User login
Sex differences in COPD symptoms predict cardiac comorbidity
Sex-specific differences in the severity of symptoms and prevalence of comorbidities in patients with chronic obstructive pulmonary disease (COPD) may point to different criteria for diagnosing cardiac comorbidities in women and men, a retrospective analysis suggests.
Among 2,046 patients in the German COSYCONET (COPD and Systemic Consequences–Comorbidities Net) cohort, most functional parameters and comorbidities and several items on the COPD Assessment Test (CAT) differed significantly between men and women.
In addition, there were sex-specific differences in the association between symptoms and cardiac disease, Franziska C. Trudzinski, MD, from the University of Heidelberg (Germany), and colleagues reported.
(Note: Although the authors used the term “gender” to distinguish male from female, this news organization has used the term “sex” in this article to refer to biological attributes of individual patients rather than personal identity.)
“[Sex]-specific differences in COPD comprised not only differences in the level of symptoms, comorbidities, and functional alterations but also differences in their mutual relationships. This was reflected in different sets of predictors for cardiac disease,” they wrote in a thematic poster presented at the American Thoracic Society’s virtual international conference.
GOLD standard
The investigators conducted an analysis of data on 795 women and 1,251 men with GOLD (Global Initiative for Chronic Obstructive Lung Disease) class 1-3 disease from the COSYCONET COPD cohort.
They looked at the patients’ clinical history, comorbidities, lung function, CAT scores, and modified Medical Research Council (mMRC) dyspnea score.
The authors used multivariate regression analysis to model potential sex-related differences in the relationship between symptoms in general and CAT items in particular, and the pattern of comorbidities and functional alterations.
They also performed logistic regression analyses to identify predictors for cardiac disease, defined as myocardial infarction, heart failure, or coronary artery disease. The analyses were controlled for age, body mass index (BMI), smoking status, mMRC, CAT items, and z scores of forced expiratory volume in 1 second/forced vital capacity ratio.
and for CAT items of cough (item 1), phlegm (item 2), and energy (item 8; P < .05 for all comparisons).
In logistic regression analysis, predictors for cardiac disease in men were energy (CAT item 8), mMRC score, smoking status, BMI, age, and spirometric lung function.
In women, however, only age was significantly predictive for cardiac disease.
“Our findings give hints how diagnostic information might be used differently in men and women,” Dr. Trudzinski and colleagues wrote.
Reassuring data
David Mannino, MD, medical director of the COPD Foundation, who was not involved in the study, said in an interview that sex differences in COPD presentation and severity are common.
“In general, men and women report symptoms differently. For example, women don’t report a whole lot of chronic bronchitis and phlegm, although they may have it,” he said, “whereas men may report less dyspnea. It varies, but in general we know that men and women, even with the same type of disease, report symptoms differently.”
Comorbidities also differ between the sexes, he noted. Women more frequently have osteoporosis, and men more frequently have heart disease, as borne out in the study. The prevalence of heart disease among patients in the study was approximately 2.5 times higher in men than women.
“It’s reassuring, because what we’re seeing is similar to what we’ve seen in other [studies] with regards to comorbidities,” he said.
The study was sponsored by Philipps University Marburg Medical Center, Germany. The authors and Dr. Mannino have reported no relevant financial relationships.
A version of the article first appeared on Medscape.com.
Sex-specific differences in the severity of symptoms and prevalence of comorbidities in patients with chronic obstructive pulmonary disease (COPD) may point to different criteria for diagnosing cardiac comorbidities in women and men, a retrospective analysis suggests.
Among 2,046 patients in the German COSYCONET (COPD and Systemic Consequences–Comorbidities Net) cohort, most functional parameters and comorbidities and several items on the COPD Assessment Test (CAT) differed significantly between men and women.
In addition, there were sex-specific differences in the association between symptoms and cardiac disease, Franziska C. Trudzinski, MD, from the University of Heidelberg (Germany), and colleagues reported.
(Note: Although the authors used the term “gender” to distinguish male from female, this news organization has used the term “sex” in this article to refer to biological attributes of individual patients rather than personal identity.)
“[Sex]-specific differences in COPD comprised not only differences in the level of symptoms, comorbidities, and functional alterations but also differences in their mutual relationships. This was reflected in different sets of predictors for cardiac disease,” they wrote in a thematic poster presented at the American Thoracic Society’s virtual international conference.
GOLD standard
The investigators conducted an analysis of data on 795 women and 1,251 men with GOLD (Global Initiative for Chronic Obstructive Lung Disease) class 1-3 disease from the COSYCONET COPD cohort.
They looked at the patients’ clinical history, comorbidities, lung function, CAT scores, and modified Medical Research Council (mMRC) dyspnea score.
The authors used multivariate regression analysis to model potential sex-related differences in the relationship between symptoms in general and CAT items in particular, and the pattern of comorbidities and functional alterations.
They also performed logistic regression analyses to identify predictors for cardiac disease, defined as myocardial infarction, heart failure, or coronary artery disease. The analyses were controlled for age, body mass index (BMI), smoking status, mMRC, CAT items, and z scores of forced expiratory volume in 1 second/forced vital capacity ratio.
and for CAT items of cough (item 1), phlegm (item 2), and energy (item 8; P < .05 for all comparisons).
In logistic regression analysis, predictors for cardiac disease in men were energy (CAT item 8), mMRC score, smoking status, BMI, age, and spirometric lung function.
In women, however, only age was significantly predictive for cardiac disease.
“Our findings give hints how diagnostic information might be used differently in men and women,” Dr. Trudzinski and colleagues wrote.
Reassuring data
David Mannino, MD, medical director of the COPD Foundation, who was not involved in the study, said in an interview that sex differences in COPD presentation and severity are common.
“In general, men and women report symptoms differently. For example, women don’t report a whole lot of chronic bronchitis and phlegm, although they may have it,” he said, “whereas men may report less dyspnea. It varies, but in general we know that men and women, even with the same type of disease, report symptoms differently.”
Comorbidities also differ between the sexes, he noted. Women more frequently have osteoporosis, and men more frequently have heart disease, as borne out in the study. The prevalence of heart disease among patients in the study was approximately 2.5 times higher in men than women.
“It’s reassuring, because what we’re seeing is similar to what we’ve seen in other [studies] with regards to comorbidities,” he said.
The study was sponsored by Philipps University Marburg Medical Center, Germany. The authors and Dr. Mannino have reported no relevant financial relationships.
A version of the article first appeared on Medscape.com.
Sex-specific differences in the severity of symptoms and prevalence of comorbidities in patients with chronic obstructive pulmonary disease (COPD) may point to different criteria for diagnosing cardiac comorbidities in women and men, a retrospective analysis suggests.
Among 2,046 patients in the German COSYCONET (COPD and Systemic Consequences–Comorbidities Net) cohort, most functional parameters and comorbidities and several items on the COPD Assessment Test (CAT) differed significantly between men and women.
In addition, there were sex-specific differences in the association between symptoms and cardiac disease, Franziska C. Trudzinski, MD, from the University of Heidelberg (Germany), and colleagues reported.
(Note: Although the authors used the term “gender” to distinguish male from female, this news organization has used the term “sex” in this article to refer to biological attributes of individual patients rather than personal identity.)
“[Sex]-specific differences in COPD comprised not only differences in the level of symptoms, comorbidities, and functional alterations but also differences in their mutual relationships. This was reflected in different sets of predictors for cardiac disease,” they wrote in a thematic poster presented at the American Thoracic Society’s virtual international conference.
GOLD standard
The investigators conducted an analysis of data on 795 women and 1,251 men with GOLD (Global Initiative for Chronic Obstructive Lung Disease) class 1-3 disease from the COSYCONET COPD cohort.
They looked at the patients’ clinical history, comorbidities, lung function, CAT scores, and modified Medical Research Council (mMRC) dyspnea score.
The authors used multivariate regression analysis to model potential sex-related differences in the relationship between symptoms in general and CAT items in particular, and the pattern of comorbidities and functional alterations.
They also performed logistic regression analyses to identify predictors for cardiac disease, defined as myocardial infarction, heart failure, or coronary artery disease. The analyses were controlled for age, body mass index (BMI), smoking status, mMRC, CAT items, and z scores of forced expiratory volume in 1 second/forced vital capacity ratio.
and for CAT items of cough (item 1), phlegm (item 2), and energy (item 8; P < .05 for all comparisons).
In logistic regression analysis, predictors for cardiac disease in men were energy (CAT item 8), mMRC score, smoking status, BMI, age, and spirometric lung function.
In women, however, only age was significantly predictive for cardiac disease.
“Our findings give hints how diagnostic information might be used differently in men and women,” Dr. Trudzinski and colleagues wrote.
Reassuring data
David Mannino, MD, medical director of the COPD Foundation, who was not involved in the study, said in an interview that sex differences in COPD presentation and severity are common.
“In general, men and women report symptoms differently. For example, women don’t report a whole lot of chronic bronchitis and phlegm, although they may have it,” he said, “whereas men may report less dyspnea. It varies, but in general we know that men and women, even with the same type of disease, report symptoms differently.”
Comorbidities also differ between the sexes, he noted. Women more frequently have osteoporosis, and men more frequently have heart disease, as borne out in the study. The prevalence of heart disease among patients in the study was approximately 2.5 times higher in men than women.
“It’s reassuring, because what we’re seeing is similar to what we’ve seen in other [studies] with regards to comorbidities,” he said.
The study was sponsored by Philipps University Marburg Medical Center, Germany. The authors and Dr. Mannino have reported no relevant financial relationships.
A version of the article first appeared on Medscape.com.
Worse outcomes for patients with COPD and COVID-19
A study of COVID-19 outcomes across the United States bolsters reports from China and Europe that indicate that patients with chronic obstructive pulmonary disease (COPD) and SARS-CoV-2 infection have worse outcomes than those of patients with COVID-19 who do not have COPD.
Investigators at the University of Texas Medical Branch at Galveston, Texas, combed through electronic health records from four geographic regions of the United States and identified a cohort of 6,056 patients with COPD among 150,775 patients whose records indicate either a diagnostic code or a positive laboratory test result for COVID-19.
Their findings indicate that patients with both COPD and COVID-19 “have worse outcomes compared to non-COPD COVID-19 patients, including 14-day hospitalization, length of stay, ICU admission, 30-day mortality, and use of mechanical ventilation,” Daniel Puebla Neira, MD, and colleagues from the University of Texas Medical Branch reported in a thematic poster presented during the American Thoracic Society (ATS) 2021 virtual international conference.
A critical care specialist who was not involved in the study said that the results are concerning but not surprising.
“If you already have a lung disease and you develop an additional lung disease on top of that, you don’t have as much reserve and you’re not going to tolerate the acute COVID infection,” said ATS expert Marc Moss, MD, Roger S. Mitchell Professor of Medicine in the division of pulmonary sciences and critical care medicine at the University of Colorado, Aurora.
The evidence shows that “patients with COPD should be even more cautious, because if they get sick and develop, they could do worse,” he said in an interview.
Retrospective analysis
Dr. Neira and colleagues assessed the characteristics and outcomes of patients with COPD who were treated for COVID-19 in the United States from March through August 2020.
Baseline demographics of the patients with and those without COPD were similar except that the mean age was higher among patients with COPD (68.62 vs. 47.08 years).
In addition, a significantly higher proportion of patients with COPD had comorbidities compared with those without COPD. Comorbidities included diabetes, hypertension, asthma, chronic kidney disease, end-stage renal disease, stroke, heart failure, cancer, coronary artery disease, and liver disease (P < .0001 for all comparisons).
Among patients with COPD, percentages were higher with respect to the following parameters: 14-day hospitalization for any cause (28.7% vs. 10.4%), COVID-19-related 14-day hospitalization (28.1% vs. 9.9%), ICU use (26.3% vs. 17.9%), mechanical ventilation use (26.3% vs. 16.1%), and 30-day mortality (13.6% vs. 7.2%; P < .0001 for all comparisons).
‘Mechanisms unclear’
“It is unclear what mechanisms drive the association between COPD and mortality in hospitalized patients with COVID-19,” the investigators wrote. “Several biological factors have been proposed, including chronic lung inflammation, oxidative stress, protease-antiprotease imbalance, and increased airway mediators.”
They recommend use of multivariable logistic regression to tease out the effects of covariates among patients with COPD and COVID-19 and call for research into long-term outcomes for these patients, “as survivors of critical illness are increasingly recognized to have cognitive, psychological, and physical consequences.”
Dr. Moss said that in general, the management of patients with COPD and COVID-19 is similar to that for patients with COVID-19 who do not have COPD, although there may be “subtle” differences, such as ventilator settings for patients with COPD.
No source of funding for the study has been disclosed. The investigators and Dr. Moss have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A study of COVID-19 outcomes across the United States bolsters reports from China and Europe that indicate that patients with chronic obstructive pulmonary disease (COPD) and SARS-CoV-2 infection have worse outcomes than those of patients with COVID-19 who do not have COPD.
Investigators at the University of Texas Medical Branch at Galveston, Texas, combed through electronic health records from four geographic regions of the United States and identified a cohort of 6,056 patients with COPD among 150,775 patients whose records indicate either a diagnostic code or a positive laboratory test result for COVID-19.
Their findings indicate that patients with both COPD and COVID-19 “have worse outcomes compared to non-COPD COVID-19 patients, including 14-day hospitalization, length of stay, ICU admission, 30-day mortality, and use of mechanical ventilation,” Daniel Puebla Neira, MD, and colleagues from the University of Texas Medical Branch reported in a thematic poster presented during the American Thoracic Society (ATS) 2021 virtual international conference.
A critical care specialist who was not involved in the study said that the results are concerning but not surprising.
“If you already have a lung disease and you develop an additional lung disease on top of that, you don’t have as much reserve and you’re not going to tolerate the acute COVID infection,” said ATS expert Marc Moss, MD, Roger S. Mitchell Professor of Medicine in the division of pulmonary sciences and critical care medicine at the University of Colorado, Aurora.
The evidence shows that “patients with COPD should be even more cautious, because if they get sick and develop, they could do worse,” he said in an interview.
Retrospective analysis
Dr. Neira and colleagues assessed the characteristics and outcomes of patients with COPD who were treated for COVID-19 in the United States from March through August 2020.
Baseline demographics of the patients with and those without COPD were similar except that the mean age was higher among patients with COPD (68.62 vs. 47.08 years).
In addition, a significantly higher proportion of patients with COPD had comorbidities compared with those without COPD. Comorbidities included diabetes, hypertension, asthma, chronic kidney disease, end-stage renal disease, stroke, heart failure, cancer, coronary artery disease, and liver disease (P < .0001 for all comparisons).
Among patients with COPD, percentages were higher with respect to the following parameters: 14-day hospitalization for any cause (28.7% vs. 10.4%), COVID-19-related 14-day hospitalization (28.1% vs. 9.9%), ICU use (26.3% vs. 17.9%), mechanical ventilation use (26.3% vs. 16.1%), and 30-day mortality (13.6% vs. 7.2%; P < .0001 for all comparisons).
‘Mechanisms unclear’
“It is unclear what mechanisms drive the association between COPD and mortality in hospitalized patients with COVID-19,” the investigators wrote. “Several biological factors have been proposed, including chronic lung inflammation, oxidative stress, protease-antiprotease imbalance, and increased airway mediators.”
They recommend use of multivariable logistic regression to tease out the effects of covariates among patients with COPD and COVID-19 and call for research into long-term outcomes for these patients, “as survivors of critical illness are increasingly recognized to have cognitive, psychological, and physical consequences.”
Dr. Moss said that in general, the management of patients with COPD and COVID-19 is similar to that for patients with COVID-19 who do not have COPD, although there may be “subtle” differences, such as ventilator settings for patients with COPD.
No source of funding for the study has been disclosed. The investigators and Dr. Moss have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A study of COVID-19 outcomes across the United States bolsters reports from China and Europe that indicate that patients with chronic obstructive pulmonary disease (COPD) and SARS-CoV-2 infection have worse outcomes than those of patients with COVID-19 who do not have COPD.
Investigators at the University of Texas Medical Branch at Galveston, Texas, combed through electronic health records from four geographic regions of the United States and identified a cohort of 6,056 patients with COPD among 150,775 patients whose records indicate either a diagnostic code or a positive laboratory test result for COVID-19.
Their findings indicate that patients with both COPD and COVID-19 “have worse outcomes compared to non-COPD COVID-19 patients, including 14-day hospitalization, length of stay, ICU admission, 30-day mortality, and use of mechanical ventilation,” Daniel Puebla Neira, MD, and colleagues from the University of Texas Medical Branch reported in a thematic poster presented during the American Thoracic Society (ATS) 2021 virtual international conference.
A critical care specialist who was not involved in the study said that the results are concerning but not surprising.
“If you already have a lung disease and you develop an additional lung disease on top of that, you don’t have as much reserve and you’re not going to tolerate the acute COVID infection,” said ATS expert Marc Moss, MD, Roger S. Mitchell Professor of Medicine in the division of pulmonary sciences and critical care medicine at the University of Colorado, Aurora.
The evidence shows that “patients with COPD should be even more cautious, because if they get sick and develop, they could do worse,” he said in an interview.
Retrospective analysis
Dr. Neira and colleagues assessed the characteristics and outcomes of patients with COPD who were treated for COVID-19 in the United States from March through August 2020.
Baseline demographics of the patients with and those without COPD were similar except that the mean age was higher among patients with COPD (68.62 vs. 47.08 years).
In addition, a significantly higher proportion of patients with COPD had comorbidities compared with those without COPD. Comorbidities included diabetes, hypertension, asthma, chronic kidney disease, end-stage renal disease, stroke, heart failure, cancer, coronary artery disease, and liver disease (P < .0001 for all comparisons).
Among patients with COPD, percentages were higher with respect to the following parameters: 14-day hospitalization for any cause (28.7% vs. 10.4%), COVID-19-related 14-day hospitalization (28.1% vs. 9.9%), ICU use (26.3% vs. 17.9%), mechanical ventilation use (26.3% vs. 16.1%), and 30-day mortality (13.6% vs. 7.2%; P < .0001 for all comparisons).
‘Mechanisms unclear’
“It is unclear what mechanisms drive the association between COPD and mortality in hospitalized patients with COVID-19,” the investigators wrote. “Several biological factors have been proposed, including chronic lung inflammation, oxidative stress, protease-antiprotease imbalance, and increased airway mediators.”
They recommend use of multivariable logistic regression to tease out the effects of covariates among patients with COPD and COVID-19 and call for research into long-term outcomes for these patients, “as survivors of critical illness are increasingly recognized to have cognitive, psychological, and physical consequences.”
Dr. Moss said that in general, the management of patients with COPD and COVID-19 is similar to that for patients with COVID-19 who do not have COPD, although there may be “subtle” differences, such as ventilator settings for patients with COPD.
No source of funding for the study has been disclosed. The investigators and Dr. Moss have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New guidance for those fully vaccinated against COVID-19
As has been dominating the headlines, the Centers for Disease Control and Prevention recently released updated public health guidance for those who are fully vaccinated against COVID-19.
This new guidance applies to those who are fully vaccinated as indicated by 2 weeks after the second dose in a 2-dose series or 2 weeks after a single-dose vaccine. Those who meet these criteria no longer need to wear a mask or physically distance themselves from others in both indoor and outdoor settings. For those not fully vaccinated, masking and social distancing should continue to be practiced.
The new guidance indicates that quarantine after a known exposure is no longer necessary.
Unless required by local, state, or territorial health authorities, testing is no longer required following domestic travel for fully vaccinated individuals. A negative test is still required prior to boarding an international flight to the United States and testing 3-5 days after arrival is still recommended. Self-quarantine is no longer required after international travel for fully vaccinated individuals.
The new guidance recommends that individuals who are fully vaccinated not participate in routine screening programs when feasible. Finally, if an individual has tested positive for COVID-19, regardless of vaccination status, that person should isolate and not visit public or private settings for a minimum of ten days.1
Updated guidance for health care facilities
In addition to changes for the general public in all settings, the CDC updated guidance for health care facilities on April 27, 2021. These updated guidelines allow for communal dining and visitation for fully vaccinated patients and their visitors. The guidelines indicate that fully vaccinated health care personnel (HCP) do not require quarantine after exposure to patients who have tested positive for COVID-19 as long as the HCP remains asymptomatic. They should, however, continue to utilize personal protective equipment as previously recommended. HCPs are able to be in break and meeting rooms unmasked if all HCPs are vaccinated.2
There are some important caveats to these updated guidelines. They do not apply to those who have immunocompromising conditions, including those using immunosuppressant agents. They also do not apply to locations subject to federal, state, local, tribal, or territorial laws, rules, and regulations, including local business and workplace guidance.
Those who work or reside in correction or detention facilities and homeless shelters are also still required to test after known exposures. Masking is still required by all travelers on all forms of public transportation into and within the United States.
Most importantly, the guidelines apply only to those who are fully vaccinated. Finally, no vaccine is perfect. As such, anyone who experiences symptoms indicative of COVID-19, regardless of vaccination status, should obtain viral testing and isolate themselves from others.1,2
Pros and cons to new guidance
Both sets of updated guidelines are a great example of public health guidance that is changing as the evidence is gathered and changes. This guidance is also a welcome encouragement that the vaccines are effective at decreasing transmission of this virus that has upended our world.
These guidelines leave room for change as evidence is gathered on emerging novel variants. There are, however, a few remaining concerns.
My first concern is for those who are not yet able to be vaccinated, including children under the age of 12. For families with members who are not fully vaccinated, they may have first heard the headlines of “you do not have to mask” to then read the fine print that remains. When truly following these guidelines, many social situations in both the public and private setting should still include both masking and social distancing.
There is no clarity on how these guidelines are enforced. Within the guidance, it is clear that individuals’ privacy is of utmost importance. In the absence of knowledge, that means that the assumption should be that all are not yet vaccinated. Unless there is a way to reliably demonstrate vaccination status, it would likely still be safer to assume that there are individuals who are not fully vaccinated within the setting.
Finally, although this is great news surrounding the efficacy of the vaccine, some are concerned that local mask mandates that have already started to be lifted will be completely removed. As there is still a large portion of the population not yet fully vaccinated, it seems premature for local, state, and territorial authorities to lift these mandates.
How to continue exercising caution
With the outstanding concerns, I will continue to mask in settings, particularly indoors, where I do not definitely know that everyone is vaccinated. I will continue to do this to protect my children and my patients who are not yet vaccinated, and my patients who are immunosuppressed for whom we do not yet have enough information.
I will continue to advise my patients to be thoughtful about the risk for themselves and their families as well.
There has been more benefit to these public health measures then just decreased transmission of COVID-19. I hope that this year has reinforced within us the benefits of masking and self-isolation in the cases of any contagious illnesses.
Although I am looking forward to the opportunities to interact in person with more colleagues and friends, I think we should continue to do this with caution and thoughtfulness. We must be prepared for the possibility of vaccines having decreased efficacy against novel variants as well as eventually the possibility of waning immunity. If these should occur, we need to be prepared for additional recommendation changes and tightening of restrictions.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program at Humboldt Park, Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. Centers for Disease Control and Prevention. Interim Public Health Recommendations for Fully Vaccinated People. U.S. Department of Health & Human Services, May 13, 2021.
2. Centers for Disease Control and Prevention. Updated Healthcare Infection Prevention and Control Recommendations in Response to COVID-19 Vaccination. U.S. Department of Health and Human Services, April 27, 2021.
As has been dominating the headlines, the Centers for Disease Control and Prevention recently released updated public health guidance for those who are fully vaccinated against COVID-19.
This new guidance applies to those who are fully vaccinated as indicated by 2 weeks after the second dose in a 2-dose series or 2 weeks after a single-dose vaccine. Those who meet these criteria no longer need to wear a mask or physically distance themselves from others in both indoor and outdoor settings. For those not fully vaccinated, masking and social distancing should continue to be practiced.
The new guidance indicates that quarantine after a known exposure is no longer necessary.
Unless required by local, state, or territorial health authorities, testing is no longer required following domestic travel for fully vaccinated individuals. A negative test is still required prior to boarding an international flight to the United States and testing 3-5 days after arrival is still recommended. Self-quarantine is no longer required after international travel for fully vaccinated individuals.
The new guidance recommends that individuals who are fully vaccinated not participate in routine screening programs when feasible. Finally, if an individual has tested positive for COVID-19, regardless of vaccination status, that person should isolate and not visit public or private settings for a minimum of ten days.1
Updated guidance for health care facilities
In addition to changes for the general public in all settings, the CDC updated guidance for health care facilities on April 27, 2021. These updated guidelines allow for communal dining and visitation for fully vaccinated patients and their visitors. The guidelines indicate that fully vaccinated health care personnel (HCP) do not require quarantine after exposure to patients who have tested positive for COVID-19 as long as the HCP remains asymptomatic. They should, however, continue to utilize personal protective equipment as previously recommended. HCPs are able to be in break and meeting rooms unmasked if all HCPs are vaccinated.2
There are some important caveats to these updated guidelines. They do not apply to those who have immunocompromising conditions, including those using immunosuppressant agents. They also do not apply to locations subject to federal, state, local, tribal, or territorial laws, rules, and regulations, including local business and workplace guidance.
Those who work or reside in correction or detention facilities and homeless shelters are also still required to test after known exposures. Masking is still required by all travelers on all forms of public transportation into and within the United States.
Most importantly, the guidelines apply only to those who are fully vaccinated. Finally, no vaccine is perfect. As such, anyone who experiences symptoms indicative of COVID-19, regardless of vaccination status, should obtain viral testing and isolate themselves from others.1,2
Pros and cons to new guidance
Both sets of updated guidelines are a great example of public health guidance that is changing as the evidence is gathered and changes. This guidance is also a welcome encouragement that the vaccines are effective at decreasing transmission of this virus that has upended our world.
These guidelines leave room for change as evidence is gathered on emerging novel variants. There are, however, a few remaining concerns.
My first concern is for those who are not yet able to be vaccinated, including children under the age of 12. For families with members who are not fully vaccinated, they may have first heard the headlines of “you do not have to mask” to then read the fine print that remains. When truly following these guidelines, many social situations in both the public and private setting should still include both masking and social distancing.
There is no clarity on how these guidelines are enforced. Within the guidance, it is clear that individuals’ privacy is of utmost importance. In the absence of knowledge, that means that the assumption should be that all are not yet vaccinated. Unless there is a way to reliably demonstrate vaccination status, it would likely still be safer to assume that there are individuals who are not fully vaccinated within the setting.
Finally, although this is great news surrounding the efficacy of the vaccine, some are concerned that local mask mandates that have already started to be lifted will be completely removed. As there is still a large portion of the population not yet fully vaccinated, it seems premature for local, state, and territorial authorities to lift these mandates.
How to continue exercising caution
With the outstanding concerns, I will continue to mask in settings, particularly indoors, where I do not definitely know that everyone is vaccinated. I will continue to do this to protect my children and my patients who are not yet vaccinated, and my patients who are immunosuppressed for whom we do not yet have enough information.
I will continue to advise my patients to be thoughtful about the risk for themselves and their families as well.
There has been more benefit to these public health measures then just decreased transmission of COVID-19. I hope that this year has reinforced within us the benefits of masking and self-isolation in the cases of any contagious illnesses.
Although I am looking forward to the opportunities to interact in person with more colleagues and friends, I think we should continue to do this with caution and thoughtfulness. We must be prepared for the possibility of vaccines having decreased efficacy against novel variants as well as eventually the possibility of waning immunity. If these should occur, we need to be prepared for additional recommendation changes and tightening of restrictions.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program at Humboldt Park, Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. Centers for Disease Control and Prevention. Interim Public Health Recommendations for Fully Vaccinated People. U.S. Department of Health & Human Services, May 13, 2021.
2. Centers for Disease Control and Prevention. Updated Healthcare Infection Prevention and Control Recommendations in Response to COVID-19 Vaccination. U.S. Department of Health and Human Services, April 27, 2021.
As has been dominating the headlines, the Centers for Disease Control and Prevention recently released updated public health guidance for those who are fully vaccinated against COVID-19.
This new guidance applies to those who are fully vaccinated as indicated by 2 weeks after the second dose in a 2-dose series or 2 weeks after a single-dose vaccine. Those who meet these criteria no longer need to wear a mask or physically distance themselves from others in both indoor and outdoor settings. For those not fully vaccinated, masking and social distancing should continue to be practiced.
The new guidance indicates that quarantine after a known exposure is no longer necessary.
Unless required by local, state, or territorial health authorities, testing is no longer required following domestic travel for fully vaccinated individuals. A negative test is still required prior to boarding an international flight to the United States and testing 3-5 days after arrival is still recommended. Self-quarantine is no longer required after international travel for fully vaccinated individuals.
The new guidance recommends that individuals who are fully vaccinated not participate in routine screening programs when feasible. Finally, if an individual has tested positive for COVID-19, regardless of vaccination status, that person should isolate and not visit public or private settings for a minimum of ten days.1
Updated guidance for health care facilities
In addition to changes for the general public in all settings, the CDC updated guidance for health care facilities on April 27, 2021. These updated guidelines allow for communal dining and visitation for fully vaccinated patients and their visitors. The guidelines indicate that fully vaccinated health care personnel (HCP) do not require quarantine after exposure to patients who have tested positive for COVID-19 as long as the HCP remains asymptomatic. They should, however, continue to utilize personal protective equipment as previously recommended. HCPs are able to be in break and meeting rooms unmasked if all HCPs are vaccinated.2
There are some important caveats to these updated guidelines. They do not apply to those who have immunocompromising conditions, including those using immunosuppressant agents. They also do not apply to locations subject to federal, state, local, tribal, or territorial laws, rules, and regulations, including local business and workplace guidance.
Those who work or reside in correction or detention facilities and homeless shelters are also still required to test after known exposures. Masking is still required by all travelers on all forms of public transportation into and within the United States.
Most importantly, the guidelines apply only to those who are fully vaccinated. Finally, no vaccine is perfect. As such, anyone who experiences symptoms indicative of COVID-19, regardless of vaccination status, should obtain viral testing and isolate themselves from others.1,2
Pros and cons to new guidance
Both sets of updated guidelines are a great example of public health guidance that is changing as the evidence is gathered and changes. This guidance is also a welcome encouragement that the vaccines are effective at decreasing transmission of this virus that has upended our world.
These guidelines leave room for change as evidence is gathered on emerging novel variants. There are, however, a few remaining concerns.
My first concern is for those who are not yet able to be vaccinated, including children under the age of 12. For families with members who are not fully vaccinated, they may have first heard the headlines of “you do not have to mask” to then read the fine print that remains. When truly following these guidelines, many social situations in both the public and private setting should still include both masking and social distancing.
There is no clarity on how these guidelines are enforced. Within the guidance, it is clear that individuals’ privacy is of utmost importance. In the absence of knowledge, that means that the assumption should be that all are not yet vaccinated. Unless there is a way to reliably demonstrate vaccination status, it would likely still be safer to assume that there are individuals who are not fully vaccinated within the setting.
Finally, although this is great news surrounding the efficacy of the vaccine, some are concerned that local mask mandates that have already started to be lifted will be completely removed. As there is still a large portion of the population not yet fully vaccinated, it seems premature for local, state, and territorial authorities to lift these mandates.
How to continue exercising caution
With the outstanding concerns, I will continue to mask in settings, particularly indoors, where I do not definitely know that everyone is vaccinated. I will continue to do this to protect my children and my patients who are not yet vaccinated, and my patients who are immunosuppressed for whom we do not yet have enough information.
I will continue to advise my patients to be thoughtful about the risk for themselves and their families as well.
There has been more benefit to these public health measures then just decreased transmission of COVID-19. I hope that this year has reinforced within us the benefits of masking and self-isolation in the cases of any contagious illnesses.
Although I am looking forward to the opportunities to interact in person with more colleagues and friends, I think we should continue to do this with caution and thoughtfulness. We must be prepared for the possibility of vaccines having decreased efficacy against novel variants as well as eventually the possibility of waning immunity. If these should occur, we need to be prepared for additional recommendation changes and tightening of restrictions.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program at Humboldt Park, Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. Centers for Disease Control and Prevention. Interim Public Health Recommendations for Fully Vaccinated People. U.S. Department of Health & Human Services, May 13, 2021.
2. Centers for Disease Control and Prevention. Updated Healthcare Infection Prevention and Control Recommendations in Response to COVID-19 Vaccination. U.S. Department of Health and Human Services, April 27, 2021.
Long-term use of prescription sleep meds unsupported by new research
a new study shows.
“While there are good data from [randomized, controlled trials] that these medications improve sleep disturbances in the short term,” few studies have examined whether they provide long-term benefits, stated the authors of the paper, which was published in BMJ Open.
“The current observational study does not support use of sleep medications over the long term, as there were no self-reported differences at 1 or 2 years of follow-up comparing sleep medication users with nonusers,” author Daniel H. Solomon, MD, MPH, from Brigham and Women’s Hospital, Boston, and colleagues wrote.
Women included in the analysis were drawn from the Study of Women’s Health Across the Nation (SWAN), an ongoing multicenter, longitudinal study examining women during the menopausal transition. The average age of the women included in the cohort was 49.5 years and approximately half were White. All women reported a sleep disturbance on at least 3 nights per week during a 2-week interval. At follow up, women were asked to use a Likert scale to rate three aspects of sleep: difficulty initiating sleep, frequent awakening, and waking up early. On the scale, 1 represented having no difficulties on any nights, 3 represented having difficulties 1-2 nights per week, and 5 represented having difficulty 5-7 nights per week.
Women already using prescription sleep medication at their baseline visit were excluded from the study. Medications used included benzodiazepines, selective BZD receptor agonists, and other hypnotics.
Over the 21 years of follow-up in the SWAN study (1995-2016), Dr. Solomon and colleagues identified 238 women using sleep medication and these were compared with a cohort of 447 propensity score–matched non–sleep medication uses. Overall, the 685 women included were similar in characteristics to each other as well as to the other potentially eligible women not included in the analysis.
Sleep disturbance patterns compared
At baseline, sleep disturbance patterns were similar between the two groups. Among medication users, the mean score for difficulty initiating sleep was 2.7 (95% confidence interval, 2.5-2.9), waking frequently 3.8 (95% CI, 3.6-3.9), and waking early 2.9 (95% CI, 2.7-3.1). Among the nonusers, the baseline scores were 2.6 (95% CI, 2.5-2.7), 3.7 (95% CI, 3.6-3.8), and 2.7 (95% CI, 2.5-2.8), respectively. After 1 year, there was no statistically significant difference in scores between the two groups. The average ratings for medication users were 2.6 (95% CI, 2.3-2.8) for difficulty initiating sleep, 3.8 (95% CI, 3.6-4.0) for waking frequently, and 2.8 (95% CI, 2.6-3.0) for waking early.
Average ratings among nonusers were 2.3 (95% CI, 2.2-2.4), 3.5 (95% CI, 3.3-3.6), and 2.5 (95% CI, 2.3-2.6), respectively.
After 2 years, there were still no statistically significant reductions in sleep disturbances among those taking prescription sleep medications, compared with those not taking medication.
The researchers noted that approximately half of the women in this cohort were current or past tobacco users and that 20% were moderate to heavy alcohol users.
More work-up, not more medication, needed
The study authors acknowledged the limitations of an observational study and noted that, since participants only reported medication use and sleep disturbances at annual visits, they did not know whether patients’ medication use was intermittent or of any interim outcomes. Additionally, the authors pointed out that those classified as “nonusers” may have been using over-the-counter medication.
“Investigations should look at detailed-use patterns, on a daily or weekly basis, with frequent outcomes data,” Dr. Solomon said in an interview. “While our data shed new light on chronic use, we only had data collected on an annual basis; daily or weekly data would provide more granular information.”
Regarding clinician prescribing practices, Dr. Solomon said, “short-term, intermittent use can be helpful, but use these agents sparingly” and “educate patients that chronic regular use of medications for sleep is not associated with improvement in sleep disturbances.”
Commenting on the study, Andrea Matsumura, MD, a sleep specialist at the Oregon Clinic in Portland, echoed this sentiment: “When someone says they are having trouble sleeping this is the tip of the iceberg and it warrants an evaluation to determine if someone has a breathing disorder, a circadian disorder, a life situation, or a type of insomnia that is driving the sleeplessness.”
“I think this study supports what we all should know,” Dr. Matsumura concluded. “Sleep aids are not meant to be used long term” and should not be used for longer than 2 weeks without further work-up.
Funding for this study was provided through a grant from the National Institutes of Health. Dr. Solomon has received salary support from research grants to Brigham and Women’s Hospital for unrelated work from AbbVie, Amgen, Corrona, Genentech and Pfizer. The other authors and Dr. Matsumura have reported no relevant financial relationships.
a new study shows.
“While there are good data from [randomized, controlled trials] that these medications improve sleep disturbances in the short term,” few studies have examined whether they provide long-term benefits, stated the authors of the paper, which was published in BMJ Open.
“The current observational study does not support use of sleep medications over the long term, as there were no self-reported differences at 1 or 2 years of follow-up comparing sleep medication users with nonusers,” author Daniel H. Solomon, MD, MPH, from Brigham and Women’s Hospital, Boston, and colleagues wrote.
Women included in the analysis were drawn from the Study of Women’s Health Across the Nation (SWAN), an ongoing multicenter, longitudinal study examining women during the menopausal transition. The average age of the women included in the cohort was 49.5 years and approximately half were White. All women reported a sleep disturbance on at least 3 nights per week during a 2-week interval. At follow up, women were asked to use a Likert scale to rate three aspects of sleep: difficulty initiating sleep, frequent awakening, and waking up early. On the scale, 1 represented having no difficulties on any nights, 3 represented having difficulties 1-2 nights per week, and 5 represented having difficulty 5-7 nights per week.
Women already using prescription sleep medication at their baseline visit were excluded from the study. Medications used included benzodiazepines, selective BZD receptor agonists, and other hypnotics.
Over the 21 years of follow-up in the SWAN study (1995-2016), Dr. Solomon and colleagues identified 238 women using sleep medication and these were compared with a cohort of 447 propensity score–matched non–sleep medication uses. Overall, the 685 women included were similar in characteristics to each other as well as to the other potentially eligible women not included in the analysis.
Sleep disturbance patterns compared
At baseline, sleep disturbance patterns were similar between the two groups. Among medication users, the mean score for difficulty initiating sleep was 2.7 (95% confidence interval, 2.5-2.9), waking frequently 3.8 (95% CI, 3.6-3.9), and waking early 2.9 (95% CI, 2.7-3.1). Among the nonusers, the baseline scores were 2.6 (95% CI, 2.5-2.7), 3.7 (95% CI, 3.6-3.8), and 2.7 (95% CI, 2.5-2.8), respectively. After 1 year, there was no statistically significant difference in scores between the two groups. The average ratings for medication users were 2.6 (95% CI, 2.3-2.8) for difficulty initiating sleep, 3.8 (95% CI, 3.6-4.0) for waking frequently, and 2.8 (95% CI, 2.6-3.0) for waking early.
Average ratings among nonusers were 2.3 (95% CI, 2.2-2.4), 3.5 (95% CI, 3.3-3.6), and 2.5 (95% CI, 2.3-2.6), respectively.
After 2 years, there were still no statistically significant reductions in sleep disturbances among those taking prescription sleep medications, compared with those not taking medication.
The researchers noted that approximately half of the women in this cohort were current or past tobacco users and that 20% were moderate to heavy alcohol users.
More work-up, not more medication, needed
The study authors acknowledged the limitations of an observational study and noted that, since participants only reported medication use and sleep disturbances at annual visits, they did not know whether patients’ medication use was intermittent or of any interim outcomes. Additionally, the authors pointed out that those classified as “nonusers” may have been using over-the-counter medication.
“Investigations should look at detailed-use patterns, on a daily or weekly basis, with frequent outcomes data,” Dr. Solomon said in an interview. “While our data shed new light on chronic use, we only had data collected on an annual basis; daily or weekly data would provide more granular information.”
Regarding clinician prescribing practices, Dr. Solomon said, “short-term, intermittent use can be helpful, but use these agents sparingly” and “educate patients that chronic regular use of medications for sleep is not associated with improvement in sleep disturbances.”
Commenting on the study, Andrea Matsumura, MD, a sleep specialist at the Oregon Clinic in Portland, echoed this sentiment: “When someone says they are having trouble sleeping this is the tip of the iceberg and it warrants an evaluation to determine if someone has a breathing disorder, a circadian disorder, a life situation, or a type of insomnia that is driving the sleeplessness.”
“I think this study supports what we all should know,” Dr. Matsumura concluded. “Sleep aids are not meant to be used long term” and should not be used for longer than 2 weeks without further work-up.
Funding for this study was provided through a grant from the National Institutes of Health. Dr. Solomon has received salary support from research grants to Brigham and Women’s Hospital for unrelated work from AbbVie, Amgen, Corrona, Genentech and Pfizer. The other authors and Dr. Matsumura have reported no relevant financial relationships.
a new study shows.
“While there are good data from [randomized, controlled trials] that these medications improve sleep disturbances in the short term,” few studies have examined whether they provide long-term benefits, stated the authors of the paper, which was published in BMJ Open.
“The current observational study does not support use of sleep medications over the long term, as there were no self-reported differences at 1 or 2 years of follow-up comparing sleep medication users with nonusers,” author Daniel H. Solomon, MD, MPH, from Brigham and Women’s Hospital, Boston, and colleagues wrote.
Women included in the analysis were drawn from the Study of Women’s Health Across the Nation (SWAN), an ongoing multicenter, longitudinal study examining women during the menopausal transition. The average age of the women included in the cohort was 49.5 years and approximately half were White. All women reported a sleep disturbance on at least 3 nights per week during a 2-week interval. At follow up, women were asked to use a Likert scale to rate three aspects of sleep: difficulty initiating sleep, frequent awakening, and waking up early. On the scale, 1 represented having no difficulties on any nights, 3 represented having difficulties 1-2 nights per week, and 5 represented having difficulty 5-7 nights per week.
Women already using prescription sleep medication at their baseline visit were excluded from the study. Medications used included benzodiazepines, selective BZD receptor agonists, and other hypnotics.
Over the 21 years of follow-up in the SWAN study (1995-2016), Dr. Solomon and colleagues identified 238 women using sleep medication and these were compared with a cohort of 447 propensity score–matched non–sleep medication uses. Overall, the 685 women included were similar in characteristics to each other as well as to the other potentially eligible women not included in the analysis.
Sleep disturbance patterns compared
At baseline, sleep disturbance patterns were similar between the two groups. Among medication users, the mean score for difficulty initiating sleep was 2.7 (95% confidence interval, 2.5-2.9), waking frequently 3.8 (95% CI, 3.6-3.9), and waking early 2.9 (95% CI, 2.7-3.1). Among the nonusers, the baseline scores were 2.6 (95% CI, 2.5-2.7), 3.7 (95% CI, 3.6-3.8), and 2.7 (95% CI, 2.5-2.8), respectively. After 1 year, there was no statistically significant difference in scores between the two groups. The average ratings for medication users were 2.6 (95% CI, 2.3-2.8) for difficulty initiating sleep, 3.8 (95% CI, 3.6-4.0) for waking frequently, and 2.8 (95% CI, 2.6-3.0) for waking early.
Average ratings among nonusers were 2.3 (95% CI, 2.2-2.4), 3.5 (95% CI, 3.3-3.6), and 2.5 (95% CI, 2.3-2.6), respectively.
After 2 years, there were still no statistically significant reductions in sleep disturbances among those taking prescription sleep medications, compared with those not taking medication.
The researchers noted that approximately half of the women in this cohort were current or past tobacco users and that 20% were moderate to heavy alcohol users.
More work-up, not more medication, needed
The study authors acknowledged the limitations of an observational study and noted that, since participants only reported medication use and sleep disturbances at annual visits, they did not know whether patients’ medication use was intermittent or of any interim outcomes. Additionally, the authors pointed out that those classified as “nonusers” may have been using over-the-counter medication.
“Investigations should look at detailed-use patterns, on a daily or weekly basis, with frequent outcomes data,” Dr. Solomon said in an interview. “While our data shed new light on chronic use, we only had data collected on an annual basis; daily or weekly data would provide more granular information.”
Regarding clinician prescribing practices, Dr. Solomon said, “short-term, intermittent use can be helpful, but use these agents sparingly” and “educate patients that chronic regular use of medications for sleep is not associated with improvement in sleep disturbances.”
Commenting on the study, Andrea Matsumura, MD, a sleep specialist at the Oregon Clinic in Portland, echoed this sentiment: “When someone says they are having trouble sleeping this is the tip of the iceberg and it warrants an evaluation to determine if someone has a breathing disorder, a circadian disorder, a life situation, or a type of insomnia that is driving the sleeplessness.”
“I think this study supports what we all should know,” Dr. Matsumura concluded. “Sleep aids are not meant to be used long term” and should not be used for longer than 2 weeks without further work-up.
Funding for this study was provided through a grant from the National Institutes of Health. Dr. Solomon has received salary support from research grants to Brigham and Women’s Hospital for unrelated work from AbbVie, Amgen, Corrona, Genentech and Pfizer. The other authors and Dr. Matsumura have reported no relevant financial relationships.
FROM BMJ OPEN
E-cigarettes linked to wheeze, shortness of breath
The use of e-cigarettes is linked to a higher frequency of self-reported wheezing and shortness of breath in adolescents and young adults, according to an online survey. The association was present even after controlling for cigarette and cannabis use.
Previous studies of adolescents and young adults have shown associations between e-cigarette use and wheeze, shortness of breath, and asthma. The Youth Risk Behavior Surveillance (YRBS) survey by the Centers for Disease Control and Prevention and other health agencies, conducted from 2015 to 2017, found that 63.5% of youth who used e-cigarettes also used some combination of cigarettes and cannabis. Combined use was associated with a 55%-65% increased odds of self-reported asthma.
The Population Assessment of Tobacco and Health (PATH) study, which was published in October 2020, had similar findings, though it did not find an association between e-cigarette use alone and wheezing.
“The findings from the current study highlight that we need to keep asking young people about respiratory symptoms, couse of other tobacco products, as well as cannabis use. As more products, including cannabis and various e-cigarette devices, enter the market, assessing respiratory health will be important both where adolescents and young adults receive their health care and in research,” Alayna Tackett, PhD, said in an interview. Dr. Tackett presented the study at the American Thoracic Society’s virtual international conference. She is an assistant professor of preventive medicine at the University of Southern California, Los Angeles.
“I found [the study] very interesting because it seems to be identifying a physiologic response to these e-cigarettes,” said Christopher Pascoe, MD, who was asked to comment. “And they were so young [age 14-21 years]. The fact that these symptoms of wheezing and shortness of breath are coming from people who are this young suggests that there may be chronic problems showing up later with continued use of these devices.”
Dr. Pascoe is an assistant professor of physiology and pathophysiology at the University of Manitoba, Winnipeg, where he also works with the Children’s Hospital Research Institute of Manitoba. His own research examines lung tissue harvested from pneumothorax surgeries in smokers and e-cigarette users to identify markers of inflammation.
He called the research a “good start” at unraveling the impacts of e-cigarettes and smoking, since some people use both products. “The fact that there was still a twofold increase in odds for wheezing, shortness of breath among people who use these e-cigarettes, but weren’t using cannabis and weren’t using cigarettes. I think it’s novel, and it suggests that there is an effect [of e-cigarettes alone].”
The study is based on a self-reported data, which is a significant limitation, especially considering that asthma is often overreported. “Self-report can be fraught with things, but I think it’s an interesting starting point for trying to recruit people who are just e-cigarette users and following them up further,” said Dr. Pascoe.
The researchers surveyed 2,931 individuals aged 14-21 years between Aug. 6 and Aug.30, 2020, with an average age of 18.9 years. Of the respondents, 80% were women and girls, and 75% were White. The high percentage of women and girls was unusual. Dr. Tackett provided no explanation for the atypical demographic but noted that the current study used convenience sampling.
The survey asked about use of e-cigarettes, cigarettes, and cannabis in the past 30 days, as well as asthma diagnosis and respiratory symptoms over the same period. The methodology employed survey management company Lucid, which recruited, collected data from, and provided compensation to participants.
A total of 24% of participants reported asthma, 13% reported wheeze, and 20% reported shortness of breath. Among 1,414 respondents who reported e-cigarette use in the past 30 days, 15% also said they had used cigarettes, and 37% said they had used cannabis.
After controlling for age, birth sex, and race/ethnicity, compared with self-reported never e-cigarette users, there was an association between past 30-day e-cigarette use and self-reported asthma (odds ratio, 1.4; 95% CI, 1.1-1.7), wheeze (OR, 3.1; 95% CI, 2.3-4.2), and shortness of breath (OR, 2.9; 95% CI, 2.3-3.6). After the researchers controlled for past 30-day cigarette cannabis use, the association with asthma was no longer statistically significant (OR, 1.11; 95% CI, 0.87-1.41), but the association with wheeze (OR, 2.3; 95% CI, 1.6-3.0) and shortness of breath (OR, 2.1; 95% CI, 1.6-2.8) remained.
Dr. Tackett noted that wheeze and shortness of breath are only two indicators of respiratory health, and more research needs to be done. Her team is conducting follow-up studies using objective measurement tools such as home-based spirometry in adolescents and young adults who exclusively use e-cigarettes and who have never used e-cigarettes.
“We need to better understand the complex relationships between use of these products and whether multiple product use is associated with worse respiratory outcomes,” said Dr. Tackett.
Dr. Pascoe and Dr. Tackett disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The use of e-cigarettes is linked to a higher frequency of self-reported wheezing and shortness of breath in adolescents and young adults, according to an online survey. The association was present even after controlling for cigarette and cannabis use.
Previous studies of adolescents and young adults have shown associations between e-cigarette use and wheeze, shortness of breath, and asthma. The Youth Risk Behavior Surveillance (YRBS) survey by the Centers for Disease Control and Prevention and other health agencies, conducted from 2015 to 2017, found that 63.5% of youth who used e-cigarettes also used some combination of cigarettes and cannabis. Combined use was associated with a 55%-65% increased odds of self-reported asthma.
The Population Assessment of Tobacco and Health (PATH) study, which was published in October 2020, had similar findings, though it did not find an association between e-cigarette use alone and wheezing.
“The findings from the current study highlight that we need to keep asking young people about respiratory symptoms, couse of other tobacco products, as well as cannabis use. As more products, including cannabis and various e-cigarette devices, enter the market, assessing respiratory health will be important both where adolescents and young adults receive their health care and in research,” Alayna Tackett, PhD, said in an interview. Dr. Tackett presented the study at the American Thoracic Society’s virtual international conference. She is an assistant professor of preventive medicine at the University of Southern California, Los Angeles.
“I found [the study] very interesting because it seems to be identifying a physiologic response to these e-cigarettes,” said Christopher Pascoe, MD, who was asked to comment. “And they were so young [age 14-21 years]. The fact that these symptoms of wheezing and shortness of breath are coming from people who are this young suggests that there may be chronic problems showing up later with continued use of these devices.”
Dr. Pascoe is an assistant professor of physiology and pathophysiology at the University of Manitoba, Winnipeg, where he also works with the Children’s Hospital Research Institute of Manitoba. His own research examines lung tissue harvested from pneumothorax surgeries in smokers and e-cigarette users to identify markers of inflammation.
He called the research a “good start” at unraveling the impacts of e-cigarettes and smoking, since some people use both products. “The fact that there was still a twofold increase in odds for wheezing, shortness of breath among people who use these e-cigarettes, but weren’t using cannabis and weren’t using cigarettes. I think it’s novel, and it suggests that there is an effect [of e-cigarettes alone].”
The study is based on a self-reported data, which is a significant limitation, especially considering that asthma is often overreported. “Self-report can be fraught with things, but I think it’s an interesting starting point for trying to recruit people who are just e-cigarette users and following them up further,” said Dr. Pascoe.
The researchers surveyed 2,931 individuals aged 14-21 years between Aug. 6 and Aug.30, 2020, with an average age of 18.9 years. Of the respondents, 80% were women and girls, and 75% were White. The high percentage of women and girls was unusual. Dr. Tackett provided no explanation for the atypical demographic but noted that the current study used convenience sampling.
The survey asked about use of e-cigarettes, cigarettes, and cannabis in the past 30 days, as well as asthma diagnosis and respiratory symptoms over the same period. The methodology employed survey management company Lucid, which recruited, collected data from, and provided compensation to participants.
A total of 24% of participants reported asthma, 13% reported wheeze, and 20% reported shortness of breath. Among 1,414 respondents who reported e-cigarette use in the past 30 days, 15% also said they had used cigarettes, and 37% said they had used cannabis.
After controlling for age, birth sex, and race/ethnicity, compared with self-reported never e-cigarette users, there was an association between past 30-day e-cigarette use and self-reported asthma (odds ratio, 1.4; 95% CI, 1.1-1.7), wheeze (OR, 3.1; 95% CI, 2.3-4.2), and shortness of breath (OR, 2.9; 95% CI, 2.3-3.6). After the researchers controlled for past 30-day cigarette cannabis use, the association with asthma was no longer statistically significant (OR, 1.11; 95% CI, 0.87-1.41), but the association with wheeze (OR, 2.3; 95% CI, 1.6-3.0) and shortness of breath (OR, 2.1; 95% CI, 1.6-2.8) remained.
Dr. Tackett noted that wheeze and shortness of breath are only two indicators of respiratory health, and more research needs to be done. Her team is conducting follow-up studies using objective measurement tools such as home-based spirometry in adolescents and young adults who exclusively use e-cigarettes and who have never used e-cigarettes.
“We need to better understand the complex relationships between use of these products and whether multiple product use is associated with worse respiratory outcomes,” said Dr. Tackett.
Dr. Pascoe and Dr. Tackett disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The use of e-cigarettes is linked to a higher frequency of self-reported wheezing and shortness of breath in adolescents and young adults, according to an online survey. The association was present even after controlling for cigarette and cannabis use.
Previous studies of adolescents and young adults have shown associations between e-cigarette use and wheeze, shortness of breath, and asthma. The Youth Risk Behavior Surveillance (YRBS) survey by the Centers for Disease Control and Prevention and other health agencies, conducted from 2015 to 2017, found that 63.5% of youth who used e-cigarettes also used some combination of cigarettes and cannabis. Combined use was associated with a 55%-65% increased odds of self-reported asthma.
The Population Assessment of Tobacco and Health (PATH) study, which was published in October 2020, had similar findings, though it did not find an association between e-cigarette use alone and wheezing.
“The findings from the current study highlight that we need to keep asking young people about respiratory symptoms, couse of other tobacco products, as well as cannabis use. As more products, including cannabis and various e-cigarette devices, enter the market, assessing respiratory health will be important both where adolescents and young adults receive their health care and in research,” Alayna Tackett, PhD, said in an interview. Dr. Tackett presented the study at the American Thoracic Society’s virtual international conference. She is an assistant professor of preventive medicine at the University of Southern California, Los Angeles.
“I found [the study] very interesting because it seems to be identifying a physiologic response to these e-cigarettes,” said Christopher Pascoe, MD, who was asked to comment. “And they were so young [age 14-21 years]. The fact that these symptoms of wheezing and shortness of breath are coming from people who are this young suggests that there may be chronic problems showing up later with continued use of these devices.”
Dr. Pascoe is an assistant professor of physiology and pathophysiology at the University of Manitoba, Winnipeg, where he also works with the Children’s Hospital Research Institute of Manitoba. His own research examines lung tissue harvested from pneumothorax surgeries in smokers and e-cigarette users to identify markers of inflammation.
He called the research a “good start” at unraveling the impacts of e-cigarettes and smoking, since some people use both products. “The fact that there was still a twofold increase in odds for wheezing, shortness of breath among people who use these e-cigarettes, but weren’t using cannabis and weren’t using cigarettes. I think it’s novel, and it suggests that there is an effect [of e-cigarettes alone].”
The study is based on a self-reported data, which is a significant limitation, especially considering that asthma is often overreported. “Self-report can be fraught with things, but I think it’s an interesting starting point for trying to recruit people who are just e-cigarette users and following them up further,” said Dr. Pascoe.
The researchers surveyed 2,931 individuals aged 14-21 years between Aug. 6 and Aug.30, 2020, with an average age of 18.9 years. Of the respondents, 80% were women and girls, and 75% were White. The high percentage of women and girls was unusual. Dr. Tackett provided no explanation for the atypical demographic but noted that the current study used convenience sampling.
The survey asked about use of e-cigarettes, cigarettes, and cannabis in the past 30 days, as well as asthma diagnosis and respiratory symptoms over the same period. The methodology employed survey management company Lucid, which recruited, collected data from, and provided compensation to participants.
A total of 24% of participants reported asthma, 13% reported wheeze, and 20% reported shortness of breath. Among 1,414 respondents who reported e-cigarette use in the past 30 days, 15% also said they had used cigarettes, and 37% said they had used cannabis.
After controlling for age, birth sex, and race/ethnicity, compared with self-reported never e-cigarette users, there was an association between past 30-day e-cigarette use and self-reported asthma (odds ratio, 1.4; 95% CI, 1.1-1.7), wheeze (OR, 3.1; 95% CI, 2.3-4.2), and shortness of breath (OR, 2.9; 95% CI, 2.3-3.6). After the researchers controlled for past 30-day cigarette cannabis use, the association with asthma was no longer statistically significant (OR, 1.11; 95% CI, 0.87-1.41), but the association with wheeze (OR, 2.3; 95% CI, 1.6-3.0) and shortness of breath (OR, 2.1; 95% CI, 1.6-2.8) remained.
Dr. Tackett noted that wheeze and shortness of breath are only two indicators of respiratory health, and more research needs to be done. Her team is conducting follow-up studies using objective measurement tools such as home-based spirometry in adolescents and young adults who exclusively use e-cigarettes and who have never used e-cigarettes.
“We need to better understand the complex relationships between use of these products and whether multiple product use is associated with worse respiratory outcomes,” said Dr. Tackett.
Dr. Pascoe and Dr. Tackett disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Checkpoint inhibitors earn spot in new ESMO SCLC guidelines
In new small cell lung cancer guidelines, the European Society of Medical Oncology calls for upfront atezolizumab or durvalumab in combination with four to six cycles of etoposide and a platinum for stage 4 disease.
The strong recommendation is based on two phase 3 trials that showed improved overall survival when the checkpoint inhibitors were added to standard chemotherapy. “With very similar results, and in the context of a severe unmet need, both trials justify the need for immunotherapy in the frontline setting” and established “new standards of care” for stage 4 disease, the group said. “Atezolizumab or durvalumab in combination with a platinum plus etoposide should be offered to all eligible chemotherapy-naive patients” with a performance status of 0-1, said the group led by Anne-Marie Dingemans, MD, PhD, a pulmonology professor at Maastricht (the Netherlands) University Medical Center.
Alessio Cortellini, MD, a consulting oncologist and visiting researcher at Imperial College London, said he strongly endorses the recommendation when asked for comment.
“The addition of a PD-L1 inhibitor to a platinum/etoposide backbone is the first strategy that has led to a significant benefit in terms of overall survival. After decades of disappointing results, the bar has” been raised, said Dr. Cortellini.
New inhibitor
EMSO also incorporated the new RNA polymerase II inhibitor lurbinectedin into their guidelines as an option for patients progressing on or after first-line platinum-based chemotherapy.
The agent was approved by the U.S. Food and Drug Administration in June 2020 for metastatic small cell lung cancer (SCLC) with disease progression on or after platinum-based chemotherapy.
The recommendations – more than 50 in all – are based on a literature review and expert opinion, and cover SCLC diagnosis, staging, treatment, and follow-up, with flowcharts outlining treatment pathways.
Atezolizumab earned the endorsement following the IMpower133 trial, which showed a median overall survival of 12.3 months for atezolizumab in combination with carboplatin and etoposide, versus 10.3 months on chemotherapy alone; 34% of the atezolizumab group was alive at 18 months versus 21% in the placebo arm.
The durvalumab recommendation is based on the CASPIAN trial, in which the addition of durvalumab to platinum plus etoposide improved median overall survival from 10.5 to 12.9 months; 32% of durvalumab patients were alive at 18 months versus 24.8% in the chemotherapy-alone arm.
ESMO said “it is important to stress that, in both trials,” patients were in good clinical condition with a median age in the early 60s, so relatively young for SCLC. Also, the modest benefits “clearly emphasize the need for” biomarkers that predict response in order to better select patients.
Immunotherapy has improved cancer treatment across many malignancies and continues to be actively investigated in SCLC, but so far only atezolizumab and durvalumab have phase 3 evidence of benefit.
Makers of the blockbuster checkpoint inhibitors nivolumab and pembrolizumab recently withdrew their FDA approval for stage 4 SCLC that’s progressed after platinum-based chemotherapy and at least one other line of therapy; phase 3 trials failed to confirm the modest survival benefit found in early studies.
Lurbinectedin earned its place in the guidelines based on a single-arm study with 105 relapsed patients that showed an overall response rate of 22.2% in platinum-resistant and 45% in platinum-sensitive patients, with a median overall survival of 9.3 months.
The jury is still out, however. A phase 3 trial of lurbinectedin plus doxorubicin versus topotecan or CAV [cyclophosphamide, adriamycin, and vincristine] for advanced recurrent disease failed to meet its endpoint of superior overall survival, according to a recent press release from the its maker.
“It might be a bit early to discuss” routine use of lurbinectedin, although having it available is good “since literally nothing works in the second line setting,” Dr. Cortellini said.
There was no external funding for the work. The authors had numerous ties to pharmaceutical companies, including Dr. Dingemans who reported adviser and speakers fees and/or research funding from Roche, Lilly, Bristol-Myers Squib, and others. Dr. Costellini reported speakers fees from Novartis, Astrazeneca, and Astellas and consultant payments from Bristol-Myers Squibb, Roche, MSD, and AstraZeneca.
In new small cell lung cancer guidelines, the European Society of Medical Oncology calls for upfront atezolizumab or durvalumab in combination with four to six cycles of etoposide and a platinum for stage 4 disease.
The strong recommendation is based on two phase 3 trials that showed improved overall survival when the checkpoint inhibitors were added to standard chemotherapy. “With very similar results, and in the context of a severe unmet need, both trials justify the need for immunotherapy in the frontline setting” and established “new standards of care” for stage 4 disease, the group said. “Atezolizumab or durvalumab in combination with a platinum plus etoposide should be offered to all eligible chemotherapy-naive patients” with a performance status of 0-1, said the group led by Anne-Marie Dingemans, MD, PhD, a pulmonology professor at Maastricht (the Netherlands) University Medical Center.
Alessio Cortellini, MD, a consulting oncologist and visiting researcher at Imperial College London, said he strongly endorses the recommendation when asked for comment.
“The addition of a PD-L1 inhibitor to a platinum/etoposide backbone is the first strategy that has led to a significant benefit in terms of overall survival. After decades of disappointing results, the bar has” been raised, said Dr. Cortellini.
New inhibitor
EMSO also incorporated the new RNA polymerase II inhibitor lurbinectedin into their guidelines as an option for patients progressing on or after first-line platinum-based chemotherapy.
The agent was approved by the U.S. Food and Drug Administration in June 2020 for metastatic small cell lung cancer (SCLC) with disease progression on or after platinum-based chemotherapy.
The recommendations – more than 50 in all – are based on a literature review and expert opinion, and cover SCLC diagnosis, staging, treatment, and follow-up, with flowcharts outlining treatment pathways.
Atezolizumab earned the endorsement following the IMpower133 trial, which showed a median overall survival of 12.3 months for atezolizumab in combination with carboplatin and etoposide, versus 10.3 months on chemotherapy alone; 34% of the atezolizumab group was alive at 18 months versus 21% in the placebo arm.
The durvalumab recommendation is based on the CASPIAN trial, in which the addition of durvalumab to platinum plus etoposide improved median overall survival from 10.5 to 12.9 months; 32% of durvalumab patients were alive at 18 months versus 24.8% in the chemotherapy-alone arm.
ESMO said “it is important to stress that, in both trials,” patients were in good clinical condition with a median age in the early 60s, so relatively young for SCLC. Also, the modest benefits “clearly emphasize the need for” biomarkers that predict response in order to better select patients.
Immunotherapy has improved cancer treatment across many malignancies and continues to be actively investigated in SCLC, but so far only atezolizumab and durvalumab have phase 3 evidence of benefit.
Makers of the blockbuster checkpoint inhibitors nivolumab and pembrolizumab recently withdrew their FDA approval for stage 4 SCLC that’s progressed after platinum-based chemotherapy and at least one other line of therapy; phase 3 trials failed to confirm the modest survival benefit found in early studies.
Lurbinectedin earned its place in the guidelines based on a single-arm study with 105 relapsed patients that showed an overall response rate of 22.2% in platinum-resistant and 45% in platinum-sensitive patients, with a median overall survival of 9.3 months.
The jury is still out, however. A phase 3 trial of lurbinectedin plus doxorubicin versus topotecan or CAV [cyclophosphamide, adriamycin, and vincristine] for advanced recurrent disease failed to meet its endpoint of superior overall survival, according to a recent press release from the its maker.
“It might be a bit early to discuss” routine use of lurbinectedin, although having it available is good “since literally nothing works in the second line setting,” Dr. Cortellini said.
There was no external funding for the work. The authors had numerous ties to pharmaceutical companies, including Dr. Dingemans who reported adviser and speakers fees and/or research funding from Roche, Lilly, Bristol-Myers Squib, and others. Dr. Costellini reported speakers fees from Novartis, Astrazeneca, and Astellas and consultant payments from Bristol-Myers Squibb, Roche, MSD, and AstraZeneca.
In new small cell lung cancer guidelines, the European Society of Medical Oncology calls for upfront atezolizumab or durvalumab in combination with four to six cycles of etoposide and a platinum for stage 4 disease.
The strong recommendation is based on two phase 3 trials that showed improved overall survival when the checkpoint inhibitors were added to standard chemotherapy. “With very similar results, and in the context of a severe unmet need, both trials justify the need for immunotherapy in the frontline setting” and established “new standards of care” for stage 4 disease, the group said. “Atezolizumab or durvalumab in combination with a platinum plus etoposide should be offered to all eligible chemotherapy-naive patients” with a performance status of 0-1, said the group led by Anne-Marie Dingemans, MD, PhD, a pulmonology professor at Maastricht (the Netherlands) University Medical Center.
Alessio Cortellini, MD, a consulting oncologist and visiting researcher at Imperial College London, said he strongly endorses the recommendation when asked for comment.
“The addition of a PD-L1 inhibitor to a platinum/etoposide backbone is the first strategy that has led to a significant benefit in terms of overall survival. After decades of disappointing results, the bar has” been raised, said Dr. Cortellini.
New inhibitor
EMSO also incorporated the new RNA polymerase II inhibitor lurbinectedin into their guidelines as an option for patients progressing on or after first-line platinum-based chemotherapy.
The agent was approved by the U.S. Food and Drug Administration in June 2020 for metastatic small cell lung cancer (SCLC) with disease progression on or after platinum-based chemotherapy.
The recommendations – more than 50 in all – are based on a literature review and expert opinion, and cover SCLC diagnosis, staging, treatment, and follow-up, with flowcharts outlining treatment pathways.
Atezolizumab earned the endorsement following the IMpower133 trial, which showed a median overall survival of 12.3 months for atezolizumab in combination with carboplatin and etoposide, versus 10.3 months on chemotherapy alone; 34% of the atezolizumab group was alive at 18 months versus 21% in the placebo arm.
The durvalumab recommendation is based on the CASPIAN trial, in which the addition of durvalumab to platinum plus etoposide improved median overall survival from 10.5 to 12.9 months; 32% of durvalumab patients were alive at 18 months versus 24.8% in the chemotherapy-alone arm.
ESMO said “it is important to stress that, in both trials,” patients were in good clinical condition with a median age in the early 60s, so relatively young for SCLC. Also, the modest benefits “clearly emphasize the need for” biomarkers that predict response in order to better select patients.
Immunotherapy has improved cancer treatment across many malignancies and continues to be actively investigated in SCLC, but so far only atezolizumab and durvalumab have phase 3 evidence of benefit.
Makers of the blockbuster checkpoint inhibitors nivolumab and pembrolizumab recently withdrew their FDA approval for stage 4 SCLC that’s progressed after platinum-based chemotherapy and at least one other line of therapy; phase 3 trials failed to confirm the modest survival benefit found in early studies.
Lurbinectedin earned its place in the guidelines based on a single-arm study with 105 relapsed patients that showed an overall response rate of 22.2% in platinum-resistant and 45% in platinum-sensitive patients, with a median overall survival of 9.3 months.
The jury is still out, however. A phase 3 trial of lurbinectedin plus doxorubicin versus topotecan or CAV [cyclophosphamide, adriamycin, and vincristine] for advanced recurrent disease failed to meet its endpoint of superior overall survival, according to a recent press release from the its maker.
“It might be a bit early to discuss” routine use of lurbinectedin, although having it available is good “since literally nothing works in the second line setting,” Dr. Cortellini said.
There was no external funding for the work. The authors had numerous ties to pharmaceutical companies, including Dr. Dingemans who reported adviser and speakers fees and/or research funding from Roche, Lilly, Bristol-Myers Squib, and others. Dr. Costellini reported speakers fees from Novartis, Astrazeneca, and Astellas and consultant payments from Bristol-Myers Squibb, Roche, MSD, and AstraZeneca.
FROM ANNALS OF ONCOLOGY
18-year-old woman • chest pain • shortness of breath • electrocardiogram abnormality • Dx?
THE CASE
An 18-year-old woman with no significant past medical history presented to the emergency department complaining of midsternal chest pain and mild shortness of breath, which had been intermittent for the past several months. She denied any history of deep vein thrombosis or pulmonary embolism risk factors, such as oral contraceptive use.
Laboratory values were within normal limits. An electrocardiogram (EKG), however, showed T-wave inversions in leads V1 and V2, and physical examination revealed decreased breath sounds in the right lung base. A chest radiograph and subsequent chest computed tomography (CT) were ordered.
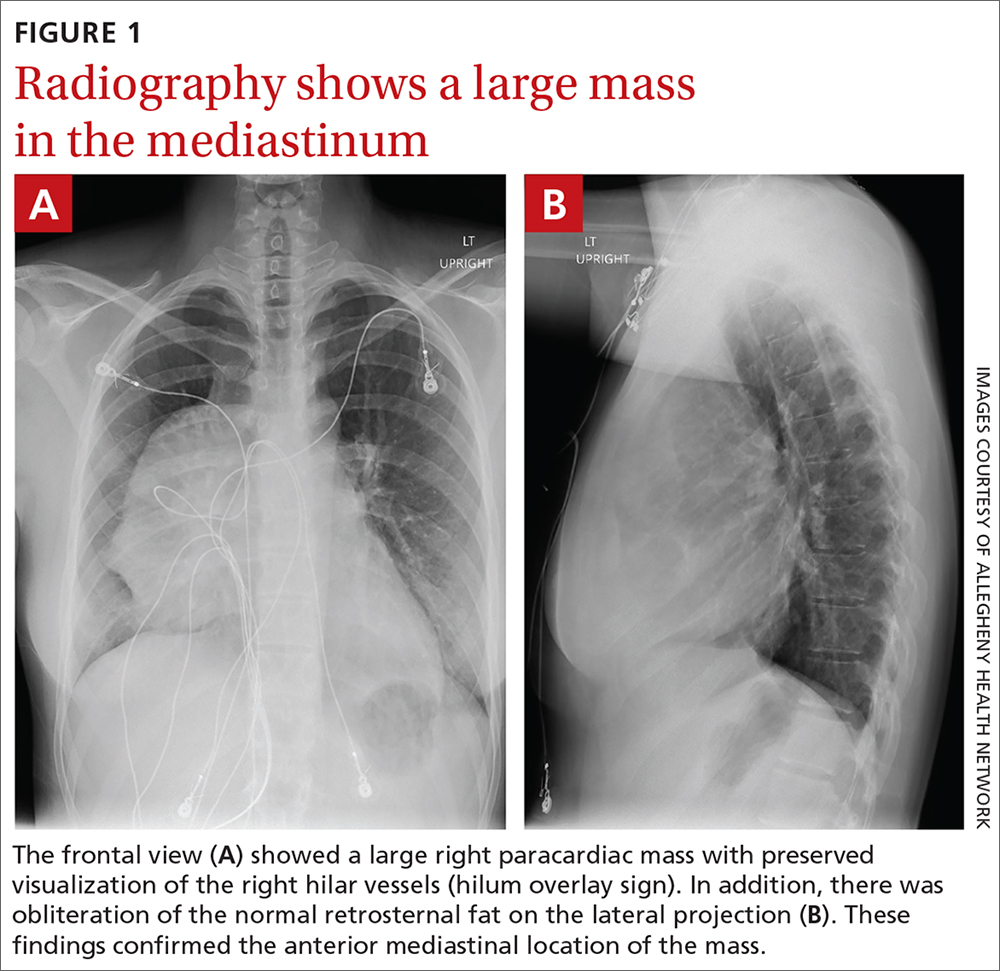
The initial radiograph (FIGURE 1) showed a large right anterior mediastinal mass; the CT revealed fat, fluid, soft tissue, and ossification within the mass (FIGURE 2). The CT also showed evidence of local mass effect on the right atrium, as well as compressive atelectasis in the adjacent right lung, contributing to the patient’s EKG abnormality and physical exam findings.
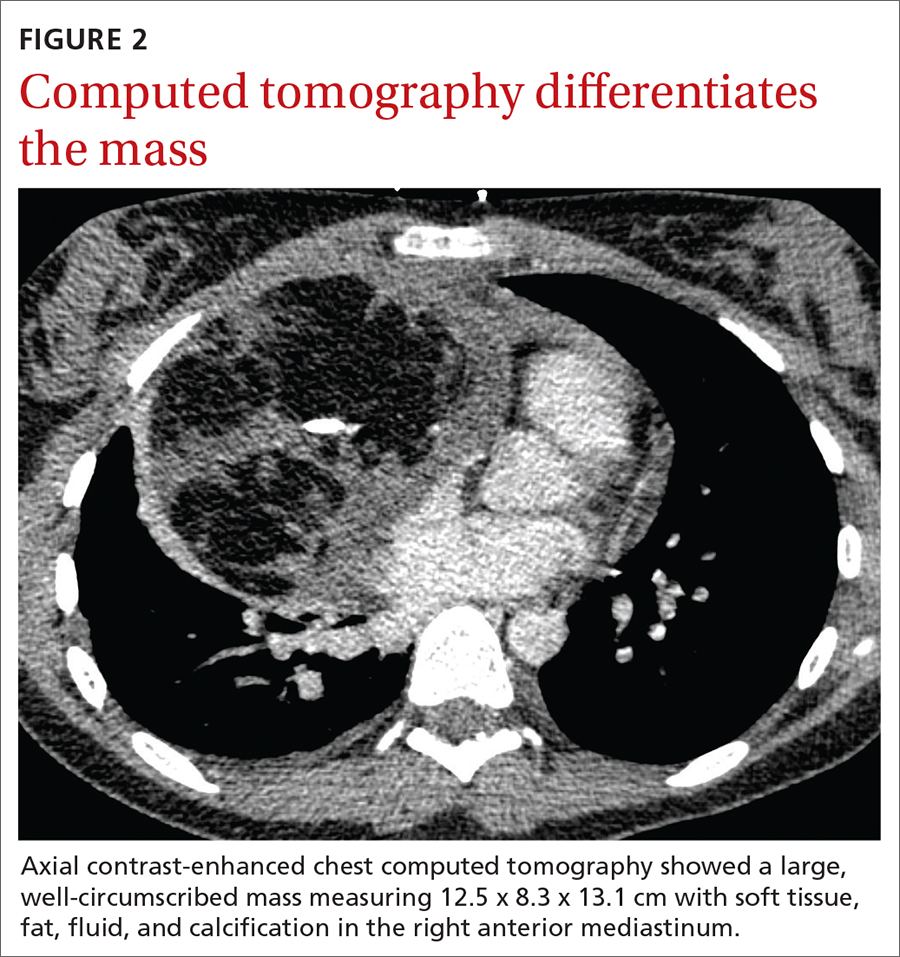
THE DIAGNOSIS
Based on the patient’s clinical history and imaging findings, which were consistent with a benign well-differentiated teratoma, she was given a diagnosis of anterior mediastinal teratoma.
DISCUSSION
Teratomas are tumors composed of pluripotent stem cells that carry elements from all 3 of the embryologic layers (ectoderm, mesoderm, and endoderm).1 There are 3 classifications of teratomas: mature (well-differentiated), immature (poorly differentiated), and malignant.
Tumors of germ cell origin are rare within the anterior mediastinum, accounting for 1% to 3% of total reported cases.2 Among anterior mediastinal masses, germ cell tumors such as teratomas, seminomas, and nonseminomatous tumors comprise approximately 15% of adult and 24% of pediatric anterior mediastinal tumors.3
It is reported that up to 60% of patients with mediastinal teratomas present with no signs or symptoms upon diagnosis.4 When the mass is large, patients can develop chest pain or shortness of breath relating to tumor mass effect. In rare instances, there can be hemoptysis or trichoptysis, pathognomonic for teratomas with bronchial communication.5 Physical exam findings are also nonspecific and may include decreased breath sounds secondary to compressive atelectasis with large tumor burden.
Continue to: Radiographic imaging...
Radiographic imaging is essential to elucidate the diagnosis. Chest radiograph can show an intrathoracic mass, and CT can provide further characterization, such as density and precise location.
Location of mass guides differential
Localizing an intrathoracic mass in the anterior, middle, or posterior mediastinum allows for narrowing of the differential diagnosis (TABLE6). The main diagnostic consideration for a middle mediastinal or hilar mass is primary carcinoma. Posterior mediastinal masses, on the other hand, are generally of benign etiology and may include neurogenic tumors, foregut duplication cysts, or, in rare cases, extramedullary hematopoiesis.
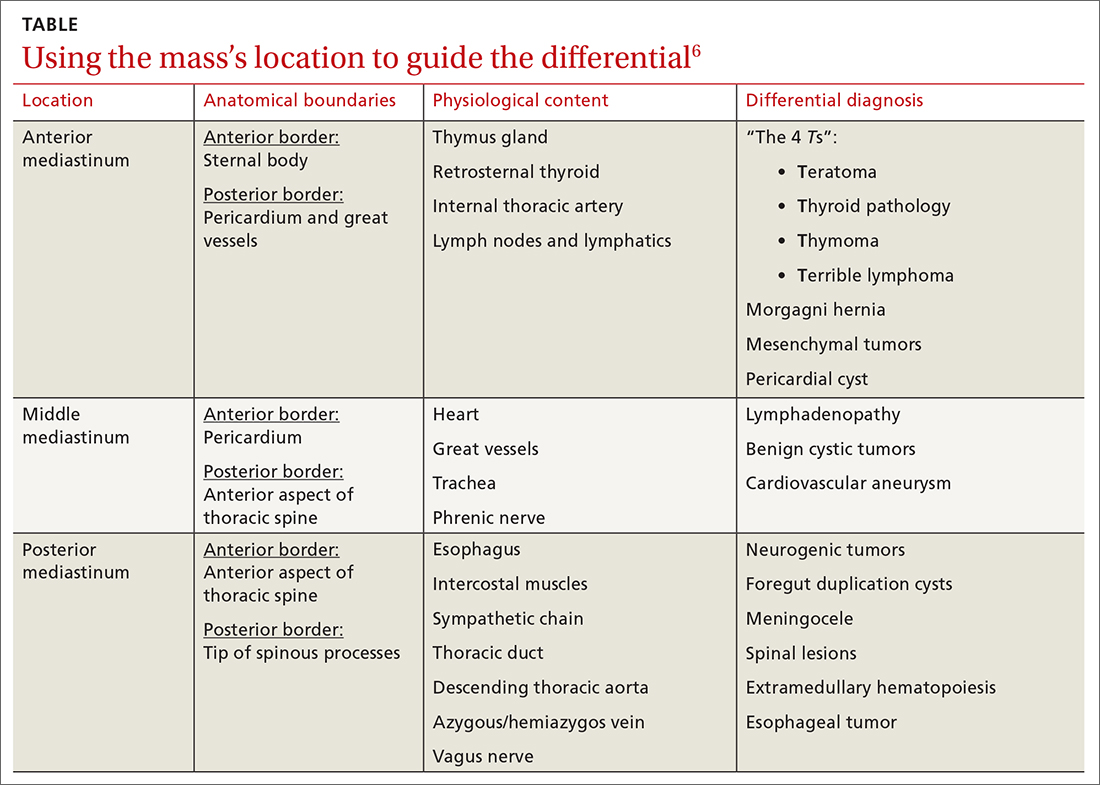
The differential diagnosis of anterior mediastinal masses can be separated into 4 main categories of disease, colloquially known as the “4 Ts”:
Teratoma. Mixed tissue densities seen on CT relate to the multiple tissue types originating from the embryologic germ cell layers.Frequently, there will be fat, fluid, and calcifications.
Thyroid pathology. A goiter or thyroid cancer can manifest with endocrine dysfunction, such as thyroid-stimulating hormone and T3/T4 abnormalities. A thyroid mass tends to sit more superiorly than do other anterior mediastinal masses and may be confirmed using a nuclear scan looking for increased radioactive iodine uptake.
Continue to: Thymoma
Thymoma. The diagnostic features include parathymic syndromes such as myasthenia gravis (30%-50% of thymoma cases7,8) and pure red cell aplasia (5% of thymoma cases9).
“Terrible” lymphoma. The most effective way to differentiate an anterior mediastinal mass due to lymphadenopathy (secondary to lymphoma) is to perform a tissue sample biopsy.
Also consider, as part of the differential for anterior mediastinal masses, such things as mesenchymal tumors, Morgagni hernia (anterior diaphragmatic defect), and pericardial cysts (fluid attenuating and usually located at the right cardiophrenic angle).
Surgical resection is effective
The treatment for anterior mediastinal teratoma is surgical resection.10 A complete surgical resection is typically curative and provides adequate therapy for symptom resolution.
The standard surgical approach involves gaining access to the anterior mediastinum via a median sternotomy. When there is extensive tumor involvement of the hemithorax, clamshell thoracotomy is preferred, requiring incisions in both the left and right hemithoraxes.11
Continue to: Our patient
Our patient underwent resection of the tumor; the subsequent pathology report for the specimen (FIGURE 3) confirmed the diagnosis. There was no abnormal enhancement or vascular invasion to suggest aggressive or malignant potential.
THE TAKEAWAY
Patients frequently present with nonspecific and vague chest complaints. This case points to the importance of obtaining a thorough clinical history and conducting a complete physical examination to guide additional work-up and radiographic imaging.
CORRESPONDENCE
Cassie Tran, MD, 320 E North Avenue, Pittsburgh, PA 15212; [email protected]
1. Chen C, Zheng H, Jiang S. An unusual case of giant mediastinal teratoma with malignant transformation. Ann Thorac Surg. 2008;86:302-304.
2. Nichols CR. Mediastinal germ cell tumors: clinical features and biologic correlates. Chest. 1991;99:472. doi: 10.1378/chest.99.2.472
3. Mulen B, Richardson JD. Primary anterior mediastinal tumors in children and adults. Ann Thorac Surg. 1986;42:338. doi: 10.1016/S0003-4975(10)62751-8
4. Carter B, Okumura M, Detterbeck F, et al. Approaching the patient with an anterior mediastinal mass: a guide for radiologists. J Thorac Oncol. 2014;9 (9 suppl 2):S100-S118.
5. Dar RA, Mushtaque M, Wani SH, et al. Giant intrapulmonary teratoma: a rare case. Case Rep Pulmonol. 2011;2011:298653.
6. Whitten C, Khan S, Munneke G, et al. A diagnostic approach to mediastinal abnormalities. RadioGraphics. 2007;27:657-672.
7. Osserman KE, Genkins G. Studies in myasthenia gravis: review of a 20-year experience in over 1200 patients. Mt Sinai J Med. 1971;38:497-537.
8. Marx A, Muller-Hermelink HK, Strobel P. The role of thymomas in the development of myasthenia gravis. Ann NY Acad Sci. 2003;998:223-236.
9. Rosai J, Levine GD. Tumors of the thymus. In: Firminger HI, ed. Atlas of Tumor Pathology. Washington, DC: Armed Forces Institute of Pathology; 1976: 34-212.
10. Yendamuri S. Resection of a giant mediastinal teratoma. Ann Thorac Surg. 2016;102:e401-e402.
11. Yokoyama Y, Chen F, Date H. Surgical resection of a giant mediastinal teratoma occupying the entire left hemithorax. Gen Thorac Cardiovasc Surg. 2014;62:255-257.
THE CASE
An 18-year-old woman with no significant past medical history presented to the emergency department complaining of midsternal chest pain and mild shortness of breath, which had been intermittent for the past several months. She denied any history of deep vein thrombosis or pulmonary embolism risk factors, such as oral contraceptive use.
Laboratory values were within normal limits. An electrocardiogram (EKG), however, showed T-wave inversions in leads V1 and V2, and physical examination revealed decreased breath sounds in the right lung base. A chest radiograph and subsequent chest computed tomography (CT) were ordered.

The initial radiograph (FIGURE 1) showed a large right anterior mediastinal mass; the CT revealed fat, fluid, soft tissue, and ossification within the mass (FIGURE 2). The CT also showed evidence of local mass effect on the right atrium, as well as compressive atelectasis in the adjacent right lung, contributing to the patient’s EKG abnormality and physical exam findings.

THE DIAGNOSIS
Based on the patient’s clinical history and imaging findings, which were consistent with a benign well-differentiated teratoma, she was given a diagnosis of anterior mediastinal teratoma.
DISCUSSION
Teratomas are tumors composed of pluripotent stem cells that carry elements from all 3 of the embryologic layers (ectoderm, mesoderm, and endoderm).1 There are 3 classifications of teratomas: mature (well-differentiated), immature (poorly differentiated), and malignant.
Tumors of germ cell origin are rare within the anterior mediastinum, accounting for 1% to 3% of total reported cases.2 Among anterior mediastinal masses, germ cell tumors such as teratomas, seminomas, and nonseminomatous tumors comprise approximately 15% of adult and 24% of pediatric anterior mediastinal tumors.3
It is reported that up to 60% of patients with mediastinal teratomas present with no signs or symptoms upon diagnosis.4 When the mass is large, patients can develop chest pain or shortness of breath relating to tumor mass effect. In rare instances, there can be hemoptysis or trichoptysis, pathognomonic for teratomas with bronchial communication.5 Physical exam findings are also nonspecific and may include decreased breath sounds secondary to compressive atelectasis with large tumor burden.
Continue to: Radiographic imaging...
Radiographic imaging is essential to elucidate the diagnosis. Chest radiograph can show an intrathoracic mass, and CT can provide further characterization, such as density and precise location.
Location of mass guides differential
Localizing an intrathoracic mass in the anterior, middle, or posterior mediastinum allows for narrowing of the differential diagnosis (TABLE6). The main diagnostic consideration for a middle mediastinal or hilar mass is primary carcinoma. Posterior mediastinal masses, on the other hand, are generally of benign etiology and may include neurogenic tumors, foregut duplication cysts, or, in rare cases, extramedullary hematopoiesis.

The differential diagnosis of anterior mediastinal masses can be separated into 4 main categories of disease, colloquially known as the “4 Ts”:
Teratoma. Mixed tissue densities seen on CT relate to the multiple tissue types originating from the embryologic germ cell layers.Frequently, there will be fat, fluid, and calcifications.
Thyroid pathology. A goiter or thyroid cancer can manifest with endocrine dysfunction, such as thyroid-stimulating hormone and T3/T4 abnormalities. A thyroid mass tends to sit more superiorly than do other anterior mediastinal masses and may be confirmed using a nuclear scan looking for increased radioactive iodine uptake.
Continue to: Thymoma
Thymoma. The diagnostic features include parathymic syndromes such as myasthenia gravis (30%-50% of thymoma cases7,8) and pure red cell aplasia (5% of thymoma cases9).
“Terrible” lymphoma. The most effective way to differentiate an anterior mediastinal mass due to lymphadenopathy (secondary to lymphoma) is to perform a tissue sample biopsy.
Also consider, as part of the differential for anterior mediastinal masses, such things as mesenchymal tumors, Morgagni hernia (anterior diaphragmatic defect), and pericardial cysts (fluid attenuating and usually located at the right cardiophrenic angle).
Surgical resection is effective
The treatment for anterior mediastinal teratoma is surgical resection.10 A complete surgical resection is typically curative and provides adequate therapy for symptom resolution.
The standard surgical approach involves gaining access to the anterior mediastinum via a median sternotomy. When there is extensive tumor involvement of the hemithorax, clamshell thoracotomy is preferred, requiring incisions in both the left and right hemithoraxes.11
Continue to: Our patient
Our patient underwent resection of the tumor; the subsequent pathology report for the specimen (FIGURE 3) confirmed the diagnosis. There was no abnormal enhancement or vascular invasion to suggest aggressive or malignant potential.

THE TAKEAWAY
Patients frequently present with nonspecific and vague chest complaints. This case points to the importance of obtaining a thorough clinical history and conducting a complete physical examination to guide additional work-up and radiographic imaging.
CORRESPONDENCE
Cassie Tran, MD, 320 E North Avenue, Pittsburgh, PA 15212; [email protected]
THE CASE
An 18-year-old woman with no significant past medical history presented to the emergency department complaining of midsternal chest pain and mild shortness of breath, which had been intermittent for the past several months. She denied any history of deep vein thrombosis or pulmonary embolism risk factors, such as oral contraceptive use.
Laboratory values were within normal limits. An electrocardiogram (EKG), however, showed T-wave inversions in leads V1 and V2, and physical examination revealed decreased breath sounds in the right lung base. A chest radiograph and subsequent chest computed tomography (CT) were ordered.

The initial radiograph (FIGURE 1) showed a large right anterior mediastinal mass; the CT revealed fat, fluid, soft tissue, and ossification within the mass (FIGURE 2). The CT also showed evidence of local mass effect on the right atrium, as well as compressive atelectasis in the adjacent right lung, contributing to the patient’s EKG abnormality and physical exam findings.

THE DIAGNOSIS
Based on the patient’s clinical history and imaging findings, which were consistent with a benign well-differentiated teratoma, she was given a diagnosis of anterior mediastinal teratoma.
DISCUSSION
Teratomas are tumors composed of pluripotent stem cells that carry elements from all 3 of the embryologic layers (ectoderm, mesoderm, and endoderm).1 There are 3 classifications of teratomas: mature (well-differentiated), immature (poorly differentiated), and malignant.
Tumors of germ cell origin are rare within the anterior mediastinum, accounting for 1% to 3% of total reported cases.2 Among anterior mediastinal masses, germ cell tumors such as teratomas, seminomas, and nonseminomatous tumors comprise approximately 15% of adult and 24% of pediatric anterior mediastinal tumors.3
It is reported that up to 60% of patients with mediastinal teratomas present with no signs or symptoms upon diagnosis.4 When the mass is large, patients can develop chest pain or shortness of breath relating to tumor mass effect. In rare instances, there can be hemoptysis or trichoptysis, pathognomonic for teratomas with bronchial communication.5 Physical exam findings are also nonspecific and may include decreased breath sounds secondary to compressive atelectasis with large tumor burden.
Continue to: Radiographic imaging...
Radiographic imaging is essential to elucidate the diagnosis. Chest radiograph can show an intrathoracic mass, and CT can provide further characterization, such as density and precise location.
Location of mass guides differential
Localizing an intrathoracic mass in the anterior, middle, or posterior mediastinum allows for narrowing of the differential diagnosis (TABLE6). The main diagnostic consideration for a middle mediastinal or hilar mass is primary carcinoma. Posterior mediastinal masses, on the other hand, are generally of benign etiology and may include neurogenic tumors, foregut duplication cysts, or, in rare cases, extramedullary hematopoiesis.

The differential diagnosis of anterior mediastinal masses can be separated into 4 main categories of disease, colloquially known as the “4 Ts”:
Teratoma. Mixed tissue densities seen on CT relate to the multiple tissue types originating from the embryologic germ cell layers.Frequently, there will be fat, fluid, and calcifications.
Thyroid pathology. A goiter or thyroid cancer can manifest with endocrine dysfunction, such as thyroid-stimulating hormone and T3/T4 abnormalities. A thyroid mass tends to sit more superiorly than do other anterior mediastinal masses and may be confirmed using a nuclear scan looking for increased radioactive iodine uptake.
Continue to: Thymoma
Thymoma. The diagnostic features include parathymic syndromes such as myasthenia gravis (30%-50% of thymoma cases7,8) and pure red cell aplasia (5% of thymoma cases9).
“Terrible” lymphoma. The most effective way to differentiate an anterior mediastinal mass due to lymphadenopathy (secondary to lymphoma) is to perform a tissue sample biopsy.
Also consider, as part of the differential for anterior mediastinal masses, such things as mesenchymal tumors, Morgagni hernia (anterior diaphragmatic defect), and pericardial cysts (fluid attenuating and usually located at the right cardiophrenic angle).
Surgical resection is effective
The treatment for anterior mediastinal teratoma is surgical resection.10 A complete surgical resection is typically curative and provides adequate therapy for symptom resolution.
The standard surgical approach involves gaining access to the anterior mediastinum via a median sternotomy. When there is extensive tumor involvement of the hemithorax, clamshell thoracotomy is preferred, requiring incisions in both the left and right hemithoraxes.11
Continue to: Our patient
Our patient underwent resection of the tumor; the subsequent pathology report for the specimen (FIGURE 3) confirmed the diagnosis. There was no abnormal enhancement or vascular invasion to suggest aggressive or malignant potential.

THE TAKEAWAY
Patients frequently present with nonspecific and vague chest complaints. This case points to the importance of obtaining a thorough clinical history and conducting a complete physical examination to guide additional work-up and radiographic imaging.
CORRESPONDENCE
Cassie Tran, MD, 320 E North Avenue, Pittsburgh, PA 15212; [email protected]
1. Chen C, Zheng H, Jiang S. An unusual case of giant mediastinal teratoma with malignant transformation. Ann Thorac Surg. 2008;86:302-304.
2. Nichols CR. Mediastinal germ cell tumors: clinical features and biologic correlates. Chest. 1991;99:472. doi: 10.1378/chest.99.2.472
3. Mulen B, Richardson JD. Primary anterior mediastinal tumors in children and adults. Ann Thorac Surg. 1986;42:338. doi: 10.1016/S0003-4975(10)62751-8
4. Carter B, Okumura M, Detterbeck F, et al. Approaching the patient with an anterior mediastinal mass: a guide for radiologists. J Thorac Oncol. 2014;9 (9 suppl 2):S100-S118.
5. Dar RA, Mushtaque M, Wani SH, et al. Giant intrapulmonary teratoma: a rare case. Case Rep Pulmonol. 2011;2011:298653.
6. Whitten C, Khan S, Munneke G, et al. A diagnostic approach to mediastinal abnormalities. RadioGraphics. 2007;27:657-672.
7. Osserman KE, Genkins G. Studies in myasthenia gravis: review of a 20-year experience in over 1200 patients. Mt Sinai J Med. 1971;38:497-537.
8. Marx A, Muller-Hermelink HK, Strobel P. The role of thymomas in the development of myasthenia gravis. Ann NY Acad Sci. 2003;998:223-236.
9. Rosai J, Levine GD. Tumors of the thymus. In: Firminger HI, ed. Atlas of Tumor Pathology. Washington, DC: Armed Forces Institute of Pathology; 1976: 34-212.
10. Yendamuri S. Resection of a giant mediastinal teratoma. Ann Thorac Surg. 2016;102:e401-e402.
11. Yokoyama Y, Chen F, Date H. Surgical resection of a giant mediastinal teratoma occupying the entire left hemithorax. Gen Thorac Cardiovasc Surg. 2014;62:255-257.
1. Chen C, Zheng H, Jiang S. An unusual case of giant mediastinal teratoma with malignant transformation. Ann Thorac Surg. 2008;86:302-304.
2. Nichols CR. Mediastinal germ cell tumors: clinical features and biologic correlates. Chest. 1991;99:472. doi: 10.1378/chest.99.2.472
3. Mulen B, Richardson JD. Primary anterior mediastinal tumors in children and adults. Ann Thorac Surg. 1986;42:338. doi: 10.1016/S0003-4975(10)62751-8
4. Carter B, Okumura M, Detterbeck F, et al. Approaching the patient with an anterior mediastinal mass: a guide for radiologists. J Thorac Oncol. 2014;9 (9 suppl 2):S100-S118.
5. Dar RA, Mushtaque M, Wani SH, et al. Giant intrapulmonary teratoma: a rare case. Case Rep Pulmonol. 2011;2011:298653.
6. Whitten C, Khan S, Munneke G, et al. A diagnostic approach to mediastinal abnormalities. RadioGraphics. 2007;27:657-672.
7. Osserman KE, Genkins G. Studies in myasthenia gravis: review of a 20-year experience in over 1200 patients. Mt Sinai J Med. 1971;38:497-537.
8. Marx A, Muller-Hermelink HK, Strobel P. The role of thymomas in the development of myasthenia gravis. Ann NY Acad Sci. 2003;998:223-236.
9. Rosai J, Levine GD. Tumors of the thymus. In: Firminger HI, ed. Atlas of Tumor Pathology. Washington, DC: Armed Forces Institute of Pathology; 1976: 34-212.
10. Yendamuri S. Resection of a giant mediastinal teratoma. Ann Thorac Surg. 2016;102:e401-e402.
11. Yokoyama Y, Chen F, Date H. Surgical resection of a giant mediastinal teratoma occupying the entire left hemithorax. Gen Thorac Cardiovasc Surg. 2014;62:255-257.
Palliative care in the pandemic: How one hospital met the challenge
Clarissa Johnston, MD, said during a virtual presentation at the annual meeting of the Society of General Internal Medicine.
Dr. Johnston, of the University of Texas at Austin, and colleagues experienced an extreme COVID-19 surge when they reopened after initial closure in the first weeks of the pandemic.
“Our hospital and clinics are the health care safety net in Austin, and we serve a predominantly uninsured and Hispanic population that experienced a greater burden of COVID-19 than other populations in our area,” she said in the presentation.
The rapid onset and spread of COVID-19 locally required physicians and staff to innovate quickly, and “we developed and implemented collaborative and novel partnerships between generalists and palliative care specialists to help ensure that our core humanizing values were not lost in the pandemic,” Dr. Johnston emphasized.
Collaboration between internal medicine and palliative care involved developing relationship-centered communication for families and health care workers, as well as engaging medical students in a Transitions of Care elective, Dr. Johnston said.
The early weeks of the pandemic impacted families with the no visitor policy and the loss of death rituals, she said. Health care providers suffered, too, as nurses experienced an overload of work, fears for their own health and safety, and feelings of disconnect from their patients. Physicians dealt with the challenges of a unique illness, and their own fears and uncertainty, Dr. Johnston said.
Meeting communication challenges
One of the strategies used to bridge the communication gap caused by the lack of visitors and family contact was the adoption of the Meet My Loved One program, adapted from a similar program at the University of Alabama, said Dr. Johnston. Meet My Loved One was a collaborative effort focused on ICU patients, Dr. Johnston said. Members of the primary care team, including medical students in the Transitions of Care elective, called family members of ICU patients to collect personal details and humanizing information about the patient, such as preferred name, favorite foods, favorite activities, and some personal history (i.e. played basketball when he was young), and this information was collated, summarized, and posted on the door of the patient’s room.
Advanced care conversations
Advanced care planning (ACP) benefits include not only the promotion of patient-centered care, but also decreases in ICU admissions, length of stay, and cost. Dr. Johnston and colleagues developed a multipronged curriculum that trainees could use to have ACP conversations with clinic patients who would be considered high risk if they developed COVID-19 infections, Dr. Johnston explained. As part of the elective, medical students were trained to have ACP conversations with patients via telehealth; students practiced role-playing conversations with each other via Zoom and met virtually as a group to review the conversations, she said.
Maintaining Humanity
“COVID-19 has changed the way we interact with patients and families,” Dr. Johnston said in an interview. The inability to rely on face-to-face discussions means that “we really need to think carefully how we maintain humanity and the human touch,” she said.
Challenges in providing palliative care during the pandemic include “maintaining humanity, remembering that there is a person behind the prone, paralyzed patient, with family members who love them, and are desperate to be with them but unable,” Dr. Johnston said.
“The Meet My Loved One program helped, as well as multidisciplinary rounds, chaplain services, and frequent check ins with the bedside nurses,” she said.
“I tried hard to call families every day to start to build that trust and rapport that was lost by all the distancing and lack of visits. I didn’t realize how much the day in and day out care of ICU patients is witnessed by families when they are in the room,” she noted. “During COVID-19, it was so much harder to build trust, especially when you add in the inequities and structural racism problems in our health care system,” she said.
“Why would a family member believe and trust some random doctor calling them on the phone? Were we really trying our hardest? Families didn’t have a way to assess that, at least not like they do when they are at bedside and see how hard everyone works,” Dr. Johnston said. “Video visits helped but were not the same.”
Some key lessons about palliative care Dr. Johnson said she learned from the pandemic were how important it is to remember the patient and family, “how we need to work to build trust,” and that clinicians should be mindful that video visits don’t work for everyone, and to “ask, ask, ask about what you don’t know, including death rituals.”
Additional research needs in palliative care in the wake of COVID-19 include more information on what works and what doesn’t work, from the patient and family perspective, said Dr. Johnston. Communication strategies are important, and “we need to address how we can better communicate around serious illness and end-of-life issues with Black and Brown communities,” she said.
Challenges of COVID care
One of the main challenges to providing palliative care in the early days of the pandemic was navigating the constantly evolving science of COVID-19, Aziz Ansari, DO, of Loyola University Chicago, Maywood, Ill., said in an interview.
“It was, and remains, very hard to prognosticate on how a patient will do having respiratory failure with COVID,” said Dr. Ansari, who was the leader of the Palliative Care interest group at the SGIM meeting.
“So, the challenge was how to have a conversation on goals, values, and preferences when we really did not know the disease entity,” Dr. Ansari noted.
“We were surprised many times [when patients with COVID-19] recovered though it took a long time, so we could not really say that in the acute phase of COVID, it was a terminal illness,” he noted.
“Regardless, it still behooves us to have conversations with our patients and families about what are they willing to go through, and how they define a quality of life,” he said.
Strategies such as those used at the University of Texas show the importance of primary care palliative skill development, said Dr. Ansari. “Every physician should have the skill set of having conversations with patients and families on goals, values, and preferences even in unknown situations,” he said. That lifelong skill set development begins in medical school, he added.
Dr. Johnston and Dr. Ansari had no financial conflicts to disclose.
Clarissa Johnston, MD, said during a virtual presentation at the annual meeting of the Society of General Internal Medicine.
Dr. Johnston, of the University of Texas at Austin, and colleagues experienced an extreme COVID-19 surge when they reopened after initial closure in the first weeks of the pandemic.
“Our hospital and clinics are the health care safety net in Austin, and we serve a predominantly uninsured and Hispanic population that experienced a greater burden of COVID-19 than other populations in our area,” she said in the presentation.
The rapid onset and spread of COVID-19 locally required physicians and staff to innovate quickly, and “we developed and implemented collaborative and novel partnerships between generalists and palliative care specialists to help ensure that our core humanizing values were not lost in the pandemic,” Dr. Johnston emphasized.
Collaboration between internal medicine and palliative care involved developing relationship-centered communication for families and health care workers, as well as engaging medical students in a Transitions of Care elective, Dr. Johnston said.
The early weeks of the pandemic impacted families with the no visitor policy and the loss of death rituals, she said. Health care providers suffered, too, as nurses experienced an overload of work, fears for their own health and safety, and feelings of disconnect from their patients. Physicians dealt with the challenges of a unique illness, and their own fears and uncertainty, Dr. Johnston said.
Meeting communication challenges
One of the strategies used to bridge the communication gap caused by the lack of visitors and family contact was the adoption of the Meet My Loved One program, adapted from a similar program at the University of Alabama, said Dr. Johnston. Meet My Loved One was a collaborative effort focused on ICU patients, Dr. Johnston said. Members of the primary care team, including medical students in the Transitions of Care elective, called family members of ICU patients to collect personal details and humanizing information about the patient, such as preferred name, favorite foods, favorite activities, and some personal history (i.e. played basketball when he was young), and this information was collated, summarized, and posted on the door of the patient’s room.
Advanced care conversations
Advanced care planning (ACP) benefits include not only the promotion of patient-centered care, but also decreases in ICU admissions, length of stay, and cost. Dr. Johnston and colleagues developed a multipronged curriculum that trainees could use to have ACP conversations with clinic patients who would be considered high risk if they developed COVID-19 infections, Dr. Johnston explained. As part of the elective, medical students were trained to have ACP conversations with patients via telehealth; students practiced role-playing conversations with each other via Zoom and met virtually as a group to review the conversations, she said.
Maintaining Humanity
“COVID-19 has changed the way we interact with patients and families,” Dr. Johnston said in an interview. The inability to rely on face-to-face discussions means that “we really need to think carefully how we maintain humanity and the human touch,” she said.
Challenges in providing palliative care during the pandemic include “maintaining humanity, remembering that there is a person behind the prone, paralyzed patient, with family members who love them, and are desperate to be with them but unable,” Dr. Johnston said.
“The Meet My Loved One program helped, as well as multidisciplinary rounds, chaplain services, and frequent check ins with the bedside nurses,” she said.
“I tried hard to call families every day to start to build that trust and rapport that was lost by all the distancing and lack of visits. I didn’t realize how much the day in and day out care of ICU patients is witnessed by families when they are in the room,” she noted. “During COVID-19, it was so much harder to build trust, especially when you add in the inequities and structural racism problems in our health care system,” she said.
“Why would a family member believe and trust some random doctor calling them on the phone? Were we really trying our hardest? Families didn’t have a way to assess that, at least not like they do when they are at bedside and see how hard everyone works,” Dr. Johnston said. “Video visits helped but were not the same.”
Some key lessons about palliative care Dr. Johnson said she learned from the pandemic were how important it is to remember the patient and family, “how we need to work to build trust,” and that clinicians should be mindful that video visits don’t work for everyone, and to “ask, ask, ask about what you don’t know, including death rituals.”
Additional research needs in palliative care in the wake of COVID-19 include more information on what works and what doesn’t work, from the patient and family perspective, said Dr. Johnston. Communication strategies are important, and “we need to address how we can better communicate around serious illness and end-of-life issues with Black and Brown communities,” she said.
Challenges of COVID care
One of the main challenges to providing palliative care in the early days of the pandemic was navigating the constantly evolving science of COVID-19, Aziz Ansari, DO, of Loyola University Chicago, Maywood, Ill., said in an interview.
“It was, and remains, very hard to prognosticate on how a patient will do having respiratory failure with COVID,” said Dr. Ansari, who was the leader of the Palliative Care interest group at the SGIM meeting.
“So, the challenge was how to have a conversation on goals, values, and preferences when we really did not know the disease entity,” Dr. Ansari noted.
“We were surprised many times [when patients with COVID-19] recovered though it took a long time, so we could not really say that in the acute phase of COVID, it was a terminal illness,” he noted.
“Regardless, it still behooves us to have conversations with our patients and families about what are they willing to go through, and how they define a quality of life,” he said.
Strategies such as those used at the University of Texas show the importance of primary care palliative skill development, said Dr. Ansari. “Every physician should have the skill set of having conversations with patients and families on goals, values, and preferences even in unknown situations,” he said. That lifelong skill set development begins in medical school, he added.
Dr. Johnston and Dr. Ansari had no financial conflicts to disclose.
Clarissa Johnston, MD, said during a virtual presentation at the annual meeting of the Society of General Internal Medicine.
Dr. Johnston, of the University of Texas at Austin, and colleagues experienced an extreme COVID-19 surge when they reopened after initial closure in the first weeks of the pandemic.
“Our hospital and clinics are the health care safety net in Austin, and we serve a predominantly uninsured and Hispanic population that experienced a greater burden of COVID-19 than other populations in our area,” she said in the presentation.
The rapid onset and spread of COVID-19 locally required physicians and staff to innovate quickly, and “we developed and implemented collaborative and novel partnerships between generalists and palliative care specialists to help ensure that our core humanizing values were not lost in the pandemic,” Dr. Johnston emphasized.
Collaboration between internal medicine and palliative care involved developing relationship-centered communication for families and health care workers, as well as engaging medical students in a Transitions of Care elective, Dr. Johnston said.
The early weeks of the pandemic impacted families with the no visitor policy and the loss of death rituals, she said. Health care providers suffered, too, as nurses experienced an overload of work, fears for their own health and safety, and feelings of disconnect from their patients. Physicians dealt with the challenges of a unique illness, and their own fears and uncertainty, Dr. Johnston said.
Meeting communication challenges
One of the strategies used to bridge the communication gap caused by the lack of visitors and family contact was the adoption of the Meet My Loved One program, adapted from a similar program at the University of Alabama, said Dr. Johnston. Meet My Loved One was a collaborative effort focused on ICU patients, Dr. Johnston said. Members of the primary care team, including medical students in the Transitions of Care elective, called family members of ICU patients to collect personal details and humanizing information about the patient, such as preferred name, favorite foods, favorite activities, and some personal history (i.e. played basketball when he was young), and this information was collated, summarized, and posted on the door of the patient’s room.
Advanced care conversations
Advanced care planning (ACP) benefits include not only the promotion of patient-centered care, but also decreases in ICU admissions, length of stay, and cost. Dr. Johnston and colleagues developed a multipronged curriculum that trainees could use to have ACP conversations with clinic patients who would be considered high risk if they developed COVID-19 infections, Dr. Johnston explained. As part of the elective, medical students were trained to have ACP conversations with patients via telehealth; students practiced role-playing conversations with each other via Zoom and met virtually as a group to review the conversations, she said.
Maintaining Humanity
“COVID-19 has changed the way we interact with patients and families,” Dr. Johnston said in an interview. The inability to rely on face-to-face discussions means that “we really need to think carefully how we maintain humanity and the human touch,” she said.
Challenges in providing palliative care during the pandemic include “maintaining humanity, remembering that there is a person behind the prone, paralyzed patient, with family members who love them, and are desperate to be with them but unable,” Dr. Johnston said.
“The Meet My Loved One program helped, as well as multidisciplinary rounds, chaplain services, and frequent check ins with the bedside nurses,” she said.
“I tried hard to call families every day to start to build that trust and rapport that was lost by all the distancing and lack of visits. I didn’t realize how much the day in and day out care of ICU patients is witnessed by families when they are in the room,” she noted. “During COVID-19, it was so much harder to build trust, especially when you add in the inequities and structural racism problems in our health care system,” she said.
“Why would a family member believe and trust some random doctor calling them on the phone? Were we really trying our hardest? Families didn’t have a way to assess that, at least not like they do when they are at bedside and see how hard everyone works,” Dr. Johnston said. “Video visits helped but were not the same.”
Some key lessons about palliative care Dr. Johnson said she learned from the pandemic were how important it is to remember the patient and family, “how we need to work to build trust,” and that clinicians should be mindful that video visits don’t work for everyone, and to “ask, ask, ask about what you don’t know, including death rituals.”
Additional research needs in palliative care in the wake of COVID-19 include more information on what works and what doesn’t work, from the patient and family perspective, said Dr. Johnston. Communication strategies are important, and “we need to address how we can better communicate around serious illness and end-of-life issues with Black and Brown communities,” she said.
Challenges of COVID care
One of the main challenges to providing palliative care in the early days of the pandemic was navigating the constantly evolving science of COVID-19, Aziz Ansari, DO, of Loyola University Chicago, Maywood, Ill., said in an interview.
“It was, and remains, very hard to prognosticate on how a patient will do having respiratory failure with COVID,” said Dr. Ansari, who was the leader of the Palliative Care interest group at the SGIM meeting.
“So, the challenge was how to have a conversation on goals, values, and preferences when we really did not know the disease entity,” Dr. Ansari noted.
“We were surprised many times [when patients with COVID-19] recovered though it took a long time, so we could not really say that in the acute phase of COVID, it was a terminal illness,” he noted.
“Regardless, it still behooves us to have conversations with our patients and families about what are they willing to go through, and how they define a quality of life,” he said.
Strategies such as those used at the University of Texas show the importance of primary care palliative skill development, said Dr. Ansari. “Every physician should have the skill set of having conversations with patients and families on goals, values, and preferences even in unknown situations,” he said. That lifelong skill set development begins in medical school, he added.
Dr. Johnston and Dr. Ansari had no financial conflicts to disclose.
FROM SGIM 2021
Operational changes in primary care linked with improved smoking, blood pressure outcomes
The qualitative analysis, published in Annals of Family Medicine , included smoking and blood pressure as separate outcome measures.
The outcomes were calculated using Clinical Quality Measure improvements, with targets of at least 10-point absolute improvements in the proportion of patients with smoking screening, if relevant, counseling, and in the proportion of hypertensive patients with adequately controlled BP. The results were obtained from practices participating in Evidence-NOW, a multisite cardiovascular disease prevention initiative. Configurational Comparative Methods were used to evaluate the joint effects of multiple factors on outcomes.
The majority of practices in the analysis were clinician owned, small (fewer than six clinicians), and/or in an urban location. The researchers sampled and interviewed practice staff from a subset of 104 primary care practices across 7 Cooperatives and 12 states, ranging from small to medium in size, having 10 or fewer clinicians. The interview data were analyzed to identify operational changes, then transformed into numeric data.
Operational changes led to improvements in specific contexts
In clinician-owned practices, process improvement, documentation, and referral to resources, combined with a moderate level of facilitation support, led to an improvement of at least 10 points in smoking outcomes.
However, the researchers found that these patterns were not observed in system–owned practices or Federally Qualified Health Centers.
In solo practices, training medical assistants to take an accurate blood pressure led to an improvement of at least 10 points in blood pressure outcomes.
Among larger, clinician-owned practices, measurement of blood pressure a second time when the first was elevated, and documentation of this reading in the electronic heath record, also led to a 10-point or greater improvement in BP outcome when combined with a large amount (50 hours or more) of facilitation.
“There was no magic bullet for improving smoking cessation counseling and blood pressure outcomes across the diverse primary care practices studied,” lead author Deborah J. Cohen, PhD, of Oregon Health & Science University, Portland, said in an interview. “Combinations of operational changes among practice sizes and types led to improvements.”
Smaller practices more nimble, experts say
Results of the qualitative data analysis suggest that smaller and clinician-owned practices are more likely to have the capacity for change and improvement compared with larger, hospital/health system–owned practices.
Commenting on the study, Noel Deep, MD, regional medical director at Aspirus Clinics, Ironwood, Mich., said solo or small private practices have a distinct advantage over larger hospital or system-owned practices when implementing new operational changes to improve clinical outcomes.
“A smaller independent practice is nimble, with the physician [or physicians] able to make a quick decision at analyzing the scientific data, planning the changes, implementing them quickly, and doing a rapid cycle review of the results and tweaking the program to attain the targets,” said Dr. Deep, a member of the editorial advisory board of Internal Medicine News.
Kate Rowland, MD, MS, assistant professor in the department of family medicine at Rush Medical College, Chicago, also noted that smaller practices have unique advantages over larger health organizations.
“Larger organizations should replicate the benefits of the smaller office, providing as much local decision-making and autonomy as possible to the site where the changes are happening,” Dr. Rowland explained in an interview.
“The clinicians at these sites are mostly likely to know what is going to be successful for achieving measurable change in the patients they care for,” she added.
The study was funded by the Agency for Healthcare Research and Quality. The authors and other experts interviewed for this piece reported having no conflicts of interest.
The qualitative analysis, published in Annals of Family Medicine , included smoking and blood pressure as separate outcome measures.
The outcomes were calculated using Clinical Quality Measure improvements, with targets of at least 10-point absolute improvements in the proportion of patients with smoking screening, if relevant, counseling, and in the proportion of hypertensive patients with adequately controlled BP. The results were obtained from practices participating in Evidence-NOW, a multisite cardiovascular disease prevention initiative. Configurational Comparative Methods were used to evaluate the joint effects of multiple factors on outcomes.
The majority of practices in the analysis were clinician owned, small (fewer than six clinicians), and/or in an urban location. The researchers sampled and interviewed practice staff from a subset of 104 primary care practices across 7 Cooperatives and 12 states, ranging from small to medium in size, having 10 or fewer clinicians. The interview data were analyzed to identify operational changes, then transformed into numeric data.
Operational changes led to improvements in specific contexts
In clinician-owned practices, process improvement, documentation, and referral to resources, combined with a moderate level of facilitation support, led to an improvement of at least 10 points in smoking outcomes.
However, the researchers found that these patterns were not observed in system–owned practices or Federally Qualified Health Centers.
In solo practices, training medical assistants to take an accurate blood pressure led to an improvement of at least 10 points in blood pressure outcomes.
Among larger, clinician-owned practices, measurement of blood pressure a second time when the first was elevated, and documentation of this reading in the electronic heath record, also led to a 10-point or greater improvement in BP outcome when combined with a large amount (50 hours or more) of facilitation.
“There was no magic bullet for improving smoking cessation counseling and blood pressure outcomes across the diverse primary care practices studied,” lead author Deborah J. Cohen, PhD, of Oregon Health & Science University, Portland, said in an interview. “Combinations of operational changes among practice sizes and types led to improvements.”
Smaller practices more nimble, experts say
Results of the qualitative data analysis suggest that smaller and clinician-owned practices are more likely to have the capacity for change and improvement compared with larger, hospital/health system–owned practices.
Commenting on the study, Noel Deep, MD, regional medical director at Aspirus Clinics, Ironwood, Mich., said solo or small private practices have a distinct advantage over larger hospital or system-owned practices when implementing new operational changes to improve clinical outcomes.
“A smaller independent practice is nimble, with the physician [or physicians] able to make a quick decision at analyzing the scientific data, planning the changes, implementing them quickly, and doing a rapid cycle review of the results and tweaking the program to attain the targets,” said Dr. Deep, a member of the editorial advisory board of Internal Medicine News.
Kate Rowland, MD, MS, assistant professor in the department of family medicine at Rush Medical College, Chicago, also noted that smaller practices have unique advantages over larger health organizations.
“Larger organizations should replicate the benefits of the smaller office, providing as much local decision-making and autonomy as possible to the site where the changes are happening,” Dr. Rowland explained in an interview.
“The clinicians at these sites are mostly likely to know what is going to be successful for achieving measurable change in the patients they care for,” she added.
The study was funded by the Agency for Healthcare Research and Quality. The authors and other experts interviewed for this piece reported having no conflicts of interest.
The qualitative analysis, published in Annals of Family Medicine , included smoking and blood pressure as separate outcome measures.
The outcomes were calculated using Clinical Quality Measure improvements, with targets of at least 10-point absolute improvements in the proportion of patients with smoking screening, if relevant, counseling, and in the proportion of hypertensive patients with adequately controlled BP. The results were obtained from practices participating in Evidence-NOW, a multisite cardiovascular disease prevention initiative. Configurational Comparative Methods were used to evaluate the joint effects of multiple factors on outcomes.
The majority of practices in the analysis were clinician owned, small (fewer than six clinicians), and/or in an urban location. The researchers sampled and interviewed practice staff from a subset of 104 primary care practices across 7 Cooperatives and 12 states, ranging from small to medium in size, having 10 or fewer clinicians. The interview data were analyzed to identify operational changes, then transformed into numeric data.
Operational changes led to improvements in specific contexts
In clinician-owned practices, process improvement, documentation, and referral to resources, combined with a moderate level of facilitation support, led to an improvement of at least 10 points in smoking outcomes.
However, the researchers found that these patterns were not observed in system–owned practices or Federally Qualified Health Centers.
In solo practices, training medical assistants to take an accurate blood pressure led to an improvement of at least 10 points in blood pressure outcomes.
Among larger, clinician-owned practices, measurement of blood pressure a second time when the first was elevated, and documentation of this reading in the electronic heath record, also led to a 10-point or greater improvement in BP outcome when combined with a large amount (50 hours or more) of facilitation.
“There was no magic bullet for improving smoking cessation counseling and blood pressure outcomes across the diverse primary care practices studied,” lead author Deborah J. Cohen, PhD, of Oregon Health & Science University, Portland, said in an interview. “Combinations of operational changes among practice sizes and types led to improvements.”
Smaller practices more nimble, experts say
Results of the qualitative data analysis suggest that smaller and clinician-owned practices are more likely to have the capacity for change and improvement compared with larger, hospital/health system–owned practices.
Commenting on the study, Noel Deep, MD, regional medical director at Aspirus Clinics, Ironwood, Mich., said solo or small private practices have a distinct advantage over larger hospital or system-owned practices when implementing new operational changes to improve clinical outcomes.
“A smaller independent practice is nimble, with the physician [or physicians] able to make a quick decision at analyzing the scientific data, planning the changes, implementing them quickly, and doing a rapid cycle review of the results and tweaking the program to attain the targets,” said Dr. Deep, a member of the editorial advisory board of Internal Medicine News.
Kate Rowland, MD, MS, assistant professor in the department of family medicine at Rush Medical College, Chicago, also noted that smaller practices have unique advantages over larger health organizations.
“Larger organizations should replicate the benefits of the smaller office, providing as much local decision-making and autonomy as possible to the site where the changes are happening,” Dr. Rowland explained in an interview.
“The clinicians at these sites are mostly likely to know what is going to be successful for achieving measurable change in the patients they care for,” she added.
The study was funded by the Agency for Healthcare Research and Quality. The authors and other experts interviewed for this piece reported having no conflicts of interest.
FROM ANNALS OF FAMILY MEDICINE
Carbon monoxide diffusion with COPD declines more in women
Single breath diffusion capacity for carbon monoxide shows greater decline over time in COPD patients compared with controls, but declines significantly more in women compared with men, according to data from 602 adults with a history of smoking.
In previous studies, diffusion capacity for carbon monoxide (DLco) has been associated with decreased exercise capacity and poor health status in patients with COPD, but its association as a measure of disease progression has not been well studied, wrote Ciro Casanova, MD, of Hospital Universitario La Candelaria, Spain, and colleagues.
In a study published in the journal CHEST®, the researchers identified 506 adult smokers with COPD and 96 adult smoker controls without COPD. Lung function based on single breath DLco was measured each year for 5 years. The study population was part of the COPH History Assessment in SpaiN (CHAIN), an ongoing observational study of adults with COPD. COPD was defined as a history of at least 10 pack-years of smoking and a post-bronchodilator FEV1/FVC greater than 0.7 after 400 micrograms of albuterol, the researchers said.
During the 5-year period, the average overall annual decline in DLco was 1.34% in COPD patients, compared with .04% in non-COPD controls (P = .004). Among COPD patients, age, body mass index, FEV1%, and active smoking were not associated with longitudinal change in DLco values, the researchers said.
Notably, women with COPD at baseline had lower baseline DLco values compared with men (11.37%) and a significantly steeper decline in DLco (.89%) compared with men (P = .039). “Being a woman was the only factor that related to the annual rate of change in DLco,” the researchers said.
In a subgroup analysis, the researchers identified 305 COPD patients and 69 non-COPD controls who had at least 3 DLco measurements over the 5-year study period. In this group, 16.4% patients with COPD and 4.3% smokers without COPD showed significant yearly declines in DLco of –4.139% and –4.440%, respectively. Among COPD patients, significantly more women than men showed significant DLco declines (26% vs. 14%, P = .005). No significant differences were observed in mortality or hospitalizations per patient-year for COPD patients with and without DLco decline, the researchers said.
The study findings were limited by several factors including the lack of annual measurements of DLco among some patients, potential variability in the instruments used to measure DLco, and the absence of computerized tomography data for the chest, the researchers noted. However, the results support the value of the test for COPD progression when conducted at 3- to 4-year intervals, given the slow pace of the decline, they said. More research is needed, but “women seem to have a different susceptibility to cigarette smoke in the alveolar or pulmonary vascular domains,” they added.
DLco remains a valuable marker
The study is important because the usual longitudinal decline of diffusion capacity, an important physiological parameter in patients with COPD, was unknown, Juan P. de Torres, MD, of Queen’s University, Kingston, Ont., said in an interview.
“The finding of a different longitudinal decline of DLco in women was a surprise,” said Dr. de Torres, who was a coauthor on the study. “We knew from previous works from our group that COPD has a different clinical and prognostic behavior in women with COPD, but this specific finding is novel and important,” he said.
“These results provide information about the testing frequency (3-4 years) needed to use DLco as a marker of COPD progression in clinical practice,” Dr. de Torres added.
“What is the driving cause of this sex difference is unknown. We speculate that different causes of low DLco in COPD such as degree of emphysema, interstitial lung abnormalities, and pulmonary hypertension, may have a different prevalence and progression in women with COPD,” he said.
Looking ahead, “Large studies including an adequate sample of women with COPD is urgently needed because they will be the main face of COPD in the near future,” said Dr. de Torres. “Sex difference in their physiological characteristics, the reason to explain those differences and how they behave longitudinally is also urgently needed,” he added.
The study was supported in part by AstraZeneca and by the COPD research program of the Spanish Respiratory Society. The researchers and Dr. de Torres had no financial conflicts to disclose.
Single breath diffusion capacity for carbon monoxide shows greater decline over time in COPD patients compared with controls, but declines significantly more in women compared with men, according to data from 602 adults with a history of smoking.
In previous studies, diffusion capacity for carbon monoxide (DLco) has been associated with decreased exercise capacity and poor health status in patients with COPD, but its association as a measure of disease progression has not been well studied, wrote Ciro Casanova, MD, of Hospital Universitario La Candelaria, Spain, and colleagues.
In a study published in the journal CHEST®, the researchers identified 506 adult smokers with COPD and 96 adult smoker controls without COPD. Lung function based on single breath DLco was measured each year for 5 years. The study population was part of the COPH History Assessment in SpaiN (CHAIN), an ongoing observational study of adults with COPD. COPD was defined as a history of at least 10 pack-years of smoking and a post-bronchodilator FEV1/FVC greater than 0.7 after 400 micrograms of albuterol, the researchers said.
During the 5-year period, the average overall annual decline in DLco was 1.34% in COPD patients, compared with .04% in non-COPD controls (P = .004). Among COPD patients, age, body mass index, FEV1%, and active smoking were not associated with longitudinal change in DLco values, the researchers said.
Notably, women with COPD at baseline had lower baseline DLco values compared with men (11.37%) and a significantly steeper decline in DLco (.89%) compared with men (P = .039). “Being a woman was the only factor that related to the annual rate of change in DLco,” the researchers said.
In a subgroup analysis, the researchers identified 305 COPD patients and 69 non-COPD controls who had at least 3 DLco measurements over the 5-year study period. In this group, 16.4% patients with COPD and 4.3% smokers without COPD showed significant yearly declines in DLco of –4.139% and –4.440%, respectively. Among COPD patients, significantly more women than men showed significant DLco declines (26% vs. 14%, P = .005). No significant differences were observed in mortality or hospitalizations per patient-year for COPD patients with and without DLco decline, the researchers said.
The study findings were limited by several factors including the lack of annual measurements of DLco among some patients, potential variability in the instruments used to measure DLco, and the absence of computerized tomography data for the chest, the researchers noted. However, the results support the value of the test for COPD progression when conducted at 3- to 4-year intervals, given the slow pace of the decline, they said. More research is needed, but “women seem to have a different susceptibility to cigarette smoke in the alveolar or pulmonary vascular domains,” they added.
DLco remains a valuable marker
The study is important because the usual longitudinal decline of diffusion capacity, an important physiological parameter in patients with COPD, was unknown, Juan P. de Torres, MD, of Queen’s University, Kingston, Ont., said in an interview.
“The finding of a different longitudinal decline of DLco in women was a surprise,” said Dr. de Torres, who was a coauthor on the study. “We knew from previous works from our group that COPD has a different clinical and prognostic behavior in women with COPD, but this specific finding is novel and important,” he said.
“These results provide information about the testing frequency (3-4 years) needed to use DLco as a marker of COPD progression in clinical practice,” Dr. de Torres added.
“What is the driving cause of this sex difference is unknown. We speculate that different causes of low DLco in COPD such as degree of emphysema, interstitial lung abnormalities, and pulmonary hypertension, may have a different prevalence and progression in women with COPD,” he said.
Looking ahead, “Large studies including an adequate sample of women with COPD is urgently needed because they will be the main face of COPD in the near future,” said Dr. de Torres. “Sex difference in their physiological characteristics, the reason to explain those differences and how they behave longitudinally is also urgently needed,” he added.
The study was supported in part by AstraZeneca and by the COPD research program of the Spanish Respiratory Society. The researchers and Dr. de Torres had no financial conflicts to disclose.
Single breath diffusion capacity for carbon monoxide shows greater decline over time in COPD patients compared with controls, but declines significantly more in women compared with men, according to data from 602 adults with a history of smoking.
In previous studies, diffusion capacity for carbon monoxide (DLco) has been associated with decreased exercise capacity and poor health status in patients with COPD, but its association as a measure of disease progression has not been well studied, wrote Ciro Casanova, MD, of Hospital Universitario La Candelaria, Spain, and colleagues.
In a study published in the journal CHEST®, the researchers identified 506 adult smokers with COPD and 96 adult smoker controls without COPD. Lung function based on single breath DLco was measured each year for 5 years. The study population was part of the COPH History Assessment in SpaiN (CHAIN), an ongoing observational study of adults with COPD. COPD was defined as a history of at least 10 pack-years of smoking and a post-bronchodilator FEV1/FVC greater than 0.7 after 400 micrograms of albuterol, the researchers said.
During the 5-year period, the average overall annual decline in DLco was 1.34% in COPD patients, compared with .04% in non-COPD controls (P = .004). Among COPD patients, age, body mass index, FEV1%, and active smoking were not associated with longitudinal change in DLco values, the researchers said.
Notably, women with COPD at baseline had lower baseline DLco values compared with men (11.37%) and a significantly steeper decline in DLco (.89%) compared with men (P = .039). “Being a woman was the only factor that related to the annual rate of change in DLco,” the researchers said.
In a subgroup analysis, the researchers identified 305 COPD patients and 69 non-COPD controls who had at least 3 DLco measurements over the 5-year study period. In this group, 16.4% patients with COPD and 4.3% smokers without COPD showed significant yearly declines in DLco of –4.139% and –4.440%, respectively. Among COPD patients, significantly more women than men showed significant DLco declines (26% vs. 14%, P = .005). No significant differences were observed in mortality or hospitalizations per patient-year for COPD patients with and without DLco decline, the researchers said.
The study findings were limited by several factors including the lack of annual measurements of DLco among some patients, potential variability in the instruments used to measure DLco, and the absence of computerized tomography data for the chest, the researchers noted. However, the results support the value of the test for COPD progression when conducted at 3- to 4-year intervals, given the slow pace of the decline, they said. More research is needed, but “women seem to have a different susceptibility to cigarette smoke in the alveolar or pulmonary vascular domains,” they added.
DLco remains a valuable marker
The study is important because the usual longitudinal decline of diffusion capacity, an important physiological parameter in patients with COPD, was unknown, Juan P. de Torres, MD, of Queen’s University, Kingston, Ont., said in an interview.
“The finding of a different longitudinal decline of DLco in women was a surprise,” said Dr. de Torres, who was a coauthor on the study. “We knew from previous works from our group that COPD has a different clinical and prognostic behavior in women with COPD, but this specific finding is novel and important,” he said.
“These results provide information about the testing frequency (3-4 years) needed to use DLco as a marker of COPD progression in clinical practice,” Dr. de Torres added.
“What is the driving cause of this sex difference is unknown. We speculate that different causes of low DLco in COPD such as degree of emphysema, interstitial lung abnormalities, and pulmonary hypertension, may have a different prevalence and progression in women with COPD,” he said.
Looking ahead, “Large studies including an adequate sample of women with COPD is urgently needed because they will be the main face of COPD in the near future,” said Dr. de Torres. “Sex difference in their physiological characteristics, the reason to explain those differences and how they behave longitudinally is also urgently needed,” he added.
The study was supported in part by AstraZeneca and by the COPD research program of the Spanish Respiratory Society. The researchers and Dr. de Torres had no financial conflicts to disclose.
FROM CHEST