User login
ColCORONA: More questions than answers for colchicine in COVID-19
Science by press release and preprint has cooled clinician enthusiasm for the use of colchicine in nonhospitalized patients with COVID-19, despite a pressing need for early treatments.
As previously reported by this news organization, a Jan. 22 press release announced that the massive ColCORONA study missed its primary endpoint of hospitalization or death among 4,488 newly diagnosed patients at increased risk for hospitalization.
But it also touted that use of the anti-inflammatory drug significantly reduced the primary endpoint in 4,159 of those patients with polymerase chain reaction–confirmed COVID and led to reductions of 25%, 50%, and 44%, respectively, for hospitalizations, ventilations, and death.
Lead investigator Jean-Claude Tardif, MD, director of the Montreal Heart Institute Research Centre, deemed the findings a “medical breakthrough.”
When the preprint released a few days later, however, newly revealed confidence intervals showed colchicine did not meaningfully reduce the need for mechanical ventilation (odds ratio, 0.50; 95% confidence interval, 0.23-1.07) or death alone (OR, 0.56; 95% CI, 0.19-1.66).
Further, the significant benefit on the primary outcome came at the cost of a fivefold increase in pulmonary embolism (11 vs. 2; P = .01), which was not mentioned in the press release.
“Whether this represents a real phenomenon or simply the play of chance is not known,” Dr. Tardif and colleagues noted later in the preprint.
“I read the preprint on colchicine and I have so many questions,” Aaron E. Glatt, MD, spokesperson for the Infectious Diseases Society of America and chief of infectious diseases, Mount Sinai South Nassau, Hewlett, N.Y., said in an interview. “I’ve been burned too many times with COVID and prefer to see better data.
“People sometimes say if you wait for perfect data, people are going to die,” he said. “Yeah, but we have no idea if people are going to die from getting this drug more than not getting it. That’s what concerns me. How many pulmonary emboli are going to be fatal versus the slight benefit that the study showed?”
The pushback to the non–peer-reviewed data on social media and via emails was so strong that Dr. Tardif posted a nearly 2,000-word letter responding to the many questions at play.
Chief among them was why the trial, originally planned for 6,000 patients, was stopped early by the investigators without consultation with the data safety monitoring board (DSMB).
The explanation in the letter that logistical issues like running the study call center, budget constraints, and a perceived need to quickly communicate the results left some calling foul that the study wasn’t allowed to finish and come to a more definitive conclusion.
“I can be a little bit sympathetic to their cause but at the same time the DSMB should have said no,” said David Boulware, MD, MPH, who led a recent hydroxychloroquine trial in COVID-19. “The problem is we’re sort of left in limbo, where some people kind of believe it and some say it’s not really a thing. So it’s not really moving the needle, as far as guidelines go.”
Indeed, a Twitter poll by cardiologist James Januzzi Jr., MD, captured the uncertainty, with 28% of respondents saying the trial was “neutral,” 58% saying “maybe but meh,” and 14% saying “colchicine for all.”
Another poll cheekily asked whether ColCORONA was the Gamestop/Reddit equivalent of COVID.
“The press release really didn’t help things because it very much oversold the effect. That, I think, poisoned the well,” said Dr. Boulware, professor of medicine in infectious diseases at the University of Minnesota, Minneapolis.
“The question I’m left with is not whether colchicine works, but who does it work in,” he said. “That’s really the fundamental question because it does seem that there are probably high-risk groups in their trial and others where they benefit, whereas other groups don’t benefit. In the subgroup analysis, there was absolutely no beneficial effect in women.”
According to the authors, the number needed to treat to prevent one death or hospitalization was 71 overall, but 29 for patients with diabetes, 31 for those aged 70 years and older, 53 for patients with respiratory disease, and 25 for those with coronary disease or heart failure.
Men are at higher risk overall for poor outcomes. But “the authors didn’t present a multivariable analysis, so it is unclear if another factor, such as a differential prevalence of smoking or cardiovascular risk factors, contributed to the differential benefit,” Rachel Bender Ignacio, MD, MPH, infectious disease specialist, University of Washington, Seattle, said in an interview.
Importantly, in this pragmatic study, duration and severity of symptoms were not reported, observed Dr. Bender Ignacio, who is also a STOP-COVID-2 investigator. “We don’t yet have data as to whether colchicine shortens duration or severity of symptoms or prevents long COVID, so we need more data on that.”
The overall risk for serious adverse events was lower in the colchicine group, but the difference in pulmonary embolism (PE) was striking, she said. This could be caused by a real biologic effect, or it’s possible that persons with shortness of breath and hypoxia, without evident viral pneumonia on chest x-ray after a positive COVID-19 test, were more likely to receive a CT-PE study.
The press release also failed to include information, later noted in the preprint, that the MHI has submitted two patents related to colchicine: “Methods of treating a coronavirus infection using colchicine” and “Early administration of low-dose colchicine after myocardial infarction.”
Reached for clarification, MHI communications adviser Camille Turbide said in an interview that the first patent “simply refers to the novel concept of preventing complications of COVID-19, such as admission to the hospital, with colchicine as tested in the ColCORONA study.”
The second patent, she said, refers to the “novel concept that administering colchicine early after a major adverse cardiovascular event is better than waiting several days,” as supported by the COLCOT study, which Dr. Tardif also led.
The patents are being reviewed by authorities and “Dr. Tardif has waived his rights in these patents and does not stand to benefit financially at all if colchicine becomes used as a treatment for COVID-19,” Ms. Turbide said.
Dr. Tardif did not respond to interview requests for this story. Dr. Glatt said conflicts of interest must be assessed and are “something that is of great concern in any scientific study.”
Cardiologist Steve Nissen, MD, of the Cleveland Clinic said in an interview that, “despite the negative results, the study does suggest that colchicine might have a benefit and should be studied in future trials. These findings are not sufficient evidence to suggest use of the drug in patients infected with COVID-19.”
He noted that adverse effects like diarrhea were expected but that the excess PE was unexpected and needs greater clarification.
“Stopping the trial for administrative reasons is puzzling and undermined the ability of the trial to give a reliable answer,” Dr. Nissen said. “This is a reasonable pilot study that should be viewed as hypothesis generating but inconclusive.”
Several sources said a new trial is unlikely, particularly given the cost and 28 trials already evaluating colchicine. Among these are RECOVERY and COLCOVID, testing whether colchicine can reduce the duration of hospitalization or death in hospitalized patients with COVID-19.
Because there are so many trials ongoing right now, including for antivirals and other immunomodulators, it’s important that, if colchicine comes to routine clinical use, it provides access to treatment for those not able or willing to access clinical trials, rather than impeding clinical trial enrollment, Dr. Bender Ignacio suggested.
“We have already learned the lesson in the pandemic that early adoption of potentially promising therapies can negatively impact our ability to study and develop other promising treatments,” she said.
The trial was coordinated by the Montreal Heart Institute and funded by the government of Quebec; the National Heart, Lung, and Blood Institute of the National Institutes of Health; Montreal philanthropist Sophie Desmarais, and the COVID-19 Therapeutics Accelerator launched by the Bill & Melinda Gates Foundation, Wellcome, and Mastercard. CGI, Dacima, and Pharmascience of Montreal were also collaborators. Dr. Glatt reported no conflicts of interest. Dr. Boulware reported receiving $18 in food and beverages from Gilead Sciences in 2018.
A version of this article first appeared on Medscape.com.
Science by press release and preprint has cooled clinician enthusiasm for the use of colchicine in nonhospitalized patients with COVID-19, despite a pressing need for early treatments.
As previously reported by this news organization, a Jan. 22 press release announced that the massive ColCORONA study missed its primary endpoint of hospitalization or death among 4,488 newly diagnosed patients at increased risk for hospitalization.
But it also touted that use of the anti-inflammatory drug significantly reduced the primary endpoint in 4,159 of those patients with polymerase chain reaction–confirmed COVID and led to reductions of 25%, 50%, and 44%, respectively, for hospitalizations, ventilations, and death.
Lead investigator Jean-Claude Tardif, MD, director of the Montreal Heart Institute Research Centre, deemed the findings a “medical breakthrough.”
When the preprint released a few days later, however, newly revealed confidence intervals showed colchicine did not meaningfully reduce the need for mechanical ventilation (odds ratio, 0.50; 95% confidence interval, 0.23-1.07) or death alone (OR, 0.56; 95% CI, 0.19-1.66).
Further, the significant benefit on the primary outcome came at the cost of a fivefold increase in pulmonary embolism (11 vs. 2; P = .01), which was not mentioned in the press release.
“Whether this represents a real phenomenon or simply the play of chance is not known,” Dr. Tardif and colleagues noted later in the preprint.
“I read the preprint on colchicine and I have so many questions,” Aaron E. Glatt, MD, spokesperson for the Infectious Diseases Society of America and chief of infectious diseases, Mount Sinai South Nassau, Hewlett, N.Y., said in an interview. “I’ve been burned too many times with COVID and prefer to see better data.
“People sometimes say if you wait for perfect data, people are going to die,” he said. “Yeah, but we have no idea if people are going to die from getting this drug more than not getting it. That’s what concerns me. How many pulmonary emboli are going to be fatal versus the slight benefit that the study showed?”
The pushback to the non–peer-reviewed data on social media and via emails was so strong that Dr. Tardif posted a nearly 2,000-word letter responding to the many questions at play.
Chief among them was why the trial, originally planned for 6,000 patients, was stopped early by the investigators without consultation with the data safety monitoring board (DSMB).
The explanation in the letter that logistical issues like running the study call center, budget constraints, and a perceived need to quickly communicate the results left some calling foul that the study wasn’t allowed to finish and come to a more definitive conclusion.
“I can be a little bit sympathetic to their cause but at the same time the DSMB should have said no,” said David Boulware, MD, MPH, who led a recent hydroxychloroquine trial in COVID-19. “The problem is we’re sort of left in limbo, where some people kind of believe it and some say it’s not really a thing. So it’s not really moving the needle, as far as guidelines go.”
Indeed, a Twitter poll by cardiologist James Januzzi Jr., MD, captured the uncertainty, with 28% of respondents saying the trial was “neutral,” 58% saying “maybe but meh,” and 14% saying “colchicine for all.”
Another poll cheekily asked whether ColCORONA was the Gamestop/Reddit equivalent of COVID.
“The press release really didn’t help things because it very much oversold the effect. That, I think, poisoned the well,” said Dr. Boulware, professor of medicine in infectious diseases at the University of Minnesota, Minneapolis.
“The question I’m left with is not whether colchicine works, but who does it work in,” he said. “That’s really the fundamental question because it does seem that there are probably high-risk groups in their trial and others where they benefit, whereas other groups don’t benefit. In the subgroup analysis, there was absolutely no beneficial effect in women.”
According to the authors, the number needed to treat to prevent one death or hospitalization was 71 overall, but 29 for patients with diabetes, 31 for those aged 70 years and older, 53 for patients with respiratory disease, and 25 for those with coronary disease or heart failure.
Men are at higher risk overall for poor outcomes. But “the authors didn’t present a multivariable analysis, so it is unclear if another factor, such as a differential prevalence of smoking or cardiovascular risk factors, contributed to the differential benefit,” Rachel Bender Ignacio, MD, MPH, infectious disease specialist, University of Washington, Seattle, said in an interview.
Importantly, in this pragmatic study, duration and severity of symptoms were not reported, observed Dr. Bender Ignacio, who is also a STOP-COVID-2 investigator. “We don’t yet have data as to whether colchicine shortens duration or severity of symptoms or prevents long COVID, so we need more data on that.”
The overall risk for serious adverse events was lower in the colchicine group, but the difference in pulmonary embolism (PE) was striking, she said. This could be caused by a real biologic effect, or it’s possible that persons with shortness of breath and hypoxia, without evident viral pneumonia on chest x-ray after a positive COVID-19 test, were more likely to receive a CT-PE study.
The press release also failed to include information, later noted in the preprint, that the MHI has submitted two patents related to colchicine: “Methods of treating a coronavirus infection using colchicine” and “Early administration of low-dose colchicine after myocardial infarction.”
Reached for clarification, MHI communications adviser Camille Turbide said in an interview that the first patent “simply refers to the novel concept of preventing complications of COVID-19, such as admission to the hospital, with colchicine as tested in the ColCORONA study.”
The second patent, she said, refers to the “novel concept that administering colchicine early after a major adverse cardiovascular event is better than waiting several days,” as supported by the COLCOT study, which Dr. Tardif also led.
The patents are being reviewed by authorities and “Dr. Tardif has waived his rights in these patents and does not stand to benefit financially at all if colchicine becomes used as a treatment for COVID-19,” Ms. Turbide said.
Dr. Tardif did not respond to interview requests for this story. Dr. Glatt said conflicts of interest must be assessed and are “something that is of great concern in any scientific study.”
Cardiologist Steve Nissen, MD, of the Cleveland Clinic said in an interview that, “despite the negative results, the study does suggest that colchicine might have a benefit and should be studied in future trials. These findings are not sufficient evidence to suggest use of the drug in patients infected with COVID-19.”
He noted that adverse effects like diarrhea were expected but that the excess PE was unexpected and needs greater clarification.
“Stopping the trial for administrative reasons is puzzling and undermined the ability of the trial to give a reliable answer,” Dr. Nissen said. “This is a reasonable pilot study that should be viewed as hypothesis generating but inconclusive.”
Several sources said a new trial is unlikely, particularly given the cost and 28 trials already evaluating colchicine. Among these are RECOVERY and COLCOVID, testing whether colchicine can reduce the duration of hospitalization or death in hospitalized patients with COVID-19.
Because there are so many trials ongoing right now, including for antivirals and other immunomodulators, it’s important that, if colchicine comes to routine clinical use, it provides access to treatment for those not able or willing to access clinical trials, rather than impeding clinical trial enrollment, Dr. Bender Ignacio suggested.
“We have already learned the lesson in the pandemic that early adoption of potentially promising therapies can negatively impact our ability to study and develop other promising treatments,” she said.
The trial was coordinated by the Montreal Heart Institute and funded by the government of Quebec; the National Heart, Lung, and Blood Institute of the National Institutes of Health; Montreal philanthropist Sophie Desmarais, and the COVID-19 Therapeutics Accelerator launched by the Bill & Melinda Gates Foundation, Wellcome, and Mastercard. CGI, Dacima, and Pharmascience of Montreal were also collaborators. Dr. Glatt reported no conflicts of interest. Dr. Boulware reported receiving $18 in food and beverages from Gilead Sciences in 2018.
A version of this article first appeared on Medscape.com.
Science by press release and preprint has cooled clinician enthusiasm for the use of colchicine in nonhospitalized patients with COVID-19, despite a pressing need for early treatments.
As previously reported by this news organization, a Jan. 22 press release announced that the massive ColCORONA study missed its primary endpoint of hospitalization or death among 4,488 newly diagnosed patients at increased risk for hospitalization.
But it also touted that use of the anti-inflammatory drug significantly reduced the primary endpoint in 4,159 of those patients with polymerase chain reaction–confirmed COVID and led to reductions of 25%, 50%, and 44%, respectively, for hospitalizations, ventilations, and death.
Lead investigator Jean-Claude Tardif, MD, director of the Montreal Heart Institute Research Centre, deemed the findings a “medical breakthrough.”
When the preprint released a few days later, however, newly revealed confidence intervals showed colchicine did not meaningfully reduce the need for mechanical ventilation (odds ratio, 0.50; 95% confidence interval, 0.23-1.07) or death alone (OR, 0.56; 95% CI, 0.19-1.66).
Further, the significant benefit on the primary outcome came at the cost of a fivefold increase in pulmonary embolism (11 vs. 2; P = .01), which was not mentioned in the press release.
“Whether this represents a real phenomenon or simply the play of chance is not known,” Dr. Tardif and colleagues noted later in the preprint.
“I read the preprint on colchicine and I have so many questions,” Aaron E. Glatt, MD, spokesperson for the Infectious Diseases Society of America and chief of infectious diseases, Mount Sinai South Nassau, Hewlett, N.Y., said in an interview. “I’ve been burned too many times with COVID and prefer to see better data.
“People sometimes say if you wait for perfect data, people are going to die,” he said. “Yeah, but we have no idea if people are going to die from getting this drug more than not getting it. That’s what concerns me. How many pulmonary emboli are going to be fatal versus the slight benefit that the study showed?”
The pushback to the non–peer-reviewed data on social media and via emails was so strong that Dr. Tardif posted a nearly 2,000-word letter responding to the many questions at play.
Chief among them was why the trial, originally planned for 6,000 patients, was stopped early by the investigators without consultation with the data safety monitoring board (DSMB).
The explanation in the letter that logistical issues like running the study call center, budget constraints, and a perceived need to quickly communicate the results left some calling foul that the study wasn’t allowed to finish and come to a more definitive conclusion.
“I can be a little bit sympathetic to their cause but at the same time the DSMB should have said no,” said David Boulware, MD, MPH, who led a recent hydroxychloroquine trial in COVID-19. “The problem is we’re sort of left in limbo, where some people kind of believe it and some say it’s not really a thing. So it’s not really moving the needle, as far as guidelines go.”
Indeed, a Twitter poll by cardiologist James Januzzi Jr., MD, captured the uncertainty, with 28% of respondents saying the trial was “neutral,” 58% saying “maybe but meh,” and 14% saying “colchicine for all.”
Another poll cheekily asked whether ColCORONA was the Gamestop/Reddit equivalent of COVID.
“The press release really didn’t help things because it very much oversold the effect. That, I think, poisoned the well,” said Dr. Boulware, professor of medicine in infectious diseases at the University of Minnesota, Minneapolis.
“The question I’m left with is not whether colchicine works, but who does it work in,” he said. “That’s really the fundamental question because it does seem that there are probably high-risk groups in their trial and others where they benefit, whereas other groups don’t benefit. In the subgroup analysis, there was absolutely no beneficial effect in women.”
According to the authors, the number needed to treat to prevent one death or hospitalization was 71 overall, but 29 for patients with diabetes, 31 for those aged 70 years and older, 53 for patients with respiratory disease, and 25 for those with coronary disease or heart failure.
Men are at higher risk overall for poor outcomes. But “the authors didn’t present a multivariable analysis, so it is unclear if another factor, such as a differential prevalence of smoking or cardiovascular risk factors, contributed to the differential benefit,” Rachel Bender Ignacio, MD, MPH, infectious disease specialist, University of Washington, Seattle, said in an interview.
Importantly, in this pragmatic study, duration and severity of symptoms were not reported, observed Dr. Bender Ignacio, who is also a STOP-COVID-2 investigator. “We don’t yet have data as to whether colchicine shortens duration or severity of symptoms or prevents long COVID, so we need more data on that.”
The overall risk for serious adverse events was lower in the colchicine group, but the difference in pulmonary embolism (PE) was striking, she said. This could be caused by a real biologic effect, or it’s possible that persons with shortness of breath and hypoxia, without evident viral pneumonia on chest x-ray after a positive COVID-19 test, were more likely to receive a CT-PE study.
The press release also failed to include information, later noted in the preprint, that the MHI has submitted two patents related to colchicine: “Methods of treating a coronavirus infection using colchicine” and “Early administration of low-dose colchicine after myocardial infarction.”
Reached for clarification, MHI communications adviser Camille Turbide said in an interview that the first patent “simply refers to the novel concept of preventing complications of COVID-19, such as admission to the hospital, with colchicine as tested in the ColCORONA study.”
The second patent, she said, refers to the “novel concept that administering colchicine early after a major adverse cardiovascular event is better than waiting several days,” as supported by the COLCOT study, which Dr. Tardif also led.
The patents are being reviewed by authorities and “Dr. Tardif has waived his rights in these patents and does not stand to benefit financially at all if colchicine becomes used as a treatment for COVID-19,” Ms. Turbide said.
Dr. Tardif did not respond to interview requests for this story. Dr. Glatt said conflicts of interest must be assessed and are “something that is of great concern in any scientific study.”
Cardiologist Steve Nissen, MD, of the Cleveland Clinic said in an interview that, “despite the negative results, the study does suggest that colchicine might have a benefit and should be studied in future trials. These findings are not sufficient evidence to suggest use of the drug in patients infected with COVID-19.”
He noted that adverse effects like diarrhea were expected but that the excess PE was unexpected and needs greater clarification.
“Stopping the trial for administrative reasons is puzzling and undermined the ability of the trial to give a reliable answer,” Dr. Nissen said. “This is a reasonable pilot study that should be viewed as hypothesis generating but inconclusive.”
Several sources said a new trial is unlikely, particularly given the cost and 28 trials already evaluating colchicine. Among these are RECOVERY and COLCOVID, testing whether colchicine can reduce the duration of hospitalization or death in hospitalized patients with COVID-19.
Because there are so many trials ongoing right now, including for antivirals and other immunomodulators, it’s important that, if colchicine comes to routine clinical use, it provides access to treatment for those not able or willing to access clinical trials, rather than impeding clinical trial enrollment, Dr. Bender Ignacio suggested.
“We have already learned the lesson in the pandemic that early adoption of potentially promising therapies can negatively impact our ability to study and develop other promising treatments,” she said.
The trial was coordinated by the Montreal Heart Institute and funded by the government of Quebec; the National Heart, Lung, and Blood Institute of the National Institutes of Health; Montreal philanthropist Sophie Desmarais, and the COVID-19 Therapeutics Accelerator launched by the Bill & Melinda Gates Foundation, Wellcome, and Mastercard. CGI, Dacima, and Pharmascience of Montreal were also collaborators. Dr. Glatt reported no conflicts of interest. Dr. Boulware reported receiving $18 in food and beverages from Gilead Sciences in 2018.
A version of this article first appeared on Medscape.com.
Bronchiolitis: Rare diseases, diagnostic challenges, and few proven therapies
What’s in a name?
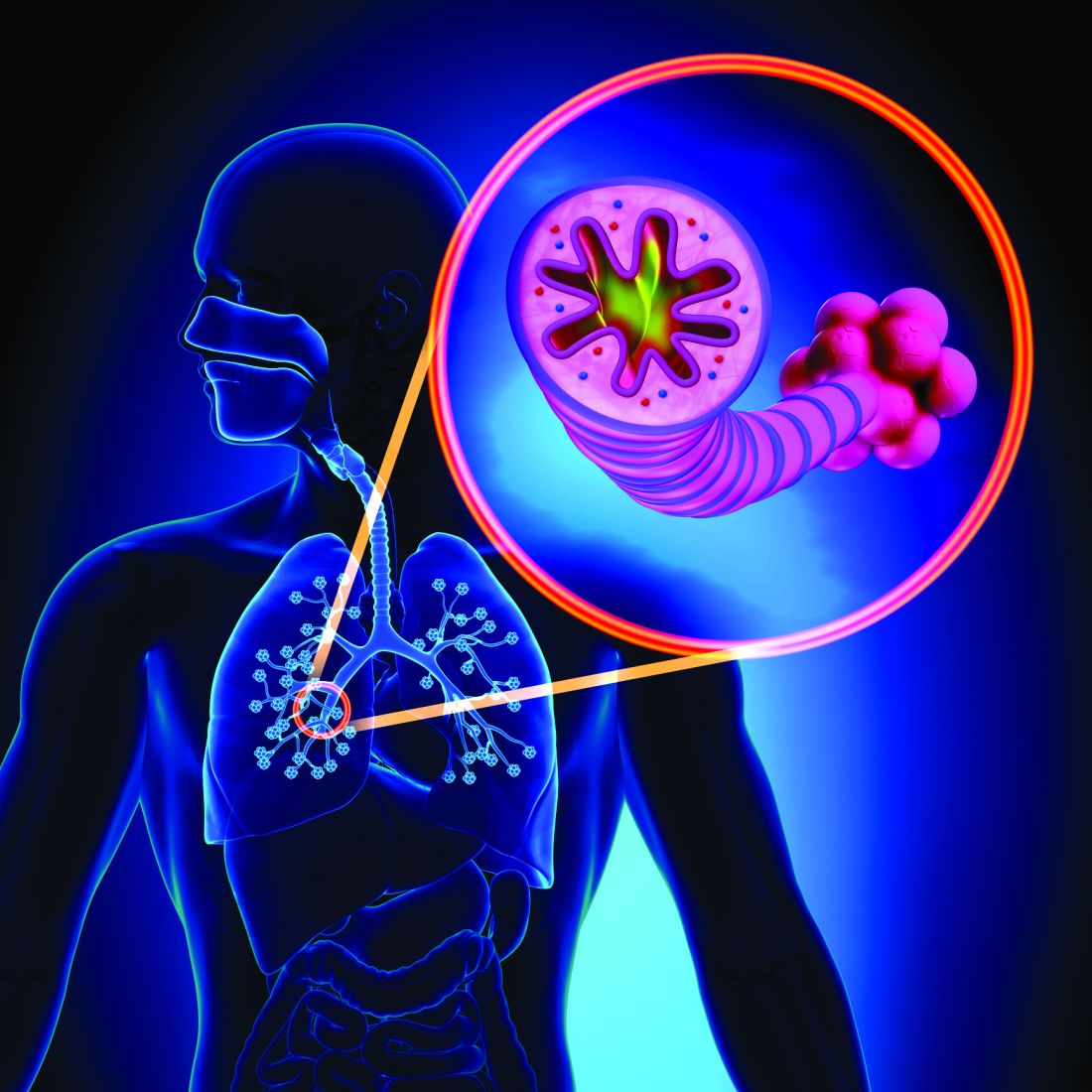
Bronchiolitis, a group of diseases also referred to as “small airways diseases,” is characterized by inflammation and/or fibrosis in airways less than 2 mm in diameter. In pediatric patients, it is most commonly related to acute viral infections, while in adults, it is often associated with chronic diseases. Bronchiolitis is a well-recognized complication in a significant number of patients who have undergone lung or stem cell transplantation. Common associations also include connective tissue diseases, environmental or occupational inhalation exposures, aspiration, drug toxicity, and infections. Diagnosing bronchiolitis can be challenging for clinicians, and few treatment options exist apart from treating identifiable underlying etiologies. More research is needed into noninvasive diagnostic techniques and treatment modalities.
The terminology used to describe bronchiolitis has evolved over time. Bronchiolitis is now used to describe conditions where the primary pathologic condition is damage to the bronchiolar epithelium not attributable to a larger parenchymal disease (such as hypersensitivity pneumonitis). This change in nomenclature explains why the condition formerly known as “bronchiolitis obliterans organizing pneumonia” (BOOP) is now simply recognized as “organizing pneumonia.” Despite several proposed classification schemes focusing on histopathology, there is no consensus regarding the different subtypes of bronchiolitis, leading to confusion in some cases. Recently, authors have attempted to distinguish cases based on three main histologic patterns (Urisman A, et al. Surg Pathol Clin. 2020;13[1]:189).
- Obliterative/constrictive bronchiolitis (OB) – the terms “obliterative” and “constrictive” are used interchangeably throughout pulmonary literature. It is characterized by fibroblast-rich tissue accumulation in the sub-epithelium of bronchioles leading to progressive narrowing of the lumen. In addition to the transplant setting, it is often seen in patients with rheumatoid arthritis or other connective tissue diseases, inhalational exposures, or acute respiratory infections. More recently, clinicians have recognized diffuse idiopathic pulmonary neuroendocrine cell hyperplasia (DIPNECH) as a rare condition causing OB with potentially effective treatment.
- Follicular bronchiolitis (FB) – features peribronchiolar inflammation with subepithelial lymphoid deposits leading to luminal obstruction. FB is chiefly associated with conditions of impaired immunity or chronic airway infection, such as autoimmune connective tissues diseases (especially rheumatoid arthritis and Sjogren’s), severe combined immunodeficiency, HIV, cystic fibrosis, and primary ciliary dyskinesia.
- Diffuse panbronchiolitis (DBP) – features bilateral bronchiolar lesions with lymphocytic inflammation of the bronchiolar wall, as well as peribronchiolar inflammation and accumulation of interstitial foamy macrophages. Patients afflicted with DBP may suffer repeated bacterial colonization or infection. There is a higher prevalence of DBP in Asia where it was first identified in the 1960s, potentially due to several HLA alleles that are more common in Asia.
In addition to the above terminology, the transplant-setting diagnosis “bronchiolitis obliterans syndrome” (BOS) is used to denote progressive obstructive lung disease for which there is not another cause aside from chronic graft rejection. For these patients, clinicians assume the underlying disease entity is OB, but they often lack histopathologic confirmation.
Diagnosis is challenging
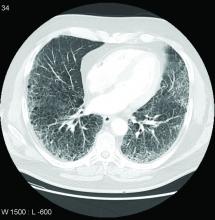
Symptoms of bronchiolitis are typically dyspnea and cough, and patients may often be diagnosed with asthma or COPD initially. Pulmonary function testing may show signs of obstruction, restriction, or mixed disease with or without a reduction in Dlco. Chest radiography often appears normal, but high-resolution CT may show expiratory air trapping and centrilobular nodules. Advanced imaging modalities may augment or replace CT imaging in diagnosing bronchiolitis: investigators are evaluating pulmonary MRI and fluoroscopy with computerized ventilation analysis in clinical trials (NCT04080232).
Currently, open or thoracoscopic lung biopsy is typically required to make a definitive diagnosis. Because bronchiolitis is a patchy and heterogeneous process, transbronchial biopsy may provide insufficient yield, with a sensitivity of 29% to 70% reported in lung transplant literature (Urisman A, et al. Surg Pathol Clin. 2020;13[1]:189).
Recent studies have demonstrated transbronchial cryobiopsy to be a promising alternative to surgical biopsy, owing to larger tissue samples than conventional transbronchial lung biopsies. For example, in a recent case series four patients underwent transbronchial cryobiopsy. The procedure yielded adequate tissue for diagnosis of a chronic bronchiolitis in each case (Yamakawa H, et al. Internal Med Advance Publication. doi: 10.2169/internalmedicine.6028-20.
Treatment options are growing
Evidence for treatment of bronchiolitis remains limited. Options are extrapolated from lung transplant patients, where incidence of BOS ranges from 50% at 5 years to 76% at 10 years post transplant. Guidelines recommend a 3-month minimum trial of azithromycin, which has been shown to slow or reverse decline of lung function in some patients. Modification of immunosuppression is also recommended. In patients who have continued lung function decline, a systematic review concluded that extracorporeal photopheresis had the most robust evidence for efficacy with stabilized lung function and improved overall survival (Benden C, et al. J Heart Lung Transplant. 2017;36[9]:921). Other salvage therapies that have lower-quality evidence of benefit include total lymphoid irradiation, montelukast, and aerosolized cyclosporine.
In patients who have undergone hematopoietic stem cell transplant, steroids are typically the first line treatment for OB as it is thought to be a form of chronic graft-vs-host disease (GVHD). Ruxolitinib, a selective JAK1/2 inhibitor, demonstrated significant improvement overall in patients with steroid-refractory acute GVHD in a recent randomized clinical trial, although the trial did not examine its effect on pulmonary manifestations (Zeiser R, et al. N Engl J Med. 2020;382[19]:1800). To date, retrospective observational studies of ruxolitinib in patients with lung GVHD have shown conflicting results regarding benefit. Investigators are currently studying ruxolitinib in a phase II trial for patients with BOS following stem cell transplant (NCT03674047).
DIPNECH is unique from other bronchiolitis entities, as small airways dysfunction develops as a result of neuroendocrine cell proliferation in the airway mucosa, ultimately leading to bronchial narrowing. It most commonly presents in middle-aged nonsmoking women with years of chronic cough and dyspnea. While it has an indolent course in many patients, some patients develop progressive symptoms and obstructive lung disease. DIPNECH is considered a precursor to other pulmonary neuroendocrine tumors. The lesions demonstrate somatostatin receptor expression in many cases, prompting the use of somatostatin analogues as treatment. In the largest published case series, 42 patients from three different institutions were identified who were treated with somatostatin analogues for a mean of 38.8 months at the time of review. Symptomatic improvement was seen in 33 of the 42 (79%), and of the 15 with posttreatment PFT data, 14 (93%) showed improvement in PFTs (Al-Toubah, T, et al. Chest. 2020;158[1]:401). Other small studies have demonstrated varying results with symptomatic improvement in 29% to 76% of patients and improvement or stability of PFTs in 50% to 100% of patients (Samhouri BF, et al. ERJ Open Res. 2020;6[4]:527).
For patients who have not undergone lung transplant, and who do not have an identifiable exposure or underlying rheumatologic condition, a similar 3-month minimum trial of macrolide antibiotics is reasonable. Macrolides have been shown to double long-term survival rates to over 90% in patients with DPB. Evidence in this patient population is quite limited, and further research is needed to determine effective therapies for patients.
What’s next for bronchiolitis
While clinicians currently have few tools for diagnosing and treating these uncommon diseases, in the coming years, we should learn whether novel imaging modalities or less invasive procedures can aid in the diagnosis. Physicians hope these advances will preclude the need for invasive biopsies in more patients going forward. We should also learn whether newer, targeted agents like ruxolitinib are effective for BOS in patients with stem cell transplant. If so, this finding may open it and similar agents to investigation in other forms of bronchiolitis.
Dr. Poole and Dr. Callahan are with University of Utah Health, Salt Lake City, Utah.
What’s in a name?
Bronchiolitis, a group of diseases also referred to as “small airways diseases,” is characterized by inflammation and/or fibrosis in airways less than 2 mm in diameter. In pediatric patients, it is most commonly related to acute viral infections, while in adults, it is often associated with chronic diseases. Bronchiolitis is a well-recognized complication in a significant number of patients who have undergone lung or stem cell transplantation. Common associations also include connective tissue diseases, environmental or occupational inhalation exposures, aspiration, drug toxicity, and infections. Diagnosing bronchiolitis can be challenging for clinicians, and few treatment options exist apart from treating identifiable underlying etiologies. More research is needed into noninvasive diagnostic techniques and treatment modalities.
The terminology used to describe bronchiolitis has evolved over time. Bronchiolitis is now used to describe conditions where the primary pathologic condition is damage to the bronchiolar epithelium not attributable to a larger parenchymal disease (such as hypersensitivity pneumonitis). This change in nomenclature explains why the condition formerly known as “bronchiolitis obliterans organizing pneumonia” (BOOP) is now simply recognized as “organizing pneumonia.” Despite several proposed classification schemes focusing on histopathology, there is no consensus regarding the different subtypes of bronchiolitis, leading to confusion in some cases. Recently, authors have attempted to distinguish cases based on three main histologic patterns (Urisman A, et al. Surg Pathol Clin. 2020;13[1]:189).
- Obliterative/constrictive bronchiolitis (OB) – the terms “obliterative” and “constrictive” are used interchangeably throughout pulmonary literature. It is characterized by fibroblast-rich tissue accumulation in the sub-epithelium of bronchioles leading to progressive narrowing of the lumen. In addition to the transplant setting, it is often seen in patients with rheumatoid arthritis or other connective tissue diseases, inhalational exposures, or acute respiratory infections. More recently, clinicians have recognized diffuse idiopathic pulmonary neuroendocrine cell hyperplasia (DIPNECH) as a rare condition causing OB with potentially effective treatment.
- Follicular bronchiolitis (FB) – features peribronchiolar inflammation with subepithelial lymphoid deposits leading to luminal obstruction. FB is chiefly associated with conditions of impaired immunity or chronic airway infection, such as autoimmune connective tissues diseases (especially rheumatoid arthritis and Sjogren’s), severe combined immunodeficiency, HIV, cystic fibrosis, and primary ciliary dyskinesia.
- Diffuse panbronchiolitis (DBP) – features bilateral bronchiolar lesions with lymphocytic inflammation of the bronchiolar wall, as well as peribronchiolar inflammation and accumulation of interstitial foamy macrophages. Patients afflicted with DBP may suffer repeated bacterial colonization or infection. There is a higher prevalence of DBP in Asia where it was first identified in the 1960s, potentially due to several HLA alleles that are more common in Asia.
In addition to the above terminology, the transplant-setting diagnosis “bronchiolitis obliterans syndrome” (BOS) is used to denote progressive obstructive lung disease for which there is not another cause aside from chronic graft rejection. For these patients, clinicians assume the underlying disease entity is OB, but they often lack histopathologic confirmation.
Diagnosis is challenging
Symptoms of bronchiolitis are typically dyspnea and cough, and patients may often be diagnosed with asthma or COPD initially. Pulmonary function testing may show signs of obstruction, restriction, or mixed disease with or without a reduction in Dlco. Chest radiography often appears normal, but high-resolution CT may show expiratory air trapping and centrilobular nodules. Advanced imaging modalities may augment or replace CT imaging in diagnosing bronchiolitis: investigators are evaluating pulmonary MRI and fluoroscopy with computerized ventilation analysis in clinical trials (NCT04080232).
Currently, open or thoracoscopic lung biopsy is typically required to make a definitive diagnosis. Because bronchiolitis is a patchy and heterogeneous process, transbronchial biopsy may provide insufficient yield, with a sensitivity of 29% to 70% reported in lung transplant literature (Urisman A, et al. Surg Pathol Clin. 2020;13[1]:189).
Recent studies have demonstrated transbronchial cryobiopsy to be a promising alternative to surgical biopsy, owing to larger tissue samples than conventional transbronchial lung biopsies. For example, in a recent case series four patients underwent transbronchial cryobiopsy. The procedure yielded adequate tissue for diagnosis of a chronic bronchiolitis in each case (Yamakawa H, et al. Internal Med Advance Publication. doi: 10.2169/internalmedicine.6028-20.
Treatment options are growing
Evidence for treatment of bronchiolitis remains limited. Options are extrapolated from lung transplant patients, where incidence of BOS ranges from 50% at 5 years to 76% at 10 years post transplant. Guidelines recommend a 3-month minimum trial of azithromycin, which has been shown to slow or reverse decline of lung function in some patients. Modification of immunosuppression is also recommended. In patients who have continued lung function decline, a systematic review concluded that extracorporeal photopheresis had the most robust evidence for efficacy with stabilized lung function and improved overall survival (Benden C, et al. J Heart Lung Transplant. 2017;36[9]:921). Other salvage therapies that have lower-quality evidence of benefit include total lymphoid irradiation, montelukast, and aerosolized cyclosporine.
In patients who have undergone hematopoietic stem cell transplant, steroids are typically the first line treatment for OB as it is thought to be a form of chronic graft-vs-host disease (GVHD). Ruxolitinib, a selective JAK1/2 inhibitor, demonstrated significant improvement overall in patients with steroid-refractory acute GVHD in a recent randomized clinical trial, although the trial did not examine its effect on pulmonary manifestations (Zeiser R, et al. N Engl J Med. 2020;382[19]:1800). To date, retrospective observational studies of ruxolitinib in patients with lung GVHD have shown conflicting results regarding benefit. Investigators are currently studying ruxolitinib in a phase II trial for patients with BOS following stem cell transplant (NCT03674047).
DIPNECH is unique from other bronchiolitis entities, as small airways dysfunction develops as a result of neuroendocrine cell proliferation in the airway mucosa, ultimately leading to bronchial narrowing. It most commonly presents in middle-aged nonsmoking women with years of chronic cough and dyspnea. While it has an indolent course in many patients, some patients develop progressive symptoms and obstructive lung disease. DIPNECH is considered a precursor to other pulmonary neuroendocrine tumors. The lesions demonstrate somatostatin receptor expression in many cases, prompting the use of somatostatin analogues as treatment. In the largest published case series, 42 patients from three different institutions were identified who were treated with somatostatin analogues for a mean of 38.8 months at the time of review. Symptomatic improvement was seen in 33 of the 42 (79%), and of the 15 with posttreatment PFT data, 14 (93%) showed improvement in PFTs (Al-Toubah, T, et al. Chest. 2020;158[1]:401). Other small studies have demonstrated varying results with symptomatic improvement in 29% to 76% of patients and improvement or stability of PFTs in 50% to 100% of patients (Samhouri BF, et al. ERJ Open Res. 2020;6[4]:527).
For patients who have not undergone lung transplant, and who do not have an identifiable exposure or underlying rheumatologic condition, a similar 3-month minimum trial of macrolide antibiotics is reasonable. Macrolides have been shown to double long-term survival rates to over 90% in patients with DPB. Evidence in this patient population is quite limited, and further research is needed to determine effective therapies for patients.
What’s next for bronchiolitis
While clinicians currently have few tools for diagnosing and treating these uncommon diseases, in the coming years, we should learn whether novel imaging modalities or less invasive procedures can aid in the diagnosis. Physicians hope these advances will preclude the need for invasive biopsies in more patients going forward. We should also learn whether newer, targeted agents like ruxolitinib are effective for BOS in patients with stem cell transplant. If so, this finding may open it and similar agents to investigation in other forms of bronchiolitis.
Dr. Poole and Dr. Callahan are with University of Utah Health, Salt Lake City, Utah.
What’s in a name?
Bronchiolitis, a group of diseases also referred to as “small airways diseases,” is characterized by inflammation and/or fibrosis in airways less than 2 mm in diameter. In pediatric patients, it is most commonly related to acute viral infections, while in adults, it is often associated with chronic diseases. Bronchiolitis is a well-recognized complication in a significant number of patients who have undergone lung or stem cell transplantation. Common associations also include connective tissue diseases, environmental or occupational inhalation exposures, aspiration, drug toxicity, and infections. Diagnosing bronchiolitis can be challenging for clinicians, and few treatment options exist apart from treating identifiable underlying etiologies. More research is needed into noninvasive diagnostic techniques and treatment modalities.
The terminology used to describe bronchiolitis has evolved over time. Bronchiolitis is now used to describe conditions where the primary pathologic condition is damage to the bronchiolar epithelium not attributable to a larger parenchymal disease (such as hypersensitivity pneumonitis). This change in nomenclature explains why the condition formerly known as “bronchiolitis obliterans organizing pneumonia” (BOOP) is now simply recognized as “organizing pneumonia.” Despite several proposed classification schemes focusing on histopathology, there is no consensus regarding the different subtypes of bronchiolitis, leading to confusion in some cases. Recently, authors have attempted to distinguish cases based on three main histologic patterns (Urisman A, et al. Surg Pathol Clin. 2020;13[1]:189).
- Obliterative/constrictive bronchiolitis (OB) – the terms “obliterative” and “constrictive” are used interchangeably throughout pulmonary literature. It is characterized by fibroblast-rich tissue accumulation in the sub-epithelium of bronchioles leading to progressive narrowing of the lumen. In addition to the transplant setting, it is often seen in patients with rheumatoid arthritis or other connective tissue diseases, inhalational exposures, or acute respiratory infections. More recently, clinicians have recognized diffuse idiopathic pulmonary neuroendocrine cell hyperplasia (DIPNECH) as a rare condition causing OB with potentially effective treatment.
- Follicular bronchiolitis (FB) – features peribronchiolar inflammation with subepithelial lymphoid deposits leading to luminal obstruction. FB is chiefly associated with conditions of impaired immunity or chronic airway infection, such as autoimmune connective tissues diseases (especially rheumatoid arthritis and Sjogren’s), severe combined immunodeficiency, HIV, cystic fibrosis, and primary ciliary dyskinesia.
- Diffuse panbronchiolitis (DBP) – features bilateral bronchiolar lesions with lymphocytic inflammation of the bronchiolar wall, as well as peribronchiolar inflammation and accumulation of interstitial foamy macrophages. Patients afflicted with DBP may suffer repeated bacterial colonization or infection. There is a higher prevalence of DBP in Asia where it was first identified in the 1960s, potentially due to several HLA alleles that are more common in Asia.
In addition to the above terminology, the transplant-setting diagnosis “bronchiolitis obliterans syndrome” (BOS) is used to denote progressive obstructive lung disease for which there is not another cause aside from chronic graft rejection. For these patients, clinicians assume the underlying disease entity is OB, but they often lack histopathologic confirmation.
Diagnosis is challenging
Symptoms of bronchiolitis are typically dyspnea and cough, and patients may often be diagnosed with asthma or COPD initially. Pulmonary function testing may show signs of obstruction, restriction, or mixed disease with or without a reduction in Dlco. Chest radiography often appears normal, but high-resolution CT may show expiratory air trapping and centrilobular nodules. Advanced imaging modalities may augment or replace CT imaging in diagnosing bronchiolitis: investigators are evaluating pulmonary MRI and fluoroscopy with computerized ventilation analysis in clinical trials (NCT04080232).
Currently, open or thoracoscopic lung biopsy is typically required to make a definitive diagnosis. Because bronchiolitis is a patchy and heterogeneous process, transbronchial biopsy may provide insufficient yield, with a sensitivity of 29% to 70% reported in lung transplant literature (Urisman A, et al. Surg Pathol Clin. 2020;13[1]:189).
Recent studies have demonstrated transbronchial cryobiopsy to be a promising alternative to surgical biopsy, owing to larger tissue samples than conventional transbronchial lung biopsies. For example, in a recent case series four patients underwent transbronchial cryobiopsy. The procedure yielded adequate tissue for diagnosis of a chronic bronchiolitis in each case (Yamakawa H, et al. Internal Med Advance Publication. doi: 10.2169/internalmedicine.6028-20.
Treatment options are growing
Evidence for treatment of bronchiolitis remains limited. Options are extrapolated from lung transplant patients, where incidence of BOS ranges from 50% at 5 years to 76% at 10 years post transplant. Guidelines recommend a 3-month minimum trial of azithromycin, which has been shown to slow or reverse decline of lung function in some patients. Modification of immunosuppression is also recommended. In patients who have continued lung function decline, a systematic review concluded that extracorporeal photopheresis had the most robust evidence for efficacy with stabilized lung function and improved overall survival (Benden C, et al. J Heart Lung Transplant. 2017;36[9]:921). Other salvage therapies that have lower-quality evidence of benefit include total lymphoid irradiation, montelukast, and aerosolized cyclosporine.
In patients who have undergone hematopoietic stem cell transplant, steroids are typically the first line treatment for OB as it is thought to be a form of chronic graft-vs-host disease (GVHD). Ruxolitinib, a selective JAK1/2 inhibitor, demonstrated significant improvement overall in patients with steroid-refractory acute GVHD in a recent randomized clinical trial, although the trial did not examine its effect on pulmonary manifestations (Zeiser R, et al. N Engl J Med. 2020;382[19]:1800). To date, retrospective observational studies of ruxolitinib in patients with lung GVHD have shown conflicting results regarding benefit. Investigators are currently studying ruxolitinib in a phase II trial for patients with BOS following stem cell transplant (NCT03674047).
DIPNECH is unique from other bronchiolitis entities, as small airways dysfunction develops as a result of neuroendocrine cell proliferation in the airway mucosa, ultimately leading to bronchial narrowing. It most commonly presents in middle-aged nonsmoking women with years of chronic cough and dyspnea. While it has an indolent course in many patients, some patients develop progressive symptoms and obstructive lung disease. DIPNECH is considered a precursor to other pulmonary neuroendocrine tumors. The lesions demonstrate somatostatin receptor expression in many cases, prompting the use of somatostatin analogues as treatment. In the largest published case series, 42 patients from three different institutions were identified who were treated with somatostatin analogues for a mean of 38.8 months at the time of review. Symptomatic improvement was seen in 33 of the 42 (79%), and of the 15 with posttreatment PFT data, 14 (93%) showed improvement in PFTs (Al-Toubah, T, et al. Chest. 2020;158[1]:401). Other small studies have demonstrated varying results with symptomatic improvement in 29% to 76% of patients and improvement or stability of PFTs in 50% to 100% of patients (Samhouri BF, et al. ERJ Open Res. 2020;6[4]:527).
For patients who have not undergone lung transplant, and who do not have an identifiable exposure or underlying rheumatologic condition, a similar 3-month minimum trial of macrolide antibiotics is reasonable. Macrolides have been shown to double long-term survival rates to over 90% in patients with DPB. Evidence in this patient population is quite limited, and further research is needed to determine effective therapies for patients.
What’s next for bronchiolitis
While clinicians currently have few tools for diagnosing and treating these uncommon diseases, in the coming years, we should learn whether novel imaging modalities or less invasive procedures can aid in the diagnosis. Physicians hope these advances will preclude the need for invasive biopsies in more patients going forward. We should also learn whether newer, targeted agents like ruxolitinib are effective for BOS in patients with stem cell transplant. If so, this finding may open it and similar agents to investigation in other forms of bronchiolitis.
Dr. Poole and Dr. Callahan are with University of Utah Health, Salt Lake City, Utah.
Teenagers get in the queue for COVID-19 vaccines
The vaccinations can’t come soon enough for parents like Stacy Hillenburg, a developmental therapist in Aurora, Ill., whose 9-year-old son takes immunosuppressants because he had a heart transplant when he was 7 weeks old. Although school-age children aren’t yet included in clinical trials, if her 12- and 13-year-old daughters could get vaccinated, along with both parents, then the family could relax some of the protocols they currently follow to prevent infection.
Whenever they are around other people, even masked and socially distanced, they come home and immediately shower and change their clothes. So far, no one in the family has been infected with COVID, but the anxiety is ever-present. “I can’t wait for it to come out,” Ms. Hillenburg said of a pediatric COVID vaccine. “It will ease my mind so much.”
She isn’t alone in that anticipation. In the fall, the American Academy of Pediatrics and other pediatric vaccine experts urged faster action on pediatric vaccine trials and worried that children would be left behind as adults gained protection from COVID. But recent developments have eased those concerns.
“Over the next couple of months, we will be doing trials in an age-deescalation manner,” with studies moving gradually to younger children, Anthony S. Fauci, MD, chief medical adviser on COVID-19 to the president, said in a coronavirus response team briefing on Jan. 29. “So that hopefully, as we get to the late spring and summer, we will have children being able to be vaccinated.”
Pfizer completed enrollment of 2,259 teens aged 12-15 years in late January and expects to move forward with a separate pediatric trial of children aged 5-11 years by this spring, Keanna Ghazvini, senior associate for global media relations at Pfizer, said in an interview.
Enrollment in Moderna’s TeenCove study of adolescents ages 12-17 years began slowly in late December, but the pace has since picked up, said company spokesperson Colleen Hussey. “We continue to bring clinical trial sites online, and we are on track to provide updated data around mid-year 2021.” A trial extension in children 11 years and younger is expected to begin later in 2021.
Johnson & Johnson and AstraZeneca said they expect to begin adolescent trials in early 2021, according to data shared by the Advisory Committee on Immunization Practices. An interim analysis of J&J’s Janssen COVID-19 vaccine trial data, released on Jan. 29, showed it was 72% effective in US participants aged 18 years or older. AstraZeneca’s U.S. trial in adults is ongoing.
Easing the burden
Vaccination could lessen children’s risk of severe disease as well as the social and emotional burdens of the pandemic, says James Campbell, MD, a pediatric infectious disease specialist at the University of Maryland’s Center for Vaccine Development in Baltimore, which was involved in the Moderna and early-phase Pfizer trials. He coauthored a September 2020 article in Clinical Infectious Diseases titled: “Warp Speed for COVID-19 vaccines: Why are children stuck in neutral?”
The adolescent trials are a vital step to ensure timely vaccine access for teens and younger children. “It is reasonable, when you have limited vaccine, that your rollout goes to the highest priority and then moves to lower and lower priorities. In adults, we’re just saying: ‘Wait your turn,’ ” he said of the current vaccination effort. “If we didn’t have the [vaccine trial] data in children, we’d be saying: ‘You don’t have a turn.’ ”
As the pandemic has worn on, the burden on children has grown. As of Tuesday, 269 children had died of COVID-19. That is well above the highest annual death toll recorded during a regular flu season – 188 flu deaths among children and adolescents under 18 in the 2019-2020 and 2017-2018 flu seasons.
Children are less likely to transmit COVID-19 in their household than adults, according to a meta-analysis of 54 studies published in JAMA Network Open. But that does not necessarily mean children are less infectious, the authors said, noting that unmeasured factors could have affected the spread of infection among adults.
Moreover, children and adolescents need protection from COVID infection – and from the potential for severe disease or lingering effects – and, given that there are 74 million children and teens in the United States, their vaccination is an important part of stopping the pandemic, said Grace Lee, MD, professor of pediatrics at Stanford (Calif.) University, and cochair of ACIP’s COVID-19 Vaccine Safety Technical Subgroup.
“In order to interrupt transmission, I don’t see how we’re going to do that without vaccinating children and adolescents,” she said.
Dr. Lee said her 16-year-old daughter misses the normal teenage social life and is excited about getting the vaccine when she is eligible. (Adolescents without high-risk conditions are in the lowest vaccination tier, according to ACIP recommendations.) “There is truly individual protection to be gained,” Dr. Lee said.
She noted that researchers continue to assess the immune responses to the adult vaccines – even looking at immune characteristics of the small percentage of people who aren’t protected from infection – and that information helps in the evaluation of the pediatric immune responses. As the trials expand to younger children and infants, dosing will be a major focus. “How many doses do they need they need to receive the same immunity? Safety considerations will be critically important,” she said.
Teen trials underway
Pfizer/BioNTech extended its adult trial to 16- and 17-year-olds in October, which enabled older teens to be included in its emergency-use authorization. They and younger teens, ages 12-15, receive the same dose as adults.
The ongoing trials with Pfizer and Moderna vaccines are immunobridging trials, designed to study safety and immunogenicity. Investigators will compare the teens’ immune response with the findings from the larger adult trials. When the trials expand to school-age children (6-12 years), protocols call for testing the safety and immunogenicity of a half-dose vaccine as well as the full dose.
Children ages 2-5 years and infants and toddlers will be enrolled in future trials, studying safety and immunogenicity of full, half, or even quarter dosages. The Pediatric Research Equity Act of 2003 requires licensed vaccines to be tested for safety and efficacy in children, unless they are not appropriate for a pediatric population.
Demand for the teen trials has been strong. At Cincinnati Children’s Hospital Medical Center, 259 teenagers joined the Pfizer/BioNTech trial, but some teenagers were turned away when the trial’s national enrollment closed in late January.
“Many of the children are having no side effects, and if they are, they’re having the same [effects] as the young adults – local redness or pain, fatigue, and headaches,” said Robert Frenck, MD, director of the Cincinnati Children’s Gamble Program for Clinical Studies.
Parents may share some of the vaccine hesitancy that has affected adult vaccination. But that is balanced by the hope that vaccines will end the pandemic and usher in a new normal. “If it looks like [vaccines] will increase the likelihood of children returning to school safely, that may be a motivating factor,” Dr. Frenck said.
Cody Meissner, MD, chief of the pediatric infectious disease service at Tufts Medical Center, Boston, was initially cautious about the extension of vaccination to adolescents. A member of the Vaccine and Related Biological Products Advisory Committee, which evaluates data and makes recommendations to the Food and Drug Administration, Dr. Meissner initially abstained in the vote on the Pfizer/BioNTech emergency-use authorization for people 16 and older.
He noted that, at the time the committee reviewed the Pfizer vaccine, the company had data available for just 134 teenagers, half of whom received a placebo. But the vaccination of 34 million adults has provided robust data about the vaccine’s safety, and the trial expansion into adolescents is important.
“I’m comfortable with the way these trials are going now,” he said. “This is the way I was hoping they would go.”
Ms. Hillenburg is on the parent advisory board of Voices for Vaccines, an organization of parents supporting vaccination that is affiliated with the Task Force for Global Health, an Atlanta-based independent public health organization. Dr. Campbell’s institution has received funds to conduct clinical trials from the National Institutes of Health and several companies, including Merck, GlaxoSmithKline, Sanofi, Pfizer, and Moderna. He has served pro bono on many safety and data monitoring committees. Dr. Frenck’s institution is receiving funds to conduct the Pfizer trial. In the past 5 years, he has also participated in clinical trials for GlaxoSmithKline, Merck, and Meissa vaccines. Dr. Lee and Dr. Meissner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The vaccinations can’t come soon enough for parents like Stacy Hillenburg, a developmental therapist in Aurora, Ill., whose 9-year-old son takes immunosuppressants because he had a heart transplant when he was 7 weeks old. Although school-age children aren’t yet included in clinical trials, if her 12- and 13-year-old daughters could get vaccinated, along with both parents, then the family could relax some of the protocols they currently follow to prevent infection.
Whenever they are around other people, even masked and socially distanced, they come home and immediately shower and change their clothes. So far, no one in the family has been infected with COVID, but the anxiety is ever-present. “I can’t wait for it to come out,” Ms. Hillenburg said of a pediatric COVID vaccine. “It will ease my mind so much.”
She isn’t alone in that anticipation. In the fall, the American Academy of Pediatrics and other pediatric vaccine experts urged faster action on pediatric vaccine trials and worried that children would be left behind as adults gained protection from COVID. But recent developments have eased those concerns.
“Over the next couple of months, we will be doing trials in an age-deescalation manner,” with studies moving gradually to younger children, Anthony S. Fauci, MD, chief medical adviser on COVID-19 to the president, said in a coronavirus response team briefing on Jan. 29. “So that hopefully, as we get to the late spring and summer, we will have children being able to be vaccinated.”
Pfizer completed enrollment of 2,259 teens aged 12-15 years in late January and expects to move forward with a separate pediatric trial of children aged 5-11 years by this spring, Keanna Ghazvini, senior associate for global media relations at Pfizer, said in an interview.
Enrollment in Moderna’s TeenCove study of adolescents ages 12-17 years began slowly in late December, but the pace has since picked up, said company spokesperson Colleen Hussey. “We continue to bring clinical trial sites online, and we are on track to provide updated data around mid-year 2021.” A trial extension in children 11 years and younger is expected to begin later in 2021.
Johnson & Johnson and AstraZeneca said they expect to begin adolescent trials in early 2021, according to data shared by the Advisory Committee on Immunization Practices. An interim analysis of J&J’s Janssen COVID-19 vaccine trial data, released on Jan. 29, showed it was 72% effective in US participants aged 18 years or older. AstraZeneca’s U.S. trial in adults is ongoing.
Easing the burden
Vaccination could lessen children’s risk of severe disease as well as the social and emotional burdens of the pandemic, says James Campbell, MD, a pediatric infectious disease specialist at the University of Maryland’s Center for Vaccine Development in Baltimore, which was involved in the Moderna and early-phase Pfizer trials. He coauthored a September 2020 article in Clinical Infectious Diseases titled: “Warp Speed for COVID-19 vaccines: Why are children stuck in neutral?”
The adolescent trials are a vital step to ensure timely vaccine access for teens and younger children. “It is reasonable, when you have limited vaccine, that your rollout goes to the highest priority and then moves to lower and lower priorities. In adults, we’re just saying: ‘Wait your turn,’ ” he said of the current vaccination effort. “If we didn’t have the [vaccine trial] data in children, we’d be saying: ‘You don’t have a turn.’ ”
As the pandemic has worn on, the burden on children has grown. As of Tuesday, 269 children had died of COVID-19. That is well above the highest annual death toll recorded during a regular flu season – 188 flu deaths among children and adolescents under 18 in the 2019-2020 and 2017-2018 flu seasons.
Children are less likely to transmit COVID-19 in their household than adults, according to a meta-analysis of 54 studies published in JAMA Network Open. But that does not necessarily mean children are less infectious, the authors said, noting that unmeasured factors could have affected the spread of infection among adults.
Moreover, children and adolescents need protection from COVID infection – and from the potential for severe disease or lingering effects – and, given that there are 74 million children and teens in the United States, their vaccination is an important part of stopping the pandemic, said Grace Lee, MD, professor of pediatrics at Stanford (Calif.) University, and cochair of ACIP’s COVID-19 Vaccine Safety Technical Subgroup.
“In order to interrupt transmission, I don’t see how we’re going to do that without vaccinating children and adolescents,” she said.
Dr. Lee said her 16-year-old daughter misses the normal teenage social life and is excited about getting the vaccine when she is eligible. (Adolescents without high-risk conditions are in the lowest vaccination tier, according to ACIP recommendations.) “There is truly individual protection to be gained,” Dr. Lee said.
She noted that researchers continue to assess the immune responses to the adult vaccines – even looking at immune characteristics of the small percentage of people who aren’t protected from infection – and that information helps in the evaluation of the pediatric immune responses. As the trials expand to younger children and infants, dosing will be a major focus. “How many doses do they need they need to receive the same immunity? Safety considerations will be critically important,” she said.
Teen trials underway
Pfizer/BioNTech extended its adult trial to 16- and 17-year-olds in October, which enabled older teens to be included in its emergency-use authorization. They and younger teens, ages 12-15, receive the same dose as adults.
The ongoing trials with Pfizer and Moderna vaccines are immunobridging trials, designed to study safety and immunogenicity. Investigators will compare the teens’ immune response with the findings from the larger adult trials. When the trials expand to school-age children (6-12 years), protocols call for testing the safety and immunogenicity of a half-dose vaccine as well as the full dose.
Children ages 2-5 years and infants and toddlers will be enrolled in future trials, studying safety and immunogenicity of full, half, or even quarter dosages. The Pediatric Research Equity Act of 2003 requires licensed vaccines to be tested for safety and efficacy in children, unless they are not appropriate for a pediatric population.
Demand for the teen trials has been strong. At Cincinnati Children’s Hospital Medical Center, 259 teenagers joined the Pfizer/BioNTech trial, but some teenagers were turned away when the trial’s national enrollment closed in late January.
“Many of the children are having no side effects, and if they are, they’re having the same [effects] as the young adults – local redness or pain, fatigue, and headaches,” said Robert Frenck, MD, director of the Cincinnati Children’s Gamble Program for Clinical Studies.
Parents may share some of the vaccine hesitancy that has affected adult vaccination. But that is balanced by the hope that vaccines will end the pandemic and usher in a new normal. “If it looks like [vaccines] will increase the likelihood of children returning to school safely, that may be a motivating factor,” Dr. Frenck said.
Cody Meissner, MD, chief of the pediatric infectious disease service at Tufts Medical Center, Boston, was initially cautious about the extension of vaccination to adolescents. A member of the Vaccine and Related Biological Products Advisory Committee, which evaluates data and makes recommendations to the Food and Drug Administration, Dr. Meissner initially abstained in the vote on the Pfizer/BioNTech emergency-use authorization for people 16 and older.
He noted that, at the time the committee reviewed the Pfizer vaccine, the company had data available for just 134 teenagers, half of whom received a placebo. But the vaccination of 34 million adults has provided robust data about the vaccine’s safety, and the trial expansion into adolescents is important.
“I’m comfortable with the way these trials are going now,” he said. “This is the way I was hoping they would go.”
Ms. Hillenburg is on the parent advisory board of Voices for Vaccines, an organization of parents supporting vaccination that is affiliated with the Task Force for Global Health, an Atlanta-based independent public health organization. Dr. Campbell’s institution has received funds to conduct clinical trials from the National Institutes of Health and several companies, including Merck, GlaxoSmithKline, Sanofi, Pfizer, and Moderna. He has served pro bono on many safety and data monitoring committees. Dr. Frenck’s institution is receiving funds to conduct the Pfizer trial. In the past 5 years, he has also participated in clinical trials for GlaxoSmithKline, Merck, and Meissa vaccines. Dr. Lee and Dr. Meissner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The vaccinations can’t come soon enough for parents like Stacy Hillenburg, a developmental therapist in Aurora, Ill., whose 9-year-old son takes immunosuppressants because he had a heart transplant when he was 7 weeks old. Although school-age children aren’t yet included in clinical trials, if her 12- and 13-year-old daughters could get vaccinated, along with both parents, then the family could relax some of the protocols they currently follow to prevent infection.
Whenever they are around other people, even masked and socially distanced, they come home and immediately shower and change their clothes. So far, no one in the family has been infected with COVID, but the anxiety is ever-present. “I can’t wait for it to come out,” Ms. Hillenburg said of a pediatric COVID vaccine. “It will ease my mind so much.”
She isn’t alone in that anticipation. In the fall, the American Academy of Pediatrics and other pediatric vaccine experts urged faster action on pediatric vaccine trials and worried that children would be left behind as adults gained protection from COVID. But recent developments have eased those concerns.
“Over the next couple of months, we will be doing trials in an age-deescalation manner,” with studies moving gradually to younger children, Anthony S. Fauci, MD, chief medical adviser on COVID-19 to the president, said in a coronavirus response team briefing on Jan. 29. “So that hopefully, as we get to the late spring and summer, we will have children being able to be vaccinated.”
Pfizer completed enrollment of 2,259 teens aged 12-15 years in late January and expects to move forward with a separate pediatric trial of children aged 5-11 years by this spring, Keanna Ghazvini, senior associate for global media relations at Pfizer, said in an interview.
Enrollment in Moderna’s TeenCove study of adolescents ages 12-17 years began slowly in late December, but the pace has since picked up, said company spokesperson Colleen Hussey. “We continue to bring clinical trial sites online, and we are on track to provide updated data around mid-year 2021.” A trial extension in children 11 years and younger is expected to begin later in 2021.
Johnson & Johnson and AstraZeneca said they expect to begin adolescent trials in early 2021, according to data shared by the Advisory Committee on Immunization Practices. An interim analysis of J&J’s Janssen COVID-19 vaccine trial data, released on Jan. 29, showed it was 72% effective in US participants aged 18 years or older. AstraZeneca’s U.S. trial in adults is ongoing.
Easing the burden
Vaccination could lessen children’s risk of severe disease as well as the social and emotional burdens of the pandemic, says James Campbell, MD, a pediatric infectious disease specialist at the University of Maryland’s Center for Vaccine Development in Baltimore, which was involved in the Moderna and early-phase Pfizer trials. He coauthored a September 2020 article in Clinical Infectious Diseases titled: “Warp Speed for COVID-19 vaccines: Why are children stuck in neutral?”
The adolescent trials are a vital step to ensure timely vaccine access for teens and younger children. “It is reasonable, when you have limited vaccine, that your rollout goes to the highest priority and then moves to lower and lower priorities. In adults, we’re just saying: ‘Wait your turn,’ ” he said of the current vaccination effort. “If we didn’t have the [vaccine trial] data in children, we’d be saying: ‘You don’t have a turn.’ ”
As the pandemic has worn on, the burden on children has grown. As of Tuesday, 269 children had died of COVID-19. That is well above the highest annual death toll recorded during a regular flu season – 188 flu deaths among children and adolescents under 18 in the 2019-2020 and 2017-2018 flu seasons.
Children are less likely to transmit COVID-19 in their household than adults, according to a meta-analysis of 54 studies published in JAMA Network Open. But that does not necessarily mean children are less infectious, the authors said, noting that unmeasured factors could have affected the spread of infection among adults.
Moreover, children and adolescents need protection from COVID infection – and from the potential for severe disease or lingering effects – and, given that there are 74 million children and teens in the United States, their vaccination is an important part of stopping the pandemic, said Grace Lee, MD, professor of pediatrics at Stanford (Calif.) University, and cochair of ACIP’s COVID-19 Vaccine Safety Technical Subgroup.
“In order to interrupt transmission, I don’t see how we’re going to do that without vaccinating children and adolescents,” she said.
Dr. Lee said her 16-year-old daughter misses the normal teenage social life and is excited about getting the vaccine when she is eligible. (Adolescents without high-risk conditions are in the lowest vaccination tier, according to ACIP recommendations.) “There is truly individual protection to be gained,” Dr. Lee said.
She noted that researchers continue to assess the immune responses to the adult vaccines – even looking at immune characteristics of the small percentage of people who aren’t protected from infection – and that information helps in the evaluation of the pediatric immune responses. As the trials expand to younger children and infants, dosing will be a major focus. “How many doses do they need they need to receive the same immunity? Safety considerations will be critically important,” she said.
Teen trials underway
Pfizer/BioNTech extended its adult trial to 16- and 17-year-olds in October, which enabled older teens to be included in its emergency-use authorization. They and younger teens, ages 12-15, receive the same dose as adults.
The ongoing trials with Pfizer and Moderna vaccines are immunobridging trials, designed to study safety and immunogenicity. Investigators will compare the teens’ immune response with the findings from the larger adult trials. When the trials expand to school-age children (6-12 years), protocols call for testing the safety and immunogenicity of a half-dose vaccine as well as the full dose.
Children ages 2-5 years and infants and toddlers will be enrolled in future trials, studying safety and immunogenicity of full, half, or even quarter dosages. The Pediatric Research Equity Act of 2003 requires licensed vaccines to be tested for safety and efficacy in children, unless they are not appropriate for a pediatric population.
Demand for the teen trials has been strong. At Cincinnati Children’s Hospital Medical Center, 259 teenagers joined the Pfizer/BioNTech trial, but some teenagers were turned away when the trial’s national enrollment closed in late January.
“Many of the children are having no side effects, and if they are, they’re having the same [effects] as the young adults – local redness or pain, fatigue, and headaches,” said Robert Frenck, MD, director of the Cincinnati Children’s Gamble Program for Clinical Studies.
Parents may share some of the vaccine hesitancy that has affected adult vaccination. But that is balanced by the hope that vaccines will end the pandemic and usher in a new normal. “If it looks like [vaccines] will increase the likelihood of children returning to school safely, that may be a motivating factor,” Dr. Frenck said.
Cody Meissner, MD, chief of the pediatric infectious disease service at Tufts Medical Center, Boston, was initially cautious about the extension of vaccination to adolescents. A member of the Vaccine and Related Biological Products Advisory Committee, which evaluates data and makes recommendations to the Food and Drug Administration, Dr. Meissner initially abstained in the vote on the Pfizer/BioNTech emergency-use authorization for people 16 and older.
He noted that, at the time the committee reviewed the Pfizer vaccine, the company had data available for just 134 teenagers, half of whom received a placebo. But the vaccination of 34 million adults has provided robust data about the vaccine’s safety, and the trial expansion into adolescents is important.
“I’m comfortable with the way these trials are going now,” he said. “This is the way I was hoping they would go.”
Ms. Hillenburg is on the parent advisory board of Voices for Vaccines, an organization of parents supporting vaccination that is affiliated with the Task Force for Global Health, an Atlanta-based independent public health organization. Dr. Campbell’s institution has received funds to conduct clinical trials from the National Institutes of Health and several companies, including Merck, GlaxoSmithKline, Sanofi, Pfizer, and Moderna. He has served pro bono on many safety and data monitoring committees. Dr. Frenck’s institution is receiving funds to conduct the Pfizer trial. In the past 5 years, he has also participated in clinical trials for GlaxoSmithKline, Merck, and Meissa vaccines. Dr. Lee and Dr. Meissner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Inhaled hyaluronan may bring sigh of relief to COPD patients
(COPD), findings of a new study suggest.
HMW-HA was associated with a significantly shorter duration of noninvasive positive-pressure ventilation (NIPPV), lower systemic inflammatory markers, and lower measured peak airway pressure, compared with placebo, reported lead author Flavia Galdi, MD, of Campus Bio-Medico University Hospital, Rome, and colleagues.
“HMW-HA is a naturally occurring sugar that is abundant in the extracellular matrix, including in the lung,” the investigators wrote in Respiratory Research. “[It] has been used routinely, together with hypertonic saline, in cystic fibrosis patients [for several years] with no reported side effects; rather, it improves tolerability and decreases the need for bronchodilators in these patients.”
According to Robert A. Sandhaus, MD, PhD, FCCP, of National Jewish Health, Denver, the role of hyaluronan in lung disease was first recognized decades ago.
“Data stretching back into the 1970s has identified decreases in hyaluronan content in emphysematous lung tissue, protection of lung connective tissue from proteolysis by hyaluronan, and potential therapeutic roles for hyaluronan in a variety of disease, especially of the lungs,” he said in an interview.
For patients with COPD, treatment with HMW-HA may provide benefit by counteracting an imbalance in diseased lung tissue, wrote Dr. Galdi and colleagues.
“Emerging evidence suggests that imbalance between declining HMW-HA levels, and increasing smaller fragments of hyaluronan may contribute to chronic airway disease pathogenesis,” they wrote. “This has led to the hypothesis that exogenous supplementation of HMW-HA may restore hyaluronan homeostasis in favor of undegraded molecules, inhibit inflammation and loss of lung function, and ameliorate COPD progression.”
To test this hypothesis, the investigators screened 44 patients with a history of acute exacerbations of COPD necessitating NIPPV, ultimately excluding 3 patients because of heart failure. Following 1:1 randomization, 20 patients received HMW-HA while 21 received placebo, each twice daily, in conjunction with NIPPV and standard medical therapy. Treatment continued until NIPPV failure or liberation from NIPPV. Most patients received NIPPV in the hospital; however, home/chronic NIPPV was given to four patients in the placebo group and three patients in the HMW-HA group.
The primary outcome was duration of NIPPV. Secondary outcomes included markers of systemic inflammation associated with acute exacerbations of COPD and respiratory physiology parameters. Adverse events were also reported.
Results showed that patients treated with HMW-HA were liberated sooner from NIPPV than were those who received placebo (mean, 5.2 vs 6.4 days; P < .037). Similarly, patients in the HMW-HA group had significantly shorter hospital stay, on average, than those in the placebo group (mean, 7.2 vs 10.2 days; P = .039). Median values followed a similar pattern.
“These data suggest that HMW-HA shortened the duration of acute respiratory failure, need for NIPPV and, consequently, hospital length of stay in these patients,” the investigators wrote.
Secondary outcomes further supported these therapeutic benefits. Compared with placebo, HMW-HA was associated with significantly lower peak pressure and greater improvements in both pCO2/FiO2 ratio and inflammatory markers. No adverse events were reported.
Further analyses involving human bronchial epithelial cell cultures offered some mechanistic insight. Using micro-optical coherence tomography imaging, the investigators found that HMW-HA treatment was associated with “a prominent effect on mucociliary transport” in cell cultures derived from COPD patients and in healthy nonsmoker cell cultures exposed to cigarette smoke extract.
“Our study shows for the first time the therapeutic potential of an extracellular matrix molecule in acute exacerbation of human lung disease,” the investigators concluded, noting a “clinically meaningful salutary effect” on duration of NIPPV.
Dr. Galdi and colleagues went on to predict that benefits in a real-world patient population could be even more meaningful.
“Since the serum samples were collected at the end of NIPPV, HMW-HA–treated patients were on average sampled a day earlier than placebo-treated patients (because they were liberated from NIPPV a day earlier on average),” the investigators wrote. “Thus, HMW-HA treatment effects may have been underestimated in our study.”
According to Dr. Sandhaus, “The current report, while a relatively small single-center study, is well controlled and the results suggest that inhaled hyaluronan decreased time on noninvasive ventilation, decreased hospital stay duration, and decreased some mediators of inflammation.”
He also suggested that HMW-HA may have a role in the prophylactic setting.
“The limitations of this pilot study are appropriately explored by the authors but do not dampen the exciting possibility that this therapeutic approach may hold promise not only in severe exacerbations of COPD but potentially for the prevention of such exacerbations,” Dr. Sandhaus said.
Jerome O. Cantor, MD, FCCP, of St. John’s University, New York, who previously conducted a pilot study for using lower molecular weight hyaluronan in COPD and published a review on the subject, said that more studies are necessary.
“Further clinical trials are needed to better determine the role of hyaluronan as an adjunct to existing therapies for COPD exacerbations,” he said.
The study was supported by the National Institutes of Health. The investigators and Dr. Sandhaus declared no conflicts of interest. Dr. Cantor disclosed a relationship with MatRx Therapeutics.
(COPD), findings of a new study suggest.
HMW-HA was associated with a significantly shorter duration of noninvasive positive-pressure ventilation (NIPPV), lower systemic inflammatory markers, and lower measured peak airway pressure, compared with placebo, reported lead author Flavia Galdi, MD, of Campus Bio-Medico University Hospital, Rome, and colleagues.
“HMW-HA is a naturally occurring sugar that is abundant in the extracellular matrix, including in the lung,” the investigators wrote in Respiratory Research. “[It] has been used routinely, together with hypertonic saline, in cystic fibrosis patients [for several years] with no reported side effects; rather, it improves tolerability and decreases the need for bronchodilators in these patients.”
According to Robert A. Sandhaus, MD, PhD, FCCP, of National Jewish Health, Denver, the role of hyaluronan in lung disease was first recognized decades ago.
“Data stretching back into the 1970s has identified decreases in hyaluronan content in emphysematous lung tissue, protection of lung connective tissue from proteolysis by hyaluronan, and potential therapeutic roles for hyaluronan in a variety of disease, especially of the lungs,” he said in an interview.
For patients with COPD, treatment with HMW-HA may provide benefit by counteracting an imbalance in diseased lung tissue, wrote Dr. Galdi and colleagues.
“Emerging evidence suggests that imbalance between declining HMW-HA levels, and increasing smaller fragments of hyaluronan may contribute to chronic airway disease pathogenesis,” they wrote. “This has led to the hypothesis that exogenous supplementation of HMW-HA may restore hyaluronan homeostasis in favor of undegraded molecules, inhibit inflammation and loss of lung function, and ameliorate COPD progression.”
To test this hypothesis, the investigators screened 44 patients with a history of acute exacerbations of COPD necessitating NIPPV, ultimately excluding 3 patients because of heart failure. Following 1:1 randomization, 20 patients received HMW-HA while 21 received placebo, each twice daily, in conjunction with NIPPV and standard medical therapy. Treatment continued until NIPPV failure or liberation from NIPPV. Most patients received NIPPV in the hospital; however, home/chronic NIPPV was given to four patients in the placebo group and three patients in the HMW-HA group.
The primary outcome was duration of NIPPV. Secondary outcomes included markers of systemic inflammation associated with acute exacerbations of COPD and respiratory physiology parameters. Adverse events were also reported.
Results showed that patients treated with HMW-HA were liberated sooner from NIPPV than were those who received placebo (mean, 5.2 vs 6.4 days; P < .037). Similarly, patients in the HMW-HA group had significantly shorter hospital stay, on average, than those in the placebo group (mean, 7.2 vs 10.2 days; P = .039). Median values followed a similar pattern.
“These data suggest that HMW-HA shortened the duration of acute respiratory failure, need for NIPPV and, consequently, hospital length of stay in these patients,” the investigators wrote.
Secondary outcomes further supported these therapeutic benefits. Compared with placebo, HMW-HA was associated with significantly lower peak pressure and greater improvements in both pCO2/FiO2 ratio and inflammatory markers. No adverse events were reported.
Further analyses involving human bronchial epithelial cell cultures offered some mechanistic insight. Using micro-optical coherence tomography imaging, the investigators found that HMW-HA treatment was associated with “a prominent effect on mucociliary transport” in cell cultures derived from COPD patients and in healthy nonsmoker cell cultures exposed to cigarette smoke extract.
“Our study shows for the first time the therapeutic potential of an extracellular matrix molecule in acute exacerbation of human lung disease,” the investigators concluded, noting a “clinically meaningful salutary effect” on duration of NIPPV.
Dr. Galdi and colleagues went on to predict that benefits in a real-world patient population could be even more meaningful.
“Since the serum samples were collected at the end of NIPPV, HMW-HA–treated patients were on average sampled a day earlier than placebo-treated patients (because they were liberated from NIPPV a day earlier on average),” the investigators wrote. “Thus, HMW-HA treatment effects may have been underestimated in our study.”
According to Dr. Sandhaus, “The current report, while a relatively small single-center study, is well controlled and the results suggest that inhaled hyaluronan decreased time on noninvasive ventilation, decreased hospital stay duration, and decreased some mediators of inflammation.”
He also suggested that HMW-HA may have a role in the prophylactic setting.
“The limitations of this pilot study are appropriately explored by the authors but do not dampen the exciting possibility that this therapeutic approach may hold promise not only in severe exacerbations of COPD but potentially for the prevention of such exacerbations,” Dr. Sandhaus said.
Jerome O. Cantor, MD, FCCP, of St. John’s University, New York, who previously conducted a pilot study for using lower molecular weight hyaluronan in COPD and published a review on the subject, said that more studies are necessary.
“Further clinical trials are needed to better determine the role of hyaluronan as an adjunct to existing therapies for COPD exacerbations,” he said.
The study was supported by the National Institutes of Health. The investigators and Dr. Sandhaus declared no conflicts of interest. Dr. Cantor disclosed a relationship with MatRx Therapeutics.
(COPD), findings of a new study suggest.
HMW-HA was associated with a significantly shorter duration of noninvasive positive-pressure ventilation (NIPPV), lower systemic inflammatory markers, and lower measured peak airway pressure, compared with placebo, reported lead author Flavia Galdi, MD, of Campus Bio-Medico University Hospital, Rome, and colleagues.
“HMW-HA is a naturally occurring sugar that is abundant in the extracellular matrix, including in the lung,” the investigators wrote in Respiratory Research. “[It] has been used routinely, together with hypertonic saline, in cystic fibrosis patients [for several years] with no reported side effects; rather, it improves tolerability and decreases the need for bronchodilators in these patients.”
According to Robert A. Sandhaus, MD, PhD, FCCP, of National Jewish Health, Denver, the role of hyaluronan in lung disease was first recognized decades ago.
“Data stretching back into the 1970s has identified decreases in hyaluronan content in emphysematous lung tissue, protection of lung connective tissue from proteolysis by hyaluronan, and potential therapeutic roles for hyaluronan in a variety of disease, especially of the lungs,” he said in an interview.
For patients with COPD, treatment with HMW-HA may provide benefit by counteracting an imbalance in diseased lung tissue, wrote Dr. Galdi and colleagues.
“Emerging evidence suggests that imbalance between declining HMW-HA levels, and increasing smaller fragments of hyaluronan may contribute to chronic airway disease pathogenesis,” they wrote. “This has led to the hypothesis that exogenous supplementation of HMW-HA may restore hyaluronan homeostasis in favor of undegraded molecules, inhibit inflammation and loss of lung function, and ameliorate COPD progression.”
To test this hypothesis, the investigators screened 44 patients with a history of acute exacerbations of COPD necessitating NIPPV, ultimately excluding 3 patients because of heart failure. Following 1:1 randomization, 20 patients received HMW-HA while 21 received placebo, each twice daily, in conjunction with NIPPV and standard medical therapy. Treatment continued until NIPPV failure or liberation from NIPPV. Most patients received NIPPV in the hospital; however, home/chronic NIPPV was given to four patients in the placebo group and three patients in the HMW-HA group.
The primary outcome was duration of NIPPV. Secondary outcomes included markers of systemic inflammation associated with acute exacerbations of COPD and respiratory physiology parameters. Adverse events were also reported.
Results showed that patients treated with HMW-HA were liberated sooner from NIPPV than were those who received placebo (mean, 5.2 vs 6.4 days; P < .037). Similarly, patients in the HMW-HA group had significantly shorter hospital stay, on average, than those in the placebo group (mean, 7.2 vs 10.2 days; P = .039). Median values followed a similar pattern.
“These data suggest that HMW-HA shortened the duration of acute respiratory failure, need for NIPPV and, consequently, hospital length of stay in these patients,” the investigators wrote.
Secondary outcomes further supported these therapeutic benefits. Compared with placebo, HMW-HA was associated with significantly lower peak pressure and greater improvements in both pCO2/FiO2 ratio and inflammatory markers. No adverse events were reported.
Further analyses involving human bronchial epithelial cell cultures offered some mechanistic insight. Using micro-optical coherence tomography imaging, the investigators found that HMW-HA treatment was associated with “a prominent effect on mucociliary transport” in cell cultures derived from COPD patients and in healthy nonsmoker cell cultures exposed to cigarette smoke extract.
“Our study shows for the first time the therapeutic potential of an extracellular matrix molecule in acute exacerbation of human lung disease,” the investigators concluded, noting a “clinically meaningful salutary effect” on duration of NIPPV.
Dr. Galdi and colleagues went on to predict that benefits in a real-world patient population could be even more meaningful.
“Since the serum samples were collected at the end of NIPPV, HMW-HA–treated patients were on average sampled a day earlier than placebo-treated patients (because they were liberated from NIPPV a day earlier on average),” the investigators wrote. “Thus, HMW-HA treatment effects may have been underestimated in our study.”
According to Dr. Sandhaus, “The current report, while a relatively small single-center study, is well controlled and the results suggest that inhaled hyaluronan decreased time on noninvasive ventilation, decreased hospital stay duration, and decreased some mediators of inflammation.”
He also suggested that HMW-HA may have a role in the prophylactic setting.
“The limitations of this pilot study are appropriately explored by the authors but do not dampen the exciting possibility that this therapeutic approach may hold promise not only in severe exacerbations of COPD but potentially for the prevention of such exacerbations,” Dr. Sandhaus said.
Jerome O. Cantor, MD, FCCP, of St. John’s University, New York, who previously conducted a pilot study for using lower molecular weight hyaluronan in COPD and published a review on the subject, said that more studies are necessary.
“Further clinical trials are needed to better determine the role of hyaluronan as an adjunct to existing therapies for COPD exacerbations,” he said.
The study was supported by the National Institutes of Health. The investigators and Dr. Sandhaus declared no conflicts of interest. Dr. Cantor disclosed a relationship with MatRx Therapeutics.
FROM RESPIRATORY RESEARCH
FDA alert confirms heart and cancer risks with tofacitinib (Xeljanz)
The Food and Drug Administration has alerted the public to an increased risk of serious heart-related problems and cancer risk associated with the Janus kinase inhibitor tofacitinib (Xeljanz, Xeljanz XR), based on early results from a safety clinical trial comparing tofacitinib and tumor necrosis factor inhibitors in patients with rheumatoid arthritis (RA).
The FDA is awaiting further results from the trial, but in a safety communication issued on Feb. 4, the agency advised patients not to discontinue tofacitinib without consulting their health care providers and advised health care professionals to weigh the risks and benefits when prescribing the drug and continue to follow the current prescribing information.
Tofacitinib was approved for treatment of RA in 2012 at a 5-mg dose. After this approval, the FDA required drug manufacturer Pfizer to conduct a safety clinical trial that included the 5-mg twice-daily dose and a 10-mg twice-daily dose that is currently approved only for ulcerative colitis. In addition to RA and ulcerative colitis, tofacitinib is approved for adults with active psoriatic arthritis and patients aged 2 years or older with active polyarticular course juvenile idiopathic arthritis.
Pfizer announced partial results of the study, known as the ORAL Surveillance trial, in a press release on Jan. 27. The randomized trial included 4,362 RA patients aged 50 years and older who received either 5-mg or 10-mg doses of tofacitinib or a TNF inhibitor (adalimumab or etanercept).
The full results have yet to be released, but based on data from approximately 10,000 person-years for the combined tofacitinib groups and approximately 5,000 person-years for the TNF inhibitor group, the rate of major cardiovascular adverse events was significantly higher in the combined tofacitinib group, compared with the TNF inhibitor group (0.98 vs. 0.73 per 100 person-years; hazard ratio, 1.33). In addition, the rate of adjudicated malignancies was significantly higher in the tofacitinib group, compared with the TNF inhibitor group (1.13 vs. 0.77 per 100 person-years; HR, 1.48).
In February 2019, the FDA issued a warning stating an increased risk of pulmonary embolism and death associated with the 10-mg twice-daily dose of tofacitinib, following interims results from the safety study.
In July 2019, the FDA added a boxed warning to tofacitinib advising of the increased risk for pulmonary embolism and death associated with the 10-mg twice-daily dose.
The FDA encouraged health care professionals and patients to report any side effects from tofacitinib or other medications through the FDA MedWatch program online or by phone at 1-800-332-1088.
Until nuances revealed, no change in practice
The preliminary study findings contain some nuances that are a bit complicated from a statistical standpoint, according to Daniel Furst, MD, professor emeritus of medicine at the University of California, Los Angeles; adjunct professor at the University of Washington, Seattle; and research professor at the University of Florence (Italy).
This is supposed to be a noninferiority study, so something might not be noninferior, “but that doesn’t mean it is inferior,” explained Dr. Furst, who is also a member of the MDedge Rheumatology Editorial Advisory Board.
Dr. Furst said he was surprised by the study findings, because “I didn’t expect there to be any differences, and in fact it is not clear how great the differences are” among the groups in the study, he said.
When the complete findings are released, in one of the instances, “the statistics may show a very small statistical difference that indicates we may have to be more careful in this particularly high-risk group,” Dr. Furst noted.
“When we understand the data more closely, we may find that there are some nuances we need to be careful about,” he said. However, “until those data are out, I would not make any changes in my practice.”
Whether the current study findings represent a class effect is “impossible to say,” since tofacitinib affects three enzymes, while other JAK inhibitors affect only one or two, he noted.
Dr. Furst disclosed receiving grant/research support from and/or consulting for AbbVie, Actelion, Amgen, Bristol-Myers Squibb, Corbus, the National Institutes of Health, Novartis, Pfizer, and Roche/Genentech.
Updated on 2/8/2021.
The Food and Drug Administration has alerted the public to an increased risk of serious heart-related problems and cancer risk associated with the Janus kinase inhibitor tofacitinib (Xeljanz, Xeljanz XR), based on early results from a safety clinical trial comparing tofacitinib and tumor necrosis factor inhibitors in patients with rheumatoid arthritis (RA).
The FDA is awaiting further results from the trial, but in a safety communication issued on Feb. 4, the agency advised patients not to discontinue tofacitinib without consulting their health care providers and advised health care professionals to weigh the risks and benefits when prescribing the drug and continue to follow the current prescribing information.
Tofacitinib was approved for treatment of RA in 2012 at a 5-mg dose. After this approval, the FDA required drug manufacturer Pfizer to conduct a safety clinical trial that included the 5-mg twice-daily dose and a 10-mg twice-daily dose that is currently approved only for ulcerative colitis. In addition to RA and ulcerative colitis, tofacitinib is approved for adults with active psoriatic arthritis and patients aged 2 years or older with active polyarticular course juvenile idiopathic arthritis.
Pfizer announced partial results of the study, known as the ORAL Surveillance trial, in a press release on Jan. 27. The randomized trial included 4,362 RA patients aged 50 years and older who received either 5-mg or 10-mg doses of tofacitinib or a TNF inhibitor (adalimumab or etanercept).
The full results have yet to be released, but based on data from approximately 10,000 person-years for the combined tofacitinib groups and approximately 5,000 person-years for the TNF inhibitor group, the rate of major cardiovascular adverse events was significantly higher in the combined tofacitinib group, compared with the TNF inhibitor group (0.98 vs. 0.73 per 100 person-years; hazard ratio, 1.33). In addition, the rate of adjudicated malignancies was significantly higher in the tofacitinib group, compared with the TNF inhibitor group (1.13 vs. 0.77 per 100 person-years; HR, 1.48).
In February 2019, the FDA issued a warning stating an increased risk of pulmonary embolism and death associated with the 10-mg twice-daily dose of tofacitinib, following interims results from the safety study.
In July 2019, the FDA added a boxed warning to tofacitinib advising of the increased risk for pulmonary embolism and death associated with the 10-mg twice-daily dose.
The FDA encouraged health care professionals and patients to report any side effects from tofacitinib or other medications through the FDA MedWatch program online or by phone at 1-800-332-1088.
Until nuances revealed, no change in practice
The preliminary study findings contain some nuances that are a bit complicated from a statistical standpoint, according to Daniel Furst, MD, professor emeritus of medicine at the University of California, Los Angeles; adjunct professor at the University of Washington, Seattle; and research professor at the University of Florence (Italy).
This is supposed to be a noninferiority study, so something might not be noninferior, “but that doesn’t mean it is inferior,” explained Dr. Furst, who is also a member of the MDedge Rheumatology Editorial Advisory Board.
Dr. Furst said he was surprised by the study findings, because “I didn’t expect there to be any differences, and in fact it is not clear how great the differences are” among the groups in the study, he said.
When the complete findings are released, in one of the instances, “the statistics may show a very small statistical difference that indicates we may have to be more careful in this particularly high-risk group,” Dr. Furst noted.
“When we understand the data more closely, we may find that there are some nuances we need to be careful about,” he said. However, “until those data are out, I would not make any changes in my practice.”
Whether the current study findings represent a class effect is “impossible to say,” since tofacitinib affects three enzymes, while other JAK inhibitors affect only one or two, he noted.
Dr. Furst disclosed receiving grant/research support from and/or consulting for AbbVie, Actelion, Amgen, Bristol-Myers Squibb, Corbus, the National Institutes of Health, Novartis, Pfizer, and Roche/Genentech.
Updated on 2/8/2021.
The Food and Drug Administration has alerted the public to an increased risk of serious heart-related problems and cancer risk associated with the Janus kinase inhibitor tofacitinib (Xeljanz, Xeljanz XR), based on early results from a safety clinical trial comparing tofacitinib and tumor necrosis factor inhibitors in patients with rheumatoid arthritis (RA).
The FDA is awaiting further results from the trial, but in a safety communication issued on Feb. 4, the agency advised patients not to discontinue tofacitinib without consulting their health care providers and advised health care professionals to weigh the risks and benefits when prescribing the drug and continue to follow the current prescribing information.
Tofacitinib was approved for treatment of RA in 2012 at a 5-mg dose. After this approval, the FDA required drug manufacturer Pfizer to conduct a safety clinical trial that included the 5-mg twice-daily dose and a 10-mg twice-daily dose that is currently approved only for ulcerative colitis. In addition to RA and ulcerative colitis, tofacitinib is approved for adults with active psoriatic arthritis and patients aged 2 years or older with active polyarticular course juvenile idiopathic arthritis.
Pfizer announced partial results of the study, known as the ORAL Surveillance trial, in a press release on Jan. 27. The randomized trial included 4,362 RA patients aged 50 years and older who received either 5-mg or 10-mg doses of tofacitinib or a TNF inhibitor (adalimumab or etanercept).
The full results have yet to be released, but based on data from approximately 10,000 person-years for the combined tofacitinib groups and approximately 5,000 person-years for the TNF inhibitor group, the rate of major cardiovascular adverse events was significantly higher in the combined tofacitinib group, compared with the TNF inhibitor group (0.98 vs. 0.73 per 100 person-years; hazard ratio, 1.33). In addition, the rate of adjudicated malignancies was significantly higher in the tofacitinib group, compared with the TNF inhibitor group (1.13 vs. 0.77 per 100 person-years; HR, 1.48).
In February 2019, the FDA issued a warning stating an increased risk of pulmonary embolism and death associated with the 10-mg twice-daily dose of tofacitinib, following interims results from the safety study.
In July 2019, the FDA added a boxed warning to tofacitinib advising of the increased risk for pulmonary embolism and death associated with the 10-mg twice-daily dose.
The FDA encouraged health care professionals and patients to report any side effects from tofacitinib or other medications through the FDA MedWatch program online or by phone at 1-800-332-1088.
Until nuances revealed, no change in practice
The preliminary study findings contain some nuances that are a bit complicated from a statistical standpoint, according to Daniel Furst, MD, professor emeritus of medicine at the University of California, Los Angeles; adjunct professor at the University of Washington, Seattle; and research professor at the University of Florence (Italy).
This is supposed to be a noninferiority study, so something might not be noninferior, “but that doesn’t mean it is inferior,” explained Dr. Furst, who is also a member of the MDedge Rheumatology Editorial Advisory Board.
Dr. Furst said he was surprised by the study findings, because “I didn’t expect there to be any differences, and in fact it is not clear how great the differences are” among the groups in the study, he said.
When the complete findings are released, in one of the instances, “the statistics may show a very small statistical difference that indicates we may have to be more careful in this particularly high-risk group,” Dr. Furst noted.
“When we understand the data more closely, we may find that there are some nuances we need to be careful about,” he said. However, “until those data are out, I would not make any changes in my practice.”
Whether the current study findings represent a class effect is “impossible to say,” since tofacitinib affects three enzymes, while other JAK inhibitors affect only one or two, he noted.
Dr. Furst disclosed receiving grant/research support from and/or consulting for AbbVie, Actelion, Amgen, Bristol-Myers Squibb, Corbus, the National Institutes of Health, Novartis, Pfizer, and Roche/Genentech.
Updated on 2/8/2021.
Dexmedetomidine, propofol similar in ventilated adults with sepsis
Outcomes for mechanically ventilated adults with sepsis receiving light sedation were the same whether they received dexmedetomidine or propofol, according to data from a 13-center randomized, controlled, double-blind study published online Feb. 2 in the New England Journal of Medicine.
Dexmedetomidine (an alpha2-receptor agonist) and propofol (a gamma-aminobutyric acid [GABA]–receptor agonist) have similar safety profiles.
The findings from the Maximizing the Efficacy of Sedation and Reducing Neurological Dysfunction and Mortality in Septic Patients with Acute Respiratory Failure (MENDS2) trial were published on an accelerated schedule to coincide with the Critical Care Congress sponsored by the Society of Critical Care Medicine.
Lead author Christopher G. Hughes, MD, chief of anesthesiology in critical care medicine at Vanderbilt University Medical Center, Nashville, Tenn., told this news organization that previous trials have shown that dexmedetomidine is likely superior to benzodiazepines, especially in improving delirium, coma, and time on a ventilator. Until this trial, dexmedetomidine’s performance in a head-to-head comparison with propofol – the current standard-of-care agent – was not clear.
Researchers discovered that, “despite theoretical advantages of dexmedetomidine, that did not translate into the clinical realm when patients were receiving up-to-date sedation care,” he said.
Guidelines currently recommend either drug when light sedation is needed for adults on ventilators. The drugs are different in the way they affect arousability, immunity, and inflammation, but a comparison of outcomes in adults with sepsis – in terms of days alive without brain dysfunction – had never before been performed in a randomized, controlled trial.
In this trial, 422 patients were randomly assigned to receive either dexmedetomidine (0.15-1.5 mcg/kg of body weight per hour) or propofol (5-50 mcg/kg per minute). Doses were adjusted by bedside nurses (who were unblinded) to achieve specified sedation goals.
The primary outcome was days alive without delirium or coma in the 14 days of intervention. The researchers found no difference between the two groups (adjusted median, 10.7 vs. 10.8 days; odds ratio, 0.96; 95% confidence interval, 0.74-1.26).
There was also little difference in three secondary outcomes: ventilator-free days (adjusted median, 23.7 vs. 24.0 days; OR, 0.98); death at 90 days (38% vs. 39%; hazard ratio, 1.06); or the Telephone Interview for Cognitive Status (TICS) Total score measuring global cognition at 6 months (adjusted median score, 40.9 vs. 41.4; OR, 0.94).
Dr. Hughes said the researchers “specifically went with a high-severity-of-illness cohort that would be most likely to see an effect.”
He said the drugs have different adverse-effect profiles, so a clinician can consider those in deciding between the two, but either should be fine at baseline.
The researchers note that at least 20 million patients each year develop sepsis with severe organ dysfunction, and more than 20% receive mechanical ventilation.
Confirmation of current guidelines
Sandra Kane-Gill, PharmD, president-elect of SCCM, stated in an interview that she is impressed with the study design and said the results give definitive confirmation of current guidelines.
“The rigorous study design is different from previous comparative-effectiveness trials on the drugs in this group of patients,” she said.
As to what clinicians think about when choosing one over the other, Dr. Kane-Gill said that with dexmedetomidine, there may be more concern about bradycardia, whereas propofol may be associated with concerns of high triglycerides.
“There may be more comfort with use of propofol,” and dexmedetomidine can be more costly than propofol, she added, so those could be factors in decision-making as well.
Dr. Hughes said this study offers a robust look at cognition after the ICU, which is getting increasing attention.
“We had a much more extensive cognitive battery we performed on patients than in previous studies,” Dr. Hughes said, “and it’s important that we did not find a difference in either the main cognition or the other cognitive scores between the two agents.”
Enrollment was completed before the pandemic, but he said the results are relevant to COVID-19 patients because those who are on ventilators in the ICU are in a sick, septic-shock cohort.
“COVID patients would be the type of patients we enrolled in this study,” he said, “with the high severity of illness and the infection on top of being on a ventilator. We know that sedation regimens have been challenging in COVID patients.”
Dr. Hughes and Dr. Kane-Gill have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Outcomes for mechanically ventilated adults with sepsis receiving light sedation were the same whether they received dexmedetomidine or propofol, according to data from a 13-center randomized, controlled, double-blind study published online Feb. 2 in the New England Journal of Medicine.
Dexmedetomidine (an alpha2-receptor agonist) and propofol (a gamma-aminobutyric acid [GABA]–receptor agonist) have similar safety profiles.
The findings from the Maximizing the Efficacy of Sedation and Reducing Neurological Dysfunction and Mortality in Septic Patients with Acute Respiratory Failure (MENDS2) trial were published on an accelerated schedule to coincide with the Critical Care Congress sponsored by the Society of Critical Care Medicine.
Lead author Christopher G. Hughes, MD, chief of anesthesiology in critical care medicine at Vanderbilt University Medical Center, Nashville, Tenn., told this news organization that previous trials have shown that dexmedetomidine is likely superior to benzodiazepines, especially in improving delirium, coma, and time on a ventilator. Until this trial, dexmedetomidine’s performance in a head-to-head comparison with propofol – the current standard-of-care agent – was not clear.
Researchers discovered that, “despite theoretical advantages of dexmedetomidine, that did not translate into the clinical realm when patients were receiving up-to-date sedation care,” he said.
Guidelines currently recommend either drug when light sedation is needed for adults on ventilators. The drugs are different in the way they affect arousability, immunity, and inflammation, but a comparison of outcomes in adults with sepsis – in terms of days alive without brain dysfunction – had never before been performed in a randomized, controlled trial.
In this trial, 422 patients were randomly assigned to receive either dexmedetomidine (0.15-1.5 mcg/kg of body weight per hour) or propofol (5-50 mcg/kg per minute). Doses were adjusted by bedside nurses (who were unblinded) to achieve specified sedation goals.
The primary outcome was days alive without delirium or coma in the 14 days of intervention. The researchers found no difference between the two groups (adjusted median, 10.7 vs. 10.8 days; odds ratio, 0.96; 95% confidence interval, 0.74-1.26).
There was also little difference in three secondary outcomes: ventilator-free days (adjusted median, 23.7 vs. 24.0 days; OR, 0.98); death at 90 days (38% vs. 39%; hazard ratio, 1.06); or the Telephone Interview for Cognitive Status (TICS) Total score measuring global cognition at 6 months (adjusted median score, 40.9 vs. 41.4; OR, 0.94).
Dr. Hughes said the researchers “specifically went with a high-severity-of-illness cohort that would be most likely to see an effect.”
He said the drugs have different adverse-effect profiles, so a clinician can consider those in deciding between the two, but either should be fine at baseline.
The researchers note that at least 20 million patients each year develop sepsis with severe organ dysfunction, and more than 20% receive mechanical ventilation.
Confirmation of current guidelines
Sandra Kane-Gill, PharmD, president-elect of SCCM, stated in an interview that she is impressed with the study design and said the results give definitive confirmation of current guidelines.
“The rigorous study design is different from previous comparative-effectiveness trials on the drugs in this group of patients,” she said.
As to what clinicians think about when choosing one over the other, Dr. Kane-Gill said that with dexmedetomidine, there may be more concern about bradycardia, whereas propofol may be associated with concerns of high triglycerides.
“There may be more comfort with use of propofol,” and dexmedetomidine can be more costly than propofol, she added, so those could be factors in decision-making as well.
Dr. Hughes said this study offers a robust look at cognition after the ICU, which is getting increasing attention.
“We had a much more extensive cognitive battery we performed on patients than in previous studies,” Dr. Hughes said, “and it’s important that we did not find a difference in either the main cognition or the other cognitive scores between the two agents.”
Enrollment was completed before the pandemic, but he said the results are relevant to COVID-19 patients because those who are on ventilators in the ICU are in a sick, septic-shock cohort.
“COVID patients would be the type of patients we enrolled in this study,” he said, “with the high severity of illness and the infection on top of being on a ventilator. We know that sedation regimens have been challenging in COVID patients.”
Dr. Hughes and Dr. Kane-Gill have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Outcomes for mechanically ventilated adults with sepsis receiving light sedation were the same whether they received dexmedetomidine or propofol, according to data from a 13-center randomized, controlled, double-blind study published online Feb. 2 in the New England Journal of Medicine.
Dexmedetomidine (an alpha2-receptor agonist) and propofol (a gamma-aminobutyric acid [GABA]–receptor agonist) have similar safety profiles.
The findings from the Maximizing the Efficacy of Sedation and Reducing Neurological Dysfunction and Mortality in Septic Patients with Acute Respiratory Failure (MENDS2) trial were published on an accelerated schedule to coincide with the Critical Care Congress sponsored by the Society of Critical Care Medicine.
Lead author Christopher G. Hughes, MD, chief of anesthesiology in critical care medicine at Vanderbilt University Medical Center, Nashville, Tenn., told this news organization that previous trials have shown that dexmedetomidine is likely superior to benzodiazepines, especially in improving delirium, coma, and time on a ventilator. Until this trial, dexmedetomidine’s performance in a head-to-head comparison with propofol – the current standard-of-care agent – was not clear.
Researchers discovered that, “despite theoretical advantages of dexmedetomidine, that did not translate into the clinical realm when patients were receiving up-to-date sedation care,” he said.
Guidelines currently recommend either drug when light sedation is needed for adults on ventilators. The drugs are different in the way they affect arousability, immunity, and inflammation, but a comparison of outcomes in adults with sepsis – in terms of days alive without brain dysfunction – had never before been performed in a randomized, controlled trial.
In this trial, 422 patients were randomly assigned to receive either dexmedetomidine (0.15-1.5 mcg/kg of body weight per hour) or propofol (5-50 mcg/kg per minute). Doses were adjusted by bedside nurses (who were unblinded) to achieve specified sedation goals.
The primary outcome was days alive without delirium or coma in the 14 days of intervention. The researchers found no difference between the two groups (adjusted median, 10.7 vs. 10.8 days; odds ratio, 0.96; 95% confidence interval, 0.74-1.26).
There was also little difference in three secondary outcomes: ventilator-free days (adjusted median, 23.7 vs. 24.0 days; OR, 0.98); death at 90 days (38% vs. 39%; hazard ratio, 1.06); or the Telephone Interview for Cognitive Status (TICS) Total score measuring global cognition at 6 months (adjusted median score, 40.9 vs. 41.4; OR, 0.94).
Dr. Hughes said the researchers “specifically went with a high-severity-of-illness cohort that would be most likely to see an effect.”
He said the drugs have different adverse-effect profiles, so a clinician can consider those in deciding between the two, but either should be fine at baseline.
The researchers note that at least 20 million patients each year develop sepsis with severe organ dysfunction, and more than 20% receive mechanical ventilation.
Confirmation of current guidelines
Sandra Kane-Gill, PharmD, president-elect of SCCM, stated in an interview that she is impressed with the study design and said the results give definitive confirmation of current guidelines.
“The rigorous study design is different from previous comparative-effectiveness trials on the drugs in this group of patients,” she said.
As to what clinicians think about when choosing one over the other, Dr. Kane-Gill said that with dexmedetomidine, there may be more concern about bradycardia, whereas propofol may be associated with concerns of high triglycerides.
“There may be more comfort with use of propofol,” and dexmedetomidine can be more costly than propofol, she added, so those could be factors in decision-making as well.
Dr. Hughes said this study offers a robust look at cognition after the ICU, which is getting increasing attention.
“We had a much more extensive cognitive battery we performed on patients than in previous studies,” Dr. Hughes said, “and it’s important that we did not find a difference in either the main cognition or the other cognitive scores between the two agents.”
Enrollment was completed before the pandemic, but he said the results are relevant to COVID-19 patients because those who are on ventilators in the ICU are in a sick, septic-shock cohort.
“COVID patients would be the type of patients we enrolled in this study,” he said, “with the high severity of illness and the infection on top of being on a ventilator. We know that sedation regimens have been challenging in COVID patients.”
Dr. Hughes and Dr. Kane-Gill have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Microthrombi, necrosis seen in COVID-19 hearts on autopsy
Autopsies on patients who died from COVID-19 are providing important clues on how to treat the disease. In an analysis of 40 hearts from COVID-19 patients who died early in the pandemic, myocyte necrosis was seen in 14 hearts, or 35%.
In the majority of these hearts, pathologists found both small areas of focal necrosis and cardiac thrombi, most of which were microthrombi in myocardial capillaries, arterioles, and small muscular cells.
In an interview, senior author Aloke V. Finn, MD, CVPath Institute, Gaithersburg, Md., stressed the importance of understanding what they saw, but also what they didn’t see.
“What we saw in the majority of patients with myocardial injury were these small areas of infarct and microthrombi in small vessels. What we didn’t see was any evidence of myocarditis and or huge infarcts in, like, the LAD artery,” he said.
“What we’re seeing here is not clinically detectable. ... There is no test that will tell you there are microthrombi and no imaging tests that will show these focal areas of necrosis, but that doesn’t mean it’s not there,” he added.
The finding of myocyte necrosis in about one-third of samples is consistent with another study that showed that 30%-40% of patients hospitalized with COVID-19 have elevated troponins, noted Dr. Finn. The investigators were unable to obtain troponin levels on their patients, which could limit the clinical translation of myocardial necrosis detected at autopsy.
Dr. Finn and colleagues, including first author Dario Pellegrini, MD, from Ospedale Papa Giovanni XXIII in Bergamo, Italy, published their findings online in Circulation on Jan. 22, 2020.
The report is a follow-up to another just published by Dr. Finn’s group in the Journal of the American College of Cardiology, which showed that myocarditis is a very rare finding in COVID-19 autopsies.
Only three of 14 individuals (21.4%) with evidence of myocyte necrosis showed evidence of acute MI, which Dr. Finn and colleagues define as an area of necrosis at least 1 cm2 in size. The remaining 11 (78.6%) had only discrete areas of myocyte necrosis (>20 necrotic myocytes with an area of ≥0.05 mm2, but <1 cm2).
“This makes sense when we saw what type of thrombus there was in these cases; it wasn’t thrombus in major epicardial vessels but microthombi in small vessels,” said Dr. Finn.
In those with necrosis, cardiac thrombi were present in 11 of 14 (78.6%) cases, with 2 of 14 (14.2%) having epicardial coronary artery thrombi and 0 of 14 (64.3%) having microthrombi in myocardial capillaries, arterioles, and small muscular arteries.
Further supporting the role of COVID-19–related hypercoagulability as the cause of myocardial injury in many patients, the investigators noted that the incidence of severe coronary artery disease (defined as >75% cross sectional narrowing) did not differ significantly between those with and without necrosis.
COVID-19 vs. non–COVID-19 thrombi
Going one step further, Dr. Finn’s team compared cardiac microthrombi from their COVID-19–positive autopsy cases with intramyocardial thromboemboli from COVID-19 cases. They also compared the samples with aspirated thrombi obtained during primary percutaneous coronary intervention from uninfected and COVID-19–infected patients presenting with ST-segment elevation MI (STEMI).
The autopsy-obtained microthrombi had significantly more fibrin and terminal complement C5b-9 immunostaining than intramyocardial thromboemboli from COVID-19–negative subjects and than aspirated thrombi from either COVID-positive or COVID-negative STEMI patients.
“Basically, what we’re seeing in these thrombi is evidence of an immune-mediated reaction,” said Dr. Finn, explaining that complement C5b-9 is an innate immune system protein that circulates in the blood in response to any kind of activation of the immune system. “It is nonspecific but can also lead to coagulation problems,” he said.
Anticoagulation, yes, but dose unclear
These findings clearly support the use of anticoagulation in hospitalized COVID patients, said Jeffrey Weitz, MD, director of the Thrombosis & Atherosclerosis Research Institute, McMaster University, Hamilton, Ont. But the details of how much anticoagulation, what kind, and for whom are still a moving target.
“I think what we can say at this point is that these autopsy findings fit with previous studies that have shown microthrombi in the lungs and thrombi in the legs and gut, and support the notion that these patients should receive prophylactic doses of anticoagulants if they’re sick enough to be hospitalized,” said Dr. Weitz.
“But it’s not as simple as to say that this study shows clots form in the heart of COVID patients and therefore more anticoagulation is going to be better than less anticoagulation,” he said in an interview.
Recent top-line findings from three linked clinical trials – REMAP-CAP, ACTIV-4, and ATTACC – show that full-dose anticoagulation was beneficial in moderately ill patients hospitalized for COVID-19 and reduced the need for mechanical ventilation.
Moderately ill patients are those not in intensive care and who did not require organ support, such as mechanical ventilation, at the time of enrollment.
However, the same group reported findings in December that showed that routine use of full-dose anticoagulation when started in the ICU in critically ill patients was not beneficial and possibly harmful.
Dr. Weitz was only a little bit surprised by this finding of potential harm in the sickest patients. “I figured everybody should get prophylaxis but I wasn’t sure that everybody should get intensified anticoagulant. But my assumption was that if anybody is going to benefit from it, it would be the ICU patients.”
It was notable, said Dr. Weitz, that levels of D-dimer, a fibrin degradation product, were not associated with outcomes. “So, it doesn’t seem to be that patients with evidence of more clotting are more likely to benefit, which might indicate that it’s not the anticoagulant effect of the heparin that’s helping, but maybe the anti-inflammatory effect. At this point, we just don’t know.”
All three studies have paused enrollment of the critically ill subgroup, but are continuing to enroll patients with moderate illness and expect to publish results in the coming months, according to previous coverage from this news organization.
The study was funded by CVPath, a nonprofit institute that receives funding from a number of different industry entities. Dr. Finn and Dr. Weitz reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Autopsies on patients who died from COVID-19 are providing important clues on how to treat the disease. In an analysis of 40 hearts from COVID-19 patients who died early in the pandemic, myocyte necrosis was seen in 14 hearts, or 35%.
In the majority of these hearts, pathologists found both small areas of focal necrosis and cardiac thrombi, most of which were microthrombi in myocardial capillaries, arterioles, and small muscular cells.
In an interview, senior author Aloke V. Finn, MD, CVPath Institute, Gaithersburg, Md., stressed the importance of understanding what they saw, but also what they didn’t see.
“What we saw in the majority of patients with myocardial injury were these small areas of infarct and microthrombi in small vessels. What we didn’t see was any evidence of myocarditis and or huge infarcts in, like, the LAD artery,” he said.
“What we’re seeing here is not clinically detectable. ... There is no test that will tell you there are microthrombi and no imaging tests that will show these focal areas of necrosis, but that doesn’t mean it’s not there,” he added.
The finding of myocyte necrosis in about one-third of samples is consistent with another study that showed that 30%-40% of patients hospitalized with COVID-19 have elevated troponins, noted Dr. Finn. The investigators were unable to obtain troponin levels on their patients, which could limit the clinical translation of myocardial necrosis detected at autopsy.
Dr. Finn and colleagues, including first author Dario Pellegrini, MD, from Ospedale Papa Giovanni XXIII in Bergamo, Italy, published their findings online in Circulation on Jan. 22, 2020.
The report is a follow-up to another just published by Dr. Finn’s group in the Journal of the American College of Cardiology, which showed that myocarditis is a very rare finding in COVID-19 autopsies.
Only three of 14 individuals (21.4%) with evidence of myocyte necrosis showed evidence of acute MI, which Dr. Finn and colleagues define as an area of necrosis at least 1 cm2 in size. The remaining 11 (78.6%) had only discrete areas of myocyte necrosis (>20 necrotic myocytes with an area of ≥0.05 mm2, but <1 cm2).
“This makes sense when we saw what type of thrombus there was in these cases; it wasn’t thrombus in major epicardial vessels but microthombi in small vessels,” said Dr. Finn.
In those with necrosis, cardiac thrombi were present in 11 of 14 (78.6%) cases, with 2 of 14 (14.2%) having epicardial coronary artery thrombi and 0 of 14 (64.3%) having microthrombi in myocardial capillaries, arterioles, and small muscular arteries.
Further supporting the role of COVID-19–related hypercoagulability as the cause of myocardial injury in many patients, the investigators noted that the incidence of severe coronary artery disease (defined as >75% cross sectional narrowing) did not differ significantly between those with and without necrosis.
COVID-19 vs. non–COVID-19 thrombi
Going one step further, Dr. Finn’s team compared cardiac microthrombi from their COVID-19–positive autopsy cases with intramyocardial thromboemboli from COVID-19 cases. They also compared the samples with aspirated thrombi obtained during primary percutaneous coronary intervention from uninfected and COVID-19–infected patients presenting with ST-segment elevation MI (STEMI).
The autopsy-obtained microthrombi had significantly more fibrin and terminal complement C5b-9 immunostaining than intramyocardial thromboemboli from COVID-19–negative subjects and than aspirated thrombi from either COVID-positive or COVID-negative STEMI patients.
“Basically, what we’re seeing in these thrombi is evidence of an immune-mediated reaction,” said Dr. Finn, explaining that complement C5b-9 is an innate immune system protein that circulates in the blood in response to any kind of activation of the immune system. “It is nonspecific but can also lead to coagulation problems,” he said.
Anticoagulation, yes, but dose unclear
These findings clearly support the use of anticoagulation in hospitalized COVID patients, said Jeffrey Weitz, MD, director of the Thrombosis & Atherosclerosis Research Institute, McMaster University, Hamilton, Ont. But the details of how much anticoagulation, what kind, and for whom are still a moving target.
“I think what we can say at this point is that these autopsy findings fit with previous studies that have shown microthrombi in the lungs and thrombi in the legs and gut, and support the notion that these patients should receive prophylactic doses of anticoagulants if they’re sick enough to be hospitalized,” said Dr. Weitz.
“But it’s not as simple as to say that this study shows clots form in the heart of COVID patients and therefore more anticoagulation is going to be better than less anticoagulation,” he said in an interview.
Recent top-line findings from three linked clinical trials – REMAP-CAP, ACTIV-4, and ATTACC – show that full-dose anticoagulation was beneficial in moderately ill patients hospitalized for COVID-19 and reduced the need for mechanical ventilation.
Moderately ill patients are those not in intensive care and who did not require organ support, such as mechanical ventilation, at the time of enrollment.
However, the same group reported findings in December that showed that routine use of full-dose anticoagulation when started in the ICU in critically ill patients was not beneficial and possibly harmful.
Dr. Weitz was only a little bit surprised by this finding of potential harm in the sickest patients. “I figured everybody should get prophylaxis but I wasn’t sure that everybody should get intensified anticoagulant. But my assumption was that if anybody is going to benefit from it, it would be the ICU patients.”
It was notable, said Dr. Weitz, that levels of D-dimer, a fibrin degradation product, were not associated with outcomes. “So, it doesn’t seem to be that patients with evidence of more clotting are more likely to benefit, which might indicate that it’s not the anticoagulant effect of the heparin that’s helping, but maybe the anti-inflammatory effect. At this point, we just don’t know.”
All three studies have paused enrollment of the critically ill subgroup, but are continuing to enroll patients with moderate illness and expect to publish results in the coming months, according to previous coverage from this news organization.
The study was funded by CVPath, a nonprofit institute that receives funding from a number of different industry entities. Dr. Finn and Dr. Weitz reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Autopsies on patients who died from COVID-19 are providing important clues on how to treat the disease. In an analysis of 40 hearts from COVID-19 patients who died early in the pandemic, myocyte necrosis was seen in 14 hearts, or 35%.
In the majority of these hearts, pathologists found both small areas of focal necrosis and cardiac thrombi, most of which were microthrombi in myocardial capillaries, arterioles, and small muscular cells.
In an interview, senior author Aloke V. Finn, MD, CVPath Institute, Gaithersburg, Md., stressed the importance of understanding what they saw, but also what they didn’t see.
“What we saw in the majority of patients with myocardial injury were these small areas of infarct and microthrombi in small vessels. What we didn’t see was any evidence of myocarditis and or huge infarcts in, like, the LAD artery,” he said.
“What we’re seeing here is not clinically detectable. ... There is no test that will tell you there are microthrombi and no imaging tests that will show these focal areas of necrosis, but that doesn’t mean it’s not there,” he added.
The finding of myocyte necrosis in about one-third of samples is consistent with another study that showed that 30%-40% of patients hospitalized with COVID-19 have elevated troponins, noted Dr. Finn. The investigators were unable to obtain troponin levels on their patients, which could limit the clinical translation of myocardial necrosis detected at autopsy.
Dr. Finn and colleagues, including first author Dario Pellegrini, MD, from Ospedale Papa Giovanni XXIII in Bergamo, Italy, published their findings online in Circulation on Jan. 22, 2020.
The report is a follow-up to another just published by Dr. Finn’s group in the Journal of the American College of Cardiology, which showed that myocarditis is a very rare finding in COVID-19 autopsies.
Only three of 14 individuals (21.4%) with evidence of myocyte necrosis showed evidence of acute MI, which Dr. Finn and colleagues define as an area of necrosis at least 1 cm2 in size. The remaining 11 (78.6%) had only discrete areas of myocyte necrosis (>20 necrotic myocytes with an area of ≥0.05 mm2, but <1 cm2).
“This makes sense when we saw what type of thrombus there was in these cases; it wasn’t thrombus in major epicardial vessels but microthombi in small vessels,” said Dr. Finn.
In those with necrosis, cardiac thrombi were present in 11 of 14 (78.6%) cases, with 2 of 14 (14.2%) having epicardial coronary artery thrombi and 0 of 14 (64.3%) having microthrombi in myocardial capillaries, arterioles, and small muscular arteries.
Further supporting the role of COVID-19–related hypercoagulability as the cause of myocardial injury in many patients, the investigators noted that the incidence of severe coronary artery disease (defined as >75% cross sectional narrowing) did not differ significantly between those with and without necrosis.
COVID-19 vs. non–COVID-19 thrombi
Going one step further, Dr. Finn’s team compared cardiac microthrombi from their COVID-19–positive autopsy cases with intramyocardial thromboemboli from COVID-19 cases. They also compared the samples with aspirated thrombi obtained during primary percutaneous coronary intervention from uninfected and COVID-19–infected patients presenting with ST-segment elevation MI (STEMI).
The autopsy-obtained microthrombi had significantly more fibrin and terminal complement C5b-9 immunostaining than intramyocardial thromboemboli from COVID-19–negative subjects and than aspirated thrombi from either COVID-positive or COVID-negative STEMI patients.
“Basically, what we’re seeing in these thrombi is evidence of an immune-mediated reaction,” said Dr. Finn, explaining that complement C5b-9 is an innate immune system protein that circulates in the blood in response to any kind of activation of the immune system. “It is nonspecific but can also lead to coagulation problems,” he said.
Anticoagulation, yes, but dose unclear
These findings clearly support the use of anticoagulation in hospitalized COVID patients, said Jeffrey Weitz, MD, director of the Thrombosis & Atherosclerosis Research Institute, McMaster University, Hamilton, Ont. But the details of how much anticoagulation, what kind, and for whom are still a moving target.
“I think what we can say at this point is that these autopsy findings fit with previous studies that have shown microthrombi in the lungs and thrombi in the legs and gut, and support the notion that these patients should receive prophylactic doses of anticoagulants if they’re sick enough to be hospitalized,” said Dr. Weitz.
“But it’s not as simple as to say that this study shows clots form in the heart of COVID patients and therefore more anticoagulation is going to be better than less anticoagulation,” he said in an interview.
Recent top-line findings from three linked clinical trials – REMAP-CAP, ACTIV-4, and ATTACC – show that full-dose anticoagulation was beneficial in moderately ill patients hospitalized for COVID-19 and reduced the need for mechanical ventilation.
Moderately ill patients are those not in intensive care and who did not require organ support, such as mechanical ventilation, at the time of enrollment.
However, the same group reported findings in December that showed that routine use of full-dose anticoagulation when started in the ICU in critically ill patients was not beneficial and possibly harmful.
Dr. Weitz was only a little bit surprised by this finding of potential harm in the sickest patients. “I figured everybody should get prophylaxis but I wasn’t sure that everybody should get intensified anticoagulant. But my assumption was that if anybody is going to benefit from it, it would be the ICU patients.”
It was notable, said Dr. Weitz, that levels of D-dimer, a fibrin degradation product, were not associated with outcomes. “So, it doesn’t seem to be that patients with evidence of more clotting are more likely to benefit, which might indicate that it’s not the anticoagulant effect of the heparin that’s helping, but maybe the anti-inflammatory effect. At this point, we just don’t know.”
All three studies have paused enrollment of the critically ill subgroup, but are continuing to enroll patients with moderate illness and expect to publish results in the coming months, according to previous coverage from this news organization.
The study was funded by CVPath, a nonprofit institute that receives funding from a number of different industry entities. Dr. Finn and Dr. Weitz reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
New COPD mortality risk model includes imaging-derived variables
All-cause mortality in patients with COPD over 10 years of follow-up was accurately predicted by a newly developed model based on a point system incorporating imaging-derived variables.
Identifying risk factors is important to develop treatments and preventive strategies, but the role of imaging variables in COPD mortality among smokers has not been well studied, wrote investigator Matthew Strand, PhD, of National Jewish Health in Denver, and colleagues.
An established risk model is the body mass index–airflow Obstruction-Dyspnea-Exercise capacity (BODE) index, developed to predict mortality in COPD patients over a 4-year period. The investigators noted that while models such as BODE provide useful information about predictors of mortality in COPD, they were developed using participants in the Global initiative for obstructive Lung Disease (GOLD) spirometry grades 1-4, and have been largely constructed without quantitative computed tomography (CT) imaging variables until recently.
“The BODE index was created as a simple point scoring system to predict risk of all-cause mortality within 4 years, and is based on FEV1 [forced expiratory volume at 1 second], [6-minute walk test], dyspnea and BMI, a subset of predictors we considered in our model,” the investigators noted. The new model includes data from pulmonary function tests and volumetric CT scans.
In a study published in Chronic Obstructive Pulmonary Diseases, the researchers identified 9,074 current and past smokers in the COPD Genetic Epidemiology study (COPDGene) for whom complete data were available. They developed a point system to determine mortality risk in current and former smokers after controlling for multiple risk factors. The average age of the study population was 60 years. All participants were current or former smokers with a smoking history of at least 10 pack-years.
Assessments of the study participants included a medical history, pre- and post-bronchodilator spirometry, a 6-minute walk distance test, and inspiratory and expiratory CT scans. The researchers analyzed mortality risk in the context of Global Initiative for Obstructive Lung Disease (GOLD) classifications of patients in the sample.
Overall, the average 10-year mortality risk was 18% for women and 25% for men. Performance on the 6-minute walk test (distances less than 500 feet), FEV1 (less than 20), and older age (80 years and older) were the strongest predictors of mortality.
The model showed strong predictive accuracy, with an area under the receiver operating characteristic curve averaging 0.797 that was validated in an external cohort, the researchers said.
The study findings were limited by the observational design that does not allow for estimating the causal effects of such modifiable factors as smoking cessation, that might impact the walking test and FEV1 values, the researchers noted. In addition, the model did not allow for testing the effects of smoking vs. not smoking.
However, the model developed in the study “will allow physicians and patients to better understand factors affecting risk of an adverse event, some of which may be modifiable,” the researchers said. “The risk estimates can be used to target groups of individuals for future clinical trials, including those not currently classified as having COPD based on GOLD criteria,” they said.
The study was supported by the National Heart, Lung, and Blood Institute and by the COPD Foundation through contributions to an industry advisory committee including AstraZeneca, Boehringer-Ingelheim, Genentech, GlaxoSmithKline, Novartis, Pfizer, Siemens, and Sunovion.
All-cause mortality in patients with COPD over 10 years of follow-up was accurately predicted by a newly developed model based on a point system incorporating imaging-derived variables.
Identifying risk factors is important to develop treatments and preventive strategies, but the role of imaging variables in COPD mortality among smokers has not been well studied, wrote investigator Matthew Strand, PhD, of National Jewish Health in Denver, and colleagues.
An established risk model is the body mass index–airflow Obstruction-Dyspnea-Exercise capacity (BODE) index, developed to predict mortality in COPD patients over a 4-year period. The investigators noted that while models such as BODE provide useful information about predictors of mortality in COPD, they were developed using participants in the Global initiative for obstructive Lung Disease (GOLD) spirometry grades 1-4, and have been largely constructed without quantitative computed tomography (CT) imaging variables until recently.
“The BODE index was created as a simple point scoring system to predict risk of all-cause mortality within 4 years, and is based on FEV1 [forced expiratory volume at 1 second], [6-minute walk test], dyspnea and BMI, a subset of predictors we considered in our model,” the investigators noted. The new model includes data from pulmonary function tests and volumetric CT scans.
In a study published in Chronic Obstructive Pulmonary Diseases, the researchers identified 9,074 current and past smokers in the COPD Genetic Epidemiology study (COPDGene) for whom complete data were available. They developed a point system to determine mortality risk in current and former smokers after controlling for multiple risk factors. The average age of the study population was 60 years. All participants were current or former smokers with a smoking history of at least 10 pack-years.
Assessments of the study participants included a medical history, pre- and post-bronchodilator spirometry, a 6-minute walk distance test, and inspiratory and expiratory CT scans. The researchers analyzed mortality risk in the context of Global Initiative for Obstructive Lung Disease (GOLD) classifications of patients in the sample.
Overall, the average 10-year mortality risk was 18% for women and 25% for men. Performance on the 6-minute walk test (distances less than 500 feet), FEV1 (less than 20), and older age (80 years and older) were the strongest predictors of mortality.
The model showed strong predictive accuracy, with an area under the receiver operating characteristic curve averaging 0.797 that was validated in an external cohort, the researchers said.
The study findings were limited by the observational design that does not allow for estimating the causal effects of such modifiable factors as smoking cessation, that might impact the walking test and FEV1 values, the researchers noted. In addition, the model did not allow for testing the effects of smoking vs. not smoking.
However, the model developed in the study “will allow physicians and patients to better understand factors affecting risk of an adverse event, some of which may be modifiable,” the researchers said. “The risk estimates can be used to target groups of individuals for future clinical trials, including those not currently classified as having COPD based on GOLD criteria,” they said.
The study was supported by the National Heart, Lung, and Blood Institute and by the COPD Foundation through contributions to an industry advisory committee including AstraZeneca, Boehringer-Ingelheim, Genentech, GlaxoSmithKline, Novartis, Pfizer, Siemens, and Sunovion.
All-cause mortality in patients with COPD over 10 years of follow-up was accurately predicted by a newly developed model based on a point system incorporating imaging-derived variables.
Identifying risk factors is important to develop treatments and preventive strategies, but the role of imaging variables in COPD mortality among smokers has not been well studied, wrote investigator Matthew Strand, PhD, of National Jewish Health in Denver, and colleagues.
An established risk model is the body mass index–airflow Obstruction-Dyspnea-Exercise capacity (BODE) index, developed to predict mortality in COPD patients over a 4-year period. The investigators noted that while models such as BODE provide useful information about predictors of mortality in COPD, they were developed using participants in the Global initiative for obstructive Lung Disease (GOLD) spirometry grades 1-4, and have been largely constructed without quantitative computed tomography (CT) imaging variables until recently.
“The BODE index was created as a simple point scoring system to predict risk of all-cause mortality within 4 years, and is based on FEV1 [forced expiratory volume at 1 second], [6-minute walk test], dyspnea and BMI, a subset of predictors we considered in our model,” the investigators noted. The new model includes data from pulmonary function tests and volumetric CT scans.
In a study published in Chronic Obstructive Pulmonary Diseases, the researchers identified 9,074 current and past smokers in the COPD Genetic Epidemiology study (COPDGene) for whom complete data were available. They developed a point system to determine mortality risk in current and former smokers after controlling for multiple risk factors. The average age of the study population was 60 years. All participants were current or former smokers with a smoking history of at least 10 pack-years.
Assessments of the study participants included a medical history, pre- and post-bronchodilator spirometry, a 6-minute walk distance test, and inspiratory and expiratory CT scans. The researchers analyzed mortality risk in the context of Global Initiative for Obstructive Lung Disease (GOLD) classifications of patients in the sample.
Overall, the average 10-year mortality risk was 18% for women and 25% for men. Performance on the 6-minute walk test (distances less than 500 feet), FEV1 (less than 20), and older age (80 years and older) were the strongest predictors of mortality.
The model showed strong predictive accuracy, with an area under the receiver operating characteristic curve averaging 0.797 that was validated in an external cohort, the researchers said.
The study findings were limited by the observational design that does not allow for estimating the causal effects of such modifiable factors as smoking cessation, that might impact the walking test and FEV1 values, the researchers noted. In addition, the model did not allow for testing the effects of smoking vs. not smoking.
However, the model developed in the study “will allow physicians and patients to better understand factors affecting risk of an adverse event, some of which may be modifiable,” the researchers said. “The risk estimates can be used to target groups of individuals for future clinical trials, including those not currently classified as having COPD based on GOLD criteria,” they said.
The study was supported by the National Heart, Lung, and Blood Institute and by the COPD Foundation through contributions to an industry advisory committee including AstraZeneca, Boehringer-Ingelheim, Genentech, GlaxoSmithKline, Novartis, Pfizer, Siemens, and Sunovion.
FROM CHRONIC OBSTRUCTIVE PULMONARY DISEASES
Lung disease raises mortality risk in older RA patients
Patients with rheumatoid arthritis–associated interstitial lung disease showed increases in overall mortality, respiratory mortality, and cancer mortality, compared with RA patients without interstitial lung disease, based on data from more than 500,000 patients in a nationwide cohort study.
RA-associated interstitial lung disease (RA-ILD) has been associated with worse survival rates as well as reduced quality of life, functional impairment, and increased health care use and costs, wrote Jeffrey A. Sparks, MD, of Brigham and Women’s Hospital, Boston, and colleagues. However, data on the incidence and prevalence of RA-ILD have been inconsistent and large studies are lacking.
In a study published online in Rheumatology, the researchers identified 509,787 RA patients aged 65 years and older from Medicare claims data. The average age of the patients was 72.6 years, and 76.2% were women.
At baseline, 10,306 (2%) of the study population had RA-ILD, and 13,372 (2.7%) developed RA-ILD over an average of 3.8 years’ follow-up per person (total of 1,873,127 person-years of follow-up). The overall incidence of RA-ILD was 7.14 per 1,000 person-years.
Overall mortality was significantly higher among RA-ILD patients than in those with RA alone in a multivariate analysis (38.7% vs. 20.7%; hazard ratio, 1.66).
In addition, RA-ILD was associated with an increased risk of respiratory mortality (HR, 4.39) and cancer mortality (HR, 1.56), compared with RA without ILD. For these hazard regression analyses, the researchers used Fine and Gray subdistribution HRs “to handle competing risks of alternative causes of mortality. For example, the risk of respiratory mortality for patients with RA-ILD, compared with RA without ILD also accounted for the competing risk of cardiovascular, cancer, infection and other types of mortality.”
In another multivariate analysis, male gender, smoking, asthma, chronic obstructive pulmonary disorder, and medication use (specifically biologic disease-modifying antirheumatic drugs, targeted synthetic DMARDs, and glucocorticoids) were independently associated with increased incident RA-ILD at baseline. However, “the associations of RA-related medications with incident RA-ILD risk should be interpreted with caution since they may be explained by unmeasured factors, including RA disease activity, severity, comorbidities, and prior or concomitant medication use,” the researchers noted.
The study findings were limited by several factors, including the lack of data on disease activity, disease duration, disease severity, and RA-related autoantibodies, the researchers noted. However, the results support data from previous studies and were strengthened by the large sample size and data on demographics and health care use.
“Ours is the first to study the epidemiology and mortality outcomes of RA-ILD using a validated claims algorithm to identify RA and RA-ILD,” and “to quantify the mortality burden of RA-ILD and to identify a potentially novel association of RA-ILD with cancer mortality,” they noted.
The study was supported by an investigator-initiated grant from Bristol-Myers Squibb. Lead author Dr. Sparks disclosed support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Rheumatology Research Foundation, the Brigham Research Institute, and the R. Bruce and Joan M. Mickey Research Scholar Fund. Dr. Sparks also disclosed serving as a consultant to Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer for work unrelated to the current study. Other authors reported research funding from Bristol-Myers Squibb, involvement in a clinical trial funded by Genentech and Bristol-Myers Squibb, and receiving research support to Brigham and Women’s Hospital for other studies from AbbVie, Bayer, Bristol-Myers Squibb, Novartis, Pfizer, Roche, and Vertex.
Patients with rheumatoid arthritis–associated interstitial lung disease showed increases in overall mortality, respiratory mortality, and cancer mortality, compared with RA patients without interstitial lung disease, based on data from more than 500,000 patients in a nationwide cohort study.
RA-associated interstitial lung disease (RA-ILD) has been associated with worse survival rates as well as reduced quality of life, functional impairment, and increased health care use and costs, wrote Jeffrey A. Sparks, MD, of Brigham and Women’s Hospital, Boston, and colleagues. However, data on the incidence and prevalence of RA-ILD have been inconsistent and large studies are lacking.
In a study published online in Rheumatology, the researchers identified 509,787 RA patients aged 65 years and older from Medicare claims data. The average age of the patients was 72.6 years, and 76.2% were women.
At baseline, 10,306 (2%) of the study population had RA-ILD, and 13,372 (2.7%) developed RA-ILD over an average of 3.8 years’ follow-up per person (total of 1,873,127 person-years of follow-up). The overall incidence of RA-ILD was 7.14 per 1,000 person-years.
Overall mortality was significantly higher among RA-ILD patients than in those with RA alone in a multivariate analysis (38.7% vs. 20.7%; hazard ratio, 1.66).
In addition, RA-ILD was associated with an increased risk of respiratory mortality (HR, 4.39) and cancer mortality (HR, 1.56), compared with RA without ILD. For these hazard regression analyses, the researchers used Fine and Gray subdistribution HRs “to handle competing risks of alternative causes of mortality. For example, the risk of respiratory mortality for patients with RA-ILD, compared with RA without ILD also accounted for the competing risk of cardiovascular, cancer, infection and other types of mortality.”
In another multivariate analysis, male gender, smoking, asthma, chronic obstructive pulmonary disorder, and medication use (specifically biologic disease-modifying antirheumatic drugs, targeted synthetic DMARDs, and glucocorticoids) were independently associated with increased incident RA-ILD at baseline. However, “the associations of RA-related medications with incident RA-ILD risk should be interpreted with caution since they may be explained by unmeasured factors, including RA disease activity, severity, comorbidities, and prior or concomitant medication use,” the researchers noted.
The study findings were limited by several factors, including the lack of data on disease activity, disease duration, disease severity, and RA-related autoantibodies, the researchers noted. However, the results support data from previous studies and were strengthened by the large sample size and data on demographics and health care use.
“Ours is the first to study the epidemiology and mortality outcomes of RA-ILD using a validated claims algorithm to identify RA and RA-ILD,” and “to quantify the mortality burden of RA-ILD and to identify a potentially novel association of RA-ILD with cancer mortality,” they noted.
The study was supported by an investigator-initiated grant from Bristol-Myers Squibb. Lead author Dr. Sparks disclosed support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Rheumatology Research Foundation, the Brigham Research Institute, and the R. Bruce and Joan M. Mickey Research Scholar Fund. Dr. Sparks also disclosed serving as a consultant to Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer for work unrelated to the current study. Other authors reported research funding from Bristol-Myers Squibb, involvement in a clinical trial funded by Genentech and Bristol-Myers Squibb, and receiving research support to Brigham and Women’s Hospital for other studies from AbbVie, Bayer, Bristol-Myers Squibb, Novartis, Pfizer, Roche, and Vertex.
Patients with rheumatoid arthritis–associated interstitial lung disease showed increases in overall mortality, respiratory mortality, and cancer mortality, compared with RA patients without interstitial lung disease, based on data from more than 500,000 patients in a nationwide cohort study.
RA-associated interstitial lung disease (RA-ILD) has been associated with worse survival rates as well as reduced quality of life, functional impairment, and increased health care use and costs, wrote Jeffrey A. Sparks, MD, of Brigham and Women’s Hospital, Boston, and colleagues. However, data on the incidence and prevalence of RA-ILD have been inconsistent and large studies are lacking.
In a study published online in Rheumatology, the researchers identified 509,787 RA patients aged 65 years and older from Medicare claims data. The average age of the patients was 72.6 years, and 76.2% were women.
At baseline, 10,306 (2%) of the study population had RA-ILD, and 13,372 (2.7%) developed RA-ILD over an average of 3.8 years’ follow-up per person (total of 1,873,127 person-years of follow-up). The overall incidence of RA-ILD was 7.14 per 1,000 person-years.
Overall mortality was significantly higher among RA-ILD patients than in those with RA alone in a multivariate analysis (38.7% vs. 20.7%; hazard ratio, 1.66).
In addition, RA-ILD was associated with an increased risk of respiratory mortality (HR, 4.39) and cancer mortality (HR, 1.56), compared with RA without ILD. For these hazard regression analyses, the researchers used Fine and Gray subdistribution HRs “to handle competing risks of alternative causes of mortality. For example, the risk of respiratory mortality for patients with RA-ILD, compared with RA without ILD also accounted for the competing risk of cardiovascular, cancer, infection and other types of mortality.”
In another multivariate analysis, male gender, smoking, asthma, chronic obstructive pulmonary disorder, and medication use (specifically biologic disease-modifying antirheumatic drugs, targeted synthetic DMARDs, and glucocorticoids) were independently associated with increased incident RA-ILD at baseline. However, “the associations of RA-related medications with incident RA-ILD risk should be interpreted with caution since they may be explained by unmeasured factors, including RA disease activity, severity, comorbidities, and prior or concomitant medication use,” the researchers noted.
The study findings were limited by several factors, including the lack of data on disease activity, disease duration, disease severity, and RA-related autoantibodies, the researchers noted. However, the results support data from previous studies and were strengthened by the large sample size and data on demographics and health care use.
“Ours is the first to study the epidemiology and mortality outcomes of RA-ILD using a validated claims algorithm to identify RA and RA-ILD,” and “to quantify the mortality burden of RA-ILD and to identify a potentially novel association of RA-ILD with cancer mortality,” they noted.
The study was supported by an investigator-initiated grant from Bristol-Myers Squibb. Lead author Dr. Sparks disclosed support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Rheumatology Research Foundation, the Brigham Research Institute, and the R. Bruce and Joan M. Mickey Research Scholar Fund. Dr. Sparks also disclosed serving as a consultant to Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer for work unrelated to the current study. Other authors reported research funding from Bristol-Myers Squibb, involvement in a clinical trial funded by Genentech and Bristol-Myers Squibb, and receiving research support to Brigham and Women’s Hospital for other studies from AbbVie, Bayer, Bristol-Myers Squibb, Novartis, Pfizer, Roche, and Vertex.
FROM RHEUMATOLOGY
Noninvasive Ventilation Use Among Medicare Beneficiaries at the End of Life
Study Overview
Objective. To examine the trend of noninvasive and invasive mechanical ventilation at the end of life from 2000 to 2017.
Design. Observational population-based cohort study.
Setting and participants. The study was a population-based cohort study to examine the use of noninvasive and invasive mechanical ventilation among decedents. The study included a random 20% sample of Medicare beneficiaries older than 65 years who were hospitalized in the last 30 days of life and died between January 1, 2000, and December 31, 2017, except for the period October 1, 2015, to December 31, 2015, when the transition from International Classification of Diseases, Ninth Revision (ICD-9) to ICD-10 occurred. Beneficiaries with the primary admitting diagnosis of cardiac arrest or with preexisting tracheostomy were excluded because of expected requirements for ventilatory support. The sample included a total of 2,470,735 Medicare beneficiaries; mean age was 82.2 years, and 54.8% were female. Primary admitting diagnosis codes were used to identify 3 subcohorts: congestive heart failure, chronic obstructive pulmonary disease, and cancer; a fourth subcohort of dementia was identified using the primary admitting diagnosis code or the first 9 secondary diagnosis codes.
Main outcome measures. The study used procedure codes to identify the use of noninvasive ventilation, invasive mechanical ventilation, or none among decedents who were hospitalized in the last 30 days of life. Descriptive statistics to characterize variables by year of hospitalization and ventilatory support were calculated, and the rates of noninvasive and invasive mechanical ventilation use were tabulated. Other outcomes of interest include site of death (in-hospital death), hospice enrollment at death, and hospice enrollment in the last 3 days of life as measures of end-of- life care use. Multivariable logistic regressions were used to examine noninvasive and invasive mechanical ventilation use among decedents, and time trends were examined, with the pattern of use in year 2000 as reference. Subgroup analysis with the subcohort of patients with different diagnoses were conducted to examine trends.
Main results. From 2000 to 2017, 16.3% of decedents had invasive mechanical ventilation, 3.7% had noninvasive ventilation, and 1.0% had both noninvasive and invasive ventilation during their hospital stay. Compared to the reference year 2000, there was a 9-fold increase in noninvasive ventilation use, from 0.8% to 7.1% in 2017, and invasive mechanical ventilation use also increased slightly, from 15.0% to 18.5%. Compared to year 2000, decedents were 2.63 times and 1.04 times (adjusted odds ratio [OR]) more likely to receive noninvasive ventilation and invasive mechanical ventilation, respectively, in 2005, 7.87 times and 1.39 times more likely in 2011, and 11.84 times and 1.63 times more likely in 2017.
Subgroup analysis showed that for congestive heart failure and chronic obstructive pulmonary disease, the increase in noninvasive ventilation use mirrored the trend observed for the overall population, but the use of invasive mechanical ventilation did not increase from 2000 to 2017, with a rate of use of 11.1% versus 7.8% (adjusted OR, 1.07; 95% confidence interval [CI], 0.95-1.19) for congestive heart failure and 17.4% vs 13.2% (OR 1.03, 95% CI, 0.88-1.21) for chronic obstructive pulmonary disease. For the cancer and dementia subgroups, the increase in noninvasive ventilation use from 2000 to 2017 was accompanied by an increase in the use of invasive mechanical ventilation, with a rate of 6.2% versus 7.4% (OR, 1.40; 95% CI, 1.26-1.55) for decedents with cancer and a rate of 5.7% versus 6.2% (OR, 1.28; 95% CI, 1.17-1.41) for decedents with dementia. For other measures of end-of-life care, noninvasive ventilation use when compared to invasive mechanical ventilation use was associated with lower rates of in-hospital (acute care) deaths (50.3% vs 76.7%), hospice enrollment in the last 3 days of life (late hospice enrollment; 57.7% vs 63.0%), and higher rates of hospice enrollment at death (41.3% vs 20.0%).
Conclusion. There was an increase in the use of noninvasive ventilation from 2000 through 2017 among Medicare beneficiaries who died. The findings also suggest that the use of invasive mechanical ventilation did not increase among decedents with congestive heart failure and chronic obstructive pulmonary disease but increased among decedents with cancer and dementia.
Commentary
Noninvasive ventilation offers an alternative to invasive mechanical ventilation for providing ventilatory support for respiratory failure, and may offer benefits as it could avert adverse effects associated with invasive mechanical ventilation, particularly in the management of respiratory failure due to congestive heart failure and chronic obstructive pulmonary disease.1 There is evidence for potential benefits of use of noninvasive ventilation in other clinical scenarios, such as pneumonia in older adults with comorbidities, though its clinical utility is not as well established for other diseases.2
As noninvasive ventilation is introduced into clinical practice, it is not surprising that over the period of the study (2000 to 2017) that its use increased substantially. Advance directives that involve discussion of life-sustaining treatments, including in scenarios with respiratory failure, may also result in physician orders that specify whether an individual desires invasive mechanical ventilation versus other medical treatments, including noninvasive ventilation.3,4 By examining the temporal trends of use of noninvasive and invasive ventilation, this study reveals that invasive mechanical ventilation use among decedents with dementia and cancer has increased, despite increases in the use of noninvasive ventilation. It is important to understand further what would explain these temporal trends and whether the use of noninvasive and also invasive mechanical ventilation at the end of life represents appropriate care with clear goals or whether it may represent overuse. It is also less clear in the end-of-life care scenario what the goals of treatment with noninvasive ventilation would be, especially if it does not avert the use of invasive mechanical ventilation.
The study includes decedents only, thus limiting the ability to draw conclusions about clinically appropriate care.5 Further studies should examine a cohort of patients who have serious and life-threatening illness to examine the trends and potential effects of noninvasive ventilation on outcomes and utilization, as individuals who have improved and survived would not be included in this present decedent cohort.
Applications for Clinical Practice
This study highlights changes in the use of noninvasive and invasive ventilation over time and the different trends seen among subgroups with different diagnoses. For older adults with serious comorbid illness such as dementia, it is especially important to have discussions on advance directives so that care at the end of life is concordant with the patient’s wishes and that unnecessary, burdensome care can be averted. Further studies to understand and define the appropriate use of noninvasive and invasive mechanical ventilation for older adults with significant comorbidities who have serious, life-threatening illness are needed to ensure appropriate clinical treatment at the end of life.
–William W. Hung, MD, MPH
1. Lindenauer PK, Stefan MS, Shieh M et al. Outcomes associated with invasive and noninvasive ventilation a mong patients hospitalized with exacerbations of chronic obstructive pulmonary disease. JAMA Intern Med. 2014;174:1982-993.
2. Johnson CS, Frei CR, Metersky ML, et al. Non-invasive mechanical ventilation and mortality in elderly immunocompromised patients hospitalized with pneumonia: a retrospective cohort study. BMC Pulm Med. 2014;14:7. Published 2014 Jan 27. doi:10.1186/1471-2466-14-7
3. Lee R, Brumbeck L, Sathitratanacheewin S, et al. Association of physician orders for life-sustaining treatment with icu admission among patients hospitalized near the end of life. JAMA. 2020;323:950-60.
4. Bomba P, Kemp M, Black J. POLST: An improvement over traditional advance directives. Cleveland Clinic J Med. 2012;79:457-464.
5. Duncan I, Ahmed T, Dove H, Maxwell TL. Medicare cost at end of life. Am J Hosp Palliat Care. 2019;36:705-710.
Study Overview
Objective. To examine the trend of noninvasive and invasive mechanical ventilation at the end of life from 2000 to 2017.
Design. Observational population-based cohort study.
Setting and participants. The study was a population-based cohort study to examine the use of noninvasive and invasive mechanical ventilation among decedents. The study included a random 20% sample of Medicare beneficiaries older than 65 years who were hospitalized in the last 30 days of life and died between January 1, 2000, and December 31, 2017, except for the period October 1, 2015, to December 31, 2015, when the transition from International Classification of Diseases, Ninth Revision (ICD-9) to ICD-10 occurred. Beneficiaries with the primary admitting diagnosis of cardiac arrest or with preexisting tracheostomy were excluded because of expected requirements for ventilatory support. The sample included a total of 2,470,735 Medicare beneficiaries; mean age was 82.2 years, and 54.8% were female. Primary admitting diagnosis codes were used to identify 3 subcohorts: congestive heart failure, chronic obstructive pulmonary disease, and cancer; a fourth subcohort of dementia was identified using the primary admitting diagnosis code or the first 9 secondary diagnosis codes.
Main outcome measures. The study used procedure codes to identify the use of noninvasive ventilation, invasive mechanical ventilation, or none among decedents who were hospitalized in the last 30 days of life. Descriptive statistics to characterize variables by year of hospitalization and ventilatory support were calculated, and the rates of noninvasive and invasive mechanical ventilation use were tabulated. Other outcomes of interest include site of death (in-hospital death), hospice enrollment at death, and hospice enrollment in the last 3 days of life as measures of end-of- life care use. Multivariable logistic regressions were used to examine noninvasive and invasive mechanical ventilation use among decedents, and time trends were examined, with the pattern of use in year 2000 as reference. Subgroup analysis with the subcohort of patients with different diagnoses were conducted to examine trends.
Main results. From 2000 to 2017, 16.3% of decedents had invasive mechanical ventilation, 3.7% had noninvasive ventilation, and 1.0% had both noninvasive and invasive ventilation during their hospital stay. Compared to the reference year 2000, there was a 9-fold increase in noninvasive ventilation use, from 0.8% to 7.1% in 2017, and invasive mechanical ventilation use also increased slightly, from 15.0% to 18.5%. Compared to year 2000, decedents were 2.63 times and 1.04 times (adjusted odds ratio [OR]) more likely to receive noninvasive ventilation and invasive mechanical ventilation, respectively, in 2005, 7.87 times and 1.39 times more likely in 2011, and 11.84 times and 1.63 times more likely in 2017.
Subgroup analysis showed that for congestive heart failure and chronic obstructive pulmonary disease, the increase in noninvasive ventilation use mirrored the trend observed for the overall population, but the use of invasive mechanical ventilation did not increase from 2000 to 2017, with a rate of use of 11.1% versus 7.8% (adjusted OR, 1.07; 95% confidence interval [CI], 0.95-1.19) for congestive heart failure and 17.4% vs 13.2% (OR 1.03, 95% CI, 0.88-1.21) for chronic obstructive pulmonary disease. For the cancer and dementia subgroups, the increase in noninvasive ventilation use from 2000 to 2017 was accompanied by an increase in the use of invasive mechanical ventilation, with a rate of 6.2% versus 7.4% (OR, 1.40; 95% CI, 1.26-1.55) for decedents with cancer and a rate of 5.7% versus 6.2% (OR, 1.28; 95% CI, 1.17-1.41) for decedents with dementia. For other measures of end-of-life care, noninvasive ventilation use when compared to invasive mechanical ventilation use was associated with lower rates of in-hospital (acute care) deaths (50.3% vs 76.7%), hospice enrollment in the last 3 days of life (late hospice enrollment; 57.7% vs 63.0%), and higher rates of hospice enrollment at death (41.3% vs 20.0%).
Conclusion. There was an increase in the use of noninvasive ventilation from 2000 through 2017 among Medicare beneficiaries who died. The findings also suggest that the use of invasive mechanical ventilation did not increase among decedents with congestive heart failure and chronic obstructive pulmonary disease but increased among decedents with cancer and dementia.
Commentary
Noninvasive ventilation offers an alternative to invasive mechanical ventilation for providing ventilatory support for respiratory failure, and may offer benefits as it could avert adverse effects associated with invasive mechanical ventilation, particularly in the management of respiratory failure due to congestive heart failure and chronic obstructive pulmonary disease.1 There is evidence for potential benefits of use of noninvasive ventilation in other clinical scenarios, such as pneumonia in older adults with comorbidities, though its clinical utility is not as well established for other diseases.2
As noninvasive ventilation is introduced into clinical practice, it is not surprising that over the period of the study (2000 to 2017) that its use increased substantially. Advance directives that involve discussion of life-sustaining treatments, including in scenarios with respiratory failure, may also result in physician orders that specify whether an individual desires invasive mechanical ventilation versus other medical treatments, including noninvasive ventilation.3,4 By examining the temporal trends of use of noninvasive and invasive ventilation, this study reveals that invasive mechanical ventilation use among decedents with dementia and cancer has increased, despite increases in the use of noninvasive ventilation. It is important to understand further what would explain these temporal trends and whether the use of noninvasive and also invasive mechanical ventilation at the end of life represents appropriate care with clear goals or whether it may represent overuse. It is also less clear in the end-of-life care scenario what the goals of treatment with noninvasive ventilation would be, especially if it does not avert the use of invasive mechanical ventilation.
The study includes decedents only, thus limiting the ability to draw conclusions about clinically appropriate care.5 Further studies should examine a cohort of patients who have serious and life-threatening illness to examine the trends and potential effects of noninvasive ventilation on outcomes and utilization, as individuals who have improved and survived would not be included in this present decedent cohort.
Applications for Clinical Practice
This study highlights changes in the use of noninvasive and invasive ventilation over time and the different trends seen among subgroups with different diagnoses. For older adults with serious comorbid illness such as dementia, it is especially important to have discussions on advance directives so that care at the end of life is concordant with the patient’s wishes and that unnecessary, burdensome care can be averted. Further studies to understand and define the appropriate use of noninvasive and invasive mechanical ventilation for older adults with significant comorbidities who have serious, life-threatening illness are needed to ensure appropriate clinical treatment at the end of life.
–William W. Hung, MD, MPH
Study Overview
Objective. To examine the trend of noninvasive and invasive mechanical ventilation at the end of life from 2000 to 2017.
Design. Observational population-based cohort study.
Setting and participants. The study was a population-based cohort study to examine the use of noninvasive and invasive mechanical ventilation among decedents. The study included a random 20% sample of Medicare beneficiaries older than 65 years who were hospitalized in the last 30 days of life and died between January 1, 2000, and December 31, 2017, except for the period October 1, 2015, to December 31, 2015, when the transition from International Classification of Diseases, Ninth Revision (ICD-9) to ICD-10 occurred. Beneficiaries with the primary admitting diagnosis of cardiac arrest or with preexisting tracheostomy were excluded because of expected requirements for ventilatory support. The sample included a total of 2,470,735 Medicare beneficiaries; mean age was 82.2 years, and 54.8% were female. Primary admitting diagnosis codes were used to identify 3 subcohorts: congestive heart failure, chronic obstructive pulmonary disease, and cancer; a fourth subcohort of dementia was identified using the primary admitting diagnosis code or the first 9 secondary diagnosis codes.
Main outcome measures. The study used procedure codes to identify the use of noninvasive ventilation, invasive mechanical ventilation, or none among decedents who were hospitalized in the last 30 days of life. Descriptive statistics to characterize variables by year of hospitalization and ventilatory support were calculated, and the rates of noninvasive and invasive mechanical ventilation use were tabulated. Other outcomes of interest include site of death (in-hospital death), hospice enrollment at death, and hospice enrollment in the last 3 days of life as measures of end-of- life care use. Multivariable logistic regressions were used to examine noninvasive and invasive mechanical ventilation use among decedents, and time trends were examined, with the pattern of use in year 2000 as reference. Subgroup analysis with the subcohort of patients with different diagnoses were conducted to examine trends.
Main results. From 2000 to 2017, 16.3% of decedents had invasive mechanical ventilation, 3.7% had noninvasive ventilation, and 1.0% had both noninvasive and invasive ventilation during their hospital stay. Compared to the reference year 2000, there was a 9-fold increase in noninvasive ventilation use, from 0.8% to 7.1% in 2017, and invasive mechanical ventilation use also increased slightly, from 15.0% to 18.5%. Compared to year 2000, decedents were 2.63 times and 1.04 times (adjusted odds ratio [OR]) more likely to receive noninvasive ventilation and invasive mechanical ventilation, respectively, in 2005, 7.87 times and 1.39 times more likely in 2011, and 11.84 times and 1.63 times more likely in 2017.
Subgroup analysis showed that for congestive heart failure and chronic obstructive pulmonary disease, the increase in noninvasive ventilation use mirrored the trend observed for the overall population, but the use of invasive mechanical ventilation did not increase from 2000 to 2017, with a rate of use of 11.1% versus 7.8% (adjusted OR, 1.07; 95% confidence interval [CI], 0.95-1.19) for congestive heart failure and 17.4% vs 13.2% (OR 1.03, 95% CI, 0.88-1.21) for chronic obstructive pulmonary disease. For the cancer and dementia subgroups, the increase in noninvasive ventilation use from 2000 to 2017 was accompanied by an increase in the use of invasive mechanical ventilation, with a rate of 6.2% versus 7.4% (OR, 1.40; 95% CI, 1.26-1.55) for decedents with cancer and a rate of 5.7% versus 6.2% (OR, 1.28; 95% CI, 1.17-1.41) for decedents with dementia. For other measures of end-of-life care, noninvasive ventilation use when compared to invasive mechanical ventilation use was associated with lower rates of in-hospital (acute care) deaths (50.3% vs 76.7%), hospice enrollment in the last 3 days of life (late hospice enrollment; 57.7% vs 63.0%), and higher rates of hospice enrollment at death (41.3% vs 20.0%).
Conclusion. There was an increase in the use of noninvasive ventilation from 2000 through 2017 among Medicare beneficiaries who died. The findings also suggest that the use of invasive mechanical ventilation did not increase among decedents with congestive heart failure and chronic obstructive pulmonary disease but increased among decedents with cancer and dementia.
Commentary
Noninvasive ventilation offers an alternative to invasive mechanical ventilation for providing ventilatory support for respiratory failure, and may offer benefits as it could avert adverse effects associated with invasive mechanical ventilation, particularly in the management of respiratory failure due to congestive heart failure and chronic obstructive pulmonary disease.1 There is evidence for potential benefits of use of noninvasive ventilation in other clinical scenarios, such as pneumonia in older adults with comorbidities, though its clinical utility is not as well established for other diseases.2
As noninvasive ventilation is introduced into clinical practice, it is not surprising that over the period of the study (2000 to 2017) that its use increased substantially. Advance directives that involve discussion of life-sustaining treatments, including in scenarios with respiratory failure, may also result in physician orders that specify whether an individual desires invasive mechanical ventilation versus other medical treatments, including noninvasive ventilation.3,4 By examining the temporal trends of use of noninvasive and invasive ventilation, this study reveals that invasive mechanical ventilation use among decedents with dementia and cancer has increased, despite increases in the use of noninvasive ventilation. It is important to understand further what would explain these temporal trends and whether the use of noninvasive and also invasive mechanical ventilation at the end of life represents appropriate care with clear goals or whether it may represent overuse. It is also less clear in the end-of-life care scenario what the goals of treatment with noninvasive ventilation would be, especially if it does not avert the use of invasive mechanical ventilation.
The study includes decedents only, thus limiting the ability to draw conclusions about clinically appropriate care.5 Further studies should examine a cohort of patients who have serious and life-threatening illness to examine the trends and potential effects of noninvasive ventilation on outcomes and utilization, as individuals who have improved and survived would not be included in this present decedent cohort.
Applications for Clinical Practice
This study highlights changes in the use of noninvasive and invasive ventilation over time and the different trends seen among subgroups with different diagnoses. For older adults with serious comorbid illness such as dementia, it is especially important to have discussions on advance directives so that care at the end of life is concordant with the patient’s wishes and that unnecessary, burdensome care can be averted. Further studies to understand and define the appropriate use of noninvasive and invasive mechanical ventilation for older adults with significant comorbidities who have serious, life-threatening illness are needed to ensure appropriate clinical treatment at the end of life.
–William W. Hung, MD, MPH
1. Lindenauer PK, Stefan MS, Shieh M et al. Outcomes associated with invasive and noninvasive ventilation a mong patients hospitalized with exacerbations of chronic obstructive pulmonary disease. JAMA Intern Med. 2014;174:1982-993.
2. Johnson CS, Frei CR, Metersky ML, et al. Non-invasive mechanical ventilation and mortality in elderly immunocompromised patients hospitalized with pneumonia: a retrospective cohort study. BMC Pulm Med. 2014;14:7. Published 2014 Jan 27. doi:10.1186/1471-2466-14-7
3. Lee R, Brumbeck L, Sathitratanacheewin S, et al. Association of physician orders for life-sustaining treatment with icu admission among patients hospitalized near the end of life. JAMA. 2020;323:950-60.
4. Bomba P, Kemp M, Black J. POLST: An improvement over traditional advance directives. Cleveland Clinic J Med. 2012;79:457-464.
5. Duncan I, Ahmed T, Dove H, Maxwell TL. Medicare cost at end of life. Am J Hosp Palliat Care. 2019;36:705-710.
1. Lindenauer PK, Stefan MS, Shieh M et al. Outcomes associated with invasive and noninvasive ventilation a mong patients hospitalized with exacerbations of chronic obstructive pulmonary disease. JAMA Intern Med. 2014;174:1982-993.
2. Johnson CS, Frei CR, Metersky ML, et al. Non-invasive mechanical ventilation and mortality in elderly immunocompromised patients hospitalized with pneumonia: a retrospective cohort study. BMC Pulm Med. 2014;14:7. Published 2014 Jan 27. doi:10.1186/1471-2466-14-7
3. Lee R, Brumbeck L, Sathitratanacheewin S, et al. Association of physician orders for life-sustaining treatment with icu admission among patients hospitalized near the end of life. JAMA. 2020;323:950-60.
4. Bomba P, Kemp M, Black J. POLST: An improvement over traditional advance directives. Cleveland Clinic J Med. 2012;79:457-464.
5. Duncan I, Ahmed T, Dove H, Maxwell TL. Medicare cost at end of life. Am J Hosp Palliat Care. 2019;36:705-710.