, Dordrecht; 2008:226.
26. Diaz JH, Leblanc KE. Common spider bites. Am Fam Physician. 2007;75:869-873.
27. The National Institute for Occupational Safety and Health (NIOSH), Centers for Disease Control and Prevention. Venomous spiders. www.cdc.gov/niosh/topics/spiders/. Accessed November 25, 2020.
28. Starr S. What you need to know to prevent a poisonous spider bite. AAP News. 2013;34:42. www.aappublications.org/content/aapnews/34/9/42.5.full.pdf. Accessed November 25, 2020.
29. Spider bites. Mayo Clinic Web site. www.mayoclinic.org/diseases-conditions/spider-bites/symptoms-causes/syc-20352371. Accessed November 25, 2020.
30. Barish RA, Arnold T. Spider bites. In: Merck Manual (Professional Version). Merck Sharp & Dohme Corp.; 2016. www.merckmanuals.com/professional/injuries-poisoning/bites-and-stings/spider-bites. Accessed November 25, 2020.
31. Juckett G. Arthropod bites. Am Fam Physician. 2013;88:841-847.
32. Clark RF, Wethern-Kestner S, Vance MV, et al. Clinical presentation and treatment of black widow spider envenomation: a review of 163 cases. Ann Emerg Med. 1992;21:782-787.
33. Koehler PG, Pereira RM, Diclaro JW II. Fleas. Publication ENY-025. University of Florida IFAS Extension. Revised January 2012. https://edis.ifas.ufl.edu/ig087. Accessed November 25, 2020.
34. Bitam I, Dittmar K, Parola P, et al. Fleas and flea-borne diseases. Int J Infect Dis. 2010;14:e667-e676.
35. Leulmi H, Socolovschi C, Laudisoit A, et al. Detection of Rickettsia felis, Rickettsia typhi, Bartonella species and Yersinia pestis in fleas (Siphonaptera) from Africa. PLoS Negl Trop Dis. 2014;8:e3152.
36. Naimer SA, Cohen AD, Mumcuoglu KY, et al. Household papular urticaria. Isr Med Assoc J. 2002;4(11 suppl):911-913.
37. Golomb MR, Golomb HS. What’s eating you? Cat flea (Ctenocephalides felis). Cutis. 2010;85:10-11.
38. Dryden MW. Flea and tick control in the 21st century: challenges and opportunities. Vet Dermatol. 2009;20:435-440.
39. Dryden MW. Fleas in dogs and cats. Merck Sharp & Dohme Corporation: Merck Manual Veterinary Manual. Updated December 2014. www.merckvetmanual.com/integumentary-system/fleas-and-flea-allergy-dermatitis/fleas-in-dogs-and-cats. Accessed November 25, 2020.
40. Centers for Disease Control and Prevention. Getting rid of fleas. www.cdc.gov/fleas/getting_rid.html. Accessed November 25, 2020.
41. Chattopadhyay P, Goyary D, Dhiman S, et al. Immunomodulating effects and hypersensitivity reactions caused by Northeast Indian black fly salivary gland extract. J Immunotoxicol. 2014;11:126-132.
42. Hrabak TM, Dice JP. Use of immunotherapy in the management of presumed anaphylaxis to the deer fly. Ann Allergy Asthma Immunol. 2003;90:351-354.
43. Royden A, Wedley A, Merga JY, et al. A role for flies (Diptera) in the transmission of Campylobacter to broilers? Epidemiol Infect. 2016;144:3326-3334.
44. Fradin MS, Day JF. Comparative efficacy of insect repellents against mosquito bites. N Engl J Med. 2002;347:13-18.

45. Carpenter S, Groschup MH, Garros C, et al. Culicoides biting midges, arboviruses and public health in Europe. Antiviral Res. 2013;100:102-113.
46. Peng Z, Yang M, Simons FE. Immunologic mechanisms in mosquito allergy: correlation of skin reactions with specific IgE and IgG anti-bodies and lymphocyte proliferation response to mosquito antigens. Ann Allergy Asthma Immunol. 1996;77:238-244.
47. Simons FE, Peng Z. Skeeter syndrome. J Allergy Clin Immunol. 1999;104:705-707.
48. Centers for Disease Control and Prevention. Travelers’ health. Clinician resources. wwwnc.cdc.gov/travel/page/clinician-information-center. Accessed November 25, 2020.
49. Gauci M, Loh RK, Stone BF, et al. Allergic reactions to the Australian paralysis tick, Ixodes holocyclus: diagnostic evaluation by skin test and radioimmunoassay. Clin Exp Allergy. 1989;19:279-283.
50. Centers for Disease Control and Prevention. Ticks. Removing a tick. www.cdc.gov/ticks/removing_a_tick.html. Accessed November 25, 2020.
51. Golden DB, Kagey-Sobotka A, Norman PS, et al. Insect sting allergy with negative venom skin test responses. J Allergy Clin Immunol. 2001;107:897-901.
52. Arzt L, Bokanovic D, Schrautzer C, et al. Immunological differences between insect venom-allergic patients with and without immunotherapy and asymptomatically sensitized subjects. Allergy. 2018;73:1223-1231.
53. Heddle R, Golden DBK. Allergy to insect stings and bites. World Allergy Organization Web site. Updated August 2015. www.worldallergy.org/education-and-programs/education/allergic-disease-resource-center/professionals/allergy-to-insect-stings-and-bites. Accessed November 25, 2020.
54. RuëffF, Przybilla B, Müller U, et al. The sting challenge test in Hymenoptera venom allergy. Position paper of the Subcommittee on Insect Venom Allergy of the European Academy of Allergology and Clinical Immunology. Allergy. 1996;51:216-225.
55. Management of simple insect bites: where’s the evidence? Drug Ther Bull. 2012;50:45-48.
56. Tracy JM. Insect allergy. Mt Sinai J Med. 2011;78:773-783.
57. Golden DBK. Insect sting allergy and venom immunotherapy: a model and a mystery. J Allergy Clin Immunol. 2005;115:439-447.
58. Winther L, Arnved J, Malling H-J, et al. Side-effects of allergen-specific immunotherapy: a prospective multi-centre study. Clin Exp Allergy. 2006;36:254-260.
59. Mellerup MT, Hahn GW, Poulsen LK, et al. Safety of allergen-specific immunotherapy. Relation between dosage regimen, allergen extract, disease and systemic side-effects during induction treatment. Clin Exp Allergy. 2000;30:1423-1429.
60. Anaphylaxis and insect stings and bites. Med Lett Drugs Ther. 2017;59:e79-e82.
61. Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report—second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. Ann Emerg Med. 2006;47:373-380.
62. Pflipsen MC, Vega Colon KM. Anaphylaxis: recognition and management. Am Fam Physician. 2020;102:355-362. Accessed November 25, 2020.
PRACTICE RECOMMENDATIONS
❯ Recommend that patients use an insect repellent, such as an over-the-counter formulation that contains DEET, picaridin, or PMD (a chemical constituent of Eucalyptus citriodora oil) to prevent flea bites. C
❯ Prescribe nonsedating oral antihistamines as first-line symptomatic treatment of mild-to-moderate pruritus secondary to an insect bite. C
❯ When indicated, refer patients for venom immunotherapy, which is approximately 95% effective in preventing or reducing severe systemic reactions and reduces the risk of anaphylaxis and death. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Home visits: A practical approach
CASE
Mr. A is a 30-year-old man with neurofibromatosis and myelopathy with associated quadriplegia, complicated by dysphasia and chronic hypercapnic respiratory failure requiring a tracheostomy. He is cared for at home by his very competent mother but requires regular visits with his medical providers for assistance with his complex care needs. Due to logistical challenges, he had been receiving regular home visits even before the COVID-19 pandemic.
After estimating the risk of exposure to the patient, Mr. A’s family and his physician’s office staff scheduled a home visit. Before the appointment, the doctor conducted a virtual visit with the patient and family members to screen for COVID-19 infection, which proved negative. The doctor arranged a visit to coincide with Mr. A’s regular appointment with the home health nurse. He invited the patient’s social worker to attend, as well.
The providers donned masks, face shields, and gloves before entering the home. Mr. A’s temperature was checked and was normal. The team completed a physical exam, assessed the patient’s current needs, and refilled prescriptions. The doctor, nurse, and social worker met afterward in the family’s driveway to coordinate plans for the patient’s future care.
This encounter allowed a vulnerable patient with special needs to have access to care while reducing his risk of undesirable exposure. Also, his health care team’s provision of care in the home setting reduced Mr. A’s anxiety and that of his family members.
Home visits have long been an integral part of what it means to be a family physician. In 1930, roughly 40% of all patient-physician encounters in the United States occurred in patients’ homes. By 1980, this number had dropped to < 1%.1 Still, a 1994 survey of American doctors in 3 primary care specialties revealed that 63% of family physicians, more than the other 2 specialties, still made house calls.2 A 2016 analysis of Medicare claims data showed that between 2006 and 2011, only 5% of American doctors overall made house calls on Medicare recipients, but interestingly, the total number of home visits was increasing.3
This resurgence of interest in home health care is due in part to the increasing number of homebound patients in America, which exceeds the number of those in nursing homes.4 Further, a growing body of evidence indicates that home visits improve patient outcomes. And finally, many family physicians whose work lives have been centered around a busy office or hospital practice have found satisfaction in once again seeing patients in their own homes.
The COVID-19 pandemic has of course presented unique challenges—and opportunities, too—for home visits, which we discuss at the end of the article.
Why aren’t more of us making home visits?
For most of us, the decision not to make home visits is simply a matter of time and money. Although Medicare reimbursement for a home visit is typically about 150% that of a comparable office visit,5 it’s difficult, if not impossible, to make 2 home visits in the time you could see 3 patients in the office. So, economically it’s a net loss. Furthermore, we tend to feel less comfortable in our patients’ homes than in our offices. We have less control outside our own environment, and what happens away from our office is often less predictable—sometimes to the point that we may be concerned for our safety.
Continue to: So why make home visits at all?
So why make home visits at all?
First and foremost, home visits improve patient outcomes. This is most evident in our more vulnerable patients: newborns and the elderly, those who have been recently hospitalized, and those at risk because of their particular home situation. Multiple studies have shown that, for elders, home visits reduce functional decline, nursing home admissions, and mortality by around 25% to 33%.6-8 For those at risk of abuse, a recent systematic review showed that home visits reduce intimate partner violence and child abuse.9 Another systematic review demonstrated that patients with diabetes who received home visits vs usual care were more likely to show improvements in quality of life.10 These patients were also more likely to have lower HbA1c levels and lower systolic blood pressure readings.10 A few caveats apply to these studies:
- all of them targeted “vulnerable” patients
- most studies enlisted interdisciplinary teams and had regular team meetings
- most findings reached significance only after multiple home visits.
A further reason for choosing to become involved in home care is that it builds relationships, understanding, and empathy with our patients. “There is deep symbolism in the home visit.... It says, ‘I care enough about you to leave my power base … to come and see you on your own ground.’”11 And this benefit is 2-way; we also grow to understand and appreciate our patients better, especially if they are different from us culturally or socioeconomically.
Home visits allow the medical team to see challenges the patient has grown accustomed to, and perhaps ones that the patient has deemed too insignificant to mention. For the patient, home visits foster a strong sense of trust with the individual doctor and our health delivery network, and they decrease the need to seek emergency services. Finally, it has been demonstrated that provider satisfaction improves when home visits are incorporated into the work week.12
What is the role of community health workers in home-based care?
Community health workers (CHWs), defined as “frontline public health workers who are trusted members of and/or have an unusually close understanding of the community they serve,”13 can be an integral part of the home-based care team. Although CHWs have variable amounts of formal training, they have a unique perspective on local health beliefs and practices, which can assist the home-care team in providing culturally competent health care services and reduce health care costs.
In a study of children with asthma in Seattle, Washington, patients were randomized to a group that had 4 home visits by CHWs and a group that received usual care. The group that received home visits demonstrated more asthma symptom–free days, improved quality-of-life scores, and fewer urgent care visits.14 Furthermore, the intervention was estimated to save approximately $1300 per patient, resulting in a return on investment of 190%. Similarly, in a study comparing inappropriate emergency department (ED) visits between children who received CHW visits and those who did not, patients in the intervention group were significantly less likely to visit the ED for ambulatory complaints (18.2% vs 35.1%; P = .004).15
Continue to: What is the role of social workersin home-based care?
What is the role of social workersin home-based care?
Social workers can help meet the complex medical and biopsychosocial needs of the homebound population.16 A study by Cohen et al based in Israel concluded that homebound participants had a significantly higher risk for mortality, higher rates of depression, and difficulty completing instrumental activities of daily living when compared with their non-homebound counterparts.17
The Mount Sinai (New York) Visiting Doctors Program (MSVD) is a home-based care team that uses social workers to meet the needs of their complex patients.18 The social workers in the MSVD program provide direct counseling, make referrals to government and community resources, and monitor caregiver burden. Using a combination of measurement tools to assess caregiver burden, Ornstein et al demonstrated that the MSVD program led to a decrease in unmet needs and in caregiver burden.19,20 Caregiver burnout can be assessed using the Caregiver Burden Inventory, a validated 24-item questionnaire.21
What electronic tools are availableto monitor patients at home?
Although expensive in terms of both dollars and personnel time, telemonitoring allows home care providers to receive real-time, updated information regarding their patients.
Chronic obstructive pulmonary disease (COPD). One systematic review showed that although telemonitoring of patients with COPD improved quality of life and decreased COPD exacerbations, it did not reduce the risk of hospitalization and, therefore, did not reduce health care costs.22 Telemonitoring in COPD can include transmission of data about spirometry parameters, weight, temperature, blood pressure, sputum color, and 6-minute walk distance.23,24
Congestive heart failure (CHF). A 2010 Cochrane review found that telemonitoring of patients with CHF reduced all-cause mortality (risk ratio [RR] = 0.66; P < .0001).25 The Telemedical Interventional Management in Heart Failure II (TIM-HF2) trial,conducted from 2013 to 2017, compared usual care for CHF patients with care incorporating daily transmission of body weight, blood pressure, heart rate, electrocardiogram tracings, pulse oximetry, and self-rated health status.26 This study showed that the average number of days lost per year due to hospital admission was less in the telemonitoring group than in the usual care group (17.8 days vs. 24.2 days; P = .046). All-cause mortality was also reduced in the telemonitoring group (hazard ratio = 0.70; P = .028).
Continue to: What role do “home hospitals” play?
What role do “home hospitals” play?
Home hospitals provide acute or subacute treatment in a patient’s home for a condition that would normally require hospitalization.27 In a meta-analysis of 61 studies evaluating the effectiveness of home hospitals, this option was more likely to reduce mortality (odds ratio [OR] = 0.81; P = .008) and to reduce readmission rates (OR = 0.75; P = .02).28 In a study of 455 older adults, Leff et al found that hospital-at-home was associated with a shorter length of stay (3.2 vs. 4.9 days; P = .004) and that the mean cost was lower for hospital-at-home vs traditional hospital care.29
However, a 2016 Cochrane review of 16 randomized controlled trials comparing hospital-at-home with traditional hospital care showed that while care in a hospital-at-home may decrease formal costs, if costs for caregivers are taken into account, any difference in cost may disappear.30
Although the evidence for cost saving is variable, hospital-at-home admission has been shown to reduce the likelihood of living in a residential care facility at 6 months (RR = 0.35; P < .0001).30 Further, the same Cochrane review showed that admission avoidance may increase patient satisfaction with the care provided.30
Finally, a recent randomized trial in a Boston-area hospital system showed that patients cared for in hospital-at-home were significantly less likely to be readmitted within 30 days and that adjusted cost was about two-thirds the cost of traditional hospital care.31
What is the physician’s rolein home health care?
While home health care is a team effort, the physician has several crucial roles. First, he or she must make the determination that home care is appropriate and feasible for a particular patient. Appropriate, meaning there is evidence that this patient is likely to benefit from home care. Feasible, meaning there are resources available in the community and family to safely care for the patient at home. “Often a house call will serve as the first step in developing a home-based-management plan.”32
Continue to: Second, the physician serves...
Second, the physician serves an important role in directing and coordinating the team of professionals involved. This primarily means helping the team to communicate with one another. Before home visits begin, the physician’s office should reach out not only to the patient and family, but also to any other health care personnel involved in the patient’s home care. Otherwise, many of the health care providers involved will never have face-to-face interaction with the physician. Creation of the coordinated health team minimizes duplication and miscommunication; it also builds a valuable bond.
How does one go about making a home visit?
Scheduling. What often works best in a busy practice is to schedule home visits for the end of the workday or to devote an entire afternoon to making home visits to several patients in one locale. Also important is scheduling times, if possible, when important family members or other caregivers are at home or when other members of the home care team can accompany you.
What to bring along. Carry a “home visit bag” that includes equipment you’re likely to need and that is not available away from your office. A minimally equipped visit bag would include different-sized blood pressure cuffs, a glucometer, a pulse oximeter, thermometers, and patient education materials. Other suggested contents are listed in TABLE 1.
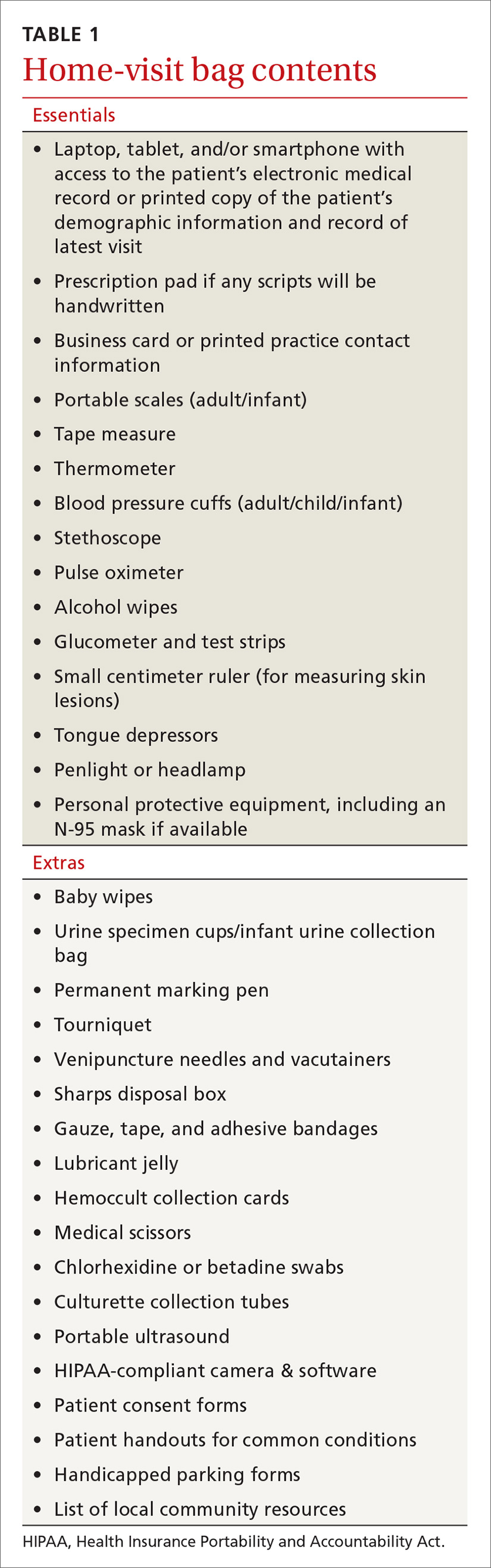
Dos and don’ts. Take a few minutes when you first arrive to simply visit with the patient. Sit down and introduce yourself and any members of the home care team that the patient has not met. Take an interim history. While you’re doing this, be observant: Is the home neat or cluttered? Is the indoor temperature comfortable? Are there fall hazards? Is there a smell of cigarette smoke? Are there any indoor combustion sources (eg, wood stove or kerosene heater)? Ask questions such as: Who lives here with you? Can you show me where you keep your medicines? (If the patient keeps insulin or any other medicines in the refrigerator, ask to see it. Note any apparent food scarcity.)
During your exam, pay particular attention to whether vital signs are appreciably different than those measured in the office or hospital. Pay special attention to the patient’s functional abilities. “A subtle, but critical distinction between medical management in the home and medical management in the hospital, clinic, or office is the emphasis on the patient’s functional abilities, family assistance, and environmental factors.”33
Observe the patient’s use of any home technology, if possible; this can be as simple as home oxygenation or as complex as home hemodialysis. Assess for any apparent caregiver stress. Finally, don’t neglect to offer appropriate emotional and spiritual support to the patient and family and to schedule the next follow-up visit before you leave.
Continue to: Documentation and reimbursement.
Documentation and reimbursement. While individual electronic medical records may require use of particular forms of documentation, using a home visit template when possible can be extremely helpful (TABLE 2). A template not only assures thoroughness and consistency (pharmacy, home health contacts, billing information) but also serves as a prompt to survey the patient and the caregivers about nonmedical, but essential, social and well-being services. The document should be as simple and user-friendly as possible.
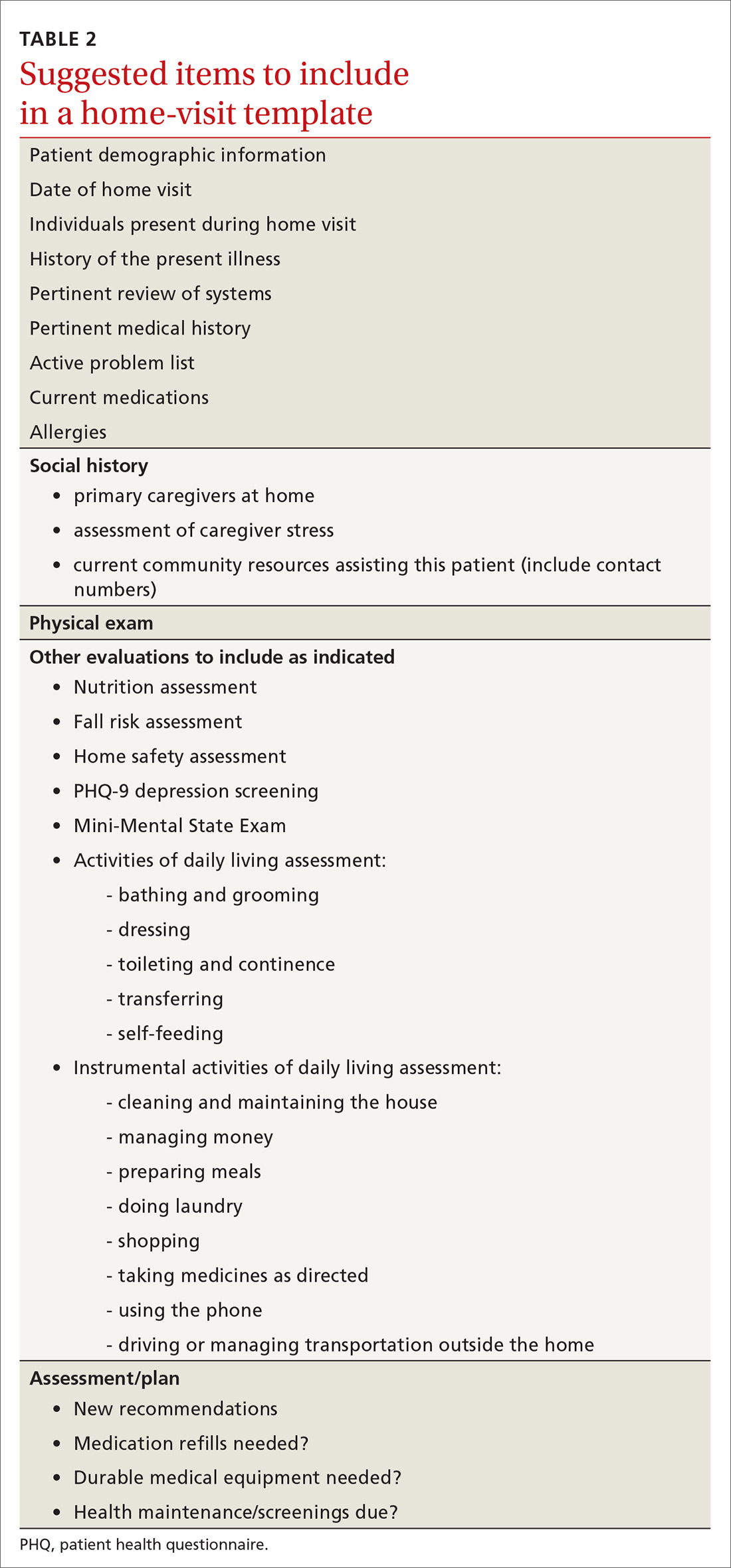
Not all assessments will be able to be done at each visit but seeing them listed in the template can be helpful. Billing follows the same principles as for office visits and has similar requirements for documentation. Codes for the most common types of home visits are listed in TABLE 3.
Where can I get help?
Graduates of family medicine residency programs are required to receive training in home visits by the Accreditation Council for Graduate Medical Education (ACGME). Current ACGME program requirements stipulate that “residents must demonstrate competence to independently diagnose, manage, and integrate the care of patients of all ages in various outpatient settings, including the FMP [family medicine practice] site and home environment,” and “residents must be primarily responsible for a panel of continuity patients, integrating each patient’s care across all settings, including the home ...” [emphasis added].34
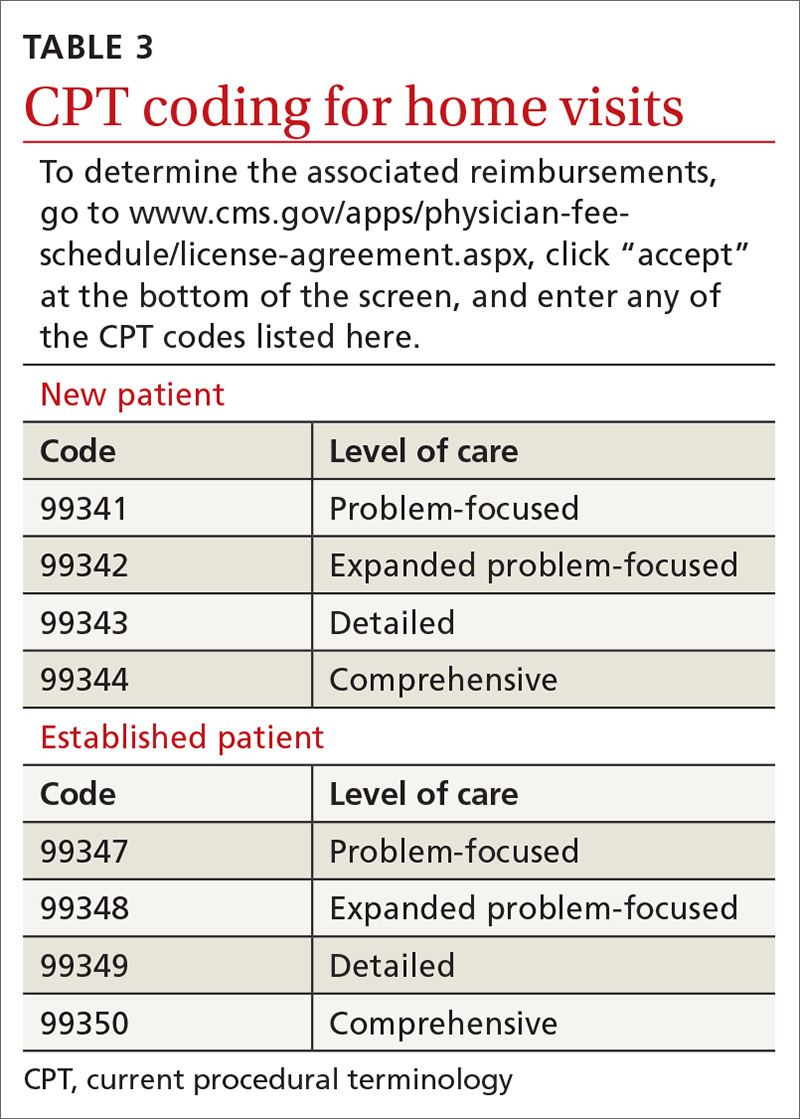
For those already in practice, one of the hardest parts of doing home visits is feeling alone, especially if few other providers in your community engage in home care. As you run into questions and challenges with incorporating home care of patients into your practice, one excellent resource is the American Academy of Home Care Medicine (www.aahcm.org/). Founded in 1988 and headquartered in Chicago, it not only provides numerous helpful resources, but serves as a networking tool for physicians involved in home care.
This unprecedented pandemichas allowed home visits to shine
As depicted in our opening patient case, patients who have high-risk conditions and those who are older than 65 years of age may be cared for more appropriately in a home visit rather than having them come to the office. Home visits may also be a way for providers to “lay eyes” on patients who do not have technology available to participate in virtual visits.
Before performing a home visit, inquire as to whether the patient has symptoms of COVID-19. Adequate PPE should be donned at all times and social distancing should be practiced when appropriate. With adequate PPE, home visits may also allow providers to care for low-risk patients known to have COVID-19 and thereby minimize risks to staff and other patients in the office. JFP
CORRESPONDENCE
Curt Elliott, MD, Prisma Health USC Family Medicine Center, 3209 Colonial Drive, Columbia, SC 29203; [email protected].
1. Unwin BK, Tatum PE. House calls. Am Fam Physician. 2011;83:925-938.
3. Sairenji T, Jetty A, Peterson LE. Shifting patterns of physician home visits. J Prim Care Community Health. 2016;7:71-75.
4. Ornstein KA, Leff B, Covinsky K, et al. Epidemiology of the homebound population in the United States. JAMA Intern Med. 2015;175;1180-1186.
5. CMS. Current Procedural Terminology, Fourth Edition ("CPT®"). www.cms.gov/apps/physician-fee-schedule/license-agreement.aspx. Accessed November 30, 2020.
6. Elkan R, Kendrick D, Dewey M, et al. Effectiveness of home based support for older people: systematic review and meta-analysis. BMJ. 2001;323:719-725.
7. Stuck AE, Egger M, Hammer A, et al. Home visits to prevent nursing home admission and functional decline in elderly people: systematic review and meta-regression analysis. JAMA. 2002;287:1022-1028.
8. Stall N, Nowaczynski M, Sinha SK. Systematic review of outcomes from home-based primary care programs for homebound older adults. J Am Geriatr Soc. 2014;62:2243-2251.
9. Prosman GJ, Lo Fo Wong SH, van der Wouden JC, et al. Effectiveness of home visiting in reducing partner violence for families experiencing abuse: a systematic review. Fam Pract. 2015;32:247-256.
10. Han L, Ma Y, Wei S, et al. Are home visits an effective method for diabetes management? A quantitative systematic review and meta-analysis. J Diabetes Investig. 2017;8:701-708.
11. McWhinney IR. Fourth annual Nicholas J. Pisacano Lecture. The doctor, the patient, and the home: returning to our roots. J Am Board Fam Pract. 1997;10:430-435.
12. Kao H, Conant R, Soriano T, et al. The past, present, and future of house calls. Clin Geriatr Med. 2009;25:19-34.
13. American Public Health Association. Community health workers. www.apha.org/apha-communities/member-sections/community-health-workers. Accessed November 30, 2020.
14. Campbell JD, Brooks M, Hosokawa P, et al. Community health worker home visits for Medicaid-enrolled children with asthma: effects on asthma outcomes and costs. Am J Public Health. 2015;105:2366-2372.
15. Anugu M, Braksmajer A, Huang J, et al. Enriched medical home intervention using community health worker home visitation and ED use. Pediatrics. 2017;139:e20161849.
16. Reckrey JM, Gettenberg G, Ross H, et al. The critical role of social workers in home-based primary care. Soc Work in Health Care. 2014;53:330-343.
17. Cohen-Mansfield J, Shmotkin D, Hazan H. The effect of homebound status on older persons. J Am Geriatr Soc. 2010;58:2358-2362.
18. Mt. Sinai Visiting Doctors Program. www.mountsinai.org/care/primary-care/upper-east-side/visiting-doctors/about. Accessed November 30, 2020.
19. Ornstein K, Hernandez CR, DeCherrie LV, et al. The Mount Sinai (New York) Visiting Doctors Program: meeting the needs of the urban homebound population. Care Manag J. 2011;12:159-163.
20. Ornstein K, Smith K, Boal J. Understanding and improving the burden and unmet needs of informal caregivers of homebound patients enrolled in a home-based primary care program. J Appl Gerontol. 2009;28:482-503.
21. Novak M, Guest C. Application of a multidimensional caregiver burden inventory. Gerontologist. 1989;29:798-803.
22. Cruz J, Brooks D, Marques A. Home telemonitoring effectiveness in COPD: a systematic review. Int J Clin Pract. 2014;68:369-378.
23. Antoniades NC, Rochford PD, Pretto JJ, et al. Pilot study of remote telemonitoring in COPD. Telemed J E Health. 2012;18:634-640.
24. Koff PB, Jones RH, Cashman JM, et al. Proactive integrated care improves quality of life in patients with COPD. Eur Respir J. 2009;33:1031-1038.
25. Inglis SC, Clark RA, McAlister FA, et al. Which components of heart failure programmes are effective? A systematic review and meta-analysis of the outcomes of structured telephone support or telemonitoring as the primary component of chronic heart failure management in 8323 patients: abridged Cochrane review. Eur J Heart Fail. 2011;13:1028-1040.
26. Koehler F, Koehler K, Deckwart O, et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet. 2018;392:1047-1057.
27. Ticona L, Schulman KA. Extreme home makeover–the role of intensive home health care. New Eng J Med. 2016;375:1707-1709.
28. Caplan GA. A meta-analysis of “hospital in the home.” Med J Aust. 2013;198:195-196.
29. Leff B, Burton L, Mader SL, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med. 2005;143:798-808.
30. Shepperd S, Iliffe S, Doll HA, et al. Admission avoidance hospital at home. Cochrane Database Syst Rev. 2016;9:CD007491.
31. Levine DM, Ouchi K, Blanchfield B, et al. Hospital-level care at home for acutely ill adults: a randomized controlled trial. Ann Intern Med. 2020;172:77-85.
32. Cornwell T and Schwartzberg JG, eds. Medical Management of the Home Care Patient: Guidelines for Physicians. 4th ed. Chicago, IL: American Medical Association and American Academy of Home Care Physicians; 2012:p18.
33. Cornwell T and Schwartzberg JG, eds. Medical Management of the Home Care Patient: Guidelines for Physicians. 4th ed. Chicago, IL: American Medical Association and American Academy of Home Care Physicians; 2012:p19.
34. Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Family Medicine. www.acgme.org/Portals/0/PFAssets/ProgramRequirements/120_FamilyMedicine_2020.pdf. (section IV.C.1.b). Accessed November 30, 2020.
CASE
Mr. A is a 30-year-old man with neurofibromatosis and myelopathy with associated quadriplegia, complicated by dysphasia and chronic hypercapnic respiratory failure requiring a tracheostomy. He is cared for at home by his very competent mother but requires regular visits with his medical providers for assistance with his complex care needs. Due to logistical challenges, he had been receiving regular home visits even before the COVID-19 pandemic.
After estimating the risk of exposure to the patient, Mr. A’s family and his physician’s office staff scheduled a home visit. Before the appointment, the doctor conducted a virtual visit with the patient and family members to screen for COVID-19 infection, which proved negative. The doctor arranged a visit to coincide with Mr. A’s regular appointment with the home health nurse. He invited the patient’s social worker to attend, as well.
The providers donned masks, face shields, and gloves before entering the home. Mr. A’s temperature was checked and was normal. The team completed a physical exam, assessed the patient’s current needs, and refilled prescriptions. The doctor, nurse, and social worker met afterward in the family’s driveway to coordinate plans for the patient’s future care.
This encounter allowed a vulnerable patient with special needs to have access to care while reducing his risk of undesirable exposure. Also, his health care team’s provision of care in the home setting reduced Mr. A’s anxiety and that of his family members.
Home visits have long been an integral part of what it means to be a family physician. In 1930, roughly 40% of all patient-physician encounters in the United States occurred in patients’ homes. By 1980, this number had dropped to < 1%.1 Still, a 1994 survey of American doctors in 3 primary care specialties revealed that 63% of family physicians, more than the other 2 specialties, still made house calls.2 A 2016 analysis of Medicare claims data showed that between 2006 and 2011, only 5% of American doctors overall made house calls on Medicare recipients, but interestingly, the total number of home visits was increasing.3
This resurgence of interest in home health care is due in part to the increasing number of homebound patients in America, which exceeds the number of those in nursing homes.4 Further, a growing body of evidence indicates that home visits improve patient outcomes. And finally, many family physicians whose work lives have been centered around a busy office or hospital practice have found satisfaction in once again seeing patients in their own homes.
The COVID-19 pandemic has of course presented unique challenges—and opportunities, too—for home visits, which we discuss at the end of the article.
Why aren’t more of us making home visits?
For most of us, the decision not to make home visits is simply a matter of time and money. Although Medicare reimbursement for a home visit is typically about 150% that of a comparable office visit,5 it’s difficult, if not impossible, to make 2 home visits in the time you could see 3 patients in the office. So, economically it’s a net loss. Furthermore, we tend to feel less comfortable in our patients’ homes than in our offices. We have less control outside our own environment, and what happens away from our office is often less predictable—sometimes to the point that we may be concerned for our safety.
Continue to: So why make home visits at all?
So why make home visits at all?
First and foremost, home visits improve patient outcomes. This is most evident in our more vulnerable patients: newborns and the elderly, those who have been recently hospitalized, and those at risk because of their particular home situation. Multiple studies have shown that, for elders, home visits reduce functional decline, nursing home admissions, and mortality by around 25% to 33%.6-8 For those at risk of abuse, a recent systematic review showed that home visits reduce intimate partner violence and child abuse.9 Another systematic review demonstrated that patients with diabetes who received home visits vs usual care were more likely to show improvements in quality of life.10 These patients were also more likely to have lower HbA1c levels and lower systolic blood pressure readings.10 A few caveats apply to these studies:
- all of them targeted “vulnerable” patients
- most studies enlisted interdisciplinary teams and had regular team meetings
- most findings reached significance only after multiple home visits.
A further reason for choosing to become involved in home care is that it builds relationships, understanding, and empathy with our patients. “There is deep symbolism in the home visit.... It says, ‘I care enough about you to leave my power base … to come and see you on your own ground.’”11 And this benefit is 2-way; we also grow to understand and appreciate our patients better, especially if they are different from us culturally or socioeconomically.
Home visits allow the medical team to see challenges the patient has grown accustomed to, and perhaps ones that the patient has deemed too insignificant to mention. For the patient, home visits foster a strong sense of trust with the individual doctor and our health delivery network, and they decrease the need to seek emergency services. Finally, it has been demonstrated that provider satisfaction improves when home visits are incorporated into the work week.12
What is the role of community health workers in home-based care?
Community health workers (CHWs), defined as “frontline public health workers who are trusted members of and/or have an unusually close understanding of the community they serve,”13 can be an integral part of the home-based care team. Although CHWs have variable amounts of formal training, they have a unique perspective on local health beliefs and practices, which can assist the home-care team in providing culturally competent health care services and reduce health care costs.
In a study of children with asthma in Seattle, Washington, patients were randomized to a group that had 4 home visits by CHWs and a group that received usual care. The group that received home visits demonstrated more asthma symptom–free days, improved quality-of-life scores, and fewer urgent care visits.14 Furthermore, the intervention was estimated to save approximately $1300 per patient, resulting in a return on investment of 190%. Similarly, in a study comparing inappropriate emergency department (ED) visits between children who received CHW visits and those who did not, patients in the intervention group were significantly less likely to visit the ED for ambulatory complaints (18.2% vs 35.1%; P = .004).15
Continue to: What is the role of social workersin home-based care?
What is the role of social workersin home-based care?
Social workers can help meet the complex medical and biopsychosocial needs of the homebound population.16 A study by Cohen et al based in Israel concluded that homebound participants had a significantly higher risk for mortality, higher rates of depression, and difficulty completing instrumental activities of daily living when compared with their non-homebound counterparts.17
The Mount Sinai (New York) Visiting Doctors Program (MSVD) is a home-based care team that uses social workers to meet the needs of their complex patients.18 The social workers in the MSVD program provide direct counseling, make referrals to government and community resources, and monitor caregiver burden. Using a combination of measurement tools to assess caregiver burden, Ornstein et al demonstrated that the MSVD program led to a decrease in unmet needs and in caregiver burden.19,20 Caregiver burnout can be assessed using the Caregiver Burden Inventory, a validated 24-item questionnaire.21
What electronic tools are availableto monitor patients at home?
Although expensive in terms of both dollars and personnel time, telemonitoring allows home care providers to receive real-time, updated information regarding their patients.
Chronic obstructive pulmonary disease (COPD). One systematic review showed that although telemonitoring of patients with COPD improved quality of life and decreased COPD exacerbations, it did not reduce the risk of hospitalization and, therefore, did not reduce health care costs.22 Telemonitoring in COPD can include transmission of data about spirometry parameters, weight, temperature, blood pressure, sputum color, and 6-minute walk distance.23,24
Congestive heart failure (CHF). A 2010 Cochrane review found that telemonitoring of patients with CHF reduced all-cause mortality (risk ratio [RR] = 0.66; P < .0001).25 The Telemedical Interventional Management in Heart Failure II (TIM-HF2) trial,conducted from 2013 to 2017, compared usual care for CHF patients with care incorporating daily transmission of body weight, blood pressure, heart rate, electrocardiogram tracings, pulse oximetry, and self-rated health status.26 This study showed that the average number of days lost per year due to hospital admission was less in the telemonitoring group than in the usual care group (17.8 days vs. 24.2 days; P = .046). All-cause mortality was also reduced in the telemonitoring group (hazard ratio = 0.70; P = .028).
Continue to: What role do “home hospitals” play?
What role do “home hospitals” play?
Home hospitals provide acute or subacute treatment in a patient’s home for a condition that would normally require hospitalization.27 In a meta-analysis of 61 studies evaluating the effectiveness of home hospitals, this option was more likely to reduce mortality (odds ratio [OR] = 0.81; P = .008) and to reduce readmission rates (OR = 0.75; P = .02).28 In a study of 455 older adults, Leff et al found that hospital-at-home was associated with a shorter length of stay (3.2 vs. 4.9 days; P = .004) and that the mean cost was lower for hospital-at-home vs traditional hospital care.29
However, a 2016 Cochrane review of 16 randomized controlled trials comparing hospital-at-home with traditional hospital care showed that while care in a hospital-at-home may decrease formal costs, if costs for caregivers are taken into account, any difference in cost may disappear.30
Although the evidence for cost saving is variable, hospital-at-home admission has been shown to reduce the likelihood of living in a residential care facility at 6 months (RR = 0.35; P < .0001).30 Further, the same Cochrane review showed that admission avoidance may increase patient satisfaction with the care provided.30
Finally, a recent randomized trial in a Boston-area hospital system showed that patients cared for in hospital-at-home were significantly less likely to be readmitted within 30 days and that adjusted cost was about two-thirds the cost of traditional hospital care.31
What is the physician’s rolein home health care?
While home health care is a team effort, the physician has several crucial roles. First, he or she must make the determination that home care is appropriate and feasible for a particular patient. Appropriate, meaning there is evidence that this patient is likely to benefit from home care. Feasible, meaning there are resources available in the community and family to safely care for the patient at home. “Often a house call will serve as the first step in developing a home-based-management plan.”32
Continue to: Second, the physician serves...
Second, the physician serves an important role in directing and coordinating the team of professionals involved. This primarily means helping the team to communicate with one another. Before home visits begin, the physician’s office should reach out not only to the patient and family, but also to any other health care personnel involved in the patient’s home care. Otherwise, many of the health care providers involved will never have face-to-face interaction with the physician. Creation of the coordinated health team minimizes duplication and miscommunication; it also builds a valuable bond.
How does one go about making a home visit?
Scheduling. What often works best in a busy practice is to schedule home visits for the end of the workday or to devote an entire afternoon to making home visits to several patients in one locale. Also important is scheduling times, if possible, when important family members or other caregivers are at home or when other members of the home care team can accompany you.
What to bring along. Carry a “home visit bag” that includes equipment you’re likely to need and that is not available away from your office. A minimally equipped visit bag would include different-sized blood pressure cuffs, a glucometer, a pulse oximeter, thermometers, and patient education materials. Other suggested contents are listed in TABLE 1.

Dos and don’ts. Take a few minutes when you first arrive to simply visit with the patient. Sit down and introduce yourself and any members of the home care team that the patient has not met. Take an interim history. While you’re doing this, be observant: Is the home neat or cluttered? Is the indoor temperature comfortable? Are there fall hazards? Is there a smell of cigarette smoke? Are there any indoor combustion sources (eg, wood stove or kerosene heater)? Ask questions such as: Who lives here with you? Can you show me where you keep your medicines? (If the patient keeps insulin or any other medicines in the refrigerator, ask to see it. Note any apparent food scarcity.)
During your exam, pay particular attention to whether vital signs are appreciably different than those measured in the office or hospital. Pay special attention to the patient’s functional abilities. “A subtle, but critical distinction between medical management in the home and medical management in the hospital, clinic, or office is the emphasis on the patient’s functional abilities, family assistance, and environmental factors.”33
Observe the patient’s use of any home technology, if possible; this can be as simple as home oxygenation or as complex as home hemodialysis. Assess for any apparent caregiver stress. Finally, don’t neglect to offer appropriate emotional and spiritual support to the patient and family and to schedule the next follow-up visit before you leave.
Continue to: Documentation and reimbursement.
Documentation and reimbursement. While individual electronic medical records may require use of particular forms of documentation, using a home visit template when possible can be extremely helpful (TABLE 2). A template not only assures thoroughness and consistency (pharmacy, home health contacts, billing information) but also serves as a prompt to survey the patient and the caregivers about nonmedical, but essential, social and well-being services. The document should be as simple and user-friendly as possible.

Not all assessments will be able to be done at each visit but seeing them listed in the template can be helpful. Billing follows the same principles as for office visits and has similar requirements for documentation. Codes for the most common types of home visits are listed in TABLE 3.
Where can I get help?
Graduates of family medicine residency programs are required to receive training in home visits by the Accreditation Council for Graduate Medical Education (ACGME). Current ACGME program requirements stipulate that “residents must demonstrate competence to independently diagnose, manage, and integrate the care of patients of all ages in various outpatient settings, including the FMP [family medicine practice] site and home environment,” and “residents must be primarily responsible for a panel of continuity patients, integrating each patient’s care across all settings, including the home ...” [emphasis added].34

For those already in practice, one of the hardest parts of doing home visits is feeling alone, especially if few other providers in your community engage in home care. As you run into questions and challenges with incorporating home care of patients into your practice, one excellent resource is the American Academy of Home Care Medicine (www.aahcm.org/). Founded in 1988 and headquartered in Chicago, it not only provides numerous helpful resources, but serves as a networking tool for physicians involved in home care.
This unprecedented pandemichas allowed home visits to shine
As depicted in our opening patient case, patients who have high-risk conditions and those who are older than 65 years of age may be cared for more appropriately in a home visit rather than having them come to the office. Home visits may also be a way for providers to “lay eyes” on patients who do not have technology available to participate in virtual visits.
Before performing a home visit, inquire as to whether the patient has symptoms of COVID-19. Adequate PPE should be donned at all times and social distancing should be practiced when appropriate. With adequate PPE, home visits may also allow providers to care for low-risk patients known to have COVID-19 and thereby minimize risks to staff and other patients in the office. JFP
CORRESPONDENCE
Curt Elliott, MD, Prisma Health USC Family Medicine Center, 3209 Colonial Drive, Columbia, SC 29203; [email protected].
CASE
Mr. A is a 30-year-old man with neurofibromatosis and myelopathy with associated quadriplegia, complicated by dysphasia and chronic hypercapnic respiratory failure requiring a tracheostomy. He is cared for at home by his very competent mother but requires regular visits with his medical providers for assistance with his complex care needs. Due to logistical challenges, he had been receiving regular home visits even before the COVID-19 pandemic.
After estimating the risk of exposure to the patient, Mr. A’s family and his physician’s office staff scheduled a home visit. Before the appointment, the doctor conducted a virtual visit with the patient and family members to screen for COVID-19 infection, which proved negative. The doctor arranged a visit to coincide with Mr. A’s regular appointment with the home health nurse. He invited the patient’s social worker to attend, as well.
The providers donned masks, face shields, and gloves before entering the home. Mr. A’s temperature was checked and was normal. The team completed a physical exam, assessed the patient’s current needs, and refilled prescriptions. The doctor, nurse, and social worker met afterward in the family’s driveway to coordinate plans for the patient’s future care.
This encounter allowed a vulnerable patient with special needs to have access to care while reducing his risk of undesirable exposure. Also, his health care team’s provision of care in the home setting reduced Mr. A’s anxiety and that of his family members.
Home visits have long been an integral part of what it means to be a family physician. In 1930, roughly 40% of all patient-physician encounters in the United States occurred in patients’ homes. By 1980, this number had dropped to < 1%.1 Still, a 1994 survey of American doctors in 3 primary care specialties revealed that 63% of family physicians, more than the other 2 specialties, still made house calls.2 A 2016 analysis of Medicare claims data showed that between 2006 and 2011, only 5% of American doctors overall made house calls on Medicare recipients, but interestingly, the total number of home visits was increasing.3
This resurgence of interest in home health care is due in part to the increasing number of homebound patients in America, which exceeds the number of those in nursing homes.4 Further, a growing body of evidence indicates that home visits improve patient outcomes. And finally, many family physicians whose work lives have been centered around a busy office or hospital practice have found satisfaction in once again seeing patients in their own homes.
The COVID-19 pandemic has of course presented unique challenges—and opportunities, too—for home visits, which we discuss at the end of the article.
Why aren’t more of us making home visits?
For most of us, the decision not to make home visits is simply a matter of time and money. Although Medicare reimbursement for a home visit is typically about 150% that of a comparable office visit,5 it’s difficult, if not impossible, to make 2 home visits in the time you could see 3 patients in the office. So, economically it’s a net loss. Furthermore, we tend to feel less comfortable in our patients’ homes than in our offices. We have less control outside our own environment, and what happens away from our office is often less predictable—sometimes to the point that we may be concerned for our safety.
Continue to: So why make home visits at all?
So why make home visits at all?
First and foremost, home visits improve patient outcomes. This is most evident in our more vulnerable patients: newborns and the elderly, those who have been recently hospitalized, and those at risk because of their particular home situation. Multiple studies have shown that, for elders, home visits reduce functional decline, nursing home admissions, and mortality by around 25% to 33%.6-8 For those at risk of abuse, a recent systematic review showed that home visits reduce intimate partner violence and child abuse.9 Another systematic review demonstrated that patients with diabetes who received home visits vs usual care were more likely to show improvements in quality of life.10 These patients were also more likely to have lower HbA1c levels and lower systolic blood pressure readings.10 A few caveats apply to these studies:
- all of them targeted “vulnerable” patients
- most studies enlisted interdisciplinary teams and had regular team meetings
- most findings reached significance only after multiple home visits.
A further reason for choosing to become involved in home care is that it builds relationships, understanding, and empathy with our patients. “There is deep symbolism in the home visit.... It says, ‘I care enough about you to leave my power base … to come and see you on your own ground.’”11 And this benefit is 2-way; we also grow to understand and appreciate our patients better, especially if they are different from us culturally or socioeconomically.
Home visits allow the medical team to see challenges the patient has grown accustomed to, and perhaps ones that the patient has deemed too insignificant to mention. For the patient, home visits foster a strong sense of trust with the individual doctor and our health delivery network, and they decrease the need to seek emergency services. Finally, it has been demonstrated that provider satisfaction improves when home visits are incorporated into the work week.12
What is the role of community health workers in home-based care?
Community health workers (CHWs), defined as “frontline public health workers who are trusted members of and/or have an unusually close understanding of the community they serve,”13 can be an integral part of the home-based care team. Although CHWs have variable amounts of formal training, they have a unique perspective on local health beliefs and practices, which can assist the home-care team in providing culturally competent health care services and reduce health care costs.
In a study of children with asthma in Seattle, Washington, patients were randomized to a group that had 4 home visits by CHWs and a group that received usual care. The group that received home visits demonstrated more asthma symptom–free days, improved quality-of-life scores, and fewer urgent care visits.14 Furthermore, the intervention was estimated to save approximately $1300 per patient, resulting in a return on investment of 190%. Similarly, in a study comparing inappropriate emergency department (ED) visits between children who received CHW visits and those who did not, patients in the intervention group were significantly less likely to visit the ED for ambulatory complaints (18.2% vs 35.1%; P = .004).15
Continue to: What is the role of social workersin home-based care?
What is the role of social workersin home-based care?
Social workers can help meet the complex medical and biopsychosocial needs of the homebound population.16 A study by Cohen et al based in Israel concluded that homebound participants had a significantly higher risk for mortality, higher rates of depression, and difficulty completing instrumental activities of daily living when compared with their non-homebound counterparts.17
The Mount Sinai (New York) Visiting Doctors Program (MSVD) is a home-based care team that uses social workers to meet the needs of their complex patients.18 The social workers in the MSVD program provide direct counseling, make referrals to government and community resources, and monitor caregiver burden. Using a combination of measurement tools to assess caregiver burden, Ornstein et al demonstrated that the MSVD program led to a decrease in unmet needs and in caregiver burden.19,20 Caregiver burnout can be assessed using the Caregiver Burden Inventory, a validated 24-item questionnaire.21
What electronic tools are availableto monitor patients at home?
Although expensive in terms of both dollars and personnel time, telemonitoring allows home care providers to receive real-time, updated information regarding their patients.
Chronic obstructive pulmonary disease (COPD). One systematic review showed that although telemonitoring of patients with COPD improved quality of life and decreased COPD exacerbations, it did not reduce the risk of hospitalization and, therefore, did not reduce health care costs.22 Telemonitoring in COPD can include transmission of data about spirometry parameters, weight, temperature, blood pressure, sputum color, and 6-minute walk distance.23,24
Congestive heart failure (CHF). A 2010 Cochrane review found that telemonitoring of patients with CHF reduced all-cause mortality (risk ratio [RR] = 0.66; P < .0001).25 The Telemedical Interventional Management in Heart Failure II (TIM-HF2) trial,conducted from 2013 to 2017, compared usual care for CHF patients with care incorporating daily transmission of body weight, blood pressure, heart rate, electrocardiogram tracings, pulse oximetry, and self-rated health status.26 This study showed that the average number of days lost per year due to hospital admission was less in the telemonitoring group than in the usual care group (17.8 days vs. 24.2 days; P = .046). All-cause mortality was also reduced in the telemonitoring group (hazard ratio = 0.70; P = .028).
Continue to: What role do “home hospitals” play?
What role do “home hospitals” play?
Home hospitals provide acute or subacute treatment in a patient’s home for a condition that would normally require hospitalization.27 In a meta-analysis of 61 studies evaluating the effectiveness of home hospitals, this option was more likely to reduce mortality (odds ratio [OR] = 0.81; P = .008) and to reduce readmission rates (OR = 0.75; P = .02).28 In a study of 455 older adults, Leff et al found that hospital-at-home was associated with a shorter length of stay (3.2 vs. 4.9 days; P = .004) and that the mean cost was lower for hospital-at-home vs traditional hospital care.29
However, a 2016 Cochrane review of 16 randomized controlled trials comparing hospital-at-home with traditional hospital care showed that while care in a hospital-at-home may decrease formal costs, if costs for caregivers are taken into account, any difference in cost may disappear.30
Although the evidence for cost saving is variable, hospital-at-home admission has been shown to reduce the likelihood of living in a residential care facility at 6 months (RR = 0.35; P < .0001).30 Further, the same Cochrane review showed that admission avoidance may increase patient satisfaction with the care provided.30
Finally, a recent randomized trial in a Boston-area hospital system showed that patients cared for in hospital-at-home were significantly less likely to be readmitted within 30 days and that adjusted cost was about two-thirds the cost of traditional hospital care.31
What is the physician’s rolein home health care?
While home health care is a team effort, the physician has several crucial roles. First, he or she must make the determination that home care is appropriate and feasible for a particular patient. Appropriate, meaning there is evidence that this patient is likely to benefit from home care. Feasible, meaning there are resources available in the community and family to safely care for the patient at home. “Often a house call will serve as the first step in developing a home-based-management plan.”32
Continue to: Second, the physician serves...
Second, the physician serves an important role in directing and coordinating the team of professionals involved. This primarily means helping the team to communicate with one another. Before home visits begin, the physician’s office should reach out not only to the patient and family, but also to any other health care personnel involved in the patient’s home care. Otherwise, many of the health care providers involved will never have face-to-face interaction with the physician. Creation of the coordinated health team minimizes duplication and miscommunication; it also builds a valuable bond.
How does one go about making a home visit?
Scheduling. What often works best in a busy practice is to schedule home visits for the end of the workday or to devote an entire afternoon to making home visits to several patients in one locale. Also important is scheduling times, if possible, when important family members or other caregivers are at home or when other members of the home care team can accompany you.
What to bring along. Carry a “home visit bag” that includes equipment you’re likely to need and that is not available away from your office. A minimally equipped visit bag would include different-sized blood pressure cuffs, a glucometer, a pulse oximeter, thermometers, and patient education materials. Other suggested contents are listed in TABLE 1.

Dos and don’ts. Take a few minutes when you first arrive to simply visit with the patient. Sit down and introduce yourself and any members of the home care team that the patient has not met. Take an interim history. While you’re doing this, be observant: Is the home neat or cluttered? Is the indoor temperature comfortable? Are there fall hazards? Is there a smell of cigarette smoke? Are there any indoor combustion sources (eg, wood stove or kerosene heater)? Ask questions such as: Who lives here with you? Can you show me where you keep your medicines? (If the patient keeps insulin or any other medicines in the refrigerator, ask to see it. Note any apparent food scarcity.)
During your exam, pay particular attention to whether vital signs are appreciably different than those measured in the office or hospital. Pay special attention to the patient’s functional abilities. “A subtle, but critical distinction between medical management in the home and medical management in the hospital, clinic, or office is the emphasis on the patient’s functional abilities, family assistance, and environmental factors.”33
Observe the patient’s use of any home technology, if possible; this can be as simple as home oxygenation or as complex as home hemodialysis. Assess for any apparent caregiver stress. Finally, don’t neglect to offer appropriate emotional and spiritual support to the patient and family and to schedule the next follow-up visit before you leave.
Continue to: Documentation and reimbursement.
Documentation and reimbursement. While individual electronic medical records may require use of particular forms of documentation, using a home visit template when possible can be extremely helpful (TABLE 2). A template not only assures thoroughness and consistency (pharmacy, home health contacts, billing information) but also serves as a prompt to survey the patient and the caregivers about nonmedical, but essential, social and well-being services. The document should be as simple and user-friendly as possible.

Not all assessments will be able to be done at each visit but seeing them listed in the template can be helpful. Billing follows the same principles as for office visits and has similar requirements for documentation. Codes for the most common types of home visits are listed in TABLE 3.
Where can I get help?
Graduates of family medicine residency programs are required to receive training in home visits by the Accreditation Council for Graduate Medical Education (ACGME). Current ACGME program requirements stipulate that “residents must demonstrate competence to independently diagnose, manage, and integrate the care of patients of all ages in various outpatient settings, including the FMP [family medicine practice] site and home environment,” and “residents must be primarily responsible for a panel of continuity patients, integrating each patient’s care across all settings, including the home ...” [emphasis added].34

For those already in practice, one of the hardest parts of doing home visits is feeling alone, especially if few other providers in your community engage in home care. As you run into questions and challenges with incorporating home care of patients into your practice, one excellent resource is the American Academy of Home Care Medicine (www.aahcm.org/). Founded in 1988 and headquartered in Chicago, it not only provides numerous helpful resources, but serves as a networking tool for physicians involved in home care.
This unprecedented pandemichas allowed home visits to shine
As depicted in our opening patient case, patients who have high-risk conditions and those who are older than 65 years of age may be cared for more appropriately in a home visit rather than having them come to the office. Home visits may also be a way for providers to “lay eyes” on patients who do not have technology available to participate in virtual visits.
Before performing a home visit, inquire as to whether the patient has symptoms of COVID-19. Adequate PPE should be donned at all times and social distancing should be practiced when appropriate. With adequate PPE, home visits may also allow providers to care for low-risk patients known to have COVID-19 and thereby minimize risks to staff and other patients in the office. JFP
CORRESPONDENCE
Curt Elliott, MD, Prisma Health USC Family Medicine Center, 3209 Colonial Drive, Columbia, SC 29203; [email protected].
1. Unwin BK, Tatum PE. House calls. Am Fam Physician. 2011;83:925-938.
3. Sairenji T, Jetty A, Peterson LE. Shifting patterns of physician home visits. J Prim Care Community Health. 2016;7:71-75.
4. Ornstein KA, Leff B, Covinsky K, et al. Epidemiology of the homebound population in the United States. JAMA Intern Med. 2015;175;1180-1186.
5. CMS. Current Procedural Terminology, Fourth Edition ("CPT®"). www.cms.gov/apps/physician-fee-schedule/license-agreement.aspx. Accessed November 30, 2020.
6. Elkan R, Kendrick D, Dewey M, et al. Effectiveness of home based support for older people: systematic review and meta-analysis. BMJ. 2001;323:719-725.
7. Stuck AE, Egger M, Hammer A, et al. Home visits to prevent nursing home admission and functional decline in elderly people: systematic review and meta-regression analysis. JAMA. 2002;287:1022-1028.
8. Stall N, Nowaczynski M, Sinha SK. Systematic review of outcomes from home-based primary care programs for homebound older adults. J Am Geriatr Soc. 2014;62:2243-2251.
9. Prosman GJ, Lo Fo Wong SH, van der Wouden JC, et al. Effectiveness of home visiting in reducing partner violence for families experiencing abuse: a systematic review. Fam Pract. 2015;32:247-256.
10. Han L, Ma Y, Wei S, et al. Are home visits an effective method for diabetes management? A quantitative systematic review and meta-analysis. J Diabetes Investig. 2017;8:701-708.
11. McWhinney IR. Fourth annual Nicholas J. Pisacano Lecture. The doctor, the patient, and the home: returning to our roots. J Am Board Fam Pract. 1997;10:430-435.
12. Kao H, Conant R, Soriano T, et al. The past, present, and future of house calls. Clin Geriatr Med. 2009;25:19-34.
13. American Public Health Association. Community health workers. www.apha.org/apha-communities/member-sections/community-health-workers. Accessed November 30, 2020.
14. Campbell JD, Brooks M, Hosokawa P, et al. Community health worker home visits for Medicaid-enrolled children with asthma: effects on asthma outcomes and costs. Am J Public Health. 2015;105:2366-2372.
15. Anugu M, Braksmajer A, Huang J, et al. Enriched medical home intervention using community health worker home visitation and ED use. Pediatrics. 2017;139:e20161849.
16. Reckrey JM, Gettenberg G, Ross H, et al. The critical role of social workers in home-based primary care. Soc Work in Health Care. 2014;53:330-343.
17. Cohen-Mansfield J, Shmotkin D, Hazan H. The effect of homebound status on older persons. J Am Geriatr Soc. 2010;58:2358-2362.
18. Mt. Sinai Visiting Doctors Program. www.mountsinai.org/care/primary-care/upper-east-side/visiting-doctors/about. Accessed November 30, 2020.
19. Ornstein K, Hernandez CR, DeCherrie LV, et al. The Mount Sinai (New York) Visiting Doctors Program: meeting the needs of the urban homebound population. Care Manag J. 2011;12:159-163.
20. Ornstein K, Smith K, Boal J. Understanding and improving the burden and unmet needs of informal caregivers of homebound patients enrolled in a home-based primary care program. J Appl Gerontol. 2009;28:482-503.
21. Novak M, Guest C. Application of a multidimensional caregiver burden inventory. Gerontologist. 1989;29:798-803.
22. Cruz J, Brooks D, Marques A. Home telemonitoring effectiveness in COPD: a systematic review. Int J Clin Pract. 2014;68:369-378.
23. Antoniades NC, Rochford PD, Pretto JJ, et al. Pilot study of remote telemonitoring in COPD. Telemed J E Health. 2012;18:634-640.
24. Koff PB, Jones RH, Cashman JM, et al. Proactive integrated care improves quality of life in patients with COPD. Eur Respir J. 2009;33:1031-1038.
25. Inglis SC, Clark RA, McAlister FA, et al. Which components of heart failure programmes are effective? A systematic review and meta-analysis of the outcomes of structured telephone support or telemonitoring as the primary component of chronic heart failure management in 8323 patients: abridged Cochrane review. Eur J Heart Fail. 2011;13:1028-1040.
26. Koehler F, Koehler K, Deckwart O, et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet. 2018;392:1047-1057.
27. Ticona L, Schulman KA. Extreme home makeover–the role of intensive home health care. New Eng J Med. 2016;375:1707-1709.
28. Caplan GA. A meta-analysis of “hospital in the home.” Med J Aust. 2013;198:195-196.
29. Leff B, Burton L, Mader SL, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med. 2005;143:798-808.
30. Shepperd S, Iliffe S, Doll HA, et al. Admission avoidance hospital at home. Cochrane Database Syst Rev. 2016;9:CD007491.
31. Levine DM, Ouchi K, Blanchfield B, et al. Hospital-level care at home for acutely ill adults: a randomized controlled trial. Ann Intern Med. 2020;172:77-85.
32. Cornwell T and Schwartzberg JG, eds. Medical Management of the Home Care Patient: Guidelines for Physicians. 4th ed. Chicago, IL: American Medical Association and American Academy of Home Care Physicians; 2012:p18.
33. Cornwell T and Schwartzberg JG, eds. Medical Management of the Home Care Patient: Guidelines for Physicians. 4th ed. Chicago, IL: American Medical Association and American Academy of Home Care Physicians; 2012:p19.
34. Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Family Medicine. www.acgme.org/Portals/0/PFAssets/ProgramRequirements/120_FamilyMedicine_2020.pdf. (section IV.C.1.b). Accessed November 30, 2020.
1. Unwin BK, Tatum PE. House calls. Am Fam Physician. 2011;83:925-938.
3. Sairenji T, Jetty A, Peterson LE. Shifting patterns of physician home visits. J Prim Care Community Health. 2016;7:71-75.
4. Ornstein KA, Leff B, Covinsky K, et al. Epidemiology of the homebound population in the United States. JAMA Intern Med. 2015;175;1180-1186.
5. CMS. Current Procedural Terminology, Fourth Edition ("CPT®"). www.cms.gov/apps/physician-fee-schedule/license-agreement.aspx. Accessed November 30, 2020.
6. Elkan R, Kendrick D, Dewey M, et al. Effectiveness of home based support for older people: systematic review and meta-analysis. BMJ. 2001;323:719-725.
7. Stuck AE, Egger M, Hammer A, et al. Home visits to prevent nursing home admission and functional decline in elderly people: systematic review and meta-regression analysis. JAMA. 2002;287:1022-1028.
8. Stall N, Nowaczynski M, Sinha SK. Systematic review of outcomes from home-based primary care programs for homebound older adults. J Am Geriatr Soc. 2014;62:2243-2251.
9. Prosman GJ, Lo Fo Wong SH, van der Wouden JC, et al. Effectiveness of home visiting in reducing partner violence for families experiencing abuse: a systematic review. Fam Pract. 2015;32:247-256.
10. Han L, Ma Y, Wei S, et al. Are home visits an effective method for diabetes management? A quantitative systematic review and meta-analysis. J Diabetes Investig. 2017;8:701-708.
11. McWhinney IR. Fourth annual Nicholas J. Pisacano Lecture. The doctor, the patient, and the home: returning to our roots. J Am Board Fam Pract. 1997;10:430-435.
12. Kao H, Conant R, Soriano T, et al. The past, present, and future of house calls. Clin Geriatr Med. 2009;25:19-34.
13. American Public Health Association. Community health workers. www.apha.org/apha-communities/member-sections/community-health-workers. Accessed November 30, 2020.
14. Campbell JD, Brooks M, Hosokawa P, et al. Community health worker home visits for Medicaid-enrolled children with asthma: effects on asthma outcomes and costs. Am J Public Health. 2015;105:2366-2372.
15. Anugu M, Braksmajer A, Huang J, et al. Enriched medical home intervention using community health worker home visitation and ED use. Pediatrics. 2017;139:e20161849.
16. Reckrey JM, Gettenberg G, Ross H, et al. The critical role of social workers in home-based primary care. Soc Work in Health Care. 2014;53:330-343.
17. Cohen-Mansfield J, Shmotkin D, Hazan H. The effect of homebound status on older persons. J Am Geriatr Soc. 2010;58:2358-2362.
18. Mt. Sinai Visiting Doctors Program. www.mountsinai.org/care/primary-care/upper-east-side/visiting-doctors/about. Accessed November 30, 2020.
19. Ornstein K, Hernandez CR, DeCherrie LV, et al. The Mount Sinai (New York) Visiting Doctors Program: meeting the needs of the urban homebound population. Care Manag J. 2011;12:159-163.
20. Ornstein K, Smith K, Boal J. Understanding and improving the burden and unmet needs of informal caregivers of homebound patients enrolled in a home-based primary care program. J Appl Gerontol. 2009;28:482-503.
21. Novak M, Guest C. Application of a multidimensional caregiver burden inventory. Gerontologist. 1989;29:798-803.
22. Cruz J, Brooks D, Marques A. Home telemonitoring effectiveness in COPD: a systematic review. Int J Clin Pract. 2014;68:369-378.
23. Antoniades NC, Rochford PD, Pretto JJ, et al. Pilot study of remote telemonitoring in COPD. Telemed J E Health. 2012;18:634-640.
24. Koff PB, Jones RH, Cashman JM, et al. Proactive integrated care improves quality of life in patients with COPD. Eur Respir J. 2009;33:1031-1038.
25. Inglis SC, Clark RA, McAlister FA, et al. Which components of heart failure programmes are effective? A systematic review and meta-analysis of the outcomes of structured telephone support or telemonitoring as the primary component of chronic heart failure management in 8323 patients: abridged Cochrane review. Eur J Heart Fail. 2011;13:1028-1040.
26. Koehler F, Koehler K, Deckwart O, et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet. 2018;392:1047-1057.
27. Ticona L, Schulman KA. Extreme home makeover–the role of intensive home health care. New Eng J Med. 2016;375:1707-1709.
28. Caplan GA. A meta-analysis of “hospital in the home.” Med J Aust. 2013;198:195-196.
29. Leff B, Burton L, Mader SL, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med. 2005;143:798-808.
30. Shepperd S, Iliffe S, Doll HA, et al. Admission avoidance hospital at home. Cochrane Database Syst Rev. 2016;9:CD007491.
31. Levine DM, Ouchi K, Blanchfield B, et al. Hospital-level care at home for acutely ill adults: a randomized controlled trial. Ann Intern Med. 2020;172:77-85.
32. Cornwell T and Schwartzberg JG, eds. Medical Management of the Home Care Patient: Guidelines for Physicians. 4th ed. Chicago, IL: American Medical Association and American Academy of Home Care Physicians; 2012:p18.
33. Cornwell T and Schwartzberg JG, eds. Medical Management of the Home Care Patient: Guidelines for Physicians. 4th ed. Chicago, IL: American Medical Association and American Academy of Home Care Physicians; 2012:p19.
34. Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Family Medicine. www.acgme.org/Portals/0/PFAssets/ProgramRequirements/120_FamilyMedicine_2020.pdf. (section IV.C.1.b). Accessed November 30, 2020.
PRACTICE RECOMMENDATIONS
❯ Consider incorporating home visits into the primary care of select vulnerable patients because doing so improves clinical outcomes, including mortality rates in neonates and elders. A
❯ Employ team-based home care and include community health workers, nurses, pharmacists, social workers, chaplains, and others. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A multicenter trial of vena cava filters in severely injured patients
Background: Venous thromboembolism and pulmonary embolism are common after major trauma. Anticoagulant prophylaxis usually is not considered because of the increased risk of bleeding. Despite the limited data, many trauma centers use inferior vena cava (IVC) filters as a primary means to prevent pulmonary embolism.
Study design: Randomized, controlled, and multicenter trial.
Setting: Four tertiary hospitals in Australia.
Synopsis: 240 major trauma patients were randomly assigned to receive either IVC filter or no IVC filter within 72 hours after admission. The primary endpoint was a composite of 90-day mortality or symptomatic pulmonary embolism confirmed on imaging. There was no difference in the rate of composite outcome in those with IVC filter, compared with those with no IVC filter.
Bottom line: After major trauma, early prophylactic placement of IVC filter did not reduce the 90-day mortality or incidence of symptomatic pulmonary embolism.
Citation: Ho KM et al. A multicenter trial of vena cava filters in severely injured patients. N Engl J Med. 2019 Jul 25;381:328-37.
Dr. Hoque Sharmy is a hospitalist and assistant professor of medicine in the division of hospital medicine at St. Louis University School of Medicine.
Background: Venous thromboembolism and pulmonary embolism are common after major trauma. Anticoagulant prophylaxis usually is not considered because of the increased risk of bleeding. Despite the limited data, many trauma centers use inferior vena cava (IVC) filters as a primary means to prevent pulmonary embolism.
Study design: Randomized, controlled, and multicenter trial.
Setting: Four tertiary hospitals in Australia.
Synopsis: 240 major trauma patients were randomly assigned to receive either IVC filter or no IVC filter within 72 hours after admission. The primary endpoint was a composite of 90-day mortality or symptomatic pulmonary embolism confirmed on imaging. There was no difference in the rate of composite outcome in those with IVC filter, compared with those with no IVC filter.
Bottom line: After major trauma, early prophylactic placement of IVC filter did not reduce the 90-day mortality or incidence of symptomatic pulmonary embolism.
Citation: Ho KM et al. A multicenter trial of vena cava filters in severely injured patients. N Engl J Med. 2019 Jul 25;381:328-37.
Dr. Hoque Sharmy is a hospitalist and assistant professor of medicine in the division of hospital medicine at St. Louis University School of Medicine.
Background: Venous thromboembolism and pulmonary embolism are common after major trauma. Anticoagulant prophylaxis usually is not considered because of the increased risk of bleeding. Despite the limited data, many trauma centers use inferior vena cava (IVC) filters as a primary means to prevent pulmonary embolism.
Study design: Randomized, controlled, and multicenter trial.
Setting: Four tertiary hospitals in Australia.
Synopsis: 240 major trauma patients were randomly assigned to receive either IVC filter or no IVC filter within 72 hours after admission. The primary endpoint was a composite of 90-day mortality or symptomatic pulmonary embolism confirmed on imaging. There was no difference in the rate of composite outcome in those with IVC filter, compared with those with no IVC filter.
Bottom line: After major trauma, early prophylactic placement of IVC filter did not reduce the 90-day mortality or incidence of symptomatic pulmonary embolism.
Citation: Ho KM et al. A multicenter trial of vena cava filters in severely injured patients. N Engl J Med. 2019 Jul 25;381:328-37.
Dr. Hoque Sharmy is a hospitalist and assistant professor of medicine in the division of hospital medicine at St. Louis University School of Medicine.
COVID-19 and risk of clotting: ‘Be proactive about prevention’
The risk of arterial and venous thrombosis in patients with COVID-19 has been a major issue throughout the pandemic, and how best to manage this risk is the subject of a new review article.
The article, by Gregory Dr. Piazza, MD, and David A. Morrow, MD, Brigham and Women’s Hospital, Boston, was published online in JAMA on Nov. 23.
“Basically we’re saying: ‘Be proactive about prevention,’” Dr. Piazza told this news organization.
There is growing recognition among those on the frontline that there is an increased risk of thrombosis in COVID-19 patients, Dr. Piazza said. The risk is highest in patients in the intensive care unit, but the risk is also increased in patients hospitalized with COVID-19, even those not in ICU.
“We don’t really know what the risk is in nonhospitalized COVID-19 patients, but we think it’s much lower than in those who are hospitalized,” he said. “We are waiting for data on the optimal way of managing this increased risk of thrombosis in COVID patients, but for the time being, we believe a systematic way of addressing this risk is best, with every patient hospitalized with COVID-19 receiving some type of thromboprophylaxis. This would mainly be with anticoagulation, but in patients in whom anticoagulation is contraindicated, then mechanical methods could be used, such as pneumatic compression boots or compression stockings.”
The authors report thrombotic complication rates of 2.6% in noncritically ill hospitalized patients with COVID-19 and 35.3% in critically ill patients from a recent U.S. registry study.
Autopsy findings of microthrombi in multiple organ systems, including the lungs, heart, and kidneys, suggest that thrombosis may contribute to multisystem organ dysfunction in severe COVID-19, they note. Although the pathophysiology is not fully defined, prothrombotic abnormalities have been identified in patients with COVID-19, including elevated levels of D-dimer, fibrinogen, and factor VIII, they add.
“There are several major questions about which COVID-19 patients to treat with thromboprophylaxis, how to treat them in term of levels of anticoagulation, and there are many ongoing clinical trials to try and answer these questions,” Dr. Piazza commented. “We need results from these randomized trials to provide a better compass for COVID-19 patients at risk of clotting.”
At present, clinicians can follow two different sets of guidelines on the issue, one from the American College of Chest Physicians and the other from the International Society on Thrombosis and Hemostasis, the authors note.
“The ACCP guidelines are very conservative and basically follow the evidence base for medical patients, while the ISTH guidelines are more aggressive and recommend increased levels of anticoagulation in both ICU and hospitalized non-ICU patients and also extend prophylaxis after discharge,” Dr. Piazza said.
“There is quite a difference between the two sets of guidelines, which can be a point of confusion,” he added.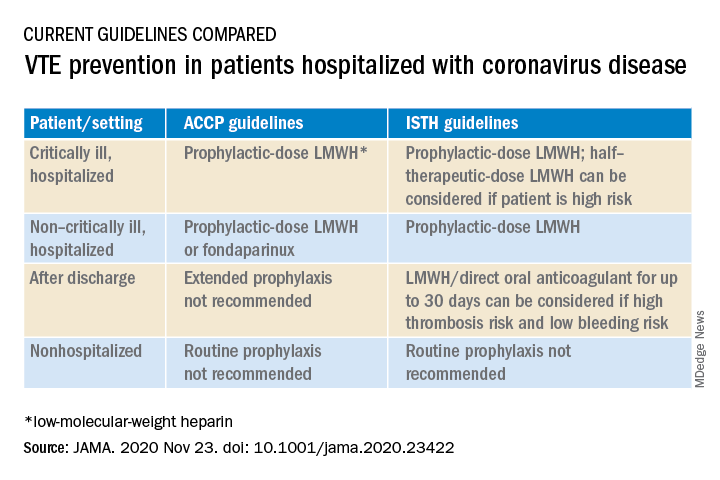
Dr. Piazza notes that at his center every hospitalized COVID patient who does not have a contraindication to anticoagulation receives a standard prophylactic dose of a once-daily low-molecular-weight heparin (for example, enoxaparin 40 mg). A once-daily product is used to minimize infection risk to staff.
While all COVID patients in the ICU should automatically receive some anticoagulation, the optimal dose is an area of active investigation, he explained. “There were several early reports of ICU patients developing blood clots despite receiving standard thromboprophylaxis so perhaps we need to use higher doses. There are trials underway looking at this, and we would advise enrolling patients into these trials.”
If patients can’t be enrolled into trials, and clinicians feel higher anticoagulation levels are needed, Dr. Piazza advises following the ISTH guidance, which allows an intermediate dose of low-molecular-weight heparin (up to 1 mg/kg enoxaparin).
“Some experts are suggesting even higher doses may be needed in some ICU patients, such as the full therapeutic dose, but I worry about the risk of bleeding with such a strategy,” he said.
Dr. Piazza says they do not routinely give anticoagulation after discharge, but if this is desired then patients could be switched to an oral agent, and some of the direct-acting oral anticoagulants are approved for prophylactic use in medically ill patients.
Dr. Piazza points out that whether thromboprophylaxis should be used for nonhospitalized COVID patients who have risk factors for clotting such as a prior history of thrombosis or obesity is a pressing question, and he encourages clinicians to enroll these patients in clinical trials evaluating this issue, such as the PREVENT-HD trial.
“If they can’t enroll patents in a trial, then they have to make a decision whether the patient is high-enough risk to justify off-label use of anticoagulant. There is a case to be made for this, but there is no evidence for or against such action at present,” he noted.
At this time, neither the ISTH nor ACCP recommend measuring D-dimer to screen for venous thromboembolism or to determine intensity of prophylaxis or treatment, the authors note.
“Ongoing investigation will determine optimal preventive regimens in COVID-19 in the intensive care unit, at hospital discharge, and in nonhospitalized patients at high risk for thrombosis,” they conclude.
Dr. Piazza reported grants from Bayer, Bristol Myers Squibb, Boston Scientific, Janssen, and Portola, and personal fees from Agile, Amgen, Pfizer, and the Prairie Education and Research Cooperative outside the submitted work. Dr. Morrow reported grants from Abbott Laboratories, Amgen, Anthos Therapeutics, Esai, GlaxoSmithKline, Takeda, and The Medicines Company; grants and personal fees from AstraZeneca, Merck, Novartis, and Roche Diagnostics; and personal fees from Bayer Pharma and InCarda outside the submitted work.
A version of this article originally appeared on Medscape.com.
The risk of arterial and venous thrombosis in patients with COVID-19 has been a major issue throughout the pandemic, and how best to manage this risk is the subject of a new review article.
The article, by Gregory Dr. Piazza, MD, and David A. Morrow, MD, Brigham and Women’s Hospital, Boston, was published online in JAMA on Nov. 23.
“Basically we’re saying: ‘Be proactive about prevention,’” Dr. Piazza told this news organization.
There is growing recognition among those on the frontline that there is an increased risk of thrombosis in COVID-19 patients, Dr. Piazza said. The risk is highest in patients in the intensive care unit, but the risk is also increased in patients hospitalized with COVID-19, even those not in ICU.
“We don’t really know what the risk is in nonhospitalized COVID-19 patients, but we think it’s much lower than in those who are hospitalized,” he said. “We are waiting for data on the optimal way of managing this increased risk of thrombosis in COVID patients, but for the time being, we believe a systematic way of addressing this risk is best, with every patient hospitalized with COVID-19 receiving some type of thromboprophylaxis. This would mainly be with anticoagulation, but in patients in whom anticoagulation is contraindicated, then mechanical methods could be used, such as pneumatic compression boots or compression stockings.”
The authors report thrombotic complication rates of 2.6% in noncritically ill hospitalized patients with COVID-19 and 35.3% in critically ill patients from a recent U.S. registry study.
Autopsy findings of microthrombi in multiple organ systems, including the lungs, heart, and kidneys, suggest that thrombosis may contribute to multisystem organ dysfunction in severe COVID-19, they note. Although the pathophysiology is not fully defined, prothrombotic abnormalities have been identified in patients with COVID-19, including elevated levels of D-dimer, fibrinogen, and factor VIII, they add.
“There are several major questions about which COVID-19 patients to treat with thromboprophylaxis, how to treat them in term of levels of anticoagulation, and there are many ongoing clinical trials to try and answer these questions,” Dr. Piazza commented. “We need results from these randomized trials to provide a better compass for COVID-19 patients at risk of clotting.”
At present, clinicians can follow two different sets of guidelines on the issue, one from the American College of Chest Physicians and the other from the International Society on Thrombosis and Hemostasis, the authors note.
“The ACCP guidelines are very conservative and basically follow the evidence base for medical patients, while the ISTH guidelines are more aggressive and recommend increased levels of anticoagulation in both ICU and hospitalized non-ICU patients and also extend prophylaxis after discharge,” Dr. Piazza said.
“There is quite a difference between the two sets of guidelines, which can be a point of confusion,” he added.
Dr. Piazza notes that at his center every hospitalized COVID patient who does not have a contraindication to anticoagulation receives a standard prophylactic dose of a once-daily low-molecular-weight heparin (for example, enoxaparin 40 mg). A once-daily product is used to minimize infection risk to staff.
While all COVID patients in the ICU should automatically receive some anticoagulation, the optimal dose is an area of active investigation, he explained. “There were several early reports of ICU patients developing blood clots despite receiving standard thromboprophylaxis so perhaps we need to use higher doses. There are trials underway looking at this, and we would advise enrolling patients into these trials.”
If patients can’t be enrolled into trials, and clinicians feel higher anticoagulation levels are needed, Dr. Piazza advises following the ISTH guidance, which allows an intermediate dose of low-molecular-weight heparin (up to 1 mg/kg enoxaparin).
“Some experts are suggesting even higher doses may be needed in some ICU patients, such as the full therapeutic dose, but I worry about the risk of bleeding with such a strategy,” he said.
Dr. Piazza says they do not routinely give anticoagulation after discharge, but if this is desired then patients could be switched to an oral agent, and some of the direct-acting oral anticoagulants are approved for prophylactic use in medically ill patients.
Dr. Piazza points out that whether thromboprophylaxis should be used for nonhospitalized COVID patients who have risk factors for clotting such as a prior history of thrombosis or obesity is a pressing question, and he encourages clinicians to enroll these patients in clinical trials evaluating this issue, such as the PREVENT-HD trial.
“If they can’t enroll patents in a trial, then they have to make a decision whether the patient is high-enough risk to justify off-label use of anticoagulant. There is a case to be made for this, but there is no evidence for or against such action at present,” he noted.
At this time, neither the ISTH nor ACCP recommend measuring D-dimer to screen for venous thromboembolism or to determine intensity of prophylaxis or treatment, the authors note.
“Ongoing investigation will determine optimal preventive regimens in COVID-19 in the intensive care unit, at hospital discharge, and in nonhospitalized patients at high risk for thrombosis,” they conclude.
Dr. Piazza reported grants from Bayer, Bristol Myers Squibb, Boston Scientific, Janssen, and Portola, and personal fees from Agile, Amgen, Pfizer, and the Prairie Education and Research Cooperative outside the submitted work. Dr. Morrow reported grants from Abbott Laboratories, Amgen, Anthos Therapeutics, Esai, GlaxoSmithKline, Takeda, and The Medicines Company; grants and personal fees from AstraZeneca, Merck, Novartis, and Roche Diagnostics; and personal fees from Bayer Pharma and InCarda outside the submitted work.
A version of this article originally appeared on Medscape.com.
The risk of arterial and venous thrombosis in patients with COVID-19 has been a major issue throughout the pandemic, and how best to manage this risk is the subject of a new review article.
The article, by Gregory Dr. Piazza, MD, and David A. Morrow, MD, Brigham and Women’s Hospital, Boston, was published online in JAMA on Nov. 23.
“Basically we’re saying: ‘Be proactive about prevention,’” Dr. Piazza told this news organization.
There is growing recognition among those on the frontline that there is an increased risk of thrombosis in COVID-19 patients, Dr. Piazza said. The risk is highest in patients in the intensive care unit, but the risk is also increased in patients hospitalized with COVID-19, even those not in ICU.
“We don’t really know what the risk is in nonhospitalized COVID-19 patients, but we think it’s much lower than in those who are hospitalized,” he said. “We are waiting for data on the optimal way of managing this increased risk of thrombosis in COVID patients, but for the time being, we believe a systematic way of addressing this risk is best, with every patient hospitalized with COVID-19 receiving some type of thromboprophylaxis. This would mainly be with anticoagulation, but in patients in whom anticoagulation is contraindicated, then mechanical methods could be used, such as pneumatic compression boots or compression stockings.”
The authors report thrombotic complication rates of 2.6% in noncritically ill hospitalized patients with COVID-19 and 35.3% in critically ill patients from a recent U.S. registry study.
Autopsy findings of microthrombi in multiple organ systems, including the lungs, heart, and kidneys, suggest that thrombosis may contribute to multisystem organ dysfunction in severe COVID-19, they note. Although the pathophysiology is not fully defined, prothrombotic abnormalities have been identified in patients with COVID-19, including elevated levels of D-dimer, fibrinogen, and factor VIII, they add.
“There are several major questions about which COVID-19 patients to treat with thromboprophylaxis, how to treat them in term of levels of anticoagulation, and there are many ongoing clinical trials to try and answer these questions,” Dr. Piazza commented. “We need results from these randomized trials to provide a better compass for COVID-19 patients at risk of clotting.”
At present, clinicians can follow two different sets of guidelines on the issue, one from the American College of Chest Physicians and the other from the International Society on Thrombosis and Hemostasis, the authors note.
“The ACCP guidelines are very conservative and basically follow the evidence base for medical patients, while the ISTH guidelines are more aggressive and recommend increased levels of anticoagulation in both ICU and hospitalized non-ICU patients and also extend prophylaxis after discharge,” Dr. Piazza said.
“There is quite a difference between the two sets of guidelines, which can be a point of confusion,” he added.
Dr. Piazza notes that at his center every hospitalized COVID patient who does not have a contraindication to anticoagulation receives a standard prophylactic dose of a once-daily low-molecular-weight heparin (for example, enoxaparin 40 mg). A once-daily product is used to minimize infection risk to staff.
While all COVID patients in the ICU should automatically receive some anticoagulation, the optimal dose is an area of active investigation, he explained. “There were several early reports of ICU patients developing blood clots despite receiving standard thromboprophylaxis so perhaps we need to use higher doses. There are trials underway looking at this, and we would advise enrolling patients into these trials.”
If patients can’t be enrolled into trials, and clinicians feel higher anticoagulation levels are needed, Dr. Piazza advises following the ISTH guidance, which allows an intermediate dose of low-molecular-weight heparin (up to 1 mg/kg enoxaparin).
“Some experts are suggesting even higher doses may be needed in some ICU patients, such as the full therapeutic dose, but I worry about the risk of bleeding with such a strategy,” he said.
Dr. Piazza says they do not routinely give anticoagulation after discharge, but if this is desired then patients could be switched to an oral agent, and some of the direct-acting oral anticoagulants are approved for prophylactic use in medically ill patients.
Dr. Piazza points out that whether thromboprophylaxis should be used for nonhospitalized COVID patients who have risk factors for clotting such as a prior history of thrombosis or obesity is a pressing question, and he encourages clinicians to enroll these patients in clinical trials evaluating this issue, such as the PREVENT-HD trial.
“If they can’t enroll patents in a trial, then they have to make a decision whether the patient is high-enough risk to justify off-label use of anticoagulant. There is a case to be made for this, but there is no evidence for or against such action at present,” he noted.
At this time, neither the ISTH nor ACCP recommend measuring D-dimer to screen for venous thromboembolism or to determine intensity of prophylaxis or treatment, the authors note.
“Ongoing investigation will determine optimal preventive regimens in COVID-19 in the intensive care unit, at hospital discharge, and in nonhospitalized patients at high risk for thrombosis,” they conclude.
Dr. Piazza reported grants from Bayer, Bristol Myers Squibb, Boston Scientific, Janssen, and Portola, and personal fees from Agile, Amgen, Pfizer, and the Prairie Education and Research Cooperative outside the submitted work. Dr. Morrow reported grants from Abbott Laboratories, Amgen, Anthos Therapeutics, Esai, GlaxoSmithKline, Takeda, and The Medicines Company; grants and personal fees from AstraZeneca, Merck, Novartis, and Roche Diagnostics; and personal fees from Bayer Pharma and InCarda outside the submitted work.
A version of this article originally appeared on Medscape.com.
Excess antibiotics and adverse events in patients with pneumonia
Background: Past surveys of providers revealed a tendency to select longer durations of antibiotics to reduce disease recurrence, but recent studies have shown that shorter courses of antibiotics are safe and equally effective in treatment for pneumonia. In addition, there has been a renewed focus on reducing unnecessary use of antibiotics to decrease adverse effects.
Study design: Retrospective cohort study.
Setting: 43 hospitals in the Michigan Hospital Medicine Safety Consortium.
Synopsis: A retrospective chart review of 6,481 patients hospitalized with pneumonia revealed that 67.8% of patients received excessive days of antibiotic treatment. On average, patients received 2 days of excessive treatment and 93.2% of the additional days came in the form of antibiotics prescribed at discharge.
Excessive treatment was defined as more than 5 days for community-acquired pneumonia (CAP) and more than 7 days for health care–associated pneumonia, methicillin-resistant Staphylococcus aureus, or gram-negative organisms. The authors adjusted for time to clinical stability when defining the expected duration of treatment.
After statistical adjustment, excess antibiotic days were not associated with increased rates of C. diff infection, emergency department visits, readmission, or 30-day mortality. Additional treatment was associated with increased patient-reported adverse effects including diarrhea, gastrointestinal distress, and mucosal candidiasis.
The impact of this study is limited by a few factors. The study was observational and relied on provider documentation and patient reporting of adverse events. Also, it was published prior to updates to the Infectious Diseases Society of America CAP guidelines, which may affect how it will be interpreted once those guidelines are released.
Bottom line: Adherence to the shortest effective duration of antibiotic treatment for pneumonia may lead to a reduction in the rates of patient reported adverse effects while not impacting treatment success.
Citation: Vaughn VM et al. Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: A multihospital cohort study. Ann Intern Med. 2019 Aug 6;171(3):153-63.
Dr. Purdy is a hospitalist and assistant professor of internal medicine at St. Louis University School of Medicine.
Background: Past surveys of providers revealed a tendency to select longer durations of antibiotics to reduce disease recurrence, but recent studies have shown that shorter courses of antibiotics are safe and equally effective in treatment for pneumonia. In addition, there has been a renewed focus on reducing unnecessary use of antibiotics to decrease adverse effects.
Study design: Retrospective cohort study.
Setting: 43 hospitals in the Michigan Hospital Medicine Safety Consortium.
Synopsis: A retrospective chart review of 6,481 patients hospitalized with pneumonia revealed that 67.8% of patients received excessive days of antibiotic treatment. On average, patients received 2 days of excessive treatment and 93.2% of the additional days came in the form of antibiotics prescribed at discharge.
Excessive treatment was defined as more than 5 days for community-acquired pneumonia (CAP) and more than 7 days for health care–associated pneumonia, methicillin-resistant Staphylococcus aureus, or gram-negative organisms. The authors adjusted for time to clinical stability when defining the expected duration of treatment.
After statistical adjustment, excess antibiotic days were not associated with increased rates of C. diff infection, emergency department visits, readmission, or 30-day mortality. Additional treatment was associated with increased patient-reported adverse effects including diarrhea, gastrointestinal distress, and mucosal candidiasis.
The impact of this study is limited by a few factors. The study was observational and relied on provider documentation and patient reporting of adverse events. Also, it was published prior to updates to the Infectious Diseases Society of America CAP guidelines, which may affect how it will be interpreted once those guidelines are released.
Bottom line: Adherence to the shortest effective duration of antibiotic treatment for pneumonia may lead to a reduction in the rates of patient reported adverse effects while not impacting treatment success.
Citation: Vaughn VM et al. Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: A multihospital cohort study. Ann Intern Med. 2019 Aug 6;171(3):153-63.
Dr. Purdy is a hospitalist and assistant professor of internal medicine at St. Louis University School of Medicine.
Background: Past surveys of providers revealed a tendency to select longer durations of antibiotics to reduce disease recurrence, but recent studies have shown that shorter courses of antibiotics are safe and equally effective in treatment for pneumonia. In addition, there has been a renewed focus on reducing unnecessary use of antibiotics to decrease adverse effects.
Study design: Retrospective cohort study.
Setting: 43 hospitals in the Michigan Hospital Medicine Safety Consortium.
Synopsis: A retrospective chart review of 6,481 patients hospitalized with pneumonia revealed that 67.8% of patients received excessive days of antibiotic treatment. On average, patients received 2 days of excessive treatment and 93.2% of the additional days came in the form of antibiotics prescribed at discharge.
Excessive treatment was defined as more than 5 days for community-acquired pneumonia (CAP) and more than 7 days for health care–associated pneumonia, methicillin-resistant Staphylococcus aureus, or gram-negative organisms. The authors adjusted for time to clinical stability when defining the expected duration of treatment.
After statistical adjustment, excess antibiotic days were not associated with increased rates of C. diff infection, emergency department visits, readmission, or 30-day mortality. Additional treatment was associated with increased patient-reported adverse effects including diarrhea, gastrointestinal distress, and mucosal candidiasis.
The impact of this study is limited by a few factors. The study was observational and relied on provider documentation and patient reporting of adverse events. Also, it was published prior to updates to the Infectious Diseases Society of America CAP guidelines, which may affect how it will be interpreted once those guidelines are released.
Bottom line: Adherence to the shortest effective duration of antibiotic treatment for pneumonia may lead to a reduction in the rates of patient reported adverse effects while not impacting treatment success.
Citation: Vaughn VM et al. Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: A multihospital cohort study. Ann Intern Med. 2019 Aug 6;171(3):153-63.
Dr. Purdy is a hospitalist and assistant professor of internal medicine at St. Louis University School of Medicine.
Assessing the impact of glucocorticoids on COVID-19 mortality
Clinical question: Is early glucocorticoid therapy associated with reduced mortality or need for mechanical ventilation in hospitalized patients with SARS-CoV-2 infection?
Background: Glucocorticoids have been used as adjunctive treatment in some infections with inflammatory responses, but their efficacy in COVID-19 infections had not been entirely clear. The RECOVERY trial found a subset of patients with COVID-19 who may benefit from treatment with glucocorticoids. The ideal role of steroids in this infection, and who the subset of patients might be for whom they would benefit, is so far unclear.
Study design: Retrospective cohort analysis.
Setting: Large academic health center in New York.
Synopsis: Researchers analyzed admissions of COVID-19 positive patients hospitalized between March 11, 2020 and April 13, 2020 who did not die or become mechanically ventilated within the first 48 hours of admission. Patients treated with glucocorticoids within 48 hours of admission were compared with patients who were not treated with glucocorticoids during this time frame. In total, 2,998 patients were examined, of whom 1,806 met inclusion criteria, and 140 (7.7%) were treated with glucocorticoids within 48 hours of admission. These treated patients were more likely to have an underlying pulmonary or rheumatologic comorbidity. Early use of glucocorticoids was not associated with in-hospital mortality or mechanical ventilation in either adjusted or unadjusted models. However, if the initial C-reactive protein (CRP) was >20mg/dL, this was associated with a reduced risk of mortality or mechanical ventilation in unadjusted (odds ratio, 0.23; 95% confidence interval, 0.08-0.70) and adjusted analyses for clinical characteristics (adjusted OR, 0.20; 95% CI, 0.06-0.67). Conversely, treatment in patients with CRP <10mg/dL was associated with significantly increased risk of mortality or ventilation during analysis.
Bottom line: Glucocorticoids can benefit patients with significantly elevated CRP but may be harmful to those with lower CRPs.
Citation: Keller MJ et al. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med. 2020;8;489-493. Published online first. 2020 Jul 22. doi:10.12788/jhm.3497.
Dr. Halpern is a med-peds hospitalist at Brigham and Women’s Hospital in Boston.
Clinical question: Is early glucocorticoid therapy associated with reduced mortality or need for mechanical ventilation in hospitalized patients with SARS-CoV-2 infection?
Background: Glucocorticoids have been used as adjunctive treatment in some infections with inflammatory responses, but their efficacy in COVID-19 infections had not been entirely clear. The RECOVERY trial found a subset of patients with COVID-19 who may benefit from treatment with glucocorticoids. The ideal role of steroids in this infection, and who the subset of patients might be for whom they would benefit, is so far unclear.
Study design: Retrospective cohort analysis.
Setting: Large academic health center in New York.
Synopsis: Researchers analyzed admissions of COVID-19 positive patients hospitalized between March 11, 2020 and April 13, 2020 who did not die or become mechanically ventilated within the first 48 hours of admission. Patients treated with glucocorticoids within 48 hours of admission were compared with patients who were not treated with glucocorticoids during this time frame. In total, 2,998 patients were examined, of whom 1,806 met inclusion criteria, and 140 (7.7%) were treated with glucocorticoids within 48 hours of admission. These treated patients were more likely to have an underlying pulmonary or rheumatologic comorbidity. Early use of glucocorticoids was not associated with in-hospital mortality or mechanical ventilation in either adjusted or unadjusted models. However, if the initial C-reactive protein (CRP) was >20mg/dL, this was associated with a reduced risk of mortality or mechanical ventilation in unadjusted (odds ratio, 0.23; 95% confidence interval, 0.08-0.70) and adjusted analyses for clinical characteristics (adjusted OR, 0.20; 95% CI, 0.06-0.67). Conversely, treatment in patients with CRP <10mg/dL was associated with significantly increased risk of mortality or ventilation during analysis.
Bottom line: Glucocorticoids can benefit patients with significantly elevated CRP but may be harmful to those with lower CRPs.
Citation: Keller MJ et al. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med. 2020;8;489-493. Published online first. 2020 Jul 22. doi:10.12788/jhm.3497.
Dr. Halpern is a med-peds hospitalist at Brigham and Women’s Hospital in Boston.
Clinical question: Is early glucocorticoid therapy associated with reduced mortality or need for mechanical ventilation in hospitalized patients with SARS-CoV-2 infection?
Background: Glucocorticoids have been used as adjunctive treatment in some infections with inflammatory responses, but their efficacy in COVID-19 infections had not been entirely clear. The RECOVERY trial found a subset of patients with COVID-19 who may benefit from treatment with glucocorticoids. The ideal role of steroids in this infection, and who the subset of patients might be for whom they would benefit, is so far unclear.
Study design: Retrospective cohort analysis.
Setting: Large academic health center in New York.
Synopsis: Researchers analyzed admissions of COVID-19 positive patients hospitalized between March 11, 2020 and April 13, 2020 who did not die or become mechanically ventilated within the first 48 hours of admission. Patients treated with glucocorticoids within 48 hours of admission were compared with patients who were not treated with glucocorticoids during this time frame. In total, 2,998 patients were examined, of whom 1,806 met inclusion criteria, and 140 (7.7%) were treated with glucocorticoids within 48 hours of admission. These treated patients were more likely to have an underlying pulmonary or rheumatologic comorbidity. Early use of glucocorticoids was not associated with in-hospital mortality or mechanical ventilation in either adjusted or unadjusted models. However, if the initial C-reactive protein (CRP) was >20mg/dL, this was associated with a reduced risk of mortality or mechanical ventilation in unadjusted (odds ratio, 0.23; 95% confidence interval, 0.08-0.70) and adjusted analyses for clinical characteristics (adjusted OR, 0.20; 95% CI, 0.06-0.67). Conversely, treatment in patients with CRP <10mg/dL was associated with significantly increased risk of mortality or ventilation during analysis.
Bottom line: Glucocorticoids can benefit patients with significantly elevated CRP but may be harmful to those with lower CRPs.
Citation: Keller MJ et al. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med. 2020;8;489-493. Published online first. 2020 Jul 22. doi:10.12788/jhm.3497.
Dr. Halpern is a med-peds hospitalist at Brigham and Women’s Hospital in Boston.
FROM THE JOURNAL OF HOSPITAL MEDICINE
Obesity, hypoxia predict severity in children with COVID-19
based on data from 281 patients at 8 locations.
Manifestations of COVID-19 in children include respiratory disease similar to that seen in adults, but the full spectrum of disease in children has been studied mainly in single settings or with a focus on one clinical manifestation, wrote Danielle M. Fernandes, MD, of Albert Einstein College of Medicine, New York, and colleagues.
In a study published in the Journal of Pediatrics, the researchers identified 281 children hospitalized with COVID-19 and/or multisystem inflammatory syndrome in children (MIS-C) at 8 sites in Connecticut, New Jersey, and New York. A total of 143 (51%) had respiratory disease, 69 (25%) had MIS-C, and 69 (25%) had other manifestations of illness including 32 patients with gastrointestinal problems, 21 infants with fever, 6 cases of neurologic disease, 6 cases of diabetic ketoacidosis, and 4 patients with other indications. The median age of the patients was 10 years, 60% were male, 51% were Hispanic, and 23% were non-Hispanic Black. The most common comorbidities were obesity (34%) and asthma (14%).
Independent predictors of disease severity in children found
After controlling for multiple variables, obesity and hypoxia at hospital admission were significant independent predictors of severe respiratory disease, with odds ratios of 3.39 and 4.01, respectively. In addition, lower absolute lymphocyte count (OR, 8.33 per unit decrease in 109 cells/L) and higher C-reactive protein (OR, 1.06 per unit increase in mg/dL) were significantly predictive of severe MIS-C (P = .001 and P = .017, respectively).
“The association between weight and severe respiratory COVID-19 is consistent with the adult literature; however, the mechanisms of this association require further study,” Dr. Fernandes and associates noted.
Overall, children with MIS-C were significantly more likely to be non-Hispanic Black, compared with children with respiratory disease, an 18% difference. However, neither race/ethnicity nor socioeconomic status were significant predictors of disease severity, the researchers wrote.
During the study period, 7 patients (2%) died and 114 (41%) were admitted to the ICU.
“We found a wide array of clinical manifestations in children and youth hospitalized with SARS-CoV-2,” Dr. Fernandes and associates wrote. Notably, gastrointestinal symptoms, ocular symptoms, and dermatologic symptoms have rarely been noted in adults with COVID-19, but occurred in more than 30% of the pediatric patients.
“We also found that SARS-CoV-2 can be an incidental finding in a substantial number of hospitalized pediatric patients,” the researchers said.
The findings were limited by several factors including a population of patients only from Connecticut, New Jersey, and New York, and the possibility that decisions on hospital and ICU admission may have varied by location, the researchers said. In addition, approaches may have varied in the absence of data on the optimal treatment of MIS-C.
“This study builds on the growing body of evidence showing that mortality in hospitalized pediatric patients is low, compared with adults,” Dr. Fernandes and associates said. “However, it highlights that the young population is not universally spared from morbidity, and that even previously healthy children and youth can develop severe disease requiring supportive therapy.”
Findings confirm other clinical experience
The study was important to show that, “although most children are spared severe illness from COVID-19, some children are hospitalized both with acute COVID-19 respiratory disease, with MIS-C and with a range of other complications,” Adrienne Randolph, MD, of Boston Children’s Hospital and Harvard Medical School, Boston, said in an interview.
Dr. Randolph said she was not surprised by the study findings, “as we are also seeing these types of complications at Boston Children’s Hospital where I work.”
Additional research is needed on the outcomes of these patients, “especially the longer-term sequelae of having COVID-19 or MIS-C early in life,” she emphasized.
The take-home message to clinicians from the findings at this time is to be aware that children and adolescents can become severely ill from COVID-19–related complications, said Dr. Randolph. “Some of the laboratory values on presentation appear to be associated with disease severity.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Randolph disclosed funding from the Centers for Disease Control and Prevention to lead the Overcoming COVID-19 Study in U.S. Children and Adults.
SOURCE: Fernandes DM et al. J Pediatr. 2020 Nov 13. doi: 10.1016/j.jpeds.2020.11.016.
based on data from 281 patients at 8 locations.
Manifestations of COVID-19 in children include respiratory disease similar to that seen in adults, but the full spectrum of disease in children has been studied mainly in single settings or with a focus on one clinical manifestation, wrote Danielle M. Fernandes, MD, of Albert Einstein College of Medicine, New York, and colleagues.
In a study published in the Journal of Pediatrics, the researchers identified 281 children hospitalized with COVID-19 and/or multisystem inflammatory syndrome in children (MIS-C) at 8 sites in Connecticut, New Jersey, and New York. A total of 143 (51%) had respiratory disease, 69 (25%) had MIS-C, and 69 (25%) had other manifestations of illness including 32 patients with gastrointestinal problems, 21 infants with fever, 6 cases of neurologic disease, 6 cases of diabetic ketoacidosis, and 4 patients with other indications. The median age of the patients was 10 years, 60% were male, 51% were Hispanic, and 23% were non-Hispanic Black. The most common comorbidities were obesity (34%) and asthma (14%).
Independent predictors of disease severity in children found
After controlling for multiple variables, obesity and hypoxia at hospital admission were significant independent predictors of severe respiratory disease, with odds ratios of 3.39 and 4.01, respectively. In addition, lower absolute lymphocyte count (OR, 8.33 per unit decrease in 109 cells/L) and higher C-reactive protein (OR, 1.06 per unit increase in mg/dL) were significantly predictive of severe MIS-C (P = .001 and P = .017, respectively).
“The association between weight and severe respiratory COVID-19 is consistent with the adult literature; however, the mechanisms of this association require further study,” Dr. Fernandes and associates noted.
Overall, children with MIS-C were significantly more likely to be non-Hispanic Black, compared with children with respiratory disease, an 18% difference. However, neither race/ethnicity nor socioeconomic status were significant predictors of disease severity, the researchers wrote.
During the study period, 7 patients (2%) died and 114 (41%) were admitted to the ICU.
“We found a wide array of clinical manifestations in children and youth hospitalized with SARS-CoV-2,” Dr. Fernandes and associates wrote. Notably, gastrointestinal symptoms, ocular symptoms, and dermatologic symptoms have rarely been noted in adults with COVID-19, but occurred in more than 30% of the pediatric patients.
“We also found that SARS-CoV-2 can be an incidental finding in a substantial number of hospitalized pediatric patients,” the researchers said.
The findings were limited by several factors including a population of patients only from Connecticut, New Jersey, and New York, and the possibility that decisions on hospital and ICU admission may have varied by location, the researchers said. In addition, approaches may have varied in the absence of data on the optimal treatment of MIS-C.
“This study builds on the growing body of evidence showing that mortality in hospitalized pediatric patients is low, compared with adults,” Dr. Fernandes and associates said. “However, it highlights that the young population is not universally spared from morbidity, and that even previously healthy children and youth can develop severe disease requiring supportive therapy.”
Findings confirm other clinical experience
The study was important to show that, “although most children are spared severe illness from COVID-19, some children are hospitalized both with acute COVID-19 respiratory disease, with MIS-C and with a range of other complications,” Adrienne Randolph, MD, of Boston Children’s Hospital and Harvard Medical School, Boston, said in an interview.
Dr. Randolph said she was not surprised by the study findings, “as we are also seeing these types of complications at Boston Children’s Hospital where I work.”
Additional research is needed on the outcomes of these patients, “especially the longer-term sequelae of having COVID-19 or MIS-C early in life,” she emphasized.
The take-home message to clinicians from the findings at this time is to be aware that children and adolescents can become severely ill from COVID-19–related complications, said Dr. Randolph. “Some of the laboratory values on presentation appear to be associated with disease severity.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Randolph disclosed funding from the Centers for Disease Control and Prevention to lead the Overcoming COVID-19 Study in U.S. Children and Adults.
SOURCE: Fernandes DM et al. J Pediatr. 2020 Nov 13. doi: 10.1016/j.jpeds.2020.11.016.
based on data from 281 patients at 8 locations.
Manifestations of COVID-19 in children include respiratory disease similar to that seen in adults, but the full spectrum of disease in children has been studied mainly in single settings or with a focus on one clinical manifestation, wrote Danielle M. Fernandes, MD, of Albert Einstein College of Medicine, New York, and colleagues.
In a study published in the Journal of Pediatrics, the researchers identified 281 children hospitalized with COVID-19 and/or multisystem inflammatory syndrome in children (MIS-C) at 8 sites in Connecticut, New Jersey, and New York. A total of 143 (51%) had respiratory disease, 69 (25%) had MIS-C, and 69 (25%) had other manifestations of illness including 32 patients with gastrointestinal problems, 21 infants with fever, 6 cases of neurologic disease, 6 cases of diabetic ketoacidosis, and 4 patients with other indications. The median age of the patients was 10 years, 60% were male, 51% were Hispanic, and 23% were non-Hispanic Black. The most common comorbidities were obesity (34%) and asthma (14%).
Independent predictors of disease severity in children found
After controlling for multiple variables, obesity and hypoxia at hospital admission were significant independent predictors of severe respiratory disease, with odds ratios of 3.39 and 4.01, respectively. In addition, lower absolute lymphocyte count (OR, 8.33 per unit decrease in 109 cells/L) and higher C-reactive protein (OR, 1.06 per unit increase in mg/dL) were significantly predictive of severe MIS-C (P = .001 and P = .017, respectively).
“The association between weight and severe respiratory COVID-19 is consistent with the adult literature; however, the mechanisms of this association require further study,” Dr. Fernandes and associates noted.
Overall, children with MIS-C were significantly more likely to be non-Hispanic Black, compared with children with respiratory disease, an 18% difference. However, neither race/ethnicity nor socioeconomic status were significant predictors of disease severity, the researchers wrote.
During the study period, 7 patients (2%) died and 114 (41%) were admitted to the ICU.
“We found a wide array of clinical manifestations in children and youth hospitalized with SARS-CoV-2,” Dr. Fernandes and associates wrote. Notably, gastrointestinal symptoms, ocular symptoms, and dermatologic symptoms have rarely been noted in adults with COVID-19, but occurred in more than 30% of the pediatric patients.
“We also found that SARS-CoV-2 can be an incidental finding in a substantial number of hospitalized pediatric patients,” the researchers said.
The findings were limited by several factors including a population of patients only from Connecticut, New Jersey, and New York, and the possibility that decisions on hospital and ICU admission may have varied by location, the researchers said. In addition, approaches may have varied in the absence of data on the optimal treatment of MIS-C.
“This study builds on the growing body of evidence showing that mortality in hospitalized pediatric patients is low, compared with adults,” Dr. Fernandes and associates said. “However, it highlights that the young population is not universally spared from morbidity, and that even previously healthy children and youth can develop severe disease requiring supportive therapy.”
Findings confirm other clinical experience
The study was important to show that, “although most children are spared severe illness from COVID-19, some children are hospitalized both with acute COVID-19 respiratory disease, with MIS-C and with a range of other complications,” Adrienne Randolph, MD, of Boston Children’s Hospital and Harvard Medical School, Boston, said in an interview.
Dr. Randolph said she was not surprised by the study findings, “as we are also seeing these types of complications at Boston Children’s Hospital where I work.”
Additional research is needed on the outcomes of these patients, “especially the longer-term sequelae of having COVID-19 or MIS-C early in life,” she emphasized.
The take-home message to clinicians from the findings at this time is to be aware that children and adolescents can become severely ill from COVID-19–related complications, said Dr. Randolph. “Some of the laboratory values on presentation appear to be associated with disease severity.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Randolph disclosed funding from the Centers for Disease Control and Prevention to lead the Overcoming COVID-19 Study in U.S. Children and Adults.
SOURCE: Fernandes DM et al. J Pediatr. 2020 Nov 13. doi: 10.1016/j.jpeds.2020.11.016.
FROM THE JOURNAL OF PEDIATRICS
CRP testing in acute COPD exacerbations cuts antibiotic use without compromising outcomes
Background: A previous study has shown that patients with acute COPD exacerbations had little difference in rate of clinical cure with placebo or antibiotics when CRP is less than 40 mg/L.
Study design: Multicenter, open-label, randomized controlled trial.
Setting: 86 general medical practices in the United Kingdom from January 2015 through September 2017.
Synopsis: More than 600 patients who presented to a primary care physician with an acute COPD exacerbation were randomized to point of care CRP testing vs. usual care. Clinicians in the CRP testing group were provided with a point-of-care testing unit along with an algorithm for results. If the CRP was greater than 40 mg/L, antibiotics were thought to be beneficial; but they were urged not to prescribe antibiotics if the level was less than 20 mg/L. For levels between 20 mg/L and 40 mg/L, it was suggested that antibiotics might be beneficial if the sputum is purulent.
The primary outcomes were patient-reported use of antibiotics for an acute COPD exacerbation within 4 weeks of randomization along with measurement of COPD-related health status on the Clinical COPD Questionnaire at 2 weeks of randomization. Fewer antibiotics were prescribed in the CRP testing group over the usual care group (57% vs. 77%). The adjusted mean difference in the Clinical COPD Questionnaire total score at 2 weeks was –0.19 points, in favor of the CRP-guided group.
Bottom line: The use of point-of-care testing CRP as an adjunctive guide to antibiotic use in acute COPD exacerbations may lower the amount of antibiotic prescribing without compromising clinical outcomes.
Citation: Butler CC et al. C-reactive protein testing to guide antibiotics prescribing for COPD exacerbations. N Engl J Med. 2019 Jul 11; 381:111-120.
Dr. Choksi is a hospitalist and associate professor of internal medicine at Saint Louis University, where she is assistant dean of admissions. She is president of the SHM St. Louis Chapter.
Background: A previous study has shown that patients with acute COPD exacerbations had little difference in rate of clinical cure with placebo or antibiotics when CRP is less than 40 mg/L.
Study design: Multicenter, open-label, randomized controlled trial.
Setting: 86 general medical practices in the United Kingdom from January 2015 through September 2017.
Synopsis: More than 600 patients who presented to a primary care physician with an acute COPD exacerbation were randomized to point of care CRP testing vs. usual care. Clinicians in the CRP testing group were provided with a point-of-care testing unit along with an algorithm for results. If the CRP was greater than 40 mg/L, antibiotics were thought to be beneficial; but they were urged not to prescribe antibiotics if the level was less than 20 mg/L. For levels between 20 mg/L and 40 mg/L, it was suggested that antibiotics might be beneficial if the sputum is purulent.
The primary outcomes were patient-reported use of antibiotics for an acute COPD exacerbation within 4 weeks of randomization along with measurement of COPD-related health status on the Clinical COPD Questionnaire at 2 weeks of randomization. Fewer antibiotics were prescribed in the CRP testing group over the usual care group (57% vs. 77%). The adjusted mean difference in the Clinical COPD Questionnaire total score at 2 weeks was –0.19 points, in favor of the CRP-guided group.
Bottom line: The use of point-of-care testing CRP as an adjunctive guide to antibiotic use in acute COPD exacerbations may lower the amount of antibiotic prescribing without compromising clinical outcomes.
Citation: Butler CC et al. C-reactive protein testing to guide antibiotics prescribing for COPD exacerbations. N Engl J Med. 2019 Jul 11; 381:111-120.
Dr. Choksi is a hospitalist and associate professor of internal medicine at Saint Louis University, where she is assistant dean of admissions. She is president of the SHM St. Louis Chapter.
Background: A previous study has shown that patients with acute COPD exacerbations had little difference in rate of clinical cure with placebo or antibiotics when CRP is less than 40 mg/L.
Study design: Multicenter, open-label, randomized controlled trial.
Setting: 86 general medical practices in the United Kingdom from January 2015 through September 2017.
Synopsis: More than 600 patients who presented to a primary care physician with an acute COPD exacerbation were randomized to point of care CRP testing vs. usual care. Clinicians in the CRP testing group were provided with a point-of-care testing unit along with an algorithm for results. If the CRP was greater than 40 mg/L, antibiotics were thought to be beneficial; but they were urged not to prescribe antibiotics if the level was less than 20 mg/L. For levels between 20 mg/L and 40 mg/L, it was suggested that antibiotics might be beneficial if the sputum is purulent.
The primary outcomes were patient-reported use of antibiotics for an acute COPD exacerbation within 4 weeks of randomization along with measurement of COPD-related health status on the Clinical COPD Questionnaire at 2 weeks of randomization. Fewer antibiotics were prescribed in the CRP testing group over the usual care group (57% vs. 77%). The adjusted mean difference in the Clinical COPD Questionnaire total score at 2 weeks was –0.19 points, in favor of the CRP-guided group.
Bottom line: The use of point-of-care testing CRP as an adjunctive guide to antibiotic use in acute COPD exacerbations may lower the amount of antibiotic prescribing without compromising clinical outcomes.
Citation: Butler CC et al. C-reactive protein testing to guide antibiotics prescribing for COPD exacerbations. N Engl J Med. 2019 Jul 11; 381:111-120.
Dr. Choksi is a hospitalist and associate professor of internal medicine at Saint Louis University, where she is assistant dean of admissions. She is president of the SHM St. Louis Chapter.
CMS launches hospital-at-home program to free up hospital capacity
As an increasing number of health systems implement “hospital-at-home” (HaH) programs to increase their traditional hospital capacity, the Centers for Medicare & Medicaid Services has given the movement a boost by changing its regulations to allow acute care to be provided in a patient’s home under certain conditions.
The CMS announced Nov. 25 that it was launching its Acute Hospital Care at Home program “to increase the capacity of the American health care system” during the COVID-19 pandemic.
At the same time, the agency announced it was giving more flexibility to ambulatory surgery centers (ASCs) to provide hospital-level care.
The CMS said its new HaH program is an expansion of the Hospitals Without Walls initiative that was unveiled last March. Hospitals Without Walls is a set of “temporary new rules” that provide flexibility for hospitals to provide acute care outside of inpatient settings. Under those rules, hospitals are able to transfer patients to outside facilities, such as ASCs, inpatient rehabilitation hospitals, hotels, and dormitories, while still receiving Medicare hospital payments.
Under CMS’ new Acute Hospital Care at Home, which is not described as temporary, patients can be transferred from emergency departments or inpatient wards to hospital-level care at home. The CMS said the HaH program is designed for people with conditions such as the acute phases of asthma, heart failure, pneumonia, and chronic obstructive pulmonary disease. Altogether, the agency said, more than 60 acute conditions can be treated safely at home.
However, the agency didn’t say that facilities can’t admit COVID-19 patients to the hospital at home. Rami Karjian, MBA, cofounder and CEO of Medically Home, a firm that supplies health systems with technical services and software for HaH programs, said in an interview that several Medically Home clients plan to treat both COVID-19 and non-COVID-19 patients at home when they begin to participate in the CMS program in the near future.
The CMS said it consulted extensively with academic and private industry leaders in building its HaH program. Before rolling out the initiative, the agency noted, it conducted successful pilot programs in leading hospitals and health systems. The results of some of these pilots have been reported in academic journals.
Participating hospitals will be required to have specified screening protocols in place before beginning acute care at home, the CMS announced. An in-person physician evaluation will be required before starting care at home. A nurse will evaluate each patient once daily in person or remotely, and either nurses or paramedics will visit the patient in person twice a day.
In contrast, Medicare regulations require nursing staff to be available around the clock in traditional hospitals. So the CMS has to grant waivers to hospitals for HaH programs.
While not going into detail on the telemonitoring capabilities that will be required in the acute hospital care at home, the release said, “Today’s announcement builds upon the critical work by CMS to expand telehealth coverage to keep beneficiaries safe and prevent the spread of COVID-19.”
More flexibility for ASCs
The agency is also giving ASCs the flexibility to provide 24-hour nursing services only when one or more patients are receiving care on site. This flexibility will be available to any of the 5,700 ASCs that wish to participate, and will be immediately effective for the 85 ASCs currently participating in the Hospital Without Walls initiative, the CMS said.
The new ASC regulations, the CMS said, are aimed at allowing communities “to maintain surgical capacity and other life-saving non-COVID-19 [care], like cancer surgeries.” Patients who need such procedures will be able to receive them in ASCs without being exposed to known COVID-19 cases.
Similarly, the CMS said patients and families not diagnosed with COVID-19 may prefer to receive acute care at home if local hospitals are full of COVID-19 patients. In addition, the CMS said it anticipates patients may value the ability to be treated at home without the visitation restrictions of hospitals.
Early HaH participants
Six health systems with extensive experience in providing acute hospital care at home have been approved for the new HaH waivers from Medicare rules. They include Brigham and Women’s Hospital (Massachusetts); Huntsman Cancer Institute (Utah); Massachusetts General Hospital (Massachusetts); Mount Sinai Health System (New York City); Presbyterian Healthcare Services (New Mexico); and UnityPoint Health (Iowa).
The CMS said that it’s in discussions with other health care systems and expects new applications to be submitted soon.
To support these efforts, the CMS has launched an online portal to streamline the waiver request process. The agency said it will closely monitor the program to safeguard beneficiaries and will require participating hospitals to report quality and safety data on a regular basis.
Support from hospitals
The first health systems participating in the CMS HaH appear to be supportive of the program, with some hospital leaders submitting comments to the CMS about their view of the initiative.
“The CMS has taken an extraordinary step today, facilitating the rapid expansion of Hospitalization at Home, an innovative care model with proven results,” said Kenneth L. Davis, MD, president and CEO of the Mount Sinai Health System in New York City. “This important and timely move will enable hospitals across the country to use effective tools to safely care for patients during this pandemic.”
David Levine, MD, assistant professor of medicine and medical director of strategy and innovation for Brigham Health Home Hospital in Boston, was similarly laudatory: “Our research at Brigham Health Home has shown that we can deliver hospital-level care in our patients’ homes with lower readmission rates, more physical mobility, and a positive patient experience,” he said. “During these challenging times, a focus on the home is critical. We are so encouraged that CMS is taking this important step, which will allow hospitals across the country to increase their capacity while delivering the care all patients deserve.”
Scaling up quickly
If other hospitals and health systems recognize the value of HaH, how long might it take them to develop and implement these programs in the midst of a pandemic?
Atrium Health, a large health system in the Southeast, ramped up a hospital-at-home initiative last spring for its 10 hospitals in the Charlotte, N.C., area, in just 2 weeks. However, it had been working on the project for some time before the pandemic struck. Focusing mostly on COVID-19 patients, the initiative reduced the COVID-19 patient load by 20%-25% in Atrium’s hospitals.
Medically Home, the HaH infrastructure company, said in a news release that it “enables health systems to establish new hospital-at-home services in as little as 30 days.” Medically Home has partnered in this venture with Huron Consulting Group, which has about 200 HaH-trained consultants, and Cardinal Health, a large global medical supplies distributor.
Mr. Karjian said in an interview that he expects private insurers to follow CMS’ example, as they often do. “We think this decision will cause not only CMS but private insurers to cover hospital at home after the pandemic, if it becomes the standard of care, because patients have better outcomes when treated at home,” he said.
Asked for his view on why the CMS specified that patients could be admitted to an HaH only from emergency departments or inpatient settings, Mr. Karjian said that the CMS wants to make sure that patients have access to brick-and-mortar hospital care if that’s what they need. Also, he noted, this model is new to most hospitals, so the CMS wants to make sure it starts “with all the safety guardrails” in place.
Overall, Mr. Karjian said, “This is an exciting development for patients across the country. What CMS has done is terrific in terms of letting patients get the care they want, where they want it, and get the benefit of better outcomes while the nation is going through this capacity crunch for hospital beds.”
A version of this article originally appeared on Medscape.com.
As an increasing number of health systems implement “hospital-at-home” (HaH) programs to increase their traditional hospital capacity, the Centers for Medicare & Medicaid Services has given the movement a boost by changing its regulations to allow acute care to be provided in a patient’s home under certain conditions.
The CMS announced Nov. 25 that it was launching its Acute Hospital Care at Home program “to increase the capacity of the American health care system” during the COVID-19 pandemic.
At the same time, the agency announced it was giving more flexibility to ambulatory surgery centers (ASCs) to provide hospital-level care.
The CMS said its new HaH program is an expansion of the Hospitals Without Walls initiative that was unveiled last March. Hospitals Without Walls is a set of “temporary new rules” that provide flexibility for hospitals to provide acute care outside of inpatient settings. Under those rules, hospitals are able to transfer patients to outside facilities, such as ASCs, inpatient rehabilitation hospitals, hotels, and dormitories, while still receiving Medicare hospital payments.
Under CMS’ new Acute Hospital Care at Home, which is not described as temporary, patients can be transferred from emergency departments or inpatient wards to hospital-level care at home. The CMS said the HaH program is designed for people with conditions such as the acute phases of asthma, heart failure, pneumonia, and chronic obstructive pulmonary disease. Altogether, the agency said, more than 60 acute conditions can be treated safely at home.
However, the agency didn’t say that facilities can’t admit COVID-19 patients to the hospital at home. Rami Karjian, MBA, cofounder and CEO of Medically Home, a firm that supplies health systems with technical services and software for HaH programs, said in an interview that several Medically Home clients plan to treat both COVID-19 and non-COVID-19 patients at home when they begin to participate in the CMS program in the near future.
The CMS said it consulted extensively with academic and private industry leaders in building its HaH program. Before rolling out the initiative, the agency noted, it conducted successful pilot programs in leading hospitals and health systems. The results of some of these pilots have been reported in academic journals.
Participating hospitals will be required to have specified screening protocols in place before beginning acute care at home, the CMS announced. An in-person physician evaluation will be required before starting care at home. A nurse will evaluate each patient once daily in person or remotely, and either nurses or paramedics will visit the patient in person twice a day.
In contrast, Medicare regulations require nursing staff to be available around the clock in traditional hospitals. So the CMS has to grant waivers to hospitals for HaH programs.
While not going into detail on the telemonitoring capabilities that will be required in the acute hospital care at home, the release said, “Today’s announcement builds upon the critical work by CMS to expand telehealth coverage to keep beneficiaries safe and prevent the spread of COVID-19.”
More flexibility for ASCs
The agency is also giving ASCs the flexibility to provide 24-hour nursing services only when one or more patients are receiving care on site. This flexibility will be available to any of the 5,700 ASCs that wish to participate, and will be immediately effective for the 85 ASCs currently participating in the Hospital Without Walls initiative, the CMS said.
The new ASC regulations, the CMS said, are aimed at allowing communities “to maintain surgical capacity and other life-saving non-COVID-19 [care], like cancer surgeries.” Patients who need such procedures will be able to receive them in ASCs without being exposed to known COVID-19 cases.
Similarly, the CMS said patients and families not diagnosed with COVID-19 may prefer to receive acute care at home if local hospitals are full of COVID-19 patients. In addition, the CMS said it anticipates patients may value the ability to be treated at home without the visitation restrictions of hospitals.
Early HaH participants
Six health systems with extensive experience in providing acute hospital care at home have been approved for the new HaH waivers from Medicare rules. They include Brigham and Women’s Hospital (Massachusetts); Huntsman Cancer Institute (Utah); Massachusetts General Hospital (Massachusetts); Mount Sinai Health System (New York City); Presbyterian Healthcare Services (New Mexico); and UnityPoint Health (Iowa).
The CMS said that it’s in discussions with other health care systems and expects new applications to be submitted soon.
To support these efforts, the CMS has launched an online portal to streamline the waiver request process. The agency said it will closely monitor the program to safeguard beneficiaries and will require participating hospitals to report quality and safety data on a regular basis.
Support from hospitals
The first health systems participating in the CMS HaH appear to be supportive of the program, with some hospital leaders submitting comments to the CMS about their view of the initiative.
“The CMS has taken an extraordinary step today, facilitating the rapid expansion of Hospitalization at Home, an innovative care model with proven results,” said Kenneth L. Davis, MD, president and CEO of the Mount Sinai Health System in New York City. “This important and timely move will enable hospitals across the country to use effective tools to safely care for patients during this pandemic.”
David Levine, MD, assistant professor of medicine and medical director of strategy and innovation for Brigham Health Home Hospital in Boston, was similarly laudatory: “Our research at Brigham Health Home has shown that we can deliver hospital-level care in our patients’ homes with lower readmission rates, more physical mobility, and a positive patient experience,” he said. “During these challenging times, a focus on the home is critical. We are so encouraged that CMS is taking this important step, which will allow hospitals across the country to increase their capacity while delivering the care all patients deserve.”
Scaling up quickly
If other hospitals and health systems recognize the value of HaH, how long might it take them to develop and implement these programs in the midst of a pandemic?
Atrium Health, a large health system in the Southeast, ramped up a hospital-at-home initiative last spring for its 10 hospitals in the Charlotte, N.C., area, in just 2 weeks. However, it had been working on the project for some time before the pandemic struck. Focusing mostly on COVID-19 patients, the initiative reduced the COVID-19 patient load by 20%-25% in Atrium’s hospitals.
Medically Home, the HaH infrastructure company, said in a news release that it “enables health systems to establish new hospital-at-home services in as little as 30 days.” Medically Home has partnered in this venture with Huron Consulting Group, which has about 200 HaH-trained consultants, and Cardinal Health, a large global medical supplies distributor.
Mr. Karjian said in an interview that he expects private insurers to follow CMS’ example, as they often do. “We think this decision will cause not only CMS but private insurers to cover hospital at home after the pandemic, if it becomes the standard of care, because patients have better outcomes when treated at home,” he said.
Asked for his view on why the CMS specified that patients could be admitted to an HaH only from emergency departments or inpatient settings, Mr. Karjian said that the CMS wants to make sure that patients have access to brick-and-mortar hospital care if that’s what they need. Also, he noted, this model is new to most hospitals, so the CMS wants to make sure it starts “with all the safety guardrails” in place.
Overall, Mr. Karjian said, “This is an exciting development for patients across the country. What CMS has done is terrific in terms of letting patients get the care they want, where they want it, and get the benefit of better outcomes while the nation is going through this capacity crunch for hospital beds.”
A version of this article originally appeared on Medscape.com.
As an increasing number of health systems implement “hospital-at-home” (HaH) programs to increase their traditional hospital capacity, the Centers for Medicare & Medicaid Services has given the movement a boost by changing its regulations to allow acute care to be provided in a patient’s home under certain conditions.
The CMS announced Nov. 25 that it was launching its Acute Hospital Care at Home program “to increase the capacity of the American health care system” during the COVID-19 pandemic.
At the same time, the agency announced it was giving more flexibility to ambulatory surgery centers (ASCs) to provide hospital-level care.
The CMS said its new HaH program is an expansion of the Hospitals Without Walls initiative that was unveiled last March. Hospitals Without Walls is a set of “temporary new rules” that provide flexibility for hospitals to provide acute care outside of inpatient settings. Under those rules, hospitals are able to transfer patients to outside facilities, such as ASCs, inpatient rehabilitation hospitals, hotels, and dormitories, while still receiving Medicare hospital payments.
Under CMS’ new Acute Hospital Care at Home, which is not described as temporary, patients can be transferred from emergency departments or inpatient wards to hospital-level care at home. The CMS said the HaH program is designed for people with conditions such as the acute phases of asthma, heart failure, pneumonia, and chronic obstructive pulmonary disease. Altogether, the agency said, more than 60 acute conditions can be treated safely at home.
However, the agency didn’t say that facilities can’t admit COVID-19 patients to the hospital at home. Rami Karjian, MBA, cofounder and CEO of Medically Home, a firm that supplies health systems with technical services and software for HaH programs, said in an interview that several Medically Home clients plan to treat both COVID-19 and non-COVID-19 patients at home when they begin to participate in the CMS program in the near future.
The CMS said it consulted extensively with academic and private industry leaders in building its HaH program. Before rolling out the initiative, the agency noted, it conducted successful pilot programs in leading hospitals and health systems. The results of some of these pilots have been reported in academic journals.
Participating hospitals will be required to have specified screening protocols in place before beginning acute care at home, the CMS announced. An in-person physician evaluation will be required before starting care at home. A nurse will evaluate each patient once daily in person or remotely, and either nurses or paramedics will visit the patient in person twice a day.
In contrast, Medicare regulations require nursing staff to be available around the clock in traditional hospitals. So the CMS has to grant waivers to hospitals for HaH programs.
While not going into detail on the telemonitoring capabilities that will be required in the acute hospital care at home, the release said, “Today’s announcement builds upon the critical work by CMS to expand telehealth coverage to keep beneficiaries safe and prevent the spread of COVID-19.”
More flexibility for ASCs
The agency is also giving ASCs the flexibility to provide 24-hour nursing services only when one or more patients are receiving care on site. This flexibility will be available to any of the 5,700 ASCs that wish to participate, and will be immediately effective for the 85 ASCs currently participating in the Hospital Without Walls initiative, the CMS said.
The new ASC regulations, the CMS said, are aimed at allowing communities “to maintain surgical capacity and other life-saving non-COVID-19 [care], like cancer surgeries.” Patients who need such procedures will be able to receive them in ASCs without being exposed to known COVID-19 cases.
Similarly, the CMS said patients and families not diagnosed with COVID-19 may prefer to receive acute care at home if local hospitals are full of COVID-19 patients. In addition, the CMS said it anticipates patients may value the ability to be treated at home without the visitation restrictions of hospitals.
Early HaH participants
Six health systems with extensive experience in providing acute hospital care at home have been approved for the new HaH waivers from Medicare rules. They include Brigham and Women’s Hospital (Massachusetts); Huntsman Cancer Institute (Utah); Massachusetts General Hospital (Massachusetts); Mount Sinai Health System (New York City); Presbyterian Healthcare Services (New Mexico); and UnityPoint Health (Iowa).
The CMS said that it’s in discussions with other health care systems and expects new applications to be submitted soon.
To support these efforts, the CMS has launched an online portal to streamline the waiver request process. The agency said it will closely monitor the program to safeguard beneficiaries and will require participating hospitals to report quality and safety data on a regular basis.
Support from hospitals
The first health systems participating in the CMS HaH appear to be supportive of the program, with some hospital leaders submitting comments to the CMS about their view of the initiative.
“The CMS has taken an extraordinary step today, facilitating the rapid expansion of Hospitalization at Home, an innovative care model with proven results,” said Kenneth L. Davis, MD, president and CEO of the Mount Sinai Health System in New York City. “This important and timely move will enable hospitals across the country to use effective tools to safely care for patients during this pandemic.”
David Levine, MD, assistant professor of medicine and medical director of strategy and innovation for Brigham Health Home Hospital in Boston, was similarly laudatory: “Our research at Brigham Health Home has shown that we can deliver hospital-level care in our patients’ homes with lower readmission rates, more physical mobility, and a positive patient experience,” he said. “During these challenging times, a focus on the home is critical. We are so encouraged that CMS is taking this important step, which will allow hospitals across the country to increase their capacity while delivering the care all patients deserve.”
Scaling up quickly
If other hospitals and health systems recognize the value of HaH, how long might it take them to develop and implement these programs in the midst of a pandemic?
Atrium Health, a large health system in the Southeast, ramped up a hospital-at-home initiative last spring for its 10 hospitals in the Charlotte, N.C., area, in just 2 weeks. However, it had been working on the project for some time before the pandemic struck. Focusing mostly on COVID-19 patients, the initiative reduced the COVID-19 patient load by 20%-25% in Atrium’s hospitals.
Medically Home, the HaH infrastructure company, said in a news release that it “enables health systems to establish new hospital-at-home services in as little as 30 days.” Medically Home has partnered in this venture with Huron Consulting Group, which has about 200 HaH-trained consultants, and Cardinal Health, a large global medical supplies distributor.
Mr. Karjian said in an interview that he expects private insurers to follow CMS’ example, as they often do. “We think this decision will cause not only CMS but private insurers to cover hospital at home after the pandemic, if it becomes the standard of care, because patients have better outcomes when treated at home,” he said.
Asked for his view on why the CMS specified that patients could be admitted to an HaH only from emergency departments or inpatient settings, Mr. Karjian said that the CMS wants to make sure that patients have access to brick-and-mortar hospital care if that’s what they need. Also, he noted, this model is new to most hospitals, so the CMS wants to make sure it starts “with all the safety guardrails” in place.
Overall, Mr. Karjian said, “This is an exciting development for patients across the country. What CMS has done is terrific in terms of letting patients get the care they want, where they want it, and get the benefit of better outcomes while the nation is going through this capacity crunch for hospital beds.”
A version of this article originally appeared on Medscape.com.








